User login
VA Urges All Veterans to Get Tested
The US Department of Veterans Affairs (VA) has a well-established National HIV Program, says Dr. Richard Stone, executive in charge of the VHA. In fact, he notes, the VA is the single largest provider of HIV care in America and has treated 31,000 veterans for HIV.
Thus, the VA plays a critical role in the effort to establish tools and resources to eradicate HIV in the US, Stone says, “one veteran at a time.” To realize this “ambitious but achievable target,” the VA is:
- Offering HIV testing at least once to every veteran and more often to those at risk;
- Rapidly linking those who are diagnosed to effective treatment;
- Deploying an HIV health force to hard-hit areas of the country, expanding timely access to high-quality HIV care and prevention across the VA’s integrated network, with both face-to-face encounters and telehealth; and
- Offering pre-exposure prophylaxis (PrEP) when clinically appropriate.
The primary goal, Stone says, is for veterans with HIV or at risk for HIV to be able to access the best care “safely and free from stigma and discrimination.”
Resources and educational tools are available at www.hiv.va.gov, including recently updated fact sheets and videos for patients about PrEP
The US Department of Veterans Affairs (VA) has a well-established National HIV Program, says Dr. Richard Stone, executive in charge of the VHA. In fact, he notes, the VA is the single largest provider of HIV care in America and has treated 31,000 veterans for HIV.
Thus, the VA plays a critical role in the effort to establish tools and resources to eradicate HIV in the US, Stone says, “one veteran at a time.” To realize this “ambitious but achievable target,” the VA is:
- Offering HIV testing at least once to every veteran and more often to those at risk;
- Rapidly linking those who are diagnosed to effective treatment;
- Deploying an HIV health force to hard-hit areas of the country, expanding timely access to high-quality HIV care and prevention across the VA’s integrated network, with both face-to-face encounters and telehealth; and
- Offering pre-exposure prophylaxis (PrEP) when clinically appropriate.
The primary goal, Stone says, is for veterans with HIV or at risk for HIV to be able to access the best care “safely and free from stigma and discrimination.”
Resources and educational tools are available at www.hiv.va.gov, including recently updated fact sheets and videos for patients about PrEP
The US Department of Veterans Affairs (VA) has a well-established National HIV Program, says Dr. Richard Stone, executive in charge of the VHA. In fact, he notes, the VA is the single largest provider of HIV care in America and has treated 31,000 veterans for HIV.
Thus, the VA plays a critical role in the effort to establish tools and resources to eradicate HIV in the US, Stone says, “one veteran at a time.” To realize this “ambitious but achievable target,” the VA is:
- Offering HIV testing at least once to every veteran and more often to those at risk;
- Rapidly linking those who are diagnosed to effective treatment;
- Deploying an HIV health force to hard-hit areas of the country, expanding timely access to high-quality HIV care and prevention across the VA’s integrated network, with both face-to-face encounters and telehealth; and
- Offering pre-exposure prophylaxis (PrEP) when clinically appropriate.
The primary goal, Stone says, is for veterans with HIV or at risk for HIV to be able to access the best care “safely and free from stigma and discrimination.”
Resources and educational tools are available at www.hiv.va.gov, including recently updated fact sheets and videos for patients about PrEP
IHS and Cherokee Nation Launch HIV-Prevention Project
The Indian Health Service (IHS) and the Cherokee Nation Health Service are launching a new pilot project to help “accelerate progress” toward ending the HIV epidemic in native communities.
The project, which will investigate the most effective prevention strategies and share the findings locally, is part of the initiative Ending the HIV Epidemic: A Plan for America. That plan focuses prevention and treatment efforts on 48 counties and 7 southern states with a higher proportion of HIV diagnosis in rural areas. The Cherokee Nation is in Oklahoma, which has the highest American Indian population among the 7 southern states.
Recent data show new HIV infections at the lowest level yet, but progress in prevention has slowed, in part due to new threats such as the opioid crisis: 10% of new HIV infections are among injectable-drug users.
The Cherokee Nation’s proven track record in hepatitis C prevention and treatment makes it a valuable partner in the project. Half of its health services patients have been screened, and among the 3.2% testing positive, 90% have been cured. The pilot project will use a similar model, IHS says. Current statistics show that 35% of Cherokee National patients using the tribe’s health centers have been screened for HIV, with < 1% testing positive. Of the patients diagnosed with HIV, 90% are receiving care and 90% of those are virally suppressed. The pilot project is aimed at boosting the screening numbers.
The pilot is one of several HHS efforts to jumpstart key activities in select communities using resources from the Minority HIV/AIDS fund. The CDC also is launching projects in select communities.
“Improved health care over multiple generations is our top priority,” said Cherokee Nation Principal Chief Bill John Baker. “If we can collaborate with our federal partners at IHS to raise awareness, increase education, and actively work to prevent new cases of HIV, then we will be creating a healthier future for northeast Oklahoma.”
The Indian Health Service (IHS) and the Cherokee Nation Health Service are launching a new pilot project to help “accelerate progress” toward ending the HIV epidemic in native communities.
The project, which will investigate the most effective prevention strategies and share the findings locally, is part of the initiative Ending the HIV Epidemic: A Plan for America. That plan focuses prevention and treatment efforts on 48 counties and 7 southern states with a higher proportion of HIV diagnosis in rural areas. The Cherokee Nation is in Oklahoma, which has the highest American Indian population among the 7 southern states.
Recent data show new HIV infections at the lowest level yet, but progress in prevention has slowed, in part due to new threats such as the opioid crisis: 10% of new HIV infections are among injectable-drug users.
The Cherokee Nation’s proven track record in hepatitis C prevention and treatment makes it a valuable partner in the project. Half of its health services patients have been screened, and among the 3.2% testing positive, 90% have been cured. The pilot project will use a similar model, IHS says. Current statistics show that 35% of Cherokee National patients using the tribe’s health centers have been screened for HIV, with < 1% testing positive. Of the patients diagnosed with HIV, 90% are receiving care and 90% of those are virally suppressed. The pilot project is aimed at boosting the screening numbers.
The pilot is one of several HHS efforts to jumpstart key activities in select communities using resources from the Minority HIV/AIDS fund. The CDC also is launching projects in select communities.
“Improved health care over multiple generations is our top priority,” said Cherokee Nation Principal Chief Bill John Baker. “If we can collaborate with our federal partners at IHS to raise awareness, increase education, and actively work to prevent new cases of HIV, then we will be creating a healthier future for northeast Oklahoma.”
The Indian Health Service (IHS) and the Cherokee Nation Health Service are launching a new pilot project to help “accelerate progress” toward ending the HIV epidemic in native communities.
The project, which will investigate the most effective prevention strategies and share the findings locally, is part of the initiative Ending the HIV Epidemic: A Plan for America. That plan focuses prevention and treatment efforts on 48 counties and 7 southern states with a higher proportion of HIV diagnosis in rural areas. The Cherokee Nation is in Oklahoma, which has the highest American Indian population among the 7 southern states.
Recent data show new HIV infections at the lowest level yet, but progress in prevention has slowed, in part due to new threats such as the opioid crisis: 10% of new HIV infections are among injectable-drug users.
The Cherokee Nation’s proven track record in hepatitis C prevention and treatment makes it a valuable partner in the project. Half of its health services patients have been screened, and among the 3.2% testing positive, 90% have been cured. The pilot project will use a similar model, IHS says. Current statistics show that 35% of Cherokee National patients using the tribe’s health centers have been screened for HIV, with < 1% testing positive. Of the patients diagnosed with HIV, 90% are receiving care and 90% of those are virally suppressed. The pilot project is aimed at boosting the screening numbers.
The pilot is one of several HHS efforts to jumpstart key activities in select communities using resources from the Minority HIV/AIDS fund. The CDC also is launching projects in select communities.
“Improved health care over multiple generations is our top priority,” said Cherokee Nation Principal Chief Bill John Baker. “If we can collaborate with our federal partners at IHS to raise awareness, increase education, and actively work to prevent new cases of HIV, then we will be creating a healthier future for northeast Oklahoma.”
Click for Credit: Roux-en-Y for diabetes; Exercise & fall prevention; more
Here are 5 articles from the July issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Cloud of inconsistency hangs over cannabis data
To take the posttest, go to: https://bit.ly/2NfjaDS
Expires February 6, 2020
2. Roux-en-Y achieves diabetes remission in majority of patients
To take the posttest, go to: https://bit.ly/2x9hLnE
Expires February 6, 2020
3. Socioeconomic status, race found to impact CPAP compliance
To take the posttest, go to: https://bit.ly/2RBpLa9
Expires February 8, 2020
4. Exercise type matters for fall prevention among elderly
To take the posttest, go to: https://bit.ly/2X26OUh
Expires February 12, 2020
5. Adult HIV patients should receive standard vaccinations, with caveats
To take the posttest, go to: https://bit.ly/2X1S7LV
Expires February 12, 2020
Here are 5 articles from the July issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Cloud of inconsistency hangs over cannabis data
To take the posttest, go to: https://bit.ly/2NfjaDS
Expires February 6, 2020
2. Roux-en-Y achieves diabetes remission in majority of patients
To take the posttest, go to: https://bit.ly/2x9hLnE
Expires February 6, 2020
3. Socioeconomic status, race found to impact CPAP compliance
To take the posttest, go to: https://bit.ly/2RBpLa9
Expires February 8, 2020
4. Exercise type matters for fall prevention among elderly
To take the posttest, go to: https://bit.ly/2X26OUh
Expires February 12, 2020
5. Adult HIV patients should receive standard vaccinations, with caveats
To take the posttest, go to: https://bit.ly/2X1S7LV
Expires February 12, 2020
Here are 5 articles from the July issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Cloud of inconsistency hangs over cannabis data
To take the posttest, go to: https://bit.ly/2NfjaDS
Expires February 6, 2020
2. Roux-en-Y achieves diabetes remission in majority of patients
To take the posttest, go to: https://bit.ly/2x9hLnE
Expires February 6, 2020
3. Socioeconomic status, race found to impact CPAP compliance
To take the posttest, go to: https://bit.ly/2RBpLa9
Expires February 8, 2020
4. Exercise type matters for fall prevention among elderly
To take the posttest, go to: https://bit.ly/2X26OUh
Expires February 12, 2020
5. Adult HIV patients should receive standard vaccinations, with caveats
To take the posttest, go to: https://bit.ly/2X1S7LV
Expires February 12, 2020
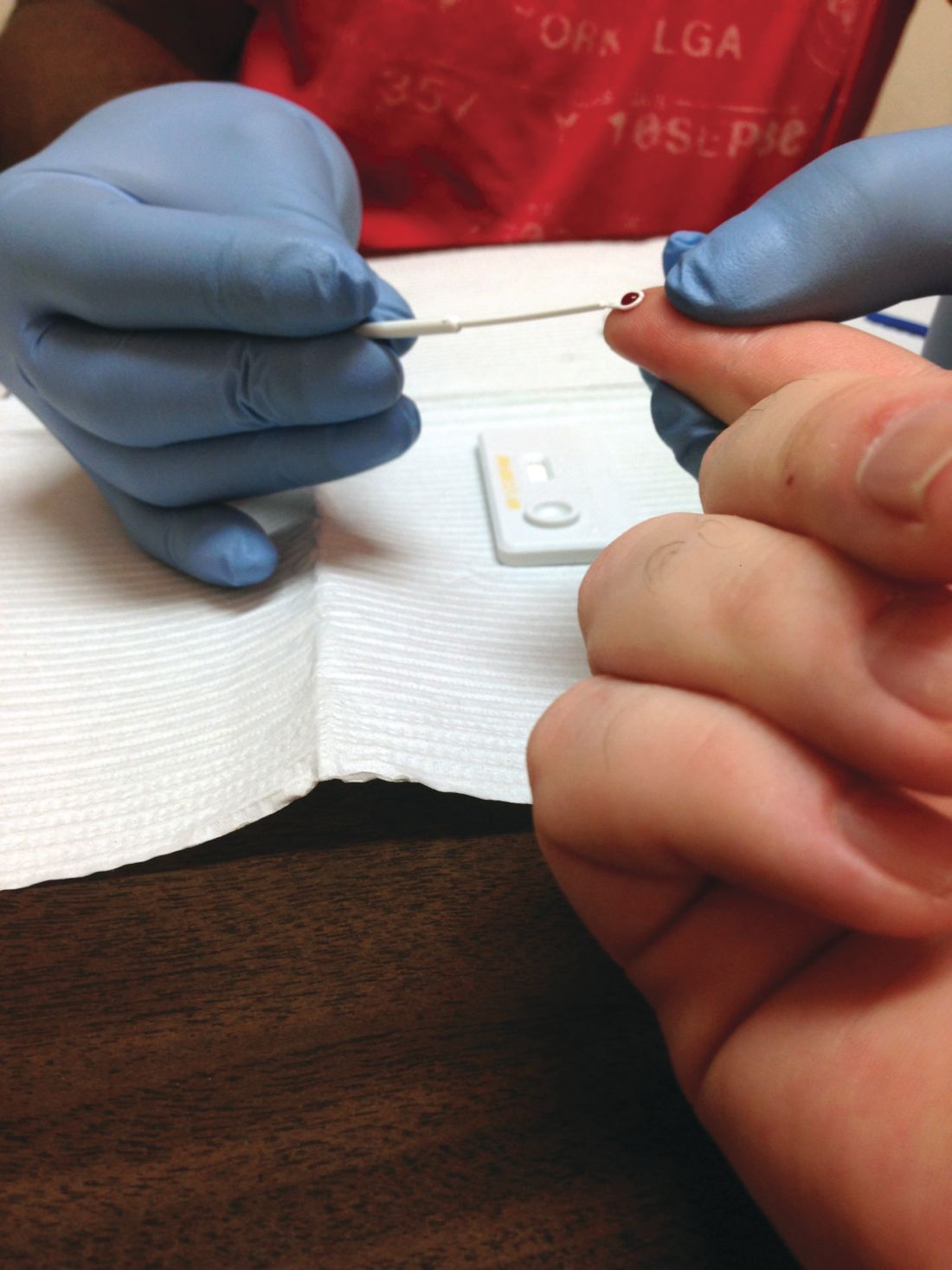
More than half of U.S. adults have never received HIV screening
Despite a 2006 recommendation by the Centers for Disease Control and Prevention recommending universal screening for HIV, less than half of U.S. adults have ever been tested for the disease, a new study found.
“HIV screening is a critical entry point to a range of HIV prevention and treatment options. For persons at ongoing risk for HIV infection exposure, annual screening also offers the opportunity to discuss options to reduce risk, including HIV preexposure prophylaxis,” Marc A. Pitasi, MPH, and investigators from the CDC wrote in the Morbidity and Mortality Weekly Report.
The CDC investigators analyzed data collected from the Behavioral Risk Factor Surveillance System in 2016-2017 to assess the percentage of adults screened for HIV ever and in the past year across the entirety of the United States, in 50 local jurisdictions where the majority of new U.S. HIV cases occur, and in seven states with a disproportionately high rate of HIV in rural populations.
The rate of ever testing was 38.9% across the entire country, 46.9% in the 50 local jurisdictions, and 35.5% in the seven states. The percentage of adults who had undergone HIV screening in the past year was significantly smaller at 10.1% across the entire country, 14.5% in the 50 local jurisdictions, and 9.3% in the seven states. This improved to 29.2%, 34.3%, and 26.2%, respectively, in adults who were tested in the past year who were also at risk for the disease.
The rate of ever testing varied widely among the 50 jurisdictions, ranging from 36.5% in Maricopa County, Ariz., to 70.7% in Washington, D.C. However, in addition to D.C., only Baltimore, Bronx County (N.Y.), and New York County reached or exceeded 60% coverage. Bronx County also had the highest rate of HIV screening in the past year at 31.3%, while Alameda County, Calif., had the lowest rate at 8.1%.
Of the seven states (Alabama, Arkansas, Kentucky, Mississippi, Missouri, Oklahoma, and South Carolina), Mississippi had the highest overall ever-testing rate at 40.2%, and Oklahoma had the lowest at 29.7%. People living in rural areas in those states were less likely to have ever been tested (32.1%) than those living in urban areas (37.2%). The difference between rural and urban areas increased for people at risk for HIV who had been screened in the past year, with 18.4% and 29.0%, respectively, reporting undergoing screening.
“These data provide a baseline from which to measure changes in screening in these jurisdictions and other parts of the United States over time. To achieve national goals and end the HIV epidemic in the United States, innovative and novel screening approaches might be needed to reach segments of the population that have never been tested for HIV,” the investigators concluded.
The investigators reported no conflicts of interest.
SOURCE: Pitasi MA et al. MMWR Morb Mortal Wkly Rep. 2019;68:561-7.
Despite a 2006 recommendation by the Centers for Disease Control and Prevention recommending universal screening for HIV, less than half of U.S. adults have ever been tested for the disease, a new study found.
“HIV screening is a critical entry point to a range of HIV prevention and treatment options. For persons at ongoing risk for HIV infection exposure, annual screening also offers the opportunity to discuss options to reduce risk, including HIV preexposure prophylaxis,” Marc A. Pitasi, MPH, and investigators from the CDC wrote in the Morbidity and Mortality Weekly Report.
The CDC investigators analyzed data collected from the Behavioral Risk Factor Surveillance System in 2016-2017 to assess the percentage of adults screened for HIV ever and in the past year across the entirety of the United States, in 50 local jurisdictions where the majority of new U.S. HIV cases occur, and in seven states with a disproportionately high rate of HIV in rural populations.
The rate of ever testing was 38.9% across the entire country, 46.9% in the 50 local jurisdictions, and 35.5% in the seven states. The percentage of adults who had undergone HIV screening in the past year was significantly smaller at 10.1% across the entire country, 14.5% in the 50 local jurisdictions, and 9.3% in the seven states. This improved to 29.2%, 34.3%, and 26.2%, respectively, in adults who were tested in the past year who were also at risk for the disease.
The rate of ever testing varied widely among the 50 jurisdictions, ranging from 36.5% in Maricopa County, Ariz., to 70.7% in Washington, D.C. However, in addition to D.C., only Baltimore, Bronx County (N.Y.), and New York County reached or exceeded 60% coverage. Bronx County also had the highest rate of HIV screening in the past year at 31.3%, while Alameda County, Calif., had the lowest rate at 8.1%.
Of the seven states (Alabama, Arkansas, Kentucky, Mississippi, Missouri, Oklahoma, and South Carolina), Mississippi had the highest overall ever-testing rate at 40.2%, and Oklahoma had the lowest at 29.7%. People living in rural areas in those states were less likely to have ever been tested (32.1%) than those living in urban areas (37.2%). The difference between rural and urban areas increased for people at risk for HIV who had been screened in the past year, with 18.4% and 29.0%, respectively, reporting undergoing screening.
“These data provide a baseline from which to measure changes in screening in these jurisdictions and other parts of the United States over time. To achieve national goals and end the HIV epidemic in the United States, innovative and novel screening approaches might be needed to reach segments of the population that have never been tested for HIV,” the investigators concluded.
The investigators reported no conflicts of interest.
SOURCE: Pitasi MA et al. MMWR Morb Mortal Wkly Rep. 2019;68:561-7.
Despite a 2006 recommendation by the Centers for Disease Control and Prevention recommending universal screening for HIV, less than half of U.S. adults have ever been tested for the disease, a new study found.
“HIV screening is a critical entry point to a range of HIV prevention and treatment options. For persons at ongoing risk for HIV infection exposure, annual screening also offers the opportunity to discuss options to reduce risk, including HIV preexposure prophylaxis,” Marc A. Pitasi, MPH, and investigators from the CDC wrote in the Morbidity and Mortality Weekly Report.
The CDC investigators analyzed data collected from the Behavioral Risk Factor Surveillance System in 2016-2017 to assess the percentage of adults screened for HIV ever and in the past year across the entirety of the United States, in 50 local jurisdictions where the majority of new U.S. HIV cases occur, and in seven states with a disproportionately high rate of HIV in rural populations.
The rate of ever testing was 38.9% across the entire country, 46.9% in the 50 local jurisdictions, and 35.5% in the seven states. The percentage of adults who had undergone HIV screening in the past year was significantly smaller at 10.1% across the entire country, 14.5% in the 50 local jurisdictions, and 9.3% in the seven states. This improved to 29.2%, 34.3%, and 26.2%, respectively, in adults who were tested in the past year who were also at risk for the disease.
The rate of ever testing varied widely among the 50 jurisdictions, ranging from 36.5% in Maricopa County, Ariz., to 70.7% in Washington, D.C. However, in addition to D.C., only Baltimore, Bronx County (N.Y.), and New York County reached or exceeded 60% coverage. Bronx County also had the highest rate of HIV screening in the past year at 31.3%, while Alameda County, Calif., had the lowest rate at 8.1%.
Of the seven states (Alabama, Arkansas, Kentucky, Mississippi, Missouri, Oklahoma, and South Carolina), Mississippi had the highest overall ever-testing rate at 40.2%, and Oklahoma had the lowest at 29.7%. People living in rural areas in those states were less likely to have ever been tested (32.1%) than those living in urban areas (37.2%). The difference between rural and urban areas increased for people at risk for HIV who had been screened in the past year, with 18.4% and 29.0%, respectively, reporting undergoing screening.
“These data provide a baseline from which to measure changes in screening in these jurisdictions and other parts of the United States over time. To achieve national goals and end the HIV epidemic in the United States, innovative and novel screening approaches might be needed to reach segments of the population that have never been tested for HIV,” the investigators concluded.
The investigators reported no conflicts of interest.
SOURCE: Pitasi MA et al. MMWR Morb Mortal Wkly Rep. 2019;68:561-7.
FROM MMWR
USPSTF reaffirms HIV screening recommendations
According to the task force, screening is recommended for all patients aged 15-65 years. Screening also is recommended for adolescents and older adults at increased risk for acquiring HIV infection and for all pregnant patients, including those in labor whose HIV status is unknown (JAMA. 2019. doi: 10.1001/jama.2019.6587).
Patients who are considered at increased risk for acquiring HIV include the following: Men who have sex with men, those who inject drugs, those who have receptive sex without a condom, those with at least one partner whose HIV status is positive or unknown, those who have transactional sex, and those who request testing for sexually transmitted infection, including HIV. All recommendations are A-level, meaning the task force recommends the service,with high certainty that the net benefit is substantial.
In a systematic review created for the task force, Roger Chou, MD, of Oregon Health & Science University, Portland, and colleagues found there continued to be no studies that examined the benefits and harms of HIV screening for HIV infections, compared with no screening, but new evidence found beginning antiretroviral therapy (ART) for patients with CD4 cell counts greater than 500/mm3 who are otherwise asymptomatic was associated with a reduced risk of mortality, compared with waiting for ART in cases of CD4 cell counts less than 350/mm3 (JAMA. 2019. doi: 10.1001/jama.2019.2592).
A second systematic review of pregnant patients by Shelley S. Selph, MD, also of Oregon Health & Science University, Portland, and colleagues found no studies examining the effectiveness of prenatal screening on mother-to-child HIV transmission, but combination ART was significantly effective at reducing transmission between mother and child, while ART that includes a boosted protease inhibitor may result in preterm delivery (JAMA. 2019. doi: 10.1001/jama.2019.2593).
Although no studies have been conducted that compare the benefits of screening with not screening for HIV, the task force concluded with “high certainty” that early HIV detection and treatment has “substantial benefits.”
“Clinicians can make a real difference toward reducing the burden of HIV in the United States,” Douglas K. Owens, MD, task force chairman, said in a statement. “HIV screening and HIV prevention work to reduce new HIV infections and ultimately save lives.”
The USPSTF is a voluntary, independent body, with operations supported by the U.S. Agency for Healthcare Research and Quality. Task force members received travel reimbursement and an honorarium for attending meetings. Dr. Owens reports financial disclosures with relation to HIV infection screening, preexposure prophylaxis for HIV prevention, and hepatitis C screening. Other task force members reported no relevant conflicts of interest.
SOURCE: JAMA. 2019. doi: 10.1001/jama.2019.6587.
According to the task force, screening is recommended for all patients aged 15-65 years. Screening also is recommended for adolescents and older adults at increased risk for acquiring HIV infection and for all pregnant patients, including those in labor whose HIV status is unknown (JAMA. 2019. doi: 10.1001/jama.2019.6587).
Patients who are considered at increased risk for acquiring HIV include the following: Men who have sex with men, those who inject drugs, those who have receptive sex without a condom, those with at least one partner whose HIV status is positive or unknown, those who have transactional sex, and those who request testing for sexually transmitted infection, including HIV. All recommendations are A-level, meaning the task force recommends the service,with high certainty that the net benefit is substantial.
In a systematic review created for the task force, Roger Chou, MD, of Oregon Health & Science University, Portland, and colleagues found there continued to be no studies that examined the benefits and harms of HIV screening for HIV infections, compared with no screening, but new evidence found beginning antiretroviral therapy (ART) for patients with CD4 cell counts greater than 500/mm3 who are otherwise asymptomatic was associated with a reduced risk of mortality, compared with waiting for ART in cases of CD4 cell counts less than 350/mm3 (JAMA. 2019. doi: 10.1001/jama.2019.2592).
A second systematic review of pregnant patients by Shelley S. Selph, MD, also of Oregon Health & Science University, Portland, and colleagues found no studies examining the effectiveness of prenatal screening on mother-to-child HIV transmission, but combination ART was significantly effective at reducing transmission between mother and child, while ART that includes a boosted protease inhibitor may result in preterm delivery (JAMA. 2019. doi: 10.1001/jama.2019.2593).
Although no studies have been conducted that compare the benefits of screening with not screening for HIV, the task force concluded with “high certainty” that early HIV detection and treatment has “substantial benefits.”
“Clinicians can make a real difference toward reducing the burden of HIV in the United States,” Douglas K. Owens, MD, task force chairman, said in a statement. “HIV screening and HIV prevention work to reduce new HIV infections and ultimately save lives.”
The USPSTF is a voluntary, independent body, with operations supported by the U.S. Agency for Healthcare Research and Quality. Task force members received travel reimbursement and an honorarium for attending meetings. Dr. Owens reports financial disclosures with relation to HIV infection screening, preexposure prophylaxis for HIV prevention, and hepatitis C screening. Other task force members reported no relevant conflicts of interest.
SOURCE: JAMA. 2019. doi: 10.1001/jama.2019.6587.
According to the task force, screening is recommended for all patients aged 15-65 years. Screening also is recommended for adolescents and older adults at increased risk for acquiring HIV infection and for all pregnant patients, including those in labor whose HIV status is unknown (JAMA. 2019. doi: 10.1001/jama.2019.6587).
Patients who are considered at increased risk for acquiring HIV include the following: Men who have sex with men, those who inject drugs, those who have receptive sex without a condom, those with at least one partner whose HIV status is positive or unknown, those who have transactional sex, and those who request testing for sexually transmitted infection, including HIV. All recommendations are A-level, meaning the task force recommends the service,with high certainty that the net benefit is substantial.
In a systematic review created for the task force, Roger Chou, MD, of Oregon Health & Science University, Portland, and colleagues found there continued to be no studies that examined the benefits and harms of HIV screening for HIV infections, compared with no screening, but new evidence found beginning antiretroviral therapy (ART) for patients with CD4 cell counts greater than 500/mm3 who are otherwise asymptomatic was associated with a reduced risk of mortality, compared with waiting for ART in cases of CD4 cell counts less than 350/mm3 (JAMA. 2019. doi: 10.1001/jama.2019.2592).
A second systematic review of pregnant patients by Shelley S. Selph, MD, also of Oregon Health & Science University, Portland, and colleagues found no studies examining the effectiveness of prenatal screening on mother-to-child HIV transmission, but combination ART was significantly effective at reducing transmission between mother and child, while ART that includes a boosted protease inhibitor may result in preterm delivery (JAMA. 2019. doi: 10.1001/jama.2019.2593).
Although no studies have been conducted that compare the benefits of screening with not screening for HIV, the task force concluded with “high certainty” that early HIV detection and treatment has “substantial benefits.”
“Clinicians can make a real difference toward reducing the burden of HIV in the United States,” Douglas K. Owens, MD, task force chairman, said in a statement. “HIV screening and HIV prevention work to reduce new HIV infections and ultimately save lives.”
The USPSTF is a voluntary, independent body, with operations supported by the U.S. Agency for Healthcare Research and Quality. Task force members received travel reimbursement and an honorarium for attending meetings. Dr. Owens reports financial disclosures with relation to HIV infection screening, preexposure prophylaxis for HIV prevention, and hepatitis C screening. Other task force members reported no relevant conflicts of interest.
SOURCE: JAMA. 2019. doi: 10.1001/jama.2019.6587.
FROM JAMA
USPSTF recommends PrEP combo for adults at high risk of HIV infection
Pre-exposure prophylaxis (PrEP) plus effective antiretroviral therapy should be offered to people at high risk of HIV acquisition, according to a new recommendation from the U.S. Preventive Services Task Force (USPSTF).
“The USPSTF concludes with high certainty that the net benefit of the use of PrEP to reduce the risk of acquisition of HIV infection in persons at high risk of HIV infection is substantial,” wrote first author Douglas K. Owens, MD, of Stanford (Calif.) University and fellow members of the USPSTF. The recommendation was published in JAMA.
In various at-risk groups – including men who have sex with men, people at risk through heterosexual contact, and people who inject drugs – the USPSTF recommends a Food and Drug Adminstration–approved, once-daily oral treatment with combined tenofovir disoproxil fumarate and emtricitabine.
This recommendation was developed after a systematic review of PrEP’s effects on HIV, adherence to the treatment, and accuracy in identifying potential treatment candidates. “The findings of this review are generally consistent with those from other recent meta-analyses that found PrEP to be effective at reducing risk of HIV infection and found greater effectiveness in trials reporting higher adherence,” wrote Roger Chou, MD, of Oregon Health & Science University in Portland and coauthors. Their study was also published in JAMA.
To comprehensively assess PrEP and thus inform the USPSTF’s HIV prevention recommendations, the researchers reviewed criteria-meeting studies on oral PrEP with tenofovir disoproxil fumarate/emtricitabine or tenofovir disoproxil fumarate monotherapy; on the diagnostic accuracy of instruments to predict HIV infection; and on PrEP adherence. The final analysis included 14 randomized clinical trials, 8 observational studies, and 7 studies of diagnostic accuracy.
In 11 of the trials, PrEP was associated with reduced risk of HIV infection versus placebo or no PrEP (relative risk, 0.46; 95% confidence interval, 0.33-0.66). In 6 trials with adherence 70% or greater, the relative risk was 0.27 (95% CI, 0.19-0.39). In 7 studies on risk assessment tools for HIV infection, the instruments had moderate discrimination, though several of the studies had methodological shortcomings. As for serious adverse events, an analysis of 12 trials found no significant difference between PrEP and placebo (RR, 0.93; 95% CI, 0.77-1.12).
Dr. Chou and coauthors noted their study’s limitations, including analyzing English-language articles only and the random-effects model used to pool studies potentially returning narrow CIs. They did note, however, that the “analyses were repeated using the profile likelihood method,” which produced similar findings.
All members of the USPSTF receive travel reimbursement and an honorarium for participating in meetings. The study was funded by the Department of Health and Human Services. One of the authors reported receiving grants from the National Institutes of Health/National Institute on Drug Abuse and serving as principal investigator of NIH-funded clinical trials that received donated drugs from two pharmaceutical companies. No other conflicts of interest were reported.
SOURCE: Owens DK et al. JAMA. 2019 Jun 11. doi: 10.1001/jama.2019.6390; Chou R et al. JAMA. 2019 Jun 11. doi: 10.1001/jama.2019.2591.
To end HIV, guidelines like this one that reflect and promote advances in treatment are needed, according to Hyman Scott, MD, MPH, of the San Francisco Department of Public Health and Paul A. Volberding, MD, of the University of California, San Francisco.
With less than 10% of individuals with an indication for PrEP currently receiving the medication, it is now time to support policies aimed at broadening the access of PrEP to people at risk, the coauthors wrote. They noted that recent USPSTF guidelines show that evidence and policy in HIV medicine has matured not only in the United States but across the globe.
That said, sometimes the simplest solutions are also the best. Though the systematic review from Roger Chou, MD, and associates notes the necessity and importance of adherence, if a clinician thinks that a candidate for PrEP might be nonadherent, that clinicians should not withhold the medication, they wrote. Averting new HIV infections is the goal, and fully endorsing treatments like PrEP is an important step in that direction.*
These comments are adapted from an accompanying editorial (JAMA. 2019 Jun 11. doi: 10.1001/jama.2019.2590). Dr. Volberding reported serving on a data and safety monitoring board for Merck.
*This article was updated on 6/11/2019.
To end HIV, guidelines like this one that reflect and promote advances in treatment are needed, according to Hyman Scott, MD, MPH, of the San Francisco Department of Public Health and Paul A. Volberding, MD, of the University of California, San Francisco.
With less than 10% of individuals with an indication for PrEP currently receiving the medication, it is now time to support policies aimed at broadening the access of PrEP to people at risk, the coauthors wrote. They noted that recent USPSTF guidelines show that evidence and policy in HIV medicine has matured not only in the United States but across the globe.
That said, sometimes the simplest solutions are also the best. Though the systematic review from Roger Chou, MD, and associates notes the necessity and importance of adherence, if a clinician thinks that a candidate for PrEP might be nonadherent, that clinicians should not withhold the medication, they wrote. Averting new HIV infections is the goal, and fully endorsing treatments like PrEP is an important step in that direction.*
These comments are adapted from an accompanying editorial (JAMA. 2019 Jun 11. doi: 10.1001/jama.2019.2590). Dr. Volberding reported serving on a data and safety monitoring board for Merck.
*This article was updated on 6/11/2019.
To end HIV, guidelines like this one that reflect and promote advances in treatment are needed, according to Hyman Scott, MD, MPH, of the San Francisco Department of Public Health and Paul A. Volberding, MD, of the University of California, San Francisco.
With less than 10% of individuals with an indication for PrEP currently receiving the medication, it is now time to support policies aimed at broadening the access of PrEP to people at risk, the coauthors wrote. They noted that recent USPSTF guidelines show that evidence and policy in HIV medicine has matured not only in the United States but across the globe.
That said, sometimes the simplest solutions are also the best. Though the systematic review from Roger Chou, MD, and associates notes the necessity and importance of adherence, if a clinician thinks that a candidate for PrEP might be nonadherent, that clinicians should not withhold the medication, they wrote. Averting new HIV infections is the goal, and fully endorsing treatments like PrEP is an important step in that direction.*
These comments are adapted from an accompanying editorial (JAMA. 2019 Jun 11. doi: 10.1001/jama.2019.2590). Dr. Volberding reported serving on a data and safety monitoring board for Merck.
*This article was updated on 6/11/2019.
Pre-exposure prophylaxis (PrEP) plus effective antiretroviral therapy should be offered to people at high risk of HIV acquisition, according to a new recommendation from the U.S. Preventive Services Task Force (USPSTF).
“The USPSTF concludes with high certainty that the net benefit of the use of PrEP to reduce the risk of acquisition of HIV infection in persons at high risk of HIV infection is substantial,” wrote first author Douglas K. Owens, MD, of Stanford (Calif.) University and fellow members of the USPSTF. The recommendation was published in JAMA.
In various at-risk groups – including men who have sex with men, people at risk through heterosexual contact, and people who inject drugs – the USPSTF recommends a Food and Drug Adminstration–approved, once-daily oral treatment with combined tenofovir disoproxil fumarate and emtricitabine.
This recommendation was developed after a systematic review of PrEP’s effects on HIV, adherence to the treatment, and accuracy in identifying potential treatment candidates. “The findings of this review are generally consistent with those from other recent meta-analyses that found PrEP to be effective at reducing risk of HIV infection and found greater effectiveness in trials reporting higher adherence,” wrote Roger Chou, MD, of Oregon Health & Science University in Portland and coauthors. Their study was also published in JAMA.
To comprehensively assess PrEP and thus inform the USPSTF’s HIV prevention recommendations, the researchers reviewed criteria-meeting studies on oral PrEP with tenofovir disoproxil fumarate/emtricitabine or tenofovir disoproxil fumarate monotherapy; on the diagnostic accuracy of instruments to predict HIV infection; and on PrEP adherence. The final analysis included 14 randomized clinical trials, 8 observational studies, and 7 studies of diagnostic accuracy.
In 11 of the trials, PrEP was associated with reduced risk of HIV infection versus placebo or no PrEP (relative risk, 0.46; 95% confidence interval, 0.33-0.66). In 6 trials with adherence 70% or greater, the relative risk was 0.27 (95% CI, 0.19-0.39). In 7 studies on risk assessment tools for HIV infection, the instruments had moderate discrimination, though several of the studies had methodological shortcomings. As for serious adverse events, an analysis of 12 trials found no significant difference between PrEP and placebo (RR, 0.93; 95% CI, 0.77-1.12).
Dr. Chou and coauthors noted their study’s limitations, including analyzing English-language articles only and the random-effects model used to pool studies potentially returning narrow CIs. They did note, however, that the “analyses were repeated using the profile likelihood method,” which produced similar findings.
All members of the USPSTF receive travel reimbursement and an honorarium for participating in meetings. The study was funded by the Department of Health and Human Services. One of the authors reported receiving grants from the National Institutes of Health/National Institute on Drug Abuse and serving as principal investigator of NIH-funded clinical trials that received donated drugs from two pharmaceutical companies. No other conflicts of interest were reported.
SOURCE: Owens DK et al. JAMA. 2019 Jun 11. doi: 10.1001/jama.2019.6390; Chou R et al. JAMA. 2019 Jun 11. doi: 10.1001/jama.2019.2591.
Pre-exposure prophylaxis (PrEP) plus effective antiretroviral therapy should be offered to people at high risk of HIV acquisition, according to a new recommendation from the U.S. Preventive Services Task Force (USPSTF).
“The USPSTF concludes with high certainty that the net benefit of the use of PrEP to reduce the risk of acquisition of HIV infection in persons at high risk of HIV infection is substantial,” wrote first author Douglas K. Owens, MD, of Stanford (Calif.) University and fellow members of the USPSTF. The recommendation was published in JAMA.
In various at-risk groups – including men who have sex with men, people at risk through heterosexual contact, and people who inject drugs – the USPSTF recommends a Food and Drug Adminstration–approved, once-daily oral treatment with combined tenofovir disoproxil fumarate and emtricitabine.
This recommendation was developed after a systematic review of PrEP’s effects on HIV, adherence to the treatment, and accuracy in identifying potential treatment candidates. “The findings of this review are generally consistent with those from other recent meta-analyses that found PrEP to be effective at reducing risk of HIV infection and found greater effectiveness in trials reporting higher adherence,” wrote Roger Chou, MD, of Oregon Health & Science University in Portland and coauthors. Their study was also published in JAMA.
To comprehensively assess PrEP and thus inform the USPSTF’s HIV prevention recommendations, the researchers reviewed criteria-meeting studies on oral PrEP with tenofovir disoproxil fumarate/emtricitabine or tenofovir disoproxil fumarate monotherapy; on the diagnostic accuracy of instruments to predict HIV infection; and on PrEP adherence. The final analysis included 14 randomized clinical trials, 8 observational studies, and 7 studies of diagnostic accuracy.
In 11 of the trials, PrEP was associated with reduced risk of HIV infection versus placebo or no PrEP (relative risk, 0.46; 95% confidence interval, 0.33-0.66). In 6 trials with adherence 70% or greater, the relative risk was 0.27 (95% CI, 0.19-0.39). In 7 studies on risk assessment tools for HIV infection, the instruments had moderate discrimination, though several of the studies had methodological shortcomings. As for serious adverse events, an analysis of 12 trials found no significant difference between PrEP and placebo (RR, 0.93; 95% CI, 0.77-1.12).
Dr. Chou and coauthors noted their study’s limitations, including analyzing English-language articles only and the random-effects model used to pool studies potentially returning narrow CIs. They did note, however, that the “analyses were repeated using the profile likelihood method,” which produced similar findings.
All members of the USPSTF receive travel reimbursement and an honorarium for participating in meetings. The study was funded by the Department of Health and Human Services. One of the authors reported receiving grants from the National Institutes of Health/National Institute on Drug Abuse and serving as principal investigator of NIH-funded clinical trials that received donated drugs from two pharmaceutical companies. No other conflicts of interest were reported.
SOURCE: Owens DK et al. JAMA. 2019 Jun 11. doi: 10.1001/jama.2019.6390; Chou R et al. JAMA. 2019 Jun 11. doi: 10.1001/jama.2019.2591.
FROM JAMA
Consider measles vaccine booster in HIV-positive patients
LJUBLJANA, SLOVENIA – A “surprisingly low” prevalence of protective antibodies against measles is present in adolescents and adults living with HIV infection despite their prior vaccination against the resurgent disease, Raquel M. Simakawa, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“With the present concern about the global reemergence of measles, we should consider measuring measles antibodies in people living with HIV, especially those who acquired the infection vertically, and then revaccinating those with low titers,” said Dr. Simakawa of the Federal University of São Paolo.
She presented interim findings of an ongoing study of the measles immunologic status of persons living with HIV, which for this analysis included 57 patients who acquired HIV from their mother via vertical transmission and 24 with horizontally acquired HIV. The vertical-transmission group was significantly younger, with a median age of 20 years, compared with 31 years in the horizontal group, who were diagnosed with HIV infection at an average age of 24 years. The vast majority of subjects were on combination antiretroviral therapy. No detectable HIV viral load had been present for a median of 70 months in the vertical group and 25 months in the horizontal group.
Only a mere 7% of the vertical transmission group had protective levels of measles IgG antibodies as measured by enzyme-linked immunosorbent assay, as did 29% of the horizontal group. The likely explanation for the higher rate of protection in the horizontal group, she said, is that they received their routine measles vaccination before they acquired HIV infection, and some of them didn’t lose their protective antibodies during their immune system’s fight against HIV infection.
Session chair Nico G. Hartwig, MD, of Franciscus Hospital in Rotterdam, the Netherlands, posed a question: Given the sky-high rate of measles seronegativity status among the vertically transmitted HIV-positive group – the patient population pediatricians focus on – why bother to measure their measles antibody level? Why not just give them all a measles booster?
Dr. Simakawa replied that that’s worth considering in routine clinical practice now that her study has shown that this group is more vulnerable to measles because of their poor response to immunization. But the study is ongoing, with larger numbers of patients to be enrolled. Also, in the second phase of the study, which will include a control group, measles IgG antibodies will be remeasured 1 month after administration of a new dose of measles vaccine.
She reported having no financial conflicts regarding this study, conducted free of commercial support.
LJUBLJANA, SLOVENIA – A “surprisingly low” prevalence of protective antibodies against measles is present in adolescents and adults living with HIV infection despite their prior vaccination against the resurgent disease, Raquel M. Simakawa, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“With the present concern about the global reemergence of measles, we should consider measuring measles antibodies in people living with HIV, especially those who acquired the infection vertically, and then revaccinating those with low titers,” said Dr. Simakawa of the Federal University of São Paolo.
She presented interim findings of an ongoing study of the measles immunologic status of persons living with HIV, which for this analysis included 57 patients who acquired HIV from their mother via vertical transmission and 24 with horizontally acquired HIV. The vertical-transmission group was significantly younger, with a median age of 20 years, compared with 31 years in the horizontal group, who were diagnosed with HIV infection at an average age of 24 years. The vast majority of subjects were on combination antiretroviral therapy. No detectable HIV viral load had been present for a median of 70 months in the vertical group and 25 months in the horizontal group.
Only a mere 7% of the vertical transmission group had protective levels of measles IgG antibodies as measured by enzyme-linked immunosorbent assay, as did 29% of the horizontal group. The likely explanation for the higher rate of protection in the horizontal group, she said, is that they received their routine measles vaccination before they acquired HIV infection, and some of them didn’t lose their protective antibodies during their immune system’s fight against HIV infection.
Session chair Nico G. Hartwig, MD, of Franciscus Hospital in Rotterdam, the Netherlands, posed a question: Given the sky-high rate of measles seronegativity status among the vertically transmitted HIV-positive group – the patient population pediatricians focus on – why bother to measure their measles antibody level? Why not just give them all a measles booster?
Dr. Simakawa replied that that’s worth considering in routine clinical practice now that her study has shown that this group is more vulnerable to measles because of their poor response to immunization. But the study is ongoing, with larger numbers of patients to be enrolled. Also, in the second phase of the study, which will include a control group, measles IgG antibodies will be remeasured 1 month after administration of a new dose of measles vaccine.
She reported having no financial conflicts regarding this study, conducted free of commercial support.
LJUBLJANA, SLOVENIA – A “surprisingly low” prevalence of protective antibodies against measles is present in adolescents and adults living with HIV infection despite their prior vaccination against the resurgent disease, Raquel M. Simakawa, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“With the present concern about the global reemergence of measles, we should consider measuring measles antibodies in people living with HIV, especially those who acquired the infection vertically, and then revaccinating those with low titers,” said Dr. Simakawa of the Federal University of São Paolo.
She presented interim findings of an ongoing study of the measles immunologic status of persons living with HIV, which for this analysis included 57 patients who acquired HIV from their mother via vertical transmission and 24 with horizontally acquired HIV. The vertical-transmission group was significantly younger, with a median age of 20 years, compared with 31 years in the horizontal group, who were diagnosed with HIV infection at an average age of 24 years. The vast majority of subjects were on combination antiretroviral therapy. No detectable HIV viral load had been present for a median of 70 months in the vertical group and 25 months in the horizontal group.
Only a mere 7% of the vertical transmission group had protective levels of measles IgG antibodies as measured by enzyme-linked immunosorbent assay, as did 29% of the horizontal group. The likely explanation for the higher rate of protection in the horizontal group, she said, is that they received their routine measles vaccination before they acquired HIV infection, and some of them didn’t lose their protective antibodies during their immune system’s fight against HIV infection.
Session chair Nico G. Hartwig, MD, of Franciscus Hospital in Rotterdam, the Netherlands, posed a question: Given the sky-high rate of measles seronegativity status among the vertically transmitted HIV-positive group – the patient population pediatricians focus on – why bother to measure their measles antibody level? Why not just give them all a measles booster?
Dr. Simakawa replied that that’s worth considering in routine clinical practice now that her study has shown that this group is more vulnerable to measles because of their poor response to immunization. But the study is ongoing, with larger numbers of patients to be enrolled. Also, in the second phase of the study, which will include a control group, measles IgG antibodies will be remeasured 1 month after administration of a new dose of measles vaccine.
She reported having no financial conflicts regarding this study, conducted free of commercial support.
REPORTING FROM ESPID 2019
Babesiosis HIV
According to the CDC, the number of reported tickborne diseases more than doubled between 2004-2016 and accounted for > 60% of all reported mosquito-borne, tickborne, and fleaborne disease cases. Which is why it is important to keep an eye out for anyone who has a history of being in a tick-promoting environment. Clinicians from Lehigh Valley Health Network Pocono and Geisinger Commonwealth School of Medicine, both in East Stroudsburg, Pennsylvania, report on a patient whose diagnosis turned on that fact.
The patient, a 71-year-old man, had fever, weakness, headaches, near syncope, and nausea for 4 days. He also had not been eating well.
A complete blood count showed pancytopenia with an excess of band cells, an indicator of inflammation and infection. The patient’s aspartate transaminase levels were elevated. The diagnostic dilemma centered on these findings: Serology tests for HIV 1 and 2 were positive, and a peripheral blood smear showed 0.5% parasitemia consistent with Babesia microti. Both babesiosis and HIV were among the possible diagnoses. Two important factors the clinicians had to consider: The patient had recently been bitten by ticks and was homosexual.
The clinicians note that a variety of infections can lead to false-positive HIV serology, such as malaria, Mycobacterium tuberculosis or Rickettsia species, influenza and hepatitis B vaccinations. Moreover, the Ixodes tick, the same vector that transmits Borrelia burgdorferi, which causes Lyme disease, also transmits B microti. Conversely, HIV infection can exacerbate Lyme disease or babesiosis.
The tests showing B microti were the clincher for the clinicians, who started treatment with fluids, atovaquone, and azithromycin. The patient recovered completely. Repeat HIV serology was negative.
The authors of the report note that babesiosis can be a life-threatening infection in patients with reduced immunity. It is possible that, like malaria and HIV serologies, Babesia and HIV serologies cross-react, the clinicians say. Thus, it is important to screen for both in both infections.
This is the first case, to the clinician’s knowledge, of HIV associated with active babesiosis
According to the CDC, the number of reported tickborne diseases more than doubled between 2004-2016 and accounted for > 60% of all reported mosquito-borne, tickborne, and fleaborne disease cases. Which is why it is important to keep an eye out for anyone who has a history of being in a tick-promoting environment. Clinicians from Lehigh Valley Health Network Pocono and Geisinger Commonwealth School of Medicine, both in East Stroudsburg, Pennsylvania, report on a patient whose diagnosis turned on that fact.
The patient, a 71-year-old man, had fever, weakness, headaches, near syncope, and nausea for 4 days. He also had not been eating well.
A complete blood count showed pancytopenia with an excess of band cells, an indicator of inflammation and infection. The patient’s aspartate transaminase levels were elevated. The diagnostic dilemma centered on these findings: Serology tests for HIV 1 and 2 were positive, and a peripheral blood smear showed 0.5% parasitemia consistent with Babesia microti. Both babesiosis and HIV were among the possible diagnoses. Two important factors the clinicians had to consider: The patient had recently been bitten by ticks and was homosexual.
The clinicians note that a variety of infections can lead to false-positive HIV serology, such as malaria, Mycobacterium tuberculosis or Rickettsia species, influenza and hepatitis B vaccinations. Moreover, the Ixodes tick, the same vector that transmits Borrelia burgdorferi, which causes Lyme disease, also transmits B microti. Conversely, HIV infection can exacerbate Lyme disease or babesiosis.
The tests showing B microti were the clincher for the clinicians, who started treatment with fluids, atovaquone, and azithromycin. The patient recovered completely. Repeat HIV serology was negative.
The authors of the report note that babesiosis can be a life-threatening infection in patients with reduced immunity. It is possible that, like malaria and HIV serologies, Babesia and HIV serologies cross-react, the clinicians say. Thus, it is important to screen for both in both infections.
This is the first case, to the clinician’s knowledge, of HIV associated with active babesiosis
According to the CDC, the number of reported tickborne diseases more than doubled between 2004-2016 and accounted for > 60% of all reported mosquito-borne, tickborne, and fleaborne disease cases. Which is why it is important to keep an eye out for anyone who has a history of being in a tick-promoting environment. Clinicians from Lehigh Valley Health Network Pocono and Geisinger Commonwealth School of Medicine, both in East Stroudsburg, Pennsylvania, report on a patient whose diagnosis turned on that fact.
The patient, a 71-year-old man, had fever, weakness, headaches, near syncope, and nausea for 4 days. He also had not been eating well.
A complete blood count showed pancytopenia with an excess of band cells, an indicator of inflammation and infection. The patient’s aspartate transaminase levels were elevated. The diagnostic dilemma centered on these findings: Serology tests for HIV 1 and 2 were positive, and a peripheral blood smear showed 0.5% parasitemia consistent with Babesia microti. Both babesiosis and HIV were among the possible diagnoses. Two important factors the clinicians had to consider: The patient had recently been bitten by ticks and was homosexual.
The clinicians note that a variety of infections can lead to false-positive HIV serology, such as malaria, Mycobacterium tuberculosis or Rickettsia species, influenza and hepatitis B vaccinations. Moreover, the Ixodes tick, the same vector that transmits Borrelia burgdorferi, which causes Lyme disease, also transmits B microti. Conversely, HIV infection can exacerbate Lyme disease or babesiosis.
The tests showing B microti were the clincher for the clinicians, who started treatment with fluids, atovaquone, and azithromycin. The patient recovered completely. Repeat HIV serology was negative.
The authors of the report note that babesiosis can be a life-threatening infection in patients with reduced immunity. It is possible that, like malaria and HIV serologies, Babesia and HIV serologies cross-react, the clinicians say. Thus, it is important to screen for both in both infections.
This is the first case, to the clinician’s knowledge, of HIV associated with active babesiosis
Zero HIV transmission rate when viral load suppressed
according to a new paper published in the Lancet.
The prospective observational PARTNER2 study (Lancet 2019 May 2. doi: 10.1016/S0140-6736[19]304018-0) followed 782 serodiscordant gay couples from 14 European countries, where the HIV-positive partner had to be virally suppressed to below 200 copies of HIV-1 RNA per milliliter, and the couple was engaging in condomless sex without using pre- or postexposure prophylaxis.
After a median follow-up of 2 years, there was not a single case of HIV transmission between couples, representing a transmission rate of zero. The study did record 15 new HIV infections during follow-up but these were all phylogenetically linked to sources other than the HIV-positive partner.
The HIV-positive partners had been on antiretroviral therapy for a median of 4.3 years – most on regimens of three or more drugs – with high levels of adherence. During the follow-up period, 37 HIV-positive partners (5%) reported missing their antiretroviral therapy for more than 4 consecutive days.
“Our results give equivalence of evidence for gay men as for heterosexual couples and indicate that the risk of HIV transmission when HIV viral load is suppressed is effectively zero for both anal and vaginal sex,” wrote Dr. Alison J. Rodger of the Institute for Global Health at University College London, and coauthors. “These findings emphasize the importance of regular monitoring to ensure HIV viral load remains suppressed and supporting HIV-positive people with long-term adherence.”
Around one-quarter of the HIV-negative men and 27% of the HIV-positive men reported a sexually-transmitted infection during follow-up; the most common infections were syphilis, gonorrhea, and chlamydia.
The study noted six additional cases of seroconversion of HIV-negative partners. However, these occurred outside the eligible couple-years of the follow-up period. They were ineligible due to no questionnaire about sexual behavior having been completed by either partner in the couple, a lack of condomless sex between the couple, use of postexposure prophylaxis, or a lack of viral load measurement for the HIV-positive partner in the past year.
The authors did note that the study population was predominantly white and with a median age of 38 years, while most HIV transmission occurs in young people aged under 25 years.
“The results from the PARTNER studies support wider dissemination of the message of the U=U [Undetectable equals Untransmittable] campaign that risk of transmission of HIV in the context of virally suppressive ART is zero,” they wrote.
The study was supported by the National Institute for Health Research, the British HIV Association, the Danish National Research Foundation, ViiV Healthcare, Gilead Sciences, Augustinus Fonden, and A P Møller Fonden. Fourteen authors declared grants, personal fees and other support from the pharmaceutical sector.
SOURCE: Rodger A et al. Lancet 2019, May 2. doi: 10.1016/S0140-6736[19]304018-0.
The results of the PARTNER and PARTNER2 trials show that timely diagnosis and effective treatment can virtually eliminate the risk of HIV transmission. However, access to HIV testing and care is not always easy, and fear, stigma, homophobia, and other forces continue to limit access to HIV treatment. This study also highlights the impact of sexual relations outside the bounds of a couple’s relationship.
While the use of preexposure prophylaxis was a criterion for exclusion from this study, this intervention should also be recognized as an important part of HIV prevention. A recent survey found that men who have sex with men are more likely to trust preexposure prophylaxis for HIV prevention than antiretroviral therapy.
Dr. Myron S Cohen is from the departments of medicine, microbiology, immunology, and epidemiology, University of North Carolina at Chapel Hill, and the UNC Institute for Global Health and Infectious Diseases in Chapel Hill. These comments are adapted from an editorial (Lancet 2019, May 2. doi: 10.1016/S0140-6736[19]30701-9). Dr. Cohen reported advisory board travel fees from Merck and Gilead unrelated to this work.
The results of the PARTNER and PARTNER2 trials show that timely diagnosis and effective treatment can virtually eliminate the risk of HIV transmission. However, access to HIV testing and care is not always easy, and fear, stigma, homophobia, and other forces continue to limit access to HIV treatment. This study also highlights the impact of sexual relations outside the bounds of a couple’s relationship.
While the use of preexposure prophylaxis was a criterion for exclusion from this study, this intervention should also be recognized as an important part of HIV prevention. A recent survey found that men who have sex with men are more likely to trust preexposure prophylaxis for HIV prevention than antiretroviral therapy.
Dr. Myron S Cohen is from the departments of medicine, microbiology, immunology, and epidemiology, University of North Carolina at Chapel Hill, and the UNC Institute for Global Health and Infectious Diseases in Chapel Hill. These comments are adapted from an editorial (Lancet 2019, May 2. doi: 10.1016/S0140-6736[19]30701-9). Dr. Cohen reported advisory board travel fees from Merck and Gilead unrelated to this work.
The results of the PARTNER and PARTNER2 trials show that timely diagnosis and effective treatment can virtually eliminate the risk of HIV transmission. However, access to HIV testing and care is not always easy, and fear, stigma, homophobia, and other forces continue to limit access to HIV treatment. This study also highlights the impact of sexual relations outside the bounds of a couple’s relationship.
While the use of preexposure prophylaxis was a criterion for exclusion from this study, this intervention should also be recognized as an important part of HIV prevention. A recent survey found that men who have sex with men are more likely to trust preexposure prophylaxis for HIV prevention than antiretroviral therapy.
Dr. Myron S Cohen is from the departments of medicine, microbiology, immunology, and epidemiology, University of North Carolina at Chapel Hill, and the UNC Institute for Global Health and Infectious Diseases in Chapel Hill. These comments are adapted from an editorial (Lancet 2019, May 2. doi: 10.1016/S0140-6736[19]30701-9). Dr. Cohen reported advisory board travel fees from Merck and Gilead unrelated to this work.
according to a new paper published in the Lancet.
The prospective observational PARTNER2 study (Lancet 2019 May 2. doi: 10.1016/S0140-6736[19]304018-0) followed 782 serodiscordant gay couples from 14 European countries, where the HIV-positive partner had to be virally suppressed to below 200 copies of HIV-1 RNA per milliliter, and the couple was engaging in condomless sex without using pre- or postexposure prophylaxis.
After a median follow-up of 2 years, there was not a single case of HIV transmission between couples, representing a transmission rate of zero. The study did record 15 new HIV infections during follow-up but these were all phylogenetically linked to sources other than the HIV-positive partner.
The HIV-positive partners had been on antiretroviral therapy for a median of 4.3 years – most on regimens of three or more drugs – with high levels of adherence. During the follow-up period, 37 HIV-positive partners (5%) reported missing their antiretroviral therapy for more than 4 consecutive days.
“Our results give equivalence of evidence for gay men as for heterosexual couples and indicate that the risk of HIV transmission when HIV viral load is suppressed is effectively zero for both anal and vaginal sex,” wrote Dr. Alison J. Rodger of the Institute for Global Health at University College London, and coauthors. “These findings emphasize the importance of regular monitoring to ensure HIV viral load remains suppressed and supporting HIV-positive people with long-term adherence.”
Around one-quarter of the HIV-negative men and 27% of the HIV-positive men reported a sexually-transmitted infection during follow-up; the most common infections were syphilis, gonorrhea, and chlamydia.
The study noted six additional cases of seroconversion of HIV-negative partners. However, these occurred outside the eligible couple-years of the follow-up period. They were ineligible due to no questionnaire about sexual behavior having been completed by either partner in the couple, a lack of condomless sex between the couple, use of postexposure prophylaxis, or a lack of viral load measurement for the HIV-positive partner in the past year.
The authors did note that the study population was predominantly white and with a median age of 38 years, while most HIV transmission occurs in young people aged under 25 years.
“The results from the PARTNER studies support wider dissemination of the message of the U=U [Undetectable equals Untransmittable] campaign that risk of transmission of HIV in the context of virally suppressive ART is zero,” they wrote.
The study was supported by the National Institute for Health Research, the British HIV Association, the Danish National Research Foundation, ViiV Healthcare, Gilead Sciences, Augustinus Fonden, and A P Møller Fonden. Fourteen authors declared grants, personal fees and other support from the pharmaceutical sector.
SOURCE: Rodger A et al. Lancet 2019, May 2. doi: 10.1016/S0140-6736[19]304018-0.
according to a new paper published in the Lancet.
The prospective observational PARTNER2 study (Lancet 2019 May 2. doi: 10.1016/S0140-6736[19]304018-0) followed 782 serodiscordant gay couples from 14 European countries, where the HIV-positive partner had to be virally suppressed to below 200 copies of HIV-1 RNA per milliliter, and the couple was engaging in condomless sex without using pre- or postexposure prophylaxis.
After a median follow-up of 2 years, there was not a single case of HIV transmission between couples, representing a transmission rate of zero. The study did record 15 new HIV infections during follow-up but these were all phylogenetically linked to sources other than the HIV-positive partner.
The HIV-positive partners had been on antiretroviral therapy for a median of 4.3 years – most on regimens of three or more drugs – with high levels of adherence. During the follow-up period, 37 HIV-positive partners (5%) reported missing their antiretroviral therapy for more than 4 consecutive days.
“Our results give equivalence of evidence for gay men as for heterosexual couples and indicate that the risk of HIV transmission when HIV viral load is suppressed is effectively zero for both anal and vaginal sex,” wrote Dr. Alison J. Rodger of the Institute for Global Health at University College London, and coauthors. “These findings emphasize the importance of regular monitoring to ensure HIV viral load remains suppressed and supporting HIV-positive people with long-term adherence.”
Around one-quarter of the HIV-negative men and 27% of the HIV-positive men reported a sexually-transmitted infection during follow-up; the most common infections were syphilis, gonorrhea, and chlamydia.
The study noted six additional cases of seroconversion of HIV-negative partners. However, these occurred outside the eligible couple-years of the follow-up period. They were ineligible due to no questionnaire about sexual behavior having been completed by either partner in the couple, a lack of condomless sex between the couple, use of postexposure prophylaxis, or a lack of viral load measurement for the HIV-positive partner in the past year.
The authors did note that the study population was predominantly white and with a median age of 38 years, while most HIV transmission occurs in young people aged under 25 years.
“The results from the PARTNER studies support wider dissemination of the message of the U=U [Undetectable equals Untransmittable] campaign that risk of transmission of HIV in the context of virally suppressive ART is zero,” they wrote.
The study was supported by the National Institute for Health Research, the British HIV Association, the Danish National Research Foundation, ViiV Healthcare, Gilead Sciences, Augustinus Fonden, and A P Møller Fonden. Fourteen authors declared grants, personal fees and other support from the pharmaceutical sector.
SOURCE: Rodger A et al. Lancet 2019, May 2. doi: 10.1016/S0140-6736[19]304018-0.
FROM THE LANCET
ICYMI: Anti-CD4 antibody maintains viral suppression in HIV patients post ART
according to results from a small, nonrandomized, open-label, phase 2 trial published in the New England Journal of Medicine (2019 Apr 17. doi: 10.1056/NEJMoa1802264).
We reported on this story at the 2017 Conference on Retroviruses & Opportunistic Infections before it was published in the journal. Find our coverage at the link below.
according to results from a small, nonrandomized, open-label, phase 2 trial published in the New England Journal of Medicine (2019 Apr 17. doi: 10.1056/NEJMoa1802264).
We reported on this story at the 2017 Conference on Retroviruses & Opportunistic Infections before it was published in the journal. Find our coverage at the link below.
according to results from a small, nonrandomized, open-label, phase 2 trial published in the New England Journal of Medicine (2019 Apr 17. doi: 10.1056/NEJMoa1802264).
We reported on this story at the 2017 Conference on Retroviruses & Opportunistic Infections before it was published in the journal. Find our coverage at the link below.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE