User login
Genetic composition of HCV changes with HIV coinfection
Marked differences were seen in the composition of hepatitis C virus hypervariable region 1 (HVR1) when comparing HIV-coinfected (CIP) with HCV-monoinfected (MIP) individuals, according to the results of a genetic analysis of nearly 300 patients.
Intrahost HCV HVR1 evolution varies between these two groups, which suggests that HIV-inflicted changes in the host environment exact a strong HCV genetic response, according to a report published online in Infection, Genetics, and Evolution.
“A high prevalence of HIV-HCV coinfection and its impact on mortality among such populations groups as PWID [people who inject drugs] and MSM [men who have sex with men] is of major concern to public health,” according to the researchers.
Previous studies using the Global Hepatitis Outbreak and Surveillance Technology, a Web-based system for the detection of HCV transmission developed by the researchers, analyzed sequences of intrahost variants of the HVR1 region using next-generation sequencing. They found that genetic variation in the HVR1 of intrahost HCV variants was strongly associated with host sex and ethnicity, resistance to interferon, and stages of HCV infection.
In this particular study, the researchers assessed 28,622 nucleotide sequences of intrahost HCV HVR1 variants from 113 CIP and 176 MIP individuals.
They examined 148 physical-chemical indexes of DNA nucleotide dimers and found that there were significant differences in the means and frequency distributions of seven physical-chemical properties between HVR1 variants from both groups.
The significant majority of these profiles (98%-99%) were found to be specific to CIP or MIP, indicating that coevolution among HVR1 sites reflects HCV adaptation to HIV among coinfected individuals. “This observation suggests substantial differences in fitness between HVR1 variants circulating in infected hosts in the presence or absence of HIV, according to the researchers from the Centers for Disease Control and Prevention.
“HCV strains circulating in high-risk groups need to be carefully monitored for the identification of potentially new traits of clinical and public health relevance,” the researchers concluded.
This study was supported by CDC intramural funding. The authors reported that they had no disclosures.
SOURCE: Lara J et al. doi: 10.1016/j.meegid.2018.07.039.
Marked differences were seen in the composition of hepatitis C virus hypervariable region 1 (HVR1) when comparing HIV-coinfected (CIP) with HCV-monoinfected (MIP) individuals, according to the results of a genetic analysis of nearly 300 patients.
Intrahost HCV HVR1 evolution varies between these two groups, which suggests that HIV-inflicted changes in the host environment exact a strong HCV genetic response, according to a report published online in Infection, Genetics, and Evolution.
“A high prevalence of HIV-HCV coinfection and its impact on mortality among such populations groups as PWID [people who inject drugs] and MSM [men who have sex with men] is of major concern to public health,” according to the researchers.
Previous studies using the Global Hepatitis Outbreak and Surveillance Technology, a Web-based system for the detection of HCV transmission developed by the researchers, analyzed sequences of intrahost variants of the HVR1 region using next-generation sequencing. They found that genetic variation in the HVR1 of intrahost HCV variants was strongly associated with host sex and ethnicity, resistance to interferon, and stages of HCV infection.
In this particular study, the researchers assessed 28,622 nucleotide sequences of intrahost HCV HVR1 variants from 113 CIP and 176 MIP individuals.
They examined 148 physical-chemical indexes of DNA nucleotide dimers and found that there were significant differences in the means and frequency distributions of seven physical-chemical properties between HVR1 variants from both groups.
The significant majority of these profiles (98%-99%) were found to be specific to CIP or MIP, indicating that coevolution among HVR1 sites reflects HCV adaptation to HIV among coinfected individuals. “This observation suggests substantial differences in fitness between HVR1 variants circulating in infected hosts in the presence or absence of HIV, according to the researchers from the Centers for Disease Control and Prevention.
“HCV strains circulating in high-risk groups need to be carefully monitored for the identification of potentially new traits of clinical and public health relevance,” the researchers concluded.
This study was supported by CDC intramural funding. The authors reported that they had no disclosures.
SOURCE: Lara J et al. doi: 10.1016/j.meegid.2018.07.039.
Marked differences were seen in the composition of hepatitis C virus hypervariable region 1 (HVR1) when comparing HIV-coinfected (CIP) with HCV-monoinfected (MIP) individuals, according to the results of a genetic analysis of nearly 300 patients.
Intrahost HCV HVR1 evolution varies between these two groups, which suggests that HIV-inflicted changes in the host environment exact a strong HCV genetic response, according to a report published online in Infection, Genetics, and Evolution.
“A high prevalence of HIV-HCV coinfection and its impact on mortality among such populations groups as PWID [people who inject drugs] and MSM [men who have sex with men] is of major concern to public health,” according to the researchers.
Previous studies using the Global Hepatitis Outbreak and Surveillance Technology, a Web-based system for the detection of HCV transmission developed by the researchers, analyzed sequences of intrahost variants of the HVR1 region using next-generation sequencing. They found that genetic variation in the HVR1 of intrahost HCV variants was strongly associated with host sex and ethnicity, resistance to interferon, and stages of HCV infection.
In this particular study, the researchers assessed 28,622 nucleotide sequences of intrahost HCV HVR1 variants from 113 CIP and 176 MIP individuals.
They examined 148 physical-chemical indexes of DNA nucleotide dimers and found that there were significant differences in the means and frequency distributions of seven physical-chemical properties between HVR1 variants from both groups.
The significant majority of these profiles (98%-99%) were found to be specific to CIP or MIP, indicating that coevolution among HVR1 sites reflects HCV adaptation to HIV among coinfected individuals. “This observation suggests substantial differences in fitness between HVR1 variants circulating in infected hosts in the presence or absence of HIV, according to the researchers from the Centers for Disease Control and Prevention.
“HCV strains circulating in high-risk groups need to be carefully monitored for the identification of potentially new traits of clinical and public health relevance,” the researchers concluded.
This study was supported by CDC intramural funding. The authors reported that they had no disclosures.
SOURCE: Lara J et al. doi: 10.1016/j.meegid.2018.07.039.
FROM INFECTION, GENETICS, AND EVOLUTION
Real-time microarrays can simultaneously detect HCV and HIV-1, -2 infections
The use of TaqMan Array Card (TAC) microarrays has been extended to permit simultaneous detection of HIV-1, HIV-2, and five hepatitis viruses from a small amount of extracted nucleic acid, according to a study by Timothy C. Granade, MD, and his colleagues at the Centers for Disease Control and Prevention, Atlanta.
This is particularly important for dealing with HIV-infected individuals, because HIV-1 and HIV-2 require different treatment interventions, and approximately one-third of HIV-infected patients have been found to be coinfected with hepatitis C or hepatitis B, according to the study report, published in the Journal of Virological Methods (J Virol Methods. 2018 Sep;259:60-5).
HIV-1-positive plasma samples from a variety of subtypes as well as whole blood specimens were confirmed for HIV-1-infection serologically or by nucleic amplification methods. HIV-2 whole blood and plasma specimens were also obtained.
TAC cards contained one positive control, one negative control, three HIV-1 replicates, and two HIV-2 replicates. In addition, the five common hepatitis viruses (A-E) were each replicated three times on each card. The cards were used to test the RNA isolates obtained from the various samples.
Ninety-five of the 104 known HIV-1-positive specimens were assayed positive using TAC; 23 of 26 HIV-2-seeded specimens were detectable using TAC and no cross-reactivity was seen between HIV-1-positive and HIV-2-positive specimens.
Eighteen of the HIV-1-positive specimens were also reactive in triplicate for HCV; three of the HIV-1-positive specimens were reactive to HBV and one specimen was reactive to HIV-1, HBV, and HCV.
“The TAC assay could be invaluable in large-scale screening environments or in surveying local outbreaks such as the recent HIV cluster found in Indiana. Many of these individuals were later determined to be infected with hepatitis C. The use of TAC could shorten the time to identifying and confirming such cases and permit the detection of multiple blood-borne infections in a single test. Application of TAC technology to general population surveillance could identify problem areas for both HIV prevention and intervention efforts in a variety of global environs,” the researchers concluded.
The authors were employed by the Centers for Disease Control and Prevention, Atlanta, which funded the study.
The use of TaqMan Array Card (TAC) microarrays has been extended to permit simultaneous detection of HIV-1, HIV-2, and five hepatitis viruses from a small amount of extracted nucleic acid, according to a study by Timothy C. Granade, MD, and his colleagues at the Centers for Disease Control and Prevention, Atlanta.
This is particularly important for dealing with HIV-infected individuals, because HIV-1 and HIV-2 require different treatment interventions, and approximately one-third of HIV-infected patients have been found to be coinfected with hepatitis C or hepatitis B, according to the study report, published in the Journal of Virological Methods (J Virol Methods. 2018 Sep;259:60-5).
HIV-1-positive plasma samples from a variety of subtypes as well as whole blood specimens were confirmed for HIV-1-infection serologically or by nucleic amplification methods. HIV-2 whole blood and plasma specimens were also obtained.
TAC cards contained one positive control, one negative control, three HIV-1 replicates, and two HIV-2 replicates. In addition, the five common hepatitis viruses (A-E) were each replicated three times on each card. The cards were used to test the RNA isolates obtained from the various samples.
Ninety-five of the 104 known HIV-1-positive specimens were assayed positive using TAC; 23 of 26 HIV-2-seeded specimens were detectable using TAC and no cross-reactivity was seen between HIV-1-positive and HIV-2-positive specimens.
Eighteen of the HIV-1-positive specimens were also reactive in triplicate for HCV; three of the HIV-1-positive specimens were reactive to HBV and one specimen was reactive to HIV-1, HBV, and HCV.
“The TAC assay could be invaluable in large-scale screening environments or in surveying local outbreaks such as the recent HIV cluster found in Indiana. Many of these individuals were later determined to be infected with hepatitis C. The use of TAC could shorten the time to identifying and confirming such cases and permit the detection of multiple blood-borne infections in a single test. Application of TAC technology to general population surveillance could identify problem areas for both HIV prevention and intervention efforts in a variety of global environs,” the researchers concluded.
The authors were employed by the Centers for Disease Control and Prevention, Atlanta, which funded the study.
The use of TaqMan Array Card (TAC) microarrays has been extended to permit simultaneous detection of HIV-1, HIV-2, and five hepatitis viruses from a small amount of extracted nucleic acid, according to a study by Timothy C. Granade, MD, and his colleagues at the Centers for Disease Control and Prevention, Atlanta.
This is particularly important for dealing with HIV-infected individuals, because HIV-1 and HIV-2 require different treatment interventions, and approximately one-third of HIV-infected patients have been found to be coinfected with hepatitis C or hepatitis B, according to the study report, published in the Journal of Virological Methods (J Virol Methods. 2018 Sep;259:60-5).
HIV-1-positive plasma samples from a variety of subtypes as well as whole blood specimens were confirmed for HIV-1-infection serologically or by nucleic amplification methods. HIV-2 whole blood and plasma specimens were also obtained.
TAC cards contained one positive control, one negative control, three HIV-1 replicates, and two HIV-2 replicates. In addition, the five common hepatitis viruses (A-E) were each replicated three times on each card. The cards were used to test the RNA isolates obtained from the various samples.
Ninety-five of the 104 known HIV-1-positive specimens were assayed positive using TAC; 23 of 26 HIV-2-seeded specimens were detectable using TAC and no cross-reactivity was seen between HIV-1-positive and HIV-2-positive specimens.
Eighteen of the HIV-1-positive specimens were also reactive in triplicate for HCV; three of the HIV-1-positive specimens were reactive to HBV and one specimen was reactive to HIV-1, HBV, and HCV.
“The TAC assay could be invaluable in large-scale screening environments or in surveying local outbreaks such as the recent HIV cluster found in Indiana. Many of these individuals were later determined to be infected with hepatitis C. The use of TAC could shorten the time to identifying and confirming such cases and permit the detection of multiple blood-borne infections in a single test. Application of TAC technology to general population surveillance could identify problem areas for both HIV prevention and intervention efforts in a variety of global environs,” the researchers concluded.
The authors were employed by the Centers for Disease Control and Prevention, Atlanta, which funded the study.
FROM THE JOURNAL OF VIROLOGICAL METHODS
Study quantifies occupational exposure risks of EDT
For trauma patients who are in extremis, but with the high rates of HIV/hepatitis among trauma patients, one that also carries what had been an unknown exposure risk for emergency staff.
“The most important findings of this prospective, multicenter study are that occupational exposures were reported in 7.2% of EDT resuscitations and 1.6% of EDT resuscitation participants and that occupational exposure risk appears to be further mitigated with strict PPE [personal protective equipment] compliance to universal precautions,” lead author Andrew Nunn, MD, and his colleagues wrote. Dr. Nunn is a trauma surgeon with Wake Forest Baptist Health in Winston-Salem, N.C.
The researchers surveyed 1,360 emergency department (ED) personnel after they performed 305 EDTs at 16 academic and community trauma centers nationwide in 2015 and 2016. The patients who had an EDT were mostly men ranging in age from 24 to 41 years (90.5%) with penetrating injuries (77.4%) and arrived at the ED after prehospital CPR (56.7%). Twenty-two occupational exposures occurred during 22 of the EDT resuscitations, with trainees sustaining most of them (68.2%). The most common source of injury was sharps, accounting for 86.4% (scalpels, 38.9%; fractured bone, 27.8%; needles, 16.7%; and scissors, 3%).
“Occupational exposures correlated with PPE utilization, as universal precautions during EDT were more often observed in providers who did not sustain occupational exposures, compared with those sustaining exposures,” Dr. Nunn and his coauthors wrote. For example, 98% of those reporting no exposure were gloved versus 91% of those who were exposed (P greater than .05).
Dr. Nunn and his coauthors called the risk of HIV or hepatitis C virus (HCV) transmission during EDT “extraordinarily low.” Based on data from their study, they determined the risk of blood-borne pathogen transmission during an EDT resuscitation is 6 in 1 million for HIV and 1 in 10,000 for HCV, and the individual risk is 1 in 1 million and 3 in 100,000, respectively. Compliance with PPE precautions further limited exposure risk, but the study found that more than 10% of surveyed personnel did not utilize one of the four components of PPE besides gloves – eyewear, mask, gown or hat.
Most – but not all – survey responders followed up after the incidence of exposure. “[A total of] 91.7% of providers reporting their exposures also reported following up with their institution specific occupational exposure protocol,” the investigators wrote.
“Our findings have particular implications for trainees,” the study authors noted, citing the high percentage of injuries in this group. The findings emphasized the need for universal PPE compliance and enforcement by resuscitation team leaders. Nonetheless, the study found that the exposure rates during EDT are no greater than other surgical procedures.
“Regardless of the lifesaving nature of the procedure, improved universal precaution compliance with PPE is paramount and would further minimize occupational exposure risks to providers during EDT,” Dr. Nunn and his coauthors said.
Dr. Nunn and his coauthors reported having no financial relationships.
SOURCE: Nunn A et al. J Trauma Acute Care Surg. 2018:85;78-84.
For trauma patients who are in extremis, but with the high rates of HIV/hepatitis among trauma patients, one that also carries what had been an unknown exposure risk for emergency staff.
“The most important findings of this prospective, multicenter study are that occupational exposures were reported in 7.2% of EDT resuscitations and 1.6% of EDT resuscitation participants and that occupational exposure risk appears to be further mitigated with strict PPE [personal protective equipment] compliance to universal precautions,” lead author Andrew Nunn, MD, and his colleagues wrote. Dr. Nunn is a trauma surgeon with Wake Forest Baptist Health in Winston-Salem, N.C.
The researchers surveyed 1,360 emergency department (ED) personnel after they performed 305 EDTs at 16 academic and community trauma centers nationwide in 2015 and 2016. The patients who had an EDT were mostly men ranging in age from 24 to 41 years (90.5%) with penetrating injuries (77.4%) and arrived at the ED after prehospital CPR (56.7%). Twenty-two occupational exposures occurred during 22 of the EDT resuscitations, with trainees sustaining most of them (68.2%). The most common source of injury was sharps, accounting for 86.4% (scalpels, 38.9%; fractured bone, 27.8%; needles, 16.7%; and scissors, 3%).
“Occupational exposures correlated with PPE utilization, as universal precautions during EDT were more often observed in providers who did not sustain occupational exposures, compared with those sustaining exposures,” Dr. Nunn and his coauthors wrote. For example, 98% of those reporting no exposure were gloved versus 91% of those who were exposed (P greater than .05).
Dr. Nunn and his coauthors called the risk of HIV or hepatitis C virus (HCV) transmission during EDT “extraordinarily low.” Based on data from their study, they determined the risk of blood-borne pathogen transmission during an EDT resuscitation is 6 in 1 million for HIV and 1 in 10,000 for HCV, and the individual risk is 1 in 1 million and 3 in 100,000, respectively. Compliance with PPE precautions further limited exposure risk, but the study found that more than 10% of surveyed personnel did not utilize one of the four components of PPE besides gloves – eyewear, mask, gown or hat.
Most – but not all – survey responders followed up after the incidence of exposure. “[A total of] 91.7% of providers reporting their exposures also reported following up with their institution specific occupational exposure protocol,” the investigators wrote.
“Our findings have particular implications for trainees,” the study authors noted, citing the high percentage of injuries in this group. The findings emphasized the need for universal PPE compliance and enforcement by resuscitation team leaders. Nonetheless, the study found that the exposure rates during EDT are no greater than other surgical procedures.
“Regardless of the lifesaving nature of the procedure, improved universal precaution compliance with PPE is paramount and would further minimize occupational exposure risks to providers during EDT,” Dr. Nunn and his coauthors said.
Dr. Nunn and his coauthors reported having no financial relationships.
SOURCE: Nunn A et al. J Trauma Acute Care Surg. 2018:85;78-84.
For trauma patients who are in extremis, but with the high rates of HIV/hepatitis among trauma patients, one that also carries what had been an unknown exposure risk for emergency staff.
“The most important findings of this prospective, multicenter study are that occupational exposures were reported in 7.2% of EDT resuscitations and 1.6% of EDT resuscitation participants and that occupational exposure risk appears to be further mitigated with strict PPE [personal protective equipment] compliance to universal precautions,” lead author Andrew Nunn, MD, and his colleagues wrote. Dr. Nunn is a trauma surgeon with Wake Forest Baptist Health in Winston-Salem, N.C.
The researchers surveyed 1,360 emergency department (ED) personnel after they performed 305 EDTs at 16 academic and community trauma centers nationwide in 2015 and 2016. The patients who had an EDT were mostly men ranging in age from 24 to 41 years (90.5%) with penetrating injuries (77.4%) and arrived at the ED after prehospital CPR (56.7%). Twenty-two occupational exposures occurred during 22 of the EDT resuscitations, with trainees sustaining most of them (68.2%). The most common source of injury was sharps, accounting for 86.4% (scalpels, 38.9%; fractured bone, 27.8%; needles, 16.7%; and scissors, 3%).
“Occupational exposures correlated with PPE utilization, as universal precautions during EDT were more often observed in providers who did not sustain occupational exposures, compared with those sustaining exposures,” Dr. Nunn and his coauthors wrote. For example, 98% of those reporting no exposure were gloved versus 91% of those who were exposed (P greater than .05).
Dr. Nunn and his coauthors called the risk of HIV or hepatitis C virus (HCV) transmission during EDT “extraordinarily low.” Based on data from their study, they determined the risk of blood-borne pathogen transmission during an EDT resuscitation is 6 in 1 million for HIV and 1 in 10,000 for HCV, and the individual risk is 1 in 1 million and 3 in 100,000, respectively. Compliance with PPE precautions further limited exposure risk, but the study found that more than 10% of surveyed personnel did not utilize one of the four components of PPE besides gloves – eyewear, mask, gown or hat.
Most – but not all – survey responders followed up after the incidence of exposure. “[A total of] 91.7% of providers reporting their exposures also reported following up with their institution specific occupational exposure protocol,” the investigators wrote.
“Our findings have particular implications for trainees,” the study authors noted, citing the high percentage of injuries in this group. The findings emphasized the need for universal PPE compliance and enforcement by resuscitation team leaders. Nonetheless, the study found that the exposure rates during EDT are no greater than other surgical procedures.
“Regardless of the lifesaving nature of the procedure, improved universal precaution compliance with PPE is paramount and would further minimize occupational exposure risks to providers during EDT,” Dr. Nunn and his coauthors said.
Dr. Nunn and his coauthors reported having no financial relationships.
SOURCE: Nunn A et al. J Trauma Acute Care Surg. 2018:85;78-84.
FROM THE JOURNAL OF TRAUMA AND ACUTE CARE SURGERY
Key clinical point: Occupational exposure risk of emergency department thoracotomy (EDT) is low for personnel.
Major finding: Occupational exposure rate to HIV/hepatitis in trauma undergoing EDT is 7.2% for personnel.
Study details: Prospective, observational study that included 1,360 personnel surveyed after they performed 305 EDTs in 2015 and 2016.
Disclosures: Dr. Nunn and his coauthors reported having no financial relationships.
Source: Nunn A et al. J Trauma Acute Care Surg. 2018:85;78-84.
Hepatitis C Federal Health Data Trends (FULL)
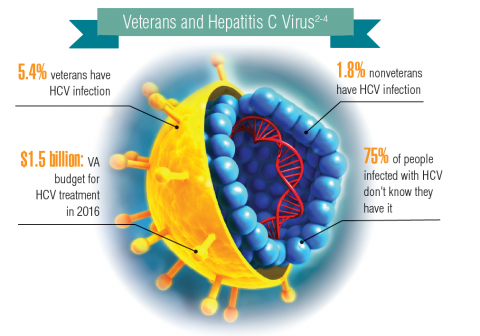
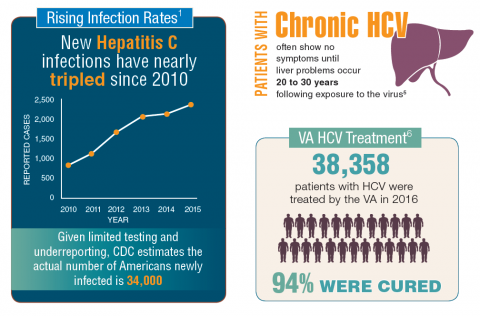
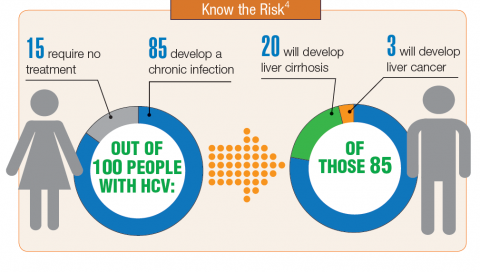
About 3.5 million people in the U.S. have chronic hepatitis C virus (HCV) infection.1 Because veterans are at greater risk of infection than is the civilian population, the VA recommends that all veterans born between 1945 and 1965 receive testing for HCV infection. Veterans also should be tested if they have ever shared a needle to inject drugs, have HIV infection, were on long-term kidney dialysis, had a blood transfusion prior to 1992, or had tattoos/body piercings in an unregulated place.
In fiscal year 2015, VA spent $696 million on treatment for patients with HCV infection, which accounted for 17% of the pharmacy budget. In 2016, the spending more than doubled to $1.5 billion, in part because the VA began offering treatment to all veterans with HCV in February 2016, regardless of degree of fibrosis or severity of the underlying liver disease. In 2016, more than 38,000 patients were treated for HCV infection, and 94% were cured. In all, 174,842 veterans have been diagnosed with HCV infection at the VA, more than 76,000 have been treated, and more than 60,000 have been cured.2
It is expected that another 40,000 patients may be infected with HCV but have not been diagnosed. Because HCV infection often has few noticeable symptoms, many veterans may not be aware that they have the disease.
Click to read the digital edition.
About 3.5 million people in the U.S. have chronic hepatitis C virus (HCV) infection.1 Because veterans are at greater risk of infection than is the civilian population, the VA recommends that all veterans born between 1945 and 1965 receive testing for HCV infection. Veterans also should be tested if they have ever shared a needle to inject drugs, have HIV infection, were on long-term kidney dialysis, had a blood transfusion prior to 1992, or had tattoos/body piercings in an unregulated place.
In fiscal year 2015, VA spent $696 million on treatment for patients with HCV infection, which accounted for 17% of the pharmacy budget. In 2016, the spending more than doubled to $1.5 billion, in part because the VA began offering treatment to all veterans with HCV in February 2016, regardless of degree of fibrosis or severity of the underlying liver disease. In 2016, more than 38,000 patients were treated for HCV infection, and 94% were cured. In all, 174,842 veterans have been diagnosed with HCV infection at the VA, more than 76,000 have been treated, and more than 60,000 have been cured.2
It is expected that another 40,000 patients may be infected with HCV but have not been diagnosed. Because HCV infection often has few noticeable symptoms, many veterans may not be aware that they have the disease.
Click to read the digital edition.
About 3.5 million people in the U.S. have chronic hepatitis C virus (HCV) infection.1 Because veterans are at greater risk of infection than is the civilian population, the VA recommends that all veterans born between 1945 and 1965 receive testing for HCV infection. Veterans also should be tested if they have ever shared a needle to inject drugs, have HIV infection, were on long-term kidney dialysis, had a blood transfusion prior to 1992, or had tattoos/body piercings in an unregulated place.
In fiscal year 2015, VA spent $696 million on treatment for patients with HCV infection, which accounted for 17% of the pharmacy budget. In 2016, the spending more than doubled to $1.5 billion, in part because the VA began offering treatment to all veterans with HCV in February 2016, regardless of degree of fibrosis or severity of the underlying liver disease. In 2016, more than 38,000 patients were treated for HCV infection, and 94% were cured. In all, 174,842 veterans have been diagnosed with HCV infection at the VA, more than 76,000 have been treated, and more than 60,000 have been cured.2
It is expected that another 40,000 patients may be infected with HCV but have not been diagnosed. Because HCV infection often has few noticeable symptoms, many veterans may not be aware that they have the disease.
Click to read the digital edition.
National Academies issues 5-step plan to address infections linked to opioid use disorder
Widespread opioid use disorder (OUD) has spawned new epidemics of hepatitis C virus (HCV) and HIV infections as well as increased hospitalizations for bacteremia, endocarditis, skin and soft tissue infections, and osteomyelitis, according to a report arising from a National Academies of Science, Engineering and Medicine (NASEM) workshop titled Integrating Infectious Disease Considerations with Response to the Opioid Epidemic.
Optimal treatment of these infections is often impeded by untreated OUD, Sandra A. Springer, MD, and her colleagues wrote in an article published online in the Annals of Internal Medicine. Failing to address OUD can result in longer hospital stays; frequent readmissions because of a lack of adherence to antibiotic regimens; or reinfection, morbidity, and high costs. “Medical settings that manage such infections offer a potential means of engaging people in treatment of OUD; however, few providers and hospitals treating such infections have the needed resources and capabilities,” Dr. Springer, director, infectious disease outpatient clinic, Veterans Administration, Newington, and of Yale University, New Haven, both in Conn., and her colleagues wrote.
The authors outlined five action steps resulting from the NASEM workshop:
- Implement screening for OUD in all relevant health care settings.
- For patients with positive screening results, immediately prescribe effective medication for OUD and/or opioid withdrawal symptoms.
- Develop hospital-based protocols that facilitate OUD treatment initiation and linkage to community-based treatment upon discharge.
- Hospitals, medical schools, physician assistant schools, nursing schools, and residency programs should increase training to identify and treat OUD.
- Increase access to addiction care and funding to states to provide effective medications to treat OUD.
Opioid withdrawal and pain syndromes should be addressed with opioid agonist therapies to optimize infectious disease (ID) treatment and relieve pain, according to Dr. Springer and her colleagues. In addition, “Because ID specialists are likely to be consulted for anyone requiring long-term antibiotic therapy or patients with HIV and HCV infection, OUD screening should be a standard part of an ID consult assessment,” the authors wrote.
“All health care providers have a role in combating the OUD epidemic and its ID consequences. Those who treat infectious complications of OUD are well suited to screen for OUD and begin treatment with effective FDA-approved medications,” the authors concluded.
The workshop was held in March 2018 in Washington and videos and slide presentations from the meeting are available.
Dr. Springer and her colleagues reported grant funding from the National Institutes of Health, but no commercial conflicts.
SOURCE: Springer SA et al. Ann Intern Med. 2018 Jul 13. doi: 10.7326/M18-1203.
Widespread opioid use disorder (OUD) has spawned new epidemics of hepatitis C virus (HCV) and HIV infections as well as increased hospitalizations for bacteremia, endocarditis, skin and soft tissue infections, and osteomyelitis, according to a report arising from a National Academies of Science, Engineering and Medicine (NASEM) workshop titled Integrating Infectious Disease Considerations with Response to the Opioid Epidemic.
Optimal treatment of these infections is often impeded by untreated OUD, Sandra A. Springer, MD, and her colleagues wrote in an article published online in the Annals of Internal Medicine. Failing to address OUD can result in longer hospital stays; frequent readmissions because of a lack of adherence to antibiotic regimens; or reinfection, morbidity, and high costs. “Medical settings that manage such infections offer a potential means of engaging people in treatment of OUD; however, few providers and hospitals treating such infections have the needed resources and capabilities,” Dr. Springer, director, infectious disease outpatient clinic, Veterans Administration, Newington, and of Yale University, New Haven, both in Conn., and her colleagues wrote.
The authors outlined five action steps resulting from the NASEM workshop:
- Implement screening for OUD in all relevant health care settings.
- For patients with positive screening results, immediately prescribe effective medication for OUD and/or opioid withdrawal symptoms.
- Develop hospital-based protocols that facilitate OUD treatment initiation and linkage to community-based treatment upon discharge.
- Hospitals, medical schools, physician assistant schools, nursing schools, and residency programs should increase training to identify and treat OUD.
- Increase access to addiction care and funding to states to provide effective medications to treat OUD.
Opioid withdrawal and pain syndromes should be addressed with opioid agonist therapies to optimize infectious disease (ID) treatment and relieve pain, according to Dr. Springer and her colleagues. In addition, “Because ID specialists are likely to be consulted for anyone requiring long-term antibiotic therapy or patients with HIV and HCV infection, OUD screening should be a standard part of an ID consult assessment,” the authors wrote.
“All health care providers have a role in combating the OUD epidemic and its ID consequences. Those who treat infectious complications of OUD are well suited to screen for OUD and begin treatment with effective FDA-approved medications,” the authors concluded.
The workshop was held in March 2018 in Washington and videos and slide presentations from the meeting are available.
Dr. Springer and her colleagues reported grant funding from the National Institutes of Health, but no commercial conflicts.
SOURCE: Springer SA et al. Ann Intern Med. 2018 Jul 13. doi: 10.7326/M18-1203.
Widespread opioid use disorder (OUD) has spawned new epidemics of hepatitis C virus (HCV) and HIV infections as well as increased hospitalizations for bacteremia, endocarditis, skin and soft tissue infections, and osteomyelitis, according to a report arising from a National Academies of Science, Engineering and Medicine (NASEM) workshop titled Integrating Infectious Disease Considerations with Response to the Opioid Epidemic.
Optimal treatment of these infections is often impeded by untreated OUD, Sandra A. Springer, MD, and her colleagues wrote in an article published online in the Annals of Internal Medicine. Failing to address OUD can result in longer hospital stays; frequent readmissions because of a lack of adherence to antibiotic regimens; or reinfection, morbidity, and high costs. “Medical settings that manage such infections offer a potential means of engaging people in treatment of OUD; however, few providers and hospitals treating such infections have the needed resources and capabilities,” Dr. Springer, director, infectious disease outpatient clinic, Veterans Administration, Newington, and of Yale University, New Haven, both in Conn., and her colleagues wrote.
The authors outlined five action steps resulting from the NASEM workshop:
- Implement screening for OUD in all relevant health care settings.
- For patients with positive screening results, immediately prescribe effective medication for OUD and/or opioid withdrawal symptoms.
- Develop hospital-based protocols that facilitate OUD treatment initiation and linkage to community-based treatment upon discharge.
- Hospitals, medical schools, physician assistant schools, nursing schools, and residency programs should increase training to identify and treat OUD.
- Increase access to addiction care and funding to states to provide effective medications to treat OUD.
Opioid withdrawal and pain syndromes should be addressed with opioid agonist therapies to optimize infectious disease (ID) treatment and relieve pain, according to Dr. Springer and her colleagues. In addition, “Because ID specialists are likely to be consulted for anyone requiring long-term antibiotic therapy or patients with HIV and HCV infection, OUD screening should be a standard part of an ID consult assessment,” the authors wrote.
“All health care providers have a role in combating the OUD epidemic and its ID consequences. Those who treat infectious complications of OUD are well suited to screen for OUD and begin treatment with effective FDA-approved medications,” the authors concluded.
The workshop was held in March 2018 in Washington and videos and slide presentations from the meeting are available.
Dr. Springer and her colleagues reported grant funding from the National Institutes of Health, but no commercial conflicts.
SOURCE: Springer SA et al. Ann Intern Med. 2018 Jul 13. doi: 10.7326/M18-1203.
FROM ANNALS OF INTERNAL MEDICINE
AGA Clinical Practice Update: Statins are safe, effective, and important for most patients with liver disease and dyslipidemia
The medications are only contraindicated in patients with decompensated cirrhosis and statin-induced liver injury, Elizabeth Speliotes, MD, PhD, MPH, and her colleagues wrote in an expert review published in Clinical Gastroenterology and Hepatology. In these patients, statin treatment can compound liver damage and should be avoided, wrote Dr. Speliotes and her coauthors.
Because the liver plays a central role in cholesterol production, many clinicians shy away from treating hyperlipidemia in patients with liver disease. But studies consistently show that lipid-lowering drugs improve dyslipidemia in these patients, which significantly improves both high- and low-density lipoproteins and thereby reduces the long-term risk of cardiovascular disease, the authors wrote.
“Furthermore, the liver plays a role in the metabolism of many drugs, including those that are used to treat dyslipidemia,” wrote Dr. Speliotes of the University of Michigan, Ann Arbor. “It is not surprising, therefore, that many practitioners are hesitant to prescribe medicines to treat dyslipidemia in the setting of liver disease.”
Cholesterol targets described in the 2013 American College of Cardiology/American Heart Association guidelines can safely be applied to patients with liver disease. “The guidelines recommend that adults with cardiovascular disease or LDL of 190 mg/dL or higher be treated with high-intensity statins with the goal of reducing LDL levels by 50%,” they said. Patients whose LDL is 189 mg/dL or lower will benefit from moderate-intensity statins, with a target of a 30%-50% decrease in LDL.
The authors described best practice advice for dyslipidemia treatment in six liver diseases: drug-induced liver injury (DILI), nonalcoholic fatty liver disease (NAFLD), viral hepatitis B and C (HBV and HCV), primary biliary cholangitis (PBC), cirrhosis, and posttransplant dyslipidemia.
DILI
DILI is characterized by elevations of threefold or more in serum alanine aminotransferase (ALT) or aspartate aminotransferase and at least a doubling of total serum bilirubin with no other identifiable cause of these aberrations except the suspect drug. Statins rarely cause a DILI (1 in 100,000 patients), but can cause transient, benign ALT elevations. Statins should be discontinued if ALT or aspartate aminotransferase levels exceed a tripling of the upper limit of normal with concomitant bilirubin elevations. They should not be prescribed to patients with acute liver failure or decompensated liver disease, but otherwise they are safe for most patients with liver disease.
NAFLD
Many patients with NAFLD also have dyslipidemia. All NAFLD patients have an increased risk of cardiovascular disease, although NAFLD and nonalcoholic steatohepatitis are not traditional cardiovascular risk factors. Nevertheless, statins and the accompanying improvement in dyslipidemia have been shown to decrease cardiovascular mortality in these patients. The IDEAL study, for example, showed that moderate statin treatment with 80 mg atorvastatin was associated with a 44% decreased risk in secondary cardiovascular events. Other studies show similar results.
NAFLD patients with elevated LDL may benefit from ezetimibe as primary or add-on therapy. However, none of the drugs used to treat dyslipidemia will improve NAFLD or nonalcoholic steatohepatitis histology.
Viral hepatitis
Hepatitis C virus
Patients with HCV infection often experience decreased serum LDL and total cholesterol. However, these are virally mediated and don’t confer cardiovascular protection. In fact, HCV infections are associated with an increased risk of myocardial infarction. If the patient spontaneously clears the virus, lipids may rebound, so levels should be regularly monitored even if the patient does not need statin therapy.
Hepatitis B virus
HBV also interacts with lipid metabolism and can lead to hyperlipidemia. The American College of Cardiology/American Heart Association guidelines for cardiovascular risk assessment and statin therapy apply to these patients. Statins are safe in patients with either HCV or HBV, who tolerate them well.
PBC
PBC is a chronic autoimmune inflammatory cholestatic disease that is associated with dyslipidemia. These patients exhibit increased serum triglyceride and HDL levels that vary according to PBC stage. About 10% have a significant risk of cardiovascular disease. PBC patients with compensated liver disease can safely tolerate statin treatment, but the drugs should not be given to PBC patients with decompensated liver disease.
Obeticholic acid (OCA) is sometimes used as second-line therapy for PBC; it affects genes that regulate bile acid synthesis, transport, and action. However, the POISE study showed that, while OCA improved PBC symptoms, it was associated with an increase in LDL and total cholesterol and a decrease in HDL. No follow-up studies have determined cardiovascular implications of that change, but OCA should be avoided in patients with active cardiovascular disease or with cardiovascular risk factors.
Cirrhosis
Recent work suggests that patients with cirrhosis may face a higher risk of coronary artery disease than was previously thought, although that risk varies widely according to the etiology of the cirrhosis.
Statins are safe and effective in patients with Child-Pugh class A cirrhosis; there are few data on their safety in patients with decompensated cirrhosis. Some guidance for these patients exists in the 2014 recommendations of the Liver Expert Panel, which advised against statin use in patients with Child-Pugh class B or C cirrhosis.
There’s some evidence that statins reduce portal pressure and may reduce the risk of decompensation in patients whose cirrhosis is caused by HCV or HBV infections, but they should not be used for this purpose.
Posttransplant dyslipidemia
After liver transplant, more than 60% of patients will develop dyslipidemia; these patients often have obesity or diabetes.
Statins are safe for patients with liver transplant. Concomitant use of calcineurin inhibitors and statins that are metabolized by cytochrome P450 may increase the risk of statin-associated myopathy. Pravastatin and fluvastatin are preferable, because they are metabolized by cytochrome P450 34A.
Neither Dr. Speliotes nor her coauthors had any financial disclosures.
SOURCE: Speliotes EK et al. Clin Gastroenterol Hepatol. 2018 Apr 21. doi: 10.1016/j.cgh.2018.04.023.
The medications are only contraindicated in patients with decompensated cirrhosis and statin-induced liver injury, Elizabeth Speliotes, MD, PhD, MPH, and her colleagues wrote in an expert review published in Clinical Gastroenterology and Hepatology. In these patients, statin treatment can compound liver damage and should be avoided, wrote Dr. Speliotes and her coauthors.
Because the liver plays a central role in cholesterol production, many clinicians shy away from treating hyperlipidemia in patients with liver disease. But studies consistently show that lipid-lowering drugs improve dyslipidemia in these patients, which significantly improves both high- and low-density lipoproteins and thereby reduces the long-term risk of cardiovascular disease, the authors wrote.
“Furthermore, the liver plays a role in the metabolism of many drugs, including those that are used to treat dyslipidemia,” wrote Dr. Speliotes of the University of Michigan, Ann Arbor. “It is not surprising, therefore, that many practitioners are hesitant to prescribe medicines to treat dyslipidemia in the setting of liver disease.”
Cholesterol targets described in the 2013 American College of Cardiology/American Heart Association guidelines can safely be applied to patients with liver disease. “The guidelines recommend that adults with cardiovascular disease or LDL of 190 mg/dL or higher be treated with high-intensity statins with the goal of reducing LDL levels by 50%,” they said. Patients whose LDL is 189 mg/dL or lower will benefit from moderate-intensity statins, with a target of a 30%-50% decrease in LDL.
The authors described best practice advice for dyslipidemia treatment in six liver diseases: drug-induced liver injury (DILI), nonalcoholic fatty liver disease (NAFLD), viral hepatitis B and C (HBV and HCV), primary biliary cholangitis (PBC), cirrhosis, and posttransplant dyslipidemia.
DILI
DILI is characterized by elevations of threefold or more in serum alanine aminotransferase (ALT) or aspartate aminotransferase and at least a doubling of total serum bilirubin with no other identifiable cause of these aberrations except the suspect drug. Statins rarely cause a DILI (1 in 100,000 patients), but can cause transient, benign ALT elevations. Statins should be discontinued if ALT or aspartate aminotransferase levels exceed a tripling of the upper limit of normal with concomitant bilirubin elevations. They should not be prescribed to patients with acute liver failure or decompensated liver disease, but otherwise they are safe for most patients with liver disease.
NAFLD
Many patients with NAFLD also have dyslipidemia. All NAFLD patients have an increased risk of cardiovascular disease, although NAFLD and nonalcoholic steatohepatitis are not traditional cardiovascular risk factors. Nevertheless, statins and the accompanying improvement in dyslipidemia have been shown to decrease cardiovascular mortality in these patients. The IDEAL study, for example, showed that moderate statin treatment with 80 mg atorvastatin was associated with a 44% decreased risk in secondary cardiovascular events. Other studies show similar results.
NAFLD patients with elevated LDL may benefit from ezetimibe as primary or add-on therapy. However, none of the drugs used to treat dyslipidemia will improve NAFLD or nonalcoholic steatohepatitis histology.
Viral hepatitis
Hepatitis C virus
Patients with HCV infection often experience decreased serum LDL and total cholesterol. However, these are virally mediated and don’t confer cardiovascular protection. In fact, HCV infections are associated with an increased risk of myocardial infarction. If the patient spontaneously clears the virus, lipids may rebound, so levels should be regularly monitored even if the patient does not need statin therapy.
Hepatitis B virus
HBV also interacts with lipid metabolism and can lead to hyperlipidemia. The American College of Cardiology/American Heart Association guidelines for cardiovascular risk assessment and statin therapy apply to these patients. Statins are safe in patients with either HCV or HBV, who tolerate them well.
PBC
PBC is a chronic autoimmune inflammatory cholestatic disease that is associated with dyslipidemia. These patients exhibit increased serum triglyceride and HDL levels that vary according to PBC stage. About 10% have a significant risk of cardiovascular disease. PBC patients with compensated liver disease can safely tolerate statin treatment, but the drugs should not be given to PBC patients with decompensated liver disease.
Obeticholic acid (OCA) is sometimes used as second-line therapy for PBC; it affects genes that regulate bile acid synthesis, transport, and action. However, the POISE study showed that, while OCA improved PBC symptoms, it was associated with an increase in LDL and total cholesterol and a decrease in HDL. No follow-up studies have determined cardiovascular implications of that change, but OCA should be avoided in patients with active cardiovascular disease or with cardiovascular risk factors.
Cirrhosis
Recent work suggests that patients with cirrhosis may face a higher risk of coronary artery disease than was previously thought, although that risk varies widely according to the etiology of the cirrhosis.
Statins are safe and effective in patients with Child-Pugh class A cirrhosis; there are few data on their safety in patients with decompensated cirrhosis. Some guidance for these patients exists in the 2014 recommendations of the Liver Expert Panel, which advised against statin use in patients with Child-Pugh class B or C cirrhosis.
There’s some evidence that statins reduce portal pressure and may reduce the risk of decompensation in patients whose cirrhosis is caused by HCV or HBV infections, but they should not be used for this purpose.
Posttransplant dyslipidemia
After liver transplant, more than 60% of patients will develop dyslipidemia; these patients often have obesity or diabetes.
Statins are safe for patients with liver transplant. Concomitant use of calcineurin inhibitors and statins that are metabolized by cytochrome P450 may increase the risk of statin-associated myopathy. Pravastatin and fluvastatin are preferable, because they are metabolized by cytochrome P450 34A.
Neither Dr. Speliotes nor her coauthors had any financial disclosures.
SOURCE: Speliotes EK et al. Clin Gastroenterol Hepatol. 2018 Apr 21. doi: 10.1016/j.cgh.2018.04.023.
The medications are only contraindicated in patients with decompensated cirrhosis and statin-induced liver injury, Elizabeth Speliotes, MD, PhD, MPH, and her colleagues wrote in an expert review published in Clinical Gastroenterology and Hepatology. In these patients, statin treatment can compound liver damage and should be avoided, wrote Dr. Speliotes and her coauthors.
Because the liver plays a central role in cholesterol production, many clinicians shy away from treating hyperlipidemia in patients with liver disease. But studies consistently show that lipid-lowering drugs improve dyslipidemia in these patients, which significantly improves both high- and low-density lipoproteins and thereby reduces the long-term risk of cardiovascular disease, the authors wrote.
“Furthermore, the liver plays a role in the metabolism of many drugs, including those that are used to treat dyslipidemia,” wrote Dr. Speliotes of the University of Michigan, Ann Arbor. “It is not surprising, therefore, that many practitioners are hesitant to prescribe medicines to treat dyslipidemia in the setting of liver disease.”
Cholesterol targets described in the 2013 American College of Cardiology/American Heart Association guidelines can safely be applied to patients with liver disease. “The guidelines recommend that adults with cardiovascular disease or LDL of 190 mg/dL or higher be treated with high-intensity statins with the goal of reducing LDL levels by 50%,” they said. Patients whose LDL is 189 mg/dL or lower will benefit from moderate-intensity statins, with a target of a 30%-50% decrease in LDL.
The authors described best practice advice for dyslipidemia treatment in six liver diseases: drug-induced liver injury (DILI), nonalcoholic fatty liver disease (NAFLD), viral hepatitis B and C (HBV and HCV), primary biliary cholangitis (PBC), cirrhosis, and posttransplant dyslipidemia.
DILI
DILI is characterized by elevations of threefold or more in serum alanine aminotransferase (ALT) or aspartate aminotransferase and at least a doubling of total serum bilirubin with no other identifiable cause of these aberrations except the suspect drug. Statins rarely cause a DILI (1 in 100,000 patients), but can cause transient, benign ALT elevations. Statins should be discontinued if ALT or aspartate aminotransferase levels exceed a tripling of the upper limit of normal with concomitant bilirubin elevations. They should not be prescribed to patients with acute liver failure or decompensated liver disease, but otherwise they are safe for most patients with liver disease.
NAFLD
Many patients with NAFLD also have dyslipidemia. All NAFLD patients have an increased risk of cardiovascular disease, although NAFLD and nonalcoholic steatohepatitis are not traditional cardiovascular risk factors. Nevertheless, statins and the accompanying improvement in dyslipidemia have been shown to decrease cardiovascular mortality in these patients. The IDEAL study, for example, showed that moderate statin treatment with 80 mg atorvastatin was associated with a 44% decreased risk in secondary cardiovascular events. Other studies show similar results.
NAFLD patients with elevated LDL may benefit from ezetimibe as primary or add-on therapy. However, none of the drugs used to treat dyslipidemia will improve NAFLD or nonalcoholic steatohepatitis histology.
Viral hepatitis
Hepatitis C virus
Patients with HCV infection often experience decreased serum LDL and total cholesterol. However, these are virally mediated and don’t confer cardiovascular protection. In fact, HCV infections are associated with an increased risk of myocardial infarction. If the patient spontaneously clears the virus, lipids may rebound, so levels should be regularly monitored even if the patient does not need statin therapy.
Hepatitis B virus
HBV also interacts with lipid metabolism and can lead to hyperlipidemia. The American College of Cardiology/American Heart Association guidelines for cardiovascular risk assessment and statin therapy apply to these patients. Statins are safe in patients with either HCV or HBV, who tolerate them well.
PBC
PBC is a chronic autoimmune inflammatory cholestatic disease that is associated with dyslipidemia. These patients exhibit increased serum triglyceride and HDL levels that vary according to PBC stage. About 10% have a significant risk of cardiovascular disease. PBC patients with compensated liver disease can safely tolerate statin treatment, but the drugs should not be given to PBC patients with decompensated liver disease.
Obeticholic acid (OCA) is sometimes used as second-line therapy for PBC; it affects genes that regulate bile acid synthesis, transport, and action. However, the POISE study showed that, while OCA improved PBC symptoms, it was associated with an increase in LDL and total cholesterol and a decrease in HDL. No follow-up studies have determined cardiovascular implications of that change, but OCA should be avoided in patients with active cardiovascular disease or with cardiovascular risk factors.
Cirrhosis
Recent work suggests that patients with cirrhosis may face a higher risk of coronary artery disease than was previously thought, although that risk varies widely according to the etiology of the cirrhosis.
Statins are safe and effective in patients with Child-Pugh class A cirrhosis; there are few data on their safety in patients with decompensated cirrhosis. Some guidance for these patients exists in the 2014 recommendations of the Liver Expert Panel, which advised against statin use in patients with Child-Pugh class B or C cirrhosis.
There’s some evidence that statins reduce portal pressure and may reduce the risk of decompensation in patients whose cirrhosis is caused by HCV or HBV infections, but they should not be used for this purpose.
Posttransplant dyslipidemia
After liver transplant, more than 60% of patients will develop dyslipidemia; these patients often have obesity or diabetes.
Statins are safe for patients with liver transplant. Concomitant use of calcineurin inhibitors and statins that are metabolized by cytochrome P450 may increase the risk of statin-associated myopathy. Pravastatin and fluvastatin are preferable, because they are metabolized by cytochrome P450 34A.
Neither Dr. Speliotes nor her coauthors had any financial disclosures.
SOURCE: Speliotes EK et al. Clin Gastroenterol Hepatol. 2018 Apr 21. doi: 10.1016/j.cgh.2018.04.023.
EXPERT ANALYSIS FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Transplanting HCV-infected kidneys in HCV-infected patients showed positive outcomes, costs
Transplanting a kidney infected with hepatitis C into individuals infected with HCV, followed by treatment, was more effective and less costly than transplanting an uninfected kidney, preceded by HCV treatment, according to Mark H. Eckman, MD, of the University of Cincinnati and his colleagues.
Largely because of the longer wait times for uninfected kidneys, a typical patient aged 58 years on hemodialysis would gain an average of 0.5 quality-adjusted life-years at a lifetime cost savings of $41,591 dollars, according to the model.
“In an era of increasing success for kidney transplants and demand that far outstrips supply, deferring antiviral therapy until after transplant of HCV-infected kidneys, when available, should be both cost saving and effective,” the researchers wrote.
The study was funded by grants from Merck Sharpe & Dohme and the National Center for Advancing Translational Science. Several of the authors reported having grants from Merck and grants and personal fees from a variety of other pharmaceutical companies.
SOURCE: Eckman MH et al. Ann Intern Med. 2018 Jul 10. doi: 10.7326/M17-3088.
Transplanting a kidney infected with hepatitis C into individuals infected with HCV, followed by treatment, was more effective and less costly than transplanting an uninfected kidney, preceded by HCV treatment, according to Mark H. Eckman, MD, of the University of Cincinnati and his colleagues.
Largely because of the longer wait times for uninfected kidneys, a typical patient aged 58 years on hemodialysis would gain an average of 0.5 quality-adjusted life-years at a lifetime cost savings of $41,591 dollars, according to the model.
“In an era of increasing success for kidney transplants and demand that far outstrips supply, deferring antiviral therapy until after transplant of HCV-infected kidneys, when available, should be both cost saving and effective,” the researchers wrote.
The study was funded by grants from Merck Sharpe & Dohme and the National Center for Advancing Translational Science. Several of the authors reported having grants from Merck and grants and personal fees from a variety of other pharmaceutical companies.
SOURCE: Eckman MH et al. Ann Intern Med. 2018 Jul 10. doi: 10.7326/M17-3088.
Transplanting a kidney infected with hepatitis C into individuals infected with HCV, followed by treatment, was more effective and less costly than transplanting an uninfected kidney, preceded by HCV treatment, according to Mark H. Eckman, MD, of the University of Cincinnati and his colleagues.
Largely because of the longer wait times for uninfected kidneys, a typical patient aged 58 years on hemodialysis would gain an average of 0.5 quality-adjusted life-years at a lifetime cost savings of $41,591 dollars, according to the model.
“In an era of increasing success for kidney transplants and demand that far outstrips supply, deferring antiviral therapy until after transplant of HCV-infected kidneys, when available, should be both cost saving and effective,” the researchers wrote.
The study was funded by grants from Merck Sharpe & Dohme and the National Center for Advancing Translational Science. Several of the authors reported having grants from Merck and grants and personal fees from a variety of other pharmaceutical companies.
SOURCE: Eckman MH et al. Ann Intern Med. 2018 Jul 10. doi: 10.7326/M17-3088.
FROM ANNALS OF INTERNAL MEDICINE
Antibodies to HCV core protein can be expressed in E. coli and silkworms
Intrabody 2H9-L, which can function as a hepatitis C virus (HCV) inhibitor in human hepatic cells, was expressed at high levels in Escherichia coli and silkworm pupae and was successfully purified in soluble form, according to a report published in Protein Expression and Purification by Tatsuya Kato, an assistant professor at Shizuoka (Japan) University, and his colleagues.
In their study, 171 mcg of purified intrabody was obtainable from 100 mL of E. coli culture, and 132 mcg could be obtained from 10 silkworm pupae.
The intrabodies were capable of binding to all HCV core protein variants tested. These purified intrabodies can be used in biochemical analyses and provide a potential pathway to developing a new type of therapy, according to the researchers.
“The structural basis of HCV core–intrabody interfaces would allow a novel strategy to design and generate chemical drugs with antiviral activities,” they stated. In addition, “to analyze the HCV core protein in detail, this intrabody can be used to keep the HCV core protein soluble, even when its concentration is high.”
No funding source or disclosures were reported in the paper.
SOURCE: Kato T et al. Protein Expression and Purification. 2018 October;150:61-6.
Intrabody 2H9-L, which can function as a hepatitis C virus (HCV) inhibitor in human hepatic cells, was expressed at high levels in Escherichia coli and silkworm pupae and was successfully purified in soluble form, according to a report published in Protein Expression and Purification by Tatsuya Kato, an assistant professor at Shizuoka (Japan) University, and his colleagues.
In their study, 171 mcg of purified intrabody was obtainable from 100 mL of E. coli culture, and 132 mcg could be obtained from 10 silkworm pupae.
The intrabodies were capable of binding to all HCV core protein variants tested. These purified intrabodies can be used in biochemical analyses and provide a potential pathway to developing a new type of therapy, according to the researchers.
“The structural basis of HCV core–intrabody interfaces would allow a novel strategy to design and generate chemical drugs with antiviral activities,” they stated. In addition, “to analyze the HCV core protein in detail, this intrabody can be used to keep the HCV core protein soluble, even when its concentration is high.”
No funding source or disclosures were reported in the paper.
SOURCE: Kato T et al. Protein Expression and Purification. 2018 October;150:61-6.
Intrabody 2H9-L, which can function as a hepatitis C virus (HCV) inhibitor in human hepatic cells, was expressed at high levels in Escherichia coli and silkworm pupae and was successfully purified in soluble form, according to a report published in Protein Expression and Purification by Tatsuya Kato, an assistant professor at Shizuoka (Japan) University, and his colleagues.
In their study, 171 mcg of purified intrabody was obtainable from 100 mL of E. coli culture, and 132 mcg could be obtained from 10 silkworm pupae.
The intrabodies were capable of binding to all HCV core protein variants tested. These purified intrabodies can be used in biochemical analyses and provide a potential pathway to developing a new type of therapy, according to the researchers.
“The structural basis of HCV core–intrabody interfaces would allow a novel strategy to design and generate chemical drugs with antiviral activities,” they stated. In addition, “to analyze the HCV core protein in detail, this intrabody can be used to keep the HCV core protein soluble, even when its concentration is high.”
No funding source or disclosures were reported in the paper.
SOURCE: Kato T et al. Protein Expression and Purification. 2018 October;150:61-6.
FROM PROTEIN EXPRESSION AND PURIFICATION
‘Reverse transitions’ from injecting to noninjecting drug use studied
SAN DIEGO – The transition from noninjecting to injecting drug use can be a reversible process, results from a long-term study suggest.
“There’s a common stereotype in popular culture and in academic research that once people start injecting drugs, that will be their dominant route of administration for the rest of their lives,” lead study author Don C. Des Jarlais, PhD, said in an interview at the annual meeting of the College on Problems of Drug Dependence. “We found that people who started injecting injected for several years, but then went back to noninjecting drug use. That so-called ‘reverse transition’ leads to a very big difference in infection with hepatitis C virus as well as reduced risk of overdose and reduced risk of other bacterial infections. Even though these people continued to use drugs, they were doing it in a way that was much safer.”
In an effort to examine the prevalence and characteristics of reverse transitions, Dr. Des Jarlais and his associates recruited injecting and noninjecting drug users aged 18 years and older from the Mount Sinai Beth Israel detoxification and methadone maintenance programs in New York City from 2000 to 2017. The researchers obtained informed consent and conducted a structured interview that included questions on why former injectors had ceased injecting drugs, along with testing for HIV and HCV.
People who were currently injecting were defined as those whose first injection was in 2000 or later and who had injected heroin or cocaine during the 6 months prior to treatment entry. People who formerly injected drugs were defined as those whose first injection was in 2000 or later but who had used heroin or cocaine without injecting drugs during the 6 months prior to treatment entry.
Dr. Des Jarlais, professor of psychiatry at the Icahn School of Medicine at Mount Sinai, New York, reported results from 937 current and 104 former injection drug users. Compared with current injection drug users, former users were older (a mean age of 41 vs. 35, respectively), more likely to be African American (28% vs. 12%), more likely to have received previous methadone treatment (68% vs. 54%), and less likely to be HCV positive (30% vs. 47%; P less than 0.05 for all associations).
The researchers found that 11% of former injection drug users had reverse transitioned to noninjection drug use. Among former injection drug users, the most common reasons for ceasing to inject were “don’t like needles” (30%), “got tired of injecting” (29%), “afraid of overdose” (17%), “concerns about stigma” (16%), and other health concerns (14%).
Dr. Des Jarlais said. “Injecting is messy and it requires private space; it’s not a pleasant way of using drugs compared to just sniffing it. If we’re going to control hepatitis C, we need to find ways of encouraging people that if they’re going to continue to use drugs, they should try to stop injecting.”
The next step in this research area is to develop a more complete understanding of reverse transitions. “These are people doing it on their own, but we don’t have any programs to help them,” he said. “We have needle exchange programs for people to inject safely, but we don’t have any programs where people go from injecting to noninjecting.”
The study received funding support from the National Institute on Drug Abuse. Dr. Des Jarlais reported having no financial disclosures.
SAN DIEGO – The transition from noninjecting to injecting drug use can be a reversible process, results from a long-term study suggest.
“There’s a common stereotype in popular culture and in academic research that once people start injecting drugs, that will be their dominant route of administration for the rest of their lives,” lead study author Don C. Des Jarlais, PhD, said in an interview at the annual meeting of the College on Problems of Drug Dependence. “We found that people who started injecting injected for several years, but then went back to noninjecting drug use. That so-called ‘reverse transition’ leads to a very big difference in infection with hepatitis C virus as well as reduced risk of overdose and reduced risk of other bacterial infections. Even though these people continued to use drugs, they were doing it in a way that was much safer.”
In an effort to examine the prevalence and characteristics of reverse transitions, Dr. Des Jarlais and his associates recruited injecting and noninjecting drug users aged 18 years and older from the Mount Sinai Beth Israel detoxification and methadone maintenance programs in New York City from 2000 to 2017. The researchers obtained informed consent and conducted a structured interview that included questions on why former injectors had ceased injecting drugs, along with testing for HIV and HCV.
People who were currently injecting were defined as those whose first injection was in 2000 or later and who had injected heroin or cocaine during the 6 months prior to treatment entry. People who formerly injected drugs were defined as those whose first injection was in 2000 or later but who had used heroin or cocaine without injecting drugs during the 6 months prior to treatment entry.
Dr. Des Jarlais, professor of psychiatry at the Icahn School of Medicine at Mount Sinai, New York, reported results from 937 current and 104 former injection drug users. Compared with current injection drug users, former users were older (a mean age of 41 vs. 35, respectively), more likely to be African American (28% vs. 12%), more likely to have received previous methadone treatment (68% vs. 54%), and less likely to be HCV positive (30% vs. 47%; P less than 0.05 for all associations).
The researchers found that 11% of former injection drug users had reverse transitioned to noninjection drug use. Among former injection drug users, the most common reasons for ceasing to inject were “don’t like needles” (30%), “got tired of injecting” (29%), “afraid of overdose” (17%), “concerns about stigma” (16%), and other health concerns (14%).
Dr. Des Jarlais said. “Injecting is messy and it requires private space; it’s not a pleasant way of using drugs compared to just sniffing it. If we’re going to control hepatitis C, we need to find ways of encouraging people that if they’re going to continue to use drugs, they should try to stop injecting.”
The next step in this research area is to develop a more complete understanding of reverse transitions. “These are people doing it on their own, but we don’t have any programs to help them,” he said. “We have needle exchange programs for people to inject safely, but we don’t have any programs where people go from injecting to noninjecting.”
The study received funding support from the National Institute on Drug Abuse. Dr. Des Jarlais reported having no financial disclosures.
SAN DIEGO – The transition from noninjecting to injecting drug use can be a reversible process, results from a long-term study suggest.
“There’s a common stereotype in popular culture and in academic research that once people start injecting drugs, that will be their dominant route of administration for the rest of their lives,” lead study author Don C. Des Jarlais, PhD, said in an interview at the annual meeting of the College on Problems of Drug Dependence. “We found that people who started injecting injected for several years, but then went back to noninjecting drug use. That so-called ‘reverse transition’ leads to a very big difference in infection with hepatitis C virus as well as reduced risk of overdose and reduced risk of other bacterial infections. Even though these people continued to use drugs, they were doing it in a way that was much safer.”
In an effort to examine the prevalence and characteristics of reverse transitions, Dr. Des Jarlais and his associates recruited injecting and noninjecting drug users aged 18 years and older from the Mount Sinai Beth Israel detoxification and methadone maintenance programs in New York City from 2000 to 2017. The researchers obtained informed consent and conducted a structured interview that included questions on why former injectors had ceased injecting drugs, along with testing for HIV and HCV.
People who were currently injecting were defined as those whose first injection was in 2000 or later and who had injected heroin or cocaine during the 6 months prior to treatment entry. People who formerly injected drugs were defined as those whose first injection was in 2000 or later but who had used heroin or cocaine without injecting drugs during the 6 months prior to treatment entry.
Dr. Des Jarlais, professor of psychiatry at the Icahn School of Medicine at Mount Sinai, New York, reported results from 937 current and 104 former injection drug users. Compared with current injection drug users, former users were older (a mean age of 41 vs. 35, respectively), more likely to be African American (28% vs. 12%), more likely to have received previous methadone treatment (68% vs. 54%), and less likely to be HCV positive (30% vs. 47%; P less than 0.05 for all associations).
The researchers found that 11% of former injection drug users had reverse transitioned to noninjection drug use. Among former injection drug users, the most common reasons for ceasing to inject were “don’t like needles” (30%), “got tired of injecting” (29%), “afraid of overdose” (17%), “concerns about stigma” (16%), and other health concerns (14%).
Dr. Des Jarlais said. “Injecting is messy and it requires private space; it’s not a pleasant way of using drugs compared to just sniffing it. If we’re going to control hepatitis C, we need to find ways of encouraging people that if they’re going to continue to use drugs, they should try to stop injecting.”
The next step in this research area is to develop a more complete understanding of reverse transitions. “These are people doing it on their own, but we don’t have any programs to help them,” he said. “We have needle exchange programs for people to inject safely, but we don’t have any programs where people go from injecting to noninjecting.”
The study received funding support from the National Institute on Drug Abuse. Dr. Des Jarlais reported having no financial disclosures.
AT CPDD 2018
Key clinical point: To control hepatitis C, patients who continue to use drugs should be encouraged to stop injecting.
Major finding: Among former injection drug users, 11% had reverse transitioned to noninjection drug use.
Study details: A study of 937 current and 104 former injection drug users between 2000 and 2017.
Disclosures: The study received funding support from the National Institute on Drug Abuse. Dr. Des Jarlais reported having no financial disclosures.
MicroRNAs flag liver damage in HIV-, HCV-infected persons
BOSTON – In persons infected with HIV-1, with or without hepatitis C coinfections, specific circulating microRNAs may signal the presence of liver injury and progression, investigators stated.
An analysis of small RNA expression in plasma samples from 144 HIV-infected patients showed that two microRNAs (miRNAs) in the same family of RNA fragments were significantly upregulated in patients with HIV-1 and HCV coinfections that progressed to liver cirrhosis, despite the patients having no evidence of liver fibrosis at the time of plasma sampling, reported Miguel Angel Martinez, PhD, of IrsiCaixa AIDS Research Institute in Badalona, Spain.
“Our results reveal that HIV-1 infection impacts liver miRNA metabolism and upregulated plasma levels of miRNAs that were previously associated with liver damage, even in the absence of an HCV coinfection,” he said at the Conference on Retroviruses & Opportunistic Infections. He reported the results in a themed discussion and scientific poster session.
Dr. Martinez and his colleagues performed large-scale deep sequencing analyses of miRNAs in plasma from 144 patients with HIV-1 who had elevated alanine aminotransferase (ALT), focal nodular hyperplasia, or HCV coinfections, and compared results with those from healthy blood donors and HCV mono-infected persons.
They identified 1,425 different mature miRNAs in the study samples. Compared with healthy donors, patients with HIV infections showed significantly dysregulated expression of 25 miRNAs, and 19 of these miRNAs were also found in patients with HCV monoinfection. All but 1 of 14 upregulated miRNAs in patients with HCV monoinfections were also upregulated in patients with HIV monoinfections.
Of these 13 upregulated miRNAs, 11 significantly and positively correlated with ALT and aspartate aminotransferase (AST) levels in most of the study samples, including those from healthy donors, Dr. Martinez noted.
“These results indicate that HIV mono-infection is able to dysregulate microRNAs related with liver injury and damage,” he said.
Of the 13 miRNAs, two, labeled miR-99a-5p and miR-100-5p, which belong to the same family of miRNAs, were found to be significantly upregulated in patients with HIV and HCV coinfections that later progressed to liver cirrhosis “even those these patients exhibited no liver fibrosis at the time of sampling,” he said
The two culprit miRNAs were significantly correlated with ALT and AST levels, as well as the degree of liver fibrosis.
A comparison of samples from patients with HIV monoinfection who had elevated ALT or focal nodular hyperplasia with those of patients with HIV infection but normal ALT levels showed that two other miRNAs, miR-122-3p and miR-193b-5p, were highly and significantly upregulated, and correlated with both aminotransferase and liver fibrosis levels.
“This study demonstrates the potential of microRNAs as biomarkers of liver injury progression in HIV-1 infected patients,” Dr. Martinez concluded.
The Spanish Instituto de Salud Carlos III and the Spanish AIDS network funded the study. Dr. Martinez reported having no conflicts of interest.
SOURCE: Martinez MA et al. CROI 2018, abstract 639.
BOSTON – In persons infected with HIV-1, with or without hepatitis C coinfections, specific circulating microRNAs may signal the presence of liver injury and progression, investigators stated.
An analysis of small RNA expression in plasma samples from 144 HIV-infected patients showed that two microRNAs (miRNAs) in the same family of RNA fragments were significantly upregulated in patients with HIV-1 and HCV coinfections that progressed to liver cirrhosis, despite the patients having no evidence of liver fibrosis at the time of plasma sampling, reported Miguel Angel Martinez, PhD, of IrsiCaixa AIDS Research Institute in Badalona, Spain.
“Our results reveal that HIV-1 infection impacts liver miRNA metabolism and upregulated plasma levels of miRNAs that were previously associated with liver damage, even in the absence of an HCV coinfection,” he said at the Conference on Retroviruses & Opportunistic Infections. He reported the results in a themed discussion and scientific poster session.
Dr. Martinez and his colleagues performed large-scale deep sequencing analyses of miRNAs in plasma from 144 patients with HIV-1 who had elevated alanine aminotransferase (ALT), focal nodular hyperplasia, or HCV coinfections, and compared results with those from healthy blood donors and HCV mono-infected persons.
They identified 1,425 different mature miRNAs in the study samples. Compared with healthy donors, patients with HIV infections showed significantly dysregulated expression of 25 miRNAs, and 19 of these miRNAs were also found in patients with HCV monoinfection. All but 1 of 14 upregulated miRNAs in patients with HCV monoinfections were also upregulated in patients with HIV monoinfections.
Of these 13 upregulated miRNAs, 11 significantly and positively correlated with ALT and aspartate aminotransferase (AST) levels in most of the study samples, including those from healthy donors, Dr. Martinez noted.
“These results indicate that HIV mono-infection is able to dysregulate microRNAs related with liver injury and damage,” he said.
Of the 13 miRNAs, two, labeled miR-99a-5p and miR-100-5p, which belong to the same family of miRNAs, were found to be significantly upregulated in patients with HIV and HCV coinfections that later progressed to liver cirrhosis “even those these patients exhibited no liver fibrosis at the time of sampling,” he said
The two culprit miRNAs were significantly correlated with ALT and AST levels, as well as the degree of liver fibrosis.
A comparison of samples from patients with HIV monoinfection who had elevated ALT or focal nodular hyperplasia with those of patients with HIV infection but normal ALT levels showed that two other miRNAs, miR-122-3p and miR-193b-5p, were highly and significantly upregulated, and correlated with both aminotransferase and liver fibrosis levels.
“This study demonstrates the potential of microRNAs as biomarkers of liver injury progression in HIV-1 infected patients,” Dr. Martinez concluded.
The Spanish Instituto de Salud Carlos III and the Spanish AIDS network funded the study. Dr. Martinez reported having no conflicts of interest.
SOURCE: Martinez MA et al. CROI 2018, abstract 639.
BOSTON – In persons infected with HIV-1, with or without hepatitis C coinfections, specific circulating microRNAs may signal the presence of liver injury and progression, investigators stated.
An analysis of small RNA expression in plasma samples from 144 HIV-infected patients showed that two microRNAs (miRNAs) in the same family of RNA fragments were significantly upregulated in patients with HIV-1 and HCV coinfections that progressed to liver cirrhosis, despite the patients having no evidence of liver fibrosis at the time of plasma sampling, reported Miguel Angel Martinez, PhD, of IrsiCaixa AIDS Research Institute in Badalona, Spain.
“Our results reveal that HIV-1 infection impacts liver miRNA metabolism and upregulated plasma levels of miRNAs that were previously associated with liver damage, even in the absence of an HCV coinfection,” he said at the Conference on Retroviruses & Opportunistic Infections. He reported the results in a themed discussion and scientific poster session.
Dr. Martinez and his colleagues performed large-scale deep sequencing analyses of miRNAs in plasma from 144 patients with HIV-1 who had elevated alanine aminotransferase (ALT), focal nodular hyperplasia, or HCV coinfections, and compared results with those from healthy blood donors and HCV mono-infected persons.
They identified 1,425 different mature miRNAs in the study samples. Compared with healthy donors, patients with HIV infections showed significantly dysregulated expression of 25 miRNAs, and 19 of these miRNAs were also found in patients with HCV monoinfection. All but 1 of 14 upregulated miRNAs in patients with HCV monoinfections were also upregulated in patients with HIV monoinfections.
Of these 13 upregulated miRNAs, 11 significantly and positively correlated with ALT and aspartate aminotransferase (AST) levels in most of the study samples, including those from healthy donors, Dr. Martinez noted.
“These results indicate that HIV mono-infection is able to dysregulate microRNAs related with liver injury and damage,” he said.
Of the 13 miRNAs, two, labeled miR-99a-5p and miR-100-5p, which belong to the same family of miRNAs, were found to be significantly upregulated in patients with HIV and HCV coinfections that later progressed to liver cirrhosis “even those these patients exhibited no liver fibrosis at the time of sampling,” he said
The two culprit miRNAs were significantly correlated with ALT and AST levels, as well as the degree of liver fibrosis.
A comparison of samples from patients with HIV monoinfection who had elevated ALT or focal nodular hyperplasia with those of patients with HIV infection but normal ALT levels showed that two other miRNAs, miR-122-3p and miR-193b-5p, were highly and significantly upregulated, and correlated with both aminotransferase and liver fibrosis levels.
“This study demonstrates the potential of microRNAs as biomarkers of liver injury progression in HIV-1 infected patients,” Dr. Martinez concluded.
The Spanish Instituto de Salud Carlos III and the Spanish AIDS network funded the study. Dr. Martinez reported having no conflicts of interest.
SOURCE: Martinez MA et al. CROI 2018, abstract 639.
REPORTING FROM CROI
Key clinical point: Specific circulating microRNAs appear to be biomarkers for liver injury and progression in persons with HIV and/or HCV infections.
Major finding: Two microRNAs correlated with elevated liver enzymes and liver fibrosis in patients with HIV and HCV coinfection, and two correlated with liver injury in patients with HIV monoinfection.
Study details: Analysis of plasma samples from 144 persons with HIV with elevated ALT, focal nodular hyperplasia, or HCV coinfections, with control samples from healthy donors and HCV monoinfected individuals.
Disclosures: The Spanish Instituto de Salud Carlos III and the Spanish AIDS network funded the study. Dr. Martinez reported having no conflicts of interest.
Source: Martinez MA et al. CROI 2018, abstract 639.