User login
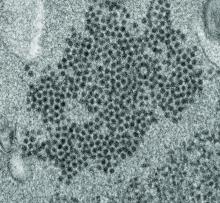
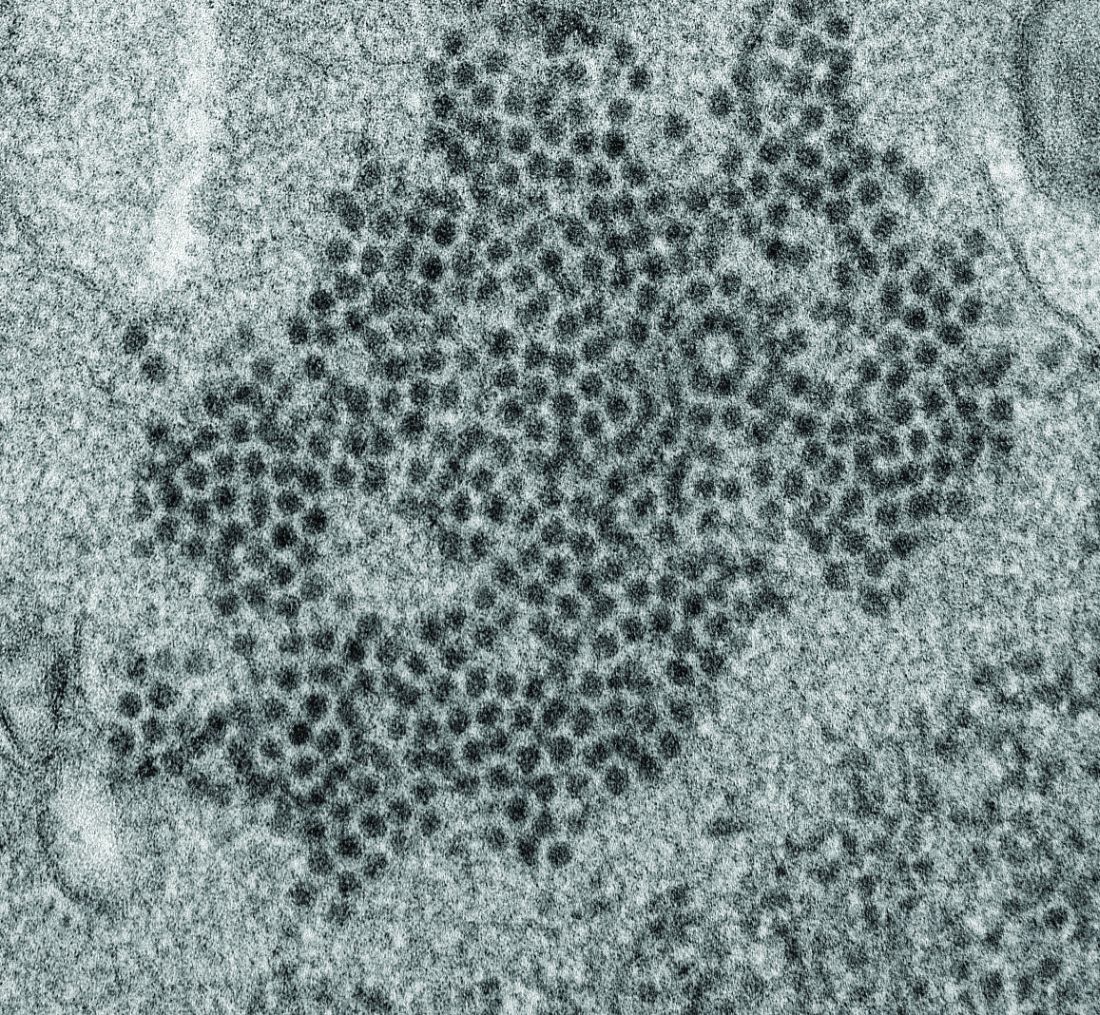
Possible role of enterovirus infection in acute flaccid myelitis cases detected
High levels of enterovirus (EV) peptides were found in the cerebrospinal fluid (CSF) and serum samples of individuals with acute flaccid myelitis (AFM) that were not present in a variety of control individuals, according to the results of a small study of patients with and without AFM published online in mBio.
In 2018, CSF samples from AFM patients were investigated by viral-capture high-throughput sequencing. These CSF and serum samples, as well as those from multiple controls, were tested for antibodies to human EVs using peptide microarrays, according to Nischay Mishra, PhD, of Columbia University, New York, and colleagues.
Although EV RNA was confirmed in CSF from only 1 adult AFM case and 1 non-AFM case, antibodies to EV peptides were present in 11 of 14 AFM patients (79%), which was a significantly higher rate than in control groups, including non-AFM patients (1 of 5, or 20%), children with Kawasaki disease (0 of 10), and adults with non-AFM CNS diseases (2 of 11, 18%), according to the authors.
In addition, 6 of 14 (43%) CSF samples and 8 of 11 (73%) serum samples from AFM patients were immunoreactive to an EV-D68–specific peptide, whereas samples from the three control groups were not immunoreactive in either CSF or sera. Previous studies have suggested that infection with EV-D68 and EV-A71 may contribute to AFM.
“There have been 570 confirmed cases since CDC began tracking AFM in August 2014. AFM outbreaks were reported to the CDC in 2014, 2016, and 2018. AFM affects the spinal cord and is characterized by the sudden onset of muscle weakness in one or more limbs. Spikes in AFM cases, primarily in children, have coincided in time and location with outbreaks of EV-D68 and a related enterovirus, EV-A71,” according to an NIH media advisory discussing the article.
In particular, as the study authors point out, a potential link to EV-D68 has also been based on the presence of viral RNA in some respiratory and stool specimens and the observation that EV-D68 infection can result in spinal cord infection.
“While other etiologies of AFM continue to be investigated, our study provides further evidence that EV infection may be a factor in AFM. In the absence of direct detection of a pathogen, antibody evidence of pathogen exposure within the CNS can be an important indicator of the underlying cause of disease,” Dr. Mishra and his colleagues added.
“These initial results may provide avenues to further explore how exposure to EV may contribute to AFM as well as the development of diagnostic tools and treatments,” the researchers concluded.
The study was funded by the National Institutes of Health. The authors reported that they had no competing financial interests.
SOURCE: Mishra N et al. mBio. 2019 Aug;10(4):e01903-19.
High levels of enterovirus (EV) peptides were found in the cerebrospinal fluid (CSF) and serum samples of individuals with acute flaccid myelitis (AFM) that were not present in a variety of control individuals, according to the results of a small study of patients with and without AFM published online in mBio.
In 2018, CSF samples from AFM patients were investigated by viral-capture high-throughput sequencing. These CSF and serum samples, as well as those from multiple controls, were tested for antibodies to human EVs using peptide microarrays, according to Nischay Mishra, PhD, of Columbia University, New York, and colleagues.
Although EV RNA was confirmed in CSF from only 1 adult AFM case and 1 non-AFM case, antibodies to EV peptides were present in 11 of 14 AFM patients (79%), which was a significantly higher rate than in control groups, including non-AFM patients (1 of 5, or 20%), children with Kawasaki disease (0 of 10), and adults with non-AFM CNS diseases (2 of 11, 18%), according to the authors.
In addition, 6 of 14 (43%) CSF samples and 8 of 11 (73%) serum samples from AFM patients were immunoreactive to an EV-D68–specific peptide, whereas samples from the three control groups were not immunoreactive in either CSF or sera. Previous studies have suggested that infection with EV-D68 and EV-A71 may contribute to AFM.
“There have been 570 confirmed cases since CDC began tracking AFM in August 2014. AFM outbreaks were reported to the CDC in 2014, 2016, and 2018. AFM affects the spinal cord and is characterized by the sudden onset of muscle weakness in one or more limbs. Spikes in AFM cases, primarily in children, have coincided in time and location with outbreaks of EV-D68 and a related enterovirus, EV-A71,” according to an NIH media advisory discussing the article.
In particular, as the study authors point out, a potential link to EV-D68 has also been based on the presence of viral RNA in some respiratory and stool specimens and the observation that EV-D68 infection can result in spinal cord infection.
“While other etiologies of AFM continue to be investigated, our study provides further evidence that EV infection may be a factor in AFM. In the absence of direct detection of a pathogen, antibody evidence of pathogen exposure within the CNS can be an important indicator of the underlying cause of disease,” Dr. Mishra and his colleagues added.
“These initial results may provide avenues to further explore how exposure to EV may contribute to AFM as well as the development of diagnostic tools and treatments,” the researchers concluded.
The study was funded by the National Institutes of Health. The authors reported that they had no competing financial interests.
SOURCE: Mishra N et al. mBio. 2019 Aug;10(4):e01903-19.
High levels of enterovirus (EV) peptides were found in the cerebrospinal fluid (CSF) and serum samples of individuals with acute flaccid myelitis (AFM) that were not present in a variety of control individuals, according to the results of a small study of patients with and without AFM published online in mBio.
In 2018, CSF samples from AFM patients were investigated by viral-capture high-throughput sequencing. These CSF and serum samples, as well as those from multiple controls, were tested for antibodies to human EVs using peptide microarrays, according to Nischay Mishra, PhD, of Columbia University, New York, and colleagues.
Although EV RNA was confirmed in CSF from only 1 adult AFM case and 1 non-AFM case, antibodies to EV peptides were present in 11 of 14 AFM patients (79%), which was a significantly higher rate than in control groups, including non-AFM patients (1 of 5, or 20%), children with Kawasaki disease (0 of 10), and adults with non-AFM CNS diseases (2 of 11, 18%), according to the authors.
In addition, 6 of 14 (43%) CSF samples and 8 of 11 (73%) serum samples from AFM patients were immunoreactive to an EV-D68–specific peptide, whereas samples from the three control groups were not immunoreactive in either CSF or sera. Previous studies have suggested that infection with EV-D68 and EV-A71 may contribute to AFM.
“There have been 570 confirmed cases since CDC began tracking AFM in August 2014. AFM outbreaks were reported to the CDC in 2014, 2016, and 2018. AFM affects the spinal cord and is characterized by the sudden onset of muscle weakness in one or more limbs. Spikes in AFM cases, primarily in children, have coincided in time and location with outbreaks of EV-D68 and a related enterovirus, EV-A71,” according to an NIH media advisory discussing the article.
In particular, as the study authors point out, a potential link to EV-D68 has also been based on the presence of viral RNA in some respiratory and stool specimens and the observation that EV-D68 infection can result in spinal cord infection.
“While other etiologies of AFM continue to be investigated, our study provides further evidence that EV infection may be a factor in AFM. In the absence of direct detection of a pathogen, antibody evidence of pathogen exposure within the CNS can be an important indicator of the underlying cause of disease,” Dr. Mishra and his colleagues added.
“These initial results may provide avenues to further explore how exposure to EV may contribute to AFM as well as the development of diagnostic tools and treatments,” the researchers concluded.
The study was funded by the National Institutes of Health. The authors reported that they had no competing financial interests.
SOURCE: Mishra N et al. mBio. 2019 Aug;10(4):e01903-19.
FROM MBIO
Key clinical point:
Major finding: EV peptide antibodies were present in 11 of 14 AFM patients (79%), significantly higher than in controls.
Study details: A peptide microarray analysis was performed on CSF and sera from 14 AFM patients, as well as three control groups of 5 pediatric and adult patients with a non-AFM CNS diseases, 10 children with Kawasaki disease, and 10 adult patients with non-AFM CNS diseases.
Disclosures: The study was funded by the National Institutes of Health. The authors reported that they had no conflicts.
Source: Mishra N et al. mBio. 2019 Aug;10(4):e01903-19.
Study: Cardiac biomarkers predicted CV events in CAP
in a recently conducted study.
These biomarkers were also used to predict late cardiovascular events at day 30 of community-acquired pneumonia (CAP) in patients who did not have a history of cardiovascular disease, according to Rosario Menéndez, MD, from the Hospital Universitario y Politécnico La Fe and Instituto de Investigación Sanitaria La Fe in Valencia, Spain, and colleagues.
“Some patients have still high levels of inflammatory and cardiac biomarkers at 30 days, when they are usually referred to primary care without receiving any specific additional recommendations,” Dr. Menéndez and colleagues wrote in CHEST. “Our results suggest that a change in usual practice is needed to reduce current and further cardiovascular CAP complications.”
Dr. Menéndez and colleagues prospectively followed 730 patients for 1 year who were hospitalized for CAP, measuring the cardiac biomarkers proadrenomedullin (proADM), pro b-type natriuretic peptide (proBNP), proendothelin-1, and troponin T, and the inflammatory biomarkers interleukin 6 (IL-6), C-reactive protein (CRP), and procalcitonin (PCT). The researchers also collected data on age, gender, smoking status, and vaccination history, as well as whether patients had any cardiac, renal, pulmonary, neurological or diabetes-related comorbidities.
Overall, 95 patients experienced early cardiovascular events, 67 patients had long-term cardiovascular events, and 20 patients experienced both early and late events. In hospital, the mortality rate was 4.7%; the 30-day mortality rate was 5.3%, and the 1-year mortality rate was 9.9%.
With regard to biomarkers, patients who experienced both early and late cardiovascular events had significantly higher initial levels of proADM, proendothelin-1, troponin, proBNP, and IL-6. Patients who experienced later events had consistent levels of these biomarkers until day 30, except for a decrease at day 4 or day 5.
After adjustment for age, sepsis, previous cardiac disease, and a partial pressure of oxygen in the alveoli to fractional inspired oxygen ratio (PaO2/FiO2) of less than 250mm Hg, cardiac biomarkers proendothelin-1 (odds ratio, 2.25; 95% confidence interval, 1.34-3.79), proADM (OR, 2.53; 95% CI, 1.53-4.20), proBNP (OR, 2.67; 95% CI, 1.59-4.49), and troponin T (OR, 2.70; 95% CI, 1.62-4.49) significantly predicted early cardiovascular events, while proendothelin-1 (OR, 3.13; 95% CI, 1.41-7.80), proADM (2.29; 95% CI, 1.01-5.19) and proBNP (OR, 2.34; 95% CI, 1.01-5.56) significantly predicted late cardiovascular events. For day 30 results, when researchers added IL-6 levels to proendothelin-1, the odds ratio for late events increased to 3.53, and when they added IL-6 levels to proADM, the odds ratio increased to 2.80.
Researchers noted the limitations of the study included that they did not analyze cardiac biomarkers to predict specific cardiovascular events, did not identify the cause for mortality at 1 year in most patients, and did not include a control group.
This study was supported in part by funding from Instituto de Salud Carlos III, Sociedad Española de Neumología y Cirugía Torácica, and the Center for Biomedical Research Network in Respiratory Diseases. The authors reported no relevant conflicts of interest.
SOURCE: Menéndez R et al. Chest. 2019 Aug 2. doi: 10.1016/j.chest.2019.06.040.
in a recently conducted study.
These biomarkers were also used to predict late cardiovascular events at day 30 of community-acquired pneumonia (CAP) in patients who did not have a history of cardiovascular disease, according to Rosario Menéndez, MD, from the Hospital Universitario y Politécnico La Fe and Instituto de Investigación Sanitaria La Fe in Valencia, Spain, and colleagues.
“Some patients have still high levels of inflammatory and cardiac biomarkers at 30 days, when they are usually referred to primary care without receiving any specific additional recommendations,” Dr. Menéndez and colleagues wrote in CHEST. “Our results suggest that a change in usual practice is needed to reduce current and further cardiovascular CAP complications.”
Dr. Menéndez and colleagues prospectively followed 730 patients for 1 year who were hospitalized for CAP, measuring the cardiac biomarkers proadrenomedullin (proADM), pro b-type natriuretic peptide (proBNP), proendothelin-1, and troponin T, and the inflammatory biomarkers interleukin 6 (IL-6), C-reactive protein (CRP), and procalcitonin (PCT). The researchers also collected data on age, gender, smoking status, and vaccination history, as well as whether patients had any cardiac, renal, pulmonary, neurological or diabetes-related comorbidities.
Overall, 95 patients experienced early cardiovascular events, 67 patients had long-term cardiovascular events, and 20 patients experienced both early and late events. In hospital, the mortality rate was 4.7%; the 30-day mortality rate was 5.3%, and the 1-year mortality rate was 9.9%.
With regard to biomarkers, patients who experienced both early and late cardiovascular events had significantly higher initial levels of proADM, proendothelin-1, troponin, proBNP, and IL-6. Patients who experienced later events had consistent levels of these biomarkers until day 30, except for a decrease at day 4 or day 5.
After adjustment for age, sepsis, previous cardiac disease, and a partial pressure of oxygen in the alveoli to fractional inspired oxygen ratio (PaO2/FiO2) of less than 250mm Hg, cardiac biomarkers proendothelin-1 (odds ratio, 2.25; 95% confidence interval, 1.34-3.79), proADM (OR, 2.53; 95% CI, 1.53-4.20), proBNP (OR, 2.67; 95% CI, 1.59-4.49), and troponin T (OR, 2.70; 95% CI, 1.62-4.49) significantly predicted early cardiovascular events, while proendothelin-1 (OR, 3.13; 95% CI, 1.41-7.80), proADM (2.29; 95% CI, 1.01-5.19) and proBNP (OR, 2.34; 95% CI, 1.01-5.56) significantly predicted late cardiovascular events. For day 30 results, when researchers added IL-6 levels to proendothelin-1, the odds ratio for late events increased to 3.53, and when they added IL-6 levels to proADM, the odds ratio increased to 2.80.
Researchers noted the limitations of the study included that they did not analyze cardiac biomarkers to predict specific cardiovascular events, did not identify the cause for mortality at 1 year in most patients, and did not include a control group.
This study was supported in part by funding from Instituto de Salud Carlos III, Sociedad Española de Neumología y Cirugía Torácica, and the Center for Biomedical Research Network in Respiratory Diseases. The authors reported no relevant conflicts of interest.
SOURCE: Menéndez R et al. Chest. 2019 Aug 2. doi: 10.1016/j.chest.2019.06.040.
in a recently conducted study.
These biomarkers were also used to predict late cardiovascular events at day 30 of community-acquired pneumonia (CAP) in patients who did not have a history of cardiovascular disease, according to Rosario Menéndez, MD, from the Hospital Universitario y Politécnico La Fe and Instituto de Investigación Sanitaria La Fe in Valencia, Spain, and colleagues.
“Some patients have still high levels of inflammatory and cardiac biomarkers at 30 days, when they are usually referred to primary care without receiving any specific additional recommendations,” Dr. Menéndez and colleagues wrote in CHEST. “Our results suggest that a change in usual practice is needed to reduce current and further cardiovascular CAP complications.”
Dr. Menéndez and colleagues prospectively followed 730 patients for 1 year who were hospitalized for CAP, measuring the cardiac biomarkers proadrenomedullin (proADM), pro b-type natriuretic peptide (proBNP), proendothelin-1, and troponin T, and the inflammatory biomarkers interleukin 6 (IL-6), C-reactive protein (CRP), and procalcitonin (PCT). The researchers also collected data on age, gender, smoking status, and vaccination history, as well as whether patients had any cardiac, renal, pulmonary, neurological or diabetes-related comorbidities.
Overall, 95 patients experienced early cardiovascular events, 67 patients had long-term cardiovascular events, and 20 patients experienced both early and late events. In hospital, the mortality rate was 4.7%; the 30-day mortality rate was 5.3%, and the 1-year mortality rate was 9.9%.
With regard to biomarkers, patients who experienced both early and late cardiovascular events had significantly higher initial levels of proADM, proendothelin-1, troponin, proBNP, and IL-6. Patients who experienced later events had consistent levels of these biomarkers until day 30, except for a decrease at day 4 or day 5.
After adjustment for age, sepsis, previous cardiac disease, and a partial pressure of oxygen in the alveoli to fractional inspired oxygen ratio (PaO2/FiO2) of less than 250mm Hg, cardiac biomarkers proendothelin-1 (odds ratio, 2.25; 95% confidence interval, 1.34-3.79), proADM (OR, 2.53; 95% CI, 1.53-4.20), proBNP (OR, 2.67; 95% CI, 1.59-4.49), and troponin T (OR, 2.70; 95% CI, 1.62-4.49) significantly predicted early cardiovascular events, while proendothelin-1 (OR, 3.13; 95% CI, 1.41-7.80), proADM (2.29; 95% CI, 1.01-5.19) and proBNP (OR, 2.34; 95% CI, 1.01-5.56) significantly predicted late cardiovascular events. For day 30 results, when researchers added IL-6 levels to proendothelin-1, the odds ratio for late events increased to 3.53, and when they added IL-6 levels to proADM, the odds ratio increased to 2.80.
Researchers noted the limitations of the study included that they did not analyze cardiac biomarkers to predict specific cardiovascular events, did not identify the cause for mortality at 1 year in most patients, and did not include a control group.
This study was supported in part by funding from Instituto de Salud Carlos III, Sociedad Española de Neumología y Cirugía Torácica, and the Center for Biomedical Research Network in Respiratory Diseases. The authors reported no relevant conflicts of interest.
SOURCE: Menéndez R et al. Chest. 2019 Aug 2. doi: 10.1016/j.chest.2019.06.040.
FROM CHEST
Novel score spots high-risk febrile children in ED
LJUBLJANA, SLOVENIA – A new age-adjusted quick Sequential Organ Failure Assessment (qSOFA) score designed for use in children presenting to the ED with fever showed good predictive value for admission to critical care within the next 48 hours, Aakash Khanijau, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“In the needle-in-a-haystack scenario that’s seen in pediatric emergency departments, our novel, age-adjusted qSOFA score could potentially improve the rapid identification and treatment of children with suspected sepsis presenting to the ED,” said Dr. Khanijau of the University of Liverpool (England).
He presented an exceptionally large retrospective validation study of the score’s performance in 12,393 children (median age, 2.5 years) who presented to EDs with fever, of whom 1,521 were admitted for suspected sepsis. Of the hospitalized children, 145 were admitted to critical care within the first 48 hours.
The pediatric qSOFA score had 72% sensitivity and 85% specificity for critical care admission within 48 hours, with a positive predictive value of 5.4% and, more importantly, a whopping negative predictive value of 99.6%.
“That very high negative predictive value underlines the powerful discriminatory nature of our tool in the emergency department setting,” Dr. Khanijau observed, adding that the score’s area under the receiver operating characteristic curve was 0.81, which is considered a good predictive value.
The impetus for developing an age-adjusted pediatric qSOFA score stems from the fact that the original qSOFA score was designed for rapid assessment of adults with suspected sepsis and isn’t applicable in children. Other existing scores, including SIRS (the Systemic Inflammatory Response Syndrome criteria), the full SOFA, and PELOD-2 (the Pediatric Logistic Organ Dysfunction score), take longer to determine than the adapted qSOFA in a setting where speed is of the essence, he explained.
The original qSOFA components are altered mentation, systolic blood pressure, and respiratory rate. The novel score developed by Dr. Khanijau and coworkers swaps out systolic BP in favor of capillary refill time and age-adjusted heart rate using the thresholds previously established in a landmark study from the Children’s Hospital of Philadelphia (Pediatrics. 2013 Apr;131[4]:e1150-7.)
“Our reasoning here is that arterial hypertension is known to be a much later sign of circulatory compromise in children and may provide less discriminatory value than signs such as delayed capillary refill time and tachycardia early in presentation in the emergency department,” according to Dr. Khanijau.
The novel scoring system features four criteria. One point each is given for a capillary refill time of 3 seconds or longer; anything less than “Alert” on the Alert, Responds to Voice, Respond to Pain, and Unresponsive scale; a heart rate above the 99th percentile on the age-adjusted curves; and a respiratory rate above the age-adjusted 99th percentile. Thus, scores can range from 0 to 4. In the validation study, a score of 2 or more spelled a 890% increased likelihood of being admitted to a critical care setting within 48 hours. It was also associated with a 100-fold increased likelihood of death during the hospitalization, which occurred in 10 children.
Asked how the new predictive score could change clinical management, Dr. Khanijau replied, “I think the key thing it does here is it identifies the children at risk of requiring critical care and should therefore motivate us in the children achieving that threshold to promptly investigate thoroughly for suspected sepsis using the more comprehensive tools, like the full SOFA.”
He reported having no financial conflicts of interest regarding his study.
SOURCE: Khanijau A et al. ESPID 2019, Abstract.
LJUBLJANA, SLOVENIA – A new age-adjusted quick Sequential Organ Failure Assessment (qSOFA) score designed for use in children presenting to the ED with fever showed good predictive value for admission to critical care within the next 48 hours, Aakash Khanijau, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“In the needle-in-a-haystack scenario that’s seen in pediatric emergency departments, our novel, age-adjusted qSOFA score could potentially improve the rapid identification and treatment of children with suspected sepsis presenting to the ED,” said Dr. Khanijau of the University of Liverpool (England).
He presented an exceptionally large retrospective validation study of the score’s performance in 12,393 children (median age, 2.5 years) who presented to EDs with fever, of whom 1,521 were admitted for suspected sepsis. Of the hospitalized children, 145 were admitted to critical care within the first 48 hours.
The pediatric qSOFA score had 72% sensitivity and 85% specificity for critical care admission within 48 hours, with a positive predictive value of 5.4% and, more importantly, a whopping negative predictive value of 99.6%.
“That very high negative predictive value underlines the powerful discriminatory nature of our tool in the emergency department setting,” Dr. Khanijau observed, adding that the score’s area under the receiver operating characteristic curve was 0.81, which is considered a good predictive value.
The impetus for developing an age-adjusted pediatric qSOFA score stems from the fact that the original qSOFA score was designed for rapid assessment of adults with suspected sepsis and isn’t applicable in children. Other existing scores, including SIRS (the Systemic Inflammatory Response Syndrome criteria), the full SOFA, and PELOD-2 (the Pediatric Logistic Organ Dysfunction score), take longer to determine than the adapted qSOFA in a setting where speed is of the essence, he explained.
The original qSOFA components are altered mentation, systolic blood pressure, and respiratory rate. The novel score developed by Dr. Khanijau and coworkers swaps out systolic BP in favor of capillary refill time and age-adjusted heart rate using the thresholds previously established in a landmark study from the Children’s Hospital of Philadelphia (Pediatrics. 2013 Apr;131[4]:e1150-7.)
“Our reasoning here is that arterial hypertension is known to be a much later sign of circulatory compromise in children and may provide less discriminatory value than signs such as delayed capillary refill time and tachycardia early in presentation in the emergency department,” according to Dr. Khanijau.
The novel scoring system features four criteria. One point each is given for a capillary refill time of 3 seconds or longer; anything less than “Alert” on the Alert, Responds to Voice, Respond to Pain, and Unresponsive scale; a heart rate above the 99th percentile on the age-adjusted curves; and a respiratory rate above the age-adjusted 99th percentile. Thus, scores can range from 0 to 4. In the validation study, a score of 2 or more spelled a 890% increased likelihood of being admitted to a critical care setting within 48 hours. It was also associated with a 100-fold increased likelihood of death during the hospitalization, which occurred in 10 children.
Asked how the new predictive score could change clinical management, Dr. Khanijau replied, “I think the key thing it does here is it identifies the children at risk of requiring critical care and should therefore motivate us in the children achieving that threshold to promptly investigate thoroughly for suspected sepsis using the more comprehensive tools, like the full SOFA.”
He reported having no financial conflicts of interest regarding his study.
SOURCE: Khanijau A et al. ESPID 2019, Abstract.
LJUBLJANA, SLOVENIA – A new age-adjusted quick Sequential Organ Failure Assessment (qSOFA) score designed for use in children presenting to the ED with fever showed good predictive value for admission to critical care within the next 48 hours, Aakash Khanijau, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“In the needle-in-a-haystack scenario that’s seen in pediatric emergency departments, our novel, age-adjusted qSOFA score could potentially improve the rapid identification and treatment of children with suspected sepsis presenting to the ED,” said Dr. Khanijau of the University of Liverpool (England).
He presented an exceptionally large retrospective validation study of the score’s performance in 12,393 children (median age, 2.5 years) who presented to EDs with fever, of whom 1,521 were admitted for suspected sepsis. Of the hospitalized children, 145 were admitted to critical care within the first 48 hours.
The pediatric qSOFA score had 72% sensitivity and 85% specificity for critical care admission within 48 hours, with a positive predictive value of 5.4% and, more importantly, a whopping negative predictive value of 99.6%.
“That very high negative predictive value underlines the powerful discriminatory nature of our tool in the emergency department setting,” Dr. Khanijau observed, adding that the score’s area under the receiver operating characteristic curve was 0.81, which is considered a good predictive value.
The impetus for developing an age-adjusted pediatric qSOFA score stems from the fact that the original qSOFA score was designed for rapid assessment of adults with suspected sepsis and isn’t applicable in children. Other existing scores, including SIRS (the Systemic Inflammatory Response Syndrome criteria), the full SOFA, and PELOD-2 (the Pediatric Logistic Organ Dysfunction score), take longer to determine than the adapted qSOFA in a setting where speed is of the essence, he explained.
The original qSOFA components are altered mentation, systolic blood pressure, and respiratory rate. The novel score developed by Dr. Khanijau and coworkers swaps out systolic BP in favor of capillary refill time and age-adjusted heart rate using the thresholds previously established in a landmark study from the Children’s Hospital of Philadelphia (Pediatrics. 2013 Apr;131[4]:e1150-7.)
“Our reasoning here is that arterial hypertension is known to be a much later sign of circulatory compromise in children and may provide less discriminatory value than signs such as delayed capillary refill time and tachycardia early in presentation in the emergency department,” according to Dr. Khanijau.
The novel scoring system features four criteria. One point each is given for a capillary refill time of 3 seconds or longer; anything less than “Alert” on the Alert, Responds to Voice, Respond to Pain, and Unresponsive scale; a heart rate above the 99th percentile on the age-adjusted curves; and a respiratory rate above the age-adjusted 99th percentile. Thus, scores can range from 0 to 4. In the validation study, a score of 2 or more spelled a 890% increased likelihood of being admitted to a critical care setting within 48 hours. It was also associated with a 100-fold increased likelihood of death during the hospitalization, which occurred in 10 children.
Asked how the new predictive score could change clinical management, Dr. Khanijau replied, “I think the key thing it does here is it identifies the children at risk of requiring critical care and should therefore motivate us in the children achieving that threshold to promptly investigate thoroughly for suspected sepsis using the more comprehensive tools, like the full SOFA.”
He reported having no financial conflicts of interest regarding his study.
SOURCE: Khanijau A et al. ESPID 2019, Abstract.
REPORTING FROM ESPID 2019
Procalcitonin advocated to help rule out bacterial infections
SEATTLE – Procalcitonin, a marker of bacterial infection, rises and peaks sooner than C-reactive protein (CRP), and is especially useful to help rule out invasive bacterial infections in young infants and pediatric community acquired pneumonia due to typical bacteria, according to a presentation at the 2019 Pediatric Hospital Medicine Conference.
It’s “excellent for identifying low risk patients” and has the potential to decrease lumbar punctures and antibiotic exposure, but “the specificity isn’t great,” so there’s the potential for false positives, said Russell McCulloh, MD, a pediatric infectious disease specialist at the University of Nebraska Medical Center, Omaha.
There was great interest in procalcitonin at the meeting; the presentation room was packed, with a line out the door. It’s used mostly in Europe at this point. Testing is available in many U.S. hospitals, but a large majority of audience members, when polled, said they don’t currently use it in clinical practice, and that it’s not a part of diagnostic algorithms at their institutions.
Levels of procalcitonin, a calcitonin precursor normally produced by the thyroid, are low or undetectable in healthy people, but inflammation, be it from infectious or noninfectious causes, triggers production by parenchymal cells throughout the body.
Levels began to rise as early as 2.5 hours after healthy subjects in one study were injected with bacterial endotoxins, and peaked as early as 6 hours; CRP, in contrast, started to rise after 12 hours, and peaked at 30 hours. Procalcitonin levels also seem to correlate with bacterial load and severity of infection, said Nivedita Srinivas, MD, a pediatric infectious disease specialist at Stanford (Calif.) University (J Pediatr Intensive Care. 2016 Dec;5[4]:162-71).
Due to time, the presenters focused their talk on community acquired pneumonia (CAP) and invasive bacterial infections (IBI) in young infants, meaning essentially bacteremia and meningitis.
Different studies use different cutoffs, but a procalcitonin below, for instance, 0.5 ng/mL is “certainly more sensitive [for IBI] than any single biomarker we currently use,” including CRP, white blood cells, and absolute neutrophil count (ANC). “If it’s negative, you’re really confident it’s negative,” but “a positive test does not necessarily indicate the presence of IBI,” Dr. McCulloh said (Pediatrics. 2012 Nov;130[5]:815-22).
“Procalcitonin works really well as part of a validated step-wise rule” that includes, for instance, CRP and ANC; “I think that’s where its utility is. On its own, it is not a substitute for you examining the patient and doing your basic risk stratification, but it may enhance your decision making incrementally above what we currently have,” he said.
Meanwhile, in a study of 532 children a median age of 2.4 years with radiographically confirmed CAP, procalcitonin levels were a median of 6.1 ng/mL in children whose pneumonia was caused by Streptococcus pneumoniae or other typical bacteria, and no child infected with typical bacteria had a level under 0.1 ng/mL. Below that level, “you can be very sure you do not have typical bacteria pneumonia,” said Marie Wang, MD, also a pediatric infectious disease specialist at Stanford (J Pediatric Infect Dis Soc. 2018 Feb 19;7[1]:46-53).
As procalcitonin levels went up, the likelihood of having bacterial pneumonia increased; at 2 ng/mL, 26% of subjects were infected with typical bacteria, “but even in that group, 58% still had viral infection, so you are still detecting a lot of viral” disease, she said.
Prolcalcitonin-guided therapy – antibiotics until patients fall below a level of 0.25 ng/ml, for instance – has also been associated with decreased antibiotic exposure (Respir Med. 2011 Dec;105[12]:1939-45).
The speakers had no disclosures. The meeting was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
SEATTLE – Procalcitonin, a marker of bacterial infection, rises and peaks sooner than C-reactive protein (CRP), and is especially useful to help rule out invasive bacterial infections in young infants and pediatric community acquired pneumonia due to typical bacteria, according to a presentation at the 2019 Pediatric Hospital Medicine Conference.
It’s “excellent for identifying low risk patients” and has the potential to decrease lumbar punctures and antibiotic exposure, but “the specificity isn’t great,” so there’s the potential for false positives, said Russell McCulloh, MD, a pediatric infectious disease specialist at the University of Nebraska Medical Center, Omaha.
There was great interest in procalcitonin at the meeting; the presentation room was packed, with a line out the door. It’s used mostly in Europe at this point. Testing is available in many U.S. hospitals, but a large majority of audience members, when polled, said they don’t currently use it in clinical practice, and that it’s not a part of diagnostic algorithms at their institutions.
Levels of procalcitonin, a calcitonin precursor normally produced by the thyroid, are low or undetectable in healthy people, but inflammation, be it from infectious or noninfectious causes, triggers production by parenchymal cells throughout the body.
Levels began to rise as early as 2.5 hours after healthy subjects in one study were injected with bacterial endotoxins, and peaked as early as 6 hours; CRP, in contrast, started to rise after 12 hours, and peaked at 30 hours. Procalcitonin levels also seem to correlate with bacterial load and severity of infection, said Nivedita Srinivas, MD, a pediatric infectious disease specialist at Stanford (Calif.) University (J Pediatr Intensive Care. 2016 Dec;5[4]:162-71).
Due to time, the presenters focused their talk on community acquired pneumonia (CAP) and invasive bacterial infections (IBI) in young infants, meaning essentially bacteremia and meningitis.
Different studies use different cutoffs, but a procalcitonin below, for instance, 0.5 ng/mL is “certainly more sensitive [for IBI] than any single biomarker we currently use,” including CRP, white blood cells, and absolute neutrophil count (ANC). “If it’s negative, you’re really confident it’s negative,” but “a positive test does not necessarily indicate the presence of IBI,” Dr. McCulloh said (Pediatrics. 2012 Nov;130[5]:815-22).
“Procalcitonin works really well as part of a validated step-wise rule” that includes, for instance, CRP and ANC; “I think that’s where its utility is. On its own, it is not a substitute for you examining the patient and doing your basic risk stratification, but it may enhance your decision making incrementally above what we currently have,” he said.
Meanwhile, in a study of 532 children a median age of 2.4 years with radiographically confirmed CAP, procalcitonin levels were a median of 6.1 ng/mL in children whose pneumonia was caused by Streptococcus pneumoniae or other typical bacteria, and no child infected with typical bacteria had a level under 0.1 ng/mL. Below that level, “you can be very sure you do not have typical bacteria pneumonia,” said Marie Wang, MD, also a pediatric infectious disease specialist at Stanford (J Pediatric Infect Dis Soc. 2018 Feb 19;7[1]:46-53).
As procalcitonin levels went up, the likelihood of having bacterial pneumonia increased; at 2 ng/mL, 26% of subjects were infected with typical bacteria, “but even in that group, 58% still had viral infection, so you are still detecting a lot of viral” disease, she said.
Prolcalcitonin-guided therapy – antibiotics until patients fall below a level of 0.25 ng/ml, for instance – has also been associated with decreased antibiotic exposure (Respir Med. 2011 Dec;105[12]:1939-45).
The speakers had no disclosures. The meeting was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
SEATTLE – Procalcitonin, a marker of bacterial infection, rises and peaks sooner than C-reactive protein (CRP), and is especially useful to help rule out invasive bacterial infections in young infants and pediatric community acquired pneumonia due to typical bacteria, according to a presentation at the 2019 Pediatric Hospital Medicine Conference.
It’s “excellent for identifying low risk patients” and has the potential to decrease lumbar punctures and antibiotic exposure, but “the specificity isn’t great,” so there’s the potential for false positives, said Russell McCulloh, MD, a pediatric infectious disease specialist at the University of Nebraska Medical Center, Omaha.
There was great interest in procalcitonin at the meeting; the presentation room was packed, with a line out the door. It’s used mostly in Europe at this point. Testing is available in many U.S. hospitals, but a large majority of audience members, when polled, said they don’t currently use it in clinical practice, and that it’s not a part of diagnostic algorithms at their institutions.
Levels of procalcitonin, a calcitonin precursor normally produced by the thyroid, are low or undetectable in healthy people, but inflammation, be it from infectious or noninfectious causes, triggers production by parenchymal cells throughout the body.
Levels began to rise as early as 2.5 hours after healthy subjects in one study were injected with bacterial endotoxins, and peaked as early as 6 hours; CRP, in contrast, started to rise after 12 hours, and peaked at 30 hours. Procalcitonin levels also seem to correlate with bacterial load and severity of infection, said Nivedita Srinivas, MD, a pediatric infectious disease specialist at Stanford (Calif.) University (J Pediatr Intensive Care. 2016 Dec;5[4]:162-71).
Due to time, the presenters focused their talk on community acquired pneumonia (CAP) and invasive bacterial infections (IBI) in young infants, meaning essentially bacteremia and meningitis.
Different studies use different cutoffs, but a procalcitonin below, for instance, 0.5 ng/mL is “certainly more sensitive [for IBI] than any single biomarker we currently use,” including CRP, white blood cells, and absolute neutrophil count (ANC). “If it’s negative, you’re really confident it’s negative,” but “a positive test does not necessarily indicate the presence of IBI,” Dr. McCulloh said (Pediatrics. 2012 Nov;130[5]:815-22).
“Procalcitonin works really well as part of a validated step-wise rule” that includes, for instance, CRP and ANC; “I think that’s where its utility is. On its own, it is not a substitute for you examining the patient and doing your basic risk stratification, but it may enhance your decision making incrementally above what we currently have,” he said.
Meanwhile, in a study of 532 children a median age of 2.4 years with radiographically confirmed CAP, procalcitonin levels were a median of 6.1 ng/mL in children whose pneumonia was caused by Streptococcus pneumoniae or other typical bacteria, and no child infected with typical bacteria had a level under 0.1 ng/mL. Below that level, “you can be very sure you do not have typical bacteria pneumonia,” said Marie Wang, MD, also a pediatric infectious disease specialist at Stanford (J Pediatric Infect Dis Soc. 2018 Feb 19;7[1]:46-53).
As procalcitonin levels went up, the likelihood of having bacterial pneumonia increased; at 2 ng/mL, 26% of subjects were infected with typical bacteria, “but even in that group, 58% still had viral infection, so you are still detecting a lot of viral” disease, she said.
Prolcalcitonin-guided therapy – antibiotics until patients fall below a level of 0.25 ng/ml, for instance – has also been associated with decreased antibiotic exposure (Respir Med. 2011 Dec;105[12]:1939-45).
The speakers had no disclosures. The meeting was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
EXPERT ANALYSIS FROM PHM 2019
Favorable Ebola results lead to drug trial termination, new focus
An investigational agent known as REGN-EB3 has met an early stopping criterion in the protocol of an Ebola therapeutics trial, according to a National Institutes of Health media advisory.
Preliminary results in 499 study participants showed that individuals receiving either of two treatments, REGN-EB3 or mAb114, had a greater chance of survival, compared with participants in the other two study arms.
The randomized, controlled Pamoja Tulinde Maisha (PALM) study, which began Nov. 20, 2018, was designed to evaluate four investigational agents (ZMapp, remdesivir, mAb114, and REGN-EB3) for the treatment of patients with Ebola virus disease in the Democratic Republic of the Congo (DRC) as part of the emergency response to an ongoing outbreak in the North Kivu and Ituri provinces.
As of Aug. 9, 2019, the trial had enrolled 681 patients at four Ebola treatment centers in live outbreak regions of the DRC, with the goal of enrolling 725 patients in total.
The trial investigators and study cosponsors accepted the recommendation for early termination, and staff at the trial sites in the DRC were promptly informed, according to the media advisory. Additional patient randomizations in the now-revised trial will be limited to treatment either with REGN-EB3 or mAb114. Patients randomized to the ZMapp or remdesivir arms in the last 10 days of the original trial will be given the option, at the discretion of their treating physician, to receive either of the two more effective treatments, according to the NIH.
“While the final analysis of the data can occur only after all the data are generated and collected (likely late September/early October 2019), the DSMB [Data and Safety Monitoring Board] and the study leadership felt the preliminary analysis of the existing data was compelling enough to recommend and implement these changes in the trial immediately. The complete results will be submitted for publication in the peer-reviewed medical literature as soon as possible,” the NIH stated.
The study is cosponsored and funded by the NIH, carried out by an international research consortium coordinated by the World Health Organization, and supported by four pharmaceutical companies (MappBio, Gilead, Regeneron, and Ridgeback Biotherapeutics).
An investigational agent known as REGN-EB3 has met an early stopping criterion in the protocol of an Ebola therapeutics trial, according to a National Institutes of Health media advisory.
Preliminary results in 499 study participants showed that individuals receiving either of two treatments, REGN-EB3 or mAb114, had a greater chance of survival, compared with participants in the other two study arms.
The randomized, controlled Pamoja Tulinde Maisha (PALM) study, which began Nov. 20, 2018, was designed to evaluate four investigational agents (ZMapp, remdesivir, mAb114, and REGN-EB3) for the treatment of patients with Ebola virus disease in the Democratic Republic of the Congo (DRC) as part of the emergency response to an ongoing outbreak in the North Kivu and Ituri provinces.
As of Aug. 9, 2019, the trial had enrolled 681 patients at four Ebola treatment centers in live outbreak regions of the DRC, with the goal of enrolling 725 patients in total.
The trial investigators and study cosponsors accepted the recommendation for early termination, and staff at the trial sites in the DRC were promptly informed, according to the media advisory. Additional patient randomizations in the now-revised trial will be limited to treatment either with REGN-EB3 or mAb114. Patients randomized to the ZMapp or remdesivir arms in the last 10 days of the original trial will be given the option, at the discretion of their treating physician, to receive either of the two more effective treatments, according to the NIH.
“While the final analysis of the data can occur only after all the data are generated and collected (likely late September/early October 2019), the DSMB [Data and Safety Monitoring Board] and the study leadership felt the preliminary analysis of the existing data was compelling enough to recommend and implement these changes in the trial immediately. The complete results will be submitted for publication in the peer-reviewed medical literature as soon as possible,” the NIH stated.
The study is cosponsored and funded by the NIH, carried out by an international research consortium coordinated by the World Health Organization, and supported by four pharmaceutical companies (MappBio, Gilead, Regeneron, and Ridgeback Biotherapeutics).
An investigational agent known as REGN-EB3 has met an early stopping criterion in the protocol of an Ebola therapeutics trial, according to a National Institutes of Health media advisory.
Preliminary results in 499 study participants showed that individuals receiving either of two treatments, REGN-EB3 or mAb114, had a greater chance of survival, compared with participants in the other two study arms.
The randomized, controlled Pamoja Tulinde Maisha (PALM) study, which began Nov. 20, 2018, was designed to evaluate four investigational agents (ZMapp, remdesivir, mAb114, and REGN-EB3) for the treatment of patients with Ebola virus disease in the Democratic Republic of the Congo (DRC) as part of the emergency response to an ongoing outbreak in the North Kivu and Ituri provinces.
As of Aug. 9, 2019, the trial had enrolled 681 patients at four Ebola treatment centers in live outbreak regions of the DRC, with the goal of enrolling 725 patients in total.
The trial investigators and study cosponsors accepted the recommendation for early termination, and staff at the trial sites in the DRC were promptly informed, according to the media advisory. Additional patient randomizations in the now-revised trial will be limited to treatment either with REGN-EB3 or mAb114. Patients randomized to the ZMapp or remdesivir arms in the last 10 days of the original trial will be given the option, at the discretion of their treating physician, to receive either of the two more effective treatments, according to the NIH.
“While the final analysis of the data can occur only after all the data are generated and collected (likely late September/early October 2019), the DSMB [Data and Safety Monitoring Board] and the study leadership felt the preliminary analysis of the existing data was compelling enough to recommend and implement these changes in the trial immediately. The complete results will be submitted for publication in the peer-reviewed medical literature as soon as possible,” the NIH stated.
The study is cosponsored and funded by the NIH, carried out by an international research consortium coordinated by the World Health Organization, and supported by four pharmaceutical companies (MappBio, Gilead, Regeneron, and Ridgeback Biotherapeutics).
In newborns, concentrated urine helps rule out UTI
SEATTLE – according to investigators at the University of Texas Health Science Center, Houston.
The researchers found that urine testing negative for nitrites with a specific gravity above 1.015 in children up to 2 months old had a sensitivity of 53% for ruling out UTIs, but that urine with a specific gravity below that mark had a sensitivity of just 14%. The finding “should be taken into account when interpreting nitrite results ... in this high-risk population,” they concluded.
Bacteria in the bladder convert nitrates to nitrites, so positive results are pretty much pathognomonic for UTIs, with a specificity of nearly 100%, according to the researchers.
Negative results, however, don’t reliably rule out infection, and are even less reliable in infants because they urinate frequently, which means they usually flush out bacteria before they have enough time to make the conversion, which takes several hours, they said.
The lead investigator Raymond Parlar-Chun, MD, an assistant professor of pediatrics at the University of Texas McGovern Medical School in Houston, said he had a hunch that negative results might be more reliable when newborns urinate less frequently and have more concentrated urine.
He and his team reviewed data collected on 413 infants up to 2 months old who were admitted for fever workup and treated for UTIs both in the hospital and after discharge. Nitrite results were stratified by urine concentration. A specific gravity of 1.015 was used as the cutoff between concentrated and dilute urine, which was “midway between the parameters reported” in every urinalysis, Dr. Parlar-Chun said.
Although the sensitivity of concentrated urine was only 53%, “it’s a stark difference from” the 14% in dilute urine, he said.“You should take a look at specific gravity to interpret nitrites. If urine is concentrated, you have [more confidence] that you don’t have a UTI if you’re negative. It’s better than taking [nitrites] at face value.”
The subjects were 31 days old, on average, and 62% were boys; 112 had a specific gravity above 1.015, and 301 below.
There was no external funding, and Dr. Parlar-Chun didn’t have any disclosures.
SEATTLE – according to investigators at the University of Texas Health Science Center, Houston.
The researchers found that urine testing negative for nitrites with a specific gravity above 1.015 in children up to 2 months old had a sensitivity of 53% for ruling out UTIs, but that urine with a specific gravity below that mark had a sensitivity of just 14%. The finding “should be taken into account when interpreting nitrite results ... in this high-risk population,” they concluded.
Bacteria in the bladder convert nitrates to nitrites, so positive results are pretty much pathognomonic for UTIs, with a specificity of nearly 100%, according to the researchers.
Negative results, however, don’t reliably rule out infection, and are even less reliable in infants because they urinate frequently, which means they usually flush out bacteria before they have enough time to make the conversion, which takes several hours, they said.
The lead investigator Raymond Parlar-Chun, MD, an assistant professor of pediatrics at the University of Texas McGovern Medical School in Houston, said he had a hunch that negative results might be more reliable when newborns urinate less frequently and have more concentrated urine.
He and his team reviewed data collected on 413 infants up to 2 months old who were admitted for fever workup and treated for UTIs both in the hospital and after discharge. Nitrite results were stratified by urine concentration. A specific gravity of 1.015 was used as the cutoff between concentrated and dilute urine, which was “midway between the parameters reported” in every urinalysis, Dr. Parlar-Chun said.
Although the sensitivity of concentrated urine was only 53%, “it’s a stark difference from” the 14% in dilute urine, he said.“You should take a look at specific gravity to interpret nitrites. If urine is concentrated, you have [more confidence] that you don’t have a UTI if you’re negative. It’s better than taking [nitrites] at face value.”
The subjects were 31 days old, on average, and 62% were boys; 112 had a specific gravity above 1.015, and 301 below.
There was no external funding, and Dr. Parlar-Chun didn’t have any disclosures.
SEATTLE – according to investigators at the University of Texas Health Science Center, Houston.
The researchers found that urine testing negative for nitrites with a specific gravity above 1.015 in children up to 2 months old had a sensitivity of 53% for ruling out UTIs, but that urine with a specific gravity below that mark had a sensitivity of just 14%. The finding “should be taken into account when interpreting nitrite results ... in this high-risk population,” they concluded.
Bacteria in the bladder convert nitrates to nitrites, so positive results are pretty much pathognomonic for UTIs, with a specificity of nearly 100%, according to the researchers.
Negative results, however, don’t reliably rule out infection, and are even less reliable in infants because they urinate frequently, which means they usually flush out bacteria before they have enough time to make the conversion, which takes several hours, they said.
The lead investigator Raymond Parlar-Chun, MD, an assistant professor of pediatrics at the University of Texas McGovern Medical School in Houston, said he had a hunch that negative results might be more reliable when newborns urinate less frequently and have more concentrated urine.
He and his team reviewed data collected on 413 infants up to 2 months old who were admitted for fever workup and treated for UTIs both in the hospital and after discharge. Nitrite results were stratified by urine concentration. A specific gravity of 1.015 was used as the cutoff between concentrated and dilute urine, which was “midway between the parameters reported” in every urinalysis, Dr. Parlar-Chun said.
Although the sensitivity of concentrated urine was only 53%, “it’s a stark difference from” the 14% in dilute urine, he said.“You should take a look at specific gravity to interpret nitrites. If urine is concentrated, you have [more confidence] that you don’t have a UTI if you’re negative. It’s better than taking [nitrites] at face value.”
The subjects were 31 days old, on average, and 62% were boys; 112 had a specific gravity above 1.015, and 301 below.
There was no external funding, and Dr. Parlar-Chun didn’t have any disclosures.
REPORTING FROM PHM 2019
FDA panel backs Descovy as HIV PrEP for men and transgender women who have sex with men
The Food and Drug Administration’s Antimicrobial Drugs Advisory Committee backed the fixed dose combination of emtricitabine and tenofovir alafenamide (TAF; Descovy, Gilead) for pre-exposure prophylaxis (PrEP) against HIV for men and transgender women who have sex with men.
In a discussion after a 16-2 vote, committee members cited analysis by the study’s sponsor and the FDA showing efficacy and a generally good safety profile in the DISCOVER trial, the single new clinical trial conducted to support TAF’s use for pre-exposure prophylaxis (PrEP).
However, this trial included no cisgender women; the sponsor asked for approval based primarily on extrapolation from the DISCOVER results and previous results with tenofovir disoproxil fumarate (TDF) in cisgender women. Both formulations of tenofovir are prodrugs and converted to tenofovir diphosphate intracellularly in peripheral blood mononuclear cells, though many aspects of their pharmacokinetics differ.
The committee voted 10-8 against the proposition that these data supported an indication of TAF for PrEP in cisgender women, in a narrowly worded question from the FDA.
Many members who voted on either side of the question had strongly worded reservations about the lack of data for cisgender women. Said committee chair Lindsey R. Baden, MD, director of the infectious disease service at Dana-Farber Cancer Institute, Boston, who voted against the indication for cisgender women, “We’ve failed women. To be at this point and not have the data to guide decision-making is a shame on all of us.”
Ighovwerha Ofotokun, MD, who voted yes, concurred: “I agree it is a terrible failure that the agency, as well as the sponsor, would come to this committee with a lack of data on women.” But for Dr. Ofotokun, a professor of infectious diseases at Emory University, Atlanta, not including cisgender women in the approval was a distasteful proposition. “Creating a two-tier prevention and treatment hierarchy would not be helpful. We should remind ourselves that there are more women living with HIV in the world than there are men, and the risk of new HIV infection is higher among women than among men, if you look at this globally,” he said.
“I find it disrespectful and an issue of research equity. Women deserve the same quality of data about the safety and efficacy of the drugs they are exposed to that men get and that is not the situation we find ourselves in at the moment,” said Dawn K. Smith, MD, MPH, a lead scientist at the Centers for Disease Control and Prevention (CDC), Atlanta, who voted against approval for cisgender women.
Michael Green, MD, MPH, professor of pediatrics, surgery and clinical and translational science at the University of Pittsburgh, echoed the frustration of many committee members when he said, “I voted yes, almost abstained, then almost voted no.” He, along with all who voted yes, emphasized the importance of mandatory postmarketing studies in cisgender women to ensure efficacy data are obtained.
Transgender women made up only about 1% of the DISCOVER population, a fact that also gave many committee members pause.
If TAF is approved, labeling and package materials should be clear that the data support only noninferiority, not superiority, compared with TDF, said several advisory committee members who voted for approval for men and transgender women who have sex with men. “My expectation of this approval is that it should be marketed responsibly from the perspective of not creating these disparities and having Truvada be a drug for poor people and Descovy be a drug for rich people,” said Demetre Dasklalakis, MD, assistant commissioner of the Bureau of HIV/AIDS Prevention and Control at the city of New York’s Department of Health and Hygiene, and of the Icahn School of Medicine at Mount Sinai, N.Y. Truvada is slated to be offered as a generic drug in 2020, according to a Securities and Exchange Commission filing by Gilead Sciences.
The CDC reported earlier in 2019 that rates of new HIV infections have plateaued in recent years. Uptake of PrEP has been particularly low among at-risk members of minority populations, in rural areas, and in the South, according to a CDC report.
The DISCOVER trial is a 96-week ongoing trial to test TAF’s noninferiority to a fixed-drug combination of emcitrabine and tenofovir dimethyl fumarate (TDF; Truvada, Gilead) for PrEP. Both drugs are already approved to treat HIV infection, and TDF is approved for PrEP. Non-inferiority was preestablished at a rate ratio of HIV incidence of 1.62 (TAF:TDF) between the two study arms.
DISCOVER has enrolled 5,387 men and transgender women who have sex with men and are deemed at high risk for HIV, and found an incidence rate ratio of 0.47, with the upper bound of the confidence interval at 1.15. Since this figure was less than the prespecified noninferiority margin, both Gilead presenters and the FDA agreed, TAF’s noninferiority for efficacy was established.
Characteristics were similar between patients in the TAF arm (N = 2,694) and the TDF arm (N = 2,693). About 60% of patients in each arm reported having receptive anal sex with at least two partners in the previous 12 weeks, and recent rectal gonorrhea, syphilis, and chlamydia rates were 9-13% at baseline. Two thirds of participants reported recreational drug use, and about one in four reported binge drinking.
Sexual behavior and sexually transmitted infection rates continued generally unchanged from baseline during the study period.
The median age was 34 years, and most participants (84%) were white. Black participants made up 9% of the study population, and about 25% were of Hispanic or Latin ethnic origin.
Known decreases in bone mineral density occur with TDF; these were not seen with TAF, and bone mineral density increased while on TAF for the DISCOVER population aged 19-25 years.
Renal biomarkers of concern with TDF included two proteins linked with proximal tubule dysfunction, as well as estimated glomerular filtration rate. According to the sponsor’s analysis, eGFR fell by 2.3 mL/min for the TAF group, compared with a 1.8 mL/min rise while on TDF (P less than .001). Changes of similar statistical significance were seen for proximal tubular proteinuria. Also, improvements were seen in renal measures for the subset of patients enrolled who were on TDF PrEP at baseline but switched to TAF, in a prespecified subgroup analysis.
However, patients who were on TDF had a significant decrease in total cholesterol and both low- and high-density lipoprotein cholesterol compared with those on TAF, who had minimal changes or slight increases in lipids (P less than .001 for all). Triglycerides rose for those on TAF and remained unchanged for those on TDF (P = .002).
The PrEP indication sought by Gilead includes adults and adolescents, defined as those who weigh more than 35 kg. A nonvoting question put before the committee asked whether the totality of tenofovir data supported an indication of TAF for cisgender men who have insertive vaginal sex; though this extrapolation didn’t give the committee as much pause as the request for approval in cisgender women, they cited similar concerns and noted that cervicovaginal mucosa are different in many ways from rectal mucosa.
The study included no cisgender women, for a host of reasons cited by the sponsor and the FDA. These included high nonadherence rates among this population, relatively lower HIV infection rates among cisgender women in the United States, and mixed efficacy results in previous tenofovir clinical trials; the latter point made establishing a noninferiority margin problematic, according to the FDA.
For Dr. Baden, “The optics of approval for population A but not for population B are problematic.” Speaking to both the sponsor and the FDA, he said, “Everyone agrees there needs to be actual data. Please do the study as quickly as possible.” What’s needed is the collective will to make it happen, he added: “I don’t accept that it’s too big, too hard, too difficult.”
The FDA usually follows the recommendations of its advisory committees.
This article was updated 8/8/19.
The Food and Drug Administration’s Antimicrobial Drugs Advisory Committee backed the fixed dose combination of emtricitabine and tenofovir alafenamide (TAF; Descovy, Gilead) for pre-exposure prophylaxis (PrEP) against HIV for men and transgender women who have sex with men.
In a discussion after a 16-2 vote, committee members cited analysis by the study’s sponsor and the FDA showing efficacy and a generally good safety profile in the DISCOVER trial, the single new clinical trial conducted to support TAF’s use for pre-exposure prophylaxis (PrEP).
However, this trial included no cisgender women; the sponsor asked for approval based primarily on extrapolation from the DISCOVER results and previous results with tenofovir disoproxil fumarate (TDF) in cisgender women. Both formulations of tenofovir are prodrugs and converted to tenofovir diphosphate intracellularly in peripheral blood mononuclear cells, though many aspects of their pharmacokinetics differ.
The committee voted 10-8 against the proposition that these data supported an indication of TAF for PrEP in cisgender women, in a narrowly worded question from the FDA.
Many members who voted on either side of the question had strongly worded reservations about the lack of data for cisgender women. Said committee chair Lindsey R. Baden, MD, director of the infectious disease service at Dana-Farber Cancer Institute, Boston, who voted against the indication for cisgender women, “We’ve failed women. To be at this point and not have the data to guide decision-making is a shame on all of us.”
Ighovwerha Ofotokun, MD, who voted yes, concurred: “I agree it is a terrible failure that the agency, as well as the sponsor, would come to this committee with a lack of data on women.” But for Dr. Ofotokun, a professor of infectious diseases at Emory University, Atlanta, not including cisgender women in the approval was a distasteful proposition. “Creating a two-tier prevention and treatment hierarchy would not be helpful. We should remind ourselves that there are more women living with HIV in the world than there are men, and the risk of new HIV infection is higher among women than among men, if you look at this globally,” he said.
“I find it disrespectful and an issue of research equity. Women deserve the same quality of data about the safety and efficacy of the drugs they are exposed to that men get and that is not the situation we find ourselves in at the moment,” said Dawn K. Smith, MD, MPH, a lead scientist at the Centers for Disease Control and Prevention (CDC), Atlanta, who voted against approval for cisgender women.
Michael Green, MD, MPH, professor of pediatrics, surgery and clinical and translational science at the University of Pittsburgh, echoed the frustration of many committee members when he said, “I voted yes, almost abstained, then almost voted no.” He, along with all who voted yes, emphasized the importance of mandatory postmarketing studies in cisgender women to ensure efficacy data are obtained.
Transgender women made up only about 1% of the DISCOVER population, a fact that also gave many committee members pause.
If TAF is approved, labeling and package materials should be clear that the data support only noninferiority, not superiority, compared with TDF, said several advisory committee members who voted for approval for men and transgender women who have sex with men. “My expectation of this approval is that it should be marketed responsibly from the perspective of not creating these disparities and having Truvada be a drug for poor people and Descovy be a drug for rich people,” said Demetre Dasklalakis, MD, assistant commissioner of the Bureau of HIV/AIDS Prevention and Control at the city of New York’s Department of Health and Hygiene, and of the Icahn School of Medicine at Mount Sinai, N.Y. Truvada is slated to be offered as a generic drug in 2020, according to a Securities and Exchange Commission filing by Gilead Sciences.
The CDC reported earlier in 2019 that rates of new HIV infections have plateaued in recent years. Uptake of PrEP has been particularly low among at-risk members of minority populations, in rural areas, and in the South, according to a CDC report.
The DISCOVER trial is a 96-week ongoing trial to test TAF’s noninferiority to a fixed-drug combination of emcitrabine and tenofovir dimethyl fumarate (TDF; Truvada, Gilead) for PrEP. Both drugs are already approved to treat HIV infection, and TDF is approved for PrEP. Non-inferiority was preestablished at a rate ratio of HIV incidence of 1.62 (TAF:TDF) between the two study arms.
DISCOVER has enrolled 5,387 men and transgender women who have sex with men and are deemed at high risk for HIV, and found an incidence rate ratio of 0.47, with the upper bound of the confidence interval at 1.15. Since this figure was less than the prespecified noninferiority margin, both Gilead presenters and the FDA agreed, TAF’s noninferiority for efficacy was established.
Characteristics were similar between patients in the TAF arm (N = 2,694) and the TDF arm (N = 2,693). About 60% of patients in each arm reported having receptive anal sex with at least two partners in the previous 12 weeks, and recent rectal gonorrhea, syphilis, and chlamydia rates were 9-13% at baseline. Two thirds of participants reported recreational drug use, and about one in four reported binge drinking.
Sexual behavior and sexually transmitted infection rates continued generally unchanged from baseline during the study period.
The median age was 34 years, and most participants (84%) were white. Black participants made up 9% of the study population, and about 25% were of Hispanic or Latin ethnic origin.
Known decreases in bone mineral density occur with TDF; these were not seen with TAF, and bone mineral density increased while on TAF for the DISCOVER population aged 19-25 years.
Renal biomarkers of concern with TDF included two proteins linked with proximal tubule dysfunction, as well as estimated glomerular filtration rate. According to the sponsor’s analysis, eGFR fell by 2.3 mL/min for the TAF group, compared with a 1.8 mL/min rise while on TDF (P less than .001). Changes of similar statistical significance were seen for proximal tubular proteinuria. Also, improvements were seen in renal measures for the subset of patients enrolled who were on TDF PrEP at baseline but switched to TAF, in a prespecified subgroup analysis.
However, patients who were on TDF had a significant decrease in total cholesterol and both low- and high-density lipoprotein cholesterol compared with those on TAF, who had minimal changes or slight increases in lipids (P less than .001 for all). Triglycerides rose for those on TAF and remained unchanged for those on TDF (P = .002).
The PrEP indication sought by Gilead includes adults and adolescents, defined as those who weigh more than 35 kg. A nonvoting question put before the committee asked whether the totality of tenofovir data supported an indication of TAF for cisgender men who have insertive vaginal sex; though this extrapolation didn’t give the committee as much pause as the request for approval in cisgender women, they cited similar concerns and noted that cervicovaginal mucosa are different in many ways from rectal mucosa.
The study included no cisgender women, for a host of reasons cited by the sponsor and the FDA. These included high nonadherence rates among this population, relatively lower HIV infection rates among cisgender women in the United States, and mixed efficacy results in previous tenofovir clinical trials; the latter point made establishing a noninferiority margin problematic, according to the FDA.
For Dr. Baden, “The optics of approval for population A but not for population B are problematic.” Speaking to both the sponsor and the FDA, he said, “Everyone agrees there needs to be actual data. Please do the study as quickly as possible.” What’s needed is the collective will to make it happen, he added: “I don’t accept that it’s too big, too hard, too difficult.”
The FDA usually follows the recommendations of its advisory committees.
This article was updated 8/8/19.
The Food and Drug Administration’s Antimicrobial Drugs Advisory Committee backed the fixed dose combination of emtricitabine and tenofovir alafenamide (TAF; Descovy, Gilead) for pre-exposure prophylaxis (PrEP) against HIV for men and transgender women who have sex with men.
In a discussion after a 16-2 vote, committee members cited analysis by the study’s sponsor and the FDA showing efficacy and a generally good safety profile in the DISCOVER trial, the single new clinical trial conducted to support TAF’s use for pre-exposure prophylaxis (PrEP).
However, this trial included no cisgender women; the sponsor asked for approval based primarily on extrapolation from the DISCOVER results and previous results with tenofovir disoproxil fumarate (TDF) in cisgender women. Both formulations of tenofovir are prodrugs and converted to tenofovir diphosphate intracellularly in peripheral blood mononuclear cells, though many aspects of their pharmacokinetics differ.
The committee voted 10-8 against the proposition that these data supported an indication of TAF for PrEP in cisgender women, in a narrowly worded question from the FDA.
Many members who voted on either side of the question had strongly worded reservations about the lack of data for cisgender women. Said committee chair Lindsey R. Baden, MD, director of the infectious disease service at Dana-Farber Cancer Institute, Boston, who voted against the indication for cisgender women, “We’ve failed women. To be at this point and not have the data to guide decision-making is a shame on all of us.”
Ighovwerha Ofotokun, MD, who voted yes, concurred: “I agree it is a terrible failure that the agency, as well as the sponsor, would come to this committee with a lack of data on women.” But for Dr. Ofotokun, a professor of infectious diseases at Emory University, Atlanta, not including cisgender women in the approval was a distasteful proposition. “Creating a two-tier prevention and treatment hierarchy would not be helpful. We should remind ourselves that there are more women living with HIV in the world than there are men, and the risk of new HIV infection is higher among women than among men, if you look at this globally,” he said.
“I find it disrespectful and an issue of research equity. Women deserve the same quality of data about the safety and efficacy of the drugs they are exposed to that men get and that is not the situation we find ourselves in at the moment,” said Dawn K. Smith, MD, MPH, a lead scientist at the Centers for Disease Control and Prevention (CDC), Atlanta, who voted against approval for cisgender women.
Michael Green, MD, MPH, professor of pediatrics, surgery and clinical and translational science at the University of Pittsburgh, echoed the frustration of many committee members when he said, “I voted yes, almost abstained, then almost voted no.” He, along with all who voted yes, emphasized the importance of mandatory postmarketing studies in cisgender women to ensure efficacy data are obtained.
Transgender women made up only about 1% of the DISCOVER population, a fact that also gave many committee members pause.
If TAF is approved, labeling and package materials should be clear that the data support only noninferiority, not superiority, compared with TDF, said several advisory committee members who voted for approval for men and transgender women who have sex with men. “My expectation of this approval is that it should be marketed responsibly from the perspective of not creating these disparities and having Truvada be a drug for poor people and Descovy be a drug for rich people,” said Demetre Dasklalakis, MD, assistant commissioner of the Bureau of HIV/AIDS Prevention and Control at the city of New York’s Department of Health and Hygiene, and of the Icahn School of Medicine at Mount Sinai, N.Y. Truvada is slated to be offered as a generic drug in 2020, according to a Securities and Exchange Commission filing by Gilead Sciences.
The CDC reported earlier in 2019 that rates of new HIV infections have plateaued in recent years. Uptake of PrEP has been particularly low among at-risk members of minority populations, in rural areas, and in the South, according to a CDC report.
The DISCOVER trial is a 96-week ongoing trial to test TAF’s noninferiority to a fixed-drug combination of emcitrabine and tenofovir dimethyl fumarate (TDF; Truvada, Gilead) for PrEP. Both drugs are already approved to treat HIV infection, and TDF is approved for PrEP. Non-inferiority was preestablished at a rate ratio of HIV incidence of 1.62 (TAF:TDF) between the two study arms.
DISCOVER has enrolled 5,387 men and transgender women who have sex with men and are deemed at high risk for HIV, and found an incidence rate ratio of 0.47, with the upper bound of the confidence interval at 1.15. Since this figure was less than the prespecified noninferiority margin, both Gilead presenters and the FDA agreed, TAF’s noninferiority for efficacy was established.
Characteristics were similar between patients in the TAF arm (N = 2,694) and the TDF arm (N = 2,693). About 60% of patients in each arm reported having receptive anal sex with at least two partners in the previous 12 weeks, and recent rectal gonorrhea, syphilis, and chlamydia rates were 9-13% at baseline. Two thirds of participants reported recreational drug use, and about one in four reported binge drinking.
Sexual behavior and sexually transmitted infection rates continued generally unchanged from baseline during the study period.
The median age was 34 years, and most participants (84%) were white. Black participants made up 9% of the study population, and about 25% were of Hispanic or Latin ethnic origin.
Known decreases in bone mineral density occur with TDF; these were not seen with TAF, and bone mineral density increased while on TAF for the DISCOVER population aged 19-25 years.
Renal biomarkers of concern with TDF included two proteins linked with proximal tubule dysfunction, as well as estimated glomerular filtration rate. According to the sponsor’s analysis, eGFR fell by 2.3 mL/min for the TAF group, compared with a 1.8 mL/min rise while on TDF (P less than .001). Changes of similar statistical significance were seen for proximal tubular proteinuria. Also, improvements were seen in renal measures for the subset of patients enrolled who were on TDF PrEP at baseline but switched to TAF, in a prespecified subgroup analysis.
However, patients who were on TDF had a significant decrease in total cholesterol and both low- and high-density lipoprotein cholesterol compared with those on TAF, who had minimal changes or slight increases in lipids (P less than .001 for all). Triglycerides rose for those on TAF and remained unchanged for those on TDF (P = .002).
The PrEP indication sought by Gilead includes adults and adolescents, defined as those who weigh more than 35 kg. A nonvoting question put before the committee asked whether the totality of tenofovir data supported an indication of TAF for cisgender men who have insertive vaginal sex; though this extrapolation didn’t give the committee as much pause as the request for approval in cisgender women, they cited similar concerns and noted that cervicovaginal mucosa are different in many ways from rectal mucosa.
The study included no cisgender women, for a host of reasons cited by the sponsor and the FDA. These included high nonadherence rates among this population, relatively lower HIV infection rates among cisgender women in the United States, and mixed efficacy results in previous tenofovir clinical trials; the latter point made establishing a noninferiority margin problematic, according to the FDA.
For Dr. Baden, “The optics of approval for population A but not for population B are problematic.” Speaking to both the sponsor and the FDA, he said, “Everyone agrees there needs to be actual data. Please do the study as quickly as possible.” What’s needed is the collective will to make it happen, he added: “I don’t accept that it’s too big, too hard, too difficult.”
The FDA usually follows the recommendations of its advisory committees.
This article was updated 8/8/19.
FROM AN FDA ADVISORY COMMITTEE MEETING
New RSV vaccine immunogenicity improved with protein engineering
Development of an effective respiratory syncytial virus (RSV) vaccine is feasible using a new technology that can contribute to development of other vaccines as well, according to results of a proof-of-concept study in Science.
The new method of protein engineering preserves the RSV antigen protein’s prefusion structure, including the epitope, thereby inducing antibodies that better “match,” and neutralize, the actual pathogen.
“Protein-based RSV vaccines have had a particularly complicated history, especially those in which the primary immunogen has been the fusion (F) glycoprotein, which exists in two major conformational states: prefusion (pre-F) and postfusion (post-F),” lead author Michelle Crank, MD, of the Vaccine Research Center at the National Institute of Allergy and Infectious Diseases in Bethesda, Md., and her colleagues explained in the paper.
Since the failure of the whole-inactivated RSV vaccine in the 1960s, researchers have focused on F subunit vaccine candidates, but these contain only post-F or “structurally undefined” F protein.
“Although the products are immunogenic, a substantial proportion of antibodies elicited are non- or poorly neutralizing, and field trials have shown no or minimal efficacy,” the authors wrote.
But now researchers have an “atomic-level understanding of F conformational states, antigenic sites, and the specificity of the human B cell repertoire and serum antibody response to infection.” Having developed a way to engineer proteins to retain the F protein’s prefusion conformation, the researchers developed the DS-Cav1 vaccine with an F protein from RSV subtype A.
In their phase 1, randomized, open-label clinical trial, the researchers tested the safety, tolerability and immunogenicity of DS-Cav1. The trial involved 90 healthy adults, aged 18-50, who had no abnormal findings in clinical lab tests, their medical history, or a physical exam.
The participants received two intramuscular doses, 12 weeks apart, of either 50 mcg, 150 mcg or 500 mcg of the vaccine. In each of these dosage groups, half the participants received a vaccine with 0.5 mcg of alum as an adjuvant, and half received a vaccine without any adjuvants. Each of the six randomized dosage-adjuvant groups had 15 participants.
The investigators report on safety and immunogenicity through 28 days after the first vaccine dose among the first 40 participants enrolled, each randomly assigned into four groups of 10 for the 50 mcg and 150 mcg doses with and without the adjuvant. Their primary immunogenicity endpoint was neutralizing activity from the vaccine.
Neutralizing activity with RSV A was seven times higher with 50 mcg and 12-15 times higher with 150 mcg at week 4 than at baseline (P less than .001).
“These increases in neutralizing activity were higher than those previously reported for F protein subunit vaccines and exceeded the threefold increase in neutralization reported after experimental human challenge with RSV,” the authors noted. Neutralization levels remained 5-10 times higher than baseline at week 12 (P less than .001).
Even with RSV B, neutralizing activity from DS-Cav1 was 4-6 times greater with 50 mcg and 9 times greater with 150 mcg, both with and without alum (P less than .001).
“The boost in neutralizing activity to subtype B after a single immunization with a subtype A–based F vaccine reflected the high conservation of F between subtypes and suggested that multiple prior infections by both RSV A and B subtypes establishes a broad preexisting B-cell repertoire,” the authors wrote.
The adjuvant had no clinically significant effect on immunogenicity, and no serious adverse events occurred in the groups.
The findings reveal that DS-Cav1 induces antibodies far more functionally effective than seen in previous RSV vaccines while opening the door to using similar techniques with other vaccines, the authors wrote. “We are now entering an era of vaccinology in which new technologies provide avenues to define the structural basis of antigenicity and to rapidly isolate and characterize human monoclonal antibodies,” the researchers wrote, marking “a step toward a future of precision vaccines.”
The research was funded by the National Institutes of Health and the Bill & Melinda Gates Foundation. Several of the study authors are inventors on patents for stabilizing the RSV F protein.
Development of an effective respiratory syncytial virus (RSV) vaccine is feasible using a new technology that can contribute to development of other vaccines as well, according to results of a proof-of-concept study in Science.
The new method of protein engineering preserves the RSV antigen protein’s prefusion structure, including the epitope, thereby inducing antibodies that better “match,” and neutralize, the actual pathogen.
“Protein-based RSV vaccines have had a particularly complicated history, especially those in which the primary immunogen has been the fusion (F) glycoprotein, which exists in two major conformational states: prefusion (pre-F) and postfusion (post-F),” lead author Michelle Crank, MD, of the Vaccine Research Center at the National Institute of Allergy and Infectious Diseases in Bethesda, Md., and her colleagues explained in the paper.
Since the failure of the whole-inactivated RSV vaccine in the 1960s, researchers have focused on F subunit vaccine candidates, but these contain only post-F or “structurally undefined” F protein.
“Although the products are immunogenic, a substantial proportion of antibodies elicited are non- or poorly neutralizing, and field trials have shown no or minimal efficacy,” the authors wrote.
But now researchers have an “atomic-level understanding of F conformational states, antigenic sites, and the specificity of the human B cell repertoire and serum antibody response to infection.” Having developed a way to engineer proteins to retain the F protein’s prefusion conformation, the researchers developed the DS-Cav1 vaccine with an F protein from RSV subtype A.
In their phase 1, randomized, open-label clinical trial, the researchers tested the safety, tolerability and immunogenicity of DS-Cav1. The trial involved 90 healthy adults, aged 18-50, who had no abnormal findings in clinical lab tests, their medical history, or a physical exam.
The participants received two intramuscular doses, 12 weeks apart, of either 50 mcg, 150 mcg or 500 mcg of the vaccine. In each of these dosage groups, half the participants received a vaccine with 0.5 mcg of alum as an adjuvant, and half received a vaccine without any adjuvants. Each of the six randomized dosage-adjuvant groups had 15 participants.
The investigators report on safety and immunogenicity through 28 days after the first vaccine dose among the first 40 participants enrolled, each randomly assigned into four groups of 10 for the 50 mcg and 150 mcg doses with and without the adjuvant. Their primary immunogenicity endpoint was neutralizing activity from the vaccine.
Neutralizing activity with RSV A was seven times higher with 50 mcg and 12-15 times higher with 150 mcg at week 4 than at baseline (P less than .001).
“These increases in neutralizing activity were higher than those previously reported for F protein subunit vaccines and exceeded the threefold increase in neutralization reported after experimental human challenge with RSV,” the authors noted. Neutralization levels remained 5-10 times higher than baseline at week 12 (P less than .001).
Even with RSV B, neutralizing activity from DS-Cav1 was 4-6 times greater with 50 mcg and 9 times greater with 150 mcg, both with and without alum (P less than .001).
“The boost in neutralizing activity to subtype B after a single immunization with a subtype A–based F vaccine reflected the high conservation of F between subtypes and suggested that multiple prior infections by both RSV A and B subtypes establishes a broad preexisting B-cell repertoire,” the authors wrote.
The adjuvant had no clinically significant effect on immunogenicity, and no serious adverse events occurred in the groups.
The findings reveal that DS-Cav1 induces antibodies far more functionally effective than seen in previous RSV vaccines while opening the door to using similar techniques with other vaccines, the authors wrote. “We are now entering an era of vaccinology in which new technologies provide avenues to define the structural basis of antigenicity and to rapidly isolate and characterize human monoclonal antibodies,” the researchers wrote, marking “a step toward a future of precision vaccines.”
The research was funded by the National Institutes of Health and the Bill & Melinda Gates Foundation. Several of the study authors are inventors on patents for stabilizing the RSV F protein.
Development of an effective respiratory syncytial virus (RSV) vaccine is feasible using a new technology that can contribute to development of other vaccines as well, according to results of a proof-of-concept study in Science.
The new method of protein engineering preserves the RSV antigen protein’s prefusion structure, including the epitope, thereby inducing antibodies that better “match,” and neutralize, the actual pathogen.
“Protein-based RSV vaccines have had a particularly complicated history, especially those in which the primary immunogen has been the fusion (F) glycoprotein, which exists in two major conformational states: prefusion (pre-F) and postfusion (post-F),” lead author Michelle Crank, MD, of the Vaccine Research Center at the National Institute of Allergy and Infectious Diseases in Bethesda, Md., and her colleagues explained in the paper.
Since the failure of the whole-inactivated RSV vaccine in the 1960s, researchers have focused on F subunit vaccine candidates, but these contain only post-F or “structurally undefined” F protein.
“Although the products are immunogenic, a substantial proportion of antibodies elicited are non- or poorly neutralizing, and field trials have shown no or minimal efficacy,” the authors wrote.
But now researchers have an “atomic-level understanding of F conformational states, antigenic sites, and the specificity of the human B cell repertoire and serum antibody response to infection.” Having developed a way to engineer proteins to retain the F protein’s prefusion conformation, the researchers developed the DS-Cav1 vaccine with an F protein from RSV subtype A.
In their phase 1, randomized, open-label clinical trial, the researchers tested the safety, tolerability and immunogenicity of DS-Cav1. The trial involved 90 healthy adults, aged 18-50, who had no abnormal findings in clinical lab tests, their medical history, or a physical exam.
The participants received two intramuscular doses, 12 weeks apart, of either 50 mcg, 150 mcg or 500 mcg of the vaccine. In each of these dosage groups, half the participants received a vaccine with 0.5 mcg of alum as an adjuvant, and half received a vaccine without any adjuvants. Each of the six randomized dosage-adjuvant groups had 15 participants.
The investigators report on safety and immunogenicity through 28 days after the first vaccine dose among the first 40 participants enrolled, each randomly assigned into four groups of 10 for the 50 mcg and 150 mcg doses with and without the adjuvant. Their primary immunogenicity endpoint was neutralizing activity from the vaccine.
Neutralizing activity with RSV A was seven times higher with 50 mcg and 12-15 times higher with 150 mcg at week 4 than at baseline (P less than .001).
“These increases in neutralizing activity were higher than those previously reported for F protein subunit vaccines and exceeded the threefold increase in neutralization reported after experimental human challenge with RSV,” the authors noted. Neutralization levels remained 5-10 times higher than baseline at week 12 (P less than .001).
Even with RSV B, neutralizing activity from DS-Cav1 was 4-6 times greater with 50 mcg and 9 times greater with 150 mcg, both with and without alum (P less than .001).
“The boost in neutralizing activity to subtype B after a single immunization with a subtype A–based F vaccine reflected the high conservation of F between subtypes and suggested that multiple prior infections by both RSV A and B subtypes establishes a broad preexisting B-cell repertoire,” the authors wrote.
The adjuvant had no clinically significant effect on immunogenicity, and no serious adverse events occurred in the groups.
The findings reveal that DS-Cav1 induces antibodies far more functionally effective than seen in previous RSV vaccines while opening the door to using similar techniques with other vaccines, the authors wrote. “We are now entering an era of vaccinology in which new technologies provide avenues to define the structural basis of antigenicity and to rapidly isolate and characterize human monoclonal antibodies,” the researchers wrote, marking “a step toward a future of precision vaccines.”
The research was funded by the National Institutes of Health and the Bill & Melinda Gates Foundation. Several of the study authors are inventors on patents for stabilizing the RSV F protein.
FROM SCIENCE
Key clinical point:
Major finding: Epitope-neutralizing activity is 5-10 times greater 12 weeks after baseline with a 50 mcg or 150 mcg with and without alum adjuvant.
Study details: The findings are based on a prespecified interim analysis of 90 healthy adult participants in a phase 1, randomized, trial of DS-Cav1.
Disclosures: The research was funded by the National Institutes of Health and the Bill & Melinda Gates Foundation. Several authors are inventors on patents for stabilizing the RSV F protein.
Source: Crank MC et al. Science. 2019;365(6452):505-9.
Hospital slashes S. aureus vancomycin resistance
LJUBLJANA, SLOVENIA – , Johannes Huebner, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
He presented a retrospective analysis of S. aureus isolates obtained from 540 patients at the Dr. von Hauner Children’s Hospital, Munich, from 2002 to 2017. All were either newly identified methicillin-resistant S. aureus (MRSA) or specimens from bacteremic children with invasive MRSA or methicillin-sensitive S. aureus (MSSA). The strains were tested for vancomycin resistance and minimum inhibitory concentration (MIC). The results from the 200 isolates obtained from 2002 to 2009 were then compared to the 340 specimens from 2010 to 2017, when antibiotic stewardship programs rose to the fore at the pediatric hospital.
All samples proved to be vancomycin sensitive. The further good news was there was absolutely no evidence of the worrisome vancomycin MIC creep that has been described at some centers. On the contrary, the MIC was significantly lower in the later samples, at 0.99 mcg/mL, compared with 1.11 mcg/mL in the earlier period. Moreover, the prevalence of heterogeneous glycopeptide-intermediate S. aureus (hGISA) – a phenotype that has been associated with increased rates of treatment failure – improved from 25% in the earlier period to 6% during the later period, reported Dr. Huebner, head of the division of pediatric infectious diseases at the children’s hospital, part of the University of Munich.
Vancomycin MICs weren’t significantly different between the MRSA and MSSA samples.
Based upon this favorable institutional experience, vancomycin remains the first-line treatment for suspected severe gram-positive cocci infections as well as proven infections involving MRSA at Dr. von Hauner Children’s Hospital.
These vancomycin MIC and hGISA data underscore the importance of periodically monitoring local S. aureus antimicrobial susceptibilities, which, as in this case, can differ from the broader global trends. The vancomycin MIC creep issue hadn’t been studied previously in German hospitals, according to Dr. Huebner.
He and his coworkers have published details of the elements of pediatric antibiotic stewardship programs they have found to be most effective (Infection. 2017 Aug;45[4]:493-504) as well as a systematic review of studies on the favorable economic impact of such programs (J Hosp Infect. 2019 Aug;102[4]:369-376).
Dr. Huebner reported having no financial conflicts regarding his study, which was conducted free of commercial support.
LJUBLJANA, SLOVENIA – , Johannes Huebner, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
He presented a retrospective analysis of S. aureus isolates obtained from 540 patients at the Dr. von Hauner Children’s Hospital, Munich, from 2002 to 2017. All were either newly identified methicillin-resistant S. aureus (MRSA) or specimens from bacteremic children with invasive MRSA or methicillin-sensitive S. aureus (MSSA). The strains were tested for vancomycin resistance and minimum inhibitory concentration (MIC). The results from the 200 isolates obtained from 2002 to 2009 were then compared to the 340 specimens from 2010 to 2017, when antibiotic stewardship programs rose to the fore at the pediatric hospital.
All samples proved to be vancomycin sensitive. The further good news was there was absolutely no evidence of the worrisome vancomycin MIC creep that has been described at some centers. On the contrary, the MIC was significantly lower in the later samples, at 0.99 mcg/mL, compared with 1.11 mcg/mL in the earlier period. Moreover, the prevalence of heterogeneous glycopeptide-intermediate S. aureus (hGISA) – a phenotype that has been associated with increased rates of treatment failure – improved from 25% in the earlier period to 6% during the later period, reported Dr. Huebner, head of the division of pediatric infectious diseases at the children’s hospital, part of the University of Munich.
Vancomycin MICs weren’t significantly different between the MRSA and MSSA samples.
Based upon this favorable institutional experience, vancomycin remains the first-line treatment for suspected severe gram-positive cocci infections as well as proven infections involving MRSA at Dr. von Hauner Children’s Hospital.
These vancomycin MIC and hGISA data underscore the importance of periodically monitoring local S. aureus antimicrobial susceptibilities, which, as in this case, can differ from the broader global trends. The vancomycin MIC creep issue hadn’t been studied previously in German hospitals, according to Dr. Huebner.
He and his coworkers have published details of the elements of pediatric antibiotic stewardship programs they have found to be most effective (Infection. 2017 Aug;45[4]:493-504) as well as a systematic review of studies on the favorable economic impact of such programs (J Hosp Infect. 2019 Aug;102[4]:369-376).
Dr. Huebner reported having no financial conflicts regarding his study, which was conducted free of commercial support.
LJUBLJANA, SLOVENIA – , Johannes Huebner, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
He presented a retrospective analysis of S. aureus isolates obtained from 540 patients at the Dr. von Hauner Children’s Hospital, Munich, from 2002 to 2017. All were either newly identified methicillin-resistant S. aureus (MRSA) or specimens from bacteremic children with invasive MRSA or methicillin-sensitive S. aureus (MSSA). The strains were tested for vancomycin resistance and minimum inhibitory concentration (MIC). The results from the 200 isolates obtained from 2002 to 2009 were then compared to the 340 specimens from 2010 to 2017, when antibiotic stewardship programs rose to the fore at the pediatric hospital.
All samples proved to be vancomycin sensitive. The further good news was there was absolutely no evidence of the worrisome vancomycin MIC creep that has been described at some centers. On the contrary, the MIC was significantly lower in the later samples, at 0.99 mcg/mL, compared with 1.11 mcg/mL in the earlier period. Moreover, the prevalence of heterogeneous glycopeptide-intermediate S. aureus (hGISA) – a phenotype that has been associated with increased rates of treatment failure – improved from 25% in the earlier period to 6% during the later period, reported Dr. Huebner, head of the division of pediatric infectious diseases at the children’s hospital, part of the University of Munich.
Vancomycin MICs weren’t significantly different between the MRSA and MSSA samples.
Based upon this favorable institutional experience, vancomycin remains the first-line treatment for suspected severe gram-positive cocci infections as well as proven infections involving MRSA at Dr. von Hauner Children’s Hospital.
These vancomycin MIC and hGISA data underscore the importance of periodically monitoring local S. aureus antimicrobial susceptibilities, which, as in this case, can differ from the broader global trends. The vancomycin MIC creep issue hadn’t been studied previously in German hospitals, according to Dr. Huebner.
He and his coworkers have published details of the elements of pediatric antibiotic stewardship programs they have found to be most effective (Infection. 2017 Aug;45[4]:493-504) as well as a systematic review of studies on the favorable economic impact of such programs (J Hosp Infect. 2019 Aug;102[4]:369-376).
Dr. Huebner reported having no financial conflicts regarding his study, which was conducted free of commercial support.
REPORTING FROM ESPID 2019
Key clinical point: Staphylococcus aureus vancomycin MIC creep is reversible through dedicated antimicrobial stewardship.
Major finding: The prevalence of hGISA in MRSA and MSSA specimens improved from 25% during 2002-2009 to 6% during 2010-2017 at one German tertiary children’s hospital.
Study details: This was a retrospective single-center analysis of vancomycin resistance trends over time in 540 S. aureus specimens gathered in 2002-2017.
Disclosures: The presenter reported having no financial conflicts regarding this study, which was conducted free of commercial support.
mRNA technology for respiratory vaccines impresses
LJUBLJANA, SLOVENIA – Encouraging safety and immunogenicity results reported from phase 1 studies of the first mRNA vaccines against the potentially pandemic H10N8 avian influenza and H7N9 influenza viruses suggest a bright future for what appears to be a breakthrough technology in vaccine development.
“We have developed an mRNA platform that has the potential to be quite applicable to the vaccine space. It’s an agile platform with the potential for relatively rapid development of vaccine antigen without the use of dedicated facilities, or growth in eggs, or insects, or mammalian cells,” Lori Panther, MD, said at the annual meeting of the European Society for Paediatric Infectious Diseases.
“We now have a platform that is relatively plug and play. If one has the mRNA sequence that you’re after to produce the protein that you’re after, it is a relatively repetitive process somewhat irrespective of the goal of the protein that you’re going to manufacture. We’re introducing an mRNA into our cellular machinery – the destination is the cellular ribosome – where it hopefully is able to be translated with fidelity into the target protein. Essentially it’s like the biological equivalent of a software hack for our own cells,” explained Dr. Panther, who is director of clinical development for infectious diseases at Moderna, in Cambridge, Mass.
Indeed, Moderna has numerous ongoing or recently completed phase 1 clinical trials of mRNA vaccines developed to protect against a raft of viral infections: respiratory syncytial virus, cytomegalovirus (NCT03382405), zika, chikungunya (NCT03829384), human metapneumovirus, and parainfluenza virus 3, as well as the aforementioned H10N8 and H7N9 influenza viruses. And an mRNA varicella zoster virus vaccine is in preclinical studies.
The mRNA vaccines closely mimic native viral infections, eliciting both B- and T-cell responses.
Moreover, the company also has ongoing phase 1 studies of mRNA-based cancer vaccines – therapies targeting solid tumors and lymphomas – as well as mRNA-directed increased production of relaxin as a treatment for heart failure and of vascular endothelial growth factor to treat myocardial ischemia.
“For the purposes of my company, the desired protein at this juncture could be an antibody, it could be a tumor antigen, it could be an enzyme that will replace an enzyme that’s lacking in somebody with an inborn error of metabolism. Or it could be a vaccine antigen target,” Dr. Panther said.
In addition to highlighting the results of the two phase 1 proof-of-concept studies of mRNA vaccines targeting the feared H10N8 and H7N9 influenza viruses, she presented interim results of an ongoing 1-year study of an mRNA vaccine that contains two antigens simultaneously targeting human metapneumovirus (hMPV) and parainfluenza virus 3 (PIV3).
“The rationale behind this study is that, taken together, these are two viruses that are responsible for a fair bit of disease burden in terms of lower respiratory tract infections and hospitalizations in children [younger] than 12 months of age, which will be the target population,” the infectious disease specialist noted.
The early positive results of the mRNA influenza vaccine studies were of particular interest to her audience of pediatric infectious disease specialists. Since the first human H7N9 infections were reported in China in 2013, five outbreaks have occurred involving more than 1,500 documented infections, resulting in more than 600 deaths. And ever since the virulent H10N8 avian influenza virus popped up on the radar in 2013, infectious disease physicians the world over have been waiting for the other shoe to drop.
There is obvious appeal to a novel, precise, and rapidly scalable technology such as that promised by intracellular delivery of mRNA in order to ramp up high-volume production of effective vaccines in the face of a looming pandemic threat. Elsewhere at the meeting, it was noted that, during the H1N1 pandemic of 2009, it took 6 months for the first vaccine doses to become available using current antiquated egg-based production methods. Another 2 months elapsed before the necessary millions of doses were produced.
The details of the two phase 1 studies of the mRNA vaccines against H7N9 and H10N8 influenza have recently been published (Vaccine. 2019 May 31;37[25]:3326-34). The vaccines, delivered in the conventional manner via injection into the deltoid muscle, were well tolerated, with the most common adverse events being the familiar ones: injection site pain, erythema, headache, fatigue, and myalgia. The immune response was robust and durable.
In response to an audience question, Dr. Panther said the mRNA vaccines are amenable to development as intranasal formulations.
The ongoing 12-month, phase 1, dose-ranging study of the mRNA hMPV/PIV3 virus vaccine includes 124 healthy adults at three U.S. sites who received two vaccinations on days 1 and 28. One month after a single vaccination, hMPV neutralizing antibody titers were 6.2-6.4 times those in the placebo arm; PIV3 neutralization titers were increased 3.3-fold. The second injection didn’t further boost antibody titers, suggesting that, at least in this study population of preexposed adults, a single vaccination is sufficient.
The use of mRNA technology has been a long time in coming. Dr. Panther explained why: “It’s a big trick to take an mRNA that by its own nature is a pretty fragile molecule and to get it past the degrading enzymes, like RNAses, that are out to chew it up immediately, and then to sneak it across the cellular membrane and into the cytoplasm, all the while avoiding the innate immune responses that exist solely to recognize RNA that looks foreign and chew it up.”
Moderna has accomplished this using a proprietary lipid nanoparticle delivery system.
“Essentially it’s a lipid shield that surrounds the mRNAs and ushers them past those enzymes and past the innate immune response that would otherwise destroy them,” according to Dr. Panther.
She and her colleagues believe they may eventually be able to change the nucleotide sequence of their manufactured mRNAs in order to expand the immunogenicity epitope and achieve a stronger immune response than would result from natural infection.
LJUBLJANA, SLOVENIA – Encouraging safety and immunogenicity results reported from phase 1 studies of the first mRNA vaccines against the potentially pandemic H10N8 avian influenza and H7N9 influenza viruses suggest a bright future for what appears to be a breakthrough technology in vaccine development.
“We have developed an mRNA platform that has the potential to be quite applicable to the vaccine space. It’s an agile platform with the potential for relatively rapid development of vaccine antigen without the use of dedicated facilities, or growth in eggs, or insects, or mammalian cells,” Lori Panther, MD, said at the annual meeting of the European Society for Paediatric Infectious Diseases.
“We now have a platform that is relatively plug and play. If one has the mRNA sequence that you’re after to produce the protein that you’re after, it is a relatively repetitive process somewhat irrespective of the goal of the protein that you’re going to manufacture. We’re introducing an mRNA into our cellular machinery – the destination is the cellular ribosome – where it hopefully is able to be translated with fidelity into the target protein. Essentially it’s like the biological equivalent of a software hack for our own cells,” explained Dr. Panther, who is director of clinical development for infectious diseases at Moderna, in Cambridge, Mass.
Indeed, Moderna has numerous ongoing or recently completed phase 1 clinical trials of mRNA vaccines developed to protect against a raft of viral infections: respiratory syncytial virus, cytomegalovirus (NCT03382405), zika, chikungunya (NCT03829384), human metapneumovirus, and parainfluenza virus 3, as well as the aforementioned H10N8 and H7N9 influenza viruses. And an mRNA varicella zoster virus vaccine is in preclinical studies.
The mRNA vaccines closely mimic native viral infections, eliciting both B- and T-cell responses.
Moreover, the company also has ongoing phase 1 studies of mRNA-based cancer vaccines – therapies targeting solid tumors and lymphomas – as well as mRNA-directed increased production of relaxin as a treatment for heart failure and of vascular endothelial growth factor to treat myocardial ischemia.
“For the purposes of my company, the desired protein at this juncture could be an antibody, it could be a tumor antigen, it could be an enzyme that will replace an enzyme that’s lacking in somebody with an inborn error of metabolism. Or it could be a vaccine antigen target,” Dr. Panther said.
In addition to highlighting the results of the two phase 1 proof-of-concept studies of mRNA vaccines targeting the feared H10N8 and H7N9 influenza viruses, she presented interim results of an ongoing 1-year study of an mRNA vaccine that contains two antigens simultaneously targeting human metapneumovirus (hMPV) and parainfluenza virus 3 (PIV3).
“The rationale behind this study is that, taken together, these are two viruses that are responsible for a fair bit of disease burden in terms of lower respiratory tract infections and hospitalizations in children [younger] than 12 months of age, which will be the target population,” the infectious disease specialist noted.
The early positive results of the mRNA influenza vaccine studies were of particular interest to her audience of pediatric infectious disease specialists. Since the first human H7N9 infections were reported in China in 2013, five outbreaks have occurred involving more than 1,500 documented infections, resulting in more than 600 deaths. And ever since the virulent H10N8 avian influenza virus popped up on the radar in 2013, infectious disease physicians the world over have been waiting for the other shoe to drop.
There is obvious appeal to a novel, precise, and rapidly scalable technology such as that promised by intracellular delivery of mRNA in order to ramp up high-volume production of effective vaccines in the face of a looming pandemic threat. Elsewhere at the meeting, it was noted that, during the H1N1 pandemic of 2009, it took 6 months for the first vaccine doses to become available using current antiquated egg-based production methods. Another 2 months elapsed before the necessary millions of doses were produced.
The details of the two phase 1 studies of the mRNA vaccines against H7N9 and H10N8 influenza have recently been published (Vaccine. 2019 May 31;37[25]:3326-34). The vaccines, delivered in the conventional manner via injection into the deltoid muscle, were well tolerated, with the most common adverse events being the familiar ones: injection site pain, erythema, headache, fatigue, and myalgia. The immune response was robust and durable.
In response to an audience question, Dr. Panther said the mRNA vaccines are amenable to development as intranasal formulations.
The ongoing 12-month, phase 1, dose-ranging study of the mRNA hMPV/PIV3 virus vaccine includes 124 healthy adults at three U.S. sites who received two vaccinations on days 1 and 28. One month after a single vaccination, hMPV neutralizing antibody titers were 6.2-6.4 times those in the placebo arm; PIV3 neutralization titers were increased 3.3-fold. The second injection didn’t further boost antibody titers, suggesting that, at least in this study population of preexposed adults, a single vaccination is sufficient.
The use of mRNA technology has been a long time in coming. Dr. Panther explained why: “It’s a big trick to take an mRNA that by its own nature is a pretty fragile molecule and to get it past the degrading enzymes, like RNAses, that are out to chew it up immediately, and then to sneak it across the cellular membrane and into the cytoplasm, all the while avoiding the innate immune responses that exist solely to recognize RNA that looks foreign and chew it up.”
Moderna has accomplished this using a proprietary lipid nanoparticle delivery system.
“Essentially it’s a lipid shield that surrounds the mRNAs and ushers them past those enzymes and past the innate immune response that would otherwise destroy them,” according to Dr. Panther.
She and her colleagues believe they may eventually be able to change the nucleotide sequence of their manufactured mRNAs in order to expand the immunogenicity epitope and achieve a stronger immune response than would result from natural infection.
LJUBLJANA, SLOVENIA – Encouraging safety and immunogenicity results reported from phase 1 studies of the first mRNA vaccines against the potentially pandemic H10N8 avian influenza and H7N9 influenza viruses suggest a bright future for what appears to be a breakthrough technology in vaccine development.
“We have developed an mRNA platform that has the potential to be quite applicable to the vaccine space. It’s an agile platform with the potential for relatively rapid development of vaccine antigen without the use of dedicated facilities, or growth in eggs, or insects, or mammalian cells,” Lori Panther, MD, said at the annual meeting of the European Society for Paediatric Infectious Diseases.
“We now have a platform that is relatively plug and play. If one has the mRNA sequence that you’re after to produce the protein that you’re after, it is a relatively repetitive process somewhat irrespective of the goal of the protein that you’re going to manufacture. We’re introducing an mRNA into our cellular machinery – the destination is the cellular ribosome – where it hopefully is able to be translated with fidelity into the target protein. Essentially it’s like the biological equivalent of a software hack for our own cells,” explained Dr. Panther, who is director of clinical development for infectious diseases at Moderna, in Cambridge, Mass.
Indeed, Moderna has numerous ongoing or recently completed phase 1 clinical trials of mRNA vaccines developed to protect against a raft of viral infections: respiratory syncytial virus, cytomegalovirus (NCT03382405), zika, chikungunya (NCT03829384), human metapneumovirus, and parainfluenza virus 3, as well as the aforementioned H10N8 and H7N9 influenza viruses. And an mRNA varicella zoster virus vaccine is in preclinical studies.
The mRNA vaccines closely mimic native viral infections, eliciting both B- and T-cell responses.
Moreover, the company also has ongoing phase 1 studies of mRNA-based cancer vaccines – therapies targeting solid tumors and lymphomas – as well as mRNA-directed increased production of relaxin as a treatment for heart failure and of vascular endothelial growth factor to treat myocardial ischemia.
“For the purposes of my company, the desired protein at this juncture could be an antibody, it could be a tumor antigen, it could be an enzyme that will replace an enzyme that’s lacking in somebody with an inborn error of metabolism. Or it could be a vaccine antigen target,” Dr. Panther said.
In addition to highlighting the results of the two phase 1 proof-of-concept studies of mRNA vaccines targeting the feared H10N8 and H7N9 influenza viruses, she presented interim results of an ongoing 1-year study of an mRNA vaccine that contains two antigens simultaneously targeting human metapneumovirus (hMPV) and parainfluenza virus 3 (PIV3).
“The rationale behind this study is that, taken together, these are two viruses that are responsible for a fair bit of disease burden in terms of lower respiratory tract infections and hospitalizations in children [younger] than 12 months of age, which will be the target population,” the infectious disease specialist noted.
The early positive results of the mRNA influenza vaccine studies were of particular interest to her audience of pediatric infectious disease specialists. Since the first human H7N9 infections were reported in China in 2013, five outbreaks have occurred involving more than 1,500 documented infections, resulting in more than 600 deaths. And ever since the virulent H10N8 avian influenza virus popped up on the radar in 2013, infectious disease physicians the world over have been waiting for the other shoe to drop.
There is obvious appeal to a novel, precise, and rapidly scalable technology such as that promised by intracellular delivery of mRNA in order to ramp up high-volume production of effective vaccines in the face of a looming pandemic threat. Elsewhere at the meeting, it was noted that, during the H1N1 pandemic of 2009, it took 6 months for the first vaccine doses to become available using current antiquated egg-based production methods. Another 2 months elapsed before the necessary millions of doses were produced.
The details of the two phase 1 studies of the mRNA vaccines against H7N9 and H10N8 influenza have recently been published (Vaccine. 2019 May 31;37[25]:3326-34). The vaccines, delivered in the conventional manner via injection into the deltoid muscle, were well tolerated, with the most common adverse events being the familiar ones: injection site pain, erythema, headache, fatigue, and myalgia. The immune response was robust and durable.
In response to an audience question, Dr. Panther said the mRNA vaccines are amenable to development as intranasal formulations.
The ongoing 12-month, phase 1, dose-ranging study of the mRNA hMPV/PIV3 virus vaccine includes 124 healthy adults at three U.S. sites who received two vaccinations on days 1 and 28. One month after a single vaccination, hMPV neutralizing antibody titers were 6.2-6.4 times those in the placebo arm; PIV3 neutralization titers were increased 3.3-fold. The second injection didn’t further boost antibody titers, suggesting that, at least in this study population of preexposed adults, a single vaccination is sufficient.
The use of mRNA technology has been a long time in coming. Dr. Panther explained why: “It’s a big trick to take an mRNA that by its own nature is a pretty fragile molecule and to get it past the degrading enzymes, like RNAses, that are out to chew it up immediately, and then to sneak it across the cellular membrane and into the cytoplasm, all the while avoiding the innate immune responses that exist solely to recognize RNA that looks foreign and chew it up.”
Moderna has accomplished this using a proprietary lipid nanoparticle delivery system.
“Essentially it’s a lipid shield that surrounds the mRNAs and ushers them past those enzymes and past the innate immune response that would otherwise destroy them,” according to Dr. Panther.
She and her colleagues believe they may eventually be able to change the nucleotide sequence of their manufactured mRNAs in order to expand the immunogenicity epitope and achieve a stronger immune response than would result from natural infection.
REPORTING FROM ESPID 2019