User login
Advanced Melanoma: Treatment After Progression on First-line Therapy
The past decade has brought rapid advancements in treatment with immune checkpoint inhibitors and molecularly targeted agents, which have significantly improved objective response rates (ORRs), progression-free survival (PFS), and overall survival (OS) for patients with metastatic melanoma. This article reviews current evidence for immune checkpoint blockade and molecularly targeted agents in the treatment of metastatic melanoma after progression on first-line therapy. The selection of first-line therapy for metastatic melanoma is reviewed in a separate article.
Case Presentation
A 62-year-old man was diagnosed with stage IIA melanoma after undergoing wide local excision of a right scalp lesion (final staging was consistent with pT3aN0M0). After 3.5 years of follow-up, he developed symptoms of vertigo, diplopia, and recurrent falls prompting medical attention. Magnetic resonance imaging (MRI) brain revealed multiple supratentorial and infratentorial lesions concerning for intracranial metastases and computed tomography (CT) chest/abdomen/pelvis revealed a right lower lobe pulmonary mass with right hilar and subcarinal lymphadenopathy. He was treated with intravenous dexamethasone and further evaluation with an endobronchial ultrasound-guided fine-needle aspiration of the right lower lobe mass revealed metastatic melanoma. The patient underwent whole brain radiation therapy for symptomatic relief prior to initiating systemic therapy. Testing showed the melanoma was positive for a BRAF V600K mutation. He was started on combination molecularly targeted therapy with dabrafenib and trametinib. He initially did well, with a partial response noted by resolution of symptoms and decreased size of his intracranial metastases and decreased size of the right lower lobe mass.
After 3 months of therapy, surveillance PET-CT notes increasing size and FDG avidity of the right lower lobe mass. MRI brain reveals resolution of several previously noted metastases, but with interval development of a new left frontal lobe mass concerning for progressive disease.
What is the general approach to treatment of metastatic melanoma after progression on first-line therapy?
Based on the current evidence, there is no definitive algorithm for the treatment of metastatic melanoma after progression on first-line therapy. Enrollment in clinical trials is encouraged to further elucidate the best sequencing of treatment. The current practice is to typically switch class of agents after progression on front-line therapy to either immunotherapy that has not yet been tried or to molecularly targeted therapy in patients harboring a BRAF V600 mutation. After further progression of disease, retreatment with a previously received agent is possible, and this may be combined with investigational therapies.
Immune Checkpoint Inhibitors in Progressive Disease
The 2 major populations of patients to consider are those with BRAF wild-type melanomas who progress on first-line immunotherapy and those with BRAF V600 mutation–positive melanoma who progress on molecularly targeted therapy with BRAF and MEK inhibitors. There is relatively limited data on the efficacy of immune checkpoint inhibition after progression on anti-programmed cell death 1 (PD-1) monotherapy. A small retrospective study of patients who progressed on anti-PD-1 monotherapy were treated with ipilimumab, with a 10% ORR and another 8% having stable disease for more than 6 months; however, 35% of patients experienced grade 3 to 5 immune-related adverse events.1 The only prospective data supports the efficacy of anti-PD-1 therapy after progression on ipilimumab, as supported by the CheckMate 037 trial (nivolumab versus chemotherapy)2 and KEYNOTE-002 trial (pembrolizumab versus chemotherapy)3,4; however, this is no longer applicable as ipilimumab is no longer given in the first-line setting and has been replaced by anti-PD-1 monotherapy or combination immunotherapy.
Another interesting facet of PD-1 monotherapy is the idea of treatment beyond progression. The concept of pseudoprogression—whereby patients receiving PD-1 inhibitors initially meet Response Evaluation Criteria in Solid Tumors (RECIST) criteria for progression, but then later go on to demonstrate significant decreases in tumor burden on subsequent imaging studies—has been described in melanoma patients receiving such immunotherapies. It is thought that pseudoprogression occurs due to either an initial delay in anti-tumor response to the immunotherapy or from the measured target lesion appearing larger due to surrounding immune/inflammatory infiltrate. In an analysis of individual patient data pooled from 8 multicenter clinical trials, 19% of patients were treated beyond initially documented RECIST progression and had subsequent imaging to evaluate the tumor burden; in these patients, the same target lesion later met RECIST criteria for response, with a greater than 30% reduction in tumor size. Furthermore, of the evaluable cohort, the median OS in patients who did receive treatment beyond progression was 24.4 months compared to 11.2 months in those who did not receive treatment beyond progression.5 While further randomized studies are warranted to characterize the potential benefit, the existing data suggests that selected patients who are doing well clinically despite evidence of radiographic progressive disease may benefit from continued treatment with PD-1 inhibitors.
Combination immunotherapy with both PD-1 and CTLA-4 blockade has been studied retrospectively in the second-line setting. A retrospective analysis of patients who had progressive disease on PD-1 inhibitor monotherapy compared the outcomes of patients who received just ipilimumab to those of patients who received both ipilimumab and nivolumab. The ORR (16% ipilimumab vs 21% combination group) and 1-year OS (54% vs 55%) were similar in both groups,6 and this demonstrated significantly less efficacy with combination therapy when compared to use in the first-line setting, albeit in a separate prospective trial.7 A multicenter, retrospective study by Tétu and colleagues compared outcomes with ipilimumab plus nivolumab across 3 groups that included previously untreated patients, patients who had progressed on single-agent immunotherapy, and patients who had progressed on prior molecularly targeted therapy.8 Despite clearly inferior efficacy in previously treated patients, the results support combination immunotherapy as a viable treatment option in the second-line setting. Outcomes are reported in Table 1 below. Of note, there is an ongoing phase 2 trial to assess the use of combined PD-1 and CTLA-4 inhibitors versus CTLA-4 inhibition alone after progression on first-line PD-1 inhibitor monotherapy (NCT03033576).
For patients with BRAF V600–mutation positive melanoma who progress on front-line molecularly targeted therapy, immune checkpoint inhibitor therapy with either anti-PD-1 monotherapy or combination anti-PD-1 and ipilimumab should be considered. The KEYNOTE-006 trial that demonstrated superiority of pembrolizumab compared to ipilimumab included patients who had received up to 1 prior systemic therapy that was not a PD-1 or CTLA-4 inhibitor, and subgroup analysis demonstrated efficacy with pembrolizumab in patients who had received prior treatment with a BRAF inhibitor.9 The retrospective analysis by Tétu et al (Table 1) noted efficacy of combination nivolumab and ipilimumab in patients treated with prior molecularly targeted therapy, as evidenced by an ORR of 35% and median OS of 16.5 months.8
A retrospective trial by Ackerman et al analyzed ORR, median PFS, and median OS from the time of commencement of BRAF inhibitor therapy (with or without a MEK inhibitor), and the comparison was made between those who received ipilimumab before or after molecularly targeted therapy. While ipilimumab is no longer the first-line immunotherapy agent used in advanced melanoma, the study did highlight some important concepts. First, ORRs to BRAF inhibitors were similar between the 2 treatment groups. The conclusions of the analysis were that there was no significant difference in median PFS or OS in regard to which therapy was given first, but median OS after BRAF inhibitors were discontinued was very short and patients had poor responses to ipilimumab after stopping a BRAF inhibitor. This highlights the concern that patients who have progressive disease on molecularly targeted therapy often have a poor performance status and undergo too rapid of a clinical decline to derive benefit from immunotherapy, which can often take weeks to months to take effect.10
A more recent retrospective study by Johnson et al compared efficacy outcomes in patients who received single-agent anti-PD-1 therapy prior to molecularly targeted therapy (BRAF inhibitor with or without MEK inhibitor) to those who received molecularly targeted therapy prior to anti-PD-1 therapy. The difference in median OS was not statistically significant (27.5 months with PD-1 inhibitor first vs 40.3 months with molecularly targeted therapy first). Both treatments demonstrated second-line efficacy, but outcomes were inferior to those reported when either type of therapy was used in the first-line setting. Interestingly, patients who were maintained on molecularly targeted therapy for more than 6 months prior to progression demonstrated an improved ORR to subsequent anti-PD-1 therapy (34% vs 15%).11
Molecularly Targeted Therapy in Progressive Disease
When melanoma patients with a BRAF V600 mutation are treated initially with immunotherapy and demonstrate progressive disease, molecularly targeted therapy with combined BRAF and MEK inhibition should be considered for second-line therapy. While there are no dedicated prospective trial results with BRAF/MEK inhibitors after progression on immune checkpoint inhibitors, for practical purposes, it may be reasonable to extrapolate outcomes from the currently available first-line studies.12-16 An ongoing study (NCT02224781) in which patients are randomized to receive ipilimumab/nivolumab followed by dabrafenib/trametinib at progression versus the reverse order is designed to help answer the question of optimal sequencing and timing of therapy. Johnson et al’s retrospective analysis of patients receiving single-agent anti-PD-1 therapy prior to molecularly targeted therapy compared to the reverse order concluded that there was no statistically significant difference in median OS.11 Ackerman et al’s retrospective study of patients who had received ipilimumab before or after molecularly targeted therapy noted similar response rates to molecularly targeted therapy in each treatment group.10
The issue of re-treatment with a BRAF/MEK inhibitor in a patient already progressing on targeted therapy is a more challenging situation, and currently available data suggests there is limited benefit. However, select patients may be considered for this approach. The combination of dabrafenib/trametinib demonstrated an ORR of approximately 15% in a cohort of patients who progressed on single-agent BRAF inhibitor therapy, with a suggestion that those patients who had previously derived benefit for more than 6 months may have a more favorable outcome.17
Based on the hypothesis that acquired resistance to BRAF/MEK inhibition may be reversible if the selective pressure of the medication is held for a period of time, a phase 2 trial analyzed outcomes with retreatment. The study included patients with BRAF V600–mutant melanoma who had progressed on prior BRAF inhibition (with or without MEK inhibitor) and required that they had been off of therapy for at least 12 weeks. Of the 25 patients who received dabrafenib plus trametinib as retreatment, 32% demonstrated a partial response and 40% had stable disease.18 While further studies are warranted, retreatment with molecularly targeted therapy may be a viable option, especially in light of the multiple approved BRAF and MEK inhibitor combinations.
Another concept that has been studied is treatment beyond disease progression with molecularly targeted therapy. In a retrospective analysis of patients who had progressed on a single-agent BRAF inhibitor, 39% of those patients were continued on the same BRAF inhibitor and compared to patients who received no subsequent therapy or changed to an alternative systemic therapy. In the multivariable analysis adjusting for other prognostic factors, continued treatment with the BRAF inhibitor was associated with prolonged OS.19
Case Conclusion
The patient is started on second-line therapy with nivolumab and ipilimumab and demonstrates a partial response. One year later he continues to feel well with decreased size of the intracranial and right lower lobe lesions, and without any interval development of new areas of metastatic disease.
Special Considerations
Intralesional Therapies
Talimogene laherparepvec (T-VEC) is a genetically modified herpesvirus-1 oncolytic virus that is injected into melanoma skin lesions and leads to the expression of granulocyte-macrophage colony-stimulating factor. While T-VEC is currently approved for local treatment of unresectable cutaneous, subcutaneous, or nodal recurrences,20 it has also been investigated in combination with other therapies for patients with advanced disease. In patients with previously treated melanoma, T-VEC plus ipilimumab demonstrated superior ORR to ipilimumab alone (39% vs 18%), and the tumor response was not limited to the injected lesions. The observation of systemic response suggests synergy between T-VEC and immune checkpoint blockade in enhancing the anti-tumor immune response.21 The phase 1b MASTERKEY-265 trial combining pembrolizumab and T-VEC led to an ORR of 62% and CR of 33%.22 A phase 3 trial comparing pembrolizumab plus T-VEC to pembrolizumab alone is ongoing (NCT02263508).
Melanoma Brain Metastases
The presence of brain metastases is a common event in patients with metastatic melanoma, and often confers a poor prognosis.23 The approach to the management of brain metastases should be multidisciplinary among medical oncology, neurosurgery, and radiation oncology providers, as treatment algorithms continue to rapidly evolve. Historically, there has been little prospective clinical trial data regarding optimal systemic therapy, and local therapies such as surgery or stereotactic radiation have long been the mainstay of therapy for intracranial disease.24 However, recent data with both immunotherapy and molecularly targeted therapy has demonstrated efficacy with intracranial metastases.
A recent trial of combined nivolumab and ipilimumab as frontline therapy in patients with asymptomatic melanoma brain metastases demonstrated a complete response rate of 26% and partial response rate of 30% in patients with a median follow-up of 14 months.25 In a separate study, ipilimumab plus nivolumab demonstrated better intracranial ORR when compared to nivolumab alone in asymptomatic, previously untreated patients. Outcomes were better in patients presenting with asymptomatic versus symptomatic brain metastases.26 Collectively, these results suggest that systemic immunotherapy alone may be adequate for patients with asymptomatic, previously untreated brain metastases.
For molecularly targeted therapy in patients with BRAF mutations and brain metastases, the BREAK-MB trial demonstrated that an intracranial response was attainable with dabrafenib regardless of whether the patient had previously received local therapy in the form of surgery or radiation.27 The COMBI-MB trial enhanced the preexisting data by testing the intracranial efficacy of dabrafenib plus trametinib in 4 different cohorts of patients, further supporting that systemic molecularly targeted therapy can provide significant intracranial activity in patients with both symptomatic and asymptomatic brain lesions and regardless of prior local therapy (Table 2).28
Conclusion
The treatment of advanced melanoma has been drastically improved over the past decade by the development and study of immune checkpoint inhibitors and molecularly targeted agents. There is still much to learn regarding the optimal combination and sequencing of therapies. Many of these trials are ongoing and will provide additional evidence to guide treatment decisions moving forward.
1. Bowyer S, Prithviraj P, Lorigan P, et al. Efficacy and toxicity of treatment with the anti-CTLA-4 antibody ipilimumab in patients with metastatic melanoma after prior anti-PD-1 therapy. Br J Cancer. 2016;114:1084-1089.
2. Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375-384.
3. Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16:908-918.
4. Hamid O, Puzanov I, Dummer R, et al. Final analysis of a randomised trial comparing pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory advanced melanoma. Eur J Cancer. 2017;86:37-45.
5. Beaver JA, Hazarika M, Mulkey F, et al. Patients with melanoma treated with an anti-PD-1 antibody beyond RECIST progression: a US Food and Drug Administration pooled analysis. Lancet Oncol. 2018;19:229-239.
6. Zimmer L, Apuri S, Eroglu Z, et al. Ipilimumab alone or in combination with nivolumab after progression on anti-PD-1 therapy in advanced melanoma. Eur J Cancer. 2017;75:47-55.
7. Larkin J, Chiarion-Sileni V, Gonazalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23-34.
8. Tétu P, Mangana J, Dummer R, et al. Benefit of the nivolumab and ipilimumab combination in pretreated advanced melanoma. Eur J Cancer. 2018;93:147-149.
9. Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2522-2532.
10. Ackerman A, Klein O, McDermott D, et al. Outcomes of patients with metastatic melanoma treated with immunotherapy prior to or after BRAF inhibitors. Cancer. 2014;120:1695-1701.
11. Johnson DB, Pectasides E, Feld E, et al. Sequencing treatment in BRAFV600 mutant melanoma: anti-pd-1 before and after BRAF inhibition. J Immunother. 2017;40:31-35.
12. Long GV, Stroyakovskiy D, Gogas H, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicenter, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2015;386:444-451.
13. Long GV, Flaherty KT, Stroyakovskiy D, et al. Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: long-term survival and safety analysis of a phase 3 study. Ann Oncol. 2017;28:1631-1639.
14. Ascierto PA, McArthur GA, Dréno B, et al. Cobimetinib combined with vemurafenib in advanced BRAFV600-mutant melanoma (coBRIM): updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol. 2016;17:1248-1260.
15. Dummer R, Ascierto PA, Gogas HJ, et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): a multicenter, open-label, randomised phase 3 trial. Lancet Oncol. 2018;19:603-615.
16. Dummer R, Ascierto PA, Gogas HJ, et al. Overall survival in patients with BRAF-mutant melanoma receiving encorafenib plus binimetinib versus vemurafenib or encorafenib (COLUMBUS): a multicenter, open-label, randomised, phase 3 trial. Lancet Oncol. 2018;19:1315-1327.
17. Johnson DB, Flaherty KT, Weber, JS et al. Combined BRAF (Dabrafenib) and MEK inhibition (Trametinib) in patients with BRAFV600-mutant melanoma experiencing progression with single-agent BRAF inhibitor. J Clin Oncol. 2014;32:3697-3704.
18. Schreuer M, Jansen Y, Planken S, et al. Combination of dabrafenib plus trametinib for BRAF and MEK inhibitor pretreated patients with advanced BRAFV600-mutant melanoma: an open-label, single arm, dual-centre, phase 2 clinical trial. Lancet Oncol. 2017;18:464-472.
19. Chan MM, Haydu LE, Azer MW, et al. The nature and management of metastatic melanoma after progression on BRAF inhibitors: effects of extended BRAF inhibition. Cancer. 2014;120:3142-3153.
20. Imlygic (talimogene laherparepvec) suspension for intralesional injection [package insert]. Thousand Oaks, CA: BioVex; 2015.
21. Chesney J, Puzanov I, Collichio F, et al. Randomized, open-label phase ii study evaluating the efficacy and safety of talimogene laherparepvec in combination with ipilimumab versus ipilimumab alone in patients with advanced, unresectable melanoma. J Clin Oncol. 2018;36:1658-1667.
22. Ribas A, Dummer R, Puzanov I, et al. Oncolytic virotherapy promotes intratumoral t cell infiltration and improves anti-PD-1 immunotherapy. Cell. 2018;174:1031-1032.
23. Sampson JH, Carter Jr. JH, Friedman AH, Seigler HF. Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. J Neurosurg. 1998;88:11-20.
24. Yamamoto M, Serizawa T, Shuto T, et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol. 2014;15:387-395.
25. Tawbi HA, Forsyth PA, Hamid O, et al. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med. 2018;379:722-730.
26. Long GV, Atkinson V, La S, et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicenter randomised phase 2 study. Lancet Oncol. 2018;19:672-681.
27. Long GV, Trefzer U, Davies MA, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicenter, open-label, phase 2 trial. Lancet Oncol. 2012;13:1087-1095.
28. Davies MA, Saiag P, Robert C, et al. Dabrafenib plus trametinib in patients with BRAFV600-mutant melanoma brain metastases (COMBI-MB): a multicenter, multicohort, open-label, phase 2 trial. Lancet Oncol. 2017;18:863-873.
The past decade has brought rapid advancements in treatment with immune checkpoint inhibitors and molecularly targeted agents, which have significantly improved objective response rates (ORRs), progression-free survival (PFS), and overall survival (OS) for patients with metastatic melanoma. This article reviews current evidence for immune checkpoint blockade and molecularly targeted agents in the treatment of metastatic melanoma after progression on first-line therapy. The selection of first-line therapy for metastatic melanoma is reviewed in a separate article.
Case Presentation
A 62-year-old man was diagnosed with stage IIA melanoma after undergoing wide local excision of a right scalp lesion (final staging was consistent with pT3aN0M0). After 3.5 years of follow-up, he developed symptoms of vertigo, diplopia, and recurrent falls prompting medical attention. Magnetic resonance imaging (MRI) brain revealed multiple supratentorial and infratentorial lesions concerning for intracranial metastases and computed tomography (CT) chest/abdomen/pelvis revealed a right lower lobe pulmonary mass with right hilar and subcarinal lymphadenopathy. He was treated with intravenous dexamethasone and further evaluation with an endobronchial ultrasound-guided fine-needle aspiration of the right lower lobe mass revealed metastatic melanoma. The patient underwent whole brain radiation therapy for symptomatic relief prior to initiating systemic therapy. Testing showed the melanoma was positive for a BRAF V600K mutation. He was started on combination molecularly targeted therapy with dabrafenib and trametinib. He initially did well, with a partial response noted by resolution of symptoms and decreased size of his intracranial metastases and decreased size of the right lower lobe mass.
After 3 months of therapy, surveillance PET-CT notes increasing size and FDG avidity of the right lower lobe mass. MRI brain reveals resolution of several previously noted metastases, but with interval development of a new left frontal lobe mass concerning for progressive disease.
What is the general approach to treatment of metastatic melanoma after progression on first-line therapy?
Based on the current evidence, there is no definitive algorithm for the treatment of metastatic melanoma after progression on first-line therapy. Enrollment in clinical trials is encouraged to further elucidate the best sequencing of treatment. The current practice is to typically switch class of agents after progression on front-line therapy to either immunotherapy that has not yet been tried or to molecularly targeted therapy in patients harboring a BRAF V600 mutation. After further progression of disease, retreatment with a previously received agent is possible, and this may be combined with investigational therapies.
Immune Checkpoint Inhibitors in Progressive Disease
The 2 major populations of patients to consider are those with BRAF wild-type melanomas who progress on first-line immunotherapy and those with BRAF V600 mutation–positive melanoma who progress on molecularly targeted therapy with BRAF and MEK inhibitors. There is relatively limited data on the efficacy of immune checkpoint inhibition after progression on anti-programmed cell death 1 (PD-1) monotherapy. A small retrospective study of patients who progressed on anti-PD-1 monotherapy were treated with ipilimumab, with a 10% ORR and another 8% having stable disease for more than 6 months; however, 35% of patients experienced grade 3 to 5 immune-related adverse events.1 The only prospective data supports the efficacy of anti-PD-1 therapy after progression on ipilimumab, as supported by the CheckMate 037 trial (nivolumab versus chemotherapy)2 and KEYNOTE-002 trial (pembrolizumab versus chemotherapy)3,4; however, this is no longer applicable as ipilimumab is no longer given in the first-line setting and has been replaced by anti-PD-1 monotherapy or combination immunotherapy.
Another interesting facet of PD-1 monotherapy is the idea of treatment beyond progression. The concept of pseudoprogression—whereby patients receiving PD-1 inhibitors initially meet Response Evaluation Criteria in Solid Tumors (RECIST) criteria for progression, but then later go on to demonstrate significant decreases in tumor burden on subsequent imaging studies—has been described in melanoma patients receiving such immunotherapies. It is thought that pseudoprogression occurs due to either an initial delay in anti-tumor response to the immunotherapy or from the measured target lesion appearing larger due to surrounding immune/inflammatory infiltrate. In an analysis of individual patient data pooled from 8 multicenter clinical trials, 19% of patients were treated beyond initially documented RECIST progression and had subsequent imaging to evaluate the tumor burden; in these patients, the same target lesion later met RECIST criteria for response, with a greater than 30% reduction in tumor size. Furthermore, of the evaluable cohort, the median OS in patients who did receive treatment beyond progression was 24.4 months compared to 11.2 months in those who did not receive treatment beyond progression.5 While further randomized studies are warranted to characterize the potential benefit, the existing data suggests that selected patients who are doing well clinically despite evidence of radiographic progressive disease may benefit from continued treatment with PD-1 inhibitors.
Combination immunotherapy with both PD-1 and CTLA-4 blockade has been studied retrospectively in the second-line setting. A retrospective analysis of patients who had progressive disease on PD-1 inhibitor monotherapy compared the outcomes of patients who received just ipilimumab to those of patients who received both ipilimumab and nivolumab. The ORR (16% ipilimumab vs 21% combination group) and 1-year OS (54% vs 55%) were similar in both groups,6 and this demonstrated significantly less efficacy with combination therapy when compared to use in the first-line setting, albeit in a separate prospective trial.7 A multicenter, retrospective study by Tétu and colleagues compared outcomes with ipilimumab plus nivolumab across 3 groups that included previously untreated patients, patients who had progressed on single-agent immunotherapy, and patients who had progressed on prior molecularly targeted therapy.8 Despite clearly inferior efficacy in previously treated patients, the results support combination immunotherapy as a viable treatment option in the second-line setting. Outcomes are reported in Table 1 below. Of note, there is an ongoing phase 2 trial to assess the use of combined PD-1 and CTLA-4 inhibitors versus CTLA-4 inhibition alone after progression on first-line PD-1 inhibitor monotherapy (NCT03033576).

For patients with BRAF V600–mutation positive melanoma who progress on front-line molecularly targeted therapy, immune checkpoint inhibitor therapy with either anti-PD-1 monotherapy or combination anti-PD-1 and ipilimumab should be considered. The KEYNOTE-006 trial that demonstrated superiority of pembrolizumab compared to ipilimumab included patients who had received up to 1 prior systemic therapy that was not a PD-1 or CTLA-4 inhibitor, and subgroup analysis demonstrated efficacy with pembrolizumab in patients who had received prior treatment with a BRAF inhibitor.9 The retrospective analysis by Tétu et al (Table 1) noted efficacy of combination nivolumab and ipilimumab in patients treated with prior molecularly targeted therapy, as evidenced by an ORR of 35% and median OS of 16.5 months.8
A retrospective trial by Ackerman et al analyzed ORR, median PFS, and median OS from the time of commencement of BRAF inhibitor therapy (with or without a MEK inhibitor), and the comparison was made between those who received ipilimumab before or after molecularly targeted therapy. While ipilimumab is no longer the first-line immunotherapy agent used in advanced melanoma, the study did highlight some important concepts. First, ORRs to BRAF inhibitors were similar between the 2 treatment groups. The conclusions of the analysis were that there was no significant difference in median PFS or OS in regard to which therapy was given first, but median OS after BRAF inhibitors were discontinued was very short and patients had poor responses to ipilimumab after stopping a BRAF inhibitor. This highlights the concern that patients who have progressive disease on molecularly targeted therapy often have a poor performance status and undergo too rapid of a clinical decline to derive benefit from immunotherapy, which can often take weeks to months to take effect.10
A more recent retrospective study by Johnson et al compared efficacy outcomes in patients who received single-agent anti-PD-1 therapy prior to molecularly targeted therapy (BRAF inhibitor with or without MEK inhibitor) to those who received molecularly targeted therapy prior to anti-PD-1 therapy. The difference in median OS was not statistically significant (27.5 months with PD-1 inhibitor first vs 40.3 months with molecularly targeted therapy first). Both treatments demonstrated second-line efficacy, but outcomes were inferior to those reported when either type of therapy was used in the first-line setting. Interestingly, patients who were maintained on molecularly targeted therapy for more than 6 months prior to progression demonstrated an improved ORR to subsequent anti-PD-1 therapy (34% vs 15%).11
Molecularly Targeted Therapy in Progressive Disease
When melanoma patients with a BRAF V600 mutation are treated initially with immunotherapy and demonstrate progressive disease, molecularly targeted therapy with combined BRAF and MEK inhibition should be considered for second-line therapy. While there are no dedicated prospective trial results with BRAF/MEK inhibitors after progression on immune checkpoint inhibitors, for practical purposes, it may be reasonable to extrapolate outcomes from the currently available first-line studies.12-16 An ongoing study (NCT02224781) in which patients are randomized to receive ipilimumab/nivolumab followed by dabrafenib/trametinib at progression versus the reverse order is designed to help answer the question of optimal sequencing and timing of therapy. Johnson et al’s retrospective analysis of patients receiving single-agent anti-PD-1 therapy prior to molecularly targeted therapy compared to the reverse order concluded that there was no statistically significant difference in median OS.11 Ackerman et al’s retrospective study of patients who had received ipilimumab before or after molecularly targeted therapy noted similar response rates to molecularly targeted therapy in each treatment group.10
The issue of re-treatment with a BRAF/MEK inhibitor in a patient already progressing on targeted therapy is a more challenging situation, and currently available data suggests there is limited benefit. However, select patients may be considered for this approach. The combination of dabrafenib/trametinib demonstrated an ORR of approximately 15% in a cohort of patients who progressed on single-agent BRAF inhibitor therapy, with a suggestion that those patients who had previously derived benefit for more than 6 months may have a more favorable outcome.17
Based on the hypothesis that acquired resistance to BRAF/MEK inhibition may be reversible if the selective pressure of the medication is held for a period of time, a phase 2 trial analyzed outcomes with retreatment. The study included patients with BRAF V600–mutant melanoma who had progressed on prior BRAF inhibition (with or without MEK inhibitor) and required that they had been off of therapy for at least 12 weeks. Of the 25 patients who received dabrafenib plus trametinib as retreatment, 32% demonstrated a partial response and 40% had stable disease.18 While further studies are warranted, retreatment with molecularly targeted therapy may be a viable option, especially in light of the multiple approved BRAF and MEK inhibitor combinations.
Another concept that has been studied is treatment beyond disease progression with molecularly targeted therapy. In a retrospective analysis of patients who had progressed on a single-agent BRAF inhibitor, 39% of those patients were continued on the same BRAF inhibitor and compared to patients who received no subsequent therapy or changed to an alternative systemic therapy. In the multivariable analysis adjusting for other prognostic factors, continued treatment with the BRAF inhibitor was associated with prolonged OS.19
Case Conclusion
The patient is started on second-line therapy with nivolumab and ipilimumab and demonstrates a partial response. One year later he continues to feel well with decreased size of the intracranial and right lower lobe lesions, and without any interval development of new areas of metastatic disease.
Special Considerations
Intralesional Therapies
Talimogene laherparepvec (T-VEC) is a genetically modified herpesvirus-1 oncolytic virus that is injected into melanoma skin lesions and leads to the expression of granulocyte-macrophage colony-stimulating factor. While T-VEC is currently approved for local treatment of unresectable cutaneous, subcutaneous, or nodal recurrences,20 it has also been investigated in combination with other therapies for patients with advanced disease. In patients with previously treated melanoma, T-VEC plus ipilimumab demonstrated superior ORR to ipilimumab alone (39% vs 18%), and the tumor response was not limited to the injected lesions. The observation of systemic response suggests synergy between T-VEC and immune checkpoint blockade in enhancing the anti-tumor immune response.21 The phase 1b MASTERKEY-265 trial combining pembrolizumab and T-VEC led to an ORR of 62% and CR of 33%.22 A phase 3 trial comparing pembrolizumab plus T-VEC to pembrolizumab alone is ongoing (NCT02263508).
Melanoma Brain Metastases
The presence of brain metastases is a common event in patients with metastatic melanoma, and often confers a poor prognosis.23 The approach to the management of brain metastases should be multidisciplinary among medical oncology, neurosurgery, and radiation oncology providers, as treatment algorithms continue to rapidly evolve. Historically, there has been little prospective clinical trial data regarding optimal systemic therapy, and local therapies such as surgery or stereotactic radiation have long been the mainstay of therapy for intracranial disease.24 However, recent data with both immunotherapy and molecularly targeted therapy has demonstrated efficacy with intracranial metastases.
A recent trial of combined nivolumab and ipilimumab as frontline therapy in patients with asymptomatic melanoma brain metastases demonstrated a complete response rate of 26% and partial response rate of 30% in patients with a median follow-up of 14 months.25 In a separate study, ipilimumab plus nivolumab demonstrated better intracranial ORR when compared to nivolumab alone in asymptomatic, previously untreated patients. Outcomes were better in patients presenting with asymptomatic versus symptomatic brain metastases.26 Collectively, these results suggest that systemic immunotherapy alone may be adequate for patients with asymptomatic, previously untreated brain metastases.
For molecularly targeted therapy in patients with BRAF mutations and brain metastases, the BREAK-MB trial demonstrated that an intracranial response was attainable with dabrafenib regardless of whether the patient had previously received local therapy in the form of surgery or radiation.27 The COMBI-MB trial enhanced the preexisting data by testing the intracranial efficacy of dabrafenib plus trametinib in 4 different cohorts of patients, further supporting that systemic molecularly targeted therapy can provide significant intracranial activity in patients with both symptomatic and asymptomatic brain lesions and regardless of prior local therapy (Table 2).28

Conclusion
The treatment of advanced melanoma has been drastically improved over the past decade by the development and study of immune checkpoint inhibitors and molecularly targeted agents. There is still much to learn regarding the optimal combination and sequencing of therapies. Many of these trials are ongoing and will provide additional evidence to guide treatment decisions moving forward.
The past decade has brought rapid advancements in treatment with immune checkpoint inhibitors and molecularly targeted agents, which have significantly improved objective response rates (ORRs), progression-free survival (PFS), and overall survival (OS) for patients with metastatic melanoma. This article reviews current evidence for immune checkpoint blockade and molecularly targeted agents in the treatment of metastatic melanoma after progression on first-line therapy. The selection of first-line therapy for metastatic melanoma is reviewed in a separate article.
Case Presentation
A 62-year-old man was diagnosed with stage IIA melanoma after undergoing wide local excision of a right scalp lesion (final staging was consistent with pT3aN0M0). After 3.5 years of follow-up, he developed symptoms of vertigo, diplopia, and recurrent falls prompting medical attention. Magnetic resonance imaging (MRI) brain revealed multiple supratentorial and infratentorial lesions concerning for intracranial metastases and computed tomography (CT) chest/abdomen/pelvis revealed a right lower lobe pulmonary mass with right hilar and subcarinal lymphadenopathy. He was treated with intravenous dexamethasone and further evaluation with an endobronchial ultrasound-guided fine-needle aspiration of the right lower lobe mass revealed metastatic melanoma. The patient underwent whole brain radiation therapy for symptomatic relief prior to initiating systemic therapy. Testing showed the melanoma was positive for a BRAF V600K mutation. He was started on combination molecularly targeted therapy with dabrafenib and trametinib. He initially did well, with a partial response noted by resolution of symptoms and decreased size of his intracranial metastases and decreased size of the right lower lobe mass.
After 3 months of therapy, surveillance PET-CT notes increasing size and FDG avidity of the right lower lobe mass. MRI brain reveals resolution of several previously noted metastases, but with interval development of a new left frontal lobe mass concerning for progressive disease.
What is the general approach to treatment of metastatic melanoma after progression on first-line therapy?
Based on the current evidence, there is no definitive algorithm for the treatment of metastatic melanoma after progression on first-line therapy. Enrollment in clinical trials is encouraged to further elucidate the best sequencing of treatment. The current practice is to typically switch class of agents after progression on front-line therapy to either immunotherapy that has not yet been tried or to molecularly targeted therapy in patients harboring a BRAF V600 mutation. After further progression of disease, retreatment with a previously received agent is possible, and this may be combined with investigational therapies.
Immune Checkpoint Inhibitors in Progressive Disease
The 2 major populations of patients to consider are those with BRAF wild-type melanomas who progress on first-line immunotherapy and those with BRAF V600 mutation–positive melanoma who progress on molecularly targeted therapy with BRAF and MEK inhibitors. There is relatively limited data on the efficacy of immune checkpoint inhibition after progression on anti-programmed cell death 1 (PD-1) monotherapy. A small retrospective study of patients who progressed on anti-PD-1 monotherapy were treated with ipilimumab, with a 10% ORR and another 8% having stable disease for more than 6 months; however, 35% of patients experienced grade 3 to 5 immune-related adverse events.1 The only prospective data supports the efficacy of anti-PD-1 therapy after progression on ipilimumab, as supported by the CheckMate 037 trial (nivolumab versus chemotherapy)2 and KEYNOTE-002 trial (pembrolizumab versus chemotherapy)3,4; however, this is no longer applicable as ipilimumab is no longer given in the first-line setting and has been replaced by anti-PD-1 monotherapy or combination immunotherapy.
Another interesting facet of PD-1 monotherapy is the idea of treatment beyond progression. The concept of pseudoprogression—whereby patients receiving PD-1 inhibitors initially meet Response Evaluation Criteria in Solid Tumors (RECIST) criteria for progression, but then later go on to demonstrate significant decreases in tumor burden on subsequent imaging studies—has been described in melanoma patients receiving such immunotherapies. It is thought that pseudoprogression occurs due to either an initial delay in anti-tumor response to the immunotherapy or from the measured target lesion appearing larger due to surrounding immune/inflammatory infiltrate. In an analysis of individual patient data pooled from 8 multicenter clinical trials, 19% of patients were treated beyond initially documented RECIST progression and had subsequent imaging to evaluate the tumor burden; in these patients, the same target lesion later met RECIST criteria for response, with a greater than 30% reduction in tumor size. Furthermore, of the evaluable cohort, the median OS in patients who did receive treatment beyond progression was 24.4 months compared to 11.2 months in those who did not receive treatment beyond progression.5 While further randomized studies are warranted to characterize the potential benefit, the existing data suggests that selected patients who are doing well clinically despite evidence of radiographic progressive disease may benefit from continued treatment with PD-1 inhibitors.
Combination immunotherapy with both PD-1 and CTLA-4 blockade has been studied retrospectively in the second-line setting. A retrospective analysis of patients who had progressive disease on PD-1 inhibitor monotherapy compared the outcomes of patients who received just ipilimumab to those of patients who received both ipilimumab and nivolumab. The ORR (16% ipilimumab vs 21% combination group) and 1-year OS (54% vs 55%) were similar in both groups,6 and this demonstrated significantly less efficacy with combination therapy when compared to use in the first-line setting, albeit in a separate prospective trial.7 A multicenter, retrospective study by Tétu and colleagues compared outcomes with ipilimumab plus nivolumab across 3 groups that included previously untreated patients, patients who had progressed on single-agent immunotherapy, and patients who had progressed on prior molecularly targeted therapy.8 Despite clearly inferior efficacy in previously treated patients, the results support combination immunotherapy as a viable treatment option in the second-line setting. Outcomes are reported in Table 1 below. Of note, there is an ongoing phase 2 trial to assess the use of combined PD-1 and CTLA-4 inhibitors versus CTLA-4 inhibition alone after progression on first-line PD-1 inhibitor monotherapy (NCT03033576).

For patients with BRAF V600–mutation positive melanoma who progress on front-line molecularly targeted therapy, immune checkpoint inhibitor therapy with either anti-PD-1 monotherapy or combination anti-PD-1 and ipilimumab should be considered. The KEYNOTE-006 trial that demonstrated superiority of pembrolizumab compared to ipilimumab included patients who had received up to 1 prior systemic therapy that was not a PD-1 or CTLA-4 inhibitor, and subgroup analysis demonstrated efficacy with pembrolizumab in patients who had received prior treatment with a BRAF inhibitor.9 The retrospective analysis by Tétu et al (Table 1) noted efficacy of combination nivolumab and ipilimumab in patients treated with prior molecularly targeted therapy, as evidenced by an ORR of 35% and median OS of 16.5 months.8
A retrospective trial by Ackerman et al analyzed ORR, median PFS, and median OS from the time of commencement of BRAF inhibitor therapy (with or without a MEK inhibitor), and the comparison was made between those who received ipilimumab before or after molecularly targeted therapy. While ipilimumab is no longer the first-line immunotherapy agent used in advanced melanoma, the study did highlight some important concepts. First, ORRs to BRAF inhibitors were similar between the 2 treatment groups. The conclusions of the analysis were that there was no significant difference in median PFS or OS in regard to which therapy was given first, but median OS after BRAF inhibitors were discontinued was very short and patients had poor responses to ipilimumab after stopping a BRAF inhibitor. This highlights the concern that patients who have progressive disease on molecularly targeted therapy often have a poor performance status and undergo too rapid of a clinical decline to derive benefit from immunotherapy, which can often take weeks to months to take effect.10
A more recent retrospective study by Johnson et al compared efficacy outcomes in patients who received single-agent anti-PD-1 therapy prior to molecularly targeted therapy (BRAF inhibitor with or without MEK inhibitor) to those who received molecularly targeted therapy prior to anti-PD-1 therapy. The difference in median OS was not statistically significant (27.5 months with PD-1 inhibitor first vs 40.3 months with molecularly targeted therapy first). Both treatments demonstrated second-line efficacy, but outcomes were inferior to those reported when either type of therapy was used in the first-line setting. Interestingly, patients who were maintained on molecularly targeted therapy for more than 6 months prior to progression demonstrated an improved ORR to subsequent anti-PD-1 therapy (34% vs 15%).11
Molecularly Targeted Therapy in Progressive Disease
When melanoma patients with a BRAF V600 mutation are treated initially with immunotherapy and demonstrate progressive disease, molecularly targeted therapy with combined BRAF and MEK inhibition should be considered for second-line therapy. While there are no dedicated prospective trial results with BRAF/MEK inhibitors after progression on immune checkpoint inhibitors, for practical purposes, it may be reasonable to extrapolate outcomes from the currently available first-line studies.12-16 An ongoing study (NCT02224781) in which patients are randomized to receive ipilimumab/nivolumab followed by dabrafenib/trametinib at progression versus the reverse order is designed to help answer the question of optimal sequencing and timing of therapy. Johnson et al’s retrospective analysis of patients receiving single-agent anti-PD-1 therapy prior to molecularly targeted therapy compared to the reverse order concluded that there was no statistically significant difference in median OS.11 Ackerman et al’s retrospective study of patients who had received ipilimumab before or after molecularly targeted therapy noted similar response rates to molecularly targeted therapy in each treatment group.10
The issue of re-treatment with a BRAF/MEK inhibitor in a patient already progressing on targeted therapy is a more challenging situation, and currently available data suggests there is limited benefit. However, select patients may be considered for this approach. The combination of dabrafenib/trametinib demonstrated an ORR of approximately 15% in a cohort of patients who progressed on single-agent BRAF inhibitor therapy, with a suggestion that those patients who had previously derived benefit for more than 6 months may have a more favorable outcome.17
Based on the hypothesis that acquired resistance to BRAF/MEK inhibition may be reversible if the selective pressure of the medication is held for a period of time, a phase 2 trial analyzed outcomes with retreatment. The study included patients with BRAF V600–mutant melanoma who had progressed on prior BRAF inhibition (with or without MEK inhibitor) and required that they had been off of therapy for at least 12 weeks. Of the 25 patients who received dabrafenib plus trametinib as retreatment, 32% demonstrated a partial response and 40% had stable disease.18 While further studies are warranted, retreatment with molecularly targeted therapy may be a viable option, especially in light of the multiple approved BRAF and MEK inhibitor combinations.
Another concept that has been studied is treatment beyond disease progression with molecularly targeted therapy. In a retrospective analysis of patients who had progressed on a single-agent BRAF inhibitor, 39% of those patients were continued on the same BRAF inhibitor and compared to patients who received no subsequent therapy or changed to an alternative systemic therapy. In the multivariable analysis adjusting for other prognostic factors, continued treatment with the BRAF inhibitor was associated with prolonged OS.19
Case Conclusion
The patient is started on second-line therapy with nivolumab and ipilimumab and demonstrates a partial response. One year later he continues to feel well with decreased size of the intracranial and right lower lobe lesions, and without any interval development of new areas of metastatic disease.
Special Considerations
Intralesional Therapies
Talimogene laherparepvec (T-VEC) is a genetically modified herpesvirus-1 oncolytic virus that is injected into melanoma skin lesions and leads to the expression of granulocyte-macrophage colony-stimulating factor. While T-VEC is currently approved for local treatment of unresectable cutaneous, subcutaneous, or nodal recurrences,20 it has also been investigated in combination with other therapies for patients with advanced disease. In patients with previously treated melanoma, T-VEC plus ipilimumab demonstrated superior ORR to ipilimumab alone (39% vs 18%), and the tumor response was not limited to the injected lesions. The observation of systemic response suggests synergy between T-VEC and immune checkpoint blockade in enhancing the anti-tumor immune response.21 The phase 1b MASTERKEY-265 trial combining pembrolizumab and T-VEC led to an ORR of 62% and CR of 33%.22 A phase 3 trial comparing pembrolizumab plus T-VEC to pembrolizumab alone is ongoing (NCT02263508).
Melanoma Brain Metastases
The presence of brain metastases is a common event in patients with metastatic melanoma, and often confers a poor prognosis.23 The approach to the management of brain metastases should be multidisciplinary among medical oncology, neurosurgery, and radiation oncology providers, as treatment algorithms continue to rapidly evolve. Historically, there has been little prospective clinical trial data regarding optimal systemic therapy, and local therapies such as surgery or stereotactic radiation have long been the mainstay of therapy for intracranial disease.24 However, recent data with both immunotherapy and molecularly targeted therapy has demonstrated efficacy with intracranial metastases.
A recent trial of combined nivolumab and ipilimumab as frontline therapy in patients with asymptomatic melanoma brain metastases demonstrated a complete response rate of 26% and partial response rate of 30% in patients with a median follow-up of 14 months.25 In a separate study, ipilimumab plus nivolumab demonstrated better intracranial ORR when compared to nivolumab alone in asymptomatic, previously untreated patients. Outcomes were better in patients presenting with asymptomatic versus symptomatic brain metastases.26 Collectively, these results suggest that systemic immunotherapy alone may be adequate for patients with asymptomatic, previously untreated brain metastases.
For molecularly targeted therapy in patients with BRAF mutations and brain metastases, the BREAK-MB trial demonstrated that an intracranial response was attainable with dabrafenib regardless of whether the patient had previously received local therapy in the form of surgery or radiation.27 The COMBI-MB trial enhanced the preexisting data by testing the intracranial efficacy of dabrafenib plus trametinib in 4 different cohorts of patients, further supporting that systemic molecularly targeted therapy can provide significant intracranial activity in patients with both symptomatic and asymptomatic brain lesions and regardless of prior local therapy (Table 2).28

Conclusion
The treatment of advanced melanoma has been drastically improved over the past decade by the development and study of immune checkpoint inhibitors and molecularly targeted agents. There is still much to learn regarding the optimal combination and sequencing of therapies. Many of these trials are ongoing and will provide additional evidence to guide treatment decisions moving forward.
1. Bowyer S, Prithviraj P, Lorigan P, et al. Efficacy and toxicity of treatment with the anti-CTLA-4 antibody ipilimumab in patients with metastatic melanoma after prior anti-PD-1 therapy. Br J Cancer. 2016;114:1084-1089.
2. Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375-384.
3. Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16:908-918.
4. Hamid O, Puzanov I, Dummer R, et al. Final analysis of a randomised trial comparing pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory advanced melanoma. Eur J Cancer. 2017;86:37-45.
5. Beaver JA, Hazarika M, Mulkey F, et al. Patients with melanoma treated with an anti-PD-1 antibody beyond RECIST progression: a US Food and Drug Administration pooled analysis. Lancet Oncol. 2018;19:229-239.
6. Zimmer L, Apuri S, Eroglu Z, et al. Ipilimumab alone or in combination with nivolumab after progression on anti-PD-1 therapy in advanced melanoma. Eur J Cancer. 2017;75:47-55.
7. Larkin J, Chiarion-Sileni V, Gonazalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23-34.
8. Tétu P, Mangana J, Dummer R, et al. Benefit of the nivolumab and ipilimumab combination in pretreated advanced melanoma. Eur J Cancer. 2018;93:147-149.
9. Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2522-2532.
10. Ackerman A, Klein O, McDermott D, et al. Outcomes of patients with metastatic melanoma treated with immunotherapy prior to or after BRAF inhibitors. Cancer. 2014;120:1695-1701.
11. Johnson DB, Pectasides E, Feld E, et al. Sequencing treatment in BRAFV600 mutant melanoma: anti-pd-1 before and after BRAF inhibition. J Immunother. 2017;40:31-35.
12. Long GV, Stroyakovskiy D, Gogas H, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicenter, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2015;386:444-451.
13. Long GV, Flaherty KT, Stroyakovskiy D, et al. Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: long-term survival and safety analysis of a phase 3 study. Ann Oncol. 2017;28:1631-1639.
14. Ascierto PA, McArthur GA, Dréno B, et al. Cobimetinib combined with vemurafenib in advanced BRAFV600-mutant melanoma (coBRIM): updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol. 2016;17:1248-1260.
15. Dummer R, Ascierto PA, Gogas HJ, et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): a multicenter, open-label, randomised phase 3 trial. Lancet Oncol. 2018;19:603-615.
16. Dummer R, Ascierto PA, Gogas HJ, et al. Overall survival in patients with BRAF-mutant melanoma receiving encorafenib plus binimetinib versus vemurafenib or encorafenib (COLUMBUS): a multicenter, open-label, randomised, phase 3 trial. Lancet Oncol. 2018;19:1315-1327.
17. Johnson DB, Flaherty KT, Weber, JS et al. Combined BRAF (Dabrafenib) and MEK inhibition (Trametinib) in patients with BRAFV600-mutant melanoma experiencing progression with single-agent BRAF inhibitor. J Clin Oncol. 2014;32:3697-3704.
18. Schreuer M, Jansen Y, Planken S, et al. Combination of dabrafenib plus trametinib for BRAF and MEK inhibitor pretreated patients with advanced BRAFV600-mutant melanoma: an open-label, single arm, dual-centre, phase 2 clinical trial. Lancet Oncol. 2017;18:464-472.
19. Chan MM, Haydu LE, Azer MW, et al. The nature and management of metastatic melanoma after progression on BRAF inhibitors: effects of extended BRAF inhibition. Cancer. 2014;120:3142-3153.
20. Imlygic (talimogene laherparepvec) suspension for intralesional injection [package insert]. Thousand Oaks, CA: BioVex; 2015.
21. Chesney J, Puzanov I, Collichio F, et al. Randomized, open-label phase ii study evaluating the efficacy and safety of talimogene laherparepvec in combination with ipilimumab versus ipilimumab alone in patients with advanced, unresectable melanoma. J Clin Oncol. 2018;36:1658-1667.
22. Ribas A, Dummer R, Puzanov I, et al. Oncolytic virotherapy promotes intratumoral t cell infiltration and improves anti-PD-1 immunotherapy. Cell. 2018;174:1031-1032.
23. Sampson JH, Carter Jr. JH, Friedman AH, Seigler HF. Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. J Neurosurg. 1998;88:11-20.
24. Yamamoto M, Serizawa T, Shuto T, et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol. 2014;15:387-395.
25. Tawbi HA, Forsyth PA, Hamid O, et al. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med. 2018;379:722-730.
26. Long GV, Atkinson V, La S, et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicenter randomised phase 2 study. Lancet Oncol. 2018;19:672-681.
27. Long GV, Trefzer U, Davies MA, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicenter, open-label, phase 2 trial. Lancet Oncol. 2012;13:1087-1095.
28. Davies MA, Saiag P, Robert C, et al. Dabrafenib plus trametinib in patients with BRAFV600-mutant melanoma brain metastases (COMBI-MB): a multicenter, multicohort, open-label, phase 2 trial. Lancet Oncol. 2017;18:863-873.
1. Bowyer S, Prithviraj P, Lorigan P, et al. Efficacy and toxicity of treatment with the anti-CTLA-4 antibody ipilimumab in patients with metastatic melanoma after prior anti-PD-1 therapy. Br J Cancer. 2016;114:1084-1089.
2. Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375-384.
3. Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16:908-918.
4. Hamid O, Puzanov I, Dummer R, et al. Final analysis of a randomised trial comparing pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory advanced melanoma. Eur J Cancer. 2017;86:37-45.
5. Beaver JA, Hazarika M, Mulkey F, et al. Patients with melanoma treated with an anti-PD-1 antibody beyond RECIST progression: a US Food and Drug Administration pooled analysis. Lancet Oncol. 2018;19:229-239.
6. Zimmer L, Apuri S, Eroglu Z, et al. Ipilimumab alone or in combination with nivolumab after progression on anti-PD-1 therapy in advanced melanoma. Eur J Cancer. 2017;75:47-55.
7. Larkin J, Chiarion-Sileni V, Gonazalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23-34.
8. Tétu P, Mangana J, Dummer R, et al. Benefit of the nivolumab and ipilimumab combination in pretreated advanced melanoma. Eur J Cancer. 2018;93:147-149.
9. Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2522-2532.
10. Ackerman A, Klein O, McDermott D, et al. Outcomes of patients with metastatic melanoma treated with immunotherapy prior to or after BRAF inhibitors. Cancer. 2014;120:1695-1701.
11. Johnson DB, Pectasides E, Feld E, et al. Sequencing treatment in BRAFV600 mutant melanoma: anti-pd-1 before and after BRAF inhibition. J Immunother. 2017;40:31-35.
12. Long GV, Stroyakovskiy D, Gogas H, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicenter, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2015;386:444-451.
13. Long GV, Flaherty KT, Stroyakovskiy D, et al. Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: long-term survival and safety analysis of a phase 3 study. Ann Oncol. 2017;28:1631-1639.
14. Ascierto PA, McArthur GA, Dréno B, et al. Cobimetinib combined with vemurafenib in advanced BRAFV600-mutant melanoma (coBRIM): updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol. 2016;17:1248-1260.
15. Dummer R, Ascierto PA, Gogas HJ, et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): a multicenter, open-label, randomised phase 3 trial. Lancet Oncol. 2018;19:603-615.
16. Dummer R, Ascierto PA, Gogas HJ, et al. Overall survival in patients with BRAF-mutant melanoma receiving encorafenib plus binimetinib versus vemurafenib or encorafenib (COLUMBUS): a multicenter, open-label, randomised, phase 3 trial. Lancet Oncol. 2018;19:1315-1327.
17. Johnson DB, Flaherty KT, Weber, JS et al. Combined BRAF (Dabrafenib) and MEK inhibition (Trametinib) in patients with BRAFV600-mutant melanoma experiencing progression with single-agent BRAF inhibitor. J Clin Oncol. 2014;32:3697-3704.
18. Schreuer M, Jansen Y, Planken S, et al. Combination of dabrafenib plus trametinib for BRAF and MEK inhibitor pretreated patients with advanced BRAFV600-mutant melanoma: an open-label, single arm, dual-centre, phase 2 clinical trial. Lancet Oncol. 2017;18:464-472.
19. Chan MM, Haydu LE, Azer MW, et al. The nature and management of metastatic melanoma after progression on BRAF inhibitors: effects of extended BRAF inhibition. Cancer. 2014;120:3142-3153.
20. Imlygic (talimogene laherparepvec) suspension for intralesional injection [package insert]. Thousand Oaks, CA: BioVex; 2015.
21. Chesney J, Puzanov I, Collichio F, et al. Randomized, open-label phase ii study evaluating the efficacy and safety of talimogene laherparepvec in combination with ipilimumab versus ipilimumab alone in patients with advanced, unresectable melanoma. J Clin Oncol. 2018;36:1658-1667.
22. Ribas A, Dummer R, Puzanov I, et al. Oncolytic virotherapy promotes intratumoral t cell infiltration and improves anti-PD-1 immunotherapy. Cell. 2018;174:1031-1032.
23. Sampson JH, Carter Jr. JH, Friedman AH, Seigler HF. Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. J Neurosurg. 1998;88:11-20.
24. Yamamoto M, Serizawa T, Shuto T, et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol. 2014;15:387-395.
25. Tawbi HA, Forsyth PA, Hamid O, et al. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med. 2018;379:722-730.
26. Long GV, Atkinson V, La S, et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicenter randomised phase 2 study. Lancet Oncol. 2018;19:672-681.
27. Long GV, Trefzer U, Davies MA, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicenter, open-label, phase 2 trial. Lancet Oncol. 2012;13:1087-1095.
28. Davies MA, Saiag P, Robert C, et al. Dabrafenib plus trametinib in patients with BRAFV600-mutant melanoma brain metastases (COMBI-MB): a multicenter, multicohort, open-label, phase 2 trial. Lancet Oncol. 2017;18:863-873.
Advanced Melanoma: First-line Therapy
Malignant melanoma is the most serious form of primary skin cancer and one of the only malignancies in which the incidence rate has been rising. It is estimated that in 2018 there were 91,270 newly diagnosed cases and 9320 deaths from advanced melanoma in the United States. Melanoma is the fifth most common cancer type in males and the sixth most common in females. Despite rising incidence rates, improvement in the treatment of advanced melanoma has resulted in declining death rates over the past decade.1 Although most melanoma is diagnosed at an early stage and can be cured with surgical excision, the prognosis for metastatic melanoma had been historically poor prior to recent advancements in treatment. Conventional chemotherapy treatment with dacarbazine or temozolomide resulted in response rates ranging from 7.5% to 12.1%, but without much impact on median overall survival (OS), with reported OS ranging from 6.4 to 7.8 months. Combination approaches with interferon alfa-2B and low-dose interleukin-2 resulted in improved response rates compared with traditional chemotherapy, but again without survival benefit.2
Immunotherapy in the form of high-dose interleukin-2 emerged as the first therapy to alter the natural history of advanced melanoma, with both improved response rates (objective response rate [ORR], 16%) and median OS (2 months), with some patients achieving durable responses lasting more than 30 months. However, significant systemic toxicity limited its application to carefully selected patients.3 The past decade has brought rapid advancements in treatment with immune checkpoint inhibitors and molecularly targeted agents, which have significantly improved ORRs, progression-free survival (PFS), and OS for patients with metastatic melanoma.4-8
This review is the first of 2 articles focusing on the treatment and sequencing of therapies in advanced melanoma. Here, we review the selection of first-line therapy for metastatic melanoma. Current evidence for immune checkpoint blockade and molecularly targeted agents in the treatment of metastatic melanoma after progression on first-line therapy is discussed in a separate article.
Pathogenesis
The incidence of melanoma is strongly associated with ultraviolet light–mediated DNA damage related to sun exposure. Specifically, melanoma is associated to a greater degree with intense intermittent sun exposure and sunburn, but not associated with higher occupational exposure.9 Ultraviolet radiation can induce DNA damage by a number of mechanisms, and deficient DNA repair leads to somatic mutations that drive the progression from normal melanocyte to melanoma.10
The most commonly identified genetic mutations in cutaneous melanomas are alterations in the mitogen-activated protein kinase (MAPK) pathway. Typically, an extracellular growth factor causes dimerization of the growth factor receptor, which activates the intracellular RAS GTPase protein. Subsequently BRAF is phosphorylated within the kinase domain, which leads to downstream activation of the MEK and ERK kinases through phosphorylation. Activated ERK leads to phosphorylation of various cytoplasmic and nuclear targets, and the downstream effects of these changes promote cellular proliferation. While activation of this pathway usually requires phosphorylation of BRAF by RAS, mutations placing an acidic amino acid near the kinase domain mimics phosphorylation and leads to constitutive activation of the BRAF serine/threonine kinase in the absence of upstream signaling from extracellular growth factors mediated through RAS.11 One study of tumor samples of 71 patients with cutaneous melanoma detected NRAS mutations in 30% and BRAF mutations in 59% of all tumors tested. Of the BRAF mutation–positive tumors, 88% harbored the Val599Glu mutation, now commonly referred to as the BRAF V600E mutation. The same study demonstrated that the vast majority of BRAF mutations were seen in the primary tumor and were preserved when metastases were analyzed. Additionally, both NRAS and BRAF mutations were detected in the radial growth phase of the melanoma tumor. These findings indicate that alterations in the MAPK pathway occur early in the pathogenesis of advanced melanoma.11 Another group demonstrated that 66% of malignant melanoma tumor samples harbored BRAF mutations, of which 80% were specifically the V600E mutation. In vitro assays showed that the BRAF V600E–mutated kinase had greater than 10-fold kinase activity compared to wild-type BRAF, and that this kinase enhanced cellular proliferation even when upstream NRAS signaling was inhibited.12
The Cancer Genome Atlas Network performed a large analysis of tumor samples from 331 different melanoma patients and studied variations at the DNA, RNA, and protein levels. The study established a framework of 4 notable genomic subtypes, including mutant BRAF (52%), mutant RAS (28%), mutant NF1 (14%), and triple wild-type (6%). Additionally, mRNA transcriptomic analysis of overexpressed genes identified 3 different subclasses, which were labeled as “immune,” “keratin,” and “MITF-low.” The immune subclass was characterized by increased expression of proteins found in immune cells, immune signaling molecules, immune checkpoint proteins, cytokines, and chemokines, and correlated with increased lymphocyte invasion within the tumor. Interestingly, in the post-accession survival analysis, the “immune” transcriptomic subclass was statistically correlated with an improved prognosis.13 Having an understanding of the molecular pathogenesis of advanced melanoma helps to create a framework for understanding the mechanisms of current standard of care therapies for the disease.
Case Presentation
A 62-year-old Caucasian man with a history of well-controlled type 2 diabetes mellitus and hypertension is being followed by his dermatologist for surveillance of melanocytic nevi. On follow-up he is noted to have an asymmetrical melanocytic lesion over the right scalp with irregular borders and variegated color. He is asymptomatic and the remainder of physical examination is unremarkable, as he has no other concerning skin lesions and no cervical, axillary, or inguinal lymphadenopathy.
How is melanoma diagnosed?
Detailed discussion about diagnosis and staging will be deferred in this review of treatment of advanced melanoma. In brief, melanoma is best diagnosed by excisional biopsy and histopathology. Staging of melanoma is done according to the American Joint Committee on Cancer’s (AJCC) Cancer Staging Manual, 8th edition, using a TNM staging system that incorporates tumor thickness (Breslow depth); ulceration; number of involved regional lymph nodes; presence of in-transit, satellite, and/or microsatellite metastases; distant metastases; and serum lactate dehydrogenase level.14
Case Continued
The patient undergoes a wide excisional biopsy of the right scalp lesion, which is consistent with malignant melanoma. Pathology demonstrates a Breslow depth of 2.6 mm, 2 mitotic figures/mm2, and no evidence of ulceration. He subsequently undergoes wide local excision with 0/3 sentinel lymph nodes positive for malignancy. His final staging is consistent with pT3aN0M0, stage IIA melanoma.
He is seen in follow-up with medical oncology for the next 3.5 years without any evidence of disease recurrence. He then develops symptoms of vertigo, diplopia, and recurrent falls, prompting medical attention. Magnetic resonance imaging (MRI) brain reveals multiple supratentorial and infratentorial lesions concerning for intracranial metastases. Further imaging with computed tomography (CT) chest/abdomen/pelvis reveals a right lower lobe pulmonary mass with right hilar and subcarinal lymphadenopathy. He is admitted for treatment with intravenous dexamethasone and further evaluation with endobronchial ultrasound-guided fine-needle aspiration of the right lower lobe mass, which reveals metastatic melanoma. Given the extent of his intracranial metastases, he is treated with whole brain radiation therapy for symptomatic relief prior to initiating systemic therapy.
What is the general approach to first-line treatment for metastatic melanoma?
The past decade has brought an abundance of data supporting the use of immunotherapy with immune checkpoint inhibitors or molecularly targeted therapy with combined BRAF/MEK inhibitors in the first-line setting.4-8 After the diagnosis of metastatic melanoma has been made, molecular testing is recommended to determine the BRAF status of the tumor. Immunotherapy is the clear choice for first-line therapy in the absence of an activating BRAF V600 mutation. When a BRAF V600 mutation is present, current evidence supports the use of either immunotherapy or molecularly targeted therapy as first-line therapy.
To date, there have been no prospective clinical trials comparing the sequencing of immunotherapy and molecularly targeted therapy in the first-line setting. An ongoing clinical trial (NCT02224781) is comparing dabrafenib and trametinib followed by ipilimumab and nivolumab at time of progression to ipilimumab and nivolumab followed by dabrafenib and trametinib in patients with newly diagnosed stage III/IV BRAF V600 mutation–positive melanoma. The primary outcome measure is 2-year OS. Until completion of that trial, current practice regarding which type of therapy to use in the first-line setting is based on a number of factors including clinical characteristics and provider preferences.
Data suggest that immunotherapies can produce durable responses, especially after treatment completion or discontinuation, albeit at the expense of taking a longer time to achieve clinical benefit and the risk of potentially serious immune-related adverse effects. This idea of a durable, off-treatment response is highlighted by a study that followed 105 patients who had achieved a complete response (CR) and found that 24-month disease-free survival from the time of CR was 90.9% in all patients and 89.9% in the 67 patients who had discontinued pembrolizumab after attaining CR.15 BRAF/MEK inhibition has the potential for rapid clinical responses, though concerns exist about the development of resistance to therapy. The following sections explore the evidence supporting the use of these therapies.
Immunotherapy with Immune Checkpoint Inhibitors
Immunotherapy via immune checkpoint blockade has revolutionized the treatment of many solid tumors over the past decade. The promise of immunotherapy revolves around the potential for achieving a dynamic and durable systemic response against cancer by augmenting the antitumor effects of the immune system. T-cells are central to mounting a systemic antitumor response, and, in addition to antigen recognition, their function depends heavily on fine tuning between co-stimulatory and co-inhibitory signaling. The cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) expressed on T-cells was the first discovered co-inhibitory receptor of T-cell activation.16 Later, it was discovered that the programmed cell death 1 receptor (PD-1), expressed on T-cells, and its ligands PD-L1 and PD-L2, expressed on antigen presenting cells, tumor cells, or other cells in the tumor microenvironment, also served as a potent negative regulator of T-cell function.17
Together, these 2 signaling pathways help to maintain peripheral immune tolerance, whereby autoreactive T-cells that have escaped from the thymus are silenced to prevent autoimmunity. However, these pathways can also be utilized by cancer cells to escape immune surveillance. Monoclonal antibodies that inhibit the aforementioned co-inhibitory signaling pathways, and thus augment the immune response, have proven to be an effective anticancer therapy capable of producing profound and durable responses in certain malignancies.16,17
Ipilimumab
Ipilimumab is a monoclonal antibody that inhibits the function of the CTLA-4 co-inhibitory immune checkpoint. In a phase 3 randomized controlled trial of 676 patients with previously treated metastatic melanoma, ipilimumab at a dose of 3 mg/kg every 3 weeks for 4 cycles, with or without a gp100 peptide vaccine, resulted in an improved median OS of 10.0 and 10.1 months, respectively, compared to 6.4 months in those receiving the peptide vaccine alone, meeting the primary endpoint.4 Subsequently, a phase 3 trial of 502 patients with untreated metastatic melanoma compared ipilimumab at a dose of 10 mg/kg every 3 weeks for 4 cycles plus dacarbazine to dacarbazine plus placebo and found a significant increase in median OS (11.2 months vs 9.1 months), with no additive benefit of chemotherapy. There was a higher reported rate of grade 3 or 4 adverse events in this trial with ipilimumab dosed at 10 mg/kg, which was felt to be dose-related.18 These trials were the first to show improved OS with any systemic therapy in metastatic melanoma and led to US Food and Drug Administration approval of ipilimumab for this indication in 2011.
PD-1 Inhibitor Monotherapy
The PD-1 inhibitors nivolumab and pembrolizumab were initially approved for metastatic melanoma after progression on ipilimumab. In the phase 1 trial of patients with previously treated metastatic melanoma, nivolumab therapy resulted in an ORR of 28%.19 The subsequent phase 2 trial conducted in pretreated patients, including patients who had progressed on ipilimumab, confirmed a similar ORR of 31%, as well as a median PFS of 3.7 months and a median OS of 16.8 months. The estimated response duration in patients who did achieve a response to therapy was 2 years.20 A phase 3 trial (CheckMate 037) comparing nivolumab (n = 120) to investigator’s choice chemotherapy (n = 47) in those with melanoma refractory to ipilimumab demonstrated that nivolumab was superior for the primary endpoint of ORR (31.7% vs 10.6%), had less toxicity (5% rate of grade 3 or 4 adverse events versus 9%), and increased median duration of response (32 months vs 13 months).21
The phase 1 trial (KEYNOTE-001) testing the efficacy of pembrolizumab demonstrated an ORR of 33% in the total population of patients treated and an ORR of 45% in those who were treatment-naive. Additionally, the median OS was 23 months for the total population and 31 months for treatment-naive patients, with only 14% of patients experiencing a grade 3 or 4 adverse event.22 The KEYNOTE-002 phase 2 trial compared 2 different pembrolizumab doses (2 mg/kg and 10 mg/kg every 3 weeks) to investigator’s choice chemotherapy (paclitaxel plus carboplatin, paclitaxel, carboplatin, dacarbazine, or oral temozolomide) in 540 patients with advanced melanoma with documented progression on ipilimumab with or without prior progression on molecularly targeted therapy if positive for a BRAF V600 mutation. The final analysis demonstrated significantly improved ORR with pembrolizumab (22% at 2 mg/kg vs 26% at 10 mg/kg vs 4% chemotherapy) and significantly improved 24-month PFS (16% vs 22% vs 0.6%, respectively). There was a nonstatistically significant improvement in median OS (13.4 months vs 14.7 months vs 10 months), although 55% of the patients initially assigned to the chemotherapy arm crossed over and received pembrolizumab after documentation of progressive disease.23,24
Because PD-1 inhibition improved efficacy with less toxicity than chemotherapy when studied in progressive disease, subsequent studies focused on PD-1 inhibition in the frontline setting. CheckMate 066 was a phase 3 trial comparing nivolumab to dacarbazine as first-line therapy for 418 patients with untreated metastatic melanoma who did not have a BRAF mutation. For the primary end point of 1-year OS, nivolumab was superior to dacarbazine (72.9% vs 42.1%; hazard ratio [HR], 0.42; P < 0.001). Treatment with nivolumab also resulted in superior ORR (40% vs 14%) and PFS (5.1 months vs 2.2 months). Additionally, nivolumab therapy had a lower rate of grade 3 or 4 toxicity compared to dacarbazine (11.7% vs 17.6%).25
The KEYNOTE-006 trial compared 2 separate dosing schedules of pembrolizumab (10 mg/kg every 2 weeks versus every 3 weeks) to ipilimumab (3 mg/kg every 3 weeks for 4 cycles) in a 1:1:1 ratio in 834 patients with metastatic melanoma who had received up to 1 prior systemic therapy, but no prior CTLA-4 or PD-1 inhibitors. The first published data reported statistically significant outcomes for the co-primary end points of 6-month PFS (47.3% for pembrolizumab every 2 weeks vs 46.4% for pembrolizumab every 3 weeks vs 26.5% for ipilimumab; HR, 0.58 for both pembrolizumab groups compared to ipilimumab; P < 0.001) and 12-month OS (74.1% vs 68.4% vs 58.2%) with pembrolizumab compared to ipilimumab. Compared to ipilimumab, pembrolizumab every 2 weeks had a hazard ratio of 0.63 (P = 0.0005) and pembrolizumab every 3 weeks had a hazard ratio of 0.69 (P = 0.0036). The pembrolizumab groups was also had lower rates of grade 3 to 5 toxicity (13.3% vs 10.1% vs 19.9%).5 Updated outcomes demonstrated improved ORR compared to the first analysis (37% vs 36% vs 13%), and improved OS (median OS, not reached for the pembrolizumab groups vs 16.0 months for the ipilimumab group; HR, 0.68, P = 0.0009 for pembrolizumab every 2 weeks versus HR 0.68, P = 0.0008 for pembrolizumab every 3 weeks).26 In addition, 24-month OS was 55% in both pembrolizumab groups compared to 43% in the ipilimumab group. Grade 3 or 4 toxicity occurred less frequently with pembrolizumab (17% vs 17% vs 20%).
Further analysis from the KEYNOTE-006 trial data demonstrated improved ORR, PFS, and OS with pembrolizumab compared to ipilimumab in tumors positive for PD-L1 expression. For PD-L1-negative tumors, response rate was higher, and PFS and OS rates were similar with pembrolizumab compared to ipilimumab. Given that pembrolizumab was associated with similar survival outcomes in PD-L1-negative tumors and with less toxicity than ipilimumab, the superiority of PD-L1 inhibitors over ipilimumab was further supported, regardless of tumor PD-L1 status.27
In sum, PD-1 inhibition should be considered the first-line immunotherapy in advanced melanoma, either alone or in combination with ipilimumab, as discussed in the following section. There is no longer a role for ipilimumab monotherapy in the first-line setting, based on evidence from direct comparison to single-agent PD-1 inhibition in clinical trials that demonstrated superior efficacy and less serious toxicity with PD-1 inhibitors.5,26 The finding that ORR and OS outcomes with single-agent PD-1 inhibitors are higher in treatment-naive patients compared to those receiving prior therapies also supports this approach.22
Combination CTLA-4 and PD-1 Therapy
Despite the potential for durable responses, the majority of patients fail to respond to single-agent PD-1 therapy. Given that preclinical data had suggested the potential for synergy between dual inhibition of CTLA-4 and PD-1, clinical trials were designed to test this approach. The first randomized phase 2 trial that established superior efficacy with combination therapy was the CheckMate 069 trial comparing nivolumab plus ipilimumab to ipilimumab monotherapy. Combination therapy resulted in increased ORR (59% vs 11%), median PFS (not reached vs 3.0 months), 2-year PFS (51.3% vs 12.0%), and 2-year OS (63.8% vs 53.6%).28 Similarly, a phase 1b trial of pembrolizumab plus reduced-dose ipilimumab demonstrated an ORR of 61%, with a 1-year PFS of 69% and 1-year OS of 89%.29
The landmark phase 3 CheckMate 067 trial analyzed efficacy outcomes for 3 different treatment regimens including nivolumab plus ipilimumab, nivolumab monotherapy, and ipilimumab monotherapy in previously untreated patients with unresectable stage III or IV melanoma. The trial was powered to compare survival outcomes for both the combination therapy arm against ipilimumab and the nivolumab monotherapy arm against ipilimumab, but not to compare combination therapy to nivolumab monotherapy. The initial analysis demonstrated a median PFS of 11.5 months with combination therapy versus 6.9 months with nivolumab and 2.9 months with ipilimumab, as well as an ORR of 58% versus 44% and 19%, respectively (Table 1).6 The updated 3-year survival outcomes from CheckMate 067 were notable for superior median OS with combination therapy (not reached in combination vs 37.6 months for nivolumab vs 19.9 months ipilimumab), improved 3-year OS (58% vs 52% vs 34%), and improved 3-year PFS (39% vs 32% vs 10%).7 In the reported 4-year survival outcomes, median OS was not reached in the combination therapy group, and was 36.9 months in the nivolumab monotherapy group and 19.9 months in the ipilimumab monotherapy group. Rates of grade 3 or 4 adverse events were significantly higher in the combination therapy group, at 59% compared to 22% with nivolumab monotherapy and 28% with ipilimumab alone.30 The 3- and 4-year OS outcomes (58% and 54%, respectively) with combination therapy were the highest seen in any phase 3 trial for treatment of advanced melanoma, supporting its use as the best approved first-line therapy in those who can tolerate the potential toxicity of combination therapy7,30 The conclusions from this landmark trial were that both combination therapy and nivolumab monotherapy resulted in statistically significant improvement in OS compared to ipilimumab.
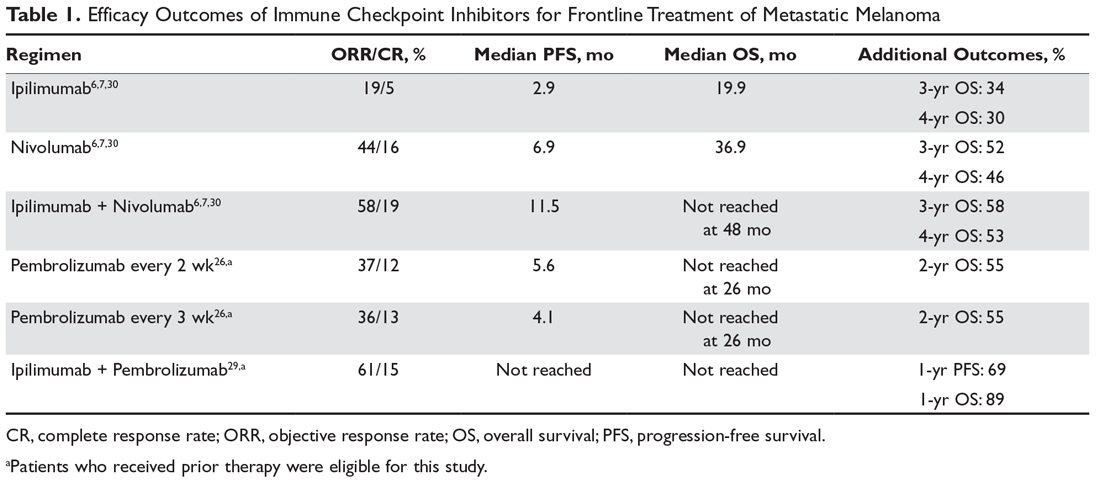
Toxicity Associated with Immune Checkpoint Inhibitors
While immune checkpoint inhibitors have revolutionized the treatment of many solid tumor malignancies, this new class of cancer therapy has brought about a new type of toxicity for clinicians to be aware of, termed immune-related adverse events (irAEs). As immune checkpoint inhibitors amplify the immune response against malignancy, they also increase the likelihood that autoreactive T-cells persist and proliferate within the circulation. Therefore, these therapies can result in almost any type of autoimmune side effect. The most commonly reported irAEs in large clinical trials studying CTLA-4 and PD-1 inhibitors include rash/pruritus, diarrhea/colitis, hepatitis, endocrinopathies (thyroiditis, hypophysitis, adrenalitis), and pneumonitis. Other more rare toxicities include pancreatitis, autoimmune hematologic toxicities, cardiac toxicity (myocarditis, heart failure), and neurologic toxicities (neuropathies, myasthenia gravis-like syndrome, Guillain-Barré syndrome). It has been observed that PD-1 inhibitors have a lower incidence of irAEs than CTLA-4 inhibitors, and that the combined use of PD-1 and CTLA-4 inhibitors is associated with a greater incidence of irAEs compared to monotherapy with either agent.31 Toxicities associated with ipilimumab have been noted to be dose dependent.18 Generally, these toxicities are treated with immunosuppression in the form of glucocorticoids and are often reversible.31 There are several published guidelines that include algorithms for the management of irAEs by organizations such as the National Comprehensive Cancer Network.32
For example, previously untreated patients treated with ipilimumab plus dacarbazine as compared to dacarbazine plus placebo had greater grade 3 or 4 adverse events (56.3% vs 27.5%), and 77.7% of patients experiencing an irAE of any grade.18 In the CheckMate 066 trial comparing frontline nivolumab to dacarbazine, nivolumab had a lower rate of grade 3 or 4 toxicity (11.7% vs 17.6%) and irAEs were relatively infrequent, with diarrhea and elevated alanine aminotransferase level each being the most prominent irAE (affecting 1.0% of patients).25 In the KEYNOTE-006 trial, irAEs seen in more than 1% of patients treated with pembrolizumab included colitis, hepatitis, hypothyroidism, and hyperthyroidism, whereas those occurring in more than 1% of patients treated with ipilimumab included colitis and hypophysitis. Overall, there were lower rates of grade 3 to 5 toxicity with the 2 pembrolizumab doses compared to ipilimumab (13.3% pembrolizumab every 2 weeks vs 10.1% pembrolizumab every 3 weeks vs 19.9% ipilimumab).5 In the CheckMate 067 trial comparing nivolumab plus ipilimumab, nivolumab monotherapy, and ipilimumab monotherapy, rates of treatment-related adverse events of any grade were higher in the combination group (96% combination vs 86% nivolumab vs 86% ipilimumab), as were rates of grade 3 or 4 adverse events (59% vs 21% vs 28%, respectively). The irAE profile was similar to that demonstrated in prior studies: rash/pruritus were the most common, and diarrhea/colitis, elevated aminotransferases, and endocrinopathies were among the more common irAEs.7
Alternative dosing strategies have been investigated in an effort to preserve efficacy and minimize toxicity. A phase 1b trial of pembrolizumab plus reduced-dose ipilimumab demonstrated an ORR of 61%, with a 1-year PFS of 69% and a 1-year OS of 89%. This combination led to 45% of patients having a grade 3 or 4 adverse event, 60% having irAEs of any grade, and only 27% having grade 3 or 4 irAEs.29 The CheckMate 067 trial studied the combination of nivolumab 1 mg/kg plus ipilimumab 3 mg/kg.6 The CheckMate 511 trial compared different combination dosing strategies (nivolumab 3 mg/kg + ipilimumab 1 mg/kg versus nivolumab 1 mg/kg + ipilimumab 3 mg/kg) to assess for safety benefit. In the results published in abstract form, the reduced ipilimumab dose (nivolumab 3 mg/kg + ipilimumab 1 mg/kg arm) resulted in significantly decreased grade 3 to 5 adverse events (33.9% vs 48.3%) without significant differences in ORR, PFS, or OS.33
The question about the efficacy of checkpoint inhibitors in patients who discontinue treatment due to irAEs has been raised, as one hypothesis suggests that such toxicities may also indicate that the antitumor immune response has been activated. In a retrospective pooled analysis of phase 2 and 3 trials where patients received combination therapy with ipilimumab and nivolumab and discontinued therapy during the induction phase due to irAEs, outcomes did not appear to be inferior. Median PFS was 8.4 months in those who discontinued therapy compared to 10.8 months in those who continued therapy, but this did not reach statistical significance. Median OS had not been reached in either group and ORR was actually higher in those who discontinued due to adverse events (58.3% vs 50.2%). While this retrospective analysis needs to be validated, it does suggest that patients likely derive antitumor benefit from immunotherapy even if they have to discontinue therapy due to irAEs. Of note, patients in this analysis were not trialed on nivolumab monotherapy after receiving immunosuppressive treatment for toxicity related to combination therapy, which has since been deemed a reasonable treatment option.34
Molecularly Targeted Therapy for Metastatic Melanoma
As previously mentioned, the MAPK pathway is frequently altered in metastatic melanoma and thus serves as a target for therapy. Mutations in BRAF can cause constitutive activation of the protein’s kinase function, which subsequently phosphorylates/activates MEK in the absence of extracellular growth signals and causes increased cellular proliferation. For the roughly half of patients diagnosed with metastatic melanoma who harbor a BRAF V600 mutation, molecularly targeted therapy with BRAF/MEK inhibitors has emerged as a standard of care treatment option. As such, all patients with advanced disease should be tested for BRAF mutations.
After early phase 1 studies of the BRAF inhibitor vemurafenib demonstrated successful inhibition of mutated BRAF,35 subsequent studies confirmed the benefit of BRAF targeted therapy. In the phase 3 randomized controlled BRIM-3 trial comparing vemurafenib with dacarbazine for treatment of 675 patients with previously untreated metastatic melanoma positive for a BRAF V600E mutation, the vemurafenib group had superior ORR and 6-month OS during the first analysis.36 In a subsequent analysis, median PFS and median OS were also superior with vemurafenib compared to dacarbazine, as vemurafenib had a median OS of 13.6 months compared to 9.7 months with dacarbazine (HR, 0.70; P = 0.0008).37 Dabrafenib was the next BRAF inhibitor to demonstrate clinical efficacy with superior PFS compared to dacarbazine.38
Despite tumor shrinkage in the majority of patients, the development of resistance to therapy was an issue early on. The development of acquired resistance emerged as a heterogeneous process, though many of the identified resistance mechanisms involved reactivation of the MAPK pathway.39 A phase 3 trial of 322 patients with metastatic melanoma comparing the MEK inhibitor trametinib as monotherapy against chemotherapy demonstrated a modest improvement in both median PFS and OS.40 As a result, subsequent efforts focused on a strategy of concurrent MEK inhibition as a means to overcome resistance to molecularly targeted monotherapy
At least 4 large phase 3 randomized controlled trials of combination therapy with BRAF plus MEK inhibitors showed an improved ORR, PFS, and OS when compared to BRAF inhibition alone. The COMBI-d trial comparing dabrafenib plus trametinib versus dabrafenib alone was the first to demonstrate the superiority of combined BRAF/MEK inhibition and made combination therapy the current standard of care for patients with metastatic melanoma and a BRAF V600 mutation. In the final analysis of this trial, 3-year PFS was 22% with combination therapy compared to 12% with dabrafenib alone, and 3-year OS was 44% compared to 32%.8,41,42 A second trial with the combination of dabrafenib and trametinib (COMBI-V) also demonstrated superior efficacy when compared to single-agent vemurafenib without increased toxicity.43 Subsequently, the combination of vemurafenib with MEK inhibitor cobimetinib demonstrated superiority compared to vemurafenib alone,44 followed by the newest combination encorafenib (BRAF inhibitor) and binimetinib (MEK inhibitor) proving superior to either vemurafenib or encorafenib alone.45,46
It is important to note that there have been no studies directly comparing the efficacy of the 3 approved BRAF/MEK inhibitor combinations, but the 3 different regimens have some differences in their toxicity profiles (Table 2). Of note, single-agent BRAF inhibition was associated with increased cutaneous toxicity, including secondary squamous cell carcinoma and keratoacanthoma,47 which was demonstrated to be driven by paradoxical activation of the MAPK pathway.48 The concerning cutaneous toxicities such as squamous cell carcinoma were substantially reduced by combination BRAF/MEK inhibitor therapy.47 Collectively, the higher efficacy along with manageable toxicity profile established combination BRAF/MEK inhibition as the preferred regimen for patients with BRAF-mutated metastatic melanoma who are being considered for molecularly targeted therapy. BRAF inhibitor monotherapy should only be used when there is a specific concern regarding the use of a MEK inhibitor in certain clinical circumstances.
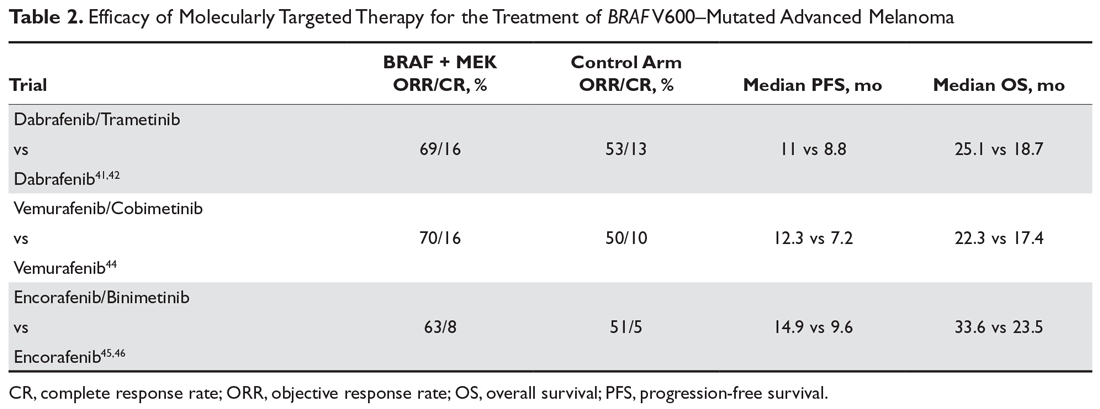
Other driver mutations associated with metastatic melanoma such as NRAS-mutated tumors have proven more difficult to effectively treat with molecularly targeted therapy, with one study showing that the MEK inhibitor binimetinib resulted in a modest improvement in ORR and median PFS without OS benefit compared to dacarbazine.49 Several phase 2 trials involving metastatic melanoma harboring a c-Kit alteration have demonstrated some efficacy with the tyrosine kinase inhibitor imatinib. The largest phase 2 trial of 43 patients treated with imatinib resulted in a 53.5% disease control rate (23.3% partial response and 30.2% stable disease), with 9 of the 10 patients who achieved partial response having a mutation in either exon 11 or 13. Median PFS was 3.5 months and 1-year OS was 51.0%.50
Case Conclusion
Prior to initiation of systemic therapy, the patient’s melanoma is tested and is found to be positive for a BRAF V600K mutation. At his follow-up appointment, the patient continues to endorse generalized weakness, fatigue, issues with balance, and residual pulmonary symptoms after being treated for post-obstructive pneumonia. Given his current symptoms and extent of metastatic disease, immunotherapy is deferred and he is started on combination molecularly targeted therapy with dabrafenib and trametinib. He initially does well, with a partial response noted by resolution of symptoms and decreased size of his intracranial metastases and decreased size of the right lower lobe mass. Further follow-up of this patient is presented in the second article in this 2-part review of advanced melanoma.
1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2018. CA Cancer J Clin. 2018;68:7-30.
2. Ives NJ, Stowe RL, Lorigan P, Wheatley K. Chemotherapy compared with biochemotherapy for the treatment of metastatic melanoma: a meta-analysis of 18 trials involving 2621 patients. J Clin Oncol. 2007;25:5426-34.
3. Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105-16.
4. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711-23.
5. Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2522-2532.
6. Larkin J, Chiarion-Sileni V, Gonazalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23-34.
7. Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377:1345-1356.
8. Long GV, Stroyakovskiy D, Gogas H, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med. 2014;371:1877-1888.
9. Elwood JM, Jopson J. Melanoma and sun exposure: an overview of published studies. Int J Cancer. 1997;73:198-203.
10. Gilchrest BA, Eller MS, Geller AC, Yaar M. The pathogenesis of melanoma induced by ultraviolet radiation. N Engl J Med. 199;340:1341-1348.
11. Omholt K, Platz A, Kanter L, et al. NRAS and BRAF mutations arise early during melanoma pathogenesis and are preserved throughout tumor progression. Clin Cancer Res. 2003;9:6483-8.
12. Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949-54.
13. Cancer Genome Atlas Network. Genomic classification of cutaneous melanoma. Cell 2015;161:1681-96.
14. Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: evidence-based changes in the American Joint Committee on Cancer Eighth Edition Cancer Staging Manual. CA Cancer J Clin. 2017;67:472-492.
15. Robert C, Ribas A, Hamid O, et al. Durable complete response after discontinuation of pembrolizumab in patients with metastatic melanoma. J Clin Oncol. 2018;36:1668-1674.
16. Salama AKS, Hodi FS. Cytotoxic T-lymphocyte-associated antigen-4. Clin Cancer Res. 2011;17:4622-8.
17. Boussiotis VA. Molecular and biochemical aspects of the PD-1 checkpoint pathway. N Engl J Med. 2016;375:1767-1778.
18. Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517-2526.
19. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443-2454.
20. Topalian S, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020-30.
21. Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375-84.
22. Ribas A, Hamid O, Daud A, et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA. 2016;315:1600-1609.
23. Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16:908-18.
24. Hamid O, Puzanov I, Dummer R, et al. Final analysis of a randomised trial comparing pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory advanced melanoma. Eur J Cancer. 2017;86:37-45.
25. Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320-330.
26. Schachter J, Ribas A, Long GV, et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicenter, randomised, open-label phase 3 study (KEYNOTE-006). Lancet Oncol. 2017;390:1853-1862.
27. Carlino MS, Long GV, Schadendorf D, et al. Outcomes by line of therapy and programmed death ligand 1 expression in patients with advanced melanoma treated with pembrolizumab or ipilimumab in KEYNOTE-006. A randomised clinical trial. Eur J Cancer. 2018;101:236-243.
28. Hodi FS, Chesney J, Pavlick AC, et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2016;17:1558-1568.
29. Long GV, Atkinson V, Cebon JS, et al. Standard-dose pembrolizumab in combination with reduced-dose ipilimumab for patients with advanced melanoma (KEYNOTE-029): an open-label, phase 1b trial. Lancet Oncol. 2017;18:1202-10.
30. Hodi FS, Chiarion-Sileni V, Gonzalez R, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19:1480-1492.
31. Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: a review. JAMA Oncol. 2016;2:1346-1353.
32. National Comprehensive Cancer Network. Management of immunotherapy-related toxicities (version 2.2019). www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf. Accessed April 8, 2019.
33. Lebbé C, Meyer N, Mortier L, et al. Initial results from a phase IIIb/IV study evaluating two dosing regimens of nivolumab (NIVO) in combination with ipilimumab (IPI) in patients with advanced melanoma (CheckMate 511) [Abstract LBA47]. Ann Oncol. 2018;29:mdy424.057.
34. Schadendorf D, Wolchok JD, Hodi FS, et al. Efficacy and safety outcomes in patients with advanced melanoma who discontinued treatment with nivolumab and ipilimumab because of adverse events: a pooled analysis of randomized phase ii and iii trials. J Clin Oncol. 2017;35:3807-3814.
35. Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809-819.
36. Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507-2516.
37. McArthur GA, Chapman PB, Robert C, et al. Safety and efficacy of vemurafenib in BRAFV600E and BRAFV600K mutation-positive melanoma (BRIM-3): extended follow up of a phase 3, randomised, open-label study. Lancet Oncol. 2014;15:323-332.
38. Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicenter, open-label, phase 3 randomised controlled trial. Lancet Oncol. 2012;380:358-365.
39. Rizos H, Menzies AM, Pupo GM, et al. BRAF inhibitor resistance mechanisms in metastatic melanoma: spectrum and clinical impact. Clin Cancer Res. 2014;20:1965-1977.
40. Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367:107-114.
41. Long GV, Stroyakovskiy D, Gogas H, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicenter, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2015;386:444-451.
42. Long GV, Flaherty KT, Stroyakovskiy D, et al. Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: long-term survival and safety analysis of a phase 3 study. Ann Oncol. 2017;28:1631-1639.
43. Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30-39.
44. Ascierto PA, McArthur GA, Dréno B, et al. Cobimetinib combined with vemurafenib in advanced BRAFV600-mutant melanoma (coBRIM): updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol. 2016;17:1248-260.
45. Dummer R, Ascierto PA, Gogas HJ, et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): a multicenter, open-label, randomised phase 3 trial. Lancet Oncol. 2018;19:603-615.
46. Dummer R, Ascierto PA, Gogas HJ, et al. Overall survival in patients with BRAF-mutant melanoma receiving encorafenib plus binimetinib versus vemurafenib or encorafenib (COLUMBUS): a multicenter, open-label, randomised, phase 3 trial. Lancet Oncol. 2018;19:1315-1327.
47. Carlos G, Anforth R, Clements A, et al. Cutaneous toxic effects of BRAF inhibitors alone and in combination with MEK inhibitors for metastatic melanoma. JAMA. Dermatol 2015;151:1103-1109.
48. Su F, Viros A, Milagre C, et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med. 2012;366:207-215.
49. Dummer R, Schadendorf D, Ascierto P, et al. Binimetinib versus dacarbazine in patients with advanced NRAS-mutant melanoma (NEMO): a multicenter, open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18:435-445.
50. Guo J, Si L, Kong Y, et al. Phase II, open-label, single-arm trial of imatinib mesylate in patients with metastatic melanoma harboring c-Kit mutation or amplification. J Clin Oncol. 2011;29:2904-2909.
Malignant melanoma is the most serious form of primary skin cancer and one of the only malignancies in which the incidence rate has been rising. It is estimated that in 2018 there were 91,270 newly diagnosed cases and 9320 deaths from advanced melanoma in the United States. Melanoma is the fifth most common cancer type in males and the sixth most common in females. Despite rising incidence rates, improvement in the treatment of advanced melanoma has resulted in declining death rates over the past decade.1 Although most melanoma is diagnosed at an early stage and can be cured with surgical excision, the prognosis for metastatic melanoma had been historically poor prior to recent advancements in treatment. Conventional chemotherapy treatment with dacarbazine or temozolomide resulted in response rates ranging from 7.5% to 12.1%, but without much impact on median overall survival (OS), with reported OS ranging from 6.4 to 7.8 months. Combination approaches with interferon alfa-2B and low-dose interleukin-2 resulted in improved response rates compared with traditional chemotherapy, but again without survival benefit.2
Immunotherapy in the form of high-dose interleukin-2 emerged as the first therapy to alter the natural history of advanced melanoma, with both improved response rates (objective response rate [ORR], 16%) and median OS (2 months), with some patients achieving durable responses lasting more than 30 months. However, significant systemic toxicity limited its application to carefully selected patients.3 The past decade has brought rapid advancements in treatment with immune checkpoint inhibitors and molecularly targeted agents, which have significantly improved ORRs, progression-free survival (PFS), and OS for patients with metastatic melanoma.4-8
This review is the first of 2 articles focusing on the treatment and sequencing of therapies in advanced melanoma. Here, we review the selection of first-line therapy for metastatic melanoma. Current evidence for immune checkpoint blockade and molecularly targeted agents in the treatment of metastatic melanoma after progression on first-line therapy is discussed in a separate article.
Pathogenesis
The incidence of melanoma is strongly associated with ultraviolet light–mediated DNA damage related to sun exposure. Specifically, melanoma is associated to a greater degree with intense intermittent sun exposure and sunburn, but not associated with higher occupational exposure.9 Ultraviolet radiation can induce DNA damage by a number of mechanisms, and deficient DNA repair leads to somatic mutations that drive the progression from normal melanocyte to melanoma.10
The most commonly identified genetic mutations in cutaneous melanomas are alterations in the mitogen-activated protein kinase (MAPK) pathway. Typically, an extracellular growth factor causes dimerization of the growth factor receptor, which activates the intracellular RAS GTPase protein. Subsequently BRAF is phosphorylated within the kinase domain, which leads to downstream activation of the MEK and ERK kinases through phosphorylation. Activated ERK leads to phosphorylation of various cytoplasmic and nuclear targets, and the downstream effects of these changes promote cellular proliferation. While activation of this pathway usually requires phosphorylation of BRAF by RAS, mutations placing an acidic amino acid near the kinase domain mimics phosphorylation and leads to constitutive activation of the BRAF serine/threonine kinase in the absence of upstream signaling from extracellular growth factors mediated through RAS.11 One study of tumor samples of 71 patients with cutaneous melanoma detected NRAS mutations in 30% and BRAF mutations in 59% of all tumors tested. Of the BRAF mutation–positive tumors, 88% harbored the Val599Glu mutation, now commonly referred to as the BRAF V600E mutation. The same study demonstrated that the vast majority of BRAF mutations were seen in the primary tumor and were preserved when metastases were analyzed. Additionally, both NRAS and BRAF mutations were detected in the radial growth phase of the melanoma tumor. These findings indicate that alterations in the MAPK pathway occur early in the pathogenesis of advanced melanoma.11 Another group demonstrated that 66% of malignant melanoma tumor samples harbored BRAF mutations, of which 80% were specifically the V600E mutation. In vitro assays showed that the BRAF V600E–mutated kinase had greater than 10-fold kinase activity compared to wild-type BRAF, and that this kinase enhanced cellular proliferation even when upstream NRAS signaling was inhibited.12
The Cancer Genome Atlas Network performed a large analysis of tumor samples from 331 different melanoma patients and studied variations at the DNA, RNA, and protein levels. The study established a framework of 4 notable genomic subtypes, including mutant BRAF (52%), mutant RAS (28%), mutant NF1 (14%), and triple wild-type (6%). Additionally, mRNA transcriptomic analysis of overexpressed genes identified 3 different subclasses, which were labeled as “immune,” “keratin,” and “MITF-low.” The immune subclass was characterized by increased expression of proteins found in immune cells, immune signaling molecules, immune checkpoint proteins, cytokines, and chemokines, and correlated with increased lymphocyte invasion within the tumor. Interestingly, in the post-accession survival analysis, the “immune” transcriptomic subclass was statistically correlated with an improved prognosis.13 Having an understanding of the molecular pathogenesis of advanced melanoma helps to create a framework for understanding the mechanisms of current standard of care therapies for the disease.
Case Presentation
A 62-year-old Caucasian man with a history of well-controlled type 2 diabetes mellitus and hypertension is being followed by his dermatologist for surveillance of melanocytic nevi. On follow-up he is noted to have an asymmetrical melanocytic lesion over the right scalp with irregular borders and variegated color. He is asymptomatic and the remainder of physical examination is unremarkable, as he has no other concerning skin lesions and no cervical, axillary, or inguinal lymphadenopathy.
How is melanoma diagnosed?
Detailed discussion about diagnosis and staging will be deferred in this review of treatment of advanced melanoma. In brief, melanoma is best diagnosed by excisional biopsy and histopathology. Staging of melanoma is done according to the American Joint Committee on Cancer’s (AJCC) Cancer Staging Manual, 8th edition, using a TNM staging system that incorporates tumor thickness (Breslow depth); ulceration; number of involved regional lymph nodes; presence of in-transit, satellite, and/or microsatellite metastases; distant metastases; and serum lactate dehydrogenase level.14
Case Continued
The patient undergoes a wide excisional biopsy of the right scalp lesion, which is consistent with malignant melanoma. Pathology demonstrates a Breslow depth of 2.6 mm, 2 mitotic figures/mm2, and no evidence of ulceration. He subsequently undergoes wide local excision with 0/3 sentinel lymph nodes positive for malignancy. His final staging is consistent with pT3aN0M0, stage IIA melanoma.
He is seen in follow-up with medical oncology for the next 3.5 years without any evidence of disease recurrence. He then develops symptoms of vertigo, diplopia, and recurrent falls, prompting medical attention. Magnetic resonance imaging (MRI) brain reveals multiple supratentorial and infratentorial lesions concerning for intracranial metastases. Further imaging with computed tomography (CT) chest/abdomen/pelvis reveals a right lower lobe pulmonary mass with right hilar and subcarinal lymphadenopathy. He is admitted for treatment with intravenous dexamethasone and further evaluation with endobronchial ultrasound-guided fine-needle aspiration of the right lower lobe mass, which reveals metastatic melanoma. Given the extent of his intracranial metastases, he is treated with whole brain radiation therapy for symptomatic relief prior to initiating systemic therapy.
What is the general approach to first-line treatment for metastatic melanoma?
The past decade has brought an abundance of data supporting the use of immunotherapy with immune checkpoint inhibitors or molecularly targeted therapy with combined BRAF/MEK inhibitors in the first-line setting.4-8 After the diagnosis of metastatic melanoma has been made, molecular testing is recommended to determine the BRAF status of the tumor. Immunotherapy is the clear choice for first-line therapy in the absence of an activating BRAF V600 mutation. When a BRAF V600 mutation is present, current evidence supports the use of either immunotherapy or molecularly targeted therapy as first-line therapy.
To date, there have been no prospective clinical trials comparing the sequencing of immunotherapy and molecularly targeted therapy in the first-line setting. An ongoing clinical trial (NCT02224781) is comparing dabrafenib and trametinib followed by ipilimumab and nivolumab at time of progression to ipilimumab and nivolumab followed by dabrafenib and trametinib in patients with newly diagnosed stage III/IV BRAF V600 mutation–positive melanoma. The primary outcome measure is 2-year OS. Until completion of that trial, current practice regarding which type of therapy to use in the first-line setting is based on a number of factors including clinical characteristics and provider preferences.
Data suggest that immunotherapies can produce durable responses, especially after treatment completion or discontinuation, albeit at the expense of taking a longer time to achieve clinical benefit and the risk of potentially serious immune-related adverse effects. This idea of a durable, off-treatment response is highlighted by a study that followed 105 patients who had achieved a complete response (CR) and found that 24-month disease-free survival from the time of CR was 90.9% in all patients and 89.9% in the 67 patients who had discontinued pembrolizumab after attaining CR.15 BRAF/MEK inhibition has the potential for rapid clinical responses, though concerns exist about the development of resistance to therapy. The following sections explore the evidence supporting the use of these therapies.
Immunotherapy with Immune Checkpoint Inhibitors
Immunotherapy via immune checkpoint blockade has revolutionized the treatment of many solid tumors over the past decade. The promise of immunotherapy revolves around the potential for achieving a dynamic and durable systemic response against cancer by augmenting the antitumor effects of the immune system. T-cells are central to mounting a systemic antitumor response, and, in addition to antigen recognition, their function depends heavily on fine tuning between co-stimulatory and co-inhibitory signaling. The cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) expressed on T-cells was the first discovered co-inhibitory receptor of T-cell activation.16 Later, it was discovered that the programmed cell death 1 receptor (PD-1), expressed on T-cells, and its ligands PD-L1 and PD-L2, expressed on antigen presenting cells, tumor cells, or other cells in the tumor microenvironment, also served as a potent negative regulator of T-cell function.17
Together, these 2 signaling pathways help to maintain peripheral immune tolerance, whereby autoreactive T-cells that have escaped from the thymus are silenced to prevent autoimmunity. However, these pathways can also be utilized by cancer cells to escape immune surveillance. Monoclonal antibodies that inhibit the aforementioned co-inhibitory signaling pathways, and thus augment the immune response, have proven to be an effective anticancer therapy capable of producing profound and durable responses in certain malignancies.16,17
Ipilimumab
Ipilimumab is a monoclonal antibody that inhibits the function of the CTLA-4 co-inhibitory immune checkpoint. In a phase 3 randomized controlled trial of 676 patients with previously treated metastatic melanoma, ipilimumab at a dose of 3 mg/kg every 3 weeks for 4 cycles, with or without a gp100 peptide vaccine, resulted in an improved median OS of 10.0 and 10.1 months, respectively, compared to 6.4 months in those receiving the peptide vaccine alone, meeting the primary endpoint.4 Subsequently, a phase 3 trial of 502 patients with untreated metastatic melanoma compared ipilimumab at a dose of 10 mg/kg every 3 weeks for 4 cycles plus dacarbazine to dacarbazine plus placebo and found a significant increase in median OS (11.2 months vs 9.1 months), with no additive benefit of chemotherapy. There was a higher reported rate of grade 3 or 4 adverse events in this trial with ipilimumab dosed at 10 mg/kg, which was felt to be dose-related.18 These trials were the first to show improved OS with any systemic therapy in metastatic melanoma and led to US Food and Drug Administration approval of ipilimumab for this indication in 2011.
PD-1 Inhibitor Monotherapy
The PD-1 inhibitors nivolumab and pembrolizumab were initially approved for metastatic melanoma after progression on ipilimumab. In the phase 1 trial of patients with previously treated metastatic melanoma, nivolumab therapy resulted in an ORR of 28%.19 The subsequent phase 2 trial conducted in pretreated patients, including patients who had progressed on ipilimumab, confirmed a similar ORR of 31%, as well as a median PFS of 3.7 months and a median OS of 16.8 months. The estimated response duration in patients who did achieve a response to therapy was 2 years.20 A phase 3 trial (CheckMate 037) comparing nivolumab (n = 120) to investigator’s choice chemotherapy (n = 47) in those with melanoma refractory to ipilimumab demonstrated that nivolumab was superior for the primary endpoint of ORR (31.7% vs 10.6%), had less toxicity (5% rate of grade 3 or 4 adverse events versus 9%), and increased median duration of response (32 months vs 13 months).21
The phase 1 trial (KEYNOTE-001) testing the efficacy of pembrolizumab demonstrated an ORR of 33% in the total population of patients treated and an ORR of 45% in those who were treatment-naive. Additionally, the median OS was 23 months for the total population and 31 months for treatment-naive patients, with only 14% of patients experiencing a grade 3 or 4 adverse event.22 The KEYNOTE-002 phase 2 trial compared 2 different pembrolizumab doses (2 mg/kg and 10 mg/kg every 3 weeks) to investigator’s choice chemotherapy (paclitaxel plus carboplatin, paclitaxel, carboplatin, dacarbazine, or oral temozolomide) in 540 patients with advanced melanoma with documented progression on ipilimumab with or without prior progression on molecularly targeted therapy if positive for a BRAF V600 mutation. The final analysis demonstrated significantly improved ORR with pembrolizumab (22% at 2 mg/kg vs 26% at 10 mg/kg vs 4% chemotherapy) and significantly improved 24-month PFS (16% vs 22% vs 0.6%, respectively). There was a nonstatistically significant improvement in median OS (13.4 months vs 14.7 months vs 10 months), although 55% of the patients initially assigned to the chemotherapy arm crossed over and received pembrolizumab after documentation of progressive disease.23,24
Because PD-1 inhibition improved efficacy with less toxicity than chemotherapy when studied in progressive disease, subsequent studies focused on PD-1 inhibition in the frontline setting. CheckMate 066 was a phase 3 trial comparing nivolumab to dacarbazine as first-line therapy for 418 patients with untreated metastatic melanoma who did not have a BRAF mutation. For the primary end point of 1-year OS, nivolumab was superior to dacarbazine (72.9% vs 42.1%; hazard ratio [HR], 0.42; P < 0.001). Treatment with nivolumab also resulted in superior ORR (40% vs 14%) and PFS (5.1 months vs 2.2 months). Additionally, nivolumab therapy had a lower rate of grade 3 or 4 toxicity compared to dacarbazine (11.7% vs 17.6%).25
The KEYNOTE-006 trial compared 2 separate dosing schedules of pembrolizumab (10 mg/kg every 2 weeks versus every 3 weeks) to ipilimumab (3 mg/kg every 3 weeks for 4 cycles) in a 1:1:1 ratio in 834 patients with metastatic melanoma who had received up to 1 prior systemic therapy, but no prior CTLA-4 or PD-1 inhibitors. The first published data reported statistically significant outcomes for the co-primary end points of 6-month PFS (47.3% for pembrolizumab every 2 weeks vs 46.4% for pembrolizumab every 3 weeks vs 26.5% for ipilimumab; HR, 0.58 for both pembrolizumab groups compared to ipilimumab; P < 0.001) and 12-month OS (74.1% vs 68.4% vs 58.2%) with pembrolizumab compared to ipilimumab. Compared to ipilimumab, pembrolizumab every 2 weeks had a hazard ratio of 0.63 (P = 0.0005) and pembrolizumab every 3 weeks had a hazard ratio of 0.69 (P = 0.0036). The pembrolizumab groups was also had lower rates of grade 3 to 5 toxicity (13.3% vs 10.1% vs 19.9%).5 Updated outcomes demonstrated improved ORR compared to the first analysis (37% vs 36% vs 13%), and improved OS (median OS, not reached for the pembrolizumab groups vs 16.0 months for the ipilimumab group; HR, 0.68, P = 0.0009 for pembrolizumab every 2 weeks versus HR 0.68, P = 0.0008 for pembrolizumab every 3 weeks).26 In addition, 24-month OS was 55% in both pembrolizumab groups compared to 43% in the ipilimumab group. Grade 3 or 4 toxicity occurred less frequently with pembrolizumab (17% vs 17% vs 20%).
Further analysis from the KEYNOTE-006 trial data demonstrated improved ORR, PFS, and OS with pembrolizumab compared to ipilimumab in tumors positive for PD-L1 expression. For PD-L1-negative tumors, response rate was higher, and PFS and OS rates were similar with pembrolizumab compared to ipilimumab. Given that pembrolizumab was associated with similar survival outcomes in PD-L1-negative tumors and with less toxicity than ipilimumab, the superiority of PD-L1 inhibitors over ipilimumab was further supported, regardless of tumor PD-L1 status.27
In sum, PD-1 inhibition should be considered the first-line immunotherapy in advanced melanoma, either alone or in combination with ipilimumab, as discussed in the following section. There is no longer a role for ipilimumab monotherapy in the first-line setting, based on evidence from direct comparison to single-agent PD-1 inhibition in clinical trials that demonstrated superior efficacy and less serious toxicity with PD-1 inhibitors.5,26 The finding that ORR and OS outcomes with single-agent PD-1 inhibitors are higher in treatment-naive patients compared to those receiving prior therapies also supports this approach.22
Combination CTLA-4 and PD-1 Therapy
Despite the potential for durable responses, the majority of patients fail to respond to single-agent PD-1 therapy. Given that preclinical data had suggested the potential for synergy between dual inhibition of CTLA-4 and PD-1, clinical trials were designed to test this approach. The first randomized phase 2 trial that established superior efficacy with combination therapy was the CheckMate 069 trial comparing nivolumab plus ipilimumab to ipilimumab monotherapy. Combination therapy resulted in increased ORR (59% vs 11%), median PFS (not reached vs 3.0 months), 2-year PFS (51.3% vs 12.0%), and 2-year OS (63.8% vs 53.6%).28 Similarly, a phase 1b trial of pembrolizumab plus reduced-dose ipilimumab demonstrated an ORR of 61%, with a 1-year PFS of 69% and 1-year OS of 89%.29
The landmark phase 3 CheckMate 067 trial analyzed efficacy outcomes for 3 different treatment regimens including nivolumab plus ipilimumab, nivolumab monotherapy, and ipilimumab monotherapy in previously untreated patients with unresectable stage III or IV melanoma. The trial was powered to compare survival outcomes for both the combination therapy arm against ipilimumab and the nivolumab monotherapy arm against ipilimumab, but not to compare combination therapy to nivolumab monotherapy. The initial analysis demonstrated a median PFS of 11.5 months with combination therapy versus 6.9 months with nivolumab and 2.9 months with ipilimumab, as well as an ORR of 58% versus 44% and 19%, respectively (Table 1).6 The updated 3-year survival outcomes from CheckMate 067 were notable for superior median OS with combination therapy (not reached in combination vs 37.6 months for nivolumab vs 19.9 months ipilimumab), improved 3-year OS (58% vs 52% vs 34%), and improved 3-year PFS (39% vs 32% vs 10%).7 In the reported 4-year survival outcomes, median OS was not reached in the combination therapy group, and was 36.9 months in the nivolumab monotherapy group and 19.9 months in the ipilimumab monotherapy group. Rates of grade 3 or 4 adverse events were significantly higher in the combination therapy group, at 59% compared to 22% with nivolumab monotherapy and 28% with ipilimumab alone.30 The 3- and 4-year OS outcomes (58% and 54%, respectively) with combination therapy were the highest seen in any phase 3 trial for treatment of advanced melanoma, supporting its use as the best approved first-line therapy in those who can tolerate the potential toxicity of combination therapy7,30 The conclusions from this landmark trial were that both combination therapy and nivolumab monotherapy resulted in statistically significant improvement in OS compared to ipilimumab.

Toxicity Associated with Immune Checkpoint Inhibitors
While immune checkpoint inhibitors have revolutionized the treatment of many solid tumor malignancies, this new class of cancer therapy has brought about a new type of toxicity for clinicians to be aware of, termed immune-related adverse events (irAEs). As immune checkpoint inhibitors amplify the immune response against malignancy, they also increase the likelihood that autoreactive T-cells persist and proliferate within the circulation. Therefore, these therapies can result in almost any type of autoimmune side effect. The most commonly reported irAEs in large clinical trials studying CTLA-4 and PD-1 inhibitors include rash/pruritus, diarrhea/colitis, hepatitis, endocrinopathies (thyroiditis, hypophysitis, adrenalitis), and pneumonitis. Other more rare toxicities include pancreatitis, autoimmune hematologic toxicities, cardiac toxicity (myocarditis, heart failure), and neurologic toxicities (neuropathies, myasthenia gravis-like syndrome, Guillain-Barré syndrome). It has been observed that PD-1 inhibitors have a lower incidence of irAEs than CTLA-4 inhibitors, and that the combined use of PD-1 and CTLA-4 inhibitors is associated with a greater incidence of irAEs compared to monotherapy with either agent.31 Toxicities associated with ipilimumab have been noted to be dose dependent.18 Generally, these toxicities are treated with immunosuppression in the form of glucocorticoids and are often reversible.31 There are several published guidelines that include algorithms for the management of irAEs by organizations such as the National Comprehensive Cancer Network.32
For example, previously untreated patients treated with ipilimumab plus dacarbazine as compared to dacarbazine plus placebo had greater grade 3 or 4 adverse events (56.3% vs 27.5%), and 77.7% of patients experiencing an irAE of any grade.18 In the CheckMate 066 trial comparing frontline nivolumab to dacarbazine, nivolumab had a lower rate of grade 3 or 4 toxicity (11.7% vs 17.6%) and irAEs were relatively infrequent, with diarrhea and elevated alanine aminotransferase level each being the most prominent irAE (affecting 1.0% of patients).25 In the KEYNOTE-006 trial, irAEs seen in more than 1% of patients treated with pembrolizumab included colitis, hepatitis, hypothyroidism, and hyperthyroidism, whereas those occurring in more than 1% of patients treated with ipilimumab included colitis and hypophysitis. Overall, there were lower rates of grade 3 to 5 toxicity with the 2 pembrolizumab doses compared to ipilimumab (13.3% pembrolizumab every 2 weeks vs 10.1% pembrolizumab every 3 weeks vs 19.9% ipilimumab).5 In the CheckMate 067 trial comparing nivolumab plus ipilimumab, nivolumab monotherapy, and ipilimumab monotherapy, rates of treatment-related adverse events of any grade were higher in the combination group (96% combination vs 86% nivolumab vs 86% ipilimumab), as were rates of grade 3 or 4 adverse events (59% vs 21% vs 28%, respectively). The irAE profile was similar to that demonstrated in prior studies: rash/pruritus were the most common, and diarrhea/colitis, elevated aminotransferases, and endocrinopathies were among the more common irAEs.7
Alternative dosing strategies have been investigated in an effort to preserve efficacy and minimize toxicity. A phase 1b trial of pembrolizumab plus reduced-dose ipilimumab demonstrated an ORR of 61%, with a 1-year PFS of 69% and a 1-year OS of 89%. This combination led to 45% of patients having a grade 3 or 4 adverse event, 60% having irAEs of any grade, and only 27% having grade 3 or 4 irAEs.29 The CheckMate 067 trial studied the combination of nivolumab 1 mg/kg plus ipilimumab 3 mg/kg.6 The CheckMate 511 trial compared different combination dosing strategies (nivolumab 3 mg/kg + ipilimumab 1 mg/kg versus nivolumab 1 mg/kg + ipilimumab 3 mg/kg) to assess for safety benefit. In the results published in abstract form, the reduced ipilimumab dose (nivolumab 3 mg/kg + ipilimumab 1 mg/kg arm) resulted in significantly decreased grade 3 to 5 adverse events (33.9% vs 48.3%) without significant differences in ORR, PFS, or OS.33
The question about the efficacy of checkpoint inhibitors in patients who discontinue treatment due to irAEs has been raised, as one hypothesis suggests that such toxicities may also indicate that the antitumor immune response has been activated. In a retrospective pooled analysis of phase 2 and 3 trials where patients received combination therapy with ipilimumab and nivolumab and discontinued therapy during the induction phase due to irAEs, outcomes did not appear to be inferior. Median PFS was 8.4 months in those who discontinued therapy compared to 10.8 months in those who continued therapy, but this did not reach statistical significance. Median OS had not been reached in either group and ORR was actually higher in those who discontinued due to adverse events (58.3% vs 50.2%). While this retrospective analysis needs to be validated, it does suggest that patients likely derive antitumor benefit from immunotherapy even if they have to discontinue therapy due to irAEs. Of note, patients in this analysis were not trialed on nivolumab monotherapy after receiving immunosuppressive treatment for toxicity related to combination therapy, which has since been deemed a reasonable treatment option.34
Molecularly Targeted Therapy for Metastatic Melanoma
As previously mentioned, the MAPK pathway is frequently altered in metastatic melanoma and thus serves as a target for therapy. Mutations in BRAF can cause constitutive activation of the protein’s kinase function, which subsequently phosphorylates/activates MEK in the absence of extracellular growth signals and causes increased cellular proliferation. For the roughly half of patients diagnosed with metastatic melanoma who harbor a BRAF V600 mutation, molecularly targeted therapy with BRAF/MEK inhibitors has emerged as a standard of care treatment option. As such, all patients with advanced disease should be tested for BRAF mutations.
After early phase 1 studies of the BRAF inhibitor vemurafenib demonstrated successful inhibition of mutated BRAF,35 subsequent studies confirmed the benefit of BRAF targeted therapy. In the phase 3 randomized controlled BRIM-3 trial comparing vemurafenib with dacarbazine for treatment of 675 patients with previously untreated metastatic melanoma positive for a BRAF V600E mutation, the vemurafenib group had superior ORR and 6-month OS during the first analysis.36 In a subsequent analysis, median PFS and median OS were also superior with vemurafenib compared to dacarbazine, as vemurafenib had a median OS of 13.6 months compared to 9.7 months with dacarbazine (HR, 0.70; P = 0.0008).37 Dabrafenib was the next BRAF inhibitor to demonstrate clinical efficacy with superior PFS compared to dacarbazine.38
Despite tumor shrinkage in the majority of patients, the development of resistance to therapy was an issue early on. The development of acquired resistance emerged as a heterogeneous process, though many of the identified resistance mechanisms involved reactivation of the MAPK pathway.39 A phase 3 trial of 322 patients with metastatic melanoma comparing the MEK inhibitor trametinib as monotherapy against chemotherapy demonstrated a modest improvement in both median PFS and OS.40 As a result, subsequent efforts focused on a strategy of concurrent MEK inhibition as a means to overcome resistance to molecularly targeted monotherapy
At least 4 large phase 3 randomized controlled trials of combination therapy with BRAF plus MEK inhibitors showed an improved ORR, PFS, and OS when compared to BRAF inhibition alone. The COMBI-d trial comparing dabrafenib plus trametinib versus dabrafenib alone was the first to demonstrate the superiority of combined BRAF/MEK inhibition and made combination therapy the current standard of care for patients with metastatic melanoma and a BRAF V600 mutation. In the final analysis of this trial, 3-year PFS was 22% with combination therapy compared to 12% with dabrafenib alone, and 3-year OS was 44% compared to 32%.8,41,42 A second trial with the combination of dabrafenib and trametinib (COMBI-V) also demonstrated superior efficacy when compared to single-agent vemurafenib without increased toxicity.43 Subsequently, the combination of vemurafenib with MEK inhibitor cobimetinib demonstrated superiority compared to vemurafenib alone,44 followed by the newest combination encorafenib (BRAF inhibitor) and binimetinib (MEK inhibitor) proving superior to either vemurafenib or encorafenib alone.45,46
It is important to note that there have been no studies directly comparing the efficacy of the 3 approved BRAF/MEK inhibitor combinations, but the 3 different regimens have some differences in their toxicity profiles (Table 2). Of note, single-agent BRAF inhibition was associated with increased cutaneous toxicity, including secondary squamous cell carcinoma and keratoacanthoma,47 which was demonstrated to be driven by paradoxical activation of the MAPK pathway.48 The concerning cutaneous toxicities such as squamous cell carcinoma were substantially reduced by combination BRAF/MEK inhibitor therapy.47 Collectively, the higher efficacy along with manageable toxicity profile established combination BRAF/MEK inhibition as the preferred regimen for patients with BRAF-mutated metastatic melanoma who are being considered for molecularly targeted therapy. BRAF inhibitor monotherapy should only be used when there is a specific concern regarding the use of a MEK inhibitor in certain clinical circumstances.

Other driver mutations associated with metastatic melanoma such as NRAS-mutated tumors have proven more difficult to effectively treat with molecularly targeted therapy, with one study showing that the MEK inhibitor binimetinib resulted in a modest improvement in ORR and median PFS without OS benefit compared to dacarbazine.49 Several phase 2 trials involving metastatic melanoma harboring a c-Kit alteration have demonstrated some efficacy with the tyrosine kinase inhibitor imatinib. The largest phase 2 trial of 43 patients treated with imatinib resulted in a 53.5% disease control rate (23.3% partial response and 30.2% stable disease), with 9 of the 10 patients who achieved partial response having a mutation in either exon 11 or 13. Median PFS was 3.5 months and 1-year OS was 51.0%.50
Case Conclusion
Prior to initiation of systemic therapy, the patient’s melanoma is tested and is found to be positive for a BRAF V600K mutation. At his follow-up appointment, the patient continues to endorse generalized weakness, fatigue, issues with balance, and residual pulmonary symptoms after being treated for post-obstructive pneumonia. Given his current symptoms and extent of metastatic disease, immunotherapy is deferred and he is started on combination molecularly targeted therapy with dabrafenib and trametinib. He initially does well, with a partial response noted by resolution of symptoms and decreased size of his intracranial metastases and decreased size of the right lower lobe mass. Further follow-up of this patient is presented in the second article in this 2-part review of advanced melanoma.
Malignant melanoma is the most serious form of primary skin cancer and one of the only malignancies in which the incidence rate has been rising. It is estimated that in 2018 there were 91,270 newly diagnosed cases and 9320 deaths from advanced melanoma in the United States. Melanoma is the fifth most common cancer type in males and the sixth most common in females. Despite rising incidence rates, improvement in the treatment of advanced melanoma has resulted in declining death rates over the past decade.1 Although most melanoma is diagnosed at an early stage and can be cured with surgical excision, the prognosis for metastatic melanoma had been historically poor prior to recent advancements in treatment. Conventional chemotherapy treatment with dacarbazine or temozolomide resulted in response rates ranging from 7.5% to 12.1%, but without much impact on median overall survival (OS), with reported OS ranging from 6.4 to 7.8 months. Combination approaches with interferon alfa-2B and low-dose interleukin-2 resulted in improved response rates compared with traditional chemotherapy, but again without survival benefit.2
Immunotherapy in the form of high-dose interleukin-2 emerged as the first therapy to alter the natural history of advanced melanoma, with both improved response rates (objective response rate [ORR], 16%) and median OS (2 months), with some patients achieving durable responses lasting more than 30 months. However, significant systemic toxicity limited its application to carefully selected patients.3 The past decade has brought rapid advancements in treatment with immune checkpoint inhibitors and molecularly targeted agents, which have significantly improved ORRs, progression-free survival (PFS), and OS for patients with metastatic melanoma.4-8
This review is the first of 2 articles focusing on the treatment and sequencing of therapies in advanced melanoma. Here, we review the selection of first-line therapy for metastatic melanoma. Current evidence for immune checkpoint blockade and molecularly targeted agents in the treatment of metastatic melanoma after progression on first-line therapy is discussed in a separate article.
Pathogenesis
The incidence of melanoma is strongly associated with ultraviolet light–mediated DNA damage related to sun exposure. Specifically, melanoma is associated to a greater degree with intense intermittent sun exposure and sunburn, but not associated with higher occupational exposure.9 Ultraviolet radiation can induce DNA damage by a number of mechanisms, and deficient DNA repair leads to somatic mutations that drive the progression from normal melanocyte to melanoma.10
The most commonly identified genetic mutations in cutaneous melanomas are alterations in the mitogen-activated protein kinase (MAPK) pathway. Typically, an extracellular growth factor causes dimerization of the growth factor receptor, which activates the intracellular RAS GTPase protein. Subsequently BRAF is phosphorylated within the kinase domain, which leads to downstream activation of the MEK and ERK kinases through phosphorylation. Activated ERK leads to phosphorylation of various cytoplasmic and nuclear targets, and the downstream effects of these changes promote cellular proliferation. While activation of this pathway usually requires phosphorylation of BRAF by RAS, mutations placing an acidic amino acid near the kinase domain mimics phosphorylation and leads to constitutive activation of the BRAF serine/threonine kinase in the absence of upstream signaling from extracellular growth factors mediated through RAS.11 One study of tumor samples of 71 patients with cutaneous melanoma detected NRAS mutations in 30% and BRAF mutations in 59% of all tumors tested. Of the BRAF mutation–positive tumors, 88% harbored the Val599Glu mutation, now commonly referred to as the BRAF V600E mutation. The same study demonstrated that the vast majority of BRAF mutations were seen in the primary tumor and were preserved when metastases were analyzed. Additionally, both NRAS and BRAF mutations were detected in the radial growth phase of the melanoma tumor. These findings indicate that alterations in the MAPK pathway occur early in the pathogenesis of advanced melanoma.11 Another group demonstrated that 66% of malignant melanoma tumor samples harbored BRAF mutations, of which 80% were specifically the V600E mutation. In vitro assays showed that the BRAF V600E–mutated kinase had greater than 10-fold kinase activity compared to wild-type BRAF, and that this kinase enhanced cellular proliferation even when upstream NRAS signaling was inhibited.12
The Cancer Genome Atlas Network performed a large analysis of tumor samples from 331 different melanoma patients and studied variations at the DNA, RNA, and protein levels. The study established a framework of 4 notable genomic subtypes, including mutant BRAF (52%), mutant RAS (28%), mutant NF1 (14%), and triple wild-type (6%). Additionally, mRNA transcriptomic analysis of overexpressed genes identified 3 different subclasses, which were labeled as “immune,” “keratin,” and “MITF-low.” The immune subclass was characterized by increased expression of proteins found in immune cells, immune signaling molecules, immune checkpoint proteins, cytokines, and chemokines, and correlated with increased lymphocyte invasion within the tumor. Interestingly, in the post-accession survival analysis, the “immune” transcriptomic subclass was statistically correlated with an improved prognosis.13 Having an understanding of the molecular pathogenesis of advanced melanoma helps to create a framework for understanding the mechanisms of current standard of care therapies for the disease.
Case Presentation
A 62-year-old Caucasian man with a history of well-controlled type 2 diabetes mellitus and hypertension is being followed by his dermatologist for surveillance of melanocytic nevi. On follow-up he is noted to have an asymmetrical melanocytic lesion over the right scalp with irregular borders and variegated color. He is asymptomatic and the remainder of physical examination is unremarkable, as he has no other concerning skin lesions and no cervical, axillary, or inguinal lymphadenopathy.
How is melanoma diagnosed?
Detailed discussion about diagnosis and staging will be deferred in this review of treatment of advanced melanoma. In brief, melanoma is best diagnosed by excisional biopsy and histopathology. Staging of melanoma is done according to the American Joint Committee on Cancer’s (AJCC) Cancer Staging Manual, 8th edition, using a TNM staging system that incorporates tumor thickness (Breslow depth); ulceration; number of involved regional lymph nodes; presence of in-transit, satellite, and/or microsatellite metastases; distant metastases; and serum lactate dehydrogenase level.14
Case Continued
The patient undergoes a wide excisional biopsy of the right scalp lesion, which is consistent with malignant melanoma. Pathology demonstrates a Breslow depth of 2.6 mm, 2 mitotic figures/mm2, and no evidence of ulceration. He subsequently undergoes wide local excision with 0/3 sentinel lymph nodes positive for malignancy. His final staging is consistent with pT3aN0M0, stage IIA melanoma.
He is seen in follow-up with medical oncology for the next 3.5 years without any evidence of disease recurrence. He then develops symptoms of vertigo, diplopia, and recurrent falls, prompting medical attention. Magnetic resonance imaging (MRI) brain reveals multiple supratentorial and infratentorial lesions concerning for intracranial metastases. Further imaging with computed tomography (CT) chest/abdomen/pelvis reveals a right lower lobe pulmonary mass with right hilar and subcarinal lymphadenopathy. He is admitted for treatment with intravenous dexamethasone and further evaluation with endobronchial ultrasound-guided fine-needle aspiration of the right lower lobe mass, which reveals metastatic melanoma. Given the extent of his intracranial metastases, he is treated with whole brain radiation therapy for symptomatic relief prior to initiating systemic therapy.
What is the general approach to first-line treatment for metastatic melanoma?
The past decade has brought an abundance of data supporting the use of immunotherapy with immune checkpoint inhibitors or molecularly targeted therapy with combined BRAF/MEK inhibitors in the first-line setting.4-8 After the diagnosis of metastatic melanoma has been made, molecular testing is recommended to determine the BRAF status of the tumor. Immunotherapy is the clear choice for first-line therapy in the absence of an activating BRAF V600 mutation. When a BRAF V600 mutation is present, current evidence supports the use of either immunotherapy or molecularly targeted therapy as first-line therapy.
To date, there have been no prospective clinical trials comparing the sequencing of immunotherapy and molecularly targeted therapy in the first-line setting. An ongoing clinical trial (NCT02224781) is comparing dabrafenib and trametinib followed by ipilimumab and nivolumab at time of progression to ipilimumab and nivolumab followed by dabrafenib and trametinib in patients with newly diagnosed stage III/IV BRAF V600 mutation–positive melanoma. The primary outcome measure is 2-year OS. Until completion of that trial, current practice regarding which type of therapy to use in the first-line setting is based on a number of factors including clinical characteristics and provider preferences.
Data suggest that immunotherapies can produce durable responses, especially after treatment completion or discontinuation, albeit at the expense of taking a longer time to achieve clinical benefit and the risk of potentially serious immune-related adverse effects. This idea of a durable, off-treatment response is highlighted by a study that followed 105 patients who had achieved a complete response (CR) and found that 24-month disease-free survival from the time of CR was 90.9% in all patients and 89.9% in the 67 patients who had discontinued pembrolizumab after attaining CR.15 BRAF/MEK inhibition has the potential for rapid clinical responses, though concerns exist about the development of resistance to therapy. The following sections explore the evidence supporting the use of these therapies.
Immunotherapy with Immune Checkpoint Inhibitors
Immunotherapy via immune checkpoint blockade has revolutionized the treatment of many solid tumors over the past decade. The promise of immunotherapy revolves around the potential for achieving a dynamic and durable systemic response against cancer by augmenting the antitumor effects of the immune system. T-cells are central to mounting a systemic antitumor response, and, in addition to antigen recognition, their function depends heavily on fine tuning between co-stimulatory and co-inhibitory signaling. The cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) expressed on T-cells was the first discovered co-inhibitory receptor of T-cell activation.16 Later, it was discovered that the programmed cell death 1 receptor (PD-1), expressed on T-cells, and its ligands PD-L1 and PD-L2, expressed on antigen presenting cells, tumor cells, or other cells in the tumor microenvironment, also served as a potent negative regulator of T-cell function.17
Together, these 2 signaling pathways help to maintain peripheral immune tolerance, whereby autoreactive T-cells that have escaped from the thymus are silenced to prevent autoimmunity. However, these pathways can also be utilized by cancer cells to escape immune surveillance. Monoclonal antibodies that inhibit the aforementioned co-inhibitory signaling pathways, and thus augment the immune response, have proven to be an effective anticancer therapy capable of producing profound and durable responses in certain malignancies.16,17
Ipilimumab
Ipilimumab is a monoclonal antibody that inhibits the function of the CTLA-4 co-inhibitory immune checkpoint. In a phase 3 randomized controlled trial of 676 patients with previously treated metastatic melanoma, ipilimumab at a dose of 3 mg/kg every 3 weeks for 4 cycles, with or without a gp100 peptide vaccine, resulted in an improved median OS of 10.0 and 10.1 months, respectively, compared to 6.4 months in those receiving the peptide vaccine alone, meeting the primary endpoint.4 Subsequently, a phase 3 trial of 502 patients with untreated metastatic melanoma compared ipilimumab at a dose of 10 mg/kg every 3 weeks for 4 cycles plus dacarbazine to dacarbazine plus placebo and found a significant increase in median OS (11.2 months vs 9.1 months), with no additive benefit of chemotherapy. There was a higher reported rate of grade 3 or 4 adverse events in this trial with ipilimumab dosed at 10 mg/kg, which was felt to be dose-related.18 These trials were the first to show improved OS with any systemic therapy in metastatic melanoma and led to US Food and Drug Administration approval of ipilimumab for this indication in 2011.
PD-1 Inhibitor Monotherapy
The PD-1 inhibitors nivolumab and pembrolizumab were initially approved for metastatic melanoma after progression on ipilimumab. In the phase 1 trial of patients with previously treated metastatic melanoma, nivolumab therapy resulted in an ORR of 28%.19 The subsequent phase 2 trial conducted in pretreated patients, including patients who had progressed on ipilimumab, confirmed a similar ORR of 31%, as well as a median PFS of 3.7 months and a median OS of 16.8 months. The estimated response duration in patients who did achieve a response to therapy was 2 years.20 A phase 3 trial (CheckMate 037) comparing nivolumab (n = 120) to investigator’s choice chemotherapy (n = 47) in those with melanoma refractory to ipilimumab demonstrated that nivolumab was superior for the primary endpoint of ORR (31.7% vs 10.6%), had less toxicity (5% rate of grade 3 or 4 adverse events versus 9%), and increased median duration of response (32 months vs 13 months).21
The phase 1 trial (KEYNOTE-001) testing the efficacy of pembrolizumab demonstrated an ORR of 33% in the total population of patients treated and an ORR of 45% in those who were treatment-naive. Additionally, the median OS was 23 months for the total population and 31 months for treatment-naive patients, with only 14% of patients experiencing a grade 3 or 4 adverse event.22 The KEYNOTE-002 phase 2 trial compared 2 different pembrolizumab doses (2 mg/kg and 10 mg/kg every 3 weeks) to investigator’s choice chemotherapy (paclitaxel plus carboplatin, paclitaxel, carboplatin, dacarbazine, or oral temozolomide) in 540 patients with advanced melanoma with documented progression on ipilimumab with or without prior progression on molecularly targeted therapy if positive for a BRAF V600 mutation. The final analysis demonstrated significantly improved ORR with pembrolizumab (22% at 2 mg/kg vs 26% at 10 mg/kg vs 4% chemotherapy) and significantly improved 24-month PFS (16% vs 22% vs 0.6%, respectively). There was a nonstatistically significant improvement in median OS (13.4 months vs 14.7 months vs 10 months), although 55% of the patients initially assigned to the chemotherapy arm crossed over and received pembrolizumab after documentation of progressive disease.23,24
Because PD-1 inhibition improved efficacy with less toxicity than chemotherapy when studied in progressive disease, subsequent studies focused on PD-1 inhibition in the frontline setting. CheckMate 066 was a phase 3 trial comparing nivolumab to dacarbazine as first-line therapy for 418 patients with untreated metastatic melanoma who did not have a BRAF mutation. For the primary end point of 1-year OS, nivolumab was superior to dacarbazine (72.9% vs 42.1%; hazard ratio [HR], 0.42; P < 0.001). Treatment with nivolumab also resulted in superior ORR (40% vs 14%) and PFS (5.1 months vs 2.2 months). Additionally, nivolumab therapy had a lower rate of grade 3 or 4 toxicity compared to dacarbazine (11.7% vs 17.6%).25
The KEYNOTE-006 trial compared 2 separate dosing schedules of pembrolizumab (10 mg/kg every 2 weeks versus every 3 weeks) to ipilimumab (3 mg/kg every 3 weeks for 4 cycles) in a 1:1:1 ratio in 834 patients with metastatic melanoma who had received up to 1 prior systemic therapy, but no prior CTLA-4 or PD-1 inhibitors. The first published data reported statistically significant outcomes for the co-primary end points of 6-month PFS (47.3% for pembrolizumab every 2 weeks vs 46.4% for pembrolizumab every 3 weeks vs 26.5% for ipilimumab; HR, 0.58 for both pembrolizumab groups compared to ipilimumab; P < 0.001) and 12-month OS (74.1% vs 68.4% vs 58.2%) with pembrolizumab compared to ipilimumab. Compared to ipilimumab, pembrolizumab every 2 weeks had a hazard ratio of 0.63 (P = 0.0005) and pembrolizumab every 3 weeks had a hazard ratio of 0.69 (P = 0.0036). The pembrolizumab groups was also had lower rates of grade 3 to 5 toxicity (13.3% vs 10.1% vs 19.9%).5 Updated outcomes demonstrated improved ORR compared to the first analysis (37% vs 36% vs 13%), and improved OS (median OS, not reached for the pembrolizumab groups vs 16.0 months for the ipilimumab group; HR, 0.68, P = 0.0009 for pembrolizumab every 2 weeks versus HR 0.68, P = 0.0008 for pembrolizumab every 3 weeks).26 In addition, 24-month OS was 55% in both pembrolizumab groups compared to 43% in the ipilimumab group. Grade 3 or 4 toxicity occurred less frequently with pembrolizumab (17% vs 17% vs 20%).
Further analysis from the KEYNOTE-006 trial data demonstrated improved ORR, PFS, and OS with pembrolizumab compared to ipilimumab in tumors positive for PD-L1 expression. For PD-L1-negative tumors, response rate was higher, and PFS and OS rates were similar with pembrolizumab compared to ipilimumab. Given that pembrolizumab was associated with similar survival outcomes in PD-L1-negative tumors and with less toxicity than ipilimumab, the superiority of PD-L1 inhibitors over ipilimumab was further supported, regardless of tumor PD-L1 status.27
In sum, PD-1 inhibition should be considered the first-line immunotherapy in advanced melanoma, either alone or in combination with ipilimumab, as discussed in the following section. There is no longer a role for ipilimumab monotherapy in the first-line setting, based on evidence from direct comparison to single-agent PD-1 inhibition in clinical trials that demonstrated superior efficacy and less serious toxicity with PD-1 inhibitors.5,26 The finding that ORR and OS outcomes with single-agent PD-1 inhibitors are higher in treatment-naive patients compared to those receiving prior therapies also supports this approach.22
Combination CTLA-4 and PD-1 Therapy
Despite the potential for durable responses, the majority of patients fail to respond to single-agent PD-1 therapy. Given that preclinical data had suggested the potential for synergy between dual inhibition of CTLA-4 and PD-1, clinical trials were designed to test this approach. The first randomized phase 2 trial that established superior efficacy with combination therapy was the CheckMate 069 trial comparing nivolumab plus ipilimumab to ipilimumab monotherapy. Combination therapy resulted in increased ORR (59% vs 11%), median PFS (not reached vs 3.0 months), 2-year PFS (51.3% vs 12.0%), and 2-year OS (63.8% vs 53.6%).28 Similarly, a phase 1b trial of pembrolizumab plus reduced-dose ipilimumab demonstrated an ORR of 61%, with a 1-year PFS of 69% and 1-year OS of 89%.29
The landmark phase 3 CheckMate 067 trial analyzed efficacy outcomes for 3 different treatment regimens including nivolumab plus ipilimumab, nivolumab monotherapy, and ipilimumab monotherapy in previously untreated patients with unresectable stage III or IV melanoma. The trial was powered to compare survival outcomes for both the combination therapy arm against ipilimumab and the nivolumab monotherapy arm against ipilimumab, but not to compare combination therapy to nivolumab monotherapy. The initial analysis demonstrated a median PFS of 11.5 months with combination therapy versus 6.9 months with nivolumab and 2.9 months with ipilimumab, as well as an ORR of 58% versus 44% and 19%, respectively (Table 1).6 The updated 3-year survival outcomes from CheckMate 067 were notable for superior median OS with combination therapy (not reached in combination vs 37.6 months for nivolumab vs 19.9 months ipilimumab), improved 3-year OS (58% vs 52% vs 34%), and improved 3-year PFS (39% vs 32% vs 10%).7 In the reported 4-year survival outcomes, median OS was not reached in the combination therapy group, and was 36.9 months in the nivolumab monotherapy group and 19.9 months in the ipilimumab monotherapy group. Rates of grade 3 or 4 adverse events were significantly higher in the combination therapy group, at 59% compared to 22% with nivolumab monotherapy and 28% with ipilimumab alone.30 The 3- and 4-year OS outcomes (58% and 54%, respectively) with combination therapy were the highest seen in any phase 3 trial for treatment of advanced melanoma, supporting its use as the best approved first-line therapy in those who can tolerate the potential toxicity of combination therapy7,30 The conclusions from this landmark trial were that both combination therapy and nivolumab monotherapy resulted in statistically significant improvement in OS compared to ipilimumab.

Toxicity Associated with Immune Checkpoint Inhibitors
While immune checkpoint inhibitors have revolutionized the treatment of many solid tumor malignancies, this new class of cancer therapy has brought about a new type of toxicity for clinicians to be aware of, termed immune-related adverse events (irAEs). As immune checkpoint inhibitors amplify the immune response against malignancy, they also increase the likelihood that autoreactive T-cells persist and proliferate within the circulation. Therefore, these therapies can result in almost any type of autoimmune side effect. The most commonly reported irAEs in large clinical trials studying CTLA-4 and PD-1 inhibitors include rash/pruritus, diarrhea/colitis, hepatitis, endocrinopathies (thyroiditis, hypophysitis, adrenalitis), and pneumonitis. Other more rare toxicities include pancreatitis, autoimmune hematologic toxicities, cardiac toxicity (myocarditis, heart failure), and neurologic toxicities (neuropathies, myasthenia gravis-like syndrome, Guillain-Barré syndrome). It has been observed that PD-1 inhibitors have a lower incidence of irAEs than CTLA-4 inhibitors, and that the combined use of PD-1 and CTLA-4 inhibitors is associated with a greater incidence of irAEs compared to monotherapy with either agent.31 Toxicities associated with ipilimumab have been noted to be dose dependent.18 Generally, these toxicities are treated with immunosuppression in the form of glucocorticoids and are often reversible.31 There are several published guidelines that include algorithms for the management of irAEs by organizations such as the National Comprehensive Cancer Network.32
For example, previously untreated patients treated with ipilimumab plus dacarbazine as compared to dacarbazine plus placebo had greater grade 3 or 4 adverse events (56.3% vs 27.5%), and 77.7% of patients experiencing an irAE of any grade.18 In the CheckMate 066 trial comparing frontline nivolumab to dacarbazine, nivolumab had a lower rate of grade 3 or 4 toxicity (11.7% vs 17.6%) and irAEs were relatively infrequent, with diarrhea and elevated alanine aminotransferase level each being the most prominent irAE (affecting 1.0% of patients).25 In the KEYNOTE-006 trial, irAEs seen in more than 1% of patients treated with pembrolizumab included colitis, hepatitis, hypothyroidism, and hyperthyroidism, whereas those occurring in more than 1% of patients treated with ipilimumab included colitis and hypophysitis. Overall, there were lower rates of grade 3 to 5 toxicity with the 2 pembrolizumab doses compared to ipilimumab (13.3% pembrolizumab every 2 weeks vs 10.1% pembrolizumab every 3 weeks vs 19.9% ipilimumab).5 In the CheckMate 067 trial comparing nivolumab plus ipilimumab, nivolumab monotherapy, and ipilimumab monotherapy, rates of treatment-related adverse events of any grade were higher in the combination group (96% combination vs 86% nivolumab vs 86% ipilimumab), as were rates of grade 3 or 4 adverse events (59% vs 21% vs 28%, respectively). The irAE profile was similar to that demonstrated in prior studies: rash/pruritus were the most common, and diarrhea/colitis, elevated aminotransferases, and endocrinopathies were among the more common irAEs.7
Alternative dosing strategies have been investigated in an effort to preserve efficacy and minimize toxicity. A phase 1b trial of pembrolizumab plus reduced-dose ipilimumab demonstrated an ORR of 61%, with a 1-year PFS of 69% and a 1-year OS of 89%. This combination led to 45% of patients having a grade 3 or 4 adverse event, 60% having irAEs of any grade, and only 27% having grade 3 or 4 irAEs.29 The CheckMate 067 trial studied the combination of nivolumab 1 mg/kg plus ipilimumab 3 mg/kg.6 The CheckMate 511 trial compared different combination dosing strategies (nivolumab 3 mg/kg + ipilimumab 1 mg/kg versus nivolumab 1 mg/kg + ipilimumab 3 mg/kg) to assess for safety benefit. In the results published in abstract form, the reduced ipilimumab dose (nivolumab 3 mg/kg + ipilimumab 1 mg/kg arm) resulted in significantly decreased grade 3 to 5 adverse events (33.9% vs 48.3%) without significant differences in ORR, PFS, or OS.33
The question about the efficacy of checkpoint inhibitors in patients who discontinue treatment due to irAEs has been raised, as one hypothesis suggests that such toxicities may also indicate that the antitumor immune response has been activated. In a retrospective pooled analysis of phase 2 and 3 trials where patients received combination therapy with ipilimumab and nivolumab and discontinued therapy during the induction phase due to irAEs, outcomes did not appear to be inferior. Median PFS was 8.4 months in those who discontinued therapy compared to 10.8 months in those who continued therapy, but this did not reach statistical significance. Median OS had not been reached in either group and ORR was actually higher in those who discontinued due to adverse events (58.3% vs 50.2%). While this retrospective analysis needs to be validated, it does suggest that patients likely derive antitumor benefit from immunotherapy even if they have to discontinue therapy due to irAEs. Of note, patients in this analysis were not trialed on nivolumab monotherapy after receiving immunosuppressive treatment for toxicity related to combination therapy, which has since been deemed a reasonable treatment option.34
Molecularly Targeted Therapy for Metastatic Melanoma
As previously mentioned, the MAPK pathway is frequently altered in metastatic melanoma and thus serves as a target for therapy. Mutations in BRAF can cause constitutive activation of the protein’s kinase function, which subsequently phosphorylates/activates MEK in the absence of extracellular growth signals and causes increased cellular proliferation. For the roughly half of patients diagnosed with metastatic melanoma who harbor a BRAF V600 mutation, molecularly targeted therapy with BRAF/MEK inhibitors has emerged as a standard of care treatment option. As such, all patients with advanced disease should be tested for BRAF mutations.
After early phase 1 studies of the BRAF inhibitor vemurafenib demonstrated successful inhibition of mutated BRAF,35 subsequent studies confirmed the benefit of BRAF targeted therapy. In the phase 3 randomized controlled BRIM-3 trial comparing vemurafenib with dacarbazine for treatment of 675 patients with previously untreated metastatic melanoma positive for a BRAF V600E mutation, the vemurafenib group had superior ORR and 6-month OS during the first analysis.36 In a subsequent analysis, median PFS and median OS were also superior with vemurafenib compared to dacarbazine, as vemurafenib had a median OS of 13.6 months compared to 9.7 months with dacarbazine (HR, 0.70; P = 0.0008).37 Dabrafenib was the next BRAF inhibitor to demonstrate clinical efficacy with superior PFS compared to dacarbazine.38
Despite tumor shrinkage in the majority of patients, the development of resistance to therapy was an issue early on. The development of acquired resistance emerged as a heterogeneous process, though many of the identified resistance mechanisms involved reactivation of the MAPK pathway.39 A phase 3 trial of 322 patients with metastatic melanoma comparing the MEK inhibitor trametinib as monotherapy against chemotherapy demonstrated a modest improvement in both median PFS and OS.40 As a result, subsequent efforts focused on a strategy of concurrent MEK inhibition as a means to overcome resistance to molecularly targeted monotherapy
At least 4 large phase 3 randomized controlled trials of combination therapy with BRAF plus MEK inhibitors showed an improved ORR, PFS, and OS when compared to BRAF inhibition alone. The COMBI-d trial comparing dabrafenib plus trametinib versus dabrafenib alone was the first to demonstrate the superiority of combined BRAF/MEK inhibition and made combination therapy the current standard of care for patients with metastatic melanoma and a BRAF V600 mutation. In the final analysis of this trial, 3-year PFS was 22% with combination therapy compared to 12% with dabrafenib alone, and 3-year OS was 44% compared to 32%.8,41,42 A second trial with the combination of dabrafenib and trametinib (COMBI-V) also demonstrated superior efficacy when compared to single-agent vemurafenib without increased toxicity.43 Subsequently, the combination of vemurafenib with MEK inhibitor cobimetinib demonstrated superiority compared to vemurafenib alone,44 followed by the newest combination encorafenib (BRAF inhibitor) and binimetinib (MEK inhibitor) proving superior to either vemurafenib or encorafenib alone.45,46
It is important to note that there have been no studies directly comparing the efficacy of the 3 approved BRAF/MEK inhibitor combinations, but the 3 different regimens have some differences in their toxicity profiles (Table 2). Of note, single-agent BRAF inhibition was associated with increased cutaneous toxicity, including secondary squamous cell carcinoma and keratoacanthoma,47 which was demonstrated to be driven by paradoxical activation of the MAPK pathway.48 The concerning cutaneous toxicities such as squamous cell carcinoma were substantially reduced by combination BRAF/MEK inhibitor therapy.47 Collectively, the higher efficacy along with manageable toxicity profile established combination BRAF/MEK inhibition as the preferred regimen for patients with BRAF-mutated metastatic melanoma who are being considered for molecularly targeted therapy. BRAF inhibitor monotherapy should only be used when there is a specific concern regarding the use of a MEK inhibitor in certain clinical circumstances.

Other driver mutations associated with metastatic melanoma such as NRAS-mutated tumors have proven more difficult to effectively treat with molecularly targeted therapy, with one study showing that the MEK inhibitor binimetinib resulted in a modest improvement in ORR and median PFS without OS benefit compared to dacarbazine.49 Several phase 2 trials involving metastatic melanoma harboring a c-Kit alteration have demonstrated some efficacy with the tyrosine kinase inhibitor imatinib. The largest phase 2 trial of 43 patients treated with imatinib resulted in a 53.5% disease control rate (23.3% partial response and 30.2% stable disease), with 9 of the 10 patients who achieved partial response having a mutation in either exon 11 or 13. Median PFS was 3.5 months and 1-year OS was 51.0%.50
Case Conclusion
Prior to initiation of systemic therapy, the patient’s melanoma is tested and is found to be positive for a BRAF V600K mutation. At his follow-up appointment, the patient continues to endorse generalized weakness, fatigue, issues with balance, and residual pulmonary symptoms after being treated for post-obstructive pneumonia. Given his current symptoms and extent of metastatic disease, immunotherapy is deferred and he is started on combination molecularly targeted therapy with dabrafenib and trametinib. He initially does well, with a partial response noted by resolution of symptoms and decreased size of his intracranial metastases and decreased size of the right lower lobe mass. Further follow-up of this patient is presented in the second article in this 2-part review of advanced melanoma.
1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2018. CA Cancer J Clin. 2018;68:7-30.
2. Ives NJ, Stowe RL, Lorigan P, Wheatley K. Chemotherapy compared with biochemotherapy for the treatment of metastatic melanoma: a meta-analysis of 18 trials involving 2621 patients. J Clin Oncol. 2007;25:5426-34.
3. Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105-16.
4. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711-23.
5. Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2522-2532.
6. Larkin J, Chiarion-Sileni V, Gonazalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23-34.
7. Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377:1345-1356.
8. Long GV, Stroyakovskiy D, Gogas H, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med. 2014;371:1877-1888.
9. Elwood JM, Jopson J. Melanoma and sun exposure: an overview of published studies. Int J Cancer. 1997;73:198-203.
10. Gilchrest BA, Eller MS, Geller AC, Yaar M. The pathogenesis of melanoma induced by ultraviolet radiation. N Engl J Med. 199;340:1341-1348.
11. Omholt K, Platz A, Kanter L, et al. NRAS and BRAF mutations arise early during melanoma pathogenesis and are preserved throughout tumor progression. Clin Cancer Res. 2003;9:6483-8.
12. Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949-54.
13. Cancer Genome Atlas Network. Genomic classification of cutaneous melanoma. Cell 2015;161:1681-96.
14. Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: evidence-based changes in the American Joint Committee on Cancer Eighth Edition Cancer Staging Manual. CA Cancer J Clin. 2017;67:472-492.
15. Robert C, Ribas A, Hamid O, et al. Durable complete response after discontinuation of pembrolizumab in patients with metastatic melanoma. J Clin Oncol. 2018;36:1668-1674.
16. Salama AKS, Hodi FS. Cytotoxic T-lymphocyte-associated antigen-4. Clin Cancer Res. 2011;17:4622-8.
17. Boussiotis VA. Molecular and biochemical aspects of the PD-1 checkpoint pathway. N Engl J Med. 2016;375:1767-1778.
18. Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517-2526.
19. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443-2454.
20. Topalian S, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020-30.
21. Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375-84.
22. Ribas A, Hamid O, Daud A, et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA. 2016;315:1600-1609.
23. Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16:908-18.
24. Hamid O, Puzanov I, Dummer R, et al. Final analysis of a randomised trial comparing pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory advanced melanoma. Eur J Cancer. 2017;86:37-45.
25. Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320-330.
26. Schachter J, Ribas A, Long GV, et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicenter, randomised, open-label phase 3 study (KEYNOTE-006). Lancet Oncol. 2017;390:1853-1862.
27. Carlino MS, Long GV, Schadendorf D, et al. Outcomes by line of therapy and programmed death ligand 1 expression in patients with advanced melanoma treated with pembrolizumab or ipilimumab in KEYNOTE-006. A randomised clinical trial. Eur J Cancer. 2018;101:236-243.
28. Hodi FS, Chesney J, Pavlick AC, et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2016;17:1558-1568.
29. Long GV, Atkinson V, Cebon JS, et al. Standard-dose pembrolizumab in combination with reduced-dose ipilimumab for patients with advanced melanoma (KEYNOTE-029): an open-label, phase 1b trial. Lancet Oncol. 2017;18:1202-10.
30. Hodi FS, Chiarion-Sileni V, Gonzalez R, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19:1480-1492.
31. Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: a review. JAMA Oncol. 2016;2:1346-1353.
32. National Comprehensive Cancer Network. Management of immunotherapy-related toxicities (version 2.2019). www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf. Accessed April 8, 2019.
33. Lebbé C, Meyer N, Mortier L, et al. Initial results from a phase IIIb/IV study evaluating two dosing regimens of nivolumab (NIVO) in combination with ipilimumab (IPI) in patients with advanced melanoma (CheckMate 511) [Abstract LBA47]. Ann Oncol. 2018;29:mdy424.057.
34. Schadendorf D, Wolchok JD, Hodi FS, et al. Efficacy and safety outcomes in patients with advanced melanoma who discontinued treatment with nivolumab and ipilimumab because of adverse events: a pooled analysis of randomized phase ii and iii trials. J Clin Oncol. 2017;35:3807-3814.
35. Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809-819.
36. Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507-2516.
37. McArthur GA, Chapman PB, Robert C, et al. Safety and efficacy of vemurafenib in BRAFV600E and BRAFV600K mutation-positive melanoma (BRIM-3): extended follow up of a phase 3, randomised, open-label study. Lancet Oncol. 2014;15:323-332.
38. Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicenter, open-label, phase 3 randomised controlled trial. Lancet Oncol. 2012;380:358-365.
39. Rizos H, Menzies AM, Pupo GM, et al. BRAF inhibitor resistance mechanisms in metastatic melanoma: spectrum and clinical impact. Clin Cancer Res. 2014;20:1965-1977.
40. Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367:107-114.
41. Long GV, Stroyakovskiy D, Gogas H, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicenter, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2015;386:444-451.
42. Long GV, Flaherty KT, Stroyakovskiy D, et al. Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: long-term survival and safety analysis of a phase 3 study. Ann Oncol. 2017;28:1631-1639.
43. Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30-39.
44. Ascierto PA, McArthur GA, Dréno B, et al. Cobimetinib combined with vemurafenib in advanced BRAFV600-mutant melanoma (coBRIM): updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol. 2016;17:1248-260.
45. Dummer R, Ascierto PA, Gogas HJ, et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): a multicenter, open-label, randomised phase 3 trial. Lancet Oncol. 2018;19:603-615.
46. Dummer R, Ascierto PA, Gogas HJ, et al. Overall survival in patients with BRAF-mutant melanoma receiving encorafenib plus binimetinib versus vemurafenib or encorafenib (COLUMBUS): a multicenter, open-label, randomised, phase 3 trial. Lancet Oncol. 2018;19:1315-1327.
47. Carlos G, Anforth R, Clements A, et al. Cutaneous toxic effects of BRAF inhibitors alone and in combination with MEK inhibitors for metastatic melanoma. JAMA. Dermatol 2015;151:1103-1109.
48. Su F, Viros A, Milagre C, et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med. 2012;366:207-215.
49. Dummer R, Schadendorf D, Ascierto P, et al. Binimetinib versus dacarbazine in patients with advanced NRAS-mutant melanoma (NEMO): a multicenter, open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18:435-445.
50. Guo J, Si L, Kong Y, et al. Phase II, open-label, single-arm trial of imatinib mesylate in patients with metastatic melanoma harboring c-Kit mutation or amplification. J Clin Oncol. 2011;29:2904-2909.
1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2018. CA Cancer J Clin. 2018;68:7-30.
2. Ives NJ, Stowe RL, Lorigan P, Wheatley K. Chemotherapy compared with biochemotherapy for the treatment of metastatic melanoma: a meta-analysis of 18 trials involving 2621 patients. J Clin Oncol. 2007;25:5426-34.
3. Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105-16.
4. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711-23.
5. Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2522-2532.
6. Larkin J, Chiarion-Sileni V, Gonazalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23-34.
7. Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377:1345-1356.
8. Long GV, Stroyakovskiy D, Gogas H, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med. 2014;371:1877-1888.
9. Elwood JM, Jopson J. Melanoma and sun exposure: an overview of published studies. Int J Cancer. 1997;73:198-203.
10. Gilchrest BA, Eller MS, Geller AC, Yaar M. The pathogenesis of melanoma induced by ultraviolet radiation. N Engl J Med. 199;340:1341-1348.
11. Omholt K, Platz A, Kanter L, et al. NRAS and BRAF mutations arise early during melanoma pathogenesis and are preserved throughout tumor progression. Clin Cancer Res. 2003;9:6483-8.
12. Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949-54.
13. Cancer Genome Atlas Network. Genomic classification of cutaneous melanoma. Cell 2015;161:1681-96.
14. Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: evidence-based changes in the American Joint Committee on Cancer Eighth Edition Cancer Staging Manual. CA Cancer J Clin. 2017;67:472-492.
15. Robert C, Ribas A, Hamid O, et al. Durable complete response after discontinuation of pembrolizumab in patients with metastatic melanoma. J Clin Oncol. 2018;36:1668-1674.
16. Salama AKS, Hodi FS. Cytotoxic T-lymphocyte-associated antigen-4. Clin Cancer Res. 2011;17:4622-8.
17. Boussiotis VA. Molecular and biochemical aspects of the PD-1 checkpoint pathway. N Engl J Med. 2016;375:1767-1778.
18. Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517-2526.
19. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443-2454.
20. Topalian S, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020-30.
21. Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375-84.
22. Ribas A, Hamid O, Daud A, et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA. 2016;315:1600-1609.
23. Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16:908-18.
24. Hamid O, Puzanov I, Dummer R, et al. Final analysis of a randomised trial comparing pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory advanced melanoma. Eur J Cancer. 2017;86:37-45.
25. Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320-330.
26. Schachter J, Ribas A, Long GV, et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicenter, randomised, open-label phase 3 study (KEYNOTE-006). Lancet Oncol. 2017;390:1853-1862.
27. Carlino MS, Long GV, Schadendorf D, et al. Outcomes by line of therapy and programmed death ligand 1 expression in patients with advanced melanoma treated with pembrolizumab or ipilimumab in KEYNOTE-006. A randomised clinical trial. Eur J Cancer. 2018;101:236-243.
28. Hodi FS, Chesney J, Pavlick AC, et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2016;17:1558-1568.
29. Long GV, Atkinson V, Cebon JS, et al. Standard-dose pembrolizumab in combination with reduced-dose ipilimumab for patients with advanced melanoma (KEYNOTE-029): an open-label, phase 1b trial. Lancet Oncol. 2017;18:1202-10.
30. Hodi FS, Chiarion-Sileni V, Gonzalez R, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19:1480-1492.
31. Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: a review. JAMA Oncol. 2016;2:1346-1353.
32. National Comprehensive Cancer Network. Management of immunotherapy-related toxicities (version 2.2019). www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf. Accessed April 8, 2019.
33. Lebbé C, Meyer N, Mortier L, et al. Initial results from a phase IIIb/IV study evaluating two dosing regimens of nivolumab (NIVO) in combination with ipilimumab (IPI) in patients with advanced melanoma (CheckMate 511) [Abstract LBA47]. Ann Oncol. 2018;29:mdy424.057.
34. Schadendorf D, Wolchok JD, Hodi FS, et al. Efficacy and safety outcomes in patients with advanced melanoma who discontinued treatment with nivolumab and ipilimumab because of adverse events: a pooled analysis of randomized phase ii and iii trials. J Clin Oncol. 2017;35:3807-3814.
35. Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809-819.
36. Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507-2516.
37. McArthur GA, Chapman PB, Robert C, et al. Safety and efficacy of vemurafenib in BRAFV600E and BRAFV600K mutation-positive melanoma (BRIM-3): extended follow up of a phase 3, randomised, open-label study. Lancet Oncol. 2014;15:323-332.
38. Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicenter, open-label, phase 3 randomised controlled trial. Lancet Oncol. 2012;380:358-365.
39. Rizos H, Menzies AM, Pupo GM, et al. BRAF inhibitor resistance mechanisms in metastatic melanoma: spectrum and clinical impact. Clin Cancer Res. 2014;20:1965-1977.
40. Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367:107-114.
41. Long GV, Stroyakovskiy D, Gogas H, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicenter, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2015;386:444-451.
42. Long GV, Flaherty KT, Stroyakovskiy D, et al. Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: long-term survival and safety analysis of a phase 3 study. Ann Oncol. 2017;28:1631-1639.
43. Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30-39.
44. Ascierto PA, McArthur GA, Dréno B, et al. Cobimetinib combined with vemurafenib in advanced BRAFV600-mutant melanoma (coBRIM): updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol. 2016;17:1248-260.
45. Dummer R, Ascierto PA, Gogas HJ, et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): a multicenter, open-label, randomised phase 3 trial. Lancet Oncol. 2018;19:603-615.
46. Dummer R, Ascierto PA, Gogas HJ, et al. Overall survival in patients with BRAF-mutant melanoma receiving encorafenib plus binimetinib versus vemurafenib or encorafenib (COLUMBUS): a multicenter, open-label, randomised, phase 3 trial. Lancet Oncol. 2018;19:1315-1327.
47. Carlos G, Anforth R, Clements A, et al. Cutaneous toxic effects of BRAF inhibitors alone and in combination with MEK inhibitors for metastatic melanoma. JAMA. Dermatol 2015;151:1103-1109.
48. Su F, Viros A, Milagre C, et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med. 2012;366:207-215.
49. Dummer R, Schadendorf D, Ascierto P, et al. Binimetinib versus dacarbazine in patients with advanced NRAS-mutant melanoma (NEMO): a multicenter, open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18:435-445.
50. Guo J, Si L, Kong Y, et al. Phase II, open-label, single-arm trial of imatinib mesylate in patients with metastatic melanoma harboring c-Kit mutation or amplification. J Clin Oncol. 2011;29:2904-2909.
ICYMI: NIH renames, streamlines gene therapy committee
The National Institutes of Health has released an amended guideline on research involving gene therapy.
As part of the streamlining process, the Recombinant DNA Advisory Committee has been renamed as the Novel and Exceptional Technology and Research Advisory Committee to better align with the committee’s original intention – following and providing advice on safety and ethical issues associated with emerging biotechnologies, according to a statement from Francis S. Collins, MD, PhD, director of the NIH.
We previously covered this story; find our coverage at the link below.
The National Institutes of Health has released an amended guideline on research involving gene therapy.
As part of the streamlining process, the Recombinant DNA Advisory Committee has been renamed as the Novel and Exceptional Technology and Research Advisory Committee to better align with the committee’s original intention – following and providing advice on safety and ethical issues associated with emerging biotechnologies, according to a statement from Francis S. Collins, MD, PhD, director of the NIH.
We previously covered this story; find our coverage at the link below.
The National Institutes of Health has released an amended guideline on research involving gene therapy.
As part of the streamlining process, the Recombinant DNA Advisory Committee has been renamed as the Novel and Exceptional Technology and Research Advisory Committee to better align with the committee’s original intention – following and providing advice on safety and ethical issues associated with emerging biotechnologies, according to a statement from Francis S. Collins, MD, PhD, director of the NIH.
We previously covered this story; find our coverage at the link below.
Monitoring, early intervention key to CAR T safety
GLASGOW – Constant patient monitoring and early intervention with tocilizumab and steroids are essential to the safe delivery of chimeric antigen receptor (CAR) T-cell therapy in patients with non-Hodgkin lymphoma (NHL), according to a leading expert.
As a clinical researcher at MD Anderson Cancer Center in Houston, Loretta Nastoupil, MD has played an active role in the evolution of CAR T-cell therapy, from early trials to ongoing development of treatment protocols. During a presentation at the annual meeting of the British Society for Haematology, Dr. Nastoupil discussed leading topics in CAR T-cell therapy, with an emphasis on safe delivery.
“[Toxicity] is something we don’t talk about as much as we should, partly because this therapy works and it’s really exciting,” Dr. Nastoupil said. “But the toxicity is not something that I minimize, and it’s very challenging. It’s led us to restructure our inpatient services. It’s led to a lot of sleepless nights. These patients can do very, very well, or they can do very, very poorly in terms of toxicity and I think the most important strategy is recognition and early intervention.”
Monitoring
Early recognition depends on close monitoring, Dr. Nastoupil said, which is carried out by highly trained nursing staff who follow therapy-specific decision algorithms.
“We have nurses that are on the front line,” Dr. Nastoupil said. “They’re the most important group. We have staff that round on [patients] daily, but the nurses are there 24 hours a day. We have a flow sheet where they grade cytokine release syndrome and neurotoxicity every 8 hours, or if there is an acute change in symptoms or toxicity, they’ll do it in real time.”
Dr. Nastoupil said that if these toxicities are detected, intervention is occurring sooner than it did with some of the first patients to receive CAR-T cell therapy.
“Initially there was a lot of fear surrounding anything that would abort the CAR-T cell therapy,” Dr. Nastoupil said. “There was concern that if you were trying to mitigate some of the toxicity you might have a negative impact on efficacy ... [W]ith the first iteration of studies, generally we were waiting until grade 3 or higher cytokine release syndrome before initiating either tocilizumab and/or steroids. As the studies evolved, it started to move into grade 2 toxicity that we started using therapy, mostly because we started to see that those patients were still responding.”
At MD Anderson, these earlier interventions have decreased severity of adverse events.
“It’s rare nowadays to have grade 3 or 4 cytokine release syndrome because we are generally introducing abortive therapy at grade 2,” Dr. Nastoupil said, citing increased use of steroids and tocilizumab.
Currently, no consensus exists for managing these events, partly because clinicians are still learning about best management practices.
“There will be a consensus on management,” Dr. Nastoupil said. “I think that’s needed. The problem is, it will probably evolve as we get more experience with managing these patients. I think there’s been a little hesitation to put something out on paper knowing that a year from now that might change.”
Grading toxicity
In contrast, Dr. Nastoupil said that a consensus has been reached for grading acute toxicity. Of note, fever is now considered an essential element of cytokine release syndrome.
“The first thing we see [with cytokine release syndrome] is fever, generally speaking,” Dr. Nastoupil said. “That will prompt a workup for infection because these patients are going to be neutropenic. And we initiate broad spectrum antimicrobials.”
She said that some patients treated with CAR T-cell therapy have had disseminated fungal infections, so clinicians need to be on the lookout for septic shock.
To assess neurotoxicity, the team at MD Anderson uses an objective scoring system, called “CARTOX.” This helps maintain consistency when facing broadly different neurological presentations.
“There’s such a wide ranging spectrum of patients that are undergoing neurotoxicity you can’t expect someone, even myself, to be consistent when you are trying to tease out how serious it is,” Dr. Nastoupil said.
With CARTOX, nurses can easily score patients and call clinicians with results. Still, this doesn’t eliminate difficulties inherent to managing neurotoxicity, particularly when it is severe.
“I’d say one of the areas that is still very challenging is when [patients with neurotoxicity] are no longer responding,” Dr. Nastoupil said. “You have to be very mindful of seizure activity. We’ve had a couple of patients with status [epilepticus]. You don’t see seizure activity physically, but when you do an EEG, you pick it up.”
Dr. Nastoupil added that most centers are now giving patients prophylactic levetiracetam (Keppra) to lower seizure risk.
Choosing therapy
When selecting between the two therapies currently approved by the Food and Drug Administration – tisagenlecleucel (Kymriah) and axicabtagene ciloleucel (Yescarta) – based on safety, Dr. Nastoupil said that rates of cytokine release syndrome appear similar, but neurotoxicity rates may differ.
“Cytokine release syndrome in my opinion is probably more similar than different in terms of grade 3 or higher because tocilizumab and steroids work quite well in aborting those toxicities,” Dr. Nastoupil said. “But neurotoxicity still sticks out in my mind as the most striking difference, where with axicabtagene you see more grade 3 or higher neurotoxicity, though very, very few deaths as a result of this. But it’s very challenging in terms of management.”
According to Dr. Nastoupil, comparisons between CAR T-cell therapies have been complicated by differences in clinical trial methodologies. However, she offered a general conclusion regarding efficacy.
“[W]hat I’ll tell you, at the end of the day, is [that existing CAR T-cell therapies] all seem to sort of settle out around 30%-40% in terms of durable responses,” Dr. Nastoupil said.
Dr. Nastoupil concluded her presentation with an overview and look to the future.
“I do think [CAR T-cell therapy] is transformative, particularly for our chemo refractory patients,” she said. “There is nothing else like it. The problem right now is that it is only durable in 40% of patients. So can we be better at selecting out patients that are more likely to respond? Does introducing this in earlier lines of therapy increase that fraction of patients that are potentially cured?”
Considering these questions, she said: “We need more patients. We need more data. We need longer follow-up to understand the nuances of this therapy.”
Dr. Nastoupil previously reported financial relationships with Celgene, Genentech, Gilead, Merck, Novartis, Spectrum, and TG Therapeutics.
GLASGOW – Constant patient monitoring and early intervention with tocilizumab and steroids are essential to the safe delivery of chimeric antigen receptor (CAR) T-cell therapy in patients with non-Hodgkin lymphoma (NHL), according to a leading expert.
As a clinical researcher at MD Anderson Cancer Center in Houston, Loretta Nastoupil, MD has played an active role in the evolution of CAR T-cell therapy, from early trials to ongoing development of treatment protocols. During a presentation at the annual meeting of the British Society for Haematology, Dr. Nastoupil discussed leading topics in CAR T-cell therapy, with an emphasis on safe delivery.
“[Toxicity] is something we don’t talk about as much as we should, partly because this therapy works and it’s really exciting,” Dr. Nastoupil said. “But the toxicity is not something that I minimize, and it’s very challenging. It’s led us to restructure our inpatient services. It’s led to a lot of sleepless nights. These patients can do very, very well, or they can do very, very poorly in terms of toxicity and I think the most important strategy is recognition and early intervention.”
Monitoring
Early recognition depends on close monitoring, Dr. Nastoupil said, which is carried out by highly trained nursing staff who follow therapy-specific decision algorithms.
“We have nurses that are on the front line,” Dr. Nastoupil said. “They’re the most important group. We have staff that round on [patients] daily, but the nurses are there 24 hours a day. We have a flow sheet where they grade cytokine release syndrome and neurotoxicity every 8 hours, or if there is an acute change in symptoms or toxicity, they’ll do it in real time.”
Dr. Nastoupil said that if these toxicities are detected, intervention is occurring sooner than it did with some of the first patients to receive CAR-T cell therapy.
“Initially there was a lot of fear surrounding anything that would abort the CAR-T cell therapy,” Dr. Nastoupil said. “There was concern that if you were trying to mitigate some of the toxicity you might have a negative impact on efficacy ... [W]ith the first iteration of studies, generally we were waiting until grade 3 or higher cytokine release syndrome before initiating either tocilizumab and/or steroids. As the studies evolved, it started to move into grade 2 toxicity that we started using therapy, mostly because we started to see that those patients were still responding.”
At MD Anderson, these earlier interventions have decreased severity of adverse events.
“It’s rare nowadays to have grade 3 or 4 cytokine release syndrome because we are generally introducing abortive therapy at grade 2,” Dr. Nastoupil said, citing increased use of steroids and tocilizumab.
Currently, no consensus exists for managing these events, partly because clinicians are still learning about best management practices.
“There will be a consensus on management,” Dr. Nastoupil said. “I think that’s needed. The problem is, it will probably evolve as we get more experience with managing these patients. I think there’s been a little hesitation to put something out on paper knowing that a year from now that might change.”
Grading toxicity
In contrast, Dr. Nastoupil said that a consensus has been reached for grading acute toxicity. Of note, fever is now considered an essential element of cytokine release syndrome.
“The first thing we see [with cytokine release syndrome] is fever, generally speaking,” Dr. Nastoupil said. “That will prompt a workup for infection because these patients are going to be neutropenic. And we initiate broad spectrum antimicrobials.”
She said that some patients treated with CAR T-cell therapy have had disseminated fungal infections, so clinicians need to be on the lookout for septic shock.
To assess neurotoxicity, the team at MD Anderson uses an objective scoring system, called “CARTOX.” This helps maintain consistency when facing broadly different neurological presentations.
“There’s such a wide ranging spectrum of patients that are undergoing neurotoxicity you can’t expect someone, even myself, to be consistent when you are trying to tease out how serious it is,” Dr. Nastoupil said.
With CARTOX, nurses can easily score patients and call clinicians with results. Still, this doesn’t eliminate difficulties inherent to managing neurotoxicity, particularly when it is severe.
“I’d say one of the areas that is still very challenging is when [patients with neurotoxicity] are no longer responding,” Dr. Nastoupil said. “You have to be very mindful of seizure activity. We’ve had a couple of patients with status [epilepticus]. You don’t see seizure activity physically, but when you do an EEG, you pick it up.”
Dr. Nastoupil added that most centers are now giving patients prophylactic levetiracetam (Keppra) to lower seizure risk.
Choosing therapy
When selecting between the two therapies currently approved by the Food and Drug Administration – tisagenlecleucel (Kymriah) and axicabtagene ciloleucel (Yescarta) – based on safety, Dr. Nastoupil said that rates of cytokine release syndrome appear similar, but neurotoxicity rates may differ.
“Cytokine release syndrome in my opinion is probably more similar than different in terms of grade 3 or higher because tocilizumab and steroids work quite well in aborting those toxicities,” Dr. Nastoupil said. “But neurotoxicity still sticks out in my mind as the most striking difference, where with axicabtagene you see more grade 3 or higher neurotoxicity, though very, very few deaths as a result of this. But it’s very challenging in terms of management.”
According to Dr. Nastoupil, comparisons between CAR T-cell therapies have been complicated by differences in clinical trial methodologies. However, she offered a general conclusion regarding efficacy.
“[W]hat I’ll tell you, at the end of the day, is [that existing CAR T-cell therapies] all seem to sort of settle out around 30%-40% in terms of durable responses,” Dr. Nastoupil said.
Dr. Nastoupil concluded her presentation with an overview and look to the future.
“I do think [CAR T-cell therapy] is transformative, particularly for our chemo refractory patients,” she said. “There is nothing else like it. The problem right now is that it is only durable in 40% of patients. So can we be better at selecting out patients that are more likely to respond? Does introducing this in earlier lines of therapy increase that fraction of patients that are potentially cured?”
Considering these questions, she said: “We need more patients. We need more data. We need longer follow-up to understand the nuances of this therapy.”
Dr. Nastoupil previously reported financial relationships with Celgene, Genentech, Gilead, Merck, Novartis, Spectrum, and TG Therapeutics.
GLASGOW – Constant patient monitoring and early intervention with tocilizumab and steroids are essential to the safe delivery of chimeric antigen receptor (CAR) T-cell therapy in patients with non-Hodgkin lymphoma (NHL), according to a leading expert.
As a clinical researcher at MD Anderson Cancer Center in Houston, Loretta Nastoupil, MD has played an active role in the evolution of CAR T-cell therapy, from early trials to ongoing development of treatment protocols. During a presentation at the annual meeting of the British Society for Haematology, Dr. Nastoupil discussed leading topics in CAR T-cell therapy, with an emphasis on safe delivery.
“[Toxicity] is something we don’t talk about as much as we should, partly because this therapy works and it’s really exciting,” Dr. Nastoupil said. “But the toxicity is not something that I minimize, and it’s very challenging. It’s led us to restructure our inpatient services. It’s led to a lot of sleepless nights. These patients can do very, very well, or they can do very, very poorly in terms of toxicity and I think the most important strategy is recognition and early intervention.”
Monitoring
Early recognition depends on close monitoring, Dr. Nastoupil said, which is carried out by highly trained nursing staff who follow therapy-specific decision algorithms.
“We have nurses that are on the front line,” Dr. Nastoupil said. “They’re the most important group. We have staff that round on [patients] daily, but the nurses are there 24 hours a day. We have a flow sheet where they grade cytokine release syndrome and neurotoxicity every 8 hours, or if there is an acute change in symptoms or toxicity, they’ll do it in real time.”
Dr. Nastoupil said that if these toxicities are detected, intervention is occurring sooner than it did with some of the first patients to receive CAR-T cell therapy.
“Initially there was a lot of fear surrounding anything that would abort the CAR-T cell therapy,” Dr. Nastoupil said. “There was concern that if you were trying to mitigate some of the toxicity you might have a negative impact on efficacy ... [W]ith the first iteration of studies, generally we were waiting until grade 3 or higher cytokine release syndrome before initiating either tocilizumab and/or steroids. As the studies evolved, it started to move into grade 2 toxicity that we started using therapy, mostly because we started to see that those patients were still responding.”
At MD Anderson, these earlier interventions have decreased severity of adverse events.
“It’s rare nowadays to have grade 3 or 4 cytokine release syndrome because we are generally introducing abortive therapy at grade 2,” Dr. Nastoupil said, citing increased use of steroids and tocilizumab.
Currently, no consensus exists for managing these events, partly because clinicians are still learning about best management practices.
“There will be a consensus on management,” Dr. Nastoupil said. “I think that’s needed. The problem is, it will probably evolve as we get more experience with managing these patients. I think there’s been a little hesitation to put something out on paper knowing that a year from now that might change.”
Grading toxicity
In contrast, Dr. Nastoupil said that a consensus has been reached for grading acute toxicity. Of note, fever is now considered an essential element of cytokine release syndrome.
“The first thing we see [with cytokine release syndrome] is fever, generally speaking,” Dr. Nastoupil said. “That will prompt a workup for infection because these patients are going to be neutropenic. And we initiate broad spectrum antimicrobials.”
She said that some patients treated with CAR T-cell therapy have had disseminated fungal infections, so clinicians need to be on the lookout for septic shock.
To assess neurotoxicity, the team at MD Anderson uses an objective scoring system, called “CARTOX.” This helps maintain consistency when facing broadly different neurological presentations.
“There’s such a wide ranging spectrum of patients that are undergoing neurotoxicity you can’t expect someone, even myself, to be consistent when you are trying to tease out how serious it is,” Dr. Nastoupil said.
With CARTOX, nurses can easily score patients and call clinicians with results. Still, this doesn’t eliminate difficulties inherent to managing neurotoxicity, particularly when it is severe.
“I’d say one of the areas that is still very challenging is when [patients with neurotoxicity] are no longer responding,” Dr. Nastoupil said. “You have to be very mindful of seizure activity. We’ve had a couple of patients with status [epilepticus]. You don’t see seizure activity physically, but when you do an EEG, you pick it up.”
Dr. Nastoupil added that most centers are now giving patients prophylactic levetiracetam (Keppra) to lower seizure risk.
Choosing therapy
When selecting between the two therapies currently approved by the Food and Drug Administration – tisagenlecleucel (Kymriah) and axicabtagene ciloleucel (Yescarta) – based on safety, Dr. Nastoupil said that rates of cytokine release syndrome appear similar, but neurotoxicity rates may differ.
“Cytokine release syndrome in my opinion is probably more similar than different in terms of grade 3 or higher because tocilizumab and steroids work quite well in aborting those toxicities,” Dr. Nastoupil said. “But neurotoxicity still sticks out in my mind as the most striking difference, where with axicabtagene you see more grade 3 or higher neurotoxicity, though very, very few deaths as a result of this. But it’s very challenging in terms of management.”
According to Dr. Nastoupil, comparisons between CAR T-cell therapies have been complicated by differences in clinical trial methodologies. However, she offered a general conclusion regarding efficacy.
“[W]hat I’ll tell you, at the end of the day, is [that existing CAR T-cell therapies] all seem to sort of settle out around 30%-40% in terms of durable responses,” Dr. Nastoupil said.
Dr. Nastoupil concluded her presentation with an overview and look to the future.
“I do think [CAR T-cell therapy] is transformative, particularly for our chemo refractory patients,” she said. “There is nothing else like it. The problem right now is that it is only durable in 40% of patients. So can we be better at selecting out patients that are more likely to respond? Does introducing this in earlier lines of therapy increase that fraction of patients that are potentially cured?”
Considering these questions, she said: “We need more patients. We need more data. We need longer follow-up to understand the nuances of this therapy.”
Dr. Nastoupil previously reported financial relationships with Celgene, Genentech, Gilead, Merck, Novartis, Spectrum, and TG Therapeutics.
EXPERT ANALYSIS FROM BSH 2019
Factors emerge for mitigating CD19 CAR T toxicity
HOUSTON – Cytokine release syndrome and neurotoxicity frequently occur with CD19-directed chimeric antigen receptor (CAR) T-cell immunotherapies, but targetable factors for mitigating the risk and effects of these complications are emerging, according to Cameron Turtle, MBBS, PhD.
These factors include infused CAR T-cell dose, bone marrow disease burden, immune response, and the lymphodepletion regimen used, Dr. Turtle, of Fred Hutchinson Cancer Research Center, Seattle, said at the Transplantation & Cellular Therapies Meetings. This list is based on an analysis of several studies that included a total of 195 patients with B-cell malignancies who were treated with defined-composition CD19 CAR T cells.
In a 2016 study included in the analysis, for instance, Dr. Turtle and his colleagues found that CD19 CAR T cells administered to adults with B-cell acute lymphoblastic leukemia (B-ALL) after lymphodepletion chemotherapy were “remarkably potent.” Remission was achieved in 27 of 29 patients (J Clin Invest. 2016 Jun 1;126[6]:2123-38).
However, the study also established that high CAR T-cell doses and tumor burden increased the risk of severe cytokine release syndrome (CRS) and neurotoxicity, Dr. Turtle said at the meeting, held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research. At its meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy (ASTCT).
“Importantly, we identified serum biomarkers that allow testing of early intervention strategies in the patients who have the highest risk of toxicity,” he said.
Dr. Turtle explained that significantly higher peak interleuken-6 (IL-6) and interferon (IFN)-gamma levels were seen after CAR T-cell infusion in patients with high bone marrow tumor burden and in patients requiring treatment in an intensive care unit (ICU).
ICU care correlated with a higher percentage of bone marrow blasts before lymphodepletion chemotherapy, he added.
Elevations of serum C-reactive protein (CRP) and ferritin also correlated with bone marrow disease burden and with the occurrence of severe CRS requiring ICU care, he said, noting that ferritin and CRP levels declined after tocilizumab or corticosteroid therapy.
In addition, all patients in the study who developed neurotoxicity had evidence of CRS. Peak levels of IL-6, IFN-gamma, ferritin, and CRP were significantly higher in those who developed grade 3 or higher neurotoxicity. Further, serum IL-6 and IFN-gamma concentrations on day 1 after infusion were significantly higher in those who required ICU care and in those who subsequently developed grade 4 neurotoxicity than in patients who developed grade 3 neurotoxicity.
Multivariate analysis indicated that serum IL-6 concentration of more than 30 pg/mL on day 1 and the total number of CD19+ cells in bone marrow before therapy were independent predictors of subsequent development of grade 3 or higher neurotoxicity.
Notably, serum IL-6 of more than 30 pg/mL on day 1 identified all patients in the study who subsequently developed grade 4 or higher neurotoxicity, Dr. Turtle and his colleagues noted.
“The findings suggested that evaluation of serum IL-6 concentration early after CAR T-cell infusion might be useful for identifying patients at high risk of severe neurotoxicity and to evaluate early intervention approaches,” he said.
Neurotoxicity
In a 2017 study from Juliane Gust, MD, PhD, and her colleagues, bone marrow disease burden, lymphodepletion regimen, and CAR T-cell dose were found to be significantly associated with neurotoxicity during multivariate analysis (Cancer Discov. 2017 Dec;7[12]:1404-19).
Patients with severe neurotoxicity in that study demonstrated evidence of endothelial activation, including disseminated intravascular coagulation, capillary leak, and increased blood-brain barrier permeability – with the latter leading to a failure to protect the cerebrospinal fluid from high concentrations of systemic cytokines, including IFN-gamma. These high levels of cytokines may cause vascular pericyte activation and stress, Dr. Turtle explained.
Patients who subsequently developed grade 4 or higher neurotoxicity had higher pretreatment levels of endothelial activation biomarkers.
“Endothelial cells and pericytes contribute to the integrity of the blood-brain barrier; this suggests a potential role for IL-6 and vascular endothelial growth factor from pericytes to augment endothelial permeability,” Dr. Turtle said.
CRS
In another 2017 study, from Kevin A. Hay, MD, and his colleagues, similar factors were found to be associated with CRS (Blood. 2017 Nov 23;130[21]:2295-306).
Multivariable analysis identified high marrow tumor burden, lymphodepletion using cyclophosphamide and fludarabine, higher CAR T-cell dose, thrombocytopenia before lymphodepletion, and manufacturing of CAR T cells without selection of CD8+ central memory T cells as independent predictors of CRS.
Severe CRS was characterized by hemodynamic instability, capillary leak, and consumptive coagulopathy. As in the study by Dr. Gust and her colleagues, biomarkers of endothelial activation, including angiopoietin-2 and von Willebrand factor, were increased during severe CRS and before lymphodepletion in patients who subsequently developed CRS.
Potential modifications
The findings to date suggest that risk stratification, prophylaxis, early intervention and therapeutic intervention are among potential strategies for mitigating the risk of CD19-directed CAR T toxicity, Dr. Turtle said. Steroids, tocilizumab, siltuximab, anakinra, anti-GM-CSF, small molecules, plasma exchange, angiopoietin-1, and hypertransfusion are among candidates under consideration for such interventions, he noted.
Other approaches that have been tested in small studies, and which may reduce toxicity and improve the therapeutic index of CD19 CAR T-cell therapy for B-ALL, include split dosing and risk-adapted dosing.
“These approaches do appear to mitigate toxicity, but larger studies are needed to confirm that treatment efficacy is maintained,” Dr. Turtle said.
Toxicity prediction and early intervention to maintain the CAR T-cell dose while avoiding grade 4 or greater toxicities would be helpful and is within reach, he said, noting that the findings by Dr. Hay and his colleagues led to the development of “day-1 cytokine combination algorithms that predict grade 4-5 CRS and could direct preemptive intervention.”
One algorithm based on three cytokines had high sensitivity and specificity, but would require screening of all patients.
Early intervention in patients in whom toxicity is predicted has not been extensively evaluated in clinical studies, he said.
Dr. Hay and his colleagues did, however, develop a “classification tree model of early intervention strategies” using their findings.
A complicating factor in predicting risk and intervening is that each CAR T-cell product is associated with differing levels of toxicity risk. The varying rates of toxicity suggest that promising approaches for addressing CAR T toxicity require validation for each product with respect to cutpoints, efficacy, and maintenance of response, Dr. Turtle said.
“The findings to date are encouraging and show that potentially targetable factors for mitigating the toxicity of CAR T-cell therapy can be identified,” he said. “But clinical studies have yet to convincingly establish the best approach.”
Dr. Turtle has served on advisory boards for Juno/Celgene, Kite/Gilead, Novartis, Precision Biosciences, Eureka Therapeutics, Caribou Biosciences, Nektar Therapeutics, Humanigen, and Aptevo; has intellectual property rights licensed to Juno; has stock options with Precision Biosciences, Eureka Therapeutics, and Caribou Biosciences; and has received research funding from Juno and Nektar Therapeutics.
HOUSTON – Cytokine release syndrome and neurotoxicity frequently occur with CD19-directed chimeric antigen receptor (CAR) T-cell immunotherapies, but targetable factors for mitigating the risk and effects of these complications are emerging, according to Cameron Turtle, MBBS, PhD.
These factors include infused CAR T-cell dose, bone marrow disease burden, immune response, and the lymphodepletion regimen used, Dr. Turtle, of Fred Hutchinson Cancer Research Center, Seattle, said at the Transplantation & Cellular Therapies Meetings. This list is based on an analysis of several studies that included a total of 195 patients with B-cell malignancies who were treated with defined-composition CD19 CAR T cells.
In a 2016 study included in the analysis, for instance, Dr. Turtle and his colleagues found that CD19 CAR T cells administered to adults with B-cell acute lymphoblastic leukemia (B-ALL) after lymphodepletion chemotherapy were “remarkably potent.” Remission was achieved in 27 of 29 patients (J Clin Invest. 2016 Jun 1;126[6]:2123-38).
However, the study also established that high CAR T-cell doses and tumor burden increased the risk of severe cytokine release syndrome (CRS) and neurotoxicity, Dr. Turtle said at the meeting, held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research. At its meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy (ASTCT).
“Importantly, we identified serum biomarkers that allow testing of early intervention strategies in the patients who have the highest risk of toxicity,” he said.
Dr. Turtle explained that significantly higher peak interleuken-6 (IL-6) and interferon (IFN)-gamma levels were seen after CAR T-cell infusion in patients with high bone marrow tumor burden and in patients requiring treatment in an intensive care unit (ICU).
ICU care correlated with a higher percentage of bone marrow blasts before lymphodepletion chemotherapy, he added.
Elevations of serum C-reactive protein (CRP) and ferritin also correlated with bone marrow disease burden and with the occurrence of severe CRS requiring ICU care, he said, noting that ferritin and CRP levels declined after tocilizumab or corticosteroid therapy.
In addition, all patients in the study who developed neurotoxicity had evidence of CRS. Peak levels of IL-6, IFN-gamma, ferritin, and CRP were significantly higher in those who developed grade 3 or higher neurotoxicity. Further, serum IL-6 and IFN-gamma concentrations on day 1 after infusion were significantly higher in those who required ICU care and in those who subsequently developed grade 4 neurotoxicity than in patients who developed grade 3 neurotoxicity.
Multivariate analysis indicated that serum IL-6 concentration of more than 30 pg/mL on day 1 and the total number of CD19+ cells in bone marrow before therapy were independent predictors of subsequent development of grade 3 or higher neurotoxicity.
Notably, serum IL-6 of more than 30 pg/mL on day 1 identified all patients in the study who subsequently developed grade 4 or higher neurotoxicity, Dr. Turtle and his colleagues noted.
“The findings suggested that evaluation of serum IL-6 concentration early after CAR T-cell infusion might be useful for identifying patients at high risk of severe neurotoxicity and to evaluate early intervention approaches,” he said.
Neurotoxicity
In a 2017 study from Juliane Gust, MD, PhD, and her colleagues, bone marrow disease burden, lymphodepletion regimen, and CAR T-cell dose were found to be significantly associated with neurotoxicity during multivariate analysis (Cancer Discov. 2017 Dec;7[12]:1404-19).
Patients with severe neurotoxicity in that study demonstrated evidence of endothelial activation, including disseminated intravascular coagulation, capillary leak, and increased blood-brain barrier permeability – with the latter leading to a failure to protect the cerebrospinal fluid from high concentrations of systemic cytokines, including IFN-gamma. These high levels of cytokines may cause vascular pericyte activation and stress, Dr. Turtle explained.
Patients who subsequently developed grade 4 or higher neurotoxicity had higher pretreatment levels of endothelial activation biomarkers.
“Endothelial cells and pericytes contribute to the integrity of the blood-brain barrier; this suggests a potential role for IL-6 and vascular endothelial growth factor from pericytes to augment endothelial permeability,” Dr. Turtle said.
CRS
In another 2017 study, from Kevin A. Hay, MD, and his colleagues, similar factors were found to be associated with CRS (Blood. 2017 Nov 23;130[21]:2295-306).
Multivariable analysis identified high marrow tumor burden, lymphodepletion using cyclophosphamide and fludarabine, higher CAR T-cell dose, thrombocytopenia before lymphodepletion, and manufacturing of CAR T cells without selection of CD8+ central memory T cells as independent predictors of CRS.
Severe CRS was characterized by hemodynamic instability, capillary leak, and consumptive coagulopathy. As in the study by Dr. Gust and her colleagues, biomarkers of endothelial activation, including angiopoietin-2 and von Willebrand factor, were increased during severe CRS and before lymphodepletion in patients who subsequently developed CRS.
Potential modifications
The findings to date suggest that risk stratification, prophylaxis, early intervention and therapeutic intervention are among potential strategies for mitigating the risk of CD19-directed CAR T toxicity, Dr. Turtle said. Steroids, tocilizumab, siltuximab, anakinra, anti-GM-CSF, small molecules, plasma exchange, angiopoietin-1, and hypertransfusion are among candidates under consideration for such interventions, he noted.
Other approaches that have been tested in small studies, and which may reduce toxicity and improve the therapeutic index of CD19 CAR T-cell therapy for B-ALL, include split dosing and risk-adapted dosing.
“These approaches do appear to mitigate toxicity, but larger studies are needed to confirm that treatment efficacy is maintained,” Dr. Turtle said.
Toxicity prediction and early intervention to maintain the CAR T-cell dose while avoiding grade 4 or greater toxicities would be helpful and is within reach, he said, noting that the findings by Dr. Hay and his colleagues led to the development of “day-1 cytokine combination algorithms that predict grade 4-5 CRS and could direct preemptive intervention.”
One algorithm based on three cytokines had high sensitivity and specificity, but would require screening of all patients.
Early intervention in patients in whom toxicity is predicted has not been extensively evaluated in clinical studies, he said.
Dr. Hay and his colleagues did, however, develop a “classification tree model of early intervention strategies” using their findings.
A complicating factor in predicting risk and intervening is that each CAR T-cell product is associated with differing levels of toxicity risk. The varying rates of toxicity suggest that promising approaches for addressing CAR T toxicity require validation for each product with respect to cutpoints, efficacy, and maintenance of response, Dr. Turtle said.
“The findings to date are encouraging and show that potentially targetable factors for mitigating the toxicity of CAR T-cell therapy can be identified,” he said. “But clinical studies have yet to convincingly establish the best approach.”
Dr. Turtle has served on advisory boards for Juno/Celgene, Kite/Gilead, Novartis, Precision Biosciences, Eureka Therapeutics, Caribou Biosciences, Nektar Therapeutics, Humanigen, and Aptevo; has intellectual property rights licensed to Juno; has stock options with Precision Biosciences, Eureka Therapeutics, and Caribou Biosciences; and has received research funding from Juno and Nektar Therapeutics.
HOUSTON – Cytokine release syndrome and neurotoxicity frequently occur with CD19-directed chimeric antigen receptor (CAR) T-cell immunotherapies, but targetable factors for mitigating the risk and effects of these complications are emerging, according to Cameron Turtle, MBBS, PhD.
These factors include infused CAR T-cell dose, bone marrow disease burden, immune response, and the lymphodepletion regimen used, Dr. Turtle, of Fred Hutchinson Cancer Research Center, Seattle, said at the Transplantation & Cellular Therapies Meetings. This list is based on an analysis of several studies that included a total of 195 patients with B-cell malignancies who were treated with defined-composition CD19 CAR T cells.
In a 2016 study included in the analysis, for instance, Dr. Turtle and his colleagues found that CD19 CAR T cells administered to adults with B-cell acute lymphoblastic leukemia (B-ALL) after lymphodepletion chemotherapy were “remarkably potent.” Remission was achieved in 27 of 29 patients (J Clin Invest. 2016 Jun 1;126[6]:2123-38).
However, the study also established that high CAR T-cell doses and tumor burden increased the risk of severe cytokine release syndrome (CRS) and neurotoxicity, Dr. Turtle said at the meeting, held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research. At its meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy (ASTCT).
“Importantly, we identified serum biomarkers that allow testing of early intervention strategies in the patients who have the highest risk of toxicity,” he said.
Dr. Turtle explained that significantly higher peak interleuken-6 (IL-6) and interferon (IFN)-gamma levels were seen after CAR T-cell infusion in patients with high bone marrow tumor burden and in patients requiring treatment in an intensive care unit (ICU).
ICU care correlated with a higher percentage of bone marrow blasts before lymphodepletion chemotherapy, he added.
Elevations of serum C-reactive protein (CRP) and ferritin also correlated with bone marrow disease burden and with the occurrence of severe CRS requiring ICU care, he said, noting that ferritin and CRP levels declined after tocilizumab or corticosteroid therapy.
In addition, all patients in the study who developed neurotoxicity had evidence of CRS. Peak levels of IL-6, IFN-gamma, ferritin, and CRP were significantly higher in those who developed grade 3 or higher neurotoxicity. Further, serum IL-6 and IFN-gamma concentrations on day 1 after infusion were significantly higher in those who required ICU care and in those who subsequently developed grade 4 neurotoxicity than in patients who developed grade 3 neurotoxicity.
Multivariate analysis indicated that serum IL-6 concentration of more than 30 pg/mL on day 1 and the total number of CD19+ cells in bone marrow before therapy were independent predictors of subsequent development of grade 3 or higher neurotoxicity.
Notably, serum IL-6 of more than 30 pg/mL on day 1 identified all patients in the study who subsequently developed grade 4 or higher neurotoxicity, Dr. Turtle and his colleagues noted.
“The findings suggested that evaluation of serum IL-6 concentration early after CAR T-cell infusion might be useful for identifying patients at high risk of severe neurotoxicity and to evaluate early intervention approaches,” he said.
Neurotoxicity
In a 2017 study from Juliane Gust, MD, PhD, and her colleagues, bone marrow disease burden, lymphodepletion regimen, and CAR T-cell dose were found to be significantly associated with neurotoxicity during multivariate analysis (Cancer Discov. 2017 Dec;7[12]:1404-19).
Patients with severe neurotoxicity in that study demonstrated evidence of endothelial activation, including disseminated intravascular coagulation, capillary leak, and increased blood-brain barrier permeability – with the latter leading to a failure to protect the cerebrospinal fluid from high concentrations of systemic cytokines, including IFN-gamma. These high levels of cytokines may cause vascular pericyte activation and stress, Dr. Turtle explained.
Patients who subsequently developed grade 4 or higher neurotoxicity had higher pretreatment levels of endothelial activation biomarkers.
“Endothelial cells and pericytes contribute to the integrity of the blood-brain barrier; this suggests a potential role for IL-6 and vascular endothelial growth factor from pericytes to augment endothelial permeability,” Dr. Turtle said.
CRS
In another 2017 study, from Kevin A. Hay, MD, and his colleagues, similar factors were found to be associated with CRS (Blood. 2017 Nov 23;130[21]:2295-306).
Multivariable analysis identified high marrow tumor burden, lymphodepletion using cyclophosphamide and fludarabine, higher CAR T-cell dose, thrombocytopenia before lymphodepletion, and manufacturing of CAR T cells without selection of CD8+ central memory T cells as independent predictors of CRS.
Severe CRS was characterized by hemodynamic instability, capillary leak, and consumptive coagulopathy. As in the study by Dr. Gust and her colleagues, biomarkers of endothelial activation, including angiopoietin-2 and von Willebrand factor, were increased during severe CRS and before lymphodepletion in patients who subsequently developed CRS.
Potential modifications
The findings to date suggest that risk stratification, prophylaxis, early intervention and therapeutic intervention are among potential strategies for mitigating the risk of CD19-directed CAR T toxicity, Dr. Turtle said. Steroids, tocilizumab, siltuximab, anakinra, anti-GM-CSF, small molecules, plasma exchange, angiopoietin-1, and hypertransfusion are among candidates under consideration for such interventions, he noted.
Other approaches that have been tested in small studies, and which may reduce toxicity and improve the therapeutic index of CD19 CAR T-cell therapy for B-ALL, include split dosing and risk-adapted dosing.
“These approaches do appear to mitigate toxicity, but larger studies are needed to confirm that treatment efficacy is maintained,” Dr. Turtle said.
Toxicity prediction and early intervention to maintain the CAR T-cell dose while avoiding grade 4 or greater toxicities would be helpful and is within reach, he said, noting that the findings by Dr. Hay and his colleagues led to the development of “day-1 cytokine combination algorithms that predict grade 4-5 CRS and could direct preemptive intervention.”
One algorithm based on three cytokines had high sensitivity and specificity, but would require screening of all patients.
Early intervention in patients in whom toxicity is predicted has not been extensively evaluated in clinical studies, he said.
Dr. Hay and his colleagues did, however, develop a “classification tree model of early intervention strategies” using their findings.
A complicating factor in predicting risk and intervening is that each CAR T-cell product is associated with differing levels of toxicity risk. The varying rates of toxicity suggest that promising approaches for addressing CAR T toxicity require validation for each product with respect to cutpoints, efficacy, and maintenance of response, Dr. Turtle said.
“The findings to date are encouraging and show that potentially targetable factors for mitigating the toxicity of CAR T-cell therapy can be identified,” he said. “But clinical studies have yet to convincingly establish the best approach.”
Dr. Turtle has served on advisory boards for Juno/Celgene, Kite/Gilead, Novartis, Precision Biosciences, Eureka Therapeutics, Caribou Biosciences, Nektar Therapeutics, Humanigen, and Aptevo; has intellectual property rights licensed to Juno; has stock options with Precision Biosciences, Eureka Therapeutics, and Caribou Biosciences; and has received research funding from Juno and Nektar Therapeutics.
REPORTING FROM TCT 2019
Creating CAR T-cell therapies for T-cell malignancies
NEWPORT BEACH, CALIF. – Preclinical research has revealed workarounds that may make chimeric antigen receptor (CAR) T-cell therapy feasible for patients with T-cell malignancies.
Researchers have found that using allogeneic cells for CAR T-cell therapy can eliminate contamination by malignant T cells, and editing those allogeneic T cells to delete the target antigen and the T-cell receptor alpha chain (TRAC) can prevent fratricide and graft-versus-host disease (GVHD).
Additionally, an interleukin-7 molecule called NT-I7 has been shown to enhance CAR T-cell proliferation, differentiation, and tumor killing in a mouse model of a T-cell malignancy.
John F. DiPersio, MD, PhD, of Washington University in St. Louis, described this work in a presentation at the Acute Leukemia Forum of Hemedicus.
Obstacles to development
“The primary obstacle for targeting T-cell malignancies with a T cell is that all of the targets that are on the [malignant] T cells are also expressed on the normal T cells,” Dr. DiPersio said. “So when you put a CAR into a normal T cell, it just kills itself. It’s called fratricide.”
A second issue that has limited development is that the phenotype of the malignant T cell in the blood is similar to a normal T cell, so they can’t be separated, he explained.
“So if you were to do anything to a normal T cell, you would also be doing it, in theory, to the malignant T cell – in theory, making it resistant to therapy,” he said.
A third obstacle, which has been seen in patients with B-cell malignancies as well, is the inability to harvest enough T cells to generate effective CAR T-cell therapy.
And a fourth obstacle is that T cells from patients with malignancies may not function normally because they have been exposed to prior therapies.
Dr. DiPersio and his colleagues believe these obstacles can be overcome by creating CAR T-cell therapies using T cells derived from healthy donors or cord blood, using gene editing to remove the target antigen and TRAC, and using NT-I7 to enhance the efficacy of these universal, “off-the-shelf” CAR T cells.
The researchers have tested these theories, and achieved successes, in preclinical models. The team is now planning a clinical trial in patients at Washington University. Dr. DiPersio and his colleagues also created a company called WUGEN that will develop the universal CAR T-cell therapies if the initial proof-of-principle trial proves successful.
UCART7
One of the universal CAR T-cell therapies Dr. DiPersio and his colleagues have tested is UCART7, which targets CD7. Dr. DiPersio noted that CD7 is expressed on 98% of T-cell acute lymphoblastic leukemias (T-ALLs), 24% of acute myeloid leukemias, natural killer (NK) cells, and T cells.
The researchers created UCART7 by using CRISPR/Cas9 to delete CD7 and TRAC from allogeneic T cells and following this with lentiviral transduction with a third-generation CD7-CAR. The team found a way to delete both TRAC and CD7 in a single day with 95% efficiency, Dr. DiPersio noted.
“Knocking out CD7 doesn’t seem to have any impact on the expansion or trafficking of these T cells in vivo,” Dr. DiPersio said. “So we think that deleting that target in a normal T cell will not affect its overall ability to kill a target when we put a CAR into those T cells.”
In fact, the researchers’ experiments showed that UCART7 can kill T-ALL cells in vitro and target primary T-ALL in vivo without inducing GVHD (Leukemia. 2018 Sep;32[9]:1970-83.)
UCART2 and NT-I7
Dr. DiPersio and his colleagues have also tested UCART2, an allogeneic CAR T-cell therapy in which CD2 and TRAC are deleted. The therapy targets CD2 because this antigen is expressed on T-ALL and other T-cell and NK-cell malignancies. Experiments showed that UCART2 targets T-cell malignancies, including T-ALL and cutaneous T-cell lymphoma, in vitro.
The researchers also tested UCART2 in a mouse model of Sézary syndrome. In these experiments, UCART2 was combined with NT-I7.
NT-I7 enhanced the proliferation, persistence, and tumor killing ability of UCART2. Sézary mice that received UCART2 and NT-I7 had “virtually no tumor burden,” according to researchers, and survived longer than mice treated with UCART2 alone (Blood. 2018;132:340).
Dr. DiPersio noted that there was no cytokine release syndrome because these were immunodeficient mice. However, cytokine release syndrome may be a side effect of NT-I7 in patients as NT-I7 induces rapid expansion of CAR T cells.
Dr. DiPersio reported ownership and investment in WUGEN and Magenta Therapeutics. He also has relationships with Cellworks Group, Tioma Therapeutics, RiverVest Venture Partners, Bioline, Asterias Biotherapeutics, Amphivena Therapeutics, Bluebird Bio, Celgene, Incyte, NeoImuneTech, and MacroGenics.
The Acute Leukemia Forum is organized by Hemedicus, which is owned by the same company as this news organization.
NEWPORT BEACH, CALIF. – Preclinical research has revealed workarounds that may make chimeric antigen receptor (CAR) T-cell therapy feasible for patients with T-cell malignancies.
Researchers have found that using allogeneic cells for CAR T-cell therapy can eliminate contamination by malignant T cells, and editing those allogeneic T cells to delete the target antigen and the T-cell receptor alpha chain (TRAC) can prevent fratricide and graft-versus-host disease (GVHD).
Additionally, an interleukin-7 molecule called NT-I7 has been shown to enhance CAR T-cell proliferation, differentiation, and tumor killing in a mouse model of a T-cell malignancy.
John F. DiPersio, MD, PhD, of Washington University in St. Louis, described this work in a presentation at the Acute Leukemia Forum of Hemedicus.
Obstacles to development
“The primary obstacle for targeting T-cell malignancies with a T cell is that all of the targets that are on the [malignant] T cells are also expressed on the normal T cells,” Dr. DiPersio said. “So when you put a CAR into a normal T cell, it just kills itself. It’s called fratricide.”
A second issue that has limited development is that the phenotype of the malignant T cell in the blood is similar to a normal T cell, so they can’t be separated, he explained.
“So if you were to do anything to a normal T cell, you would also be doing it, in theory, to the malignant T cell – in theory, making it resistant to therapy,” he said.
A third obstacle, which has been seen in patients with B-cell malignancies as well, is the inability to harvest enough T cells to generate effective CAR T-cell therapy.
And a fourth obstacle is that T cells from patients with malignancies may not function normally because they have been exposed to prior therapies.
Dr. DiPersio and his colleagues believe these obstacles can be overcome by creating CAR T-cell therapies using T cells derived from healthy donors or cord blood, using gene editing to remove the target antigen and TRAC, and using NT-I7 to enhance the efficacy of these universal, “off-the-shelf” CAR T cells.
The researchers have tested these theories, and achieved successes, in preclinical models. The team is now planning a clinical trial in patients at Washington University. Dr. DiPersio and his colleagues also created a company called WUGEN that will develop the universal CAR T-cell therapies if the initial proof-of-principle trial proves successful.
UCART7
One of the universal CAR T-cell therapies Dr. DiPersio and his colleagues have tested is UCART7, which targets CD7. Dr. DiPersio noted that CD7 is expressed on 98% of T-cell acute lymphoblastic leukemias (T-ALLs), 24% of acute myeloid leukemias, natural killer (NK) cells, and T cells.
The researchers created UCART7 by using CRISPR/Cas9 to delete CD7 and TRAC from allogeneic T cells and following this with lentiviral transduction with a third-generation CD7-CAR. The team found a way to delete both TRAC and CD7 in a single day with 95% efficiency, Dr. DiPersio noted.
“Knocking out CD7 doesn’t seem to have any impact on the expansion or trafficking of these T cells in vivo,” Dr. DiPersio said. “So we think that deleting that target in a normal T cell will not affect its overall ability to kill a target when we put a CAR into those T cells.”
In fact, the researchers’ experiments showed that UCART7 can kill T-ALL cells in vitro and target primary T-ALL in vivo without inducing GVHD (Leukemia. 2018 Sep;32[9]:1970-83.)
UCART2 and NT-I7
Dr. DiPersio and his colleagues have also tested UCART2, an allogeneic CAR T-cell therapy in which CD2 and TRAC are deleted. The therapy targets CD2 because this antigen is expressed on T-ALL and other T-cell and NK-cell malignancies. Experiments showed that UCART2 targets T-cell malignancies, including T-ALL and cutaneous T-cell lymphoma, in vitro.
The researchers also tested UCART2 in a mouse model of Sézary syndrome. In these experiments, UCART2 was combined with NT-I7.
NT-I7 enhanced the proliferation, persistence, and tumor killing ability of UCART2. Sézary mice that received UCART2 and NT-I7 had “virtually no tumor burden,” according to researchers, and survived longer than mice treated with UCART2 alone (Blood. 2018;132:340).
Dr. DiPersio noted that there was no cytokine release syndrome because these were immunodeficient mice. However, cytokine release syndrome may be a side effect of NT-I7 in patients as NT-I7 induces rapid expansion of CAR T cells.
Dr. DiPersio reported ownership and investment in WUGEN and Magenta Therapeutics. He also has relationships with Cellworks Group, Tioma Therapeutics, RiverVest Venture Partners, Bioline, Asterias Biotherapeutics, Amphivena Therapeutics, Bluebird Bio, Celgene, Incyte, NeoImuneTech, and MacroGenics.
The Acute Leukemia Forum is organized by Hemedicus, which is owned by the same company as this news organization.
NEWPORT BEACH, CALIF. – Preclinical research has revealed workarounds that may make chimeric antigen receptor (CAR) T-cell therapy feasible for patients with T-cell malignancies.
Researchers have found that using allogeneic cells for CAR T-cell therapy can eliminate contamination by malignant T cells, and editing those allogeneic T cells to delete the target antigen and the T-cell receptor alpha chain (TRAC) can prevent fratricide and graft-versus-host disease (GVHD).
Additionally, an interleukin-7 molecule called NT-I7 has been shown to enhance CAR T-cell proliferation, differentiation, and tumor killing in a mouse model of a T-cell malignancy.
John F. DiPersio, MD, PhD, of Washington University in St. Louis, described this work in a presentation at the Acute Leukemia Forum of Hemedicus.
Obstacles to development
“The primary obstacle for targeting T-cell malignancies with a T cell is that all of the targets that are on the [malignant] T cells are also expressed on the normal T cells,” Dr. DiPersio said. “So when you put a CAR into a normal T cell, it just kills itself. It’s called fratricide.”
A second issue that has limited development is that the phenotype of the malignant T cell in the blood is similar to a normal T cell, so they can’t be separated, he explained.
“So if you were to do anything to a normal T cell, you would also be doing it, in theory, to the malignant T cell – in theory, making it resistant to therapy,” he said.
A third obstacle, which has been seen in patients with B-cell malignancies as well, is the inability to harvest enough T cells to generate effective CAR T-cell therapy.
And a fourth obstacle is that T cells from patients with malignancies may not function normally because they have been exposed to prior therapies.
Dr. DiPersio and his colleagues believe these obstacles can be overcome by creating CAR T-cell therapies using T cells derived from healthy donors or cord blood, using gene editing to remove the target antigen and TRAC, and using NT-I7 to enhance the efficacy of these universal, “off-the-shelf” CAR T cells.
The researchers have tested these theories, and achieved successes, in preclinical models. The team is now planning a clinical trial in patients at Washington University. Dr. DiPersio and his colleagues also created a company called WUGEN that will develop the universal CAR T-cell therapies if the initial proof-of-principle trial proves successful.
UCART7
One of the universal CAR T-cell therapies Dr. DiPersio and his colleagues have tested is UCART7, which targets CD7. Dr. DiPersio noted that CD7 is expressed on 98% of T-cell acute lymphoblastic leukemias (T-ALLs), 24% of acute myeloid leukemias, natural killer (NK) cells, and T cells.
The researchers created UCART7 by using CRISPR/Cas9 to delete CD7 and TRAC from allogeneic T cells and following this with lentiviral transduction with a third-generation CD7-CAR. The team found a way to delete both TRAC and CD7 in a single day with 95% efficiency, Dr. DiPersio noted.
“Knocking out CD7 doesn’t seem to have any impact on the expansion or trafficking of these T cells in vivo,” Dr. DiPersio said. “So we think that deleting that target in a normal T cell will not affect its overall ability to kill a target when we put a CAR into those T cells.”
In fact, the researchers’ experiments showed that UCART7 can kill T-ALL cells in vitro and target primary T-ALL in vivo without inducing GVHD (Leukemia. 2018 Sep;32[9]:1970-83.)
UCART2 and NT-I7
Dr. DiPersio and his colleagues have also tested UCART2, an allogeneic CAR T-cell therapy in which CD2 and TRAC are deleted. The therapy targets CD2 because this antigen is expressed on T-ALL and other T-cell and NK-cell malignancies. Experiments showed that UCART2 targets T-cell malignancies, including T-ALL and cutaneous T-cell lymphoma, in vitro.
The researchers also tested UCART2 in a mouse model of Sézary syndrome. In these experiments, UCART2 was combined with NT-I7.
NT-I7 enhanced the proliferation, persistence, and tumor killing ability of UCART2. Sézary mice that received UCART2 and NT-I7 had “virtually no tumor burden,” according to researchers, and survived longer than mice treated with UCART2 alone (Blood. 2018;132:340).
Dr. DiPersio noted that there was no cytokine release syndrome because these were immunodeficient mice. However, cytokine release syndrome may be a side effect of NT-I7 in patients as NT-I7 induces rapid expansion of CAR T cells.
Dr. DiPersio reported ownership and investment in WUGEN and Magenta Therapeutics. He also has relationships with Cellworks Group, Tioma Therapeutics, RiverVest Venture Partners, Bioline, Asterias Biotherapeutics, Amphivena Therapeutics, Bluebird Bio, Celgene, Incyte, NeoImuneTech, and MacroGenics.
The Acute Leukemia Forum is organized by Hemedicus, which is owned by the same company as this news organization.
EXPERT ANALYSIS FROM ALF 2019
Higher dose of checkpoint inhibitor every 4 weeks feasible in NSCLC
SAN FRANCISCO – For patients with advanced non–small cell lung cancer (NSCLC) who previously had disease control with the checkpoint inhibitor nivolumab (Opdivo), second-line nivolumab at a higher dose every 4 weeks appeared to be comparable in efficacy and safety with standard-dose nivolumab every 2 weeks.
The key word in that last sentence is “appeared,” because the Checkmate 384 trial that was designed to show noninferiority of the every-4-weeks regimen lacked the statistical muscle to get the job done, reported Edward B. Garon, MD, from the University of California, Los Angeles.
“In many respects, extending the dosing frequency of nivolumab fulfills some of the promise of immunotherapy: The idea that we would be able to decrease the medicalization of the lives of our patients. For some people this would lead to them being able to resume a more normal work schedule, and for other people it would allow them to do things for fun, like travel on trips that would take longer than a couple of weeks,” he said at the American Society of Clinical Oncology (ASCO) – Society for Immunotherapy of Cancer (SITC): Clinical Immuno-Oncology Symposium.
However, because of difficulties in recruitment, the investigators had to stop enrollment early and settle for a sample size of 363 patients, instead of the 600 planned that would be necessary to meet a 10% noninferiority margin and one-sided 95% confidence interval. Thus, the trial analysis can only be reported as descriptive rather than definitive, Dr. Garon acknowledged.
Nivolumab is approved at a fixed dose of 240 mg every 2 weeks for the treatment of multiple tumor types in several different nations, and in the United States and Canada it is approved at a dose of 480 mg every 4 weeks for the treatment of NSCLC.
The CheckMate 384 study enrolled patients with advanced or metastatic NSCLC who had received 3 mg/kg or 240 mg of nivolumab every 2 weeks for up to 1 year. The patients had to have had relatively good performance status (Eastern Cooperative Oncology Group 0-2) and two consecutive assessments of either complete response, partial response, or stable disease.
The patients were stratified by tumor histology (squamous or nonsquamous) and response to prior nivolumab therapy at randomization, and were then randomized to receive nivolumab 240 mg every 2 weeks or 480 mg every 4 weeks until disease progression or unacceptable toxicity for up to 2 years.
Dr. Garon presented an interim analysis including data on 329 of the 363 patients; the final analysis will occur after all patients have had a minimum of 12 months of follow-up. Here, he reported on 6-month progression-free survival, a coprimary endpoint with 12-month PFS.
After a median follow-up of 9.5 months in the Q4-week group and 10.2 months in the Q2-week group, the 6-month PFS rates were identical between the two dosing strategies, at 72%. The median PFS was 12.1 months and 12.2 months, respectively.
“Although the study is no longer formally powered to show noninferiority, there’s certainly nothing in these curves that makes me concerned that this 480 mg every-4-week dose would be inferior,” Dr. Garon said.
There was a slightly higher rate of treatment-related adverse events of any grade in the lower, more frequent dose group: 48% in the Q4-week versus 61% in the Q2-week arm. The respective rates of grade 3 or 4 adverse events were 8% and 12%. Rates of serious adverse events and events leading to treatment discontinuation were similar between the group; there were no treatment-related deaths.
The investigators hypothesize that the higher rate of overall events in the lower-dose group may be attributable to more frequent visits and more opportunities to report adverse events, Dr. Garon said.
“Overall, the clinical data are in agreement with the pharmacokinetic modeling and give further evidence for this 480 mg every 4 week nivolumab dosing option,” he concluded.
The study was supported by Bristol-Myers Squibb. Dr. Garon reported receiving research support from Bristol-Myers Squibb and others and consulting fees from Dracen Pharmaceuticals.
SOURCE: Garon EB et al. ASCO-SITC, Abstract 100.
SAN FRANCISCO – For patients with advanced non–small cell lung cancer (NSCLC) who previously had disease control with the checkpoint inhibitor nivolumab (Opdivo), second-line nivolumab at a higher dose every 4 weeks appeared to be comparable in efficacy and safety with standard-dose nivolumab every 2 weeks.
The key word in that last sentence is “appeared,” because the Checkmate 384 trial that was designed to show noninferiority of the every-4-weeks regimen lacked the statistical muscle to get the job done, reported Edward B. Garon, MD, from the University of California, Los Angeles.
“In many respects, extending the dosing frequency of nivolumab fulfills some of the promise of immunotherapy: The idea that we would be able to decrease the medicalization of the lives of our patients. For some people this would lead to them being able to resume a more normal work schedule, and for other people it would allow them to do things for fun, like travel on trips that would take longer than a couple of weeks,” he said at the American Society of Clinical Oncology (ASCO) – Society for Immunotherapy of Cancer (SITC): Clinical Immuno-Oncology Symposium.
However, because of difficulties in recruitment, the investigators had to stop enrollment early and settle for a sample size of 363 patients, instead of the 600 planned that would be necessary to meet a 10% noninferiority margin and one-sided 95% confidence interval. Thus, the trial analysis can only be reported as descriptive rather than definitive, Dr. Garon acknowledged.
Nivolumab is approved at a fixed dose of 240 mg every 2 weeks for the treatment of multiple tumor types in several different nations, and in the United States and Canada it is approved at a dose of 480 mg every 4 weeks for the treatment of NSCLC.
The CheckMate 384 study enrolled patients with advanced or metastatic NSCLC who had received 3 mg/kg or 240 mg of nivolumab every 2 weeks for up to 1 year. The patients had to have had relatively good performance status (Eastern Cooperative Oncology Group 0-2) and two consecutive assessments of either complete response, partial response, or stable disease.
The patients were stratified by tumor histology (squamous or nonsquamous) and response to prior nivolumab therapy at randomization, and were then randomized to receive nivolumab 240 mg every 2 weeks or 480 mg every 4 weeks until disease progression or unacceptable toxicity for up to 2 years.
Dr. Garon presented an interim analysis including data on 329 of the 363 patients; the final analysis will occur after all patients have had a minimum of 12 months of follow-up. Here, he reported on 6-month progression-free survival, a coprimary endpoint with 12-month PFS.
After a median follow-up of 9.5 months in the Q4-week group and 10.2 months in the Q2-week group, the 6-month PFS rates were identical between the two dosing strategies, at 72%. The median PFS was 12.1 months and 12.2 months, respectively.
“Although the study is no longer formally powered to show noninferiority, there’s certainly nothing in these curves that makes me concerned that this 480 mg every-4-week dose would be inferior,” Dr. Garon said.
There was a slightly higher rate of treatment-related adverse events of any grade in the lower, more frequent dose group: 48% in the Q4-week versus 61% in the Q2-week arm. The respective rates of grade 3 or 4 adverse events were 8% and 12%. Rates of serious adverse events and events leading to treatment discontinuation were similar between the group; there were no treatment-related deaths.
The investigators hypothesize that the higher rate of overall events in the lower-dose group may be attributable to more frequent visits and more opportunities to report adverse events, Dr. Garon said.
“Overall, the clinical data are in agreement with the pharmacokinetic modeling and give further evidence for this 480 mg every 4 week nivolumab dosing option,” he concluded.
The study was supported by Bristol-Myers Squibb. Dr. Garon reported receiving research support from Bristol-Myers Squibb and others and consulting fees from Dracen Pharmaceuticals.
SOURCE: Garon EB et al. ASCO-SITC, Abstract 100.
SAN FRANCISCO – For patients with advanced non–small cell lung cancer (NSCLC) who previously had disease control with the checkpoint inhibitor nivolumab (Opdivo), second-line nivolumab at a higher dose every 4 weeks appeared to be comparable in efficacy and safety with standard-dose nivolumab every 2 weeks.
The key word in that last sentence is “appeared,” because the Checkmate 384 trial that was designed to show noninferiority of the every-4-weeks regimen lacked the statistical muscle to get the job done, reported Edward B. Garon, MD, from the University of California, Los Angeles.
“In many respects, extending the dosing frequency of nivolumab fulfills some of the promise of immunotherapy: The idea that we would be able to decrease the medicalization of the lives of our patients. For some people this would lead to them being able to resume a more normal work schedule, and for other people it would allow them to do things for fun, like travel on trips that would take longer than a couple of weeks,” he said at the American Society of Clinical Oncology (ASCO) – Society for Immunotherapy of Cancer (SITC): Clinical Immuno-Oncology Symposium.
However, because of difficulties in recruitment, the investigators had to stop enrollment early and settle for a sample size of 363 patients, instead of the 600 planned that would be necessary to meet a 10% noninferiority margin and one-sided 95% confidence interval. Thus, the trial analysis can only be reported as descriptive rather than definitive, Dr. Garon acknowledged.
Nivolumab is approved at a fixed dose of 240 mg every 2 weeks for the treatment of multiple tumor types in several different nations, and in the United States and Canada it is approved at a dose of 480 mg every 4 weeks for the treatment of NSCLC.
The CheckMate 384 study enrolled patients with advanced or metastatic NSCLC who had received 3 mg/kg or 240 mg of nivolumab every 2 weeks for up to 1 year. The patients had to have had relatively good performance status (Eastern Cooperative Oncology Group 0-2) and two consecutive assessments of either complete response, partial response, or stable disease.
The patients were stratified by tumor histology (squamous or nonsquamous) and response to prior nivolumab therapy at randomization, and were then randomized to receive nivolumab 240 mg every 2 weeks or 480 mg every 4 weeks until disease progression or unacceptable toxicity for up to 2 years.
Dr. Garon presented an interim analysis including data on 329 of the 363 patients; the final analysis will occur after all patients have had a minimum of 12 months of follow-up. Here, he reported on 6-month progression-free survival, a coprimary endpoint with 12-month PFS.
After a median follow-up of 9.5 months in the Q4-week group and 10.2 months in the Q2-week group, the 6-month PFS rates were identical between the two dosing strategies, at 72%. The median PFS was 12.1 months and 12.2 months, respectively.
“Although the study is no longer formally powered to show noninferiority, there’s certainly nothing in these curves that makes me concerned that this 480 mg every-4-week dose would be inferior,” Dr. Garon said.
There was a slightly higher rate of treatment-related adverse events of any grade in the lower, more frequent dose group: 48% in the Q4-week versus 61% in the Q2-week arm. The respective rates of grade 3 or 4 adverse events were 8% and 12%. Rates of serious adverse events and events leading to treatment discontinuation were similar between the group; there were no treatment-related deaths.
The investigators hypothesize that the higher rate of overall events in the lower-dose group may be attributable to more frequent visits and more opportunities to report adverse events, Dr. Garon said.
“Overall, the clinical data are in agreement with the pharmacokinetic modeling and give further evidence for this 480 mg every 4 week nivolumab dosing option,” he concluded.
The study was supported by Bristol-Myers Squibb. Dr. Garon reported receiving research support from Bristol-Myers Squibb and others and consulting fees from Dracen Pharmaceuticals.
SOURCE: Garon EB et al. ASCO-SITC, Abstract 100.
REPORTING FROM ASCO-SITC
Barriers to CAR T use in the spotlight at first European meeting
outcomes data suggest.
For that reason, and because bone marrow units are profit centers and CAR T-cell therapy reimbursement remains problematic, CAR T in the United States is “effectively being used as a bridge to transplant” – at a cost of more than $1 million per dose, economist Duane Schulthess told attendees at a recent, first-of-its-kind joint European CAR T-cell meeting in Paris, which was cosponsored by the European Hematology Association (EHA) and the European Society for Blood and Marrow Transplantation (EBMT).
“This is the way clinical practice is evolving right now; the price is not allowing enough experimentation for CAR T to flow up and be used in the less-diseased population,” said Mr. Schulthess, managing director of Vital Transformation, a consulting company based in Wezembeek-Oppem, Belgium.
In Europe, there is a slightly different problem in that health technology assessment bodies (HTAs) “have to figure out what they want to do” given the 2018 approvals of the first CAR T therapies there, he said, explaining that the data he presented was from a study commissioned by the Dutch government to help determine “what [CAR T] looks like from an effectiveness standpoint while they’re trying to figure out how much it’s worth and what they should pay.”
“Increasingly these are the big issues,” Mr. Schulthess said.
In August, the European Commission approved tisagenlecleucel (Kymriah) and axicabtagene ciloleucel (Yescarta) on the recommendation of the European Medicines Agency. Kymriah was approved for pediatric and young adult patients up to age 25 years with refractory B-cell acute lymphoblastic leukemia in relapse after transplant or in second or later relapse, as well as for adults patients with relapsed/refractory diffuse large B-cell lymphoma after failing at least two lines of systemic therapy, and Yescarta was approved for the latter and for the treatment of primary refractory mediastinal large B-cell lymphoma after at least two lines of systemic therapy.
The approvals have researchers and clinicians there clamoring for information about the therapy, which is revolutionizing the field of hematologic malignancies, according to Christian Chabannon, MD, PhD, chair of the EBMT Cellular Therapy & Immunobiology Working Party and vice-chair of the EBMT Scientific Council.
“An increasing number of European institutions are starting to administer this new category of medicinal products and increasingly contribute to ongoing clinical protocols and preclinical studies,” Dr. Chabannon said in an interview, explaining the urgency in planning the 1st European CAR T Cell Meeting just 6 months after the CAR T approvals in Europe.
EHA and EBMT brought together patient advocates, young investigators, and experts from across the globe to present the latest relevant information and data on topics ranging from current trials and experience, CAR T implementation and management, the preclinical and clinical pipelines, various CAR T applications, industry perspectives, and relevant economic issues, he said.
The latter is where Mr. Schulthess came in.
His research involved patient-level treatment pathway data from a database of more than 3 million patients treated with either allogeneic hematopoietic stem cell transplant (allo-HCT) or CAR T therapy across 5 years of experience. The data showed up to 85% response rates for each in the first-line setting. He and his colleagues then looked at therapy choices for those who failed to respond to second-line therapies and at how decisions were made regarding transplant and CAR T therapy – and specifically whether CAR T can be a substitute for transplant.
Ultimately, they looked at 29 allo-HCT recipients and 14 CAR T therapy recipients for a head-to-head comparison of the two treatments and performed an in-depth cost-efficacy analysis using a novel “visual pathology” methodology to account for limitations in the data.
The 3-year relapse-free survival probability was nearly 68% in the transplant recipients and 46% with CAR T.
“Now why is that? [Because] ... these populations are not the same; the CAR T population has a much higher disease burden,” Mr. Schulthess said. “So what we’re seeing [among] actual clinical doctors doing this for real – they are defaulting to bone marrow transplants, except in those cases where they do not have enough time or the patient does not respond. Then and only then are they giving CAR T.”
And that comes back to the fact that bone marrow units make money, he said.
CAR T is costly, and reimbursement can be problematic; these are disincentives for doctors to use CAR T therapy, at least in the United States, and while this is currently “being worked out,” the choice more often is “giving bone marrow transplant first and seeing what happens,” Mr. Schulthess said.
In Europe, that creates “a tough choice” for the HTAs, he said, noting that, in the absence of evidence of CAR T being curative in the subpopulation of patients with high disease burden who fail transplant and given the high cost, there is a push to determine at what point it begins to make sense economically.
“We think that you gain efficiency at ... roughly $277,000 [per dose] because [at that cost] you can do more CAR Ts than you can do bone marrow transplants. [CAR T] is less invasive, it’s lighter touch, it’s more efficient,” he said. “So if we were to see an efficiency cost of between $222,000 and $277,000, we think that works.”
Another recent study came to similar conclusions based on quality assessments, he said (J Clin Oncol. 2018 Sep 13. doi: 10.1200/JCO.2018.79.0642).
“We think that’s where this is going to end up, so we think that, if someone starts producing this for a couple hundred thousand bucks, then – certainly in Europe – it will make sense for this to start drifting up and being used as a substitute [to transplant],” he added.
Mr. Schulthess was one of scores of experts and investigators who presented at the EHA/EBMT joint meeting, which included numerous U.S. pioneers in the field and young European investigators, among others, Dr. Chabannon said.
Attesting to the enthusiasm in Europe regarding CAR T, Dr. Chabannon said that there were “more requests for registration than the venue could safely accommodate, a long waiting list, and a high number of individuals on the waiting list who registered for the live streaming” of the event.
“The field of CAR T cells is growing at a fast pace since the first clinical successes reported in the early 2010s, and one can wonder whether the expectations are not in excess of what reality will deliver,” he said. “Nevertheless, CAR T cells represent an essential innovation, not an incremental progress in biomedical sciences. They combine new mechanisms of action, clinical activity in advanced malignancies (and possibly beyond the field of cancer), transfer of manufacturing of human cell-based therapeutics to the industry, and potentially the first commercial success for a gene therapy.”
Surveys conducted by various professional associations, including EBMT, have clearly identified the potential for clinical successes that CAR T cells represent and the tremendous challenges raised by these innovations, he said, noting that “these include fulfilling specific educational needs.”
Therefore, EBMT and EHA have already announced that a second edition of the meeting is planned for Jan. 30 – Feb. 1, 2020, he noted.
Mr. Schulthess reported that his research was funded by the Dutch government.
outcomes data suggest.
For that reason, and because bone marrow units are profit centers and CAR T-cell therapy reimbursement remains problematic, CAR T in the United States is “effectively being used as a bridge to transplant” – at a cost of more than $1 million per dose, economist Duane Schulthess told attendees at a recent, first-of-its-kind joint European CAR T-cell meeting in Paris, which was cosponsored by the European Hematology Association (EHA) and the European Society for Blood and Marrow Transplantation (EBMT).
“This is the way clinical practice is evolving right now; the price is not allowing enough experimentation for CAR T to flow up and be used in the less-diseased population,” said Mr. Schulthess, managing director of Vital Transformation, a consulting company based in Wezembeek-Oppem, Belgium.
In Europe, there is a slightly different problem in that health technology assessment bodies (HTAs) “have to figure out what they want to do” given the 2018 approvals of the first CAR T therapies there, he said, explaining that the data he presented was from a study commissioned by the Dutch government to help determine “what [CAR T] looks like from an effectiveness standpoint while they’re trying to figure out how much it’s worth and what they should pay.”
“Increasingly these are the big issues,” Mr. Schulthess said.
In August, the European Commission approved tisagenlecleucel (Kymriah) and axicabtagene ciloleucel (Yescarta) on the recommendation of the European Medicines Agency. Kymriah was approved for pediatric and young adult patients up to age 25 years with refractory B-cell acute lymphoblastic leukemia in relapse after transplant or in second or later relapse, as well as for adults patients with relapsed/refractory diffuse large B-cell lymphoma after failing at least two lines of systemic therapy, and Yescarta was approved for the latter and for the treatment of primary refractory mediastinal large B-cell lymphoma after at least two lines of systemic therapy.
The approvals have researchers and clinicians there clamoring for information about the therapy, which is revolutionizing the field of hematologic malignancies, according to Christian Chabannon, MD, PhD, chair of the EBMT Cellular Therapy & Immunobiology Working Party and vice-chair of the EBMT Scientific Council.
“An increasing number of European institutions are starting to administer this new category of medicinal products and increasingly contribute to ongoing clinical protocols and preclinical studies,” Dr. Chabannon said in an interview, explaining the urgency in planning the 1st European CAR T Cell Meeting just 6 months after the CAR T approvals in Europe.
EHA and EBMT brought together patient advocates, young investigators, and experts from across the globe to present the latest relevant information and data on topics ranging from current trials and experience, CAR T implementation and management, the preclinical and clinical pipelines, various CAR T applications, industry perspectives, and relevant economic issues, he said.
The latter is where Mr. Schulthess came in.
His research involved patient-level treatment pathway data from a database of more than 3 million patients treated with either allogeneic hematopoietic stem cell transplant (allo-HCT) or CAR T therapy across 5 years of experience. The data showed up to 85% response rates for each in the first-line setting. He and his colleagues then looked at therapy choices for those who failed to respond to second-line therapies and at how decisions were made regarding transplant and CAR T therapy – and specifically whether CAR T can be a substitute for transplant.
Ultimately, they looked at 29 allo-HCT recipients and 14 CAR T therapy recipients for a head-to-head comparison of the two treatments and performed an in-depth cost-efficacy analysis using a novel “visual pathology” methodology to account for limitations in the data.
The 3-year relapse-free survival probability was nearly 68% in the transplant recipients and 46% with CAR T.
“Now why is that? [Because] ... these populations are not the same; the CAR T population has a much higher disease burden,” Mr. Schulthess said. “So what we’re seeing [among] actual clinical doctors doing this for real – they are defaulting to bone marrow transplants, except in those cases where they do not have enough time or the patient does not respond. Then and only then are they giving CAR T.”
And that comes back to the fact that bone marrow units make money, he said.
CAR T is costly, and reimbursement can be problematic; these are disincentives for doctors to use CAR T therapy, at least in the United States, and while this is currently “being worked out,” the choice more often is “giving bone marrow transplant first and seeing what happens,” Mr. Schulthess said.
In Europe, that creates “a tough choice” for the HTAs, he said, noting that, in the absence of evidence of CAR T being curative in the subpopulation of patients with high disease burden who fail transplant and given the high cost, there is a push to determine at what point it begins to make sense economically.
“We think that you gain efficiency at ... roughly $277,000 [per dose] because [at that cost] you can do more CAR Ts than you can do bone marrow transplants. [CAR T] is less invasive, it’s lighter touch, it’s more efficient,” he said. “So if we were to see an efficiency cost of between $222,000 and $277,000, we think that works.”
Another recent study came to similar conclusions based on quality assessments, he said (J Clin Oncol. 2018 Sep 13. doi: 10.1200/JCO.2018.79.0642).
“We think that’s where this is going to end up, so we think that, if someone starts producing this for a couple hundred thousand bucks, then – certainly in Europe – it will make sense for this to start drifting up and being used as a substitute [to transplant],” he added.
Mr. Schulthess was one of scores of experts and investigators who presented at the EHA/EBMT joint meeting, which included numerous U.S. pioneers in the field and young European investigators, among others, Dr. Chabannon said.
Attesting to the enthusiasm in Europe regarding CAR T, Dr. Chabannon said that there were “more requests for registration than the venue could safely accommodate, a long waiting list, and a high number of individuals on the waiting list who registered for the live streaming” of the event.
“The field of CAR T cells is growing at a fast pace since the first clinical successes reported in the early 2010s, and one can wonder whether the expectations are not in excess of what reality will deliver,” he said. “Nevertheless, CAR T cells represent an essential innovation, not an incremental progress in biomedical sciences. They combine new mechanisms of action, clinical activity in advanced malignancies (and possibly beyond the field of cancer), transfer of manufacturing of human cell-based therapeutics to the industry, and potentially the first commercial success for a gene therapy.”
Surveys conducted by various professional associations, including EBMT, have clearly identified the potential for clinical successes that CAR T cells represent and the tremendous challenges raised by these innovations, he said, noting that “these include fulfilling specific educational needs.”
Therefore, EBMT and EHA have already announced that a second edition of the meeting is planned for Jan. 30 – Feb. 1, 2020, he noted.
Mr. Schulthess reported that his research was funded by the Dutch government.
outcomes data suggest.
For that reason, and because bone marrow units are profit centers and CAR T-cell therapy reimbursement remains problematic, CAR T in the United States is “effectively being used as a bridge to transplant” – at a cost of more than $1 million per dose, economist Duane Schulthess told attendees at a recent, first-of-its-kind joint European CAR T-cell meeting in Paris, which was cosponsored by the European Hematology Association (EHA) and the European Society for Blood and Marrow Transplantation (EBMT).
“This is the way clinical practice is evolving right now; the price is not allowing enough experimentation for CAR T to flow up and be used in the less-diseased population,” said Mr. Schulthess, managing director of Vital Transformation, a consulting company based in Wezembeek-Oppem, Belgium.
In Europe, there is a slightly different problem in that health technology assessment bodies (HTAs) “have to figure out what they want to do” given the 2018 approvals of the first CAR T therapies there, he said, explaining that the data he presented was from a study commissioned by the Dutch government to help determine “what [CAR T] looks like from an effectiveness standpoint while they’re trying to figure out how much it’s worth and what they should pay.”
“Increasingly these are the big issues,” Mr. Schulthess said.
In August, the European Commission approved tisagenlecleucel (Kymriah) and axicabtagene ciloleucel (Yescarta) on the recommendation of the European Medicines Agency. Kymriah was approved for pediatric and young adult patients up to age 25 years with refractory B-cell acute lymphoblastic leukemia in relapse after transplant or in second or later relapse, as well as for adults patients with relapsed/refractory diffuse large B-cell lymphoma after failing at least two lines of systemic therapy, and Yescarta was approved for the latter and for the treatment of primary refractory mediastinal large B-cell lymphoma after at least two lines of systemic therapy.
The approvals have researchers and clinicians there clamoring for information about the therapy, which is revolutionizing the field of hematologic malignancies, according to Christian Chabannon, MD, PhD, chair of the EBMT Cellular Therapy & Immunobiology Working Party and vice-chair of the EBMT Scientific Council.
“An increasing number of European institutions are starting to administer this new category of medicinal products and increasingly contribute to ongoing clinical protocols and preclinical studies,” Dr. Chabannon said in an interview, explaining the urgency in planning the 1st European CAR T Cell Meeting just 6 months after the CAR T approvals in Europe.
EHA and EBMT brought together patient advocates, young investigators, and experts from across the globe to present the latest relevant information and data on topics ranging from current trials and experience, CAR T implementation and management, the preclinical and clinical pipelines, various CAR T applications, industry perspectives, and relevant economic issues, he said.
The latter is where Mr. Schulthess came in.
His research involved patient-level treatment pathway data from a database of more than 3 million patients treated with either allogeneic hematopoietic stem cell transplant (allo-HCT) or CAR T therapy across 5 years of experience. The data showed up to 85% response rates for each in the first-line setting. He and his colleagues then looked at therapy choices for those who failed to respond to second-line therapies and at how decisions were made regarding transplant and CAR T therapy – and specifically whether CAR T can be a substitute for transplant.
Ultimately, they looked at 29 allo-HCT recipients and 14 CAR T therapy recipients for a head-to-head comparison of the two treatments and performed an in-depth cost-efficacy analysis using a novel “visual pathology” methodology to account for limitations in the data.
The 3-year relapse-free survival probability was nearly 68% in the transplant recipients and 46% with CAR T.
“Now why is that? [Because] ... these populations are not the same; the CAR T population has a much higher disease burden,” Mr. Schulthess said. “So what we’re seeing [among] actual clinical doctors doing this for real – they are defaulting to bone marrow transplants, except in those cases where they do not have enough time or the patient does not respond. Then and only then are they giving CAR T.”
And that comes back to the fact that bone marrow units make money, he said.
CAR T is costly, and reimbursement can be problematic; these are disincentives for doctors to use CAR T therapy, at least in the United States, and while this is currently “being worked out,” the choice more often is “giving bone marrow transplant first and seeing what happens,” Mr. Schulthess said.
In Europe, that creates “a tough choice” for the HTAs, he said, noting that, in the absence of evidence of CAR T being curative in the subpopulation of patients with high disease burden who fail transplant and given the high cost, there is a push to determine at what point it begins to make sense economically.
“We think that you gain efficiency at ... roughly $277,000 [per dose] because [at that cost] you can do more CAR Ts than you can do bone marrow transplants. [CAR T] is less invasive, it’s lighter touch, it’s more efficient,” he said. “So if we were to see an efficiency cost of between $222,000 and $277,000, we think that works.”
Another recent study came to similar conclusions based on quality assessments, he said (J Clin Oncol. 2018 Sep 13. doi: 10.1200/JCO.2018.79.0642).
“We think that’s where this is going to end up, so we think that, if someone starts producing this for a couple hundred thousand bucks, then – certainly in Europe – it will make sense for this to start drifting up and being used as a substitute [to transplant],” he added.
Mr. Schulthess was one of scores of experts and investigators who presented at the EHA/EBMT joint meeting, which included numerous U.S. pioneers in the field and young European investigators, among others, Dr. Chabannon said.
Attesting to the enthusiasm in Europe regarding CAR T, Dr. Chabannon said that there were “more requests for registration than the venue could safely accommodate, a long waiting list, and a high number of individuals on the waiting list who registered for the live streaming” of the event.
“The field of CAR T cells is growing at a fast pace since the first clinical successes reported in the early 2010s, and one can wonder whether the expectations are not in excess of what reality will deliver,” he said. “Nevertheless, CAR T cells represent an essential innovation, not an incremental progress in biomedical sciences. They combine new mechanisms of action, clinical activity in advanced malignancies (and possibly beyond the field of cancer), transfer of manufacturing of human cell-based therapeutics to the industry, and potentially the first commercial success for a gene therapy.”
Surveys conducted by various professional associations, including EBMT, have clearly identified the potential for clinical successes that CAR T cells represent and the tremendous challenges raised by these innovations, he said, noting that “these include fulfilling specific educational needs.”
Therefore, EBMT and EHA have already announced that a second edition of the meeting is planned for Jan. 30 – Feb. 1, 2020, he noted.
Mr. Schulthess reported that his research was funded by the Dutch government.
CMS proposes coverage of CAR T-cell therapy in trials
The Centers for Medicare & Medicaid Services has proposed to cover chimeric antigen receptor (CAR) T-cell therapy for cancer patients participating in clinical trials that study the treatment’s effectiveness, according to a Feb. 15 announcement.
The proposed national coverage determination would require CMS to cover CAR T-cell therapies nationwide when the treatment is offered in CMS-approved registries or clinical studies in which patients are monitored for 2 or more years following treatment.
Results from the studies would help CMS identify which patients benefit most from CAR T-cell therapies and inform future coverage decisions, CMS Administrator Seema Verma said.
“CAR T-cell therapy was the first FDA-approved gene therapy, marking the beginning of an entirely new approach to treating serious and even life-threatening diseases,” Ms. Verma said in a statement. “Today’s proposed coverage decision would improve access to this therapy while deepening CMS’s understanding of how patients in Medicare respond to it, so the agency can ensure that it is paying for CAR T-cell therapy for cases in which the benefits outweigh the risks.”
As part of the proposal, CMS would cover autologous treatment with T cells expressing at least one chimeric antigen receptor (CAR) through coverage with evidence development when prescribed by a treating oncologist and performed in a hospital, according to a summary of the proposal.
The patient and hospital must meet specific criteria to be eligible for coverage, including that patients have relapsed or refractory cancer and do not have a comorbidity that would otherwise preclude patient benefit.
Hospitals, meanwhile, must have a cellular therapy program consisting of an integrated medical team that includes a clinical program director, a quality manager, and at least one physician experienced in cellular therapy, among other requirements.
CMS also would require that treatment is an FDA-approved biologic, providing targeted therapy for a known antigen expressed in the patient’s cancer according to an FDA indication. Repeat treatment would be covered only when a new primary cancer diagnosis is made by the treating oncologist and certain patient conditions are met.
Both inpatient and outpatient settings for the CAR T-cell therapy treatment are acceptable under the proposal. In either case, the patient and the hospital must be participating in a prospective, national, audited registry that consecutively enrolls patients, accepts all manufactured products, follows the patient for at least 2 years, and addresses a set of approved evidence-development questions. Additionally, all registries must be reviewed and approved by CMS.
The proposed national coverage determination was the result of an Aug. 22, 2018 meeting of the Medicare Evidence Development & Coverage Advisory Committee. The committee provides CMS with an external assessment of the appropriateness of therapies under review.
Public comments about the CAR T-cell therapy proposal will be accepted online here until March 15. A final decision on the proposal is expected by May 2019.
The agency’s proposal follows an Aug. 17 final rule by CMS that sets a new payment scheme for inpatient administration of two CAR T-cell therapies. The rule categorizes CAR T-cell therapies under the umbrella of the renamed Medicare Severity–Diagnosis Related Groups 016 – Autologous Bone Marrow Transplant with CC/MCC or T-cell Immunotherapy – and assigns ICD-10 PCS procedure codes XW033C3 and XW043C3 to the use of axicabtagene ciloleucel (Yescarta) and tisagenlecleucel (Kymriah) in the inpatient setting for fiscal year 2019, which began in October 2018. CMS also approved a temporary New Technology Add-On Payment for use of the therapies with a maximum threshold of $186,500.
In April 2018, CMS announced payment rates for outpatient administration of the two drugs, settling on $395,380 for axicabtagene ciloleucel and $500,839 for tisagenlecleucel. The two medications have list prices of $373,000 and $475,000, respectively.
The Centers for Medicare & Medicaid Services has proposed to cover chimeric antigen receptor (CAR) T-cell therapy for cancer patients participating in clinical trials that study the treatment’s effectiveness, according to a Feb. 15 announcement.
The proposed national coverage determination would require CMS to cover CAR T-cell therapies nationwide when the treatment is offered in CMS-approved registries or clinical studies in which patients are monitored for 2 or more years following treatment.
Results from the studies would help CMS identify which patients benefit most from CAR T-cell therapies and inform future coverage decisions, CMS Administrator Seema Verma said.
“CAR T-cell therapy was the first FDA-approved gene therapy, marking the beginning of an entirely new approach to treating serious and even life-threatening diseases,” Ms. Verma said in a statement. “Today’s proposed coverage decision would improve access to this therapy while deepening CMS’s understanding of how patients in Medicare respond to it, so the agency can ensure that it is paying for CAR T-cell therapy for cases in which the benefits outweigh the risks.”
As part of the proposal, CMS would cover autologous treatment with T cells expressing at least one chimeric antigen receptor (CAR) through coverage with evidence development when prescribed by a treating oncologist and performed in a hospital, according to a summary of the proposal.
The patient and hospital must meet specific criteria to be eligible for coverage, including that patients have relapsed or refractory cancer and do not have a comorbidity that would otherwise preclude patient benefit.
Hospitals, meanwhile, must have a cellular therapy program consisting of an integrated medical team that includes a clinical program director, a quality manager, and at least one physician experienced in cellular therapy, among other requirements.
CMS also would require that treatment is an FDA-approved biologic, providing targeted therapy for a known antigen expressed in the patient’s cancer according to an FDA indication. Repeat treatment would be covered only when a new primary cancer diagnosis is made by the treating oncologist and certain patient conditions are met.
Both inpatient and outpatient settings for the CAR T-cell therapy treatment are acceptable under the proposal. In either case, the patient and the hospital must be participating in a prospective, national, audited registry that consecutively enrolls patients, accepts all manufactured products, follows the patient for at least 2 years, and addresses a set of approved evidence-development questions. Additionally, all registries must be reviewed and approved by CMS.
The proposed national coverage determination was the result of an Aug. 22, 2018 meeting of the Medicare Evidence Development & Coverage Advisory Committee. The committee provides CMS with an external assessment of the appropriateness of therapies under review.
Public comments about the CAR T-cell therapy proposal will be accepted online here until March 15. A final decision on the proposal is expected by May 2019.
The agency’s proposal follows an Aug. 17 final rule by CMS that sets a new payment scheme for inpatient administration of two CAR T-cell therapies. The rule categorizes CAR T-cell therapies under the umbrella of the renamed Medicare Severity–Diagnosis Related Groups 016 – Autologous Bone Marrow Transplant with CC/MCC or T-cell Immunotherapy – and assigns ICD-10 PCS procedure codes XW033C3 and XW043C3 to the use of axicabtagene ciloleucel (Yescarta) and tisagenlecleucel (Kymriah) in the inpatient setting for fiscal year 2019, which began in October 2018. CMS also approved a temporary New Technology Add-On Payment for use of the therapies with a maximum threshold of $186,500.
In April 2018, CMS announced payment rates for outpatient administration of the two drugs, settling on $395,380 for axicabtagene ciloleucel and $500,839 for tisagenlecleucel. The two medications have list prices of $373,000 and $475,000, respectively.
The Centers for Medicare & Medicaid Services has proposed to cover chimeric antigen receptor (CAR) T-cell therapy for cancer patients participating in clinical trials that study the treatment’s effectiveness, according to a Feb. 15 announcement.
The proposed national coverage determination would require CMS to cover CAR T-cell therapies nationwide when the treatment is offered in CMS-approved registries or clinical studies in which patients are monitored for 2 or more years following treatment.
Results from the studies would help CMS identify which patients benefit most from CAR T-cell therapies and inform future coverage decisions, CMS Administrator Seema Verma said.
“CAR T-cell therapy was the first FDA-approved gene therapy, marking the beginning of an entirely new approach to treating serious and even life-threatening diseases,” Ms. Verma said in a statement. “Today’s proposed coverage decision would improve access to this therapy while deepening CMS’s understanding of how patients in Medicare respond to it, so the agency can ensure that it is paying for CAR T-cell therapy for cases in which the benefits outweigh the risks.”
As part of the proposal, CMS would cover autologous treatment with T cells expressing at least one chimeric antigen receptor (CAR) through coverage with evidence development when prescribed by a treating oncologist and performed in a hospital, according to a summary of the proposal.
The patient and hospital must meet specific criteria to be eligible for coverage, including that patients have relapsed or refractory cancer and do not have a comorbidity that would otherwise preclude patient benefit.
Hospitals, meanwhile, must have a cellular therapy program consisting of an integrated medical team that includes a clinical program director, a quality manager, and at least one physician experienced in cellular therapy, among other requirements.
CMS also would require that treatment is an FDA-approved biologic, providing targeted therapy for a known antigen expressed in the patient’s cancer according to an FDA indication. Repeat treatment would be covered only when a new primary cancer diagnosis is made by the treating oncologist and certain patient conditions are met.
Both inpatient and outpatient settings for the CAR T-cell therapy treatment are acceptable under the proposal. In either case, the patient and the hospital must be participating in a prospective, national, audited registry that consecutively enrolls patients, accepts all manufactured products, follows the patient for at least 2 years, and addresses a set of approved evidence-development questions. Additionally, all registries must be reviewed and approved by CMS.
The proposed national coverage determination was the result of an Aug. 22, 2018 meeting of the Medicare Evidence Development & Coverage Advisory Committee. The committee provides CMS with an external assessment of the appropriateness of therapies under review.
Public comments about the CAR T-cell therapy proposal will be accepted online here until March 15. A final decision on the proposal is expected by May 2019.
The agency’s proposal follows an Aug. 17 final rule by CMS that sets a new payment scheme for inpatient administration of two CAR T-cell therapies. The rule categorizes CAR T-cell therapies under the umbrella of the renamed Medicare Severity–Diagnosis Related Groups 016 – Autologous Bone Marrow Transplant with CC/MCC or T-cell Immunotherapy – and assigns ICD-10 PCS procedure codes XW033C3 and XW043C3 to the use of axicabtagene ciloleucel (Yescarta) and tisagenlecleucel (Kymriah) in the inpatient setting for fiscal year 2019, which began in October 2018. CMS also approved a temporary New Technology Add-On Payment for use of the therapies with a maximum threshold of $186,500.
In April 2018, CMS announced payment rates for outpatient administration of the two drugs, settling on $395,380 for axicabtagene ciloleucel and $500,839 for tisagenlecleucel. The two medications have list prices of $373,000 and $475,000, respectively.
Immunotherapy’s cardiac effects require early monitoring, management
WASHINGTON – Unquestionably, immunotherapy is revolutionizing the care of patients with various solid tumors and hematologic malignancies.
But it’s equally true that there’s no such thing as either a free lunch or a cancer therapy free of side effects, whether it’s increased risk for heart failure associated with anthracycline-based chemotherapy, or inflammatory conditions, arrhythmias, and thromboembolic events associated with immune checkpoint inhibitors, said R. Frank Cornell, MD, of Vanderbilt University Medical Center in Nashville, Tenn.
“Early awareness and intervention is critical for improved outcomes, and a multidisciplinary approach between oncology, cardiology, the clinic nurse, and other health care providers is critical in managing these patients with these complicated therapies,” he said at the American College of Cardiology’s Advancing the Cardiovascular Care of the Oncology Patient meeting.
Checkpoint inhibitors and the heart
Toxicities associated with immune checkpoint inhibitors such as the programmed death 1/ligand 1 (PD-1/PD-L1) inhibitors nivolumab (Opdivo) and pembrolizumab (Keytruda) and the cytotoxic T-lymphocyte antigen 4 antibody ipilimumab (Yervoy) tend to mimic autoimmune conditions, Dr. Cornell said.
Cardiovascular events associated with these agents, while uncommon, include myocarditis, pericarditis, arrhythmias, impaired ventricular function with heart failure, vasculitis, and venous thromboembolism, he said, citing an American Society of Clinical Oncology (ASCO) clinical practice guideline (J Clin Oncol 2018;36[17]:1714-68).
Dr. Cornell described the case of a 63-year-old woman with disseminated metastatic melanoma who presented to the emergency department 10 days after starting on combination therapy with ipilimumab and nivolumab. She had developed shortness of breath, pleuritic chest pain, and a mild cough for 1 or 2 days.
Her cardiac laboratory markers had been normal at baseline, but were markedly elevated on presentation, and electrocardiograms showed complete heart block and subsequent ventricular tachycardia.
The patient was started on high-dose prednisone, but she died in hospital, and an autopsy showed that the cause of death was infiltration into the myocardium of CD3-positive and CD8-positive T lymphocytes.
“So how do we manage this? This is a good opportunity, I think, for further cardiology and oncology collaboration to develop more robust guidelines for what we can do to best prevent this,” Dr. Cornell said.
Patients started on the ipilimumab/nivolumab combination should be tested weekly for cardiac troponin, creatine kinase (CK) and CK-muscle/brain (CK-MB) weekly for the first 3-4 weeks of therapy. Therapy should be stopped if troponin levels continue to rise, and the patient should be started on high-dose steroids, he said.
The role of other anti-inflammatory agents such as infliximab (Remicade and biosimilars) is unclear and needs further study, he added.
Dr. Cornell cited a 2018 letter to The Lancet by Javid J. Moslehi, MD, and colleagues from Vanderbilt describing an increase in reports of fatal myocarditis among patients treated with checkpoint inhibitors.
“We highlight the high mortality rate with severe immune checkpoint inhibitor–related myocarditis, which is more frequent with combination PD-1 and CTLA-4 blockade, but can also occur with monotherapy. Myocarditis was observed across immune checkpoint inhibitor regimens, although it remains too early to determine whether the incidence differs between use of anti-PD1 and anti-PD-L1 drugs. Furthermore, this condition occurs early on during therapy and across cancer types,” they wrote.
Most of the patients had no preexisting cardiovascular disease, and most were not taking medications for hypertension, cardiovascular disease, or diabetes.
CAR-T cells and cardiac disease
The primary cardiac complications associated with CAR-T cell therapy are related to the cytokine release syndrome (CRS), a condition marked by progressive elevation in inflammatory cytokines that in turn leads to marked elevations in C-reactive protein (CRP), interferon gamma, tumor necrosis factor al, and release of pro-inflammatory cytokines including interleukin (IL) 6, IL-10, IL-12, and IL-1 beta.
In rare instances, CRS can lead to disseminated intravascular coagulation (DIC), capillary leak syndrome, and a hemophagocytic lymphohistiocytosis-like (HLH) syndrome, Dr. Cornell said.
Package inserts for the two Food and Drug Administration–approved CAR-T cell products, axicabtagene ciloleucel (Yescarta) and tisagenlecleucel (Kymriah) show that each was associated in clinical trials with a high incidence of CRS.
Among patients treated with axicabtagene ciloleucel, 94% developed CRS, which was grade 3 or greater in severity in 13%. The median time to onset was 2 days, and the median duration was 7 days. Cardiovascular adverse events included grade 3 or greater tachycardia in 2%, arrhythmias in 7%, edema in 1%, dyspnea in 3%, pleural effusion in 2%, hypotension in 15%, hypertension in 6%, and thrombosis in 1%.
Among patients treated with tisagenlecleucel, 79% treated for B-cell acute lymphoblastic leukemia (B-ALL) and 74% treated for diffuse large B cell lymphoma (DLBCL) developed CRS, which was grade 3 or greater in 49% and 23% of patients, respectively. The median time to onset was 3 days, and the median duration of CRS was 8 days.
Cardiovascular adverse events of grade 3 or greater among these patients included tachycardia in 4%, fluid overload in 7%, edema in 1%, dyspnea in 12%, pulmonary edema in 4%, hypotension in 22%, and hypertension in 6%.
Risk factors for CRS include high pre-infusion tumor burden, active infections, and concurrent inflammatory processes, Dr. Cornell said.
Prevention of cardiovascular complications of CAR-T cell therapy requires management of CRS. Patients with grade 2 or greater CRS should receive the anti-IL-6 agent tocilizumab (Actemra) 8 mg/kg intravenously over 1 hour to a maximum dose of 800 mg. Tocilizumab infusions can be repeated every 8 hours as needed if the patient is not responsive to intravenous fluids or increasing supplement oxygen, but should be limited to a maximum of three doses over 24 hours, and a maximum total of four doses.
Patients with grade 3 CRS should also receive intravenous methylprednisolone 1 mg/kg twice daily or the equivalent amount of dexamethasone, with corticosteroids continued until the severity of CRS is grade 1 or less, then tapered over 3 days,
Patients with grade 4 CRS should also receive IV methylprednisolone 1,000 mg per day for 3 days, and if symptoms improve, continue management as per grade 3, Dr. Cornell said.
Dr. Cornell reported having nothing to disclose.
WASHINGTON – Unquestionably, immunotherapy is revolutionizing the care of patients with various solid tumors and hematologic malignancies.
But it’s equally true that there’s no such thing as either a free lunch or a cancer therapy free of side effects, whether it’s increased risk for heart failure associated with anthracycline-based chemotherapy, or inflammatory conditions, arrhythmias, and thromboembolic events associated with immune checkpoint inhibitors, said R. Frank Cornell, MD, of Vanderbilt University Medical Center in Nashville, Tenn.
“Early awareness and intervention is critical for improved outcomes, and a multidisciplinary approach between oncology, cardiology, the clinic nurse, and other health care providers is critical in managing these patients with these complicated therapies,” he said at the American College of Cardiology’s Advancing the Cardiovascular Care of the Oncology Patient meeting.
Checkpoint inhibitors and the heart
Toxicities associated with immune checkpoint inhibitors such as the programmed death 1/ligand 1 (PD-1/PD-L1) inhibitors nivolumab (Opdivo) and pembrolizumab (Keytruda) and the cytotoxic T-lymphocyte antigen 4 antibody ipilimumab (Yervoy) tend to mimic autoimmune conditions, Dr. Cornell said.
Cardiovascular events associated with these agents, while uncommon, include myocarditis, pericarditis, arrhythmias, impaired ventricular function with heart failure, vasculitis, and venous thromboembolism, he said, citing an American Society of Clinical Oncology (ASCO) clinical practice guideline (J Clin Oncol 2018;36[17]:1714-68).
Dr. Cornell described the case of a 63-year-old woman with disseminated metastatic melanoma who presented to the emergency department 10 days after starting on combination therapy with ipilimumab and nivolumab. She had developed shortness of breath, pleuritic chest pain, and a mild cough for 1 or 2 days.
Her cardiac laboratory markers had been normal at baseline, but were markedly elevated on presentation, and electrocardiograms showed complete heart block and subsequent ventricular tachycardia.
The patient was started on high-dose prednisone, but she died in hospital, and an autopsy showed that the cause of death was infiltration into the myocardium of CD3-positive and CD8-positive T lymphocytes.
“So how do we manage this? This is a good opportunity, I think, for further cardiology and oncology collaboration to develop more robust guidelines for what we can do to best prevent this,” Dr. Cornell said.
Patients started on the ipilimumab/nivolumab combination should be tested weekly for cardiac troponin, creatine kinase (CK) and CK-muscle/brain (CK-MB) weekly for the first 3-4 weeks of therapy. Therapy should be stopped if troponin levels continue to rise, and the patient should be started on high-dose steroids, he said.
The role of other anti-inflammatory agents such as infliximab (Remicade and biosimilars) is unclear and needs further study, he added.
Dr. Cornell cited a 2018 letter to The Lancet by Javid J. Moslehi, MD, and colleagues from Vanderbilt describing an increase in reports of fatal myocarditis among patients treated with checkpoint inhibitors.
“We highlight the high mortality rate with severe immune checkpoint inhibitor–related myocarditis, which is more frequent with combination PD-1 and CTLA-4 blockade, but can also occur with monotherapy. Myocarditis was observed across immune checkpoint inhibitor regimens, although it remains too early to determine whether the incidence differs between use of anti-PD1 and anti-PD-L1 drugs. Furthermore, this condition occurs early on during therapy and across cancer types,” they wrote.
Most of the patients had no preexisting cardiovascular disease, and most were not taking medications for hypertension, cardiovascular disease, or diabetes.
CAR-T cells and cardiac disease
The primary cardiac complications associated with CAR-T cell therapy are related to the cytokine release syndrome (CRS), a condition marked by progressive elevation in inflammatory cytokines that in turn leads to marked elevations in C-reactive protein (CRP), interferon gamma, tumor necrosis factor al, and release of pro-inflammatory cytokines including interleukin (IL) 6, IL-10, IL-12, and IL-1 beta.
In rare instances, CRS can lead to disseminated intravascular coagulation (DIC), capillary leak syndrome, and a hemophagocytic lymphohistiocytosis-like (HLH) syndrome, Dr. Cornell said.
Package inserts for the two Food and Drug Administration–approved CAR-T cell products, axicabtagene ciloleucel (Yescarta) and tisagenlecleucel (Kymriah) show that each was associated in clinical trials with a high incidence of CRS.
Among patients treated with axicabtagene ciloleucel, 94% developed CRS, which was grade 3 or greater in severity in 13%. The median time to onset was 2 days, and the median duration was 7 days. Cardiovascular adverse events included grade 3 or greater tachycardia in 2%, arrhythmias in 7%, edema in 1%, dyspnea in 3%, pleural effusion in 2%, hypotension in 15%, hypertension in 6%, and thrombosis in 1%.
Among patients treated with tisagenlecleucel, 79% treated for B-cell acute lymphoblastic leukemia (B-ALL) and 74% treated for diffuse large B cell lymphoma (DLBCL) developed CRS, which was grade 3 or greater in 49% and 23% of patients, respectively. The median time to onset was 3 days, and the median duration of CRS was 8 days.
Cardiovascular adverse events of grade 3 or greater among these patients included tachycardia in 4%, fluid overload in 7%, edema in 1%, dyspnea in 12%, pulmonary edema in 4%, hypotension in 22%, and hypertension in 6%.
Risk factors for CRS include high pre-infusion tumor burden, active infections, and concurrent inflammatory processes, Dr. Cornell said.
Prevention of cardiovascular complications of CAR-T cell therapy requires management of CRS. Patients with grade 2 or greater CRS should receive the anti-IL-6 agent tocilizumab (Actemra) 8 mg/kg intravenously over 1 hour to a maximum dose of 800 mg. Tocilizumab infusions can be repeated every 8 hours as needed if the patient is not responsive to intravenous fluids or increasing supplement oxygen, but should be limited to a maximum of three doses over 24 hours, and a maximum total of four doses.
Patients with grade 3 CRS should also receive intravenous methylprednisolone 1 mg/kg twice daily or the equivalent amount of dexamethasone, with corticosteroids continued until the severity of CRS is grade 1 or less, then tapered over 3 days,
Patients with grade 4 CRS should also receive IV methylprednisolone 1,000 mg per day for 3 days, and if symptoms improve, continue management as per grade 3, Dr. Cornell said.
Dr. Cornell reported having nothing to disclose.
WASHINGTON – Unquestionably, immunotherapy is revolutionizing the care of patients with various solid tumors and hematologic malignancies.
But it’s equally true that there’s no such thing as either a free lunch or a cancer therapy free of side effects, whether it’s increased risk for heart failure associated with anthracycline-based chemotherapy, or inflammatory conditions, arrhythmias, and thromboembolic events associated with immune checkpoint inhibitors, said R. Frank Cornell, MD, of Vanderbilt University Medical Center in Nashville, Tenn.
“Early awareness and intervention is critical for improved outcomes, and a multidisciplinary approach between oncology, cardiology, the clinic nurse, and other health care providers is critical in managing these patients with these complicated therapies,” he said at the American College of Cardiology’s Advancing the Cardiovascular Care of the Oncology Patient meeting.
Checkpoint inhibitors and the heart
Toxicities associated with immune checkpoint inhibitors such as the programmed death 1/ligand 1 (PD-1/PD-L1) inhibitors nivolumab (Opdivo) and pembrolizumab (Keytruda) and the cytotoxic T-lymphocyte antigen 4 antibody ipilimumab (Yervoy) tend to mimic autoimmune conditions, Dr. Cornell said.
Cardiovascular events associated with these agents, while uncommon, include myocarditis, pericarditis, arrhythmias, impaired ventricular function with heart failure, vasculitis, and venous thromboembolism, he said, citing an American Society of Clinical Oncology (ASCO) clinical practice guideline (J Clin Oncol 2018;36[17]:1714-68).
Dr. Cornell described the case of a 63-year-old woman with disseminated metastatic melanoma who presented to the emergency department 10 days after starting on combination therapy with ipilimumab and nivolumab. She had developed shortness of breath, pleuritic chest pain, and a mild cough for 1 or 2 days.
Her cardiac laboratory markers had been normal at baseline, but were markedly elevated on presentation, and electrocardiograms showed complete heart block and subsequent ventricular tachycardia.
The patient was started on high-dose prednisone, but she died in hospital, and an autopsy showed that the cause of death was infiltration into the myocardium of CD3-positive and CD8-positive T lymphocytes.
“So how do we manage this? This is a good opportunity, I think, for further cardiology and oncology collaboration to develop more robust guidelines for what we can do to best prevent this,” Dr. Cornell said.
Patients started on the ipilimumab/nivolumab combination should be tested weekly for cardiac troponin, creatine kinase (CK) and CK-muscle/brain (CK-MB) weekly for the first 3-4 weeks of therapy. Therapy should be stopped if troponin levels continue to rise, and the patient should be started on high-dose steroids, he said.
The role of other anti-inflammatory agents such as infliximab (Remicade and biosimilars) is unclear and needs further study, he added.
Dr. Cornell cited a 2018 letter to The Lancet by Javid J. Moslehi, MD, and colleagues from Vanderbilt describing an increase in reports of fatal myocarditis among patients treated with checkpoint inhibitors.
“We highlight the high mortality rate with severe immune checkpoint inhibitor–related myocarditis, which is more frequent with combination PD-1 and CTLA-4 blockade, but can also occur with monotherapy. Myocarditis was observed across immune checkpoint inhibitor regimens, although it remains too early to determine whether the incidence differs between use of anti-PD1 and anti-PD-L1 drugs. Furthermore, this condition occurs early on during therapy and across cancer types,” they wrote.
Most of the patients had no preexisting cardiovascular disease, and most were not taking medications for hypertension, cardiovascular disease, or diabetes.
CAR-T cells and cardiac disease
The primary cardiac complications associated with CAR-T cell therapy are related to the cytokine release syndrome (CRS), a condition marked by progressive elevation in inflammatory cytokines that in turn leads to marked elevations in C-reactive protein (CRP), interferon gamma, tumor necrosis factor al, and release of pro-inflammatory cytokines including interleukin (IL) 6, IL-10, IL-12, and IL-1 beta.
In rare instances, CRS can lead to disseminated intravascular coagulation (DIC), capillary leak syndrome, and a hemophagocytic lymphohistiocytosis-like (HLH) syndrome, Dr. Cornell said.
Package inserts for the two Food and Drug Administration–approved CAR-T cell products, axicabtagene ciloleucel (Yescarta) and tisagenlecleucel (Kymriah) show that each was associated in clinical trials with a high incidence of CRS.
Among patients treated with axicabtagene ciloleucel, 94% developed CRS, which was grade 3 or greater in severity in 13%. The median time to onset was 2 days, and the median duration was 7 days. Cardiovascular adverse events included grade 3 or greater tachycardia in 2%, arrhythmias in 7%, edema in 1%, dyspnea in 3%, pleural effusion in 2%, hypotension in 15%, hypertension in 6%, and thrombosis in 1%.
Among patients treated with tisagenlecleucel, 79% treated for B-cell acute lymphoblastic leukemia (B-ALL) and 74% treated for diffuse large B cell lymphoma (DLBCL) developed CRS, which was grade 3 or greater in 49% and 23% of patients, respectively. The median time to onset was 3 days, and the median duration of CRS was 8 days.
Cardiovascular adverse events of grade 3 or greater among these patients included tachycardia in 4%, fluid overload in 7%, edema in 1%, dyspnea in 12%, pulmonary edema in 4%, hypotension in 22%, and hypertension in 6%.
Risk factors for CRS include high pre-infusion tumor burden, active infections, and concurrent inflammatory processes, Dr. Cornell said.
Prevention of cardiovascular complications of CAR-T cell therapy requires management of CRS. Patients with grade 2 or greater CRS should receive the anti-IL-6 agent tocilizumab (Actemra) 8 mg/kg intravenously over 1 hour to a maximum dose of 800 mg. Tocilizumab infusions can be repeated every 8 hours as needed if the patient is not responsive to intravenous fluids or increasing supplement oxygen, but should be limited to a maximum of three doses over 24 hours, and a maximum total of four doses.
Patients with grade 3 CRS should also receive intravenous methylprednisolone 1 mg/kg twice daily or the equivalent amount of dexamethasone, with corticosteroids continued until the severity of CRS is grade 1 or less, then tapered over 3 days,
Patients with grade 4 CRS should also receive IV methylprednisolone 1,000 mg per day for 3 days, and if symptoms improve, continue management as per grade 3, Dr. Cornell said.
Dr. Cornell reported having nothing to disclose.
REPORTING FROM ACC CARDIO-ONCOLOGY
Key clinical point: Monitor for cardiac symptoms and treat or interrupt immunotherapy as needed.
Major finding: Immune checkpoint inhibitors and CAR T-cell therapies are associated with distinct cardiovascular adverse events.
Study details: Review of strategies for managing the cardiovascular consequences of cancer immunotherapies.
Disclosures: Dr. Cornell reported having nothing to disclose.