User login
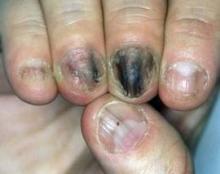
Several Conditions Mimic Nail Fungus in Children
As a pediatric dermatologist, Dr. Robert A. Silverman is all too familiar with this scenario: A child is referred to him with a diagnosis of nail fungus, and the parents are frustrated that the oral antifungal agents did not work.
What bothers him the most is not the antifungals, but that the patients didn’t need them to begin with because the child didn’t have a fungal infection, he said at the annual Hawaii Dermatology Seminar, sponsored by Skin Disease Education Foundation (SDEF). Several conditions of the nail in children can easily be mistaken for fungal infections.
Dr. Silverman of the department of pediatrics at Georgetown University Medical Center in Washington discussed how to distinguish fungal disease mimics from other pediatric nail conditions in children.
Onychomycosis, the most common nail infection in adults, is not all that common in children, Dr. Silverman said. Studies have shown the prevalence in children to be less than 3% in developed countries, although it is increased among children who have Down syndrome and HIV, or children from households with moccasintype Trichophyton rubrum. Clinical variants are similar to those that occur in adults, such as white superficial onychomycosis, distal lateral subungual onychomycosis, proximal subungual onychomycosis, and endonyx onychomycosis.
Other conditions that may be mistaken for fungal infections include:
• Psoriasis. Nail findings in patients who have psoriasis can be misinterpreted as fungal disease. Telltale signs of psoriasis in the nails, however, are large, irregular pits and the oil spot sign.
• Subungual tumors. These include subungual exostosis and onychomatricoma. These benign growths push on the skin surface, leading to separation of the nail. "Some people think fungus when it’s really a tumor of the underlying bone," Dr. Silverman said.
• Pachyonychia congenita. Though often confused with fungal disease, this condition, which involves a single thickened toenail, is somewhat rare. "If someone came in with a thick toenail, I’d culture him or her," Dr. Silverman said. "If the culture is negative, then you have to start thinking of these other conditions."
• Alopecia areata. Children may have nail signs of alopecia areata before hair loss occurs. In alopecia areata, the nail surface is studded to near confluence with tiny pits, also known as Scotch plaid nails. Also, the nail will have lost its luster and has a sandpaperlike texture.
"If you see what looks like alopecia areata of the nail, but don’t see any hair findings, you ought to scrape the nail to rule out fungus, because fungus can look like alopecia areata of the nails," Dr. Silverman said. "And, of course, then you would want to treat it."
Treatment for fungal infections requires the use of an oral agent for 6-12 weeks, so Dr. Silverman emphasized the importance of obtaining a culture. "If you’re going to treat someone for that length of time, it makes sense to know exactly what you’re treating," he said.
Also, Dr. Silverman said, any time he sees that a child’s parent appears to have a fungal infection, he considers that to be a red flag when trying to diagnose the patient.
As a pediatric dermatologist, Dr. Robert A. Silverman is all too familiar with this scenario: A child is referred to him with a diagnosis of nail fungus, and the parents are frustrated that the oral antifungal agents did not work.
What bothers him the most is not the antifungals, but that the patients didn’t need them to begin with because the child didn’t have a fungal infection, he said at the annual Hawaii Dermatology Seminar, sponsored by Skin Disease Education Foundation (SDEF). Several conditions of the nail in children can easily be mistaken for fungal infections.
Dr. Silverman of the department of pediatrics at Georgetown University Medical Center in Washington discussed how to distinguish fungal disease mimics from other pediatric nail conditions in children.
Onychomycosis, the most common nail infection in adults, is not all that common in children, Dr. Silverman said. Studies have shown the prevalence in children to be less than 3% in developed countries, although it is increased among children who have Down syndrome and HIV, or children from households with moccasintype Trichophyton rubrum. Clinical variants are similar to those that occur in adults, such as white superficial onychomycosis, distal lateral subungual onychomycosis, proximal subungual onychomycosis, and endonyx onychomycosis.
Other conditions that may be mistaken for fungal infections include:
• Psoriasis. Nail findings in patients who have psoriasis can be misinterpreted as fungal disease. Telltale signs of psoriasis in the nails, however, are large, irregular pits and the oil spot sign.
• Subungual tumors. These include subungual exostosis and onychomatricoma. These benign growths push on the skin surface, leading to separation of the nail. "Some people think fungus when it’s really a tumor of the underlying bone," Dr. Silverman said.
• Pachyonychia congenita. Though often confused with fungal disease, this condition, which involves a single thickened toenail, is somewhat rare. "If someone came in with a thick toenail, I’d culture him or her," Dr. Silverman said. "If the culture is negative, then you have to start thinking of these other conditions."
• Alopecia areata. Children may have nail signs of alopecia areata before hair loss occurs. In alopecia areata, the nail surface is studded to near confluence with tiny pits, also known as Scotch plaid nails. Also, the nail will have lost its luster and has a sandpaperlike texture.
"If you see what looks like alopecia areata of the nail, but don’t see any hair findings, you ought to scrape the nail to rule out fungus, because fungus can look like alopecia areata of the nails," Dr. Silverman said. "And, of course, then you would want to treat it."
Treatment for fungal infections requires the use of an oral agent for 6-12 weeks, so Dr. Silverman emphasized the importance of obtaining a culture. "If you’re going to treat someone for that length of time, it makes sense to know exactly what you’re treating," he said.
Also, Dr. Silverman said, any time he sees that a child’s parent appears to have a fungal infection, he considers that to be a red flag when trying to diagnose the patient.
As a pediatric dermatologist, Dr. Robert A. Silverman is all too familiar with this scenario: A child is referred to him with a diagnosis of nail fungus, and the parents are frustrated that the oral antifungal agents did not work.
What bothers him the most is not the antifungals, but that the patients didn’t need them to begin with because the child didn’t have a fungal infection, he said at the annual Hawaii Dermatology Seminar, sponsored by Skin Disease Education Foundation (SDEF). Several conditions of the nail in children can easily be mistaken for fungal infections.
Dr. Silverman of the department of pediatrics at Georgetown University Medical Center in Washington discussed how to distinguish fungal disease mimics from other pediatric nail conditions in children.
Onychomycosis, the most common nail infection in adults, is not all that common in children, Dr. Silverman said. Studies have shown the prevalence in children to be less than 3% in developed countries, although it is increased among children who have Down syndrome and HIV, or children from households with moccasintype Trichophyton rubrum. Clinical variants are similar to those that occur in adults, such as white superficial onychomycosis, distal lateral subungual onychomycosis, proximal subungual onychomycosis, and endonyx onychomycosis.
Other conditions that may be mistaken for fungal infections include:
• Psoriasis. Nail findings in patients who have psoriasis can be misinterpreted as fungal disease. Telltale signs of psoriasis in the nails, however, are large, irregular pits and the oil spot sign.
• Subungual tumors. These include subungual exostosis and onychomatricoma. These benign growths push on the skin surface, leading to separation of the nail. "Some people think fungus when it’s really a tumor of the underlying bone," Dr. Silverman said.
• Pachyonychia congenita. Though often confused with fungal disease, this condition, which involves a single thickened toenail, is somewhat rare. "If someone came in with a thick toenail, I’d culture him or her," Dr. Silverman said. "If the culture is negative, then you have to start thinking of these other conditions."
• Alopecia areata. Children may have nail signs of alopecia areata before hair loss occurs. In alopecia areata, the nail surface is studded to near confluence with tiny pits, also known as Scotch plaid nails. Also, the nail will have lost its luster and has a sandpaperlike texture.
"If you see what looks like alopecia areata of the nail, but don’t see any hair findings, you ought to scrape the nail to rule out fungus, because fungus can look like alopecia areata of the nails," Dr. Silverman said. "And, of course, then you would want to treat it."
Treatment for fungal infections requires the use of an oral agent for 6-12 weeks, so Dr. Silverman emphasized the importance of obtaining a culture. "If you’re going to treat someone for that length of time, it makes sense to know exactly what you’re treating," he said.
Also, Dr. Silverman said, any time he sees that a child’s parent appears to have a fungal infection, he considers that to be a red flag when trying to diagnose the patient.
EXPERT ANALYSIS FROM THE SDEF HAWAII DERMATOLOGY SEMINAR
Blog: Top 10 Stories of 2011
For those of you who have had a busy year and haven't had the chance to regularly read the latest dermatology news on Skin and Allergy News Digital Network, we have you covered. As we ring in the new year, here's a rundown of last year's most-viewed stories:
10. Experts: Medical Dermatology Is Losing Ground, By Bruce Jancin: Experts in medical dermatology predicted the specialty will become narrower and less medically oriented by 2020. As we enter 2012, some experts said they were concerned about the emphasis on aesthetic dermatology and dermatologic surgery.
9. Mohs Surgery in Medicare Patients Skyrocketing, By Sherry Boschert: Several Mohs surgery experts found that the rate of Mohs surgery per 1,000 Medicare beneficiaries increased by 236% between 1999 and 2009. Dr. Matthew Donaldson and his associates presented the data at the annual meeting of the American College of Mohs Surgery.
8. Blog: New Isotretinoin Drug May Address Safety Concerns, By Amy Pfeiffer: This much-viewed blog post highlighted an investigational isotretinoin drug that may eliminate safety concerns associated with the drug, like IBD and depression. The gelatin capsules of CIP-iisotretinoin help reduce GI irritation and the drug is less food dependent.
7. Dosing Isotretinoin: Go Big to Avoid Second Course, By Jeffrey Eisenberg: In another isotretinoin study, investigators found that patients receiving a higher cumulative dose of the drug were no less likely to experience an acne relapse than those who received a lower cumulative dose. However, the investigators found that patients treated with a higher dose were less likely to need a second course of treatment.
6. Knifelike Vulvar Ulcers May Signal Crohn's Disease, By Kate Johnson: Knifelike vulvar ulcers could be a sign of Crohn's disease in women, according to experts at a conference on vulvovaginal diseases. For some patients, ulcers may be the only manifestation of the disorder.
5. Biologics Up Cardiovascular Risk, New Analysis Finds, By Sherry Boschert: Biologic therapies used to treat psoriasis have been linked to an increase in major cardiovascular events, according to researchers. One patient on placebo developed a major cardiovascular event in a study of etanercept. Five patients on ustekinumab, five on briakinumab, and one on adalimumab also developed major cardiovascular events.
4. Future Technologies Hold Promise for Hair Restoration, By Damian McNamara: At an annual meeting of dermatologic surgeons, Dr. Ricardo Mejia discussed technological advancements in hair restoration. He said the future for hair restoration could include technologies like robotic hair transfer, hair cloning, and technologies to optimize new growth.
3. AAD: Potential Doxycycline, IBD Link Considered Worrisome, By Bruce Jancin: In more acne news, a retrospective cohort study linked tetracycline-class antibiotics with an increase in inflammatory bowel disease. The highly controversial findings were one of the hottest topics at the annual meeting of the American Academy of Dermatology and on this website.
2. Bimatoprost Repigments Vitiligo Patient Skin, By Bruce Jancin: A topical bimatoprost ophthalmic solution could serve as treatment for focal vitiligo, according to a pilot study presented at the World Congress of Dermatology. Researchers said 7 out of 10 patients exhibited pronounced repigmentation after 2 months of treatment.
1. Marijuana Allergies "Fairly Common," Expert Says, By M. Alexander Otto: A heads up to physicians: allergy experts said marijuana allergies are more common than most people think. Patients with with a marijuana allergy exhibit symptoms including wheezing, sinusitis, throat swelling, and inhalation issues.
Best wishes for 2012!
-- Frances Correa (FMCReporting)
For those of you who have had a busy year and haven't had the chance to regularly read the latest dermatology news on Skin and Allergy News Digital Network, we have you covered. As we ring in the new year, here's a rundown of last year's most-viewed stories:
10. Experts: Medical Dermatology Is Losing Ground, By Bruce Jancin: Experts in medical dermatology predicted the specialty will become narrower and less medically oriented by 2020. As we enter 2012, some experts said they were concerned about the emphasis on aesthetic dermatology and dermatologic surgery.
9. Mohs Surgery in Medicare Patients Skyrocketing, By Sherry Boschert: Several Mohs surgery experts found that the rate of Mohs surgery per 1,000 Medicare beneficiaries increased by 236% between 1999 and 2009. Dr. Matthew Donaldson and his associates presented the data at the annual meeting of the American College of Mohs Surgery.
8. Blog: New Isotretinoin Drug May Address Safety Concerns, By Amy Pfeiffer: This much-viewed blog post highlighted an investigational isotretinoin drug that may eliminate safety concerns associated with the drug, like IBD and depression. The gelatin capsules of CIP-iisotretinoin help reduce GI irritation and the drug is less food dependent.
7. Dosing Isotretinoin: Go Big to Avoid Second Course, By Jeffrey Eisenberg: In another isotretinoin study, investigators found that patients receiving a higher cumulative dose of the drug were no less likely to experience an acne relapse than those who received a lower cumulative dose. However, the investigators found that patients treated with a higher dose were less likely to need a second course of treatment.
6. Knifelike Vulvar Ulcers May Signal Crohn's Disease, By Kate Johnson: Knifelike vulvar ulcers could be a sign of Crohn's disease in women, according to experts at a conference on vulvovaginal diseases. For some patients, ulcers may be the only manifestation of the disorder.
5. Biologics Up Cardiovascular Risk, New Analysis Finds, By Sherry Boschert: Biologic therapies used to treat psoriasis have been linked to an increase in major cardiovascular events, according to researchers. One patient on placebo developed a major cardiovascular event in a study of etanercept. Five patients on ustekinumab, five on briakinumab, and one on adalimumab also developed major cardiovascular events.
4. Future Technologies Hold Promise for Hair Restoration, By Damian McNamara: At an annual meeting of dermatologic surgeons, Dr. Ricardo Mejia discussed technological advancements in hair restoration. He said the future for hair restoration could include technologies like robotic hair transfer, hair cloning, and technologies to optimize new growth.
3. AAD: Potential Doxycycline, IBD Link Considered Worrisome, By Bruce Jancin: In more acne news, a retrospective cohort study linked tetracycline-class antibiotics with an increase in inflammatory bowel disease. The highly controversial findings were one of the hottest topics at the annual meeting of the American Academy of Dermatology and on this website.
2. Bimatoprost Repigments Vitiligo Patient Skin, By Bruce Jancin: A topical bimatoprost ophthalmic solution could serve as treatment for focal vitiligo, according to a pilot study presented at the World Congress of Dermatology. Researchers said 7 out of 10 patients exhibited pronounced repigmentation after 2 months of treatment.
1. Marijuana Allergies "Fairly Common," Expert Says, By M. Alexander Otto: A heads up to physicians: allergy experts said marijuana allergies are more common than most people think. Patients with with a marijuana allergy exhibit symptoms including wheezing, sinusitis, throat swelling, and inhalation issues.
Best wishes for 2012!
-- Frances Correa (FMCReporting)
For those of you who have had a busy year and haven't had the chance to regularly read the latest dermatology news on Skin and Allergy News Digital Network, we have you covered. As we ring in the new year, here's a rundown of last year's most-viewed stories:
10. Experts: Medical Dermatology Is Losing Ground, By Bruce Jancin: Experts in medical dermatology predicted the specialty will become narrower and less medically oriented by 2020. As we enter 2012, some experts said they were concerned about the emphasis on aesthetic dermatology and dermatologic surgery.
9. Mohs Surgery in Medicare Patients Skyrocketing, By Sherry Boschert: Several Mohs surgery experts found that the rate of Mohs surgery per 1,000 Medicare beneficiaries increased by 236% between 1999 and 2009. Dr. Matthew Donaldson and his associates presented the data at the annual meeting of the American College of Mohs Surgery.
8. Blog: New Isotretinoin Drug May Address Safety Concerns, By Amy Pfeiffer: This much-viewed blog post highlighted an investigational isotretinoin drug that may eliminate safety concerns associated with the drug, like IBD and depression. The gelatin capsules of CIP-iisotretinoin help reduce GI irritation and the drug is less food dependent.
7. Dosing Isotretinoin: Go Big to Avoid Second Course, By Jeffrey Eisenberg: In another isotretinoin study, investigators found that patients receiving a higher cumulative dose of the drug were no less likely to experience an acne relapse than those who received a lower cumulative dose. However, the investigators found that patients treated with a higher dose were less likely to need a second course of treatment.
6. Knifelike Vulvar Ulcers May Signal Crohn's Disease, By Kate Johnson: Knifelike vulvar ulcers could be a sign of Crohn's disease in women, according to experts at a conference on vulvovaginal diseases. For some patients, ulcers may be the only manifestation of the disorder.
5. Biologics Up Cardiovascular Risk, New Analysis Finds, By Sherry Boschert: Biologic therapies used to treat psoriasis have been linked to an increase in major cardiovascular events, according to researchers. One patient on placebo developed a major cardiovascular event in a study of etanercept. Five patients on ustekinumab, five on briakinumab, and one on adalimumab also developed major cardiovascular events.
4. Future Technologies Hold Promise for Hair Restoration, By Damian McNamara: At an annual meeting of dermatologic surgeons, Dr. Ricardo Mejia discussed technological advancements in hair restoration. He said the future for hair restoration could include technologies like robotic hair transfer, hair cloning, and technologies to optimize new growth.
3. AAD: Potential Doxycycline, IBD Link Considered Worrisome, By Bruce Jancin: In more acne news, a retrospective cohort study linked tetracycline-class antibiotics with an increase in inflammatory bowel disease. The highly controversial findings were one of the hottest topics at the annual meeting of the American Academy of Dermatology and on this website.
2. Bimatoprost Repigments Vitiligo Patient Skin, By Bruce Jancin: A topical bimatoprost ophthalmic solution could serve as treatment for focal vitiligo, according to a pilot study presented at the World Congress of Dermatology. Researchers said 7 out of 10 patients exhibited pronounced repigmentation after 2 months of treatment.
1. Marijuana Allergies "Fairly Common," Expert Says, By M. Alexander Otto: A heads up to physicians: allergy experts said marijuana allergies are more common than most people think. Patients with with a marijuana allergy exhibit symptoms including wheezing, sinusitis, throat swelling, and inhalation issues.
Best wishes for 2012!
-- Frances Correa (FMCReporting)
Pulsed Dye Laser Zaps Nail Psoriasis in Small Study
LISBON – Pulsed dye laser therapy may be an attractive new option for treating nail psoriasis, according to Dr. Veronique Blatiere.
Nail psoriasis is challenging to treat because the psoriatic disease process damages the nails while they are still being formed. But Turkish investigators have reported positive results with three once-monthly pulsed dye laser (PDL) treatment sessions in a small uncontrolled patient series, Dr. Blatiere reported at the annual congress of the European Academy of Dermatology and Venereology.
Dr. Yasemin Oram and coworkers at the American Hospital in Istanbul, Turkey, reported on five patients with nail psoriasis treated using PDL. The laser therapy was applied at 595 nm with a pulse duration of 1.5 milliseconds, a beam diameter of 7 mm, and an energy fluence of 8-10 J/cm2. A treatment session was continued until a purple discoloration appeared.
The hypothesized mechanism of action involves destruction of the abnormal vasculature, according to the investigators (Dermatol. Surg. 2010;36:377-81).
Nail bed lesions, particularly onycholysis and subungual hyperkeratosis, responded to PDL better than did nail matrix lesions. After three treatment sessions, the average Nail Psoriasis Severity Index (NAPSI) score for nail bed lesions dropped from 14.8 to 8.
While the Turkish report is certainly encouraging, it should be viewed as a proof of concept pilot study, said Dr. Blatiere of Saint Eloi University Hospital in Montpellier, France. It needs confirmation with larger numbers of patients, a control arm, and blinded investigator assessment.
Dr. Blatiere noted that interest has been mounting in evaluating biologic agents for nail psoriasis. Favorable clinical experiences, albeit all of them open label, have recently been reported for the use of infliximab (J. Eur. Acad. Dermatol. Venereol. 2011;25:549-53); adalimumab (J. Eur. Acad. Dermatol. Venereol. 2010;24:530-4); ustekinumab (Arch. Dermatol. 2010;146:1315-6); and etanercept for nail psoriasis (J. Eur. Acad. Dermatol. Venereol. 2009;23:896-904).
But important questions remain about biologics for nail psoriasis, such as the appropriate duration of treatment, length of response, and whether they will help prevent psoriatic arthritis. And then there are the still incompletely answered questions regarding the long-term safety of the agents, as well as the issue of their considerable expense, Dr. Blatiere said.
She reported having no relevant financial disclosures.
psoriatic disease, nail damage, PDL, the European Academy of Dermatology and Venereology, Dr. Yasemin Oram Nail Psoriasis Severity Index, NAPSI, nail bed lesions
LISBON – Pulsed dye laser therapy may be an attractive new option for treating nail psoriasis, according to Dr. Veronique Blatiere.
Nail psoriasis is challenging to treat because the psoriatic disease process damages the nails while they are still being formed. But Turkish investigators have reported positive results with three once-monthly pulsed dye laser (PDL) treatment sessions in a small uncontrolled patient series, Dr. Blatiere reported at the annual congress of the European Academy of Dermatology and Venereology.
Dr. Yasemin Oram and coworkers at the American Hospital in Istanbul, Turkey, reported on five patients with nail psoriasis treated using PDL. The laser therapy was applied at 595 nm with a pulse duration of 1.5 milliseconds, a beam diameter of 7 mm, and an energy fluence of 8-10 J/cm2. A treatment session was continued until a purple discoloration appeared.
The hypothesized mechanism of action involves destruction of the abnormal vasculature, according to the investigators (Dermatol. Surg. 2010;36:377-81).
Nail bed lesions, particularly onycholysis and subungual hyperkeratosis, responded to PDL better than did nail matrix lesions. After three treatment sessions, the average Nail Psoriasis Severity Index (NAPSI) score for nail bed lesions dropped from 14.8 to 8.
While the Turkish report is certainly encouraging, it should be viewed as a proof of concept pilot study, said Dr. Blatiere of Saint Eloi University Hospital in Montpellier, France. It needs confirmation with larger numbers of patients, a control arm, and blinded investigator assessment.
Dr. Blatiere noted that interest has been mounting in evaluating biologic agents for nail psoriasis. Favorable clinical experiences, albeit all of them open label, have recently been reported for the use of infliximab (J. Eur. Acad. Dermatol. Venereol. 2011;25:549-53); adalimumab (J. Eur. Acad. Dermatol. Venereol. 2010;24:530-4); ustekinumab (Arch. Dermatol. 2010;146:1315-6); and etanercept for nail psoriasis (J. Eur. Acad. Dermatol. Venereol. 2009;23:896-904).
But important questions remain about biologics for nail psoriasis, such as the appropriate duration of treatment, length of response, and whether they will help prevent psoriatic arthritis. And then there are the still incompletely answered questions regarding the long-term safety of the agents, as well as the issue of their considerable expense, Dr. Blatiere said.
She reported having no relevant financial disclosures.
LISBON – Pulsed dye laser therapy may be an attractive new option for treating nail psoriasis, according to Dr. Veronique Blatiere.
Nail psoriasis is challenging to treat because the psoriatic disease process damages the nails while they are still being formed. But Turkish investigators have reported positive results with three once-monthly pulsed dye laser (PDL) treatment sessions in a small uncontrolled patient series, Dr. Blatiere reported at the annual congress of the European Academy of Dermatology and Venereology.
Dr. Yasemin Oram and coworkers at the American Hospital in Istanbul, Turkey, reported on five patients with nail psoriasis treated using PDL. The laser therapy was applied at 595 nm with a pulse duration of 1.5 milliseconds, a beam diameter of 7 mm, and an energy fluence of 8-10 J/cm2. A treatment session was continued until a purple discoloration appeared.
The hypothesized mechanism of action involves destruction of the abnormal vasculature, according to the investigators (Dermatol. Surg. 2010;36:377-81).
Nail bed lesions, particularly onycholysis and subungual hyperkeratosis, responded to PDL better than did nail matrix lesions. After three treatment sessions, the average Nail Psoriasis Severity Index (NAPSI) score for nail bed lesions dropped from 14.8 to 8.
While the Turkish report is certainly encouraging, it should be viewed as a proof of concept pilot study, said Dr. Blatiere of Saint Eloi University Hospital in Montpellier, France. It needs confirmation with larger numbers of patients, a control arm, and blinded investigator assessment.
Dr. Blatiere noted that interest has been mounting in evaluating biologic agents for nail psoriasis. Favorable clinical experiences, albeit all of them open label, have recently been reported for the use of infliximab (J. Eur. Acad. Dermatol. Venereol. 2011;25:549-53); adalimumab (J. Eur. Acad. Dermatol. Venereol. 2010;24:530-4); ustekinumab (Arch. Dermatol. 2010;146:1315-6); and etanercept for nail psoriasis (J. Eur. Acad. Dermatol. Venereol. 2009;23:896-904).
But important questions remain about biologics for nail psoriasis, such as the appropriate duration of treatment, length of response, and whether they will help prevent psoriatic arthritis. And then there are the still incompletely answered questions regarding the long-term safety of the agents, as well as the issue of their considerable expense, Dr. Blatiere said.
She reported having no relevant financial disclosures.
psoriatic disease, nail damage, PDL, the European Academy of Dermatology and Venereology, Dr. Yasemin Oram Nail Psoriasis Severity Index, NAPSI, nail bed lesions
psoriatic disease, nail damage, PDL, the European Academy of Dermatology and Venereology, Dr. Yasemin Oram Nail Psoriasis Severity Index, NAPSI, nail bed lesions
EXPERT ANALYSIS FROM THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Instant Glue Thwarts Onychotillomania
LISBON – Applying a fast-drying cyanoacrylate glue to the proximal nail fold once or twice weekly is an inexpensive and effective treatment for the habit-tic condition of onychotillomania.
Onychotillomania is often categorized as a compulsive psychiatric disorder. The same psychiatric medications employed in cases of obsessive-compulsive disorder, including selective serotonin reuptake inhibitors, are sometimes prescribed to good effect.
Gluing the problem nail – it is most often a thumbnail – can work, and at negligible cost, with low risk, and no risk of systemic side effects stemming from psychiatric medications, Dr. Veronique Blatiere said at the annual congress of the European Academy of Dermatology and Venereology.
The likely mechanisms of benefit from the cyanoacrylate glue, such as Super Glue or Krazy Glue, are twofold: the built-up layer of glue creates a physical barrier that helps protect the proximal nail fold against the patient’s mindless repetitive picking, and, at the same time, the artificial layer promotes self-awareness of the tic habit, said Dr. Blatiere, a dermatologist at the University of Montpellier (France).
She credited the instant nail-gluing therapy to Dr. Daniel S. Ring, a Chesterfield, Mo., dermatologist who presented the approach in the Archives of Dermatology (2010;146:1222-3).
Dr. Ring described onychotillomania as a common condition given little attention in most dermatologic textbooks. Onset is typically in adulthood, and patients frequently bring up the problem as an "oh, by the way" afterthought during an office visit scheduled for another reason.
Patients typically display parallel transverse ridges running from the proximal nail fold to the distal nail plate along with a lack of cuticle. These changes result from years of picking at the cuticle or pushing it back.
In his report, Dr. Ring described two cases in detail and mentioned 10 others successfully treated with glue. The affected nails typically normalized after 3-6 months of weekly gluing.
Dr. Blatiere said that instant-drying cyanoacrylate glues may also be worth investigating for the treatment of chronic paronychia.
However, she noted that a potential hazard of applying this therapy for months at a time is development of an allergic contact dermatitis to the acrylate.
Neither Dr. Ring nor Dr. Blatiere reported having any financial conflicts.
LISBON – Applying a fast-drying cyanoacrylate glue to the proximal nail fold once or twice weekly is an inexpensive and effective treatment for the habit-tic condition of onychotillomania.
Onychotillomania is often categorized as a compulsive psychiatric disorder. The same psychiatric medications employed in cases of obsessive-compulsive disorder, including selective serotonin reuptake inhibitors, are sometimes prescribed to good effect.
Gluing the problem nail – it is most often a thumbnail – can work, and at negligible cost, with low risk, and no risk of systemic side effects stemming from psychiatric medications, Dr. Veronique Blatiere said at the annual congress of the European Academy of Dermatology and Venereology.
The likely mechanisms of benefit from the cyanoacrylate glue, such as Super Glue or Krazy Glue, are twofold: the built-up layer of glue creates a physical barrier that helps protect the proximal nail fold against the patient’s mindless repetitive picking, and, at the same time, the artificial layer promotes self-awareness of the tic habit, said Dr. Blatiere, a dermatologist at the University of Montpellier (France).
She credited the instant nail-gluing therapy to Dr. Daniel S. Ring, a Chesterfield, Mo., dermatologist who presented the approach in the Archives of Dermatology (2010;146:1222-3).
Dr. Ring described onychotillomania as a common condition given little attention in most dermatologic textbooks. Onset is typically in adulthood, and patients frequently bring up the problem as an "oh, by the way" afterthought during an office visit scheduled for another reason.
Patients typically display parallel transverse ridges running from the proximal nail fold to the distal nail plate along with a lack of cuticle. These changes result from years of picking at the cuticle or pushing it back.
In his report, Dr. Ring described two cases in detail and mentioned 10 others successfully treated with glue. The affected nails typically normalized after 3-6 months of weekly gluing.
Dr. Blatiere said that instant-drying cyanoacrylate glues may also be worth investigating for the treatment of chronic paronychia.
However, she noted that a potential hazard of applying this therapy for months at a time is development of an allergic contact dermatitis to the acrylate.
Neither Dr. Ring nor Dr. Blatiere reported having any financial conflicts.
LISBON – Applying a fast-drying cyanoacrylate glue to the proximal nail fold once or twice weekly is an inexpensive and effective treatment for the habit-tic condition of onychotillomania.
Onychotillomania is often categorized as a compulsive psychiatric disorder. The same psychiatric medications employed in cases of obsessive-compulsive disorder, including selective serotonin reuptake inhibitors, are sometimes prescribed to good effect.
Gluing the problem nail – it is most often a thumbnail – can work, and at negligible cost, with low risk, and no risk of systemic side effects stemming from psychiatric medications, Dr. Veronique Blatiere said at the annual congress of the European Academy of Dermatology and Venereology.
The likely mechanisms of benefit from the cyanoacrylate glue, such as Super Glue or Krazy Glue, are twofold: the built-up layer of glue creates a physical barrier that helps protect the proximal nail fold against the patient’s mindless repetitive picking, and, at the same time, the artificial layer promotes self-awareness of the tic habit, said Dr. Blatiere, a dermatologist at the University of Montpellier (France).
She credited the instant nail-gluing therapy to Dr. Daniel S. Ring, a Chesterfield, Mo., dermatologist who presented the approach in the Archives of Dermatology (2010;146:1222-3).
Dr. Ring described onychotillomania as a common condition given little attention in most dermatologic textbooks. Onset is typically in adulthood, and patients frequently bring up the problem as an "oh, by the way" afterthought during an office visit scheduled for another reason.
Patients typically display parallel transverse ridges running from the proximal nail fold to the distal nail plate along with a lack of cuticle. These changes result from years of picking at the cuticle or pushing it back.
In his report, Dr. Ring described two cases in detail and mentioned 10 others successfully treated with glue. The affected nails typically normalized after 3-6 months of weekly gluing.
Dr. Blatiere said that instant-drying cyanoacrylate glues may also be worth investigating for the treatment of chronic paronychia.
However, she noted that a potential hazard of applying this therapy for months at a time is development of an allergic contact dermatitis to the acrylate.
Neither Dr. Ring nor Dr. Blatiere reported having any financial conflicts.
EXPERT ANALYSIS FROM THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Hair Weathering, Part 2: Clinical Features, Diagnosis, Prevention, and Treatment
Skin of Color: Advances in Laser Hair Removal
In the November issue of Journal of Drugs in Dermatology (2011;10:1235-9), Dr. Eliot F. Battle Jr., gives an excellent review of "Advances in Laser Hair Removal in Skin of Color."
Dr. Battle summarizes that "Laser hair removal, previously contraindicated in patients with ethnically dark (phototypes IV-VI) or sun-tanned skin, is now recognized as a safe and effective method of permanent hair reduction in all patients. Longer wavelengths, conservative fluences, longer pulse durations and appropriate cooling methods are necessary to minimize untoward side effects and maximize efficacy. The longer wavelength Nd:YAG laser is considered safest in treating darker skin of color. An added benefit of laser epilation is that side effects of conventional hair removal such as pseudo-folliculitis barbae and post inflammatory dyspigmentation, more commonly seen in skin of color, may also respond favorably to the laser, thus increasing the potential for patient satisfaction."
The mechanism of laser hair reduction (LHR) is based on the theory of selective photothermolysis, whereby thermal injury to a desired chromophore can be achieved with the appropriate wavelength, pulse duration, and fluence.
In LHR, the target chromophore is the pigment in the hair follicle and bulb. However, Dr. Battle notes that destruction of the non-pigmented progenitor stem cells is also required to achieve permanent hair reduction. Therefore, a modified theory of selective photothermolysis has been proposed for the mechanism of LHR where appropriate wavelengths, as well as longer pulse durations, must be used to allow heat to effectively destroy the melanocytic hair follicle and bulb, as well as the amelanotic hair follicle and stem cell.
In darker skin types, longer wavelength lasers must be used to bypass absorption of epidermal pigment to prevent untoward side effects of dyspigmentation.
Currently the 810-nm diode and 1064-nm Nd:YAG lasers are Food and Drug Administration approved for skin types IV-VI. The Nd:YAG is inherently the safer of the two devices because of the longer wavelength; however, long pulse durations with the diode laser with appropriate cooling have been shown to increase its safety profile.
Epidermal damage from lasers occurs when the epidermal temperature equals or exceeds 45 degrees Celsius, thus appropriate cooling mechanisms are essential for safe and effective LHR. Excessive cooling, however, can lead to dyspigmentation in darker skin.
Initiating LHR in darker skin should be done conservatively with longer wavelengths, lower fluences, and longer pulse durations. If test spots are performed, it is recommended to wait 48 hours before proceeding with therapy as patients with darker skin may manifest delayed dyspigmentation.
Patients with skin types IV-VI may also be at increased risk for paradoxical hypertrichosis. While it has been reported in most ethnic origins, those of Mediterranean and Pacific Asian descent may be particularly affected. Paradoxical hypertrichosis mainly occurs on the face and neck, and has been reported both within and outside the treatment area. While the exact cause is unknown, possible causes include the effect of inflammatory mediators and subtherapeutic thermal injury causing induction of the hair cycle. Current treatment for paradoxical hypertrichosis is laser therapy of the affected area.
The only contraindications for LHR are gold therapy and St. John’s Wort, which should be discontinued for 3 months prior to therapy. While not contraindicated, LHR is not recommended in pregnant women.
There is no evidence supporting increased LHR side effects in patients recently receiving Accutane; however, until there is more data, it is recommended to wait 3 months after discontinuing Accutane before initiating LHR. Anti-viral prophylaxis may be taken 2-3 days prior to LHR and for 5-7 days after treatment for patients with a history of recurrent herpetic infections in the treatment area.
With each treatment, patients may expect a 10%-20% decrease in hair count, color, and diameter of the hair. In patients of darker color, a minimum of eight treatments may be required to achieve results, with treatments typically scheduled 4-8 weeks apart.
Dr. Battle also noted that LHR not only treats unwanted hair, but also effectively diminishes inflammation and dyspigmentation from pseudofolliculitis barbae and acne keloidalis nuchae, as these conditions are due to ingrown and/or tufted coarse curled hairs in darker skin types.
In the November issue of Journal of Drugs in Dermatology (2011;10:1235-9), Dr. Eliot F. Battle Jr., gives an excellent review of "Advances in Laser Hair Removal in Skin of Color."
Dr. Battle summarizes that "Laser hair removal, previously contraindicated in patients with ethnically dark (phototypes IV-VI) or sun-tanned skin, is now recognized as a safe and effective method of permanent hair reduction in all patients. Longer wavelengths, conservative fluences, longer pulse durations and appropriate cooling methods are necessary to minimize untoward side effects and maximize efficacy. The longer wavelength Nd:YAG laser is considered safest in treating darker skin of color. An added benefit of laser epilation is that side effects of conventional hair removal such as pseudo-folliculitis barbae and post inflammatory dyspigmentation, more commonly seen in skin of color, may also respond favorably to the laser, thus increasing the potential for patient satisfaction."
The mechanism of laser hair reduction (LHR) is based on the theory of selective photothermolysis, whereby thermal injury to a desired chromophore can be achieved with the appropriate wavelength, pulse duration, and fluence.
In LHR, the target chromophore is the pigment in the hair follicle and bulb. However, Dr. Battle notes that destruction of the non-pigmented progenitor stem cells is also required to achieve permanent hair reduction. Therefore, a modified theory of selective photothermolysis has been proposed for the mechanism of LHR where appropriate wavelengths, as well as longer pulse durations, must be used to allow heat to effectively destroy the melanocytic hair follicle and bulb, as well as the amelanotic hair follicle and stem cell.
In darker skin types, longer wavelength lasers must be used to bypass absorption of epidermal pigment to prevent untoward side effects of dyspigmentation.
Currently the 810-nm diode and 1064-nm Nd:YAG lasers are Food and Drug Administration approved for skin types IV-VI. The Nd:YAG is inherently the safer of the two devices because of the longer wavelength; however, long pulse durations with the diode laser with appropriate cooling have been shown to increase its safety profile.
Epidermal damage from lasers occurs when the epidermal temperature equals or exceeds 45 degrees Celsius, thus appropriate cooling mechanisms are essential for safe and effective LHR. Excessive cooling, however, can lead to dyspigmentation in darker skin.
Initiating LHR in darker skin should be done conservatively with longer wavelengths, lower fluences, and longer pulse durations. If test spots are performed, it is recommended to wait 48 hours before proceeding with therapy as patients with darker skin may manifest delayed dyspigmentation.
Patients with skin types IV-VI may also be at increased risk for paradoxical hypertrichosis. While it has been reported in most ethnic origins, those of Mediterranean and Pacific Asian descent may be particularly affected. Paradoxical hypertrichosis mainly occurs on the face and neck, and has been reported both within and outside the treatment area. While the exact cause is unknown, possible causes include the effect of inflammatory mediators and subtherapeutic thermal injury causing induction of the hair cycle. Current treatment for paradoxical hypertrichosis is laser therapy of the affected area.
The only contraindications for LHR are gold therapy and St. John’s Wort, which should be discontinued for 3 months prior to therapy. While not contraindicated, LHR is not recommended in pregnant women.
There is no evidence supporting increased LHR side effects in patients recently receiving Accutane; however, until there is more data, it is recommended to wait 3 months after discontinuing Accutane before initiating LHR. Anti-viral prophylaxis may be taken 2-3 days prior to LHR and for 5-7 days after treatment for patients with a history of recurrent herpetic infections in the treatment area.
With each treatment, patients may expect a 10%-20% decrease in hair count, color, and diameter of the hair. In patients of darker color, a minimum of eight treatments may be required to achieve results, with treatments typically scheduled 4-8 weeks apart.
Dr. Battle also noted that LHR not only treats unwanted hair, but also effectively diminishes inflammation and dyspigmentation from pseudofolliculitis barbae and acne keloidalis nuchae, as these conditions are due to ingrown and/or tufted coarse curled hairs in darker skin types.
In the November issue of Journal of Drugs in Dermatology (2011;10:1235-9), Dr. Eliot F. Battle Jr., gives an excellent review of "Advances in Laser Hair Removal in Skin of Color."
Dr. Battle summarizes that "Laser hair removal, previously contraindicated in patients with ethnically dark (phototypes IV-VI) or sun-tanned skin, is now recognized as a safe and effective method of permanent hair reduction in all patients. Longer wavelengths, conservative fluences, longer pulse durations and appropriate cooling methods are necessary to minimize untoward side effects and maximize efficacy. The longer wavelength Nd:YAG laser is considered safest in treating darker skin of color. An added benefit of laser epilation is that side effects of conventional hair removal such as pseudo-folliculitis barbae and post inflammatory dyspigmentation, more commonly seen in skin of color, may also respond favorably to the laser, thus increasing the potential for patient satisfaction."
The mechanism of laser hair reduction (LHR) is based on the theory of selective photothermolysis, whereby thermal injury to a desired chromophore can be achieved with the appropriate wavelength, pulse duration, and fluence.
In LHR, the target chromophore is the pigment in the hair follicle and bulb. However, Dr. Battle notes that destruction of the non-pigmented progenitor stem cells is also required to achieve permanent hair reduction. Therefore, a modified theory of selective photothermolysis has been proposed for the mechanism of LHR where appropriate wavelengths, as well as longer pulse durations, must be used to allow heat to effectively destroy the melanocytic hair follicle and bulb, as well as the amelanotic hair follicle and stem cell.
In darker skin types, longer wavelength lasers must be used to bypass absorption of epidermal pigment to prevent untoward side effects of dyspigmentation.
Currently the 810-nm diode and 1064-nm Nd:YAG lasers are Food and Drug Administration approved for skin types IV-VI. The Nd:YAG is inherently the safer of the two devices because of the longer wavelength; however, long pulse durations with the diode laser with appropriate cooling have been shown to increase its safety profile.
Epidermal damage from lasers occurs when the epidermal temperature equals or exceeds 45 degrees Celsius, thus appropriate cooling mechanisms are essential for safe and effective LHR. Excessive cooling, however, can lead to dyspigmentation in darker skin.
Initiating LHR in darker skin should be done conservatively with longer wavelengths, lower fluences, and longer pulse durations. If test spots are performed, it is recommended to wait 48 hours before proceeding with therapy as patients with darker skin may manifest delayed dyspigmentation.
Patients with skin types IV-VI may also be at increased risk for paradoxical hypertrichosis. While it has been reported in most ethnic origins, those of Mediterranean and Pacific Asian descent may be particularly affected. Paradoxical hypertrichosis mainly occurs on the face and neck, and has been reported both within and outside the treatment area. While the exact cause is unknown, possible causes include the effect of inflammatory mediators and subtherapeutic thermal injury causing induction of the hair cycle. Current treatment for paradoxical hypertrichosis is laser therapy of the affected area.
The only contraindications for LHR are gold therapy and St. John’s Wort, which should be discontinued for 3 months prior to therapy. While not contraindicated, LHR is not recommended in pregnant women.
There is no evidence supporting increased LHR side effects in patients recently receiving Accutane; however, until there is more data, it is recommended to wait 3 months after discontinuing Accutane before initiating LHR. Anti-viral prophylaxis may be taken 2-3 days prior to LHR and for 5-7 days after treatment for patients with a history of recurrent herpetic infections in the treatment area.
With each treatment, patients may expect a 10%-20% decrease in hair count, color, and diameter of the hair. In patients of darker color, a minimum of eight treatments may be required to achieve results, with treatments typically scheduled 4-8 weeks apart.
Dr. Battle also noted that LHR not only treats unwanted hair, but also effectively diminishes inflammation and dyspigmentation from pseudofolliculitis barbae and acne keloidalis nuchae, as these conditions are due to ingrown and/or tufted coarse curled hairs in darker skin types.
Hair Weathering, Part 1: Hair Structure and Pathogenesis
Hair Stylists Report Looking for Suspicious Lesions
Hair stylists are on the lookout for suspicious lesions on their customers’ scalps, necks, and faces, and are "very" interested in receiving formal skin cancer education, according to a study published Oct. 17 in the Archives of Dermatology.
Although only 28% of hair professionals have received any formal skin cancer education, many reported routinely looking for problematic spots or changing moles, according to Dr. Elizabeth E. Bailey of the department of medicine at Brigham and Women’s Hospital in Boston.
Dr. Bailey and her colleagues received 203 completed surveys from hair professionals in January 2010 on skin cancer practice and knowledge. The hair professionals were from a chain of 17 salons in the greater Houston area.
Hair stylists who frequently talked with their customers about health issues, including personal skin protection practices, were more likely to scan for suspicious lesions. However, it didn’t seem to matter whether or not the stylists had basic skin cancer knowledge, possibly because most already knew the basics, the investigators reported (Arch. Dermatol. 2011;147:1159-65).
According to the study, about 90% agreed or strongly agreed that a customer should see a health professional for a mole that is changing in size or frequently bleeds. A total of 89% said customers should see a health professional if they have a mole that is changing in color, and 78% said moles that itch frequently should be checked out.
A total of 37% of the hair professionals surveyed had scanned more than half of their customers’ scalps, 29% had scanned more than half of their customers’ necks, and 15% had scanned more than half of their customers’ faces for suspicious lesions in the past month.
Survey participants who knew the ABCD rule for melanoma were more likely to look at customers’ skin, and "understanding the difference between melanoma and ordinary skin growths and disagreeing that skin cancer was more difficult to detect than other types of cancer was also associated with a higher likelihood of customer observation," the investigators wrote.
Hair stylists who were confident looking at their own moles also tended to look at their customers’ skin, and stylists who had a personal history of skin cancer or experience with a friend or family member’s skin cancer also looked for potential problem spots on customers’ skin more often, the study found.
About half (49%) of survey participants said they were "very" or "extremely" interested in participating in a skin cancer education program, indicating that dermatologists should consider investigating hair stylists’ potential role in skin cancer prevention and detection.
"Hair professionals are currently acting as lay health advisors for skin cancer detection and prevention and are willing to become more involved in skin cancer education in the salon," said the investigators. "As professionals who have a natural view of difficult-to-see areas and who develop a close rapport with their customers, hair professionals are ideally suited to this role."
Melanoma of the scalp and neck accounted for 6% of all melanoma and for 10% of melanoma deaths in the United States between 1973 and 2003, likely because it’s difficult to find suspicious lesions in these locations during self-examinations by patients and routine exams by physicians, the investigators noted.
Therefore, hair stylists – who typically see areas of the head and scalp that patients and physicians might miss – are in a unique position to detect skin cancers that might otherwise go unnoticed.
"Through the many active professional education venues within the hair industry, the infrastructure exists to educate them," wrote Dr. Bailey and her colleagues. "Future research should focus on creating a program that provides hair professionals with expert training and effective health communication tools to become confident and skilled lay skin cancer educators."
The investigators did not report having any conflicts of interest.
Hair stylists are on the lookout for suspicious lesions on their customers’ scalps, necks, and faces, and are "very" interested in receiving formal skin cancer education, according to a study published Oct. 17 in the Archives of Dermatology.
Although only 28% of hair professionals have received any formal skin cancer education, many reported routinely looking for problematic spots or changing moles, according to Dr. Elizabeth E. Bailey of the department of medicine at Brigham and Women’s Hospital in Boston.
Dr. Bailey and her colleagues received 203 completed surveys from hair professionals in January 2010 on skin cancer practice and knowledge. The hair professionals were from a chain of 17 salons in the greater Houston area.
Hair stylists who frequently talked with their customers about health issues, including personal skin protection practices, were more likely to scan for suspicious lesions. However, it didn’t seem to matter whether or not the stylists had basic skin cancer knowledge, possibly because most already knew the basics, the investigators reported (Arch. Dermatol. 2011;147:1159-65).
According to the study, about 90% agreed or strongly agreed that a customer should see a health professional for a mole that is changing in size or frequently bleeds. A total of 89% said customers should see a health professional if they have a mole that is changing in color, and 78% said moles that itch frequently should be checked out.
A total of 37% of the hair professionals surveyed had scanned more than half of their customers’ scalps, 29% had scanned more than half of their customers’ necks, and 15% had scanned more than half of their customers’ faces for suspicious lesions in the past month.
Survey participants who knew the ABCD rule for melanoma were more likely to look at customers’ skin, and "understanding the difference between melanoma and ordinary skin growths and disagreeing that skin cancer was more difficult to detect than other types of cancer was also associated with a higher likelihood of customer observation," the investigators wrote.
Hair stylists who were confident looking at their own moles also tended to look at their customers’ skin, and stylists who had a personal history of skin cancer or experience with a friend or family member’s skin cancer also looked for potential problem spots on customers’ skin more often, the study found.
About half (49%) of survey participants said they were "very" or "extremely" interested in participating in a skin cancer education program, indicating that dermatologists should consider investigating hair stylists’ potential role in skin cancer prevention and detection.
"Hair professionals are currently acting as lay health advisors for skin cancer detection and prevention and are willing to become more involved in skin cancer education in the salon," said the investigators. "As professionals who have a natural view of difficult-to-see areas and who develop a close rapport with their customers, hair professionals are ideally suited to this role."
Melanoma of the scalp and neck accounted for 6% of all melanoma and for 10% of melanoma deaths in the United States between 1973 and 2003, likely because it’s difficult to find suspicious lesions in these locations during self-examinations by patients and routine exams by physicians, the investigators noted.
Therefore, hair stylists – who typically see areas of the head and scalp that patients and physicians might miss – are in a unique position to detect skin cancers that might otherwise go unnoticed.
"Through the many active professional education venues within the hair industry, the infrastructure exists to educate them," wrote Dr. Bailey and her colleagues. "Future research should focus on creating a program that provides hair professionals with expert training and effective health communication tools to become confident and skilled lay skin cancer educators."
The investigators did not report having any conflicts of interest.
Hair stylists are on the lookout for suspicious lesions on their customers’ scalps, necks, and faces, and are "very" interested in receiving formal skin cancer education, according to a study published Oct. 17 in the Archives of Dermatology.
Although only 28% of hair professionals have received any formal skin cancer education, many reported routinely looking for problematic spots or changing moles, according to Dr. Elizabeth E. Bailey of the department of medicine at Brigham and Women’s Hospital in Boston.
Dr. Bailey and her colleagues received 203 completed surveys from hair professionals in January 2010 on skin cancer practice and knowledge. The hair professionals were from a chain of 17 salons in the greater Houston area.
Hair stylists who frequently talked with their customers about health issues, including personal skin protection practices, were more likely to scan for suspicious lesions. However, it didn’t seem to matter whether or not the stylists had basic skin cancer knowledge, possibly because most already knew the basics, the investigators reported (Arch. Dermatol. 2011;147:1159-65).
According to the study, about 90% agreed or strongly agreed that a customer should see a health professional for a mole that is changing in size or frequently bleeds. A total of 89% said customers should see a health professional if they have a mole that is changing in color, and 78% said moles that itch frequently should be checked out.
A total of 37% of the hair professionals surveyed had scanned more than half of their customers’ scalps, 29% had scanned more than half of their customers’ necks, and 15% had scanned more than half of their customers’ faces for suspicious lesions in the past month.
Survey participants who knew the ABCD rule for melanoma were more likely to look at customers’ skin, and "understanding the difference between melanoma and ordinary skin growths and disagreeing that skin cancer was more difficult to detect than other types of cancer was also associated with a higher likelihood of customer observation," the investigators wrote.
Hair stylists who were confident looking at their own moles also tended to look at their customers’ skin, and stylists who had a personal history of skin cancer or experience with a friend or family member’s skin cancer also looked for potential problem spots on customers’ skin more often, the study found.
About half (49%) of survey participants said they were "very" or "extremely" interested in participating in a skin cancer education program, indicating that dermatologists should consider investigating hair stylists’ potential role in skin cancer prevention and detection.
"Hair professionals are currently acting as lay health advisors for skin cancer detection and prevention and are willing to become more involved in skin cancer education in the salon," said the investigators. "As professionals who have a natural view of difficult-to-see areas and who develop a close rapport with their customers, hair professionals are ideally suited to this role."
Melanoma of the scalp and neck accounted for 6% of all melanoma and for 10% of melanoma deaths in the United States between 1973 and 2003, likely because it’s difficult to find suspicious lesions in these locations during self-examinations by patients and routine exams by physicians, the investigators noted.
Therefore, hair stylists – who typically see areas of the head and scalp that patients and physicians might miss – are in a unique position to detect skin cancers that might otherwise go unnoticed.
"Through the many active professional education venues within the hair industry, the infrastructure exists to educate them," wrote Dr. Bailey and her colleagues. "Future research should focus on creating a program that provides hair professionals with expert training and effective health communication tools to become confident and skilled lay skin cancer educators."
The investigators did not report having any conflicts of interest.
FROM ARCHIVES OF DERMATOLOGY
Major Finding: A total of 37% of the hair professionals surveyed had scanned more than half of their customers’ scalps, 29% had scanned more than half of their customers’ necks, and 15% had scanned more than half of their customers’ faces for suspicious lesions in the past month.
Data Source: Survey of hair professionals (n = 203) from a chain of 17 salons in the greater Houston area, conducted in January 2010.
Disclosures: The investigators did not report having any conflicts of interest.
Genetic Basis of Alopecia Areata Leads to Abatacept Trial
PHILADELPHIA – New findings that show T-cell activation plays a critical role in the development of alopecia areata has opened new doors to treatment.
A report last year from a genome-wide association study involving 1,054 patients with alopecia areata (AA) and more than 3,000 controls, identified eight genes strongly linked to the disease (Nature 2010;466:113-7). One of the gene’s codes for a ligand, ULBP3, appears in the dermal sheath of hair follicles in patients with AA. The ULBP3 ligand appears responsible for attracting the cluster of T cells that produce the characteristic histopathology of affected hair follicles, Angela M. Christiano, Ph.D., said at the meeting.
"Normally the hair follicle is immune privileged, but when the ULBP3 ligand is increased, T cells attack" the follicle and cause its destruction and the hair loss that is pathognomonic for AA, said Dr. Christiano, professor of dermatology and of genetics and development at Columbia University Medical Center in New York.
The ULBP3 finding led to a search for possible treatments that could interfere with the T-cell attack, guiding Dr. Christiano and her associates to the drug abatacept (Orencia). The agent suppresses T-cell activation and activity and is approved for treating rheumatoid arthritis (RA) and juvenile idiopathic arthritis. Study results reported almost a decade ago showed that abatacept worked in a mouse model of AA, she said.
Testing of a drug such as abatacept represents a new direction for AA treatment, which until now has usually been treated with agents developed for psoriasis, a strategy that has been unsuccessful.
Dr. Christiano and her coinvestigators designed a pilot study to test the efficacy of abatacept in patients with moderately severe AA, 6-12 months after diagnosis. They set these parameters because the patients will have established disease that is unlikely to spontaneously remit, but not so severe as to be too advanced to respond to T-cell based treatment.
Their planned study will randomize 56 patients to either a subcutaneous injection of abatacept or placebo at baseline, weeks 2 and 4, and then every 4 weeks for five cycles for a total treatment duration of 6 months. The study’s primary endpoint will be a 30%-40% improvement on the severity of alopecia tool after the first 6 months of treatment, and then after an additional 6 months of untreated follow-up, she said in an interview.
"I don’t think we could have been more shocked by what we found" in the genetic study, said Dr. Christiano at the meeting, sponsored by the Drug Information Association. "We fully expected to be aligned with other skin autoimmune diseases, like psoriasis." Instead, the eight genes linked to AA closely overlapped with type 1 diabetes, RA, and celiac disease, disorders that "we never considered."
But like the ULBP3 ligand found in the hair-follicle dermal sheaths of patients with AA, these autoimmune diseases also feature upregulated ligands that attract T cells to cellular targets and cause the disease: synoviocytes in RA, gut epithelial cells in celiac disease, and pancreatic islet cells in a mouse model of type 1 diabetes.
Dr. Christiano said that she had no disclosures.
PHILADELPHIA – New findings that show T-cell activation plays a critical role in the development of alopecia areata has opened new doors to treatment.
A report last year from a genome-wide association study involving 1,054 patients with alopecia areata (AA) and more than 3,000 controls, identified eight genes strongly linked to the disease (Nature 2010;466:113-7). One of the gene’s codes for a ligand, ULBP3, appears in the dermal sheath of hair follicles in patients with AA. The ULBP3 ligand appears responsible for attracting the cluster of T cells that produce the characteristic histopathology of affected hair follicles, Angela M. Christiano, Ph.D., said at the meeting.
"Normally the hair follicle is immune privileged, but when the ULBP3 ligand is increased, T cells attack" the follicle and cause its destruction and the hair loss that is pathognomonic for AA, said Dr. Christiano, professor of dermatology and of genetics and development at Columbia University Medical Center in New York.
The ULBP3 finding led to a search for possible treatments that could interfere with the T-cell attack, guiding Dr. Christiano and her associates to the drug abatacept (Orencia). The agent suppresses T-cell activation and activity and is approved for treating rheumatoid arthritis (RA) and juvenile idiopathic arthritis. Study results reported almost a decade ago showed that abatacept worked in a mouse model of AA, she said.
Testing of a drug such as abatacept represents a new direction for AA treatment, which until now has usually been treated with agents developed for psoriasis, a strategy that has been unsuccessful.
Dr. Christiano and her coinvestigators designed a pilot study to test the efficacy of abatacept in patients with moderately severe AA, 6-12 months after diagnosis. They set these parameters because the patients will have established disease that is unlikely to spontaneously remit, but not so severe as to be too advanced to respond to T-cell based treatment.
Their planned study will randomize 56 patients to either a subcutaneous injection of abatacept or placebo at baseline, weeks 2 and 4, and then every 4 weeks for five cycles for a total treatment duration of 6 months. The study’s primary endpoint will be a 30%-40% improvement on the severity of alopecia tool after the first 6 months of treatment, and then after an additional 6 months of untreated follow-up, she said in an interview.
"I don’t think we could have been more shocked by what we found" in the genetic study, said Dr. Christiano at the meeting, sponsored by the Drug Information Association. "We fully expected to be aligned with other skin autoimmune diseases, like psoriasis." Instead, the eight genes linked to AA closely overlapped with type 1 diabetes, RA, and celiac disease, disorders that "we never considered."
But like the ULBP3 ligand found in the hair-follicle dermal sheaths of patients with AA, these autoimmune diseases also feature upregulated ligands that attract T cells to cellular targets and cause the disease: synoviocytes in RA, gut epithelial cells in celiac disease, and pancreatic islet cells in a mouse model of type 1 diabetes.
Dr. Christiano said that she had no disclosures.
PHILADELPHIA – New findings that show T-cell activation plays a critical role in the development of alopecia areata has opened new doors to treatment.
A report last year from a genome-wide association study involving 1,054 patients with alopecia areata (AA) and more than 3,000 controls, identified eight genes strongly linked to the disease (Nature 2010;466:113-7). One of the gene’s codes for a ligand, ULBP3, appears in the dermal sheath of hair follicles in patients with AA. The ULBP3 ligand appears responsible for attracting the cluster of T cells that produce the characteristic histopathology of affected hair follicles, Angela M. Christiano, Ph.D., said at the meeting.
"Normally the hair follicle is immune privileged, but when the ULBP3 ligand is increased, T cells attack" the follicle and cause its destruction and the hair loss that is pathognomonic for AA, said Dr. Christiano, professor of dermatology and of genetics and development at Columbia University Medical Center in New York.
The ULBP3 finding led to a search for possible treatments that could interfere with the T-cell attack, guiding Dr. Christiano and her associates to the drug abatacept (Orencia). The agent suppresses T-cell activation and activity and is approved for treating rheumatoid arthritis (RA) and juvenile idiopathic arthritis. Study results reported almost a decade ago showed that abatacept worked in a mouse model of AA, she said.
Testing of a drug such as abatacept represents a new direction for AA treatment, which until now has usually been treated with agents developed for psoriasis, a strategy that has been unsuccessful.
Dr. Christiano and her coinvestigators designed a pilot study to test the efficacy of abatacept in patients with moderately severe AA, 6-12 months after diagnosis. They set these parameters because the patients will have established disease that is unlikely to spontaneously remit, but not so severe as to be too advanced to respond to T-cell based treatment.
Their planned study will randomize 56 patients to either a subcutaneous injection of abatacept or placebo at baseline, weeks 2 and 4, and then every 4 weeks for five cycles for a total treatment duration of 6 months. The study’s primary endpoint will be a 30%-40% improvement on the severity of alopecia tool after the first 6 months of treatment, and then after an additional 6 months of untreated follow-up, she said in an interview.
"I don’t think we could have been more shocked by what we found" in the genetic study, said Dr. Christiano at the meeting, sponsored by the Drug Information Association. "We fully expected to be aligned with other skin autoimmune diseases, like psoriasis." Instead, the eight genes linked to AA closely overlapped with type 1 diabetes, RA, and celiac disease, disorders that "we never considered."
But like the ULBP3 ligand found in the hair-follicle dermal sheaths of patients with AA, these autoimmune diseases also feature upregulated ligands that attract T cells to cellular targets and cause the disease: synoviocytes in RA, gut epithelial cells in celiac disease, and pancreatic islet cells in a mouse model of type 1 diabetes.
Dr. Christiano said that she had no disclosures.
EXPERT ANALYSIS FROM A MEETING ON IMPROVING CLINICAL TRIAL SAMPLING FOR FUTURE RESEARCH