User login
Probe linked to smartphone found effective in diagnosing oral cancer
DALLAS – A low-cost , in a clinical study of 92 people.
“Oral cancer is the sixth most common cancer in the world, but it’s the only major cancer whose outcome has not improved in the last 50 years,” study author Petra Wilder-Smith DDS, PhD, said in an interview following the annual conference of the American Society for Laser Medicine and Surgery Inc. “The main challenge is that over two-thirds of oral cancers are detected after they’ve metastasized. When you get spread like that, your survival is about 20% at 5 years, whereas if you detect it before spread, your survival is about 80% at 5 years.”
At the meeting, Vania Firmalino, an undergraduate student at the University of California, Irvine, discussed efforts by Dr. Wilder-Smith, Rongguang Liang, PhD, of the College of Optical Sciences at the University of Arizona, Tucson, and their colleagues to develop and evaluate the screening performance of a novel, low-cost smartphone-based mini probe for oral cancer screening and oral potentially premalignant lesions (OPMLs). The device provides high-resolution polarized white light images in combination with autofluorescence (AF) imaging capability.
The researchers found that inter-subject variation at each location was small, but inter-site differences were considerable. For example, optical data from OPMLs and oral cancer sites differed from normal with regard to white-light reflectance intensities, vascular homogeneity, and standard deviation. The AF signal in OPMLs and oral cancers shifted progressively to the red, together with a diminished green fluorescence signal. The cloud-based diagnostic algorithm based on these properties performed well, with an agreement with standard-of-care diagnosis of 80.6%.
“Artificial intelligence improves with data,” said Dr. Wilder-Smith, who is also a senior fellow at the university’s Chao Family Comprehensive Cancer Center. “We trained this system on about 200 images. When you’re up to 1,000 images per condition, that’s when you really start to get the benefits of artificial intelligence and machine learning. There’s huge potential here, especially when you think that 40% of the world’s risk for oral cancer is in India, which has good cell phone coverage. India also has a government-financed public health program whereby they already send health care workers to the remote areas of India to screen for basic diseases.”
The study won an award for best overall clinical abstract at the meeting. Dr. Wilder-Smith reported having no financial disclosures. The project was supported with funding from the National Institute of Biomedical Imaging and Bioengineering and the Beckman Foundation.
DALLAS – A low-cost , in a clinical study of 92 people.
“Oral cancer is the sixth most common cancer in the world, but it’s the only major cancer whose outcome has not improved in the last 50 years,” study author Petra Wilder-Smith DDS, PhD, said in an interview following the annual conference of the American Society for Laser Medicine and Surgery Inc. “The main challenge is that over two-thirds of oral cancers are detected after they’ve metastasized. When you get spread like that, your survival is about 20% at 5 years, whereas if you detect it before spread, your survival is about 80% at 5 years.”
At the meeting, Vania Firmalino, an undergraduate student at the University of California, Irvine, discussed efforts by Dr. Wilder-Smith, Rongguang Liang, PhD, of the College of Optical Sciences at the University of Arizona, Tucson, and their colleagues to develop and evaluate the screening performance of a novel, low-cost smartphone-based mini probe for oral cancer screening and oral potentially premalignant lesions (OPMLs). The device provides high-resolution polarized white light images in combination with autofluorescence (AF) imaging capability.
The researchers found that inter-subject variation at each location was small, but inter-site differences were considerable. For example, optical data from OPMLs and oral cancer sites differed from normal with regard to white-light reflectance intensities, vascular homogeneity, and standard deviation. The AF signal in OPMLs and oral cancers shifted progressively to the red, together with a diminished green fluorescence signal. The cloud-based diagnostic algorithm based on these properties performed well, with an agreement with standard-of-care diagnosis of 80.6%.
“Artificial intelligence improves with data,” said Dr. Wilder-Smith, who is also a senior fellow at the university’s Chao Family Comprehensive Cancer Center. “We trained this system on about 200 images. When you’re up to 1,000 images per condition, that’s when you really start to get the benefits of artificial intelligence and machine learning. There’s huge potential here, especially when you think that 40% of the world’s risk for oral cancer is in India, which has good cell phone coverage. India also has a government-financed public health program whereby they already send health care workers to the remote areas of India to screen for basic diseases.”
The study won an award for best overall clinical abstract at the meeting. Dr. Wilder-Smith reported having no financial disclosures. The project was supported with funding from the National Institute of Biomedical Imaging and Bioengineering and the Beckman Foundation.
DALLAS – A low-cost , in a clinical study of 92 people.
“Oral cancer is the sixth most common cancer in the world, but it’s the only major cancer whose outcome has not improved in the last 50 years,” study author Petra Wilder-Smith DDS, PhD, said in an interview following the annual conference of the American Society for Laser Medicine and Surgery Inc. “The main challenge is that over two-thirds of oral cancers are detected after they’ve metastasized. When you get spread like that, your survival is about 20% at 5 years, whereas if you detect it before spread, your survival is about 80% at 5 years.”
At the meeting, Vania Firmalino, an undergraduate student at the University of California, Irvine, discussed efforts by Dr. Wilder-Smith, Rongguang Liang, PhD, of the College of Optical Sciences at the University of Arizona, Tucson, and their colleagues to develop and evaluate the screening performance of a novel, low-cost smartphone-based mini probe for oral cancer screening and oral potentially premalignant lesions (OPMLs). The device provides high-resolution polarized white light images in combination with autofluorescence (AF) imaging capability.
The researchers found that inter-subject variation at each location was small, but inter-site differences were considerable. For example, optical data from OPMLs and oral cancer sites differed from normal with regard to white-light reflectance intensities, vascular homogeneity, and standard deviation. The AF signal in OPMLs and oral cancers shifted progressively to the red, together with a diminished green fluorescence signal. The cloud-based diagnostic algorithm based on these properties performed well, with an agreement with standard-of-care diagnosis of 80.6%.
“Artificial intelligence improves with data,” said Dr. Wilder-Smith, who is also a senior fellow at the university’s Chao Family Comprehensive Cancer Center. “We trained this system on about 200 images. When you’re up to 1,000 images per condition, that’s when you really start to get the benefits of artificial intelligence and machine learning. There’s huge potential here, especially when you think that 40% of the world’s risk for oral cancer is in India, which has good cell phone coverage. India also has a government-financed public health program whereby they already send health care workers to the remote areas of India to screen for basic diseases.”
The study won an award for best overall clinical abstract at the meeting. Dr. Wilder-Smith reported having no financial disclosures. The project was supported with funding from the National Institute of Biomedical Imaging and Bioengineering and the Beckman Foundation.
REPORTING FROM ASLMS 2018
Key clinical point: A compact oral probe that links to a smartphone was able to detect oral cancer.
Major finding: The optical diagnostic probe had a high rate of agreement (80.6%) with standard-of-care diagnosis.
Study details: A clinical analysis of 92 people with visually healthy oral mucosa or oral leukoplakia, erythroplakia, or ulceration.
Disclosures: Dr. Wilder-Smith reported having no financial disclosures. The National Institute of Biomedical Imaging and Bioengineering and the Beckman Foundation funded the project.
Early Intervention Has Long-Term Benefits for Oral Cancer Survivors
Exercise and physical therapy in the first weeks after surgery can have a positive impact on health, physical function, and quality of life (QOL) in patients with oral cancer. The changes persist for months afterward, say researchers from Chang Gung Memorial Hospital in Taiwan.
The study involved 65 patients who had undergone reconstructive microsurgery for oral cavity squamous cell carcinoma. The time of intervention had 3 phases: early (8 days to within a month after surgery), middle (1- 3 months after surgery), and late (> 3 months after surgery). The program included pain management, temporomandibular joint exercise, and shoulder and neck exercises.
In the early phase, the main goal was to help participants deal with pain, edema, shoulder dysfunction, and other consequences of surgery. Transcutaneous electrical stimulation for 15 minutes in each treatment session was followed by gentle massage and exercise. During the middle phase, the intervention focused on impairment from surgery or radiation therapy and intensified exercise. The late phase goal was to recover residual function as much as possible.
At 1 month, 40% of patients were on a soft diet. By 6 months, all nasogastric tubes had been removed, and 53% of patients had returned to a normal diet. The researchers note that early intervention to exercise the temporomandibular joint exercise may improve mouth opening. In the advanced stage group, the maximum mouth opening reached its highest at 3 months.
Scapular muscle strength and shoulder range of motion improved progressively during the 6-month follow-up. At 1 month, the mean DASH (Disability of the Arms, Shoulder, and Hand) score showed significant improvement (dropping from 34 to 17). Health-related QOL also showed significant improvement. The predicted return-to-work rate was 80% at 1 year: Patients in skilled or semiskilled work and the self-employed had the highest rates (88% and 87%, respectively).
Source:
Chen YH, Liang WA, Hsu CY, et al. PeerJ. 2018;6e4419.
doi: 10.7717/peerj.4419.
Exercise and physical therapy in the first weeks after surgery can have a positive impact on health, physical function, and quality of life (QOL) in patients with oral cancer. The changes persist for months afterward, say researchers from Chang Gung Memorial Hospital in Taiwan.
The study involved 65 patients who had undergone reconstructive microsurgery for oral cavity squamous cell carcinoma. The time of intervention had 3 phases: early (8 days to within a month after surgery), middle (1- 3 months after surgery), and late (> 3 months after surgery). The program included pain management, temporomandibular joint exercise, and shoulder and neck exercises.
In the early phase, the main goal was to help participants deal with pain, edema, shoulder dysfunction, and other consequences of surgery. Transcutaneous electrical stimulation for 15 minutes in each treatment session was followed by gentle massage and exercise. During the middle phase, the intervention focused on impairment from surgery or radiation therapy and intensified exercise. The late phase goal was to recover residual function as much as possible.
At 1 month, 40% of patients were on a soft diet. By 6 months, all nasogastric tubes had been removed, and 53% of patients had returned to a normal diet. The researchers note that early intervention to exercise the temporomandibular joint exercise may improve mouth opening. In the advanced stage group, the maximum mouth opening reached its highest at 3 months.
Scapular muscle strength and shoulder range of motion improved progressively during the 6-month follow-up. At 1 month, the mean DASH (Disability of the Arms, Shoulder, and Hand) score showed significant improvement (dropping from 34 to 17). Health-related QOL also showed significant improvement. The predicted return-to-work rate was 80% at 1 year: Patients in skilled or semiskilled work and the self-employed had the highest rates (88% and 87%, respectively).
Source:
Chen YH, Liang WA, Hsu CY, et al. PeerJ. 2018;6e4419.
doi: 10.7717/peerj.4419.
Exercise and physical therapy in the first weeks after surgery can have a positive impact on health, physical function, and quality of life (QOL) in patients with oral cancer. The changes persist for months afterward, say researchers from Chang Gung Memorial Hospital in Taiwan.
The study involved 65 patients who had undergone reconstructive microsurgery for oral cavity squamous cell carcinoma. The time of intervention had 3 phases: early (8 days to within a month after surgery), middle (1- 3 months after surgery), and late (> 3 months after surgery). The program included pain management, temporomandibular joint exercise, and shoulder and neck exercises.
In the early phase, the main goal was to help participants deal with pain, edema, shoulder dysfunction, and other consequences of surgery. Transcutaneous electrical stimulation for 15 minutes in each treatment session was followed by gentle massage and exercise. During the middle phase, the intervention focused on impairment from surgery or radiation therapy and intensified exercise. The late phase goal was to recover residual function as much as possible.
At 1 month, 40% of patients were on a soft diet. By 6 months, all nasogastric tubes had been removed, and 53% of patients had returned to a normal diet. The researchers note that early intervention to exercise the temporomandibular joint exercise may improve mouth opening. In the advanced stage group, the maximum mouth opening reached its highest at 3 months.
Scapular muscle strength and shoulder range of motion improved progressively during the 6-month follow-up. At 1 month, the mean DASH (Disability of the Arms, Shoulder, and Hand) score showed significant improvement (dropping from 34 to 17). Health-related QOL also showed significant improvement. The predicted return-to-work rate was 80% at 1 year: Patients in skilled or semiskilled work and the self-employed had the highest rates (88% and 87%, respectively).
Source:
Chen YH, Liang WA, Hsu CY, et al. PeerJ. 2018;6e4419.
doi: 10.7717/peerj.4419.
When it comes to thyroid cancer follow-up, serum microRNA profiles have earned new respect
CHICAGO –
The usual tool for trying to detect recurrence while following patients with papillary thyroid cancer after surgery has been the serum thyroglobulin assay. However, management of papillary thyroid cancer has become more conservative, involving lobectomy and isthmusectomy on the affected side rather than total gland resection. The benefit of the conservative approach is to avoid complications while maintaining an overall survival rate equivalent to the more extensive approach.
The investigators measured 754 miRNAs in serum samples of 11 patients with papillary thyroid cancer both before and 30 days after surgical thyroidectomy. They re-evaluated major candidate miRNAs using absolute quantitative polymerase chain reaction analysis in an independent cohort of 44 other patients with papillary thyroid cancer or benign nodules or 20 healthy controls, Dr. Rosignolo said at the annual meeting of the Endocrine Society.
The 2 miRNAs most significantly associated with thyroid tumors were then assessed in matched serum samples (before and 30 days, and 1 to 2 years after surgery) from the 20 PTC patients with complete follow-up datasets and results correlated with American Thyroid Association (ATA) responses to therapy.
Serum levels of both miRNAs after 1 to 2 years of follow-up were consistent with ATA responses to therapy in all patients, including two patients who developed structural evidence of disease whose thyroglobulin assay results remained negative (less than 1 ng/mL) for cancer recurrence.
Fifteen of the 20 patients had excellent or indeterminate responses to therapy as defined by 2015 ATA guidelines. In these 15 cases, and in the single patient with a biochemical incomplete response, expression levels of miR-146a-5p and miR-221-3p decreased after surgery and remained low at the 1- to 2-year visit.
It was a very different story for the 4 patients with structural incomplete responses at 1 to 2 years. In this subgroup, initial postoperative declines in serum miR-146a-5p and miR-221-3p levels were followed by increases to levels at the 1- to 2-year visit that were similar to or higher than those found prior to surgery.
The study was funded by the Umberto Di Mario Foundation, Banca d’Italia, University of Rome Sapienza, the program of Biotechnologies and Clinical Medicine of the University of Rome Sapienza, the European Medical Writers Association, and the Umberto Di Mario Foundation.
SOURCE: Rosignolo F et al. J Endo Soc. 2017;1(1)3-13. ENDO 2018, Abstract OR17-1.
CHICAGO –
The usual tool for trying to detect recurrence while following patients with papillary thyroid cancer after surgery has been the serum thyroglobulin assay. However, management of papillary thyroid cancer has become more conservative, involving lobectomy and isthmusectomy on the affected side rather than total gland resection. The benefit of the conservative approach is to avoid complications while maintaining an overall survival rate equivalent to the more extensive approach.
The investigators measured 754 miRNAs in serum samples of 11 patients with papillary thyroid cancer both before and 30 days after surgical thyroidectomy. They re-evaluated major candidate miRNAs using absolute quantitative polymerase chain reaction analysis in an independent cohort of 44 other patients with papillary thyroid cancer or benign nodules or 20 healthy controls, Dr. Rosignolo said at the annual meeting of the Endocrine Society.
The 2 miRNAs most significantly associated with thyroid tumors were then assessed in matched serum samples (before and 30 days, and 1 to 2 years after surgery) from the 20 PTC patients with complete follow-up datasets and results correlated with American Thyroid Association (ATA) responses to therapy.
Serum levels of both miRNAs after 1 to 2 years of follow-up were consistent with ATA responses to therapy in all patients, including two patients who developed structural evidence of disease whose thyroglobulin assay results remained negative (less than 1 ng/mL) for cancer recurrence.
Fifteen of the 20 patients had excellent or indeterminate responses to therapy as defined by 2015 ATA guidelines. In these 15 cases, and in the single patient with a biochemical incomplete response, expression levels of miR-146a-5p and miR-221-3p decreased after surgery and remained low at the 1- to 2-year visit.
It was a very different story for the 4 patients with structural incomplete responses at 1 to 2 years. In this subgroup, initial postoperative declines in serum miR-146a-5p and miR-221-3p levels were followed by increases to levels at the 1- to 2-year visit that were similar to or higher than those found prior to surgery.
The study was funded by the Umberto Di Mario Foundation, Banca d’Italia, University of Rome Sapienza, the program of Biotechnologies and Clinical Medicine of the University of Rome Sapienza, the European Medical Writers Association, and the Umberto Di Mario Foundation.
SOURCE: Rosignolo F et al. J Endo Soc. 2017;1(1)3-13. ENDO 2018, Abstract OR17-1.
CHICAGO –
The usual tool for trying to detect recurrence while following patients with papillary thyroid cancer after surgery has been the serum thyroglobulin assay. However, management of papillary thyroid cancer has become more conservative, involving lobectomy and isthmusectomy on the affected side rather than total gland resection. The benefit of the conservative approach is to avoid complications while maintaining an overall survival rate equivalent to the more extensive approach.
The investigators measured 754 miRNAs in serum samples of 11 patients with papillary thyroid cancer both before and 30 days after surgical thyroidectomy. They re-evaluated major candidate miRNAs using absolute quantitative polymerase chain reaction analysis in an independent cohort of 44 other patients with papillary thyroid cancer or benign nodules or 20 healthy controls, Dr. Rosignolo said at the annual meeting of the Endocrine Society.
The 2 miRNAs most significantly associated with thyroid tumors were then assessed in matched serum samples (before and 30 days, and 1 to 2 years after surgery) from the 20 PTC patients with complete follow-up datasets and results correlated with American Thyroid Association (ATA) responses to therapy.
Serum levels of both miRNAs after 1 to 2 years of follow-up were consistent with ATA responses to therapy in all patients, including two patients who developed structural evidence of disease whose thyroglobulin assay results remained negative (less than 1 ng/mL) for cancer recurrence.
Fifteen of the 20 patients had excellent or indeterminate responses to therapy as defined by 2015 ATA guidelines. In these 15 cases, and in the single patient with a biochemical incomplete response, expression levels of miR-146a-5p and miR-221-3p decreased after surgery and remained low at the 1- to 2-year visit.
It was a very different story for the 4 patients with structural incomplete responses at 1 to 2 years. In this subgroup, initial postoperative declines in serum miR-146a-5p and miR-221-3p levels were followed by increases to levels at the 1- to 2-year visit that were similar to or higher than those found prior to surgery.
The study was funded by the Umberto Di Mario Foundation, Banca d’Italia, University of Rome Sapienza, the program of Biotechnologies and Clinical Medicine of the University of Rome Sapienza, the European Medical Writers Association, and the Umberto Di Mario Foundation.
SOURCE: Rosignolo F et al. J Endo Soc. 2017;1(1)3-13. ENDO 2018, Abstract OR17-1.
REPORTING FROM ENDO 2018
Key clinical point: Serum microRNA profiles hold promise for postsurgical monitoring of patients with papillary thyroid cancer.
Major finding: Of eight tested, two serum microRNA profiles – miR-146a-5p and miR-221-3p – were the most promising thyroid tumor biomarkers.
Study details: Prospective analysis of the blood of 31 patients with papillary thyroid cancer before and after surgery to assess which markers were most sensitive to cancer.
Disclosures: The study was funded by the Umberto Di Mario Foundation, Banca d’Italia, University of Rome Sapienza, the program of Biotechnologies and Clinical Medicine of the University of Rome Sapienza, the European Medical Writers Association, and the Umberto Di Mario Foundation.
Source: Rosignolo F et al. J Endo Soc. 2017;1(1):3-13. ENDO 2018, Abstract OR17-1.
Recurrent head and neck cancer presenting as a large retroperitoneal mass
Worldwide, head and neck cancers account for more than half a million cases annually and nearly 400,000 deaths.1 Although the exact incidence of metastatic disease of these primarily squamous cell tumors is difficult to determine, the incidence is thought to be much lower than that of other solid tumors.2 When the different sites of metastatic disease of these tumors have been studied previously, the most common have been (in descending order of frequency) the lungs, bones, liver, skin, mediastinum, and bone marrow.2,3 It is extremely rare area for head and neck squamous cell cancers to metastasize to the retroperitoneum. To our knowledge, only 2 other such cases have been reported in the literature.4,5 In those two cases, the metastatic recurrence occurred at 6 and 13 months after definitive treatment of the primary cancer.
Case presentation and summary
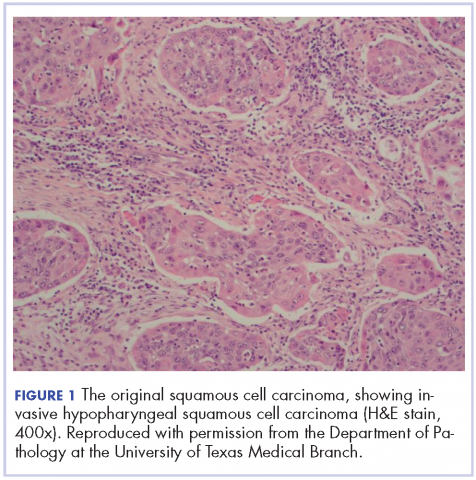
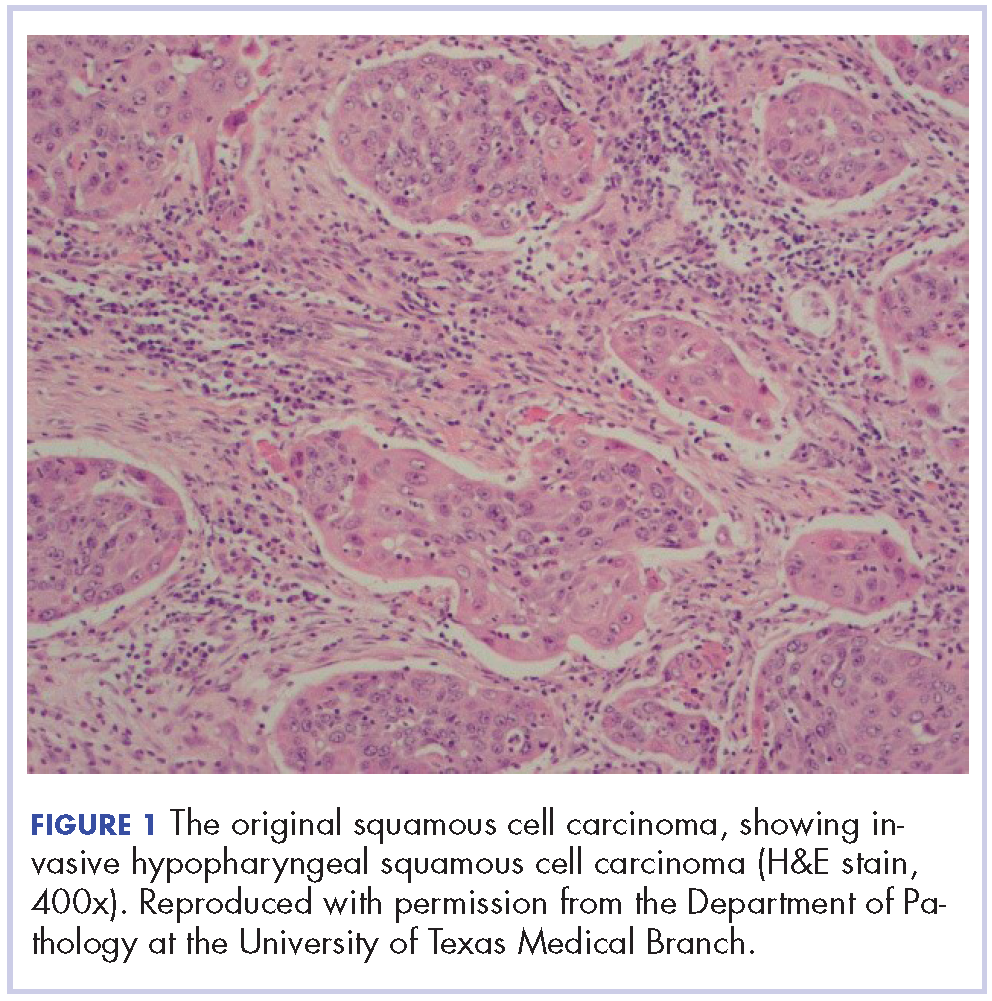
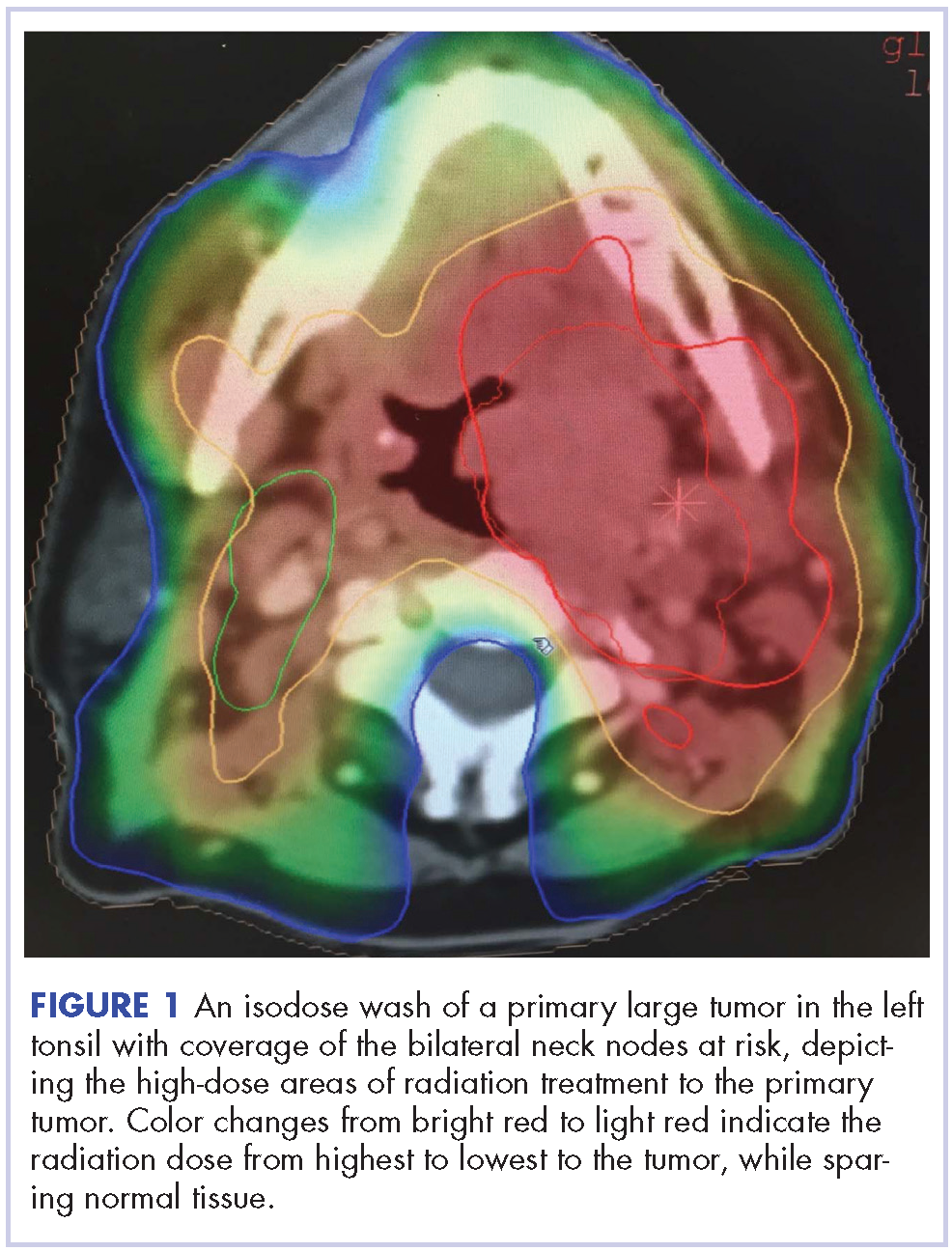
The patient in this case is a 60-year-old man with a history of stage IV moderately differentiated invasive squamous cell carcinoma (p16 negative, Bcl-2 negative, EGFR positive) of the hypopharynx that had been initially diagnosed in 2012. At that time, he underwent a total laryngectomy, partial pharyngectomy, and total thyroidectomy. A 2-centimeter mediastinal mass was also identified on a computed-tomography scan of the thorax and resected during the initial curative surgery. Final surgical pathology on the primary hypopharygeal tumor revealed a 4.1-cm moderately differentiated squamous cell carcinoma with negative margins, but positive lymphovascular invasion (Figure 1). The 2-cm mediastinal mass also revealed the same squamous cell carcinoma as the hypopharyngeal primary. Final surgical margins were negative.
The patient went on to receive adjuvant treatment in the form of concurrent chemoradiation with cisplatin (100 mg/m2 every 21 days for 3 doses, with 70 Gy of radiation]. After his initial treatment, he was followed closely by a multidisciplinary team, including otolaryngology, radiation oncology, and medical oncology specialists. He underwent a positron-emission tomography–CT scan 1 year after the conclusion of adjuvant therapy that showed no evidence of local or distant disease. The patient underwent 12 fiberoptic pharyngoscopy procedures over the course of 4 years without any evidence of local disease recurrence. He underwent a CT scan of the neck in October of 2016 without any evidence of local disease recurrence.
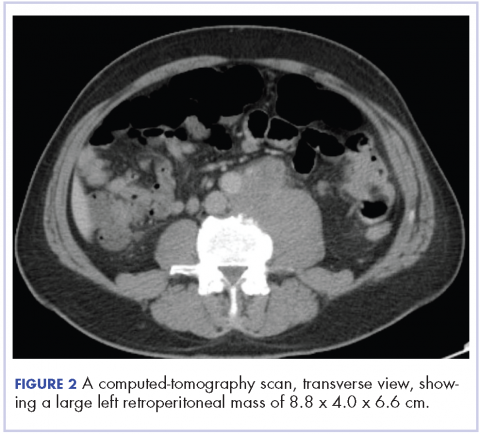
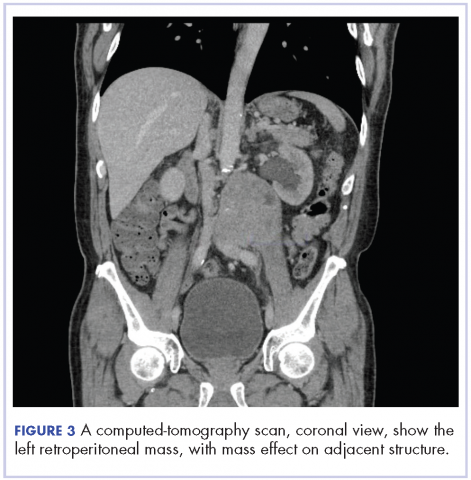
In early 2017, the patient presented with fatigue, abdominal pain, and back pain during the previous month. CT imaging revealed a left retroperitoneal mass of 8.8 x 4.0 x 6.6 cm, with bony destruction of L3-L4 causing left hydronephrosis (Figure 2 and Figure 3). Other staging work-up and imaging did not reveal any other distant disease or locoregional disease recurrence in the head and neck. Lab work was significant for an acute kidney injury that was likely secondary to mass effect from the tumor.
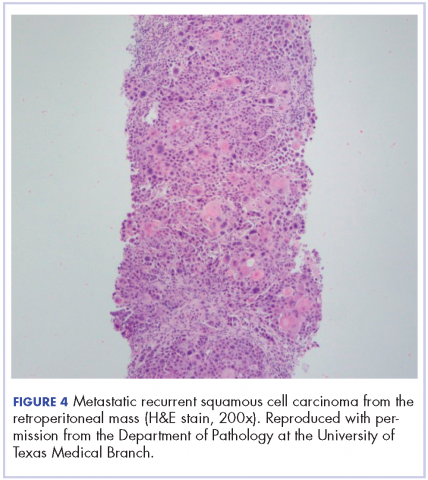
The mass was biopsied, with pathology revealing squamous cell carcinoma consistent with metastatic, recurrent disease from the previously known head and neck primary, and it was also p16 negative, Bcl-2 negative, and EGFR positive (Figure 4).
After a multidisciplinary discussion it was determined that the best front-line treatment option would be to treat with definitive concurrent chemoradiation. However, due to the size and location of the mass, it was not possible to deliver an effective therapeutic dose of radiation without unacceptable toxicity to the adjacent structures. Therefore, palliative systemic therapy was the only option. These treatment options, including systemic chemotherapy and immunotherapy, were discussed with the patient. However, he did not want to pursue any further cancer treatment and wanted instead to focus on palliation (pain control, antiemetics and nephrostomy to relieve obstruction) and hospice. He passed away 3 months later.
Discussion
Masses of the retroperitoneum have a wide differential diagnosis.6 Primary malignancies including lymphomas, sarcomas, neurogenic tumors, and germ cell tumors may all present primarily as retroperitoneal masses.6,7 Nonmalignant processes such as retroperitoneal fibrosis may also present in this manner.7 Certain tumors are known to metastasize to the retroperitoneum, namely carcinomas of the gastrointestinal tract and ovary as well as lung cancer or melanoma.5,8 Some primary retroperitoneal masses in women have been described in the literature as being HPV-associated squamous cell cancers of unknown primaries.9
When head and neck cancers metastasize they typically metastasize to the lungs, bone, liver, mediastinum, skin, and bone marrow. Most metastasis is pulmonary in origin, with the literature indicating it accounts for 52%-66% of head and neck cancer metastases, with bone metastases next in frequency at 12%-22%.2,3,10 In general, the incidence of distant metastatic disease in head and neck cancers is not as common as its other solid tumor counterparts, and even metastasis to other lymph node groups other than locoregional cervical nodes is rare.11 Furthermore, late metastasis occurring more than 2 years after definitive treatment is also an infrequent occurrence.12
When discussing distant metastatic disease in head and neck cancer, previous literature has described an increasing likelihood of distant metastases when there is locoregional disease recurrence.13 Moreover, the retroperitoneum is an exceedingly rare site of distant metastatic disease for head and neck cancer. There have been only 2 previous cases that have described this phenomenon, and in both cases the metastases occurred within or close to 1 year of definitive locoregional treatment.4,5
Conclusion
We present our case to present an exceedingly rare case of distant metastatic, recurrent disease from head and neck cancer to the retroperitoneum (without locoregional recurrence) that occurred 4 years after definitive treatment. We believe this to be the first case of its kind to be described when taking into consideration the site of metastases, when the metastatic recurrence occurred and that it happened without loco-regional disease recurrence. This case highlights the importance of keeping a wide differential diagnosis when encountering a retroperitoneal mass in a patient with even a remote history of head and neck cancer.
Acknowledgments
The authors thank the following members of the Department of Pathology at the University of Texas Medical Branch: Asad Ahmad, MD; Eduardo Eyzaguirre, MD; Timothy C Allen, MD, JD, FACP; and Suimmin Qiu, MD, PHD.
1. Fitzmaurice C, Allen C, Barber RM, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3:524-548.
2. Ferlito A, Shaha AR, Silver CE, Rinaldo A, Mondin V. Incidence and sites of distant metastases from head and neck cancer. ORL J Otorhinolaryngol Relat Spec. 2001;63:202-207.
3. Wiegand S, Zimmermann A, Wilhelm T, Werner JA. Survival after distant metastasis in head and neck cancer. Anticancer Res. 2015;35:5499-5502.
4. Hofmann U, O’Connor JP, Biyani CS, Harnden P, Selby P, Weston PM. Retroperitoneal metastatic squamous cell carcinoma of the tonsil (with elevated beta human chorionic gonadotrophin): a misdiagnosis as extra-gonadal germ cell tumour. J Laryngol Otol. 2006;120:885-887.
5. Purkayastha A, Sharma N, Suhag V. Extremely rare and unusual case of retroperitoneal and pelvic metastasis from squamous cell carcinoma of vallecula. Int J Cancer Ther Oncol. 2016;4(2):1-4.
6. Rajiah P, Sinha R, Cuevas C, Dubinsky TJ, Bush WH, Kolokythas O. Imaging of uncommon retroperitoneal masses. Radiographics 2011;31:949-976.
7. Scali EP, Chandler TM, Heffernan EJ, Coyle J, Harris AC, Chang SD. Primary retroperitoneal masses: what is the differential diagnosis? Abdom Imaging. 2015;40:1887-1903.
8. Levy AD, Shaw JC, Sobin LH. Secondary tumors and tumorlike lesions of the peritoneal cavity: imaging features with pathologic correlation. Radiographics. 2009;29:347-373.
9. Isbell A, Fields EC. Three cases of women with HPV-related squamous cell carcinoma of unknown primary in the pelvis and retroperitoneum: a case series. Gynecol Oncol Rep. 2016;16:5-8.
10. León X, Quer M, Orús C, del Prado Venegas M, López M. Distant metastases in head and neck cancer patients who achieved loco-regional control. Head Neck. 2000;22:680-686.
11. Alavi S, Namazie A, Sercarz JA, Wang MB, Blackwell KE. Distant lymphatic metastasis from head and neck cancer. Ann Otol Rhinol Laryngol. 1999;108:860-863.
12. Krishnatry R, Gupta T, Murthy V, et al. Factors predicting ‘time to distant metastasis’ in radically treated head and neck cancer. Indian J Cancer. 2014;51:231-235.
13. Goodwin WJ. Distant metastases from oropharyngeal cancer. ORL J Otorhinolaryngol Relat Spec. 2001;63:222-223.
Worldwide, head and neck cancers account for more than half a million cases annually and nearly 400,000 deaths.1 Although the exact incidence of metastatic disease of these primarily squamous cell tumors is difficult to determine, the incidence is thought to be much lower than that of other solid tumors.2 When the different sites of metastatic disease of these tumors have been studied previously, the most common have been (in descending order of frequency) the lungs, bones, liver, skin, mediastinum, and bone marrow.2,3 It is extremely rare area for head and neck squamous cell cancers to metastasize to the retroperitoneum. To our knowledge, only 2 other such cases have been reported in the literature.4,5 In those two cases, the metastatic recurrence occurred at 6 and 13 months after definitive treatment of the primary cancer.
Case presentation and summary
The patient in this case is a 60-year-old man with a history of stage IV moderately differentiated invasive squamous cell carcinoma (p16 negative, Bcl-2 negative, EGFR positive) of the hypopharynx that had been initially diagnosed in 2012. At that time, he underwent a total laryngectomy, partial pharyngectomy, and total thyroidectomy. A 2-centimeter mediastinal mass was also identified on a computed-tomography scan of the thorax and resected during the initial curative surgery. Final surgical pathology on the primary hypopharygeal tumor revealed a 4.1-cm moderately differentiated squamous cell carcinoma with negative margins, but positive lymphovascular invasion (Figure 1). The 2-cm mediastinal mass also revealed the same squamous cell carcinoma as the hypopharyngeal primary. Final surgical margins were negative.
The patient went on to receive adjuvant treatment in the form of concurrent chemoradiation with cisplatin (100 mg/m2 every 21 days for 3 doses, with 70 Gy of radiation]. After his initial treatment, he was followed closely by a multidisciplinary team, including otolaryngology, radiation oncology, and medical oncology specialists. He underwent a positron-emission tomography–CT scan 1 year after the conclusion of adjuvant therapy that showed no evidence of local or distant disease. The patient underwent 12 fiberoptic pharyngoscopy procedures over the course of 4 years without any evidence of local disease recurrence. He underwent a CT scan of the neck in October of 2016 without any evidence of local disease recurrence.
In early 2017, the patient presented with fatigue, abdominal pain, and back pain during the previous month. CT imaging revealed a left retroperitoneal mass of 8.8 x 4.0 x 6.6 cm, with bony destruction of L3-L4 causing left hydronephrosis (Figure 2 and Figure 3). Other staging work-up and imaging did not reveal any other distant disease or locoregional disease recurrence in the head and neck. Lab work was significant for an acute kidney injury that was likely secondary to mass effect from the tumor.
The mass was biopsied, with pathology revealing squamous cell carcinoma consistent with metastatic, recurrent disease from the previously known head and neck primary, and it was also p16 negative, Bcl-2 negative, and EGFR positive (Figure 4).
After a multidisciplinary discussion it was determined that the best front-line treatment option would be to treat with definitive concurrent chemoradiation. However, due to the size and location of the mass, it was not possible to deliver an effective therapeutic dose of radiation without unacceptable toxicity to the adjacent structures. Therefore, palliative systemic therapy was the only option. These treatment options, including systemic chemotherapy and immunotherapy, were discussed with the patient. However, he did not want to pursue any further cancer treatment and wanted instead to focus on palliation (pain control, antiemetics and nephrostomy to relieve obstruction) and hospice. He passed away 3 months later.
Discussion
Masses of the retroperitoneum have a wide differential diagnosis.6 Primary malignancies including lymphomas, sarcomas, neurogenic tumors, and germ cell tumors may all present primarily as retroperitoneal masses.6,7 Nonmalignant processes such as retroperitoneal fibrosis may also present in this manner.7 Certain tumors are known to metastasize to the retroperitoneum, namely carcinomas of the gastrointestinal tract and ovary as well as lung cancer or melanoma.5,8 Some primary retroperitoneal masses in women have been described in the literature as being HPV-associated squamous cell cancers of unknown primaries.9
When head and neck cancers metastasize they typically metastasize to the lungs, bone, liver, mediastinum, skin, and bone marrow. Most metastasis is pulmonary in origin, with the literature indicating it accounts for 52%-66% of head and neck cancer metastases, with bone metastases next in frequency at 12%-22%.2,3,10 In general, the incidence of distant metastatic disease in head and neck cancers is not as common as its other solid tumor counterparts, and even metastasis to other lymph node groups other than locoregional cervical nodes is rare.11 Furthermore, late metastasis occurring more than 2 years after definitive treatment is also an infrequent occurrence.12
When discussing distant metastatic disease in head and neck cancer, previous literature has described an increasing likelihood of distant metastases when there is locoregional disease recurrence.13 Moreover, the retroperitoneum is an exceedingly rare site of distant metastatic disease for head and neck cancer. There have been only 2 previous cases that have described this phenomenon, and in both cases the metastases occurred within or close to 1 year of definitive locoregional treatment.4,5
Conclusion
We present our case to present an exceedingly rare case of distant metastatic, recurrent disease from head and neck cancer to the retroperitoneum (without locoregional recurrence) that occurred 4 years after definitive treatment. We believe this to be the first case of its kind to be described when taking into consideration the site of metastases, when the metastatic recurrence occurred and that it happened without loco-regional disease recurrence. This case highlights the importance of keeping a wide differential diagnosis when encountering a retroperitoneal mass in a patient with even a remote history of head and neck cancer.
Acknowledgments
The authors thank the following members of the Department of Pathology at the University of Texas Medical Branch: Asad Ahmad, MD; Eduardo Eyzaguirre, MD; Timothy C Allen, MD, JD, FACP; and Suimmin Qiu, MD, PHD.
Worldwide, head and neck cancers account for more than half a million cases annually and nearly 400,000 deaths.1 Although the exact incidence of metastatic disease of these primarily squamous cell tumors is difficult to determine, the incidence is thought to be much lower than that of other solid tumors.2 When the different sites of metastatic disease of these tumors have been studied previously, the most common have been (in descending order of frequency) the lungs, bones, liver, skin, mediastinum, and bone marrow.2,3 It is extremely rare area for head and neck squamous cell cancers to metastasize to the retroperitoneum. To our knowledge, only 2 other such cases have been reported in the literature.4,5 In those two cases, the metastatic recurrence occurred at 6 and 13 months after definitive treatment of the primary cancer.
Case presentation and summary
The patient in this case is a 60-year-old man with a history of stage IV moderately differentiated invasive squamous cell carcinoma (p16 negative, Bcl-2 negative, EGFR positive) of the hypopharynx that had been initially diagnosed in 2012. At that time, he underwent a total laryngectomy, partial pharyngectomy, and total thyroidectomy. A 2-centimeter mediastinal mass was also identified on a computed-tomography scan of the thorax and resected during the initial curative surgery. Final surgical pathology on the primary hypopharygeal tumor revealed a 4.1-cm moderately differentiated squamous cell carcinoma with negative margins, but positive lymphovascular invasion (Figure 1). The 2-cm mediastinal mass also revealed the same squamous cell carcinoma as the hypopharyngeal primary. Final surgical margins were negative.
The patient went on to receive adjuvant treatment in the form of concurrent chemoradiation with cisplatin (100 mg/m2 every 21 days for 3 doses, with 70 Gy of radiation]. After his initial treatment, he was followed closely by a multidisciplinary team, including otolaryngology, radiation oncology, and medical oncology specialists. He underwent a positron-emission tomography–CT scan 1 year after the conclusion of adjuvant therapy that showed no evidence of local or distant disease. The patient underwent 12 fiberoptic pharyngoscopy procedures over the course of 4 years without any evidence of local disease recurrence. He underwent a CT scan of the neck in October of 2016 without any evidence of local disease recurrence.
In early 2017, the patient presented with fatigue, abdominal pain, and back pain during the previous month. CT imaging revealed a left retroperitoneal mass of 8.8 x 4.0 x 6.6 cm, with bony destruction of L3-L4 causing left hydronephrosis (Figure 2 and Figure 3). Other staging work-up and imaging did not reveal any other distant disease or locoregional disease recurrence in the head and neck. Lab work was significant for an acute kidney injury that was likely secondary to mass effect from the tumor.
The mass was biopsied, with pathology revealing squamous cell carcinoma consistent with metastatic, recurrent disease from the previously known head and neck primary, and it was also p16 negative, Bcl-2 negative, and EGFR positive (Figure 4).
After a multidisciplinary discussion it was determined that the best front-line treatment option would be to treat with definitive concurrent chemoradiation. However, due to the size and location of the mass, it was not possible to deliver an effective therapeutic dose of radiation without unacceptable toxicity to the adjacent structures. Therefore, palliative systemic therapy was the only option. These treatment options, including systemic chemotherapy and immunotherapy, were discussed with the patient. However, he did not want to pursue any further cancer treatment and wanted instead to focus on palliation (pain control, antiemetics and nephrostomy to relieve obstruction) and hospice. He passed away 3 months later.
Discussion
Masses of the retroperitoneum have a wide differential diagnosis.6 Primary malignancies including lymphomas, sarcomas, neurogenic tumors, and germ cell tumors may all present primarily as retroperitoneal masses.6,7 Nonmalignant processes such as retroperitoneal fibrosis may also present in this manner.7 Certain tumors are known to metastasize to the retroperitoneum, namely carcinomas of the gastrointestinal tract and ovary as well as lung cancer or melanoma.5,8 Some primary retroperitoneal masses in women have been described in the literature as being HPV-associated squamous cell cancers of unknown primaries.9
When head and neck cancers metastasize they typically metastasize to the lungs, bone, liver, mediastinum, skin, and bone marrow. Most metastasis is pulmonary in origin, with the literature indicating it accounts for 52%-66% of head and neck cancer metastases, with bone metastases next in frequency at 12%-22%.2,3,10 In general, the incidence of distant metastatic disease in head and neck cancers is not as common as its other solid tumor counterparts, and even metastasis to other lymph node groups other than locoregional cervical nodes is rare.11 Furthermore, late metastasis occurring more than 2 years after definitive treatment is also an infrequent occurrence.12
When discussing distant metastatic disease in head and neck cancer, previous literature has described an increasing likelihood of distant metastases when there is locoregional disease recurrence.13 Moreover, the retroperitoneum is an exceedingly rare site of distant metastatic disease for head and neck cancer. There have been only 2 previous cases that have described this phenomenon, and in both cases the metastases occurred within or close to 1 year of definitive locoregional treatment.4,5
Conclusion
We present our case to present an exceedingly rare case of distant metastatic, recurrent disease from head and neck cancer to the retroperitoneum (without locoregional recurrence) that occurred 4 years after definitive treatment. We believe this to be the first case of its kind to be described when taking into consideration the site of metastases, when the metastatic recurrence occurred and that it happened without loco-regional disease recurrence. This case highlights the importance of keeping a wide differential diagnosis when encountering a retroperitoneal mass in a patient with even a remote history of head and neck cancer.
Acknowledgments
The authors thank the following members of the Department of Pathology at the University of Texas Medical Branch: Asad Ahmad, MD; Eduardo Eyzaguirre, MD; Timothy C Allen, MD, JD, FACP; and Suimmin Qiu, MD, PHD.
1. Fitzmaurice C, Allen C, Barber RM, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3:524-548.
2. Ferlito A, Shaha AR, Silver CE, Rinaldo A, Mondin V. Incidence and sites of distant metastases from head and neck cancer. ORL J Otorhinolaryngol Relat Spec. 2001;63:202-207.
3. Wiegand S, Zimmermann A, Wilhelm T, Werner JA. Survival after distant metastasis in head and neck cancer. Anticancer Res. 2015;35:5499-5502.
4. Hofmann U, O’Connor JP, Biyani CS, Harnden P, Selby P, Weston PM. Retroperitoneal metastatic squamous cell carcinoma of the tonsil (with elevated beta human chorionic gonadotrophin): a misdiagnosis as extra-gonadal germ cell tumour. J Laryngol Otol. 2006;120:885-887.
5. Purkayastha A, Sharma N, Suhag V. Extremely rare and unusual case of retroperitoneal and pelvic metastasis from squamous cell carcinoma of vallecula. Int J Cancer Ther Oncol. 2016;4(2):1-4.
6. Rajiah P, Sinha R, Cuevas C, Dubinsky TJ, Bush WH, Kolokythas O. Imaging of uncommon retroperitoneal masses. Radiographics 2011;31:949-976.
7. Scali EP, Chandler TM, Heffernan EJ, Coyle J, Harris AC, Chang SD. Primary retroperitoneal masses: what is the differential diagnosis? Abdom Imaging. 2015;40:1887-1903.
8. Levy AD, Shaw JC, Sobin LH. Secondary tumors and tumorlike lesions of the peritoneal cavity: imaging features with pathologic correlation. Radiographics. 2009;29:347-373.
9. Isbell A, Fields EC. Three cases of women with HPV-related squamous cell carcinoma of unknown primary in the pelvis and retroperitoneum: a case series. Gynecol Oncol Rep. 2016;16:5-8.
10. León X, Quer M, Orús C, del Prado Venegas M, López M. Distant metastases in head and neck cancer patients who achieved loco-regional control. Head Neck. 2000;22:680-686.
11. Alavi S, Namazie A, Sercarz JA, Wang MB, Blackwell KE. Distant lymphatic metastasis from head and neck cancer. Ann Otol Rhinol Laryngol. 1999;108:860-863.
12. Krishnatry R, Gupta T, Murthy V, et al. Factors predicting ‘time to distant metastasis’ in radically treated head and neck cancer. Indian J Cancer. 2014;51:231-235.
13. Goodwin WJ. Distant metastases from oropharyngeal cancer. ORL J Otorhinolaryngol Relat Spec. 2001;63:222-223.
1. Fitzmaurice C, Allen C, Barber RM, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3:524-548.
2. Ferlito A, Shaha AR, Silver CE, Rinaldo A, Mondin V. Incidence and sites of distant metastases from head and neck cancer. ORL J Otorhinolaryngol Relat Spec. 2001;63:202-207.
3. Wiegand S, Zimmermann A, Wilhelm T, Werner JA. Survival after distant metastasis in head and neck cancer. Anticancer Res. 2015;35:5499-5502.
4. Hofmann U, O’Connor JP, Biyani CS, Harnden P, Selby P, Weston PM. Retroperitoneal metastatic squamous cell carcinoma of the tonsil (with elevated beta human chorionic gonadotrophin): a misdiagnosis as extra-gonadal germ cell tumour. J Laryngol Otol. 2006;120:885-887.
5. Purkayastha A, Sharma N, Suhag V. Extremely rare and unusual case of retroperitoneal and pelvic metastasis from squamous cell carcinoma of vallecula. Int J Cancer Ther Oncol. 2016;4(2):1-4.
6. Rajiah P, Sinha R, Cuevas C, Dubinsky TJ, Bush WH, Kolokythas O. Imaging of uncommon retroperitoneal masses. Radiographics 2011;31:949-976.
7. Scali EP, Chandler TM, Heffernan EJ, Coyle J, Harris AC, Chang SD. Primary retroperitoneal masses: what is the differential diagnosis? Abdom Imaging. 2015;40:1887-1903.
8. Levy AD, Shaw JC, Sobin LH. Secondary tumors and tumorlike lesions of the peritoneal cavity: imaging features with pathologic correlation. Radiographics. 2009;29:347-373.
9. Isbell A, Fields EC. Three cases of women with HPV-related squamous cell carcinoma of unknown primary in the pelvis and retroperitoneum: a case series. Gynecol Oncol Rep. 2016;16:5-8.
10. León X, Quer M, Orús C, del Prado Venegas M, López M. Distant metastases in head and neck cancer patients who achieved loco-regional control. Head Neck. 2000;22:680-686.
11. Alavi S, Namazie A, Sercarz JA, Wang MB, Blackwell KE. Distant lymphatic metastasis from head and neck cancer. Ann Otol Rhinol Laryngol. 1999;108:860-863.
12. Krishnatry R, Gupta T, Murthy V, et al. Factors predicting ‘time to distant metastasis’ in radically treated head and neck cancer. Indian J Cancer. 2014;51:231-235.
13. Goodwin WJ. Distant metastases from oropharyngeal cancer. ORL J Otorhinolaryngol Relat Spec. 2001;63:222-223.
Pembrolizumab plus SBRT shows promise for advanced solid tumors
SAN FRANCISCO – Pembrolizumab immunotherapy with multi-site stereotactic body radiotherapy (SBRT) appears to be a safe and effective treatment in patients with advanced solid tumors, according to findings from a phase 1 study.
Of 79 patients with metastatic solid tumors who progressed on standard treatment and who were enrolled in the study, 68 underwent multi-site SBRT, received at least one cycle of pembrolizumab (Keytruda), and had imaging follow-up. The overall objective response rate in those 68 patients was 13.2%, Jeffrey Lemons, MD, reported at the ASCO-SITC Clinical Immuno-Oncology Symposium.
When responses in the non-irradiated lesions (out-of-field responses) were measured based on a 30% reduction in any single lesion, the rate was 26.9%. But when defined by a 30% reduction in aggregate diameter of the non-irradiated measurable lesions, the rate was 13.5%, he said. While both approaches for measuring response are acceptable, Dr. Lemons noted, it’s important to be sure which one is being used in a given study.
Overall, 73 patients received both SBRT and pembrolizumab (5 had no imaging follow-up). They had a mean age of 62 years and a median of five prior therapies. Cancer types included ovarian/fallopian tube cancer (12.3%), non–small cell lung cancer (9.6%), breast cancer (8.2%), cholangiocarcinoma (8.2%), endometrial cancer (8.2%), colorectal cancer (6.8%), head and neck cancer (5.5%), and other tumors, each with less than 5% accrual (41.2%).
The number of sites treated with SBRT was two in 94.5% of patients, three in 4.1%, and four in 1.3%; 151 lesions in total were treated.
The premise for combining pembrolizumab and SBRT is that response to anti-programmed cell death-1 (PD1) therapy seems to correspond with interferon-gamma signaling, and that SBRT can stimulate innate and adaptive immunity to potentially augment immunotherapy, Dr. Lemons explained. In addition, anti-PD1 treatment outcomes are improved with lower disease burden.
Multi-site radiation is an emerging paradigm for eradicating metastatic disease, he said.
Patients included in the study had metastatic solid tumors and had progressed on standard treatment. They had measurable disease by RECIST, and metastases amenable to SBRT with 0.25 cc to 65 cc of viable tumor.
Tumors larger than 65 cc were partially targeted with radiotherapy. Radiation doses were adapted from recently completed and ongoing National Cancer Institute trials and ranged from 30-50 Gy (3-5 fractions) based on anatomic location.
Pembrolizumab was initiated within 7 days of the final SBRT treatment.
Dose-limiting toxicities, all grade 3, occurred in six patients during a median follow-up of 5.5 months, and included pneumonitis in three patients, hepatic failure in one patient, and colitis in two patients, but there were no radiation dose reductions, Dr. Lemons said.
“This is the first and largest prospective trial to determine the safety of this combination,” he explained. “There was some intriguing clinical activity ... and we feel that this justifies further randomized studies
The University of Chicago sponsored the study. Dr. Lemons reported having no disclosures.
SOURCE: Lemons J et al., ASCO-SITC abstract #20.
SAN FRANCISCO – Pembrolizumab immunotherapy with multi-site stereotactic body radiotherapy (SBRT) appears to be a safe and effective treatment in patients with advanced solid tumors, according to findings from a phase 1 study.
Of 79 patients with metastatic solid tumors who progressed on standard treatment and who were enrolled in the study, 68 underwent multi-site SBRT, received at least one cycle of pembrolizumab (Keytruda), and had imaging follow-up. The overall objective response rate in those 68 patients was 13.2%, Jeffrey Lemons, MD, reported at the ASCO-SITC Clinical Immuno-Oncology Symposium.
When responses in the non-irradiated lesions (out-of-field responses) were measured based on a 30% reduction in any single lesion, the rate was 26.9%. But when defined by a 30% reduction in aggregate diameter of the non-irradiated measurable lesions, the rate was 13.5%, he said. While both approaches for measuring response are acceptable, Dr. Lemons noted, it’s important to be sure which one is being used in a given study.
Overall, 73 patients received both SBRT and pembrolizumab (5 had no imaging follow-up). They had a mean age of 62 years and a median of five prior therapies. Cancer types included ovarian/fallopian tube cancer (12.3%), non–small cell lung cancer (9.6%), breast cancer (8.2%), cholangiocarcinoma (8.2%), endometrial cancer (8.2%), colorectal cancer (6.8%), head and neck cancer (5.5%), and other tumors, each with less than 5% accrual (41.2%).
The number of sites treated with SBRT was two in 94.5% of patients, three in 4.1%, and four in 1.3%; 151 lesions in total were treated.
The premise for combining pembrolizumab and SBRT is that response to anti-programmed cell death-1 (PD1) therapy seems to correspond with interferon-gamma signaling, and that SBRT can stimulate innate and adaptive immunity to potentially augment immunotherapy, Dr. Lemons explained. In addition, anti-PD1 treatment outcomes are improved with lower disease burden.
Multi-site radiation is an emerging paradigm for eradicating metastatic disease, he said.
Patients included in the study had metastatic solid tumors and had progressed on standard treatment. They had measurable disease by RECIST, and metastases amenable to SBRT with 0.25 cc to 65 cc of viable tumor.
Tumors larger than 65 cc were partially targeted with radiotherapy. Radiation doses were adapted from recently completed and ongoing National Cancer Institute trials and ranged from 30-50 Gy (3-5 fractions) based on anatomic location.
Pembrolizumab was initiated within 7 days of the final SBRT treatment.
Dose-limiting toxicities, all grade 3, occurred in six patients during a median follow-up of 5.5 months, and included pneumonitis in three patients, hepatic failure in one patient, and colitis in two patients, but there were no radiation dose reductions, Dr. Lemons said.
“This is the first and largest prospective trial to determine the safety of this combination,” he explained. “There was some intriguing clinical activity ... and we feel that this justifies further randomized studies
The University of Chicago sponsored the study. Dr. Lemons reported having no disclosures.
SOURCE: Lemons J et al., ASCO-SITC abstract #20.
SAN FRANCISCO – Pembrolizumab immunotherapy with multi-site stereotactic body radiotherapy (SBRT) appears to be a safe and effective treatment in patients with advanced solid tumors, according to findings from a phase 1 study.
Of 79 patients with metastatic solid tumors who progressed on standard treatment and who were enrolled in the study, 68 underwent multi-site SBRT, received at least one cycle of pembrolizumab (Keytruda), and had imaging follow-up. The overall objective response rate in those 68 patients was 13.2%, Jeffrey Lemons, MD, reported at the ASCO-SITC Clinical Immuno-Oncology Symposium.
When responses in the non-irradiated lesions (out-of-field responses) were measured based on a 30% reduction in any single lesion, the rate was 26.9%. But when defined by a 30% reduction in aggregate diameter of the non-irradiated measurable lesions, the rate was 13.5%, he said. While both approaches for measuring response are acceptable, Dr. Lemons noted, it’s important to be sure which one is being used in a given study.
Overall, 73 patients received both SBRT and pembrolizumab (5 had no imaging follow-up). They had a mean age of 62 years and a median of five prior therapies. Cancer types included ovarian/fallopian tube cancer (12.3%), non–small cell lung cancer (9.6%), breast cancer (8.2%), cholangiocarcinoma (8.2%), endometrial cancer (8.2%), colorectal cancer (6.8%), head and neck cancer (5.5%), and other tumors, each with less than 5% accrual (41.2%).
The number of sites treated with SBRT was two in 94.5% of patients, three in 4.1%, and four in 1.3%; 151 lesions in total were treated.
The premise for combining pembrolizumab and SBRT is that response to anti-programmed cell death-1 (PD1) therapy seems to correspond with interferon-gamma signaling, and that SBRT can stimulate innate and adaptive immunity to potentially augment immunotherapy, Dr. Lemons explained. In addition, anti-PD1 treatment outcomes are improved with lower disease burden.
Multi-site radiation is an emerging paradigm for eradicating metastatic disease, he said.
Patients included in the study had metastatic solid tumors and had progressed on standard treatment. They had measurable disease by RECIST, and metastases amenable to SBRT with 0.25 cc to 65 cc of viable tumor.
Tumors larger than 65 cc were partially targeted with radiotherapy. Radiation doses were adapted from recently completed and ongoing National Cancer Institute trials and ranged from 30-50 Gy (3-5 fractions) based on anatomic location.
Pembrolizumab was initiated within 7 days of the final SBRT treatment.
Dose-limiting toxicities, all grade 3, occurred in six patients during a median follow-up of 5.5 months, and included pneumonitis in three patients, hepatic failure in one patient, and colitis in two patients, but there were no radiation dose reductions, Dr. Lemons said.
“This is the first and largest prospective trial to determine the safety of this combination,” he explained. “There was some intriguing clinical activity ... and we feel that this justifies further randomized studies
The University of Chicago sponsored the study. Dr. Lemons reported having no disclosures.
SOURCE: Lemons J et al., ASCO-SITC abstract #20.
REPORTING FROM THE CLINICAL IMMUNO-ONCOLOGY SYMPOSIUM
Key clinical point: Pembrolizumab plus multi-site SBRT appears safe and effective for advanced solid tumors.
Major finding: The overall objective response rate was 13.2%.
Study details: A phase 1 study of 79 patients.
Disclosures: The University of Chicago sponsored the study. Dr. Lemons reported having no disclosures
Source: Lemons J et al. ASCO-SITC abstract #20.
New and improved classifiers may sharpen thyroid nodule diagnosis
VICTORIA, B.C. – Several new and improved molecular classifiers show good performance for preoperatively assessing the nature of thyroid nodules, including histologic subsets that continue to pose diagnostic challenges, according to a trio of studies reported at the annual meeting of the American Thyroid Association.
ThyroSeq v3 classifier
In a prospective, blinded, multi-institutional study, investigators validated the ThyroSeq v3 genomic classifier, which uses next-generation sequencing to test for mutations, fusions, gene expression alterations, and copy number variations in 112 genes.
The validation cohort consisted of 234 patients from 10 centers who had thyroid nodules with Bethesda III to V cytology and known surgical outcome, with central pathology review, and successful molecular testing. In total, they had 257 fine needle aspiration samples.
Of the 247 samples from nodules having Bethesda III or IV cytology – those of greatest interest – 28% were cancer or noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP), reported senior author Yuri Nikiforov, MD, PhD, professor of pathology and director of the division of molecular & genomic pathology at the University of Pittsburgh Medical Center. “Both cancer and NIFTP are surgical diseases, so we felt they belong in one group,” he noted.
Among the Bethesda III or IV samples, ThyroSeq v3 had a sensitivity of 94%, a specificity of 82%, a positive predictive value of 66%, and a negative predictive value of 97%. Additional analyses showed that the test would still have a negative predictive value of 95% or better up to a cancer/NIFTP prevalence of 44%.
All five false-negative cases in the entire study cohort were intrathyroidal nodules of low stage and without aggressive histology.
Of the 33 false-positive cases, 68% were diagnosed on pathology as Hurthle cell or follicular adenomas, 10% were initially diagnosed by local pathologists as cancer or NIFTP, and 94% harbored clonal oncogenic molecular alterations.
“So, these are not actually hyperplasia; these are true tumors. Probably at least some of them would have the potential to progress,” said Dr. Nikiforov. “I believe that this so-called false-positive rate may not be really false positive. This is a rate of detection of precancerous tumors, not hyperplasia, that still may require surgical excision.”
In this study, “we found very high sensitivity and negative predictive value of ThyroSeq v3, with robust negative predictive value in populations with different disease prevalence,” he concluded. “Robust performance was achieved in many thyroid cancer types, including Hurthle cell cancer.”
All study patients underwent surgery, so it is not clear how the classifier would perform in the context of surveillance, he acknowledged. But the 97% negative predictive value gives confidence for patients having a negative result.
“Those patients very likely can be observed – not necessarily dismissed from medical surveillance, but observed – and could probably avoid surgery,” he said. “If patients have a positive test, it will depend on the type of mutation, because some of them confer a high risk and others confer low risk. So, there may be a spectrum of management based on combination of clinical parameters and molecular testing. But those are more likely to be surgical candidates.”
“This is a study that is desperately needed in this field,” session attendee Bryan McIver, MD, PhD, an endocrinologist and deputy physician-in-chief at the Moffitt Cancer Center in Tampa, said in an interview. “These are very challenging studies to do, because the marketing of these molecular tests has run ahead of a lot of the clinical studies.
“It’s very hard in the United States, at least, to find patients who are truly naive to molecular testing whom you can take to the operating room,” he explained. “And if you can’t take patients with a negative molecular test to the operating room, then you can’t actually calculate the true sensitivity and specificity of the test, and the whole evaluation of the test starts to become skewed.”
According to Dr. McIver, this study is noteworthy in that it largely fulfills four key criteria: There were no post hoc sample exclusions after unblinding of data, both pathology evaluation and decision to operate were blinded to classifier results, and patients were generally unselected, with little to no prior molecular testing.
“So, we actually have a proper high-quality validation study now available for this new test, the ThyroSeq v3,” he noted. “That sets the bar where it needed to be set a long time ago, and I can’t begin to tell you how excited I am to finally have a test that passed that bar. The fact that it shows a negative predictive value of 97% in this clinical study and a positive predictive value in the mid-60% range means that there is a potential for a clinical utility there that is backed by solid science. In this field, that’s almost unique.”
Afirma GSC with Hurthle classifiers
In a second study, investigators led by Quan-Yang Duh, MD, professor of surgery, division of general surgery, and chief, section of endocrine surgery, University of California, San Francisco, developed and validated a pair of classifiers to enhance performance of the Afirma platform among Hurthle cell specimens.
“The Hurthle cell lesions tend to give us trouble,” Dr. Duh said. On molecular analysis, those that are malignant seldom harbor mutations that would aid diagnosis, whereas those that are benign are usually classified as suspicious by the original Afirma Gene Expression Classifier (GEC).
“The specific group that is causing trouble are those that are Hurthle cell but not neoplasm, because they are the ones that give you the false positives,” Dr. Duh said. Therefore, it makes sense to stratify lesions on both of these factors, and then subject that specific subset to a more stringent threshold.
The investigators developed two classifiers that work with the Afirma core Genomic Sequencing Classifier (GSC), which uses RNA sequencing and machine learning algorithms.
The first classifier uses differential expression of 1,408 genes to determine whether a sample contains Hurthle cells. The second classifier, applied only to lesions containing Hurthle cells, uses differential expression of 2,041 genes and assesses loss of heterozygosity – which is prevalent in Hurthle cell neoplasms – to determine whether a Hurthle cell lesion is a neoplasm.
The ensemble model then makes a final classification, using a higher threshold for suspicious lesions determined to be Hurthle cell but not neoplasm, and a normal threshold for all the rest.
The investigators validated the Afirma GSC with the two classifiers in blinded fashion using 186 thyroid lesion samples having Bethesda III or IV cytology that had been part of the overall multicenter validation of the original Afirma GEC (N Engl J Med. 2012 Aug 23;367[8]:705-15).
Among the 26 Hurthle cell lesions, specificity for identifying benign lesions improved from 11.8% with the original Afirma GEC to 58.8% with the Afirma GSC and new classifiers. That was an absolute gain of 47% (P = .012), Dr. Duh reported. Sensitivity for identifying cancer was 88.9%.
There were also smaller absolute gains in specificity of 18% among all lesions in the cohort (P = .0028) and 14% among non-Hurthle lesions (P = .028).
“The new GSC test has significantly improved specificity in the patients with Bethesda III and IV specimens with Hurthle cells, and this may reduce unnecessary diagnostic surgery,” said Dr. Duh. “Basically, there are fewer false positives and more patients who can be called benign in the Hurthle cell group who would not need an operation.”
Further validation is needed, he acknowledged. “For a while, I wouldn’t send my Hurthle cell aspirate patients for Afirma, because I knew it was going to come back suspicious. I think I will start to do it now, but we need to see what the answers look like” with additional validation.
Afirma GSC with medullary thyroid cancer classifier
In a third study, investigators developed and validated a classifier for medullary thyroid cancer to be used with the Afirma GSC. They were led by Gregory Randolph, MD, professor of otolaryngology and the Claire and John Bertucci Endowed Chair in Thyroid Surgical Oncology at Harvard Medical School, and division chief of the general and thyroid/parathyroid endocrine surgical divisions at the Massachusetts Eye and Ear Infirmary, Boston.
Better preoperative identification of this cancer is key for several reasons, he maintained.
Establishing the diagnosis from needle biopsy is challenging, because some features overlap with those of other thyroid lesions, according to Dr. Randolph. In about a third of patients with medullary thyroid cancer brought to the operating room, the diagnosis is unknown at the time, and that often results in inadequate initial surgery.
The investigators developed a medullary thyroid cancer classifier cassette that assesses differential expression of 108 genes. They then performed blinded, independent validation in a cohort of 211 fine-needle aspiration samples from thyroid nodules: 21 medullary thyroid cancers and 190 other benign and malignant neoplasms.
Results showed that the Afirma GSC with the medullary thyroid cancer classifier had sensitivity of 100% and specificity of 100%, reported Dr. Randolph.
“The Afirma GSC medullary thyroid cancer testing cassette, within the larger GSC system, uses RNA sequencing and advanced machine learning to improve the diagnostic detection of medullary thyroid cancer, which currently misses approximately a third of medullary thyroid cancer patients,” he said.
Session attendees wondered which patients are appropriate candidates and how much the test will cost.
“We have to have a discussion about that, because the missed medullaries are, frankly, widely distributed – they can be in any of the Bethesda categories, basically,” Dr. Randolph said. “So, there are cytopathologic mistakes made uniformly, including in the suspicious and frankly malignant Bethesda categories. In terms of cost, this is embedded in the GSC classifier; so, if you order that test, you will obtain this medullary cassette.”
Actual sensitivity of the classifier may ultimately be less than 100% with use in larger samples, he acknowledged. “I think a greater number of validation tests is absolutely in order. I imagine this classifier may not be perfect, but it is way better than the third we miss with just cytopathology.”
Dr. Nikiforov disclosed that he is owner of an IP for ThyroSeq, and that his laboratory has a contract to offer the test commercially. Dr. Duh disclosed that he had no relevant conflicts of interest. Dr. Randolph disclosed that he had no relevant conflicts of interest.
VICTORIA, B.C. – Several new and improved molecular classifiers show good performance for preoperatively assessing the nature of thyroid nodules, including histologic subsets that continue to pose diagnostic challenges, according to a trio of studies reported at the annual meeting of the American Thyroid Association.
ThyroSeq v3 classifier
In a prospective, blinded, multi-institutional study, investigators validated the ThyroSeq v3 genomic classifier, which uses next-generation sequencing to test for mutations, fusions, gene expression alterations, and copy number variations in 112 genes.
The validation cohort consisted of 234 patients from 10 centers who had thyroid nodules with Bethesda III to V cytology and known surgical outcome, with central pathology review, and successful molecular testing. In total, they had 257 fine needle aspiration samples.
Of the 247 samples from nodules having Bethesda III or IV cytology – those of greatest interest – 28% were cancer or noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP), reported senior author Yuri Nikiforov, MD, PhD, professor of pathology and director of the division of molecular & genomic pathology at the University of Pittsburgh Medical Center. “Both cancer and NIFTP are surgical diseases, so we felt they belong in one group,” he noted.
Among the Bethesda III or IV samples, ThyroSeq v3 had a sensitivity of 94%, a specificity of 82%, a positive predictive value of 66%, and a negative predictive value of 97%. Additional analyses showed that the test would still have a negative predictive value of 95% or better up to a cancer/NIFTP prevalence of 44%.
All five false-negative cases in the entire study cohort were intrathyroidal nodules of low stage and without aggressive histology.
Of the 33 false-positive cases, 68% were diagnosed on pathology as Hurthle cell or follicular adenomas, 10% were initially diagnosed by local pathologists as cancer or NIFTP, and 94% harbored clonal oncogenic molecular alterations.
“So, these are not actually hyperplasia; these are true tumors. Probably at least some of them would have the potential to progress,” said Dr. Nikiforov. “I believe that this so-called false-positive rate may not be really false positive. This is a rate of detection of precancerous tumors, not hyperplasia, that still may require surgical excision.”
In this study, “we found very high sensitivity and negative predictive value of ThyroSeq v3, with robust negative predictive value in populations with different disease prevalence,” he concluded. “Robust performance was achieved in many thyroid cancer types, including Hurthle cell cancer.”
All study patients underwent surgery, so it is not clear how the classifier would perform in the context of surveillance, he acknowledged. But the 97% negative predictive value gives confidence for patients having a negative result.
“Those patients very likely can be observed – not necessarily dismissed from medical surveillance, but observed – and could probably avoid surgery,” he said. “If patients have a positive test, it will depend on the type of mutation, because some of them confer a high risk and others confer low risk. So, there may be a spectrum of management based on combination of clinical parameters and molecular testing. But those are more likely to be surgical candidates.”
“This is a study that is desperately needed in this field,” session attendee Bryan McIver, MD, PhD, an endocrinologist and deputy physician-in-chief at the Moffitt Cancer Center in Tampa, said in an interview. “These are very challenging studies to do, because the marketing of these molecular tests has run ahead of a lot of the clinical studies.
“It’s very hard in the United States, at least, to find patients who are truly naive to molecular testing whom you can take to the operating room,” he explained. “And if you can’t take patients with a negative molecular test to the operating room, then you can’t actually calculate the true sensitivity and specificity of the test, and the whole evaluation of the test starts to become skewed.”
According to Dr. McIver, this study is noteworthy in that it largely fulfills four key criteria: There were no post hoc sample exclusions after unblinding of data, both pathology evaluation and decision to operate were blinded to classifier results, and patients were generally unselected, with little to no prior molecular testing.
“So, we actually have a proper high-quality validation study now available for this new test, the ThyroSeq v3,” he noted. “That sets the bar where it needed to be set a long time ago, and I can’t begin to tell you how excited I am to finally have a test that passed that bar. The fact that it shows a negative predictive value of 97% in this clinical study and a positive predictive value in the mid-60% range means that there is a potential for a clinical utility there that is backed by solid science. In this field, that’s almost unique.”
Afirma GSC with Hurthle classifiers
In a second study, investigators led by Quan-Yang Duh, MD, professor of surgery, division of general surgery, and chief, section of endocrine surgery, University of California, San Francisco, developed and validated a pair of classifiers to enhance performance of the Afirma platform among Hurthle cell specimens.
“The Hurthle cell lesions tend to give us trouble,” Dr. Duh said. On molecular analysis, those that are malignant seldom harbor mutations that would aid diagnosis, whereas those that are benign are usually classified as suspicious by the original Afirma Gene Expression Classifier (GEC).
“The specific group that is causing trouble are those that are Hurthle cell but not neoplasm, because they are the ones that give you the false positives,” Dr. Duh said. Therefore, it makes sense to stratify lesions on both of these factors, and then subject that specific subset to a more stringent threshold.
The investigators developed two classifiers that work with the Afirma core Genomic Sequencing Classifier (GSC), which uses RNA sequencing and machine learning algorithms.
The first classifier uses differential expression of 1,408 genes to determine whether a sample contains Hurthle cells. The second classifier, applied only to lesions containing Hurthle cells, uses differential expression of 2,041 genes and assesses loss of heterozygosity – which is prevalent in Hurthle cell neoplasms – to determine whether a Hurthle cell lesion is a neoplasm.
The ensemble model then makes a final classification, using a higher threshold for suspicious lesions determined to be Hurthle cell but not neoplasm, and a normal threshold for all the rest.
The investigators validated the Afirma GSC with the two classifiers in blinded fashion using 186 thyroid lesion samples having Bethesda III or IV cytology that had been part of the overall multicenter validation of the original Afirma GEC (N Engl J Med. 2012 Aug 23;367[8]:705-15).
Among the 26 Hurthle cell lesions, specificity for identifying benign lesions improved from 11.8% with the original Afirma GEC to 58.8% with the Afirma GSC and new classifiers. That was an absolute gain of 47% (P = .012), Dr. Duh reported. Sensitivity for identifying cancer was 88.9%.
There were also smaller absolute gains in specificity of 18% among all lesions in the cohort (P = .0028) and 14% among non-Hurthle lesions (P = .028).
“The new GSC test has significantly improved specificity in the patients with Bethesda III and IV specimens with Hurthle cells, and this may reduce unnecessary diagnostic surgery,” said Dr. Duh. “Basically, there are fewer false positives and more patients who can be called benign in the Hurthle cell group who would not need an operation.”
Further validation is needed, he acknowledged. “For a while, I wouldn’t send my Hurthle cell aspirate patients for Afirma, because I knew it was going to come back suspicious. I think I will start to do it now, but we need to see what the answers look like” with additional validation.
Afirma GSC with medullary thyroid cancer classifier
In a third study, investigators developed and validated a classifier for medullary thyroid cancer to be used with the Afirma GSC. They were led by Gregory Randolph, MD, professor of otolaryngology and the Claire and John Bertucci Endowed Chair in Thyroid Surgical Oncology at Harvard Medical School, and division chief of the general and thyroid/parathyroid endocrine surgical divisions at the Massachusetts Eye and Ear Infirmary, Boston.
Better preoperative identification of this cancer is key for several reasons, he maintained.
Establishing the diagnosis from needle biopsy is challenging, because some features overlap with those of other thyroid lesions, according to Dr. Randolph. In about a third of patients with medullary thyroid cancer brought to the operating room, the diagnosis is unknown at the time, and that often results in inadequate initial surgery.
The investigators developed a medullary thyroid cancer classifier cassette that assesses differential expression of 108 genes. They then performed blinded, independent validation in a cohort of 211 fine-needle aspiration samples from thyroid nodules: 21 medullary thyroid cancers and 190 other benign and malignant neoplasms.
Results showed that the Afirma GSC with the medullary thyroid cancer classifier had sensitivity of 100% and specificity of 100%, reported Dr. Randolph.
“The Afirma GSC medullary thyroid cancer testing cassette, within the larger GSC system, uses RNA sequencing and advanced machine learning to improve the diagnostic detection of medullary thyroid cancer, which currently misses approximately a third of medullary thyroid cancer patients,” he said.
Session attendees wondered which patients are appropriate candidates and how much the test will cost.
“We have to have a discussion about that, because the missed medullaries are, frankly, widely distributed – they can be in any of the Bethesda categories, basically,” Dr. Randolph said. “So, there are cytopathologic mistakes made uniformly, including in the suspicious and frankly malignant Bethesda categories. In terms of cost, this is embedded in the GSC classifier; so, if you order that test, you will obtain this medullary cassette.”
Actual sensitivity of the classifier may ultimately be less than 100% with use in larger samples, he acknowledged. “I think a greater number of validation tests is absolutely in order. I imagine this classifier may not be perfect, but it is way better than the third we miss with just cytopathology.”
Dr. Nikiforov disclosed that he is owner of an IP for ThyroSeq, and that his laboratory has a contract to offer the test commercially. Dr. Duh disclosed that he had no relevant conflicts of interest. Dr. Randolph disclosed that he had no relevant conflicts of interest.
VICTORIA, B.C. – Several new and improved molecular classifiers show good performance for preoperatively assessing the nature of thyroid nodules, including histologic subsets that continue to pose diagnostic challenges, according to a trio of studies reported at the annual meeting of the American Thyroid Association.
ThyroSeq v3 classifier
In a prospective, blinded, multi-institutional study, investigators validated the ThyroSeq v3 genomic classifier, which uses next-generation sequencing to test for mutations, fusions, gene expression alterations, and copy number variations in 112 genes.
The validation cohort consisted of 234 patients from 10 centers who had thyroid nodules with Bethesda III to V cytology and known surgical outcome, with central pathology review, and successful molecular testing. In total, they had 257 fine needle aspiration samples.
Of the 247 samples from nodules having Bethesda III or IV cytology – those of greatest interest – 28% were cancer or noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP), reported senior author Yuri Nikiforov, MD, PhD, professor of pathology and director of the division of molecular & genomic pathology at the University of Pittsburgh Medical Center. “Both cancer and NIFTP are surgical diseases, so we felt they belong in one group,” he noted.
Among the Bethesda III or IV samples, ThyroSeq v3 had a sensitivity of 94%, a specificity of 82%, a positive predictive value of 66%, and a negative predictive value of 97%. Additional analyses showed that the test would still have a negative predictive value of 95% or better up to a cancer/NIFTP prevalence of 44%.
All five false-negative cases in the entire study cohort were intrathyroidal nodules of low stage and without aggressive histology.
Of the 33 false-positive cases, 68% were diagnosed on pathology as Hurthle cell or follicular adenomas, 10% were initially diagnosed by local pathologists as cancer or NIFTP, and 94% harbored clonal oncogenic molecular alterations.
“So, these are not actually hyperplasia; these are true tumors. Probably at least some of them would have the potential to progress,” said Dr. Nikiforov. “I believe that this so-called false-positive rate may not be really false positive. This is a rate of detection of precancerous tumors, not hyperplasia, that still may require surgical excision.”
In this study, “we found very high sensitivity and negative predictive value of ThyroSeq v3, with robust negative predictive value in populations with different disease prevalence,” he concluded. “Robust performance was achieved in many thyroid cancer types, including Hurthle cell cancer.”
All study patients underwent surgery, so it is not clear how the classifier would perform in the context of surveillance, he acknowledged. But the 97% negative predictive value gives confidence for patients having a negative result.
“Those patients very likely can be observed – not necessarily dismissed from medical surveillance, but observed – and could probably avoid surgery,” he said. “If patients have a positive test, it will depend on the type of mutation, because some of them confer a high risk and others confer low risk. So, there may be a spectrum of management based on combination of clinical parameters and molecular testing. But those are more likely to be surgical candidates.”
“This is a study that is desperately needed in this field,” session attendee Bryan McIver, MD, PhD, an endocrinologist and deputy physician-in-chief at the Moffitt Cancer Center in Tampa, said in an interview. “These are very challenging studies to do, because the marketing of these molecular tests has run ahead of a lot of the clinical studies.
“It’s very hard in the United States, at least, to find patients who are truly naive to molecular testing whom you can take to the operating room,” he explained. “And if you can’t take patients with a negative molecular test to the operating room, then you can’t actually calculate the true sensitivity and specificity of the test, and the whole evaluation of the test starts to become skewed.”
According to Dr. McIver, this study is noteworthy in that it largely fulfills four key criteria: There were no post hoc sample exclusions after unblinding of data, both pathology evaluation and decision to operate were blinded to classifier results, and patients were generally unselected, with little to no prior molecular testing.
“So, we actually have a proper high-quality validation study now available for this new test, the ThyroSeq v3,” he noted. “That sets the bar where it needed to be set a long time ago, and I can’t begin to tell you how excited I am to finally have a test that passed that bar. The fact that it shows a negative predictive value of 97% in this clinical study and a positive predictive value in the mid-60% range means that there is a potential for a clinical utility there that is backed by solid science. In this field, that’s almost unique.”
Afirma GSC with Hurthle classifiers
In a second study, investigators led by Quan-Yang Duh, MD, professor of surgery, division of general surgery, and chief, section of endocrine surgery, University of California, San Francisco, developed and validated a pair of classifiers to enhance performance of the Afirma platform among Hurthle cell specimens.
“The Hurthle cell lesions tend to give us trouble,” Dr. Duh said. On molecular analysis, those that are malignant seldom harbor mutations that would aid diagnosis, whereas those that are benign are usually classified as suspicious by the original Afirma Gene Expression Classifier (GEC).
“The specific group that is causing trouble are those that are Hurthle cell but not neoplasm, because they are the ones that give you the false positives,” Dr. Duh said. Therefore, it makes sense to stratify lesions on both of these factors, and then subject that specific subset to a more stringent threshold.
The investigators developed two classifiers that work with the Afirma core Genomic Sequencing Classifier (GSC), which uses RNA sequencing and machine learning algorithms.
The first classifier uses differential expression of 1,408 genes to determine whether a sample contains Hurthle cells. The second classifier, applied only to lesions containing Hurthle cells, uses differential expression of 2,041 genes and assesses loss of heterozygosity – which is prevalent in Hurthle cell neoplasms – to determine whether a Hurthle cell lesion is a neoplasm.
The ensemble model then makes a final classification, using a higher threshold for suspicious lesions determined to be Hurthle cell but not neoplasm, and a normal threshold for all the rest.
The investigators validated the Afirma GSC with the two classifiers in blinded fashion using 186 thyroid lesion samples having Bethesda III or IV cytology that had been part of the overall multicenter validation of the original Afirma GEC (N Engl J Med. 2012 Aug 23;367[8]:705-15).
Among the 26 Hurthle cell lesions, specificity for identifying benign lesions improved from 11.8% with the original Afirma GEC to 58.8% with the Afirma GSC and new classifiers. That was an absolute gain of 47% (P = .012), Dr. Duh reported. Sensitivity for identifying cancer was 88.9%.
There were also smaller absolute gains in specificity of 18% among all lesions in the cohort (P = .0028) and 14% among non-Hurthle lesions (P = .028).
“The new GSC test has significantly improved specificity in the patients with Bethesda III and IV specimens with Hurthle cells, and this may reduce unnecessary diagnostic surgery,” said Dr. Duh. “Basically, there are fewer false positives and more patients who can be called benign in the Hurthle cell group who would not need an operation.”
Further validation is needed, he acknowledged. “For a while, I wouldn’t send my Hurthle cell aspirate patients for Afirma, because I knew it was going to come back suspicious. I think I will start to do it now, but we need to see what the answers look like” with additional validation.
Afirma GSC with medullary thyroid cancer classifier
In a third study, investigators developed and validated a classifier for medullary thyroid cancer to be used with the Afirma GSC. They were led by Gregory Randolph, MD, professor of otolaryngology and the Claire and John Bertucci Endowed Chair in Thyroid Surgical Oncology at Harvard Medical School, and division chief of the general and thyroid/parathyroid endocrine surgical divisions at the Massachusetts Eye and Ear Infirmary, Boston.
Better preoperative identification of this cancer is key for several reasons, he maintained.
Establishing the diagnosis from needle biopsy is challenging, because some features overlap with those of other thyroid lesions, according to Dr. Randolph. In about a third of patients with medullary thyroid cancer brought to the operating room, the diagnosis is unknown at the time, and that often results in inadequate initial surgery.
The investigators developed a medullary thyroid cancer classifier cassette that assesses differential expression of 108 genes. They then performed blinded, independent validation in a cohort of 211 fine-needle aspiration samples from thyroid nodules: 21 medullary thyroid cancers and 190 other benign and malignant neoplasms.
Results showed that the Afirma GSC with the medullary thyroid cancer classifier had sensitivity of 100% and specificity of 100%, reported Dr. Randolph.
“The Afirma GSC medullary thyroid cancer testing cassette, within the larger GSC system, uses RNA sequencing and advanced machine learning to improve the diagnostic detection of medullary thyroid cancer, which currently misses approximately a third of medullary thyroid cancer patients,” he said.
Session attendees wondered which patients are appropriate candidates and how much the test will cost.
“We have to have a discussion about that, because the missed medullaries are, frankly, widely distributed – they can be in any of the Bethesda categories, basically,” Dr. Randolph said. “So, there are cytopathologic mistakes made uniformly, including in the suspicious and frankly malignant Bethesda categories. In terms of cost, this is embedded in the GSC classifier; so, if you order that test, you will obtain this medullary cassette.”
Actual sensitivity of the classifier may ultimately be less than 100% with use in larger samples, he acknowledged. “I think a greater number of validation tests is absolutely in order. I imagine this classifier may not be perfect, but it is way better than the third we miss with just cytopathology.”
Dr. Nikiforov disclosed that he is owner of an IP for ThyroSeq, and that his laboratory has a contract to offer the test commercially. Dr. Duh disclosed that he had no relevant conflicts of interest. Dr. Randolph disclosed that he had no relevant conflicts of interest.
AT ATA 2017
Key clinical point:
Major finding: ThyroSeq v3 had a negative predictive value of 97%. Specificity was an absolute 47% greater for Afirma GSC with Hurthle-specific classifiers than for Afirma GEC. The Afirma GSC with a medullary thyroid cancer classifier had 100% sensitivity and specificity.
Data source: Validation studies of the ThyroSeq v3 classifier (257 samples), the Afirma GSC with Hurthle-specific classifiers (186 samples), and the Afirma GSC with a medullary thyroid cancer classifier (211 samples).
Disclosures: Dr. Nikiforov disclosed that he is owner of an IP for ThyroSeq, and that his laboratory has a contract to offer the test commercially. Dr. Duh disclosed that he had no relevant conflicts of interest. Dr. Randolph disclosed that he had no relevant conflicts of interest.
ASCO larynx-preservation guidelines reflect important practice changes
The latest edition of the clinical practice guideline on larynx preservation strategies for the treatment of laryngeal cancer from the American Society of Clinical Oncology (ASCO) emphasizes that larynx preservation in patients with early stage disease does not compromise survival compared with total laryngectomy.
“The nuances of treatment selection, assessments of pretreatment voice and swallowing, and public awareness of new organ-preservation treatment and decision making have increased to the point that careful and individualized discussion with patients and families with the multidisciplinary treatment team is a critical element of modern care,” wrote Arlene A. Forastiere, MD, of Johns Hopkins Medicine in Baltimore, and her colleagues. The report was published in the Journal of Clinical Oncology.
Changes since the last guideline on the subject, issued in 2006, include evidence-based support for the use of endoscopic resection in patients with limited stage (T1 and T2) disease, and as an initial total laryngectomy therapy both in patients with stage T4a disease, and in those with severe laryngeal dysfunction prior to treatment.
Also new since the last guideline are recommendations for the use of positron-emission tomography imaging for evaluating the status of regional nodes after treatment, as well as guidance on the best techniques for evaluating voice and swallowing function.
While the initial recommendation that all patients with T1 and T2 laryngeal cancer should be treated with the intent to preserve the larynx has not changed, there is a new recommendation (1.3) stating that surgery may be more effective than radiotherapy for initial larynx preservation therapy, although this recommendation is based on retrospective data and may be affected by patient selection factors, the authors acknowledged. The new recommendation also notes that in an experienced operator’s hands, endoscopic resections can have outcomes that are equal to or better than those with open partial laryngectomy.
The initial recommendation stating that “[e]very effort should be made to avoid combining surgery with radiation therapy because functional outcomes may be compromised by combined-modality therapy; single-modality treatment is effective for limited-stage, invasive cancer of the larynx” remains unchanged.
There is also an updated recommendation that tumor-free margins should be the goal when surgery with larynx preservation intent is performed (1.4).
“Surgery that anticipates the need for postoperative [radiation therapy] to treat close or involved tumor margins or widespread dysplasia is not an acceptable treatment approach,” the guideline authors noted.
There are two other new recommendations including the opinion, based on evidence of benefits vs. harms, that total laryngectomy rather than larynx preservation may be associated with better survival and quality of life in patients with extensive T3 lesions, large T4 lesions, or in those who have poor pretreatment laryngeal function.
The third new recommendation is that “[a]s part of a comprehensive pretreatment evaluation, all patients should undergo a baseline assessment of voice and swallowing function, voice (use and requirements), and counseling with regard to the potential effect of treatment options on voice, swallowing, and quality of life.”
Among the updated recommendations are the following:
• An emphasis on the importance of considering a multiplicity of factors when choosing therapy for patients with limited-stage disease (1.7).
• The option of specialized organ-preservation procedures for a small number of patients with T3 or T4 primary site disease (2.4).
• A strong recommendation for the use of concurrent chemoradiotherapy compared with radiotherapy alone or sequential therapy (2.5).
• Elective neck dissection is not required for patients with clinically involved regional cervical nodes treated with definitive radiotherapy of chemoradiotherapy who have complete clinical, radiologic, and metabolic imaging (3.3).
• “Selection of therapy for an individual patient requires assessment by the multidisciplinary team as well as consideration of voice and swallowing function; patient comorbidity, psychosocial situation, and preferences; and local therapeutic expertise” (4.2).
The guideline development process was supported by ASCO. Dr, Forastiere disclosed employment and stock ownership in NantHealth. Many of her coauthors disclosed institutional funding, consultation/advising, travel support and expenses, honoraria, and or patents/royalties with multiple entities.
The latest edition of the clinical practice guideline on larynx preservation strategies for the treatment of laryngeal cancer from the American Society of Clinical Oncology (ASCO) emphasizes that larynx preservation in patients with early stage disease does not compromise survival compared with total laryngectomy.
“The nuances of treatment selection, assessments of pretreatment voice and swallowing, and public awareness of new organ-preservation treatment and decision making have increased to the point that careful and individualized discussion with patients and families with the multidisciplinary treatment team is a critical element of modern care,” wrote Arlene A. Forastiere, MD, of Johns Hopkins Medicine in Baltimore, and her colleagues. The report was published in the Journal of Clinical Oncology.
Changes since the last guideline on the subject, issued in 2006, include evidence-based support for the use of endoscopic resection in patients with limited stage (T1 and T2) disease, and as an initial total laryngectomy therapy both in patients with stage T4a disease, and in those with severe laryngeal dysfunction prior to treatment.
Also new since the last guideline are recommendations for the use of positron-emission tomography imaging for evaluating the status of regional nodes after treatment, as well as guidance on the best techniques for evaluating voice and swallowing function.
While the initial recommendation that all patients with T1 and T2 laryngeal cancer should be treated with the intent to preserve the larynx has not changed, there is a new recommendation (1.3) stating that surgery may be more effective than radiotherapy for initial larynx preservation therapy, although this recommendation is based on retrospective data and may be affected by patient selection factors, the authors acknowledged. The new recommendation also notes that in an experienced operator’s hands, endoscopic resections can have outcomes that are equal to or better than those with open partial laryngectomy.
The initial recommendation stating that “[e]very effort should be made to avoid combining surgery with radiation therapy because functional outcomes may be compromised by combined-modality therapy; single-modality treatment is effective for limited-stage, invasive cancer of the larynx” remains unchanged.
There is also an updated recommendation that tumor-free margins should be the goal when surgery with larynx preservation intent is performed (1.4).
“Surgery that anticipates the need for postoperative [radiation therapy] to treat close or involved tumor margins or widespread dysplasia is not an acceptable treatment approach,” the guideline authors noted.
There are two other new recommendations including the opinion, based on evidence of benefits vs. harms, that total laryngectomy rather than larynx preservation may be associated with better survival and quality of life in patients with extensive T3 lesions, large T4 lesions, or in those who have poor pretreatment laryngeal function.
The third new recommendation is that “[a]s part of a comprehensive pretreatment evaluation, all patients should undergo a baseline assessment of voice and swallowing function, voice (use and requirements), and counseling with regard to the potential effect of treatment options on voice, swallowing, and quality of life.”
Among the updated recommendations are the following:
• An emphasis on the importance of considering a multiplicity of factors when choosing therapy for patients with limited-stage disease (1.7).
• The option of specialized organ-preservation procedures for a small number of patients with T3 or T4 primary site disease (2.4).
• A strong recommendation for the use of concurrent chemoradiotherapy compared with radiotherapy alone or sequential therapy (2.5).
• Elective neck dissection is not required for patients with clinically involved regional cervical nodes treated with definitive radiotherapy of chemoradiotherapy who have complete clinical, radiologic, and metabolic imaging (3.3).
• “Selection of therapy for an individual patient requires assessment by the multidisciplinary team as well as consideration of voice and swallowing function; patient comorbidity, psychosocial situation, and preferences; and local therapeutic expertise” (4.2).
The guideline development process was supported by ASCO. Dr, Forastiere disclosed employment and stock ownership in NantHealth. Many of her coauthors disclosed institutional funding, consultation/advising, travel support and expenses, honoraria, and or patents/royalties with multiple entities.
The latest edition of the clinical practice guideline on larynx preservation strategies for the treatment of laryngeal cancer from the American Society of Clinical Oncology (ASCO) emphasizes that larynx preservation in patients with early stage disease does not compromise survival compared with total laryngectomy.
“The nuances of treatment selection, assessments of pretreatment voice and swallowing, and public awareness of new organ-preservation treatment and decision making have increased to the point that careful and individualized discussion with patients and families with the multidisciplinary treatment team is a critical element of modern care,” wrote Arlene A. Forastiere, MD, of Johns Hopkins Medicine in Baltimore, and her colleagues. The report was published in the Journal of Clinical Oncology.
Changes since the last guideline on the subject, issued in 2006, include evidence-based support for the use of endoscopic resection in patients with limited stage (T1 and T2) disease, and as an initial total laryngectomy therapy both in patients with stage T4a disease, and in those with severe laryngeal dysfunction prior to treatment.
Also new since the last guideline are recommendations for the use of positron-emission tomography imaging for evaluating the status of regional nodes after treatment, as well as guidance on the best techniques for evaluating voice and swallowing function.
While the initial recommendation that all patients with T1 and T2 laryngeal cancer should be treated with the intent to preserve the larynx has not changed, there is a new recommendation (1.3) stating that surgery may be more effective than radiotherapy for initial larynx preservation therapy, although this recommendation is based on retrospective data and may be affected by patient selection factors, the authors acknowledged. The new recommendation also notes that in an experienced operator’s hands, endoscopic resections can have outcomes that are equal to or better than those with open partial laryngectomy.
The initial recommendation stating that “[e]very effort should be made to avoid combining surgery with radiation therapy because functional outcomes may be compromised by combined-modality therapy; single-modality treatment is effective for limited-stage, invasive cancer of the larynx” remains unchanged.
There is also an updated recommendation that tumor-free margins should be the goal when surgery with larynx preservation intent is performed (1.4).
“Surgery that anticipates the need for postoperative [radiation therapy] to treat close or involved tumor margins or widespread dysplasia is not an acceptable treatment approach,” the guideline authors noted.
There are two other new recommendations including the opinion, based on evidence of benefits vs. harms, that total laryngectomy rather than larynx preservation may be associated with better survival and quality of life in patients with extensive T3 lesions, large T4 lesions, or in those who have poor pretreatment laryngeal function.
The third new recommendation is that “[a]s part of a comprehensive pretreatment evaluation, all patients should undergo a baseline assessment of voice and swallowing function, voice (use and requirements), and counseling with regard to the potential effect of treatment options on voice, swallowing, and quality of life.”
Among the updated recommendations are the following:
• An emphasis on the importance of considering a multiplicity of factors when choosing therapy for patients with limited-stage disease (1.7).
• The option of specialized organ-preservation procedures for a small number of patients with T3 or T4 primary site disease (2.4).
• A strong recommendation for the use of concurrent chemoradiotherapy compared with radiotherapy alone or sequential therapy (2.5).
• Elective neck dissection is not required for patients with clinically involved regional cervical nodes treated with definitive radiotherapy of chemoradiotherapy who have complete clinical, radiologic, and metabolic imaging (3.3).
• “Selection of therapy for an individual patient requires assessment by the multidisciplinary team as well as consideration of voice and swallowing function; patient comorbidity, psychosocial situation, and preferences; and local therapeutic expertise” (4.2).
The guideline development process was supported by ASCO. Dr, Forastiere disclosed employment and stock ownership in NantHealth. Many of her coauthors disclosed institutional funding, consultation/advising, travel support and expenses, honoraria, and or patents/royalties with multiple entities.
FROM JCO
Management of tonsillar carcinoma with advanced radiation therapy and chemotherapy techniques
Tonsillar carcinoma is the most common of the oropharyngeal malignancies of the head and neck region after thyroid and laryngeal carcinoma. Squamous cell carcinoma is the most frequent histologic type of these tumors.1 Tonsillar tumors may originate in the oral cavity, oropharynx, hypopharynx, or larynx. In the United States, more than 5,000 new cases of oropharynx cancer are diagnosed annually.2 Men are affected three to four times more often than are women, and the rate of incidence increases after the 4th decade of life.3 Surveillance, Epidemiology, and End Results data from 1975-2004 show that tonsillar squamous cell carcinoma has had one of the largest increases in the male-to-female incidence rate ratios.4 The overall incidence of tonsillar carcinoma is increasing, especially in the younger population, and this may be attributed to increasing rates of human papilloma virus.5,6
Squamous cell carcinoma in the head and neck originate from subsites within the oral cavity, oropharynx, hypopharynx, larynx, and nasopharynx.7 Traditionally, alcohol consumption and tobacco use were considered the most significant risk factors for the development of tonsillar cancer.8 More recently, however, the high-risk oncogenic human papilloma virus has emerged as a clinical entity in the pathogenesis of squamous cell carcinoma in the head and neck. Other risk factors include poor oral hygiene, mechanical irritation, chewing of betel quid preparations, and a lack of vegetables and fruits in the diet.9-11 Squamous cell carcinoma of the oropharynx often presents late with lymph node involvement at the time of diagnosis. Nonspecific symptoms such as a sore throat and dysphagia can allow head and neck cancer to evade early detection. Many patients with tonsillar carcinoma present with advanced disease because early lesions are generally asymptomatic when small. This absence of symptoms is responsible for 67%-77% of patients presenting with tumors larger than 2.0 cm and often with regional nodal metastasis. At presentation, 45% of anterior tonsillar pillar lesions and 76% of tonsillar fossa lesions have clinically positive necks.12
Despite significant treatment advances, the management of advanced squamous cell carcinoma of the tonsil remains challenging. Historically, surgery was considered the standard of care for patients with tonsillar carcinoma with or without postoperative adjuvant radiotherapy. In locally advanced tonsillar carcinoma, extensive surgery with major tissue reconstruction was necessary, leading to speech dysfunction, cosmetic deformities, and difficulties in swallowing, all of which are detrimental to patient quality of life.13 Given the critical role of the oropharynx in speech and swallowing, nonsurgical therapy with organ-preserving chemoradiation has gained a greater role in the treatment of tonsil carcinoma.13 Over the past decade, innovations in radiation therapy techniques have led to the introduction of intensity-modulated radiation therapy (IMRT) and image-guided radiation therapy (IGRT) for the treatment of various cancers including tonsillar carcinoma.14,15 IMRT is an advanced mode of conformal high-precision radiotherapy that uses computer-controlled multiple small radiation beams of varying intensities to deliver precise radiation doses to the target tissues while sparing adjacent healthy tissues.14 By incorporating three-dimensional computed-tomography (CT) or positron-emission–tomography (PET) imaging technology, IMRT allows the radiation dose to conform more precisely to the three-dimensional shape of the tumor while modulating the intensity of the radiation beam and minimizing its dose to those adjacent sensitive and unaffected organs. IGRT uses a range of two-, three-, and four-dimensional imaging techniques that improve the precision and accuracy of the delivery of the radiation dose to the targeted tumor tissue while minimizing the dose to the surrounding normal tissue during the course of radiation therapy (Figure 1). In this report, we present challenging cases of advanced tonsillar carcinoma and describe our experience in managing the disease using a hyperfractionated IMRT-IGRT based three-dimensional conformal radiation therapy protocol with concurrent chemotherapy.
Case presentations and summaries
Case 1
A 52-year-old white, nonsmoking man who worked in a research chemical laboratory, presented with complaints of throat pain and difficulty in swallowing. The patient had a history of asthma and allergies and had been seen by an ear, nose, and throat (ENT) specialist prior to his visit to our oncology center. A biopsy was performed on a right tonsillar mass measuring 2.7 x 3.6 cm. A computed-tomography (CT) scan showed 2 enlarged inhomogeneous lymph nodes measuring 2.9 cm and 1.7 cm. The nodes were well defined with no soft tissue edema. Neoplasm was favored as a diagnosis and biopsy of the mass was carried out. A biopsy specimen measuring 1.0 x 0.4 x 0.3 cm revealed a moderately differentiated infiltrating squamous cell carcinoma, which extended to the edge of the biopsy specimen. The patient’s Karnofsky performance status was 90% (ie, able to carry on normal activity; minor signs or symptoms of disease).
A CT scan of the chest was clear with no evidence of malignant involvement. A subsequent CT scan of the neck revealed a primary neoplasm of the right faucial tonsil measuring 3.3 x 3.0 cm and associated with right level II, level III, and level IV pathological lymphadenopathy. Positron-emission tomography (PET) imaging of the neck revealed a right tonsillar lesion of 2.7 x 3.0 cm involving the right parapharyngeal space (Figure 2, Case 1). The standardized uptake value (SUV) of the PET scan of the primary lesion was measured at 7.3. A cluster of right level II cervical nodes measuring 3.2 x 2.5 cm had an SUV of 3.5. A 1.0-cm right level III jugular node was also seen with an SUV of 1.6, and a right level IV lymph node measuring 1.5 x 1.0 cm was seen with an SUV of 1.8. No other lesions were noted. The tumor stage was T2N2bM0, a stage IVa disease.
The patient had a percutaneous endoscopic gastrostomy (PEG) tube placement before starting radiation. He underwent a course of hyperfractionated intensity-modulated radiation therapy with image guidance (IMRT-IGRT) in 67 fractions of 120 cGy twice a day to a final tumor dose of 8,040 cGy.16 Concurrently, the patient received systemic chemotherapy with carboplatin at a dose of 240 mg weekly. To optimize the treatment, molecular profiling was performed to identify the sensitive genetic targets to systemic chemotherapy drugs.17, 18 Targets sensitive to paclitaxel and docetaxel were identified by molecular profiling of the tumor tissue, then chemotherapy with paclitaxel or docetaxel (25 mg/m2 weekly for 3 weeks and 1 week off) was also administered to the patient.
The follow-up after 41 months indicated that the patient had no evidence of recurrent disease (Figure 2, Case 1). Posttreatment magnetic-resonance imaging (MRI) of the neck also indicated no evidence of residual tonsillar cancer. The patient’s demographics, tumor characteristics, and the treatment details are summarized in the Table.
Case 2
A 49-year-old black male presented with throat pain and a mass seen initially by his family physician. The patient had a history of tobacco use (at least 1 cigar a day) periodically for about 10 years and had quit cigar smoking 15 years prior to developing his disease. An initial evaluation indicated that the patient had a hypopharyngeal mass in the left inferior pole of his tonsil with near occlusion of the hypopharyngeal airway. His larynx could not be visualized because of the obstructive mass. A neck lymph node measuring 3.0 cm in the left jugulodigastric region was also noted. The patient’s Karnofsky performance status was 90%. Subsequently, the patient underwent excision of the right tonsil and left tonsillar region.
The pathology of the right tonsil was found to be benign. Histology of the left tonsil revealed invasive squamous cell carcinoma. The resected tumor size measured 3.7 x 2.7 x 2.5 cm. The tumor was moderately differentiated involving the deep surgical margins. No lymphovascular invasion was seen. A PET scan revealed a mass arising from the left tonsillar pillar measuring 3.6 x 2.6 x 3.3 cm with deviation of the epiglottis posteriorly nearing the left vallecula. In addition, multiple large cervical nodal lesions in the left level II nodal chain were seen, with the largest measuring 3.1 x 3.0 x 4.5 cm with an SUV of 3.4. Displacement of the left submandibular gland with several further enlarged level II lymph nodes was observed. In the region of left vallecula, there was soft tissue thickening with increased activity measuring 2.7 x 1.5 cm, likely crossing the midline with an SUV of 5.5. The rest of the neck was negative for metastatic involvement (Figure 2, Case 2). The tumor stage was T3N2Mx, a stage IVa disease.
The patient had a Port-A-Cath placed, which caused a hemothorax after placement of the port and delayed initiating his treatment. A pretreatment MRI scan of the neck revealed multiple conglomerate hypodense peripherally enhancing nodular areas in the left neck posterior to the left submandibular gland deep to the parotid tail worrisome for necrotic lymphadenopathy. The patient underwent a course of hyperfractionated IMRT-IGRT in 67 fractions of 120 cGy twice daily for a total dose of 8,040 cGy to the primary tumor site.16 The patient had a port and PEG tube prior to initiating his radiation therapy. He received IMRT-IGRT with concurrent chemotherapy that was selected based on the recommendation of his genomic testing.17,18 The chemotherapy regimen used included carboplatin (300 mg weekly) and docetaxel (400 mg weekly). The patient had a treatment break because he was hospitalized for anemia and pancytopenia from his chemotherapy and he received supportive cancer care with epoetin alfa.A post therapy PET scan was negative for evidence of hypermetabolic malignancy; however, a 3.3 x 2.7 cm calcified lesion representing likely level III jugular lymph node exhibited no measurable activity at that time. The follow-up after 40 months indicated that the patient had no reported recurrence of the disease (Figure 2, Case 2). The patient’s demographics, tumor characteristics, and the treatment details are summarized in the Table.
Case 3
A 53-year-old white man, who had no smoking or tobacco history but who was exposed to chemicals including sulfuric acid, hydrogen chloride gas, and glycols at work, presented initially with a sore throat that became more painful over time. His ENT specialist referred him for a CT scan of the neck, which revealed a left-sided neck mass measuring 2.5 cm in diameter posterior to the submandibular gland and lateral to carotid sheath and anterior to the triangle (Figure 2, Case 3). The mass appeared to be encapsulated. There was a lobulated spherical mass in the left supraglottic area with formation of the airway of the pyriform sinus and additional anterior vascular involvement was noted. The mass measured 3.6 cm in transverse diameter.
A left tonsillar biopsy specimen measuring 1.4 x 0.6 x 0.2 cm was obtained, and its pathology revealed that the patient had a metastatic squamous cell carcinoma. The left neck lymph node mass aspiration also revealed the presence of squamous cell carcinoma. A PET-CT scan staging showed a dominant tonsillar fossa mass extending from the soft palate down to the pyriform sinus measuring 4.2 x 3.8 cm, with an SUV uptake of 7.3. There was a dominant left level II necrotic lymph node presence measuring 5.0 x 3.7 cm, with an SUV of 3.0. The patient’s Karnofsky performance status was 90%. The tumor stage was T4N2M0, a stage IVa disease. The patient received a course of conformal hyperfractionated IMRT-IGRT delivered to the primary tumor in 67 fractions at 120 cGy twice daily for a total dose of 8,040 cGy16 and concurrent carboplatin chemotherapy at a weekly dose of 200 mg.
After completion of his radiation therapy, chemotherapy was changed based on genomic testing from single agent to doublet with carboplatin (area under the curve (AUC) dose of 2 or 200 mg, weekly) plus docetaxel (25 mg/m2 weekly for 3 weeks and 1 week off ).17,18 A PET scan after chemoradiation therapy revealed a marked anatomical improvement in the primary neoplastic disease seen in the faucial tonsil. The tonsillar mass noted previously had almost completely resolved over the interval, with only a mild persistent asymmetrical thickening of around 1.5 cm, with a peak SUV of 2.0. A lymph node of 2.8 x 2.0 cm was present anterior to the left sternocleidomastoid muscle exhibiting SUV of only 1.8. No other abnormal lesions were noted (Figure 2, Case 3). The patient continues to do extremely well without local recurrence of the disease 46 months after radiation therapy (see Table for patient demographics, tumor characteristics, and therapy details.)
Discussion
The management of patients with primary squamous cell carcinoma of the oropharyngeal remains controversial. Traditionally, early-stage tonsillar squamous cell carcinoma was managed by a single modality treatment, either by surgery or radiation therapy, each showing similar efficacy and outcomes.19 For late-stage disease, a combined approach using surgery and radiation therapy was found to be superior to single modality treatment. However, surgery in conjugation with radiation therapy has been associated with significant toxicities compared with the radiation therapy alone.13Therefore, the use of radiation therapy without surgery is becoming more common with increasingly sophisticated radiation therapy techniques and organ preservation approach in patients with squamous cell carcinoma of the tonsil.
Findings from several studies have shown that in stage I or II oropharyngeal cancer, single modality treatment with radiation therapy achieves 80%-90% of local control of the disease, but poorer outcomes are reported for locally advanced stages III/IV with a local control rate of 63%-74%.20 These findings and others have led to a shift to evaluate the clinical benefits of radiation therapy given with concurrent chemotherapy for the primary treatment of advanced stage oropharyngeal squamous cell carcinoma.20,21 Findings from a number of studies have since reported comparable efficacy and toxicity outcomes using this regimen with concurrent chemotherapy in patients with locally advanced head and neck squamous cell cancer.22-24 Synchronous carboplatin chemotherapy was used effectively as an alternative to cisplatin with fewer potential adverse effects in the good prognosis group of patients with oropharyngeal squamous cell carcinoma.25,26 For our 3 patients, we used carboplatin-based chemotherapy with concurrent advanced hyperfractionated radiation therapy techniques to successfully manage tonsillar squamous cell carcinoma and reduce renal toxicity and neuropathy.
Advanced radiation therapy techniques such as IMRT-IGRT are used routinely at the University Cancer and Diagnostic Centers in Houston, Texas, to manage a range of malignant cancers.27 These innovative techniques have the potential to deliver highly conformal dose-intense radiation to targeted regions of disease, while sparing adjacent critical nonmalignant tissue. The improved shaping of high-dose distributions with IMRT-IGRT could mitigate treatment-related toxicities. For example, the use of advanced radiation therapy techniques has been associated with increased preservation of parotid salivary flow.28-30 The use of advanced radiation therapy techniques in head and neck squamous cell carcinoma is growing, and early evidence confirms its ability to secure excellent local and regional disease control.31,32 In this study, we have demonstrated that by using hyperfractionated conformal three-dimensional IMRT-IGRT we were able not only to manage advanced tonsillar squamous cell carcinoma and treat the malignant metastasis, but also spare adjacent critical organs that were not involved in the disease, thus reducing many of the detrimental side effects associated with hyperfractionated chemoradiation.
All 3 patients were followed for between 40 and 46 months. They continue to do extremely well without local recurrence of their disease, indicating a 100% disease control and overall survival rate. The disease control and survival outcomes for our patients with stage IVA disease compare favorably to other published reports in the literature.33,34 Findings from a study by Prestwich and colleagues33 of 41 patients with stage IV tonsillar carcinoma showed that the radiation therapy with concurrent chemotherapy achieved local and regional disease control in 91% of complete responders and an overall survival rate of 66% at 3 years. Similarly, Setton and colleagues34 reported on 442 patients – 50% with tonsillar cancer, 46% with base-of-tongue cancer – who underwent IMRT and concurrent chemotherapy and who achieved a 3-year overall survival of 84.9%. Our study findings demonstrate that hyperfractionated conformal three-dimensional IMRT-IGRT with concurrent chemotherapy can be delivered safely and effectively to patients with advanced tonsillar squamous cell carcinoma.
Acknowledgment
The authors thank Ms June Lyliston, LVN, for editing and proofreading the manuscript.
1. Stambuk HE, Karimi S, Lee N, Patel SG. Oral cavity and oropharynx tumors. Radiol Clin North Am. 2007;45(1):1-20.
2. Lin DT, Cohen SM, Coppit GL, Burkey BB. Squamous cell carcinoma of the oropharynx and hypopharynx. Otolaryngol Clin North Am. 2005;38(1):59-74, viii.
3. Golas SM. Trends in palatine tonsillar cancer incidence and mortality rates in the United States. Community Dent Oral Epidemiol. 2007;35(2):98-108.
4. Cook MB, Dawsey SM, Freedman ND, et al. Sex disparities in cancer incidence by period and age. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1174-1182.
5. Enomoto LM, Bann DV, Hollenbeak CS, Goldenberg D. Trends in the Incidence of oropharyngeal cancers in the United States. Otolaryngol Head Neck Surg. 2016.
6. Shiboski CH, Schmidt BL, Jordan RC. Tongue and tonsil carcinoma: increasing trends in the U.S. population ages 20-44 years. Cancer. 2005;103(9):1843-1849.
7. Marur S, Forastiere AA. Head and neck cancer: changing epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2008;83(4):489-501.
8. Hong AM, Martin A, Chatfield M, et al. Human papillomavirus, smoking status and outcomes in tonsillar squamous cell carcinoma. Int J Cancer. 2013;132(12):2748-2754.
9. Velly AM, Franco EL, Schlecht N, et al. Relationship between dental factors and risk of upper aerodigestive tract cancer. Oral Oncol. 1998;34(4):284-291.
10. Farrow DC, Vaughan TL, Berwick M, et al. Diet and nasopharyngeal cancer in a low-risk population. Int J Cancer. 1998;78(6):675-679.
11. Freedman ND, Park Y, Subar AF, et al. Fruit and vegetable intake and head and neck cancer risk in a large United States prospective cohort study. Int J Cancer. 2008;122(10):2330-2336.
12. Guay ME, Lavertu P. Tonsillar carcinoma. Eur Arch Otorhinolaryngol. 1995;252(5):259-264.
13. Parsons JT, Mendenhall WM, Stringer SP, et al. Squamous cell carcinoma of the oropharynx: surgery, radiation therapy, or both. Cancer. 2002;94(11):2967-2980.
14. Yao M, Dornfeld KJ, Buatti JM, et al. Intensity-modulated radiation treatment for head-and-neck squamous cell carcinoma--the University of Iowa experience. Int J Radiat Oncol Biol Phys. 2005;63(2):410-421.
15. Yang ES, Murphy BM, Chung CH, et al. Evolution of clinical trials in head and neck cancer. Crit Rev Oncol Hematol. 2009;71(1):29-42.
16. Beitler JJ, Zhang Q, Fu KK, et al. Final results of local-regional control and late toxicity of RTOG 9003: a randomized trial of altered fractionation radiation for locally advanced head and neck cancer. Int J Radiat Oncol Biol Phys. 2014;89(1):13-20.
17. Tomkiewicz C, Hans S, Mucchielli MH, et al. A head and neck cancer tumor response-specific gene signature for cisplatin, 5-fluorouracil induction chemotherapy fails with added taxanes. PLoS One. 2012;7(10):e47170.
18. Feldman R, Gatalica Z, Knezetic J, et al. Molecular profiling of head and neck squamous cell carcinoma. Head Neck. 2016;38 Suppl 1:E1625-1638.
19. Moose BD, Kelly MD, Levine PA, et al. Definitive radiotherapy for T1 and T2 squamous cell carcinoma of the tonsil. Head Neck. 1995;17(4):334-338.
20. Chen AY, Schrag N, Hao Y, Stewart A, Ward E. Changes in treatment of advanced oropharyngeal cancer, 1985-2001. Laryngoscope. 2007;117(1):16-21.
21. Machtay M, Rosenthal DI, Hershock D, et al. Organ preservation therapy using induction plus concurrent chemoradiation for advanced resectable oropharyngeal carcinoma: a University of Pennsylvania Phase II Trial. J Clin Oncol. 2002;20(19):3964-3971.
22. Jegannathen A, Swindell R, Yap B, et al. Can synchronous chemotherapy be added to accelerated hypofractionated radiotherapy in patients with base of tongue cancer? Clin Oncol (R Coll Radiol). 2010;22(3):185-191.
23. Budach V, Becker ET, Boehmer D, et al. Concurrent hyperfractionated accelerated radiotherapy with 5-FU and once weekly cisplatin in locally advanced head and neck cancer. The 10-year results of a prospective phase II trial. Strahlenther Onkol. 2014;190(3):250-255.
24. Tobias JS, Monson K, Gupta N, et al. Chemoradiotherapy for locally advanced head and neck cancer: 10-year follow-up of the UK Head and Neck (UKHAN1) trial. Lancet Oncol. 2010;11(1):66-74.
25. Wilkins AC, Rosenfelder N, Schick U, et al. Equivalence of cisplatin and carboplatin-based chemoradiation for locally advanced squamous cell carcinoma of the head and neck: a matched-pair analysis. Oral Oncol. 2013;49(6):615-619.
26. Benghiat H, Sanghera P, Cashmore1 J, et al. Four week hypofractionated accelerated intensity modulated radiotherapy and synchronous carboplatin or cetuximab in biologically staged oropharyngeal carcinoma. Cancer and Clinical Oncology. 2014;3:1-9.
27. D’Andrea MA, Reddy GK. Management of metastatic malignant thymoma with advanced radiation and chemotherapy techniques: report of a rare case. World J Surg Oncol. 2015;13:77.
28. Little M, Schipper M, Feng FY, et al. Reducing xerostomia after chemo-IMRT for head-and-neck cancer: beyond sparing the parotid glands. Int J Radiat Oncol Biol Phys. 2012;83(3):1007-1014.
29. Eisbruch A. Reducing xerostomia by IMRT: what may, and may not, be achieved. J Clin Oncol. 2007;25(31):4863-4864.
30. Pow EH, Kwong DL, McMillan AS, et al. Xerostomia and quality of life after intensity-modulated radiotherapy vs. conventional radiotherapy for early-stage nasopharyngeal carcinoma: initial report on a randomized controlled clinical trial. Int J Radiat Oncol Biol Phys. 2006;66(4):981-991.
31. Lee NY, de Arruda FF, Puri DR, et al. A comparison of intensity-modulated radiation therapy and concomitant boost radiotherapy in the setting of concurrent chemotherapy for locally advanced oropharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2006;66(4):966-974.
32. Daly ME, Lieskovsky Y, Pawlicki T, et al. Evaluation of patterns of failure and subjective salivary function in patients treated with intensity modulated radiotherapy for head and neck squamous cell carcinoma. Head Neck. 2007;29(3):211-220.
33. Prestwich RJ, Kancherla K, Oksuz DC, et al. A single centre experience with sequential and concomitant chemoradiotherapy in locally advanced stage IV tonsillar cancer. Radiat Oncol. 2010;5:121.
34. Setton J, Caria N, Romanyshyn J, et al. Intensity-modulated radiotherapy in the treatment of oropharyngeal cancer: an update of the Memorial Sloan-Kettering Cancer Center experience. Int J Radiat Oncol Biol Phys. 2012;82(1):291-298.
Tonsillar carcinoma is the most common of the oropharyngeal malignancies of the head and neck region after thyroid and laryngeal carcinoma. Squamous cell carcinoma is the most frequent histologic type of these tumors.1 Tonsillar tumors may originate in the oral cavity, oropharynx, hypopharynx, or larynx. In the United States, more than 5,000 new cases of oropharynx cancer are diagnosed annually.2 Men are affected three to four times more often than are women, and the rate of incidence increases after the 4th decade of life.3 Surveillance, Epidemiology, and End Results data from 1975-2004 show that tonsillar squamous cell carcinoma has had one of the largest increases in the male-to-female incidence rate ratios.4 The overall incidence of tonsillar carcinoma is increasing, especially in the younger population, and this may be attributed to increasing rates of human papilloma virus.5,6
Squamous cell carcinoma in the head and neck originate from subsites within the oral cavity, oropharynx, hypopharynx, larynx, and nasopharynx.7 Traditionally, alcohol consumption and tobacco use were considered the most significant risk factors for the development of tonsillar cancer.8 More recently, however, the high-risk oncogenic human papilloma virus has emerged as a clinical entity in the pathogenesis of squamous cell carcinoma in the head and neck. Other risk factors include poor oral hygiene, mechanical irritation, chewing of betel quid preparations, and a lack of vegetables and fruits in the diet.9-11 Squamous cell carcinoma of the oropharynx often presents late with lymph node involvement at the time of diagnosis. Nonspecific symptoms such as a sore throat and dysphagia can allow head and neck cancer to evade early detection. Many patients with tonsillar carcinoma present with advanced disease because early lesions are generally asymptomatic when small. This absence of symptoms is responsible for 67%-77% of patients presenting with tumors larger than 2.0 cm and often with regional nodal metastasis. At presentation, 45% of anterior tonsillar pillar lesions and 76% of tonsillar fossa lesions have clinically positive necks.12
Despite significant treatment advances, the management of advanced squamous cell carcinoma of the tonsil remains challenging. Historically, surgery was considered the standard of care for patients with tonsillar carcinoma with or without postoperative adjuvant radiotherapy. In locally advanced tonsillar carcinoma, extensive surgery with major tissue reconstruction was necessary, leading to speech dysfunction, cosmetic deformities, and difficulties in swallowing, all of which are detrimental to patient quality of life.13 Given the critical role of the oropharynx in speech and swallowing, nonsurgical therapy with organ-preserving chemoradiation has gained a greater role in the treatment of tonsil carcinoma.13 Over the past decade, innovations in radiation therapy techniques have led to the introduction of intensity-modulated radiation therapy (IMRT) and image-guided radiation therapy (IGRT) for the treatment of various cancers including tonsillar carcinoma.14,15 IMRT is an advanced mode of conformal high-precision radiotherapy that uses computer-controlled multiple small radiation beams of varying intensities to deliver precise radiation doses to the target tissues while sparing adjacent healthy tissues.14 By incorporating three-dimensional computed-tomography (CT) or positron-emission–tomography (PET) imaging technology, IMRT allows the radiation dose to conform more precisely to the three-dimensional shape of the tumor while modulating the intensity of the radiation beam and minimizing its dose to those adjacent sensitive and unaffected organs. IGRT uses a range of two-, three-, and four-dimensional imaging techniques that improve the precision and accuracy of the delivery of the radiation dose to the targeted tumor tissue while minimizing the dose to the surrounding normal tissue during the course of radiation therapy (Figure 1). In this report, we present challenging cases of advanced tonsillar carcinoma and describe our experience in managing the disease using a hyperfractionated IMRT-IGRT based three-dimensional conformal radiation therapy protocol with concurrent chemotherapy.
Case presentations and summaries
Case 1
A 52-year-old white, nonsmoking man who worked in a research chemical laboratory, presented with complaints of throat pain and difficulty in swallowing. The patient had a history of asthma and allergies and had been seen by an ear, nose, and throat (ENT) specialist prior to his visit to our oncology center. A biopsy was performed on a right tonsillar mass measuring 2.7 x 3.6 cm. A computed-tomography (CT) scan showed 2 enlarged inhomogeneous lymph nodes measuring 2.9 cm and 1.7 cm. The nodes were well defined with no soft tissue edema. Neoplasm was favored as a diagnosis and biopsy of the mass was carried out. A biopsy specimen measuring 1.0 x 0.4 x 0.3 cm revealed a moderately differentiated infiltrating squamous cell carcinoma, which extended to the edge of the biopsy specimen. The patient’s Karnofsky performance status was 90% (ie, able to carry on normal activity; minor signs or symptoms of disease).
A CT scan of the chest was clear with no evidence of malignant involvement. A subsequent CT scan of the neck revealed a primary neoplasm of the right faucial tonsil measuring 3.3 x 3.0 cm and associated with right level II, level III, and level IV pathological lymphadenopathy. Positron-emission tomography (PET) imaging of the neck revealed a right tonsillar lesion of 2.7 x 3.0 cm involving the right parapharyngeal space (Figure 2, Case 1). The standardized uptake value (SUV) of the PET scan of the primary lesion was measured at 7.3. A cluster of right level II cervical nodes measuring 3.2 x 2.5 cm had an SUV of 3.5. A 1.0-cm right level III jugular node was also seen with an SUV of 1.6, and a right level IV lymph node measuring 1.5 x 1.0 cm was seen with an SUV of 1.8. No other lesions were noted. The tumor stage was T2N2bM0, a stage IVa disease.
The patient had a percutaneous endoscopic gastrostomy (PEG) tube placement before starting radiation. He underwent a course of hyperfractionated intensity-modulated radiation therapy with image guidance (IMRT-IGRT) in 67 fractions of 120 cGy twice a day to a final tumor dose of 8,040 cGy.16 Concurrently, the patient received systemic chemotherapy with carboplatin at a dose of 240 mg weekly. To optimize the treatment, molecular profiling was performed to identify the sensitive genetic targets to systemic chemotherapy drugs.17, 18 Targets sensitive to paclitaxel and docetaxel were identified by molecular profiling of the tumor tissue, then chemotherapy with paclitaxel or docetaxel (25 mg/m2 weekly for 3 weeks and 1 week off) was also administered to the patient.
The follow-up after 41 months indicated that the patient had no evidence of recurrent disease (Figure 2, Case 1). Posttreatment magnetic-resonance imaging (MRI) of the neck also indicated no evidence of residual tonsillar cancer. The patient’s demographics, tumor characteristics, and the treatment details are summarized in the Table.
Case 2
A 49-year-old black male presented with throat pain and a mass seen initially by his family physician. The patient had a history of tobacco use (at least 1 cigar a day) periodically for about 10 years and had quit cigar smoking 15 years prior to developing his disease. An initial evaluation indicated that the patient had a hypopharyngeal mass in the left inferior pole of his tonsil with near occlusion of the hypopharyngeal airway. His larynx could not be visualized because of the obstructive mass. A neck lymph node measuring 3.0 cm in the left jugulodigastric region was also noted. The patient’s Karnofsky performance status was 90%. Subsequently, the patient underwent excision of the right tonsil and left tonsillar region.
The pathology of the right tonsil was found to be benign. Histology of the left tonsil revealed invasive squamous cell carcinoma. The resected tumor size measured 3.7 x 2.7 x 2.5 cm. The tumor was moderately differentiated involving the deep surgical margins. No lymphovascular invasion was seen. A PET scan revealed a mass arising from the left tonsillar pillar measuring 3.6 x 2.6 x 3.3 cm with deviation of the epiglottis posteriorly nearing the left vallecula. In addition, multiple large cervical nodal lesions in the left level II nodal chain were seen, with the largest measuring 3.1 x 3.0 x 4.5 cm with an SUV of 3.4. Displacement of the left submandibular gland with several further enlarged level II lymph nodes was observed. In the region of left vallecula, there was soft tissue thickening with increased activity measuring 2.7 x 1.5 cm, likely crossing the midline with an SUV of 5.5. The rest of the neck was negative for metastatic involvement (Figure 2, Case 2). The tumor stage was T3N2Mx, a stage IVa disease.
The patient had a Port-A-Cath placed, which caused a hemothorax after placement of the port and delayed initiating his treatment. A pretreatment MRI scan of the neck revealed multiple conglomerate hypodense peripherally enhancing nodular areas in the left neck posterior to the left submandibular gland deep to the parotid tail worrisome for necrotic lymphadenopathy. The patient underwent a course of hyperfractionated IMRT-IGRT in 67 fractions of 120 cGy twice daily for a total dose of 8,040 cGy to the primary tumor site.16 The patient had a port and PEG tube prior to initiating his radiation therapy. He received IMRT-IGRT with concurrent chemotherapy that was selected based on the recommendation of his genomic testing.17,18 The chemotherapy regimen used included carboplatin (300 mg weekly) and docetaxel (400 mg weekly). The patient had a treatment break because he was hospitalized for anemia and pancytopenia from his chemotherapy and he received supportive cancer care with epoetin alfa.A post therapy PET scan was negative for evidence of hypermetabolic malignancy; however, a 3.3 x 2.7 cm calcified lesion representing likely level III jugular lymph node exhibited no measurable activity at that time. The follow-up after 40 months indicated that the patient had no reported recurrence of the disease (Figure 2, Case 2). The patient’s demographics, tumor characteristics, and the treatment details are summarized in the Table.
Case 3
A 53-year-old white man, who had no smoking or tobacco history but who was exposed to chemicals including sulfuric acid, hydrogen chloride gas, and glycols at work, presented initially with a sore throat that became more painful over time. His ENT specialist referred him for a CT scan of the neck, which revealed a left-sided neck mass measuring 2.5 cm in diameter posterior to the submandibular gland and lateral to carotid sheath and anterior to the triangle (Figure 2, Case 3). The mass appeared to be encapsulated. There was a lobulated spherical mass in the left supraglottic area with formation of the airway of the pyriform sinus and additional anterior vascular involvement was noted. The mass measured 3.6 cm in transverse diameter.
A left tonsillar biopsy specimen measuring 1.4 x 0.6 x 0.2 cm was obtained, and its pathology revealed that the patient had a metastatic squamous cell carcinoma. The left neck lymph node mass aspiration also revealed the presence of squamous cell carcinoma. A PET-CT scan staging showed a dominant tonsillar fossa mass extending from the soft palate down to the pyriform sinus measuring 4.2 x 3.8 cm, with an SUV uptake of 7.3. There was a dominant left level II necrotic lymph node presence measuring 5.0 x 3.7 cm, with an SUV of 3.0. The patient’s Karnofsky performance status was 90%. The tumor stage was T4N2M0, a stage IVa disease. The patient received a course of conformal hyperfractionated IMRT-IGRT delivered to the primary tumor in 67 fractions at 120 cGy twice daily for a total dose of 8,040 cGy16 and concurrent carboplatin chemotherapy at a weekly dose of 200 mg.
After completion of his radiation therapy, chemotherapy was changed based on genomic testing from single agent to doublet with carboplatin (area under the curve (AUC) dose of 2 or 200 mg, weekly) plus docetaxel (25 mg/m2 weekly for 3 weeks and 1 week off ).17,18 A PET scan after chemoradiation therapy revealed a marked anatomical improvement in the primary neoplastic disease seen in the faucial tonsil. The tonsillar mass noted previously had almost completely resolved over the interval, with only a mild persistent asymmetrical thickening of around 1.5 cm, with a peak SUV of 2.0. A lymph node of 2.8 x 2.0 cm was present anterior to the left sternocleidomastoid muscle exhibiting SUV of only 1.8. No other abnormal lesions were noted (Figure 2, Case 3). The patient continues to do extremely well without local recurrence of the disease 46 months after radiation therapy (see Table for patient demographics, tumor characteristics, and therapy details.)
Discussion
The management of patients with primary squamous cell carcinoma of the oropharyngeal remains controversial. Traditionally, early-stage tonsillar squamous cell carcinoma was managed by a single modality treatment, either by surgery or radiation therapy, each showing similar efficacy and outcomes.19 For late-stage disease, a combined approach using surgery and radiation therapy was found to be superior to single modality treatment. However, surgery in conjugation with radiation therapy has been associated with significant toxicities compared with the radiation therapy alone.13Therefore, the use of radiation therapy without surgery is becoming more common with increasingly sophisticated radiation therapy techniques and organ preservation approach in patients with squamous cell carcinoma of the tonsil.
Findings from several studies have shown that in stage I or II oropharyngeal cancer, single modality treatment with radiation therapy achieves 80%-90% of local control of the disease, but poorer outcomes are reported for locally advanced stages III/IV with a local control rate of 63%-74%.20 These findings and others have led to a shift to evaluate the clinical benefits of radiation therapy given with concurrent chemotherapy for the primary treatment of advanced stage oropharyngeal squamous cell carcinoma.20,21 Findings from a number of studies have since reported comparable efficacy and toxicity outcomes using this regimen with concurrent chemotherapy in patients with locally advanced head and neck squamous cell cancer.22-24 Synchronous carboplatin chemotherapy was used effectively as an alternative to cisplatin with fewer potential adverse effects in the good prognosis group of patients with oropharyngeal squamous cell carcinoma.25,26 For our 3 patients, we used carboplatin-based chemotherapy with concurrent advanced hyperfractionated radiation therapy techniques to successfully manage tonsillar squamous cell carcinoma and reduce renal toxicity and neuropathy.
Advanced radiation therapy techniques such as IMRT-IGRT are used routinely at the University Cancer and Diagnostic Centers in Houston, Texas, to manage a range of malignant cancers.27 These innovative techniques have the potential to deliver highly conformal dose-intense radiation to targeted regions of disease, while sparing adjacent critical nonmalignant tissue. The improved shaping of high-dose distributions with IMRT-IGRT could mitigate treatment-related toxicities. For example, the use of advanced radiation therapy techniques has been associated with increased preservation of parotid salivary flow.28-30 The use of advanced radiation therapy techniques in head and neck squamous cell carcinoma is growing, and early evidence confirms its ability to secure excellent local and regional disease control.31,32 In this study, we have demonstrated that by using hyperfractionated conformal three-dimensional IMRT-IGRT we were able not only to manage advanced tonsillar squamous cell carcinoma and treat the malignant metastasis, but also spare adjacent critical organs that were not involved in the disease, thus reducing many of the detrimental side effects associated with hyperfractionated chemoradiation.
All 3 patients were followed for between 40 and 46 months. They continue to do extremely well without local recurrence of their disease, indicating a 100% disease control and overall survival rate. The disease control and survival outcomes for our patients with stage IVA disease compare favorably to other published reports in the literature.33,34 Findings from a study by Prestwich and colleagues33 of 41 patients with stage IV tonsillar carcinoma showed that the radiation therapy with concurrent chemotherapy achieved local and regional disease control in 91% of complete responders and an overall survival rate of 66% at 3 years. Similarly, Setton and colleagues34 reported on 442 patients – 50% with tonsillar cancer, 46% with base-of-tongue cancer – who underwent IMRT and concurrent chemotherapy and who achieved a 3-year overall survival of 84.9%. Our study findings demonstrate that hyperfractionated conformal three-dimensional IMRT-IGRT with concurrent chemotherapy can be delivered safely and effectively to patients with advanced tonsillar squamous cell carcinoma.
Acknowledgment
The authors thank Ms June Lyliston, LVN, for editing and proofreading the manuscript.
Tonsillar carcinoma is the most common of the oropharyngeal malignancies of the head and neck region after thyroid and laryngeal carcinoma. Squamous cell carcinoma is the most frequent histologic type of these tumors.1 Tonsillar tumors may originate in the oral cavity, oropharynx, hypopharynx, or larynx. In the United States, more than 5,000 new cases of oropharynx cancer are diagnosed annually.2 Men are affected three to four times more often than are women, and the rate of incidence increases after the 4th decade of life.3 Surveillance, Epidemiology, and End Results data from 1975-2004 show that tonsillar squamous cell carcinoma has had one of the largest increases in the male-to-female incidence rate ratios.4 The overall incidence of tonsillar carcinoma is increasing, especially in the younger population, and this may be attributed to increasing rates of human papilloma virus.5,6
Squamous cell carcinoma in the head and neck originate from subsites within the oral cavity, oropharynx, hypopharynx, larynx, and nasopharynx.7 Traditionally, alcohol consumption and tobacco use were considered the most significant risk factors for the development of tonsillar cancer.8 More recently, however, the high-risk oncogenic human papilloma virus has emerged as a clinical entity in the pathogenesis of squamous cell carcinoma in the head and neck. Other risk factors include poor oral hygiene, mechanical irritation, chewing of betel quid preparations, and a lack of vegetables and fruits in the diet.9-11 Squamous cell carcinoma of the oropharynx often presents late with lymph node involvement at the time of diagnosis. Nonspecific symptoms such as a sore throat and dysphagia can allow head and neck cancer to evade early detection. Many patients with tonsillar carcinoma present with advanced disease because early lesions are generally asymptomatic when small. This absence of symptoms is responsible for 67%-77% of patients presenting with tumors larger than 2.0 cm and often with regional nodal metastasis. At presentation, 45% of anterior tonsillar pillar lesions and 76% of tonsillar fossa lesions have clinically positive necks.12
Despite significant treatment advances, the management of advanced squamous cell carcinoma of the tonsil remains challenging. Historically, surgery was considered the standard of care for patients with tonsillar carcinoma with or without postoperative adjuvant radiotherapy. In locally advanced tonsillar carcinoma, extensive surgery with major tissue reconstruction was necessary, leading to speech dysfunction, cosmetic deformities, and difficulties in swallowing, all of which are detrimental to patient quality of life.13 Given the critical role of the oropharynx in speech and swallowing, nonsurgical therapy with organ-preserving chemoradiation has gained a greater role in the treatment of tonsil carcinoma.13 Over the past decade, innovations in radiation therapy techniques have led to the introduction of intensity-modulated radiation therapy (IMRT) and image-guided radiation therapy (IGRT) for the treatment of various cancers including tonsillar carcinoma.14,15 IMRT is an advanced mode of conformal high-precision radiotherapy that uses computer-controlled multiple small radiation beams of varying intensities to deliver precise radiation doses to the target tissues while sparing adjacent healthy tissues.14 By incorporating three-dimensional computed-tomography (CT) or positron-emission–tomography (PET) imaging technology, IMRT allows the radiation dose to conform more precisely to the three-dimensional shape of the tumor while modulating the intensity of the radiation beam and minimizing its dose to those adjacent sensitive and unaffected organs. IGRT uses a range of two-, three-, and four-dimensional imaging techniques that improve the precision and accuracy of the delivery of the radiation dose to the targeted tumor tissue while minimizing the dose to the surrounding normal tissue during the course of radiation therapy (Figure 1). In this report, we present challenging cases of advanced tonsillar carcinoma and describe our experience in managing the disease using a hyperfractionated IMRT-IGRT based three-dimensional conformal radiation therapy protocol with concurrent chemotherapy.
Case presentations and summaries
Case 1
A 52-year-old white, nonsmoking man who worked in a research chemical laboratory, presented with complaints of throat pain and difficulty in swallowing. The patient had a history of asthma and allergies and had been seen by an ear, nose, and throat (ENT) specialist prior to his visit to our oncology center. A biopsy was performed on a right tonsillar mass measuring 2.7 x 3.6 cm. A computed-tomography (CT) scan showed 2 enlarged inhomogeneous lymph nodes measuring 2.9 cm and 1.7 cm. The nodes were well defined with no soft tissue edema. Neoplasm was favored as a diagnosis and biopsy of the mass was carried out. A biopsy specimen measuring 1.0 x 0.4 x 0.3 cm revealed a moderately differentiated infiltrating squamous cell carcinoma, which extended to the edge of the biopsy specimen. The patient’s Karnofsky performance status was 90% (ie, able to carry on normal activity; minor signs or symptoms of disease).
A CT scan of the chest was clear with no evidence of malignant involvement. A subsequent CT scan of the neck revealed a primary neoplasm of the right faucial tonsil measuring 3.3 x 3.0 cm and associated with right level II, level III, and level IV pathological lymphadenopathy. Positron-emission tomography (PET) imaging of the neck revealed a right tonsillar lesion of 2.7 x 3.0 cm involving the right parapharyngeal space (Figure 2, Case 1). The standardized uptake value (SUV) of the PET scan of the primary lesion was measured at 7.3. A cluster of right level II cervical nodes measuring 3.2 x 2.5 cm had an SUV of 3.5. A 1.0-cm right level III jugular node was also seen with an SUV of 1.6, and a right level IV lymph node measuring 1.5 x 1.0 cm was seen with an SUV of 1.8. No other lesions were noted. The tumor stage was T2N2bM0, a stage IVa disease.
The patient had a percutaneous endoscopic gastrostomy (PEG) tube placement before starting radiation. He underwent a course of hyperfractionated intensity-modulated radiation therapy with image guidance (IMRT-IGRT) in 67 fractions of 120 cGy twice a day to a final tumor dose of 8,040 cGy.16 Concurrently, the patient received systemic chemotherapy with carboplatin at a dose of 240 mg weekly. To optimize the treatment, molecular profiling was performed to identify the sensitive genetic targets to systemic chemotherapy drugs.17, 18 Targets sensitive to paclitaxel and docetaxel were identified by molecular profiling of the tumor tissue, then chemotherapy with paclitaxel or docetaxel (25 mg/m2 weekly for 3 weeks and 1 week off) was also administered to the patient.
The follow-up after 41 months indicated that the patient had no evidence of recurrent disease (Figure 2, Case 1). Posttreatment magnetic-resonance imaging (MRI) of the neck also indicated no evidence of residual tonsillar cancer. The patient’s demographics, tumor characteristics, and the treatment details are summarized in the Table.
Case 2
A 49-year-old black male presented with throat pain and a mass seen initially by his family physician. The patient had a history of tobacco use (at least 1 cigar a day) periodically for about 10 years and had quit cigar smoking 15 years prior to developing his disease. An initial evaluation indicated that the patient had a hypopharyngeal mass in the left inferior pole of his tonsil with near occlusion of the hypopharyngeal airway. His larynx could not be visualized because of the obstructive mass. A neck lymph node measuring 3.0 cm in the left jugulodigastric region was also noted. The patient’s Karnofsky performance status was 90%. Subsequently, the patient underwent excision of the right tonsil and left tonsillar region.
The pathology of the right tonsil was found to be benign. Histology of the left tonsil revealed invasive squamous cell carcinoma. The resected tumor size measured 3.7 x 2.7 x 2.5 cm. The tumor was moderately differentiated involving the deep surgical margins. No lymphovascular invasion was seen. A PET scan revealed a mass arising from the left tonsillar pillar measuring 3.6 x 2.6 x 3.3 cm with deviation of the epiglottis posteriorly nearing the left vallecula. In addition, multiple large cervical nodal lesions in the left level II nodal chain were seen, with the largest measuring 3.1 x 3.0 x 4.5 cm with an SUV of 3.4. Displacement of the left submandibular gland with several further enlarged level II lymph nodes was observed. In the region of left vallecula, there was soft tissue thickening with increased activity measuring 2.7 x 1.5 cm, likely crossing the midline with an SUV of 5.5. The rest of the neck was negative for metastatic involvement (Figure 2, Case 2). The tumor stage was T3N2Mx, a stage IVa disease.
The patient had a Port-A-Cath placed, which caused a hemothorax after placement of the port and delayed initiating his treatment. A pretreatment MRI scan of the neck revealed multiple conglomerate hypodense peripherally enhancing nodular areas in the left neck posterior to the left submandibular gland deep to the parotid tail worrisome for necrotic lymphadenopathy. The patient underwent a course of hyperfractionated IMRT-IGRT in 67 fractions of 120 cGy twice daily for a total dose of 8,040 cGy to the primary tumor site.16 The patient had a port and PEG tube prior to initiating his radiation therapy. He received IMRT-IGRT with concurrent chemotherapy that was selected based on the recommendation of his genomic testing.17,18 The chemotherapy regimen used included carboplatin (300 mg weekly) and docetaxel (400 mg weekly). The patient had a treatment break because he was hospitalized for anemia and pancytopenia from his chemotherapy and he received supportive cancer care with epoetin alfa.A post therapy PET scan was negative for evidence of hypermetabolic malignancy; however, a 3.3 x 2.7 cm calcified lesion representing likely level III jugular lymph node exhibited no measurable activity at that time. The follow-up after 40 months indicated that the patient had no reported recurrence of the disease (Figure 2, Case 2). The patient’s demographics, tumor characteristics, and the treatment details are summarized in the Table.
Case 3
A 53-year-old white man, who had no smoking or tobacco history but who was exposed to chemicals including sulfuric acid, hydrogen chloride gas, and glycols at work, presented initially with a sore throat that became more painful over time. His ENT specialist referred him for a CT scan of the neck, which revealed a left-sided neck mass measuring 2.5 cm in diameter posterior to the submandibular gland and lateral to carotid sheath and anterior to the triangle (Figure 2, Case 3). The mass appeared to be encapsulated. There was a lobulated spherical mass in the left supraglottic area with formation of the airway of the pyriform sinus and additional anterior vascular involvement was noted. The mass measured 3.6 cm in transverse diameter.
A left tonsillar biopsy specimen measuring 1.4 x 0.6 x 0.2 cm was obtained, and its pathology revealed that the patient had a metastatic squamous cell carcinoma. The left neck lymph node mass aspiration also revealed the presence of squamous cell carcinoma. A PET-CT scan staging showed a dominant tonsillar fossa mass extending from the soft palate down to the pyriform sinus measuring 4.2 x 3.8 cm, with an SUV uptake of 7.3. There was a dominant left level II necrotic lymph node presence measuring 5.0 x 3.7 cm, with an SUV of 3.0. The patient’s Karnofsky performance status was 90%. The tumor stage was T4N2M0, a stage IVa disease. The patient received a course of conformal hyperfractionated IMRT-IGRT delivered to the primary tumor in 67 fractions at 120 cGy twice daily for a total dose of 8,040 cGy16 and concurrent carboplatin chemotherapy at a weekly dose of 200 mg.
After completion of his radiation therapy, chemotherapy was changed based on genomic testing from single agent to doublet with carboplatin (area under the curve (AUC) dose of 2 or 200 mg, weekly) plus docetaxel (25 mg/m2 weekly for 3 weeks and 1 week off ).17,18 A PET scan after chemoradiation therapy revealed a marked anatomical improvement in the primary neoplastic disease seen in the faucial tonsil. The tonsillar mass noted previously had almost completely resolved over the interval, with only a mild persistent asymmetrical thickening of around 1.5 cm, with a peak SUV of 2.0. A lymph node of 2.8 x 2.0 cm was present anterior to the left sternocleidomastoid muscle exhibiting SUV of only 1.8. No other abnormal lesions were noted (Figure 2, Case 3). The patient continues to do extremely well without local recurrence of the disease 46 months after radiation therapy (see Table for patient demographics, tumor characteristics, and therapy details.)
Discussion
The management of patients with primary squamous cell carcinoma of the oropharyngeal remains controversial. Traditionally, early-stage tonsillar squamous cell carcinoma was managed by a single modality treatment, either by surgery or radiation therapy, each showing similar efficacy and outcomes.19 For late-stage disease, a combined approach using surgery and radiation therapy was found to be superior to single modality treatment. However, surgery in conjugation with radiation therapy has been associated with significant toxicities compared with the radiation therapy alone.13Therefore, the use of radiation therapy without surgery is becoming more common with increasingly sophisticated radiation therapy techniques and organ preservation approach in patients with squamous cell carcinoma of the tonsil.
Findings from several studies have shown that in stage I or II oropharyngeal cancer, single modality treatment with radiation therapy achieves 80%-90% of local control of the disease, but poorer outcomes are reported for locally advanced stages III/IV with a local control rate of 63%-74%.20 These findings and others have led to a shift to evaluate the clinical benefits of radiation therapy given with concurrent chemotherapy for the primary treatment of advanced stage oropharyngeal squamous cell carcinoma.20,21 Findings from a number of studies have since reported comparable efficacy and toxicity outcomes using this regimen with concurrent chemotherapy in patients with locally advanced head and neck squamous cell cancer.22-24 Synchronous carboplatin chemotherapy was used effectively as an alternative to cisplatin with fewer potential adverse effects in the good prognosis group of patients with oropharyngeal squamous cell carcinoma.25,26 For our 3 patients, we used carboplatin-based chemotherapy with concurrent advanced hyperfractionated radiation therapy techniques to successfully manage tonsillar squamous cell carcinoma and reduce renal toxicity and neuropathy.
Advanced radiation therapy techniques such as IMRT-IGRT are used routinely at the University Cancer and Diagnostic Centers in Houston, Texas, to manage a range of malignant cancers.27 These innovative techniques have the potential to deliver highly conformal dose-intense radiation to targeted regions of disease, while sparing adjacent critical nonmalignant tissue. The improved shaping of high-dose distributions with IMRT-IGRT could mitigate treatment-related toxicities. For example, the use of advanced radiation therapy techniques has been associated with increased preservation of parotid salivary flow.28-30 The use of advanced radiation therapy techniques in head and neck squamous cell carcinoma is growing, and early evidence confirms its ability to secure excellent local and regional disease control.31,32 In this study, we have demonstrated that by using hyperfractionated conformal three-dimensional IMRT-IGRT we were able not only to manage advanced tonsillar squamous cell carcinoma and treat the malignant metastasis, but also spare adjacent critical organs that were not involved in the disease, thus reducing many of the detrimental side effects associated with hyperfractionated chemoradiation.
All 3 patients were followed for between 40 and 46 months. They continue to do extremely well without local recurrence of their disease, indicating a 100% disease control and overall survival rate. The disease control and survival outcomes for our patients with stage IVA disease compare favorably to other published reports in the literature.33,34 Findings from a study by Prestwich and colleagues33 of 41 patients with stage IV tonsillar carcinoma showed that the radiation therapy with concurrent chemotherapy achieved local and regional disease control in 91% of complete responders and an overall survival rate of 66% at 3 years. Similarly, Setton and colleagues34 reported on 442 patients – 50% with tonsillar cancer, 46% with base-of-tongue cancer – who underwent IMRT and concurrent chemotherapy and who achieved a 3-year overall survival of 84.9%. Our study findings demonstrate that hyperfractionated conformal three-dimensional IMRT-IGRT with concurrent chemotherapy can be delivered safely and effectively to patients with advanced tonsillar squamous cell carcinoma.
Acknowledgment
The authors thank Ms June Lyliston, LVN, for editing and proofreading the manuscript.
1. Stambuk HE, Karimi S, Lee N, Patel SG. Oral cavity and oropharynx tumors. Radiol Clin North Am. 2007;45(1):1-20.
2. Lin DT, Cohen SM, Coppit GL, Burkey BB. Squamous cell carcinoma of the oropharynx and hypopharynx. Otolaryngol Clin North Am. 2005;38(1):59-74, viii.
3. Golas SM. Trends in palatine tonsillar cancer incidence and mortality rates in the United States. Community Dent Oral Epidemiol. 2007;35(2):98-108.
4. Cook MB, Dawsey SM, Freedman ND, et al. Sex disparities in cancer incidence by period and age. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1174-1182.
5. Enomoto LM, Bann DV, Hollenbeak CS, Goldenberg D. Trends in the Incidence of oropharyngeal cancers in the United States. Otolaryngol Head Neck Surg. 2016.
6. Shiboski CH, Schmidt BL, Jordan RC. Tongue and tonsil carcinoma: increasing trends in the U.S. population ages 20-44 years. Cancer. 2005;103(9):1843-1849.
7. Marur S, Forastiere AA. Head and neck cancer: changing epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2008;83(4):489-501.
8. Hong AM, Martin A, Chatfield M, et al. Human papillomavirus, smoking status and outcomes in tonsillar squamous cell carcinoma. Int J Cancer. 2013;132(12):2748-2754.
9. Velly AM, Franco EL, Schlecht N, et al. Relationship between dental factors and risk of upper aerodigestive tract cancer. Oral Oncol. 1998;34(4):284-291.
10. Farrow DC, Vaughan TL, Berwick M, et al. Diet and nasopharyngeal cancer in a low-risk population. Int J Cancer. 1998;78(6):675-679.
11. Freedman ND, Park Y, Subar AF, et al. Fruit and vegetable intake and head and neck cancer risk in a large United States prospective cohort study. Int J Cancer. 2008;122(10):2330-2336.
12. Guay ME, Lavertu P. Tonsillar carcinoma. Eur Arch Otorhinolaryngol. 1995;252(5):259-264.
13. Parsons JT, Mendenhall WM, Stringer SP, et al. Squamous cell carcinoma of the oropharynx: surgery, radiation therapy, or both. Cancer. 2002;94(11):2967-2980.
14. Yao M, Dornfeld KJ, Buatti JM, et al. Intensity-modulated radiation treatment for head-and-neck squamous cell carcinoma--the University of Iowa experience. Int J Radiat Oncol Biol Phys. 2005;63(2):410-421.
15. Yang ES, Murphy BM, Chung CH, et al. Evolution of clinical trials in head and neck cancer. Crit Rev Oncol Hematol. 2009;71(1):29-42.
16. Beitler JJ, Zhang Q, Fu KK, et al. Final results of local-regional control and late toxicity of RTOG 9003: a randomized trial of altered fractionation radiation for locally advanced head and neck cancer. Int J Radiat Oncol Biol Phys. 2014;89(1):13-20.
17. Tomkiewicz C, Hans S, Mucchielli MH, et al. A head and neck cancer tumor response-specific gene signature for cisplatin, 5-fluorouracil induction chemotherapy fails with added taxanes. PLoS One. 2012;7(10):e47170.
18. Feldman R, Gatalica Z, Knezetic J, et al. Molecular profiling of head and neck squamous cell carcinoma. Head Neck. 2016;38 Suppl 1:E1625-1638.
19. Moose BD, Kelly MD, Levine PA, et al. Definitive radiotherapy for T1 and T2 squamous cell carcinoma of the tonsil. Head Neck. 1995;17(4):334-338.
20. Chen AY, Schrag N, Hao Y, Stewart A, Ward E. Changes in treatment of advanced oropharyngeal cancer, 1985-2001. Laryngoscope. 2007;117(1):16-21.
21. Machtay M, Rosenthal DI, Hershock D, et al. Organ preservation therapy using induction plus concurrent chemoradiation for advanced resectable oropharyngeal carcinoma: a University of Pennsylvania Phase II Trial. J Clin Oncol. 2002;20(19):3964-3971.
22. Jegannathen A, Swindell R, Yap B, et al. Can synchronous chemotherapy be added to accelerated hypofractionated radiotherapy in patients with base of tongue cancer? Clin Oncol (R Coll Radiol). 2010;22(3):185-191.
23. Budach V, Becker ET, Boehmer D, et al. Concurrent hyperfractionated accelerated radiotherapy with 5-FU and once weekly cisplatin in locally advanced head and neck cancer. The 10-year results of a prospective phase II trial. Strahlenther Onkol. 2014;190(3):250-255.
24. Tobias JS, Monson K, Gupta N, et al. Chemoradiotherapy for locally advanced head and neck cancer: 10-year follow-up of the UK Head and Neck (UKHAN1) trial. Lancet Oncol. 2010;11(1):66-74.
25. Wilkins AC, Rosenfelder N, Schick U, et al. Equivalence of cisplatin and carboplatin-based chemoradiation for locally advanced squamous cell carcinoma of the head and neck: a matched-pair analysis. Oral Oncol. 2013;49(6):615-619.
26. Benghiat H, Sanghera P, Cashmore1 J, et al. Four week hypofractionated accelerated intensity modulated radiotherapy and synchronous carboplatin or cetuximab in biologically staged oropharyngeal carcinoma. Cancer and Clinical Oncology. 2014;3:1-9.
27. D’Andrea MA, Reddy GK. Management of metastatic malignant thymoma with advanced radiation and chemotherapy techniques: report of a rare case. World J Surg Oncol. 2015;13:77.
28. Little M, Schipper M, Feng FY, et al. Reducing xerostomia after chemo-IMRT for head-and-neck cancer: beyond sparing the parotid glands. Int J Radiat Oncol Biol Phys. 2012;83(3):1007-1014.
29. Eisbruch A. Reducing xerostomia by IMRT: what may, and may not, be achieved. J Clin Oncol. 2007;25(31):4863-4864.
30. Pow EH, Kwong DL, McMillan AS, et al. Xerostomia and quality of life after intensity-modulated radiotherapy vs. conventional radiotherapy for early-stage nasopharyngeal carcinoma: initial report on a randomized controlled clinical trial. Int J Radiat Oncol Biol Phys. 2006;66(4):981-991.
31. Lee NY, de Arruda FF, Puri DR, et al. A comparison of intensity-modulated radiation therapy and concomitant boost radiotherapy in the setting of concurrent chemotherapy for locally advanced oropharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2006;66(4):966-974.
32. Daly ME, Lieskovsky Y, Pawlicki T, et al. Evaluation of patterns of failure and subjective salivary function in patients treated with intensity modulated radiotherapy for head and neck squamous cell carcinoma. Head Neck. 2007;29(3):211-220.
33. Prestwich RJ, Kancherla K, Oksuz DC, et al. A single centre experience with sequential and concomitant chemoradiotherapy in locally advanced stage IV tonsillar cancer. Radiat Oncol. 2010;5:121.
34. Setton J, Caria N, Romanyshyn J, et al. Intensity-modulated radiotherapy in the treatment of oropharyngeal cancer: an update of the Memorial Sloan-Kettering Cancer Center experience. Int J Radiat Oncol Biol Phys. 2012;82(1):291-298.
1. Stambuk HE, Karimi S, Lee N, Patel SG. Oral cavity and oropharynx tumors. Radiol Clin North Am. 2007;45(1):1-20.
2. Lin DT, Cohen SM, Coppit GL, Burkey BB. Squamous cell carcinoma of the oropharynx and hypopharynx. Otolaryngol Clin North Am. 2005;38(1):59-74, viii.
3. Golas SM. Trends in palatine tonsillar cancer incidence and mortality rates in the United States. Community Dent Oral Epidemiol. 2007;35(2):98-108.
4. Cook MB, Dawsey SM, Freedman ND, et al. Sex disparities in cancer incidence by period and age. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1174-1182.
5. Enomoto LM, Bann DV, Hollenbeak CS, Goldenberg D. Trends in the Incidence of oropharyngeal cancers in the United States. Otolaryngol Head Neck Surg. 2016.
6. Shiboski CH, Schmidt BL, Jordan RC. Tongue and tonsil carcinoma: increasing trends in the U.S. population ages 20-44 years. Cancer. 2005;103(9):1843-1849.
7. Marur S, Forastiere AA. Head and neck cancer: changing epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2008;83(4):489-501.
8. Hong AM, Martin A, Chatfield M, et al. Human papillomavirus, smoking status and outcomes in tonsillar squamous cell carcinoma. Int J Cancer. 2013;132(12):2748-2754.
9. Velly AM, Franco EL, Schlecht N, et al. Relationship between dental factors and risk of upper aerodigestive tract cancer. Oral Oncol. 1998;34(4):284-291.
10. Farrow DC, Vaughan TL, Berwick M, et al. Diet and nasopharyngeal cancer in a low-risk population. Int J Cancer. 1998;78(6):675-679.
11. Freedman ND, Park Y, Subar AF, et al. Fruit and vegetable intake and head and neck cancer risk in a large United States prospective cohort study. Int J Cancer. 2008;122(10):2330-2336.
12. Guay ME, Lavertu P. Tonsillar carcinoma. Eur Arch Otorhinolaryngol. 1995;252(5):259-264.
13. Parsons JT, Mendenhall WM, Stringer SP, et al. Squamous cell carcinoma of the oropharynx: surgery, radiation therapy, or both. Cancer. 2002;94(11):2967-2980.
14. Yao M, Dornfeld KJ, Buatti JM, et al. Intensity-modulated radiation treatment for head-and-neck squamous cell carcinoma--the University of Iowa experience. Int J Radiat Oncol Biol Phys. 2005;63(2):410-421.
15. Yang ES, Murphy BM, Chung CH, et al. Evolution of clinical trials in head and neck cancer. Crit Rev Oncol Hematol. 2009;71(1):29-42.
16. Beitler JJ, Zhang Q, Fu KK, et al. Final results of local-regional control and late toxicity of RTOG 9003: a randomized trial of altered fractionation radiation for locally advanced head and neck cancer. Int J Radiat Oncol Biol Phys. 2014;89(1):13-20.
17. Tomkiewicz C, Hans S, Mucchielli MH, et al. A head and neck cancer tumor response-specific gene signature for cisplatin, 5-fluorouracil induction chemotherapy fails with added taxanes. PLoS One. 2012;7(10):e47170.
18. Feldman R, Gatalica Z, Knezetic J, et al. Molecular profiling of head and neck squamous cell carcinoma. Head Neck. 2016;38 Suppl 1:E1625-1638.
19. Moose BD, Kelly MD, Levine PA, et al. Definitive radiotherapy for T1 and T2 squamous cell carcinoma of the tonsil. Head Neck. 1995;17(4):334-338.
20. Chen AY, Schrag N, Hao Y, Stewart A, Ward E. Changes in treatment of advanced oropharyngeal cancer, 1985-2001. Laryngoscope. 2007;117(1):16-21.
21. Machtay M, Rosenthal DI, Hershock D, et al. Organ preservation therapy using induction plus concurrent chemoradiation for advanced resectable oropharyngeal carcinoma: a University of Pennsylvania Phase II Trial. J Clin Oncol. 2002;20(19):3964-3971.
22. Jegannathen A, Swindell R, Yap B, et al. Can synchronous chemotherapy be added to accelerated hypofractionated radiotherapy in patients with base of tongue cancer? Clin Oncol (R Coll Radiol). 2010;22(3):185-191.
23. Budach V, Becker ET, Boehmer D, et al. Concurrent hyperfractionated accelerated radiotherapy with 5-FU and once weekly cisplatin in locally advanced head and neck cancer. The 10-year results of a prospective phase II trial. Strahlenther Onkol. 2014;190(3):250-255.
24. Tobias JS, Monson K, Gupta N, et al. Chemoradiotherapy for locally advanced head and neck cancer: 10-year follow-up of the UK Head and Neck (UKHAN1) trial. Lancet Oncol. 2010;11(1):66-74.
25. Wilkins AC, Rosenfelder N, Schick U, et al. Equivalence of cisplatin and carboplatin-based chemoradiation for locally advanced squamous cell carcinoma of the head and neck: a matched-pair analysis. Oral Oncol. 2013;49(6):615-619.
26. Benghiat H, Sanghera P, Cashmore1 J, et al. Four week hypofractionated accelerated intensity modulated radiotherapy and synchronous carboplatin or cetuximab in biologically staged oropharyngeal carcinoma. Cancer and Clinical Oncology. 2014;3:1-9.
27. D’Andrea MA, Reddy GK. Management of metastatic malignant thymoma with advanced radiation and chemotherapy techniques: report of a rare case. World J Surg Oncol. 2015;13:77.
28. Little M, Schipper M, Feng FY, et al. Reducing xerostomia after chemo-IMRT for head-and-neck cancer: beyond sparing the parotid glands. Int J Radiat Oncol Biol Phys. 2012;83(3):1007-1014.
29. Eisbruch A. Reducing xerostomia by IMRT: what may, and may not, be achieved. J Clin Oncol. 2007;25(31):4863-4864.
30. Pow EH, Kwong DL, McMillan AS, et al. Xerostomia and quality of life after intensity-modulated radiotherapy vs. conventional radiotherapy for early-stage nasopharyngeal carcinoma: initial report on a randomized controlled clinical trial. Int J Radiat Oncol Biol Phys. 2006;66(4):981-991.
31. Lee NY, de Arruda FF, Puri DR, et al. A comparison of intensity-modulated radiation therapy and concomitant boost radiotherapy in the setting of concurrent chemotherapy for locally advanced oropharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2006;66(4):966-974.
32. Daly ME, Lieskovsky Y, Pawlicki T, et al. Evaluation of patterns of failure and subjective salivary function in patients treated with intensity modulated radiotherapy for head and neck squamous cell carcinoma. Head Neck. 2007;29(3):211-220.
33. Prestwich RJ, Kancherla K, Oksuz DC, et al. A single centre experience with sequential and concomitant chemoradiotherapy in locally advanced stage IV tonsillar cancer. Radiat Oncol. 2010;5:121.
34. Setton J, Caria N, Romanyshyn J, et al. Intensity-modulated radiotherapy in the treatment of oropharyngeal cancer: an update of the Memorial Sloan-Kettering Cancer Center experience. Int J Radiat Oncol Biol Phys. 2012;82(1):291-298.
Is It Safe to Manage Thyroid Cancer by Surveillance?
Research groups have suggested that small papillary thyroid cancers (PTCs) (< 2 cm) have been overdiagnosed and overtreated. But then the question is how to manage patients with small PTCs and the even smaller (< 1 cm) papillary microcarcinomas (PMCs).
In 1993, physicians from Kuma Hospital, Kobe, Japan, initiated an active surveillance trial for patients with low-risk PMCs without worrisome features. They observed that only a minority of patients showed disease progression, and those patients were successfully treated with rescue surgery. The Cancer Institute Hospital in Tokyo reported similar promising data from a trial 2 years later. The 2015 American Thyroid Association guidelines now acknowledge that active surveillance can be an alternative to immediate surgery in patients with low-risk PMCs.
At Kuma Hospital, the researchers noted that over time disease progression differed according to patient age. Using data on 1,211 patients in their surveillance program from 1993 to 2011, they estimated the lifetime probabilities of disease progression during active surveillance.
The estimated trend curves of disease progression “varied markedly,” the researchers found, depending on the patient’s age at presentation. Patients in their 20s and 30s had a steep increase for the first 10 to 20 years, with a gradual increase thereafter. Patients in their 40s or older showed a milder increase. The estimated lifetime probability of disease progression fell with each decade of age at presentation.
Related: Hashimoto’s Thyroiditis and Lymphoma
The researchers propose that surveillance be continued for the patient’s lifetime because tumors progress in a “small but noteworthy” percentage of patients. When patients with low-risk PMCs are treated surgically at presentation, they should still be followed after the surgery. Two- thirds of the patients who underwent immediate surgery still needed l-thyroxine and most likely will for their lifetime, the researchers note. However, PMCs that may progress after the 10-year point of active surveillance could be expected to have a very mild progressive nature, the researchers conclude, and the outcome of surgery should be excellent.
Source:
Miyauchi A, Kudo T, Ito Y, et al. Surgery. 2017. http://dx.doi.org/10.1016/j.surg.2017.03.028. In press.
Research groups have suggested that small papillary thyroid cancers (PTCs) (< 2 cm) have been overdiagnosed and overtreated. But then the question is how to manage patients with small PTCs and the even smaller (< 1 cm) papillary microcarcinomas (PMCs).
In 1993, physicians from Kuma Hospital, Kobe, Japan, initiated an active surveillance trial for patients with low-risk PMCs without worrisome features. They observed that only a minority of patients showed disease progression, and those patients were successfully treated with rescue surgery. The Cancer Institute Hospital in Tokyo reported similar promising data from a trial 2 years later. The 2015 American Thyroid Association guidelines now acknowledge that active surveillance can be an alternative to immediate surgery in patients with low-risk PMCs.
At Kuma Hospital, the researchers noted that over time disease progression differed according to patient age. Using data on 1,211 patients in their surveillance program from 1993 to 2011, they estimated the lifetime probabilities of disease progression during active surveillance.
The estimated trend curves of disease progression “varied markedly,” the researchers found, depending on the patient’s age at presentation. Patients in their 20s and 30s had a steep increase for the first 10 to 20 years, with a gradual increase thereafter. Patients in their 40s or older showed a milder increase. The estimated lifetime probability of disease progression fell with each decade of age at presentation.
Related: Hashimoto’s Thyroiditis and Lymphoma
The researchers propose that surveillance be continued for the patient’s lifetime because tumors progress in a “small but noteworthy” percentage of patients. When patients with low-risk PMCs are treated surgically at presentation, they should still be followed after the surgery. Two- thirds of the patients who underwent immediate surgery still needed l-thyroxine and most likely will for their lifetime, the researchers note. However, PMCs that may progress after the 10-year point of active surveillance could be expected to have a very mild progressive nature, the researchers conclude, and the outcome of surgery should be excellent.
Source:
Miyauchi A, Kudo T, Ito Y, et al. Surgery. 2017. http://dx.doi.org/10.1016/j.surg.2017.03.028. In press.
Research groups have suggested that small papillary thyroid cancers (PTCs) (< 2 cm) have been overdiagnosed and overtreated. But then the question is how to manage patients with small PTCs and the even smaller (< 1 cm) papillary microcarcinomas (PMCs).
In 1993, physicians from Kuma Hospital, Kobe, Japan, initiated an active surveillance trial for patients with low-risk PMCs without worrisome features. They observed that only a minority of patients showed disease progression, and those patients were successfully treated with rescue surgery. The Cancer Institute Hospital in Tokyo reported similar promising data from a trial 2 years later. The 2015 American Thyroid Association guidelines now acknowledge that active surveillance can be an alternative to immediate surgery in patients with low-risk PMCs.
At Kuma Hospital, the researchers noted that over time disease progression differed according to patient age. Using data on 1,211 patients in their surveillance program from 1993 to 2011, they estimated the lifetime probabilities of disease progression during active surveillance.
The estimated trend curves of disease progression “varied markedly,” the researchers found, depending on the patient’s age at presentation. Patients in their 20s and 30s had a steep increase for the first 10 to 20 years, with a gradual increase thereafter. Patients in their 40s or older showed a milder increase. The estimated lifetime probability of disease progression fell with each decade of age at presentation.
Related: Hashimoto’s Thyroiditis and Lymphoma
The researchers propose that surveillance be continued for the patient’s lifetime because tumors progress in a “small but noteworthy” percentage of patients. When patients with low-risk PMCs are treated surgically at presentation, they should still be followed after the surgery. Two- thirds of the patients who underwent immediate surgery still needed l-thyroxine and most likely will for their lifetime, the researchers note. However, PMCs that may progress after the 10-year point of active surveillance could be expected to have a very mild progressive nature, the researchers conclude, and the outcome of surgery should be excellent.
Source:
Miyauchi A, Kudo T, Ito Y, et al. Surgery. 2017. http://dx.doi.org/10.1016/j.surg.2017.03.028. In press.
Cancer patients with TKI-induced hypothyroidism had better survival rates
VICTORIA, B.C. – When it comes to the adverse effects of tyrosine kinase inhibitors (TKIs), hypothyroidism appears to have a bright side, according to a retrospective cohort study among patients with nonthyroid cancers.
While taking one of these targeted agents, roughly a quarter of patients became overtly hypothyroid, an adverse effect that appears to be due in part to immune destruction. Risk was higher for women and earlier in therapy.
Relative to counterparts who remained euthyroid, overtly hypothyroid patients were 44% less likely to die after other factors were taken into account.
Hypothyroidism may reflect changes in immune activation, Dr. Angell proposed. “Additional studies may be helpful, both prospectively looking at the clinical importance of this finding [of survival benefit], and also potentially mechanistically, to understand the relationship between hypothyroidism and survival in these patients.”
“This is an innovative study that looked at an interesting clinical question,” observed session cochair Angela M. Leung, MD, of the University of California, Los Angeles, and an endocrinologist at both UCLA and the VA Greater Los Angeles Healthcare System.
Thyroid dysfunction is a well-known, common side effect of TKI therapy, Dr. Angell noted. “The possible mechanisms that have been suggested for this are direct toxicity on the thyroid gland, destructive thyroiditis, increased thyroid hormone clearance, and vascular endothelial growth factor (VEGF) inhibition, among others.”
Some previous research has suggested a possible survival benefit of TKI-induced hypothyroidism. But “there are limitations in our understanding of hypothyroidism in this setting, including the timing of onset, what risk factors there may be, and the effect of additional clinical variables on the survival effect seen,” Dr. Angell pointed out.
He and his coinvestigators studied 538 adult patients with nonthyroid cancers (mostly stage III or IV) who received a first TKI during 2000-2013 and were followed up through 2017. They excluded those who had preexisting thyroid disease or were on thyroid-related medications.
During TKI therapy, 26.7% of patients developed overt hypothyroidism, and another 13.2% developed subclinical hypothyroidism.
“For a given drug, patients were less likely to develop hypothyroidism when they were given it subsequent to another TKI, as opposed to it being the initial TKI,” Dr. Angell reported. But median time to onset of hypothyroidism was about 2.5 months, regardless.
Cumulative months of all TKI exposure during cancer treatment were not significantly associated with development of hypothyroidism.
In a multivariate analysis, patients were significantly more likely to develop hypothyroidism if they were female (odds ratio, 1.99) and significantly less likely if they had a longer total time on treatment (OR, 0.98) or received a non-TKI VEGF inhibitor (OR, 0.43). Age, race, and cumulative TKI exposure did not influence the outcome.
In a second multivariate analysis, patients’ risk of death was significantly lower if they developed overt hypothyroidism (hazard ratio, 0.56; P less than .0001), but not if they developed subclinical hypothyroidism (HR, 0.79; P = .1655).
Treatment of hypothyroidism did not appear to influence survival, according to Dr. Angell. However, “there wasn’t a specific decision on who was treated, how they were treated, [or] when they were treated,” he said. “So, it is difficult within this cohort to look specifically at which cutoff would be ideal” for initiating treatment.
Similarly, thyroid function testing was not standardized in this retrospectively identified cohort, so it was not possible to determine how long patients were hypothyroid and whether that had an impact, according to Dr. Angell.
Dr. Angell had no relevant conflicts of interest.
VICTORIA, B.C. – When it comes to the adverse effects of tyrosine kinase inhibitors (TKIs), hypothyroidism appears to have a bright side, according to a retrospective cohort study among patients with nonthyroid cancers.
While taking one of these targeted agents, roughly a quarter of patients became overtly hypothyroid, an adverse effect that appears to be due in part to immune destruction. Risk was higher for women and earlier in therapy.
Relative to counterparts who remained euthyroid, overtly hypothyroid patients were 44% less likely to die after other factors were taken into account.
Hypothyroidism may reflect changes in immune activation, Dr. Angell proposed. “Additional studies may be helpful, both prospectively looking at the clinical importance of this finding [of survival benefit], and also potentially mechanistically, to understand the relationship between hypothyroidism and survival in these patients.”
“This is an innovative study that looked at an interesting clinical question,” observed session cochair Angela M. Leung, MD, of the University of California, Los Angeles, and an endocrinologist at both UCLA and the VA Greater Los Angeles Healthcare System.
Thyroid dysfunction is a well-known, common side effect of TKI therapy, Dr. Angell noted. “The possible mechanisms that have been suggested for this are direct toxicity on the thyroid gland, destructive thyroiditis, increased thyroid hormone clearance, and vascular endothelial growth factor (VEGF) inhibition, among others.”
Some previous research has suggested a possible survival benefit of TKI-induced hypothyroidism. But “there are limitations in our understanding of hypothyroidism in this setting, including the timing of onset, what risk factors there may be, and the effect of additional clinical variables on the survival effect seen,” Dr. Angell pointed out.
He and his coinvestigators studied 538 adult patients with nonthyroid cancers (mostly stage III or IV) who received a first TKI during 2000-2013 and were followed up through 2017. They excluded those who had preexisting thyroid disease or were on thyroid-related medications.
During TKI therapy, 26.7% of patients developed overt hypothyroidism, and another 13.2% developed subclinical hypothyroidism.
“For a given drug, patients were less likely to develop hypothyroidism when they were given it subsequent to another TKI, as opposed to it being the initial TKI,” Dr. Angell reported. But median time to onset of hypothyroidism was about 2.5 months, regardless.
Cumulative months of all TKI exposure during cancer treatment were not significantly associated with development of hypothyroidism.
In a multivariate analysis, patients were significantly more likely to develop hypothyroidism if they were female (odds ratio, 1.99) and significantly less likely if they had a longer total time on treatment (OR, 0.98) or received a non-TKI VEGF inhibitor (OR, 0.43). Age, race, and cumulative TKI exposure did not influence the outcome.
In a second multivariate analysis, patients’ risk of death was significantly lower if they developed overt hypothyroidism (hazard ratio, 0.56; P less than .0001), but not if they developed subclinical hypothyroidism (HR, 0.79; P = .1655).
Treatment of hypothyroidism did not appear to influence survival, according to Dr. Angell. However, “there wasn’t a specific decision on who was treated, how they were treated, [or] when they were treated,” he said. “So, it is difficult within this cohort to look specifically at which cutoff would be ideal” for initiating treatment.
Similarly, thyroid function testing was not standardized in this retrospectively identified cohort, so it was not possible to determine how long patients were hypothyroid and whether that had an impact, according to Dr. Angell.
Dr. Angell had no relevant conflicts of interest.
VICTORIA, B.C. – When it comes to the adverse effects of tyrosine kinase inhibitors (TKIs), hypothyroidism appears to have a bright side, according to a retrospective cohort study among patients with nonthyroid cancers.
While taking one of these targeted agents, roughly a quarter of patients became overtly hypothyroid, an adverse effect that appears to be due in part to immune destruction. Risk was higher for women and earlier in therapy.
Relative to counterparts who remained euthyroid, overtly hypothyroid patients were 44% less likely to die after other factors were taken into account.
Hypothyroidism may reflect changes in immune activation, Dr. Angell proposed. “Additional studies may be helpful, both prospectively looking at the clinical importance of this finding [of survival benefit], and also potentially mechanistically, to understand the relationship between hypothyroidism and survival in these patients.”
“This is an innovative study that looked at an interesting clinical question,” observed session cochair Angela M. Leung, MD, of the University of California, Los Angeles, and an endocrinologist at both UCLA and the VA Greater Los Angeles Healthcare System.
Thyroid dysfunction is a well-known, common side effect of TKI therapy, Dr. Angell noted. “The possible mechanisms that have been suggested for this are direct toxicity on the thyroid gland, destructive thyroiditis, increased thyroid hormone clearance, and vascular endothelial growth factor (VEGF) inhibition, among others.”
Some previous research has suggested a possible survival benefit of TKI-induced hypothyroidism. But “there are limitations in our understanding of hypothyroidism in this setting, including the timing of onset, what risk factors there may be, and the effect of additional clinical variables on the survival effect seen,” Dr. Angell pointed out.
He and his coinvestigators studied 538 adult patients with nonthyroid cancers (mostly stage III or IV) who received a first TKI during 2000-2013 and were followed up through 2017. They excluded those who had preexisting thyroid disease or were on thyroid-related medications.
During TKI therapy, 26.7% of patients developed overt hypothyroidism, and another 13.2% developed subclinical hypothyroidism.
“For a given drug, patients were less likely to develop hypothyroidism when they were given it subsequent to another TKI, as opposed to it being the initial TKI,” Dr. Angell reported. But median time to onset of hypothyroidism was about 2.5 months, regardless.
Cumulative months of all TKI exposure during cancer treatment were not significantly associated with development of hypothyroidism.
In a multivariate analysis, patients were significantly more likely to develop hypothyroidism if they were female (odds ratio, 1.99) and significantly less likely if they had a longer total time on treatment (OR, 0.98) or received a non-TKI VEGF inhibitor (OR, 0.43). Age, race, and cumulative TKI exposure did not influence the outcome.
In a second multivariate analysis, patients’ risk of death was significantly lower if they developed overt hypothyroidism (hazard ratio, 0.56; P less than .0001), but not if they developed subclinical hypothyroidism (HR, 0.79; P = .1655).
Treatment of hypothyroidism did not appear to influence survival, according to Dr. Angell. However, “there wasn’t a specific decision on who was treated, how they were treated, [or] when they were treated,” he said. “So, it is difficult within this cohort to look specifically at which cutoff would be ideal” for initiating treatment.
Similarly, thyroid function testing was not standardized in this retrospectively identified cohort, so it was not possible to determine how long patients were hypothyroid and whether that had an impact, according to Dr. Angell.
Dr. Angell had no relevant conflicts of interest.
AT ATA 2017
Key clinical point:
Major finding: Relative to peers who remained euthyroid, patients who developed overt hypothyroidism had a reduced risk of death (HR, 0.56; P less than .0001).
Data source: A retrospective cohort study of 538 adult patients with mainly advanced nonthyroid cancers treated with a tyrosine kinase inhibitor.
Disclosures: Dr. Angell had no relevant conflicts of interest.