User login
Stick with the full 12-week DAA course for acute HCV
The first randomized trial to see if a short course of a direct-acting antiviral works as well for acute hepatitis C virus (HCV) infection as the standard 12-week course was stopped early after it became clear that it did not, according to a report at the Conference on Retroviruses & Opportunistic Infections.
In the end, 6 weeks of sofosbuvir-velpatasvir (Epclusa) “was inferior” to 12 weeks, said investigators led by Gail Matthews, MD, PhD, an associate professor in the Viral Hepatitis Clinical Research Program at the Kirby Institute, in Sydney, New South Wales, Australia.
Guidelines recommend 12 weeks of direct-acting antiviral treatment, but a few observational studies have suggested that 6 weeks might be enough. Since that would make it easier for physicians and patients, and would save money, Dr. Matthews and her team set out to resolve the uncertainty with a randomized trial.
Enrollment was halted short of the 250 target because of an “unacceptably high” relapse rate of 9.7% among 93 people randomized to 6 weeks of sofosbuvir-velpatasvir versus 2% among 99 subjects randomized to the standard 12-week regimen. All the relapse patients except for one in the 12-week arm were more than 95% adherent to treatment, she at the meeting, which was scheduled to be in Boston, but was held online this year because of concerns about spreading the COVID-19 virus.
There were 17 treatment failures (18.3%) in the short arm: two deaths, three reinfections, three lost to follow-up, and the nine relapses 12 weeks out from the end of treatment. There were eight failures (8%) in the long arm, including two reinfections, two lost to follow-up, and the two relapses, but no deaths. Excluding patients with no virologic reason for failure, Dr. Matthews said, “we see the difference in the two arms even more clearly,” with viral RNA undetectable in 98% of the 12-week patients – which is in keeping with label data – versus 89% in the short arm.
The groups were well balanced. Almost all the subjects were men and the majority were white; the median age was 43 years. Almost two-thirds had a primary infection at baseline and HCV genotype 1 a/b was the most common in both groups. Patients had been infected for a year or less, with a median of 25 weeks.
The majority of subjects picked up the virus through homosexual sex, but about 20% by injection drug use. Over two-thirds had well-controlled HIV. There were no treatment related discontinuations, and all the relapsed patients were successfully treated with subsequent therapy, Dr. Matthews said.
The study was conducted in the United States, Europe, Canada, New Zealand, and Australia, and funded by the National Institutes of Health. Dr. Matthews reported research grants to her institution form Abbvie and Gilead, maker of Epclusa.
SOURCE: Matthews G. CROI 2020 abstract 121.
The first randomized trial to see if a short course of a direct-acting antiviral works as well for acute hepatitis C virus (HCV) infection as the standard 12-week course was stopped early after it became clear that it did not, according to a report at the Conference on Retroviruses & Opportunistic Infections.
In the end, 6 weeks of sofosbuvir-velpatasvir (Epclusa) “was inferior” to 12 weeks, said investigators led by Gail Matthews, MD, PhD, an associate professor in the Viral Hepatitis Clinical Research Program at the Kirby Institute, in Sydney, New South Wales, Australia.
Guidelines recommend 12 weeks of direct-acting antiviral treatment, but a few observational studies have suggested that 6 weeks might be enough. Since that would make it easier for physicians and patients, and would save money, Dr. Matthews and her team set out to resolve the uncertainty with a randomized trial.
Enrollment was halted short of the 250 target because of an “unacceptably high” relapse rate of 9.7% among 93 people randomized to 6 weeks of sofosbuvir-velpatasvir versus 2% among 99 subjects randomized to the standard 12-week regimen. All the relapse patients except for one in the 12-week arm were more than 95% adherent to treatment, she at the meeting, which was scheduled to be in Boston, but was held online this year because of concerns about spreading the COVID-19 virus.
There were 17 treatment failures (18.3%) in the short arm: two deaths, three reinfections, three lost to follow-up, and the nine relapses 12 weeks out from the end of treatment. There were eight failures (8%) in the long arm, including two reinfections, two lost to follow-up, and the two relapses, but no deaths. Excluding patients with no virologic reason for failure, Dr. Matthews said, “we see the difference in the two arms even more clearly,” with viral RNA undetectable in 98% of the 12-week patients – which is in keeping with label data – versus 89% in the short arm.
The groups were well balanced. Almost all the subjects were men and the majority were white; the median age was 43 years. Almost two-thirds had a primary infection at baseline and HCV genotype 1 a/b was the most common in both groups. Patients had been infected for a year or less, with a median of 25 weeks.
The majority of subjects picked up the virus through homosexual sex, but about 20% by injection drug use. Over two-thirds had well-controlled HIV. There were no treatment related discontinuations, and all the relapsed patients were successfully treated with subsequent therapy, Dr. Matthews said.
The study was conducted in the United States, Europe, Canada, New Zealand, and Australia, and funded by the National Institutes of Health. Dr. Matthews reported research grants to her institution form Abbvie and Gilead, maker of Epclusa.
SOURCE: Matthews G. CROI 2020 abstract 121.
The first randomized trial to see if a short course of a direct-acting antiviral works as well for acute hepatitis C virus (HCV) infection as the standard 12-week course was stopped early after it became clear that it did not, according to a report at the Conference on Retroviruses & Opportunistic Infections.
In the end, 6 weeks of sofosbuvir-velpatasvir (Epclusa) “was inferior” to 12 weeks, said investigators led by Gail Matthews, MD, PhD, an associate professor in the Viral Hepatitis Clinical Research Program at the Kirby Institute, in Sydney, New South Wales, Australia.
Guidelines recommend 12 weeks of direct-acting antiviral treatment, but a few observational studies have suggested that 6 weeks might be enough. Since that would make it easier for physicians and patients, and would save money, Dr. Matthews and her team set out to resolve the uncertainty with a randomized trial.
Enrollment was halted short of the 250 target because of an “unacceptably high” relapse rate of 9.7% among 93 people randomized to 6 weeks of sofosbuvir-velpatasvir versus 2% among 99 subjects randomized to the standard 12-week regimen. All the relapse patients except for one in the 12-week arm were more than 95% adherent to treatment, she at the meeting, which was scheduled to be in Boston, but was held online this year because of concerns about spreading the COVID-19 virus.
There were 17 treatment failures (18.3%) in the short arm: two deaths, three reinfections, three lost to follow-up, and the nine relapses 12 weeks out from the end of treatment. There were eight failures (8%) in the long arm, including two reinfections, two lost to follow-up, and the two relapses, but no deaths. Excluding patients with no virologic reason for failure, Dr. Matthews said, “we see the difference in the two arms even more clearly,” with viral RNA undetectable in 98% of the 12-week patients – which is in keeping with label data – versus 89% in the short arm.
The groups were well balanced. Almost all the subjects were men and the majority were white; the median age was 43 years. Almost two-thirds had a primary infection at baseline and HCV genotype 1 a/b was the most common in both groups. Patients had been infected for a year or less, with a median of 25 weeks.
The majority of subjects picked up the virus through homosexual sex, but about 20% by injection drug use. Over two-thirds had well-controlled HIV. There were no treatment related discontinuations, and all the relapsed patients were successfully treated with subsequent therapy, Dr. Matthews said.
The study was conducted in the United States, Europe, Canada, New Zealand, and Australia, and funded by the National Institutes of Health. Dr. Matthews reported research grants to her institution form Abbvie and Gilead, maker of Epclusa.
SOURCE: Matthews G. CROI 2020 abstract 121.
FROM CROI 2020
Screen all adults for hepatitis C, says USPSTF
Adults aged 18-79 years should be screened for hepatitis C virus infection, according to an updated grade B recommendation from the U.S. Preventive Services Task Force.
Cases of acute hepatitis C virus (HCV) infection have spiked in the last decade, in part because of increased use of injection drugs and in part because of better surveillance, Douglas K. Owens, MD, of Stanford (Calif.) University, and colleagues wrote in the recommendation statement published in JAMA.
The recommendation applies to all asymptomatic adults aged 18-79 years without known liver disease, and expands on the 2013 recommendation to screen adults born between 1945 and 1965. The grade B designation means that the task force concluded with moderate certainty that HCV screening for adults aged 18-79 years had “substantial net benefit.”
The recommendations are based on an evidence report including 8 randomized, controlled trials, 48 other treatment studies, and 33 cohort studies published through February 2019 for a total of 179,230 individuals.
The screening is a one-time procedure for most adults, according to the task force, but clinicians should periodically screen individuals at increased risk, such as those with a past or current history of injection drug use. In addition, clinicians should consider screening individuals at increased risk who are above or below the recommended age range.
Although the task force identified no direct evidence on the benefit of screening for HCV infection in asymptomatic adults, a notable finding was that the newer direct-acting antiviral (DAA) regimens are sufficiently effective to support the expanded screening recommendation, they said. However, clinicians should inform patients that screening is voluntary and conducted only with the patient’s knowledge. Clinicians should educate patients about hepatitis C and give them an opportunity to ask questions and to make a decision about screening, according to the task force.
In the evidence report, a total of 49 studies including 10,181 individuals showed DAA treatment associated with pooled sustained virologic response rates greater than 95% across all virus genotypes, and a short-term serious adverse event rate of 1.9%. In addition, sustained virologic response following an antiviral therapy was associated with a reduction in risk of all-cause mortality (pooled hazard ratio 0.40) and of hepatocellular carcinoma (pooled HR 0.29) compared with cases of no sustained virologic response.
The evidence report findings were limited by several factors, including the relatively small number of randomized trials involving current DAA treatments, limited data on baseline symptoms, limited data on adolescents, and limited evidence on potential long-term harms of DAA therapy, noted Richard Chou, MD, of Oregon Health & Science University, Portland, and colleagues. However, new pooled evidence “indicates that SVR rates with currently recommended all-oral DAA regimens are substantially higher (more than 95%) than with interferon-based therapies evaluated in the prior review (68%-78%),” they said.
Several editorials were published concurrently with the recommendation.
In an editorial published in JAMA, Camilla S. Graham, MD, of Harvard Medical School, Boston, and Stacey Trooskin, MD, of the University of Pennsylvania, Philadelphia, wrote that the new recommendation reflects changes in hepatitis C virus management.
“With the approvals of sofosbuvir and simeprevir in 2013, patients with hepatitis C, a chronic viral illness associated with the deaths of more U.S. patients than the next 60 reportable infectious diseases combined, including HIV and tuberculosis, could expect a greater than 90% rate of achieving sustained virologic response (SVR, defined as undetectable HCV levels 12 weeks or longer after treatment completion, which is consistent with virologic cure of HCV infection) following 12 weeks of treatment,” they said.
These medications are effective but expensive; however, the combination of the availability of generic medications and the ongoing opioid epidemic in the United States are important contributors to the expanded recommendations, which “are welcome,” and may help meeting WHO 2030 targets for reducing new HCV infections, they said.
Dr. Graham disclosed personal fees from UpToDate. Dr. Trooskin disclosed grants from Gilead Sciences and personal fees from Merck, AbbVie, and Gilead Sciences.
In an editorial published in JAMA Internal Medicine, Jennifer C. Price, MD, and Danielle Brandman, MD, both of the University of California, San Francisco, wrote that “the advancements in HCV diagnosis and treatment have been extraordinary,” but that the new recommendation does not go far enough. “Implementation of HCV screening and linkage to treatment requires large-scale coordinated efforts, innovation, and resources. For example, point-of-care HCV RNA testing would enable scale-up of HCV screening and confirmatory testing among individuals at greatest risk of HCV infection,” they said. “Additionally, barriers remain between diagnosis and treatment, such as access to a health care provider who can treat HCV and authorization to receive affordable DAAs,” they noted. “Although the USPSTF HCV screening recommendation is a step forward for controlling HCV infection in the U.S., it will take a coordinated and funded effort to ensure that the anticipated benefits are realized,” they concluded.
Dr. Price disclosed research funding from Gilead Sciences and Merck. Dr. Brandman disclosed research funding from Gilead Sciences, Pfizer, Conatus, Allergan, and Grifols, as well as personal fees from Alnylam.
In an editorial published in JAMA Network Open, Eli S. Rosenberg, PhD, of the University at Albany (N.Y.) School of Public Health, and Joshua A. Barocas, MD, of Boston University, emphasized the need to change the stigma surrounding HCV infection in the United States.
“Given the changing epidemiology of HCV infection, new public health priorities, advancements in treatment, and unmet diagnostic needs, it is wise to periodically reevaluate screening recommendations to ensure that they are maximally addressing these areas and patients’ individual needs,” they said. “The Affordable Care Act requires private insurers and Medicaid to cover preventive services recommended by the USPSTF with a grade of A or B with no cost sharing (i.e., no deductible or copayment),” they noted. Although the new recommendation for one-time screening will likely identify more cases, improve outcomes, and reduce deaths, the editorialists cautioned that “one-time screening should not be interpreted like catch-up vaccinations, whereby we immunize someone at any age for hepatitis B virus, for example, and they are then immunized for the remainder of their life,” and that reassessments are needed, especially for younger adults.
In addition, they emphasized the need to reduce the stigma surrounding HCV and allow for recommendations based on risk, rather than age. “We have forced the USPSTF to adopt age-based screening recommendations because we, as a society, have created a culture in which we have stigmatized these behaviors and we, as practitioners, have proven to be inadequate at eliciting HCV risk behaviors,” they said. “Our responsibility as a society and practice community is to address structural and individual factors that limit our ability to most precisely address the needs of our patients and truly move toward HCV elimination,” they concluded.
The USPSTF is supported by the Agency for Healthcare Research and Quality. The task force researchers had no financial conflicts to disclose.
SOURCES: Owens DK et al. JAMA. 2020 Mar 2. doi: 10.1001/jama.2020.1123; Chou R et al. JAMA. 2020 Mar 2. doi: 10.1001/jama.2019.20788; Graham CS, Trooskin S. JAMA. 2020 Mar 2. doi: 10.1001/jama.2019.22313; Price JC and Brandman D. JAMA Intern Med. 2020 Mar 2. doi: 10.1001/jamainternmed.2019.7334; Rosenberg ES, Barocas JA. JAMA Network Open. 2020 Mar 2. doi: 10.1001/jamanetworkopen.2020.0538.
Adults aged 18-79 years should be screened for hepatitis C virus infection, according to an updated grade B recommendation from the U.S. Preventive Services Task Force.
Cases of acute hepatitis C virus (HCV) infection have spiked in the last decade, in part because of increased use of injection drugs and in part because of better surveillance, Douglas K. Owens, MD, of Stanford (Calif.) University, and colleagues wrote in the recommendation statement published in JAMA.
The recommendation applies to all asymptomatic adults aged 18-79 years without known liver disease, and expands on the 2013 recommendation to screen adults born between 1945 and 1965. The grade B designation means that the task force concluded with moderate certainty that HCV screening for adults aged 18-79 years had “substantial net benefit.”
The recommendations are based on an evidence report including 8 randomized, controlled trials, 48 other treatment studies, and 33 cohort studies published through February 2019 for a total of 179,230 individuals.
The screening is a one-time procedure for most adults, according to the task force, but clinicians should periodically screen individuals at increased risk, such as those with a past or current history of injection drug use. In addition, clinicians should consider screening individuals at increased risk who are above or below the recommended age range.
Although the task force identified no direct evidence on the benefit of screening for HCV infection in asymptomatic adults, a notable finding was that the newer direct-acting antiviral (DAA) regimens are sufficiently effective to support the expanded screening recommendation, they said. However, clinicians should inform patients that screening is voluntary and conducted only with the patient’s knowledge. Clinicians should educate patients about hepatitis C and give them an opportunity to ask questions and to make a decision about screening, according to the task force.
In the evidence report, a total of 49 studies including 10,181 individuals showed DAA treatment associated with pooled sustained virologic response rates greater than 95% across all virus genotypes, and a short-term serious adverse event rate of 1.9%. In addition, sustained virologic response following an antiviral therapy was associated with a reduction in risk of all-cause mortality (pooled hazard ratio 0.40) and of hepatocellular carcinoma (pooled HR 0.29) compared with cases of no sustained virologic response.
The evidence report findings were limited by several factors, including the relatively small number of randomized trials involving current DAA treatments, limited data on baseline symptoms, limited data on adolescents, and limited evidence on potential long-term harms of DAA therapy, noted Richard Chou, MD, of Oregon Health & Science University, Portland, and colleagues. However, new pooled evidence “indicates that SVR rates with currently recommended all-oral DAA regimens are substantially higher (more than 95%) than with interferon-based therapies evaluated in the prior review (68%-78%),” they said.
Several editorials were published concurrently with the recommendation.
In an editorial published in JAMA, Camilla S. Graham, MD, of Harvard Medical School, Boston, and Stacey Trooskin, MD, of the University of Pennsylvania, Philadelphia, wrote that the new recommendation reflects changes in hepatitis C virus management.
“With the approvals of sofosbuvir and simeprevir in 2013, patients with hepatitis C, a chronic viral illness associated with the deaths of more U.S. patients than the next 60 reportable infectious diseases combined, including HIV and tuberculosis, could expect a greater than 90% rate of achieving sustained virologic response (SVR, defined as undetectable HCV levels 12 weeks or longer after treatment completion, which is consistent with virologic cure of HCV infection) following 12 weeks of treatment,” they said.
These medications are effective but expensive; however, the combination of the availability of generic medications and the ongoing opioid epidemic in the United States are important contributors to the expanded recommendations, which “are welcome,” and may help meeting WHO 2030 targets for reducing new HCV infections, they said.
Dr. Graham disclosed personal fees from UpToDate. Dr. Trooskin disclosed grants from Gilead Sciences and personal fees from Merck, AbbVie, and Gilead Sciences.
In an editorial published in JAMA Internal Medicine, Jennifer C. Price, MD, and Danielle Brandman, MD, both of the University of California, San Francisco, wrote that “the advancements in HCV diagnosis and treatment have been extraordinary,” but that the new recommendation does not go far enough. “Implementation of HCV screening and linkage to treatment requires large-scale coordinated efforts, innovation, and resources. For example, point-of-care HCV RNA testing would enable scale-up of HCV screening and confirmatory testing among individuals at greatest risk of HCV infection,” they said. “Additionally, barriers remain between diagnosis and treatment, such as access to a health care provider who can treat HCV and authorization to receive affordable DAAs,” they noted. “Although the USPSTF HCV screening recommendation is a step forward for controlling HCV infection in the U.S., it will take a coordinated and funded effort to ensure that the anticipated benefits are realized,” they concluded.
Dr. Price disclosed research funding from Gilead Sciences and Merck. Dr. Brandman disclosed research funding from Gilead Sciences, Pfizer, Conatus, Allergan, and Grifols, as well as personal fees from Alnylam.
In an editorial published in JAMA Network Open, Eli S. Rosenberg, PhD, of the University at Albany (N.Y.) School of Public Health, and Joshua A. Barocas, MD, of Boston University, emphasized the need to change the stigma surrounding HCV infection in the United States.
“Given the changing epidemiology of HCV infection, new public health priorities, advancements in treatment, and unmet diagnostic needs, it is wise to periodically reevaluate screening recommendations to ensure that they are maximally addressing these areas and patients’ individual needs,” they said. “The Affordable Care Act requires private insurers and Medicaid to cover preventive services recommended by the USPSTF with a grade of A or B with no cost sharing (i.e., no deductible or copayment),” they noted. Although the new recommendation for one-time screening will likely identify more cases, improve outcomes, and reduce deaths, the editorialists cautioned that “one-time screening should not be interpreted like catch-up vaccinations, whereby we immunize someone at any age for hepatitis B virus, for example, and they are then immunized for the remainder of their life,” and that reassessments are needed, especially for younger adults.
In addition, they emphasized the need to reduce the stigma surrounding HCV and allow for recommendations based on risk, rather than age. “We have forced the USPSTF to adopt age-based screening recommendations because we, as a society, have created a culture in which we have stigmatized these behaviors and we, as practitioners, have proven to be inadequate at eliciting HCV risk behaviors,” they said. “Our responsibility as a society and practice community is to address structural and individual factors that limit our ability to most precisely address the needs of our patients and truly move toward HCV elimination,” they concluded.
The USPSTF is supported by the Agency for Healthcare Research and Quality. The task force researchers had no financial conflicts to disclose.
SOURCES: Owens DK et al. JAMA. 2020 Mar 2. doi: 10.1001/jama.2020.1123; Chou R et al. JAMA. 2020 Mar 2. doi: 10.1001/jama.2019.20788; Graham CS, Trooskin S. JAMA. 2020 Mar 2. doi: 10.1001/jama.2019.22313; Price JC and Brandman D. JAMA Intern Med. 2020 Mar 2. doi: 10.1001/jamainternmed.2019.7334; Rosenberg ES, Barocas JA. JAMA Network Open. 2020 Mar 2. doi: 10.1001/jamanetworkopen.2020.0538.
Adults aged 18-79 years should be screened for hepatitis C virus infection, according to an updated grade B recommendation from the U.S. Preventive Services Task Force.
Cases of acute hepatitis C virus (HCV) infection have spiked in the last decade, in part because of increased use of injection drugs and in part because of better surveillance, Douglas K. Owens, MD, of Stanford (Calif.) University, and colleagues wrote in the recommendation statement published in JAMA.
The recommendation applies to all asymptomatic adults aged 18-79 years without known liver disease, and expands on the 2013 recommendation to screen adults born between 1945 and 1965. The grade B designation means that the task force concluded with moderate certainty that HCV screening for adults aged 18-79 years had “substantial net benefit.”
The recommendations are based on an evidence report including 8 randomized, controlled trials, 48 other treatment studies, and 33 cohort studies published through February 2019 for a total of 179,230 individuals.
The screening is a one-time procedure for most adults, according to the task force, but clinicians should periodically screen individuals at increased risk, such as those with a past or current history of injection drug use. In addition, clinicians should consider screening individuals at increased risk who are above or below the recommended age range.
Although the task force identified no direct evidence on the benefit of screening for HCV infection in asymptomatic adults, a notable finding was that the newer direct-acting antiviral (DAA) regimens are sufficiently effective to support the expanded screening recommendation, they said. However, clinicians should inform patients that screening is voluntary and conducted only with the patient’s knowledge. Clinicians should educate patients about hepatitis C and give them an opportunity to ask questions and to make a decision about screening, according to the task force.
In the evidence report, a total of 49 studies including 10,181 individuals showed DAA treatment associated with pooled sustained virologic response rates greater than 95% across all virus genotypes, and a short-term serious adverse event rate of 1.9%. In addition, sustained virologic response following an antiviral therapy was associated with a reduction in risk of all-cause mortality (pooled hazard ratio 0.40) and of hepatocellular carcinoma (pooled HR 0.29) compared with cases of no sustained virologic response.
The evidence report findings were limited by several factors, including the relatively small number of randomized trials involving current DAA treatments, limited data on baseline symptoms, limited data on adolescents, and limited evidence on potential long-term harms of DAA therapy, noted Richard Chou, MD, of Oregon Health & Science University, Portland, and colleagues. However, new pooled evidence “indicates that SVR rates with currently recommended all-oral DAA regimens are substantially higher (more than 95%) than with interferon-based therapies evaluated in the prior review (68%-78%),” they said.
Several editorials were published concurrently with the recommendation.
In an editorial published in JAMA, Camilla S. Graham, MD, of Harvard Medical School, Boston, and Stacey Trooskin, MD, of the University of Pennsylvania, Philadelphia, wrote that the new recommendation reflects changes in hepatitis C virus management.
“With the approvals of sofosbuvir and simeprevir in 2013, patients with hepatitis C, a chronic viral illness associated with the deaths of more U.S. patients than the next 60 reportable infectious diseases combined, including HIV and tuberculosis, could expect a greater than 90% rate of achieving sustained virologic response (SVR, defined as undetectable HCV levels 12 weeks or longer after treatment completion, which is consistent with virologic cure of HCV infection) following 12 weeks of treatment,” they said.
These medications are effective but expensive; however, the combination of the availability of generic medications and the ongoing opioid epidemic in the United States are important contributors to the expanded recommendations, which “are welcome,” and may help meeting WHO 2030 targets for reducing new HCV infections, they said.
Dr. Graham disclosed personal fees from UpToDate. Dr. Trooskin disclosed grants from Gilead Sciences and personal fees from Merck, AbbVie, and Gilead Sciences.
In an editorial published in JAMA Internal Medicine, Jennifer C. Price, MD, and Danielle Brandman, MD, both of the University of California, San Francisco, wrote that “the advancements in HCV diagnosis and treatment have been extraordinary,” but that the new recommendation does not go far enough. “Implementation of HCV screening and linkage to treatment requires large-scale coordinated efforts, innovation, and resources. For example, point-of-care HCV RNA testing would enable scale-up of HCV screening and confirmatory testing among individuals at greatest risk of HCV infection,” they said. “Additionally, barriers remain between diagnosis and treatment, such as access to a health care provider who can treat HCV and authorization to receive affordable DAAs,” they noted. “Although the USPSTF HCV screening recommendation is a step forward for controlling HCV infection in the U.S., it will take a coordinated and funded effort to ensure that the anticipated benefits are realized,” they concluded.
Dr. Price disclosed research funding from Gilead Sciences and Merck. Dr. Brandman disclosed research funding from Gilead Sciences, Pfizer, Conatus, Allergan, and Grifols, as well as personal fees from Alnylam.
In an editorial published in JAMA Network Open, Eli S. Rosenberg, PhD, of the University at Albany (N.Y.) School of Public Health, and Joshua A. Barocas, MD, of Boston University, emphasized the need to change the stigma surrounding HCV infection in the United States.
“Given the changing epidemiology of HCV infection, new public health priorities, advancements in treatment, and unmet diagnostic needs, it is wise to periodically reevaluate screening recommendations to ensure that they are maximally addressing these areas and patients’ individual needs,” they said. “The Affordable Care Act requires private insurers and Medicaid to cover preventive services recommended by the USPSTF with a grade of A or B with no cost sharing (i.e., no deductible or copayment),” they noted. Although the new recommendation for one-time screening will likely identify more cases, improve outcomes, and reduce deaths, the editorialists cautioned that “one-time screening should not be interpreted like catch-up vaccinations, whereby we immunize someone at any age for hepatitis B virus, for example, and they are then immunized for the remainder of their life,” and that reassessments are needed, especially for younger adults.
In addition, they emphasized the need to reduce the stigma surrounding HCV and allow for recommendations based on risk, rather than age. “We have forced the USPSTF to adopt age-based screening recommendations because we, as a society, have created a culture in which we have stigmatized these behaviors and we, as practitioners, have proven to be inadequate at eliciting HCV risk behaviors,” they said. “Our responsibility as a society and practice community is to address structural and individual factors that limit our ability to most precisely address the needs of our patients and truly move toward HCV elimination,” they concluded.
The USPSTF is supported by the Agency for Healthcare Research and Quality. The task force researchers had no financial conflicts to disclose.
SOURCES: Owens DK et al. JAMA. 2020 Mar 2. doi: 10.1001/jama.2020.1123; Chou R et al. JAMA. 2020 Mar 2. doi: 10.1001/jama.2019.20788; Graham CS, Trooskin S. JAMA. 2020 Mar 2. doi: 10.1001/jama.2019.22313; Price JC and Brandman D. JAMA Intern Med. 2020 Mar 2. doi: 10.1001/jamainternmed.2019.7334; Rosenberg ES, Barocas JA. JAMA Network Open. 2020 Mar 2. doi: 10.1001/jamanetworkopen.2020.0538.
FROM JAMA
Expert: Eliminating HCV ‘sounds ambitious, but I think it’s possible’
LAS VEGAS – Between 2010 and 2017, the proportion of newly diagnosed cases of acute hepatitis C virus infection rose threefold, driven largely by the concomitant opioid epidemic.
That makes efforts to screen, diagnose, and cure high-risk populations more important than ever, Stevan A. Gonzalez, MD, said at an annual psychopharmacology update held by the Nevada Psychiatric Association.
About 70% of HCV cases are related to injection drug use,” said Dr. Gonzalez, medical director of liver transplantation at the Baylor Simmons Transplant Institute at the Baylor Scott & White All Saints Medical Center in Fort Worth, Tex. “This is affecting whites as much as blacks and Hispanics, females as much as males, and in nonurban areas as much as in urban areas.”
Data from the Centers for Disease Control and Prevention and the Substance Abuse and Mental Health Services Administration indicate that during 2004-2014, the number of acute HCV cases among those aged 18-29 years increased 400%, and the use of injection opioids rose 600%.
At the same time, the number of HCV cases among those aged 30-39 years increased 325%, and the use of injection opioids rose 83%.
“We’re starting to see a pattern overlapping between HCV exposure and opioid injection,” Dr. Gonzalez said. Other high-risk populations include homeless and incarcerated individuals.
More than 70 million people worldwide have chronic HCV infection, Dr. Gonzalez noted, with possibly as many as 5 million cases in the United States. It remains the nation’s most common blood-borne infection.
Chronic disease develops in up to 85% of people who are exposed, infection is asymptomatic, and HCV remains one of the leading indications for liver transplantation and causes of liver cancer.
From a geographic standpoint, the prevalence of HCV in young adults is eclipsing that of Baby Boomers in several states in the Appalachian region and in Northeast, which have long been trouble spots for opioid use disorder (Gastroenterol. 2018 May;154[6]:1850-1).
Surprising exposure risk
The primary risk of transmission is through contaminated blood and the exposure through needles.
“It really doesn’t matter whether it’s a needle that has a small amount of dead space where a little bit of blood can remain or needles that have a larger amount of blood,” Dr. Gonzalez said.
“I’ve had patients who come to me and say, ‘I can’t believe I have HCV. It’s impossible. I always use my own needles. They’re always brand new; I’ve never shared with anybody,’” he continued.
“This is where education and awareness is so critical, because it’s not just the needles,” Dr. Gonzalez explained. “HCV can survive on inanimate objects. For example, on a tabletop surface or a water container, HCV can remain viable up to 3 weeks. In a syringe, 2 months. For that reason, HCV can also be transmitted through crack pipes and nasal drug use, where the prevalence can be up to 35%.”
The duration of a person’s HCV infection drives the transmission.
“That’s important to think about, because people who have chronic hepatitis C are infectious until they’re treated,” Dr. Gonzalez said. “If they don’t know that they have hepatitis C, they continue to transmit the virus to others.”
One study found that half of people living with HCV are unaware of their infection (PLoS One. 2014 Jul 2;9[7]:e101554). According to Dr. Gonzalez, forthcoming guidelines from the U.S. Preventive Services Task Force are expected to recommend a one-time screening for HCV infection in all adults aged 18-79 years, a Grade B recommendation. “That’s a big deal,” he said. (The draft recommendations are available here.)
HCV infection disproportionately affects individuals in correctional institutions. In fact, an estimated one in three inmates in the United States has chronic HCV.
“This is sort of a forgotten population with a lot of substance use and mental illness,” Dr. Gonzalez said. “Injection drug use in that setting is the most common risk factor: It’s about 60% in terms of the risk of transmission within correctional settings. HCV-associated liver disease has now surpassed HIV as a cause of death within correctional settings.”
Weighing treatment options
The most common oral regimens for chronic HCV include sofosbuvir/ledipasvir, sofosbuvir/velpatasvir, and glecaprevir/pibrentasvir. They achieve cure in 93%-100% of cases.
“HCV can be cured; it can be eradicated from the body long term,” Dr. Gonzalez said. “The choice of regimen, treatment duration, and use of ribavirin depends on the presence/absence of cirrhosis, prior treatment experience, and the genotype.”
All six forms of the HCV genotype can be treated with oral medication, he added, and methadone, bupropion, and naloxone are safe to use during therapy.
Reinfection following HCV treatment occurs infrequently. Dr. Gonzalez cited a randomized, controlled trial presented as an abstract at the 2018 annual meeting of the American Association for the Study of Liver Diseases. That study’s researchers found that – among 199 patients on opioid-replacement therapy who were receiving direct-acting antiviral therapy, in whom greater than 50% were actively using drugs – the rate of reinfection at 3 years was 1.8 reinfections/100 person-years.
“That’s lower than people expect,” Dr. Gonzalez said.
How to boost screening
Electronic health record systems can be used as an important tool to increase HCV screening in health care settings.
In 2017, researchers published an analysis of three randomized trials carried out at three separate primary care settings to improve screening for HCV: repeated mailings, an EHR best practice alert (BPA), and patient solicitation (Hepatology 2017 Jan;65[1]:44-53). They evaluated HCV antibody testing, diagnosis, and costs for each of the interventions, compared with standard-of-care testing.
The investigators found that the BPA intervention had the lowest incremental cost per completed test – $24 with fixed start-up costs, including technical design and development of the BPA system; $3 without fixed start-up costs. The BPA intervention also had the lowest incremental cost per new case identified.
Other efforts to expand access to screening and treatment are underway.
In 2019, Louisiana health officials negotiated a one-time fee for unlimited access for 5 years to sofosbuvir/velpatasvir (Epclusa) to treat the estimated 30,000 patients on Louisiana Medicaid and in that state’s department of corrections who have HCV.
“The goal is 90% cure; the burden is on the state health department to screen, diagnose, and dispense medication,” Dr. Gonzalez said.
Also in 2019, the state of Washington used an open bidding process to negotiate access to glecaprevir/pibrentasvir (Mavyret) for the state’s Medicaid population who have HCV.
“Those states are setting the pace,” Dr. Gonzalez said. “They are showing examples of how we can start implementing a process to treat these vulnerable populations.”
Meanwhile, the World Health Organization set a goal of eliminating viral hepatitis as a major public health threat by 2030.
“That sounds ambitious, but I think it’s possible,” Dr. Gonzalez said. “It’s important to address these high-risk populations: the incarcerated, people who use drugs, and the homeless, because those are the groups that have a high prevalence of HCV – mainly through injection drug use.
“If we don’t address that population, and we only target the general population, we’re going to have a continual source of transmission,” Dr. Gonzalez warned. “In that case, we would never be able to achieve elimination.”
Dr. Gonzalez disclosed that he is a member of the speakers bureau for AbbVie and Salix.
LAS VEGAS – Between 2010 and 2017, the proportion of newly diagnosed cases of acute hepatitis C virus infection rose threefold, driven largely by the concomitant opioid epidemic.
That makes efforts to screen, diagnose, and cure high-risk populations more important than ever, Stevan A. Gonzalez, MD, said at an annual psychopharmacology update held by the Nevada Psychiatric Association.
About 70% of HCV cases are related to injection drug use,” said Dr. Gonzalez, medical director of liver transplantation at the Baylor Simmons Transplant Institute at the Baylor Scott & White All Saints Medical Center in Fort Worth, Tex. “This is affecting whites as much as blacks and Hispanics, females as much as males, and in nonurban areas as much as in urban areas.”
Data from the Centers for Disease Control and Prevention and the Substance Abuse and Mental Health Services Administration indicate that during 2004-2014, the number of acute HCV cases among those aged 18-29 years increased 400%, and the use of injection opioids rose 600%.
At the same time, the number of HCV cases among those aged 30-39 years increased 325%, and the use of injection opioids rose 83%.
“We’re starting to see a pattern overlapping between HCV exposure and opioid injection,” Dr. Gonzalez said. Other high-risk populations include homeless and incarcerated individuals.
More than 70 million people worldwide have chronic HCV infection, Dr. Gonzalez noted, with possibly as many as 5 million cases in the United States. It remains the nation’s most common blood-borne infection.
Chronic disease develops in up to 85% of people who are exposed, infection is asymptomatic, and HCV remains one of the leading indications for liver transplantation and causes of liver cancer.
From a geographic standpoint, the prevalence of HCV in young adults is eclipsing that of Baby Boomers in several states in the Appalachian region and in Northeast, which have long been trouble spots for opioid use disorder (Gastroenterol. 2018 May;154[6]:1850-1).
Surprising exposure risk
The primary risk of transmission is through contaminated blood and the exposure through needles.
“It really doesn’t matter whether it’s a needle that has a small amount of dead space where a little bit of blood can remain or needles that have a larger amount of blood,” Dr. Gonzalez said.
“I’ve had patients who come to me and say, ‘I can’t believe I have HCV. It’s impossible. I always use my own needles. They’re always brand new; I’ve never shared with anybody,’” he continued.
“This is where education and awareness is so critical, because it’s not just the needles,” Dr. Gonzalez explained. “HCV can survive on inanimate objects. For example, on a tabletop surface or a water container, HCV can remain viable up to 3 weeks. In a syringe, 2 months. For that reason, HCV can also be transmitted through crack pipes and nasal drug use, where the prevalence can be up to 35%.”
The duration of a person’s HCV infection drives the transmission.
“That’s important to think about, because people who have chronic hepatitis C are infectious until they’re treated,” Dr. Gonzalez said. “If they don’t know that they have hepatitis C, they continue to transmit the virus to others.”
One study found that half of people living with HCV are unaware of their infection (PLoS One. 2014 Jul 2;9[7]:e101554). According to Dr. Gonzalez, forthcoming guidelines from the U.S. Preventive Services Task Force are expected to recommend a one-time screening for HCV infection in all adults aged 18-79 years, a Grade B recommendation. “That’s a big deal,” he said. (The draft recommendations are available here.)
HCV infection disproportionately affects individuals in correctional institutions. In fact, an estimated one in three inmates in the United States has chronic HCV.
“This is sort of a forgotten population with a lot of substance use and mental illness,” Dr. Gonzalez said. “Injection drug use in that setting is the most common risk factor: It’s about 60% in terms of the risk of transmission within correctional settings. HCV-associated liver disease has now surpassed HIV as a cause of death within correctional settings.”
Weighing treatment options
The most common oral regimens for chronic HCV include sofosbuvir/ledipasvir, sofosbuvir/velpatasvir, and glecaprevir/pibrentasvir. They achieve cure in 93%-100% of cases.
“HCV can be cured; it can be eradicated from the body long term,” Dr. Gonzalez said. “The choice of regimen, treatment duration, and use of ribavirin depends on the presence/absence of cirrhosis, prior treatment experience, and the genotype.”
All six forms of the HCV genotype can be treated with oral medication, he added, and methadone, bupropion, and naloxone are safe to use during therapy.
Reinfection following HCV treatment occurs infrequently. Dr. Gonzalez cited a randomized, controlled trial presented as an abstract at the 2018 annual meeting of the American Association for the Study of Liver Diseases. That study’s researchers found that – among 199 patients on opioid-replacement therapy who were receiving direct-acting antiviral therapy, in whom greater than 50% were actively using drugs – the rate of reinfection at 3 years was 1.8 reinfections/100 person-years.
“That’s lower than people expect,” Dr. Gonzalez said.
How to boost screening
Electronic health record systems can be used as an important tool to increase HCV screening in health care settings.
In 2017, researchers published an analysis of three randomized trials carried out at three separate primary care settings to improve screening for HCV: repeated mailings, an EHR best practice alert (BPA), and patient solicitation (Hepatology 2017 Jan;65[1]:44-53). They evaluated HCV antibody testing, diagnosis, and costs for each of the interventions, compared with standard-of-care testing.
The investigators found that the BPA intervention had the lowest incremental cost per completed test – $24 with fixed start-up costs, including technical design and development of the BPA system; $3 without fixed start-up costs. The BPA intervention also had the lowest incremental cost per new case identified.
Other efforts to expand access to screening and treatment are underway.
In 2019, Louisiana health officials negotiated a one-time fee for unlimited access for 5 years to sofosbuvir/velpatasvir (Epclusa) to treat the estimated 30,000 patients on Louisiana Medicaid and in that state’s department of corrections who have HCV.
“The goal is 90% cure; the burden is on the state health department to screen, diagnose, and dispense medication,” Dr. Gonzalez said.
Also in 2019, the state of Washington used an open bidding process to negotiate access to glecaprevir/pibrentasvir (Mavyret) for the state’s Medicaid population who have HCV.
“Those states are setting the pace,” Dr. Gonzalez said. “They are showing examples of how we can start implementing a process to treat these vulnerable populations.”
Meanwhile, the World Health Organization set a goal of eliminating viral hepatitis as a major public health threat by 2030.
“That sounds ambitious, but I think it’s possible,” Dr. Gonzalez said. “It’s important to address these high-risk populations: the incarcerated, people who use drugs, and the homeless, because those are the groups that have a high prevalence of HCV – mainly through injection drug use.
“If we don’t address that population, and we only target the general population, we’re going to have a continual source of transmission,” Dr. Gonzalez warned. “In that case, we would never be able to achieve elimination.”
Dr. Gonzalez disclosed that he is a member of the speakers bureau for AbbVie and Salix.
LAS VEGAS – Between 2010 and 2017, the proportion of newly diagnosed cases of acute hepatitis C virus infection rose threefold, driven largely by the concomitant opioid epidemic.
That makes efforts to screen, diagnose, and cure high-risk populations more important than ever, Stevan A. Gonzalez, MD, said at an annual psychopharmacology update held by the Nevada Psychiatric Association.
About 70% of HCV cases are related to injection drug use,” said Dr. Gonzalez, medical director of liver transplantation at the Baylor Simmons Transplant Institute at the Baylor Scott & White All Saints Medical Center in Fort Worth, Tex. “This is affecting whites as much as blacks and Hispanics, females as much as males, and in nonurban areas as much as in urban areas.”
Data from the Centers for Disease Control and Prevention and the Substance Abuse and Mental Health Services Administration indicate that during 2004-2014, the number of acute HCV cases among those aged 18-29 years increased 400%, and the use of injection opioids rose 600%.
At the same time, the number of HCV cases among those aged 30-39 years increased 325%, and the use of injection opioids rose 83%.
“We’re starting to see a pattern overlapping between HCV exposure and opioid injection,” Dr. Gonzalez said. Other high-risk populations include homeless and incarcerated individuals.
More than 70 million people worldwide have chronic HCV infection, Dr. Gonzalez noted, with possibly as many as 5 million cases in the United States. It remains the nation’s most common blood-borne infection.
Chronic disease develops in up to 85% of people who are exposed, infection is asymptomatic, and HCV remains one of the leading indications for liver transplantation and causes of liver cancer.
From a geographic standpoint, the prevalence of HCV in young adults is eclipsing that of Baby Boomers in several states in the Appalachian region and in Northeast, which have long been trouble spots for opioid use disorder (Gastroenterol. 2018 May;154[6]:1850-1).
Surprising exposure risk
The primary risk of transmission is through contaminated blood and the exposure through needles.
“It really doesn’t matter whether it’s a needle that has a small amount of dead space where a little bit of blood can remain or needles that have a larger amount of blood,” Dr. Gonzalez said.
“I’ve had patients who come to me and say, ‘I can’t believe I have HCV. It’s impossible. I always use my own needles. They’re always brand new; I’ve never shared with anybody,’” he continued.
“This is where education and awareness is so critical, because it’s not just the needles,” Dr. Gonzalez explained. “HCV can survive on inanimate objects. For example, on a tabletop surface or a water container, HCV can remain viable up to 3 weeks. In a syringe, 2 months. For that reason, HCV can also be transmitted through crack pipes and nasal drug use, where the prevalence can be up to 35%.”
The duration of a person’s HCV infection drives the transmission.
“That’s important to think about, because people who have chronic hepatitis C are infectious until they’re treated,” Dr. Gonzalez said. “If they don’t know that they have hepatitis C, they continue to transmit the virus to others.”
One study found that half of people living with HCV are unaware of their infection (PLoS One. 2014 Jul 2;9[7]:e101554). According to Dr. Gonzalez, forthcoming guidelines from the U.S. Preventive Services Task Force are expected to recommend a one-time screening for HCV infection in all adults aged 18-79 years, a Grade B recommendation. “That’s a big deal,” he said. (The draft recommendations are available here.)
HCV infection disproportionately affects individuals in correctional institutions. In fact, an estimated one in three inmates in the United States has chronic HCV.
“This is sort of a forgotten population with a lot of substance use and mental illness,” Dr. Gonzalez said. “Injection drug use in that setting is the most common risk factor: It’s about 60% in terms of the risk of transmission within correctional settings. HCV-associated liver disease has now surpassed HIV as a cause of death within correctional settings.”
Weighing treatment options
The most common oral regimens for chronic HCV include sofosbuvir/ledipasvir, sofosbuvir/velpatasvir, and glecaprevir/pibrentasvir. They achieve cure in 93%-100% of cases.
“HCV can be cured; it can be eradicated from the body long term,” Dr. Gonzalez said. “The choice of regimen, treatment duration, and use of ribavirin depends on the presence/absence of cirrhosis, prior treatment experience, and the genotype.”
All six forms of the HCV genotype can be treated with oral medication, he added, and methadone, bupropion, and naloxone are safe to use during therapy.
Reinfection following HCV treatment occurs infrequently. Dr. Gonzalez cited a randomized, controlled trial presented as an abstract at the 2018 annual meeting of the American Association for the Study of Liver Diseases. That study’s researchers found that – among 199 patients on opioid-replacement therapy who were receiving direct-acting antiviral therapy, in whom greater than 50% were actively using drugs – the rate of reinfection at 3 years was 1.8 reinfections/100 person-years.
“That’s lower than people expect,” Dr. Gonzalez said.
How to boost screening
Electronic health record systems can be used as an important tool to increase HCV screening in health care settings.
In 2017, researchers published an analysis of three randomized trials carried out at three separate primary care settings to improve screening for HCV: repeated mailings, an EHR best practice alert (BPA), and patient solicitation (Hepatology 2017 Jan;65[1]:44-53). They evaluated HCV antibody testing, diagnosis, and costs for each of the interventions, compared with standard-of-care testing.
The investigators found that the BPA intervention had the lowest incremental cost per completed test – $24 with fixed start-up costs, including technical design and development of the BPA system; $3 without fixed start-up costs. The BPA intervention also had the lowest incremental cost per new case identified.
Other efforts to expand access to screening and treatment are underway.
In 2019, Louisiana health officials negotiated a one-time fee for unlimited access for 5 years to sofosbuvir/velpatasvir (Epclusa) to treat the estimated 30,000 patients on Louisiana Medicaid and in that state’s department of corrections who have HCV.
“The goal is 90% cure; the burden is on the state health department to screen, diagnose, and dispense medication,” Dr. Gonzalez said.
Also in 2019, the state of Washington used an open bidding process to negotiate access to glecaprevir/pibrentasvir (Mavyret) for the state’s Medicaid population who have HCV.
“Those states are setting the pace,” Dr. Gonzalez said. “They are showing examples of how we can start implementing a process to treat these vulnerable populations.”
Meanwhile, the World Health Organization set a goal of eliminating viral hepatitis as a major public health threat by 2030.
“That sounds ambitious, but I think it’s possible,” Dr. Gonzalez said. “It’s important to address these high-risk populations: the incarcerated, people who use drugs, and the homeless, because those are the groups that have a high prevalence of HCV – mainly through injection drug use.
“If we don’t address that population, and we only target the general population, we’re going to have a continual source of transmission,” Dr. Gonzalez warned. “In that case, we would never be able to achieve elimination.”
Dr. Gonzalez disclosed that he is a member of the speakers bureau for AbbVie and Salix.
REPORTING FROM NPA 2020
Blistering Disease During the Treatment of Chronic Hepatitis C With Ledipasvir/Sofosbuvir (FULL)
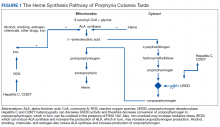
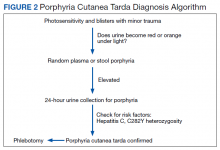
Porphyria cutanea tarda (PCT) is the most common type of porphyria. The accumulation of porphyrin in various organ systems results from a deficiency of uroporphyrinogen decarboxylase (UROD).1-3 Chronic hepatitis C virus (HCV) causes a hepatic decrease in hepcidin production, resulting in increased iron absorption. Iron loading and increased oxidative stress in the liver leads to nonporphyrin inhibition of UROD production and to oxidation of porphyrinogens to porphyrins.4 This in turn leads to accumulation of uroporphyrins and carboxylated metabolites that can be detected in urine.4
Signs of PCT include blisters, vesicles, and possibly milia developing on sun-exposed areas of the skin, such as the face, forearms, and dorsal hands.4 Case reports have demonstrated a resolution of PCT in patients with chronic HCV with treatment with direct-acting antivirals (DAAs), such as ledipasvir/sofosbuvir.1,3 However, here we present 2 cases of patients who developed blistering diseases during treatment of chronic HCV with ledipasvir/sofosbuvir. Neither demonstrated complete resolution of symptoms during the treatment regimen.
Cases
Patient 1
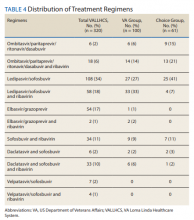
A 63-year-old white male with a history of chronic HCV (genotype 1a), bipolar disorder, hyperlipidemia, tobacco dependence, and cirrhosis (F4 by elastography) presented with minimally to moderately painful blisters on his bilateral dorsal hands that had developed around weeks 8 to 9 of treatment with ledipasvir/sofosbuvir. The patient reported that no new blisters had appeared following completion of 12 weeks of treatment and that his current blisters were in various stages of healing. He reported alcohol use of 1 to 2 twelve-ounce beers daily and no history of dioxin exposure. His medications included doxepin, hydralazine, hydrochlorothiazide, quetiapine, folic acid, and thiamine. His hepatitis C viral load was 440,000 IU/mL prior to treatment. Tests for hepatitis B surface antigen and HIV antibodies were negative. His iron level was 135 µg/dL, total iron-binding capacity (TIBC) was 323 µg/dL, and ferritin was 299.0 ng/mL. His HFE
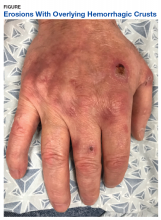
A physical examination on presentation revealed erosions with overlying hemorrhagic crusts on the bilateral dorsal hands (Figure).
At the 4-month follow-up, the patient reported no new blister formations. A physical examination revealed well-healed scars and several clustered milia on bilateral dorsal hands with no active vesicles or bullae noted.
Patient 2
An African American male aged 63 years presented with a 1-month history of moderately painful blisters on his bilateral dorsal hands during treatment of chronic HCV (genotype 1a) with ledipasvir/sofosbuvir. His medical history included gout, tobacco and alcohol addiction, osteoarthritis, and hepatic fibrosis (F3 by elastography). The patient’s medications included allopurinol, lisinopril, and hydrochlorothiazide. He reported no history of dioxin exposure. On the day of presentation, he was on week 9 of the 12-week treatment ledipasvir/sofosbuvir regimen. Laboratory results included an initial HCV viral load of 1,618,605 IU/mL. Tests for hepatitis B surface antigen and HIV antibodies were negative. His iron was 191 µg/dL, TIBC 388 µg/dL, and ferritin 459.0 ng/mL. After 4 weeks of treatment, the patient’s hepatitis C viral load was undetectable.
A physical examination revealed several resolving erosions to his bilateral dorsal hands, some of which had overlying crusting along with one small hemorrhagic vesicle on the right dorsal hand. A punch biopsy of the hemorrhagic vesicle was performed and demonstrated a cell-poor subepidermal blister with festooning of the dermal papilla. A direct immunofluorescence study showed immunoglobulin (Ig) G fluorescence along the dermal-epidermal junction and within vessel walls in the superficial dermis. Weak IgM and C3 fluorescence also was noted within vessel walls in the superficial dermis. All of the patient findings and history were consistent with PCT, although pseudo-PCT also was a consideration. A 24-hour urine sample yielded negative results for porphobilinogen. Urine porphyrin test results were not available, leading to a presumptive histological diagnosis of PCT.
The patient completed 11 of the prescribed 12 weeks of ledipasvir/sofosbuvir. The blisters resolved shortly thereafter.
Discussion
PCT has a well-established association with chronic HCV infection.4 We present 2 cases of a blistering disease clinically and histologically compatible with PCT that developed in patients only after initiation of treatment for chronic HCV with ledipasvir/sofosbuvir. One case was confirmed as PCT on the basis of compatible histopathologic findings and a urine porphyrin assay that showed elevated levels of uroporphyrins and carboxylated metabolites. The second case was clinically and histologically suggestive of PCT but not confirmed by urine porphyrin testing. In both patients, after 8 to 9 weeks of a 12-week course of antiviral therapy, the blistering lesions were noted but appeared to be resolving, and no new lesions were noted after discontinuation of therapy. It appeared that the antiviral treatment temporally triggered the initiation of the blistering skin disease, and as the chronic HCV infection cleared after treatment, the blistering lesions also began to resolve.
Mechanistically, it is known that the virally-induced hepatic damage leads to inhibition of uroporphyrinogen decarboxylase, and the subsequent oxidation of porphyrinogens to porphyrins. Cofactors such as HIV infection also may contribute to development of PCT.5
De novo PCT has been documented during therapy using interferon and ribavirin.6 The hemolytic anemia and increased hepatic iron were implicated as potential etiologies.6 Patients with HCV and PCT treated with the newer direct-acting antiviral therapies have been described to have experienced improvement in PCT symptoms.3
Although there were rare reports of deterioration in renal and liver function,7 reactivation of HBV infection,8 and Stevens-Johnson syndrome9 with antiviral therapy, these complications were not observed in these patients. Both patients also had successful resolution of HCV infection, and by completion of the antiviral therapy, the blistering also resolved.
Conclusion
PCT is an extrahepatic manifestation of HCV infection. Health care providers should be aware of the association of chronic HCV infection with PCT. The findings of PCT should not result in the delay or discontinuation of antiviral therapy.
1. Combalia A, To-Figueras J, Laguno M, Martinez-Rebollar M, Aguilera P. Direct-acting antivirals for hepatitis C virus induce a rapid clinical and biochemical remission of porphyria cutanea tarda. Br J Dermatol. 2017;177(5):e183-e184.
2. Younossi Z, Park H, Henry L, Adeyemi A, Stepanova M. Extrahepatic manifestations of hepatitis C: a meta-analysis of prevalence, quality of life, and economic burden. Gastroenterology. 2016;150(7):1599-1608.
3. Tong Y, Song YK, Tyring S. Resolution of porphyria cutanea tarda in patients with hepatitis C following ledipasvir/sofosbuvir combination therapy. JAMA Dermatol. 2016;152(12):1393-1395.
4. Ryan Caballes F, Sendi H, Bonkovsky H. Hepatitis C, porphyria cutanea tarda and liver iron: an update. Liver Int. 2012;32(6):880-893.
5. Quansah R, Cooper CJ, Said S, Bizet J, Paez D, Hernandez GT. Hepatitis C- and HIV-induced porphyria cutanea tarda. Am J Case Rep. 2014;15:35-40.
6. Azim J, McCurdy H, Moseley RH. Porphyria cutanea tarda as a complication of therapy for chronic hepatitis C. World J Gastroenterol. 2008;14(38):5913-5915.
7. Ahmed M. Harvoni-induced deterioration of renal and liver function. Adv Res Gastroentero Hepatol. 2017;2(3):555588.
8. De Monte A, Courion J, Anty R, et al. Direct-acting antiviral treatment in adults infected with hepatitis C virus: reactivation of hepatitis B virus coinfection as a further challenge. J Clin Virol. 2016;78:27-30.
9. Verma N, Singh S, Sawatkar G, Singh V. Sofosbuvir induced Steven Johnson Syndrome in a patient with hepatitis C virus-related cirrhosis. Hepatol Commun. 2017;2(1):16-20.
Porphyria cutanea tarda (PCT) is the most common type of porphyria. The accumulation of porphyrin in various organ systems results from a deficiency of uroporphyrinogen decarboxylase (UROD).1-3 Chronic hepatitis C virus (HCV) causes a hepatic decrease in hepcidin production, resulting in increased iron absorption. Iron loading and increased oxidative stress in the liver leads to nonporphyrin inhibition of UROD production and to oxidation of porphyrinogens to porphyrins.4 This in turn leads to accumulation of uroporphyrins and carboxylated metabolites that can be detected in urine.4
Signs of PCT include blisters, vesicles, and possibly milia developing on sun-exposed areas of the skin, such as the face, forearms, and dorsal hands.4 Case reports have demonstrated a resolution of PCT in patients with chronic HCV with treatment with direct-acting antivirals (DAAs), such as ledipasvir/sofosbuvir.1,3 However, here we present 2 cases of patients who developed blistering diseases during treatment of chronic HCV with ledipasvir/sofosbuvir. Neither demonstrated complete resolution of symptoms during the treatment regimen.
Cases
Patient 1
A 63-year-old white male with a history of chronic HCV (genotype 1a), bipolar disorder, hyperlipidemia, tobacco dependence, and cirrhosis (F4 by elastography) presented with minimally to moderately painful blisters on his bilateral dorsal hands that had developed around weeks 8 to 9 of treatment with ledipasvir/sofosbuvir. The patient reported that no new blisters had appeared following completion of 12 weeks of treatment and that his current blisters were in various stages of healing. He reported alcohol use of 1 to 2 twelve-ounce beers daily and no history of dioxin exposure. His medications included doxepin, hydralazine, hydrochlorothiazide, quetiapine, folic acid, and thiamine. His hepatitis C viral load was 440,000 IU/mL prior to treatment. Tests for hepatitis B surface antigen and HIV antibodies were negative. His iron level was 135 µg/dL, total iron-binding capacity (TIBC) was 323 µg/dL, and ferritin was 299.0 ng/mL. His HFE
A physical examination on presentation revealed erosions with overlying hemorrhagic crusts on the bilateral dorsal hands (Figure).
At the 4-month follow-up, the patient reported no new blister formations. A physical examination revealed well-healed scars and several clustered milia on bilateral dorsal hands with no active vesicles or bullae noted.
Patient 2
An African American male aged 63 years presented with a 1-month history of moderately painful blisters on his bilateral dorsal hands during treatment of chronic HCV (genotype 1a) with ledipasvir/sofosbuvir. His medical history included gout, tobacco and alcohol addiction, osteoarthritis, and hepatic fibrosis (F3 by elastography). The patient’s medications included allopurinol, lisinopril, and hydrochlorothiazide. He reported no history of dioxin exposure. On the day of presentation, he was on week 9 of the 12-week treatment ledipasvir/sofosbuvir regimen. Laboratory results included an initial HCV viral load of 1,618,605 IU/mL. Tests for hepatitis B surface antigen and HIV antibodies were negative. His iron was 191 µg/dL, TIBC 388 µg/dL, and ferritin 459.0 ng/mL. After 4 weeks of treatment, the patient’s hepatitis C viral load was undetectable.
A physical examination revealed several resolving erosions to his bilateral dorsal hands, some of which had overlying crusting along with one small hemorrhagic vesicle on the right dorsal hand. A punch biopsy of the hemorrhagic vesicle was performed and demonstrated a cell-poor subepidermal blister with festooning of the dermal papilla. A direct immunofluorescence study showed immunoglobulin (Ig) G fluorescence along the dermal-epidermal junction and within vessel walls in the superficial dermis. Weak IgM and C3 fluorescence also was noted within vessel walls in the superficial dermis. All of the patient findings and history were consistent with PCT, although pseudo-PCT also was a consideration. A 24-hour urine sample yielded negative results for porphobilinogen. Urine porphyrin test results were not available, leading to a presumptive histological diagnosis of PCT.
The patient completed 11 of the prescribed 12 weeks of ledipasvir/sofosbuvir. The blisters resolved shortly thereafter.
Discussion
PCT has a well-established association with chronic HCV infection.4 We present 2 cases of a blistering disease clinically and histologically compatible with PCT that developed in patients only after initiation of treatment for chronic HCV with ledipasvir/sofosbuvir. One case was confirmed as PCT on the basis of compatible histopathologic findings and a urine porphyrin assay that showed elevated levels of uroporphyrins and carboxylated metabolites. The second case was clinically and histologically suggestive of PCT but not confirmed by urine porphyrin testing. In both patients, after 8 to 9 weeks of a 12-week course of antiviral therapy, the blistering lesions were noted but appeared to be resolving, and no new lesions were noted after discontinuation of therapy. It appeared that the antiviral treatment temporally triggered the initiation of the blistering skin disease, and as the chronic HCV infection cleared after treatment, the blistering lesions also began to resolve.
Mechanistically, it is known that the virally-induced hepatic damage leads to inhibition of uroporphyrinogen decarboxylase, and the subsequent oxidation of porphyrinogens to porphyrins. Cofactors such as HIV infection also may contribute to development of PCT.5
De novo PCT has been documented during therapy using interferon and ribavirin.6 The hemolytic anemia and increased hepatic iron were implicated as potential etiologies.6 Patients with HCV and PCT treated with the newer direct-acting antiviral therapies have been described to have experienced improvement in PCT symptoms.3
Although there were rare reports of deterioration in renal and liver function,7 reactivation of HBV infection,8 and Stevens-Johnson syndrome9 with antiviral therapy, these complications were not observed in these patients. Both patients also had successful resolution of HCV infection, and by completion of the antiviral therapy, the blistering also resolved.
Conclusion
PCT is an extrahepatic manifestation of HCV infection. Health care providers should be aware of the association of chronic HCV infection with PCT. The findings of PCT should not result in the delay or discontinuation of antiviral therapy.
Porphyria cutanea tarda (PCT) is the most common type of porphyria. The accumulation of porphyrin in various organ systems results from a deficiency of uroporphyrinogen decarboxylase (UROD).1-3 Chronic hepatitis C virus (HCV) causes a hepatic decrease in hepcidin production, resulting in increased iron absorption. Iron loading and increased oxidative stress in the liver leads to nonporphyrin inhibition of UROD production and to oxidation of porphyrinogens to porphyrins.4 This in turn leads to accumulation of uroporphyrins and carboxylated metabolites that can be detected in urine.4
Signs of PCT include blisters, vesicles, and possibly milia developing on sun-exposed areas of the skin, such as the face, forearms, and dorsal hands.4 Case reports have demonstrated a resolution of PCT in patients with chronic HCV with treatment with direct-acting antivirals (DAAs), such as ledipasvir/sofosbuvir.1,3 However, here we present 2 cases of patients who developed blistering diseases during treatment of chronic HCV with ledipasvir/sofosbuvir. Neither demonstrated complete resolution of symptoms during the treatment regimen.
Cases
Patient 1
A 63-year-old white male with a history of chronic HCV (genotype 1a), bipolar disorder, hyperlipidemia, tobacco dependence, and cirrhosis (F4 by elastography) presented with minimally to moderately painful blisters on his bilateral dorsal hands that had developed around weeks 8 to 9 of treatment with ledipasvir/sofosbuvir. The patient reported that no new blisters had appeared following completion of 12 weeks of treatment and that his current blisters were in various stages of healing. He reported alcohol use of 1 to 2 twelve-ounce beers daily and no history of dioxin exposure. His medications included doxepin, hydralazine, hydrochlorothiazide, quetiapine, folic acid, and thiamine. His hepatitis C viral load was 440,000 IU/mL prior to treatment. Tests for hepatitis B surface antigen and HIV antibodies were negative. His iron level was 135 µg/dL, total iron-binding capacity (TIBC) was 323 µg/dL, and ferritin was 299.0 ng/mL. His HFE
A physical examination on presentation revealed erosions with overlying hemorrhagic crusts on the bilateral dorsal hands (Figure).
At the 4-month follow-up, the patient reported no new blister formations. A physical examination revealed well-healed scars and several clustered milia on bilateral dorsal hands with no active vesicles or bullae noted.
Patient 2
An African American male aged 63 years presented with a 1-month history of moderately painful blisters on his bilateral dorsal hands during treatment of chronic HCV (genotype 1a) with ledipasvir/sofosbuvir. His medical history included gout, tobacco and alcohol addiction, osteoarthritis, and hepatic fibrosis (F3 by elastography). The patient’s medications included allopurinol, lisinopril, and hydrochlorothiazide. He reported no history of dioxin exposure. On the day of presentation, he was on week 9 of the 12-week treatment ledipasvir/sofosbuvir regimen. Laboratory results included an initial HCV viral load of 1,618,605 IU/mL. Tests for hepatitis B surface antigen and HIV antibodies were negative. His iron was 191 µg/dL, TIBC 388 µg/dL, and ferritin 459.0 ng/mL. After 4 weeks of treatment, the patient’s hepatitis C viral load was undetectable.
A physical examination revealed several resolving erosions to his bilateral dorsal hands, some of which had overlying crusting along with one small hemorrhagic vesicle on the right dorsal hand. A punch biopsy of the hemorrhagic vesicle was performed and demonstrated a cell-poor subepidermal blister with festooning of the dermal papilla. A direct immunofluorescence study showed immunoglobulin (Ig) G fluorescence along the dermal-epidermal junction and within vessel walls in the superficial dermis. Weak IgM and C3 fluorescence also was noted within vessel walls in the superficial dermis. All of the patient findings and history were consistent with PCT, although pseudo-PCT also was a consideration. A 24-hour urine sample yielded negative results for porphobilinogen. Urine porphyrin test results were not available, leading to a presumptive histological diagnosis of PCT.
The patient completed 11 of the prescribed 12 weeks of ledipasvir/sofosbuvir. The blisters resolved shortly thereafter.
Discussion
PCT has a well-established association with chronic HCV infection.4 We present 2 cases of a blistering disease clinically and histologically compatible with PCT that developed in patients only after initiation of treatment for chronic HCV with ledipasvir/sofosbuvir. One case was confirmed as PCT on the basis of compatible histopathologic findings and a urine porphyrin assay that showed elevated levels of uroporphyrins and carboxylated metabolites. The second case was clinically and histologically suggestive of PCT but not confirmed by urine porphyrin testing. In both patients, after 8 to 9 weeks of a 12-week course of antiviral therapy, the blistering lesions were noted but appeared to be resolving, and no new lesions were noted after discontinuation of therapy. It appeared that the antiviral treatment temporally triggered the initiation of the blistering skin disease, and as the chronic HCV infection cleared after treatment, the blistering lesions also began to resolve.
Mechanistically, it is known that the virally-induced hepatic damage leads to inhibition of uroporphyrinogen decarboxylase, and the subsequent oxidation of porphyrinogens to porphyrins. Cofactors such as HIV infection also may contribute to development of PCT.5
De novo PCT has been documented during therapy using interferon and ribavirin.6 The hemolytic anemia and increased hepatic iron were implicated as potential etiologies.6 Patients with HCV and PCT treated with the newer direct-acting antiviral therapies have been described to have experienced improvement in PCT symptoms.3
Although there were rare reports of deterioration in renal and liver function,7 reactivation of HBV infection,8 and Stevens-Johnson syndrome9 with antiviral therapy, these complications were not observed in these patients. Both patients also had successful resolution of HCV infection, and by completion of the antiviral therapy, the blistering also resolved.
Conclusion
PCT is an extrahepatic manifestation of HCV infection. Health care providers should be aware of the association of chronic HCV infection with PCT. The findings of PCT should not result in the delay or discontinuation of antiviral therapy.
1. Combalia A, To-Figueras J, Laguno M, Martinez-Rebollar M, Aguilera P. Direct-acting antivirals for hepatitis C virus induce a rapid clinical and biochemical remission of porphyria cutanea tarda. Br J Dermatol. 2017;177(5):e183-e184.
2. Younossi Z, Park H, Henry L, Adeyemi A, Stepanova M. Extrahepatic manifestations of hepatitis C: a meta-analysis of prevalence, quality of life, and economic burden. Gastroenterology. 2016;150(7):1599-1608.
3. Tong Y, Song YK, Tyring S. Resolution of porphyria cutanea tarda in patients with hepatitis C following ledipasvir/sofosbuvir combination therapy. JAMA Dermatol. 2016;152(12):1393-1395.
4. Ryan Caballes F, Sendi H, Bonkovsky H. Hepatitis C, porphyria cutanea tarda and liver iron: an update. Liver Int. 2012;32(6):880-893.
5. Quansah R, Cooper CJ, Said S, Bizet J, Paez D, Hernandez GT. Hepatitis C- and HIV-induced porphyria cutanea tarda. Am J Case Rep. 2014;15:35-40.
6. Azim J, McCurdy H, Moseley RH. Porphyria cutanea tarda as a complication of therapy for chronic hepatitis C. World J Gastroenterol. 2008;14(38):5913-5915.
7. Ahmed M. Harvoni-induced deterioration of renal and liver function. Adv Res Gastroentero Hepatol. 2017;2(3):555588.
8. De Monte A, Courion J, Anty R, et al. Direct-acting antiviral treatment in adults infected with hepatitis C virus: reactivation of hepatitis B virus coinfection as a further challenge. J Clin Virol. 2016;78:27-30.
9. Verma N, Singh S, Sawatkar G, Singh V. Sofosbuvir induced Steven Johnson Syndrome in a patient with hepatitis C virus-related cirrhosis. Hepatol Commun. 2017;2(1):16-20.
1. Combalia A, To-Figueras J, Laguno M, Martinez-Rebollar M, Aguilera P. Direct-acting antivirals for hepatitis C virus induce a rapid clinical and biochemical remission of porphyria cutanea tarda. Br J Dermatol. 2017;177(5):e183-e184.
2. Younossi Z, Park H, Henry L, Adeyemi A, Stepanova M. Extrahepatic manifestations of hepatitis C: a meta-analysis of prevalence, quality of life, and economic burden. Gastroenterology. 2016;150(7):1599-1608.
3. Tong Y, Song YK, Tyring S. Resolution of porphyria cutanea tarda in patients with hepatitis C following ledipasvir/sofosbuvir combination therapy. JAMA Dermatol. 2016;152(12):1393-1395.
4. Ryan Caballes F, Sendi H, Bonkovsky H. Hepatitis C, porphyria cutanea tarda and liver iron: an update. Liver Int. 2012;32(6):880-893.
5. Quansah R, Cooper CJ, Said S, Bizet J, Paez D, Hernandez GT. Hepatitis C- and HIV-induced porphyria cutanea tarda. Am J Case Rep. 2014;15:35-40.
6. Azim J, McCurdy H, Moseley RH. Porphyria cutanea tarda as a complication of therapy for chronic hepatitis C. World J Gastroenterol. 2008;14(38):5913-5915.
7. Ahmed M. Harvoni-induced deterioration of renal and liver function. Adv Res Gastroentero Hepatol. 2017;2(3):555588.
8. De Monte A, Courion J, Anty R, et al. Direct-acting antiviral treatment in adults infected with hepatitis C virus: reactivation of hepatitis B virus coinfection as a further challenge. J Clin Virol. 2016;78:27-30.
9. Verma N, Singh S, Sawatkar G, Singh V. Sofosbuvir induced Steven Johnson Syndrome in a patient with hepatitis C virus-related cirrhosis. Hepatol Commun. 2017;2(1):16-20.
HBV: Rethink the free pass for immune tolerant patients
MAUI, HAWAII – There might well be a cure for hepatitis B in coming years, just like there is now for hepatitis C, according to Norah Terrault, MD, chief of the division of GI and liver at the University of Southern California, Los Angeles.
“We are going to have a laundry list of new drugs” that are in the pipeline now. Phase 2 results “look encouraging. You will hear much more about this in the years ahead,” said Dr. Terrault, lead author of the 2018 American Association for the Study of Liver Diseases (AASLD) hepatitis B guidance.
For now, though, the field is largely limited to the nucleoside analogues tenofovir and entecavir. Treatment is often indefinite because, although hepatitis B virus (HBV) e-antigen is cleared, it usually doesn’t clear the HBV surface antigen, which is linked to liver cancer. “Even with e-antigen–negative patients, we feel that indefinite therapy is really the way to go,” Dr. Terrault said at the Gastroenterology Updates, IBD, Liver Disease Conference.
One of the biggest problems with that strategy is what to do when HBV does not seem to be much of a problem for carriers. Such patients are referred to as immune tolerant.
A newly recognized cancer risk
Immune tolerant patients tend to be young and have extremely high viral loads but no apparent ill effects, with normal ALT levels, normal histology, and no sign of cirrhosis. Although the AASLD recommends not treating these patients until they are 40 years old, waiting makes people nervous. “You have a hammer, you want to hit a nail,” Dr. Terrault said.
A recent review (Gut. 2018 May;67[5]:945-52) suggests that hitting the nail might be the way to go. South Korean investigators found that 413 untreated immune tolerate patients with a mean age of 38 years had more than twice the risk of liver cancer over 10 years than did almost 1,500 treated patients with active disease.
The study investigators concluded that “unnecessary deaths could be prevented through earlier antiviral intervention in select [immune tolerate] patients.”
This finding is one reason “we [AASLD] are rethinking the mantra of not treating the immune tolerant. There is a group that is transitioning” to active disease. “I’m thinking we should really [lower] the age cutoff” to 30 years, as some other groups [European Association for the Study of the Liver and Asian Pacific Association for the Study of the Liver] have done, plus “patients feel really good when they know the virus is controlled, and so do physicians,” Dr. Terrault said.
Entecavir versus tenofovir
Meanwhile, recent studies have raised the question of whether tenofovir is better than entecavir at preventing liver cancer.
A JAMA Oncology (JAMA Oncol. 2019 Jan 1;5[1]:30-6) study of some 25,000 patients in South Korea found a 32% lower risk of liver cancer when they were treated with tenofovir instead of entecavir. “This led to a lot of concern that maybe we should be moving all our patients to tenofovir,” she said.
Another study, a meta-analysis published earlier this year (Hepatol Int. 2020 Jan;14[1]:105-14), confirmed the difference in cancer risk when it combined those findings with other research. After adjustment for potential confounders, including disease stage and length of follow-up, “the difference disappeared” (hazard ration, 0.87; 95% confidence interval, 0.73-1.04), authors of the meta-analysis reported.
Study patients who received entecavir tended to be “treated many years ago and tended to have more severe [baseline] disease,” Dr. Terrault said.
So “while we see this difference, there’s not enough data yet for us to make a recommendation for our patients to switch from” entecavir to tenofovir. “Until a randomized controlled trial is done, this may remain an issue,” she said.
A drug holiday?
Dr. Terrault also reviewed research that suggests nucleoside analogue treatment can be stopped in e-antigen–negative patients after at least 3 years.
“The evidence is increasing that a finite NA [nucleoside analogue] treatment approach leads to higher HBsAg [hepatitis B surface antigen] loss rates, compared with the current long-term NA strategy, and can be considered a rational strategy to induce a functional cure in selected HBeAg-negative patients without cirrhosis who are willing to comply with close follow-up monitoring. ... The current observed functional cure rates” – perhaps about 40% – “would be well worth the effort,” editorialists commenting on the research concluded (Hepatology. 2018 Aug;68[2]:397-400).
It’s an interesting idea, Dr. Terrault said, but the virus will flare 8-12 weeks after treatment withdrawal, which is why it shouldn’t be considered in patients with cirrhosis.
Dr. Terrault is a consultant for AbbVie, Merck, Gilead, and other companies and disclosed grants from those companies and others.
MAUI, HAWAII – There might well be a cure for hepatitis B in coming years, just like there is now for hepatitis C, according to Norah Terrault, MD, chief of the division of GI and liver at the University of Southern California, Los Angeles.
“We are going to have a laundry list of new drugs” that are in the pipeline now. Phase 2 results “look encouraging. You will hear much more about this in the years ahead,” said Dr. Terrault, lead author of the 2018 American Association for the Study of Liver Diseases (AASLD) hepatitis B guidance.
For now, though, the field is largely limited to the nucleoside analogues tenofovir and entecavir. Treatment is often indefinite because, although hepatitis B virus (HBV) e-antigen is cleared, it usually doesn’t clear the HBV surface antigen, which is linked to liver cancer. “Even with e-antigen–negative patients, we feel that indefinite therapy is really the way to go,” Dr. Terrault said at the Gastroenterology Updates, IBD, Liver Disease Conference.
One of the biggest problems with that strategy is what to do when HBV does not seem to be much of a problem for carriers. Such patients are referred to as immune tolerant.
A newly recognized cancer risk
Immune tolerant patients tend to be young and have extremely high viral loads but no apparent ill effects, with normal ALT levels, normal histology, and no sign of cirrhosis. Although the AASLD recommends not treating these patients until they are 40 years old, waiting makes people nervous. “You have a hammer, you want to hit a nail,” Dr. Terrault said.
A recent review (Gut. 2018 May;67[5]:945-52) suggests that hitting the nail might be the way to go. South Korean investigators found that 413 untreated immune tolerate patients with a mean age of 38 years had more than twice the risk of liver cancer over 10 years than did almost 1,500 treated patients with active disease.
The study investigators concluded that “unnecessary deaths could be prevented through earlier antiviral intervention in select [immune tolerate] patients.”
This finding is one reason “we [AASLD] are rethinking the mantra of not treating the immune tolerant. There is a group that is transitioning” to active disease. “I’m thinking we should really [lower] the age cutoff” to 30 years, as some other groups [European Association for the Study of the Liver and Asian Pacific Association for the Study of the Liver] have done, plus “patients feel really good when they know the virus is controlled, and so do physicians,” Dr. Terrault said.
Entecavir versus tenofovir
Meanwhile, recent studies have raised the question of whether tenofovir is better than entecavir at preventing liver cancer.
A JAMA Oncology (JAMA Oncol. 2019 Jan 1;5[1]:30-6) study of some 25,000 patients in South Korea found a 32% lower risk of liver cancer when they were treated with tenofovir instead of entecavir. “This led to a lot of concern that maybe we should be moving all our patients to tenofovir,” she said.
Another study, a meta-analysis published earlier this year (Hepatol Int. 2020 Jan;14[1]:105-14), confirmed the difference in cancer risk when it combined those findings with other research. After adjustment for potential confounders, including disease stage and length of follow-up, “the difference disappeared” (hazard ration, 0.87; 95% confidence interval, 0.73-1.04), authors of the meta-analysis reported.
Study patients who received entecavir tended to be “treated many years ago and tended to have more severe [baseline] disease,” Dr. Terrault said.
So “while we see this difference, there’s not enough data yet for us to make a recommendation for our patients to switch from” entecavir to tenofovir. “Until a randomized controlled trial is done, this may remain an issue,” she said.
A drug holiday?
Dr. Terrault also reviewed research that suggests nucleoside analogue treatment can be stopped in e-antigen–negative patients after at least 3 years.
“The evidence is increasing that a finite NA [nucleoside analogue] treatment approach leads to higher HBsAg [hepatitis B surface antigen] loss rates, compared with the current long-term NA strategy, and can be considered a rational strategy to induce a functional cure in selected HBeAg-negative patients without cirrhosis who are willing to comply with close follow-up monitoring. ... The current observed functional cure rates” – perhaps about 40% – “would be well worth the effort,” editorialists commenting on the research concluded (Hepatology. 2018 Aug;68[2]:397-400).
It’s an interesting idea, Dr. Terrault said, but the virus will flare 8-12 weeks after treatment withdrawal, which is why it shouldn’t be considered in patients with cirrhosis.
Dr. Terrault is a consultant for AbbVie, Merck, Gilead, and other companies and disclosed grants from those companies and others.
MAUI, HAWAII – There might well be a cure for hepatitis B in coming years, just like there is now for hepatitis C, according to Norah Terrault, MD, chief of the division of GI and liver at the University of Southern California, Los Angeles.
“We are going to have a laundry list of new drugs” that are in the pipeline now. Phase 2 results “look encouraging. You will hear much more about this in the years ahead,” said Dr. Terrault, lead author of the 2018 American Association for the Study of Liver Diseases (AASLD) hepatitis B guidance.
For now, though, the field is largely limited to the nucleoside analogues tenofovir and entecavir. Treatment is often indefinite because, although hepatitis B virus (HBV) e-antigen is cleared, it usually doesn’t clear the HBV surface antigen, which is linked to liver cancer. “Even with e-antigen–negative patients, we feel that indefinite therapy is really the way to go,” Dr. Terrault said at the Gastroenterology Updates, IBD, Liver Disease Conference.
One of the biggest problems with that strategy is what to do when HBV does not seem to be much of a problem for carriers. Such patients are referred to as immune tolerant.
A newly recognized cancer risk
Immune tolerant patients tend to be young and have extremely high viral loads but no apparent ill effects, with normal ALT levels, normal histology, and no sign of cirrhosis. Although the AASLD recommends not treating these patients until they are 40 years old, waiting makes people nervous. “You have a hammer, you want to hit a nail,” Dr. Terrault said.
A recent review (Gut. 2018 May;67[5]:945-52) suggests that hitting the nail might be the way to go. South Korean investigators found that 413 untreated immune tolerate patients with a mean age of 38 years had more than twice the risk of liver cancer over 10 years than did almost 1,500 treated patients with active disease.
The study investigators concluded that “unnecessary deaths could be prevented through earlier antiviral intervention in select [immune tolerate] patients.”
This finding is one reason “we [AASLD] are rethinking the mantra of not treating the immune tolerant. There is a group that is transitioning” to active disease. “I’m thinking we should really [lower] the age cutoff” to 30 years, as some other groups [European Association for the Study of the Liver and Asian Pacific Association for the Study of the Liver] have done, plus “patients feel really good when they know the virus is controlled, and so do physicians,” Dr. Terrault said.
Entecavir versus tenofovir
Meanwhile, recent studies have raised the question of whether tenofovir is better than entecavir at preventing liver cancer.
A JAMA Oncology (JAMA Oncol. 2019 Jan 1;5[1]:30-6) study of some 25,000 patients in South Korea found a 32% lower risk of liver cancer when they were treated with tenofovir instead of entecavir. “This led to a lot of concern that maybe we should be moving all our patients to tenofovir,” she said.
Another study, a meta-analysis published earlier this year (Hepatol Int. 2020 Jan;14[1]:105-14), confirmed the difference in cancer risk when it combined those findings with other research. After adjustment for potential confounders, including disease stage and length of follow-up, “the difference disappeared” (hazard ration, 0.87; 95% confidence interval, 0.73-1.04), authors of the meta-analysis reported.
Study patients who received entecavir tended to be “treated many years ago and tended to have more severe [baseline] disease,” Dr. Terrault said.
So “while we see this difference, there’s not enough data yet for us to make a recommendation for our patients to switch from” entecavir to tenofovir. “Until a randomized controlled trial is done, this may remain an issue,” she said.
A drug holiday?
Dr. Terrault also reviewed research that suggests nucleoside analogue treatment can be stopped in e-antigen–negative patients after at least 3 years.
“The evidence is increasing that a finite NA [nucleoside analogue] treatment approach leads to higher HBsAg [hepatitis B surface antigen] loss rates, compared with the current long-term NA strategy, and can be considered a rational strategy to induce a functional cure in selected HBeAg-negative patients without cirrhosis who are willing to comply with close follow-up monitoring. ... The current observed functional cure rates” – perhaps about 40% – “would be well worth the effort,” editorialists commenting on the research concluded (Hepatology. 2018 Aug;68[2]:397-400).
It’s an interesting idea, Dr. Terrault said, but the virus will flare 8-12 weeks after treatment withdrawal, which is why it shouldn’t be considered in patients with cirrhosis.
Dr. Terrault is a consultant for AbbVie, Merck, Gilead, and other companies and disclosed grants from those companies and others.
EXPERT ANALYSIS FROM GUILD 2020
FDA approves weekly contraceptive patch Twirla
in women whose body mass index is less than 30 kg/m2 and for whom a combined hormonal contraceptive is appropriate.
Applied weekly to the abdomen, buttock, or upper torso (excluding the breasts), Twirla delivers a 30-mcg daily dose of ethinyl estradiol and 120-mcg daily dose of levonorgestrel.
“Twirla is an important addition to available hormonal contraceptive methods, allowing prescribers to now offer appropriate U.S. women a weekly transdermal option that delivers estrogen levels in line with labeled doses of many commonly prescribed oral contraceptives, David Portman, MD, an obstetrician/gynecologist in Columbus, Ohio, and a primary investigator of the SECURE trial, said in a news release issued by the company.
Twirla was evaluated in “a diverse population providing important data to prescribers and to women seeking contraception. It is vital to expand the full range of contraceptive methods and inform the choices that fit an individual’s family planning needs and lifestyle,” Dr. Portman added.
As part of approval, the FDA will require Agile Therapeutics to conduct a long-term, prospective, observational postmarketing study to assess risks for venous thromboembolism and arterial thromboembolism in new users of Twirla, compared with new users of other combined hormonal contraceptives.
Twirla is contraindicated in women at high risk for arterial or venous thrombotic disease, including women with a BMI equal to or greater than 30 kg/m2; women who have headaches with focal neurologic symptoms or migraine with aura; and women older than 35 years who have any migraine headache.
Twirla also should be avoided in women who have liver tumors, acute viral hepatitis, decompensated cirrhosis, liver disease, or undiagnosed abnormal uterine bleeding. It also should be avoided during pregnancy; in women who currently have or who have history of breast cancer or other estrogen- or progestin-sensitive cancer; in women who are hypersensitivity to any components of Twirla; and in women who use hepatitis C drug combinations containing ombitasvir/paraparesis/ritonavir, with or without dasabuvir.
Because cigarette smoking increases the risk for serious cardiovascular events from combined hormonal contraceptive use, Twirla also is contraindicated in women older than 35 who smoke.
Twirla will contain a boxed warning that will include these risks about cigarette smoking and the serious cardiovascular events, and it will stipulate that Twirla is contraindicated in women with a BMI greater than 30 kg/m2.
This article first appeared on Medscape.com.
in women whose body mass index is less than 30 kg/m2 and for whom a combined hormonal contraceptive is appropriate.
Applied weekly to the abdomen, buttock, or upper torso (excluding the breasts), Twirla delivers a 30-mcg daily dose of ethinyl estradiol and 120-mcg daily dose of levonorgestrel.
“Twirla is an important addition to available hormonal contraceptive methods, allowing prescribers to now offer appropriate U.S. women a weekly transdermal option that delivers estrogen levels in line with labeled doses of many commonly prescribed oral contraceptives, David Portman, MD, an obstetrician/gynecologist in Columbus, Ohio, and a primary investigator of the SECURE trial, said in a news release issued by the company.
Twirla was evaluated in “a diverse population providing important data to prescribers and to women seeking contraception. It is vital to expand the full range of contraceptive methods and inform the choices that fit an individual’s family planning needs and lifestyle,” Dr. Portman added.
As part of approval, the FDA will require Agile Therapeutics to conduct a long-term, prospective, observational postmarketing study to assess risks for venous thromboembolism and arterial thromboembolism in new users of Twirla, compared with new users of other combined hormonal contraceptives.
Twirla is contraindicated in women at high risk for arterial or venous thrombotic disease, including women with a BMI equal to or greater than 30 kg/m2; women who have headaches with focal neurologic symptoms or migraine with aura; and women older than 35 years who have any migraine headache.
Twirla also should be avoided in women who have liver tumors, acute viral hepatitis, decompensated cirrhosis, liver disease, or undiagnosed abnormal uterine bleeding. It also should be avoided during pregnancy; in women who currently have or who have history of breast cancer or other estrogen- or progestin-sensitive cancer; in women who are hypersensitivity to any components of Twirla; and in women who use hepatitis C drug combinations containing ombitasvir/paraparesis/ritonavir, with or without dasabuvir.
Because cigarette smoking increases the risk for serious cardiovascular events from combined hormonal contraceptive use, Twirla also is contraindicated in women older than 35 who smoke.
Twirla will contain a boxed warning that will include these risks about cigarette smoking and the serious cardiovascular events, and it will stipulate that Twirla is contraindicated in women with a BMI greater than 30 kg/m2.
This article first appeared on Medscape.com.
in women whose body mass index is less than 30 kg/m2 and for whom a combined hormonal contraceptive is appropriate.
Applied weekly to the abdomen, buttock, or upper torso (excluding the breasts), Twirla delivers a 30-mcg daily dose of ethinyl estradiol and 120-mcg daily dose of levonorgestrel.
“Twirla is an important addition to available hormonal contraceptive methods, allowing prescribers to now offer appropriate U.S. women a weekly transdermal option that delivers estrogen levels in line with labeled doses of many commonly prescribed oral contraceptives, David Portman, MD, an obstetrician/gynecologist in Columbus, Ohio, and a primary investigator of the SECURE trial, said in a news release issued by the company.
Twirla was evaluated in “a diverse population providing important data to prescribers and to women seeking contraception. It is vital to expand the full range of contraceptive methods and inform the choices that fit an individual’s family planning needs and lifestyle,” Dr. Portman added.
As part of approval, the FDA will require Agile Therapeutics to conduct a long-term, prospective, observational postmarketing study to assess risks for venous thromboembolism and arterial thromboembolism in new users of Twirla, compared with new users of other combined hormonal contraceptives.
Twirla is contraindicated in women at high risk for arterial or venous thrombotic disease, including women with a BMI equal to or greater than 30 kg/m2; women who have headaches with focal neurologic symptoms or migraine with aura; and women older than 35 years who have any migraine headache.
Twirla also should be avoided in women who have liver tumors, acute viral hepatitis, decompensated cirrhosis, liver disease, or undiagnosed abnormal uterine bleeding. It also should be avoided during pregnancy; in women who currently have or who have history of breast cancer or other estrogen- or progestin-sensitive cancer; in women who are hypersensitivity to any components of Twirla; and in women who use hepatitis C drug combinations containing ombitasvir/paraparesis/ritonavir, with or without dasabuvir.
Because cigarette smoking increases the risk for serious cardiovascular events from combined hormonal contraceptive use, Twirla also is contraindicated in women older than 35 who smoke.
Twirla will contain a boxed warning that will include these risks about cigarette smoking and the serious cardiovascular events, and it will stipulate that Twirla is contraindicated in women with a BMI greater than 30 kg/m2.
This article first appeared on Medscape.com.
HBV: Surface antigen titer and ALT predict seroconversion
Among patients with hepatitis B virus (HBV) infection who are not receiving antiviral therapy, surface antigen titers and alanine aminotransferase (ALT) levels may independently predict spontaneous seroconversion, based on a recent case-control study.
, reported principal author Sammy Saab, MD, of the University of California, Los Angeles, and colleagues.
While the predictive value of HBsAg titers has been demonstrated for patients undergoing antiviral therapy, data are limited for spontaneous seroconversion, the investigators wrote in Journal of Clinical Gastroenterology.
To learn more about this scenario, the investigators reviewed medical records from 2,126 patients who visited a large community practice in the Los Angeles area between 2014 and 2019. Cases were defined by HBV infection with seroconversion, whereas matched controls were defined by HBV without seroconversion. A variety of demographic and clinical data were also evaluated, including age, ethnicity, sex, HBsAg titer, ALT, HBV DNA, total cholesterol, presence of fatty liver, and other factors.
The investigators identified 167 patients with HBV who were not on antiviral therapy. Of these, 14 underwent seroconversion, and were matched with 70 patients who did not seroconvert. All patients were of Asian descent, most were women, and none had cirrhosis.
Across all demographic and clinical parameters, the two factors that significantly differed between cases and controls were ALT and HBsAg titer. The mean ALT for patients who seroconverted was 17.6 U/L, versus 25.1 U/L in those who did not undergo seroconversion (P less than .01). Similarly, mean titer was lower in the seroconversion group (459.8 vs. 782.0 IU/mL; P = .01).
The investigators noted that seroconversion was more common among patients with an HBsAg titer level less than 1,000 IU/mL. Specifically, 79% of patients who seroconverted had a titer less than 1,000 IU/mL, compared with just 16% of patients who did not seroconvert (P = .001).
HBV DNA levels were not predictive of seroconversion, the investigators noted, which aligns with most, but not all, previous research.
The investigators reported no disclosures.
SOURCE: Wu CF et al. J Clin Gastroenterol. 2020 Feb 11. doi: 10.1097/MCG.0000000000001324.
Among patients with hepatitis B virus (HBV) infection who are not receiving antiviral therapy, surface antigen titers and alanine aminotransferase (ALT) levels may independently predict spontaneous seroconversion, based on a recent case-control study.
, reported principal author Sammy Saab, MD, of the University of California, Los Angeles, and colleagues.
While the predictive value of HBsAg titers has been demonstrated for patients undergoing antiviral therapy, data are limited for spontaneous seroconversion, the investigators wrote in Journal of Clinical Gastroenterology.
To learn more about this scenario, the investigators reviewed medical records from 2,126 patients who visited a large community practice in the Los Angeles area between 2014 and 2019. Cases were defined by HBV infection with seroconversion, whereas matched controls were defined by HBV without seroconversion. A variety of demographic and clinical data were also evaluated, including age, ethnicity, sex, HBsAg titer, ALT, HBV DNA, total cholesterol, presence of fatty liver, and other factors.
The investigators identified 167 patients with HBV who were not on antiviral therapy. Of these, 14 underwent seroconversion, and were matched with 70 patients who did not seroconvert. All patients were of Asian descent, most were women, and none had cirrhosis.
Across all demographic and clinical parameters, the two factors that significantly differed between cases and controls were ALT and HBsAg titer. The mean ALT for patients who seroconverted was 17.6 U/L, versus 25.1 U/L in those who did not undergo seroconversion (P less than .01). Similarly, mean titer was lower in the seroconversion group (459.8 vs. 782.0 IU/mL; P = .01).
The investigators noted that seroconversion was more common among patients with an HBsAg titer level less than 1,000 IU/mL. Specifically, 79% of patients who seroconverted had a titer less than 1,000 IU/mL, compared with just 16% of patients who did not seroconvert (P = .001).
HBV DNA levels were not predictive of seroconversion, the investigators noted, which aligns with most, but not all, previous research.
The investigators reported no disclosures.
SOURCE: Wu CF et al. J Clin Gastroenterol. 2020 Feb 11. doi: 10.1097/MCG.0000000000001324.
Among patients with hepatitis B virus (HBV) infection who are not receiving antiviral therapy, surface antigen titers and alanine aminotransferase (ALT) levels may independently predict spontaneous seroconversion, based on a recent case-control study.
, reported principal author Sammy Saab, MD, of the University of California, Los Angeles, and colleagues.
While the predictive value of HBsAg titers has been demonstrated for patients undergoing antiviral therapy, data are limited for spontaneous seroconversion, the investigators wrote in Journal of Clinical Gastroenterology.
To learn more about this scenario, the investigators reviewed medical records from 2,126 patients who visited a large community practice in the Los Angeles area between 2014 and 2019. Cases were defined by HBV infection with seroconversion, whereas matched controls were defined by HBV without seroconversion. A variety of demographic and clinical data were also evaluated, including age, ethnicity, sex, HBsAg titer, ALT, HBV DNA, total cholesterol, presence of fatty liver, and other factors.
The investigators identified 167 patients with HBV who were not on antiviral therapy. Of these, 14 underwent seroconversion, and were matched with 70 patients who did not seroconvert. All patients were of Asian descent, most were women, and none had cirrhosis.
Across all demographic and clinical parameters, the two factors that significantly differed between cases and controls were ALT and HBsAg titer. The mean ALT for patients who seroconverted was 17.6 U/L, versus 25.1 U/L in those who did not undergo seroconversion (P less than .01). Similarly, mean titer was lower in the seroconversion group (459.8 vs. 782.0 IU/mL; P = .01).
The investigators noted that seroconversion was more common among patients with an HBsAg titer level less than 1,000 IU/mL. Specifically, 79% of patients who seroconverted had a titer less than 1,000 IU/mL, compared with just 16% of patients who did not seroconvert (P = .001).
HBV DNA levels were not predictive of seroconversion, the investigators noted, which aligns with most, but not all, previous research.
The investigators reported no disclosures.
SOURCE: Wu CF et al. J Clin Gastroenterol. 2020 Feb 11. doi: 10.1097/MCG.0000000000001324.
FROM JOURNAL OF CLINICAL GASTROENTEROLOGY
Outcomes Comparison of the Veterans’ Choice Program With the Veterans Affairs Healthcare System for Hepatitis C Treatment
Population studies show high prevalence of chronic hepatitis C virus (HCV) infection among veterans, especially Vietnam War era veterans.1,2 The development of safe and efficacious direct-acting antiviral (DAA) medications to treat HCV infection made the majority of those infected eligible for treatment. However, the large number of veterans needing DAA treatment stressed the resources of the US Department of Veterans Affairs (VA) health care system. This occurred while Congress was focused on reducing wait times for veterans receiving care at the VA.
Congress passed the Veterans Access, Choice, and Accountability Act (Choice) on August 7, 2014, leading to the creation of the Veterans Choice Program. Legislators felt there were inappropriate delays in care at the VA, and the Choice program was meant to address this problem and provide an “apples-to-apples comparison [of the VA] with non-VA hospitals.”3
Congress acknowledged the importance of curing HCV in the veteran population and allocated $1.5 billion for fiscal year (FY) 2016 for DAAs. The VA Central Office (VACO) carefully monitored these resources. The first policy memorandum from VACO for HCV care, issued on May 21, 2015, recommended that the sickest patients who will benefit from the treatment “receive priority over those who are less ill.”4,5 Those who met criteria for advanced liver disease were prioritized for treatment at the VA, while those who did not meet criteria were given the option of receiving treatment through Choice, or waiting for a change in policy.6 Over time, revisions to the guidelines relaxed the criteria for VA treatment eligibility, and on February 24, 2016, all restrictions on HCV treatment at the VA were lifted.7,8
The aim of this study was to provide a comparison of VA and non-VA care, specifically to determine whether care provided through Choice was timelier and more cost effective than care provided by the VA, and whether there was a quality difference. The high prevalence among veterans, well-established standards of care, and finite treatment course with clear indicators of success and failure makes HCV treatment an ideal disease with which to make this comparison.
Methods
We retrospectively analyzed the VA electronic health records of all veterans seen in the VA Loma Linda Healthcare System (VALLHCS) Hepatology clinic for chronic HCV infection during FY 2016 who were referred to Choice for HCV treatment. One hundred veterans met these criteria, encompassing the Choice population; 71 were seen at least once by a non-VA (Choice) health care provider (HCP) and 61 completed a treatment regimen through Choice. Treatment completion was defined as cessation of medication after the planned duration of therapy, or early termination of medication without resumption by that HCP. The Choice population was matched to an equal number of veterans who received HCV treatment from VALLHCS HCPs.
Data collected included age, gender, HCV genotype, determinants of liver fibrosis, and treatment success (defined as sustained virologic response at 12 weeks after the last dose of medication [SVR12]). Determinants of liver fibrosis included documented cirrhosis or complications of cirrhosis, Fibrosis-4 score (Fib-4), and platelet count.
Treatment failures were categorized as nonresponse (defined as detectable HCV viral load at the end of treatment), relapse (defined as an undetectable HCV viral load at the end of treatment with a subsequent positive test), and early termination (defined as a failure to complete the planned treatment regimen). Documented patient nonadherence, medical comorbidities that affected the treatment protocol, mental health diagnoses, and active social issues (defined as active or history of heavy alcohol use, active or history of illicit drug use, lack of social support, and homelessness) were noted.
Timeliness of delivery of care was measured in days. For the VA group, the wait time was defined as the date the consult for HCV treatment was placed to the date of the initial appointment with the HCV treatment provider. For the Choice group, the wait time was defined as the date the referral to the Choice program was made to the date of the initial appointment with the Choice HCP. Treatment regimens were evaluated for appropriateness based on guidelines from VACO and the American Association for the Study of Liver Diseases.9-11
Tests performed by Choice providers were evaluated for whether they were relevant to HCV care and whether similar data already were available from VALLHCS. Tests that were not indicated were identified as unnecessary costs incurred by the Choice program, as were tests that had to be repeated at the VA because of a lack of documentation from the Choice provider. All medications given inappropriately were considered added costs. Medicare reimbursement rates for the most applicable Current Procedural Terminology (CPT) code and VA national contract pricing for medications were used for calculations. This study was approved by the VALLHCS institutional review board.
Statistical Analysis
IBM (Armonk, NY) Statistical Package for Social Sciences software was used to evaluate for differences in Fib-4, platelet count, prevalence of cirrhosis, prevalence of medical comorbidities, prevalence of mental health comorbidities, prevalence of the social issues defined in the Methods section, time from referral to time of appointment date, and SVR12 rate between the VA and Choice groups.
Exclusions
There were 15 veterans in the VA group who had a wait time of > 100 days. Of these, 5 (33%) were initially Choice referrals, but due to negative interactions with the Choice provider, the veterans returned to VALLHCS for care. Two of the 15 (13%) did not keep appointments and were lost to follow up. Six of the 15 (40%) had medical comorbidities that required more immediate attention, so HCV treatment initiation was deliberately moved back. The final 2 veterans scheduled their appointments unusually far apart, artificially increasing their wait time. Given that these were unique situations and some of the veterans received care from both Choice and VA providers, a decision was made to exclude these individuals from the study.
It has been shown that platelet count correlates with degree of liver fibrosis, a concept that is the basis for the Fib-4 scoring system.12 Studies have shown that platelet count is a survival predictor in those with cirrhosis, and thrombocytopenia is a negative predictor of HCV treatment success using peginterferon and ribavirin.13,14 Therefore, the VA memorandum automatically assigned the sickest individuals to the VA for HCV treatment. The goal of this study was to compare the impact of factors other than stage of fibrosis on HCV treatment success, which is why the 12 veterans with platelet count < 100,000 in the VA group were excluded. There were no veterans with platelet count < 100,000 in the Choice group.
When comparing SVR12 rates between the VA and Choice groups, every veteran treated at VALLHCS in FY 2016 was included, increasing the number in the VA group from 100 to 320 and therefore the power of this comparison.
Results
A summary of the statistical analysis can be found in Table 1. The genotype distribution was consistent with epidemiological studies, including those specific to veterans.15,16 There were statistically significant differences (P < .001) in mean Fib-4 and mean platelet count. The VA group had a higher Fib-4 and lower platelet count. Seventy-four percent of the VA population was defined as cirrhotic, while only 3% of the Choice population met similar criteria (P < .001). The VA and Choice groups were similar in terms of age, gender, and genotype distribution (Table 2).
The VA group was found to have a higher prevalence of comorbidities that affected HCV treatment. These conditions included but were not limited to: chronic kidney disease that precluded the use of certain medications, any condition that required medication with a known interaction with DAAs (ie, proton pump inhibitors, statins, and amiodarone), and cirrhosis if it impacted the treatment regimen. The difference in the prevalence of mental health comorbidities was not significant (P = .39), but there was a higher prevalence of social issues among the VA group (P = .002).
The mean wait time from referral to appointment was 28.6 days for the VA group and 42.3 days for the Choice group (P < .001), indicating that a Choice referral took longer to complete than a referral within the VA for HCV treatment. Thirty of the 71 (42%) veterans seen by a Choice provider accrued extraneous cost, with a mean additional cost of $8,561.40 per veteran. In the Choice group, 61 veterans completed a treatment regimen with the Choice HCP. Fifty-five veterans completed treatment and had available SVR12 data (6 were lost to follow up without SVR12 testing) and 50 (91%) had confirmed SVR12. The charts of the 5 treatment failures were reviewed to discern the cause for failure. Two cases involved early termination of therapy, 3 involved relapse and 2 failed to comply with medication instructions. There was 1 case of the Choice HCP not addressing simultaneous use of ledipasvir and a proton pump inhibitor, potentially causing an interaction, and 1 case where both the VA and Choice providers failed to recognize indicators of cirrhosis, which impacted the regimen used.
In the VALLHCS group, records of 320 veterans who completed treatment and had SVR12 testing were reviewed. While the Choice memorandum was active, veterans selected to be treated at VALLHCS had advanced liver fibrosis or cirrhosis, medical and mental health comorbidities that increased the risk of treatment complications or were considered to have difficulty adhering to the medication regimen. For this group, 296 (93%) had confirmed SVR12. Eighteen of the 24 (75%) treatment failures were complicated by nonadherence, including all 13 cases of early termination. One patient died from complications of decompensated cirrhosis before completing treatment, and 1 did not receive HCV medications during a hospital admission due to poor coordination of care between the VA inpatient and outpatient pharmacy services, leading to multiple missed doses.
The difference in SVR12 rates (ie, treatment failure rates), between the VA and Choice groups was not statistically significant (P = .61). None of the specific reasons for treatment failure had a statistically significant difference between groups. A treatment failure analysis is shown in Table 3, and Table 4 indicates the breakdown of treatment regimens.
Discussion
The Veterans Health Administration (VHA) is the largest integrated health care system in the US, consisting of 152 medical centers and > 1,700 sites of care. The VA has the potential to meet the health care needs of 21.6 million veterans. About 9 million veterans are enrolled in the VA system and 5.9 million received health care through VHA.17 However, every medical service cannot realistically be made available at every facility, and some veterans have difficulty gaining access to VHA care; distance and wait times have been well-publicized issues that need further exploration.18,19 The Choice program is an attempt to meet gaps in VA coverage using non-VA HCPs.
HCV infection is a specific diagnosis with national treatment guidelines and well-studied treatments; it can be cured, with an evidence-based definition of cure. The VACO policy memorandum to refer less sick veterans to Choice while treating sicker veterans at the VA provided the opportunity to directly compare the quality of the 2 programs. The SVR12 rates of VALLHCS and Choice providers were comparable to the national average at the time, and while the difference in SVR12 rate was not significant, VALLHCS treated a significantly higher number of patients with cirrhosis because of the referral criteria.20
The significant difference in medical comorbidities between the VA and Choice groups was not surprising, partly because of the referral criteria. Cirrhosis can impact the treatment regimen, especially in regard to use of ribavirin. Since the presence of mental health comorbidities did not affect selection into the Choice group, it makes sense that there was no significant difference in prevalence between the groups.
VACO allowed veterans with HCV treatment plans that VA HCPs felt were too complicated for the Choice program to be treated by VHA HCPs.9 VALLHCS exercised this right for veterans at risk for nonadherence, because in HCV treatment, nonadherence leads to treatment failure and development of drug resistant virus strains. Therefore, veterans who would have difficulty traveling to VALLHCS to pick up medications, those who lacked means of communication (such as those who were homeless), and those who had active substance abuse were treated at the VA, where closer monitoring and immediate access to a wide range of services was possible. Studies have confirmed the impact of these types of issues on HCV treatment adherence and success.21 This explains the higher prevalence of social issues in the VA group.
For an internal referral for HCV treatment at VALLHCS, the hepatology provider submits a consult request to the HCV treatment provider, who works in the same office, making direct communication simple. The main administrative limiting factor to minimizing wait times is the number of HCPs, which is dependent on hiring allowances.
When a veteran is referred to Choice, the VA provider places a consult for non-VA care, which the VA Office of Community Care processes by compiling relevant documents and sending the package to Triwest Healthcare Alliance, a private insurance processor contracted with the VA. Triwest selects the Choice provider, often without any input from the VA, and arranges the veteran’s initial appointment.22 Geographic distance to the veteran’s address is the main selection criteria for Triwest. Documents sent between the Choice and VA HCPs go through the Office of Community Care and Triwest. This significantly increases the potential for delays and failed communication. Triwest had a comprehensive list of providers deemed to be qualified to treat HCV within the geographic catchment of VALLHCS. This list was reviewed, and all veterans referred to Choice had HCPs near their home address; therefore, availability of Choice HCPs was not an issue.
The VA can provide guidance on management of the veteran in the form of bundle packages containing a list of services for which the Choice provider is authorized to provide, and ones the Choice provider is not authorized to provide. Some Choice HCPs ordered tests that were not authorized for HCV treatment such as esophagogastroduodenoscopy, colonoscopy, and liver biopsy. In all, 17 of 71 (24%) veterans seen by Choice HCPs had tests performed or ordered that VA HCPs would not have obtained for the purpose of HCV treatment (Table 5).
In order to prevent veterans from receiving unnecessary tests, a VALLHCS hepatologist asked to be notified by VA administrators overseeing Choice referrals whenever a secondary authorization request (SAR) was submitted by a Choice HCP. This strategy is not standard VA practice, therefore at many VA sites these requested tests would have been performed by the Choice HCP, which is why SARs were factored into cost analysis.
SVR12 test results that were drawn too early and had to be repeated at VALLHCS were a cost unique to the Choice program. Duplicate tests, particularly imaging studies and blood work, were extraneous costs. The largest extraneous costs were treatment regimens prescribed by Choice HCPs that did not follow standard of care and required VA provider intervention. Thirty of the 71 (42%) veterans seen by a Choice provider accrued a mean $8,561.40 in extra costs. As a result, the Choice program cost VHA $250,000 more to provide care for 30 veterans (enough to pay for a physician’s annual salary).
Some inappropriate treatment regimens were the result of Choice HCP error, such as 1 case in which a veteran was inadvertently switched from ledipasvir/sofosbuvir to ombitasvir/paritaprevir/ritonavir/dasabuvir after week 4. The veteran had to start therapy over but still achieved SVR12. Other cases saw veterans receive regimens for which they had clear contraindications, such as creatinine clearance < 30 mL/min/1.73m2 for sofosbuvir or a positive resistance panel for specific medications. Eleven of 62 (18%) veterans who were started on HCV treatment by a Choice HCP received a regimen not consistent with VA guidelines—an alarming result.
Follow up for veterans referred to Choice was extremely labor intensive, and assessment of personnel requirements in a Choice-based VA system must take this into consideration. The Choice HCP has no obligation to communicate with the VA HCP. At the time of chart review, 57 of 71 (80%) Choice veterans had inadequate documentation to make a confident assessment of the treatment outcome. Multiple calls to the offices of the Choice HCP were needed to acquire records, and veterans had to be tracked down for additional tests. Veterans often would complete treatment and stop following up with the Choice provider before SVR12 confirmation. The VA hepatology provider reviewing Choice referrals served as clinician, case manager, and clerk in order to get veterans to an appropriate end point in their hepatitis C treatment, with mostly unmeasured hours of work.
Limitations
The study population size was limited by the number of veterans able to complete treatment through Choice. The parameters in the VACO policy memos automatically selected the VA and Choice groups but made them clinically distinct populations. New treatment medications were released during the study period, which impacted management strategy. Occasionally, VA and non-VA HCPs preferred different treatment regimens, leading to variation in the distribution of regimens used despite similar genotype distribution (Tables 2 and 4). In addition, a retrospective study is at risk for recall bias. A prospective study randomizing veterans to the Choice and VA groups is an important future endeavor. Comparing veteran satisfaction for Choice and VA services is also crucial.
Conclusions
This study demonstrates that the VA was able to provide more cost-effective and more timely care for HCV treatment to a relatively sicker population with no reduction in treatment success when compared with non-VA HCPs through the Choice program. While the Choice program can help veterans receive services they may otherwise not have access to and reduce travel time, the current system introduces inefficiencies that delay care and decrease cost-effectiveness. The Choice HCP selection process is based on proximity rather than quality, which may place the veteran at risk for receiving substandard care. Large-scale quality of care studies that compare efficiency measures, clinical outcomes, patient demographics, travel distance, cost efficacy and patient satisfaction for veterans receiving similar services at a VA facility and through Choice should be performed to ensure that veterans receive the best care available.
1. Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160(5):293-300.
2. Dominitz JA, Boyko EJ, Koepsell TD, et al. Elevated prevalence of hepatitis C infection in users of United States veterans medical centers. Hepatology. 2005;41(1):88-96.
3. Veterans Access, Choice, and Accountability Act of 2014. 42 USC §1395 (2014).
4. Tuchschmidt J. Attachment C: Provision of hepatitis C treatment. US Department of Veterans Affairs Central Office Memorandum from the Principal Deputy Under Secretary for Health. http://vaww.hepatitis.va.gov/education/choice-provision-hcv-treatment.asp. Published May 21, 2015. [Nonpublic site.]
5. Tuchschmidt J. Attachment A: Provision of hepatitis C (HCV) treatment through the Choice program. US Department of Veterans Affairs Central Office Memorandum from the Principal Deputy Under Secretary for Health. http://vaww.hepatitis.va.gov/pdf/choice-attachment-a-FY16.pdf. Published May 21, 2015. [Nonpublic site.]
6. Tuchschmidt J. Attachment B: Initiation of hepatitis C virus (HCV) treatment: protocol for prioritization. US Department of Veterans Affairs Central Office Memorandum from the Principal Deputy Under Secretary for Health. http://vaww.hepatitis.va.gov/pdf/provision-HCV-treatment-attachment-b.pdf. Published May 21, 2015. [Nonpublic site.]
7. Murphy, JP. Hepatitis C virus funding and prioritization status. US Department of Veterans Affairs Central Office Memorandum from the Assistant Deputy Under Secretary for Health for Clinical Operations. http://vaww.hepatitis.va.gov/education/choice-memo-hcv-funding-and-prioritization-status-01272016.asp. Published January 27, 2016. [Nonpublic site.]
8. Lynch TJ, McCarthy MF. Hepatitis C virus funding and prioritization status update. US Department of Veterans Affairs Central Office Memorandum from the Assistant Deputy Under Secretary for Health for Clinical Operations and Acting Assistant Deputy Under Secretary for Health for Patient Care Services. http://vaww.hepatitis.va.gov/education/choice-funding-update-feb-2016.asp. Published February 24, 2016. [Nonpublic site.]
9. Morgan TR, Yee H; US Department of Veterans Affairs National Hepatitis C Resource Center Program and the National Viral Hepatitis Program in the Office of Patient Care Services. Chronic hepatitis C virus (HCV) infection: treatment considerations. http://vaww.hepatitis.va.gov/pdf/treatment-considerations-2016-03-28.pdf. Published March 28, 2016. [Nonpublic site.]
10. American Association for the Study of Liver Diseases; Infectious Diseases Society of America. Initial treatment box. http://hcvguidelines.org/full-report/initial-treatment-box-summary-recommendations-patients-who-are-initiating-therapy-hcv. Updated November 6, 2019. Accessed September 27, 2016
11. AASLD/IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62(3): 932-954.
12. Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006; 43(6):1317-1325.
13. Realdi G, Fattovich G, Hadziyannis S, et al. Survival and prognostic factors in 366 patients with compensated cirrhosis type B: a multicenter study. The Investigators of the European Concerted Action on Viral Hepatitis (EUROHEP). J Hepatol. 1994;21(4):656-666.
14. Kanda T, Kato K, Tsubota A, et al. Platelet count and sustained virological response in hepatitis C treatment. World J Hepatol. 2013;5(4):182-188.
15. Manos MM, Shvachko VA, Murphy RC, Arduino JM, Shire NJ. Distribution of hepatitis C virus genotypes in a diverse US integrated health care population. J Med Virol. 2012;84(11):1744-1750.
16. Cheung RC. Epidemiology of hepatitis C virus infection in American veterans. Am J Gastroenterol. 2000;95(3):740-747.
17. Bagalman E. The number of Veterans that use VA health care services: a fact sheet. Congressional Research Service Report R43579. https://fas.org/sgp/crs/misc/R43579.pdf. Published June 3, 2014. Accessed November 25, 2019.
18. US General Accounting Office. Report to the Ranking Minority Member, Subcommittee on Compensation, Pension, Insurance, and Memorial Affairs, Committee on Veterans’ Affairs, US House of Representatives. How distance from VA facilities affects veterans’ use of VA services. https://www.gao.gov/assets/230/221992.pdf. Published December 1995. Accessed November 25, 2019.
19. Bronstein S, Griffin D. A fatal wait: Veterans languish and die on a VA hospital’s secret list. http://www.cnn.com/2014/04/23/health/veterans-dying-health-care-delays/index.html. Published April 23, 2014. Accessed November 25, 2019.
20. Ioannou GN, Beste LA, Chang MF, et al. Effectiveness of sofosbuvir, ledipasvir/sofosbuvir, or paritaprevir/ritonavir/ombitasvir and dasabuvir regimens for treatment of patients with hepatitis C in the Veterans Affairs national health care system. Gastroenterology. 2016;151(3):457-471.
21. Malespin MH, Harris C, Kanar O, et al. Barriers to treatment of chronic hepatitis C with direct acting antivirals in an urban clinic. Ann Hepatol. 2019;18(2):304–309.
22. Tuchschmidt J. Attachment D: Hepatitis C virus (HCV) fact sheet for Veterans Choice Program for both VA and Choice providers. US Department of Veterans Affairs Central Office Memorandum from the Deputy Under Secretary for Health for Policy and Services and the Acting Deputy Undersecretary for Health for Operations and Management. http://vaww.hepatitis.va.gov/educatiochoice-provision-HCV-treatment-additional.asp. [Nonpublic site.]
Population studies show high prevalence of chronic hepatitis C virus (HCV) infection among veterans, especially Vietnam War era veterans.1,2 The development of safe and efficacious direct-acting antiviral (DAA) medications to treat HCV infection made the majority of those infected eligible for treatment. However, the large number of veterans needing DAA treatment stressed the resources of the US Department of Veterans Affairs (VA) health care system. This occurred while Congress was focused on reducing wait times for veterans receiving care at the VA.
Congress passed the Veterans Access, Choice, and Accountability Act (Choice) on August 7, 2014, leading to the creation of the Veterans Choice Program. Legislators felt there were inappropriate delays in care at the VA, and the Choice program was meant to address this problem and provide an “apples-to-apples comparison [of the VA] with non-VA hospitals.”3
Congress acknowledged the importance of curing HCV in the veteran population and allocated $1.5 billion for fiscal year (FY) 2016 for DAAs. The VA Central Office (VACO) carefully monitored these resources. The first policy memorandum from VACO for HCV care, issued on May 21, 2015, recommended that the sickest patients who will benefit from the treatment “receive priority over those who are less ill.”4,5 Those who met criteria for advanced liver disease were prioritized for treatment at the VA, while those who did not meet criteria were given the option of receiving treatment through Choice, or waiting for a change in policy.6 Over time, revisions to the guidelines relaxed the criteria for VA treatment eligibility, and on February 24, 2016, all restrictions on HCV treatment at the VA were lifted.7,8
The aim of this study was to provide a comparison of VA and non-VA care, specifically to determine whether care provided through Choice was timelier and more cost effective than care provided by the VA, and whether there was a quality difference. The high prevalence among veterans, well-established standards of care, and finite treatment course with clear indicators of success and failure makes HCV treatment an ideal disease with which to make this comparison.
Methods
We retrospectively analyzed the VA electronic health records of all veterans seen in the VA Loma Linda Healthcare System (VALLHCS) Hepatology clinic for chronic HCV infection during FY 2016 who were referred to Choice for HCV treatment. One hundred veterans met these criteria, encompassing the Choice population; 71 were seen at least once by a non-VA (Choice) health care provider (HCP) and 61 completed a treatment regimen through Choice. Treatment completion was defined as cessation of medication after the planned duration of therapy, or early termination of medication without resumption by that HCP. The Choice population was matched to an equal number of veterans who received HCV treatment from VALLHCS HCPs.
Data collected included age, gender, HCV genotype, determinants of liver fibrosis, and treatment success (defined as sustained virologic response at 12 weeks after the last dose of medication [SVR12]). Determinants of liver fibrosis included documented cirrhosis or complications of cirrhosis, Fibrosis-4 score (Fib-4), and platelet count.
Treatment failures were categorized as nonresponse (defined as detectable HCV viral load at the end of treatment), relapse (defined as an undetectable HCV viral load at the end of treatment with a subsequent positive test), and early termination (defined as a failure to complete the planned treatment regimen). Documented patient nonadherence, medical comorbidities that affected the treatment protocol, mental health diagnoses, and active social issues (defined as active or history of heavy alcohol use, active or history of illicit drug use, lack of social support, and homelessness) were noted.
Timeliness of delivery of care was measured in days. For the VA group, the wait time was defined as the date the consult for HCV treatment was placed to the date of the initial appointment with the HCV treatment provider. For the Choice group, the wait time was defined as the date the referral to the Choice program was made to the date of the initial appointment with the Choice HCP. Treatment regimens were evaluated for appropriateness based on guidelines from VACO and the American Association for the Study of Liver Diseases.9-11
Tests performed by Choice providers were evaluated for whether they were relevant to HCV care and whether similar data already were available from VALLHCS. Tests that were not indicated were identified as unnecessary costs incurred by the Choice program, as were tests that had to be repeated at the VA because of a lack of documentation from the Choice provider. All medications given inappropriately were considered added costs. Medicare reimbursement rates for the most applicable Current Procedural Terminology (CPT) code and VA national contract pricing for medications were used for calculations. This study was approved by the VALLHCS institutional review board.
Statistical Analysis
IBM (Armonk, NY) Statistical Package for Social Sciences software was used to evaluate for differences in Fib-4, platelet count, prevalence of cirrhosis, prevalence of medical comorbidities, prevalence of mental health comorbidities, prevalence of the social issues defined in the Methods section, time from referral to time of appointment date, and SVR12 rate between the VA and Choice groups.
Exclusions
There were 15 veterans in the VA group who had a wait time of > 100 days. Of these, 5 (33%) were initially Choice referrals, but due to negative interactions with the Choice provider, the veterans returned to VALLHCS for care. Two of the 15 (13%) did not keep appointments and were lost to follow up. Six of the 15 (40%) had medical comorbidities that required more immediate attention, so HCV treatment initiation was deliberately moved back. The final 2 veterans scheduled their appointments unusually far apart, artificially increasing their wait time. Given that these were unique situations and some of the veterans received care from both Choice and VA providers, a decision was made to exclude these individuals from the study.
It has been shown that platelet count correlates with degree of liver fibrosis, a concept that is the basis for the Fib-4 scoring system.12 Studies have shown that platelet count is a survival predictor in those with cirrhosis, and thrombocytopenia is a negative predictor of HCV treatment success using peginterferon and ribavirin.13,14 Therefore, the VA memorandum automatically assigned the sickest individuals to the VA for HCV treatment. The goal of this study was to compare the impact of factors other than stage of fibrosis on HCV treatment success, which is why the 12 veterans with platelet count < 100,000 in the VA group were excluded. There were no veterans with platelet count < 100,000 in the Choice group.
When comparing SVR12 rates between the VA and Choice groups, every veteran treated at VALLHCS in FY 2016 was included, increasing the number in the VA group from 100 to 320 and therefore the power of this comparison.
Results
A summary of the statistical analysis can be found in Table 1. The genotype distribution was consistent with epidemiological studies, including those specific to veterans.15,16 There were statistically significant differences (P < .001) in mean Fib-4 and mean platelet count. The VA group had a higher Fib-4 and lower platelet count. Seventy-four percent of the VA population was defined as cirrhotic, while only 3% of the Choice population met similar criteria (P < .001). The VA and Choice groups were similar in terms of age, gender, and genotype distribution (Table 2).
The VA group was found to have a higher prevalence of comorbidities that affected HCV treatment. These conditions included but were not limited to: chronic kidney disease that precluded the use of certain medications, any condition that required medication with a known interaction with DAAs (ie, proton pump inhibitors, statins, and amiodarone), and cirrhosis if it impacted the treatment regimen. The difference in the prevalence of mental health comorbidities was not significant (P = .39), but there was a higher prevalence of social issues among the VA group (P = .002).
The mean wait time from referral to appointment was 28.6 days for the VA group and 42.3 days for the Choice group (P < .001), indicating that a Choice referral took longer to complete than a referral within the VA for HCV treatment. Thirty of the 71 (42%) veterans seen by a Choice provider accrued extraneous cost, with a mean additional cost of $8,561.40 per veteran. In the Choice group, 61 veterans completed a treatment regimen with the Choice HCP. Fifty-five veterans completed treatment and had available SVR12 data (6 were lost to follow up without SVR12 testing) and 50 (91%) had confirmed SVR12. The charts of the 5 treatment failures were reviewed to discern the cause for failure. Two cases involved early termination of therapy, 3 involved relapse and 2 failed to comply with medication instructions. There was 1 case of the Choice HCP not addressing simultaneous use of ledipasvir and a proton pump inhibitor, potentially causing an interaction, and 1 case where both the VA and Choice providers failed to recognize indicators of cirrhosis, which impacted the regimen used.
In the VALLHCS group, records of 320 veterans who completed treatment and had SVR12 testing were reviewed. While the Choice memorandum was active, veterans selected to be treated at VALLHCS had advanced liver fibrosis or cirrhosis, medical and mental health comorbidities that increased the risk of treatment complications or were considered to have difficulty adhering to the medication regimen. For this group, 296 (93%) had confirmed SVR12. Eighteen of the 24 (75%) treatment failures were complicated by nonadherence, including all 13 cases of early termination. One patient died from complications of decompensated cirrhosis before completing treatment, and 1 did not receive HCV medications during a hospital admission due to poor coordination of care between the VA inpatient and outpatient pharmacy services, leading to multiple missed doses.
The difference in SVR12 rates (ie, treatment failure rates), between the VA and Choice groups was not statistically significant (P = .61). None of the specific reasons for treatment failure had a statistically significant difference between groups. A treatment failure analysis is shown in Table 3, and Table 4 indicates the breakdown of treatment regimens.
Discussion
The Veterans Health Administration (VHA) is the largest integrated health care system in the US, consisting of 152 medical centers and > 1,700 sites of care. The VA has the potential to meet the health care needs of 21.6 million veterans. About 9 million veterans are enrolled in the VA system and 5.9 million received health care through VHA.17 However, every medical service cannot realistically be made available at every facility, and some veterans have difficulty gaining access to VHA care; distance and wait times have been well-publicized issues that need further exploration.18,19 The Choice program is an attempt to meet gaps in VA coverage using non-VA HCPs.
HCV infection is a specific diagnosis with national treatment guidelines and well-studied treatments; it can be cured, with an evidence-based definition of cure. The VACO policy memorandum to refer less sick veterans to Choice while treating sicker veterans at the VA provided the opportunity to directly compare the quality of the 2 programs. The SVR12 rates of VALLHCS and Choice providers were comparable to the national average at the time, and while the difference in SVR12 rate was not significant, VALLHCS treated a significantly higher number of patients with cirrhosis because of the referral criteria.20
The significant difference in medical comorbidities between the VA and Choice groups was not surprising, partly because of the referral criteria. Cirrhosis can impact the treatment regimen, especially in regard to use of ribavirin. Since the presence of mental health comorbidities did not affect selection into the Choice group, it makes sense that there was no significant difference in prevalence between the groups.
VACO allowed veterans with HCV treatment plans that VA HCPs felt were too complicated for the Choice program to be treated by VHA HCPs.9 VALLHCS exercised this right for veterans at risk for nonadherence, because in HCV treatment, nonadherence leads to treatment failure and development of drug resistant virus strains. Therefore, veterans who would have difficulty traveling to VALLHCS to pick up medications, those who lacked means of communication (such as those who were homeless), and those who had active substance abuse were treated at the VA, where closer monitoring and immediate access to a wide range of services was possible. Studies have confirmed the impact of these types of issues on HCV treatment adherence and success.21 This explains the higher prevalence of social issues in the VA group.
For an internal referral for HCV treatment at VALLHCS, the hepatology provider submits a consult request to the HCV treatment provider, who works in the same office, making direct communication simple. The main administrative limiting factor to minimizing wait times is the number of HCPs, which is dependent on hiring allowances.
When a veteran is referred to Choice, the VA provider places a consult for non-VA care, which the VA Office of Community Care processes by compiling relevant documents and sending the package to Triwest Healthcare Alliance, a private insurance processor contracted with the VA. Triwest selects the Choice provider, often without any input from the VA, and arranges the veteran’s initial appointment.22 Geographic distance to the veteran’s address is the main selection criteria for Triwest. Documents sent between the Choice and VA HCPs go through the Office of Community Care and Triwest. This significantly increases the potential for delays and failed communication. Triwest had a comprehensive list of providers deemed to be qualified to treat HCV within the geographic catchment of VALLHCS. This list was reviewed, and all veterans referred to Choice had HCPs near their home address; therefore, availability of Choice HCPs was not an issue.
The VA can provide guidance on management of the veteran in the form of bundle packages containing a list of services for which the Choice provider is authorized to provide, and ones the Choice provider is not authorized to provide. Some Choice HCPs ordered tests that were not authorized for HCV treatment such as esophagogastroduodenoscopy, colonoscopy, and liver biopsy. In all, 17 of 71 (24%) veterans seen by Choice HCPs had tests performed or ordered that VA HCPs would not have obtained for the purpose of HCV treatment (Table 5).
In order to prevent veterans from receiving unnecessary tests, a VALLHCS hepatologist asked to be notified by VA administrators overseeing Choice referrals whenever a secondary authorization request (SAR) was submitted by a Choice HCP. This strategy is not standard VA practice, therefore at many VA sites these requested tests would have been performed by the Choice HCP, which is why SARs were factored into cost analysis.
SVR12 test results that were drawn too early and had to be repeated at VALLHCS were a cost unique to the Choice program. Duplicate tests, particularly imaging studies and blood work, were extraneous costs. The largest extraneous costs were treatment regimens prescribed by Choice HCPs that did not follow standard of care and required VA provider intervention. Thirty of the 71 (42%) veterans seen by a Choice provider accrued a mean $8,561.40 in extra costs. As a result, the Choice program cost VHA $250,000 more to provide care for 30 veterans (enough to pay for a physician’s annual salary).
Some inappropriate treatment regimens were the result of Choice HCP error, such as 1 case in which a veteran was inadvertently switched from ledipasvir/sofosbuvir to ombitasvir/paritaprevir/ritonavir/dasabuvir after week 4. The veteran had to start therapy over but still achieved SVR12. Other cases saw veterans receive regimens for which they had clear contraindications, such as creatinine clearance < 30 mL/min/1.73m2 for sofosbuvir or a positive resistance panel for specific medications. Eleven of 62 (18%) veterans who were started on HCV treatment by a Choice HCP received a regimen not consistent with VA guidelines—an alarming result.
Follow up for veterans referred to Choice was extremely labor intensive, and assessment of personnel requirements in a Choice-based VA system must take this into consideration. The Choice HCP has no obligation to communicate with the VA HCP. At the time of chart review, 57 of 71 (80%) Choice veterans had inadequate documentation to make a confident assessment of the treatment outcome. Multiple calls to the offices of the Choice HCP were needed to acquire records, and veterans had to be tracked down for additional tests. Veterans often would complete treatment and stop following up with the Choice provider before SVR12 confirmation. The VA hepatology provider reviewing Choice referrals served as clinician, case manager, and clerk in order to get veterans to an appropriate end point in their hepatitis C treatment, with mostly unmeasured hours of work.
Limitations
The study population size was limited by the number of veterans able to complete treatment through Choice. The parameters in the VACO policy memos automatically selected the VA and Choice groups but made them clinically distinct populations. New treatment medications were released during the study period, which impacted management strategy. Occasionally, VA and non-VA HCPs preferred different treatment regimens, leading to variation in the distribution of regimens used despite similar genotype distribution (Tables 2 and 4). In addition, a retrospective study is at risk for recall bias. A prospective study randomizing veterans to the Choice and VA groups is an important future endeavor. Comparing veteran satisfaction for Choice and VA services is also crucial.
Conclusions
This study demonstrates that the VA was able to provide more cost-effective and more timely care for HCV treatment to a relatively sicker population with no reduction in treatment success when compared with non-VA HCPs through the Choice program. While the Choice program can help veterans receive services they may otherwise not have access to and reduce travel time, the current system introduces inefficiencies that delay care and decrease cost-effectiveness. The Choice HCP selection process is based on proximity rather than quality, which may place the veteran at risk for receiving substandard care. Large-scale quality of care studies that compare efficiency measures, clinical outcomes, patient demographics, travel distance, cost efficacy and patient satisfaction for veterans receiving similar services at a VA facility and through Choice should be performed to ensure that veterans receive the best care available.
Population studies show high prevalence of chronic hepatitis C virus (HCV) infection among veterans, especially Vietnam War era veterans.1,2 The development of safe and efficacious direct-acting antiviral (DAA) medications to treat HCV infection made the majority of those infected eligible for treatment. However, the large number of veterans needing DAA treatment stressed the resources of the US Department of Veterans Affairs (VA) health care system. This occurred while Congress was focused on reducing wait times for veterans receiving care at the VA.
Congress passed the Veterans Access, Choice, and Accountability Act (Choice) on August 7, 2014, leading to the creation of the Veterans Choice Program. Legislators felt there were inappropriate delays in care at the VA, and the Choice program was meant to address this problem and provide an “apples-to-apples comparison [of the VA] with non-VA hospitals.”3
Congress acknowledged the importance of curing HCV in the veteran population and allocated $1.5 billion for fiscal year (FY) 2016 for DAAs. The VA Central Office (VACO) carefully monitored these resources. The first policy memorandum from VACO for HCV care, issued on May 21, 2015, recommended that the sickest patients who will benefit from the treatment “receive priority over those who are less ill.”4,5 Those who met criteria for advanced liver disease were prioritized for treatment at the VA, while those who did not meet criteria were given the option of receiving treatment through Choice, or waiting for a change in policy.6 Over time, revisions to the guidelines relaxed the criteria for VA treatment eligibility, and on February 24, 2016, all restrictions on HCV treatment at the VA were lifted.7,8
The aim of this study was to provide a comparison of VA and non-VA care, specifically to determine whether care provided through Choice was timelier and more cost effective than care provided by the VA, and whether there was a quality difference. The high prevalence among veterans, well-established standards of care, and finite treatment course with clear indicators of success and failure makes HCV treatment an ideal disease with which to make this comparison.
Methods
We retrospectively analyzed the VA electronic health records of all veterans seen in the VA Loma Linda Healthcare System (VALLHCS) Hepatology clinic for chronic HCV infection during FY 2016 who were referred to Choice for HCV treatment. One hundred veterans met these criteria, encompassing the Choice population; 71 were seen at least once by a non-VA (Choice) health care provider (HCP) and 61 completed a treatment regimen through Choice. Treatment completion was defined as cessation of medication after the planned duration of therapy, or early termination of medication without resumption by that HCP. The Choice population was matched to an equal number of veterans who received HCV treatment from VALLHCS HCPs.
Data collected included age, gender, HCV genotype, determinants of liver fibrosis, and treatment success (defined as sustained virologic response at 12 weeks after the last dose of medication [SVR12]). Determinants of liver fibrosis included documented cirrhosis or complications of cirrhosis, Fibrosis-4 score (Fib-4), and platelet count.
Treatment failures were categorized as nonresponse (defined as detectable HCV viral load at the end of treatment), relapse (defined as an undetectable HCV viral load at the end of treatment with a subsequent positive test), and early termination (defined as a failure to complete the planned treatment regimen). Documented patient nonadherence, medical comorbidities that affected the treatment protocol, mental health diagnoses, and active social issues (defined as active or history of heavy alcohol use, active or history of illicit drug use, lack of social support, and homelessness) were noted.
Timeliness of delivery of care was measured in days. For the VA group, the wait time was defined as the date the consult for HCV treatment was placed to the date of the initial appointment with the HCV treatment provider. For the Choice group, the wait time was defined as the date the referral to the Choice program was made to the date of the initial appointment with the Choice HCP. Treatment regimens were evaluated for appropriateness based on guidelines from VACO and the American Association for the Study of Liver Diseases.9-11
Tests performed by Choice providers were evaluated for whether they were relevant to HCV care and whether similar data already were available from VALLHCS. Tests that were not indicated were identified as unnecessary costs incurred by the Choice program, as were tests that had to be repeated at the VA because of a lack of documentation from the Choice provider. All medications given inappropriately were considered added costs. Medicare reimbursement rates for the most applicable Current Procedural Terminology (CPT) code and VA national contract pricing for medications were used for calculations. This study was approved by the VALLHCS institutional review board.
Statistical Analysis
IBM (Armonk, NY) Statistical Package for Social Sciences software was used to evaluate for differences in Fib-4, platelet count, prevalence of cirrhosis, prevalence of medical comorbidities, prevalence of mental health comorbidities, prevalence of the social issues defined in the Methods section, time from referral to time of appointment date, and SVR12 rate between the VA and Choice groups.
Exclusions
There were 15 veterans in the VA group who had a wait time of > 100 days. Of these, 5 (33%) were initially Choice referrals, but due to negative interactions with the Choice provider, the veterans returned to VALLHCS for care. Two of the 15 (13%) did not keep appointments and were lost to follow up. Six of the 15 (40%) had medical comorbidities that required more immediate attention, so HCV treatment initiation was deliberately moved back. The final 2 veterans scheduled their appointments unusually far apart, artificially increasing their wait time. Given that these were unique situations and some of the veterans received care from both Choice and VA providers, a decision was made to exclude these individuals from the study.
It has been shown that platelet count correlates with degree of liver fibrosis, a concept that is the basis for the Fib-4 scoring system.12 Studies have shown that platelet count is a survival predictor in those with cirrhosis, and thrombocytopenia is a negative predictor of HCV treatment success using peginterferon and ribavirin.13,14 Therefore, the VA memorandum automatically assigned the sickest individuals to the VA for HCV treatment. The goal of this study was to compare the impact of factors other than stage of fibrosis on HCV treatment success, which is why the 12 veterans with platelet count < 100,000 in the VA group were excluded. There were no veterans with platelet count < 100,000 in the Choice group.
When comparing SVR12 rates between the VA and Choice groups, every veteran treated at VALLHCS in FY 2016 was included, increasing the number in the VA group from 100 to 320 and therefore the power of this comparison.
Results
A summary of the statistical analysis can be found in Table 1. The genotype distribution was consistent with epidemiological studies, including those specific to veterans.15,16 There were statistically significant differences (P < .001) in mean Fib-4 and mean platelet count. The VA group had a higher Fib-4 and lower platelet count. Seventy-four percent of the VA population was defined as cirrhotic, while only 3% of the Choice population met similar criteria (P < .001). The VA and Choice groups were similar in terms of age, gender, and genotype distribution (Table 2).
The VA group was found to have a higher prevalence of comorbidities that affected HCV treatment. These conditions included but were not limited to: chronic kidney disease that precluded the use of certain medications, any condition that required medication with a known interaction with DAAs (ie, proton pump inhibitors, statins, and amiodarone), and cirrhosis if it impacted the treatment regimen. The difference in the prevalence of mental health comorbidities was not significant (P = .39), but there was a higher prevalence of social issues among the VA group (P = .002).
The mean wait time from referral to appointment was 28.6 days for the VA group and 42.3 days for the Choice group (P < .001), indicating that a Choice referral took longer to complete than a referral within the VA for HCV treatment. Thirty of the 71 (42%) veterans seen by a Choice provider accrued extraneous cost, with a mean additional cost of $8,561.40 per veteran. In the Choice group, 61 veterans completed a treatment regimen with the Choice HCP. Fifty-five veterans completed treatment and had available SVR12 data (6 were lost to follow up without SVR12 testing) and 50 (91%) had confirmed SVR12. The charts of the 5 treatment failures were reviewed to discern the cause for failure. Two cases involved early termination of therapy, 3 involved relapse and 2 failed to comply with medication instructions. There was 1 case of the Choice HCP not addressing simultaneous use of ledipasvir and a proton pump inhibitor, potentially causing an interaction, and 1 case where both the VA and Choice providers failed to recognize indicators of cirrhosis, which impacted the regimen used.
In the VALLHCS group, records of 320 veterans who completed treatment and had SVR12 testing were reviewed. While the Choice memorandum was active, veterans selected to be treated at VALLHCS had advanced liver fibrosis or cirrhosis, medical and mental health comorbidities that increased the risk of treatment complications or were considered to have difficulty adhering to the medication regimen. For this group, 296 (93%) had confirmed SVR12. Eighteen of the 24 (75%) treatment failures were complicated by nonadherence, including all 13 cases of early termination. One patient died from complications of decompensated cirrhosis before completing treatment, and 1 did not receive HCV medications during a hospital admission due to poor coordination of care between the VA inpatient and outpatient pharmacy services, leading to multiple missed doses.
The difference in SVR12 rates (ie, treatment failure rates), between the VA and Choice groups was not statistically significant (P = .61). None of the specific reasons for treatment failure had a statistically significant difference between groups. A treatment failure analysis is shown in Table 3, and Table 4 indicates the breakdown of treatment regimens.
Discussion
The Veterans Health Administration (VHA) is the largest integrated health care system in the US, consisting of 152 medical centers and > 1,700 sites of care. The VA has the potential to meet the health care needs of 21.6 million veterans. About 9 million veterans are enrolled in the VA system and 5.9 million received health care through VHA.17 However, every medical service cannot realistically be made available at every facility, and some veterans have difficulty gaining access to VHA care; distance and wait times have been well-publicized issues that need further exploration.18,19 The Choice program is an attempt to meet gaps in VA coverage using non-VA HCPs.
HCV infection is a specific diagnosis with national treatment guidelines and well-studied treatments; it can be cured, with an evidence-based definition of cure. The VACO policy memorandum to refer less sick veterans to Choice while treating sicker veterans at the VA provided the opportunity to directly compare the quality of the 2 programs. The SVR12 rates of VALLHCS and Choice providers were comparable to the national average at the time, and while the difference in SVR12 rate was not significant, VALLHCS treated a significantly higher number of patients with cirrhosis because of the referral criteria.20
The significant difference in medical comorbidities between the VA and Choice groups was not surprising, partly because of the referral criteria. Cirrhosis can impact the treatment regimen, especially in regard to use of ribavirin. Since the presence of mental health comorbidities did not affect selection into the Choice group, it makes sense that there was no significant difference in prevalence between the groups.
VACO allowed veterans with HCV treatment plans that VA HCPs felt were too complicated for the Choice program to be treated by VHA HCPs.9 VALLHCS exercised this right for veterans at risk for nonadherence, because in HCV treatment, nonadherence leads to treatment failure and development of drug resistant virus strains. Therefore, veterans who would have difficulty traveling to VALLHCS to pick up medications, those who lacked means of communication (such as those who were homeless), and those who had active substance abuse were treated at the VA, where closer monitoring and immediate access to a wide range of services was possible. Studies have confirmed the impact of these types of issues on HCV treatment adherence and success.21 This explains the higher prevalence of social issues in the VA group.
For an internal referral for HCV treatment at VALLHCS, the hepatology provider submits a consult request to the HCV treatment provider, who works in the same office, making direct communication simple. The main administrative limiting factor to minimizing wait times is the number of HCPs, which is dependent on hiring allowances.
When a veteran is referred to Choice, the VA provider places a consult for non-VA care, which the VA Office of Community Care processes by compiling relevant documents and sending the package to Triwest Healthcare Alliance, a private insurance processor contracted with the VA. Triwest selects the Choice provider, often without any input from the VA, and arranges the veteran’s initial appointment.22 Geographic distance to the veteran’s address is the main selection criteria for Triwest. Documents sent between the Choice and VA HCPs go through the Office of Community Care and Triwest. This significantly increases the potential for delays and failed communication. Triwest had a comprehensive list of providers deemed to be qualified to treat HCV within the geographic catchment of VALLHCS. This list was reviewed, and all veterans referred to Choice had HCPs near their home address; therefore, availability of Choice HCPs was not an issue.
The VA can provide guidance on management of the veteran in the form of bundle packages containing a list of services for which the Choice provider is authorized to provide, and ones the Choice provider is not authorized to provide. Some Choice HCPs ordered tests that were not authorized for HCV treatment such as esophagogastroduodenoscopy, colonoscopy, and liver biopsy. In all, 17 of 71 (24%) veterans seen by Choice HCPs had tests performed or ordered that VA HCPs would not have obtained for the purpose of HCV treatment (Table 5).
In order to prevent veterans from receiving unnecessary tests, a VALLHCS hepatologist asked to be notified by VA administrators overseeing Choice referrals whenever a secondary authorization request (SAR) was submitted by a Choice HCP. This strategy is not standard VA practice, therefore at many VA sites these requested tests would have been performed by the Choice HCP, which is why SARs were factored into cost analysis.
SVR12 test results that were drawn too early and had to be repeated at VALLHCS were a cost unique to the Choice program. Duplicate tests, particularly imaging studies and blood work, were extraneous costs. The largest extraneous costs were treatment regimens prescribed by Choice HCPs that did not follow standard of care and required VA provider intervention. Thirty of the 71 (42%) veterans seen by a Choice provider accrued a mean $8,561.40 in extra costs. As a result, the Choice program cost VHA $250,000 more to provide care for 30 veterans (enough to pay for a physician’s annual salary).
Some inappropriate treatment regimens were the result of Choice HCP error, such as 1 case in which a veteran was inadvertently switched from ledipasvir/sofosbuvir to ombitasvir/paritaprevir/ritonavir/dasabuvir after week 4. The veteran had to start therapy over but still achieved SVR12. Other cases saw veterans receive regimens for which they had clear contraindications, such as creatinine clearance < 30 mL/min/1.73m2 for sofosbuvir or a positive resistance panel for specific medications. Eleven of 62 (18%) veterans who were started on HCV treatment by a Choice HCP received a regimen not consistent with VA guidelines—an alarming result.
Follow up for veterans referred to Choice was extremely labor intensive, and assessment of personnel requirements in a Choice-based VA system must take this into consideration. The Choice HCP has no obligation to communicate with the VA HCP. At the time of chart review, 57 of 71 (80%) Choice veterans had inadequate documentation to make a confident assessment of the treatment outcome. Multiple calls to the offices of the Choice HCP were needed to acquire records, and veterans had to be tracked down for additional tests. Veterans often would complete treatment and stop following up with the Choice provider before SVR12 confirmation. The VA hepatology provider reviewing Choice referrals served as clinician, case manager, and clerk in order to get veterans to an appropriate end point in their hepatitis C treatment, with mostly unmeasured hours of work.
Limitations
The study population size was limited by the number of veterans able to complete treatment through Choice. The parameters in the VACO policy memos automatically selected the VA and Choice groups but made them clinically distinct populations. New treatment medications were released during the study period, which impacted management strategy. Occasionally, VA and non-VA HCPs preferred different treatment regimens, leading to variation in the distribution of regimens used despite similar genotype distribution (Tables 2 and 4). In addition, a retrospective study is at risk for recall bias. A prospective study randomizing veterans to the Choice and VA groups is an important future endeavor. Comparing veteran satisfaction for Choice and VA services is also crucial.
Conclusions
This study demonstrates that the VA was able to provide more cost-effective and more timely care for HCV treatment to a relatively sicker population with no reduction in treatment success when compared with non-VA HCPs through the Choice program. While the Choice program can help veterans receive services they may otherwise not have access to and reduce travel time, the current system introduces inefficiencies that delay care and decrease cost-effectiveness. The Choice HCP selection process is based on proximity rather than quality, which may place the veteran at risk for receiving substandard care. Large-scale quality of care studies that compare efficiency measures, clinical outcomes, patient demographics, travel distance, cost efficacy and patient satisfaction for veterans receiving similar services at a VA facility and through Choice should be performed to ensure that veterans receive the best care available.
1. Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160(5):293-300.
2. Dominitz JA, Boyko EJ, Koepsell TD, et al. Elevated prevalence of hepatitis C infection in users of United States veterans medical centers. Hepatology. 2005;41(1):88-96.
3. Veterans Access, Choice, and Accountability Act of 2014. 42 USC §1395 (2014).
4. Tuchschmidt J. Attachment C: Provision of hepatitis C treatment. US Department of Veterans Affairs Central Office Memorandum from the Principal Deputy Under Secretary for Health. http://vaww.hepatitis.va.gov/education/choice-provision-hcv-treatment.asp. Published May 21, 2015. [Nonpublic site.]
5. Tuchschmidt J. Attachment A: Provision of hepatitis C (HCV) treatment through the Choice program. US Department of Veterans Affairs Central Office Memorandum from the Principal Deputy Under Secretary for Health. http://vaww.hepatitis.va.gov/pdf/choice-attachment-a-FY16.pdf. Published May 21, 2015. [Nonpublic site.]
6. Tuchschmidt J. Attachment B: Initiation of hepatitis C virus (HCV) treatment: protocol for prioritization. US Department of Veterans Affairs Central Office Memorandum from the Principal Deputy Under Secretary for Health. http://vaww.hepatitis.va.gov/pdf/provision-HCV-treatment-attachment-b.pdf. Published May 21, 2015. [Nonpublic site.]
7. Murphy, JP. Hepatitis C virus funding and prioritization status. US Department of Veterans Affairs Central Office Memorandum from the Assistant Deputy Under Secretary for Health for Clinical Operations. http://vaww.hepatitis.va.gov/education/choice-memo-hcv-funding-and-prioritization-status-01272016.asp. Published January 27, 2016. [Nonpublic site.]
8. Lynch TJ, McCarthy MF. Hepatitis C virus funding and prioritization status update. US Department of Veterans Affairs Central Office Memorandum from the Assistant Deputy Under Secretary for Health for Clinical Operations and Acting Assistant Deputy Under Secretary for Health for Patient Care Services. http://vaww.hepatitis.va.gov/education/choice-funding-update-feb-2016.asp. Published February 24, 2016. [Nonpublic site.]
9. Morgan TR, Yee H; US Department of Veterans Affairs National Hepatitis C Resource Center Program and the National Viral Hepatitis Program in the Office of Patient Care Services. Chronic hepatitis C virus (HCV) infection: treatment considerations. http://vaww.hepatitis.va.gov/pdf/treatment-considerations-2016-03-28.pdf. Published March 28, 2016. [Nonpublic site.]
10. American Association for the Study of Liver Diseases; Infectious Diseases Society of America. Initial treatment box. http://hcvguidelines.org/full-report/initial-treatment-box-summary-recommendations-patients-who-are-initiating-therapy-hcv. Updated November 6, 2019. Accessed September 27, 2016
11. AASLD/IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62(3): 932-954.
12. Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006; 43(6):1317-1325.
13. Realdi G, Fattovich G, Hadziyannis S, et al. Survival and prognostic factors in 366 patients with compensated cirrhosis type B: a multicenter study. The Investigators of the European Concerted Action on Viral Hepatitis (EUROHEP). J Hepatol. 1994;21(4):656-666.
14. Kanda T, Kato K, Tsubota A, et al. Platelet count and sustained virological response in hepatitis C treatment. World J Hepatol. 2013;5(4):182-188.
15. Manos MM, Shvachko VA, Murphy RC, Arduino JM, Shire NJ. Distribution of hepatitis C virus genotypes in a diverse US integrated health care population. J Med Virol. 2012;84(11):1744-1750.
16. Cheung RC. Epidemiology of hepatitis C virus infection in American veterans. Am J Gastroenterol. 2000;95(3):740-747.
17. Bagalman E. The number of Veterans that use VA health care services: a fact sheet. Congressional Research Service Report R43579. https://fas.org/sgp/crs/misc/R43579.pdf. Published June 3, 2014. Accessed November 25, 2019.
18. US General Accounting Office. Report to the Ranking Minority Member, Subcommittee on Compensation, Pension, Insurance, and Memorial Affairs, Committee on Veterans’ Affairs, US House of Representatives. How distance from VA facilities affects veterans’ use of VA services. https://www.gao.gov/assets/230/221992.pdf. Published December 1995. Accessed November 25, 2019.
19. Bronstein S, Griffin D. A fatal wait: Veterans languish and die on a VA hospital’s secret list. http://www.cnn.com/2014/04/23/health/veterans-dying-health-care-delays/index.html. Published April 23, 2014. Accessed November 25, 2019.
20. Ioannou GN, Beste LA, Chang MF, et al. Effectiveness of sofosbuvir, ledipasvir/sofosbuvir, or paritaprevir/ritonavir/ombitasvir and dasabuvir regimens for treatment of patients with hepatitis C in the Veterans Affairs national health care system. Gastroenterology. 2016;151(3):457-471.
21. Malespin MH, Harris C, Kanar O, et al. Barriers to treatment of chronic hepatitis C with direct acting antivirals in an urban clinic. Ann Hepatol. 2019;18(2):304–309.
22. Tuchschmidt J. Attachment D: Hepatitis C virus (HCV) fact sheet for Veterans Choice Program for both VA and Choice providers. US Department of Veterans Affairs Central Office Memorandum from the Deputy Under Secretary for Health for Policy and Services and the Acting Deputy Undersecretary for Health for Operations and Management. http://vaww.hepatitis.va.gov/educatiochoice-provision-HCV-treatment-additional.asp. [Nonpublic site.]
1. Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160(5):293-300.
2. Dominitz JA, Boyko EJ, Koepsell TD, et al. Elevated prevalence of hepatitis C infection in users of United States veterans medical centers. Hepatology. 2005;41(1):88-96.
3. Veterans Access, Choice, and Accountability Act of 2014. 42 USC §1395 (2014).
4. Tuchschmidt J. Attachment C: Provision of hepatitis C treatment. US Department of Veterans Affairs Central Office Memorandum from the Principal Deputy Under Secretary for Health. http://vaww.hepatitis.va.gov/education/choice-provision-hcv-treatment.asp. Published May 21, 2015. [Nonpublic site.]
5. Tuchschmidt J. Attachment A: Provision of hepatitis C (HCV) treatment through the Choice program. US Department of Veterans Affairs Central Office Memorandum from the Principal Deputy Under Secretary for Health. http://vaww.hepatitis.va.gov/pdf/choice-attachment-a-FY16.pdf. Published May 21, 2015. [Nonpublic site.]
6. Tuchschmidt J. Attachment B: Initiation of hepatitis C virus (HCV) treatment: protocol for prioritization. US Department of Veterans Affairs Central Office Memorandum from the Principal Deputy Under Secretary for Health. http://vaww.hepatitis.va.gov/pdf/provision-HCV-treatment-attachment-b.pdf. Published May 21, 2015. [Nonpublic site.]
7. Murphy, JP. Hepatitis C virus funding and prioritization status. US Department of Veterans Affairs Central Office Memorandum from the Assistant Deputy Under Secretary for Health for Clinical Operations. http://vaww.hepatitis.va.gov/education/choice-memo-hcv-funding-and-prioritization-status-01272016.asp. Published January 27, 2016. [Nonpublic site.]
8. Lynch TJ, McCarthy MF. Hepatitis C virus funding and prioritization status update. US Department of Veterans Affairs Central Office Memorandum from the Assistant Deputy Under Secretary for Health for Clinical Operations and Acting Assistant Deputy Under Secretary for Health for Patient Care Services. http://vaww.hepatitis.va.gov/education/choice-funding-update-feb-2016.asp. Published February 24, 2016. [Nonpublic site.]
9. Morgan TR, Yee H; US Department of Veterans Affairs National Hepatitis C Resource Center Program and the National Viral Hepatitis Program in the Office of Patient Care Services. Chronic hepatitis C virus (HCV) infection: treatment considerations. http://vaww.hepatitis.va.gov/pdf/treatment-considerations-2016-03-28.pdf. Published March 28, 2016. [Nonpublic site.]
10. American Association for the Study of Liver Diseases; Infectious Diseases Society of America. Initial treatment box. http://hcvguidelines.org/full-report/initial-treatment-box-summary-recommendations-patients-who-are-initiating-therapy-hcv. Updated November 6, 2019. Accessed September 27, 2016
11. AASLD/IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62(3): 932-954.
12. Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006; 43(6):1317-1325.
13. Realdi G, Fattovich G, Hadziyannis S, et al. Survival and prognostic factors in 366 patients with compensated cirrhosis type B: a multicenter study. The Investigators of the European Concerted Action on Viral Hepatitis (EUROHEP). J Hepatol. 1994;21(4):656-666.
14. Kanda T, Kato K, Tsubota A, et al. Platelet count and sustained virological response in hepatitis C treatment. World J Hepatol. 2013;5(4):182-188.
15. Manos MM, Shvachko VA, Murphy RC, Arduino JM, Shire NJ. Distribution of hepatitis C virus genotypes in a diverse US integrated health care population. J Med Virol. 2012;84(11):1744-1750.
16. Cheung RC. Epidemiology of hepatitis C virus infection in American veterans. Am J Gastroenterol. 2000;95(3):740-747.
17. Bagalman E. The number of Veterans that use VA health care services: a fact sheet. Congressional Research Service Report R43579. https://fas.org/sgp/crs/misc/R43579.pdf. Published June 3, 2014. Accessed November 25, 2019.
18. US General Accounting Office. Report to the Ranking Minority Member, Subcommittee on Compensation, Pension, Insurance, and Memorial Affairs, Committee on Veterans’ Affairs, US House of Representatives. How distance from VA facilities affects veterans’ use of VA services. https://www.gao.gov/assets/230/221992.pdf. Published December 1995. Accessed November 25, 2019.
19. Bronstein S, Griffin D. A fatal wait: Veterans languish and die on a VA hospital’s secret list. http://www.cnn.com/2014/04/23/health/veterans-dying-health-care-delays/index.html. Published April 23, 2014. Accessed November 25, 2019.
20. Ioannou GN, Beste LA, Chang MF, et al. Effectiveness of sofosbuvir, ledipasvir/sofosbuvir, or paritaprevir/ritonavir/ombitasvir and dasabuvir regimens for treatment of patients with hepatitis C in the Veterans Affairs national health care system. Gastroenterology. 2016;151(3):457-471.
21. Malespin MH, Harris C, Kanar O, et al. Barriers to treatment of chronic hepatitis C with direct acting antivirals in an urban clinic. Ann Hepatol. 2019;18(2):304–309.
22. Tuchschmidt J. Attachment D: Hepatitis C virus (HCV) fact sheet for Veterans Choice Program for both VA and Choice providers. US Department of Veterans Affairs Central Office Memorandum from the Deputy Under Secretary for Health for Policy and Services and the Acting Deputy Undersecretary for Health for Operations and Management. http://vaww.hepatitis.va.gov/educatiochoice-provision-HCV-treatment-additional.asp. [Nonpublic site.]
A Case-Based Review of Iron Overload With an Emphasis on Porphyria Cutanea Tarda, Hepatitis C, C282Y Heterozygosity, and Coronary Artery Disease
Sporadic porphyria cutanea tarda (PCT) is the most common cause of porphyria worldwide.1,2 Unlike other forms of porphyria, PCT usually is an acquired disease precipitated by extrinsic risk factors that commonly include excessive alcohol consumption, smoking, and chronic hepatitis C virus (HCV) infection. Additional risk factors include myeloproliferative disorders, exposure to polyhalogenated compounds, estrogen therapy, diseases of iron overload like hereditary hemochromatosis (HH), and potentially, HIV infection.1-3
In this case report, we present a patient with an iron overload (due in part to an HFE gene mutation) and concomitant PCT,
Case Presentation
Mr. M is a 59-year-old white male of Irish background with a medical history that includes coronary artery disease. He is status post ST-elevation myocardial infarction and percutaneous coronary intervention with placement of 2 drug-eluting stents. Additional medical issues include PCT and HCV infection with cirrhosis. He is an active smoker.
The patient has a long history of developing blisters with minor trauma, such as rubbing against his mattress/bed sheets or bumping into doors. These blisters primarily occur on his upper extremities, but also can occur on his face after shaving. Mr. M was diagnosed with HCV infection in 1979 while on active military duty. At that time, he had an acute HCV infection and jaundice that required a prolonged hospitalization. He reported no IV drug use and that many others on his military base had similar manifestations. He drinks 1 to 2 beers daily, but reports no binge drinking.
His laboratory studies were notable for ferritin, 2,069 ng/mL; serum iron, 317 mcg/dL; total iron binding capacity, 320 mcg/dL; transferrin, 239 mg/dl; liver function test alanine aminotransferase, 151 U/L; aspartate aminotransferase, 159 U/L; total bilirubin, 1.73 mg/dL; albumin, 3.6 g/dL; alkaline phosphatase, 119 U/L; INR, 1.1; and transferrin saturation, 99%. Mr. M’s HCV viral load was 28,700 IU/L with genotype 1b. Hemochromatosis genetic studies were notable for a heterozygous C282Y gene mutation and negative for H63D and S65C mutations. He repeatedly declined completing a 24-hour urine study of porphyrins. Ultrasonography was consistent with cirrhosis and splenomegaly. The patient was treatment naïve for HCV. He declined multiple offers for treatment of his HCV, citing financial considerations.
Porphyria Cutanea Tarda
The pathogenesis of PCT is related to the intrahepatic deficiency of uroporphyrinogen decarboxylase (UROD), an enzyme in the heme biosynthetic pathway (Figure 1). Decreased activity of UROD leads to accumulation of uroporphyrinogen and its derivatives, which most likely are oxidized in presence of cytochrome P450 1A2. Up to 80% of PCT cases are sporadic, in which the deficiency of UROD is acquired by exogenous risk factors as mentioned above. However, the remaining 20% of PCT cases are due to an autosomal dominant mutation of UROD that causes the partial deficiency (up to 50%) of UROD. In these cases, additional risk factors are needed to decrease UROD activity to < 75% for symptoms to occur.
Clinical Manifestation
Patients with PCT typically develop blisters, skin fragility, and peeling with sun exposure or minor trauma. They also may experience delayed wound healing in sun-exposed skin.3 The photosensitivity of PCT is believed to be related to the saturation of highly carboxylated uroporphyrins in the liver, which are then released into the circulation. Sun exposure then activates these products facilitating an immune reaction and subsequent skin damage.2 In chronic cases, fibrotic reactions and scaring occur which can be mistaken for scleroderma. Other skin manifestations include hyperpigmentation, hypertrichosis, alopecia due to scaring and purplish heliotrope suffusion of periorbital areas.
Patients can develop cirrhosis due to accumulation of porphyria in the hepatocytes and subsequent parenchymal damage. Hepatocellular carcinoma surveillance is recommended for patients with PCT, although its incidence is rare in those patients.
Diagnosis and Treatment
PCT is mainly a clinical diagnosis. Physicians should consider PCT in patients with photosensitivity and blisters after minor trauma (Figure 2). The urine of a patient with PCT is often pink or red when exposed to air or light due to its high concentration of porphyrin products. Mild elevation of liver enzymes and fatty liver on ultrasound are also noted. Evidence of iron overload is seen in most cases. Screening for risk factors like HCV, HIV, hepatitis B virus, and HH is recommended. Confirmation of PCT typically requires measurement of the porphyria level in a 24-hour urine collection.
Avoiding sun exposure is fundamental in decreasing the development of skin lesions and scaring. Additionally, patients should be advised about the adverse effects of alcohol, smoking, and estrogen therapy on PCT. Treatment of PCT is frequently focused on iron overload and subsequent increased porphyrin oxidation.1,2 Iron can increase reactive oxygen species (ROS), which, in turn, increases the rate of oxidation of uroporphyrinogens. Excess iron also decreases the activity of UROD and increases δ-aminolevulinic acid (ALA) production (the precursor of uroporphyrinogen). Phlebotomy to treat iron overload should be done to a target ferritin level of 20 ng/mL. Clinical manifestations, including skin lesions, typically will normalize before the laboratory findings. Therapeutic remission is expected after 6 to 7 phlebotomy attempts, while clinical improvement can occur after 2 to 3 phlebotomies.
In addition to phlebotomy, 4-aminoquinoline medications (chloroquine and hydroxychloroquine) can be used effectively to treat PCT. Hydroxychloroquine is generally preferred due to its better safety profile. Although the exact mechanism of action of 4-aminoquinolines is not clear, it has been suggested that they bind to porphyrins and form water-soluble products, which are then excreted in the urine. Again, clinical remission occurs much sooner than chemical remission, (3 months vs 12 months). A 4-aminoquinoline should not be used in patients with severe liver disease, renal insufficiency, pregnancy, or G6PD deficiency. When used, they should be used in lower than typical doses due to the rapid removal of accumulated porphyrin from the hepatocytes potentially causing necrosis and acute hepatitis.
Iron chelation also is effective, but it is slower in achieving remission and more expensive than phlebotomy. Treatment of PCT should be individualized. For example, 4-aminoquinolines are contraindicated for patients with end-stage renal disease (ESRD), while phlebotomy could present a problem for patients with preexisting anemia. In this instance, removing 50 cc of blood every 2 weeks may be safe and effective. Furthermore, 4-aminoquinolines in patients with severe iron overload and phlebotomy have been used together. Plasmapheresis is still another option in patients with ESRD.
The use of direct antiviral agents (DAA) in the treatment of HCV has shown promising results in maintaining undetectable viral loads and concurrent remission of PCT. Several studies have shown that treatment of HCV with a DAA obviates the need for treatment PCT.3-5 Treatment of HCV with interferon (IFN) and ribavirin have shown mixed results in controlling PCT, possibly due to their ineffectiveness in maintaining a suppressed viral load. Some studies even showed worsening of PCT with IFN/ribavirin.6
Hemochromatosis
Human cells need iron for aerobic respiration. The intestinal mucosa controls iron uptake and its transfer to the blood stream. Aside from variations in intestinal absorption with fecal excretion, humans do not have another pathway to excrete excess iron. HH is the most common genetic disorder in whites.7 It is an autosomal recessive disorder that increases the intestinal absorption of iron. The most common mutation in the hemochromatosis (HFE) gene results in a substitution of tyrosine for cysteine at amino acid number 282 and is referred to as the C282Y mutation. A second mutation changes histidine at position 63 to aspartic acid and is referred to as a H63D mutation. H63D is present in a minority of the patients with phenotypically expressed HH and its clinical impact is unknown.
Homozygosity of the C282Y mutation is the most common genotype associated with clinical hemochromatosis. While carriers of the C282Y gene heterozygote mutation typically do not develop enough iron overload to cause clinical hemochromatosis, they can if other risk factors, such as PCT, excess alcohol use, liver disease, or HCV, are present.8 Additionally, an associated genetic defect, like a compound heterozygotes C282Y/H63D mutation, a private HFE mutation in trans, or other iron-related genes, can cause manifestations of iron overload. Lastly, about 20% of patients that are heterozygous for both mutations can express the HH phenotype.8
Clinical Manifestation
Patients with HH absorb only a few extra milligrams of iron daily. The clinical manifestation begins to occur when the total body iron store reaches 15-40 g (normal, 4 g). While the genetic mutation is present from birth, iron stores start to rise slowly to around 10 g > age 15 years, at which point serum iron levels are elevated. After age 20 years, the speed with which the iron is stored increases, and by 30 years, liver damage and tissue injury will occur. Cirrhosis is possible by 40 years.7 Age, sex, dietary iron intake, blood loss (menstruation), pregnancy, and other unknown factors greatly influence the disease progression. Homozygote C282Y mutation is as common in women as it is in men, but women are less likely to express the HH phenotype, presumably due, in part, to menstruation. When diagnosed early, most of the clinical manifestations of HH are preventable. Additional manifestations of HH include hyperpigmentation, cardiomyopathy, diabetes mellitus, hypogonadism, hypothyroidism, and arthropathy due to pseudogout.
Iron overload due to HH should be distinguished from other causes of iron overload including exogenous iron overload, anemia (thalassemia, sideroblastic), and chronic liver diseases like PCT, viral hepatitis, nonalcoholic steatohepatitis, and alcoholic liver disease.
Diagnosis
HH should be suspected in patients with a high serum transferrin saturation and elevated serum ferritin concentrations. Typically, transferrin saturation is > 50% and ferritin levels are > 300 ng/mL in men and > 200 ng/mL in women. In early stages of the disease, transferrin saturation can be normal. Additionally, in patients with chronic inflammation, ferritin may be high due to acute-phase reactants and the iron panel should be interpreted with caution. When the secondary causes of abnormalities in a patient’s iron studies are excluded, genetic testing for HFE gene is recommended.
The majority of patients (60-93%) with clinically evident hemochromatosis are homozygous for C282Y mutation. In a heterozygous C282Y mutation with a high transferrin saturation and HH phenotype, additional genetic testing for a heterozygous compound mutation C282Y/H63D is recommended.8 Additional studies could include evaluation for a private HFE mutation in trans or other iron-related genes. Liver biopsy is the gold standard for assessing the degree of hepatic fibrosis. Determining the degree of fibrosis by some means is needed due to the increased risk of hepatocellular carcinoma (HCC) in HH patients with advanced fibrosis and cirrhosis.9
Treatment
Iron depletion with phlebotomy is the cornerstone of treatment in HH. Phlebotomy initially is done weekly with goal of achieving a transferrin saturation < 50%, a serum ferritin level < 50 ng/mL, and a hemoglobin of 12 to 13 ng/mL. When these goals are achieved, patients typically need 4 to 8 phlebotomies per year to maintain a transferrin saturation < 50% (Figure 3).
Hemochromatosis and PCT
Many studies have investigated the relevance of C282Y and/or H63D mutations in patients with PCT.9,10 It appears that ≥ 1 mutation of the HFE gene in PCT may be an important susceptibility factor in the development of clinical PCT. Various studies have shown an incidence of C282Y mutations of 44 to 47% in patients with PCT, compared with 9 to 12% in control populations.9,10 The incidence of the H63D mutation in PCT has been more variable, with some studies showing no difference between patients with PCT and a control group, while other studies showed 31% incidence of H63D mutation in patients with PCT.9,10 A higher incidence of C282Y and H63D mutations in PCT may be a sign that the HFE mutation could be an important factor in developing PCT.
Hemochromatosis and Hepatitis C
Transferrin saturation is frequently elevated in patients with HCV. It is yet unclear whether the pathology of liver disease in patients with HCV is influenced by iron overload or limited to the direct cell damage from replication of the virus and subsequent inflammation. It is believed that the pathology of iron overload in the patients with HCV is different from HH. Like other secondary causes of iron overload, the excess iron is stored in the Kupffer cells of patients with HCV. In HH, excess iron is stored in hepatocytes.
The prevalence of the HFE mutation is the same in the patients with chronic HCV and healthy individuals.10,11 However, HFE mutations are more prevalent in 30 to 60% of the patients with chronic HCV who have elevated transferrin saturations. Alone, C282Y heterozygosity, H63D heterozygosity, or C282Y/H63D compound heterozygosity could not lead to clinically significant iron overload in otherwise healthy individuals; however, these could be a significant cause of iron overload in patients with chronic HCV. Theoretically, the combination of iron overload and HFE gene mutations could increase the rate of advanced fibrosis/cirrhosis in chronic HCV. An increase serum ferritin level of 200 ng/dL in women and 250 ng/dL in men has been observed in 32% of patients with chronic HCV. In this subset of patients, phlebotomy reduced the progression of their liver disease and reduction in their liver enzymes.
Iron Overload and Cardiovascular Risk
In 1987, a Framingham cohort of > 2,800 patients showed a higher incident of CAD in postmenopausal women when compared with premenopausal women.12 In the 1980s, Sullivan hypothesized that the reason for higher incidence of CAD in men when compared with premenopausal women was due to their higher body iron storage.13-16 A study of 1,930 Finnish men reported that the men with ferritin level ≥ 200 ng/dL had a risk 2.2 times higher of acute myocardial infarction when compared to men with lower serum ferritin level.17
A prospective study published in 1997 by Klechl showed the role of iron stores in early atherogenesis via promotion of lipid oxidation.18 Other epidemiological studies have shown a decreased risk of myocardial infarction in blood donors, and while arguments have been made that the blood donors tend to be healthier individuals, 2 studies were published in 1997 matching healthy blood donors to healthy nonblood donors, and both showed a lower risk of CVD in the donors when compared with nondonors.19,20 Furthermore, in an animal model of atherosclerosis, an iron depleted diet showed a reduction of atherosclerosis progression.21 Multiple studies have shown that the heterozygosity for HFE is significantly linked to the risk of cardiovascular events, including the fact that heterozygosity for C282Y has been shown to be a risk factor for myocardial infarction in men and cerebrovascular death in women.22-25
Conclusion
Multiple studies have shown an association between the elevated iron levels associated with the HFE genotype and the disease states of our patient. These include an increased risk of CAD, the increased risk of cirrhosis in HCV and the development of PCT. Indeed, in this case, our patient likely acquired PCT from the combined risks of HCV and his heterozygous HFE genetic mutation.
With regard to Mr. M’s treatment, the use of an antiviral agent in the treatment of his HCV is fundamental, along with avoidance of alcohol and smoking. If he were to accept HCV treatment, we would anticipate resolution of the PCT, but the ongoing progression of his liver and cardiovascular conditions, due perhaps in part, to relative iron overload from his heterozygous HFE mutation. In this situation, we expect that an ongoing course of therapeutic phlebotomy could help to delay the progression of his chronic liver and cardiovascular diseases.
1. Singal AK. Porphyria cutanea tarda: Recent update. Mol Genet Metab. 2019;128(3):271-281.
2. Ryan Caballes F, Sendi H, Bonkovsky HL. Hepatitis C, porphyria cutanea tarda and liver iron: an update. Liver Int. 2012;32(6):880-893.
3. Wiznia LE, Laird ME, Franks AG Jr. Hepatitis C virus and its cutaneous manifestations: treatment in the direct-acting antiviral era. J Eur Acad Dermatol Venereol. 2017;31(8):1260-1270.
4. Nihei T, Kiniwa Y, Mikoshiba Y, Joshita S, Okuyama R. Improvement of porphyria cutanea tarda following treatment of hepatitis C virus by direct-acting antivirals: a case report. J Dermatol. 2019;46(5):e149-e151.
5. Combalia A, To-Figueras J, Laguno M, Martínez-Rebollar M, Aguilera P. Direct-acting antivirals for hepatitis C virus induce a rapid clinical and biochemical remission of porphyria cutanea tarda. Br J Dermatol. 2017;177(5):e183-e184. 6. Singal AK, Venkata KVR, Jampana S, Islam FU, Anderson KE. Hepatitis C treatment in patients with porphyria cutanea tarda. Am J Med Sci. 2017;353 (6):523-528.
7. Brandhagen DJ, Fairbanks VF, Baldus W. Recognition and management of hereditary hemochromatosis. Am Fam Physician. 2002;65(5):853-860.
8. Aguilar-Martinez P, Grandchamp B, Cunat S, et al. Iron overload in HFE C282Y heterozygotes at first genetic testing: a strategy for identifying rare HFE variants. Haematologica. 2011;96(4):507-514.
9. Erhardt A, Maschner-Olberg A, Mellenthin C, et al. HFE mutations and chronic hepatitis C: H63D and C282Y heterozygosity are independent risk factors for liver fibrosis and cirrhosis. J Hepatol. 2003;38(3):335-342.
10. Mehrany K, Drage LA, Brandhagen DJ, Pittelkow MR. Association of porphyria cutanea tarda with hereditary hemochromatosis. J Am Acad Dermatol. 2004;51(2):205-211.
11. Pietrangelo A. Hemochromatosis gene modifies course of hepatitis C viral infection. Gastroenterology. 2003;124(5):1509-1523.
12. Gordon T, Kannel WB, Hjortland MC, McNamara PM. Menopause and coronary heart disease. The Framingham Study. Ann Intern Med. 1978;89(2):157-161.
13. Sullivan JL. Iron and the sex difference in heart disease risk. Lancet. 1981;1(8233):1293-1294.
14. Sullivan JL. The sex difference in ischemic heart disease. Perspect Biol Med. 1983;26(4):657-671.
15. Sullivan JL. The iron paradigm of ischemic heart disease. Am Heart J. 1989;117(5):1177-1188.
16. Sullivan JL. Stored iron and ischemic heart disease: empirical support for a new paradigm. Circulation. 1992;86(3):1036-1037.
17. Salonen JT, Nyyssönen K, Korpela H, Tuomilehto J, Seppänen R, Salonen R. High stored iron levels are associated with excess risk of myocardial infarction in eastern Finnish men. Circulation. 1992;86(3):803-811.
18. Kiechl S, Willeit J, Egger G, Poewe W, Oberhollenzer F. Body iron stores and the risk of carotid atherosclerosis: prospective results from the Bruneck study. Circulation. 1997;96(10):3300-3307.
19. Tuomainen TP, Salonen R, Nyyssönen K, Salonen JT. Cohort study of relation between donating blood and risk of myocardial infarction in 2682 men in eastern Finland. BMJ. 1997;314(7083):793-794.
20. Meyers DG, Strickland D, Maloley PA, Seburg JK, Wilson JE, McManus BF. Possible association of a reduction in cardiovascular events with blood donation. Heart. 1997;78(2):188-193.
21. Lee TS, Shiao MS, Pan CC, Chau LY. Iron-deficient diet reduces atherosclerotic lesions in apoE-deficient mice. Circulation. 1999;99(9):1222-1229.
22. Surber R, Sigusch HH, Kuehnert H, Figulla HR. Haemochromatosis (HFE) gene C282Y mutation and the risk of coronary artery disease and myocardial infarction: a study in 1279 patients undergoing coronary angiography. J Med Genet. 2003;40(5):e58.
23. Tuomainen TP, Kontula K, Nyyssönen K, Lakka TA, Heliö T, Salonen JT. Increased risk of acute myocardial infarction in carriers of the hemochromatosis gene Cys282Tyr mutation: a prospective cohort study in men in eastern Finland. Circulation. 1999;100(12):1274-1279.
24. Roest M, van der Schouw YT, de Valk B, et al. Heterozygosity for a hereditary hemochromatosis gene is associated with cardiovascular death in women. Circulation. 1999;100(12):1268-1273.
25. Pourmoghaddas A, Sanei H, Garakyaraghi M, Esteki-Ghashghaei F, Gharaati M. The relation between body iron store and ferritin, and coronary artery disease. ARYA Atheroscler. 2014;10(1):32-36.
Sporadic porphyria cutanea tarda (PCT) is the most common cause of porphyria worldwide.1,2 Unlike other forms of porphyria, PCT usually is an acquired disease precipitated by extrinsic risk factors that commonly include excessive alcohol consumption, smoking, and chronic hepatitis C virus (HCV) infection. Additional risk factors include myeloproliferative disorders, exposure to polyhalogenated compounds, estrogen therapy, diseases of iron overload like hereditary hemochromatosis (HH), and potentially, HIV infection.1-3
In this case report, we present a patient with an iron overload (due in part to an HFE gene mutation) and concomitant PCT,
Case Presentation
Mr. M is a 59-year-old white male of Irish background with a medical history that includes coronary artery disease. He is status post ST-elevation myocardial infarction and percutaneous coronary intervention with placement of 2 drug-eluting stents. Additional medical issues include PCT and HCV infection with cirrhosis. He is an active smoker.
The patient has a long history of developing blisters with minor trauma, such as rubbing against his mattress/bed sheets or bumping into doors. These blisters primarily occur on his upper extremities, but also can occur on his face after shaving. Mr. M was diagnosed with HCV infection in 1979 while on active military duty. At that time, he had an acute HCV infection and jaundice that required a prolonged hospitalization. He reported no IV drug use and that many others on his military base had similar manifestations. He drinks 1 to 2 beers daily, but reports no binge drinking.
His laboratory studies were notable for ferritin, 2,069 ng/mL; serum iron, 317 mcg/dL; total iron binding capacity, 320 mcg/dL; transferrin, 239 mg/dl; liver function test alanine aminotransferase, 151 U/L; aspartate aminotransferase, 159 U/L; total bilirubin, 1.73 mg/dL; albumin, 3.6 g/dL; alkaline phosphatase, 119 U/L; INR, 1.1; and transferrin saturation, 99%. Mr. M’s HCV viral load was 28,700 IU/L with genotype 1b. Hemochromatosis genetic studies were notable for a heterozygous C282Y gene mutation and negative for H63D and S65C mutations. He repeatedly declined completing a 24-hour urine study of porphyrins. Ultrasonography was consistent with cirrhosis and splenomegaly. The patient was treatment naïve for HCV. He declined multiple offers for treatment of his HCV, citing financial considerations.
Porphyria Cutanea Tarda
The pathogenesis of PCT is related to the intrahepatic deficiency of uroporphyrinogen decarboxylase (UROD), an enzyme in the heme biosynthetic pathway (Figure 1). Decreased activity of UROD leads to accumulation of uroporphyrinogen and its derivatives, which most likely are oxidized in presence of cytochrome P450 1A2. Up to 80% of PCT cases are sporadic, in which the deficiency of UROD is acquired by exogenous risk factors as mentioned above. However, the remaining 20% of PCT cases are due to an autosomal dominant mutation of UROD that causes the partial deficiency (up to 50%) of UROD. In these cases, additional risk factors are needed to decrease UROD activity to < 75% for symptoms to occur.
Clinical Manifestation
Patients with PCT typically develop blisters, skin fragility, and peeling with sun exposure or minor trauma. They also may experience delayed wound healing in sun-exposed skin.3 The photosensitivity of PCT is believed to be related to the saturation of highly carboxylated uroporphyrins in the liver, which are then released into the circulation. Sun exposure then activates these products facilitating an immune reaction and subsequent skin damage.2 In chronic cases, fibrotic reactions and scaring occur which can be mistaken for scleroderma. Other skin manifestations include hyperpigmentation, hypertrichosis, alopecia due to scaring and purplish heliotrope suffusion of periorbital areas.
Patients can develop cirrhosis due to accumulation of porphyria in the hepatocytes and subsequent parenchymal damage. Hepatocellular carcinoma surveillance is recommended for patients with PCT, although its incidence is rare in those patients.
Diagnosis and Treatment
PCT is mainly a clinical diagnosis. Physicians should consider PCT in patients with photosensitivity and blisters after minor trauma (Figure 2). The urine of a patient with PCT is often pink or red when exposed to air or light due to its high concentration of porphyrin products. Mild elevation of liver enzymes and fatty liver on ultrasound are also noted. Evidence of iron overload is seen in most cases. Screening for risk factors like HCV, HIV, hepatitis B virus, and HH is recommended. Confirmation of PCT typically requires measurement of the porphyria level in a 24-hour urine collection.
Avoiding sun exposure is fundamental in decreasing the development of skin lesions and scaring. Additionally, patients should be advised about the adverse effects of alcohol, smoking, and estrogen therapy on PCT. Treatment of PCT is frequently focused on iron overload and subsequent increased porphyrin oxidation.1,2 Iron can increase reactive oxygen species (ROS), which, in turn, increases the rate of oxidation of uroporphyrinogens. Excess iron also decreases the activity of UROD and increases δ-aminolevulinic acid (ALA) production (the precursor of uroporphyrinogen). Phlebotomy to treat iron overload should be done to a target ferritin level of 20 ng/mL. Clinical manifestations, including skin lesions, typically will normalize before the laboratory findings. Therapeutic remission is expected after 6 to 7 phlebotomy attempts, while clinical improvement can occur after 2 to 3 phlebotomies.
In addition to phlebotomy, 4-aminoquinoline medications (chloroquine and hydroxychloroquine) can be used effectively to treat PCT. Hydroxychloroquine is generally preferred due to its better safety profile. Although the exact mechanism of action of 4-aminoquinolines is not clear, it has been suggested that they bind to porphyrins and form water-soluble products, which are then excreted in the urine. Again, clinical remission occurs much sooner than chemical remission, (3 months vs 12 months). A 4-aminoquinoline should not be used in patients with severe liver disease, renal insufficiency, pregnancy, or G6PD deficiency. When used, they should be used in lower than typical doses due to the rapid removal of accumulated porphyrin from the hepatocytes potentially causing necrosis and acute hepatitis.
Iron chelation also is effective, but it is slower in achieving remission and more expensive than phlebotomy. Treatment of PCT should be individualized. For example, 4-aminoquinolines are contraindicated for patients with end-stage renal disease (ESRD), while phlebotomy could present a problem for patients with preexisting anemia. In this instance, removing 50 cc of blood every 2 weeks may be safe and effective. Furthermore, 4-aminoquinolines in patients with severe iron overload and phlebotomy have been used together. Plasmapheresis is still another option in patients with ESRD.
The use of direct antiviral agents (DAA) in the treatment of HCV has shown promising results in maintaining undetectable viral loads and concurrent remission of PCT. Several studies have shown that treatment of HCV with a DAA obviates the need for treatment PCT.3-5 Treatment of HCV with interferon (IFN) and ribavirin have shown mixed results in controlling PCT, possibly due to their ineffectiveness in maintaining a suppressed viral load. Some studies even showed worsening of PCT with IFN/ribavirin.6
Hemochromatosis
Human cells need iron for aerobic respiration. The intestinal mucosa controls iron uptake and its transfer to the blood stream. Aside from variations in intestinal absorption with fecal excretion, humans do not have another pathway to excrete excess iron. HH is the most common genetic disorder in whites.7 It is an autosomal recessive disorder that increases the intestinal absorption of iron. The most common mutation in the hemochromatosis (HFE) gene results in a substitution of tyrosine for cysteine at amino acid number 282 and is referred to as the C282Y mutation. A second mutation changes histidine at position 63 to aspartic acid and is referred to as a H63D mutation. H63D is present in a minority of the patients with phenotypically expressed HH and its clinical impact is unknown.
Homozygosity of the C282Y mutation is the most common genotype associated with clinical hemochromatosis. While carriers of the C282Y gene heterozygote mutation typically do not develop enough iron overload to cause clinical hemochromatosis, they can if other risk factors, such as PCT, excess alcohol use, liver disease, or HCV, are present.8 Additionally, an associated genetic defect, like a compound heterozygotes C282Y/H63D mutation, a private HFE mutation in trans, or other iron-related genes, can cause manifestations of iron overload. Lastly, about 20% of patients that are heterozygous for both mutations can express the HH phenotype.8
Clinical Manifestation
Patients with HH absorb only a few extra milligrams of iron daily. The clinical manifestation begins to occur when the total body iron store reaches 15-40 g (normal, 4 g). While the genetic mutation is present from birth, iron stores start to rise slowly to around 10 g > age 15 years, at which point serum iron levels are elevated. After age 20 years, the speed with which the iron is stored increases, and by 30 years, liver damage and tissue injury will occur. Cirrhosis is possible by 40 years.7 Age, sex, dietary iron intake, blood loss (menstruation), pregnancy, and other unknown factors greatly influence the disease progression. Homozygote C282Y mutation is as common in women as it is in men, but women are less likely to express the HH phenotype, presumably due, in part, to menstruation. When diagnosed early, most of the clinical manifestations of HH are preventable. Additional manifestations of HH include hyperpigmentation, cardiomyopathy, diabetes mellitus, hypogonadism, hypothyroidism, and arthropathy due to pseudogout.
Iron overload due to HH should be distinguished from other causes of iron overload including exogenous iron overload, anemia (thalassemia, sideroblastic), and chronic liver diseases like PCT, viral hepatitis, nonalcoholic steatohepatitis, and alcoholic liver disease.
Diagnosis
HH should be suspected in patients with a high serum transferrin saturation and elevated serum ferritin concentrations. Typically, transferrin saturation is > 50% and ferritin levels are > 300 ng/mL in men and > 200 ng/mL in women. In early stages of the disease, transferrin saturation can be normal. Additionally, in patients with chronic inflammation, ferritin may be high due to acute-phase reactants and the iron panel should be interpreted with caution. When the secondary causes of abnormalities in a patient’s iron studies are excluded, genetic testing for HFE gene is recommended.
The majority of patients (60-93%) with clinically evident hemochromatosis are homozygous for C282Y mutation. In a heterozygous C282Y mutation with a high transferrin saturation and HH phenotype, additional genetic testing for a heterozygous compound mutation C282Y/H63D is recommended.8 Additional studies could include evaluation for a private HFE mutation in trans or other iron-related genes. Liver biopsy is the gold standard for assessing the degree of hepatic fibrosis. Determining the degree of fibrosis by some means is needed due to the increased risk of hepatocellular carcinoma (HCC) in HH patients with advanced fibrosis and cirrhosis.9
Treatment
Iron depletion with phlebotomy is the cornerstone of treatment in HH. Phlebotomy initially is done weekly with goal of achieving a transferrin saturation < 50%, a serum ferritin level < 50 ng/mL, and a hemoglobin of 12 to 13 ng/mL. When these goals are achieved, patients typically need 4 to 8 phlebotomies per year to maintain a transferrin saturation < 50% (Figure 3).
Hemochromatosis and PCT
Many studies have investigated the relevance of C282Y and/or H63D mutations in patients with PCT.9,10 It appears that ≥ 1 mutation of the HFE gene in PCT may be an important susceptibility factor in the development of clinical PCT. Various studies have shown an incidence of C282Y mutations of 44 to 47% in patients with PCT, compared with 9 to 12% in control populations.9,10 The incidence of the H63D mutation in PCT has been more variable, with some studies showing no difference between patients with PCT and a control group, while other studies showed 31% incidence of H63D mutation in patients with PCT.9,10 A higher incidence of C282Y and H63D mutations in PCT may be a sign that the HFE mutation could be an important factor in developing PCT.
Hemochromatosis and Hepatitis C
Transferrin saturation is frequently elevated in patients with HCV. It is yet unclear whether the pathology of liver disease in patients with HCV is influenced by iron overload or limited to the direct cell damage from replication of the virus and subsequent inflammation. It is believed that the pathology of iron overload in the patients with HCV is different from HH. Like other secondary causes of iron overload, the excess iron is stored in the Kupffer cells of patients with HCV. In HH, excess iron is stored in hepatocytes.
The prevalence of the HFE mutation is the same in the patients with chronic HCV and healthy individuals.10,11 However, HFE mutations are more prevalent in 30 to 60% of the patients with chronic HCV who have elevated transferrin saturations. Alone, C282Y heterozygosity, H63D heterozygosity, or C282Y/H63D compound heterozygosity could not lead to clinically significant iron overload in otherwise healthy individuals; however, these could be a significant cause of iron overload in patients with chronic HCV. Theoretically, the combination of iron overload and HFE gene mutations could increase the rate of advanced fibrosis/cirrhosis in chronic HCV. An increase serum ferritin level of 200 ng/dL in women and 250 ng/dL in men has been observed in 32% of patients with chronic HCV. In this subset of patients, phlebotomy reduced the progression of their liver disease and reduction in their liver enzymes.
Iron Overload and Cardiovascular Risk
In 1987, a Framingham cohort of > 2,800 patients showed a higher incident of CAD in postmenopausal women when compared with premenopausal women.12 In the 1980s, Sullivan hypothesized that the reason for higher incidence of CAD in men when compared with premenopausal women was due to their higher body iron storage.13-16 A study of 1,930 Finnish men reported that the men with ferritin level ≥ 200 ng/dL had a risk 2.2 times higher of acute myocardial infarction when compared to men with lower serum ferritin level.17
A prospective study published in 1997 by Klechl showed the role of iron stores in early atherogenesis via promotion of lipid oxidation.18 Other epidemiological studies have shown a decreased risk of myocardial infarction in blood donors, and while arguments have been made that the blood donors tend to be healthier individuals, 2 studies were published in 1997 matching healthy blood donors to healthy nonblood donors, and both showed a lower risk of CVD in the donors when compared with nondonors.19,20 Furthermore, in an animal model of atherosclerosis, an iron depleted diet showed a reduction of atherosclerosis progression.21 Multiple studies have shown that the heterozygosity for HFE is significantly linked to the risk of cardiovascular events, including the fact that heterozygosity for C282Y has been shown to be a risk factor for myocardial infarction in men and cerebrovascular death in women.22-25
Conclusion
Multiple studies have shown an association between the elevated iron levels associated with the HFE genotype and the disease states of our patient. These include an increased risk of CAD, the increased risk of cirrhosis in HCV and the development of PCT. Indeed, in this case, our patient likely acquired PCT from the combined risks of HCV and his heterozygous HFE genetic mutation.
With regard to Mr. M’s treatment, the use of an antiviral agent in the treatment of his HCV is fundamental, along with avoidance of alcohol and smoking. If he were to accept HCV treatment, we would anticipate resolution of the PCT, but the ongoing progression of his liver and cardiovascular conditions, due perhaps in part, to relative iron overload from his heterozygous HFE mutation. In this situation, we expect that an ongoing course of therapeutic phlebotomy could help to delay the progression of his chronic liver and cardiovascular diseases.
Sporadic porphyria cutanea tarda (PCT) is the most common cause of porphyria worldwide.1,2 Unlike other forms of porphyria, PCT usually is an acquired disease precipitated by extrinsic risk factors that commonly include excessive alcohol consumption, smoking, and chronic hepatitis C virus (HCV) infection. Additional risk factors include myeloproliferative disorders, exposure to polyhalogenated compounds, estrogen therapy, diseases of iron overload like hereditary hemochromatosis (HH), and potentially, HIV infection.1-3
In this case report, we present a patient with an iron overload (due in part to an HFE gene mutation) and concomitant PCT,
Case Presentation
Mr. M is a 59-year-old white male of Irish background with a medical history that includes coronary artery disease. He is status post ST-elevation myocardial infarction and percutaneous coronary intervention with placement of 2 drug-eluting stents. Additional medical issues include PCT and HCV infection with cirrhosis. He is an active smoker.
The patient has a long history of developing blisters with minor trauma, such as rubbing against his mattress/bed sheets or bumping into doors. These blisters primarily occur on his upper extremities, but also can occur on his face after shaving. Mr. M was diagnosed with HCV infection in 1979 while on active military duty. At that time, he had an acute HCV infection and jaundice that required a prolonged hospitalization. He reported no IV drug use and that many others on his military base had similar manifestations. He drinks 1 to 2 beers daily, but reports no binge drinking.
His laboratory studies were notable for ferritin, 2,069 ng/mL; serum iron, 317 mcg/dL; total iron binding capacity, 320 mcg/dL; transferrin, 239 mg/dl; liver function test alanine aminotransferase, 151 U/L; aspartate aminotransferase, 159 U/L; total bilirubin, 1.73 mg/dL; albumin, 3.6 g/dL; alkaline phosphatase, 119 U/L; INR, 1.1; and transferrin saturation, 99%. Mr. M’s HCV viral load was 28,700 IU/L with genotype 1b. Hemochromatosis genetic studies were notable for a heterozygous C282Y gene mutation and negative for H63D and S65C mutations. He repeatedly declined completing a 24-hour urine study of porphyrins. Ultrasonography was consistent with cirrhosis and splenomegaly. The patient was treatment naïve for HCV. He declined multiple offers for treatment of his HCV, citing financial considerations.
Porphyria Cutanea Tarda
The pathogenesis of PCT is related to the intrahepatic deficiency of uroporphyrinogen decarboxylase (UROD), an enzyme in the heme biosynthetic pathway (Figure 1). Decreased activity of UROD leads to accumulation of uroporphyrinogen and its derivatives, which most likely are oxidized in presence of cytochrome P450 1A2. Up to 80% of PCT cases are sporadic, in which the deficiency of UROD is acquired by exogenous risk factors as mentioned above. However, the remaining 20% of PCT cases are due to an autosomal dominant mutation of UROD that causes the partial deficiency (up to 50%) of UROD. In these cases, additional risk factors are needed to decrease UROD activity to < 75% for symptoms to occur.
Clinical Manifestation
Patients with PCT typically develop blisters, skin fragility, and peeling with sun exposure or minor trauma. They also may experience delayed wound healing in sun-exposed skin.3 The photosensitivity of PCT is believed to be related to the saturation of highly carboxylated uroporphyrins in the liver, which are then released into the circulation. Sun exposure then activates these products facilitating an immune reaction and subsequent skin damage.2 In chronic cases, fibrotic reactions and scaring occur which can be mistaken for scleroderma. Other skin manifestations include hyperpigmentation, hypertrichosis, alopecia due to scaring and purplish heliotrope suffusion of periorbital areas.
Patients can develop cirrhosis due to accumulation of porphyria in the hepatocytes and subsequent parenchymal damage. Hepatocellular carcinoma surveillance is recommended for patients with PCT, although its incidence is rare in those patients.
Diagnosis and Treatment
PCT is mainly a clinical diagnosis. Physicians should consider PCT in patients with photosensitivity and blisters after minor trauma (Figure 2). The urine of a patient with PCT is often pink or red when exposed to air or light due to its high concentration of porphyrin products. Mild elevation of liver enzymes and fatty liver on ultrasound are also noted. Evidence of iron overload is seen in most cases. Screening for risk factors like HCV, HIV, hepatitis B virus, and HH is recommended. Confirmation of PCT typically requires measurement of the porphyria level in a 24-hour urine collection.
Avoiding sun exposure is fundamental in decreasing the development of skin lesions and scaring. Additionally, patients should be advised about the adverse effects of alcohol, smoking, and estrogen therapy on PCT. Treatment of PCT is frequently focused on iron overload and subsequent increased porphyrin oxidation.1,2 Iron can increase reactive oxygen species (ROS), which, in turn, increases the rate of oxidation of uroporphyrinogens. Excess iron also decreases the activity of UROD and increases δ-aminolevulinic acid (ALA) production (the precursor of uroporphyrinogen). Phlebotomy to treat iron overload should be done to a target ferritin level of 20 ng/mL. Clinical manifestations, including skin lesions, typically will normalize before the laboratory findings. Therapeutic remission is expected after 6 to 7 phlebotomy attempts, while clinical improvement can occur after 2 to 3 phlebotomies.
In addition to phlebotomy, 4-aminoquinoline medications (chloroquine and hydroxychloroquine) can be used effectively to treat PCT. Hydroxychloroquine is generally preferred due to its better safety profile. Although the exact mechanism of action of 4-aminoquinolines is not clear, it has been suggested that they bind to porphyrins and form water-soluble products, which are then excreted in the urine. Again, clinical remission occurs much sooner than chemical remission, (3 months vs 12 months). A 4-aminoquinoline should not be used in patients with severe liver disease, renal insufficiency, pregnancy, or G6PD deficiency. When used, they should be used in lower than typical doses due to the rapid removal of accumulated porphyrin from the hepatocytes potentially causing necrosis and acute hepatitis.
Iron chelation also is effective, but it is slower in achieving remission and more expensive than phlebotomy. Treatment of PCT should be individualized. For example, 4-aminoquinolines are contraindicated for patients with end-stage renal disease (ESRD), while phlebotomy could present a problem for patients with preexisting anemia. In this instance, removing 50 cc of blood every 2 weeks may be safe and effective. Furthermore, 4-aminoquinolines in patients with severe iron overload and phlebotomy have been used together. Plasmapheresis is still another option in patients with ESRD.
The use of direct antiviral agents (DAA) in the treatment of HCV has shown promising results in maintaining undetectable viral loads and concurrent remission of PCT. Several studies have shown that treatment of HCV with a DAA obviates the need for treatment PCT.3-5 Treatment of HCV with interferon (IFN) and ribavirin have shown mixed results in controlling PCT, possibly due to their ineffectiveness in maintaining a suppressed viral load. Some studies even showed worsening of PCT with IFN/ribavirin.6
Hemochromatosis
Human cells need iron for aerobic respiration. The intestinal mucosa controls iron uptake and its transfer to the blood stream. Aside from variations in intestinal absorption with fecal excretion, humans do not have another pathway to excrete excess iron. HH is the most common genetic disorder in whites.7 It is an autosomal recessive disorder that increases the intestinal absorption of iron. The most common mutation in the hemochromatosis (HFE) gene results in a substitution of tyrosine for cysteine at amino acid number 282 and is referred to as the C282Y mutation. A second mutation changes histidine at position 63 to aspartic acid and is referred to as a H63D mutation. H63D is present in a minority of the patients with phenotypically expressed HH and its clinical impact is unknown.
Homozygosity of the C282Y mutation is the most common genotype associated with clinical hemochromatosis. While carriers of the C282Y gene heterozygote mutation typically do not develop enough iron overload to cause clinical hemochromatosis, they can if other risk factors, such as PCT, excess alcohol use, liver disease, or HCV, are present.8 Additionally, an associated genetic defect, like a compound heterozygotes C282Y/H63D mutation, a private HFE mutation in trans, or other iron-related genes, can cause manifestations of iron overload. Lastly, about 20% of patients that are heterozygous for both mutations can express the HH phenotype.8
Clinical Manifestation
Patients with HH absorb only a few extra milligrams of iron daily. The clinical manifestation begins to occur when the total body iron store reaches 15-40 g (normal, 4 g). While the genetic mutation is present from birth, iron stores start to rise slowly to around 10 g > age 15 years, at which point serum iron levels are elevated. After age 20 years, the speed with which the iron is stored increases, and by 30 years, liver damage and tissue injury will occur. Cirrhosis is possible by 40 years.7 Age, sex, dietary iron intake, blood loss (menstruation), pregnancy, and other unknown factors greatly influence the disease progression. Homozygote C282Y mutation is as common in women as it is in men, but women are less likely to express the HH phenotype, presumably due, in part, to menstruation. When diagnosed early, most of the clinical manifestations of HH are preventable. Additional manifestations of HH include hyperpigmentation, cardiomyopathy, diabetes mellitus, hypogonadism, hypothyroidism, and arthropathy due to pseudogout.
Iron overload due to HH should be distinguished from other causes of iron overload including exogenous iron overload, anemia (thalassemia, sideroblastic), and chronic liver diseases like PCT, viral hepatitis, nonalcoholic steatohepatitis, and alcoholic liver disease.
Diagnosis
HH should be suspected in patients with a high serum transferrin saturation and elevated serum ferritin concentrations. Typically, transferrin saturation is > 50% and ferritin levels are > 300 ng/mL in men and > 200 ng/mL in women. In early stages of the disease, transferrin saturation can be normal. Additionally, in patients with chronic inflammation, ferritin may be high due to acute-phase reactants and the iron panel should be interpreted with caution. When the secondary causes of abnormalities in a patient’s iron studies are excluded, genetic testing for HFE gene is recommended.
The majority of patients (60-93%) with clinically evident hemochromatosis are homozygous for C282Y mutation. In a heterozygous C282Y mutation with a high transferrin saturation and HH phenotype, additional genetic testing for a heterozygous compound mutation C282Y/H63D is recommended.8 Additional studies could include evaluation for a private HFE mutation in trans or other iron-related genes. Liver biopsy is the gold standard for assessing the degree of hepatic fibrosis. Determining the degree of fibrosis by some means is needed due to the increased risk of hepatocellular carcinoma (HCC) in HH patients with advanced fibrosis and cirrhosis.9
Treatment
Iron depletion with phlebotomy is the cornerstone of treatment in HH. Phlebotomy initially is done weekly with goal of achieving a transferrin saturation < 50%, a serum ferritin level < 50 ng/mL, and a hemoglobin of 12 to 13 ng/mL. When these goals are achieved, patients typically need 4 to 8 phlebotomies per year to maintain a transferrin saturation < 50% (Figure 3).
Hemochromatosis and PCT
Many studies have investigated the relevance of C282Y and/or H63D mutations in patients with PCT.9,10 It appears that ≥ 1 mutation of the HFE gene in PCT may be an important susceptibility factor in the development of clinical PCT. Various studies have shown an incidence of C282Y mutations of 44 to 47% in patients with PCT, compared with 9 to 12% in control populations.9,10 The incidence of the H63D mutation in PCT has been more variable, with some studies showing no difference between patients with PCT and a control group, while other studies showed 31% incidence of H63D mutation in patients with PCT.9,10 A higher incidence of C282Y and H63D mutations in PCT may be a sign that the HFE mutation could be an important factor in developing PCT.
Hemochromatosis and Hepatitis C
Transferrin saturation is frequently elevated in patients with HCV. It is yet unclear whether the pathology of liver disease in patients with HCV is influenced by iron overload or limited to the direct cell damage from replication of the virus and subsequent inflammation. It is believed that the pathology of iron overload in the patients with HCV is different from HH. Like other secondary causes of iron overload, the excess iron is stored in the Kupffer cells of patients with HCV. In HH, excess iron is stored in hepatocytes.
The prevalence of the HFE mutation is the same in the patients with chronic HCV and healthy individuals.10,11 However, HFE mutations are more prevalent in 30 to 60% of the patients with chronic HCV who have elevated transferrin saturations. Alone, C282Y heterozygosity, H63D heterozygosity, or C282Y/H63D compound heterozygosity could not lead to clinically significant iron overload in otherwise healthy individuals; however, these could be a significant cause of iron overload in patients with chronic HCV. Theoretically, the combination of iron overload and HFE gene mutations could increase the rate of advanced fibrosis/cirrhosis in chronic HCV. An increase serum ferritin level of 200 ng/dL in women and 250 ng/dL in men has been observed in 32% of patients with chronic HCV. In this subset of patients, phlebotomy reduced the progression of their liver disease and reduction in their liver enzymes.
Iron Overload and Cardiovascular Risk
In 1987, a Framingham cohort of > 2,800 patients showed a higher incident of CAD in postmenopausal women when compared with premenopausal women.12 In the 1980s, Sullivan hypothesized that the reason for higher incidence of CAD in men when compared with premenopausal women was due to their higher body iron storage.13-16 A study of 1,930 Finnish men reported that the men with ferritin level ≥ 200 ng/dL had a risk 2.2 times higher of acute myocardial infarction when compared to men with lower serum ferritin level.17
A prospective study published in 1997 by Klechl showed the role of iron stores in early atherogenesis via promotion of lipid oxidation.18 Other epidemiological studies have shown a decreased risk of myocardial infarction in blood donors, and while arguments have been made that the blood donors tend to be healthier individuals, 2 studies were published in 1997 matching healthy blood donors to healthy nonblood donors, and both showed a lower risk of CVD in the donors when compared with nondonors.19,20 Furthermore, in an animal model of atherosclerosis, an iron depleted diet showed a reduction of atherosclerosis progression.21 Multiple studies have shown that the heterozygosity for HFE is significantly linked to the risk of cardiovascular events, including the fact that heterozygosity for C282Y has been shown to be a risk factor for myocardial infarction in men and cerebrovascular death in women.22-25
Conclusion
Multiple studies have shown an association between the elevated iron levels associated with the HFE genotype and the disease states of our patient. These include an increased risk of CAD, the increased risk of cirrhosis in HCV and the development of PCT. Indeed, in this case, our patient likely acquired PCT from the combined risks of HCV and his heterozygous HFE genetic mutation.
With regard to Mr. M’s treatment, the use of an antiviral agent in the treatment of his HCV is fundamental, along with avoidance of alcohol and smoking. If he were to accept HCV treatment, we would anticipate resolution of the PCT, but the ongoing progression of his liver and cardiovascular conditions, due perhaps in part, to relative iron overload from his heterozygous HFE mutation. In this situation, we expect that an ongoing course of therapeutic phlebotomy could help to delay the progression of his chronic liver and cardiovascular diseases.
1. Singal AK. Porphyria cutanea tarda: Recent update. Mol Genet Metab. 2019;128(3):271-281.
2. Ryan Caballes F, Sendi H, Bonkovsky HL. Hepatitis C, porphyria cutanea tarda and liver iron: an update. Liver Int. 2012;32(6):880-893.
3. Wiznia LE, Laird ME, Franks AG Jr. Hepatitis C virus and its cutaneous manifestations: treatment in the direct-acting antiviral era. J Eur Acad Dermatol Venereol. 2017;31(8):1260-1270.
4. Nihei T, Kiniwa Y, Mikoshiba Y, Joshita S, Okuyama R. Improvement of porphyria cutanea tarda following treatment of hepatitis C virus by direct-acting antivirals: a case report. J Dermatol. 2019;46(5):e149-e151.
5. Combalia A, To-Figueras J, Laguno M, Martínez-Rebollar M, Aguilera P. Direct-acting antivirals for hepatitis C virus induce a rapid clinical and biochemical remission of porphyria cutanea tarda. Br J Dermatol. 2017;177(5):e183-e184. 6. Singal AK, Venkata KVR, Jampana S, Islam FU, Anderson KE. Hepatitis C treatment in patients with porphyria cutanea tarda. Am J Med Sci. 2017;353 (6):523-528.
7. Brandhagen DJ, Fairbanks VF, Baldus W. Recognition and management of hereditary hemochromatosis. Am Fam Physician. 2002;65(5):853-860.
8. Aguilar-Martinez P, Grandchamp B, Cunat S, et al. Iron overload in HFE C282Y heterozygotes at first genetic testing: a strategy for identifying rare HFE variants. Haematologica. 2011;96(4):507-514.
9. Erhardt A, Maschner-Olberg A, Mellenthin C, et al. HFE mutations and chronic hepatitis C: H63D and C282Y heterozygosity are independent risk factors for liver fibrosis and cirrhosis. J Hepatol. 2003;38(3):335-342.
10. Mehrany K, Drage LA, Brandhagen DJ, Pittelkow MR. Association of porphyria cutanea tarda with hereditary hemochromatosis. J Am Acad Dermatol. 2004;51(2):205-211.
11. Pietrangelo A. Hemochromatosis gene modifies course of hepatitis C viral infection. Gastroenterology. 2003;124(5):1509-1523.
12. Gordon T, Kannel WB, Hjortland MC, McNamara PM. Menopause and coronary heart disease. The Framingham Study. Ann Intern Med. 1978;89(2):157-161.
13. Sullivan JL. Iron and the sex difference in heart disease risk. Lancet. 1981;1(8233):1293-1294.
14. Sullivan JL. The sex difference in ischemic heart disease. Perspect Biol Med. 1983;26(4):657-671.
15. Sullivan JL. The iron paradigm of ischemic heart disease. Am Heart J. 1989;117(5):1177-1188.
16. Sullivan JL. Stored iron and ischemic heart disease: empirical support for a new paradigm. Circulation. 1992;86(3):1036-1037.
17. Salonen JT, Nyyssönen K, Korpela H, Tuomilehto J, Seppänen R, Salonen R. High stored iron levels are associated with excess risk of myocardial infarction in eastern Finnish men. Circulation. 1992;86(3):803-811.
18. Kiechl S, Willeit J, Egger G, Poewe W, Oberhollenzer F. Body iron stores and the risk of carotid atherosclerosis: prospective results from the Bruneck study. Circulation. 1997;96(10):3300-3307.
19. Tuomainen TP, Salonen R, Nyyssönen K, Salonen JT. Cohort study of relation between donating blood and risk of myocardial infarction in 2682 men in eastern Finland. BMJ. 1997;314(7083):793-794.
20. Meyers DG, Strickland D, Maloley PA, Seburg JK, Wilson JE, McManus BF. Possible association of a reduction in cardiovascular events with blood donation. Heart. 1997;78(2):188-193.
21. Lee TS, Shiao MS, Pan CC, Chau LY. Iron-deficient diet reduces atherosclerotic lesions in apoE-deficient mice. Circulation. 1999;99(9):1222-1229.
22. Surber R, Sigusch HH, Kuehnert H, Figulla HR. Haemochromatosis (HFE) gene C282Y mutation and the risk of coronary artery disease and myocardial infarction: a study in 1279 patients undergoing coronary angiography. J Med Genet. 2003;40(5):e58.
23. Tuomainen TP, Kontula K, Nyyssönen K, Lakka TA, Heliö T, Salonen JT. Increased risk of acute myocardial infarction in carriers of the hemochromatosis gene Cys282Tyr mutation: a prospective cohort study in men in eastern Finland. Circulation. 1999;100(12):1274-1279.
24. Roest M, van der Schouw YT, de Valk B, et al. Heterozygosity for a hereditary hemochromatosis gene is associated with cardiovascular death in women. Circulation. 1999;100(12):1268-1273.
25. Pourmoghaddas A, Sanei H, Garakyaraghi M, Esteki-Ghashghaei F, Gharaati M. The relation between body iron store and ferritin, and coronary artery disease. ARYA Atheroscler. 2014;10(1):32-36.
1. Singal AK. Porphyria cutanea tarda: Recent update. Mol Genet Metab. 2019;128(3):271-281.
2. Ryan Caballes F, Sendi H, Bonkovsky HL. Hepatitis C, porphyria cutanea tarda and liver iron: an update. Liver Int. 2012;32(6):880-893.
3. Wiznia LE, Laird ME, Franks AG Jr. Hepatitis C virus and its cutaneous manifestations: treatment in the direct-acting antiviral era. J Eur Acad Dermatol Venereol. 2017;31(8):1260-1270.
4. Nihei T, Kiniwa Y, Mikoshiba Y, Joshita S, Okuyama R. Improvement of porphyria cutanea tarda following treatment of hepatitis C virus by direct-acting antivirals: a case report. J Dermatol. 2019;46(5):e149-e151.
5. Combalia A, To-Figueras J, Laguno M, Martínez-Rebollar M, Aguilera P. Direct-acting antivirals for hepatitis C virus induce a rapid clinical and biochemical remission of porphyria cutanea tarda. Br J Dermatol. 2017;177(5):e183-e184. 6. Singal AK, Venkata KVR, Jampana S, Islam FU, Anderson KE. Hepatitis C treatment in patients with porphyria cutanea tarda. Am J Med Sci. 2017;353 (6):523-528.
7. Brandhagen DJ, Fairbanks VF, Baldus W. Recognition and management of hereditary hemochromatosis. Am Fam Physician. 2002;65(5):853-860.
8. Aguilar-Martinez P, Grandchamp B, Cunat S, et al. Iron overload in HFE C282Y heterozygotes at first genetic testing: a strategy for identifying rare HFE variants. Haematologica. 2011;96(4):507-514.
9. Erhardt A, Maschner-Olberg A, Mellenthin C, et al. HFE mutations and chronic hepatitis C: H63D and C282Y heterozygosity are independent risk factors for liver fibrosis and cirrhosis. J Hepatol. 2003;38(3):335-342.
10. Mehrany K, Drage LA, Brandhagen DJ, Pittelkow MR. Association of porphyria cutanea tarda with hereditary hemochromatosis. J Am Acad Dermatol. 2004;51(2):205-211.
11. Pietrangelo A. Hemochromatosis gene modifies course of hepatitis C viral infection. Gastroenterology. 2003;124(5):1509-1523.
12. Gordon T, Kannel WB, Hjortland MC, McNamara PM. Menopause and coronary heart disease. The Framingham Study. Ann Intern Med. 1978;89(2):157-161.
13. Sullivan JL. Iron and the sex difference in heart disease risk. Lancet. 1981;1(8233):1293-1294.
14. Sullivan JL. The sex difference in ischemic heart disease. Perspect Biol Med. 1983;26(4):657-671.
15. Sullivan JL. The iron paradigm of ischemic heart disease. Am Heart J. 1989;117(5):1177-1188.
16. Sullivan JL. Stored iron and ischemic heart disease: empirical support for a new paradigm. Circulation. 1992;86(3):1036-1037.
17. Salonen JT, Nyyssönen K, Korpela H, Tuomilehto J, Seppänen R, Salonen R. High stored iron levels are associated with excess risk of myocardial infarction in eastern Finnish men. Circulation. 1992;86(3):803-811.
18. Kiechl S, Willeit J, Egger G, Poewe W, Oberhollenzer F. Body iron stores and the risk of carotid atherosclerosis: prospective results from the Bruneck study. Circulation. 1997;96(10):3300-3307.
19. Tuomainen TP, Salonen R, Nyyssönen K, Salonen JT. Cohort study of relation between donating blood and risk of myocardial infarction in 2682 men in eastern Finland. BMJ. 1997;314(7083):793-794.
20. Meyers DG, Strickland D, Maloley PA, Seburg JK, Wilson JE, McManus BF. Possible association of a reduction in cardiovascular events with blood donation. Heart. 1997;78(2):188-193.
21. Lee TS, Shiao MS, Pan CC, Chau LY. Iron-deficient diet reduces atherosclerotic lesions in apoE-deficient mice. Circulation. 1999;99(9):1222-1229.
22. Surber R, Sigusch HH, Kuehnert H, Figulla HR. Haemochromatosis (HFE) gene C282Y mutation and the risk of coronary artery disease and myocardial infarction: a study in 1279 patients undergoing coronary angiography. J Med Genet. 2003;40(5):e58.
23. Tuomainen TP, Kontula K, Nyyssönen K, Lakka TA, Heliö T, Salonen JT. Increased risk of acute myocardial infarction in carriers of the hemochromatosis gene Cys282Tyr mutation: a prospective cohort study in men in eastern Finland. Circulation. 1999;100(12):1274-1279.
24. Roest M, van der Schouw YT, de Valk B, et al. Heterozygosity for a hereditary hemochromatosis gene is associated with cardiovascular death in women. Circulation. 1999;100(12):1268-1273.
25. Pourmoghaddas A, Sanei H, Garakyaraghi M, Esteki-Ghashghaei F, Gharaati M. The relation between body iron store and ferritin, and coronary artery disease. ARYA Atheroscler. 2014;10(1):32-36.
Occult HCV infection is correlated to unfavorable genotypes in hemophilia patients
The presence of occult hepatitis C virus infection is determined by finding HCV RNA in the liver and peripheral blood mononuclear cells, with no HCV RNA in the serum. Researchers have shown that the presence of occult HCV infection (OCI) was correlated with unfavorable polymorphisms near interferon lambda-3/4 (IFNL3/4), which has been associated with spontaneous HCV clearance.
This study was conducted to assess the frequency of OCI in 450 hemophilia patients in Iran with negative HCV markers, and to evaluate the association of three IFNL3 single nucleotide polymorphisms (rs8099917, rs12979860, and rs12980275) and the IFNL4 ss469415590 SNP with OCI positivity.
The estimated OCI rate was 10.2%. Among the 46 OCI patients, 56.5%, 23.9%, and 19.6% were infected with HCV-1b, HCV-1a, and HCV-3a, respectively. The researchers found that, compared with patients without OCI, unfavorable IFNL3 rs12979860, IFNL3 rs8099917, IFNL3 rs12980275, and IFNL4 ss469415590 genotypes were more frequently found in OCI patients. Multivariate analysis showed that ALT, cholesterol, triglyceride, as well as the aforementioned unfavorable interferon SNP geneotypes were associated with OCI positivity.
“10.2% of anti-HCV seronegative Iranian patients with hemophilia had OCI in our study; therefore, risk of this infection should be taken into consideration. We also showed that patients with unfavorable IFNL3 SNPs and IFNL4 ss469415590 genotypes were exposed to a higher risk of OCI, compared to hemophilia patients with other genotypes,” the researchers concluded.
The authors reported that they had no disclosures.
SOURCE: Nafari AH et al. Infect Genet Evol. 2019 Dec 13. doi: 10.1016/j.meegid.2019.104144.
The presence of occult hepatitis C virus infection is determined by finding HCV RNA in the liver and peripheral blood mononuclear cells, with no HCV RNA in the serum. Researchers have shown that the presence of occult HCV infection (OCI) was correlated with unfavorable polymorphisms near interferon lambda-3/4 (IFNL3/4), which has been associated with spontaneous HCV clearance.
This study was conducted to assess the frequency of OCI in 450 hemophilia patients in Iran with negative HCV markers, and to evaluate the association of three IFNL3 single nucleotide polymorphisms (rs8099917, rs12979860, and rs12980275) and the IFNL4 ss469415590 SNP with OCI positivity.
The estimated OCI rate was 10.2%. Among the 46 OCI patients, 56.5%, 23.9%, and 19.6% were infected with HCV-1b, HCV-1a, and HCV-3a, respectively. The researchers found that, compared with patients without OCI, unfavorable IFNL3 rs12979860, IFNL3 rs8099917, IFNL3 rs12980275, and IFNL4 ss469415590 genotypes were more frequently found in OCI patients. Multivariate analysis showed that ALT, cholesterol, triglyceride, as well as the aforementioned unfavorable interferon SNP geneotypes were associated with OCI positivity.
“10.2% of anti-HCV seronegative Iranian patients with hemophilia had OCI in our study; therefore, risk of this infection should be taken into consideration. We also showed that patients with unfavorable IFNL3 SNPs and IFNL4 ss469415590 genotypes were exposed to a higher risk of OCI, compared to hemophilia patients with other genotypes,” the researchers concluded.
The authors reported that they had no disclosures.
SOURCE: Nafari AH et al. Infect Genet Evol. 2019 Dec 13. doi: 10.1016/j.meegid.2019.104144.
The presence of occult hepatitis C virus infection is determined by finding HCV RNA in the liver and peripheral blood mononuclear cells, with no HCV RNA in the serum. Researchers have shown that the presence of occult HCV infection (OCI) was correlated with unfavorable polymorphisms near interferon lambda-3/4 (IFNL3/4), which has been associated with spontaneous HCV clearance.
This study was conducted to assess the frequency of OCI in 450 hemophilia patients in Iran with negative HCV markers, and to evaluate the association of three IFNL3 single nucleotide polymorphisms (rs8099917, rs12979860, and rs12980275) and the IFNL4 ss469415590 SNP with OCI positivity.
The estimated OCI rate was 10.2%. Among the 46 OCI patients, 56.5%, 23.9%, and 19.6% were infected with HCV-1b, HCV-1a, and HCV-3a, respectively. The researchers found that, compared with patients without OCI, unfavorable IFNL3 rs12979860, IFNL3 rs8099917, IFNL3 rs12980275, and IFNL4 ss469415590 genotypes were more frequently found in OCI patients. Multivariate analysis showed that ALT, cholesterol, triglyceride, as well as the aforementioned unfavorable interferon SNP geneotypes were associated with OCI positivity.
“10.2% of anti-HCV seronegative Iranian patients with hemophilia had OCI in our study; therefore, risk of this infection should be taken into consideration. We also showed that patients with unfavorable IFNL3 SNPs and IFNL4 ss469415590 genotypes were exposed to a higher risk of OCI, compared to hemophilia patients with other genotypes,” the researchers concluded.
The authors reported that they had no disclosures.
SOURCE: Nafari AH et al. Infect Genet Evol. 2019 Dec 13. doi: 10.1016/j.meegid.2019.104144.
FROM INFECTION, GENETICS AND EVOLUTION