User login
ART traces in donor samples show risk to blood supply
SAN ANTONIO, TEX. – Some HIV-positive individuals on antiretroviral therapy continue to attempt blood donation, for reasons that may include ignorance of the risks of transmission, test-seeking behavior, or other unknown motivations to donate despite knowing their viral status, caution investigators who monitor the nation’s blood supply.
Additionally, evidence of pre-exposure prophylaxis (PrEP) with antiretroviral agents (ARV) in HIV-negative donors raises concerns about possible risk of transmission from HIV-breakthrough infections, reported Brian S. Custer, PhD, of Vitalant Research Institute.
Dr. Custer and colleagues in the U.S. Transfusion Transmissible Infections Monitoring System (TTIMS) evaluated plasma samples from four large blood collection organizations and found metabolites of drugs typically used in ARV regimens, at concentrations indicating that they had been taken within a week of blood donation.
“What are the motivations of these individuals who are ARV-positive and HIV-positive? Are they using the blood center as a place to monitor their infection status? We just do not know the answer to that, but it does bring back this issue of whether there a form of test seeking going on here,” he said at the annual meeting of AABB, the group formerly known as the American Association of Blood Banks.
The findings suggest that current donor health questionnaires and pre-donation screening are not adequate for ascertaining disclosure of HIV status or behaviors that could put the safety of the blood supply in doubt.
Does ‘U’ really equal ‘U’?
The public health message that “undetectable” equals “untransmittable” may help to prevent new infections, but may not translate into transfusion safety, Dr. Custer said.
Current HIV treatment guidelines recommend that infected individuals start on ARV immediately upon receiving a diagnosis. ARV drugs both suppress viremia and alter biomarkers of HIV progression, and may delay the time to antibody seroconversion, which could affect the ability to detect HIV through blood donation screening.
To see whether there is renewed cause for concern about safety of the blood supply, the TTIMS investigators tested for ARV metabolites in donated blood samples as a surrogate for HIV infection.
They looked at 299 HIV-positive plasma samples and 300 samples negative for all testable infections using liquid chromatography–mass spectometry for metabolites of 13 ARVs.
No ARV metabolites could be detected in the HIV-negative samples, but in 299 samples from 463 HIV-positive donors they found that 46 (15.4%) tested positive for ARVs. Of these 46 specimens, 43 were from first-time donors, and 34 were from males. Three samples were from repeat donors.
In all, 41 of the 46 ARV-positive donors would have been considered as potential “elite controllers” – HIV-infected individuals with no detectable viral loads in the absence of therapy – based on HIV-positive serology but negative nucleic acid test (NAT) results.
Samples from five first-time donors tested positive for HIV by both serology and NAT, and also contained ARV metabolites, suggesting that the donors had started ARVs recently, had suboptimal therapy, or were poorly adherent to their regimens.
PrEP use among HIV-negative donors
The investigators also looked at PrEP use in 1494 samples from first-time male donors that tested negative for all markers routinely screened: HIV 1/2, hepatitis B virus, hepatitis C virus, HTLV-I/II, West Nile virus, ZIKV, Treponema pallidum and Trypanosoma cruzi.
The donors came from Boston, Los Angeles, Miami, New York, San Francisco, and Washington, all cities with elevated HIV prevalence and active PrEP rollout campaigns.
They tested for analytes to emtricitabine and tenofovir, the two ARV components of the PrEP drug Truvada, and found that nine samples (0.6%) tested positive for PrEP. Of these, three donors had taken PrEP approximately 1 day before donation, two took it 2 days before, and four took it about 4 days before donation.
“There is quite a bit of concern about what this might mean for blood safety.” Dr. Custer said. “There’s a possibility of a breakthrough infection if someone is not PrEP adherent, and would we be able to detect them? Could this be an additional indicator for the risk of transfusion-transmissible infections? Could we identify subgroups of PrEP use in the donor population, and would we see any evidence?”
Mitigation Efforts
The National Heart, Lung, and Blood Institute is supporting a 7-year research project to study the potential impact of antiretroviral therapy and PrEP in the United States and Brazil under the Recipient Epidemiology and Donor Evaluation Study-IV-Pediatric (REDS-IV-P) project.
In addition, an AABB task force has drafted new questions for the donor health questionnaire intended to focus the attention of potential donors on PrEP, postexposure prophylaxis (PEP), and antiretroviral therapy.
In addition to asking about the use of medications on the deferral list, the proposed additions would ask, “In the past 16 weeks, have you taken any medication to prevent an HIV infection?” and “Have you EVER taken any medication to treat an HIV infection?”
TTIMS is supported by the U.S. Food and Drug Administration, NHLBI, and the Office of the Assistant Secretary for Health. Dr. Custer and all coinvestigators reported having no relevant disclosures.
SOURCE: : wit AABB 2019. Abstract PL5-MN4-32
SAN ANTONIO, TEX. – Some HIV-positive individuals on antiretroviral therapy continue to attempt blood donation, for reasons that may include ignorance of the risks of transmission, test-seeking behavior, or other unknown motivations to donate despite knowing their viral status, caution investigators who monitor the nation’s blood supply.
Additionally, evidence of pre-exposure prophylaxis (PrEP) with antiretroviral agents (ARV) in HIV-negative donors raises concerns about possible risk of transmission from HIV-breakthrough infections, reported Brian S. Custer, PhD, of Vitalant Research Institute.
Dr. Custer and colleagues in the U.S. Transfusion Transmissible Infections Monitoring System (TTIMS) evaluated plasma samples from four large blood collection organizations and found metabolites of drugs typically used in ARV regimens, at concentrations indicating that they had been taken within a week of blood donation.
“What are the motivations of these individuals who are ARV-positive and HIV-positive? Are they using the blood center as a place to monitor their infection status? We just do not know the answer to that, but it does bring back this issue of whether there a form of test seeking going on here,” he said at the annual meeting of AABB, the group formerly known as the American Association of Blood Banks.
The findings suggest that current donor health questionnaires and pre-donation screening are not adequate for ascertaining disclosure of HIV status or behaviors that could put the safety of the blood supply in doubt.
Does ‘U’ really equal ‘U’?
The public health message that “undetectable” equals “untransmittable” may help to prevent new infections, but may not translate into transfusion safety, Dr. Custer said.
Current HIV treatment guidelines recommend that infected individuals start on ARV immediately upon receiving a diagnosis. ARV drugs both suppress viremia and alter biomarkers of HIV progression, and may delay the time to antibody seroconversion, which could affect the ability to detect HIV through blood donation screening.
To see whether there is renewed cause for concern about safety of the blood supply, the TTIMS investigators tested for ARV metabolites in donated blood samples as a surrogate for HIV infection.
They looked at 299 HIV-positive plasma samples and 300 samples negative for all testable infections using liquid chromatography–mass spectometry for metabolites of 13 ARVs.
No ARV metabolites could be detected in the HIV-negative samples, but in 299 samples from 463 HIV-positive donors they found that 46 (15.4%) tested positive for ARVs. Of these 46 specimens, 43 were from first-time donors, and 34 were from males. Three samples were from repeat donors.
In all, 41 of the 46 ARV-positive donors would have been considered as potential “elite controllers” – HIV-infected individuals with no detectable viral loads in the absence of therapy – based on HIV-positive serology but negative nucleic acid test (NAT) results.
Samples from five first-time donors tested positive for HIV by both serology and NAT, and also contained ARV metabolites, suggesting that the donors had started ARVs recently, had suboptimal therapy, or were poorly adherent to their regimens.
PrEP use among HIV-negative donors
The investigators also looked at PrEP use in 1494 samples from first-time male donors that tested negative for all markers routinely screened: HIV 1/2, hepatitis B virus, hepatitis C virus, HTLV-I/II, West Nile virus, ZIKV, Treponema pallidum and Trypanosoma cruzi.
The donors came from Boston, Los Angeles, Miami, New York, San Francisco, and Washington, all cities with elevated HIV prevalence and active PrEP rollout campaigns.
They tested for analytes to emtricitabine and tenofovir, the two ARV components of the PrEP drug Truvada, and found that nine samples (0.6%) tested positive for PrEP. Of these, three donors had taken PrEP approximately 1 day before donation, two took it 2 days before, and four took it about 4 days before donation.
“There is quite a bit of concern about what this might mean for blood safety.” Dr. Custer said. “There’s a possibility of a breakthrough infection if someone is not PrEP adherent, and would we be able to detect them? Could this be an additional indicator for the risk of transfusion-transmissible infections? Could we identify subgroups of PrEP use in the donor population, and would we see any evidence?”
Mitigation Efforts
The National Heart, Lung, and Blood Institute is supporting a 7-year research project to study the potential impact of antiretroviral therapy and PrEP in the United States and Brazil under the Recipient Epidemiology and Donor Evaluation Study-IV-Pediatric (REDS-IV-P) project.
In addition, an AABB task force has drafted new questions for the donor health questionnaire intended to focus the attention of potential donors on PrEP, postexposure prophylaxis (PEP), and antiretroviral therapy.
In addition to asking about the use of medications on the deferral list, the proposed additions would ask, “In the past 16 weeks, have you taken any medication to prevent an HIV infection?” and “Have you EVER taken any medication to treat an HIV infection?”
TTIMS is supported by the U.S. Food and Drug Administration, NHLBI, and the Office of the Assistant Secretary for Health. Dr. Custer and all coinvestigators reported having no relevant disclosures.
SOURCE: : wit AABB 2019. Abstract PL5-MN4-32
SAN ANTONIO, TEX. – Some HIV-positive individuals on antiretroviral therapy continue to attempt blood donation, for reasons that may include ignorance of the risks of transmission, test-seeking behavior, or other unknown motivations to donate despite knowing their viral status, caution investigators who monitor the nation’s blood supply.
Additionally, evidence of pre-exposure prophylaxis (PrEP) with antiretroviral agents (ARV) in HIV-negative donors raises concerns about possible risk of transmission from HIV-breakthrough infections, reported Brian S. Custer, PhD, of Vitalant Research Institute.
Dr. Custer and colleagues in the U.S. Transfusion Transmissible Infections Monitoring System (TTIMS) evaluated plasma samples from four large blood collection organizations and found metabolites of drugs typically used in ARV regimens, at concentrations indicating that they had been taken within a week of blood donation.
“What are the motivations of these individuals who are ARV-positive and HIV-positive? Are they using the blood center as a place to monitor their infection status? We just do not know the answer to that, but it does bring back this issue of whether there a form of test seeking going on here,” he said at the annual meeting of AABB, the group formerly known as the American Association of Blood Banks.
The findings suggest that current donor health questionnaires and pre-donation screening are not adequate for ascertaining disclosure of HIV status or behaviors that could put the safety of the blood supply in doubt.
Does ‘U’ really equal ‘U’?
The public health message that “undetectable” equals “untransmittable” may help to prevent new infections, but may not translate into transfusion safety, Dr. Custer said.
Current HIV treatment guidelines recommend that infected individuals start on ARV immediately upon receiving a diagnosis. ARV drugs both suppress viremia and alter biomarkers of HIV progression, and may delay the time to antibody seroconversion, which could affect the ability to detect HIV through blood donation screening.
To see whether there is renewed cause for concern about safety of the blood supply, the TTIMS investigators tested for ARV metabolites in donated blood samples as a surrogate for HIV infection.
They looked at 299 HIV-positive plasma samples and 300 samples negative for all testable infections using liquid chromatography–mass spectometry for metabolites of 13 ARVs.
No ARV metabolites could be detected in the HIV-negative samples, but in 299 samples from 463 HIV-positive donors they found that 46 (15.4%) tested positive for ARVs. Of these 46 specimens, 43 were from first-time donors, and 34 were from males. Three samples were from repeat donors.
In all, 41 of the 46 ARV-positive donors would have been considered as potential “elite controllers” – HIV-infected individuals with no detectable viral loads in the absence of therapy – based on HIV-positive serology but negative nucleic acid test (NAT) results.
Samples from five first-time donors tested positive for HIV by both serology and NAT, and also contained ARV metabolites, suggesting that the donors had started ARVs recently, had suboptimal therapy, or were poorly adherent to their regimens.
PrEP use among HIV-negative donors
The investigators also looked at PrEP use in 1494 samples from first-time male donors that tested negative for all markers routinely screened: HIV 1/2, hepatitis B virus, hepatitis C virus, HTLV-I/II, West Nile virus, ZIKV, Treponema pallidum and Trypanosoma cruzi.
The donors came from Boston, Los Angeles, Miami, New York, San Francisco, and Washington, all cities with elevated HIV prevalence and active PrEP rollout campaigns.
They tested for analytes to emtricitabine and tenofovir, the two ARV components of the PrEP drug Truvada, and found that nine samples (0.6%) tested positive for PrEP. Of these, three donors had taken PrEP approximately 1 day before donation, two took it 2 days before, and four took it about 4 days before donation.
“There is quite a bit of concern about what this might mean for blood safety.” Dr. Custer said. “There’s a possibility of a breakthrough infection if someone is not PrEP adherent, and would we be able to detect them? Could this be an additional indicator for the risk of transfusion-transmissible infections? Could we identify subgroups of PrEP use in the donor population, and would we see any evidence?”
Mitigation Efforts
The National Heart, Lung, and Blood Institute is supporting a 7-year research project to study the potential impact of antiretroviral therapy and PrEP in the United States and Brazil under the Recipient Epidemiology and Donor Evaluation Study-IV-Pediatric (REDS-IV-P) project.
In addition, an AABB task force has drafted new questions for the donor health questionnaire intended to focus the attention of potential donors on PrEP, postexposure prophylaxis (PEP), and antiretroviral therapy.
In addition to asking about the use of medications on the deferral list, the proposed additions would ask, “In the past 16 weeks, have you taken any medication to prevent an HIV infection?” and “Have you EVER taken any medication to treat an HIV infection?”
TTIMS is supported by the U.S. Food and Drug Administration, NHLBI, and the Office of the Assistant Secretary for Health. Dr. Custer and all coinvestigators reported having no relevant disclosures.
SOURCE: : wit AABB 2019. Abstract PL5-MN4-32
REPORTING FROM AABB 2019
NIH seeks gene-based cures for HIV, sickle cell disease
The National Institutes of Health and the Bill & Melinda Gates Foundation have announced that they plan to invest $100 million each over the next 4 years to develop affordable, gene-based cures for sickle cell disease (SCD) and HIV.
The initiative follows an announcement from President Trump that set a goal of ending the HIV epidemic in the United States in the next 10 years, seeking to reduce the number of diagnoses by 90% by 2030. The Trump administration has also identified SCD as an “intractable health challenge with the potential for dramatic advances in the coming years,” the NIH said in a statement.
Gene-based therapy has become a reality in recent years thanks to dramatic advances, but the cost is prohibitive in many parts of the world. “The collaboration between the NIH and the Gates Foundation sets out a bold goal of advancing safe, effective, and durable gene-based cures to clinical trials in the United States and relevant countries in sub-Saharan Africa within the next 7-10 years. The ultimate goal is to scale and implement these treatments globally in areas hardest hit by these diseases,” the NIH said.
Both diseases are a significant burden on low- and middle-income countries, as 95% of the 38 million people living with HIV globally are in the developing world, with 67% living in sub-Saharan Africa; about half of the HIV-infected population receives no treatment for the disease. An estimated 15 million children will be born with SCD over the next 30 years, with three-quarters of those births occurring in sub-Saharan Africa. About 50%-90% of children born with SCD will die before age 5 years.
The collaboration will focus on coordination in two areas: identifying potential candidate cures for SCD and HIV for preclinical and clinical evaluation, and defining long-term opportunities to work together and with African partners on advancing promising candidates to late-phase clinical trials, with funding to be determined as candidates progress.
“In recent years, gene-based treatments have been groundbreaking for rare genetic disorders and infectious diseases. While these treatments are exciting, people in low- and middle-income countries do not have access to these breakthroughs. By working with the NIH and scientists across Africa, we aim to ensure these approaches will improve the lives of those most in need and bring the incredible promise of gene-based treatments to the world of public health,” said Trevor Mundel, MD, PhD, president of the global health program at the Bill & Melinda Gates Foundation.
The National Institutes of Health and the Bill & Melinda Gates Foundation have announced that they plan to invest $100 million each over the next 4 years to develop affordable, gene-based cures for sickle cell disease (SCD) and HIV.
The initiative follows an announcement from President Trump that set a goal of ending the HIV epidemic in the United States in the next 10 years, seeking to reduce the number of diagnoses by 90% by 2030. The Trump administration has also identified SCD as an “intractable health challenge with the potential for dramatic advances in the coming years,” the NIH said in a statement.
Gene-based therapy has become a reality in recent years thanks to dramatic advances, but the cost is prohibitive in many parts of the world. “The collaboration between the NIH and the Gates Foundation sets out a bold goal of advancing safe, effective, and durable gene-based cures to clinical trials in the United States and relevant countries in sub-Saharan Africa within the next 7-10 years. The ultimate goal is to scale and implement these treatments globally in areas hardest hit by these diseases,” the NIH said.
Both diseases are a significant burden on low- and middle-income countries, as 95% of the 38 million people living with HIV globally are in the developing world, with 67% living in sub-Saharan Africa; about half of the HIV-infected population receives no treatment for the disease. An estimated 15 million children will be born with SCD over the next 30 years, with three-quarters of those births occurring in sub-Saharan Africa. About 50%-90% of children born with SCD will die before age 5 years.
The collaboration will focus on coordination in two areas: identifying potential candidate cures for SCD and HIV for preclinical and clinical evaluation, and defining long-term opportunities to work together and with African partners on advancing promising candidates to late-phase clinical trials, with funding to be determined as candidates progress.
“In recent years, gene-based treatments have been groundbreaking for rare genetic disorders and infectious diseases. While these treatments are exciting, people in low- and middle-income countries do not have access to these breakthroughs. By working with the NIH and scientists across Africa, we aim to ensure these approaches will improve the lives of those most in need and bring the incredible promise of gene-based treatments to the world of public health,” said Trevor Mundel, MD, PhD, president of the global health program at the Bill & Melinda Gates Foundation.
The National Institutes of Health and the Bill & Melinda Gates Foundation have announced that they plan to invest $100 million each over the next 4 years to develop affordable, gene-based cures for sickle cell disease (SCD) and HIV.
The initiative follows an announcement from President Trump that set a goal of ending the HIV epidemic in the United States in the next 10 years, seeking to reduce the number of diagnoses by 90% by 2030. The Trump administration has also identified SCD as an “intractable health challenge with the potential for dramatic advances in the coming years,” the NIH said in a statement.
Gene-based therapy has become a reality in recent years thanks to dramatic advances, but the cost is prohibitive in many parts of the world. “The collaboration between the NIH and the Gates Foundation sets out a bold goal of advancing safe, effective, and durable gene-based cures to clinical trials in the United States and relevant countries in sub-Saharan Africa within the next 7-10 years. The ultimate goal is to scale and implement these treatments globally in areas hardest hit by these diseases,” the NIH said.
Both diseases are a significant burden on low- and middle-income countries, as 95% of the 38 million people living with HIV globally are in the developing world, with 67% living in sub-Saharan Africa; about half of the HIV-infected population receives no treatment for the disease. An estimated 15 million children will be born with SCD over the next 30 years, with three-quarters of those births occurring in sub-Saharan Africa. About 50%-90% of children born with SCD will die before age 5 years.
The collaboration will focus on coordination in two areas: identifying potential candidate cures for SCD and HIV for preclinical and clinical evaluation, and defining long-term opportunities to work together and with African partners on advancing promising candidates to late-phase clinical trials, with funding to be determined as candidates progress.
“In recent years, gene-based treatments have been groundbreaking for rare genetic disorders and infectious diseases. While these treatments are exciting, people in low- and middle-income countries do not have access to these breakthroughs. By working with the NIH and scientists across Africa, we aim to ensure these approaches will improve the lives of those most in need and bring the incredible promise of gene-based treatments to the world of public health,” said Trevor Mundel, MD, PhD, president of the global health program at the Bill & Melinda Gates Foundation.
HIV-Positive Kidney Transplantations Offer Hope
People with HIV could be a safe kidney donation source for other people with HIV, according to researchers from the National Institute of Allergy and Infectious Diseases (NIAID) and from the University of Cape Town, South Africa.
Their study followed 51 study participants with HIV who received kidney transplants from deceased donors with HIV in South Africa.
Five years after kidney transplantation, 83% of the South African cohort survived; 79% still had a functioning transplanted kidney. Those findings are similar to findings from a 2010 US NIAID-funded study, with kidneys from both living and deceased donors that reported an 88% survival rate and 74% kidney graft survival rate after 3 years.
All participants in the South African cohort were virally suppressed at the time of transplantation. The researchers did not observe any increases in viral load among those who adhered to antiretroviral therapy (ART). While 10 participants changed their ART regimens during the study, none did so because of drug resistance.
Deceased donors had strains of HIV genetically distinct from those of the transplant recipients. The investigators watched closely for signs of possible superinfections with strains of HIV that might be resistant to the recipient’s ART regimen. They identified only 1 potential case of transient superinfection, but further analyses determined that it was most likely residual virus carried over from the donor during the transplant and not a true sustained superinfection. “By using the most advanced laboratory techniques available, our team showed that HIV superinfection is of limited risk in these patients,” said study author Andrew Redd, PhD, of the NIAID Laboratory of Immunoregulation.
Such studies were illegal in the US before the HIV Organ Policy Equity (HOPE) Act passed in 2013. The law intended to increase the availability of organs for transplantation for people with HIV and permits US transplant teams with an approved research protocol to transplant organs from donors with HIV into qualified recipients with HIV and end-stage organ failure.
Two ongoing NIAID-funded clinical trials, the HOPE in Action Multicenter Kidney Study and the HOPE in Action Multicenter Liver Study, are comparing clinical outcomes among people living with HIV who receive organs from deceased donors with HIV to those who receive HIV-negative organs.
People with HIV could be a safe kidney donation source for other people with HIV, according to researchers from the National Institute of Allergy and Infectious Diseases (NIAID) and from the University of Cape Town, South Africa.
Their study followed 51 study participants with HIV who received kidney transplants from deceased donors with HIV in South Africa.
Five years after kidney transplantation, 83% of the South African cohort survived; 79% still had a functioning transplanted kidney. Those findings are similar to findings from a 2010 US NIAID-funded study, with kidneys from both living and deceased donors that reported an 88% survival rate and 74% kidney graft survival rate after 3 years.
All participants in the South African cohort were virally suppressed at the time of transplantation. The researchers did not observe any increases in viral load among those who adhered to antiretroviral therapy (ART). While 10 participants changed their ART regimens during the study, none did so because of drug resistance.
Deceased donors had strains of HIV genetically distinct from those of the transplant recipients. The investigators watched closely for signs of possible superinfections with strains of HIV that might be resistant to the recipient’s ART regimen. They identified only 1 potential case of transient superinfection, but further analyses determined that it was most likely residual virus carried over from the donor during the transplant and not a true sustained superinfection. “By using the most advanced laboratory techniques available, our team showed that HIV superinfection is of limited risk in these patients,” said study author Andrew Redd, PhD, of the NIAID Laboratory of Immunoregulation.
Such studies were illegal in the US before the HIV Organ Policy Equity (HOPE) Act passed in 2013. The law intended to increase the availability of organs for transplantation for people with HIV and permits US transplant teams with an approved research protocol to transplant organs from donors with HIV into qualified recipients with HIV and end-stage organ failure.
Two ongoing NIAID-funded clinical trials, the HOPE in Action Multicenter Kidney Study and the HOPE in Action Multicenter Liver Study, are comparing clinical outcomes among people living with HIV who receive organs from deceased donors with HIV to those who receive HIV-negative organs.
People with HIV could be a safe kidney donation source for other people with HIV, according to researchers from the National Institute of Allergy and Infectious Diseases (NIAID) and from the University of Cape Town, South Africa.
Their study followed 51 study participants with HIV who received kidney transplants from deceased donors with HIV in South Africa.
Five years after kidney transplantation, 83% of the South African cohort survived; 79% still had a functioning transplanted kidney. Those findings are similar to findings from a 2010 US NIAID-funded study, with kidneys from both living and deceased donors that reported an 88% survival rate and 74% kidney graft survival rate after 3 years.
All participants in the South African cohort were virally suppressed at the time of transplantation. The researchers did not observe any increases in viral load among those who adhered to antiretroviral therapy (ART). While 10 participants changed their ART regimens during the study, none did so because of drug resistance.
Deceased donors had strains of HIV genetically distinct from those of the transplant recipients. The investigators watched closely for signs of possible superinfections with strains of HIV that might be resistant to the recipient’s ART regimen. They identified only 1 potential case of transient superinfection, but further analyses determined that it was most likely residual virus carried over from the donor during the transplant and not a true sustained superinfection. “By using the most advanced laboratory techniques available, our team showed that HIV superinfection is of limited risk in these patients,” said study author Andrew Redd, PhD, of the NIAID Laboratory of Immunoregulation.
Such studies were illegal in the US before the HIV Organ Policy Equity (HOPE) Act passed in 2013. The law intended to increase the availability of organs for transplantation for people with HIV and permits US transplant teams with an approved research protocol to transplant organs from donors with HIV into qualified recipients with HIV and end-stage organ failure.
Two ongoing NIAID-funded clinical trials, the HOPE in Action Multicenter Kidney Study and the HOPE in Action Multicenter Liver Study, are comparing clinical outcomes among people living with HIV who receive organs from deceased donors with HIV to those who receive HIV-negative organs.
California bans “Pay for Delay,” promotes black maternal health, PrEP access
AB 824, the Pay for Delay bill, bans pharmaceutical companies from keeping cheaper generic drugs off the market. The bill prohibits agreements between brand name and generic drug manufacturers to delay the release of generic drugs, defining them as presumptively anticompetitive. A Federal Trade Commission study found that “these anticompetitive deals cost consumers and taxpayers $3.5 billion in higher drug costs every year,” according to a statement from the governor’s office.
The second bill, SB 464, is intended to improve black maternal health care. The bill is designed to reduce preventable maternal mortality among black women by requiring all perinatal health care providers to undergo implicit bias training to curb the effects of bias on maternal health and by improving data collection at the California Department of Public Health to better understand pregnancy-related deaths. “We know that black women have been dying at alarming rates during and after giving birth. The disproportionate effect of the maternal mortality rate on this community is a public health crisis and a major health equity issue. We must do everything in our power to take implicit bias out of the medical system – it is literally a matter of life and death,” said Gov. Newsom.
The third bill, SB 159, aims to facilitate the use of pre-exposure prophylaxis and postexposure prophylaxis against HIV infection. The bill allows pharmacists in the state to dispense PrEP and PEP without a physician’s prescription and prohibits insurance companies from requiring prior authorization for patients to obtain PrEP coverage. “All Californians deserve access to PrEP and PEP, two treatments that have transformed our fight against HIV and AIDS,” Gov. Newsom said in a statement.
AB 824, the Pay for Delay bill, bans pharmaceutical companies from keeping cheaper generic drugs off the market. The bill prohibits agreements between brand name and generic drug manufacturers to delay the release of generic drugs, defining them as presumptively anticompetitive. A Federal Trade Commission study found that “these anticompetitive deals cost consumers and taxpayers $3.5 billion in higher drug costs every year,” according to a statement from the governor’s office.
The second bill, SB 464, is intended to improve black maternal health care. The bill is designed to reduce preventable maternal mortality among black women by requiring all perinatal health care providers to undergo implicit bias training to curb the effects of bias on maternal health and by improving data collection at the California Department of Public Health to better understand pregnancy-related deaths. “We know that black women have been dying at alarming rates during and after giving birth. The disproportionate effect of the maternal mortality rate on this community is a public health crisis and a major health equity issue. We must do everything in our power to take implicit bias out of the medical system – it is literally a matter of life and death,” said Gov. Newsom.
The third bill, SB 159, aims to facilitate the use of pre-exposure prophylaxis and postexposure prophylaxis against HIV infection. The bill allows pharmacists in the state to dispense PrEP and PEP without a physician’s prescription and prohibits insurance companies from requiring prior authorization for patients to obtain PrEP coverage. “All Californians deserve access to PrEP and PEP, two treatments that have transformed our fight against HIV and AIDS,” Gov. Newsom said in a statement.
AB 824, the Pay for Delay bill, bans pharmaceutical companies from keeping cheaper generic drugs off the market. The bill prohibits agreements between brand name and generic drug manufacturers to delay the release of generic drugs, defining them as presumptively anticompetitive. A Federal Trade Commission study found that “these anticompetitive deals cost consumers and taxpayers $3.5 billion in higher drug costs every year,” according to a statement from the governor’s office.
The second bill, SB 464, is intended to improve black maternal health care. The bill is designed to reduce preventable maternal mortality among black women by requiring all perinatal health care providers to undergo implicit bias training to curb the effects of bias on maternal health and by improving data collection at the California Department of Public Health to better understand pregnancy-related deaths. “We know that black women have been dying at alarming rates during and after giving birth. The disproportionate effect of the maternal mortality rate on this community is a public health crisis and a major health equity issue. We must do everything in our power to take implicit bias out of the medical system – it is literally a matter of life and death,” said Gov. Newsom.
The third bill, SB 159, aims to facilitate the use of pre-exposure prophylaxis and postexposure prophylaxis against HIV infection. The bill allows pharmacists in the state to dispense PrEP and PEP without a physician’s prescription and prohibits insurance companies from requiring prior authorization for patients to obtain PrEP coverage. “All Californians deserve access to PrEP and PEP, two treatments that have transformed our fight against HIV and AIDS,” Gov. Newsom said in a statement.
FDA approves Descovy as HIV PrEP for men and transgender women who have sex with men
The decision, backing the earlier recommendation of the FDA’s Antimicrobial Drugs Advisory Committee, was based upon results from DISCOVER, a pivotal, multiyear, global phase 3 clinical trial that evaluated the safety and efficacy of Descovy (emtricitabine 200 mg and tenofovir alafenamide 25-mg tablets for PrEP, compared with Truvada (emtricitabine 200 mg and tenofovir disoproxil fumarate 300-mg tablets).
DISCOVER included more than 5,300 adult cisgender men who have sex with men or transgender women who have sex with men.
In the trial, Descovy achieved noninferiority to Truvada.
Descovy has a Boxed Warning in its U.S. product label regarding the risk of posttreatment acute exacerbation of hepatitis B, according to the company.
The Descovy label also includes a Boxed Warning regarding the risk of drug resistance with PrEP use in undiagnosed early HIV-1 infection. The effectiveness of Descovy for PrEP in individuals at risk of HIV-1 from receptive vaginal sex was not tested, and thus cisgender women at risk for infection from vaginal sex were not included in the population for which the drug was approved.
The Descovy label and safety information is available here.
The FDA version of the announcement is available here.
The decision, backing the earlier recommendation of the FDA’s Antimicrobial Drugs Advisory Committee, was based upon results from DISCOVER, a pivotal, multiyear, global phase 3 clinical trial that evaluated the safety and efficacy of Descovy (emtricitabine 200 mg and tenofovir alafenamide 25-mg tablets for PrEP, compared with Truvada (emtricitabine 200 mg and tenofovir disoproxil fumarate 300-mg tablets).
DISCOVER included more than 5,300 adult cisgender men who have sex with men or transgender women who have sex with men.
In the trial, Descovy achieved noninferiority to Truvada.
Descovy has a Boxed Warning in its U.S. product label regarding the risk of posttreatment acute exacerbation of hepatitis B, according to the company.
The Descovy label also includes a Boxed Warning regarding the risk of drug resistance with PrEP use in undiagnosed early HIV-1 infection. The effectiveness of Descovy for PrEP in individuals at risk of HIV-1 from receptive vaginal sex was not tested, and thus cisgender women at risk for infection from vaginal sex were not included in the population for which the drug was approved.
The Descovy label and safety information is available here.
The FDA version of the announcement is available here.
The decision, backing the earlier recommendation of the FDA’s Antimicrobial Drugs Advisory Committee, was based upon results from DISCOVER, a pivotal, multiyear, global phase 3 clinical trial that evaluated the safety and efficacy of Descovy (emtricitabine 200 mg and tenofovir alafenamide 25-mg tablets for PrEP, compared with Truvada (emtricitabine 200 mg and tenofovir disoproxil fumarate 300-mg tablets).
DISCOVER included more than 5,300 adult cisgender men who have sex with men or transgender women who have sex with men.
In the trial, Descovy achieved noninferiority to Truvada.
Descovy has a Boxed Warning in its U.S. product label regarding the risk of posttreatment acute exacerbation of hepatitis B, according to the company.
The Descovy label also includes a Boxed Warning regarding the risk of drug resistance with PrEP use in undiagnosed early HIV-1 infection. The effectiveness of Descovy for PrEP in individuals at risk of HIV-1 from receptive vaginal sex was not tested, and thus cisgender women at risk for infection from vaginal sex were not included in the population for which the drug was approved.
The Descovy label and safety information is available here.
The FDA version of the announcement is available here.
Clinical Pharmacists Improve Patient Outcomes and Expand Access to Care
The US is in the midst of a chronic disease crisis. According to the latest published data available, 60% of Americans have at least 1 chronic condition, and 42% have ≥ 2 chronic conditions.1 Estimates by the Health Resources and Services Administration (HRSA) indicate a current shortfall of 13 800 primary care physicians and a projected escalation of that shortage to be between 14 800 and 49 300 physicians by the year 2030.2
The US Public Health Service (USPHS) has used pharmacists since 1930 to provide direct patient care to underserved and vulnerable populations. Clinical pharmacists currently serve in direct patient care roles within the Indian Health Service (IHS), Federal Bureau of Prisons (BOP), Immigration and Customs Enforcement (ICE), and the United States Coast Guard (USCG) in many states (Figure). These pharmacists play a vital role in improving access to care and delivering quality care by managing acute and chronic diseases in collaborative practice settings and pharmacist-managed clinics.
It has previously been reported that in the face of physician shortages and growing demand for primary health care providers, pharmacists are well-equipped and motivated to meet this demand.3 A review of the previous 2 years of outcomes reported by clinical pharmacists certified through the USPHS National Clinical Pharmacy Specialist (NCPS) Committee are presented to demonstrate the impact of pharmacists in advancing the health of the populations they serve and to showcase a model for ameliorating the ongoing physician shortage.
Background
The USPHS NCPS Committee serves to promote uniform competency among clinical pharmacists by establishing national standards for protocols, collaborative practice agreements (CPAs), credentialing and privileging of pharmacists, and by collecting, reviewing, and publishing health care outcomes. The committee, whose constituents include pharmacist and physician subject matter experts from across USPHS agencies, reviews applications and protocols and certifies pharmacists (civilian and uniformed) to recognize an advanced scope of practice in managing various diseases and optimizing medication therapy. NCPScertified pharmacists manage a wide spectrum of diseases, including coagulopathy, asthma, diabetes mellitus (DM), hepatitis C, HIV, hypertension, pain, seizure disorders, and tobacco use disorders.
Clinical pharmacists practicing chronic disease management establish a clinical service in collaboration with 1 or more physicians, physician assistants, or nurse practitioners. In this collaborative practice, the health care practitioner(s) refer patients to be managed by a pharmacist for specific medical needs, such as anticoagulation management, or for holistic medication- focused care (eg, cardiovascular risk reduction, DM management, HIV, hepatitis, or mental health). The pharmacist may order and interpret laboratory tests, check vital signs, perform a limited physical examination, and gather other pertinent information from the patient and the medical record in order to provide the best possible care to the patient.
Medications may be started, stopped, or adjusted, education is provided, and therapeutic lifestyle interventions may be recommended. The pharmacist-run clinic provides the patient more frequent interaction with a health care professional (pharmacist) and focused disease management. As a result, pharmacists increase access to care and allow the medical team to handle a larger panel of patients as the practitioner delegates specified diseases to the pharmacist- managed clinic(s). The number of NCPS-certified pharmacists grew 46% from 2012 (n = 230) to 2017 (n = 336), reflecting an evolution of pharmacists’ practice to better meet the need of patients across the nation.
Methods
The NCPS Committee requires NCPS pharmacists to report data annually from all patients referred for pharmacist management for specific diseases in which they have been certified. The data reflect the patient’s clinical outcome goal status at the time of referral as well as the same status at the end of the reporting period or on release from the pharmacist-run clinic. These data describe the impact prescribing pharmacists have on patients reaching clinical outcome goals acting as the team member specializing in the medication selection and dosing aspect of care.
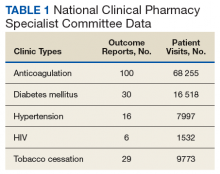
These records were reviewed for the fiscal year (FY) periods of October 1, 2015 to September 30, 2016 (FY 2016) and October 1, 2016 to September 30, 2017 (FY 2017). A systematic review of submitted reports resulted in 181 reports that included all requested data points for the disease as published here for FYs 2016 and 2017. These include 66 reports from FY 2016 and 115 reports from FY 2017; they cover 76 BOP and IHS facilities located across 24 states. Table 1 shows the number of outcome reports collected from 104 075 patient visits in pharmacist-run clinics in FYs 2016 and 2017.
Results
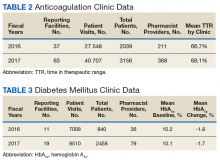
The following tables represent the standardized outcomes collected by NCPS-certified pharmacists providing direct patient care. Patients on anticoagulants (eg, warfarin) require special monitoring and education for drug interactions and adverse effects. NCPS-certified pharmacists were able to achieve a mean patient time in therapeutic range (TTR) of 67.6% (regardless of indication) over the 2 years (calculated per each facility by Rosendaal method of linear interpolation then combined in a weighted average per visit). The TTR produced by NCPS-certified pharmacists are consistent with Chest Guidelines and Expert Panel Report suggesting that TTR should be between 65% and 70%.4 Table 2 shows data from 100 reports with 68 255 patient visits for anticoagulation management.
DM management can be complex and time-intensive. NCPS data indicate pharmacist intervention resulted in a mean decrease in hemoglobin A1c (HbA1c) of 1.8% from a baseline of 10.2% (decrease calculated per each facility then combined by weighted average per visit). Table 3 shows data from 30 reports with 16 518 patient visits for DM care.
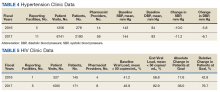
In addition to diet and exercise, medication management plays a vital role in managing hypertension. Patients managed by an NCPS-certified pharmacist experienced a mean decrease in blood pressure from 144/83 to 133/77, putting them in goal for both systolic and diastolic ranges (decrease calculated per each facility then combined by weighted average per visit). Table 4 shows data from 16 reports and 7997 patient visits for treatment of hypertension.
HIV viral suppression is vital in order to best manage patients with HIV and reduce the risk of transmission. Pharmacistled clinics have shown a 32.9% absolute improvement in patients at goal (viral load < 50 copies/mL), from a mean baseline of 46.0% to a mean final assessment of 71.6% of patients at goal (combined by weighted average visits). Table 5 shows data from 6 reports covering 1532 patient encounters for management of HIV.
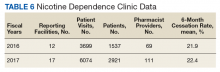
Nicotine dependence includes the use of cigarettes, cigars, pipe tobacco, chewing tobacco, and vaping products containing nicotine. NCPS-certified pharmacists have successfully helped patients improve their chance of quitting, with a 6-month quit rate of 22.2% (quit rate calculated per each facility then combined by weighted average by visits), which is higher than the national average of 9.4% as reported by the Centers for Disease and Control and Prevention. 5 Table 6 shows 29 reports covering 9773 patient visits for treatment of nicotine dependence.
Discussion
These data demonstrate the ability of advanced practice pharmacists in multiple locations within the federal sector to improve targeted clinical outcomes in patients with varying diseases. These results are strengthened by their varied origins as well as the improvements observed across the board. Limitations include the general lack of a comparable dataset, manual method of selfreporting by the individual facilities, and the relatively limited array of diseases reported. Although NCPS-certified pharmacists are currently providing care for patients with hepatitis C, asthma, seizure, pain and other diseases not reported here, there are insufficient data collected for FYs 2016 and 2017 to merit inclusion within this report.
Pharmacists are trusted, readily available medication experts. In a clinical role, NCPS-certified pharmacists have increased access to primary care services and demonstrated beneficial impact on important health outcomes as exhibited by the data reported above. Clinical pharmacy is a growing field, and NCPS has displayed continual growth in both the number of NCPS-certified pharmacists and the number of patient encounters performed by these providers. As more pharmacists in all settings collaborate with medical providers to offer high-quality clinical care, these providers will have more opportunity to delegate disease management. Continued reporting of clinical pharmacy outcomes is expected to increase confidence in pharmacists as primary care providers, increase utilization of pharmacy clinical services, and assist in easing the burden of primary care provider shortages across our nation.
Although these outcomes indicate demonstrable benefit in patient-centered outcomes, the need for ongoing assessment and continued improvement is not obviated. Future efforts may benefit from a comparison of alternative approaches to better facilitate the establishment of best practices. Alignment of clinical outcomes with the Centers for Medicare and Medicaid Services (CMS) Electronic Clinical Quality Measures, where applicable, also may prove beneficial by automating the reporting process and thereby decreasing the burden of reporting as well as providing an avenue for standard comparison across multiple populations. Clinical pharmacy interventions have positive outcomes based on the NCPS model, and the NCPS Committee invites other clinical settings to report outcomes data with which to compare.
Conclusion
The NCPS Committee has documented positive outcomes of clinical pharmacy intervention and anticipates growth of the pharmacy profession as additional states and health systems recognize the capacity of the pharmacist to provide high-quality, multidisciplinary patient care. Clinical pharmacists are prepared to address critical health care needs as the US continues to face a PCP shortage.2 The NCPS Committee challenges those participating in clinical pharmacy practice to report outcomes to amplify this body of evidence.
Acknowledgments
NCPS-certified pharmacists provided the outcomes detailed in this report. For document review and edits: Federal Bureau of Prison Publication Review Workgroup; RADM Ty Bingham, USPHS; CAPT Cindy Gunderson, USPHS; CAPT Kevin Brooks, USPHS.
1. Buttorff C, Ruder T, Bauman M. Multiple Chronic Conditions in the United States. Santa Monica, CA: Rand Corp; 2017.
2. Dall T, West T, Chakrabarti R, Reynolds R, Iacobucci W. The complexities of physician supply and demand: projections from 2016 to 2030, 2018 update. Association of American Medical Colleges. March 2018.
3. Giberson S, Yoder S, Lee MP. Improving patient and health system outcomes through advanced pharmacy practice. A report to the U.S. Surgeon General 2011. https://www .accp.com/docs/positions/misc/improving_patient_and _health_system_outcomes.pdf. Updated December 2011. Accessed September 11, 2019.
4. Lip G, Banerjee A, Boriani G, et al. Antithrombotic therapy for atrial fibrillation. CHEST guideline and Expert Panel Report. Chest. 2018;154(5):1121-1201.
5. Babb S, Marlarcher A, Schauer G, Asman K, Jamal A. Quitting smoking among adults—United States, 2000-2015. MMWR Morb Mortal Wkly Rep. 2017;65(52):1457-1464.
The US is in the midst of a chronic disease crisis. According to the latest published data available, 60% of Americans have at least 1 chronic condition, and 42% have ≥ 2 chronic conditions.1 Estimates by the Health Resources and Services Administration (HRSA) indicate a current shortfall of 13 800 primary care physicians and a projected escalation of that shortage to be between 14 800 and 49 300 physicians by the year 2030.2
The US Public Health Service (USPHS) has used pharmacists since 1930 to provide direct patient care to underserved and vulnerable populations. Clinical pharmacists currently serve in direct patient care roles within the Indian Health Service (IHS), Federal Bureau of Prisons (BOP), Immigration and Customs Enforcement (ICE), and the United States Coast Guard (USCG) in many states (Figure). These pharmacists play a vital role in improving access to care and delivering quality care by managing acute and chronic diseases in collaborative practice settings and pharmacist-managed clinics.
It has previously been reported that in the face of physician shortages and growing demand for primary health care providers, pharmacists are well-equipped and motivated to meet this demand.3 A review of the previous 2 years of outcomes reported by clinical pharmacists certified through the USPHS National Clinical Pharmacy Specialist (NCPS) Committee are presented to demonstrate the impact of pharmacists in advancing the health of the populations they serve and to showcase a model for ameliorating the ongoing physician shortage.
Background
The USPHS NCPS Committee serves to promote uniform competency among clinical pharmacists by establishing national standards for protocols, collaborative practice agreements (CPAs), credentialing and privileging of pharmacists, and by collecting, reviewing, and publishing health care outcomes. The committee, whose constituents include pharmacist and physician subject matter experts from across USPHS agencies, reviews applications and protocols and certifies pharmacists (civilian and uniformed) to recognize an advanced scope of practice in managing various diseases and optimizing medication therapy. NCPScertified pharmacists manage a wide spectrum of diseases, including coagulopathy, asthma, diabetes mellitus (DM), hepatitis C, HIV, hypertension, pain, seizure disorders, and tobacco use disorders.
Clinical pharmacists practicing chronic disease management establish a clinical service in collaboration with 1 or more physicians, physician assistants, or nurse practitioners. In this collaborative practice, the health care practitioner(s) refer patients to be managed by a pharmacist for specific medical needs, such as anticoagulation management, or for holistic medication- focused care (eg, cardiovascular risk reduction, DM management, HIV, hepatitis, or mental health). The pharmacist may order and interpret laboratory tests, check vital signs, perform a limited physical examination, and gather other pertinent information from the patient and the medical record in order to provide the best possible care to the patient.
Medications may be started, stopped, or adjusted, education is provided, and therapeutic lifestyle interventions may be recommended. The pharmacist-run clinic provides the patient more frequent interaction with a health care professional (pharmacist) and focused disease management. As a result, pharmacists increase access to care and allow the medical team to handle a larger panel of patients as the practitioner delegates specified diseases to the pharmacist- managed clinic(s). The number of NCPS-certified pharmacists grew 46% from 2012 (n = 230) to 2017 (n = 336), reflecting an evolution of pharmacists’ practice to better meet the need of patients across the nation.
Methods
The NCPS Committee requires NCPS pharmacists to report data annually from all patients referred for pharmacist management for specific diseases in which they have been certified. The data reflect the patient’s clinical outcome goal status at the time of referral as well as the same status at the end of the reporting period or on release from the pharmacist-run clinic. These data describe the impact prescribing pharmacists have on patients reaching clinical outcome goals acting as the team member specializing in the medication selection and dosing aspect of care.
These records were reviewed for the fiscal year (FY) periods of October 1, 2015 to September 30, 2016 (FY 2016) and October 1, 2016 to September 30, 2017 (FY 2017). A systematic review of submitted reports resulted in 181 reports that included all requested data points for the disease as published here for FYs 2016 and 2017. These include 66 reports from FY 2016 and 115 reports from FY 2017; they cover 76 BOP and IHS facilities located across 24 states. Table 1 shows the number of outcome reports collected from 104 075 patient visits in pharmacist-run clinics in FYs 2016 and 2017.
Results
The following tables represent the standardized outcomes collected by NCPS-certified pharmacists providing direct patient care. Patients on anticoagulants (eg, warfarin) require special monitoring and education for drug interactions and adverse effects. NCPS-certified pharmacists were able to achieve a mean patient time in therapeutic range (TTR) of 67.6% (regardless of indication) over the 2 years (calculated per each facility by Rosendaal method of linear interpolation then combined in a weighted average per visit). The TTR produced by NCPS-certified pharmacists are consistent with Chest Guidelines and Expert Panel Report suggesting that TTR should be between 65% and 70%.4 Table 2 shows data from 100 reports with 68 255 patient visits for anticoagulation management.
DM management can be complex and time-intensive. NCPS data indicate pharmacist intervention resulted in a mean decrease in hemoglobin A1c (HbA1c) of 1.8% from a baseline of 10.2% (decrease calculated per each facility then combined by weighted average per visit). Table 3 shows data from 30 reports with 16 518 patient visits for DM care.
In addition to diet and exercise, medication management plays a vital role in managing hypertension. Patients managed by an NCPS-certified pharmacist experienced a mean decrease in blood pressure from 144/83 to 133/77, putting them in goal for both systolic and diastolic ranges (decrease calculated per each facility then combined by weighted average per visit). Table 4 shows data from 16 reports and 7997 patient visits for treatment of hypertension.
HIV viral suppression is vital in order to best manage patients with HIV and reduce the risk of transmission. Pharmacistled clinics have shown a 32.9% absolute improvement in patients at goal (viral load < 50 copies/mL), from a mean baseline of 46.0% to a mean final assessment of 71.6% of patients at goal (combined by weighted average visits). Table 5 shows data from 6 reports covering 1532 patient encounters for management of HIV.
Nicotine dependence includes the use of cigarettes, cigars, pipe tobacco, chewing tobacco, and vaping products containing nicotine. NCPS-certified pharmacists have successfully helped patients improve their chance of quitting, with a 6-month quit rate of 22.2% (quit rate calculated per each facility then combined by weighted average by visits), which is higher than the national average of 9.4% as reported by the Centers for Disease and Control and Prevention. 5 Table 6 shows 29 reports covering 9773 patient visits for treatment of nicotine dependence.
Discussion
These data demonstrate the ability of advanced practice pharmacists in multiple locations within the federal sector to improve targeted clinical outcomes in patients with varying diseases. These results are strengthened by their varied origins as well as the improvements observed across the board. Limitations include the general lack of a comparable dataset, manual method of selfreporting by the individual facilities, and the relatively limited array of diseases reported. Although NCPS-certified pharmacists are currently providing care for patients with hepatitis C, asthma, seizure, pain and other diseases not reported here, there are insufficient data collected for FYs 2016 and 2017 to merit inclusion within this report.
Pharmacists are trusted, readily available medication experts. In a clinical role, NCPS-certified pharmacists have increased access to primary care services and demonstrated beneficial impact on important health outcomes as exhibited by the data reported above. Clinical pharmacy is a growing field, and NCPS has displayed continual growth in both the number of NCPS-certified pharmacists and the number of patient encounters performed by these providers. As more pharmacists in all settings collaborate with medical providers to offer high-quality clinical care, these providers will have more opportunity to delegate disease management. Continued reporting of clinical pharmacy outcomes is expected to increase confidence in pharmacists as primary care providers, increase utilization of pharmacy clinical services, and assist in easing the burden of primary care provider shortages across our nation.
Although these outcomes indicate demonstrable benefit in patient-centered outcomes, the need for ongoing assessment and continued improvement is not obviated. Future efforts may benefit from a comparison of alternative approaches to better facilitate the establishment of best practices. Alignment of clinical outcomes with the Centers for Medicare and Medicaid Services (CMS) Electronic Clinical Quality Measures, where applicable, also may prove beneficial by automating the reporting process and thereby decreasing the burden of reporting as well as providing an avenue for standard comparison across multiple populations. Clinical pharmacy interventions have positive outcomes based on the NCPS model, and the NCPS Committee invites other clinical settings to report outcomes data with which to compare.
Conclusion
The NCPS Committee has documented positive outcomes of clinical pharmacy intervention and anticipates growth of the pharmacy profession as additional states and health systems recognize the capacity of the pharmacist to provide high-quality, multidisciplinary patient care. Clinical pharmacists are prepared to address critical health care needs as the US continues to face a PCP shortage.2 The NCPS Committee challenges those participating in clinical pharmacy practice to report outcomes to amplify this body of evidence.
Acknowledgments
NCPS-certified pharmacists provided the outcomes detailed in this report. For document review and edits: Federal Bureau of Prison Publication Review Workgroup; RADM Ty Bingham, USPHS; CAPT Cindy Gunderson, USPHS; CAPT Kevin Brooks, USPHS.
The US is in the midst of a chronic disease crisis. According to the latest published data available, 60% of Americans have at least 1 chronic condition, and 42% have ≥ 2 chronic conditions.1 Estimates by the Health Resources and Services Administration (HRSA) indicate a current shortfall of 13 800 primary care physicians and a projected escalation of that shortage to be between 14 800 and 49 300 physicians by the year 2030.2
The US Public Health Service (USPHS) has used pharmacists since 1930 to provide direct patient care to underserved and vulnerable populations. Clinical pharmacists currently serve in direct patient care roles within the Indian Health Service (IHS), Federal Bureau of Prisons (BOP), Immigration and Customs Enforcement (ICE), and the United States Coast Guard (USCG) in many states (Figure). These pharmacists play a vital role in improving access to care and delivering quality care by managing acute and chronic diseases in collaborative practice settings and pharmacist-managed clinics.
It has previously been reported that in the face of physician shortages and growing demand for primary health care providers, pharmacists are well-equipped and motivated to meet this demand.3 A review of the previous 2 years of outcomes reported by clinical pharmacists certified through the USPHS National Clinical Pharmacy Specialist (NCPS) Committee are presented to demonstrate the impact of pharmacists in advancing the health of the populations they serve and to showcase a model for ameliorating the ongoing physician shortage.
Background
The USPHS NCPS Committee serves to promote uniform competency among clinical pharmacists by establishing national standards for protocols, collaborative practice agreements (CPAs), credentialing and privileging of pharmacists, and by collecting, reviewing, and publishing health care outcomes. The committee, whose constituents include pharmacist and physician subject matter experts from across USPHS agencies, reviews applications and protocols and certifies pharmacists (civilian and uniformed) to recognize an advanced scope of practice in managing various diseases and optimizing medication therapy. NCPScertified pharmacists manage a wide spectrum of diseases, including coagulopathy, asthma, diabetes mellitus (DM), hepatitis C, HIV, hypertension, pain, seizure disorders, and tobacco use disorders.
Clinical pharmacists practicing chronic disease management establish a clinical service in collaboration with 1 or more physicians, physician assistants, or nurse practitioners. In this collaborative practice, the health care practitioner(s) refer patients to be managed by a pharmacist for specific medical needs, such as anticoagulation management, or for holistic medication- focused care (eg, cardiovascular risk reduction, DM management, HIV, hepatitis, or mental health). The pharmacist may order and interpret laboratory tests, check vital signs, perform a limited physical examination, and gather other pertinent information from the patient and the medical record in order to provide the best possible care to the patient.
Medications may be started, stopped, or adjusted, education is provided, and therapeutic lifestyle interventions may be recommended. The pharmacist-run clinic provides the patient more frequent interaction with a health care professional (pharmacist) and focused disease management. As a result, pharmacists increase access to care and allow the medical team to handle a larger panel of patients as the practitioner delegates specified diseases to the pharmacist- managed clinic(s). The number of NCPS-certified pharmacists grew 46% from 2012 (n = 230) to 2017 (n = 336), reflecting an evolution of pharmacists’ practice to better meet the need of patients across the nation.
Methods
The NCPS Committee requires NCPS pharmacists to report data annually from all patients referred for pharmacist management for specific diseases in which they have been certified. The data reflect the patient’s clinical outcome goal status at the time of referral as well as the same status at the end of the reporting period or on release from the pharmacist-run clinic. These data describe the impact prescribing pharmacists have on patients reaching clinical outcome goals acting as the team member specializing in the medication selection and dosing aspect of care.
These records were reviewed for the fiscal year (FY) periods of October 1, 2015 to September 30, 2016 (FY 2016) and October 1, 2016 to September 30, 2017 (FY 2017). A systematic review of submitted reports resulted in 181 reports that included all requested data points for the disease as published here for FYs 2016 and 2017. These include 66 reports from FY 2016 and 115 reports from FY 2017; they cover 76 BOP and IHS facilities located across 24 states. Table 1 shows the number of outcome reports collected from 104 075 patient visits in pharmacist-run clinics in FYs 2016 and 2017.
Results
The following tables represent the standardized outcomes collected by NCPS-certified pharmacists providing direct patient care. Patients on anticoagulants (eg, warfarin) require special monitoring and education for drug interactions and adverse effects. NCPS-certified pharmacists were able to achieve a mean patient time in therapeutic range (TTR) of 67.6% (regardless of indication) over the 2 years (calculated per each facility by Rosendaal method of linear interpolation then combined in a weighted average per visit). The TTR produced by NCPS-certified pharmacists are consistent with Chest Guidelines and Expert Panel Report suggesting that TTR should be between 65% and 70%.4 Table 2 shows data from 100 reports with 68 255 patient visits for anticoagulation management.
DM management can be complex and time-intensive. NCPS data indicate pharmacist intervention resulted in a mean decrease in hemoglobin A1c (HbA1c) of 1.8% from a baseline of 10.2% (decrease calculated per each facility then combined by weighted average per visit). Table 3 shows data from 30 reports with 16 518 patient visits for DM care.
In addition to diet and exercise, medication management plays a vital role in managing hypertension. Patients managed by an NCPS-certified pharmacist experienced a mean decrease in blood pressure from 144/83 to 133/77, putting them in goal for both systolic and diastolic ranges (decrease calculated per each facility then combined by weighted average per visit). Table 4 shows data from 16 reports and 7997 patient visits for treatment of hypertension.
HIV viral suppression is vital in order to best manage patients with HIV and reduce the risk of transmission. Pharmacistled clinics have shown a 32.9% absolute improvement in patients at goal (viral load < 50 copies/mL), from a mean baseline of 46.0% to a mean final assessment of 71.6% of patients at goal (combined by weighted average visits). Table 5 shows data from 6 reports covering 1532 patient encounters for management of HIV.
Nicotine dependence includes the use of cigarettes, cigars, pipe tobacco, chewing tobacco, and vaping products containing nicotine. NCPS-certified pharmacists have successfully helped patients improve their chance of quitting, with a 6-month quit rate of 22.2% (quit rate calculated per each facility then combined by weighted average by visits), which is higher than the national average of 9.4% as reported by the Centers for Disease and Control and Prevention. 5 Table 6 shows 29 reports covering 9773 patient visits for treatment of nicotine dependence.
Discussion
These data demonstrate the ability of advanced practice pharmacists in multiple locations within the federal sector to improve targeted clinical outcomes in patients with varying diseases. These results are strengthened by their varied origins as well as the improvements observed across the board. Limitations include the general lack of a comparable dataset, manual method of selfreporting by the individual facilities, and the relatively limited array of diseases reported. Although NCPS-certified pharmacists are currently providing care for patients with hepatitis C, asthma, seizure, pain and other diseases not reported here, there are insufficient data collected for FYs 2016 and 2017 to merit inclusion within this report.
Pharmacists are trusted, readily available medication experts. In a clinical role, NCPS-certified pharmacists have increased access to primary care services and demonstrated beneficial impact on important health outcomes as exhibited by the data reported above. Clinical pharmacy is a growing field, and NCPS has displayed continual growth in both the number of NCPS-certified pharmacists and the number of patient encounters performed by these providers. As more pharmacists in all settings collaborate with medical providers to offer high-quality clinical care, these providers will have more opportunity to delegate disease management. Continued reporting of clinical pharmacy outcomes is expected to increase confidence in pharmacists as primary care providers, increase utilization of pharmacy clinical services, and assist in easing the burden of primary care provider shortages across our nation.
Although these outcomes indicate demonstrable benefit in patient-centered outcomes, the need for ongoing assessment and continued improvement is not obviated. Future efforts may benefit from a comparison of alternative approaches to better facilitate the establishment of best practices. Alignment of clinical outcomes with the Centers for Medicare and Medicaid Services (CMS) Electronic Clinical Quality Measures, where applicable, also may prove beneficial by automating the reporting process and thereby decreasing the burden of reporting as well as providing an avenue for standard comparison across multiple populations. Clinical pharmacy interventions have positive outcomes based on the NCPS model, and the NCPS Committee invites other clinical settings to report outcomes data with which to compare.
Conclusion
The NCPS Committee has documented positive outcomes of clinical pharmacy intervention and anticipates growth of the pharmacy profession as additional states and health systems recognize the capacity of the pharmacist to provide high-quality, multidisciplinary patient care. Clinical pharmacists are prepared to address critical health care needs as the US continues to face a PCP shortage.2 The NCPS Committee challenges those participating in clinical pharmacy practice to report outcomes to amplify this body of evidence.
Acknowledgments
NCPS-certified pharmacists provided the outcomes detailed in this report. For document review and edits: Federal Bureau of Prison Publication Review Workgroup; RADM Ty Bingham, USPHS; CAPT Cindy Gunderson, USPHS; CAPT Kevin Brooks, USPHS.
1. Buttorff C, Ruder T, Bauman M. Multiple Chronic Conditions in the United States. Santa Monica, CA: Rand Corp; 2017.
2. Dall T, West T, Chakrabarti R, Reynolds R, Iacobucci W. The complexities of physician supply and demand: projections from 2016 to 2030, 2018 update. Association of American Medical Colleges. March 2018.
3. Giberson S, Yoder S, Lee MP. Improving patient and health system outcomes through advanced pharmacy practice. A report to the U.S. Surgeon General 2011. https://www .accp.com/docs/positions/misc/improving_patient_and _health_system_outcomes.pdf. Updated December 2011. Accessed September 11, 2019.
4. Lip G, Banerjee A, Boriani G, et al. Antithrombotic therapy for atrial fibrillation. CHEST guideline and Expert Panel Report. Chest. 2018;154(5):1121-1201.
5. Babb S, Marlarcher A, Schauer G, Asman K, Jamal A. Quitting smoking among adults—United States, 2000-2015. MMWR Morb Mortal Wkly Rep. 2017;65(52):1457-1464.
1. Buttorff C, Ruder T, Bauman M. Multiple Chronic Conditions in the United States. Santa Monica, CA: Rand Corp; 2017.
2. Dall T, West T, Chakrabarti R, Reynolds R, Iacobucci W. The complexities of physician supply and demand: projections from 2016 to 2030, 2018 update. Association of American Medical Colleges. March 2018.
3. Giberson S, Yoder S, Lee MP. Improving patient and health system outcomes through advanced pharmacy practice. A report to the U.S. Surgeon General 2011. https://www .accp.com/docs/positions/misc/improving_patient_and _health_system_outcomes.pdf. Updated December 2011. Accessed September 11, 2019.
4. Lip G, Banerjee A, Boriani G, et al. Antithrombotic therapy for atrial fibrillation. CHEST guideline and Expert Panel Report. Chest. 2018;154(5):1121-1201.
5. Babb S, Marlarcher A, Schauer G, Asman K, Jamal A. Quitting smoking among adults—United States, 2000-2015. MMWR Morb Mortal Wkly Rep. 2017;65(52):1457-1464.
CAR T-cell therapy found safe, effective for HIV-associated lymphoma
HIV positivity does not preclude chimeric antigen receptor (CAR) T-cell therapy for patients with aggressive lymphoma, a report of two cases suggests. Both of the HIV-positive patients, one of whom had long-term psychiatric comorbidity, achieved durable remission on axicabtagene ciloleucel (Yescarta) without undue toxicity.
“To our knowledge, these are the first reported cases of CAR T-cell therapy administered to HIV-infected patients with lymphoma,” Jeremy S. Abramson, MD, of Massachusetts General Hospital, Boston and his colleagues wrote in Cancer. “Patients with HIV and AIDS, as well as those with preexisting mental illness, should not be considered disqualified from CAR T-cell therapy and deserve ongoing studies to optimize efficacy and safety in this population.”
The Food and Drug Administration has approved two CAR T-cell products that target the B-cell antigen CD19 for the treatment of refractory lymphoma. But their efficacy and safety in HIV-positive patients are unknown because this group has been excluded from pivotal clinical trials.
Dr. Abramson and coauthors detail the two cases of successful anti-CD19 CAR T-cell therapy with axicabtagene ciloleucel in patients with HIV-associated, refractory, high-grade B-cell lymphoma.
The first patient was an HIV-positive man with diffuse large B-cell lymphoma (DLBCL) of germinal center B-cell subtype who was intermittently adherent to antiretroviral therapy. His comorbidities included posttraumatic stress disorder and schizoaffective disorder.
Previous treatments for DLBCL included dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab (EPOCH-R), and rituximab, ifosfamide, carboplatin, and etoposide (RICE). A recurrence precluded high-dose chemotherapy with autologous stem cell support.
With close multidisciplinary management, including psychiatric consultation, the patient became a candidate for CAR T-cell therapy and received axicabtagene ciloleucel. He experienced grade 2 cytokine release syndrome and grade 3 neurologic toxicity, both of which resolved with treatment. Imaging showed complete remission at approximately 3 months that was sustained at 1 year. Additionally, he had an undetectable HIV viral load and was psychiatrically stable.
The second patient was a man with AIDS-associated, non–germinal center B-cell, Epstein-Barr virus–positive DLBCL who was adherent to antiretroviral therapy. His lymphoma had recurred rapidly after initially responding to dose-adjusted EPOCH-R and then was refractory to combination rituximab and lenalidomide. He previously had hepatitis B virus, cytomegalovirus, and Mycobacterium avium complex infections.
Because of prolonged cytopenias and infectious complications after the previous lymphoma treatments, the patient was considered a poor candidate for high-dose chemotherapy. He underwent CAR T-cell therapy with axicabtagene ciloleucel and had a complete remission on day 28. Additionally, his HIV infection remained well controlled.
“Although much remains to be learned regarding CAR T-cell therapy in patients with refractory hematologic malignancies, with or without HIV infection, the cases presented herein demonstrate that patients with chemotherapy-refractory, high-grade B-cell lymphoma can successfully undergo autologous CAR T-cell manufacturing, and subsequently can safely tolerate CAR T-cell therapy and achieve a durable complete remission,” the researchers wrote. “These cases have further demonstrated the proactive, multidisciplinary care required to navigate a patient with high-risk lymphoma through CAR T-cell therapy with attention to significant medical and psychiatric comorbidities.”
Dr. Abramson reported that he has acted as a paid member of the scientific advisory board and as a paid consultant for Kite Pharma, which markets Yescarta, and several other companies.
SOURCE: Abramson JS et al. Cancer. 2019 Sep 10. doi: 10.1002/cncr.32411.
HIV positivity does not preclude chimeric antigen receptor (CAR) T-cell therapy for patients with aggressive lymphoma, a report of two cases suggests. Both of the HIV-positive patients, one of whom had long-term psychiatric comorbidity, achieved durable remission on axicabtagene ciloleucel (Yescarta) without undue toxicity.
“To our knowledge, these are the first reported cases of CAR T-cell therapy administered to HIV-infected patients with lymphoma,” Jeremy S. Abramson, MD, of Massachusetts General Hospital, Boston and his colleagues wrote in Cancer. “Patients with HIV and AIDS, as well as those with preexisting mental illness, should not be considered disqualified from CAR T-cell therapy and deserve ongoing studies to optimize efficacy and safety in this population.”
The Food and Drug Administration has approved two CAR T-cell products that target the B-cell antigen CD19 for the treatment of refractory lymphoma. But their efficacy and safety in HIV-positive patients are unknown because this group has been excluded from pivotal clinical trials.
Dr. Abramson and coauthors detail the two cases of successful anti-CD19 CAR T-cell therapy with axicabtagene ciloleucel in patients with HIV-associated, refractory, high-grade B-cell lymphoma.
The first patient was an HIV-positive man with diffuse large B-cell lymphoma (DLBCL) of germinal center B-cell subtype who was intermittently adherent to antiretroviral therapy. His comorbidities included posttraumatic stress disorder and schizoaffective disorder.
Previous treatments for DLBCL included dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab (EPOCH-R), and rituximab, ifosfamide, carboplatin, and etoposide (RICE). A recurrence precluded high-dose chemotherapy with autologous stem cell support.
With close multidisciplinary management, including psychiatric consultation, the patient became a candidate for CAR T-cell therapy and received axicabtagene ciloleucel. He experienced grade 2 cytokine release syndrome and grade 3 neurologic toxicity, both of which resolved with treatment. Imaging showed complete remission at approximately 3 months that was sustained at 1 year. Additionally, he had an undetectable HIV viral load and was psychiatrically stable.
The second patient was a man with AIDS-associated, non–germinal center B-cell, Epstein-Barr virus–positive DLBCL who was adherent to antiretroviral therapy. His lymphoma had recurred rapidly after initially responding to dose-adjusted EPOCH-R and then was refractory to combination rituximab and lenalidomide. He previously had hepatitis B virus, cytomegalovirus, and Mycobacterium avium complex infections.
Because of prolonged cytopenias and infectious complications after the previous lymphoma treatments, the patient was considered a poor candidate for high-dose chemotherapy. He underwent CAR T-cell therapy with axicabtagene ciloleucel and had a complete remission on day 28. Additionally, his HIV infection remained well controlled.
“Although much remains to be learned regarding CAR T-cell therapy in patients with refractory hematologic malignancies, with or without HIV infection, the cases presented herein demonstrate that patients with chemotherapy-refractory, high-grade B-cell lymphoma can successfully undergo autologous CAR T-cell manufacturing, and subsequently can safely tolerate CAR T-cell therapy and achieve a durable complete remission,” the researchers wrote. “These cases have further demonstrated the proactive, multidisciplinary care required to navigate a patient with high-risk lymphoma through CAR T-cell therapy with attention to significant medical and psychiatric comorbidities.”
Dr. Abramson reported that he has acted as a paid member of the scientific advisory board and as a paid consultant for Kite Pharma, which markets Yescarta, and several other companies.
SOURCE: Abramson JS et al. Cancer. 2019 Sep 10. doi: 10.1002/cncr.32411.
HIV positivity does not preclude chimeric antigen receptor (CAR) T-cell therapy for patients with aggressive lymphoma, a report of two cases suggests. Both of the HIV-positive patients, one of whom had long-term psychiatric comorbidity, achieved durable remission on axicabtagene ciloleucel (Yescarta) without undue toxicity.
“To our knowledge, these are the first reported cases of CAR T-cell therapy administered to HIV-infected patients with lymphoma,” Jeremy S. Abramson, MD, of Massachusetts General Hospital, Boston and his colleagues wrote in Cancer. “Patients with HIV and AIDS, as well as those with preexisting mental illness, should not be considered disqualified from CAR T-cell therapy and deserve ongoing studies to optimize efficacy and safety in this population.”
The Food and Drug Administration has approved two CAR T-cell products that target the B-cell antigen CD19 for the treatment of refractory lymphoma. But their efficacy and safety in HIV-positive patients are unknown because this group has been excluded from pivotal clinical trials.
Dr. Abramson and coauthors detail the two cases of successful anti-CD19 CAR T-cell therapy with axicabtagene ciloleucel in patients with HIV-associated, refractory, high-grade B-cell lymphoma.
The first patient was an HIV-positive man with diffuse large B-cell lymphoma (DLBCL) of germinal center B-cell subtype who was intermittently adherent to antiretroviral therapy. His comorbidities included posttraumatic stress disorder and schizoaffective disorder.
Previous treatments for DLBCL included dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab (EPOCH-R), and rituximab, ifosfamide, carboplatin, and etoposide (RICE). A recurrence precluded high-dose chemotherapy with autologous stem cell support.
With close multidisciplinary management, including psychiatric consultation, the patient became a candidate for CAR T-cell therapy and received axicabtagene ciloleucel. He experienced grade 2 cytokine release syndrome and grade 3 neurologic toxicity, both of which resolved with treatment. Imaging showed complete remission at approximately 3 months that was sustained at 1 year. Additionally, he had an undetectable HIV viral load and was psychiatrically stable.
The second patient was a man with AIDS-associated, non–germinal center B-cell, Epstein-Barr virus–positive DLBCL who was adherent to antiretroviral therapy. His lymphoma had recurred rapidly after initially responding to dose-adjusted EPOCH-R and then was refractory to combination rituximab and lenalidomide. He previously had hepatitis B virus, cytomegalovirus, and Mycobacterium avium complex infections.
Because of prolonged cytopenias and infectious complications after the previous lymphoma treatments, the patient was considered a poor candidate for high-dose chemotherapy. He underwent CAR T-cell therapy with axicabtagene ciloleucel and had a complete remission on day 28. Additionally, his HIV infection remained well controlled.
“Although much remains to be learned regarding CAR T-cell therapy in patients with refractory hematologic malignancies, with or without HIV infection, the cases presented herein demonstrate that patients with chemotherapy-refractory, high-grade B-cell lymphoma can successfully undergo autologous CAR T-cell manufacturing, and subsequently can safely tolerate CAR T-cell therapy and achieve a durable complete remission,” the researchers wrote. “These cases have further demonstrated the proactive, multidisciplinary care required to navigate a patient with high-risk lymphoma through CAR T-cell therapy with attention to significant medical and psychiatric comorbidities.”
Dr. Abramson reported that he has acted as a paid member of the scientific advisory board and as a paid consultant for Kite Pharma, which markets Yescarta, and several other companies.
SOURCE: Abramson JS et al. Cancer. 2019 Sep 10. doi: 10.1002/cncr.32411.
FROM CANCER
New engineered HIV-1 vaccine candidate shows improved immunogenicity in early trial
ALVAC-HIV vaccine showed immunogenicity across several HIV clades in an early trial involving 100 healthy patients at low risk of HIV infection, according to a study by Glenda E. Gray, MBBCH, FCPaed, of the University of the Witwatersrand, Johannesburg, South Africa, and colleagues that was published online in the Sep. 18 issue of Science Translational Medicine.
ALVAC-HIV (vCP1521) is a live attenuated recombinant canarypox-derived virus that expresses gene products from the HIV-1 gp120 (92TH023/clade E), Gag (clade B), and Pro (clade B) that is cultured in chicken embryo fibroblast cells.
Four injections of ALVAC-HIV were given at months 0, 1, 3, and 6. At months 3 and 6, two booster injections were given of AIDSVAX/BE, a bivalent HIV glycoprotein 120 (gp120) that was previously studied in the RV144 trial. The HVTN 097 trial examined primary immunogenicity endpoints including the frequency and magnitude of IgG and IgG3 antibody binding, measured in serum specimens obtained at baseline, at a peak time point (2 weeks after second ALVAC/AIDSVAX vaccination), a durability time point (6 months after second ALVAC/AIDSVAX vaccination), and the response rates and magnitudes of CD4+ and CD8+ T-cell responses at the baseline, peak, and durability time points. One hundred healthy adults at low risk for HIV infection were randomized in 3:1:1 ratio to group T1 (HIV vaccines, tetanus vaccine, and hepatitis B vaccine), group T2 (HIV vaccine only), and the placebo group T3 (tetanus vaccine and hepatitis B vaccine). There were no meaningful differences in HIV immune responses between the HIV vaccine recipients with or without the tetanus and hepatitis B vaccines, so the researchers pooled the data from groups T1 and T2 in their analysis.
At the peak immunogenicity time point, the vaccine schedule predominantly induced CD4+ T cells directed to HIV-1 Env; this was measured by expression of interleukin-2 and/or interferon-gamma. The Env-specific CD4+ T-cell response rate was significantly higher in HVTN 097 vaccine recipients than it was in those in the RV144 trial (51.9% vs. 36.4%; P = .043). The HVTN 097 trial also showed significantly higher response rates for CD40L(59.3% for HVTN 097 vs. 33.7% for RV144; P less than .001) and for interferon-gamma (42.6% in HVTN 097 vs. 19.5% in RV144; P = .001).
However, durability at 6 months after the second vaccine injection remained an issue, with the frequency of circulating Env-specific CD4+ T-cell responses among vaccine recipients declining significantly; the response rate dropped from 70.8% to 36.1%.
“These data may indicate that cross-clade immune responses, especially to non-neutralizing epitopes correlated with decreased HIV-1 risk, can be achieved for a globally effective vaccine by using unique HIV Env strains,” Dr. Gray and associates concluded.
The authors declared that they had no competing interests.
SOURCE: Gray GE et al. Sci. Transl. Med. 2019 Sep 18. doi: 10.1126/scitranslmed.aax1880..
ALVAC-HIV vaccine showed immunogenicity across several HIV clades in an early trial involving 100 healthy patients at low risk of HIV infection, according to a study by Glenda E. Gray, MBBCH, FCPaed, of the University of the Witwatersrand, Johannesburg, South Africa, and colleagues that was published online in the Sep. 18 issue of Science Translational Medicine.
ALVAC-HIV (vCP1521) is a live attenuated recombinant canarypox-derived virus that expresses gene products from the HIV-1 gp120 (92TH023/clade E), Gag (clade B), and Pro (clade B) that is cultured in chicken embryo fibroblast cells.
Four injections of ALVAC-HIV were given at months 0, 1, 3, and 6. At months 3 and 6, two booster injections were given of AIDSVAX/BE, a bivalent HIV glycoprotein 120 (gp120) that was previously studied in the RV144 trial. The HVTN 097 trial examined primary immunogenicity endpoints including the frequency and magnitude of IgG and IgG3 antibody binding, measured in serum specimens obtained at baseline, at a peak time point (2 weeks after second ALVAC/AIDSVAX vaccination), a durability time point (6 months after second ALVAC/AIDSVAX vaccination), and the response rates and magnitudes of CD4+ and CD8+ T-cell responses at the baseline, peak, and durability time points. One hundred healthy adults at low risk for HIV infection were randomized in 3:1:1 ratio to group T1 (HIV vaccines, tetanus vaccine, and hepatitis B vaccine), group T2 (HIV vaccine only), and the placebo group T3 (tetanus vaccine and hepatitis B vaccine). There were no meaningful differences in HIV immune responses between the HIV vaccine recipients with or without the tetanus and hepatitis B vaccines, so the researchers pooled the data from groups T1 and T2 in their analysis.
At the peak immunogenicity time point, the vaccine schedule predominantly induced CD4+ T cells directed to HIV-1 Env; this was measured by expression of interleukin-2 and/or interferon-gamma. The Env-specific CD4+ T-cell response rate was significantly higher in HVTN 097 vaccine recipients than it was in those in the RV144 trial (51.9% vs. 36.4%; P = .043). The HVTN 097 trial also showed significantly higher response rates for CD40L(59.3% for HVTN 097 vs. 33.7% for RV144; P less than .001) and for interferon-gamma (42.6% in HVTN 097 vs. 19.5% in RV144; P = .001).
However, durability at 6 months after the second vaccine injection remained an issue, with the frequency of circulating Env-specific CD4+ T-cell responses among vaccine recipients declining significantly; the response rate dropped from 70.8% to 36.1%.
“These data may indicate that cross-clade immune responses, especially to non-neutralizing epitopes correlated with decreased HIV-1 risk, can be achieved for a globally effective vaccine by using unique HIV Env strains,” Dr. Gray and associates concluded.
The authors declared that they had no competing interests.
SOURCE: Gray GE et al. Sci. Transl. Med. 2019 Sep 18. doi: 10.1126/scitranslmed.aax1880..
ALVAC-HIV vaccine showed immunogenicity across several HIV clades in an early trial involving 100 healthy patients at low risk of HIV infection, according to a study by Glenda E. Gray, MBBCH, FCPaed, of the University of the Witwatersrand, Johannesburg, South Africa, and colleagues that was published online in the Sep. 18 issue of Science Translational Medicine.
ALVAC-HIV (vCP1521) is a live attenuated recombinant canarypox-derived virus that expresses gene products from the HIV-1 gp120 (92TH023/clade E), Gag (clade B), and Pro (clade B) that is cultured in chicken embryo fibroblast cells.
Four injections of ALVAC-HIV were given at months 0, 1, 3, and 6. At months 3 and 6, two booster injections were given of AIDSVAX/BE, a bivalent HIV glycoprotein 120 (gp120) that was previously studied in the RV144 trial. The HVTN 097 trial examined primary immunogenicity endpoints including the frequency and magnitude of IgG and IgG3 antibody binding, measured in serum specimens obtained at baseline, at a peak time point (2 weeks after second ALVAC/AIDSVAX vaccination), a durability time point (6 months after second ALVAC/AIDSVAX vaccination), and the response rates and magnitudes of CD4+ and CD8+ T-cell responses at the baseline, peak, and durability time points. One hundred healthy adults at low risk for HIV infection were randomized in 3:1:1 ratio to group T1 (HIV vaccines, tetanus vaccine, and hepatitis B vaccine), group T2 (HIV vaccine only), and the placebo group T3 (tetanus vaccine and hepatitis B vaccine). There were no meaningful differences in HIV immune responses between the HIV vaccine recipients with or without the tetanus and hepatitis B vaccines, so the researchers pooled the data from groups T1 and T2 in their analysis.
At the peak immunogenicity time point, the vaccine schedule predominantly induced CD4+ T cells directed to HIV-1 Env; this was measured by expression of interleukin-2 and/or interferon-gamma. The Env-specific CD4+ T-cell response rate was significantly higher in HVTN 097 vaccine recipients than it was in those in the RV144 trial (51.9% vs. 36.4%; P = .043). The HVTN 097 trial also showed significantly higher response rates for CD40L(59.3% for HVTN 097 vs. 33.7% for RV144; P less than .001) and for interferon-gamma (42.6% in HVTN 097 vs. 19.5% in RV144; P = .001).
However, durability at 6 months after the second vaccine injection remained an issue, with the frequency of circulating Env-specific CD4+ T-cell responses among vaccine recipients declining significantly; the response rate dropped from 70.8% to 36.1%.
“These data may indicate that cross-clade immune responses, especially to non-neutralizing epitopes correlated with decreased HIV-1 risk, can be achieved for a globally effective vaccine by using unique HIV Env strains,” Dr. Gray and associates concluded.
The authors declared that they had no competing interests.
SOURCE: Gray GE et al. Sci. Transl. Med. 2019 Sep 18. doi: 10.1126/scitranslmed.aax1880..
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: “These data may indicate that cross-clade immune responses ... can be achieved for a globally effective vaccine by using unique HIV Env strains.”
Major finding: At the peak immunogenicity time point, the vaccine schedule predominantly induced CD4+ T cells directed to HIV-1 Env .
Study details: A phase 1b randomized, double-blind, placebo-controlled trial to assess the safety and immunogenicity of the ALVAC-HIV vaccine in 100 healthy patients at low risk of HIV infection.
Disclosures: The study was supported by the National Institute of Allergy and Infectious Diseases and other global health agencies. The authors declared that they had no competing interests.
Source: Gray GE et al. Sci Transl Med. 2019 Sep 18. doi: 10.1126/scitranslmed.aax1880.
Stem cells gene edited to be HIV resistant treat ALL, but not HIV
Gene editing of donor stem cells prior to transplantation into a patient with both HIV infection and acute lymphoblastic leukemia (ALL) was safe and effectively treated the patient’s leukemia, but failed to resolve his HIV, investigators reported.
The 27-year-old man received an HLA-matched transplant of hematopoietic stem and progenitor cells (HSPCs) that had been genetically engineered to lack CCR5, a key gateway for HIV entry into cells.
Although the transplant resulted in complete remission of leukemia with full donor chimerism, only about 9% of the posttransplant lymphocytes showed disruption of CCR5, and during a brief trial of antiretroviral therapy interruption his HIV viral load rebounded, reported Hongkui Deng, PhD, and colleagues from Peking University in China.
Although the experiment did not meet its goal of a drug-free HIV remission, it serves as a proof of concept for the use of CRISPR-Cas9 (clustered regularly interspaced palindromic repeats/CRISPR-associated protein 9) gene editing to treat HIV infection, the authors contend.
“These results show the proof of principle that transplantation and long-term engraftment of CRISPR-edited allogeneic HSPCs can be achieved; however, the efficiency of the response was not adequate to achieve the target of cure of HIV-1 infection,” they wrote in a brief report published in the New England Journal of Medicine.
As previously reported, other research groups have investigated genetic editing to mimic a naturally occurring mutation that effectively disables the CCR5 HIV coreceptor, preventing the retrovirus from entering healthy cells. The mutation was first identified in a man named Timothy Brown who came to be known as “the Berlin patient” after he was apparently cured of HIV infection after a bone marrow transplant from a donor who had the mutation.
Dr. Deng and colleagues took advantage of HSPC transplantation, a standard therapy for ALL to see whether it could also have beneficial effects on concomitant HIV infection.
They treated donor HSPCs with CRISPR-Cas9 to ablate CCR5 and then delivered them to the patient along with additional CD34-depleted donor cells from mobilized peripheral blood.
The transplant was a success, with neutrophil engraftment on day 13 and platelet engraftment on day 27, and the leukemia was in morphologic complete remission at week 4 following transplantation. The patient remained in complete remission from leukemia throughout the 19-month follow-up period, with full donor chimerism .
However, when a planned interruption of antiretroviral therapy was carried out at 7 months post transplant, the serum viral load increased to 3 × 107 copies/ml at week 4 following interruption, and the patient was restarted on the drug. His viral levels gradually decreased to undetectable level during the subsequent months.
The investigators noted that 2 weeks after the drug interruption trial was started there was a small increase in the percentage of CCR5 insertion/deletions.
“The low efficiency of gene editing in the patient may be due to the competitive engraftment of the coinfused HSPCs in CD34-depleted cells and the persistence of donor T cells. To further clarify the anti-HIV effect of CCR5-ablated HSPCs, it will be essential to increase the gene-editing efficiency of our CRISPR-Cas9 system and improve the transplantation protocol,” they wrote.
The study was funded by the Beijing Municipal Science and Technology Commission and others (unspecified). All authors reported having nothing to disclose.
SOURCE: Xu L et al. N Engl J Med. 2019. doi: 10.1056/NEJMoa1817426.
Gene editing of donor stem cells prior to transplantation into a patient with both HIV infection and acute lymphoblastic leukemia (ALL) was safe and effectively treated the patient’s leukemia, but failed to resolve his HIV, investigators reported.
The 27-year-old man received an HLA-matched transplant of hematopoietic stem and progenitor cells (HSPCs) that had been genetically engineered to lack CCR5, a key gateway for HIV entry into cells.
Although the transplant resulted in complete remission of leukemia with full donor chimerism, only about 9% of the posttransplant lymphocytes showed disruption of CCR5, and during a brief trial of antiretroviral therapy interruption his HIV viral load rebounded, reported Hongkui Deng, PhD, and colleagues from Peking University in China.
Although the experiment did not meet its goal of a drug-free HIV remission, it serves as a proof of concept for the use of CRISPR-Cas9 (clustered regularly interspaced palindromic repeats/CRISPR-associated protein 9) gene editing to treat HIV infection, the authors contend.
“These results show the proof of principle that transplantation and long-term engraftment of CRISPR-edited allogeneic HSPCs can be achieved; however, the efficiency of the response was not adequate to achieve the target of cure of HIV-1 infection,” they wrote in a brief report published in the New England Journal of Medicine.
As previously reported, other research groups have investigated genetic editing to mimic a naturally occurring mutation that effectively disables the CCR5 HIV coreceptor, preventing the retrovirus from entering healthy cells. The mutation was first identified in a man named Timothy Brown who came to be known as “the Berlin patient” after he was apparently cured of HIV infection after a bone marrow transplant from a donor who had the mutation.
Dr. Deng and colleagues took advantage of HSPC transplantation, a standard therapy for ALL to see whether it could also have beneficial effects on concomitant HIV infection.
They treated donor HSPCs with CRISPR-Cas9 to ablate CCR5 and then delivered them to the patient along with additional CD34-depleted donor cells from mobilized peripheral blood.
The transplant was a success, with neutrophil engraftment on day 13 and platelet engraftment on day 27, and the leukemia was in morphologic complete remission at week 4 following transplantation. The patient remained in complete remission from leukemia throughout the 19-month follow-up period, with full donor chimerism .
However, when a planned interruption of antiretroviral therapy was carried out at 7 months post transplant, the serum viral load increased to 3 × 107 copies/ml at week 4 following interruption, and the patient was restarted on the drug. His viral levels gradually decreased to undetectable level during the subsequent months.
The investigators noted that 2 weeks after the drug interruption trial was started there was a small increase in the percentage of CCR5 insertion/deletions.
“The low efficiency of gene editing in the patient may be due to the competitive engraftment of the coinfused HSPCs in CD34-depleted cells and the persistence of donor T cells. To further clarify the anti-HIV effect of CCR5-ablated HSPCs, it will be essential to increase the gene-editing efficiency of our CRISPR-Cas9 system and improve the transplantation protocol,” they wrote.
The study was funded by the Beijing Municipal Science and Technology Commission and others (unspecified). All authors reported having nothing to disclose.
SOURCE: Xu L et al. N Engl J Med. 2019. doi: 10.1056/NEJMoa1817426.
Gene editing of donor stem cells prior to transplantation into a patient with both HIV infection and acute lymphoblastic leukemia (ALL) was safe and effectively treated the patient’s leukemia, but failed to resolve his HIV, investigators reported.
The 27-year-old man received an HLA-matched transplant of hematopoietic stem and progenitor cells (HSPCs) that had been genetically engineered to lack CCR5, a key gateway for HIV entry into cells.
Although the transplant resulted in complete remission of leukemia with full donor chimerism, only about 9% of the posttransplant lymphocytes showed disruption of CCR5, and during a brief trial of antiretroviral therapy interruption his HIV viral load rebounded, reported Hongkui Deng, PhD, and colleagues from Peking University in China.
Although the experiment did not meet its goal of a drug-free HIV remission, it serves as a proof of concept for the use of CRISPR-Cas9 (clustered regularly interspaced palindromic repeats/CRISPR-associated protein 9) gene editing to treat HIV infection, the authors contend.
“These results show the proof of principle that transplantation and long-term engraftment of CRISPR-edited allogeneic HSPCs can be achieved; however, the efficiency of the response was not adequate to achieve the target of cure of HIV-1 infection,” they wrote in a brief report published in the New England Journal of Medicine.
As previously reported, other research groups have investigated genetic editing to mimic a naturally occurring mutation that effectively disables the CCR5 HIV coreceptor, preventing the retrovirus from entering healthy cells. The mutation was first identified in a man named Timothy Brown who came to be known as “the Berlin patient” after he was apparently cured of HIV infection after a bone marrow transplant from a donor who had the mutation.
Dr. Deng and colleagues took advantage of HSPC transplantation, a standard therapy for ALL to see whether it could also have beneficial effects on concomitant HIV infection.
They treated donor HSPCs with CRISPR-Cas9 to ablate CCR5 and then delivered them to the patient along with additional CD34-depleted donor cells from mobilized peripheral blood.
The transplant was a success, with neutrophil engraftment on day 13 and platelet engraftment on day 27, and the leukemia was in morphologic complete remission at week 4 following transplantation. The patient remained in complete remission from leukemia throughout the 19-month follow-up period, with full donor chimerism .
However, when a planned interruption of antiretroviral therapy was carried out at 7 months post transplant, the serum viral load increased to 3 × 107 copies/ml at week 4 following interruption, and the patient was restarted on the drug. His viral levels gradually decreased to undetectable level during the subsequent months.
The investigators noted that 2 weeks after the drug interruption trial was started there was a small increase in the percentage of CCR5 insertion/deletions.
“The low efficiency of gene editing in the patient may be due to the competitive engraftment of the coinfused HSPCs in CD34-depleted cells and the persistence of donor T cells. To further clarify the anti-HIV effect of CCR5-ablated HSPCs, it will be essential to increase the gene-editing efficiency of our CRISPR-Cas9 system and improve the transplantation protocol,” they wrote.
The study was funded by the Beijing Municipal Science and Technology Commission and others (unspecified). All authors reported having nothing to disclose.
SOURCE: Xu L et al. N Engl J Med. 2019. doi: 10.1056/NEJMoa1817426.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Donor cells depleted of the HIV coreceptor CCR5 effectively treated ALL, but not HIV.
Major finding: The patient had a sustained complete remission of ALL, but HIV persisted after transplantation.
Study details: Case report of a 27-year-old man with ALL and HIV.
Disclosures: The study was funded by the Beijing Municipal Science and Technology Commission and others (unspecified). All authors reported having nothing to disclose.
Source: Xu L et al. N Engl J Med. 2019. doi: 10.1056/NEJMoa1817426.
Summary: Preexposure prophylaxis for the prevention of HIV infection USPSTF recommendation statement
It is estimated that there are 1.1 million people in the United States living with HIV and that 15% of those people do not know they have HIV. Although the number of new cases reported each year is decreasing, there were still 38,281 new diagnoses in 2017. New cases might be decreasing overall, but the incidence of HIV is rising in some groups including people aged 25-29 years old and American Indian/Alaska Native and Asian populations. In addition, HIV disproportionately affects men who have sex with men, black/African American populations, and Hispanic/Latino populations, according to the USPSTF statement.
Given the prevalence of HIV and rising new cases in certain groups, it is thought that preexposure prophylaxis (PrEP) is being underutilized. The CDC reported that, in 2015, 1.2 million people were candidates for PrEP, but in 2017, only 100,282 people were using PrEP. The USPSTF performed a meta-analysis of 12 RCTs comparing rates of HIV infection in groups treated with PrEP versus those treated with placebo or no treatment and found a risk ratio of 0.46 (95% confidence interval, 0.33-0.66) and absolute risk reduction of –2% (95% CI, –2.8% to –1.2%) after 4 months and 4 years.
With this epidemiologic data and the meta-analysis, the USPSTF offered the following recommendations.
Screening
In order to decrease the rates of transmission and incidence of HIV infection, we must appropriately identify those who would be good candidates for PrEP. That begins with taking a complete and thorough sexual and injection drug use history in a manner that does not make patients feel stigmatized or discriminated against. The USPSTF recommends screening for HIV infection in patients aged 15-65 years old, in younger and older patients who have increased risk factors, and all pregnant patients. PrEP is not an appropriate choice in those who have HIV because it can lead to drug resistance.
When screening for HIV and considering starting PrEP, it is recommended that clinicians also test for kidney function, hepatitis B and C, other STIs, and pregnancy. The USPSTF suggests that the following groups be considered for PrEP given the increased risk of HIV infection:
- Men who have sex with men, are sexually active, and have one of these additional characteristics: a serodiscordant sex partner, inconsistent use of condoms during receptive or insertive anal sex, or infection with syphilis, gonorrhea, or chlamydia in the past 6 months.
- Heterosexual men or women who are sexually active with one or more of these additional characteristics: a serodiscordant sex partner, inconsistent use of condoms during sex with a partner whose HIV status is unknown and who is at high risk, and infection with syphilis or gonorrhea in the past 6 months.
- Patients who inject drugs with one or more of the following characteristics: shared use of drug injection equipment and risk of sexual acquisition (as in the categories above).
The USPSTF also notes that those who engage in transactional sex (for money, drugs, or housing) and transgender patients are at an increased risk of HIV infection.
Treatment
The only FDA approved treatment for the prevention HIV infection is once daily oral combined tenofovir disoproxil fumarate and emtricitabine; however, some studies have found that tenofovir disproxil fumarate monotherapy is also effective. Considering these trials, the CDC has suggested that tenofovir disoproxil fumarate monotherapy can be used as an alternative for men and women at high risk and those who inject drugs.
Tenofovir disoproxil fumarate/emtricitabine can also be used in pregnant patients, however the USPSTF notes that no PrEP trials included pregnant women. Additionally, tenofovir disoproxil fumarate/emtricitabine can be used in adolescents who weigh more than 35 kg. It is unknown how much time it takes to achieve protection against HIV infection after starting PrEP, and there is no clear timeline for how long patients should be on PrEP. Patients may discontinue medication because of preference, decreased risk of HIV exposure, or side effects.
Side effects include renal adverse events (serum creatinine rise), gastrointestinal adverse events (mostly nausea), and bone loss and increased fracture risk, although none were statistically significant when PrEP and placebo groups were compared. The USPSTF’s recommendations note that the effectiveness of PrEP is dependent on medication adherence.
While PrEP is an important part of preventing HIV, it is always important to counsel patients on other ways to reduce risk. The USPSTF notes that consistent condom use reduces the risk of HIV infection by around 80% in addition to reducing the risk of other STIs. All trials studied by the USPSTF for these recommendations included counseling on behavior, adherence, and condom use.
Bottom Line
It is estimated that 1.1 million Americans are living with HIV and 15% are unaware that they are positive for HIV. Overall cases of new HIV diagnoses are down, but they are rising in some groups. Every patient should be screened for high-risk sexual behavior and drug use with a thorough history. Patients aged 15-65 years should be screened for HIV. If patients are negative for HIV, but participate in high-risk sexual behaviors and drug injection, they should be offered PrEP along with counseling on, medication adherence, condom use, and reduction of high-risk behaviors.
Dr. Skolnik is professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Sprogell is a second-year resident in the Family Medicine Residency Program at Abington Jefferson Health
Reference
Owens DK et al. “Preexposure prophylaxis for the prevention of HIV infection: US Preventive Services Task Force recommendation statement.” JAMA. 2019 Jun 11;321(22):2203-13.
It is estimated that there are 1.1 million people in the United States living with HIV and that 15% of those people do not know they have HIV. Although the number of new cases reported each year is decreasing, there were still 38,281 new diagnoses in 2017. New cases might be decreasing overall, but the incidence of HIV is rising in some groups including people aged 25-29 years old and American Indian/Alaska Native and Asian populations. In addition, HIV disproportionately affects men who have sex with men, black/African American populations, and Hispanic/Latino populations, according to the USPSTF statement.
Given the prevalence of HIV and rising new cases in certain groups, it is thought that preexposure prophylaxis (PrEP) is being underutilized. The CDC reported that, in 2015, 1.2 million people were candidates for PrEP, but in 2017, only 100,282 people were using PrEP. The USPSTF performed a meta-analysis of 12 RCTs comparing rates of HIV infection in groups treated with PrEP versus those treated with placebo or no treatment and found a risk ratio of 0.46 (95% confidence interval, 0.33-0.66) and absolute risk reduction of –2% (95% CI, –2.8% to –1.2%) after 4 months and 4 years.
With this epidemiologic data and the meta-analysis, the USPSTF offered the following recommendations.
Screening
In order to decrease the rates of transmission and incidence of HIV infection, we must appropriately identify those who would be good candidates for PrEP. That begins with taking a complete and thorough sexual and injection drug use history in a manner that does not make patients feel stigmatized or discriminated against. The USPSTF recommends screening for HIV infection in patients aged 15-65 years old, in younger and older patients who have increased risk factors, and all pregnant patients. PrEP is not an appropriate choice in those who have HIV because it can lead to drug resistance.
When screening for HIV and considering starting PrEP, it is recommended that clinicians also test for kidney function, hepatitis B and C, other STIs, and pregnancy. The USPSTF suggests that the following groups be considered for PrEP given the increased risk of HIV infection:
- Men who have sex with men, are sexually active, and have one of these additional characteristics: a serodiscordant sex partner, inconsistent use of condoms during receptive or insertive anal sex, or infection with syphilis, gonorrhea, or chlamydia in the past 6 months.
- Heterosexual men or women who are sexually active with one or more of these additional characteristics: a serodiscordant sex partner, inconsistent use of condoms during sex with a partner whose HIV status is unknown and who is at high risk, and infection with syphilis or gonorrhea in the past 6 months.
- Patients who inject drugs with one or more of the following characteristics: shared use of drug injection equipment and risk of sexual acquisition (as in the categories above).
The USPSTF also notes that those who engage in transactional sex (for money, drugs, or housing) and transgender patients are at an increased risk of HIV infection.
Treatment
The only FDA approved treatment for the prevention HIV infection is once daily oral combined tenofovir disoproxil fumarate and emtricitabine; however, some studies have found that tenofovir disproxil fumarate monotherapy is also effective. Considering these trials, the CDC has suggested that tenofovir disoproxil fumarate monotherapy can be used as an alternative for men and women at high risk and those who inject drugs.
Tenofovir disoproxil fumarate/emtricitabine can also be used in pregnant patients, however the USPSTF notes that no PrEP trials included pregnant women. Additionally, tenofovir disoproxil fumarate/emtricitabine can be used in adolescents who weigh more than 35 kg. It is unknown how much time it takes to achieve protection against HIV infection after starting PrEP, and there is no clear timeline for how long patients should be on PrEP. Patients may discontinue medication because of preference, decreased risk of HIV exposure, or side effects.
Side effects include renal adverse events (serum creatinine rise), gastrointestinal adverse events (mostly nausea), and bone loss and increased fracture risk, although none were statistically significant when PrEP and placebo groups were compared. The USPSTF’s recommendations note that the effectiveness of PrEP is dependent on medication adherence.
While PrEP is an important part of preventing HIV, it is always important to counsel patients on other ways to reduce risk. The USPSTF notes that consistent condom use reduces the risk of HIV infection by around 80% in addition to reducing the risk of other STIs. All trials studied by the USPSTF for these recommendations included counseling on behavior, adherence, and condom use.
Bottom Line
It is estimated that 1.1 million Americans are living with HIV and 15% are unaware that they are positive for HIV. Overall cases of new HIV diagnoses are down, but they are rising in some groups. Every patient should be screened for high-risk sexual behavior and drug use with a thorough history. Patients aged 15-65 years should be screened for HIV. If patients are negative for HIV, but participate in high-risk sexual behaviors and drug injection, they should be offered PrEP along with counseling on, medication adherence, condom use, and reduction of high-risk behaviors.
Dr. Skolnik is professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Sprogell is a second-year resident in the Family Medicine Residency Program at Abington Jefferson Health
Reference
Owens DK et al. “Preexposure prophylaxis for the prevention of HIV infection: US Preventive Services Task Force recommendation statement.” JAMA. 2019 Jun 11;321(22):2203-13.
It is estimated that there are 1.1 million people in the United States living with HIV and that 15% of those people do not know they have HIV. Although the number of new cases reported each year is decreasing, there were still 38,281 new diagnoses in 2017. New cases might be decreasing overall, but the incidence of HIV is rising in some groups including people aged 25-29 years old and American Indian/Alaska Native and Asian populations. In addition, HIV disproportionately affects men who have sex with men, black/African American populations, and Hispanic/Latino populations, according to the USPSTF statement.
Given the prevalence of HIV and rising new cases in certain groups, it is thought that preexposure prophylaxis (PrEP) is being underutilized. The CDC reported that, in 2015, 1.2 million people were candidates for PrEP, but in 2017, only 100,282 people were using PrEP. The USPSTF performed a meta-analysis of 12 RCTs comparing rates of HIV infection in groups treated with PrEP versus those treated with placebo or no treatment and found a risk ratio of 0.46 (95% confidence interval, 0.33-0.66) and absolute risk reduction of –2% (95% CI, –2.8% to –1.2%) after 4 months and 4 years.
With this epidemiologic data and the meta-analysis, the USPSTF offered the following recommendations.
Screening
In order to decrease the rates of transmission and incidence of HIV infection, we must appropriately identify those who would be good candidates for PrEP. That begins with taking a complete and thorough sexual and injection drug use history in a manner that does not make patients feel stigmatized or discriminated against. The USPSTF recommends screening for HIV infection in patients aged 15-65 years old, in younger and older patients who have increased risk factors, and all pregnant patients. PrEP is not an appropriate choice in those who have HIV because it can lead to drug resistance.
When screening for HIV and considering starting PrEP, it is recommended that clinicians also test for kidney function, hepatitis B and C, other STIs, and pregnancy. The USPSTF suggests that the following groups be considered for PrEP given the increased risk of HIV infection:
- Men who have sex with men, are sexually active, and have one of these additional characteristics: a serodiscordant sex partner, inconsistent use of condoms during receptive or insertive anal sex, or infection with syphilis, gonorrhea, or chlamydia in the past 6 months.
- Heterosexual men or women who are sexually active with one or more of these additional characteristics: a serodiscordant sex partner, inconsistent use of condoms during sex with a partner whose HIV status is unknown and who is at high risk, and infection with syphilis or gonorrhea in the past 6 months.
- Patients who inject drugs with one or more of the following characteristics: shared use of drug injection equipment and risk of sexual acquisition (as in the categories above).
The USPSTF also notes that those who engage in transactional sex (for money, drugs, or housing) and transgender patients are at an increased risk of HIV infection.
Treatment
The only FDA approved treatment for the prevention HIV infection is once daily oral combined tenofovir disoproxil fumarate and emtricitabine; however, some studies have found that tenofovir disproxil fumarate monotherapy is also effective. Considering these trials, the CDC has suggested that tenofovir disoproxil fumarate monotherapy can be used as an alternative for men and women at high risk and those who inject drugs.
Tenofovir disoproxil fumarate/emtricitabine can also be used in pregnant patients, however the USPSTF notes that no PrEP trials included pregnant women. Additionally, tenofovir disoproxil fumarate/emtricitabine can be used in adolescents who weigh more than 35 kg. It is unknown how much time it takes to achieve protection against HIV infection after starting PrEP, and there is no clear timeline for how long patients should be on PrEP. Patients may discontinue medication because of preference, decreased risk of HIV exposure, or side effects.
Side effects include renal adverse events (serum creatinine rise), gastrointestinal adverse events (mostly nausea), and bone loss and increased fracture risk, although none were statistically significant when PrEP and placebo groups were compared. The USPSTF’s recommendations note that the effectiveness of PrEP is dependent on medication adherence.
While PrEP is an important part of preventing HIV, it is always important to counsel patients on other ways to reduce risk. The USPSTF notes that consistent condom use reduces the risk of HIV infection by around 80% in addition to reducing the risk of other STIs. All trials studied by the USPSTF for these recommendations included counseling on behavior, adherence, and condom use.
Bottom Line
It is estimated that 1.1 million Americans are living with HIV and 15% are unaware that they are positive for HIV. Overall cases of new HIV diagnoses are down, but they are rising in some groups. Every patient should be screened for high-risk sexual behavior and drug use with a thorough history. Patients aged 15-65 years should be screened for HIV. If patients are negative for HIV, but participate in high-risk sexual behaviors and drug injection, they should be offered PrEP along with counseling on, medication adherence, condom use, and reduction of high-risk behaviors.
Dr. Skolnik is professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Sprogell is a second-year resident in the Family Medicine Residency Program at Abington Jefferson Health
Reference
Owens DK et al. “Preexposure prophylaxis for the prevention of HIV infection: US Preventive Services Task Force recommendation statement.” JAMA. 2019 Jun 11;321(22):2203-13.