User login
Former Smokers Motivate Quitters
In 2012, the CDC launched the “Tips from Former Smokers” campaign. It was memorable and emotionally forceful—one woman who had oral and throat cancer delivered her ad through an artificial voicebox—but did it have an impact on actual quitting rates?
No study had been done to assess the campaign’s combined, multiyear impact until CDC researchers looked at sustained (6 month) cigarette abstinence during the first 4 years of the campaign (2012-2015).
They found that the Tips campaign led to about 9.15 million total quit attempts. Based on an assumed 5.7% abstinence rate for people attempting to quit, this amounts to approximately 522,000 sustained quits.
The researchers say their findings indicate that the comprehensive approach combining evidence-based messages with the promotion of cessation resources was highly successful. Their finding of more than half-million sustained quits underscores the critical role of national tobacco education campaigns as a “counterpoint” to the substantial pro-tobacco advertising and promotion.
In 2012, the CDC launched the “Tips from Former Smokers” campaign. It was memorable and emotionally forceful—one woman who had oral and throat cancer delivered her ad through an artificial voicebox—but did it have an impact on actual quitting rates?
No study had been done to assess the campaign’s combined, multiyear impact until CDC researchers looked at sustained (6 month) cigarette abstinence during the first 4 years of the campaign (2012-2015).
They found that the Tips campaign led to about 9.15 million total quit attempts. Based on an assumed 5.7% abstinence rate for people attempting to quit, this amounts to approximately 522,000 sustained quits.
The researchers say their findings indicate that the comprehensive approach combining evidence-based messages with the promotion of cessation resources was highly successful. Their finding of more than half-million sustained quits underscores the critical role of national tobacco education campaigns as a “counterpoint” to the substantial pro-tobacco advertising and promotion.
In 2012, the CDC launched the “Tips from Former Smokers” campaign. It was memorable and emotionally forceful—one woman who had oral and throat cancer delivered her ad through an artificial voicebox—but did it have an impact on actual quitting rates?
No study had been done to assess the campaign’s combined, multiyear impact until CDC researchers looked at sustained (6 month) cigarette abstinence during the first 4 years of the campaign (2012-2015).
They found that the Tips campaign led to about 9.15 million total quit attempts. Based on an assumed 5.7% abstinence rate for people attempting to quit, this amounts to approximately 522,000 sustained quits.
The researchers say their findings indicate that the comprehensive approach combining evidence-based messages with the promotion of cessation resources was highly successful. Their finding of more than half-million sustained quits underscores the critical role of national tobacco education campaigns as a “counterpoint” to the substantial pro-tobacco advertising and promotion.
Effective management of severe radiation dermatitis after head and neck radiotherapy
Head and neck cancer is among the most prevalent cancers in developing countries.1 Most of the patients in developing countries present in locally advanced stages, and radical radiation therapy with concurrent chemotherapy is the standard treatment.1 Radiation therapy is associated with radiation dermatitis, which causes severe symptoms in the patient and can lead to disruption of treatment, diminished rates of disease control rates, and impaired patient quality of life.2 The management of advanced radiation dermatitis is difficult and can cause consequential late morbidity to patients.2 We report here the rare case of a patient with locally advanced tonsil carcinoma who developed grade 3 radiation dermatitis while receiving radical chemoradiation. The patient’s radiation dermatitis was effectively managed with the use of a silver-containing antimicrobial dressing that yielded remarkable results, so the patient was able to resume and complete radiation therapy.
Case presentation and summary
A 48-year-old man was diagnosed with squamous cell carcinoma of the right tonsil, with bilateral neck nodes (Stage T4a N2c M0; The American Joint Committee on Cancer staging manual, 7th edition). In view of the locally advanced status of his disease, the patient was scheduled for radical radiation therapy at 70 Gy in 35 fractions over 7 weeks along with weekly chemotherapy (cisplatin 40 mg/m2). During the course of radiation therapy, the patient was monitored twice a week, and symptomatic care was done for radiation-therapy–induced toxicities.
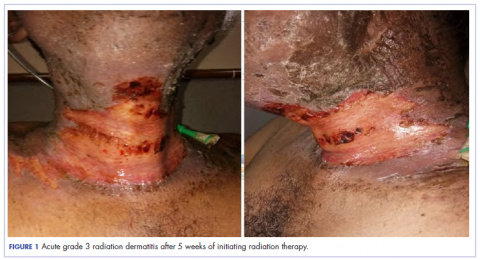
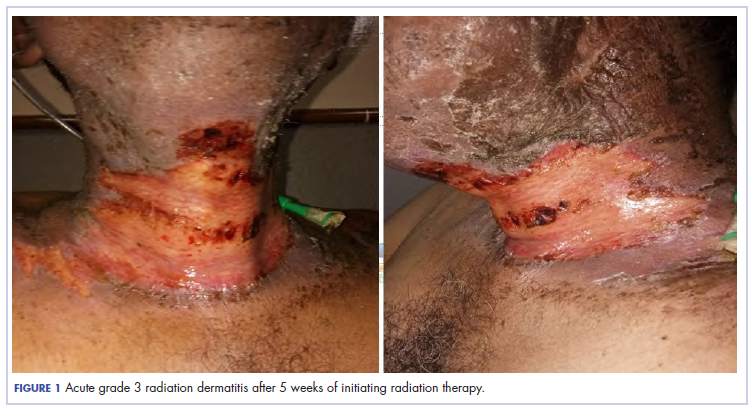
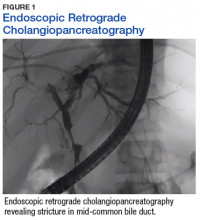
The patient presented with grade 3 radiation dermatitis after receiving 58 Gy in 29 fractions over 5 weeks (grade 0, no change; grades 3 and 4, severe change). The radiation dermatitis involved the anterior and bilateral neck with moist desquamation of the skin (Figure 1).
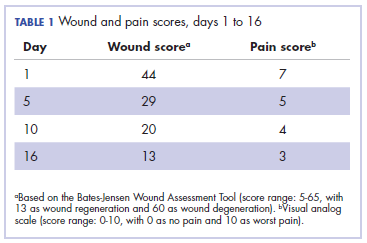
It was associated with severe pain, difficulty in swallowing, and oral mucositis. The patient was subsequently admitted to the hospital; radiation therapy was stopped, and treatment was initiated to ease the effects of the radiation dermatitis. Analgesics were administered for the pain, and adequate hydration and nutritional support was administered through a nasogastric tube. The patient’s score on the Bates-Jensen Wound Assessment Tool (BWAT) for monitoring wound status was 44, which falls in extreme severity status.
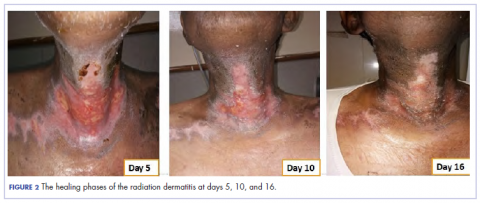
In view of the extreme severity status of the radiation dermatitis, after cleaning the wound with sterile water, we covered it with an antimicrobial dressing that contained silver salt (Mepilex AG; Mölnlycke Health Care, Norcross, GA). The dressing was changed regularly every 4 days. There was a gradual improvement in the radiation dermatitis (Figure 2).
Discussion
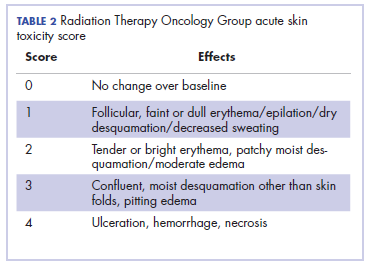
Head and neck cancer is one of the most common cancers in developing countries.1 Most patients present with locally advanced disease, so chemoradiation is the standard treatment in these patents. Radiation therapy is associated with acute and chronic toxicities. The common radiation therapy toxicities are directed at skin and mucosa, which leads to radiation dermatitis and radiation mucositis, respectively.2 These toxicities are graded as per the Radiation Therapy Oncology Group (RTOG) criteria (Table 2).3
Acute radiation dermatitis is radiation therapy dose-dependent and manifests within a few days to weeks after starting external beam radiation therapy. Its presentation varies in severity and gradually manifests as erythema, dry or moist desquamation, and ulceration when severe. These can cause severe symptoms in the patient, leading to frequent breaks in treatment, decreased rates of disease control, and impaired patient quality of life.2 Apart from RTOG grading, radiation dermatitis can also be scored using the BWAT. This tool has been validated across many studies to score initial wound status and monitor the subsequent status numerically.4 The radiation dermatitis of the index case was scored and monitored with both RTOG and BWAT scores.The management of advanced radiation dermatitis is difficult, and it causes consequential late morbidity in patients. A range of topical agents and dressings are used to treat radiation dermatitis, but there is minimal evidence to support their use.5 The Multinational Association for Supportive Care in Cancer treatment guidelines for prevention and treatment of radiation dermatitis have also concluded that there is a lack of sufficient evidence in the literature to support the superiority for any specific intervention.6 Management of radiation dermatitis varies among practitioners because of the inconclusive evidence for available treatment options.
The use of silver-based antimicrobial dressings has been reported in the literature in the prevention and treatment of radiation dermatitis, but with mixed results.7 Such dressings absorb exudate, maintain a moist environment that promotes wound healing, fight infection, and minimize the risk for maceration, according to the product information sheet.8 Clinical study findings have shown silver to be effective in fighting many different types of pathogens, including Methicillin-resistant Staphylococcus aureus and other drug-resistant bacteria.
Aquino-Parsons and colleagues studied 196 patients with breast cancer who were undergoing whole-breast radiation therapy.9 They showed that there was no benefit of silver-containing foam dressings for the prevention of acute grade 3 radiation dermatitis compared with patients who received standard skin care (with moisturizing cream, topical steroids, saline compress, and silver sulfadiazine cream). However, the incidence of itching in the last week of radiation and 1 week after treatment completion was lower among the patients who used the dressings.
Diggelmann and colleagues studied 24 patients with breast cancer who were undergoing radiation therapy.10 Each of the erythematous areas (n = 34) was randomly divided into 2 groups; 1 group was treated with Mepilex Lite dressing and the other with standard aqueous cream. There was a significant reduction in the severity of acute radiation dermatitis in the areas on which Mepilex Lite dressings were used compared with the areas on which standard aqueous cream was used.
The patient in the present case had severe grade 3 acute radiation dermatitis with a BWAT score indicative of extreme severity. After cleaning the wound with sterile water, instead of using the standard aqueous cream on the wounds, we used Mepilex AG, an antimicrobial dressing that contains silver salt. The results were remarkable (Figure 2 and Table 2). The patient was able to restart radiation therapy, and he completed his scheduled doses.
This case highlights the effectiveness of a silver-based antimicrobial dressing in the management of advanced and severe radiation dermatitis. Further large and randomized studies are needed to test the routine use of the dressing in the management of radiation dermatitis.
1. Simard EP, Torre LA, Jemal A. International trends in head and neck cancer incidence rates: differences by country, sex and anatomic site. Oral Oncol. 2014;50(5):387-403.
2. Hymes SR, Strom EA, Fife C. Radiation dermatitis: clinical presentation, pathophysiology, and treatment 2006. J Am Acad Dermatol. 2006;54(1):28-46.
3. Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31(5):1341-1346.
4. Harris C, Bates-Jensen B, Parslow N, Raizman R, Singh M, Ketchen R. Bates‐Jensen wound assessment tool: pictorial guide validation project. J Wound Ostomy Continence Nurs. 2010;37(3):253-259.
5. Lucey P, Zouzias C, Franco L, Chennupati SK, Kalnicki S, McLellan BN. Practice patterns for the prophylaxis and treatment of acute radiation dermatitis in the United States. Support Care Cancer. 2017;25(9):2857-2862.
6. Wong RK, Bensadoun RJ, Boers-Doets CB, et al. Clinical practice guidelines for the prevention and treatment of acute and late radiation reactions from the MASCC Skin Toxicity Study Group. Support Care Cancer. 2013;21(10):2933-2948.
7. Vavassis P, Gelinas M, Chabot Tr J, Nguyen-Tân PF. Phase 2 study of silver leaf dressing for treatment of radiation-induced dermatitis in patients receiving radiotherapy to the head and neck. J Otolaryngology Head Neck Surg. 2008;37(1):124-129.
8. Mepilex Ag product information. Mölnlycke Health Care website. http://www.molnlycke.us/advanced-wound-care-products/antimicrobial-products/mepilex-ag/#confirm. Accessed May 3, 2018.
9. Aquino-Parsons C, Lomas S, Smith K, et al. Phase III study of silver leaf nylon dressing vs standard care for reduction of inframammary moist desquamation in patients undergoing adjuvant whole breast radiation therapy. J Med Imaging Radiat Sci. 2010;41(4):215-221.
10. Diggelmann KV, Zytkovicz AE, Tuaine JM, Bennett NC, Kelly LE, Herst PM. Mepilex Lite dressings for the management of radiation-induced erythema: a systematic inpatient controlled clinical trial. Br J Radiol. 2010;83(995):971-978.
Head and neck cancer is among the most prevalent cancers in developing countries.1 Most of the patients in developing countries present in locally advanced stages, and radical radiation therapy with concurrent chemotherapy is the standard treatment.1 Radiation therapy is associated with radiation dermatitis, which causes severe symptoms in the patient and can lead to disruption of treatment, diminished rates of disease control rates, and impaired patient quality of life.2 The management of advanced radiation dermatitis is difficult and can cause consequential late morbidity to patients.2 We report here the rare case of a patient with locally advanced tonsil carcinoma who developed grade 3 radiation dermatitis while receiving radical chemoradiation. The patient’s radiation dermatitis was effectively managed with the use of a silver-containing antimicrobial dressing that yielded remarkable results, so the patient was able to resume and complete radiation therapy.
Case presentation and summary
A 48-year-old man was diagnosed with squamous cell carcinoma of the right tonsil, with bilateral neck nodes (Stage T4a N2c M0; The American Joint Committee on Cancer staging manual, 7th edition). In view of the locally advanced status of his disease, the patient was scheduled for radical radiation therapy at 70 Gy in 35 fractions over 7 weeks along with weekly chemotherapy (cisplatin 40 mg/m2). During the course of radiation therapy, the patient was monitored twice a week, and symptomatic care was done for radiation-therapy–induced toxicities.
The patient presented with grade 3 radiation dermatitis after receiving 58 Gy in 29 fractions over 5 weeks (grade 0, no change; grades 3 and 4, severe change). The radiation dermatitis involved the anterior and bilateral neck with moist desquamation of the skin (Figure 1).
It was associated with severe pain, difficulty in swallowing, and oral mucositis. The patient was subsequently admitted to the hospital; radiation therapy was stopped, and treatment was initiated to ease the effects of the radiation dermatitis. Analgesics were administered for the pain, and adequate hydration and nutritional support was administered through a nasogastric tube. The patient’s score on the Bates-Jensen Wound Assessment Tool (BWAT) for monitoring wound status was 44, which falls in extreme severity status.
In view of the extreme severity status of the radiation dermatitis, after cleaning the wound with sterile water, we covered it with an antimicrobial dressing that contained silver salt (Mepilex AG; Mölnlycke Health Care, Norcross, GA). The dressing was changed regularly every 4 days. There was a gradual improvement in the radiation dermatitis (Figure 2).
Discussion
Head and neck cancer is one of the most common cancers in developing countries.1 Most patients present with locally advanced disease, so chemoradiation is the standard treatment in these patents. Radiation therapy is associated with acute and chronic toxicities. The common radiation therapy toxicities are directed at skin and mucosa, which leads to radiation dermatitis and radiation mucositis, respectively.2 These toxicities are graded as per the Radiation Therapy Oncology Group (RTOG) criteria (Table 2).3
Acute radiation dermatitis is radiation therapy dose-dependent and manifests within a few days to weeks after starting external beam radiation therapy. Its presentation varies in severity and gradually manifests as erythema, dry or moist desquamation, and ulceration when severe. These can cause severe symptoms in the patient, leading to frequent breaks in treatment, decreased rates of disease control, and impaired patient quality of life.2 Apart from RTOG grading, radiation dermatitis can also be scored using the BWAT. This tool has been validated across many studies to score initial wound status and monitor the subsequent status numerically.4 The radiation dermatitis of the index case was scored and monitored with both RTOG and BWAT scores.The management of advanced radiation dermatitis is difficult, and it causes consequential late morbidity in patients. A range of topical agents and dressings are used to treat radiation dermatitis, but there is minimal evidence to support their use.5 The Multinational Association for Supportive Care in Cancer treatment guidelines for prevention and treatment of radiation dermatitis have also concluded that there is a lack of sufficient evidence in the literature to support the superiority for any specific intervention.6 Management of radiation dermatitis varies among practitioners because of the inconclusive evidence for available treatment options.
The use of silver-based antimicrobial dressings has been reported in the literature in the prevention and treatment of radiation dermatitis, but with mixed results.7 Such dressings absorb exudate, maintain a moist environment that promotes wound healing, fight infection, and minimize the risk for maceration, according to the product information sheet.8 Clinical study findings have shown silver to be effective in fighting many different types of pathogens, including Methicillin-resistant Staphylococcus aureus and other drug-resistant bacteria.
Aquino-Parsons and colleagues studied 196 patients with breast cancer who were undergoing whole-breast radiation therapy.9 They showed that there was no benefit of silver-containing foam dressings for the prevention of acute grade 3 radiation dermatitis compared with patients who received standard skin care (with moisturizing cream, topical steroids, saline compress, and silver sulfadiazine cream). However, the incidence of itching in the last week of radiation and 1 week after treatment completion was lower among the patients who used the dressings.
Diggelmann and colleagues studied 24 patients with breast cancer who were undergoing radiation therapy.10 Each of the erythematous areas (n = 34) was randomly divided into 2 groups; 1 group was treated with Mepilex Lite dressing and the other with standard aqueous cream. There was a significant reduction in the severity of acute radiation dermatitis in the areas on which Mepilex Lite dressings were used compared with the areas on which standard aqueous cream was used.
The patient in the present case had severe grade 3 acute radiation dermatitis with a BWAT score indicative of extreme severity. After cleaning the wound with sterile water, instead of using the standard aqueous cream on the wounds, we used Mepilex AG, an antimicrobial dressing that contains silver salt. The results were remarkable (Figure 2 and Table 2). The patient was able to restart radiation therapy, and he completed his scheduled doses.
This case highlights the effectiveness of a silver-based antimicrobial dressing in the management of advanced and severe radiation dermatitis. Further large and randomized studies are needed to test the routine use of the dressing in the management of radiation dermatitis.
Head and neck cancer is among the most prevalent cancers in developing countries.1 Most of the patients in developing countries present in locally advanced stages, and radical radiation therapy with concurrent chemotherapy is the standard treatment.1 Radiation therapy is associated with radiation dermatitis, which causes severe symptoms in the patient and can lead to disruption of treatment, diminished rates of disease control rates, and impaired patient quality of life.2 The management of advanced radiation dermatitis is difficult and can cause consequential late morbidity to patients.2 We report here the rare case of a patient with locally advanced tonsil carcinoma who developed grade 3 radiation dermatitis while receiving radical chemoradiation. The patient’s radiation dermatitis was effectively managed with the use of a silver-containing antimicrobial dressing that yielded remarkable results, so the patient was able to resume and complete radiation therapy.
Case presentation and summary
A 48-year-old man was diagnosed with squamous cell carcinoma of the right tonsil, with bilateral neck nodes (Stage T4a N2c M0; The American Joint Committee on Cancer staging manual, 7th edition). In view of the locally advanced status of his disease, the patient was scheduled for radical radiation therapy at 70 Gy in 35 fractions over 7 weeks along with weekly chemotherapy (cisplatin 40 mg/m2). During the course of radiation therapy, the patient was monitored twice a week, and symptomatic care was done for radiation-therapy–induced toxicities.
The patient presented with grade 3 radiation dermatitis after receiving 58 Gy in 29 fractions over 5 weeks (grade 0, no change; grades 3 and 4, severe change). The radiation dermatitis involved the anterior and bilateral neck with moist desquamation of the skin (Figure 1).
It was associated with severe pain, difficulty in swallowing, and oral mucositis. The patient was subsequently admitted to the hospital; radiation therapy was stopped, and treatment was initiated to ease the effects of the radiation dermatitis. Analgesics were administered for the pain, and adequate hydration and nutritional support was administered through a nasogastric tube. The patient’s score on the Bates-Jensen Wound Assessment Tool (BWAT) for monitoring wound status was 44, which falls in extreme severity status.
In view of the extreme severity status of the radiation dermatitis, after cleaning the wound with sterile water, we covered it with an antimicrobial dressing that contained silver salt (Mepilex AG; Mölnlycke Health Care, Norcross, GA). The dressing was changed regularly every 4 days. There was a gradual improvement in the radiation dermatitis (Figure 2).
Discussion
Head and neck cancer is one of the most common cancers in developing countries.1 Most patients present with locally advanced disease, so chemoradiation is the standard treatment in these patents. Radiation therapy is associated with acute and chronic toxicities. The common radiation therapy toxicities are directed at skin and mucosa, which leads to radiation dermatitis and radiation mucositis, respectively.2 These toxicities are graded as per the Radiation Therapy Oncology Group (RTOG) criteria (Table 2).3
Acute radiation dermatitis is radiation therapy dose-dependent and manifests within a few days to weeks after starting external beam radiation therapy. Its presentation varies in severity and gradually manifests as erythema, dry or moist desquamation, and ulceration when severe. These can cause severe symptoms in the patient, leading to frequent breaks in treatment, decreased rates of disease control, and impaired patient quality of life.2 Apart from RTOG grading, radiation dermatitis can also be scored using the BWAT. This tool has been validated across many studies to score initial wound status and monitor the subsequent status numerically.4 The radiation dermatitis of the index case was scored and monitored with both RTOG and BWAT scores.The management of advanced radiation dermatitis is difficult, and it causes consequential late morbidity in patients. A range of topical agents and dressings are used to treat radiation dermatitis, but there is minimal evidence to support their use.5 The Multinational Association for Supportive Care in Cancer treatment guidelines for prevention and treatment of radiation dermatitis have also concluded that there is a lack of sufficient evidence in the literature to support the superiority for any specific intervention.6 Management of radiation dermatitis varies among practitioners because of the inconclusive evidence for available treatment options.
The use of silver-based antimicrobial dressings has been reported in the literature in the prevention and treatment of radiation dermatitis, but with mixed results.7 Such dressings absorb exudate, maintain a moist environment that promotes wound healing, fight infection, and minimize the risk for maceration, according to the product information sheet.8 Clinical study findings have shown silver to be effective in fighting many different types of pathogens, including Methicillin-resistant Staphylococcus aureus and other drug-resistant bacteria.
Aquino-Parsons and colleagues studied 196 patients with breast cancer who were undergoing whole-breast radiation therapy.9 They showed that there was no benefit of silver-containing foam dressings for the prevention of acute grade 3 radiation dermatitis compared with patients who received standard skin care (with moisturizing cream, topical steroids, saline compress, and silver sulfadiazine cream). However, the incidence of itching in the last week of radiation and 1 week after treatment completion was lower among the patients who used the dressings.
Diggelmann and colleagues studied 24 patients with breast cancer who were undergoing radiation therapy.10 Each of the erythematous areas (n = 34) was randomly divided into 2 groups; 1 group was treated with Mepilex Lite dressing and the other with standard aqueous cream. There was a significant reduction in the severity of acute radiation dermatitis in the areas on which Mepilex Lite dressings were used compared with the areas on which standard aqueous cream was used.
The patient in the present case had severe grade 3 acute radiation dermatitis with a BWAT score indicative of extreme severity. After cleaning the wound with sterile water, instead of using the standard aqueous cream on the wounds, we used Mepilex AG, an antimicrobial dressing that contains silver salt. The results were remarkable (Figure 2 and Table 2). The patient was able to restart radiation therapy, and he completed his scheduled doses.
This case highlights the effectiveness of a silver-based antimicrobial dressing in the management of advanced and severe radiation dermatitis. Further large and randomized studies are needed to test the routine use of the dressing in the management of radiation dermatitis.
1. Simard EP, Torre LA, Jemal A. International trends in head and neck cancer incidence rates: differences by country, sex and anatomic site. Oral Oncol. 2014;50(5):387-403.
2. Hymes SR, Strom EA, Fife C. Radiation dermatitis: clinical presentation, pathophysiology, and treatment 2006. J Am Acad Dermatol. 2006;54(1):28-46.
3. Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31(5):1341-1346.
4. Harris C, Bates-Jensen B, Parslow N, Raizman R, Singh M, Ketchen R. Bates‐Jensen wound assessment tool: pictorial guide validation project. J Wound Ostomy Continence Nurs. 2010;37(3):253-259.
5. Lucey P, Zouzias C, Franco L, Chennupati SK, Kalnicki S, McLellan BN. Practice patterns for the prophylaxis and treatment of acute radiation dermatitis in the United States. Support Care Cancer. 2017;25(9):2857-2862.
6. Wong RK, Bensadoun RJ, Boers-Doets CB, et al. Clinical practice guidelines for the prevention and treatment of acute and late radiation reactions from the MASCC Skin Toxicity Study Group. Support Care Cancer. 2013;21(10):2933-2948.
7. Vavassis P, Gelinas M, Chabot Tr J, Nguyen-Tân PF. Phase 2 study of silver leaf dressing for treatment of radiation-induced dermatitis in patients receiving radiotherapy to the head and neck. J Otolaryngology Head Neck Surg. 2008;37(1):124-129.
8. Mepilex Ag product information. Mölnlycke Health Care website. http://www.molnlycke.us/advanced-wound-care-products/antimicrobial-products/mepilex-ag/#confirm. Accessed May 3, 2018.
9. Aquino-Parsons C, Lomas S, Smith K, et al. Phase III study of silver leaf nylon dressing vs standard care for reduction of inframammary moist desquamation in patients undergoing adjuvant whole breast radiation therapy. J Med Imaging Radiat Sci. 2010;41(4):215-221.
10. Diggelmann KV, Zytkovicz AE, Tuaine JM, Bennett NC, Kelly LE, Herst PM. Mepilex Lite dressings for the management of radiation-induced erythema: a systematic inpatient controlled clinical trial. Br J Radiol. 2010;83(995):971-978.
1. Simard EP, Torre LA, Jemal A. International trends in head and neck cancer incidence rates: differences by country, sex and anatomic site. Oral Oncol. 2014;50(5):387-403.
2. Hymes SR, Strom EA, Fife C. Radiation dermatitis: clinical presentation, pathophysiology, and treatment 2006. J Am Acad Dermatol. 2006;54(1):28-46.
3. Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31(5):1341-1346.
4. Harris C, Bates-Jensen B, Parslow N, Raizman R, Singh M, Ketchen R. Bates‐Jensen wound assessment tool: pictorial guide validation project. J Wound Ostomy Continence Nurs. 2010;37(3):253-259.
5. Lucey P, Zouzias C, Franco L, Chennupati SK, Kalnicki S, McLellan BN. Practice patterns for the prophylaxis and treatment of acute radiation dermatitis in the United States. Support Care Cancer. 2017;25(9):2857-2862.
6. Wong RK, Bensadoun RJ, Boers-Doets CB, et al. Clinical practice guidelines for the prevention and treatment of acute and late radiation reactions from the MASCC Skin Toxicity Study Group. Support Care Cancer. 2013;21(10):2933-2948.
7. Vavassis P, Gelinas M, Chabot Tr J, Nguyen-Tân PF. Phase 2 study of silver leaf dressing for treatment of radiation-induced dermatitis in patients receiving radiotherapy to the head and neck. J Otolaryngology Head Neck Surg. 2008;37(1):124-129.
8. Mepilex Ag product information. Mölnlycke Health Care website. http://www.molnlycke.us/advanced-wound-care-products/antimicrobial-products/mepilex-ag/#confirm. Accessed May 3, 2018.
9. Aquino-Parsons C, Lomas S, Smith K, et al. Phase III study of silver leaf nylon dressing vs standard care for reduction of inframammary moist desquamation in patients undergoing adjuvant whole breast radiation therapy. J Med Imaging Radiat Sci. 2010;41(4):215-221.
10. Diggelmann KV, Zytkovicz AE, Tuaine JM, Bennett NC, Kelly LE, Herst PM. Mepilex Lite dressings for the management of radiation-induced erythema: a systematic inpatient controlled clinical trial. Br J Radiol. 2010;83(995):971-978.
The impact of inpatient rehabilitation on outcomes for patients with cancer
The American Cancer Society reports that 1.6 million people are diagnosed with cancer each year, of whom 78% are aged 55 years or older. The 5-year survival rate for cancer is 68%.1 Almost 15.5 million living Americans have been diagnosed with cancer.2 Many patients with cancer have difficulty walking and with activities of daily living. Patients with primary brain tumors or tumors metastatic to the brain may present with focal weakness or cognitive deficits similar to patients with stroke. Patients with tumors metastatic to the spine may have the same deficits as a patient with a traumatic spinal cord injury. Patients with metastasis to bone may have pathologic fractures of the hip or long bones. Patients may develop peripheral neuropathy associated with a paraneoplastic syndrome, chemotherapy, or critical illness neuropathy. Lehmann and colleagues evaluated 805 patients admitted to hospitals affiliated with the University of Washington Medical School with a diagnosis of cancer and found that 15% had difficulty walking and 20% had difficulty with activities of daily living.3
Many patients with cancer can benefit from inpatient rehabilitation.4,5 Study findings have shown that patients with impairments in function related to cancer are often not referred for rehabilitation. Among the reasons mentioned for that are that oncologists are more focused on treating the patients’ cancer than on their functional deficits and that specialists in rehabilitation medicine do not want to be involved with patients with complex medical problems. Rehabilitation facilities may not want to incur the costs associated with caring for patients with cancer.6
The present paper looks at the outcomes of 61 consecutive patients with cancer who were admitted to an inpatient rehabilitation facility (IRF) and received radiation therapy concurrent with rehabilitation. It compares the outcomes of the cancer patients with the outcomes of patients without cancer who were admitted with stroke or spinal cord injury, conditions more commonly treated at an IRF.
Methods
We reviewed electronic medical records of all patients with cancer admitted to the IRF from 2008 through 2013 who received radiation therapy while at the facility. We also reviewed the data of all patients without cancer admitted with a diagnosis of stroke in 2013 and all patients admitted with a diagnosis of traumatic spinal cord injury in 2012 and 2013. No patients were excluded from stroke and traumatic spinal cord injury groups.
We recorded the sex, age, diagnostic group, Functional Independence Measure (FIM) admission score, FIM discharge score, length of stay (LoS) in the IRF, place of discharge of each patient (eg, home, acute care, or subacute care), and calculated the FIM efficiency score (change in FIM/LoS) for each patient. The FIM is an instrument that has 18 items measuring mobility, participation in activities of daily living, ability to communicate, and cognitive function.7 Each item is scored from 1 to 7, with 1 denoting that the patient cannot perform the task and 7 that the activity can be performed independently. The minimum score is 18 (complete dependence), and the maximum score is 126 (independent function). Thirteen items compose the motor FIM score: eating, grooming, bathing, dressing upper body, dressing lower body, toileting, bladder management, management of bowel, transfer to bed or wheelchair, transfer to toilet, tub transfer, walking (or wheelchair use), and climbing stairs. Five items – comprehension, expression, social interaction, problem solving, and memory – compose the cognitive FIM score.
We used a 1-way analysis of variance to evaluate differences between age and cancer type, age and diagnostic group, admission FIM score and cancer type, discharge FIM score and cancer type, change in FIM and cancer type, LoS and cancer type, and LoS and diagnostic group. The Pearson chi-square test was used to test the goodness of fit between the place of disposition and diagnostic group. The paired t test was used to evaluate the improvement in FIM of the patients who were in the cancer groups. The Tukey Simultaneous Tests for Differences of Means was used to compare the FIM efficiency scores of the groups. A 2-sample t test was used to evaluate the factors associated with the need for transfer from the IRF to the acute medical service.
Results
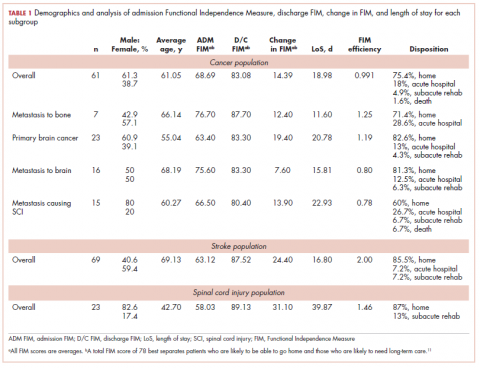
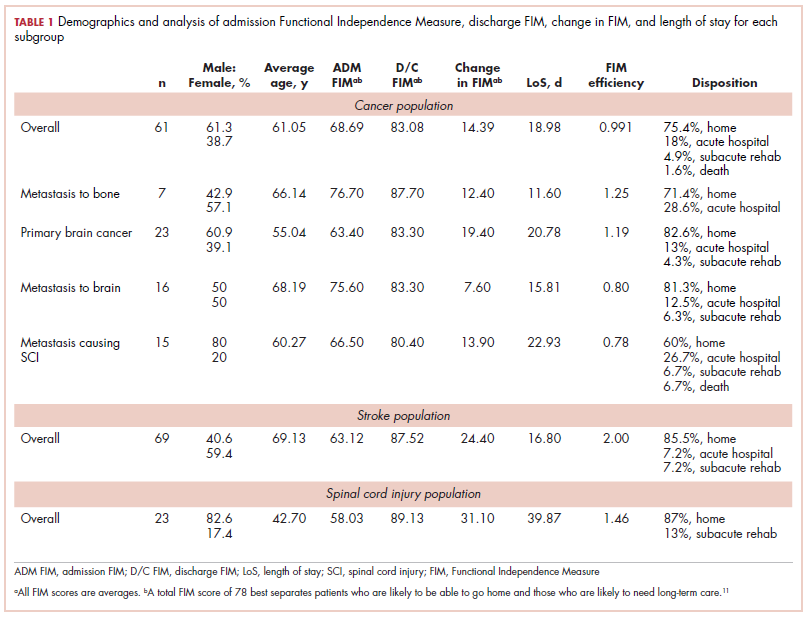
The demographic characteristics of the patients in the study and the admission and discharge FIM scores are reported in Table 1. There were initially 62 cancer patients in the radiation group, which was further divided into 4 subgroups based on the site of the primary tumor or metastasis. In all, 23 had a primary malignant brain tumor and received radiation and temozolomide. Sixteen patients had malignancies metastatic to the brain, 15 patients had tumors metastatic to the spine, and 7 had tumors metastatic to the long bones. One patient had laryngeal cancer and was excluded from the study because we did not think that we could do an analysis of a group with only 1 patient. The final number of patients in the cancer group was therefore 61. There were 69 patients in the stroke group and 23 in the spinal cord injury group.
We report improvement in total FIM, motor FIM, and cognitive FIM scores and were able to identify all 18 of the items of the FIM score on 60 of the 61 patients in the cancer group. Improvement in total FIM of the 61 patients in the cancer groups was significant at P P P = .05. Just over 75% of the patients in the cancer group had sufficient enough improvement in their level of function that they were able to return to their homes (Table 1). The average FIM score at the time of discharge was 83.08. This was not significantly different than the level of function of patients discharged after stroke (87.52) or traumatic spinal cord injury (89.13).
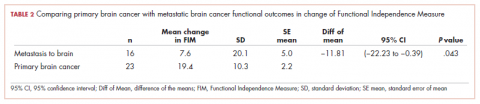
The patients with primary brain tumors were younger than the patients with cancer metastatic to the brain (P = .013). The patients with a primary brain tumor had lower admission FIM scores than patients with tumors metastatic to the brain (P = .027). The patients with a primary brain tumor had a greater increase in FIM score than patients with metastasis to the brain (P = .043; Table 2). There was not a significant difference between these 2 groups in FIM score at discharge or in the likelihood of discharge to home (Table 1). The FIM efficiency score was 1.12 for the patients in the primary brain tumor group and .80 in those with metastasis to the brain. This difference was not significant P = .96.
There were 69 patients in the stroke group. We compared the 39 patients with primary or metastatic brain lesion to the stroke group. The patients with primary or metastatic cancer of the brain were younger than the patients with stroke, 60.4 years old versus 69.1 years old (P = .004). The patients in the combined cancer group had a higher admission FIM score compared with the stroke patients (68.4 vs 63.12; P = .05). The discharge FIM scores were 83.3 in the combined cancer group and 87.5 in the stroke group (Table 1). This difference was not significant, but the improvement in the combined cancer group (14.6) was less than the improvement in the stroke group (24.40; P = .002) (Table 3).
The average LoS in the IRF was 18.7 days in the combined cancer group and 16.8 days in the stroke group. This difference was not significant. An average of 82% of the patients in the primary tumor or brain metastasis group and 85.5% of the patients in the stroke group were discharged to home. This difference was not significant. The FIM efficiency score of the patients in the stroke group was 2.0. This was significantly greater than the score for the patients in the metastasis to the brain group (0.80; P = .044) but not significantly greater than the primary brain cancer group (1.19; P = .22).
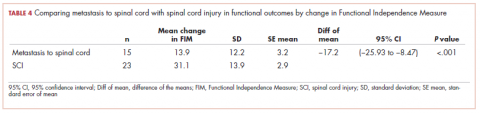
There were 23 patients in the traumatic spinal cord injury group. A comparison of the patients with tumors metastatic to the spine and patients with traumatic spinal cord injury showed that the patients in the cancer group were older (60.27 and 42.70 years, respectively; P = .001). In all, 80% of patients with tumors metastatic to the spine were men. This was not significantly different from the percentage of men in the traumatic spinal cord injury group (82.6%; Table 1). The admission FIM score of the patients with cancer was 66.5 (standard deviation [SD], 13.3) and 58.03 (SD, 15.1) in the patients with a traumatic spinal cord injury (Table 1). The FIM score at discharge was 80.4 (SD, 19.1) in the patients with cancer and 89.1 (SD, 20.3) in the patients with a traumatic spinal cord injury (Table 1). Neither of these were statistically significant. The improvement in patients with cancer was 13.9 (SD, 12.2) and 31.1 (SD, 13.9) in the traumatic spinal cord injured patients. This difference was significant (P
The median LoS was 18.98 days in the cancer metastasis to spine group (interquartile range [IQR] is the 25th-75th percentile, 12-30 days). In the traumatic group the median LoS was 23 days (IQR, 16-50 days). This difference was not significant (P = .14 Mann-Whitney test). The mean FIM efficiency score was 1.46 in the traumatic spinal cord injury group and .78 in the group with cancer metastatic to the spine. This difference was not significant (P = .72). Sixty percent of the patients in the cancer group were discharged to home, and 87% of patients in the traumatic spinal cord group were discharged to home. This difference was not significant (P = .12; Fisher exact test).
As far as we can ascertain, this is the first paper that has looked at the outcomes of patients receiving rehabilitation concurrent with radiation of the long bones. The average improvement in FIM was 12.4 (Table 1). The LoS was 11.6 days, and the FIM efficiency was 1.25. In all, 71.4% made enough progress to go home.
Of the total number of cancer patients, 18% were transferred to the acute medical service of the hospital (Table 1). Neither age, sex, type of cancer, nor admission FIM score were associated with the need for transfer to acute hospital care. Change in FIM score was inversely associated with transfer to acute hospital care (P = .027). Patients whose function did not improve with rehabilitation were most likely to be transferred back to acute hospital care.
Discussion
Radiation therapy is considered a service that is provided to people who come for treatment as an outpatient. Caregivers may have difficulty transporting patients to radiation if the patient has deficits in mobility. This may be particularly true if the patient is heavy, the caregivers are frail, or perhaps if they live in rural settings where there is no wheelchair-accessible public transportation. There are many factors that help determine whether a patient with functional deficits can be discharged to his or her home. These include sex, age, marital status, family and/or community support, income, and insurance.8 The FIM is an instrument that indicates how much help a patient needs with mobility and self-care skills. It also correlates with the amount of time that caregivers must spend helping a patient.9 Study findings have shown that the FIM score is an important determinant of whether a patient can be discharged to home. The total FIM score is as useful as an analysis of the components of the FIM score in predicting whether a patient can return to the community.10,11 Reistetter and colleagues found a total FIM score of 78 to be the score that best separates patients who are likely to be able to go home and patients who are likely to need long-term care.11 Bottemiller and colleagues10 reported that 37% of patients with total discharge FIM scores of less than 40 were discharged to home. They reported that 62% of patients with FIM scores between 40 and 79 were discharged to home, and 88% of patients with scores of 80 or above were discharged to home.10 The goal in bringing patients to the IRF was to accept and treat patients with reasonable community support and potential to achieve a functional level compatible with discharge to the community. Most patients in each of the cancer groups were able to reach an FIM score of 78 to 80 and to be discharged to home.
Most of the patients in the cancer groups had underlying problems that are not considered curable. The primary goal was to enable the patients to have some time at home with their families before requiring readmission to a hospital or hospice care. Reasonable LoS and rate of progress are now expected or required by third-party payors and hospital administrators. Physicians at the Mayo Clinic have indicated that a rehabilitation service should aim for an FIM efficiency score of at least .6 points per day.10 The FIM efficiency of patients in each of the 4 cancer subgroups in this study was higher than this level.
J. Herbert Dietz, Jr was an early advocate of the need to provide comprehensive rehabilitation services for patients with cancer. He first described his work in 1969.12 Since that time, there have been many papers that have documented the benefits of IRF for patients with cancer. O’Toole and Golden have shown outcomes of a large series of patients from an IRF. They reported that at the time of admission, 14% of patients could ambulate, but at discharge, 80% could ambulate without hands-on assistance. They reported significant improvements in continence, FIM score, and score on the Karnofsky Performance Scale.13 Marciniak,14 Hunter,15 Shin,16 and Cole,17 and their respective colleagues have all shown that patients with many different types of cancer benefit from rehabilitation at the IRF level. Gallegos-Kearin and colleagues4 reported on the care of 115,570 patients admitted to IRF with cancer from 2002 to 2014. Patients had significant improvement in function, with more than 70% of patients discharged to home.4 Ng and colleagues studied a group of 200 patients who received IRF care and found there was significant improvement in function. Ninety-four percent of patients rated their stay as either extremely good or very good.5
Metastasis to the spine is a common problem. It is found in 30% of cancer patients at autopsy. The most common sources of metastasis to the spine are breast, lung, prostate, kidney, and thyroid.18 Multiple myeloma and lymphoma may also involve the spine. Several authors have shown that these patients benefit from inpatient rehabilitation. Mckinley and colleagues19 have noted that patients with metastasis to the spine make significant improvement with care at an IRF. Compared with patients with a traumatic spinal cord injury, the cancer patients had shorter LoS, smaller improvement in FIM, equal FIM efficiency (FIM gain/LoS), and equal success in making enough progress to be discharged to home.19 Eriks and colleagues showed that patients at an IRF in Amsterdam made significant improvement in function as measured by the Barthel’s Index.20 Tang .,21 and Parsch22 and their respective colleagues, Murray,23 and New24 and colleagues have published findings confirming that patients with spinal cord injury caused by metastasis to the spine make significant progress with inpatient rehabilitation programs. The present study adds to the literature by showing that patients with metastasis to the spine who are receiving radiation can make progress and be discharged to the community.
There are 24,000 new cases of primary malignant brain tumors in the United States each year.25 The incidence of metastatic cancer to the brain has been estimated to be 100,000 cases per year in the United States. The most common cancer sources are lung, breast, melanoma, kidney, and colon.26,27 The first study of patients admitted to an IRF for treatment of brain tumors was published in 1998 by Huang and colleagues28 who compared the outcomes of 63 patients with brain tumors with the outcomes of 63 patients with stroke. They reported that the patients with the brain tumors made significant improvement in function. There was not a significant difference between the 2 groups of patients in improvement in function, FIM efficiency, or success in discharging the patients to home.28 Greenberg29 and Bartolo30 and their respective colleagues compared the outcomes of patients admitted with brain tumors and patients with stroke and found that improvement in function and discharge to home was similar in the 2 groups. In 2000, Huang and his same colleagues31 compared a group of patients with brain tumors to a group of patients with traumatic brain injury. They found significant improvement in the function of the patients with brain tumors. Patients in the traumatic brain injury group made more progress but had longer LoS. FIM efficiency was not significantly different between the groups.31
Three papers have reported outcomes of patients who received radiation concurrent with inpatient rehabilitation. Tang and colleagues32 reported 63 patients, of whom 48% percent received radiation concurrent with rehabilitation. The patients who received radiation made significant gains in function, and more than 70% were discharged to home. There was no difference in the outcomes of the patients in the radiation and nonradiation groups.32 Marciniak33 and O’Dell34 and their colleagues also reported that patients with brain tumors that required radiation therapy can benefit from inpatient rehabilitation. The present paper is the fourth (with the largest patient group) to show that patients with primary and metastatic tumors to the brain can benefit from a program that provides radiation concurrent with inpatient rehabilitation. We have shown that patients can achieve functional levels and rates of discharge to home that are not significantly different from those of the most commonly admitted group of patients to IRF – patients with stroke.
In the present study, 18% of all of the cancer patients were transferred to medical services and/or acute hospital care (Table 1). This is consistent with a paper by Asher and colleagues35 who reported that 17.4% of patients at an IRF with a diagnosis of cancer required transfer back to medical service, and that low admission motor FIM score correlated with the likelihood of transfer back to medical service. In the present paper, the total admission FIM score was not related to the likelihood of return to medical service, although a lack of improvement in the FIM score did correlate with transfer to medical service.
All of the papers we reviewed found that appropriately selected patients with cancer make significant improvement in function with treatment at an IRF. Tang and colleagues have also shown that for patients with malignant brain tumors and metastasis to the spine, improvement in function correlates with increased survival.32 Our paper confirms that patients with primary malignant brain tumors, malignant tumors metastatic to the brain or spine, and tumors metastatic to long bones may benefit from rehabilitation concurrent with radiation. Rehabilitation units are traditionally associated with treating patients with stroke and spinal cord injury. The patients in our study had cancer and were receiving radiation therapy. They had significant improvement in function and FIM efficiency scores that are not below the threshold set as expected for care at an IRF. Most patients in our study achieved a functional level consistent with what is needed to go home.
There is a prospective payment or reimbursement system for rehabilitation units.36 The payments are based on the admitting diagnosis, the admission FIM score, the age of the patient, and comorbidities. There are 4 tiers for comorbidities with no additional payments for patients in tier 0 but with additional payments for patients with conditions that qualify for tiers 1 through 3. The highest payments are for patients in tier 1. Examples of conditions that can increase payment include morbid obesity, congestive heart failure, vocal cord paralysis, and the need for hemodialysis. There is no increased payment for provision of radiation therapy. There are no reports on the feasibility, in terms of finances, of providing radiation on an IRF. We asked the finance office of the Albany Medical Center to comment on the cost to the hospital of providing radiation therapy to patients on the rehabilitation unit. The hospital’s finance department reviewed available data and reported that the variable cost of providing radiation therapy is about 6.5% of the revenue collected from third-party payors for caring for patients who receive that service (personal communication from the finance office of Albany Medical Center to George Forrest, 2015). Our findings suggest that the Centers for Medicare & Medicaid Services should make an adjustment to the payment system to support the cost of providing radiation to patients at an IRF. Even under the current payment system, for a hospital that has the equipment and personnel to provide radiation treatments, the variable cost of 6.5% of revenue should not be an absolute barrier to providing this service.
Limitations
This study reports on the experience of only 1 facility. The number of patients in the radiation group is greater than the number of patients in any previous report of people receiving radiation at an IRF, but the statistician does not think it is large enough to allow statistical analysis of covariates such as age, sex, and comorbid conditions. In addition, we did not investigate all of the factors that influence the type of care patients are offered and their LoS, such as hospital policy, insurance coverage, income, and family structure.
Conclusions
Acute care medical units are now challenged to both reduce LoS and reduce the number of patients who are readmitted to the hospital. Rehabilitation units are challenged to maintain census, as government and private payors are shifting patients from acute rehabilitation units to subacute rehabilitation units. We found that patients with cancer who need radiation are a population of patients who are seen by payors as needing to be in a facility with excellent nursing, therapy, and comprehensive physician services. A comprehensive cancer care program within a rehabilitation unit can be a great benefit to the acute care services, the IRF, and, most importantly, patients and their families.
1. American Cancer Society. Cancer facts & figures 2016. Atlanta, GA: American Cancer Society; 2016.
2. National Cancer Institute: Office of cancer survivorship: statistics. https://cancercontrol.cancer.gov/ocs/statistics/statistics.html. Updated October 17, 2016. Accessed April 21, 2018.
3. Lehmann JF, DeLisa JA, Warren CG, deLateur BJ, Bryant PL, Nicholson CG. Cancer rehabilitation: assessment of need, development and evaluation of a model of care. Arch Phys Med Rehabil. 1978;59(9):410-419.
4. Gallegos-Kearin V, Knowlton SE, Goldstein R, et al. Outcome trends of adult cancer patients receiving inpatient rehabilitation: a 13-year review [published online Feb 21, 2018]. Am J Phys Med Rehabil. doi:10.1097/PHM.0000000000000911
5. Ng AH, Gupta E, Fontillas RC, et al. Patient-reported usefulness of acute cancer rehabilitation. PM R. 2017;9(11):1135-1143.
6. Cheville AL, Kornblith AB, Basford JR. An examination of the causes for the underutilization of rehabilitation services among people with advanced cancer. Am J Phys Med Rehabil. 2011;90(5 suppl 1):S27-S37.
7. Cohen ME, Marino RJ. The tools of disability outcomes research functional status measures. Arch Phys Med Rehabil. 2000;81(12 suppl 2):S21-S29.
8. Nguyen VQ, PrvuBettger J, Guerrier T, et al. Factors associated with discharge to home versus discharge to institutional care after inpatient stroke rehabilitation. Arch Phys Med Rehabil. 2015;96(7):1297-1303.
9. Forrest G, Schwam A, Cohen E. Time of care required by patients discharged from a rehabilitation unit. Am J Phys Med Rehabil. 2002;81(1):57-62.
10. Bottemiller KL, Bieber PL, Basford JR, Harris M. FIM scores, FIM efficiency and discharge following inpatient stroke rehabilitation. Rehabil Nurs. 2006;31(1):22-25.
11. Reistetter TA, Graham JE, Deutsch A, Granger CV, Markello S, Ottenbacher KJ. Utility of functional status for classifying community versus institutional discharges after inpatient rehabilitation for stroke. Arch Phys Med Rehabil. 2010;91(3):345-350.
12. Dietz JH Jr. Rehabilitation of the cancer patient. Med Clin North Am. 1969;53(3):607-624.
13. O'Toole DM, Golden AM. Evaluating cancer patients for rehabilitation potential. West J Med. 1991;155(4):384-387.
14. Marciniak CM, Sliwa JA, Spill G, Heinemann AW, Semik PE. Functional outcome following rehabilitation of the cancer patient. Arch Phys Med Rehabil. 1996;77(1):54-57.
15. Hunter EG, Baltisberger J. Functional outcomes by age for inpatient cancer rehabilitation: a retrospective chart review. J Appl Gerontol. 2013;32(4):443-456.
16. Shin KY, Guo Y, Konzen B, Fu J, Yadav R, Bruera E. Inpatient cancer rehabilitation: the experience of a national comprehensive cancer center. Am J Phys Med Rehabil. 2011;90(5 suppl 1):S63-S68.
17. Cole RP, Scialla S, Bednarz L. Functional recovery in cancer rehabilitation. Arch Phys Med Rehabil. 2000;81(5):623-627.
18. White AP, Kwon BK, Lindskog DM, Friedlaender GE, Grauer JN. Metastatic disease of the spine. J Am Acad Orthop Surg. 2006;14(11):587-598.
19. McKinley WO, Huang ME, Tewksbury MA. Neoplastic vs traumatic spinal cord injury: an inpatient rehabilitation comparison. Am J Phys Med Rehabil. 2000;79(2):138-144.
20. Eriks IE, Angenot EL, Lankhorst GJ. Epidural metastatic spinal cord compression: functional outcome and survival after inpatient rehabilitation. Spinal Cord. 2004;42(4):235-239.
21. Tang V, Harvey D, Park Dorsay J, Jiang S, Rathbone MP. Prognostic indicators in metastatic spinal cord compression: using functional independence measure and Tokuhashi scale to optimize rehabilitation planning. Spinal Cord. 2007;45(10):671-677.
22. Parsch D, Mikut R, Abel R. Postacute management of patients with spinal cord injury due to metastatic tumor disease: survival and efficacy of rehabilitation. Spinal Cord. 2003;41:205-210.
23. Murray PK. Functional outcome and survival in spinal cord injury secondary to neoplasia. Cancer. 1985;55:197-201.
24. New PW. Functional outcomes and disability after nontraumatic spinal cord injury rehabilitation: results from a retrospective study. Arch Phys Med Rehabil. 2005;86(2):250-261
25. Central Brain Tumor Registry of the United States: 2016 CBTRUS fact sheet. www.cbtrus.org/factsheet/factsheet.html. Updated 2017. Accessed May 28, 2016.
26. Memorial Sloan Kettering Cancer Center: Metastatic brain tumors & secondary brain cancer. https://www.mskcc.org/cancer-care/types/brain-tumors-metastatic. Updated 2018. Accessed April 21, 2018.
27. Bruckner JC, Brown PD, O'Neill BP, Meyer FB, Wetmore CJ, Uhm JH. Central nervous system tumors. Mayo Clin Proc. 2007;82(10):1271-1286.
28. Huang ME, Cifu DX, Keyser-Marcus L. Functional outcome after brain tumor and acute stroke: a comparative analysis. Arch Phys Med Rehabil. 1998;79(11):1386-1390.
29. Greenberg E, Treger I, Ring H. Rehabilitation outcomes in patients with brain tumors and acute stroke: comparative study of inpatient rehabilitation. Am J Phys Med Rehabil. 2006;85(7):568-573.
30. Bartolo M, Zucchella C, Pace A, et al. Early rehabilitation after surgery improves functional outcomes in inpatients with brain tumours. J Neurooncol. 2012;107(3);537-544.
31. Huang ME, Cifu DX, Keyser-Marcus L. Functional outcomes in patients with brain tumor after inpatient rehabilitation: comparison with traumatic brain injury. Am J Phys Med Rehabil. 2000;79(4):327-335.
32. Tang V, Rathbone M, Park Dorsay J, Jiang S, Harvey D. Rehabilitation in primary and metastatic brain tumours: impact of functional outcomes on survival. J Neurol. 2008;255(6):820-827.
33. Marciniak CM, Sliwa JA, Heinemann AW, Semik PE. Functional outcomes of persons with brain tumors after inpatient rehabilitation. Arch Phys Med Rehabil. 2001;82(4):457-463.
34. O'Dell MW, Barr K, Spanier D, Warnick RE. Functional outcome of inpatient rehabilitation in persons with brain tumors. Arch Phys Med Rehabil. 1998;79(12):1530-1534.
35. Asher A, Roberts PS, Bresee C, Zabel G, Riggs RV, Rogatko A. Transferring inpatient rehabilitation facility cancer patients back to acute care (TRIPBAC). PM R. 2014;6(9):808-813.
36. Centers for Medicare and Medicaid Services: Inpatient rehabilitation facilities. https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/CertificationandComplianc/InpatientRehab.html. Published March 5, 2012. Accessed May 21, 2018.
The American Cancer Society reports that 1.6 million people are diagnosed with cancer each year, of whom 78% are aged 55 years or older. The 5-year survival rate for cancer is 68%.1 Almost 15.5 million living Americans have been diagnosed with cancer.2 Many patients with cancer have difficulty walking and with activities of daily living. Patients with primary brain tumors or tumors metastatic to the brain may present with focal weakness or cognitive deficits similar to patients with stroke. Patients with tumors metastatic to the spine may have the same deficits as a patient with a traumatic spinal cord injury. Patients with metastasis to bone may have pathologic fractures of the hip or long bones. Patients may develop peripheral neuropathy associated with a paraneoplastic syndrome, chemotherapy, or critical illness neuropathy. Lehmann and colleagues evaluated 805 patients admitted to hospitals affiliated with the University of Washington Medical School with a diagnosis of cancer and found that 15% had difficulty walking and 20% had difficulty with activities of daily living.3
Many patients with cancer can benefit from inpatient rehabilitation.4,5 Study findings have shown that patients with impairments in function related to cancer are often not referred for rehabilitation. Among the reasons mentioned for that are that oncologists are more focused on treating the patients’ cancer than on their functional deficits and that specialists in rehabilitation medicine do not want to be involved with patients with complex medical problems. Rehabilitation facilities may not want to incur the costs associated with caring for patients with cancer.6
The present paper looks at the outcomes of 61 consecutive patients with cancer who were admitted to an inpatient rehabilitation facility (IRF) and received radiation therapy concurrent with rehabilitation. It compares the outcomes of the cancer patients with the outcomes of patients without cancer who were admitted with stroke or spinal cord injury, conditions more commonly treated at an IRF.
Methods
We reviewed electronic medical records of all patients with cancer admitted to the IRF from 2008 through 2013 who received radiation therapy while at the facility. We also reviewed the data of all patients without cancer admitted with a diagnosis of stroke in 2013 and all patients admitted with a diagnosis of traumatic spinal cord injury in 2012 and 2013. No patients were excluded from stroke and traumatic spinal cord injury groups.
We recorded the sex, age, diagnostic group, Functional Independence Measure (FIM) admission score, FIM discharge score, length of stay (LoS) in the IRF, place of discharge of each patient (eg, home, acute care, or subacute care), and calculated the FIM efficiency score (change in FIM/LoS) for each patient. The FIM is an instrument that has 18 items measuring mobility, participation in activities of daily living, ability to communicate, and cognitive function.7 Each item is scored from 1 to 7, with 1 denoting that the patient cannot perform the task and 7 that the activity can be performed independently. The minimum score is 18 (complete dependence), and the maximum score is 126 (independent function). Thirteen items compose the motor FIM score: eating, grooming, bathing, dressing upper body, dressing lower body, toileting, bladder management, management of bowel, transfer to bed or wheelchair, transfer to toilet, tub transfer, walking (or wheelchair use), and climbing stairs. Five items – comprehension, expression, social interaction, problem solving, and memory – compose the cognitive FIM score.
We used a 1-way analysis of variance to evaluate differences between age and cancer type, age and diagnostic group, admission FIM score and cancer type, discharge FIM score and cancer type, change in FIM and cancer type, LoS and cancer type, and LoS and diagnostic group. The Pearson chi-square test was used to test the goodness of fit between the place of disposition and diagnostic group. The paired t test was used to evaluate the improvement in FIM of the patients who were in the cancer groups. The Tukey Simultaneous Tests for Differences of Means was used to compare the FIM efficiency scores of the groups. A 2-sample t test was used to evaluate the factors associated with the need for transfer from the IRF to the acute medical service.
Results
The demographic characteristics of the patients in the study and the admission and discharge FIM scores are reported in Table 1. There were initially 62 cancer patients in the radiation group, which was further divided into 4 subgroups based on the site of the primary tumor or metastasis. In all, 23 had a primary malignant brain tumor and received radiation and temozolomide. Sixteen patients had malignancies metastatic to the brain, 15 patients had tumors metastatic to the spine, and 7 had tumors metastatic to the long bones. One patient had laryngeal cancer and was excluded from the study because we did not think that we could do an analysis of a group with only 1 patient. The final number of patients in the cancer group was therefore 61. There were 69 patients in the stroke group and 23 in the spinal cord injury group.
We report improvement in total FIM, motor FIM, and cognitive FIM scores and were able to identify all 18 of the items of the FIM score on 60 of the 61 patients in the cancer group. Improvement in total FIM of the 61 patients in the cancer groups was significant at P P P = .05. Just over 75% of the patients in the cancer group had sufficient enough improvement in their level of function that they were able to return to their homes (Table 1). The average FIM score at the time of discharge was 83.08. This was not significantly different than the level of function of patients discharged after stroke (87.52) or traumatic spinal cord injury (89.13).
The patients with primary brain tumors were younger than the patients with cancer metastatic to the brain (P = .013). The patients with a primary brain tumor had lower admission FIM scores than patients with tumors metastatic to the brain (P = .027). The patients with a primary brain tumor had a greater increase in FIM score than patients with metastasis to the brain (P = .043; Table 2). There was not a significant difference between these 2 groups in FIM score at discharge or in the likelihood of discharge to home (Table 1). The FIM efficiency score was 1.12 for the patients in the primary brain tumor group and .80 in those with metastasis to the brain. This difference was not significant P = .96.
There were 69 patients in the stroke group. We compared the 39 patients with primary or metastatic brain lesion to the stroke group. The patients with primary or metastatic cancer of the brain were younger than the patients with stroke, 60.4 years old versus 69.1 years old (P = .004). The patients in the combined cancer group had a higher admission FIM score compared with the stroke patients (68.4 vs 63.12; P = .05). The discharge FIM scores were 83.3 in the combined cancer group and 87.5 in the stroke group (Table 1). This difference was not significant, but the improvement in the combined cancer group (14.6) was less than the improvement in the stroke group (24.40; P = .002) (Table 3).
The average LoS in the IRF was 18.7 days in the combined cancer group and 16.8 days in the stroke group. This difference was not significant. An average of 82% of the patients in the primary tumor or brain metastasis group and 85.5% of the patients in the stroke group were discharged to home. This difference was not significant. The FIM efficiency score of the patients in the stroke group was 2.0. This was significantly greater than the score for the patients in the metastasis to the brain group (0.80; P = .044) but not significantly greater than the primary brain cancer group (1.19; P = .22).
There were 23 patients in the traumatic spinal cord injury group. A comparison of the patients with tumors metastatic to the spine and patients with traumatic spinal cord injury showed that the patients in the cancer group were older (60.27 and 42.70 years, respectively; P = .001). In all, 80% of patients with tumors metastatic to the spine were men. This was not significantly different from the percentage of men in the traumatic spinal cord injury group (82.6%; Table 1). The admission FIM score of the patients with cancer was 66.5 (standard deviation [SD], 13.3) and 58.03 (SD, 15.1) in the patients with a traumatic spinal cord injury (Table 1). The FIM score at discharge was 80.4 (SD, 19.1) in the patients with cancer and 89.1 (SD, 20.3) in the patients with a traumatic spinal cord injury (Table 1). Neither of these were statistically significant. The improvement in patients with cancer was 13.9 (SD, 12.2) and 31.1 (SD, 13.9) in the traumatic spinal cord injured patients. This difference was significant (P
The median LoS was 18.98 days in the cancer metastasis to spine group (interquartile range [IQR] is the 25th-75th percentile, 12-30 days). In the traumatic group the median LoS was 23 days (IQR, 16-50 days). This difference was not significant (P = .14 Mann-Whitney test). The mean FIM efficiency score was 1.46 in the traumatic spinal cord injury group and .78 in the group with cancer metastatic to the spine. This difference was not significant (P = .72). Sixty percent of the patients in the cancer group were discharged to home, and 87% of patients in the traumatic spinal cord group were discharged to home. This difference was not significant (P = .12; Fisher exact test).
As far as we can ascertain, this is the first paper that has looked at the outcomes of patients receiving rehabilitation concurrent with radiation of the long bones. The average improvement in FIM was 12.4 (Table 1). The LoS was 11.6 days, and the FIM efficiency was 1.25. In all, 71.4% made enough progress to go home.
Of the total number of cancer patients, 18% were transferred to the acute medical service of the hospital (Table 1). Neither age, sex, type of cancer, nor admission FIM score were associated with the need for transfer to acute hospital care. Change in FIM score was inversely associated with transfer to acute hospital care (P = .027). Patients whose function did not improve with rehabilitation were most likely to be transferred back to acute hospital care.
Discussion
Radiation therapy is considered a service that is provided to people who come for treatment as an outpatient. Caregivers may have difficulty transporting patients to radiation if the patient has deficits in mobility. This may be particularly true if the patient is heavy, the caregivers are frail, or perhaps if they live in rural settings where there is no wheelchair-accessible public transportation. There are many factors that help determine whether a patient with functional deficits can be discharged to his or her home. These include sex, age, marital status, family and/or community support, income, and insurance.8 The FIM is an instrument that indicates how much help a patient needs with mobility and self-care skills. It also correlates with the amount of time that caregivers must spend helping a patient.9 Study findings have shown that the FIM score is an important determinant of whether a patient can be discharged to home. The total FIM score is as useful as an analysis of the components of the FIM score in predicting whether a patient can return to the community.10,11 Reistetter and colleagues found a total FIM score of 78 to be the score that best separates patients who are likely to be able to go home and patients who are likely to need long-term care.11 Bottemiller and colleagues10 reported that 37% of patients with total discharge FIM scores of less than 40 were discharged to home. They reported that 62% of patients with FIM scores between 40 and 79 were discharged to home, and 88% of patients with scores of 80 or above were discharged to home.10 The goal in bringing patients to the IRF was to accept and treat patients with reasonable community support and potential to achieve a functional level compatible with discharge to the community. Most patients in each of the cancer groups were able to reach an FIM score of 78 to 80 and to be discharged to home.
Most of the patients in the cancer groups had underlying problems that are not considered curable. The primary goal was to enable the patients to have some time at home with their families before requiring readmission to a hospital or hospice care. Reasonable LoS and rate of progress are now expected or required by third-party payors and hospital administrators. Physicians at the Mayo Clinic have indicated that a rehabilitation service should aim for an FIM efficiency score of at least .6 points per day.10 The FIM efficiency of patients in each of the 4 cancer subgroups in this study was higher than this level.
J. Herbert Dietz, Jr was an early advocate of the need to provide comprehensive rehabilitation services for patients with cancer. He first described his work in 1969.12 Since that time, there have been many papers that have documented the benefits of IRF for patients with cancer. O’Toole and Golden have shown outcomes of a large series of patients from an IRF. They reported that at the time of admission, 14% of patients could ambulate, but at discharge, 80% could ambulate without hands-on assistance. They reported significant improvements in continence, FIM score, and score on the Karnofsky Performance Scale.13 Marciniak,14 Hunter,15 Shin,16 and Cole,17 and their respective colleagues have all shown that patients with many different types of cancer benefit from rehabilitation at the IRF level. Gallegos-Kearin and colleagues4 reported on the care of 115,570 patients admitted to IRF with cancer from 2002 to 2014. Patients had significant improvement in function, with more than 70% of patients discharged to home.4 Ng and colleagues studied a group of 200 patients who received IRF care and found there was significant improvement in function. Ninety-four percent of patients rated their stay as either extremely good or very good.5
Metastasis to the spine is a common problem. It is found in 30% of cancer patients at autopsy. The most common sources of metastasis to the spine are breast, lung, prostate, kidney, and thyroid.18 Multiple myeloma and lymphoma may also involve the spine. Several authors have shown that these patients benefit from inpatient rehabilitation. Mckinley and colleagues19 have noted that patients with metastasis to the spine make significant improvement with care at an IRF. Compared with patients with a traumatic spinal cord injury, the cancer patients had shorter LoS, smaller improvement in FIM, equal FIM efficiency (FIM gain/LoS), and equal success in making enough progress to be discharged to home.19 Eriks and colleagues showed that patients at an IRF in Amsterdam made significant improvement in function as measured by the Barthel’s Index.20 Tang .,21 and Parsch22 and their respective colleagues, Murray,23 and New24 and colleagues have published findings confirming that patients with spinal cord injury caused by metastasis to the spine make significant progress with inpatient rehabilitation programs. The present study adds to the literature by showing that patients with metastasis to the spine who are receiving radiation can make progress and be discharged to the community.
There are 24,000 new cases of primary malignant brain tumors in the United States each year.25 The incidence of metastatic cancer to the brain has been estimated to be 100,000 cases per year in the United States. The most common cancer sources are lung, breast, melanoma, kidney, and colon.26,27 The first study of patients admitted to an IRF for treatment of brain tumors was published in 1998 by Huang and colleagues28 who compared the outcomes of 63 patients with brain tumors with the outcomes of 63 patients with stroke. They reported that the patients with the brain tumors made significant improvement in function. There was not a significant difference between the 2 groups of patients in improvement in function, FIM efficiency, or success in discharging the patients to home.28 Greenberg29 and Bartolo30 and their respective colleagues compared the outcomes of patients admitted with brain tumors and patients with stroke and found that improvement in function and discharge to home was similar in the 2 groups. In 2000, Huang and his same colleagues31 compared a group of patients with brain tumors to a group of patients with traumatic brain injury. They found significant improvement in the function of the patients with brain tumors. Patients in the traumatic brain injury group made more progress but had longer LoS. FIM efficiency was not significantly different between the groups.31
Three papers have reported outcomes of patients who received radiation concurrent with inpatient rehabilitation. Tang and colleagues32 reported 63 patients, of whom 48% percent received radiation concurrent with rehabilitation. The patients who received radiation made significant gains in function, and more than 70% were discharged to home. There was no difference in the outcomes of the patients in the radiation and nonradiation groups.32 Marciniak33 and O’Dell34 and their colleagues also reported that patients with brain tumors that required radiation therapy can benefit from inpatient rehabilitation. The present paper is the fourth (with the largest patient group) to show that patients with primary and metastatic tumors to the brain can benefit from a program that provides radiation concurrent with inpatient rehabilitation. We have shown that patients can achieve functional levels and rates of discharge to home that are not significantly different from those of the most commonly admitted group of patients to IRF – patients with stroke.
In the present study, 18% of all of the cancer patients were transferred to medical services and/or acute hospital care (Table 1). This is consistent with a paper by Asher and colleagues35 who reported that 17.4% of patients at an IRF with a diagnosis of cancer required transfer back to medical service, and that low admission motor FIM score correlated with the likelihood of transfer back to medical service. In the present paper, the total admission FIM score was not related to the likelihood of return to medical service, although a lack of improvement in the FIM score did correlate with transfer to medical service.
All of the papers we reviewed found that appropriately selected patients with cancer make significant improvement in function with treatment at an IRF. Tang and colleagues have also shown that for patients with malignant brain tumors and metastasis to the spine, improvement in function correlates with increased survival.32 Our paper confirms that patients with primary malignant brain tumors, malignant tumors metastatic to the brain or spine, and tumors metastatic to long bones may benefit from rehabilitation concurrent with radiation. Rehabilitation units are traditionally associated with treating patients with stroke and spinal cord injury. The patients in our study had cancer and were receiving radiation therapy. They had significant improvement in function and FIM efficiency scores that are not below the threshold set as expected for care at an IRF. Most patients in our study achieved a functional level consistent with what is needed to go home.
There is a prospective payment or reimbursement system for rehabilitation units.36 The payments are based on the admitting diagnosis, the admission FIM score, the age of the patient, and comorbidities. There are 4 tiers for comorbidities with no additional payments for patients in tier 0 but with additional payments for patients with conditions that qualify for tiers 1 through 3. The highest payments are for patients in tier 1. Examples of conditions that can increase payment include morbid obesity, congestive heart failure, vocal cord paralysis, and the need for hemodialysis. There is no increased payment for provision of radiation therapy. There are no reports on the feasibility, in terms of finances, of providing radiation on an IRF. We asked the finance office of the Albany Medical Center to comment on the cost to the hospital of providing radiation therapy to patients on the rehabilitation unit. The hospital’s finance department reviewed available data and reported that the variable cost of providing radiation therapy is about 6.5% of the revenue collected from third-party payors for caring for patients who receive that service (personal communication from the finance office of Albany Medical Center to George Forrest, 2015). Our findings suggest that the Centers for Medicare & Medicaid Services should make an adjustment to the payment system to support the cost of providing radiation to patients at an IRF. Even under the current payment system, for a hospital that has the equipment and personnel to provide radiation treatments, the variable cost of 6.5% of revenue should not be an absolute barrier to providing this service.
Limitations
This study reports on the experience of only 1 facility. The number of patients in the radiation group is greater than the number of patients in any previous report of people receiving radiation at an IRF, but the statistician does not think it is large enough to allow statistical analysis of covariates such as age, sex, and comorbid conditions. In addition, we did not investigate all of the factors that influence the type of care patients are offered and their LoS, such as hospital policy, insurance coverage, income, and family structure.
Conclusions
Acute care medical units are now challenged to both reduce LoS and reduce the number of patients who are readmitted to the hospital. Rehabilitation units are challenged to maintain census, as government and private payors are shifting patients from acute rehabilitation units to subacute rehabilitation units. We found that patients with cancer who need radiation are a population of patients who are seen by payors as needing to be in a facility with excellent nursing, therapy, and comprehensive physician services. A comprehensive cancer care program within a rehabilitation unit can be a great benefit to the acute care services, the IRF, and, most importantly, patients and their families.
The American Cancer Society reports that 1.6 million people are diagnosed with cancer each year, of whom 78% are aged 55 years or older. The 5-year survival rate for cancer is 68%.1 Almost 15.5 million living Americans have been diagnosed with cancer.2 Many patients with cancer have difficulty walking and with activities of daily living. Patients with primary brain tumors or tumors metastatic to the brain may present with focal weakness or cognitive deficits similar to patients with stroke. Patients with tumors metastatic to the spine may have the same deficits as a patient with a traumatic spinal cord injury. Patients with metastasis to bone may have pathologic fractures of the hip or long bones. Patients may develop peripheral neuropathy associated with a paraneoplastic syndrome, chemotherapy, or critical illness neuropathy. Lehmann and colleagues evaluated 805 patients admitted to hospitals affiliated with the University of Washington Medical School with a diagnosis of cancer and found that 15% had difficulty walking and 20% had difficulty with activities of daily living.3
Many patients with cancer can benefit from inpatient rehabilitation.4,5 Study findings have shown that patients with impairments in function related to cancer are often not referred for rehabilitation. Among the reasons mentioned for that are that oncologists are more focused on treating the patients’ cancer than on their functional deficits and that specialists in rehabilitation medicine do not want to be involved with patients with complex medical problems. Rehabilitation facilities may not want to incur the costs associated with caring for patients with cancer.6
The present paper looks at the outcomes of 61 consecutive patients with cancer who were admitted to an inpatient rehabilitation facility (IRF) and received radiation therapy concurrent with rehabilitation. It compares the outcomes of the cancer patients with the outcomes of patients without cancer who were admitted with stroke or spinal cord injury, conditions more commonly treated at an IRF.
Methods
We reviewed electronic medical records of all patients with cancer admitted to the IRF from 2008 through 2013 who received radiation therapy while at the facility. We also reviewed the data of all patients without cancer admitted with a diagnosis of stroke in 2013 and all patients admitted with a diagnosis of traumatic spinal cord injury in 2012 and 2013. No patients were excluded from stroke and traumatic spinal cord injury groups.
We recorded the sex, age, diagnostic group, Functional Independence Measure (FIM) admission score, FIM discharge score, length of stay (LoS) in the IRF, place of discharge of each patient (eg, home, acute care, or subacute care), and calculated the FIM efficiency score (change in FIM/LoS) for each patient. The FIM is an instrument that has 18 items measuring mobility, participation in activities of daily living, ability to communicate, and cognitive function.7 Each item is scored from 1 to 7, with 1 denoting that the patient cannot perform the task and 7 that the activity can be performed independently. The minimum score is 18 (complete dependence), and the maximum score is 126 (independent function). Thirteen items compose the motor FIM score: eating, grooming, bathing, dressing upper body, dressing lower body, toileting, bladder management, management of bowel, transfer to bed or wheelchair, transfer to toilet, tub transfer, walking (or wheelchair use), and climbing stairs. Five items – comprehension, expression, social interaction, problem solving, and memory – compose the cognitive FIM score.
We used a 1-way analysis of variance to evaluate differences between age and cancer type, age and diagnostic group, admission FIM score and cancer type, discharge FIM score and cancer type, change in FIM and cancer type, LoS and cancer type, and LoS and diagnostic group. The Pearson chi-square test was used to test the goodness of fit between the place of disposition and diagnostic group. The paired t test was used to evaluate the improvement in FIM of the patients who were in the cancer groups. The Tukey Simultaneous Tests for Differences of Means was used to compare the FIM efficiency scores of the groups. A 2-sample t test was used to evaluate the factors associated with the need for transfer from the IRF to the acute medical service.
Results
The demographic characteristics of the patients in the study and the admission and discharge FIM scores are reported in Table 1. There were initially 62 cancer patients in the radiation group, which was further divided into 4 subgroups based on the site of the primary tumor or metastasis. In all, 23 had a primary malignant brain tumor and received radiation and temozolomide. Sixteen patients had malignancies metastatic to the brain, 15 patients had tumors metastatic to the spine, and 7 had tumors metastatic to the long bones. One patient had laryngeal cancer and was excluded from the study because we did not think that we could do an analysis of a group with only 1 patient. The final number of patients in the cancer group was therefore 61. There were 69 patients in the stroke group and 23 in the spinal cord injury group.
We report improvement in total FIM, motor FIM, and cognitive FIM scores and were able to identify all 18 of the items of the FIM score on 60 of the 61 patients in the cancer group. Improvement in total FIM of the 61 patients in the cancer groups was significant at P P P = .05. Just over 75% of the patients in the cancer group had sufficient enough improvement in their level of function that they were able to return to their homes (Table 1). The average FIM score at the time of discharge was 83.08. This was not significantly different than the level of function of patients discharged after stroke (87.52) or traumatic spinal cord injury (89.13).
The patients with primary brain tumors were younger than the patients with cancer metastatic to the brain (P = .013). The patients with a primary brain tumor had lower admission FIM scores than patients with tumors metastatic to the brain (P = .027). The patients with a primary brain tumor had a greater increase in FIM score than patients with metastasis to the brain (P = .043; Table 2). There was not a significant difference between these 2 groups in FIM score at discharge or in the likelihood of discharge to home (Table 1). The FIM efficiency score was 1.12 for the patients in the primary brain tumor group and .80 in those with metastasis to the brain. This difference was not significant P = .96.
There were 69 patients in the stroke group. We compared the 39 patients with primary or metastatic brain lesion to the stroke group. The patients with primary or metastatic cancer of the brain were younger than the patients with stroke, 60.4 years old versus 69.1 years old (P = .004). The patients in the combined cancer group had a higher admission FIM score compared with the stroke patients (68.4 vs 63.12; P = .05). The discharge FIM scores were 83.3 in the combined cancer group and 87.5 in the stroke group (Table 1). This difference was not significant, but the improvement in the combined cancer group (14.6) was less than the improvement in the stroke group (24.40; P = .002) (Table 3).
The average LoS in the IRF was 18.7 days in the combined cancer group and 16.8 days in the stroke group. This difference was not significant. An average of 82% of the patients in the primary tumor or brain metastasis group and 85.5% of the patients in the stroke group were discharged to home. This difference was not significant. The FIM efficiency score of the patients in the stroke group was 2.0. This was significantly greater than the score for the patients in the metastasis to the brain group (0.80; P = .044) but not significantly greater than the primary brain cancer group (1.19; P = .22).
There were 23 patients in the traumatic spinal cord injury group. A comparison of the patients with tumors metastatic to the spine and patients with traumatic spinal cord injury showed that the patients in the cancer group were older (60.27 and 42.70 years, respectively; P = .001). In all, 80% of patients with tumors metastatic to the spine were men. This was not significantly different from the percentage of men in the traumatic spinal cord injury group (82.6%; Table 1). The admission FIM score of the patients with cancer was 66.5 (standard deviation [SD], 13.3) and 58.03 (SD, 15.1) in the patients with a traumatic spinal cord injury (Table 1). The FIM score at discharge was 80.4 (SD, 19.1) in the patients with cancer and 89.1 (SD, 20.3) in the patients with a traumatic spinal cord injury (Table 1). Neither of these were statistically significant. The improvement in patients with cancer was 13.9 (SD, 12.2) and 31.1 (SD, 13.9) in the traumatic spinal cord injured patients. This difference was significant (P
The median LoS was 18.98 days in the cancer metastasis to spine group (interquartile range [IQR] is the 25th-75th percentile, 12-30 days). In the traumatic group the median LoS was 23 days (IQR, 16-50 days). This difference was not significant (P = .14 Mann-Whitney test). The mean FIM efficiency score was 1.46 in the traumatic spinal cord injury group and .78 in the group with cancer metastatic to the spine. This difference was not significant (P = .72). Sixty percent of the patients in the cancer group were discharged to home, and 87% of patients in the traumatic spinal cord group were discharged to home. This difference was not significant (P = .12; Fisher exact test).
As far as we can ascertain, this is the first paper that has looked at the outcomes of patients receiving rehabilitation concurrent with radiation of the long bones. The average improvement in FIM was 12.4 (Table 1). The LoS was 11.6 days, and the FIM efficiency was 1.25. In all, 71.4% made enough progress to go home.
Of the total number of cancer patients, 18% were transferred to the acute medical service of the hospital (Table 1). Neither age, sex, type of cancer, nor admission FIM score were associated with the need for transfer to acute hospital care. Change in FIM score was inversely associated with transfer to acute hospital care (P = .027). Patients whose function did not improve with rehabilitation were most likely to be transferred back to acute hospital care.
Discussion
Radiation therapy is considered a service that is provided to people who come for treatment as an outpatient. Caregivers may have difficulty transporting patients to radiation if the patient has deficits in mobility. This may be particularly true if the patient is heavy, the caregivers are frail, or perhaps if they live in rural settings where there is no wheelchair-accessible public transportation. There are many factors that help determine whether a patient with functional deficits can be discharged to his or her home. These include sex, age, marital status, family and/or community support, income, and insurance.8 The FIM is an instrument that indicates how much help a patient needs with mobility and self-care skills. It also correlates with the amount of time that caregivers must spend helping a patient.9 Study findings have shown that the FIM score is an important determinant of whether a patient can be discharged to home. The total FIM score is as useful as an analysis of the components of the FIM score in predicting whether a patient can return to the community.10,11 Reistetter and colleagues found a total FIM score of 78 to be the score that best separates patients who are likely to be able to go home and patients who are likely to need long-term care.11 Bottemiller and colleagues10 reported that 37% of patients with total discharge FIM scores of less than 40 were discharged to home. They reported that 62% of patients with FIM scores between 40 and 79 were discharged to home, and 88% of patients with scores of 80 or above were discharged to home.10 The goal in bringing patients to the IRF was to accept and treat patients with reasonable community support and potential to achieve a functional level compatible with discharge to the community. Most patients in each of the cancer groups were able to reach an FIM score of 78 to 80 and to be discharged to home.
Most of the patients in the cancer groups had underlying problems that are not considered curable. The primary goal was to enable the patients to have some time at home with their families before requiring readmission to a hospital or hospice care. Reasonable LoS and rate of progress are now expected or required by third-party payors and hospital administrators. Physicians at the Mayo Clinic have indicated that a rehabilitation service should aim for an FIM efficiency score of at least .6 points per day.10 The FIM efficiency of patients in each of the 4 cancer subgroups in this study was higher than this level.
J. Herbert Dietz, Jr was an early advocate of the need to provide comprehensive rehabilitation services for patients with cancer. He first described his work in 1969.12 Since that time, there have been many papers that have documented the benefits of IRF for patients with cancer. O’Toole and Golden have shown outcomes of a large series of patients from an IRF. They reported that at the time of admission, 14% of patients could ambulate, but at discharge, 80% could ambulate without hands-on assistance. They reported significant improvements in continence, FIM score, and score on the Karnofsky Performance Scale.13 Marciniak,14 Hunter,15 Shin,16 and Cole,17 and their respective colleagues have all shown that patients with many different types of cancer benefit from rehabilitation at the IRF level. Gallegos-Kearin and colleagues4 reported on the care of 115,570 patients admitted to IRF with cancer from 2002 to 2014. Patients had significant improvement in function, with more than 70% of patients discharged to home.4 Ng and colleagues studied a group of 200 patients who received IRF care and found there was significant improvement in function. Ninety-four percent of patients rated their stay as either extremely good or very good.5
Metastasis to the spine is a common problem. It is found in 30% of cancer patients at autopsy. The most common sources of metastasis to the spine are breast, lung, prostate, kidney, and thyroid.18 Multiple myeloma and lymphoma may also involve the spine. Several authors have shown that these patients benefit from inpatient rehabilitation. Mckinley and colleagues19 have noted that patients with metastasis to the spine make significant improvement with care at an IRF. Compared with patients with a traumatic spinal cord injury, the cancer patients had shorter LoS, smaller improvement in FIM, equal FIM efficiency (FIM gain/LoS), and equal success in making enough progress to be discharged to home.19 Eriks and colleagues showed that patients at an IRF in Amsterdam made significant improvement in function as measured by the Barthel’s Index.20 Tang .,21 and Parsch22 and their respective colleagues, Murray,23 and New24 and colleagues have published findings confirming that patients with spinal cord injury caused by metastasis to the spine make significant progress with inpatient rehabilitation programs. The present study adds to the literature by showing that patients with metastasis to the spine who are receiving radiation can make progress and be discharged to the community.
There are 24,000 new cases of primary malignant brain tumors in the United States each year.25 The incidence of metastatic cancer to the brain has been estimated to be 100,000 cases per year in the United States. The most common cancer sources are lung, breast, melanoma, kidney, and colon.26,27 The first study of patients admitted to an IRF for treatment of brain tumors was published in 1998 by Huang and colleagues28 who compared the outcomes of 63 patients with brain tumors with the outcomes of 63 patients with stroke. They reported that the patients with the brain tumors made significant improvement in function. There was not a significant difference between the 2 groups of patients in improvement in function, FIM efficiency, or success in discharging the patients to home.28 Greenberg29 and Bartolo30 and their respective colleagues compared the outcomes of patients admitted with brain tumors and patients with stroke and found that improvement in function and discharge to home was similar in the 2 groups. In 2000, Huang and his same colleagues31 compared a group of patients with brain tumors to a group of patients with traumatic brain injury. They found significant improvement in the function of the patients with brain tumors. Patients in the traumatic brain injury group made more progress but had longer LoS. FIM efficiency was not significantly different between the groups.31
Three papers have reported outcomes of patients who received radiation concurrent with inpatient rehabilitation. Tang and colleagues32 reported 63 patients, of whom 48% percent received radiation concurrent with rehabilitation. The patients who received radiation made significant gains in function, and more than 70% were discharged to home. There was no difference in the outcomes of the patients in the radiation and nonradiation groups.32 Marciniak33 and O’Dell34 and their colleagues also reported that patients with brain tumors that required radiation therapy can benefit from inpatient rehabilitation. The present paper is the fourth (with the largest patient group) to show that patients with primary and metastatic tumors to the brain can benefit from a program that provides radiation concurrent with inpatient rehabilitation. We have shown that patients can achieve functional levels and rates of discharge to home that are not significantly different from those of the most commonly admitted group of patients to IRF – patients with stroke.
In the present study, 18% of all of the cancer patients were transferred to medical services and/or acute hospital care (Table 1). This is consistent with a paper by Asher and colleagues35 who reported that 17.4% of patients at an IRF with a diagnosis of cancer required transfer back to medical service, and that low admission motor FIM score correlated with the likelihood of transfer back to medical service. In the present paper, the total admission FIM score was not related to the likelihood of return to medical service, although a lack of improvement in the FIM score did correlate with transfer to medical service.
All of the papers we reviewed found that appropriately selected patients with cancer make significant improvement in function with treatment at an IRF. Tang and colleagues have also shown that for patients with malignant brain tumors and metastasis to the spine, improvement in function correlates with increased survival.32 Our paper confirms that patients with primary malignant brain tumors, malignant tumors metastatic to the brain or spine, and tumors metastatic to long bones may benefit from rehabilitation concurrent with radiation. Rehabilitation units are traditionally associated with treating patients with stroke and spinal cord injury. The patients in our study had cancer and were receiving radiation therapy. They had significant improvement in function and FIM efficiency scores that are not below the threshold set as expected for care at an IRF. Most patients in our study achieved a functional level consistent with what is needed to go home.
There is a prospective payment or reimbursement system for rehabilitation units.36 The payments are based on the admitting diagnosis, the admission FIM score, the age of the patient, and comorbidities. There are 4 tiers for comorbidities with no additional payments for patients in tier 0 but with additional payments for patients with conditions that qualify for tiers 1 through 3. The highest payments are for patients in tier 1. Examples of conditions that can increase payment include morbid obesity, congestive heart failure, vocal cord paralysis, and the need for hemodialysis. There is no increased payment for provision of radiation therapy. There are no reports on the feasibility, in terms of finances, of providing radiation on an IRF. We asked the finance office of the Albany Medical Center to comment on the cost to the hospital of providing radiation therapy to patients on the rehabilitation unit. The hospital’s finance department reviewed available data and reported that the variable cost of providing radiation therapy is about 6.5% of the revenue collected from third-party payors for caring for patients who receive that service (personal communication from the finance office of Albany Medical Center to George Forrest, 2015). Our findings suggest that the Centers for Medicare & Medicaid Services should make an adjustment to the payment system to support the cost of providing radiation to patients at an IRF. Even under the current payment system, for a hospital that has the equipment and personnel to provide radiation treatments, the variable cost of 6.5% of revenue should not be an absolute barrier to providing this service.
Limitations
This study reports on the experience of only 1 facility. The number of patients in the radiation group is greater than the number of patients in any previous report of people receiving radiation at an IRF, but the statistician does not think it is large enough to allow statistical analysis of covariates such as age, sex, and comorbid conditions. In addition, we did not investigate all of the factors that influence the type of care patients are offered and their LoS, such as hospital policy, insurance coverage, income, and family structure.
Conclusions
Acute care medical units are now challenged to both reduce LoS and reduce the number of patients who are readmitted to the hospital. Rehabilitation units are challenged to maintain census, as government and private payors are shifting patients from acute rehabilitation units to subacute rehabilitation units. We found that patients with cancer who need radiation are a population of patients who are seen by payors as needing to be in a facility with excellent nursing, therapy, and comprehensive physician services. A comprehensive cancer care program within a rehabilitation unit can be a great benefit to the acute care services, the IRF, and, most importantly, patients and their families.
1. American Cancer Society. Cancer facts & figures 2016. Atlanta, GA: American Cancer Society; 2016.
2. National Cancer Institute: Office of cancer survivorship: statistics. https://cancercontrol.cancer.gov/ocs/statistics/statistics.html. Updated October 17, 2016. Accessed April 21, 2018.
3. Lehmann JF, DeLisa JA, Warren CG, deLateur BJ, Bryant PL, Nicholson CG. Cancer rehabilitation: assessment of need, development and evaluation of a model of care. Arch Phys Med Rehabil. 1978;59(9):410-419.
4. Gallegos-Kearin V, Knowlton SE, Goldstein R, et al. Outcome trends of adult cancer patients receiving inpatient rehabilitation: a 13-year review [published online Feb 21, 2018]. Am J Phys Med Rehabil. doi:10.1097/PHM.0000000000000911
5. Ng AH, Gupta E, Fontillas RC, et al. Patient-reported usefulness of acute cancer rehabilitation. PM R. 2017;9(11):1135-1143.
6. Cheville AL, Kornblith AB, Basford JR. An examination of the causes for the underutilization of rehabilitation services among people with advanced cancer. Am J Phys Med Rehabil. 2011;90(5 suppl 1):S27-S37.
7. Cohen ME, Marino RJ. The tools of disability outcomes research functional status measures. Arch Phys Med Rehabil. 2000;81(12 suppl 2):S21-S29.
8. Nguyen VQ, PrvuBettger J, Guerrier T, et al. Factors associated with discharge to home versus discharge to institutional care after inpatient stroke rehabilitation. Arch Phys Med Rehabil. 2015;96(7):1297-1303.
9. Forrest G, Schwam A, Cohen E. Time of care required by patients discharged from a rehabilitation unit. Am J Phys Med Rehabil. 2002;81(1):57-62.
10. Bottemiller KL, Bieber PL, Basford JR, Harris M. FIM scores, FIM efficiency and discharge following inpatient stroke rehabilitation. Rehabil Nurs. 2006;31(1):22-25.
11. Reistetter TA, Graham JE, Deutsch A, Granger CV, Markello S, Ottenbacher KJ. Utility of functional status for classifying community versus institutional discharges after inpatient rehabilitation for stroke. Arch Phys Med Rehabil. 2010;91(3):345-350.
12. Dietz JH Jr. Rehabilitation of the cancer patient. Med Clin North Am. 1969;53(3):607-624.
13. O'Toole DM, Golden AM. Evaluating cancer patients for rehabilitation potential. West J Med. 1991;155(4):384-387.
14. Marciniak CM, Sliwa JA, Spill G, Heinemann AW, Semik PE. Functional outcome following rehabilitation of the cancer patient. Arch Phys Med Rehabil. 1996;77(1):54-57.
15. Hunter EG, Baltisberger J. Functional outcomes by age for inpatient cancer rehabilitation: a retrospective chart review. J Appl Gerontol. 2013;32(4):443-456.
16. Shin KY, Guo Y, Konzen B, Fu J, Yadav R, Bruera E. Inpatient cancer rehabilitation: the experience of a national comprehensive cancer center. Am J Phys Med Rehabil. 2011;90(5 suppl 1):S63-S68.
17. Cole RP, Scialla S, Bednarz L. Functional recovery in cancer rehabilitation. Arch Phys Med Rehabil. 2000;81(5):623-627.
18. White AP, Kwon BK, Lindskog DM, Friedlaender GE, Grauer JN. Metastatic disease of the spine. J Am Acad Orthop Surg. 2006;14(11):587-598.
19. McKinley WO, Huang ME, Tewksbury MA. Neoplastic vs traumatic spinal cord injury: an inpatient rehabilitation comparison. Am J Phys Med Rehabil. 2000;79(2):138-144.
20. Eriks IE, Angenot EL, Lankhorst GJ. Epidural metastatic spinal cord compression: functional outcome and survival after inpatient rehabilitation. Spinal Cord. 2004;42(4):235-239.
21. Tang V, Harvey D, Park Dorsay J, Jiang S, Rathbone MP. Prognostic indicators in metastatic spinal cord compression: using functional independence measure and Tokuhashi scale to optimize rehabilitation planning. Spinal Cord. 2007;45(10):671-677.
22. Parsch D, Mikut R, Abel R. Postacute management of patients with spinal cord injury due to metastatic tumor disease: survival and efficacy of rehabilitation. Spinal Cord. 2003;41:205-210.
23. Murray PK. Functional outcome and survival in spinal cord injury secondary to neoplasia. Cancer. 1985;55:197-201.
24. New PW. Functional outcomes and disability after nontraumatic spinal cord injury rehabilitation: results from a retrospective study. Arch Phys Med Rehabil. 2005;86(2):250-261
25. Central Brain Tumor Registry of the United States: 2016 CBTRUS fact sheet. www.cbtrus.org/factsheet/factsheet.html. Updated 2017. Accessed May 28, 2016.
26. Memorial Sloan Kettering Cancer Center: Metastatic brain tumors & secondary brain cancer. https://www.mskcc.org/cancer-care/types/brain-tumors-metastatic. Updated 2018. Accessed April 21, 2018.
27. Bruckner JC, Brown PD, O'Neill BP, Meyer FB, Wetmore CJ, Uhm JH. Central nervous system tumors. Mayo Clin Proc. 2007;82(10):1271-1286.
28. Huang ME, Cifu DX, Keyser-Marcus L. Functional outcome after brain tumor and acute stroke: a comparative analysis. Arch Phys Med Rehabil. 1998;79(11):1386-1390.
29. Greenberg E, Treger I, Ring H. Rehabilitation outcomes in patients with brain tumors and acute stroke: comparative study of inpatient rehabilitation. Am J Phys Med Rehabil. 2006;85(7):568-573.
30. Bartolo M, Zucchella C, Pace A, et al. Early rehabilitation after surgery improves functional outcomes in inpatients with brain tumours. J Neurooncol. 2012;107(3);537-544.
31. Huang ME, Cifu DX, Keyser-Marcus L. Functional outcomes in patients with brain tumor after inpatient rehabilitation: comparison with traumatic brain injury. Am J Phys Med Rehabil. 2000;79(4):327-335.
32. Tang V, Rathbone M, Park Dorsay J, Jiang S, Harvey D. Rehabilitation in primary and metastatic brain tumours: impact of functional outcomes on survival. J Neurol. 2008;255(6):820-827.
33. Marciniak CM, Sliwa JA, Heinemann AW, Semik PE. Functional outcomes of persons with brain tumors after inpatient rehabilitation. Arch Phys Med Rehabil. 2001;82(4):457-463.
34. O'Dell MW, Barr K, Spanier D, Warnick RE. Functional outcome of inpatient rehabilitation in persons with brain tumors. Arch Phys Med Rehabil. 1998;79(12):1530-1534.
35. Asher A, Roberts PS, Bresee C, Zabel G, Riggs RV, Rogatko A. Transferring inpatient rehabilitation facility cancer patients back to acute care (TRIPBAC). PM R. 2014;6(9):808-813.
36. Centers for Medicare and Medicaid Services: Inpatient rehabilitation facilities. https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/CertificationandComplianc/InpatientRehab.html. Published March 5, 2012. Accessed May 21, 2018.
1. American Cancer Society. Cancer facts & figures 2016. Atlanta, GA: American Cancer Society; 2016.
2. National Cancer Institute: Office of cancer survivorship: statistics. https://cancercontrol.cancer.gov/ocs/statistics/statistics.html. Updated October 17, 2016. Accessed April 21, 2018.
3. Lehmann JF, DeLisa JA, Warren CG, deLateur BJ, Bryant PL, Nicholson CG. Cancer rehabilitation: assessment of need, development and evaluation of a model of care. Arch Phys Med Rehabil. 1978;59(9):410-419.
4. Gallegos-Kearin V, Knowlton SE, Goldstein R, et al. Outcome trends of adult cancer patients receiving inpatient rehabilitation: a 13-year review [published online Feb 21, 2018]. Am J Phys Med Rehabil. doi:10.1097/PHM.0000000000000911
5. Ng AH, Gupta E, Fontillas RC, et al. Patient-reported usefulness of acute cancer rehabilitation. PM R. 2017;9(11):1135-1143.
6. Cheville AL, Kornblith AB, Basford JR. An examination of the causes for the underutilization of rehabilitation services among people with advanced cancer. Am J Phys Med Rehabil. 2011;90(5 suppl 1):S27-S37.
7. Cohen ME, Marino RJ. The tools of disability outcomes research functional status measures. Arch Phys Med Rehabil. 2000;81(12 suppl 2):S21-S29.
8. Nguyen VQ, PrvuBettger J, Guerrier T, et al. Factors associated with discharge to home versus discharge to institutional care after inpatient stroke rehabilitation. Arch Phys Med Rehabil. 2015;96(7):1297-1303.
9. Forrest G, Schwam A, Cohen E. Time of care required by patients discharged from a rehabilitation unit. Am J Phys Med Rehabil. 2002;81(1):57-62.
10. Bottemiller KL, Bieber PL, Basford JR, Harris M. FIM scores, FIM efficiency and discharge following inpatient stroke rehabilitation. Rehabil Nurs. 2006;31(1):22-25.
11. Reistetter TA, Graham JE, Deutsch A, Granger CV, Markello S, Ottenbacher KJ. Utility of functional status for classifying community versus institutional discharges after inpatient rehabilitation for stroke. Arch Phys Med Rehabil. 2010;91(3):345-350.
12. Dietz JH Jr. Rehabilitation of the cancer patient. Med Clin North Am. 1969;53(3):607-624.
13. O'Toole DM, Golden AM. Evaluating cancer patients for rehabilitation potential. West J Med. 1991;155(4):384-387.
14. Marciniak CM, Sliwa JA, Spill G, Heinemann AW, Semik PE. Functional outcome following rehabilitation of the cancer patient. Arch Phys Med Rehabil. 1996;77(1):54-57.
15. Hunter EG, Baltisberger J. Functional outcomes by age for inpatient cancer rehabilitation: a retrospective chart review. J Appl Gerontol. 2013;32(4):443-456.
16. Shin KY, Guo Y, Konzen B, Fu J, Yadav R, Bruera E. Inpatient cancer rehabilitation: the experience of a national comprehensive cancer center. Am J Phys Med Rehabil. 2011;90(5 suppl 1):S63-S68.
17. Cole RP, Scialla S, Bednarz L. Functional recovery in cancer rehabilitation. Arch Phys Med Rehabil. 2000;81(5):623-627.
18. White AP, Kwon BK, Lindskog DM, Friedlaender GE, Grauer JN. Metastatic disease of the spine. J Am Acad Orthop Surg. 2006;14(11):587-598.
19. McKinley WO, Huang ME, Tewksbury MA. Neoplastic vs traumatic spinal cord injury: an inpatient rehabilitation comparison. Am J Phys Med Rehabil. 2000;79(2):138-144.
20. Eriks IE, Angenot EL, Lankhorst GJ. Epidural metastatic spinal cord compression: functional outcome and survival after inpatient rehabilitation. Spinal Cord. 2004;42(4):235-239.
21. Tang V, Harvey D, Park Dorsay J, Jiang S, Rathbone MP. Prognostic indicators in metastatic spinal cord compression: using functional independence measure and Tokuhashi scale to optimize rehabilitation planning. Spinal Cord. 2007;45(10):671-677.
22. Parsch D, Mikut R, Abel R. Postacute management of patients with spinal cord injury due to metastatic tumor disease: survival and efficacy of rehabilitation. Spinal Cord. 2003;41:205-210.
23. Murray PK. Functional outcome and survival in spinal cord injury secondary to neoplasia. Cancer. 1985;55:197-201.
24. New PW. Functional outcomes and disability after nontraumatic spinal cord injury rehabilitation: results from a retrospective study. Arch Phys Med Rehabil. 2005;86(2):250-261
25. Central Brain Tumor Registry of the United States: 2016 CBTRUS fact sheet. www.cbtrus.org/factsheet/factsheet.html. Updated 2017. Accessed May 28, 2016.
26. Memorial Sloan Kettering Cancer Center: Metastatic brain tumors & secondary brain cancer. https://www.mskcc.org/cancer-care/types/brain-tumors-metastatic. Updated 2018. Accessed April 21, 2018.
27. Bruckner JC, Brown PD, O'Neill BP, Meyer FB, Wetmore CJ, Uhm JH. Central nervous system tumors. Mayo Clin Proc. 2007;82(10):1271-1286.
28. Huang ME, Cifu DX, Keyser-Marcus L. Functional outcome after brain tumor and acute stroke: a comparative analysis. Arch Phys Med Rehabil. 1998;79(11):1386-1390.
29. Greenberg E, Treger I, Ring H. Rehabilitation outcomes in patients with brain tumors and acute stroke: comparative study of inpatient rehabilitation. Am J Phys Med Rehabil. 2006;85(7):568-573.
30. Bartolo M, Zucchella C, Pace A, et al. Early rehabilitation after surgery improves functional outcomes in inpatients with brain tumours. J Neurooncol. 2012;107(3);537-544.
31. Huang ME, Cifu DX, Keyser-Marcus L. Functional outcomes in patients with brain tumor after inpatient rehabilitation: comparison with traumatic brain injury. Am J Phys Med Rehabil. 2000;79(4):327-335.
32. Tang V, Rathbone M, Park Dorsay J, Jiang S, Harvey D. Rehabilitation in primary and metastatic brain tumours: impact of functional outcomes on survival. J Neurol. 2008;255(6):820-827.
33. Marciniak CM, Sliwa JA, Heinemann AW, Semik PE. Functional outcomes of persons with brain tumors after inpatient rehabilitation. Arch Phys Med Rehabil. 2001;82(4):457-463.
34. O'Dell MW, Barr K, Spanier D, Warnick RE. Functional outcome of inpatient rehabilitation in persons with brain tumors. Arch Phys Med Rehabil. 1998;79(12):1530-1534.
35. Asher A, Roberts PS, Bresee C, Zabel G, Riggs RV, Rogatko A. Transferring inpatient rehabilitation facility cancer patients back to acute care (TRIPBAC). PM R. 2014;6(9):808-813.
36. Centers for Medicare and Medicaid Services: Inpatient rehabilitation facilities. https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/CertificationandComplianc/InpatientRehab.html. Published March 5, 2012. Accessed May 21, 2018.
H&N cancer may be undertreated in women
CHICAGO – Sex disparities in the treatment of head and neck cancer may be leading to poorer outcomes for women, according to a retrospective registry-based cohort study of 884 patients reported at annual meeting of the American Society of Clinical Oncology.
“The treatment of head and neck cancer often requires intensive treatment that can have lasting side effects,” senior study author Jed A. Katzel, MD, a medical oncologist at Kaiser Permanente in Santa Clara, Calif., said in a press briefing. “Our goal was to review data from a large group of patients in Northern California to determine which patients are most likely to benefit from aggressive therapy, while minimizing toxicity for those likely to die from competing events.”
The reasons for the observed sex disparities are not known, according to Dr. Katzel. However, they may include patient preferences, physician practices, and the higher proportion among men of oropharynx tumors, as those tumors are more commonly associated with human papillomavirus (HPV), which carries a more favorable prognosis.
“Further investigation is needed to determine if there is an actual difference in treatment and outcomes for women, compared with men,” he said. “To this end, we have planned a chart-by-chart review, as well as a prospective analysis that will be performed in the currently enrolling NRG HN004 clinical trial.”
“The outcome of this study was very surprising to us, the idea that there are disparities in both the treatment that women receive relative to men, but also in the rate of death from head and neck cancer for women compared to men,” commented ASCO Expert Joshua Jones, MD, MA, who is also a radiation oncologist at the Perelman Center for Advanced Medicine, University of Pennsylvania, Philadelphia.
Dr. Katzel and his colleagues used the Kaiser Permanente Northern California registry to identify patients with stage II to IVB head and neck cancer diagnosed during 2000-2015.
Analyses were based on 223 women and 661 men, relative numbers that are not surprising given the known demographics of this cancer. Oropharyngeal tumors accounted for 38% of the cancers in the former, but 55% in the latter. (HPV status was not directly ascertained.)
The rate of receipt of intensive chemotherapy was 35% for women and 46% for men (adjusted odds ratio, 0.68; 95% CI, 0.48-0.98; P = .006). Similarly, the rate of receipt of radiation therapy was 60% for women and 70% for men (AOR, 0.79; 95% CI, 0.56-1.11; P = .008). Receipt of surgery was similar for the sexes.
The investigators analyzed deaths according to type using a GCE model that controlled for age, sex, tumor site, and Charlson Comorbidity Index. “The GCE model essentially describes the degree to which cancer is the patient’s problem,” Dr. Katzel explained.
Results showed that both women and men were more likely to die from cancer than from other causes; however, the ratio was 7 for women, compared with just 3.8 for men, a difference translating to a relative hazard ratio of 1.92 (95% CI, 1.07-3.43).
In terms of potential confounding, there were only 19 noncancer deaths among the women studied, suggesting that they may have been more healthy than the men, which could have influenced the calculations, according to Dr. Katzel.
“This GCE model has been validated in head and neck cancer, but also in breast cancer, prostate cancer, and endometrial cancer, so we are using a validated model to do this evaluation,” he noted. “So I would say we are confident in our findings.”
Dr. Katzel disclosed that he had no relevant conflicts of interest. The study received funding from Kaiser Permanente Northern California Graduate Medical Education Department.
SOURCE: Park A et al. ASCO 2018 Abstract LBA6002.
CHICAGO – Sex disparities in the treatment of head and neck cancer may be leading to poorer outcomes for women, according to a retrospective registry-based cohort study of 884 patients reported at annual meeting of the American Society of Clinical Oncology.
“The treatment of head and neck cancer often requires intensive treatment that can have lasting side effects,” senior study author Jed A. Katzel, MD, a medical oncologist at Kaiser Permanente in Santa Clara, Calif., said in a press briefing. “Our goal was to review data from a large group of patients in Northern California to determine which patients are most likely to benefit from aggressive therapy, while minimizing toxicity for those likely to die from competing events.”
The reasons for the observed sex disparities are not known, according to Dr. Katzel. However, they may include patient preferences, physician practices, and the higher proportion among men of oropharynx tumors, as those tumors are more commonly associated with human papillomavirus (HPV), which carries a more favorable prognosis.
“Further investigation is needed to determine if there is an actual difference in treatment and outcomes for women, compared with men,” he said. “To this end, we have planned a chart-by-chart review, as well as a prospective analysis that will be performed in the currently enrolling NRG HN004 clinical trial.”
“The outcome of this study was very surprising to us, the idea that there are disparities in both the treatment that women receive relative to men, but also in the rate of death from head and neck cancer for women compared to men,” commented ASCO Expert Joshua Jones, MD, MA, who is also a radiation oncologist at the Perelman Center for Advanced Medicine, University of Pennsylvania, Philadelphia.
Dr. Katzel and his colleagues used the Kaiser Permanente Northern California registry to identify patients with stage II to IVB head and neck cancer diagnosed during 2000-2015.
Analyses were based on 223 women and 661 men, relative numbers that are not surprising given the known demographics of this cancer. Oropharyngeal tumors accounted for 38% of the cancers in the former, but 55% in the latter. (HPV status was not directly ascertained.)
The rate of receipt of intensive chemotherapy was 35% for women and 46% for men (adjusted odds ratio, 0.68; 95% CI, 0.48-0.98; P = .006). Similarly, the rate of receipt of radiation therapy was 60% for women and 70% for men (AOR, 0.79; 95% CI, 0.56-1.11; P = .008). Receipt of surgery was similar for the sexes.
The investigators analyzed deaths according to type using a GCE model that controlled for age, sex, tumor site, and Charlson Comorbidity Index. “The GCE model essentially describes the degree to which cancer is the patient’s problem,” Dr. Katzel explained.
Results showed that both women and men were more likely to die from cancer than from other causes; however, the ratio was 7 for women, compared with just 3.8 for men, a difference translating to a relative hazard ratio of 1.92 (95% CI, 1.07-3.43).
In terms of potential confounding, there were only 19 noncancer deaths among the women studied, suggesting that they may have been more healthy than the men, which could have influenced the calculations, according to Dr. Katzel.
“This GCE model has been validated in head and neck cancer, but also in breast cancer, prostate cancer, and endometrial cancer, so we are using a validated model to do this evaluation,” he noted. “So I would say we are confident in our findings.”
Dr. Katzel disclosed that he had no relevant conflicts of interest. The study received funding from Kaiser Permanente Northern California Graduate Medical Education Department.
SOURCE: Park A et al. ASCO 2018 Abstract LBA6002.
CHICAGO – Sex disparities in the treatment of head and neck cancer may be leading to poorer outcomes for women, according to a retrospective registry-based cohort study of 884 patients reported at annual meeting of the American Society of Clinical Oncology.
“The treatment of head and neck cancer often requires intensive treatment that can have lasting side effects,” senior study author Jed A. Katzel, MD, a medical oncologist at Kaiser Permanente in Santa Clara, Calif., said in a press briefing. “Our goal was to review data from a large group of patients in Northern California to determine which patients are most likely to benefit from aggressive therapy, while minimizing toxicity for those likely to die from competing events.”
The reasons for the observed sex disparities are not known, according to Dr. Katzel. However, they may include patient preferences, physician practices, and the higher proportion among men of oropharynx tumors, as those tumors are more commonly associated with human papillomavirus (HPV), which carries a more favorable prognosis.
“Further investigation is needed to determine if there is an actual difference in treatment and outcomes for women, compared with men,” he said. “To this end, we have planned a chart-by-chart review, as well as a prospective analysis that will be performed in the currently enrolling NRG HN004 clinical trial.”
“The outcome of this study was very surprising to us, the idea that there are disparities in both the treatment that women receive relative to men, but also in the rate of death from head and neck cancer for women compared to men,” commented ASCO Expert Joshua Jones, MD, MA, who is also a radiation oncologist at the Perelman Center for Advanced Medicine, University of Pennsylvania, Philadelphia.
Dr. Katzel and his colleagues used the Kaiser Permanente Northern California registry to identify patients with stage II to IVB head and neck cancer diagnosed during 2000-2015.
Analyses were based on 223 women and 661 men, relative numbers that are not surprising given the known demographics of this cancer. Oropharyngeal tumors accounted for 38% of the cancers in the former, but 55% in the latter. (HPV status was not directly ascertained.)
The rate of receipt of intensive chemotherapy was 35% for women and 46% for men (adjusted odds ratio, 0.68; 95% CI, 0.48-0.98; P = .006). Similarly, the rate of receipt of radiation therapy was 60% for women and 70% for men (AOR, 0.79; 95% CI, 0.56-1.11; P = .008). Receipt of surgery was similar for the sexes.
The investigators analyzed deaths according to type using a GCE model that controlled for age, sex, tumor site, and Charlson Comorbidity Index. “The GCE model essentially describes the degree to which cancer is the patient’s problem,” Dr. Katzel explained.
Results showed that both women and men were more likely to die from cancer than from other causes; however, the ratio was 7 for women, compared with just 3.8 for men, a difference translating to a relative hazard ratio of 1.92 (95% CI, 1.07-3.43).
In terms of potential confounding, there were only 19 noncancer deaths among the women studied, suggesting that they may have been more healthy than the men, which could have influenced the calculations, according to Dr. Katzel.
“This GCE model has been validated in head and neck cancer, but also in breast cancer, prostate cancer, and endometrial cancer, so we are using a validated model to do this evaluation,” he noted. “So I would say we are confident in our findings.”
Dr. Katzel disclosed that he had no relevant conflicts of interest. The study received funding from Kaiser Permanente Northern California Graduate Medical Education Department.
SOURCE: Park A et al. ASCO 2018 Abstract LBA6002.
REPORTING FROM ASCO 2018
Key clinical point: Women with head and neck cancer may be relatively undertreated and therefore are more likely to die from the disease.
Major finding: Compared with male counterparts, female patients had lower rates of receiving intensive chemotherapy (35% vs. 46%) and radiation therapy (60% vs. 70%) and a higher ratio of cancer to noncancer mortality (adjusted relative hazard ratio, 1.92).
Study details: Retrospective, registry-based, cohort study of 884 patients with stage II to IVB H&N cancer diagnosed during 2000-2015.
Disclosures: Dr. Katzel disclosed that he had no relevant conflicts of interest. The study received funding from Kaiser Permanente Northern California Graduate Medical Education Department.
Source: Park A et al. ASCO 2018, Abstract LBA6002.
Head and neck cancers: Women less commonly receive intensive chemo
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – Women with head and neck cancer less commonly receive intensive chemotherapy (35% vs. 46%) and radiation therapy (60% vs. 70%) than do their male counterparts, finds an analysis of 223 female patients and 661 male patients with stage II-IVB disease treated at Kaiser Permanente Northern California. And this apparent undertreatment may be compromising survival for women, as their ratio of cancer deaths to other deaths is nearly twice that of men (adjusted relative hazard ratio, 1.92; 95% CI, 1.07-3.43).
In this video interview from the annual meeting of the American Society of Clinical Oncology, senior study author Jed A. Katzel, MD, of Kaiser Permanente in Santa Clara, Calif., described the new statistical approach used to assess outcomes and discussed ongoing research to pin down the reasons for the apparent treatment disparities, including patient preferences and the influences of tumor site and HPV status.
Dr. Katzel reported no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – Women with head and neck cancer less commonly receive intensive chemotherapy (35% vs. 46%) and radiation therapy (60% vs. 70%) than do their male counterparts, finds an analysis of 223 female patients and 661 male patients with stage II-IVB disease treated at Kaiser Permanente Northern California. And this apparent undertreatment may be compromising survival for women, as their ratio of cancer deaths to other deaths is nearly twice that of men (adjusted relative hazard ratio, 1.92; 95% CI, 1.07-3.43).
In this video interview from the annual meeting of the American Society of Clinical Oncology, senior study author Jed A. Katzel, MD, of Kaiser Permanente in Santa Clara, Calif., described the new statistical approach used to assess outcomes and discussed ongoing research to pin down the reasons for the apparent treatment disparities, including patient preferences and the influences of tumor site and HPV status.
Dr. Katzel reported no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – Women with head and neck cancer less commonly receive intensive chemotherapy (35% vs. 46%) and radiation therapy (60% vs. 70%) than do their male counterparts, finds an analysis of 223 female patients and 661 male patients with stage II-IVB disease treated at Kaiser Permanente Northern California. And this apparent undertreatment may be compromising survival for women, as their ratio of cancer deaths to other deaths is nearly twice that of men (adjusted relative hazard ratio, 1.92; 95% CI, 1.07-3.43).
In this video interview from the annual meeting of the American Society of Clinical Oncology, senior study author Jed A. Katzel, MD, of Kaiser Permanente in Santa Clara, Calif., described the new statistical approach used to assess outcomes and discussed ongoing research to pin down the reasons for the apparent treatment disparities, including patient preferences and the influences of tumor site and HPV status.
Dr. Katzel reported no financial disclosures.
REPORTING FROM ASCO 2018
Positivity Rates in Oropharyngeal and Nonoropharyngeal Head and Neck Cancer in the VA
Head and neck cancer (HNC) continues to be a major health issue with an estimated 51,540 cases in the US in 2018, making it the eighth most common cancer among men with an estimated 4% of all new cancer diagnoses.1 Over the past decade, human papillomavirus (HPV) has emerged as a major prognostic factor for survival in squamous cell carcinomas of the oropharynx. Patients who are HPV-positive (HPV+) have a much higher survival rate than patients who have HPV-negative (HPV-) cancers of the oropharynx. The 8th edition of the American Joint Committee on Cancer (AJCC) staging manual has 2 distinct stagings for HPV+ and HPV- oropharyngeal tumors using p16-positivity (p16+) as a surrogate marker.2
Squamous cell carcinomas of the oropharynx that are HPV+ have about half the risk of death of HPV- tumors, are highly responsive to treatment, and are more often seen in younger and healthier patients with little to no tobacco use.2,3 As such, there also is a movement to de-escalate HPV+ oropharyngeal cancers with multiple trials by either replacing cytotoxic chemotherapy with a targeted agent (cisplatin vs cetuximab in RTOG 1016) or reducing the radiation dose (ECOG 1308, NRG HN002, Quarterback, and OPTIMA trials).3
The focus of many epidemiologic studies has been in the HNC general population. A recent epidemiologic analysis of the HNC general population found a p16 positivity rate of 60% in oropharyngeal squamous cell carcinomas (OPSCC) and 10% in nonoropharyngeal squamous cell carcinomas (NOPSCC).4 There has been a lack of studies focusing on the US Department of Veterans Administration (VA) population. The VA HNC population consists mostly of older white male smokers; whereas the rise of OPSCC in the general population consists primarily of males aged < 60 years often with little or no tobacco use.5 Furthermore, the importance of p16 positivity in NOPSCC also may be prognostic.6 Population data on this subset in the VA are lacking as well.This study’s purpose is to analyze the p16 positivity rate in both the OPSCC and NOPSCC in the VA population. Elucidation of epidemiologic factors that are associated with these groups may bring to light important differences between the VA and general HNC populations.
Methods
A review of the Kansas City VA Medical Center database for patients with HNC was performed from 2011 to 2017. The review consisted of 183 patient records (second primaries were scored separately), and 123 were deemed eligible for the study. Epidemiologic data were collected, including site, OPSCC vs NOPSCC, age, race, education level, tobacco use, alcohol use, TNM stage, and marital status (Table).
Results
The NOPSCC p16+ group had the greatest mean pack-year use (57). The lowest was in the OPSCC p16+ group (29). The OPSCC p16+ group had 37% never smokers compared with ≤ 10% for the other groups. Both the OPSCC and NOPSCC p16- groups had much more alcohol use per week than that of the p16+ groups. The differences in marital status included a lower rate of never married individuals in the p16+ group and a higher rate of marriage in the NOPSCC p16- group. The T stage distribution within the OPSCC groups was similar, but NOPSCC groups saw more T1 lesions in the NOPSCC p16- group (42% p16- vs 18% p16+). Conversely, more T4 lesions were found in the NOPSCC p16+ patients (7% p16- vs 29% p16+).
Discussion
The overall HPV positivity rate in the general population of patients with HNC has been reported as between 57% and 72% for OPSCC and between 1.3% and 7% for NOPSCC.6 One study, however, examined the p16 positivity rate in NOPSCC patients enrolled in major trials (RTOG 0129, 0234, and 0522 studies) and found that up to 19.3% of NOPSCC patients had p16 positivity.6 Even with the near 20% rate in those aforementioned trials that are above the reported norm, the current study found that nearly 30% of its VA population had p16+ NOPSCC. It has been shown that regardless of site, HPV-driven head and neck tumors share a similar gene expression and DNA methylation profiles (nonkeratinizing, basaloid histopathologic features, and lack of TP53 or CDKN2A alterations).5 p16+ NOPSCC has a different immune microenvironment with less lymphocyte infiltration, and there is some debate in the literature about the effects on tumor outcomes for NOPSCC cancer.5
In the aforementioned RTOG trials, p16- NOPSCC had worse outcomes compared with those of p16+ NOPSCC.6 This result is in contrast to the Danish Head and Neck Cancer Group (DAHANCA) and the combined Johns Hopkins University (JHU) and University of California, San Francisco (UCSF) data that found no difference between p16+ NOPSCC or p16- NOPSCC.7,8 In regards to race, this study did not find any differences. Another UCSF and JHU study showed lower p16+ rates in African American patients with OPSCC, but no distinction between race in the NOPSCC group. This result is consistent with the data in the current study as the distribution of race was no different among the 4 groups; however, this study's cohort was 90% white, 10% African American, and only < 1% Native American.4 This study's cohort population also was consistent with HPV-positive tumors presenting with earlier T, but higher N staging.9
Smoking is known to decrease survival in HPV-positive HNC, with the RTOG 0129 study separating head and neck tumors into low, medium, and high risk, based on HPV status, smoking, and stage.10 Although the average smoking pack-years in the current study’s OPC p16+ group was high at 29 pack-years, there was still a significant number of nonsmokers in that same group (37%). The University of Michigan conducted a study that had a similar profile of patients with an average age of 56.5 and 32.4% never smokers in their p16+ OPSCC cohort; thus, the VA p16+ OPSCC group in this study may be similar to the general population's p16+ OPSCC group.11 Nonmonogamous relationships also have been shown to be a risk factor for HPV positivity, and there was a difference in marital status (assuming it was a surrogate for monogamy) between the 4 groups; however, in contrast, the p16+ group in the current study had a high number of married patients, 45% in OPC p16+ group, and may not have been a good surrogate for monogamy in this VA population.
Limitations
Limitations of this study include all the caveats that come with a retrospective study, such as confounding variables, unbalanced groups, and selection bias. A detailed sexual history was not included, although it is well known that sexual activity is linked with oral HPV positivity.12 Human papillomavirus positivity based on p16 immunohistochemical analysis also was used as a surrogate marker for HPV instead of DNA in situ hybridization. The data also may be skewed due to the study patient’s being predominantly white and male: Both groups have a higher predilection for HPV-driven HNCs.13
Conclusion
The proportion of p16+ VA OPSCC cases was similar to that of the general population at 75% with 37% never smokers, but the percentage in NOPSCC was higher at 29% with only 10% never smokers. The p16+ NOPSCC also presented with more T4 lesions and a higher overall stage compared with p16- NOPSCC. Further studies are needed to compare these subgroups in the VA and in the general HNC populations.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30.
2. Lydiatt WM, Patel SG, O’Sullivan B, et al. Head and neck cancers major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67(2):122-137.
3. Mirghani H, Blanchard P. Treatment de-escalation for HPV-driven oropharyngeal cancer: where do we stand? Clin Transl Radiat Oncol. 2017;8:4-11.
4. D’Souza G, Westra WH, Wang SJ, et al. Differences in the prevalence of human papillomavirus (HPV) in head and neck squamous cell cancers by sex, race, anatomic tumor site, and HPV detection method. JAMA Oncol. 2017;3(2):169-177.
5. Chakravarthy A, Henderson S, Thirdborough SM, et al. Human papillomavirus drives tumor development throughout the head and neck: improved prognosis is associated with an immune response largely restricted to the oropharynx. J Clin Oncol. 2016;34(34):4132-4141.
6. Chung CH, Zhang Q, Kong CS, et al. p16 protein expression and human papillomavirus status as prognostic biomarkers of nonoropharyngeal head and neck squamous cell carcinoma. J Clin Oncol. 2014;32(35):3930-3938.
7. Lassen P, Primdahl H, Johansen J, et al; Danish Head and Neck Cancer Group (DAHANCA). Impact of HPV-associated p16-expression on radiotherapy outcome in advanced oropharynx and non-oropharynx cancer. Radiother Oncol. 2014;113(3):310-316.
8. Fakhry C, Westra WH, Wang SJ, et al. The prognostic role of sex, race, and human papillomavirus in oropharyngeal and nonoropharyngeal head and neck squamous cell cancer. Cancer. 2017;123(9):1566-1575.
9. Elrefaey S, Massaro MA, Chiocca S, Chiesa F, Ansarin M. HPV in oropharyngeal cancer: the basics to know in clinical practice. Acta Otorhinolaryngol Ital. 2014;34(5):299-309.
10. Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24-35.
11. Maxwell, JH, Kumar B, Feng FY, et al. Tobacco use in HPV-positive advanced oropharynx cancer patients related to increased risk of distant metastases and tumor recurrence. Clin Cancer Res. 2010;16(4):1226-1235.
12. Gillison ML, Broutian T, Pickard RK, et al. Prevalence of oral HPV infection in the United States, 2009-2010. JAMA. 2012;307(7):693-703.
13. Benson E, Li R, Eisele D, Fakhry C. The clinical impact of HPV tumor status upon head and neck squamous cell carcinomas. Oral Oncol. 2014;50(6):565-574.
Head and neck cancer (HNC) continues to be a major health issue with an estimated 51,540 cases in the US in 2018, making it the eighth most common cancer among men with an estimated 4% of all new cancer diagnoses.1 Over the past decade, human papillomavirus (HPV) has emerged as a major prognostic factor for survival in squamous cell carcinomas of the oropharynx. Patients who are HPV-positive (HPV+) have a much higher survival rate than patients who have HPV-negative (HPV-) cancers of the oropharynx. The 8th edition of the American Joint Committee on Cancer (AJCC) staging manual has 2 distinct stagings for HPV+ and HPV- oropharyngeal tumors using p16-positivity (p16+) as a surrogate marker.2
Squamous cell carcinomas of the oropharynx that are HPV+ have about half the risk of death of HPV- tumors, are highly responsive to treatment, and are more often seen in younger and healthier patients with little to no tobacco use.2,3 As such, there also is a movement to de-escalate HPV+ oropharyngeal cancers with multiple trials by either replacing cytotoxic chemotherapy with a targeted agent (cisplatin vs cetuximab in RTOG 1016) or reducing the radiation dose (ECOG 1308, NRG HN002, Quarterback, and OPTIMA trials).3
The focus of many epidemiologic studies has been in the HNC general population. A recent epidemiologic analysis of the HNC general population found a p16 positivity rate of 60% in oropharyngeal squamous cell carcinomas (OPSCC) and 10% in nonoropharyngeal squamous cell carcinomas (NOPSCC).4 There has been a lack of studies focusing on the US Department of Veterans Administration (VA) population. The VA HNC population consists mostly of older white male smokers; whereas the rise of OPSCC in the general population consists primarily of males aged < 60 years often with little or no tobacco use.5 Furthermore, the importance of p16 positivity in NOPSCC also may be prognostic.6 Population data on this subset in the VA are lacking as well.This study’s purpose is to analyze the p16 positivity rate in both the OPSCC and NOPSCC in the VA population. Elucidation of epidemiologic factors that are associated with these groups may bring to light important differences between the VA and general HNC populations.
Methods
A review of the Kansas City VA Medical Center database for patients with HNC was performed from 2011 to 2017. The review consisted of 183 patient records (second primaries were scored separately), and 123 were deemed eligible for the study. Epidemiologic data were collected, including site, OPSCC vs NOPSCC, age, race, education level, tobacco use, alcohol use, TNM stage, and marital status (Table).
Results
The NOPSCC p16+ group had the greatest mean pack-year use (57). The lowest was in the OPSCC p16+ group (29). The OPSCC p16+ group had 37% never smokers compared with ≤ 10% for the other groups. Both the OPSCC and NOPSCC p16- groups had much more alcohol use per week than that of the p16+ groups. The differences in marital status included a lower rate of never married individuals in the p16+ group and a higher rate of marriage in the NOPSCC p16- group. The T stage distribution within the OPSCC groups was similar, but NOPSCC groups saw more T1 lesions in the NOPSCC p16- group (42% p16- vs 18% p16+). Conversely, more T4 lesions were found in the NOPSCC p16+ patients (7% p16- vs 29% p16+).
Discussion
The overall HPV positivity rate in the general population of patients with HNC has been reported as between 57% and 72% for OPSCC and between 1.3% and 7% for NOPSCC.6 One study, however, examined the p16 positivity rate in NOPSCC patients enrolled in major trials (RTOG 0129, 0234, and 0522 studies) and found that up to 19.3% of NOPSCC patients had p16 positivity.6 Even with the near 20% rate in those aforementioned trials that are above the reported norm, the current study found that nearly 30% of its VA population had p16+ NOPSCC. It has been shown that regardless of site, HPV-driven head and neck tumors share a similar gene expression and DNA methylation profiles (nonkeratinizing, basaloid histopathologic features, and lack of TP53 or CDKN2A alterations).5 p16+ NOPSCC has a different immune microenvironment with less lymphocyte infiltration, and there is some debate in the literature about the effects on tumor outcomes for NOPSCC cancer.5
In the aforementioned RTOG trials, p16- NOPSCC had worse outcomes compared with those of p16+ NOPSCC.6 This result is in contrast to the Danish Head and Neck Cancer Group (DAHANCA) and the combined Johns Hopkins University (JHU) and University of California, San Francisco (UCSF) data that found no difference between p16+ NOPSCC or p16- NOPSCC.7,8 In regards to race, this study did not find any differences. Another UCSF and JHU study showed lower p16+ rates in African American patients with OPSCC, but no distinction between race in the NOPSCC group. This result is consistent with the data in the current study as the distribution of race was no different among the 4 groups; however, this study's cohort was 90% white, 10% African American, and only < 1% Native American.4 This study's cohort population also was consistent with HPV-positive tumors presenting with earlier T, but higher N staging.9
Smoking is known to decrease survival in HPV-positive HNC, with the RTOG 0129 study separating head and neck tumors into low, medium, and high risk, based on HPV status, smoking, and stage.10 Although the average smoking pack-years in the current study’s OPC p16+ group was high at 29 pack-years, there was still a significant number of nonsmokers in that same group (37%). The University of Michigan conducted a study that had a similar profile of patients with an average age of 56.5 and 32.4% never smokers in their p16+ OPSCC cohort; thus, the VA p16+ OPSCC group in this study may be similar to the general population's p16+ OPSCC group.11 Nonmonogamous relationships also have been shown to be a risk factor for HPV positivity, and there was a difference in marital status (assuming it was a surrogate for monogamy) between the 4 groups; however, in contrast, the p16+ group in the current study had a high number of married patients, 45% in OPC p16+ group, and may not have been a good surrogate for monogamy in this VA population.
Limitations
Limitations of this study include all the caveats that come with a retrospective study, such as confounding variables, unbalanced groups, and selection bias. A detailed sexual history was not included, although it is well known that sexual activity is linked with oral HPV positivity.12 Human papillomavirus positivity based on p16 immunohistochemical analysis also was used as a surrogate marker for HPV instead of DNA in situ hybridization. The data also may be skewed due to the study patient’s being predominantly white and male: Both groups have a higher predilection for HPV-driven HNCs.13
Conclusion
The proportion of p16+ VA OPSCC cases was similar to that of the general population at 75% with 37% never smokers, but the percentage in NOPSCC was higher at 29% with only 10% never smokers. The p16+ NOPSCC also presented with more T4 lesions and a higher overall stage compared with p16- NOPSCC. Further studies are needed to compare these subgroups in the VA and in the general HNC populations.
Head and neck cancer (HNC) continues to be a major health issue with an estimated 51,540 cases in the US in 2018, making it the eighth most common cancer among men with an estimated 4% of all new cancer diagnoses.1 Over the past decade, human papillomavirus (HPV) has emerged as a major prognostic factor for survival in squamous cell carcinomas of the oropharynx. Patients who are HPV-positive (HPV+) have a much higher survival rate than patients who have HPV-negative (HPV-) cancers of the oropharynx. The 8th edition of the American Joint Committee on Cancer (AJCC) staging manual has 2 distinct stagings for HPV+ and HPV- oropharyngeal tumors using p16-positivity (p16+) as a surrogate marker.2
Squamous cell carcinomas of the oropharynx that are HPV+ have about half the risk of death of HPV- tumors, are highly responsive to treatment, and are more often seen in younger and healthier patients with little to no tobacco use.2,3 As such, there also is a movement to de-escalate HPV+ oropharyngeal cancers with multiple trials by either replacing cytotoxic chemotherapy with a targeted agent (cisplatin vs cetuximab in RTOG 1016) or reducing the radiation dose (ECOG 1308, NRG HN002, Quarterback, and OPTIMA trials).3
The focus of many epidemiologic studies has been in the HNC general population. A recent epidemiologic analysis of the HNC general population found a p16 positivity rate of 60% in oropharyngeal squamous cell carcinomas (OPSCC) and 10% in nonoropharyngeal squamous cell carcinomas (NOPSCC).4 There has been a lack of studies focusing on the US Department of Veterans Administration (VA) population. The VA HNC population consists mostly of older white male smokers; whereas the rise of OPSCC in the general population consists primarily of males aged < 60 years often with little or no tobacco use.5 Furthermore, the importance of p16 positivity in NOPSCC also may be prognostic.6 Population data on this subset in the VA are lacking as well.This study’s purpose is to analyze the p16 positivity rate in both the OPSCC and NOPSCC in the VA population. Elucidation of epidemiologic factors that are associated with these groups may bring to light important differences between the VA and general HNC populations.
Methods
A review of the Kansas City VA Medical Center database for patients with HNC was performed from 2011 to 2017. The review consisted of 183 patient records (second primaries were scored separately), and 123 were deemed eligible for the study. Epidemiologic data were collected, including site, OPSCC vs NOPSCC, age, race, education level, tobacco use, alcohol use, TNM stage, and marital status (Table).
Results
The NOPSCC p16+ group had the greatest mean pack-year use (57). The lowest was in the OPSCC p16+ group (29). The OPSCC p16+ group had 37% never smokers compared with ≤ 10% for the other groups. Both the OPSCC and NOPSCC p16- groups had much more alcohol use per week than that of the p16+ groups. The differences in marital status included a lower rate of never married individuals in the p16+ group and a higher rate of marriage in the NOPSCC p16- group. The T stage distribution within the OPSCC groups was similar, but NOPSCC groups saw more T1 lesions in the NOPSCC p16- group (42% p16- vs 18% p16+). Conversely, more T4 lesions were found in the NOPSCC p16+ patients (7% p16- vs 29% p16+).
Discussion
The overall HPV positivity rate in the general population of patients with HNC has been reported as between 57% and 72% for OPSCC and between 1.3% and 7% for NOPSCC.6 One study, however, examined the p16 positivity rate in NOPSCC patients enrolled in major trials (RTOG 0129, 0234, and 0522 studies) and found that up to 19.3% of NOPSCC patients had p16 positivity.6 Even with the near 20% rate in those aforementioned trials that are above the reported norm, the current study found that nearly 30% of its VA population had p16+ NOPSCC. It has been shown that regardless of site, HPV-driven head and neck tumors share a similar gene expression and DNA methylation profiles (nonkeratinizing, basaloid histopathologic features, and lack of TP53 or CDKN2A alterations).5 p16+ NOPSCC has a different immune microenvironment with less lymphocyte infiltration, and there is some debate in the literature about the effects on tumor outcomes for NOPSCC cancer.5
In the aforementioned RTOG trials, p16- NOPSCC had worse outcomes compared with those of p16+ NOPSCC.6 This result is in contrast to the Danish Head and Neck Cancer Group (DAHANCA) and the combined Johns Hopkins University (JHU) and University of California, San Francisco (UCSF) data that found no difference between p16+ NOPSCC or p16- NOPSCC.7,8 In regards to race, this study did not find any differences. Another UCSF and JHU study showed lower p16+ rates in African American patients with OPSCC, but no distinction between race in the NOPSCC group. This result is consistent with the data in the current study as the distribution of race was no different among the 4 groups; however, this study's cohort was 90% white, 10% African American, and only < 1% Native American.4 This study's cohort population also was consistent with HPV-positive tumors presenting with earlier T, but higher N staging.9
Smoking is known to decrease survival in HPV-positive HNC, with the RTOG 0129 study separating head and neck tumors into low, medium, and high risk, based on HPV status, smoking, and stage.10 Although the average smoking pack-years in the current study’s OPC p16+ group was high at 29 pack-years, there was still a significant number of nonsmokers in that same group (37%). The University of Michigan conducted a study that had a similar profile of patients with an average age of 56.5 and 32.4% never smokers in their p16+ OPSCC cohort; thus, the VA p16+ OPSCC group in this study may be similar to the general population's p16+ OPSCC group.11 Nonmonogamous relationships also have been shown to be a risk factor for HPV positivity, and there was a difference in marital status (assuming it was a surrogate for monogamy) between the 4 groups; however, in contrast, the p16+ group in the current study had a high number of married patients, 45% in OPC p16+ group, and may not have been a good surrogate for monogamy in this VA population.
Limitations
Limitations of this study include all the caveats that come with a retrospective study, such as confounding variables, unbalanced groups, and selection bias. A detailed sexual history was not included, although it is well known that sexual activity is linked with oral HPV positivity.12 Human papillomavirus positivity based on p16 immunohistochemical analysis also was used as a surrogate marker for HPV instead of DNA in situ hybridization. The data also may be skewed due to the study patient’s being predominantly white and male: Both groups have a higher predilection for HPV-driven HNCs.13
Conclusion
The proportion of p16+ VA OPSCC cases was similar to that of the general population at 75% with 37% never smokers, but the percentage in NOPSCC was higher at 29% with only 10% never smokers. The p16+ NOPSCC also presented with more T4 lesions and a higher overall stage compared with p16- NOPSCC. Further studies are needed to compare these subgroups in the VA and in the general HNC populations.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30.
2. Lydiatt WM, Patel SG, O’Sullivan B, et al. Head and neck cancers major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67(2):122-137.
3. Mirghani H, Blanchard P. Treatment de-escalation for HPV-driven oropharyngeal cancer: where do we stand? Clin Transl Radiat Oncol. 2017;8:4-11.
4. D’Souza G, Westra WH, Wang SJ, et al. Differences in the prevalence of human papillomavirus (HPV) in head and neck squamous cell cancers by sex, race, anatomic tumor site, and HPV detection method. JAMA Oncol. 2017;3(2):169-177.
5. Chakravarthy A, Henderson S, Thirdborough SM, et al. Human papillomavirus drives tumor development throughout the head and neck: improved prognosis is associated with an immune response largely restricted to the oropharynx. J Clin Oncol. 2016;34(34):4132-4141.
6. Chung CH, Zhang Q, Kong CS, et al. p16 protein expression and human papillomavirus status as prognostic biomarkers of nonoropharyngeal head and neck squamous cell carcinoma. J Clin Oncol. 2014;32(35):3930-3938.
7. Lassen P, Primdahl H, Johansen J, et al; Danish Head and Neck Cancer Group (DAHANCA). Impact of HPV-associated p16-expression on radiotherapy outcome in advanced oropharynx and non-oropharynx cancer. Radiother Oncol. 2014;113(3):310-316.
8. Fakhry C, Westra WH, Wang SJ, et al. The prognostic role of sex, race, and human papillomavirus in oropharyngeal and nonoropharyngeal head and neck squamous cell cancer. Cancer. 2017;123(9):1566-1575.
9. Elrefaey S, Massaro MA, Chiocca S, Chiesa F, Ansarin M. HPV in oropharyngeal cancer: the basics to know in clinical practice. Acta Otorhinolaryngol Ital. 2014;34(5):299-309.
10. Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24-35.
11. Maxwell, JH, Kumar B, Feng FY, et al. Tobacco use in HPV-positive advanced oropharynx cancer patients related to increased risk of distant metastases and tumor recurrence. Clin Cancer Res. 2010;16(4):1226-1235.
12. Gillison ML, Broutian T, Pickard RK, et al. Prevalence of oral HPV infection in the United States, 2009-2010. JAMA. 2012;307(7):693-703.
13. Benson E, Li R, Eisele D, Fakhry C. The clinical impact of HPV tumor status upon head and neck squamous cell carcinomas. Oral Oncol. 2014;50(6):565-574.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30.
2. Lydiatt WM, Patel SG, O’Sullivan B, et al. Head and neck cancers major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67(2):122-137.
3. Mirghani H, Blanchard P. Treatment de-escalation for HPV-driven oropharyngeal cancer: where do we stand? Clin Transl Radiat Oncol. 2017;8:4-11.
4. D’Souza G, Westra WH, Wang SJ, et al. Differences in the prevalence of human papillomavirus (HPV) in head and neck squamous cell cancers by sex, race, anatomic tumor site, and HPV detection method. JAMA Oncol. 2017;3(2):169-177.
5. Chakravarthy A, Henderson S, Thirdborough SM, et al. Human papillomavirus drives tumor development throughout the head and neck: improved prognosis is associated with an immune response largely restricted to the oropharynx. J Clin Oncol. 2016;34(34):4132-4141.
6. Chung CH, Zhang Q, Kong CS, et al. p16 protein expression and human papillomavirus status as prognostic biomarkers of nonoropharyngeal head and neck squamous cell carcinoma. J Clin Oncol. 2014;32(35):3930-3938.
7. Lassen P, Primdahl H, Johansen J, et al; Danish Head and Neck Cancer Group (DAHANCA). Impact of HPV-associated p16-expression on radiotherapy outcome in advanced oropharynx and non-oropharynx cancer. Radiother Oncol. 2014;113(3):310-316.
8. Fakhry C, Westra WH, Wang SJ, et al. The prognostic role of sex, race, and human papillomavirus in oropharyngeal and nonoropharyngeal head and neck squamous cell cancer. Cancer. 2017;123(9):1566-1575.
9. Elrefaey S, Massaro MA, Chiocca S, Chiesa F, Ansarin M. HPV in oropharyngeal cancer: the basics to know in clinical practice. Acta Otorhinolaryngol Ital. 2014;34(5):299-309.
10. Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24-35.
11. Maxwell, JH, Kumar B, Feng FY, et al. Tobacco use in HPV-positive advanced oropharynx cancer patients related to increased risk of distant metastases and tumor recurrence. Clin Cancer Res. 2010;16(4):1226-1235.
12. Gillison ML, Broutian T, Pickard RK, et al. Prevalence of oral HPV infection in the United States, 2009-2010. JAMA. 2012;307(7):693-703.
13. Benson E, Li R, Eisele D, Fakhry C. The clinical impact of HPV tumor status upon head and neck squamous cell carcinomas. Oral Oncol. 2014;50(6):565-574.
New remote monitoring system lessens symptoms in head and neck cancer
A new system for remotely assessing and transmitting selected vitals and self-reported measures reduces symptom severity among patients with head and neck cancer receiving radiation therapy, in a randomized trial reported at a press briefing held before the annual meeting of the American Society of Clinical Oncology.
“Head and neck cancer patients who receive radiation treatment have a high symptom burden and also are at increased risk for dehydration during treatment,” said lead study author Susan K. Peterson, PhD, a professor in the department of behavioral science at the University of Texas MD Anderson Cancer Center, Houston. “Previously, we have shown that it was feasible to use mobile and sensor technology to identify treatment-related symptoms and early dehydration risk in patients receiving radiation treatment as part of their outpatient care.”
In the new trial, the investigators tested the CYCORE system (Cyberinfrastructure for Comparative Effectiveness Research), which consists of a Bluetooth-enabled weight scale and blood pressure cuff, and a mobile tablet with a symptom-tracking app that sends information directly to the physician each weekday. A network hub/router was set up in patients’ homes to transmit their sensor readouts, and the mobile app transmitted their symptom data to secure firewall-protected computers. “CYCORE also included a software infrastructure that enabled the analysis and viewing of data in near-real time and was compliant with safety and security and confidentiality standards,” Dr. Peterson noted.
Main trial results showed that compared with peers receiving only usual care (weekly visits with the radiation oncologist), patients receiving usual care augmented with the CYCORE system had lower mean scores on a 10-point scale for general symptoms (0.5-point difference) and for symptoms specific to head and neck cancer (0.6-point difference). In addition, daily remote tracking of patient well-being allowed clinicians to more rapidly detect and respond to symptoms.
“This is important because symptoms can affects patients’ ability to tolerate treatment and can also impact their quality of life during treatment,” Dr. Peterson said.
The mean age of the patients was 60 years; the oldest was 86 years old. “This supports the notion that the use of technology-based interventions can be feasible in older patients,” she maintained. In addition, patients in the CYCORE group showed at least 80% adherence to the daily monitoring tasks.
“We believe that good patient adherence plus the fact that this imposed minimal burden on clinicians for the monitoring supports the use of systems like CYCORE during intensive treatment periods in cancer care, and that using sensor and mobile technology to monitor patients during critical periods of outpatient care can provide a timely source of information for clinical decision making and may ultimately improve quality of life and health outcomes,” Dr. Peterson concluded. “Our next steps would be to explore ways to implement this as part of clinical care, including in community cancer centers, where most patients receive their care.”
“This is yet another application of technology-enabled sharing of information generated at home,” said ASCO President Bruce E. Johnson, MD, FASCO, noting that a similar study last year showed better patient-reported experience and overall survival.
Such technology will likely be increasingly used to obtain timely information that ultimately leads to a reduction in complications, he speculated.
“This information in head and neck cancer is particularly important because patients commonly get a lot of side effects when attempting to swallow enough fluids, such that some centers end up putting a feeding tube into the stomach because it’s so difficult to swallow,” added Dr. Johnson, who is also a professor of medicine at the Dana-Farber Cancer Institute and a leader of the Dana-Farber/Harvard Cancer Center Lung Cancer Program, Boston. “So this is a particularly important clinical application in cancer.”
Study details
The trial population consisted of 357 patients undergoing radiation therapy for head and neck cancer. “We believe that this is the first and largest study of its kind in head and neck cancer,” Dr. Peterson said.
The severity of symptoms and their interference with daily activities were assessed at serial time points with the MD Anderson Symptom Inventory.
In the CYCORE group, 87% of patients measured their blood pressure daily, 86% measured their weight daily, and 80% used the symptom-tracking app daily.
At the end of radiation therapy, the CYCORE patients had lower (i.e., better) mean scores for general symptoms (e.g., pain, nausea, fatigue) relative to usual care counterparts (2.9 vs. 3.4), with a difference still evident 6-8 weeks later (1.6 vs. 1.9) (P = .007).
In addition, the CYCORE patients had lower mean scores for symptoms specific to head and neck cancer (e.g., dysphagia, pain, rash) at the end of radiation therapy (4.2 vs. 4.8), with a difference still evident 6-8 weeks later (1.7 vs. 2.1) (P = .009).
The groups fared essentially the same with respect to scores assessing interference of symptoms with activities of daily living.
Dr. Peterson disclosed that she had no relevant conflicts of interest. The study received funding from the National Institutes of Health.
SOURCE: Peterson et al. ASCO 2018, Abstract 6063.
A new system for remotely assessing and transmitting selected vitals and self-reported measures reduces symptom severity among patients with head and neck cancer receiving radiation therapy, in a randomized trial reported at a press briefing held before the annual meeting of the American Society of Clinical Oncology.
“Head and neck cancer patients who receive radiation treatment have a high symptom burden and also are at increased risk for dehydration during treatment,” said lead study author Susan K. Peterson, PhD, a professor in the department of behavioral science at the University of Texas MD Anderson Cancer Center, Houston. “Previously, we have shown that it was feasible to use mobile and sensor technology to identify treatment-related symptoms and early dehydration risk in patients receiving radiation treatment as part of their outpatient care.”
In the new trial, the investigators tested the CYCORE system (Cyberinfrastructure for Comparative Effectiveness Research), which consists of a Bluetooth-enabled weight scale and blood pressure cuff, and a mobile tablet with a symptom-tracking app that sends information directly to the physician each weekday. A network hub/router was set up in patients’ homes to transmit their sensor readouts, and the mobile app transmitted their symptom data to secure firewall-protected computers. “CYCORE also included a software infrastructure that enabled the analysis and viewing of data in near-real time and was compliant with safety and security and confidentiality standards,” Dr. Peterson noted.
Main trial results showed that compared with peers receiving only usual care (weekly visits with the radiation oncologist), patients receiving usual care augmented with the CYCORE system had lower mean scores on a 10-point scale for general symptoms (0.5-point difference) and for symptoms specific to head and neck cancer (0.6-point difference). In addition, daily remote tracking of patient well-being allowed clinicians to more rapidly detect and respond to symptoms.
“This is important because symptoms can affects patients’ ability to tolerate treatment and can also impact their quality of life during treatment,” Dr. Peterson said.
The mean age of the patients was 60 years; the oldest was 86 years old. “This supports the notion that the use of technology-based interventions can be feasible in older patients,” she maintained. In addition, patients in the CYCORE group showed at least 80% adherence to the daily monitoring tasks.
“We believe that good patient adherence plus the fact that this imposed minimal burden on clinicians for the monitoring supports the use of systems like CYCORE during intensive treatment periods in cancer care, and that using sensor and mobile technology to monitor patients during critical periods of outpatient care can provide a timely source of information for clinical decision making and may ultimately improve quality of life and health outcomes,” Dr. Peterson concluded. “Our next steps would be to explore ways to implement this as part of clinical care, including in community cancer centers, where most patients receive their care.”
“This is yet another application of technology-enabled sharing of information generated at home,” said ASCO President Bruce E. Johnson, MD, FASCO, noting that a similar study last year showed better patient-reported experience and overall survival.
Such technology will likely be increasingly used to obtain timely information that ultimately leads to a reduction in complications, he speculated.
“This information in head and neck cancer is particularly important because patients commonly get a lot of side effects when attempting to swallow enough fluids, such that some centers end up putting a feeding tube into the stomach because it’s so difficult to swallow,” added Dr. Johnson, who is also a professor of medicine at the Dana-Farber Cancer Institute and a leader of the Dana-Farber/Harvard Cancer Center Lung Cancer Program, Boston. “So this is a particularly important clinical application in cancer.”
Study details
The trial population consisted of 357 patients undergoing radiation therapy for head and neck cancer. “We believe that this is the first and largest study of its kind in head and neck cancer,” Dr. Peterson said.
The severity of symptoms and their interference with daily activities were assessed at serial time points with the MD Anderson Symptom Inventory.
In the CYCORE group, 87% of patients measured their blood pressure daily, 86% measured their weight daily, and 80% used the symptom-tracking app daily.
At the end of radiation therapy, the CYCORE patients had lower (i.e., better) mean scores for general symptoms (e.g., pain, nausea, fatigue) relative to usual care counterparts (2.9 vs. 3.4), with a difference still evident 6-8 weeks later (1.6 vs. 1.9) (P = .007).
In addition, the CYCORE patients had lower mean scores for symptoms specific to head and neck cancer (e.g., dysphagia, pain, rash) at the end of radiation therapy (4.2 vs. 4.8), with a difference still evident 6-8 weeks later (1.7 vs. 2.1) (P = .009).
The groups fared essentially the same with respect to scores assessing interference of symptoms with activities of daily living.
Dr. Peterson disclosed that she had no relevant conflicts of interest. The study received funding from the National Institutes of Health.
SOURCE: Peterson et al. ASCO 2018, Abstract 6063.
A new system for remotely assessing and transmitting selected vitals and self-reported measures reduces symptom severity among patients with head and neck cancer receiving radiation therapy, in a randomized trial reported at a press briefing held before the annual meeting of the American Society of Clinical Oncology.
“Head and neck cancer patients who receive radiation treatment have a high symptom burden and also are at increased risk for dehydration during treatment,” said lead study author Susan K. Peterson, PhD, a professor in the department of behavioral science at the University of Texas MD Anderson Cancer Center, Houston. “Previously, we have shown that it was feasible to use mobile and sensor technology to identify treatment-related symptoms and early dehydration risk in patients receiving radiation treatment as part of their outpatient care.”
In the new trial, the investigators tested the CYCORE system (Cyberinfrastructure for Comparative Effectiveness Research), which consists of a Bluetooth-enabled weight scale and blood pressure cuff, and a mobile tablet with a symptom-tracking app that sends information directly to the physician each weekday. A network hub/router was set up in patients’ homes to transmit their sensor readouts, and the mobile app transmitted their symptom data to secure firewall-protected computers. “CYCORE also included a software infrastructure that enabled the analysis and viewing of data in near-real time and was compliant with safety and security and confidentiality standards,” Dr. Peterson noted.
Main trial results showed that compared with peers receiving only usual care (weekly visits with the radiation oncologist), patients receiving usual care augmented with the CYCORE system had lower mean scores on a 10-point scale for general symptoms (0.5-point difference) and for symptoms specific to head and neck cancer (0.6-point difference). In addition, daily remote tracking of patient well-being allowed clinicians to more rapidly detect and respond to symptoms.
“This is important because symptoms can affects patients’ ability to tolerate treatment and can also impact their quality of life during treatment,” Dr. Peterson said.
The mean age of the patients was 60 years; the oldest was 86 years old. “This supports the notion that the use of technology-based interventions can be feasible in older patients,” she maintained. In addition, patients in the CYCORE group showed at least 80% adherence to the daily monitoring tasks.
“We believe that good patient adherence plus the fact that this imposed minimal burden on clinicians for the monitoring supports the use of systems like CYCORE during intensive treatment periods in cancer care, and that using sensor and mobile technology to monitor patients during critical periods of outpatient care can provide a timely source of information for clinical decision making and may ultimately improve quality of life and health outcomes,” Dr. Peterson concluded. “Our next steps would be to explore ways to implement this as part of clinical care, including in community cancer centers, where most patients receive their care.”
“This is yet another application of technology-enabled sharing of information generated at home,” said ASCO President Bruce E. Johnson, MD, FASCO, noting that a similar study last year showed better patient-reported experience and overall survival.
Such technology will likely be increasingly used to obtain timely information that ultimately leads to a reduction in complications, he speculated.
“This information in head and neck cancer is particularly important because patients commonly get a lot of side effects when attempting to swallow enough fluids, such that some centers end up putting a feeding tube into the stomach because it’s so difficult to swallow,” added Dr. Johnson, who is also a professor of medicine at the Dana-Farber Cancer Institute and a leader of the Dana-Farber/Harvard Cancer Center Lung Cancer Program, Boston. “So this is a particularly important clinical application in cancer.”
Study details
The trial population consisted of 357 patients undergoing radiation therapy for head and neck cancer. “We believe that this is the first and largest study of its kind in head and neck cancer,” Dr. Peterson said.
The severity of symptoms and their interference with daily activities were assessed at serial time points with the MD Anderson Symptom Inventory.
In the CYCORE group, 87% of patients measured their blood pressure daily, 86% measured their weight daily, and 80% used the symptom-tracking app daily.
At the end of radiation therapy, the CYCORE patients had lower (i.e., better) mean scores for general symptoms (e.g., pain, nausea, fatigue) relative to usual care counterparts (2.9 vs. 3.4), with a difference still evident 6-8 weeks later (1.6 vs. 1.9) (P = .007).
In addition, the CYCORE patients had lower mean scores for symptoms specific to head and neck cancer (e.g., dysphagia, pain, rash) at the end of radiation therapy (4.2 vs. 4.8), with a difference still evident 6-8 weeks later (1.7 vs. 2.1) (P = .009).
The groups fared essentially the same with respect to scores assessing interference of symptoms with activities of daily living.
Dr. Peterson disclosed that she had no relevant conflicts of interest. The study received funding from the National Institutes of Health.
SOURCE: Peterson et al. ASCO 2018, Abstract 6063.
REPORTING FROM ASCO 2018
Key clinical point: .
Major finding: Compared with usual care, the system for tracking and transmitting vitals and self-reported measures through sensor and mobile devices was associated with milder general symptoms (0.5-point difference) and head and neck cancer symptoms (0.6-point difference).
Study details: A randomized controlled trial among 357 patients with head and neck cancer undergoing radiation therapy.
Disclosures: Dr. Peterson disclosed that she had no conflicts of interest. The study received funding from the National Institutes of Health.
Source: Peterson et al. ASCO 2018, Abstract 6063.
Novel Neuroendocrine Tumor in Multiple Endocrine Neoplasia Type 1 (FULL)
Neuroendocrine tumors (NETs) are uncommon and can occur in the context of genetic conditions. Multiple endocrine neoplasia type 1 (MEN1) is an autosomal dominant disorder of the tumor suppressor gene of the same name—MEN1, which encodes for the protein menin. Multiple endocrine neoplasia type 1 is characterized clinically by the presence of 2 or more of the following NETs: parathyroid, pituitary, and pancreaticoduodenal.1 Pancreaticoduodenal NETs occur in 30% to 80% of patients with MEN1 and have malignant potential. Although the majority of pancreaticoduodenal NETs are nonfunctioning, patients may present with symptoms secondary to mass effect.
Genetic testing exists for MEN1, but not all genetic mutations that cause MEN1 have been discovered. Therefore, because negative genetic testing does not rule out MEN1, a diagnosis is based on tumor type and location. Neuroendocrine tumors of the biliary tree are rare, and there
are no well-accepted guidelines on how to stage them.2-4 The following case demonstrates an unusual initial presentation of a NET in the context of MEN1.
Case Report
A 29-year-old, active-duty African-American man deployed in Kuwait presented with icterus, flank pain, and hematuria. His past medical history was significant for nephrolithiasis, and his family history was notable for hyperparathyroidism. Laboratory results showed primary hyperparathyroidism and evidence of biliary obstruction.
A sestamibi scan demonstrated uptake in a location corresponding with the right inferior parathyroid gland. A computed tomography (CT) scan showed nephrolithiasis and hepatic biliary ductal dilatation. Magnetic resonance cholangiopancreatography (MRCP) revealed both intra- and extrahepatic ductal dilatation, focal narrowing of the proximal common bile duct, and possible adenopathy that was concerning for cholangiocarcinoma. Endoscopic retrograde cholangiopancreatography (ERCP) demonstrated a 1 cm to 2 cm focal stricture within the mid-common bile duct with intra- and extrahepatic ductal dilatation (Figure 1). An endoscopy showed no masses in the duodenum, and anendoscopic ultrasound showed no masses in the pancreas. Endoscopic brushings and endoscopic, ultrasound-guided, fine-needle aspiration
cytology were nondiagnostic. Exploratory laparotomy revealed a dilated hepatic bile duct, an inflamed porta hepatis, and a mass involving the distal hepatic bile duct.
The patient underwent cholecystectomy, radical extra hepatic bile duct resection to the level of the hepatic bifurcation, and hepaticojejunostomy. Gross examination of the specimen showed a nodule centered in the distal common hepatic duct with an adjacent, 2-cm lymph node. The histologic examination revealed a neoplastic proliferation consisting of epithelioid cells with round nuclei and granular chromatin with amphophilic cytoplasm in a trabecular and nested architecture.
The tumor was centered in the submucosa, which is typical of gastrointestinal NETs (Figure 2). There was no evidence of direct tumor extension elsewhere. About 40% of the tumor cells contained eosinophilic, intracytoplasmic inclusions (Figure 3). The tumor did not involve the margins or lymph node.
Positive staining with the neuroendocrine markers synaptophysin and chromagranin A confirmed a well-differentiated NET. The intracytoplasmic inclusions stained strongly positive for cytokeratin CAM 5.2. The tumor had higher-grade features, including tumor cell necrosis, a Ki-67 labeling index of 3%, and perineural invasion. The 2010 World Health Organization (WHO) criteria for NET of the digestive system classified this tumor as a grade 2, well-differentiated NET and as stage 1a (limited to the bile duct).4
Postoperatively, octreotide scan with single-photon emission computed tomography (SPECT)-CT did not show additional masses or lesions. Serum pancreatic polypeptide was elevated, with the remaining serum and plasma NET markers—including gastrin, glucagon, insulin, chromogranin A, and vasoactive intestinal polypeptide (VIP)—being within reference ranges. Genetic testing (GeneDx, Inc, Gaithersburg, MD) showed an E563X nonsense mutation in the MEN1 gene, confirming a MEN1 disorder. The patient then underwent a 4-gland parathyroidectomy with reimplantation; the parathyroid glands demonstrated hyperplasia in all 4 glands.
Biochemical follow-up at 14 months showed that the serum pancreatic polypeptide had normalized. There was no evidence of pituitary orpancreatic hypersecretion. The patient developed hypoparathyroidism, requiring calcium and calcitriol supplementation. Radiographic follow-up using abdominal magnetic resonance imaging at 16 months showed no evidence of disease.
Discussion
This case illustrates a genetic disease with an unusual initial presentation. Primary extrahepatic bile duct NETs are rare and have been reported previously in patients without MEN1.5-9 Neuroendocrine tumors in the hepatic bile duct in patients with MEN1 also have been reported but only after these tumors first appeared in the pancreas or duodenum.10 An extensive literature search revealed no prior reports extrahepatic bile duct NETs with MEN1 as the primary site or with biliary obstruction, which is why this patient’s presentation is particularly interesting.5,6,10-13 The table summarizes select reports of NETs.
Tumor location in this patient was atypical, and genetic testing guided the management. Serum MEN1 genetic testing is indicated in patients with ≥ 2 tumors that are atypical but possibly associated with MEN1 (such as adrenal tumors, gastrinomas, and carcinoids) and in patients aged < 45 years with primary hyperparathyroidism.14,15 The patient in this study was aged 29 years and had hyperparathyroidism and an NET of the hepatic bile duct. This condition was sufficient to warrant genetic testing, the results of which affected the patient’s subsequent parathyroid surgery.15 Despite the suggestion of unifocal localization on the sestamibi scan, the patient underwent the more appropriate subtotal parathyroidectomy.14 The patient’s tumor most likely originated from a germline mutation of the MEN1 gene.
As a result of the patient’s genetic test results, his daughter also was tested. She was found to have the same mutation as her father and will undergo proper tumor surveillance for MEN1. There was no personal or family history of hemangioblastomas, renal cell carcinomas, or cystadenomas, which would have prompted testing for von Hippel-Lindau disease. Likewise, there was no personal or family history of café-au-lait macules and neurofibromas, which would have prompted testing for neurofibromatosis type 1.
Due to the paucity of cases, there are currently no well-accepted guidelines on how to stage extrahepatic biliary NETs.3-5,16 The WHO recommends staging according to adenocarcinomas of the gallbladder and bile duct.3 As such, the pathologic stage of this tumor would be stage 1a.
The significance of the intracytoplasmic inclusion in this case is unknown. Pancreatic NETs and neuroendocrine carcinomas have demonstrated intracytoplasmic inclusions that stain positively for keratin and may indicate more aggressive tumor behavior.17-19 In 1 report, electron microscopic examination demonstrated intermediate filaments with entrapped neurosecretory granules.18 In a series of 84 cases of pancreatic endocrine tumors, 14 had intracytoplasmic inclusions; of these, 5 had MEN1.17 In the present case, the patient continues to show no evidence of tumor recurrence at 16 months after resection.
Conclusion
Extrahepatic biliary neuroendocrine tumors are rare. Further investigation into biliary tree NET staging and future studies to determine the significance of intracytoplasmic inclusions may be beneficial. This case highlights the appropriate use of genetic testing and supports expanding the clinical diagnosis of MEN1 to include NETs of the extrahepatic bile duct.
Click here to read the digital edition.
1. Melmed S, Polonsky KS, Larsen PR, Kronenberg HM, eds. Williams Textbook of Endocrinology. 12th ed. Philadelphia, PA: WB Saunders; 2011.
2. American Joint Committee on Cancer. Neuroendocrine Tumors. In: Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, eds. American Joint Committee on Cancer Staging Handbook. 7th ed. From the AJCC Cancer Staging Manual. New York, NY: Springer-Verlag; 2010:227-236.
3. Komminoth P, Arnold R, Capella C, et al. Neuroendocrine neoplasms of the gallbladder and extrahepatic bile ducts. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, et al, eds. WHO Classification of Tumours of the Digestive System. 4th ed. Lyon, France: IARC Press; 2010:274-276.
4. Rindi G, Arnold R, Bosman FT. Nomenclature and classification of neuroendocrine neoplasms of the digestive system. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, et al, eds. WHO Classification of Tumours of the Digestive System. 4th ed. Lyon, France: IARC Press; 2010:13.
5. Price TN, Thompson GB, Lewis JT, Lloyd RV, Young WF. Zollinger-Ellison syndrome due to primary gastrinoma of the extrahepatic biliary tree: three case reports and review of literature. Endocr Pract. 2009;15(7):737-749.
6. Bhandarwar AH, Shaikh TA, Borisa AD, et al. Primary neuroendocrine tumor of the left hepatic duct: a case report with review of the literature. Case Rep Surg. 2012:786432.
7. Bhalla P, Powle V, Shah RC, Jagannath P. Neuroendocrine tumor of common hepatic duct. Indian J Gastroenterol. 2012;31(3):144-146.
8. Khan FA, Stevens-Chase A, Chaudhry R, Hashmi A, Edelman D, Weaver D. Extrahepatic biliary obstrution secondary to neuroendocrine tumor of the common hepatic duct. Int J Surg Case Rep. 2017;30:46-49.
9. Hong N, Kim HJ, Byun JH, et al. Neuroendocrine neoplasms of the extrahepatic bile duct: radiologic and clinical characteristics. Abdom Imaging. 2015;40(1):181-191.
10. Tonelli F, Giudici F, Nesi G, Batignani G, Brandi ML. Biliary tree gastrinomas in multiple endocrine neoplasia type 1 syndrome. World J Gastroenterol. 2013;19(45):8312-8320.
11. Gibril F, Schumann M, Pace A, Jensen RT. Multiple endocrine neoplasia type 1 and Zollinger-Ellison syndrome: a prospective study of 107 cases and comparison with 1009 cases from the literature. Medicine (Baltimore). 2004;83(1):43-83.
12. Pieterman CRC, Conemans EB, Dreijerink KMA, et al. Thoracic and duodenopancreatic neuroendocrine tumors in multiple endocrine neoplasia type 1: natural history and function of menin in tumorigenesis. Endocr Relat Cancer. 2014;21(3):R121-R142.
13. Pipeleers-Marichal M, Somers G, Willems G, et al. Gastrinomas in the duodenums of patients with multiple endocrine neoplasia type 1 and the Zollinger-Ellison syndrome. N Engl J Med. 1990;322(11):723-727.
14. Thakker RV, Newey PJ, Walls GV, et al; Endocrine Society. Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1). J Clin Endocrinol Metab. 2012;97(9):2990-3011.
15. Eastell R, Brandi ML, Costa AG, et al. Diagnosis of asymptomatic primary hyperparathyroidism: proceedings of the Fourth International Workshop. J Clin Endocrinol Metab. 2014;99(10):3570-3579.
16. Michalopoulos N, Papavramidis TS, Karayannopoulou G, Pliakos I, Papavramidis ST, Kanellos I. Neuroendocrine tumors of extrahepatic biliary tract. Pathol Oncol Res. 2014;20(4):765-775.
17. Serra S, Asa SL, Chetty R. Intracytoplasmic inclusions (including the so-called “rhabdoid” phenotype) in pancreatic endocrine tumors. Endocr Pathol. 2006;17(1):75-81.
18. Shia J, Erlandson RA, Klimstra DS. Whorls of intermediate filaments with entrapped neurosecretory granules correspond to the “rhabdoid” inclusions seen in pancreatic endocrine
neoplasms. Am J Surg Pathol. 2004;28(2):271-273.
19. Perez-Montiel MD, Frankel WL, Suster S. Neuroendocrine carcinomas of the pancreas with ‘Rhabdoid’ features. Am J Surg Pathol. 2003;27(5):642-649.
Neuroendocrine tumors (NETs) are uncommon and can occur in the context of genetic conditions. Multiple endocrine neoplasia type 1 (MEN1) is an autosomal dominant disorder of the tumor suppressor gene of the same name—MEN1, which encodes for the protein menin. Multiple endocrine neoplasia type 1 is characterized clinically by the presence of 2 or more of the following NETs: parathyroid, pituitary, and pancreaticoduodenal.1 Pancreaticoduodenal NETs occur in 30% to 80% of patients with MEN1 and have malignant potential. Although the majority of pancreaticoduodenal NETs are nonfunctioning, patients may present with symptoms secondary to mass effect.
Genetic testing exists for MEN1, but not all genetic mutations that cause MEN1 have been discovered. Therefore, because negative genetic testing does not rule out MEN1, a diagnosis is based on tumor type and location. Neuroendocrine tumors of the biliary tree are rare, and there
are no well-accepted guidelines on how to stage them.2-4 The following case demonstrates an unusual initial presentation of a NET in the context of MEN1.
Case Report
A 29-year-old, active-duty African-American man deployed in Kuwait presented with icterus, flank pain, and hematuria. His past medical history was significant for nephrolithiasis, and his family history was notable for hyperparathyroidism. Laboratory results showed primary hyperparathyroidism and evidence of biliary obstruction.
A sestamibi scan demonstrated uptake in a location corresponding with the right inferior parathyroid gland. A computed tomography (CT) scan showed nephrolithiasis and hepatic biliary ductal dilatation. Magnetic resonance cholangiopancreatography (MRCP) revealed both intra- and extrahepatic ductal dilatation, focal narrowing of the proximal common bile duct, and possible adenopathy that was concerning for cholangiocarcinoma. Endoscopic retrograde cholangiopancreatography (ERCP) demonstrated a 1 cm to 2 cm focal stricture within the mid-common bile duct with intra- and extrahepatic ductal dilatation (Figure 1). An endoscopy showed no masses in the duodenum, and anendoscopic ultrasound showed no masses in the pancreas. Endoscopic brushings and endoscopic, ultrasound-guided, fine-needle aspiration
cytology were nondiagnostic. Exploratory laparotomy revealed a dilated hepatic bile duct, an inflamed porta hepatis, and a mass involving the distal hepatic bile duct.
The patient underwent cholecystectomy, radical extra hepatic bile duct resection to the level of the hepatic bifurcation, and hepaticojejunostomy. Gross examination of the specimen showed a nodule centered in the distal common hepatic duct with an adjacent, 2-cm lymph node. The histologic examination revealed a neoplastic proliferation consisting of epithelioid cells with round nuclei and granular chromatin with amphophilic cytoplasm in a trabecular and nested architecture.
The tumor was centered in the submucosa, which is typical of gastrointestinal NETs (Figure 2). There was no evidence of direct tumor extension elsewhere. About 40% of the tumor cells contained eosinophilic, intracytoplasmic inclusions (Figure 3). The tumor did not involve the margins or lymph node.
Positive staining with the neuroendocrine markers synaptophysin and chromagranin A confirmed a well-differentiated NET. The intracytoplasmic inclusions stained strongly positive for cytokeratin CAM 5.2. The tumor had higher-grade features, including tumor cell necrosis, a Ki-67 labeling index of 3%, and perineural invasion. The 2010 World Health Organization (WHO) criteria for NET of the digestive system classified this tumor as a grade 2, well-differentiated NET and as stage 1a (limited to the bile duct).4
Postoperatively, octreotide scan with single-photon emission computed tomography (SPECT)-CT did not show additional masses or lesions. Serum pancreatic polypeptide was elevated, with the remaining serum and plasma NET markers—including gastrin, glucagon, insulin, chromogranin A, and vasoactive intestinal polypeptide (VIP)—being within reference ranges. Genetic testing (GeneDx, Inc, Gaithersburg, MD) showed an E563X nonsense mutation in the MEN1 gene, confirming a MEN1 disorder. The patient then underwent a 4-gland parathyroidectomy with reimplantation; the parathyroid glands demonstrated hyperplasia in all 4 glands.
Biochemical follow-up at 14 months showed that the serum pancreatic polypeptide had normalized. There was no evidence of pituitary orpancreatic hypersecretion. The patient developed hypoparathyroidism, requiring calcium and calcitriol supplementation. Radiographic follow-up using abdominal magnetic resonance imaging at 16 months showed no evidence of disease.
Discussion
This case illustrates a genetic disease with an unusual initial presentation. Primary extrahepatic bile duct NETs are rare and have been reported previously in patients without MEN1.5-9 Neuroendocrine tumors in the hepatic bile duct in patients with MEN1 also have been reported but only after these tumors first appeared in the pancreas or duodenum.10 An extensive literature search revealed no prior reports extrahepatic bile duct NETs with MEN1 as the primary site or with biliary obstruction, which is why this patient’s presentation is particularly interesting.5,6,10-13 The table summarizes select reports of NETs.
Tumor location in this patient was atypical, and genetic testing guided the management. Serum MEN1 genetic testing is indicated in patients with ≥ 2 tumors that are atypical but possibly associated with MEN1 (such as adrenal tumors, gastrinomas, and carcinoids) and in patients aged < 45 years with primary hyperparathyroidism.14,15 The patient in this study was aged 29 years and had hyperparathyroidism and an NET of the hepatic bile duct. This condition was sufficient to warrant genetic testing, the results of which affected the patient’s subsequent parathyroid surgery.15 Despite the suggestion of unifocal localization on the sestamibi scan, the patient underwent the more appropriate subtotal parathyroidectomy.14 The patient’s tumor most likely originated from a germline mutation of the MEN1 gene.
As a result of the patient’s genetic test results, his daughter also was tested. She was found to have the same mutation as her father and will undergo proper tumor surveillance for MEN1. There was no personal or family history of hemangioblastomas, renal cell carcinomas, or cystadenomas, which would have prompted testing for von Hippel-Lindau disease. Likewise, there was no personal or family history of café-au-lait macules and neurofibromas, which would have prompted testing for neurofibromatosis type 1.
Due to the paucity of cases, there are currently no well-accepted guidelines on how to stage extrahepatic biliary NETs.3-5,16 The WHO recommends staging according to adenocarcinomas of the gallbladder and bile duct.3 As such, the pathologic stage of this tumor would be stage 1a.
The significance of the intracytoplasmic inclusion in this case is unknown. Pancreatic NETs and neuroendocrine carcinomas have demonstrated intracytoplasmic inclusions that stain positively for keratin and may indicate more aggressive tumor behavior.17-19 In 1 report, electron microscopic examination demonstrated intermediate filaments with entrapped neurosecretory granules.18 In a series of 84 cases of pancreatic endocrine tumors, 14 had intracytoplasmic inclusions; of these, 5 had MEN1.17 In the present case, the patient continues to show no evidence of tumor recurrence at 16 months after resection.
Conclusion
Extrahepatic biliary neuroendocrine tumors are rare. Further investigation into biliary tree NET staging and future studies to determine the significance of intracytoplasmic inclusions may be beneficial. This case highlights the appropriate use of genetic testing and supports expanding the clinical diagnosis of MEN1 to include NETs of the extrahepatic bile duct.
Click here to read the digital edition.
Neuroendocrine tumors (NETs) are uncommon and can occur in the context of genetic conditions. Multiple endocrine neoplasia type 1 (MEN1) is an autosomal dominant disorder of the tumor suppressor gene of the same name—MEN1, which encodes for the protein menin. Multiple endocrine neoplasia type 1 is characterized clinically by the presence of 2 or more of the following NETs: parathyroid, pituitary, and pancreaticoduodenal.1 Pancreaticoduodenal NETs occur in 30% to 80% of patients with MEN1 and have malignant potential. Although the majority of pancreaticoduodenal NETs are nonfunctioning, patients may present with symptoms secondary to mass effect.
Genetic testing exists for MEN1, but not all genetic mutations that cause MEN1 have been discovered. Therefore, because negative genetic testing does not rule out MEN1, a diagnosis is based on tumor type and location. Neuroendocrine tumors of the biliary tree are rare, and there
are no well-accepted guidelines on how to stage them.2-4 The following case demonstrates an unusual initial presentation of a NET in the context of MEN1.
Case Report
A 29-year-old, active-duty African-American man deployed in Kuwait presented with icterus, flank pain, and hematuria. His past medical history was significant for nephrolithiasis, and his family history was notable for hyperparathyroidism. Laboratory results showed primary hyperparathyroidism and evidence of biliary obstruction.
A sestamibi scan demonstrated uptake in a location corresponding with the right inferior parathyroid gland. A computed tomography (CT) scan showed nephrolithiasis and hepatic biliary ductal dilatation. Magnetic resonance cholangiopancreatography (MRCP) revealed both intra- and extrahepatic ductal dilatation, focal narrowing of the proximal common bile duct, and possible adenopathy that was concerning for cholangiocarcinoma. Endoscopic retrograde cholangiopancreatography (ERCP) demonstrated a 1 cm to 2 cm focal stricture within the mid-common bile duct with intra- and extrahepatic ductal dilatation (Figure 1). An endoscopy showed no masses in the duodenum, and anendoscopic ultrasound showed no masses in the pancreas. Endoscopic brushings and endoscopic, ultrasound-guided, fine-needle aspiration
cytology were nondiagnostic. Exploratory laparotomy revealed a dilated hepatic bile duct, an inflamed porta hepatis, and a mass involving the distal hepatic bile duct.
The patient underwent cholecystectomy, radical extra hepatic bile duct resection to the level of the hepatic bifurcation, and hepaticojejunostomy. Gross examination of the specimen showed a nodule centered in the distal common hepatic duct with an adjacent, 2-cm lymph node. The histologic examination revealed a neoplastic proliferation consisting of epithelioid cells with round nuclei and granular chromatin with amphophilic cytoplasm in a trabecular and nested architecture.
The tumor was centered in the submucosa, which is typical of gastrointestinal NETs (Figure 2). There was no evidence of direct tumor extension elsewhere. About 40% of the tumor cells contained eosinophilic, intracytoplasmic inclusions (Figure 3). The tumor did not involve the margins or lymph node.
Positive staining with the neuroendocrine markers synaptophysin and chromagranin A confirmed a well-differentiated NET. The intracytoplasmic inclusions stained strongly positive for cytokeratin CAM 5.2. The tumor had higher-grade features, including tumor cell necrosis, a Ki-67 labeling index of 3%, and perineural invasion. The 2010 World Health Organization (WHO) criteria for NET of the digestive system classified this tumor as a grade 2, well-differentiated NET and as stage 1a (limited to the bile duct).4
Postoperatively, octreotide scan with single-photon emission computed tomography (SPECT)-CT did not show additional masses or lesions. Serum pancreatic polypeptide was elevated, with the remaining serum and plasma NET markers—including gastrin, glucagon, insulin, chromogranin A, and vasoactive intestinal polypeptide (VIP)—being within reference ranges. Genetic testing (GeneDx, Inc, Gaithersburg, MD) showed an E563X nonsense mutation in the MEN1 gene, confirming a MEN1 disorder. The patient then underwent a 4-gland parathyroidectomy with reimplantation; the parathyroid glands demonstrated hyperplasia in all 4 glands.
Biochemical follow-up at 14 months showed that the serum pancreatic polypeptide had normalized. There was no evidence of pituitary orpancreatic hypersecretion. The patient developed hypoparathyroidism, requiring calcium and calcitriol supplementation. Radiographic follow-up using abdominal magnetic resonance imaging at 16 months showed no evidence of disease.
Discussion
This case illustrates a genetic disease with an unusual initial presentation. Primary extrahepatic bile duct NETs are rare and have been reported previously in patients without MEN1.5-9 Neuroendocrine tumors in the hepatic bile duct in patients with MEN1 also have been reported but only after these tumors first appeared in the pancreas or duodenum.10 An extensive literature search revealed no prior reports extrahepatic bile duct NETs with MEN1 as the primary site or with biliary obstruction, which is why this patient’s presentation is particularly interesting.5,6,10-13 The table summarizes select reports of NETs.
Tumor location in this patient was atypical, and genetic testing guided the management. Serum MEN1 genetic testing is indicated in patients with ≥ 2 tumors that are atypical but possibly associated with MEN1 (such as adrenal tumors, gastrinomas, and carcinoids) and in patients aged < 45 years with primary hyperparathyroidism.14,15 The patient in this study was aged 29 years and had hyperparathyroidism and an NET of the hepatic bile duct. This condition was sufficient to warrant genetic testing, the results of which affected the patient’s subsequent parathyroid surgery.15 Despite the suggestion of unifocal localization on the sestamibi scan, the patient underwent the more appropriate subtotal parathyroidectomy.14 The patient’s tumor most likely originated from a germline mutation of the MEN1 gene.
As a result of the patient’s genetic test results, his daughter also was tested. She was found to have the same mutation as her father and will undergo proper tumor surveillance for MEN1. There was no personal or family history of hemangioblastomas, renal cell carcinomas, or cystadenomas, which would have prompted testing for von Hippel-Lindau disease. Likewise, there was no personal or family history of café-au-lait macules and neurofibromas, which would have prompted testing for neurofibromatosis type 1.
Due to the paucity of cases, there are currently no well-accepted guidelines on how to stage extrahepatic biliary NETs.3-5,16 The WHO recommends staging according to adenocarcinomas of the gallbladder and bile duct.3 As such, the pathologic stage of this tumor would be stage 1a.
The significance of the intracytoplasmic inclusion in this case is unknown. Pancreatic NETs and neuroendocrine carcinomas have demonstrated intracytoplasmic inclusions that stain positively for keratin and may indicate more aggressive tumor behavior.17-19 In 1 report, electron microscopic examination demonstrated intermediate filaments with entrapped neurosecretory granules.18 In a series of 84 cases of pancreatic endocrine tumors, 14 had intracytoplasmic inclusions; of these, 5 had MEN1.17 In the present case, the patient continues to show no evidence of tumor recurrence at 16 months after resection.
Conclusion
Extrahepatic biliary neuroendocrine tumors are rare. Further investigation into biliary tree NET staging and future studies to determine the significance of intracytoplasmic inclusions may be beneficial. This case highlights the appropriate use of genetic testing and supports expanding the clinical diagnosis of MEN1 to include NETs of the extrahepatic bile duct.
Click here to read the digital edition.
1. Melmed S, Polonsky KS, Larsen PR, Kronenberg HM, eds. Williams Textbook of Endocrinology. 12th ed. Philadelphia, PA: WB Saunders; 2011.
2. American Joint Committee on Cancer. Neuroendocrine Tumors. In: Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, eds. American Joint Committee on Cancer Staging Handbook. 7th ed. From the AJCC Cancer Staging Manual. New York, NY: Springer-Verlag; 2010:227-236.
3. Komminoth P, Arnold R, Capella C, et al. Neuroendocrine neoplasms of the gallbladder and extrahepatic bile ducts. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, et al, eds. WHO Classification of Tumours of the Digestive System. 4th ed. Lyon, France: IARC Press; 2010:274-276.
4. Rindi G, Arnold R, Bosman FT. Nomenclature and classification of neuroendocrine neoplasms of the digestive system. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, et al, eds. WHO Classification of Tumours of the Digestive System. 4th ed. Lyon, France: IARC Press; 2010:13.
5. Price TN, Thompson GB, Lewis JT, Lloyd RV, Young WF. Zollinger-Ellison syndrome due to primary gastrinoma of the extrahepatic biliary tree: three case reports and review of literature. Endocr Pract. 2009;15(7):737-749.
6. Bhandarwar AH, Shaikh TA, Borisa AD, et al. Primary neuroendocrine tumor of the left hepatic duct: a case report with review of the literature. Case Rep Surg. 2012:786432.
7. Bhalla P, Powle V, Shah RC, Jagannath P. Neuroendocrine tumor of common hepatic duct. Indian J Gastroenterol. 2012;31(3):144-146.
8. Khan FA, Stevens-Chase A, Chaudhry R, Hashmi A, Edelman D, Weaver D. Extrahepatic biliary obstrution secondary to neuroendocrine tumor of the common hepatic duct. Int J Surg Case Rep. 2017;30:46-49.
9. Hong N, Kim HJ, Byun JH, et al. Neuroendocrine neoplasms of the extrahepatic bile duct: radiologic and clinical characteristics. Abdom Imaging. 2015;40(1):181-191.
10. Tonelli F, Giudici F, Nesi G, Batignani G, Brandi ML. Biliary tree gastrinomas in multiple endocrine neoplasia type 1 syndrome. World J Gastroenterol. 2013;19(45):8312-8320.
11. Gibril F, Schumann M, Pace A, Jensen RT. Multiple endocrine neoplasia type 1 and Zollinger-Ellison syndrome: a prospective study of 107 cases and comparison with 1009 cases from the literature. Medicine (Baltimore). 2004;83(1):43-83.
12. Pieterman CRC, Conemans EB, Dreijerink KMA, et al. Thoracic and duodenopancreatic neuroendocrine tumors in multiple endocrine neoplasia type 1: natural history and function of menin in tumorigenesis. Endocr Relat Cancer. 2014;21(3):R121-R142.
13. Pipeleers-Marichal M, Somers G, Willems G, et al. Gastrinomas in the duodenums of patients with multiple endocrine neoplasia type 1 and the Zollinger-Ellison syndrome. N Engl J Med. 1990;322(11):723-727.
14. Thakker RV, Newey PJ, Walls GV, et al; Endocrine Society. Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1). J Clin Endocrinol Metab. 2012;97(9):2990-3011.
15. Eastell R, Brandi ML, Costa AG, et al. Diagnosis of asymptomatic primary hyperparathyroidism: proceedings of the Fourth International Workshop. J Clin Endocrinol Metab. 2014;99(10):3570-3579.
16. Michalopoulos N, Papavramidis TS, Karayannopoulou G, Pliakos I, Papavramidis ST, Kanellos I. Neuroendocrine tumors of extrahepatic biliary tract. Pathol Oncol Res. 2014;20(4):765-775.
17. Serra S, Asa SL, Chetty R. Intracytoplasmic inclusions (including the so-called “rhabdoid” phenotype) in pancreatic endocrine tumors. Endocr Pathol. 2006;17(1):75-81.
18. Shia J, Erlandson RA, Klimstra DS. Whorls of intermediate filaments with entrapped neurosecretory granules correspond to the “rhabdoid” inclusions seen in pancreatic endocrine
neoplasms. Am J Surg Pathol. 2004;28(2):271-273.
19. Perez-Montiel MD, Frankel WL, Suster S. Neuroendocrine carcinomas of the pancreas with ‘Rhabdoid’ features. Am J Surg Pathol. 2003;27(5):642-649.
1. Melmed S, Polonsky KS, Larsen PR, Kronenberg HM, eds. Williams Textbook of Endocrinology. 12th ed. Philadelphia, PA: WB Saunders; 2011.
2. American Joint Committee on Cancer. Neuroendocrine Tumors. In: Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, eds. American Joint Committee on Cancer Staging Handbook. 7th ed. From the AJCC Cancer Staging Manual. New York, NY: Springer-Verlag; 2010:227-236.
3. Komminoth P, Arnold R, Capella C, et al. Neuroendocrine neoplasms of the gallbladder and extrahepatic bile ducts. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, et al, eds. WHO Classification of Tumours of the Digestive System. 4th ed. Lyon, France: IARC Press; 2010:274-276.
4. Rindi G, Arnold R, Bosman FT. Nomenclature and classification of neuroendocrine neoplasms of the digestive system. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, et al, eds. WHO Classification of Tumours of the Digestive System. 4th ed. Lyon, France: IARC Press; 2010:13.
5. Price TN, Thompson GB, Lewis JT, Lloyd RV, Young WF. Zollinger-Ellison syndrome due to primary gastrinoma of the extrahepatic biliary tree: three case reports and review of literature. Endocr Pract. 2009;15(7):737-749.
6. Bhandarwar AH, Shaikh TA, Borisa AD, et al. Primary neuroendocrine tumor of the left hepatic duct: a case report with review of the literature. Case Rep Surg. 2012:786432.
7. Bhalla P, Powle V, Shah RC, Jagannath P. Neuroendocrine tumor of common hepatic duct. Indian J Gastroenterol. 2012;31(3):144-146.
8. Khan FA, Stevens-Chase A, Chaudhry R, Hashmi A, Edelman D, Weaver D. Extrahepatic biliary obstrution secondary to neuroendocrine tumor of the common hepatic duct. Int J Surg Case Rep. 2017;30:46-49.
9. Hong N, Kim HJ, Byun JH, et al. Neuroendocrine neoplasms of the extrahepatic bile duct: radiologic and clinical characteristics. Abdom Imaging. 2015;40(1):181-191.
10. Tonelli F, Giudici F, Nesi G, Batignani G, Brandi ML. Biliary tree gastrinomas in multiple endocrine neoplasia type 1 syndrome. World J Gastroenterol. 2013;19(45):8312-8320.
11. Gibril F, Schumann M, Pace A, Jensen RT. Multiple endocrine neoplasia type 1 and Zollinger-Ellison syndrome: a prospective study of 107 cases and comparison with 1009 cases from the literature. Medicine (Baltimore). 2004;83(1):43-83.
12. Pieterman CRC, Conemans EB, Dreijerink KMA, et al. Thoracic and duodenopancreatic neuroendocrine tumors in multiple endocrine neoplasia type 1: natural history and function of menin in tumorigenesis. Endocr Relat Cancer. 2014;21(3):R121-R142.
13. Pipeleers-Marichal M, Somers G, Willems G, et al. Gastrinomas in the duodenums of patients with multiple endocrine neoplasia type 1 and the Zollinger-Ellison syndrome. N Engl J Med. 1990;322(11):723-727.
14. Thakker RV, Newey PJ, Walls GV, et al; Endocrine Society. Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1). J Clin Endocrinol Metab. 2012;97(9):2990-3011.
15. Eastell R, Brandi ML, Costa AG, et al. Diagnosis of asymptomatic primary hyperparathyroidism: proceedings of the Fourth International Workshop. J Clin Endocrinol Metab. 2014;99(10):3570-3579.
16. Michalopoulos N, Papavramidis TS, Karayannopoulou G, Pliakos I, Papavramidis ST, Kanellos I. Neuroendocrine tumors of extrahepatic biliary tract. Pathol Oncol Res. 2014;20(4):765-775.
17. Serra S, Asa SL, Chetty R. Intracytoplasmic inclusions (including the so-called “rhabdoid” phenotype) in pancreatic endocrine tumors. Endocr Pathol. 2006;17(1):75-81.
18. Shia J, Erlandson RA, Klimstra DS. Whorls of intermediate filaments with entrapped neurosecretory granules correspond to the “rhabdoid” inclusions seen in pancreatic endocrine
neoplasms. Am J Surg Pathol. 2004;28(2):271-273.
19. Perez-Montiel MD, Frankel WL, Suster S. Neuroendocrine carcinomas of the pancreas with ‘Rhabdoid’ features. Am J Surg Pathol. 2003;27(5):642-649.
FDA approves dabrafenib/trametinib for BRAF-positive anaplastic thyroid cancer
FDA approval was based on results from an open-label clinical trial of patients with various rare, BRAF V600E–positive cancers. In a group of 23 patients, 57% experienced a partial response and 4% experienced a full response. In the response group, nine patients had no significant tumor growth for a period of at least 6 months.
The most common side effects of dabrafenib/trametinib are fever, rash, chills, headache, joint pain, cough, fatigue, nausea, vomiting, diarrhea, myalgia, dry skin, decreased appetite, edema, hemorrhage, high blood pressure, and difficulty breathing. Both drugs can cause damage to developing fetuses, and women should be advised to use proper contraception.
“This approval demonstrates that targeting the same molecular pathway in diverse diseases is an effective way to expedite the development of treatments that may help more patients,” Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence, said in the press release.
Find the full press release on the FDA website.
FDA approval was based on results from an open-label clinical trial of patients with various rare, BRAF V600E–positive cancers. In a group of 23 patients, 57% experienced a partial response and 4% experienced a full response. In the response group, nine patients had no significant tumor growth for a period of at least 6 months.
The most common side effects of dabrafenib/trametinib are fever, rash, chills, headache, joint pain, cough, fatigue, nausea, vomiting, diarrhea, myalgia, dry skin, decreased appetite, edema, hemorrhage, high blood pressure, and difficulty breathing. Both drugs can cause damage to developing fetuses, and women should be advised to use proper contraception.
“This approval demonstrates that targeting the same molecular pathway in diverse diseases is an effective way to expedite the development of treatments that may help more patients,” Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence, said in the press release.
Find the full press release on the FDA website.
FDA approval was based on results from an open-label clinical trial of patients with various rare, BRAF V600E–positive cancers. In a group of 23 patients, 57% experienced a partial response and 4% experienced a full response. In the response group, nine patients had no significant tumor growth for a period of at least 6 months.
The most common side effects of dabrafenib/trametinib are fever, rash, chills, headache, joint pain, cough, fatigue, nausea, vomiting, diarrhea, myalgia, dry skin, decreased appetite, edema, hemorrhage, high blood pressure, and difficulty breathing. Both drugs can cause damage to developing fetuses, and women should be advised to use proper contraception.
“This approval demonstrates that targeting the same molecular pathway in diverse diseases is an effective way to expedite the development of treatments that may help more patients,” Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence, said in the press release.
Find the full press release on the FDA website.
Resolution of refractory pruritus with aprepitant in a patient with microcystic adnexal carcinoma
Substance P is an important neurotransmitter implicated in itch pathways.1 After binding to its receptor, neurokinin-1 (NK-1), substance P induces release of factors including histamine, which may cause pruritus.2 Recent literature has reported successful use of aprepitant, an NK-1 antagonist that has been approved by the US Food and Drug Administration for the treatment of chemotherapy-induced nausea and vomiting, for treatment of pruritus. We report here the case of a patient with microcystic adnexal carcinoma (MAC) who presented with refractory pruritus and who had rapid and complete resolution of itch after administration of aprepitant.
Case presentation and summary
A 73-year-old man presented with a 12-year history of a small nodule on his philtrum, which had been increasing in size. He subsequently developed upper-lip numbness and nasal induration. He complained of 2.5 months of severe, debilitating, full-body pruritus. His symptoms were refractory to treatment with prednisone, gabapentin, doxycycline, doxepin, antihistamines, and topical steroids. At the time of consultation, he was being treated with hydroxyzine and topical pramocaine lotion with minimal relief.
At initial dermatologic evaluation, his tumor involved the lower two-thirds of the nose and entire upper cutaneous lip. There was a 4-mm rolled ulcer on the nasal tip and a 1-cm exophytic, smooth nodule on the left upper lip with palpable 4-cm submandibular adenopathy (Figure). Skin examination otherwise revealed linear excoriations on the upper back with no additional primary lesions. The nodule was biopsied, and the patient was diagnosed with MAC with gross nodal involvement. Laboratory findings including serum chemistries, blood urea nitrogen, complete blood cell count, thyroid, and liver function were normal. Positron emission tomography-computed tomography (PET-CT) imaging was negative for distant metastases.
Treatment was initiated with oral aprepitant – 125 mg on day 1, 80 mg on day 2, and 80 mg on day 3 –with concomitant weekly carboplatin (AUC 1.5) and paclitaxel (30 mg/m2) as well as radiation. Within hours after the first dose of aprepitant, the patient reported a notable cessation in his pruritus. He reported that after 5 hours, his skin “finally turned off” and over the hour that followed, he had complete resolution of symptoms. He completed chemoradiation with a significant disease response. Despite persistent MAC confined to the philtrum, he has been followed for over 2 years without recurrence of itch.
Discussion
MAC is an uncommon cutaneous malignancy of sweat and eccrine gland differentiation. In all, 700 cases of MAC have been described in the literature; a 2008 review estimated the incidence of metastasis at around 2.1%.3 Though metastasis is exceedingly rare, the tumor is locally aggressive and there are reports of invasion into the muscle, perichondrium, periosteum, bone marrow, as well as perineural spaces and vascular adventitia.4
The clinical presentation of MAC includes smooth, flesh-colored or yellow papules, nodules, or plaques.3 Patients often present with numbness, paresthesia, and burning in the area of involvement because of neural infiltration with tumor. Despite the rarity of MAC, pruritus has been reported as a presenting symptom in 1 other case in the literature.4 Our case represents the first report of MAC presenting with a grossly enlarging centrofacial mass, lymph node involvement, and severe full-body pruritus. Our patient responded completely, and within hours, to treatment with aprepitant after experiencing months of failure with conventional antipruritus treatments and without recurrence in symptoms in more than 2 years of follow-up.
Aprepitant blocks the binding of substance P to its receptor NK-1 and has been approved as an anti-emetic for chemotherapy patients. Substance P has been shown to be important in both nausea and itch pathways. The largest prospective study to date on aprepitant for the indication of pruritus in 45 patients with metastatic solid tumors demonstrated a 91% response rate, defined by >50% reduction in pruritus intensity, and 13% recurrence rate that occurred at a median of 7 weeks after initial treatment.5 Aprepitant treatment has been used with success for pruritus associated with both malignant and nonmalignant conditions in at least 74 patients,6 among whom the malignant conditions included cutaneous T-cell lymphoma, Hodgkin lymphoma, and metastatic solid tumors.5-7 Aprepitant has also been used for erlotinib- and nivolumab-induced pruritus in non–small cell lung cancer, which suggests a possible future role for aprepitant in the treatment of pruritus secondary to novel cancer therapies, perhaps including immune checkpoint inhibitors.8-10
However, despite those reports, and likely owing to the multifactorial nature of pruritus, aprepitant is not unviversally effective. Mechanisms of malignancy-associated itch are yet to be elucidated, and optimal patient selection for aprepitant use needs to be determined. However, our patient’s notable response supports the increasing evidence that substance P is a key mediator of pruritus and that disruption of binding to its receptor may result in significant improvement in symptoms in certain patients. It remains to be seen whether the cell type or the tendency toward neural invasion plays a role. Large, randomized studies are needed to guide patient selection and confirm the findings reported here and in the literature, with careful documentation of and close attention paid to timing of pruritus relief and improvement in patient quality of life. Aprepitant might be an important therapeutic tool for refractory, malignancy-associated pruritus, in which patient quality of life is especially critical.
Acknowledgments
This work was presented at the Multinational Association of Supportive Care and Cancer Meeting, in Miami Florida, June 26-28, 2014. The authors are indebted to Saajar Jadeja for his assistance preparing the manuscript.
1. Wallengren J. Neuroanatomy and neurophysiology of itch. Dermatol Ther. 2005;18(4):292-303.
2. Kulka M, Sheen CH, Tancowny BP, Grammer LC, Schleimer RP. Neuropeptides activate human mast cell degranulation and chemokine production. Immunology. 2008;123(3):398-410.
3. Wetter R, Goldstein GD. Microcystic adnexal carcinoma: a diagnostic and therapeutic challenge. Dermatol Ther. 2008;21(6):452-458.
4. Adamson T. Microcystic adnexal carcinoma. Dermatol Nurs. 2004;16(4):365.
5. Santini D, Vincenzi B, Guida FM, et al. Aprepitant for management of severe pruritus related to biological cancer treatments: a pilot study. Lancet Oncol. 2012;13(10):1020-1024.
6. Song JS, Tawa M, Chau NG, Kupper TS, LeBoeuf NR. Aprepitant for refractory cutaneous T-cell lymphoma-associated pruritus: 4 cases and a review of the literature. BMC Cancer. 2017;17.
7. Villafranca JJA, Siles MG, Casanova M, Goitia BT, Domínguez AR. Paraneoplastic pruritus presenting with Hodgkin’s lymphoma: a case report. J Med Case Reports. 2014;8:300.
8. Ito J, Fujimoto D, Nakamura A, et al. Aprepitant for refractory nivolumab-induced pruritus. Lung Cancer Amst Neth. 2017;109:58-61.
9. Levêque D. Aprepitant for erlotinib-induced pruritus. N Engl J Med. 2010;363(17):1680-1681; author reply 1681.
10. Gerber PA, Buhren BA, Homey B. More on aprepitant for erlotinib-induced pruritus. N Engl J Med. 2011;364(5):486-487.
Substance P is an important neurotransmitter implicated in itch pathways.1 After binding to its receptor, neurokinin-1 (NK-1), substance P induces release of factors including histamine, which may cause pruritus.2 Recent literature has reported successful use of aprepitant, an NK-1 antagonist that has been approved by the US Food and Drug Administration for the treatment of chemotherapy-induced nausea and vomiting, for treatment of pruritus. We report here the case of a patient with microcystic adnexal carcinoma (MAC) who presented with refractory pruritus and who had rapid and complete resolution of itch after administration of aprepitant.
Case presentation and summary
A 73-year-old man presented with a 12-year history of a small nodule on his philtrum, which had been increasing in size. He subsequently developed upper-lip numbness and nasal induration. He complained of 2.5 months of severe, debilitating, full-body pruritus. His symptoms were refractory to treatment with prednisone, gabapentin, doxycycline, doxepin, antihistamines, and topical steroids. At the time of consultation, he was being treated with hydroxyzine and topical pramocaine lotion with minimal relief.
At initial dermatologic evaluation, his tumor involved the lower two-thirds of the nose and entire upper cutaneous lip. There was a 4-mm rolled ulcer on the nasal tip and a 1-cm exophytic, smooth nodule on the left upper lip with palpable 4-cm submandibular adenopathy (Figure). Skin examination otherwise revealed linear excoriations on the upper back with no additional primary lesions. The nodule was biopsied, and the patient was diagnosed with MAC with gross nodal involvement. Laboratory findings including serum chemistries, blood urea nitrogen, complete blood cell count, thyroid, and liver function were normal. Positron emission tomography-computed tomography (PET-CT) imaging was negative for distant metastases.
Treatment was initiated with oral aprepitant – 125 mg on day 1, 80 mg on day 2, and 80 mg on day 3 –with concomitant weekly carboplatin (AUC 1.5) and paclitaxel (30 mg/m2) as well as radiation. Within hours after the first dose of aprepitant, the patient reported a notable cessation in his pruritus. He reported that after 5 hours, his skin “finally turned off” and over the hour that followed, he had complete resolution of symptoms. He completed chemoradiation with a significant disease response. Despite persistent MAC confined to the philtrum, he has been followed for over 2 years without recurrence of itch.
Discussion
MAC is an uncommon cutaneous malignancy of sweat and eccrine gland differentiation. In all, 700 cases of MAC have been described in the literature; a 2008 review estimated the incidence of metastasis at around 2.1%.3 Though metastasis is exceedingly rare, the tumor is locally aggressive and there are reports of invasion into the muscle, perichondrium, periosteum, bone marrow, as well as perineural spaces and vascular adventitia.4
The clinical presentation of MAC includes smooth, flesh-colored or yellow papules, nodules, or plaques.3 Patients often present with numbness, paresthesia, and burning in the area of involvement because of neural infiltration with tumor. Despite the rarity of MAC, pruritus has been reported as a presenting symptom in 1 other case in the literature.4 Our case represents the first report of MAC presenting with a grossly enlarging centrofacial mass, lymph node involvement, and severe full-body pruritus. Our patient responded completely, and within hours, to treatment with aprepitant after experiencing months of failure with conventional antipruritus treatments and without recurrence in symptoms in more than 2 years of follow-up.
Aprepitant blocks the binding of substance P to its receptor NK-1 and has been approved as an anti-emetic for chemotherapy patients. Substance P has been shown to be important in both nausea and itch pathways. The largest prospective study to date on aprepitant for the indication of pruritus in 45 patients with metastatic solid tumors demonstrated a 91% response rate, defined by >50% reduction in pruritus intensity, and 13% recurrence rate that occurred at a median of 7 weeks after initial treatment.5 Aprepitant treatment has been used with success for pruritus associated with both malignant and nonmalignant conditions in at least 74 patients,6 among whom the malignant conditions included cutaneous T-cell lymphoma, Hodgkin lymphoma, and metastatic solid tumors.5-7 Aprepitant has also been used for erlotinib- and nivolumab-induced pruritus in non–small cell lung cancer, which suggests a possible future role for aprepitant in the treatment of pruritus secondary to novel cancer therapies, perhaps including immune checkpoint inhibitors.8-10
However, despite those reports, and likely owing to the multifactorial nature of pruritus, aprepitant is not unviversally effective. Mechanisms of malignancy-associated itch are yet to be elucidated, and optimal patient selection for aprepitant use needs to be determined. However, our patient’s notable response supports the increasing evidence that substance P is a key mediator of pruritus and that disruption of binding to its receptor may result in significant improvement in symptoms in certain patients. It remains to be seen whether the cell type or the tendency toward neural invasion plays a role. Large, randomized studies are needed to guide patient selection and confirm the findings reported here and in the literature, with careful documentation of and close attention paid to timing of pruritus relief and improvement in patient quality of life. Aprepitant might be an important therapeutic tool for refractory, malignancy-associated pruritus, in which patient quality of life is especially critical.
Acknowledgments
This work was presented at the Multinational Association of Supportive Care and Cancer Meeting, in Miami Florida, June 26-28, 2014. The authors are indebted to Saajar Jadeja for his assistance preparing the manuscript.
Substance P is an important neurotransmitter implicated in itch pathways.1 After binding to its receptor, neurokinin-1 (NK-1), substance P induces release of factors including histamine, which may cause pruritus.2 Recent literature has reported successful use of aprepitant, an NK-1 antagonist that has been approved by the US Food and Drug Administration for the treatment of chemotherapy-induced nausea and vomiting, for treatment of pruritus. We report here the case of a patient with microcystic adnexal carcinoma (MAC) who presented with refractory pruritus and who had rapid and complete resolution of itch after administration of aprepitant.
Case presentation and summary
A 73-year-old man presented with a 12-year history of a small nodule on his philtrum, which had been increasing in size. He subsequently developed upper-lip numbness and nasal induration. He complained of 2.5 months of severe, debilitating, full-body pruritus. His symptoms were refractory to treatment with prednisone, gabapentin, doxycycline, doxepin, antihistamines, and topical steroids. At the time of consultation, he was being treated with hydroxyzine and topical pramocaine lotion with minimal relief.
At initial dermatologic evaluation, his tumor involved the lower two-thirds of the nose and entire upper cutaneous lip. There was a 4-mm rolled ulcer on the nasal tip and a 1-cm exophytic, smooth nodule on the left upper lip with palpable 4-cm submandibular adenopathy (Figure). Skin examination otherwise revealed linear excoriations on the upper back with no additional primary lesions. The nodule was biopsied, and the patient was diagnosed with MAC with gross nodal involvement. Laboratory findings including serum chemistries, blood urea nitrogen, complete blood cell count, thyroid, and liver function were normal. Positron emission tomography-computed tomography (PET-CT) imaging was negative for distant metastases.
Treatment was initiated with oral aprepitant – 125 mg on day 1, 80 mg on day 2, and 80 mg on day 3 –with concomitant weekly carboplatin (AUC 1.5) and paclitaxel (30 mg/m2) as well as radiation. Within hours after the first dose of aprepitant, the patient reported a notable cessation in his pruritus. He reported that after 5 hours, his skin “finally turned off” and over the hour that followed, he had complete resolution of symptoms. He completed chemoradiation with a significant disease response. Despite persistent MAC confined to the philtrum, he has been followed for over 2 years without recurrence of itch.
Discussion
MAC is an uncommon cutaneous malignancy of sweat and eccrine gland differentiation. In all, 700 cases of MAC have been described in the literature; a 2008 review estimated the incidence of metastasis at around 2.1%.3 Though metastasis is exceedingly rare, the tumor is locally aggressive and there are reports of invasion into the muscle, perichondrium, periosteum, bone marrow, as well as perineural spaces and vascular adventitia.4
The clinical presentation of MAC includes smooth, flesh-colored or yellow papules, nodules, or plaques.3 Patients often present with numbness, paresthesia, and burning in the area of involvement because of neural infiltration with tumor. Despite the rarity of MAC, pruritus has been reported as a presenting symptom in 1 other case in the literature.4 Our case represents the first report of MAC presenting with a grossly enlarging centrofacial mass, lymph node involvement, and severe full-body pruritus. Our patient responded completely, and within hours, to treatment with aprepitant after experiencing months of failure with conventional antipruritus treatments and without recurrence in symptoms in more than 2 years of follow-up.
Aprepitant blocks the binding of substance P to its receptor NK-1 and has been approved as an anti-emetic for chemotherapy patients. Substance P has been shown to be important in both nausea and itch pathways. The largest prospective study to date on aprepitant for the indication of pruritus in 45 patients with metastatic solid tumors demonstrated a 91% response rate, defined by >50% reduction in pruritus intensity, and 13% recurrence rate that occurred at a median of 7 weeks after initial treatment.5 Aprepitant treatment has been used with success for pruritus associated with both malignant and nonmalignant conditions in at least 74 patients,6 among whom the malignant conditions included cutaneous T-cell lymphoma, Hodgkin lymphoma, and metastatic solid tumors.5-7 Aprepitant has also been used for erlotinib- and nivolumab-induced pruritus in non–small cell lung cancer, which suggests a possible future role for aprepitant in the treatment of pruritus secondary to novel cancer therapies, perhaps including immune checkpoint inhibitors.8-10
However, despite those reports, and likely owing to the multifactorial nature of pruritus, aprepitant is not unviversally effective. Mechanisms of malignancy-associated itch are yet to be elucidated, and optimal patient selection for aprepitant use needs to be determined. However, our patient’s notable response supports the increasing evidence that substance P is a key mediator of pruritus and that disruption of binding to its receptor may result in significant improvement in symptoms in certain patients. It remains to be seen whether the cell type or the tendency toward neural invasion plays a role. Large, randomized studies are needed to guide patient selection and confirm the findings reported here and in the literature, with careful documentation of and close attention paid to timing of pruritus relief and improvement in patient quality of life. Aprepitant might be an important therapeutic tool for refractory, malignancy-associated pruritus, in which patient quality of life is especially critical.
Acknowledgments
This work was presented at the Multinational Association of Supportive Care and Cancer Meeting, in Miami Florida, June 26-28, 2014. The authors are indebted to Saajar Jadeja for his assistance preparing the manuscript.
1. Wallengren J. Neuroanatomy and neurophysiology of itch. Dermatol Ther. 2005;18(4):292-303.
2. Kulka M, Sheen CH, Tancowny BP, Grammer LC, Schleimer RP. Neuropeptides activate human mast cell degranulation and chemokine production. Immunology. 2008;123(3):398-410.
3. Wetter R, Goldstein GD. Microcystic adnexal carcinoma: a diagnostic and therapeutic challenge. Dermatol Ther. 2008;21(6):452-458.
4. Adamson T. Microcystic adnexal carcinoma. Dermatol Nurs. 2004;16(4):365.
5. Santini D, Vincenzi B, Guida FM, et al. Aprepitant for management of severe pruritus related to biological cancer treatments: a pilot study. Lancet Oncol. 2012;13(10):1020-1024.
6. Song JS, Tawa M, Chau NG, Kupper TS, LeBoeuf NR. Aprepitant for refractory cutaneous T-cell lymphoma-associated pruritus: 4 cases and a review of the literature. BMC Cancer. 2017;17.
7. Villafranca JJA, Siles MG, Casanova M, Goitia BT, Domínguez AR. Paraneoplastic pruritus presenting with Hodgkin’s lymphoma: a case report. J Med Case Reports. 2014;8:300.
8. Ito J, Fujimoto D, Nakamura A, et al. Aprepitant for refractory nivolumab-induced pruritus. Lung Cancer Amst Neth. 2017;109:58-61.
9. Levêque D. Aprepitant for erlotinib-induced pruritus. N Engl J Med. 2010;363(17):1680-1681; author reply 1681.
10. Gerber PA, Buhren BA, Homey B. More on aprepitant for erlotinib-induced pruritus. N Engl J Med. 2011;364(5):486-487.
1. Wallengren J. Neuroanatomy and neurophysiology of itch. Dermatol Ther. 2005;18(4):292-303.
2. Kulka M, Sheen CH, Tancowny BP, Grammer LC, Schleimer RP. Neuropeptides activate human mast cell degranulation and chemokine production. Immunology. 2008;123(3):398-410.
3. Wetter R, Goldstein GD. Microcystic adnexal carcinoma: a diagnostic and therapeutic challenge. Dermatol Ther. 2008;21(6):452-458.
4. Adamson T. Microcystic adnexal carcinoma. Dermatol Nurs. 2004;16(4):365.
5. Santini D, Vincenzi B, Guida FM, et al. Aprepitant for management of severe pruritus related to biological cancer treatments: a pilot study. Lancet Oncol. 2012;13(10):1020-1024.
6. Song JS, Tawa M, Chau NG, Kupper TS, LeBoeuf NR. Aprepitant for refractory cutaneous T-cell lymphoma-associated pruritus: 4 cases and a review of the literature. BMC Cancer. 2017;17.
7. Villafranca JJA, Siles MG, Casanova M, Goitia BT, Domínguez AR. Paraneoplastic pruritus presenting with Hodgkin’s lymphoma: a case report. J Med Case Reports. 2014;8:300.
8. Ito J, Fujimoto D, Nakamura A, et al. Aprepitant for refractory nivolumab-induced pruritus. Lung Cancer Amst Neth. 2017;109:58-61.
9. Levêque D. Aprepitant for erlotinib-induced pruritus. N Engl J Med. 2010;363(17):1680-1681; author reply 1681.
10. Gerber PA, Buhren BA, Homey B. More on aprepitant for erlotinib-induced pruritus. N Engl J Med. 2011;364(5):486-487.