User login
Bezlotoxumab may lower risk of C. difficile readmissions
Clostridium difficile infection (CDI) patients treated with bezlotoxumab were less likely to be readmitted for recurring symptoms within 30 days of discharge, according to a phase 3 trial funded by Merck.
Recurrent CDI is a burden on both patients and providers, increasing health risks with each recurrence and eating through hospital resources, according to Vimalanand S. Prabhu, PhD, associate principal scientist for Merck.
In a randomized, double-blind, placebo-controlled, study of 1,050 CDI patients, a total of 27 (5%) of 530 of those given bezlotoxumab were re-hospitalized 30 days after discharge, compared with 58 (11%) of 520 patients in the placebo group (Clin Infect Dis. 2017 Aug 11. doi. 10.1093/cid/cix523).
Patients were gathered from 322 sites across 30 countries between November 2011 and May 2015.
When measuring CDI-related readmissions, the investigators found use of bezlotoxumab reduced rCDI hospitalizations by 6%, and by approximately 8% in high-risk patients, such as those over 65 years old or with severe CDI.
Bezlotoxumab works by binding to CDI toxin B, a primary cause of CDI symptoms, according to Dr. Prabhu and fellow investigators. The researchers suggested that bezlotoxumab could be a prevailing factor in fighting the rate of CDI infections, which accounted for 29,000 deaths in 2011 (N Engl J Med. 2015 Jun 11;372[24]:2368-9).
Investigators acknowledged that patients admitted for the study may be healthier than the real-world CDI population.
All investigators reported some financial involvement, whether being a full-time employee or acting as a consultant, for Merck, which funded the study. Individually, investigators reported financial ties to similar medical companies, such as Pfizer and AstraZeneca.
[email protected]
On Twitter @eaztweets
Clostridium difficile infection (CDI) patients treated with bezlotoxumab were less likely to be readmitted for recurring symptoms within 30 days of discharge, according to a phase 3 trial funded by Merck.
Recurrent CDI is a burden on both patients and providers, increasing health risks with each recurrence and eating through hospital resources, according to Vimalanand S. Prabhu, PhD, associate principal scientist for Merck.
In a randomized, double-blind, placebo-controlled, study of 1,050 CDI patients, a total of 27 (5%) of 530 of those given bezlotoxumab were re-hospitalized 30 days after discharge, compared with 58 (11%) of 520 patients in the placebo group (Clin Infect Dis. 2017 Aug 11. doi. 10.1093/cid/cix523).
Patients were gathered from 322 sites across 30 countries between November 2011 and May 2015.
When measuring CDI-related readmissions, the investigators found use of bezlotoxumab reduced rCDI hospitalizations by 6%, and by approximately 8% in high-risk patients, such as those over 65 years old or with severe CDI.
Bezlotoxumab works by binding to CDI toxin B, a primary cause of CDI symptoms, according to Dr. Prabhu and fellow investigators. The researchers suggested that bezlotoxumab could be a prevailing factor in fighting the rate of CDI infections, which accounted for 29,000 deaths in 2011 (N Engl J Med. 2015 Jun 11;372[24]:2368-9).
Investigators acknowledged that patients admitted for the study may be healthier than the real-world CDI population.
All investigators reported some financial involvement, whether being a full-time employee or acting as a consultant, for Merck, which funded the study. Individually, investigators reported financial ties to similar medical companies, such as Pfizer and AstraZeneca.
[email protected]
On Twitter @eaztweets
Clostridium difficile infection (CDI) patients treated with bezlotoxumab were less likely to be readmitted for recurring symptoms within 30 days of discharge, according to a phase 3 trial funded by Merck.
Recurrent CDI is a burden on both patients and providers, increasing health risks with each recurrence and eating through hospital resources, according to Vimalanand S. Prabhu, PhD, associate principal scientist for Merck.
In a randomized, double-blind, placebo-controlled, study of 1,050 CDI patients, a total of 27 (5%) of 530 of those given bezlotoxumab were re-hospitalized 30 days after discharge, compared with 58 (11%) of 520 patients in the placebo group (Clin Infect Dis. 2017 Aug 11. doi. 10.1093/cid/cix523).
Patients were gathered from 322 sites across 30 countries between November 2011 and May 2015.
When measuring CDI-related readmissions, the investigators found use of bezlotoxumab reduced rCDI hospitalizations by 6%, and by approximately 8% in high-risk patients, such as those over 65 years old or with severe CDI.
Bezlotoxumab works by binding to CDI toxin B, a primary cause of CDI symptoms, according to Dr. Prabhu and fellow investigators. The researchers suggested that bezlotoxumab could be a prevailing factor in fighting the rate of CDI infections, which accounted for 29,000 deaths in 2011 (N Engl J Med. 2015 Jun 11;372[24]:2368-9).
Investigators acknowledged that patients admitted for the study may be healthier than the real-world CDI population.
All investigators reported some financial involvement, whether being a full-time employee or acting as a consultant, for Merck, which funded the study. Individually, investigators reported financial ties to similar medical companies, such as Pfizer and AstraZeneca.
[email protected]
On Twitter @eaztweets
FROM CLINICAL INFECTIOUS DISEASES
Key clinical point:
Major finding: A total of 27 of 530 (5%) bezlotoxumab patients were readmitted within 30 days of discharge compared with 58 of 520 (11%) placebo patients.
Data source: Randomized, double-blind, placebo-controlled, multicenter, global phase 3 trials conducted from November 2011-May 2015 at 322 sites in 30 countries.
Disclosures: All investigators report employment or financial support with Merck and have individually reported financial ties to similar companies like Astellas, AstraZeneca, Pfizer, and others.
Enhanced disinfection of duodenoscopes did not reduce contamination
Duodenoscopes had similar rates of contamination after double high-level disinfection, standard high-level disinfection, or standard high-level disinfection followed by ethylene oxide gas sterilization, a randomized, prospective study of 516 bacterial cultures of 18 duodenoscopes showed.
“Our results do not support the routine use of double high-level disinfection or ethylene oxide sterilization for duodenoscope reprocessing,” wrote Graham M. Snyder, MD, of Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, and his associates. They stopped the study after 3 months because none of the duodenoscopes cultured multidrug-resistant organisms, the primary endpoint. “[We] found that in the nonoutbreak setting, duodenoscope contamination by multidrug-resistant organisms is extremely uncommon,” they wrote in the October issue of Gastroenterology (doi: 10.1053/j.gastro.2017.06.052). However, 16% of duodenoscopes cultured at least one colony-forming unit (CFU) after either standard high-level or double high-level disinfection, and 23% of duodenoscopes produced at least one CFU despite standard high-level disinfection followed by ethylene gas sterilization (P = .2), the investigators reported.
Outbreaks of carbapenem-resistant Enterobacteriaceae infections have been traced to duodenoscopes, even though they were reprocessed according to manufacturer instructions. In 2015, the Food and Drug Administration responded by warning that the design of duodenoscopes might preclude effective cleaning. Reasons for residual contamination remain uncertain, but biofilms, which are notoriously resistant to standard disinfection methods, might be a culprit, Dr. Snyder and his associates noted. Accordingly, some experts have suggested repeating the reprocessing cycle or adding ethylene oxide sterilization, but these measures are costly, time intensive, and not widely available. Furthermore, their efficacy “has never been systematically studied in a nonoutbreak setting,” the researchers wrote.
In response, they studied 516 cultures of elevator mechanisms and working channels from 18 reprocessed duodenoscopes (Olympus, model TJF-Q180). Immediately after use, each duodenoscope was manually wiped with enzymatic solution (EmPower), and then was manually reprocessed within an hour before undergoing automated reprocessing (System 83 Plus 9) with ortho-phthalaldehyde disinfectant (MetriCide OPA Plus) followed by ethanol flush. One-third of the duodenoscopes were randomly assigned to undergo double high-level disinfection with two automated reprocessing cycles, and another third underwent standard high-level disinfection followed by ethylene oxide gas sterilization (Steri-Vac sterilizer/aerator). All instruments were stored by hanging them vertically in an unventilated cabinet.
Multidrug-resistant organisms were cultured from 3% of rectal swabs and duodenal aspirates, but not from any of the cultures of duodenoscopes. Therefore, the study was stopped for futility. The enhanced disinfection methods failed to prevent contamination, compared with standard high-level disinfection, the researchers noted. Ten or more CFUs grew in 2% of duodenoscopes that underwent standard high-level disinfection, 4% of those that underwent double high-level disinfection, and 4% of those that underwent high-level disinfection followed by ethylene oxide sterilization (P = .4).
“There is no consensus on what parts of the standard high-level disinfection process should be repeated,” the investigators wrote. “It is uncertain if the addition of a second cycle of manual reprocessing might have improved the effectiveness of double high-level disinfection.”
Funders included the American Society for Gastrointestinal Endoscopy and Beth Israel Deaconess Medical Center. The investigators reported having no conflicts of interest.
Duodenoscopes had similar rates of contamination after double high-level disinfection, standard high-level disinfection, or standard high-level disinfection followed by ethylene oxide gas sterilization, a randomized, prospective study of 516 bacterial cultures of 18 duodenoscopes showed.
“Our results do not support the routine use of double high-level disinfection or ethylene oxide sterilization for duodenoscope reprocessing,” wrote Graham M. Snyder, MD, of Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, and his associates. They stopped the study after 3 months because none of the duodenoscopes cultured multidrug-resistant organisms, the primary endpoint. “[We] found that in the nonoutbreak setting, duodenoscope contamination by multidrug-resistant organisms is extremely uncommon,” they wrote in the October issue of Gastroenterology (doi: 10.1053/j.gastro.2017.06.052). However, 16% of duodenoscopes cultured at least one colony-forming unit (CFU) after either standard high-level or double high-level disinfection, and 23% of duodenoscopes produced at least one CFU despite standard high-level disinfection followed by ethylene gas sterilization (P = .2), the investigators reported.
Outbreaks of carbapenem-resistant Enterobacteriaceae infections have been traced to duodenoscopes, even though they were reprocessed according to manufacturer instructions. In 2015, the Food and Drug Administration responded by warning that the design of duodenoscopes might preclude effective cleaning. Reasons for residual contamination remain uncertain, but biofilms, which are notoriously resistant to standard disinfection methods, might be a culprit, Dr. Snyder and his associates noted. Accordingly, some experts have suggested repeating the reprocessing cycle or adding ethylene oxide sterilization, but these measures are costly, time intensive, and not widely available. Furthermore, their efficacy “has never been systematically studied in a nonoutbreak setting,” the researchers wrote.
In response, they studied 516 cultures of elevator mechanisms and working channels from 18 reprocessed duodenoscopes (Olympus, model TJF-Q180). Immediately after use, each duodenoscope was manually wiped with enzymatic solution (EmPower), and then was manually reprocessed within an hour before undergoing automated reprocessing (System 83 Plus 9) with ortho-phthalaldehyde disinfectant (MetriCide OPA Plus) followed by ethanol flush. One-third of the duodenoscopes were randomly assigned to undergo double high-level disinfection with two automated reprocessing cycles, and another third underwent standard high-level disinfection followed by ethylene oxide gas sterilization (Steri-Vac sterilizer/aerator). All instruments were stored by hanging them vertically in an unventilated cabinet.
Multidrug-resistant organisms were cultured from 3% of rectal swabs and duodenal aspirates, but not from any of the cultures of duodenoscopes. Therefore, the study was stopped for futility. The enhanced disinfection methods failed to prevent contamination, compared with standard high-level disinfection, the researchers noted. Ten or more CFUs grew in 2% of duodenoscopes that underwent standard high-level disinfection, 4% of those that underwent double high-level disinfection, and 4% of those that underwent high-level disinfection followed by ethylene oxide sterilization (P = .4).
“There is no consensus on what parts of the standard high-level disinfection process should be repeated,” the investigators wrote. “It is uncertain if the addition of a second cycle of manual reprocessing might have improved the effectiveness of double high-level disinfection.”
Funders included the American Society for Gastrointestinal Endoscopy and Beth Israel Deaconess Medical Center. The investigators reported having no conflicts of interest.
Duodenoscopes had similar rates of contamination after double high-level disinfection, standard high-level disinfection, or standard high-level disinfection followed by ethylene oxide gas sterilization, a randomized, prospective study of 516 bacterial cultures of 18 duodenoscopes showed.
“Our results do not support the routine use of double high-level disinfection or ethylene oxide sterilization for duodenoscope reprocessing,” wrote Graham M. Snyder, MD, of Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, and his associates. They stopped the study after 3 months because none of the duodenoscopes cultured multidrug-resistant organisms, the primary endpoint. “[We] found that in the nonoutbreak setting, duodenoscope contamination by multidrug-resistant organisms is extremely uncommon,” they wrote in the October issue of Gastroenterology (doi: 10.1053/j.gastro.2017.06.052). However, 16% of duodenoscopes cultured at least one colony-forming unit (CFU) after either standard high-level or double high-level disinfection, and 23% of duodenoscopes produced at least one CFU despite standard high-level disinfection followed by ethylene gas sterilization (P = .2), the investigators reported.
Outbreaks of carbapenem-resistant Enterobacteriaceae infections have been traced to duodenoscopes, even though they were reprocessed according to manufacturer instructions. In 2015, the Food and Drug Administration responded by warning that the design of duodenoscopes might preclude effective cleaning. Reasons for residual contamination remain uncertain, but biofilms, which are notoriously resistant to standard disinfection methods, might be a culprit, Dr. Snyder and his associates noted. Accordingly, some experts have suggested repeating the reprocessing cycle or adding ethylene oxide sterilization, but these measures are costly, time intensive, and not widely available. Furthermore, their efficacy “has never been systematically studied in a nonoutbreak setting,” the researchers wrote.
In response, they studied 516 cultures of elevator mechanisms and working channels from 18 reprocessed duodenoscopes (Olympus, model TJF-Q180). Immediately after use, each duodenoscope was manually wiped with enzymatic solution (EmPower), and then was manually reprocessed within an hour before undergoing automated reprocessing (System 83 Plus 9) with ortho-phthalaldehyde disinfectant (MetriCide OPA Plus) followed by ethanol flush. One-third of the duodenoscopes were randomly assigned to undergo double high-level disinfection with two automated reprocessing cycles, and another third underwent standard high-level disinfection followed by ethylene oxide gas sterilization (Steri-Vac sterilizer/aerator). All instruments were stored by hanging them vertically in an unventilated cabinet.
Multidrug-resistant organisms were cultured from 3% of rectal swabs and duodenal aspirates, but not from any of the cultures of duodenoscopes. Therefore, the study was stopped for futility. The enhanced disinfection methods failed to prevent contamination, compared with standard high-level disinfection, the researchers noted. Ten or more CFUs grew in 2% of duodenoscopes that underwent standard high-level disinfection, 4% of those that underwent double high-level disinfection, and 4% of those that underwent high-level disinfection followed by ethylene oxide sterilization (P = .4).
“There is no consensus on what parts of the standard high-level disinfection process should be repeated,” the investigators wrote. “It is uncertain if the addition of a second cycle of manual reprocessing might have improved the effectiveness of double high-level disinfection.”
Funders included the American Society for Gastrointestinal Endoscopy and Beth Israel Deaconess Medical Center. The investigators reported having no conflicts of interest.
FROM GASTROENTEROLOGY
Key clinical point: Enhanced disinfection of duodenoscopes did not provide additional protection against contamination.
Major finding: No cultures were positive for multidrug-resistant organisms, but 16% of duodenoscopes had at least one colony-forming unit despite standard high-level disinfection or double high-level disinfection. Standard high-level disinfection followed by ethylene oxide gas failed to sterilize 23% of duodenoscopes (P = .2).
Data source: A single-center, prospective randomized study of 516 cultures of 18 duodenoscopes.
Disclosures: Funders included the American Society for Gastrointestinal Endoscopy and Beth Israel Deaconess Medical Center. The investigators reported having no conflicts of interest.
Consider routine penicillin allergy testing in obstetrics
PARK CITY, UTAH – When attendees at the annual scientific meeting of the Infectious Diseases Society for Obstetrics and Gynecology were asked if their institutions test to confirm alleged penicillin allergies, the only hands that went up were from clinicians at Duke University.
That’s a problem, according to Robert Heine, MD, a maternal-fetal medicine specialist at Duke, in Durham, N.C. “We, as a group, need to be doing [penicillin] allergy testing,” he said.
It’s become clear in recent years that patients who say they have a penicillin allergy often don’t have one, or remember a mild reaction from childhood that doesn’t preclude the use of beta-lactam antibiotics as adults. For decades, however, clinicians have taken those claims at face value, and duly noted them in charts and switched patients to non–beta-lactam antibiotics that don’t work as well.
That’s what happened at Duke in 2014. A total of 81 women with documented penicillin allergies were put on gentamicin and clindamycin to protect against cesarean wound infections and 16% ended up with infections anyway. Among the 864 women who received cefazolin – the first-line cesarean prophylaxis choice at Duke – the infection rate was 7%.
“Beta-lactam antibiotic prophylaxis reduced the risk of surgical site infections after cesareans by 60%,” said Benjamin Harris, MD, the lead investigator and an ob.gyn. resident at Duke, who presented the findings at the meeting.
When the investigators took a closer look at the 81 women who reported penicillin allergies, most of them had rashes and other mild reactions noted in their charts.
Findings such as those led Dr. Heine to push for routine testing. “I brought Duke into it kicking and screaming,” he said. The biggest obstacle was concern over liability, specifically that pregnant women would go into anaphylaxis and deliver prematurely, he said.
After a lot of lobbying, Dr. Heine and his colleagues started routine penicillin allergy testing in March 2016. There hasn’t been a single reaction among the 80-plus pregnant women tested so far, he reported.
Duke administrators were also concerned about reimbursement, but it hasn’t turned out to be a problem. Reimbursements from public and private payers “cover our costs,” a little over $100 per test, Dr. Heine said.
Dr. Heine said he can imagine outpatient testing at some point, but for now women are checked into triage. They get a fetal heart tone before 24 weeks, and a fetal heart rate monitor afterward. “We try to do it before 20 weeks so we don’t have to worry about the fetus,” he said.
When penicillin allergies are in the chart, or women say they are allergic, ask what type of reaction they had in the past. Type 1 reactions should be confirmed with testing. It’s okay to skip testing and give beta-lactams for non–type 1 reactions, but “if a woman has a non–type 1, and they’re already set up for testing, I’m going to do it anyway because getting the penicillin allergy off her chart is good for her and her life,” Dr. Heine said.
Dr. Heine and Dr. Harris reported having no financial disclosures.
PARK CITY, UTAH – When attendees at the annual scientific meeting of the Infectious Diseases Society for Obstetrics and Gynecology were asked if their institutions test to confirm alleged penicillin allergies, the only hands that went up were from clinicians at Duke University.
That’s a problem, according to Robert Heine, MD, a maternal-fetal medicine specialist at Duke, in Durham, N.C. “We, as a group, need to be doing [penicillin] allergy testing,” he said.
It’s become clear in recent years that patients who say they have a penicillin allergy often don’t have one, or remember a mild reaction from childhood that doesn’t preclude the use of beta-lactam antibiotics as adults. For decades, however, clinicians have taken those claims at face value, and duly noted them in charts and switched patients to non–beta-lactam antibiotics that don’t work as well.
That’s what happened at Duke in 2014. A total of 81 women with documented penicillin allergies were put on gentamicin and clindamycin to protect against cesarean wound infections and 16% ended up with infections anyway. Among the 864 women who received cefazolin – the first-line cesarean prophylaxis choice at Duke – the infection rate was 7%.
“Beta-lactam antibiotic prophylaxis reduced the risk of surgical site infections after cesareans by 60%,” said Benjamin Harris, MD, the lead investigator and an ob.gyn. resident at Duke, who presented the findings at the meeting.
When the investigators took a closer look at the 81 women who reported penicillin allergies, most of them had rashes and other mild reactions noted in their charts.
Findings such as those led Dr. Heine to push for routine testing. “I brought Duke into it kicking and screaming,” he said. The biggest obstacle was concern over liability, specifically that pregnant women would go into anaphylaxis and deliver prematurely, he said.
After a lot of lobbying, Dr. Heine and his colleagues started routine penicillin allergy testing in March 2016. There hasn’t been a single reaction among the 80-plus pregnant women tested so far, he reported.
Duke administrators were also concerned about reimbursement, but it hasn’t turned out to be a problem. Reimbursements from public and private payers “cover our costs,” a little over $100 per test, Dr. Heine said.
Dr. Heine said he can imagine outpatient testing at some point, but for now women are checked into triage. They get a fetal heart tone before 24 weeks, and a fetal heart rate monitor afterward. “We try to do it before 20 weeks so we don’t have to worry about the fetus,” he said.
When penicillin allergies are in the chart, or women say they are allergic, ask what type of reaction they had in the past. Type 1 reactions should be confirmed with testing. It’s okay to skip testing and give beta-lactams for non–type 1 reactions, but “if a woman has a non–type 1, and they’re already set up for testing, I’m going to do it anyway because getting the penicillin allergy off her chart is good for her and her life,” Dr. Heine said.
Dr. Heine and Dr. Harris reported having no financial disclosures.
PARK CITY, UTAH – When attendees at the annual scientific meeting of the Infectious Diseases Society for Obstetrics and Gynecology were asked if their institutions test to confirm alleged penicillin allergies, the only hands that went up were from clinicians at Duke University.
That’s a problem, according to Robert Heine, MD, a maternal-fetal medicine specialist at Duke, in Durham, N.C. “We, as a group, need to be doing [penicillin] allergy testing,” he said.
It’s become clear in recent years that patients who say they have a penicillin allergy often don’t have one, or remember a mild reaction from childhood that doesn’t preclude the use of beta-lactam antibiotics as adults. For decades, however, clinicians have taken those claims at face value, and duly noted them in charts and switched patients to non–beta-lactam antibiotics that don’t work as well.
That’s what happened at Duke in 2014. A total of 81 women with documented penicillin allergies were put on gentamicin and clindamycin to protect against cesarean wound infections and 16% ended up with infections anyway. Among the 864 women who received cefazolin – the first-line cesarean prophylaxis choice at Duke – the infection rate was 7%.
“Beta-lactam antibiotic prophylaxis reduced the risk of surgical site infections after cesareans by 60%,” said Benjamin Harris, MD, the lead investigator and an ob.gyn. resident at Duke, who presented the findings at the meeting.
When the investigators took a closer look at the 81 women who reported penicillin allergies, most of them had rashes and other mild reactions noted in their charts.
Findings such as those led Dr. Heine to push for routine testing. “I brought Duke into it kicking and screaming,” he said. The biggest obstacle was concern over liability, specifically that pregnant women would go into anaphylaxis and deliver prematurely, he said.
After a lot of lobbying, Dr. Heine and his colleagues started routine penicillin allergy testing in March 2016. There hasn’t been a single reaction among the 80-plus pregnant women tested so far, he reported.
Duke administrators were also concerned about reimbursement, but it hasn’t turned out to be a problem. Reimbursements from public and private payers “cover our costs,” a little over $100 per test, Dr. Heine said.
Dr. Heine said he can imagine outpatient testing at some point, but for now women are checked into triage. They get a fetal heart tone before 24 weeks, and a fetal heart rate monitor afterward. “We try to do it before 20 weeks so we don’t have to worry about the fetus,” he said.
When penicillin allergies are in the chart, or women say they are allergic, ask what type of reaction they had in the past. Type 1 reactions should be confirmed with testing. It’s okay to skip testing and give beta-lactams for non–type 1 reactions, but “if a woman has a non–type 1, and they’re already set up for testing, I’m going to do it anyway because getting the penicillin allergy off her chart is good for her and her life,” Dr. Heine said.
Dr. Heine and Dr. Harris reported having no financial disclosures.
AT IDSOG
Key clinical point:
Major finding: Among 81 women with documented penicillin allergies who received gentamicin and clindamycin, 16% developed surgical site infections. In contrast, among the 864 women who received cefazolin, the infection rate was 7%.
Data source: A single-center review at Duke University.
Disclosures: The investigators reported having no relevant financial disclosures.
Standardized infection ratio for CLABSI almost halved since 2009
The standardized infection ratio (SIR) for central line–associated bloodstream infections dropped 42% from 2009 to 2014, according to the Agency for Healthcare Research and Quality.
For acute care hospitalizations, the SIR for central line–associated bloodstream infections (CLABSIs) fell from 0.854 in 2009 to 0.495 in 2014. Over that same time period, the SIR for surgical site infections involving Surgical Care Improvement Project procedures decreased from 0.981 to 0.827 – almost 16%, the AHRQ said in its annual National Healthcare Quality and Disparities Report.
From 2010 to 2014, the SIR for catheter-associated urinary tract infections increased 6.7% from 0.937 to 1.000, but that change was not significant. For laboratory-identified hospital-onset Clostridium difficile infection, the SIR dropped from 0.963 to 0.924 – about 4% – from 2012 to 2014, the AHRQ reported using data from the National Center for Emerging and Zoonotic Infectious Diseases and the National Healthcare Safety Network.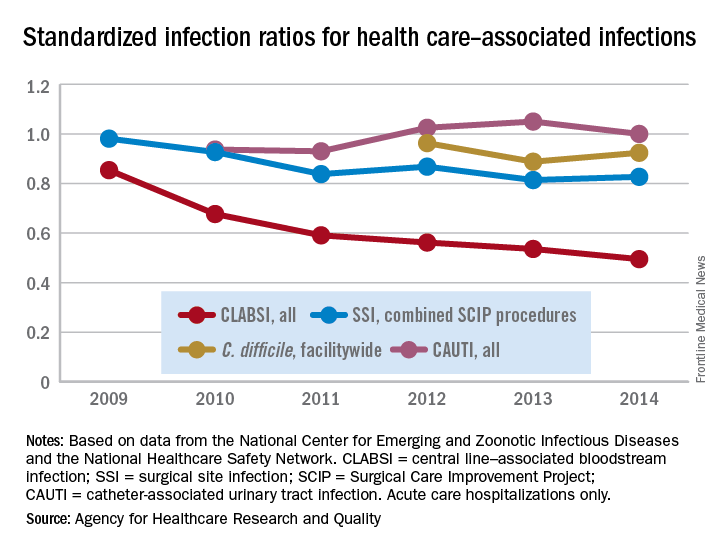
The standardized infection ratio (SIR) for central line–associated bloodstream infections dropped 42% from 2009 to 2014, according to the Agency for Healthcare Research and Quality.
For acute care hospitalizations, the SIR for central line–associated bloodstream infections (CLABSIs) fell from 0.854 in 2009 to 0.495 in 2014. Over that same time period, the SIR for surgical site infections involving Surgical Care Improvement Project procedures decreased from 0.981 to 0.827 – almost 16%, the AHRQ said in its annual National Healthcare Quality and Disparities Report.
From 2010 to 2014, the SIR for catheter-associated urinary tract infections increased 6.7% from 0.937 to 1.000, but that change was not significant. For laboratory-identified hospital-onset Clostridium difficile infection, the SIR dropped from 0.963 to 0.924 – about 4% – from 2012 to 2014, the AHRQ reported using data from the National Center for Emerging and Zoonotic Infectious Diseases and the National Healthcare Safety Network.
The standardized infection ratio (SIR) for central line–associated bloodstream infections dropped 42% from 2009 to 2014, according to the Agency for Healthcare Research and Quality.
For acute care hospitalizations, the SIR for central line–associated bloodstream infections (CLABSIs) fell from 0.854 in 2009 to 0.495 in 2014. Over that same time period, the SIR for surgical site infections involving Surgical Care Improvement Project procedures decreased from 0.981 to 0.827 – almost 16%, the AHRQ said in its annual National Healthcare Quality and Disparities Report.
From 2010 to 2014, the SIR for catheter-associated urinary tract infections increased 6.7% from 0.937 to 1.000, but that change was not significant. For laboratory-identified hospital-onset Clostridium difficile infection, the SIR dropped from 0.963 to 0.924 – about 4% – from 2012 to 2014, the AHRQ reported using data from the National Center for Emerging and Zoonotic Infectious Diseases and the National Healthcare Safety Network.
Unexplained leukocytosis in a hospitalized patient
A 70-year-old man is evaluated for a persistent leukocytosis. He was hospitalized 10 days ago for a severe exacerbation of chronic obstructive pulmonary disease. He was intubated for 3 days, was diagnosed with a left lower lobe pneumonia, and was treated with antibiotics. His white blood cell count on admission was 20,000 per mcL. It dropped as low as 15,000 on day 6 but is now 25,000, with 23,000 polymorphonuclear leukocytes (10% band forms). He is on oral prednisone 15 mg once daily. Chest x-ray shows no infiltrate. Urinalysis without WBCs.
What is the most likely cause of his leukocytosis?
A) Pulmonary embolus.
B) Lung abscess.
C) Perinephric abscess.
D) Prednisone.
E) Clostridium difficile infection.
The most likely diagnosis in otherwise unexplained leukocytosis in a hospitalized patient is C. difficile.
Anna Wanahita, MD, of the St. John Clinic in Tulsa, Okla., and her colleagues prospectively studied 60 patients admitted to a VA hospital who had unexplained leukocytosis.1 All patients had stool specimens sent for C. difficile toxin; in addition, 26 hospitalized control patients without leukocytosis also had stool sent for C. difficile toxin. For study purposes, leukocytosis was defined as a WBC greater than 15,000 per mcL. Any patient for whom C. difficile toxin was sent because of clinical suspicion and who was positive was excluded from the study results.
Almost 60% of the patients with unexplained leukocytosis (35 of 60) had a positive C. difficile toxin, compared with 12% of the controls (P less than .001). More than half of the patients with a positive C. difficile test had the onset of leukocytosis prior to any symptoms of colitis. Leukocytosis responded to treatment with metronidazole in 83% of the patients with a positive C. difficile toxin, and 75% of the patients who had leukocytosis did not have a positive C. difficile toxin.
In another study, Mamatha Bulusu, and colleagues did a retrospective study of 70 hospitalized patients who had diarrhea and underwent testing for C. difficile.2 They evaluated the pattern of white blood cell counts in patients who were positive and negative for C. difficile toxin. The mean WBC for C. difficile–positive patients was 15,800, compared with 7,700 for the patients who were C. difficile negative (P less than .01). They described three patterns: one in which leukocytosis occurred at the onset of diarrhea; a pattern in which unexplained leukocytosis occurred days prior to diarrhea; and a pattern in which patients treated for infection with leukocytosis had a worsening of their leukocytosis at the onset of diarrheal symptoms. Treatment with metronidazole led to a resolution of leukocytosis in all the C. difficile–positive patients.
Another possibility in this case was WBC elevation because of the patient’s prednisone. Prednisone can increase WBC as early as the first day of therapy.3 The elevation and rapidity of increase are dose related. The important pearl is that steroid-induced leukocytosis involves an increase of polymorphonuclear white blood cells with a rise in monocytes and a decrease in eosinophils and lymphocytes.
Pearl: Think of underlying C. difficile infection in your hospitalized patient with unexplained leukocytosis.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Am J Med. 2003 Nov;115(7):543-6.
2. Am J Gastroenterol. 2000 Nov;95(11):3137-41.
3. J Clin Invest. 1975 Oct;56(4):808-13.
4. Am J Med. 1981 Nov;71(5):773-8.
A 70-year-old man is evaluated for a persistent leukocytosis. He was hospitalized 10 days ago for a severe exacerbation of chronic obstructive pulmonary disease. He was intubated for 3 days, was diagnosed with a left lower lobe pneumonia, and was treated with antibiotics. His white blood cell count on admission was 20,000 per mcL. It dropped as low as 15,000 on day 6 but is now 25,000, with 23,000 polymorphonuclear leukocytes (10% band forms). He is on oral prednisone 15 mg once daily. Chest x-ray shows no infiltrate. Urinalysis without WBCs.
What is the most likely cause of his leukocytosis?
A) Pulmonary embolus.
B) Lung abscess.
C) Perinephric abscess.
D) Prednisone.
E) Clostridium difficile infection.
The most likely diagnosis in otherwise unexplained leukocytosis in a hospitalized patient is C. difficile.
Anna Wanahita, MD, of the St. John Clinic in Tulsa, Okla., and her colleagues prospectively studied 60 patients admitted to a VA hospital who had unexplained leukocytosis.1 All patients had stool specimens sent for C. difficile toxin; in addition, 26 hospitalized control patients without leukocytosis also had stool sent for C. difficile toxin. For study purposes, leukocytosis was defined as a WBC greater than 15,000 per mcL. Any patient for whom C. difficile toxin was sent because of clinical suspicion and who was positive was excluded from the study results.
Almost 60% of the patients with unexplained leukocytosis (35 of 60) had a positive C. difficile toxin, compared with 12% of the controls (P less than .001). More than half of the patients with a positive C. difficile test had the onset of leukocytosis prior to any symptoms of colitis. Leukocytosis responded to treatment with metronidazole in 83% of the patients with a positive C. difficile toxin, and 75% of the patients who had leukocytosis did not have a positive C. difficile toxin.
In another study, Mamatha Bulusu, and colleagues did a retrospective study of 70 hospitalized patients who had diarrhea and underwent testing for C. difficile.2 They evaluated the pattern of white blood cell counts in patients who were positive and negative for C. difficile toxin. The mean WBC for C. difficile–positive patients was 15,800, compared with 7,700 for the patients who were C. difficile negative (P less than .01). They described three patterns: one in which leukocytosis occurred at the onset of diarrhea; a pattern in which unexplained leukocytosis occurred days prior to diarrhea; and a pattern in which patients treated for infection with leukocytosis had a worsening of their leukocytosis at the onset of diarrheal symptoms. Treatment with metronidazole led to a resolution of leukocytosis in all the C. difficile–positive patients.
Another possibility in this case was WBC elevation because of the patient’s prednisone. Prednisone can increase WBC as early as the first day of therapy.3 The elevation and rapidity of increase are dose related. The important pearl is that steroid-induced leukocytosis involves an increase of polymorphonuclear white blood cells with a rise in monocytes and a decrease in eosinophils and lymphocytes.
Pearl: Think of underlying C. difficile infection in your hospitalized patient with unexplained leukocytosis.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Am J Med. 2003 Nov;115(7):543-6.
2. Am J Gastroenterol. 2000 Nov;95(11):3137-41.
3. J Clin Invest. 1975 Oct;56(4):808-13.
4. Am J Med. 1981 Nov;71(5):773-8.
A 70-year-old man is evaluated for a persistent leukocytosis. He was hospitalized 10 days ago for a severe exacerbation of chronic obstructive pulmonary disease. He was intubated for 3 days, was diagnosed with a left lower lobe pneumonia, and was treated with antibiotics. His white blood cell count on admission was 20,000 per mcL. It dropped as low as 15,000 on day 6 but is now 25,000, with 23,000 polymorphonuclear leukocytes (10% band forms). He is on oral prednisone 15 mg once daily. Chest x-ray shows no infiltrate. Urinalysis without WBCs.
What is the most likely cause of his leukocytosis?
A) Pulmonary embolus.
B) Lung abscess.
C) Perinephric abscess.
D) Prednisone.
E) Clostridium difficile infection.
The most likely diagnosis in otherwise unexplained leukocytosis in a hospitalized patient is C. difficile.
Anna Wanahita, MD, of the St. John Clinic in Tulsa, Okla., and her colleagues prospectively studied 60 patients admitted to a VA hospital who had unexplained leukocytosis.1 All patients had stool specimens sent for C. difficile toxin; in addition, 26 hospitalized control patients without leukocytosis also had stool sent for C. difficile toxin. For study purposes, leukocytosis was defined as a WBC greater than 15,000 per mcL. Any patient for whom C. difficile toxin was sent because of clinical suspicion and who was positive was excluded from the study results.
Almost 60% of the patients with unexplained leukocytosis (35 of 60) had a positive C. difficile toxin, compared with 12% of the controls (P less than .001). More than half of the patients with a positive C. difficile test had the onset of leukocytosis prior to any symptoms of colitis. Leukocytosis responded to treatment with metronidazole in 83% of the patients with a positive C. difficile toxin, and 75% of the patients who had leukocytosis did not have a positive C. difficile toxin.
In another study, Mamatha Bulusu, and colleagues did a retrospective study of 70 hospitalized patients who had diarrhea and underwent testing for C. difficile.2 They evaluated the pattern of white blood cell counts in patients who were positive and negative for C. difficile toxin. The mean WBC for C. difficile–positive patients was 15,800, compared with 7,700 for the patients who were C. difficile negative (P less than .01). They described three patterns: one in which leukocytosis occurred at the onset of diarrhea; a pattern in which unexplained leukocytosis occurred days prior to diarrhea; and a pattern in which patients treated for infection with leukocytosis had a worsening of their leukocytosis at the onset of diarrheal symptoms. Treatment with metronidazole led to a resolution of leukocytosis in all the C. difficile–positive patients.
Another possibility in this case was WBC elevation because of the patient’s prednisone. Prednisone can increase WBC as early as the first day of therapy.3 The elevation and rapidity of increase are dose related. The important pearl is that steroid-induced leukocytosis involves an increase of polymorphonuclear white blood cells with a rise in monocytes and a decrease in eosinophils and lymphocytes.
Pearl: Think of underlying C. difficile infection in your hospitalized patient with unexplained leukocytosis.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Am J Med. 2003 Nov;115(7):543-6.
2. Am J Gastroenterol. 2000 Nov;95(11):3137-41.
3. J Clin Invest. 1975 Oct;56(4):808-13.
4. Am J Med. 1981 Nov;71(5):773-8.
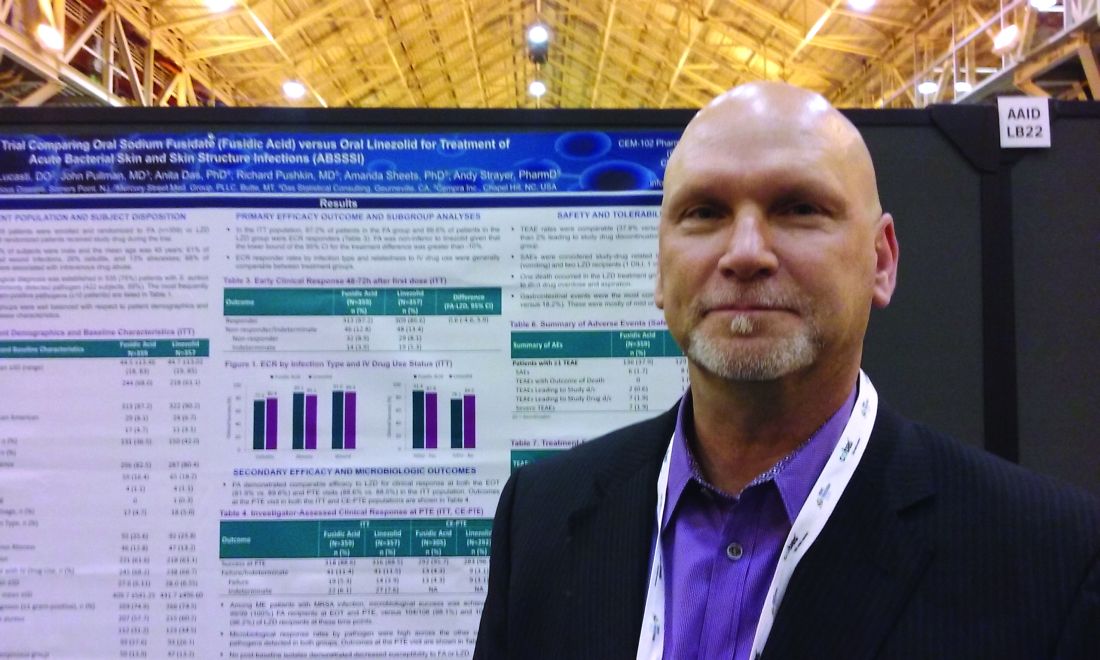
Sodium fusidate noninferior to linezolid for acute skin infections
NEW ORLEANS – An oral antibiotic in development in the United States, fusidic acid (oral formulation, sodium fusidate) was noninferior to linezolid based on early clinical response in a randomized, double-blind, multicenter trial of 716 people with acute bacterial skin and skin structure infections (ABSSSI), including cellulitis, wound infection, and major cutaneous abscesses.
Early clinical response was defined as a 20% or greater reduction from baseline in the surface area of redness, edema, or induration at 48-72 hours after starting treatment with the study drugs. In an intent-to-treat analysis, 87.2% of patients randomized to fusidic acid and 86.6% of the linezolid group met this primary endpoint of the phase 3 study.
“Fusidic acid showed similar efficacy and comparable safety” that persisted through treatment, said Andy Strayer, PharmD, vice president of clinical programs at Cempra Pharmaceuticals, which is developing sodium fusidate as an oral agent to treat ABSSSI patients in the United States. Leo Pharmaceuticals has marketed sodium fusidate outside the United States in various formulations for decades.
Fusidic acid has potent activity against gram-positive aerobic organisms, including methicillin-resistant Staphylococcus aureus (MRSA). “Strikingly, fusidic acid showed 100% success in patients with MRSA in the microbiologically evaluable population at the end of treatment and posttherapy evaluation time points,” Dr. Strayer said at the annual meeting of the American Society for Microbiology. “Fusidic acid may offer an important oral therapy alternative for MRSA infection.”
“Fusidic acid, a drug long used in other parts of the world, has been demonstrated in this first phase 3 trial, to be a potential new option for the treatment of MRSA skin and skin structure infections in the U.S.,” said Carrie Cardenas, MD, lead study author and a principal investigator at eStudySite, San Diego, and an internist in private practice in La Mesa, California.
There was a microbiological diagnosis established in 75% of patients. S. aureus was the most commonly detected pathogen (422 patients; 59%), and the study included 235 patients diagnosed with MRSA infection.
About two-thirds, 65%, of participants were men. Mean age was 45 years. Infections were classified as wounds in 61%, cellulitis in 26%, and abscess in 13%. Notably, 68% of the recruited participants had ABSSSI associated with intravenous drug use, a “sometimes overlooked consequence of the ongoing epidemic of IV drug use in the U.S.,” Dr. Strayer said.
In terms of safety, treatment-emergent adverse event rates were comparable between the two groups (37.9% with fusidic acid versus 36.1% with linezolid). Gastrointestinal events were the most common adverse events, 22.8% versus 18.2%, respectively.
“Considering complicated skin infections are one of the most rapidly growing reasons for hospitalizations and emergency department visits each year, we anticipate that fusidic acid, if approved, may help clinicians decrease the length of inpatient stay or avoid hospitalization altogether,” Dr. Strayer said.
Cempra sponsored the study. Dr. Strayer is a Cempra employee and shareholder. Dr. Carrie Cardenas is a principal investigator at eStudySite, San Diego, and performs research for Cempra, Paratek, Debiopharm, Motif, Durata, MicuRx, Bristol-Myers Squibb, and Bayer.
NEW ORLEANS – An oral antibiotic in development in the United States, fusidic acid (oral formulation, sodium fusidate) was noninferior to linezolid based on early clinical response in a randomized, double-blind, multicenter trial of 716 people with acute bacterial skin and skin structure infections (ABSSSI), including cellulitis, wound infection, and major cutaneous abscesses.
Early clinical response was defined as a 20% or greater reduction from baseline in the surface area of redness, edema, or induration at 48-72 hours after starting treatment with the study drugs. In an intent-to-treat analysis, 87.2% of patients randomized to fusidic acid and 86.6% of the linezolid group met this primary endpoint of the phase 3 study.
“Fusidic acid showed similar efficacy and comparable safety” that persisted through treatment, said Andy Strayer, PharmD, vice president of clinical programs at Cempra Pharmaceuticals, which is developing sodium fusidate as an oral agent to treat ABSSSI patients in the United States. Leo Pharmaceuticals has marketed sodium fusidate outside the United States in various formulations for decades.
Fusidic acid has potent activity against gram-positive aerobic organisms, including methicillin-resistant Staphylococcus aureus (MRSA). “Strikingly, fusidic acid showed 100% success in patients with MRSA in the microbiologically evaluable population at the end of treatment and posttherapy evaluation time points,” Dr. Strayer said at the annual meeting of the American Society for Microbiology. “Fusidic acid may offer an important oral therapy alternative for MRSA infection.”
“Fusidic acid, a drug long used in other parts of the world, has been demonstrated in this first phase 3 trial, to be a potential new option for the treatment of MRSA skin and skin structure infections in the U.S.,” said Carrie Cardenas, MD, lead study author and a principal investigator at eStudySite, San Diego, and an internist in private practice in La Mesa, California.
There was a microbiological diagnosis established in 75% of patients. S. aureus was the most commonly detected pathogen (422 patients; 59%), and the study included 235 patients diagnosed with MRSA infection.
About two-thirds, 65%, of participants were men. Mean age was 45 years. Infections were classified as wounds in 61%, cellulitis in 26%, and abscess in 13%. Notably, 68% of the recruited participants had ABSSSI associated with intravenous drug use, a “sometimes overlooked consequence of the ongoing epidemic of IV drug use in the U.S.,” Dr. Strayer said.
In terms of safety, treatment-emergent adverse event rates were comparable between the two groups (37.9% with fusidic acid versus 36.1% with linezolid). Gastrointestinal events were the most common adverse events, 22.8% versus 18.2%, respectively.
“Considering complicated skin infections are one of the most rapidly growing reasons for hospitalizations and emergency department visits each year, we anticipate that fusidic acid, if approved, may help clinicians decrease the length of inpatient stay or avoid hospitalization altogether,” Dr. Strayer said.
Cempra sponsored the study. Dr. Strayer is a Cempra employee and shareholder. Dr. Carrie Cardenas is a principal investigator at eStudySite, San Diego, and performs research for Cempra, Paratek, Debiopharm, Motif, Durata, MicuRx, Bristol-Myers Squibb, and Bayer.
NEW ORLEANS – An oral antibiotic in development in the United States, fusidic acid (oral formulation, sodium fusidate) was noninferior to linezolid based on early clinical response in a randomized, double-blind, multicenter trial of 716 people with acute bacterial skin and skin structure infections (ABSSSI), including cellulitis, wound infection, and major cutaneous abscesses.
Early clinical response was defined as a 20% or greater reduction from baseline in the surface area of redness, edema, or induration at 48-72 hours after starting treatment with the study drugs. In an intent-to-treat analysis, 87.2% of patients randomized to fusidic acid and 86.6% of the linezolid group met this primary endpoint of the phase 3 study.
“Fusidic acid showed similar efficacy and comparable safety” that persisted through treatment, said Andy Strayer, PharmD, vice president of clinical programs at Cempra Pharmaceuticals, which is developing sodium fusidate as an oral agent to treat ABSSSI patients in the United States. Leo Pharmaceuticals has marketed sodium fusidate outside the United States in various formulations for decades.
Fusidic acid has potent activity against gram-positive aerobic organisms, including methicillin-resistant Staphylococcus aureus (MRSA). “Strikingly, fusidic acid showed 100% success in patients with MRSA in the microbiologically evaluable population at the end of treatment and posttherapy evaluation time points,” Dr. Strayer said at the annual meeting of the American Society for Microbiology. “Fusidic acid may offer an important oral therapy alternative for MRSA infection.”
“Fusidic acid, a drug long used in other parts of the world, has been demonstrated in this first phase 3 trial, to be a potential new option for the treatment of MRSA skin and skin structure infections in the U.S.,” said Carrie Cardenas, MD, lead study author and a principal investigator at eStudySite, San Diego, and an internist in private practice in La Mesa, California.
There was a microbiological diagnosis established in 75% of patients. S. aureus was the most commonly detected pathogen (422 patients; 59%), and the study included 235 patients diagnosed with MRSA infection.
About two-thirds, 65%, of participants were men. Mean age was 45 years. Infections were classified as wounds in 61%, cellulitis in 26%, and abscess in 13%. Notably, 68% of the recruited participants had ABSSSI associated with intravenous drug use, a “sometimes overlooked consequence of the ongoing epidemic of IV drug use in the U.S.,” Dr. Strayer said.
In terms of safety, treatment-emergent adverse event rates were comparable between the two groups (37.9% with fusidic acid versus 36.1% with linezolid). Gastrointestinal events were the most common adverse events, 22.8% versus 18.2%, respectively.
“Considering complicated skin infections are one of the most rapidly growing reasons for hospitalizations and emergency department visits each year, we anticipate that fusidic acid, if approved, may help clinicians decrease the length of inpatient stay or avoid hospitalization altogether,” Dr. Strayer said.
Cempra sponsored the study. Dr. Strayer is a Cempra employee and shareholder. Dr. Carrie Cardenas is a principal investigator at eStudySite, San Diego, and performs research for Cempra, Paratek, Debiopharm, Motif, Durata, MicuRx, Bristol-Myers Squibb, and Bayer.
AT ASM MICROBE 2017
Key clinical point: Sodium fusidate, active as fusidic acid, showed noninferiority to linezolid for early clinical response in ABSSI patients.
Major finding: 87.2% of patients given sodium fusidate and 86.6% of those receiving linezolid achieved an early clinical response.
Data source: Randomized, controlled, double-blind, phase 3 study with 716 participants.
Disclosures: Cempra sponsored the study. Dr. Carrier Cardenas is a researcher for Cempra, Paratek, Debiopharm, Motif, Durata, MicuRx, Bristol-Myers Squibb, and Bayer. Dr. Strayer is a Cempra employee and shareholder.
VA cohort study: Individualize SSI prophylaxis based on patient factors
The combined use of vancomycin and a beta-lactam antibiotic for prophylaxis against surgical site infections is associated with both benefits and harms, according to findings from a national propensity-score–adjusted retrospective cohort study.
For example, the combination treatment reduced surgical site infections (SSIs) 30 days after cardiac surgical procedures but increased the risk of postoperative acute kidney injury (AKI) in some patients, Westyn Branch-Elliman, MD, of the VA Boston Healthcare System and her colleagues reported online July 10 in PLOS Medicine.
Among cardiac surgery patients, the incidence of surgical site infections was significantly lower for the 6,953 patients treated with both drugs vs. the 12,834 treated with a single agent (0.95% vs. 1.48%), the investigators found (PLOS Med. 2017 Jul 10. doi: 10.1371/journal.pmed.1002340).
SSI benefit with combination therapy
“After controlling for age, diabetes, ASA [American Society of Anesthesiologists] score, mupirocin administration, current smoking status, and preoperative MRSA [methicillin-resistant Staphylococcus aureus] colonization status, receipt of combination antimicrobial prophylaxis was associated with reduced SSI risk following cardiac surgical procedures (adjusted risk ratio, 0.61),” they wrote, noting that, when combination therapy was compared with either of the agents alone, the associations were similar and that no association between SSI reduction and the combination regimen was seen for the other types of surgical procedures assessed.
Secondary analyses showed that, among the cardiac patients, differences in the rates of SSIs were seen based on MRSA status in patients undergoing cardiac surgery. Among MRSA-colonized patients, SSIs occurred in 8 of 346 patients (2.3%) who received combination prophylaxis vs. 4 of 100 patients (4%) who received vancomycin alone (aRR, 0.53), and, among MRSA-negative and MRSA-unknown cardiac surgery patients, SSIs occurred in 58 of 6,607 patients (0.88%) receiving combination prophylaxis and 146 of 10,215 patients (1.4%) receiving a beta-lactam alone (aRR, 0.60).
“Among MRSA-colonized patients undergoing cardiac surgery, the associated absolute risk reduction for SSI was approximately triple that of the absolute risk reduction in MRSA-negative or -unknown patients, with a [number needed to treat] to prevent 1 SSI of 53 for the MRSA-colonized group, compared with 176 for the MRSA-negative or -unknown groups,” they wrote.
The incidence of Clostridium difficile infection was similar in both exposure groups (0.72% and 0.81% with combination and single agent prophylaxis, respectively).
Higher AKI risk with combination therapy
“In contrast, combination versus single prophylaxis was associated with higher relative risk of AKI in the 7-day postoperative period after adjusting for prophylaxis regimen duration, age, diabetes, ASA score, and smoking,” they said.
The rate of AKI was 23.75% among patients receiving combination prophylaxis, compared with 20.79% and 13.93% among those receiving vancomycin alone and a beta-lactam alone, respectively.
Significant associations between absolute risk of AKI and receipt of combination regimens were seen across all types of procedures, the investigators said.
“Overall, the NNH [number needed to harm] to cause one episode of AKI in cardiac surgery patients receiving combination therapy was 22, and, for stage 3 AKI, 167. The NNH associated with one additional episode of any postoperative AKI after receipt of combination therapy was 76 following orthopedic procedures and 25 following vascular surgical procedures,” they said.
The optimal approach for preventing SSIs is unclear. Although the multidisciplinary Clinical Practice Guidelines for Antimicrobial Prophylaxis in Surgery recommend single agent prophylaxis most often, with a beta-lactam antibiotic, for most surgical procedures, the use of vancomycin alone is a consideration in MRSA-colonized patients and in centers with a high MRSA incidence, and combination prophylaxis with a beta-lactam plus vancomycin is increasing. However, the relative risks and benefit of this strategy have not been carefully studied, the investigators said.
Thus, the investigators used a propensity-adjusted, log-binomial regression model stratified by type of surgical procedure among the cases identified in the Veterans Affairs cohort to assess the association between SSIs and receipt of combination prophylaxis versus single agent prophylaxis.
Though limited by the observational study design and by factors such as a predominantly male and slightly older and more rural population, the findings suggest that “clinicians may need to individualize prophylaxis strategy based on patient-specific factors that influence the risk-versus-benefit equation,” they said, concluding that “future studies are needed to evaluate the utility of MRSA screening protocols for optimizing and individualizing surgical prophylaxis regimen.”
This study was funded by Veterans Affairs Health Services Research and Development. Dr. Branch-Elliman reported having no disclosures. One other author, Eli Perencevich, MD, received an investigator initiated Grant from Merck Pharmaceuticals in 2013.
The combined use of vancomycin and a beta-lactam antibiotic for prophylaxis against surgical site infections is associated with both benefits and harms, according to findings from a national propensity-score–adjusted retrospective cohort study.
For example, the combination treatment reduced surgical site infections (SSIs) 30 days after cardiac surgical procedures but increased the risk of postoperative acute kidney injury (AKI) in some patients, Westyn Branch-Elliman, MD, of the VA Boston Healthcare System and her colleagues reported online July 10 in PLOS Medicine.
Among cardiac surgery patients, the incidence of surgical site infections was significantly lower for the 6,953 patients treated with both drugs vs. the 12,834 treated with a single agent (0.95% vs. 1.48%), the investigators found (PLOS Med. 2017 Jul 10. doi: 10.1371/journal.pmed.1002340).
SSI benefit with combination therapy
“After controlling for age, diabetes, ASA [American Society of Anesthesiologists] score, mupirocin administration, current smoking status, and preoperative MRSA [methicillin-resistant Staphylococcus aureus] colonization status, receipt of combination antimicrobial prophylaxis was associated with reduced SSI risk following cardiac surgical procedures (adjusted risk ratio, 0.61),” they wrote, noting that, when combination therapy was compared with either of the agents alone, the associations were similar and that no association between SSI reduction and the combination regimen was seen for the other types of surgical procedures assessed.
Secondary analyses showed that, among the cardiac patients, differences in the rates of SSIs were seen based on MRSA status in patients undergoing cardiac surgery. Among MRSA-colonized patients, SSIs occurred in 8 of 346 patients (2.3%) who received combination prophylaxis vs. 4 of 100 patients (4%) who received vancomycin alone (aRR, 0.53), and, among MRSA-negative and MRSA-unknown cardiac surgery patients, SSIs occurred in 58 of 6,607 patients (0.88%) receiving combination prophylaxis and 146 of 10,215 patients (1.4%) receiving a beta-lactam alone (aRR, 0.60).
“Among MRSA-colonized patients undergoing cardiac surgery, the associated absolute risk reduction for SSI was approximately triple that of the absolute risk reduction in MRSA-negative or -unknown patients, with a [number needed to treat] to prevent 1 SSI of 53 for the MRSA-colonized group, compared with 176 for the MRSA-negative or -unknown groups,” they wrote.
The incidence of Clostridium difficile infection was similar in both exposure groups (0.72% and 0.81% with combination and single agent prophylaxis, respectively).
Higher AKI risk with combination therapy
“In contrast, combination versus single prophylaxis was associated with higher relative risk of AKI in the 7-day postoperative period after adjusting for prophylaxis regimen duration, age, diabetes, ASA score, and smoking,” they said.
The rate of AKI was 23.75% among patients receiving combination prophylaxis, compared with 20.79% and 13.93% among those receiving vancomycin alone and a beta-lactam alone, respectively.
Significant associations between absolute risk of AKI and receipt of combination regimens were seen across all types of procedures, the investigators said.
“Overall, the NNH [number needed to harm] to cause one episode of AKI in cardiac surgery patients receiving combination therapy was 22, and, for stage 3 AKI, 167. The NNH associated with one additional episode of any postoperative AKI after receipt of combination therapy was 76 following orthopedic procedures and 25 following vascular surgical procedures,” they said.
The optimal approach for preventing SSIs is unclear. Although the multidisciplinary Clinical Practice Guidelines for Antimicrobial Prophylaxis in Surgery recommend single agent prophylaxis most often, with a beta-lactam antibiotic, for most surgical procedures, the use of vancomycin alone is a consideration in MRSA-colonized patients and in centers with a high MRSA incidence, and combination prophylaxis with a beta-lactam plus vancomycin is increasing. However, the relative risks and benefit of this strategy have not been carefully studied, the investigators said.
Thus, the investigators used a propensity-adjusted, log-binomial regression model stratified by type of surgical procedure among the cases identified in the Veterans Affairs cohort to assess the association between SSIs and receipt of combination prophylaxis versus single agent prophylaxis.
Though limited by the observational study design and by factors such as a predominantly male and slightly older and more rural population, the findings suggest that “clinicians may need to individualize prophylaxis strategy based on patient-specific factors that influence the risk-versus-benefit equation,” they said, concluding that “future studies are needed to evaluate the utility of MRSA screening protocols for optimizing and individualizing surgical prophylaxis regimen.”
This study was funded by Veterans Affairs Health Services Research and Development. Dr. Branch-Elliman reported having no disclosures. One other author, Eli Perencevich, MD, received an investigator initiated Grant from Merck Pharmaceuticals in 2013.
The combined use of vancomycin and a beta-lactam antibiotic for prophylaxis against surgical site infections is associated with both benefits and harms, according to findings from a national propensity-score–adjusted retrospective cohort study.
For example, the combination treatment reduced surgical site infections (SSIs) 30 days after cardiac surgical procedures but increased the risk of postoperative acute kidney injury (AKI) in some patients, Westyn Branch-Elliman, MD, of the VA Boston Healthcare System and her colleagues reported online July 10 in PLOS Medicine.
Among cardiac surgery patients, the incidence of surgical site infections was significantly lower for the 6,953 patients treated with both drugs vs. the 12,834 treated with a single agent (0.95% vs. 1.48%), the investigators found (PLOS Med. 2017 Jul 10. doi: 10.1371/journal.pmed.1002340).
SSI benefit with combination therapy
“After controlling for age, diabetes, ASA [American Society of Anesthesiologists] score, mupirocin administration, current smoking status, and preoperative MRSA [methicillin-resistant Staphylococcus aureus] colonization status, receipt of combination antimicrobial prophylaxis was associated with reduced SSI risk following cardiac surgical procedures (adjusted risk ratio, 0.61),” they wrote, noting that, when combination therapy was compared with either of the agents alone, the associations were similar and that no association between SSI reduction and the combination regimen was seen for the other types of surgical procedures assessed.
Secondary analyses showed that, among the cardiac patients, differences in the rates of SSIs were seen based on MRSA status in patients undergoing cardiac surgery. Among MRSA-colonized patients, SSIs occurred in 8 of 346 patients (2.3%) who received combination prophylaxis vs. 4 of 100 patients (4%) who received vancomycin alone (aRR, 0.53), and, among MRSA-negative and MRSA-unknown cardiac surgery patients, SSIs occurred in 58 of 6,607 patients (0.88%) receiving combination prophylaxis and 146 of 10,215 patients (1.4%) receiving a beta-lactam alone (aRR, 0.60).
“Among MRSA-colonized patients undergoing cardiac surgery, the associated absolute risk reduction for SSI was approximately triple that of the absolute risk reduction in MRSA-negative or -unknown patients, with a [number needed to treat] to prevent 1 SSI of 53 for the MRSA-colonized group, compared with 176 for the MRSA-negative or -unknown groups,” they wrote.
The incidence of Clostridium difficile infection was similar in both exposure groups (0.72% and 0.81% with combination and single agent prophylaxis, respectively).
Higher AKI risk with combination therapy
“In contrast, combination versus single prophylaxis was associated with higher relative risk of AKI in the 7-day postoperative period after adjusting for prophylaxis regimen duration, age, diabetes, ASA score, and smoking,” they said.
The rate of AKI was 23.75% among patients receiving combination prophylaxis, compared with 20.79% and 13.93% among those receiving vancomycin alone and a beta-lactam alone, respectively.
Significant associations between absolute risk of AKI and receipt of combination regimens were seen across all types of procedures, the investigators said.
“Overall, the NNH [number needed to harm] to cause one episode of AKI in cardiac surgery patients receiving combination therapy was 22, and, for stage 3 AKI, 167. The NNH associated with one additional episode of any postoperative AKI after receipt of combination therapy was 76 following orthopedic procedures and 25 following vascular surgical procedures,” they said.
The optimal approach for preventing SSIs is unclear. Although the multidisciplinary Clinical Practice Guidelines for Antimicrobial Prophylaxis in Surgery recommend single agent prophylaxis most often, with a beta-lactam antibiotic, for most surgical procedures, the use of vancomycin alone is a consideration in MRSA-colonized patients and in centers with a high MRSA incidence, and combination prophylaxis with a beta-lactam plus vancomycin is increasing. However, the relative risks and benefit of this strategy have not been carefully studied, the investigators said.
Thus, the investigators used a propensity-adjusted, log-binomial regression model stratified by type of surgical procedure among the cases identified in the Veterans Affairs cohort to assess the association between SSIs and receipt of combination prophylaxis versus single agent prophylaxis.
Though limited by the observational study design and by factors such as a predominantly male and slightly older and more rural population, the findings suggest that “clinicians may need to individualize prophylaxis strategy based on patient-specific factors that influence the risk-versus-benefit equation,” they said, concluding that “future studies are needed to evaluate the utility of MRSA screening protocols for optimizing and individualizing surgical prophylaxis regimen.”
This study was funded by Veterans Affairs Health Services Research and Development. Dr. Branch-Elliman reported having no disclosures. One other author, Eli Perencevich, MD, received an investigator initiated Grant from Merck Pharmaceuticals in 2013.
FROM PLOS MEDICINE
Key clinical point:
Major finding: The SSI incidence was 0.95% vs. 1.48% with combination vs. single agent–therapy in cardiac surgery patients. Acute kidney injuries occurred in 23.75% of all surgery patients receiving combination prophylaxis, compared with 20.79% and 13.93% with vancomycin or a beta-lactam, respectively.
Data source: A retrospective cohort study of more than 70,000 surgical procedures.
Disclosures: This study was funded by Veterans Affairs Health Services Research and Development. Dr. Branch-Elliman reported having no disclosures. One other author, Eli Perencevich, MD, received an investigator initiated grant from Merck Pharmaceuticals in 2013.
Multiple factors predict surgical site infection risk after lower extremity revascularization
Patient, operative, and hospital factors were found to be significant predictors of the risk of surgical site infection in patients who underwent open lower extremity bypass procedures, according to the results of a retrospective data analysis.
The study assessed the outcomes of 3,033 patients who underwent elective or urgent open LEB procedures between January 2012 and June 2015 using data from the Blue Cross Blue Shield of Michigan Cardiovascular Consortium Vascular Intervention Collaborative (BMC2 VIC), a statewide cardiovascular consortium of 35 hospitals, according to Frank M. Davis, MD, and his colleagues at the University of Michigan, Ann Arbor.
Demographic information, medical history, laboratory test results before and after the procedure, procedural indication, procedural urgency, technical details of procedures, and associated complications were assessed for each patient. Women comprised 31% of patients, the average patient age was 66 years, and 83% of the population was white (J Vasc Surg. 2017 Jun;65[6]:1769-78).
Among all of the patients treated, 320 developed SSIs and 2,713 did not. The procedural indications included one or more of the following: claudication (72%), rest pain (50.5%), ulcer/gangrene (32.4%), or acute limb ischemia (15.1%). Antibiotics were appropriately administered to 97% of the patients, according to the researchers, “demonstrating high compliance across the BMC2 VIC.”
- Patient factors: As indicated by previous studies, obesity (odds ratio, 1.78), dialysis dependence (OR, 4.33), and hypertension (OR, 4.29) conferred a significant increased risk of SSI after LEB, according to Dr. Davis and his colleagues. In addition, however, they found that previous vascular surgery (OR, 1.57), previous percutaneous coronary intervention (OR, 1.47), use of antiplatelet medication (OR, 4.29), and low Peripheral Artery Questionnaire symptom severity (OR, 1.48) were significant independent predictors of SSI.
- Operative factors: Prolonged procedural length (OR, 2.95), iodine-only antiseptic skin preparation (OR, 1.73), and high peak intraoperative glucose (defined as a peak glucose greater than 180 mg/dL; OR, 1.99) were significant independent predictors of SSI. However, concomitant stent placement was found to be significantly predictive (OR, .38), “perhaps due to improvement in regional and subcutaneous vascular flow after the intervention,” the researchers suggested.
- Hospital factors: Larger overall hospital size (OR, 2.22) and major teaching center (OR, 1.66) were associated with increased risk of SSI. “Interestingly, we did not find an association with SSI and the hospital annual volume or the hospital urgent/emergent procedure rate,” the researchers added.
SSIs were not found to be significantly associated with a difference in 30-day mortality. However, they were significantly associated with an increased rate of several postoperative morbidities, including transfusion, lymph leak, major amputation, and open surgical bypass revision at or within 30 days of the index operation, according to Dr. Davis and his colleagues.
“Although some factors, such as patients comorbidities, are not modifiable, others represent areas for quality improvement in at-risk patients,” the researchers indicated. “Diligence should be devoted to decreasing operative length, controlling intraoperative glucose levels, and avoiding iodine-only skin preparation to decrease the rate of SSIs and its numerous associate morbidities in vascular surgery patients.”
In discussing the issue of antiplatelet medication being an indicator of increased risk, the authors pointed out that it was a hitherto unreported factor in the vascular literature, and of concern because, “as expected, a high percentage of patients (78.7%) were taking antiplatelet medication at the time of their LEB.”
Because the association of antiplatelet medication with SSIs was independent of the need for operative transfusion or the need for repeat intervention, the researchers speculated that “all antiplatelet agents have the theoretical potential to diminish activation-dependent platelet immune functions.” They referred to previous studies showing that clopidogrel was associated with significantly higher clinical rates of infection, particularly pneumonia.
Limitations cited for the study were the retrospective nature of the database analysis, the possibility of confounders not assessed in the data, and the fact that outcomes were limited to 30-day events, which would not take into account longer-term graft failure or mortality.
The authors reported having no conflicts of interest with regard to the study.
Patient, operative, and hospital factors were found to be significant predictors of the risk of surgical site infection in patients who underwent open lower extremity bypass procedures, according to the results of a retrospective data analysis.
The study assessed the outcomes of 3,033 patients who underwent elective or urgent open LEB procedures between January 2012 and June 2015 using data from the Blue Cross Blue Shield of Michigan Cardiovascular Consortium Vascular Intervention Collaborative (BMC2 VIC), a statewide cardiovascular consortium of 35 hospitals, according to Frank M. Davis, MD, and his colleagues at the University of Michigan, Ann Arbor.
Demographic information, medical history, laboratory test results before and after the procedure, procedural indication, procedural urgency, technical details of procedures, and associated complications were assessed for each patient. Women comprised 31% of patients, the average patient age was 66 years, and 83% of the population was white (J Vasc Surg. 2017 Jun;65[6]:1769-78).
Among all of the patients treated, 320 developed SSIs and 2,713 did not. The procedural indications included one or more of the following: claudication (72%), rest pain (50.5%), ulcer/gangrene (32.4%), or acute limb ischemia (15.1%). Antibiotics were appropriately administered to 97% of the patients, according to the researchers, “demonstrating high compliance across the BMC2 VIC.”
- Patient factors: As indicated by previous studies, obesity (odds ratio, 1.78), dialysis dependence (OR, 4.33), and hypertension (OR, 4.29) conferred a significant increased risk of SSI after LEB, according to Dr. Davis and his colleagues. In addition, however, they found that previous vascular surgery (OR, 1.57), previous percutaneous coronary intervention (OR, 1.47), use of antiplatelet medication (OR, 4.29), and low Peripheral Artery Questionnaire symptom severity (OR, 1.48) were significant independent predictors of SSI.
- Operative factors: Prolonged procedural length (OR, 2.95), iodine-only antiseptic skin preparation (OR, 1.73), and high peak intraoperative glucose (defined as a peak glucose greater than 180 mg/dL; OR, 1.99) were significant independent predictors of SSI. However, concomitant stent placement was found to be significantly predictive (OR, .38), “perhaps due to improvement in regional and subcutaneous vascular flow after the intervention,” the researchers suggested.
- Hospital factors: Larger overall hospital size (OR, 2.22) and major teaching center (OR, 1.66) were associated with increased risk of SSI. “Interestingly, we did not find an association with SSI and the hospital annual volume or the hospital urgent/emergent procedure rate,” the researchers added.
SSIs were not found to be significantly associated with a difference in 30-day mortality. However, they were significantly associated with an increased rate of several postoperative morbidities, including transfusion, lymph leak, major amputation, and open surgical bypass revision at or within 30 days of the index operation, according to Dr. Davis and his colleagues.
“Although some factors, such as patients comorbidities, are not modifiable, others represent areas for quality improvement in at-risk patients,” the researchers indicated. “Diligence should be devoted to decreasing operative length, controlling intraoperative glucose levels, and avoiding iodine-only skin preparation to decrease the rate of SSIs and its numerous associate morbidities in vascular surgery patients.”
In discussing the issue of antiplatelet medication being an indicator of increased risk, the authors pointed out that it was a hitherto unreported factor in the vascular literature, and of concern because, “as expected, a high percentage of patients (78.7%) were taking antiplatelet medication at the time of their LEB.”
Because the association of antiplatelet medication with SSIs was independent of the need for operative transfusion or the need for repeat intervention, the researchers speculated that “all antiplatelet agents have the theoretical potential to diminish activation-dependent platelet immune functions.” They referred to previous studies showing that clopidogrel was associated with significantly higher clinical rates of infection, particularly pneumonia.
Limitations cited for the study were the retrospective nature of the database analysis, the possibility of confounders not assessed in the data, and the fact that outcomes were limited to 30-day events, which would not take into account longer-term graft failure or mortality.
The authors reported having no conflicts of interest with regard to the study.
Patient, operative, and hospital factors were found to be significant predictors of the risk of surgical site infection in patients who underwent open lower extremity bypass procedures, according to the results of a retrospective data analysis.
The study assessed the outcomes of 3,033 patients who underwent elective or urgent open LEB procedures between January 2012 and June 2015 using data from the Blue Cross Blue Shield of Michigan Cardiovascular Consortium Vascular Intervention Collaborative (BMC2 VIC), a statewide cardiovascular consortium of 35 hospitals, according to Frank M. Davis, MD, and his colleagues at the University of Michigan, Ann Arbor.
Demographic information, medical history, laboratory test results before and after the procedure, procedural indication, procedural urgency, technical details of procedures, and associated complications were assessed for each patient. Women comprised 31% of patients, the average patient age was 66 years, and 83% of the population was white (J Vasc Surg. 2017 Jun;65[6]:1769-78).
Among all of the patients treated, 320 developed SSIs and 2,713 did not. The procedural indications included one or more of the following: claudication (72%), rest pain (50.5%), ulcer/gangrene (32.4%), or acute limb ischemia (15.1%). Antibiotics were appropriately administered to 97% of the patients, according to the researchers, “demonstrating high compliance across the BMC2 VIC.”
- Patient factors: As indicated by previous studies, obesity (odds ratio, 1.78), dialysis dependence (OR, 4.33), and hypertension (OR, 4.29) conferred a significant increased risk of SSI after LEB, according to Dr. Davis and his colleagues. In addition, however, they found that previous vascular surgery (OR, 1.57), previous percutaneous coronary intervention (OR, 1.47), use of antiplatelet medication (OR, 4.29), and low Peripheral Artery Questionnaire symptom severity (OR, 1.48) were significant independent predictors of SSI.
- Operative factors: Prolonged procedural length (OR, 2.95), iodine-only antiseptic skin preparation (OR, 1.73), and high peak intraoperative glucose (defined as a peak glucose greater than 180 mg/dL; OR, 1.99) were significant independent predictors of SSI. However, concomitant stent placement was found to be significantly predictive (OR, .38), “perhaps due to improvement in regional and subcutaneous vascular flow after the intervention,” the researchers suggested.
- Hospital factors: Larger overall hospital size (OR, 2.22) and major teaching center (OR, 1.66) were associated with increased risk of SSI. “Interestingly, we did not find an association with SSI and the hospital annual volume or the hospital urgent/emergent procedure rate,” the researchers added.
SSIs were not found to be significantly associated with a difference in 30-day mortality. However, they were significantly associated with an increased rate of several postoperative morbidities, including transfusion, lymph leak, major amputation, and open surgical bypass revision at or within 30 days of the index operation, according to Dr. Davis and his colleagues.
“Although some factors, such as patients comorbidities, are not modifiable, others represent areas for quality improvement in at-risk patients,” the researchers indicated. “Diligence should be devoted to decreasing operative length, controlling intraoperative glucose levels, and avoiding iodine-only skin preparation to decrease the rate of SSIs and its numerous associate morbidities in vascular surgery patients.”
In discussing the issue of antiplatelet medication being an indicator of increased risk, the authors pointed out that it was a hitherto unreported factor in the vascular literature, and of concern because, “as expected, a high percentage of patients (78.7%) were taking antiplatelet medication at the time of their LEB.”
Because the association of antiplatelet medication with SSIs was independent of the need for operative transfusion or the need for repeat intervention, the researchers speculated that “all antiplatelet agents have the theoretical potential to diminish activation-dependent platelet immune functions.” They referred to previous studies showing that clopidogrel was associated with significantly higher clinical rates of infection, particularly pneumonia.
Limitations cited for the study were the retrospective nature of the database analysis, the possibility of confounders not assessed in the data, and the fact that outcomes were limited to 30-day events, which would not take into account longer-term graft failure or mortality.
The authors reported having no conflicts of interest with regard to the study.
FROM THE JOURNAL OF VASCULAR SURGERY
Key clinical point:
Major finding: SSIs occurred in 10.6% of lower extremity bypasses.
Data source: A retrospective analysis of 3,033 elective or urgent open LEB procedures performed over a 3.5-year period in a statewide cardiovascular consortium of 35 hospitals.
Disclosures: The authors reported having no conflicts of interest with regard to the study.
Multidisciplinary bundle drives drop in colorectal SSIs
NEW YORK – Facing an “unacceptably high” rate of surgical site infections associated with colorectal surgery at their community hospital, surgeons searched for solutions. They created a perioperative bundle of interventions that ultimately dropped their infection rates enough to achieve the highest ranking in the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP).
“The Centers for Disease Control and Prevention recommends we use a robust surveillance program to monitor surgical site infection data. The system gives us feedback, and that will [help us] reduce surgical site infection (SSI) risk,” said Christopher Wolff, MD, a PGY4 resident at the Cleveland Clinic Akron General Hospital. “NSQIP and the National Healthcare Safety Network from the CDC are two programs that do just that.”
The effort paid off, with the number of SSIs going from 16 cases in 2013 to 10 cases in 2014 and then 5 cases in 2015. Since the bundle was implemented in the last quarter of 2014, “we’ve seen a consistent downtrend since that point in our total infections, and we kept that in the background of a consistent number of cases.
“We have good outcomes by incidence, but that is not the whole story,” Dr. Wolff said. “With respect to colorectal infections, we are now performing in the ‘exemplary’ category, compared with our peers” according to the ACS NSQIP data. In addition, “we are performing at or below the SIR [standardized infection ratio] or expected number consistently since the implementation.”
The bundle addresses actions in five domains: preoperative, anesthesia, operating room, post–anesthesia care unit, and postoperative floor interventions. Preoperative elements include patient education, use of chlorhexidine wipes before surgery, and antibiotics noted on the chart, for example. Additional features include prewarming preoperatively and maintaining normothermia, requiring all surgeons scrub traditionally instead of “foaming,” use of wound protectors in the OR, and close monitoring of blood glucose in diabetics postoperatively. “There also is education of floor nurses on how to take care of these patients specifically,” Dr. Wolff noted.
To identify these areas for improvement, Dr. Wolff and his colleagues initially reviewed the literature to find individual and bundle elements demonstrated to improve outcomes. Then, a surgeon group “think tank” discussed the possibilities. However, reaching agreement was not easy, Dr. Wolff said. “They had a hard time agreeing on best practices, even within our own specialty. We did finally come to a consensus.
“We took those bundled protocols through to other areas and said ‘here are the things we want you to work on, things we want you to improve.’ That did not necessarily go over so well,” Dr. Wolff said. Because of resistance from their colleagues, they changed strategies. “We brought other people to the table and changed our work groups from being surgeons only to [being] a multidisciplinary team.”
The process took months and months of deliberation. It’s important to have a champion behind the project, said Dr. Wolff. “I have to thank my chairman, Mark C. Horattas, MD, FACS, who had the vision to see this through.
“We implemented tried-and-true measures to reduce surgical site infections. We did so in a team manner and had multidisciplinary buy-in, and that created a culture change in our program over time,” Dr. Wolff said.
This study also shows, Dr. Wolff added, that “a successful multidisciplinary quality improvement program can be implemented in a community hospital setting.”
Going forward, continuous monitoring will identify any areas that need improvement over time. The preoperative bundle also will be integrated into an Enhanced Recovery After Surgery protocol.
The Akron Hospital is now ranked by ACS NSQIP in the top 10% of hospitals for their colorectal SSI rate. “It’s nice to meet someone in the first decile,” session moderator Timothy D. Jackson, MD, FACS, of the University of Toronto said after Dr. Wolff’s presentation. “I’ve never done that before, and I took notes for what to do at my hospital.”
Dr. Wolff had no relevant financial disclosures.
NEW YORK – Facing an “unacceptably high” rate of surgical site infections associated with colorectal surgery at their community hospital, surgeons searched for solutions. They created a perioperative bundle of interventions that ultimately dropped their infection rates enough to achieve the highest ranking in the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP).
“The Centers for Disease Control and Prevention recommends we use a robust surveillance program to monitor surgical site infection data. The system gives us feedback, and that will [help us] reduce surgical site infection (SSI) risk,” said Christopher Wolff, MD, a PGY4 resident at the Cleveland Clinic Akron General Hospital. “NSQIP and the National Healthcare Safety Network from the CDC are two programs that do just that.”
The effort paid off, with the number of SSIs going from 16 cases in 2013 to 10 cases in 2014 and then 5 cases in 2015. Since the bundle was implemented in the last quarter of 2014, “we’ve seen a consistent downtrend since that point in our total infections, and we kept that in the background of a consistent number of cases.
“We have good outcomes by incidence, but that is not the whole story,” Dr. Wolff said. “With respect to colorectal infections, we are now performing in the ‘exemplary’ category, compared with our peers” according to the ACS NSQIP data. In addition, “we are performing at or below the SIR [standardized infection ratio] or expected number consistently since the implementation.”
The bundle addresses actions in five domains: preoperative, anesthesia, operating room, post–anesthesia care unit, and postoperative floor interventions. Preoperative elements include patient education, use of chlorhexidine wipes before surgery, and antibiotics noted on the chart, for example. Additional features include prewarming preoperatively and maintaining normothermia, requiring all surgeons scrub traditionally instead of “foaming,” use of wound protectors in the OR, and close monitoring of blood glucose in diabetics postoperatively. “There also is education of floor nurses on how to take care of these patients specifically,” Dr. Wolff noted.
To identify these areas for improvement, Dr. Wolff and his colleagues initially reviewed the literature to find individual and bundle elements demonstrated to improve outcomes. Then, a surgeon group “think tank” discussed the possibilities. However, reaching agreement was not easy, Dr. Wolff said. “They had a hard time agreeing on best practices, even within our own specialty. We did finally come to a consensus.
“We took those bundled protocols through to other areas and said ‘here are the things we want you to work on, things we want you to improve.’ That did not necessarily go over so well,” Dr. Wolff said. Because of resistance from their colleagues, they changed strategies. “We brought other people to the table and changed our work groups from being surgeons only to [being] a multidisciplinary team.”
The process took months and months of deliberation. It’s important to have a champion behind the project, said Dr. Wolff. “I have to thank my chairman, Mark C. Horattas, MD, FACS, who had the vision to see this through.
“We implemented tried-and-true measures to reduce surgical site infections. We did so in a team manner and had multidisciplinary buy-in, and that created a culture change in our program over time,” Dr. Wolff said.
This study also shows, Dr. Wolff added, that “a successful multidisciplinary quality improvement program can be implemented in a community hospital setting.”
Going forward, continuous monitoring will identify any areas that need improvement over time. The preoperative bundle also will be integrated into an Enhanced Recovery After Surgery protocol.
The Akron Hospital is now ranked by ACS NSQIP in the top 10% of hospitals for their colorectal SSI rate. “It’s nice to meet someone in the first decile,” session moderator Timothy D. Jackson, MD, FACS, of the University of Toronto said after Dr. Wolff’s presentation. “I’ve never done that before, and I took notes for what to do at my hospital.”
Dr. Wolff had no relevant financial disclosures.
NEW YORK – Facing an “unacceptably high” rate of surgical site infections associated with colorectal surgery at their community hospital, surgeons searched for solutions. They created a perioperative bundle of interventions that ultimately dropped their infection rates enough to achieve the highest ranking in the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP).
“The Centers for Disease Control and Prevention recommends we use a robust surveillance program to monitor surgical site infection data. The system gives us feedback, and that will [help us] reduce surgical site infection (SSI) risk,” said Christopher Wolff, MD, a PGY4 resident at the Cleveland Clinic Akron General Hospital. “NSQIP and the National Healthcare Safety Network from the CDC are two programs that do just that.”
The effort paid off, with the number of SSIs going from 16 cases in 2013 to 10 cases in 2014 and then 5 cases in 2015. Since the bundle was implemented in the last quarter of 2014, “we’ve seen a consistent downtrend since that point in our total infections, and we kept that in the background of a consistent number of cases.
“We have good outcomes by incidence, but that is not the whole story,” Dr. Wolff said. “With respect to colorectal infections, we are now performing in the ‘exemplary’ category, compared with our peers” according to the ACS NSQIP data. In addition, “we are performing at or below the SIR [standardized infection ratio] or expected number consistently since the implementation.”
The bundle addresses actions in five domains: preoperative, anesthesia, operating room, post–anesthesia care unit, and postoperative floor interventions. Preoperative elements include patient education, use of chlorhexidine wipes before surgery, and antibiotics noted on the chart, for example. Additional features include prewarming preoperatively and maintaining normothermia, requiring all surgeons scrub traditionally instead of “foaming,” use of wound protectors in the OR, and close monitoring of blood glucose in diabetics postoperatively. “There also is education of floor nurses on how to take care of these patients specifically,” Dr. Wolff noted.
To identify these areas for improvement, Dr. Wolff and his colleagues initially reviewed the literature to find individual and bundle elements demonstrated to improve outcomes. Then, a surgeon group “think tank” discussed the possibilities. However, reaching agreement was not easy, Dr. Wolff said. “They had a hard time agreeing on best practices, even within our own specialty. We did finally come to a consensus.
“We took those bundled protocols through to other areas and said ‘here are the things we want you to work on, things we want you to improve.’ That did not necessarily go over so well,” Dr. Wolff said. Because of resistance from their colleagues, they changed strategies. “We brought other people to the table and changed our work groups from being surgeons only to [being] a multidisciplinary team.”
The process took months and months of deliberation. It’s important to have a champion behind the project, said Dr. Wolff. “I have to thank my chairman, Mark C. Horattas, MD, FACS, who had the vision to see this through.
“We implemented tried-and-true measures to reduce surgical site infections. We did so in a team manner and had multidisciplinary buy-in, and that created a culture change in our program over time,” Dr. Wolff said.
This study also shows, Dr. Wolff added, that “a successful multidisciplinary quality improvement program can be implemented in a community hospital setting.”
Going forward, continuous monitoring will identify any areas that need improvement over time. The preoperative bundle also will be integrated into an Enhanced Recovery After Surgery protocol.
The Akron Hospital is now ranked by ACS NSQIP in the top 10% of hospitals for their colorectal SSI rate. “It’s nice to meet someone in the first decile,” session moderator Timothy D. Jackson, MD, FACS, of the University of Toronto said after Dr. Wolff’s presentation. “I’ve never done that before, and I took notes for what to do at my hospital.”
Dr. Wolff had no relevant financial disclosures.
AT THE ACS QUALITY AND SAFETY CONFERENCE
Key clinical point: A multidisciplinary team initiative successfully reduced surgical site infections after colorectal surgery in a community hospital.
Major finding: The number of annual SSIs dropped from 16 in the calendar year before the intervention to 5 afterward.
Data source: Comparison of SSI rates before and after a bundled intervention in late 2014.
Disclosures: Dr. Wolff had no relevant financial disclosures.
Factory contamination seen as likely source of postop endocarditis outbreak
Since 2013, over 100 cases of Mycobacterium chimaera prosthetic valve endocarditis and disseminated disease were detected in Europe and the United States, and these were presumptively linked to contaminated heater-cooler units (HCUs) used during cardiac surgery. A molecular epidemiological analysis of microbial isolate genomes detected a “remarkable clonality of isolates” in almost all of the assessed patients with M. chimaera disease, which “strongly points to a common source of infection,” as reported online in The Lancet Infectious Diseases.
The analysis comprised 250 whole-genome sequencing datasets: 24 isolates from 21 cardiac surgery–related patients in Switzerland, Germany, the Netherlands, and the United Kingdom; 36 from 35 unrelated patients; 126 from LivaNova HCUs in use (85 water cultures, 41 air cultures); 13 from LivaNova HCUs returned to the production site in Germany for disinfection; 4 from the LivaNova production site (3 from newly produced HCUs, 1 from a water source); 2 from Maquet extracorporeal membrane oxygenation (ECMO) devices in use; 14 from Maquet HCUs in use; 15 from new Maquet HCUs sampled at the production site; and 7 from hospital water supplies in Switzerland, Germany, and the Netherlands, plus one M. chimaera DSM 44623–type strain, and eight M. intracellulare strains (from four unrelated patients from Germany and four published genomes).
Isolates were analyzed by next-generation whole-genome sequencing and compared with published M. chimaera genomes, according to Jakko van Ingen, PhD, Radboud University Medical Center, Nijmegen, the Netherlands, and his colleagues. Phylogenetic analysis of these 250 isolates revealed two major M. chimaera groups. They found that all cardiac surgery–related patient isolates could be classified into group 1. They then did a subgroup analysis.
“Three distinct strains of M. chimaera appear to have contaminated the water systems of LivaNova HCUs at the production site, belonging to subgroups 1.1, 1.8, and 2.1,” the authors stated. However, most M. chimaera isolates from air samples taken near operating LivaNova HCUs and those of 23 of the 24 related patients belonged to subgroup 1.1.
“This finding further supports the presumed airborne transmission pathway leading to endocarditis, aortic graft infection, disseminated disease, and surgical site infections in the affected patients,” according to the authors (doi: 10.1016/S1473-3099[17]30324-9).
The results suggest “the possibility that the vast majority of cases of cardiothoracic surgery–related severe M. chimaera infections diagnosed in Switzerland, Germany, the Netherlands, the United Kingdom, the United States, and Australia resulted from a single common source of infection: LivaNova HCUs that were most likely contaminated during production in Germany,” the researchers concluded.
The study was partly funded by the EU Horizon 2020 program, its FP7 program, the German Center for Infection Research (DZIF), the Swiss National Science Foundation, the Swiss Federal Office of Public Health, and National Institute of Health Research Oxford Health Protection Research Units on Healthcare Associated Infection and Antimicrobial Resistance. The authors reported having no relevant conflicts.
Since 2013, over 100 cases of Mycobacterium chimaera prosthetic valve endocarditis and disseminated disease were detected in Europe and the United States, and these were presumptively linked to contaminated heater-cooler units (HCUs) used during cardiac surgery. A molecular epidemiological analysis of microbial isolate genomes detected a “remarkable clonality of isolates” in almost all of the assessed patients with M. chimaera disease, which “strongly points to a common source of infection,” as reported online in The Lancet Infectious Diseases.
The analysis comprised 250 whole-genome sequencing datasets: 24 isolates from 21 cardiac surgery–related patients in Switzerland, Germany, the Netherlands, and the United Kingdom; 36 from 35 unrelated patients; 126 from LivaNova HCUs in use (85 water cultures, 41 air cultures); 13 from LivaNova HCUs returned to the production site in Germany for disinfection; 4 from the LivaNova production site (3 from newly produced HCUs, 1 from a water source); 2 from Maquet extracorporeal membrane oxygenation (ECMO) devices in use; 14 from Maquet HCUs in use; 15 from new Maquet HCUs sampled at the production site; and 7 from hospital water supplies in Switzerland, Germany, and the Netherlands, plus one M. chimaera DSM 44623–type strain, and eight M. intracellulare strains (from four unrelated patients from Germany and four published genomes).
Isolates were analyzed by next-generation whole-genome sequencing and compared with published M. chimaera genomes, according to Jakko van Ingen, PhD, Radboud University Medical Center, Nijmegen, the Netherlands, and his colleagues. Phylogenetic analysis of these 250 isolates revealed two major M. chimaera groups. They found that all cardiac surgery–related patient isolates could be classified into group 1. They then did a subgroup analysis.
“Three distinct strains of M. chimaera appear to have contaminated the water systems of LivaNova HCUs at the production site, belonging to subgroups 1.1, 1.8, and 2.1,” the authors stated. However, most M. chimaera isolates from air samples taken near operating LivaNova HCUs and those of 23 of the 24 related patients belonged to subgroup 1.1.
“This finding further supports the presumed airborne transmission pathway leading to endocarditis, aortic graft infection, disseminated disease, and surgical site infections in the affected patients,” according to the authors (doi: 10.1016/S1473-3099[17]30324-9).
The results suggest “the possibility that the vast majority of cases of cardiothoracic surgery–related severe M. chimaera infections diagnosed in Switzerland, Germany, the Netherlands, the United Kingdom, the United States, and Australia resulted from a single common source of infection: LivaNova HCUs that were most likely contaminated during production in Germany,” the researchers concluded.
The study was partly funded by the EU Horizon 2020 program, its FP7 program, the German Center for Infection Research (DZIF), the Swiss National Science Foundation, the Swiss Federal Office of Public Health, and National Institute of Health Research Oxford Health Protection Research Units on Healthcare Associated Infection and Antimicrobial Resistance. The authors reported having no relevant conflicts.
Since 2013, over 100 cases of Mycobacterium chimaera prosthetic valve endocarditis and disseminated disease were detected in Europe and the United States, and these were presumptively linked to contaminated heater-cooler units (HCUs) used during cardiac surgery. A molecular epidemiological analysis of microbial isolate genomes detected a “remarkable clonality of isolates” in almost all of the assessed patients with M. chimaera disease, which “strongly points to a common source of infection,” as reported online in The Lancet Infectious Diseases.
The analysis comprised 250 whole-genome sequencing datasets: 24 isolates from 21 cardiac surgery–related patients in Switzerland, Germany, the Netherlands, and the United Kingdom; 36 from 35 unrelated patients; 126 from LivaNova HCUs in use (85 water cultures, 41 air cultures); 13 from LivaNova HCUs returned to the production site in Germany for disinfection; 4 from the LivaNova production site (3 from newly produced HCUs, 1 from a water source); 2 from Maquet extracorporeal membrane oxygenation (ECMO) devices in use; 14 from Maquet HCUs in use; 15 from new Maquet HCUs sampled at the production site; and 7 from hospital water supplies in Switzerland, Germany, and the Netherlands, plus one M. chimaera DSM 44623–type strain, and eight M. intracellulare strains (from four unrelated patients from Germany and four published genomes).
Isolates were analyzed by next-generation whole-genome sequencing and compared with published M. chimaera genomes, according to Jakko van Ingen, PhD, Radboud University Medical Center, Nijmegen, the Netherlands, and his colleagues. Phylogenetic analysis of these 250 isolates revealed two major M. chimaera groups. They found that all cardiac surgery–related patient isolates could be classified into group 1. They then did a subgroup analysis.
“Three distinct strains of M. chimaera appear to have contaminated the water systems of LivaNova HCUs at the production site, belonging to subgroups 1.1, 1.8, and 2.1,” the authors stated. However, most M. chimaera isolates from air samples taken near operating LivaNova HCUs and those of 23 of the 24 related patients belonged to subgroup 1.1.
“This finding further supports the presumed airborne transmission pathway leading to endocarditis, aortic graft infection, disseminated disease, and surgical site infections in the affected patients,” according to the authors (doi: 10.1016/S1473-3099[17]30324-9).
The results suggest “the possibility that the vast majority of cases of cardiothoracic surgery–related severe M. chimaera infections diagnosed in Switzerland, Germany, the Netherlands, the United Kingdom, the United States, and Australia resulted from a single common source of infection: LivaNova HCUs that were most likely contaminated during production in Germany,” the researchers concluded.
The study was partly funded by the EU Horizon 2020 program, its FP7 program, the German Center for Infection Research (DZIF), the Swiss National Science Foundation, the Swiss Federal Office of Public Health, and National Institute of Health Research Oxford Health Protection Research Units on Healthcare Associated Infection and Antimicrobial Resistance. The authors reported having no relevant conflicts.
FROM THE LANCET INFECTIOUS DISEASES
Key clinical point:
Major finding: Cardiac surgery–related patient isolates were all classified into the same group, in which all, except one, formed a distinct subgroup of Mycobacterium chimaera, which also comprised most isolates from LivaNova HCUs, and one from the equipment production site.
Data source: Phylogenetic analysis based on whole-genome sequencing of 250 M. chimaera isolates obtained from cardiac surgery patients, hospitals, and other sources.
Disclosures: Partly funded by the EU Horizon 2020 program and several German, Swiss, and U.K. infectious disease–related NGOs. The authors reported having no disclosures.