User login
Probably okay to skip SIBO breath test before starting rifaximin in IBS-D
MAUI, HAWAII – , according to IBS expert Lin Chang, MD, a professor and vice chief of the division of digestive diseases at the University of California, Los Angeles.
Rifaximin is an antibiotic that works in the intestines but is not absorbed by the body, and is approved for irritable bowel syndrome with diarrhea (IBS-D). It’s a second-line option for moderate illness when diet, OTC medications, and other easier options don’t do the trick. Endpoints were met by about 10% more patients on rifaximin than placebo in randomized trials, perhaps by resetting gut microbiota.
“It was a small therapeutic gain, but it was statistically significant,” Dr. Chang said at the Gastroenterology Updates, IBD, Liver Disease Conference. In her own practice, she said it sometimes helps even with bloating and abdominal distension, two of the most vexing problems in IBS-D.
However, symptoms come back after 3-6 months, and prices approach $2,000 for the 2-week IBS-D course. Insurance doesn’t always fully cover it, and the Food and Drug Administration has capped treatments at three.
That’s left providers wondering what to do when people ask for rifaximin after seeing it advertised and eager for some way to predict if it will work or not. Some clinicians have turned to a breath test for small-intestinal bacterial overgrowth (SIBO) before prescribing rifaximin because there is evidence that SIBO contributes to IBS-D, but others have not. It’s a contentious issue in IBS medicine, Dr. Chang said.
A recent open-label study funded by rifaximin maker Salix Pharmaceuticals made a case for testing. Among 93 IBS-D patients, 60% with a positive breath test at baseline had reduced pain, diarrhea, and other symptoms after 550 mg three times daily for 2 weeks, versus 26% with a negative test (Am J Gastroenterol. 2019 Dec;114[12]:1886-93).
Even so, when asked after her IBS presentation, Dr. Chang said she doesn’t breath test because it doesn’t make sense. Even with positive results, “you don’t really know if they have SIBO or not. Sometimes healthy controls have a positive test, and some people with IBS who end up responding to rifaximin have a negative breath test. It’s up to you, [but] it doesn’t correlate” with outcomes, she said.
Indeed, a letter in response to the study made a strong case that nine of the subjects should have been counted as having a negative, not positive, breath test, and if they had, the difference in outcome would have disappeared (Am J Gastroenterol. 2020 Mar 2. doi: 10.14309/ajg.0000000000000569).
Dr. Chang has opted for an empiric approach. “I just treat patients who meet the criteria used in the clinical trials,” such as moderate abdominal pain and bloating, she said (for example, N Engl J Med. 2011 Jan 6;364[1]:22-32).
And she doesn’t push if people can’t afford rifaximin. Some turn to other antibiotics, like metronidazole (Flagyl) or ciprofloxacin (Cipro), which are absorbed in the GI tract, but Dr. Chang said symptoms will recur after a few months regardless of the antibiotic. “You don’t want all these young women to be given antibiotics over and over again. Their symptoms are just going to come back, and you are going to have to learn how to treat them anyway,” she said.
Instead, she moves on to other options when patients are at the point where they need prescription pharmaceuticals. One of her top choices is the tricyclic antidepressant amitriptyline. It helps with the pain; the anticholinergic effects counter the diarrhea; and the sedative effects help patients who have a hard time sleeping. Sleep problems make IBS-D symptoms worse, Dr. Chang said.
MAUI, HAWAII – , according to IBS expert Lin Chang, MD, a professor and vice chief of the division of digestive diseases at the University of California, Los Angeles.
Rifaximin is an antibiotic that works in the intestines but is not absorbed by the body, and is approved for irritable bowel syndrome with diarrhea (IBS-D). It’s a second-line option for moderate illness when diet, OTC medications, and other easier options don’t do the trick. Endpoints were met by about 10% more patients on rifaximin than placebo in randomized trials, perhaps by resetting gut microbiota.
“It was a small therapeutic gain, but it was statistically significant,” Dr. Chang said at the Gastroenterology Updates, IBD, Liver Disease Conference. In her own practice, she said it sometimes helps even with bloating and abdominal distension, two of the most vexing problems in IBS-D.
However, symptoms come back after 3-6 months, and prices approach $2,000 for the 2-week IBS-D course. Insurance doesn’t always fully cover it, and the Food and Drug Administration has capped treatments at three.
That’s left providers wondering what to do when people ask for rifaximin after seeing it advertised and eager for some way to predict if it will work or not. Some clinicians have turned to a breath test for small-intestinal bacterial overgrowth (SIBO) before prescribing rifaximin because there is evidence that SIBO contributes to IBS-D, but others have not. It’s a contentious issue in IBS medicine, Dr. Chang said.
A recent open-label study funded by rifaximin maker Salix Pharmaceuticals made a case for testing. Among 93 IBS-D patients, 60% with a positive breath test at baseline had reduced pain, diarrhea, and other symptoms after 550 mg three times daily for 2 weeks, versus 26% with a negative test (Am J Gastroenterol. 2019 Dec;114[12]:1886-93).
Even so, when asked after her IBS presentation, Dr. Chang said she doesn’t breath test because it doesn’t make sense. Even with positive results, “you don’t really know if they have SIBO or not. Sometimes healthy controls have a positive test, and some people with IBS who end up responding to rifaximin have a negative breath test. It’s up to you, [but] it doesn’t correlate” with outcomes, she said.
Indeed, a letter in response to the study made a strong case that nine of the subjects should have been counted as having a negative, not positive, breath test, and if they had, the difference in outcome would have disappeared (Am J Gastroenterol. 2020 Mar 2. doi: 10.14309/ajg.0000000000000569).
Dr. Chang has opted for an empiric approach. “I just treat patients who meet the criteria used in the clinical trials,” such as moderate abdominal pain and bloating, she said (for example, N Engl J Med. 2011 Jan 6;364[1]:22-32).
And she doesn’t push if people can’t afford rifaximin. Some turn to other antibiotics, like metronidazole (Flagyl) or ciprofloxacin (Cipro), which are absorbed in the GI tract, but Dr. Chang said symptoms will recur after a few months regardless of the antibiotic. “You don’t want all these young women to be given antibiotics over and over again. Their symptoms are just going to come back, and you are going to have to learn how to treat them anyway,” she said.
Instead, she moves on to other options when patients are at the point where they need prescription pharmaceuticals. One of her top choices is the tricyclic antidepressant amitriptyline. It helps with the pain; the anticholinergic effects counter the diarrhea; and the sedative effects help patients who have a hard time sleeping. Sleep problems make IBS-D symptoms worse, Dr. Chang said.
MAUI, HAWAII – , according to IBS expert Lin Chang, MD, a professor and vice chief of the division of digestive diseases at the University of California, Los Angeles.
Rifaximin is an antibiotic that works in the intestines but is not absorbed by the body, and is approved for irritable bowel syndrome with diarrhea (IBS-D). It’s a second-line option for moderate illness when diet, OTC medications, and other easier options don’t do the trick. Endpoints were met by about 10% more patients on rifaximin than placebo in randomized trials, perhaps by resetting gut microbiota.
“It was a small therapeutic gain, but it was statistically significant,” Dr. Chang said at the Gastroenterology Updates, IBD, Liver Disease Conference. In her own practice, she said it sometimes helps even with bloating and abdominal distension, two of the most vexing problems in IBS-D.
However, symptoms come back after 3-6 months, and prices approach $2,000 for the 2-week IBS-D course. Insurance doesn’t always fully cover it, and the Food and Drug Administration has capped treatments at three.
That’s left providers wondering what to do when people ask for rifaximin after seeing it advertised and eager for some way to predict if it will work or not. Some clinicians have turned to a breath test for small-intestinal bacterial overgrowth (SIBO) before prescribing rifaximin because there is evidence that SIBO contributes to IBS-D, but others have not. It’s a contentious issue in IBS medicine, Dr. Chang said.
A recent open-label study funded by rifaximin maker Salix Pharmaceuticals made a case for testing. Among 93 IBS-D patients, 60% with a positive breath test at baseline had reduced pain, diarrhea, and other symptoms after 550 mg three times daily for 2 weeks, versus 26% with a negative test (Am J Gastroenterol. 2019 Dec;114[12]:1886-93).
Even so, when asked after her IBS presentation, Dr. Chang said she doesn’t breath test because it doesn’t make sense. Even with positive results, “you don’t really know if they have SIBO or not. Sometimes healthy controls have a positive test, and some people with IBS who end up responding to rifaximin have a negative breath test. It’s up to you, [but] it doesn’t correlate” with outcomes, she said.
Indeed, a letter in response to the study made a strong case that nine of the subjects should have been counted as having a negative, not positive, breath test, and if they had, the difference in outcome would have disappeared (Am J Gastroenterol. 2020 Mar 2. doi: 10.14309/ajg.0000000000000569).
Dr. Chang has opted for an empiric approach. “I just treat patients who meet the criteria used in the clinical trials,” such as moderate abdominal pain and bloating, she said (for example, N Engl J Med. 2011 Jan 6;364[1]:22-32).
And she doesn’t push if people can’t afford rifaximin. Some turn to other antibiotics, like metronidazole (Flagyl) or ciprofloxacin (Cipro), which are absorbed in the GI tract, but Dr. Chang said symptoms will recur after a few months regardless of the antibiotic. “You don’t want all these young women to be given antibiotics over and over again. Their symptoms are just going to come back, and you are going to have to learn how to treat them anyway,” she said.
Instead, she moves on to other options when patients are at the point where they need prescription pharmaceuticals. One of her top choices is the tricyclic antidepressant amitriptyline. It helps with the pain; the anticholinergic effects counter the diarrhea; and the sedative effects help patients who have a hard time sleeping. Sleep problems make IBS-D symptoms worse, Dr. Chang said.
REPORTING FROM GUILD 2020
Genetic variants in nudix hydrolase linked to thiopurine myelosuppression
MAUI, HAWAII – There’s a new kid on the block to worry about when it comes to thiopurine pharmacogenetics: Genetic variants in the thiopurine-metabolizing enzyme nudix hydrolase 15 have been linked to a markedly increased risk of thiopurine myelosuppression among inflammatory bowel disease patients.
The Food and Drug Administration and others already recommend screening for genetic variants in thiopurine methyltransferase (TPMT), another enzyme that metabolizes thiopurine. Polymorphisms lead to TMPT dysfunction, accumulation of cytotoxic metabolites, and increased risk of thiopurine-induced myelosuppression (TIM). Carriers are advised to use reduced doses with careful drug monitoring, or to skip thiopurines altogether.
A similar picture is emerging for nudix hydrolase 15 (NUDT15). It’s been known for several years that genetic variants are not uncommon among East Asian people and lead to TIM, but their prevalence and impact among people of European decent wasn’t clearly understood until now.
Investigators led by Gareth Walker, MBBS, of the Royal Devon and Exeter Hospital in Exeter, England, compared rates of problematic TMPT and NUDT15 variants among European inflammatory bowel disease patients who had developed TIM and those who had not, about 1,000 patients in all. The majority were on azathioprine and had Crohn’s disease. Finnish people were excluded because “their unique genetic background ... has led to the enrichment of some disease-causing gene variants and losses of others,” according to the study, which was published in JAMA.
Carriage of any of three coding NUDT15 variants greatly increased the risk of TIM (odds ratio, 27.3; 95% confidence interval, 9.3-116.7), independent of TPMT genotype and thiopurine dose. A particular variant – an in-frame deletion in NUDT15 – increased the risk 38-fold (95% CI, 5.1-286.1), and was carried by 5.8% of TIM patients.
The analysis also confirmed the importance of TPMT variants, which were found in 30.5% of TIM patients (95/311) versus 16.4% (100/608) of patients who did not develop TIM.
“Patients with variants of either NUDT15 or TPMT, or among those with variants of both genes, had a faster onset of TIM, more severe TIM, and had a greater need for granulocyte colony–stimulating factor rescue therapy. ... Our data suggest that pretreatment sequencing of the NUDT15 gene ... may be considered prior to initiation of thiopurine therapy,” the team concluded.
The prevalence of problematic NUDT15 variants among non-Finnish Europeans is about 1.6%, 6.9% among people from Finland, and almost 30% among East Asians. The team estimated NUDT15 would have to be genotyped in 95 non-Finnish Europeans to prevent one case of TIM; the number is 123 for TPMT. “Given the widespread use of thiopurines” – primarily in rheumatology and transplant medicine, in addition to gastroenterology – “these findings may have ramifications beyond the management of IBD,” the investigators wrote.
“I do think it’s worthwhile” to screen for NUDT15, said Edward Loftus, MD, a professor and consultant in the division of gastroenterology and hepatology at the Mayo Clinic in Rochester, Minn., who reviewed the study at the Gastroenterology Updates, IBD, Liver Disease Conference. “If you are a homozygote for this, your chance of getting profound leukopenia is very high, so I would probably not use a thiopurine.”
“If you are going to start low dose on everyone” with careful blood monitoring, “I suppose you could just do that, but I would say if you can get” the test and “are reassured the patient is not” carrying problematic NUDT15 or TMPT variants, “then I think you just go ahead and do full dose,” he said.
Testing for the relevant variants is available through the Mayo Clinic and several commercial labs.
“Even though the thiopurine dose is adjusted based on genotype, patients still need to be monitored closely for the development of thiopurine-induced myelosuppression as” TMPT and NUDT15 variants “do not explain all cases of” TIM, Marieke Coenen, PhD, an associate professor of pharmacogenetics at Radboud University Medical Center in Nijmegen, the Netherlands, cautioned in an editorial (Transl Gastroenterol Hepatol. 2019 Dec 12;4:81).
The prevalence of problematic NUDT15 variants is 0.7% among African and 20.7% among Hispanic people.
The work was funded by the National Institutes of Health, Crohn’s & Colitis UK, the Wellcome Trust, and others. Dr. Walker and other investigators reported numerous industry ties. Dr. Loftus is a consultant and/or has research funding from Abbott, Pfizer, and other companies. Dr. Coenen had no disclosures.
SOURCE: Walker GJ et al. JAMA. 2019 Feb 26;321(8):773-85.
MAUI, HAWAII – There’s a new kid on the block to worry about when it comes to thiopurine pharmacogenetics: Genetic variants in the thiopurine-metabolizing enzyme nudix hydrolase 15 have been linked to a markedly increased risk of thiopurine myelosuppression among inflammatory bowel disease patients.
The Food and Drug Administration and others already recommend screening for genetic variants in thiopurine methyltransferase (TPMT), another enzyme that metabolizes thiopurine. Polymorphisms lead to TMPT dysfunction, accumulation of cytotoxic metabolites, and increased risk of thiopurine-induced myelosuppression (TIM). Carriers are advised to use reduced doses with careful drug monitoring, or to skip thiopurines altogether.
A similar picture is emerging for nudix hydrolase 15 (NUDT15). It’s been known for several years that genetic variants are not uncommon among East Asian people and lead to TIM, but their prevalence and impact among people of European decent wasn’t clearly understood until now.
Investigators led by Gareth Walker, MBBS, of the Royal Devon and Exeter Hospital in Exeter, England, compared rates of problematic TMPT and NUDT15 variants among European inflammatory bowel disease patients who had developed TIM and those who had not, about 1,000 patients in all. The majority were on azathioprine and had Crohn’s disease. Finnish people were excluded because “their unique genetic background ... has led to the enrichment of some disease-causing gene variants and losses of others,” according to the study, which was published in JAMA.
Carriage of any of three coding NUDT15 variants greatly increased the risk of TIM (odds ratio, 27.3; 95% confidence interval, 9.3-116.7), independent of TPMT genotype and thiopurine dose. A particular variant – an in-frame deletion in NUDT15 – increased the risk 38-fold (95% CI, 5.1-286.1), and was carried by 5.8% of TIM patients.
The analysis also confirmed the importance of TPMT variants, which were found in 30.5% of TIM patients (95/311) versus 16.4% (100/608) of patients who did not develop TIM.
“Patients with variants of either NUDT15 or TPMT, or among those with variants of both genes, had a faster onset of TIM, more severe TIM, and had a greater need for granulocyte colony–stimulating factor rescue therapy. ... Our data suggest that pretreatment sequencing of the NUDT15 gene ... may be considered prior to initiation of thiopurine therapy,” the team concluded.
The prevalence of problematic NUDT15 variants among non-Finnish Europeans is about 1.6%, 6.9% among people from Finland, and almost 30% among East Asians. The team estimated NUDT15 would have to be genotyped in 95 non-Finnish Europeans to prevent one case of TIM; the number is 123 for TPMT. “Given the widespread use of thiopurines” – primarily in rheumatology and transplant medicine, in addition to gastroenterology – “these findings may have ramifications beyond the management of IBD,” the investigators wrote.
“I do think it’s worthwhile” to screen for NUDT15, said Edward Loftus, MD, a professor and consultant in the division of gastroenterology and hepatology at the Mayo Clinic in Rochester, Minn., who reviewed the study at the Gastroenterology Updates, IBD, Liver Disease Conference. “If you are a homozygote for this, your chance of getting profound leukopenia is very high, so I would probably not use a thiopurine.”
“If you are going to start low dose on everyone” with careful blood monitoring, “I suppose you could just do that, but I would say if you can get” the test and “are reassured the patient is not” carrying problematic NUDT15 or TMPT variants, “then I think you just go ahead and do full dose,” he said.
Testing for the relevant variants is available through the Mayo Clinic and several commercial labs.
“Even though the thiopurine dose is adjusted based on genotype, patients still need to be monitored closely for the development of thiopurine-induced myelosuppression as” TMPT and NUDT15 variants “do not explain all cases of” TIM, Marieke Coenen, PhD, an associate professor of pharmacogenetics at Radboud University Medical Center in Nijmegen, the Netherlands, cautioned in an editorial (Transl Gastroenterol Hepatol. 2019 Dec 12;4:81).
The prevalence of problematic NUDT15 variants is 0.7% among African and 20.7% among Hispanic people.
The work was funded by the National Institutes of Health, Crohn’s & Colitis UK, the Wellcome Trust, and others. Dr. Walker and other investigators reported numerous industry ties. Dr. Loftus is a consultant and/or has research funding from Abbott, Pfizer, and other companies. Dr. Coenen had no disclosures.
SOURCE: Walker GJ et al. JAMA. 2019 Feb 26;321(8):773-85.
MAUI, HAWAII – There’s a new kid on the block to worry about when it comes to thiopurine pharmacogenetics: Genetic variants in the thiopurine-metabolizing enzyme nudix hydrolase 15 have been linked to a markedly increased risk of thiopurine myelosuppression among inflammatory bowel disease patients.
The Food and Drug Administration and others already recommend screening for genetic variants in thiopurine methyltransferase (TPMT), another enzyme that metabolizes thiopurine. Polymorphisms lead to TMPT dysfunction, accumulation of cytotoxic metabolites, and increased risk of thiopurine-induced myelosuppression (TIM). Carriers are advised to use reduced doses with careful drug monitoring, or to skip thiopurines altogether.
A similar picture is emerging for nudix hydrolase 15 (NUDT15). It’s been known for several years that genetic variants are not uncommon among East Asian people and lead to TIM, but their prevalence and impact among people of European decent wasn’t clearly understood until now.
Investigators led by Gareth Walker, MBBS, of the Royal Devon and Exeter Hospital in Exeter, England, compared rates of problematic TMPT and NUDT15 variants among European inflammatory bowel disease patients who had developed TIM and those who had not, about 1,000 patients in all. The majority were on azathioprine and had Crohn’s disease. Finnish people were excluded because “their unique genetic background ... has led to the enrichment of some disease-causing gene variants and losses of others,” according to the study, which was published in JAMA.
Carriage of any of three coding NUDT15 variants greatly increased the risk of TIM (odds ratio, 27.3; 95% confidence interval, 9.3-116.7), independent of TPMT genotype and thiopurine dose. A particular variant – an in-frame deletion in NUDT15 – increased the risk 38-fold (95% CI, 5.1-286.1), and was carried by 5.8% of TIM patients.
The analysis also confirmed the importance of TPMT variants, which were found in 30.5% of TIM patients (95/311) versus 16.4% (100/608) of patients who did not develop TIM.
“Patients with variants of either NUDT15 or TPMT, or among those with variants of both genes, had a faster onset of TIM, more severe TIM, and had a greater need for granulocyte colony–stimulating factor rescue therapy. ... Our data suggest that pretreatment sequencing of the NUDT15 gene ... may be considered prior to initiation of thiopurine therapy,” the team concluded.
The prevalence of problematic NUDT15 variants among non-Finnish Europeans is about 1.6%, 6.9% among people from Finland, and almost 30% among East Asians. The team estimated NUDT15 would have to be genotyped in 95 non-Finnish Europeans to prevent one case of TIM; the number is 123 for TPMT. “Given the widespread use of thiopurines” – primarily in rheumatology and transplant medicine, in addition to gastroenterology – “these findings may have ramifications beyond the management of IBD,” the investigators wrote.
“I do think it’s worthwhile” to screen for NUDT15, said Edward Loftus, MD, a professor and consultant in the division of gastroenterology and hepatology at the Mayo Clinic in Rochester, Minn., who reviewed the study at the Gastroenterology Updates, IBD, Liver Disease Conference. “If you are a homozygote for this, your chance of getting profound leukopenia is very high, so I would probably not use a thiopurine.”
“If you are going to start low dose on everyone” with careful blood monitoring, “I suppose you could just do that, but I would say if you can get” the test and “are reassured the patient is not” carrying problematic NUDT15 or TMPT variants, “then I think you just go ahead and do full dose,” he said.
Testing for the relevant variants is available through the Mayo Clinic and several commercial labs.
“Even though the thiopurine dose is adjusted based on genotype, patients still need to be monitored closely for the development of thiopurine-induced myelosuppression as” TMPT and NUDT15 variants “do not explain all cases of” TIM, Marieke Coenen, PhD, an associate professor of pharmacogenetics at Radboud University Medical Center in Nijmegen, the Netherlands, cautioned in an editorial (Transl Gastroenterol Hepatol. 2019 Dec 12;4:81).
The prevalence of problematic NUDT15 variants is 0.7% among African and 20.7% among Hispanic people.
The work was funded by the National Institutes of Health, Crohn’s & Colitis UK, the Wellcome Trust, and others. Dr. Walker and other investigators reported numerous industry ties. Dr. Loftus is a consultant and/or has research funding from Abbott, Pfizer, and other companies. Dr. Coenen had no disclosures.
SOURCE: Walker GJ et al. JAMA. 2019 Feb 26;321(8):773-85.
REPORTING FROM GUILD 2020
HLA gene variant predicts anti-TNF antibodies in Crohn’s
MAUI, HAWAII – A variant in the human leukocyte antigen gene – DQA1*05 – almost doubled the risk of antibodies forming against tumor necrosis factor (TNF) inhibitors in Crohn’s disease patients, irrespective of concomitant immunomodulator use, according to a report in Gastroenterology.
“Pretreatment genetic testing for HLA-DQA1*05 may help personalize the choice of anti-TNF and the need for combination therapy,” concluded investigators led by Aleksejs Sazonovs, of the Wellcome Sanger Institute in Hinxton, England.
The same variant increases the risk of celiac disease, and it is included in commercial celiac genotyping assays. The allele is carried by about 40% of Europeans.
“This is turning into a hot topic; people are talking about it, [and it’s] blowing up on Twitter,” said Edward Loftus, MD, a professor and consultant in the division of gastroenterology and hepatology at the Mayo Clinic in Rochester, Minn. “It turns out this is really a significant predictor of immunogenicity. Whatever your risk of developing antibodies, it’s going to double if you have this HLA marker, and it’s common.
“I think we are going to start [stratifying] our decision on combination [or] monotherapy based on this,” added Dr. Loftus, speaking at the Gastroenterology Updates, IBD, Liver Disease Conference. “I would argue that, if your patient has this marker, it would be criminal to give that patient infliximab monotherapy.”
The finding also begs the question of whether to bypass anti-TNFs altogether if a patient has the marker, Dr. Loftus noted, and just use ustekinumab, vedolizumab, or another agent.
Checking for celiac disease in inflammatory bowel disease isn’t unusual and involves the same gene variant, he added, so payer coverage shouldn’t be much of a problem.
The investigators ran a genome-wide association study on 1,418 biologic-naive Crohn’s patients starting infliximab or adalimumab therapy. Patients were in their 30s, on average, with a disease duration of about 3 years; there were about equal numbers of men and women.
A total of 44% of patients developed antidrug antibodies within a year. Overall, the rate of immunogenicity – defined as an antidrug antibody titer of at least 10 AU/mL – was nearly doubled in HLA-DQA1*05 carriers (hazard ratio, 1.90; 95% confidence interval, 1.60-2.25).
The association was consistent for patients treated with adalimumab (HR, 1.89; 95% CI, 1.32-2.70) or infliximab (HR, 1.92; 95% CI, 1.57-2.33) and for patients treated with anti-TNF therapy alone (HR, 1.75; 95% CI, 1.37-2.22) or in combination with an immunomodulator, usually azathioprine (HR, 2.01; 95% CI, 1.57-2.58).
The highest rates of immunogenicity, 92% at 1 year, were in HLA-DQA1*05 carriers on infliximab monotherapy. The lowest rates, 10% at 1 year, were in adalimumab patients on combination therapy who didn’t carry the variant. HLA-DQA1*05 was also associated with lower drug persistence rates.
The specific alleles HLA-DQA1*05:01 and HLA-DQA1*05:05 mediated most of the risk.
The study authors advised that “all patients treated with an anti-TNF should be prescribed an immunomodulator to lower the risk of immunogenicity.” Among HLA-DQA1*05 carriers “in whom immunomodulators are contraindicated or not tolerated, clinicians might advise against the use of anti-TNF drugs, particularly infliximab.”
In contrast, “patients who do not carry HLA-DQA1*05 might be given the choice between adalimumab or infliximab combination therapy,” the investigators said. “Patients without the risk allele and a history of adverse drug reactions to thiopurines and/or methotrexate, or who are at high risk of opportunistic infections, might be spared the additional risks of combination therapy and treated with adalimumab monotherapy.”
The mechanism for the association is unknown, the authors said.
The work was funded by the British Society of Gastroenterology, AbbVie, Merck, Pfizer, and others. The authors disclosed numerous ties to those or other pharmaceutical companies. Two authors were employees of AbbVie, marketer of the branded adalimumab Humira.
SOURCE: Sazonovs A et al. Gastroenterology. 2020 Jan;158(1):189-99.
MAUI, HAWAII – A variant in the human leukocyte antigen gene – DQA1*05 – almost doubled the risk of antibodies forming against tumor necrosis factor (TNF) inhibitors in Crohn’s disease patients, irrespective of concomitant immunomodulator use, according to a report in Gastroenterology.
“Pretreatment genetic testing for HLA-DQA1*05 may help personalize the choice of anti-TNF and the need for combination therapy,” concluded investigators led by Aleksejs Sazonovs, of the Wellcome Sanger Institute in Hinxton, England.
The same variant increases the risk of celiac disease, and it is included in commercial celiac genotyping assays. The allele is carried by about 40% of Europeans.
“This is turning into a hot topic; people are talking about it, [and it’s] blowing up on Twitter,” said Edward Loftus, MD, a professor and consultant in the division of gastroenterology and hepatology at the Mayo Clinic in Rochester, Minn. “It turns out this is really a significant predictor of immunogenicity. Whatever your risk of developing antibodies, it’s going to double if you have this HLA marker, and it’s common.
“I think we are going to start [stratifying] our decision on combination [or] monotherapy based on this,” added Dr. Loftus, speaking at the Gastroenterology Updates, IBD, Liver Disease Conference. “I would argue that, if your patient has this marker, it would be criminal to give that patient infliximab monotherapy.”
The finding also begs the question of whether to bypass anti-TNFs altogether if a patient has the marker, Dr. Loftus noted, and just use ustekinumab, vedolizumab, or another agent.
Checking for celiac disease in inflammatory bowel disease isn’t unusual and involves the same gene variant, he added, so payer coverage shouldn’t be much of a problem.
The investigators ran a genome-wide association study on 1,418 biologic-naive Crohn’s patients starting infliximab or adalimumab therapy. Patients were in their 30s, on average, with a disease duration of about 3 years; there were about equal numbers of men and women.
A total of 44% of patients developed antidrug antibodies within a year. Overall, the rate of immunogenicity – defined as an antidrug antibody titer of at least 10 AU/mL – was nearly doubled in HLA-DQA1*05 carriers (hazard ratio, 1.90; 95% confidence interval, 1.60-2.25).
The association was consistent for patients treated with adalimumab (HR, 1.89; 95% CI, 1.32-2.70) or infliximab (HR, 1.92; 95% CI, 1.57-2.33) and for patients treated with anti-TNF therapy alone (HR, 1.75; 95% CI, 1.37-2.22) or in combination with an immunomodulator, usually azathioprine (HR, 2.01; 95% CI, 1.57-2.58).
The highest rates of immunogenicity, 92% at 1 year, were in HLA-DQA1*05 carriers on infliximab monotherapy. The lowest rates, 10% at 1 year, were in adalimumab patients on combination therapy who didn’t carry the variant. HLA-DQA1*05 was also associated with lower drug persistence rates.
The specific alleles HLA-DQA1*05:01 and HLA-DQA1*05:05 mediated most of the risk.
The study authors advised that “all patients treated with an anti-TNF should be prescribed an immunomodulator to lower the risk of immunogenicity.” Among HLA-DQA1*05 carriers “in whom immunomodulators are contraindicated or not tolerated, clinicians might advise against the use of anti-TNF drugs, particularly infliximab.”
In contrast, “patients who do not carry HLA-DQA1*05 might be given the choice between adalimumab or infliximab combination therapy,” the investigators said. “Patients without the risk allele and a history of adverse drug reactions to thiopurines and/or methotrexate, or who are at high risk of opportunistic infections, might be spared the additional risks of combination therapy and treated with adalimumab monotherapy.”
The mechanism for the association is unknown, the authors said.
The work was funded by the British Society of Gastroenterology, AbbVie, Merck, Pfizer, and others. The authors disclosed numerous ties to those or other pharmaceutical companies. Two authors were employees of AbbVie, marketer of the branded adalimumab Humira.
SOURCE: Sazonovs A et al. Gastroenterology. 2020 Jan;158(1):189-99.
MAUI, HAWAII – A variant in the human leukocyte antigen gene – DQA1*05 – almost doubled the risk of antibodies forming against tumor necrosis factor (TNF) inhibitors in Crohn’s disease patients, irrespective of concomitant immunomodulator use, according to a report in Gastroenterology.
“Pretreatment genetic testing for HLA-DQA1*05 may help personalize the choice of anti-TNF and the need for combination therapy,” concluded investigators led by Aleksejs Sazonovs, of the Wellcome Sanger Institute in Hinxton, England.
The same variant increases the risk of celiac disease, and it is included in commercial celiac genotyping assays. The allele is carried by about 40% of Europeans.
“This is turning into a hot topic; people are talking about it, [and it’s] blowing up on Twitter,” said Edward Loftus, MD, a professor and consultant in the division of gastroenterology and hepatology at the Mayo Clinic in Rochester, Minn. “It turns out this is really a significant predictor of immunogenicity. Whatever your risk of developing antibodies, it’s going to double if you have this HLA marker, and it’s common.
“I think we are going to start [stratifying] our decision on combination [or] monotherapy based on this,” added Dr. Loftus, speaking at the Gastroenterology Updates, IBD, Liver Disease Conference. “I would argue that, if your patient has this marker, it would be criminal to give that patient infliximab monotherapy.”
The finding also begs the question of whether to bypass anti-TNFs altogether if a patient has the marker, Dr. Loftus noted, and just use ustekinumab, vedolizumab, or another agent.
Checking for celiac disease in inflammatory bowel disease isn’t unusual and involves the same gene variant, he added, so payer coverage shouldn’t be much of a problem.
The investigators ran a genome-wide association study on 1,418 biologic-naive Crohn’s patients starting infliximab or adalimumab therapy. Patients were in their 30s, on average, with a disease duration of about 3 years; there were about equal numbers of men and women.
A total of 44% of patients developed antidrug antibodies within a year. Overall, the rate of immunogenicity – defined as an antidrug antibody titer of at least 10 AU/mL – was nearly doubled in HLA-DQA1*05 carriers (hazard ratio, 1.90; 95% confidence interval, 1.60-2.25).
The association was consistent for patients treated with adalimumab (HR, 1.89; 95% CI, 1.32-2.70) or infliximab (HR, 1.92; 95% CI, 1.57-2.33) and for patients treated with anti-TNF therapy alone (HR, 1.75; 95% CI, 1.37-2.22) or in combination with an immunomodulator, usually azathioprine (HR, 2.01; 95% CI, 1.57-2.58).
The highest rates of immunogenicity, 92% at 1 year, were in HLA-DQA1*05 carriers on infliximab monotherapy. The lowest rates, 10% at 1 year, were in adalimumab patients on combination therapy who didn’t carry the variant. HLA-DQA1*05 was also associated with lower drug persistence rates.
The specific alleles HLA-DQA1*05:01 and HLA-DQA1*05:05 mediated most of the risk.
The study authors advised that “all patients treated with an anti-TNF should be prescribed an immunomodulator to lower the risk of immunogenicity.” Among HLA-DQA1*05 carriers “in whom immunomodulators are contraindicated or not tolerated, clinicians might advise against the use of anti-TNF drugs, particularly infliximab.”
In contrast, “patients who do not carry HLA-DQA1*05 might be given the choice between adalimumab or infliximab combination therapy,” the investigators said. “Patients without the risk allele and a history of adverse drug reactions to thiopurines and/or methotrexate, or who are at high risk of opportunistic infections, might be spared the additional risks of combination therapy and treated with adalimumab monotherapy.”
The mechanism for the association is unknown, the authors said.
The work was funded by the British Society of Gastroenterology, AbbVie, Merck, Pfizer, and others. The authors disclosed numerous ties to those or other pharmaceutical companies. Two authors were employees of AbbVie, marketer of the branded adalimumab Humira.
SOURCE: Sazonovs A et al. Gastroenterology. 2020 Jan;158(1):189-99.
REPORTING FROM GUILD 2020
Secondary bile acid deficiency may be culprit in UC inflammation
Researchers have found three potential gut mechanisms linked to secondary bile acid (SBA) deficiencies implicated in intestinal inflammation in ulcerative colitis (UC), and reported that supplementation may aid in restoring bile acid levels and potentially treating intestinal inflammation, according to a study published in Cell Host & Microbe.
The study identified lower levels of the following gut components in SBA deficiency in colectomy patients with UC, compared with those with familial adenomatous polyposis (FAP): deoxycholic and lithocholic acids (DCA and LCA), the most abundant gut secondary bile acids (SBAs); expression of the genes needed to convert primary bile acids (PBAs) into SBAs; and the number of Ruminococcaceae, one of the few taxa that include bacteria that generate SBAs.
“Our findings confirm that significant changes in bacterial diversity and composition occur in UC versus FAP pouches,” wrote Sidhartha R. Sinha, MD, of Stanford (Calif.) University, and coauthors. “Notably, our finding of decreased Ruminococcaceae in UC, compared to FAP pouch stool, requires further exploration.” They added that this is the first study to identify Ruminococcaceae as a key contributor to the production of LCA or DCA from PBAs.
The study found average DCA counts of 60,957 in FAP versus 1,593 in UC (P = .002), and average LCA counts of 30,644 and 282.9, respectively (P = .001). The study profiled stools from ileal pouches in colectomy patients who had UC (17) or FAP (7), a noninflammatory disease. “Remarkably, our data identify LCA and DCA to be almost undetectable in UC pouch patients,” Dr. Sinha and coauthors wrote. “This striking finding in patients who underwent colectomy suggests that SBAs may play a role in dysregulated metabolism-induced intestinal inflammation.”
The study found that UC pouches demonstrated less bacterial diversity, or alpha-diversity, than FAP pouches, which is in line with previously reported findings (J Inflamm Res. 2017;10:63-73), and had significantly lower expression of bile-acid inducible genes that encode enzymes that transform PBA to SBA.
The researchers also demonstrated that LCA and DCA supplementation reduced intestinal inflammation in mice with UC.
“Our results show that LCA and DCA treatments caused a remarkable and significant decrease in multiple chemokines and cytokines associated with inflammation, including those often increased in intestinal inflammation,” Dr. Sinha and coauthors wrote. However, they found that LCA had no protective effect on dextran sodium sulfate–induced colitis in mice with TGR5-deficient immune cells. The TGR5 bile acid receptor influences the anti-inflammatory effect in SBA supplementation, the study reported.
The researchers have initiated a clinical study (NCT03724175) to investigate the role of SBAs in patients with pouchitis that doesn’t respond to antibiotic therapy. “Insights from this study will further inform our understanding of the role of SBAs in intestinal inflammation and hold promise to provide an effective treatment,” Dr. Sinha and coauthors wrote.
Christian Jobin, PhD, of the University of Florida, Gainesville, said in an interview these findings are consistent with a recent paper that found a correlation between production of SBAs such as DCA and disease remission (ISME J. 2020;14:702-13). Dr. Jobin coauthored a 2018 study that similarly found SBAs have a role in intestinal inflammation (Gastroenterology. 2018;154:1751-63).
“For a long time, secondary bile acid, especially DCA was linked to cell injury, toxicity and even cancer,” he said. “It’s time to rehabilitate this important signaling molecule and recognize its important role in regulating host homeostasis.” The 2018 paper he coauthored showed that injection of DCA into germ-free mice did not promote intestinal pathology. “Actually, DCA was critical in preventing Campylobacter jejune–induced colitis,” he added.
Dr. Sinha received funding from the Crohn’s & Colitis Foundation, Stanford Clinical and Translational Science Award, and Kenneth Rainin Foundation Synergy Award. Coauthors received funding from the National Institutes of Health, the Ann and Bill Swindells Charitable Trust, Leslie and Douglas Ballinger, and the Kenneth Rainin Foundation.
SOURCE: Sinha SR et al. Cell Host Microbe. 2020 Feb 25. doi: 10.1016/j.chom.2020.01.021.
Researchers have found three potential gut mechanisms linked to secondary bile acid (SBA) deficiencies implicated in intestinal inflammation in ulcerative colitis (UC), and reported that supplementation may aid in restoring bile acid levels and potentially treating intestinal inflammation, according to a study published in Cell Host & Microbe.
The study identified lower levels of the following gut components in SBA deficiency in colectomy patients with UC, compared with those with familial adenomatous polyposis (FAP): deoxycholic and lithocholic acids (DCA and LCA), the most abundant gut secondary bile acids (SBAs); expression of the genes needed to convert primary bile acids (PBAs) into SBAs; and the number of Ruminococcaceae, one of the few taxa that include bacteria that generate SBAs.
“Our findings confirm that significant changes in bacterial diversity and composition occur in UC versus FAP pouches,” wrote Sidhartha R. Sinha, MD, of Stanford (Calif.) University, and coauthors. “Notably, our finding of decreased Ruminococcaceae in UC, compared to FAP pouch stool, requires further exploration.” They added that this is the first study to identify Ruminococcaceae as a key contributor to the production of LCA or DCA from PBAs.
The study found average DCA counts of 60,957 in FAP versus 1,593 in UC (P = .002), and average LCA counts of 30,644 and 282.9, respectively (P = .001). The study profiled stools from ileal pouches in colectomy patients who had UC (17) or FAP (7), a noninflammatory disease. “Remarkably, our data identify LCA and DCA to be almost undetectable in UC pouch patients,” Dr. Sinha and coauthors wrote. “This striking finding in patients who underwent colectomy suggests that SBAs may play a role in dysregulated metabolism-induced intestinal inflammation.”
The study found that UC pouches demonstrated less bacterial diversity, or alpha-diversity, than FAP pouches, which is in line with previously reported findings (J Inflamm Res. 2017;10:63-73), and had significantly lower expression of bile-acid inducible genes that encode enzymes that transform PBA to SBA.
The researchers also demonstrated that LCA and DCA supplementation reduced intestinal inflammation in mice with UC.
“Our results show that LCA and DCA treatments caused a remarkable and significant decrease in multiple chemokines and cytokines associated with inflammation, including those often increased in intestinal inflammation,” Dr. Sinha and coauthors wrote. However, they found that LCA had no protective effect on dextran sodium sulfate–induced colitis in mice with TGR5-deficient immune cells. The TGR5 bile acid receptor influences the anti-inflammatory effect in SBA supplementation, the study reported.
The researchers have initiated a clinical study (NCT03724175) to investigate the role of SBAs in patients with pouchitis that doesn’t respond to antibiotic therapy. “Insights from this study will further inform our understanding of the role of SBAs in intestinal inflammation and hold promise to provide an effective treatment,” Dr. Sinha and coauthors wrote.
Christian Jobin, PhD, of the University of Florida, Gainesville, said in an interview these findings are consistent with a recent paper that found a correlation between production of SBAs such as DCA and disease remission (ISME J. 2020;14:702-13). Dr. Jobin coauthored a 2018 study that similarly found SBAs have a role in intestinal inflammation (Gastroenterology. 2018;154:1751-63).
“For a long time, secondary bile acid, especially DCA was linked to cell injury, toxicity and even cancer,” he said. “It’s time to rehabilitate this important signaling molecule and recognize its important role in regulating host homeostasis.” The 2018 paper he coauthored showed that injection of DCA into germ-free mice did not promote intestinal pathology. “Actually, DCA was critical in preventing Campylobacter jejune–induced colitis,” he added.
Dr. Sinha received funding from the Crohn’s & Colitis Foundation, Stanford Clinical and Translational Science Award, and Kenneth Rainin Foundation Synergy Award. Coauthors received funding from the National Institutes of Health, the Ann and Bill Swindells Charitable Trust, Leslie and Douglas Ballinger, and the Kenneth Rainin Foundation.
SOURCE: Sinha SR et al. Cell Host Microbe. 2020 Feb 25. doi: 10.1016/j.chom.2020.01.021.
Researchers have found three potential gut mechanisms linked to secondary bile acid (SBA) deficiencies implicated in intestinal inflammation in ulcerative colitis (UC), and reported that supplementation may aid in restoring bile acid levels and potentially treating intestinal inflammation, according to a study published in Cell Host & Microbe.
The study identified lower levels of the following gut components in SBA deficiency in colectomy patients with UC, compared with those with familial adenomatous polyposis (FAP): deoxycholic and lithocholic acids (DCA and LCA), the most abundant gut secondary bile acids (SBAs); expression of the genes needed to convert primary bile acids (PBAs) into SBAs; and the number of Ruminococcaceae, one of the few taxa that include bacteria that generate SBAs.
“Our findings confirm that significant changes in bacterial diversity and composition occur in UC versus FAP pouches,” wrote Sidhartha R. Sinha, MD, of Stanford (Calif.) University, and coauthors. “Notably, our finding of decreased Ruminococcaceae in UC, compared to FAP pouch stool, requires further exploration.” They added that this is the first study to identify Ruminococcaceae as a key contributor to the production of LCA or DCA from PBAs.
The study found average DCA counts of 60,957 in FAP versus 1,593 in UC (P = .002), and average LCA counts of 30,644 and 282.9, respectively (P = .001). The study profiled stools from ileal pouches in colectomy patients who had UC (17) or FAP (7), a noninflammatory disease. “Remarkably, our data identify LCA and DCA to be almost undetectable in UC pouch patients,” Dr. Sinha and coauthors wrote. “This striking finding in patients who underwent colectomy suggests that SBAs may play a role in dysregulated metabolism-induced intestinal inflammation.”
The study found that UC pouches demonstrated less bacterial diversity, or alpha-diversity, than FAP pouches, which is in line with previously reported findings (J Inflamm Res. 2017;10:63-73), and had significantly lower expression of bile-acid inducible genes that encode enzymes that transform PBA to SBA.
The researchers also demonstrated that LCA and DCA supplementation reduced intestinal inflammation in mice with UC.
“Our results show that LCA and DCA treatments caused a remarkable and significant decrease in multiple chemokines and cytokines associated with inflammation, including those often increased in intestinal inflammation,” Dr. Sinha and coauthors wrote. However, they found that LCA had no protective effect on dextran sodium sulfate–induced colitis in mice with TGR5-deficient immune cells. The TGR5 bile acid receptor influences the anti-inflammatory effect in SBA supplementation, the study reported.
The researchers have initiated a clinical study (NCT03724175) to investigate the role of SBAs in patients with pouchitis that doesn’t respond to antibiotic therapy. “Insights from this study will further inform our understanding of the role of SBAs in intestinal inflammation and hold promise to provide an effective treatment,” Dr. Sinha and coauthors wrote.
Christian Jobin, PhD, of the University of Florida, Gainesville, said in an interview these findings are consistent with a recent paper that found a correlation between production of SBAs such as DCA and disease remission (ISME J. 2020;14:702-13). Dr. Jobin coauthored a 2018 study that similarly found SBAs have a role in intestinal inflammation (Gastroenterology. 2018;154:1751-63).
“For a long time, secondary bile acid, especially DCA was linked to cell injury, toxicity and even cancer,” he said. “It’s time to rehabilitate this important signaling molecule and recognize its important role in regulating host homeostasis.” The 2018 paper he coauthored showed that injection of DCA into germ-free mice did not promote intestinal pathology. “Actually, DCA was critical in preventing Campylobacter jejune–induced colitis,” he added.
Dr. Sinha received funding from the Crohn’s & Colitis Foundation, Stanford Clinical and Translational Science Award, and Kenneth Rainin Foundation Synergy Award. Coauthors received funding from the National Institutes of Health, the Ann and Bill Swindells Charitable Trust, Leslie and Douglas Ballinger, and the Kenneth Rainin Foundation.
SOURCE: Sinha SR et al. Cell Host Microbe. 2020 Feb 25. doi: 10.1016/j.chom.2020.01.021.
FROM CELL HOST & MICROBE
Testing phagocytes might better characterize IBD dysbiosis
For patients with inflammatory bowel disease, 16S ribosomal gene sequencing of lamina propria phagocytes identified microbiota closely associated with inflamed intestinal tissue, according to the results of a pilot study.
This microbiome differed from that of the intestinal mucosa, containing a markedly higher concentration of Proteobacteria, reported Rishu Dheer, PhD, of the University of Miami, and associates. The microbiota also differed between Crohn’s disease and ulcerative colitis, while inflammatory gene expression did not. “The approach used in this study can narrow the spectrum of potentially dysbiotic bacterial populations” in patients with inflammatory bowel disease, the researchers wrote in Cellular and Molecular Gastroenterology and Hepatology.
Recent studies have confirmed intestinal dysbiosis in patients with inflammatory bowel disease, but little is known about disease susceptibility or severity or how microbiota correlate with inflammatory gene expression, the researchers said. They obtained ileal and colonic punch biopsy specimens from 32 patients with inflammatory bowel disease (20 with Crohn’s disease and 12 with ulcerative colitis) and performed 16S ribosomal RNA sequencing of CD11+ phagocytic cells from the lamina propria. They also performed innate immune gene expression profiling. For comparison, they also studied the microbiota of the intestinal mucosa of the same patients.
Compared with mucosal microbiota, the lamina propria microbiota was enriched in Proteobacteria — the “defining phyla” associated with dysbiosis in inflammatory bowel disease, the investigators wrote. Gene profiling revealed extensive functional and metabolic differences between the lamina propria microbiota and the mucosal microbiota, regardless of whether patients had Crohn’s disease or ulcerative colitis.
The microbiota associated with phagocytes was similar in inflamed and uninflamed tissue from the same patients, but it significantly differed between inflamed tissue from patients with Crohn’s disease and inflamed tissue from patients with ulcerative colitis. “These results suggest that the phagocyte-associated microbiota distinguishes Crohn’s disease and ulcerative colitis in the setting of inflammation,” the researchers wrote.
The oncostatin M (OSM) gene, which is part of the IL6 cytokine family of genes, was “highly upregulated” in inflamed CD11b+ cells from the patients, the researchers said. An adjusted analysis did not find statistically significant correlations between specific microbes and inflammatory genes, but clusters of genes were expressed at higher and lower levels in cells from inflamed versus noninflamed tissue, and these gene clusters correlated with specific bacterial genera.
“These results suggest that the variation in the abundance of specific groups of microbiota may affect gene expression levels in host lamina propria phagocyte cell types,” the researchers said. They added that their study method enabled them to “amplify and detect bacteria that are found at very low abundance in the gastrointestinal tract [and that] may participate in initiating or promoting inflammatory bowel disease.”
The study was supported by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Crohn’s & Colitis Foundation of America, Micky & Madeleine Arison Family Foundation Crohn’s & Colitis Discovery Laboratory, and the Martin Kaiser Chair in Gastroenterology at the University of Miami. The senior investigator disclosed ties to Prometheus, Takeda, Pfizer, AbbVie, Janssen, and several other companies. The other researchers reported having no conflicts of interest.
SOURCE: Dheer R et al. Cell Mol Gastroenterol Hepatol. 2020. doi: 10.1016/j.jcmgh.2019.10.013.
Dysbiosis, or pathological changes in the composition or abundance of gut microbiota, has been linked to inflammatory bowel disease in multiple studies, although cause and effect relationships are sometimes difficult to establish. One issue is whether the analysis of the microbiome from stool samples or even whole colonic biopsies is the optimal method to assess its impact of altered bacterial colonization on disease, or whether it might be more informative to analyze the microbiota that are in direct contact with lamina propria phagocytes. Phagocytes, i.e. cells of the innate immune system including macrophages, monocytes, and neutrophils, are the “first responders” to bacteria that invade the ileal or colonic epithelium and thus might be a better reflection of the disease-relevant microbes than stool or whole mucosal specimens commonly analyzed.
Indeed, major differences between phagocyte-associated microbiota and those found in whole biopsy samples were discovered. Importantly, several of the phagocyte-associated phyla, such as Prevotella species, are known to promote Th-17 mediated mucosal inflammation. Thus, it appears that selective invasion of the mucosa by inflammation-promoting bacteria could modify the immune response and thus degrees of progression. In addition, there are striking differences between the phagocyte-associated microbiome in inflamed tissue from ulcerative colitis or Crohn’s patients. Thus, for the first time it appears that microbiota are different between the two diseases in the setting of inflammation. Future research is needed to generalize these findings, and to compare the phagocyte-associated microbiome from IBD patients to that of healthy individuals.
Klaus H. Kaestner, PhD, MS, is an investigator in the department of genetics and Center for Molecular Studies in Digestive and Liver Diseases at the Perelman School of Medicine of the University of Pennsylvania in Philadelphia, codirector of Penn’s Digestive Disease Research Center, and co-Editor-in-Chief of Cellular and Molecular Gastroenterology and Hepatology. He has no conflicts.
Dysbiosis, or pathological changes in the composition or abundance of gut microbiota, has been linked to inflammatory bowel disease in multiple studies, although cause and effect relationships are sometimes difficult to establish. One issue is whether the analysis of the microbiome from stool samples or even whole colonic biopsies is the optimal method to assess its impact of altered bacterial colonization on disease, or whether it might be more informative to analyze the microbiota that are in direct contact with lamina propria phagocytes. Phagocytes, i.e. cells of the innate immune system including macrophages, monocytes, and neutrophils, are the “first responders” to bacteria that invade the ileal or colonic epithelium and thus might be a better reflection of the disease-relevant microbes than stool or whole mucosal specimens commonly analyzed.
Indeed, major differences between phagocyte-associated microbiota and those found in whole biopsy samples were discovered. Importantly, several of the phagocyte-associated phyla, such as Prevotella species, are known to promote Th-17 mediated mucosal inflammation. Thus, it appears that selective invasion of the mucosa by inflammation-promoting bacteria could modify the immune response and thus degrees of progression. In addition, there are striking differences between the phagocyte-associated microbiome in inflamed tissue from ulcerative colitis or Crohn’s patients. Thus, for the first time it appears that microbiota are different between the two diseases in the setting of inflammation. Future research is needed to generalize these findings, and to compare the phagocyte-associated microbiome from IBD patients to that of healthy individuals.
Klaus H. Kaestner, PhD, MS, is an investigator in the department of genetics and Center for Molecular Studies in Digestive and Liver Diseases at the Perelman School of Medicine of the University of Pennsylvania in Philadelphia, codirector of Penn’s Digestive Disease Research Center, and co-Editor-in-Chief of Cellular and Molecular Gastroenterology and Hepatology. He has no conflicts.
Dysbiosis, or pathological changes in the composition or abundance of gut microbiota, has been linked to inflammatory bowel disease in multiple studies, although cause and effect relationships are sometimes difficult to establish. One issue is whether the analysis of the microbiome from stool samples or even whole colonic biopsies is the optimal method to assess its impact of altered bacterial colonization on disease, or whether it might be more informative to analyze the microbiota that are in direct contact with lamina propria phagocytes. Phagocytes, i.e. cells of the innate immune system including macrophages, monocytes, and neutrophils, are the “first responders” to bacteria that invade the ileal or colonic epithelium and thus might be a better reflection of the disease-relevant microbes than stool or whole mucosal specimens commonly analyzed.
Indeed, major differences between phagocyte-associated microbiota and those found in whole biopsy samples were discovered. Importantly, several of the phagocyte-associated phyla, such as Prevotella species, are known to promote Th-17 mediated mucosal inflammation. Thus, it appears that selective invasion of the mucosa by inflammation-promoting bacteria could modify the immune response and thus degrees of progression. In addition, there are striking differences between the phagocyte-associated microbiome in inflamed tissue from ulcerative colitis or Crohn’s patients. Thus, for the first time it appears that microbiota are different between the two diseases in the setting of inflammation. Future research is needed to generalize these findings, and to compare the phagocyte-associated microbiome from IBD patients to that of healthy individuals.
Klaus H. Kaestner, PhD, MS, is an investigator in the department of genetics and Center for Molecular Studies in Digestive and Liver Diseases at the Perelman School of Medicine of the University of Pennsylvania in Philadelphia, codirector of Penn’s Digestive Disease Research Center, and co-Editor-in-Chief of Cellular and Molecular Gastroenterology and Hepatology. He has no conflicts.
For patients with inflammatory bowel disease, 16S ribosomal gene sequencing of lamina propria phagocytes identified microbiota closely associated with inflamed intestinal tissue, according to the results of a pilot study.
This microbiome differed from that of the intestinal mucosa, containing a markedly higher concentration of Proteobacteria, reported Rishu Dheer, PhD, of the University of Miami, and associates. The microbiota also differed between Crohn’s disease and ulcerative colitis, while inflammatory gene expression did not. “The approach used in this study can narrow the spectrum of potentially dysbiotic bacterial populations” in patients with inflammatory bowel disease, the researchers wrote in Cellular and Molecular Gastroenterology and Hepatology.
Recent studies have confirmed intestinal dysbiosis in patients with inflammatory bowel disease, but little is known about disease susceptibility or severity or how microbiota correlate with inflammatory gene expression, the researchers said. They obtained ileal and colonic punch biopsy specimens from 32 patients with inflammatory bowel disease (20 with Crohn’s disease and 12 with ulcerative colitis) and performed 16S ribosomal RNA sequencing of CD11+ phagocytic cells from the lamina propria. They also performed innate immune gene expression profiling. For comparison, they also studied the microbiota of the intestinal mucosa of the same patients.
Compared with mucosal microbiota, the lamina propria microbiota was enriched in Proteobacteria — the “defining phyla” associated with dysbiosis in inflammatory bowel disease, the investigators wrote. Gene profiling revealed extensive functional and metabolic differences between the lamina propria microbiota and the mucosal microbiota, regardless of whether patients had Crohn’s disease or ulcerative colitis.
The microbiota associated with phagocytes was similar in inflamed and uninflamed tissue from the same patients, but it significantly differed between inflamed tissue from patients with Crohn’s disease and inflamed tissue from patients with ulcerative colitis. “These results suggest that the phagocyte-associated microbiota distinguishes Crohn’s disease and ulcerative colitis in the setting of inflammation,” the researchers wrote.
The oncostatin M (OSM) gene, which is part of the IL6 cytokine family of genes, was “highly upregulated” in inflamed CD11b+ cells from the patients, the researchers said. An adjusted analysis did not find statistically significant correlations between specific microbes and inflammatory genes, but clusters of genes were expressed at higher and lower levels in cells from inflamed versus noninflamed tissue, and these gene clusters correlated with specific bacterial genera.
“These results suggest that the variation in the abundance of specific groups of microbiota may affect gene expression levels in host lamina propria phagocyte cell types,” the researchers said. They added that their study method enabled them to “amplify and detect bacteria that are found at very low abundance in the gastrointestinal tract [and that] may participate in initiating or promoting inflammatory bowel disease.”
The study was supported by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Crohn’s & Colitis Foundation of America, Micky & Madeleine Arison Family Foundation Crohn’s & Colitis Discovery Laboratory, and the Martin Kaiser Chair in Gastroenterology at the University of Miami. The senior investigator disclosed ties to Prometheus, Takeda, Pfizer, AbbVie, Janssen, and several other companies. The other researchers reported having no conflicts of interest.
SOURCE: Dheer R et al. Cell Mol Gastroenterol Hepatol. 2020. doi: 10.1016/j.jcmgh.2019.10.013.
For patients with inflammatory bowel disease, 16S ribosomal gene sequencing of lamina propria phagocytes identified microbiota closely associated with inflamed intestinal tissue, according to the results of a pilot study.
This microbiome differed from that of the intestinal mucosa, containing a markedly higher concentration of Proteobacteria, reported Rishu Dheer, PhD, of the University of Miami, and associates. The microbiota also differed between Crohn’s disease and ulcerative colitis, while inflammatory gene expression did not. “The approach used in this study can narrow the spectrum of potentially dysbiotic bacterial populations” in patients with inflammatory bowel disease, the researchers wrote in Cellular and Molecular Gastroenterology and Hepatology.
Recent studies have confirmed intestinal dysbiosis in patients with inflammatory bowel disease, but little is known about disease susceptibility or severity or how microbiota correlate with inflammatory gene expression, the researchers said. They obtained ileal and colonic punch biopsy specimens from 32 patients with inflammatory bowel disease (20 with Crohn’s disease and 12 with ulcerative colitis) and performed 16S ribosomal RNA sequencing of CD11+ phagocytic cells from the lamina propria. They also performed innate immune gene expression profiling. For comparison, they also studied the microbiota of the intestinal mucosa of the same patients.
Compared with mucosal microbiota, the lamina propria microbiota was enriched in Proteobacteria — the “defining phyla” associated with dysbiosis in inflammatory bowel disease, the investigators wrote. Gene profiling revealed extensive functional and metabolic differences between the lamina propria microbiota and the mucosal microbiota, regardless of whether patients had Crohn’s disease or ulcerative colitis.
The microbiota associated with phagocytes was similar in inflamed and uninflamed tissue from the same patients, but it significantly differed between inflamed tissue from patients with Crohn’s disease and inflamed tissue from patients with ulcerative colitis. “These results suggest that the phagocyte-associated microbiota distinguishes Crohn’s disease and ulcerative colitis in the setting of inflammation,” the researchers wrote.
The oncostatin M (OSM) gene, which is part of the IL6 cytokine family of genes, was “highly upregulated” in inflamed CD11b+ cells from the patients, the researchers said. An adjusted analysis did not find statistically significant correlations between specific microbes and inflammatory genes, but clusters of genes were expressed at higher and lower levels in cells from inflamed versus noninflamed tissue, and these gene clusters correlated with specific bacterial genera.
“These results suggest that the variation in the abundance of specific groups of microbiota may affect gene expression levels in host lamina propria phagocyte cell types,” the researchers said. They added that their study method enabled them to “amplify and detect bacteria that are found at very low abundance in the gastrointestinal tract [and that] may participate in initiating or promoting inflammatory bowel disease.”
The study was supported by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Crohn’s & Colitis Foundation of America, Micky & Madeleine Arison Family Foundation Crohn’s & Colitis Discovery Laboratory, and the Martin Kaiser Chair in Gastroenterology at the University of Miami. The senior investigator disclosed ties to Prometheus, Takeda, Pfizer, AbbVie, Janssen, and several other companies. The other researchers reported having no conflicts of interest.
SOURCE: Dheer R et al. Cell Mol Gastroenterol Hepatol. 2020. doi: 10.1016/j.jcmgh.2019.10.013.
FROM CELLULAR AND MOLECULAR GASTROENTEROLOGY AND HEPATOLOGY
Study links CRP, FC monitoring, more remission
AUSTIN, TEX. – A program of frequent monitoring in Crohn’s disease and ulcerative colitis that includes fecal calprotectin (FC) and C-reactive protein (CRP) testing may be cost effective to significantly reduce disease recurrence and hospitalization rates, according to a review of published studies presented at the annual congress of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
“Some data show that calprotectin levels rise months before the onset of symptoms, so it’s my practice that every 3-4 months patients should undergo CRP and calprotectin testing, if they’re willing to do so, while they’re on biologic therapy,” Frank I. Scott, MD, MSCE, of the University of Colorado in Aurora, Denver, said in an interview after the presentation.
Regular monitoring of the two levels makes sense as the practice of tight control of IBD symptoms and treating to target has emerged over the past decade, Dr. Scott said. He noted the 2015 Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) guidelines called for using CRP and FC as adjunctive targets only in symptom assessment (Am J Gastroenterol. 2015:110[9]:1324-58). “I argue that we’ve had a growing body of literature that we should be using these tests regularly as well,” he said.
STRIDE calls for endoscopic assessment 6-9 months after therapy change and consideration of cross-sectional imaging if the small bowel is involved, with assessment every 3 months until symptoms improve and then every 6-12 months thereafter.
However, Dr. Scott noted potential drawbacks to these follow-up steps. “They currently focus on clinical symptoms in the short-term follow-up, and we know from looking at our disease activity indices, such as the CDAI [Crohn’s disease activity index] or Harvey-Bradshaw index, that they don’t always perfectly correlate with actual mucosal healing or resolution of inflammation in Crohn’s or [ulcerative colitis],” he said, pointing to a 2014 study that found CDAI had an area under the curve of 0.57, “which is pretty poor correlation” (Gut. 2014;63[1]:88-95).
Whereas a study of 2,499 patients that showed CRP had an area under the curve of 0.72 and FC of 0.89 (Am J Gastroentrol. 2015;110[6]:802-19). “CRP is a really attractive potential noninvasive marker of inflammation,” he said. “It’s relatively inexpensive, it’s widely available, and the cutoff ranges are well defined.”
He noted four potential drawbacks of CRP: the false-positive rate is relatively high; as a marker of systemic inflammation it’s not specific to the GI tract; false negatives have been well described, with up to 15% of patients not registering a response; and levels can depend on disease location. “Those with isolated ileal disease, for instance, may have relatively low CRP elevations when their disease is active,” Dr. Scott said.
Stool-based FC “represents a potentially more attractive option,” Dr. Scott said. Along with an area under the curve superior to CRP, FC has a documented sensitivity and specificity of 88% and 73%, respectively, versus 49% and 92% for CRP. Drawbacks of fecal calprotectin are that it’s specific to the GI tract but not inflammatory bowel disease, it costs more, and insurance coverage is not as universal as it is for CRP, although more carriers are covering the test, he said.
“However, we do know that through clinical trial data that the use of CRP and FC, in addition to clinical symptom monitoring, does appear to improve care,” Dr. Scott said, noting that the CALM trial of tight disease control through the frequent use of biochemical markers of inflammation with anti–tumor necrosis therapy bore this out (Lancet. 2018;390[10114]:2779-89). “This trial was able to demonstrate at 48 weeks that mucosal healing rates were improved in those receiving CRP and FC monitoring, compared to symptom monitoring alone, with higher rates of steroid-free remission at each visit, which persisted over the follow-up time.”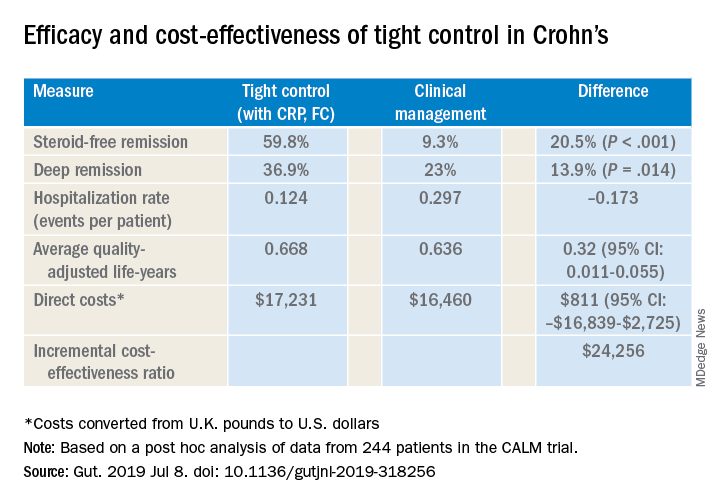
Dr. Scott also cited a post hoc analysis of CALM trial data that validated CRP and FC monitoring to improve steroid-free remission rates and other outcomes (Gut. 2019 Jul 8. doi: 10.1136/gutjnl-2019-318256). That trial reported steroid-free remission rates of 39.3% with clinical management and 59.8% with tight control, a 34% overall difference (P less than .001). “And it was cost effective to incorporate this monitoring at a cost of about $24,300 per quality-adjusted life-year, well below the typically used $50,000 willingness-to-pay threshold when considering new tests,” Dr. Scott said.
Dr. Scott acknowledged that FC testing may pose some inconvenience to patients when collecting their stool samples, but accuracy has improved. “Laboratories are becoming more reliable in terms of what the values are, and the cutoffs are becoming more defined as far as what’s positive and what’s negative, so it’s good way to monitor whether or not patients are at increased risk of a future flare,” he said.
Dr. Scott reported financial relationships with Takeda, Janssen, Merck and PRIME.
SOURCE: Scott FI et al. Crohn’s & Colitis Congress 2020, Session Sp125.
AUSTIN, TEX. – A program of frequent monitoring in Crohn’s disease and ulcerative colitis that includes fecal calprotectin (FC) and C-reactive protein (CRP) testing may be cost effective to significantly reduce disease recurrence and hospitalization rates, according to a review of published studies presented at the annual congress of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
“Some data show that calprotectin levels rise months before the onset of symptoms, so it’s my practice that every 3-4 months patients should undergo CRP and calprotectin testing, if they’re willing to do so, while they’re on biologic therapy,” Frank I. Scott, MD, MSCE, of the University of Colorado in Aurora, Denver, said in an interview after the presentation.
Regular monitoring of the two levels makes sense as the practice of tight control of IBD symptoms and treating to target has emerged over the past decade, Dr. Scott said. He noted the 2015 Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) guidelines called for using CRP and FC as adjunctive targets only in symptom assessment (Am J Gastroenterol. 2015:110[9]:1324-58). “I argue that we’ve had a growing body of literature that we should be using these tests regularly as well,” he said.
STRIDE calls for endoscopic assessment 6-9 months after therapy change and consideration of cross-sectional imaging if the small bowel is involved, with assessment every 3 months until symptoms improve and then every 6-12 months thereafter.
However, Dr. Scott noted potential drawbacks to these follow-up steps. “They currently focus on clinical symptoms in the short-term follow-up, and we know from looking at our disease activity indices, such as the CDAI [Crohn’s disease activity index] or Harvey-Bradshaw index, that they don’t always perfectly correlate with actual mucosal healing or resolution of inflammation in Crohn’s or [ulcerative colitis],” he said, pointing to a 2014 study that found CDAI had an area under the curve of 0.57, “which is pretty poor correlation” (Gut. 2014;63[1]:88-95).
Whereas a study of 2,499 patients that showed CRP had an area under the curve of 0.72 and FC of 0.89 (Am J Gastroentrol. 2015;110[6]:802-19). “CRP is a really attractive potential noninvasive marker of inflammation,” he said. “It’s relatively inexpensive, it’s widely available, and the cutoff ranges are well defined.”
He noted four potential drawbacks of CRP: the false-positive rate is relatively high; as a marker of systemic inflammation it’s not specific to the GI tract; false negatives have been well described, with up to 15% of patients not registering a response; and levels can depend on disease location. “Those with isolated ileal disease, for instance, may have relatively low CRP elevations when their disease is active,” Dr. Scott said.
Stool-based FC “represents a potentially more attractive option,” Dr. Scott said. Along with an area under the curve superior to CRP, FC has a documented sensitivity and specificity of 88% and 73%, respectively, versus 49% and 92% for CRP. Drawbacks of fecal calprotectin are that it’s specific to the GI tract but not inflammatory bowel disease, it costs more, and insurance coverage is not as universal as it is for CRP, although more carriers are covering the test, he said.
“However, we do know that through clinical trial data that the use of CRP and FC, in addition to clinical symptom monitoring, does appear to improve care,” Dr. Scott said, noting that the CALM trial of tight disease control through the frequent use of biochemical markers of inflammation with anti–tumor necrosis therapy bore this out (Lancet. 2018;390[10114]:2779-89). “This trial was able to demonstrate at 48 weeks that mucosal healing rates were improved in those receiving CRP and FC monitoring, compared to symptom monitoring alone, with higher rates of steroid-free remission at each visit, which persisted over the follow-up time.”
Dr. Scott also cited a post hoc analysis of CALM trial data that validated CRP and FC monitoring to improve steroid-free remission rates and other outcomes (Gut. 2019 Jul 8. doi: 10.1136/gutjnl-2019-318256). That trial reported steroid-free remission rates of 39.3% with clinical management and 59.8% with tight control, a 34% overall difference (P less than .001). “And it was cost effective to incorporate this monitoring at a cost of about $24,300 per quality-adjusted life-year, well below the typically used $50,000 willingness-to-pay threshold when considering new tests,” Dr. Scott said.
Dr. Scott acknowledged that FC testing may pose some inconvenience to patients when collecting their stool samples, but accuracy has improved. “Laboratories are becoming more reliable in terms of what the values are, and the cutoffs are becoming more defined as far as what’s positive and what’s negative, so it’s good way to monitor whether or not patients are at increased risk of a future flare,” he said.
Dr. Scott reported financial relationships with Takeda, Janssen, Merck and PRIME.
SOURCE: Scott FI et al. Crohn’s & Colitis Congress 2020, Session Sp125.
AUSTIN, TEX. – A program of frequent monitoring in Crohn’s disease and ulcerative colitis that includes fecal calprotectin (FC) and C-reactive protein (CRP) testing may be cost effective to significantly reduce disease recurrence and hospitalization rates, according to a review of published studies presented at the annual congress of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
“Some data show that calprotectin levels rise months before the onset of symptoms, so it’s my practice that every 3-4 months patients should undergo CRP and calprotectin testing, if they’re willing to do so, while they’re on biologic therapy,” Frank I. Scott, MD, MSCE, of the University of Colorado in Aurora, Denver, said in an interview after the presentation.
Regular monitoring of the two levels makes sense as the practice of tight control of IBD symptoms and treating to target has emerged over the past decade, Dr. Scott said. He noted the 2015 Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) guidelines called for using CRP and FC as adjunctive targets only in symptom assessment (Am J Gastroenterol. 2015:110[9]:1324-58). “I argue that we’ve had a growing body of literature that we should be using these tests regularly as well,” he said.
STRIDE calls for endoscopic assessment 6-9 months after therapy change and consideration of cross-sectional imaging if the small bowel is involved, with assessment every 3 months until symptoms improve and then every 6-12 months thereafter.
However, Dr. Scott noted potential drawbacks to these follow-up steps. “They currently focus on clinical symptoms in the short-term follow-up, and we know from looking at our disease activity indices, such as the CDAI [Crohn’s disease activity index] or Harvey-Bradshaw index, that they don’t always perfectly correlate with actual mucosal healing or resolution of inflammation in Crohn’s or [ulcerative colitis],” he said, pointing to a 2014 study that found CDAI had an area under the curve of 0.57, “which is pretty poor correlation” (Gut. 2014;63[1]:88-95).
Whereas a study of 2,499 patients that showed CRP had an area under the curve of 0.72 and FC of 0.89 (Am J Gastroentrol. 2015;110[6]:802-19). “CRP is a really attractive potential noninvasive marker of inflammation,” he said. “It’s relatively inexpensive, it’s widely available, and the cutoff ranges are well defined.”
He noted four potential drawbacks of CRP: the false-positive rate is relatively high; as a marker of systemic inflammation it’s not specific to the GI tract; false negatives have been well described, with up to 15% of patients not registering a response; and levels can depend on disease location. “Those with isolated ileal disease, for instance, may have relatively low CRP elevations when their disease is active,” Dr. Scott said.
Stool-based FC “represents a potentially more attractive option,” Dr. Scott said. Along with an area under the curve superior to CRP, FC has a documented sensitivity and specificity of 88% and 73%, respectively, versus 49% and 92% for CRP. Drawbacks of fecal calprotectin are that it’s specific to the GI tract but not inflammatory bowel disease, it costs more, and insurance coverage is not as universal as it is for CRP, although more carriers are covering the test, he said.
“However, we do know that through clinical trial data that the use of CRP and FC, in addition to clinical symptom monitoring, does appear to improve care,” Dr. Scott said, noting that the CALM trial of tight disease control through the frequent use of biochemical markers of inflammation with anti–tumor necrosis therapy bore this out (Lancet. 2018;390[10114]:2779-89). “This trial was able to demonstrate at 48 weeks that mucosal healing rates were improved in those receiving CRP and FC monitoring, compared to symptom monitoring alone, with higher rates of steroid-free remission at each visit, which persisted over the follow-up time.”
Dr. Scott also cited a post hoc analysis of CALM trial data that validated CRP and FC monitoring to improve steroid-free remission rates and other outcomes (Gut. 2019 Jul 8. doi: 10.1136/gutjnl-2019-318256). That trial reported steroid-free remission rates of 39.3% with clinical management and 59.8% with tight control, a 34% overall difference (P less than .001). “And it was cost effective to incorporate this monitoring at a cost of about $24,300 per quality-adjusted life-year, well below the typically used $50,000 willingness-to-pay threshold when considering new tests,” Dr. Scott said.
Dr. Scott acknowledged that FC testing may pose some inconvenience to patients when collecting their stool samples, but accuracy has improved. “Laboratories are becoming more reliable in terms of what the values are, and the cutoffs are becoming more defined as far as what’s positive and what’s negative, so it’s good way to monitor whether or not patients are at increased risk of a future flare,” he said.
Dr. Scott reported financial relationships with Takeda, Janssen, Merck and PRIME.
SOURCE: Scott FI et al. Crohn’s & Colitis Congress 2020, Session Sp125.
REPORTING FROM CROHN’S & COLITIS CONGRESS
Sometimes medication is enough for a Crohn’s abscess
MAUI, HAWAII – If an intra-abdominal abscess in a recently diagnosed Crohn’s disease patient is less than 6 cm across with no downstream stenosis, involves only a short segment of bowel, and the patient has no perianal disease, then infliximab and azathioprine after drainage and antibiotics might be enough to heal it, according to Miguel Regueiro, MD, chair of the department of gastroenterology, hepatology, and nutrition at the Cleveland Clinic.
That will work in about 30% of patients who hit the mark; the rest will eventually need surgery, said Dr. Regueiro, a clinical researcher who has worked extensively with surgical GI patients and is also a coauthor on the American College of Gastroenterology 2018 Crohn’s disease guidelines (Am J Gastroenterol. 2018 Apr;113[4]:481-517).
Intra-abdominal abscesses are common in Crohn’s, usually from an inflammation-induced fistula or sinus in the small intestines that spills luminal contents into the abdominal cavity. Drainage and antibiotics are first line, but then there’s the question of who needs to go to the operating room and who doesn’t.
It has to do with “how much the hole in the intestines is actually reversible. Evidence of a stricture is of paramount importance. If you have a stricture below a fistula and prestenotic dilatation, that’s a high-pressure zone.” It’s a “fixed complication that, in my opinion, no medication is ever going to treat,” he said at the Gastroenterology Updates, IBD, Liver Disease Conference.
“Infliximab is still probably the best medicine for fistulizing disease,” so Dr. Regueiro opts for that if patients haven’t been on it before, in combination with an immunomodulator, generally azathioprine at half the standard dose, to prevent patients from forming antibodies to the infliximab.
When patients do go to the operating room, there is a good chance they will end up with a temporary ostomy, and definitely so if the abscess can’t be drained completely to prevent spillage. The risk of dehiscence and other complications is too great for primary anastomosis.
“I mentally prepare my patients for that; I tell them up front. I never guarantee that they are not going to have an ostomy bag,” Dr. Regueiro said.
He also said abscess formation isn’t necessarily a sign the biologic patients were on before has failed, especially if they were only on it for 6 months or so. More likely, “the disease was too far gone at that point” for short-term treatment to have much of an effect.
So he’s often likely to continue patients on the same biologic after surgery. “We’ve done a lot of study on” this and have “actually found that” patients do well with the approach. He will switch treatment, however, if they otherwise no longer seem to respond to a biologic they have been taking a while, despite adequate serum levels.
There’s no need to delay surgery for patients on biologics. “If they get a biologic the day before, they can still go to the [operating room]. We are not seeing increased postop complications, infections, or wound dehiscence,” he said.
Dr. Regueiro generally restarts biologics 2-4 weeks after surgery, which is enough time to know if there is going to be a surgical complication but not so long that patients will have a Crohn’s relapse. He restarts the maintenance dose, as “it’s not necessary to reinduct patients after such a short break,” he said.
He also noted that opioids and steroids should be avoided with Crohn’s abscesses. Opioids increase the risk of ileus, and steroids the risk of sepsis.
Dr. Regueiro reported no relevant disclosures.
MAUI, HAWAII – If an intra-abdominal abscess in a recently diagnosed Crohn’s disease patient is less than 6 cm across with no downstream stenosis, involves only a short segment of bowel, and the patient has no perianal disease, then infliximab and azathioprine after drainage and antibiotics might be enough to heal it, according to Miguel Regueiro, MD, chair of the department of gastroenterology, hepatology, and nutrition at the Cleveland Clinic.
That will work in about 30% of patients who hit the mark; the rest will eventually need surgery, said Dr. Regueiro, a clinical researcher who has worked extensively with surgical GI patients and is also a coauthor on the American College of Gastroenterology 2018 Crohn’s disease guidelines (Am J Gastroenterol. 2018 Apr;113[4]:481-517).
Intra-abdominal abscesses are common in Crohn’s, usually from an inflammation-induced fistula or sinus in the small intestines that spills luminal contents into the abdominal cavity. Drainage and antibiotics are first line, but then there’s the question of who needs to go to the operating room and who doesn’t.
It has to do with “how much the hole in the intestines is actually reversible. Evidence of a stricture is of paramount importance. If you have a stricture below a fistula and prestenotic dilatation, that’s a high-pressure zone.” It’s a “fixed complication that, in my opinion, no medication is ever going to treat,” he said at the Gastroenterology Updates, IBD, Liver Disease Conference.
“Infliximab is still probably the best medicine for fistulizing disease,” so Dr. Regueiro opts for that if patients haven’t been on it before, in combination with an immunomodulator, generally azathioprine at half the standard dose, to prevent patients from forming antibodies to the infliximab.
When patients do go to the operating room, there is a good chance they will end up with a temporary ostomy, and definitely so if the abscess can’t be drained completely to prevent spillage. The risk of dehiscence and other complications is too great for primary anastomosis.
“I mentally prepare my patients for that; I tell them up front. I never guarantee that they are not going to have an ostomy bag,” Dr. Regueiro said.
He also said abscess formation isn’t necessarily a sign the biologic patients were on before has failed, especially if they were only on it for 6 months or so. More likely, “the disease was too far gone at that point” for short-term treatment to have much of an effect.
So he’s often likely to continue patients on the same biologic after surgery. “We’ve done a lot of study on” this and have “actually found that” patients do well with the approach. He will switch treatment, however, if they otherwise no longer seem to respond to a biologic they have been taking a while, despite adequate serum levels.
There’s no need to delay surgery for patients on biologics. “If they get a biologic the day before, they can still go to the [operating room]. We are not seeing increased postop complications, infections, or wound dehiscence,” he said.
Dr. Regueiro generally restarts biologics 2-4 weeks after surgery, which is enough time to know if there is going to be a surgical complication but not so long that patients will have a Crohn’s relapse. He restarts the maintenance dose, as “it’s not necessary to reinduct patients after such a short break,” he said.
He also noted that opioids and steroids should be avoided with Crohn’s abscesses. Opioids increase the risk of ileus, and steroids the risk of sepsis.
Dr. Regueiro reported no relevant disclosures.
MAUI, HAWAII – If an intra-abdominal abscess in a recently diagnosed Crohn’s disease patient is less than 6 cm across with no downstream stenosis, involves only a short segment of bowel, and the patient has no perianal disease, then infliximab and azathioprine after drainage and antibiotics might be enough to heal it, according to Miguel Regueiro, MD, chair of the department of gastroenterology, hepatology, and nutrition at the Cleveland Clinic.
That will work in about 30% of patients who hit the mark; the rest will eventually need surgery, said Dr. Regueiro, a clinical researcher who has worked extensively with surgical GI patients and is also a coauthor on the American College of Gastroenterology 2018 Crohn’s disease guidelines (Am J Gastroenterol. 2018 Apr;113[4]:481-517).
Intra-abdominal abscesses are common in Crohn’s, usually from an inflammation-induced fistula or sinus in the small intestines that spills luminal contents into the abdominal cavity. Drainage and antibiotics are first line, but then there’s the question of who needs to go to the operating room and who doesn’t.
It has to do with “how much the hole in the intestines is actually reversible. Evidence of a stricture is of paramount importance. If you have a stricture below a fistula and prestenotic dilatation, that’s a high-pressure zone.” It’s a “fixed complication that, in my opinion, no medication is ever going to treat,” he said at the Gastroenterology Updates, IBD, Liver Disease Conference.
“Infliximab is still probably the best medicine for fistulizing disease,” so Dr. Regueiro opts for that if patients haven’t been on it before, in combination with an immunomodulator, generally azathioprine at half the standard dose, to prevent patients from forming antibodies to the infliximab.
When patients do go to the operating room, there is a good chance they will end up with a temporary ostomy, and definitely so if the abscess can’t be drained completely to prevent spillage. The risk of dehiscence and other complications is too great for primary anastomosis.
“I mentally prepare my patients for that; I tell them up front. I never guarantee that they are not going to have an ostomy bag,” Dr. Regueiro said.
He also said abscess formation isn’t necessarily a sign the biologic patients were on before has failed, especially if they were only on it for 6 months or so. More likely, “the disease was too far gone at that point” for short-term treatment to have much of an effect.
So he’s often likely to continue patients on the same biologic after surgery. “We’ve done a lot of study on” this and have “actually found that” patients do well with the approach. He will switch treatment, however, if they otherwise no longer seem to respond to a biologic they have been taking a while, despite adequate serum levels.
There’s no need to delay surgery for patients on biologics. “If they get a biologic the day before, they can still go to the [operating room]. We are not seeing increased postop complications, infections, or wound dehiscence,” he said.
Dr. Regueiro generally restarts biologics 2-4 weeks after surgery, which is enough time to know if there is going to be a surgical complication but not so long that patients will have a Crohn’s relapse. He restarts the maintenance dose, as “it’s not necessary to reinduct patients after such a short break,” he said.
He also noted that opioids and steroids should be avoided with Crohn’s abscesses. Opioids increase the risk of ileus, and steroids the risk of sepsis.
Dr. Regueiro reported no relevant disclosures.
EXPERT ANALYSIS FROM GUILD 2020
IBD fertility has improved
AUSTIN, TEX. – Patients with inflammatory bowel disease (IBD) who want to have children can benefit from better education about recent findings that disease control, laparoscopic surgery, and in vitro fertilization (IVF) have improved their chances of conceiving, according to a review of published reports presented here at the Crohn’s & Colitis Congress, a partnership of the Crohn’s & Colitis Congress Foundation and the American Gastroenterological Association.
“Decreased fertility in IBD is due to voluntary childlessness, which we can change with education; surgery for IBD, which we can improve with laparoscopic surgery; and increased disease activity, which we can also make a difference in,” Sonia Friedman, MD, of Harvard Medical School, Boston, said in an interview.
Dr. Friedman and coauthors last year published an analysis of the Danish National Birth Cohort, which showed women with IBD had an 28% greater relative risk of taking a year or more to get pregnant than controls without IBD, and that the relative risk was even higher in women with Crohn’s disease — 54% (Clin Gastroenterol Hepatol. 2019. doi: 10.1016/j.cgh.2019.08.031). “We found that women with Crohn’s surgery had decreased fertility by 2.54 times greater relative risk,” she said.
“Fertility, pregnancy is the most important thing to patients,” Dr. Friedman said in an interview. “That’s what people ask me about the most. In the population of IBD patients, the onset is age 15-35, and these people are in the prime of their reproductive years.” Sexual function, known to be decreased in men and women with IBD, is also an overriding concern in these patients, she said. “There needs to be a lot more information out there about it.”
She said gastroenterologists should keep in mind that much of the evidence documenting reduced fertility after ileo-pouch anal anastomosis is dated and focused on open surgery, which caused profound scarring of the pelvis and fallopian tubes, thus hindering conception. Laparoscopic ileoanal J-pouch surgery (IPAA) has yielded much improved outcomes in women of child-bearing age, she said, citing a study late last year that reported women who had laparoscopic IPAA had a median time to pregnancy of 3.5 months versus 9 months for women who had open IPAA (Surgery. 2019;166:670-7).
“It’s really important to discuss the issues of fertility, especially for patients contemplating surgery,” Dr. Friedman said. “Emphasize that there are good outcomes with laparoscopic surgery, and they can have assisted reproductive technology [ART], or in vitro fertilization, if needed. Never withhold surgery based on fear of infertility.”
Her practice is to refer women with IBD in remission for IVF if they’ve tried to get pregnant every month for a year or more and to refer women with IBD surgery for IVF after trying to get pregnant for 6 months. Dr. Friedman coauthored two studies of the Danish National Birth Cohort of ART in women with Crohn’s disease and ulcerative colitis (UC) along with controls (Gut. 2016;65:767-76; Gut. 2017;66:556-58). “We found that women with Crohn’s and UC had a decreased chance of having a clinical pregnancy, but they had no problem carrying the pregnancy to term,” she said.
Those findings raised questions about the etiology of decreased fertility in IBD patients, which could include factors such as IVF technique, reproductive hormone and microbiome changes, or IBD medications. “How can we carry that forward to all women with IBD?” she said. Women with IBD have less chance of conceiving with each IVF treatment cycle than do women without IBD, she said. “The most interesting thing is that the reduced chance of live birth after IVF treatment in Crohn’s and UC is related to the stages of implantation and not to the ability to maintain the fetus throughout pregnancy,” she said.
Dr. Friedman has no financial relationships to disclose.
The AGA IBD Parenthood Project can help guide your patients with IBD throughout their pregnancy, from trying to conceive through postpartum care. Learn more at IBDParenthoodProject.org.
SOURCE: Friedman S. Crohn’s & Colitis Congress, Session Sp86.
AUSTIN, TEX. – Patients with inflammatory bowel disease (IBD) who want to have children can benefit from better education about recent findings that disease control, laparoscopic surgery, and in vitro fertilization (IVF) have improved their chances of conceiving, according to a review of published reports presented here at the Crohn’s & Colitis Congress, a partnership of the Crohn’s & Colitis Congress Foundation and the American Gastroenterological Association.
“Decreased fertility in IBD is due to voluntary childlessness, which we can change with education; surgery for IBD, which we can improve with laparoscopic surgery; and increased disease activity, which we can also make a difference in,” Sonia Friedman, MD, of Harvard Medical School, Boston, said in an interview.
Dr. Friedman and coauthors last year published an analysis of the Danish National Birth Cohort, which showed women with IBD had an 28% greater relative risk of taking a year or more to get pregnant than controls without IBD, and that the relative risk was even higher in women with Crohn’s disease — 54% (Clin Gastroenterol Hepatol. 2019. doi: 10.1016/j.cgh.2019.08.031). “We found that women with Crohn’s surgery had decreased fertility by 2.54 times greater relative risk,” she said.
“Fertility, pregnancy is the most important thing to patients,” Dr. Friedman said in an interview. “That’s what people ask me about the most. In the population of IBD patients, the onset is age 15-35, and these people are in the prime of their reproductive years.” Sexual function, known to be decreased in men and women with IBD, is also an overriding concern in these patients, she said. “There needs to be a lot more information out there about it.”
She said gastroenterologists should keep in mind that much of the evidence documenting reduced fertility after ileo-pouch anal anastomosis is dated and focused on open surgery, which caused profound scarring of the pelvis and fallopian tubes, thus hindering conception. Laparoscopic ileoanal J-pouch surgery (IPAA) has yielded much improved outcomes in women of child-bearing age, she said, citing a study late last year that reported women who had laparoscopic IPAA had a median time to pregnancy of 3.5 months versus 9 months for women who had open IPAA (Surgery. 2019;166:670-7).
“It’s really important to discuss the issues of fertility, especially for patients contemplating surgery,” Dr. Friedman said. “Emphasize that there are good outcomes with laparoscopic surgery, and they can have assisted reproductive technology [ART], or in vitro fertilization, if needed. Never withhold surgery based on fear of infertility.”
Her practice is to refer women with IBD in remission for IVF if they’ve tried to get pregnant every month for a year or more and to refer women with IBD surgery for IVF after trying to get pregnant for 6 months. Dr. Friedman coauthored two studies of the Danish National Birth Cohort of ART in women with Crohn’s disease and ulcerative colitis (UC) along with controls (Gut. 2016;65:767-76; Gut. 2017;66:556-58). “We found that women with Crohn’s and UC had a decreased chance of having a clinical pregnancy, but they had no problem carrying the pregnancy to term,” she said.
Those findings raised questions about the etiology of decreased fertility in IBD patients, which could include factors such as IVF technique, reproductive hormone and microbiome changes, or IBD medications. “How can we carry that forward to all women with IBD?” she said. Women with IBD have less chance of conceiving with each IVF treatment cycle than do women without IBD, she said. “The most interesting thing is that the reduced chance of live birth after IVF treatment in Crohn’s and UC is related to the stages of implantation and not to the ability to maintain the fetus throughout pregnancy,” she said.
Dr. Friedman has no financial relationships to disclose.
The AGA IBD Parenthood Project can help guide your patients with IBD throughout their pregnancy, from trying to conceive through postpartum care. Learn more at IBDParenthoodProject.org.
SOURCE: Friedman S. Crohn’s & Colitis Congress, Session Sp86.
AUSTIN, TEX. – Patients with inflammatory bowel disease (IBD) who want to have children can benefit from better education about recent findings that disease control, laparoscopic surgery, and in vitro fertilization (IVF) have improved their chances of conceiving, according to a review of published reports presented here at the Crohn’s & Colitis Congress, a partnership of the Crohn’s & Colitis Congress Foundation and the American Gastroenterological Association.
“Decreased fertility in IBD is due to voluntary childlessness, which we can change with education; surgery for IBD, which we can improve with laparoscopic surgery; and increased disease activity, which we can also make a difference in,” Sonia Friedman, MD, of Harvard Medical School, Boston, said in an interview.
Dr. Friedman and coauthors last year published an analysis of the Danish National Birth Cohort, which showed women with IBD had an 28% greater relative risk of taking a year or more to get pregnant than controls without IBD, and that the relative risk was even higher in women with Crohn’s disease — 54% (Clin Gastroenterol Hepatol. 2019. doi: 10.1016/j.cgh.2019.08.031). “We found that women with Crohn’s surgery had decreased fertility by 2.54 times greater relative risk,” she said.
“Fertility, pregnancy is the most important thing to patients,” Dr. Friedman said in an interview. “That’s what people ask me about the most. In the population of IBD patients, the onset is age 15-35, and these people are in the prime of their reproductive years.” Sexual function, known to be decreased in men and women with IBD, is also an overriding concern in these patients, she said. “There needs to be a lot more information out there about it.”
She said gastroenterologists should keep in mind that much of the evidence documenting reduced fertility after ileo-pouch anal anastomosis is dated and focused on open surgery, which caused profound scarring of the pelvis and fallopian tubes, thus hindering conception. Laparoscopic ileoanal J-pouch surgery (IPAA) has yielded much improved outcomes in women of child-bearing age, she said, citing a study late last year that reported women who had laparoscopic IPAA had a median time to pregnancy of 3.5 months versus 9 months for women who had open IPAA (Surgery. 2019;166:670-7).
“It’s really important to discuss the issues of fertility, especially for patients contemplating surgery,” Dr. Friedman said. “Emphasize that there are good outcomes with laparoscopic surgery, and they can have assisted reproductive technology [ART], or in vitro fertilization, if needed. Never withhold surgery based on fear of infertility.”
Her practice is to refer women with IBD in remission for IVF if they’ve tried to get pregnant every month for a year or more and to refer women with IBD surgery for IVF after trying to get pregnant for 6 months. Dr. Friedman coauthored two studies of the Danish National Birth Cohort of ART in women with Crohn’s disease and ulcerative colitis (UC) along with controls (Gut. 2016;65:767-76; Gut. 2017;66:556-58). “We found that women with Crohn’s and UC had a decreased chance of having a clinical pregnancy, but they had no problem carrying the pregnancy to term,” she said.
Those findings raised questions about the etiology of decreased fertility in IBD patients, which could include factors such as IVF technique, reproductive hormone and microbiome changes, or IBD medications. “How can we carry that forward to all women with IBD?” she said. Women with IBD have less chance of conceiving with each IVF treatment cycle than do women without IBD, she said. “The most interesting thing is that the reduced chance of live birth after IVF treatment in Crohn’s and UC is related to the stages of implantation and not to the ability to maintain the fetus throughout pregnancy,” she said.
Dr. Friedman has no financial relationships to disclose.
The AGA IBD Parenthood Project can help guide your patients with IBD throughout their pregnancy, from trying to conceive through postpartum care. Learn more at IBDParenthoodProject.org.
SOURCE: Friedman S. Crohn’s & Colitis Congress, Session Sp86.
REPORTING FROM CROHN’S & COLITIS CONGRESS
IBD quality initiative slashes ED utilization
AUSTIN, TEX. – A quality improvement initiative aimed at patients with inflammatory bowel disease (IBD) has reduced emergency department visits and hospitalizations by 20% or more and slashed opioid use by half, according to study results presented at the Crohn’s & Colitis Congress®, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
After 15 months, the quality improvement program saw emergency department visit rates decline from 18% to 14%, a 22% relative decrease, Gil Y. Melmed, MD, of Cedars-Sinai Medical Center, Los Angeles, said. Additionally, the study documented a similar decrease in the rate of hospitalization, declining from 14% to 11%, while opioid utilization rates declined from 8% to 4%. “We also found decreases in special-cause variation in other measures of interest, including CT scan utilization as well as corticosteroid use, which was reduced 29% during the course of the program,” he said.
The quality initiative was conducted through the Crohn’s & Colitis Foundation as an outgrowth of its IBD Qorus quality improvement program. The 15-month study involved 20,392 patient visits at 15 academic and 11 private/community practices from January 2018 to April 2019. “This specific project within Qorus is focused specifically around the concept of improving access during times of urgent care need,” Dr. Melmed told this news organization. The goal was to identify practice changes that can drive improvement.
The intervention consisted of 19 different strategies, called a “Change Package,” and participating sites could choose to test and implement one or more of them, Dr. Melmed said. Some examples included designating urgent care slots in the clinic schedule, installing a nurse hotline, a weekly “huddle” to review high-risk patients, and patient education on using urgent care.
One of the drivers of the program was to provide immediate care improvement to patients, Dr. Melmed said in the interview. “As opposed to investments into the cure of IBD that we need, but which can take years to develop, this research has immediate, practical applicability for patients today,” he said.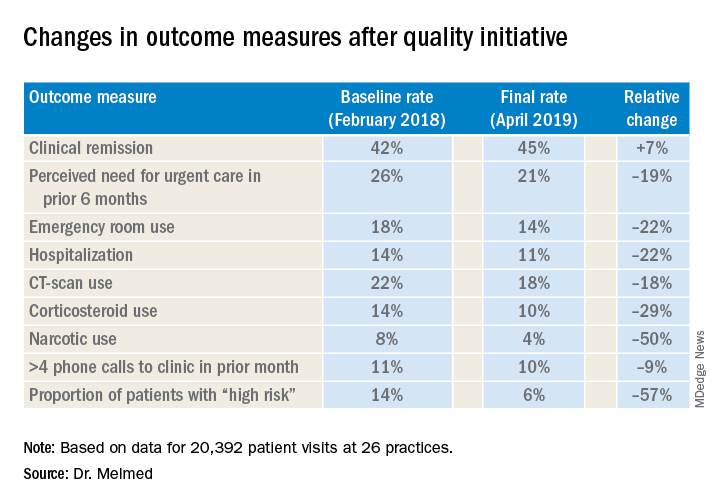
“The fact that we were able to demonstrate reduction in emergency room utilization and hospitalization, steroid use, and narcotic use has really energized the work that we were doing. We can now show that very-low-cost process changes at a site level lead to robust improvement in patient outcomes. These changes are potentially implementable in any practice setting,” Dr. Melmed said in the interview.
After Dr. Melmed’s presentation, Maria T. Abreu, MD, director of the Crohn’s and Colitis Center at the University of Miami, asked about the cost of the interventions. Dr. Melmed said the costs were nominal, such as paying for a new phone line for a patient hotline. “But overall the cost really involved in the program was the time that it took to review the high-risk list on a weekly basis with the team, and that is essentially a 15-minute huddle,” he said.
Later, Dr. Abreu said in an interview that the program was “a terrific example of how measuring outcomes and sharing ideas can make huge impacts in the lives of patients.” She added, “An enormous amount of money is spent on clinical trials of expensive biologics which have revolutionized treatment, yet the humanistic aspects of our care have just as great of an impact. In this study, each center focused on ways they could lower ER visits and hospitalizations. One size did not fit all, yet they could learn from each other. The very platform they used to conduct the study is a model for all of us.”
Corey A. Siegel, MD, of the Dartmouth-Hitchcock Medical Center, Lebanon, N.H., and Dr. Melmed's coprincipal investigator on Qorus, said the quality initiative now includes 49 GI practices across the country with plans to grow to 60 by the end of the year. "We have created this 'collaboratory' for providers from actross the country to work togetherr to learn how to best deliver high-qulaity care for patients with IBD," he said.
Another feature of the quality initiative allowed participating sites to see how they compared with others anonymously, Dr. Melmed said. “Using the data, we called out high-performing sites to teach the rest of us what they were doing that enabled them to improve, so that all of us could learn from their successes,” he said.
The researchers are aiming to evaluate costs and identify the most successful interventions, with the plan to present the latter at Digestive Disease Week® 2020 and use them to develop a toolkit practices can use. “Ultimately,” said Dr. Melmed, “this is scalable.”
Dr. Melmed disclosed financial relationships with AbbVie, Boehringer-Ingelheim, Celgene, Jannsen, GSK, Medtronic, Pfizer, Samsung Bioepis, Takeda, and Techlab; IBD Qorus receives support from Abbvie, AMAG, Helmsley Charitable Trust, Janssen, Nephoroceuticals, Pfizer, Takeda, and UCB.
SOURCE: Melmed GT et al. Crohn’s & Colitis Congress 2020, Session 28.
AUSTIN, TEX. – A quality improvement initiative aimed at patients with inflammatory bowel disease (IBD) has reduced emergency department visits and hospitalizations by 20% or more and slashed opioid use by half, according to study results presented at the Crohn’s & Colitis Congress®, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
After 15 months, the quality improvement program saw emergency department visit rates decline from 18% to 14%, a 22% relative decrease, Gil Y. Melmed, MD, of Cedars-Sinai Medical Center, Los Angeles, said. Additionally, the study documented a similar decrease in the rate of hospitalization, declining from 14% to 11%, while opioid utilization rates declined from 8% to 4%. “We also found decreases in special-cause variation in other measures of interest, including CT scan utilization as well as corticosteroid use, which was reduced 29% during the course of the program,” he said.
The quality initiative was conducted through the Crohn’s & Colitis Foundation as an outgrowth of its IBD Qorus quality improvement program. The 15-month study involved 20,392 patient visits at 15 academic and 11 private/community practices from January 2018 to April 2019. “This specific project within Qorus is focused specifically around the concept of improving access during times of urgent care need,” Dr. Melmed told this news organization. The goal was to identify practice changes that can drive improvement.
The intervention consisted of 19 different strategies, called a “Change Package,” and participating sites could choose to test and implement one or more of them, Dr. Melmed said. Some examples included designating urgent care slots in the clinic schedule, installing a nurse hotline, a weekly “huddle” to review high-risk patients, and patient education on using urgent care.
One of the drivers of the program was to provide immediate care improvement to patients, Dr. Melmed said in the interview. “As opposed to investments into the cure of IBD that we need, but which can take years to develop, this research has immediate, practical applicability for patients today,” he said.
“The fact that we were able to demonstrate reduction in emergency room utilization and hospitalization, steroid use, and narcotic use has really energized the work that we were doing. We can now show that very-low-cost process changes at a site level lead to robust improvement in patient outcomes. These changes are potentially implementable in any practice setting,” Dr. Melmed said in the interview.
After Dr. Melmed’s presentation, Maria T. Abreu, MD, director of the Crohn’s and Colitis Center at the University of Miami, asked about the cost of the interventions. Dr. Melmed said the costs were nominal, such as paying for a new phone line for a patient hotline. “But overall the cost really involved in the program was the time that it took to review the high-risk list on a weekly basis with the team, and that is essentially a 15-minute huddle,” he said.
Later, Dr. Abreu said in an interview that the program was “a terrific example of how measuring outcomes and sharing ideas can make huge impacts in the lives of patients.” She added, “An enormous amount of money is spent on clinical trials of expensive biologics which have revolutionized treatment, yet the humanistic aspects of our care have just as great of an impact. In this study, each center focused on ways they could lower ER visits and hospitalizations. One size did not fit all, yet they could learn from each other. The very platform they used to conduct the study is a model for all of us.”
Corey A. Siegel, MD, of the Dartmouth-Hitchcock Medical Center, Lebanon, N.H., and Dr. Melmed's coprincipal investigator on Qorus, said the quality initiative now includes 49 GI practices across the country with plans to grow to 60 by the end of the year. "We have created this 'collaboratory' for providers from actross the country to work togetherr to learn how to best deliver high-qulaity care for patients with IBD," he said.
Another feature of the quality initiative allowed participating sites to see how they compared with others anonymously, Dr. Melmed said. “Using the data, we called out high-performing sites to teach the rest of us what they were doing that enabled them to improve, so that all of us could learn from their successes,” he said.
The researchers are aiming to evaluate costs and identify the most successful interventions, with the plan to present the latter at Digestive Disease Week® 2020 and use them to develop a toolkit practices can use. “Ultimately,” said Dr. Melmed, “this is scalable.”
Dr. Melmed disclosed financial relationships with AbbVie, Boehringer-Ingelheim, Celgene, Jannsen, GSK, Medtronic, Pfizer, Samsung Bioepis, Takeda, and Techlab; IBD Qorus receives support from Abbvie, AMAG, Helmsley Charitable Trust, Janssen, Nephoroceuticals, Pfizer, Takeda, and UCB.
SOURCE: Melmed GT et al. Crohn’s & Colitis Congress 2020, Session 28.
AUSTIN, TEX. – A quality improvement initiative aimed at patients with inflammatory bowel disease (IBD) has reduced emergency department visits and hospitalizations by 20% or more and slashed opioid use by half, according to study results presented at the Crohn’s & Colitis Congress®, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
After 15 months, the quality improvement program saw emergency department visit rates decline from 18% to 14%, a 22% relative decrease, Gil Y. Melmed, MD, of Cedars-Sinai Medical Center, Los Angeles, said. Additionally, the study documented a similar decrease in the rate of hospitalization, declining from 14% to 11%, while opioid utilization rates declined from 8% to 4%. “We also found decreases in special-cause variation in other measures of interest, including CT scan utilization as well as corticosteroid use, which was reduced 29% during the course of the program,” he said.
The quality initiative was conducted through the Crohn’s & Colitis Foundation as an outgrowth of its IBD Qorus quality improvement program. The 15-month study involved 20,392 patient visits at 15 academic and 11 private/community practices from January 2018 to April 2019. “This specific project within Qorus is focused specifically around the concept of improving access during times of urgent care need,” Dr. Melmed told this news organization. The goal was to identify practice changes that can drive improvement.
The intervention consisted of 19 different strategies, called a “Change Package,” and participating sites could choose to test and implement one or more of them, Dr. Melmed said. Some examples included designating urgent care slots in the clinic schedule, installing a nurse hotline, a weekly “huddle” to review high-risk patients, and patient education on using urgent care.
One of the drivers of the program was to provide immediate care improvement to patients, Dr. Melmed said in the interview. “As opposed to investments into the cure of IBD that we need, but which can take years to develop, this research has immediate, practical applicability for patients today,” he said.
“The fact that we were able to demonstrate reduction in emergency room utilization and hospitalization, steroid use, and narcotic use has really energized the work that we were doing. We can now show that very-low-cost process changes at a site level lead to robust improvement in patient outcomes. These changes are potentially implementable in any practice setting,” Dr. Melmed said in the interview.
After Dr. Melmed’s presentation, Maria T. Abreu, MD, director of the Crohn’s and Colitis Center at the University of Miami, asked about the cost of the interventions. Dr. Melmed said the costs were nominal, such as paying for a new phone line for a patient hotline. “But overall the cost really involved in the program was the time that it took to review the high-risk list on a weekly basis with the team, and that is essentially a 15-minute huddle,” he said.
Later, Dr. Abreu said in an interview that the program was “a terrific example of how measuring outcomes and sharing ideas can make huge impacts in the lives of patients.” She added, “An enormous amount of money is spent on clinical trials of expensive biologics which have revolutionized treatment, yet the humanistic aspects of our care have just as great of an impact. In this study, each center focused on ways they could lower ER visits and hospitalizations. One size did not fit all, yet they could learn from each other. The very platform they used to conduct the study is a model for all of us.”
Corey A. Siegel, MD, of the Dartmouth-Hitchcock Medical Center, Lebanon, N.H., and Dr. Melmed's coprincipal investigator on Qorus, said the quality initiative now includes 49 GI practices across the country with plans to grow to 60 by the end of the year. "We have created this 'collaboratory' for providers from actross the country to work togetherr to learn how to best deliver high-qulaity care for patients with IBD," he said.
Another feature of the quality initiative allowed participating sites to see how they compared with others anonymously, Dr. Melmed said. “Using the data, we called out high-performing sites to teach the rest of us what they were doing that enabled them to improve, so that all of us could learn from their successes,” he said.
The researchers are aiming to evaluate costs and identify the most successful interventions, with the plan to present the latter at Digestive Disease Week® 2020 and use them to develop a toolkit practices can use. “Ultimately,” said Dr. Melmed, “this is scalable.”
Dr. Melmed disclosed financial relationships with AbbVie, Boehringer-Ingelheim, Celgene, Jannsen, GSK, Medtronic, Pfizer, Samsung Bioepis, Takeda, and Techlab; IBD Qorus receives support from Abbvie, AMAG, Helmsley Charitable Trust, Janssen, Nephoroceuticals, Pfizer, Takeda, and UCB.
SOURCE: Melmed GT et al. Crohn’s & Colitis Congress 2020, Session 28.
REPORTING FROM CROHN’S & COLITIS CONGRESS
ERAS takes its place in IBD surgery
AUSTIN, TEX. – Enhanced recovery after surgery (ERAS) protocols have been around for decades, but typically excluded patients having surgery for inflammatory bowel disease (IBD). However, recent studies have shown strategies to optimize these patients, including presurgery carbohydrate loading and early postsurgery feeding, can improve outcomes, according to a review of evidence presented at the annual congress of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
“It’s really important that we implement strategies to help mitigate the impact that malnutrition is going to have on our perioperative patients, and one of the ways we do that is by using an ERAS or enhanced recovery after surgery protocol,” said Kelly Issokson, MS, RD, of Cedars-Sinai Medical Center, Los Angeles. She noted that patients with IBD are five times more likely to be malnourished than non-IBD patients, and those with fistulizing Crohn’s disease and bowel resections are at greatest risk (Inflamm Bowel Dis. 2008;14:1139-46).
“I constantly see patients who are kept NPO [nothing by mouth] 12 or 24 hours before surgery, maybe even longer sometimes, unfortunately,” she said. “We should really be minimizing that NPO to help mitigate the catabolic effect that surgery has on our patients and help them recover more quickly.”
To screen surgery patients for nutrition risk, Ms. Issokson said that gastroenterologists can ask two questions from the malnutrition screening tool: Did the patient have recent unintentional weight loss, and is the patient eating less because of poor appetite? A yes to either question merits referral to a registered dietician. Malnutrition, weight loss of 5%-10% of total body weight, and sarcopenia are predictors of surgical complications for IBD patients, the latter an independent predictor in patients aged 40 years and older.
The ERAS protocol involves optimizing preoperative and postoperative nutrition, she said. It has been linked with improved outcomes in elective colorectal surgery (World J Surg. 2014;38:1531-41), although the evidence in IBD isn’t as robust. She cited a retrospective study reported at the 2019 annual Digestive Disease Week of patients with Crohn’s disease that found no difference in readmissions, complications, or reoperations between ERAS and standard-care patients.
Preoperative nutrition optimization in ERAS involves anemia and fluid management, oral nutrition supplementation, and – based on European Society for Clinical Nutrition and Metabolism (ESPEN) 2017 guidelines – delaying the operation where possible if the patient is malnourished. “Patients who receive preoperative nutrition support have been shown to have better outcomes postoperatively,” Ms. Issokson said, citing a meta-analysis of 1,111 Crohn’s disease patients that reported the complication rate was 20% in patients on nutrition support versus 60% for those on standard care; in those on enteral nutrition, the disparity was more pronounced: 21% versus 73% (Eur J Gastro Hep. 2018;30:997-1002).
Gastroenterologists should not be afraid of implementing total parenteral nutrition (TPN) perioperatively in these patients, Ms. Issokson said. “This can really help to improve outcomes and quality of life in our patients, and it’s something that we really should not shy away from,” she added in an interview. “If our patients are malnourished and meet the criteria for TPN, then we should really not be withholding it.” Patients with severe IBD who are not absorbing from their gut and can’t meet 60% of their needs by mouth are prime candidates for TPN, she said, referencing a 2019 study that reported that preoperative TPN in malnourished IBD patients resulted in a rate of overall noninfectious complications half that of no-TPN patients: 8.3% versus 16.8% (Gastroenterol Rep. 2019 Apr;7:107-14).
Carbohydrate loading before surgery is a big part of ERAS in these patients. “Surgery has a huge impact on the catabolic state of a patient,” Ms. Issokson said. “It’s similar to running a marathon; you wouldn’t go out and run a marathon without fueling up the night before with a whole bunch of carbohydrates. So we use this same strategy in our surgical patients.”
ERAS society guidelines call for 100 g of carbohydrates the night before and 50 g 2 hours before surgery in the form of a clear liquid beverage, along with permitting a light meal up to 6 hours before, with exceptions in gastroparesis, motility disorders, and emergency surgery.
Another key component of ERAS in IBD is early postoperative feeding. “Postoperatively we want to feed our patients as soon as possible,” Ms. Issokson said. ESPEN guidelines call for feeding patients with new nondiverted colorectal anastomosis within 4 hours. “Studies show that patients aren’t able to eat enough calories to help them recover postoperatively, so implementing an oral nutrition supplement might be helpful there,” she added.
Ms. Issokson is a Crohn’s & Colitis Foundation board member, and disclosed financial relationships with Orgain, RMEI, and Medscape.
SOURCE: Issokson K et al. Crohn’s & Colitis Congress 2020, Session Sp83.
AUSTIN, TEX. – Enhanced recovery after surgery (ERAS) protocols have been around for decades, but typically excluded patients having surgery for inflammatory bowel disease (IBD). However, recent studies have shown strategies to optimize these patients, including presurgery carbohydrate loading and early postsurgery feeding, can improve outcomes, according to a review of evidence presented at the annual congress of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
“It’s really important that we implement strategies to help mitigate the impact that malnutrition is going to have on our perioperative patients, and one of the ways we do that is by using an ERAS or enhanced recovery after surgery protocol,” said Kelly Issokson, MS, RD, of Cedars-Sinai Medical Center, Los Angeles. She noted that patients with IBD are five times more likely to be malnourished than non-IBD patients, and those with fistulizing Crohn’s disease and bowel resections are at greatest risk (Inflamm Bowel Dis. 2008;14:1139-46).
“I constantly see patients who are kept NPO [nothing by mouth] 12 or 24 hours before surgery, maybe even longer sometimes, unfortunately,” she said. “We should really be minimizing that NPO to help mitigate the catabolic effect that surgery has on our patients and help them recover more quickly.”
To screen surgery patients for nutrition risk, Ms. Issokson said that gastroenterologists can ask two questions from the malnutrition screening tool: Did the patient have recent unintentional weight loss, and is the patient eating less because of poor appetite? A yes to either question merits referral to a registered dietician. Malnutrition, weight loss of 5%-10% of total body weight, and sarcopenia are predictors of surgical complications for IBD patients, the latter an independent predictor in patients aged 40 years and older.
The ERAS protocol involves optimizing preoperative and postoperative nutrition, she said. It has been linked with improved outcomes in elective colorectal surgery (World J Surg. 2014;38:1531-41), although the evidence in IBD isn’t as robust. She cited a retrospective study reported at the 2019 annual Digestive Disease Week of patients with Crohn’s disease that found no difference in readmissions, complications, or reoperations between ERAS and standard-care patients.
Preoperative nutrition optimization in ERAS involves anemia and fluid management, oral nutrition supplementation, and – based on European Society for Clinical Nutrition and Metabolism (ESPEN) 2017 guidelines – delaying the operation where possible if the patient is malnourished. “Patients who receive preoperative nutrition support have been shown to have better outcomes postoperatively,” Ms. Issokson said, citing a meta-analysis of 1,111 Crohn’s disease patients that reported the complication rate was 20% in patients on nutrition support versus 60% for those on standard care; in those on enteral nutrition, the disparity was more pronounced: 21% versus 73% (Eur J Gastro Hep. 2018;30:997-1002).
Gastroenterologists should not be afraid of implementing total parenteral nutrition (TPN) perioperatively in these patients, Ms. Issokson said. “This can really help to improve outcomes and quality of life in our patients, and it’s something that we really should not shy away from,” she added in an interview. “If our patients are malnourished and meet the criteria for TPN, then we should really not be withholding it.” Patients with severe IBD who are not absorbing from their gut and can’t meet 60% of their needs by mouth are prime candidates for TPN, she said, referencing a 2019 study that reported that preoperative TPN in malnourished IBD patients resulted in a rate of overall noninfectious complications half that of no-TPN patients: 8.3% versus 16.8% (Gastroenterol Rep. 2019 Apr;7:107-14).
Carbohydrate loading before surgery is a big part of ERAS in these patients. “Surgery has a huge impact on the catabolic state of a patient,” Ms. Issokson said. “It’s similar to running a marathon; you wouldn’t go out and run a marathon without fueling up the night before with a whole bunch of carbohydrates. So we use this same strategy in our surgical patients.”
ERAS society guidelines call for 100 g of carbohydrates the night before and 50 g 2 hours before surgery in the form of a clear liquid beverage, along with permitting a light meal up to 6 hours before, with exceptions in gastroparesis, motility disorders, and emergency surgery.
Another key component of ERAS in IBD is early postoperative feeding. “Postoperatively we want to feed our patients as soon as possible,” Ms. Issokson said. ESPEN guidelines call for feeding patients with new nondiverted colorectal anastomosis within 4 hours. “Studies show that patients aren’t able to eat enough calories to help them recover postoperatively, so implementing an oral nutrition supplement might be helpful there,” she added.
Ms. Issokson is a Crohn’s & Colitis Foundation board member, and disclosed financial relationships with Orgain, RMEI, and Medscape.
SOURCE: Issokson K et al. Crohn’s & Colitis Congress 2020, Session Sp83.
AUSTIN, TEX. – Enhanced recovery after surgery (ERAS) protocols have been around for decades, but typically excluded patients having surgery for inflammatory bowel disease (IBD). However, recent studies have shown strategies to optimize these patients, including presurgery carbohydrate loading and early postsurgery feeding, can improve outcomes, according to a review of evidence presented at the annual congress of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
“It’s really important that we implement strategies to help mitigate the impact that malnutrition is going to have on our perioperative patients, and one of the ways we do that is by using an ERAS or enhanced recovery after surgery protocol,” said Kelly Issokson, MS, RD, of Cedars-Sinai Medical Center, Los Angeles. She noted that patients with IBD are five times more likely to be malnourished than non-IBD patients, and those with fistulizing Crohn’s disease and bowel resections are at greatest risk (Inflamm Bowel Dis. 2008;14:1139-46).
“I constantly see patients who are kept NPO [nothing by mouth] 12 or 24 hours before surgery, maybe even longer sometimes, unfortunately,” she said. “We should really be minimizing that NPO to help mitigate the catabolic effect that surgery has on our patients and help them recover more quickly.”
To screen surgery patients for nutrition risk, Ms. Issokson said that gastroenterologists can ask two questions from the malnutrition screening tool: Did the patient have recent unintentional weight loss, and is the patient eating less because of poor appetite? A yes to either question merits referral to a registered dietician. Malnutrition, weight loss of 5%-10% of total body weight, and sarcopenia are predictors of surgical complications for IBD patients, the latter an independent predictor in patients aged 40 years and older.
The ERAS protocol involves optimizing preoperative and postoperative nutrition, she said. It has been linked with improved outcomes in elective colorectal surgery (World J Surg. 2014;38:1531-41), although the evidence in IBD isn’t as robust. She cited a retrospective study reported at the 2019 annual Digestive Disease Week of patients with Crohn’s disease that found no difference in readmissions, complications, or reoperations between ERAS and standard-care patients.
Preoperative nutrition optimization in ERAS involves anemia and fluid management, oral nutrition supplementation, and – based on European Society for Clinical Nutrition and Metabolism (ESPEN) 2017 guidelines – delaying the operation where possible if the patient is malnourished. “Patients who receive preoperative nutrition support have been shown to have better outcomes postoperatively,” Ms. Issokson said, citing a meta-analysis of 1,111 Crohn’s disease patients that reported the complication rate was 20% in patients on nutrition support versus 60% for those on standard care; in those on enteral nutrition, the disparity was more pronounced: 21% versus 73% (Eur J Gastro Hep. 2018;30:997-1002).
Gastroenterologists should not be afraid of implementing total parenteral nutrition (TPN) perioperatively in these patients, Ms. Issokson said. “This can really help to improve outcomes and quality of life in our patients, and it’s something that we really should not shy away from,” she added in an interview. “If our patients are malnourished and meet the criteria for TPN, then we should really not be withholding it.” Patients with severe IBD who are not absorbing from their gut and can’t meet 60% of their needs by mouth are prime candidates for TPN, she said, referencing a 2019 study that reported that preoperative TPN in malnourished IBD patients resulted in a rate of overall noninfectious complications half that of no-TPN patients: 8.3% versus 16.8% (Gastroenterol Rep. 2019 Apr;7:107-14).
Carbohydrate loading before surgery is a big part of ERAS in these patients. “Surgery has a huge impact on the catabolic state of a patient,” Ms. Issokson said. “It’s similar to running a marathon; you wouldn’t go out and run a marathon without fueling up the night before with a whole bunch of carbohydrates. So we use this same strategy in our surgical patients.”
ERAS society guidelines call for 100 g of carbohydrates the night before and 50 g 2 hours before surgery in the form of a clear liquid beverage, along with permitting a light meal up to 6 hours before, with exceptions in gastroparesis, motility disorders, and emergency surgery.
Another key component of ERAS in IBD is early postoperative feeding. “Postoperatively we want to feed our patients as soon as possible,” Ms. Issokson said. ESPEN guidelines call for feeding patients with new nondiverted colorectal anastomosis within 4 hours. “Studies show that patients aren’t able to eat enough calories to help them recover postoperatively, so implementing an oral nutrition supplement might be helpful there,” she added.
Ms. Issokson is a Crohn’s & Colitis Foundation board member, and disclosed financial relationships with Orgain, RMEI, and Medscape.
SOURCE: Issokson K et al. Crohn’s & Colitis Congress 2020, Session Sp83.
REPORTING FROM THE CROHN’S & COLITIS CONGRESS















