User login
A Veteran Presenting With Leg Swelling, Dyspnea, and Proteinuria
*This article has been corrected to include a missing author.
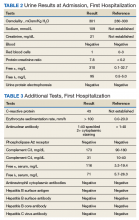
Case Presentation. A 63-year-old male with well-controlled HIV (CD4 count 757, undetectable viral load), epilepsy, and hypertension presented to the VA Boston Healthcare System (VABHS) emergency department with 1 week of bilateral leg swelling and exertional shortness of breath. He reported having no fever, cough, chest pain, pain with inspiration and orthopnea. There was no personal or family history of pulmonary embolism. He reported weight gain but was unable to quantify how much. He also reported flare up of chronic knee pain, without swelling for which he had taken up to 4 tablets of naproxen daily for several weeks. His physical examination was notable for a heart rate of 105 beats per minute and bilateral pitting edema to his knees. Laboratory testing revealed a creatinine level of 2.5 mg/dL, which was increased from a baseline of 1.0 mg/dL (Table 1), and a urine protein-to-creatinine ratio of 7.8 mg/mg (Table 2). A renal ultrasound showed normal-sized kidneys without hydronephrosis or obstructing renal calculi. The patient was admitted for further workup of his dyspnea and acute kidney injury.
► Jonathan Li, MD, Chief Medical Resident, VABHS and Beth Israel Deaconess Medical Center (BIDMC). Dr. William, based on the degree of proteinuria and edema, a diagnosis of nephrotic syndrome was made. How is nephrotic syndrome defined, and how is it distinguished from glomerulonephritis?
► Jeffrey William, MD, Nephrologist, BIDMC, Assistant Professor of Medicine, Harvard Medical School. The pathophysiology of nephrotic disease and glomerulonephritis are quite distinct, resulting in symptoms and systemic manifestations that only slightly overlap. Glomerulonephritis is characterized by inflammation of the endothelial cells of the trilayered glomerular capillary, with a resulting active urine sediment with red blood cells, white blood cells, and casts. Nephrotic syndrome mostly affects the visceral epithelial cells of the glomerular capillary, commonly referred to as podocytes, and hence, the urine sediment in nephrotic disease is often inactive. Patients with nephrotic syndrome have nephrotic-range proteinuria (excretion of > 3.5 g per 24 h or a spot urine protein-creatinine ratio > 3.5 g in the steady state) and both hypoalbuminemia (< 3 g/dL) and peripheral edema. Lipiduria and hyperlipidemia are common findings in nephrotic syndrome but are not required for a clinical diagnosis.1 In contrast, glomerulonephritis is defined by a constellation of findings that include renal insufficiency (often indicated by an elevation in blood urea nitrogen and creatinine), hypertension, hematuria, and subnephrotic range proteinuria. In practice, patients may fulfill criteria of both nephrotic and nephritic syndromes, but the preponderance of clinical evidence often points one way or the other. In this case, nephrotic syndrome was diagnosed based on the urine protein-to-creatinine ratio of 7.8 mg/mg, hypoalbuminemia, and edema.
► Dr. Li. What would be your first-line workup for evaluation of the etiology of this patient’s nephrotic syndrome?
► Dr. William. Rather than memorizing a list of etiologies of nephrotic syndrome, it is essential to consider the pathophysiology of heavy proteinuria. Though the glomerular filtration barrier is extremely complex and defects in any component can cause proteinuria, disruption of the podocyte is often involved. Common disease processes that chiefly target the podocyte include minimal change disease, primary focal and segmental glomerulosclerosis (FSGS), and membranous nephropathy, all by differing mechanisms. Minimal change disease and idiopathic/primary FSGS are increasingly thought to be at differing points on a spectrum of the same disease.2 Secondary FSGS, on the other hand, is a progressive disease, commonly resulting from longstanding hypertension, diabetes mellitus, and obesity in adults. Membranous nephropathy can also be either primary or secondary. Primary membranous nephropathy is chiefly caused by a circulating IgG4 antibody to the podocyte membrane antigen PLA2R (M-type phospholipase A2 receptor), whereas secondary membranous nephropathy can be caused by a variety of systemic etiologies, including autoimmune disease (eg, systemic lupus erythematosus), certain malignancies, chronic infections (eg, hepatitis B and C), and many medications, including nonsteroidal anti-inflammatory drugs (NSAIDs).3-5 Paraprotein deposition diseases can also cause glomerular damage leading to nephrotic-range proteinuria.
Given these potential diagnoses, a careful history should be taken to assess exposures and recent medication use. Urine sediment evaluation is essential in the evaluation of nephrotic syndrome to determine if there is an underlying nephritic process. Select serologies may be sent to look for autoimmune disease, such as systemic lupus erythematosus and common viral exposures like hepatitis B or C. Serum and urine protein electrophoreses would be appropriate initial tests of suspected paraprotein-related diseases. Other serologies, such as antineutrophil cytoplasmic antibodies or antiglomerular basement membrane antibodies, would not necessarily be indicated here given the lack of hematuria and presence of nephrotic-range proteinuria.
► Dr. Li. The initial evaluation was notable for an erythrocyte sedimentation rate > 120 (mm/h) and a weakly positive antinuclear antibody (ANA) titer of 1:40. The remainder of his initial workup did not reveal an etiology for his nephrotic syndrome (Table 3).
Dr. William, is there a role for starting urgent empiric steroids in nephrotic syndrome while workup is ongoing? If so, do the severity of proteinuria and/or symptoms play a role or is this determination based on something else?
► Dr. William. Edema is a primary symptom of nephrotic syndrome and can often be managed with diuretics alone. If a clear medication-mediated cause is suspected, discontinuation of this agent may result in spontaneous improvement without steroid treatment. However,in cases where an etiology is unclear and there are serious thrombotic complications requiring anticoagulation, and a renal biopsy is deemed to be too risky, then empiric steroid therapy may be necessary. Children with new-onset nephrotic syndrome are presumed to have minimal change disease, given its prevalence in this patient population, and are often given empiric steroids without obtaining a renal biopsy. However, in the adult population, a renal biopsy can typically be performed quickly and safely, with pathology results interpreted within days. In this patient, since a diagnosis was unclear and there was no contraindication to renal biopsy, a biopsy should be obtained before consideration of steroids.
► Dr. Li. Steroids were deferred in anticipation of renal biopsy, which showed stage I membranous nephropathy, suggestive of membranous lupus nephritis Class V. The deposits were strongly reactive for immunoglobuline G (IgG), IgA, and complement 1q (C1q), showed co-dominant staining for IgG1, IgG2, and IgG3, and were weakly positive for the PLA2 receptor. Focal intimal arteritis in a small interlobular vessel was seen.
Dr. William, the pathology returned suggestive of lupus nephritis. Does the overall clinical picture fit with lupus nephritis?
► Dr. William. Given the history and a rather low ANA, the diagnosis of lupus nephritis seems unlikely. The lack of IgG4 and PLA2R staining in the biopsy suggests that this membranous pattern on the biopsy is likely to be secondary to a systemic etiology, but further investigation should be pursued.
► Dr. Li. The patient was discharged after the biopsy with a planned outpatient nephrology follow-up to discuss results and treatment. He was prescribed an oral diuretic, and his symptoms improved. Several days after discharge, he developed blurry vision and was evaluated in the Ophthalmology clinic. On fundoscopy, he was found to have acute papillitis, a form of optic neuritis. As part of initial evaluation of infectious etiologies of papillitis, ophthalmology recommended testing for syphilis.
Dr. Strymish, when we are considering secondary syphilis, what is the recommended approach to diagnostic testing?
► Judith Strymish, MD, Infectious Diseases, BIDMC, Assistant Professor of Medicine, Harvard Medical School. The diagnosis of syphilis is usually made through serologic testing of blood specimens. Methods that detect the spirochete directly like dark-field smears are not readily available. Serologic tests include treponemal tests (eg, Treponema pallidum particle agglutination assay [TPPA]) and nontreponemal tests (eg, rapid plasma reagin [RPR]). One needs a confirmatory test because either test is associated with false positives. Either test can be done first. Most laboratories, including those at VABHS are now performing treponemal tests first as these have become more cost-effective.6 The TPPA treponemal test was found to have a lower false negative rate in primary syphilis compared with that of nontreponemal tests.7 Nontreponemal tests can be followed for response to therapy. If a patient has a history of treated syphilis, a nontreponemal test should be sent, since the treponemal test will remain positive for life.
If there is clinical concern for neurosyphilis, cerebrospinal fluid fluorescent (CSF) treponemal antibody needs to be sampled and sent for the nontreponemal venereal disease research laboratory (VDRL) test. The VDRL is highly specific for neurosyphilis but not as sensitive. Cerebrospinal fluid fluorescent treponemal antibody (CSF FTA) may also be sent; it is very sensitive but not very specific for neurosyphilis.
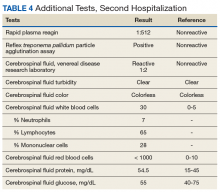
► Dr. Li. An RPR returned positive at 1:512 (was negative 14 months prior on a routine screening test), with positive reflex TPPA (Table 4). A diagnosis of secondary syphilis was made. Dr. Strymish, at this point, what additional testing and treatment is necessary?
► Dr. Strymish. With papillitis and a very high RPR, we need to assume that he has ophthalmic syphilis. This can occur in any stage of syphilis, but his eye findings and high RPR are consistent with secondary syphilis. Ophthalmic syphilis has been on the upswing, even more than is expected with recent increases in syphilis cases.8 Ophthalmic syphilis is considered a form of neurosyphilis. A lumbar puncture and treatment for neurosyphilis is recommended.9,10
► Dr. Li. A lumbar puncture was performed, and his CSF was VDRL positive. This confirmed a diagnosis of neurosyphilis (Table 4). The patient was treated for neurosyphilis with IV penicillin. The patient shared that he had episodes of unprotected oral sexual activity within the past year and approximately 1 year ago, he came in close contact (but no sexual activity) with a person who had a rash consistent with syphilis.Dr. William, syphilis would be a potential unifying diagnosis of his renal and ophthalmologic manifestations. Is syphilis known to cause membranous nephropathy?
► Dr. William. Though it is uncommon, the nephrotic syndrome is a well-described complication of secondary syphilis.11,12 Syphilis has been shown to cause nephrotic syndrome in a variety of ways. Case reports abound linking syphilis to minimal change disease and other glomerular diseases.13,14 A case report from 1993 shows a membranous pattern of glomerular disease similar to this case.15 As a form of secondary membranous nephropathy, the immunofluorescence pattern can demonstrate staining similar to the “full house” seen in lupus nephritis (IgA, IgM, and C1q, in addition to IgG and C3).16 This explains the initial interpretation of this patient’s biopsy, as lupus nephritis would be a much more common etiology of secondary membranous nephropathy than is acute syphilis with this immunofluorescence pattern. However, the data in this case are highly suggestive of a causal relationship between secondary syphilis and membranous nephropathy.
► Dr. Li. Dr. Strymish, how should this patient be screened for syphilis reinfection, and at what intervals would you recommend?
► Dr. Strymish. He will need follow-up testing to make sure that his syphilis is effectively treated. If CSF pleocytosis was present initially, a CSF examination should be repeated every 6 months until the cell count is normal. He will also need follow-up for normalization of his RPR. Persons with HIV infection and primary or secondary syphilis should be evaluated clinically and serologically for treatment failure at 3, 6, 9, 12, and 24 months after therapy according to US Centers for Disease Control and Prevention guidelines.9
His treponemal test for syphilis will likely stay positive for life. His RPR should decrease significantly with effective treatment. It makes sense to screen with RPR alone as long as he continues to have risk factors for acquiring syphilis. Routine syphilis testing is recommended for pregnant women, sexually active men who have sex with men, sexually active persons with HIV, and persons taking PrEP (pre-exposure prophylaxis) for HIV prevention. He should be screened at least yearly for syphilis.
► Dr. Li. Over the next several months, the patient’s creatinine normalized and his proteinuria resolved. His vision recovered, and he has had no further ophthalmologic complications.
Dr. William, what is his long-term renal prognosis? Do you expect that his acute episode of membranous nephropathy will have permanent effects on his renal function?
► Dr. William. His rapid response to therapy for neurosyphilis provides evidence for this etiology of his renal dysfunction and glomerulonephritis. His long-term prognosis is quite good if the syphilis is the only reason for him to have renal disease. The renal damage is often reversible in these cases. However, given his prior extensive NSAID exposure and history of hypertension, he may be at higher risk for chronic kidney disease than an otherwise healthy patient, especially after an episode of acute kidney injury. Therefore, his renal function should continue to be monitored as an outpatient.
Acknowledgments
The authors thank this veteran for sharing his story and allowing us to learn from this unusual case for the benefit of our future patients.
1. Rennke H, Denker BM. Renal Pathophysiology: The Essentials. 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2014.
2. Maas RJ, Deegens JK, Smeets B, Moeller MJ, Wetzels JF. Minimal change disease and idiopathic FSGS: manifestations of the same disease. Nat Rev Nephrol. 2016;12(12):768-776.
3. Beck LH Jr, Bonegio RG, Lambeau G, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361(1):11-21.
4. Rennke HG. Secondary membranoproliferative glomerulonephritis. Kidney Int. 1995;47(2):643-656.
5. Nawaz FA, Larsen CP, Troxell ML. Membranous nephropathy and nonsteroidal anti-inflammatory agents. Am J Kidney Dis. 2013;62(5):1012-1017.
6. Pillay A. Centers for Disease Control and Prevention Syphilis Summit—Diagnostics and laboratory issues. Sex Transm Dis. 2018;45(9S)(suppl 1):S13-S16.
7. Levett PN, Fonseca K, Tsang RS, et al. Canadian Public Health Laboratory Network laboratory guidelines for the use of serological tests (excluding point-of-care tests) for the diagnosis of syphilis in Canada. Can J Infect Dis Med Microbiol. 2015;26(suppl A):6A-12A.
8. Oliver SE, Aubin M, Atwell L, et al. Ocular syphilis—eight jurisdictions, United States, 2014-2015. MMWR Morb Mortal Wkly Rep. 2016;65(43):1185-1188.
9. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recommendations and Reports 2015;64(RR3):1-137. [Erratum in MMWR Recomm Rep. 2015;64(33):924.]
10. US Centers for Disease Control and Prevention. Clinical advisory: ocular syphilis in the United States. https://www.cdc.gov/std/syphilis/clinicaladvisoryos2015.htm. Updated March 24, 2016. Accessed August 12, 2019.
11. Braunstein GD, Lewis EJ, Galvanek EG, Hamilton A, Bell WR. The nephrotic syndrome associated with secondary syphilis: an immune deposit disease. Am J Med. 1970;48:643-648.1.
12. Handoko ML, Duijvestein M, Scheepstra CG, de Fijter CW. Syphilis: a reversible cause of nephrotic syndrome. BMJ Case Rep. 2013;2013:pii:bcr2012008279
13. Krane NK, Espenan P, Walker PD, Bergman SM, Wallin JD. Renal disease and syphilis: a report of nephrotic syndrome with minimal change disease. Am J Kidney Dis. 1987;9(2):176-179.
14. Bhorade MS, Carag HB, Lee HJ, Potter EV, Dunea G. Nephropathy of secondary syphilis: a clinical and pathological spectrum. JAMA. 1971;216(7):1159-1166.
15. Hunte W, al-Ghraoui F, Cohen RJ. Secondary syphilis and the nephrotic syndrome. J Am Soc Nephrol. 1993;3(7):1351-1355.
16. Gamble CN, Reardan JB. Immunopathogenesis of syphilitic glomerulonephritis. Elution of antitreponemal antibody from glomerular immune-complex deposits. N Engl J Med. 1975;292(9):449-454.
*This article has been corrected to include a missing author.
Case Presentation. A 63-year-old male with well-controlled HIV (CD4 count 757, undetectable viral load), epilepsy, and hypertension presented to the VA Boston Healthcare System (VABHS) emergency department with 1 week of bilateral leg swelling and exertional shortness of breath. He reported having no fever, cough, chest pain, pain with inspiration and orthopnea. There was no personal or family history of pulmonary embolism. He reported weight gain but was unable to quantify how much. He also reported flare up of chronic knee pain, without swelling for which he had taken up to 4 tablets of naproxen daily for several weeks. His physical examination was notable for a heart rate of 105 beats per minute and bilateral pitting edema to his knees. Laboratory testing revealed a creatinine level of 2.5 mg/dL, which was increased from a baseline of 1.0 mg/dL (Table 1), and a urine protein-to-creatinine ratio of 7.8 mg/mg (Table 2). A renal ultrasound showed normal-sized kidneys without hydronephrosis or obstructing renal calculi. The patient was admitted for further workup of his dyspnea and acute kidney injury.
► Jonathan Li, MD, Chief Medical Resident, VABHS and Beth Israel Deaconess Medical Center (BIDMC). Dr. William, based on the degree of proteinuria and edema, a diagnosis of nephrotic syndrome was made. How is nephrotic syndrome defined, and how is it distinguished from glomerulonephritis?
► Jeffrey William, MD, Nephrologist, BIDMC, Assistant Professor of Medicine, Harvard Medical School. The pathophysiology of nephrotic disease and glomerulonephritis are quite distinct, resulting in symptoms and systemic manifestations that only slightly overlap. Glomerulonephritis is characterized by inflammation of the endothelial cells of the trilayered glomerular capillary, with a resulting active urine sediment with red blood cells, white blood cells, and casts. Nephrotic syndrome mostly affects the visceral epithelial cells of the glomerular capillary, commonly referred to as podocytes, and hence, the urine sediment in nephrotic disease is often inactive. Patients with nephrotic syndrome have nephrotic-range proteinuria (excretion of > 3.5 g per 24 h or a spot urine protein-creatinine ratio > 3.5 g in the steady state) and both hypoalbuminemia (< 3 g/dL) and peripheral edema. Lipiduria and hyperlipidemia are common findings in nephrotic syndrome but are not required for a clinical diagnosis.1 In contrast, glomerulonephritis is defined by a constellation of findings that include renal insufficiency (often indicated by an elevation in blood urea nitrogen and creatinine), hypertension, hematuria, and subnephrotic range proteinuria. In practice, patients may fulfill criteria of both nephrotic and nephritic syndromes, but the preponderance of clinical evidence often points one way or the other. In this case, nephrotic syndrome was diagnosed based on the urine protein-to-creatinine ratio of 7.8 mg/mg, hypoalbuminemia, and edema.
► Dr. Li. What would be your first-line workup for evaluation of the etiology of this patient’s nephrotic syndrome?
► Dr. William. Rather than memorizing a list of etiologies of nephrotic syndrome, it is essential to consider the pathophysiology of heavy proteinuria. Though the glomerular filtration barrier is extremely complex and defects in any component can cause proteinuria, disruption of the podocyte is often involved. Common disease processes that chiefly target the podocyte include minimal change disease, primary focal and segmental glomerulosclerosis (FSGS), and membranous nephropathy, all by differing mechanisms. Minimal change disease and idiopathic/primary FSGS are increasingly thought to be at differing points on a spectrum of the same disease.2 Secondary FSGS, on the other hand, is a progressive disease, commonly resulting from longstanding hypertension, diabetes mellitus, and obesity in adults. Membranous nephropathy can also be either primary or secondary. Primary membranous nephropathy is chiefly caused by a circulating IgG4 antibody to the podocyte membrane antigen PLA2R (M-type phospholipase A2 receptor), whereas secondary membranous nephropathy can be caused by a variety of systemic etiologies, including autoimmune disease (eg, systemic lupus erythematosus), certain malignancies, chronic infections (eg, hepatitis B and C), and many medications, including nonsteroidal anti-inflammatory drugs (NSAIDs).3-5 Paraprotein deposition diseases can also cause glomerular damage leading to nephrotic-range proteinuria.
Given these potential diagnoses, a careful history should be taken to assess exposures and recent medication use. Urine sediment evaluation is essential in the evaluation of nephrotic syndrome to determine if there is an underlying nephritic process. Select serologies may be sent to look for autoimmune disease, such as systemic lupus erythematosus and common viral exposures like hepatitis B or C. Serum and urine protein electrophoreses would be appropriate initial tests of suspected paraprotein-related diseases. Other serologies, such as antineutrophil cytoplasmic antibodies or antiglomerular basement membrane antibodies, would not necessarily be indicated here given the lack of hematuria and presence of nephrotic-range proteinuria.
► Dr. Li. The initial evaluation was notable for an erythrocyte sedimentation rate > 120 (mm/h) and a weakly positive antinuclear antibody (ANA) titer of 1:40. The remainder of his initial workup did not reveal an etiology for his nephrotic syndrome (Table 3).
Dr. William, is there a role for starting urgent empiric steroids in nephrotic syndrome while workup is ongoing? If so, do the severity of proteinuria and/or symptoms play a role or is this determination based on something else?
► Dr. William. Edema is a primary symptom of nephrotic syndrome and can often be managed with diuretics alone. If a clear medication-mediated cause is suspected, discontinuation of this agent may result in spontaneous improvement without steroid treatment. However,in cases where an etiology is unclear and there are serious thrombotic complications requiring anticoagulation, and a renal biopsy is deemed to be too risky, then empiric steroid therapy may be necessary. Children with new-onset nephrotic syndrome are presumed to have minimal change disease, given its prevalence in this patient population, and are often given empiric steroids without obtaining a renal biopsy. However, in the adult population, a renal biopsy can typically be performed quickly and safely, with pathology results interpreted within days. In this patient, since a diagnosis was unclear and there was no contraindication to renal biopsy, a biopsy should be obtained before consideration of steroids.
► Dr. Li. Steroids were deferred in anticipation of renal biopsy, which showed stage I membranous nephropathy, suggestive of membranous lupus nephritis Class V. The deposits were strongly reactive for immunoglobuline G (IgG), IgA, and complement 1q (C1q), showed co-dominant staining for IgG1, IgG2, and IgG3, and were weakly positive for the PLA2 receptor. Focal intimal arteritis in a small interlobular vessel was seen.
Dr. William, the pathology returned suggestive of lupus nephritis. Does the overall clinical picture fit with lupus nephritis?
► Dr. William. Given the history and a rather low ANA, the diagnosis of lupus nephritis seems unlikely. The lack of IgG4 and PLA2R staining in the biopsy suggests that this membranous pattern on the biopsy is likely to be secondary to a systemic etiology, but further investigation should be pursued.
► Dr. Li. The patient was discharged after the biopsy with a planned outpatient nephrology follow-up to discuss results and treatment. He was prescribed an oral diuretic, and his symptoms improved. Several days after discharge, he developed blurry vision and was evaluated in the Ophthalmology clinic. On fundoscopy, he was found to have acute papillitis, a form of optic neuritis. As part of initial evaluation of infectious etiologies of papillitis, ophthalmology recommended testing for syphilis.
Dr. Strymish, when we are considering secondary syphilis, what is the recommended approach to diagnostic testing?
► Judith Strymish, MD, Infectious Diseases, BIDMC, Assistant Professor of Medicine, Harvard Medical School. The diagnosis of syphilis is usually made through serologic testing of blood specimens. Methods that detect the spirochete directly like dark-field smears are not readily available. Serologic tests include treponemal tests (eg, Treponema pallidum particle agglutination assay [TPPA]) and nontreponemal tests (eg, rapid plasma reagin [RPR]). One needs a confirmatory test because either test is associated with false positives. Either test can be done first. Most laboratories, including those at VABHS are now performing treponemal tests first as these have become more cost-effective.6 The TPPA treponemal test was found to have a lower false negative rate in primary syphilis compared with that of nontreponemal tests.7 Nontreponemal tests can be followed for response to therapy. If a patient has a history of treated syphilis, a nontreponemal test should be sent, since the treponemal test will remain positive for life.
If there is clinical concern for neurosyphilis, cerebrospinal fluid fluorescent (CSF) treponemal antibody needs to be sampled and sent for the nontreponemal venereal disease research laboratory (VDRL) test. The VDRL is highly specific for neurosyphilis but not as sensitive. Cerebrospinal fluid fluorescent treponemal antibody (CSF FTA) may also be sent; it is very sensitive but not very specific for neurosyphilis.
► Dr. Li. An RPR returned positive at 1:512 (was negative 14 months prior on a routine screening test), with positive reflex TPPA (Table 4). A diagnosis of secondary syphilis was made. Dr. Strymish, at this point, what additional testing and treatment is necessary?
► Dr. Strymish. With papillitis and a very high RPR, we need to assume that he has ophthalmic syphilis. This can occur in any stage of syphilis, but his eye findings and high RPR are consistent with secondary syphilis. Ophthalmic syphilis has been on the upswing, even more than is expected with recent increases in syphilis cases.8 Ophthalmic syphilis is considered a form of neurosyphilis. A lumbar puncture and treatment for neurosyphilis is recommended.9,10
► Dr. Li. A lumbar puncture was performed, and his CSF was VDRL positive. This confirmed a diagnosis of neurosyphilis (Table 4). The patient was treated for neurosyphilis with IV penicillin. The patient shared that he had episodes of unprotected oral sexual activity within the past year and approximately 1 year ago, he came in close contact (but no sexual activity) with a person who had a rash consistent with syphilis.Dr. William, syphilis would be a potential unifying diagnosis of his renal and ophthalmologic manifestations. Is syphilis known to cause membranous nephropathy?
► Dr. William. Though it is uncommon, the nephrotic syndrome is a well-described complication of secondary syphilis.11,12 Syphilis has been shown to cause nephrotic syndrome in a variety of ways. Case reports abound linking syphilis to minimal change disease and other glomerular diseases.13,14 A case report from 1993 shows a membranous pattern of glomerular disease similar to this case.15 As a form of secondary membranous nephropathy, the immunofluorescence pattern can demonstrate staining similar to the “full house” seen in lupus nephritis (IgA, IgM, and C1q, in addition to IgG and C3).16 This explains the initial interpretation of this patient’s biopsy, as lupus nephritis would be a much more common etiology of secondary membranous nephropathy than is acute syphilis with this immunofluorescence pattern. However, the data in this case are highly suggestive of a causal relationship between secondary syphilis and membranous nephropathy.
► Dr. Li. Dr. Strymish, how should this patient be screened for syphilis reinfection, and at what intervals would you recommend?
► Dr. Strymish. He will need follow-up testing to make sure that his syphilis is effectively treated. If CSF pleocytosis was present initially, a CSF examination should be repeated every 6 months until the cell count is normal. He will also need follow-up for normalization of his RPR. Persons with HIV infection and primary or secondary syphilis should be evaluated clinically and serologically for treatment failure at 3, 6, 9, 12, and 24 months after therapy according to US Centers for Disease Control and Prevention guidelines.9
His treponemal test for syphilis will likely stay positive for life. His RPR should decrease significantly with effective treatment. It makes sense to screen with RPR alone as long as he continues to have risk factors for acquiring syphilis. Routine syphilis testing is recommended for pregnant women, sexually active men who have sex with men, sexually active persons with HIV, and persons taking PrEP (pre-exposure prophylaxis) for HIV prevention. He should be screened at least yearly for syphilis.
► Dr. Li. Over the next several months, the patient’s creatinine normalized and his proteinuria resolved. His vision recovered, and he has had no further ophthalmologic complications.
Dr. William, what is his long-term renal prognosis? Do you expect that his acute episode of membranous nephropathy will have permanent effects on his renal function?
► Dr. William. His rapid response to therapy for neurosyphilis provides evidence for this etiology of his renal dysfunction and glomerulonephritis. His long-term prognosis is quite good if the syphilis is the only reason for him to have renal disease. The renal damage is often reversible in these cases. However, given his prior extensive NSAID exposure and history of hypertension, he may be at higher risk for chronic kidney disease than an otherwise healthy patient, especially after an episode of acute kidney injury. Therefore, his renal function should continue to be monitored as an outpatient.
Acknowledgments
The authors thank this veteran for sharing his story and allowing us to learn from this unusual case for the benefit of our future patients.
*This article has been corrected to include a missing author.
Case Presentation. A 63-year-old male with well-controlled HIV (CD4 count 757, undetectable viral load), epilepsy, and hypertension presented to the VA Boston Healthcare System (VABHS) emergency department with 1 week of bilateral leg swelling and exertional shortness of breath. He reported having no fever, cough, chest pain, pain with inspiration and orthopnea. There was no personal or family history of pulmonary embolism. He reported weight gain but was unable to quantify how much. He also reported flare up of chronic knee pain, without swelling for which he had taken up to 4 tablets of naproxen daily for several weeks. His physical examination was notable for a heart rate of 105 beats per minute and bilateral pitting edema to his knees. Laboratory testing revealed a creatinine level of 2.5 mg/dL, which was increased from a baseline of 1.0 mg/dL (Table 1), and a urine protein-to-creatinine ratio of 7.8 mg/mg (Table 2). A renal ultrasound showed normal-sized kidneys without hydronephrosis or obstructing renal calculi. The patient was admitted for further workup of his dyspnea and acute kidney injury.
► Jonathan Li, MD, Chief Medical Resident, VABHS and Beth Israel Deaconess Medical Center (BIDMC). Dr. William, based on the degree of proteinuria and edema, a diagnosis of nephrotic syndrome was made. How is nephrotic syndrome defined, and how is it distinguished from glomerulonephritis?
► Jeffrey William, MD, Nephrologist, BIDMC, Assistant Professor of Medicine, Harvard Medical School. The pathophysiology of nephrotic disease and glomerulonephritis are quite distinct, resulting in symptoms and systemic manifestations that only slightly overlap. Glomerulonephritis is characterized by inflammation of the endothelial cells of the trilayered glomerular capillary, with a resulting active urine sediment with red blood cells, white blood cells, and casts. Nephrotic syndrome mostly affects the visceral epithelial cells of the glomerular capillary, commonly referred to as podocytes, and hence, the urine sediment in nephrotic disease is often inactive. Patients with nephrotic syndrome have nephrotic-range proteinuria (excretion of > 3.5 g per 24 h or a spot urine protein-creatinine ratio > 3.5 g in the steady state) and both hypoalbuminemia (< 3 g/dL) and peripheral edema. Lipiduria and hyperlipidemia are common findings in nephrotic syndrome but are not required for a clinical diagnosis.1 In contrast, glomerulonephritis is defined by a constellation of findings that include renal insufficiency (often indicated by an elevation in blood urea nitrogen and creatinine), hypertension, hematuria, and subnephrotic range proteinuria. In practice, patients may fulfill criteria of both nephrotic and nephritic syndromes, but the preponderance of clinical evidence often points one way or the other. In this case, nephrotic syndrome was diagnosed based on the urine protein-to-creatinine ratio of 7.8 mg/mg, hypoalbuminemia, and edema.
► Dr. Li. What would be your first-line workup for evaluation of the etiology of this patient’s nephrotic syndrome?
► Dr. William. Rather than memorizing a list of etiologies of nephrotic syndrome, it is essential to consider the pathophysiology of heavy proteinuria. Though the glomerular filtration barrier is extremely complex and defects in any component can cause proteinuria, disruption of the podocyte is often involved. Common disease processes that chiefly target the podocyte include minimal change disease, primary focal and segmental glomerulosclerosis (FSGS), and membranous nephropathy, all by differing mechanisms. Minimal change disease and idiopathic/primary FSGS are increasingly thought to be at differing points on a spectrum of the same disease.2 Secondary FSGS, on the other hand, is a progressive disease, commonly resulting from longstanding hypertension, diabetes mellitus, and obesity in adults. Membranous nephropathy can also be either primary or secondary. Primary membranous nephropathy is chiefly caused by a circulating IgG4 antibody to the podocyte membrane antigen PLA2R (M-type phospholipase A2 receptor), whereas secondary membranous nephropathy can be caused by a variety of systemic etiologies, including autoimmune disease (eg, systemic lupus erythematosus), certain malignancies, chronic infections (eg, hepatitis B and C), and many medications, including nonsteroidal anti-inflammatory drugs (NSAIDs).3-5 Paraprotein deposition diseases can also cause glomerular damage leading to nephrotic-range proteinuria.
Given these potential diagnoses, a careful history should be taken to assess exposures and recent medication use. Urine sediment evaluation is essential in the evaluation of nephrotic syndrome to determine if there is an underlying nephritic process. Select serologies may be sent to look for autoimmune disease, such as systemic lupus erythematosus and common viral exposures like hepatitis B or C. Serum and urine protein electrophoreses would be appropriate initial tests of suspected paraprotein-related diseases. Other serologies, such as antineutrophil cytoplasmic antibodies or antiglomerular basement membrane antibodies, would not necessarily be indicated here given the lack of hematuria and presence of nephrotic-range proteinuria.
► Dr. Li. The initial evaluation was notable for an erythrocyte sedimentation rate > 120 (mm/h) and a weakly positive antinuclear antibody (ANA) titer of 1:40. The remainder of his initial workup did not reveal an etiology for his nephrotic syndrome (Table 3).
Dr. William, is there a role for starting urgent empiric steroids in nephrotic syndrome while workup is ongoing? If so, do the severity of proteinuria and/or symptoms play a role or is this determination based on something else?
► Dr. William. Edema is a primary symptom of nephrotic syndrome and can often be managed with diuretics alone. If a clear medication-mediated cause is suspected, discontinuation of this agent may result in spontaneous improvement without steroid treatment. However,in cases where an etiology is unclear and there are serious thrombotic complications requiring anticoagulation, and a renal biopsy is deemed to be too risky, then empiric steroid therapy may be necessary. Children with new-onset nephrotic syndrome are presumed to have minimal change disease, given its prevalence in this patient population, and are often given empiric steroids without obtaining a renal biopsy. However, in the adult population, a renal biopsy can typically be performed quickly and safely, with pathology results interpreted within days. In this patient, since a diagnosis was unclear and there was no contraindication to renal biopsy, a biopsy should be obtained before consideration of steroids.
► Dr. Li. Steroids were deferred in anticipation of renal biopsy, which showed stage I membranous nephropathy, suggestive of membranous lupus nephritis Class V. The deposits were strongly reactive for immunoglobuline G (IgG), IgA, and complement 1q (C1q), showed co-dominant staining for IgG1, IgG2, and IgG3, and were weakly positive for the PLA2 receptor. Focal intimal arteritis in a small interlobular vessel was seen.
Dr. William, the pathology returned suggestive of lupus nephritis. Does the overall clinical picture fit with lupus nephritis?
► Dr. William. Given the history and a rather low ANA, the diagnosis of lupus nephritis seems unlikely. The lack of IgG4 and PLA2R staining in the biopsy suggests that this membranous pattern on the biopsy is likely to be secondary to a systemic etiology, but further investigation should be pursued.
► Dr. Li. The patient was discharged after the biopsy with a planned outpatient nephrology follow-up to discuss results and treatment. He was prescribed an oral diuretic, and his symptoms improved. Several days after discharge, he developed blurry vision and was evaluated in the Ophthalmology clinic. On fundoscopy, he was found to have acute papillitis, a form of optic neuritis. As part of initial evaluation of infectious etiologies of papillitis, ophthalmology recommended testing for syphilis.
Dr. Strymish, when we are considering secondary syphilis, what is the recommended approach to diagnostic testing?
► Judith Strymish, MD, Infectious Diseases, BIDMC, Assistant Professor of Medicine, Harvard Medical School. The diagnosis of syphilis is usually made through serologic testing of blood specimens. Methods that detect the spirochete directly like dark-field smears are not readily available. Serologic tests include treponemal tests (eg, Treponema pallidum particle agglutination assay [TPPA]) and nontreponemal tests (eg, rapid plasma reagin [RPR]). One needs a confirmatory test because either test is associated with false positives. Either test can be done first. Most laboratories, including those at VABHS are now performing treponemal tests first as these have become more cost-effective.6 The TPPA treponemal test was found to have a lower false negative rate in primary syphilis compared with that of nontreponemal tests.7 Nontreponemal tests can be followed for response to therapy. If a patient has a history of treated syphilis, a nontreponemal test should be sent, since the treponemal test will remain positive for life.
If there is clinical concern for neurosyphilis, cerebrospinal fluid fluorescent (CSF) treponemal antibody needs to be sampled and sent for the nontreponemal venereal disease research laboratory (VDRL) test. The VDRL is highly specific for neurosyphilis but not as sensitive. Cerebrospinal fluid fluorescent treponemal antibody (CSF FTA) may also be sent; it is very sensitive but not very specific for neurosyphilis.
► Dr. Li. An RPR returned positive at 1:512 (was negative 14 months prior on a routine screening test), with positive reflex TPPA (Table 4). A diagnosis of secondary syphilis was made. Dr. Strymish, at this point, what additional testing and treatment is necessary?
► Dr. Strymish. With papillitis and a very high RPR, we need to assume that he has ophthalmic syphilis. This can occur in any stage of syphilis, but his eye findings and high RPR are consistent with secondary syphilis. Ophthalmic syphilis has been on the upswing, even more than is expected with recent increases in syphilis cases.8 Ophthalmic syphilis is considered a form of neurosyphilis. A lumbar puncture and treatment for neurosyphilis is recommended.9,10
► Dr. Li. A lumbar puncture was performed, and his CSF was VDRL positive. This confirmed a diagnosis of neurosyphilis (Table 4). The patient was treated for neurosyphilis with IV penicillin. The patient shared that he had episodes of unprotected oral sexual activity within the past year and approximately 1 year ago, he came in close contact (but no sexual activity) with a person who had a rash consistent with syphilis.Dr. William, syphilis would be a potential unifying diagnosis of his renal and ophthalmologic manifestations. Is syphilis known to cause membranous nephropathy?
► Dr. William. Though it is uncommon, the nephrotic syndrome is a well-described complication of secondary syphilis.11,12 Syphilis has been shown to cause nephrotic syndrome in a variety of ways. Case reports abound linking syphilis to minimal change disease and other glomerular diseases.13,14 A case report from 1993 shows a membranous pattern of glomerular disease similar to this case.15 As a form of secondary membranous nephropathy, the immunofluorescence pattern can demonstrate staining similar to the “full house” seen in lupus nephritis (IgA, IgM, and C1q, in addition to IgG and C3).16 This explains the initial interpretation of this patient’s biopsy, as lupus nephritis would be a much more common etiology of secondary membranous nephropathy than is acute syphilis with this immunofluorescence pattern. However, the data in this case are highly suggestive of a causal relationship between secondary syphilis and membranous nephropathy.
► Dr. Li. Dr. Strymish, how should this patient be screened for syphilis reinfection, and at what intervals would you recommend?
► Dr. Strymish. He will need follow-up testing to make sure that his syphilis is effectively treated. If CSF pleocytosis was present initially, a CSF examination should be repeated every 6 months until the cell count is normal. He will also need follow-up for normalization of his RPR. Persons with HIV infection and primary or secondary syphilis should be evaluated clinically and serologically for treatment failure at 3, 6, 9, 12, and 24 months after therapy according to US Centers for Disease Control and Prevention guidelines.9
His treponemal test for syphilis will likely stay positive for life. His RPR should decrease significantly with effective treatment. It makes sense to screen with RPR alone as long as he continues to have risk factors for acquiring syphilis. Routine syphilis testing is recommended for pregnant women, sexually active men who have sex with men, sexually active persons with HIV, and persons taking PrEP (pre-exposure prophylaxis) for HIV prevention. He should be screened at least yearly for syphilis.
► Dr. Li. Over the next several months, the patient’s creatinine normalized and his proteinuria resolved. His vision recovered, and he has had no further ophthalmologic complications.
Dr. William, what is his long-term renal prognosis? Do you expect that his acute episode of membranous nephropathy will have permanent effects on his renal function?
► Dr. William. His rapid response to therapy for neurosyphilis provides evidence for this etiology of his renal dysfunction and glomerulonephritis. His long-term prognosis is quite good if the syphilis is the only reason for him to have renal disease. The renal damage is often reversible in these cases. However, given his prior extensive NSAID exposure and history of hypertension, he may be at higher risk for chronic kidney disease than an otherwise healthy patient, especially after an episode of acute kidney injury. Therefore, his renal function should continue to be monitored as an outpatient.
Acknowledgments
The authors thank this veteran for sharing his story and allowing us to learn from this unusual case for the benefit of our future patients.
1. Rennke H, Denker BM. Renal Pathophysiology: The Essentials. 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2014.
2. Maas RJ, Deegens JK, Smeets B, Moeller MJ, Wetzels JF. Minimal change disease and idiopathic FSGS: manifestations of the same disease. Nat Rev Nephrol. 2016;12(12):768-776.
3. Beck LH Jr, Bonegio RG, Lambeau G, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361(1):11-21.
4. Rennke HG. Secondary membranoproliferative glomerulonephritis. Kidney Int. 1995;47(2):643-656.
5. Nawaz FA, Larsen CP, Troxell ML. Membranous nephropathy and nonsteroidal anti-inflammatory agents. Am J Kidney Dis. 2013;62(5):1012-1017.
6. Pillay A. Centers for Disease Control and Prevention Syphilis Summit—Diagnostics and laboratory issues. Sex Transm Dis. 2018;45(9S)(suppl 1):S13-S16.
7. Levett PN, Fonseca K, Tsang RS, et al. Canadian Public Health Laboratory Network laboratory guidelines for the use of serological tests (excluding point-of-care tests) for the diagnosis of syphilis in Canada. Can J Infect Dis Med Microbiol. 2015;26(suppl A):6A-12A.
8. Oliver SE, Aubin M, Atwell L, et al. Ocular syphilis—eight jurisdictions, United States, 2014-2015. MMWR Morb Mortal Wkly Rep. 2016;65(43):1185-1188.
9. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recommendations and Reports 2015;64(RR3):1-137. [Erratum in MMWR Recomm Rep. 2015;64(33):924.]
10. US Centers for Disease Control and Prevention. Clinical advisory: ocular syphilis in the United States. https://www.cdc.gov/std/syphilis/clinicaladvisoryos2015.htm. Updated March 24, 2016. Accessed August 12, 2019.
11. Braunstein GD, Lewis EJ, Galvanek EG, Hamilton A, Bell WR. The nephrotic syndrome associated with secondary syphilis: an immune deposit disease. Am J Med. 1970;48:643-648.1.
12. Handoko ML, Duijvestein M, Scheepstra CG, de Fijter CW. Syphilis: a reversible cause of nephrotic syndrome. BMJ Case Rep. 2013;2013:pii:bcr2012008279
13. Krane NK, Espenan P, Walker PD, Bergman SM, Wallin JD. Renal disease and syphilis: a report of nephrotic syndrome with minimal change disease. Am J Kidney Dis. 1987;9(2):176-179.
14. Bhorade MS, Carag HB, Lee HJ, Potter EV, Dunea G. Nephropathy of secondary syphilis: a clinical and pathological spectrum. JAMA. 1971;216(7):1159-1166.
15. Hunte W, al-Ghraoui F, Cohen RJ. Secondary syphilis and the nephrotic syndrome. J Am Soc Nephrol. 1993;3(7):1351-1355.
16. Gamble CN, Reardan JB. Immunopathogenesis of syphilitic glomerulonephritis. Elution of antitreponemal antibody from glomerular immune-complex deposits. N Engl J Med. 1975;292(9):449-454.
1. Rennke H, Denker BM. Renal Pathophysiology: The Essentials. 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2014.
2. Maas RJ, Deegens JK, Smeets B, Moeller MJ, Wetzels JF. Minimal change disease and idiopathic FSGS: manifestations of the same disease. Nat Rev Nephrol. 2016;12(12):768-776.
3. Beck LH Jr, Bonegio RG, Lambeau G, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361(1):11-21.
4. Rennke HG. Secondary membranoproliferative glomerulonephritis. Kidney Int. 1995;47(2):643-656.
5. Nawaz FA, Larsen CP, Troxell ML. Membranous nephropathy and nonsteroidal anti-inflammatory agents. Am J Kidney Dis. 2013;62(5):1012-1017.
6. Pillay A. Centers for Disease Control and Prevention Syphilis Summit—Diagnostics and laboratory issues. Sex Transm Dis. 2018;45(9S)(suppl 1):S13-S16.
7. Levett PN, Fonseca K, Tsang RS, et al. Canadian Public Health Laboratory Network laboratory guidelines for the use of serological tests (excluding point-of-care tests) for the diagnosis of syphilis in Canada. Can J Infect Dis Med Microbiol. 2015;26(suppl A):6A-12A.
8. Oliver SE, Aubin M, Atwell L, et al. Ocular syphilis—eight jurisdictions, United States, 2014-2015. MMWR Morb Mortal Wkly Rep. 2016;65(43):1185-1188.
9. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recommendations and Reports 2015;64(RR3):1-137. [Erratum in MMWR Recomm Rep. 2015;64(33):924.]
10. US Centers for Disease Control and Prevention. Clinical advisory: ocular syphilis in the United States. https://www.cdc.gov/std/syphilis/clinicaladvisoryos2015.htm. Updated March 24, 2016. Accessed August 12, 2019.
11. Braunstein GD, Lewis EJ, Galvanek EG, Hamilton A, Bell WR. The nephrotic syndrome associated with secondary syphilis: an immune deposit disease. Am J Med. 1970;48:643-648.1.
12. Handoko ML, Duijvestein M, Scheepstra CG, de Fijter CW. Syphilis: a reversible cause of nephrotic syndrome. BMJ Case Rep. 2013;2013:pii:bcr2012008279
13. Krane NK, Espenan P, Walker PD, Bergman SM, Wallin JD. Renal disease and syphilis: a report of nephrotic syndrome with minimal change disease. Am J Kidney Dis. 1987;9(2):176-179.
14. Bhorade MS, Carag HB, Lee HJ, Potter EV, Dunea G. Nephropathy of secondary syphilis: a clinical and pathological spectrum. JAMA. 1971;216(7):1159-1166.
15. Hunte W, al-Ghraoui F, Cohen RJ. Secondary syphilis and the nephrotic syndrome. J Am Soc Nephrol. 1993;3(7):1351-1355.
16. Gamble CN, Reardan JB. Immunopathogenesis of syphilitic glomerulonephritis. Elution of antitreponemal antibody from glomerular immune-complex deposits. N Engl J Med. 1975;292(9):449-454.
HIV drug may enhance efficacy of chemoradiation in locally advanced lung cancer
Administering an HIV drug concurrently with chemoradiotherapy resulted in promising local control and overall survival in patients with unresectable, locally advanced non–small cell lung cancer, researchers reported.
There was no overt exacerbation of the toxic effects of chemoradiotherapy with the addition of nelfinavir, a protease inhibitor, in the prospective, open-label, phase 1/2 study, the researchers wrote.
Nelfinavir plus chemoradiotherapy yielded a median progression-free survival of 11.7 months and median survival of 41.1 months, while the cumulative local failure incidence was 39% according to their report.
Those outcomes compare favorably with historical data, the investigators wrote in JAMA Oncology.
In benchmark results of the RTOG 0617 study of chemoradiotherapy in locally advanced non–small cell lung cancer, median overall survival was 28.7 months receiving radiotherapy at a standard dose of 60 Gy, and 20.3 months for those receiving high-dose (74 Gy) radiotherapy.
However, a randomized, phase 3 trial is needed to confirm these latest results with a protease inhibitor added to chemotherapy, according to Ramesh Rengan, MD, PhD, of the University of Washington, Seattle, and coinvestigators.
“As nelfinavir is a U.S. Food and Drug Administration–approved oral drug, this treatment approach is feasible and is potentially a readily exportable platform for daily clinical use,” Dr. Rengan and coauthors wrote.
In vitro and in vivo studies have shown that nelfinavir inhibited PI3K and Akt signaling, sensitized tumor cells to ionizing radiation, and improved tumor perfusion in animal models. “We hypothesize that it is these properties that drive the clinical results observed in this study,” Dr. Rengan and coauthors wrote.
They reported on a total of 35 patients with stage IIIA/IIIB non–small cell lung cancer who received nelfinavir at either 625 mg or 1,250 mg twice daily, starting 7-14 days before starting radiotherapy to 66.6 Gy at 1.8 Gy per fraction, and throughout the full course of radiotherapy.
There were no dose-limiting toxic effects observed in the study, and toxic effects were “acceptable,” with no grade 4 nonhematologic toxic effects seen, according to investigators. Leukopenia was the primary grade 3-4 hematologic toxic effect, observed in 2 of 5 patients receiving the lower nelfinavir dose and 18 of 30 at the higher dose.
Beyond non–small cell lung cancer, the efficacy and safety nelfinavir given concurrently with radiotherapy has been looked at in other disease settings. Data from those trials suggest that this protease inhibitor could “augment tumor response” not only in non–small cell lung cancer, but in locally advanced pancreatic cancer and glioblastoma, all of which are relatively radioresistant, according to Dr. Rengan and colleagues.
Study support came from grants from the National Institutes of Health and Abramson Cancer Center, and an American Society for Radiation Oncology training award to Dr. Rengan. Study authors reported disclosures related to Pfizer, 511 Pharma, Progenics Pharmaceuticals, Siemens, Actinium, AstraZeneca, Merck, Bristol-Myers Squibb, and others.
SOURCE: Rengan R et al. JAMA Oncol. 2019 Aug 22. doi: 10.1001/jamaoncol.2019.2095.
Administering an HIV drug concurrently with chemoradiotherapy resulted in promising local control and overall survival in patients with unresectable, locally advanced non–small cell lung cancer, researchers reported.
There was no overt exacerbation of the toxic effects of chemoradiotherapy with the addition of nelfinavir, a protease inhibitor, in the prospective, open-label, phase 1/2 study, the researchers wrote.
Nelfinavir plus chemoradiotherapy yielded a median progression-free survival of 11.7 months and median survival of 41.1 months, while the cumulative local failure incidence was 39% according to their report.
Those outcomes compare favorably with historical data, the investigators wrote in JAMA Oncology.
In benchmark results of the RTOG 0617 study of chemoradiotherapy in locally advanced non–small cell lung cancer, median overall survival was 28.7 months receiving radiotherapy at a standard dose of 60 Gy, and 20.3 months for those receiving high-dose (74 Gy) radiotherapy.
However, a randomized, phase 3 trial is needed to confirm these latest results with a protease inhibitor added to chemotherapy, according to Ramesh Rengan, MD, PhD, of the University of Washington, Seattle, and coinvestigators.
“As nelfinavir is a U.S. Food and Drug Administration–approved oral drug, this treatment approach is feasible and is potentially a readily exportable platform for daily clinical use,” Dr. Rengan and coauthors wrote.
In vitro and in vivo studies have shown that nelfinavir inhibited PI3K and Akt signaling, sensitized tumor cells to ionizing radiation, and improved tumor perfusion in animal models. “We hypothesize that it is these properties that drive the clinical results observed in this study,” Dr. Rengan and coauthors wrote.
They reported on a total of 35 patients with stage IIIA/IIIB non–small cell lung cancer who received nelfinavir at either 625 mg or 1,250 mg twice daily, starting 7-14 days before starting radiotherapy to 66.6 Gy at 1.8 Gy per fraction, and throughout the full course of radiotherapy.
There were no dose-limiting toxic effects observed in the study, and toxic effects were “acceptable,” with no grade 4 nonhematologic toxic effects seen, according to investigators. Leukopenia was the primary grade 3-4 hematologic toxic effect, observed in 2 of 5 patients receiving the lower nelfinavir dose and 18 of 30 at the higher dose.
Beyond non–small cell lung cancer, the efficacy and safety nelfinavir given concurrently with radiotherapy has been looked at in other disease settings. Data from those trials suggest that this protease inhibitor could “augment tumor response” not only in non–small cell lung cancer, but in locally advanced pancreatic cancer and glioblastoma, all of which are relatively radioresistant, according to Dr. Rengan and colleagues.
Study support came from grants from the National Institutes of Health and Abramson Cancer Center, and an American Society for Radiation Oncology training award to Dr. Rengan. Study authors reported disclosures related to Pfizer, 511 Pharma, Progenics Pharmaceuticals, Siemens, Actinium, AstraZeneca, Merck, Bristol-Myers Squibb, and others.
SOURCE: Rengan R et al. JAMA Oncol. 2019 Aug 22. doi: 10.1001/jamaoncol.2019.2095.
Administering an HIV drug concurrently with chemoradiotherapy resulted in promising local control and overall survival in patients with unresectable, locally advanced non–small cell lung cancer, researchers reported.
There was no overt exacerbation of the toxic effects of chemoradiotherapy with the addition of nelfinavir, a protease inhibitor, in the prospective, open-label, phase 1/2 study, the researchers wrote.
Nelfinavir plus chemoradiotherapy yielded a median progression-free survival of 11.7 months and median survival of 41.1 months, while the cumulative local failure incidence was 39% according to their report.
Those outcomes compare favorably with historical data, the investigators wrote in JAMA Oncology.
In benchmark results of the RTOG 0617 study of chemoradiotherapy in locally advanced non–small cell lung cancer, median overall survival was 28.7 months receiving radiotherapy at a standard dose of 60 Gy, and 20.3 months for those receiving high-dose (74 Gy) radiotherapy.
However, a randomized, phase 3 trial is needed to confirm these latest results with a protease inhibitor added to chemotherapy, according to Ramesh Rengan, MD, PhD, of the University of Washington, Seattle, and coinvestigators.
“As nelfinavir is a U.S. Food and Drug Administration–approved oral drug, this treatment approach is feasible and is potentially a readily exportable platform for daily clinical use,” Dr. Rengan and coauthors wrote.
In vitro and in vivo studies have shown that nelfinavir inhibited PI3K and Akt signaling, sensitized tumor cells to ionizing radiation, and improved tumor perfusion in animal models. “We hypothesize that it is these properties that drive the clinical results observed in this study,” Dr. Rengan and coauthors wrote.
They reported on a total of 35 patients with stage IIIA/IIIB non–small cell lung cancer who received nelfinavir at either 625 mg or 1,250 mg twice daily, starting 7-14 days before starting radiotherapy to 66.6 Gy at 1.8 Gy per fraction, and throughout the full course of radiotherapy.
There were no dose-limiting toxic effects observed in the study, and toxic effects were “acceptable,” with no grade 4 nonhematologic toxic effects seen, according to investigators. Leukopenia was the primary grade 3-4 hematologic toxic effect, observed in 2 of 5 patients receiving the lower nelfinavir dose and 18 of 30 at the higher dose.
Beyond non–small cell lung cancer, the efficacy and safety nelfinavir given concurrently with radiotherapy has been looked at in other disease settings. Data from those trials suggest that this protease inhibitor could “augment tumor response” not only in non–small cell lung cancer, but in locally advanced pancreatic cancer and glioblastoma, all of which are relatively radioresistant, according to Dr. Rengan and colleagues.
Study support came from grants from the National Institutes of Health and Abramson Cancer Center, and an American Society for Radiation Oncology training award to Dr. Rengan. Study authors reported disclosures related to Pfizer, 511 Pharma, Progenics Pharmaceuticals, Siemens, Actinium, AstraZeneca, Merck, Bristol-Myers Squibb, and others.
SOURCE: Rengan R et al. JAMA Oncol. 2019 Aug 22. doi: 10.1001/jamaoncol.2019.2095.
FROM JAMA ONCOLOGY
HCV coinfection adds to cardiovascular risk in HIV-infected patients
Hepatitis C virus (HCV) coinfection, as well as an accumulation of viral and bacterial infections, was independently associated with the risk of developing a cardiovascular event in HIV-infected patients, according to the results of a large retrospective analysis.
The study comprised 823 patients at a single institution during 1982-2018. The researchers assessed those patients who had at least two visits to the HIV clinic, data concerning herpes varicella zoster virus (VZV) reactivation, and bacterial infections. Data on HCV coinfection status (as determined by HCV antibodies and qualitative HCV-PCR) were also available, according to Miguel Genebat, MD, of Virgen del Rocío University Hospital, Seville, Spain, and colleagues.
During the observational period, 58 patients (7%) experienced a cardiovascular event at a median age of 47 years. Most of these patients (50, 86%) had effective HIV treatment, with their viral load being persistently undetectable.
In terms of standard cardiovascular disease (CVD) risk factors, hypercholesterolemia was present in 31 patients (53%) and only 11 subjects (19%) had diabetes. This left 24 “low-risk” subjects, 5 of whom (21%) developed recurrent CVD and 8 of whom (33%) died after the development of cardiovascular disease.
The most frequent cardiovascular event was acute coronary syndrome (ACS), developed by 38 patients, with 14 (24%) of these individuals having recurrent CVD events. Among the 58 patients who experienced a cardiovascular event, 21 (36%) died, 17 from cardiovascular disease, 2 from cancer, and 2 each from acute bacterial infection and end-stage liver disease.
The researchers examined other variables potentially associated with the development of cardiovascular disease. They performed a multivariate analysis considering the added burden of infections and found that advanced age at HIV-1 diagnosis (OR, 1.07), a T-CD4 nadir of less than 200 cells/mcL (OR, 2.01), a diagnosis of HIV prior to combined antiretroviral therapy availability in 1996 (OR, 2.35), and cumulative infections greater than 2 (OR, 3.63), were all significantly and independently associated with the risk of developing a cardiovascular event.
They also found that HCV coinfection (OR, 2.84) on its own in simple multivariate analysis increased the risk of developing a CVD event in HIV-infected subjects. There was insufficient power to tease out the individual risk of other infections, such as herpes zoster virus and bacterial infections, hence the use of cumulative infections reported above.
The researchers concluded that potential strategies to minimize cardiovascular risk in these subjects could be treating HCV coinfection in all subjects independently of liver fibrosis stage, starting cART as soon as possible, and immunizing for those infections for which effective vaccine are available.
The authors reported that they had no conflicts of interest.
SOURCE: Genebat M. et al. Antiviral Res. 2019 Sep;169:104527.
Hepatitis C virus (HCV) coinfection, as well as an accumulation of viral and bacterial infections, was independently associated with the risk of developing a cardiovascular event in HIV-infected patients, according to the results of a large retrospective analysis.
The study comprised 823 patients at a single institution during 1982-2018. The researchers assessed those patients who had at least two visits to the HIV clinic, data concerning herpes varicella zoster virus (VZV) reactivation, and bacterial infections. Data on HCV coinfection status (as determined by HCV antibodies and qualitative HCV-PCR) were also available, according to Miguel Genebat, MD, of Virgen del Rocío University Hospital, Seville, Spain, and colleagues.
During the observational period, 58 patients (7%) experienced a cardiovascular event at a median age of 47 years. Most of these patients (50, 86%) had effective HIV treatment, with their viral load being persistently undetectable.
In terms of standard cardiovascular disease (CVD) risk factors, hypercholesterolemia was present in 31 patients (53%) and only 11 subjects (19%) had diabetes. This left 24 “low-risk” subjects, 5 of whom (21%) developed recurrent CVD and 8 of whom (33%) died after the development of cardiovascular disease.
The most frequent cardiovascular event was acute coronary syndrome (ACS), developed by 38 patients, with 14 (24%) of these individuals having recurrent CVD events. Among the 58 patients who experienced a cardiovascular event, 21 (36%) died, 17 from cardiovascular disease, 2 from cancer, and 2 each from acute bacterial infection and end-stage liver disease.
The researchers examined other variables potentially associated with the development of cardiovascular disease. They performed a multivariate analysis considering the added burden of infections and found that advanced age at HIV-1 diagnosis (OR, 1.07), a T-CD4 nadir of less than 200 cells/mcL (OR, 2.01), a diagnosis of HIV prior to combined antiretroviral therapy availability in 1996 (OR, 2.35), and cumulative infections greater than 2 (OR, 3.63), were all significantly and independently associated with the risk of developing a cardiovascular event.
They also found that HCV coinfection (OR, 2.84) on its own in simple multivariate analysis increased the risk of developing a CVD event in HIV-infected subjects. There was insufficient power to tease out the individual risk of other infections, such as herpes zoster virus and bacterial infections, hence the use of cumulative infections reported above.
The researchers concluded that potential strategies to minimize cardiovascular risk in these subjects could be treating HCV coinfection in all subjects independently of liver fibrosis stage, starting cART as soon as possible, and immunizing for those infections for which effective vaccine are available.
The authors reported that they had no conflicts of interest.
SOURCE: Genebat M. et al. Antiviral Res. 2019 Sep;169:104527.
Hepatitis C virus (HCV) coinfection, as well as an accumulation of viral and bacterial infections, was independently associated with the risk of developing a cardiovascular event in HIV-infected patients, according to the results of a large retrospective analysis.
The study comprised 823 patients at a single institution during 1982-2018. The researchers assessed those patients who had at least two visits to the HIV clinic, data concerning herpes varicella zoster virus (VZV) reactivation, and bacterial infections. Data on HCV coinfection status (as determined by HCV antibodies and qualitative HCV-PCR) were also available, according to Miguel Genebat, MD, of Virgen del Rocío University Hospital, Seville, Spain, and colleagues.
During the observational period, 58 patients (7%) experienced a cardiovascular event at a median age of 47 years. Most of these patients (50, 86%) had effective HIV treatment, with their viral load being persistently undetectable.
In terms of standard cardiovascular disease (CVD) risk factors, hypercholesterolemia was present in 31 patients (53%) and only 11 subjects (19%) had diabetes. This left 24 “low-risk” subjects, 5 of whom (21%) developed recurrent CVD and 8 of whom (33%) died after the development of cardiovascular disease.
The most frequent cardiovascular event was acute coronary syndrome (ACS), developed by 38 patients, with 14 (24%) of these individuals having recurrent CVD events. Among the 58 patients who experienced a cardiovascular event, 21 (36%) died, 17 from cardiovascular disease, 2 from cancer, and 2 each from acute bacterial infection and end-stage liver disease.
The researchers examined other variables potentially associated with the development of cardiovascular disease. They performed a multivariate analysis considering the added burden of infections and found that advanced age at HIV-1 diagnosis (OR, 1.07), a T-CD4 nadir of less than 200 cells/mcL (OR, 2.01), a diagnosis of HIV prior to combined antiretroviral therapy availability in 1996 (OR, 2.35), and cumulative infections greater than 2 (OR, 3.63), were all significantly and independently associated with the risk of developing a cardiovascular event.
They also found that HCV coinfection (OR, 2.84) on its own in simple multivariate analysis increased the risk of developing a CVD event in HIV-infected subjects. There was insufficient power to tease out the individual risk of other infections, such as herpes zoster virus and bacterial infections, hence the use of cumulative infections reported above.
The researchers concluded that potential strategies to minimize cardiovascular risk in these subjects could be treating HCV coinfection in all subjects independently of liver fibrosis stage, starting cART as soon as possible, and immunizing for those infections for which effective vaccine are available.
The authors reported that they had no conflicts of interest.
SOURCE: Genebat M. et al. Antiviral Res. 2019 Sep;169:104527.
FROM ANTIVIRAL RESEARCH
FDA panel backs Descovy as HIV PrEP for men and transgender women who have sex with men
The Food and Drug Administration’s Antimicrobial Drugs Advisory Committee backed the fixed dose combination of emtricitabine and tenofovir alafenamide (TAF; Descovy, Gilead) for pre-exposure prophylaxis (PrEP) against HIV for men and transgender women who have sex with men.
In a discussion after a 16-2 vote, committee members cited analysis by the study’s sponsor and the FDA showing efficacy and a generally good safety profile in the DISCOVER trial, the single new clinical trial conducted to support TAF’s use for pre-exposure prophylaxis (PrEP).
However, this trial included no cisgender women; the sponsor asked for approval based primarily on extrapolation from the DISCOVER results and previous results with tenofovir disoproxil fumarate (TDF) in cisgender women. Both formulations of tenofovir are prodrugs and converted to tenofovir diphosphate intracellularly in peripheral blood mononuclear cells, though many aspects of their pharmacokinetics differ.
The committee voted 10-8 against the proposition that these data supported an indication of TAF for PrEP in cisgender women, in a narrowly worded question from the FDA.
Many members who voted on either side of the question had strongly worded reservations about the lack of data for cisgender women. Said committee chair Lindsey R. Baden, MD, director of the infectious disease service at Dana-Farber Cancer Institute, Boston, who voted against the indication for cisgender women, “We’ve failed women. To be at this point and not have the data to guide decision-making is a shame on all of us.”
Ighovwerha Ofotokun, MD, who voted yes, concurred: “I agree it is a terrible failure that the agency, as well as the sponsor, would come to this committee with a lack of data on women.” But for Dr. Ofotokun, a professor of infectious diseases at Emory University, Atlanta, not including cisgender women in the approval was a distasteful proposition. “Creating a two-tier prevention and treatment hierarchy would not be helpful. We should remind ourselves that there are more women living with HIV in the world than there are men, and the risk of new HIV infection is higher among women than among men, if you look at this globally,” he said.
“I find it disrespectful and an issue of research equity. Women deserve the same quality of data about the safety and efficacy of the drugs they are exposed to that men get and that is not the situation we find ourselves in at the moment,” said Dawn K. Smith, MD, MPH, a lead scientist at the Centers for Disease Control and Prevention (CDC), Atlanta, who voted against approval for cisgender women.
Michael Green, MD, MPH, professor of pediatrics, surgery and clinical and translational science at the University of Pittsburgh, echoed the frustration of many committee members when he said, “I voted yes, almost abstained, then almost voted no.” He, along with all who voted yes, emphasized the importance of mandatory postmarketing studies in cisgender women to ensure efficacy data are obtained.
Transgender women made up only about 1% of the DISCOVER population, a fact that also gave many committee members pause.
If TAF is approved, labeling and package materials should be clear that the data support only noninferiority, not superiority, compared with TDF, said several advisory committee members who voted for approval for men and transgender women who have sex with men. “My expectation of this approval is that it should be marketed responsibly from the perspective of not creating these disparities and having Truvada be a drug for poor people and Descovy be a drug for rich people,” said Demetre Dasklalakis, MD, assistant commissioner of the Bureau of HIV/AIDS Prevention and Control at the city of New York’s Department of Health and Hygiene, and of the Icahn School of Medicine at Mount Sinai, N.Y. Truvada is slated to be offered as a generic drug in 2020, according to a Securities and Exchange Commission filing by Gilead Sciences.
The CDC reported earlier in 2019 that rates of new HIV infections have plateaued in recent years. Uptake of PrEP has been particularly low among at-risk members of minority populations, in rural areas, and in the South, according to a CDC report.
The DISCOVER trial is a 96-week ongoing trial to test TAF’s noninferiority to a fixed-drug combination of emcitrabine and tenofovir dimethyl fumarate (TDF; Truvada, Gilead) for PrEP. Both drugs are already approved to treat HIV infection, and TDF is approved for PrEP. Non-inferiority was preestablished at a rate ratio of HIV incidence of 1.62 (TAF:TDF) between the two study arms.
DISCOVER has enrolled 5,387 men and transgender women who have sex with men and are deemed at high risk for HIV, and found an incidence rate ratio of 0.47, with the upper bound of the confidence interval at 1.15. Since this figure was less than the prespecified noninferiority margin, both Gilead presenters and the FDA agreed, TAF’s noninferiority for efficacy was established.
Characteristics were similar between patients in the TAF arm (N = 2,694) and the TDF arm (N = 2,693). About 60% of patients in each arm reported having receptive anal sex with at least two partners in the previous 12 weeks, and recent rectal gonorrhea, syphilis, and chlamydia rates were 9-13% at baseline. Two thirds of participants reported recreational drug use, and about one in four reported binge drinking.
Sexual behavior and sexually transmitted infection rates continued generally unchanged from baseline during the study period.
The median age was 34 years, and most participants (84%) were white. Black participants made up 9% of the study population, and about 25% were of Hispanic or Latin ethnic origin.
Known decreases in bone mineral density occur with TDF; these were not seen with TAF, and bone mineral density increased while on TAF for the DISCOVER population aged 19-25 years.
Renal biomarkers of concern with TDF included two proteins linked with proximal tubule dysfunction, as well as estimated glomerular filtration rate. According to the sponsor’s analysis, eGFR fell by 2.3 mL/min for the TAF group, compared with a 1.8 mL/min rise while on TDF (P less than .001). Changes of similar statistical significance were seen for proximal tubular proteinuria. Also, improvements were seen in renal measures for the subset of patients enrolled who were on TDF PrEP at baseline but switched to TAF, in a prespecified subgroup analysis.
However, patients who were on TDF had a significant decrease in total cholesterol and both low- and high-density lipoprotein cholesterol compared with those on TAF, who had minimal changes or slight increases in lipids (P less than .001 for all). Triglycerides rose for those on TAF and remained unchanged for those on TDF (P = .002).
The PrEP indication sought by Gilead includes adults and adolescents, defined as those who weigh more than 35 kg. A nonvoting question put before the committee asked whether the totality of tenofovir data supported an indication of TAF for cisgender men who have insertive vaginal sex; though this extrapolation didn’t give the committee as much pause as the request for approval in cisgender women, they cited similar concerns and noted that cervicovaginal mucosa are different in many ways from rectal mucosa.
The study included no cisgender women, for a host of reasons cited by the sponsor and the FDA. These included high nonadherence rates among this population, relatively lower HIV infection rates among cisgender women in the United States, and mixed efficacy results in previous tenofovir clinical trials; the latter point made establishing a noninferiority margin problematic, according to the FDA.
For Dr. Baden, “The optics of approval for population A but not for population B are problematic.” Speaking to both the sponsor and the FDA, he said, “Everyone agrees there needs to be actual data. Please do the study as quickly as possible.” What’s needed is the collective will to make it happen, he added: “I don’t accept that it’s too big, too hard, too difficult.”
The FDA usually follows the recommendations of its advisory committees.
This article was updated 8/8/19.
The Food and Drug Administration’s Antimicrobial Drugs Advisory Committee backed the fixed dose combination of emtricitabine and tenofovir alafenamide (TAF; Descovy, Gilead) for pre-exposure prophylaxis (PrEP) against HIV for men and transgender women who have sex with men.
In a discussion after a 16-2 vote, committee members cited analysis by the study’s sponsor and the FDA showing efficacy and a generally good safety profile in the DISCOVER trial, the single new clinical trial conducted to support TAF’s use for pre-exposure prophylaxis (PrEP).
However, this trial included no cisgender women; the sponsor asked for approval based primarily on extrapolation from the DISCOVER results and previous results with tenofovir disoproxil fumarate (TDF) in cisgender women. Both formulations of tenofovir are prodrugs and converted to tenofovir diphosphate intracellularly in peripheral blood mononuclear cells, though many aspects of their pharmacokinetics differ.
The committee voted 10-8 against the proposition that these data supported an indication of TAF for PrEP in cisgender women, in a narrowly worded question from the FDA.
Many members who voted on either side of the question had strongly worded reservations about the lack of data for cisgender women. Said committee chair Lindsey R. Baden, MD, director of the infectious disease service at Dana-Farber Cancer Institute, Boston, who voted against the indication for cisgender women, “We’ve failed women. To be at this point and not have the data to guide decision-making is a shame on all of us.”
Ighovwerha Ofotokun, MD, who voted yes, concurred: “I agree it is a terrible failure that the agency, as well as the sponsor, would come to this committee with a lack of data on women.” But for Dr. Ofotokun, a professor of infectious diseases at Emory University, Atlanta, not including cisgender women in the approval was a distasteful proposition. “Creating a two-tier prevention and treatment hierarchy would not be helpful. We should remind ourselves that there are more women living with HIV in the world than there are men, and the risk of new HIV infection is higher among women than among men, if you look at this globally,” he said.
“I find it disrespectful and an issue of research equity. Women deserve the same quality of data about the safety and efficacy of the drugs they are exposed to that men get and that is not the situation we find ourselves in at the moment,” said Dawn K. Smith, MD, MPH, a lead scientist at the Centers for Disease Control and Prevention (CDC), Atlanta, who voted against approval for cisgender women.
Michael Green, MD, MPH, professor of pediatrics, surgery and clinical and translational science at the University of Pittsburgh, echoed the frustration of many committee members when he said, “I voted yes, almost abstained, then almost voted no.” He, along with all who voted yes, emphasized the importance of mandatory postmarketing studies in cisgender women to ensure efficacy data are obtained.
Transgender women made up only about 1% of the DISCOVER population, a fact that also gave many committee members pause.
If TAF is approved, labeling and package materials should be clear that the data support only noninferiority, not superiority, compared with TDF, said several advisory committee members who voted for approval for men and transgender women who have sex with men. “My expectation of this approval is that it should be marketed responsibly from the perspective of not creating these disparities and having Truvada be a drug for poor people and Descovy be a drug for rich people,” said Demetre Dasklalakis, MD, assistant commissioner of the Bureau of HIV/AIDS Prevention and Control at the city of New York’s Department of Health and Hygiene, and of the Icahn School of Medicine at Mount Sinai, N.Y. Truvada is slated to be offered as a generic drug in 2020, according to a Securities and Exchange Commission filing by Gilead Sciences.
The CDC reported earlier in 2019 that rates of new HIV infections have plateaued in recent years. Uptake of PrEP has been particularly low among at-risk members of minority populations, in rural areas, and in the South, according to a CDC report.
The DISCOVER trial is a 96-week ongoing trial to test TAF’s noninferiority to a fixed-drug combination of emcitrabine and tenofovir dimethyl fumarate (TDF; Truvada, Gilead) for PrEP. Both drugs are already approved to treat HIV infection, and TDF is approved for PrEP. Non-inferiority was preestablished at a rate ratio of HIV incidence of 1.62 (TAF:TDF) between the two study arms.
DISCOVER has enrolled 5,387 men and transgender women who have sex with men and are deemed at high risk for HIV, and found an incidence rate ratio of 0.47, with the upper bound of the confidence interval at 1.15. Since this figure was less than the prespecified noninferiority margin, both Gilead presenters and the FDA agreed, TAF’s noninferiority for efficacy was established.
Characteristics were similar between patients in the TAF arm (N = 2,694) and the TDF arm (N = 2,693). About 60% of patients in each arm reported having receptive anal sex with at least two partners in the previous 12 weeks, and recent rectal gonorrhea, syphilis, and chlamydia rates were 9-13% at baseline. Two thirds of participants reported recreational drug use, and about one in four reported binge drinking.
Sexual behavior and sexually transmitted infection rates continued generally unchanged from baseline during the study period.
The median age was 34 years, and most participants (84%) were white. Black participants made up 9% of the study population, and about 25% were of Hispanic or Latin ethnic origin.
Known decreases in bone mineral density occur with TDF; these were not seen with TAF, and bone mineral density increased while on TAF for the DISCOVER population aged 19-25 years.
Renal biomarkers of concern with TDF included two proteins linked with proximal tubule dysfunction, as well as estimated glomerular filtration rate. According to the sponsor’s analysis, eGFR fell by 2.3 mL/min for the TAF group, compared with a 1.8 mL/min rise while on TDF (P less than .001). Changes of similar statistical significance were seen for proximal tubular proteinuria. Also, improvements were seen in renal measures for the subset of patients enrolled who were on TDF PrEP at baseline but switched to TAF, in a prespecified subgroup analysis.
However, patients who were on TDF had a significant decrease in total cholesterol and both low- and high-density lipoprotein cholesterol compared with those on TAF, who had minimal changes or slight increases in lipids (P less than .001 for all). Triglycerides rose for those on TAF and remained unchanged for those on TDF (P = .002).
The PrEP indication sought by Gilead includes adults and adolescents, defined as those who weigh more than 35 kg. A nonvoting question put before the committee asked whether the totality of tenofovir data supported an indication of TAF for cisgender men who have insertive vaginal sex; though this extrapolation didn’t give the committee as much pause as the request for approval in cisgender women, they cited similar concerns and noted that cervicovaginal mucosa are different in many ways from rectal mucosa.
The study included no cisgender women, for a host of reasons cited by the sponsor and the FDA. These included high nonadherence rates among this population, relatively lower HIV infection rates among cisgender women in the United States, and mixed efficacy results in previous tenofovir clinical trials; the latter point made establishing a noninferiority margin problematic, according to the FDA.
For Dr. Baden, “The optics of approval for population A but not for population B are problematic.” Speaking to both the sponsor and the FDA, he said, “Everyone agrees there needs to be actual data. Please do the study as quickly as possible.” What’s needed is the collective will to make it happen, he added: “I don’t accept that it’s too big, too hard, too difficult.”
The FDA usually follows the recommendations of its advisory committees.
This article was updated 8/8/19.
The Food and Drug Administration’s Antimicrobial Drugs Advisory Committee backed the fixed dose combination of emtricitabine and tenofovir alafenamide (TAF; Descovy, Gilead) for pre-exposure prophylaxis (PrEP) against HIV for men and transgender women who have sex with men.
In a discussion after a 16-2 vote, committee members cited analysis by the study’s sponsor and the FDA showing efficacy and a generally good safety profile in the DISCOVER trial, the single new clinical trial conducted to support TAF’s use for pre-exposure prophylaxis (PrEP).
However, this trial included no cisgender women; the sponsor asked for approval based primarily on extrapolation from the DISCOVER results and previous results with tenofovir disoproxil fumarate (TDF) in cisgender women. Both formulations of tenofovir are prodrugs and converted to tenofovir diphosphate intracellularly in peripheral blood mononuclear cells, though many aspects of their pharmacokinetics differ.
The committee voted 10-8 against the proposition that these data supported an indication of TAF for PrEP in cisgender women, in a narrowly worded question from the FDA.
Many members who voted on either side of the question had strongly worded reservations about the lack of data for cisgender women. Said committee chair Lindsey R. Baden, MD, director of the infectious disease service at Dana-Farber Cancer Institute, Boston, who voted against the indication for cisgender women, “We’ve failed women. To be at this point and not have the data to guide decision-making is a shame on all of us.”
Ighovwerha Ofotokun, MD, who voted yes, concurred: “I agree it is a terrible failure that the agency, as well as the sponsor, would come to this committee with a lack of data on women.” But for Dr. Ofotokun, a professor of infectious diseases at Emory University, Atlanta, not including cisgender women in the approval was a distasteful proposition. “Creating a two-tier prevention and treatment hierarchy would not be helpful. We should remind ourselves that there are more women living with HIV in the world than there are men, and the risk of new HIV infection is higher among women than among men, if you look at this globally,” he said.
“I find it disrespectful and an issue of research equity. Women deserve the same quality of data about the safety and efficacy of the drugs they are exposed to that men get and that is not the situation we find ourselves in at the moment,” said Dawn K. Smith, MD, MPH, a lead scientist at the Centers for Disease Control and Prevention (CDC), Atlanta, who voted against approval for cisgender women.
Michael Green, MD, MPH, professor of pediatrics, surgery and clinical and translational science at the University of Pittsburgh, echoed the frustration of many committee members when he said, “I voted yes, almost abstained, then almost voted no.” He, along with all who voted yes, emphasized the importance of mandatory postmarketing studies in cisgender women to ensure efficacy data are obtained.
Transgender women made up only about 1% of the DISCOVER population, a fact that also gave many committee members pause.
If TAF is approved, labeling and package materials should be clear that the data support only noninferiority, not superiority, compared with TDF, said several advisory committee members who voted for approval for men and transgender women who have sex with men. “My expectation of this approval is that it should be marketed responsibly from the perspective of not creating these disparities and having Truvada be a drug for poor people and Descovy be a drug for rich people,” said Demetre Dasklalakis, MD, assistant commissioner of the Bureau of HIV/AIDS Prevention and Control at the city of New York’s Department of Health and Hygiene, and of the Icahn School of Medicine at Mount Sinai, N.Y. Truvada is slated to be offered as a generic drug in 2020, according to a Securities and Exchange Commission filing by Gilead Sciences.
The CDC reported earlier in 2019 that rates of new HIV infections have plateaued in recent years. Uptake of PrEP has been particularly low among at-risk members of minority populations, in rural areas, and in the South, according to a CDC report.
The DISCOVER trial is a 96-week ongoing trial to test TAF’s noninferiority to a fixed-drug combination of emcitrabine and tenofovir dimethyl fumarate (TDF; Truvada, Gilead) for PrEP. Both drugs are already approved to treat HIV infection, and TDF is approved for PrEP. Non-inferiority was preestablished at a rate ratio of HIV incidence of 1.62 (TAF:TDF) between the two study arms.
DISCOVER has enrolled 5,387 men and transgender women who have sex with men and are deemed at high risk for HIV, and found an incidence rate ratio of 0.47, with the upper bound of the confidence interval at 1.15. Since this figure was less than the prespecified noninferiority margin, both Gilead presenters and the FDA agreed, TAF’s noninferiority for efficacy was established.
Characteristics were similar between patients in the TAF arm (N = 2,694) and the TDF arm (N = 2,693). About 60% of patients in each arm reported having receptive anal sex with at least two partners in the previous 12 weeks, and recent rectal gonorrhea, syphilis, and chlamydia rates were 9-13% at baseline. Two thirds of participants reported recreational drug use, and about one in four reported binge drinking.
Sexual behavior and sexually transmitted infection rates continued generally unchanged from baseline during the study period.
The median age was 34 years, and most participants (84%) were white. Black participants made up 9% of the study population, and about 25% were of Hispanic or Latin ethnic origin.
Known decreases in bone mineral density occur with TDF; these were not seen with TAF, and bone mineral density increased while on TAF for the DISCOVER population aged 19-25 years.
Renal biomarkers of concern with TDF included two proteins linked with proximal tubule dysfunction, as well as estimated glomerular filtration rate. According to the sponsor’s analysis, eGFR fell by 2.3 mL/min for the TAF group, compared with a 1.8 mL/min rise while on TDF (P less than .001). Changes of similar statistical significance were seen for proximal tubular proteinuria. Also, improvements were seen in renal measures for the subset of patients enrolled who were on TDF PrEP at baseline but switched to TAF, in a prespecified subgroup analysis.
However, patients who were on TDF had a significant decrease in total cholesterol and both low- and high-density lipoprotein cholesterol compared with those on TAF, who had minimal changes or slight increases in lipids (P less than .001 for all). Triglycerides rose for those on TAF and remained unchanged for those on TDF (P = .002).
The PrEP indication sought by Gilead includes adults and adolescents, defined as those who weigh more than 35 kg. A nonvoting question put before the committee asked whether the totality of tenofovir data supported an indication of TAF for cisgender men who have insertive vaginal sex; though this extrapolation didn’t give the committee as much pause as the request for approval in cisgender women, they cited similar concerns and noted that cervicovaginal mucosa are different in many ways from rectal mucosa.
The study included no cisgender women, for a host of reasons cited by the sponsor and the FDA. These included high nonadherence rates among this population, relatively lower HIV infection rates among cisgender women in the United States, and mixed efficacy results in previous tenofovir clinical trials; the latter point made establishing a noninferiority margin problematic, according to the FDA.
For Dr. Baden, “The optics of approval for population A but not for population B are problematic.” Speaking to both the sponsor and the FDA, he said, “Everyone agrees there needs to be actual data. Please do the study as quickly as possible.” What’s needed is the collective will to make it happen, he added: “I don’t accept that it’s too big, too hard, too difficult.”
The FDA usually follows the recommendations of its advisory committees.
This article was updated 8/8/19.
FROM AN FDA ADVISORY COMMITTEE MEETING
“Hidden” HIV in Cerebrospinal Fluid Cells May Lead to Cognitive Problems
Even when a standard HIV RNA test is negative, some patients may still have viral DNA in their cerebrospinal fluid (CSF)—and that could be a predictor of later memory and concentration problems, say researchers who conducted a National Institute of Health (NIH)-funded study of patients on long-term antiretroviral therapy (ART).
The 69 participants, enrolled in the AIDS Clinical Trials Group HIV Reservoirs Cohort Study, had infections controlled with ART for a median of 9 years. Calling it a “striking observation,” the researchers found nearly half of the patients had viral DNA in CSF cells, although the standard viral load tests of the cell-free CSF fluid were positive in only 4% of the patients.
Of the 30 patients with persistent HIV, 9 (30%) experienced neurocognitive difficulties in a 7-domain neuropsychological test battery. Among 35 participants with no detectable HIV DNA in CSF, 4 (11%) were clinically impaired.
The low rates of detectable HIV RNA in the cell-free CSF fraction and within CSF cell pellets suggest low levels of HIV transcription within cells and infrequent release into the extracellular space during systemically suppressive ART, the researchers say.
The brain is one of the first targets of the virus, and CNS manifestations are common. Neurocognitive impairment in HIV-positive patients is probably related to multiple factors, including HIV infection, age, neuroinflammation, and comorbid conditions, including substance abuse, the researchers say. There also may be a “legacy effect,” in which processes associated with long-term exposure to HIV before ART leads to irreversible neurologic injury and more extensive infection of CSF cells. Lack of an association with inflammatory biomarkers in this study suggested that current inflammation does not lead to present neurocognitive dysfunction, the researchers say, but does not rule out prior inflammation as the underlying cause of neuronal injury.
Still, given that brain tissue in living individuals is “inaccessible” (as the researchers put it), CSF offers a window into neuropathogenesis of HIV. Studies have found a range between 15% and 55% of participants develop an HIV-associated neurocognitive disorder (HAND). The new findings may support a role of persistent HIV-infected cells, but the researchers emphasize that the association does not confirm that HIV DNA causes HAND. However, they add, persistent HIV in “sanctuary sites” despite ART presents a barrier to curing the infection. Their study, they say, indicates that examination of CSF cells is important in assessing residual HIV in compartments during ART.
Even when a standard HIV RNA test is negative, some patients may still have viral DNA in their cerebrospinal fluid (CSF)—and that could be a predictor of later memory and concentration problems, say researchers who conducted a National Institute of Health (NIH)-funded study of patients on long-term antiretroviral therapy (ART).
The 69 participants, enrolled in the AIDS Clinical Trials Group HIV Reservoirs Cohort Study, had infections controlled with ART for a median of 9 years. Calling it a “striking observation,” the researchers found nearly half of the patients had viral DNA in CSF cells, although the standard viral load tests of the cell-free CSF fluid were positive in only 4% of the patients.
Of the 30 patients with persistent HIV, 9 (30%) experienced neurocognitive difficulties in a 7-domain neuropsychological test battery. Among 35 participants with no detectable HIV DNA in CSF, 4 (11%) were clinically impaired.
The low rates of detectable HIV RNA in the cell-free CSF fraction and within CSF cell pellets suggest low levels of HIV transcription within cells and infrequent release into the extracellular space during systemically suppressive ART, the researchers say.
The brain is one of the first targets of the virus, and CNS manifestations are common. Neurocognitive impairment in HIV-positive patients is probably related to multiple factors, including HIV infection, age, neuroinflammation, and comorbid conditions, including substance abuse, the researchers say. There also may be a “legacy effect,” in which processes associated with long-term exposure to HIV before ART leads to irreversible neurologic injury and more extensive infection of CSF cells. Lack of an association with inflammatory biomarkers in this study suggested that current inflammation does not lead to present neurocognitive dysfunction, the researchers say, but does not rule out prior inflammation as the underlying cause of neuronal injury.
Still, given that brain tissue in living individuals is “inaccessible” (as the researchers put it), CSF offers a window into neuropathogenesis of HIV. Studies have found a range between 15% and 55% of participants develop an HIV-associated neurocognitive disorder (HAND). The new findings may support a role of persistent HIV-infected cells, but the researchers emphasize that the association does not confirm that HIV DNA causes HAND. However, they add, persistent HIV in “sanctuary sites” despite ART presents a barrier to curing the infection. Their study, they say, indicates that examination of CSF cells is important in assessing residual HIV in compartments during ART.
Even when a standard HIV RNA test is negative, some patients may still have viral DNA in their cerebrospinal fluid (CSF)—and that could be a predictor of later memory and concentration problems, say researchers who conducted a National Institute of Health (NIH)-funded study of patients on long-term antiretroviral therapy (ART).
The 69 participants, enrolled in the AIDS Clinical Trials Group HIV Reservoirs Cohort Study, had infections controlled with ART for a median of 9 years. Calling it a “striking observation,” the researchers found nearly half of the patients had viral DNA in CSF cells, although the standard viral load tests of the cell-free CSF fluid were positive in only 4% of the patients.
Of the 30 patients with persistent HIV, 9 (30%) experienced neurocognitive difficulties in a 7-domain neuropsychological test battery. Among 35 participants with no detectable HIV DNA in CSF, 4 (11%) were clinically impaired.
The low rates of detectable HIV RNA in the cell-free CSF fraction and within CSF cell pellets suggest low levels of HIV transcription within cells and infrequent release into the extracellular space during systemically suppressive ART, the researchers say.
The brain is one of the first targets of the virus, and CNS manifestations are common. Neurocognitive impairment in HIV-positive patients is probably related to multiple factors, including HIV infection, age, neuroinflammation, and comorbid conditions, including substance abuse, the researchers say. There also may be a “legacy effect,” in which processes associated with long-term exposure to HIV before ART leads to irreversible neurologic injury and more extensive infection of CSF cells. Lack of an association with inflammatory biomarkers in this study suggested that current inflammation does not lead to present neurocognitive dysfunction, the researchers say, but does not rule out prior inflammation as the underlying cause of neuronal injury.
Still, given that brain tissue in living individuals is “inaccessible” (as the researchers put it), CSF offers a window into neuropathogenesis of HIV. Studies have found a range between 15% and 55% of participants develop an HIV-associated neurocognitive disorder (HAND). The new findings may support a role of persistent HIV-infected cells, but the researchers emphasize that the association does not confirm that HIV DNA causes HAND. However, they add, persistent HIV in “sanctuary sites” despite ART presents a barrier to curing the infection. Their study, they say, indicates that examination of CSF cells is important in assessing residual HIV in compartments during ART.
Novel translocation inhibitor shows efficacy in treatment-naive HIV-1–infected adults
The first-in-class antiretroviral therapy islatravir (Merck) was well tolerated and had promising efficacy in a phase 2B study including treatment-naive adults with HIV-1 infection, supporting plans to initiate a phase 3 trial, an investigator said at the International AIDS Society Conference on HIV Science.
The proportion of patients achieving viral suppression at week 48 with combinations including the nucleoside transcriptase translocation inhibitor (NRTTI) was comparable to what was achieved with a standard triple regimen, said investigator Jean-Michel Molina, MD, professor of infectious diseases at the University of Paris Diderot, and head of the infectious diseases department at the Saint-Louis Hospital in Paris
The treatment was effective not only as part of a three-drug combination of islatravir, doravirine, and lamivudine over 24 weeks, but also over an additional 24 weeks in patients who achieved viral suppression and were switched to dual therapy with islatravir and doravirine, according to Dr. Molina.
“These are promising data that will encourage the company to move to a phase 3 trial to see how these results can be confirmed in a larger study set, and also to assess the potency of the dual combination for maintenance therapy in the future, providing also novel options for people with a drug that has a high genetic barrier to resistance and efficacy that seems to be quite interesting,” Dr. Molina said in an IAS press conference in Mexico City.
This drug has very potent activity not only against wild-type HIV-1 viruses, but also multiresistant viruses, according to Dr. Molina.
“It has a high inhibitory quotient at a very low dose, so you give people a tiny amount of drug – in the range of 1 milligram per day, instead of a few hundred milligrams with other, regular drugs,” he said.
Another attribute of islatravir is its long half-life of approximately 120 hours, allowing not only for once-daily dosing, but potentially for evaluation as once-weekly or once-monthly dosing in the future, he said, adding that a subdermal islatravir-eluting implant under investigation for preexposure prophylaxis has potential as a once-yearly option.
The international, multicenter, 121-patient clinical trial that Dr. Molina described included adults with HIV-1 infection naive to antiretroviral therapy randomized to islatravir (in one of three doses) plus doravirine and lamivudine, or to the combination of doravirine, lamivudine, and tenofovir (Delstrigo, Merck).
After at least 24 weeks of treatment, subjects in the islatravir treatment groups were transitioned to the two-drug combination of islatravir and doravirine if they had HIV-1 RNA levels less than 50 copies/mL and did not meet any protocol-defined criteria for virologic failure.
Those participants in the islatravir arms who received 48 weeks of treatment had “very good response” and safety that was comparable to the control arm, according to Dr. Molina.
At 48 weeks, the proportion of patients with HIV-1 RNA less than 50 copies/mL were 89.7%, 90%, and 77.4% for regimens containing islatravir 0.25 mg, 0.75 mg, and 2.25 mg, respectively, and 83.9% for those receiving the standard triple therapy, according to reported data.
All patients with protocol-defined virologic failure (greater than or equal to 50 copies/mL) in the study actually had very low viral load, below 80 copies/mL, Dr. Molina said.
The study was supported by Merck. Dr. Molina has been on the Merck advisory board and speaker’s bureau.
SOURCE: Molina J-M et al. IAS 2019, Abstract WEAB0402LB.
The first-in-class antiretroviral therapy islatravir (Merck) was well tolerated and had promising efficacy in a phase 2B study including treatment-naive adults with HIV-1 infection, supporting plans to initiate a phase 3 trial, an investigator said at the International AIDS Society Conference on HIV Science.
The proportion of patients achieving viral suppression at week 48 with combinations including the nucleoside transcriptase translocation inhibitor (NRTTI) was comparable to what was achieved with a standard triple regimen, said investigator Jean-Michel Molina, MD, professor of infectious diseases at the University of Paris Diderot, and head of the infectious diseases department at the Saint-Louis Hospital in Paris
The treatment was effective not only as part of a three-drug combination of islatravir, doravirine, and lamivudine over 24 weeks, but also over an additional 24 weeks in patients who achieved viral suppression and were switched to dual therapy with islatravir and doravirine, according to Dr. Molina.
“These are promising data that will encourage the company to move to a phase 3 trial to see how these results can be confirmed in a larger study set, and also to assess the potency of the dual combination for maintenance therapy in the future, providing also novel options for people with a drug that has a high genetic barrier to resistance and efficacy that seems to be quite interesting,” Dr. Molina said in an IAS press conference in Mexico City.
This drug has very potent activity not only against wild-type HIV-1 viruses, but also multiresistant viruses, according to Dr. Molina.
“It has a high inhibitory quotient at a very low dose, so you give people a tiny amount of drug – in the range of 1 milligram per day, instead of a few hundred milligrams with other, regular drugs,” he said.
Another attribute of islatravir is its long half-life of approximately 120 hours, allowing not only for once-daily dosing, but potentially for evaluation as once-weekly or once-monthly dosing in the future, he said, adding that a subdermal islatravir-eluting implant under investigation for preexposure prophylaxis has potential as a once-yearly option.
The international, multicenter, 121-patient clinical trial that Dr. Molina described included adults with HIV-1 infection naive to antiretroviral therapy randomized to islatravir (in one of three doses) plus doravirine and lamivudine, or to the combination of doravirine, lamivudine, and tenofovir (Delstrigo, Merck).
After at least 24 weeks of treatment, subjects in the islatravir treatment groups were transitioned to the two-drug combination of islatravir and doravirine if they had HIV-1 RNA levels less than 50 copies/mL and did not meet any protocol-defined criteria for virologic failure.
Those participants in the islatravir arms who received 48 weeks of treatment had “very good response” and safety that was comparable to the control arm, according to Dr. Molina.
At 48 weeks, the proportion of patients with HIV-1 RNA less than 50 copies/mL were 89.7%, 90%, and 77.4% for regimens containing islatravir 0.25 mg, 0.75 mg, and 2.25 mg, respectively, and 83.9% for those receiving the standard triple therapy, according to reported data.
All patients with protocol-defined virologic failure (greater than or equal to 50 copies/mL) in the study actually had very low viral load, below 80 copies/mL, Dr. Molina said.
The study was supported by Merck. Dr. Molina has been on the Merck advisory board and speaker’s bureau.
SOURCE: Molina J-M et al. IAS 2019, Abstract WEAB0402LB.
The first-in-class antiretroviral therapy islatravir (Merck) was well tolerated and had promising efficacy in a phase 2B study including treatment-naive adults with HIV-1 infection, supporting plans to initiate a phase 3 trial, an investigator said at the International AIDS Society Conference on HIV Science.
The proportion of patients achieving viral suppression at week 48 with combinations including the nucleoside transcriptase translocation inhibitor (NRTTI) was comparable to what was achieved with a standard triple regimen, said investigator Jean-Michel Molina, MD, professor of infectious diseases at the University of Paris Diderot, and head of the infectious diseases department at the Saint-Louis Hospital in Paris
The treatment was effective not only as part of a three-drug combination of islatravir, doravirine, and lamivudine over 24 weeks, but also over an additional 24 weeks in patients who achieved viral suppression and were switched to dual therapy with islatravir and doravirine, according to Dr. Molina.
“These are promising data that will encourage the company to move to a phase 3 trial to see how these results can be confirmed in a larger study set, and also to assess the potency of the dual combination for maintenance therapy in the future, providing also novel options for people with a drug that has a high genetic barrier to resistance and efficacy that seems to be quite interesting,” Dr. Molina said in an IAS press conference in Mexico City.
This drug has very potent activity not only against wild-type HIV-1 viruses, but also multiresistant viruses, according to Dr. Molina.
“It has a high inhibitory quotient at a very low dose, so you give people a tiny amount of drug – in the range of 1 milligram per day, instead of a few hundred milligrams with other, regular drugs,” he said.
Another attribute of islatravir is its long half-life of approximately 120 hours, allowing not only for once-daily dosing, but potentially for evaluation as once-weekly or once-monthly dosing in the future, he said, adding that a subdermal islatravir-eluting implant under investigation for preexposure prophylaxis has potential as a once-yearly option.
The international, multicenter, 121-patient clinical trial that Dr. Molina described included adults with HIV-1 infection naive to antiretroviral therapy randomized to islatravir (in one of three doses) plus doravirine and lamivudine, or to the combination of doravirine, lamivudine, and tenofovir (Delstrigo, Merck).
After at least 24 weeks of treatment, subjects in the islatravir treatment groups were transitioned to the two-drug combination of islatravir and doravirine if they had HIV-1 RNA levels less than 50 copies/mL and did not meet any protocol-defined criteria for virologic failure.
Those participants in the islatravir arms who received 48 weeks of treatment had “very good response” and safety that was comparable to the control arm, according to Dr. Molina.
At 48 weeks, the proportion of patients with HIV-1 RNA less than 50 copies/mL were 89.7%, 90%, and 77.4% for regimens containing islatravir 0.25 mg, 0.75 mg, and 2.25 mg, respectively, and 83.9% for those receiving the standard triple therapy, according to reported data.
All patients with protocol-defined virologic failure (greater than or equal to 50 copies/mL) in the study actually had very low viral load, below 80 copies/mL, Dr. Molina said.
The study was supported by Merck. Dr. Molina has been on the Merck advisory board and speaker’s bureau.
SOURCE: Molina J-M et al. IAS 2019, Abstract WEAB0402LB.
FROM IAS 2019
Antiretroviral-eluting implant could provide HIV prophylaxis for a year or more
An implant that elutes an investigational antiretroviral agent provided drug release that should be sufficient for HIV prophylaxis for 12 months or more, according to results of a phase 1 clinical trial just presented here at the International AIDS Society Conference on HIV Science.
The islatravir-eluting arm implant was safe and generally well tolerated, with drug concentrations that remained above the target level needed for protection throughout the randomized, placebo-controlled study, said investigator Randolph P. Matthews, MD, PhD, senior principal scientist at Merck, Kenilworth, N.J.
“Based on this study, the islatravir-eluting implant appears to be a potentially important option for preexposure prophylaxis (PrEP) as an agent that could be effective with yearly dosing,” Dr. Matthews said in an IAS press conference.
This drug-eluting implant, inserted subdermally in the skin of the upper arm, could represent a “meaningful option” for many individuals at high risk of HIV infection, particularly those who have adherence challenges, said Dr. Matthews.
“Many at-risk individuals face adherence challenges with the existing daily oral PrEP therapy,” he added. “A high degree of adherence is required for it to be effective, and daily adherence is challenging for many, particularly for women.”
Islatravir, formerly known as MK-8591, is a nucleoside reverse transcriptase translocation inhibitor (NRTTI) being evaluated in clinical trials not only for PrEP, but also for treatment of HIV-1 infection in combination with other antiretrovirals.
In preclinical trials, islatravir demonstrated high potency, a high barrier to resistance, and a long half-life, according to Dr. Matthews.
The phase 1, single-site, double-blind study included a total of 16 healthy adult volunteers who received implants of islatravir at one of two doses (54 mg and 62 mg) or placebo for 12 weeks.
Both active doses of islatravir led to concentrations above the target level at 12 weeks, and based on data modeling, the higher-dose implant would still be above the target level for at least a year, Dr. Matthews said in the press conference.
The projected duration above the target ranged from 12 to 16 months for the 62-mg dose of islatravir, and from 8 to 10 months for the 54-mg dose, according to the reported data.
All drug-related adverse events were mild or moderate in severity, and none of the volunteers discontinued the study because of an adverse event, Dr. Matthews said.
Taken together, these data support the continued progression of the implant clinical development program, said Dr. Matthews, who is an employee of Merck, which sponsored the study.
SOURCE: Matthews RP et al. IAS 2019, Abstract TUAC0401LB.
An implant that elutes an investigational antiretroviral agent provided drug release that should be sufficient for HIV prophylaxis for 12 months or more, according to results of a phase 1 clinical trial just presented here at the International AIDS Society Conference on HIV Science.
The islatravir-eluting arm implant was safe and generally well tolerated, with drug concentrations that remained above the target level needed for protection throughout the randomized, placebo-controlled study, said investigator Randolph P. Matthews, MD, PhD, senior principal scientist at Merck, Kenilworth, N.J.
“Based on this study, the islatravir-eluting implant appears to be a potentially important option for preexposure prophylaxis (PrEP) as an agent that could be effective with yearly dosing,” Dr. Matthews said in an IAS press conference.
This drug-eluting implant, inserted subdermally in the skin of the upper arm, could represent a “meaningful option” for many individuals at high risk of HIV infection, particularly those who have adherence challenges, said Dr. Matthews.
“Many at-risk individuals face adherence challenges with the existing daily oral PrEP therapy,” he added. “A high degree of adherence is required for it to be effective, and daily adherence is challenging for many, particularly for women.”
Islatravir, formerly known as MK-8591, is a nucleoside reverse transcriptase translocation inhibitor (NRTTI) being evaluated in clinical trials not only for PrEP, but also for treatment of HIV-1 infection in combination with other antiretrovirals.
In preclinical trials, islatravir demonstrated high potency, a high barrier to resistance, and a long half-life, according to Dr. Matthews.
The phase 1, single-site, double-blind study included a total of 16 healthy adult volunteers who received implants of islatravir at one of two doses (54 mg and 62 mg) or placebo for 12 weeks.
Both active doses of islatravir led to concentrations above the target level at 12 weeks, and based on data modeling, the higher-dose implant would still be above the target level for at least a year, Dr. Matthews said in the press conference.
The projected duration above the target ranged from 12 to 16 months for the 62-mg dose of islatravir, and from 8 to 10 months for the 54-mg dose, according to the reported data.
All drug-related adverse events were mild or moderate in severity, and none of the volunteers discontinued the study because of an adverse event, Dr. Matthews said.
Taken together, these data support the continued progression of the implant clinical development program, said Dr. Matthews, who is an employee of Merck, which sponsored the study.
SOURCE: Matthews RP et al. IAS 2019, Abstract TUAC0401LB.
An implant that elutes an investigational antiretroviral agent provided drug release that should be sufficient for HIV prophylaxis for 12 months or more, according to results of a phase 1 clinical trial just presented here at the International AIDS Society Conference on HIV Science.
The islatravir-eluting arm implant was safe and generally well tolerated, with drug concentrations that remained above the target level needed for protection throughout the randomized, placebo-controlled study, said investigator Randolph P. Matthews, MD, PhD, senior principal scientist at Merck, Kenilworth, N.J.
“Based on this study, the islatravir-eluting implant appears to be a potentially important option for preexposure prophylaxis (PrEP) as an agent that could be effective with yearly dosing,” Dr. Matthews said in an IAS press conference.
This drug-eluting implant, inserted subdermally in the skin of the upper arm, could represent a “meaningful option” for many individuals at high risk of HIV infection, particularly those who have adherence challenges, said Dr. Matthews.
“Many at-risk individuals face adherence challenges with the existing daily oral PrEP therapy,” he added. “A high degree of adherence is required for it to be effective, and daily adherence is challenging for many, particularly for women.”
Islatravir, formerly known as MK-8591, is a nucleoside reverse transcriptase translocation inhibitor (NRTTI) being evaluated in clinical trials not only for PrEP, but also for treatment of HIV-1 infection in combination with other antiretrovirals.
In preclinical trials, islatravir demonstrated high potency, a high barrier to resistance, and a long half-life, according to Dr. Matthews.
The phase 1, single-site, double-blind study included a total of 16 healthy adult volunteers who received implants of islatravir at one of two doses (54 mg and 62 mg) or placebo for 12 weeks.
Both active doses of islatravir led to concentrations above the target level at 12 weeks, and based on data modeling, the higher-dose implant would still be above the target level for at least a year, Dr. Matthews said in the press conference.
The projected duration above the target ranged from 12 to 16 months for the 62-mg dose of islatravir, and from 8 to 10 months for the 54-mg dose, according to the reported data.
All drug-related adverse events were mild or moderate in severity, and none of the volunteers discontinued the study because of an adverse event, Dr. Matthews said.
Taken together, these data support the continued progression of the implant clinical development program, said Dr. Matthews, who is an employee of Merck, which sponsored the study.
SOURCE: Matthews RP et al. IAS 2019, Abstract TUAC0401LB.
FROM IAS 2019
Two-drug integrase inhibitor–based regimen noninferior to standard in HIV-infected adults
New studies of a two-drug, integrase inhibitor–based regimen, presented at the International AIDS Society Conference on HIV Science, provide additional data to challenge the three-drug HIV treatment paradigm.
In the results of one study, known as TANGO (NCT03446573), switching to the fixed-dose combination of dolutegravir (DTG) plus lamivudine (3TC) from a tenofovir alafenamide (TAF)–based three- or four-drug regimen maintained virologic suppression at 48 weeks in HIV-1 infected adults, an investigator said, and was noninferior to continuing TAF-based therapy.
In another presentation, updating results of the GEMINI-1 (NCT02831673) and GEMINI-2 studies (NCT02831764), a researcher said the DTG+3TC combination remained noninferior to DTG plus tenofovir/emtricitabine (TDF/FTC) at week 96 in antiretroviral (ART) treatment-naive adults with HIV-1 infection, with no treatment-emergent resistance and no increase in risk of virologic failure.
,” according to IAS Past President Pedro Cahn, MD, PhD, of Fundación Huésped, Buenos Aires, who presented the updated GEMINI study results.
“We don’t mean to say now everything should be dual therapy, and we are going to wipe out all other options, but I think we have another strong option for initiating therapy, and probably also for switch therapy,” Dr. Cahn said in a press conference.
Dr. Cahn presented an updated analysis of GEMINI-1 and GEMINI-2, two identically designed phase 3 randomized clinical trials including a total of 1,400 patients.
The 48-week results from those trials, presented in 2018, showed for the first time that a dual-therapy combination of an integrase inhibitor with 3TC was noninferior to the triple-drug regimen of DTG+TDF/FTC, Dr. Cahn said.
In the updated analysis, including 96 weeks of data, the two-drug regimen remained noninferior to the three-drug regimen, according to the investigator.
A total of 11 patients on DTG+3TC and 7 on DTG+TDF/FTC met virologic withdrawal criteria through week 96, with no treatment-emergent resistance mutations seen in either arm, according to results reported in the study abstract.
The proportion of subjects with plasma HIV-1 RNA below 50 c/mL at week 96 was 86% for the two-drug regimen and 90% for the three-drug regimen (adjusted difference, –3.4; 95% confidence interval, –6.7 to 0.0), the reported data showed.
Drug-related adverse events were numerically more common in the three-drug arm, though rates of adverse event–related withdrawal were low in both arms, according to investigators.
The strategy of switching to DTG+3TC was evaluated in the randomized, phase 3 TANGO trial, which included 741 subjects who were already virally suppressed on a TAF-based three- or four-drug regimen. After 48 weeks of therapy, the two-drug regimen was noninferior to remaining on TAF-based therapy in terms of achieving and maintaining viral suppression, according to investigator Jean van Wyk, MB, ChB, Global Medical Lead for dolutegravir at ViiV Healthcare.
Safety outcomes were similar between the arms, though the absolute number of treatment-related adverse events was higher in the DTG+3TC arm “as expected in a switch study,” Dr. van Wyk said.
The percentage of subjects withdrawing from the study because of adverse events was 4% in the DTG+3TC arm and less than 1% in the TAF-based treatment arm, according to reported data.
And with regard to safety there were similar outcomes between the two arms, with regard to overall adverse events, “but as expected in a switch study, we did see more treatment-related adverse events in the dolutegravir plus lamivudine arm, and that’s because the majority of patients were on two new agents in the form of dolutegravir plus lamivudine, so it’s completely expected,” Dr. van Wyk said.
The study met its primary endpoint for noninferiority at week 48, based on the Food and Drug Administration snapshot algorithm. Results showed that switching to DTG+3TC was noninferior to continuing the TAF-containing regimen at week 48, with virologic failure per snapshot criteria seen in less than 1% of subjects in either arm, according to reported data. The proportion of subjects with plasma HIV-1 RNA less than 50 c/mL was 93.2% and 93.0% for the DTG+3TC and TAF-based treatment arms, respectively.
Longer-term follow-up will be important to confirm the noninferiority of the two-drug approach, Dr. van Wyk and Dr. Cahn said in the press conference. The TANGO study of the switch strategy will continue through 148 weeks, while an additional year of follow-up is ongoing for the GEMINI studies of the initial therapy approach.
TANGO and both GEMINI 1 and GEMINI 2 were sponsored by ViiV Healthcare. Dr. van Wyk is an employee of ViiV Healthcare. Dr. Cahn has received research support grants, and fees as consultant and speaker from ViiV Healthcare.
SOURCE: Cahn P et al. IAS 2019, Abstract WEAB0404LB; van Wyk J et al. IAS 2019, Abstract WEAB0403LB.
New studies of a two-drug, integrase inhibitor–based regimen, presented at the International AIDS Society Conference on HIV Science, provide additional data to challenge the three-drug HIV treatment paradigm.
In the results of one study, known as TANGO (NCT03446573), switching to the fixed-dose combination of dolutegravir (DTG) plus lamivudine (3TC) from a tenofovir alafenamide (TAF)–based three- or four-drug regimen maintained virologic suppression at 48 weeks in HIV-1 infected adults, an investigator said, and was noninferior to continuing TAF-based therapy.
In another presentation, updating results of the GEMINI-1 (NCT02831673) and GEMINI-2 studies (NCT02831764), a researcher said the DTG+3TC combination remained noninferior to DTG plus tenofovir/emtricitabine (TDF/FTC) at week 96 in antiretroviral (ART) treatment-naive adults with HIV-1 infection, with no treatment-emergent resistance and no increase in risk of virologic failure.
,” according to IAS Past President Pedro Cahn, MD, PhD, of Fundación Huésped, Buenos Aires, who presented the updated GEMINI study results.
“We don’t mean to say now everything should be dual therapy, and we are going to wipe out all other options, but I think we have another strong option for initiating therapy, and probably also for switch therapy,” Dr. Cahn said in a press conference.
Dr. Cahn presented an updated analysis of GEMINI-1 and GEMINI-2, two identically designed phase 3 randomized clinical trials including a total of 1,400 patients.
The 48-week results from those trials, presented in 2018, showed for the first time that a dual-therapy combination of an integrase inhibitor with 3TC was noninferior to the triple-drug regimen of DTG+TDF/FTC, Dr. Cahn said.
In the updated analysis, including 96 weeks of data, the two-drug regimen remained noninferior to the three-drug regimen, according to the investigator.
A total of 11 patients on DTG+3TC and 7 on DTG+TDF/FTC met virologic withdrawal criteria through week 96, with no treatment-emergent resistance mutations seen in either arm, according to results reported in the study abstract.
The proportion of subjects with plasma HIV-1 RNA below 50 c/mL at week 96 was 86% for the two-drug regimen and 90% for the three-drug regimen (adjusted difference, –3.4; 95% confidence interval, –6.7 to 0.0), the reported data showed.
Drug-related adverse events were numerically more common in the three-drug arm, though rates of adverse event–related withdrawal were low in both arms, according to investigators.
The strategy of switching to DTG+3TC was evaluated in the randomized, phase 3 TANGO trial, which included 741 subjects who were already virally suppressed on a TAF-based three- or four-drug regimen. After 48 weeks of therapy, the two-drug regimen was noninferior to remaining on TAF-based therapy in terms of achieving and maintaining viral suppression, according to investigator Jean van Wyk, MB, ChB, Global Medical Lead for dolutegravir at ViiV Healthcare.
Safety outcomes were similar between the arms, though the absolute number of treatment-related adverse events was higher in the DTG+3TC arm “as expected in a switch study,” Dr. van Wyk said.
The percentage of subjects withdrawing from the study because of adverse events was 4% in the DTG+3TC arm and less than 1% in the TAF-based treatment arm, according to reported data.
And with regard to safety there were similar outcomes between the two arms, with regard to overall adverse events, “but as expected in a switch study, we did see more treatment-related adverse events in the dolutegravir plus lamivudine arm, and that’s because the majority of patients were on two new agents in the form of dolutegravir plus lamivudine, so it’s completely expected,” Dr. van Wyk said.
The study met its primary endpoint for noninferiority at week 48, based on the Food and Drug Administration snapshot algorithm. Results showed that switching to DTG+3TC was noninferior to continuing the TAF-containing regimen at week 48, with virologic failure per snapshot criteria seen in less than 1% of subjects in either arm, according to reported data. The proportion of subjects with plasma HIV-1 RNA less than 50 c/mL was 93.2% and 93.0% for the DTG+3TC and TAF-based treatment arms, respectively.
Longer-term follow-up will be important to confirm the noninferiority of the two-drug approach, Dr. van Wyk and Dr. Cahn said in the press conference. The TANGO study of the switch strategy will continue through 148 weeks, while an additional year of follow-up is ongoing for the GEMINI studies of the initial therapy approach.
TANGO and both GEMINI 1 and GEMINI 2 were sponsored by ViiV Healthcare. Dr. van Wyk is an employee of ViiV Healthcare. Dr. Cahn has received research support grants, and fees as consultant and speaker from ViiV Healthcare.
SOURCE: Cahn P et al. IAS 2019, Abstract WEAB0404LB; van Wyk J et al. IAS 2019, Abstract WEAB0403LB.
New studies of a two-drug, integrase inhibitor–based regimen, presented at the International AIDS Society Conference on HIV Science, provide additional data to challenge the three-drug HIV treatment paradigm.
In the results of one study, known as TANGO (NCT03446573), switching to the fixed-dose combination of dolutegravir (DTG) plus lamivudine (3TC) from a tenofovir alafenamide (TAF)–based three- or four-drug regimen maintained virologic suppression at 48 weeks in HIV-1 infected adults, an investigator said, and was noninferior to continuing TAF-based therapy.
In another presentation, updating results of the GEMINI-1 (NCT02831673) and GEMINI-2 studies (NCT02831764), a researcher said the DTG+3TC combination remained noninferior to DTG plus tenofovir/emtricitabine (TDF/FTC) at week 96 in antiretroviral (ART) treatment-naive adults with HIV-1 infection, with no treatment-emergent resistance and no increase in risk of virologic failure.
,” according to IAS Past President Pedro Cahn, MD, PhD, of Fundación Huésped, Buenos Aires, who presented the updated GEMINI study results.
“We don’t mean to say now everything should be dual therapy, and we are going to wipe out all other options, but I think we have another strong option for initiating therapy, and probably also for switch therapy,” Dr. Cahn said in a press conference.
Dr. Cahn presented an updated analysis of GEMINI-1 and GEMINI-2, two identically designed phase 3 randomized clinical trials including a total of 1,400 patients.
The 48-week results from those trials, presented in 2018, showed for the first time that a dual-therapy combination of an integrase inhibitor with 3TC was noninferior to the triple-drug regimen of DTG+TDF/FTC, Dr. Cahn said.
In the updated analysis, including 96 weeks of data, the two-drug regimen remained noninferior to the three-drug regimen, according to the investigator.
A total of 11 patients on DTG+3TC and 7 on DTG+TDF/FTC met virologic withdrawal criteria through week 96, with no treatment-emergent resistance mutations seen in either arm, according to results reported in the study abstract.
The proportion of subjects with plasma HIV-1 RNA below 50 c/mL at week 96 was 86% for the two-drug regimen and 90% for the three-drug regimen (adjusted difference, –3.4; 95% confidence interval, –6.7 to 0.0), the reported data showed.
Drug-related adverse events were numerically more common in the three-drug arm, though rates of adverse event–related withdrawal were low in both arms, according to investigators.
The strategy of switching to DTG+3TC was evaluated in the randomized, phase 3 TANGO trial, which included 741 subjects who were already virally suppressed on a TAF-based three- or four-drug regimen. After 48 weeks of therapy, the two-drug regimen was noninferior to remaining on TAF-based therapy in terms of achieving and maintaining viral suppression, according to investigator Jean van Wyk, MB, ChB, Global Medical Lead for dolutegravir at ViiV Healthcare.
Safety outcomes were similar between the arms, though the absolute number of treatment-related adverse events was higher in the DTG+3TC arm “as expected in a switch study,” Dr. van Wyk said.
The percentage of subjects withdrawing from the study because of adverse events was 4% in the DTG+3TC arm and less than 1% in the TAF-based treatment arm, according to reported data.
And with regard to safety there were similar outcomes between the two arms, with regard to overall adverse events, “but as expected in a switch study, we did see more treatment-related adverse events in the dolutegravir plus lamivudine arm, and that’s because the majority of patients were on two new agents in the form of dolutegravir plus lamivudine, so it’s completely expected,” Dr. van Wyk said.
The study met its primary endpoint for noninferiority at week 48, based on the Food and Drug Administration snapshot algorithm. Results showed that switching to DTG+3TC was noninferior to continuing the TAF-containing regimen at week 48, with virologic failure per snapshot criteria seen in less than 1% of subjects in either arm, according to reported data. The proportion of subjects with plasma HIV-1 RNA less than 50 c/mL was 93.2% and 93.0% for the DTG+3TC and TAF-based treatment arms, respectively.
Longer-term follow-up will be important to confirm the noninferiority of the two-drug approach, Dr. van Wyk and Dr. Cahn said in the press conference. The TANGO study of the switch strategy will continue through 148 weeks, while an additional year of follow-up is ongoing for the GEMINI studies of the initial therapy approach.
TANGO and both GEMINI 1 and GEMINI 2 were sponsored by ViiV Healthcare. Dr. van Wyk is an employee of ViiV Healthcare. Dr. Cahn has received research support grants, and fees as consultant and speaker from ViiV Healthcare.
SOURCE: Cahn P et al. IAS 2019, Abstract WEAB0404LB; van Wyk J et al. IAS 2019, Abstract WEAB0403LB.
REPORTING FROM IAS 2019
An Algorithm to Identify PrEP-Potential Patients
Health care providers who do not have the time or tools to screen patients for HIV risk also may not be prescribing preexposure prophylaxis (PrEP). But help is on the way: NIH-funded researchers have come up with novel computerized methods to identify the patients PrEP could benefit.
In 2 separate studies, the researchers developed and validated algorithms that analyze electronic health records (EHR). In the first study, Harvard researchers used machine learning to create an HIV prediction algorithm using 2007 to 2015 data from > 1 million patients in Massachusetts. The model included variables such as diagnosis codes for HIV counseling or sexually transmitted infections (STIs), laboratory tests for HIV or STIs, and prescriptions for medications related to treating STIs.
The model was validated using data from nearly 600,000 other patients treated between 2011 and 2016. The prediction algorithm successfully distinguished with high precision between patients who did or did not acquire HIV and between those who did or did not receive a PrEP prescription.
The researchers found hundreds of potential missed opportunities. They point to > 9,500 people in the 2016 dataset with particularly high-risk scores who were not prescribed PrEP. A “striking outcome,” the researchers say, is that their analysis suggests that nearly 40% of new HIV cases might have been averted had clinicians received alerts to discuss and offer PrEP to patients with the highest 2% of risk scores.
In the second study, researchers used the EHRs of > 3.7 million patients who entered the Kaiser Permanente System Northern California between 2007 and 2014 to develop a model to predict HIV incidence, then validated the model with data from between 2015 and 2017. Of the original patient group, 784 developed HIV within 3 years of baseline. The study found that the model identified nearly half of the incident HIV cases among males by flagging only 2% of the general patient population.
Embedding the algorithm into the EHR, the lead investigator says, “could prompt providers to discuss PrEP with patients who are most likely to benefit.”
Health care providers who do not have the time or tools to screen patients for HIV risk also may not be prescribing preexposure prophylaxis (PrEP). But help is on the way: NIH-funded researchers have come up with novel computerized methods to identify the patients PrEP could benefit.
In 2 separate studies, the researchers developed and validated algorithms that analyze electronic health records (EHR). In the first study, Harvard researchers used machine learning to create an HIV prediction algorithm using 2007 to 2015 data from > 1 million patients in Massachusetts. The model included variables such as diagnosis codes for HIV counseling or sexually transmitted infections (STIs), laboratory tests for HIV or STIs, and prescriptions for medications related to treating STIs.
The model was validated using data from nearly 600,000 other patients treated between 2011 and 2016. The prediction algorithm successfully distinguished with high precision between patients who did or did not acquire HIV and between those who did or did not receive a PrEP prescription.
The researchers found hundreds of potential missed opportunities. They point to > 9,500 people in the 2016 dataset with particularly high-risk scores who were not prescribed PrEP. A “striking outcome,” the researchers say, is that their analysis suggests that nearly 40% of new HIV cases might have been averted had clinicians received alerts to discuss and offer PrEP to patients with the highest 2% of risk scores.
In the second study, researchers used the EHRs of > 3.7 million patients who entered the Kaiser Permanente System Northern California between 2007 and 2014 to develop a model to predict HIV incidence, then validated the model with data from between 2015 and 2017. Of the original patient group, 784 developed HIV within 3 years of baseline. The study found that the model identified nearly half of the incident HIV cases among males by flagging only 2% of the general patient population.
Embedding the algorithm into the EHR, the lead investigator says, “could prompt providers to discuss PrEP with patients who are most likely to benefit.”
Health care providers who do not have the time or tools to screen patients for HIV risk also may not be prescribing preexposure prophylaxis (PrEP). But help is on the way: NIH-funded researchers have come up with novel computerized methods to identify the patients PrEP could benefit.
In 2 separate studies, the researchers developed and validated algorithms that analyze electronic health records (EHR). In the first study, Harvard researchers used machine learning to create an HIV prediction algorithm using 2007 to 2015 data from > 1 million patients in Massachusetts. The model included variables such as diagnosis codes for HIV counseling or sexually transmitted infections (STIs), laboratory tests for HIV or STIs, and prescriptions for medications related to treating STIs.
The model was validated using data from nearly 600,000 other patients treated between 2011 and 2016. The prediction algorithm successfully distinguished with high precision between patients who did or did not acquire HIV and between those who did or did not receive a PrEP prescription.
The researchers found hundreds of potential missed opportunities. They point to > 9,500 people in the 2016 dataset with particularly high-risk scores who were not prescribed PrEP. A “striking outcome,” the researchers say, is that their analysis suggests that nearly 40% of new HIV cases might have been averted had clinicians received alerts to discuss and offer PrEP to patients with the highest 2% of risk scores.
In the second study, researchers used the EHRs of > 3.7 million patients who entered the Kaiser Permanente System Northern California between 2007 and 2014 to develop a model to predict HIV incidence, then validated the model with data from between 2015 and 2017. Of the original patient group, 784 developed HIV within 3 years of baseline. The study found that the model identified nearly half of the incident HIV cases among males by flagging only 2% of the general patient population.
Embedding the algorithm into the EHR, the lead investigator says, “could prompt providers to discuss PrEP with patients who are most likely to benefit.”
New WHO recommendations promote dolutegravir benefits in the face of lowered risk signal for neural tube defects
The risk of neural tube defects linked to dolutegravir exposure during pregnancy is lower than previously signaled, according to new reports that have prompted the World Health Organization (WHO) to confirm that this antiviral medication should be the preferred option across all populations.
The use of dolutegravir (DTG) during pregnancy has been a pressing global health question since May 2018, when an unplanned interim analysis of the Tsepamo surveillance study of birth outcomes in Botswana showed four neural tube defects associated with dolutegravir exposure among 426 infants born to HIV-positive women (0.94%).
With follow-up for additional births, however, just one more neural tube defect was identified out of 1,683 deliveries among women who had taken DTG around the time of conception (0.30%), according to a report just presented here at the at the International AIDS Society Conference on HIV Science.
By comparison, prevalence rates of neural tube defects were 0.10% for mothers taking other antiretroviral therapies at conception, 0.04% for those specifically taking efavirenz at conception, and 0.08% in HIV-uninfected mothers, according to the report, which was simultaneously published in the New England Journal of Medicine.
“While there may be a risk for neural tube defects, this risk is small, and really importantly, needs to be weighed against the large potential benefits of dolutegravir,” investigator Rebecca M. Zash, MD, of Beth Israel Deaconess Medical Center, Boston, said here in Mexico City during an IAS 2019 video press conference.
The WHO had previously sounded a note of caution, saying that DTG could be “considered” in women of childbearing age if other first‐line antiretroviral agents such as efavirenz could not be used.
However, following release of new evidence, including the study by Dr. Zash and colleagues, the WHO has come out with a clear recommendation for HIV drug as “the preferred first-line and second-line treatment for all populations, including pregnant women and those of childbearing potential.”
The updated scientific reports and guidelines have important implications for global health. “Many countries have been working to make dolutegravir-based treatment their preferred first-line regimen, as it’s got several advantages over efavirenz, which people have been using for many years now, including its tolerability and resistance profiles, and its impact on morbidity and mortality,” IAS president Anton Pozniak, MD, said in the press conference.
Some countries paused their plans to roll out dolutegravir-based regimens after the preliminary safety signal from the Tsepamo study was reported, Dr. Pozniak added.
In another study presented at IAS looking at dolutegravir use at conception, investigators described an additional surveillance study in Botswana, conducted independently from the Tsepamo study. One neural tube defect was found among 152 deliveries in mothers who had been taking DTG at conception (0.66%), and two neural tube defects among 2,326 deliveries to HIV-negative mothers (0.09%).
Although the number of deliveries are small in this study, the results suggest a risk of neural tube defects with DTG exposure at conception of less than 1%, said Mmakgomo Mimi Raesima, MD, MPH, public health specialist, Ministry of Health and Wellness, Botswana.
Because neural-tube defects might be related to low folate levels, Dr. Raesima said “conversations are continuing” with regard to folate food fortification in Botswana, a country that does not mandate folate-fortified grains.
“We want to capitalize on the momentum from these results,” Dr. Raesima said in the press conference.
The Tsepamo study was funded by the National Institutes of Health. Dr. Zash reported grants during the conduct of the study from the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
SOURCE: Zash R et al. N Engl J Med. 2019 Jul 22. doi: 10.1056/NEJMoa1905230.
The risk of neural tube defects linked to dolutegravir exposure during pregnancy is lower than previously signaled, according to new reports that have prompted the World Health Organization (WHO) to confirm that this antiviral medication should be the preferred option across all populations.
The use of dolutegravir (DTG) during pregnancy has been a pressing global health question since May 2018, when an unplanned interim analysis of the Tsepamo surveillance study of birth outcomes in Botswana showed four neural tube defects associated with dolutegravir exposure among 426 infants born to HIV-positive women (0.94%).
With follow-up for additional births, however, just one more neural tube defect was identified out of 1,683 deliveries among women who had taken DTG around the time of conception (0.30%), according to a report just presented here at the at the International AIDS Society Conference on HIV Science.
By comparison, prevalence rates of neural tube defects were 0.10% for mothers taking other antiretroviral therapies at conception, 0.04% for those specifically taking efavirenz at conception, and 0.08% in HIV-uninfected mothers, according to the report, which was simultaneously published in the New England Journal of Medicine.
“While there may be a risk for neural tube defects, this risk is small, and really importantly, needs to be weighed against the large potential benefits of dolutegravir,” investigator Rebecca M. Zash, MD, of Beth Israel Deaconess Medical Center, Boston, said here in Mexico City during an IAS 2019 video press conference.
The WHO had previously sounded a note of caution, saying that DTG could be “considered” in women of childbearing age if other first‐line antiretroviral agents such as efavirenz could not be used.
However, following release of new evidence, including the study by Dr. Zash and colleagues, the WHO has come out with a clear recommendation for HIV drug as “the preferred first-line and second-line treatment for all populations, including pregnant women and those of childbearing potential.”
The updated scientific reports and guidelines have important implications for global health. “Many countries have been working to make dolutegravir-based treatment their preferred first-line regimen, as it’s got several advantages over efavirenz, which people have been using for many years now, including its tolerability and resistance profiles, and its impact on morbidity and mortality,” IAS president Anton Pozniak, MD, said in the press conference.
Some countries paused their plans to roll out dolutegravir-based regimens after the preliminary safety signal from the Tsepamo study was reported, Dr. Pozniak added.
In another study presented at IAS looking at dolutegravir use at conception, investigators described an additional surveillance study in Botswana, conducted independently from the Tsepamo study. One neural tube defect was found among 152 deliveries in mothers who had been taking DTG at conception (0.66%), and two neural tube defects among 2,326 deliveries to HIV-negative mothers (0.09%).
Although the number of deliveries are small in this study, the results suggest a risk of neural tube defects with DTG exposure at conception of less than 1%, said Mmakgomo Mimi Raesima, MD, MPH, public health specialist, Ministry of Health and Wellness, Botswana.
Because neural-tube defects might be related to low folate levels, Dr. Raesima said “conversations are continuing” with regard to folate food fortification in Botswana, a country that does not mandate folate-fortified grains.
“We want to capitalize on the momentum from these results,” Dr. Raesima said in the press conference.
The Tsepamo study was funded by the National Institutes of Health. Dr. Zash reported grants during the conduct of the study from the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
SOURCE: Zash R et al. N Engl J Med. 2019 Jul 22. doi: 10.1056/NEJMoa1905230.
The risk of neural tube defects linked to dolutegravir exposure during pregnancy is lower than previously signaled, according to new reports that have prompted the World Health Organization (WHO) to confirm that this antiviral medication should be the preferred option across all populations.
The use of dolutegravir (DTG) during pregnancy has been a pressing global health question since May 2018, when an unplanned interim analysis of the Tsepamo surveillance study of birth outcomes in Botswana showed four neural tube defects associated with dolutegravir exposure among 426 infants born to HIV-positive women (0.94%).
With follow-up for additional births, however, just one more neural tube defect was identified out of 1,683 deliveries among women who had taken DTG around the time of conception (0.30%), according to a report just presented here at the at the International AIDS Society Conference on HIV Science.
By comparison, prevalence rates of neural tube defects were 0.10% for mothers taking other antiretroviral therapies at conception, 0.04% for those specifically taking efavirenz at conception, and 0.08% in HIV-uninfected mothers, according to the report, which was simultaneously published in the New England Journal of Medicine.
“While there may be a risk for neural tube defects, this risk is small, and really importantly, needs to be weighed against the large potential benefits of dolutegravir,” investigator Rebecca M. Zash, MD, of Beth Israel Deaconess Medical Center, Boston, said here in Mexico City during an IAS 2019 video press conference.
The WHO had previously sounded a note of caution, saying that DTG could be “considered” in women of childbearing age if other first‐line antiretroviral agents such as efavirenz could not be used.
However, following release of new evidence, including the study by Dr. Zash and colleagues, the WHO has come out with a clear recommendation for HIV drug as “the preferred first-line and second-line treatment for all populations, including pregnant women and those of childbearing potential.”
The updated scientific reports and guidelines have important implications for global health. “Many countries have been working to make dolutegravir-based treatment their preferred first-line regimen, as it’s got several advantages over efavirenz, which people have been using for many years now, including its tolerability and resistance profiles, and its impact on morbidity and mortality,” IAS president Anton Pozniak, MD, said in the press conference.
Some countries paused their plans to roll out dolutegravir-based regimens after the preliminary safety signal from the Tsepamo study was reported, Dr. Pozniak added.
In another study presented at IAS looking at dolutegravir use at conception, investigators described an additional surveillance study in Botswana, conducted independently from the Tsepamo study. One neural tube defect was found among 152 deliveries in mothers who had been taking DTG at conception (0.66%), and two neural tube defects among 2,326 deliveries to HIV-negative mothers (0.09%).
Although the number of deliveries are small in this study, the results suggest a risk of neural tube defects with DTG exposure at conception of less than 1%, said Mmakgomo Mimi Raesima, MD, MPH, public health specialist, Ministry of Health and Wellness, Botswana.
Because neural-tube defects might be related to low folate levels, Dr. Raesima said “conversations are continuing” with regard to folate food fortification in Botswana, a country that does not mandate folate-fortified grains.
“We want to capitalize on the momentum from these results,” Dr. Raesima said in the press conference.
The Tsepamo study was funded by the National Institutes of Health. Dr. Zash reported grants during the conduct of the study from the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
SOURCE: Zash R et al. N Engl J Med. 2019 Jul 22. doi: 10.1056/NEJMoa1905230.
FROM IAS 2019