User login
Try to normalize albumin before laparoscopic hysterectomy
LAS VEGAS – Serum albumin is an everyday health marker commonly used for risk assessment in open abdominal procedures, but it’s often not checked before laparoscopic hysterectomies.
Low levels mean something is off, be it malnutrition, inflammation, chronic disease, or other problems. If it can be normalized before surgery, it should be; women probably will do better, according to investigators from the University of Kentucky, Lexington.
“In minimally invasive gynecologic procedures, we haven’t come to adopt this marker just quite yet. It could be included in the routine battery of tests” at minimal cost. “I think it’s something we should consider,” said ob.gyn. resident Suzanne Lababidi, MD.
The team was curious why serum albumin generally is not a part of routine testing for laparoscopic hysterectomy. The first step was to see if it made a difference, so they reviewed 43,289 cases in the National Surgical Quality Improvement Program database. The women were “par for the course;” 51 years old, on average; and had a mean body mass index of 31.9 kg/m2. More than one-third were hypertensive. Mean albumin was in the normal range at 4.1 g/dL, Dr. Lababidi said at the meeting, sponsored by the American Association of Gynecologic Laparoscopists.
Her team did not come up with a cut-point to delay surgery – that’s the goal of further research – but they noticed on linear regression that women with lower preop albumin had higher rates of surgical site infections and intraoperative transfusions, plus higher rates of postop pneumonia; renal failure; urinary tract infection; sepsis; and deep vein thrombosis, among other issues – and even after controlling for hypertension, diabetes, and other comorbidities. The findings met statistical significance.
It’s true that patients with low albumin might have gone into the operating room sicker, but no matter; Dr. Lababidi’s point was that preop serum albumin is something to pay attention to and correct whenever possible before laparoscopic hysterectomies.
Preop levels are something to consider for “counseling and optimizing patients to improve surgical outcomes,” she said.
The next step toward an albumin cut-point is to weed out confounders by further stratifying patients based on albumin levels, she said.
The work received no industry funding. Dr. Lababidi had no disclosures.
SOURCE: Lababidi S et al. 2018 AAGL Global Congress, Abstract 199.
LAS VEGAS – Serum albumin is an everyday health marker commonly used for risk assessment in open abdominal procedures, but it’s often not checked before laparoscopic hysterectomies.
Low levels mean something is off, be it malnutrition, inflammation, chronic disease, or other problems. If it can be normalized before surgery, it should be; women probably will do better, according to investigators from the University of Kentucky, Lexington.
“In minimally invasive gynecologic procedures, we haven’t come to adopt this marker just quite yet. It could be included in the routine battery of tests” at minimal cost. “I think it’s something we should consider,” said ob.gyn. resident Suzanne Lababidi, MD.
The team was curious why serum albumin generally is not a part of routine testing for laparoscopic hysterectomy. The first step was to see if it made a difference, so they reviewed 43,289 cases in the National Surgical Quality Improvement Program database. The women were “par for the course;” 51 years old, on average; and had a mean body mass index of 31.9 kg/m2. More than one-third were hypertensive. Mean albumin was in the normal range at 4.1 g/dL, Dr. Lababidi said at the meeting, sponsored by the American Association of Gynecologic Laparoscopists.
Her team did not come up with a cut-point to delay surgery – that’s the goal of further research – but they noticed on linear regression that women with lower preop albumin had higher rates of surgical site infections and intraoperative transfusions, plus higher rates of postop pneumonia; renal failure; urinary tract infection; sepsis; and deep vein thrombosis, among other issues – and even after controlling for hypertension, diabetes, and other comorbidities. The findings met statistical significance.
It’s true that patients with low albumin might have gone into the operating room sicker, but no matter; Dr. Lababidi’s point was that preop serum albumin is something to pay attention to and correct whenever possible before laparoscopic hysterectomies.
Preop levels are something to consider for “counseling and optimizing patients to improve surgical outcomes,” she said.
The next step toward an albumin cut-point is to weed out confounders by further stratifying patients based on albumin levels, she said.
The work received no industry funding. Dr. Lababidi had no disclosures.
SOURCE: Lababidi S et al. 2018 AAGL Global Congress, Abstract 199.
LAS VEGAS – Serum albumin is an everyday health marker commonly used for risk assessment in open abdominal procedures, but it’s often not checked before laparoscopic hysterectomies.
Low levels mean something is off, be it malnutrition, inflammation, chronic disease, or other problems. If it can be normalized before surgery, it should be; women probably will do better, according to investigators from the University of Kentucky, Lexington.
“In minimally invasive gynecologic procedures, we haven’t come to adopt this marker just quite yet. It could be included in the routine battery of tests” at minimal cost. “I think it’s something we should consider,” said ob.gyn. resident Suzanne Lababidi, MD.
The team was curious why serum albumin generally is not a part of routine testing for laparoscopic hysterectomy. The first step was to see if it made a difference, so they reviewed 43,289 cases in the National Surgical Quality Improvement Program database. The women were “par for the course;” 51 years old, on average; and had a mean body mass index of 31.9 kg/m2. More than one-third were hypertensive. Mean albumin was in the normal range at 4.1 g/dL, Dr. Lababidi said at the meeting, sponsored by the American Association of Gynecologic Laparoscopists.
Her team did not come up with a cut-point to delay surgery – that’s the goal of further research – but they noticed on linear regression that women with lower preop albumin had higher rates of surgical site infections and intraoperative transfusions, plus higher rates of postop pneumonia; renal failure; urinary tract infection; sepsis; and deep vein thrombosis, among other issues – and even after controlling for hypertension, diabetes, and other comorbidities. The findings met statistical significance.
It’s true that patients with low albumin might have gone into the operating room sicker, but no matter; Dr. Lababidi’s point was that preop serum albumin is something to pay attention to and correct whenever possible before laparoscopic hysterectomies.
Preop levels are something to consider for “counseling and optimizing patients to improve surgical outcomes,” she said.
The next step toward an albumin cut-point is to weed out confounders by further stratifying patients based on albumin levels, she said.
The work received no industry funding. Dr. Lababidi had no disclosures.
SOURCE: Lababidi S et al. 2018 AAGL Global Congress, Abstract 199.
REPORTING FROM THE AAGL GLOBAL CONGRESS
VTE risk after gynecologic surgery lower with laparoscopic procedures
according to a study published in Obstetrics & Gynecology.

The retrospective cohort study looked at data from 37,485 patients who underwent 43,751 gynecologic surgical procedures, including hysterectomy and myomectomy, at two tertiary care academic hospitals.
Overall, 96 patients (0.2%) were diagnosed with postoperative venous thromboembolism. However patients who underwent laparoscopic or vaginal surgery had a significant 78% and 93% lower risk of venous thromboembolism, respectively, than those who underwent laparotomy, even after adjusting for potential confounders such as age, cancer, race, pharmacologic thromboprophylaxis, and surgical time.
The incidence of postoperative thromboembolism was significantly higher among patients undergoing gynecologic surgery for cancer (1.1%). The incidence among those undergoing surgery for benign indications was only 0.2%, and the highest incidence was among patients with cancer who underwent laparotomy (2.2%).
“This study adds to data demonstrating that venous thromboembolism is rare in gynecologic surgery, particularly when a patient undergoes a minimally invasive procedure for benign indications,” wrote Dr. Elisa M. Jorgensen of Beth Israel Deaconess Medical Center, and her coauthors.
Among the 8,273 patients who underwent a hysterectomy, there were 55 cases of venous thromboembolism – representing an 0.7% incidence. However patients who underwent laparotomy had a 1% incidence of postoperative venous thromboembolism, while those who underwent laparoscopic hysterectomy had an 0.3% incidence and those who underwent vaginal hysterectomy had an 0.1% incidence.
Laparotomy was the most common mode of surgery for hysterectomy – accounting for 57% of operations – while 34% were laparoscopic and 9% were vaginal.
However, the authors noted that the use of laparoscopy increased and laparotomy declined over the 9 years of the study. In 2006, 12% of hysterectomies were laparoscopic, compared with 55% in 2015, while over that same period the percentage of laparotomies dropped from 74% to 41%, and the percentage of vaginal procedures declined from 14% to 4%.
“Because current practice guidelines do not account for mode of surgery, we find them to be insufficient for the modern gynecologic surgeon to counsel patients on their individual venous thromboembolism risk or to make ideal decisions regarding selection of thromboprophylaxis,” Dr. Jorgenson and her associates wrote.
Only 5 patients of the 2,851 who underwent myomectomy developed postoperative VTE – an overall incidence of 0.2% – and the authors said numbers were too small to analyze. Vaginal or hysteroscopic myomectomy was the most common surgical method, accounting for 62% of procedures, compared with 23% for laparotomies and 15% for laparoscopies.
More than 90% of patients who experienced postoperative thromboembolism had received some form of thromboprophylaxis before surgery, either mechanical, pharmacologic, or both. In comparison, only 55% of the group who didn’t experience thromboembolism had received thromboprophylaxis.
“The high rate of prophylaxis among patients who developed postoperative venous thromboembolism may reflect surgeons’ abilities to preoperatively identify patients at increased risk, guiding appropriate selection of thromboprophylaxis,” Dr. Jorgenson and her associates wrote.
Addressing the study’s limitations, the authors noted that they were not able to capture data on patients’ body mass index and also were unable to account for patients who might have been diagnosed and treated for postoperative VTE at other hospitals.
No conflicts of interest were declared.
SOURCE: Jorgensen EM et al. Obstet Gynecol. 2018 Nov;132:1275-84.
The aim of this study was to determine the 3-month postoperative incidence of venous thromboembolism among patients undergoing gynecologic surgery. The study also addressed the mode of surgery to allow a comparison between laparotomy and minimally invasive approaches.
Postoperative VTE was defined as deep venous thrombosis of the lower extremities, pulmonary embolism, or both that occurred within 90 days of surgery. A key component of the study was that clinically recognized VTEs that required treatment with anticoagulation, vena caval filter, or both were included.
The study evaluated 43,751 gynecological cases among 37,485 patients. As expected, 59% of the cases were classified as vaginal surgery, 24% were laparoscopic cases, and 17% of the cases were laparotomies.
Of the 8,273 hysterectomies, 57% were via an abdominal approach, 34% were laparoscopic, and 9 were vaginal cases.
Overall, 0.2% of patients were diagnosed with a VTE. As expected, the greatest incidence of VTE was in patients with cancer who underwent a laparotomy. Those with a VTE were significantly more likely to have had an inpatient stay (longer than 24 hours), a cancer diagnosis, a longer surgical time, and an American Society of Anesthesiologists score of 3 or more. They also were older (mean age 56 years vs. 44 years). Of note, 20% of the VTE group identified as black.
Among patients who had a hysterectomy, there were VTEs in 0.7%: 1% in the laparotomy group, 0.3% in the laparoscopic group, and only 0.1% in the vaginal hysterectomy group.
It is interesting to note that 91% of the patients diagnosed with a VTE did received preoperative VTE prophylaxis. The authors noted that the high rate of prophylaxis may have reflected the surgeon’s ability to identify patients who are at high risk.
The authors recognized that the current guidelines do not stratify VTE risk based on the mode of surgery. Further, they noted that low-risk patients undergoing low-risk surgery may be receiving pharmacologic VTE prophylaxis, thus placing these patients at risk for complications related to such therapy.
This paper by Jorgensen et al. should remind us that VTE prophylaxis should be individualized. Patients may not fit nicely into boxes on our EMR; each clinical decision should be made for each patient and for each clinical scenario. The surgeon’s responsibility is to adopt the evidence-based guidelines that serve each individual patient’s unique risk/benefit profile.
David M. Jaspan, DO, is director of minimally invasive and pelvic surgery and chairman of the department of obstetrics and gynecology at the Einstein Medical Center in Philadelphia. Dr. Jaspan, who was asked to comment on the Jorgenson et al. article, said he had no relevant financial disclosures.
The aim of this study was to determine the 3-month postoperative incidence of venous thromboembolism among patients undergoing gynecologic surgery. The study also addressed the mode of surgery to allow a comparison between laparotomy and minimally invasive approaches.
Postoperative VTE was defined as deep venous thrombosis of the lower extremities, pulmonary embolism, or both that occurred within 90 days of surgery. A key component of the study was that clinically recognized VTEs that required treatment with anticoagulation, vena caval filter, or both were included.
The study evaluated 43,751 gynecological cases among 37,485 patients. As expected, 59% of the cases were classified as vaginal surgery, 24% were laparoscopic cases, and 17% of the cases were laparotomies.
Of the 8,273 hysterectomies, 57% were via an abdominal approach, 34% were laparoscopic, and 9 were vaginal cases.
Overall, 0.2% of patients were diagnosed with a VTE. As expected, the greatest incidence of VTE was in patients with cancer who underwent a laparotomy. Those with a VTE were significantly more likely to have had an inpatient stay (longer than 24 hours), a cancer diagnosis, a longer surgical time, and an American Society of Anesthesiologists score of 3 or more. They also were older (mean age 56 years vs. 44 years). Of note, 20% of the VTE group identified as black.
Among patients who had a hysterectomy, there were VTEs in 0.7%: 1% in the laparotomy group, 0.3% in the laparoscopic group, and only 0.1% in the vaginal hysterectomy group.
It is interesting to note that 91% of the patients diagnosed with a VTE did received preoperative VTE prophylaxis. The authors noted that the high rate of prophylaxis may have reflected the surgeon’s ability to identify patients who are at high risk.
The authors recognized that the current guidelines do not stratify VTE risk based on the mode of surgery. Further, they noted that low-risk patients undergoing low-risk surgery may be receiving pharmacologic VTE prophylaxis, thus placing these patients at risk for complications related to such therapy.
This paper by Jorgensen et al. should remind us that VTE prophylaxis should be individualized. Patients may not fit nicely into boxes on our EMR; each clinical decision should be made for each patient and for each clinical scenario. The surgeon’s responsibility is to adopt the evidence-based guidelines that serve each individual patient’s unique risk/benefit profile.
David M. Jaspan, DO, is director of minimally invasive and pelvic surgery and chairman of the department of obstetrics and gynecology at the Einstein Medical Center in Philadelphia. Dr. Jaspan, who was asked to comment on the Jorgenson et al. article, said he had no relevant financial disclosures.
The aim of this study was to determine the 3-month postoperative incidence of venous thromboembolism among patients undergoing gynecologic surgery. The study also addressed the mode of surgery to allow a comparison between laparotomy and minimally invasive approaches.
Postoperative VTE was defined as deep venous thrombosis of the lower extremities, pulmonary embolism, or both that occurred within 90 days of surgery. A key component of the study was that clinically recognized VTEs that required treatment with anticoagulation, vena caval filter, or both were included.
The study evaluated 43,751 gynecological cases among 37,485 patients. As expected, 59% of the cases were classified as vaginal surgery, 24% were laparoscopic cases, and 17% of the cases were laparotomies.
Of the 8,273 hysterectomies, 57% were via an abdominal approach, 34% were laparoscopic, and 9 were vaginal cases.
Overall, 0.2% of patients were diagnosed with a VTE. As expected, the greatest incidence of VTE was in patients with cancer who underwent a laparotomy. Those with a VTE were significantly more likely to have had an inpatient stay (longer than 24 hours), a cancer diagnosis, a longer surgical time, and an American Society of Anesthesiologists score of 3 or more. They also were older (mean age 56 years vs. 44 years). Of note, 20% of the VTE group identified as black.
Among patients who had a hysterectomy, there were VTEs in 0.7%: 1% in the laparotomy group, 0.3% in the laparoscopic group, and only 0.1% in the vaginal hysterectomy group.
It is interesting to note that 91% of the patients diagnosed with a VTE did received preoperative VTE prophylaxis. The authors noted that the high rate of prophylaxis may have reflected the surgeon’s ability to identify patients who are at high risk.
The authors recognized that the current guidelines do not stratify VTE risk based on the mode of surgery. Further, they noted that low-risk patients undergoing low-risk surgery may be receiving pharmacologic VTE prophylaxis, thus placing these patients at risk for complications related to such therapy.
This paper by Jorgensen et al. should remind us that VTE prophylaxis should be individualized. Patients may not fit nicely into boxes on our EMR; each clinical decision should be made for each patient and for each clinical scenario. The surgeon’s responsibility is to adopt the evidence-based guidelines that serve each individual patient’s unique risk/benefit profile.
David M. Jaspan, DO, is director of minimally invasive and pelvic surgery and chairman of the department of obstetrics and gynecology at the Einstein Medical Center in Philadelphia. Dr. Jaspan, who was asked to comment on the Jorgenson et al. article, said he had no relevant financial disclosures.
according to a study published in Obstetrics & Gynecology.

The retrospective cohort study looked at data from 37,485 patients who underwent 43,751 gynecologic surgical procedures, including hysterectomy and myomectomy, at two tertiary care academic hospitals.
Overall, 96 patients (0.2%) were diagnosed with postoperative venous thromboembolism. However patients who underwent laparoscopic or vaginal surgery had a significant 78% and 93% lower risk of venous thromboembolism, respectively, than those who underwent laparotomy, even after adjusting for potential confounders such as age, cancer, race, pharmacologic thromboprophylaxis, and surgical time.
The incidence of postoperative thromboembolism was significantly higher among patients undergoing gynecologic surgery for cancer (1.1%). The incidence among those undergoing surgery for benign indications was only 0.2%, and the highest incidence was among patients with cancer who underwent laparotomy (2.2%).
“This study adds to data demonstrating that venous thromboembolism is rare in gynecologic surgery, particularly when a patient undergoes a minimally invasive procedure for benign indications,” wrote Dr. Elisa M. Jorgensen of Beth Israel Deaconess Medical Center, and her coauthors.
Among the 8,273 patients who underwent a hysterectomy, there were 55 cases of venous thromboembolism – representing an 0.7% incidence. However patients who underwent laparotomy had a 1% incidence of postoperative venous thromboembolism, while those who underwent laparoscopic hysterectomy had an 0.3% incidence and those who underwent vaginal hysterectomy had an 0.1% incidence.
Laparotomy was the most common mode of surgery for hysterectomy – accounting for 57% of operations – while 34% were laparoscopic and 9% were vaginal.
However, the authors noted that the use of laparoscopy increased and laparotomy declined over the 9 years of the study. In 2006, 12% of hysterectomies were laparoscopic, compared with 55% in 2015, while over that same period the percentage of laparotomies dropped from 74% to 41%, and the percentage of vaginal procedures declined from 14% to 4%.
“Because current practice guidelines do not account for mode of surgery, we find them to be insufficient for the modern gynecologic surgeon to counsel patients on their individual venous thromboembolism risk or to make ideal decisions regarding selection of thromboprophylaxis,” Dr. Jorgenson and her associates wrote.
Only 5 patients of the 2,851 who underwent myomectomy developed postoperative VTE – an overall incidence of 0.2% – and the authors said numbers were too small to analyze. Vaginal or hysteroscopic myomectomy was the most common surgical method, accounting for 62% of procedures, compared with 23% for laparotomies and 15% for laparoscopies.
More than 90% of patients who experienced postoperative thromboembolism had received some form of thromboprophylaxis before surgery, either mechanical, pharmacologic, or both. In comparison, only 55% of the group who didn’t experience thromboembolism had received thromboprophylaxis.
“The high rate of prophylaxis among patients who developed postoperative venous thromboembolism may reflect surgeons’ abilities to preoperatively identify patients at increased risk, guiding appropriate selection of thromboprophylaxis,” Dr. Jorgenson and her associates wrote.
Addressing the study’s limitations, the authors noted that they were not able to capture data on patients’ body mass index and also were unable to account for patients who might have been diagnosed and treated for postoperative VTE at other hospitals.
No conflicts of interest were declared.
SOURCE: Jorgensen EM et al. Obstet Gynecol. 2018 Nov;132:1275-84.
according to a study published in Obstetrics & Gynecology.

The retrospective cohort study looked at data from 37,485 patients who underwent 43,751 gynecologic surgical procedures, including hysterectomy and myomectomy, at two tertiary care academic hospitals.
Overall, 96 patients (0.2%) were diagnosed with postoperative venous thromboembolism. However patients who underwent laparoscopic or vaginal surgery had a significant 78% and 93% lower risk of venous thromboembolism, respectively, than those who underwent laparotomy, even after adjusting for potential confounders such as age, cancer, race, pharmacologic thromboprophylaxis, and surgical time.
The incidence of postoperative thromboembolism was significantly higher among patients undergoing gynecologic surgery for cancer (1.1%). The incidence among those undergoing surgery for benign indications was only 0.2%, and the highest incidence was among patients with cancer who underwent laparotomy (2.2%).
“This study adds to data demonstrating that venous thromboembolism is rare in gynecologic surgery, particularly when a patient undergoes a minimally invasive procedure for benign indications,” wrote Dr. Elisa M. Jorgensen of Beth Israel Deaconess Medical Center, and her coauthors.
Among the 8,273 patients who underwent a hysterectomy, there were 55 cases of venous thromboembolism – representing an 0.7% incidence. However patients who underwent laparotomy had a 1% incidence of postoperative venous thromboembolism, while those who underwent laparoscopic hysterectomy had an 0.3% incidence and those who underwent vaginal hysterectomy had an 0.1% incidence.
Laparotomy was the most common mode of surgery for hysterectomy – accounting for 57% of operations – while 34% were laparoscopic and 9% were vaginal.
However, the authors noted that the use of laparoscopy increased and laparotomy declined over the 9 years of the study. In 2006, 12% of hysterectomies were laparoscopic, compared with 55% in 2015, while over that same period the percentage of laparotomies dropped from 74% to 41%, and the percentage of vaginal procedures declined from 14% to 4%.
“Because current practice guidelines do not account for mode of surgery, we find them to be insufficient for the modern gynecologic surgeon to counsel patients on their individual venous thromboembolism risk or to make ideal decisions regarding selection of thromboprophylaxis,” Dr. Jorgenson and her associates wrote.
Only 5 patients of the 2,851 who underwent myomectomy developed postoperative VTE – an overall incidence of 0.2% – and the authors said numbers were too small to analyze. Vaginal or hysteroscopic myomectomy was the most common surgical method, accounting for 62% of procedures, compared with 23% for laparotomies and 15% for laparoscopies.
More than 90% of patients who experienced postoperative thromboembolism had received some form of thromboprophylaxis before surgery, either mechanical, pharmacologic, or both. In comparison, only 55% of the group who didn’t experience thromboembolism had received thromboprophylaxis.
“The high rate of prophylaxis among patients who developed postoperative venous thromboembolism may reflect surgeons’ abilities to preoperatively identify patients at increased risk, guiding appropriate selection of thromboprophylaxis,” Dr. Jorgenson and her associates wrote.
Addressing the study’s limitations, the authors noted that they were not able to capture data on patients’ body mass index and also were unable to account for patients who might have been diagnosed and treated for postoperative VTE at other hospitals.
No conflicts of interest were declared.
SOURCE: Jorgensen EM et al. Obstet Gynecol. 2018 Nov;132:1275-84.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point: Laparoscopic gynecologic surgery is associated with a lower risk of postoperative VTE than laparotomy.
Major finding: Laparoscopic hysterectomy was associated with a 78% lower incidence of postoperative VTE than laparotomy.
Study details: Retrospective cohort study of 37,485 patients who underwent 43,751 gynecologic surgical procedures
Disclosures: No conflicts of interest were declared.
Source: Jorgensen EM et al. Obstet Gynecol. 2018 Nov;132:1275-84.
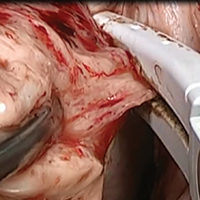
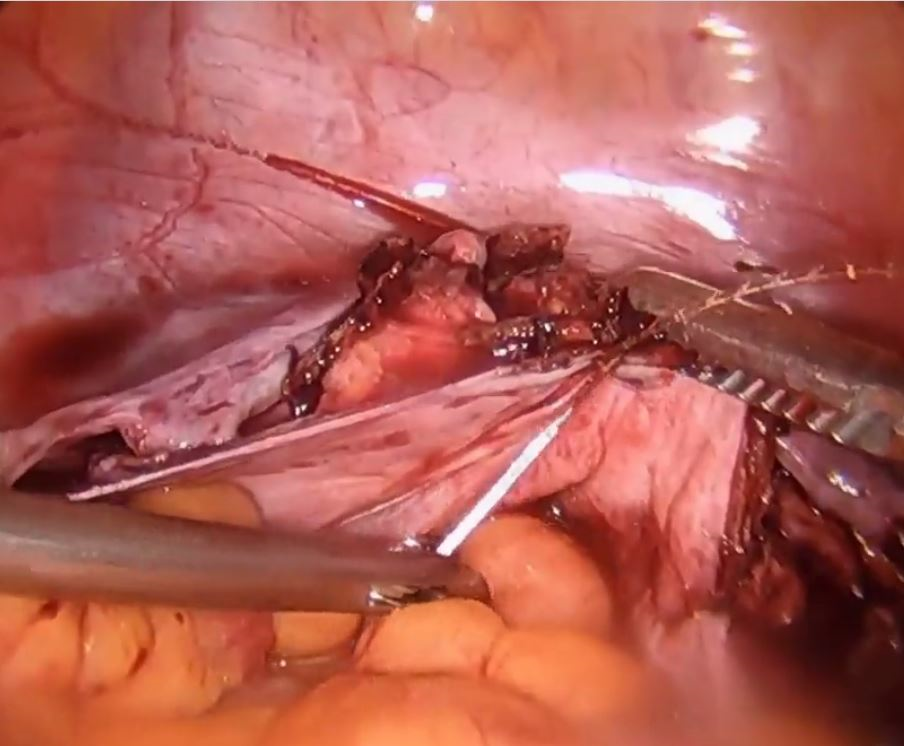
Laparoscopic suturing is an option
Laparoscopic suturing is an option
Dr. Lum presented a nicely produced video demonstrating various strategies aimed at facilitating total laparoscopic hysterectomy (TLH) of the very large uterus. Her patient’s evaluation included magnetic resonance imaging. In the video, she demonstrates a variety of interventions, including the use of a preoperative gonadotropin–releasing hormone (GNRH) agonist and immediate perioperative radial artery–uterine artery embolization. Intraoperative techniques include use of ureteral stents and securing the uterine arteries at their origins.
Clearly, TLH of a huge uterus is a technical challenge. However, I’d like to suggest that a relatively basic and important skill would greatly assist in such procedures and likely obviate the need for a GNRH agonist and/or uterine artery embolization. The vessel-sealing devices shown in the video are generally not capable of sealing such large vessels adequately, and this is what leads to the massive hemorrhaging that often occurs.
Laparoscopic suturing with extracorporeal knot tying can be used effectively to control the extremely large vessels associated with a huge uterus. The judicious placement of sutures can completely control such vessels and prevent bleeding from both proximal and distal ends when 2 sutures are placed and the vessels are transected between the stitches. Many laparoscopic surgeons have come to rely on bipolar energy or ultrasonic devices to coagulate vessels. But when dealing with huge vessels, a return to basics using laparoscopic suturing will greatly benefit the patient and the surgeon by reducing blood loss and operative time.
David L. Zisow, MD
Baltimore, Maryland
Dr. Lum responds
I thank Dr. Zisow for his thoughtful comments. I agree that laparoscopic suturing is an essential skill that can be utilized to suture ligate vessels. If we consider the basics of an open hysterectomy, the uterine artery is clamped first, then suture ligated. When approaching a very large vessel during TLH, I would be concerned that a simple suture around a large vessel might tear through and cause more bleeding. To mitigate this risk, the vessel can be clamped with a grasper first, similar to the approach in an open hysterectomy. However, once a vessel is compressed, a sealing device can usually work just as well as a suture. It becomes a matter of preference and cost.
During hysterectomy of a very large uterus, a big challenge is managing bleeding of the uterus itself during manipulation from above. Bleeding from the vascular sinuses of the myometrium can be brisk and obscure visualization, potentially leading to laparotomy conversion. A common misconception is that uterine artery embolization is equivalent to suturing the uterine arteries. In actuality, the goal of a uterine artery embolization is to embolize the distal branches of the uterine arteries, which can help with any potential bleeding from the uterus itself during hysterectomy.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Laparoscopic suturing is an option
Dr. Lum presented a nicely produced video demonstrating various strategies aimed at facilitating total laparoscopic hysterectomy (TLH) of the very large uterus. Her patient’s evaluation included magnetic resonance imaging. In the video, she demonstrates a variety of interventions, including the use of a preoperative gonadotropin–releasing hormone (GNRH) agonist and immediate perioperative radial artery–uterine artery embolization. Intraoperative techniques include use of ureteral stents and securing the uterine arteries at their origins.
Clearly, TLH of a huge uterus is a technical challenge. However, I’d like to suggest that a relatively basic and important skill would greatly assist in such procedures and likely obviate the need for a GNRH agonist and/or uterine artery embolization. The vessel-sealing devices shown in the video are generally not capable of sealing such large vessels adequately, and this is what leads to the massive hemorrhaging that often occurs.
Laparoscopic suturing with extracorporeal knot tying can be used effectively to control the extremely large vessels associated with a huge uterus. The judicious placement of sutures can completely control such vessels and prevent bleeding from both proximal and distal ends when 2 sutures are placed and the vessels are transected between the stitches. Many laparoscopic surgeons have come to rely on bipolar energy or ultrasonic devices to coagulate vessels. But when dealing with huge vessels, a return to basics using laparoscopic suturing will greatly benefit the patient and the surgeon by reducing blood loss and operative time.
David L. Zisow, MD
Baltimore, Maryland
Dr. Lum responds
I thank Dr. Zisow for his thoughtful comments. I agree that laparoscopic suturing is an essential skill that can be utilized to suture ligate vessels. If we consider the basics of an open hysterectomy, the uterine artery is clamped first, then suture ligated. When approaching a very large vessel during TLH, I would be concerned that a simple suture around a large vessel might tear through and cause more bleeding. To mitigate this risk, the vessel can be clamped with a grasper first, similar to the approach in an open hysterectomy. However, once a vessel is compressed, a sealing device can usually work just as well as a suture. It becomes a matter of preference and cost.
During hysterectomy of a very large uterus, a big challenge is managing bleeding of the uterus itself during manipulation from above. Bleeding from the vascular sinuses of the myometrium can be brisk and obscure visualization, potentially leading to laparotomy conversion. A common misconception is that uterine artery embolization is equivalent to suturing the uterine arteries. In actuality, the goal of a uterine artery embolization is to embolize the distal branches of the uterine arteries, which can help with any potential bleeding from the uterus itself during hysterectomy.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Laparoscopic suturing is an option
Dr. Lum presented a nicely produced video demonstrating various strategies aimed at facilitating total laparoscopic hysterectomy (TLH) of the very large uterus. Her patient’s evaluation included magnetic resonance imaging. In the video, she demonstrates a variety of interventions, including the use of a preoperative gonadotropin–releasing hormone (GNRH) agonist and immediate perioperative radial artery–uterine artery embolization. Intraoperative techniques include use of ureteral stents and securing the uterine arteries at their origins.
Clearly, TLH of a huge uterus is a technical challenge. However, I’d like to suggest that a relatively basic and important skill would greatly assist in such procedures and likely obviate the need for a GNRH agonist and/or uterine artery embolization. The vessel-sealing devices shown in the video are generally not capable of sealing such large vessels adequately, and this is what leads to the massive hemorrhaging that often occurs.
Laparoscopic suturing with extracorporeal knot tying can be used effectively to control the extremely large vessels associated with a huge uterus. The judicious placement of sutures can completely control such vessels and prevent bleeding from both proximal and distal ends when 2 sutures are placed and the vessels are transected between the stitches. Many laparoscopic surgeons have come to rely on bipolar energy or ultrasonic devices to coagulate vessels. But when dealing with huge vessels, a return to basics using laparoscopic suturing will greatly benefit the patient and the surgeon by reducing blood loss and operative time.
David L. Zisow, MD
Baltimore, Maryland
Dr. Lum responds
I thank Dr. Zisow for his thoughtful comments. I agree that laparoscopic suturing is an essential skill that can be utilized to suture ligate vessels. If we consider the basics of an open hysterectomy, the uterine artery is clamped first, then suture ligated. When approaching a very large vessel during TLH, I would be concerned that a simple suture around a large vessel might tear through and cause more bleeding. To mitigate this risk, the vessel can be clamped with a grasper first, similar to the approach in an open hysterectomy. However, once a vessel is compressed, a sealing device can usually work just as well as a suture. It becomes a matter of preference and cost.
During hysterectomy of a very large uterus, a big challenge is managing bleeding of the uterus itself during manipulation from above. Bleeding from the vascular sinuses of the myometrium can be brisk and obscure visualization, potentially leading to laparotomy conversion. A common misconception is that uterine artery embolization is equivalent to suturing the uterine arteries. In actuality, the goal of a uterine artery embolization is to embolize the distal branches of the uterine arteries, which can help with any potential bleeding from the uterus itself during hysterectomy.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Size can matter: Laparoscopic hysterectomy for the very large uterus

Visit the Society of Gynecologic Surgeons online: sgsonline.org
Additional videos from SGS are available here, including these recent offerings:

Visit the Society of Gynecologic Surgeons online: sgsonline.org
Additional videos from SGS are available here, including these recent offerings:

Visit the Society of Gynecologic Surgeons online: sgsonline.org
Additional videos from SGS are available here, including these recent offerings:
This video is brought to you by
Basic technique of vaginal hysterectomy

Visit the Society of Gynecologic Surgeons online: sgsonline.org
Additional videos from SGS are available here, including these recent offerings:

Visit the Society of Gynecologic Surgeons online: sgsonline.org
Additional videos from SGS are available here, including these recent offerings:

Visit the Society of Gynecologic Surgeons online: sgsonline.org
Additional videos from SGS are available here, including these recent offerings:
This video is brought to you by
What’s new in simulation training for hysterectomy
Due to an increase in minimally invasive approaches to hysterectomy, including vaginal and laparoscopic approaches, gynecologic surgeons may need to turn to simulation training to augment practice and hone skills. Simulation is useful for all surgeons, especially for low-volume surgeons, as a warm-up to sharpen technical skills prior to starting the day’s cases. Additionally, educators are uniquely poised to use simulation to teach residents and to evaluate their procedural competency.
In this article, we provide an overview of the 3 approaches to hysterectomy—vaginal, laparoscopic, abdominal—through medical modeling and simulation techniques. We focus on practical issues, including current resources available online, cost, setup time, fidelity, and limitations of some commonly available vaginal, laparoscopic, and open hysterectomy models.
Simulation directly influences patient safety. Thus, the value of simulation cannot be overstated, as it can increase the quality of health care by improving patient outcomes and lowering overall costs. In 2008, the American College of Obstetricians and Gynecologists (ACOG) founded the Simulations Working Group to establish simulation as a pillar in education for women’s health through collaboration, advocacy, research, and the development and implementation of multidisciplinary simulations-based educational resources and opportunities.
Refer to the ACOG Simulations Working Group Toolkit online to see the objectives, simulation, and videos related to each module. Under the “Hysterectomy” section, you will find how to construct the “flower pot” model for abdominal and vaginal hysterectomy, as well as the AAGL vaginal and laparoscopic hysterectomy webinars. All content is reaffirmed frequently to keep it up to date. You can access the toolkit, with your ACOG login and passcode, at https://www.acog.org/About-ACOG/ACOG-Departments/Simulations-Consortium/Simulations-Consortium-Tool-Kit.
For a comprehensive gynecology curriculum to include vaginal, laparoscopic, and abdominal approaches to hysterectomy, refer to ACOG’s Surgical Curriculum in Obstetrics and Gynecology page at https://cfweb.acog.org/scog/. This page lists the standardized surgical skills curriculum for use in training residents in obstetrics and gynecology by procedure. It includes:
- the objective, description, and assessment of the module
- a description of the simulation
- a description of the surgical procedure
- a quiz that must be passed to proceed to evaluation by a faculty member
- an evaluation form to be downloaded and printed by the learner.
Takeaway. Value of Simulation = Quality (Improved Patient Outcomes) ÷ Direct and Indirect Costs.
Simulation models for training in vaginal hysterectomy
According to the Accreditation Council for Graduate Medical Education (ACGME), the minimum number of vaginal hysterectomies is 15; this number represents the minimum accepted exposure, however, and does not imply competency. Exposure to vaginal hysterectomy in residency training has significantly declined over the years, with a mean of only 19 vaginal hysterectomies performed by the time of graduation in 2014.1
A wide range of simulation models are available that you either can construct or purchase, based on your budget. We discuss 3 such models below.
The Miya model
The Miya Model Pelvic Surgery Training Model (Miyazaki Enterprises) consists of a bony pelvic frame and multiple replaceable and realistic anatomic structures, including the uterus, cervix, and adnexa (1 structure), vagina, bladder, and a few selected muscles and ligaments for pelvic floor disorders (FIGURE 1). The model incorporates features to simulate actual surgical experiences, such as realistic cutting and puncturing tensions, palpable surgical landmarks, a pressurized vascular system with bleeding for inadequate technique, and an inflatable bladder that can leak water if damaged.

Mounted on a rotating stand with the top of the pelvis open, the Miya model is designed to provide access and visibility, enabling supervising physicians the ability to give immediate guidance and feedback. The interchangeable parts allow the learner to be challenged at the appropriate skill level with the use of a large uterus versus a smaller uterus.
New in 2018 is an “intern” uterus and vagina that have no vascular supply and a single-layer vagina; this model is one-third of the cost of the larger, high-fidelity uterus (which has a vascular supply and additional tissue layers).
The Miya model reusable bony pelvic frame has a one-time cost of a few thousand dollars. Advantages include its high fidelity, low technology, light weight, portability, and quick setup. To view a video of the Miya model, go to https://www.youtube.com/watch?time_continue=49&v=A2RjOgVRclo. To see a simulated vaginal hysterectomy, visit https://www.youtube.com/watch?time_continue=13&v=dwiQz4DTyy8.
The gynecologic surgeon and inventor, Dr. Douglas Miyazaki, has improved the vesicouterine peritoneal fold (usually the most challenging for the surgeon) to have a more realistic, slippery feel when palpated.
This model’s weaknesses are its cost (relative to low-fidelity models) and the inability to use energy devices.
Takeaway. The Miya model is a high-fidelity, portable vaginal hysterectomy model with a reusable base and consumable replacement parts
The Gynesim model
The Gynesim Vaginal Hysterectomy Model, developed by Dr. Malcolm “Kip” Mackenzie (Gynesim), is a high-fidelity surgical simulation model constructed from animal tissue to provide realistic training in pelvic surgery (FIGURE 2).

These “real tissue models” are hand-constructed from animal tissue harvested from US Department of Agriculture inspected meat processing centers. The models mimic normal and abnormal abdominal and pelvic anatomy, providing realistic feel (haptics) and response to all surgical energy modalities. The “cassette” tissues are placed within a vaginal approach platform, which is portable.
Each model (including a 120- to 240-g uterus, bladder, ureter, uterine artery, cardinal and uterosacral ligaments, and rectum) supports critical gaps in surgical techniques such as peritoneal entry and cuff closure. Gynesim staff set up the entire laboratory, including the simulation models, instruments, and/or cameras; however, surgical energy systems are secured from the host institution.
The advantages of this model are its excellent tissue haptics and the minimal preparation time required from the busy gynecologic teaching faculty, as the company performs the setup and breakdown. Disadvantages include the model’s cost (relative to low-fidelity models), that it does not bleed, its one-time use, and the need for technical assistance from the company for setup.
This model can be used for laparoscopic and open hysterectomy approaches, as well as for vaginal hysterectomy. For more information, visit the Gynesim website at https://www.gynesim.com/vaginal-hysterectomy/.
Takeaway. The high-fidelity Gynesim model can be used to practice vaginal, laparoscopic, or open hysterectomy approaches. It offers excellent tissue haptics, one-time use “cassettes” made from animal tissue, and compatibility with energy devices.
The milk jug model
The milk jug and fabric uterus model, developed by Dr. Dee Fenner, is a low-cost simulation model and an alternative to the flower pot model (described later in this article). The bony pelvis is simulated by a 1-gallon milk carton that is taped to a foam ring. Other materials used to make the uterus are fabric, stuffing, and a needle and thread (or a sewing machine). Each model costs approximately $5 and takes approximately 15 minutes to create. For instructions on how to construct this model, see the Society for Gynecologic Surgeons (SGS) award-winning video from 2012 at https://vimeo.com/123804677.
The advantages of this model are that it is inexpensive and is a good tool with which novice gynecologic surgeons can learn the basic steps of the procedure. The disadvantages are that it does not bleed, is not compatible with energy devices, and must be constructed by hand (adding considerable time) or with a sewing machine.
Takeaway. The milk jug model is a low-cost, low-fidelity model for the novice surgeon that can be quickly constructed with the use of a sewing machine.
Read about simulation models for training in laparoscopic hysterectomy.
Simulation models for training in laparoscopic hysterectomy
While overall hysterectomy numbers have remained relatively stable during the last 10 years, the proportion of laparoscopic hysterectomy procedures is increasing in residency training.1 Many toolkits and models are available for practicing skills, from low-fidelity models on which to rehearse laparoscopic techniques (suturing, instrument handling) to high-fidelity models that provide augmented reality views of the abdominal cavity as well as the operating room itself. We offer a sampling of 4 such models below.
The FLS trainer system
The Fundamentals of Laparoscopic Surgery (FLS) Trainer Box (Limbs & Things Ltd) provides hands-on manual skills practice and training for laparoscopic surgery (FIGURE 3). The FLS trainer box uses 5 skills to challenge a surgeon’s dexterity and psychomotor skills. The set includes the trainer box with a camera and light source as well as the equipment needed to perform the 5 FLS tasks (peg transfer, pattern cutting, ligating loop, and intracorporeal and extracorporeal knot tying). The kit does not include laparoscopic instruments or a monitor.

The FLS trainer box with camera costs $1,164. The advantages are that it is portable and can be used to warm-up prior to surgery or for practice to improve technical skills. It is a great tool for junior residents who are learning the basics of laparoscopic surgery. This trainer’s disadvantages are that it is a low-fidelity unit that is procedure agnostic. For more information, visit the Limbs & Things website at https://www.fls-products.com.
Notably, ObGyn residents who graduate after May 31, 2020, will be required to successfully complete the FLS program as a prerequisite for specialty board certification.2 The FLS program is endorsed by the American College of Surgeons and is run through the Society of American Gastrointestinal and Endoscopic Surgeons. The FLS test is proctored and must be taken at a testing center.
Takeaway. The FLS trainer box is readily available, portable, relatively inexpensive, low-tech, and has valid benchmarks for proficiency. The FLS test will be required for ObGyn residents by 2020.
The SimPraxis software trainer
The SimPraxis Laparoscopic Hysterectomy Trainer (Red Llama, Inc) is an interactive simulation software platform that is available in DVD or USB format (FIGURE 4). The software is designed to review anatomy, surgical instrumentation, and specific steps of the procedure. It provides formative assessments and offers summative feedback for users.

The SimPraxis training software would make a useful tool to familiarize medical students and interns with the basics of the procedure before advancing to other simulation trainers. The software costs $100. For more information, visit https://www.3-dmed.com/product/simpraxis%C3%82%C2%AE-laparoscopic-hysterectomy-trainer.
Takeaway. The SimPraxis software is ideal for novice learners and can be used on a home or office computer.
The LapSim virtual reality trainer
The LapSim Haptic System (Surgical Science) is a virtual reality skills trainer. The hysterectomy module includes right and left uterine artery dissection, vaginal cuff opening, and cuff closure (FIGURE 5). One advantage of this simulator is its haptic feedback system, which enhances the fidelity of the training.
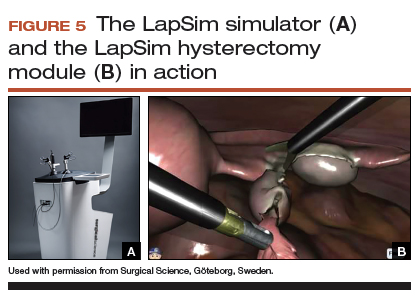
The LapSim simulator includes a training module for students and early learners and modules to improve camera handling. The virtual reality base system costs $70,720, and the hysterectomy software module is an additional $15,600.
For more information, visit the company’s website at https://surgicalscience.com/systems/lapsim/. For an informational video, go to https://surgicalscience.com/systems/lapsim/video/.
Takeaway. The LapSim is an expensive, high-fidelity, virtual reality simulator with enhanced haptics and software for practicing laparoscopic hysterectomy.
The LAP Mentor virtual reality simulator
The LAP Mentor VR (3D Systems) is another virtual reality simulator that has modules for laparoscopic hysterectomy and cuff closure (FIGURE 6). The trainee uses a virtual reality headset and becomes fully immersed in the operating room environment with audio and visual cues that mimic a real surgical experience.

The hysterectomy module allows the user to manipulate the uterus, identify the ureters, divide the superior pedicles, mobilize the bladder, expose and divide the uterine artery, and perform the colpotomy. The cuff closure module allows the user to suture the vaginal cuff using barbed suture. The module also can expose the learner to complications, such as bladder, ureteral, colon, or vascular injury.
The LAP Mentor VR base system costs $84,000 and the modules cost about $15,000. For additional information, visit the company’s website at http://simbionix.com/simulators/lap-mentor/lap-mentor-vr-or/.
Takeaway. The LAP Mentor is an expensive, high-fidelity simulation platform with a virtual reality headset that simulates a laparoscopic hysterectomy (with complications) in the operating room.
Read about simulations models for robot-assisted lap hysterectomy and abdominal hysterectomy.
Simulation models for training in robot-assisted laparoscopic hysterectomy
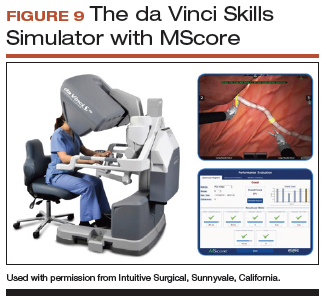
All robot-assisted simulation platforms have highly realistic graphics, and they are expensive (TABLE). However, the da Vinci Skills Simulator (backpack) platform is included with the da Vinci Si and Xi Systems. Note, though, that it can be challenging to access the surgeon console and backpack at institutions with high volumes of robot-assisted surgery.
Other options that generally reside outside of the operating room include Mimic’s FlexVR and dV-Trainer and the Robotix Mentor by 3D Systems (FIGURES 7–11). Mimic’s new technology, called MaestroAR (augmented reality), allows trainees to manipulate virtual robotic instruments to interact with anatomic regions within augmented 3D surgical video footage, with narration and instruction by Dr. Arnold Advincula.
Newer software by Simbionix allows augmented reality to assist the simulation of robot-assisted hysterectomy with the da Vinci Xi backpack and RobotiX platforms.

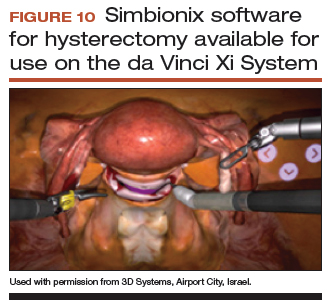
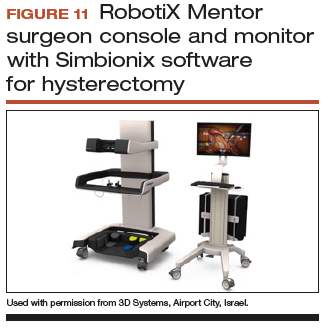
Models for training in abdominal hysterectomy
In the last 10 years, there has been a 30% decrease in the number of abdominal hysterectomies performed by residents.1 Because of this decline in operating room experience, simulation training can be an important tool to bolster residency experience.
There are not many simulation models available for teaching abdominal hysterectomy, but here we discuss 2 that we utilize in our residency program.
Adaptable task trainer
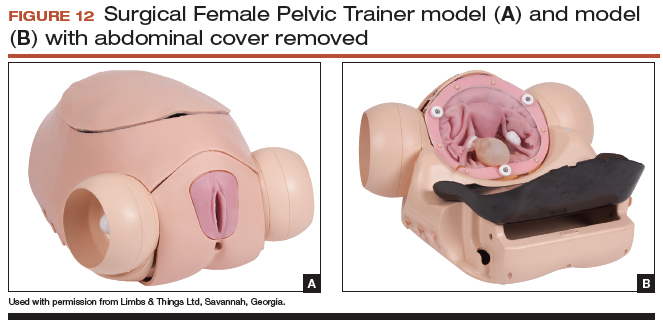
The Surgical Female Pelvic Trainer (SFPT) (Limbs & Things Ltd), a pelvic task trainer primarily used for simulation of laparoscopic hysterectomy, can be adapted for abdominal hysterectomy by removing the abdominal cover (FIGURE 12). This trainer can be used with simulated blood to increase the realism of training. The SFPT trainer costs $2,190. For more information, go to https://www.limbsandthings.com/us/our-products/details/surgical-female-pelvic-trainer-sfpt-mk-2.
Takeaway. The SFPT is a medium-fidelity task trainer with a reusable base and consumable replacement parts.
ACOG’s do-it-yourself flower pot model
The flower pot model (developed by the ACOG Simulation Working Group, Washington, DC) is a comprehensive educational package that includes learning objectives, simulation construction instructions, content review of the abdominal hysterectomy, quiz, and evaluation form.3 ACOG has endorsed this low-cost model for residency education. Each model costs approximately $20, and the base (flower pot) is reusable (FIGURE 13).Construction time for each model is 30 to 60 minutes, and learners can participate in the construction. This can aid in anatomy review and familiarization with the model prior to training in the surgical procedure.

The learning objectives, content review, quiz, and evaluation form can be used for the flower pot model or for high-fidelity models.
The advantages of this model are the low cost and that it provides enough fidelity to teach each of the critical steps of the procedure. The disadvantages include that it is a lower-fidelity model, requires a considerable amount of time for construction, does not bleed, and is not compatible with energy devices. This model also can be used for training in laparoscopic and vaginal hysterectomy. For more information, visit ACOG’s Surgical Curriculum website at https://cfweb.acog.org/scog/.
Takeaway. ACOG’s flower pot model for hysterectomy training is a comprehensive, low-cost, low-fidelity simulation model that requires significant setup time.
Simulation’s offerings
Simulation training is the present and future of medicine that bridges the gap between textbook learning and technical proficiency. Although in this article we describe only a handful of the simulation resources available, we hope that you will incorporate such tools into your practice for continuing education and skill development. Utilize peer-reviewed resources, such as the ACOG curriculum module and evaluation tools for abdominal, laparoscopic, and vaginal hysterectomy, which can be used with any simulation model to provide a comprehensive and complimentary learning experience.
The future of health care depends on the commitment and ingenuity of educators who embrace medical simulation’s purpose: improved patient safety, effectiveness, and efficiency. Join the movement!
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Washburn EE, Cohen SL, Manoucheri E, Zurawin RK, Einarsson JI. Trends in reported resident surgical experience in hysterectomy. J Minim Invasive Gynecol. 2014;21(6):1067–1070.
- American Board of Obstetrics and Gynecology. ABOG announces new eligibility requirement for board certification. https://www.abog.org/new/ABOG_FLS.aspx. Published January 22, 2018. Accessed April 10, 2018.
- Altman K, Burrell D, Chen G, Chou B, Fashokun T. Surgical curriculum in obstetrics and gynecology: vaginal hysterectomy simulation. https://cfweb.acog.org/scog/scog008/Simulation.cfm. Published December 2014. Accessed April 10, 2018.
Due to an increase in minimally invasive approaches to hysterectomy, including vaginal and laparoscopic approaches, gynecologic surgeons may need to turn to simulation training to augment practice and hone skills. Simulation is useful for all surgeons, especially for low-volume surgeons, as a warm-up to sharpen technical skills prior to starting the day’s cases. Additionally, educators are uniquely poised to use simulation to teach residents and to evaluate their procedural competency.
In this article, we provide an overview of the 3 approaches to hysterectomy—vaginal, laparoscopic, abdominal—through medical modeling and simulation techniques. We focus on practical issues, including current resources available online, cost, setup time, fidelity, and limitations of some commonly available vaginal, laparoscopic, and open hysterectomy models.
Simulation directly influences patient safety. Thus, the value of simulation cannot be overstated, as it can increase the quality of health care by improving patient outcomes and lowering overall costs. In 2008, the American College of Obstetricians and Gynecologists (ACOG) founded the Simulations Working Group to establish simulation as a pillar in education for women’s health through collaboration, advocacy, research, and the development and implementation of multidisciplinary simulations-based educational resources and opportunities.
Refer to the ACOG Simulations Working Group Toolkit online to see the objectives, simulation, and videos related to each module. Under the “Hysterectomy” section, you will find how to construct the “flower pot” model for abdominal and vaginal hysterectomy, as well as the AAGL vaginal and laparoscopic hysterectomy webinars. All content is reaffirmed frequently to keep it up to date. You can access the toolkit, with your ACOG login and passcode, at https://www.acog.org/About-ACOG/ACOG-Departments/Simulations-Consortium/Simulations-Consortium-Tool-Kit.
For a comprehensive gynecology curriculum to include vaginal, laparoscopic, and abdominal approaches to hysterectomy, refer to ACOG’s Surgical Curriculum in Obstetrics and Gynecology page at https://cfweb.acog.org/scog/. This page lists the standardized surgical skills curriculum for use in training residents in obstetrics and gynecology by procedure. It includes:
- the objective, description, and assessment of the module
- a description of the simulation
- a description of the surgical procedure
- a quiz that must be passed to proceed to evaluation by a faculty member
- an evaluation form to be downloaded and printed by the learner.
Takeaway. Value of Simulation = Quality (Improved Patient Outcomes) ÷ Direct and Indirect Costs.
Simulation models for training in vaginal hysterectomy
According to the Accreditation Council for Graduate Medical Education (ACGME), the minimum number of vaginal hysterectomies is 15; this number represents the minimum accepted exposure, however, and does not imply competency. Exposure to vaginal hysterectomy in residency training has significantly declined over the years, with a mean of only 19 vaginal hysterectomies performed by the time of graduation in 2014.1
A wide range of simulation models are available that you either can construct or purchase, based on your budget. We discuss 3 such models below.
The Miya model
The Miya Model Pelvic Surgery Training Model (Miyazaki Enterprises) consists of a bony pelvic frame and multiple replaceable and realistic anatomic structures, including the uterus, cervix, and adnexa (1 structure), vagina, bladder, and a few selected muscles and ligaments for pelvic floor disorders (FIGURE 1). The model incorporates features to simulate actual surgical experiences, such as realistic cutting and puncturing tensions, palpable surgical landmarks, a pressurized vascular system with bleeding for inadequate technique, and an inflatable bladder that can leak water if damaged.

Mounted on a rotating stand with the top of the pelvis open, the Miya model is designed to provide access and visibility, enabling supervising physicians the ability to give immediate guidance and feedback. The interchangeable parts allow the learner to be challenged at the appropriate skill level with the use of a large uterus versus a smaller uterus.
New in 2018 is an “intern” uterus and vagina that have no vascular supply and a single-layer vagina; this model is one-third of the cost of the larger, high-fidelity uterus (which has a vascular supply and additional tissue layers).
The Miya model reusable bony pelvic frame has a one-time cost of a few thousand dollars. Advantages include its high fidelity, low technology, light weight, portability, and quick setup. To view a video of the Miya model, go to https://www.youtube.com/watch?time_continue=49&v=A2RjOgVRclo. To see a simulated vaginal hysterectomy, visit https://www.youtube.com/watch?time_continue=13&v=dwiQz4DTyy8.
The gynecologic surgeon and inventor, Dr. Douglas Miyazaki, has improved the vesicouterine peritoneal fold (usually the most challenging for the surgeon) to have a more realistic, slippery feel when palpated.
This model’s weaknesses are its cost (relative to low-fidelity models) and the inability to use energy devices.
Takeaway. The Miya model is a high-fidelity, portable vaginal hysterectomy model with a reusable base and consumable replacement parts
The Gynesim model
The Gynesim Vaginal Hysterectomy Model, developed by Dr. Malcolm “Kip” Mackenzie (Gynesim), is a high-fidelity surgical simulation model constructed from animal tissue to provide realistic training in pelvic surgery (FIGURE 2).

These “real tissue models” are hand-constructed from animal tissue harvested from US Department of Agriculture inspected meat processing centers. The models mimic normal and abnormal abdominal and pelvic anatomy, providing realistic feel (haptics) and response to all surgical energy modalities. The “cassette” tissues are placed within a vaginal approach platform, which is portable.
Each model (including a 120- to 240-g uterus, bladder, ureter, uterine artery, cardinal and uterosacral ligaments, and rectum) supports critical gaps in surgical techniques such as peritoneal entry and cuff closure. Gynesim staff set up the entire laboratory, including the simulation models, instruments, and/or cameras; however, surgical energy systems are secured from the host institution.
The advantages of this model are its excellent tissue haptics and the minimal preparation time required from the busy gynecologic teaching faculty, as the company performs the setup and breakdown. Disadvantages include the model’s cost (relative to low-fidelity models), that it does not bleed, its one-time use, and the need for technical assistance from the company for setup.
This model can be used for laparoscopic and open hysterectomy approaches, as well as for vaginal hysterectomy. For more information, visit the Gynesim website at https://www.gynesim.com/vaginal-hysterectomy/.
Takeaway. The high-fidelity Gynesim model can be used to practice vaginal, laparoscopic, or open hysterectomy approaches. It offers excellent tissue haptics, one-time use “cassettes” made from animal tissue, and compatibility with energy devices.
The milk jug model
The milk jug and fabric uterus model, developed by Dr. Dee Fenner, is a low-cost simulation model and an alternative to the flower pot model (described later in this article). The bony pelvis is simulated by a 1-gallon milk carton that is taped to a foam ring. Other materials used to make the uterus are fabric, stuffing, and a needle and thread (or a sewing machine). Each model costs approximately $5 and takes approximately 15 minutes to create. For instructions on how to construct this model, see the Society for Gynecologic Surgeons (SGS) award-winning video from 2012 at https://vimeo.com/123804677.
The advantages of this model are that it is inexpensive and is a good tool with which novice gynecologic surgeons can learn the basic steps of the procedure. The disadvantages are that it does not bleed, is not compatible with energy devices, and must be constructed by hand (adding considerable time) or with a sewing machine.
Takeaway. The milk jug model is a low-cost, low-fidelity model for the novice surgeon that can be quickly constructed with the use of a sewing machine.
Read about simulation models for training in laparoscopic hysterectomy.
Simulation models for training in laparoscopic hysterectomy
While overall hysterectomy numbers have remained relatively stable during the last 10 years, the proportion of laparoscopic hysterectomy procedures is increasing in residency training.1 Many toolkits and models are available for practicing skills, from low-fidelity models on which to rehearse laparoscopic techniques (suturing, instrument handling) to high-fidelity models that provide augmented reality views of the abdominal cavity as well as the operating room itself. We offer a sampling of 4 such models below.
The FLS trainer system
The Fundamentals of Laparoscopic Surgery (FLS) Trainer Box (Limbs & Things Ltd) provides hands-on manual skills practice and training for laparoscopic surgery (FIGURE 3). The FLS trainer box uses 5 skills to challenge a surgeon’s dexterity and psychomotor skills. The set includes the trainer box with a camera and light source as well as the equipment needed to perform the 5 FLS tasks (peg transfer, pattern cutting, ligating loop, and intracorporeal and extracorporeal knot tying). The kit does not include laparoscopic instruments or a monitor.

The FLS trainer box with camera costs $1,164. The advantages are that it is portable and can be used to warm-up prior to surgery or for practice to improve technical skills. It is a great tool for junior residents who are learning the basics of laparoscopic surgery. This trainer’s disadvantages are that it is a low-fidelity unit that is procedure agnostic. For more information, visit the Limbs & Things website at https://www.fls-products.com.
Notably, ObGyn residents who graduate after May 31, 2020, will be required to successfully complete the FLS program as a prerequisite for specialty board certification.2 The FLS program is endorsed by the American College of Surgeons and is run through the Society of American Gastrointestinal and Endoscopic Surgeons. The FLS test is proctored and must be taken at a testing center.
Takeaway. The FLS trainer box is readily available, portable, relatively inexpensive, low-tech, and has valid benchmarks for proficiency. The FLS test will be required for ObGyn residents by 2020.
The SimPraxis software trainer
The SimPraxis Laparoscopic Hysterectomy Trainer (Red Llama, Inc) is an interactive simulation software platform that is available in DVD or USB format (FIGURE 4). The software is designed to review anatomy, surgical instrumentation, and specific steps of the procedure. It provides formative assessments and offers summative feedback for users.

The SimPraxis training software would make a useful tool to familiarize medical students and interns with the basics of the procedure before advancing to other simulation trainers. The software costs $100. For more information, visit https://www.3-dmed.com/product/simpraxis%C3%82%C2%AE-laparoscopic-hysterectomy-trainer.
Takeaway. The SimPraxis software is ideal for novice learners and can be used on a home or office computer.
The LapSim virtual reality trainer
The LapSim Haptic System (Surgical Science) is a virtual reality skills trainer. The hysterectomy module includes right and left uterine artery dissection, vaginal cuff opening, and cuff closure (FIGURE 5). One advantage of this simulator is its haptic feedback system, which enhances the fidelity of the training.

The LapSim simulator includes a training module for students and early learners and modules to improve camera handling. The virtual reality base system costs $70,720, and the hysterectomy software module is an additional $15,600.
For more information, visit the company’s website at https://surgicalscience.com/systems/lapsim/. For an informational video, go to https://surgicalscience.com/systems/lapsim/video/.
Takeaway. The LapSim is an expensive, high-fidelity, virtual reality simulator with enhanced haptics and software for practicing laparoscopic hysterectomy.
The LAP Mentor virtual reality simulator
The LAP Mentor VR (3D Systems) is another virtual reality simulator that has modules for laparoscopic hysterectomy and cuff closure (FIGURE 6). The trainee uses a virtual reality headset and becomes fully immersed in the operating room environment with audio and visual cues that mimic a real surgical experience.

The hysterectomy module allows the user to manipulate the uterus, identify the ureters, divide the superior pedicles, mobilize the bladder, expose and divide the uterine artery, and perform the colpotomy. The cuff closure module allows the user to suture the vaginal cuff using barbed suture. The module also can expose the learner to complications, such as bladder, ureteral, colon, or vascular injury.
The LAP Mentor VR base system costs $84,000 and the modules cost about $15,000. For additional information, visit the company’s website at http://simbionix.com/simulators/lap-mentor/lap-mentor-vr-or/.
Takeaway. The LAP Mentor is an expensive, high-fidelity simulation platform with a virtual reality headset that simulates a laparoscopic hysterectomy (with complications) in the operating room.
Read about simulations models for robot-assisted lap hysterectomy and abdominal hysterectomy.
Simulation models for training in robot-assisted laparoscopic hysterectomy
All robot-assisted simulation platforms have highly realistic graphics, and they are expensive (TABLE). However, the da Vinci Skills Simulator (backpack) platform is included with the da Vinci Si and Xi Systems. Note, though, that it can be challenging to access the surgeon console and backpack at institutions with high volumes of robot-assisted surgery.

Other options that generally reside outside of the operating room include Mimic’s FlexVR and dV-Trainer and the Robotix Mentor by 3D Systems (FIGURES 7–11). Mimic’s new technology, called MaestroAR (augmented reality), allows trainees to manipulate virtual robotic instruments to interact with anatomic regions within augmented 3D surgical video footage, with narration and instruction by Dr. Arnold Advincula.
Newer software by Simbionix allows augmented reality to assist the simulation of robot-assisted hysterectomy with the da Vinci Xi backpack and RobotiX platforms.





Models for training in abdominal hysterectomy
In the last 10 years, there has been a 30% decrease in the number of abdominal hysterectomies performed by residents.1 Because of this decline in operating room experience, simulation training can be an important tool to bolster residency experience.
There are not many simulation models available for teaching abdominal hysterectomy, but here we discuss 2 that we utilize in our residency program.
Adaptable task trainer
The Surgical Female Pelvic Trainer (SFPT) (Limbs & Things Ltd), a pelvic task trainer primarily used for simulation of laparoscopic hysterectomy, can be adapted for abdominal hysterectomy by removing the abdominal cover (FIGURE 12). This trainer can be used with simulated blood to increase the realism of training. The SFPT trainer costs $2,190. For more information, go to https://www.limbsandthings.com/us/our-products/details/surgical-female-pelvic-trainer-sfpt-mk-2.
Takeaway. The SFPT is a medium-fidelity task trainer with a reusable base and consumable replacement parts.

ACOG’s do-it-yourself flower pot model
The flower pot model (developed by the ACOG Simulation Working Group, Washington, DC) is a comprehensive educational package that includes learning objectives, simulation construction instructions, content review of the abdominal hysterectomy, quiz, and evaluation form.3 ACOG has endorsed this low-cost model for residency education. Each model costs approximately $20, and the base (flower pot) is reusable (FIGURE 13).Construction time for each model is 30 to 60 minutes, and learners can participate in the construction. This can aid in anatomy review and familiarization with the model prior to training in the surgical procedure.

The learning objectives, content review, quiz, and evaluation form can be used for the flower pot model or for high-fidelity models.
The advantages of this model are the low cost and that it provides enough fidelity to teach each of the critical steps of the procedure. The disadvantages include that it is a lower-fidelity model, requires a considerable amount of time for construction, does not bleed, and is not compatible with energy devices. This model also can be used for training in laparoscopic and vaginal hysterectomy. For more information, visit ACOG’s Surgical Curriculum website at https://cfweb.acog.org/scog/.
Takeaway. ACOG’s flower pot model for hysterectomy training is a comprehensive, low-cost, low-fidelity simulation model that requires significant setup time.
Simulation’s offerings
Simulation training is the present and future of medicine that bridges the gap between textbook learning and technical proficiency. Although in this article we describe only a handful of the simulation resources available, we hope that you will incorporate such tools into your practice for continuing education and skill development. Utilize peer-reviewed resources, such as the ACOG curriculum module and evaluation tools for abdominal, laparoscopic, and vaginal hysterectomy, which can be used with any simulation model to provide a comprehensive and complimentary learning experience.
The future of health care depends on the commitment and ingenuity of educators who embrace medical simulation’s purpose: improved patient safety, effectiveness, and efficiency. Join the movement!
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Due to an increase in minimally invasive approaches to hysterectomy, including vaginal and laparoscopic approaches, gynecologic surgeons may need to turn to simulation training to augment practice and hone skills. Simulation is useful for all surgeons, especially for low-volume surgeons, as a warm-up to sharpen technical skills prior to starting the day’s cases. Additionally, educators are uniquely poised to use simulation to teach residents and to evaluate their procedural competency.
In this article, we provide an overview of the 3 approaches to hysterectomy—vaginal, laparoscopic, abdominal—through medical modeling and simulation techniques. We focus on practical issues, including current resources available online, cost, setup time, fidelity, and limitations of some commonly available vaginal, laparoscopic, and open hysterectomy models.
Simulation directly influences patient safety. Thus, the value of simulation cannot be overstated, as it can increase the quality of health care by improving patient outcomes and lowering overall costs. In 2008, the American College of Obstetricians and Gynecologists (ACOG) founded the Simulations Working Group to establish simulation as a pillar in education for women’s health through collaboration, advocacy, research, and the development and implementation of multidisciplinary simulations-based educational resources and opportunities.
Refer to the ACOG Simulations Working Group Toolkit online to see the objectives, simulation, and videos related to each module. Under the “Hysterectomy” section, you will find how to construct the “flower pot” model for abdominal and vaginal hysterectomy, as well as the AAGL vaginal and laparoscopic hysterectomy webinars. All content is reaffirmed frequently to keep it up to date. You can access the toolkit, with your ACOG login and passcode, at https://www.acog.org/About-ACOG/ACOG-Departments/Simulations-Consortium/Simulations-Consortium-Tool-Kit.
For a comprehensive gynecology curriculum to include vaginal, laparoscopic, and abdominal approaches to hysterectomy, refer to ACOG’s Surgical Curriculum in Obstetrics and Gynecology page at https://cfweb.acog.org/scog/. This page lists the standardized surgical skills curriculum for use in training residents in obstetrics and gynecology by procedure. It includes:
- the objective, description, and assessment of the module
- a description of the simulation
- a description of the surgical procedure
- a quiz that must be passed to proceed to evaluation by a faculty member
- an evaluation form to be downloaded and printed by the learner.
Takeaway. Value of Simulation = Quality (Improved Patient Outcomes) ÷ Direct and Indirect Costs.
Simulation models for training in vaginal hysterectomy
According to the Accreditation Council for Graduate Medical Education (ACGME), the minimum number of vaginal hysterectomies is 15; this number represents the minimum accepted exposure, however, and does not imply competency. Exposure to vaginal hysterectomy in residency training has significantly declined over the years, with a mean of only 19 vaginal hysterectomies performed by the time of graduation in 2014.1
A wide range of simulation models are available that you either can construct or purchase, based on your budget. We discuss 3 such models below.
The Miya model
The Miya Model Pelvic Surgery Training Model (Miyazaki Enterprises) consists of a bony pelvic frame and multiple replaceable and realistic anatomic structures, including the uterus, cervix, and adnexa (1 structure), vagina, bladder, and a few selected muscles and ligaments for pelvic floor disorders (FIGURE 1). The model incorporates features to simulate actual surgical experiences, such as realistic cutting and puncturing tensions, palpable surgical landmarks, a pressurized vascular system with bleeding for inadequate technique, and an inflatable bladder that can leak water if damaged.

Mounted on a rotating stand with the top of the pelvis open, the Miya model is designed to provide access and visibility, enabling supervising physicians the ability to give immediate guidance and feedback. The interchangeable parts allow the learner to be challenged at the appropriate skill level with the use of a large uterus versus a smaller uterus.
New in 2018 is an “intern” uterus and vagina that have no vascular supply and a single-layer vagina; this model is one-third of the cost of the larger, high-fidelity uterus (which has a vascular supply and additional tissue layers).
The Miya model reusable bony pelvic frame has a one-time cost of a few thousand dollars. Advantages include its high fidelity, low technology, light weight, portability, and quick setup. To view a video of the Miya model, go to https://www.youtube.com/watch?time_continue=49&v=A2RjOgVRclo. To see a simulated vaginal hysterectomy, visit https://www.youtube.com/watch?time_continue=13&v=dwiQz4DTyy8.
The gynecologic surgeon and inventor, Dr. Douglas Miyazaki, has improved the vesicouterine peritoneal fold (usually the most challenging for the surgeon) to have a more realistic, slippery feel when palpated.
This model’s weaknesses are its cost (relative to low-fidelity models) and the inability to use energy devices.
Takeaway. The Miya model is a high-fidelity, portable vaginal hysterectomy model with a reusable base and consumable replacement parts
The Gynesim model
The Gynesim Vaginal Hysterectomy Model, developed by Dr. Malcolm “Kip” Mackenzie (Gynesim), is a high-fidelity surgical simulation model constructed from animal tissue to provide realistic training in pelvic surgery (FIGURE 2).

These “real tissue models” are hand-constructed from animal tissue harvested from US Department of Agriculture inspected meat processing centers. The models mimic normal and abnormal abdominal and pelvic anatomy, providing realistic feel (haptics) and response to all surgical energy modalities. The “cassette” tissues are placed within a vaginal approach platform, which is portable.
Each model (including a 120- to 240-g uterus, bladder, ureter, uterine artery, cardinal and uterosacral ligaments, and rectum) supports critical gaps in surgical techniques such as peritoneal entry and cuff closure. Gynesim staff set up the entire laboratory, including the simulation models, instruments, and/or cameras; however, surgical energy systems are secured from the host institution.
The advantages of this model are its excellent tissue haptics and the minimal preparation time required from the busy gynecologic teaching faculty, as the company performs the setup and breakdown. Disadvantages include the model’s cost (relative to low-fidelity models), that it does not bleed, its one-time use, and the need for technical assistance from the company for setup.
This model can be used for laparoscopic and open hysterectomy approaches, as well as for vaginal hysterectomy. For more information, visit the Gynesim website at https://www.gynesim.com/vaginal-hysterectomy/.
Takeaway. The high-fidelity Gynesim model can be used to practice vaginal, laparoscopic, or open hysterectomy approaches. It offers excellent tissue haptics, one-time use “cassettes” made from animal tissue, and compatibility with energy devices.
The milk jug model
The milk jug and fabric uterus model, developed by Dr. Dee Fenner, is a low-cost simulation model and an alternative to the flower pot model (described later in this article). The bony pelvis is simulated by a 1-gallon milk carton that is taped to a foam ring. Other materials used to make the uterus are fabric, stuffing, and a needle and thread (or a sewing machine). Each model costs approximately $5 and takes approximately 15 minutes to create. For instructions on how to construct this model, see the Society for Gynecologic Surgeons (SGS) award-winning video from 2012 at https://vimeo.com/123804677.
The advantages of this model are that it is inexpensive and is a good tool with which novice gynecologic surgeons can learn the basic steps of the procedure. The disadvantages are that it does not bleed, is not compatible with energy devices, and must be constructed by hand (adding considerable time) or with a sewing machine.
Takeaway. The milk jug model is a low-cost, low-fidelity model for the novice surgeon that can be quickly constructed with the use of a sewing machine.
Read about simulation models for training in laparoscopic hysterectomy.
Simulation models for training in laparoscopic hysterectomy
While overall hysterectomy numbers have remained relatively stable during the last 10 years, the proportion of laparoscopic hysterectomy procedures is increasing in residency training.1 Many toolkits and models are available for practicing skills, from low-fidelity models on which to rehearse laparoscopic techniques (suturing, instrument handling) to high-fidelity models that provide augmented reality views of the abdominal cavity as well as the operating room itself. We offer a sampling of 4 such models below.
The FLS trainer system
The Fundamentals of Laparoscopic Surgery (FLS) Trainer Box (Limbs & Things Ltd) provides hands-on manual skills practice and training for laparoscopic surgery (FIGURE 3). The FLS trainer box uses 5 skills to challenge a surgeon’s dexterity and psychomotor skills. The set includes the trainer box with a camera and light source as well as the equipment needed to perform the 5 FLS tasks (peg transfer, pattern cutting, ligating loop, and intracorporeal and extracorporeal knot tying). The kit does not include laparoscopic instruments or a monitor.

The FLS trainer box with camera costs $1,164. The advantages are that it is portable and can be used to warm-up prior to surgery or for practice to improve technical skills. It is a great tool for junior residents who are learning the basics of laparoscopic surgery. This trainer’s disadvantages are that it is a low-fidelity unit that is procedure agnostic. For more information, visit the Limbs & Things website at https://www.fls-products.com.
Notably, ObGyn residents who graduate after May 31, 2020, will be required to successfully complete the FLS program as a prerequisite for specialty board certification.2 The FLS program is endorsed by the American College of Surgeons and is run through the Society of American Gastrointestinal and Endoscopic Surgeons. The FLS test is proctored and must be taken at a testing center.
Takeaway. The FLS trainer box is readily available, portable, relatively inexpensive, low-tech, and has valid benchmarks for proficiency. The FLS test will be required for ObGyn residents by 2020.
The SimPraxis software trainer
The SimPraxis Laparoscopic Hysterectomy Trainer (Red Llama, Inc) is an interactive simulation software platform that is available in DVD or USB format (FIGURE 4). The software is designed to review anatomy, surgical instrumentation, and specific steps of the procedure. It provides formative assessments and offers summative feedback for users.

The SimPraxis training software would make a useful tool to familiarize medical students and interns with the basics of the procedure before advancing to other simulation trainers. The software costs $100. For more information, visit https://www.3-dmed.com/product/simpraxis%C3%82%C2%AE-laparoscopic-hysterectomy-trainer.
Takeaway. The SimPraxis software is ideal for novice learners and can be used on a home or office computer.
The LapSim virtual reality trainer
The LapSim Haptic System (Surgical Science) is a virtual reality skills trainer. The hysterectomy module includes right and left uterine artery dissection, vaginal cuff opening, and cuff closure (FIGURE 5). One advantage of this simulator is its haptic feedback system, which enhances the fidelity of the training.

The LapSim simulator includes a training module for students and early learners and modules to improve camera handling. The virtual reality base system costs $70,720, and the hysterectomy software module is an additional $15,600.
For more information, visit the company’s website at https://surgicalscience.com/systems/lapsim/. For an informational video, go to https://surgicalscience.com/systems/lapsim/video/.
Takeaway. The LapSim is an expensive, high-fidelity, virtual reality simulator with enhanced haptics and software for practicing laparoscopic hysterectomy.
The LAP Mentor virtual reality simulator
The LAP Mentor VR (3D Systems) is another virtual reality simulator that has modules for laparoscopic hysterectomy and cuff closure (FIGURE 6). The trainee uses a virtual reality headset and becomes fully immersed in the operating room environment with audio and visual cues that mimic a real surgical experience.

The hysterectomy module allows the user to manipulate the uterus, identify the ureters, divide the superior pedicles, mobilize the bladder, expose and divide the uterine artery, and perform the colpotomy. The cuff closure module allows the user to suture the vaginal cuff using barbed suture. The module also can expose the learner to complications, such as bladder, ureteral, colon, or vascular injury.
The LAP Mentor VR base system costs $84,000 and the modules cost about $15,000. For additional information, visit the company’s website at http://simbionix.com/simulators/lap-mentor/lap-mentor-vr-or/.
Takeaway. The LAP Mentor is an expensive, high-fidelity simulation platform with a virtual reality headset that simulates a laparoscopic hysterectomy (with complications) in the operating room.
Read about simulations models for robot-assisted lap hysterectomy and abdominal hysterectomy.
Simulation models for training in robot-assisted laparoscopic hysterectomy
All robot-assisted simulation platforms have highly realistic graphics, and they are expensive (TABLE). However, the da Vinci Skills Simulator (backpack) platform is included with the da Vinci Si and Xi Systems. Note, though, that it can be challenging to access the surgeon console and backpack at institutions with high volumes of robot-assisted surgery.

Other options that generally reside outside of the operating room include Mimic’s FlexVR and dV-Trainer and the Robotix Mentor by 3D Systems (FIGURES 7–11). Mimic’s new technology, called MaestroAR (augmented reality), allows trainees to manipulate virtual robotic instruments to interact with anatomic regions within augmented 3D surgical video footage, with narration and instruction by Dr. Arnold Advincula.
Newer software by Simbionix allows augmented reality to assist the simulation of robot-assisted hysterectomy with the da Vinci Xi backpack and RobotiX platforms.





Models for training in abdominal hysterectomy
In the last 10 years, there has been a 30% decrease in the number of abdominal hysterectomies performed by residents.1 Because of this decline in operating room experience, simulation training can be an important tool to bolster residency experience.
There are not many simulation models available for teaching abdominal hysterectomy, but here we discuss 2 that we utilize in our residency program.
Adaptable task trainer
The Surgical Female Pelvic Trainer (SFPT) (Limbs & Things Ltd), a pelvic task trainer primarily used for simulation of laparoscopic hysterectomy, can be adapted for abdominal hysterectomy by removing the abdominal cover (FIGURE 12). This trainer can be used with simulated blood to increase the realism of training. The SFPT trainer costs $2,190. For more information, go to https://www.limbsandthings.com/us/our-products/details/surgical-female-pelvic-trainer-sfpt-mk-2.
Takeaway. The SFPT is a medium-fidelity task trainer with a reusable base and consumable replacement parts.

ACOG’s do-it-yourself flower pot model
The flower pot model (developed by the ACOG Simulation Working Group, Washington, DC) is a comprehensive educational package that includes learning objectives, simulation construction instructions, content review of the abdominal hysterectomy, quiz, and evaluation form.3 ACOG has endorsed this low-cost model for residency education. Each model costs approximately $20, and the base (flower pot) is reusable (FIGURE 13).Construction time for each model is 30 to 60 minutes, and learners can participate in the construction. This can aid in anatomy review and familiarization with the model prior to training in the surgical procedure.

The learning objectives, content review, quiz, and evaluation form can be used for the flower pot model or for high-fidelity models.
The advantages of this model are the low cost and that it provides enough fidelity to teach each of the critical steps of the procedure. The disadvantages include that it is a lower-fidelity model, requires a considerable amount of time for construction, does not bleed, and is not compatible with energy devices. This model also can be used for training in laparoscopic and vaginal hysterectomy. For more information, visit ACOG’s Surgical Curriculum website at https://cfweb.acog.org/scog/.
Takeaway. ACOG’s flower pot model for hysterectomy training is a comprehensive, low-cost, low-fidelity simulation model that requires significant setup time.
Simulation’s offerings
Simulation training is the present and future of medicine that bridges the gap between textbook learning and technical proficiency. Although in this article we describe only a handful of the simulation resources available, we hope that you will incorporate such tools into your practice for continuing education and skill development. Utilize peer-reviewed resources, such as the ACOG curriculum module and evaluation tools for abdominal, laparoscopic, and vaginal hysterectomy, which can be used with any simulation model to provide a comprehensive and complimentary learning experience.
The future of health care depends on the commitment and ingenuity of educators who embrace medical simulation’s purpose: improved patient safety, effectiveness, and efficiency. Join the movement!
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Washburn EE, Cohen SL, Manoucheri E, Zurawin RK, Einarsson JI. Trends in reported resident surgical experience in hysterectomy. J Minim Invasive Gynecol. 2014;21(6):1067–1070.
- American Board of Obstetrics and Gynecology. ABOG announces new eligibility requirement for board certification. https://www.abog.org/new/ABOG_FLS.aspx. Published January 22, 2018. Accessed April 10, 2018.
- Altman K, Burrell D, Chen G, Chou B, Fashokun T. Surgical curriculum in obstetrics and gynecology: vaginal hysterectomy simulation. https://cfweb.acog.org/scog/scog008/Simulation.cfm. Published December 2014. Accessed April 10, 2018.
- Washburn EE, Cohen SL, Manoucheri E, Zurawin RK, Einarsson JI. Trends in reported resident surgical experience in hysterectomy. J Minim Invasive Gynecol. 2014;21(6):1067–1070.
- American Board of Obstetrics and Gynecology. ABOG announces new eligibility requirement for board certification. https://www.abog.org/new/ABOG_FLS.aspx. Published January 22, 2018. Accessed April 10, 2018.
- Altman K, Burrell D, Chen G, Chou B, Fashokun T. Surgical curriculum in obstetrics and gynecology: vaginal hysterectomy simulation. https://cfweb.acog.org/scog/scog008/Simulation.cfm. Published December 2014. Accessed April 10, 2018.
Technique for apical suspension at the time of total laparoscopic hysterectomy

Visit the Society of Gynecologic Surgeons online: sgsonline.org
Additional videos from SGS are available here, including these recent offerings:

Visit the Society of Gynecologic Surgeons online: sgsonline.org
Additional videos from SGS are available here, including these recent offerings:

Visit the Society of Gynecologic Surgeons online: sgsonline.org
Additional videos from SGS are available here, including these recent offerings:
This video is brought to you by
Suture found in bladder after hysterectomy
Suture found in bladder after hysterectomy
A 40-year-old woman underwent a hysterectomy due to dysmenorrhea. Despite the presence of blood in the catheter bag after the procedure, the surgeon did not consult a urologist or perform a cystoscopy. Later, when the patient reported urinary retention, urinary leakage, and dyspareunia, a urologist performed a cystoscopy and discovered a suture in the bladder wall and a vesicovaginal fistula.
PATIENTS' CLAIM:
During the procedure, the gynecologic surgeon inadvertently placed a suture in the bladder wall. The presence of blood in the Foley catheter required an immediate urology consult and cystoscopy, during which the presence of the errant suture would have been discovered. Repair surgery then would have prevented subsequent injuries.
PHYSICIANS' DEFENSE:
The surgeon used reasonable judgment, as there were explanations for the blood in the catheter due to a difficult catheter placement and lysis of bladder adhesions.
VERDICT:
A Michigan defense verdict was returned.
Related article:
How to avoid intestinal and urinary tract injuries during gynecologic laparoscopy
Bowel injury during tubal ligation
A 40-year-old woman underwent laparoscopic tubal ligation using cauterization at an outpatient surgery center. Two hours after the procedure, her BP began to drop. She was promptly transferred to a hospital and underwent emergency surgery that revealed a bowel injury. Part of the patient’s small intestine was resected.
PATIENTS' CLAIM:
The gynecologic surgeon committed a medical error when she injured the bowel during trocar insertion.
DEFENDANTS' DEFENSE:
The bowel injury was a known complication of the surgery.
VERDICT:
A Louisiana defense verdict was returned.
Related article:
How to avoid major vessel injury during gynecologic laparoscopy
Colon injured twice: $1M settlement
A 59-year-old woman underwent laparoscopic total hysterectomy and salpingectomy. Her history included an umbilical hernia repair.
Two days after surgery, the patient experienced abdominal pain, chills, abdominal distention, and a foul-smelling discharge from her umbilical suture site. She went to the emergency department where a computed tomography scan revealed 2 injuries in the bowel. Emergency laparotomy included transverse colon resection and right colon colostomy with Hartmann’s pouch. She wore an ostomy bag for 8 months. She developed an infection because of the colostomy and also required operations to resolve a bowel obstruction and repair incisional hernias.
PATIENTS' CLAIM:
The gynecologic surgeon was negligent when performing the surgery. When he inserted the Veress needle and trocar through the patient’s umbilicus, the transverse colon was injured twice with a 3-cm anterior tear and a 1-cm posterior laceration. The injuries were not discovered during the procedure. He should have been more careful knowing that she had undergone prior umbilical hernia surgery.
PHYSICIANS' DEFENSE:
The case was settled before the trial began.
VERDICT:
A $1 million Virginia settlement was reached.
Chronic pain after sling procedure: $2M verdict
A 63-year-old woman reported urinary incontinence to her gynecologist, who performed a transobturator midurethral sling procedure. After surgery, the patient experienced pelvic pain, urinary urgency, intermittent incontinence, and dyspareunia. She returned to the gynecologist twice. He performed a cystoscopy after the second visit but found nothing wrong.
The patient sought a second opinion. A gynecologic surgeon found a large mass in the patient’s bladder consisting of a crystallized piece of tape that had been used to secure the sling supporting the bladder. The mass was removed and the patient reported that, although surgery alleviated many symptoms, she was not pain-free.
PATIENTS' CLAIM:
The gynecologist negligently inserted the end of the sling through one wall of her bladder and failed to detect the malpositioning during surgery or later. He failed to diagnose and treat bladder stones that resulted from the sling’s malpositioning. He failed to perform a cystoscopy when she first reported symptoms and improperly performed cystoscopy at the second visit.
DEFENDANTS' DEFENSE:
There was no negligence on the part of the gynecologist. The patient did not report ongoing symptoms until 1 year after sling insertion.
VERDICT:
A $2 million Pennsylvania verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Suture found in bladder after hysterectomy
A 40-year-old woman underwent a hysterectomy due to dysmenorrhea. Despite the presence of blood in the catheter bag after the procedure, the surgeon did not consult a urologist or perform a cystoscopy. Later, when the patient reported urinary retention, urinary leakage, and dyspareunia, a urologist performed a cystoscopy and discovered a suture in the bladder wall and a vesicovaginal fistula.
PATIENTS' CLAIM:
During the procedure, the gynecologic surgeon inadvertently placed a suture in the bladder wall. The presence of blood in the Foley catheter required an immediate urology consult and cystoscopy, during which the presence of the errant suture would have been discovered. Repair surgery then would have prevented subsequent injuries.
PHYSICIANS' DEFENSE:
The surgeon used reasonable judgment, as there were explanations for the blood in the catheter due to a difficult catheter placement and lysis of bladder adhesions.
VERDICT:
A Michigan defense verdict was returned.
Related article:
How to avoid intestinal and urinary tract injuries during gynecologic laparoscopy
Bowel injury during tubal ligation
A 40-year-old woman underwent laparoscopic tubal ligation using cauterization at an outpatient surgery center. Two hours after the procedure, her BP began to drop. She was promptly transferred to a hospital and underwent emergency surgery that revealed a bowel injury. Part of the patient’s small intestine was resected.
PATIENTS' CLAIM:
The gynecologic surgeon committed a medical error when she injured the bowel during trocar insertion.
DEFENDANTS' DEFENSE:
The bowel injury was a known complication of the surgery.
VERDICT:
A Louisiana defense verdict was returned.
Related article:
How to avoid major vessel injury during gynecologic laparoscopy
Colon injured twice: $1M settlement
A 59-year-old woman underwent laparoscopic total hysterectomy and salpingectomy. Her history included an umbilical hernia repair.
Two days after surgery, the patient experienced abdominal pain, chills, abdominal distention, and a foul-smelling discharge from her umbilical suture site. She went to the emergency department where a computed tomography scan revealed 2 injuries in the bowel. Emergency laparotomy included transverse colon resection and right colon colostomy with Hartmann’s pouch. She wore an ostomy bag for 8 months. She developed an infection because of the colostomy and also required operations to resolve a bowel obstruction and repair incisional hernias.
PATIENTS' CLAIM:
The gynecologic surgeon was negligent when performing the surgery. When he inserted the Veress needle and trocar through the patient’s umbilicus, the transverse colon was injured twice with a 3-cm anterior tear and a 1-cm posterior laceration. The injuries were not discovered during the procedure. He should have been more careful knowing that she had undergone prior umbilical hernia surgery.
PHYSICIANS' DEFENSE:
The case was settled before the trial began.
VERDICT:
A $1 million Virginia settlement was reached.
Chronic pain after sling procedure: $2M verdict
A 63-year-old woman reported urinary incontinence to her gynecologist, who performed a transobturator midurethral sling procedure. After surgery, the patient experienced pelvic pain, urinary urgency, intermittent incontinence, and dyspareunia. She returned to the gynecologist twice. He performed a cystoscopy after the second visit but found nothing wrong.
The patient sought a second opinion. A gynecologic surgeon found a large mass in the patient’s bladder consisting of a crystallized piece of tape that had been used to secure the sling supporting the bladder. The mass was removed and the patient reported that, although surgery alleviated many symptoms, she was not pain-free.
PATIENTS' CLAIM:
The gynecologist negligently inserted the end of the sling through one wall of her bladder and failed to detect the malpositioning during surgery or later. He failed to diagnose and treat bladder stones that resulted from the sling’s malpositioning. He failed to perform a cystoscopy when she first reported symptoms and improperly performed cystoscopy at the second visit.
DEFENDANTS' DEFENSE:
There was no negligence on the part of the gynecologist. The patient did not report ongoing symptoms until 1 year after sling insertion.
VERDICT:
A $2 million Pennsylvania verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Suture found in bladder after hysterectomy
A 40-year-old woman underwent a hysterectomy due to dysmenorrhea. Despite the presence of blood in the catheter bag after the procedure, the surgeon did not consult a urologist or perform a cystoscopy. Later, when the patient reported urinary retention, urinary leakage, and dyspareunia, a urologist performed a cystoscopy and discovered a suture in the bladder wall and a vesicovaginal fistula.
PATIENTS' CLAIM:
During the procedure, the gynecologic surgeon inadvertently placed a suture in the bladder wall. The presence of blood in the Foley catheter required an immediate urology consult and cystoscopy, during which the presence of the errant suture would have been discovered. Repair surgery then would have prevented subsequent injuries.
PHYSICIANS' DEFENSE:
The surgeon used reasonable judgment, as there were explanations for the blood in the catheter due to a difficult catheter placement and lysis of bladder adhesions.
VERDICT:
A Michigan defense verdict was returned.
Related article:
How to avoid intestinal and urinary tract injuries during gynecologic laparoscopy
Bowel injury during tubal ligation
A 40-year-old woman underwent laparoscopic tubal ligation using cauterization at an outpatient surgery center. Two hours after the procedure, her BP began to drop. She was promptly transferred to a hospital and underwent emergency surgery that revealed a bowel injury. Part of the patient’s small intestine was resected.
PATIENTS' CLAIM:
The gynecologic surgeon committed a medical error when she injured the bowel during trocar insertion.
DEFENDANTS' DEFENSE:
The bowel injury was a known complication of the surgery.
VERDICT:
A Louisiana defense verdict was returned.
Related article:
How to avoid major vessel injury during gynecologic laparoscopy
Colon injured twice: $1M settlement
A 59-year-old woman underwent laparoscopic total hysterectomy and salpingectomy. Her history included an umbilical hernia repair.
Two days after surgery, the patient experienced abdominal pain, chills, abdominal distention, and a foul-smelling discharge from her umbilical suture site. She went to the emergency department where a computed tomography scan revealed 2 injuries in the bowel. Emergency laparotomy included transverse colon resection and right colon colostomy with Hartmann’s pouch. She wore an ostomy bag for 8 months. She developed an infection because of the colostomy and also required operations to resolve a bowel obstruction and repair incisional hernias.
PATIENTS' CLAIM:
The gynecologic surgeon was negligent when performing the surgery. When he inserted the Veress needle and trocar through the patient’s umbilicus, the transverse colon was injured twice with a 3-cm anterior tear and a 1-cm posterior laceration. The injuries were not discovered during the procedure. He should have been more careful knowing that she had undergone prior umbilical hernia surgery.
PHYSICIANS' DEFENSE:
The case was settled before the trial began.
VERDICT:
A $1 million Virginia settlement was reached.
Chronic pain after sling procedure: $2M verdict
A 63-year-old woman reported urinary incontinence to her gynecologist, who performed a transobturator midurethral sling procedure. After surgery, the patient experienced pelvic pain, urinary urgency, intermittent incontinence, and dyspareunia. She returned to the gynecologist twice. He performed a cystoscopy after the second visit but found nothing wrong.
The patient sought a second opinion. A gynecologic surgeon found a large mass in the patient’s bladder consisting of a crystallized piece of tape that had been used to secure the sling supporting the bladder. The mass was removed and the patient reported that, although surgery alleviated many symptoms, she was not pain-free.
PATIENTS' CLAIM:
The gynecologist negligently inserted the end of the sling through one wall of her bladder and failed to detect the malpositioning during surgery or later. He failed to diagnose and treat bladder stones that resulted from the sling’s malpositioning. He failed to perform a cystoscopy when she first reported symptoms and improperly performed cystoscopy at the second visit.
DEFENDANTS' DEFENSE:
There was no negligence on the part of the gynecologist. The patient did not report ongoing symptoms until 1 year after sling insertion.
VERDICT:
A $2 million Pennsylvania verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Woman dies after robotic hysterectomy: $5M verdict
Woman dies after robotic hysterectomy: $5M verdict
When a 36-year-old woman underwent robotic hysterectomy, the gynecologist inserted a plastic trocar and sleeve through the patient's umbilicus to access the abdominal cavity at 7:30 am.
The certified registered nurse anesthetist (CRNA) noted a significant abnormality in the patient's vital signs at 8:07 am and administered medication and fluids to treat a suspected blood loss. When the patient's heart rate became extremely elevated at 8:25 am, the CRNA administered another drug, which failed to bring the patient's heart rate down. At 8:37 am, the monitoring machine could not record the patient's blood pressure. The CRNA informed the surgeon of the patient's condition. The supervising anesthesiologist was called; he arrived at 8:45 am and determined that the patient was bleeding internally. He asked the surgeon if he could visualize any bleeding; the surgeon could not.
The patient's condition continued to deteriorate. At 9:05 am, her blood pressure was still undetectable on the monitor. A Code Blue was called at 9:30 am. Exploratory surgery and blood transfusions begun at 9:43 am were not able to counteract the patient's massive blood loss. After cardiac arrest, she was pronounced dead at 11:18 am.
ESTATE'S CLAIM:
The surgeon was negligent in lacerating the left common iliac artery when inserting the trocar, and in not detecting the injury intraoperatively.
The anesthesia staff was negligent. The CRNA did not inform the surgeon until the situation was dire. A simple procedure could have been performed at any time to check the patient's hematocrit and hemoglobin levels, but that was not done until 9:30 am. If the severity of the patient's condition had been determined earlier, blood transfusions and further treatment could have saved her life.
DEFENDANTS' DEFENSE:
There was no negligence on the part of the surgeon or anesthesia team. The standard of care was met. Arterial laceration is a known risk of the surgery.
VERDICT:
A $5,008,922 Illinois verdict was returned against all defendants except the CRNA.
A woman with MS becomes incontinent after surgery
A 43-year-old woman with multiple sclerosis (MS) underwent a hysterectomy performed by a gynecologic surgeon. During surgery, the patient's ureter was injured, requiring additional surgery. The patient is now permanently incontinent.
PATIENT'S CLAIM:
During surgery, the surgeon constricted the ureter with stitches. A second surgery was needed to remove the stitches and reimplant the ureter. The second surgery left her permanently incontinent. Although incontinence is a known complication of the second surgery, the second surgery would not have been necessary if the surgeon had not injured the ureter during the first surgery. Incontinence was not a result of her MS as she was not incontinent before the second surgery.
DEFENDANTS' DEFENSE:
There was no deviation from the standard of care. There was no stitching around the ureter. The ureter was damaged by kinking, which was addressed during the second surgery. Incontinence was a result of her MS.
VERDICT:
A $700,000 South Carolina verdict was returned.
Bowel injury during robotic procedure: $6.25M settlement
A woman in her late 60s reported minor urinary incontinence to her gynecologist. She underwent robot-assisted laparoscopic hysterectomy with a sling procedure for pelvic prolapse. During the sling procedure, the transverse colon was injured. The patient developed sepsis, requiring multiple attempts at surgical repair, including colostomy. The patient requires a permanent colostomy. She has a malabsorption disorder and needs frequent intravenous treatment for dehydration.
PATIENT'S CLAIM:
The surgeon failed to properly control the robotic device, causing injury to the patient's bowel. The surgeon deviated from the standard of care by failing to convert from the robot-assisted laparoscopic procedure to an open procedure when complications arose. The injury was not properly treated before the surgeon closed the initial surgery, causing the patient to develop sepsis.
PHYSICIAN'S DEFENSE:
The surgeon claimed that the injuries and resulting sepsis were the fault of other physicians and hospital staff. The case settled during trial.
VERDICT:
A $6.25 million New Jersey settlement was reached.
Hydrothermal ablation led to genital burns
A woman SAW AN OBGYN on October 2 to report menorrhagia. She had been treated for uterine fibroids with a Mirena intrauterine device and hydrothermal ablation. Another physician had suggested hysterectomy, which she declined.
When the ObGyn found that the patient had an enlarged uterus, he ordered ultrasonography and an endometrial biopsy. On follow-up, the ObGyn provided options of robotic hysterectomy or operative hysteroscopy with hydrothermal ablation. The patient chose hysteroscopy and the procedure was scheduled for December 28.
During surgery, an improper seal to the cervix around the hydrothermal ablation sheath was detected before heating the fluid. A tenaculum and 2 sponges were placed on the cervix to help form a seal and the fluid was heated for 4 minutes. The procedure was aborted when fluid was seen to be leaking again. Instruments were removed after a cooling period. The patient was discharged from the surgery center the same day with a prescription for oral hydrocodone bitartrate and acetaminophen for pain.
On January 4, the patient reported severe vulvar pain. The ObGyn found thermal burns on both labia with possible cellulitis. He prescribed silver sulfadiazine cream twice daily, levofloxacin 500 mg for 7 days, and warm-water soaks. When the patient called to report continued pain on January 7, the hydrocodone and acetaminophen prescription was renewed. On January 8, the ObGyn found continued evidence of labia and introitus burns with no signs of infection. The patient was told to continue taking the oral pain medication and to apply topical lidocaine gel and silver sulfadiazine cream.
Examinations on January 11, 17, 24, and 31 showed continued evidence of active healing. When new evidence of vulvar ulceration with inflammation and infection appeared, supportive care and antibiotics were given. On February 7, granulation tissue had developed at the introitus with continued healing.
On March 27, she saw a gynecologist for dyspareunia. The skin was healed but a tender band of scar tissue was noted at the burn site. She was referred for physical therapy and given estradiol vaginal cream.
On December 11, the patient reported dyspareunia and depression to the gynecologist, who prescribed medication for depression and referred her to counseling.
PATIENT'S CLAIM:
The ObGyn was negligent in failing to maintain a proper seal around the hydrothermal ablation shield. The patient sustained second-degree burns to her genital area from the hot saline solution that leaked from the uterus. The injury caused lasting dyspareunia and depression.
PHYSICIAN'S DEFENSE:
There was no negligence. Once the ObGyn realized that the seal was incomplete, the procedure was stopped and the fluid cooled before being released. Burns were treated within the standard of care.
VERDICT:
A Texas defense verdict was returned based on a no- evidence partial summary judgment: neither the patient nor the expert witness supplied evidence to support the claims of gross negligence or exemplary damages against the ObGyn.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Woman dies after robotic hysterectomy: $5M verdict
When a 36-year-old woman underwent robotic hysterectomy, the gynecologist inserted a plastic trocar and sleeve through the patient's umbilicus to access the abdominal cavity at 7:30 am.
The certified registered nurse anesthetist (CRNA) noted a significant abnormality in the patient's vital signs at 8:07 am and administered medication and fluids to treat a suspected blood loss. When the patient's heart rate became extremely elevated at 8:25 am, the CRNA administered another drug, which failed to bring the patient's heart rate down. At 8:37 am, the monitoring machine could not record the patient's blood pressure. The CRNA informed the surgeon of the patient's condition. The supervising anesthesiologist was called; he arrived at 8:45 am and determined that the patient was bleeding internally. He asked the surgeon if he could visualize any bleeding; the surgeon could not.
The patient's condition continued to deteriorate. At 9:05 am, her blood pressure was still undetectable on the monitor. A Code Blue was called at 9:30 am. Exploratory surgery and blood transfusions begun at 9:43 am were not able to counteract the patient's massive blood loss. After cardiac arrest, she was pronounced dead at 11:18 am.
ESTATE'S CLAIM:
The surgeon was negligent in lacerating the left common iliac artery when inserting the trocar, and in not detecting the injury intraoperatively.
The anesthesia staff was negligent. The CRNA did not inform the surgeon until the situation was dire. A simple procedure could have been performed at any time to check the patient's hematocrit and hemoglobin levels, but that was not done until 9:30 am. If the severity of the patient's condition had been determined earlier, blood transfusions and further treatment could have saved her life.
DEFENDANTS' DEFENSE:
There was no negligence on the part of the surgeon or anesthesia team. The standard of care was met. Arterial laceration is a known risk of the surgery.
VERDICT:
A $5,008,922 Illinois verdict was returned against all defendants except the CRNA.
A woman with MS becomes incontinent after surgery
A 43-year-old woman with multiple sclerosis (MS) underwent a hysterectomy performed by a gynecologic surgeon. During surgery, the patient's ureter was injured, requiring additional surgery. The patient is now permanently incontinent.
PATIENT'S CLAIM:
During surgery, the surgeon constricted the ureter with stitches. A second surgery was needed to remove the stitches and reimplant the ureter. The second surgery left her permanently incontinent. Although incontinence is a known complication of the second surgery, the second surgery would not have been necessary if the surgeon had not injured the ureter during the first surgery. Incontinence was not a result of her MS as she was not incontinent before the second surgery.
DEFENDANTS' DEFENSE:
There was no deviation from the standard of care. There was no stitching around the ureter. The ureter was damaged by kinking, which was addressed during the second surgery. Incontinence was a result of her MS.
VERDICT:
A $700,000 South Carolina verdict was returned.
Bowel injury during robotic procedure: $6.25M settlement
A woman in her late 60s reported minor urinary incontinence to her gynecologist. She underwent robot-assisted laparoscopic hysterectomy with a sling procedure for pelvic prolapse. During the sling procedure, the transverse colon was injured. The patient developed sepsis, requiring multiple attempts at surgical repair, including colostomy. The patient requires a permanent colostomy. She has a malabsorption disorder and needs frequent intravenous treatment for dehydration.
PATIENT'S CLAIM:
The surgeon failed to properly control the robotic device, causing injury to the patient's bowel. The surgeon deviated from the standard of care by failing to convert from the robot-assisted laparoscopic procedure to an open procedure when complications arose. The injury was not properly treated before the surgeon closed the initial surgery, causing the patient to develop sepsis.
PHYSICIAN'S DEFENSE:
The surgeon claimed that the injuries and resulting sepsis were the fault of other physicians and hospital staff. The case settled during trial.
VERDICT:
A $6.25 million New Jersey settlement was reached.
Hydrothermal ablation led to genital burns
A woman SAW AN OBGYN on October 2 to report menorrhagia. She had been treated for uterine fibroids with a Mirena intrauterine device and hydrothermal ablation. Another physician had suggested hysterectomy, which she declined.
When the ObGyn found that the patient had an enlarged uterus, he ordered ultrasonography and an endometrial biopsy. On follow-up, the ObGyn provided options of robotic hysterectomy or operative hysteroscopy with hydrothermal ablation. The patient chose hysteroscopy and the procedure was scheduled for December 28.
During surgery, an improper seal to the cervix around the hydrothermal ablation sheath was detected before heating the fluid. A tenaculum and 2 sponges were placed on the cervix to help form a seal and the fluid was heated for 4 minutes. The procedure was aborted when fluid was seen to be leaking again. Instruments were removed after a cooling period. The patient was discharged from the surgery center the same day with a prescription for oral hydrocodone bitartrate and acetaminophen for pain.
On January 4, the patient reported severe vulvar pain. The ObGyn found thermal burns on both labia with possible cellulitis. He prescribed silver sulfadiazine cream twice daily, levofloxacin 500 mg for 7 days, and warm-water soaks. When the patient called to report continued pain on January 7, the hydrocodone and acetaminophen prescription was renewed. On January 8, the ObGyn found continued evidence of labia and introitus burns with no signs of infection. The patient was told to continue taking the oral pain medication and to apply topical lidocaine gel and silver sulfadiazine cream.
Examinations on January 11, 17, 24, and 31 showed continued evidence of active healing. When new evidence of vulvar ulceration with inflammation and infection appeared, supportive care and antibiotics were given. On February 7, granulation tissue had developed at the introitus with continued healing.
On March 27, she saw a gynecologist for dyspareunia. The skin was healed but a tender band of scar tissue was noted at the burn site. She was referred for physical therapy and given estradiol vaginal cream.
On December 11, the patient reported dyspareunia and depression to the gynecologist, who prescribed medication for depression and referred her to counseling.
PATIENT'S CLAIM:
The ObGyn was negligent in failing to maintain a proper seal around the hydrothermal ablation shield. The patient sustained second-degree burns to her genital area from the hot saline solution that leaked from the uterus. The injury caused lasting dyspareunia and depression.
PHYSICIAN'S DEFENSE:
There was no negligence. Once the ObGyn realized that the seal was incomplete, the procedure was stopped and the fluid cooled before being released. Burns were treated within the standard of care.
VERDICT:
A Texas defense verdict was returned based on a no- evidence partial summary judgment: neither the patient nor the expert witness supplied evidence to support the claims of gross negligence or exemplary damages against the ObGyn.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Woman dies after robotic hysterectomy: $5M verdict
When a 36-year-old woman underwent robotic hysterectomy, the gynecologist inserted a plastic trocar and sleeve through the patient's umbilicus to access the abdominal cavity at 7:30 am.
The certified registered nurse anesthetist (CRNA) noted a significant abnormality in the patient's vital signs at 8:07 am and administered medication and fluids to treat a suspected blood loss. When the patient's heart rate became extremely elevated at 8:25 am, the CRNA administered another drug, which failed to bring the patient's heart rate down. At 8:37 am, the monitoring machine could not record the patient's blood pressure. The CRNA informed the surgeon of the patient's condition. The supervising anesthesiologist was called; he arrived at 8:45 am and determined that the patient was bleeding internally. He asked the surgeon if he could visualize any bleeding; the surgeon could not.
The patient's condition continued to deteriorate. At 9:05 am, her blood pressure was still undetectable on the monitor. A Code Blue was called at 9:30 am. Exploratory surgery and blood transfusions begun at 9:43 am were not able to counteract the patient's massive blood loss. After cardiac arrest, she was pronounced dead at 11:18 am.
ESTATE'S CLAIM:
The surgeon was negligent in lacerating the left common iliac artery when inserting the trocar, and in not detecting the injury intraoperatively.
The anesthesia staff was negligent. The CRNA did not inform the surgeon until the situation was dire. A simple procedure could have been performed at any time to check the patient's hematocrit and hemoglobin levels, but that was not done until 9:30 am. If the severity of the patient's condition had been determined earlier, blood transfusions and further treatment could have saved her life.
DEFENDANTS' DEFENSE:
There was no negligence on the part of the surgeon or anesthesia team. The standard of care was met. Arterial laceration is a known risk of the surgery.
VERDICT:
A $5,008,922 Illinois verdict was returned against all defendants except the CRNA.
A woman with MS becomes incontinent after surgery
A 43-year-old woman with multiple sclerosis (MS) underwent a hysterectomy performed by a gynecologic surgeon. During surgery, the patient's ureter was injured, requiring additional surgery. The patient is now permanently incontinent.
PATIENT'S CLAIM:
During surgery, the surgeon constricted the ureter with stitches. A second surgery was needed to remove the stitches and reimplant the ureter. The second surgery left her permanently incontinent. Although incontinence is a known complication of the second surgery, the second surgery would not have been necessary if the surgeon had not injured the ureter during the first surgery. Incontinence was not a result of her MS as she was not incontinent before the second surgery.
DEFENDANTS' DEFENSE:
There was no deviation from the standard of care. There was no stitching around the ureter. The ureter was damaged by kinking, which was addressed during the second surgery. Incontinence was a result of her MS.
VERDICT:
A $700,000 South Carolina verdict was returned.
Bowel injury during robotic procedure: $6.25M settlement
A woman in her late 60s reported minor urinary incontinence to her gynecologist. She underwent robot-assisted laparoscopic hysterectomy with a sling procedure for pelvic prolapse. During the sling procedure, the transverse colon was injured. The patient developed sepsis, requiring multiple attempts at surgical repair, including colostomy. The patient requires a permanent colostomy. She has a malabsorption disorder and needs frequent intravenous treatment for dehydration.
PATIENT'S CLAIM:
The surgeon failed to properly control the robotic device, causing injury to the patient's bowel. The surgeon deviated from the standard of care by failing to convert from the robot-assisted laparoscopic procedure to an open procedure when complications arose. The injury was not properly treated before the surgeon closed the initial surgery, causing the patient to develop sepsis.
PHYSICIAN'S DEFENSE:
The surgeon claimed that the injuries and resulting sepsis were the fault of other physicians and hospital staff. The case settled during trial.
VERDICT:
A $6.25 million New Jersey settlement was reached.
Hydrothermal ablation led to genital burns
A woman SAW AN OBGYN on October 2 to report menorrhagia. She had been treated for uterine fibroids with a Mirena intrauterine device and hydrothermal ablation. Another physician had suggested hysterectomy, which she declined.
When the ObGyn found that the patient had an enlarged uterus, he ordered ultrasonography and an endometrial biopsy. On follow-up, the ObGyn provided options of robotic hysterectomy or operative hysteroscopy with hydrothermal ablation. The patient chose hysteroscopy and the procedure was scheduled for December 28.
During surgery, an improper seal to the cervix around the hydrothermal ablation sheath was detected before heating the fluid. A tenaculum and 2 sponges were placed on the cervix to help form a seal and the fluid was heated for 4 minutes. The procedure was aborted when fluid was seen to be leaking again. Instruments were removed after a cooling period. The patient was discharged from the surgery center the same day with a prescription for oral hydrocodone bitartrate and acetaminophen for pain.
On January 4, the patient reported severe vulvar pain. The ObGyn found thermal burns on both labia with possible cellulitis. He prescribed silver sulfadiazine cream twice daily, levofloxacin 500 mg for 7 days, and warm-water soaks. When the patient called to report continued pain on January 7, the hydrocodone and acetaminophen prescription was renewed. On January 8, the ObGyn found continued evidence of labia and introitus burns with no signs of infection. The patient was told to continue taking the oral pain medication and to apply topical lidocaine gel and silver sulfadiazine cream.
Examinations on January 11, 17, 24, and 31 showed continued evidence of active healing. When new evidence of vulvar ulceration with inflammation and infection appeared, supportive care and antibiotics were given. On February 7, granulation tissue had developed at the introitus with continued healing.
On March 27, she saw a gynecologist for dyspareunia. The skin was healed but a tender band of scar tissue was noted at the burn site. She was referred for physical therapy and given estradiol vaginal cream.
On December 11, the patient reported dyspareunia and depression to the gynecologist, who prescribed medication for depression and referred her to counseling.
PATIENT'S CLAIM:
The ObGyn was negligent in failing to maintain a proper seal around the hydrothermal ablation shield. The patient sustained second-degree burns to her genital area from the hot saline solution that leaked from the uterus. The injury caused lasting dyspareunia and depression.
PHYSICIAN'S DEFENSE:
There was no negligence. Once the ObGyn realized that the seal was incomplete, the procedure was stopped and the fluid cooled before being released. Burns were treated within the standard of care.
VERDICT:
A Texas defense verdict was returned based on a no- evidence partial summary judgment: neither the patient nor the expert witness supplied evidence to support the claims of gross negligence or exemplary damages against the ObGyn.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Is the use of a containment bag at minimally invasive hysterectomy or myomectomy effective at reducing tissue spillage?
Tissue extraction during laparoscopic or robot-assisted laparoscopic gynecologic surgery raises safety concerns for dissemination of tissue during the open, or uncontained, electromechanical morcellation process. Researchers from Brigham & Women’s Hospital in Boston, Massachusetts, investigated whether contained tissue extraction using power morcellators entirely within a bag is safe and practical for preventing tissue spillage. Goggins and colleagues presented their findings in a poster at the 2015 Annual Clinical Meeting of the American College of Obstetricians and Gynecologists in San Francisco, California.
A total of 76 women at 4 institutions underwent laparoscopic or robotic multiport surgery (42 hysterectomy; 34 myomectomy). The average (SD) age and body mass index of the women were 43.16 (8.53) years and 26.47 kg/m2 (5.93), respectively. After surgical dissection, each specimen was placed into a containment bag that also included blue dye. The bag was insufflated intracorporeally and electromechanical morcellation and extraction of tissue were performed. The bag was evaluated visually for dye leakage or tears before and after the procedure.
Results
In one case, there was a tear in the bag before morcellation; no bag tears occurred during the morcellation process. Spillage of dye or tissue was noted in 7 cases, although containment bags were intact in each instance. One patient experienced intraoperative blood loss (3600 mL), and that procedure was converted to open radical hysterectomy. The most common pathologic finding was benign leiomyoma.
Conclusion
Goggins and colleagues concluded, “Contained tissue extraction using electromechanical morcellation and intracorporeally insufflated bags may provide a safe alternative to uncontained morcellation by decreasing the spread of tissue in the peritoneal cavity while allowing for the traditional benefits of laparoscopy.”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Reference
- Goggins ER, Greenberg JA, Cohen SL, Morris SN, Brown DN, Einarsson JI. Efficacy of contained tissue extraction for minimizing tissue dissemination during laparoscopic hysterectomy and myomectomy. Obstet Gynecol. 2015;125(5)(suppl):29S.
Tissue extraction during laparoscopic or robot-assisted laparoscopic gynecologic surgery raises safety concerns for dissemination of tissue during the open, or uncontained, electromechanical morcellation process. Researchers from Brigham & Women’s Hospital in Boston, Massachusetts, investigated whether contained tissue extraction using power morcellators entirely within a bag is safe and practical for preventing tissue spillage. Goggins and colleagues presented their findings in a poster at the 2015 Annual Clinical Meeting of the American College of Obstetricians and Gynecologists in San Francisco, California.
A total of 76 women at 4 institutions underwent laparoscopic or robotic multiport surgery (42 hysterectomy; 34 myomectomy). The average (SD) age and body mass index of the women were 43.16 (8.53) years and 26.47 kg/m2 (5.93), respectively. After surgical dissection, each specimen was placed into a containment bag that also included blue dye. The bag was insufflated intracorporeally and electromechanical morcellation and extraction of tissue were performed. The bag was evaluated visually for dye leakage or tears before and after the procedure.
Results
In one case, there was a tear in the bag before morcellation; no bag tears occurred during the morcellation process. Spillage of dye or tissue was noted in 7 cases, although containment bags were intact in each instance. One patient experienced intraoperative blood loss (3600 mL), and that procedure was converted to open radical hysterectomy. The most common pathologic finding was benign leiomyoma.
Conclusion
Goggins and colleagues concluded, “Contained tissue extraction using electromechanical morcellation and intracorporeally insufflated bags may provide a safe alternative to uncontained morcellation by decreasing the spread of tissue in the peritoneal cavity while allowing for the traditional benefits of laparoscopy.”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Tissue extraction during laparoscopic or robot-assisted laparoscopic gynecologic surgery raises safety concerns for dissemination of tissue during the open, or uncontained, electromechanical morcellation process. Researchers from Brigham & Women’s Hospital in Boston, Massachusetts, investigated whether contained tissue extraction using power morcellators entirely within a bag is safe and practical for preventing tissue spillage. Goggins and colleagues presented their findings in a poster at the 2015 Annual Clinical Meeting of the American College of Obstetricians and Gynecologists in San Francisco, California.
A total of 76 women at 4 institutions underwent laparoscopic or robotic multiport surgery (42 hysterectomy; 34 myomectomy). The average (SD) age and body mass index of the women were 43.16 (8.53) years and 26.47 kg/m2 (5.93), respectively. After surgical dissection, each specimen was placed into a containment bag that also included blue dye. The bag was insufflated intracorporeally and electromechanical morcellation and extraction of tissue were performed. The bag was evaluated visually for dye leakage or tears before and after the procedure.
Results
In one case, there was a tear in the bag before morcellation; no bag tears occurred during the morcellation process. Spillage of dye or tissue was noted in 7 cases, although containment bags were intact in each instance. One patient experienced intraoperative blood loss (3600 mL), and that procedure was converted to open radical hysterectomy. The most common pathologic finding was benign leiomyoma.
Conclusion
Goggins and colleagues concluded, “Contained tissue extraction using electromechanical morcellation and intracorporeally insufflated bags may provide a safe alternative to uncontained morcellation by decreasing the spread of tissue in the peritoneal cavity while allowing for the traditional benefits of laparoscopy.”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Reference
- Goggins ER, Greenberg JA, Cohen SL, Morris SN, Brown DN, Einarsson JI. Efficacy of contained tissue extraction for minimizing tissue dissemination during laparoscopic hysterectomy and myomectomy. Obstet Gynecol. 2015;125(5)(suppl):29S.
Reference
- Goggins ER, Greenberg JA, Cohen SL, Morris SN, Brown DN, Einarsson JI. Efficacy of contained tissue extraction for minimizing tissue dissemination during laparoscopic hysterectomy and myomectomy. Obstet Gynecol. 2015;125(5)(suppl):29S.