User login
Many advanced countries missing targets for HCV elimination
Eleven high-income countries are on track to meet World Health Organization targets to eliminate hepatitis C infection by 2030, compared with 9 countries 2 years ago, researchers reported. But 28 countries, including the United States, are not expected to eliminate HCV until 2050.
“In the countries making progress, the common elements are political will, a clear national plan, and easing of restrictions on the cascade of care and testing,” Yuri Sanchez Gonzalez, PhD, director of health economics and outcomes research for biopharmaceutical company AbbVie said in an interview. That would include offering hepatitis C treatment to individuals who have liver fibrosis and those struggling with sobriety, he said. “We can’t overstate how much this is a massive driver of the hepatitis C epidemic.”
His research, presented at the digital edition of the International Liver Congress this week, showed more countries on target than in a study published 2 years ago in Liver International . “But it’s not enough,” Dr. Sanchez Gonzalez said. “We know that more than 80% of infections are in people who inject drugs. Stigmatization of drug use is still a very major issue.” Despite data clearly showing that countries who have harm-reduction programs make progress, “in many countries these programs are still illegal.”
To evaluate which countries are on target to eliminate hepatitis C by 2030, researchers performed Markov disease progression models of HCV infection in 45 high-income countries. The results showed that Australia, Canada, France, Germany, Iceland, Italy, Japan, Spain, Sweden, Switzerland, and the United Kingdom are “in the green” (on target for 2030).
Austria, Malta, the Netherlands, New Zealand, and South Korea are “in the yellow” (on target for 2040), and 28 remaining countries, including the United States, are “in the red,” with targets estimated to be met by 2050.
Compared with an analysis performed 2 years ago, South Korea moved from green to yellow, while Canada, Germany, and Sweden moved from red to green.
Researchers say that the countries moving the needle are the ones addressing barriers to care.
EASL: Eliminate barriers to treatment
During this week’s Congress, the European Association for the Study of the Liver (EASL) launched a policy statement recommending breaking down all barriers that prevent people who inject drugs from getting access to hepatitis C treatment, including encouragement of laws and policies that “decriminalize drug use, drug possession and drug users themselves,” said statement coauthor Mojca Maticic, MD, PhD, University of Ljubljana, Slovenia.
“To reach the desired WHO goal, combining decriminalization of personal drug consumption and integrated interventions that include hepatitis C testing and treatment should be implemented,” she added. We need to adopt “an approach based on public health promotion, respect for human rights, and evidence.”
Although harm reduction is the top strategy for making 2030 targets, having precision data also helps a lot.
“High-quality data and harm-reduction innovation to curb the overdose crisis has moved us out of the red and into the green,” Canadian researcher Jordan Feld, MD, MPH, University of Toronto, said in an interview. He points to British Columbia, Canada’s third-most populous province, putting harm reduction programs in place as key to Canadian progress.
“Given the increasing opioid epidemic, you’re creating yourself a bigger problem if you don’t treat this population,” Dr. Feld said. When a person needs 6 months to get sober in order to be treated for HCV, that’s more potential time to pass the infection to others. His study, also presented at ILC this week, outlines anticipated timing of hepatitis C in Canada’s four most populous provinces (Ontario, Quebec, British Columbia, and Alberta), and shows British Columbia will reach targets by 2028.
Lifting all restrictions clearly helps, Dr. Sanchez Gonzalez reported. He pointed to Sweden as a good example, a country that recently lifted HCV treatment restrictions for individuals living with fibrosis. Sweden moved from a red to a green spot in this analysis and is now on target for 2030.
“As long as everyone who needs treatment gets treatment, you can make tremendous progress,” he said.
Keeping track is also essential to moving the needle. Since the WHO has no enforcement power, “these studies, which offer a report card of progress, really matter,” Dr. Sanchez Gonzalez explained. When a country knows where they stand, they are more likely to take action to change. “Nobody likes to be shown in the red.”
Still, “it’s not a shaming exercise,” he said. It’s about starting a conversation, showing who’s on track, and sharing how to get on track. “Knowing that there is something in your power to move the needle toward elimination by learning from your neighbors is powerful – often, it just takes political will.”
Dr. Feld has received consulting fees from AbbVie. Dr. Sanchez Gonzalez is on staff as the Director of Economics at AbbVie. Dr. Maticic has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Eleven high-income countries are on track to meet World Health Organization targets to eliminate hepatitis C infection by 2030, compared with 9 countries 2 years ago, researchers reported. But 28 countries, including the United States, are not expected to eliminate HCV until 2050.
“In the countries making progress, the common elements are political will, a clear national plan, and easing of restrictions on the cascade of care and testing,” Yuri Sanchez Gonzalez, PhD, director of health economics and outcomes research for biopharmaceutical company AbbVie said in an interview. That would include offering hepatitis C treatment to individuals who have liver fibrosis and those struggling with sobriety, he said. “We can’t overstate how much this is a massive driver of the hepatitis C epidemic.”
His research, presented at the digital edition of the International Liver Congress this week, showed more countries on target than in a study published 2 years ago in Liver International . “But it’s not enough,” Dr. Sanchez Gonzalez said. “We know that more than 80% of infections are in people who inject drugs. Stigmatization of drug use is still a very major issue.” Despite data clearly showing that countries who have harm-reduction programs make progress, “in many countries these programs are still illegal.”
To evaluate which countries are on target to eliminate hepatitis C by 2030, researchers performed Markov disease progression models of HCV infection in 45 high-income countries. The results showed that Australia, Canada, France, Germany, Iceland, Italy, Japan, Spain, Sweden, Switzerland, and the United Kingdom are “in the green” (on target for 2030).
Austria, Malta, the Netherlands, New Zealand, and South Korea are “in the yellow” (on target for 2040), and 28 remaining countries, including the United States, are “in the red,” with targets estimated to be met by 2050.
Compared with an analysis performed 2 years ago, South Korea moved from green to yellow, while Canada, Germany, and Sweden moved from red to green.
Researchers say that the countries moving the needle are the ones addressing barriers to care.
EASL: Eliminate barriers to treatment
During this week’s Congress, the European Association for the Study of the Liver (EASL) launched a policy statement recommending breaking down all barriers that prevent people who inject drugs from getting access to hepatitis C treatment, including encouragement of laws and policies that “decriminalize drug use, drug possession and drug users themselves,” said statement coauthor Mojca Maticic, MD, PhD, University of Ljubljana, Slovenia.
“To reach the desired WHO goal, combining decriminalization of personal drug consumption and integrated interventions that include hepatitis C testing and treatment should be implemented,” she added. We need to adopt “an approach based on public health promotion, respect for human rights, and evidence.”
Although harm reduction is the top strategy for making 2030 targets, having precision data also helps a lot.
“High-quality data and harm-reduction innovation to curb the overdose crisis has moved us out of the red and into the green,” Canadian researcher Jordan Feld, MD, MPH, University of Toronto, said in an interview. He points to British Columbia, Canada’s third-most populous province, putting harm reduction programs in place as key to Canadian progress.
“Given the increasing opioid epidemic, you’re creating yourself a bigger problem if you don’t treat this population,” Dr. Feld said. When a person needs 6 months to get sober in order to be treated for HCV, that’s more potential time to pass the infection to others. His study, also presented at ILC this week, outlines anticipated timing of hepatitis C in Canada’s four most populous provinces (Ontario, Quebec, British Columbia, and Alberta), and shows British Columbia will reach targets by 2028.
Lifting all restrictions clearly helps, Dr. Sanchez Gonzalez reported. He pointed to Sweden as a good example, a country that recently lifted HCV treatment restrictions for individuals living with fibrosis. Sweden moved from a red to a green spot in this analysis and is now on target for 2030.
“As long as everyone who needs treatment gets treatment, you can make tremendous progress,” he said.
Keeping track is also essential to moving the needle. Since the WHO has no enforcement power, “these studies, which offer a report card of progress, really matter,” Dr. Sanchez Gonzalez explained. When a country knows where they stand, they are more likely to take action to change. “Nobody likes to be shown in the red.”
Still, “it’s not a shaming exercise,” he said. It’s about starting a conversation, showing who’s on track, and sharing how to get on track. “Knowing that there is something in your power to move the needle toward elimination by learning from your neighbors is powerful – often, it just takes political will.”
Dr. Feld has received consulting fees from AbbVie. Dr. Sanchez Gonzalez is on staff as the Director of Economics at AbbVie. Dr. Maticic has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Eleven high-income countries are on track to meet World Health Organization targets to eliminate hepatitis C infection by 2030, compared with 9 countries 2 years ago, researchers reported. But 28 countries, including the United States, are not expected to eliminate HCV until 2050.
“In the countries making progress, the common elements are political will, a clear national plan, and easing of restrictions on the cascade of care and testing,” Yuri Sanchez Gonzalez, PhD, director of health economics and outcomes research for biopharmaceutical company AbbVie said in an interview. That would include offering hepatitis C treatment to individuals who have liver fibrosis and those struggling with sobriety, he said. “We can’t overstate how much this is a massive driver of the hepatitis C epidemic.”
His research, presented at the digital edition of the International Liver Congress this week, showed more countries on target than in a study published 2 years ago in Liver International . “But it’s not enough,” Dr. Sanchez Gonzalez said. “We know that more than 80% of infections are in people who inject drugs. Stigmatization of drug use is still a very major issue.” Despite data clearly showing that countries who have harm-reduction programs make progress, “in many countries these programs are still illegal.”
To evaluate which countries are on target to eliminate hepatitis C by 2030, researchers performed Markov disease progression models of HCV infection in 45 high-income countries. The results showed that Australia, Canada, France, Germany, Iceland, Italy, Japan, Spain, Sweden, Switzerland, and the United Kingdom are “in the green” (on target for 2030).
Austria, Malta, the Netherlands, New Zealand, and South Korea are “in the yellow” (on target for 2040), and 28 remaining countries, including the United States, are “in the red,” with targets estimated to be met by 2050.
Compared with an analysis performed 2 years ago, South Korea moved from green to yellow, while Canada, Germany, and Sweden moved from red to green.
Researchers say that the countries moving the needle are the ones addressing barriers to care.
EASL: Eliminate barriers to treatment
During this week’s Congress, the European Association for the Study of the Liver (EASL) launched a policy statement recommending breaking down all barriers that prevent people who inject drugs from getting access to hepatitis C treatment, including encouragement of laws and policies that “decriminalize drug use, drug possession and drug users themselves,” said statement coauthor Mojca Maticic, MD, PhD, University of Ljubljana, Slovenia.
“To reach the desired WHO goal, combining decriminalization of personal drug consumption and integrated interventions that include hepatitis C testing and treatment should be implemented,” she added. We need to adopt “an approach based on public health promotion, respect for human rights, and evidence.”
Although harm reduction is the top strategy for making 2030 targets, having precision data also helps a lot.
“High-quality data and harm-reduction innovation to curb the overdose crisis has moved us out of the red and into the green,” Canadian researcher Jordan Feld, MD, MPH, University of Toronto, said in an interview. He points to British Columbia, Canada’s third-most populous province, putting harm reduction programs in place as key to Canadian progress.
“Given the increasing opioid epidemic, you’re creating yourself a bigger problem if you don’t treat this population,” Dr. Feld said. When a person needs 6 months to get sober in order to be treated for HCV, that’s more potential time to pass the infection to others. His study, also presented at ILC this week, outlines anticipated timing of hepatitis C in Canada’s four most populous provinces (Ontario, Quebec, British Columbia, and Alberta), and shows British Columbia will reach targets by 2028.
Lifting all restrictions clearly helps, Dr. Sanchez Gonzalez reported. He pointed to Sweden as a good example, a country that recently lifted HCV treatment restrictions for individuals living with fibrosis. Sweden moved from a red to a green spot in this analysis and is now on target for 2030.
“As long as everyone who needs treatment gets treatment, you can make tremendous progress,” he said.
Keeping track is also essential to moving the needle. Since the WHO has no enforcement power, “these studies, which offer a report card of progress, really matter,” Dr. Sanchez Gonzalez explained. When a country knows where they stand, they are more likely to take action to change. “Nobody likes to be shown in the red.”
Still, “it’s not a shaming exercise,” he said. It’s about starting a conversation, showing who’s on track, and sharing how to get on track. “Knowing that there is something in your power to move the needle toward elimination by learning from your neighbors is powerful – often, it just takes political will.”
Dr. Feld has received consulting fees from AbbVie. Dr. Sanchez Gonzalez is on staff as the Director of Economics at AbbVie. Dr. Maticic has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Hepatitis screening now for all patients with cancer on therapy
All patients with cancer who are candidates for systemic anticancer therapy should be screened for hepatitis B virus (HBV) infection prior to or at the start of therapy, according to an updated provisional clinical opinion (PCO) from the American Society of Clinical Oncology.
“This is a new approach [that] will actively take system changes ... but it will ultimately be safer for patients – and that is crucial,” commented Jessica P. Hwang, MD, MPH, cochair of the American Society of Clinical Oncology HBV Screening Expert Panel and the first author of the PCO.
Uptake of this universal screening approach would streamline testing protocols and identify more patients at risk for HBV reactivation who should receive prophylactic antiviral therapy, Dr. Hwang said in an interview.
The PCO calls for antiviral prophylaxis during and for at least 12 months after therapy for those with chronic HBV infection who are receiving any systemic anticancer treatment and for those with have had HBV in the past and are receiving any therapies that pose a risk for HBV reactivation.
“Hepatitis B reactivation can cause really terrible outcomes, like organ failure and even death,” Dr. Hwang, who is also a professor at the University of Texas MD Anderson Cancer Center, Houston, commented in an interview.
“This whole [issue of] reactivation and adverse outcomes with anticancer therapies is completely preventable with good planning, good communication, comanagement with specialists, and antiviral therapy and monitoring,” she added.
The updated opinion was published online July 27 in the Journal of Clinical Oncology.
It was developed in response to new data that call into question the previously recommended risk-adaptive approach to HBV screening of cancer patients, say the authors.
ASCO PCOs are developed “to provide timely clinical guidance” on the basis of emerging practice-changing information. This is the second update to follow the initial HBV screening PCO, published in 2010. In the absence of clear consensus because of limited data, the original PCO called for a risk-based approach to screening. A 2015 update extended the recommendation for screening to patients starting anti-CD20 therapy or who are to undergo stem cell transplant and to those with risk factors for HBV exposure.
The current update provides “a clinically pragmatic approach to HBV screening and management” that is based on the latest findings, say the authors. These include findings from a multicenter prospective cohort study of more than 3000 patients. In that study, 21% of patients with chronic HBV had no known risk factors for the infection. In another large prospective observational cohort study, led by Dr. Hwang, which included more than 2100 patients with cancer, 90% had one or more significant risk factors for HBV infection, making selective screening “inefficient and impractical,” she said.
“The results of these two studies suggest that a universal screening approach, its potential harms (e.g., patient and clinician anxiety about management, financial burden associated with antiviral therapy) notwithstanding, is the most efficient, clinically pragmatic approach to HBV screening in persons anticipating systemic anticancer treatment,” the authors comment.
The screening recommended in the PCO requires three tests: hepatitis B surface antigen (HBsAg), core antibody total immunoglobulin or IgG, and antibody to HBsAg tests.
Anticancer therapy should not be delayed pending the results, they write.
Planning for monitoring and long-term prophylaxis for chronic HBV infection should involve a clinician experienced in HBV management, the authors write. Management of those with past infection should be individualized. Alternatively, patients with past infection can be carefully monitored rather than given prophylactic treatment, as long as frequent and consistent follow-up is possible to allow for rapid initiation of antiviral therapy in the event of reactivation, they say.
Hormonal therapy without systemic anticancer therapy is not likely to lead to HBV reactivation in patients with chronic or past infection; antiviral therapy and management of these patients should follow relevant national HBV guidelines, they note.
Challenges in implementing universal HBV screening
The expert panel acknowledges the challenges associated with implementation of universal HBV screening as recommended in their report and notes that electronic health record–based approaches that use alerts to prompt screening have demonstrated success. In one study of high-risk primary care patients, an EHR alert system significantly increased testing rates (odds ratio, 2.64 in comparison with a control group without alerts), and another study that used a simple “sticky-note” alert system to promote referral of HBsAg patients to hepatologists increased referrals from 28% to 73%.
In a cancer population, a “comprehensive set of multimodal interventions,” including pharmacy staff checks for screening prior to anti-CD20 therapy administration and electronic medication order reviews to assess for appropriate testing and treatment before anti-CD20 therapy, increased testing rates to greater than 90% and antiviral prophylaxis rates to more than 80%.
A study of 965 patients in Taiwan showed that a computer-assisted reminder system that prompted for testing prior to ordering anticancer therapy increased screening from 8% to 86% but was less effective for improving the rates of antiviral prophylaxis for those who tested positive for HBV, particularly among physicians treating patients with nonhematologic malignancies.
“Future studies will be needed to make universal HBV screening and linkage to care efficient and systematic, likely based in EHR systems,” the panel says. The authors note that “[o]ngoing studies of HBV tests such as ultrasensitive HBsAg, HBV RNA, and hepatitis B core antigen are being studied and may be useful in predicting risk of HBV reactivation.”
The panel also identified a research gap related to HBV reactivation risks “for the growing list of agents that deplete or modulate B cells.” It notes a need for additional research on the cost-effectiveness of HBV screening. The results of prior cost analyses have been inconsistent and vary with respect to the population studied. For example, universal screening and antiviral prophylaxis approaches have been shown to be cost-effective for patients with hematologic malignancies and high HBV reactivation risk but are less so for patients with solid tumors and lower reactivation risk, they explain.
Dr. Hwang said that not one of the more than 2100 patients in her HBV screening cohort study encountered problems with receiving insurance payment for their HBV screening.
“That’s a really strong statement that insurance payers are accepting of this kind of preventative service,” she said.
Expert panel cochair Andrew Artz, MD, commented that there is now greater acceptance of the need for HBV screening across medical specialties.
“There’s growing consensus among hepatologists, infectious disease specialists, oncologists, and HBV specialists that we need to do a better job of finding patients with hepatitis B [who are] about to receive immunocompromising treatment,” Dr. Artz said in an interview.
Dr. Artz is director of the Program for Aging and Blood Cancers and deputy director of the Center for Cancer and Aging at City of Hope Comprehensive Cancer Center, Duarte, California.
He suggested that the growing acceptance is due in part to the increasing number of anticancer therapies available and the resulting increase in the likelihood of patients receiving therapies that could cause reactivation.
More therapies – and more lines of therapy – could mean greater risk, he explained. He said that testing is easy and that universal screening is the simplest approach to determining who needs it. “There’s no question we will have to change practice,” Dr. Artz said in an interview. “But this is easier than the previous approach that essentially wasn’t being followed because it was too difficult to follow and patients were being missed.”
Most clinicians will appreciate having an approach that’s easier to follow, Dr. Artz predicted.
If there’s a challenge it will be in developing partnerships with HBV specialists, particularly in rural areas. In areas where there is a paucity of subspecialists, oncologists will have to “take some ownership of the issue,” as they often do in such settings, he said.
However, with support from pharmacists, administrators, and others in embracing this guidance, implementation can take place at a systems level rather than an individual clinician level, he added.
The recommendations in this updated PCO were all rated as “strong,” with the exception of the recommendation on hormonal therapy in the absence of systemic anticancer therapy, which was rated as “moderate.” All were based on “informal consensus,” with the exception of the key recommendation for universal HBV screening – use of three specific tests – which was “evidence based.”
The expert panel agreed that the benefits outweigh the harms for each recommendation in the update.
Dr. Hwang received research funding to her institution from Gilead Sciences and Merck Sharp & Dohme. She also has a relationship with the Asian Health Foundation. Dr. Artz received research funding from Miltenyi Biotec. All expert panel members’ disclosures are available in the PCO update.
This article first appeared on Medscape.com.
All patients with cancer who are candidates for systemic anticancer therapy should be screened for hepatitis B virus (HBV) infection prior to or at the start of therapy, according to an updated provisional clinical opinion (PCO) from the American Society of Clinical Oncology.
“This is a new approach [that] will actively take system changes ... but it will ultimately be safer for patients – and that is crucial,” commented Jessica P. Hwang, MD, MPH, cochair of the American Society of Clinical Oncology HBV Screening Expert Panel and the first author of the PCO.
Uptake of this universal screening approach would streamline testing protocols and identify more patients at risk for HBV reactivation who should receive prophylactic antiviral therapy, Dr. Hwang said in an interview.
The PCO calls for antiviral prophylaxis during and for at least 12 months after therapy for those with chronic HBV infection who are receiving any systemic anticancer treatment and for those with have had HBV in the past and are receiving any therapies that pose a risk for HBV reactivation.
“Hepatitis B reactivation can cause really terrible outcomes, like organ failure and even death,” Dr. Hwang, who is also a professor at the University of Texas MD Anderson Cancer Center, Houston, commented in an interview.
“This whole [issue of] reactivation and adverse outcomes with anticancer therapies is completely preventable with good planning, good communication, comanagement with specialists, and antiviral therapy and monitoring,” she added.
The updated opinion was published online July 27 in the Journal of Clinical Oncology.
It was developed in response to new data that call into question the previously recommended risk-adaptive approach to HBV screening of cancer patients, say the authors.
ASCO PCOs are developed “to provide timely clinical guidance” on the basis of emerging practice-changing information. This is the second update to follow the initial HBV screening PCO, published in 2010. In the absence of clear consensus because of limited data, the original PCO called for a risk-based approach to screening. A 2015 update extended the recommendation for screening to patients starting anti-CD20 therapy or who are to undergo stem cell transplant and to those with risk factors for HBV exposure.
The current update provides “a clinically pragmatic approach to HBV screening and management” that is based on the latest findings, say the authors. These include findings from a multicenter prospective cohort study of more than 3000 patients. In that study, 21% of patients with chronic HBV had no known risk factors for the infection. In another large prospective observational cohort study, led by Dr. Hwang, which included more than 2100 patients with cancer, 90% had one or more significant risk factors for HBV infection, making selective screening “inefficient and impractical,” she said.
“The results of these two studies suggest that a universal screening approach, its potential harms (e.g., patient and clinician anxiety about management, financial burden associated with antiviral therapy) notwithstanding, is the most efficient, clinically pragmatic approach to HBV screening in persons anticipating systemic anticancer treatment,” the authors comment.
The screening recommended in the PCO requires three tests: hepatitis B surface antigen (HBsAg), core antibody total immunoglobulin or IgG, and antibody to HBsAg tests.
Anticancer therapy should not be delayed pending the results, they write.
Planning for monitoring and long-term prophylaxis for chronic HBV infection should involve a clinician experienced in HBV management, the authors write. Management of those with past infection should be individualized. Alternatively, patients with past infection can be carefully monitored rather than given prophylactic treatment, as long as frequent and consistent follow-up is possible to allow for rapid initiation of antiviral therapy in the event of reactivation, they say.
Hormonal therapy without systemic anticancer therapy is not likely to lead to HBV reactivation in patients with chronic or past infection; antiviral therapy and management of these patients should follow relevant national HBV guidelines, they note.
Challenges in implementing universal HBV screening
The expert panel acknowledges the challenges associated with implementation of universal HBV screening as recommended in their report and notes that electronic health record–based approaches that use alerts to prompt screening have demonstrated success. In one study of high-risk primary care patients, an EHR alert system significantly increased testing rates (odds ratio, 2.64 in comparison with a control group without alerts), and another study that used a simple “sticky-note” alert system to promote referral of HBsAg patients to hepatologists increased referrals from 28% to 73%.
In a cancer population, a “comprehensive set of multimodal interventions,” including pharmacy staff checks for screening prior to anti-CD20 therapy administration and electronic medication order reviews to assess for appropriate testing and treatment before anti-CD20 therapy, increased testing rates to greater than 90% and antiviral prophylaxis rates to more than 80%.
A study of 965 patients in Taiwan showed that a computer-assisted reminder system that prompted for testing prior to ordering anticancer therapy increased screening from 8% to 86% but was less effective for improving the rates of antiviral prophylaxis for those who tested positive for HBV, particularly among physicians treating patients with nonhematologic malignancies.
“Future studies will be needed to make universal HBV screening and linkage to care efficient and systematic, likely based in EHR systems,” the panel says. The authors note that “[o]ngoing studies of HBV tests such as ultrasensitive HBsAg, HBV RNA, and hepatitis B core antigen are being studied and may be useful in predicting risk of HBV reactivation.”
The panel also identified a research gap related to HBV reactivation risks “for the growing list of agents that deplete or modulate B cells.” It notes a need for additional research on the cost-effectiveness of HBV screening. The results of prior cost analyses have been inconsistent and vary with respect to the population studied. For example, universal screening and antiviral prophylaxis approaches have been shown to be cost-effective for patients with hematologic malignancies and high HBV reactivation risk but are less so for patients with solid tumors and lower reactivation risk, they explain.
Dr. Hwang said that not one of the more than 2100 patients in her HBV screening cohort study encountered problems with receiving insurance payment for their HBV screening.
“That’s a really strong statement that insurance payers are accepting of this kind of preventative service,” she said.
Expert panel cochair Andrew Artz, MD, commented that there is now greater acceptance of the need for HBV screening across medical specialties.
“There’s growing consensus among hepatologists, infectious disease specialists, oncologists, and HBV specialists that we need to do a better job of finding patients with hepatitis B [who are] about to receive immunocompromising treatment,” Dr. Artz said in an interview.
Dr. Artz is director of the Program for Aging and Blood Cancers and deputy director of the Center for Cancer and Aging at City of Hope Comprehensive Cancer Center, Duarte, California.
He suggested that the growing acceptance is due in part to the increasing number of anticancer therapies available and the resulting increase in the likelihood of patients receiving therapies that could cause reactivation.
More therapies – and more lines of therapy – could mean greater risk, he explained. He said that testing is easy and that universal screening is the simplest approach to determining who needs it. “There’s no question we will have to change practice,” Dr. Artz said in an interview. “But this is easier than the previous approach that essentially wasn’t being followed because it was too difficult to follow and patients were being missed.”
Most clinicians will appreciate having an approach that’s easier to follow, Dr. Artz predicted.
If there’s a challenge it will be in developing partnerships with HBV specialists, particularly in rural areas. In areas where there is a paucity of subspecialists, oncologists will have to “take some ownership of the issue,” as they often do in such settings, he said.
However, with support from pharmacists, administrators, and others in embracing this guidance, implementation can take place at a systems level rather than an individual clinician level, he added.
The recommendations in this updated PCO were all rated as “strong,” with the exception of the recommendation on hormonal therapy in the absence of systemic anticancer therapy, which was rated as “moderate.” All were based on “informal consensus,” with the exception of the key recommendation for universal HBV screening – use of three specific tests – which was “evidence based.”
The expert panel agreed that the benefits outweigh the harms for each recommendation in the update.
Dr. Hwang received research funding to her institution from Gilead Sciences and Merck Sharp & Dohme. She also has a relationship with the Asian Health Foundation. Dr. Artz received research funding from Miltenyi Biotec. All expert panel members’ disclosures are available in the PCO update.
This article first appeared on Medscape.com.
All patients with cancer who are candidates for systemic anticancer therapy should be screened for hepatitis B virus (HBV) infection prior to or at the start of therapy, according to an updated provisional clinical opinion (PCO) from the American Society of Clinical Oncology.
“This is a new approach [that] will actively take system changes ... but it will ultimately be safer for patients – and that is crucial,” commented Jessica P. Hwang, MD, MPH, cochair of the American Society of Clinical Oncology HBV Screening Expert Panel and the first author of the PCO.
Uptake of this universal screening approach would streamline testing protocols and identify more patients at risk for HBV reactivation who should receive prophylactic antiviral therapy, Dr. Hwang said in an interview.
The PCO calls for antiviral prophylaxis during and for at least 12 months after therapy for those with chronic HBV infection who are receiving any systemic anticancer treatment and for those with have had HBV in the past and are receiving any therapies that pose a risk for HBV reactivation.
“Hepatitis B reactivation can cause really terrible outcomes, like organ failure and even death,” Dr. Hwang, who is also a professor at the University of Texas MD Anderson Cancer Center, Houston, commented in an interview.
“This whole [issue of] reactivation and adverse outcomes with anticancer therapies is completely preventable with good planning, good communication, comanagement with specialists, and antiviral therapy and monitoring,” she added.
The updated opinion was published online July 27 in the Journal of Clinical Oncology.
It was developed in response to new data that call into question the previously recommended risk-adaptive approach to HBV screening of cancer patients, say the authors.
ASCO PCOs are developed “to provide timely clinical guidance” on the basis of emerging practice-changing information. This is the second update to follow the initial HBV screening PCO, published in 2010. In the absence of clear consensus because of limited data, the original PCO called for a risk-based approach to screening. A 2015 update extended the recommendation for screening to patients starting anti-CD20 therapy or who are to undergo stem cell transplant and to those with risk factors for HBV exposure.
The current update provides “a clinically pragmatic approach to HBV screening and management” that is based on the latest findings, say the authors. These include findings from a multicenter prospective cohort study of more than 3000 patients. In that study, 21% of patients with chronic HBV had no known risk factors for the infection. In another large prospective observational cohort study, led by Dr. Hwang, which included more than 2100 patients with cancer, 90% had one or more significant risk factors for HBV infection, making selective screening “inefficient and impractical,” she said.
“The results of these two studies suggest that a universal screening approach, its potential harms (e.g., patient and clinician anxiety about management, financial burden associated with antiviral therapy) notwithstanding, is the most efficient, clinically pragmatic approach to HBV screening in persons anticipating systemic anticancer treatment,” the authors comment.
The screening recommended in the PCO requires three tests: hepatitis B surface antigen (HBsAg), core antibody total immunoglobulin or IgG, and antibody to HBsAg tests.
Anticancer therapy should not be delayed pending the results, they write.
Planning for monitoring and long-term prophylaxis for chronic HBV infection should involve a clinician experienced in HBV management, the authors write. Management of those with past infection should be individualized. Alternatively, patients with past infection can be carefully monitored rather than given prophylactic treatment, as long as frequent and consistent follow-up is possible to allow for rapid initiation of antiviral therapy in the event of reactivation, they say.
Hormonal therapy without systemic anticancer therapy is not likely to lead to HBV reactivation in patients with chronic or past infection; antiviral therapy and management of these patients should follow relevant national HBV guidelines, they note.
Challenges in implementing universal HBV screening
The expert panel acknowledges the challenges associated with implementation of universal HBV screening as recommended in their report and notes that electronic health record–based approaches that use alerts to prompt screening have demonstrated success. In one study of high-risk primary care patients, an EHR alert system significantly increased testing rates (odds ratio, 2.64 in comparison with a control group without alerts), and another study that used a simple “sticky-note” alert system to promote referral of HBsAg patients to hepatologists increased referrals from 28% to 73%.
In a cancer population, a “comprehensive set of multimodal interventions,” including pharmacy staff checks for screening prior to anti-CD20 therapy administration and electronic medication order reviews to assess for appropriate testing and treatment before anti-CD20 therapy, increased testing rates to greater than 90% and antiviral prophylaxis rates to more than 80%.
A study of 965 patients in Taiwan showed that a computer-assisted reminder system that prompted for testing prior to ordering anticancer therapy increased screening from 8% to 86% but was less effective for improving the rates of antiviral prophylaxis for those who tested positive for HBV, particularly among physicians treating patients with nonhematologic malignancies.
“Future studies will be needed to make universal HBV screening and linkage to care efficient and systematic, likely based in EHR systems,” the panel says. The authors note that “[o]ngoing studies of HBV tests such as ultrasensitive HBsAg, HBV RNA, and hepatitis B core antigen are being studied and may be useful in predicting risk of HBV reactivation.”
The panel also identified a research gap related to HBV reactivation risks “for the growing list of agents that deplete or modulate B cells.” It notes a need for additional research on the cost-effectiveness of HBV screening. The results of prior cost analyses have been inconsistent and vary with respect to the population studied. For example, universal screening and antiviral prophylaxis approaches have been shown to be cost-effective for patients with hematologic malignancies and high HBV reactivation risk but are less so for patients with solid tumors and lower reactivation risk, they explain.
Dr. Hwang said that not one of the more than 2100 patients in her HBV screening cohort study encountered problems with receiving insurance payment for their HBV screening.
“That’s a really strong statement that insurance payers are accepting of this kind of preventative service,” she said.
Expert panel cochair Andrew Artz, MD, commented that there is now greater acceptance of the need for HBV screening across medical specialties.
“There’s growing consensus among hepatologists, infectious disease specialists, oncologists, and HBV specialists that we need to do a better job of finding patients with hepatitis B [who are] about to receive immunocompromising treatment,” Dr. Artz said in an interview.
Dr. Artz is director of the Program for Aging and Blood Cancers and deputy director of the Center for Cancer and Aging at City of Hope Comprehensive Cancer Center, Duarte, California.
He suggested that the growing acceptance is due in part to the increasing number of anticancer therapies available and the resulting increase in the likelihood of patients receiving therapies that could cause reactivation.
More therapies – and more lines of therapy – could mean greater risk, he explained. He said that testing is easy and that universal screening is the simplest approach to determining who needs it. “There’s no question we will have to change practice,” Dr. Artz said in an interview. “But this is easier than the previous approach that essentially wasn’t being followed because it was too difficult to follow and patients were being missed.”
Most clinicians will appreciate having an approach that’s easier to follow, Dr. Artz predicted.
If there’s a challenge it will be in developing partnerships with HBV specialists, particularly in rural areas. In areas where there is a paucity of subspecialists, oncologists will have to “take some ownership of the issue,” as they often do in such settings, he said.
However, with support from pharmacists, administrators, and others in embracing this guidance, implementation can take place at a systems level rather than an individual clinician level, he added.
The recommendations in this updated PCO were all rated as “strong,” with the exception of the recommendation on hormonal therapy in the absence of systemic anticancer therapy, which was rated as “moderate.” All were based on “informal consensus,” with the exception of the key recommendation for universal HBV screening – use of three specific tests – which was “evidence based.”
The expert panel agreed that the benefits outweigh the harms for each recommendation in the update.
Dr. Hwang received research funding to her institution from Gilead Sciences and Merck Sharp & Dohme. She also has a relationship with the Asian Health Foundation. Dr. Artz received research funding from Miltenyi Biotec. All expert panel members’ disclosures are available in the PCO update.
This article first appeared on Medscape.com.
New CDC guidance for health care personnel exposed to HCV
The new guidance was developed in part as a result of an increase in the incidence of acute HCV infection in the United States, which increases the risk for occupational exposure among HCP. “[I]n certain health care settings, HCP might be exposed to source patients with early HCV infection before those patients develop serologic evidence of infection or symptoms indicative of viral hepatitis,” wrote the authors of the report, published online July 24 in the CDC’s Morbidity and Mortality Weekly Report.
The guidelines, which no longer recommend waiting for spontaneous resolution upon initial diagnosis, include recommendations and algorithms for baseline and follow-up testing, appropriate test type, and recommendations for clinical management. The recommendations were developed on the basis of a current literature review, expert opinion from subject matter experts, and recent guidance from the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America.
Baseline testing ASAP
Baseline testing of the source patient and the HCP should be performed as soon as possible, preferably within 48 hours of exposure. The source patient should be tested for HCV RNA using a nucleic acid test. Alternatively, screening anti-HCV serology can be performed in patients at low risk for HCV and a nucleic acid test performed if serology is positive.
Baseline testing for the HCP should include anti-HCV testing and, if positive, HCV RNA testing is recommended. HCPs who test positive for HCV RNA at baseline are considered to have a preexisting HCV infection and should be referred for treatment.
Follow-up testing
For HCPs with exposure to blood or body fluids from a patient who is anti-HCV positive but HCV RNA negative, follow-up testing is not required.
If the source patient is HCV RNA positive, or if status of the source patient is unknown, the authors recommend that exposed HCPs have HCV RNA follow-up testing at 3-6 weeks post exposure, in addition to baseline testing. A final anti-HCV test is recommended at 4-6 months post exposure as there can be potential periods of aviremia during acute HCV infection.
Exposed HCPs who develop signs of illness indicative of HCV infection at any time should be tested for HCV RNA.
HCPs with positive HCV RNA test results should be referred for care and curative antiviral therapy.
Postexposure prophylaxis is not recommended
Recent data have shown that the risk for HCV infection from percutaneous exposure is 0.2% and from mucocutaneous exposure is 0%. On the basis of this information, the CDC guidelines no longer recommend routine postexposure prophylaxis for HCPs with occupational exposure to HCV. Rather, curative antiviral regimens should be reserved for instances of documented HCV transmission.
The authors disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The new guidance was developed in part as a result of an increase in the incidence of acute HCV infection in the United States, which increases the risk for occupational exposure among HCP. “[I]n certain health care settings, HCP might be exposed to source patients with early HCV infection before those patients develop serologic evidence of infection or symptoms indicative of viral hepatitis,” wrote the authors of the report, published online July 24 in the CDC’s Morbidity and Mortality Weekly Report.
The guidelines, which no longer recommend waiting for spontaneous resolution upon initial diagnosis, include recommendations and algorithms for baseline and follow-up testing, appropriate test type, and recommendations for clinical management. The recommendations were developed on the basis of a current literature review, expert opinion from subject matter experts, and recent guidance from the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America.
Baseline testing ASAP
Baseline testing of the source patient and the HCP should be performed as soon as possible, preferably within 48 hours of exposure. The source patient should be tested for HCV RNA using a nucleic acid test. Alternatively, screening anti-HCV serology can be performed in patients at low risk for HCV and a nucleic acid test performed if serology is positive.
Baseline testing for the HCP should include anti-HCV testing and, if positive, HCV RNA testing is recommended. HCPs who test positive for HCV RNA at baseline are considered to have a preexisting HCV infection and should be referred for treatment.
Follow-up testing
For HCPs with exposure to blood or body fluids from a patient who is anti-HCV positive but HCV RNA negative, follow-up testing is not required.
If the source patient is HCV RNA positive, or if status of the source patient is unknown, the authors recommend that exposed HCPs have HCV RNA follow-up testing at 3-6 weeks post exposure, in addition to baseline testing. A final anti-HCV test is recommended at 4-6 months post exposure as there can be potential periods of aviremia during acute HCV infection.
Exposed HCPs who develop signs of illness indicative of HCV infection at any time should be tested for HCV RNA.
HCPs with positive HCV RNA test results should be referred for care and curative antiviral therapy.
Postexposure prophylaxis is not recommended
Recent data have shown that the risk for HCV infection from percutaneous exposure is 0.2% and from mucocutaneous exposure is 0%. On the basis of this information, the CDC guidelines no longer recommend routine postexposure prophylaxis for HCPs with occupational exposure to HCV. Rather, curative antiviral regimens should be reserved for instances of documented HCV transmission.
The authors disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The new guidance was developed in part as a result of an increase in the incidence of acute HCV infection in the United States, which increases the risk for occupational exposure among HCP. “[I]n certain health care settings, HCP might be exposed to source patients with early HCV infection before those patients develop serologic evidence of infection or symptoms indicative of viral hepatitis,” wrote the authors of the report, published online July 24 in the CDC’s Morbidity and Mortality Weekly Report.
The guidelines, which no longer recommend waiting for spontaneous resolution upon initial diagnosis, include recommendations and algorithms for baseline and follow-up testing, appropriate test type, and recommendations for clinical management. The recommendations were developed on the basis of a current literature review, expert opinion from subject matter experts, and recent guidance from the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America.
Baseline testing ASAP
Baseline testing of the source patient and the HCP should be performed as soon as possible, preferably within 48 hours of exposure. The source patient should be tested for HCV RNA using a nucleic acid test. Alternatively, screening anti-HCV serology can be performed in patients at low risk for HCV and a nucleic acid test performed if serology is positive.
Baseline testing for the HCP should include anti-HCV testing and, if positive, HCV RNA testing is recommended. HCPs who test positive for HCV RNA at baseline are considered to have a preexisting HCV infection and should be referred for treatment.
Follow-up testing
For HCPs with exposure to blood or body fluids from a patient who is anti-HCV positive but HCV RNA negative, follow-up testing is not required.
If the source patient is HCV RNA positive, or if status of the source patient is unknown, the authors recommend that exposed HCPs have HCV RNA follow-up testing at 3-6 weeks post exposure, in addition to baseline testing. A final anti-HCV test is recommended at 4-6 months post exposure as there can be potential periods of aviremia during acute HCV infection.
Exposed HCPs who develop signs of illness indicative of HCV infection at any time should be tested for HCV RNA.
HCPs with positive HCV RNA test results should be referred for care and curative antiviral therapy.
Postexposure prophylaxis is not recommended
Recent data have shown that the risk for HCV infection from percutaneous exposure is 0.2% and from mucocutaneous exposure is 0%. On the basis of this information, the CDC guidelines no longer recommend routine postexposure prophylaxis for HCPs with occupational exposure to HCV. Rather, curative antiviral regimens should be reserved for instances of documented HCV transmission.
The authors disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Hep C sofosbuvir/daclatasvir combo promising for COVID-19
research from an open-label Iranian study shows.
And the good news is that the treatment combination “already has a well-established safety profile in the treatment of hepatitis C,” said investigator Andrew Hill, PhD, from the University of Liverpool, United Kingdom.
But although the results look promising, they are preliminary, he cautioned. The combination could follow the path of ritonavir plus lopinavir (Kaletra, AbbVie Pharmaceuticals) or hydroxychloroquine (Plaquenil, Sanofi Pharmaceuticals), which showed promise early but did not perform as hoped in large randomized controlled trials.
“We need to remember that conducting research amidst a pandemic with overwhelmed hospitals is a clear challenge, and we cannot be sure of success,” he added.
Three Trials, 176 Patients
Data collected during a four-site trial of the combination treatment in Tehran during an early spike in cases in Iran were presented at the Virtual COVID-19 Conference 2020 by Hannah Wentzel, a masters student in public health at Imperial College London and a member of Hill’s team.
All 66 study participants were diagnosed with moderate to severe COVID-19 and were treated with standard care, which consisted of hydroxychloroquine 200 mg twice daily with or without the combination of lopinavir plus ritonavir 250 mg twice daily.
The 33 patients randomized to the treatment group also received the combination of sofosbuvir plus daclatasvir 460 mg once daily. These patients were slightly younger and more likely to be men than were those in the standard-care group, but the differences were not significant.
All participants were treated for 14 days, and then the researchers assessed fever, respiration rate, and blood oxygen saturation.
More patients in the treatment group than in the standard-care group had recovered at 14 days (88% vs 67%), but the difference was not significant.
However, median time to clinical recovery, which took into account death as a competing risk, was significantly faster in the treatment group than in the standard-care group (6 vs 11 days; P = .041).
The researchers then pooled their Tehran data with those from two other trials of the sofosbuvir plus daclatasvir combination conducted in Iran: one in the city of Sari with 48 patients and one in the city of Abadan with 62 patients.
A meta-analysis showed that clinical recovery in 14 days was 14% better in the treatment group than in the control group in the Sari study, 32% better in the Tehran study, and 82% better in the Abadan study. However, in a sensitivity analysis, because “the trial in Abadan was not properly randomized,” only the improvements in the Sari and Tehran studies were significant, Wentzel reported.
The meta-analysis also showed that patients in the treatment groups were 70% more likely than those in the standard-care groups to survive.
However, the treatment regimens in the standard-care groups of the three studies were all different, reflecting evolving national treatment guidelines in Iran at the time. And SARS-CoV-2 viral loads were not measured in any of the trials, so the effects of the different drugs on the virus itself could not be assessed.
Still, overall, “sofosbuvir and daclatasvir is associated with faster discharge from hospital and improved survival,” Wentzel said.
These findings are hopeful, “provocative, and encouraging,” said Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, and he echoed Hill’s call to “get these kinds of studies into randomized controlled trials.”
But he cautioned that more data are needed before the sofosbuvir and daclatasvir combination can be added to the National Institutes of Health COVID-19 Treatment Guidelines, which clinicians who might be under-resourced and overwhelmed with spikes in COVID-19 cases rely on.
Results from three double-blind randomized controlled trials – one each in Iran, Egypt, and South Africa – with an estimated cumulative enrollment of about 2,000 patients, are expected in October, Hill reported.
“Having gone through feeling so desperate to help people and try new things, it’s really important to do these trials,” said Kristen Marks, MD, from Weill Cornell Medicine in New York City.
“You get tempted to just kind of throw anything at people. And I think we really have to have science to guide us,” she told Medscape Medical News.
This article first appeared on Medscape.com.
research from an open-label Iranian study shows.
And the good news is that the treatment combination “already has a well-established safety profile in the treatment of hepatitis C,” said investigator Andrew Hill, PhD, from the University of Liverpool, United Kingdom.
But although the results look promising, they are preliminary, he cautioned. The combination could follow the path of ritonavir plus lopinavir (Kaletra, AbbVie Pharmaceuticals) or hydroxychloroquine (Plaquenil, Sanofi Pharmaceuticals), which showed promise early but did not perform as hoped in large randomized controlled trials.
“We need to remember that conducting research amidst a pandemic with overwhelmed hospitals is a clear challenge, and we cannot be sure of success,” he added.
Three Trials, 176 Patients
Data collected during a four-site trial of the combination treatment in Tehran during an early spike in cases in Iran were presented at the Virtual COVID-19 Conference 2020 by Hannah Wentzel, a masters student in public health at Imperial College London and a member of Hill’s team.
All 66 study participants were diagnosed with moderate to severe COVID-19 and were treated with standard care, which consisted of hydroxychloroquine 200 mg twice daily with or without the combination of lopinavir plus ritonavir 250 mg twice daily.
The 33 patients randomized to the treatment group also received the combination of sofosbuvir plus daclatasvir 460 mg once daily. These patients were slightly younger and more likely to be men than were those in the standard-care group, but the differences were not significant.
All participants were treated for 14 days, and then the researchers assessed fever, respiration rate, and blood oxygen saturation.
More patients in the treatment group than in the standard-care group had recovered at 14 days (88% vs 67%), but the difference was not significant.
However, median time to clinical recovery, which took into account death as a competing risk, was significantly faster in the treatment group than in the standard-care group (6 vs 11 days; P = .041).
The researchers then pooled their Tehran data with those from two other trials of the sofosbuvir plus daclatasvir combination conducted in Iran: one in the city of Sari with 48 patients and one in the city of Abadan with 62 patients.
A meta-analysis showed that clinical recovery in 14 days was 14% better in the treatment group than in the control group in the Sari study, 32% better in the Tehran study, and 82% better in the Abadan study. However, in a sensitivity analysis, because “the trial in Abadan was not properly randomized,” only the improvements in the Sari and Tehran studies were significant, Wentzel reported.
The meta-analysis also showed that patients in the treatment groups were 70% more likely than those in the standard-care groups to survive.
However, the treatment regimens in the standard-care groups of the three studies were all different, reflecting evolving national treatment guidelines in Iran at the time. And SARS-CoV-2 viral loads were not measured in any of the trials, so the effects of the different drugs on the virus itself could not be assessed.
Still, overall, “sofosbuvir and daclatasvir is associated with faster discharge from hospital and improved survival,” Wentzel said.
These findings are hopeful, “provocative, and encouraging,” said Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, and he echoed Hill’s call to “get these kinds of studies into randomized controlled trials.”
But he cautioned that more data are needed before the sofosbuvir and daclatasvir combination can be added to the National Institutes of Health COVID-19 Treatment Guidelines, which clinicians who might be under-resourced and overwhelmed with spikes in COVID-19 cases rely on.
Results from three double-blind randomized controlled trials – one each in Iran, Egypt, and South Africa – with an estimated cumulative enrollment of about 2,000 patients, are expected in October, Hill reported.
“Having gone through feeling so desperate to help people and try new things, it’s really important to do these trials,” said Kristen Marks, MD, from Weill Cornell Medicine in New York City.
“You get tempted to just kind of throw anything at people. And I think we really have to have science to guide us,” she told Medscape Medical News.
This article first appeared on Medscape.com.
research from an open-label Iranian study shows.
And the good news is that the treatment combination “already has a well-established safety profile in the treatment of hepatitis C,” said investigator Andrew Hill, PhD, from the University of Liverpool, United Kingdom.
But although the results look promising, they are preliminary, he cautioned. The combination could follow the path of ritonavir plus lopinavir (Kaletra, AbbVie Pharmaceuticals) or hydroxychloroquine (Plaquenil, Sanofi Pharmaceuticals), which showed promise early but did not perform as hoped in large randomized controlled trials.
“We need to remember that conducting research amidst a pandemic with overwhelmed hospitals is a clear challenge, and we cannot be sure of success,” he added.
Three Trials, 176 Patients
Data collected during a four-site trial of the combination treatment in Tehran during an early spike in cases in Iran were presented at the Virtual COVID-19 Conference 2020 by Hannah Wentzel, a masters student in public health at Imperial College London and a member of Hill’s team.
All 66 study participants were diagnosed with moderate to severe COVID-19 and were treated with standard care, which consisted of hydroxychloroquine 200 mg twice daily with or without the combination of lopinavir plus ritonavir 250 mg twice daily.
The 33 patients randomized to the treatment group also received the combination of sofosbuvir plus daclatasvir 460 mg once daily. These patients were slightly younger and more likely to be men than were those in the standard-care group, but the differences were not significant.
All participants were treated for 14 days, and then the researchers assessed fever, respiration rate, and blood oxygen saturation.
More patients in the treatment group than in the standard-care group had recovered at 14 days (88% vs 67%), but the difference was not significant.
However, median time to clinical recovery, which took into account death as a competing risk, was significantly faster in the treatment group than in the standard-care group (6 vs 11 days; P = .041).
The researchers then pooled their Tehran data with those from two other trials of the sofosbuvir plus daclatasvir combination conducted in Iran: one in the city of Sari with 48 patients and one in the city of Abadan with 62 patients.
A meta-analysis showed that clinical recovery in 14 days was 14% better in the treatment group than in the control group in the Sari study, 32% better in the Tehran study, and 82% better in the Abadan study. However, in a sensitivity analysis, because “the trial in Abadan was not properly randomized,” only the improvements in the Sari and Tehran studies were significant, Wentzel reported.
The meta-analysis also showed that patients in the treatment groups were 70% more likely than those in the standard-care groups to survive.
However, the treatment regimens in the standard-care groups of the three studies were all different, reflecting evolving national treatment guidelines in Iran at the time. And SARS-CoV-2 viral loads were not measured in any of the trials, so the effects of the different drugs on the virus itself could not be assessed.
Still, overall, “sofosbuvir and daclatasvir is associated with faster discharge from hospital and improved survival,” Wentzel said.
These findings are hopeful, “provocative, and encouraging,” said Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, and he echoed Hill’s call to “get these kinds of studies into randomized controlled trials.”
But he cautioned that more data are needed before the sofosbuvir and daclatasvir combination can be added to the National Institutes of Health COVID-19 Treatment Guidelines, which clinicians who might be under-resourced and overwhelmed with spikes in COVID-19 cases rely on.
Results from three double-blind randomized controlled trials – one each in Iran, Egypt, and South Africa – with an estimated cumulative enrollment of about 2,000 patients, are expected in October, Hill reported.
“Having gone through feeling so desperate to help people and try new things, it’s really important to do these trials,” said Kristen Marks, MD, from Weill Cornell Medicine in New York City.
“You get tempted to just kind of throw anything at people. And I think we really have to have science to guide us,” she told Medscape Medical News.
This article first appeared on Medscape.com.
Retreatment of Hepatitis C Infection With Direct-Acting Antivirals
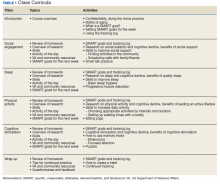
An estimated 3.5 million people in the US have chronic hepatitis C virus (HCV) infection, and between 10% and 20% of those developed cirrhosis over 20 to 30 years.1 There are at least 6 genotypes (GTs) of HCV, with GT1 being the most common in the US and previously one of the most difficult to treat.2,3 The goal of treatment is to achieve viral cure, called sustained virologic response (SVR) when HCV viral load remains undetectable several weeks after therapy completion. In the 2000s, pegylated interferon (pegIFN) and ribavirin (RBV) were the standard of care.2 For patients with GT1 infections, an SVR of 40 to 50% was commonly seen after 48 weeks of pegIFN/RBV regimens compared with 70 to 80% SVR for GT2 or GT3 after 24 weeks of pegIFN/RBV therapy.2 However, treatment has evolved rapidly (Table 1).2-17
In 2011, the US Food and Drug Administration (FDA) approved the protease inhibitors (PIs) boceprevir and telaprevir, which added a new class of agents with increased SVR for patients with GT1 infection; however, pegIFN and RBV were still needed for treatment.4 In addition, both PIs required multiple doses per day and strict adherence to an 8-hour schedule.4 Boceprevir required treatment with RBV and pegIFN for 48 weeks unless futility rule was met at 24 weeks of treatment (ie, viral load still detectable).4 The SVR in patients with GT1 infection improved to > 65% for patients in clinical trials.2 FDA approval of the direct-acting antivirals (DAAs) sofosbuvir and simeprevir in late 2013 decreased the usual duration of therapy to only 12 weeks with improved SVR rates 12 weeks posttherapy (SVR12) to 90% or higher.2,6,10
FDA approval of ledipasvir (LDV)/sofosbuvir (SOF) in October 2014 resulted in the first interferon-free all-oral regimen indicated for HCV GT1 infection.11 In December 2014, FDA approved a combination of paritaprevir, ritonavir, ombitasvir, and dasabuvir (PrOD).12 In 2015 GT-specific approvals were issued for daclastavir to be used with SOV for GT1 and GT3 and a combination similar to PrOD without dasabuvir (PrO) for GT4.13 In 2016, a combination of elbasvir (ERB) and grazoprevir (GZP) was approved for GT1 and GT4.14
In 2016, a pangenotypic DAA of SOF and velpatasvir (VEL) was approved.15 Most recently, combinations of SOF, VEL, and voxilaprevir (VOX), and glecaprevir (GLE) and pibrentasvir (PIB) were approved for patients with previous DAA treatment failures.7, 8,16,17 These oral regimens avoided the significant adverse events (AEs) associated with pegIFN and RBV (eg, thrombocytopenia, depression), were expected to improve treatment adherence and shorten duration of therapy.
The West Palm Beach Veterans Affairs has had a nurse practitioner (NP)-based HCV treatment clinic since the late 1990s. When PIs became available, a CPS started reviewing patient electronic health records (EHRs) and monitored response to therapy along with the NP to ensure discontinuation of therapy if futility criteria were met.7 Our unpublished experience showed SVR > 60% with both boceprevir and SOF regimens and > 90% with oral DAA regimens.
This review will provide the SVR rates for patients that needed retreatment for HCV infection since 2015 until December 2019. We treated all willing patients, beginning with the patients who had experienced failures with previous regimens. Patients first received education on HCV infection and treatment options in a group class then they were seen by the NP individually for specific education on treatment. The CPS reviewed the patient’s medical record to assess for appropriate therapy, possible drug-drug interactions and contraindications to therapy. In addition, patient outcomes (eg, viral load, AEs) were documented by the CPS in collaboration with the NP throughout treatment until viral load for SVR evaluation was obtained.
Methods
A retrospective EHR review of patients retreated from January 2015 to December 2019 was conducted. Data collected included age, sex, HCV GT, previous therapy, new medications prescribed, creatinine clearance, and achievement of SVR12. This retrospective review was approved by the facility’s scientific advisory committee as part of performance improvement efforts. Descriptive statistics are provided.
Results
Boceprevir
We treated 31 patients with boceprevir of which 3 met futility rule and 28 completed therapy. Eighteen of 28 responded (64%) to the treatment. The 10 patients who failed treatment were retreated with LDV/SOF, and all achieved SVR.
Sofosbuvir
A total of 53 patients were treated with SOF, RBV, and pegIFN for 12 weeks. Forty-one achieved SVR (77%). Of the 12 who failed therapy, all have been retreated and achieved SVR (Table 2).
Interferon-Free DAA Oral Regimens
More than 900 patients have been treated with interferon-free regimens since 2015 and outcomes were documented for > 800 patients. The SVR rates by GT were as follows: GT1 639 of 676 (95%); GT2 76 of 79 (96%); GT3 40 of 48 (83%); and GT4 6 of 6 (100%). Eighty-four percent of patients had GT1 infection. The median age of patient was 62 years, 72% were treatment naïve, and 35% having cirrhosis (based on liver biopsy or FIB4 score).18
Of 48 treatment failures, 30 patients were retreated; the rest of the patients were lost to follow-up (n = 9) or unable to receive retreatment (n = 9) mainly due to decompensated cirrhosis or liver cancer and short life expectancy. The median age of patient in this retreatment group was 62 years, 62% had cirrhosis, and most were infected with GT1. The average creatinine clearance was 73 mL/min. Twenty-two patients who failed therapy with ledipasvir/SOF were retreated (Table 3). A total of 13 patients out of the 19 tested eventually achieved SVR (68%). Four of the patients who had treatment failure again had GT1 infection and the other 2 GT3. All had cirrhosis.
Thirty-five patients were treated with PrOD, and 32 achieved SVR (91%). All 3 patients were retreated. One patient each achieved SVR with ERB/GZP, SOF/VEL and SOF/VEL/VOX. Fifty patients were treated with ERB/GZP and 45 achieved SVR (90%). All 5 treatment failures were retreated. Four achieved SVR and 1 was lost to follow-up (Table 4). Overall, of 30 patients who were retreated after failure with an all-oral DAA regimen, 27 patients had SVR values available and 21 achieved it (78%).
Discussion
Overall SVR was very high for patients who received oral treatment for HCV infection. A low number of patients failed therapy and were retreated. Patients who failed therapy again were similar in age but were more likely to have cirrhosis when compared with the overall interferon-free treated group. Thus, prompt treatment after HCV detection and before disease progression may improve treatment outcomes. Achieving SVR has been shown to improve fibrosis, portal hypertension, splenomegaly and cirrhosis, and reduce the risk of hepatocellular carcinoma by 70% and liver-related mortality by 90%.19-21/
Patients who failed therapy primarily had GT1—the most prevalent GT treated. A higher prevalence of GT1 is expected since it is the most common GT in the US.6 However, disease progression occurs more rapidly in those with GT3 and is more difficult to treat.22 The overall response rate was lower with this GT (83%) in this report, with only 1 of 3 patients retreated achieving an SVR.
Similar results are documented in retreatment trials.23 In the POLARIS-1 trial, treatment with SOF/VEL/VOX resulted in an overall response rate of 96% but only 91% for patients with GT3, compared with 95 to 100% for GTs 1, 2, or 4.23 In the current report, only 1 patient (GT1) failed retreatment with SOF/VEL/VOX. At this time, there are no clear treatment options for this patient. However, patients who fail GLE/PIB (none so far in the current report) may be able to receive SOF/VEL/VOX.24 In a small study, 29 of 31 patients achieved SVR with SOF/VEL/VOX after GLE/PIB failure (12 of 13 GT1 and 17 of 18 GT3).24
Limitations
This review was an observational, nonrandomized design, and only 1 medical center was involved. These results may not be applicable to other patient populations without a clinic set up with routine follow-ups to encourage adherence and completion of therapy.
Conclusions
Treatment of HCV infection has improved significantly over the past 10 years. Use of DAAs results in SVR for > 90% of patients, especially if the disease had not progressed to cirrhosis. Failure after retreatment for HCV infection was rare as well. Given that cirrhosis seems to increase the chance of treatment failure, it is imperative to identify candidates for treatment before the infection has progressed to cirrhosis. Patients infected with GT3 in particular should be more aggressively identified and treated.
Acknowledgments
The authors thank Nick P. Becky, PharmD, for his contributions to the identification of patients needing treatment for their HCV infection and review of initial manuscript information.
1. Centers for Disease Control and Prevention. Viral hepatitis: hepatitis C information. https://www.cdc.gov/hepatitis/hcv/index.htm. Reviewed April 14, 2020. Accessed June 16, 2020.
2. American Association for the Study of Liver Disease, Infectious Diseases Society of America. HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C. https://www.hcvguidelines.org. Accessed June 16, 2020.
3. Lingala S, Ghany MG. Natural history of hepatitis C. Gastroenterol Clin N Am. 2015;44(4):717-734. doi:10.1016/j.gtc.2015.07.003
4. Foote BS, Spooner LM, Belliveau PP. Boceprevir: a protease inhibitor for the treatment of chronic hepatitis C. Ann Pharmacother. 2011;45(9):1085-1093. doi:10.1345/aph.1P744
5. Kayali Z, Schmidt WN. Finally sofosbuvir: an oral anti-HCV drug with wide performance capability. Pharmgenomics Pers Med. 2014:7:387-398. doi:10.2147/PGPM.S52629
6. Falade-Nwulis O, Suarez-Cuervo C, Nelson DR, Fried MW, Segal JB, Sulkowski MS. Oral direct-acting agent therapy for hepatitis C virus infection: a systematic review. Ann Intern Med. 2017;166(9):637-648. doi:10.7326/M16-2575
7. Carrion AF, Martin P. Glecaprevir + pibrentasvir for treatment of hepatitis C. Expert Opinion Pharmacother. 2018;19(4):413-419. doi:10.1080/14656566.2018.1444030
8. Chahine EB, Kelley D, Childs-Kean LM. Sofosbuvir/velpatasvir/voxilaprevir: a pan-genotypic direct-acting antiviral combination for hepatitis C. Ann Pharmacother. 2018;52(4):352-363. doi:10.1177/1060028017741508
9. Lagasca AM, Kan VL. Hepatitis C treatment at a Veteran Affairs medical center after the availability of direct-acting agents: things are looking up. Clin Infect Dis. 2015:61(8):1347-1349. doi:10.1093/cid/civ573
10. Sovaldi (sofosbuvir) [package insert]. Foster City, CA: Gilead Sciences Inc; 2017.
11. Harvoni (ledipasvir and sofosbuvir) [package insert]. Foster City, CA: Gilead Sciences Inc; 2017.
12. Viekira Pak (ombitasvir, paritaprevir and ritonavir; dasabuvir) [package insert]. North Chicago, IL: AbbVie Inc; 2018.
13. Technivie (ombitasvir, paritaprevir and ritonavir) [package insert]. North Chicago, IL: AbbVie Inc; 2018.
14. Zepatier (elbasvir and grazoprevir) [package insert]. Whitehouse Station, NJ: Merck & Co Inc; 2018.
15. Epclusa (sofosbuvir and velpatasvir) [package insert]. Foster City, CA: Gilead Sciences Inc; 2017.
16. Mavyret (glecaprevir and pibrentasvir) [package insert]. North Chicago, IL: AbbVie Inc; 2019.
17. Vosevi (sofosbuvir, velpatasvir and voxilaprevir) [package insert]. Foster City, CA: Gilead Sciences Inc; 2017.
18. Vallet-Pichard A, Mallet V, Nalpas V, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and FibroTest. Hepatology. 2017;46(1):32-36. doi:10.1002/hep.21669
19. Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158(5, pt 1):329-337. doi:10.7326/0003-4819-158-5-201303050-00005
20. van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308(24):2584-2593. doi:10.1001/jama.2012.144878
21. Veldt BJ, Heathcote EJ, Wedemeyer H, et al. Sustained virologic response and clinical outcomes in patients with chronic hepatitis C and advanced fibrosis. Ann Intern Med. 2007;147(10):677-684. doi:10.7326/0003-4819-147-10-200711200-00003
22. Chen A, Patel K, Naggie S. Genotype 3 infection: the last stand of hepatitis C virus. Drugs. 2017;77(2):131-144. doi:10.1007/s40265-016-0685-x
23. Bourlière M, Gordon SC, Flamm SL, et al; POLARIS-1 and POLARIS-4 Investigators. Sofosbuvir, velpatasvir and voxilaprevir for previously treated HCV infection. N Engl J Med. 2017;376(22):2136-2146. doi:10.1056/NEJMoa1613512
24. Pearlman B, Perrys M, Hinds A. Sofosbuvir/velpatasvir/voxilaprevir for previous treatment failures with glecaprevir/pibrentasvir in chronic hepatitis C infection. Am J Gastroenterol. 2019;114(9):1550-1552. doi:10.14309/ajg.0000000000000248
An estimated 3.5 million people in the US have chronic hepatitis C virus (HCV) infection, and between 10% and 20% of those developed cirrhosis over 20 to 30 years.1 There are at least 6 genotypes (GTs) of HCV, with GT1 being the most common in the US and previously one of the most difficult to treat.2,3 The goal of treatment is to achieve viral cure, called sustained virologic response (SVR) when HCV viral load remains undetectable several weeks after therapy completion. In the 2000s, pegylated interferon (pegIFN) and ribavirin (RBV) were the standard of care.2 For patients with GT1 infections, an SVR of 40 to 50% was commonly seen after 48 weeks of pegIFN/RBV regimens compared with 70 to 80% SVR for GT2 or GT3 after 24 weeks of pegIFN/RBV therapy.2 However, treatment has evolved rapidly (Table 1).2-17
In 2011, the US Food and Drug Administration (FDA) approved the protease inhibitors (PIs) boceprevir and telaprevir, which added a new class of agents with increased SVR for patients with GT1 infection; however, pegIFN and RBV were still needed for treatment.4 In addition, both PIs required multiple doses per day and strict adherence to an 8-hour schedule.4 Boceprevir required treatment with RBV and pegIFN for 48 weeks unless futility rule was met at 24 weeks of treatment (ie, viral load still detectable).4 The SVR in patients with GT1 infection improved to > 65% for patients in clinical trials.2 FDA approval of the direct-acting antivirals (DAAs) sofosbuvir and simeprevir in late 2013 decreased the usual duration of therapy to only 12 weeks with improved SVR rates 12 weeks posttherapy (SVR12) to 90% or higher.2,6,10
FDA approval of ledipasvir (LDV)/sofosbuvir (SOF) in October 2014 resulted in the first interferon-free all-oral regimen indicated for HCV GT1 infection.11 In December 2014, FDA approved a combination of paritaprevir, ritonavir, ombitasvir, and dasabuvir (PrOD).12 In 2015 GT-specific approvals were issued for daclastavir to be used with SOV for GT1 and GT3 and a combination similar to PrOD without dasabuvir (PrO) for GT4.13 In 2016, a combination of elbasvir (ERB) and grazoprevir (GZP) was approved for GT1 and GT4.14
In 2016, a pangenotypic DAA of SOF and velpatasvir (VEL) was approved.15 Most recently, combinations of SOF, VEL, and voxilaprevir (VOX), and glecaprevir (GLE) and pibrentasvir (PIB) were approved for patients with previous DAA treatment failures.7, 8,16,17 These oral regimens avoided the significant adverse events (AEs) associated with pegIFN and RBV (eg, thrombocytopenia, depression), were expected to improve treatment adherence and shorten duration of therapy.
The West Palm Beach Veterans Affairs has had a nurse practitioner (NP)-based HCV treatment clinic since the late 1990s. When PIs became available, a CPS started reviewing patient electronic health records (EHRs) and monitored response to therapy along with the NP to ensure discontinuation of therapy if futility criteria were met.7 Our unpublished experience showed SVR > 60% with both boceprevir and SOF regimens and > 90% with oral DAA regimens.
This review will provide the SVR rates for patients that needed retreatment for HCV infection since 2015 until December 2019. We treated all willing patients, beginning with the patients who had experienced failures with previous regimens. Patients first received education on HCV infection and treatment options in a group class then they were seen by the NP individually for specific education on treatment. The CPS reviewed the patient’s medical record to assess for appropriate therapy, possible drug-drug interactions and contraindications to therapy. In addition, patient outcomes (eg, viral load, AEs) were documented by the CPS in collaboration with the NP throughout treatment until viral load for SVR evaluation was obtained.
Methods
A retrospective EHR review of patients retreated from January 2015 to December 2019 was conducted. Data collected included age, sex, HCV GT, previous therapy, new medications prescribed, creatinine clearance, and achievement of SVR12. This retrospective review was approved by the facility’s scientific advisory committee as part of performance improvement efforts. Descriptive statistics are provided.
Results
Boceprevir
We treated 31 patients with boceprevir of which 3 met futility rule and 28 completed therapy. Eighteen of 28 responded (64%) to the treatment. The 10 patients who failed treatment were retreated with LDV/SOF, and all achieved SVR.
Sofosbuvir
A total of 53 patients were treated with SOF, RBV, and pegIFN for 12 weeks. Forty-one achieved SVR (77%). Of the 12 who failed therapy, all have been retreated and achieved SVR (Table 2).
Interferon-Free DAA Oral Regimens
More than 900 patients have been treated with interferon-free regimens since 2015 and outcomes were documented for > 800 patients. The SVR rates by GT were as follows: GT1 639 of 676 (95%); GT2 76 of 79 (96%); GT3 40 of 48 (83%); and GT4 6 of 6 (100%). Eighty-four percent of patients had GT1 infection. The median age of patient was 62 years, 72% were treatment naïve, and 35% having cirrhosis (based on liver biopsy or FIB4 score).18
Of 48 treatment failures, 30 patients were retreated; the rest of the patients were lost to follow-up (n = 9) or unable to receive retreatment (n = 9) mainly due to decompensated cirrhosis or liver cancer and short life expectancy. The median age of patient in this retreatment group was 62 years, 62% had cirrhosis, and most were infected with GT1. The average creatinine clearance was 73 mL/min. Twenty-two patients who failed therapy with ledipasvir/SOF were retreated (Table 3). A total of 13 patients out of the 19 tested eventually achieved SVR (68%). Four of the patients who had treatment failure again had GT1 infection and the other 2 GT3. All had cirrhosis.
Thirty-five patients were treated with PrOD, and 32 achieved SVR (91%). All 3 patients were retreated. One patient each achieved SVR with ERB/GZP, SOF/VEL and SOF/VEL/VOX. Fifty patients were treated with ERB/GZP and 45 achieved SVR (90%). All 5 treatment failures were retreated. Four achieved SVR and 1 was lost to follow-up (Table 4). Overall, of 30 patients who were retreated after failure with an all-oral DAA regimen, 27 patients had SVR values available and 21 achieved it (78%).
Discussion
Overall SVR was very high for patients who received oral treatment for HCV infection. A low number of patients failed therapy and were retreated. Patients who failed therapy again were similar in age but were more likely to have cirrhosis when compared with the overall interferon-free treated group. Thus, prompt treatment after HCV detection and before disease progression may improve treatment outcomes. Achieving SVR has been shown to improve fibrosis, portal hypertension, splenomegaly and cirrhosis, and reduce the risk of hepatocellular carcinoma by 70% and liver-related mortality by 90%.19-21/
Patients who failed therapy primarily had GT1—the most prevalent GT treated. A higher prevalence of GT1 is expected since it is the most common GT in the US.6 However, disease progression occurs more rapidly in those with GT3 and is more difficult to treat.22 The overall response rate was lower with this GT (83%) in this report, with only 1 of 3 patients retreated achieving an SVR.
Similar results are documented in retreatment trials.23 In the POLARIS-1 trial, treatment with SOF/VEL/VOX resulted in an overall response rate of 96% but only 91% for patients with GT3, compared with 95 to 100% for GTs 1, 2, or 4.23 In the current report, only 1 patient (GT1) failed retreatment with SOF/VEL/VOX. At this time, there are no clear treatment options for this patient. However, patients who fail GLE/PIB (none so far in the current report) may be able to receive SOF/VEL/VOX.24 In a small study, 29 of 31 patients achieved SVR with SOF/VEL/VOX after GLE/PIB failure (12 of 13 GT1 and 17 of 18 GT3).24
Limitations
This review was an observational, nonrandomized design, and only 1 medical center was involved. These results may not be applicable to other patient populations without a clinic set up with routine follow-ups to encourage adherence and completion of therapy.
Conclusions
Treatment of HCV infection has improved significantly over the past 10 years. Use of DAAs results in SVR for > 90% of patients, especially if the disease had not progressed to cirrhosis. Failure after retreatment for HCV infection was rare as well. Given that cirrhosis seems to increase the chance of treatment failure, it is imperative to identify candidates for treatment before the infection has progressed to cirrhosis. Patients infected with GT3 in particular should be more aggressively identified and treated.
Acknowledgments
The authors thank Nick P. Becky, PharmD, for his contributions to the identification of patients needing treatment for their HCV infection and review of initial manuscript information.
An estimated 3.5 million people in the US have chronic hepatitis C virus (HCV) infection, and between 10% and 20% of those developed cirrhosis over 20 to 30 years.1 There are at least 6 genotypes (GTs) of HCV, with GT1 being the most common in the US and previously one of the most difficult to treat.2,3 The goal of treatment is to achieve viral cure, called sustained virologic response (SVR) when HCV viral load remains undetectable several weeks after therapy completion. In the 2000s, pegylated interferon (pegIFN) and ribavirin (RBV) were the standard of care.2 For patients with GT1 infections, an SVR of 40 to 50% was commonly seen after 48 weeks of pegIFN/RBV regimens compared with 70 to 80% SVR for GT2 or GT3 after 24 weeks of pegIFN/RBV therapy.2 However, treatment has evolved rapidly (Table 1).2-17
In 2011, the US Food and Drug Administration (FDA) approved the protease inhibitors (PIs) boceprevir and telaprevir, which added a new class of agents with increased SVR for patients with GT1 infection; however, pegIFN and RBV were still needed for treatment.4 In addition, both PIs required multiple doses per day and strict adherence to an 8-hour schedule.4 Boceprevir required treatment with RBV and pegIFN for 48 weeks unless futility rule was met at 24 weeks of treatment (ie, viral load still detectable).4 The SVR in patients with GT1 infection improved to > 65% for patients in clinical trials.2 FDA approval of the direct-acting antivirals (DAAs) sofosbuvir and simeprevir in late 2013 decreased the usual duration of therapy to only 12 weeks with improved SVR rates 12 weeks posttherapy (SVR12) to 90% or higher.2,6,10
FDA approval of ledipasvir (LDV)/sofosbuvir (SOF) in October 2014 resulted in the first interferon-free all-oral regimen indicated for HCV GT1 infection.11 In December 2014, FDA approved a combination of paritaprevir, ritonavir, ombitasvir, and dasabuvir (PrOD).12 In 2015 GT-specific approvals were issued for daclastavir to be used with SOV for GT1 and GT3 and a combination similar to PrOD without dasabuvir (PrO) for GT4.13 In 2016, a combination of elbasvir (ERB) and grazoprevir (GZP) was approved for GT1 and GT4.14
In 2016, a pangenotypic DAA of SOF and velpatasvir (VEL) was approved.15 Most recently, combinations of SOF, VEL, and voxilaprevir (VOX), and glecaprevir (GLE) and pibrentasvir (PIB) were approved for patients with previous DAA treatment failures.7, 8,16,17 These oral regimens avoided the significant adverse events (AEs) associated with pegIFN and RBV (eg, thrombocytopenia, depression), were expected to improve treatment adherence and shorten duration of therapy.
The West Palm Beach Veterans Affairs has had a nurse practitioner (NP)-based HCV treatment clinic since the late 1990s. When PIs became available, a CPS started reviewing patient electronic health records (EHRs) and monitored response to therapy along with the NP to ensure discontinuation of therapy if futility criteria were met.7 Our unpublished experience showed SVR > 60% with both boceprevir and SOF regimens and > 90% with oral DAA regimens.
This review will provide the SVR rates for patients that needed retreatment for HCV infection since 2015 until December 2019. We treated all willing patients, beginning with the patients who had experienced failures with previous regimens. Patients first received education on HCV infection and treatment options in a group class then they were seen by the NP individually for specific education on treatment. The CPS reviewed the patient’s medical record to assess for appropriate therapy, possible drug-drug interactions and contraindications to therapy. In addition, patient outcomes (eg, viral load, AEs) were documented by the CPS in collaboration with the NP throughout treatment until viral load for SVR evaluation was obtained.
Methods
A retrospective EHR review of patients retreated from January 2015 to December 2019 was conducted. Data collected included age, sex, HCV GT, previous therapy, new medications prescribed, creatinine clearance, and achievement of SVR12. This retrospective review was approved by the facility’s scientific advisory committee as part of performance improvement efforts. Descriptive statistics are provided.
Results
Boceprevir
We treated 31 patients with boceprevir of which 3 met futility rule and 28 completed therapy. Eighteen of 28 responded (64%) to the treatment. The 10 patients who failed treatment were retreated with LDV/SOF, and all achieved SVR.
Sofosbuvir
A total of 53 patients were treated with SOF, RBV, and pegIFN for 12 weeks. Forty-one achieved SVR (77%). Of the 12 who failed therapy, all have been retreated and achieved SVR (Table 2).
Interferon-Free DAA Oral Regimens
More than 900 patients have been treated with interferon-free regimens since 2015 and outcomes were documented for > 800 patients. The SVR rates by GT were as follows: GT1 639 of 676 (95%); GT2 76 of 79 (96%); GT3 40 of 48 (83%); and GT4 6 of 6 (100%). Eighty-four percent of patients had GT1 infection. The median age of patient was 62 years, 72% were treatment naïve, and 35% having cirrhosis (based on liver biopsy or FIB4 score).18
Of 48 treatment failures, 30 patients were retreated; the rest of the patients were lost to follow-up (n = 9) or unable to receive retreatment (n = 9) mainly due to decompensated cirrhosis or liver cancer and short life expectancy. The median age of patient in this retreatment group was 62 years, 62% had cirrhosis, and most were infected with GT1. The average creatinine clearance was 73 mL/min. Twenty-two patients who failed therapy with ledipasvir/SOF were retreated (Table 3). A total of 13 patients out of the 19 tested eventually achieved SVR (68%). Four of the patients who had treatment failure again had GT1 infection and the other 2 GT3. All had cirrhosis.
Thirty-five patients were treated with PrOD, and 32 achieved SVR (91%). All 3 patients were retreated. One patient each achieved SVR with ERB/GZP, SOF/VEL and SOF/VEL/VOX. Fifty patients were treated with ERB/GZP and 45 achieved SVR (90%). All 5 treatment failures were retreated. Four achieved SVR and 1 was lost to follow-up (Table 4). Overall, of 30 patients who were retreated after failure with an all-oral DAA regimen, 27 patients had SVR values available and 21 achieved it (78%).
Discussion
Overall SVR was very high for patients who received oral treatment for HCV infection. A low number of patients failed therapy and were retreated. Patients who failed therapy again were similar in age but were more likely to have cirrhosis when compared with the overall interferon-free treated group. Thus, prompt treatment after HCV detection and before disease progression may improve treatment outcomes. Achieving SVR has been shown to improve fibrosis, portal hypertension, splenomegaly and cirrhosis, and reduce the risk of hepatocellular carcinoma by 70% and liver-related mortality by 90%.19-21/
Patients who failed therapy primarily had GT1—the most prevalent GT treated. A higher prevalence of GT1 is expected since it is the most common GT in the US.6 However, disease progression occurs more rapidly in those with GT3 and is more difficult to treat.22 The overall response rate was lower with this GT (83%) in this report, with only 1 of 3 patients retreated achieving an SVR.
Similar results are documented in retreatment trials.23 In the POLARIS-1 trial, treatment with SOF/VEL/VOX resulted in an overall response rate of 96% but only 91% for patients with GT3, compared with 95 to 100% for GTs 1, 2, or 4.23 In the current report, only 1 patient (GT1) failed retreatment with SOF/VEL/VOX. At this time, there are no clear treatment options for this patient. However, patients who fail GLE/PIB (none so far in the current report) may be able to receive SOF/VEL/VOX.24 In a small study, 29 of 31 patients achieved SVR with SOF/VEL/VOX after GLE/PIB failure (12 of 13 GT1 and 17 of 18 GT3).24
Limitations
This review was an observational, nonrandomized design, and only 1 medical center was involved. These results may not be applicable to other patient populations without a clinic set up with routine follow-ups to encourage adherence and completion of therapy.
Conclusions
Treatment of HCV infection has improved significantly over the past 10 years. Use of DAAs results in SVR for > 90% of patients, especially if the disease had not progressed to cirrhosis. Failure after retreatment for HCV infection was rare as well. Given that cirrhosis seems to increase the chance of treatment failure, it is imperative to identify candidates for treatment before the infection has progressed to cirrhosis. Patients infected with GT3 in particular should be more aggressively identified and treated.
Acknowledgments
The authors thank Nick P. Becky, PharmD, for his contributions to the identification of patients needing treatment for their HCV infection and review of initial manuscript information.
1. Centers for Disease Control and Prevention. Viral hepatitis: hepatitis C information. https://www.cdc.gov/hepatitis/hcv/index.htm. Reviewed April 14, 2020. Accessed June 16, 2020.
2. American Association for the Study of Liver Disease, Infectious Diseases Society of America. HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C. https://www.hcvguidelines.org. Accessed June 16, 2020.
3. Lingala S, Ghany MG. Natural history of hepatitis C. Gastroenterol Clin N Am. 2015;44(4):717-734. doi:10.1016/j.gtc.2015.07.003
4. Foote BS, Spooner LM, Belliveau PP. Boceprevir: a protease inhibitor for the treatment of chronic hepatitis C. Ann Pharmacother. 2011;45(9):1085-1093. doi:10.1345/aph.1P744
5. Kayali Z, Schmidt WN. Finally sofosbuvir: an oral anti-HCV drug with wide performance capability. Pharmgenomics Pers Med. 2014:7:387-398. doi:10.2147/PGPM.S52629
6. Falade-Nwulis O, Suarez-Cuervo C, Nelson DR, Fried MW, Segal JB, Sulkowski MS. Oral direct-acting agent therapy for hepatitis C virus infection: a systematic review. Ann Intern Med. 2017;166(9):637-648. doi:10.7326/M16-2575
7. Carrion AF, Martin P. Glecaprevir + pibrentasvir for treatment of hepatitis C. Expert Opinion Pharmacother. 2018;19(4):413-419. doi:10.1080/14656566.2018.1444030
8. Chahine EB, Kelley D, Childs-Kean LM. Sofosbuvir/velpatasvir/voxilaprevir: a pan-genotypic direct-acting antiviral combination for hepatitis C. Ann Pharmacother. 2018;52(4):352-363. doi:10.1177/1060028017741508
9. Lagasca AM, Kan VL. Hepatitis C treatment at a Veteran Affairs medical center after the availability of direct-acting agents: things are looking up. Clin Infect Dis. 2015:61(8):1347-1349. doi:10.1093/cid/civ573
10. Sovaldi (sofosbuvir) [package insert]. Foster City, CA: Gilead Sciences Inc; 2017.
11. Harvoni (ledipasvir and sofosbuvir) [package insert]. Foster City, CA: Gilead Sciences Inc; 2017.
12. Viekira Pak (ombitasvir, paritaprevir and ritonavir; dasabuvir) [package insert]. North Chicago, IL: AbbVie Inc; 2018.
13. Technivie (ombitasvir, paritaprevir and ritonavir) [package insert]. North Chicago, IL: AbbVie Inc; 2018.
14. Zepatier (elbasvir and grazoprevir) [package insert]. Whitehouse Station, NJ: Merck & Co Inc; 2018.
15. Epclusa (sofosbuvir and velpatasvir) [package insert]. Foster City, CA: Gilead Sciences Inc; 2017.
16. Mavyret (glecaprevir and pibrentasvir) [package insert]. North Chicago, IL: AbbVie Inc; 2019.
17. Vosevi (sofosbuvir, velpatasvir and voxilaprevir) [package insert]. Foster City, CA: Gilead Sciences Inc; 2017.
18. Vallet-Pichard A, Mallet V, Nalpas V, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and FibroTest. Hepatology. 2017;46(1):32-36. doi:10.1002/hep.21669
19. Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158(5, pt 1):329-337. doi:10.7326/0003-4819-158-5-201303050-00005
20. van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308(24):2584-2593. doi:10.1001/jama.2012.144878
21. Veldt BJ, Heathcote EJ, Wedemeyer H, et al. Sustained virologic response and clinical outcomes in patients with chronic hepatitis C and advanced fibrosis. Ann Intern Med. 2007;147(10):677-684. doi:10.7326/0003-4819-147-10-200711200-00003
22. Chen A, Patel K, Naggie S. Genotype 3 infection: the last stand of hepatitis C virus. Drugs. 2017;77(2):131-144. doi:10.1007/s40265-016-0685-x
23. Bourlière M, Gordon SC, Flamm SL, et al; POLARIS-1 and POLARIS-4 Investigators. Sofosbuvir, velpatasvir and voxilaprevir for previously treated HCV infection. N Engl J Med. 2017;376(22):2136-2146. doi:10.1056/NEJMoa1613512
24. Pearlman B, Perrys M, Hinds A. Sofosbuvir/velpatasvir/voxilaprevir for previous treatment failures with glecaprevir/pibrentasvir in chronic hepatitis C infection. Am J Gastroenterol. 2019;114(9):1550-1552. doi:10.14309/ajg.0000000000000248
1. Centers for Disease Control and Prevention. Viral hepatitis: hepatitis C information. https://www.cdc.gov/hepatitis/hcv/index.htm. Reviewed April 14, 2020. Accessed June 16, 2020.
2. American Association for the Study of Liver Disease, Infectious Diseases Society of America. HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C. https://www.hcvguidelines.org. Accessed June 16, 2020.
3. Lingala S, Ghany MG. Natural history of hepatitis C. Gastroenterol Clin N Am. 2015;44(4):717-734. doi:10.1016/j.gtc.2015.07.003
4. Foote BS, Spooner LM, Belliveau PP. Boceprevir: a protease inhibitor for the treatment of chronic hepatitis C. Ann Pharmacother. 2011;45(9):1085-1093. doi:10.1345/aph.1P744
5. Kayali Z, Schmidt WN. Finally sofosbuvir: an oral anti-HCV drug with wide performance capability. Pharmgenomics Pers Med. 2014:7:387-398. doi:10.2147/PGPM.S52629
6. Falade-Nwulis O, Suarez-Cuervo C, Nelson DR, Fried MW, Segal JB, Sulkowski MS. Oral direct-acting agent therapy for hepatitis C virus infection: a systematic review. Ann Intern Med. 2017;166(9):637-648. doi:10.7326/M16-2575
7. Carrion AF, Martin P. Glecaprevir + pibrentasvir for treatment of hepatitis C. Expert Opinion Pharmacother. 2018;19(4):413-419. doi:10.1080/14656566.2018.1444030
8. Chahine EB, Kelley D, Childs-Kean LM. Sofosbuvir/velpatasvir/voxilaprevir: a pan-genotypic direct-acting antiviral combination for hepatitis C. Ann Pharmacother. 2018;52(4):352-363. doi:10.1177/1060028017741508
9. Lagasca AM, Kan VL. Hepatitis C treatment at a Veteran Affairs medical center after the availability of direct-acting agents: things are looking up. Clin Infect Dis. 2015:61(8):1347-1349. doi:10.1093/cid/civ573
10. Sovaldi (sofosbuvir) [package insert]. Foster City, CA: Gilead Sciences Inc; 2017.
11. Harvoni (ledipasvir and sofosbuvir) [package insert]. Foster City, CA: Gilead Sciences Inc; 2017.
12. Viekira Pak (ombitasvir, paritaprevir and ritonavir; dasabuvir) [package insert]. North Chicago, IL: AbbVie Inc; 2018.
13. Technivie (ombitasvir, paritaprevir and ritonavir) [package insert]. North Chicago, IL: AbbVie Inc; 2018.
14. Zepatier (elbasvir and grazoprevir) [package insert]. Whitehouse Station, NJ: Merck & Co Inc; 2018.
15. Epclusa (sofosbuvir and velpatasvir) [package insert]. Foster City, CA: Gilead Sciences Inc; 2017.
16. Mavyret (glecaprevir and pibrentasvir) [package insert]. North Chicago, IL: AbbVie Inc; 2019.
17. Vosevi (sofosbuvir, velpatasvir and voxilaprevir) [package insert]. Foster City, CA: Gilead Sciences Inc; 2017.
18. Vallet-Pichard A, Mallet V, Nalpas V, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and FibroTest. Hepatology. 2017;46(1):32-36. doi:10.1002/hep.21669
19. Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158(5, pt 1):329-337. doi:10.7326/0003-4819-158-5-201303050-00005
20. van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308(24):2584-2593. doi:10.1001/jama.2012.144878
21. Veldt BJ, Heathcote EJ, Wedemeyer H, et al. Sustained virologic response and clinical outcomes in patients with chronic hepatitis C and advanced fibrosis. Ann Intern Med. 2007;147(10):677-684. doi:10.7326/0003-4819-147-10-200711200-00003
22. Chen A, Patel K, Naggie S. Genotype 3 infection: the last stand of hepatitis C virus. Drugs. 2017;77(2):131-144. doi:10.1007/s40265-016-0685-x
23. Bourlière M, Gordon SC, Flamm SL, et al; POLARIS-1 and POLARIS-4 Investigators. Sofosbuvir, velpatasvir and voxilaprevir for previously treated HCV infection. N Engl J Med. 2017;376(22):2136-2146. doi:10.1056/NEJMoa1613512
24. Pearlman B, Perrys M, Hinds A. Sofosbuvir/velpatasvir/voxilaprevir for previous treatment failures with glecaprevir/pibrentasvir in chronic hepatitis C infection. Am J Gastroenterol. 2019;114(9):1550-1552. doi:10.14309/ajg.0000000000000248
Outcomes Comparison of the Veterans’ Choice Program With the Veterans Affairs Health Care System for Hepatitis C Treatment
Population studies show high prevalence of chronic hepatitis C virus (HCV) infection among veterans, especially Vietnam War era veterans.1,2 The development of safe and efficacious direct-acting antiviral (DAA) medications to treat HCV infection made the majority of those infected eligible for treatment. However, the large number of veterans needing DAA treatment stressed the resources of the US Department of Veterans Affairs (VA) health care system. This occurred while Congress was focused on reducing wait times for veterans receiving care at the VA.
Congress passed the Veterans Access, Choice, and Accountability Act on August 7, 2014, leading to the creation of the Veterans Choice Program. Legislators felt there were inappropriate delays in care at the VA, and the Choice program was meant to address this problem and provide an “apples-to-apples comparison [of the VA] with non-VA hospitals.”3
Congress acknowledged the importance of curing HCV in the veteran population and allocated $1.5 billion for fiscal year (FY) 2016 for DAAs. The VA Central Office (VACO) carefully monitored these resources. The first policy memorandum from VACO for HCV care, issued on May 21, 2015, recommended that the sickest patients who will benefit from the treatment “receive priority over those who are less ill.”4,5 Those who met criteria for advanced liver disease were prioritized for treatment at the VA, while those who did not meet criteria were given the option of receiving treatment through Choice, or waiting for a change in policy.6 Over time, revisions to the guidelines relaxed the criteria for VA treatment eligibility, and on February 24, 2016, all restrictions on HCV treatment at the VA were lifted.7,8
The aim of this study was to provide a comparison of VA and non-VA care, specifically to determine whether care provided through Choice was timelier and more cost effective than care provided by the VA, and whether there was a quality difference. The high prevalence among veterans, wellestablished standards of care, and finite treatment course with clear indicators of success and failure makes HCV treatment an ideal disease with which to make this comparison.
Methods
We retrospectively analyzed the VA electronic health records of all veterans seen in the VA Loma Linda Healthcare System (VALLHCS) Hepatology clinic for chronic HCV infection during FY 2016 who were referred to Choice for HCV treatment. One hundred veterans met these criteria, encompassing the Choice population; 71 were seen at least once by a non-VA (Choice) health care provider (HCP) and 61 completed a treatment regimen through Choice. Treatment completion was defined as cessation of medication after the planned duration of therapy, or early termination of medication without resumption by that HCP. The Choice population was matched to an equal number of veterans who received HCV treatment from VALLHCS HCPs.
Data collected included age, gender, HCV genotype, determinants of liver fibrosis, and treatment success (defined as sustained virologic response at 12 weeks after the last dose of medication [SVR12]). Determinants of liver fibrosis included documented cirrhosis or complications of cirrhosis, Fibrosis-4 score (Fib-4), and platelet count.
Treatment failures were categorized as nonresponse (defined as detectable HCV viral load at the end of treatment), relapse (defined as an undetectable HCV viral load at the end of treatment with a subsequent positive test), and early termination (defined as a failure to complete the planned treatment regimen). Documented patient nonadherence, medical comorbidities that affected the treatment protocol, mental health diagnoses, and active social issues (defined as active or history of heavy alcohol use, active or history of illicit drug use, lack of social support, and homelessness) were noted.
Timeliness of delivery of care was measured in days. For the VA group, the wait time was defined as the date the consult for HCV treatment was placed to the date of the initial appointment with the HCV treatment provider. For the Choice group, the wait time was defined as the date the referral to the Choice program was made to the date of the initial appointment with the Choice HCP. Treatment regimens were evaluated for appropriateness based on guidelines from VACO and the American Association for the Study of Liver Diseases.9-11
Tests performed by Choice providers were evaluated for whether they were relevant to HCV care and whether similar data already were available from VALLHCS. Tests that were not indicated were identified as unnecessary costs incurred by the Choice program, as were tests that had to be repeated at the VA because of a lack of documentation from the Choice provider. All medications given inappropriately were considered added costs. Medicare reimbursement rates for the most applicable Current Procedural Terminology (CPT) code and VA national contract pricing for medications were used for calculations. This study was approved by the VALLHCS institutional review board.
Statistical Analysis
IBM (Armonk, NY) Statistical Package for Social Sciences software was used to evaluate for differences in Fib-4, platelet count, prevalence of cirrhosis, prevalence of medical comorbidities, prevalence of mental health comorbidities, prevalence of the social issues defined in the Methods section, time from referral to time of appointment date, and SVR12 rate between the VA and Choice groups.
Exclusions
There were 15 veterans in the VA group who had a wait time of > 100 days. Of these, 5 (33%) were initially Choice referrals, but due to negative interactions with the Choice provider, the veterans returned to VALLHCS for care. Two of the 15 (13%) did not keep appointments and were lost to follow up. Six of the 15 (40%) had medical comorbidities that required more immediate attention, so HCV treatment initiation was deliberately moved back. The final 2 veterans scheduled their appointments unusually far apart, artificially increasing their wait time. Given that these were unique situations and some of the veterans received care from both Choice and VA providers, a decision was made to exclude these individuals from the study.
It has been shown that platelet count correlates with degree of liver fibrosis, a concept that is the basis for the Fib-4 scoring system.12 Studies have shown that platelet count is a survival predictor in those with cirrhosis, and thrombocytopenia is a negative predictor of HCV treatment success using peginterferon and ribavirin13,14 Therefore, the VA memorandum automatically assigned the sickest individuals to the VA for HCV treatment. The goal of this study was to compare the impact of factors other than stage of fibrosis on HCV treatment success, which is why the 12 veterans with platelet count < 100,000 in the VA group were excluded. There were no veterans with platelet count < 100,000 in the Choice group.
When comparing SVR12 rates between the VA and Choice groups, every veteran treated at VALLHCS in FY 2016 was included, increasing the number in the VA group from 100 to 320 and therefore the power of this comparison.
Results
A summary of the statistical analysis can be found in Table 1. The genotype distribution was consistent with epidemiological studies, including those specific to veterans.15,16 There were statistically significant differences (P < .001) in mean Fib-4 and mean platelet count. The VA group had a higher Fib-4 and lower platelet count. Seventy-four percent of the VA population was defined as cirrhotic, while only 3% of the Choice population met similar criteria (P < .001). The VA and Choice groups were similar in terms of age, gender, and genotype distribution (Table 2).
The VA group was found to have a higher prevalence of comorbidities that affected HCV treatment. These conditions included but were not limited to: chronic kidney disease that precluded the use of certain medications, any condition that required medication with a known interaction with DAAs (ie, proton pump inhibitors, statins, and amiodarone), and cirrhosis if it impacted the treatment regimen. The difference in the prevalence of mental health comorbidities was not significant (P = .39), but there was a higher prevalence of social issues among the VA group (P = .002).
The mean wait time from referral to appointment was 28.6 days for the VA group and 42.3 days for the Choice group (P < .001), indicating that a Choice referral took longer to complete than a referral within the VA for HCV treatment. Thirty of the 71 (42%) veterans seen by a Choice provider accrued extraneous cost, with a mean additional cost of $8,561.40 per veteran. In the Choice group, 61 veterans completed a treatment regimen with the Choice HCP. Fifty-five veterans completed treatment and had available SVR12 data (6 were lost to follow up without SVR12 testing) and 50 (91%) had confirmed SVR12. The charts of the 5 treatment failures were reviewed to discern the cause for failure. Two cases involved early termination of therapy, 3 involved relapse and 2 failed to comply with medication instructions. There was 1 case of the Choice HCP not addressing simultaneous use of ledipasvir and a proton pump inhibitor, potentially causing an interaction, and 1 case where both the VA and Choice providers failed to recognize indicators of cirrhosis, which impacted the regimen used.
In the VALLHCS group, records of 320 veterans who completed treatment and had SVR12 testing were reviewed. While the Choice memorandum was active, veterans selected to be treated at VALLHCS had advanced liver fibrosis or cirrhosis, medical and mental health comorbidities that increased the risk of treatment complications or were considered to have difficulty adhering to the medication regimen. For this group, 296 (93%) had confirmed SVR12. Eighteen of the 24 (75%) treatment failures were complicated by nonadherence, including all 13 cases of early termination. One patient died from complications of decompensated cirrhosis before completing treatment, and 1 did not receive HCV medications during a hospital admission due to poor coordination of care between the VA inpatient and outpatient pharmacy services, leading to multiple missed doses.
The difference in SVR12 rates (ie, treatment failure rates), between the VA and Choice groups was not statistically significant (P = .61). None of the specific reasons for treatment failure had a statistically significant difference between groups. A treatment failure analysis is shown in Table 3, and Table 4 indicates the breakdown of treatment regimens.
Discussion
The Veterans Health Administration (VHA) is the largest integrated health care system in the US, consisting of 152 medical centers and > 1,700 sites of care. The VA has the potential to meet the health care needs of 21.6 million veterans. About 9 million veterans are enrolled in the VA system and 5.9 million received health care through VHA.17 However, every medical service cannot realistically be made available at every facility, and some veterans have difficulty gaining access to VHA care; distance and wait times have been well-publicized issues that need further exploration.18,19 The Choice program is an attempt to meet gaps in VA coverage using non-VA HCPs.
HCV infection is a specific diagnosis with national treatment guidelines and wellstudied treatments; it can be cured, with an evidence- based definition of cure. The VACO policy memorandum to refer less sick veterans to Choice while treating sicker veterans at the VA provided the opportunity to directly compare the quality of the 2 programs. The SVR12 rates of VALLHCS and Choice providers were comparable to the national average at the time, and while the difference in SVR12 rate was not significant, VALLHCS treated a significantly higher number of patients with cirrhosis because of the referral criteria.20
The significant difference in medical comorbidities between the VA and Choice groups was not surprising, partly because of the referral criteria. Cirrhosis can impact the treatment regimen, especially in regard to use of ribavirin. Since the presence of mental health comorbidities did not affect selection into the Choice group, it makes sense that there was no significant difference in prevalence between the groups.
VACO allowed veterans with HCV treatment plans that VA HCPs felt were too complicated for the Choice program to be treated by VHA HCPs.9 VALLHCS exercised this right for veterans at risk for nonadherence, because in HCV treatment, nonadherence leads to treatment failure and development of drug resistant virus strains. Therefore, veterans who would have difficulty traveling to VALLHCS to pick up medications, those who lacked means of communication (such as those who were homeless), and those who had active substance abuse were treated at the VA, where closer monitoring and immediate access to a wide range of services was possible. Studies have confirmed the impact of these types of issues on HCV treatment adherence and success. 21 This explains the higher prevalence of social issues in the VA group.
For an internal referral for HCV treatment at VALLHCS, the hepatology provider submits a consult request to the HCV treatment provider, who works in the same office, making direct communication simple. The main administrative limiting factor to minimizing wait times is the number of HCPs, which is dependent on hiring allowances.
When a veteran is referred to Choice, the VA provider places a consult for non-VA care, which the VA Office of Community Care processes by compiling relevant documents and sending the package to Triwest Healthcare Alliance, a private insurance processor contracted with the VA. Triwest selects the Choice provider, often without any input from the VA, and arranges the veteran’s initial appointment.22 Geographic distance to the veteran’s address is the main selection criteria for Triwest. Documents sent between the Choice and VA HCPs go through the Office of Community Care and Triwest. This significantly increases the potential for delays and failed communication. Triwest had a comprehensive list of providers deemed to be qualified to treat HCV within the geographic catchment of VALLHCS. This list was reviewed, and all veterans referred to Choice had HCPs near their home address; therefore, availability of Choice HCPs was not an issue.
The VA can provide guidance on management of the veteran in the form of bundle packages containing a list of services for which the Choice provider is authorized to provide, and ones the Choice provider is not authorized to provide. Some Choice HCPs ordered tests that were not authorized for HCV treatment such as esophagogastroduodenoscopy, colonoscopy, and liver biopsy. In all, 17 of 71 (24%) veterans seen by Choice HCPs had tests performed or ordered that VA HCPs would not have obtained for the purpose of HCV treatment (Table 5).
In order to prevent veterans from receiving unnecessary tests, a VALLHCS hepatologist asked to be notified by VA administrators overseeing Choice referrals whenever a secondary authorization request (SAR) was submitted by a Choice HCP. This strategy is not standard VA practice, therefore at many VA sites these requested tests would have been performed by the Choice HCP, which is why SARs were factored into cost analysis.
SVR12 test results that were drawn too early and had to be repeated at VALLHCS were a cost unique to the Choice program. Duplicate tests, particularly imaging studies and blood work, were extraneous costs. The largest extraneous costs were treatment regimens prescribed by Choice HCPs that did not follow standard of care and required VA provider intervention. Thirty of the 71 (42%) veterans seen by a Choice provider accrued a mean $8,561.40 in extra costs. As a result, the Choice program cost VHA $250,000 more to provide care for 30 veterans (enough to pay for a physician’s annual salary).
Some inappropriate treatment regimens were the result of Choice HCP error, such as 1 case in which a veteran was inadvertently switched from ledipasvir/sofosbuvir to ombitasvir/ paritaprevir/ritonavir/dasabuvir after week 4. The veteran had to start therapy over but still achieved SVR12. Other cases saw veterans receive regimens for which they had clear contraindications, such as creatinine clearance < 30 mL/min/1.73m2 for sofosbuvir or a positive resistance panel for specific medications. Eleven of 62 (18%) veterans who were started on HCV treatment by a Choice HCP received a regimen not consistent with VA guidelines—an alarming result.
Follow up for veterans referred to Choice was extremely labor intensive, and assessment of personnel requirements in a Choice-based VA system must take this into consideration. The Choice HCP has no obligation to communicate with the VA HCP. At the time of chart review, 57 of 71 (80%) Choice veterans had inadequate documentation to make a confident assessment of the treatment outcome. Multiple calls to the offices of the Choice HCP were needed to acquire records, and veterans had to be tracked down for additional tests. Veterans often would complete treatment and stop following up with the Choice provider before SVR12 confirmation. The VA hepatology provider reviewing Choice referrals served as clinician, case manager, and clerk in order to get veterans to an appropriate end point in their hepatitis C treatment, with mostly unmeasured hours of work.
Limitations
The study population size was limited by the number of veterans able to complete treatment through Choice. The parameters in the VACO policy memos automatically selected the VA and Choice groups but made them clinically distinct populations. New treatment medications were released during the study period, which impacted management strategy. Occasionally, VA and non- VA HCPs preferred different treatment regimens, leading to variation in the distribution of regimens used despite similar genotype distribution (Tables 2 and 4). In addition, a retrospective study is at risk for recall bias. A prospective study randomizing veterans to the Choice and VA groups is an important future endeavor. Comparing veteran satisfaction for Choice and VA services is also crucial.
Conclusions
This study demonstrates that the VA was able to provide more cost-effective and more timely care for HCV treatment to a relatively sicker population with no reduction in treatment success when compared with non-VA HCPs through the Choice program. While the Choice program can help veterans receive services they may otherwise not have access to and reduce travel time, the current system introduces inefficiencies that delay care and decrease cost-effectiveness. The Choice HCP selection process is based on proximity rather than quality, which may place the veteran at risk for receiving substandard care. Large-scale quality of care studies that compare efficiency measures, clinical outcomes, patient demographics, travel distance, cost efficacy and patient satisfaction for veterans receiving similar services at a VA facility and through Choice should be performed to ensure that veterans receive the best care available.
1. Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160(5):293-300. doi:10.7326/M13-1133
2. Dominitz JA, Boyko EJ, Koepsell TD, et al. Elevated prevalence of hepatitis C infection in users of United States veterans medical centers. Hepatology. 2005;41(1):88-96. doi:10.1002/hep.20502
3. Veterans Access, Choice, and Accountability Act of 2014. 42 USC §1395 (2014).
4. Tuchschmidt J. Attachment C: Provision of hepatitis C treatment. US Department of Veterans Affairs Central Office Memorandum from the Principal Deputy Under Secretary for Health. http://vaww.hepatitis.va.gov/education /choice-provision-hcv-treatment.asp. Published May 21, 2015. [Nonpublic site.]
5. Tuchschmidt J. Attachment A: Provision of hepatitis C (HCV) treatment through the Choice program. US Department of Veterans Affairs Central Office Memorandum from the Principal Deputy Under Secretary for Health. http:// vaww.hepatitis.va.gov/pdf/choice-attachment-a-FY16 .pdf. Published May 21, 2015. [Nonpublic site.]
6. Tuchschmidt J. Attachment B: Initiation of hepatitis C virus (HCV) treatment: protocol for prioritization. US Department of Veterans Affairs Central Office Memorandum from the Principal Deputy Under Secretary for Health. http://vaww .hepatitis.va.gov/pdf/provision-HCV-treatment-attach ment-b.pdf. Published May 21, 2015. [Nonpublic site.]
7. Murphy, JP. Hepatitis C virus funding and prioritization status. US Department of Veterans Affairs Central Office Memorandum from the Assistant Deputy Under Secretary for Health for Clinical Operations. http://vaww.hepatitis .va.gov/education/choice-memo-hcv-funding-and -prioritization-status-01272016.asp. Published January 27, 2016. [Nonpublic site.]
8. Lynch TJ, McCarthy MF. Hepatitis C virus funding and prioritization status update. US Department of Veterans Affairs Central Office Memorandum from the Assistant Deputy Under Secretary for Health for Clinical Operations and Acting Assistant Deputy Under Secretary for Health for Patient Care Services. http://vaww.hepatitis.va.gov /education/choice-funding-update-feb-2016.asp. Published February 24, 2016. [Nonpublic site.]
9. Morgan TR, Yee H; US Department of Veterans Affairs National Hepatitis C Resource Center Program and the National Viral Hepatitis Program in the Office of Patient Care Services. Chronic hepatitis C virus (HCV) infection: treatment considerations. http://vaww.hepatitis.va.gov /pdf/treatment-considerations-2016-03-28.pdf. Published March 28, 2016. [Nonpublic site.]
10. American Association for the Study of Liver Diseases; Infectious Diseases Society of America. Initial Treatment Box. http://hcvguidelines.org/full-report/initial-treatment -box-summary-recommendations-patients-who-are -initiating-therapy-hcv. Updated November 6, 2019. Accessed May 11, 2020.
11. AASLD/IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62(3): 932-954. doi:10.1002/hep.27950
12. Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006; 43(6):1317-1325. doi:10.1002/hep.21178
13. Realdi G, Fattovich G, Hadziyannis S, et al. Survival and prognostic factors in 366 patients with compensated cirrhosis type B: a multicenter study. The Investigators of the European Concerted Action on Viral Hepatitis (EUROHEP). J Hepatol. 1994;21(4):656-666. doi:10.1016/s0168 -8278(94)80115-0
14. Kanda T, Kato K, Tsubota A, et al. Platelet count and sustained virological response in hepatitis C treatment. World J Hepatol. 2013;5(4):182-188. doi:10.4254/wjh.v5.i4.182
15. Manos MM, Shvachko VA, Murphy RC, Arduino JM, Shire NJ. Distribution of hepatitis C virus genotypes in a diverse US integrated health care population. J Med Virol. 2012;84(11):1744-1750. doi:10.1002/jmv.23399
16. Cheung RC. Epidemiology of hepatitis C virus infection in American veterans. Am J Gastroenterol. 2000;95(3): 740-747. doi:10.1111/j.1572-0241.2000.01854.x
17. Bagalman E. The number of Veterans that use VA health care services: a fact sheet. Congressional Research Service Report R43579. https://fas.org/sgp/crs/misc/R43579.pdf. Published June 3, 2014. Accessed May 11, 2020.
18. US General Accounting Office. Report to the Ranking Minority Member, Subcommittee on Compensation, Pension, Insurance, and Memorial Affairs, Committee on Veterans’ Affairs, US House of Representatives. How distance from VA facilities affects veterans’ use of VA services. https:// www.gao.gov/assets/230/221992.pdf. Published December 1995. Accessed May 11, 2020.
19. Bronstein S, Griffin D. A fatal wait: Veterans languish and die on a VA hospital’s secret list. http://www.cnn .com/2014/04/23/health/veterans-dying-health-care -delays/index.html. Published April 23, 2014. Accessed May 11, 2020.
20. Ioannou GN, Beste LA, Chang MF, et al. Effectiveness of sofosbuvir, ledipasvir/sofosbuvir, or paritaprevir/ritonavir/ ombitasvir and dasabuvir regimens for treatment of patients with hepatitis C in the Veterans Affairs national health care system. Gastroenterology. 2016;151(3):457- 471. doi:10.1053/j.gastro.2016.05.049
21. Malespin MH, Harris C, Kanar O, et al. Barriers to treatment of chronic hepatitis C with direct acting antivirals in an urban clinic. Ann Hepatol. 2019;18(2):304-309. doi:10.1016/j.aohep.2018.06.001
22. Tuchschmidt J. Attachment D: Hepatitis C virus (HCV) fact sheet for Veterans Choice Program for both VA and Choice providers. US Department of Veterans Affairs Central Office Memorandum from the Deputy Under Secretary for Health for Policy and Services and the Acting Deputy Undersecretary for Health for Operations and Management. http://vaww .hepatitis.va.gov/educatiochoice-provision-HCV-treatment -additional.asp. [Nonpublic site.]
Population studies show high prevalence of chronic hepatitis C virus (HCV) infection among veterans, especially Vietnam War era veterans.1,2 The development of safe and efficacious direct-acting antiviral (DAA) medications to treat HCV infection made the majority of those infected eligible for treatment. However, the large number of veterans needing DAA treatment stressed the resources of the US Department of Veterans Affairs (VA) health care system. This occurred while Congress was focused on reducing wait times for veterans receiving care at the VA.
Congress passed the Veterans Access, Choice, and Accountability Act on August 7, 2014, leading to the creation of the Veterans Choice Program. Legislators felt there were inappropriate delays in care at the VA, and the Choice program was meant to address this problem and provide an “apples-to-apples comparison [of the VA] with non-VA hospitals.”3
Congress acknowledged the importance of curing HCV in the veteran population and allocated $1.5 billion for fiscal year (FY) 2016 for DAAs. The VA Central Office (VACO) carefully monitored these resources. The first policy memorandum from VACO for HCV care, issued on May 21, 2015, recommended that the sickest patients who will benefit from the treatment “receive priority over those who are less ill.”4,5 Those who met criteria for advanced liver disease were prioritized for treatment at the VA, while those who did not meet criteria were given the option of receiving treatment through Choice, or waiting for a change in policy.6 Over time, revisions to the guidelines relaxed the criteria for VA treatment eligibility, and on February 24, 2016, all restrictions on HCV treatment at the VA were lifted.7,8
The aim of this study was to provide a comparison of VA and non-VA care, specifically to determine whether care provided through Choice was timelier and more cost effective than care provided by the VA, and whether there was a quality difference. The high prevalence among veterans, wellestablished standards of care, and finite treatment course with clear indicators of success and failure makes HCV treatment an ideal disease with which to make this comparison.
Methods
We retrospectively analyzed the VA electronic health records of all veterans seen in the VA Loma Linda Healthcare System (VALLHCS) Hepatology clinic for chronic HCV infection during FY 2016 who were referred to Choice for HCV treatment. One hundred veterans met these criteria, encompassing the Choice population; 71 were seen at least once by a non-VA (Choice) health care provider (HCP) and 61 completed a treatment regimen through Choice. Treatment completion was defined as cessation of medication after the planned duration of therapy, or early termination of medication without resumption by that HCP. The Choice population was matched to an equal number of veterans who received HCV treatment from VALLHCS HCPs.
Data collected included age, gender, HCV genotype, determinants of liver fibrosis, and treatment success (defined as sustained virologic response at 12 weeks after the last dose of medication [SVR12]). Determinants of liver fibrosis included documented cirrhosis or complications of cirrhosis, Fibrosis-4 score (Fib-4), and platelet count.
Treatment failures were categorized as nonresponse (defined as detectable HCV viral load at the end of treatment), relapse (defined as an undetectable HCV viral load at the end of treatment with a subsequent positive test), and early termination (defined as a failure to complete the planned treatment regimen). Documented patient nonadherence, medical comorbidities that affected the treatment protocol, mental health diagnoses, and active social issues (defined as active or history of heavy alcohol use, active or history of illicit drug use, lack of social support, and homelessness) were noted.
Timeliness of delivery of care was measured in days. For the VA group, the wait time was defined as the date the consult for HCV treatment was placed to the date of the initial appointment with the HCV treatment provider. For the Choice group, the wait time was defined as the date the referral to the Choice program was made to the date of the initial appointment with the Choice HCP. Treatment regimens were evaluated for appropriateness based on guidelines from VACO and the American Association for the Study of Liver Diseases.9-11
Tests performed by Choice providers were evaluated for whether they were relevant to HCV care and whether similar data already were available from VALLHCS. Tests that were not indicated were identified as unnecessary costs incurred by the Choice program, as were tests that had to be repeated at the VA because of a lack of documentation from the Choice provider. All medications given inappropriately were considered added costs. Medicare reimbursement rates for the most applicable Current Procedural Terminology (CPT) code and VA national contract pricing for medications were used for calculations. This study was approved by the VALLHCS institutional review board.
Statistical Analysis
IBM (Armonk, NY) Statistical Package for Social Sciences software was used to evaluate for differences in Fib-4, platelet count, prevalence of cirrhosis, prevalence of medical comorbidities, prevalence of mental health comorbidities, prevalence of the social issues defined in the Methods section, time from referral to time of appointment date, and SVR12 rate between the VA and Choice groups.
Exclusions
There were 15 veterans in the VA group who had a wait time of > 100 days. Of these, 5 (33%) were initially Choice referrals, but due to negative interactions with the Choice provider, the veterans returned to VALLHCS for care. Two of the 15 (13%) did not keep appointments and were lost to follow up. Six of the 15 (40%) had medical comorbidities that required more immediate attention, so HCV treatment initiation was deliberately moved back. The final 2 veterans scheduled their appointments unusually far apart, artificially increasing their wait time. Given that these were unique situations and some of the veterans received care from both Choice and VA providers, a decision was made to exclude these individuals from the study.
It has been shown that platelet count correlates with degree of liver fibrosis, a concept that is the basis for the Fib-4 scoring system.12 Studies have shown that platelet count is a survival predictor in those with cirrhosis, and thrombocytopenia is a negative predictor of HCV treatment success using peginterferon and ribavirin13,14 Therefore, the VA memorandum automatically assigned the sickest individuals to the VA for HCV treatment. The goal of this study was to compare the impact of factors other than stage of fibrosis on HCV treatment success, which is why the 12 veterans with platelet count < 100,000 in the VA group were excluded. There were no veterans with platelet count < 100,000 in the Choice group.
When comparing SVR12 rates between the VA and Choice groups, every veteran treated at VALLHCS in FY 2016 was included, increasing the number in the VA group from 100 to 320 and therefore the power of this comparison.
Results
A summary of the statistical analysis can be found in Table 1. The genotype distribution was consistent with epidemiological studies, including those specific to veterans.15,16 There were statistically significant differences (P < .001) in mean Fib-4 and mean platelet count. The VA group had a higher Fib-4 and lower platelet count. Seventy-four percent of the VA population was defined as cirrhotic, while only 3% of the Choice population met similar criteria (P < .001). The VA and Choice groups were similar in terms of age, gender, and genotype distribution (Table 2).
The VA group was found to have a higher prevalence of comorbidities that affected HCV treatment. These conditions included but were not limited to: chronic kidney disease that precluded the use of certain medications, any condition that required medication with a known interaction with DAAs (ie, proton pump inhibitors, statins, and amiodarone), and cirrhosis if it impacted the treatment regimen. The difference in the prevalence of mental health comorbidities was not significant (P = .39), but there was a higher prevalence of social issues among the VA group (P = .002).
The mean wait time from referral to appointment was 28.6 days for the VA group and 42.3 days for the Choice group (P < .001), indicating that a Choice referral took longer to complete than a referral within the VA for HCV treatment. Thirty of the 71 (42%) veterans seen by a Choice provider accrued extraneous cost, with a mean additional cost of $8,561.40 per veteran. In the Choice group, 61 veterans completed a treatment regimen with the Choice HCP. Fifty-five veterans completed treatment and had available SVR12 data (6 were lost to follow up without SVR12 testing) and 50 (91%) had confirmed SVR12. The charts of the 5 treatment failures were reviewed to discern the cause for failure. Two cases involved early termination of therapy, 3 involved relapse and 2 failed to comply with medication instructions. There was 1 case of the Choice HCP not addressing simultaneous use of ledipasvir and a proton pump inhibitor, potentially causing an interaction, and 1 case where both the VA and Choice providers failed to recognize indicators of cirrhosis, which impacted the regimen used.
In the VALLHCS group, records of 320 veterans who completed treatment and had SVR12 testing were reviewed. While the Choice memorandum was active, veterans selected to be treated at VALLHCS had advanced liver fibrosis or cirrhosis, medical and mental health comorbidities that increased the risk of treatment complications or were considered to have difficulty adhering to the medication regimen. For this group, 296 (93%) had confirmed SVR12. Eighteen of the 24 (75%) treatment failures were complicated by nonadherence, including all 13 cases of early termination. One patient died from complications of decompensated cirrhosis before completing treatment, and 1 did not receive HCV medications during a hospital admission due to poor coordination of care between the VA inpatient and outpatient pharmacy services, leading to multiple missed doses.
The difference in SVR12 rates (ie, treatment failure rates), between the VA and Choice groups was not statistically significant (P = .61). None of the specific reasons for treatment failure had a statistically significant difference between groups. A treatment failure analysis is shown in Table 3, and Table 4 indicates the breakdown of treatment regimens.
Discussion
The Veterans Health Administration (VHA) is the largest integrated health care system in the US, consisting of 152 medical centers and > 1,700 sites of care. The VA has the potential to meet the health care needs of 21.6 million veterans. About 9 million veterans are enrolled in the VA system and 5.9 million received health care through VHA.17 However, every medical service cannot realistically be made available at every facility, and some veterans have difficulty gaining access to VHA care; distance and wait times have been well-publicized issues that need further exploration.18,19 The Choice program is an attempt to meet gaps in VA coverage using non-VA HCPs.
HCV infection is a specific diagnosis with national treatment guidelines and wellstudied treatments; it can be cured, with an evidence- based definition of cure. The VACO policy memorandum to refer less sick veterans to Choice while treating sicker veterans at the VA provided the opportunity to directly compare the quality of the 2 programs. The SVR12 rates of VALLHCS and Choice providers were comparable to the national average at the time, and while the difference in SVR12 rate was not significant, VALLHCS treated a significantly higher number of patients with cirrhosis because of the referral criteria.20
The significant difference in medical comorbidities between the VA and Choice groups was not surprising, partly because of the referral criteria. Cirrhosis can impact the treatment regimen, especially in regard to use of ribavirin. Since the presence of mental health comorbidities did not affect selection into the Choice group, it makes sense that there was no significant difference in prevalence between the groups.
VACO allowed veterans with HCV treatment plans that VA HCPs felt were too complicated for the Choice program to be treated by VHA HCPs.9 VALLHCS exercised this right for veterans at risk for nonadherence, because in HCV treatment, nonadherence leads to treatment failure and development of drug resistant virus strains. Therefore, veterans who would have difficulty traveling to VALLHCS to pick up medications, those who lacked means of communication (such as those who were homeless), and those who had active substance abuse were treated at the VA, where closer monitoring and immediate access to a wide range of services was possible. Studies have confirmed the impact of these types of issues on HCV treatment adherence and success. 21 This explains the higher prevalence of social issues in the VA group.
For an internal referral for HCV treatment at VALLHCS, the hepatology provider submits a consult request to the HCV treatment provider, who works in the same office, making direct communication simple. The main administrative limiting factor to minimizing wait times is the number of HCPs, which is dependent on hiring allowances.
When a veteran is referred to Choice, the VA provider places a consult for non-VA care, which the VA Office of Community Care processes by compiling relevant documents and sending the package to Triwest Healthcare Alliance, a private insurance processor contracted with the VA. Triwest selects the Choice provider, often without any input from the VA, and arranges the veteran’s initial appointment.22 Geographic distance to the veteran’s address is the main selection criteria for Triwest. Documents sent between the Choice and VA HCPs go through the Office of Community Care and Triwest. This significantly increases the potential for delays and failed communication. Triwest had a comprehensive list of providers deemed to be qualified to treat HCV within the geographic catchment of VALLHCS. This list was reviewed, and all veterans referred to Choice had HCPs near their home address; therefore, availability of Choice HCPs was not an issue.
The VA can provide guidance on management of the veteran in the form of bundle packages containing a list of services for which the Choice provider is authorized to provide, and ones the Choice provider is not authorized to provide. Some Choice HCPs ordered tests that were not authorized for HCV treatment such as esophagogastroduodenoscopy, colonoscopy, and liver biopsy. In all, 17 of 71 (24%) veterans seen by Choice HCPs had tests performed or ordered that VA HCPs would not have obtained for the purpose of HCV treatment (Table 5).
In order to prevent veterans from receiving unnecessary tests, a VALLHCS hepatologist asked to be notified by VA administrators overseeing Choice referrals whenever a secondary authorization request (SAR) was submitted by a Choice HCP. This strategy is not standard VA practice, therefore at many VA sites these requested tests would have been performed by the Choice HCP, which is why SARs were factored into cost analysis.
SVR12 test results that were drawn too early and had to be repeated at VALLHCS were a cost unique to the Choice program. Duplicate tests, particularly imaging studies and blood work, were extraneous costs. The largest extraneous costs were treatment regimens prescribed by Choice HCPs that did not follow standard of care and required VA provider intervention. Thirty of the 71 (42%) veterans seen by a Choice provider accrued a mean $8,561.40 in extra costs. As a result, the Choice program cost VHA $250,000 more to provide care for 30 veterans (enough to pay for a physician’s annual salary).
Some inappropriate treatment regimens were the result of Choice HCP error, such as 1 case in which a veteran was inadvertently switched from ledipasvir/sofosbuvir to ombitasvir/ paritaprevir/ritonavir/dasabuvir after week 4. The veteran had to start therapy over but still achieved SVR12. Other cases saw veterans receive regimens for which they had clear contraindications, such as creatinine clearance < 30 mL/min/1.73m2 for sofosbuvir or a positive resistance panel for specific medications. Eleven of 62 (18%) veterans who were started on HCV treatment by a Choice HCP received a regimen not consistent with VA guidelines—an alarming result.
Follow up for veterans referred to Choice was extremely labor intensive, and assessment of personnel requirements in a Choice-based VA system must take this into consideration. The Choice HCP has no obligation to communicate with the VA HCP. At the time of chart review, 57 of 71 (80%) Choice veterans had inadequate documentation to make a confident assessment of the treatment outcome. Multiple calls to the offices of the Choice HCP were needed to acquire records, and veterans had to be tracked down for additional tests. Veterans often would complete treatment and stop following up with the Choice provider before SVR12 confirmation. The VA hepatology provider reviewing Choice referrals served as clinician, case manager, and clerk in order to get veterans to an appropriate end point in their hepatitis C treatment, with mostly unmeasured hours of work.
Limitations
The study population size was limited by the number of veterans able to complete treatment through Choice. The parameters in the VACO policy memos automatically selected the VA and Choice groups but made them clinically distinct populations. New treatment medications were released during the study period, which impacted management strategy. Occasionally, VA and non- VA HCPs preferred different treatment regimens, leading to variation in the distribution of regimens used despite similar genotype distribution (Tables 2 and 4). In addition, a retrospective study is at risk for recall bias. A prospective study randomizing veterans to the Choice and VA groups is an important future endeavor. Comparing veteran satisfaction for Choice and VA services is also crucial.
Conclusions
This study demonstrates that the VA was able to provide more cost-effective and more timely care for HCV treatment to a relatively sicker population with no reduction in treatment success when compared with non-VA HCPs through the Choice program. While the Choice program can help veterans receive services they may otherwise not have access to and reduce travel time, the current system introduces inefficiencies that delay care and decrease cost-effectiveness. The Choice HCP selection process is based on proximity rather than quality, which may place the veteran at risk for receiving substandard care. Large-scale quality of care studies that compare efficiency measures, clinical outcomes, patient demographics, travel distance, cost efficacy and patient satisfaction for veterans receiving similar services at a VA facility and through Choice should be performed to ensure that veterans receive the best care available.
Population studies show high prevalence of chronic hepatitis C virus (HCV) infection among veterans, especially Vietnam War era veterans.1,2 The development of safe and efficacious direct-acting antiviral (DAA) medications to treat HCV infection made the majority of those infected eligible for treatment. However, the large number of veterans needing DAA treatment stressed the resources of the US Department of Veterans Affairs (VA) health care system. This occurred while Congress was focused on reducing wait times for veterans receiving care at the VA.
Congress passed the Veterans Access, Choice, and Accountability Act on August 7, 2014, leading to the creation of the Veterans Choice Program. Legislators felt there were inappropriate delays in care at the VA, and the Choice program was meant to address this problem and provide an “apples-to-apples comparison [of the VA] with non-VA hospitals.”3
Congress acknowledged the importance of curing HCV in the veteran population and allocated $1.5 billion for fiscal year (FY) 2016 for DAAs. The VA Central Office (VACO) carefully monitored these resources. The first policy memorandum from VACO for HCV care, issued on May 21, 2015, recommended that the sickest patients who will benefit from the treatment “receive priority over those who are less ill.”4,5 Those who met criteria for advanced liver disease were prioritized for treatment at the VA, while those who did not meet criteria were given the option of receiving treatment through Choice, or waiting for a change in policy.6 Over time, revisions to the guidelines relaxed the criteria for VA treatment eligibility, and on February 24, 2016, all restrictions on HCV treatment at the VA were lifted.7,8
The aim of this study was to provide a comparison of VA and non-VA care, specifically to determine whether care provided through Choice was timelier and more cost effective than care provided by the VA, and whether there was a quality difference. The high prevalence among veterans, wellestablished standards of care, and finite treatment course with clear indicators of success and failure makes HCV treatment an ideal disease with which to make this comparison.
Methods
We retrospectively analyzed the VA electronic health records of all veterans seen in the VA Loma Linda Healthcare System (VALLHCS) Hepatology clinic for chronic HCV infection during FY 2016 who were referred to Choice for HCV treatment. One hundred veterans met these criteria, encompassing the Choice population; 71 were seen at least once by a non-VA (Choice) health care provider (HCP) and 61 completed a treatment regimen through Choice. Treatment completion was defined as cessation of medication after the planned duration of therapy, or early termination of medication without resumption by that HCP. The Choice population was matched to an equal number of veterans who received HCV treatment from VALLHCS HCPs.
Data collected included age, gender, HCV genotype, determinants of liver fibrosis, and treatment success (defined as sustained virologic response at 12 weeks after the last dose of medication [SVR12]). Determinants of liver fibrosis included documented cirrhosis or complications of cirrhosis, Fibrosis-4 score (Fib-4), and platelet count.
Treatment failures were categorized as nonresponse (defined as detectable HCV viral load at the end of treatment), relapse (defined as an undetectable HCV viral load at the end of treatment with a subsequent positive test), and early termination (defined as a failure to complete the planned treatment regimen). Documented patient nonadherence, medical comorbidities that affected the treatment protocol, mental health diagnoses, and active social issues (defined as active or history of heavy alcohol use, active or history of illicit drug use, lack of social support, and homelessness) were noted.
Timeliness of delivery of care was measured in days. For the VA group, the wait time was defined as the date the consult for HCV treatment was placed to the date of the initial appointment with the HCV treatment provider. For the Choice group, the wait time was defined as the date the referral to the Choice program was made to the date of the initial appointment with the Choice HCP. Treatment regimens were evaluated for appropriateness based on guidelines from VACO and the American Association for the Study of Liver Diseases.9-11
Tests performed by Choice providers were evaluated for whether they were relevant to HCV care and whether similar data already were available from VALLHCS. Tests that were not indicated were identified as unnecessary costs incurred by the Choice program, as were tests that had to be repeated at the VA because of a lack of documentation from the Choice provider. All medications given inappropriately were considered added costs. Medicare reimbursement rates for the most applicable Current Procedural Terminology (CPT) code and VA national contract pricing for medications were used for calculations. This study was approved by the VALLHCS institutional review board.
Statistical Analysis
IBM (Armonk, NY) Statistical Package for Social Sciences software was used to evaluate for differences in Fib-4, platelet count, prevalence of cirrhosis, prevalence of medical comorbidities, prevalence of mental health comorbidities, prevalence of the social issues defined in the Methods section, time from referral to time of appointment date, and SVR12 rate between the VA and Choice groups.
Exclusions
There were 15 veterans in the VA group who had a wait time of > 100 days. Of these, 5 (33%) were initially Choice referrals, but due to negative interactions with the Choice provider, the veterans returned to VALLHCS for care. Two of the 15 (13%) did not keep appointments and were lost to follow up. Six of the 15 (40%) had medical comorbidities that required more immediate attention, so HCV treatment initiation was deliberately moved back. The final 2 veterans scheduled their appointments unusually far apart, artificially increasing their wait time. Given that these were unique situations and some of the veterans received care from both Choice and VA providers, a decision was made to exclude these individuals from the study.
It has been shown that platelet count correlates with degree of liver fibrosis, a concept that is the basis for the Fib-4 scoring system.12 Studies have shown that platelet count is a survival predictor in those with cirrhosis, and thrombocytopenia is a negative predictor of HCV treatment success using peginterferon and ribavirin13,14 Therefore, the VA memorandum automatically assigned the sickest individuals to the VA for HCV treatment. The goal of this study was to compare the impact of factors other than stage of fibrosis on HCV treatment success, which is why the 12 veterans with platelet count < 100,000 in the VA group were excluded. There were no veterans with platelet count < 100,000 in the Choice group.
When comparing SVR12 rates between the VA and Choice groups, every veteran treated at VALLHCS in FY 2016 was included, increasing the number in the VA group from 100 to 320 and therefore the power of this comparison.
Results
A summary of the statistical analysis can be found in Table 1. The genotype distribution was consistent with epidemiological studies, including those specific to veterans.15,16 There were statistically significant differences (P < .001) in mean Fib-4 and mean platelet count. The VA group had a higher Fib-4 and lower platelet count. Seventy-four percent of the VA population was defined as cirrhotic, while only 3% of the Choice population met similar criteria (P < .001). The VA and Choice groups were similar in terms of age, gender, and genotype distribution (Table 2).
The VA group was found to have a higher prevalence of comorbidities that affected HCV treatment. These conditions included but were not limited to: chronic kidney disease that precluded the use of certain medications, any condition that required medication with a known interaction with DAAs (ie, proton pump inhibitors, statins, and amiodarone), and cirrhosis if it impacted the treatment regimen. The difference in the prevalence of mental health comorbidities was not significant (P = .39), but there was a higher prevalence of social issues among the VA group (P = .002).
The mean wait time from referral to appointment was 28.6 days for the VA group and 42.3 days for the Choice group (P < .001), indicating that a Choice referral took longer to complete than a referral within the VA for HCV treatment. Thirty of the 71 (42%) veterans seen by a Choice provider accrued extraneous cost, with a mean additional cost of $8,561.40 per veteran. In the Choice group, 61 veterans completed a treatment regimen with the Choice HCP. Fifty-five veterans completed treatment and had available SVR12 data (6 were lost to follow up without SVR12 testing) and 50 (91%) had confirmed SVR12. The charts of the 5 treatment failures were reviewed to discern the cause for failure. Two cases involved early termination of therapy, 3 involved relapse and 2 failed to comply with medication instructions. There was 1 case of the Choice HCP not addressing simultaneous use of ledipasvir and a proton pump inhibitor, potentially causing an interaction, and 1 case where both the VA and Choice providers failed to recognize indicators of cirrhosis, which impacted the regimen used.
In the VALLHCS group, records of 320 veterans who completed treatment and had SVR12 testing were reviewed. While the Choice memorandum was active, veterans selected to be treated at VALLHCS had advanced liver fibrosis or cirrhosis, medical and mental health comorbidities that increased the risk of treatment complications or were considered to have difficulty adhering to the medication regimen. For this group, 296 (93%) had confirmed SVR12. Eighteen of the 24 (75%) treatment failures were complicated by nonadherence, including all 13 cases of early termination. One patient died from complications of decompensated cirrhosis before completing treatment, and 1 did not receive HCV medications during a hospital admission due to poor coordination of care between the VA inpatient and outpatient pharmacy services, leading to multiple missed doses.
The difference in SVR12 rates (ie, treatment failure rates), between the VA and Choice groups was not statistically significant (P = .61). None of the specific reasons for treatment failure had a statistically significant difference between groups. A treatment failure analysis is shown in Table 3, and Table 4 indicates the breakdown of treatment regimens.
Discussion
The Veterans Health Administration (VHA) is the largest integrated health care system in the US, consisting of 152 medical centers and > 1,700 sites of care. The VA has the potential to meet the health care needs of 21.6 million veterans. About 9 million veterans are enrolled in the VA system and 5.9 million received health care through VHA.17 However, every medical service cannot realistically be made available at every facility, and some veterans have difficulty gaining access to VHA care; distance and wait times have been well-publicized issues that need further exploration.18,19 The Choice program is an attempt to meet gaps in VA coverage using non-VA HCPs.
HCV infection is a specific diagnosis with national treatment guidelines and wellstudied treatments; it can be cured, with an evidence- based definition of cure. The VACO policy memorandum to refer less sick veterans to Choice while treating sicker veterans at the VA provided the opportunity to directly compare the quality of the 2 programs. The SVR12 rates of VALLHCS and Choice providers were comparable to the national average at the time, and while the difference in SVR12 rate was not significant, VALLHCS treated a significantly higher number of patients with cirrhosis because of the referral criteria.20
The significant difference in medical comorbidities between the VA and Choice groups was not surprising, partly because of the referral criteria. Cirrhosis can impact the treatment regimen, especially in regard to use of ribavirin. Since the presence of mental health comorbidities did not affect selection into the Choice group, it makes sense that there was no significant difference in prevalence between the groups.
VACO allowed veterans with HCV treatment plans that VA HCPs felt were too complicated for the Choice program to be treated by VHA HCPs.9 VALLHCS exercised this right for veterans at risk for nonadherence, because in HCV treatment, nonadherence leads to treatment failure and development of drug resistant virus strains. Therefore, veterans who would have difficulty traveling to VALLHCS to pick up medications, those who lacked means of communication (such as those who were homeless), and those who had active substance abuse were treated at the VA, where closer monitoring and immediate access to a wide range of services was possible. Studies have confirmed the impact of these types of issues on HCV treatment adherence and success. 21 This explains the higher prevalence of social issues in the VA group.
For an internal referral for HCV treatment at VALLHCS, the hepatology provider submits a consult request to the HCV treatment provider, who works in the same office, making direct communication simple. The main administrative limiting factor to minimizing wait times is the number of HCPs, which is dependent on hiring allowances.
When a veteran is referred to Choice, the VA provider places a consult for non-VA care, which the VA Office of Community Care processes by compiling relevant documents and sending the package to Triwest Healthcare Alliance, a private insurance processor contracted with the VA. Triwest selects the Choice provider, often without any input from the VA, and arranges the veteran’s initial appointment.22 Geographic distance to the veteran’s address is the main selection criteria for Triwest. Documents sent between the Choice and VA HCPs go through the Office of Community Care and Triwest. This significantly increases the potential for delays and failed communication. Triwest had a comprehensive list of providers deemed to be qualified to treat HCV within the geographic catchment of VALLHCS. This list was reviewed, and all veterans referred to Choice had HCPs near their home address; therefore, availability of Choice HCPs was not an issue.
The VA can provide guidance on management of the veteran in the form of bundle packages containing a list of services for which the Choice provider is authorized to provide, and ones the Choice provider is not authorized to provide. Some Choice HCPs ordered tests that were not authorized for HCV treatment such as esophagogastroduodenoscopy, colonoscopy, and liver biopsy. In all, 17 of 71 (24%) veterans seen by Choice HCPs had tests performed or ordered that VA HCPs would not have obtained for the purpose of HCV treatment (Table 5).
In order to prevent veterans from receiving unnecessary tests, a VALLHCS hepatologist asked to be notified by VA administrators overseeing Choice referrals whenever a secondary authorization request (SAR) was submitted by a Choice HCP. This strategy is not standard VA practice, therefore at many VA sites these requested tests would have been performed by the Choice HCP, which is why SARs were factored into cost analysis.
SVR12 test results that were drawn too early and had to be repeated at VALLHCS were a cost unique to the Choice program. Duplicate tests, particularly imaging studies and blood work, were extraneous costs. The largest extraneous costs were treatment regimens prescribed by Choice HCPs that did not follow standard of care and required VA provider intervention. Thirty of the 71 (42%) veterans seen by a Choice provider accrued a mean $8,561.40 in extra costs. As a result, the Choice program cost VHA $250,000 more to provide care for 30 veterans (enough to pay for a physician’s annual salary).
Some inappropriate treatment regimens were the result of Choice HCP error, such as 1 case in which a veteran was inadvertently switched from ledipasvir/sofosbuvir to ombitasvir/ paritaprevir/ritonavir/dasabuvir after week 4. The veteran had to start therapy over but still achieved SVR12. Other cases saw veterans receive regimens for which they had clear contraindications, such as creatinine clearance < 30 mL/min/1.73m2 for sofosbuvir or a positive resistance panel for specific medications. Eleven of 62 (18%) veterans who were started on HCV treatment by a Choice HCP received a regimen not consistent with VA guidelines—an alarming result.
Follow up for veterans referred to Choice was extremely labor intensive, and assessment of personnel requirements in a Choice-based VA system must take this into consideration. The Choice HCP has no obligation to communicate with the VA HCP. At the time of chart review, 57 of 71 (80%) Choice veterans had inadequate documentation to make a confident assessment of the treatment outcome. Multiple calls to the offices of the Choice HCP were needed to acquire records, and veterans had to be tracked down for additional tests. Veterans often would complete treatment and stop following up with the Choice provider before SVR12 confirmation. The VA hepatology provider reviewing Choice referrals served as clinician, case manager, and clerk in order to get veterans to an appropriate end point in their hepatitis C treatment, with mostly unmeasured hours of work.
Limitations
The study population size was limited by the number of veterans able to complete treatment through Choice. The parameters in the VACO policy memos automatically selected the VA and Choice groups but made them clinically distinct populations. New treatment medications were released during the study period, which impacted management strategy. Occasionally, VA and non- VA HCPs preferred different treatment regimens, leading to variation in the distribution of regimens used despite similar genotype distribution (Tables 2 and 4). In addition, a retrospective study is at risk for recall bias. A prospective study randomizing veterans to the Choice and VA groups is an important future endeavor. Comparing veteran satisfaction for Choice and VA services is also crucial.
Conclusions
This study demonstrates that the VA was able to provide more cost-effective and more timely care for HCV treatment to a relatively sicker population with no reduction in treatment success when compared with non-VA HCPs through the Choice program. While the Choice program can help veterans receive services they may otherwise not have access to and reduce travel time, the current system introduces inefficiencies that delay care and decrease cost-effectiveness. The Choice HCP selection process is based on proximity rather than quality, which may place the veteran at risk for receiving substandard care. Large-scale quality of care studies that compare efficiency measures, clinical outcomes, patient demographics, travel distance, cost efficacy and patient satisfaction for veterans receiving similar services at a VA facility and through Choice should be performed to ensure that veterans receive the best care available.
1. Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160(5):293-300. doi:10.7326/M13-1133
2. Dominitz JA, Boyko EJ, Koepsell TD, et al. Elevated prevalence of hepatitis C infection in users of United States veterans medical centers. Hepatology. 2005;41(1):88-96. doi:10.1002/hep.20502
3. Veterans Access, Choice, and Accountability Act of 2014. 42 USC §1395 (2014).
4. Tuchschmidt J. Attachment C: Provision of hepatitis C treatment. US Department of Veterans Affairs Central Office Memorandum from the Principal Deputy Under Secretary for Health. http://vaww.hepatitis.va.gov/education /choice-provision-hcv-treatment.asp. Published May 21, 2015. [Nonpublic site.]
5. Tuchschmidt J. Attachment A: Provision of hepatitis C (HCV) treatment through the Choice program. US Department of Veterans Affairs Central Office Memorandum from the Principal Deputy Under Secretary for Health. http:// vaww.hepatitis.va.gov/pdf/choice-attachment-a-FY16 .pdf. Published May 21, 2015. [Nonpublic site.]
6. Tuchschmidt J. Attachment B: Initiation of hepatitis C virus (HCV) treatment: protocol for prioritization. US Department of Veterans Affairs Central Office Memorandum from the Principal Deputy Under Secretary for Health. http://vaww .hepatitis.va.gov/pdf/provision-HCV-treatment-attach ment-b.pdf. Published May 21, 2015. [Nonpublic site.]
7. Murphy, JP. Hepatitis C virus funding and prioritization status. US Department of Veterans Affairs Central Office Memorandum from the Assistant Deputy Under Secretary for Health for Clinical Operations. http://vaww.hepatitis .va.gov/education/choice-memo-hcv-funding-and -prioritization-status-01272016.asp. Published January 27, 2016. [Nonpublic site.]
8. Lynch TJ, McCarthy MF. Hepatitis C virus funding and prioritization status update. US Department of Veterans Affairs Central Office Memorandum from the Assistant Deputy Under Secretary for Health for Clinical Operations and Acting Assistant Deputy Under Secretary for Health for Patient Care Services. http://vaww.hepatitis.va.gov /education/choice-funding-update-feb-2016.asp. Published February 24, 2016. [Nonpublic site.]
9. Morgan TR, Yee H; US Department of Veterans Affairs National Hepatitis C Resource Center Program and the National Viral Hepatitis Program in the Office of Patient Care Services. Chronic hepatitis C virus (HCV) infection: treatment considerations. http://vaww.hepatitis.va.gov /pdf/treatment-considerations-2016-03-28.pdf. Published March 28, 2016. [Nonpublic site.]
10. American Association for the Study of Liver Diseases; Infectious Diseases Society of America. Initial Treatment Box. http://hcvguidelines.org/full-report/initial-treatment -box-summary-recommendations-patients-who-are -initiating-therapy-hcv. Updated November 6, 2019. Accessed May 11, 2020.
11. AASLD/IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62(3): 932-954. doi:10.1002/hep.27950
12. Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006; 43(6):1317-1325. doi:10.1002/hep.21178
13. Realdi G, Fattovich G, Hadziyannis S, et al. Survival and prognostic factors in 366 patients with compensated cirrhosis type B: a multicenter study. The Investigators of the European Concerted Action on Viral Hepatitis (EUROHEP). J Hepatol. 1994;21(4):656-666. doi:10.1016/s0168 -8278(94)80115-0
14. Kanda T, Kato K, Tsubota A, et al. Platelet count and sustained virological response in hepatitis C treatment. World J Hepatol. 2013;5(4):182-188. doi:10.4254/wjh.v5.i4.182
15. Manos MM, Shvachko VA, Murphy RC, Arduino JM, Shire NJ. Distribution of hepatitis C virus genotypes in a diverse US integrated health care population. J Med Virol. 2012;84(11):1744-1750. doi:10.1002/jmv.23399
16. Cheung RC. Epidemiology of hepatitis C virus infection in American veterans. Am J Gastroenterol. 2000;95(3): 740-747. doi:10.1111/j.1572-0241.2000.01854.x
17. Bagalman E. The number of Veterans that use VA health care services: a fact sheet. Congressional Research Service Report R43579. https://fas.org/sgp/crs/misc/R43579.pdf. Published June 3, 2014. Accessed May 11, 2020.
18. US General Accounting Office. Report to the Ranking Minority Member, Subcommittee on Compensation, Pension, Insurance, and Memorial Affairs, Committee on Veterans’ Affairs, US House of Representatives. How distance from VA facilities affects veterans’ use of VA services. https:// www.gao.gov/assets/230/221992.pdf. Published December 1995. Accessed May 11, 2020.
19. Bronstein S, Griffin D. A fatal wait: Veterans languish and die on a VA hospital’s secret list. http://www.cnn .com/2014/04/23/health/veterans-dying-health-care -delays/index.html. Published April 23, 2014. Accessed May 11, 2020.
20. Ioannou GN, Beste LA, Chang MF, et al. Effectiveness of sofosbuvir, ledipasvir/sofosbuvir, or paritaprevir/ritonavir/ ombitasvir and dasabuvir regimens for treatment of patients with hepatitis C in the Veterans Affairs national health care system. Gastroenterology. 2016;151(3):457- 471. doi:10.1053/j.gastro.2016.05.049
21. Malespin MH, Harris C, Kanar O, et al. Barriers to treatment of chronic hepatitis C with direct acting antivirals in an urban clinic. Ann Hepatol. 2019;18(2):304-309. doi:10.1016/j.aohep.2018.06.001
22. Tuchschmidt J. Attachment D: Hepatitis C virus (HCV) fact sheet for Veterans Choice Program for both VA and Choice providers. US Department of Veterans Affairs Central Office Memorandum from the Deputy Under Secretary for Health for Policy and Services and the Acting Deputy Undersecretary for Health for Operations and Management. http://vaww .hepatitis.va.gov/educatiochoice-provision-HCV-treatment -additional.asp. [Nonpublic site.]
1. Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160(5):293-300. doi:10.7326/M13-1133
2. Dominitz JA, Boyko EJ, Koepsell TD, et al. Elevated prevalence of hepatitis C infection in users of United States veterans medical centers. Hepatology. 2005;41(1):88-96. doi:10.1002/hep.20502
3. Veterans Access, Choice, and Accountability Act of 2014. 42 USC §1395 (2014).
4. Tuchschmidt J. Attachment C: Provision of hepatitis C treatment. US Department of Veterans Affairs Central Office Memorandum from the Principal Deputy Under Secretary for Health. http://vaww.hepatitis.va.gov/education /choice-provision-hcv-treatment.asp. Published May 21, 2015. [Nonpublic site.]
5. Tuchschmidt J. Attachment A: Provision of hepatitis C (HCV) treatment through the Choice program. US Department of Veterans Affairs Central Office Memorandum from the Principal Deputy Under Secretary for Health. http:// vaww.hepatitis.va.gov/pdf/choice-attachment-a-FY16 .pdf. Published May 21, 2015. [Nonpublic site.]
6. Tuchschmidt J. Attachment B: Initiation of hepatitis C virus (HCV) treatment: protocol for prioritization. US Department of Veterans Affairs Central Office Memorandum from the Principal Deputy Under Secretary for Health. http://vaww .hepatitis.va.gov/pdf/provision-HCV-treatment-attach ment-b.pdf. Published May 21, 2015. [Nonpublic site.]
7. Murphy, JP. Hepatitis C virus funding and prioritization status. US Department of Veterans Affairs Central Office Memorandum from the Assistant Deputy Under Secretary for Health for Clinical Operations. http://vaww.hepatitis .va.gov/education/choice-memo-hcv-funding-and -prioritization-status-01272016.asp. Published January 27, 2016. [Nonpublic site.]
8. Lynch TJ, McCarthy MF. Hepatitis C virus funding and prioritization status update. US Department of Veterans Affairs Central Office Memorandum from the Assistant Deputy Under Secretary for Health for Clinical Operations and Acting Assistant Deputy Under Secretary for Health for Patient Care Services. http://vaww.hepatitis.va.gov /education/choice-funding-update-feb-2016.asp. Published February 24, 2016. [Nonpublic site.]
9. Morgan TR, Yee H; US Department of Veterans Affairs National Hepatitis C Resource Center Program and the National Viral Hepatitis Program in the Office of Patient Care Services. Chronic hepatitis C virus (HCV) infection: treatment considerations. http://vaww.hepatitis.va.gov /pdf/treatment-considerations-2016-03-28.pdf. Published March 28, 2016. [Nonpublic site.]
10. American Association for the Study of Liver Diseases; Infectious Diseases Society of America. Initial Treatment Box. http://hcvguidelines.org/full-report/initial-treatment -box-summary-recommendations-patients-who-are -initiating-therapy-hcv. Updated November 6, 2019. Accessed May 11, 2020.
11. AASLD/IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62(3): 932-954. doi:10.1002/hep.27950
12. Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006; 43(6):1317-1325. doi:10.1002/hep.21178
13. Realdi G, Fattovich G, Hadziyannis S, et al. Survival and prognostic factors in 366 patients with compensated cirrhosis type B: a multicenter study. The Investigators of the European Concerted Action on Viral Hepatitis (EUROHEP). J Hepatol. 1994;21(4):656-666. doi:10.1016/s0168 -8278(94)80115-0
14. Kanda T, Kato K, Tsubota A, et al. Platelet count and sustained virological response in hepatitis C treatment. World J Hepatol. 2013;5(4):182-188. doi:10.4254/wjh.v5.i4.182
15. Manos MM, Shvachko VA, Murphy RC, Arduino JM, Shire NJ. Distribution of hepatitis C virus genotypes in a diverse US integrated health care population. J Med Virol. 2012;84(11):1744-1750. doi:10.1002/jmv.23399
16. Cheung RC. Epidemiology of hepatitis C virus infection in American veterans. Am J Gastroenterol. 2000;95(3): 740-747. doi:10.1111/j.1572-0241.2000.01854.x
17. Bagalman E. The number of Veterans that use VA health care services: a fact sheet. Congressional Research Service Report R43579. https://fas.org/sgp/crs/misc/R43579.pdf. Published June 3, 2014. Accessed May 11, 2020.
18. US General Accounting Office. Report to the Ranking Minority Member, Subcommittee on Compensation, Pension, Insurance, and Memorial Affairs, Committee on Veterans’ Affairs, US House of Representatives. How distance from VA facilities affects veterans’ use of VA services. https:// www.gao.gov/assets/230/221992.pdf. Published December 1995. Accessed May 11, 2020.
19. Bronstein S, Griffin D. A fatal wait: Veterans languish and die on a VA hospital’s secret list. http://www.cnn .com/2014/04/23/health/veterans-dying-health-care -delays/index.html. Published April 23, 2014. Accessed May 11, 2020.
20. Ioannou GN, Beste LA, Chang MF, et al. Effectiveness of sofosbuvir, ledipasvir/sofosbuvir, or paritaprevir/ritonavir/ ombitasvir and dasabuvir regimens for treatment of patients with hepatitis C in the Veterans Affairs national health care system. Gastroenterology. 2016;151(3):457- 471. doi:10.1053/j.gastro.2016.05.049
21. Malespin MH, Harris C, Kanar O, et al. Barriers to treatment of chronic hepatitis C with direct acting antivirals in an urban clinic. Ann Hepatol. 2019;18(2):304-309. doi:10.1016/j.aohep.2018.06.001
22. Tuchschmidt J. Attachment D: Hepatitis C virus (HCV) fact sheet for Veterans Choice Program for both VA and Choice providers. US Department of Veterans Affairs Central Office Memorandum from the Deputy Under Secretary for Health for Policy and Services and the Acting Deputy Undersecretary for Health for Operations and Management. http://vaww .hepatitis.va.gov/educatiochoice-provision-HCV-treatment -additional.asp. [Nonpublic site.]
Nucleoside polymers show early promise in HBV
For patients with chronic hepatitis B virus (HBV) infection, triple-combination therapy with tenofovir disoproxil fumarate, pegylated interferon alfa-2a (TDF-pegIFN), and either of two investigational nucleic acid polymers was tolerable and led to long-term functional cures in an open-label phase 2 trial.
The addition of either REP 2139 or REP 2165 to backbone TDF-pegIFN therapy produced functional cures in 39% of patients without lessening HBV DNA control or exacerbating treatment-induced neutropenia or thrombocytopenia, said Michel Bazinet, MD, of Replicor in Montreal and his associates. “Increases in levels of transaminases were significantly more frequent (P < .001 vs. controls) and greater (P = .002 vs. controls) in the nucleic acid polymer groups but did not produce symptoms, correlated with [an] initial decrease in hepatitis B surface antigen [HBsAg], and normalized during therapy and follow-up,” the investigators wrote in Gastroenterology.
Nucleic acid polymers (NAPs) suppress the assembly and secretion of HBV subviral particles. NAP monotherapy is active against HBV but usually does not provide long-term virologic control. In a small study, adding pegIFN or thymosin alpha-1 to an investigational NAP achieved functional control (HBsAg positive, HBV DNA ≤ 2000 IU/mL, and normal alanine aminotransferase levels) in eight of nine patients.
Building on these findings, two triple-combination NAP regimens were evaluated in 40 noncirrhotic HB envelope antigen–negative adults with chronic HBV infection. After 24 weeks of TDF monotherapy, participants were randomly assigned to either 48 weeks of REP 2139 or REP 2165 plus backbone therapy with TDF and pegIFN, or 24 weeks of backbone therapy followed by 48 weeks of triple-combination treatment. Patients were then followed without treatment for 24-48 weeks.
Backbone TDF-pegIFN therapy produced no HBsAg seroconversions, and HBsAg levels dropped by more than 1 log10 IU/mL in only three patients. In contrast, triple-combination NAP therapy produced undetectable HBsAg and HBsAg seroconversions (up to 233,055 mIU/mL) for 60% of patients. Among 36 patients followed for 24-48 weeks after completing treatment, 78% maintained virologic control and 39% showed functional cures (HBsAg < 0.05 IU/mL, undetectable HBV DNA, and normal ALT). “Additional follow-up is planned to confirm the long-term stability of [these] outcomes,” the researchers said.
Both NAPs were formulated with chelated magnesium to improve their tolerability. Although 95% of patients experienced transaminase flares, these “self-resolved or declined during continuing NAP therapy and normalized in 32 of 34 (94%) of participants completing 48 weeks of follow-up,” the researchers said. In keeping with prior studies, transaminase flares were associated with early declines in HBsAg but not with altered liver function or liver disease symptoms.
The study was conducted at three sites in Maldova. Most participants were men with HBV genotype D infection. “During follow-up, viral rebound occurred in participants [in whom] HBsAg was still detectable at the end of 48 weeks of combination therapy (≥ 57.9 IU/mL), who did not complete therapy, or [for whom] HBsAg clearance occurred very late in therapy,” the researchers wrote. Thus, “persistent exposure to pegIFN while HBsAg is cleared may be important for the establishment of virologic control and functional cure.” They recommended evaluating NAP plus nucleos(t)ide analogue (NUC) therapy to assess response in the absence of pegIFN. Such studies should enroll “NUC-experienced participants with well-controlled HBV DNA.”
Replicor provided funding. Dr. Bazinet and the senior investigator reported that they are employees and shareholders of Replicor and have invented patents that Replicor holds. One coinvestigator reported compensation from Replicor to his institution. The remaining 11 coinvestigators reported having no relevant disclosures.
SOURCE: Bazinet M et al. Gastroenterology. 2020 Mar 5. doi: 0.1053/j.gastro.2020.02.058.
Manhal Izzy, MD, is assistant professor of medicine, Vanderbilt University Medical Center, division of gastroenterology, hepatology, and nutrition, and transplant hepatology at the Vanderbilt Clinic, Nashville, Tenn. He has no conflicts.
Manhal Izzy, MD, is assistant professor of medicine, Vanderbilt University Medical Center, division of gastroenterology, hepatology, and nutrition, and transplant hepatology at the Vanderbilt Clinic, Nashville, Tenn. He has no conflicts.
Manhal Izzy, MD, is assistant professor of medicine, Vanderbilt University Medical Center, division of gastroenterology, hepatology, and nutrition, and transplant hepatology at the Vanderbilt Clinic, Nashville, Tenn. He has no conflicts.
For patients with chronic hepatitis B virus (HBV) infection, triple-combination therapy with tenofovir disoproxil fumarate, pegylated interferon alfa-2a (TDF-pegIFN), and either of two investigational nucleic acid polymers was tolerable and led to long-term functional cures in an open-label phase 2 trial.
The addition of either REP 2139 or REP 2165 to backbone TDF-pegIFN therapy produced functional cures in 39% of patients without lessening HBV DNA control or exacerbating treatment-induced neutropenia or thrombocytopenia, said Michel Bazinet, MD, of Replicor in Montreal and his associates. “Increases in levels of transaminases were significantly more frequent (P < .001 vs. controls) and greater (P = .002 vs. controls) in the nucleic acid polymer groups but did not produce symptoms, correlated with [an] initial decrease in hepatitis B surface antigen [HBsAg], and normalized during therapy and follow-up,” the investigators wrote in Gastroenterology.
Nucleic acid polymers (NAPs) suppress the assembly and secretion of HBV subviral particles. NAP monotherapy is active against HBV but usually does not provide long-term virologic control. In a small study, adding pegIFN or thymosin alpha-1 to an investigational NAP achieved functional control (HBsAg positive, HBV DNA ≤ 2000 IU/mL, and normal alanine aminotransferase levels) in eight of nine patients.
Building on these findings, two triple-combination NAP regimens were evaluated in 40 noncirrhotic HB envelope antigen–negative adults with chronic HBV infection. After 24 weeks of TDF monotherapy, participants were randomly assigned to either 48 weeks of REP 2139 or REP 2165 plus backbone therapy with TDF and pegIFN, or 24 weeks of backbone therapy followed by 48 weeks of triple-combination treatment. Patients were then followed without treatment for 24-48 weeks.
Backbone TDF-pegIFN therapy produced no HBsAg seroconversions, and HBsAg levels dropped by more than 1 log10 IU/mL in only three patients. In contrast, triple-combination NAP therapy produced undetectable HBsAg and HBsAg seroconversions (up to 233,055 mIU/mL) for 60% of patients. Among 36 patients followed for 24-48 weeks after completing treatment, 78% maintained virologic control and 39% showed functional cures (HBsAg < 0.05 IU/mL, undetectable HBV DNA, and normal ALT). “Additional follow-up is planned to confirm the long-term stability of [these] outcomes,” the researchers said.
Both NAPs were formulated with chelated magnesium to improve their tolerability. Although 95% of patients experienced transaminase flares, these “self-resolved or declined during continuing NAP therapy and normalized in 32 of 34 (94%) of participants completing 48 weeks of follow-up,” the researchers said. In keeping with prior studies, transaminase flares were associated with early declines in HBsAg but not with altered liver function or liver disease symptoms.
The study was conducted at three sites in Maldova. Most participants were men with HBV genotype D infection. “During follow-up, viral rebound occurred in participants [in whom] HBsAg was still detectable at the end of 48 weeks of combination therapy (≥ 57.9 IU/mL), who did not complete therapy, or [for whom] HBsAg clearance occurred very late in therapy,” the researchers wrote. Thus, “persistent exposure to pegIFN while HBsAg is cleared may be important for the establishment of virologic control and functional cure.” They recommended evaluating NAP plus nucleos(t)ide analogue (NUC) therapy to assess response in the absence of pegIFN. Such studies should enroll “NUC-experienced participants with well-controlled HBV DNA.”
Replicor provided funding. Dr. Bazinet and the senior investigator reported that they are employees and shareholders of Replicor and have invented patents that Replicor holds. One coinvestigator reported compensation from Replicor to his institution. The remaining 11 coinvestigators reported having no relevant disclosures.
SOURCE: Bazinet M et al. Gastroenterology. 2020 Mar 5. doi: 0.1053/j.gastro.2020.02.058.
For patients with chronic hepatitis B virus (HBV) infection, triple-combination therapy with tenofovir disoproxil fumarate, pegylated interferon alfa-2a (TDF-pegIFN), and either of two investigational nucleic acid polymers was tolerable and led to long-term functional cures in an open-label phase 2 trial.
The addition of either REP 2139 or REP 2165 to backbone TDF-pegIFN therapy produced functional cures in 39% of patients without lessening HBV DNA control or exacerbating treatment-induced neutropenia or thrombocytopenia, said Michel Bazinet, MD, of Replicor in Montreal and his associates. “Increases in levels of transaminases were significantly more frequent (P < .001 vs. controls) and greater (P = .002 vs. controls) in the nucleic acid polymer groups but did not produce symptoms, correlated with [an] initial decrease in hepatitis B surface antigen [HBsAg], and normalized during therapy and follow-up,” the investigators wrote in Gastroenterology.
Nucleic acid polymers (NAPs) suppress the assembly and secretion of HBV subviral particles. NAP monotherapy is active against HBV but usually does not provide long-term virologic control. In a small study, adding pegIFN or thymosin alpha-1 to an investigational NAP achieved functional control (HBsAg positive, HBV DNA ≤ 2000 IU/mL, and normal alanine aminotransferase levels) in eight of nine patients.
Building on these findings, two triple-combination NAP regimens were evaluated in 40 noncirrhotic HB envelope antigen–negative adults with chronic HBV infection. After 24 weeks of TDF monotherapy, participants were randomly assigned to either 48 weeks of REP 2139 or REP 2165 plus backbone therapy with TDF and pegIFN, or 24 weeks of backbone therapy followed by 48 weeks of triple-combination treatment. Patients were then followed without treatment for 24-48 weeks.
Backbone TDF-pegIFN therapy produced no HBsAg seroconversions, and HBsAg levels dropped by more than 1 log10 IU/mL in only three patients. In contrast, triple-combination NAP therapy produced undetectable HBsAg and HBsAg seroconversions (up to 233,055 mIU/mL) for 60% of patients. Among 36 patients followed for 24-48 weeks after completing treatment, 78% maintained virologic control and 39% showed functional cures (HBsAg < 0.05 IU/mL, undetectable HBV DNA, and normal ALT). “Additional follow-up is planned to confirm the long-term stability of [these] outcomes,” the researchers said.
Both NAPs were formulated with chelated magnesium to improve their tolerability. Although 95% of patients experienced transaminase flares, these “self-resolved or declined during continuing NAP therapy and normalized in 32 of 34 (94%) of participants completing 48 weeks of follow-up,” the researchers said. In keeping with prior studies, transaminase flares were associated with early declines in HBsAg but not with altered liver function or liver disease symptoms.
The study was conducted at three sites in Maldova. Most participants were men with HBV genotype D infection. “During follow-up, viral rebound occurred in participants [in whom] HBsAg was still detectable at the end of 48 weeks of combination therapy (≥ 57.9 IU/mL), who did not complete therapy, or [for whom] HBsAg clearance occurred very late in therapy,” the researchers wrote. Thus, “persistent exposure to pegIFN while HBsAg is cleared may be important for the establishment of virologic control and functional cure.” They recommended evaluating NAP plus nucleos(t)ide analogue (NUC) therapy to assess response in the absence of pegIFN. Such studies should enroll “NUC-experienced participants with well-controlled HBV DNA.”
Replicor provided funding. Dr. Bazinet and the senior investigator reported that they are employees and shareholders of Replicor and have invented patents that Replicor holds. One coinvestigator reported compensation from Replicor to his institution. The remaining 11 coinvestigators reported having no relevant disclosures.
SOURCE: Bazinet M et al. Gastroenterology. 2020 Mar 5. doi: 0.1053/j.gastro.2020.02.058.
FROM GASTROENTEROLOGY
CDC: Screen nearly all adults, including pregnant women, for HCV
In the latest issue of the Morbidity and Mortality Weekly Report, the Centers for Disease Control and Prevention recommended hepatitis C virus screening for all adults and all pregnant women – during each of their pregnancies – in areas where prevalence of the infection is 0.1% or greater.
That’s essentially the entire United States; there’s no state with a statewide adult prevalence below 0.1%, and “few settings are known to exist” otherwise, the CDC noted (MMWR Recomm Rep. 2020 Apr 10;69(2):1-17).
The agency encouraged providers to consult state or local health departments or the CDC directly to determine local HCV prevalence. “As a general guide ... approximately 59% of anti-HCV positive persons are HCV RNA positive,” indicating active infection, the agency noted.
The advice was an expansion from the CDC’s last universal screening recommendation in 2012, which was limited to people born from 1945 to 1965; the incidence of acute infections has climbed since then and is highest now among younger people, so the guideline needed to be revisited, explained authors led by Sarah Schillie, MD, of the CDC’s Division of Viral Hepatitis, Atlanta.
The U.S. Preventive Services Task Force also recently recommended universal adult screening after previously limiting it to baby boomers.
As for pregnancy, the CDC’s past advice was to screen pregnant women with known risk factors, but that needed to be revisited as well. For one thing, the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America have since recommended testing all pregnant women.
But also, the CDC said, it’s an opportune time for screening because “many women only have access to health care during pregnancy and the immediate postpartum period,” when treatment, if needed, can be started. Plus, HCV status is important for management decisions, such as using amniocentesis in positive women instead of chorionic villus sampling.
The rest of CDC’s 2012 recommendations stand, including screening all people with risk factors and repeating screening while they persist. Also, “any person who requests hepatitis C testing should receive it, regardless of disclosure of risk,” because people might be reluctant to report things like IV drug use, the authors said.
Screening in the guidelines means an HCV antibody test, followed by a nucleic acid test to check for active infection. The CDC encouraged automatic reflex testing, meaning immediately checking antibody positive samples for HCV RNA. RNA in the blood indicates active, replicating virus.
The new recommendations penciled out in modeling, with an incremental cost-effectiveness ratio (ICER) for universal adult screening of approximately $36,000 per quality-adjusted life year (QALY) gained, and an ICER of approximately $15,000 per QALY gained for pregnancy screening, where HCV prevalence is 0.1%; the 0.1% cost/benefit cutpoint was one of the reasons it was chosen as the prevalence threshold. An ICER under $50,000 is the conservative benchmark for cost-effectiveness, the authors noted.
There was no external funding, and the authors had no disclosures.
SOURCE: Schillie S et al. MMWR Recomm Rep. 2020 Apr 10;69[2]:1-17).
In the latest issue of the Morbidity and Mortality Weekly Report, the Centers for Disease Control and Prevention recommended hepatitis C virus screening for all adults and all pregnant women – during each of their pregnancies – in areas where prevalence of the infection is 0.1% or greater.
That’s essentially the entire United States; there’s no state with a statewide adult prevalence below 0.1%, and “few settings are known to exist” otherwise, the CDC noted (MMWR Recomm Rep. 2020 Apr 10;69(2):1-17).
The agency encouraged providers to consult state or local health departments or the CDC directly to determine local HCV prevalence. “As a general guide ... approximately 59% of anti-HCV positive persons are HCV RNA positive,” indicating active infection, the agency noted.
The advice was an expansion from the CDC’s last universal screening recommendation in 2012, which was limited to people born from 1945 to 1965; the incidence of acute infections has climbed since then and is highest now among younger people, so the guideline needed to be revisited, explained authors led by Sarah Schillie, MD, of the CDC’s Division of Viral Hepatitis, Atlanta.
The U.S. Preventive Services Task Force also recently recommended universal adult screening after previously limiting it to baby boomers.
As for pregnancy, the CDC’s past advice was to screen pregnant women with known risk factors, but that needed to be revisited as well. For one thing, the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America have since recommended testing all pregnant women.
But also, the CDC said, it’s an opportune time for screening because “many women only have access to health care during pregnancy and the immediate postpartum period,” when treatment, if needed, can be started. Plus, HCV status is important for management decisions, such as using amniocentesis in positive women instead of chorionic villus sampling.
The rest of CDC’s 2012 recommendations stand, including screening all people with risk factors and repeating screening while they persist. Also, “any person who requests hepatitis C testing should receive it, regardless of disclosure of risk,” because people might be reluctant to report things like IV drug use, the authors said.
Screening in the guidelines means an HCV antibody test, followed by a nucleic acid test to check for active infection. The CDC encouraged automatic reflex testing, meaning immediately checking antibody positive samples for HCV RNA. RNA in the blood indicates active, replicating virus.
The new recommendations penciled out in modeling, with an incremental cost-effectiveness ratio (ICER) for universal adult screening of approximately $36,000 per quality-adjusted life year (QALY) gained, and an ICER of approximately $15,000 per QALY gained for pregnancy screening, where HCV prevalence is 0.1%; the 0.1% cost/benefit cutpoint was one of the reasons it was chosen as the prevalence threshold. An ICER under $50,000 is the conservative benchmark for cost-effectiveness, the authors noted.
There was no external funding, and the authors had no disclosures.
SOURCE: Schillie S et al. MMWR Recomm Rep. 2020 Apr 10;69[2]:1-17).
In the latest issue of the Morbidity and Mortality Weekly Report, the Centers for Disease Control and Prevention recommended hepatitis C virus screening for all adults and all pregnant women – during each of their pregnancies – in areas where prevalence of the infection is 0.1% or greater.
That’s essentially the entire United States; there’s no state with a statewide adult prevalence below 0.1%, and “few settings are known to exist” otherwise, the CDC noted (MMWR Recomm Rep. 2020 Apr 10;69(2):1-17).
The agency encouraged providers to consult state or local health departments or the CDC directly to determine local HCV prevalence. “As a general guide ... approximately 59% of anti-HCV positive persons are HCV RNA positive,” indicating active infection, the agency noted.
The advice was an expansion from the CDC’s last universal screening recommendation in 2012, which was limited to people born from 1945 to 1965; the incidence of acute infections has climbed since then and is highest now among younger people, so the guideline needed to be revisited, explained authors led by Sarah Schillie, MD, of the CDC’s Division of Viral Hepatitis, Atlanta.
The U.S. Preventive Services Task Force also recently recommended universal adult screening after previously limiting it to baby boomers.
As for pregnancy, the CDC’s past advice was to screen pregnant women with known risk factors, but that needed to be revisited as well. For one thing, the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America have since recommended testing all pregnant women.
But also, the CDC said, it’s an opportune time for screening because “many women only have access to health care during pregnancy and the immediate postpartum period,” when treatment, if needed, can be started. Plus, HCV status is important for management decisions, such as using amniocentesis in positive women instead of chorionic villus sampling.
The rest of CDC’s 2012 recommendations stand, including screening all people with risk factors and repeating screening while they persist. Also, “any person who requests hepatitis C testing should receive it, regardless of disclosure of risk,” because people might be reluctant to report things like IV drug use, the authors said.
Screening in the guidelines means an HCV antibody test, followed by a nucleic acid test to check for active infection. The CDC encouraged automatic reflex testing, meaning immediately checking antibody positive samples for HCV RNA. RNA in the blood indicates active, replicating virus.
The new recommendations penciled out in modeling, with an incremental cost-effectiveness ratio (ICER) for universal adult screening of approximately $36,000 per quality-adjusted life year (QALY) gained, and an ICER of approximately $15,000 per QALY gained for pregnancy screening, where HCV prevalence is 0.1%; the 0.1% cost/benefit cutpoint was one of the reasons it was chosen as the prevalence threshold. An ICER under $50,000 is the conservative benchmark for cost-effectiveness, the authors noted.
There was no external funding, and the authors had no disclosures.
SOURCE: Schillie S et al. MMWR Recomm Rep. 2020 Apr 10;69[2]:1-17).
AASLD: Liver transplants should proceed despite COVID-19
In liver transplant recipients or patients with autoimmune hepatitis on immunosuppressive therapy, acute cellular rejection or disease flare should not be presumed in the face of active coronavirus disease 2019 (COVID-19), according to the American Association for the Study of Liver Diseases (AASLD).
Signs that would normally be interpreted as flare or rejection need to be considered more cautiously now because the virus attacks the liver, and elevated aspartate aminotransferase, alanine aminotransferase, and slightly elevated bilirubin are common, ranging from a prevalence of 14% to 53% in COVID-19 patients. Acute liver injury is possible, especially in more severe cases, the group said.
The advice comes from a recently released document from AASLD, called “Clinical Insights for Hepatology and Liver Transplant Providers During the Covid-19 Pandemic,” to help hepatologists and liver transplant providers negotiate the pandemic, according to the latest data. It’s a far-ranging work that contains a lot of now familiar steps for providers to take to protect themselves and patients from the virus, but also much advice specific to liver medicine.
For instance, the group said it’s important to keep in mind that experimental treatments for the infection, including statins, remdesivir, and tocilizumab, can be hepatotoxic. Abnormal liver biochemistries are not a contraindication, but liver biochemistries need to be followed regularly in COVID-19 patients, especially those treated with remdesivir or tocilizumab, regardless of baseline values.
Also, lopinavir/ritonavir is a potent inhibitor of cytochrome P450 enzymes involved with calcineurin inhibitor metabolism, so if it’s used, AASLD said to reduce tacrolimus dosages to 1/20–1/50 of baseline.
The group cautioned against anticipatory adjustments to immunosuppressive drugs or dosages in patients without COVID-19, but if immunosuppressed liver disease patients do get the infection, prednisone doses should be reduced but kept above 10 mg/day to avoid adrenal insufficiency. In the setting of lymphopenia, fever, or worsening COVID-19 pneumonia, it advised reduction of azathioprine and mycophenolate dosages and reduction of, but not stopping, calcineurin inhibitors.
Liver transplants should not be postponed. However, to minimize exposure to the hospital environment, AASLD advised to “consider evaluating only patients with HCC [hepatocellular carcinoma] or those patients with severe disease and high MELD [model for end-stage liver disease] scores who are likely to benefit from immediate liver transplant.”
“An argument that has been put forward to justify deferring some transplants is concern about immunosuppressing patients during the COVID-19 pandemic,” the group said, but “data suggest the innate immune response may be the main driver for pulmonary injury due to COVID-19 and [that] immunosuppression may be protective. ... Posttransplant immunosuppression was not a risk factor for mortality associated with” the severe acute respiratory syndrome pandemic in 2003-2004 or the ongoing Middle East respiratory syndrome pandemic, both also caused by coronaviruses.
AASLD advised against reducing immunosuppression or stopping mycophenolate for asymptomatic patients after transplant, but COVID-19 prevention measures should be emphasized, including frequent hand washing and staying away from large crowds.
People who test positive for COVID-19 are ineligible for organ donation. Bronchoalveolar lavage is the most sensitive test (93%), followed by nasal swabs (63%) and pharyngeal swabs (32%).
In general, the group said elective procedures should be postponed, but urgent ones, such as biliary surgery and transjugular intrahepatic portosystemic shunts for bleeding varices, in addition to liver transplants, should not.
Also, HCC patients “should not wait until the pandemic abates to undergo [surveillance] imaging because the prospective duration of the pandemic is unknown. ... An arbitrary delay of 2 months is reasonable” for imaging based on patient and facility circumstances, but otherwise, “proceed with HCC treatments rather than delaying them due to the pandemic,” the group said.
As for who to bring into the office for an initial consult, “consider seeing in person only new adult and pediatric patients with urgent issues and clinically significant liver disease (e.g., jaundice, elevated ALT or AST above 500 U/L, recent onset of hepatic decompensation),” AASLD said.
In liver transplant recipients or patients with autoimmune hepatitis on immunosuppressive therapy, acute cellular rejection or disease flare should not be presumed in the face of active coronavirus disease 2019 (COVID-19), according to the American Association for the Study of Liver Diseases (AASLD).
Signs that would normally be interpreted as flare or rejection need to be considered more cautiously now because the virus attacks the liver, and elevated aspartate aminotransferase, alanine aminotransferase, and slightly elevated bilirubin are common, ranging from a prevalence of 14% to 53% in COVID-19 patients. Acute liver injury is possible, especially in more severe cases, the group said.
The advice comes from a recently released document from AASLD, called “Clinical Insights for Hepatology and Liver Transplant Providers During the Covid-19 Pandemic,” to help hepatologists and liver transplant providers negotiate the pandemic, according to the latest data. It’s a far-ranging work that contains a lot of now familiar steps for providers to take to protect themselves and patients from the virus, but also much advice specific to liver medicine.
For instance, the group said it’s important to keep in mind that experimental treatments for the infection, including statins, remdesivir, and tocilizumab, can be hepatotoxic. Abnormal liver biochemistries are not a contraindication, but liver biochemistries need to be followed regularly in COVID-19 patients, especially those treated with remdesivir or tocilizumab, regardless of baseline values.
Also, lopinavir/ritonavir is a potent inhibitor of cytochrome P450 enzymes involved with calcineurin inhibitor metabolism, so if it’s used, AASLD said to reduce tacrolimus dosages to 1/20–1/50 of baseline.
The group cautioned against anticipatory adjustments to immunosuppressive drugs or dosages in patients without COVID-19, but if immunosuppressed liver disease patients do get the infection, prednisone doses should be reduced but kept above 10 mg/day to avoid adrenal insufficiency. In the setting of lymphopenia, fever, or worsening COVID-19 pneumonia, it advised reduction of azathioprine and mycophenolate dosages and reduction of, but not stopping, calcineurin inhibitors.
Liver transplants should not be postponed. However, to minimize exposure to the hospital environment, AASLD advised to “consider evaluating only patients with HCC [hepatocellular carcinoma] or those patients with severe disease and high MELD [model for end-stage liver disease] scores who are likely to benefit from immediate liver transplant.”
“An argument that has been put forward to justify deferring some transplants is concern about immunosuppressing patients during the COVID-19 pandemic,” the group said, but “data suggest the innate immune response may be the main driver for pulmonary injury due to COVID-19 and [that] immunosuppression may be protective. ... Posttransplant immunosuppression was not a risk factor for mortality associated with” the severe acute respiratory syndrome pandemic in 2003-2004 or the ongoing Middle East respiratory syndrome pandemic, both also caused by coronaviruses.
AASLD advised against reducing immunosuppression or stopping mycophenolate for asymptomatic patients after transplant, but COVID-19 prevention measures should be emphasized, including frequent hand washing and staying away from large crowds.
People who test positive for COVID-19 are ineligible for organ donation. Bronchoalveolar lavage is the most sensitive test (93%), followed by nasal swabs (63%) and pharyngeal swabs (32%).
In general, the group said elective procedures should be postponed, but urgent ones, such as biliary surgery and transjugular intrahepatic portosystemic shunts for bleeding varices, in addition to liver transplants, should not.
Also, HCC patients “should not wait until the pandemic abates to undergo [surveillance] imaging because the prospective duration of the pandemic is unknown. ... An arbitrary delay of 2 months is reasonable” for imaging based on patient and facility circumstances, but otherwise, “proceed with HCC treatments rather than delaying them due to the pandemic,” the group said.
As for who to bring into the office for an initial consult, “consider seeing in person only new adult and pediatric patients with urgent issues and clinically significant liver disease (e.g., jaundice, elevated ALT or AST above 500 U/L, recent onset of hepatic decompensation),” AASLD said.
In liver transplant recipients or patients with autoimmune hepatitis on immunosuppressive therapy, acute cellular rejection or disease flare should not be presumed in the face of active coronavirus disease 2019 (COVID-19), according to the American Association for the Study of Liver Diseases (AASLD).
Signs that would normally be interpreted as flare or rejection need to be considered more cautiously now because the virus attacks the liver, and elevated aspartate aminotransferase, alanine aminotransferase, and slightly elevated bilirubin are common, ranging from a prevalence of 14% to 53% in COVID-19 patients. Acute liver injury is possible, especially in more severe cases, the group said.
The advice comes from a recently released document from AASLD, called “Clinical Insights for Hepatology and Liver Transplant Providers During the Covid-19 Pandemic,” to help hepatologists and liver transplant providers negotiate the pandemic, according to the latest data. It’s a far-ranging work that contains a lot of now familiar steps for providers to take to protect themselves and patients from the virus, but also much advice specific to liver medicine.
For instance, the group said it’s important to keep in mind that experimental treatments for the infection, including statins, remdesivir, and tocilizumab, can be hepatotoxic. Abnormal liver biochemistries are not a contraindication, but liver biochemistries need to be followed regularly in COVID-19 patients, especially those treated with remdesivir or tocilizumab, regardless of baseline values.
Also, lopinavir/ritonavir is a potent inhibitor of cytochrome P450 enzymes involved with calcineurin inhibitor metabolism, so if it’s used, AASLD said to reduce tacrolimus dosages to 1/20–1/50 of baseline.
The group cautioned against anticipatory adjustments to immunosuppressive drugs or dosages in patients without COVID-19, but if immunosuppressed liver disease patients do get the infection, prednisone doses should be reduced but kept above 10 mg/day to avoid adrenal insufficiency. In the setting of lymphopenia, fever, or worsening COVID-19 pneumonia, it advised reduction of azathioprine and mycophenolate dosages and reduction of, but not stopping, calcineurin inhibitors.
Liver transplants should not be postponed. However, to minimize exposure to the hospital environment, AASLD advised to “consider evaluating only patients with HCC [hepatocellular carcinoma] or those patients with severe disease and high MELD [model for end-stage liver disease] scores who are likely to benefit from immediate liver transplant.”
“An argument that has been put forward to justify deferring some transplants is concern about immunosuppressing patients during the COVID-19 pandemic,” the group said, but “data suggest the innate immune response may be the main driver for pulmonary injury due to COVID-19 and [that] immunosuppression may be protective. ... Posttransplant immunosuppression was not a risk factor for mortality associated with” the severe acute respiratory syndrome pandemic in 2003-2004 or the ongoing Middle East respiratory syndrome pandemic, both also caused by coronaviruses.
AASLD advised against reducing immunosuppression or stopping mycophenolate for asymptomatic patients after transplant, but COVID-19 prevention measures should be emphasized, including frequent hand washing and staying away from large crowds.
People who test positive for COVID-19 are ineligible for organ donation. Bronchoalveolar lavage is the most sensitive test (93%), followed by nasal swabs (63%) and pharyngeal swabs (32%).
In general, the group said elective procedures should be postponed, but urgent ones, such as biliary surgery and transjugular intrahepatic portosystemic shunts for bleeding varices, in addition to liver transplants, should not.
Also, HCC patients “should not wait until the pandemic abates to undergo [surveillance] imaging because the prospective duration of the pandemic is unknown. ... An arbitrary delay of 2 months is reasonable” for imaging based on patient and facility circumstances, but otherwise, “proceed with HCC treatments rather than delaying them due to the pandemic,” the group said.
As for who to bring into the office for an initial consult, “consider seeing in person only new adult and pediatric patients with urgent issues and clinically significant liver disease (e.g., jaundice, elevated ALT or AST above 500 U/L, recent onset of hepatic decompensation),” AASLD said.
HCV screening risk factors in pregnant women need updating
“Because risk-factor screening has obvious limitations, universal screening in pregnancy has been suggested to allow for linkage to postpartum care and identification of children for future testing and treatment,” wrote Mona Prasad, DO, of Ohio State University, Columbus, and colleagues.
In a study published in Obstetrics & Gynecology, the researchers reviewed data from women with singleton pregnancies presenting for prenatal care prior to 23 weeks’ gestation during 2012-2015. Of these, 254 tested positive for the hepatitis C virus (HCV) antibody, for a seroprevalence rate of 2.4 cases per 1,000 women.
The researchers conducted a case-control analysis of 131 women who tested positive and 251 controls to identify HCV infection risk factors based on interviews and chart reviews. They found that risk factors significantly associated with positive HCV antibodies included injection drug use (adjusted odds ratio, 22.9), a history of blood transfusion (aOR, 3.7), having an HCV-infected partner (aOR, 6.3), having had more than three sexual partners (aOR, 5.3), and smoking during pregnancy (aOR, 2.4).
In an unadjusted analysis, the researchers confirmed two of the risk factors currently recommended by the Centers for Disease Control and Prevention for screening for HCV: injection drug use and being born to a mother with HCV infection, but not dialysis, organ transplantation, or HIV infection.
“Our results demonstrate that current risk factors could be contemporized,” Dr. Prasad and colleagues noted. “The currently accepted risk factors such as exposure to clotting factors, dialysis, and organ transplants are unlikely to be found. A thorough assessment of injection drug use history, smoking, transfusions, number of sexual partners, and partners with HCV infection is more sensitive in an obstetric population.”
The study findings were limited by several factors including possible selection bias and inclusion of only 65% of eligible women who were HCV positive, as well as a lack of screening data from 2016 to the present, which may not reflect the impact of the recent opioid epidemic, the researchers noted. However, the results were strengthened by the large sample size, and the generalizability of the study population.
“Our results regarding prevalence rates and risk factors of HCV antibody among pregnant women in the United States will be valuable to policymakers as they weigh the costs and benefits of universal screening,” Dr. Prasad and associates concluded.
Although universal screening has the potential to be more cost effective, given the small population of pregnant women eligible for treatment and lack of an available treatment, “the rationale is weaker for unique universal HCV screening recommendations for pregnant women,” they said.
By contrast, Sammy Saab, MD, MPH, of the University of California, Los Angeles; Ravina Kullar, PharmD, MPH, of Gilead Sciences, Foster City, Calif.; and Prabhu Gounder, MD, MPH, of the Los Angeles Department of Public Health, wrote an accompanying commentary in favor of universal HCV screening for pregnant women, in part because of the increase in HCV in the younger population overall.
“For many women of reproductive age, pregnancy is one of their few points of contact with their health care provider; therefore, pregnancy could provide a crucial time for targeting this population,” they noted.
Risk-based screening is of limited effectiveness because patients are not identified by way of current screening tools or they decline to reveal risk factors that providers might miss, the editorialists said. Pregnancy has not been shown to affect the accuracy of HCV tests, and identifying infections in mothers allows for screening in children as well.
“The perinatal hepatitis B virus infection program, which has been implemented in several state and local public health departments, could serve as an example for how to conduct surveillance for mothers with HCV infection and to ensure that HCV-exposed children receive appropriate follow-up testing and linkage to care,” the editorialists concluded.
The study was supported in part by multiple grants from the National Institute of Child Health and Human Development. Dr. Prasad disclosed funding from Ohio State University and from Gilead. Coauthors had links with pharmaceutical companies, associations, and organizations – most unrelated to this study. The editorialists had no financial conflicts to disclose.
SOURCES: Prasad M et al. Obstet Gynecol. 2020;135:778-88; Saab S et al. Obstet Gynecol. 2020;135:773-7.
“Because risk-factor screening has obvious limitations, universal screening in pregnancy has been suggested to allow for linkage to postpartum care and identification of children for future testing and treatment,” wrote Mona Prasad, DO, of Ohio State University, Columbus, and colleagues.
In a study published in Obstetrics & Gynecology, the researchers reviewed data from women with singleton pregnancies presenting for prenatal care prior to 23 weeks’ gestation during 2012-2015. Of these, 254 tested positive for the hepatitis C virus (HCV) antibody, for a seroprevalence rate of 2.4 cases per 1,000 women.
The researchers conducted a case-control analysis of 131 women who tested positive and 251 controls to identify HCV infection risk factors based on interviews and chart reviews. They found that risk factors significantly associated with positive HCV antibodies included injection drug use (adjusted odds ratio, 22.9), a history of blood transfusion (aOR, 3.7), having an HCV-infected partner (aOR, 6.3), having had more than three sexual partners (aOR, 5.3), and smoking during pregnancy (aOR, 2.4).
In an unadjusted analysis, the researchers confirmed two of the risk factors currently recommended by the Centers for Disease Control and Prevention for screening for HCV: injection drug use and being born to a mother with HCV infection, but not dialysis, organ transplantation, or HIV infection.
“Our results demonstrate that current risk factors could be contemporized,” Dr. Prasad and colleagues noted. “The currently accepted risk factors such as exposure to clotting factors, dialysis, and organ transplants are unlikely to be found. A thorough assessment of injection drug use history, smoking, transfusions, number of sexual partners, and partners with HCV infection is more sensitive in an obstetric population.”
The study findings were limited by several factors including possible selection bias and inclusion of only 65% of eligible women who were HCV positive, as well as a lack of screening data from 2016 to the present, which may not reflect the impact of the recent opioid epidemic, the researchers noted. However, the results were strengthened by the large sample size, and the generalizability of the study population.
“Our results regarding prevalence rates and risk factors of HCV antibody among pregnant women in the United States will be valuable to policymakers as they weigh the costs and benefits of universal screening,” Dr. Prasad and associates concluded.
Although universal screening has the potential to be more cost effective, given the small population of pregnant women eligible for treatment and lack of an available treatment, “the rationale is weaker for unique universal HCV screening recommendations for pregnant women,” they said.
By contrast, Sammy Saab, MD, MPH, of the University of California, Los Angeles; Ravina Kullar, PharmD, MPH, of Gilead Sciences, Foster City, Calif.; and Prabhu Gounder, MD, MPH, of the Los Angeles Department of Public Health, wrote an accompanying commentary in favor of universal HCV screening for pregnant women, in part because of the increase in HCV in the younger population overall.
“For many women of reproductive age, pregnancy is one of their few points of contact with their health care provider; therefore, pregnancy could provide a crucial time for targeting this population,” they noted.
Risk-based screening is of limited effectiveness because patients are not identified by way of current screening tools or they decline to reveal risk factors that providers might miss, the editorialists said. Pregnancy has not been shown to affect the accuracy of HCV tests, and identifying infections in mothers allows for screening in children as well.
“The perinatal hepatitis B virus infection program, which has been implemented in several state and local public health departments, could serve as an example for how to conduct surveillance for mothers with HCV infection and to ensure that HCV-exposed children receive appropriate follow-up testing and linkage to care,” the editorialists concluded.
The study was supported in part by multiple grants from the National Institute of Child Health and Human Development. Dr. Prasad disclosed funding from Ohio State University and from Gilead. Coauthors had links with pharmaceutical companies, associations, and organizations – most unrelated to this study. The editorialists had no financial conflicts to disclose.
SOURCES: Prasad M et al. Obstet Gynecol. 2020;135:778-88; Saab S et al. Obstet Gynecol. 2020;135:773-7.
“Because risk-factor screening has obvious limitations, universal screening in pregnancy has been suggested to allow for linkage to postpartum care and identification of children for future testing and treatment,” wrote Mona Prasad, DO, of Ohio State University, Columbus, and colleagues.
In a study published in Obstetrics & Gynecology, the researchers reviewed data from women with singleton pregnancies presenting for prenatal care prior to 23 weeks’ gestation during 2012-2015. Of these, 254 tested positive for the hepatitis C virus (HCV) antibody, for a seroprevalence rate of 2.4 cases per 1,000 women.
The researchers conducted a case-control analysis of 131 women who tested positive and 251 controls to identify HCV infection risk factors based on interviews and chart reviews. They found that risk factors significantly associated with positive HCV antibodies included injection drug use (adjusted odds ratio, 22.9), a history of blood transfusion (aOR, 3.7), having an HCV-infected partner (aOR, 6.3), having had more than three sexual partners (aOR, 5.3), and smoking during pregnancy (aOR, 2.4).
In an unadjusted analysis, the researchers confirmed two of the risk factors currently recommended by the Centers for Disease Control and Prevention for screening for HCV: injection drug use and being born to a mother with HCV infection, but not dialysis, organ transplantation, or HIV infection.
“Our results demonstrate that current risk factors could be contemporized,” Dr. Prasad and colleagues noted. “The currently accepted risk factors such as exposure to clotting factors, dialysis, and organ transplants are unlikely to be found. A thorough assessment of injection drug use history, smoking, transfusions, number of sexual partners, and partners with HCV infection is more sensitive in an obstetric population.”
The study findings were limited by several factors including possible selection bias and inclusion of only 65% of eligible women who were HCV positive, as well as a lack of screening data from 2016 to the present, which may not reflect the impact of the recent opioid epidemic, the researchers noted. However, the results were strengthened by the large sample size, and the generalizability of the study population.
“Our results regarding prevalence rates and risk factors of HCV antibody among pregnant women in the United States will be valuable to policymakers as they weigh the costs and benefits of universal screening,” Dr. Prasad and associates concluded.
Although universal screening has the potential to be more cost effective, given the small population of pregnant women eligible for treatment and lack of an available treatment, “the rationale is weaker for unique universal HCV screening recommendations for pregnant women,” they said.
By contrast, Sammy Saab, MD, MPH, of the University of California, Los Angeles; Ravina Kullar, PharmD, MPH, of Gilead Sciences, Foster City, Calif.; and Prabhu Gounder, MD, MPH, of the Los Angeles Department of Public Health, wrote an accompanying commentary in favor of universal HCV screening for pregnant women, in part because of the increase in HCV in the younger population overall.
“For many women of reproductive age, pregnancy is one of their few points of contact with their health care provider; therefore, pregnancy could provide a crucial time for targeting this population,” they noted.
Risk-based screening is of limited effectiveness because patients are not identified by way of current screening tools or they decline to reveal risk factors that providers might miss, the editorialists said. Pregnancy has not been shown to affect the accuracy of HCV tests, and identifying infections in mothers allows for screening in children as well.
“The perinatal hepatitis B virus infection program, which has been implemented in several state and local public health departments, could serve as an example for how to conduct surveillance for mothers with HCV infection and to ensure that HCV-exposed children receive appropriate follow-up testing and linkage to care,” the editorialists concluded.
The study was supported in part by multiple grants from the National Institute of Child Health and Human Development. Dr. Prasad disclosed funding from Ohio State University and from Gilead. Coauthors had links with pharmaceutical companies, associations, and organizations – most unrelated to this study. The editorialists had no financial conflicts to disclose.
SOURCES: Prasad M et al. Obstet Gynecol. 2020;135:778-88; Saab S et al. Obstet Gynecol. 2020;135:773-7.
FROM OBSTETRICS & GYNECOLOGY