User login
Biopsy not required to diagnose most cases of pediatric celiac disease
(ESPGHAN). The authors recommend that the diagnosis be established with a two-stage blood test instead of an endoscopy, which children often find distressing.
The guidance was published in the Journal of Pediatric Gastroenterology and Nutrition. The document is an update of ESPGHAN’s 2012 guidance.
About half of children with suspected celiac disease undergo a biopsy to confirm the diagnosis. By reducing the number of biopsies, and the anesthesia required to perform them, the new guidelines could reduce European health care costs.
Steffen Husby, MD, of Odense (Denmark) University Hospital, and colleagues recommend testing for total IgA and anti-intestinal transglutaminase 2 (TGA-IgA) antibodies as initial screening in children with suspected celiac disease. An IgG-based test is indicated only when total IgA is low or undetectable, according to the authors. Physicians should refer children with positive results to a pediatric gastroenterologist. If the level of TGA-IgA is 10 or more times the upper limit of normal, and the family agrees, the physician may diagnose celiac disease without a biopsy, provided that endomysial antibodies test positive in a second blood sample, according to the guidance. For children with a positive TGA-IgA level of less than 10 times the upper limit of normal, however, at least four biopsies from the distal duodenum and at least one from the bulb are required to establish the diagnosis.
Physicians can diagnose celiac disease in children with no symptoms without the need for a biopsy using the same criteria as they use for symptomatic children, wrote Dr. Husby and colleagues. Clinicians, parents, and, when appropriate, children should participate in the decision about whether to perform a biopsy.
Celiac disease is the most prevalent food-related chronic disease in European children, but as much as 80% of children with celiac disease are undiagnosed. The prevalence of celiac disease is increasing, and undiagnosed children with this disease are at risk of nutritional and developmental problems, as well as long-term health complications. Although celiac disease is easy to detect and treat, 10-13 years may elapse between symptom onset and the time of diagnosis. The new guidelines are intended to facilitate diagnosis and increase its accuracy, thus enabling earlier diagnosis and improved detection, according to ESPGHAN.
“These new guidelines mean that more than half of all children being investigated for celiac disease will no longer need to have an invasive biopsy,” said Luisa Mearin Manrique, MD, PhD, professor of pediatrics at Leiden (the Netherlands) University and senior author of the guidelines, in a press release. “This is a big step forward in our mission to ensure that children can be diagnosed and effectively treated for celiac disease. It is scandalous that so many children go so long, often up to 10 years, without diagnosis. Removing the need for biopsy in order to achieve diagnosis will reduce the stresses associated with such an invasive procedure and mean that diagnoses are quicker and cheaper for health care systems.”
No conflicts of interest were reported.
SOURCE: Husby S et al. J Pediatr Gastroenterol Nutr. 2020;70(1):141-56.
(ESPGHAN). The authors recommend that the diagnosis be established with a two-stage blood test instead of an endoscopy, which children often find distressing.
The guidance was published in the Journal of Pediatric Gastroenterology and Nutrition. The document is an update of ESPGHAN’s 2012 guidance.
About half of children with suspected celiac disease undergo a biopsy to confirm the diagnosis. By reducing the number of biopsies, and the anesthesia required to perform them, the new guidelines could reduce European health care costs.
Steffen Husby, MD, of Odense (Denmark) University Hospital, and colleagues recommend testing for total IgA and anti-intestinal transglutaminase 2 (TGA-IgA) antibodies as initial screening in children with suspected celiac disease. An IgG-based test is indicated only when total IgA is low or undetectable, according to the authors. Physicians should refer children with positive results to a pediatric gastroenterologist. If the level of TGA-IgA is 10 or more times the upper limit of normal, and the family agrees, the physician may diagnose celiac disease without a biopsy, provided that endomysial antibodies test positive in a second blood sample, according to the guidance. For children with a positive TGA-IgA level of less than 10 times the upper limit of normal, however, at least four biopsies from the distal duodenum and at least one from the bulb are required to establish the diagnosis.
Physicians can diagnose celiac disease in children with no symptoms without the need for a biopsy using the same criteria as they use for symptomatic children, wrote Dr. Husby and colleagues. Clinicians, parents, and, when appropriate, children should participate in the decision about whether to perform a biopsy.
Celiac disease is the most prevalent food-related chronic disease in European children, but as much as 80% of children with celiac disease are undiagnosed. The prevalence of celiac disease is increasing, and undiagnosed children with this disease are at risk of nutritional and developmental problems, as well as long-term health complications. Although celiac disease is easy to detect and treat, 10-13 years may elapse between symptom onset and the time of diagnosis. The new guidelines are intended to facilitate diagnosis and increase its accuracy, thus enabling earlier diagnosis and improved detection, according to ESPGHAN.
“These new guidelines mean that more than half of all children being investigated for celiac disease will no longer need to have an invasive biopsy,” said Luisa Mearin Manrique, MD, PhD, professor of pediatrics at Leiden (the Netherlands) University and senior author of the guidelines, in a press release. “This is a big step forward in our mission to ensure that children can be diagnosed and effectively treated for celiac disease. It is scandalous that so many children go so long, often up to 10 years, without diagnosis. Removing the need for biopsy in order to achieve diagnosis will reduce the stresses associated with such an invasive procedure and mean that diagnoses are quicker and cheaper for health care systems.”
No conflicts of interest were reported.
SOURCE: Husby S et al. J Pediatr Gastroenterol Nutr. 2020;70(1):141-56.
(ESPGHAN). The authors recommend that the diagnosis be established with a two-stage blood test instead of an endoscopy, which children often find distressing.
The guidance was published in the Journal of Pediatric Gastroenterology and Nutrition. The document is an update of ESPGHAN’s 2012 guidance.
About half of children with suspected celiac disease undergo a biopsy to confirm the diagnosis. By reducing the number of biopsies, and the anesthesia required to perform them, the new guidelines could reduce European health care costs.
Steffen Husby, MD, of Odense (Denmark) University Hospital, and colleagues recommend testing for total IgA and anti-intestinal transglutaminase 2 (TGA-IgA) antibodies as initial screening in children with suspected celiac disease. An IgG-based test is indicated only when total IgA is low or undetectable, according to the authors. Physicians should refer children with positive results to a pediatric gastroenterologist. If the level of TGA-IgA is 10 or more times the upper limit of normal, and the family agrees, the physician may diagnose celiac disease without a biopsy, provided that endomysial antibodies test positive in a second blood sample, according to the guidance. For children with a positive TGA-IgA level of less than 10 times the upper limit of normal, however, at least four biopsies from the distal duodenum and at least one from the bulb are required to establish the diagnosis.
Physicians can diagnose celiac disease in children with no symptoms without the need for a biopsy using the same criteria as they use for symptomatic children, wrote Dr. Husby and colleagues. Clinicians, parents, and, when appropriate, children should participate in the decision about whether to perform a biopsy.
Celiac disease is the most prevalent food-related chronic disease in European children, but as much as 80% of children with celiac disease are undiagnosed. The prevalence of celiac disease is increasing, and undiagnosed children with this disease are at risk of nutritional and developmental problems, as well as long-term health complications. Although celiac disease is easy to detect and treat, 10-13 years may elapse between symptom onset and the time of diagnosis. The new guidelines are intended to facilitate diagnosis and increase its accuracy, thus enabling earlier diagnosis and improved detection, according to ESPGHAN.
“These new guidelines mean that more than half of all children being investigated for celiac disease will no longer need to have an invasive biopsy,” said Luisa Mearin Manrique, MD, PhD, professor of pediatrics at Leiden (the Netherlands) University and senior author of the guidelines, in a press release. “This is a big step forward in our mission to ensure that children can be diagnosed and effectively treated for celiac disease. It is scandalous that so many children go so long, often up to 10 years, without diagnosis. Removing the need for biopsy in order to achieve diagnosis will reduce the stresses associated with such an invasive procedure and mean that diagnoses are quicker and cheaper for health care systems.”
No conflicts of interest were reported.
SOURCE: Husby S et al. J Pediatr Gastroenterol Nutr. 2020;70(1):141-56.
FROM THE JOURNAL OF PEDIATRIC GASTROENTEROLOGY AND NUTRITION
Can diet, microbiome personalization reverse IBD increase?
AUSTIN, TEX. – With the increase in the prevalence of inflammatory bowel disease worldwide approaching pandemic proportions, personalized medicine targeting diet and the microbiome may contribute to a halt or even a reversal of the trend, a leading researcher from Israel and proponent of the Mediterranean diet reported at the Crohn’s & Colitis Congress®, a partnership of the Crohn's & Colitis Foundation and the American Gastroenterological Association.
“Inflammatory bowel disease is turning into a pandemic around the world,” said Iris Dotan, MD, of the Rabin Medical Center in Petah Tikva, Israel, citing research reported at the 2015 meeting of the European Crohn’s & Colitis Organization that showed the prevalence of inflammatory bowel disease (IBD) in Israel increasing almost 400% from 1991 to 2015, up to 460 per 100,000. “The rising incidence highlights the role of environmental factors,” she added.
She reported on her work with the Rabin Medical Center Pouch Cohort, which is studying the ileal pouch as a man-made model of IBD to get answers. “We believe that when patients progress from having a pouch that is normal to having pouchitis it actually resembles Crohn’s disease and can be used as a model to identify processes that are ongoing in these patients,” she said.
The study is following an unspecified number of ileal pouch patients prospectively, evaluating their biomaterial with stool samples and mucosal biopsies, and tracking their diet and psychosocial status to gain insight into what sets off episodes of pouchitis.
Already, the study has provided insight into how antibiotics may contribute to IBD. “Pouch disease is a very antibiotic-responsive disease,” she said in an interview. “Antibiotics are clinically effective. However, as we dive deeper into this and understand the mechanistics of it, we see that antibiotics cause more dysbiosis, which is something that is unwanted in patients with a pouch.”
A similar response has been shown in Crohn’s disease, she said. With antibiotics, “you’re doing something that might be helpful clinically for the short term; however, it might be harmful in the long term.”
When these patients stop antibiotic therapy, their dysbiosis increases and resistance wanes, she said. “These patients are replenished by other bacteria, probably some from the oral cavity, causing this circle so they would need recurrent courses of antibiotics.”
Evidence is accumulating that the Mediterranean diet can break that cycle, Dr. Dotan said, citing unpublished findings from her group that showed pouchitis patients consumed less fruit than counterparts without the disease. Dr. Dotan also cited a study published this year that found the Mediterranean diet is associated with a lower risk of Crohn’s disease later in life (Gut. 2020 Jan 3. doi: 10.1136/gutjnl-2019-319505).
Dr. Dotan’s research has shown that patients don’t have to adhere completely to the Mediterranean diet but can make less-drastic but significant changes. “You don’t need the whole program,” she said. “If you tell patients to start with something – increase fruits and vegetables – that’s not too complex. Then try to change some of the protein with legumes or other protein sources; that also would be helpful.” Another strategy is to direct patients away from processed foods with additives and preservatives.
“Microbial modifications, including antibiotics, probiotics, diet, and fecal microbial transplantation may have variable effects on specific patient populations, so we can’t be too long simplistic about these options,” she said.
Personalization of diet and microbial manipulations may do more than provide short-term treatment for patients, she said. “It might contribute to halting or even reversing the global increase we’ve seen in IBD recurrence over the past few years.”
Dr. Dotan disclosed financial relationships with AbbVie, Takeda, Pfizer, Genentech/Roche, Neopharm, and Gilead.
SOURCE: Dotan I. Crohn’s & Colitis Congress 2020, Presentation Sp75.
AUSTIN, TEX. – With the increase in the prevalence of inflammatory bowel disease worldwide approaching pandemic proportions, personalized medicine targeting diet and the microbiome may contribute to a halt or even a reversal of the trend, a leading researcher from Israel and proponent of the Mediterranean diet reported at the Crohn’s & Colitis Congress®, a partnership of the Crohn's & Colitis Foundation and the American Gastroenterological Association.
“Inflammatory bowel disease is turning into a pandemic around the world,” said Iris Dotan, MD, of the Rabin Medical Center in Petah Tikva, Israel, citing research reported at the 2015 meeting of the European Crohn’s & Colitis Organization that showed the prevalence of inflammatory bowel disease (IBD) in Israel increasing almost 400% from 1991 to 2015, up to 460 per 100,000. “The rising incidence highlights the role of environmental factors,” she added.
She reported on her work with the Rabin Medical Center Pouch Cohort, which is studying the ileal pouch as a man-made model of IBD to get answers. “We believe that when patients progress from having a pouch that is normal to having pouchitis it actually resembles Crohn’s disease and can be used as a model to identify processes that are ongoing in these patients,” she said.
The study is following an unspecified number of ileal pouch patients prospectively, evaluating their biomaterial with stool samples and mucosal biopsies, and tracking their diet and psychosocial status to gain insight into what sets off episodes of pouchitis.
Already, the study has provided insight into how antibiotics may contribute to IBD. “Pouch disease is a very antibiotic-responsive disease,” she said in an interview. “Antibiotics are clinically effective. However, as we dive deeper into this and understand the mechanistics of it, we see that antibiotics cause more dysbiosis, which is something that is unwanted in patients with a pouch.”
A similar response has been shown in Crohn’s disease, she said. With antibiotics, “you’re doing something that might be helpful clinically for the short term; however, it might be harmful in the long term.”
When these patients stop antibiotic therapy, their dysbiosis increases and resistance wanes, she said. “These patients are replenished by other bacteria, probably some from the oral cavity, causing this circle so they would need recurrent courses of antibiotics.”
Evidence is accumulating that the Mediterranean diet can break that cycle, Dr. Dotan said, citing unpublished findings from her group that showed pouchitis patients consumed less fruit than counterparts without the disease. Dr. Dotan also cited a study published this year that found the Mediterranean diet is associated with a lower risk of Crohn’s disease later in life (Gut. 2020 Jan 3. doi: 10.1136/gutjnl-2019-319505).
Dr. Dotan’s research has shown that patients don’t have to adhere completely to the Mediterranean diet but can make less-drastic but significant changes. “You don’t need the whole program,” she said. “If you tell patients to start with something – increase fruits and vegetables – that’s not too complex. Then try to change some of the protein with legumes or other protein sources; that also would be helpful.” Another strategy is to direct patients away from processed foods with additives and preservatives.
“Microbial modifications, including antibiotics, probiotics, diet, and fecal microbial transplantation may have variable effects on specific patient populations, so we can’t be too long simplistic about these options,” she said.
Personalization of diet and microbial manipulations may do more than provide short-term treatment for patients, she said. “It might contribute to halting or even reversing the global increase we’ve seen in IBD recurrence over the past few years.”
Dr. Dotan disclosed financial relationships with AbbVie, Takeda, Pfizer, Genentech/Roche, Neopharm, and Gilead.
SOURCE: Dotan I. Crohn’s & Colitis Congress 2020, Presentation Sp75.
AUSTIN, TEX. – With the increase in the prevalence of inflammatory bowel disease worldwide approaching pandemic proportions, personalized medicine targeting diet and the microbiome may contribute to a halt or even a reversal of the trend, a leading researcher from Israel and proponent of the Mediterranean diet reported at the Crohn’s & Colitis Congress®, a partnership of the Crohn's & Colitis Foundation and the American Gastroenterological Association.
“Inflammatory bowel disease is turning into a pandemic around the world,” said Iris Dotan, MD, of the Rabin Medical Center in Petah Tikva, Israel, citing research reported at the 2015 meeting of the European Crohn’s & Colitis Organization that showed the prevalence of inflammatory bowel disease (IBD) in Israel increasing almost 400% from 1991 to 2015, up to 460 per 100,000. “The rising incidence highlights the role of environmental factors,” she added.
She reported on her work with the Rabin Medical Center Pouch Cohort, which is studying the ileal pouch as a man-made model of IBD to get answers. “We believe that when patients progress from having a pouch that is normal to having pouchitis it actually resembles Crohn’s disease and can be used as a model to identify processes that are ongoing in these patients,” she said.
The study is following an unspecified number of ileal pouch patients prospectively, evaluating their biomaterial with stool samples and mucosal biopsies, and tracking their diet and psychosocial status to gain insight into what sets off episodes of pouchitis.
Already, the study has provided insight into how antibiotics may contribute to IBD. “Pouch disease is a very antibiotic-responsive disease,” she said in an interview. “Antibiotics are clinically effective. However, as we dive deeper into this and understand the mechanistics of it, we see that antibiotics cause more dysbiosis, which is something that is unwanted in patients with a pouch.”
A similar response has been shown in Crohn’s disease, she said. With antibiotics, “you’re doing something that might be helpful clinically for the short term; however, it might be harmful in the long term.”
When these patients stop antibiotic therapy, their dysbiosis increases and resistance wanes, she said. “These patients are replenished by other bacteria, probably some from the oral cavity, causing this circle so they would need recurrent courses of antibiotics.”
Evidence is accumulating that the Mediterranean diet can break that cycle, Dr. Dotan said, citing unpublished findings from her group that showed pouchitis patients consumed less fruit than counterparts without the disease. Dr. Dotan also cited a study published this year that found the Mediterranean diet is associated with a lower risk of Crohn’s disease later in life (Gut. 2020 Jan 3. doi: 10.1136/gutjnl-2019-319505).
Dr. Dotan’s research has shown that patients don’t have to adhere completely to the Mediterranean diet but can make less-drastic but significant changes. “You don’t need the whole program,” she said. “If you tell patients to start with something – increase fruits and vegetables – that’s not too complex. Then try to change some of the protein with legumes or other protein sources; that also would be helpful.” Another strategy is to direct patients away from processed foods with additives and preservatives.
“Microbial modifications, including antibiotics, probiotics, diet, and fecal microbial transplantation may have variable effects on specific patient populations, so we can’t be too long simplistic about these options,” she said.
Personalization of diet and microbial manipulations may do more than provide short-term treatment for patients, she said. “It might contribute to halting or even reversing the global increase we’ve seen in IBD recurrence over the past few years.”
Dr. Dotan disclosed financial relationships with AbbVie, Takeda, Pfizer, Genentech/Roche, Neopharm, and Gilead.
SOURCE: Dotan I. Crohn’s & Colitis Congress 2020, Presentation Sp75.
REPORTING FROM THE CROHN’S & COLITIS CONGRESS
High stress linked to UC flare risk
AUSTIN, TEX. – Early results from a cohort study that aims to characterize the brain-gut relationship in ulcerative colitis (UC) have identified potential structural and functional brain changes consistent with the effect chronic bowel inflammation has on the brain and found two subgroups of patients that differed in how they respond to stress. These findings may provide further insight into the role of the brain in symptom flares, the study leader reported the Crohn’s & Colitis Congress®, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
So far, the study has shown that, based on validated measures of perceived stress or neuroticism, patients with UC in clinical remission are clustered on two ends of the stress spectrum, with high and low stress responsiveness, said Emeran A. Mayer, MD, who is coprincipal investigator of the study with Jenny Sauk, MD, both of the University of California, Los Angeles.
The goal of the longitudinal follow-up study is to identify brain, gut microbiome, and stress signatures that predict the risk of flares for up to 2 years in UC patients in clinical remission, he said. Patients’ clinical, microbiome, and stress-psychological measures are evaluated quarterly. The intent is to enroll 100- 120 patients between ages 18 years and 65 years. Questionnaire and symptom data on 70 patients have been analyzed so far.
“What we found so far is that, at baseline using the questionnaire data and clustering analysis, you can identify two distinct subgroups: one characterized by stress hyperresponsiveness and one that does not have that feature,” Dr. Mayer said in an interview. “Then we found in the stress hyperresponsive group there are more flares reported and documented during a mean follow-up of 8.1 months.”
The findings so far have also determined that the differences in stress responsiveness and flare frequency don’t seem to be related to baseline fecal calprotectin levels, said Dr. Mayer, who is director of the G. Oppenheimer Center for Neurobiology of Stress and Resilience (CNSR) and codirector of the CURE: Digestive Diseases Research Center.
Early findings show the incidence of clinical flares in the high stress responsiveness group was 27.4% vs. 9.3% in the low-stress group, and the rate of symptomatic flares was 11.8% vs. 4.6%, respectively.
With regard to baseline biological measures, there were no significant differences in cardiovagal tone or morning salivary cortisol measures between the two clusters, Dr. Mayer noted, although the high stress responsiveness cluster had higher sympathetic tone before, during, and after a brief psychological stress.
He noted that the same clustering into low and high stress responsiveness was confirmed in a different data set of 66 UC subjects and that the two clusters showed significant differences in anatomical connectivity of the default mode network in the brain, a set of regions involved in chronic pain and emotion regulation.
By identifying factors that contribute to inflammatory bowel disease, the study may ultimately simplify the process of identifying IBD patients in remission who are at highest risk of flares, Dr. Mayer said. “Patients won’t need to undergo brain imaging or assessment of microbiome parameters; they can just answer a short questionnaire,” he said. Another potential benefit of the study would be to identify changes in the brain–gut microbiome interactions associated with flares, he said.
Full study results would be available in about 2 years, Dr. Mayer said, with more data on biological parameters expected next year.
The study is funded with a Crohn’s & Colitis Foundation grant. Dr. Mayer disclosed financial relationships with Amare, Axial Biotherapeutics, Bloom Science, Danone, Mahana Therapeutics, Pendulum, Ubiome, and Viome.
SOURCE: Mayer EA et al. Crohn’s & Colitis Congress 2020, Session Sp74.
AUSTIN, TEX. – Early results from a cohort study that aims to characterize the brain-gut relationship in ulcerative colitis (UC) have identified potential structural and functional brain changes consistent with the effect chronic bowel inflammation has on the brain and found two subgroups of patients that differed in how they respond to stress. These findings may provide further insight into the role of the brain in symptom flares, the study leader reported the Crohn’s & Colitis Congress®, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
So far, the study has shown that, based on validated measures of perceived stress or neuroticism, patients with UC in clinical remission are clustered on two ends of the stress spectrum, with high and low stress responsiveness, said Emeran A. Mayer, MD, who is coprincipal investigator of the study with Jenny Sauk, MD, both of the University of California, Los Angeles.
The goal of the longitudinal follow-up study is to identify brain, gut microbiome, and stress signatures that predict the risk of flares for up to 2 years in UC patients in clinical remission, he said. Patients’ clinical, microbiome, and stress-psychological measures are evaluated quarterly. The intent is to enroll 100- 120 patients between ages 18 years and 65 years. Questionnaire and symptom data on 70 patients have been analyzed so far.
“What we found so far is that, at baseline using the questionnaire data and clustering analysis, you can identify two distinct subgroups: one characterized by stress hyperresponsiveness and one that does not have that feature,” Dr. Mayer said in an interview. “Then we found in the stress hyperresponsive group there are more flares reported and documented during a mean follow-up of 8.1 months.”
The findings so far have also determined that the differences in stress responsiveness and flare frequency don’t seem to be related to baseline fecal calprotectin levels, said Dr. Mayer, who is director of the G. Oppenheimer Center for Neurobiology of Stress and Resilience (CNSR) and codirector of the CURE: Digestive Diseases Research Center.
Early findings show the incidence of clinical flares in the high stress responsiveness group was 27.4% vs. 9.3% in the low-stress group, and the rate of symptomatic flares was 11.8% vs. 4.6%, respectively.
With regard to baseline biological measures, there were no significant differences in cardiovagal tone or morning salivary cortisol measures between the two clusters, Dr. Mayer noted, although the high stress responsiveness cluster had higher sympathetic tone before, during, and after a brief psychological stress.
He noted that the same clustering into low and high stress responsiveness was confirmed in a different data set of 66 UC subjects and that the two clusters showed significant differences in anatomical connectivity of the default mode network in the brain, a set of regions involved in chronic pain and emotion regulation.
By identifying factors that contribute to inflammatory bowel disease, the study may ultimately simplify the process of identifying IBD patients in remission who are at highest risk of flares, Dr. Mayer said. “Patients won’t need to undergo brain imaging or assessment of microbiome parameters; they can just answer a short questionnaire,” he said. Another potential benefit of the study would be to identify changes in the brain–gut microbiome interactions associated with flares, he said.
Full study results would be available in about 2 years, Dr. Mayer said, with more data on biological parameters expected next year.
The study is funded with a Crohn’s & Colitis Foundation grant. Dr. Mayer disclosed financial relationships with Amare, Axial Biotherapeutics, Bloom Science, Danone, Mahana Therapeutics, Pendulum, Ubiome, and Viome.
SOURCE: Mayer EA et al. Crohn’s & Colitis Congress 2020, Session Sp74.
AUSTIN, TEX. – Early results from a cohort study that aims to characterize the brain-gut relationship in ulcerative colitis (UC) have identified potential structural and functional brain changes consistent with the effect chronic bowel inflammation has on the brain and found two subgroups of patients that differed in how they respond to stress. These findings may provide further insight into the role of the brain in symptom flares, the study leader reported the Crohn’s & Colitis Congress®, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
So far, the study has shown that, based on validated measures of perceived stress or neuroticism, patients with UC in clinical remission are clustered on two ends of the stress spectrum, with high and low stress responsiveness, said Emeran A. Mayer, MD, who is coprincipal investigator of the study with Jenny Sauk, MD, both of the University of California, Los Angeles.
The goal of the longitudinal follow-up study is to identify brain, gut microbiome, and stress signatures that predict the risk of flares for up to 2 years in UC patients in clinical remission, he said. Patients’ clinical, microbiome, and stress-psychological measures are evaluated quarterly. The intent is to enroll 100- 120 patients between ages 18 years and 65 years. Questionnaire and symptom data on 70 patients have been analyzed so far.
“What we found so far is that, at baseline using the questionnaire data and clustering analysis, you can identify two distinct subgroups: one characterized by stress hyperresponsiveness and one that does not have that feature,” Dr. Mayer said in an interview. “Then we found in the stress hyperresponsive group there are more flares reported and documented during a mean follow-up of 8.1 months.”
The findings so far have also determined that the differences in stress responsiveness and flare frequency don’t seem to be related to baseline fecal calprotectin levels, said Dr. Mayer, who is director of the G. Oppenheimer Center for Neurobiology of Stress and Resilience (CNSR) and codirector of the CURE: Digestive Diseases Research Center.
Early findings show the incidence of clinical flares in the high stress responsiveness group was 27.4% vs. 9.3% in the low-stress group, and the rate of symptomatic flares was 11.8% vs. 4.6%, respectively.
With regard to baseline biological measures, there were no significant differences in cardiovagal tone or morning salivary cortisol measures between the two clusters, Dr. Mayer noted, although the high stress responsiveness cluster had higher sympathetic tone before, during, and after a brief psychological stress.
He noted that the same clustering into low and high stress responsiveness was confirmed in a different data set of 66 UC subjects and that the two clusters showed significant differences in anatomical connectivity of the default mode network in the brain, a set of regions involved in chronic pain and emotion regulation.
By identifying factors that contribute to inflammatory bowel disease, the study may ultimately simplify the process of identifying IBD patients in remission who are at highest risk of flares, Dr. Mayer said. “Patients won’t need to undergo brain imaging or assessment of microbiome parameters; they can just answer a short questionnaire,” he said. Another potential benefit of the study would be to identify changes in the brain–gut microbiome interactions associated with flares, he said.
Full study results would be available in about 2 years, Dr. Mayer said, with more data on biological parameters expected next year.
The study is funded with a Crohn’s & Colitis Foundation grant. Dr. Mayer disclosed financial relationships with Amare, Axial Biotherapeutics, Bloom Science, Danone, Mahana Therapeutics, Pendulum, Ubiome, and Viome.
SOURCE: Mayer EA et al. Crohn’s & Colitis Congress 2020, Session Sp74.
REPORTING FROM CROHN’S & COLITIS CONGRESS
FDA strengthens warning regarding clozapine, serious bowel complication risk
The Food and Drug Administration is strengthening a previous warning regarding the uncommon risk of serious bowel complications associated with the schizophrenia medication clozapine (Clozaril, FazaClo ODT, Versacloz).
According to the FDA press release, dated Jan. 28, clozapine affects bowel function in a majority of patients, and constipation is a common adverse event associated with clozapine use. This can uncommonly progress to serious bowel complications, including complete bowel blockage, and can result in hospitalization or even death if the constipation is not diagnosed and treated quickly.
Patients should contact their health care clinician if their bowel movements are less frequent, they have a bowel movement less than three times a week, they have hard or dry stool, or they have difficulty passing gas. Urgent care is needed if patients are experiencing nausea, vomiting, belly pain, or bloating, according to the FDA.
In addition, , avoid coprescribing with other anticholinergic medicines, advise and question patients about the risks of clozapine and their bowel movements, monitor patients for complications, and consider prophylactic laxative treatment in patients with a history of constipation or bowel obstruction, the FDA added.
The Food and Drug Administration is strengthening a previous warning regarding the uncommon risk of serious bowel complications associated with the schizophrenia medication clozapine (Clozaril, FazaClo ODT, Versacloz).
According to the FDA press release, dated Jan. 28, clozapine affects bowel function in a majority of patients, and constipation is a common adverse event associated with clozapine use. This can uncommonly progress to serious bowel complications, including complete bowel blockage, and can result in hospitalization or even death if the constipation is not diagnosed and treated quickly.
Patients should contact their health care clinician if their bowel movements are less frequent, they have a bowel movement less than three times a week, they have hard or dry stool, or they have difficulty passing gas. Urgent care is needed if patients are experiencing nausea, vomiting, belly pain, or bloating, according to the FDA.
In addition, , avoid coprescribing with other anticholinergic medicines, advise and question patients about the risks of clozapine and their bowel movements, monitor patients for complications, and consider prophylactic laxative treatment in patients with a history of constipation or bowel obstruction, the FDA added.
The Food and Drug Administration is strengthening a previous warning regarding the uncommon risk of serious bowel complications associated with the schizophrenia medication clozapine (Clozaril, FazaClo ODT, Versacloz).
According to the FDA press release, dated Jan. 28, clozapine affects bowel function in a majority of patients, and constipation is a common adverse event associated with clozapine use. This can uncommonly progress to serious bowel complications, including complete bowel blockage, and can result in hospitalization or even death if the constipation is not diagnosed and treated quickly.
Patients should contact their health care clinician if their bowel movements are less frequent, they have a bowel movement less than three times a week, they have hard or dry stool, or they have difficulty passing gas. Urgent care is needed if patients are experiencing nausea, vomiting, belly pain, or bloating, according to the FDA.
In addition, , avoid coprescribing with other anticholinergic medicines, advise and question patients about the risks of clozapine and their bowel movements, monitor patients for complications, and consider prophylactic laxative treatment in patients with a history of constipation or bowel obstruction, the FDA added.
FDA approves fidaxomicin for treatment of C. difficile–associated diarrhea
The Food and Drug Administration has approved fidaxomicin (Dificid) for the treatment of Clostridioides difficile–associated diarrhea in children aged 6 months and older.
Approval was based on results from SUNSHINE, a phase 3, multicenter, investigator-blind, randomized, parallel-group study in 142 pediatric patients aged between 6 months and 18 years with confirmed C. difficile infection who received either fidaxomicin or vancomycin for 10 days. Clinical response 2 days after the conclusion of treatment was similar in both groups (77.6% for fidaxomicin vs. 70.5% for vancomycin), and fidaxomicin had a superior sustained response 30 days after the conclusion of treatment (68.4% vs. 50.0%).
The safety of fidaxomicin was assessed in a pair of clinical trials involving 136 patients; the most common adverse events were pyrexia, abdominal pain, vomiting, diarrhea, constipation, increased aminotransferases, and rash. Four patients discontinued fidaxomicin treatment because of adverse events, and four patients died during the trials, though all deaths were in patients aged younger than 2 years and seemed to be related to other comorbidities.
“C. difficile is an important cause of health care– and community-associated diarrheal illness in children, and sustained cure is difficult to achieve in some patients. The fidaxomicin pediatric trial was the first randomized, controlled trial of C. difficile infection treatment in children,” Larry K. Kociolek, MD, associate medical director of infection prevention and control at Ann & Robert H. Lurie Children’s Hospital of Chicago, said in the press release from Merck, manufacturer of fidaxomicin.
*This story was updated on 1/27/2020.
The Food and Drug Administration has approved fidaxomicin (Dificid) for the treatment of Clostridioides difficile–associated diarrhea in children aged 6 months and older.
Approval was based on results from SUNSHINE, a phase 3, multicenter, investigator-blind, randomized, parallel-group study in 142 pediatric patients aged between 6 months and 18 years with confirmed C. difficile infection who received either fidaxomicin or vancomycin for 10 days. Clinical response 2 days after the conclusion of treatment was similar in both groups (77.6% for fidaxomicin vs. 70.5% for vancomycin), and fidaxomicin had a superior sustained response 30 days after the conclusion of treatment (68.4% vs. 50.0%).
The safety of fidaxomicin was assessed in a pair of clinical trials involving 136 patients; the most common adverse events were pyrexia, abdominal pain, vomiting, diarrhea, constipation, increased aminotransferases, and rash. Four patients discontinued fidaxomicin treatment because of adverse events, and four patients died during the trials, though all deaths were in patients aged younger than 2 years and seemed to be related to other comorbidities.
“C. difficile is an important cause of health care– and community-associated diarrheal illness in children, and sustained cure is difficult to achieve in some patients. The fidaxomicin pediatric trial was the first randomized, controlled trial of C. difficile infection treatment in children,” Larry K. Kociolek, MD, associate medical director of infection prevention and control at Ann & Robert H. Lurie Children’s Hospital of Chicago, said in the press release from Merck, manufacturer of fidaxomicin.
*This story was updated on 1/27/2020.
The Food and Drug Administration has approved fidaxomicin (Dificid) for the treatment of Clostridioides difficile–associated diarrhea in children aged 6 months and older.
Approval was based on results from SUNSHINE, a phase 3, multicenter, investigator-blind, randomized, parallel-group study in 142 pediatric patients aged between 6 months and 18 years with confirmed C. difficile infection who received either fidaxomicin or vancomycin for 10 days. Clinical response 2 days after the conclusion of treatment was similar in both groups (77.6% for fidaxomicin vs. 70.5% for vancomycin), and fidaxomicin had a superior sustained response 30 days after the conclusion of treatment (68.4% vs. 50.0%).
The safety of fidaxomicin was assessed in a pair of clinical trials involving 136 patients; the most common adverse events were pyrexia, abdominal pain, vomiting, diarrhea, constipation, increased aminotransferases, and rash. Four patients discontinued fidaxomicin treatment because of adverse events, and four patients died during the trials, though all deaths were in patients aged younger than 2 years and seemed to be related to other comorbidities.
“C. difficile is an important cause of health care– and community-associated diarrheal illness in children, and sustained cure is difficult to achieve in some patients. The fidaxomicin pediatric trial was the first randomized, controlled trial of C. difficile infection treatment in children,” Larry K. Kociolek, MD, associate medical director of infection prevention and control at Ann & Robert H. Lurie Children’s Hospital of Chicago, said in the press release from Merck, manufacturer of fidaxomicin.
*This story was updated on 1/27/2020.
IBD: Inpatient opioids linked with outpatient use
, based on a retrospective analysis of more than 800 patients.
Awareness of this dose-dependent relationship and IBD-related risks of opioid use should encourage physicians to consider alternative analgesics, according to lead author Rahul S. Dalal, MD, of Brigham and Women’s Hospital, Boston, and colleagues.
“Recent evidence has demonstrated that opioid use is associated with severe infections and increased mortality among IBD patients,” the investigators wrote in Clinical Gastroenterology and Hepatology. “Despite these concerns, opioids are commonly prescribed to IBD patients in the outpatient setting and to as many as 70% of IBD patients who are hospitalized.”
To look for a possible relationship between inpatient and outpatient opioid use, the investigators reviewed electronic medical records of 862 IBD patients who were treated at three urban hospitals in the University of Pennsylvania Health System. The primary outcome was opioid prescription within 12 months of discharge, including prescriptions at time of hospital dismissal.
During hospitalization, about two-thirds (67.6%) of patients received intravenous opioids. Of the total population, slightly more than half (54.6%) received intravenous hydromorphone and about one-quarter (25.9%) received intravenous morphine. Following discharge, almost half of the population (44.7%) was prescribed opioids, and about 3 out of 4 patients (77.9%) received an additional opioid prescription within the same year.
After accounting for confounders such as IBD severity, preadmission opioid use, pain scores, and psychiatric conditions, data analysis showed that inpatients who received intravenous opioids had a threefold (odds ratio [OR], 3.3) increased likelihood of receiving postdischarge opioid prescription, compared with patients who received no opioids while hospitalized. This association was stronger among those who had IBD flares (OR, 5.4). Furthermore, intravenous dose was positively correlated with postdischarge opioid prescription.
Avoiding intravenous opioids had no impact on the relationship between inpatient and outpatient opioid use. Among inpatients who received only oral or transdermal opioids, a similarly increased likelihood of postdischarge opioid prescription was observed (OR, 4.2), although this was a small cohort (n = 67).
Compared with other physicians, gastroenterologists were the least likely to prescribe opioids. Considering that gastroenterologists were also most likely aware of IBD-related risks of opioid use, the investigators concluded that more interdisciplinary communication and education are needed.
“Alternative analgesics such as acetaminophen, dicyclomine, hyoscyamine, and celecoxib could be advised, as many of these therapies have been deemed relatively safe and effective in this population,” they wrote.The investigators disclosed relationships with Abbott, Gilead, Romark, and others.
SOURCE: Dalal RS et al. Clin Gastro Hepatol. 2019 Dec 27. doi: 10.1016/j.cgh.2019.12.024.
, based on a retrospective analysis of more than 800 patients.
Awareness of this dose-dependent relationship and IBD-related risks of opioid use should encourage physicians to consider alternative analgesics, according to lead author Rahul S. Dalal, MD, of Brigham and Women’s Hospital, Boston, and colleagues.
“Recent evidence has demonstrated that opioid use is associated with severe infections and increased mortality among IBD patients,” the investigators wrote in Clinical Gastroenterology and Hepatology. “Despite these concerns, opioids are commonly prescribed to IBD patients in the outpatient setting and to as many as 70% of IBD patients who are hospitalized.”
To look for a possible relationship between inpatient and outpatient opioid use, the investigators reviewed electronic medical records of 862 IBD patients who were treated at three urban hospitals in the University of Pennsylvania Health System. The primary outcome was opioid prescription within 12 months of discharge, including prescriptions at time of hospital dismissal.
During hospitalization, about two-thirds (67.6%) of patients received intravenous opioids. Of the total population, slightly more than half (54.6%) received intravenous hydromorphone and about one-quarter (25.9%) received intravenous morphine. Following discharge, almost half of the population (44.7%) was prescribed opioids, and about 3 out of 4 patients (77.9%) received an additional opioid prescription within the same year.
After accounting for confounders such as IBD severity, preadmission opioid use, pain scores, and psychiatric conditions, data analysis showed that inpatients who received intravenous opioids had a threefold (odds ratio [OR], 3.3) increased likelihood of receiving postdischarge opioid prescription, compared with patients who received no opioids while hospitalized. This association was stronger among those who had IBD flares (OR, 5.4). Furthermore, intravenous dose was positively correlated with postdischarge opioid prescription.
Avoiding intravenous opioids had no impact on the relationship between inpatient and outpatient opioid use. Among inpatients who received only oral or transdermal opioids, a similarly increased likelihood of postdischarge opioid prescription was observed (OR, 4.2), although this was a small cohort (n = 67).
Compared with other physicians, gastroenterologists were the least likely to prescribe opioids. Considering that gastroenterologists were also most likely aware of IBD-related risks of opioid use, the investigators concluded that more interdisciplinary communication and education are needed.
“Alternative analgesics such as acetaminophen, dicyclomine, hyoscyamine, and celecoxib could be advised, as many of these therapies have been deemed relatively safe and effective in this population,” they wrote.The investigators disclosed relationships with Abbott, Gilead, Romark, and others.
SOURCE: Dalal RS et al. Clin Gastro Hepatol. 2019 Dec 27. doi: 10.1016/j.cgh.2019.12.024.
, based on a retrospective analysis of more than 800 patients.
Awareness of this dose-dependent relationship and IBD-related risks of opioid use should encourage physicians to consider alternative analgesics, according to lead author Rahul S. Dalal, MD, of Brigham and Women’s Hospital, Boston, and colleagues.
“Recent evidence has demonstrated that opioid use is associated with severe infections and increased mortality among IBD patients,” the investigators wrote in Clinical Gastroenterology and Hepatology. “Despite these concerns, opioids are commonly prescribed to IBD patients in the outpatient setting and to as many as 70% of IBD patients who are hospitalized.”
To look for a possible relationship between inpatient and outpatient opioid use, the investigators reviewed electronic medical records of 862 IBD patients who were treated at three urban hospitals in the University of Pennsylvania Health System. The primary outcome was opioid prescription within 12 months of discharge, including prescriptions at time of hospital dismissal.
During hospitalization, about two-thirds (67.6%) of patients received intravenous opioids. Of the total population, slightly more than half (54.6%) received intravenous hydromorphone and about one-quarter (25.9%) received intravenous morphine. Following discharge, almost half of the population (44.7%) was prescribed opioids, and about 3 out of 4 patients (77.9%) received an additional opioid prescription within the same year.
After accounting for confounders such as IBD severity, preadmission opioid use, pain scores, and psychiatric conditions, data analysis showed that inpatients who received intravenous opioids had a threefold (odds ratio [OR], 3.3) increased likelihood of receiving postdischarge opioid prescription, compared with patients who received no opioids while hospitalized. This association was stronger among those who had IBD flares (OR, 5.4). Furthermore, intravenous dose was positively correlated with postdischarge opioid prescription.
Avoiding intravenous opioids had no impact on the relationship between inpatient and outpatient opioid use. Among inpatients who received only oral or transdermal opioids, a similarly increased likelihood of postdischarge opioid prescription was observed (OR, 4.2), although this was a small cohort (n = 67).
Compared with other physicians, gastroenterologists were the least likely to prescribe opioids. Considering that gastroenterologists were also most likely aware of IBD-related risks of opioid use, the investigators concluded that more interdisciplinary communication and education are needed.
“Alternative analgesics such as acetaminophen, dicyclomine, hyoscyamine, and celecoxib could be advised, as many of these therapies have been deemed relatively safe and effective in this population,” they wrote.The investigators disclosed relationships with Abbott, Gilead, Romark, and others.
SOURCE: Dalal RS et al. Clin Gastro Hepatol. 2019 Dec 27. doi: 10.1016/j.cgh.2019.12.024.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Patients with inflammatory bowel disease (IBD) who receive opioids while hospitalized are three times as likely to be prescribed opioids after discharge.
Major finding: Patients who were given intravenous opioids while hospitalized were three times as likely to receive a postdischarge opioid prescription, compared with patients who did not receive inpatient intravenous opioids (odds ratio, 3.3).
Study details: A retrospective cohort study involving 862 patients with inflammatory bowel disease.
Disclosures: The investigators disclosed relationships Abbott, Gilead, Romark, and others.
Source: Dalal RS et al. Clin Gastro Hepatol. 2019 Dec 27. doi: 10.1016/j.cgh.2019.12.024.
Consider hyperbaric oxygen for inflammatory ileal pouchitis
SAN ANTONIO – , Hamna Fahad, MD, reported at the annual meeting of the American College of Gastroenterology.
Dr. Fahad, of the Cleveland Clinic, presented a retrospective case series of 21 consecutive clinic patients who presented with inflammatory bowel disease, a surgically created ileal pouch–anal anastomosis, and medically refractory pouchitis. All patients received 30 hyperbaric oxygen treatment sessions, each an hour long, over the course of 2 months. This intensive regimen worked out to 3-5 sessions per week involving 100% oxygen pressurized to 2.4-3.0 ATA.
Overall, 19 of 21 patients experienced improvement in their modified Pouchitis Disease Activity Index (mPDAI) score. The mean total mPDAI at baseline was 8.71, improving significantly to 5 post treatment. The mPDAI symptoms subscore also showed significant improvement in response to a course of hyperbaric oxygen therapy, decreasing from 4 points to 2. The cuff subscore fell from 3 to 0, and the pouch body subscore improved from 3 to 2.
Thirteen of 21 patients reported subjective symptomatic improvement in stool frequency, bleeding, urgency, and fevers, including 6 with complete symptomatic remission. Seventeen patients demonstrated significant endoscopic improvement upon blinded assessment. Seven of 9 patients with fistulae experienced healing of the fistula tract.
The treatment entailed no side effects. However, the benefits weren’t uniformly durable. Several patients underwent a second 30-session round of hyperbaric oxygen therapy within a year because of recurrent pouchitis symptoms refractory to corticosteroids, biologics, and other medications.
Dr. Fahad said the mechanism of benefit for hyperbaric oxygen in the treatment of chronic inflammatory pouchitis is probably severalfold: reversal of a disordered microbiome through inhibition of the growth of anaerobes, reduced production of tumor necrosis factor–alpha and other inflammatory cytokines, and increased plasma oxygen, which reduces ischemia at the tissue level, thereby promoting tissue healing.
Audience members had a practical question: How can they get this treatment paid for? One gastroenterologist said she has encountered considerable payer resistance when she has sought coverage of hyperbaric oxygen for patients with ulcerative colitis and fistulae, even though there is already published evidence of benefit. But Dr. Fahad’s groundbreaking study provides the first such evidence in pouchitis. So how did she and her coworkers do it? Eighty percent of the pouchitis patients obtained payer approval only upon appeal, which was readily granted, she explained.
Dr. Fahad reported having no financial conflicts regarding her study, conducted without commercial support.
SOURCE: Fahad H. ACG 2019 Abstract 38.
SAN ANTONIO – , Hamna Fahad, MD, reported at the annual meeting of the American College of Gastroenterology.
Dr. Fahad, of the Cleveland Clinic, presented a retrospective case series of 21 consecutive clinic patients who presented with inflammatory bowel disease, a surgically created ileal pouch–anal anastomosis, and medically refractory pouchitis. All patients received 30 hyperbaric oxygen treatment sessions, each an hour long, over the course of 2 months. This intensive regimen worked out to 3-5 sessions per week involving 100% oxygen pressurized to 2.4-3.0 ATA.
Overall, 19 of 21 patients experienced improvement in their modified Pouchitis Disease Activity Index (mPDAI) score. The mean total mPDAI at baseline was 8.71, improving significantly to 5 post treatment. The mPDAI symptoms subscore also showed significant improvement in response to a course of hyperbaric oxygen therapy, decreasing from 4 points to 2. The cuff subscore fell from 3 to 0, and the pouch body subscore improved from 3 to 2.
Thirteen of 21 patients reported subjective symptomatic improvement in stool frequency, bleeding, urgency, and fevers, including 6 with complete symptomatic remission. Seventeen patients demonstrated significant endoscopic improvement upon blinded assessment. Seven of 9 patients with fistulae experienced healing of the fistula tract.
The treatment entailed no side effects. However, the benefits weren’t uniformly durable. Several patients underwent a second 30-session round of hyperbaric oxygen therapy within a year because of recurrent pouchitis symptoms refractory to corticosteroids, biologics, and other medications.
Dr. Fahad said the mechanism of benefit for hyperbaric oxygen in the treatment of chronic inflammatory pouchitis is probably severalfold: reversal of a disordered microbiome through inhibition of the growth of anaerobes, reduced production of tumor necrosis factor–alpha and other inflammatory cytokines, and increased plasma oxygen, which reduces ischemia at the tissue level, thereby promoting tissue healing.
Audience members had a practical question: How can they get this treatment paid for? One gastroenterologist said she has encountered considerable payer resistance when she has sought coverage of hyperbaric oxygen for patients with ulcerative colitis and fistulae, even though there is already published evidence of benefit. But Dr. Fahad’s groundbreaking study provides the first such evidence in pouchitis. So how did she and her coworkers do it? Eighty percent of the pouchitis patients obtained payer approval only upon appeal, which was readily granted, she explained.
Dr. Fahad reported having no financial conflicts regarding her study, conducted without commercial support.
SOURCE: Fahad H. ACG 2019 Abstract 38.
SAN ANTONIO – , Hamna Fahad, MD, reported at the annual meeting of the American College of Gastroenterology.
Dr. Fahad, of the Cleveland Clinic, presented a retrospective case series of 21 consecutive clinic patients who presented with inflammatory bowel disease, a surgically created ileal pouch–anal anastomosis, and medically refractory pouchitis. All patients received 30 hyperbaric oxygen treatment sessions, each an hour long, over the course of 2 months. This intensive regimen worked out to 3-5 sessions per week involving 100% oxygen pressurized to 2.4-3.0 ATA.
Overall, 19 of 21 patients experienced improvement in their modified Pouchitis Disease Activity Index (mPDAI) score. The mean total mPDAI at baseline was 8.71, improving significantly to 5 post treatment. The mPDAI symptoms subscore also showed significant improvement in response to a course of hyperbaric oxygen therapy, decreasing from 4 points to 2. The cuff subscore fell from 3 to 0, and the pouch body subscore improved from 3 to 2.
Thirteen of 21 patients reported subjective symptomatic improvement in stool frequency, bleeding, urgency, and fevers, including 6 with complete symptomatic remission. Seventeen patients demonstrated significant endoscopic improvement upon blinded assessment. Seven of 9 patients with fistulae experienced healing of the fistula tract.
The treatment entailed no side effects. However, the benefits weren’t uniformly durable. Several patients underwent a second 30-session round of hyperbaric oxygen therapy within a year because of recurrent pouchitis symptoms refractory to corticosteroids, biologics, and other medications.
Dr. Fahad said the mechanism of benefit for hyperbaric oxygen in the treatment of chronic inflammatory pouchitis is probably severalfold: reversal of a disordered microbiome through inhibition of the growth of anaerobes, reduced production of tumor necrosis factor–alpha and other inflammatory cytokines, and increased plasma oxygen, which reduces ischemia at the tissue level, thereby promoting tissue healing.
Audience members had a practical question: How can they get this treatment paid for? One gastroenterologist said she has encountered considerable payer resistance when she has sought coverage of hyperbaric oxygen for patients with ulcerative colitis and fistulae, even though there is already published evidence of benefit. But Dr. Fahad’s groundbreaking study provides the first such evidence in pouchitis. So how did she and her coworkers do it? Eighty percent of the pouchitis patients obtained payer approval only upon appeal, which was readily granted, she explained.
Dr. Fahad reported having no financial conflicts regarding her study, conducted without commercial support.
SOURCE: Fahad H. ACG 2019 Abstract 38.
REPORTING FROM ACG 2019
Eluxadoline effective for IBS in loperamide nonresponders
SAN ANTONIO – Darren M. Brenner, MD, reported at the annual meeting of the American College of Gastroenterology.
“From the totality of the clinical trials data we have now, we believe that eluxadoline can be effective both in patients who are naive to other treatments and in patients who have failed loperamide therapy,” concluded Dr. Brenner, a gastroenterologist at Northwestern University, Chicago.
Eluxadoline (Viberzi) is a novel mixed mu- and kappa-opioid receptor agonist and delta-opioid receptor antagonist approved by the Food and Drug Administration for treatment of irritable bowel syndrome with diarrhea (IBS-D) in adults. In contrast, loperamide, a mu-opioid receptor agonist, is not approved for that indication. Yet loperamide is widely prescribed for this purpose, despite the fact that both the Canadian Association of Gastroenterology and the ACG now recommend against this practice.
“There is a lack of conclusive evidence to support the use of loperamide for the relief of global IBS-D symptoms. It works on the stool symptoms – stool frequency and texture – but has never been shown to be beneficial for the abdominal pain symptoms or discomfort or bloating. That being said, as practitioners we continue to see loperamide used as a first-line agent,” Dr. Brenner noted.
RELIEF was a multicenter, prospective, double-blind study which randomized 346 patients with moderate to severe IBS-D to eluxadoline at 100 mg twice daily or placebo for 12 weeks. All participants were required to have an intact gallbladder as per the drug’s labeling guidance, and all had a self-reported recent inadequate response to loperamide.
The primary composite endpoint in the RELIEF trial was a 40% or greater improvement from baseline in the 11-point Daily Worst Abdominal Pain score plus a Bristol Stool Form score below 5 on the same day for at least 50% of study days. At baseline, participants had an average Worst Abdominal Pain score of 6.2 on the 0-10 scale and a Bristol score of 6.2. The primary endpoint was achieved at week 12 in 23% of the eluxadoline group, significantly better than the 10% rate in controls. The eluxadoline group also showed significantly greater improvement on the many secondary endpoints having to do with urgency-free days, stool consistency, bowel movement frequency, abdominal discomfort, bloating, and the experience of adequate relief of symptoms.
The safety profile of eluxadoline mirrored that of placebo, with no serious adverse events recorded in either study arm and a 2.9% study discontinuation rate because of treatment-emergent adverse events in the eluxadoline group. Asked why he thinks eluxadoline was effective in improving the full range of IBS-D symptoms when loperamide wasn’t, even though both drugs are mu-opioid receptor agonists, Dr. Brenner replied, “The problem is mu receptors line the entire GI tract, so you can actually push somebody from diarrhea to opioid-induced constipation – and that’s not the goal. What delta does is alleviate some of the adverse events by binding to the receptor, which results in increased transit time, reduced secretion, and increased absorption. Delta brings things back towards the center. We also believe antagonism of delta potentiates analgesic effects at the mu receptor, improves the pain component, gut symptoms, and stool symptoms.”
Dr. Brenner reported serving as a consultant to and member of a speaker’s bureau for Allergan, which markets eluxadoline and sponsored the RELIEF trial.
SAN ANTONIO – Darren M. Brenner, MD, reported at the annual meeting of the American College of Gastroenterology.
“From the totality of the clinical trials data we have now, we believe that eluxadoline can be effective both in patients who are naive to other treatments and in patients who have failed loperamide therapy,” concluded Dr. Brenner, a gastroenterologist at Northwestern University, Chicago.
Eluxadoline (Viberzi) is a novel mixed mu- and kappa-opioid receptor agonist and delta-opioid receptor antagonist approved by the Food and Drug Administration for treatment of irritable bowel syndrome with diarrhea (IBS-D) in adults. In contrast, loperamide, a mu-opioid receptor agonist, is not approved for that indication. Yet loperamide is widely prescribed for this purpose, despite the fact that both the Canadian Association of Gastroenterology and the ACG now recommend against this practice.
“There is a lack of conclusive evidence to support the use of loperamide for the relief of global IBS-D symptoms. It works on the stool symptoms – stool frequency and texture – but has never been shown to be beneficial for the abdominal pain symptoms or discomfort or bloating. That being said, as practitioners we continue to see loperamide used as a first-line agent,” Dr. Brenner noted.
RELIEF was a multicenter, prospective, double-blind study which randomized 346 patients with moderate to severe IBS-D to eluxadoline at 100 mg twice daily or placebo for 12 weeks. All participants were required to have an intact gallbladder as per the drug’s labeling guidance, and all had a self-reported recent inadequate response to loperamide.
The primary composite endpoint in the RELIEF trial was a 40% or greater improvement from baseline in the 11-point Daily Worst Abdominal Pain score plus a Bristol Stool Form score below 5 on the same day for at least 50% of study days. At baseline, participants had an average Worst Abdominal Pain score of 6.2 on the 0-10 scale and a Bristol score of 6.2. The primary endpoint was achieved at week 12 in 23% of the eluxadoline group, significantly better than the 10% rate in controls. The eluxadoline group also showed significantly greater improvement on the many secondary endpoints having to do with urgency-free days, stool consistency, bowel movement frequency, abdominal discomfort, bloating, and the experience of adequate relief of symptoms.
The safety profile of eluxadoline mirrored that of placebo, with no serious adverse events recorded in either study arm and a 2.9% study discontinuation rate because of treatment-emergent adverse events in the eluxadoline group. Asked why he thinks eluxadoline was effective in improving the full range of IBS-D symptoms when loperamide wasn’t, even though both drugs are mu-opioid receptor agonists, Dr. Brenner replied, “The problem is mu receptors line the entire GI tract, so you can actually push somebody from diarrhea to opioid-induced constipation – and that’s not the goal. What delta does is alleviate some of the adverse events by binding to the receptor, which results in increased transit time, reduced secretion, and increased absorption. Delta brings things back towards the center. We also believe antagonism of delta potentiates analgesic effects at the mu receptor, improves the pain component, gut symptoms, and stool symptoms.”
Dr. Brenner reported serving as a consultant to and member of a speaker’s bureau for Allergan, which markets eluxadoline and sponsored the RELIEF trial.
SAN ANTONIO – Darren M. Brenner, MD, reported at the annual meeting of the American College of Gastroenterology.
“From the totality of the clinical trials data we have now, we believe that eluxadoline can be effective both in patients who are naive to other treatments and in patients who have failed loperamide therapy,” concluded Dr. Brenner, a gastroenterologist at Northwestern University, Chicago.
Eluxadoline (Viberzi) is a novel mixed mu- and kappa-opioid receptor agonist and delta-opioid receptor antagonist approved by the Food and Drug Administration for treatment of irritable bowel syndrome with diarrhea (IBS-D) in adults. In contrast, loperamide, a mu-opioid receptor agonist, is not approved for that indication. Yet loperamide is widely prescribed for this purpose, despite the fact that both the Canadian Association of Gastroenterology and the ACG now recommend against this practice.
“There is a lack of conclusive evidence to support the use of loperamide for the relief of global IBS-D symptoms. It works on the stool symptoms – stool frequency and texture – but has never been shown to be beneficial for the abdominal pain symptoms or discomfort or bloating. That being said, as practitioners we continue to see loperamide used as a first-line agent,” Dr. Brenner noted.
RELIEF was a multicenter, prospective, double-blind study which randomized 346 patients with moderate to severe IBS-D to eluxadoline at 100 mg twice daily or placebo for 12 weeks. All participants were required to have an intact gallbladder as per the drug’s labeling guidance, and all had a self-reported recent inadequate response to loperamide.
The primary composite endpoint in the RELIEF trial was a 40% or greater improvement from baseline in the 11-point Daily Worst Abdominal Pain score plus a Bristol Stool Form score below 5 on the same day for at least 50% of study days. At baseline, participants had an average Worst Abdominal Pain score of 6.2 on the 0-10 scale and a Bristol score of 6.2. The primary endpoint was achieved at week 12 in 23% of the eluxadoline group, significantly better than the 10% rate in controls. The eluxadoline group also showed significantly greater improvement on the many secondary endpoints having to do with urgency-free days, stool consistency, bowel movement frequency, abdominal discomfort, bloating, and the experience of adequate relief of symptoms.
The safety profile of eluxadoline mirrored that of placebo, with no serious adverse events recorded in either study arm and a 2.9% study discontinuation rate because of treatment-emergent adverse events in the eluxadoline group. Asked why he thinks eluxadoline was effective in improving the full range of IBS-D symptoms when loperamide wasn’t, even though both drugs are mu-opioid receptor agonists, Dr. Brenner replied, “The problem is mu receptors line the entire GI tract, so you can actually push somebody from diarrhea to opioid-induced constipation – and that’s not the goal. What delta does is alleviate some of the adverse events by binding to the receptor, which results in increased transit time, reduced secretion, and increased absorption. Delta brings things back towards the center. We also believe antagonism of delta potentiates analgesic effects at the mu receptor, improves the pain component, gut symptoms, and stool symptoms.”
Dr. Brenner reported serving as a consultant to and member of a speaker’s bureau for Allergan, which markets eluxadoline and sponsored the RELIEF trial.
REPORTING FROM ACG 2019
New ustekinumab response predictor in Crohn’s called ‘brilliant’
SAN ANTONIO – The probability of achieving clinical remission of Crohn’s disease in response to ustekinumab can now be readily estimated by using a clinical prediction tool, Parambir S. Dulai, MBBS, announced at the annual meeting of the American College of Gastroenterology.
This new clinical decision support tool also provides individualized stratification of the rapidity with which symptoms will be reduced in response to the anti-interleukin-12/23 biologic, added Dr. Dulai, a gastroenterologist at the University of California, San Diego.
He and his coinvestigators developed the prediction tool through analysis of detailed data on 781 patients with active Crohn’s disease treated with ustekinumab (Stelara) during both the induction and maintenance portions of the phase 3 UNITI randomized trials conducted in the biologic’s development program. The researchers identified a series of baseline features associated with clinical remission as defined by a Crohn’s Disease Activity Index (CDAI) score below 150 by week 16 of treatment. Through statistical manipulation, they transformed the data into a predictive model and then went one step further by turning the model into a decision support tool with points given for the individual predictive variables (see graphic).
Patients with 5 or more total points were categorized as having a high probability of week-16 clinical remission. Patients with 0 or 1 point were deemed low probability, and a score of 2-4 indicated an intermediate likelihood of clinical remission.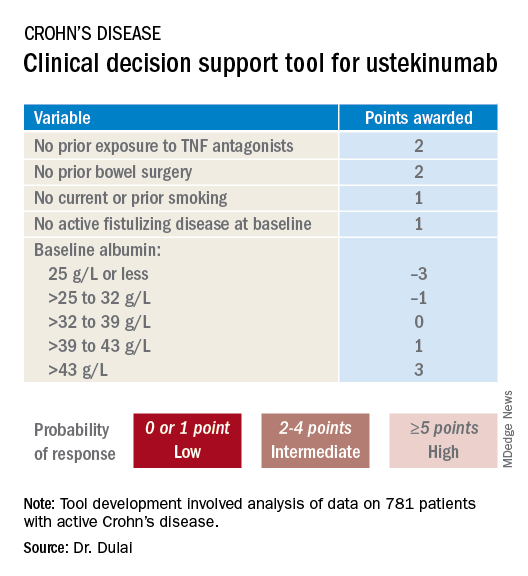
Next, the investigators applied their new clinical decision support tool to the 781 ustekinumab-treated patients included in the derivation analysis. The tool performed well: The high-probability group had a 57% clinical remission rate, significantly better than the 34% rate in the intermediate-probability group, which in turn was significantly better than the 21% rate of clinical remission in the group with a baseline score of 0 or 1.
In addition, onset of treatment benefit was significantly faster in the group having a score of 5 or more. They had a significantly higher clinical remission rate than the intermediate- and low-probability groups at all scheduled assessments, which were conducted at weeks 3, 6, 8, and 16. Indeed, by week 3 the high-probability group experienced a mean 69-point drop from baseline in CDAI and a 94-point drop by week 8, as compared with week-8 reductions of 54 and 40 points in the intermediate- and low-probability groups, respectively.
In an exploratory analysis involving the 122 patients who underwent week-8 endoscopy, endoscopic remission was documented in 12% of patients whose baseline scores placed them in the high-probability group, 10% in the intermediate group, and 8% of those in the low-probability group.
The high-probability group had significantly higher ustekinumab trough concentrations than did the intermediate- and low-probability groups when measured at weeks 3, 6, 8, and 16.
An external validation study conducted in a large cohort of Crohn’s disease patients seen in routine clinical practice has recently been completed, with the results now being analyzed, according to Dr. Dulai.
Miguel Requeiro, MD, chairman of gastroenterology and hepatology at the Cleveland Clinic, rose from the audience to declare the creation of the decision support tool to be “brilliant work.” He asked if it has changed clinical practice for Dr. Dulai and his coworkers.
“We’ve begun doing two things differently,” Dr. Dulai replied. “First, we’ve built a similar model for vedolizumab and Crohn’s. That means we can use both tools together to discriminate between a patient who should get vedolizumab versus ustekinumab because the variables and their weighting differ between the two. And the other thing we’ve been able to do is argue with payers for positioning of the treatments when we have evidence to support that we can use them earlier in the treatment course to optimize outcomes.”
Another audience member, David T. Rubin, MD, also praised the decision support tool as “brilliant” and “definitely needed.”
“Thank you for hitting the sweet spot of what we’ve all been waiting for,” added Dr. Rubin, professor of medicine and codirector of the Digestive Diseases Center at the University of Chicago.
Dr. Dulai reported receiving a research grant for the project from Janssen, which markets ustekinumab.
Help your patients better understand their Crohn’s disease treatment options by sharing AGA patient education at https://www.gastro.org/
SAN ANTONIO – The probability of achieving clinical remission of Crohn’s disease in response to ustekinumab can now be readily estimated by using a clinical prediction tool, Parambir S. Dulai, MBBS, announced at the annual meeting of the American College of Gastroenterology.
This new clinical decision support tool also provides individualized stratification of the rapidity with which symptoms will be reduced in response to the anti-interleukin-12/23 biologic, added Dr. Dulai, a gastroenterologist at the University of California, San Diego.
He and his coinvestigators developed the prediction tool through analysis of detailed data on 781 patients with active Crohn’s disease treated with ustekinumab (Stelara) during both the induction and maintenance portions of the phase 3 UNITI randomized trials conducted in the biologic’s development program. The researchers identified a series of baseline features associated with clinical remission as defined by a Crohn’s Disease Activity Index (CDAI) score below 150 by week 16 of treatment. Through statistical manipulation, they transformed the data into a predictive model and then went one step further by turning the model into a decision support tool with points given for the individual predictive variables (see graphic).
Patients with 5 or more total points were categorized as having a high probability of week-16 clinical remission. Patients with 0 or 1 point were deemed low probability, and a score of 2-4 indicated an intermediate likelihood of clinical remission.
Next, the investigators applied their new clinical decision support tool to the 781 ustekinumab-treated patients included in the derivation analysis. The tool performed well: The high-probability group had a 57% clinical remission rate, significantly better than the 34% rate in the intermediate-probability group, which in turn was significantly better than the 21% rate of clinical remission in the group with a baseline score of 0 or 1.
In addition, onset of treatment benefit was significantly faster in the group having a score of 5 or more. They had a significantly higher clinical remission rate than the intermediate- and low-probability groups at all scheduled assessments, which were conducted at weeks 3, 6, 8, and 16. Indeed, by week 3 the high-probability group experienced a mean 69-point drop from baseline in CDAI and a 94-point drop by week 8, as compared with week-8 reductions of 54 and 40 points in the intermediate- and low-probability groups, respectively.
In an exploratory analysis involving the 122 patients who underwent week-8 endoscopy, endoscopic remission was documented in 12% of patients whose baseline scores placed them in the high-probability group, 10% in the intermediate group, and 8% of those in the low-probability group.
The high-probability group had significantly higher ustekinumab trough concentrations than did the intermediate- and low-probability groups when measured at weeks 3, 6, 8, and 16.
An external validation study conducted in a large cohort of Crohn’s disease patients seen in routine clinical practice has recently been completed, with the results now being analyzed, according to Dr. Dulai.
Miguel Requeiro, MD, chairman of gastroenterology and hepatology at the Cleveland Clinic, rose from the audience to declare the creation of the decision support tool to be “brilliant work.” He asked if it has changed clinical practice for Dr. Dulai and his coworkers.
“We’ve begun doing two things differently,” Dr. Dulai replied. “First, we’ve built a similar model for vedolizumab and Crohn’s. That means we can use both tools together to discriminate between a patient who should get vedolizumab versus ustekinumab because the variables and their weighting differ between the two. And the other thing we’ve been able to do is argue with payers for positioning of the treatments when we have evidence to support that we can use them earlier in the treatment course to optimize outcomes.”
Another audience member, David T. Rubin, MD, also praised the decision support tool as “brilliant” and “definitely needed.”
“Thank you for hitting the sweet spot of what we’ve all been waiting for,” added Dr. Rubin, professor of medicine and codirector of the Digestive Diseases Center at the University of Chicago.
Dr. Dulai reported receiving a research grant for the project from Janssen, which markets ustekinumab.
Help your patients better understand their Crohn’s disease treatment options by sharing AGA patient education at https://www.gastro.org/
SAN ANTONIO – The probability of achieving clinical remission of Crohn’s disease in response to ustekinumab can now be readily estimated by using a clinical prediction tool, Parambir S. Dulai, MBBS, announced at the annual meeting of the American College of Gastroenterology.
This new clinical decision support tool also provides individualized stratification of the rapidity with which symptoms will be reduced in response to the anti-interleukin-12/23 biologic, added Dr. Dulai, a gastroenterologist at the University of California, San Diego.
He and his coinvestigators developed the prediction tool through analysis of detailed data on 781 patients with active Crohn’s disease treated with ustekinumab (Stelara) during both the induction and maintenance portions of the phase 3 UNITI randomized trials conducted in the biologic’s development program. The researchers identified a series of baseline features associated with clinical remission as defined by a Crohn’s Disease Activity Index (CDAI) score below 150 by week 16 of treatment. Through statistical manipulation, they transformed the data into a predictive model and then went one step further by turning the model into a decision support tool with points given for the individual predictive variables (see graphic).
Patients with 5 or more total points were categorized as having a high probability of week-16 clinical remission. Patients with 0 or 1 point were deemed low probability, and a score of 2-4 indicated an intermediate likelihood of clinical remission.
Next, the investigators applied their new clinical decision support tool to the 781 ustekinumab-treated patients included in the derivation analysis. The tool performed well: The high-probability group had a 57% clinical remission rate, significantly better than the 34% rate in the intermediate-probability group, which in turn was significantly better than the 21% rate of clinical remission in the group with a baseline score of 0 or 1.
In addition, onset of treatment benefit was significantly faster in the group having a score of 5 or more. They had a significantly higher clinical remission rate than the intermediate- and low-probability groups at all scheduled assessments, which were conducted at weeks 3, 6, 8, and 16. Indeed, by week 3 the high-probability group experienced a mean 69-point drop from baseline in CDAI and a 94-point drop by week 8, as compared with week-8 reductions of 54 and 40 points in the intermediate- and low-probability groups, respectively.
In an exploratory analysis involving the 122 patients who underwent week-8 endoscopy, endoscopic remission was documented in 12% of patients whose baseline scores placed them in the high-probability group, 10% in the intermediate group, and 8% of those in the low-probability group.
The high-probability group had significantly higher ustekinumab trough concentrations than did the intermediate- and low-probability groups when measured at weeks 3, 6, 8, and 16.
An external validation study conducted in a large cohort of Crohn’s disease patients seen in routine clinical practice has recently been completed, with the results now being analyzed, according to Dr. Dulai.
Miguel Requeiro, MD, chairman of gastroenterology and hepatology at the Cleveland Clinic, rose from the audience to declare the creation of the decision support tool to be “brilliant work.” He asked if it has changed clinical practice for Dr. Dulai and his coworkers.
“We’ve begun doing two things differently,” Dr. Dulai replied. “First, we’ve built a similar model for vedolizumab and Crohn’s. That means we can use both tools together to discriminate between a patient who should get vedolizumab versus ustekinumab because the variables and their weighting differ between the two. And the other thing we’ve been able to do is argue with payers for positioning of the treatments when we have evidence to support that we can use them earlier in the treatment course to optimize outcomes.”
Another audience member, David T. Rubin, MD, also praised the decision support tool as “brilliant” and “definitely needed.”
“Thank you for hitting the sweet spot of what we’ve all been waiting for,” added Dr. Rubin, professor of medicine and codirector of the Digestive Diseases Center at the University of Chicago.
Dr. Dulai reported receiving a research grant for the project from Janssen, which markets ustekinumab.
Help your patients better understand their Crohn’s disease treatment options by sharing AGA patient education at https://www.gastro.org/
REPORTING FROM ACG 2019
Human milk oligosaccharides quell IBS symptoms
SAN ANTONIO – Oral supplementation with a proprietary blend of human milk oligosaccharides improved all of the core symptoms of irritable bowel syndrome in a large open-label study, Magnus Simren, MD, PhD, reported at the annual meeting of the American College of Gastroenterology.
The human milk oligosaccharides (HMOs) were well tolerated, too. Only 2.5% of 317 study participants at 17 U.S. sites discontinued the 12-week study because of side effects, which consisted of flatulence and other mild gastrointestinal symptoms, noted Dr. Simren, a gastroenterologist and professor of medicine at the University of Gothenburg (Sweden).
These positive study results are consistent with the notion that an altered gut microbiota plays a pathophysiological role in irritable bowel syndrome (IBS).
“The challenge is to identify suitable interventions that restore intestinal microbiota composition and functioning,” Dr. Simren observed.
Oral HMOs show promise as one such intervention. In prior small proof-of-concept studies, Dr. Simren and his coworkers demonstrated that HMOs increased gut levels of Bifidobacteria, which are microorganisms important to a healthy gut and are depleted in IBS. The investigators also established that HMOs increased levels of metabolites essential for the gut’s barrier and immune functions.
HMOs are the third-largest constituent in human breast milk. Interest in their potential therapeutic application in IBS grew out of earlier pediatric work demonstrating that HMOs are of great importance in infant health: They bind pathogens and promote gut barrier maturation and immune function.
Dr. Simren reported on 317 patients who met Rome IV criteria for IBS. Nearly two-thirds of them had severe IBS based upon an IBS–Symptom Severity Score above 300. Another third had moderate IBS. Subjects were instructed to take 5 g/day of a 4:1 mix of the HMOs 2’-fucosyllactose and lacto-N-neotetraose, a proprietary nutritional support product available over the counter as Holigos. Participants remained on stable background medications throughout the 12-week study, during which they were evaluated every 4 weeks.
The primary outcome was the effect of daily oral consumption of HMOs on stool consistency as assessed using the Bristol Stool Form Scale. At baseline, 50.3% of subjects had IBS constipation as defined by Bristol type 1-2 stools. By week 4, the proportion of patients with constipation dropped to 32.9%, and at weeks 8 and 12, just under 31%. Similarly, the proportion of patients with diarrhea as reflected in Bristol type 6-7 stools quickly improved from 40.4% at baseline to 27.5% at week 4 and 26% thereafter. Meanwhile, the proportion of patients with normal stools on the Bristol scale jumped from 9.3% at baseline to 39.6% at week 4 and nearly 43% thereafter.
About 77% of patients reported a significant reduction in symptom severity within 4 weeks, and 87% did so by 12 weeks. Bloating decreased by 59%, as did abdominal pain severity. In addition, scores on the IBS Quality of Life Scale improved by 48%.
The observed improvements in symptoms and quality of life were consistent across all IBS subtypes.
“Of course, the next step now is to perform a randomized, placebo-controlled, double-blind study to see if these encouraging results can be confirmed in that setting,” Dr. Simren commented.
Session comoderator Brooks D. Cash, MD, of the University of Texas, Houston, called the HMO study “very provocative” and declared he is looking forward to the randomized, controlled trial, which he hopes will assess the long-term durability of the treatment benefits. That trial is still in the planning stages.
Dr. Simren reported serving on an advisory board for Glycom, the Danish company which markets Holigos and sponsored the open-label U.S. study.
SAN ANTONIO – Oral supplementation with a proprietary blend of human milk oligosaccharides improved all of the core symptoms of irritable bowel syndrome in a large open-label study, Magnus Simren, MD, PhD, reported at the annual meeting of the American College of Gastroenterology.
The human milk oligosaccharides (HMOs) were well tolerated, too. Only 2.5% of 317 study participants at 17 U.S. sites discontinued the 12-week study because of side effects, which consisted of flatulence and other mild gastrointestinal symptoms, noted Dr. Simren, a gastroenterologist and professor of medicine at the University of Gothenburg (Sweden).
These positive study results are consistent with the notion that an altered gut microbiota plays a pathophysiological role in irritable bowel syndrome (IBS).
“The challenge is to identify suitable interventions that restore intestinal microbiota composition and functioning,” Dr. Simren observed.
Oral HMOs show promise as one such intervention. In prior small proof-of-concept studies, Dr. Simren and his coworkers demonstrated that HMOs increased gut levels of Bifidobacteria, which are microorganisms important to a healthy gut and are depleted in IBS. The investigators also established that HMOs increased levels of metabolites essential for the gut’s barrier and immune functions.
HMOs are the third-largest constituent in human breast milk. Interest in their potential therapeutic application in IBS grew out of earlier pediatric work demonstrating that HMOs are of great importance in infant health: They bind pathogens and promote gut barrier maturation and immune function.
Dr. Simren reported on 317 patients who met Rome IV criteria for IBS. Nearly two-thirds of them had severe IBS based upon an IBS–Symptom Severity Score above 300. Another third had moderate IBS. Subjects were instructed to take 5 g/day of a 4:1 mix of the HMOs 2’-fucosyllactose and lacto-N-neotetraose, a proprietary nutritional support product available over the counter as Holigos. Participants remained on stable background medications throughout the 12-week study, during which they were evaluated every 4 weeks.
The primary outcome was the effect of daily oral consumption of HMOs on stool consistency as assessed using the Bristol Stool Form Scale. At baseline, 50.3% of subjects had IBS constipation as defined by Bristol type 1-2 stools. By week 4, the proportion of patients with constipation dropped to 32.9%, and at weeks 8 and 12, just under 31%. Similarly, the proportion of patients with diarrhea as reflected in Bristol type 6-7 stools quickly improved from 40.4% at baseline to 27.5% at week 4 and 26% thereafter. Meanwhile, the proportion of patients with normal stools on the Bristol scale jumped from 9.3% at baseline to 39.6% at week 4 and nearly 43% thereafter.
About 77% of patients reported a significant reduction in symptom severity within 4 weeks, and 87% did so by 12 weeks. Bloating decreased by 59%, as did abdominal pain severity. In addition, scores on the IBS Quality of Life Scale improved by 48%.
The observed improvements in symptoms and quality of life were consistent across all IBS subtypes.
“Of course, the next step now is to perform a randomized, placebo-controlled, double-blind study to see if these encouraging results can be confirmed in that setting,” Dr. Simren commented.
Session comoderator Brooks D. Cash, MD, of the University of Texas, Houston, called the HMO study “very provocative” and declared he is looking forward to the randomized, controlled trial, which he hopes will assess the long-term durability of the treatment benefits. That trial is still in the planning stages.
Dr. Simren reported serving on an advisory board for Glycom, the Danish company which markets Holigos and sponsored the open-label U.S. study.
SAN ANTONIO – Oral supplementation with a proprietary blend of human milk oligosaccharides improved all of the core symptoms of irritable bowel syndrome in a large open-label study, Magnus Simren, MD, PhD, reported at the annual meeting of the American College of Gastroenterology.
The human milk oligosaccharides (HMOs) were well tolerated, too. Only 2.5% of 317 study participants at 17 U.S. sites discontinued the 12-week study because of side effects, which consisted of flatulence and other mild gastrointestinal symptoms, noted Dr. Simren, a gastroenterologist and professor of medicine at the University of Gothenburg (Sweden).
These positive study results are consistent with the notion that an altered gut microbiota plays a pathophysiological role in irritable bowel syndrome (IBS).
“The challenge is to identify suitable interventions that restore intestinal microbiota composition and functioning,” Dr. Simren observed.
Oral HMOs show promise as one such intervention. In prior small proof-of-concept studies, Dr. Simren and his coworkers demonstrated that HMOs increased gut levels of Bifidobacteria, which are microorganisms important to a healthy gut and are depleted in IBS. The investigators also established that HMOs increased levels of metabolites essential for the gut’s barrier and immune functions.
HMOs are the third-largest constituent in human breast milk. Interest in their potential therapeutic application in IBS grew out of earlier pediatric work demonstrating that HMOs are of great importance in infant health: They bind pathogens and promote gut barrier maturation and immune function.
Dr. Simren reported on 317 patients who met Rome IV criteria for IBS. Nearly two-thirds of them had severe IBS based upon an IBS–Symptom Severity Score above 300. Another third had moderate IBS. Subjects were instructed to take 5 g/day of a 4:1 mix of the HMOs 2’-fucosyllactose and lacto-N-neotetraose, a proprietary nutritional support product available over the counter as Holigos. Participants remained on stable background medications throughout the 12-week study, during which they were evaluated every 4 weeks.
The primary outcome was the effect of daily oral consumption of HMOs on stool consistency as assessed using the Bristol Stool Form Scale. At baseline, 50.3% of subjects had IBS constipation as defined by Bristol type 1-2 stools. By week 4, the proportion of patients with constipation dropped to 32.9%, and at weeks 8 and 12, just under 31%. Similarly, the proportion of patients with diarrhea as reflected in Bristol type 6-7 stools quickly improved from 40.4% at baseline to 27.5% at week 4 and 26% thereafter. Meanwhile, the proportion of patients with normal stools on the Bristol scale jumped from 9.3% at baseline to 39.6% at week 4 and nearly 43% thereafter.
About 77% of patients reported a significant reduction in symptom severity within 4 weeks, and 87% did so by 12 weeks. Bloating decreased by 59%, as did abdominal pain severity. In addition, scores on the IBS Quality of Life Scale improved by 48%.
The observed improvements in symptoms and quality of life were consistent across all IBS subtypes.
“Of course, the next step now is to perform a randomized, placebo-controlled, double-blind study to see if these encouraging results can be confirmed in that setting,” Dr. Simren commented.
Session comoderator Brooks D. Cash, MD, of the University of Texas, Houston, called the HMO study “very provocative” and declared he is looking forward to the randomized, controlled trial, which he hopes will assess the long-term durability of the treatment benefits. That trial is still in the planning stages.
Dr. Simren reported serving on an advisory board for Glycom, the Danish company which markets Holigos and sponsored the open-label U.S. study.
REPORTING FROM ACG 2019











