User login
Ulcerative colitis: Donor-derived strains predict response in FMT
The Odoribacter splanchnicus strain of human donor-derived bacteria correlated with clinical response to ulcerative colitis in study in which mouse models were colonized with patient-derived strains.
Although some recent trials have shown the effectiveness of fecal microbiota transplantation (FMT) for patients with ulcerative colitis (UC), the current process is limited by the used of crude donor fecal material, which increases the risk of infection and decreases potential effectiveness, Svetlana Lima, MD, of Weill Cornell Medicine, New York, and colleagues wrote.
“Rational selection and production of specific microbial strains or communities could improve efficacy, minimize the risk of adverse reactions as well as increase the acceptance of microbiome-based therapies,” the researchers wrote.
In a study published in Gastroenterology, the researchers used metagenomic analysis and IgA sequencing (for sorting and sequencing IgA-coated microbiota) to identify a core of transferable and IgA-coated microbiota. They conducted metagenomic sequencing on 60 stool samples, including 20 recipient-participants with active UC who were treated with FMT, and another 20 FMT recipients with data from 4 weeks after FMT from a previously reported trial.
The core transferable microbiota (CTM) included 22 species of bacteria at 4 weeks after FMT. To determine a relationship between CTM and clinical response to FMT, the researchers defined clinical response as a decrease in Mayo score of 3 or greater with a rectal bleeding score of 1 or less by 4 weeks after FMT; 35% of study participants met this endpoint.* A total of 20 species were unique to the responders. “Of the donor-derived genera, only the relative abundance of Odoribacter at [week 4] post FMT and its increase post FMT was found to significantly correlate with decrease in Mayo score,” the researchers noted.
The researchers then colonized germ-free or genetically engineered mice with patient-derived bacterial strains.
O. splanchnicus also increased induction of interleukin-10, and increased the production of short-chain fatty acids. Taken together, these factors allowed for O. splanchnicus to limit colitis in the mice.
The study findings represent the first strain-level analysis of FMT in UC participants, and define a transferable microbiota associated with clinical response that could serve as a prognostic biomarker, the researchers noted in their discussion section. Although analysis revealed 12 donor-derived bacterial species that predicted clinical response, further IgA analysis identified O. splanchnicus as “the only microbe within the responders core that correlates with clinical response and highlights the potential impact of this taxa seen in independent cohorts, as well as mouse models of colitis and colorectal cancer,” the researchers emphasized.
The study findings were limited by the small sample size and the lack of prospective data. However, “collectively, this work provides the first evidence of transferable, donor-derived strains that correlate with clinical response to FMT in UC and reveals O. splanchnicus as a key component, which mechanistically promotes protection through both cellular and metabolic function,” the researchers said. “These mechanistic features will help enable desperately needed strategies to enhance therapeutic efficacy of microbial therapy for UC.”
Study strains improve effectiveness
“There is an accumulating body of evidence that suggests that gut dysbiosis, or the imbalance between good and bad microbes, plays an important role in the pathogenesis and progression of ulcerative colitis,” Jeffrey Berinstein, MD, of the University of Michigan, Ann Arbor, said in an interview. “It is for this reason that therapeutic manipulation of gut microbiota with fecal microbiota transplant is being explored as a potential treatment option for UC. FMT has demonstrated promise for ulcerative colitis, however little is known about the specific microbiota strains contributing to this observed improvement. In this study, the authors aimed to better understand the mechanisms and the specific strains in FMT contributing to this observed improvement, which is an important step toward improving efficacy and minimizing the risk of adverse events related to FMT in the future.”
Dr. Berinstein was surprised that O. splanchnicus was the only microbe identified that correlated with clinical response. “Previous studies have suggested that microbial diversity is a key factor in successful response to FMT,” he noted. “FMT remains an important potential nonpharmacologic treatment strategy for ulcerative colitis, however more research is needed to understand the mechanism and to develop safer and more efficacious methods for delivering FMT.” Specifically, prospective studies are needed to explore the efficacy and safety of FMT enriched in strains of O. splanchnicus to confirm the current study findings.
The current study is important at this time because, although microbial transferability has emerged as a potential to treat IBD, “the mechanistic understanding of microbial transferability and engraftment has been lacking,” Atsushi Sakuraba, MD, PhD of the University of Chicago, said in an interview. “I was surprised that the effectiveness of FMT in UC could be narrowed down to O. splanchnicus.” The current take-home message for clinicians is that, although FMT currently uses crude donor fecal material, it may soon use more selected microbial strains. However, “whether transfer of O. splanchnicus alone or enriched fecal material provide improved efficacy and safety need to be analyzed,” he added.
The study was supported by Boehringer Ingelheim, the National Institutes of Health, the Kenneth Rainin Foundation, and the Charina Foundation. One coauthor disclosed grant support from Boehringer Ingelheim for this study, and several coauthors are employees of Boehringer Ingelheim. Neither Dr. Berinstein nor Dr. Sakuraba had no financial conflicts to disclose.
This article was updated Dec. 1, 2021.
*Correction, 4/11/22: An earlier version of this article misstated the definition of clinical response.
The Odoribacter splanchnicus strain of human donor-derived bacteria correlated with clinical response to ulcerative colitis in study in which mouse models were colonized with patient-derived strains.
Although some recent trials have shown the effectiveness of fecal microbiota transplantation (FMT) for patients with ulcerative colitis (UC), the current process is limited by the used of crude donor fecal material, which increases the risk of infection and decreases potential effectiveness, Svetlana Lima, MD, of Weill Cornell Medicine, New York, and colleagues wrote.
“Rational selection and production of specific microbial strains or communities could improve efficacy, minimize the risk of adverse reactions as well as increase the acceptance of microbiome-based therapies,” the researchers wrote.
In a study published in Gastroenterology, the researchers used metagenomic analysis and IgA sequencing (for sorting and sequencing IgA-coated microbiota) to identify a core of transferable and IgA-coated microbiota. They conducted metagenomic sequencing on 60 stool samples, including 20 recipient-participants with active UC who were treated with FMT, and another 20 FMT recipients with data from 4 weeks after FMT from a previously reported trial.
The core transferable microbiota (CTM) included 22 species of bacteria at 4 weeks after FMT. To determine a relationship between CTM and clinical response to FMT, the researchers defined clinical response as a decrease in Mayo score of 3 or greater with a rectal bleeding score of 1 or less by 4 weeks after FMT; 35% of study participants met this endpoint.* A total of 20 species were unique to the responders. “Of the donor-derived genera, only the relative abundance of Odoribacter at [week 4] post FMT and its increase post FMT was found to significantly correlate with decrease in Mayo score,” the researchers noted.
The researchers then colonized germ-free or genetically engineered mice with patient-derived bacterial strains.
O. splanchnicus also increased induction of interleukin-10, and increased the production of short-chain fatty acids. Taken together, these factors allowed for O. splanchnicus to limit colitis in the mice.
The study findings represent the first strain-level analysis of FMT in UC participants, and define a transferable microbiota associated with clinical response that could serve as a prognostic biomarker, the researchers noted in their discussion section. Although analysis revealed 12 donor-derived bacterial species that predicted clinical response, further IgA analysis identified O. splanchnicus as “the only microbe within the responders core that correlates with clinical response and highlights the potential impact of this taxa seen in independent cohorts, as well as mouse models of colitis and colorectal cancer,” the researchers emphasized.
The study findings were limited by the small sample size and the lack of prospective data. However, “collectively, this work provides the first evidence of transferable, donor-derived strains that correlate with clinical response to FMT in UC and reveals O. splanchnicus as a key component, which mechanistically promotes protection through both cellular and metabolic function,” the researchers said. “These mechanistic features will help enable desperately needed strategies to enhance therapeutic efficacy of microbial therapy for UC.”
Study strains improve effectiveness
“There is an accumulating body of evidence that suggests that gut dysbiosis, or the imbalance between good and bad microbes, plays an important role in the pathogenesis and progression of ulcerative colitis,” Jeffrey Berinstein, MD, of the University of Michigan, Ann Arbor, said in an interview. “It is for this reason that therapeutic manipulation of gut microbiota with fecal microbiota transplant is being explored as a potential treatment option for UC. FMT has demonstrated promise for ulcerative colitis, however little is known about the specific microbiota strains contributing to this observed improvement. In this study, the authors aimed to better understand the mechanisms and the specific strains in FMT contributing to this observed improvement, which is an important step toward improving efficacy and minimizing the risk of adverse events related to FMT in the future.”
Dr. Berinstein was surprised that O. splanchnicus was the only microbe identified that correlated with clinical response. “Previous studies have suggested that microbial diversity is a key factor in successful response to FMT,” he noted. “FMT remains an important potential nonpharmacologic treatment strategy for ulcerative colitis, however more research is needed to understand the mechanism and to develop safer and more efficacious methods for delivering FMT.” Specifically, prospective studies are needed to explore the efficacy and safety of FMT enriched in strains of O. splanchnicus to confirm the current study findings.
The current study is important at this time because, although microbial transferability has emerged as a potential to treat IBD, “the mechanistic understanding of microbial transferability and engraftment has been lacking,” Atsushi Sakuraba, MD, PhD of the University of Chicago, said in an interview. “I was surprised that the effectiveness of FMT in UC could be narrowed down to O. splanchnicus.” The current take-home message for clinicians is that, although FMT currently uses crude donor fecal material, it may soon use more selected microbial strains. However, “whether transfer of O. splanchnicus alone or enriched fecal material provide improved efficacy and safety need to be analyzed,” he added.
The study was supported by Boehringer Ingelheim, the National Institutes of Health, the Kenneth Rainin Foundation, and the Charina Foundation. One coauthor disclosed grant support from Boehringer Ingelheim for this study, and several coauthors are employees of Boehringer Ingelheim. Neither Dr. Berinstein nor Dr. Sakuraba had no financial conflicts to disclose.
This article was updated Dec. 1, 2021.
*Correction, 4/11/22: An earlier version of this article misstated the definition of clinical response.
The Odoribacter splanchnicus strain of human donor-derived bacteria correlated with clinical response to ulcerative colitis in study in which mouse models were colonized with patient-derived strains.
Although some recent trials have shown the effectiveness of fecal microbiota transplantation (FMT) for patients with ulcerative colitis (UC), the current process is limited by the used of crude donor fecal material, which increases the risk of infection and decreases potential effectiveness, Svetlana Lima, MD, of Weill Cornell Medicine, New York, and colleagues wrote.
“Rational selection and production of specific microbial strains or communities could improve efficacy, minimize the risk of adverse reactions as well as increase the acceptance of microbiome-based therapies,” the researchers wrote.
In a study published in Gastroenterology, the researchers used metagenomic analysis and IgA sequencing (for sorting and sequencing IgA-coated microbiota) to identify a core of transferable and IgA-coated microbiota. They conducted metagenomic sequencing on 60 stool samples, including 20 recipient-participants with active UC who were treated with FMT, and another 20 FMT recipients with data from 4 weeks after FMT from a previously reported trial.
The core transferable microbiota (CTM) included 22 species of bacteria at 4 weeks after FMT. To determine a relationship between CTM and clinical response to FMT, the researchers defined clinical response as a decrease in Mayo score of 3 or greater with a rectal bleeding score of 1 or less by 4 weeks after FMT; 35% of study participants met this endpoint.* A total of 20 species were unique to the responders. “Of the donor-derived genera, only the relative abundance of Odoribacter at [week 4] post FMT and its increase post FMT was found to significantly correlate with decrease in Mayo score,” the researchers noted.
The researchers then colonized germ-free or genetically engineered mice with patient-derived bacterial strains.
O. splanchnicus also increased induction of interleukin-10, and increased the production of short-chain fatty acids. Taken together, these factors allowed for O. splanchnicus to limit colitis in the mice.
The study findings represent the first strain-level analysis of FMT in UC participants, and define a transferable microbiota associated with clinical response that could serve as a prognostic biomarker, the researchers noted in their discussion section. Although analysis revealed 12 donor-derived bacterial species that predicted clinical response, further IgA analysis identified O. splanchnicus as “the only microbe within the responders core that correlates with clinical response and highlights the potential impact of this taxa seen in independent cohorts, as well as mouse models of colitis and colorectal cancer,” the researchers emphasized.
The study findings were limited by the small sample size and the lack of prospective data. However, “collectively, this work provides the first evidence of transferable, donor-derived strains that correlate with clinical response to FMT in UC and reveals O. splanchnicus as a key component, which mechanistically promotes protection through both cellular and metabolic function,” the researchers said. “These mechanistic features will help enable desperately needed strategies to enhance therapeutic efficacy of microbial therapy for UC.”
Study strains improve effectiveness
“There is an accumulating body of evidence that suggests that gut dysbiosis, or the imbalance between good and bad microbes, plays an important role in the pathogenesis and progression of ulcerative colitis,” Jeffrey Berinstein, MD, of the University of Michigan, Ann Arbor, said in an interview. “It is for this reason that therapeutic manipulation of gut microbiota with fecal microbiota transplant is being explored as a potential treatment option for UC. FMT has demonstrated promise for ulcerative colitis, however little is known about the specific microbiota strains contributing to this observed improvement. In this study, the authors aimed to better understand the mechanisms and the specific strains in FMT contributing to this observed improvement, which is an important step toward improving efficacy and minimizing the risk of adverse events related to FMT in the future.”
Dr. Berinstein was surprised that O. splanchnicus was the only microbe identified that correlated with clinical response. “Previous studies have suggested that microbial diversity is a key factor in successful response to FMT,” he noted. “FMT remains an important potential nonpharmacologic treatment strategy for ulcerative colitis, however more research is needed to understand the mechanism and to develop safer and more efficacious methods for delivering FMT.” Specifically, prospective studies are needed to explore the efficacy and safety of FMT enriched in strains of O. splanchnicus to confirm the current study findings.
The current study is important at this time because, although microbial transferability has emerged as a potential to treat IBD, “the mechanistic understanding of microbial transferability and engraftment has been lacking,” Atsushi Sakuraba, MD, PhD of the University of Chicago, said in an interview. “I was surprised that the effectiveness of FMT in UC could be narrowed down to O. splanchnicus.” The current take-home message for clinicians is that, although FMT currently uses crude donor fecal material, it may soon use more selected microbial strains. However, “whether transfer of O. splanchnicus alone or enriched fecal material provide improved efficacy and safety need to be analyzed,” he added.
The study was supported by Boehringer Ingelheim, the National Institutes of Health, the Kenneth Rainin Foundation, and the Charina Foundation. One coauthor disclosed grant support from Boehringer Ingelheim for this study, and several coauthors are employees of Boehringer Ingelheim. Neither Dr. Berinstein nor Dr. Sakuraba had no financial conflicts to disclose.
This article was updated Dec. 1, 2021.
*Correction, 4/11/22: An earlier version of this article misstated the definition of clinical response.
FROM GASTROENTEROLOGY
People of color missing in inflammatory bowel disease trials
LAS VEGAS – Clinical trials of treatments for inflammatory bowel disease (IBD) have disproportionately enrolled White people, researchers say.
These skewed demographics could result in researchers overlooking differences in how the disease and its treatments might affect other racial and ethnic groups, said Jellyana Peraza, MD, a chief resident at Albert Einstein College of Medicine, New York.
“The only way we can determine that therapies work differently in different populations is by including those populations in these clinical trials,” she said in an interview. “We think that diversity should be present, and that will answer some questions about the pathogenesis of the disease in general.”
Dr. Peraza presented the findings at the annual meeting of the American College of Gastroenterology.
Previous studies have found that, in trials of other conditions, such as cancer and cardiovascular disease, White people have been disproportionately represented. However, little research has been conducted regarding race and ethnicity in IBD trials.
To fill that gap, Dr. Peraza and colleagues analyzed data from completed trials through the U.S. National Library of Medicine’s registry, ClinicalTrials.gov, for the period from 2000 to 2020.
They found 22 trials conducted exclusively in the United States and 56 conducted in other countries that reported the race or ethnicity of participants; 54 trials did not include this information.
With regard to the prevalence of IBD in White people and Asian people, these populations were overrepresented in U.S. clinical trials. All other groups were underrepresented.
The researchers calculated the odds ratio of being included in an IBD clinical trial for each group. Compared with White people, all the other groups were less likely to be included except for Asian people, who were 85% more likely to be included. These ORs were all statistically significant (P < .03) except for Hispanic people (OR, 0.81; 95% confidence interval, 0.65-1.01; P = .06).
It’s not clear why Asian people are overrepresented, Dr. Peraza said. “Honestly, that was kind of surprising for us. We initially thought that could be related to where these studies were conducted, for example, if some of them were conducted on the West Coast, where maybe more Asian communities are located. However, we didn’t find any specific association between location and Asian representation.”
IBD is more prevalent among White people, although its prevalence is increasing among other groups, Dr. Peraza said. However, that is not reflected in the trials. In an analysis of data in 5-year increments, the researchers found that the participation of White and Hispanic people in IBD trials had not changed much, whereas the participation of Black people had declined, and the participation of Asian and Native American people had increased.
On the basis of work of other researchers, Dr. Peraza said that people of color are as willing to participate in trials as White people. “There is not so much a mistrust as a lack of education and a lack of access to the tertiary centers or the centers where these studies are conducted,” she said.
Clinical trial investigators should recruit more participants from community centers, and health care practitioners should talk about the trials with people in underrepresented groups, she said. “They should have the conversation with their patients about how these clinical trials can benefit the evolution of their diseases.”
One research center that is working hard to diversify its IBD trials is the Ohio State University IBD Center, Columbus, said Anita Afzali, MD, its medical director.
“We have a great team that works actively on the recruitment of all patients,” she said in an interview. “Oftentimes, it just takes a little bit of education and spending time with the patient on discussing what the options are for them.”
Some research indicates that Black people with IBD are more likely to have fistulizing disease, Dr. Afzali said. “However, it doesn’t come so much of their differences in phenotype that we’re seeing but more so the differences in access to care and getting the appropriate therapy in a timely way.”
Dr. Peraza and Dr. Afzali disclosed no relevant financial relationships.
AGA applauds researchers who are working to raise our awareness of health disparities in digestive diseases. AGA is committed to addressing this important societal issue head on. Learn more about AGA’s commitment through the AGA Equity Project.
A version of this article first appeared on Medscape.com.
LAS VEGAS – Clinical trials of treatments for inflammatory bowel disease (IBD) have disproportionately enrolled White people, researchers say.
These skewed demographics could result in researchers overlooking differences in how the disease and its treatments might affect other racial and ethnic groups, said Jellyana Peraza, MD, a chief resident at Albert Einstein College of Medicine, New York.
“The only way we can determine that therapies work differently in different populations is by including those populations in these clinical trials,” she said in an interview. “We think that diversity should be present, and that will answer some questions about the pathogenesis of the disease in general.”
Dr. Peraza presented the findings at the annual meeting of the American College of Gastroenterology.
Previous studies have found that, in trials of other conditions, such as cancer and cardiovascular disease, White people have been disproportionately represented. However, little research has been conducted regarding race and ethnicity in IBD trials.
To fill that gap, Dr. Peraza and colleagues analyzed data from completed trials through the U.S. National Library of Medicine’s registry, ClinicalTrials.gov, for the period from 2000 to 2020.
They found 22 trials conducted exclusively in the United States and 56 conducted in other countries that reported the race or ethnicity of participants; 54 trials did not include this information.
With regard to the prevalence of IBD in White people and Asian people, these populations were overrepresented in U.S. clinical trials. All other groups were underrepresented.
The researchers calculated the odds ratio of being included in an IBD clinical trial for each group. Compared with White people, all the other groups were less likely to be included except for Asian people, who were 85% more likely to be included. These ORs were all statistically significant (P < .03) except for Hispanic people (OR, 0.81; 95% confidence interval, 0.65-1.01; P = .06).
It’s not clear why Asian people are overrepresented, Dr. Peraza said. “Honestly, that was kind of surprising for us. We initially thought that could be related to where these studies were conducted, for example, if some of them were conducted on the West Coast, where maybe more Asian communities are located. However, we didn’t find any specific association between location and Asian representation.”
IBD is more prevalent among White people, although its prevalence is increasing among other groups, Dr. Peraza said. However, that is not reflected in the trials. In an analysis of data in 5-year increments, the researchers found that the participation of White and Hispanic people in IBD trials had not changed much, whereas the participation of Black people had declined, and the participation of Asian and Native American people had increased.
On the basis of work of other researchers, Dr. Peraza said that people of color are as willing to participate in trials as White people. “There is not so much a mistrust as a lack of education and a lack of access to the tertiary centers or the centers where these studies are conducted,” she said.
Clinical trial investigators should recruit more participants from community centers, and health care practitioners should talk about the trials with people in underrepresented groups, she said. “They should have the conversation with their patients about how these clinical trials can benefit the evolution of their diseases.”
One research center that is working hard to diversify its IBD trials is the Ohio State University IBD Center, Columbus, said Anita Afzali, MD, its medical director.
“We have a great team that works actively on the recruitment of all patients,” she said in an interview. “Oftentimes, it just takes a little bit of education and spending time with the patient on discussing what the options are for them.”
Some research indicates that Black people with IBD are more likely to have fistulizing disease, Dr. Afzali said. “However, it doesn’t come so much of their differences in phenotype that we’re seeing but more so the differences in access to care and getting the appropriate therapy in a timely way.”
Dr. Peraza and Dr. Afzali disclosed no relevant financial relationships.
AGA applauds researchers who are working to raise our awareness of health disparities in digestive diseases. AGA is committed to addressing this important societal issue head on. Learn more about AGA’s commitment through the AGA Equity Project.
A version of this article first appeared on Medscape.com.
LAS VEGAS – Clinical trials of treatments for inflammatory bowel disease (IBD) have disproportionately enrolled White people, researchers say.
These skewed demographics could result in researchers overlooking differences in how the disease and its treatments might affect other racial and ethnic groups, said Jellyana Peraza, MD, a chief resident at Albert Einstein College of Medicine, New York.
“The only way we can determine that therapies work differently in different populations is by including those populations in these clinical trials,” she said in an interview. “We think that diversity should be present, and that will answer some questions about the pathogenesis of the disease in general.”
Dr. Peraza presented the findings at the annual meeting of the American College of Gastroenterology.
Previous studies have found that, in trials of other conditions, such as cancer and cardiovascular disease, White people have been disproportionately represented. However, little research has been conducted regarding race and ethnicity in IBD trials.
To fill that gap, Dr. Peraza and colleagues analyzed data from completed trials through the U.S. National Library of Medicine’s registry, ClinicalTrials.gov, for the period from 2000 to 2020.
They found 22 trials conducted exclusively in the United States and 56 conducted in other countries that reported the race or ethnicity of participants; 54 trials did not include this information.
With regard to the prevalence of IBD in White people and Asian people, these populations were overrepresented in U.S. clinical trials. All other groups were underrepresented.
The researchers calculated the odds ratio of being included in an IBD clinical trial for each group. Compared with White people, all the other groups were less likely to be included except for Asian people, who were 85% more likely to be included. These ORs were all statistically significant (P < .03) except for Hispanic people (OR, 0.81; 95% confidence interval, 0.65-1.01; P = .06).
It’s not clear why Asian people are overrepresented, Dr. Peraza said. “Honestly, that was kind of surprising for us. We initially thought that could be related to where these studies were conducted, for example, if some of them were conducted on the West Coast, where maybe more Asian communities are located. However, we didn’t find any specific association between location and Asian representation.”
IBD is more prevalent among White people, although its prevalence is increasing among other groups, Dr. Peraza said. However, that is not reflected in the trials. In an analysis of data in 5-year increments, the researchers found that the participation of White and Hispanic people in IBD trials had not changed much, whereas the participation of Black people had declined, and the participation of Asian and Native American people had increased.
On the basis of work of other researchers, Dr. Peraza said that people of color are as willing to participate in trials as White people. “There is not so much a mistrust as a lack of education and a lack of access to the tertiary centers or the centers where these studies are conducted,” she said.
Clinical trial investigators should recruit more participants from community centers, and health care practitioners should talk about the trials with people in underrepresented groups, she said. “They should have the conversation with their patients about how these clinical trials can benefit the evolution of their diseases.”
One research center that is working hard to diversify its IBD trials is the Ohio State University IBD Center, Columbus, said Anita Afzali, MD, its medical director.
“We have a great team that works actively on the recruitment of all patients,” she said in an interview. “Oftentimes, it just takes a little bit of education and spending time with the patient on discussing what the options are for them.”
Some research indicates that Black people with IBD are more likely to have fistulizing disease, Dr. Afzali said. “However, it doesn’t come so much of their differences in phenotype that we’re seeing but more so the differences in access to care and getting the appropriate therapy in a timely way.”
Dr. Peraza and Dr. Afzali disclosed no relevant financial relationships.
AGA applauds researchers who are working to raise our awareness of health disparities in digestive diseases. AGA is committed to addressing this important societal issue head on. Learn more about AGA’s commitment through the AGA Equity Project.
A version of this article first appeared on Medscape.com.
AT ACG 2021
Whole-food exclusion diet effective for adult Crohn’s disease
The Crohn’s disease exclusion diet (CDED), with or without partial enteral nutrition (PEN), effectively induced and maintained remission in adults with mild to moderate, biologic-naive CD, a randomized Israeli pilot study found.
The authors, led by Henit Yanai, MD, MBA, chief of the IBD Center at Rabin Medical Center in Petah Tikva, Israel, also suggested that dietary monotherapy might lead to durable endoscopic remission.
The study, published in The Lancet Gastroenterology & Hepatology, found about 60% of patients on CDED achieved clinical remission by week 6 without adding medications.
Furthermore, 80% of patients in remission at week 6 maintained clinical remission at week 24 on dietary monotherapy alone, allowing more than 50% of the intention-to-treat (ITT) population to achieve sustained remission at 6 months.
Dietary therapy resulted in a significant and progressive reduction in inflammatory markers such as C-reactive protein and fecal calprotectin. The benefit extended to mucosal healing, with 35% of the ITT population achieving endoscopic remission at 24 weeks.
Dr. Yanai explained the clinical context in which her group designed the study. “There were preliminary data regarding the efficacy of the dietary strategy for the induction of remission of mild CD in the pediatric population,” she said in an interview. “Additionally, there was an anecdotal experience in adults who reported benefits. Facing this and the lack of attractive alternatives for mild CD, we decided to examine the effectiveness of this therapeutic strategy in adults.”
Given the costs and side effects of medical treatment, interest in dietary monotherapy for IBD has been growing. As Dr. Yanai said, the CDED, a whole-foods regimen, plus PEN, has been found to help children with CD.
The CDED excludes proinflammatory food components associated with intestinal microbial dysbiosis, altered innate immunity, and impaired gut barrier function.
It involves increased consumption of fruits and vegetables, high-quality lean protein, complex carbohydrates, healthy oils, and fiber, while decreasing intake of inflammation-driving components such as animal and saturated vegetable fats, wheat, and dairy, as well as food additives such as emulsifiers, maltodextrin, and sulfites.
“The realization that exclusive enteral nutrition, which is based on liquid formulas, is effective for inducting remission in children PEN, which is a combination of liquid formulas and food, was less effective, led to the hypothesis that the mechanism might be the exclusion of dietary components that may lead to inflammation,” Dr. Yanai said. This theory, she added, derived from animal models showing that specific dietary components potentially drive inflammation.
The only current guideline-recommended nutritional therapy for remission induction is exclusive enteral nutrition (EEN) in pediatric CD.
The study
The open-label pilot trial led by Dr. Yanai, conducted at three medical centers, enrolled biologic-naive adults ages 18-55 with uncomplicated mild to moderate CD, with a disease duration of no more than 5 years. They had active disease on imaging and elevated C-reactive protein or fecal calprotectin.
During the period January 2017 to May 2020, eligible patients were randomly assigned 1 to 1 to CDED plus PEN enteral (n = 20) or CDED alone (n = 24) for 24 weeks. The primary endpoint was clinical remission, defined as a Harvey–Bradshaw Index score of less than 5 at week 6, an outcome was assessed in the ITT population, which consisted of those who used dietary therapy for at least 48 hours.
At week 6, 13 (68%) of 19 remaining patients in the CDED-plus-PEN group and 12 (57%) of 21 patients in the CDED-alone group had achieved clinical remission (P = .4618).
Among the 25 patients in remission 6 weeks out, 20 (80%) remained in sustained remission at week 24: 12 in the CDED-plus-PEN group and 8 in the CDED-alone group.
Moreover, 14 (35%) of 40 patients were in endoscopic remission at week 24: 8 on CDED plus PEN and 6 on CDED alone.
“CDED with or without partial enteral nutrition was effective for induction and maintenance of remission in adults with mild to moderate biologic-naive Crohn’s disease and might lead to endoscopic remission,” the authors wrote, adding that CDED for mild to moderate active disease should be assessed in a powered randomized controlled trial.
Compliance and adherence are obstacles to dietary therapies. Data for adults using exclusive enteral nutrition are conflicting, with poor compliance postulated to drive an inadequate response in some studies.
“Like every dietary treatment, adherence is challenging,” Dr. Yanai said. “However, when patients feel that it helps them, they have more incentive to follow the diet in the long run, and also once they quit and fare worse, they can go back and follow the first stage of CDED.”
She and her colleagues stressed the need for adequately powered randomized trials and recommended that the personalization of therapeutic diets in the future should take into account the need to deliver energy tailored to the nutritional and therapeutic goals of the patient.
Diets alone are not therapy
In an accompanying editorial, Alexa N. Sasson, MD, an IBD fellow at Massachusetts General Hospital and T.H. Chan Harvard School of Public Health, both in Boston, called diet “a promising and potentially modifiable risk factor with mounting evidence supporting its therapeutic benefit.”
She concurred that the Israeli findings indicate that CDED with or without PEN appears effective for inducing and maintaining remission in this cohort of patients. “Assessment of composite diets such as the CDED is important since they can be incorporated into daily life. The relative efficacy of each of the included and excluded foods, however, is not clear,” she wrote.
She cautioned, however, that dietary therapy does not constitute maintenance therapy and its effects are not sustained after the reintroduction of whole foods. “Identification of sustainable dietary interventions for the prevention and treatment of IBD is increasingly a focus of research,” she wrote.
Dr. Sasson agreed that dietary therapy for CD should be centered on patient interests, goals, and disease states. “Adjunctive dietary measures might be considered in all interested patients as a method of improving gastrointestinal-related symptoms and quality of life, with the potential to achieve a higher and more sustained level of remission,” she wrote.
Both the authors and the commentator agreed on the need for larger randomized trials with long-term follow-up to guide treatment decisions and identify patients who might benefit from dietary intervention.
This study was funded by the Azrieli Foundation and Nestlé Health Science. Dr. Yanai reported financial relationships with Pfizer, AbbVie, Ferring, Janssen, Neopharm, Pfizer, and Takeda. Several coauthors disclosed financial ties to multiple private-sector companies. Dr. Sasson had no competing interests to declare.
This article was updated Dec. 1, 2021.
The Crohn’s disease exclusion diet (CDED), with or without partial enteral nutrition (PEN), effectively induced and maintained remission in adults with mild to moderate, biologic-naive CD, a randomized Israeli pilot study found.
The authors, led by Henit Yanai, MD, MBA, chief of the IBD Center at Rabin Medical Center in Petah Tikva, Israel, also suggested that dietary monotherapy might lead to durable endoscopic remission.
The study, published in The Lancet Gastroenterology & Hepatology, found about 60% of patients on CDED achieved clinical remission by week 6 without adding medications.
Furthermore, 80% of patients in remission at week 6 maintained clinical remission at week 24 on dietary monotherapy alone, allowing more than 50% of the intention-to-treat (ITT) population to achieve sustained remission at 6 months.
Dietary therapy resulted in a significant and progressive reduction in inflammatory markers such as C-reactive protein and fecal calprotectin. The benefit extended to mucosal healing, with 35% of the ITT population achieving endoscopic remission at 24 weeks.
Dr. Yanai explained the clinical context in which her group designed the study. “There were preliminary data regarding the efficacy of the dietary strategy for the induction of remission of mild CD in the pediatric population,” she said in an interview. “Additionally, there was an anecdotal experience in adults who reported benefits. Facing this and the lack of attractive alternatives for mild CD, we decided to examine the effectiveness of this therapeutic strategy in adults.”
Given the costs and side effects of medical treatment, interest in dietary monotherapy for IBD has been growing. As Dr. Yanai said, the CDED, a whole-foods regimen, plus PEN, has been found to help children with CD.
The CDED excludes proinflammatory food components associated with intestinal microbial dysbiosis, altered innate immunity, and impaired gut barrier function.
It involves increased consumption of fruits and vegetables, high-quality lean protein, complex carbohydrates, healthy oils, and fiber, while decreasing intake of inflammation-driving components such as animal and saturated vegetable fats, wheat, and dairy, as well as food additives such as emulsifiers, maltodextrin, and sulfites.
“The realization that exclusive enteral nutrition, which is based on liquid formulas, is effective for inducting remission in children PEN, which is a combination of liquid formulas and food, was less effective, led to the hypothesis that the mechanism might be the exclusion of dietary components that may lead to inflammation,” Dr. Yanai said. This theory, she added, derived from animal models showing that specific dietary components potentially drive inflammation.
The only current guideline-recommended nutritional therapy for remission induction is exclusive enteral nutrition (EEN) in pediatric CD.
The study
The open-label pilot trial led by Dr. Yanai, conducted at three medical centers, enrolled biologic-naive adults ages 18-55 with uncomplicated mild to moderate CD, with a disease duration of no more than 5 years. They had active disease on imaging and elevated C-reactive protein or fecal calprotectin.
During the period January 2017 to May 2020, eligible patients were randomly assigned 1 to 1 to CDED plus PEN enteral (n = 20) or CDED alone (n = 24) for 24 weeks. The primary endpoint was clinical remission, defined as a Harvey–Bradshaw Index score of less than 5 at week 6, an outcome was assessed in the ITT population, which consisted of those who used dietary therapy for at least 48 hours.
At week 6, 13 (68%) of 19 remaining patients in the CDED-plus-PEN group and 12 (57%) of 21 patients in the CDED-alone group had achieved clinical remission (P = .4618).
Among the 25 patients in remission 6 weeks out, 20 (80%) remained in sustained remission at week 24: 12 in the CDED-plus-PEN group and 8 in the CDED-alone group.
Moreover, 14 (35%) of 40 patients were in endoscopic remission at week 24: 8 on CDED plus PEN and 6 on CDED alone.
“CDED with or without partial enteral nutrition was effective for induction and maintenance of remission in adults with mild to moderate biologic-naive Crohn’s disease and might lead to endoscopic remission,” the authors wrote, adding that CDED for mild to moderate active disease should be assessed in a powered randomized controlled trial.
Compliance and adherence are obstacles to dietary therapies. Data for adults using exclusive enteral nutrition are conflicting, with poor compliance postulated to drive an inadequate response in some studies.
“Like every dietary treatment, adherence is challenging,” Dr. Yanai said. “However, when patients feel that it helps them, they have more incentive to follow the diet in the long run, and also once they quit and fare worse, they can go back and follow the first stage of CDED.”
She and her colleagues stressed the need for adequately powered randomized trials and recommended that the personalization of therapeutic diets in the future should take into account the need to deliver energy tailored to the nutritional and therapeutic goals of the patient.
Diets alone are not therapy
In an accompanying editorial, Alexa N. Sasson, MD, an IBD fellow at Massachusetts General Hospital and T.H. Chan Harvard School of Public Health, both in Boston, called diet “a promising and potentially modifiable risk factor with mounting evidence supporting its therapeutic benefit.”
She concurred that the Israeli findings indicate that CDED with or without PEN appears effective for inducing and maintaining remission in this cohort of patients. “Assessment of composite diets such as the CDED is important since they can be incorporated into daily life. The relative efficacy of each of the included and excluded foods, however, is not clear,” she wrote.
She cautioned, however, that dietary therapy does not constitute maintenance therapy and its effects are not sustained after the reintroduction of whole foods. “Identification of sustainable dietary interventions for the prevention and treatment of IBD is increasingly a focus of research,” she wrote.
Dr. Sasson agreed that dietary therapy for CD should be centered on patient interests, goals, and disease states. “Adjunctive dietary measures might be considered in all interested patients as a method of improving gastrointestinal-related symptoms and quality of life, with the potential to achieve a higher and more sustained level of remission,” she wrote.
Both the authors and the commentator agreed on the need for larger randomized trials with long-term follow-up to guide treatment decisions and identify patients who might benefit from dietary intervention.
This study was funded by the Azrieli Foundation and Nestlé Health Science. Dr. Yanai reported financial relationships with Pfizer, AbbVie, Ferring, Janssen, Neopharm, Pfizer, and Takeda. Several coauthors disclosed financial ties to multiple private-sector companies. Dr. Sasson had no competing interests to declare.
This article was updated Dec. 1, 2021.
The Crohn’s disease exclusion diet (CDED), with or without partial enteral nutrition (PEN), effectively induced and maintained remission in adults with mild to moderate, biologic-naive CD, a randomized Israeli pilot study found.
The authors, led by Henit Yanai, MD, MBA, chief of the IBD Center at Rabin Medical Center in Petah Tikva, Israel, also suggested that dietary monotherapy might lead to durable endoscopic remission.
The study, published in The Lancet Gastroenterology & Hepatology, found about 60% of patients on CDED achieved clinical remission by week 6 without adding medications.
Furthermore, 80% of patients in remission at week 6 maintained clinical remission at week 24 on dietary monotherapy alone, allowing more than 50% of the intention-to-treat (ITT) population to achieve sustained remission at 6 months.
Dietary therapy resulted in a significant and progressive reduction in inflammatory markers such as C-reactive protein and fecal calprotectin. The benefit extended to mucosal healing, with 35% of the ITT population achieving endoscopic remission at 24 weeks.
Dr. Yanai explained the clinical context in which her group designed the study. “There were preliminary data regarding the efficacy of the dietary strategy for the induction of remission of mild CD in the pediatric population,” she said in an interview. “Additionally, there was an anecdotal experience in adults who reported benefits. Facing this and the lack of attractive alternatives for mild CD, we decided to examine the effectiveness of this therapeutic strategy in adults.”
Given the costs and side effects of medical treatment, interest in dietary monotherapy for IBD has been growing. As Dr. Yanai said, the CDED, a whole-foods regimen, plus PEN, has been found to help children with CD.
The CDED excludes proinflammatory food components associated with intestinal microbial dysbiosis, altered innate immunity, and impaired gut barrier function.
It involves increased consumption of fruits and vegetables, high-quality lean protein, complex carbohydrates, healthy oils, and fiber, while decreasing intake of inflammation-driving components such as animal and saturated vegetable fats, wheat, and dairy, as well as food additives such as emulsifiers, maltodextrin, and sulfites.
“The realization that exclusive enteral nutrition, which is based on liquid formulas, is effective for inducting remission in children PEN, which is a combination of liquid formulas and food, was less effective, led to the hypothesis that the mechanism might be the exclusion of dietary components that may lead to inflammation,” Dr. Yanai said. This theory, she added, derived from animal models showing that specific dietary components potentially drive inflammation.
The only current guideline-recommended nutritional therapy for remission induction is exclusive enteral nutrition (EEN) in pediatric CD.
The study
The open-label pilot trial led by Dr. Yanai, conducted at three medical centers, enrolled biologic-naive adults ages 18-55 with uncomplicated mild to moderate CD, with a disease duration of no more than 5 years. They had active disease on imaging and elevated C-reactive protein or fecal calprotectin.
During the period January 2017 to May 2020, eligible patients were randomly assigned 1 to 1 to CDED plus PEN enteral (n = 20) or CDED alone (n = 24) for 24 weeks. The primary endpoint was clinical remission, defined as a Harvey–Bradshaw Index score of less than 5 at week 6, an outcome was assessed in the ITT population, which consisted of those who used dietary therapy for at least 48 hours.
At week 6, 13 (68%) of 19 remaining patients in the CDED-plus-PEN group and 12 (57%) of 21 patients in the CDED-alone group had achieved clinical remission (P = .4618).
Among the 25 patients in remission 6 weeks out, 20 (80%) remained in sustained remission at week 24: 12 in the CDED-plus-PEN group and 8 in the CDED-alone group.
Moreover, 14 (35%) of 40 patients were in endoscopic remission at week 24: 8 on CDED plus PEN and 6 on CDED alone.
“CDED with or without partial enteral nutrition was effective for induction and maintenance of remission in adults with mild to moderate biologic-naive Crohn’s disease and might lead to endoscopic remission,” the authors wrote, adding that CDED for mild to moderate active disease should be assessed in a powered randomized controlled trial.
Compliance and adherence are obstacles to dietary therapies. Data for adults using exclusive enteral nutrition are conflicting, with poor compliance postulated to drive an inadequate response in some studies.
“Like every dietary treatment, adherence is challenging,” Dr. Yanai said. “However, when patients feel that it helps them, they have more incentive to follow the diet in the long run, and also once they quit and fare worse, they can go back and follow the first stage of CDED.”
She and her colleagues stressed the need for adequately powered randomized trials and recommended that the personalization of therapeutic diets in the future should take into account the need to deliver energy tailored to the nutritional and therapeutic goals of the patient.
Diets alone are not therapy
In an accompanying editorial, Alexa N. Sasson, MD, an IBD fellow at Massachusetts General Hospital and T.H. Chan Harvard School of Public Health, both in Boston, called diet “a promising and potentially modifiable risk factor with mounting evidence supporting its therapeutic benefit.”
She concurred that the Israeli findings indicate that CDED with or without PEN appears effective for inducing and maintaining remission in this cohort of patients. “Assessment of composite diets such as the CDED is important since they can be incorporated into daily life. The relative efficacy of each of the included and excluded foods, however, is not clear,” she wrote.
She cautioned, however, that dietary therapy does not constitute maintenance therapy and its effects are not sustained after the reintroduction of whole foods. “Identification of sustainable dietary interventions for the prevention and treatment of IBD is increasingly a focus of research,” she wrote.
Dr. Sasson agreed that dietary therapy for CD should be centered on patient interests, goals, and disease states. “Adjunctive dietary measures might be considered in all interested patients as a method of improving gastrointestinal-related symptoms and quality of life, with the potential to achieve a higher and more sustained level of remission,” she wrote.
Both the authors and the commentator agreed on the need for larger randomized trials with long-term follow-up to guide treatment decisions and identify patients who might benefit from dietary intervention.
This study was funded by the Azrieli Foundation and Nestlé Health Science. Dr. Yanai reported financial relationships with Pfizer, AbbVie, Ferring, Janssen, Neopharm, Pfizer, and Takeda. Several coauthors disclosed financial ties to multiple private-sector companies. Dr. Sasson had no competing interests to declare.
This article was updated Dec. 1, 2021.
FROM LANCET GASTROENTEROLOGY & HEPATOLOGY
Upadacitinib delivers rapid response in ulcerative colitis
Induction therapy with Janus kinase inhibitor upadacitinib is superior to placebo for patients with moderately to severely active ulcerative colitis (UC), regardless of prior biologic treatments, based on results of the phase 3 U-ACHIEVE trial.
Clinical responses in the upadacitinib group occurred as soon as 2 weeks and were sustained through the 8-week study period, reported lead author Silvio Danese, MD, PhD, of Humanitas Clinical and Research Center IRCCS and Hunimed, Milan.
“Despite availability of multiple treatment options, many patients with ulcerative colitis do not achieve disease remission with current therapies and unmet therapeutic need remains, especially in patients with moderate to severe disease,” said coauthor Peter Higgins, MD, PhD, of the University of Michigan, Ann Arbor, who presented findings at the annual meeting of the American College of Gastroenterology.
The U-ACHIEVE trial involved 474 patients with moderate to severe UC randomized to receive either upadacitinib induction therapy (45 mg once daily; n = 319) or placebo (n = 155). The primary endpoint was clinical remission at week 8. Secondary endpoints included endoscopic improvement at week 8, endoscopic remission at week 8, clinical response at week 8, clinical response at week 2, histologic-endoscopic mucosal improvement at week 8, and adverse events.
The study population was “very sick” and “very experienced,” Dr. Higgins said, noting that approximately half of the patients had inadequate responses to prior biologics, and within this subgroup of inadequate responders, approximately two-thirds of the patients had received more than one prior biologic. According to Dr. Higgins, this helps explain why 12.3% of the patients in the placebo group discontinued therapy, compared with just 3.8% in the upadacitinib group – because most patients involved were “quite ill.”
At week 8, 26.1% of the patients in the upadacitinib group had achieved clinical remission, versus 4.8% of the patients given placebo (26.1% vs. 4.8%; P < .0001). Clinical response at week 2 followed a similar pattern (60.1% vs. 27.3%; P < .001), as did clinical response at week 8 (72.6% vs. 27.3%; P < .0001).
All other 8-week secondary endpoints also significantly favored upadacitinib, including endoscopic improvement (36.3% vs 7.4%), endoscopic remission (13.7% vs 1.3%), and histologic-endoscopic mucosal improvement (29.9% vs. 6.5%).
Serious and severe adverse events were more common in the placebo group, and patients in the placebo group more frequently discontinued therapy because of treatment-related adverse events. While rates of serious infection were similar between groups, patients taking upadacitinib had higher rates of neutropenia and lymphopenia.
Based on these findings, the investigators concluded that upadacitinib induction therapy is superior to placebo for clinical remission and clinical response regardless of previous treatment failure.
According to Jordan E. Axelrad, MD, of New York University Langone Health, the findings reflect a real-world setting and clinicians should take note of the rapid response observed with upadacitinib.
“This was a relatively sick group, so you know this reflects what we’re seeing in clinical practice,” Dr. Axelrad said in an interview. “Clinical response was detected as early week 2, and that’s extremely important to highlight, because a lot of our drugs that we have on the market – some of these biologics – may take a little time to work. Having a drug that can work fast and is effective is critical.”
Dr. Axelrad suggested that second-line JAK inhibitors like upadacitinib, which target JAK proteins more selectively than first-generation agents, may alleviate some lingering concerns about JAK inhibitor safety; still, optimal treatment sequencing remains unclear.
“With more selective inhibition, you’re getting less of that side-effect profile,” Dr. Axelrad said, noting that long-term data is needed to confirm this likelihood. “The real question moving forward is: Will upadacitinib replace first-generation JAK inhibitors as a category, or, because of the broader safety profile, will it come earlier in the positioning of where we put our drugs for colitis?”
Dr. Axelrad suggested that the answer may ultimately come from regulators, although patients could also guide decision-making.
“Oral drugs are a really important mode of administration that we’re missing for the moderate to severe group,” he said. “Should [further clinical trials] demonstrate superior safety to nonselective JAK inhibitors, upadacitinib could be a first-line option for patients who don’t want to be taking an infusion or injection, more especially so for those that are already biologically experienced, or need something fast.”
Siddharth Singh, MD, director of the IBD Center at the University of California, San Diego, called U-ACHIEVE a “pivotal trial” that demonstrated the “remarkable efficacy” of upadacitinib for moderate to severe ulcerative colitis; still, he noted that drug sequencing remains undetermined.
“It’s unclear whether or not it’ll be the best in class for JAK inhibitors right now,” Dr. Singh said in an interview. “A lot of that hinges on the safety of this drug. In terms of positioning, it depends on whether the [Food and Drug Administration] requires patients to have failed anti–[tumor necrosis factor] therapy before using this drug, like tofacitinib.”
That may depend on long-term data, he suggested.
“Right now, it is hard to comment on the relative safety of upadacitinib versus tofacitinib,” Dr. Singh said. “While the JAK1 selectivity may contribute to efficacy by allowing us to use a higher dose, it’s unclear whether the higher dose of this medication is any safer than tofacitinib. Longer term, 5- to 7-year registry studies of real-world data are warranted to examine risk of cardiovascular disease, thromboembolism, malignancy, and mortality with upadacitinib.
“How to sequence and position these therapies in real-world practice is a key question,” he concluded.
The study was supported by AbbVie. The investigators disclosed additional affiliations with Genentech, Ferring, AstraZeneca, and others. Dr. Axelrad has previously consulted for AbbVie. Dr. Singh has received research funding from AbbVie, Pfizer, and Janssen in the last 24 months, as well as personal fees from Pfizer for an ad hoc grant review.
Induction therapy with Janus kinase inhibitor upadacitinib is superior to placebo for patients with moderately to severely active ulcerative colitis (UC), regardless of prior biologic treatments, based on results of the phase 3 U-ACHIEVE trial.
Clinical responses in the upadacitinib group occurred as soon as 2 weeks and were sustained through the 8-week study period, reported lead author Silvio Danese, MD, PhD, of Humanitas Clinical and Research Center IRCCS and Hunimed, Milan.
“Despite availability of multiple treatment options, many patients with ulcerative colitis do not achieve disease remission with current therapies and unmet therapeutic need remains, especially in patients with moderate to severe disease,” said coauthor Peter Higgins, MD, PhD, of the University of Michigan, Ann Arbor, who presented findings at the annual meeting of the American College of Gastroenterology.
The U-ACHIEVE trial involved 474 patients with moderate to severe UC randomized to receive either upadacitinib induction therapy (45 mg once daily; n = 319) or placebo (n = 155). The primary endpoint was clinical remission at week 8. Secondary endpoints included endoscopic improvement at week 8, endoscopic remission at week 8, clinical response at week 8, clinical response at week 2, histologic-endoscopic mucosal improvement at week 8, and adverse events.
The study population was “very sick” and “very experienced,” Dr. Higgins said, noting that approximately half of the patients had inadequate responses to prior biologics, and within this subgroup of inadequate responders, approximately two-thirds of the patients had received more than one prior biologic. According to Dr. Higgins, this helps explain why 12.3% of the patients in the placebo group discontinued therapy, compared with just 3.8% in the upadacitinib group – because most patients involved were “quite ill.”
At week 8, 26.1% of the patients in the upadacitinib group had achieved clinical remission, versus 4.8% of the patients given placebo (26.1% vs. 4.8%; P < .0001). Clinical response at week 2 followed a similar pattern (60.1% vs. 27.3%; P < .001), as did clinical response at week 8 (72.6% vs. 27.3%; P < .0001).
All other 8-week secondary endpoints also significantly favored upadacitinib, including endoscopic improvement (36.3% vs 7.4%), endoscopic remission (13.7% vs 1.3%), and histologic-endoscopic mucosal improvement (29.9% vs. 6.5%).
Serious and severe adverse events were more common in the placebo group, and patients in the placebo group more frequently discontinued therapy because of treatment-related adverse events. While rates of serious infection were similar between groups, patients taking upadacitinib had higher rates of neutropenia and lymphopenia.
Based on these findings, the investigators concluded that upadacitinib induction therapy is superior to placebo for clinical remission and clinical response regardless of previous treatment failure.
According to Jordan E. Axelrad, MD, of New York University Langone Health, the findings reflect a real-world setting and clinicians should take note of the rapid response observed with upadacitinib.
“This was a relatively sick group, so you know this reflects what we’re seeing in clinical practice,” Dr. Axelrad said in an interview. “Clinical response was detected as early week 2, and that’s extremely important to highlight, because a lot of our drugs that we have on the market – some of these biologics – may take a little time to work. Having a drug that can work fast and is effective is critical.”
Dr. Axelrad suggested that second-line JAK inhibitors like upadacitinib, which target JAK proteins more selectively than first-generation agents, may alleviate some lingering concerns about JAK inhibitor safety; still, optimal treatment sequencing remains unclear.
“With more selective inhibition, you’re getting less of that side-effect profile,” Dr. Axelrad said, noting that long-term data is needed to confirm this likelihood. “The real question moving forward is: Will upadacitinib replace first-generation JAK inhibitors as a category, or, because of the broader safety profile, will it come earlier in the positioning of where we put our drugs for colitis?”
Dr. Axelrad suggested that the answer may ultimately come from regulators, although patients could also guide decision-making.
“Oral drugs are a really important mode of administration that we’re missing for the moderate to severe group,” he said. “Should [further clinical trials] demonstrate superior safety to nonselective JAK inhibitors, upadacitinib could be a first-line option for patients who don’t want to be taking an infusion or injection, more especially so for those that are already biologically experienced, or need something fast.”
Siddharth Singh, MD, director of the IBD Center at the University of California, San Diego, called U-ACHIEVE a “pivotal trial” that demonstrated the “remarkable efficacy” of upadacitinib for moderate to severe ulcerative colitis; still, he noted that drug sequencing remains undetermined.
“It’s unclear whether or not it’ll be the best in class for JAK inhibitors right now,” Dr. Singh said in an interview. “A lot of that hinges on the safety of this drug. In terms of positioning, it depends on whether the [Food and Drug Administration] requires patients to have failed anti–[tumor necrosis factor] therapy before using this drug, like tofacitinib.”
That may depend on long-term data, he suggested.
“Right now, it is hard to comment on the relative safety of upadacitinib versus tofacitinib,” Dr. Singh said. “While the JAK1 selectivity may contribute to efficacy by allowing us to use a higher dose, it’s unclear whether the higher dose of this medication is any safer than tofacitinib. Longer term, 5- to 7-year registry studies of real-world data are warranted to examine risk of cardiovascular disease, thromboembolism, malignancy, and mortality with upadacitinib.
“How to sequence and position these therapies in real-world practice is a key question,” he concluded.
The study was supported by AbbVie. The investigators disclosed additional affiliations with Genentech, Ferring, AstraZeneca, and others. Dr. Axelrad has previously consulted for AbbVie. Dr. Singh has received research funding from AbbVie, Pfizer, and Janssen in the last 24 months, as well as personal fees from Pfizer for an ad hoc grant review.
Induction therapy with Janus kinase inhibitor upadacitinib is superior to placebo for patients with moderately to severely active ulcerative colitis (UC), regardless of prior biologic treatments, based on results of the phase 3 U-ACHIEVE trial.
Clinical responses in the upadacitinib group occurred as soon as 2 weeks and were sustained through the 8-week study period, reported lead author Silvio Danese, MD, PhD, of Humanitas Clinical and Research Center IRCCS and Hunimed, Milan.
“Despite availability of multiple treatment options, many patients with ulcerative colitis do not achieve disease remission with current therapies and unmet therapeutic need remains, especially in patients with moderate to severe disease,” said coauthor Peter Higgins, MD, PhD, of the University of Michigan, Ann Arbor, who presented findings at the annual meeting of the American College of Gastroenterology.
The U-ACHIEVE trial involved 474 patients with moderate to severe UC randomized to receive either upadacitinib induction therapy (45 mg once daily; n = 319) or placebo (n = 155). The primary endpoint was clinical remission at week 8. Secondary endpoints included endoscopic improvement at week 8, endoscopic remission at week 8, clinical response at week 8, clinical response at week 2, histologic-endoscopic mucosal improvement at week 8, and adverse events.
The study population was “very sick” and “very experienced,” Dr. Higgins said, noting that approximately half of the patients had inadequate responses to prior biologics, and within this subgroup of inadequate responders, approximately two-thirds of the patients had received more than one prior biologic. According to Dr. Higgins, this helps explain why 12.3% of the patients in the placebo group discontinued therapy, compared with just 3.8% in the upadacitinib group – because most patients involved were “quite ill.”
At week 8, 26.1% of the patients in the upadacitinib group had achieved clinical remission, versus 4.8% of the patients given placebo (26.1% vs. 4.8%; P < .0001). Clinical response at week 2 followed a similar pattern (60.1% vs. 27.3%; P < .001), as did clinical response at week 8 (72.6% vs. 27.3%; P < .0001).
All other 8-week secondary endpoints also significantly favored upadacitinib, including endoscopic improvement (36.3% vs 7.4%), endoscopic remission (13.7% vs 1.3%), and histologic-endoscopic mucosal improvement (29.9% vs. 6.5%).
Serious and severe adverse events were more common in the placebo group, and patients in the placebo group more frequently discontinued therapy because of treatment-related adverse events. While rates of serious infection were similar between groups, patients taking upadacitinib had higher rates of neutropenia and lymphopenia.
Based on these findings, the investigators concluded that upadacitinib induction therapy is superior to placebo for clinical remission and clinical response regardless of previous treatment failure.
According to Jordan E. Axelrad, MD, of New York University Langone Health, the findings reflect a real-world setting and clinicians should take note of the rapid response observed with upadacitinib.
“This was a relatively sick group, so you know this reflects what we’re seeing in clinical practice,” Dr. Axelrad said in an interview. “Clinical response was detected as early week 2, and that’s extremely important to highlight, because a lot of our drugs that we have on the market – some of these biologics – may take a little time to work. Having a drug that can work fast and is effective is critical.”
Dr. Axelrad suggested that second-line JAK inhibitors like upadacitinib, which target JAK proteins more selectively than first-generation agents, may alleviate some lingering concerns about JAK inhibitor safety; still, optimal treatment sequencing remains unclear.
“With more selective inhibition, you’re getting less of that side-effect profile,” Dr. Axelrad said, noting that long-term data is needed to confirm this likelihood. “The real question moving forward is: Will upadacitinib replace first-generation JAK inhibitors as a category, or, because of the broader safety profile, will it come earlier in the positioning of where we put our drugs for colitis?”
Dr. Axelrad suggested that the answer may ultimately come from regulators, although patients could also guide decision-making.
“Oral drugs are a really important mode of administration that we’re missing for the moderate to severe group,” he said. “Should [further clinical trials] demonstrate superior safety to nonselective JAK inhibitors, upadacitinib could be a first-line option for patients who don’t want to be taking an infusion or injection, more especially so for those that are already biologically experienced, or need something fast.”
Siddharth Singh, MD, director of the IBD Center at the University of California, San Diego, called U-ACHIEVE a “pivotal trial” that demonstrated the “remarkable efficacy” of upadacitinib for moderate to severe ulcerative colitis; still, he noted that drug sequencing remains undetermined.
“It’s unclear whether or not it’ll be the best in class for JAK inhibitors right now,” Dr. Singh said in an interview. “A lot of that hinges on the safety of this drug. In terms of positioning, it depends on whether the [Food and Drug Administration] requires patients to have failed anti–[tumor necrosis factor] therapy before using this drug, like tofacitinib.”
That may depend on long-term data, he suggested.
“Right now, it is hard to comment on the relative safety of upadacitinib versus tofacitinib,” Dr. Singh said. “While the JAK1 selectivity may contribute to efficacy by allowing us to use a higher dose, it’s unclear whether the higher dose of this medication is any safer than tofacitinib. Longer term, 5- to 7-year registry studies of real-world data are warranted to examine risk of cardiovascular disease, thromboembolism, malignancy, and mortality with upadacitinib.
“How to sequence and position these therapies in real-world practice is a key question,” he concluded.
The study was supported by AbbVie. The investigators disclosed additional affiliations with Genentech, Ferring, AstraZeneca, and others. Dr. Axelrad has previously consulted for AbbVie. Dr. Singh has received research funding from AbbVie, Pfizer, and Janssen in the last 24 months, as well as personal fees from Pfizer for an ad hoc grant review.
FROM ACG 2021
Risankizumab has early and lasting benefits in Crohn’s disease
LAS VEGAS – Risankizumab (Skyrizi, AbbVie) provides early and lasting benefits for patients with Crohn’s disease, phase 3 trials indicate.
Based on these and other recent findings, the drug could be used as a first-line treatment and even displace ustekinumab (Stelara, Janssen), which itself was approved by the Food and Drug Administration for Crohn’s disease in 2016, according to David Rubin, MD, the Joseph B. Kirsner Professor of Medicine at the University of Chicago.
“The drug works fast,” Dr. Rubin said in an interview. “If you start this therapy in patients with moderate to severe Crohn’s disease, they’re likely to feel better within the first few weeks.”
Dr. Rubin presented the findings on the drug’s early onset at the annual meeting of the American College of Gastroenterology. A related trial presented at the meeting showed the drug continuing to perform well up to 52 weeks.
Advances in immunomodulation have allowed drug companies to feed multiple new therapies into the pipeline for Crohn’s disease and related conditions in recent years, giving hope to the many patients who have not been able to benefit from older classes of drugs, such as biologics.
A humanized immunoglobulin G1 (IgG1) monoclonal antibody, risankizumab blocks interleukin (IL) 23 by binding to its p19 subunit. IL-23 is a cytokine implicated in several chronic immune disorders, including Crohn’s disease and psoriasis. Researchers hope that risankizumab will prove more selective, with a better safety profile, than previous drugs in its class. The FDA approved risankizumab in April 2019 for the treatment of moderate to severe plaque psoriasis.
MOTIVATE and ADVANCE studies
The two induction trials for Crohn’s disease enrolled slightly different populations.
The MOTIVATE study enrolled patients who had responded inadequately or were intolerant to biologic therapy. In this trial, the investigators assigned 205 patients to 1,200 mg of risankizumab, 206 patients to 600 mg of risankizumab, and 207 patients to placebo.
The ADVANCE study enrolled patients who had responded inadequately or could not tolerate either biologic or conventional therapy. In this trial, investigators randomly assigned 372 patients to 1,200 mg of risankizumab, 373 patients to 600 mg of risankizumab, and 186 patients to placebo.
In both trials, intravenous injections were given at weeks 0, 4, and 8.
The researchers defined a Crohn’s Disease Activity Index (CDAI) clinical remission as a score less than 150. They defined a Stool Frequency and Abdominal Pain Score (SF/APS) clinical remission as a soft stool frequency of no more than 2.8, and an abdominal pain score of no more than 1 and not worse than baseline.
A CDAI clinical response was at least a 100-point decrease from baseline. The SF/APS enhanced clinical response was at least a 60% decrease in average daily stool frequency or at least a 35% decrease in average daily abdominal pain, with both not worse than baseline.
At 4 weeks, the researchers found that the percentage of patients who achieved CDAI clinical remission in both risankizumab groups of both studies was greater than in the placebo group. The difference was statistically significant (P ≤ .01 in ADVANCE and P ≤ .05 in MOTIVATE), and it continued to grow at 8 weeks and 12 weeks.
By 12 weeks in the ADVANCE trial, according to a press release from AbbVie, 45% of patients on the 600-mg dose of risankizumab and 42% on the 1,200-mg dose of risankizumab had achieved CDAI clinical remission, compared with 25% of those on placebo, which was statistically significant (P < .001). For the MOTIVATE trial, the results were significantly better for patients in the risankizumab groups than for those in the placebo group.
In both trials, the treated groups continued to improve faster than the placebo groups through 12 weeks. Improvements in SF/APS enhanced clinical response largely paralleled those for CDAI clinical remission.
“It did show very good results,” session moderator Jonathan Leighton, MD, professor of medicine and chair of the division of gastroenterology at Mayo Clinic in Phoenix, Ariz., said in an interview with Medscape Medical News. “But basically, it’s so early that we don’t have all the data.” In particular, he would have liked to see whether patients responded to the drug before week 4.
FORTIFY study
In FORTIFY, the maintenance trial that followed, the researchers rerandomized those patients who had responded to risankizumab into three groups. Two groups received subcutaneous injections of risankizumab, with 179 patients getting 360 mg and another 179 patients getting 180 mg. The placebo group included the remaining 184 patients.
At week 52, 40.9% of patients in the placebo group were in clinical remission, compared with 52.2% in the 360-mg group and 55.4% in the 180-mg group, which was statistically significant (P = .005 for 360 mg, and P = .003 for 180 mg.)
“It showed us that [risankizumab] could achieve deep remission, which means patients achieving remission endoscopically in combination with clinical remission,” the presenter, Marla Dubinsky, MD, professor of pediatrics and medicine in the division of pediatric gastroenterology at Icahn School of Medicine at Mount Sinai in New York, said in an interview.
Over the 52 weeks, deep remission and endoscopic remission rates increased in the 360-mg group, held steady in the 180-mg group, and decreased in the placebo group. Mean fecal calprotectin and C-reactive protein levels decreased in the risankizumab groups and increased in the placebo group.
There were more total treatment-emergent adverse events per 100 patient-years in the placebo group (339.7) than in the 360-mg group (269.3) or the 180-mg group (283.5). The same difference between groups was true of severe treatment-emergent adverse events. Serious events and events leading to discontinuation were similar in the three groups.
Dr. Leighton reports financial relationships to Olympus and Pfizer. Dr. Rubin reports financial relationships to AbbVie, AltruBio, Allergan, Arena Pharmaceuticals, Athos Therapeutics, Bellatrix, Boehringer Ingelheim, Bristol Myers Squibb, Celgene/Syneos, Connect Biopharma, GalenPharma/Atlantica, Genentech/Roche, Gilead, InDex Pharmaceuticals, Ironwood, Iterative Scopes, Janssen, Lilly, Materia Prima Farmaceutica, Pfizer, Prometheus Biosciences, Reistone, Takeda, and TECHLAB. Dr. Dubinsky reports financial relationships to all or most of the companies making drugs for inflammatory bowel disease. The studies were funded by AbbVie.
A version of this article first appeared on Medscape.com.
LAS VEGAS – Risankizumab (Skyrizi, AbbVie) provides early and lasting benefits for patients with Crohn’s disease, phase 3 trials indicate.
Based on these and other recent findings, the drug could be used as a first-line treatment and even displace ustekinumab (Stelara, Janssen), which itself was approved by the Food and Drug Administration for Crohn’s disease in 2016, according to David Rubin, MD, the Joseph B. Kirsner Professor of Medicine at the University of Chicago.
“The drug works fast,” Dr. Rubin said in an interview. “If you start this therapy in patients with moderate to severe Crohn’s disease, they’re likely to feel better within the first few weeks.”
Dr. Rubin presented the findings on the drug’s early onset at the annual meeting of the American College of Gastroenterology. A related trial presented at the meeting showed the drug continuing to perform well up to 52 weeks.
Advances in immunomodulation have allowed drug companies to feed multiple new therapies into the pipeline for Crohn’s disease and related conditions in recent years, giving hope to the many patients who have not been able to benefit from older classes of drugs, such as biologics.
A humanized immunoglobulin G1 (IgG1) monoclonal antibody, risankizumab blocks interleukin (IL) 23 by binding to its p19 subunit. IL-23 is a cytokine implicated in several chronic immune disorders, including Crohn’s disease and psoriasis. Researchers hope that risankizumab will prove more selective, with a better safety profile, than previous drugs in its class. The FDA approved risankizumab in April 2019 for the treatment of moderate to severe plaque psoriasis.
MOTIVATE and ADVANCE studies
The two induction trials for Crohn’s disease enrolled slightly different populations.
The MOTIVATE study enrolled patients who had responded inadequately or were intolerant to biologic therapy. In this trial, the investigators assigned 205 patients to 1,200 mg of risankizumab, 206 patients to 600 mg of risankizumab, and 207 patients to placebo.
The ADVANCE study enrolled patients who had responded inadequately or could not tolerate either biologic or conventional therapy. In this trial, investigators randomly assigned 372 patients to 1,200 mg of risankizumab, 373 patients to 600 mg of risankizumab, and 186 patients to placebo.
In both trials, intravenous injections were given at weeks 0, 4, and 8.
The researchers defined a Crohn’s Disease Activity Index (CDAI) clinical remission as a score less than 150. They defined a Stool Frequency and Abdominal Pain Score (SF/APS) clinical remission as a soft stool frequency of no more than 2.8, and an abdominal pain score of no more than 1 and not worse than baseline.
A CDAI clinical response was at least a 100-point decrease from baseline. The SF/APS enhanced clinical response was at least a 60% decrease in average daily stool frequency or at least a 35% decrease in average daily abdominal pain, with both not worse than baseline.
At 4 weeks, the researchers found that the percentage of patients who achieved CDAI clinical remission in both risankizumab groups of both studies was greater than in the placebo group. The difference was statistically significant (P ≤ .01 in ADVANCE and P ≤ .05 in MOTIVATE), and it continued to grow at 8 weeks and 12 weeks.
By 12 weeks in the ADVANCE trial, according to a press release from AbbVie, 45% of patients on the 600-mg dose of risankizumab and 42% on the 1,200-mg dose of risankizumab had achieved CDAI clinical remission, compared with 25% of those on placebo, which was statistically significant (P < .001). For the MOTIVATE trial, the results were significantly better for patients in the risankizumab groups than for those in the placebo group.
In both trials, the treated groups continued to improve faster than the placebo groups through 12 weeks. Improvements in SF/APS enhanced clinical response largely paralleled those for CDAI clinical remission.
“It did show very good results,” session moderator Jonathan Leighton, MD, professor of medicine and chair of the division of gastroenterology at Mayo Clinic in Phoenix, Ariz., said in an interview with Medscape Medical News. “But basically, it’s so early that we don’t have all the data.” In particular, he would have liked to see whether patients responded to the drug before week 4.
FORTIFY study
In FORTIFY, the maintenance trial that followed, the researchers rerandomized those patients who had responded to risankizumab into three groups. Two groups received subcutaneous injections of risankizumab, with 179 patients getting 360 mg and another 179 patients getting 180 mg. The placebo group included the remaining 184 patients.
At week 52, 40.9% of patients in the placebo group were in clinical remission, compared with 52.2% in the 360-mg group and 55.4% in the 180-mg group, which was statistically significant (P = .005 for 360 mg, and P = .003 for 180 mg.)
“It showed us that [risankizumab] could achieve deep remission, which means patients achieving remission endoscopically in combination with clinical remission,” the presenter, Marla Dubinsky, MD, professor of pediatrics and medicine in the division of pediatric gastroenterology at Icahn School of Medicine at Mount Sinai in New York, said in an interview.
Over the 52 weeks, deep remission and endoscopic remission rates increased in the 360-mg group, held steady in the 180-mg group, and decreased in the placebo group. Mean fecal calprotectin and C-reactive protein levels decreased in the risankizumab groups and increased in the placebo group.
There were more total treatment-emergent adverse events per 100 patient-years in the placebo group (339.7) than in the 360-mg group (269.3) or the 180-mg group (283.5). The same difference between groups was true of severe treatment-emergent adverse events. Serious events and events leading to discontinuation were similar in the three groups.
Dr. Leighton reports financial relationships to Olympus and Pfizer. Dr. Rubin reports financial relationships to AbbVie, AltruBio, Allergan, Arena Pharmaceuticals, Athos Therapeutics, Bellatrix, Boehringer Ingelheim, Bristol Myers Squibb, Celgene/Syneos, Connect Biopharma, GalenPharma/Atlantica, Genentech/Roche, Gilead, InDex Pharmaceuticals, Ironwood, Iterative Scopes, Janssen, Lilly, Materia Prima Farmaceutica, Pfizer, Prometheus Biosciences, Reistone, Takeda, and TECHLAB. Dr. Dubinsky reports financial relationships to all or most of the companies making drugs for inflammatory bowel disease. The studies were funded by AbbVie.
A version of this article first appeared on Medscape.com.
LAS VEGAS – Risankizumab (Skyrizi, AbbVie) provides early and lasting benefits for patients with Crohn’s disease, phase 3 trials indicate.
Based on these and other recent findings, the drug could be used as a first-line treatment and even displace ustekinumab (Stelara, Janssen), which itself was approved by the Food and Drug Administration for Crohn’s disease in 2016, according to David Rubin, MD, the Joseph B. Kirsner Professor of Medicine at the University of Chicago.
“The drug works fast,” Dr. Rubin said in an interview. “If you start this therapy in patients with moderate to severe Crohn’s disease, they’re likely to feel better within the first few weeks.”
Dr. Rubin presented the findings on the drug’s early onset at the annual meeting of the American College of Gastroenterology. A related trial presented at the meeting showed the drug continuing to perform well up to 52 weeks.
Advances in immunomodulation have allowed drug companies to feed multiple new therapies into the pipeline for Crohn’s disease and related conditions in recent years, giving hope to the many patients who have not been able to benefit from older classes of drugs, such as biologics.
A humanized immunoglobulin G1 (IgG1) monoclonal antibody, risankizumab blocks interleukin (IL) 23 by binding to its p19 subunit. IL-23 is a cytokine implicated in several chronic immune disorders, including Crohn’s disease and psoriasis. Researchers hope that risankizumab will prove more selective, with a better safety profile, than previous drugs in its class. The FDA approved risankizumab in April 2019 for the treatment of moderate to severe plaque psoriasis.
MOTIVATE and ADVANCE studies
The two induction trials for Crohn’s disease enrolled slightly different populations.
The MOTIVATE study enrolled patients who had responded inadequately or were intolerant to biologic therapy. In this trial, the investigators assigned 205 patients to 1,200 mg of risankizumab, 206 patients to 600 mg of risankizumab, and 207 patients to placebo.
The ADVANCE study enrolled patients who had responded inadequately or could not tolerate either biologic or conventional therapy. In this trial, investigators randomly assigned 372 patients to 1,200 mg of risankizumab, 373 patients to 600 mg of risankizumab, and 186 patients to placebo.
In both trials, intravenous injections were given at weeks 0, 4, and 8.
The researchers defined a Crohn’s Disease Activity Index (CDAI) clinical remission as a score less than 150. They defined a Stool Frequency and Abdominal Pain Score (SF/APS) clinical remission as a soft stool frequency of no more than 2.8, and an abdominal pain score of no more than 1 and not worse than baseline.
A CDAI clinical response was at least a 100-point decrease from baseline. The SF/APS enhanced clinical response was at least a 60% decrease in average daily stool frequency or at least a 35% decrease in average daily abdominal pain, with both not worse than baseline.
At 4 weeks, the researchers found that the percentage of patients who achieved CDAI clinical remission in both risankizumab groups of both studies was greater than in the placebo group. The difference was statistically significant (P ≤ .01 in ADVANCE and P ≤ .05 in MOTIVATE), and it continued to grow at 8 weeks and 12 weeks.
By 12 weeks in the ADVANCE trial, according to a press release from AbbVie, 45% of patients on the 600-mg dose of risankizumab and 42% on the 1,200-mg dose of risankizumab had achieved CDAI clinical remission, compared with 25% of those on placebo, which was statistically significant (P < .001). For the MOTIVATE trial, the results were significantly better for patients in the risankizumab groups than for those in the placebo group.
In both trials, the treated groups continued to improve faster than the placebo groups through 12 weeks. Improvements in SF/APS enhanced clinical response largely paralleled those for CDAI clinical remission.
“It did show very good results,” session moderator Jonathan Leighton, MD, professor of medicine and chair of the division of gastroenterology at Mayo Clinic in Phoenix, Ariz., said in an interview with Medscape Medical News. “But basically, it’s so early that we don’t have all the data.” In particular, he would have liked to see whether patients responded to the drug before week 4.
FORTIFY study
In FORTIFY, the maintenance trial that followed, the researchers rerandomized those patients who had responded to risankizumab into three groups. Two groups received subcutaneous injections of risankizumab, with 179 patients getting 360 mg and another 179 patients getting 180 mg. The placebo group included the remaining 184 patients.
At week 52, 40.9% of patients in the placebo group were in clinical remission, compared with 52.2% in the 360-mg group and 55.4% in the 180-mg group, which was statistically significant (P = .005 for 360 mg, and P = .003 for 180 mg.)
“It showed us that [risankizumab] could achieve deep remission, which means patients achieving remission endoscopically in combination with clinical remission,” the presenter, Marla Dubinsky, MD, professor of pediatrics and medicine in the division of pediatric gastroenterology at Icahn School of Medicine at Mount Sinai in New York, said in an interview.
Over the 52 weeks, deep remission and endoscopic remission rates increased in the 360-mg group, held steady in the 180-mg group, and decreased in the placebo group. Mean fecal calprotectin and C-reactive protein levels decreased in the risankizumab groups and increased in the placebo group.
There were more total treatment-emergent adverse events per 100 patient-years in the placebo group (339.7) than in the 360-mg group (269.3) or the 180-mg group (283.5). The same difference between groups was true of severe treatment-emergent adverse events. Serious events and events leading to discontinuation were similar in the three groups.
Dr. Leighton reports financial relationships to Olympus and Pfizer. Dr. Rubin reports financial relationships to AbbVie, AltruBio, Allergan, Arena Pharmaceuticals, Athos Therapeutics, Bellatrix, Boehringer Ingelheim, Bristol Myers Squibb, Celgene/Syneos, Connect Biopharma, GalenPharma/Atlantica, Genentech/Roche, Gilead, InDex Pharmaceuticals, Ironwood, Iterative Scopes, Janssen, Lilly, Materia Prima Farmaceutica, Pfizer, Prometheus Biosciences, Reistone, Takeda, and TECHLAB. Dr. Dubinsky reports financial relationships to all or most of the companies making drugs for inflammatory bowel disease. The studies were funded by AbbVie.
A version of this article first appeared on Medscape.com.
AT ACG 2021
Newly discovered vascular barrier in the brain may explain IBD-related anxiety, depression
A newly discovered vascular brain barrier that blocks the passage of inflammatory molecules triggered by gut bacteria may be why patients with inflammatory bowel disease (IBD) are at increased risk for certain mental health disorders, including anxiety and depression, early research suggests.
The discovery, which was based on a preclinical model, could lead to new therapeutic targets that could have applications for both gastrointestinal and psychiatric conditions, investigators note.
The research team, which was led by immunologist Maria Rescigno, PhD, and neuroscientist Simona Lodato, PhD, both from Humanitas University, Milan, notes that the barrier resides in the choroid plexus, a region of the brain that is involved in filtering cerebrospinal fluid. The researchers found that the region closes in response to inflammatory molecules produced in reaction to the presence of intestinal bacteria in patients with gut disorders.
Dr. Lodato said in an interview that the brain’s choroid plexus vascular barrier, along with another barrier between the gut and liver, known as the gut vascular barrier, appear to control the movement of molecules along the gut-brain axis.
“We show that in addition to the epithelial barrier in the choroid plexus, there is a functional vascular barrier that only becomes evident in blocking entry of various inflammatory molecules under conditions of systemic inflammation,” Dr. Lodato said.
“This interruption of the gut-brain interaction has developed to protect the brain from inflammation. Why this happens is not yet known, but it is likely to prevent epileptic seizures and imbalanced neuronal activity,” added Dr. Rescigno.
The study was published online October 22 in Science.
The gut a root cause of mental illness?
Nearly 40% of patients with IBD also experience depression and anxiety. It was once thought that these conditions arose because of patients’ difficulties in coping with their disease, said Dr. Rescigno.
“People with these disorders conventionally thought to be caused by an imbalance in the brain may actually find the root cause is located in the intestine. This is the first time these symptoms have been associated with the choroid plexus vascular brain barrier and its closure,” she noted.
Dr. Rescigno added that subtle, rather than overt, inflammation may be all that’s required for closure of the choroid plexus and the subsequent effects on mental health.
In 2015, Dr. Rescigno’s group first described the gut vascular barrier that protects the systemic circulation from gut bacteria or associated bacteria-derived molecules. During intestinal inflammation, such as occurs in IBD, this barrier is compromised and becomes more permeable. This allows microbes to pass across the epithelium of the gut barrier and enter the systemic circulation, including the liver and spleen, explained Dr. Rescigno.
Dr. Rescigno and Dr. Lodato then explored whether this systemic inflammatory condition was connected to the brain along a gut-brain axis and found that it was.
The researchers tested the hypothesis that central nervous system symptoms may be due to vascular changes at the interface between the gut or the brain and elsewhere in the body.
“We set out to test whether opening of the gut vascular barrier would allow gut bacteria to trigger the release of inflammatory molecules that spread to more distant areas, possibly leading to a deficiency of certain nutrients and precipitating mental disorders,” they said.
An experimental preclinical model of the choroid plexus vascular barrier closure led to anxiety-like behavior, as well as short-term memory loss. That this behavior occurred independently of inflammation suggested that it was likely a response to closure itself, they note.
In the noninflammatory state, the epithelium of the choroid plexus filters molecules. Those that are ≤70 kDa are allowed to pass through to the brain. However, the investigators found that during systemic inflammation, this filtration stops, and the blood capillaries of the choroid plexus prevent entry of inflammatory molecules such as cytokines.
Dr. Lodato speculated that when the vascular barrier of the choroid plexus shuts off during the systemic inflammatory state, it responds by bathing the brain in cerebrospinal fluid.
“When the choroid plexus closes, like a door slamming shut, then communication between the brain and the rest of the body is halted. This means that the brain is deprived of certain nutrients and other beneficial molecules that usually enter via the cerebrospinal fluid or enriched of potentially dangerous ones, as drainage could also be affected,” she said.
If confirmed in further studies, these results may open the way to new interventions.
‘A significant leap forward’
Commenting on the findings, David T. Rubin, MD, professor of medicine at the University of Chicago, noted that the study’s results represent “a significant leap forward” and that it highlights “another important cost to uncontrolled gut inflammation that is the potential for worsened mental health disorders.”
Dr. Rubin, whose research involves measuring metabolites of the dietary amino acid tryptophan, including melatonin and serotonin, in patients with IBD, added that the findings offer a possible explanation for the association of both Crohn’s disease and ulcerative colitis with anxiety and depressive disorders.
“There was a belief that the association was in the opposite direction, that the mental health disorder was causing or worsening the gut inflammation, but this has been disavowed,” Dr. Rubin said.
“Most recently, the recognition that the major sources of serotonin and other metabolites of tryptophan that come from the gut microbiome has led to the hypothesis that the inflamed bowel and dysbiotic gut biome may in fact be driving the mental health disorders due to the effect of neurotransmitter imbalance,” he added. Dr. Rubin also suggested that the shutdown of the choroid plexus vascular barrier may contribute to this imbalance but that this needs additional study.
“This further supports my ongoing contention that the gut really is the center of the universe,” said Dr. Rubin.
Also commenting on the findings, Miguel Rigueiro, MD, professor in the department of medicine in the Cleveland Clinic Lerner College of Medicine of Case Western Reserve University, Cleveland, said, “There’s an implication that anxiety and depression and other behavioral health disorders may be explained by this mechanism. If that is the case, there may be a way to target medications against the choroid plexus and potentially treat depression or anxiety.”
This prospect was echoed by Dr. Rubin, who said, “The clinical implication is that treatment of gut inflammation may restore a balance to the neurotransmitters and resolve anxiety or depressive disorders.”
To identify new therapeutic targets, investigators will study the regions and circuits of the brain that are more susceptible to this closure of the choroid plexus, said Dr. Lodato.
“If these regions are associated with depression or other psychosocial disorders, then this new understanding around the choroid plexus vascular barrier might eventually have implications for helping treat such disorders,” she noted.
Reflecting a general shift from a brain-centric view of some psychosocial disorders to an intestinal-centric one, Dr. Lodato added, “The brain cannot be considered in isolation. It is part of a much larger body, and we need to think this way.”
Dr. Rescigno, Dr. Lodato, and Dr. Rubin report no relevant financial relationships. Dr. Rigueiro has served on advisory boards and as consultant for AbbVie, Janssen, UCB, Takeda, Pfizer, Miraca Labs, Amgen, Celgene, Seres, Allergan, Genentech, Gilead, Salix, Prometheus, Lilly, TARGET Pharma Solutions, ALFASIGMA, SpA, and Bristol-Meyer Squibb.
A version of this article first appeared on Medscape.com.
A newly discovered vascular brain barrier that blocks the passage of inflammatory molecules triggered by gut bacteria may be why patients with inflammatory bowel disease (IBD) are at increased risk for certain mental health disorders, including anxiety and depression, early research suggests.
The discovery, which was based on a preclinical model, could lead to new therapeutic targets that could have applications for both gastrointestinal and psychiatric conditions, investigators note.
The research team, which was led by immunologist Maria Rescigno, PhD, and neuroscientist Simona Lodato, PhD, both from Humanitas University, Milan, notes that the barrier resides in the choroid plexus, a region of the brain that is involved in filtering cerebrospinal fluid. The researchers found that the region closes in response to inflammatory molecules produced in reaction to the presence of intestinal bacteria in patients with gut disorders.
Dr. Lodato said in an interview that the brain’s choroid plexus vascular barrier, along with another barrier between the gut and liver, known as the gut vascular barrier, appear to control the movement of molecules along the gut-brain axis.
“We show that in addition to the epithelial barrier in the choroid plexus, there is a functional vascular barrier that only becomes evident in blocking entry of various inflammatory molecules under conditions of systemic inflammation,” Dr. Lodato said.
“This interruption of the gut-brain interaction has developed to protect the brain from inflammation. Why this happens is not yet known, but it is likely to prevent epileptic seizures and imbalanced neuronal activity,” added Dr. Rescigno.
The study was published online October 22 in Science.
The gut a root cause of mental illness?
Nearly 40% of patients with IBD also experience depression and anxiety. It was once thought that these conditions arose because of patients’ difficulties in coping with their disease, said Dr. Rescigno.
“People with these disorders conventionally thought to be caused by an imbalance in the brain may actually find the root cause is located in the intestine. This is the first time these symptoms have been associated with the choroid plexus vascular brain barrier and its closure,” she noted.
Dr. Rescigno added that subtle, rather than overt, inflammation may be all that’s required for closure of the choroid plexus and the subsequent effects on mental health.
In 2015, Dr. Rescigno’s group first described the gut vascular barrier that protects the systemic circulation from gut bacteria or associated bacteria-derived molecules. During intestinal inflammation, such as occurs in IBD, this barrier is compromised and becomes more permeable. This allows microbes to pass across the epithelium of the gut barrier and enter the systemic circulation, including the liver and spleen, explained Dr. Rescigno.
Dr. Rescigno and Dr. Lodato then explored whether this systemic inflammatory condition was connected to the brain along a gut-brain axis and found that it was.
The researchers tested the hypothesis that central nervous system symptoms may be due to vascular changes at the interface between the gut or the brain and elsewhere in the body.
“We set out to test whether opening of the gut vascular barrier would allow gut bacteria to trigger the release of inflammatory molecules that spread to more distant areas, possibly leading to a deficiency of certain nutrients and precipitating mental disorders,” they said.
An experimental preclinical model of the choroid plexus vascular barrier closure led to anxiety-like behavior, as well as short-term memory loss. That this behavior occurred independently of inflammation suggested that it was likely a response to closure itself, they note.
In the noninflammatory state, the epithelium of the choroid plexus filters molecules. Those that are ≤70 kDa are allowed to pass through to the brain. However, the investigators found that during systemic inflammation, this filtration stops, and the blood capillaries of the choroid plexus prevent entry of inflammatory molecules such as cytokines.
Dr. Lodato speculated that when the vascular barrier of the choroid plexus shuts off during the systemic inflammatory state, it responds by bathing the brain in cerebrospinal fluid.
“When the choroid plexus closes, like a door slamming shut, then communication between the brain and the rest of the body is halted. This means that the brain is deprived of certain nutrients and other beneficial molecules that usually enter via the cerebrospinal fluid or enriched of potentially dangerous ones, as drainage could also be affected,” she said.
If confirmed in further studies, these results may open the way to new interventions.
‘A significant leap forward’
Commenting on the findings, David T. Rubin, MD, professor of medicine at the University of Chicago, noted that the study’s results represent “a significant leap forward” and that it highlights “another important cost to uncontrolled gut inflammation that is the potential for worsened mental health disorders.”
Dr. Rubin, whose research involves measuring metabolites of the dietary amino acid tryptophan, including melatonin and serotonin, in patients with IBD, added that the findings offer a possible explanation for the association of both Crohn’s disease and ulcerative colitis with anxiety and depressive disorders.
“There was a belief that the association was in the opposite direction, that the mental health disorder was causing or worsening the gut inflammation, but this has been disavowed,” Dr. Rubin said.
“Most recently, the recognition that the major sources of serotonin and other metabolites of tryptophan that come from the gut microbiome has led to the hypothesis that the inflamed bowel and dysbiotic gut biome may in fact be driving the mental health disorders due to the effect of neurotransmitter imbalance,” he added. Dr. Rubin also suggested that the shutdown of the choroid plexus vascular barrier may contribute to this imbalance but that this needs additional study.
“This further supports my ongoing contention that the gut really is the center of the universe,” said Dr. Rubin.
Also commenting on the findings, Miguel Rigueiro, MD, professor in the department of medicine in the Cleveland Clinic Lerner College of Medicine of Case Western Reserve University, Cleveland, said, “There’s an implication that anxiety and depression and other behavioral health disorders may be explained by this mechanism. If that is the case, there may be a way to target medications against the choroid plexus and potentially treat depression or anxiety.”
This prospect was echoed by Dr. Rubin, who said, “The clinical implication is that treatment of gut inflammation may restore a balance to the neurotransmitters and resolve anxiety or depressive disorders.”
To identify new therapeutic targets, investigators will study the regions and circuits of the brain that are more susceptible to this closure of the choroid plexus, said Dr. Lodato.
“If these regions are associated with depression or other psychosocial disorders, then this new understanding around the choroid plexus vascular barrier might eventually have implications for helping treat such disorders,” she noted.
Reflecting a general shift from a brain-centric view of some psychosocial disorders to an intestinal-centric one, Dr. Lodato added, “The brain cannot be considered in isolation. It is part of a much larger body, and we need to think this way.”
Dr. Rescigno, Dr. Lodato, and Dr. Rubin report no relevant financial relationships. Dr. Rigueiro has served on advisory boards and as consultant for AbbVie, Janssen, UCB, Takeda, Pfizer, Miraca Labs, Amgen, Celgene, Seres, Allergan, Genentech, Gilead, Salix, Prometheus, Lilly, TARGET Pharma Solutions, ALFASIGMA, SpA, and Bristol-Meyer Squibb.
A version of this article first appeared on Medscape.com.
A newly discovered vascular brain barrier that blocks the passage of inflammatory molecules triggered by gut bacteria may be why patients with inflammatory bowel disease (IBD) are at increased risk for certain mental health disorders, including anxiety and depression, early research suggests.
The discovery, which was based on a preclinical model, could lead to new therapeutic targets that could have applications for both gastrointestinal and psychiatric conditions, investigators note.
The research team, which was led by immunologist Maria Rescigno, PhD, and neuroscientist Simona Lodato, PhD, both from Humanitas University, Milan, notes that the barrier resides in the choroid plexus, a region of the brain that is involved in filtering cerebrospinal fluid. The researchers found that the region closes in response to inflammatory molecules produced in reaction to the presence of intestinal bacteria in patients with gut disorders.
Dr. Lodato said in an interview that the brain’s choroid plexus vascular barrier, along with another barrier between the gut and liver, known as the gut vascular barrier, appear to control the movement of molecules along the gut-brain axis.
“We show that in addition to the epithelial barrier in the choroid plexus, there is a functional vascular barrier that only becomes evident in blocking entry of various inflammatory molecules under conditions of systemic inflammation,” Dr. Lodato said.
“This interruption of the gut-brain interaction has developed to protect the brain from inflammation. Why this happens is not yet known, but it is likely to prevent epileptic seizures and imbalanced neuronal activity,” added Dr. Rescigno.
The study was published online October 22 in Science.
The gut a root cause of mental illness?
Nearly 40% of patients with IBD also experience depression and anxiety. It was once thought that these conditions arose because of patients’ difficulties in coping with their disease, said Dr. Rescigno.
“People with these disorders conventionally thought to be caused by an imbalance in the brain may actually find the root cause is located in the intestine. This is the first time these symptoms have been associated with the choroid plexus vascular brain barrier and its closure,” she noted.
Dr. Rescigno added that subtle, rather than overt, inflammation may be all that’s required for closure of the choroid plexus and the subsequent effects on mental health.
In 2015, Dr. Rescigno’s group first described the gut vascular barrier that protects the systemic circulation from gut bacteria or associated bacteria-derived molecules. During intestinal inflammation, such as occurs in IBD, this barrier is compromised and becomes more permeable. This allows microbes to pass across the epithelium of the gut barrier and enter the systemic circulation, including the liver and spleen, explained Dr. Rescigno.
Dr. Rescigno and Dr. Lodato then explored whether this systemic inflammatory condition was connected to the brain along a gut-brain axis and found that it was.
The researchers tested the hypothesis that central nervous system symptoms may be due to vascular changes at the interface between the gut or the brain and elsewhere in the body.
“We set out to test whether opening of the gut vascular barrier would allow gut bacteria to trigger the release of inflammatory molecules that spread to more distant areas, possibly leading to a deficiency of certain nutrients and precipitating mental disorders,” they said.
An experimental preclinical model of the choroid plexus vascular barrier closure led to anxiety-like behavior, as well as short-term memory loss. That this behavior occurred independently of inflammation suggested that it was likely a response to closure itself, they note.
In the noninflammatory state, the epithelium of the choroid plexus filters molecules. Those that are ≤70 kDa are allowed to pass through to the brain. However, the investigators found that during systemic inflammation, this filtration stops, and the blood capillaries of the choroid plexus prevent entry of inflammatory molecules such as cytokines.
Dr. Lodato speculated that when the vascular barrier of the choroid plexus shuts off during the systemic inflammatory state, it responds by bathing the brain in cerebrospinal fluid.
“When the choroid plexus closes, like a door slamming shut, then communication between the brain and the rest of the body is halted. This means that the brain is deprived of certain nutrients and other beneficial molecules that usually enter via the cerebrospinal fluid or enriched of potentially dangerous ones, as drainage could also be affected,” she said.
If confirmed in further studies, these results may open the way to new interventions.
‘A significant leap forward’
Commenting on the findings, David T. Rubin, MD, professor of medicine at the University of Chicago, noted that the study’s results represent “a significant leap forward” and that it highlights “another important cost to uncontrolled gut inflammation that is the potential for worsened mental health disorders.”
Dr. Rubin, whose research involves measuring metabolites of the dietary amino acid tryptophan, including melatonin and serotonin, in patients with IBD, added that the findings offer a possible explanation for the association of both Crohn’s disease and ulcerative colitis with anxiety and depressive disorders.
“There was a belief that the association was in the opposite direction, that the mental health disorder was causing or worsening the gut inflammation, but this has been disavowed,” Dr. Rubin said.
“Most recently, the recognition that the major sources of serotonin and other metabolites of tryptophan that come from the gut microbiome has led to the hypothesis that the inflamed bowel and dysbiotic gut biome may in fact be driving the mental health disorders due to the effect of neurotransmitter imbalance,” he added. Dr. Rubin also suggested that the shutdown of the choroid plexus vascular barrier may contribute to this imbalance but that this needs additional study.
“This further supports my ongoing contention that the gut really is the center of the universe,” said Dr. Rubin.
Also commenting on the findings, Miguel Rigueiro, MD, professor in the department of medicine in the Cleveland Clinic Lerner College of Medicine of Case Western Reserve University, Cleveland, said, “There’s an implication that anxiety and depression and other behavioral health disorders may be explained by this mechanism. If that is the case, there may be a way to target medications against the choroid plexus and potentially treat depression or anxiety.”
This prospect was echoed by Dr. Rubin, who said, “The clinical implication is that treatment of gut inflammation may restore a balance to the neurotransmitters and resolve anxiety or depressive disorders.”
To identify new therapeutic targets, investigators will study the regions and circuits of the brain that are more susceptible to this closure of the choroid plexus, said Dr. Lodato.
“If these regions are associated with depression or other psychosocial disorders, then this new understanding around the choroid plexus vascular barrier might eventually have implications for helping treat such disorders,” she noted.
Reflecting a general shift from a brain-centric view of some psychosocial disorders to an intestinal-centric one, Dr. Lodato added, “The brain cannot be considered in isolation. It is part of a much larger body, and we need to think this way.”
Dr. Rescigno, Dr. Lodato, and Dr. Rubin report no relevant financial relationships. Dr. Rigueiro has served on advisory boards and as consultant for AbbVie, Janssen, UCB, Takeda, Pfizer, Miraca Labs, Amgen, Celgene, Seres, Allergan, Genentech, Gilead, Salix, Prometheus, Lilly, TARGET Pharma Solutions, ALFASIGMA, SpA, and Bristol-Meyer Squibb.
A version of this article first appeared on Medscape.com.
FROM SCIENCE
AGA Clinical Practice Update: Managing pain in gut-brain interaction disorders
An American Gastroenterological Association clinical practice update for gastrointestinal pain in disorders of gut-brain interaction (DGBI), published in Clinical Gastroenterology and Hepatology, emphasizes patient-physician collaboration and improvement of patient understanding of the pathways and mechanisms of pain sensations. It is aimed at management of patients in whom pain persists after first-line therapies fail to resolve visceral causes of pain.
DGBIs include irritable bowel syndrome, functional dyspepsia, and centrally mediated abdominal pain syndrome, according to Laurie Keefer, PhD, AGAF, of the division of gastroenterology at Icahn School of Medicine at Mount Sinai, New York, and colleagues. Initial treatment usually focuses on visceral triggers of pain such as food and bowel movements, but this approach is ineffective for many.
Cognitive, affective, and behavioral factors can impact the treatment of these patients, making it a complex clinical problem that calls for a collaborative approach between the patient and clinician. Opioids and other drugs that could be misused should be avoided, according to the authors. Both pharmacologic and nonpharmacologic approaches can be considered, but the update did not address use of marijuana or other complementary or alternative therapies.
Effective management requires empathy and collaboration. The patient has often seen various other clinicians with suboptimal results, which has left them dissatisfied with their care. Cultural sensitivity is crucial because the understanding and interpretation of pain, and preferred management approaches, vary across cultures.
The first step is a nonjudgmental patient history using open-ended questions. Examples include: “How do your symptoms interfere with your ability to do what you want in your daily life?” or “How are these symptoms impacting your life the most?” These types of questions may identify patients who could benefit from behavioral health interventions.
Questions about symptom-related anxiety can improve understanding of patient concerns and offer an opportunity to address fears. Additional understanding of the patient’s perspective can come from questions like: “What do you think is causing your symptoms,” “Why are you coming to see me now?” and “What are you most concerned about with your symptoms?”
The initial assessment should ideally result in shared goals and expectations for pain management.
Providers should educate the patient about the pathogenesis of pain and how it can be modified. Pain signals can result from innocuous signals from the gut that are misinterpreted by the vigilant brain as it scans for injury or illness. That model might explain why some patients with similar diagnoses have widely differing pain experiences, and offers hope that a change in how one approaches pain might lead to improvements. Patients should be encouraged to avoid too much focus on the cause or a solution to pain, because it can interfere with acceptance of pain or, when needed, treatment.
Opioids should not be prescribed for these patients, and if they are already taking them on referral, it’s important to manage them within a multidisciplinary framework until the opioids can be discontinued. Long-term use of opioids can lead to narcotic bowel syndrome, which results in chronic and often heightened abdominal pain even with escalating opioid doses. Opioid stoppage often must be accompanied by behavioral and psychiatric therapies to ensure success.
Nonpharmacological therapies such as brain-gut psychotherapies should be brought up as potential options early in treatment, even though many patients won’t require this type of care. Early mention is likely to keep the patient more open to trying them because they’re less likely to think of it as a sign of failure or a “last-ditch” approach. Cognitive-behavioral therapy works to improve pain management skills and bolster skill deficits, with attention to pain catastrophizing, pain hypervigilance, and visceral anxiety through different techniques.
Gut-directed hypnotherapy deals with somatic awareness and the use of imagery and suggestion to reduce pain sensations. Mindfulness-based stress reduction has been shown to be effective in inflammatory bowel disease and musculoskeletal pain syndromes. The provider should be familiar with these available methods, but should leave choice of interventions to partner mental health providers.
It’s important to distinguish between gastrointestinal pain with visceral causes and centrally mediated pain. Central sensitization can cause intermittent pain to become persistent even in the absence of ongoing peripheral causes of pain.
Peripheral acting agents affect gastrointestinal pain, and a network meta-analysis identified the top three drugs for pain relief in irritable bowel syndrome as tricyclic antidepressants, antispasmodics, and peppermint oil.
Neuromodulator drugs are an option for DGBI pain because the gut nervous system shares embryonic developmental pathways with the brain and spinal cord, which helps explains some of the benefits of low-dose antidepressants, now termed gut-brain neuromodulators. These drugs should be started at a low dose and gradually titrated according to symptom response and tolerability.
The authors have financial relationships with various pharmaceutical companies.
An American Gastroenterological Association clinical practice update for gastrointestinal pain in disorders of gut-brain interaction (DGBI), published in Clinical Gastroenterology and Hepatology, emphasizes patient-physician collaboration and improvement of patient understanding of the pathways and mechanisms of pain sensations. It is aimed at management of patients in whom pain persists after first-line therapies fail to resolve visceral causes of pain.
DGBIs include irritable bowel syndrome, functional dyspepsia, and centrally mediated abdominal pain syndrome, according to Laurie Keefer, PhD, AGAF, of the division of gastroenterology at Icahn School of Medicine at Mount Sinai, New York, and colleagues. Initial treatment usually focuses on visceral triggers of pain such as food and bowel movements, but this approach is ineffective for many.
Cognitive, affective, and behavioral factors can impact the treatment of these patients, making it a complex clinical problem that calls for a collaborative approach between the patient and clinician. Opioids and other drugs that could be misused should be avoided, according to the authors. Both pharmacologic and nonpharmacologic approaches can be considered, but the update did not address use of marijuana or other complementary or alternative therapies.
Effective management requires empathy and collaboration. The patient has often seen various other clinicians with suboptimal results, which has left them dissatisfied with their care. Cultural sensitivity is crucial because the understanding and interpretation of pain, and preferred management approaches, vary across cultures.
The first step is a nonjudgmental patient history using open-ended questions. Examples include: “How do your symptoms interfere with your ability to do what you want in your daily life?” or “How are these symptoms impacting your life the most?” These types of questions may identify patients who could benefit from behavioral health interventions.
Questions about symptom-related anxiety can improve understanding of patient concerns and offer an opportunity to address fears. Additional understanding of the patient’s perspective can come from questions like: “What do you think is causing your symptoms,” “Why are you coming to see me now?” and “What are you most concerned about with your symptoms?”
The initial assessment should ideally result in shared goals and expectations for pain management.
Providers should educate the patient about the pathogenesis of pain and how it can be modified. Pain signals can result from innocuous signals from the gut that are misinterpreted by the vigilant brain as it scans for injury or illness. That model might explain why some patients with similar diagnoses have widely differing pain experiences, and offers hope that a change in how one approaches pain might lead to improvements. Patients should be encouraged to avoid too much focus on the cause or a solution to pain, because it can interfere with acceptance of pain or, when needed, treatment.
Opioids should not be prescribed for these patients, and if they are already taking them on referral, it’s important to manage them within a multidisciplinary framework until the opioids can be discontinued. Long-term use of opioids can lead to narcotic bowel syndrome, which results in chronic and often heightened abdominal pain even with escalating opioid doses. Opioid stoppage often must be accompanied by behavioral and psychiatric therapies to ensure success.
Nonpharmacological therapies such as brain-gut psychotherapies should be brought up as potential options early in treatment, even though many patients won’t require this type of care. Early mention is likely to keep the patient more open to trying them because they’re less likely to think of it as a sign of failure or a “last-ditch” approach. Cognitive-behavioral therapy works to improve pain management skills and bolster skill deficits, with attention to pain catastrophizing, pain hypervigilance, and visceral anxiety through different techniques.
Gut-directed hypnotherapy deals with somatic awareness and the use of imagery and suggestion to reduce pain sensations. Mindfulness-based stress reduction has been shown to be effective in inflammatory bowel disease and musculoskeletal pain syndromes. The provider should be familiar with these available methods, but should leave choice of interventions to partner mental health providers.
It’s important to distinguish between gastrointestinal pain with visceral causes and centrally mediated pain. Central sensitization can cause intermittent pain to become persistent even in the absence of ongoing peripheral causes of pain.
Peripheral acting agents affect gastrointestinal pain, and a network meta-analysis identified the top three drugs for pain relief in irritable bowel syndrome as tricyclic antidepressants, antispasmodics, and peppermint oil.
Neuromodulator drugs are an option for DGBI pain because the gut nervous system shares embryonic developmental pathways with the brain and spinal cord, which helps explains some of the benefits of low-dose antidepressants, now termed gut-brain neuromodulators. These drugs should be started at a low dose and gradually titrated according to symptom response and tolerability.
The authors have financial relationships with various pharmaceutical companies.
An American Gastroenterological Association clinical practice update for gastrointestinal pain in disorders of gut-brain interaction (DGBI), published in Clinical Gastroenterology and Hepatology, emphasizes patient-physician collaboration and improvement of patient understanding of the pathways and mechanisms of pain sensations. It is aimed at management of patients in whom pain persists after first-line therapies fail to resolve visceral causes of pain.
DGBIs include irritable bowel syndrome, functional dyspepsia, and centrally mediated abdominal pain syndrome, according to Laurie Keefer, PhD, AGAF, of the division of gastroenterology at Icahn School of Medicine at Mount Sinai, New York, and colleagues. Initial treatment usually focuses on visceral triggers of pain such as food and bowel movements, but this approach is ineffective for many.
Cognitive, affective, and behavioral factors can impact the treatment of these patients, making it a complex clinical problem that calls for a collaborative approach between the patient and clinician. Opioids and other drugs that could be misused should be avoided, according to the authors. Both pharmacologic and nonpharmacologic approaches can be considered, but the update did not address use of marijuana or other complementary or alternative therapies.
Effective management requires empathy and collaboration. The patient has often seen various other clinicians with suboptimal results, which has left them dissatisfied with their care. Cultural sensitivity is crucial because the understanding and interpretation of pain, and preferred management approaches, vary across cultures.
The first step is a nonjudgmental patient history using open-ended questions. Examples include: “How do your symptoms interfere with your ability to do what you want in your daily life?” or “How are these symptoms impacting your life the most?” These types of questions may identify patients who could benefit from behavioral health interventions.
Questions about symptom-related anxiety can improve understanding of patient concerns and offer an opportunity to address fears. Additional understanding of the patient’s perspective can come from questions like: “What do you think is causing your symptoms,” “Why are you coming to see me now?” and “What are you most concerned about with your symptoms?”
The initial assessment should ideally result in shared goals and expectations for pain management.
Providers should educate the patient about the pathogenesis of pain and how it can be modified. Pain signals can result from innocuous signals from the gut that are misinterpreted by the vigilant brain as it scans for injury or illness. That model might explain why some patients with similar diagnoses have widely differing pain experiences, and offers hope that a change in how one approaches pain might lead to improvements. Patients should be encouraged to avoid too much focus on the cause or a solution to pain, because it can interfere with acceptance of pain or, when needed, treatment.
Opioids should not be prescribed for these patients, and if they are already taking them on referral, it’s important to manage them within a multidisciplinary framework until the opioids can be discontinued. Long-term use of opioids can lead to narcotic bowel syndrome, which results in chronic and often heightened abdominal pain even with escalating opioid doses. Opioid stoppage often must be accompanied by behavioral and psychiatric therapies to ensure success.
Nonpharmacological therapies such as brain-gut psychotherapies should be brought up as potential options early in treatment, even though many patients won’t require this type of care. Early mention is likely to keep the patient more open to trying them because they’re less likely to think of it as a sign of failure or a “last-ditch” approach. Cognitive-behavioral therapy works to improve pain management skills and bolster skill deficits, with attention to pain catastrophizing, pain hypervigilance, and visceral anxiety through different techniques.
Gut-directed hypnotherapy deals with somatic awareness and the use of imagery and suggestion to reduce pain sensations. Mindfulness-based stress reduction has been shown to be effective in inflammatory bowel disease and musculoskeletal pain syndromes. The provider should be familiar with these available methods, but should leave choice of interventions to partner mental health providers.
It’s important to distinguish between gastrointestinal pain with visceral causes and centrally mediated pain. Central sensitization can cause intermittent pain to become persistent even in the absence of ongoing peripheral causes of pain.
Peripheral acting agents affect gastrointestinal pain, and a network meta-analysis identified the top three drugs for pain relief in irritable bowel syndrome as tricyclic antidepressants, antispasmodics, and peppermint oil.
Neuromodulator drugs are an option for DGBI pain because the gut nervous system shares embryonic developmental pathways with the brain and spinal cord, which helps explains some of the benefits of low-dose antidepressants, now termed gut-brain neuromodulators. These drugs should be started at a low dose and gradually titrated according to symptom response and tolerability.
The authors have financial relationships with various pharmaceutical companies.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Definitive diverticular hemorrhage: Diagnosis and management
Diverticular hemorrhage is the most common cause of colonic bleeding, accounting for 20%-65% of cases of severe lower intestinal bleeding in adults.1 Urgent colonoscopy after purging the colon of blood, clots, and stool is the most accurate method of diagnosing and guiding treatment of definitive diverticular hemorrhage.2-5 The diagnosis of definitive diverticular hemorrhage depends upon identification of some stigmata of recent hemorrhage (SRH) in a single diverticulum (TIC), which can include active arterial bleeding, oozing, non-bleeding visible vessel, adherent clot, or flat spot.2-4 Although other approaches, such as nuclear medicine scans and angiography of various types (CT, MRI, or standard angiography), for the early diagnosis of patients with severe hematochezia are utilized in many medical centers, only active bleeding can be detected by these techniques. However, as subsequently discussed, this SRH is documented in only 26% of definitive diverticular bleeds found on urgent colonoscopy, so diagnostic yields of these techniques will be low.2-5
The diagnosis of patients with severe hematochezia and diverticulosis, as well as triage of all of them to specific medical, endoscopic, radiologic, or surgical management, is facilitated by an urgent endoscopic approach.2-5 Patients who are diagnosed with definitive diverticular hemorrhage on colonoscopy represent about 30% of all true TIC bleeds when urgent colonoscopy is the management approach.2-5 That is because approximately 50% of all patients with colon diverticulosis and first presentation of severe hematochezia have incidental diverticulosis; they have colonic diverticulosis, but another site of bleeding is identified as the cause of hemorrhage in the gastrointestinal tract.2-4 Presumptive diverticular hemorrhage is diagnosed when colonic diverticulosis without TIC stigmata are found but no other GI bleeding source is found on colonoscopy, anoscopy, enteroscopy, or capsule endoscopy.2-5 In our experience with urgent colonoscopy, the presumptive diverticular bleed group accounts for about 70% of patients with documented diverticular hemorrhage (e.g., not including incidental diverticulosis bleeds but combining subgroups of patients with either definitive or presumptive TIC diagnoses as documented TIC hemorrhage).
Clinical presentation
Patients with diverticular hemorrhage present with severe, painless large volume hematochezia. Hematochezia may be self-limited and spontaneously resolve in 75%-80% of all patients but with high rebleeding rates up to 40%.5-7 Of all patients with diverticulosis, only about 3%-5% develop diverticular hemorrhage.8 Risk factors for diverticular hemorrhage include medications (e.g., nonsteroidal anti-inflammatory drugs – NSAIDs, antiplatelet drugs, and anticoagulants) and other clinical factors, such as older age, low-fiber diet, and chronic constipation.9,10 On urgent colonoscopy, more than 70% of diverticulosis in U.S. patients are located anatomically in the descending colon or more distally. In contrast, about 60% of definitive diverticular hemorrhage cases in our experience had diverticula with stigmata identified at or proximal to the splenic flexure.2,4,11
Pathophysiology
Colonic diverticula are herniations of mucosa and submucosa with colonic arteries that penetrate the muscular wall. Bleeding can occur when there is asymmetric rupture of the vasa recta at either the base of the diverticulum or the neck.4 Thinning of the mucosa on the luminal surface (such as that resulting from impacted fecaliths and stool) can cause injury to the site of the penetrating vessels, resulting in hemorrhage.12
Initial management
Patients with acute, severe hematochezia should be triaged to an inpatient setting with a monitored bed. Admission to an intensive care unit should be considered for patients with hemodynamic instability, persistent bleeding, and/or significant comorbidities. Patients with TIC hemorrhage often require resuscitation with crystalloids and packed red blood cell transfusions for hemoglobin less than 8 g/dl.4 Unlike upper GI hemorrhage, which has been extensively reported on, data regarding a more restrictive transfusion threshold, compared with a liberal transfusion threshold, in lower intestinal bleeding are very limited. Correction of underlying coagulopathies is recommended but should be individualized, particularly in those patients on antithrombotic agents or with underlying bleeding disorders.
Urgent diagnosis and hemostasis
Urgent colonoscopy within 24 hours is the most accurate way to make a diagnosis of definitive diverticular hemorrhage and to effectively and safely treat them.2-4,10,11 For patients with severe hematochezia, when the colonoscopy is either not available in a medical center or does not reveal the source of bleeding, nuclear scintigraphy or angiography (CT, MRI, or interventional radiology [IR]) are recommended. CT angiography may be particularly helpful to diagnose patients with hemodynamic instability who are suspected to have active TIC bleeding and are not able to complete a bowel preparation. However, these imaging techniques require active bleeding at the time of the study to be diagnostic. This SRH is also uncommon for definitive diverticular hemorrhage, so the diagnostic yield is usually quite low.2-5,10,11 An additional limitation of scintigraphy and CT or MRI angiography is that, if active bleeding is found, some other type of treatment, such as colonoscopy, IR angiography, or surgery, will be required for definitive hemostasis.
For urgent colonoscopy, adequate colon preparation with a large volume preparation (6-8 liters of polyethylene glycol-based solution) is recommended to clear stool, blood, and clots to allow endoscopic visualization and localization of the bleeding source. Use of a nasogastric tube should be considered if the patient is unable to drink enough prep.2-4,13 Additionally, administration of a prokinetic agent, such as Metoclopramide, may improve gastric emptying and tolerance of the prep. During colonoscopy, careful inspection of the colonic mucosa during insertion and withdrawal is important since lesions may bleed intermittently and SRH can be missed. An adult or pediatric colonoscope with a large working channel (at least 3.3 mm) is recommended to facilitate suctioning of blood clots and stool, as well as allow the passage of endoscopic hemostasis accessories. Targeted water-jet irrigation, an expert colonoscopist, a cap attachment, and adequate colon preparation are all predictors for improved diagnosis of definitive diverticular hemorrhage.4,14
SRH in definitive TIC bleeds all have a high risk of TIC rebleeding,2-4,10,11 including active bleeding, nonbleeding visible vessel, adherent clot, and a flat spot (See Figure).
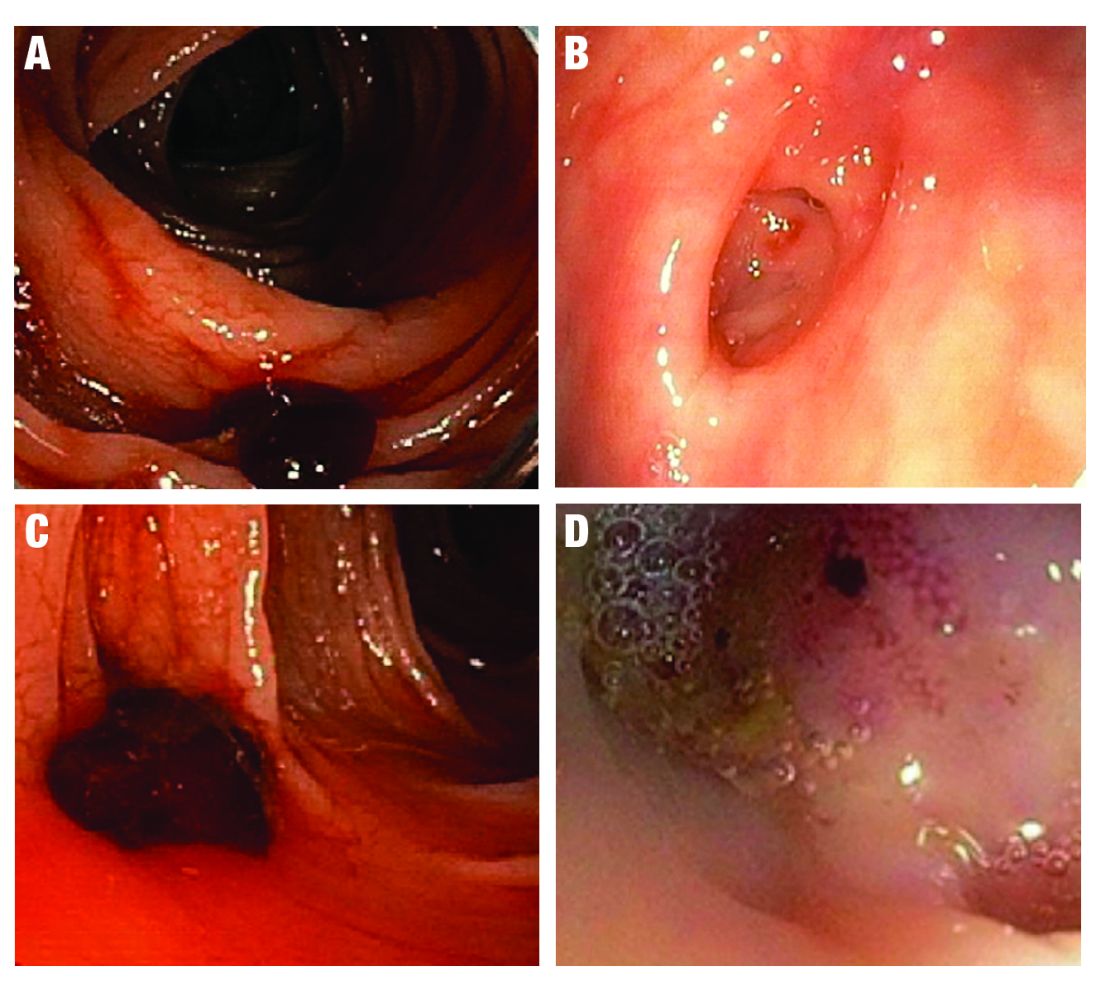
Based on CURE Hemostasis Group data of 118 definitive TIC bleeds, 26% had active bleeding, 24% had a nonbleeding visible vessel, 37% had an adherent clot, and 13% had a flat spot (with underlying arterial blood flow by Doppler probe monitoring).4 Approximately 50% of the SRH were found in the neck of the TIC and 50% at the base, with actively bleeding cases more often from the base. In CURE Doppler endoscopic probe studies, 90% of all stigmata had an underlying arterial blood flow detected with the Doppler probe.4,10 The Doppler probe is reported to be very useful for risk stratification and to confirm obliteration of the arterial blood flow underlying SRH for definitive hemostasis.4,10
Endoscopic treatment
Given high rates of rebleeding with medical management alone, definitive TIC hemorrhage can be effectively and safely treated with endoscopic therapies once SRH are localized.4,10 Endoscopic therapies that have been reported in the literature include electrocoagulation, hemoclip, band ligation, and over-the-scope clip. Four-quadrant injection of 1:20,000 epinephrine around the SRH can improve visualization of SRH and provide temporary control of bleeding, but it should be combined with other modalities because of risk of rebleeding with epinephrine alone.15 Results from studies reporting rates of both early rebleeding (occurring within 30 days) and late rebleeding (occurring after 30 days) are listed in the Table. 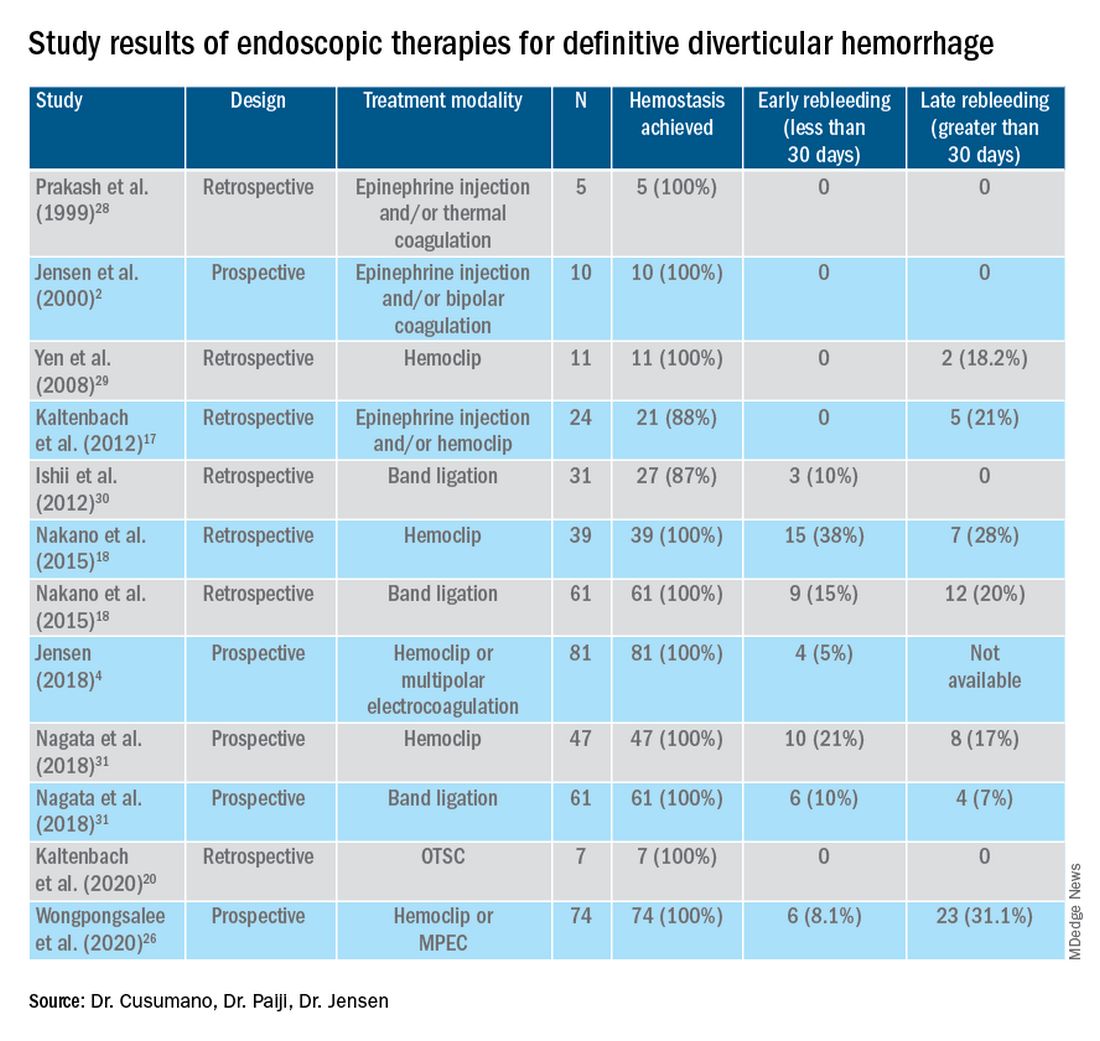
Multipolar electrocoagulation (MPEC), which utilizes a focal electric current to generate heat, can coaptively coagulate small TIC arteries.16 For SRH in the neck of TIC, MPEC is effective for coaptive coagulation at a power of 12-15 watts in 1-2 second pulses with moderate laterally applied tamponade pressure. MPEC should be avoided for treating SRH at the TIC base because of lack of muscularis propria and higher risk of perforation.
Hemoclip therapy has been reported to be safe and efficacious in treatment of definitive TIC hemorrhage, by causing mechanical hemostasis with occlusion of the bleeding artery.16 Hemoclips are recommended to treat stigmata in the base of TICs and should be targeted on either side of visible vessel in order to occlude the artery underneath it.4,10 With a cap on the tip of the colonoscope, suctioning can evert TICs, allowing more precise placement of hemoclip on SRH in the base of the TIC.17 Hemoclip retention rates vary with different models and can range from less than 7 days to more than 4 weeks. Hemoclips can also mark the site if early rebleeding occurs; then, reintervention (e.g., repeat endoscopy or angioembolization) is facilitated.
Another treatment is endoscopic band ligation, which provides mechanical hemostasis. Endoscopic band ligation has been reported to be efficacious for TIC hemorrhage.18 Suctioning the TIC with the SRH into the distal cap and deploying a band leads to obliteration of vessels and potentially necrosis and disappearance of banded TIC.16 This technique carries a risk of perforation because of the thin walls of TICs. This risk may be higher for right-sided colon lesions since an exvivo colon specimen study reported serosal entrapment and inclusion of muscularis propria postband ligation, both of which may result in ischemia of intestinal wall and delayed perforation.19
Over-the-scope clip (OTSC) has been reported in case series for treatment of definitive TIC hemorrhage. With a distal cap and large clip, suctioning can evert TICs and facilitate deployment over the SRH.20,21 OTSC can grasp an entire TIC with the SRH and obliterate the arterial blood flow with a single clip.20,21 No complications have been reported yet for treatment of TIC hemorrhage. However, the OTSC system is relatively expensive when compared with other modalities.
After endoscopic treatment is performed, four-quadrant spot tattooing is recommended adjacent to the TIC with the SRH. This step will facilitate localization and treatment in the case of TIC rebleeding.4,10
Outcomes following endoscopic treatment
Following endoscopic treatment, patients should be monitored for early and late rebleeding. In a pooled analysis of case series composed of 847 patients with TIC bleeding, among the 137 patients in which endoscopic hemostasis was initially achieved, early rebleeding occurred in 8% and late rebleeding occurred in 12% of patients.22 Risk factors for TIC rebleeding within 30 days were residual arterial blood flow following hemostasis and early reinitiation of antiplatelet agents.
Remote treatment of TIC hemorrhage distant from the SRH is a significant risk factor for early TIC rebleeding.4, 10 For example, using hemoclips to close the mouth of a TIC when active bleeding or an SRH is located in the TIC base often fails because arterial flow remains open in the base and the artery is larger there.4,10 This example highlights the importance of focal obliteration of arterial blood flow underlying SRH in order to achieve definitive hemostasis.4,10
Salvage treatments
For TIC hemorrhage that is not controlled by endoscopic therapy, transcatheter arterial embolization (TAE) is recommended. If bleeding rate is high enough (at least 0.5 milliliters per minute) to be detected by angiography, TAE can serve as an effective method of diagnosis and immediate hemostasis.23 However, the most common major complication of embolization is intestinal ischemia. The incidence of intestinal ischemia has been reported as high as 10%, with highest risk with embolization of at least three vasa recta.24
Surgery is also recommended if TIC hemorrhage cannot be controlled with endoscopic therapy or TAE. Segmental colectomy is recommended if the bleeding site can be localized before surgery with colonoscopy or angiography resulting from significantly lower perioperative morbidity than subtotal colectomy.25 However, subtotal colectomy may be necessary if preoperative localization of bleeding is unsuccessful.
There are very few reports of short- or long-term results that compare endoscopy, TAE, and surgery for management of TIC bleeding. However, a recent retrospective study reported better outcomes with endoscopic treatment of definitive TIC bleeding.26 Patients who underwent endoscopic treatment had fewer RBC transfusions, shorter hospitalizations, and lower rates of postprocedure complications.
Management after cessation of hemorrhage
Medical management is important following an episode of TIC hemorrhage. A mainstay is daily fiber supplementation every morning and stool softener in the evening. Furthermore, patients are advised to drink an extra liter of fluids (not containing alcohol or caffeine) daily. By reducing colon transit time and increasing stool weight, these measures can help control constipation and prevent future complications of TIC disease.27
Patients with recurrent TIC hemorrhage should undergo evaluation for elective surgery, provided they are appropriate surgical candidates. If preoperative localization of bleeding site is successful, segmental colectomy is preferred. Segmental resection is associated with significantly decreased rebleeding rate, with lower rates of morbidity compared with subtotal colectomy.32
Chronic NSAIDs, aspirin, and antiplatelet drugs are risk factors for recurrent TIC hemorrhage, and avoiding these medications is recommended if possible.33,34 Although anticoagulants have shown to be associated with increased risk of all-cause gastrointestinal bleeding, these agents have not been shown to increase risk of recurrent TIC hemorrhage in recent large retrospective studies. Since antiplatelet and anticoagulation agents serve to reduce risk of thromboembolic events, the clinician who recommended these medications should be consulted after a TIC bleed to re-evaluate whether these medications can be discontinued or reduced in dose.
Conclusion
The most effective way to diagnose and treat definitive TIC hemorrhage is to perform an urgent colonoscopy within 24 hours to identify and treat TIC SRH. This procedure requires thoroughly cleansing the colon first, as well as an experienced colonoscopist who can identify and treat TIC SRH to obliterate arterial blood flow underneath SRH and achieve definitive TIC hemostasis. Other approaches to early diagnosis include nuclear medicine scintigraphy or angiography (CT, MRI, or IR). However, these techniques can only detect active bleeding which is documented in only 26% of colonoscopically diagnosed definitive TIC hemorrhages. So, the expected diagnostic yield of these tests will be low. When urgent colonoscopy fails to make a diagnosis or TIC bleeding continues, TAE and/or surgery are recommended. After definitive hemostasis of TIC hemorrhage and for long term management, control of constipation and discontinuation of chronic NSAIDs and antiplatelet drugs (if possible) are recommended to prevent recurrent TIC hemorrhage.
Dr. Cusumano and Dr. Paiji are fellow physicians in the Vatche and Tamar Manoukian Division of Digestive Diseases at University of California Los Angeles. Dr. Jensen is a professor of medicine in Vatche and Tamar Manoukian Division of Digestive Diseases and is with the CURE Digestive Diseases Research Center at the VA Greater Los Angeles Healthcare System, Calif. All authors declare that they have no competing interests or disclosures.
References
1. Longstreth GF. Am J Gastroenterol. 1997;92(3):419-24.
2. Jensen DM et al. The New England Journal of Medicine. 2000;342(2):78-82.
3. Jensen DM et al. Techniques in Gastrointestinal Endoscopy. 2001;3(4):192-8.
4. Jensen DM. Am J Gastroenterol. 2018;113(11):1570-3.
5. Zuckerman GR et al. Gastrointestinal Endoscopy. 1999;49(2):228-38.
6. Stollman N et al. Lancet. 2004;363(9409):631-9.
7. McGuire HH et al. Ann Surg. 1994;220(5):653-6.
8. McGuire HH et al. Ann Surg. 1972;175(6):847-55.
9. Strate LL et al. Clinical gastroenterology and hepatol. 2008;6(9):1004-10.
10. Jensen DM et al. Gastrointestinal endoscopy. 2016;83(2):416-23.
11. Jensen DM et al. Gastrointest Endosc Clin N Am. 1997;7(3):477-98.
12. Maykel JA et al. Clin Colon Rectal Surg. 2004;17(3):195-204.
13. Green BT et al. Am J Gastroenterol. 2005;100(11):2395-402.
14. Niikura R et al. Journal of Clinical Gastroenterol. 2015;49(3):e24-30.
15. Bloomfeld RS et al. Am J Gastroenterol. 2001;96(8):2367-72.
16. Parsi MA,et al. VideoGIE. 2019;4(7):285-99.
17. Kaltenbach T et al. Clinical Gastroenterology and Hepatol. 2012;10(2):131-7.
18. Nakano K et al. Endosc Int Open. 2015;3(5):E529-33.
19. Barker KB et al. Gastrointestinal Endoscopy. 2005;62(2):224-7.
20. Kaltenbach T et al. Gastrointest Endosc Clin N Am. 2020;30(1):13-23.
21. Yamazaki K et al. VideoGIE. 2020;5(6):252-4.
22. Strate LL et al. Clinical Gastroenterology and Hepatol. 2010;8(4):333-43.
23. Evangelista et al. J Vasc Interv Radiol. 2000;11(5):601-6.
24. Kodani M et al. J Vasc Interv Radiol. 2016;27(6):824-30.
25. Mohammed et al. Clin Colon Rectal Surg. 2018;31(4):243-50.
26. Wongpongsalee T et al. Gastrointestinal Endoscopy. 2020;91(6):AB471-2.
27. Böhm SK. Viszeralmedizin. 2015;31(2):84-94.
28. Prakash C et al. Endoscopy. 1999;31(6):460-3.
29. Yen EF et al. Digestive Diseases and Sciences. 2008;53(9):2480-5.
30. Ishii N et al. Gastrointestinal Endoscopy. 2012;75(2):382-7.
31. Nagata N et al. Gastrointestinal Endoscopy. 2018;88(5):841-53.e4.
32. Parkes BM et al. Am Surg. 1993;59(10):676-8.
33. Vajravelu RK et al. Gastroenterology. 2018;155(5):1416-27.
34. Oakland K et al. Clin Gastroenterol Hepatol. 2019;17(7):1276-84.e3.
35. Yamada A et al. Dis Colon Rectum. 2008;51(1):116-20.
36. Coleman CI et al. Int J Clin Pract. 2012;66(1):53-63.
37. Holster IL et al. Gastroenterology. 2013;145(1):105-12.e15.
Diverticular hemorrhage is the most common cause of colonic bleeding, accounting for 20%-65% of cases of severe lower intestinal bleeding in adults.1 Urgent colonoscopy after purging the colon of blood, clots, and stool is the most accurate method of diagnosing and guiding treatment of definitive diverticular hemorrhage.2-5 The diagnosis of definitive diverticular hemorrhage depends upon identification of some stigmata of recent hemorrhage (SRH) in a single diverticulum (TIC), which can include active arterial bleeding, oozing, non-bleeding visible vessel, adherent clot, or flat spot.2-4 Although other approaches, such as nuclear medicine scans and angiography of various types (CT, MRI, or standard angiography), for the early diagnosis of patients with severe hematochezia are utilized in many medical centers, only active bleeding can be detected by these techniques. However, as subsequently discussed, this SRH is documented in only 26% of definitive diverticular bleeds found on urgent colonoscopy, so diagnostic yields of these techniques will be low.2-5
The diagnosis of patients with severe hematochezia and diverticulosis, as well as triage of all of them to specific medical, endoscopic, radiologic, or surgical management, is facilitated by an urgent endoscopic approach.2-5 Patients who are diagnosed with definitive diverticular hemorrhage on colonoscopy represent about 30% of all true TIC bleeds when urgent colonoscopy is the management approach.2-5 That is because approximately 50% of all patients with colon diverticulosis and first presentation of severe hematochezia have incidental diverticulosis; they have colonic diverticulosis, but another site of bleeding is identified as the cause of hemorrhage in the gastrointestinal tract.2-4 Presumptive diverticular hemorrhage is diagnosed when colonic diverticulosis without TIC stigmata are found but no other GI bleeding source is found on colonoscopy, anoscopy, enteroscopy, or capsule endoscopy.2-5 In our experience with urgent colonoscopy, the presumptive diverticular bleed group accounts for about 70% of patients with documented diverticular hemorrhage (e.g., not including incidental diverticulosis bleeds but combining subgroups of patients with either definitive or presumptive TIC diagnoses as documented TIC hemorrhage).
Clinical presentation
Patients with diverticular hemorrhage present with severe, painless large volume hematochezia. Hematochezia may be self-limited and spontaneously resolve in 75%-80% of all patients but with high rebleeding rates up to 40%.5-7 Of all patients with diverticulosis, only about 3%-5% develop diverticular hemorrhage.8 Risk factors for diverticular hemorrhage include medications (e.g., nonsteroidal anti-inflammatory drugs – NSAIDs, antiplatelet drugs, and anticoagulants) and other clinical factors, such as older age, low-fiber diet, and chronic constipation.9,10 On urgent colonoscopy, more than 70% of diverticulosis in U.S. patients are located anatomically in the descending colon or more distally. In contrast, about 60% of definitive diverticular hemorrhage cases in our experience had diverticula with stigmata identified at or proximal to the splenic flexure.2,4,11
Pathophysiology
Colonic diverticula are herniations of mucosa and submucosa with colonic arteries that penetrate the muscular wall. Bleeding can occur when there is asymmetric rupture of the vasa recta at either the base of the diverticulum or the neck.4 Thinning of the mucosa on the luminal surface (such as that resulting from impacted fecaliths and stool) can cause injury to the site of the penetrating vessels, resulting in hemorrhage.12
Initial management
Patients with acute, severe hematochezia should be triaged to an inpatient setting with a monitored bed. Admission to an intensive care unit should be considered for patients with hemodynamic instability, persistent bleeding, and/or significant comorbidities. Patients with TIC hemorrhage often require resuscitation with crystalloids and packed red blood cell transfusions for hemoglobin less than 8 g/dl.4 Unlike upper GI hemorrhage, which has been extensively reported on, data regarding a more restrictive transfusion threshold, compared with a liberal transfusion threshold, in lower intestinal bleeding are very limited. Correction of underlying coagulopathies is recommended but should be individualized, particularly in those patients on antithrombotic agents or with underlying bleeding disorders.
Urgent diagnosis and hemostasis
Urgent colonoscopy within 24 hours is the most accurate way to make a diagnosis of definitive diverticular hemorrhage and to effectively and safely treat them.2-4,10,11 For patients with severe hematochezia, when the colonoscopy is either not available in a medical center or does not reveal the source of bleeding, nuclear scintigraphy or angiography (CT, MRI, or interventional radiology [IR]) are recommended. CT angiography may be particularly helpful to diagnose patients with hemodynamic instability who are suspected to have active TIC bleeding and are not able to complete a bowel preparation. However, these imaging techniques require active bleeding at the time of the study to be diagnostic. This SRH is also uncommon for definitive diverticular hemorrhage, so the diagnostic yield is usually quite low.2-5,10,11 An additional limitation of scintigraphy and CT or MRI angiography is that, if active bleeding is found, some other type of treatment, such as colonoscopy, IR angiography, or surgery, will be required for definitive hemostasis.
For urgent colonoscopy, adequate colon preparation with a large volume preparation (6-8 liters of polyethylene glycol-based solution) is recommended to clear stool, blood, and clots to allow endoscopic visualization and localization of the bleeding source. Use of a nasogastric tube should be considered if the patient is unable to drink enough prep.2-4,13 Additionally, administration of a prokinetic agent, such as Metoclopramide, may improve gastric emptying and tolerance of the prep. During colonoscopy, careful inspection of the colonic mucosa during insertion and withdrawal is important since lesions may bleed intermittently and SRH can be missed. An adult or pediatric colonoscope with a large working channel (at least 3.3 mm) is recommended to facilitate suctioning of blood clots and stool, as well as allow the passage of endoscopic hemostasis accessories. Targeted water-jet irrigation, an expert colonoscopist, a cap attachment, and adequate colon preparation are all predictors for improved diagnosis of definitive diverticular hemorrhage.4,14
SRH in definitive TIC bleeds all have a high risk of TIC rebleeding,2-4,10,11 including active bleeding, nonbleeding visible vessel, adherent clot, and a flat spot (See Figure).

Based on CURE Hemostasis Group data of 118 definitive TIC bleeds, 26% had active bleeding, 24% had a nonbleeding visible vessel, 37% had an adherent clot, and 13% had a flat spot (with underlying arterial blood flow by Doppler probe monitoring).4 Approximately 50% of the SRH were found in the neck of the TIC and 50% at the base, with actively bleeding cases more often from the base. In CURE Doppler endoscopic probe studies, 90% of all stigmata had an underlying arterial blood flow detected with the Doppler probe.4,10 The Doppler probe is reported to be very useful for risk stratification and to confirm obliteration of the arterial blood flow underlying SRH for definitive hemostasis.4,10
Endoscopic treatment
Given high rates of rebleeding with medical management alone, definitive TIC hemorrhage can be effectively and safely treated with endoscopic therapies once SRH are localized.4,10 Endoscopic therapies that have been reported in the literature include electrocoagulation, hemoclip, band ligation, and over-the-scope clip. Four-quadrant injection of 1:20,000 epinephrine around the SRH can improve visualization of SRH and provide temporary control of bleeding, but it should be combined with other modalities because of risk of rebleeding with epinephrine alone.15 Results from studies reporting rates of both early rebleeding (occurring within 30 days) and late rebleeding (occurring after 30 days) are listed in the Table. 
Multipolar electrocoagulation (MPEC), which utilizes a focal electric current to generate heat, can coaptively coagulate small TIC arteries.16 For SRH in the neck of TIC, MPEC is effective for coaptive coagulation at a power of 12-15 watts in 1-2 second pulses with moderate laterally applied tamponade pressure. MPEC should be avoided for treating SRH at the TIC base because of lack of muscularis propria and higher risk of perforation.
Hemoclip therapy has been reported to be safe and efficacious in treatment of definitive TIC hemorrhage, by causing mechanical hemostasis with occlusion of the bleeding artery.16 Hemoclips are recommended to treat stigmata in the base of TICs and should be targeted on either side of visible vessel in order to occlude the artery underneath it.4,10 With a cap on the tip of the colonoscope, suctioning can evert TICs, allowing more precise placement of hemoclip on SRH in the base of the TIC.17 Hemoclip retention rates vary with different models and can range from less than 7 days to more than 4 weeks. Hemoclips can also mark the site if early rebleeding occurs; then, reintervention (e.g., repeat endoscopy or angioembolization) is facilitated.
Another treatment is endoscopic band ligation, which provides mechanical hemostasis. Endoscopic band ligation has been reported to be efficacious for TIC hemorrhage.18 Suctioning the TIC with the SRH into the distal cap and deploying a band leads to obliteration of vessels and potentially necrosis and disappearance of banded TIC.16 This technique carries a risk of perforation because of the thin walls of TICs. This risk may be higher for right-sided colon lesions since an exvivo colon specimen study reported serosal entrapment and inclusion of muscularis propria postband ligation, both of which may result in ischemia of intestinal wall and delayed perforation.19
Over-the-scope clip (OTSC) has been reported in case series for treatment of definitive TIC hemorrhage. With a distal cap and large clip, suctioning can evert TICs and facilitate deployment over the SRH.20,21 OTSC can grasp an entire TIC with the SRH and obliterate the arterial blood flow with a single clip.20,21 No complications have been reported yet for treatment of TIC hemorrhage. However, the OTSC system is relatively expensive when compared with other modalities.
After endoscopic treatment is performed, four-quadrant spot tattooing is recommended adjacent to the TIC with the SRH. This step will facilitate localization and treatment in the case of TIC rebleeding.4,10
Outcomes following endoscopic treatment
Following endoscopic treatment, patients should be monitored for early and late rebleeding. In a pooled analysis of case series composed of 847 patients with TIC bleeding, among the 137 patients in which endoscopic hemostasis was initially achieved, early rebleeding occurred in 8% and late rebleeding occurred in 12% of patients.22 Risk factors for TIC rebleeding within 30 days were residual arterial blood flow following hemostasis and early reinitiation of antiplatelet agents.
Remote treatment of TIC hemorrhage distant from the SRH is a significant risk factor for early TIC rebleeding.4, 10 For example, using hemoclips to close the mouth of a TIC when active bleeding or an SRH is located in the TIC base often fails because arterial flow remains open in the base and the artery is larger there.4,10 This example highlights the importance of focal obliteration of arterial blood flow underlying SRH in order to achieve definitive hemostasis.4,10
Salvage treatments
For TIC hemorrhage that is not controlled by endoscopic therapy, transcatheter arterial embolization (TAE) is recommended. If bleeding rate is high enough (at least 0.5 milliliters per minute) to be detected by angiography, TAE can serve as an effective method of diagnosis and immediate hemostasis.23 However, the most common major complication of embolization is intestinal ischemia. The incidence of intestinal ischemia has been reported as high as 10%, with highest risk with embolization of at least three vasa recta.24
Surgery is also recommended if TIC hemorrhage cannot be controlled with endoscopic therapy or TAE. Segmental colectomy is recommended if the bleeding site can be localized before surgery with colonoscopy or angiography resulting from significantly lower perioperative morbidity than subtotal colectomy.25 However, subtotal colectomy may be necessary if preoperative localization of bleeding is unsuccessful.
There are very few reports of short- or long-term results that compare endoscopy, TAE, and surgery for management of TIC bleeding. However, a recent retrospective study reported better outcomes with endoscopic treatment of definitive TIC bleeding.26 Patients who underwent endoscopic treatment had fewer RBC transfusions, shorter hospitalizations, and lower rates of postprocedure complications.
Management after cessation of hemorrhage
Medical management is important following an episode of TIC hemorrhage. A mainstay is daily fiber supplementation every morning and stool softener in the evening. Furthermore, patients are advised to drink an extra liter of fluids (not containing alcohol or caffeine) daily. By reducing colon transit time and increasing stool weight, these measures can help control constipation and prevent future complications of TIC disease.27
Patients with recurrent TIC hemorrhage should undergo evaluation for elective surgery, provided they are appropriate surgical candidates. If preoperative localization of bleeding site is successful, segmental colectomy is preferred. Segmental resection is associated with significantly decreased rebleeding rate, with lower rates of morbidity compared with subtotal colectomy.32
Chronic NSAIDs, aspirin, and antiplatelet drugs are risk factors for recurrent TIC hemorrhage, and avoiding these medications is recommended if possible.33,34 Although anticoagulants have shown to be associated with increased risk of all-cause gastrointestinal bleeding, these agents have not been shown to increase risk of recurrent TIC hemorrhage in recent large retrospective studies. Since antiplatelet and anticoagulation agents serve to reduce risk of thromboembolic events, the clinician who recommended these medications should be consulted after a TIC bleed to re-evaluate whether these medications can be discontinued or reduced in dose.
Conclusion
The most effective way to diagnose and treat definitive TIC hemorrhage is to perform an urgent colonoscopy within 24 hours to identify and treat TIC SRH. This procedure requires thoroughly cleansing the colon first, as well as an experienced colonoscopist who can identify and treat TIC SRH to obliterate arterial blood flow underneath SRH and achieve definitive TIC hemostasis. Other approaches to early diagnosis include nuclear medicine scintigraphy or angiography (CT, MRI, or IR). However, these techniques can only detect active bleeding which is documented in only 26% of colonoscopically diagnosed definitive TIC hemorrhages. So, the expected diagnostic yield of these tests will be low. When urgent colonoscopy fails to make a diagnosis or TIC bleeding continues, TAE and/or surgery are recommended. After definitive hemostasis of TIC hemorrhage and for long term management, control of constipation and discontinuation of chronic NSAIDs and antiplatelet drugs (if possible) are recommended to prevent recurrent TIC hemorrhage.
Dr. Cusumano and Dr. Paiji are fellow physicians in the Vatche and Tamar Manoukian Division of Digestive Diseases at University of California Los Angeles. Dr. Jensen is a professor of medicine in Vatche and Tamar Manoukian Division of Digestive Diseases and is with the CURE Digestive Diseases Research Center at the VA Greater Los Angeles Healthcare System, Calif. All authors declare that they have no competing interests or disclosures.
References
1. Longstreth GF. Am J Gastroenterol. 1997;92(3):419-24.
2. Jensen DM et al. The New England Journal of Medicine. 2000;342(2):78-82.
3. Jensen DM et al. Techniques in Gastrointestinal Endoscopy. 2001;3(4):192-8.
4. Jensen DM. Am J Gastroenterol. 2018;113(11):1570-3.
5. Zuckerman GR et al. Gastrointestinal Endoscopy. 1999;49(2):228-38.
6. Stollman N et al. Lancet. 2004;363(9409):631-9.
7. McGuire HH et al. Ann Surg. 1994;220(5):653-6.
8. McGuire HH et al. Ann Surg. 1972;175(6):847-55.
9. Strate LL et al. Clinical gastroenterology and hepatol. 2008;6(9):1004-10.
10. Jensen DM et al. Gastrointestinal endoscopy. 2016;83(2):416-23.
11. Jensen DM et al. Gastrointest Endosc Clin N Am. 1997;7(3):477-98.
12. Maykel JA et al. Clin Colon Rectal Surg. 2004;17(3):195-204.
13. Green BT et al. Am J Gastroenterol. 2005;100(11):2395-402.
14. Niikura R et al. Journal of Clinical Gastroenterol. 2015;49(3):e24-30.
15. Bloomfeld RS et al. Am J Gastroenterol. 2001;96(8):2367-72.
16. Parsi MA,et al. VideoGIE. 2019;4(7):285-99.
17. Kaltenbach T et al. Clinical Gastroenterology and Hepatol. 2012;10(2):131-7.
18. Nakano K et al. Endosc Int Open. 2015;3(5):E529-33.
19. Barker KB et al. Gastrointestinal Endoscopy. 2005;62(2):224-7.
20. Kaltenbach T et al. Gastrointest Endosc Clin N Am. 2020;30(1):13-23.
21. Yamazaki K et al. VideoGIE. 2020;5(6):252-4.
22. Strate LL et al. Clinical Gastroenterology and Hepatol. 2010;8(4):333-43.
23. Evangelista et al. J Vasc Interv Radiol. 2000;11(5):601-6.
24. Kodani M et al. J Vasc Interv Radiol. 2016;27(6):824-30.
25. Mohammed et al. Clin Colon Rectal Surg. 2018;31(4):243-50.
26. Wongpongsalee T et al. Gastrointestinal Endoscopy. 2020;91(6):AB471-2.
27. Böhm SK. Viszeralmedizin. 2015;31(2):84-94.
28. Prakash C et al. Endoscopy. 1999;31(6):460-3.
29. Yen EF et al. Digestive Diseases and Sciences. 2008;53(9):2480-5.
30. Ishii N et al. Gastrointestinal Endoscopy. 2012;75(2):382-7.
31. Nagata N et al. Gastrointestinal Endoscopy. 2018;88(5):841-53.e4.
32. Parkes BM et al. Am Surg. 1993;59(10):676-8.
33. Vajravelu RK et al. Gastroenterology. 2018;155(5):1416-27.
34. Oakland K et al. Clin Gastroenterol Hepatol. 2019;17(7):1276-84.e3.
35. Yamada A et al. Dis Colon Rectum. 2008;51(1):116-20.
36. Coleman CI et al. Int J Clin Pract. 2012;66(1):53-63.
37. Holster IL et al. Gastroenterology. 2013;145(1):105-12.e15.
Diverticular hemorrhage is the most common cause of colonic bleeding, accounting for 20%-65% of cases of severe lower intestinal bleeding in adults.1 Urgent colonoscopy after purging the colon of blood, clots, and stool is the most accurate method of diagnosing and guiding treatment of definitive diverticular hemorrhage.2-5 The diagnosis of definitive diverticular hemorrhage depends upon identification of some stigmata of recent hemorrhage (SRH) in a single diverticulum (TIC), which can include active arterial bleeding, oozing, non-bleeding visible vessel, adherent clot, or flat spot.2-4 Although other approaches, such as nuclear medicine scans and angiography of various types (CT, MRI, or standard angiography), for the early diagnosis of patients with severe hematochezia are utilized in many medical centers, only active bleeding can be detected by these techniques. However, as subsequently discussed, this SRH is documented in only 26% of definitive diverticular bleeds found on urgent colonoscopy, so diagnostic yields of these techniques will be low.2-5
The diagnosis of patients with severe hematochezia and diverticulosis, as well as triage of all of them to specific medical, endoscopic, radiologic, or surgical management, is facilitated by an urgent endoscopic approach.2-5 Patients who are diagnosed with definitive diverticular hemorrhage on colonoscopy represent about 30% of all true TIC bleeds when urgent colonoscopy is the management approach.2-5 That is because approximately 50% of all patients with colon diverticulosis and first presentation of severe hematochezia have incidental diverticulosis; they have colonic diverticulosis, but another site of bleeding is identified as the cause of hemorrhage in the gastrointestinal tract.2-4 Presumptive diverticular hemorrhage is diagnosed when colonic diverticulosis without TIC stigmata are found but no other GI bleeding source is found on colonoscopy, anoscopy, enteroscopy, or capsule endoscopy.2-5 In our experience with urgent colonoscopy, the presumptive diverticular bleed group accounts for about 70% of patients with documented diverticular hemorrhage (e.g., not including incidental diverticulosis bleeds but combining subgroups of patients with either definitive or presumptive TIC diagnoses as documented TIC hemorrhage).
Clinical presentation
Patients with diverticular hemorrhage present with severe, painless large volume hematochezia. Hematochezia may be self-limited and spontaneously resolve in 75%-80% of all patients but with high rebleeding rates up to 40%.5-7 Of all patients with diverticulosis, only about 3%-5% develop diverticular hemorrhage.8 Risk factors for diverticular hemorrhage include medications (e.g., nonsteroidal anti-inflammatory drugs – NSAIDs, antiplatelet drugs, and anticoagulants) and other clinical factors, such as older age, low-fiber diet, and chronic constipation.9,10 On urgent colonoscopy, more than 70% of diverticulosis in U.S. patients are located anatomically in the descending colon or more distally. In contrast, about 60% of definitive diverticular hemorrhage cases in our experience had diverticula with stigmata identified at or proximal to the splenic flexure.2,4,11
Pathophysiology
Colonic diverticula are herniations of mucosa and submucosa with colonic arteries that penetrate the muscular wall. Bleeding can occur when there is asymmetric rupture of the vasa recta at either the base of the diverticulum or the neck.4 Thinning of the mucosa on the luminal surface (such as that resulting from impacted fecaliths and stool) can cause injury to the site of the penetrating vessels, resulting in hemorrhage.12
Initial management
Patients with acute, severe hematochezia should be triaged to an inpatient setting with a monitored bed. Admission to an intensive care unit should be considered for patients with hemodynamic instability, persistent bleeding, and/or significant comorbidities. Patients with TIC hemorrhage often require resuscitation with crystalloids and packed red blood cell transfusions for hemoglobin less than 8 g/dl.4 Unlike upper GI hemorrhage, which has been extensively reported on, data regarding a more restrictive transfusion threshold, compared with a liberal transfusion threshold, in lower intestinal bleeding are very limited. Correction of underlying coagulopathies is recommended but should be individualized, particularly in those patients on antithrombotic agents or with underlying bleeding disorders.
Urgent diagnosis and hemostasis
Urgent colonoscopy within 24 hours is the most accurate way to make a diagnosis of definitive diverticular hemorrhage and to effectively and safely treat them.2-4,10,11 For patients with severe hematochezia, when the colonoscopy is either not available in a medical center or does not reveal the source of bleeding, nuclear scintigraphy or angiography (CT, MRI, or interventional radiology [IR]) are recommended. CT angiography may be particularly helpful to diagnose patients with hemodynamic instability who are suspected to have active TIC bleeding and are not able to complete a bowel preparation. However, these imaging techniques require active bleeding at the time of the study to be diagnostic. This SRH is also uncommon for definitive diverticular hemorrhage, so the diagnostic yield is usually quite low.2-5,10,11 An additional limitation of scintigraphy and CT or MRI angiography is that, if active bleeding is found, some other type of treatment, such as colonoscopy, IR angiography, or surgery, will be required for definitive hemostasis.
For urgent colonoscopy, adequate colon preparation with a large volume preparation (6-8 liters of polyethylene glycol-based solution) is recommended to clear stool, blood, and clots to allow endoscopic visualization and localization of the bleeding source. Use of a nasogastric tube should be considered if the patient is unable to drink enough prep.2-4,13 Additionally, administration of a prokinetic agent, such as Metoclopramide, may improve gastric emptying and tolerance of the prep. During colonoscopy, careful inspection of the colonic mucosa during insertion and withdrawal is important since lesions may bleed intermittently and SRH can be missed. An adult or pediatric colonoscope with a large working channel (at least 3.3 mm) is recommended to facilitate suctioning of blood clots and stool, as well as allow the passage of endoscopic hemostasis accessories. Targeted water-jet irrigation, an expert colonoscopist, a cap attachment, and adequate colon preparation are all predictors for improved diagnosis of definitive diverticular hemorrhage.4,14
SRH in definitive TIC bleeds all have a high risk of TIC rebleeding,2-4,10,11 including active bleeding, nonbleeding visible vessel, adherent clot, and a flat spot (See Figure).

Based on CURE Hemostasis Group data of 118 definitive TIC bleeds, 26% had active bleeding, 24% had a nonbleeding visible vessel, 37% had an adherent clot, and 13% had a flat spot (with underlying arterial blood flow by Doppler probe monitoring).4 Approximately 50% of the SRH were found in the neck of the TIC and 50% at the base, with actively bleeding cases more often from the base. In CURE Doppler endoscopic probe studies, 90% of all stigmata had an underlying arterial blood flow detected with the Doppler probe.4,10 The Doppler probe is reported to be very useful for risk stratification and to confirm obliteration of the arterial blood flow underlying SRH for definitive hemostasis.4,10
Endoscopic treatment
Given high rates of rebleeding with medical management alone, definitive TIC hemorrhage can be effectively and safely treated with endoscopic therapies once SRH are localized.4,10 Endoscopic therapies that have been reported in the literature include electrocoagulation, hemoclip, band ligation, and over-the-scope clip. Four-quadrant injection of 1:20,000 epinephrine around the SRH can improve visualization of SRH and provide temporary control of bleeding, but it should be combined with other modalities because of risk of rebleeding with epinephrine alone.15 Results from studies reporting rates of both early rebleeding (occurring within 30 days) and late rebleeding (occurring after 30 days) are listed in the Table. 
Multipolar electrocoagulation (MPEC), which utilizes a focal electric current to generate heat, can coaptively coagulate small TIC arteries.16 For SRH in the neck of TIC, MPEC is effective for coaptive coagulation at a power of 12-15 watts in 1-2 second pulses with moderate laterally applied tamponade pressure. MPEC should be avoided for treating SRH at the TIC base because of lack of muscularis propria and higher risk of perforation.
Hemoclip therapy has been reported to be safe and efficacious in treatment of definitive TIC hemorrhage, by causing mechanical hemostasis with occlusion of the bleeding artery.16 Hemoclips are recommended to treat stigmata in the base of TICs and should be targeted on either side of visible vessel in order to occlude the artery underneath it.4,10 With a cap on the tip of the colonoscope, suctioning can evert TICs, allowing more precise placement of hemoclip on SRH in the base of the TIC.17 Hemoclip retention rates vary with different models and can range from less than 7 days to more than 4 weeks. Hemoclips can also mark the site if early rebleeding occurs; then, reintervention (e.g., repeat endoscopy or angioembolization) is facilitated.
Another treatment is endoscopic band ligation, which provides mechanical hemostasis. Endoscopic band ligation has been reported to be efficacious for TIC hemorrhage.18 Suctioning the TIC with the SRH into the distal cap and deploying a band leads to obliteration of vessels and potentially necrosis and disappearance of banded TIC.16 This technique carries a risk of perforation because of the thin walls of TICs. This risk may be higher for right-sided colon lesions since an exvivo colon specimen study reported serosal entrapment and inclusion of muscularis propria postband ligation, both of which may result in ischemia of intestinal wall and delayed perforation.19
Over-the-scope clip (OTSC) has been reported in case series for treatment of definitive TIC hemorrhage. With a distal cap and large clip, suctioning can evert TICs and facilitate deployment over the SRH.20,21 OTSC can grasp an entire TIC with the SRH and obliterate the arterial blood flow with a single clip.20,21 No complications have been reported yet for treatment of TIC hemorrhage. However, the OTSC system is relatively expensive when compared with other modalities.
After endoscopic treatment is performed, four-quadrant spot tattooing is recommended adjacent to the TIC with the SRH. This step will facilitate localization and treatment in the case of TIC rebleeding.4,10
Outcomes following endoscopic treatment
Following endoscopic treatment, patients should be monitored for early and late rebleeding. In a pooled analysis of case series composed of 847 patients with TIC bleeding, among the 137 patients in which endoscopic hemostasis was initially achieved, early rebleeding occurred in 8% and late rebleeding occurred in 12% of patients.22 Risk factors for TIC rebleeding within 30 days were residual arterial blood flow following hemostasis and early reinitiation of antiplatelet agents.
Remote treatment of TIC hemorrhage distant from the SRH is a significant risk factor for early TIC rebleeding.4, 10 For example, using hemoclips to close the mouth of a TIC when active bleeding or an SRH is located in the TIC base often fails because arterial flow remains open in the base and the artery is larger there.4,10 This example highlights the importance of focal obliteration of arterial blood flow underlying SRH in order to achieve definitive hemostasis.4,10
Salvage treatments
For TIC hemorrhage that is not controlled by endoscopic therapy, transcatheter arterial embolization (TAE) is recommended. If bleeding rate is high enough (at least 0.5 milliliters per minute) to be detected by angiography, TAE can serve as an effective method of diagnosis and immediate hemostasis.23 However, the most common major complication of embolization is intestinal ischemia. The incidence of intestinal ischemia has been reported as high as 10%, with highest risk with embolization of at least three vasa recta.24
Surgery is also recommended if TIC hemorrhage cannot be controlled with endoscopic therapy or TAE. Segmental colectomy is recommended if the bleeding site can be localized before surgery with colonoscopy or angiography resulting from significantly lower perioperative morbidity than subtotal colectomy.25 However, subtotal colectomy may be necessary if preoperative localization of bleeding is unsuccessful.
There are very few reports of short- or long-term results that compare endoscopy, TAE, and surgery for management of TIC bleeding. However, a recent retrospective study reported better outcomes with endoscopic treatment of definitive TIC bleeding.26 Patients who underwent endoscopic treatment had fewer RBC transfusions, shorter hospitalizations, and lower rates of postprocedure complications.
Management after cessation of hemorrhage
Medical management is important following an episode of TIC hemorrhage. A mainstay is daily fiber supplementation every morning and stool softener in the evening. Furthermore, patients are advised to drink an extra liter of fluids (not containing alcohol or caffeine) daily. By reducing colon transit time and increasing stool weight, these measures can help control constipation and prevent future complications of TIC disease.27
Patients with recurrent TIC hemorrhage should undergo evaluation for elective surgery, provided they are appropriate surgical candidates. If preoperative localization of bleeding site is successful, segmental colectomy is preferred. Segmental resection is associated with significantly decreased rebleeding rate, with lower rates of morbidity compared with subtotal colectomy.32
Chronic NSAIDs, aspirin, and antiplatelet drugs are risk factors for recurrent TIC hemorrhage, and avoiding these medications is recommended if possible.33,34 Although anticoagulants have shown to be associated with increased risk of all-cause gastrointestinal bleeding, these agents have not been shown to increase risk of recurrent TIC hemorrhage in recent large retrospective studies. Since antiplatelet and anticoagulation agents serve to reduce risk of thromboembolic events, the clinician who recommended these medications should be consulted after a TIC bleed to re-evaluate whether these medications can be discontinued or reduced in dose.
Conclusion
The most effective way to diagnose and treat definitive TIC hemorrhage is to perform an urgent colonoscopy within 24 hours to identify and treat TIC SRH. This procedure requires thoroughly cleansing the colon first, as well as an experienced colonoscopist who can identify and treat TIC SRH to obliterate arterial blood flow underneath SRH and achieve definitive TIC hemostasis. Other approaches to early diagnosis include nuclear medicine scintigraphy or angiography (CT, MRI, or IR). However, these techniques can only detect active bleeding which is documented in only 26% of colonoscopically diagnosed definitive TIC hemorrhages. So, the expected diagnostic yield of these tests will be low. When urgent colonoscopy fails to make a diagnosis or TIC bleeding continues, TAE and/or surgery are recommended. After definitive hemostasis of TIC hemorrhage and for long term management, control of constipation and discontinuation of chronic NSAIDs and antiplatelet drugs (if possible) are recommended to prevent recurrent TIC hemorrhage.
Dr. Cusumano and Dr. Paiji are fellow physicians in the Vatche and Tamar Manoukian Division of Digestive Diseases at University of California Los Angeles. Dr. Jensen is a professor of medicine in Vatche and Tamar Manoukian Division of Digestive Diseases and is with the CURE Digestive Diseases Research Center at the VA Greater Los Angeles Healthcare System, Calif. All authors declare that they have no competing interests or disclosures.
References
1. Longstreth GF. Am J Gastroenterol. 1997;92(3):419-24.
2. Jensen DM et al. The New England Journal of Medicine. 2000;342(2):78-82.
3. Jensen DM et al. Techniques in Gastrointestinal Endoscopy. 2001;3(4):192-8.
4. Jensen DM. Am J Gastroenterol. 2018;113(11):1570-3.
5. Zuckerman GR et al. Gastrointestinal Endoscopy. 1999;49(2):228-38.
6. Stollman N et al. Lancet. 2004;363(9409):631-9.
7. McGuire HH et al. Ann Surg. 1994;220(5):653-6.
8. McGuire HH et al. Ann Surg. 1972;175(6):847-55.
9. Strate LL et al. Clinical gastroenterology and hepatol. 2008;6(9):1004-10.
10. Jensen DM et al. Gastrointestinal endoscopy. 2016;83(2):416-23.
11. Jensen DM et al. Gastrointest Endosc Clin N Am. 1997;7(3):477-98.
12. Maykel JA et al. Clin Colon Rectal Surg. 2004;17(3):195-204.
13. Green BT et al. Am J Gastroenterol. 2005;100(11):2395-402.
14. Niikura R et al. Journal of Clinical Gastroenterol. 2015;49(3):e24-30.
15. Bloomfeld RS et al. Am J Gastroenterol. 2001;96(8):2367-72.
16. Parsi MA,et al. VideoGIE. 2019;4(7):285-99.
17. Kaltenbach T et al. Clinical Gastroenterology and Hepatol. 2012;10(2):131-7.
18. Nakano K et al. Endosc Int Open. 2015;3(5):E529-33.
19. Barker KB et al. Gastrointestinal Endoscopy. 2005;62(2):224-7.
20. Kaltenbach T et al. Gastrointest Endosc Clin N Am. 2020;30(1):13-23.
21. Yamazaki K et al. VideoGIE. 2020;5(6):252-4.
22. Strate LL et al. Clinical Gastroenterology and Hepatol. 2010;8(4):333-43.
23. Evangelista et al. J Vasc Interv Radiol. 2000;11(5):601-6.
24. Kodani M et al. J Vasc Interv Radiol. 2016;27(6):824-30.
25. Mohammed et al. Clin Colon Rectal Surg. 2018;31(4):243-50.
26. Wongpongsalee T et al. Gastrointestinal Endoscopy. 2020;91(6):AB471-2.
27. Böhm SK. Viszeralmedizin. 2015;31(2):84-94.
28. Prakash C et al. Endoscopy. 1999;31(6):460-3.
29. Yen EF et al. Digestive Diseases and Sciences. 2008;53(9):2480-5.
30. Ishii N et al. Gastrointestinal Endoscopy. 2012;75(2):382-7.
31. Nagata N et al. Gastrointestinal Endoscopy. 2018;88(5):841-53.e4.
32. Parkes BM et al. Am Surg. 1993;59(10):676-8.
33. Vajravelu RK et al. Gastroenterology. 2018;155(5):1416-27.
34. Oakland K et al. Clin Gastroenterol Hepatol. 2019;17(7):1276-84.e3.
35. Yamada A et al. Dis Colon Rectum. 2008;51(1):116-20.
36. Coleman CI et al. Int J Clin Pract. 2012;66(1):53-63.
37. Holster IL et al. Gastroenterology. 2013;145(1):105-12.e15.
Immune response detected in most IBD patients after COVID vaccines
Most patients with inflammatory bowel disease (IBD) develop a humoral immune response after completing an mRNA SARS-CoV-2 vaccine series, according to data from almost 800 patients.
Anti–receptor binding domain IgG antibodies specific to SARS-CoV-2 were detectable in 95% of patients, with “generally similar” results across vaccine type, age group, and medication class, apart from corticosteroid users, who had an 86% antibody detection rate, reported lead author Kimberly N. Weaver, MD, of the University of North Carolina at Chapel Hill, and colleagues.
“Patients with IBD on immunosuppressive medications have the potential for attenuated response to the SARS-CoV-2 vaccination,” Dr. Weaver said at the annual meeting of the American College of Gastroenterology.
In support of this possibility, Dr. Weaver cited two recent trials from earlier in 2021: one demonstrated blunted antibody responses in IBD patients taking infliximab, while the other showed that full vaccination was less effective at preventing SARS-CoV-2 infection among patients with IBD than nonimmunosuppressed individuals.
To better characterize antibody responses after receiving an mRNA vaccination series, Dr. Weaver and colleagues launched the PREVENT-COVID trial, including the present dataset of 787 patients with IBD older than 12 years, all of whom provided serum samples 8 weeks after completing an mRNA vaccine series. Patients with positive nucleocapsid antibody (indicating prior infection), and/or those who reported prior COVID-19 infection, were excluded. Most patients were White (95%) and female (73%), with an average age of 48 years. Slightly more patients received the BNT162b2 vaccine than the mRNA-1273 vaccine (58% vs. 42%).
At 8 weeks, 752 out of 787 patients had detectable antibodies (95%). Antibody rates were highest among patients receiving vedolizumab monotherapy (n = 83; 99%) or ustekinumab monotherapy (n = 102; 99%), followed by mercaptopurine, azathioprine, or methotrexate monotherapy (n = 67; 97%); anti–tumor necrosis factor monotherapy (n = 270; 96%); mesalamine, sulfasalazine, or budesonide monotherapy or no medication (n = 143; 95%); and finally anti-TNF/immunosuppressive combination therapy (n = 75; 86%). Median and mean antibody titers were lowest for anti-TNF combination therapy and highest for vedolizumab.
Thirty-five patients taking corticosteroids had an antibody detection rate of 85.7% (95% CI, 70.6-93.7), compared with 95.9% (95% CI, 94.2-97.1) among nonsteroid users. In contrast, antibody detection rates were not significantly affected by age or vaccine type.
“Reassuringly, most IBD medications do not prevent an initial antibody response after SARS-CoV-2 vaccination, and this is unlike other classes of immune suppression such as B-cell depletion therapy,” Dr. Weaver concluded. “Additional data are forthcoming on a larger subset of participants in the PREVENT-COVID study which will allow for analysis of factors associated with humoral immune response and potential optimization of immunization strategies.” She described a dataset of about 500 IBD patients in which booster vaccines overcame poor antibody responses to the initial vaccine series.
‘The data we need’
Serre-yu Wong, MD, PhD, of Icahn School of Medicine at Mount Sinai, New York, agreed that the findings should offer some reassurance to patients with IBD and their care providers.
“At the end of the day we have really nice seroconversion rates for the IBD population,” Dr. Wong said.
In April 2021, Dr. Wong and the ICARUS-IBD Working Group published a similar report of 48 patients with IBD receiving biologic therapies, among whom the seroconversion rate was 100%.
“A lot of the early data, including ours, are on infusion medications, and that’s sort of a practical thing because those were the only patients we could get samples from, but [Dr. Weaver and colleagues] were able to get samples from patients not on medications, on oral medications, and on other injection medications that people can take at home, and these are really the data we need for all of our other IBD patients,” Dr. Wong said.
Dr. Wong highlighted that both trials showed some IBD patients generating “very, very high” titers, many of them above the threshold needed for donating convalescent plasma for COVID-19 treatment; still, exact titer levels needed to protect against SARS-CoV-2 infection remain unclear.
“This is going to require longitudinal studies,” Dr. Wong said. “We can’t answer that perfectly right now. We don’t know the magic level of antibodies. I don’t know if you need a titer of 1:100 or 1:1,000.”
Although postvaccination antibody testing is not recommended by the Centers for Disease Control and Prevention, Dr. Wong said that “many patients” check their titers anyway, leading to anxiety if antibodies are low or undetectable.
“I know that it’s very disconcerting sometimes when you don’t see an antibody response, and this is one of the hardest things to try to explain to patients,” Dr. Wong said. “[It’s necessary] to have a frank discussion about the fact that we don’t know the magic level of antibodies, and that there are also other parts of the immune system that we haven’t tested with antibodies. We haven’t tested the T-cell response, and we do know you can have a T-cell response even if you don’t have a B-cell response.”
Dr. Wong suggested that more work is needed to determine the impact of the IBD disease process on susceptibility to SARS-CoV-2 infection, and the rates of antibody responses for the various other vaccines being used around the world.
The PREVENT-COVID study was supported by the Leona M. and Harry B. Helmsley Charitable Trust. The investigators disclosed additional relationships with AbbVie, Johnson & Johnson, Genentech, and others. Dr. Wong reported no relevant conflicts of interest.
This article was updated Oct. 28, 2021.
Most patients with inflammatory bowel disease (IBD) develop a humoral immune response after completing an mRNA SARS-CoV-2 vaccine series, according to data from almost 800 patients.
Anti–receptor binding domain IgG antibodies specific to SARS-CoV-2 were detectable in 95% of patients, with “generally similar” results across vaccine type, age group, and medication class, apart from corticosteroid users, who had an 86% antibody detection rate, reported lead author Kimberly N. Weaver, MD, of the University of North Carolina at Chapel Hill, and colleagues.
“Patients with IBD on immunosuppressive medications have the potential for attenuated response to the SARS-CoV-2 vaccination,” Dr. Weaver said at the annual meeting of the American College of Gastroenterology.
In support of this possibility, Dr. Weaver cited two recent trials from earlier in 2021: one demonstrated blunted antibody responses in IBD patients taking infliximab, while the other showed that full vaccination was less effective at preventing SARS-CoV-2 infection among patients with IBD than nonimmunosuppressed individuals.
To better characterize antibody responses after receiving an mRNA vaccination series, Dr. Weaver and colleagues launched the PREVENT-COVID trial, including the present dataset of 787 patients with IBD older than 12 years, all of whom provided serum samples 8 weeks after completing an mRNA vaccine series. Patients with positive nucleocapsid antibody (indicating prior infection), and/or those who reported prior COVID-19 infection, were excluded. Most patients were White (95%) and female (73%), with an average age of 48 years. Slightly more patients received the BNT162b2 vaccine than the mRNA-1273 vaccine (58% vs. 42%).
At 8 weeks, 752 out of 787 patients had detectable antibodies (95%). Antibody rates were highest among patients receiving vedolizumab monotherapy (n = 83; 99%) or ustekinumab monotherapy (n = 102; 99%), followed by mercaptopurine, azathioprine, or methotrexate monotherapy (n = 67; 97%); anti–tumor necrosis factor monotherapy (n = 270; 96%); mesalamine, sulfasalazine, or budesonide monotherapy or no medication (n = 143; 95%); and finally anti-TNF/immunosuppressive combination therapy (n = 75; 86%). Median and mean antibody titers were lowest for anti-TNF combination therapy and highest for vedolizumab.
Thirty-five patients taking corticosteroids had an antibody detection rate of 85.7% (95% CI, 70.6-93.7), compared with 95.9% (95% CI, 94.2-97.1) among nonsteroid users. In contrast, antibody detection rates were not significantly affected by age or vaccine type.
“Reassuringly, most IBD medications do not prevent an initial antibody response after SARS-CoV-2 vaccination, and this is unlike other classes of immune suppression such as B-cell depletion therapy,” Dr. Weaver concluded. “Additional data are forthcoming on a larger subset of participants in the PREVENT-COVID study which will allow for analysis of factors associated with humoral immune response and potential optimization of immunization strategies.” She described a dataset of about 500 IBD patients in which booster vaccines overcame poor antibody responses to the initial vaccine series.
‘The data we need’
Serre-yu Wong, MD, PhD, of Icahn School of Medicine at Mount Sinai, New York, agreed that the findings should offer some reassurance to patients with IBD and their care providers.
“At the end of the day we have really nice seroconversion rates for the IBD population,” Dr. Wong said.
In April 2021, Dr. Wong and the ICARUS-IBD Working Group published a similar report of 48 patients with IBD receiving biologic therapies, among whom the seroconversion rate was 100%.
“A lot of the early data, including ours, are on infusion medications, and that’s sort of a practical thing because those were the only patients we could get samples from, but [Dr. Weaver and colleagues] were able to get samples from patients not on medications, on oral medications, and on other injection medications that people can take at home, and these are really the data we need for all of our other IBD patients,” Dr. Wong said.
Dr. Wong highlighted that both trials showed some IBD patients generating “very, very high” titers, many of them above the threshold needed for donating convalescent plasma for COVID-19 treatment; still, exact titer levels needed to protect against SARS-CoV-2 infection remain unclear.
“This is going to require longitudinal studies,” Dr. Wong said. “We can’t answer that perfectly right now. We don’t know the magic level of antibodies. I don’t know if you need a titer of 1:100 or 1:1,000.”
Although postvaccination antibody testing is not recommended by the Centers for Disease Control and Prevention, Dr. Wong said that “many patients” check their titers anyway, leading to anxiety if antibodies are low or undetectable.
“I know that it’s very disconcerting sometimes when you don’t see an antibody response, and this is one of the hardest things to try to explain to patients,” Dr. Wong said. “[It’s necessary] to have a frank discussion about the fact that we don’t know the magic level of antibodies, and that there are also other parts of the immune system that we haven’t tested with antibodies. We haven’t tested the T-cell response, and we do know you can have a T-cell response even if you don’t have a B-cell response.”
Dr. Wong suggested that more work is needed to determine the impact of the IBD disease process on susceptibility to SARS-CoV-2 infection, and the rates of antibody responses for the various other vaccines being used around the world.
The PREVENT-COVID study was supported by the Leona M. and Harry B. Helmsley Charitable Trust. The investigators disclosed additional relationships with AbbVie, Johnson & Johnson, Genentech, and others. Dr. Wong reported no relevant conflicts of interest.
This article was updated Oct. 28, 2021.
Most patients with inflammatory bowel disease (IBD) develop a humoral immune response after completing an mRNA SARS-CoV-2 vaccine series, according to data from almost 800 patients.
Anti–receptor binding domain IgG antibodies specific to SARS-CoV-2 were detectable in 95% of patients, with “generally similar” results across vaccine type, age group, and medication class, apart from corticosteroid users, who had an 86% antibody detection rate, reported lead author Kimberly N. Weaver, MD, of the University of North Carolina at Chapel Hill, and colleagues.
“Patients with IBD on immunosuppressive medications have the potential for attenuated response to the SARS-CoV-2 vaccination,” Dr. Weaver said at the annual meeting of the American College of Gastroenterology.
In support of this possibility, Dr. Weaver cited two recent trials from earlier in 2021: one demonstrated blunted antibody responses in IBD patients taking infliximab, while the other showed that full vaccination was less effective at preventing SARS-CoV-2 infection among patients with IBD than nonimmunosuppressed individuals.
To better characterize antibody responses after receiving an mRNA vaccination series, Dr. Weaver and colleagues launched the PREVENT-COVID trial, including the present dataset of 787 patients with IBD older than 12 years, all of whom provided serum samples 8 weeks after completing an mRNA vaccine series. Patients with positive nucleocapsid antibody (indicating prior infection), and/or those who reported prior COVID-19 infection, were excluded. Most patients were White (95%) and female (73%), with an average age of 48 years. Slightly more patients received the BNT162b2 vaccine than the mRNA-1273 vaccine (58% vs. 42%).
At 8 weeks, 752 out of 787 patients had detectable antibodies (95%). Antibody rates were highest among patients receiving vedolizumab monotherapy (n = 83; 99%) or ustekinumab monotherapy (n = 102; 99%), followed by mercaptopurine, azathioprine, or methotrexate monotherapy (n = 67; 97%); anti–tumor necrosis factor monotherapy (n = 270; 96%); mesalamine, sulfasalazine, or budesonide monotherapy or no medication (n = 143; 95%); and finally anti-TNF/immunosuppressive combination therapy (n = 75; 86%). Median and mean antibody titers were lowest for anti-TNF combination therapy and highest for vedolizumab.
Thirty-five patients taking corticosteroids had an antibody detection rate of 85.7% (95% CI, 70.6-93.7), compared with 95.9% (95% CI, 94.2-97.1) among nonsteroid users. In contrast, antibody detection rates were not significantly affected by age or vaccine type.
“Reassuringly, most IBD medications do not prevent an initial antibody response after SARS-CoV-2 vaccination, and this is unlike other classes of immune suppression such as B-cell depletion therapy,” Dr. Weaver concluded. “Additional data are forthcoming on a larger subset of participants in the PREVENT-COVID study which will allow for analysis of factors associated with humoral immune response and potential optimization of immunization strategies.” She described a dataset of about 500 IBD patients in which booster vaccines overcame poor antibody responses to the initial vaccine series.
‘The data we need’
Serre-yu Wong, MD, PhD, of Icahn School of Medicine at Mount Sinai, New York, agreed that the findings should offer some reassurance to patients with IBD and their care providers.
“At the end of the day we have really nice seroconversion rates for the IBD population,” Dr. Wong said.
In April 2021, Dr. Wong and the ICARUS-IBD Working Group published a similar report of 48 patients with IBD receiving biologic therapies, among whom the seroconversion rate was 100%.
“A lot of the early data, including ours, are on infusion medications, and that’s sort of a practical thing because those were the only patients we could get samples from, but [Dr. Weaver and colleagues] were able to get samples from patients not on medications, on oral medications, and on other injection medications that people can take at home, and these are really the data we need for all of our other IBD patients,” Dr. Wong said.
Dr. Wong highlighted that both trials showed some IBD patients generating “very, very high” titers, many of them above the threshold needed for donating convalescent plasma for COVID-19 treatment; still, exact titer levels needed to protect against SARS-CoV-2 infection remain unclear.
“This is going to require longitudinal studies,” Dr. Wong said. “We can’t answer that perfectly right now. We don’t know the magic level of antibodies. I don’t know if you need a titer of 1:100 or 1:1,000.”
Although postvaccination antibody testing is not recommended by the Centers for Disease Control and Prevention, Dr. Wong said that “many patients” check their titers anyway, leading to anxiety if antibodies are low or undetectable.
“I know that it’s very disconcerting sometimes when you don’t see an antibody response, and this is one of the hardest things to try to explain to patients,” Dr. Wong said. “[It’s necessary] to have a frank discussion about the fact that we don’t know the magic level of antibodies, and that there are also other parts of the immune system that we haven’t tested with antibodies. We haven’t tested the T-cell response, and we do know you can have a T-cell response even if you don’t have a B-cell response.”
Dr. Wong suggested that more work is needed to determine the impact of the IBD disease process on susceptibility to SARS-CoV-2 infection, and the rates of antibody responses for the various other vaccines being used around the world.
The PREVENT-COVID study was supported by the Leona M. and Harry B. Helmsley Charitable Trust. The investigators disclosed additional relationships with AbbVie, Johnson & Johnson, Genentech, and others. Dr. Wong reported no relevant conflicts of interest.
This article was updated Oct. 28, 2021.
AT ACG 2021
A pill for C. difficile works by increasing microbiome diversity
CP101, under development by Finch Therapeutics, proved more effective than a placebo in preventing recurrent infections for up to 24 weeks.
The CP101 capsules contain a powder of freeze-dried human stools from screened donors. They restore natural diversity that has been disrupted by antibiotics, said Jessica Allegretti, MD, MPH a gastroenterologist at Brigham and Women’s Hospital in Boston.
The treatment offers an alternative to fecal microbiota transplant, which can effectively treat antibiotic-resistant C. difficile infections but is difficult to standardize and administer – and doesn’t have full approval from the U.S. Food and Drug Administration, she added.
“I think this marks a moment in this space where we’re going to have better, safer, and more available options for patients,” she said in an interview. “It’s exciting.”
Dr. Allegretti is an author on three presentations of results from PRISM3, a phase 2 trial of CP101. They will be presented this week at the annual meeting of the American College of Gastroenterology. These results extend out to 24 weeks, whereas the 8-week results of this trial were presented a year ago at the same meeting.
Study details
The study enrolled 198 people who received antibiotics for recurrent C. difficile infections. Some patients had two or more recurrences, while others had only one recurrence but were 65 years of age or older.
“That was a unique aspect of this study, to see the effect of bringing a therapy like CP101 earlier in the treatment paradigm,” said Dr. Allegretti. “You can imagine for an older, frail, or more fragile patient that you would want to get rid of this [infection] earlier.”
After waiting 2-6 days for the antibiotics to wash out, the researchers randomly assigned 102 of these patients to take the CP101 pills orally and 96 to take placebo pills, both without bowel preparation.
The two groups were not significantly different in age, gender, comorbidities, the number of C. difficile recurrences, or the type of test used to diagnose the infection (PCR-based vs. toxin EIA-based).
After 8 weeks, 74.5% of those given the CP101 pills had not had a recurrence, compared with 61.5% of those given the placebo. The difference was just barely statistically significant (P = .0488).
Sixteen weeks later, the effect endured, with 73.5% of the CP101 group and 59.4% of the placebo group still free of recurrence. The statistical significance of the difference improved slightly (P = .0347).
Drug-related emergent adverse events were similar between the two groups: 16.3% for the CP101 group vs. 19.2% for the placebo group. These were mostly gastrointestinal symptoms, and none were serious.
Some of the patients received vancomycin as a first-line treatment for C. difficile infections, and the researchers wondered if the washout period was not sufficient to purge that antibiotic, leaving enough to interfere with the effectiveness of CP101.
Therefore, they separately analyzed 40 patients treated with fidaxomicin, which they expected to wash out more quickly. Among these patients, 81% who received CP101 were free of recurrences, at 8 weeks and 24 weeks. This compared with 42.1% of those who received the placebo, at both time points. This difference was more statistically significant (P = .0211).
Understanding how it works
To understand better how CP101 achieves its effects, the researchers collected stool samples from the patients and counted the number of different kinds organisms in each sample.
At baseline, the patients had about the same number, but after a week the diversity was greater in the patients treated with CP101, and that difference had increased at week 8. The researchers also found much less diversity of organisms in the stools of those patients who had recurrences of C. difficile infection.
The diversity of microbes in the successfully treated patients appeared to have been introduced by CP101. Dr. Allegretti and colleagues measured the number of organisms in the stool samples that came from CP101. They found that 96% of patients colonized by the CP101 organisms had avoided recurrence of the C. difficile infections, compared with 54.2% of those patients not colonized by these microbes.
“We now have some microbiome-based markers that show us as early as week 1 that the patient is going to be cured or not,” Dr. Allegretti said.
Based on these results, Finch plans to launch a phase 3 trial soon, she said.
The data on colonization is interesting because it has not been found with fecal microbiota transplants, said Purna Kashyap, MBBS, codirector of the Microbiome Program at the Mayo Clinic College of Medicine in Rochester, Minn., who was not involved in the study.
But to better interpret the data, it would be helpful to know more about how the placebo and CP101 groups compared at baseline with regard to medications, immunosuppression, and antibiotics used to treat the C. difficile infections, Dr. Kashyap said. He was struck by the lower cure rate in the portion of the placebo group treated with fidaxomicin.
“Overall, I think these are exciting observations based on the data but require careful review of the entire data to make sense of [them], which will happen when it goes through peer review,” he told this news organization in an email.
Several other standardized microbiota restoration products are under development, including at least two other capsules. In contrast to CP101, which is made up of whole stool, VE303 (Vedanta Biosciences) is a “rationally defined bacterial consortium,” and SER-109 (Seres Therapeutics) is a “consortium of highly purified Firmicutes spores.” VE303 has completed a phase 2 trial, and SER-109 has completed a phase 3 trial.
Dr. Allegretti is a consultant for Finch Therapeutics, which funded the trial. Dr. Kashyap has disclosed no relevant financial relationships.
Help your patients understand their C. difficile diagnosis by sharing patient education from the AGA GI Patient Center: www.gastro.org/Cdiff.
A version of this article first appeared on Medscape.com.
CP101, under development by Finch Therapeutics, proved more effective than a placebo in preventing recurrent infections for up to 24 weeks.
The CP101 capsules contain a powder of freeze-dried human stools from screened donors. They restore natural diversity that has been disrupted by antibiotics, said Jessica Allegretti, MD, MPH a gastroenterologist at Brigham and Women’s Hospital in Boston.
The treatment offers an alternative to fecal microbiota transplant, which can effectively treat antibiotic-resistant C. difficile infections but is difficult to standardize and administer – and doesn’t have full approval from the U.S. Food and Drug Administration, she added.
“I think this marks a moment in this space where we’re going to have better, safer, and more available options for patients,” she said in an interview. “It’s exciting.”
Dr. Allegretti is an author on three presentations of results from PRISM3, a phase 2 trial of CP101. They will be presented this week at the annual meeting of the American College of Gastroenterology. These results extend out to 24 weeks, whereas the 8-week results of this trial were presented a year ago at the same meeting.
Study details
The study enrolled 198 people who received antibiotics for recurrent C. difficile infections. Some patients had two or more recurrences, while others had only one recurrence but were 65 years of age or older.
“That was a unique aspect of this study, to see the effect of bringing a therapy like CP101 earlier in the treatment paradigm,” said Dr. Allegretti. “You can imagine for an older, frail, or more fragile patient that you would want to get rid of this [infection] earlier.”
After waiting 2-6 days for the antibiotics to wash out, the researchers randomly assigned 102 of these patients to take the CP101 pills orally and 96 to take placebo pills, both without bowel preparation.
The two groups were not significantly different in age, gender, comorbidities, the number of C. difficile recurrences, or the type of test used to diagnose the infection (PCR-based vs. toxin EIA-based).
After 8 weeks, 74.5% of those given the CP101 pills had not had a recurrence, compared with 61.5% of those given the placebo. The difference was just barely statistically significant (P = .0488).
Sixteen weeks later, the effect endured, with 73.5% of the CP101 group and 59.4% of the placebo group still free of recurrence. The statistical significance of the difference improved slightly (P = .0347).
Drug-related emergent adverse events were similar between the two groups: 16.3% for the CP101 group vs. 19.2% for the placebo group. These were mostly gastrointestinal symptoms, and none were serious.
Some of the patients received vancomycin as a first-line treatment for C. difficile infections, and the researchers wondered if the washout period was not sufficient to purge that antibiotic, leaving enough to interfere with the effectiveness of CP101.
Therefore, they separately analyzed 40 patients treated with fidaxomicin, which they expected to wash out more quickly. Among these patients, 81% who received CP101 were free of recurrences, at 8 weeks and 24 weeks. This compared with 42.1% of those who received the placebo, at both time points. This difference was more statistically significant (P = .0211).
Understanding how it works
To understand better how CP101 achieves its effects, the researchers collected stool samples from the patients and counted the number of different kinds organisms in each sample.
At baseline, the patients had about the same number, but after a week the diversity was greater in the patients treated with CP101, and that difference had increased at week 8. The researchers also found much less diversity of organisms in the stools of those patients who had recurrences of C. difficile infection.
The diversity of microbes in the successfully treated patients appeared to have been introduced by CP101. Dr. Allegretti and colleagues measured the number of organisms in the stool samples that came from CP101. They found that 96% of patients colonized by the CP101 organisms had avoided recurrence of the C. difficile infections, compared with 54.2% of those patients not colonized by these microbes.
“We now have some microbiome-based markers that show us as early as week 1 that the patient is going to be cured or not,” Dr. Allegretti said.
Based on these results, Finch plans to launch a phase 3 trial soon, she said.
The data on colonization is interesting because it has not been found with fecal microbiota transplants, said Purna Kashyap, MBBS, codirector of the Microbiome Program at the Mayo Clinic College of Medicine in Rochester, Minn., who was not involved in the study.
But to better interpret the data, it would be helpful to know more about how the placebo and CP101 groups compared at baseline with regard to medications, immunosuppression, and antibiotics used to treat the C. difficile infections, Dr. Kashyap said. He was struck by the lower cure rate in the portion of the placebo group treated with fidaxomicin.
“Overall, I think these are exciting observations based on the data but require careful review of the entire data to make sense of [them], which will happen when it goes through peer review,” he told this news organization in an email.
Several other standardized microbiota restoration products are under development, including at least two other capsules. In contrast to CP101, which is made up of whole stool, VE303 (Vedanta Biosciences) is a “rationally defined bacterial consortium,” and SER-109 (Seres Therapeutics) is a “consortium of highly purified Firmicutes spores.” VE303 has completed a phase 2 trial, and SER-109 has completed a phase 3 trial.
Dr. Allegretti is a consultant for Finch Therapeutics, which funded the trial. Dr. Kashyap has disclosed no relevant financial relationships.
Help your patients understand their C. difficile diagnosis by sharing patient education from the AGA GI Patient Center: www.gastro.org/Cdiff.
A version of this article first appeared on Medscape.com.
CP101, under development by Finch Therapeutics, proved more effective than a placebo in preventing recurrent infections for up to 24 weeks.
The CP101 capsules contain a powder of freeze-dried human stools from screened donors. They restore natural diversity that has been disrupted by antibiotics, said Jessica Allegretti, MD, MPH a gastroenterologist at Brigham and Women’s Hospital in Boston.
The treatment offers an alternative to fecal microbiota transplant, which can effectively treat antibiotic-resistant C. difficile infections but is difficult to standardize and administer – and doesn’t have full approval from the U.S. Food and Drug Administration, she added.
“I think this marks a moment in this space where we’re going to have better, safer, and more available options for patients,” she said in an interview. “It’s exciting.”
Dr. Allegretti is an author on three presentations of results from PRISM3, a phase 2 trial of CP101. They will be presented this week at the annual meeting of the American College of Gastroenterology. These results extend out to 24 weeks, whereas the 8-week results of this trial were presented a year ago at the same meeting.
Study details
The study enrolled 198 people who received antibiotics for recurrent C. difficile infections. Some patients had two or more recurrences, while others had only one recurrence but were 65 years of age or older.
“That was a unique aspect of this study, to see the effect of bringing a therapy like CP101 earlier in the treatment paradigm,” said Dr. Allegretti. “You can imagine for an older, frail, or more fragile patient that you would want to get rid of this [infection] earlier.”
After waiting 2-6 days for the antibiotics to wash out, the researchers randomly assigned 102 of these patients to take the CP101 pills orally and 96 to take placebo pills, both without bowel preparation.
The two groups were not significantly different in age, gender, comorbidities, the number of C. difficile recurrences, or the type of test used to diagnose the infection (PCR-based vs. toxin EIA-based).
After 8 weeks, 74.5% of those given the CP101 pills had not had a recurrence, compared with 61.5% of those given the placebo. The difference was just barely statistically significant (P = .0488).
Sixteen weeks later, the effect endured, with 73.5% of the CP101 group and 59.4% of the placebo group still free of recurrence. The statistical significance of the difference improved slightly (P = .0347).
Drug-related emergent adverse events were similar between the two groups: 16.3% for the CP101 group vs. 19.2% for the placebo group. These were mostly gastrointestinal symptoms, and none were serious.
Some of the patients received vancomycin as a first-line treatment for C. difficile infections, and the researchers wondered if the washout period was not sufficient to purge that antibiotic, leaving enough to interfere with the effectiveness of CP101.
Therefore, they separately analyzed 40 patients treated with fidaxomicin, which they expected to wash out more quickly. Among these patients, 81% who received CP101 were free of recurrences, at 8 weeks and 24 weeks. This compared with 42.1% of those who received the placebo, at both time points. This difference was more statistically significant (P = .0211).
Understanding how it works
To understand better how CP101 achieves its effects, the researchers collected stool samples from the patients and counted the number of different kinds organisms in each sample.
At baseline, the patients had about the same number, but after a week the diversity was greater in the patients treated with CP101, and that difference had increased at week 8. The researchers also found much less diversity of organisms in the stools of those patients who had recurrences of C. difficile infection.
The diversity of microbes in the successfully treated patients appeared to have been introduced by CP101. Dr. Allegretti and colleagues measured the number of organisms in the stool samples that came from CP101. They found that 96% of patients colonized by the CP101 organisms had avoided recurrence of the C. difficile infections, compared with 54.2% of those patients not colonized by these microbes.
“We now have some microbiome-based markers that show us as early as week 1 that the patient is going to be cured or not,” Dr. Allegretti said.
Based on these results, Finch plans to launch a phase 3 trial soon, she said.
The data on colonization is interesting because it has not been found with fecal microbiota transplants, said Purna Kashyap, MBBS, codirector of the Microbiome Program at the Mayo Clinic College of Medicine in Rochester, Minn., who was not involved in the study.
But to better interpret the data, it would be helpful to know more about how the placebo and CP101 groups compared at baseline with regard to medications, immunosuppression, and antibiotics used to treat the C. difficile infections, Dr. Kashyap said. He was struck by the lower cure rate in the portion of the placebo group treated with fidaxomicin.
“Overall, I think these are exciting observations based on the data but require careful review of the entire data to make sense of [them], which will happen when it goes through peer review,” he told this news organization in an email.
Several other standardized microbiota restoration products are under development, including at least two other capsules. In contrast to CP101, which is made up of whole stool, VE303 (Vedanta Biosciences) is a “rationally defined bacterial consortium,” and SER-109 (Seres Therapeutics) is a “consortium of highly purified Firmicutes spores.” VE303 has completed a phase 2 trial, and SER-109 has completed a phase 3 trial.
Dr. Allegretti is a consultant for Finch Therapeutics, which funded the trial. Dr. Kashyap has disclosed no relevant financial relationships.
Help your patients understand their C. difficile diagnosis by sharing patient education from the AGA GI Patient Center: www.gastro.org/Cdiff.
A version of this article first appeared on Medscape.com.
FROM ACG 2021