User login
Scrubbing Often During Nail Removal Reduces Infection Rates
LAS VEGAS – Preoperative scrubs before nail avulsion fail to reduce postoperative infection, recent data suggest.
However, studies have identified best practices for reducing bacterial counts – such as irrigating after nail plate avulsion though there is no evidence that the measures reduce postoperative infection rates.
"Every study that has looked at rates of colonization or recolonization after your scrub showed significant bacterial presence. As good as our scrubs are, they're probably not good enough," Dr. Nathaniel J. Jellinek said at the annual meeting of the American College of Mohs Surgery.
A recent study measured bacterial counts after a 7-minute surgical scrub and again after avulsion of the nail plate. The bacterial counts were essentially the same. "It's as if the surgical scrub didn't do any good," said Dr. Jellinek, who was not involved in the study (Dermatol. Surg. 2010;36:1258-65).
The investigators then irrigated the nail bed with saline, which reduced bacterial counts by 95%. That's "an easy thing to do intraoperatively that you might consider for your practice," said Dr. Jellinek of the department of dermatology at Brown University, Providence, R.I.
Studies published mainly in the podiatric and orthopedic literature show that postoperative infection rates are higher after nail surgery than after skin surgery. Rates of bacterial colonization before and after surgical preparation of nails make the term "sterile surgery" inaccurate for these procedures, which might better be considered clean-contaminated or even contaminated surgery, he said in an interview after his presentation.
Other studies have shown that applying alcohol and chlorhexidine may be superior to chloroxylenol and povidone-iodine to reduce bacterial counts before nail surgery. Bacterial persistence and recolonization also can be reduced by the use of bristled brushes, soaked gauze pads, scrubbing to the interdigital webs, and repeat scrubs.
"At this point, it's all hypothetical whether it decreases infection. But we know that infection rates are unacceptably high, so it can only help if you decrease the bacterial count," Dr. Jellinek said.
He has added multiple prophylactic measures to his practice, where he performs a lot of nail surgery.
First, Dr. Jellinek informs the patient that the nail is a dirty site, he reviews wound care, and educates the patient to pay attention to risk factors for infection and avoid putting fingers or toes in dirty places.
He recommends strict sterile preoperative and intraoperative techniques. A nurse or medical assistant should scrub the surgical area for several minutes. "We're talking not 10 or 30 seconds, but 2, 3, 5 minutes," he said.
The scrub should use multiple agents. He prefers using a bristle brush to scrub with chlorhexidine, gauze pads soaked in 70% isopropyl alcohol, and maybe even povidone-iodine paint. He lets all that sit, and applies a sterile glove over the digit, hand, or foot after the scrub.
During surgery, once the nail plate is avulsed, he recommends either performing a repeat scrub or at least irrigating the nail bed with sterile saline.
Dr. Jellinek said recent data have lowered his threshold for using prophylactic antibiotics. He has come to appreciate that nail infections may be caused by different organisms than those that cause most skin infections and require different antibiotics for treatment.
As a result, "I actually have very few nail infections, but I can't pinpoint which of those five or six things that I've done give me those results," he said.
Studies of prophylactic measures to avoid postoperative nail infections include:
- Preoperative skin and nail preparation of the foot: Comparison of the efficacy of 4 different methods in reducing bacterial load (J. Am. Acad. Dermatol. 2009;61:986-92).
- Chlorhexidine Provides Superior Skin Decontamination in Foot and Ankle Surgery: A Prospective Randomized Study (Clin. Orthop. Relat. Res. 2005;438:204-8).
- Preoperative Skin Preparation of the Foot and Ankle: Bristles and Alcohol Are Better (J. Bone Joint Surg. 2005;87:986-92).
- Efficacy of Surgical Preparation in Solutions in Foot and Ankle Surgery (J. Bone Joint Surg. 2005;87:980-5).
Dr. Jellinek said he had no relevant financial disclosures.
LAS VEGAS – Preoperative scrubs before nail avulsion fail to reduce postoperative infection, recent data suggest.
However, studies have identified best practices for reducing bacterial counts – such as irrigating after nail plate avulsion though there is no evidence that the measures reduce postoperative infection rates.
"Every study that has looked at rates of colonization or recolonization after your scrub showed significant bacterial presence. As good as our scrubs are, they're probably not good enough," Dr. Nathaniel J. Jellinek said at the annual meeting of the American College of Mohs Surgery.
A recent study measured bacterial counts after a 7-minute surgical scrub and again after avulsion of the nail plate. The bacterial counts were essentially the same. "It's as if the surgical scrub didn't do any good," said Dr. Jellinek, who was not involved in the study (Dermatol. Surg. 2010;36:1258-65).
The investigators then irrigated the nail bed with saline, which reduced bacterial counts by 95%. That's "an easy thing to do intraoperatively that you might consider for your practice," said Dr. Jellinek of the department of dermatology at Brown University, Providence, R.I.
Studies published mainly in the podiatric and orthopedic literature show that postoperative infection rates are higher after nail surgery than after skin surgery. Rates of bacterial colonization before and after surgical preparation of nails make the term "sterile surgery" inaccurate for these procedures, which might better be considered clean-contaminated or even contaminated surgery, he said in an interview after his presentation.
Other studies have shown that applying alcohol and chlorhexidine may be superior to chloroxylenol and povidone-iodine to reduce bacterial counts before nail surgery. Bacterial persistence and recolonization also can be reduced by the use of bristled brushes, soaked gauze pads, scrubbing to the interdigital webs, and repeat scrubs.
"At this point, it's all hypothetical whether it decreases infection. But we know that infection rates are unacceptably high, so it can only help if you decrease the bacterial count," Dr. Jellinek said.
He has added multiple prophylactic measures to his practice, where he performs a lot of nail surgery.
First, Dr. Jellinek informs the patient that the nail is a dirty site, he reviews wound care, and educates the patient to pay attention to risk factors for infection and avoid putting fingers or toes in dirty places.
He recommends strict sterile preoperative and intraoperative techniques. A nurse or medical assistant should scrub the surgical area for several minutes. "We're talking not 10 or 30 seconds, but 2, 3, 5 minutes," he said.
The scrub should use multiple agents. He prefers using a bristle brush to scrub with chlorhexidine, gauze pads soaked in 70% isopropyl alcohol, and maybe even povidone-iodine paint. He lets all that sit, and applies a sterile glove over the digit, hand, or foot after the scrub.
During surgery, once the nail plate is avulsed, he recommends either performing a repeat scrub or at least irrigating the nail bed with sterile saline.
Dr. Jellinek said recent data have lowered his threshold for using prophylactic antibiotics. He has come to appreciate that nail infections may be caused by different organisms than those that cause most skin infections and require different antibiotics for treatment.
As a result, "I actually have very few nail infections, but I can't pinpoint which of those five or six things that I've done give me those results," he said.
Studies of prophylactic measures to avoid postoperative nail infections include:
- Preoperative skin and nail preparation of the foot: Comparison of the efficacy of 4 different methods in reducing bacterial load (J. Am. Acad. Dermatol. 2009;61:986-92).
- Chlorhexidine Provides Superior Skin Decontamination in Foot and Ankle Surgery: A Prospective Randomized Study (Clin. Orthop. Relat. Res. 2005;438:204-8).
- Preoperative Skin Preparation of the Foot and Ankle: Bristles and Alcohol Are Better (J. Bone Joint Surg. 2005;87:986-92).
- Efficacy of Surgical Preparation in Solutions in Foot and Ankle Surgery (J. Bone Joint Surg. 2005;87:980-5).
Dr. Jellinek said he had no relevant financial disclosures.
LAS VEGAS – Preoperative scrubs before nail avulsion fail to reduce postoperative infection, recent data suggest.
However, studies have identified best practices for reducing bacterial counts – such as irrigating after nail plate avulsion though there is no evidence that the measures reduce postoperative infection rates.
"Every study that has looked at rates of colonization or recolonization after your scrub showed significant bacterial presence. As good as our scrubs are, they're probably not good enough," Dr. Nathaniel J. Jellinek said at the annual meeting of the American College of Mohs Surgery.
A recent study measured bacterial counts after a 7-minute surgical scrub and again after avulsion of the nail plate. The bacterial counts were essentially the same. "It's as if the surgical scrub didn't do any good," said Dr. Jellinek, who was not involved in the study (Dermatol. Surg. 2010;36:1258-65).
The investigators then irrigated the nail bed with saline, which reduced bacterial counts by 95%. That's "an easy thing to do intraoperatively that you might consider for your practice," said Dr. Jellinek of the department of dermatology at Brown University, Providence, R.I.
Studies published mainly in the podiatric and orthopedic literature show that postoperative infection rates are higher after nail surgery than after skin surgery. Rates of bacterial colonization before and after surgical preparation of nails make the term "sterile surgery" inaccurate for these procedures, which might better be considered clean-contaminated or even contaminated surgery, he said in an interview after his presentation.
Other studies have shown that applying alcohol and chlorhexidine may be superior to chloroxylenol and povidone-iodine to reduce bacterial counts before nail surgery. Bacterial persistence and recolonization also can be reduced by the use of bristled brushes, soaked gauze pads, scrubbing to the interdigital webs, and repeat scrubs.
"At this point, it's all hypothetical whether it decreases infection. But we know that infection rates are unacceptably high, so it can only help if you decrease the bacterial count," Dr. Jellinek said.
He has added multiple prophylactic measures to his practice, where he performs a lot of nail surgery.
First, Dr. Jellinek informs the patient that the nail is a dirty site, he reviews wound care, and educates the patient to pay attention to risk factors for infection and avoid putting fingers or toes in dirty places.
He recommends strict sterile preoperative and intraoperative techniques. A nurse or medical assistant should scrub the surgical area for several minutes. "We're talking not 10 or 30 seconds, but 2, 3, 5 minutes," he said.
The scrub should use multiple agents. He prefers using a bristle brush to scrub with chlorhexidine, gauze pads soaked in 70% isopropyl alcohol, and maybe even povidone-iodine paint. He lets all that sit, and applies a sterile glove over the digit, hand, or foot after the scrub.
During surgery, once the nail plate is avulsed, he recommends either performing a repeat scrub or at least irrigating the nail bed with sterile saline.
Dr. Jellinek said recent data have lowered his threshold for using prophylactic antibiotics. He has come to appreciate that nail infections may be caused by different organisms than those that cause most skin infections and require different antibiotics for treatment.
As a result, "I actually have very few nail infections, but I can't pinpoint which of those five or six things that I've done give me those results," he said.
Studies of prophylactic measures to avoid postoperative nail infections include:
- Preoperative skin and nail preparation of the foot: Comparison of the efficacy of 4 different methods in reducing bacterial load (J. Am. Acad. Dermatol. 2009;61:986-92).
- Chlorhexidine Provides Superior Skin Decontamination in Foot and Ankle Surgery: A Prospective Randomized Study (Clin. Orthop. Relat. Res. 2005;438:204-8).
- Preoperative Skin Preparation of the Foot and Ankle: Bristles and Alcohol Are Better (J. Bone Joint Surg. 2005;87:986-92).
- Efficacy of Surgical Preparation in Solutions in Foot and Ankle Surgery (J. Bone Joint Surg. 2005;87:980-5).
Dr. Jellinek said he had no relevant financial disclosures.
EXPERT ANALYSIS FROM THE ANNUAL MEETING OF THE AMERICAN COLLEGE OF MOHS SURGERY
Curing Acne: The Skinny Podcast
In this month's episode reporters discuss a possible cure for acne based on research being conducted by Dr. R. Rox Anderson.
Dr. Albert C. Yan goes head to head with lice. He talks about a regimen that uses Cetaphil to suffocate the bugs.
Tips for reducing infection rates during nail surgery are given by Dr. Nathaniel Jellinek.
Dr. Lily Talakoub talks all things sunscreen.
And, last but not least, Dr. Alan Rockoff closes this month's episode with a story about a patient with very strong opinions, and he does a little singing too.
In this month's episode reporters discuss a possible cure for acne based on research being conducted by Dr. R. Rox Anderson.
Dr. Albert C. Yan goes head to head with lice. He talks about a regimen that uses Cetaphil to suffocate the bugs.
Tips for reducing infection rates during nail surgery are given by Dr. Nathaniel Jellinek.
Dr. Lily Talakoub talks all things sunscreen.
And, last but not least, Dr. Alan Rockoff closes this month's episode with a story about a patient with very strong opinions, and he does a little singing too.
In this month's episode reporters discuss a possible cure for acne based on research being conducted by Dr. R. Rox Anderson.
Dr. Albert C. Yan goes head to head with lice. He talks about a regimen that uses Cetaphil to suffocate the bugs.
Tips for reducing infection rates during nail surgery are given by Dr. Nathaniel Jellinek.
Dr. Lily Talakoub talks all things sunscreen.
And, last but not least, Dr. Alan Rockoff closes this month's episode with a story about a patient with very strong opinions, and he does a little singing too.
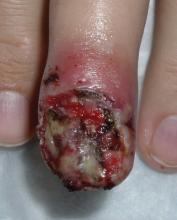
Ischemic Onycholysis of the Hands
House Members Seek Recall of Formaldehyde-Containing Hair Straighteners
Ten members of Congress have written to the Food and Drug Administration asking that the agency recall several brands of hair-straightening products that they say contain formaldehyde.
In a May 6 letter to FDA Commissioner Margaret Hamburg, U.S. House of Representative members wrote that the Brazilian Blowout Solution and Acai Professional Smoothing Solution both contain formaldehyde, but the chemical is not listed on the product labels as an ingredient.
Rep. Earl Blumenauer (D-Ore.) had written to the agency last fall outlining complaints from Oregon hair stylists who had been experiencing acute reactions, such as nosebleeds, while working with the hair straighteners. The congressman also noted testing conducted by the Oregon Occupational Safety and Health Division and by Oregon Health and Science University found the two products contained between 6% and 12% formaldehyde.
He said that the federal Occupational Safety and Health Administration (OSHA) subsequently issued a hazard alert warning salons to steer clear of formaldehyde-based straighteners.
Formaldehyde has been recognized as a possible carcinogen and has been the subject of 47 complaints to the Environmental Working Group, members of Congress noted in the letter.
"It is clear that the FDA needs to take decisive action," they wrote.
In addition to a recall, they are seeking a warning for products that contain formaldehyde, an investigation into the companies that are claiming their products are formaldehyde free, and a review of whether the chemical should be banned from hair straighteners.
According to the May 6 letter to Dr. Hamburg, the FDA responded in late November to Rep. Blumenauer's initial letter and said that it was investigating whether the products were being marketed directly to consumers. "If so, failure to comply with the ingredient declaration requirement would constitute misbranding," responded the agency.
Ten members of Congress have written to the Food and Drug Administration asking that the agency recall several brands of hair-straightening products that they say contain formaldehyde.
In a May 6 letter to FDA Commissioner Margaret Hamburg, U.S. House of Representative members wrote that the Brazilian Blowout Solution and Acai Professional Smoothing Solution both contain formaldehyde, but the chemical is not listed on the product labels as an ingredient.
Rep. Earl Blumenauer (D-Ore.) had written to the agency last fall outlining complaints from Oregon hair stylists who had been experiencing acute reactions, such as nosebleeds, while working with the hair straighteners. The congressman also noted testing conducted by the Oregon Occupational Safety and Health Division and by Oregon Health and Science University found the two products contained between 6% and 12% formaldehyde.
He said that the federal Occupational Safety and Health Administration (OSHA) subsequently issued a hazard alert warning salons to steer clear of formaldehyde-based straighteners.
Formaldehyde has been recognized as a possible carcinogen and has been the subject of 47 complaints to the Environmental Working Group, members of Congress noted in the letter.
"It is clear that the FDA needs to take decisive action," they wrote.
In addition to a recall, they are seeking a warning for products that contain formaldehyde, an investigation into the companies that are claiming their products are formaldehyde free, and a review of whether the chemical should be banned from hair straighteners.
According to the May 6 letter to Dr. Hamburg, the FDA responded in late November to Rep. Blumenauer's initial letter and said that it was investigating whether the products were being marketed directly to consumers. "If so, failure to comply with the ingredient declaration requirement would constitute misbranding," responded the agency.
Ten members of Congress have written to the Food and Drug Administration asking that the agency recall several brands of hair-straightening products that they say contain formaldehyde.
In a May 6 letter to FDA Commissioner Margaret Hamburg, U.S. House of Representative members wrote that the Brazilian Blowout Solution and Acai Professional Smoothing Solution both contain formaldehyde, but the chemical is not listed on the product labels as an ingredient.
Rep. Earl Blumenauer (D-Ore.) had written to the agency last fall outlining complaints from Oregon hair stylists who had been experiencing acute reactions, such as nosebleeds, while working with the hair straighteners. The congressman also noted testing conducted by the Oregon Occupational Safety and Health Division and by Oregon Health and Science University found the two products contained between 6% and 12% formaldehyde.
He said that the federal Occupational Safety and Health Administration (OSHA) subsequently issued a hazard alert warning salons to steer clear of formaldehyde-based straighteners.
Formaldehyde has been recognized as a possible carcinogen and has been the subject of 47 complaints to the Environmental Working Group, members of Congress noted in the letter.
"It is clear that the FDA needs to take decisive action," they wrote.
In addition to a recall, they are seeking a warning for products that contain formaldehyde, an investigation into the companies that are claiming their products are formaldehyde free, and a review of whether the chemical should be banned from hair straighteners.
According to the May 6 letter to Dr. Hamburg, the FDA responded in late November to Rep. Blumenauer's initial letter and said that it was investigating whether the products were being marketed directly to consumers. "If so, failure to comply with the ingredient declaration requirement would constitute misbranding," responded the agency.
The Field Effect [editorial]
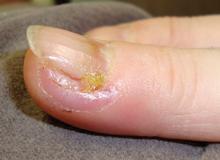
Simple Onycholysis
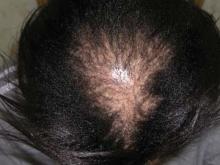
Scarring Alopecia in Black Women Still Not Understood
Despite being the most common pattern of scarring hair loss among African American women, central centrifugal cicatricial alopecia is still poorly understood, according to a report published online April 11 in Archives of Dermatology.
The etiology of and risk factors for the disorder have not been established. And although African American women commonly present with the condition, the exact prevalence remains unknown, said Dr. Angela Kyei and her associates at the Cleveland Clinic Institute of Dermatology and Plastic Surgery.
Central centrifugal cicatricial alopecia (CCCA) – previously known as hot comb alopecia or follicular degeneration syndrome – is a scarring hair loss centered on the vertex of the scalp and spreading peripherally, which is described almost exclusively in African American women. In the late 1960s it was linked to the use of hot combs, but since then almost every method of hair grooming in this population has been associated, albeit weakly, with the disorder. Few studies have examined other possible etiologies, such as immunologic, dermatologic, or genetic factors.
"Given the lack of epidemiologic data, the main goal of [our] study was to elucidate environmental as well as medical risk factors that may be associated with CCCA," the researchers wrote.
They administered questionnaires to 326 African American women attending two churches and a health fair in the Cleveland area. Sixteen of the women ended up being excluded from the analysis.
The questionnaires asked detailed information about family history of male- and female-pattern hair loss; personal medical history, including bacterial and fungal skin infections, autoimmune conditions, and hormonal dysregulation; and hair grooming methods used. The study subjects also underwent a scalp examination that included the grading of hair loss.
A total of 86 of the 310 women (28%) were judged to have central hair loss. Of these, 52 women – 17% of the total study population – had CCCA.
It appeared that hair grooming practices that cause traction, such as weaves and braids, may contribute to the development of CCCA, as women with the most severe central hair loss used these styles more frequently than did those without hair loss. "This has some clinical bearing because traction can clinically produce folliculitis of the scalp, which can cause scarring if this inflammation is prolonged," noted Dr. Kyei and her colleagues (Arch. Dermatol. 2011 April 11 [doi:10.1001/archdermatol.2011.66]).
After paying hundreds of dollars for such hair styling, women often maintain weaves and braids for weeks to months, so any reactive inflammation usually is prolonged, the investigators added.
However, "the relationship between chemical relaxer use and the development of CCCA continues to be murky," they noted. These products clearly weaken the hair shaft, which can cause breakage, but that does not necessarily lead to CCCA. "The fact that most African Americans use chemical relaxers in combination with braiding and other hair grooming practices makes it even more difficult to tease out a relationship," Dr. Kyei and her associates added.
It is difficult to find subjects who do not use chemical relaxers for comparison, since most African Americans begin the practice in childhood. In this study, 91% of the women reported using chemical relaxers, and began doing so at an average age of 10 years.
Nevertheless, "we feel that it is not unreasonable to assume that the scalp may absorb some of the caustic chemicals found in relaxers, which in time leads to damage of the scalp in the form of scarring," they wrote.
Although the prevalence of bacterial skin infections in these study subjects was only 11%, there was a significant elevation among women with CCCA, compared with women who did not have CCCA. The rate of acne also was elevated in affected subjects, but not to a significant degree. Thus, the researchers said that their study "demonstrates that inflammation in the form of bacterial infection and acne may also be contributing to the development of CCCA, a finding consistent with the histopathologic characteristics of this disease," which show a lymphocytic perifollicular infiltrate in its early stages.
In contrast, there was no such association with fungal infections of the scalp, ringworm, or vaginal yeast infections, which was "surprising given how prone this population is to fungal infection," they added.
"One of the most surprising findings ... was the overrepresentation of type 2 [diabetes mellitus] in those with CCCA," the authors noted. Again, the prevalence of type 2 diabetes was low, at 8%, but it was significantly elevated in women with CCCA, compared with those without CCCA.
"These are important data that need further study because CCCA may be a marker of metabolic dysfunction and, when present, can prompt clinicians to do further testing for diabetes mellitus," Dr. Kyei and her colleagues wrote.
Only 9% of the study subjects had thyroid abnormalities, and three-fourths of them had no or minimal hair loss.
A history of male-pattern baldness in the maternal grandfather was found to be a risk factor for CCCA, which suggests that genetics may play a role.
Hormonal dysregulation did not appear to be associated with development of CCCA. Neither were scarring disorders, since only 6% of the subjects reported a history of keloids, and the rate was no higher in women with CCCA than in those without CCCA.
Similarly, there were relatively high prevalences of seborrheic dermatitis (24%), eczema (13%), and contact dermatitis from chemical relaxers (9%), as would be expected in an African American population. However, these conditions were unrelated to CCCA.
This study was supported in part by the North American Hair Research Society and Procter & Gamble. No financial conflicts of interest were reported.
Despite being the most common pattern of scarring hair loss among African American women, central centrifugal cicatricial alopecia is still poorly understood, according to a report published online April 11 in Archives of Dermatology.
The etiology of and risk factors for the disorder have not been established. And although African American women commonly present with the condition, the exact prevalence remains unknown, said Dr. Angela Kyei and her associates at the Cleveland Clinic Institute of Dermatology and Plastic Surgery.
Central centrifugal cicatricial alopecia (CCCA) – previously known as hot comb alopecia or follicular degeneration syndrome – is a scarring hair loss centered on the vertex of the scalp and spreading peripherally, which is described almost exclusively in African American women. In the late 1960s it was linked to the use of hot combs, but since then almost every method of hair grooming in this population has been associated, albeit weakly, with the disorder. Few studies have examined other possible etiologies, such as immunologic, dermatologic, or genetic factors.
"Given the lack of epidemiologic data, the main goal of [our] study was to elucidate environmental as well as medical risk factors that may be associated with CCCA," the researchers wrote.
They administered questionnaires to 326 African American women attending two churches and a health fair in the Cleveland area. Sixteen of the women ended up being excluded from the analysis.
The questionnaires asked detailed information about family history of male- and female-pattern hair loss; personal medical history, including bacterial and fungal skin infections, autoimmune conditions, and hormonal dysregulation; and hair grooming methods used. The study subjects also underwent a scalp examination that included the grading of hair loss.
A total of 86 of the 310 women (28%) were judged to have central hair loss. Of these, 52 women – 17% of the total study population – had CCCA.
It appeared that hair grooming practices that cause traction, such as weaves and braids, may contribute to the development of CCCA, as women with the most severe central hair loss used these styles more frequently than did those without hair loss. "This has some clinical bearing because traction can clinically produce folliculitis of the scalp, which can cause scarring if this inflammation is prolonged," noted Dr. Kyei and her colleagues (Arch. Dermatol. 2011 April 11 [doi:10.1001/archdermatol.2011.66]).
After paying hundreds of dollars for such hair styling, women often maintain weaves and braids for weeks to months, so any reactive inflammation usually is prolonged, the investigators added.
However, "the relationship between chemical relaxer use and the development of CCCA continues to be murky," they noted. These products clearly weaken the hair shaft, which can cause breakage, but that does not necessarily lead to CCCA. "The fact that most African Americans use chemical relaxers in combination with braiding and other hair grooming practices makes it even more difficult to tease out a relationship," Dr. Kyei and her associates added.
It is difficult to find subjects who do not use chemical relaxers for comparison, since most African Americans begin the practice in childhood. In this study, 91% of the women reported using chemical relaxers, and began doing so at an average age of 10 years.
Nevertheless, "we feel that it is not unreasonable to assume that the scalp may absorb some of the caustic chemicals found in relaxers, which in time leads to damage of the scalp in the form of scarring," they wrote.
Although the prevalence of bacterial skin infections in these study subjects was only 11%, there was a significant elevation among women with CCCA, compared with women who did not have CCCA. The rate of acne also was elevated in affected subjects, but not to a significant degree. Thus, the researchers said that their study "demonstrates that inflammation in the form of bacterial infection and acne may also be contributing to the development of CCCA, a finding consistent with the histopathologic characteristics of this disease," which show a lymphocytic perifollicular infiltrate in its early stages.
In contrast, there was no such association with fungal infections of the scalp, ringworm, or vaginal yeast infections, which was "surprising given how prone this population is to fungal infection," they added.
"One of the most surprising findings ... was the overrepresentation of type 2 [diabetes mellitus] in those with CCCA," the authors noted. Again, the prevalence of type 2 diabetes was low, at 8%, but it was significantly elevated in women with CCCA, compared with those without CCCA.
"These are important data that need further study because CCCA may be a marker of metabolic dysfunction and, when present, can prompt clinicians to do further testing for diabetes mellitus," Dr. Kyei and her colleagues wrote.
Only 9% of the study subjects had thyroid abnormalities, and three-fourths of them had no or minimal hair loss.
A history of male-pattern baldness in the maternal grandfather was found to be a risk factor for CCCA, which suggests that genetics may play a role.
Hormonal dysregulation did not appear to be associated with development of CCCA. Neither were scarring disorders, since only 6% of the subjects reported a history of keloids, and the rate was no higher in women with CCCA than in those without CCCA.
Similarly, there were relatively high prevalences of seborrheic dermatitis (24%), eczema (13%), and contact dermatitis from chemical relaxers (9%), as would be expected in an African American population. However, these conditions were unrelated to CCCA.
This study was supported in part by the North American Hair Research Society and Procter & Gamble. No financial conflicts of interest were reported.
Despite being the most common pattern of scarring hair loss among African American women, central centrifugal cicatricial alopecia is still poorly understood, according to a report published online April 11 in Archives of Dermatology.
The etiology of and risk factors for the disorder have not been established. And although African American women commonly present with the condition, the exact prevalence remains unknown, said Dr. Angela Kyei and her associates at the Cleveland Clinic Institute of Dermatology and Plastic Surgery.
Central centrifugal cicatricial alopecia (CCCA) – previously known as hot comb alopecia or follicular degeneration syndrome – is a scarring hair loss centered on the vertex of the scalp and spreading peripherally, which is described almost exclusively in African American women. In the late 1960s it was linked to the use of hot combs, but since then almost every method of hair grooming in this population has been associated, albeit weakly, with the disorder. Few studies have examined other possible etiologies, such as immunologic, dermatologic, or genetic factors.
"Given the lack of epidemiologic data, the main goal of [our] study was to elucidate environmental as well as medical risk factors that may be associated with CCCA," the researchers wrote.
They administered questionnaires to 326 African American women attending two churches and a health fair in the Cleveland area. Sixteen of the women ended up being excluded from the analysis.
The questionnaires asked detailed information about family history of male- and female-pattern hair loss; personal medical history, including bacterial and fungal skin infections, autoimmune conditions, and hormonal dysregulation; and hair grooming methods used. The study subjects also underwent a scalp examination that included the grading of hair loss.
A total of 86 of the 310 women (28%) were judged to have central hair loss. Of these, 52 women – 17% of the total study population – had CCCA.
It appeared that hair grooming practices that cause traction, such as weaves and braids, may contribute to the development of CCCA, as women with the most severe central hair loss used these styles more frequently than did those without hair loss. "This has some clinical bearing because traction can clinically produce folliculitis of the scalp, which can cause scarring if this inflammation is prolonged," noted Dr. Kyei and her colleagues (Arch. Dermatol. 2011 April 11 [doi:10.1001/archdermatol.2011.66]).
After paying hundreds of dollars for such hair styling, women often maintain weaves and braids for weeks to months, so any reactive inflammation usually is prolonged, the investigators added.
However, "the relationship between chemical relaxer use and the development of CCCA continues to be murky," they noted. These products clearly weaken the hair shaft, which can cause breakage, but that does not necessarily lead to CCCA. "The fact that most African Americans use chemical relaxers in combination with braiding and other hair grooming practices makes it even more difficult to tease out a relationship," Dr. Kyei and her associates added.
It is difficult to find subjects who do not use chemical relaxers for comparison, since most African Americans begin the practice in childhood. In this study, 91% of the women reported using chemical relaxers, and began doing so at an average age of 10 years.
Nevertheless, "we feel that it is not unreasonable to assume that the scalp may absorb some of the caustic chemicals found in relaxers, which in time leads to damage of the scalp in the form of scarring," they wrote.
Although the prevalence of bacterial skin infections in these study subjects was only 11%, there was a significant elevation among women with CCCA, compared with women who did not have CCCA. The rate of acne also was elevated in affected subjects, but not to a significant degree. Thus, the researchers said that their study "demonstrates that inflammation in the form of bacterial infection and acne may also be contributing to the development of CCCA, a finding consistent with the histopathologic characteristics of this disease," which show a lymphocytic perifollicular infiltrate in its early stages.
In contrast, there was no such association with fungal infections of the scalp, ringworm, or vaginal yeast infections, which was "surprising given how prone this population is to fungal infection," they added.
"One of the most surprising findings ... was the overrepresentation of type 2 [diabetes mellitus] in those with CCCA," the authors noted. Again, the prevalence of type 2 diabetes was low, at 8%, but it was significantly elevated in women with CCCA, compared with those without CCCA.
"These are important data that need further study because CCCA may be a marker of metabolic dysfunction and, when present, can prompt clinicians to do further testing for diabetes mellitus," Dr. Kyei and her colleagues wrote.
Only 9% of the study subjects had thyroid abnormalities, and three-fourths of them had no or minimal hair loss.
A history of male-pattern baldness in the maternal grandfather was found to be a risk factor for CCCA, which suggests that genetics may play a role.
Hormonal dysregulation did not appear to be associated with development of CCCA. Neither were scarring disorders, since only 6% of the subjects reported a history of keloids, and the rate was no higher in women with CCCA than in those without CCCA.
Similarly, there were relatively high prevalences of seborrheic dermatitis (24%), eczema (13%), and contact dermatitis from chemical relaxers (9%), as would be expected in an African American population. However, these conditions were unrelated to CCCA.
This study was supported in part by the North American Hair Research Society and Procter & Gamble. No financial conflicts of interest were reported.
FROM ARCHIVES OF DERMATOLOGY
Major Finding: A total of 86 of the 310 women (28%) were judged to have central hair loss. Of these, 52 women – 17% of the total study population – had CCCA.
Data Source: A community-based cross-sectional survey of 326 African American women.
Disclosures: This study was supported in part by the North American Hair Research Society and Procter & Gamble. No financial conflicts of interest were reported.
Picture Review: Dermoscopy Distinguishes Hair Disorders
ORLANDO - Dermoscopy can help with the diagnosis and management of hair and scale disorders.
In some instances, dermoscopy very rapidly reveal a characteristic sign of a hair or scalp condition. In presentations where dermoscopy only narrows down your differential diagnosis, further work-up with histology might be required, said Dr. Antonella Tosti, professor of clinical dermatology at the University of Miami.
How well do you know your dermoscopy diagnoses?

|
All photos courtesy Dr. Antonella Tosti Normal Scalp. Normally, hairs are arranged in follicular units, with up to three hair shafts from the same orifice. "You also see single hairs coming out from the scalp," Dr. Tosti said. Most hair disorders feature a high number of single hairs. A normal vascular pattern presents as interfollicular red loops and arborizing red lines. |
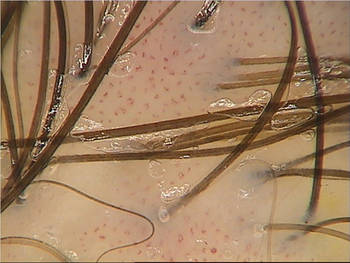
|
Psoriasis. Twisted, coiled loops are a sign of scalp psoriasis. Folliculitis decalvans is the only other scalp condition with a similar loop presentation. If you observe white scales, use high-magnification dermoscopy to look at the vessels before diagnosing psoriasis. White scales can also be seen in cicatricial alopecias such as lupus erythematosus and lichen planopilaris. "So you may need to take a biopsy when patient presents with wide, white scales. It's not always psoriasis," Dr. Tosti said. |
|
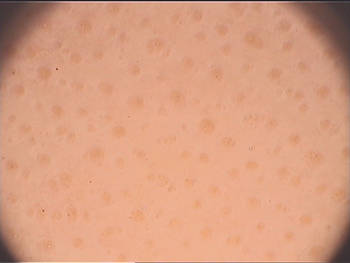
Alopecia areata. Alopecia areata and androgenetic alopecia each will feature yellow dots that correspond to follicular openings. "How often you see the yellow dots depends on the patients. They are seen often in [whites] and less commonly seen in darker-skinned patients," Dr. Tosti said. Hair diameter variability, a sign of androgenetic alopecia, can help you to distinguish these conditions. More than 20% variability in shaft thickness and an increase in the number of thinner hairs with advanced disease are diagnostic. "My suggestion is always use your dermatoscope when women complain of hair loss," Dr. Tosti said. "It is an easy and quick way to distinguish androgenetic alopecia." |
|
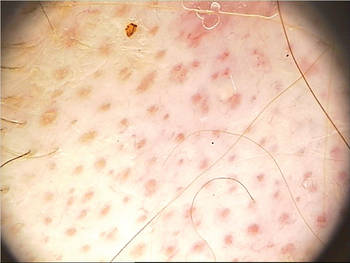
Discoid lupus erythematosus. A follicular red dot pattern is characteristic of this condition on dermoscopy. An atrophic epidermis can be seen in active lesions. The red dots are important to recognize because, if treated, hair can regrow in the affected area. "Make the diagnosis early and treat the patient immediately," Dr. Tosti said. Also, the red dot pattern will help you to distinguish this disorder from other conditions that can cause cicatricial alopecia. |

|
Cicatricial alopecia. A loss of follicular openings is an important clue on dermoscopy that indicates cicatricial alopecias |

|
Traction alopecia. Hair casts are a useful dermoscopy clue for this condition. "You can see them immediately," Dr. Tosti said. "Hair casts are also useful in African Americans if their hairstyle is associated with traction, even if they don't have alopecia. Sometimes they will deny a traction hairstyle." |
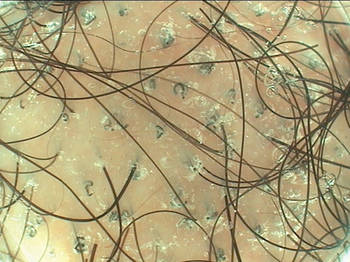
|
Tinea capitis. Dermoscopy facilitates this diagnosis. Look for comma-shaped hairs, a very specific sign seen in both ectothrix and endothrix T. capitis infections. "This is something you should look for in children. It's very fast," Dr. Tosti said. The comma hairs will be more numerous in recent infections, she added. |
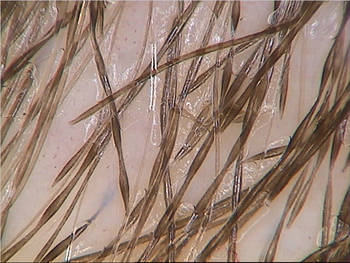
|
Monilethrix. Dermoscopy can help you diagnose this condition very, very fast, Dr. Tosti said. The condition features beading of the hair shafts. The autosomal recessive form of monilethrix will appear much more severe on dermoscopy (J. Invest. Dermatol. 2006;126:1292-6). |
Dr. Tosti said that she had no relevant disclosures.
ORLANDO - Dermoscopy can help with the diagnosis and management of hair and scale disorders.
In some instances, dermoscopy very rapidly reveal a characteristic sign of a hair or scalp condition. In presentations where dermoscopy only narrows down your differential diagnosis, further work-up with histology might be required, said Dr. Antonella Tosti, professor of clinical dermatology at the University of Miami.
How well do you know your dermoscopy diagnoses?

|
All photos courtesy Dr. Antonella Tosti Normal Scalp. Normally, hairs are arranged in follicular units, with up to three hair shafts from the same orifice. "You also see single hairs coming out from the scalp," Dr. Tosti said. Most hair disorders feature a high number of single hairs. A normal vascular pattern presents as interfollicular red loops and arborizing red lines. |

|
Psoriasis. Twisted, coiled loops are a sign of scalp psoriasis. Folliculitis decalvans is the only other scalp condition with a similar loop presentation. If you observe white scales, use high-magnification dermoscopy to look at the vessels before diagnosing psoriasis. White scales can also be seen in cicatricial alopecias such as lupus erythematosus and lichen planopilaris. "So you may need to take a biopsy when patient presents with wide, white scales. It's not always psoriasis," Dr. Tosti said. |

|
Alopecia areata. Alopecia areata and androgenetic alopecia each will feature yellow dots that correspond to follicular openings. "How often you see the yellow dots depends on the patients. They are seen often in [whites] and less commonly seen in darker-skinned patients," Dr. Tosti said. Hair diameter variability, a sign of androgenetic alopecia, can help you to distinguish these conditions. More than 20% variability in shaft thickness and an increase in the number of thinner hairs with advanced disease are diagnostic. "My suggestion is always use your dermatoscope when women complain of hair loss," Dr. Tosti said. "It is an easy and quick way to distinguish androgenetic alopecia." |

|
Discoid lupus erythematosus. A follicular red dot pattern is characteristic of this condition on dermoscopy. An atrophic epidermis can be seen in active lesions. The red dots are important to recognize because, if treated, hair can regrow in the affected area. "Make the diagnosis early and treat the patient immediately," Dr. Tosti said. Also, the red dot pattern will help you to distinguish this disorder from other conditions that can cause cicatricial alopecia. |

|
Cicatricial alopecia. A loss of follicular openings is an important clue on dermoscopy that indicates cicatricial alopecias |

|
Traction alopecia. Hair casts are a useful dermoscopy clue for this condition. "You can see them immediately," Dr. Tosti said. "Hair casts are also useful in African Americans if their hairstyle is associated with traction, even if they don't have alopecia. Sometimes they will deny a traction hairstyle." |

|
Tinea capitis. Dermoscopy facilitates this diagnosis. Look for comma-shaped hairs, a very specific sign seen in both ectothrix and endothrix T. capitis infections. "This is something you should look for in children. It's very fast," Dr. Tosti said. The comma hairs will be more numerous in recent infections, she added. |

|
Monilethrix. Dermoscopy can help you diagnose this condition very, very fast, Dr. Tosti said. The condition features beading of the hair shafts. The autosomal recessive form of monilethrix will appear much more severe on dermoscopy (J. Invest. Dermatol. 2006;126:1292-6). |
Dr. Tosti said that she had no relevant disclosures.
ORLANDO - Dermoscopy can help with the diagnosis and management of hair and scale disorders.
In some instances, dermoscopy very rapidly reveal a characteristic sign of a hair or scalp condition. In presentations where dermoscopy only narrows down your differential diagnosis, further work-up with histology might be required, said Dr. Antonella Tosti, professor of clinical dermatology at the University of Miami.
How well do you know your dermoscopy diagnoses?

|
All photos courtesy Dr. Antonella Tosti Normal Scalp. Normally, hairs are arranged in follicular units, with up to three hair shafts from the same orifice. "You also see single hairs coming out from the scalp," Dr. Tosti said. Most hair disorders feature a high number of single hairs. A normal vascular pattern presents as interfollicular red loops and arborizing red lines. |

|
Psoriasis. Twisted, coiled loops are a sign of scalp psoriasis. Folliculitis decalvans is the only other scalp condition with a similar loop presentation. If you observe white scales, use high-magnification dermoscopy to look at the vessels before diagnosing psoriasis. White scales can also be seen in cicatricial alopecias such as lupus erythematosus and lichen planopilaris. "So you may need to take a biopsy when patient presents with wide, white scales. It's not always psoriasis," Dr. Tosti said. |

|
Alopecia areata. Alopecia areata and androgenetic alopecia each will feature yellow dots that correspond to follicular openings. "How often you see the yellow dots depends on the patients. They are seen often in [whites] and less commonly seen in darker-skinned patients," Dr. Tosti said. Hair diameter variability, a sign of androgenetic alopecia, can help you to distinguish these conditions. More than 20% variability in shaft thickness and an increase in the number of thinner hairs with advanced disease are diagnostic. "My suggestion is always use your dermatoscope when women complain of hair loss," Dr. Tosti said. "It is an easy and quick way to distinguish androgenetic alopecia." |

|
Discoid lupus erythematosus. A follicular red dot pattern is characteristic of this condition on dermoscopy. An atrophic epidermis can be seen in active lesions. The red dots are important to recognize because, if treated, hair can regrow in the affected area. "Make the diagnosis early and treat the patient immediately," Dr. Tosti said. Also, the red dot pattern will help you to distinguish this disorder from other conditions that can cause cicatricial alopecia. |

|
Cicatricial alopecia. A loss of follicular openings is an important clue on dermoscopy that indicates cicatricial alopecias |

|
Traction alopecia. Hair casts are a useful dermoscopy clue for this condition. "You can see them immediately," Dr. Tosti said. "Hair casts are also useful in African Americans if their hairstyle is associated with traction, even if they don't have alopecia. Sometimes they will deny a traction hairstyle." |

|
Tinea capitis. Dermoscopy facilitates this diagnosis. Look for comma-shaped hairs, a very specific sign seen in both ectothrix and endothrix T. capitis infections. "This is something you should look for in children. It's very fast," Dr. Tosti said. The comma hairs will be more numerous in recent infections, she added. |

|
Monilethrix. Dermoscopy can help you diagnose this condition very, very fast, Dr. Tosti said. The condition features beading of the hair shafts. The autosomal recessive form of monilethrix will appear much more severe on dermoscopy (J. Invest. Dermatol. 2006;126:1292-6). |
Dr. Tosti said that she had no relevant disclosures.
FROM THE ANNUAL MEETING OF THE FLORIDA SOCIETY OF DERMATOLOGIC SURGEONS
Dermatologic Manifestations of Musicians: A Case Report and Review of Skin Conditions in Musicians
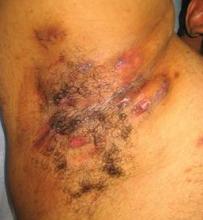
AAD: Adalimumab Improves Life Quality for Hidradenitis Suppurativa Patients
NEW ORLEANS – Adalimumab demonstrated significant efficacy for moderate to severe hidradenitis suppurativa in an interim 16-week analysis of a 52-week placebo-controlled randomized trial.
"Overall I was really pleased with seeing these results in terms of moving the science forward and managing these patients. ...This is a disease that I think has really been under-appreciated and under-managed and under-researched," Dr. Alexa B. Kimball said in presenting the interim phase II study findings at the annual meeting of the American Academy of Dermatology.
Anti–tumor necrosis factor (TNF) therapy is of considerable research interest because of the disease's prominent inflammatory component and the shortcomings of current therapies, explained Dr. Kimball of Harvard Medical School, Boston.
The clinical trial involved 154 patients with moderate to severe hidradenitis suppurativa who were randomized to 16 weeks of double-blind adalimumab (Humira) at 40 mg per week, 40 mg every other week, or to placebo. After 16 weeks, everyone was placed on 36 weeks of open-label adalimumab at 40 mg every other week, the standard psoriasis dosing. The loading dose was 80 mg at week 0 in the every-other-week group and 160 mg at week 0 and 80 mg at week 2 in the weekly therapy arm.
Because no practical instrument existed for grading the severity of hidradenitis suppurativa and scoring changes over time, Dr. Kimball and her coinvestigators created one: the Hidradenitis Suppurativa Physician Global Assessment (HS-PGA).
At baseline, two-thirds of participants had moderate disease as defined by the HS-PGA. Another 20% had very severe disease, with more than five abscesses or draining fistulas. The rest had severe disease.
At 16 weeks, 49% of patients on weekly adalimumab had improved to clear, mild, or minimal disease status. This was a significantly better result than the 21% rate in patients on alternate-week adalimumab or the 24% on placebo.
The primary study end point required achieving an HS-PGA score of clear, minimal, or mild plus at least a two-grade improvement. However, the 20% of subjects with baseline severe disease could only reach the primary end point by improving at least three grades to mild disease, and this proved a high bar for the severely affected subgroup, who often have scarring and tracts that are irreversible. Nonetheless, 18% of patients on weekly adalimumab achieved the primary end point, a significantly higher proportion than the 10% on every-other-week anti-TNF therapy or the 4% on placebo, Dr. Kimball noted.
Skin pain was a prominent feature among study participants. Their mean baseline pain score was roughly 55 points on a 0-100 visual analog scale. At week 16, however, 48% of patients in the weekly adalimumab arm had a 30% or greater reduction in pain score, compared with baseline, which is widely accepted in pain studies as a clinically meaningful improvement. This was a significantly higher proportion than the 36% rate among patients on alternate-week adalimumab or the 27% rate among controls.
Treatment-emergent side effects were what dermatologists have come to expect with anti-TNF therapy for psoriasis. Serious adverse events occurred in two patients in the placebo arm, three on alternate-week adalimumab, and four on weekly therapy with the biologic agent.
Dr. Kimball said she suspects that minor skin infections, which were fairly common in the first 16 weeks of the study, will become less of an issue with longer-term adalimumab therapy, as inflammation diminishes and the substantial baseline microbiologic load decreases. In the future, she added, it might be useful to prescribe antibiotics during the first 12-16 weeks of adalimumab therapy while waiting for the TNF inhibitor to help patients gain good control of the disease.
She noted that the hidradenitis suppurativa population in this study differed in several key respects from the psoriasis patients for whom dermatologists are accustomed to prescribing anti-TNF biologics. Seventy percent of participants in the hidradenitis suppurativa study were women, and the mean body weight across the three study arms was 95-100 kg. In contrast, typically two-thirds of participants in studies of anti-TNF therapy for psoriasis are men, with a mean weight of 85-90 kg.
Effective new therapy for hidradenitis suppurativa is sorely needed because affected patients often end up undergoing surgery, which entails removing large areas of skin and a 4- to 6-month recovery. "While surgery does result in significant improvements in quality of life overall, it’s a really long haul and incredibly disfiguring," the dermatologist observed.
Dr. Kimball declared that she serves as an investigator for and consultant to Abbott Laboratories, which is sponsoring the phase II trial, and in similar capacities with other pharmaceutical companies.
NEW ORLEANS – Adalimumab demonstrated significant efficacy for moderate to severe hidradenitis suppurativa in an interim 16-week analysis of a 52-week placebo-controlled randomized trial.
"Overall I was really pleased with seeing these results in terms of moving the science forward and managing these patients. ...This is a disease that I think has really been under-appreciated and under-managed and under-researched," Dr. Alexa B. Kimball said in presenting the interim phase II study findings at the annual meeting of the American Academy of Dermatology.
Anti–tumor necrosis factor (TNF) therapy is of considerable research interest because of the disease's prominent inflammatory component and the shortcomings of current therapies, explained Dr. Kimball of Harvard Medical School, Boston.
The clinical trial involved 154 patients with moderate to severe hidradenitis suppurativa who were randomized to 16 weeks of double-blind adalimumab (Humira) at 40 mg per week, 40 mg every other week, or to placebo. After 16 weeks, everyone was placed on 36 weeks of open-label adalimumab at 40 mg every other week, the standard psoriasis dosing. The loading dose was 80 mg at week 0 in the every-other-week group and 160 mg at week 0 and 80 mg at week 2 in the weekly therapy arm.
Because no practical instrument existed for grading the severity of hidradenitis suppurativa and scoring changes over time, Dr. Kimball and her coinvestigators created one: the Hidradenitis Suppurativa Physician Global Assessment (HS-PGA).
At baseline, two-thirds of participants had moderate disease as defined by the HS-PGA. Another 20% had very severe disease, with more than five abscesses or draining fistulas. The rest had severe disease.
At 16 weeks, 49% of patients on weekly adalimumab had improved to clear, mild, or minimal disease status. This was a significantly better result than the 21% rate in patients on alternate-week adalimumab or the 24% on placebo.
The primary study end point required achieving an HS-PGA score of clear, minimal, or mild plus at least a two-grade improvement. However, the 20% of subjects with baseline severe disease could only reach the primary end point by improving at least three grades to mild disease, and this proved a high bar for the severely affected subgroup, who often have scarring and tracts that are irreversible. Nonetheless, 18% of patients on weekly adalimumab achieved the primary end point, a significantly higher proportion than the 10% on every-other-week anti-TNF therapy or the 4% on placebo, Dr. Kimball noted.
Skin pain was a prominent feature among study participants. Their mean baseline pain score was roughly 55 points on a 0-100 visual analog scale. At week 16, however, 48% of patients in the weekly adalimumab arm had a 30% or greater reduction in pain score, compared with baseline, which is widely accepted in pain studies as a clinically meaningful improvement. This was a significantly higher proportion than the 36% rate among patients on alternate-week adalimumab or the 27% rate among controls.
Treatment-emergent side effects were what dermatologists have come to expect with anti-TNF therapy for psoriasis. Serious adverse events occurred in two patients in the placebo arm, three on alternate-week adalimumab, and four on weekly therapy with the biologic agent.
Dr. Kimball said she suspects that minor skin infections, which were fairly common in the first 16 weeks of the study, will become less of an issue with longer-term adalimumab therapy, as inflammation diminishes and the substantial baseline microbiologic load decreases. In the future, she added, it might be useful to prescribe antibiotics during the first 12-16 weeks of adalimumab therapy while waiting for the TNF inhibitor to help patients gain good control of the disease.
She noted that the hidradenitis suppurativa population in this study differed in several key respects from the psoriasis patients for whom dermatologists are accustomed to prescribing anti-TNF biologics. Seventy percent of participants in the hidradenitis suppurativa study were women, and the mean body weight across the three study arms was 95-100 kg. In contrast, typically two-thirds of participants in studies of anti-TNF therapy for psoriasis are men, with a mean weight of 85-90 kg.
Effective new therapy for hidradenitis suppurativa is sorely needed because affected patients often end up undergoing surgery, which entails removing large areas of skin and a 4- to 6-month recovery. "While surgery does result in significant improvements in quality of life overall, it’s a really long haul and incredibly disfiguring," the dermatologist observed.
Dr. Kimball declared that she serves as an investigator for and consultant to Abbott Laboratories, which is sponsoring the phase II trial, and in similar capacities with other pharmaceutical companies.
NEW ORLEANS – Adalimumab demonstrated significant efficacy for moderate to severe hidradenitis suppurativa in an interim 16-week analysis of a 52-week placebo-controlled randomized trial.
"Overall I was really pleased with seeing these results in terms of moving the science forward and managing these patients. ...This is a disease that I think has really been under-appreciated and under-managed and under-researched," Dr. Alexa B. Kimball said in presenting the interim phase II study findings at the annual meeting of the American Academy of Dermatology.
Anti–tumor necrosis factor (TNF) therapy is of considerable research interest because of the disease's prominent inflammatory component and the shortcomings of current therapies, explained Dr. Kimball of Harvard Medical School, Boston.
The clinical trial involved 154 patients with moderate to severe hidradenitis suppurativa who were randomized to 16 weeks of double-blind adalimumab (Humira) at 40 mg per week, 40 mg every other week, or to placebo. After 16 weeks, everyone was placed on 36 weeks of open-label adalimumab at 40 mg every other week, the standard psoriasis dosing. The loading dose was 80 mg at week 0 in the every-other-week group and 160 mg at week 0 and 80 mg at week 2 in the weekly therapy arm.
Because no practical instrument existed for grading the severity of hidradenitis suppurativa and scoring changes over time, Dr. Kimball and her coinvestigators created one: the Hidradenitis Suppurativa Physician Global Assessment (HS-PGA).
At baseline, two-thirds of participants had moderate disease as defined by the HS-PGA. Another 20% had very severe disease, with more than five abscesses or draining fistulas. The rest had severe disease.
At 16 weeks, 49% of patients on weekly adalimumab had improved to clear, mild, or minimal disease status. This was a significantly better result than the 21% rate in patients on alternate-week adalimumab or the 24% on placebo.
The primary study end point required achieving an HS-PGA score of clear, minimal, or mild plus at least a two-grade improvement. However, the 20% of subjects with baseline severe disease could only reach the primary end point by improving at least three grades to mild disease, and this proved a high bar for the severely affected subgroup, who often have scarring and tracts that are irreversible. Nonetheless, 18% of patients on weekly adalimumab achieved the primary end point, a significantly higher proportion than the 10% on every-other-week anti-TNF therapy or the 4% on placebo, Dr. Kimball noted.
Skin pain was a prominent feature among study participants. Their mean baseline pain score was roughly 55 points on a 0-100 visual analog scale. At week 16, however, 48% of patients in the weekly adalimumab arm had a 30% or greater reduction in pain score, compared with baseline, which is widely accepted in pain studies as a clinically meaningful improvement. This was a significantly higher proportion than the 36% rate among patients on alternate-week adalimumab or the 27% rate among controls.
Treatment-emergent side effects were what dermatologists have come to expect with anti-TNF therapy for psoriasis. Serious adverse events occurred in two patients in the placebo arm, three on alternate-week adalimumab, and four on weekly therapy with the biologic agent.
Dr. Kimball said she suspects that minor skin infections, which were fairly common in the first 16 weeks of the study, will become less of an issue with longer-term adalimumab therapy, as inflammation diminishes and the substantial baseline microbiologic load decreases. In the future, she added, it might be useful to prescribe antibiotics during the first 12-16 weeks of adalimumab therapy while waiting for the TNF inhibitor to help patients gain good control of the disease.
She noted that the hidradenitis suppurativa population in this study differed in several key respects from the psoriasis patients for whom dermatologists are accustomed to prescribing anti-TNF biologics. Seventy percent of participants in the hidradenitis suppurativa study were women, and the mean body weight across the three study arms was 95-100 kg. In contrast, typically two-thirds of participants in studies of anti-TNF therapy for psoriasis are men, with a mean weight of 85-90 kg.
Effective new therapy for hidradenitis suppurativa is sorely needed because affected patients often end up undergoing surgery, which entails removing large areas of skin and a 4- to 6-month recovery. "While surgery does result in significant improvements in quality of life overall, it’s a really long haul and incredibly disfiguring," the dermatologist observed.
Dr. Kimball declared that she serves as an investigator for and consultant to Abbott Laboratories, which is sponsoring the phase II trial, and in similar capacities with other pharmaceutical companies.
FROM THE ANNUAL MEETING OF THE AMERICAN ACADEMY OF DERMATOLOGY
Major Finding: At 16 weeks, 49% of patients on weekly adalimumab had improved to clear, mild, or minimal disease status. This was a significantly better result than the 21% rate in patients on alternate-week adalimumab or the 24% on placebo.
Data Source: A clinical trial involving 154 patients with moderate to severe hidradenitis suppurativa who were randomized to 16 weeks of double-blind adalimumab (Humira) at 40 mg per week, 40 mg every other week, or to placebo. After 16 weeks, everyone was placed on 36 weeks of open-label adalimumab at 40 mg every other week.
Disclosures: Dr. Kimball declared that she serves as an investigator for and consultant to Abbott Laboratories, which is sponsoring the Phase II trial, and in similar capacities with other pharmaceutical companies.