User login
Acalabrutinib shows less off-target activity in mantle cell lymphoma
A new Bruton tyrosine kinase inhibitor has shown a high response rate and favorable safety profile in the treatment of patients with mantle cell lymphoma.
Researchers reported the results of an open-label, phase 2 study of oral acalabrutinib (100 mg, twice daily) in 124 patients with relapsed or refractory mantle cell lymphoma in The Lancet. Acalabrutinib (Calquence) received accelerated approval from the Food and Drug Administration in October 2017 for treatment of adults with mantle cell lymphoma who have received at least one prior therapy.
The Bruton tyrosine kinase (BTK) inhibitor ibrutinib (Imbruvica), which was approved in 2013 for the treatment of mantle cell lymphoma, has been associated with side effects including atrial fibrillation, infections and bleeding, likely due to its off-target activity against other kinases. But acalabrutinib (ACP-196) “is a highly selective, potent BTK inhibitor developed to minimise off-target activity,” wrote Michael Wang, MD, of the department of lymphoma and myeloma at the University of Texas MD Anderson Cancer Center, Houston, and his colleagues.
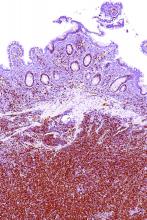
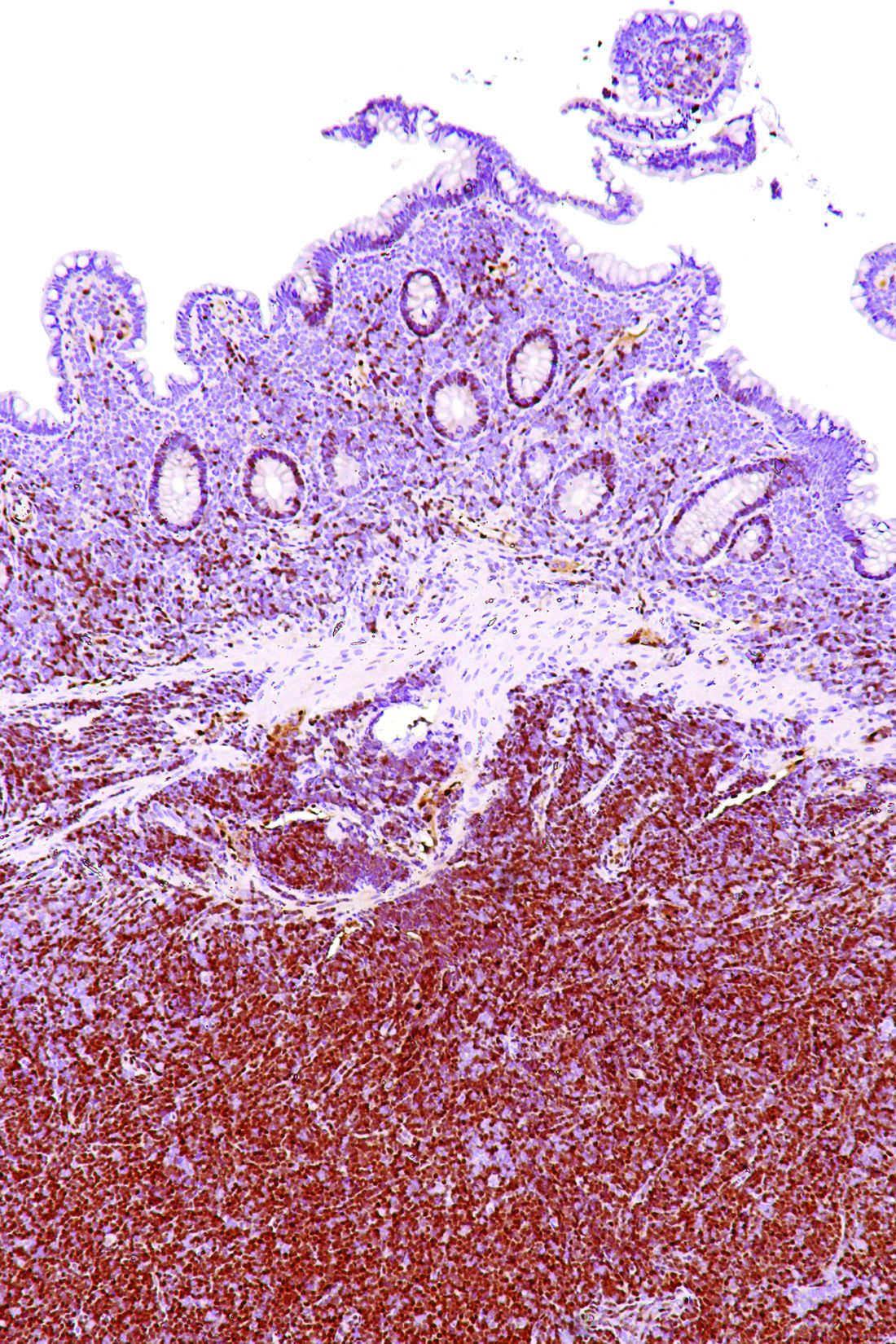
After a median follow-up of 15.2 months, 81% of patients in the study achieved an investigator-assessed overall response based on Lugano classification, with 40% achieving a complete response. The results were similar according to an independent review committee evaluation of responses based on CT and PET scans, bone-marrow biopsy specimens, endoscopy results, and clinical data.
There was also a reduction in lymphadenopathy seen in 94% of patients. The patients who showed a complete response took a median of 3.4 months to get there, and the median time to best response was 1.9 months.
The researchers also looked at response rates across a number of prespecified subgroups. Patients with Ann Arbor stage IV disease, those with bone marrow involvement, and those with extranodal disease were less likely to achieve a complete response (29%, 14% and 28% respectively). Patients with lymph nodes 5 cm or larger in diameter showed a 78% response rate.
While the Kaplan-Meier estimated medians for duration of response, progression-free survival, and overall survival were not reached, 87% of patients achieved 12-month overall survival and 67% of patients achieved progression-free survival at 12 months.
Most of the adverse events were grade 1 or 2, and included headache, diarrhea, fatigue, and myalgia.
There were no grade 4 or 5 adverse events, but 10% of patients experienced neutropenia, 9% experienced anemia and 5% experienced pneumonia. There was also one case of grade 3 or worse hemorrhage, but no cases of atrial fibrillation. Lymphocytosis was seen in 31% of patients.
Nearly half of patients (44%) discontinued treatment, mostly because of progressive disease (31%) but 6% discontinued the treatment because of adverse events.
“Overall, treatment with acalabrutinib demonstrated a favourable benefit-risk profile and represents a promising treatment option for patients with relapsed or refractory mantle cell lymphoma,” the researchers wrote. “Data from the ongoing ACE-CL-006 trial directly comparing acalabrutinib with ibrutinib in previously treated patients with high-risk chronic lymphocytic leukaemia will further differentiate the safety profiles of the two treatments.”
The researchers noted a decrease in plasma levels of tumor necrosis factor alpha, the cytokine CXCL13, and other cytokines known to be involved in inflammation and cell trafficking.
“These findings add to the growing body of evidence indicating that BTK inhibition disrupts the tumour microenvironment, limiting the supply of cytokines and chemokines necessary for complex interactions with stromal and accessory cells important for tumour growth and survival.”
The study was sponsored by Acerta Pharma, a member of the AstraZeneca Group. Several study authors reported grants, personal fees and other support from the pharmaceutical industry, including Acerta Pharma, most outside the submitted work. Several authors were also employees of Acerta and some had acalabrutinib patents pending or issued.
SOURCE: Wang M et al., Lancet. 2018;391:659-67.
A new Bruton tyrosine kinase inhibitor has shown a high response rate and favorable safety profile in the treatment of patients with mantle cell lymphoma.
Researchers reported the results of an open-label, phase 2 study of oral acalabrutinib (100 mg, twice daily) in 124 patients with relapsed or refractory mantle cell lymphoma in The Lancet. Acalabrutinib (Calquence) received accelerated approval from the Food and Drug Administration in October 2017 for treatment of adults with mantle cell lymphoma who have received at least one prior therapy.
The Bruton tyrosine kinase (BTK) inhibitor ibrutinib (Imbruvica), which was approved in 2013 for the treatment of mantle cell lymphoma, has been associated with side effects including atrial fibrillation, infections and bleeding, likely due to its off-target activity against other kinases. But acalabrutinib (ACP-196) “is a highly selective, potent BTK inhibitor developed to minimise off-target activity,” wrote Michael Wang, MD, of the department of lymphoma and myeloma at the University of Texas MD Anderson Cancer Center, Houston, and his colleagues.
After a median follow-up of 15.2 months, 81% of patients in the study achieved an investigator-assessed overall response based on Lugano classification, with 40% achieving a complete response. The results were similar according to an independent review committee evaluation of responses based on CT and PET scans, bone-marrow biopsy specimens, endoscopy results, and clinical data.
There was also a reduction in lymphadenopathy seen in 94% of patients. The patients who showed a complete response took a median of 3.4 months to get there, and the median time to best response was 1.9 months.
The researchers also looked at response rates across a number of prespecified subgroups. Patients with Ann Arbor stage IV disease, those with bone marrow involvement, and those with extranodal disease were less likely to achieve a complete response (29%, 14% and 28% respectively). Patients with lymph nodes 5 cm or larger in diameter showed a 78% response rate.
While the Kaplan-Meier estimated medians for duration of response, progression-free survival, and overall survival were not reached, 87% of patients achieved 12-month overall survival and 67% of patients achieved progression-free survival at 12 months.
Most of the adverse events were grade 1 or 2, and included headache, diarrhea, fatigue, and myalgia.
There were no grade 4 or 5 adverse events, but 10% of patients experienced neutropenia, 9% experienced anemia and 5% experienced pneumonia. There was also one case of grade 3 or worse hemorrhage, but no cases of atrial fibrillation. Lymphocytosis was seen in 31% of patients.
Nearly half of patients (44%) discontinued treatment, mostly because of progressive disease (31%) but 6% discontinued the treatment because of adverse events.
“Overall, treatment with acalabrutinib demonstrated a favourable benefit-risk profile and represents a promising treatment option for patients with relapsed or refractory mantle cell lymphoma,” the researchers wrote. “Data from the ongoing ACE-CL-006 trial directly comparing acalabrutinib with ibrutinib in previously treated patients with high-risk chronic lymphocytic leukaemia will further differentiate the safety profiles of the two treatments.”
The researchers noted a decrease in plasma levels of tumor necrosis factor alpha, the cytokine CXCL13, and other cytokines known to be involved in inflammation and cell trafficking.
“These findings add to the growing body of evidence indicating that BTK inhibition disrupts the tumour microenvironment, limiting the supply of cytokines and chemokines necessary for complex interactions with stromal and accessory cells important for tumour growth and survival.”
The study was sponsored by Acerta Pharma, a member of the AstraZeneca Group. Several study authors reported grants, personal fees and other support from the pharmaceutical industry, including Acerta Pharma, most outside the submitted work. Several authors were also employees of Acerta and some had acalabrutinib patents pending or issued.
SOURCE: Wang M et al., Lancet. 2018;391:659-67.
A new Bruton tyrosine kinase inhibitor has shown a high response rate and favorable safety profile in the treatment of patients with mantle cell lymphoma.
Researchers reported the results of an open-label, phase 2 study of oral acalabrutinib (100 mg, twice daily) in 124 patients with relapsed or refractory mantle cell lymphoma in The Lancet. Acalabrutinib (Calquence) received accelerated approval from the Food and Drug Administration in October 2017 for treatment of adults with mantle cell lymphoma who have received at least one prior therapy.
The Bruton tyrosine kinase (BTK) inhibitor ibrutinib (Imbruvica), which was approved in 2013 for the treatment of mantle cell lymphoma, has been associated with side effects including atrial fibrillation, infections and bleeding, likely due to its off-target activity against other kinases. But acalabrutinib (ACP-196) “is a highly selective, potent BTK inhibitor developed to minimise off-target activity,” wrote Michael Wang, MD, of the department of lymphoma and myeloma at the University of Texas MD Anderson Cancer Center, Houston, and his colleagues.
After a median follow-up of 15.2 months, 81% of patients in the study achieved an investigator-assessed overall response based on Lugano classification, with 40% achieving a complete response. The results were similar according to an independent review committee evaluation of responses based on CT and PET scans, bone-marrow biopsy specimens, endoscopy results, and clinical data.
There was also a reduction in lymphadenopathy seen in 94% of patients. The patients who showed a complete response took a median of 3.4 months to get there, and the median time to best response was 1.9 months.
The researchers also looked at response rates across a number of prespecified subgroups. Patients with Ann Arbor stage IV disease, those with bone marrow involvement, and those with extranodal disease were less likely to achieve a complete response (29%, 14% and 28% respectively). Patients with lymph nodes 5 cm or larger in diameter showed a 78% response rate.
While the Kaplan-Meier estimated medians for duration of response, progression-free survival, and overall survival were not reached, 87% of patients achieved 12-month overall survival and 67% of patients achieved progression-free survival at 12 months.
Most of the adverse events were grade 1 or 2, and included headache, diarrhea, fatigue, and myalgia.
There were no grade 4 or 5 adverse events, but 10% of patients experienced neutropenia, 9% experienced anemia and 5% experienced pneumonia. There was also one case of grade 3 or worse hemorrhage, but no cases of atrial fibrillation. Lymphocytosis was seen in 31% of patients.
Nearly half of patients (44%) discontinued treatment, mostly because of progressive disease (31%) but 6% discontinued the treatment because of adverse events.
“Overall, treatment with acalabrutinib demonstrated a favourable benefit-risk profile and represents a promising treatment option for patients with relapsed or refractory mantle cell lymphoma,” the researchers wrote. “Data from the ongoing ACE-CL-006 trial directly comparing acalabrutinib with ibrutinib in previously treated patients with high-risk chronic lymphocytic leukaemia will further differentiate the safety profiles of the two treatments.”
The researchers noted a decrease in plasma levels of tumor necrosis factor alpha, the cytokine CXCL13, and other cytokines known to be involved in inflammation and cell trafficking.
“These findings add to the growing body of evidence indicating that BTK inhibition disrupts the tumour microenvironment, limiting the supply of cytokines and chemokines necessary for complex interactions with stromal and accessory cells important for tumour growth and survival.”
The study was sponsored by Acerta Pharma, a member of the AstraZeneca Group. Several study authors reported grants, personal fees and other support from the pharmaceutical industry, including Acerta Pharma, most outside the submitted work. Several authors were also employees of Acerta and some had acalabrutinib patents pending or issued.
SOURCE: Wang M et al., Lancet. 2018;391:659-67.
FROM THE LANCET
Key clinical point:
Major finding: Eighty-one percent of patients with relapsed or refractory mantle cell lymphoma showed a partial or complete response to Bruton tyrosine kinase inhibitor acalabrutinib.
Study details: An open-label, phase 2 study in 124 patients with relapsed or refractory mantle cell lymphoma.
Disclosures: The study was sponsored by Acerta Pharma, a member of the AstraZeneca Group. Several study authors reported grants, personal fees and other support from the pharmaceutical industry, including Acerta Pharma, most outside the submitted work. Several authors were also employees of Acerta and some had acalabrutinib patents pending or issued.
Source: Wang M et al. Lancet. 2018;391:659-67.
Baseline stress signals need for psychological help in CLL
, according to a prospective study of 152 patients.
“These findings suggest that integration of psychological intervention for patients who have high cancer-specific stress at baseline might be appropriate for this population,” wrote investigators led by Neha G. Goyal, PhD, a research fellow at Wake Forest University, Winston-Salem, N.C.
The subjects all had relapsed/refractory chronic lymphocytic leukemia (CLL). They filled out mental and physical function questionnaires at baseline, then at months 1, 2, and 5 during a nonrandomized phase 2 trial of ibrutinib (Imbruvica). The findings were published in the Annals of Behavioral Medicine.
Cancer-specific stress – essentially traumatic stress associated with cancer diagnosis, recurrence, and treatment – was assessed by the Impact of Event Scale, a common cancer research tool in which patients rate the intensity of intrusive thoughts and avoidant thoughts and behaviors. A score of 8 – out of a range of 0-64 – was the cut point used to separate patients with low versus high stress; higher scores mean worse symptoms.
“At treatment initiation, cancer-specific stress was associated with higher levels of cognitive-affective depressive symptoms, negative mood, fatigue interference, and sleep problems, and lower mental health quality of life. While patients with higher cancer-specific stress at baseline improved more rapidly on these outcomes ... higher cancer-specific stress at baseline was still associated with poorer psychological outcomes, but not physical outcomes, at 5 months,” the investigators said (Ann Behav Med. 2018 Feb 9. doi: 10.1093/abm/kax004).
For instance, high-stress patients started the trial with mean scores of about 4.5 on the 42-point cognitive-affective subscale of the Beck Depression Inventory; scores improved to about 2.5 after 5 months of treatment. Low-stress patients, however, started and ended the study with scores of about 1.5.
Cancer-specific stress has been associated with poorer outcomes in previous cancer studies, but its impact on CLL hasn’t been clear until now. It might be a particularly bad problem in CLL, because the disease is incurable and patients go through multiple relapses and treatment cycles.
“There has been a call to screen cancer patients to determine those who may be at risk for poor outcomes, and assessment of cancer-specific stress may have clinical utility as an individual difference predictor of psychological responses,” the team noted.
The mean age in the study was 64.1 years; 71% of the subjects were men. The majority were educated beyond high school, and almost all reported significant, supportive relationships. Patients had a median of three prior therapies, but one patients had been through 16.
Dr. Goyal reported having no financial disclosures. One author disclosed ties to Pharmacyclics and Janssen, marketers of ibrutinib. The work was supported by the National Cancer Institute and Pharmacyclics, among others.
, according to a prospective study of 152 patients.
“These findings suggest that integration of psychological intervention for patients who have high cancer-specific stress at baseline might be appropriate for this population,” wrote investigators led by Neha G. Goyal, PhD, a research fellow at Wake Forest University, Winston-Salem, N.C.
The subjects all had relapsed/refractory chronic lymphocytic leukemia (CLL). They filled out mental and physical function questionnaires at baseline, then at months 1, 2, and 5 during a nonrandomized phase 2 trial of ibrutinib (Imbruvica). The findings were published in the Annals of Behavioral Medicine.
Cancer-specific stress – essentially traumatic stress associated with cancer diagnosis, recurrence, and treatment – was assessed by the Impact of Event Scale, a common cancer research tool in which patients rate the intensity of intrusive thoughts and avoidant thoughts and behaviors. A score of 8 – out of a range of 0-64 – was the cut point used to separate patients with low versus high stress; higher scores mean worse symptoms.
“At treatment initiation, cancer-specific stress was associated with higher levels of cognitive-affective depressive symptoms, negative mood, fatigue interference, and sleep problems, and lower mental health quality of life. While patients with higher cancer-specific stress at baseline improved more rapidly on these outcomes ... higher cancer-specific stress at baseline was still associated with poorer psychological outcomes, but not physical outcomes, at 5 months,” the investigators said (Ann Behav Med. 2018 Feb 9. doi: 10.1093/abm/kax004).
For instance, high-stress patients started the trial with mean scores of about 4.5 on the 42-point cognitive-affective subscale of the Beck Depression Inventory; scores improved to about 2.5 after 5 months of treatment. Low-stress patients, however, started and ended the study with scores of about 1.5.
Cancer-specific stress has been associated with poorer outcomes in previous cancer studies, but its impact on CLL hasn’t been clear until now. It might be a particularly bad problem in CLL, because the disease is incurable and patients go through multiple relapses and treatment cycles.
“There has been a call to screen cancer patients to determine those who may be at risk for poor outcomes, and assessment of cancer-specific stress may have clinical utility as an individual difference predictor of psychological responses,” the team noted.
The mean age in the study was 64.1 years; 71% of the subjects were men. The majority were educated beyond high school, and almost all reported significant, supportive relationships. Patients had a median of three prior therapies, but one patients had been through 16.
Dr. Goyal reported having no financial disclosures. One author disclosed ties to Pharmacyclics and Janssen, marketers of ibrutinib. The work was supported by the National Cancer Institute and Pharmacyclics, among others.
, according to a prospective study of 152 patients.
“These findings suggest that integration of psychological intervention for patients who have high cancer-specific stress at baseline might be appropriate for this population,” wrote investigators led by Neha G. Goyal, PhD, a research fellow at Wake Forest University, Winston-Salem, N.C.
The subjects all had relapsed/refractory chronic lymphocytic leukemia (CLL). They filled out mental and physical function questionnaires at baseline, then at months 1, 2, and 5 during a nonrandomized phase 2 trial of ibrutinib (Imbruvica). The findings were published in the Annals of Behavioral Medicine.
Cancer-specific stress – essentially traumatic stress associated with cancer diagnosis, recurrence, and treatment – was assessed by the Impact of Event Scale, a common cancer research tool in which patients rate the intensity of intrusive thoughts and avoidant thoughts and behaviors. A score of 8 – out of a range of 0-64 – was the cut point used to separate patients with low versus high stress; higher scores mean worse symptoms.
“At treatment initiation, cancer-specific stress was associated with higher levels of cognitive-affective depressive symptoms, negative mood, fatigue interference, and sleep problems, and lower mental health quality of life. While patients with higher cancer-specific stress at baseline improved more rapidly on these outcomes ... higher cancer-specific stress at baseline was still associated with poorer psychological outcomes, but not physical outcomes, at 5 months,” the investigators said (Ann Behav Med. 2018 Feb 9. doi: 10.1093/abm/kax004).
For instance, high-stress patients started the trial with mean scores of about 4.5 on the 42-point cognitive-affective subscale of the Beck Depression Inventory; scores improved to about 2.5 after 5 months of treatment. Low-stress patients, however, started and ended the study with scores of about 1.5.
Cancer-specific stress has been associated with poorer outcomes in previous cancer studies, but its impact on CLL hasn’t been clear until now. It might be a particularly bad problem in CLL, because the disease is incurable and patients go through multiple relapses and treatment cycles.
“There has been a call to screen cancer patients to determine those who may be at risk for poor outcomes, and assessment of cancer-specific stress may have clinical utility as an individual difference predictor of psychological responses,” the team noted.
The mean age in the study was 64.1 years; 71% of the subjects were men. The majority were educated beyond high school, and almost all reported significant, supportive relationships. Patients had a median of three prior therapies, but one patients had been through 16.
Dr. Goyal reported having no financial disclosures. One author disclosed ties to Pharmacyclics and Janssen, marketers of ibrutinib. The work was supported by the National Cancer Institute and Pharmacyclics, among others.
FROM ANNALS OF BEHAVIORAL MEDICINE
Treatment and Management of Multiple Myeloma (FULL)
Early Treatment and Diagnosis
Dr. Ascensão. An area that is becoming very important is identifying and separating smoldering multiple myeloma (SMM) from multiple myeloma (MM) and determining when to start treatment. At the Washington DC VAMC (DCVAMC) we started early on bisphosphonates and thalidomide without much benefit, but perhaps we were treating the wrong disease.
Dr. Mehta. Identifying patients as early as possible is often the best way to start. Treating early disease is easier than treating late disease, and it avoids all the complications. The problem is we don’t want to treat too many people because some of the people with SMM will never develop overt MM and, therefore, may not need treatment. We don’t have benign treatment yet. Whatever treatment we decide to use is going to carry adverse effects and toxicity.
So the trick is identifying those patients with SMM who are likely to progress in a finite period and, therefore, can be helped by treating early to avoid the complications of late diagnosis. We know that early treatment for patients with high-risk SMM helps. In a report from Lancet Oncology, early treatment with lenalidomide and dexamethasone reduces time to progression.1 There are other reports that treating early reduces time to progression.
So how do we identify those patients who are going to progress? We have a few clues. We know that patients who have a myeloma spike of > 1.5 g/dL are more likely to progress than others…The more discordant the / ratio from 1:1, the higher the risk for progression. And if that ratio is 1:100 or more, that would be a risk factor for progression.
We know from the work of Mayo Clinic researchers that if there are ≥ 60% of plasma cells in the bone marrow then it is a risk factor for progression. And we know from early studies that magnetic resonance imaging (MRI) detection of bone lesions, even long before they become detectable by X-ray, also is a risk factor for rapid development to myeloma.
...Methods such as genotyping, which we do here at the University of Arkansas for Medical Sciences, even in patients with MGUS (monoclonal gammopathy of undetermined significance), can identify high-risk patients, but that is not the standard of care yet. But it may become the standard of care in the days to come.
Another thing to think about for MGUS patients: Are there ways to identify what causes MGUS patients to evolve to SMM and then to overt myeloma, and to develop means of interrupting the progression cascade? There have been clinical trials on treatments (eg, bisphosphonates, thalidomide, aspirin, and cyclooxygenase inhibitors), but we haven’t found any safe, good treatment to prevent progression yet. With better technologies, we may be able to do that.
Dr. Ascensão. At the DCVAMC often we receive consults for a patient who had a little anemia, diabetes, renal disease, and the serum protein electrophoresis reveals a very small peak. How often should you follow patients? Do you do a complete workup the moment you see an MGUS or do you wait until they reach SMM?
Dr. Mehta. I don’t think every patient needs a complete workup. If you have obviously identifiable reasons for the anemia or the renal failure, then it’s less likely to be suspicious for myeloma. But patients with M spikes > 1 g/dL deserve a workup with a bone marrow aspirate and biopsy and at least bone X-rays, although MRIs would be even better.
I would differentiate based on the amount of M protein. Higher M protein patients deserve to have at least a bone marrow aspirate and bone study. Patients
with M protein > 1g/dL deserve to be seen every 3 to 4 months. I see patients with tiny little peaks every 6 months. And then, after 1 or 2 years, I turn over their care to the primary care doctor to follow. If we had research protocols to look at those patients and find the methods for progression, which I had at one point, then of course, we could see them more often and try to unravel the mystery.
Use of Imaging
Dr. Ascensão. That’s pretty close to what we do at DCVAMC. What do you think is the role for a bone survey as opposed to MRIs and positron emission tomography (PET) scans in this setting?
Dr. Mehta. In the real world X-rays are more accessible and much less expensive. So for the patient with very low risk who doesn’t have any complaints and
who has a low M spike, I think a bone survey is adequate. But you need about 30% to 40% bone destruction before you’re going to find anything on the X-ray.
MRIs are much more sensitive, plus they tell you about bone marrow involvement, but that should be reserved for the patient who has symptoms or a high
M protein. At Central Arkansas Veterans Healthcare System we simply can’t get PET scans for myeloma patients. At the myeloma center across the street from us, PET scans are used for routine evaluations.
Dr. Chauncey. I agree with Dr. Mehta. At VA Puget Sound Healthcare System (VAPSHCS) there isn’t a problem getting PET scans, but we probably get far fewer
scans than Arkansas. I still like the skeletal survey because it directs you where to look for potential pathologic fracture. It’s definitely not as sensitive as the dedicated myeloma MRI, but it’s a lot easier to get at VAPSHCS, especially as a screening tool.
Dr. Ascensão. Right, I believe there are some issues about the number of osteolytic lesions that may drive diagnosis.
Dr. Mehta. For patients with high M protein, I always request MRI. But the correlation is poorer in patients who have lower M protein. I try to limit it to the patients who have symptoms or high M protein, but I don’t have any evidence-based data to prove that’s the right way.
Dr. Ascensão. If you were going to start treatment of SMM that you believe is evolving to a more regular myeloma, do you do anything different than you would for any of the patients that you have identified as having active myeloma? Do you have different protocols for those patients as opposed to patients who present de novo with active myeloma?
Dr. Mehta. Those patients should be treated with the same drugs, an IMiD and a steroid. And the question is plus or minus a proteasome inhibitor. Studies have shown that an IMiD with a steroid gets much better results than using observation alone. Whether you would get even better results with the proteasome inhibitor remains to be seen. Maybe we can do that study.
Dr. Chauncey. We strive to identify high-risk SMM patients and treat them accordingly. Alternatively, physicians are pulling the trigger for therapy earlier and earlier and when they come for transplant with a diagnosis of MM, it is critical to review the initial diagnostic information. Most transplant centers have experience with this phenomena and know that they don’t want to transplant a non-high-risk SMM or any MGUS. However, by the time the patient is referred for transplantation, the initial clinical data are sometimes obscured or inaccessible.
Dr. Ascensão. We also look into the bone bearing areas, which allows us to make sure that if the patient has hip problems, we can work on how to approach them, whether we want to radiate those patients to prevent fractures.
Use of Bisphosphonates
Dr. Cosgriff. Myeloma metastasizes to bone, and it is one of the common sites of metastatic disease. It poses some interesting complications, whether it is from hypercalcemia due to metastatic sites, or pain syndromes. Bisphosphonates are indicated for myeloma, and they have been for years. Interestingly, unlike some of the other disease, the use of bisphosphonates induces apoptosis in myeloma. So we have seen some disease control with these agents.
The 2 bisphosphonates that are available for use are pamidronate and zoledronic acid. At the VA Portland Health Care System (VAPORHCS), we have been
using pamidronate exclusively for individuals with myeloma. There was a 2003 paper that evaluated the use of bisphosphonates for skeletal-related events in myeloma and in patients with metastatic breast cancer.2 In the subset analysis of myeloma patients with the bisphosphonates, there was no difference between pamidronate and zoledronic acid.
At the time, zoledronic acid was significantly more expensive than pamidronate, and so VAPORHCS opted to use pamidronate as a cost-saving measure. But there are the other reasons for picking pamidronate: Zoledronic acid has some dose recommendations and guidelines for individuals with renal failure, which is often a significant problem in patients with myeloma as well. To get around dose adjustments that need to be made for zoledronic acid, VAPORHCS switched to pamidronate, which is looser with the recommendations on renal failure.
Earlier use criteria, like the National Comprehensive Cancer Network guidelines, stated that if the renal failure was due to the disease itself and not some other outlying factor, a full 90-mg dose of pamidronate could still be used. That comment has since been removed. We still pay attention to it and reduce pamidronate dosing to 60 mg for patients with renal failure.
The prices for zoledronic acid have dropped significantly since it became a generic. The nice thing about zoledronic acid is that it has a short infusion time of 15 minutes. As chair space becomes a problem—VAPHCS has significant issues with that—zoledronic acid looks more and more attractive. The FDA label states that pamidronate should be infused over 4 hours, but VAPHCS typically has been infusing it for 3 hours.
It should be noted that denosumab (XGEVA), a monoclonal antibody that also is targeted for hypercalcemia, has been specifically excluded for myeloma. It
has no FDA indication for myeloma. It does have an indication for hypercalcemia. Whether or not you can state that the patient with myeloma is hypercalcemic, and that’s the reason you want to use it, it starts crossing into some gray area. The drug is still significantly more expensive and it seems to have similar efficacy rates compared with both pamidronate and zoledronic acid, so VAPHCS limits its use to individuals who would otherwise be contraindicated to zoledronic acid or pamidronate due to renal failure.
Dr. Ascensão. How often do you give it, every month, every 3 months?
Dr. Cosgriff. Currently, VAPORHCS is giving bisphosphonates every month whether in the chemotherapy unit or in the short stay unit. We are starting to reevaluate that. I have heard some emerging data that suggest we can use it once a quarter and get the same results. Those data are still emerging. It would be nice to be able to reduce the infusion frequency. But bisphosphonates adhere to bone and get incorporated into the bone matrix and stay there for an extended period of time, upwards of 6 months to a year, as with zoledronic acid.
Osteonecrosis
Dr. Ascensão. Do you require dental clearance prior to first dose?
Dr. Cosgriff. Bisphosphonates have a warning for 2% incidence of osteonecrosis of the jaw. Risk factors for the development of osteonecrosis of the jaw include poor dentition or major dental work, like extractions and illfitting dentures but not necessarily root canals. Ill-fitting dentures tend to rub on the gums and irritate the bone layer underneath. It’s the irritation of the bone that’s the biggest risk factor for osteonecrosis of the jaw.
We require that patients see the dentist because we’ve had individuals develop osteonecrosis eventhough we thought they had good dentition. If a patient is seeing a dentist outside of the VA system, we ask them to notify their dentist that they’re receiving bisphosphonates. Because of the risk and because we’ve had some individuals with good dentition develop it, VAPORHCS requires all patients, particularly those who are receiving zoledronic acid, to have dental evaluations. Denosumab also has a listed 2% incidence of osteonecrosis of the jaw, so those individuals also need to be evaluated by our dental service.
Dr. Ascensão. The DCVAMC has the same problem. I have a patient that presented primarily with a plasmacytoma, and we tried to get him to see the dentist. The dentist said, ‘You’ve got to get your teeth pulled.’ The patient has tried to see outside dentists and is finding all kinds of excuses because he would like to have implants.
Dr. Cosgriff. Anytime that you somehow damage or irritate that bone, that becomes a risk factor for the development of osteonecrosis. And for those individuals, we delay the bisphosphonate. If they’re having pain syndrome, we try to support them with opiates. We would love to be able to use nonsteroidal anti-inflammatory drugs—they have really good efficacy against bone pain—but renal function and renal failures prevent the use of those in a majority of patients. We start bisphosphonates as soon as dental clears them.
Dr. Mehta. Isn’t there a contraindication for denosumab and some evidence that it may worsen MM outcomes?
Dr. Cosgriff. When the drug first came on the market, it specifically stated in the package insert that it is not to be used in MM (it doesn’t state it specifically anymore). There is a thought that maybe some underlying mechanism exists that might stimulate some of the myeloma problems, which is why I get a little concerned when people say, “Well, I’m using it for hypercalcemia, I’m not using it to treat or to prevent a skeletal-related event in patients with myeloma.” That becomes a gray area and in that type of situation, I would recommend treating the hypercalcemia with a single dose and then switching the
patient to a bisphosphonate.
Dr. Mehta. And of course, bisphosphonates also lower calcium. They can be used to treat hypercalcemia.
Dr. Cosgriff. Yes. Zoledronic acid does have limitations in renal failure, though pamidronate doesn’t have quite the same limitations. The VAPORHCS tries to
use exclusively for hypercalcemia as well. The data show that when using zoledronic acid compared with pamidronate, you end up with the same outcomes as far as hypercalcemia. The zoledronic acid onset of action is a little faster, around 12 to 24 hours vs 48 to 72 hours with pamidronate, but you can get around that by using calcitonin over a short period; 48 hours is typically the maximum efficacy for calcitonin in treating hypercalcemia. So we use pamidronate in place of that, supplementing with calcitonin.
The result is that at 7 days, pamidronate and zoledronic acid show the same efficacy rates for treating hypercalcemia. But the renal function sometimes prevents us from doing that. Denosumab does become an option for hypercalcemia, but again, I caution against its use for treating hypercalcemia in patients with myeloma due to the risk of advancing the myeloma.
Bone Marrow Transplant
Dr. Ascensão. Do you transplant for 1 or 2 bone marrows? What’s the best maintenance regimen postallograft, and when do you start? Do you use lenalidomide the first month of the transplant or do you wait until day 100?
Dr. Chauncey. From my perspective, hematopoietic stem cell transplantation has never really lost prominence. It is true that the concept of marrow transplantation for MM has been around for more than 20 years for those patients with first best response (Note that I’ll use best response rather than first remission). The concept was developed in an era when we had much less effective therapy, and in comparative trials, progressionfree survival was consistently superior and occasionally, overall survival was better with transplantation. As treatments got better, responses got better, and there were regular questions as to whether we still needed transplantation. But the data show that as responses got better, the progression-free survivals continued to improve, and transplantation still adds something to initial therapy.
Probably the most current data are from the Dana Farber- IFM trial for which Nikhil Munshi, MD, is an investigator. The trial includes induction with lenalidomide/bortezomib/dexamethasone, which is one of the more aggressive induction regimens. When upfront transplant vs delayed transplant are compared, it seems the preliminary data still favor having an upfront transplant after initial induction therapy.
The consensus is that autologous transplantation adds to the better response that we see with better induction therapy. Overall survival has become a less accessible endpoint since the initial trials, and that’s really a consequence of having better salvage therapy, and the confounding effects of subsequent treatments. We have so many options for salvage therapy that it’s now very hard to look at overall survival as an endpoint in trials of initial therapy.
A sometimes contentious question when it comes to payers, and less so in the VA, is how many transplants to do as part of initial therapy? Little Rock and the French did some of the pioneering work on tandem transplants. The BMT CTN 0702–StaMINA trial looks at this directly, and is mature and should be presented soon [Editorial Note: Preliminary results were presented at the American Society of Hematology meeting on December 6, 2016].
The approach at VAPSHCS and most other transplant centers has typically been to harvest a sufficient quantity of peripheral blood stem cells to do 2 transplants. If less than a very good partial response is achieved after the first transplant, then we do a second transplant in tandem fashion.
One exception would be for plasma cell leukemia, which is very aggressive. In that case, we would routinely perform tandem transplantation. We are unlikely to ever have a randomized trial that compares 1 vs 2 transplants in that particular setting.
Another question is whether a second autologous transplantation can be useful in a nontandem fashion, and there is a large amount of retrospective data about its use as salvage treatment. In eras when there were not as many effective therapies, salvage autologous transplant was more attractive. As new therapies came along, its use has somewhat waned, but there’s been renewed interest because dose-intensive melphalan with autologous rescue is relatively safe and not cross-resistant to other therapies. It also offers the option of a drug holiday after the transplant, whereas salvage drug therapy is typically continuous.
There is no universal agreement on nontandem second transplantation, there are no consistent algorithms to say when it is appropriate, but it’s worth discussing with the transplant programs, especially if there is a lot of toxicity with current salvage therapy.
The last question is the role of allogeneic transplantation, and while I’m generally a proponent of allogeneic transplantation for many diseases, in spite of some really significant efforts, the majority of allogeneic data for MM has not been very positive. The large BMT-CTN 0102 trial compared tandem autologous transplant at first response to a single autologous transplantation followed by reduced-intensity autologous transplantation from a matched sibling. This study was limited in part by enrollment bias, but the published results did not favor an allogeneic approach.3 Although there was less relapse in the allogeneic setting, the mortality of allogeneic transplant was not overcome by the decrease in relapse. Neither progression-free or overall survivals at 3 years were better in the allogeneic group.
Despite small studies showing feasibility and promising results, it’s currently very hard to advocate for allogeneic transplantation in MM. There are certainly centers that continue to have their own approach, with some in the U.S. that are pioneering tweaks on allogeneic regimens and graft engineering, but the data are typically small and anecdotal. That doesn’t mean that there won’t ultimately be a better way to do allogeneic transplantation in MM, but rather that we don’t currently know the best way to approach this strategy.
Next Steps in Myeloma Treatment
Dr. Ascensão. There are some people who are now starting to talk about a cure for myeloma. I’m not sure we’re there yet. Certainly, it’s a chronic disease that, if we can take care of the complications and maybe by starting treatment early. I’m not sure Agent Orange-exposed patients do better or worse. That’s something that needs to be researched if we can find a way to compare within this group and within the type of treatment that patients get.
Is it reasonable to start looking for minimal residual disease in cells? Should we shoot for the best response? I think one of the points that Dr. Chauncey made a number of times, and I agree, is that our patient population may not be able to tolerate some of the more aggressive therapies. Perhaps we need to find a slightly different version of this algorithm for VA patients.
Dr. Chauncey. There’s a diverse biology for both veterans and nonveterans alike. There are patients for whom a deeper response will lead to longer remission and better survival, and there are others whose disease will smolder with a lower tumor burden and not progress quickly. A lot of the early gene expression profiling data on this comes from Little Rock. Unfortunately, determination of an individual’s biology is not readily accessible in the clinic, and we are typically unable to clearly define each patient’s inherent disease biology.
Dr. Mehta. We just don’t have the answers as to exactly what to do with the information that we get except watch more closely and treat a little bit earlier. We don’t even know the significance of minimal residue disease and how often to test for it and if it correlates truly with longer-term survival. These are great research questions. We need to accumulate the data and try to analyze it. We need to participate in the big data programs.
Dr. Ascensão. The other thing, of course, is now we have new immunotherapy approaches beyond transplant, which includes some of the checkpoint inhibitors and there’s some exciting data coming out. So I think the future looks good.
We all are committed to treating our patients, our veterans, to the best of our abilities. And I think the VA has done a very good job in allowing us to do this for our patients and allowing us to provide the best treatments available out there.
Click here to read the digital edition.
1. Mateos MV, Hernández MT, Giraldo P, et al. Lenalidomide plus dexamethasone versus observation in patients with high-risk smouldering multiple myeloma (QuiRedex): long-term follow-up of a randomised, controlled, phase 3 trial. Lancet Oncol. 2016;17(8):1127-1136.
2. Rosen LS, Gordon D, Kaminski M, et al. Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma: a randomized, double-blind, multicenter, comparative trial. Cancer. 2003;98(8):1735-1744.
3. Krishnan A, Pasquini MC, Logan B, et al; Blood Marrow Transplant Clinical Trials Network (BMT CTN). Autologous haemopoietic stem-cell transplantation followed by allogeneic or autologous haemopoietic stem-cell transplantation in patients with multiple myeloma (BMT CTN 0102): a phase 3 biological assignment trial. Lancet Oncol. 2011;12(13):1195-11203.
Early Treatment and Diagnosis
Dr. Ascensão. An area that is becoming very important is identifying and separating smoldering multiple myeloma (SMM) from multiple myeloma (MM) and determining when to start treatment. At the Washington DC VAMC (DCVAMC) we started early on bisphosphonates and thalidomide without much benefit, but perhaps we were treating the wrong disease.
Dr. Mehta. Identifying patients as early as possible is often the best way to start. Treating early disease is easier than treating late disease, and it avoids all the complications. The problem is we don’t want to treat too many people because some of the people with SMM will never develop overt MM and, therefore, may not need treatment. We don’t have benign treatment yet. Whatever treatment we decide to use is going to carry adverse effects and toxicity.
So the trick is identifying those patients with SMM who are likely to progress in a finite period and, therefore, can be helped by treating early to avoid the complications of late diagnosis. We know that early treatment for patients with high-risk SMM helps. In a report from Lancet Oncology, early treatment with lenalidomide and dexamethasone reduces time to progression.1 There are other reports that treating early reduces time to progression.
So how do we identify those patients who are going to progress? We have a few clues. We know that patients who have a myeloma spike of > 1.5 g/dL are more likely to progress than others…The more discordant the / ratio from 1:1, the higher the risk for progression. And if that ratio is 1:100 or more, that would be a risk factor for progression.
We know from the work of Mayo Clinic researchers that if there are ≥ 60% of plasma cells in the bone marrow then it is a risk factor for progression. And we know from early studies that magnetic resonance imaging (MRI) detection of bone lesions, even long before they become detectable by X-ray, also is a risk factor for rapid development to myeloma.
...Methods such as genotyping, which we do here at the University of Arkansas for Medical Sciences, even in patients with MGUS (monoclonal gammopathy of undetermined significance), can identify high-risk patients, but that is not the standard of care yet. But it may become the standard of care in the days to come.
Another thing to think about for MGUS patients: Are there ways to identify what causes MGUS patients to evolve to SMM and then to overt myeloma, and to develop means of interrupting the progression cascade? There have been clinical trials on treatments (eg, bisphosphonates, thalidomide, aspirin, and cyclooxygenase inhibitors), but we haven’t found any safe, good treatment to prevent progression yet. With better technologies, we may be able to do that.
Dr. Ascensão. At the DCVAMC often we receive consults for a patient who had a little anemia, diabetes, renal disease, and the serum protein electrophoresis reveals a very small peak. How often should you follow patients? Do you do a complete workup the moment you see an MGUS or do you wait until they reach SMM?
Dr. Mehta. I don’t think every patient needs a complete workup. If you have obviously identifiable reasons for the anemia or the renal failure, then it’s less likely to be suspicious for myeloma. But patients with M spikes > 1 g/dL deserve a workup with a bone marrow aspirate and biopsy and at least bone X-rays, although MRIs would be even better.
I would differentiate based on the amount of M protein. Higher M protein patients deserve to have at least a bone marrow aspirate and bone study. Patients
with M protein > 1g/dL deserve to be seen every 3 to 4 months. I see patients with tiny little peaks every 6 months. And then, after 1 or 2 years, I turn over their care to the primary care doctor to follow. If we had research protocols to look at those patients and find the methods for progression, which I had at one point, then of course, we could see them more often and try to unravel the mystery.
Use of Imaging
Dr. Ascensão. That’s pretty close to what we do at DCVAMC. What do you think is the role for a bone survey as opposed to MRIs and positron emission tomography (PET) scans in this setting?
Dr. Mehta. In the real world X-rays are more accessible and much less expensive. So for the patient with very low risk who doesn’t have any complaints and
who has a low M spike, I think a bone survey is adequate. But you need about 30% to 40% bone destruction before you’re going to find anything on the X-ray.
MRIs are much more sensitive, plus they tell you about bone marrow involvement, but that should be reserved for the patient who has symptoms or a high
M protein. At Central Arkansas Veterans Healthcare System we simply can’t get PET scans for myeloma patients. At the myeloma center across the street from us, PET scans are used for routine evaluations.
Dr. Chauncey. I agree with Dr. Mehta. At VA Puget Sound Healthcare System (VAPSHCS) there isn’t a problem getting PET scans, but we probably get far fewer
scans than Arkansas. I still like the skeletal survey because it directs you where to look for potential pathologic fracture. It’s definitely not as sensitive as the dedicated myeloma MRI, but it’s a lot easier to get at VAPSHCS, especially as a screening tool.
Dr. Ascensão. Right, I believe there are some issues about the number of osteolytic lesions that may drive diagnosis.
Dr. Mehta. For patients with high M protein, I always request MRI. But the correlation is poorer in patients who have lower M protein. I try to limit it to the patients who have symptoms or high M protein, but I don’t have any evidence-based data to prove that’s the right way.
Dr. Ascensão. If you were going to start treatment of SMM that you believe is evolving to a more regular myeloma, do you do anything different than you would for any of the patients that you have identified as having active myeloma? Do you have different protocols for those patients as opposed to patients who present de novo with active myeloma?
Dr. Mehta. Those patients should be treated with the same drugs, an IMiD and a steroid. And the question is plus or minus a proteasome inhibitor. Studies have shown that an IMiD with a steroid gets much better results than using observation alone. Whether you would get even better results with the proteasome inhibitor remains to be seen. Maybe we can do that study.
Dr. Chauncey. We strive to identify high-risk SMM patients and treat them accordingly. Alternatively, physicians are pulling the trigger for therapy earlier and earlier and when they come for transplant with a diagnosis of MM, it is critical to review the initial diagnostic information. Most transplant centers have experience with this phenomena and know that they don’t want to transplant a non-high-risk SMM or any MGUS. However, by the time the patient is referred for transplantation, the initial clinical data are sometimes obscured or inaccessible.
Dr. Ascensão. We also look into the bone bearing areas, which allows us to make sure that if the patient has hip problems, we can work on how to approach them, whether we want to radiate those patients to prevent fractures.
Use of Bisphosphonates
Dr. Cosgriff. Myeloma metastasizes to bone, and it is one of the common sites of metastatic disease. It poses some interesting complications, whether it is from hypercalcemia due to metastatic sites, or pain syndromes. Bisphosphonates are indicated for myeloma, and they have been for years. Interestingly, unlike some of the other disease, the use of bisphosphonates induces apoptosis in myeloma. So we have seen some disease control with these agents.
The 2 bisphosphonates that are available for use are pamidronate and zoledronic acid. At the VA Portland Health Care System (VAPORHCS), we have been
using pamidronate exclusively for individuals with myeloma. There was a 2003 paper that evaluated the use of bisphosphonates for skeletal-related events in myeloma and in patients with metastatic breast cancer.2 In the subset analysis of myeloma patients with the bisphosphonates, there was no difference between pamidronate and zoledronic acid.
At the time, zoledronic acid was significantly more expensive than pamidronate, and so VAPORHCS opted to use pamidronate as a cost-saving measure. But there are the other reasons for picking pamidronate: Zoledronic acid has some dose recommendations and guidelines for individuals with renal failure, which is often a significant problem in patients with myeloma as well. To get around dose adjustments that need to be made for zoledronic acid, VAPORHCS switched to pamidronate, which is looser with the recommendations on renal failure.
Earlier use criteria, like the National Comprehensive Cancer Network guidelines, stated that if the renal failure was due to the disease itself and not some other outlying factor, a full 90-mg dose of pamidronate could still be used. That comment has since been removed. We still pay attention to it and reduce pamidronate dosing to 60 mg for patients with renal failure.
The prices for zoledronic acid have dropped significantly since it became a generic. The nice thing about zoledronic acid is that it has a short infusion time of 15 minutes. As chair space becomes a problem—VAPHCS has significant issues with that—zoledronic acid looks more and more attractive. The FDA label states that pamidronate should be infused over 4 hours, but VAPHCS typically has been infusing it for 3 hours.
It should be noted that denosumab (XGEVA), a monoclonal antibody that also is targeted for hypercalcemia, has been specifically excluded for myeloma. It
has no FDA indication for myeloma. It does have an indication for hypercalcemia. Whether or not you can state that the patient with myeloma is hypercalcemic, and that’s the reason you want to use it, it starts crossing into some gray area. The drug is still significantly more expensive and it seems to have similar efficacy rates compared with both pamidronate and zoledronic acid, so VAPHCS limits its use to individuals who would otherwise be contraindicated to zoledronic acid or pamidronate due to renal failure.
Dr. Ascensão. How often do you give it, every month, every 3 months?
Dr. Cosgriff. Currently, VAPORHCS is giving bisphosphonates every month whether in the chemotherapy unit or in the short stay unit. We are starting to reevaluate that. I have heard some emerging data that suggest we can use it once a quarter and get the same results. Those data are still emerging. It would be nice to be able to reduce the infusion frequency. But bisphosphonates adhere to bone and get incorporated into the bone matrix and stay there for an extended period of time, upwards of 6 months to a year, as with zoledronic acid.
Osteonecrosis
Dr. Ascensão. Do you require dental clearance prior to first dose?
Dr. Cosgriff. Bisphosphonates have a warning for 2% incidence of osteonecrosis of the jaw. Risk factors for the development of osteonecrosis of the jaw include poor dentition or major dental work, like extractions and illfitting dentures but not necessarily root canals. Ill-fitting dentures tend to rub on the gums and irritate the bone layer underneath. It’s the irritation of the bone that’s the biggest risk factor for osteonecrosis of the jaw.
We require that patients see the dentist because we’ve had individuals develop osteonecrosis eventhough we thought they had good dentition. If a patient is seeing a dentist outside of the VA system, we ask them to notify their dentist that they’re receiving bisphosphonates. Because of the risk and because we’ve had some individuals with good dentition develop it, VAPORHCS requires all patients, particularly those who are receiving zoledronic acid, to have dental evaluations. Denosumab also has a listed 2% incidence of osteonecrosis of the jaw, so those individuals also need to be evaluated by our dental service.
Dr. Ascensão. The DCVAMC has the same problem. I have a patient that presented primarily with a plasmacytoma, and we tried to get him to see the dentist. The dentist said, ‘You’ve got to get your teeth pulled.’ The patient has tried to see outside dentists and is finding all kinds of excuses because he would like to have implants.
Dr. Cosgriff. Anytime that you somehow damage or irritate that bone, that becomes a risk factor for the development of osteonecrosis. And for those individuals, we delay the bisphosphonate. If they’re having pain syndrome, we try to support them with opiates. We would love to be able to use nonsteroidal anti-inflammatory drugs—they have really good efficacy against bone pain—but renal function and renal failures prevent the use of those in a majority of patients. We start bisphosphonates as soon as dental clears them.
Dr. Mehta. Isn’t there a contraindication for denosumab and some evidence that it may worsen MM outcomes?
Dr. Cosgriff. When the drug first came on the market, it specifically stated in the package insert that it is not to be used in MM (it doesn’t state it specifically anymore). There is a thought that maybe some underlying mechanism exists that might stimulate some of the myeloma problems, which is why I get a little concerned when people say, “Well, I’m using it for hypercalcemia, I’m not using it to treat or to prevent a skeletal-related event in patients with myeloma.” That becomes a gray area and in that type of situation, I would recommend treating the hypercalcemia with a single dose and then switching the
patient to a bisphosphonate.
Dr. Mehta. And of course, bisphosphonates also lower calcium. They can be used to treat hypercalcemia.
Dr. Cosgriff. Yes. Zoledronic acid does have limitations in renal failure, though pamidronate doesn’t have quite the same limitations. The VAPORHCS tries to
use exclusively for hypercalcemia as well. The data show that when using zoledronic acid compared with pamidronate, you end up with the same outcomes as far as hypercalcemia. The zoledronic acid onset of action is a little faster, around 12 to 24 hours vs 48 to 72 hours with pamidronate, but you can get around that by using calcitonin over a short period; 48 hours is typically the maximum efficacy for calcitonin in treating hypercalcemia. So we use pamidronate in place of that, supplementing with calcitonin.
The result is that at 7 days, pamidronate and zoledronic acid show the same efficacy rates for treating hypercalcemia. But the renal function sometimes prevents us from doing that. Denosumab does become an option for hypercalcemia, but again, I caution against its use for treating hypercalcemia in patients with myeloma due to the risk of advancing the myeloma.
Bone Marrow Transplant
Dr. Ascensão. Do you transplant for 1 or 2 bone marrows? What’s the best maintenance regimen postallograft, and when do you start? Do you use lenalidomide the first month of the transplant or do you wait until day 100?
Dr. Chauncey. From my perspective, hematopoietic stem cell transplantation has never really lost prominence. It is true that the concept of marrow transplantation for MM has been around for more than 20 years for those patients with first best response (Note that I’ll use best response rather than first remission). The concept was developed in an era when we had much less effective therapy, and in comparative trials, progressionfree survival was consistently superior and occasionally, overall survival was better with transplantation. As treatments got better, responses got better, and there were regular questions as to whether we still needed transplantation. But the data show that as responses got better, the progression-free survivals continued to improve, and transplantation still adds something to initial therapy.
Probably the most current data are from the Dana Farber- IFM trial for which Nikhil Munshi, MD, is an investigator. The trial includes induction with lenalidomide/bortezomib/dexamethasone, which is one of the more aggressive induction regimens. When upfront transplant vs delayed transplant are compared, it seems the preliminary data still favor having an upfront transplant after initial induction therapy.
The consensus is that autologous transplantation adds to the better response that we see with better induction therapy. Overall survival has become a less accessible endpoint since the initial trials, and that’s really a consequence of having better salvage therapy, and the confounding effects of subsequent treatments. We have so many options for salvage therapy that it’s now very hard to look at overall survival as an endpoint in trials of initial therapy.
A sometimes contentious question when it comes to payers, and less so in the VA, is how many transplants to do as part of initial therapy? Little Rock and the French did some of the pioneering work on tandem transplants. The BMT CTN 0702–StaMINA trial looks at this directly, and is mature and should be presented soon [Editorial Note: Preliminary results were presented at the American Society of Hematology meeting on December 6, 2016].
The approach at VAPSHCS and most other transplant centers has typically been to harvest a sufficient quantity of peripheral blood stem cells to do 2 transplants. If less than a very good partial response is achieved after the first transplant, then we do a second transplant in tandem fashion.
One exception would be for plasma cell leukemia, which is very aggressive. In that case, we would routinely perform tandem transplantation. We are unlikely to ever have a randomized trial that compares 1 vs 2 transplants in that particular setting.
Another question is whether a second autologous transplantation can be useful in a nontandem fashion, and there is a large amount of retrospective data about its use as salvage treatment. In eras when there were not as many effective therapies, salvage autologous transplant was more attractive. As new therapies came along, its use has somewhat waned, but there’s been renewed interest because dose-intensive melphalan with autologous rescue is relatively safe and not cross-resistant to other therapies. It also offers the option of a drug holiday after the transplant, whereas salvage drug therapy is typically continuous.
There is no universal agreement on nontandem second transplantation, there are no consistent algorithms to say when it is appropriate, but it’s worth discussing with the transplant programs, especially if there is a lot of toxicity with current salvage therapy.
The last question is the role of allogeneic transplantation, and while I’m generally a proponent of allogeneic transplantation for many diseases, in spite of some really significant efforts, the majority of allogeneic data for MM has not been very positive. The large BMT-CTN 0102 trial compared tandem autologous transplant at first response to a single autologous transplantation followed by reduced-intensity autologous transplantation from a matched sibling. This study was limited in part by enrollment bias, but the published results did not favor an allogeneic approach.3 Although there was less relapse in the allogeneic setting, the mortality of allogeneic transplant was not overcome by the decrease in relapse. Neither progression-free or overall survivals at 3 years were better in the allogeneic group.
Despite small studies showing feasibility and promising results, it’s currently very hard to advocate for allogeneic transplantation in MM. There are certainly centers that continue to have their own approach, with some in the U.S. that are pioneering tweaks on allogeneic regimens and graft engineering, but the data are typically small and anecdotal. That doesn’t mean that there won’t ultimately be a better way to do allogeneic transplantation in MM, but rather that we don’t currently know the best way to approach this strategy.
Next Steps in Myeloma Treatment
Dr. Ascensão. There are some people who are now starting to talk about a cure for myeloma. I’m not sure we’re there yet. Certainly, it’s a chronic disease that, if we can take care of the complications and maybe by starting treatment early. I’m not sure Agent Orange-exposed patients do better or worse. That’s something that needs to be researched if we can find a way to compare within this group and within the type of treatment that patients get.
Is it reasonable to start looking for minimal residual disease in cells? Should we shoot for the best response? I think one of the points that Dr. Chauncey made a number of times, and I agree, is that our patient population may not be able to tolerate some of the more aggressive therapies. Perhaps we need to find a slightly different version of this algorithm for VA patients.
Dr. Chauncey. There’s a diverse biology for both veterans and nonveterans alike. There are patients for whom a deeper response will lead to longer remission and better survival, and there are others whose disease will smolder with a lower tumor burden and not progress quickly. A lot of the early gene expression profiling data on this comes from Little Rock. Unfortunately, determination of an individual’s biology is not readily accessible in the clinic, and we are typically unable to clearly define each patient’s inherent disease biology.
Dr. Mehta. We just don’t have the answers as to exactly what to do with the information that we get except watch more closely and treat a little bit earlier. We don’t even know the significance of minimal residue disease and how often to test for it and if it correlates truly with longer-term survival. These are great research questions. We need to accumulate the data and try to analyze it. We need to participate in the big data programs.
Dr. Ascensão. The other thing, of course, is now we have new immunotherapy approaches beyond transplant, which includes some of the checkpoint inhibitors and there’s some exciting data coming out. So I think the future looks good.
We all are committed to treating our patients, our veterans, to the best of our abilities. And I think the VA has done a very good job in allowing us to do this for our patients and allowing us to provide the best treatments available out there.
Click here to read the digital edition.
Early Treatment and Diagnosis
Dr. Ascensão. An area that is becoming very important is identifying and separating smoldering multiple myeloma (SMM) from multiple myeloma (MM) and determining when to start treatment. At the Washington DC VAMC (DCVAMC) we started early on bisphosphonates and thalidomide without much benefit, but perhaps we were treating the wrong disease.
Dr. Mehta. Identifying patients as early as possible is often the best way to start. Treating early disease is easier than treating late disease, and it avoids all the complications. The problem is we don’t want to treat too many people because some of the people with SMM will never develop overt MM and, therefore, may not need treatment. We don’t have benign treatment yet. Whatever treatment we decide to use is going to carry adverse effects and toxicity.
So the trick is identifying those patients with SMM who are likely to progress in a finite period and, therefore, can be helped by treating early to avoid the complications of late diagnosis. We know that early treatment for patients with high-risk SMM helps. In a report from Lancet Oncology, early treatment with lenalidomide and dexamethasone reduces time to progression.1 There are other reports that treating early reduces time to progression.
So how do we identify those patients who are going to progress? We have a few clues. We know that patients who have a myeloma spike of > 1.5 g/dL are more likely to progress than others…The more discordant the / ratio from 1:1, the higher the risk for progression. And if that ratio is 1:100 or more, that would be a risk factor for progression.
We know from the work of Mayo Clinic researchers that if there are ≥ 60% of plasma cells in the bone marrow then it is a risk factor for progression. And we know from early studies that magnetic resonance imaging (MRI) detection of bone lesions, even long before they become detectable by X-ray, also is a risk factor for rapid development to myeloma.
...Methods such as genotyping, which we do here at the University of Arkansas for Medical Sciences, even in patients with MGUS (monoclonal gammopathy of undetermined significance), can identify high-risk patients, but that is not the standard of care yet. But it may become the standard of care in the days to come.
Another thing to think about for MGUS patients: Are there ways to identify what causes MGUS patients to evolve to SMM and then to overt myeloma, and to develop means of interrupting the progression cascade? There have been clinical trials on treatments (eg, bisphosphonates, thalidomide, aspirin, and cyclooxygenase inhibitors), but we haven’t found any safe, good treatment to prevent progression yet. With better technologies, we may be able to do that.
Dr. Ascensão. At the DCVAMC often we receive consults for a patient who had a little anemia, diabetes, renal disease, and the serum protein electrophoresis reveals a very small peak. How often should you follow patients? Do you do a complete workup the moment you see an MGUS or do you wait until they reach SMM?
Dr. Mehta. I don’t think every patient needs a complete workup. If you have obviously identifiable reasons for the anemia or the renal failure, then it’s less likely to be suspicious for myeloma. But patients with M spikes > 1 g/dL deserve a workup with a bone marrow aspirate and biopsy and at least bone X-rays, although MRIs would be even better.
I would differentiate based on the amount of M protein. Higher M protein patients deserve to have at least a bone marrow aspirate and bone study. Patients
with M protein > 1g/dL deserve to be seen every 3 to 4 months. I see patients with tiny little peaks every 6 months. And then, after 1 or 2 years, I turn over their care to the primary care doctor to follow. If we had research protocols to look at those patients and find the methods for progression, which I had at one point, then of course, we could see them more often and try to unravel the mystery.
Use of Imaging
Dr. Ascensão. That’s pretty close to what we do at DCVAMC. What do you think is the role for a bone survey as opposed to MRIs and positron emission tomography (PET) scans in this setting?
Dr. Mehta. In the real world X-rays are more accessible and much less expensive. So for the patient with very low risk who doesn’t have any complaints and
who has a low M spike, I think a bone survey is adequate. But you need about 30% to 40% bone destruction before you’re going to find anything on the X-ray.
MRIs are much more sensitive, plus they tell you about bone marrow involvement, but that should be reserved for the patient who has symptoms or a high
M protein. At Central Arkansas Veterans Healthcare System we simply can’t get PET scans for myeloma patients. At the myeloma center across the street from us, PET scans are used for routine evaluations.
Dr. Chauncey. I agree with Dr. Mehta. At VA Puget Sound Healthcare System (VAPSHCS) there isn’t a problem getting PET scans, but we probably get far fewer
scans than Arkansas. I still like the skeletal survey because it directs you where to look for potential pathologic fracture. It’s definitely not as sensitive as the dedicated myeloma MRI, but it’s a lot easier to get at VAPSHCS, especially as a screening tool.
Dr. Ascensão. Right, I believe there are some issues about the number of osteolytic lesions that may drive diagnosis.
Dr. Mehta. For patients with high M protein, I always request MRI. But the correlation is poorer in patients who have lower M protein. I try to limit it to the patients who have symptoms or high M protein, but I don’t have any evidence-based data to prove that’s the right way.
Dr. Ascensão. If you were going to start treatment of SMM that you believe is evolving to a more regular myeloma, do you do anything different than you would for any of the patients that you have identified as having active myeloma? Do you have different protocols for those patients as opposed to patients who present de novo with active myeloma?
Dr. Mehta. Those patients should be treated with the same drugs, an IMiD and a steroid. And the question is plus or minus a proteasome inhibitor. Studies have shown that an IMiD with a steroid gets much better results than using observation alone. Whether you would get even better results with the proteasome inhibitor remains to be seen. Maybe we can do that study.
Dr. Chauncey. We strive to identify high-risk SMM patients and treat them accordingly. Alternatively, physicians are pulling the trigger for therapy earlier and earlier and when they come for transplant with a diagnosis of MM, it is critical to review the initial diagnostic information. Most transplant centers have experience with this phenomena and know that they don’t want to transplant a non-high-risk SMM or any MGUS. However, by the time the patient is referred for transplantation, the initial clinical data are sometimes obscured or inaccessible.
Dr. Ascensão. We also look into the bone bearing areas, which allows us to make sure that if the patient has hip problems, we can work on how to approach them, whether we want to radiate those patients to prevent fractures.
Use of Bisphosphonates
Dr. Cosgriff. Myeloma metastasizes to bone, and it is one of the common sites of metastatic disease. It poses some interesting complications, whether it is from hypercalcemia due to metastatic sites, or pain syndromes. Bisphosphonates are indicated for myeloma, and they have been for years. Interestingly, unlike some of the other disease, the use of bisphosphonates induces apoptosis in myeloma. So we have seen some disease control with these agents.
The 2 bisphosphonates that are available for use are pamidronate and zoledronic acid. At the VA Portland Health Care System (VAPORHCS), we have been
using pamidronate exclusively for individuals with myeloma. There was a 2003 paper that evaluated the use of bisphosphonates for skeletal-related events in myeloma and in patients with metastatic breast cancer.2 In the subset analysis of myeloma patients with the bisphosphonates, there was no difference between pamidronate and zoledronic acid.
At the time, zoledronic acid was significantly more expensive than pamidronate, and so VAPORHCS opted to use pamidronate as a cost-saving measure. But there are the other reasons for picking pamidronate: Zoledronic acid has some dose recommendations and guidelines for individuals with renal failure, which is often a significant problem in patients with myeloma as well. To get around dose adjustments that need to be made for zoledronic acid, VAPORHCS switched to pamidronate, which is looser with the recommendations on renal failure.
Earlier use criteria, like the National Comprehensive Cancer Network guidelines, stated that if the renal failure was due to the disease itself and not some other outlying factor, a full 90-mg dose of pamidronate could still be used. That comment has since been removed. We still pay attention to it and reduce pamidronate dosing to 60 mg for patients with renal failure.
The prices for zoledronic acid have dropped significantly since it became a generic. The nice thing about zoledronic acid is that it has a short infusion time of 15 minutes. As chair space becomes a problem—VAPHCS has significant issues with that—zoledronic acid looks more and more attractive. The FDA label states that pamidronate should be infused over 4 hours, but VAPHCS typically has been infusing it for 3 hours.
It should be noted that denosumab (XGEVA), a monoclonal antibody that also is targeted for hypercalcemia, has been specifically excluded for myeloma. It
has no FDA indication for myeloma. It does have an indication for hypercalcemia. Whether or not you can state that the patient with myeloma is hypercalcemic, and that’s the reason you want to use it, it starts crossing into some gray area. The drug is still significantly more expensive and it seems to have similar efficacy rates compared with both pamidronate and zoledronic acid, so VAPHCS limits its use to individuals who would otherwise be contraindicated to zoledronic acid or pamidronate due to renal failure.
Dr. Ascensão. How often do you give it, every month, every 3 months?
Dr. Cosgriff. Currently, VAPORHCS is giving bisphosphonates every month whether in the chemotherapy unit or in the short stay unit. We are starting to reevaluate that. I have heard some emerging data that suggest we can use it once a quarter and get the same results. Those data are still emerging. It would be nice to be able to reduce the infusion frequency. But bisphosphonates adhere to bone and get incorporated into the bone matrix and stay there for an extended period of time, upwards of 6 months to a year, as with zoledronic acid.
Osteonecrosis
Dr. Ascensão. Do you require dental clearance prior to first dose?
Dr. Cosgriff. Bisphosphonates have a warning for 2% incidence of osteonecrosis of the jaw. Risk factors for the development of osteonecrosis of the jaw include poor dentition or major dental work, like extractions and illfitting dentures but not necessarily root canals. Ill-fitting dentures tend to rub on the gums and irritate the bone layer underneath. It’s the irritation of the bone that’s the biggest risk factor for osteonecrosis of the jaw.
We require that patients see the dentist because we’ve had individuals develop osteonecrosis eventhough we thought they had good dentition. If a patient is seeing a dentist outside of the VA system, we ask them to notify their dentist that they’re receiving bisphosphonates. Because of the risk and because we’ve had some individuals with good dentition develop it, VAPORHCS requires all patients, particularly those who are receiving zoledronic acid, to have dental evaluations. Denosumab also has a listed 2% incidence of osteonecrosis of the jaw, so those individuals also need to be evaluated by our dental service.
Dr. Ascensão. The DCVAMC has the same problem. I have a patient that presented primarily with a plasmacytoma, and we tried to get him to see the dentist. The dentist said, ‘You’ve got to get your teeth pulled.’ The patient has tried to see outside dentists and is finding all kinds of excuses because he would like to have implants.
Dr. Cosgriff. Anytime that you somehow damage or irritate that bone, that becomes a risk factor for the development of osteonecrosis. And for those individuals, we delay the bisphosphonate. If they’re having pain syndrome, we try to support them with opiates. We would love to be able to use nonsteroidal anti-inflammatory drugs—they have really good efficacy against bone pain—but renal function and renal failures prevent the use of those in a majority of patients. We start bisphosphonates as soon as dental clears them.
Dr. Mehta. Isn’t there a contraindication for denosumab and some evidence that it may worsen MM outcomes?
Dr. Cosgriff. When the drug first came on the market, it specifically stated in the package insert that it is not to be used in MM (it doesn’t state it specifically anymore). There is a thought that maybe some underlying mechanism exists that might stimulate some of the myeloma problems, which is why I get a little concerned when people say, “Well, I’m using it for hypercalcemia, I’m not using it to treat or to prevent a skeletal-related event in patients with myeloma.” That becomes a gray area and in that type of situation, I would recommend treating the hypercalcemia with a single dose and then switching the
patient to a bisphosphonate.
Dr. Mehta. And of course, bisphosphonates also lower calcium. They can be used to treat hypercalcemia.
Dr. Cosgriff. Yes. Zoledronic acid does have limitations in renal failure, though pamidronate doesn’t have quite the same limitations. The VAPORHCS tries to
use exclusively for hypercalcemia as well. The data show that when using zoledronic acid compared with pamidronate, you end up with the same outcomes as far as hypercalcemia. The zoledronic acid onset of action is a little faster, around 12 to 24 hours vs 48 to 72 hours with pamidronate, but you can get around that by using calcitonin over a short period; 48 hours is typically the maximum efficacy for calcitonin in treating hypercalcemia. So we use pamidronate in place of that, supplementing with calcitonin.
The result is that at 7 days, pamidronate and zoledronic acid show the same efficacy rates for treating hypercalcemia. But the renal function sometimes prevents us from doing that. Denosumab does become an option for hypercalcemia, but again, I caution against its use for treating hypercalcemia in patients with myeloma due to the risk of advancing the myeloma.
Bone Marrow Transplant
Dr. Ascensão. Do you transplant for 1 or 2 bone marrows? What’s the best maintenance regimen postallograft, and when do you start? Do you use lenalidomide the first month of the transplant or do you wait until day 100?
Dr. Chauncey. From my perspective, hematopoietic stem cell transplantation has never really lost prominence. It is true that the concept of marrow transplantation for MM has been around for more than 20 years for those patients with first best response (Note that I’ll use best response rather than first remission). The concept was developed in an era when we had much less effective therapy, and in comparative trials, progressionfree survival was consistently superior and occasionally, overall survival was better with transplantation. As treatments got better, responses got better, and there were regular questions as to whether we still needed transplantation. But the data show that as responses got better, the progression-free survivals continued to improve, and transplantation still adds something to initial therapy.
Probably the most current data are from the Dana Farber- IFM trial for which Nikhil Munshi, MD, is an investigator. The trial includes induction with lenalidomide/bortezomib/dexamethasone, which is one of the more aggressive induction regimens. When upfront transplant vs delayed transplant are compared, it seems the preliminary data still favor having an upfront transplant after initial induction therapy.
The consensus is that autologous transplantation adds to the better response that we see with better induction therapy. Overall survival has become a less accessible endpoint since the initial trials, and that’s really a consequence of having better salvage therapy, and the confounding effects of subsequent treatments. We have so many options for salvage therapy that it’s now very hard to look at overall survival as an endpoint in trials of initial therapy.
A sometimes contentious question when it comes to payers, and less so in the VA, is how many transplants to do as part of initial therapy? Little Rock and the French did some of the pioneering work on tandem transplants. The BMT CTN 0702–StaMINA trial looks at this directly, and is mature and should be presented soon [Editorial Note: Preliminary results were presented at the American Society of Hematology meeting on December 6, 2016].
The approach at VAPSHCS and most other transplant centers has typically been to harvest a sufficient quantity of peripheral blood stem cells to do 2 transplants. If less than a very good partial response is achieved after the first transplant, then we do a second transplant in tandem fashion.
One exception would be for plasma cell leukemia, which is very aggressive. In that case, we would routinely perform tandem transplantation. We are unlikely to ever have a randomized trial that compares 1 vs 2 transplants in that particular setting.
Another question is whether a second autologous transplantation can be useful in a nontandem fashion, and there is a large amount of retrospective data about its use as salvage treatment. In eras when there were not as many effective therapies, salvage autologous transplant was more attractive. As new therapies came along, its use has somewhat waned, but there’s been renewed interest because dose-intensive melphalan with autologous rescue is relatively safe and not cross-resistant to other therapies. It also offers the option of a drug holiday after the transplant, whereas salvage drug therapy is typically continuous.
There is no universal agreement on nontandem second transplantation, there are no consistent algorithms to say when it is appropriate, but it’s worth discussing with the transplant programs, especially if there is a lot of toxicity with current salvage therapy.
The last question is the role of allogeneic transplantation, and while I’m generally a proponent of allogeneic transplantation for many diseases, in spite of some really significant efforts, the majority of allogeneic data for MM has not been very positive. The large BMT-CTN 0102 trial compared tandem autologous transplant at first response to a single autologous transplantation followed by reduced-intensity autologous transplantation from a matched sibling. This study was limited in part by enrollment bias, but the published results did not favor an allogeneic approach.3 Although there was less relapse in the allogeneic setting, the mortality of allogeneic transplant was not overcome by the decrease in relapse. Neither progression-free or overall survivals at 3 years were better in the allogeneic group.
Despite small studies showing feasibility and promising results, it’s currently very hard to advocate for allogeneic transplantation in MM. There are certainly centers that continue to have their own approach, with some in the U.S. that are pioneering tweaks on allogeneic regimens and graft engineering, but the data are typically small and anecdotal. That doesn’t mean that there won’t ultimately be a better way to do allogeneic transplantation in MM, but rather that we don’t currently know the best way to approach this strategy.
Next Steps in Myeloma Treatment
Dr. Ascensão. There are some people who are now starting to talk about a cure for myeloma. I’m not sure we’re there yet. Certainly, it’s a chronic disease that, if we can take care of the complications and maybe by starting treatment early. I’m not sure Agent Orange-exposed patients do better or worse. That’s something that needs to be researched if we can find a way to compare within this group and within the type of treatment that patients get.
Is it reasonable to start looking for minimal residual disease in cells? Should we shoot for the best response? I think one of the points that Dr. Chauncey made a number of times, and I agree, is that our patient population may not be able to tolerate some of the more aggressive therapies. Perhaps we need to find a slightly different version of this algorithm for VA patients.
Dr. Chauncey. There’s a diverse biology for both veterans and nonveterans alike. There are patients for whom a deeper response will lead to longer remission and better survival, and there are others whose disease will smolder with a lower tumor burden and not progress quickly. A lot of the early gene expression profiling data on this comes from Little Rock. Unfortunately, determination of an individual’s biology is not readily accessible in the clinic, and we are typically unable to clearly define each patient’s inherent disease biology.
Dr. Mehta. We just don’t have the answers as to exactly what to do with the information that we get except watch more closely and treat a little bit earlier. We don’t even know the significance of minimal residue disease and how often to test for it and if it correlates truly with longer-term survival. These are great research questions. We need to accumulate the data and try to analyze it. We need to participate in the big data programs.
Dr. Ascensão. The other thing, of course, is now we have new immunotherapy approaches beyond transplant, which includes some of the checkpoint inhibitors and there’s some exciting data coming out. So I think the future looks good.
We all are committed to treating our patients, our veterans, to the best of our abilities. And I think the VA has done a very good job in allowing us to do this for our patients and allowing us to provide the best treatments available out there.
Click here to read the digital edition.
1. Mateos MV, Hernández MT, Giraldo P, et al. Lenalidomide plus dexamethasone versus observation in patients with high-risk smouldering multiple myeloma (QuiRedex): long-term follow-up of a randomised, controlled, phase 3 trial. Lancet Oncol. 2016;17(8):1127-1136.
2. Rosen LS, Gordon D, Kaminski M, et al. Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma: a randomized, double-blind, multicenter, comparative trial. Cancer. 2003;98(8):1735-1744.
3. Krishnan A, Pasquini MC, Logan B, et al; Blood Marrow Transplant Clinical Trials Network (BMT CTN). Autologous haemopoietic stem-cell transplantation followed by allogeneic or autologous haemopoietic stem-cell transplantation in patients with multiple myeloma (BMT CTN 0102): a phase 3 biological assignment trial. Lancet Oncol. 2011;12(13):1195-11203.
1. Mateos MV, Hernández MT, Giraldo P, et al. Lenalidomide plus dexamethasone versus observation in patients with high-risk smouldering multiple myeloma (QuiRedex): long-term follow-up of a randomised, controlled, phase 3 trial. Lancet Oncol. 2016;17(8):1127-1136.
2. Rosen LS, Gordon D, Kaminski M, et al. Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma: a randomized, double-blind, multicenter, comparative trial. Cancer. 2003;98(8):1735-1744.
3. Krishnan A, Pasquini MC, Logan B, et al; Blood Marrow Transplant Clinical Trials Network (BMT CTN). Autologous haemopoietic stem-cell transplantation followed by allogeneic or autologous haemopoietic stem-cell transplantation in patients with multiple myeloma (BMT CTN 0102): a phase 3 biological assignment trial. Lancet Oncol. 2011;12(13):1195-11203.
Getting hematologic cancer drugs on the fast track
LA JOLLA, CALIF. – The words “rapid approval” and “Food and Drug Administration” rarely appear in the same sentence. But despite that perception, the pace of hematologic drug development has been accelerating over the last several years, according to an agency staffer.
“FDA is committed toward the expedited development of safe and effective therapies for serious and life-threatening diseases,” R. Angelo de Claro, MD, of the FDA’s Office of Hematology and Oncology Products said at the annual T-cell Lymphoma Forum. Dr. de Claro outlined his agency’s efforts to accelerate approval of drugs for treatment of T-cell malignancies.
Hematologic drug bonanza
In 2017 alone, the FDA approved 17 agents for new or expanded indications for hematologic malignancies, including brentuximab vedotin (Adcetris) for anaplastic large cell lymphoma (ALCL) and CD30-positive mycosis fungoides (MF).
Approval was based on a 56% objective response rate for brentuximab vedotin versus 12% for physician’s choice in a phase 3 trial (ALCANZA) of 131 patients with mycosis fungoides or primary cutaneous ALCL. All patients had received one prior systemic therapy and were randomized (1:1) to receive either brentuximab vedotin or the physician’s choice of methotrexate or bexarotene.
Dr. de Claro noted that in the ALCANZA trial, patients were required to have one or more biopsy samples with at least 10% CD30 expression, but among 184 patients with MF screened for the trial, 32% were ineligible because of less than 10% CD30 expression. The FDA therefore requested additional efficacy data for patients with MF with less than 10% CD30 expression and accepted data from two investigator-sponsored trials showing that 35 patients with MF expressing CD30 on 1%-9% of cells had a 31% overall response rate, whereas two patients with no CD30 expression did not have responses.
Who minds the store
Hematology products are under the aegis of the FDA’s Oncology Center of Excellence. Oversight includes benign hematology products, as well as products for hematologic cancers and hematologic support. Hematology and oncology toxicology is monitored by pharmacologists and toxicologists in a separate division, he explained.
“The Oncology Center of Excellence was formally launched in 2017 as part of the 21st century CURES Act. The mission of the Oncology Center of Excellence is to achieve patient-centered regulatory decision making through innovation and collaboration,” he said.
Getting the nod
To get approved, a new therapy requires “substantial” evidence of efficacy and safety. Regular approvals are based on either direct measures of clinical benefits – how a patient “feels, functions, or survives” – or a measure of the effect of a drug on an established surrogate endpoint.
For an accelerated approval, developers must be able to show evidence on either a surrogate or intermediate clinical endpoint that the agent is reasonably likely to offer a benefit and be a meaningful improvement over available therapies. Postapproval trials may be needed to verify the proposed benefits.
FDA accelerated approval programs include:
- Fast track. The pathway requires nonclinical or clinical data demonstrating the potential for addressing an unmet need.
- Breakthrough therapy. This pathway requires preliminary clinical evidence demonstrating substantial improvement over existing available therapies.
- Priority review. These are agents that, if approved, would provide significant improvements in safety or effectiveness.
- Accelerated approval. The drug must demonstrate an effect on an “endpoint reasonably likely to predict clinical benefit over available therapies.”
Dr. de Claro is employed by the FDA. The T-Cell Lymphoma Forum is held by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. – The words “rapid approval” and “Food and Drug Administration” rarely appear in the same sentence. But despite that perception, the pace of hematologic drug development has been accelerating over the last several years, according to an agency staffer.
“FDA is committed toward the expedited development of safe and effective therapies for serious and life-threatening diseases,” R. Angelo de Claro, MD, of the FDA’s Office of Hematology and Oncology Products said at the annual T-cell Lymphoma Forum. Dr. de Claro outlined his agency’s efforts to accelerate approval of drugs for treatment of T-cell malignancies.
Hematologic drug bonanza
In 2017 alone, the FDA approved 17 agents for new or expanded indications for hematologic malignancies, including brentuximab vedotin (Adcetris) for anaplastic large cell lymphoma (ALCL) and CD30-positive mycosis fungoides (MF).
Approval was based on a 56% objective response rate for brentuximab vedotin versus 12% for physician’s choice in a phase 3 trial (ALCANZA) of 131 patients with mycosis fungoides or primary cutaneous ALCL. All patients had received one prior systemic therapy and were randomized (1:1) to receive either brentuximab vedotin or the physician’s choice of methotrexate or bexarotene.
Dr. de Claro noted that in the ALCANZA trial, patients were required to have one or more biopsy samples with at least 10% CD30 expression, but among 184 patients with MF screened for the trial, 32% were ineligible because of less than 10% CD30 expression. The FDA therefore requested additional efficacy data for patients with MF with less than 10% CD30 expression and accepted data from two investigator-sponsored trials showing that 35 patients with MF expressing CD30 on 1%-9% of cells had a 31% overall response rate, whereas two patients with no CD30 expression did not have responses.
Who minds the store
Hematology products are under the aegis of the FDA’s Oncology Center of Excellence. Oversight includes benign hematology products, as well as products for hematologic cancers and hematologic support. Hematology and oncology toxicology is monitored by pharmacologists and toxicologists in a separate division, he explained.
“The Oncology Center of Excellence was formally launched in 2017 as part of the 21st century CURES Act. The mission of the Oncology Center of Excellence is to achieve patient-centered regulatory decision making through innovation and collaboration,” he said.
Getting the nod
To get approved, a new therapy requires “substantial” evidence of efficacy and safety. Regular approvals are based on either direct measures of clinical benefits – how a patient “feels, functions, or survives” – or a measure of the effect of a drug on an established surrogate endpoint.
For an accelerated approval, developers must be able to show evidence on either a surrogate or intermediate clinical endpoint that the agent is reasonably likely to offer a benefit and be a meaningful improvement over available therapies. Postapproval trials may be needed to verify the proposed benefits.
FDA accelerated approval programs include:
- Fast track. The pathway requires nonclinical or clinical data demonstrating the potential for addressing an unmet need.
- Breakthrough therapy. This pathway requires preliminary clinical evidence demonstrating substantial improvement over existing available therapies.
- Priority review. These are agents that, if approved, would provide significant improvements in safety or effectiveness.
- Accelerated approval. The drug must demonstrate an effect on an “endpoint reasonably likely to predict clinical benefit over available therapies.”
Dr. de Claro is employed by the FDA. The T-Cell Lymphoma Forum is held by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. – The words “rapid approval” and “Food and Drug Administration” rarely appear in the same sentence. But despite that perception, the pace of hematologic drug development has been accelerating over the last several years, according to an agency staffer.
“FDA is committed toward the expedited development of safe and effective therapies for serious and life-threatening diseases,” R. Angelo de Claro, MD, of the FDA’s Office of Hematology and Oncology Products said at the annual T-cell Lymphoma Forum. Dr. de Claro outlined his agency’s efforts to accelerate approval of drugs for treatment of T-cell malignancies.
Hematologic drug bonanza
In 2017 alone, the FDA approved 17 agents for new or expanded indications for hematologic malignancies, including brentuximab vedotin (Adcetris) for anaplastic large cell lymphoma (ALCL) and CD30-positive mycosis fungoides (MF).
Approval was based on a 56% objective response rate for brentuximab vedotin versus 12% for physician’s choice in a phase 3 trial (ALCANZA) of 131 patients with mycosis fungoides or primary cutaneous ALCL. All patients had received one prior systemic therapy and were randomized (1:1) to receive either brentuximab vedotin or the physician’s choice of methotrexate or bexarotene.
Dr. de Claro noted that in the ALCANZA trial, patients were required to have one or more biopsy samples with at least 10% CD30 expression, but among 184 patients with MF screened for the trial, 32% were ineligible because of less than 10% CD30 expression. The FDA therefore requested additional efficacy data for patients with MF with less than 10% CD30 expression and accepted data from two investigator-sponsored trials showing that 35 patients with MF expressing CD30 on 1%-9% of cells had a 31% overall response rate, whereas two patients with no CD30 expression did not have responses.
Who minds the store
Hematology products are under the aegis of the FDA’s Oncology Center of Excellence. Oversight includes benign hematology products, as well as products for hematologic cancers and hematologic support. Hematology and oncology toxicology is monitored by pharmacologists and toxicologists in a separate division, he explained.
“The Oncology Center of Excellence was formally launched in 2017 as part of the 21st century CURES Act. The mission of the Oncology Center of Excellence is to achieve patient-centered regulatory decision making through innovation and collaboration,” he said.
Getting the nod
To get approved, a new therapy requires “substantial” evidence of efficacy and safety. Regular approvals are based on either direct measures of clinical benefits – how a patient “feels, functions, or survives” – or a measure of the effect of a drug on an established surrogate endpoint.
For an accelerated approval, developers must be able to show evidence on either a surrogate or intermediate clinical endpoint that the agent is reasonably likely to offer a benefit and be a meaningful improvement over available therapies. Postapproval trials may be needed to verify the proposed benefits.
FDA accelerated approval programs include:
- Fast track. The pathway requires nonclinical or clinical data demonstrating the potential for addressing an unmet need.
- Breakthrough therapy. This pathway requires preliminary clinical evidence demonstrating substantial improvement over existing available therapies.
- Priority review. These are agents that, if approved, would provide significant improvements in safety or effectiveness.
- Accelerated approval. The drug must demonstrate an effect on an “endpoint reasonably likely to predict clinical benefit over available therapies.”
Dr. de Claro is employed by the FDA. The T-Cell Lymphoma Forum is held by Jonathan Wood & Associates, which is owned by the same company as this news organization.
EXPERT ANALYSIS FROM TCLF 2018
Meningococcal Arthritis Masking as Possible Myeloma
For a group of clinicians in Australia, the diagnosis of meningococcal arthritis was “straightforward” except for abnormal serum total protein, anemia, and immunoglobulin results, which suggested their patient might have a hematological disorder such as myeloma.
The patient came to the hospital after 4 days of worsening knee and arm pain so severe he could not stand. His knees and both wrists showed swelling but no palpable lymphadenopathy or hepatosplenomegaly. The patient’s medical history showed he was taking no regular medications.
Joint aspiration grew Neisseria meningitidis. The patient’s blood tests showed hemoglobin 126 g/dL, white blood cell count 15.3 x 109/L, an unusually high total protein level (100 g/L), and an IgM kappa paraprotein band of 45 g/L on protein electrophoresis. A computed tomography scan showed widespread lymphadenopathy, hepatosplenomegaly, and multilevel thoracic vertebral collapse. A bone marrow biopsy showed evidence of a lymphocytic infiltrate, with lymphoplasmacytoid differentiation.
The histology best fitted a diagnosis of nodal marginal zone lymphoma with plasmacytic differentiation, the clinicians say. Having ruled out other possibilities, they settled on non-Hodgkin lymphoma.
Initially, the suspicion was that the patient had septic arthritis due to Staphylococcus aureus (the most common organism isolated in septic arthritis), and he was given piperacillin/tazobactam. That was changed to flucloxacillin and then to ceftriaxone after the result of N meningitidis. The patient also was treated with rituximab and bendamustine for the lymphoma with a complete remission.
Meningococcal infection presenting as septic arthritis in the case of invasive meningococcemia is rare, the clinicians say, but primary meningococcal arthritis is even rarer. The case highlights the important aspect that “diagnosis of one condition can lead to diagnosis of another”—in this case, the lymphoma-weakened immune system led to the symptoms of polyarthropathy and the diagnosis of primary meningococcal arthritis. The clinicians also cited a case of a patient who presented with meningococcal meningitis and arthritis who was found to have an underlying Waldenström disease, and a patient whose HIV was diagnosed again after the patient presented with meningococcal arthritis symptoms.
The clinicans say such cases underscore the importance of screening for an underlying impaired immune response in patients presenting with rare conditions such as meningococcal arthritis.
For a group of clinicians in Australia, the diagnosis of meningococcal arthritis was “straightforward” except for abnormal serum total protein, anemia, and immunoglobulin results, which suggested their patient might have a hematological disorder such as myeloma.
The patient came to the hospital after 4 days of worsening knee and arm pain so severe he could not stand. His knees and both wrists showed swelling but no palpable lymphadenopathy or hepatosplenomegaly. The patient’s medical history showed he was taking no regular medications.
Joint aspiration grew Neisseria meningitidis. The patient’s blood tests showed hemoglobin 126 g/dL, white blood cell count 15.3 x 109/L, an unusually high total protein level (100 g/L), and an IgM kappa paraprotein band of 45 g/L on protein electrophoresis. A computed tomography scan showed widespread lymphadenopathy, hepatosplenomegaly, and multilevel thoracic vertebral collapse. A bone marrow biopsy showed evidence of a lymphocytic infiltrate, with lymphoplasmacytoid differentiation.
The histology best fitted a diagnosis of nodal marginal zone lymphoma with plasmacytic differentiation, the clinicians say. Having ruled out other possibilities, they settled on non-Hodgkin lymphoma.
Initially, the suspicion was that the patient had septic arthritis due to Staphylococcus aureus (the most common organism isolated in septic arthritis), and he was given piperacillin/tazobactam. That was changed to flucloxacillin and then to ceftriaxone after the result of N meningitidis. The patient also was treated with rituximab and bendamustine for the lymphoma with a complete remission.
Meningococcal infection presenting as septic arthritis in the case of invasive meningococcemia is rare, the clinicians say, but primary meningococcal arthritis is even rarer. The case highlights the important aspect that “diagnosis of one condition can lead to diagnosis of another”—in this case, the lymphoma-weakened immune system led to the symptoms of polyarthropathy and the diagnosis of primary meningococcal arthritis. The clinicians also cited a case of a patient who presented with meningococcal meningitis and arthritis who was found to have an underlying Waldenström disease, and a patient whose HIV was diagnosed again after the patient presented with meningococcal arthritis symptoms.
The clinicans say such cases underscore the importance of screening for an underlying impaired immune response in patients presenting with rare conditions such as meningococcal arthritis.
For a group of clinicians in Australia, the diagnosis of meningococcal arthritis was “straightforward” except for abnormal serum total protein, anemia, and immunoglobulin results, which suggested their patient might have a hematological disorder such as myeloma.
The patient came to the hospital after 4 days of worsening knee and arm pain so severe he could not stand. His knees and both wrists showed swelling but no palpable lymphadenopathy or hepatosplenomegaly. The patient’s medical history showed he was taking no regular medications.
Joint aspiration grew Neisseria meningitidis. The patient’s blood tests showed hemoglobin 126 g/dL, white blood cell count 15.3 x 109/L, an unusually high total protein level (100 g/L), and an IgM kappa paraprotein band of 45 g/L on protein electrophoresis. A computed tomography scan showed widespread lymphadenopathy, hepatosplenomegaly, and multilevel thoracic vertebral collapse. A bone marrow biopsy showed evidence of a lymphocytic infiltrate, with lymphoplasmacytoid differentiation.
The histology best fitted a diagnosis of nodal marginal zone lymphoma with plasmacytic differentiation, the clinicians say. Having ruled out other possibilities, they settled on non-Hodgkin lymphoma.
Initially, the suspicion was that the patient had septic arthritis due to Staphylococcus aureus (the most common organism isolated in septic arthritis), and he was given piperacillin/tazobactam. That was changed to flucloxacillin and then to ceftriaxone after the result of N meningitidis. The patient also was treated with rituximab and bendamustine for the lymphoma with a complete remission.
Meningococcal infection presenting as septic arthritis in the case of invasive meningococcemia is rare, the clinicians say, but primary meningococcal arthritis is even rarer. The case highlights the important aspect that “diagnosis of one condition can lead to diagnosis of another”—in this case, the lymphoma-weakened immune system led to the symptoms of polyarthropathy and the diagnosis of primary meningococcal arthritis. The clinicians also cited a case of a patient who presented with meningococcal meningitis and arthritis who was found to have an underlying Waldenström disease, and a patient whose HIV was diagnosed again after the patient presented with meningococcal arthritis symptoms.
The clinicans say such cases underscore the importance of screening for an underlying impaired immune response in patients presenting with rare conditions such as meningococcal arthritis.
Triple therapy ups response in refractory mantle cell lymphoma
A combination of ibrutinib, lenalidomide, and rituximab produced an overall response rate of 76% at 17.8 months median follow-up among 50 adults with relapsed or refractory mantle cell lymphoma, according to an open-label, single-arm, phase 2 trial.
There were complete responses in 28 patients (56%) and partial responses in 10 (20%). Median progression-free survival was 16 months and median overall survival was 22 months. Similar proportions of patients, with and without TP53 mutations, had overall and complete responses, suggesting that triple therapy might be particularly useful in patients with high-risk genetic features.
“Our results provide preliminary evidence that the triplet combination of ibrutinib, lenalidomide, and rituximab is an active regimen in patients with relapsed or refractory mantle cell lymphoma, and should be evaluated in a prospective randomized controlled trial,” wrote Mats Jerkeman, MD, of Lund University, Sweden, and colleagues. The report was published in The Lancet Haematology.
“Addition of lenalidomide to ibrutinib and rituximab might increase the proportion of patients who have complete remission ... Previous studies reported complete responses in 44% of patients on ibrutinib and rituximab, in 36% of patients on rituximab and lenalidomide, and in 19% of patients on ibrutinib alone,” they wrote.
Treatment was divided into an induction phase of 12 cycles of 28 days with all three drugs and a maintenance phase with ibrutinib and rituximab only, given until disease progression or unacceptable toxicity. All the patients had previously been treated with at least one rituximab-containing regimen.
Janssen and Celgene funded the work. Dr. Jerkeman reported ties to Janssen and Celgene, as well as AbbVie and Gilead.
SOURCE: Jerkeman M et al. Lancet Haematol. 2018 Jan 29. doi: 10.1016/S2352-3026(18)30018-8.
A combination of ibrutinib, lenalidomide, and rituximab produced an overall response rate of 76% at 17.8 months median follow-up among 50 adults with relapsed or refractory mantle cell lymphoma, according to an open-label, single-arm, phase 2 trial.
There were complete responses in 28 patients (56%) and partial responses in 10 (20%). Median progression-free survival was 16 months and median overall survival was 22 months. Similar proportions of patients, with and without TP53 mutations, had overall and complete responses, suggesting that triple therapy might be particularly useful in patients with high-risk genetic features.
“Our results provide preliminary evidence that the triplet combination of ibrutinib, lenalidomide, and rituximab is an active regimen in patients with relapsed or refractory mantle cell lymphoma, and should be evaluated in a prospective randomized controlled trial,” wrote Mats Jerkeman, MD, of Lund University, Sweden, and colleagues. The report was published in The Lancet Haematology.
“Addition of lenalidomide to ibrutinib and rituximab might increase the proportion of patients who have complete remission ... Previous studies reported complete responses in 44% of patients on ibrutinib and rituximab, in 36% of patients on rituximab and lenalidomide, and in 19% of patients on ibrutinib alone,” they wrote.
Treatment was divided into an induction phase of 12 cycles of 28 days with all three drugs and a maintenance phase with ibrutinib and rituximab only, given until disease progression or unacceptable toxicity. All the patients had previously been treated with at least one rituximab-containing regimen.
Janssen and Celgene funded the work. Dr. Jerkeman reported ties to Janssen and Celgene, as well as AbbVie and Gilead.
SOURCE: Jerkeman M et al. Lancet Haematol. 2018 Jan 29. doi: 10.1016/S2352-3026(18)30018-8.
A combination of ibrutinib, lenalidomide, and rituximab produced an overall response rate of 76% at 17.8 months median follow-up among 50 adults with relapsed or refractory mantle cell lymphoma, according to an open-label, single-arm, phase 2 trial.
There were complete responses in 28 patients (56%) and partial responses in 10 (20%). Median progression-free survival was 16 months and median overall survival was 22 months. Similar proportions of patients, with and without TP53 mutations, had overall and complete responses, suggesting that triple therapy might be particularly useful in patients with high-risk genetic features.
“Our results provide preliminary evidence that the triplet combination of ibrutinib, lenalidomide, and rituximab is an active regimen in patients with relapsed or refractory mantle cell lymphoma, and should be evaluated in a prospective randomized controlled trial,” wrote Mats Jerkeman, MD, of Lund University, Sweden, and colleagues. The report was published in The Lancet Haematology.
“Addition of lenalidomide to ibrutinib and rituximab might increase the proportion of patients who have complete remission ... Previous studies reported complete responses in 44% of patients on ibrutinib and rituximab, in 36% of patients on rituximab and lenalidomide, and in 19% of patients on ibrutinib alone,” they wrote.
Treatment was divided into an induction phase of 12 cycles of 28 days with all three drugs and a maintenance phase with ibrutinib and rituximab only, given until disease progression or unacceptable toxicity. All the patients had previously been treated with at least one rituximab-containing regimen.
Janssen and Celgene funded the work. Dr. Jerkeman reported ties to Janssen and Celgene, as well as AbbVie and Gilead.
SOURCE: Jerkeman M et al. Lancet Haematol. 2018 Jan 29. doi: 10.1016/S2352-3026(18)30018-8.
FROM THE LANCET HAEMATOLOGY
Key clinical point:
Major finding: The overall response from for the combination of the three drugs was 76% at 17.8 months median follow-up.
Study details: An open-label, single-arm, phase 2 trial of 50 adults with relapsed/refractory MCL.
Disclosures: Janssen and Celgene funded the work. Dr. Jerkeman reported ties to Janssen and Celgene, as well as AbbVie and Gilead.
Source: Jerkeman M et al. Lancet Haematol. 2018 Jan 29. doi: 10.1016/S2352-3026(18)30018-8.
Rituximab Still Proves Safe Long Term
Rituximab, a B-cell–depleting agent, has been found safe and effective in clinical trials of patients with multiple sclerosis and patients with rheumatoid arthritis, among others. However, progressive multifocal leukoencephalopathy (PML) and malignancies have been reported in patients with lymphoma, rheumatoid arthritis, and lupus who also received multiple immunosuppressive therapies, say researchers from Wayne State University in Michigan and University of Chicago in Illinois. In studies with ocrelizumab, which also depletes B-cells, adverse effects (AEs) have included infections, such as, herpes virus-associated infection, and neoplasms.
Although most research has found rituximab and ocrelizumab safe and effective, there is a “paucity of literature” on the safety of continuous B-cell depletion over a long period, the researchers say. They conducted a retrospective study involving 29 patients with immune-mediated neurologic disorders who received continuous cycles of rituximab infusions every 6 to 9 months for up to 7 years. Although small, the study was longer than the trials with ocrelizumab in multiple sclerosis . The mean duration of treatment was 51 months; with a mean of 9 treatment cycles.
The researchers found a low incidence of adverse events and prolonged rituximab-induced B-cell depletion did not lead to any life-threatening AEs, including malignancy. Overall, 32 AEs were reported. Four were serious; 3 were noted after 9 cycles (48 months), and 1 after 11 cycles (60 months). There were no cases of PML or malignancies. Repeated rituximab infusions were well tolerated The rate of AEs remained low over the 7-year observation period.
Source:
Memon AB, Javed A, Caon C, et al. PLoS ONE. 2018;13(1):e0190425.
doi: 10.1371/journal.pone.0190425.
Rituximab, a B-cell–depleting agent, has been found safe and effective in clinical trials of patients with multiple sclerosis and patients with rheumatoid arthritis, among others. However, progressive multifocal leukoencephalopathy (PML) and malignancies have been reported in patients with lymphoma, rheumatoid arthritis, and lupus who also received multiple immunosuppressive therapies, say researchers from Wayne State University in Michigan and University of Chicago in Illinois. In studies with ocrelizumab, which also depletes B-cells, adverse effects (AEs) have included infections, such as, herpes virus-associated infection, and neoplasms.
Although most research has found rituximab and ocrelizumab safe and effective, there is a “paucity of literature” on the safety of continuous B-cell depletion over a long period, the researchers say. They conducted a retrospective study involving 29 patients with immune-mediated neurologic disorders who received continuous cycles of rituximab infusions every 6 to 9 months for up to 7 years. Although small, the study was longer than the trials with ocrelizumab in multiple sclerosis . The mean duration of treatment was 51 months; with a mean of 9 treatment cycles.
The researchers found a low incidence of adverse events and prolonged rituximab-induced B-cell depletion did not lead to any life-threatening AEs, including malignancy. Overall, 32 AEs were reported. Four were serious; 3 were noted after 9 cycles (48 months), and 1 after 11 cycles (60 months). There were no cases of PML or malignancies. Repeated rituximab infusions were well tolerated The rate of AEs remained low over the 7-year observation period.
Source:
Memon AB, Javed A, Caon C, et al. PLoS ONE. 2018;13(1):e0190425.
doi: 10.1371/journal.pone.0190425.
Rituximab, a B-cell–depleting agent, has been found safe and effective in clinical trials of patients with multiple sclerosis and patients with rheumatoid arthritis, among others. However, progressive multifocal leukoencephalopathy (PML) and malignancies have been reported in patients with lymphoma, rheumatoid arthritis, and lupus who also received multiple immunosuppressive therapies, say researchers from Wayne State University in Michigan and University of Chicago in Illinois. In studies with ocrelizumab, which also depletes B-cells, adverse effects (AEs) have included infections, such as, herpes virus-associated infection, and neoplasms.
Although most research has found rituximab and ocrelizumab safe and effective, there is a “paucity of literature” on the safety of continuous B-cell depletion over a long period, the researchers say. They conducted a retrospective study involving 29 patients with immune-mediated neurologic disorders who received continuous cycles of rituximab infusions every 6 to 9 months for up to 7 years. Although small, the study was longer than the trials with ocrelizumab in multiple sclerosis . The mean duration of treatment was 51 months; with a mean of 9 treatment cycles.
The researchers found a low incidence of adverse events and prolonged rituximab-induced B-cell depletion did not lead to any life-threatening AEs, including malignancy. Overall, 32 AEs were reported. Four were serious; 3 were noted after 9 cycles (48 months), and 1 after 11 cycles (60 months). There were no cases of PML or malignancies. Repeated rituximab infusions were well tolerated The rate of AEs remained low over the 7-year observation period.
Source:
Memon AB, Javed A, Caon C, et al. PLoS ONE. 2018;13(1):e0190425.
doi: 10.1371/journal.pone.0190425.
CLL drug in limited supply outside U.S.
Ofatumumab (Arzerra), a monoclonal antibody treatment for chronic lymphocytic leukemia, will soon be available outside the United States through compassionate use programs only. The drug will continue to be widely available in the United States.
Novartis announced in January that it would begin limiting the availability of the drug outside of the United States and would work with regulatory authorities to set up compassionate use programs for patients who are currently being treated with the drug. Patients who use these programs will receive the drug for free.
The decision was driven by the surge in CLL drugs that have become available over the last 5 years, according to Novartis.
The decision to pull the drug from international markets will not affect its use in ongoing clinical trials, particularly two phase 3 studies in relapsing multiple sclerosis and indolent non-Hodgkin lymphoma.
Ofatumumab (Arzerra), a monoclonal antibody treatment for chronic lymphocytic leukemia, will soon be available outside the United States through compassionate use programs only. The drug will continue to be widely available in the United States.
Novartis announced in January that it would begin limiting the availability of the drug outside of the United States and would work with regulatory authorities to set up compassionate use programs for patients who are currently being treated with the drug. Patients who use these programs will receive the drug for free.
The decision was driven by the surge in CLL drugs that have become available over the last 5 years, according to Novartis.
The decision to pull the drug from international markets will not affect its use in ongoing clinical trials, particularly two phase 3 studies in relapsing multiple sclerosis and indolent non-Hodgkin lymphoma.
Ofatumumab (Arzerra), a monoclonal antibody treatment for chronic lymphocytic leukemia, will soon be available outside the United States through compassionate use programs only. The drug will continue to be widely available in the United States.
Novartis announced in January that it would begin limiting the availability of the drug outside of the United States and would work with regulatory authorities to set up compassionate use programs for patients who are currently being treated with the drug. Patients who use these programs will receive the drug for free.
The decision was driven by the surge in CLL drugs that have become available over the last 5 years, according to Novartis.
The decision to pull the drug from international markets will not affect its use in ongoing clinical trials, particularly two phase 3 studies in relapsing multiple sclerosis and indolent non-Hodgkin lymphoma.
Continue to opt for HDT/ASCT for multiple myeloma
High-dose therapy with melphalan followed by autologous stem cell transplant (HDT/ASCT) is still the best option for multiple myeloma even after almost 2 decades with newer and highly effective induction agents, according to a recent systematic review and two meta-analyses.
Given the “unprecedented efficacy” of “modern induction therapy with immunomodulatory drugs and proteasome inhibitors (also called ‘novel agents’),” investigators “have sought to reevaluate the role of HDT/ASCT,” wrote Binod Dhakal, MD, of the Medical College of Wisconsin, and his colleagues. The report is in JAMA Oncology.
To solve the issue, they analyzed five randomized controlled trials conducted since 2000 and concluded that HDT/ASCT is still the preferred treatment approach.
Despite a lack of demonstrable overall survival benefit, there is a significant progression-free survival (PFS) benefit, low treatment-related mortality, and potential high minimal residual disease-negative rates conferred by HDT/ASCT in newly-diagnosed multiple myeloma, the researchers noted.
The combined odds for complete response were 1.27 (95% confidence interval, 0.97-1.65, P = .07) with HDT/ASCT, compared with standard-dose therapy (SDT). The combined hazard ratio (HR) for PFS was 0.55 (95% CI, 0.41-0.7, P less than .001) and 0.76 for overall survival (95% CI, 0.42-1.36, P = .20) in favor of HDT.
PFS was best with tandem HDT/ASCT (HR, 0.49, 95% CI, 0.37-0.65) followed by single HDT/ASCT with bortezomib, lenalidomide, and dexamethasone consolidation (HR, 0.53, 95% CI, 0.37-0.76) and single HDT/ASCT alone (HR, 0.68, 95% CI, 0.53-0.87), compared with SDT. However, none of the HDT/ASCT approaches had a significant impact on overall survival.
Meanwhile, treatment-related mortality with HDT/ASCT was minimal, at less than 1%.
“The achievement of high [minimal residual disease] rates with HDT/ASCT may render this approach the ideal platform for testing novel approaches (e.g., immunotherapy) aiming at disease eradication and cures,” the researchers wrote.
The researchers reported relationships with a number of companies, including Takeda, Celgene, and Amgen, that make novel induction agents.
SOURCE: Dhakal B et al. JAMA Oncol. 2018 Jan 4. doi: 10.1001/jamaoncol.2017.4600.
High-dose therapy with melphalan followed by autologous stem cell transplant (HDT/ASCT) is still the best option for multiple myeloma even after almost 2 decades with newer and highly effective induction agents, according to a recent systematic review and two meta-analyses.
Given the “unprecedented efficacy” of “modern induction therapy with immunomodulatory drugs and proteasome inhibitors (also called ‘novel agents’),” investigators “have sought to reevaluate the role of HDT/ASCT,” wrote Binod Dhakal, MD, of the Medical College of Wisconsin, and his colleagues. The report is in JAMA Oncology.
To solve the issue, they analyzed five randomized controlled trials conducted since 2000 and concluded that HDT/ASCT is still the preferred treatment approach.
Despite a lack of demonstrable overall survival benefit, there is a significant progression-free survival (PFS) benefit, low treatment-related mortality, and potential high minimal residual disease-negative rates conferred by HDT/ASCT in newly-diagnosed multiple myeloma, the researchers noted.
The combined odds for complete response were 1.27 (95% confidence interval, 0.97-1.65, P = .07) with HDT/ASCT, compared with standard-dose therapy (SDT). The combined hazard ratio (HR) for PFS was 0.55 (95% CI, 0.41-0.7, P less than .001) and 0.76 for overall survival (95% CI, 0.42-1.36, P = .20) in favor of HDT.
PFS was best with tandem HDT/ASCT (HR, 0.49, 95% CI, 0.37-0.65) followed by single HDT/ASCT with bortezomib, lenalidomide, and dexamethasone consolidation (HR, 0.53, 95% CI, 0.37-0.76) and single HDT/ASCT alone (HR, 0.68, 95% CI, 0.53-0.87), compared with SDT. However, none of the HDT/ASCT approaches had a significant impact on overall survival.
Meanwhile, treatment-related mortality with HDT/ASCT was minimal, at less than 1%.
“The achievement of high [minimal residual disease] rates with HDT/ASCT may render this approach the ideal platform for testing novel approaches (e.g., immunotherapy) aiming at disease eradication and cures,” the researchers wrote.
The researchers reported relationships with a number of companies, including Takeda, Celgene, and Amgen, that make novel induction agents.
SOURCE: Dhakal B et al. JAMA Oncol. 2018 Jan 4. doi: 10.1001/jamaoncol.2017.4600.
High-dose therapy with melphalan followed by autologous stem cell transplant (HDT/ASCT) is still the best option for multiple myeloma even after almost 2 decades with newer and highly effective induction agents, according to a recent systematic review and two meta-analyses.
Given the “unprecedented efficacy” of “modern induction therapy with immunomodulatory drugs and proteasome inhibitors (also called ‘novel agents’),” investigators “have sought to reevaluate the role of HDT/ASCT,” wrote Binod Dhakal, MD, of the Medical College of Wisconsin, and his colleagues. The report is in JAMA Oncology.
To solve the issue, they analyzed five randomized controlled trials conducted since 2000 and concluded that HDT/ASCT is still the preferred treatment approach.
Despite a lack of demonstrable overall survival benefit, there is a significant progression-free survival (PFS) benefit, low treatment-related mortality, and potential high minimal residual disease-negative rates conferred by HDT/ASCT in newly-diagnosed multiple myeloma, the researchers noted.
The combined odds for complete response were 1.27 (95% confidence interval, 0.97-1.65, P = .07) with HDT/ASCT, compared with standard-dose therapy (SDT). The combined hazard ratio (HR) for PFS was 0.55 (95% CI, 0.41-0.7, P less than .001) and 0.76 for overall survival (95% CI, 0.42-1.36, P = .20) in favor of HDT.
PFS was best with tandem HDT/ASCT (HR, 0.49, 95% CI, 0.37-0.65) followed by single HDT/ASCT with bortezomib, lenalidomide, and dexamethasone consolidation (HR, 0.53, 95% CI, 0.37-0.76) and single HDT/ASCT alone (HR, 0.68, 95% CI, 0.53-0.87), compared with SDT. However, none of the HDT/ASCT approaches had a significant impact on overall survival.
Meanwhile, treatment-related mortality with HDT/ASCT was minimal, at less than 1%.
“The achievement of high [minimal residual disease] rates with HDT/ASCT may render this approach the ideal platform for testing novel approaches (e.g., immunotherapy) aiming at disease eradication and cures,” the researchers wrote.
The researchers reported relationships with a number of companies, including Takeda, Celgene, and Amgen, that make novel induction agents.
SOURCE: Dhakal B et al. JAMA Oncol. 2018 Jan 4. doi: 10.1001/jamaoncol.2017.4600.
FROM JAMA ONCOLOGY
Key clinical point:
Major finding: The combined odds for complete response were 1.27 (95% CI 0.97-1.65, P = .07) with HDT/ASCT, compared with standard-dose therapy (SDT).
Study details: A systematic review and two meta-analyses examining five phase 3 clinical trials reported since 2000.
Disclosures: The researchers reported relationships with a number of companies, including Takeda, Celgene, and Amgen, that make novel induction agents.
Source: Dhakal B et al. JAMA Oncol. 2018 Jan 4. doi: 10.1001/jamaoncol.2017.4600.
Higher Risk of Secondary Cancers for Patients With Mycosis Fungoides
Adult patients with mycosis fungoides (MF) have a higher risk of secondary malignancies, according to a 20-year population-based cohort study. The researchers, from Bezmialem Vakif University in Istanbul, Turkey, say their findings support earlier research about a higher risk of, for instance, Hodgkin lymphoma, chronic leukemia, and lung cancer.
Between 1998 and 2015, the researchers documented 143 cases of cutaneous T-cell lymphoma (CTCL). The majority of patients had early-stage disease.
The researchers also documented 13 cases (9%) of secondary malignancy diagnosed at least 3 months after the diagnosis of CTCL. The cancers included bladder cancer, nasopharynx cancer, renal cell carcinoma, lung cancer, and superficial spreading malignant melanoma.
Older age, stage IV disease, lymphomatoid papulosis, and having CTCL for > 10 years raised the chances of developing secondary solid tumors. In 60% of patients, the secondary malignancies occurred during the first year of diagnosis.
Research has suggested that antilymphoma drugs, particularly alkylating agents, may lead to leukemia, the researchers note. In this study, 6 patients with secondary cancers were getting systemic treatment with interferon or acitretin; 7 were getting no systemic treatment.
The researchers add that MF and hematologic malignancies may share genetic origin, carcinogens, or viruses that affect lymphocyte precursors. They also note that the first neoplasm may produce cytokines that induce development of the secondary neoplasm. The researchers cite research that found MF is a T helper cell 2–mediated disease associated with human leukocyte antigen 2 alleles. Viruses such as Epstein-Barr and herpes simplex also have been implicated.
“Extensive evaluation” for secondary malignancies in adult patients with MF is wise, the researchers advise, particularly if the patient has lymphomatoid papulosis.
Source:
Cengiz FP, Emiroğlu N, Onsun N. Turk J Haematol. 2017;34(4):378-379.
doi: 10.4274/tjh.2017.0234.
Adult patients with mycosis fungoides (MF) have a higher risk of secondary malignancies, according to a 20-year population-based cohort study. The researchers, from Bezmialem Vakif University in Istanbul, Turkey, say their findings support earlier research about a higher risk of, for instance, Hodgkin lymphoma, chronic leukemia, and lung cancer.
Between 1998 and 2015, the researchers documented 143 cases of cutaneous T-cell lymphoma (CTCL). The majority of patients had early-stage disease.
The researchers also documented 13 cases (9%) of secondary malignancy diagnosed at least 3 months after the diagnosis of CTCL. The cancers included bladder cancer, nasopharynx cancer, renal cell carcinoma, lung cancer, and superficial spreading malignant melanoma.
Older age, stage IV disease, lymphomatoid papulosis, and having CTCL for > 10 years raised the chances of developing secondary solid tumors. In 60% of patients, the secondary malignancies occurred during the first year of diagnosis.
Research has suggested that antilymphoma drugs, particularly alkylating agents, may lead to leukemia, the researchers note. In this study, 6 patients with secondary cancers were getting systemic treatment with interferon or acitretin; 7 were getting no systemic treatment.
The researchers add that MF and hematologic malignancies may share genetic origin, carcinogens, or viruses that affect lymphocyte precursors. They also note that the first neoplasm may produce cytokines that induce development of the secondary neoplasm. The researchers cite research that found MF is a T helper cell 2–mediated disease associated with human leukocyte antigen 2 alleles. Viruses such as Epstein-Barr and herpes simplex also have been implicated.
“Extensive evaluation” for secondary malignancies in adult patients with MF is wise, the researchers advise, particularly if the patient has lymphomatoid papulosis.
Source:
Cengiz FP, Emiroğlu N, Onsun N. Turk J Haematol. 2017;34(4):378-379.
doi: 10.4274/tjh.2017.0234.
Adult patients with mycosis fungoides (MF) have a higher risk of secondary malignancies, according to a 20-year population-based cohort study. The researchers, from Bezmialem Vakif University in Istanbul, Turkey, say their findings support earlier research about a higher risk of, for instance, Hodgkin lymphoma, chronic leukemia, and lung cancer.
Between 1998 and 2015, the researchers documented 143 cases of cutaneous T-cell lymphoma (CTCL). The majority of patients had early-stage disease.
The researchers also documented 13 cases (9%) of secondary malignancy diagnosed at least 3 months after the diagnosis of CTCL. The cancers included bladder cancer, nasopharynx cancer, renal cell carcinoma, lung cancer, and superficial spreading malignant melanoma.
Older age, stage IV disease, lymphomatoid papulosis, and having CTCL for > 10 years raised the chances of developing secondary solid tumors. In 60% of patients, the secondary malignancies occurred during the first year of diagnosis.
Research has suggested that antilymphoma drugs, particularly alkylating agents, may lead to leukemia, the researchers note. In this study, 6 patients with secondary cancers were getting systemic treatment with interferon or acitretin; 7 were getting no systemic treatment.
The researchers add that MF and hematologic malignancies may share genetic origin, carcinogens, or viruses that affect lymphocyte precursors. They also note that the first neoplasm may produce cytokines that induce development of the secondary neoplasm. The researchers cite research that found MF is a T helper cell 2–mediated disease associated with human leukocyte antigen 2 alleles. Viruses such as Epstein-Barr and herpes simplex also have been implicated.
“Extensive evaluation” for secondary malignancies in adult patients with MF is wise, the researchers advise, particularly if the patient has lymphomatoid papulosis.
Source:
Cengiz FP, Emiroğlu N, Onsun N. Turk J Haematol. 2017;34(4):378-379.
doi: 10.4274/tjh.2017.0234.