User login
IBD classification needs an upgrade
The current clinical classification tools for inflammatory bowel disease (IBD) are suboptimal, and revision beyond the broad categories of Crohn’s disease (CD) and ulcerative colitis (UC) could improve trial design, research, and ultimately patient outcomes, according to the authors of a recent review.
“Despite clear improvements in our understanding of disease biology and increasing treatment options, we still face an important therapeutic ceiling [in IBD],” wrote Bram Verstockt, MD, of University Hospitals Leuven, Belgium, and colleagues.
“In part, our limited therapeutic successes can be attributed to the disease heterogeneity of IBD: There is not one CD, nor a single UC phenotype,” therefore, a revision of the current systems based on better understanding of IBD is needed, the researchers said.
In a review article published in Gastroenterology, the researchers identified clinical features important to IBD heterogeneity, examined limitations of the current classifications, and proposed improvements.
Characterizing a complex condition
IBD diagnosis is challenging not only because of the overlapping phenotypes, but because other pathologies, including infections, can mimic IBD, the authors noted.
Age of onset should be considered in characterizing IBD, they wrote. Notably, patients with late-onset CD should be distinguished from elderly patients who have had CD for years. The authors cited research showing that “the development of IBD at extremes of age are specific sub-groups that require a different clinical recognition and clinical management,” and that large sample sizes and unbiased statistical methods are needed to define subgroups of IBD patients.
Current CD classification (the Montreal Classification) involves disease location, disease behavior, and age at diagnosis, and considers four phenotypes within disease location: involvement of the ileum, involvement of the colon, involvement of both the ileum and the colon, or isolated upper disease.* “Recently, there has been notable interest in the differential response rates among ileal predominant CD compared to colonic CD,” the authors wrote. Consequently, they proposed a revision of CD classification based on location. Genetic data appear to support this revision. In an IBD genotype-phenotype study including nearly 30,000 patients, three loci (NOD2, MHC, MST1 3p21) were strongly associated with disease location, they said. Other emerging evidence suggests that gut microbiota may vary according to disease location. The authors identified clinical aspects of CD classification based on disease location that distinguish small bowel predominant CD versus colonic predominant CD. Ileal disease patients have shown an increased risk for undergoing surgery, while those with colonic involvement have an increased risk for developing extraintestinal manifestations.
They also emphasized the value of considering rectal inflammation, which significantly impacts surgical procedures in CD.
Standard UC classification is based on macroscopic disease in the colon at the time of inflammation, the authors said. Although this approach allows for quick assessment of a patient’s risk of colectomy, the authors proposed improvements, including the use of serum biomarkers (C-reactive protein or erythrocyte sedimentation rate) to identify patients at highest risk for colectomy and colon cancer based on inflammation. The authors also suggested that patients with refractory proctitis be enrolled in UC clinical trials or in studies focusing on refractory proctitis in particular.
The pelvic pouch has become the most often performed surgical procedure for patients undergoing colectomy, but there is no agreement on classification of inflammatory pelvic pouch disorders, and studies of etiology and treatment are lacking, the authors noted. They advised a clinical assessment based on symptoms, including stool frequency, urgency, and incontinence. They also suggested that afferent limb ulcers of erosions should be classified separately from pouch inflammation.
The authors ended by noting that extraintestinal manifestations (EIMs) that occur in up to half of IBD patients may or may not be directly related to intestinal disease, and may represent a different phenotype, and the presence and type of EIM should be included in a revised IBD classification system, they said.
The authors emphasized that continuing to refer to IBD as only CD and UC “does a great disservice to our attempts to better understand IBD pathogenesis and to improve clinical patient management.”
They concluded: “Although revised clinical classification tools alone will not be sufficient and should be complemented by deeper and more detailed study into molecular subclassification of disease, the considerations here could be used as a springboard toward improved trial design, future translational research approaches and better treatment outcomes for patients.”
Review reflects complexity of IBD and challenges of change
The review is important at this time because of the growing recognition that IBD, while traditionally categorized as either UC or CD, is most likely composed of a range of heterogeneous conditions involving inflammation of the gastrointestinal tract, Jatin Roper, MD, of Duke University in Durham, N.C., said in an interview.
“Evidence that our current classification of IBD is suboptimal comes from both the wide range of clinical phenotypes as well as complexity in genetic markers that are associated with IBD,” he said. “It is well accepted in the gastroenterology community that IBD is a complex condition; so it is surprising to me that the dichotomy of UC vs. Crohn’s disease has rarely been challenged,” said Dr. Roper. “The authors of this review should be commended for raising the question of whether IBD deserves a more nuanced classification system that reflects the growing recognition of the wide heterogeneity of patient presentations and genetics,” he said.
“Challenging medical definitions is inherently difficult because patient diagnoses, treatment plans, as well as decades of clinical research have been based on well-accepted disease categories. Another major challenge in reclassification is that the course of IBD can vary greatly over time in the same patient in severity, range, and complexity, and potentially includes many disease subtypes noted by the authors of this review,” he added. “Therefore, I believe that the current system of dividing IBD in UC and CD is here to stay until subtypes based on mechanisms of disease pathogenesis are discovered.
“Additional research is needed to understand the molecular basis of IBD,” Dr. Roper emphasized. “Recent advances in RNA expression and proteomics at the single cell level may reveal distinct cell types or cell functions in tissues from IBD patients that may help us understand clinical phenotype or response to therapy.”
The study received no outside funding. The authors disclosed financial relationships with AbbVie, Biogen, Chiesi, Falk, Ferring, Galapagos, Janssen, MSD, Pfizer, R-Biopharm, Takeda, and Truvion. Dr. Roper had no financial conflicts to disclose.
Help your patients better understand their IBD treatment options by sharing AGA’s patient education, “Living with IBD,” in the AGA GI Patient Center at www.gastro.org/IBD.
*Correction, 4/11/22: An earlier version of this article misstated the Montreal Classification.
The current clinical classification tools for inflammatory bowel disease (IBD) are suboptimal, and revision beyond the broad categories of Crohn’s disease (CD) and ulcerative colitis (UC) could improve trial design, research, and ultimately patient outcomes, according to the authors of a recent review.
“Despite clear improvements in our understanding of disease biology and increasing treatment options, we still face an important therapeutic ceiling [in IBD],” wrote Bram Verstockt, MD, of University Hospitals Leuven, Belgium, and colleagues.
“In part, our limited therapeutic successes can be attributed to the disease heterogeneity of IBD: There is not one CD, nor a single UC phenotype,” therefore, a revision of the current systems based on better understanding of IBD is needed, the researchers said.
In a review article published in Gastroenterology, the researchers identified clinical features important to IBD heterogeneity, examined limitations of the current classifications, and proposed improvements.
Characterizing a complex condition
IBD diagnosis is challenging not only because of the overlapping phenotypes, but because other pathologies, including infections, can mimic IBD, the authors noted.
Age of onset should be considered in characterizing IBD, they wrote. Notably, patients with late-onset CD should be distinguished from elderly patients who have had CD for years. The authors cited research showing that “the development of IBD at extremes of age are specific sub-groups that require a different clinical recognition and clinical management,” and that large sample sizes and unbiased statistical methods are needed to define subgroups of IBD patients.
Current CD classification (the Montreal Classification) involves disease location, disease behavior, and age at diagnosis, and considers four phenotypes within disease location: involvement of the ileum, involvement of the colon, involvement of both the ileum and the colon, or isolated upper disease.* “Recently, there has been notable interest in the differential response rates among ileal predominant CD compared to colonic CD,” the authors wrote. Consequently, they proposed a revision of CD classification based on location. Genetic data appear to support this revision. In an IBD genotype-phenotype study including nearly 30,000 patients, three loci (NOD2, MHC, MST1 3p21) were strongly associated with disease location, they said. Other emerging evidence suggests that gut microbiota may vary according to disease location. The authors identified clinical aspects of CD classification based on disease location that distinguish small bowel predominant CD versus colonic predominant CD. Ileal disease patients have shown an increased risk for undergoing surgery, while those with colonic involvement have an increased risk for developing extraintestinal manifestations.
They also emphasized the value of considering rectal inflammation, which significantly impacts surgical procedures in CD.
Standard UC classification is based on macroscopic disease in the colon at the time of inflammation, the authors said. Although this approach allows for quick assessment of a patient’s risk of colectomy, the authors proposed improvements, including the use of serum biomarkers (C-reactive protein or erythrocyte sedimentation rate) to identify patients at highest risk for colectomy and colon cancer based on inflammation. The authors also suggested that patients with refractory proctitis be enrolled in UC clinical trials or in studies focusing on refractory proctitis in particular.
The pelvic pouch has become the most often performed surgical procedure for patients undergoing colectomy, but there is no agreement on classification of inflammatory pelvic pouch disorders, and studies of etiology and treatment are lacking, the authors noted. They advised a clinical assessment based on symptoms, including stool frequency, urgency, and incontinence. They also suggested that afferent limb ulcers of erosions should be classified separately from pouch inflammation.
The authors ended by noting that extraintestinal manifestations (EIMs) that occur in up to half of IBD patients may or may not be directly related to intestinal disease, and may represent a different phenotype, and the presence and type of EIM should be included in a revised IBD classification system, they said.
The authors emphasized that continuing to refer to IBD as only CD and UC “does a great disservice to our attempts to better understand IBD pathogenesis and to improve clinical patient management.”
They concluded: “Although revised clinical classification tools alone will not be sufficient and should be complemented by deeper and more detailed study into molecular subclassification of disease, the considerations here could be used as a springboard toward improved trial design, future translational research approaches and better treatment outcomes for patients.”
Review reflects complexity of IBD and challenges of change
The review is important at this time because of the growing recognition that IBD, while traditionally categorized as either UC or CD, is most likely composed of a range of heterogeneous conditions involving inflammation of the gastrointestinal tract, Jatin Roper, MD, of Duke University in Durham, N.C., said in an interview.
“Evidence that our current classification of IBD is suboptimal comes from both the wide range of clinical phenotypes as well as complexity in genetic markers that are associated with IBD,” he said. “It is well accepted in the gastroenterology community that IBD is a complex condition; so it is surprising to me that the dichotomy of UC vs. Crohn’s disease has rarely been challenged,” said Dr. Roper. “The authors of this review should be commended for raising the question of whether IBD deserves a more nuanced classification system that reflects the growing recognition of the wide heterogeneity of patient presentations and genetics,” he said.
“Challenging medical definitions is inherently difficult because patient diagnoses, treatment plans, as well as decades of clinical research have been based on well-accepted disease categories. Another major challenge in reclassification is that the course of IBD can vary greatly over time in the same patient in severity, range, and complexity, and potentially includes many disease subtypes noted by the authors of this review,” he added. “Therefore, I believe that the current system of dividing IBD in UC and CD is here to stay until subtypes based on mechanisms of disease pathogenesis are discovered.
“Additional research is needed to understand the molecular basis of IBD,” Dr. Roper emphasized. “Recent advances in RNA expression and proteomics at the single cell level may reveal distinct cell types or cell functions in tissues from IBD patients that may help us understand clinical phenotype or response to therapy.”
The study received no outside funding. The authors disclosed financial relationships with AbbVie, Biogen, Chiesi, Falk, Ferring, Galapagos, Janssen, MSD, Pfizer, R-Biopharm, Takeda, and Truvion. Dr. Roper had no financial conflicts to disclose.
Help your patients better understand their IBD treatment options by sharing AGA’s patient education, “Living with IBD,” in the AGA GI Patient Center at www.gastro.org/IBD.
*Correction, 4/11/22: An earlier version of this article misstated the Montreal Classification.
The current clinical classification tools for inflammatory bowel disease (IBD) are suboptimal, and revision beyond the broad categories of Crohn’s disease (CD) and ulcerative colitis (UC) could improve trial design, research, and ultimately patient outcomes, according to the authors of a recent review.
“Despite clear improvements in our understanding of disease biology and increasing treatment options, we still face an important therapeutic ceiling [in IBD],” wrote Bram Verstockt, MD, of University Hospitals Leuven, Belgium, and colleagues.
“In part, our limited therapeutic successes can be attributed to the disease heterogeneity of IBD: There is not one CD, nor a single UC phenotype,” therefore, a revision of the current systems based on better understanding of IBD is needed, the researchers said.
In a review article published in Gastroenterology, the researchers identified clinical features important to IBD heterogeneity, examined limitations of the current classifications, and proposed improvements.
Characterizing a complex condition
IBD diagnosis is challenging not only because of the overlapping phenotypes, but because other pathologies, including infections, can mimic IBD, the authors noted.
Age of onset should be considered in characterizing IBD, they wrote. Notably, patients with late-onset CD should be distinguished from elderly patients who have had CD for years. The authors cited research showing that “the development of IBD at extremes of age are specific sub-groups that require a different clinical recognition and clinical management,” and that large sample sizes and unbiased statistical methods are needed to define subgroups of IBD patients.
Current CD classification (the Montreal Classification) involves disease location, disease behavior, and age at diagnosis, and considers four phenotypes within disease location: involvement of the ileum, involvement of the colon, involvement of both the ileum and the colon, or isolated upper disease.* “Recently, there has been notable interest in the differential response rates among ileal predominant CD compared to colonic CD,” the authors wrote. Consequently, they proposed a revision of CD classification based on location. Genetic data appear to support this revision. In an IBD genotype-phenotype study including nearly 30,000 patients, three loci (NOD2, MHC, MST1 3p21) were strongly associated with disease location, they said. Other emerging evidence suggests that gut microbiota may vary according to disease location. The authors identified clinical aspects of CD classification based on disease location that distinguish small bowel predominant CD versus colonic predominant CD. Ileal disease patients have shown an increased risk for undergoing surgery, while those with colonic involvement have an increased risk for developing extraintestinal manifestations.
They also emphasized the value of considering rectal inflammation, which significantly impacts surgical procedures in CD.
Standard UC classification is based on macroscopic disease in the colon at the time of inflammation, the authors said. Although this approach allows for quick assessment of a patient’s risk of colectomy, the authors proposed improvements, including the use of serum biomarkers (C-reactive protein or erythrocyte sedimentation rate) to identify patients at highest risk for colectomy and colon cancer based on inflammation. The authors also suggested that patients with refractory proctitis be enrolled in UC clinical trials or in studies focusing on refractory proctitis in particular.
The pelvic pouch has become the most often performed surgical procedure for patients undergoing colectomy, but there is no agreement on classification of inflammatory pelvic pouch disorders, and studies of etiology and treatment are lacking, the authors noted. They advised a clinical assessment based on symptoms, including stool frequency, urgency, and incontinence. They also suggested that afferent limb ulcers of erosions should be classified separately from pouch inflammation.
The authors ended by noting that extraintestinal manifestations (EIMs) that occur in up to half of IBD patients may or may not be directly related to intestinal disease, and may represent a different phenotype, and the presence and type of EIM should be included in a revised IBD classification system, they said.
The authors emphasized that continuing to refer to IBD as only CD and UC “does a great disservice to our attempts to better understand IBD pathogenesis and to improve clinical patient management.”
They concluded: “Although revised clinical classification tools alone will not be sufficient and should be complemented by deeper and more detailed study into molecular subclassification of disease, the considerations here could be used as a springboard toward improved trial design, future translational research approaches and better treatment outcomes for patients.”
Review reflects complexity of IBD and challenges of change
The review is important at this time because of the growing recognition that IBD, while traditionally categorized as either UC or CD, is most likely composed of a range of heterogeneous conditions involving inflammation of the gastrointestinal tract, Jatin Roper, MD, of Duke University in Durham, N.C., said in an interview.
“Evidence that our current classification of IBD is suboptimal comes from both the wide range of clinical phenotypes as well as complexity in genetic markers that are associated with IBD,” he said. “It is well accepted in the gastroenterology community that IBD is a complex condition; so it is surprising to me that the dichotomy of UC vs. Crohn’s disease has rarely been challenged,” said Dr. Roper. “The authors of this review should be commended for raising the question of whether IBD deserves a more nuanced classification system that reflects the growing recognition of the wide heterogeneity of patient presentations and genetics,” he said.
“Challenging medical definitions is inherently difficult because patient diagnoses, treatment plans, as well as decades of clinical research have been based on well-accepted disease categories. Another major challenge in reclassification is that the course of IBD can vary greatly over time in the same patient in severity, range, and complexity, and potentially includes many disease subtypes noted by the authors of this review,” he added. “Therefore, I believe that the current system of dividing IBD in UC and CD is here to stay until subtypes based on mechanisms of disease pathogenesis are discovered.
“Additional research is needed to understand the molecular basis of IBD,” Dr. Roper emphasized. “Recent advances in RNA expression and proteomics at the single cell level may reveal distinct cell types or cell functions in tissues from IBD patients that may help us understand clinical phenotype or response to therapy.”
The study received no outside funding. The authors disclosed financial relationships with AbbVie, Biogen, Chiesi, Falk, Ferring, Galapagos, Janssen, MSD, Pfizer, R-Biopharm, Takeda, and Truvion. Dr. Roper had no financial conflicts to disclose.
Help your patients better understand their IBD treatment options by sharing AGA’s patient education, “Living with IBD,” in the AGA GI Patient Center at www.gastro.org/IBD.
*Correction, 4/11/22: An earlier version of this article misstated the Montreal Classification.
FROM GASTROENTEROLOGY
ARFID or reasonable food restriction? The jury is out
Problems with eating and nutrition are common among patients with inflammatory bowel disease (IBD) and other gastrointestinal disorders, but clinicians who treat them should be careful not to automatically assume that patients have eating disorders, according to a psychologist who specializes in the psychological and social aspects of chronic digestive diseases.
On the other hand, clinicians must also be aware of the possibility that patients could have a recently identified syndrome cluster called avoidant restrictive food intake disorder (ARFID), said Tiffany Taft, PsyD, a research associate professor of medicine (gastroenterology and hepatology), medical social sciences, and psychiatry and behavioral sciences at Northwestern University, Chicago. In a recent study, she and her colleagues defined ARFID as “failure to meet one’s nutritional needs owing to sensory hypersensitivity, lack of interest in eating, or fear of aversive consequences from eating, and is associated with negative medical and psychosocial outcomes.”
ARFID “is a hot topic that we really don’t understand,” she said in an online presentation at the annual Crohn’s & Colitis Congress®, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
Nutritional deficiencies
Nutritional deficiencies are common among patients with IBD, “and nutritional deficiencies themselves can lead to symptoms or side effects that can cause people to eat less,” she said.
“As our vitamin B12 goes down, our cognitive functioning starts to decline, and we might not be making clear decisions in how we’re deciding what to eat, when to eat, if we should be eating at all – just something to think about in your patients who have nutritional deficiencies,” she told the audience.
Other common nutritional deficiencies that can affect eating and food choice among patients with IBD include low folate (B9) levels associated with sore tongue and weight loss, low iron levels leading to nausea and loss of appetite, and zinc deficiency leading to loss of appetite and alterations in taste and/or smell, she said.
Newly recognized in GI
She noted that “ARFID actually originates in the pediatric psychiatric literature, mostly in children with sensory issues [such as] autism spectrum disorder, so this is not a construct that started in digestive disease, but has been adapted and applied to patients with digestive disease, including IBD.”
The DSM-5 lists four criteria for ARFID: significant weight loss, significant nutritional deficiency, dependence on enteral nutrition or oral supplements, and marked interference with psychosocial functions.
Helen Burton Murray, PhD, director of the gastrointestinal behavioral health program in the Center for Neurointestinal Health at Massachusetts General Hospital, Boston, who is familiar with Dr. Taft’s work, said in an interview that inclusion of ARFID in DSM-5 has put a name to a syndrome or symptom cluster that in all likelihood already existed.
However, “the jury is still out about whether, if we do diagnose patients who have digestive diseases with ARFID, that then helps them get to a treatment that improves their relationship with food and improves nutritional issues that may have occurred as a result of a restricted food intake,” she said.
“We don’t know yet if the diagnosis will actually improve things. In our clinical practice, anecdotally, it has, both for patients with IBDs and for patients with other GI conditions, particularly GI functional motility disorders. We’re a little bit more confident about making the diagnosis of ARFID in GI functional motility disorders than we are in IBD of course,” she said.
Screening measures
To get a better sense of the prevalence of ARFID, compared with reasonable responses to digestive diseases, Dr. Taft and colleagues conducted their cross-sectional study in 289 adults with achalasia, celiac, eosinophilic esophagitis, or IBD.
They found that 51.3% of the total sample met the diagnostic criteria for ARFID based on the Nine-Item ARFID Screen (NIAS), including 75.7 % of patients with achalasia. But Dr. Taft had cautions
“I can tell you, working with achalasia patients, 75% do not have ARFID,” Dr. Taft said.
She noted that the 51.3% of patients with IBD identified by NIAS or the 53% identified by the ARFID+ scale as having ARFID was also highly doubtful.
Dr. Taft and colleagues determined that nearly half of the variance in the NIAS could be accounted for by GI symptoms rather than psychosocial factors, making it less than ideal for use in the clinic or by researchers.
She also noted, however, that she received an email from one of the creators of NIAS, Hana F. Zickgraf, PhD, from the University of South Alabama, Mobile. Dr. Zickgraf agreed that the scale had drawbacks when applied to patients with GI disease, and pointed instead to the Fear of Food Questionnaire, a newly developed 18-item GI disease-specific instrument. Dr. Taft recommended the new questionnaire for research purposes, and expressed hope that a shorter version could be made available for screening patients in clinic.
Dr. Burton Murray said that while the Fear of Food Questionnaire, perhaps in combination with NIAS, has the potential to be a useful screening tool, cutoffs for it have yet to be established.
“At the end of the day, the diagnosis would be made by a clinician who is able to determine whether the life impairment or if the nutritional impairment or restricted food intake are reasonable in the realm of their digestive disease, or could a treatment for ARFID be warranted to help them to make changes to improve their quality of life and nutrition,” she said.
Check biases at the door
Before arriving at a diagnosis of ARFID, clinicians should also consider biases, Dr. Taft said.
“Eating disorders are highly stigmatized and stereotyped diagnoses,” more often attributed to young White women than to either men or to people of racial or ethnic minorities, she said.
Cultural background may contribute to food restrictions, and the risk may increase with age, with 68% of patients with later-onset IBD restricting diets to control the disease. It’s also possible that beliefs about food and “clean and healthy” eating may influence food and eating choices after a patient receives an IBD diagnosis.
Dr. Taft also pointed out that clinicians and patients may have different ideas about what constitutes significant food avoidance. Clinicians may expect patients with IBD to eat despite feeling nauseated, having abdominal pains, or diarrhea, for example, when the same food avoidance might be deemed reasonable in patients with short-term GI infections.
“Severe IBD symptoms are a significant predictor of posttraumatic stress disorder symptoms, and PTSD is hallmarked by avoidance behaviors,” she added.
She emphasized the need for clinicians to ask the right questions of patients to get at the roots of their nutritional deficiency or eating behavior, and to refer patients to mental health professionals with expertise in disordered eating or GI psychology.
Dr. Taft and Dr. Burton Murray reported having no conflicts of interest to disclose.
This article was updated on Feb. 4, 2022.
Problems with eating and nutrition are common among patients with inflammatory bowel disease (IBD) and other gastrointestinal disorders, but clinicians who treat them should be careful not to automatically assume that patients have eating disorders, according to a psychologist who specializes in the psychological and social aspects of chronic digestive diseases.
On the other hand, clinicians must also be aware of the possibility that patients could have a recently identified syndrome cluster called avoidant restrictive food intake disorder (ARFID), said Tiffany Taft, PsyD, a research associate professor of medicine (gastroenterology and hepatology), medical social sciences, and psychiatry and behavioral sciences at Northwestern University, Chicago. In a recent study, she and her colleagues defined ARFID as “failure to meet one’s nutritional needs owing to sensory hypersensitivity, lack of interest in eating, or fear of aversive consequences from eating, and is associated with negative medical and psychosocial outcomes.”
ARFID “is a hot topic that we really don’t understand,” she said in an online presentation at the annual Crohn’s & Colitis Congress®, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
Nutritional deficiencies
Nutritional deficiencies are common among patients with IBD, “and nutritional deficiencies themselves can lead to symptoms or side effects that can cause people to eat less,” she said.
“As our vitamin B12 goes down, our cognitive functioning starts to decline, and we might not be making clear decisions in how we’re deciding what to eat, when to eat, if we should be eating at all – just something to think about in your patients who have nutritional deficiencies,” she told the audience.
Other common nutritional deficiencies that can affect eating and food choice among patients with IBD include low folate (B9) levels associated with sore tongue and weight loss, low iron levels leading to nausea and loss of appetite, and zinc deficiency leading to loss of appetite and alterations in taste and/or smell, she said.
Newly recognized in GI
She noted that “ARFID actually originates in the pediatric psychiatric literature, mostly in children with sensory issues [such as] autism spectrum disorder, so this is not a construct that started in digestive disease, but has been adapted and applied to patients with digestive disease, including IBD.”
The DSM-5 lists four criteria for ARFID: significant weight loss, significant nutritional deficiency, dependence on enteral nutrition or oral supplements, and marked interference with psychosocial functions.
Helen Burton Murray, PhD, director of the gastrointestinal behavioral health program in the Center for Neurointestinal Health at Massachusetts General Hospital, Boston, who is familiar with Dr. Taft’s work, said in an interview that inclusion of ARFID in DSM-5 has put a name to a syndrome or symptom cluster that in all likelihood already existed.
However, “the jury is still out about whether, if we do diagnose patients who have digestive diseases with ARFID, that then helps them get to a treatment that improves their relationship with food and improves nutritional issues that may have occurred as a result of a restricted food intake,” she said.
“We don’t know yet if the diagnosis will actually improve things. In our clinical practice, anecdotally, it has, both for patients with IBDs and for patients with other GI conditions, particularly GI functional motility disorders. We’re a little bit more confident about making the diagnosis of ARFID in GI functional motility disorders than we are in IBD of course,” she said.
Screening measures
To get a better sense of the prevalence of ARFID, compared with reasonable responses to digestive diseases, Dr. Taft and colleagues conducted their cross-sectional study in 289 adults with achalasia, celiac, eosinophilic esophagitis, or IBD.
They found that 51.3% of the total sample met the diagnostic criteria for ARFID based on the Nine-Item ARFID Screen (NIAS), including 75.7 % of patients with achalasia. But Dr. Taft had cautions
“I can tell you, working with achalasia patients, 75% do not have ARFID,” Dr. Taft said.
She noted that the 51.3% of patients with IBD identified by NIAS or the 53% identified by the ARFID+ scale as having ARFID was also highly doubtful.
Dr. Taft and colleagues determined that nearly half of the variance in the NIAS could be accounted for by GI symptoms rather than psychosocial factors, making it less than ideal for use in the clinic or by researchers.
She also noted, however, that she received an email from one of the creators of NIAS, Hana F. Zickgraf, PhD, from the University of South Alabama, Mobile. Dr. Zickgraf agreed that the scale had drawbacks when applied to patients with GI disease, and pointed instead to the Fear of Food Questionnaire, a newly developed 18-item GI disease-specific instrument. Dr. Taft recommended the new questionnaire for research purposes, and expressed hope that a shorter version could be made available for screening patients in clinic.
Dr. Burton Murray said that while the Fear of Food Questionnaire, perhaps in combination with NIAS, has the potential to be a useful screening tool, cutoffs for it have yet to be established.
“At the end of the day, the diagnosis would be made by a clinician who is able to determine whether the life impairment or if the nutritional impairment or restricted food intake are reasonable in the realm of their digestive disease, or could a treatment for ARFID be warranted to help them to make changes to improve their quality of life and nutrition,” she said.
Check biases at the door
Before arriving at a diagnosis of ARFID, clinicians should also consider biases, Dr. Taft said.
“Eating disorders are highly stigmatized and stereotyped diagnoses,” more often attributed to young White women than to either men or to people of racial or ethnic minorities, she said.
Cultural background may contribute to food restrictions, and the risk may increase with age, with 68% of patients with later-onset IBD restricting diets to control the disease. It’s also possible that beliefs about food and “clean and healthy” eating may influence food and eating choices after a patient receives an IBD diagnosis.
Dr. Taft also pointed out that clinicians and patients may have different ideas about what constitutes significant food avoidance. Clinicians may expect patients with IBD to eat despite feeling nauseated, having abdominal pains, or diarrhea, for example, when the same food avoidance might be deemed reasonable in patients with short-term GI infections.
“Severe IBD symptoms are a significant predictor of posttraumatic stress disorder symptoms, and PTSD is hallmarked by avoidance behaviors,” she added.
She emphasized the need for clinicians to ask the right questions of patients to get at the roots of their nutritional deficiency or eating behavior, and to refer patients to mental health professionals with expertise in disordered eating or GI psychology.
Dr. Taft and Dr. Burton Murray reported having no conflicts of interest to disclose.
This article was updated on Feb. 4, 2022.
Problems with eating and nutrition are common among patients with inflammatory bowel disease (IBD) and other gastrointestinal disorders, but clinicians who treat them should be careful not to automatically assume that patients have eating disorders, according to a psychologist who specializes in the psychological and social aspects of chronic digestive diseases.
On the other hand, clinicians must also be aware of the possibility that patients could have a recently identified syndrome cluster called avoidant restrictive food intake disorder (ARFID), said Tiffany Taft, PsyD, a research associate professor of medicine (gastroenterology and hepatology), medical social sciences, and psychiatry and behavioral sciences at Northwestern University, Chicago. In a recent study, she and her colleagues defined ARFID as “failure to meet one’s nutritional needs owing to sensory hypersensitivity, lack of interest in eating, or fear of aversive consequences from eating, and is associated with negative medical and psychosocial outcomes.”
ARFID “is a hot topic that we really don’t understand,” she said in an online presentation at the annual Crohn’s & Colitis Congress®, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
Nutritional deficiencies
Nutritional deficiencies are common among patients with IBD, “and nutritional deficiencies themselves can lead to symptoms or side effects that can cause people to eat less,” she said.
“As our vitamin B12 goes down, our cognitive functioning starts to decline, and we might not be making clear decisions in how we’re deciding what to eat, when to eat, if we should be eating at all – just something to think about in your patients who have nutritional deficiencies,” she told the audience.
Other common nutritional deficiencies that can affect eating and food choice among patients with IBD include low folate (B9) levels associated with sore tongue and weight loss, low iron levels leading to nausea and loss of appetite, and zinc deficiency leading to loss of appetite and alterations in taste and/or smell, she said.
Newly recognized in GI
She noted that “ARFID actually originates in the pediatric psychiatric literature, mostly in children with sensory issues [such as] autism spectrum disorder, so this is not a construct that started in digestive disease, but has been adapted and applied to patients with digestive disease, including IBD.”
The DSM-5 lists four criteria for ARFID: significant weight loss, significant nutritional deficiency, dependence on enteral nutrition or oral supplements, and marked interference with psychosocial functions.
Helen Burton Murray, PhD, director of the gastrointestinal behavioral health program in the Center for Neurointestinal Health at Massachusetts General Hospital, Boston, who is familiar with Dr. Taft’s work, said in an interview that inclusion of ARFID in DSM-5 has put a name to a syndrome or symptom cluster that in all likelihood already existed.
However, “the jury is still out about whether, if we do diagnose patients who have digestive diseases with ARFID, that then helps them get to a treatment that improves their relationship with food and improves nutritional issues that may have occurred as a result of a restricted food intake,” she said.
“We don’t know yet if the diagnosis will actually improve things. In our clinical practice, anecdotally, it has, both for patients with IBDs and for patients with other GI conditions, particularly GI functional motility disorders. We’re a little bit more confident about making the diagnosis of ARFID in GI functional motility disorders than we are in IBD of course,” she said.
Screening measures
To get a better sense of the prevalence of ARFID, compared with reasonable responses to digestive diseases, Dr. Taft and colleagues conducted their cross-sectional study in 289 adults with achalasia, celiac, eosinophilic esophagitis, or IBD.
They found that 51.3% of the total sample met the diagnostic criteria for ARFID based on the Nine-Item ARFID Screen (NIAS), including 75.7 % of patients with achalasia. But Dr. Taft had cautions
“I can tell you, working with achalasia patients, 75% do not have ARFID,” Dr. Taft said.
She noted that the 51.3% of patients with IBD identified by NIAS or the 53% identified by the ARFID+ scale as having ARFID was also highly doubtful.
Dr. Taft and colleagues determined that nearly half of the variance in the NIAS could be accounted for by GI symptoms rather than psychosocial factors, making it less than ideal for use in the clinic or by researchers.
She also noted, however, that she received an email from one of the creators of NIAS, Hana F. Zickgraf, PhD, from the University of South Alabama, Mobile. Dr. Zickgraf agreed that the scale had drawbacks when applied to patients with GI disease, and pointed instead to the Fear of Food Questionnaire, a newly developed 18-item GI disease-specific instrument. Dr. Taft recommended the new questionnaire for research purposes, and expressed hope that a shorter version could be made available for screening patients in clinic.
Dr. Burton Murray said that while the Fear of Food Questionnaire, perhaps in combination with NIAS, has the potential to be a useful screening tool, cutoffs for it have yet to be established.
“At the end of the day, the diagnosis would be made by a clinician who is able to determine whether the life impairment or if the nutritional impairment or restricted food intake are reasonable in the realm of their digestive disease, or could a treatment for ARFID be warranted to help them to make changes to improve their quality of life and nutrition,” she said.
Check biases at the door
Before arriving at a diagnosis of ARFID, clinicians should also consider biases, Dr. Taft said.
“Eating disorders are highly stigmatized and stereotyped diagnoses,” more often attributed to young White women than to either men or to people of racial or ethnic minorities, she said.
Cultural background may contribute to food restrictions, and the risk may increase with age, with 68% of patients with later-onset IBD restricting diets to control the disease. It’s also possible that beliefs about food and “clean and healthy” eating may influence food and eating choices after a patient receives an IBD diagnosis.
Dr. Taft also pointed out that clinicians and patients may have different ideas about what constitutes significant food avoidance. Clinicians may expect patients with IBD to eat despite feeling nauseated, having abdominal pains, or diarrhea, for example, when the same food avoidance might be deemed reasonable in patients with short-term GI infections.
“Severe IBD symptoms are a significant predictor of posttraumatic stress disorder symptoms, and PTSD is hallmarked by avoidance behaviors,” she added.
She emphasized the need for clinicians to ask the right questions of patients to get at the roots of their nutritional deficiency or eating behavior, and to refer patients to mental health professionals with expertise in disordered eating or GI psychology.
Dr. Taft and Dr. Burton Murray reported having no conflicts of interest to disclose.
This article was updated on Feb. 4, 2022.
FROM CROHN’S & COLITIS CONGRESS
The management of inflammatory bowel disease in pregnancy
Inflammatory bowel disease (IBD) incidence is rising globally.1-3 In the United States, we have seen a 123% increase in prevalence of IBD among adults and a 133% increase among children from 2007 to 2016, with an annual percentage change of 9.9%.1 The rise of IBD in young people, and the overall higher prevalence in women compared with men, make pregnancy and IBD a topic of increasing importance for gastroenterologists.1 Here, we will discuss management and expectations in women with IBD before conception, during pregnancy, and post partum.
Preconception
Disease activity
Achieving both clinical and endoscopic remission of disease prior to conception is the key to ensuring the best maternal and fetal outcomes. Patients with IBD who conceive while in remission remain in remission 80% of the time.4,5 On the other hand, those who conceive while their disease is active may continue to have active or worsening disease in nearly 70% of cases.4 Active disease has been associated with an increased incidence of preterm birth, low birth weight, and small-for-gestational-age birth.6-8 Active disease can also exacerbate malnutrition and result in poor maternal weight gain, which is associated with intrauterine growth restriction.9,7 Pregnancy outcomes in patients with IBD and quiescent disease are similar to those in the general population.10,11
Health care maintenance
Optimizing maternal health prior to conception is critical. Alcohol, tobacco, recreational drugs, and marijuana should all be avoided. Opioids should be tapered off prior to conception, as continued use may result in neonatal opioid withdrawal syndrome and long-term neurodevelopmental consequences.12,13 In addition, aiming for a healthy body mass index between 18 and 25 months prior to conception allows for better overall pregnancy outcomes.13 Appropriate cancer screening includes colon cancer screening in those with more than 8 years of colitis, regular pap smear for cervical cancer, and annual total body skin cancer examinations for patients on thiopurines and biologic therapies.14
Nutrition
Folic acid supplementation with at least 400 micrograms (mcg) daily is necessary for all women planning pregnancy. Patients with small bowel involvement or history of small bowel resection should have a folate intake of a minimum of 2 grams per day. Adequate vitamin D levels (at least 20 ng/mL) are recommended in all women with IBD. Those with malabsorption should be screened for deficiencies in vitamin B12, folate, and iron.13 These nutritional markers should be evaluated prepregnancy, during the first trimester, and thereafter as needed.15-18
Preconception counseling
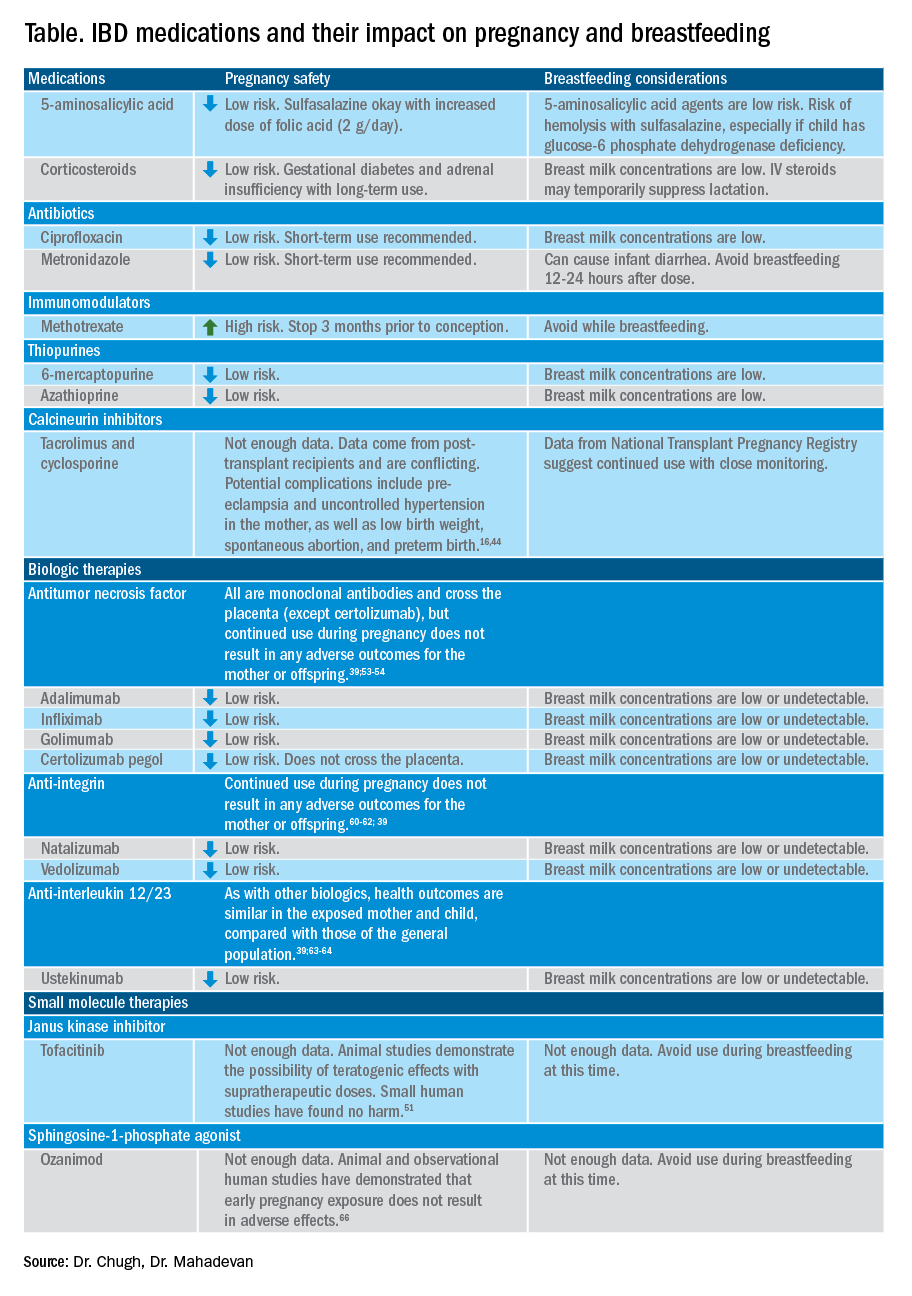
Steroid-free remission for at least 3 months prior to conception is recommended and is associated with reduced risk of flare during pregnancy.16,19 IBD medications needed to control disease activity are generally safe preconception and during pregnancy, with some exception (Table).
Misconceptions regarding heritability of IBD have sometimes discouraged men and women from having children. While genetics may increase susceptibility, environmental and other factors are involved as well. The concordance rates for monozygotic twins range from 33.3%-58.3% for Crohn’s disease and 13.4%-27.9% for ulcerative colitis (UC).20 The risk of a child developing IBD is higher in those who have multiple relatives with IBD and whose parents had IBD at the time of conception.21 While genetic testing for IBD loci is available, it is not commonly performed at this time as many genes are involved.22
Pregnancy
Coordinated care
A complete team of specialists with coordinated care among all providers is needed for optimal maternal and fetal outcomes.23,24 A gastroenterologist, ideally an IBD specialist, should follow the patient throughout pregnancy, seeing the patient at least once during the first or second trimester and as needed during pregnancy.16 A high-risk obstetrician or maternal-fetal medicine specialist should be involved early in pregnancy, as well. Open communication among all disciplines ensures that a common message is conveyed to the patient.16,24 A nutritionist, mental health provider, and lactation specialist knowledgeable about IBD drugs may be of assistance, as well.16
Disease activity
While women with IBD are at increased risk of spontaneous abortion, preterm birth, and labor complications, this risk is mitigated by controlling disease activity.25 The risk of preterm birth, small-for-gestational-age birth, and delivery via C-section is much higher in women with moderate-to-high disease activity, compared with those with low disease activity.26 The presence of active perianal disease mandates C-section over vaginal delivery. Fourth-degree lacerations following vaginal delivery are most common among those patients with perianal disease.26,27 Stillbirths were shown to be increased only in those with active IBD when compared with non-IBD comparators and inactive IBD.28-31;11
Noninvasive methods for disease monitoring are preferred in pregnancy, but serum markers such as erythrocyte sedimentation rate and C-reactive protein may not be reliable in the pregnant patient (Figure).32 Fecal calprotectin does rise in correlation with disease activity, but exact thresholds have not been validated in pregnancy.33,34
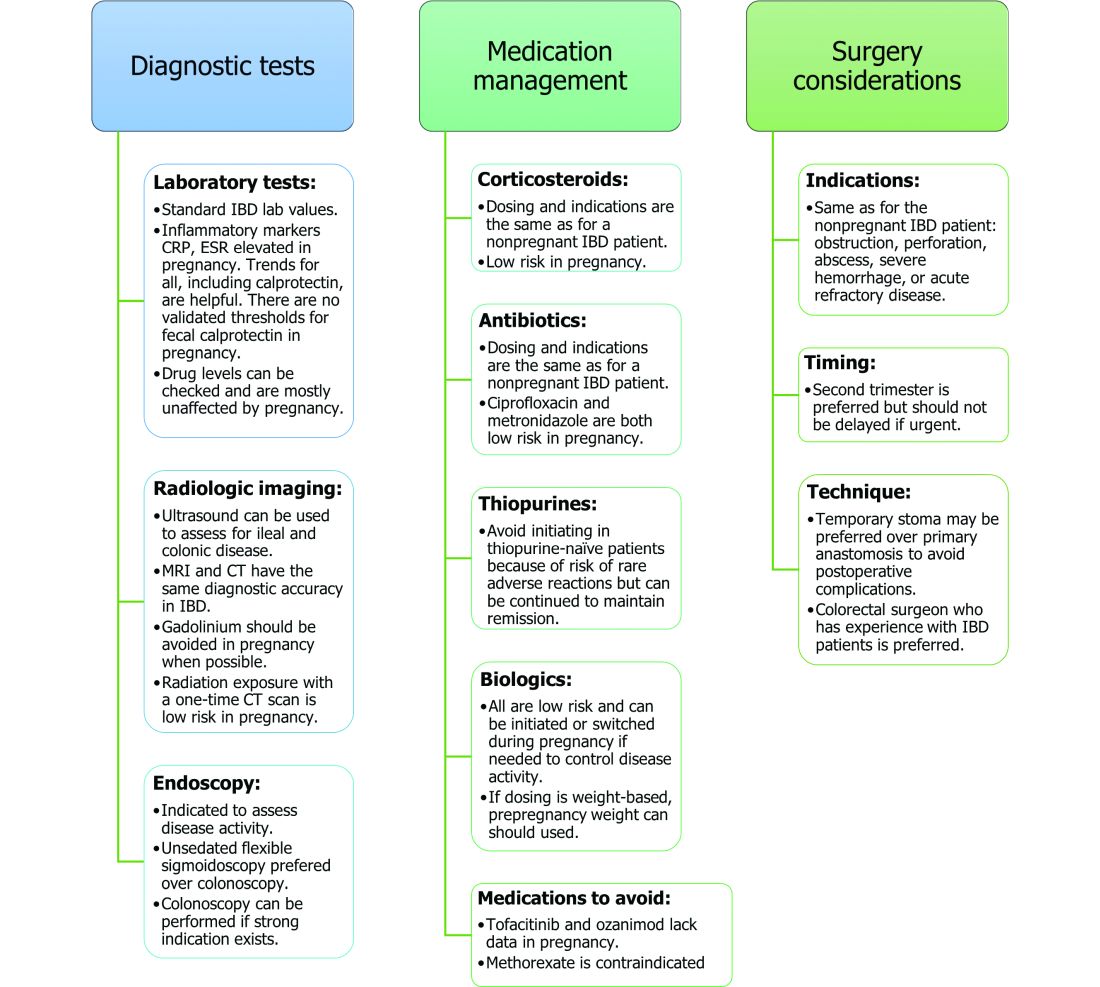
An unsedated, unprepped flexible sigmoidoscopy can be safely performed throughout pregnancy.35 When there is a strong indication, a complete colonoscopy can be performed in the pregnant patient as well.36 Current American Society for Gastrointestinal Endoscopy (ASGE) guidelines suggest placing the patient in the left lateral tilt position to avoid decreased maternal and placental perfusion via compression of the aorta or inferior vena cava and performing endoscopy during the second trimester, although trimester-specific timing is not always feasible by indication.37
Medication use and safety
IBD medications are a priority topic of concern among pregnant patients or those considering conception.38 Comprehensive data from the PIANO (Pregnancy in Inflammatory Bowel Disease and Neonatal Outcomes) registry has shown that most IBD drugs do not result in adverse pregnancy outcomes and should be continued.39 The use of biologics and thiopurines, either in combination or alone, is not related to an increased risk of congenital malformations, spontaneous abortion, preterm birth, low birth weight, or infections during the child’s first year of life.7,39 Developmental milestones also remain unaffected.39 Here, we will discuss safety considerations during pregnancy (see Table).
5-aminosalycylic acid. 5-aminosalicylic acid (5-ASA) agents are generally low risk during pregnancy and should be continued.40-41 Sulfasalazine does interfere with folate metabolism, but by increasing folic acid supplementation to 2 grams per day, sulfasalazine can be continued throughout pregnancy, as well.42
Corticosteroids. Intrapartum corticosteroid use is associated with an increased risk of gestational diabetes and adrenal insufficiency when used long term.43-45 Short-term use may, however, be necessary to control an acute flare. The lowest dose for the shortest duration possible is recommended. Because of its high first-pass metabolism, budesonide is considered low risk in pregnancy.
Methotrexate. Methotrexate needs to be stopped at least 3 months prior to conception and should be avoided throughout pregnancy. Use during pregnancy can result in spontaneous abortions, as well as embryotoxicity.46
Thiopurines (6-mercaptopurine and azathioprine). Patients who are taking thiopurines prior to conception to maintain remission can continue to do so. Data on thiopurines from the PIANO registry has shown no increase in spontaneous abortions, congenital malformations, low birth weight, preterm birth, rates of infection in the child, or developmental delays.47-51
Calcineurin inhibitors (cyclosporine and tacrolimus). Calcineurin inhibitors are reserved for the management of acute severe UC. Safety data on calcineurin inhibitors is conflicting, and there is not enough information at this time to identify risk during pregnancy. Cyclosporine can be used for salvage therapy if absolutely needed, and there are case reports of its successful using during pregnancy.16,52
Biologic therapies. With the exception of certolizumab, all of the currently used biologics are actively transported across the placenta.39,53,54 Intrapartum use of biologic therapies does not worsen pregnancy or neonatal outcomes, including the risk for intensive care unit admission, infections, and developmental milestones.39,47
While drug concentrations may vary slightly during pregnancy, these changes are not substantial enough to warrant more frequent monitoring or dose adjustments, and prepregnancy weight should be used for dosing.55,56
Antitumor necrosis factor agents used in IBD include infliximab, adalimumab, certolizumab, and golimumab.57 All are low risk for pregnant patients and their offspring. Dosage timings can be adjusted, but not stopped, to minimize exposure to the child; however, it cannot be adjusted for certolizumab pegol because of its lack of placental transfer.58-59
Natalizumab and vedolizumab are integrin receptor antagonists and are also low risk in pregnancy.57;60-62;39
Ustekinumab, an interleukin-12/23 antagonist, can be found in infant serum and cord blood, as well. Health outcomes are similar in the exposed mother and child, however, compared with those of the general population.39;63-64
Small molecule drugs. Unlike monoclonal antibodies, which do not cross the placenta in large amounts until early in the second trimester, small molecules can cross in the first trimester during the critical period of organogenesis.
The two small molecule agents currently approved for use in UC are tofacitinib, a janus kinase inhibitor, and ozanimod, a sphingosine-1-phosphate receptor agonist.65-66 Further data are still needed to make recommendations on the use of tofacitinib and ozanimod in pregnancy. At this time, we recommend weighing the risks (unknown risk to human pregnancy) vs. benefits (controlled disease activity with clear risk of harm to mother and baby from flare) in the individual patient before counseling on use in pregnancy.
Delivery
Mode of delivery
The mode of delivery should be determined by the obstetrician. C-section is recommended for patients with active perianal disease or, in some cases, a history of ileal pouch anal anastomosis (IPAA).67-68 Vaginal delivery in the setting of perianal disease has been shown to increase the risk of fourth-degree laceration and anal sphincter dysfunction in the future.26-27 Anorectal motility may be impacted by IPAA construction and vaginal delivery independently of each other. It is therefore suggested that vaginal delivery be avoided in patients with a history of IPAA to avoid compounding the risk. Some studies do not show clear harm from vaginal delivery in the setting of IPAA, however, and informed decision making among all stakeholders should be had.27;69-70
Anticoagulation
The incidence of venous thromboembolism (VTE) is elevated in patients with IBD during pregnancy, and up to 12 weeks postpartum, compared with pregnant patients without IBD.71-72 VTE for prophylaxis is indicated in the pregnant patient while hospitalized and potentially thereafter depending on the patient’s risk factors, which may include obesity, prior personal history of VTE, heart failure, and prolonged immobility. Unfractionated heparin, low molecular weight heparin, and warfarin are safe for breastfeeding women.16,73
Postpartum care of mother
There is a risk of postpartum flare, occurring in about one third of patients in the first 6 months postpartum.74-75 De-escalating therapy during delivery or immediately postpartum is a predictor of a postpartum flare.75 If no infection is present and the timing interval is appropriate, biologic therapies should be continued and can be resumed 24 hours after a vaginal delivery and 48 hours after a C-section.16,76
NSAIDs and opioids can be used for pain relief but should be avoided in the long-term to prevent flares (NSAIDs) and infant sedation (associated with opioids) when used while breastfeeding.77 The LactMed database is an excellent resource for clarification on risk of medication use while breastfeeding.78
In particular, contraception should be addressed postpartum. Exogenous estrogen use increases the risk of VTE, which is already increased in IBD; nonestrogen containing, long-acting reversible contraception is preferred.79-80 Progestin-only implants or intrauterine devices may be used first line. The efficacy of oral contraceptives is theoretically reduced in those with rapid bowel transit, active small bowel inflammation, and prior small bowel resection, so adding another form of contraception is recommended.16,81
Source: American Gastroenterological Association
Postdelivery care of baby
Breastfeeding
Guidelines regarding medication use during breastfeeding are similar to those in pregnancy (see Table). Breastfeeding on biologics and thiopurines can continue without interruption in the child. Thiopurine concentrations in breast milk are low or undetectable.82,78 TNF receptor antagonists, anti-integrin therapies, and ustekinumab are found in low to undetectable levels in breast milk, as well.78
On the other hand, the active metabolite of methotrexate is detectable in breast milk and most sources recommend not breastfeeding on methotrexate. At doses used in IBD (15-25 milligrams per week), some experts have suggested avoiding breastfeeding for 24 hours following a dose.57,78 It is the practice of this author to recommend not breastfeeding at all on methotrexate.
5-ASA therapies are low risk for breastfeeding, but alternatives to sulfasalazine are preferred. The sulfapyridine metabolite transfers to breast milk and may cause hemolysis in infants born with a glucose-6-phosphate dehydrogenase deficiency.78
With regards to calcineurin inhibitors, tacrolimus appears in breast milk in low quantities, while cyclosporine levels are variable. Data from the National Transplantation Pregnancy Registry suggest that these medications can be used at the time of breastfeeding with close monitoring.78
There is not enough data on small molecule therapies at this time to support breastfeeding safety, and it is our practice to not recommend breastfeeding in this scenario.
The transfer of steroids to the child via breast milk does occur but at subtherapeutic levels.16 Budesonide has high first pass metabolism and is low risk during breastfeeding.83-84 As far as is known, IBD maintenance medications do not suppress lactation. The use of intravenous corticosteroids can, however, temporarily decrease milk production.16,85
Vaccines
Vaccination of infants can proceed as indicated by the Center for Disease Control and Prevention guidelines, with one exception. If the child’s mother was exposed to any biologic agents (not including certolizumab) during the third trimester, any live vaccines should be withheld in the first 6 months of life. In the United States, this restriction currently only applies to the rotavirus vaccine, which is administered starting at the age of 2 months.16,86 Notably, inadvertent administration of the rotavirus vaccine in the biologic-exposed child does not appear to result in any adverse effects.87 Immunity is achieved even if the child is exposed to IBD therapies through breast milk.88
Developmental milestones
Infant exposure to biologics and thiopurines has not been shown to result in any developmental delays. The PIANO study measured developmental milestones at 48 months from birth and found no differences when compared with validated population norms.39 A separate study observing childhood development up to 7 years of age in patients born to mothers with IBD found similar cognitive scores and motor development when compared with those born to mothers without IBD.89
Conclusion
Women considering conception should be optimized prior to pregnancy and maintained on appropriate medications throughout pregnancy and lactation to achieve a healthy pregnancy for both mother and baby. To date, biologics and thiopurines are not associated with adverse pregnancy outcomes. More data are needed for small molecules.
Dr. Chugh is an advanced inflammatory bowel disease fellow in the division of gastroenterology at the University of California San Francisco. Dr. Mahadevan is professor of medicine and codirector at the Center for Colitis and Crohn’s Disease in the division of gastroenterology at the University of California San Francisco. Dr. Mahadevan has potential conflicts related to AbbVie, Janssen, BMS, Takeda, Pfizer, Lilly, Gilead, Arena, and Prometheus Biosciences.
References
1. Ye Y et al. Inflamm Bowel Dis. 2020;26:619-25.
2. Sykora J et al. World J Gastroenterol. 2018;24:2741-63.
3. Murakami Y et al. J Gastroenterol 2019;54:1070-7.
4. Hashash JG and Kane S. Gastroenterol Hepatol. (N Y) 2015;11:96-102.
5. Miller JP. J R Soc Med. 1986;79:221-5.
6. Cornish J et al. Gut. 2007;56:830-7.
7. Leung KK et al. Inflamm Bowel Dis. 2021;27:550-62.
8. O’Toole A et al. Dig Dis Sci. 2015;60:2750-61.
9. Nguyen GC et al. Inflamm Bowel Dis. 2008;14:1105-11.
10. Lee HH et al. Aliment Pharmacol Ther. 2020;51:861-9.
11. Kim MA et al. J Crohns Colitis. 2021;15:719-32.
12. Conradt E et al. Pediatrics. 2019;144.
13. ACOG Committee Opinion No. 762: Prepregnancy Counseling. Obstet Gynecol. 2019;133:e78-e89.
14. Farraye FA et al. Am J Gastroenterol. 2017;112:241-58.
15. Lee S et al. J Crohns Colitis. 2018;12:702-9.
16. Mahadevan U et al. Inflamm Bowel Dis. 2019;25:627-41.
17. Ward MG et al. Inflamm. Bowel Dis 2015;21:2839-47.
18. Battat R et al. Inflamm Bowel Dis. 2014;20:1120-8.
19. Pedersen N et al. Aliment Pharmacol Ther. 2013;38:501-12.
20. Annese V. Pharmacol Res. 2020;159:104892.
21. Bennett RA et al. Gastroenterology. 1991;100:1638-43.
22. Turpin W et al. Inflamm Bowel Dis. 2018;24:1133-48.
23. de Lima A et al. Clin Gastroenterol Hepatol. 2016;14:1285-92 e1.
24. Selinger C et al. Frontline Gastroenterol. 2021;12:182-7.
25. Mahadevan U et al. Gastroenterology. 2007;133:1106-12.
26. Hatch Q et al. Dis Colon Rectum. 2014;57:174-8.
27. Foulon A et al. Inflamm Bowel Dis. 2017;23:712-20.
28. Norgard B et al. Am J Gastroenterol. 2007;102:1947-54.
29. Broms G et al. Scand J Gastroenterol 2016;51:1462-9.
30. Meyer A et al. Aliment Pharmacol Ther. 2020;52:1480-90.
31. Kammerlander H et al. Inflamm Bowel Dis. 2017;23:1011-8.
32. Tandon P et al. J Clin Gastroenterol. 2019;53:574-81.
33. Kammerlander H et al. Inflamm Bowel Dis. 2018;24:839-48.
34. Julsgaard M et al. Inflamm Bowel Dis. 2017;23:1240-6.
35. Ko MS et al. Dig Dis Sci. 2020;65:2979-85.
36. Cappell MS et al. J Reprod Med. 2010;55:115-23.
37. Committee ASoP et al. Gastrointest Endosc. 2012;76:18-24.
38. Aboubakr A et al. Dig Dis Sci. 2021;66:1829-35.
39. Mahadevan U et al. Gastroenterology. 2021;160:1131-9.
40. Diav-Citrin O et al. Gastroenterology. 1998;114:23-8.
41. Rahimi R et al. Reprod Toxicol. 2008;25:271-5.
42. Norgard B et al. Aliment Pharmacol Ther. 2001;15:483-6.
43. Leung YP et al. J Crohns Colitis. 2015;9:223-30.
44. Schulze H et al. Aliment Pharmacol Ther. 2014;40:991-1008.
45. Szymanska E et al. J Gynecol Obstet Hum Reprod. 2021;50:101777.
46. Weber-Schoendorfer C et al. Arthritis Rheumatol. 2014;66:1101-10.
47. Nielsen OH et al. Clin Gastroenterol Hepatol. 2022 Jan;20(1):74-87.e3.
48. Coelho J et al. Gut. 2011;60:198-203.
49. Sheikh M et al. J Crohns Colitis. 2015;9:680-4.
50. Kanis SL et al. Clin Gastroenterol Hepatol. 2017;15:1232-41 e1.
51. Mahadevan U et al. Inflamm Bowel Dis. 2018;24:2494-500.
52. Rosen MH et al. Inflamm Bowel Dis. 2020;26:971-3.
53. Porter C et al. J Reprod Immunol. 2016;116:7-12.
54. Mahadevan U et al. Clin Gastroenterol Hepatol. 2013;11:286-92; quiz e24.
55. Picardo S and Seow CH. Best Pract Res Clin Gastroenterol. 2020;44-5:101670.
56. Flanagan E et al. Aliment Pharmacol Ther. 2020;52:1551-62.
57. Singh S et al. Gastroenterology. 2021;160:2512-56 e9.
58. de Lima A et al. Gut. 2016;65:1261-8.
59. Julsgaard M et al. Inflamm Bowel Dis. 2020;26:93-102.
60. Wils P et al. Aliment Pharmacol Ther. 2021;53:460-70.
61. Mahadevan U et al. Aliment Pharmacol Ther. 2017;45:941-50.
62. Bar-Gil Shitrit A et al. Am J Gastroenterol. 2019;114:1172-5.
63. Klenske E et al. J Crohns Colitis. 2019;13:267-9.
64. Matro R et al. Gastroenterology. 2018;155:696-704.
65. Feuerstein JD et al. Gastroenterology. 2020;158:1450-61.
66. Sandborn WJ et al. J Crohns Colitis. 2021 Jul 5;15(7):1120-1129.
67. Lamb CA et al. Gut. 2019;68:s1-s106.
68. Nguyen GC et al. Gastroenterology. 2016;150:734-57 e1.
69. Ravid A et al. Dis Colon Rectum. 2002;45:1283-8.
70. Seligman NS et al. J Matern Fetal Neonatal Med. 2011;24:525-30.
71. Kim YH et al. Medicine (Baltimore). 2019;98:e17309.
72. Hansen AT et al. J Thromb Haemost. 2017;15:702-8.
73. Bates SM et al. J Thromb Thrombolysis. 2016;41:92-128.
74. Bennett A et al. Inflamm Bowel Dis. 2021 May 17;izab104.
75. Yu A et al. Inflamm Bowel Dis. 2020;26:1926-32.
76. Mahadevan U et al. Gastroenterology. 2017;152:451-62 e2.
77. Long MD et al. J Clin Gastroenterol. 2016;50:152-6.
78. Drugs and Lactation Database (LactMed). 2006 ed. Bethesda, MD: National Library of Medicine (US), 2006-2021.
79. Khalili H et al. Gut. 2013;62:1153-9.
80. Long MD and Hutfless S. Gastroenterology. 2016;150:1518-20.
81. Centers for Disease Control and Prevention. U S. Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR Recomm Rep. 2010;59:1-86.
82. Angelberger S et al. J Crohns Colitis. 2011;5:95-100.
83. Vestergaard T et al. Scand J Gastroenterol. 2018;53:1459-62.
84. Beaulieu DB et al. Inflamm Bowel Dis. 2009;15:25-8.
85. Anderson PO. Breastfeed Med. 2017;12:199-201.
86. Wodi AP et al. MMWR Morb Mortal Wkly Rep. 2021;70:189-92.
87. Chiarella-Redfern H et al. Inflamm Bowel Dis. 2022 Jan 5;28(1):79-86.
88. Beaulieu DB et al. Clin Gastroenterol Hepatol. 2018;16:99-105.
89. Friedman S et al. J Crohns Colitis. 2020 Dec 2;14(12):1709-1716.
Inflammatory bowel disease (IBD) incidence is rising globally.1-3 In the United States, we have seen a 123% increase in prevalence of IBD among adults and a 133% increase among children from 2007 to 2016, with an annual percentage change of 9.9%.1 The rise of IBD in young people, and the overall higher prevalence in women compared with men, make pregnancy and IBD a topic of increasing importance for gastroenterologists.1 Here, we will discuss management and expectations in women with IBD before conception, during pregnancy, and post partum.
Preconception
Disease activity
Achieving both clinical and endoscopic remission of disease prior to conception is the key to ensuring the best maternal and fetal outcomes. Patients with IBD who conceive while in remission remain in remission 80% of the time.4,5 On the other hand, those who conceive while their disease is active may continue to have active or worsening disease in nearly 70% of cases.4 Active disease has been associated with an increased incidence of preterm birth, low birth weight, and small-for-gestational-age birth.6-8 Active disease can also exacerbate malnutrition and result in poor maternal weight gain, which is associated with intrauterine growth restriction.9,7 Pregnancy outcomes in patients with IBD and quiescent disease are similar to those in the general population.10,11
Health care maintenance
Optimizing maternal health prior to conception is critical. Alcohol, tobacco, recreational drugs, and marijuana should all be avoided. Opioids should be tapered off prior to conception, as continued use may result in neonatal opioid withdrawal syndrome and long-term neurodevelopmental consequences.12,13 In addition, aiming for a healthy body mass index between 18 and 25 months prior to conception allows for better overall pregnancy outcomes.13 Appropriate cancer screening includes colon cancer screening in those with more than 8 years of colitis, regular pap smear for cervical cancer, and annual total body skin cancer examinations for patients on thiopurines and biologic therapies.14
Nutrition
Folic acid supplementation with at least 400 micrograms (mcg) daily is necessary for all women planning pregnancy. Patients with small bowel involvement or history of small bowel resection should have a folate intake of a minimum of 2 grams per day. Adequate vitamin D levels (at least 20 ng/mL) are recommended in all women with IBD. Those with malabsorption should be screened for deficiencies in vitamin B12, folate, and iron.13 These nutritional markers should be evaluated prepregnancy, during the first trimester, and thereafter as needed.15-18
Preconception counseling
Steroid-free remission for at least 3 months prior to conception is recommended and is associated with reduced risk of flare during pregnancy.16,19 IBD medications needed to control disease activity are generally safe preconception and during pregnancy, with some exception (Table).

Misconceptions regarding heritability of IBD have sometimes discouraged men and women from having children. While genetics may increase susceptibility, environmental and other factors are involved as well. The concordance rates for monozygotic twins range from 33.3%-58.3% for Crohn’s disease and 13.4%-27.9% for ulcerative colitis (UC).20 The risk of a child developing IBD is higher in those who have multiple relatives with IBD and whose parents had IBD at the time of conception.21 While genetic testing for IBD loci is available, it is not commonly performed at this time as many genes are involved.22
Pregnancy
Coordinated care
A complete team of specialists with coordinated care among all providers is needed for optimal maternal and fetal outcomes.23,24 A gastroenterologist, ideally an IBD specialist, should follow the patient throughout pregnancy, seeing the patient at least once during the first or second trimester and as needed during pregnancy.16 A high-risk obstetrician or maternal-fetal medicine specialist should be involved early in pregnancy, as well. Open communication among all disciplines ensures that a common message is conveyed to the patient.16,24 A nutritionist, mental health provider, and lactation specialist knowledgeable about IBD drugs may be of assistance, as well.16
Disease activity
While women with IBD are at increased risk of spontaneous abortion, preterm birth, and labor complications, this risk is mitigated by controlling disease activity.25 The risk of preterm birth, small-for-gestational-age birth, and delivery via C-section is much higher in women with moderate-to-high disease activity, compared with those with low disease activity.26 The presence of active perianal disease mandates C-section over vaginal delivery. Fourth-degree lacerations following vaginal delivery are most common among those patients with perianal disease.26,27 Stillbirths were shown to be increased only in those with active IBD when compared with non-IBD comparators and inactive IBD.28-31;11
Noninvasive methods for disease monitoring are preferred in pregnancy, but serum markers such as erythrocyte sedimentation rate and C-reactive protein may not be reliable in the pregnant patient (Figure).32 Fecal calprotectin does rise in correlation with disease activity, but exact thresholds have not been validated in pregnancy.33,34

An unsedated, unprepped flexible sigmoidoscopy can be safely performed throughout pregnancy.35 When there is a strong indication, a complete colonoscopy can be performed in the pregnant patient as well.36 Current American Society for Gastrointestinal Endoscopy (ASGE) guidelines suggest placing the patient in the left lateral tilt position to avoid decreased maternal and placental perfusion via compression of the aorta or inferior vena cava and performing endoscopy during the second trimester, although trimester-specific timing is not always feasible by indication.37
Medication use and safety
IBD medications are a priority topic of concern among pregnant patients or those considering conception.38 Comprehensive data from the PIANO (Pregnancy in Inflammatory Bowel Disease and Neonatal Outcomes) registry has shown that most IBD drugs do not result in adverse pregnancy outcomes and should be continued.39 The use of biologics and thiopurines, either in combination or alone, is not related to an increased risk of congenital malformations, spontaneous abortion, preterm birth, low birth weight, or infections during the child’s first year of life.7,39 Developmental milestones also remain unaffected.39 Here, we will discuss safety considerations during pregnancy (see Table).
5-aminosalycylic acid. 5-aminosalicylic acid (5-ASA) agents are generally low risk during pregnancy and should be continued.40-41 Sulfasalazine does interfere with folate metabolism, but by increasing folic acid supplementation to 2 grams per day, sulfasalazine can be continued throughout pregnancy, as well.42
Corticosteroids. Intrapartum corticosteroid use is associated with an increased risk of gestational diabetes and adrenal insufficiency when used long term.43-45 Short-term use may, however, be necessary to control an acute flare. The lowest dose for the shortest duration possible is recommended. Because of its high first-pass metabolism, budesonide is considered low risk in pregnancy.
Methotrexate. Methotrexate needs to be stopped at least 3 months prior to conception and should be avoided throughout pregnancy. Use during pregnancy can result in spontaneous abortions, as well as embryotoxicity.46
Thiopurines (6-mercaptopurine and azathioprine). Patients who are taking thiopurines prior to conception to maintain remission can continue to do so. Data on thiopurines from the PIANO registry has shown no increase in spontaneous abortions, congenital malformations, low birth weight, preterm birth, rates of infection in the child, or developmental delays.47-51
Calcineurin inhibitors (cyclosporine and tacrolimus). Calcineurin inhibitors are reserved for the management of acute severe UC. Safety data on calcineurin inhibitors is conflicting, and there is not enough information at this time to identify risk during pregnancy. Cyclosporine can be used for salvage therapy if absolutely needed, and there are case reports of its successful using during pregnancy.16,52
Biologic therapies. With the exception of certolizumab, all of the currently used biologics are actively transported across the placenta.39,53,54 Intrapartum use of biologic therapies does not worsen pregnancy or neonatal outcomes, including the risk for intensive care unit admission, infections, and developmental milestones.39,47
While drug concentrations may vary slightly during pregnancy, these changes are not substantial enough to warrant more frequent monitoring or dose adjustments, and prepregnancy weight should be used for dosing.55,56
Antitumor necrosis factor agents used in IBD include infliximab, adalimumab, certolizumab, and golimumab.57 All are low risk for pregnant patients and their offspring. Dosage timings can be adjusted, but not stopped, to minimize exposure to the child; however, it cannot be adjusted for certolizumab pegol because of its lack of placental transfer.58-59
Natalizumab and vedolizumab are integrin receptor antagonists and are also low risk in pregnancy.57;60-62;39
Ustekinumab, an interleukin-12/23 antagonist, can be found in infant serum and cord blood, as well. Health outcomes are similar in the exposed mother and child, however, compared with those of the general population.39;63-64
Small molecule drugs. Unlike monoclonal antibodies, which do not cross the placenta in large amounts until early in the second trimester, small molecules can cross in the first trimester during the critical period of organogenesis.
The two small molecule agents currently approved for use in UC are tofacitinib, a janus kinase inhibitor, and ozanimod, a sphingosine-1-phosphate receptor agonist.65-66 Further data are still needed to make recommendations on the use of tofacitinib and ozanimod in pregnancy. At this time, we recommend weighing the risks (unknown risk to human pregnancy) vs. benefits (controlled disease activity with clear risk of harm to mother and baby from flare) in the individual patient before counseling on use in pregnancy.
Delivery
Mode of delivery
The mode of delivery should be determined by the obstetrician. C-section is recommended for patients with active perianal disease or, in some cases, a history of ileal pouch anal anastomosis (IPAA).67-68 Vaginal delivery in the setting of perianal disease has been shown to increase the risk of fourth-degree laceration and anal sphincter dysfunction in the future.26-27 Anorectal motility may be impacted by IPAA construction and vaginal delivery independently of each other. It is therefore suggested that vaginal delivery be avoided in patients with a history of IPAA to avoid compounding the risk. Some studies do not show clear harm from vaginal delivery in the setting of IPAA, however, and informed decision making among all stakeholders should be had.27;69-70
Anticoagulation
The incidence of venous thromboembolism (VTE) is elevated in patients with IBD during pregnancy, and up to 12 weeks postpartum, compared with pregnant patients without IBD.71-72 VTE for prophylaxis is indicated in the pregnant patient while hospitalized and potentially thereafter depending on the patient’s risk factors, which may include obesity, prior personal history of VTE, heart failure, and prolonged immobility. Unfractionated heparin, low molecular weight heparin, and warfarin are safe for breastfeeding women.16,73
Postpartum care of mother
There is a risk of postpartum flare, occurring in about one third of patients in the first 6 months postpartum.74-75 De-escalating therapy during delivery or immediately postpartum is a predictor of a postpartum flare.75 If no infection is present and the timing interval is appropriate, biologic therapies should be continued and can be resumed 24 hours after a vaginal delivery and 48 hours after a C-section.16,76
NSAIDs and opioids can be used for pain relief but should be avoided in the long-term to prevent flares (NSAIDs) and infant sedation (associated with opioids) when used while breastfeeding.77 The LactMed database is an excellent resource for clarification on risk of medication use while breastfeeding.78
In particular, contraception should be addressed postpartum. Exogenous estrogen use increases the risk of VTE, which is already increased in IBD; nonestrogen containing, long-acting reversible contraception is preferred.79-80 Progestin-only implants or intrauterine devices may be used first line. The efficacy of oral contraceptives is theoretically reduced in those with rapid bowel transit, active small bowel inflammation, and prior small bowel resection, so adding another form of contraception is recommended.16,81
Source: American Gastroenterological Association
Postdelivery care of baby
Breastfeeding
Guidelines regarding medication use during breastfeeding are similar to those in pregnancy (see Table). Breastfeeding on biologics and thiopurines can continue without interruption in the child. Thiopurine concentrations in breast milk are low or undetectable.82,78 TNF receptor antagonists, anti-integrin therapies, and ustekinumab are found in low to undetectable levels in breast milk, as well.78
On the other hand, the active metabolite of methotrexate is detectable in breast milk and most sources recommend not breastfeeding on methotrexate. At doses used in IBD (15-25 milligrams per week), some experts have suggested avoiding breastfeeding for 24 hours following a dose.57,78 It is the practice of this author to recommend not breastfeeding at all on methotrexate.
5-ASA therapies are low risk for breastfeeding, but alternatives to sulfasalazine are preferred. The sulfapyridine metabolite transfers to breast milk and may cause hemolysis in infants born with a glucose-6-phosphate dehydrogenase deficiency.78
With regards to calcineurin inhibitors, tacrolimus appears in breast milk in low quantities, while cyclosporine levels are variable. Data from the National Transplantation Pregnancy Registry suggest that these medications can be used at the time of breastfeeding with close monitoring.78
There is not enough data on small molecule therapies at this time to support breastfeeding safety, and it is our practice to not recommend breastfeeding in this scenario.
The transfer of steroids to the child via breast milk does occur but at subtherapeutic levels.16 Budesonide has high first pass metabolism and is low risk during breastfeeding.83-84 As far as is known, IBD maintenance medications do not suppress lactation. The use of intravenous corticosteroids can, however, temporarily decrease milk production.16,85
Vaccines
Vaccination of infants can proceed as indicated by the Center for Disease Control and Prevention guidelines, with one exception. If the child’s mother was exposed to any biologic agents (not including certolizumab) during the third trimester, any live vaccines should be withheld in the first 6 months of life. In the United States, this restriction currently only applies to the rotavirus vaccine, which is administered starting at the age of 2 months.16,86 Notably, inadvertent administration of the rotavirus vaccine in the biologic-exposed child does not appear to result in any adverse effects.87 Immunity is achieved even if the child is exposed to IBD therapies through breast milk.88
Developmental milestones
Infant exposure to biologics and thiopurines has not been shown to result in any developmental delays. The PIANO study measured developmental milestones at 48 months from birth and found no differences when compared with validated population norms.39 A separate study observing childhood development up to 7 years of age in patients born to mothers with IBD found similar cognitive scores and motor development when compared with those born to mothers without IBD.89
Conclusion
Women considering conception should be optimized prior to pregnancy and maintained on appropriate medications throughout pregnancy and lactation to achieve a healthy pregnancy for both mother and baby. To date, biologics and thiopurines are not associated with adverse pregnancy outcomes. More data are needed for small molecules.
Dr. Chugh is an advanced inflammatory bowel disease fellow in the division of gastroenterology at the University of California San Francisco. Dr. Mahadevan is professor of medicine and codirector at the Center for Colitis and Crohn’s Disease in the division of gastroenterology at the University of California San Francisco. Dr. Mahadevan has potential conflicts related to AbbVie, Janssen, BMS, Takeda, Pfizer, Lilly, Gilead, Arena, and Prometheus Biosciences.
References
1. Ye Y et al. Inflamm Bowel Dis. 2020;26:619-25.
2. Sykora J et al. World J Gastroenterol. 2018;24:2741-63.
3. Murakami Y et al. J Gastroenterol 2019;54:1070-7.
4. Hashash JG and Kane S. Gastroenterol Hepatol. (N Y) 2015;11:96-102.
5. Miller JP. J R Soc Med. 1986;79:221-5.
6. Cornish J et al. Gut. 2007;56:830-7.
7. Leung KK et al. Inflamm Bowel Dis. 2021;27:550-62.
8. O’Toole A et al. Dig Dis Sci. 2015;60:2750-61.
9. Nguyen GC et al. Inflamm Bowel Dis. 2008;14:1105-11.
10. Lee HH et al. Aliment Pharmacol Ther. 2020;51:861-9.
11. Kim MA et al. J Crohns Colitis. 2021;15:719-32.
12. Conradt E et al. Pediatrics. 2019;144.
13. ACOG Committee Opinion No. 762: Prepregnancy Counseling. Obstet Gynecol. 2019;133:e78-e89.
14. Farraye FA et al. Am J Gastroenterol. 2017;112:241-58.
15. Lee S et al. J Crohns Colitis. 2018;12:702-9.
16. Mahadevan U et al. Inflamm Bowel Dis. 2019;25:627-41.
17. Ward MG et al. Inflamm. Bowel Dis 2015;21:2839-47.
18. Battat R et al. Inflamm Bowel Dis. 2014;20:1120-8.
19. Pedersen N et al. Aliment Pharmacol Ther. 2013;38:501-12.
20. Annese V. Pharmacol Res. 2020;159:104892.
21. Bennett RA et al. Gastroenterology. 1991;100:1638-43.
22. Turpin W et al. Inflamm Bowel Dis. 2018;24:1133-48.
23. de Lima A et al. Clin Gastroenterol Hepatol. 2016;14:1285-92 e1.
24. Selinger C et al. Frontline Gastroenterol. 2021;12:182-7.
25. Mahadevan U et al. Gastroenterology. 2007;133:1106-12.
26. Hatch Q et al. Dis Colon Rectum. 2014;57:174-8.
27. Foulon A et al. Inflamm Bowel Dis. 2017;23:712-20.
28. Norgard B et al. Am J Gastroenterol. 2007;102:1947-54.
29. Broms G et al. Scand J Gastroenterol 2016;51:1462-9.
30. Meyer A et al. Aliment Pharmacol Ther. 2020;52:1480-90.
31. Kammerlander H et al. Inflamm Bowel Dis. 2017;23:1011-8.
32. Tandon P et al. J Clin Gastroenterol. 2019;53:574-81.
33. Kammerlander H et al. Inflamm Bowel Dis. 2018;24:839-48.
34. Julsgaard M et al. Inflamm Bowel Dis. 2017;23:1240-6.
35. Ko MS et al. Dig Dis Sci. 2020;65:2979-85.
36. Cappell MS et al. J Reprod Med. 2010;55:115-23.
37. Committee ASoP et al. Gastrointest Endosc. 2012;76:18-24.
38. Aboubakr A et al. Dig Dis Sci. 2021;66:1829-35.
39. Mahadevan U et al. Gastroenterology. 2021;160:1131-9.
40. Diav-Citrin O et al. Gastroenterology. 1998;114:23-8.
41. Rahimi R et al. Reprod Toxicol. 2008;25:271-5.
42. Norgard B et al. Aliment Pharmacol Ther. 2001;15:483-6.
43. Leung YP et al. J Crohns Colitis. 2015;9:223-30.
44. Schulze H et al. Aliment Pharmacol Ther. 2014;40:991-1008.
45. Szymanska E et al. J Gynecol Obstet Hum Reprod. 2021;50:101777.
46. Weber-Schoendorfer C et al. Arthritis Rheumatol. 2014;66:1101-10.
47. Nielsen OH et al. Clin Gastroenterol Hepatol. 2022 Jan;20(1):74-87.e3.
48. Coelho J et al. Gut. 2011;60:198-203.
49. Sheikh M et al. J Crohns Colitis. 2015;9:680-4.
50. Kanis SL et al. Clin Gastroenterol Hepatol. 2017;15:1232-41 e1.
51. Mahadevan U et al. Inflamm Bowel Dis. 2018;24:2494-500.
52. Rosen MH et al. Inflamm Bowel Dis. 2020;26:971-3.
53. Porter C et al. J Reprod Immunol. 2016;116:7-12.
54. Mahadevan U et al. Clin Gastroenterol Hepatol. 2013;11:286-92; quiz e24.
55. Picardo S and Seow CH. Best Pract Res Clin Gastroenterol. 2020;44-5:101670.
56. Flanagan E et al. Aliment Pharmacol Ther. 2020;52:1551-62.
57. Singh S et al. Gastroenterology. 2021;160:2512-56 e9.
58. de Lima A et al. Gut. 2016;65:1261-8.
59. Julsgaard M et al. Inflamm Bowel Dis. 2020;26:93-102.
60. Wils P et al. Aliment Pharmacol Ther. 2021;53:460-70.
61. Mahadevan U et al. Aliment Pharmacol Ther. 2017;45:941-50.
62. Bar-Gil Shitrit A et al. Am J Gastroenterol. 2019;114:1172-5.
63. Klenske E et al. J Crohns Colitis. 2019;13:267-9.
64. Matro R et al. Gastroenterology. 2018;155:696-704.
65. Feuerstein JD et al. Gastroenterology. 2020;158:1450-61.
66. Sandborn WJ et al. J Crohns Colitis. 2021 Jul 5;15(7):1120-1129.
67. Lamb CA et al. Gut. 2019;68:s1-s106.
68. Nguyen GC et al. Gastroenterology. 2016;150:734-57 e1.
69. Ravid A et al. Dis Colon Rectum. 2002;45:1283-8.
70. Seligman NS et al. J Matern Fetal Neonatal Med. 2011;24:525-30.
71. Kim YH et al. Medicine (Baltimore). 2019;98:e17309.
72. Hansen AT et al. J Thromb Haemost. 2017;15:702-8.
73. Bates SM et al. J Thromb Thrombolysis. 2016;41:92-128.
74. Bennett A et al. Inflamm Bowel Dis. 2021 May 17;izab104.
75. Yu A et al. Inflamm Bowel Dis. 2020;26:1926-32.
76. Mahadevan U et al. Gastroenterology. 2017;152:451-62 e2.
77. Long MD et al. J Clin Gastroenterol. 2016;50:152-6.
78. Drugs and Lactation Database (LactMed). 2006 ed. Bethesda, MD: National Library of Medicine (US), 2006-2021.
79. Khalili H et al. Gut. 2013;62:1153-9.
80. Long MD and Hutfless S. Gastroenterology. 2016;150:1518-20.
81. Centers for Disease Control and Prevention. U S. Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR Recomm Rep. 2010;59:1-86.
82. Angelberger S et al. J Crohns Colitis. 2011;5:95-100.
83. Vestergaard T et al. Scand J Gastroenterol. 2018;53:1459-62.
84. Beaulieu DB et al. Inflamm Bowel Dis. 2009;15:25-8.
85. Anderson PO. Breastfeed Med. 2017;12:199-201.
86. Wodi AP et al. MMWR Morb Mortal Wkly Rep. 2021;70:189-92.
87. Chiarella-Redfern H et al. Inflamm Bowel Dis. 2022 Jan 5;28(1):79-86.
88. Beaulieu DB et al. Clin Gastroenterol Hepatol. 2018;16:99-105.
89. Friedman S et al. J Crohns Colitis. 2020 Dec 2;14(12):1709-1716.
Inflammatory bowel disease (IBD) incidence is rising globally.1-3 In the United States, we have seen a 123% increase in prevalence of IBD among adults and a 133% increase among children from 2007 to 2016, with an annual percentage change of 9.9%.1 The rise of IBD in young people, and the overall higher prevalence in women compared with men, make pregnancy and IBD a topic of increasing importance for gastroenterologists.1 Here, we will discuss management and expectations in women with IBD before conception, during pregnancy, and post partum.
Preconception
Disease activity
Achieving both clinical and endoscopic remission of disease prior to conception is the key to ensuring the best maternal and fetal outcomes. Patients with IBD who conceive while in remission remain in remission 80% of the time.4,5 On the other hand, those who conceive while their disease is active may continue to have active or worsening disease in nearly 70% of cases.4 Active disease has been associated with an increased incidence of preterm birth, low birth weight, and small-for-gestational-age birth.6-8 Active disease can also exacerbate malnutrition and result in poor maternal weight gain, which is associated with intrauterine growth restriction.9,7 Pregnancy outcomes in patients with IBD and quiescent disease are similar to those in the general population.10,11
Health care maintenance
Optimizing maternal health prior to conception is critical. Alcohol, tobacco, recreational drugs, and marijuana should all be avoided. Opioids should be tapered off prior to conception, as continued use may result in neonatal opioid withdrawal syndrome and long-term neurodevelopmental consequences.12,13 In addition, aiming for a healthy body mass index between 18 and 25 months prior to conception allows for better overall pregnancy outcomes.13 Appropriate cancer screening includes colon cancer screening in those with more than 8 years of colitis, regular pap smear for cervical cancer, and annual total body skin cancer examinations for patients on thiopurines and biologic therapies.14
Nutrition
Folic acid supplementation with at least 400 micrograms (mcg) daily is necessary for all women planning pregnancy. Patients with small bowel involvement or history of small bowel resection should have a folate intake of a minimum of 2 grams per day. Adequate vitamin D levels (at least 20 ng/mL) are recommended in all women with IBD. Those with malabsorption should be screened for deficiencies in vitamin B12, folate, and iron.13 These nutritional markers should be evaluated prepregnancy, during the first trimester, and thereafter as needed.15-18
Preconception counseling
Steroid-free remission for at least 3 months prior to conception is recommended and is associated with reduced risk of flare during pregnancy.16,19 IBD medications needed to control disease activity are generally safe preconception and during pregnancy, with some exception (Table).

Misconceptions regarding heritability of IBD have sometimes discouraged men and women from having children. While genetics may increase susceptibility, environmental and other factors are involved as well. The concordance rates for monozygotic twins range from 33.3%-58.3% for Crohn’s disease and 13.4%-27.9% for ulcerative colitis (UC).20 The risk of a child developing IBD is higher in those who have multiple relatives with IBD and whose parents had IBD at the time of conception.21 While genetic testing for IBD loci is available, it is not commonly performed at this time as many genes are involved.22
Pregnancy
Coordinated care
A complete team of specialists with coordinated care among all providers is needed for optimal maternal and fetal outcomes.23,24 A gastroenterologist, ideally an IBD specialist, should follow the patient throughout pregnancy, seeing the patient at least once during the first or second trimester and as needed during pregnancy.16 A high-risk obstetrician or maternal-fetal medicine specialist should be involved early in pregnancy, as well. Open communication among all disciplines ensures that a common message is conveyed to the patient.16,24 A nutritionist, mental health provider, and lactation specialist knowledgeable about IBD drugs may be of assistance, as well.16
Disease activity
While women with IBD are at increased risk of spontaneous abortion, preterm birth, and labor complications, this risk is mitigated by controlling disease activity.25 The risk of preterm birth, small-for-gestational-age birth, and delivery via C-section is much higher in women with moderate-to-high disease activity, compared with those with low disease activity.26 The presence of active perianal disease mandates C-section over vaginal delivery. Fourth-degree lacerations following vaginal delivery are most common among those patients with perianal disease.26,27 Stillbirths were shown to be increased only in those with active IBD when compared with non-IBD comparators and inactive IBD.28-31;11
Noninvasive methods for disease monitoring are preferred in pregnancy, but serum markers such as erythrocyte sedimentation rate and C-reactive protein may not be reliable in the pregnant patient (Figure).32 Fecal calprotectin does rise in correlation with disease activity, but exact thresholds have not been validated in pregnancy.33,34

An unsedated, unprepped flexible sigmoidoscopy can be safely performed throughout pregnancy.35 When there is a strong indication, a complete colonoscopy can be performed in the pregnant patient as well.36 Current American Society for Gastrointestinal Endoscopy (ASGE) guidelines suggest placing the patient in the left lateral tilt position to avoid decreased maternal and placental perfusion via compression of the aorta or inferior vena cava and performing endoscopy during the second trimester, although trimester-specific timing is not always feasible by indication.37
Medication use and safety
IBD medications are a priority topic of concern among pregnant patients or those considering conception.38 Comprehensive data from the PIANO (Pregnancy in Inflammatory Bowel Disease and Neonatal Outcomes) registry has shown that most IBD drugs do not result in adverse pregnancy outcomes and should be continued.39 The use of biologics and thiopurines, either in combination or alone, is not related to an increased risk of congenital malformations, spontaneous abortion, preterm birth, low birth weight, or infections during the child’s first year of life.7,39 Developmental milestones also remain unaffected.39 Here, we will discuss safety considerations during pregnancy (see Table).
5-aminosalycylic acid. 5-aminosalicylic acid (5-ASA) agents are generally low risk during pregnancy and should be continued.40-41 Sulfasalazine does interfere with folate metabolism, but by increasing folic acid supplementation to 2 grams per day, sulfasalazine can be continued throughout pregnancy, as well.42
Corticosteroids. Intrapartum corticosteroid use is associated with an increased risk of gestational diabetes and adrenal insufficiency when used long term.43-45 Short-term use may, however, be necessary to control an acute flare. The lowest dose for the shortest duration possible is recommended. Because of its high first-pass metabolism, budesonide is considered low risk in pregnancy.
Methotrexate. Methotrexate needs to be stopped at least 3 months prior to conception and should be avoided throughout pregnancy. Use during pregnancy can result in spontaneous abortions, as well as embryotoxicity.46
Thiopurines (6-mercaptopurine and azathioprine). Patients who are taking thiopurines prior to conception to maintain remission can continue to do so. Data on thiopurines from the PIANO registry has shown no increase in spontaneous abortions, congenital malformations, low birth weight, preterm birth, rates of infection in the child, or developmental delays.47-51
Calcineurin inhibitors (cyclosporine and tacrolimus). Calcineurin inhibitors are reserved for the management of acute severe UC. Safety data on calcineurin inhibitors is conflicting, and there is not enough information at this time to identify risk during pregnancy. Cyclosporine can be used for salvage therapy if absolutely needed, and there are case reports of its successful using during pregnancy.16,52
Biologic therapies. With the exception of certolizumab, all of the currently used biologics are actively transported across the placenta.39,53,54 Intrapartum use of biologic therapies does not worsen pregnancy or neonatal outcomes, including the risk for intensive care unit admission, infections, and developmental milestones.39,47
While drug concentrations may vary slightly during pregnancy, these changes are not substantial enough to warrant more frequent monitoring or dose adjustments, and prepregnancy weight should be used for dosing.55,56
Antitumor necrosis factor agents used in IBD include infliximab, adalimumab, certolizumab, and golimumab.57 All are low risk for pregnant patients and their offspring. Dosage timings can be adjusted, but not stopped, to minimize exposure to the child; however, it cannot be adjusted for certolizumab pegol because of its lack of placental transfer.58-59
Natalizumab and vedolizumab are integrin receptor antagonists and are also low risk in pregnancy.57;60-62;39
Ustekinumab, an interleukin-12/23 antagonist, can be found in infant serum and cord blood, as well. Health outcomes are similar in the exposed mother and child, however, compared with those of the general population.39;63-64
Small molecule drugs. Unlike monoclonal antibodies, which do not cross the placenta in large amounts until early in the second trimester, small molecules can cross in the first trimester during the critical period of organogenesis.
The two small molecule agents currently approved for use in UC are tofacitinib, a janus kinase inhibitor, and ozanimod, a sphingosine-1-phosphate receptor agonist.65-66 Further data are still needed to make recommendations on the use of tofacitinib and ozanimod in pregnancy. At this time, we recommend weighing the risks (unknown risk to human pregnancy) vs. benefits (controlled disease activity with clear risk of harm to mother and baby from flare) in the individual patient before counseling on use in pregnancy.
Delivery
Mode of delivery
The mode of delivery should be determined by the obstetrician. C-section is recommended for patients with active perianal disease or, in some cases, a history of ileal pouch anal anastomosis (IPAA).67-68 Vaginal delivery in the setting of perianal disease has been shown to increase the risk of fourth-degree laceration and anal sphincter dysfunction in the future.26-27 Anorectal motility may be impacted by IPAA construction and vaginal delivery independently of each other. It is therefore suggested that vaginal delivery be avoided in patients with a history of IPAA to avoid compounding the risk. Some studies do not show clear harm from vaginal delivery in the setting of IPAA, however, and informed decision making among all stakeholders should be had.27;69-70
Anticoagulation
The incidence of venous thromboembolism (VTE) is elevated in patients with IBD during pregnancy, and up to 12 weeks postpartum, compared with pregnant patients without IBD.71-72 VTE for prophylaxis is indicated in the pregnant patient while hospitalized and potentially thereafter depending on the patient’s risk factors, which may include obesity, prior personal history of VTE, heart failure, and prolonged immobility. Unfractionated heparin, low molecular weight heparin, and warfarin are safe for breastfeeding women.16,73
Postpartum care of mother
There is a risk of postpartum flare, occurring in about one third of patients in the first 6 months postpartum.74-75 De-escalating therapy during delivery or immediately postpartum is a predictor of a postpartum flare.75 If no infection is present and the timing interval is appropriate, biologic therapies should be continued and can be resumed 24 hours after a vaginal delivery and 48 hours after a C-section.16,76
NSAIDs and opioids can be used for pain relief but should be avoided in the long-term to prevent flares (NSAIDs) and infant sedation (associated with opioids) when used while breastfeeding.77 The LactMed database is an excellent resource for clarification on risk of medication use while breastfeeding.78
In particular, contraception should be addressed postpartum. Exogenous estrogen use increases the risk of VTE, which is already increased in IBD; nonestrogen containing, long-acting reversible contraception is preferred.79-80 Progestin-only implants or intrauterine devices may be used first line. The efficacy of oral contraceptives is theoretically reduced in those with rapid bowel transit, active small bowel inflammation, and prior small bowel resection, so adding another form of contraception is recommended.16,81
Source: American Gastroenterological Association
Postdelivery care of baby
Breastfeeding
Guidelines regarding medication use during breastfeeding are similar to those in pregnancy (see Table). Breastfeeding on biologics and thiopurines can continue without interruption in the child. Thiopurine concentrations in breast milk are low or undetectable.82,78 TNF receptor antagonists, anti-integrin therapies, and ustekinumab are found in low to undetectable levels in breast milk, as well.78
On the other hand, the active metabolite of methotrexate is detectable in breast milk and most sources recommend not breastfeeding on methotrexate. At doses used in IBD (15-25 milligrams per week), some experts have suggested avoiding breastfeeding for 24 hours following a dose.57,78 It is the practice of this author to recommend not breastfeeding at all on methotrexate.
5-ASA therapies are low risk for breastfeeding, but alternatives to sulfasalazine are preferred. The sulfapyridine metabolite transfers to breast milk and may cause hemolysis in infants born with a glucose-6-phosphate dehydrogenase deficiency.78
With regards to calcineurin inhibitors, tacrolimus appears in breast milk in low quantities, while cyclosporine levels are variable. Data from the National Transplantation Pregnancy Registry suggest that these medications can be used at the time of breastfeeding with close monitoring.78
There is not enough data on small molecule therapies at this time to support breastfeeding safety, and it is our practice to not recommend breastfeeding in this scenario.
The transfer of steroids to the child via breast milk does occur but at subtherapeutic levels.16 Budesonide has high first pass metabolism and is low risk during breastfeeding.83-84 As far as is known, IBD maintenance medications do not suppress lactation. The use of intravenous corticosteroids can, however, temporarily decrease milk production.16,85
Vaccines
Vaccination of infants can proceed as indicated by the Center for Disease Control and Prevention guidelines, with one exception. If the child’s mother was exposed to any biologic agents (not including certolizumab) during the third trimester, any live vaccines should be withheld in the first 6 months of life. In the United States, this restriction currently only applies to the rotavirus vaccine, which is administered starting at the age of 2 months.16,86 Notably, inadvertent administration of the rotavirus vaccine in the biologic-exposed child does not appear to result in any adverse effects.87 Immunity is achieved even if the child is exposed to IBD therapies through breast milk.88
Developmental milestones
Infant exposure to biologics and thiopurines has not been shown to result in any developmental delays. The PIANO study measured developmental milestones at 48 months from birth and found no differences when compared with validated population norms.39 A separate study observing childhood development up to 7 years of age in patients born to mothers with IBD found similar cognitive scores and motor development when compared with those born to mothers without IBD.89
Conclusion
Women considering conception should be optimized prior to pregnancy and maintained on appropriate medications throughout pregnancy and lactation to achieve a healthy pregnancy for both mother and baby. To date, biologics and thiopurines are not associated with adverse pregnancy outcomes. More data are needed for small molecules.
Dr. Chugh is an advanced inflammatory bowel disease fellow in the division of gastroenterology at the University of California San Francisco. Dr. Mahadevan is professor of medicine and codirector at the Center for Colitis and Crohn’s Disease in the division of gastroenterology at the University of California San Francisco. Dr. Mahadevan has potential conflicts related to AbbVie, Janssen, BMS, Takeda, Pfizer, Lilly, Gilead, Arena, and Prometheus Biosciences.
References
1. Ye Y et al. Inflamm Bowel Dis. 2020;26:619-25.
2. Sykora J et al. World J Gastroenterol. 2018;24:2741-63.
3. Murakami Y et al. J Gastroenterol 2019;54:1070-7.
4. Hashash JG and Kane S. Gastroenterol Hepatol. (N Y) 2015;11:96-102.
5. Miller JP. J R Soc Med. 1986;79:221-5.
6. Cornish J et al. Gut. 2007;56:830-7.
7. Leung KK et al. Inflamm Bowel Dis. 2021;27:550-62.
8. O’Toole A et al. Dig Dis Sci. 2015;60:2750-61.
9. Nguyen GC et al. Inflamm Bowel Dis. 2008;14:1105-11.
10. Lee HH et al. Aliment Pharmacol Ther. 2020;51:861-9.
11. Kim MA et al. J Crohns Colitis. 2021;15:719-32.
12. Conradt E et al. Pediatrics. 2019;144.
13. ACOG Committee Opinion No. 762: Prepregnancy Counseling. Obstet Gynecol. 2019;133:e78-e89.
14. Farraye FA et al. Am J Gastroenterol. 2017;112:241-58.
15. Lee S et al. J Crohns Colitis. 2018;12:702-9.
16. Mahadevan U et al. Inflamm Bowel Dis. 2019;25:627-41.
17. Ward MG et al. Inflamm. Bowel Dis 2015;21:2839-47.
18. Battat R et al. Inflamm Bowel Dis. 2014;20:1120-8.
19. Pedersen N et al. Aliment Pharmacol Ther. 2013;38:501-12.
20. Annese V. Pharmacol Res. 2020;159:104892.
21. Bennett RA et al. Gastroenterology. 1991;100:1638-43.
22. Turpin W et al. Inflamm Bowel Dis. 2018;24:1133-48.
23. de Lima A et al. Clin Gastroenterol Hepatol. 2016;14:1285-92 e1.
24. Selinger C et al. Frontline Gastroenterol. 2021;12:182-7.
25. Mahadevan U et al. Gastroenterology. 2007;133:1106-12.
26. Hatch Q et al. Dis Colon Rectum. 2014;57:174-8.
27. Foulon A et al. Inflamm Bowel Dis. 2017;23:712-20.
28. Norgard B et al. Am J Gastroenterol. 2007;102:1947-54.
29. Broms G et al. Scand J Gastroenterol 2016;51:1462-9.
30. Meyer A et al. Aliment Pharmacol Ther. 2020;52:1480-90.
31. Kammerlander H et al. Inflamm Bowel Dis. 2017;23:1011-8.
32. Tandon P et al. J Clin Gastroenterol. 2019;53:574-81.
33. Kammerlander H et al. Inflamm Bowel Dis. 2018;24:839-48.
34. Julsgaard M et al. Inflamm Bowel Dis. 2017;23:1240-6.
35. Ko MS et al. Dig Dis Sci. 2020;65:2979-85.
36. Cappell MS et al. J Reprod Med. 2010;55:115-23.
37. Committee ASoP et al. Gastrointest Endosc. 2012;76:18-24.
38. Aboubakr A et al. Dig Dis Sci. 2021;66:1829-35.
39. Mahadevan U et al. Gastroenterology. 2021;160:1131-9.
40. Diav-Citrin O et al. Gastroenterology. 1998;114:23-8.
41. Rahimi R et al. Reprod Toxicol. 2008;25:271-5.
42. Norgard B et al. Aliment Pharmacol Ther. 2001;15:483-6.
43. Leung YP et al. J Crohns Colitis. 2015;9:223-30.
44. Schulze H et al. Aliment Pharmacol Ther. 2014;40:991-1008.
45. Szymanska E et al. J Gynecol Obstet Hum Reprod. 2021;50:101777.
46. Weber-Schoendorfer C et al. Arthritis Rheumatol. 2014;66:1101-10.
47. Nielsen OH et al. Clin Gastroenterol Hepatol. 2022 Jan;20(1):74-87.e3.
48. Coelho J et al. Gut. 2011;60:198-203.
49. Sheikh M et al. J Crohns Colitis. 2015;9:680-4.
50. Kanis SL et al. Clin Gastroenterol Hepatol. 2017;15:1232-41 e1.
51. Mahadevan U et al. Inflamm Bowel Dis. 2018;24:2494-500.
52. Rosen MH et al. Inflamm Bowel Dis. 2020;26:971-3.
53. Porter C et al. J Reprod Immunol. 2016;116:7-12.
54. Mahadevan U et al. Clin Gastroenterol Hepatol. 2013;11:286-92; quiz e24.
55. Picardo S and Seow CH. Best Pract Res Clin Gastroenterol. 2020;44-5:101670.
56. Flanagan E et al. Aliment Pharmacol Ther. 2020;52:1551-62.
57. Singh S et al. Gastroenterology. 2021;160:2512-56 e9.
58. de Lima A et al. Gut. 2016;65:1261-8.
59. Julsgaard M et al. Inflamm Bowel Dis. 2020;26:93-102.
60. Wils P et al. Aliment Pharmacol Ther. 2021;53:460-70.
61. Mahadevan U et al. Aliment Pharmacol Ther. 2017;45:941-50.
62. Bar-Gil Shitrit A et al. Am J Gastroenterol. 2019;114:1172-5.
63. Klenske E et al. J Crohns Colitis. 2019;13:267-9.
64. Matro R et al. Gastroenterology. 2018;155:696-704.
65. Feuerstein JD et al. Gastroenterology. 2020;158:1450-61.
66. Sandborn WJ et al. J Crohns Colitis. 2021 Jul 5;15(7):1120-1129.
67. Lamb CA et al. Gut. 2019;68:s1-s106.
68. Nguyen GC et al. Gastroenterology. 2016;150:734-57 e1.
69. Ravid A et al. Dis Colon Rectum. 2002;45:1283-8.
70. Seligman NS et al. J Matern Fetal Neonatal Med. 2011;24:525-30.
71. Kim YH et al. Medicine (Baltimore). 2019;98:e17309.
72. Hansen AT et al. J Thromb Haemost. 2017;15:702-8.
73. Bates SM et al. J Thromb Thrombolysis. 2016;41:92-128.
74. Bennett A et al. Inflamm Bowel Dis. 2021 May 17;izab104.
75. Yu A et al. Inflamm Bowel Dis. 2020;26:1926-32.
76. Mahadevan U et al. Gastroenterology. 2017;152:451-62 e2.
77. Long MD et al. J Clin Gastroenterol. 2016;50:152-6.
78. Drugs and Lactation Database (LactMed). 2006 ed. Bethesda, MD: National Library of Medicine (US), 2006-2021.
79. Khalili H et al. Gut. 2013;62:1153-9.
80. Long MD and Hutfless S. Gastroenterology. 2016;150:1518-20.
81. Centers for Disease Control and Prevention. U S. Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR Recomm Rep. 2010;59:1-86.
82. Angelberger S et al. J Crohns Colitis. 2011;5:95-100.
83. Vestergaard T et al. Scand J Gastroenterol. 2018;53:1459-62.
84. Beaulieu DB et al. Inflamm Bowel Dis. 2009;15:25-8.
85. Anderson PO. Breastfeed Med. 2017;12:199-201.
86. Wodi AP et al. MMWR Morb Mortal Wkly Rep. 2021;70:189-92.
87. Chiarella-Redfern H et al. Inflamm Bowel Dis. 2022 Jan 5;28(1):79-86.
88. Beaulieu DB et al. Clin Gastroenterol Hepatol. 2018;16:99-105.
89. Friedman S et al. J Crohns Colitis. 2020 Dec 2;14(12):1709-1716.
Steroid-free remission doesn’t decrease risk of Crohn’s disease progression
Achieving steroid-free clinical and endoscopic remission does not decrease the risk of Crohn’s disease progression measured by surgery, hospitalization, or initiation of new treatments, according to a post hoc analysis of data from a prospective RCT.
The therapeutic goals of Crohn’s disease have evolved from controlling symptoms to blocking disease progression and reducing complications, wrote David Laharie, MD, of Hôpital Haut-Lévêque, Pessac, France, and colleagues. The goal of steroid-free remission has been used as an endpoint of treatment, but data on the impact of such remission on long-term disease are limited, the researchers noted in a retrospective study published in Clinical Gastroenterology and Hepatology.
In the study, the researchers reviewed data from 95 adults with early Crohn’s disease (CD) who participated in the TAILORIX trial involving treatment with infliximab and immunomodulators. The primary endpoint of the TAILORIX trial was sustained corticosteroid-free remission from week 22 to 54. In the current study, the primary endpoint was progression-free survival at 1, 3, and 5 years in patients who did or did not meet the TAILORIX primary endpoint. The median disease duration was 4.5 months, and the median follow-up was 64.2 months.
Progression-free survival was defined as a composite of luminal surgery, anal surgery, hospitalization, and the need for a new CD treatment during the follow-up period.
In the study population, 45 patients achieved corticosteroid-free remission and 50 did not. At 54 weeks, 17 patients with corticosteroid-free remission (38%) and 28 patients without remission (56%) achieved complete mucosal healing, and progression-free survival rates were similar between these groups.
Overall, the rates of progression-free survival at 1, 3, and 5 years were not significantly different between the remission and nonremission groups: 86% versus 91%, respectively, at 1 year; 70% for both groups at 3 years; and 64% and 61%, respectively, at 5 years.
The researchers also compared individual components of the primary endpoint (luminal surgery, anal surgery, hospitalization, and the need for a new CD treatment), and found no significant differences in survival rates in patients who had and had not achieved steroid-free remission.
Survival rates without luminal surgery at 1, 3, and 5 years were 97% versus 96%, 93% versus 90%, and 87% versus 82%, respectively, for remission and nonremission groups. Similarly, survival rates without anal surgery were 93%, 86%, and 86% versus 96%, 88%, and 85%, respectively, for the two groups. Rates of hospitalization-free survival at 1, 3, and 5 years were 90% versus 92%, 81% versus 81%, and 78% versus 69%, respectively, in the remission and nonremission groups. Survival rates without a new systemic CD treatment also were similar at 1, 3, and 5 years: 93% versus 95%, 71% versus 93%, and 60% versus 51%, respectively, for the remission and nonremission groups.
CD progression was not associated with not achieving corticosteroid-free remission (hazard ratio, 0.861). Other factors that were not associated with disease progression in this study included CRP greater than 5 mg/L, age older than 30 years, active smoking, and B1 phenotype.
The researchers noted that, although endoscopic and clinical remission is currently recommended for CD, “there is no validated or standardized definition of this endoscopic goal.” The high rates of survival without major abdominal surgery, regardless of remission status, suggest a significant impact of early combination therapy for CD patients who were biologic naive. Other studies have shown similar improved outcomes for CD patients with early treatment.
The study findings were limited by several factors including the retrospective design and lack of power to compare long-term progression-free survival, the researchers noted. However, the results were strengthened by the robust data on hospitalizations and surgeries from the TAILORIX trial.
The results support a more flexible strategy for CD, “recommending endoscopic and clinical remission in early diagnosed patients and less stringent objectives in those with more refractory or advanced disease,” they concluded.
Findings may guide patient management
The current study is important to help clinicians know whether CD patients who achieve a short-term, steroid-free clinical and endoscopic remission go on to experience better long-term disease outcomes than those who do not achieve this short-term remission, Atsushi Sakuraba, MD, of the University of Chicago said in an interview.
Dr. Sakuraba said that he was surprised by the study findings. “Achieving a clinical remission off steroids with complete endoscopic remission, i.e., deep remission, is considered a treatment goal, but the fact that it did not result in less disease progression was surprising.”
The take-home message for clinicians from the study is that CD patients may still experience disease progression after achieving a single time of clinical and endoscopic remission “mainly due to loss of response to infliximab, so continued long-term disease monitoring and control are required,” Dr. Sakuraba said.
The current study was a post hoc follow-up analysis of a previous trial, Dr. Sakuraba noted. Therefore, studies primarily focused on changing the disease progression and natural course of CD are warranted.
Dr. Laharie disclosed counseling, boards, transportation, or fees from AbbVie, Biogaran, Biogen, Ferring, HAC-pharma, Janssen, MSD, Novartis, Pfizer, Prometheus, Roche, Takeda, Theradiag, and Tillots. Dr. Sakuraba had no relevant financial conflicts to disclose.
This article was updated Feb. 10, 2022.
Achieving steroid-free clinical and endoscopic remission does not decrease the risk of Crohn’s disease progression measured by surgery, hospitalization, or initiation of new treatments, according to a post hoc analysis of data from a prospective RCT.
The therapeutic goals of Crohn’s disease have evolved from controlling symptoms to blocking disease progression and reducing complications, wrote David Laharie, MD, of Hôpital Haut-Lévêque, Pessac, France, and colleagues. The goal of steroid-free remission has been used as an endpoint of treatment, but data on the impact of such remission on long-term disease are limited, the researchers noted in a retrospective study published in Clinical Gastroenterology and Hepatology.
In the study, the researchers reviewed data from 95 adults with early Crohn’s disease (CD) who participated in the TAILORIX trial involving treatment with infliximab and immunomodulators. The primary endpoint of the TAILORIX trial was sustained corticosteroid-free remission from week 22 to 54. In the current study, the primary endpoint was progression-free survival at 1, 3, and 5 years in patients who did or did not meet the TAILORIX primary endpoint. The median disease duration was 4.5 months, and the median follow-up was 64.2 months.
Progression-free survival was defined as a composite of luminal surgery, anal surgery, hospitalization, and the need for a new CD treatment during the follow-up period.
In the study population, 45 patients achieved corticosteroid-free remission and 50 did not. At 54 weeks, 17 patients with corticosteroid-free remission (38%) and 28 patients without remission (56%) achieved complete mucosal healing, and progression-free survival rates were similar between these groups.
Overall, the rates of progression-free survival at 1, 3, and 5 years were not significantly different between the remission and nonremission groups: 86% versus 91%, respectively, at 1 year; 70% for both groups at 3 years; and 64% and 61%, respectively, at 5 years.
The researchers also compared individual components of the primary endpoint (luminal surgery, anal surgery, hospitalization, and the need for a new CD treatment), and found no significant differences in survival rates in patients who had and had not achieved steroid-free remission.
Survival rates without luminal surgery at 1, 3, and 5 years were 97% versus 96%, 93% versus 90%, and 87% versus 82%, respectively, for remission and nonremission groups. Similarly, survival rates without anal surgery were 93%, 86%, and 86% versus 96%, 88%, and 85%, respectively, for the two groups. Rates of hospitalization-free survival at 1, 3, and 5 years were 90% versus 92%, 81% versus 81%, and 78% versus 69%, respectively, in the remission and nonremission groups. Survival rates without a new systemic CD treatment also were similar at 1, 3, and 5 years: 93% versus 95%, 71% versus 93%, and 60% versus 51%, respectively, for the remission and nonremission groups.
CD progression was not associated with not achieving corticosteroid-free remission (hazard ratio, 0.861). Other factors that were not associated with disease progression in this study included CRP greater than 5 mg/L, age older than 30 years, active smoking, and B1 phenotype.
The researchers noted that, although endoscopic and clinical remission is currently recommended for CD, “there is no validated or standardized definition of this endoscopic goal.” The high rates of survival without major abdominal surgery, regardless of remission status, suggest a significant impact of early combination therapy for CD patients who were biologic naive. Other studies have shown similar improved outcomes for CD patients with early treatment.
The study findings were limited by several factors including the retrospective design and lack of power to compare long-term progression-free survival, the researchers noted. However, the results were strengthened by the robust data on hospitalizations and surgeries from the TAILORIX trial.
The results support a more flexible strategy for CD, “recommending endoscopic and clinical remission in early diagnosed patients and less stringent objectives in those with more refractory or advanced disease,” they concluded.
Findings may guide patient management
The current study is important to help clinicians know whether CD patients who achieve a short-term, steroid-free clinical and endoscopic remission go on to experience better long-term disease outcomes than those who do not achieve this short-term remission, Atsushi Sakuraba, MD, of the University of Chicago said in an interview.
Dr. Sakuraba said that he was surprised by the study findings. “Achieving a clinical remission off steroids with complete endoscopic remission, i.e., deep remission, is considered a treatment goal, but the fact that it did not result in less disease progression was surprising.”
The take-home message for clinicians from the study is that CD patients may still experience disease progression after achieving a single time of clinical and endoscopic remission “mainly due to loss of response to infliximab, so continued long-term disease monitoring and control are required,” Dr. Sakuraba said.
The current study was a post hoc follow-up analysis of a previous trial, Dr. Sakuraba noted. Therefore, studies primarily focused on changing the disease progression and natural course of CD are warranted.
Dr. Laharie disclosed counseling, boards, transportation, or fees from AbbVie, Biogaran, Biogen, Ferring, HAC-pharma, Janssen, MSD, Novartis, Pfizer, Prometheus, Roche, Takeda, Theradiag, and Tillots. Dr. Sakuraba had no relevant financial conflicts to disclose.
This article was updated Feb. 10, 2022.
Achieving steroid-free clinical and endoscopic remission does not decrease the risk of Crohn’s disease progression measured by surgery, hospitalization, or initiation of new treatments, according to a post hoc analysis of data from a prospective RCT.
The therapeutic goals of Crohn’s disease have evolved from controlling symptoms to blocking disease progression and reducing complications, wrote David Laharie, MD, of Hôpital Haut-Lévêque, Pessac, France, and colleagues. The goal of steroid-free remission has been used as an endpoint of treatment, but data on the impact of such remission on long-term disease are limited, the researchers noted in a retrospective study published in Clinical Gastroenterology and Hepatology.
In the study, the researchers reviewed data from 95 adults with early Crohn’s disease (CD) who participated in the TAILORIX trial involving treatment with infliximab and immunomodulators. The primary endpoint of the TAILORIX trial was sustained corticosteroid-free remission from week 22 to 54. In the current study, the primary endpoint was progression-free survival at 1, 3, and 5 years in patients who did or did not meet the TAILORIX primary endpoint. The median disease duration was 4.5 months, and the median follow-up was 64.2 months.
Progression-free survival was defined as a composite of luminal surgery, anal surgery, hospitalization, and the need for a new CD treatment during the follow-up period.
In the study population, 45 patients achieved corticosteroid-free remission and 50 did not. At 54 weeks, 17 patients with corticosteroid-free remission (38%) and 28 patients without remission (56%) achieved complete mucosal healing, and progression-free survival rates were similar between these groups.
Overall, the rates of progression-free survival at 1, 3, and 5 years were not significantly different between the remission and nonremission groups: 86% versus 91%, respectively, at 1 year; 70% for both groups at 3 years; and 64% and 61%, respectively, at 5 years.
The researchers also compared individual components of the primary endpoint (luminal surgery, anal surgery, hospitalization, and the need for a new CD treatment), and found no significant differences in survival rates in patients who had and had not achieved steroid-free remission.
Survival rates without luminal surgery at 1, 3, and 5 years were 97% versus 96%, 93% versus 90%, and 87% versus 82%, respectively, for remission and nonremission groups. Similarly, survival rates without anal surgery were 93%, 86%, and 86% versus 96%, 88%, and 85%, respectively, for the two groups. Rates of hospitalization-free survival at 1, 3, and 5 years were 90% versus 92%, 81% versus 81%, and 78% versus 69%, respectively, in the remission and nonremission groups. Survival rates without a new systemic CD treatment also were similar at 1, 3, and 5 years: 93% versus 95%, 71% versus 93%, and 60% versus 51%, respectively, for the remission and nonremission groups.
CD progression was not associated with not achieving corticosteroid-free remission (hazard ratio, 0.861). Other factors that were not associated with disease progression in this study included CRP greater than 5 mg/L, age older than 30 years, active smoking, and B1 phenotype.
The researchers noted that, although endoscopic and clinical remission is currently recommended for CD, “there is no validated or standardized definition of this endoscopic goal.” The high rates of survival without major abdominal surgery, regardless of remission status, suggest a significant impact of early combination therapy for CD patients who were biologic naive. Other studies have shown similar improved outcomes for CD patients with early treatment.
The study findings were limited by several factors including the retrospective design and lack of power to compare long-term progression-free survival, the researchers noted. However, the results were strengthened by the robust data on hospitalizations and surgeries from the TAILORIX trial.
The results support a more flexible strategy for CD, “recommending endoscopic and clinical remission in early diagnosed patients and less stringent objectives in those with more refractory or advanced disease,” they concluded.
Findings may guide patient management
The current study is important to help clinicians know whether CD patients who achieve a short-term, steroid-free clinical and endoscopic remission go on to experience better long-term disease outcomes than those who do not achieve this short-term remission, Atsushi Sakuraba, MD, of the University of Chicago said in an interview.
Dr. Sakuraba said that he was surprised by the study findings. “Achieving a clinical remission off steroids with complete endoscopic remission, i.e., deep remission, is considered a treatment goal, but the fact that it did not result in less disease progression was surprising.”
The take-home message for clinicians from the study is that CD patients may still experience disease progression after achieving a single time of clinical and endoscopic remission “mainly due to loss of response to infliximab, so continued long-term disease monitoring and control are required,” Dr. Sakuraba said.
The current study was a post hoc follow-up analysis of a previous trial, Dr. Sakuraba noted. Therefore, studies primarily focused on changing the disease progression and natural course of CD are warranted.
Dr. Laharie disclosed counseling, boards, transportation, or fees from AbbVie, Biogaran, Biogen, Ferring, HAC-pharma, Janssen, MSD, Novartis, Pfizer, Prometheus, Roche, Takeda, Theradiag, and Tillots. Dr. Sakuraba had no relevant financial conflicts to disclose.
This article was updated Feb. 10, 2022.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Therapeutic drug monitoring with infliximab improves IBD disease control
A proactive approach to therapeutic drug monitoring during maintenance therapy with infliximab in the treatment of inflammatory bowel diseases (IBDs) and other chronic immune-mediated inflammatory diseases significantly improves sustained disease control, compared with standard care, which was defined as no therapeutic drug monitoring, new research shows.
“This trial showed that therapeutic drug monitoring improved infliximab [maintenance] treatment by preventing disease flares without increasing drug consumption,” reported the authors of the study published in JAMA.
Infliximab and other tumor necrosis factor (TNF)–inhibitor drugs offer significant benefits in the treatment of IBDs and other chronic inflammatory diseases; however, up to 50% of patients become nonresponsive to the therapy within the first years of treatment, posing risks of disease worsening and possible organ damage.
To address the problem, therapeutic drug monitoring has been recommended, more so with IBD guidelines than rheumatoid arthritis; these have included measures such as dose adjustment or drug switching when there is evidence that disease control is not being maintained.
However, with low serum drug concentrations and the development of antidrug antibodies believed to be key indicators of a waning response, there is growing interest in a more proactive monitoring approach, involving scheduled assessments of serum and antibody levels, performed regardless of any signs of disease activity changes, and the provision of dosing adjustments, if needed.
In the earlier Norwegian Drug Monitoring (NORDRUM) A trial, first author Silje Watterdal Syversen, MD, PhD, of the division of rheumatology and research, Diakonhjemmet Hospital, Oslo, Norway, and colleagues evaluated the effects of the proactive drug monitoring approach during the initiation phase of infliximab treatment, but found no significant improvement in the study of 411 patients in terms of the primary outcome of remission rates.
For the current NORDRUM B trial, they sought to instead determine if benefits of the proactive therapeutic drug monitoring may be more apparent during the maintenance phase of infliximab treatment.
The trial involved 458 adults at 20 hospitals in Norway who had immune-mediated inflammatory diseases (IMIDs), including rheumatoid arthritis (n = 80), spondyloarthritis (n = 138), psoriatic arthritis (n = 54), ulcerative colitis (n = 81), Crohn’s disease (n = 68), or psoriasis (n = 37), and who were undergoing maintenance therapy with infliximab.
The patients, who had a median of about 40 weeks’ prior infliximab therapy, were randomized 1:1 to receive either proactive therapeutic drug monitoring, consisting of scheduled monitoring of serum drug levels and antidrug antibodies and adjustments in dose and intervals as needed according to a trial algorithm (n = 228), or standard infliximab therapy, without the regular drug and antibody level monitoring (n = 230).
Over a 52-week follow-up, the primary outcome of sustained disease control without disease worsening was significantly higher in the proactive therapeutic drug monitoring group (73.6%; n = 167) versus standard care (55.9%; n = 127; P < .001). The risk of disease worsening was meanwhile significantly greater with standard care versus proactive drug monitoring (hazard ratio, 2.1).
Serum infliximab levels remained within the therapeutic range throughout the study period in 30% of patients receiving proactive therapeutic drug monitoring, compared with 17% in the standard care group, and low serum infliximab levels (≤2 mg/L) occurred at least once during the study period among 19% and 27% of the two groups, respectively.
Clinically significant levels of antidrug antibodies (≥50 mcg/L) occurred in 9.2% of the therapeutic drug monitoring patients and 15.0% of the standard care group.
About 55% of patients were also using concomitant immunosuppressive therapy, and the findings were consistent among those who did and did not use the drugs. There no significant differences in adverse events between the therapeutic drug-monitoring (60%) and standard care groups (63%).
Importantly, while the mean dose of infliximab during the trial was 4.8 mg/kg in both groups, an increase in dosage after disease worsening was more common in the standard therapy group (51%) than in the therapeutic drug monitoring group (31.6%), underscoring the improved dose control provided with the proactive therapeutic drug monitoring, the authors note.
The findings suggest “proactive therapeutic drug monitoring might be more important during maintenance therapy, a period during which low drug levels could be an important risk factor for therapeutic failure,” the authors conclude.
In commenting to this news organizatin, Dr. Syversen noted that, despite the variety of IMIDs, there were no significant differences with IBDs or other disorders.
“Our data show consistent findings across all diseases included,” she said.
Furthermore, “in the present study, and [prior research] using the same definition of disease worsening, IBD patients in general had a comparable flare rate as compared to inflammatory arthritis.”
Caveats include severe illness, potential cost challenges
Commenting on the research, Stephen B. Hanauer, MD, noted that a potential exception to the lack of benefit previously observed during treatment induction could be patients with severe illness.
“In patients with more severe disease and in particular with low albumin, [which is] more common in IBD than other immune-mediated inflammatory diseases, there is rapid metabolism and clearance of monoclonal antibodies early that limit efficacy of standard induction dosing,” said Dr. Hanauer, a professor of medicine and medical director of the Digestive Health Center at Northwestern University, Chicago.
Noting that the average duration of treatment in the study prior to randomization was nearly a year (about 40 weeks), he added that a key question is “How to initiate therapeutic drug monitoring earlier in course to further optimize induction and prevent loss of response in maintenance.”
Dr. Hanauer shared that, “in our practice, we use a combination of proactive and reactive therapeutic drug monitoring based on individual patients and their history with prior biologics.”
Findings may usher in ‘new era’ in immune-mediated inflammatory disease treatment
In an editorial published in JAMA with the study, Zachary S. Wallace, MD, and Jeffrey A. Sparks, MD, both of Harvard Medical School in Boston, further commented that “maintaining disease control in nearly 3 of 4 patients represents a meaningful improvement over standard care.”
However, key challenges with the approach include the potential need for additional nurses and others to help monitor patients, and associated costs, which insurance providers may not always cover, they noted.
Another consideration is a lack of effective tools for monitoring measures including antidrug antibodies, they added, and “additional clinical trials within specific disease subgroups are needed.”
However, addressing such barriers “may help introduce a new era in treatment approach to maintenance therapy for patients with immune-mediated inflammatory diseases,” the editorialists write.
Niels Vande Casteele, PharmD, PhD, an associate professor at the department of medicine, University of California, San Diego, and affiliate faculty, Skaggs School of Pharmacy & Pharmaceutical Sciences, commented that the study is “an important milestone in the field of therapeutic drug monitoring of biologics for immunoinflammatory diseases.”
“The ability of proactive therapeutic drug monitoring to achieve sustained disease control without increased drug consumption is [a] notable finding,” he said in an interview.
Noting a limitation, Dr. Casteele suggested the inclusion of more specific measures of disease activity could have provided clearer insights.
“In particular for gastrointestinal diseases, we know that symptoms do not correlate well with inflammatory disease activity,” he said. “As such, I would have preferred to see clinical symptoms being complemented with endoscopic and histologic finds to confirm disease activity.”
Ultimately, he said that the results suggest “proactive therapeutic drug monitoring is not required for all patients, but it is beneficial in some to achieve sustained disease control over a prolonged period of time.”
This study received funding by grants from the Norwegian Regional Health Authorities (interregional KLINBEFORSK grants) and the South-Eastern Norway Regional Health Authorities; study authors reported relationships with various pharmaceutical companies, including Pfizer, which makes infliximab. Dr. Wallace reported research support from Bristol Myers Squibb and Principia/Sanofi and consulting fees from Viela Bio, Zenas Biopharma, and MedPace. Dr. Sparks reported consultancy fees from AbbVie, Bristol Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer. Dr. Hanauer is a consultant and lecturer for Abbvie, Janssen, and Takeda and consultant for Pfizer, Celltrion, Amgen, Samsung Bioepis. Dr. Casteele has received research grants and personal fees from R-Biopharm, Takeda and UCB; and personal fees from AcelaBio, Alimentiv, Celltrion, Prometheus, Procise DX, and Vividion for activities that were all outside of the reviewed study.
Help your patients better understand their IBD treatment options by sharing AGA’s patient education, “Living with IBD,” in the AGA GI Patient Center at www.gastro.org/IBD.
A proactive approach to therapeutic drug monitoring during maintenance therapy with infliximab in the treatment of inflammatory bowel diseases (IBDs) and other chronic immune-mediated inflammatory diseases significantly improves sustained disease control, compared with standard care, which was defined as no therapeutic drug monitoring, new research shows.
“This trial showed that therapeutic drug monitoring improved infliximab [maintenance] treatment by preventing disease flares without increasing drug consumption,” reported the authors of the study published in JAMA.
Infliximab and other tumor necrosis factor (TNF)–inhibitor drugs offer significant benefits in the treatment of IBDs and other chronic inflammatory diseases; however, up to 50% of patients become nonresponsive to the therapy within the first years of treatment, posing risks of disease worsening and possible organ damage.
To address the problem, therapeutic drug monitoring has been recommended, more so with IBD guidelines than rheumatoid arthritis; these have included measures such as dose adjustment or drug switching when there is evidence that disease control is not being maintained.
However, with low serum drug concentrations and the development of antidrug antibodies believed to be key indicators of a waning response, there is growing interest in a more proactive monitoring approach, involving scheduled assessments of serum and antibody levels, performed regardless of any signs of disease activity changes, and the provision of dosing adjustments, if needed.
In the earlier Norwegian Drug Monitoring (NORDRUM) A trial, first author Silje Watterdal Syversen, MD, PhD, of the division of rheumatology and research, Diakonhjemmet Hospital, Oslo, Norway, and colleagues evaluated the effects of the proactive drug monitoring approach during the initiation phase of infliximab treatment, but found no significant improvement in the study of 411 patients in terms of the primary outcome of remission rates.
For the current NORDRUM B trial, they sought to instead determine if benefits of the proactive therapeutic drug monitoring may be more apparent during the maintenance phase of infliximab treatment.
The trial involved 458 adults at 20 hospitals in Norway who had immune-mediated inflammatory diseases (IMIDs), including rheumatoid arthritis (n = 80), spondyloarthritis (n = 138), psoriatic arthritis (n = 54), ulcerative colitis (n = 81), Crohn’s disease (n = 68), or psoriasis (n = 37), and who were undergoing maintenance therapy with infliximab.
The patients, who had a median of about 40 weeks’ prior infliximab therapy, were randomized 1:1 to receive either proactive therapeutic drug monitoring, consisting of scheduled monitoring of serum drug levels and antidrug antibodies and adjustments in dose and intervals as needed according to a trial algorithm (n = 228), or standard infliximab therapy, without the regular drug and antibody level monitoring (n = 230).
Over a 52-week follow-up, the primary outcome of sustained disease control without disease worsening was significantly higher in the proactive therapeutic drug monitoring group (73.6%; n = 167) versus standard care (55.9%; n = 127; P < .001). The risk of disease worsening was meanwhile significantly greater with standard care versus proactive drug monitoring (hazard ratio, 2.1).
Serum infliximab levels remained within the therapeutic range throughout the study period in 30% of patients receiving proactive therapeutic drug monitoring, compared with 17% in the standard care group, and low serum infliximab levels (≤2 mg/L) occurred at least once during the study period among 19% and 27% of the two groups, respectively.
Clinically significant levels of antidrug antibodies (≥50 mcg/L) occurred in 9.2% of the therapeutic drug monitoring patients and 15.0% of the standard care group.
About 55% of patients were also using concomitant immunosuppressive therapy, and the findings were consistent among those who did and did not use the drugs. There no significant differences in adverse events between the therapeutic drug-monitoring (60%) and standard care groups (63%).
Importantly, while the mean dose of infliximab during the trial was 4.8 mg/kg in both groups, an increase in dosage after disease worsening was more common in the standard therapy group (51%) than in the therapeutic drug monitoring group (31.6%), underscoring the improved dose control provided with the proactive therapeutic drug monitoring, the authors note.
The findings suggest “proactive therapeutic drug monitoring might be more important during maintenance therapy, a period during which low drug levels could be an important risk factor for therapeutic failure,” the authors conclude.
In commenting to this news organizatin, Dr. Syversen noted that, despite the variety of IMIDs, there were no significant differences with IBDs or other disorders.
“Our data show consistent findings across all diseases included,” she said.
Furthermore, “in the present study, and [prior research] using the same definition of disease worsening, IBD patients in general had a comparable flare rate as compared to inflammatory arthritis.”
Caveats include severe illness, potential cost challenges
Commenting on the research, Stephen B. Hanauer, MD, noted that a potential exception to the lack of benefit previously observed during treatment induction could be patients with severe illness.
“In patients with more severe disease and in particular with low albumin, [which is] more common in IBD than other immune-mediated inflammatory diseases, there is rapid metabolism and clearance of monoclonal antibodies early that limit efficacy of standard induction dosing,” said Dr. Hanauer, a professor of medicine and medical director of the Digestive Health Center at Northwestern University, Chicago.
Noting that the average duration of treatment in the study prior to randomization was nearly a year (about 40 weeks), he added that a key question is “How to initiate therapeutic drug monitoring earlier in course to further optimize induction and prevent loss of response in maintenance.”
Dr. Hanauer shared that, “in our practice, we use a combination of proactive and reactive therapeutic drug monitoring based on individual patients and their history with prior biologics.”
Findings may usher in ‘new era’ in immune-mediated inflammatory disease treatment
In an editorial published in JAMA with the study, Zachary S. Wallace, MD, and Jeffrey A. Sparks, MD, both of Harvard Medical School in Boston, further commented that “maintaining disease control in nearly 3 of 4 patients represents a meaningful improvement over standard care.”
However, key challenges with the approach include the potential need for additional nurses and others to help monitor patients, and associated costs, which insurance providers may not always cover, they noted.
Another consideration is a lack of effective tools for monitoring measures including antidrug antibodies, they added, and “additional clinical trials within specific disease subgroups are needed.”
However, addressing such barriers “may help introduce a new era in treatment approach to maintenance therapy for patients with immune-mediated inflammatory diseases,” the editorialists write.
Niels Vande Casteele, PharmD, PhD, an associate professor at the department of medicine, University of California, San Diego, and affiliate faculty, Skaggs School of Pharmacy & Pharmaceutical Sciences, commented that the study is “an important milestone in the field of therapeutic drug monitoring of biologics for immunoinflammatory diseases.”
“The ability of proactive therapeutic drug monitoring to achieve sustained disease control without increased drug consumption is [a] notable finding,” he said in an interview.
Noting a limitation, Dr. Casteele suggested the inclusion of more specific measures of disease activity could have provided clearer insights.
“In particular for gastrointestinal diseases, we know that symptoms do not correlate well with inflammatory disease activity,” he said. “As such, I would have preferred to see clinical symptoms being complemented with endoscopic and histologic finds to confirm disease activity.”
Ultimately, he said that the results suggest “proactive therapeutic drug monitoring is not required for all patients, but it is beneficial in some to achieve sustained disease control over a prolonged period of time.”
This study received funding by grants from the Norwegian Regional Health Authorities (interregional KLINBEFORSK grants) and the South-Eastern Norway Regional Health Authorities; study authors reported relationships with various pharmaceutical companies, including Pfizer, which makes infliximab. Dr. Wallace reported research support from Bristol Myers Squibb and Principia/Sanofi and consulting fees from Viela Bio, Zenas Biopharma, and MedPace. Dr. Sparks reported consultancy fees from AbbVie, Bristol Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer. Dr. Hanauer is a consultant and lecturer for Abbvie, Janssen, and Takeda and consultant for Pfizer, Celltrion, Amgen, Samsung Bioepis. Dr. Casteele has received research grants and personal fees from R-Biopharm, Takeda and UCB; and personal fees from AcelaBio, Alimentiv, Celltrion, Prometheus, Procise DX, and Vividion for activities that were all outside of the reviewed study.
Help your patients better understand their IBD treatment options by sharing AGA’s patient education, “Living with IBD,” in the AGA GI Patient Center at www.gastro.org/IBD.
A proactive approach to therapeutic drug monitoring during maintenance therapy with infliximab in the treatment of inflammatory bowel diseases (IBDs) and other chronic immune-mediated inflammatory diseases significantly improves sustained disease control, compared with standard care, which was defined as no therapeutic drug monitoring, new research shows.
“This trial showed that therapeutic drug monitoring improved infliximab [maintenance] treatment by preventing disease flares without increasing drug consumption,” reported the authors of the study published in JAMA.
Infliximab and other tumor necrosis factor (TNF)–inhibitor drugs offer significant benefits in the treatment of IBDs and other chronic inflammatory diseases; however, up to 50% of patients become nonresponsive to the therapy within the first years of treatment, posing risks of disease worsening and possible organ damage.
To address the problem, therapeutic drug monitoring has been recommended, more so with IBD guidelines than rheumatoid arthritis; these have included measures such as dose adjustment or drug switching when there is evidence that disease control is not being maintained.
However, with low serum drug concentrations and the development of antidrug antibodies believed to be key indicators of a waning response, there is growing interest in a more proactive monitoring approach, involving scheduled assessments of serum and antibody levels, performed regardless of any signs of disease activity changes, and the provision of dosing adjustments, if needed.
In the earlier Norwegian Drug Monitoring (NORDRUM) A trial, first author Silje Watterdal Syversen, MD, PhD, of the division of rheumatology and research, Diakonhjemmet Hospital, Oslo, Norway, and colleagues evaluated the effects of the proactive drug monitoring approach during the initiation phase of infliximab treatment, but found no significant improvement in the study of 411 patients in terms of the primary outcome of remission rates.
For the current NORDRUM B trial, they sought to instead determine if benefits of the proactive therapeutic drug monitoring may be more apparent during the maintenance phase of infliximab treatment.
The trial involved 458 adults at 20 hospitals in Norway who had immune-mediated inflammatory diseases (IMIDs), including rheumatoid arthritis (n = 80), spondyloarthritis (n = 138), psoriatic arthritis (n = 54), ulcerative colitis (n = 81), Crohn’s disease (n = 68), or psoriasis (n = 37), and who were undergoing maintenance therapy with infliximab.
The patients, who had a median of about 40 weeks’ prior infliximab therapy, were randomized 1:1 to receive either proactive therapeutic drug monitoring, consisting of scheduled monitoring of serum drug levels and antidrug antibodies and adjustments in dose and intervals as needed according to a trial algorithm (n = 228), or standard infliximab therapy, without the regular drug and antibody level monitoring (n = 230).
Over a 52-week follow-up, the primary outcome of sustained disease control without disease worsening was significantly higher in the proactive therapeutic drug monitoring group (73.6%; n = 167) versus standard care (55.9%; n = 127; P < .001). The risk of disease worsening was meanwhile significantly greater with standard care versus proactive drug monitoring (hazard ratio, 2.1).
Serum infliximab levels remained within the therapeutic range throughout the study period in 30% of patients receiving proactive therapeutic drug monitoring, compared with 17% in the standard care group, and low serum infliximab levels (≤2 mg/L) occurred at least once during the study period among 19% and 27% of the two groups, respectively.
Clinically significant levels of antidrug antibodies (≥50 mcg/L) occurred in 9.2% of the therapeutic drug monitoring patients and 15.0% of the standard care group.
About 55% of patients were also using concomitant immunosuppressive therapy, and the findings were consistent among those who did and did not use the drugs. There no significant differences in adverse events between the therapeutic drug-monitoring (60%) and standard care groups (63%).
Importantly, while the mean dose of infliximab during the trial was 4.8 mg/kg in both groups, an increase in dosage after disease worsening was more common in the standard therapy group (51%) than in the therapeutic drug monitoring group (31.6%), underscoring the improved dose control provided with the proactive therapeutic drug monitoring, the authors note.
The findings suggest “proactive therapeutic drug monitoring might be more important during maintenance therapy, a period during which low drug levels could be an important risk factor for therapeutic failure,” the authors conclude.
In commenting to this news organizatin, Dr. Syversen noted that, despite the variety of IMIDs, there were no significant differences with IBDs or other disorders.
“Our data show consistent findings across all diseases included,” she said.
Furthermore, “in the present study, and [prior research] using the same definition of disease worsening, IBD patients in general had a comparable flare rate as compared to inflammatory arthritis.”
Caveats include severe illness, potential cost challenges
Commenting on the research, Stephen B. Hanauer, MD, noted that a potential exception to the lack of benefit previously observed during treatment induction could be patients with severe illness.
“In patients with more severe disease and in particular with low albumin, [which is] more common in IBD than other immune-mediated inflammatory diseases, there is rapid metabolism and clearance of monoclonal antibodies early that limit efficacy of standard induction dosing,” said Dr. Hanauer, a professor of medicine and medical director of the Digestive Health Center at Northwestern University, Chicago.
Noting that the average duration of treatment in the study prior to randomization was nearly a year (about 40 weeks), he added that a key question is “How to initiate therapeutic drug monitoring earlier in course to further optimize induction and prevent loss of response in maintenance.”
Dr. Hanauer shared that, “in our practice, we use a combination of proactive and reactive therapeutic drug monitoring based on individual patients and their history with prior biologics.”
Findings may usher in ‘new era’ in immune-mediated inflammatory disease treatment
In an editorial published in JAMA with the study, Zachary S. Wallace, MD, and Jeffrey A. Sparks, MD, both of Harvard Medical School in Boston, further commented that “maintaining disease control in nearly 3 of 4 patients represents a meaningful improvement over standard care.”
However, key challenges with the approach include the potential need for additional nurses and others to help monitor patients, and associated costs, which insurance providers may not always cover, they noted.
Another consideration is a lack of effective tools for monitoring measures including antidrug antibodies, they added, and “additional clinical trials within specific disease subgroups are needed.”
However, addressing such barriers “may help introduce a new era in treatment approach to maintenance therapy for patients with immune-mediated inflammatory diseases,” the editorialists write.
Niels Vande Casteele, PharmD, PhD, an associate professor at the department of medicine, University of California, San Diego, and affiliate faculty, Skaggs School of Pharmacy & Pharmaceutical Sciences, commented that the study is “an important milestone in the field of therapeutic drug monitoring of biologics for immunoinflammatory diseases.”
“The ability of proactive therapeutic drug monitoring to achieve sustained disease control without increased drug consumption is [a] notable finding,” he said in an interview.
Noting a limitation, Dr. Casteele suggested the inclusion of more specific measures of disease activity could have provided clearer insights.
“In particular for gastrointestinal diseases, we know that symptoms do not correlate well with inflammatory disease activity,” he said. “As such, I would have preferred to see clinical symptoms being complemented with endoscopic and histologic finds to confirm disease activity.”
Ultimately, he said that the results suggest “proactive therapeutic drug monitoring is not required for all patients, but it is beneficial in some to achieve sustained disease control over a prolonged period of time.”
This study received funding by grants from the Norwegian Regional Health Authorities (interregional KLINBEFORSK grants) and the South-Eastern Norway Regional Health Authorities; study authors reported relationships with various pharmaceutical companies, including Pfizer, which makes infliximab. Dr. Wallace reported research support from Bristol Myers Squibb and Principia/Sanofi and consulting fees from Viela Bio, Zenas Biopharma, and MedPace. Dr. Sparks reported consultancy fees from AbbVie, Bristol Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer. Dr. Hanauer is a consultant and lecturer for Abbvie, Janssen, and Takeda and consultant for Pfizer, Celltrion, Amgen, Samsung Bioepis. Dr. Casteele has received research grants and personal fees from R-Biopharm, Takeda and UCB; and personal fees from AcelaBio, Alimentiv, Celltrion, Prometheus, Procise DX, and Vividion for activities that were all outside of the reviewed study.
Help your patients better understand their IBD treatment options by sharing AGA’s patient education, “Living with IBD,” in the AGA GI Patient Center at www.gastro.org/IBD.
FROM JAMA
Gut bacteria linked with long COVID
While links have been found between the gut’s microbiome and COVID-19, as well as other diseases, this is the first published research to show a link specifically to COVID’s long-term effects, the investigators, based at the Chinese University of Hong Kong, wrote in Gut.
“To our knowledge, this is the first study to show that altered gut microbiome composition is strongly associated with persistent symptoms in patients with COVID-19 up to 6 months after clearance of SARS-CoV-2 virus,” said Siew Ng, MBBS, PhD, associate director at the university’s Center for Gut Microbiota Research.
At three hospitals, the researchers enrolled 106 patients with COVID-19 from February to August 2020 with stool samples at admission and at 1 month and 6 months after discharge, and compared them with people who did not have COVID, recruited in 2019. The severity of COVID in the enrolled patients was mostly mild to moderate.
At 3 months, 86 of the patients with COVID had post–acute COVID-19 syndrome (PACS) – defined as at least one persistent, otherwise unexplained symptom 4 weeks after clearance of the virus. And 81 patients had PACS at 6 months, most commonly fatigue, poor memory, hair loss, anxiety, and trouble sleeping.
Using stool samples for their analysis, the researchers found that, broadly, the diversity of the types of bacteria, and the abundance of these bacteria, were significantly lower at 6 months for those with PACS, compared with those without PACS and with controls (P < .05 and P < .0001, respectively). Among those with PACS, 28 bacteria species were diminished and 14 were enriched, both at baseline and follow-up. Those patients who had COVID but not PACS showed just 25 alterations of bacteria species at the time of hospital admission, and they all normalized by 6 months.
Having respiratory symptoms at 6 months was linked with higher levels of opportunistic pathogens such as Streptococcus anginosus and S. vestibularis. Neuropsychiatric symptoms and fatigue were associated with nosocomial pathogens that are linked to opportunistic infections, such as Clostridium innocuum and Actinomyces naeslundii (P < .05).
Bacteria known for producing butyrate, a beneficial fatty acid, were significantly depleted in those patients with hair loss. And certain of these bacteria, including Bifidobacterium pseudocatenulatum and Faecalibacterium prausnitzii, had the largest inverse correlations with PACS at 6 months (P < .05), the researchers found.
“Particular gut microbial profiles may indicate heightened susceptibility,” Dr. Ng said.
Although the findings were drawn from patients with earlier strains of the COVID-19 virus, the findings still apply to new variants, including Omicron, since these pose the same problem of persistent disruption of the immune system, Dr. Ng said.
Her group is conducting trials to look at how modulating the microbiome might prevent long COVID and boost antibodies after vaccination in high-risk people, she said.
“Gut microbiota influences the health of the host,” Dr. Ng said. “It provides crucial benefits in the form of immune system development, prevention of infections, nutrient acquisition, and brain and nervous system functionality. Considering the millions of people infected during the ongoing pandemic, our findings are a strong impetus for consideration of microbiota modulation to facilitate timely recovery and reduce the burden of post–acute COVID-19 syndrome.”
John Haran, MD, PhD, associate professor of microbiology and physiological systems and emergency medicine at the University of Massachusetts, Worcester, said the research adds to the evidence base on the gut microbiome’s links to COVID, but there was likely be no clinical impact yet. Still, he said the findings linking specific species to specific symptoms was particularly interesting.
“Very early on during hospitalization, [the researchers] saw these differences and correlated out with people who have longer symptoms, and especially different groups of people that have longer symptoms, too,” said Dr. Haran, who has done research on the topic. “It’s very different if you have different symptoms, for example, you keep coughing for months versus you have brain fog and fatigue, or other debilitating symptoms.”
Dr. Haran noted that the findings didn’t identify bacteria types especially linked to COVID, but rather species that have already been found to be associated with a “bad” microbiome. He also pointed out that the patients enrolled in the study were not vaccinated, because vaccines weren’t available at the time. Still, further study to see whether modulation of gut bacteria can be a therapy seems worthwhile.
“Microbiome modulation is pretty safe, and that’s really the next big step that needs to be taken in this,” he said.
For now, the findings don’t give the clinician much new ammunition for treatment.
“We’re not there yet,” he added. “It’s not as if clinicians are going to tell their COVID patients: ‘Go out and buy some kale.’ ”
Eugene Chang, MD, professor of medicine at the University of Chicago, who has studied the gut microbiome and gastrointestinal disease, said it’s “too preliminary” to say whether the findings could lead to a clinical impact. The measures used merely identify the microbes present, but not what they are doing.
“These measures are unlikely to perform well enough to be useful for risk assessment or predicting clinical outcomes,” he said. “That being said, advances in technology are being made where next generations of metrics could be developed and useful as stratifiers and predictors of risk.”
Seeing shifting patterns associated with certain symptoms, he said, is “notable because it suggests that the disturbances of the gut microbiota in PACS are significant.”
But he said it’s important to know whether these changes are a cause of PACS in some way or just an effect of it.
“If causative or contributory – this has to be proven – then ‘microbiota modulation’ would make sense and could be a priority for development,” he said. “If merely an effect, these metrics and better ones to come could be useful as predictors or measures of the patient’s general state of health.”
As seen in his group’s work and other work, he said, “the gut microbiota is highly sensitive to changes in their ecosystem, which is influenced by the health state of the patient.”
Dr. Ng, Dr. Haran, and Dr. Chang reported no relevant disclosures.
This article was updated Jan. 27, 2022.
While links have been found between the gut’s microbiome and COVID-19, as well as other diseases, this is the first published research to show a link specifically to COVID’s long-term effects, the investigators, based at the Chinese University of Hong Kong, wrote in Gut.
“To our knowledge, this is the first study to show that altered gut microbiome composition is strongly associated with persistent symptoms in patients with COVID-19 up to 6 months after clearance of SARS-CoV-2 virus,” said Siew Ng, MBBS, PhD, associate director at the university’s Center for Gut Microbiota Research.
At three hospitals, the researchers enrolled 106 patients with COVID-19 from February to August 2020 with stool samples at admission and at 1 month and 6 months after discharge, and compared them with people who did not have COVID, recruited in 2019. The severity of COVID in the enrolled patients was mostly mild to moderate.
At 3 months, 86 of the patients with COVID had post–acute COVID-19 syndrome (PACS) – defined as at least one persistent, otherwise unexplained symptom 4 weeks after clearance of the virus. And 81 patients had PACS at 6 months, most commonly fatigue, poor memory, hair loss, anxiety, and trouble sleeping.
Using stool samples for their analysis, the researchers found that, broadly, the diversity of the types of bacteria, and the abundance of these bacteria, were significantly lower at 6 months for those with PACS, compared with those without PACS and with controls (P < .05 and P < .0001, respectively). Among those with PACS, 28 bacteria species were diminished and 14 were enriched, both at baseline and follow-up. Those patients who had COVID but not PACS showed just 25 alterations of bacteria species at the time of hospital admission, and they all normalized by 6 months.
Having respiratory symptoms at 6 months was linked with higher levels of opportunistic pathogens such as Streptococcus anginosus and S. vestibularis. Neuropsychiatric symptoms and fatigue were associated with nosocomial pathogens that are linked to opportunistic infections, such as Clostridium innocuum and Actinomyces naeslundii (P < .05).
Bacteria known for producing butyrate, a beneficial fatty acid, were significantly depleted in those patients with hair loss. And certain of these bacteria, including Bifidobacterium pseudocatenulatum and Faecalibacterium prausnitzii, had the largest inverse correlations with PACS at 6 months (P < .05), the researchers found.
“Particular gut microbial profiles may indicate heightened susceptibility,” Dr. Ng said.
Although the findings were drawn from patients with earlier strains of the COVID-19 virus, the findings still apply to new variants, including Omicron, since these pose the same problem of persistent disruption of the immune system, Dr. Ng said.
Her group is conducting trials to look at how modulating the microbiome might prevent long COVID and boost antibodies after vaccination in high-risk people, she said.
“Gut microbiota influences the health of the host,” Dr. Ng said. “It provides crucial benefits in the form of immune system development, prevention of infections, nutrient acquisition, and brain and nervous system functionality. Considering the millions of people infected during the ongoing pandemic, our findings are a strong impetus for consideration of microbiota modulation to facilitate timely recovery and reduce the burden of post–acute COVID-19 syndrome.”
John Haran, MD, PhD, associate professor of microbiology and physiological systems and emergency medicine at the University of Massachusetts, Worcester, said the research adds to the evidence base on the gut microbiome’s links to COVID, but there was likely be no clinical impact yet. Still, he said the findings linking specific species to specific symptoms was particularly interesting.
“Very early on during hospitalization, [the researchers] saw these differences and correlated out with people who have longer symptoms, and especially different groups of people that have longer symptoms, too,” said Dr. Haran, who has done research on the topic. “It’s very different if you have different symptoms, for example, you keep coughing for months versus you have brain fog and fatigue, or other debilitating symptoms.”
Dr. Haran noted that the findings didn’t identify bacteria types especially linked to COVID, but rather species that have already been found to be associated with a “bad” microbiome. He also pointed out that the patients enrolled in the study were not vaccinated, because vaccines weren’t available at the time. Still, further study to see whether modulation of gut bacteria can be a therapy seems worthwhile.
“Microbiome modulation is pretty safe, and that’s really the next big step that needs to be taken in this,” he said.
For now, the findings don’t give the clinician much new ammunition for treatment.
“We’re not there yet,” he added. “It’s not as if clinicians are going to tell their COVID patients: ‘Go out and buy some kale.’ ”
Eugene Chang, MD, professor of medicine at the University of Chicago, who has studied the gut microbiome and gastrointestinal disease, said it’s “too preliminary” to say whether the findings could lead to a clinical impact. The measures used merely identify the microbes present, but not what they are doing.
“These measures are unlikely to perform well enough to be useful for risk assessment or predicting clinical outcomes,” he said. “That being said, advances in technology are being made where next generations of metrics could be developed and useful as stratifiers and predictors of risk.”
Seeing shifting patterns associated with certain symptoms, he said, is “notable because it suggests that the disturbances of the gut microbiota in PACS are significant.”
But he said it’s important to know whether these changes are a cause of PACS in some way or just an effect of it.
“If causative or contributory – this has to be proven – then ‘microbiota modulation’ would make sense and could be a priority for development,” he said. “If merely an effect, these metrics and better ones to come could be useful as predictors or measures of the patient’s general state of health.”
As seen in his group’s work and other work, he said, “the gut microbiota is highly sensitive to changes in their ecosystem, which is influenced by the health state of the patient.”
Dr. Ng, Dr. Haran, and Dr. Chang reported no relevant disclosures.
This article was updated Jan. 27, 2022.
While links have been found between the gut’s microbiome and COVID-19, as well as other diseases, this is the first published research to show a link specifically to COVID’s long-term effects, the investigators, based at the Chinese University of Hong Kong, wrote in Gut.
“To our knowledge, this is the first study to show that altered gut microbiome composition is strongly associated with persistent symptoms in patients with COVID-19 up to 6 months after clearance of SARS-CoV-2 virus,” said Siew Ng, MBBS, PhD, associate director at the university’s Center for Gut Microbiota Research.
At three hospitals, the researchers enrolled 106 patients with COVID-19 from February to August 2020 with stool samples at admission and at 1 month and 6 months after discharge, and compared them with people who did not have COVID, recruited in 2019. The severity of COVID in the enrolled patients was mostly mild to moderate.
At 3 months, 86 of the patients with COVID had post–acute COVID-19 syndrome (PACS) – defined as at least one persistent, otherwise unexplained symptom 4 weeks after clearance of the virus. And 81 patients had PACS at 6 months, most commonly fatigue, poor memory, hair loss, anxiety, and trouble sleeping.
Using stool samples for their analysis, the researchers found that, broadly, the diversity of the types of bacteria, and the abundance of these bacteria, were significantly lower at 6 months for those with PACS, compared with those without PACS and with controls (P < .05 and P < .0001, respectively). Among those with PACS, 28 bacteria species were diminished and 14 were enriched, both at baseline and follow-up. Those patients who had COVID but not PACS showed just 25 alterations of bacteria species at the time of hospital admission, and they all normalized by 6 months.
Having respiratory symptoms at 6 months was linked with higher levels of opportunistic pathogens such as Streptococcus anginosus and S. vestibularis. Neuropsychiatric symptoms and fatigue were associated with nosocomial pathogens that are linked to opportunistic infections, such as Clostridium innocuum and Actinomyces naeslundii (P < .05).
Bacteria known for producing butyrate, a beneficial fatty acid, were significantly depleted in those patients with hair loss. And certain of these bacteria, including Bifidobacterium pseudocatenulatum and Faecalibacterium prausnitzii, had the largest inverse correlations with PACS at 6 months (P < .05), the researchers found.
“Particular gut microbial profiles may indicate heightened susceptibility,” Dr. Ng said.
Although the findings were drawn from patients with earlier strains of the COVID-19 virus, the findings still apply to new variants, including Omicron, since these pose the same problem of persistent disruption of the immune system, Dr. Ng said.
Her group is conducting trials to look at how modulating the microbiome might prevent long COVID and boost antibodies after vaccination in high-risk people, she said.
“Gut microbiota influences the health of the host,” Dr. Ng said. “It provides crucial benefits in the form of immune system development, prevention of infections, nutrient acquisition, and brain and nervous system functionality. Considering the millions of people infected during the ongoing pandemic, our findings are a strong impetus for consideration of microbiota modulation to facilitate timely recovery and reduce the burden of post–acute COVID-19 syndrome.”
John Haran, MD, PhD, associate professor of microbiology and physiological systems and emergency medicine at the University of Massachusetts, Worcester, said the research adds to the evidence base on the gut microbiome’s links to COVID, but there was likely be no clinical impact yet. Still, he said the findings linking specific species to specific symptoms was particularly interesting.
“Very early on during hospitalization, [the researchers] saw these differences and correlated out with people who have longer symptoms, and especially different groups of people that have longer symptoms, too,” said Dr. Haran, who has done research on the topic. “It’s very different if you have different symptoms, for example, you keep coughing for months versus you have brain fog and fatigue, or other debilitating symptoms.”
Dr. Haran noted that the findings didn’t identify bacteria types especially linked to COVID, but rather species that have already been found to be associated with a “bad” microbiome. He also pointed out that the patients enrolled in the study were not vaccinated, because vaccines weren’t available at the time. Still, further study to see whether modulation of gut bacteria can be a therapy seems worthwhile.
“Microbiome modulation is pretty safe, and that’s really the next big step that needs to be taken in this,” he said.
For now, the findings don’t give the clinician much new ammunition for treatment.
“We’re not there yet,” he added. “It’s not as if clinicians are going to tell their COVID patients: ‘Go out and buy some kale.’ ”
Eugene Chang, MD, professor of medicine at the University of Chicago, who has studied the gut microbiome and gastrointestinal disease, said it’s “too preliminary” to say whether the findings could lead to a clinical impact. The measures used merely identify the microbes present, but not what they are doing.
“These measures are unlikely to perform well enough to be useful for risk assessment or predicting clinical outcomes,” he said. “That being said, advances in technology are being made where next generations of metrics could be developed and useful as stratifiers and predictors of risk.”
Seeing shifting patterns associated with certain symptoms, he said, is “notable because it suggests that the disturbances of the gut microbiota in PACS are significant.”
But he said it’s important to know whether these changes are a cause of PACS in some way or just an effect of it.
“If causative or contributory – this has to be proven – then ‘microbiota modulation’ would make sense and could be a priority for development,” he said. “If merely an effect, these metrics and better ones to come could be useful as predictors or measures of the patient’s general state of health.”
As seen in his group’s work and other work, he said, “the gut microbiota is highly sensitive to changes in their ecosystem, which is influenced by the health state of the patient.”
Dr. Ng, Dr. Haran, and Dr. Chang reported no relevant disclosures.
This article was updated Jan. 27, 2022.
FROM GUT
Steroid-free remission fails to impact Crohn’s disease
A steroid-free clinical response had no impact on multiple components of Crohn’s disease progression, based on data from 95 adults.
The therapeutic goals of Crohn’s disease have evolved from controlling symptoms to blocking disease progression and reducing complications, wrote David Laharie, MD, of Hôpital Haut-Lévêque, Pessac, France, and colleagues. The goal of steroid-free remission has been used as an endpoint of treatment, but data on the impact of such remission on long-term disease are limited, the researchers noted in a retrospective study published in Clinical Gastroenterology and Hepatology.
In the study, the researchers reviewed data from 95 adults with early Crohn’s disease (CD) who participated in the TAILORIX trial involving treatment with infliximab and immunosuppressants. The primary endpoint of the TAILORIX trial was sustained corticosteroid-free remission from week 22 to 54. In the current study, the primary endpoint was progression-free survival at 1, 3, and 5 years in patients who did or did not meet the TAILORIX primary endpoint. The median disease duration was 4.5 months, and the median follow-up was 64.2 months.
Progression-free survival was defined as a composite of luminal surgery, anal surgery, hospitalization, and the need for a new CD treatment during the follow-up period.
In the study population, 45 patients achieved corticosteroid-free remission and 50 did not. At 54 weeks, 17 patients with corticosteroid-free remission (38%) and 28 patients without remission (56%) achieved complete mucosal healing, and progression-free survival rates were similar between these groups.
Overall, the rates of progression-free survival at 1, 3, and 5 years were not significantly different between the remission and nonremission groups: 86% versus 91%, respectively, at 1 year; 70% for both groups at 3 years; and 64% and 61%, respectively, at 5 years.
The researchers also compared individual components of the primary endpoint (luminal surgery, anal surgery, hospitalization, and the need for a new CD treatment), and found no significant differences in survival rates in patients who had and had not achieved steroid-free remission.
Survival rates without luminal surgery at 1, 3, and 5 years were 97% versus 96%, 93% versus 90%, and 87% versus 82%, respectively, for remission and nonremission groups. Similarly, survival rates without anal surgery were 93%, 86%, and 86% versus 96%, 88%, and 85%, respectively, for the two groups. Rates of hospitalization-free survival at 1, 3, and 5 years were 90% versus 92%, 81% versus 81%, and 78% versus 69%, respectively, in the remission and nonremission groups. Survival rates without a new systemic CD treatment also were similar at 1, 3, and 5 years: 93% versus 95%, 71% versus 93%, and 60% versus 51%, respectively, for the remission and nonremission groups.
CD progression was not associated with not achieving corticosteroid-free remission (hazard ratio, 0.861). Other factors that were not associated with disease progression in this study included CRP greater than 5 mg/L, age older than 30 years, active smoking, and B1 phenotype.
The researchers noted that, although endoscopic and clinical remission is currently recommended for CD, “there is no validated or standardized definition of this endoscopic goal.” The high rates of survival without major abdominal surgery, regardless of remission status, suggest a significant impact of early combination therapy for CD patients who were biologic naive. Other studies have shown similar improved outcomes for CD patients with early treatment.
The study findings were limited by several factors including the retrospective design and lack of power to compare long-term progression-free survival, the researchers noted. However, the results were strengthened by the robust data on hospitalizations and surgeries from the TAILORIX trial.
The results support a more flexible strategy for CD, “recommending endoscopic and clinical remission in early diagnosed patients and less stringent objectives in those with more refractory or advanced disease,” they concluded.
Findings may guide patient management
The current study is important to help clinicians know whether CD patients who achieve a short-term, steroid-free clinical and endoscopic remission go on to experience better long-term disease outcomes than those who do not achieve this short-term remission, Atsushi Sakuraba, MD, of the University of Chicago said in an interview.
Dr. Sakuraba said that he was surprised by the study findings. “Achieving a clinical remission off steroids with complete endoscopic remission, i.e., deep remission, is considered a treatment goal, but the fact that it did not result in less disease progression was surprising.”
The take-home message for clinicians from the study is that CD patients may still experience disease progression after achieving a single time of clinical and endoscopic remission “mainly due to loss of response to infliximab, so continued long-term disease monitoring and control are required,” Dr. Sakuraba said.
The current study was a post hoc follow-up analysis of a previous trial, Dr. Sakuraba noted. Therefore, studies primarily focused on changing the disease progression and natural course of CD are warranted.
Dr. Laharie disclosed counseling, boards, transportation, or fees from AbbVie, Biogaran, Biogen, Ferring, HAC-pharma, Janssen, MSD, Novartis, Pfizer, Prometheus, Roche, Takeda, Theradiag, and Tillots. Dr. Sakuraba had no relevant financial conflicts to disclose.
A steroid-free clinical response had no impact on multiple components of Crohn’s disease progression, based on data from 95 adults.
The therapeutic goals of Crohn’s disease have evolved from controlling symptoms to blocking disease progression and reducing complications, wrote David Laharie, MD, of Hôpital Haut-Lévêque, Pessac, France, and colleagues. The goal of steroid-free remission has been used as an endpoint of treatment, but data on the impact of such remission on long-term disease are limited, the researchers noted in a retrospective study published in Clinical Gastroenterology and Hepatology.
In the study, the researchers reviewed data from 95 adults with early Crohn’s disease (CD) who participated in the TAILORIX trial involving treatment with infliximab and immunosuppressants. The primary endpoint of the TAILORIX trial was sustained corticosteroid-free remission from week 22 to 54. In the current study, the primary endpoint was progression-free survival at 1, 3, and 5 years in patients who did or did not meet the TAILORIX primary endpoint. The median disease duration was 4.5 months, and the median follow-up was 64.2 months.
Progression-free survival was defined as a composite of luminal surgery, anal surgery, hospitalization, and the need for a new CD treatment during the follow-up period.
In the study population, 45 patients achieved corticosteroid-free remission and 50 did not. At 54 weeks, 17 patients with corticosteroid-free remission (38%) and 28 patients without remission (56%) achieved complete mucosal healing, and progression-free survival rates were similar between these groups.
Overall, the rates of progression-free survival at 1, 3, and 5 years were not significantly different between the remission and nonremission groups: 86% versus 91%, respectively, at 1 year; 70% for both groups at 3 years; and 64% and 61%, respectively, at 5 years.
The researchers also compared individual components of the primary endpoint (luminal surgery, anal surgery, hospitalization, and the need for a new CD treatment), and found no significant differences in survival rates in patients who had and had not achieved steroid-free remission.
Survival rates without luminal surgery at 1, 3, and 5 years were 97% versus 96%, 93% versus 90%, and 87% versus 82%, respectively, for remission and nonremission groups. Similarly, survival rates without anal surgery were 93%, 86%, and 86% versus 96%, 88%, and 85%, respectively, for the two groups. Rates of hospitalization-free survival at 1, 3, and 5 years were 90% versus 92%, 81% versus 81%, and 78% versus 69%, respectively, in the remission and nonremission groups. Survival rates without a new systemic CD treatment also were similar at 1, 3, and 5 years: 93% versus 95%, 71% versus 93%, and 60% versus 51%, respectively, for the remission and nonremission groups.
CD progression was not associated with not achieving corticosteroid-free remission (hazard ratio, 0.861). Other factors that were not associated with disease progression in this study included CRP greater than 5 mg/L, age older than 30 years, active smoking, and B1 phenotype.
The researchers noted that, although endoscopic and clinical remission is currently recommended for CD, “there is no validated or standardized definition of this endoscopic goal.” The high rates of survival without major abdominal surgery, regardless of remission status, suggest a significant impact of early combination therapy for CD patients who were biologic naive. Other studies have shown similar improved outcomes for CD patients with early treatment.
The study findings were limited by several factors including the retrospective design and lack of power to compare long-term progression-free survival, the researchers noted. However, the results were strengthened by the robust data on hospitalizations and surgeries from the TAILORIX trial.
The results support a more flexible strategy for CD, “recommending endoscopic and clinical remission in early diagnosed patients and less stringent objectives in those with more refractory or advanced disease,” they concluded.
Findings may guide patient management
The current study is important to help clinicians know whether CD patients who achieve a short-term, steroid-free clinical and endoscopic remission go on to experience better long-term disease outcomes than those who do not achieve this short-term remission, Atsushi Sakuraba, MD, of the University of Chicago said in an interview.
Dr. Sakuraba said that he was surprised by the study findings. “Achieving a clinical remission off steroids with complete endoscopic remission, i.e., deep remission, is considered a treatment goal, but the fact that it did not result in less disease progression was surprising.”
The take-home message for clinicians from the study is that CD patients may still experience disease progression after achieving a single time of clinical and endoscopic remission “mainly due to loss of response to infliximab, so continued long-term disease monitoring and control are required,” Dr. Sakuraba said.
The current study was a post hoc follow-up analysis of a previous trial, Dr. Sakuraba noted. Therefore, studies primarily focused on changing the disease progression and natural course of CD are warranted.
Dr. Laharie disclosed counseling, boards, transportation, or fees from AbbVie, Biogaran, Biogen, Ferring, HAC-pharma, Janssen, MSD, Novartis, Pfizer, Prometheus, Roche, Takeda, Theradiag, and Tillots. Dr. Sakuraba had no relevant financial conflicts to disclose.
A steroid-free clinical response had no impact on multiple components of Crohn’s disease progression, based on data from 95 adults.
The therapeutic goals of Crohn’s disease have evolved from controlling symptoms to blocking disease progression and reducing complications, wrote David Laharie, MD, of Hôpital Haut-Lévêque, Pessac, France, and colleagues. The goal of steroid-free remission has been used as an endpoint of treatment, but data on the impact of such remission on long-term disease are limited, the researchers noted in a retrospective study published in Clinical Gastroenterology and Hepatology.
In the study, the researchers reviewed data from 95 adults with early Crohn’s disease (CD) who participated in the TAILORIX trial involving treatment with infliximab and immunosuppressants. The primary endpoint of the TAILORIX trial was sustained corticosteroid-free remission from week 22 to 54. In the current study, the primary endpoint was progression-free survival at 1, 3, and 5 years in patients who did or did not meet the TAILORIX primary endpoint. The median disease duration was 4.5 months, and the median follow-up was 64.2 months.
Progression-free survival was defined as a composite of luminal surgery, anal surgery, hospitalization, and the need for a new CD treatment during the follow-up period.
In the study population, 45 patients achieved corticosteroid-free remission and 50 did not. At 54 weeks, 17 patients with corticosteroid-free remission (38%) and 28 patients without remission (56%) achieved complete mucosal healing, and progression-free survival rates were similar between these groups.
Overall, the rates of progression-free survival at 1, 3, and 5 years were not significantly different between the remission and nonremission groups: 86% versus 91%, respectively, at 1 year; 70% for both groups at 3 years; and 64% and 61%, respectively, at 5 years.
The researchers also compared individual components of the primary endpoint (luminal surgery, anal surgery, hospitalization, and the need for a new CD treatment), and found no significant differences in survival rates in patients who had and had not achieved steroid-free remission.
Survival rates without luminal surgery at 1, 3, and 5 years were 97% versus 96%, 93% versus 90%, and 87% versus 82%, respectively, for remission and nonremission groups. Similarly, survival rates without anal surgery were 93%, 86%, and 86% versus 96%, 88%, and 85%, respectively, for the two groups. Rates of hospitalization-free survival at 1, 3, and 5 years were 90% versus 92%, 81% versus 81%, and 78% versus 69%, respectively, in the remission and nonremission groups. Survival rates without a new systemic CD treatment also were similar at 1, 3, and 5 years: 93% versus 95%, 71% versus 93%, and 60% versus 51%, respectively, for the remission and nonremission groups.
CD progression was not associated with not achieving corticosteroid-free remission (hazard ratio, 0.861). Other factors that were not associated with disease progression in this study included CRP greater than 5 mg/L, age older than 30 years, active smoking, and B1 phenotype.
The researchers noted that, although endoscopic and clinical remission is currently recommended for CD, “there is no validated or standardized definition of this endoscopic goal.” The high rates of survival without major abdominal surgery, regardless of remission status, suggest a significant impact of early combination therapy for CD patients who were biologic naive. Other studies have shown similar improved outcomes for CD patients with early treatment.
The study findings were limited by several factors including the retrospective design and lack of power to compare long-term progression-free survival, the researchers noted. However, the results were strengthened by the robust data on hospitalizations and surgeries from the TAILORIX trial.
The results support a more flexible strategy for CD, “recommending endoscopic and clinical remission in early diagnosed patients and less stringent objectives in those with more refractory or advanced disease,” they concluded.
Findings may guide patient management
The current study is important to help clinicians know whether CD patients who achieve a short-term, steroid-free clinical and endoscopic remission go on to experience better long-term disease outcomes than those who do not achieve this short-term remission, Atsushi Sakuraba, MD, of the University of Chicago said in an interview.
Dr. Sakuraba said that he was surprised by the study findings. “Achieving a clinical remission off steroids with complete endoscopic remission, i.e., deep remission, is considered a treatment goal, but the fact that it did not result in less disease progression was surprising.”
The take-home message for clinicians from the study is that CD patients may still experience disease progression after achieving a single time of clinical and endoscopic remission “mainly due to loss of response to infliximab, so continued long-term disease monitoring and control are required,” Dr. Sakuraba said.
The current study was a post hoc follow-up analysis of a previous trial, Dr. Sakuraba noted. Therefore, studies primarily focused on changing the disease progression and natural course of CD are warranted.
Dr. Laharie disclosed counseling, boards, transportation, or fees from AbbVie, Biogaran, Biogen, Ferring, HAC-pharma, Janssen, MSD, Novartis, Pfizer, Prometheus, Roche, Takeda, Theradiag, and Tillots. Dr. Sakuraba had no relevant financial conflicts to disclose.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
ACE index predicts steroid response in acute severe UC
The recently developed ACE index – which incorporates three variables at hospital admission (C-reactive protein [CRP], albumin, and endoscopic severity) – accurately predicts steroid response at hospital admission in patients with acute severe ulcerative colitis (ASUC). This is according to study findings presented at the annual Advances in Inflammatory Bowel Diseases conference by Marta Freitas, MD, of the Senhora da Oliveira Hospital in Guimarães, Portugal.*
Although intravenous steroids represent the first-line medical therapy for patients admitted to the hospital with acute UC, one study found that approximately 30% of patients with ASUC do not respond to this treatment approach and therefore require more advanced management options.
In patients with ASUC, delays in initiating therapy may be associated with an increased risk of mortality, explained Dr. Freitas and colleagues. Given this risk, there is a need for sensitive and accurate tools that can identify patients at admission who are at high risk of steroid nonresponse and who may likewise receive benefit from surgical intervention or earlier second-line therapy.
Early prediction of response to steroids in patients with ASUC at time of admission could also be helpful for prioritizing further assessment and counseling. The ACE index was recently developed to identify these patients to help improve risk stratification and facilitate earlier treatment delivery. A combination of three parameters is found within the ACE index: albumin ≤30 g/L; CRP ≥50 mg/L; and increased endoscopic severity as defined by a Mayo endoscopic score of 3.
Dr. Freitas and researchers retrospectively evaluated the performance of the ACE index in predicting steroid response in 65 patients with ASUC (mean age, 34 years). The study included a review of admissions for the disease between 2005 and 2020. The accuracy of the ACE index score was evaluated through the area under the curve.
Approximately 78.5% of patients in the retrospective cohort study had responded to steroids. Compared with nonresponders, responders had significantly different mean CRP (108.0 ± 60.0 vs. 66.0 ± 53.2 mg/dL, respectively; P = .01), mean albumin (2.9 ± 0.66 vs. 3.4 ± 0.71 g/L; P = .02), and median endoscopic severity score (3 vs. 3; interquartile range, 1 vs. 0; P = .005) at admission. In contrast, no statistically significant difference was found between responders versus nonresponders in regard to the median UC Endoscopic Index of Severity (UCEIS) score (8 vs. 7; P = .28).
Overall, the median ACE index score was 2. Steroid nonresponders had a significantly higher ACE index score (2.5 vs. 1; P = .001). The researchers noted that the ACE index score was a significant predictor of steroid response (AUC, 0.789; P = .001). Half (50.0%) of patients with an ACE index score of 3 had no response to steroids, while 86.3% of patients who had an ACE index score lower than 3 experienced a steroid response.
In a poster presentation by Hartman Brunt, MD, of the Louisiana State University Health Sciences Center in Baton Rouge, real-world data suggest there exists several inconsistencies in the use of UC-monitoring strategies recommended by clinical practice guidelines. According to a single-center retrospective chart review of adult patients with moderate to severe UC, Dr. Brunt and colleagues found that measurement of CRP decreased over time as did measurement of fecal calprotectin.
Given the lack of standardization for IBD monitoring, Dr. Hartman and colleagues noted “there is inevitably increased variability in provider care.” Consequently, this variability and lack of guideline adherence may lead to heterogeneous effects among the IBD patient population, including those that may drive suboptimal long-term outcomes.
In addition to disease monitoring, assessment of treatment response remains highly valuable, yet no clinical guidance currently exists on the use of the ACE index score in ASUC. Further research is needed to determine the validity of the ACE Index in a larger patient population to inform future clinical practice guidelines and expert consensus statements.
Ashwin Ananthakrishnan, MBBS, a gastroenterologist from Massachusetts General Hospital in Boston, said in an email to this news organization that there is an urgent need for tools that “accurately predict treatment response in severe UC because of the higher morbidity and rate of surgery in this population.” Dr. Ananthakrishnan is a co–primary investigator of the MASCC (Multi-center Acute Severe UC Cohort Study), sponsored by Johns Hopkins University, which is investigating the ACE index and other predictors of outcomes in severe UC.
“In addition, treatment decisions need to be made fairly quickly as clinical condition may change day to day,” further emphasizing the need for these predictors, added Dr. Ananthakrishnan. “At this point, the ACE index and other prediction scores have been described from observational studies, but the key is to prospectively incorporate this into a treatment algorithm.”
Dr. Ananthakrishnan explained that patients with a high ACE index, or those with UC who satisfy the high-risk criteria of other prediction models, may benefit from early or even upfront rescue therapy rather than trying steroids for 3-5 days first. He added that “the field is not quite there, and we need more study of this” approach.
Dr. Freitas, Dr. Brunt, and Dr. Ananthakrishnan declared no relevant conflicts of interest.
This article was updated 1/11/22.
*Correction, 1/11/22: An earlier version of this article misstated Dr. Martha Freitas' name.
The recently developed ACE index – which incorporates three variables at hospital admission (C-reactive protein [CRP], albumin, and endoscopic severity) – accurately predicts steroid response at hospital admission in patients with acute severe ulcerative colitis (ASUC). This is according to study findings presented at the annual Advances in Inflammatory Bowel Diseases conference by Marta Freitas, MD, of the Senhora da Oliveira Hospital in Guimarães, Portugal.*
Although intravenous steroids represent the first-line medical therapy for patients admitted to the hospital with acute UC, one study found that approximately 30% of patients with ASUC do not respond to this treatment approach and therefore require more advanced management options.
In patients with ASUC, delays in initiating therapy may be associated with an increased risk of mortality, explained Dr. Freitas and colleagues. Given this risk, there is a need for sensitive and accurate tools that can identify patients at admission who are at high risk of steroid nonresponse and who may likewise receive benefit from surgical intervention or earlier second-line therapy.
Early prediction of response to steroids in patients with ASUC at time of admission could also be helpful for prioritizing further assessment and counseling. The ACE index was recently developed to identify these patients to help improve risk stratification and facilitate earlier treatment delivery. A combination of three parameters is found within the ACE index: albumin ≤30 g/L; CRP ≥50 mg/L; and increased endoscopic severity as defined by a Mayo endoscopic score of 3.
Dr. Freitas and researchers retrospectively evaluated the performance of the ACE index in predicting steroid response in 65 patients with ASUC (mean age, 34 years). The study included a review of admissions for the disease between 2005 and 2020. The accuracy of the ACE index score was evaluated through the area under the curve.
Approximately 78.5% of patients in the retrospective cohort study had responded to steroids. Compared with nonresponders, responders had significantly different mean CRP (108.0 ± 60.0 vs. 66.0 ± 53.2 mg/dL, respectively; P = .01), mean albumin (2.9 ± 0.66 vs. 3.4 ± 0.71 g/L; P = .02), and median endoscopic severity score (3 vs. 3; interquartile range, 1 vs. 0; P = .005) at admission. In contrast, no statistically significant difference was found between responders versus nonresponders in regard to the median UC Endoscopic Index of Severity (UCEIS) score (8 vs. 7; P = .28).
Overall, the median ACE index score was 2. Steroid nonresponders had a significantly higher ACE index score (2.5 vs. 1; P = .001). The researchers noted that the ACE index score was a significant predictor of steroid response (AUC, 0.789; P = .001). Half (50.0%) of patients with an ACE index score of 3 had no response to steroids, while 86.3% of patients who had an ACE index score lower than 3 experienced a steroid response.
In a poster presentation by Hartman Brunt, MD, of the Louisiana State University Health Sciences Center in Baton Rouge, real-world data suggest there exists several inconsistencies in the use of UC-monitoring strategies recommended by clinical practice guidelines. According to a single-center retrospective chart review of adult patients with moderate to severe UC, Dr. Brunt and colleagues found that measurement of CRP decreased over time as did measurement of fecal calprotectin.
Given the lack of standardization for IBD monitoring, Dr. Hartman and colleagues noted “there is inevitably increased variability in provider care.” Consequently, this variability and lack of guideline adherence may lead to heterogeneous effects among the IBD patient population, including those that may drive suboptimal long-term outcomes.
In addition to disease monitoring, assessment of treatment response remains highly valuable, yet no clinical guidance currently exists on the use of the ACE index score in ASUC. Further research is needed to determine the validity of the ACE Index in a larger patient population to inform future clinical practice guidelines and expert consensus statements.
Ashwin Ananthakrishnan, MBBS, a gastroenterologist from Massachusetts General Hospital in Boston, said in an email to this news organization that there is an urgent need for tools that “accurately predict treatment response in severe UC because of the higher morbidity and rate of surgery in this population.” Dr. Ananthakrishnan is a co–primary investigator of the MASCC (Multi-center Acute Severe UC Cohort Study), sponsored by Johns Hopkins University, which is investigating the ACE index and other predictors of outcomes in severe UC.
“In addition, treatment decisions need to be made fairly quickly as clinical condition may change day to day,” further emphasizing the need for these predictors, added Dr. Ananthakrishnan. “At this point, the ACE index and other prediction scores have been described from observational studies, but the key is to prospectively incorporate this into a treatment algorithm.”
Dr. Ananthakrishnan explained that patients with a high ACE index, or those with UC who satisfy the high-risk criteria of other prediction models, may benefit from early or even upfront rescue therapy rather than trying steroids for 3-5 days first. He added that “the field is not quite there, and we need more study of this” approach.
Dr. Freitas, Dr. Brunt, and Dr. Ananthakrishnan declared no relevant conflicts of interest.
This article was updated 1/11/22.
*Correction, 1/11/22: An earlier version of this article misstated Dr. Martha Freitas' name.
The recently developed ACE index – which incorporates three variables at hospital admission (C-reactive protein [CRP], albumin, and endoscopic severity) – accurately predicts steroid response at hospital admission in patients with acute severe ulcerative colitis (ASUC). This is according to study findings presented at the annual Advances in Inflammatory Bowel Diseases conference by Marta Freitas, MD, of the Senhora da Oliveira Hospital in Guimarães, Portugal.*
Although intravenous steroids represent the first-line medical therapy for patients admitted to the hospital with acute UC, one study found that approximately 30% of patients with ASUC do not respond to this treatment approach and therefore require more advanced management options.
In patients with ASUC, delays in initiating therapy may be associated with an increased risk of mortality, explained Dr. Freitas and colleagues. Given this risk, there is a need for sensitive and accurate tools that can identify patients at admission who are at high risk of steroid nonresponse and who may likewise receive benefit from surgical intervention or earlier second-line therapy.
Early prediction of response to steroids in patients with ASUC at time of admission could also be helpful for prioritizing further assessment and counseling. The ACE index was recently developed to identify these patients to help improve risk stratification and facilitate earlier treatment delivery. A combination of three parameters is found within the ACE index: albumin ≤30 g/L; CRP ≥50 mg/L; and increased endoscopic severity as defined by a Mayo endoscopic score of 3.
Dr. Freitas and researchers retrospectively evaluated the performance of the ACE index in predicting steroid response in 65 patients with ASUC (mean age, 34 years). The study included a review of admissions for the disease between 2005 and 2020. The accuracy of the ACE index score was evaluated through the area under the curve.
Approximately 78.5% of patients in the retrospective cohort study had responded to steroids. Compared with nonresponders, responders had significantly different mean CRP (108.0 ± 60.0 vs. 66.0 ± 53.2 mg/dL, respectively; P = .01), mean albumin (2.9 ± 0.66 vs. 3.4 ± 0.71 g/L; P = .02), and median endoscopic severity score (3 vs. 3; interquartile range, 1 vs. 0; P = .005) at admission. In contrast, no statistically significant difference was found between responders versus nonresponders in regard to the median UC Endoscopic Index of Severity (UCEIS) score (8 vs. 7; P = .28).
Overall, the median ACE index score was 2. Steroid nonresponders had a significantly higher ACE index score (2.5 vs. 1; P = .001). The researchers noted that the ACE index score was a significant predictor of steroid response (AUC, 0.789; P = .001). Half (50.0%) of patients with an ACE index score of 3 had no response to steroids, while 86.3% of patients who had an ACE index score lower than 3 experienced a steroid response.
In a poster presentation by Hartman Brunt, MD, of the Louisiana State University Health Sciences Center in Baton Rouge, real-world data suggest there exists several inconsistencies in the use of UC-monitoring strategies recommended by clinical practice guidelines. According to a single-center retrospective chart review of adult patients with moderate to severe UC, Dr. Brunt and colleagues found that measurement of CRP decreased over time as did measurement of fecal calprotectin.
Given the lack of standardization for IBD monitoring, Dr. Hartman and colleagues noted “there is inevitably increased variability in provider care.” Consequently, this variability and lack of guideline adherence may lead to heterogeneous effects among the IBD patient population, including those that may drive suboptimal long-term outcomes.
In addition to disease monitoring, assessment of treatment response remains highly valuable, yet no clinical guidance currently exists on the use of the ACE index score in ASUC. Further research is needed to determine the validity of the ACE Index in a larger patient population to inform future clinical practice guidelines and expert consensus statements.
Ashwin Ananthakrishnan, MBBS, a gastroenterologist from Massachusetts General Hospital in Boston, said in an email to this news organization that there is an urgent need for tools that “accurately predict treatment response in severe UC because of the higher morbidity and rate of surgery in this population.” Dr. Ananthakrishnan is a co–primary investigator of the MASCC (Multi-center Acute Severe UC Cohort Study), sponsored by Johns Hopkins University, which is investigating the ACE index and other predictors of outcomes in severe UC.
“In addition, treatment decisions need to be made fairly quickly as clinical condition may change day to day,” further emphasizing the need for these predictors, added Dr. Ananthakrishnan. “At this point, the ACE index and other prediction scores have been described from observational studies, but the key is to prospectively incorporate this into a treatment algorithm.”
Dr. Ananthakrishnan explained that patients with a high ACE index, or those with UC who satisfy the high-risk criteria of other prediction models, may benefit from early or even upfront rescue therapy rather than trying steroids for 3-5 days first. He added that “the field is not quite there, and we need more study of this” approach.
Dr. Freitas, Dr. Brunt, and Dr. Ananthakrishnan declared no relevant conflicts of interest.
This article was updated 1/11/22.
*Correction, 1/11/22: An earlier version of this article misstated Dr. Martha Freitas' name.
FROM AIBD 2021
Vedolizumab does not increase risk of C. diff infection in UC
Vedolizumab does not seem to increase the risk of Clostridioides difficile infection (CDI), compared with anti–tumor necrosis factor (TNF) therapies in biologic-naive patients with ulcerative colitis (UC), despite concerns that the gut-selective monoclonal antibody treatment may increase gastrointestinal infections at a greater rate than other biologics in this patient population.
Perturbations of the gut microbiota that occur in IBD predispose patients to CDI. Given that treatment with monoclonal antibody vedolizumab exerts an inhibitory action on lymphocyte trafficking to the intestines, questions have been raised on whether this action could increase the risk of CDI in an already vulnerable population.
In patients with UC, the incidence of CDI typically confers a higher risk of adverse outcomes. Unfortunately, CDI is a common complication associated with inflammatory bowel disease (IBD) that can lead to disease flares, further adding to the physical and psychological burden associated with the condition, according to recent studies.
These concerns, however, may not be warranted in patients with UC, according to findings from a retrospective study presented at the annual Advances in Inflammatory Bowel Diseases conference by Rahul Dalal, MD, a gastroenterology fellow at Brigham and Women’s Hospital in Boston.
In the study, Dr. Dalal and colleagues retrospectively analyzed electronic medical records of adult patients with UC who initiated infliximab, adalimumab, or vedolizumab between June 2014 and December 2020. Patients in this retrospective cohort were followed until there was a documented occurrence of CDI, colectomy, or biologic discontinuation/switch, or until the last recorded gastroenterology encounter.
The researchers analyzed the time from biologic initiation to first CDI, which was characterized by a positive stool for C. difficile toxin or toxigenic C. difficile polymerase chain reaction with CDI-specific antibiotic prescriptions. Additionally, the investigators evaluated rates of CDI-related hospitalization, colectomy, or death within a 30-day period of CDI. The primary analysis compared patients with UC who initiated vedolizumab (n = 195) versus anti-TNF therapy (n = 610).
Compared with those treated with anti-TNF agents, patients who initiated vedolizumab were older and less frequently received systemic corticosteroids or had UC-related hospitalization within 12 months prior to starting biologics.
Over 1,436 patient-years’ worth of follow-up, the investigators observed 43 CDIs. Patients treated with vedolizumab less frequently had CDI (1.0% vs. 6.7%; P =.001) and CDI hospitalization (1.0% vs. 3.8%; P =.042), compared with those treated with anti-TNF therapies. The investigators reported no significant differences in the rates of colectomies or deaths or rates of exposure to antibiotics/corticosteroids during the follow-up period or within 30 days prior to CDI onset.
In the unadjusted Cox model, the researchers reported that vedolizumab featured a lower hazard of CDI, compared with anti-TNF (hazard ratio, 0.17; 95% confidence interval, 0.04-0.71). The multivariable Cox model found no significant difference in hazard of CDI for vedolizumab when compared with anti-TNF therapy (HR, 0.33; 95% CI, 0.05-2.03) or immunomodulator exposure (HR, 1.01; 95% CI, 0.41-2.40). The incidence of CDI prior to biologic initiation was associated with an increased hazard of subsequent CDI (HR, 5.95; 95% CI, 2.93-12.09). In the subgroup of patients who experienced a CDI, approximately 39.5% had CDI before biologic initiation at a median of 227 days preceding the subsequent event.
“Vedolizumab is one of the safest biologics that we have in the clinic,” said Jean-Frederic Colombel, MD, who was asked to comment on the study. Dr. Colombel, who wasn’t involved in the study, is a gastroenterologist and serves as director of the Feinstein IBD Center at Mount Sinai Hospital and professor of medicine (division of gastroenterology) at the Icahn School of Medicine at Mount Sinai, both in New York. “Findings from this study reinforce the safety profile of vedolizumab” despite the potential concerns regarding gastroenterological infection with the agent, he added.
Recurrence worries
Recurrent CDI is an issue in patients with IBD, many of whom are considered at high risk for initial and recurrent infection. During a session on CDI and recurrence at the AIBD meeting, Sahil Khanna, MBBS, of the Mayo Clinic, explained that there are three different treatment guidelines to manage initial CDI in patients with IBD.
Predominantly, these guidelines also suggest human monoclonal antibody bezlotoxumab could be used for prevention of CDI recurrence in patients at high risk of recurrence, including those who had experienced severe CDI. “One can argue that anyone with IBD who has C. difficile can be a severe CDI patient because of the bad outcomes we can see,” he explained.
“We do know that IBD is a state of chronic microbial dysbiosis compared to our patients without IBD who get C. difficile because of antibiotic exposure, and that’s why these patients have a high risk of recurrence, compared with non-IBD patients,” said Dr. Khanna. He noted that the bezlotoxumab studies showed numerically lower CDI recurrence rates compared with other treatments in patients with IBD who were initially treated with the monoclonal antibody, but this difference was not statistically significant. “But again, this agent has been shown to be safe in this patient population.”
Dr. Dalal reported having no relevant conflicts of interest. Dr. Colombel has consulted for Takeda, which markets Entyvio for UC. Dr. Khanna has research grants from Rebiotix, as well as consulting fees from Shire Plc, Premier, Facile Therapeutics, and ProbioTech.
Help your patients understand their C. difficile diagnosis by sharing patient education from the AGA GI Patient Center: www.gastro.org/Cdiff .
Vedolizumab does not seem to increase the risk of Clostridioides difficile infection (CDI), compared with anti–tumor necrosis factor (TNF) therapies in biologic-naive patients with ulcerative colitis (UC), despite concerns that the gut-selective monoclonal antibody treatment may increase gastrointestinal infections at a greater rate than other biologics in this patient population.
Perturbations of the gut microbiota that occur in IBD predispose patients to CDI. Given that treatment with monoclonal antibody vedolizumab exerts an inhibitory action on lymphocyte trafficking to the intestines, questions have been raised on whether this action could increase the risk of CDI in an already vulnerable population.
In patients with UC, the incidence of CDI typically confers a higher risk of adverse outcomes. Unfortunately, CDI is a common complication associated with inflammatory bowel disease (IBD) that can lead to disease flares, further adding to the physical and psychological burden associated with the condition, according to recent studies.
These concerns, however, may not be warranted in patients with UC, according to findings from a retrospective study presented at the annual Advances in Inflammatory Bowel Diseases conference by Rahul Dalal, MD, a gastroenterology fellow at Brigham and Women’s Hospital in Boston.
In the study, Dr. Dalal and colleagues retrospectively analyzed electronic medical records of adult patients with UC who initiated infliximab, adalimumab, or vedolizumab between June 2014 and December 2020. Patients in this retrospective cohort were followed until there was a documented occurrence of CDI, colectomy, or biologic discontinuation/switch, or until the last recorded gastroenterology encounter.
The researchers analyzed the time from biologic initiation to first CDI, which was characterized by a positive stool for C. difficile toxin or toxigenic C. difficile polymerase chain reaction with CDI-specific antibiotic prescriptions. Additionally, the investigators evaluated rates of CDI-related hospitalization, colectomy, or death within a 30-day period of CDI. The primary analysis compared patients with UC who initiated vedolizumab (n = 195) versus anti-TNF therapy (n = 610).
Compared with those treated with anti-TNF agents, patients who initiated vedolizumab were older and less frequently received systemic corticosteroids or had UC-related hospitalization within 12 months prior to starting biologics.
Over 1,436 patient-years’ worth of follow-up, the investigators observed 43 CDIs. Patients treated with vedolizumab less frequently had CDI (1.0% vs. 6.7%; P =.001) and CDI hospitalization (1.0% vs. 3.8%; P =.042), compared with those treated with anti-TNF therapies. The investigators reported no significant differences in the rates of colectomies or deaths or rates of exposure to antibiotics/corticosteroids during the follow-up period or within 30 days prior to CDI onset.
In the unadjusted Cox model, the researchers reported that vedolizumab featured a lower hazard of CDI, compared with anti-TNF (hazard ratio, 0.17; 95% confidence interval, 0.04-0.71). The multivariable Cox model found no significant difference in hazard of CDI for vedolizumab when compared with anti-TNF therapy (HR, 0.33; 95% CI, 0.05-2.03) or immunomodulator exposure (HR, 1.01; 95% CI, 0.41-2.40). The incidence of CDI prior to biologic initiation was associated with an increased hazard of subsequent CDI (HR, 5.95; 95% CI, 2.93-12.09). In the subgroup of patients who experienced a CDI, approximately 39.5% had CDI before biologic initiation at a median of 227 days preceding the subsequent event.
“Vedolizumab is one of the safest biologics that we have in the clinic,” said Jean-Frederic Colombel, MD, who was asked to comment on the study. Dr. Colombel, who wasn’t involved in the study, is a gastroenterologist and serves as director of the Feinstein IBD Center at Mount Sinai Hospital and professor of medicine (division of gastroenterology) at the Icahn School of Medicine at Mount Sinai, both in New York. “Findings from this study reinforce the safety profile of vedolizumab” despite the potential concerns regarding gastroenterological infection with the agent, he added.
Recurrence worries
Recurrent CDI is an issue in patients with IBD, many of whom are considered at high risk for initial and recurrent infection. During a session on CDI and recurrence at the AIBD meeting, Sahil Khanna, MBBS, of the Mayo Clinic, explained that there are three different treatment guidelines to manage initial CDI in patients with IBD.
Predominantly, these guidelines also suggest human monoclonal antibody bezlotoxumab could be used for prevention of CDI recurrence in patients at high risk of recurrence, including those who had experienced severe CDI. “One can argue that anyone with IBD who has C. difficile can be a severe CDI patient because of the bad outcomes we can see,” he explained.
“We do know that IBD is a state of chronic microbial dysbiosis compared to our patients without IBD who get C. difficile because of antibiotic exposure, and that’s why these patients have a high risk of recurrence, compared with non-IBD patients,” said Dr. Khanna. He noted that the bezlotoxumab studies showed numerically lower CDI recurrence rates compared with other treatments in patients with IBD who were initially treated with the monoclonal antibody, but this difference was not statistically significant. “But again, this agent has been shown to be safe in this patient population.”
Dr. Dalal reported having no relevant conflicts of interest. Dr. Colombel has consulted for Takeda, which markets Entyvio for UC. Dr. Khanna has research grants from Rebiotix, as well as consulting fees from Shire Plc, Premier, Facile Therapeutics, and ProbioTech.
Help your patients understand their C. difficile diagnosis by sharing patient education from the AGA GI Patient Center: www.gastro.org/Cdiff .
Vedolizumab does not seem to increase the risk of Clostridioides difficile infection (CDI), compared with anti–tumor necrosis factor (TNF) therapies in biologic-naive patients with ulcerative colitis (UC), despite concerns that the gut-selective monoclonal antibody treatment may increase gastrointestinal infections at a greater rate than other biologics in this patient population.
Perturbations of the gut microbiota that occur in IBD predispose patients to CDI. Given that treatment with monoclonal antibody vedolizumab exerts an inhibitory action on lymphocyte trafficking to the intestines, questions have been raised on whether this action could increase the risk of CDI in an already vulnerable population.
In patients with UC, the incidence of CDI typically confers a higher risk of adverse outcomes. Unfortunately, CDI is a common complication associated with inflammatory bowel disease (IBD) that can lead to disease flares, further adding to the physical and psychological burden associated with the condition, according to recent studies.
These concerns, however, may not be warranted in patients with UC, according to findings from a retrospective study presented at the annual Advances in Inflammatory Bowel Diseases conference by Rahul Dalal, MD, a gastroenterology fellow at Brigham and Women’s Hospital in Boston.
In the study, Dr. Dalal and colleagues retrospectively analyzed electronic medical records of adult patients with UC who initiated infliximab, adalimumab, or vedolizumab between June 2014 and December 2020. Patients in this retrospective cohort were followed until there was a documented occurrence of CDI, colectomy, or biologic discontinuation/switch, or until the last recorded gastroenterology encounter.
The researchers analyzed the time from biologic initiation to first CDI, which was characterized by a positive stool for C. difficile toxin or toxigenic C. difficile polymerase chain reaction with CDI-specific antibiotic prescriptions. Additionally, the investigators evaluated rates of CDI-related hospitalization, colectomy, or death within a 30-day period of CDI. The primary analysis compared patients with UC who initiated vedolizumab (n = 195) versus anti-TNF therapy (n = 610).
Compared with those treated with anti-TNF agents, patients who initiated vedolizumab were older and less frequently received systemic corticosteroids or had UC-related hospitalization within 12 months prior to starting biologics.
Over 1,436 patient-years’ worth of follow-up, the investigators observed 43 CDIs. Patients treated with vedolizumab less frequently had CDI (1.0% vs. 6.7%; P =.001) and CDI hospitalization (1.0% vs. 3.8%; P =.042), compared with those treated with anti-TNF therapies. The investigators reported no significant differences in the rates of colectomies or deaths or rates of exposure to antibiotics/corticosteroids during the follow-up period or within 30 days prior to CDI onset.
In the unadjusted Cox model, the researchers reported that vedolizumab featured a lower hazard of CDI, compared with anti-TNF (hazard ratio, 0.17; 95% confidence interval, 0.04-0.71). The multivariable Cox model found no significant difference in hazard of CDI for vedolizumab when compared with anti-TNF therapy (HR, 0.33; 95% CI, 0.05-2.03) or immunomodulator exposure (HR, 1.01; 95% CI, 0.41-2.40). The incidence of CDI prior to biologic initiation was associated with an increased hazard of subsequent CDI (HR, 5.95; 95% CI, 2.93-12.09). In the subgroup of patients who experienced a CDI, approximately 39.5% had CDI before biologic initiation at a median of 227 days preceding the subsequent event.
“Vedolizumab is one of the safest biologics that we have in the clinic,” said Jean-Frederic Colombel, MD, who was asked to comment on the study. Dr. Colombel, who wasn’t involved in the study, is a gastroenterologist and serves as director of the Feinstein IBD Center at Mount Sinai Hospital and professor of medicine (division of gastroenterology) at the Icahn School of Medicine at Mount Sinai, both in New York. “Findings from this study reinforce the safety profile of vedolizumab” despite the potential concerns regarding gastroenterological infection with the agent, he added.
Recurrence worries
Recurrent CDI is an issue in patients with IBD, many of whom are considered at high risk for initial and recurrent infection. During a session on CDI and recurrence at the AIBD meeting, Sahil Khanna, MBBS, of the Mayo Clinic, explained that there are three different treatment guidelines to manage initial CDI in patients with IBD.
Predominantly, these guidelines also suggest human monoclonal antibody bezlotoxumab could be used for prevention of CDI recurrence in patients at high risk of recurrence, including those who had experienced severe CDI. “One can argue that anyone with IBD who has C. difficile can be a severe CDI patient because of the bad outcomes we can see,” he explained.
“We do know that IBD is a state of chronic microbial dysbiosis compared to our patients without IBD who get C. difficile because of antibiotic exposure, and that’s why these patients have a high risk of recurrence, compared with non-IBD patients,” said Dr. Khanna. He noted that the bezlotoxumab studies showed numerically lower CDI recurrence rates compared with other treatments in patients with IBD who were initially treated with the monoclonal antibody, but this difference was not statistically significant. “But again, this agent has been shown to be safe in this patient population.”
Dr. Dalal reported having no relevant conflicts of interest. Dr. Colombel has consulted for Takeda, which markets Entyvio for UC. Dr. Khanna has research grants from Rebiotix, as well as consulting fees from Shire Plc, Premier, Facile Therapeutics, and ProbioTech.
Help your patients understand their C. difficile diagnosis by sharing patient education from the AGA GI Patient Center: www.gastro.org/Cdiff .
FROM AIBD 2021
Microbiota may predict success on low FODMAP diet
Two distinct gut microbiota subtypes showed an enhanced clinical response to a low FODMAP diet in an analysis of 41 adults with irritable bowel syndrome and household controls.
Irritable bowel syndrome (IBS) has a significant impact on quality of life, and some patients find relief on a low FODMAP (fermentable oligosaccharides, disaccharides, monosaccharides and polyols) diet, wrote Kevin Vervier, PhD, of Wellcome Sanger Institute, Hinxton, England, and colleagues. However, the mechanism of action for the success of low FODMAP diets remains unclear, the diet is hard for many patients to follow, and the long-term impact on health is unknown. Therefore, research is needed to identify patients who would derive the most benefit, they said.
In a study published in Gut, the researchers used metagenomics and functional analysis to identify potential biomarkers of response to a low FODMAP diet. They analyzed stool samples from 41 pairs of IBS patients and household contacts. Stool samples were collected at baseline while on usual diets, and again after 4 weeks and 12 weeks on a low FODMAP diet. The patients were divided into two groups based on microbiota clusters; baseline demographics and clinical characteristics were similar between the clusters. In addition, symptom severity was measured using the IBS Severity Scoring System (IBS-SSS).
Cluster 1 was referred to as IBSP microbiome type because of its pathogenic properties, and cluster 2 as IBSH microbiome type because of its resemblance to the microbiome of healthy household controls, the researchers wrote.
“We found a significant enrichment of 109 functional pathways and significant depletion of 13 functional pathways in IBSP microbiomes compared with IBSH microbiomes,” the researchers said.
More specifically, the IBSP microbiomes were enriched in Firmicutes and in genes for amino acid and carbohydrate metabolism, at baseline, while the IBSH microbiomes were similar to healthy controls.
After 4 weeks on the low FODMAP diet, the IBSP microbiomes normalized, with increased levels of Bacterioides and decreased levels of pathobionts (including Clostridium difficile, Streptococcus parasanguinis, and Paeniclostridium sordellii) to create a microbiome profile resembling the IBSH microbiomes and healthy controls. The taxonomic profile of microbiomes observed in IBSH and healthy controls did not demonstrate a significant shift.
Although both microbiome groups showed improvement in IBS-SSS scores from baseline on the low FODMAP diet, decreasing from a mean baseline score of 278 to a diet score of 128, the improvement was greater in the IBSP group than the IBSH group (delta, 194 vs. 114, respectively; P = .02), the researchers noted. “The shift in the IBSP microbiota to a healthy profile appeared stable for at least 3 months and correlated with continuing symptomatic well-being,” they wrote.
The distinct responses of the IBSP and IBSH microbiomes to the low FODMAP diet suggest a potential mode of action, the researchers said in their discussion. Based on their findings, “it is possible that removal of the eliciting dietary component starves the pathobionts, leading to reduction in their growth and metabolism and a consequent decrease in symptoms, accompanied by an expansion of commensal or symbiotic species leading to a health-associated microbiome,” but more research is needed to prove causality, they said.
The study findings were limited by several factors, including the relatively small sample size, strict inclusion criteria, restriction of medications, and need for participation by household controls, the researchers noted. Other limitations include the inability to control for other factors that could have impacted the gut microbiota, such as the placebo effect and psychological factors, they said.
However, the findings provide a foundation for more research and should be validated in other populations involving different geographical regions and dietary habits, they said. “The identification of a microbial signature ‘biomarker’ that correlates with improved response to a low FODMAP diet may, if validated, allow better stratification and selection of patients likely to benefit from the diet,” they concluded.
Setting the stage for focused studies
The low FODMAP diet has demonstrated effectiveness for symptom relief in IBS, although potential risks include exacerbation of disordered eating, nutrition deficiencies, and disrupting gut microbiota, wrote Peter R. Gibson, MD, and Emma P. Halmos, MD, of Monash University and Alfred Health, Melbourne, in an accompanying editorial. However, the current study takes a new step on the journey to identifying patients most likely to respond to a low FODMAP diet, they said.
The editorialists noted three key takeaway points. First, the fecal microbiome may predict response to a low FODMAP diet. Second, the correction of the microbiome through the low FODMAP diet appeared to continue even after the diet was discontinued. “The other intriguing finding was that trehalose metabolic pathways were ‘activated’ in those with dysbiosis,” suggesting that trehalose might be an unrecognized FODMAP, the researchers noted. Trehalose has not been well studied but has been associated with pathogenicity, they said.
Although the study may overemphasize the impact of the low FODMAP diet given the relatively poor assessment of FODMAP intake, “the beauty of Vervier’s work is not in its definitive nature but in that it enables the creation of feasible innovative hypotheses that can be examined by focused studies,” they concluded.
The current study is important because IBS and related disorders of gut-brain interaction are common and greatly impact the quality of life of affected individuals, Jatin Roper, MD, of Duke University in Durham, N.C., said in an interview. Although the mechanisms for improvement are unknown, he said, “The low FODMAP diet is widely used to treat IBS, based on the hypothesis that this diet modifies the gut microbiome in a beneficial way.”
The study authors made two important discoveries, said Dr. Roper. “First, they found that they were able to distinguish IBS versus household controls based on their gut microbial signatures as well expression of key metabolic genes,” he said. “Second, they identified a unique microbiota subtype that was associated with a significant clinical response to the low FODMAP diet in IBS patients; IBS patients with a ‘pathogenic’ microbiome consisting of high Firmicutes and low Bacteroidetes responded to a greater degree to the low FODMAP diet compared to IBS patients with a ‘healthy’ microbiome that was similar to controls,” he explained. “Furthermore, after time on the low FODMAP diet, the IBS patients with pathogenic microbiome signatures developed a microbiome with low Firmicutes and high Bacteroidetes, which is thought to be healthy,” he added.
“These findings are exciting because they suggest that a patient’s microbial signature might be used clinically to predict response to the low FODMAP diet,” said Dr. Roper. “The surprising aspect of these results is that the microbial signature alone was able to predict response to a low FODMAP diet, despite the complex effects of the diet on host physiology and metabolism and the multifactorial etiology of IBS,” he noted.
However, larger clinical studies are needed to confirm the study findings results in larger patient cohorts and to show that standardized clinical assays can be used to prospectively predict response to dietary interventions such as low FODMAP in IBS, Dr. Roper emphasized.
“This paper provides preliminary and provocative findings that suggest that gut microbiota metabolites may play a role in the pathogenesis of IBS,” said Dr. Roper. “Future basic science and translational research is needed to study the mechanisms by which specific bacterial metabolites regulate intestinal function and disorders such as IBS. I hope that this research will eventually lead to metabolite-based therapies for IBS and other gastrointestinal disorders,” he said.
The study received no outside funding. Lead author Dr. Vervier had no financial conflicts to disclose. Dr. Gibson disclosed authoring two educational/recipe books on the low FODMAP diet, and Monash University financially benefits from the sales of a digital application, booklets, and online courses on the low FODMAP diet. Dr. Halmos had no financial conflicts to disclose. Dr. Roper had no financial conflicts to disclose.
Through the AGA Center for Gut Microbiome Research and Education, AGA is committed to keeping you up-to-speed on the latest news, research and policy updates related to the gut microbiome: www.gastro.org/microbiome.
Two distinct gut microbiota subtypes showed an enhanced clinical response to a low FODMAP diet in an analysis of 41 adults with irritable bowel syndrome and household controls.
Irritable bowel syndrome (IBS) has a significant impact on quality of life, and some patients find relief on a low FODMAP (fermentable oligosaccharides, disaccharides, monosaccharides and polyols) diet, wrote Kevin Vervier, PhD, of Wellcome Sanger Institute, Hinxton, England, and colleagues. However, the mechanism of action for the success of low FODMAP diets remains unclear, the diet is hard for many patients to follow, and the long-term impact on health is unknown. Therefore, research is needed to identify patients who would derive the most benefit, they said.
In a study published in Gut, the researchers used metagenomics and functional analysis to identify potential biomarkers of response to a low FODMAP diet. They analyzed stool samples from 41 pairs of IBS patients and household contacts. Stool samples were collected at baseline while on usual diets, and again after 4 weeks and 12 weeks on a low FODMAP diet. The patients were divided into two groups based on microbiota clusters; baseline demographics and clinical characteristics were similar between the clusters. In addition, symptom severity was measured using the IBS Severity Scoring System (IBS-SSS).
Cluster 1 was referred to as IBSP microbiome type because of its pathogenic properties, and cluster 2 as IBSH microbiome type because of its resemblance to the microbiome of healthy household controls, the researchers wrote.
“We found a significant enrichment of 109 functional pathways and significant depletion of 13 functional pathways in IBSP microbiomes compared with IBSH microbiomes,” the researchers said.
More specifically, the IBSP microbiomes were enriched in Firmicutes and in genes for amino acid and carbohydrate metabolism, at baseline, while the IBSH microbiomes were similar to healthy controls.
After 4 weeks on the low FODMAP diet, the IBSP microbiomes normalized, with increased levels of Bacterioides and decreased levels of pathobionts (including Clostridium difficile, Streptococcus parasanguinis, and Paeniclostridium sordellii) to create a microbiome profile resembling the IBSH microbiomes and healthy controls. The taxonomic profile of microbiomes observed in IBSH and healthy controls did not demonstrate a significant shift.
Although both microbiome groups showed improvement in IBS-SSS scores from baseline on the low FODMAP diet, decreasing from a mean baseline score of 278 to a diet score of 128, the improvement was greater in the IBSP group than the IBSH group (delta, 194 vs. 114, respectively; P = .02), the researchers noted. “The shift in the IBSP microbiota to a healthy profile appeared stable for at least 3 months and correlated with continuing symptomatic well-being,” they wrote.
The distinct responses of the IBSP and IBSH microbiomes to the low FODMAP diet suggest a potential mode of action, the researchers said in their discussion. Based on their findings, “it is possible that removal of the eliciting dietary component starves the pathobionts, leading to reduction in their growth and metabolism and a consequent decrease in symptoms, accompanied by an expansion of commensal or symbiotic species leading to a health-associated microbiome,” but more research is needed to prove causality, they said.
The study findings were limited by several factors, including the relatively small sample size, strict inclusion criteria, restriction of medications, and need for participation by household controls, the researchers noted. Other limitations include the inability to control for other factors that could have impacted the gut microbiota, such as the placebo effect and psychological factors, they said.
However, the findings provide a foundation for more research and should be validated in other populations involving different geographical regions and dietary habits, they said. “The identification of a microbial signature ‘biomarker’ that correlates with improved response to a low FODMAP diet may, if validated, allow better stratification and selection of patients likely to benefit from the diet,” they concluded.
Setting the stage for focused studies
The low FODMAP diet has demonstrated effectiveness for symptom relief in IBS, although potential risks include exacerbation of disordered eating, nutrition deficiencies, and disrupting gut microbiota, wrote Peter R. Gibson, MD, and Emma P. Halmos, MD, of Monash University and Alfred Health, Melbourne, in an accompanying editorial. However, the current study takes a new step on the journey to identifying patients most likely to respond to a low FODMAP diet, they said.
The editorialists noted three key takeaway points. First, the fecal microbiome may predict response to a low FODMAP diet. Second, the correction of the microbiome through the low FODMAP diet appeared to continue even after the diet was discontinued. “The other intriguing finding was that trehalose metabolic pathways were ‘activated’ in those with dysbiosis,” suggesting that trehalose might be an unrecognized FODMAP, the researchers noted. Trehalose has not been well studied but has been associated with pathogenicity, they said.
Although the study may overemphasize the impact of the low FODMAP diet given the relatively poor assessment of FODMAP intake, “the beauty of Vervier’s work is not in its definitive nature but in that it enables the creation of feasible innovative hypotheses that can be examined by focused studies,” they concluded.
The current study is important because IBS and related disorders of gut-brain interaction are common and greatly impact the quality of life of affected individuals, Jatin Roper, MD, of Duke University in Durham, N.C., said in an interview. Although the mechanisms for improvement are unknown, he said, “The low FODMAP diet is widely used to treat IBS, based on the hypothesis that this diet modifies the gut microbiome in a beneficial way.”
The study authors made two important discoveries, said Dr. Roper. “First, they found that they were able to distinguish IBS versus household controls based on their gut microbial signatures as well expression of key metabolic genes,” he said. “Second, they identified a unique microbiota subtype that was associated with a significant clinical response to the low FODMAP diet in IBS patients; IBS patients with a ‘pathogenic’ microbiome consisting of high Firmicutes and low Bacteroidetes responded to a greater degree to the low FODMAP diet compared to IBS patients with a ‘healthy’ microbiome that was similar to controls,” he explained. “Furthermore, after time on the low FODMAP diet, the IBS patients with pathogenic microbiome signatures developed a microbiome with low Firmicutes and high Bacteroidetes, which is thought to be healthy,” he added.
“These findings are exciting because they suggest that a patient’s microbial signature might be used clinically to predict response to the low FODMAP diet,” said Dr. Roper. “The surprising aspect of these results is that the microbial signature alone was able to predict response to a low FODMAP diet, despite the complex effects of the diet on host physiology and metabolism and the multifactorial etiology of IBS,” he noted.
However, larger clinical studies are needed to confirm the study findings results in larger patient cohorts and to show that standardized clinical assays can be used to prospectively predict response to dietary interventions such as low FODMAP in IBS, Dr. Roper emphasized.
“This paper provides preliminary and provocative findings that suggest that gut microbiota metabolites may play a role in the pathogenesis of IBS,” said Dr. Roper. “Future basic science and translational research is needed to study the mechanisms by which specific bacterial metabolites regulate intestinal function and disorders such as IBS. I hope that this research will eventually lead to metabolite-based therapies for IBS and other gastrointestinal disorders,” he said.
The study received no outside funding. Lead author Dr. Vervier had no financial conflicts to disclose. Dr. Gibson disclosed authoring two educational/recipe books on the low FODMAP diet, and Monash University financially benefits from the sales of a digital application, booklets, and online courses on the low FODMAP diet. Dr. Halmos had no financial conflicts to disclose. Dr. Roper had no financial conflicts to disclose.
Through the AGA Center for Gut Microbiome Research and Education, AGA is committed to keeping you up-to-speed on the latest news, research and policy updates related to the gut microbiome: www.gastro.org/microbiome.
Two distinct gut microbiota subtypes showed an enhanced clinical response to a low FODMAP diet in an analysis of 41 adults with irritable bowel syndrome and household controls.
Irritable bowel syndrome (IBS) has a significant impact on quality of life, and some patients find relief on a low FODMAP (fermentable oligosaccharides, disaccharides, monosaccharides and polyols) diet, wrote Kevin Vervier, PhD, of Wellcome Sanger Institute, Hinxton, England, and colleagues. However, the mechanism of action for the success of low FODMAP diets remains unclear, the diet is hard for many patients to follow, and the long-term impact on health is unknown. Therefore, research is needed to identify patients who would derive the most benefit, they said.
In a study published in Gut, the researchers used metagenomics and functional analysis to identify potential biomarkers of response to a low FODMAP diet. They analyzed stool samples from 41 pairs of IBS patients and household contacts. Stool samples were collected at baseline while on usual diets, and again after 4 weeks and 12 weeks on a low FODMAP diet. The patients were divided into two groups based on microbiota clusters; baseline demographics and clinical characteristics were similar between the clusters. In addition, symptom severity was measured using the IBS Severity Scoring System (IBS-SSS).
Cluster 1 was referred to as IBSP microbiome type because of its pathogenic properties, and cluster 2 as IBSH microbiome type because of its resemblance to the microbiome of healthy household controls, the researchers wrote.
“We found a significant enrichment of 109 functional pathways and significant depletion of 13 functional pathways in IBSP microbiomes compared with IBSH microbiomes,” the researchers said.
More specifically, the IBSP microbiomes were enriched in Firmicutes and in genes for amino acid and carbohydrate metabolism, at baseline, while the IBSH microbiomes were similar to healthy controls.
After 4 weeks on the low FODMAP diet, the IBSP microbiomes normalized, with increased levels of Bacterioides and decreased levels of pathobionts (including Clostridium difficile, Streptococcus parasanguinis, and Paeniclostridium sordellii) to create a microbiome profile resembling the IBSH microbiomes and healthy controls. The taxonomic profile of microbiomes observed in IBSH and healthy controls did not demonstrate a significant shift.
Although both microbiome groups showed improvement in IBS-SSS scores from baseline on the low FODMAP diet, decreasing from a mean baseline score of 278 to a diet score of 128, the improvement was greater in the IBSP group than the IBSH group (delta, 194 vs. 114, respectively; P = .02), the researchers noted. “The shift in the IBSP microbiota to a healthy profile appeared stable for at least 3 months and correlated with continuing symptomatic well-being,” they wrote.
The distinct responses of the IBSP and IBSH microbiomes to the low FODMAP diet suggest a potential mode of action, the researchers said in their discussion. Based on their findings, “it is possible that removal of the eliciting dietary component starves the pathobionts, leading to reduction in their growth and metabolism and a consequent decrease in symptoms, accompanied by an expansion of commensal or symbiotic species leading to a health-associated microbiome,” but more research is needed to prove causality, they said.
The study findings were limited by several factors, including the relatively small sample size, strict inclusion criteria, restriction of medications, and need for participation by household controls, the researchers noted. Other limitations include the inability to control for other factors that could have impacted the gut microbiota, such as the placebo effect and psychological factors, they said.
However, the findings provide a foundation for more research and should be validated in other populations involving different geographical regions and dietary habits, they said. “The identification of a microbial signature ‘biomarker’ that correlates with improved response to a low FODMAP diet may, if validated, allow better stratification and selection of patients likely to benefit from the diet,” they concluded.
Setting the stage for focused studies
The low FODMAP diet has demonstrated effectiveness for symptom relief in IBS, although potential risks include exacerbation of disordered eating, nutrition deficiencies, and disrupting gut microbiota, wrote Peter R. Gibson, MD, and Emma P. Halmos, MD, of Monash University and Alfred Health, Melbourne, in an accompanying editorial. However, the current study takes a new step on the journey to identifying patients most likely to respond to a low FODMAP diet, they said.
The editorialists noted three key takeaway points. First, the fecal microbiome may predict response to a low FODMAP diet. Second, the correction of the microbiome through the low FODMAP diet appeared to continue even after the diet was discontinued. “The other intriguing finding was that trehalose metabolic pathways were ‘activated’ in those with dysbiosis,” suggesting that trehalose might be an unrecognized FODMAP, the researchers noted. Trehalose has not been well studied but has been associated with pathogenicity, they said.
Although the study may overemphasize the impact of the low FODMAP diet given the relatively poor assessment of FODMAP intake, “the beauty of Vervier’s work is not in its definitive nature but in that it enables the creation of feasible innovative hypotheses that can be examined by focused studies,” they concluded.
The current study is important because IBS and related disorders of gut-brain interaction are common and greatly impact the quality of life of affected individuals, Jatin Roper, MD, of Duke University in Durham, N.C., said in an interview. Although the mechanisms for improvement are unknown, he said, “The low FODMAP diet is widely used to treat IBS, based on the hypothesis that this diet modifies the gut microbiome in a beneficial way.”
The study authors made two important discoveries, said Dr. Roper. “First, they found that they were able to distinguish IBS versus household controls based on their gut microbial signatures as well expression of key metabolic genes,” he said. “Second, they identified a unique microbiota subtype that was associated with a significant clinical response to the low FODMAP diet in IBS patients; IBS patients with a ‘pathogenic’ microbiome consisting of high Firmicutes and low Bacteroidetes responded to a greater degree to the low FODMAP diet compared to IBS patients with a ‘healthy’ microbiome that was similar to controls,” he explained. “Furthermore, after time on the low FODMAP diet, the IBS patients with pathogenic microbiome signatures developed a microbiome with low Firmicutes and high Bacteroidetes, which is thought to be healthy,” he added.
“These findings are exciting because they suggest that a patient’s microbial signature might be used clinically to predict response to the low FODMAP diet,” said Dr. Roper. “The surprising aspect of these results is that the microbial signature alone was able to predict response to a low FODMAP diet, despite the complex effects of the diet on host physiology and metabolism and the multifactorial etiology of IBS,” he noted.
However, larger clinical studies are needed to confirm the study findings results in larger patient cohorts and to show that standardized clinical assays can be used to prospectively predict response to dietary interventions such as low FODMAP in IBS, Dr. Roper emphasized.
“This paper provides preliminary and provocative findings that suggest that gut microbiota metabolites may play a role in the pathogenesis of IBS,” said Dr. Roper. “Future basic science and translational research is needed to study the mechanisms by which specific bacterial metabolites regulate intestinal function and disorders such as IBS. I hope that this research will eventually lead to metabolite-based therapies for IBS and other gastrointestinal disorders,” he said.
The study received no outside funding. Lead author Dr. Vervier had no financial conflicts to disclose. Dr. Gibson disclosed authoring two educational/recipe books on the low FODMAP diet, and Monash University financially benefits from the sales of a digital application, booklets, and online courses on the low FODMAP diet. Dr. Halmos had no financial conflicts to disclose. Dr. Roper had no financial conflicts to disclose.
Through the AGA Center for Gut Microbiome Research and Education, AGA is committed to keeping you up-to-speed on the latest news, research and policy updates related to the gut microbiome: www.gastro.org/microbiome.
FROM GUT