User login
Effect of time of admission to treatment initiation on outcomes of patients with acute myeloid leukemia: a tertiary care referral center experience
Acute myeloid leukemia (AML) is the most common acute leukemia in adults in the United States.1 In 2018, the estimated annual incidence of AML is 19,520 (32.4% of all new leukemia cases), with 10,670 projected deaths (43.8% of all leukemia deaths).1 New molecularly targeted treatments are increasingly being used in treating AML, and some of them have shown improved health outcomes. In general, age, white blood cell (WBC) count at presentation, cytogenetics, and molecular characteristics are the major determinants of prognosis and treatment outcome. Studies analyzing the Surveillance Epidemiology and End Results database have also shown racial differences in outcomes.2 It is well known to the oncology community that patients with similar characteristics may respond differently to treatment and that outcome is not uniformly related to the well-defined clinical and laboratory characteristics. Issues related to health care disparities and access to health care are also known to affect the outcome in patients with cancer.3-9
AML is generally considered by the medical community as a time-sensitive condition. Treatment of patients with AML usually consists of induction chemotherapy followed by consolidation treatment with consideration for stem cell transplant. The duration of time from admission to treatment (TAT) of AML with induction chemotherapy is dependent on multiple factors. These may include the assessment of comorbid conditions and the availability of molecular studies at the time of treatment, which can be time consuming. The effect of treatment delays after AML diagnosis has been investigated, but with conflicting results. One study showed that time from diagnosis to treatment initiation affects survival in younger patients, and another showed it has no effect on survival regardless of patient age.10,11 We describe here the results of a retrospective analysis evaluating the impact of TAT and day of admission on outcomes of patients with AML who received treatment at a tertiary care referral center.
Methods and materials
We did a retrospective medical record review of all newly diagnosed AML patients at the Oklahoma University Health Sciences Center (OUHSC). Our sample was composed of 154 adult patients. Our inclusion criteria were an age of 18 years or older with complete insurance data, a diagnosis of AML, and having received treatment at our institution from January 2000 through June 2015. Data were obtained on laboratory values at diagnosis, pathology data including cytogenetics, molecular data, and bone marrow biopsies. Data on patient characteristics such as age, race and/or ethnicity, and comorbidities were obtained from the electronic medical records. Treatment data on type and dose of chemotherapy during induction, subsequent treatment phases, and number of treatments to achieve complete response (CR) as well as response data of CR achievement, relapse, date of CR, date of relapse, stem cell transplantation data, date of death, and date of last follow-up visit were recorded retrospectively from the electronic medical record. The study was approved by the OUHSC Institutional Review Board.
Statistical analysis
TAT was analyzed categorically (0-4 days vs >4 days), and day of admission was analyzed categorically (Monday to Thursday vs Friday to Sunday). Descriptive statistics were calculated overall and by TAT group. The chi-square test was used to compare the association between our covariates and TAT. Kaplan-Meier estimates (with a log-rank test) were used to assess the unadjusted effect of TAT with overall survival (OS) and event-free survival (EFS). Median OS and EFS and 95% confidence intervals (CIs) were also calculated. We used the Cox proportional hazards regression modeling to evaluate the relationship between OS and TAT. The initial model was built by including covariates, with P < .25 for the association between the covariates with OS. TAT was maintained in the final model because it was the primary variable of interest, whereas age and risk group were also included in the final model because those covariates are known prognostic risk factors in AML. Among the set of variables screened in, all 2-way interactions were assessed using P < .05. No significant interactions were found. Backward elimination was then performed. During the backward elimination, confounding was deemed to have been present if the measure of association of significant variables in the model changed by more than 20% and the P-value of the confounding variable was less than .30. Variables with P-values of less than .05 or deemed a confounder would then be retained. A similar modeling approach was used to examine EFS and CR. To evaluate the association between CR with potential predictors, binary logistic regression was used, whereby day of admission and time to treatment were explored unadjusted and then adjusted for age, WBC count, risk group, and undergoing allogeneic stem cell transplant (AlloSCT). SAS version 9.4 (SAS Institute Inc, Cary, North Carolina) was used for all analyses. A final alpha of 0.05 was used unless otherwise noted.
Results
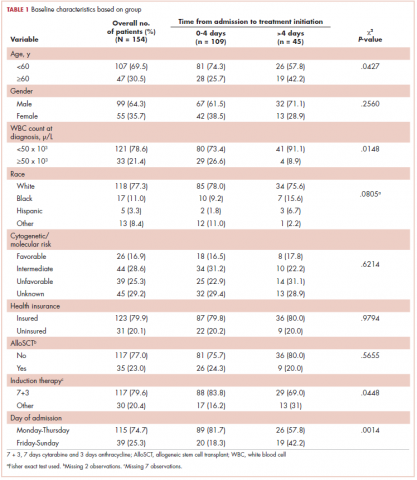
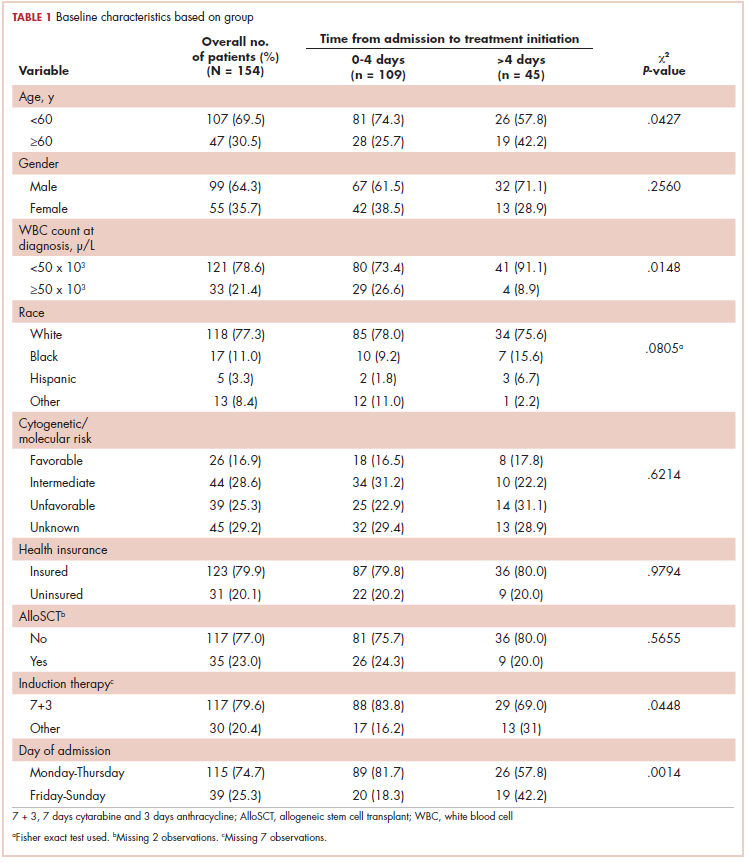
Baseline characteristics are presented in Table 1. Treatment was initiated within 4 days for 71% (109/154) of patients. Most patients in our study were younger than 60 years (70%), male (64%), and white (77%). Most patients were admitted to the hospital for treatment between Monday and Thursday (75%). A higher proportion of patients in the 0-4 days TAT group were <60 years of age compared with patients in the >4 days TAT group (P = .0427). A higher proportion of patients in the 0-4 days TAT group had a WBC count of ≥50 x 103 μ/L compared with patients in the >4 days TAT group (27% vs 9%, respectively; P = .0148). A higher proportion of patients were admitted Friday to Sunday in the TAT >4 days group. Insured and uninsured patients were equally distributed between the 2 groups (P = .0014). Cytogenetic and/or molecular risk was not statistically different between the 0-4 days and >4 days TAT groups (unfavorable risk, 25% vs 23%, respectively; P = .6214). A higher proportion of patients received 7 + 3 induction chemotherapy (7 days cytarabine and 3 days anthracycline) in the TAT 0-4 days group compared with the >4 days TAT group (84% vs 69%, respectively; P = .0448). The most common intensive chemotherapy regimen used was 7 + 3 (80%). The rest of the patients (20%) received high-dose cytarabine clofarabine-based chemotherapy, hypomethylating agents, or other treatments. The proportion of patients who received an AlloSCT did not differ between the 0-4 days and >4 days TAT groups (24% vs 20%, respectively; P = .5655).
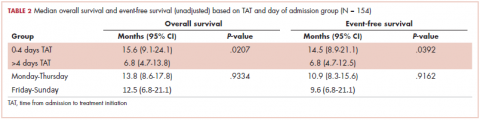
The median OS for all patients was 10.9 months (95% CI, 8.3-15.1), and the median EFS was 9.1 months (95% CI, 7.4-13.8). Median follow-up time was 8.6 months (95% CI, 6.7-11). We found a significant association between TAT and both OS and EFS without any adjustment (Table 2).
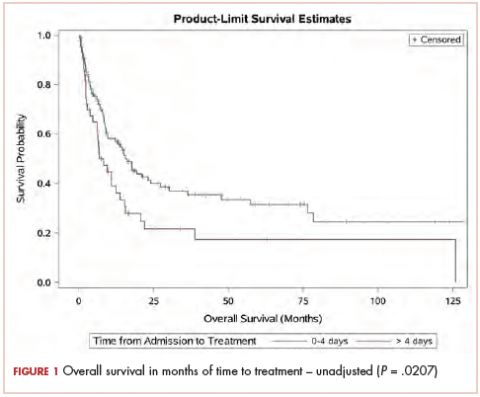
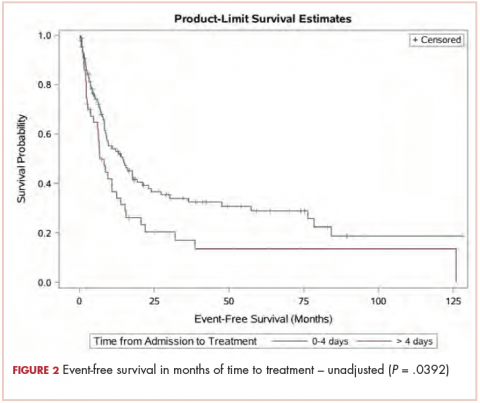
The median OS for the TAT 0-4 days group was 15.6 months, and for the TAT >4 days group, it was 6.8 months (P = .0207; Figure 1). The median EFS for the TAT 0-4 days group was 14.5 months, and for the TAT >4 days group, it was 6.8 months (P = .0240; Figure 2).
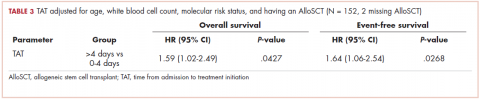
We found no association between the day of admission to hospital (Monday-Thursday vs Friday-Sunday) and either OS or EFS. After adjusting for age, WBC count, molecular risk status, and undergoing AlloSCT, the OS was shorter for those who received treatment >4 days after admission compared with those who received treatment within 0 to 4 days, with a hazard ratio (HR) of 1.59 (95% CI, 1.02-2.49; P = .0427; Table 3).
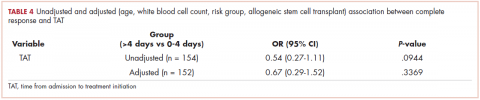
There was no association between day of admission with OS in the multivariable analysis. Similarly, after adjusting for age, WBC count, molecular risk status, and undergoing AlloSCT, EFS was shorter in patients who received treatment >4 days after admission compared with those who received treatment within 0 to 4 days (HR, 1.64; 95% CI, 1.06-2.54; P = .0268). There was no association between day of admission with EFS in the multivariable model. Although there was a trend for a higher CR rate with earlier treatment, this was not statistically significant (Table 4).
Discussion
Treatment outcomes for patients with AML are known to be affected by several patient- and disease-related factors. Patient-related factors can include age, performance status, comorbidities, and availability of a stem cell donor. Examples of disease-related factors include molecular alterations and site of disease involvement. Little is known about whether the timing of treatment initiation affects patient outcomes. Short-term treatment delays after the diagnosis of leukemia are not uncommon. Generally, patients are treated with anthracycline-based induction chemotherapy, but the response rate and survival are particularly poor in the older age group.12 Moreover, increasing comorbidities with aging are expected to lead to lower treatment tolerability.13 Therefore, elderly patients are particularly prone to treatment delays while providers await the results of the molecular studies to guide the use of less intensive targeted therapies.10 Other reasons for treatment delays may also include transfers between hospitals, suspected or documented infections, and evaluation of chronic illnesses. Our analysis also indicates that admission to the hospital on the weekend contributes to a delay in therapy compared with admission on a weekday.
We found a decreased OS and EFS in patients who received treatment >4 days after admission to the hospital compared with patients who received treatment within 0 to 4 days of admission. This association was statistically significant in a bivariate analysis as well as in a multivariable analysis with adjustment for age, WBC count on presentation, molecular risk group, and undergoing AlloSCT. A previous large retrospective study showed that the time from diagnosis to treatment initiation predicts survival in younger, but not older, patients with AML.10 This remained true after adjusting for age, performance status, WBC count, and the type of AML in a multivariable analysis. In our study, the declines in overall survival and event-free survival were evident after a delay of more than 4 days.
Another retrospective study that included 599 newly diagnosed AML patients, with a median time from diagnosis to treatment of 8 days, did not show any impact of treatment delay on overall survival, early death, or response rate.11 These differences in the effect of treatment delay on outcomes could be related to the differences in baseline characteristics of patients in these studies. Our study had a higher proportion of patients younger than 60 years, for example. We hypothesize that treatment delays, especially in patients with a high WBC count on presentation, might lead to further organ compromise and poorer outcomes with chemotherapy.
In our study, a higher proportion of patients were admitted over the weekend in the >4 days TAT group, but when we analyzed the day of admission to hospital separately, it was not associated with OS or EFS. Admission over the weekend was also not associated with clinical outcomes including 30-day mortality in a larger study that included 422 patients treated at a large teaching referral hospital.14
Limitations of our study include a small sample size and a short median follow-up time. Most of our patients were young and white, which may not be representative of the general population.
In conclusion, we found that treatment delays are associated with inferior outcomes in AML patients. It remains to be elucidated whether the benefit gained from using targeted and less-intensive chemotherapy, especially in elderly patients, outweighs the potential harm from delaying treatment. Additional studies are needed to confirm our findings in different settings and patient populations.
Acknowledgment
Statistical support was provided by the Stephenson Cancer Center Biostatistics and Research Design Shared Resource.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30.
2. Patel MI, Ma Y, Mitchell B, Rhoads KF. How do differences in treatment impact racial and ethnic disparities in acute myeloid leukemia? Cancer Epidemiol Biomarkers Prev. 2015;24(2):344-349.
3. Weber JJ, Kachare SD, Vohra NA, Fitzgerald TF, Wong JH. Regional disparities in breast cancer outcomes and the process of care. Am Surg. 2014;80(7):669-674.
4. Shippee TP, Kozhimannil KB, Rowan K, Virnig BA. Health insurance coverage and racial disparities in breast reconstruction after mastectomy. Womens Health Issues. 2014;24(3):e261-e269.
5. Dickens C, Joffe M, Jacobson J, et al. Stage at breast cancer diagnosis and distance from diagnostic hospital in a periurban setting: a South African public hospital case series of over 1,000 women. Int J Cancer. 2014;135(9):2173-2182.
6. Nguyen-Pham S, Leung J, McLaughlin D. Disparities in breast cancer stage at diagnosis in urban and rural adult women: a systematic review and meta-analysis. Ann Epidemiol. 2014;24(3):228-235.
7. Gong G, Belasco E, Hargrave KA, Lyford CP, Philips BU Jr. Determinants of delayed detection of cancers in Texas counties in the United States of America. Int J Equity Health. 2012;11:29.
8. Erhunmwunsee L, Joshi MB, Conlon DH, Harpole DH Jr. Neighborhood‐level socioeconomic determinants impact outcomes in nonsmall cell lung cancer patients in the Southeastern United States. Cancer. 2012;118(20):5117-5123.
9. Steele CB, Pisu M, Richardson LC. Urban/rural patterns in receipt of treatment for non–small cell lung cancer among black and white Medicare beneficiaries, 2000-2003. J Natl Med Assoc. 2011;103(8):711-718.
10. Sekeres MA, Elson P, Kalaycio ME, et al. Time from diagnosis to treatment initiation predicts survival in younger, but not older, acute myeloid leukemia patients. Blood. 2009;113(1):28-36.
11. Bertoli S, Bérard E, Huguet F, et al. Time from diagnosis to intensive chemotherapy initiation does not adversely impact the outcome of patients with acute myeloid leukemia. Blood. 2013:121(14):2618-2626.
12. Shah A, Andersson TM, Rachet B, Björkholm M, Lambert PC. Survival and cure of acute myeloid leukaemia in England, 1971-2006: a population-based study. Br J Haematol. 2013;162(4):509-516.
13. Mohammadi M, Cao Y, Glimelius I, Bottai M, Eloranta S, Smedby KE. The impact of comorbid disease history on all-cause and cancer-specific mortality in myeloid leukemia and myeloma – a Swedish population-based study. BMC Cancer. 2015;15:850.
14. Bejanyan N, Fu AZ, Lazaryan A, et al. Impact of weekend admissions on quality of care and outcomes in patients with acute myeloid leukemia. Cancer. 2010;116(15):3614-3620.
Acute myeloid leukemia (AML) is the most common acute leukemia in adults in the United States.1 In 2018, the estimated annual incidence of AML is 19,520 (32.4% of all new leukemia cases), with 10,670 projected deaths (43.8% of all leukemia deaths).1 New molecularly targeted treatments are increasingly being used in treating AML, and some of them have shown improved health outcomes. In general, age, white blood cell (WBC) count at presentation, cytogenetics, and molecular characteristics are the major determinants of prognosis and treatment outcome. Studies analyzing the Surveillance Epidemiology and End Results database have also shown racial differences in outcomes.2 It is well known to the oncology community that patients with similar characteristics may respond differently to treatment and that outcome is not uniformly related to the well-defined clinical and laboratory characteristics. Issues related to health care disparities and access to health care are also known to affect the outcome in patients with cancer.3-9
AML is generally considered by the medical community as a time-sensitive condition. Treatment of patients with AML usually consists of induction chemotherapy followed by consolidation treatment with consideration for stem cell transplant. The duration of time from admission to treatment (TAT) of AML with induction chemotherapy is dependent on multiple factors. These may include the assessment of comorbid conditions and the availability of molecular studies at the time of treatment, which can be time consuming. The effect of treatment delays after AML diagnosis has been investigated, but with conflicting results. One study showed that time from diagnosis to treatment initiation affects survival in younger patients, and another showed it has no effect on survival regardless of patient age.10,11 We describe here the results of a retrospective analysis evaluating the impact of TAT and day of admission on outcomes of patients with AML who received treatment at a tertiary care referral center.
Methods and materials
We did a retrospective medical record review of all newly diagnosed AML patients at the Oklahoma University Health Sciences Center (OUHSC). Our sample was composed of 154 adult patients. Our inclusion criteria were an age of 18 years or older with complete insurance data, a diagnosis of AML, and having received treatment at our institution from January 2000 through June 2015. Data were obtained on laboratory values at diagnosis, pathology data including cytogenetics, molecular data, and bone marrow biopsies. Data on patient characteristics such as age, race and/or ethnicity, and comorbidities were obtained from the electronic medical records. Treatment data on type and dose of chemotherapy during induction, subsequent treatment phases, and number of treatments to achieve complete response (CR) as well as response data of CR achievement, relapse, date of CR, date of relapse, stem cell transplantation data, date of death, and date of last follow-up visit were recorded retrospectively from the electronic medical record. The study was approved by the OUHSC Institutional Review Board.
Statistical analysis
TAT was analyzed categorically (0-4 days vs >4 days), and day of admission was analyzed categorically (Monday to Thursday vs Friday to Sunday). Descriptive statistics were calculated overall and by TAT group. The chi-square test was used to compare the association between our covariates and TAT. Kaplan-Meier estimates (with a log-rank test) were used to assess the unadjusted effect of TAT with overall survival (OS) and event-free survival (EFS). Median OS and EFS and 95% confidence intervals (CIs) were also calculated. We used the Cox proportional hazards regression modeling to evaluate the relationship between OS and TAT. The initial model was built by including covariates, with P < .25 for the association between the covariates with OS. TAT was maintained in the final model because it was the primary variable of interest, whereas age and risk group were also included in the final model because those covariates are known prognostic risk factors in AML. Among the set of variables screened in, all 2-way interactions were assessed using P < .05. No significant interactions were found. Backward elimination was then performed. During the backward elimination, confounding was deemed to have been present if the measure of association of significant variables in the model changed by more than 20% and the P-value of the confounding variable was less than .30. Variables with P-values of less than .05 or deemed a confounder would then be retained. A similar modeling approach was used to examine EFS and CR. To evaluate the association between CR with potential predictors, binary logistic regression was used, whereby day of admission and time to treatment were explored unadjusted and then adjusted for age, WBC count, risk group, and undergoing allogeneic stem cell transplant (AlloSCT). SAS version 9.4 (SAS Institute Inc, Cary, North Carolina) was used for all analyses. A final alpha of 0.05 was used unless otherwise noted.
Results
Baseline characteristics are presented in Table 1. Treatment was initiated within 4 days for 71% (109/154) of patients. Most patients in our study were younger than 60 years (70%), male (64%), and white (77%). Most patients were admitted to the hospital for treatment between Monday and Thursday (75%). A higher proportion of patients in the 0-4 days TAT group were <60 years of age compared with patients in the >4 days TAT group (P = .0427). A higher proportion of patients in the 0-4 days TAT group had a WBC count of ≥50 x 103 μ/L compared with patients in the >4 days TAT group (27% vs 9%, respectively; P = .0148). A higher proportion of patients were admitted Friday to Sunday in the TAT >4 days group. Insured and uninsured patients were equally distributed between the 2 groups (P = .0014). Cytogenetic and/or molecular risk was not statistically different between the 0-4 days and >4 days TAT groups (unfavorable risk, 25% vs 23%, respectively; P = .6214). A higher proportion of patients received 7 + 3 induction chemotherapy (7 days cytarabine and 3 days anthracycline) in the TAT 0-4 days group compared with the >4 days TAT group (84% vs 69%, respectively; P = .0448). The most common intensive chemotherapy regimen used was 7 + 3 (80%). The rest of the patients (20%) received high-dose cytarabine clofarabine-based chemotherapy, hypomethylating agents, or other treatments. The proportion of patients who received an AlloSCT did not differ between the 0-4 days and >4 days TAT groups (24% vs 20%, respectively; P = .5655).
The median OS for all patients was 10.9 months (95% CI, 8.3-15.1), and the median EFS was 9.1 months (95% CI, 7.4-13.8). Median follow-up time was 8.6 months (95% CI, 6.7-11). We found a significant association between TAT and both OS and EFS without any adjustment (Table 2).
The median OS for the TAT 0-4 days group was 15.6 months, and for the TAT >4 days group, it was 6.8 months (P = .0207; Figure 1). The median EFS for the TAT 0-4 days group was 14.5 months, and for the TAT >4 days group, it was 6.8 months (P = .0240; Figure 2).
We found no association between the day of admission to hospital (Monday-Thursday vs Friday-Sunday) and either OS or EFS. After adjusting for age, WBC count, molecular risk status, and undergoing AlloSCT, the OS was shorter for those who received treatment >4 days after admission compared with those who received treatment within 0 to 4 days, with a hazard ratio (HR) of 1.59 (95% CI, 1.02-2.49; P = .0427; Table 3).
There was no association between day of admission with OS in the multivariable analysis. Similarly, after adjusting for age, WBC count, molecular risk status, and undergoing AlloSCT, EFS was shorter in patients who received treatment >4 days after admission compared with those who received treatment within 0 to 4 days (HR, 1.64; 95% CI, 1.06-2.54; P = .0268). There was no association between day of admission with EFS in the multivariable model. Although there was a trend for a higher CR rate with earlier treatment, this was not statistically significant (Table 4).
Discussion
Treatment outcomes for patients with AML are known to be affected by several patient- and disease-related factors. Patient-related factors can include age, performance status, comorbidities, and availability of a stem cell donor. Examples of disease-related factors include molecular alterations and site of disease involvement. Little is known about whether the timing of treatment initiation affects patient outcomes. Short-term treatment delays after the diagnosis of leukemia are not uncommon. Generally, patients are treated with anthracycline-based induction chemotherapy, but the response rate and survival are particularly poor in the older age group.12 Moreover, increasing comorbidities with aging are expected to lead to lower treatment tolerability.13 Therefore, elderly patients are particularly prone to treatment delays while providers await the results of the molecular studies to guide the use of less intensive targeted therapies.10 Other reasons for treatment delays may also include transfers between hospitals, suspected or documented infections, and evaluation of chronic illnesses. Our analysis also indicates that admission to the hospital on the weekend contributes to a delay in therapy compared with admission on a weekday.
We found a decreased OS and EFS in patients who received treatment >4 days after admission to the hospital compared with patients who received treatment within 0 to 4 days of admission. This association was statistically significant in a bivariate analysis as well as in a multivariable analysis with adjustment for age, WBC count on presentation, molecular risk group, and undergoing AlloSCT. A previous large retrospective study showed that the time from diagnosis to treatment initiation predicts survival in younger, but not older, patients with AML.10 This remained true after adjusting for age, performance status, WBC count, and the type of AML in a multivariable analysis. In our study, the declines in overall survival and event-free survival were evident after a delay of more than 4 days.
Another retrospective study that included 599 newly diagnosed AML patients, with a median time from diagnosis to treatment of 8 days, did not show any impact of treatment delay on overall survival, early death, or response rate.11 These differences in the effect of treatment delay on outcomes could be related to the differences in baseline characteristics of patients in these studies. Our study had a higher proportion of patients younger than 60 years, for example. We hypothesize that treatment delays, especially in patients with a high WBC count on presentation, might lead to further organ compromise and poorer outcomes with chemotherapy.
In our study, a higher proportion of patients were admitted over the weekend in the >4 days TAT group, but when we analyzed the day of admission to hospital separately, it was not associated with OS or EFS. Admission over the weekend was also not associated with clinical outcomes including 30-day mortality in a larger study that included 422 patients treated at a large teaching referral hospital.14
Limitations of our study include a small sample size and a short median follow-up time. Most of our patients were young and white, which may not be representative of the general population.
In conclusion, we found that treatment delays are associated with inferior outcomes in AML patients. It remains to be elucidated whether the benefit gained from using targeted and less-intensive chemotherapy, especially in elderly patients, outweighs the potential harm from delaying treatment. Additional studies are needed to confirm our findings in different settings and patient populations.
Acknowledgment
Statistical support was provided by the Stephenson Cancer Center Biostatistics and Research Design Shared Resource.
Acute myeloid leukemia (AML) is the most common acute leukemia in adults in the United States.1 In 2018, the estimated annual incidence of AML is 19,520 (32.4% of all new leukemia cases), with 10,670 projected deaths (43.8% of all leukemia deaths).1 New molecularly targeted treatments are increasingly being used in treating AML, and some of them have shown improved health outcomes. In general, age, white blood cell (WBC) count at presentation, cytogenetics, and molecular characteristics are the major determinants of prognosis and treatment outcome. Studies analyzing the Surveillance Epidemiology and End Results database have also shown racial differences in outcomes.2 It is well known to the oncology community that patients with similar characteristics may respond differently to treatment and that outcome is not uniformly related to the well-defined clinical and laboratory characteristics. Issues related to health care disparities and access to health care are also known to affect the outcome in patients with cancer.3-9
AML is generally considered by the medical community as a time-sensitive condition. Treatment of patients with AML usually consists of induction chemotherapy followed by consolidation treatment with consideration for stem cell transplant. The duration of time from admission to treatment (TAT) of AML with induction chemotherapy is dependent on multiple factors. These may include the assessment of comorbid conditions and the availability of molecular studies at the time of treatment, which can be time consuming. The effect of treatment delays after AML diagnosis has been investigated, but with conflicting results. One study showed that time from diagnosis to treatment initiation affects survival in younger patients, and another showed it has no effect on survival regardless of patient age.10,11 We describe here the results of a retrospective analysis evaluating the impact of TAT and day of admission on outcomes of patients with AML who received treatment at a tertiary care referral center.
Methods and materials
We did a retrospective medical record review of all newly diagnosed AML patients at the Oklahoma University Health Sciences Center (OUHSC). Our sample was composed of 154 adult patients. Our inclusion criteria were an age of 18 years or older with complete insurance data, a diagnosis of AML, and having received treatment at our institution from January 2000 through June 2015. Data were obtained on laboratory values at diagnosis, pathology data including cytogenetics, molecular data, and bone marrow biopsies. Data on patient characteristics such as age, race and/or ethnicity, and comorbidities were obtained from the electronic medical records. Treatment data on type and dose of chemotherapy during induction, subsequent treatment phases, and number of treatments to achieve complete response (CR) as well as response data of CR achievement, relapse, date of CR, date of relapse, stem cell transplantation data, date of death, and date of last follow-up visit were recorded retrospectively from the electronic medical record. The study was approved by the OUHSC Institutional Review Board.
Statistical analysis
TAT was analyzed categorically (0-4 days vs >4 days), and day of admission was analyzed categorically (Monday to Thursday vs Friday to Sunday). Descriptive statistics were calculated overall and by TAT group. The chi-square test was used to compare the association between our covariates and TAT. Kaplan-Meier estimates (with a log-rank test) were used to assess the unadjusted effect of TAT with overall survival (OS) and event-free survival (EFS). Median OS and EFS and 95% confidence intervals (CIs) were also calculated. We used the Cox proportional hazards regression modeling to evaluate the relationship between OS and TAT. The initial model was built by including covariates, with P < .25 for the association between the covariates with OS. TAT was maintained in the final model because it was the primary variable of interest, whereas age and risk group were also included in the final model because those covariates are known prognostic risk factors in AML. Among the set of variables screened in, all 2-way interactions were assessed using P < .05. No significant interactions were found. Backward elimination was then performed. During the backward elimination, confounding was deemed to have been present if the measure of association of significant variables in the model changed by more than 20% and the P-value of the confounding variable was less than .30. Variables with P-values of less than .05 or deemed a confounder would then be retained. A similar modeling approach was used to examine EFS and CR. To evaluate the association between CR with potential predictors, binary logistic regression was used, whereby day of admission and time to treatment were explored unadjusted and then adjusted for age, WBC count, risk group, and undergoing allogeneic stem cell transplant (AlloSCT). SAS version 9.4 (SAS Institute Inc, Cary, North Carolina) was used for all analyses. A final alpha of 0.05 was used unless otherwise noted.
Results
Baseline characteristics are presented in Table 1. Treatment was initiated within 4 days for 71% (109/154) of patients. Most patients in our study were younger than 60 years (70%), male (64%), and white (77%). Most patients were admitted to the hospital for treatment between Monday and Thursday (75%). A higher proportion of patients in the 0-4 days TAT group were <60 years of age compared with patients in the >4 days TAT group (P = .0427). A higher proportion of patients in the 0-4 days TAT group had a WBC count of ≥50 x 103 μ/L compared with patients in the >4 days TAT group (27% vs 9%, respectively; P = .0148). A higher proportion of patients were admitted Friday to Sunday in the TAT >4 days group. Insured and uninsured patients were equally distributed between the 2 groups (P = .0014). Cytogenetic and/or molecular risk was not statistically different between the 0-4 days and >4 days TAT groups (unfavorable risk, 25% vs 23%, respectively; P = .6214). A higher proportion of patients received 7 + 3 induction chemotherapy (7 days cytarabine and 3 days anthracycline) in the TAT 0-4 days group compared with the >4 days TAT group (84% vs 69%, respectively; P = .0448). The most common intensive chemotherapy regimen used was 7 + 3 (80%). The rest of the patients (20%) received high-dose cytarabine clofarabine-based chemotherapy, hypomethylating agents, or other treatments. The proportion of patients who received an AlloSCT did not differ between the 0-4 days and >4 days TAT groups (24% vs 20%, respectively; P = .5655).
The median OS for all patients was 10.9 months (95% CI, 8.3-15.1), and the median EFS was 9.1 months (95% CI, 7.4-13.8). Median follow-up time was 8.6 months (95% CI, 6.7-11). We found a significant association between TAT and both OS and EFS without any adjustment (Table 2).
The median OS for the TAT 0-4 days group was 15.6 months, and for the TAT >4 days group, it was 6.8 months (P = .0207; Figure 1). The median EFS for the TAT 0-4 days group was 14.5 months, and for the TAT >4 days group, it was 6.8 months (P = .0240; Figure 2).
We found no association between the day of admission to hospital (Monday-Thursday vs Friday-Sunday) and either OS or EFS. After adjusting for age, WBC count, molecular risk status, and undergoing AlloSCT, the OS was shorter for those who received treatment >4 days after admission compared with those who received treatment within 0 to 4 days, with a hazard ratio (HR) of 1.59 (95% CI, 1.02-2.49; P = .0427; Table 3).
There was no association between day of admission with OS in the multivariable analysis. Similarly, after adjusting for age, WBC count, molecular risk status, and undergoing AlloSCT, EFS was shorter in patients who received treatment >4 days after admission compared with those who received treatment within 0 to 4 days (HR, 1.64; 95% CI, 1.06-2.54; P = .0268). There was no association between day of admission with EFS in the multivariable model. Although there was a trend for a higher CR rate with earlier treatment, this was not statistically significant (Table 4).
Discussion
Treatment outcomes for patients with AML are known to be affected by several patient- and disease-related factors. Patient-related factors can include age, performance status, comorbidities, and availability of a stem cell donor. Examples of disease-related factors include molecular alterations and site of disease involvement. Little is known about whether the timing of treatment initiation affects patient outcomes. Short-term treatment delays after the diagnosis of leukemia are not uncommon. Generally, patients are treated with anthracycline-based induction chemotherapy, but the response rate and survival are particularly poor in the older age group.12 Moreover, increasing comorbidities with aging are expected to lead to lower treatment tolerability.13 Therefore, elderly patients are particularly prone to treatment delays while providers await the results of the molecular studies to guide the use of less intensive targeted therapies.10 Other reasons for treatment delays may also include transfers between hospitals, suspected or documented infections, and evaluation of chronic illnesses. Our analysis also indicates that admission to the hospital on the weekend contributes to a delay in therapy compared with admission on a weekday.
We found a decreased OS and EFS in patients who received treatment >4 days after admission to the hospital compared with patients who received treatment within 0 to 4 days of admission. This association was statistically significant in a bivariate analysis as well as in a multivariable analysis with adjustment for age, WBC count on presentation, molecular risk group, and undergoing AlloSCT. A previous large retrospective study showed that the time from diagnosis to treatment initiation predicts survival in younger, but not older, patients with AML.10 This remained true after adjusting for age, performance status, WBC count, and the type of AML in a multivariable analysis. In our study, the declines in overall survival and event-free survival were evident after a delay of more than 4 days.
Another retrospective study that included 599 newly diagnosed AML patients, with a median time from diagnosis to treatment of 8 days, did not show any impact of treatment delay on overall survival, early death, or response rate.11 These differences in the effect of treatment delay on outcomes could be related to the differences in baseline characteristics of patients in these studies. Our study had a higher proportion of patients younger than 60 years, for example. We hypothesize that treatment delays, especially in patients with a high WBC count on presentation, might lead to further organ compromise and poorer outcomes with chemotherapy.
In our study, a higher proportion of patients were admitted over the weekend in the >4 days TAT group, but when we analyzed the day of admission to hospital separately, it was not associated with OS or EFS. Admission over the weekend was also not associated with clinical outcomes including 30-day mortality in a larger study that included 422 patients treated at a large teaching referral hospital.14
Limitations of our study include a small sample size and a short median follow-up time. Most of our patients were young and white, which may not be representative of the general population.
In conclusion, we found that treatment delays are associated with inferior outcomes in AML patients. It remains to be elucidated whether the benefit gained from using targeted and less-intensive chemotherapy, especially in elderly patients, outweighs the potential harm from delaying treatment. Additional studies are needed to confirm our findings in different settings and patient populations.
Acknowledgment
Statistical support was provided by the Stephenson Cancer Center Biostatistics and Research Design Shared Resource.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30.
2. Patel MI, Ma Y, Mitchell B, Rhoads KF. How do differences in treatment impact racial and ethnic disparities in acute myeloid leukemia? Cancer Epidemiol Biomarkers Prev. 2015;24(2):344-349.
3. Weber JJ, Kachare SD, Vohra NA, Fitzgerald TF, Wong JH. Regional disparities in breast cancer outcomes and the process of care. Am Surg. 2014;80(7):669-674.
4. Shippee TP, Kozhimannil KB, Rowan K, Virnig BA. Health insurance coverage and racial disparities in breast reconstruction after mastectomy. Womens Health Issues. 2014;24(3):e261-e269.
5. Dickens C, Joffe M, Jacobson J, et al. Stage at breast cancer diagnosis and distance from diagnostic hospital in a periurban setting: a South African public hospital case series of over 1,000 women. Int J Cancer. 2014;135(9):2173-2182.
6. Nguyen-Pham S, Leung J, McLaughlin D. Disparities in breast cancer stage at diagnosis in urban and rural adult women: a systematic review and meta-analysis. Ann Epidemiol. 2014;24(3):228-235.
7. Gong G, Belasco E, Hargrave KA, Lyford CP, Philips BU Jr. Determinants of delayed detection of cancers in Texas counties in the United States of America. Int J Equity Health. 2012;11:29.
8. Erhunmwunsee L, Joshi MB, Conlon DH, Harpole DH Jr. Neighborhood‐level socioeconomic determinants impact outcomes in nonsmall cell lung cancer patients in the Southeastern United States. Cancer. 2012;118(20):5117-5123.
9. Steele CB, Pisu M, Richardson LC. Urban/rural patterns in receipt of treatment for non–small cell lung cancer among black and white Medicare beneficiaries, 2000-2003. J Natl Med Assoc. 2011;103(8):711-718.
10. Sekeres MA, Elson P, Kalaycio ME, et al. Time from diagnosis to treatment initiation predicts survival in younger, but not older, acute myeloid leukemia patients. Blood. 2009;113(1):28-36.
11. Bertoli S, Bérard E, Huguet F, et al. Time from diagnosis to intensive chemotherapy initiation does not adversely impact the outcome of patients with acute myeloid leukemia. Blood. 2013:121(14):2618-2626.
12. Shah A, Andersson TM, Rachet B, Björkholm M, Lambert PC. Survival and cure of acute myeloid leukaemia in England, 1971-2006: a population-based study. Br J Haematol. 2013;162(4):509-516.
13. Mohammadi M, Cao Y, Glimelius I, Bottai M, Eloranta S, Smedby KE. The impact of comorbid disease history on all-cause and cancer-specific mortality in myeloid leukemia and myeloma – a Swedish population-based study. BMC Cancer. 2015;15:850.
14. Bejanyan N, Fu AZ, Lazaryan A, et al. Impact of weekend admissions on quality of care and outcomes in patients with acute myeloid leukemia. Cancer. 2010;116(15):3614-3620.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30.
2. Patel MI, Ma Y, Mitchell B, Rhoads KF. How do differences in treatment impact racial and ethnic disparities in acute myeloid leukemia? Cancer Epidemiol Biomarkers Prev. 2015;24(2):344-349.
3. Weber JJ, Kachare SD, Vohra NA, Fitzgerald TF, Wong JH. Regional disparities in breast cancer outcomes and the process of care. Am Surg. 2014;80(7):669-674.
4. Shippee TP, Kozhimannil KB, Rowan K, Virnig BA. Health insurance coverage and racial disparities in breast reconstruction after mastectomy. Womens Health Issues. 2014;24(3):e261-e269.
5. Dickens C, Joffe M, Jacobson J, et al. Stage at breast cancer diagnosis and distance from diagnostic hospital in a periurban setting: a South African public hospital case series of over 1,000 women. Int J Cancer. 2014;135(9):2173-2182.
6. Nguyen-Pham S, Leung J, McLaughlin D. Disparities in breast cancer stage at diagnosis in urban and rural adult women: a systematic review and meta-analysis. Ann Epidemiol. 2014;24(3):228-235.
7. Gong G, Belasco E, Hargrave KA, Lyford CP, Philips BU Jr. Determinants of delayed detection of cancers in Texas counties in the United States of America. Int J Equity Health. 2012;11:29.
8. Erhunmwunsee L, Joshi MB, Conlon DH, Harpole DH Jr. Neighborhood‐level socioeconomic determinants impact outcomes in nonsmall cell lung cancer patients in the Southeastern United States. Cancer. 2012;118(20):5117-5123.
9. Steele CB, Pisu M, Richardson LC. Urban/rural patterns in receipt of treatment for non–small cell lung cancer among black and white Medicare beneficiaries, 2000-2003. J Natl Med Assoc. 2011;103(8):711-718.
10. Sekeres MA, Elson P, Kalaycio ME, et al. Time from diagnosis to treatment initiation predicts survival in younger, but not older, acute myeloid leukemia patients. Blood. 2009;113(1):28-36.
11. Bertoli S, Bérard E, Huguet F, et al. Time from diagnosis to intensive chemotherapy initiation does not adversely impact the outcome of patients with acute myeloid leukemia. Blood. 2013:121(14):2618-2626.
12. Shah A, Andersson TM, Rachet B, Björkholm M, Lambert PC. Survival and cure of acute myeloid leukaemia in England, 1971-2006: a population-based study. Br J Haematol. 2013;162(4):509-516.
13. Mohammadi M, Cao Y, Glimelius I, Bottai M, Eloranta S, Smedby KE. The impact of comorbid disease history on all-cause and cancer-specific mortality in myeloid leukemia and myeloma – a Swedish population-based study. BMC Cancer. 2015;15:850.
14. Bejanyan N, Fu AZ, Lazaryan A, et al. Impact of weekend admissions on quality of care and outcomes in patients with acute myeloid leukemia. Cancer. 2010;116(15):3614-3620.
‘Mechanoprimed’ MSCs aid hematopoietic recovery
Specially grown mesenchymal stromal cells (MSCs) can improve hematopoietic recovery, according to preclinical research published in Stem Cell Research and Therapy.
Researchers grew MSCs on a surface with mechanical properties similar to those of bone marrow, which prompted the MSCs to secrete growth factors that aid hematopoietic recovery.
When implanted in irradiated mice, these “mechanoprimed” MSCs sped recovery of all hematopoietic lineages and improved the animals’ survival.
“[MSCs] act like drug factories,” explained study author Krystyn Van Vliet, PhD, of the Massachusetts Institute of Technology in Cambridge.
“They can become tissue lineage cells, but they also pump out a lot of factors that change the environment that the hematopoietic stem cells are operating in.”
Dr. Van Vliet and her colleagues noted that MSCs play an important role in supporting, maintaining, and expanding hematopoietic stem and progenitor cells (HSPCs). However, in a given population of MSCs, usually only about 20% of the cells produce the factors needed to stimulate hematopoietic recovery.
In an earlier study, Dr. Van Vliet and her colleagues showed they could sort MSCs with a microfluidic device that can identify the 20% of cells that promote hematopoietic recovery.
However, the researchers wanted to improve on that by finding a way to stimulate an entire population of MSCs to produce the necessary factors. To do that, they first had to discover which factors were the most important.
Analyses in mice suggested eight factors were associated with improved survival after irradiation—IL-6, IL-8, BMP2, EGF, FGF1, RANTES, VEGF-A, and ANG-1.
Mechanopriming
Having identified factors associated with hematopoietic recovery, Dr. Van Vliet and her colleagues explored the idea of mechanopriming MSCs so they would produce more of these factors.
Over the past decade, researchers have shown that varying the mechanical properties of surfaces on which stem cells are grown can affect their differentiation into mature cell types. However, in this study, the researchers showed that mechanical properties can also affect the factors stem cells secrete before committing to a specific lineage.
For the growth surface, Dr. Van Vliet and her colleagues tested a polymer called polydimethylsiloxane (PDMS). The team varied the mechanical stiffness of the PDMS surface to see how this would affect the MSCs.
MSCs grown on the least stiff PDMS surface produced the greatest number of factors necessary to induce differentiation in HSPCs. These MSCs were able to promote hematopoiesis in an in vitro co-culture model with HSPCs.
Testing in mice
The researchers then tested the mechanoprimed MSCs by implanting them into irradiated mice.
The mechanoprimed MSCs quickly repopulated the animals’ blood cells and helped them recover more quickly than mice treated with MSCs grown on traditional glass surfaces.
Mice that received mechanoprimed MSCs also recovered faster than mice treated with factor-producing MSCs selected by the microfluidic sorting device.
Dr. Van Vliet’s lab is now performing more animal studies in hopes of developing a combination treatment of MSCs and HSPCs that could be tested in humans.
The current research was funded by the National Institutes of Health and the BioSystems and Micromechanics Interdisciplinary Research Group of the Singapore-MIT Alliance for Research and Technology through the Singapore National Research Foundation.
The researchers said they had no competing interests.
Specially grown mesenchymal stromal cells (MSCs) can improve hematopoietic recovery, according to preclinical research published in Stem Cell Research and Therapy.
Researchers grew MSCs on a surface with mechanical properties similar to those of bone marrow, which prompted the MSCs to secrete growth factors that aid hematopoietic recovery.
When implanted in irradiated mice, these “mechanoprimed” MSCs sped recovery of all hematopoietic lineages and improved the animals’ survival.
“[MSCs] act like drug factories,” explained study author Krystyn Van Vliet, PhD, of the Massachusetts Institute of Technology in Cambridge.
“They can become tissue lineage cells, but they also pump out a lot of factors that change the environment that the hematopoietic stem cells are operating in.”
Dr. Van Vliet and her colleagues noted that MSCs play an important role in supporting, maintaining, and expanding hematopoietic stem and progenitor cells (HSPCs). However, in a given population of MSCs, usually only about 20% of the cells produce the factors needed to stimulate hematopoietic recovery.
In an earlier study, Dr. Van Vliet and her colleagues showed they could sort MSCs with a microfluidic device that can identify the 20% of cells that promote hematopoietic recovery.
However, the researchers wanted to improve on that by finding a way to stimulate an entire population of MSCs to produce the necessary factors. To do that, they first had to discover which factors were the most important.
Analyses in mice suggested eight factors were associated with improved survival after irradiation—IL-6, IL-8, BMP2, EGF, FGF1, RANTES, VEGF-A, and ANG-1.
Mechanopriming
Having identified factors associated with hematopoietic recovery, Dr. Van Vliet and her colleagues explored the idea of mechanopriming MSCs so they would produce more of these factors.
Over the past decade, researchers have shown that varying the mechanical properties of surfaces on which stem cells are grown can affect their differentiation into mature cell types. However, in this study, the researchers showed that mechanical properties can also affect the factors stem cells secrete before committing to a specific lineage.
For the growth surface, Dr. Van Vliet and her colleagues tested a polymer called polydimethylsiloxane (PDMS). The team varied the mechanical stiffness of the PDMS surface to see how this would affect the MSCs.
MSCs grown on the least stiff PDMS surface produced the greatest number of factors necessary to induce differentiation in HSPCs. These MSCs were able to promote hematopoiesis in an in vitro co-culture model with HSPCs.
Testing in mice
The researchers then tested the mechanoprimed MSCs by implanting them into irradiated mice.
The mechanoprimed MSCs quickly repopulated the animals’ blood cells and helped them recover more quickly than mice treated with MSCs grown on traditional glass surfaces.
Mice that received mechanoprimed MSCs also recovered faster than mice treated with factor-producing MSCs selected by the microfluidic sorting device.
Dr. Van Vliet’s lab is now performing more animal studies in hopes of developing a combination treatment of MSCs and HSPCs that could be tested in humans.
The current research was funded by the National Institutes of Health and the BioSystems and Micromechanics Interdisciplinary Research Group of the Singapore-MIT Alliance for Research and Technology through the Singapore National Research Foundation.
The researchers said they had no competing interests.
Specially grown mesenchymal stromal cells (MSCs) can improve hematopoietic recovery, according to preclinical research published in Stem Cell Research and Therapy.
Researchers grew MSCs on a surface with mechanical properties similar to those of bone marrow, which prompted the MSCs to secrete growth factors that aid hematopoietic recovery.
When implanted in irradiated mice, these “mechanoprimed” MSCs sped recovery of all hematopoietic lineages and improved the animals’ survival.
“[MSCs] act like drug factories,” explained study author Krystyn Van Vliet, PhD, of the Massachusetts Institute of Technology in Cambridge.
“They can become tissue lineage cells, but they also pump out a lot of factors that change the environment that the hematopoietic stem cells are operating in.”
Dr. Van Vliet and her colleagues noted that MSCs play an important role in supporting, maintaining, and expanding hematopoietic stem and progenitor cells (HSPCs). However, in a given population of MSCs, usually only about 20% of the cells produce the factors needed to stimulate hematopoietic recovery.
In an earlier study, Dr. Van Vliet and her colleagues showed they could sort MSCs with a microfluidic device that can identify the 20% of cells that promote hematopoietic recovery.
However, the researchers wanted to improve on that by finding a way to stimulate an entire population of MSCs to produce the necessary factors. To do that, they first had to discover which factors were the most important.
Analyses in mice suggested eight factors were associated with improved survival after irradiation—IL-6, IL-8, BMP2, EGF, FGF1, RANTES, VEGF-A, and ANG-1.
Mechanopriming
Having identified factors associated with hematopoietic recovery, Dr. Van Vliet and her colleagues explored the idea of mechanopriming MSCs so they would produce more of these factors.
Over the past decade, researchers have shown that varying the mechanical properties of surfaces on which stem cells are grown can affect their differentiation into mature cell types. However, in this study, the researchers showed that mechanical properties can also affect the factors stem cells secrete before committing to a specific lineage.
For the growth surface, Dr. Van Vliet and her colleagues tested a polymer called polydimethylsiloxane (PDMS). The team varied the mechanical stiffness of the PDMS surface to see how this would affect the MSCs.
MSCs grown on the least stiff PDMS surface produced the greatest number of factors necessary to induce differentiation in HSPCs. These MSCs were able to promote hematopoiesis in an in vitro co-culture model with HSPCs.
Testing in mice
The researchers then tested the mechanoprimed MSCs by implanting them into irradiated mice.
The mechanoprimed MSCs quickly repopulated the animals’ blood cells and helped them recover more quickly than mice treated with MSCs grown on traditional glass surfaces.
Mice that received mechanoprimed MSCs also recovered faster than mice treated with factor-producing MSCs selected by the microfluidic sorting device.
Dr. Van Vliet’s lab is now performing more animal studies in hopes of developing a combination treatment of MSCs and HSPCs that could be tested in humans.
The current research was funded by the National Institutes of Health and the BioSystems and Micromechanics Interdisciplinary Research Group of the Singapore-MIT Alliance for Research and Technology through the Singapore National Research Foundation.
The researchers said they had no competing interests.
MDM2 inhibitors could treat resistant AML
Preclinical research has revealed a potential treatment for chemotherapy-resistant acute myeloid leukemia (AML).
Researchers characterized a mechanism of chemotherapy resistance in AML and found that MDM2 is a key player in this dysregulated signaling pathway.
They tested MDM2 inhibitors and found these drugs could sensitize resistant AML to chemotherapy in vitro and in vivo.
In fact, mice with refractory AML responded to standard induction therapy when combined with an MDM2 inhibitor, showing no signs of disease and prolonged survival.
These results were published in Cancer Discovery.
“We were blown away when we saw the results,” said study author William Stanford, PhD, of Ottawa Hospital Research Institute in Ontario, Canada.
“If these findings hold up in clinical trials, we could have a new treatment for people who would almost certainly die of their disease today.”
Mechanism of resistance
Dr. Stanford’s research began with the protein MTF2. He and his colleagues previously found that MTF2 plays a role in erythropoiesis, and the team wanted to determine if MTF2 also plays a role in AML.
Using AML samples from patients treated at The Ottawa Hospital, the researchers found the mean survival was three times longer in patients with normal MTF2 activity than in patients with low MTF2 activity.
“Initially, we thought that MTF2 could be an important biomarker to identify patients who might benefit from experimental therapies,” Dr. Stanford said. “But then we started thinking that if we could understand what MTF2 was doing, maybe we could use this information to develop a new treatment.”
Dr. Stanford and his colleagues discovered that MTF2 represses MDM2, a protein that helps cells resist chemotherapy.
The team found that MTF2-deficient cells overexpress MDM2, which inhibits p53, and this leads to defects in cell-cycle regulation and apoptosis that enable resistance to chemotherapy.
Testing MDM2 inhibitors
Since MDM2 inhibitors are already being tested in clinical trials for other cancers, Dr. Stanford and his colleagues tested these inhibitors in vitro and in mouse models of chemotherapy-resistant AML.
The in vitro experiments included two MDM2 inhibitors—Nutlin3A and MI-773—combined with daunorubicin or cytarabine.
The researchers found that refractory, MTF2-deficient AML cells underwent apoptosis when treated with either daunorubicin or cytarabine in combination with Nutlin3A or MI-773. The effect was comparable to that observed in AML cells with normal MTF2.
The team found that Nutlin3A was more efficient at sensitizing refractory, MTF2-deficient AML cells to daunorubicin, so they used Nutlin3A in the in vivo experiments.
For these experiments, the researchers tested Nutlin3A in mice injected with either chemotherapy-responsive AML cells (with normal MTF2) or refractory, MTF2-deficient AML cells.
Once the mice had “a substantial leukemic burden” (≥ 20% CD45+CD33+ leukemic blasts in their peripheral blood), they were randomized to receive vehicle control, Nutlin3A, standard induction therapy, or induction plus Nutlin3A.
The mice engrafted with chemotherapy-responsive AML cells did not respond to vehicle control or Nutlin3A alone. However, they did respond to standard induction and induction plus Nutlin3A, surviving until the end of the experiment at 16 weeks.
Among the mice engrafted with refractory, MTF2-deficient AML cells, only those animals treated with induction plus Nutlin3A survived until the end of the experiment.
The researchers also noted a “dramatic loss” in the blast-containing CD45+CD33+ and CD34+CD38− populations in mice treated with induction plus Nutlin3A.
To assess residual disease, the researchers performed secondary transplants with cells from mice that had engrafted with refractory, MTF2-deficient AML cells but responded to induction plus Nutlin3A.
The recipient mice had no evidence of AML at 16 weeks after transplant when the experiment ended.
Dr. Stanford and his colleagues are now trying to obtain pharmaceutical-grade MDM2 inhibitors to conduct trials in AML patients at The Ottawa Hospital.
The researchers are also screening libraries of approved drugs to see if any of these can block MDM2, and they are working with a biotech company to develop a test to identify chemotherapy-resistant AML patients who would respond to MDM2 inhibitors.
The current research was supported by grants from the Canadian Cancer Society Research Institute, Canadian Institutes of Health Research, Cancer Research Society, National Institutes of Health, and a Tier 1 Canada Research Chair in Integrative Stem Cell Biology. One study author reported a relationship with Epicypher, Inc. No other conflicts of interest were reported.
Preclinical research has revealed a potential treatment for chemotherapy-resistant acute myeloid leukemia (AML).
Researchers characterized a mechanism of chemotherapy resistance in AML and found that MDM2 is a key player in this dysregulated signaling pathway.
They tested MDM2 inhibitors and found these drugs could sensitize resistant AML to chemotherapy in vitro and in vivo.
In fact, mice with refractory AML responded to standard induction therapy when combined with an MDM2 inhibitor, showing no signs of disease and prolonged survival.
These results were published in Cancer Discovery.
“We were blown away when we saw the results,” said study author William Stanford, PhD, of Ottawa Hospital Research Institute in Ontario, Canada.
“If these findings hold up in clinical trials, we could have a new treatment for people who would almost certainly die of their disease today.”
Mechanism of resistance
Dr. Stanford’s research began with the protein MTF2. He and his colleagues previously found that MTF2 plays a role in erythropoiesis, and the team wanted to determine if MTF2 also plays a role in AML.
Using AML samples from patients treated at The Ottawa Hospital, the researchers found the mean survival was three times longer in patients with normal MTF2 activity than in patients with low MTF2 activity.
“Initially, we thought that MTF2 could be an important biomarker to identify patients who might benefit from experimental therapies,” Dr. Stanford said. “But then we started thinking that if we could understand what MTF2 was doing, maybe we could use this information to develop a new treatment.”
Dr. Stanford and his colleagues discovered that MTF2 represses MDM2, a protein that helps cells resist chemotherapy.
The team found that MTF2-deficient cells overexpress MDM2, which inhibits p53, and this leads to defects in cell-cycle regulation and apoptosis that enable resistance to chemotherapy.
Testing MDM2 inhibitors
Since MDM2 inhibitors are already being tested in clinical trials for other cancers, Dr. Stanford and his colleagues tested these inhibitors in vitro and in mouse models of chemotherapy-resistant AML.
The in vitro experiments included two MDM2 inhibitors—Nutlin3A and MI-773—combined with daunorubicin or cytarabine.
The researchers found that refractory, MTF2-deficient AML cells underwent apoptosis when treated with either daunorubicin or cytarabine in combination with Nutlin3A or MI-773. The effect was comparable to that observed in AML cells with normal MTF2.
The team found that Nutlin3A was more efficient at sensitizing refractory, MTF2-deficient AML cells to daunorubicin, so they used Nutlin3A in the in vivo experiments.
For these experiments, the researchers tested Nutlin3A in mice injected with either chemotherapy-responsive AML cells (with normal MTF2) or refractory, MTF2-deficient AML cells.
Once the mice had “a substantial leukemic burden” (≥ 20% CD45+CD33+ leukemic blasts in their peripheral blood), they were randomized to receive vehicle control, Nutlin3A, standard induction therapy, or induction plus Nutlin3A.
The mice engrafted with chemotherapy-responsive AML cells did not respond to vehicle control or Nutlin3A alone. However, they did respond to standard induction and induction plus Nutlin3A, surviving until the end of the experiment at 16 weeks.
Among the mice engrafted with refractory, MTF2-deficient AML cells, only those animals treated with induction plus Nutlin3A survived until the end of the experiment.
The researchers also noted a “dramatic loss” in the blast-containing CD45+CD33+ and CD34+CD38− populations in mice treated with induction plus Nutlin3A.
To assess residual disease, the researchers performed secondary transplants with cells from mice that had engrafted with refractory, MTF2-deficient AML cells but responded to induction plus Nutlin3A.
The recipient mice had no evidence of AML at 16 weeks after transplant when the experiment ended.
Dr. Stanford and his colleagues are now trying to obtain pharmaceutical-grade MDM2 inhibitors to conduct trials in AML patients at The Ottawa Hospital.
The researchers are also screening libraries of approved drugs to see if any of these can block MDM2, and they are working with a biotech company to develop a test to identify chemotherapy-resistant AML patients who would respond to MDM2 inhibitors.
The current research was supported by grants from the Canadian Cancer Society Research Institute, Canadian Institutes of Health Research, Cancer Research Society, National Institutes of Health, and a Tier 1 Canada Research Chair in Integrative Stem Cell Biology. One study author reported a relationship with Epicypher, Inc. No other conflicts of interest were reported.
Preclinical research has revealed a potential treatment for chemotherapy-resistant acute myeloid leukemia (AML).
Researchers characterized a mechanism of chemotherapy resistance in AML and found that MDM2 is a key player in this dysregulated signaling pathway.
They tested MDM2 inhibitors and found these drugs could sensitize resistant AML to chemotherapy in vitro and in vivo.
In fact, mice with refractory AML responded to standard induction therapy when combined with an MDM2 inhibitor, showing no signs of disease and prolonged survival.
These results were published in Cancer Discovery.
“We were blown away when we saw the results,” said study author William Stanford, PhD, of Ottawa Hospital Research Institute in Ontario, Canada.
“If these findings hold up in clinical trials, we could have a new treatment for people who would almost certainly die of their disease today.”
Mechanism of resistance
Dr. Stanford’s research began with the protein MTF2. He and his colleagues previously found that MTF2 plays a role in erythropoiesis, and the team wanted to determine if MTF2 also plays a role in AML.
Using AML samples from patients treated at The Ottawa Hospital, the researchers found the mean survival was three times longer in patients with normal MTF2 activity than in patients with low MTF2 activity.
“Initially, we thought that MTF2 could be an important biomarker to identify patients who might benefit from experimental therapies,” Dr. Stanford said. “But then we started thinking that if we could understand what MTF2 was doing, maybe we could use this information to develop a new treatment.”
Dr. Stanford and his colleagues discovered that MTF2 represses MDM2, a protein that helps cells resist chemotherapy.
The team found that MTF2-deficient cells overexpress MDM2, which inhibits p53, and this leads to defects in cell-cycle regulation and apoptosis that enable resistance to chemotherapy.
Testing MDM2 inhibitors
Since MDM2 inhibitors are already being tested in clinical trials for other cancers, Dr. Stanford and his colleagues tested these inhibitors in vitro and in mouse models of chemotherapy-resistant AML.
The in vitro experiments included two MDM2 inhibitors—Nutlin3A and MI-773—combined with daunorubicin or cytarabine.
The researchers found that refractory, MTF2-deficient AML cells underwent apoptosis when treated with either daunorubicin or cytarabine in combination with Nutlin3A or MI-773. The effect was comparable to that observed in AML cells with normal MTF2.
The team found that Nutlin3A was more efficient at sensitizing refractory, MTF2-deficient AML cells to daunorubicin, so they used Nutlin3A in the in vivo experiments.
For these experiments, the researchers tested Nutlin3A in mice injected with either chemotherapy-responsive AML cells (with normal MTF2) or refractory, MTF2-deficient AML cells.
Once the mice had “a substantial leukemic burden” (≥ 20% CD45+CD33+ leukemic blasts in their peripheral blood), they were randomized to receive vehicle control, Nutlin3A, standard induction therapy, or induction plus Nutlin3A.
The mice engrafted with chemotherapy-responsive AML cells did not respond to vehicle control or Nutlin3A alone. However, they did respond to standard induction and induction plus Nutlin3A, surviving until the end of the experiment at 16 weeks.
Among the mice engrafted with refractory, MTF2-deficient AML cells, only those animals treated with induction plus Nutlin3A survived until the end of the experiment.
The researchers also noted a “dramatic loss” in the blast-containing CD45+CD33+ and CD34+CD38− populations in mice treated with induction plus Nutlin3A.
To assess residual disease, the researchers performed secondary transplants with cells from mice that had engrafted with refractory, MTF2-deficient AML cells but responded to induction plus Nutlin3A.
The recipient mice had no evidence of AML at 16 weeks after transplant when the experiment ended.
Dr. Stanford and his colleagues are now trying to obtain pharmaceutical-grade MDM2 inhibitors to conduct trials in AML patients at The Ottawa Hospital.
The researchers are also screening libraries of approved drugs to see if any of these can block MDM2, and they are working with a biotech company to develop a test to identify chemotherapy-resistant AML patients who would respond to MDM2 inhibitors.
The current research was supported by grants from the Canadian Cancer Society Research Institute, Canadian Institutes of Health Research, Cancer Research Society, National Institutes of Health, and a Tier 1 Canada Research Chair in Integrative Stem Cell Biology. One study author reported a relationship with Epicypher, Inc. No other conflicts of interest were reported.
Cost-effectiveness of CAR T-cell therapy
Tisagenlecleucel has the potential to be cost-effective for pediatric B-cell acute lymphoblastic leukemia (B-ALL) patients in the United States, according to researchers.
The group found evidence to suggest the chimeric antigen receptor (CAR) T-cell therapy—which has a list price of $475,000—may prove cost-effective if long-term survival benefits are realized.
An analysis indicated that the incremental cost-effectiveness ratio for tisagenlecleucel compared to clofarabine ranged from $37,000 to $78,000 per quality-adjusted life year (QALY) gained.
Melanie D. Whittington, PhD, of the University of Colorado at Denver, Aurora, and her colleagues described this work in JAMA Pediatrics.
For this study, the researchers used a decision analytic model that extrapolated the evidence from clinical trials over a patient’s lifetime to assess life-years gained, QALYs gained, and incremental costs per life-year and QALY gained. The researchers compared tisagenlecleucel to the antineoplastic agent clofarabine.
While tisagenlecleucel has a list price of $475,000, researchers discounted the price by 3% and added several additional costs, such as hospital administration, pretreatment, and potential adverse events, to get to a total discounted cost of about $667,000.
The team estimated that 42.6% of B-ALL patients would be long-term survivors with tisagenlecleucel, 10.34 life-years would be gained, and 9.28 QALYs would be gained.
In comparison, clofarabine had a total discounted cost of approximately $337,000, which included an initial discounted price of $164,000 plus additional treatment and administrative costs.
With clofarabine, 10.8% of B-ALL patients were long-term survivors, 2.43 life-years were gained, and 2.10 QALYs were gained in the model.
Overall, the mean incremental cost-effectiveness ratio was about $46,000 per QALY gained in this base-case model.
In analyses of different scenarios, such as a deeper discount, a different treatment start, or a different calculation of future treatment costs, the cost-effectiveness ratio varied from $37,000 to $78,000 per QALY gained.
The researchers noted that clinical trial evidence for tisagenlecleucel came from single-arm trials, which made the selection of a comparator challenging. Clofarabine was chosen because it had the most similar baseline population characteristics, but the researchers acknowledged that blinatumomab is also frequently used as a treatment for these patients.
“We suspect that tisagenlecleucel would remain cost-effective compared with blinatumomab,” the researchers wrote in JAMA Pediatrics. “A study conducted by other researchers found the incremental cost-effectiveness ratio of tisagenlecleucel versus blinatumomab was similar to the incremental cost-effectiveness ratio of tisagenlecleucel versus clofarabine [i.e., $3,000 more per QALY].”
The researchers suggested that uncertainties in the evidence should be considered as payers are negotiating coverage and payment for tisagenlecleucel.
This study was funded by the Institute for Clinical and Economic Review, which receives some funding from the pharmaceutical industry. Four study authors are employees of the Institute for Clinical and Economic Review.
Tisagenlecleucel has the potential to be cost-effective for pediatric B-cell acute lymphoblastic leukemia (B-ALL) patients in the United States, according to researchers.
The group found evidence to suggest the chimeric antigen receptor (CAR) T-cell therapy—which has a list price of $475,000—may prove cost-effective if long-term survival benefits are realized.
An analysis indicated that the incremental cost-effectiveness ratio for tisagenlecleucel compared to clofarabine ranged from $37,000 to $78,000 per quality-adjusted life year (QALY) gained.
Melanie D. Whittington, PhD, of the University of Colorado at Denver, Aurora, and her colleagues described this work in JAMA Pediatrics.
For this study, the researchers used a decision analytic model that extrapolated the evidence from clinical trials over a patient’s lifetime to assess life-years gained, QALYs gained, and incremental costs per life-year and QALY gained. The researchers compared tisagenlecleucel to the antineoplastic agent clofarabine.
While tisagenlecleucel has a list price of $475,000, researchers discounted the price by 3% and added several additional costs, such as hospital administration, pretreatment, and potential adverse events, to get to a total discounted cost of about $667,000.
The team estimated that 42.6% of B-ALL patients would be long-term survivors with tisagenlecleucel, 10.34 life-years would be gained, and 9.28 QALYs would be gained.
In comparison, clofarabine had a total discounted cost of approximately $337,000, which included an initial discounted price of $164,000 plus additional treatment and administrative costs.
With clofarabine, 10.8% of B-ALL patients were long-term survivors, 2.43 life-years were gained, and 2.10 QALYs were gained in the model.
Overall, the mean incremental cost-effectiveness ratio was about $46,000 per QALY gained in this base-case model.
In analyses of different scenarios, such as a deeper discount, a different treatment start, or a different calculation of future treatment costs, the cost-effectiveness ratio varied from $37,000 to $78,000 per QALY gained.
The researchers noted that clinical trial evidence for tisagenlecleucel came from single-arm trials, which made the selection of a comparator challenging. Clofarabine was chosen because it had the most similar baseline population characteristics, but the researchers acknowledged that blinatumomab is also frequently used as a treatment for these patients.
“We suspect that tisagenlecleucel would remain cost-effective compared with blinatumomab,” the researchers wrote in JAMA Pediatrics. “A study conducted by other researchers found the incremental cost-effectiveness ratio of tisagenlecleucel versus blinatumomab was similar to the incremental cost-effectiveness ratio of tisagenlecleucel versus clofarabine [i.e., $3,000 more per QALY].”
The researchers suggested that uncertainties in the evidence should be considered as payers are negotiating coverage and payment for tisagenlecleucel.
This study was funded by the Institute for Clinical and Economic Review, which receives some funding from the pharmaceutical industry. Four study authors are employees of the Institute for Clinical and Economic Review.
Tisagenlecleucel has the potential to be cost-effective for pediatric B-cell acute lymphoblastic leukemia (B-ALL) patients in the United States, according to researchers.
The group found evidence to suggest the chimeric antigen receptor (CAR) T-cell therapy—which has a list price of $475,000—may prove cost-effective if long-term survival benefits are realized.
An analysis indicated that the incremental cost-effectiveness ratio for tisagenlecleucel compared to clofarabine ranged from $37,000 to $78,000 per quality-adjusted life year (QALY) gained.
Melanie D. Whittington, PhD, of the University of Colorado at Denver, Aurora, and her colleagues described this work in JAMA Pediatrics.
For this study, the researchers used a decision analytic model that extrapolated the evidence from clinical trials over a patient’s lifetime to assess life-years gained, QALYs gained, and incremental costs per life-year and QALY gained. The researchers compared tisagenlecleucel to the antineoplastic agent clofarabine.
While tisagenlecleucel has a list price of $475,000, researchers discounted the price by 3% and added several additional costs, such as hospital administration, pretreatment, and potential adverse events, to get to a total discounted cost of about $667,000.
The team estimated that 42.6% of B-ALL patients would be long-term survivors with tisagenlecleucel, 10.34 life-years would be gained, and 9.28 QALYs would be gained.
In comparison, clofarabine had a total discounted cost of approximately $337,000, which included an initial discounted price of $164,000 plus additional treatment and administrative costs.
With clofarabine, 10.8% of B-ALL patients were long-term survivors, 2.43 life-years were gained, and 2.10 QALYs were gained in the model.
Overall, the mean incremental cost-effectiveness ratio was about $46,000 per QALY gained in this base-case model.
In analyses of different scenarios, such as a deeper discount, a different treatment start, or a different calculation of future treatment costs, the cost-effectiveness ratio varied from $37,000 to $78,000 per QALY gained.
The researchers noted that clinical trial evidence for tisagenlecleucel came from single-arm trials, which made the selection of a comparator challenging. Clofarabine was chosen because it had the most similar baseline population characteristics, but the researchers acknowledged that blinatumomab is also frequently used as a treatment for these patients.
“We suspect that tisagenlecleucel would remain cost-effective compared with blinatumomab,” the researchers wrote in JAMA Pediatrics. “A study conducted by other researchers found the incremental cost-effectiveness ratio of tisagenlecleucel versus blinatumomab was similar to the incremental cost-effectiveness ratio of tisagenlecleucel versus clofarabine [i.e., $3,000 more per QALY].”
The researchers suggested that uncertainties in the evidence should be considered as payers are negotiating coverage and payment for tisagenlecleucel.
This study was funded by the Institute for Clinical and Economic Review, which receives some funding from the pharmaceutical industry. Four study authors are employees of the Institute for Clinical and Economic Review.
CDK8 inhibitor shows activity against AML
DUBROVNIK, CROATIA – The (AML), but the agent’s mechanism of action is still unclear.
Researchers found that several AML cell lines were “highly sensitive” to SEL120, and the inhibitor was active in primary patient samples. SEL120 also reduced tumor growth in mouse models of AML and demonstrated synergy with venetoclax.
The researchers suggest that SEL120 works by affecting the maintenance of AML cells and leukemic stem cells (LSCs), inducing differentiation and, sometimes, apoptosis. However, the mechanism is not well defined.
Eliza Majewska, PhD, of Selvita S.A. in Krakow, Poland, discussed research on SEL120 at Leukemia and Lymphoma, a meeting jointly sponsored by the University of Texas MD Anderson Cancer Center and the School of Medicine at the University of Zagreb, Croatia.
Dr. Majewska explained that CDK8 is a transcriptional kinase working in the context of the Mediator complex, and previous research indicated that CDK8 drives oncogenic transcription in AML (Nature. 2015 Oct 8;526[7572]:273-6).
In a prior study, researchers found that SEL120 inhibits CDK8 activity in AML cells with high levels of STAT phosphorylation (Oncotarget. 2017 May 16;8[20]:33779-95).
Dr. Majewska said the MV4-11 cell line responds particularly well to SEL120, and other sensitive cell lines include SKNO-1, Oci-AML5, GDM-1, KG-1, MOLM-16, and Oci-AML3.
“The fact that STAT signaling was upregulated in those cell lines that were very sensitive to SEL120 gave us the hint that perhaps we are looking at a mechanism of action of the compound that has something to do with leukemic stem cells,” Dr. Majewska said.
In fact, she and her colleagues found that cell lines sensitive to SEL120 had upregulation of genes linked to LSCs and high levels of CD34 surface expression.
Experiments in CD34+ TEX cells showed that SEL120 specifically depletes CD34+ cells, leads to downregulation of stemness-related genes, and induces myeloid differentiation.
After 6 days of treatment with SEL120, TEX cells showed decreased expression of the LSC-linked genes MEIS1 and LILRB2, enrichment of gene sets downregulated in LSCs and linked to differentiation, and increased expression of differentiation markers and immune response genes.
SEL120 also demonstrated antileukemic activity in vivo. The researchers tested SEL120 in a CD34+ model of AML (KG-1) and a FLT3-ITD model of AML (MV4-11).
In both models, SEL120 induced “significant tumor regression” of about 80%. In some cases, the researchers observed apoptosis.
Toxicities observed in the mice included weight loss and upregulation of inflammation.
The researchers also found that SEL120 was synergistic with venetoclax. In fact, the combination of these drugs resulted in “almost complete remission cures” in the MV4-11 model, according to Dr. Majewska.
Finally, she and her colleagues discovered that SEL120 was active against primary patient cells. Samples from three of four patients had a significant reduction in cell numbers after 7 days of treatment with SEL120. For one patient, there were no viable cells on day 7.
Dr. Majewska said a phase 1 trial of SEL120 is planned for 2019 or 2020, and SEL120’s mechanism of action is still under investigation.
“The mechanism of action ... is, in our mind – at least in some cases – linked to the fact that CDK8 functions within the context of the Mediator complex, which contributes to gene expression related to leukemic stem cells,” Dr. Majewska said.
“And when we inhibit this specific transcription, of course, the Mediator complex still works because this is just one of the components of the complex. However, the function that it has is suddenly very different, and it’s actually linked to lack of maintenance of leukemic stem cells, resulting in differentiation [and], in some cases, the induction of apoptosis, but we do not fully understand the mechanism of this induction.”
Dr. Majewska works for Selvita, the company developing SEL120. This research was funded by Selvita, the Leukemia & Lymphoma Society, and the National Centre for Research and Development.
The Leukemia and Lymphoma meeting is organized by Jonathan Wood & Association, which is owned by the parent company of this news organization.
DUBROVNIK, CROATIA – The (AML), but the agent’s mechanism of action is still unclear.
Researchers found that several AML cell lines were “highly sensitive” to SEL120, and the inhibitor was active in primary patient samples. SEL120 also reduced tumor growth in mouse models of AML and demonstrated synergy with venetoclax.
The researchers suggest that SEL120 works by affecting the maintenance of AML cells and leukemic stem cells (LSCs), inducing differentiation and, sometimes, apoptosis. However, the mechanism is not well defined.
Eliza Majewska, PhD, of Selvita S.A. in Krakow, Poland, discussed research on SEL120 at Leukemia and Lymphoma, a meeting jointly sponsored by the University of Texas MD Anderson Cancer Center and the School of Medicine at the University of Zagreb, Croatia.
Dr. Majewska explained that CDK8 is a transcriptional kinase working in the context of the Mediator complex, and previous research indicated that CDK8 drives oncogenic transcription in AML (Nature. 2015 Oct 8;526[7572]:273-6).
In a prior study, researchers found that SEL120 inhibits CDK8 activity in AML cells with high levels of STAT phosphorylation (Oncotarget. 2017 May 16;8[20]:33779-95).
Dr. Majewska said the MV4-11 cell line responds particularly well to SEL120, and other sensitive cell lines include SKNO-1, Oci-AML5, GDM-1, KG-1, MOLM-16, and Oci-AML3.
“The fact that STAT signaling was upregulated in those cell lines that were very sensitive to SEL120 gave us the hint that perhaps we are looking at a mechanism of action of the compound that has something to do with leukemic stem cells,” Dr. Majewska said.
In fact, she and her colleagues found that cell lines sensitive to SEL120 had upregulation of genes linked to LSCs and high levels of CD34 surface expression.
Experiments in CD34+ TEX cells showed that SEL120 specifically depletes CD34+ cells, leads to downregulation of stemness-related genes, and induces myeloid differentiation.
After 6 days of treatment with SEL120, TEX cells showed decreased expression of the LSC-linked genes MEIS1 and LILRB2, enrichment of gene sets downregulated in LSCs and linked to differentiation, and increased expression of differentiation markers and immune response genes.
SEL120 also demonstrated antileukemic activity in vivo. The researchers tested SEL120 in a CD34+ model of AML (KG-1) and a FLT3-ITD model of AML (MV4-11).
In both models, SEL120 induced “significant tumor regression” of about 80%. In some cases, the researchers observed apoptosis.
Toxicities observed in the mice included weight loss and upregulation of inflammation.
The researchers also found that SEL120 was synergistic with venetoclax. In fact, the combination of these drugs resulted in “almost complete remission cures” in the MV4-11 model, according to Dr. Majewska.
Finally, she and her colleagues discovered that SEL120 was active against primary patient cells. Samples from three of four patients had a significant reduction in cell numbers after 7 days of treatment with SEL120. For one patient, there were no viable cells on day 7.
Dr. Majewska said a phase 1 trial of SEL120 is planned for 2019 or 2020, and SEL120’s mechanism of action is still under investigation.
“The mechanism of action ... is, in our mind – at least in some cases – linked to the fact that CDK8 functions within the context of the Mediator complex, which contributes to gene expression related to leukemic stem cells,” Dr. Majewska said.
“And when we inhibit this specific transcription, of course, the Mediator complex still works because this is just one of the components of the complex. However, the function that it has is suddenly very different, and it’s actually linked to lack of maintenance of leukemic stem cells, resulting in differentiation [and], in some cases, the induction of apoptosis, but we do not fully understand the mechanism of this induction.”
Dr. Majewska works for Selvita, the company developing SEL120. This research was funded by Selvita, the Leukemia & Lymphoma Society, and the National Centre for Research and Development.
The Leukemia and Lymphoma meeting is organized by Jonathan Wood & Association, which is owned by the parent company of this news organization.
DUBROVNIK, CROATIA – The (AML), but the agent’s mechanism of action is still unclear.
Researchers found that several AML cell lines were “highly sensitive” to SEL120, and the inhibitor was active in primary patient samples. SEL120 also reduced tumor growth in mouse models of AML and demonstrated synergy with venetoclax.
The researchers suggest that SEL120 works by affecting the maintenance of AML cells and leukemic stem cells (LSCs), inducing differentiation and, sometimes, apoptosis. However, the mechanism is not well defined.
Eliza Majewska, PhD, of Selvita S.A. in Krakow, Poland, discussed research on SEL120 at Leukemia and Lymphoma, a meeting jointly sponsored by the University of Texas MD Anderson Cancer Center and the School of Medicine at the University of Zagreb, Croatia.
Dr. Majewska explained that CDK8 is a transcriptional kinase working in the context of the Mediator complex, and previous research indicated that CDK8 drives oncogenic transcription in AML (Nature. 2015 Oct 8;526[7572]:273-6).
In a prior study, researchers found that SEL120 inhibits CDK8 activity in AML cells with high levels of STAT phosphorylation (Oncotarget. 2017 May 16;8[20]:33779-95).
Dr. Majewska said the MV4-11 cell line responds particularly well to SEL120, and other sensitive cell lines include SKNO-1, Oci-AML5, GDM-1, KG-1, MOLM-16, and Oci-AML3.
“The fact that STAT signaling was upregulated in those cell lines that were very sensitive to SEL120 gave us the hint that perhaps we are looking at a mechanism of action of the compound that has something to do with leukemic stem cells,” Dr. Majewska said.
In fact, she and her colleagues found that cell lines sensitive to SEL120 had upregulation of genes linked to LSCs and high levels of CD34 surface expression.
Experiments in CD34+ TEX cells showed that SEL120 specifically depletes CD34+ cells, leads to downregulation of stemness-related genes, and induces myeloid differentiation.
After 6 days of treatment with SEL120, TEX cells showed decreased expression of the LSC-linked genes MEIS1 and LILRB2, enrichment of gene sets downregulated in LSCs and linked to differentiation, and increased expression of differentiation markers and immune response genes.
SEL120 also demonstrated antileukemic activity in vivo. The researchers tested SEL120 in a CD34+ model of AML (KG-1) and a FLT3-ITD model of AML (MV4-11).
In both models, SEL120 induced “significant tumor regression” of about 80%. In some cases, the researchers observed apoptosis.
Toxicities observed in the mice included weight loss and upregulation of inflammation.
The researchers also found that SEL120 was synergistic with venetoclax. In fact, the combination of these drugs resulted in “almost complete remission cures” in the MV4-11 model, according to Dr. Majewska.
Finally, she and her colleagues discovered that SEL120 was active against primary patient cells. Samples from three of four patients had a significant reduction in cell numbers after 7 days of treatment with SEL120. For one patient, there were no viable cells on day 7.
Dr. Majewska said a phase 1 trial of SEL120 is planned for 2019 or 2020, and SEL120’s mechanism of action is still under investigation.
“The mechanism of action ... is, in our mind – at least in some cases – linked to the fact that CDK8 functions within the context of the Mediator complex, which contributes to gene expression related to leukemic stem cells,” Dr. Majewska said.
“And when we inhibit this specific transcription, of course, the Mediator complex still works because this is just one of the components of the complex. However, the function that it has is suddenly very different, and it’s actually linked to lack of maintenance of leukemic stem cells, resulting in differentiation [and], in some cases, the induction of apoptosis, but we do not fully understand the mechanism of this induction.”
Dr. Majewska works for Selvita, the company developing SEL120. This research was funded by Selvita, the Leukemia & Lymphoma Society, and the National Centre for Research and Development.
The Leukemia and Lymphoma meeting is organized by Jonathan Wood & Association, which is owned by the parent company of this news organization.
EXPERT ANALYSIS FROM LEUKEMIA AND LYMPHOMA 2018
When is it CMML?
DUBROVNIK, CROATIA – in 2018.
Even with updated World Health Organization (WHO) criteria, karyotyping, and genetic analyses, it can be difficult to distinguish CMML from other conditions, according to Nadira Durakovic, MD, PhD, of the University Hospital Centre Zagreb (Croatia).
However, there are characteristics that differentiate CMML from myelodysplastic syndromes (MDS), myeloproliferative neoplasms (MPNs), and atypical chronic myeloid leukemia (CML), Dr. Durakovic said at Leukemia and Lymphoma, a meeting jointly sponsored by the University of Texas MD Anderson Cancer Center and the School of Medicine at the University of Zagreb, Croatia.
Studies have suggested that monocyte subset distribution analysis can be useful for diagnosing CMML.
According to the 2016 WHO classification, patients have CMML if:
- They have persistent peripheral blood monocytosis (1×109/L), with monocytes accounting for 10% of the white blood cell count.
- They do not meet WHO criteria for BCR-ABL1-positive CML, primary myelofibrosis, polycythemia vera, or essential thrombocythemia.
- There is no evidence of PCM1-JAK2 or PDGFRA, PDGFRB, or FGFR1 rearrangement.
- They have fewer than 20% blasts in the blood and bone marrow they have dysplasia in one or more myeloid lineages.
If myelodysplasia is absent or minimal, an acquired clonal cytogenetic or molecular genetic abnormality must be present. Alternatively, if patients have monocytosis that has persisted for at least 3 months, and all other causes of monocytosis have been excluded, “you can say that your patient has CMML,” Dr. Durakovic said.
Other causes of monocytosis include infections, malignancies, medications, inflammatory conditions, and other conditions, such as pregnancy.
However, Dr. Durakovic pointed out that the cause of monocytosis cannot always be determined, and, in some cases, CMML patients may not meet the WHO criteria.
“There are cases where there just aren’t enough monocytes to fulfill the WHO criteria,” Dr. Durakovic said. “You can have a patient with peripheral blood cytopenia and monocytosis who does not have 1,000 monocytes. Patients can have progressive dysplasia, can have splenomegaly, be really sick, but fail to meet WHO criteria.”
Differential diagnosis
“Differentiating CMML from myelodysplastic syndromes can be tough,” Dr. Durakovic said. “There are dysplastic features that are present in CMML ... but, in CMML, they are more subtle, and they are more difficult to appreciate than in myelodysplastic syndromes.”
The ratio of myeloid to erythroid cells is elevated in CMML, and patients may have atypical monocytes (paramyeloid cells) that are unique to CMML.
Dr. Durakovic noted that megakaryocyte dysplasia in CMML can be characterized by “myeloproliferative megakaryocytes,” which are large cells that cluster and have hyperlobulated nuclei, or “MDS megakaryocytes,” which are small, solitary cells with hypolobulated nuclei.
She noted that “MPN phenotype” CMML is characterized by leukocytosis, monocytosis, hepatomegaly, splenomegaly, and clinical features of myeloproliferation (fatigue, night sweats, bone pain, weight loss, etc.).
Thirty percent of cases are associated with splenomegaly, and 30% of patients can have an increase in bone marrow reticulin fibrosis.
Dr. Durakovic also noted that a prior MPN diagnosis excludes CMML. The presence of common MPN mutations, such as JAK2, CALR, or MPL, suggests a patient has an MPN with monocytosis rather than CMML.
Patients who have unclassified MPNs or MDS, rather than CMML, either do not have 1,000 monocytes or the monocytes do not represent more than 10% of the differential, Dr. Durakovic said.
It can also be difficult to differentiate CMML from atypical CML.
“Atypical CML is characterized by profound dysgranulopoiesis, absence of the BCR-ABL1 fusion gene, and neutrophilia,” Dr. Durakovic explained. “Those patients [commonly] have monocytosis, but, here, that 10% rule is valuable because their monocytes comprise less than 10% of the entire white blood cell count.”
Karyotyping, genotyping, and immunophenotyping
“There is no disease-defining karyotype abnormality [in CMML],” Dr. Durakovic said.
She said 30% of patients have abnormal karyotype, and the most common abnormality is trisomy 8. Unlike in patients with MDS, del(5q) and monosomal karyotypes are infrequent in patients with CMML.
Similarly, there are no “disease-defining” mutations or genetic changes in CMML, although CMML is genetically distinct from MDS, Dr. Durakovic said.
For instance, SRSF2 encodes a component of the spliceosome that is mutated in almost half of CMML patients and less than 10% of MDS patients. Likewise, ASLX1 and TET2 are “much more frequently involved” in CMML than in MDS, Dr. Durakovic said.
In a 2012 study of 275 CMML patients, researchers found that 93% of patients had at least one somatic mutation in nine recurrently mutated genes – SRFS2, ASXL1, CBL, EZH2, JAK2V617F, KRAS, NRAS, RUNX1, and TET2 (Blood. 2012;120:3080-8).
However, Dr. Durakovic noted that these mutations are found in other disorders as well, so this information may not be helpful in differentiating CMML from other disorders.
A 2015 study revealed a technique that does appear useful for identifying CMML – monocyte subset distribution analysis. For this analysis, monocytes are divided into the following categories:
- Classical/MO1 (CD14bright/CD16−).
- Intermediate/MO2 (CD14bright/CD16+).
- Nonclassical/MO3 (CD14dim/CD16+).
The researchers found that CMML patients had an increase in the fraction of classical monocytes (with a cutoff value of 94%), as compared to healthy control subjects, patients with another hematologic disorder, and patients with reactive monocytosis (Blood. 2015 Jun 4;125[23]:3618-26).
A 2018 study confirmed that monocyte subset distribution analysis could differentiate CMML from other hematologic disorders, with the exception of atypical CML. This study also suggested that a decreased percentage of non-classical monocytes was more sensitive than an increased percentage of classical monocytes (Am J Clin Pathol. 2018 Aug 30;150[4]:293-302).
Despite the differences between these studies, “monocyte subset distribution analysis is showing promise as a method of identifying hard-to-identify CMML patients with ease and affordability,” Dr. Durakovic said.
She added that the technique can be implemented in clinical practice using the Hematoflow solution.
Dr. Durakovic did not report any conflicts of interest.
The Leukemia and Lymphoma meeting is organized by Jonathan Wood & Association, which is owned by the parent company of this news organization.
DUBROVNIK, CROATIA – in 2018.
Even with updated World Health Organization (WHO) criteria, karyotyping, and genetic analyses, it can be difficult to distinguish CMML from other conditions, according to Nadira Durakovic, MD, PhD, of the University Hospital Centre Zagreb (Croatia).
However, there are characteristics that differentiate CMML from myelodysplastic syndromes (MDS), myeloproliferative neoplasms (MPNs), and atypical chronic myeloid leukemia (CML), Dr. Durakovic said at Leukemia and Lymphoma, a meeting jointly sponsored by the University of Texas MD Anderson Cancer Center and the School of Medicine at the University of Zagreb, Croatia.
Studies have suggested that monocyte subset distribution analysis can be useful for diagnosing CMML.
According to the 2016 WHO classification, patients have CMML if:
- They have persistent peripheral blood monocytosis (1×109/L), with monocytes accounting for 10% of the white blood cell count.
- They do not meet WHO criteria for BCR-ABL1-positive CML, primary myelofibrosis, polycythemia vera, or essential thrombocythemia.
- There is no evidence of PCM1-JAK2 or PDGFRA, PDGFRB, or FGFR1 rearrangement.
- They have fewer than 20% blasts in the blood and bone marrow they have dysplasia in one or more myeloid lineages.
If myelodysplasia is absent or minimal, an acquired clonal cytogenetic or molecular genetic abnormality must be present. Alternatively, if patients have monocytosis that has persisted for at least 3 months, and all other causes of monocytosis have been excluded, “you can say that your patient has CMML,” Dr. Durakovic said.
Other causes of monocytosis include infections, malignancies, medications, inflammatory conditions, and other conditions, such as pregnancy.
However, Dr. Durakovic pointed out that the cause of monocytosis cannot always be determined, and, in some cases, CMML patients may not meet the WHO criteria.
“There are cases where there just aren’t enough monocytes to fulfill the WHO criteria,” Dr. Durakovic said. “You can have a patient with peripheral blood cytopenia and monocytosis who does not have 1,000 monocytes. Patients can have progressive dysplasia, can have splenomegaly, be really sick, but fail to meet WHO criteria.”
Differential diagnosis
“Differentiating CMML from myelodysplastic syndromes can be tough,” Dr. Durakovic said. “There are dysplastic features that are present in CMML ... but, in CMML, they are more subtle, and they are more difficult to appreciate than in myelodysplastic syndromes.”
The ratio of myeloid to erythroid cells is elevated in CMML, and patients may have atypical monocytes (paramyeloid cells) that are unique to CMML.
Dr. Durakovic noted that megakaryocyte dysplasia in CMML can be characterized by “myeloproliferative megakaryocytes,” which are large cells that cluster and have hyperlobulated nuclei, or “MDS megakaryocytes,” which are small, solitary cells with hypolobulated nuclei.
She noted that “MPN phenotype” CMML is characterized by leukocytosis, monocytosis, hepatomegaly, splenomegaly, and clinical features of myeloproliferation (fatigue, night sweats, bone pain, weight loss, etc.).
Thirty percent of cases are associated with splenomegaly, and 30% of patients can have an increase in bone marrow reticulin fibrosis.
Dr. Durakovic also noted that a prior MPN diagnosis excludes CMML. The presence of common MPN mutations, such as JAK2, CALR, or MPL, suggests a patient has an MPN with monocytosis rather than CMML.
Patients who have unclassified MPNs or MDS, rather than CMML, either do not have 1,000 monocytes or the monocytes do not represent more than 10% of the differential, Dr. Durakovic said.
It can also be difficult to differentiate CMML from atypical CML.
“Atypical CML is characterized by profound dysgranulopoiesis, absence of the BCR-ABL1 fusion gene, and neutrophilia,” Dr. Durakovic explained. “Those patients [commonly] have monocytosis, but, here, that 10% rule is valuable because their monocytes comprise less than 10% of the entire white blood cell count.”
Karyotyping, genotyping, and immunophenotyping
“There is no disease-defining karyotype abnormality [in CMML],” Dr. Durakovic said.
She said 30% of patients have abnormal karyotype, and the most common abnormality is trisomy 8. Unlike in patients with MDS, del(5q) and monosomal karyotypes are infrequent in patients with CMML.
Similarly, there are no “disease-defining” mutations or genetic changes in CMML, although CMML is genetically distinct from MDS, Dr. Durakovic said.
For instance, SRSF2 encodes a component of the spliceosome that is mutated in almost half of CMML patients and less than 10% of MDS patients. Likewise, ASLX1 and TET2 are “much more frequently involved” in CMML than in MDS, Dr. Durakovic said.
In a 2012 study of 275 CMML patients, researchers found that 93% of patients had at least one somatic mutation in nine recurrently mutated genes – SRFS2, ASXL1, CBL, EZH2, JAK2V617F, KRAS, NRAS, RUNX1, and TET2 (Blood. 2012;120:3080-8).
However, Dr. Durakovic noted that these mutations are found in other disorders as well, so this information may not be helpful in differentiating CMML from other disorders.
A 2015 study revealed a technique that does appear useful for identifying CMML – monocyte subset distribution analysis. For this analysis, monocytes are divided into the following categories:
- Classical/MO1 (CD14bright/CD16−).
- Intermediate/MO2 (CD14bright/CD16+).
- Nonclassical/MO3 (CD14dim/CD16+).
The researchers found that CMML patients had an increase in the fraction of classical monocytes (with a cutoff value of 94%), as compared to healthy control subjects, patients with another hematologic disorder, and patients with reactive monocytosis (Blood. 2015 Jun 4;125[23]:3618-26).
A 2018 study confirmed that monocyte subset distribution analysis could differentiate CMML from other hematologic disorders, with the exception of atypical CML. This study also suggested that a decreased percentage of non-classical monocytes was more sensitive than an increased percentage of classical monocytes (Am J Clin Pathol. 2018 Aug 30;150[4]:293-302).
Despite the differences between these studies, “monocyte subset distribution analysis is showing promise as a method of identifying hard-to-identify CMML patients with ease and affordability,” Dr. Durakovic said.
She added that the technique can be implemented in clinical practice using the Hematoflow solution.
Dr. Durakovic did not report any conflicts of interest.
The Leukemia and Lymphoma meeting is organized by Jonathan Wood & Association, which is owned by the parent company of this news organization.
DUBROVNIK, CROATIA – in 2018.
Even with updated World Health Organization (WHO) criteria, karyotyping, and genetic analyses, it can be difficult to distinguish CMML from other conditions, according to Nadira Durakovic, MD, PhD, of the University Hospital Centre Zagreb (Croatia).
However, there are characteristics that differentiate CMML from myelodysplastic syndromes (MDS), myeloproliferative neoplasms (MPNs), and atypical chronic myeloid leukemia (CML), Dr. Durakovic said at Leukemia and Lymphoma, a meeting jointly sponsored by the University of Texas MD Anderson Cancer Center and the School of Medicine at the University of Zagreb, Croatia.
Studies have suggested that monocyte subset distribution analysis can be useful for diagnosing CMML.
According to the 2016 WHO classification, patients have CMML if:
- They have persistent peripheral blood monocytosis (1×109/L), with monocytes accounting for 10% of the white blood cell count.
- They do not meet WHO criteria for BCR-ABL1-positive CML, primary myelofibrosis, polycythemia vera, or essential thrombocythemia.
- There is no evidence of PCM1-JAK2 or PDGFRA, PDGFRB, or FGFR1 rearrangement.
- They have fewer than 20% blasts in the blood and bone marrow they have dysplasia in one or more myeloid lineages.
If myelodysplasia is absent or minimal, an acquired clonal cytogenetic or molecular genetic abnormality must be present. Alternatively, if patients have monocytosis that has persisted for at least 3 months, and all other causes of monocytosis have been excluded, “you can say that your patient has CMML,” Dr. Durakovic said.
Other causes of monocytosis include infections, malignancies, medications, inflammatory conditions, and other conditions, such as pregnancy.
However, Dr. Durakovic pointed out that the cause of monocytosis cannot always be determined, and, in some cases, CMML patients may not meet the WHO criteria.
“There are cases where there just aren’t enough monocytes to fulfill the WHO criteria,” Dr. Durakovic said. “You can have a patient with peripheral blood cytopenia and monocytosis who does not have 1,000 monocytes. Patients can have progressive dysplasia, can have splenomegaly, be really sick, but fail to meet WHO criteria.”
Differential diagnosis
“Differentiating CMML from myelodysplastic syndromes can be tough,” Dr. Durakovic said. “There are dysplastic features that are present in CMML ... but, in CMML, they are more subtle, and they are more difficult to appreciate than in myelodysplastic syndromes.”
The ratio of myeloid to erythroid cells is elevated in CMML, and patients may have atypical monocytes (paramyeloid cells) that are unique to CMML.
Dr. Durakovic noted that megakaryocyte dysplasia in CMML can be characterized by “myeloproliferative megakaryocytes,” which are large cells that cluster and have hyperlobulated nuclei, or “MDS megakaryocytes,” which are small, solitary cells with hypolobulated nuclei.
She noted that “MPN phenotype” CMML is characterized by leukocytosis, monocytosis, hepatomegaly, splenomegaly, and clinical features of myeloproliferation (fatigue, night sweats, bone pain, weight loss, etc.).
Thirty percent of cases are associated with splenomegaly, and 30% of patients can have an increase in bone marrow reticulin fibrosis.
Dr. Durakovic also noted that a prior MPN diagnosis excludes CMML. The presence of common MPN mutations, such as JAK2, CALR, or MPL, suggests a patient has an MPN with monocytosis rather than CMML.
Patients who have unclassified MPNs or MDS, rather than CMML, either do not have 1,000 monocytes or the monocytes do not represent more than 10% of the differential, Dr. Durakovic said.
It can also be difficult to differentiate CMML from atypical CML.
“Atypical CML is characterized by profound dysgranulopoiesis, absence of the BCR-ABL1 fusion gene, and neutrophilia,” Dr. Durakovic explained. “Those patients [commonly] have monocytosis, but, here, that 10% rule is valuable because their monocytes comprise less than 10% of the entire white blood cell count.”
Karyotyping, genotyping, and immunophenotyping
“There is no disease-defining karyotype abnormality [in CMML],” Dr. Durakovic said.
She said 30% of patients have abnormal karyotype, and the most common abnormality is trisomy 8. Unlike in patients with MDS, del(5q) and monosomal karyotypes are infrequent in patients with CMML.
Similarly, there are no “disease-defining” mutations or genetic changes in CMML, although CMML is genetically distinct from MDS, Dr. Durakovic said.
For instance, SRSF2 encodes a component of the spliceosome that is mutated in almost half of CMML patients and less than 10% of MDS patients. Likewise, ASLX1 and TET2 are “much more frequently involved” in CMML than in MDS, Dr. Durakovic said.
In a 2012 study of 275 CMML patients, researchers found that 93% of patients had at least one somatic mutation in nine recurrently mutated genes – SRFS2, ASXL1, CBL, EZH2, JAK2V617F, KRAS, NRAS, RUNX1, and TET2 (Blood. 2012;120:3080-8).
However, Dr. Durakovic noted that these mutations are found in other disorders as well, so this information may not be helpful in differentiating CMML from other disorders.
A 2015 study revealed a technique that does appear useful for identifying CMML – monocyte subset distribution analysis. For this analysis, monocytes are divided into the following categories:
- Classical/MO1 (CD14bright/CD16−).
- Intermediate/MO2 (CD14bright/CD16+).
- Nonclassical/MO3 (CD14dim/CD16+).
The researchers found that CMML patients had an increase in the fraction of classical monocytes (with a cutoff value of 94%), as compared to healthy control subjects, patients with another hematologic disorder, and patients with reactive monocytosis (Blood. 2015 Jun 4;125[23]:3618-26).
A 2018 study confirmed that monocyte subset distribution analysis could differentiate CMML from other hematologic disorders, with the exception of atypical CML. This study also suggested that a decreased percentage of non-classical monocytes was more sensitive than an increased percentage of classical monocytes (Am J Clin Pathol. 2018 Aug 30;150[4]:293-302).
Despite the differences between these studies, “monocyte subset distribution analysis is showing promise as a method of identifying hard-to-identify CMML patients with ease and affordability,” Dr. Durakovic said.
She added that the technique can be implemented in clinical practice using the Hematoflow solution.
Dr. Durakovic did not report any conflicts of interest.
The Leukemia and Lymphoma meeting is organized by Jonathan Wood & Association, which is owned by the parent company of this news organization.
EXPERT ANALYSIS FROM LEUKEMIA AND LYMPHOMA 2018
Study supports sequencing in kids with cancer
SAN DIEGO—Comprehensive next-generation sequencing is both feasible and clinically useful in pediatric cancer patients, a new study suggests.
Researchers sequenced samples from 253 pediatric cancer patients and found that, in 79% of cases, there was at least one finding that could help guide care.
Scott Newman, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee, presented these findings at the American Society of Human Genetics (ASHG) 2018 Annual Meeting (abstract 52).
The researchers conducted whole-genome, exome, and transcriptome sequencing of the patients’ tumors, as well as sequencing non-cancerous tissues from the same patients.
Of the 253 patients studied, 123 had hematologic malignancies.
The researchers found a mean of four pathogenic or likely pathogenic variants per patient (range, 0-18). This included prognostic (21.8%) and diagnostic (15.1%) variants as well as variants that could be targeted therapeutically (6.8%).
In all, 79% of the patients had at least one variant that was targetable, diagnostic, or prognostic. And test results were available within about 40 days, quickly enough that they could be used to guide care.
“With results available in a clinically relevant time frame, and pricing becoming increasingly comparable to the radiology and pathology tests, WGS [whole-genome sequencing] is becoming more accessible to pediatric oncology patients,” Dr. Newman said.
This work was part of the Genomes for Kids study (G4K), an effort to understand how best to use genetic data for pediatric cancer diagnosis and treatment. St. Jude has compiled the information from G4K into a publicly accessible online database.
The researchers have continued to perform sequencing on current patients, and, since the original study ended, have successfully used this method on roughly 300 additional patients. The team plans to continue studying sequencing methods in hopes of producing clinically applicable data more quickly.
G4K was sponsored by St. Jude.
SAN DIEGO—Comprehensive next-generation sequencing is both feasible and clinically useful in pediatric cancer patients, a new study suggests.
Researchers sequenced samples from 253 pediatric cancer patients and found that, in 79% of cases, there was at least one finding that could help guide care.
Scott Newman, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee, presented these findings at the American Society of Human Genetics (ASHG) 2018 Annual Meeting (abstract 52).
The researchers conducted whole-genome, exome, and transcriptome sequencing of the patients’ tumors, as well as sequencing non-cancerous tissues from the same patients.
Of the 253 patients studied, 123 had hematologic malignancies.
The researchers found a mean of four pathogenic or likely pathogenic variants per patient (range, 0-18). This included prognostic (21.8%) and diagnostic (15.1%) variants as well as variants that could be targeted therapeutically (6.8%).
In all, 79% of the patients had at least one variant that was targetable, diagnostic, or prognostic. And test results were available within about 40 days, quickly enough that they could be used to guide care.
“With results available in a clinically relevant time frame, and pricing becoming increasingly comparable to the radiology and pathology tests, WGS [whole-genome sequencing] is becoming more accessible to pediatric oncology patients,” Dr. Newman said.
This work was part of the Genomes for Kids study (G4K), an effort to understand how best to use genetic data for pediatric cancer diagnosis and treatment. St. Jude has compiled the information from G4K into a publicly accessible online database.
The researchers have continued to perform sequencing on current patients, and, since the original study ended, have successfully used this method on roughly 300 additional patients. The team plans to continue studying sequencing methods in hopes of producing clinically applicable data more quickly.
G4K was sponsored by St. Jude.
SAN DIEGO—Comprehensive next-generation sequencing is both feasible and clinically useful in pediatric cancer patients, a new study suggests.
Researchers sequenced samples from 253 pediatric cancer patients and found that, in 79% of cases, there was at least one finding that could help guide care.
Scott Newman, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee, presented these findings at the American Society of Human Genetics (ASHG) 2018 Annual Meeting (abstract 52).
The researchers conducted whole-genome, exome, and transcriptome sequencing of the patients’ tumors, as well as sequencing non-cancerous tissues from the same patients.
Of the 253 patients studied, 123 had hematologic malignancies.
The researchers found a mean of four pathogenic or likely pathogenic variants per patient (range, 0-18). This included prognostic (21.8%) and diagnostic (15.1%) variants as well as variants that could be targeted therapeutically (6.8%).
In all, 79% of the patients had at least one variant that was targetable, diagnostic, or prognostic. And test results were available within about 40 days, quickly enough that they could be used to guide care.
“With results available in a clinically relevant time frame, and pricing becoming increasingly comparable to the radiology and pathology tests, WGS [whole-genome sequencing] is becoming more accessible to pediatric oncology patients,” Dr. Newman said.
This work was part of the Genomes for Kids study (G4K), an effort to understand how best to use genetic data for pediatric cancer diagnosis and treatment. St. Jude has compiled the information from G4K into a publicly accessible online database.
The researchers have continued to perform sequencing on current patients, and, since the original study ended, have successfully used this method on roughly 300 additional patients. The team plans to continue studying sequencing methods in hopes of producing clinically applicable data more quickly.
G4K was sponsored by St. Jude.
‘Intense’ end-of-life care may be common in HSCT recipients
Patients who die within a year of allogeneic hematopoietic stem cell transplant (HSCT) tend to receive “medically intense” end-of-life care, an analysis suggests.
Researchers studied more than 2,000 patients who died within a year of allogeneic HSCT and found that a majority of the patients died in the hospital, and about half of them were admitted to the intensive care unit (ICU).
However, patient age, underlying diagnosis, and other factors influenced the likelihood of receiving intense end-of-life care.
For example, patients diagnosed with acute myeloid leukemia (AML) or myelodysplastic syndromes (MDS) were less likely than patients with acute lymphoblastic leukemia (ALL) to receive medically intense care.
Emily Johnston, MD, of the University of Alabama at Birmingham, and her colleagues reported these findings in the Journal of Clinical Oncology.
The researchers studied 2,135 patients in California who underwent inpatient HSCT and died within a year of the transplant (not as a result of peripartum events or trauma) between 2000 and 2013.
Fifty-three percent of the patients received some type of medically intense intervention, and 57% had at least two types of intense interventions.
Eighty-three percent of patients died in hospital, and 43% spent all of their last 30 days in the hospital.
Forty-nine percent of patients were admitted to the ICU, 45% were intubated, 22% underwent hemodialysis, and 8% received cardiopulmonary resuscitation.
Factors associated with intense care
The researchers said receipt of a medically intense intervention varied by age at death, underlying diagnosis, year of HSCT, location of care, and comorbidities. However, use of intense interventions did not vary according to sex, race/ethnicity, insurance type, or income.
Compared to patients age 60 and older, patients in the following age groups were more likely to receive medically intense interventions:
- Ages 15 to 21—odds ratio (OR)=2.6 (P<0.001)
- Ages 30 to 39—OR=1.8 (P<0.01)
- Ages 40 to 49—OR=1.4 (P<0.05).
Patients with comorbidities were more likely to receive intense interventions as well. The OR was 1.6 (P<0.01) for patients with one comorbidity and 2.5 (P<0.001) for patients with two or more comorbidities.
Patients with AML or MDS were less likely than patients with ALL to receive a medically intense intervention—OR=0.7 (P<0.05).
Patients who were transplanted between 2000 and 2004 were less likely to receive an intense intervention than patients transplanted between 2010 and 2013—OR=0.7 (P<0.01).
Patients who changed hospitals between HSCT and death were less likely to receive an intense intervention than patients who stayed at the same hospital. The OR was 0.3 if they transferred to a community hospital and 0.4 if they transferred to a specialty hospital (P<0.001 for both).
Patients living in rural areas were less likely than urban patients to receive a medically intense intervention—OR=0.6 (P<0.05).
“From our data, we understand there is a correlation with high-intensity end-of-life care in patients who die within one year after receiving a stem cell transplant, but we are still unsure if that was the care they wanted,” Dr. Johnston said.
“The findings suggest that, as oncologists, we need to start having end-of-life care conversations earlier with patients to determine if a high-intensity treatment plan is consistent with their goals or if a lower-intensity treatment plan is best. It’s not a one-size-fits-all approach in end-of-life care.”
This research was supported by Stanford University. One study author reported relationships with Corvus Pharmaceuticals, Shire Pharmaceuticals, and Adaptive Biotechnologies. All other authors reported no conflicts.
Patients who die within a year of allogeneic hematopoietic stem cell transplant (HSCT) tend to receive “medically intense” end-of-life care, an analysis suggests.
Researchers studied more than 2,000 patients who died within a year of allogeneic HSCT and found that a majority of the patients died in the hospital, and about half of them were admitted to the intensive care unit (ICU).
However, patient age, underlying diagnosis, and other factors influenced the likelihood of receiving intense end-of-life care.
For example, patients diagnosed with acute myeloid leukemia (AML) or myelodysplastic syndromes (MDS) were less likely than patients with acute lymphoblastic leukemia (ALL) to receive medically intense care.
Emily Johnston, MD, of the University of Alabama at Birmingham, and her colleagues reported these findings in the Journal of Clinical Oncology.
The researchers studied 2,135 patients in California who underwent inpatient HSCT and died within a year of the transplant (not as a result of peripartum events or trauma) between 2000 and 2013.
Fifty-three percent of the patients received some type of medically intense intervention, and 57% had at least two types of intense interventions.
Eighty-three percent of patients died in hospital, and 43% spent all of their last 30 days in the hospital.
Forty-nine percent of patients were admitted to the ICU, 45% were intubated, 22% underwent hemodialysis, and 8% received cardiopulmonary resuscitation.
Factors associated with intense care
The researchers said receipt of a medically intense intervention varied by age at death, underlying diagnosis, year of HSCT, location of care, and comorbidities. However, use of intense interventions did not vary according to sex, race/ethnicity, insurance type, or income.
Compared to patients age 60 and older, patients in the following age groups were more likely to receive medically intense interventions:
- Ages 15 to 21—odds ratio (OR)=2.6 (P<0.001)
- Ages 30 to 39—OR=1.8 (P<0.01)
- Ages 40 to 49—OR=1.4 (P<0.05).
Patients with comorbidities were more likely to receive intense interventions as well. The OR was 1.6 (P<0.01) for patients with one comorbidity and 2.5 (P<0.001) for patients with two or more comorbidities.
Patients with AML or MDS were less likely than patients with ALL to receive a medically intense intervention—OR=0.7 (P<0.05).
Patients who were transplanted between 2000 and 2004 were less likely to receive an intense intervention than patients transplanted between 2010 and 2013—OR=0.7 (P<0.01).
Patients who changed hospitals between HSCT and death were less likely to receive an intense intervention than patients who stayed at the same hospital. The OR was 0.3 if they transferred to a community hospital and 0.4 if they transferred to a specialty hospital (P<0.001 for both).
Patients living in rural areas were less likely than urban patients to receive a medically intense intervention—OR=0.6 (P<0.05).
“From our data, we understand there is a correlation with high-intensity end-of-life care in patients who die within one year after receiving a stem cell transplant, but we are still unsure if that was the care they wanted,” Dr. Johnston said.
“The findings suggest that, as oncologists, we need to start having end-of-life care conversations earlier with patients to determine if a high-intensity treatment plan is consistent with their goals or if a lower-intensity treatment plan is best. It’s not a one-size-fits-all approach in end-of-life care.”
This research was supported by Stanford University. One study author reported relationships with Corvus Pharmaceuticals, Shire Pharmaceuticals, and Adaptive Biotechnologies. All other authors reported no conflicts.
Patients who die within a year of allogeneic hematopoietic stem cell transplant (HSCT) tend to receive “medically intense” end-of-life care, an analysis suggests.
Researchers studied more than 2,000 patients who died within a year of allogeneic HSCT and found that a majority of the patients died in the hospital, and about half of them were admitted to the intensive care unit (ICU).
However, patient age, underlying diagnosis, and other factors influenced the likelihood of receiving intense end-of-life care.
For example, patients diagnosed with acute myeloid leukemia (AML) or myelodysplastic syndromes (MDS) were less likely than patients with acute lymphoblastic leukemia (ALL) to receive medically intense care.
Emily Johnston, MD, of the University of Alabama at Birmingham, and her colleagues reported these findings in the Journal of Clinical Oncology.
The researchers studied 2,135 patients in California who underwent inpatient HSCT and died within a year of the transplant (not as a result of peripartum events or trauma) between 2000 and 2013.
Fifty-three percent of the patients received some type of medically intense intervention, and 57% had at least two types of intense interventions.
Eighty-three percent of patients died in hospital, and 43% spent all of their last 30 days in the hospital.
Forty-nine percent of patients were admitted to the ICU, 45% were intubated, 22% underwent hemodialysis, and 8% received cardiopulmonary resuscitation.
Factors associated with intense care
The researchers said receipt of a medically intense intervention varied by age at death, underlying diagnosis, year of HSCT, location of care, and comorbidities. However, use of intense interventions did not vary according to sex, race/ethnicity, insurance type, or income.
Compared to patients age 60 and older, patients in the following age groups were more likely to receive medically intense interventions:
- Ages 15 to 21—odds ratio (OR)=2.6 (P<0.001)
- Ages 30 to 39—OR=1.8 (P<0.01)
- Ages 40 to 49—OR=1.4 (P<0.05).
Patients with comorbidities were more likely to receive intense interventions as well. The OR was 1.6 (P<0.01) for patients with one comorbidity and 2.5 (P<0.001) for patients with two or more comorbidities.
Patients with AML or MDS were less likely than patients with ALL to receive a medically intense intervention—OR=0.7 (P<0.05).
Patients who were transplanted between 2000 and 2004 were less likely to receive an intense intervention than patients transplanted between 2010 and 2013—OR=0.7 (P<0.01).
Patients who changed hospitals between HSCT and death were less likely to receive an intense intervention than patients who stayed at the same hospital. The OR was 0.3 if they transferred to a community hospital and 0.4 if they transferred to a specialty hospital (P<0.001 for both).
Patients living in rural areas were less likely than urban patients to receive a medically intense intervention—OR=0.6 (P<0.05).
“From our data, we understand there is a correlation with high-intensity end-of-life care in patients who die within one year after receiving a stem cell transplant, but we are still unsure if that was the care they wanted,” Dr. Johnston said.
“The findings suggest that, as oncologists, we need to start having end-of-life care conversations earlier with patients to determine if a high-intensity treatment plan is consistent with their goals or if a lower-intensity treatment plan is best. It’s not a one-size-fits-all approach in end-of-life care.”
This research was supported by Stanford University. One study author reported relationships with Corvus Pharmaceuticals, Shire Pharmaceuticals, and Adaptive Biotechnologies. All other authors reported no conflicts.
Adoptive T-cell therapy treats PML
Adoptive T-cell therapy has proven effective for treating progressive multifocal leukoencephalopathy (PML), according to research published in The New England Journal of Medicine.
Researchers observed substantial improvements in three PML patients infused with donor T cells targeting the BK virus.
Although one patient ultimately died, two had complete clearance of the JC virus and no clinical signs of PML after treatment.
“The JC and BK viruses are genetically similar and share proteins that can be targeted by the immune system,” said study author Katy Rezvani, MD, PhD, of The University of Texas MD Anderson Cancer Center in Houston.
“Because of these similarities, we hypothesized that T cells developed against BK virus may also be effective against JC virus infection.”
Dr. Rezvani’s team developed a novel approach for the generation of BK virus-specific T cells from healthy donors and established a bank of viral-specific T cells for immediate clinical use.
The researchers treated three patients with third-party, partially human leukocyte antigen (HLA)-matched, BK virus-specific T cells.
Patient 1 was a 32-year-old female with acute myeloid leukemia (AML) who previously received a double cord blood transplant.
Patient 2 was a 73-year-old female with JAK2-positive polycythemia rubra vera (PV) who had been treated with ruxolitinib.
Patient 3 was a 35-year-old man with AIDS who had discontinued highly active antiretroviral therapy due to side effects and who was no longer able to walk.
Following the first infusion, all three patients had a reduction in JC viral load in their cerebrospinal fluid. Viral loads dropped from:
- 700 to 78 copies in the AML patient
- 230,000 to 5,200 in the PV patient
- 4,300 to 1,300 in the AIDS patient.
“After infusion of viral-specific T cells, patients 1 and 3 had clinical improvement with significant reduction in JC virus in their cerebrospinal fluid,” Dr. Rezvani said.
“Both patients responded despite persistent T-cell immunodeficiency, supporting the concept that the response was mediated by the adoptively infused viral-specific T cells, and there were no infusion-related reactions.”
The AML patient received two additional infusions, which resulted in clearance of the virus in the cerebrospinal fluid and no signs of PML 27 months after the first infusion.
The PV patient received a second infusion that further reduced JC viral load, but no additional improvement was seen. The patient died 8 months after the first infusion.
The AIDS patient received additional infusions, resulting in complete clearance of the JC virus. This patient has regained mobility, and, 9 months after the first infusion, he is able to walk with a cane.
“We are encouraged that off-the-shelf, third-party, partially HLA-matched BK viral-specific T cells may provide a therapy for PML,” Dr. Rezvani said. “Further study in a larger group of patients is required to determine the success rate, durability, and longer-term adverse events with this treatment.”
This study was supported with funding from the Myelodysplastic Syndromes and Acute Myeloid Leukemia Moon Shot, part of MD Anderson’s Moon Shots Program, as well as the National Institutes of Health.
Adoptive T-cell therapy has proven effective for treating progressive multifocal leukoencephalopathy (PML), according to research published in The New England Journal of Medicine.
Researchers observed substantial improvements in three PML patients infused with donor T cells targeting the BK virus.
Although one patient ultimately died, two had complete clearance of the JC virus and no clinical signs of PML after treatment.
“The JC and BK viruses are genetically similar and share proteins that can be targeted by the immune system,” said study author Katy Rezvani, MD, PhD, of The University of Texas MD Anderson Cancer Center in Houston.
“Because of these similarities, we hypothesized that T cells developed against BK virus may also be effective against JC virus infection.”
Dr. Rezvani’s team developed a novel approach for the generation of BK virus-specific T cells from healthy donors and established a bank of viral-specific T cells for immediate clinical use.
The researchers treated three patients with third-party, partially human leukocyte antigen (HLA)-matched, BK virus-specific T cells.
Patient 1 was a 32-year-old female with acute myeloid leukemia (AML) who previously received a double cord blood transplant.
Patient 2 was a 73-year-old female with JAK2-positive polycythemia rubra vera (PV) who had been treated with ruxolitinib.
Patient 3 was a 35-year-old man with AIDS who had discontinued highly active antiretroviral therapy due to side effects and who was no longer able to walk.
Following the first infusion, all three patients had a reduction in JC viral load in their cerebrospinal fluid. Viral loads dropped from:
- 700 to 78 copies in the AML patient
- 230,000 to 5,200 in the PV patient
- 4,300 to 1,300 in the AIDS patient.
“After infusion of viral-specific T cells, patients 1 and 3 had clinical improvement with significant reduction in JC virus in their cerebrospinal fluid,” Dr. Rezvani said.
“Both patients responded despite persistent T-cell immunodeficiency, supporting the concept that the response was mediated by the adoptively infused viral-specific T cells, and there were no infusion-related reactions.”
The AML patient received two additional infusions, which resulted in clearance of the virus in the cerebrospinal fluid and no signs of PML 27 months after the first infusion.
The PV patient received a second infusion that further reduced JC viral load, but no additional improvement was seen. The patient died 8 months after the first infusion.
The AIDS patient received additional infusions, resulting in complete clearance of the JC virus. This patient has regained mobility, and, 9 months after the first infusion, he is able to walk with a cane.
“We are encouraged that off-the-shelf, third-party, partially HLA-matched BK viral-specific T cells may provide a therapy for PML,” Dr. Rezvani said. “Further study in a larger group of patients is required to determine the success rate, durability, and longer-term adverse events with this treatment.”
This study was supported with funding from the Myelodysplastic Syndromes and Acute Myeloid Leukemia Moon Shot, part of MD Anderson’s Moon Shots Program, as well as the National Institutes of Health.
Adoptive T-cell therapy has proven effective for treating progressive multifocal leukoencephalopathy (PML), according to research published in The New England Journal of Medicine.
Researchers observed substantial improvements in three PML patients infused with donor T cells targeting the BK virus.
Although one patient ultimately died, two had complete clearance of the JC virus and no clinical signs of PML after treatment.
“The JC and BK viruses are genetically similar and share proteins that can be targeted by the immune system,” said study author Katy Rezvani, MD, PhD, of The University of Texas MD Anderson Cancer Center in Houston.
“Because of these similarities, we hypothesized that T cells developed against BK virus may also be effective against JC virus infection.”
Dr. Rezvani’s team developed a novel approach for the generation of BK virus-specific T cells from healthy donors and established a bank of viral-specific T cells for immediate clinical use.
The researchers treated three patients with third-party, partially human leukocyte antigen (HLA)-matched, BK virus-specific T cells.
Patient 1 was a 32-year-old female with acute myeloid leukemia (AML) who previously received a double cord blood transplant.
Patient 2 was a 73-year-old female with JAK2-positive polycythemia rubra vera (PV) who had been treated with ruxolitinib.
Patient 3 was a 35-year-old man with AIDS who had discontinued highly active antiretroviral therapy due to side effects and who was no longer able to walk.
Following the first infusion, all three patients had a reduction in JC viral load in their cerebrospinal fluid. Viral loads dropped from:
- 700 to 78 copies in the AML patient
- 230,000 to 5,200 in the PV patient
- 4,300 to 1,300 in the AIDS patient.
“After infusion of viral-specific T cells, patients 1 and 3 had clinical improvement with significant reduction in JC virus in their cerebrospinal fluid,” Dr. Rezvani said.
“Both patients responded despite persistent T-cell immunodeficiency, supporting the concept that the response was mediated by the adoptively infused viral-specific T cells, and there were no infusion-related reactions.”
The AML patient received two additional infusions, which resulted in clearance of the virus in the cerebrospinal fluid and no signs of PML 27 months after the first infusion.
The PV patient received a second infusion that further reduced JC viral load, but no additional improvement was seen. The patient died 8 months after the first infusion.
The AIDS patient received additional infusions, resulting in complete clearance of the JC virus. This patient has regained mobility, and, 9 months after the first infusion, he is able to walk with a cane.
“We are encouraged that off-the-shelf, third-party, partially HLA-matched BK viral-specific T cells may provide a therapy for PML,” Dr. Rezvani said. “Further study in a larger group of patients is required to determine the success rate, durability, and longer-term adverse events with this treatment.”
This study was supported with funding from the Myelodysplastic Syndromes and Acute Myeloid Leukemia Moon Shot, part of MD Anderson’s Moon Shots Program, as well as the National Institutes of Health.
Dataset could reveal better therapies for AML
Researchers have released a dataset detailing the molecular makeup of tumor cells from more than 500 patients with acute myeloid leukemia (AML).
The team discovered mutations not previously observed in AML and found associations between mutations and responses to certain therapies.
For instance, AML cases with FLT3, NPM1, and DNMT3A mutations proved sensitive to the BTK inhibitor ibrutinib.
The researchers described their findings in Nature.
The team also made their dataset available via Vizome, an online data viewer. Other researchers can use Vizome to find out which targeted therapies might be most effective against specific subsets of AML cells.
“People can get online, search our database, and very quickly get answers to ‘Is this a good drug?’ or ‘Is there a patient population my drug can work in?’” said study author Brian Druker, MD, of Oregon Health & Science University (OHSU) in Portland, Oregon.
Newly identified mutations
For this study, part of the Beat AML initiative, Dr. Druker and his colleagues performed whole-exome and RNA sequencing on 672 samples from 562 AML patients.
The team identified mutations in 11 genes that were called in 1% or more of patients in this dataset but had not been observed in previous AML sequencing studies. The genes were:
- CUB and Sushi multiple domains 2 (CSMD2)
- NAC alpha domain containing (NACAD)
- Teneurin transmembrane protein 2 (TENM2)
- Aggrecan (ACAN)
- ADAM metallopeptidase with thrombospondin type 1 motif 7 (ADAMTS7)
- Immunoglobulin-like and fibronectin type III domain containing 1 (IGFN1)
- Neurobeachin-like 2 (NBEAL2)
- Poly(U) binding splicing factor 60 (PUF60)
- Zinc-finger protein 687 (ZNF687)
- Cadherin EGF LAG sevenpass G-type receptor 2 (CELSR2)
- Glutamate ionotropic receptor NMDA type subunit 2B (GRIN2B).
Testing therapies
The researchers also assessed how AML cells from 409 of the patient samples responded to each of 122 targeted therapies.
The team found that mutations in TP53, ASXL1, NRAS, and KRAS caused “a broad pattern of drug resistance.”
However, cases with TP53 mutations were sensitive to elesclomol (a drug that targets cancer cell metabolism), cases with ASXL1 mutations were sensitive to the HDAC inhibitor panobinostat, and cases with KRAS/NRAS mutations were sensitive to MAPK inhibitors (with NRAS-mutated cases demonstrating greater sensitivity).
The researchers also found that IDH2 mutations “conferred sensitivity to a broad spectrum of drugs,” but IDH1 mutations were associated with resistance to most drugs.
As previously mentioned, the researchers found a significant association between mutations in FLT3, NPM1, and DNMT3A and sensitivity to ibrutinib. However, the team found that cases with DNMT3A mutations alone or mutations in DNMT3A and FLT3 were not significantly different from cases with wild-type genes.
On the other hand, cases with FLT3-ITD alone or any combination with a mutation in NPM1 (including mutations in all three genes) were significantly more sensitive to ibrutinib than cases with wild-type genes.
Cases with FLT3-ITD and mutations in NPM1 were sensitive to another kinase inhibitor, entospletinib, as well.
The researchers also found that mutations in both BCOR and RUNX1 correlated with increased sensitivity to four JAK inhibitors—momelotinib, ruxolitinib, tofacitinib, and JAK inhibitor I.
However, cases with BCOR mutations alone or mutations in BCOR and DNMT3A or SRSF2 showed no difference in sensitivity to the JAK inhibitors from cases with wild-type genes.
Next steps
“We’re just starting to scratch the surface of what we can do when we analyze the data,” Dr. Druker said. “The real power comes when you start to integrate all that data. You can analyze what drug worked and why it worked.”
In fact, the researchers are already developing and initiating clinical trials to test hypotheses generated by this study.
“You can start to sense some momentum building with new, better therapeutics for AML patients, and, hopefully, this dataset will help fuel that momentum even further,” said study author Jeff Tyner, PhD, of the OHSU School of Medicine.
“We want to parlay this information into clinical trials as much as we can, and we also want the broader community to use this dataset to accelerate their own work.”
Funding for the current study was provided by grants from The Leukemia & Lymphoma Society, the National Cancer Institute, the National Library of Medicine, and other groups.
Researchers have released a dataset detailing the molecular makeup of tumor cells from more than 500 patients with acute myeloid leukemia (AML).
The team discovered mutations not previously observed in AML and found associations between mutations and responses to certain therapies.
For instance, AML cases with FLT3, NPM1, and DNMT3A mutations proved sensitive to the BTK inhibitor ibrutinib.
The researchers described their findings in Nature.
The team also made their dataset available via Vizome, an online data viewer. Other researchers can use Vizome to find out which targeted therapies might be most effective against specific subsets of AML cells.
“People can get online, search our database, and very quickly get answers to ‘Is this a good drug?’ or ‘Is there a patient population my drug can work in?’” said study author Brian Druker, MD, of Oregon Health & Science University (OHSU) in Portland, Oregon.
Newly identified mutations
For this study, part of the Beat AML initiative, Dr. Druker and his colleagues performed whole-exome and RNA sequencing on 672 samples from 562 AML patients.
The team identified mutations in 11 genes that were called in 1% or more of patients in this dataset but had not been observed in previous AML sequencing studies. The genes were:
- CUB and Sushi multiple domains 2 (CSMD2)
- NAC alpha domain containing (NACAD)
- Teneurin transmembrane protein 2 (TENM2)
- Aggrecan (ACAN)
- ADAM metallopeptidase with thrombospondin type 1 motif 7 (ADAMTS7)
- Immunoglobulin-like and fibronectin type III domain containing 1 (IGFN1)
- Neurobeachin-like 2 (NBEAL2)
- Poly(U) binding splicing factor 60 (PUF60)
- Zinc-finger protein 687 (ZNF687)
- Cadherin EGF LAG sevenpass G-type receptor 2 (CELSR2)
- Glutamate ionotropic receptor NMDA type subunit 2B (GRIN2B).
Testing therapies
The researchers also assessed how AML cells from 409 of the patient samples responded to each of 122 targeted therapies.
The team found that mutations in TP53, ASXL1, NRAS, and KRAS caused “a broad pattern of drug resistance.”
However, cases with TP53 mutations were sensitive to elesclomol (a drug that targets cancer cell metabolism), cases with ASXL1 mutations were sensitive to the HDAC inhibitor panobinostat, and cases with KRAS/NRAS mutations were sensitive to MAPK inhibitors (with NRAS-mutated cases demonstrating greater sensitivity).
The researchers also found that IDH2 mutations “conferred sensitivity to a broad spectrum of drugs,” but IDH1 mutations were associated with resistance to most drugs.
As previously mentioned, the researchers found a significant association between mutations in FLT3, NPM1, and DNMT3A and sensitivity to ibrutinib. However, the team found that cases with DNMT3A mutations alone or mutations in DNMT3A and FLT3 were not significantly different from cases with wild-type genes.
On the other hand, cases with FLT3-ITD alone or any combination with a mutation in NPM1 (including mutations in all three genes) were significantly more sensitive to ibrutinib than cases with wild-type genes.
Cases with FLT3-ITD and mutations in NPM1 were sensitive to another kinase inhibitor, entospletinib, as well.
The researchers also found that mutations in both BCOR and RUNX1 correlated with increased sensitivity to four JAK inhibitors—momelotinib, ruxolitinib, tofacitinib, and JAK inhibitor I.
However, cases with BCOR mutations alone or mutations in BCOR and DNMT3A or SRSF2 showed no difference in sensitivity to the JAK inhibitors from cases with wild-type genes.
Next steps
“We’re just starting to scratch the surface of what we can do when we analyze the data,” Dr. Druker said. “The real power comes when you start to integrate all that data. You can analyze what drug worked and why it worked.”
In fact, the researchers are already developing and initiating clinical trials to test hypotheses generated by this study.
“You can start to sense some momentum building with new, better therapeutics for AML patients, and, hopefully, this dataset will help fuel that momentum even further,” said study author Jeff Tyner, PhD, of the OHSU School of Medicine.
“We want to parlay this information into clinical trials as much as we can, and we also want the broader community to use this dataset to accelerate their own work.”
Funding for the current study was provided by grants from The Leukemia & Lymphoma Society, the National Cancer Institute, the National Library of Medicine, and other groups.
Researchers have released a dataset detailing the molecular makeup of tumor cells from more than 500 patients with acute myeloid leukemia (AML).
The team discovered mutations not previously observed in AML and found associations between mutations and responses to certain therapies.
For instance, AML cases with FLT3, NPM1, and DNMT3A mutations proved sensitive to the BTK inhibitor ibrutinib.
The researchers described their findings in Nature.
The team also made their dataset available via Vizome, an online data viewer. Other researchers can use Vizome to find out which targeted therapies might be most effective against specific subsets of AML cells.
“People can get online, search our database, and very quickly get answers to ‘Is this a good drug?’ or ‘Is there a patient population my drug can work in?’” said study author Brian Druker, MD, of Oregon Health & Science University (OHSU) in Portland, Oregon.
Newly identified mutations
For this study, part of the Beat AML initiative, Dr. Druker and his colleagues performed whole-exome and RNA sequencing on 672 samples from 562 AML patients.
The team identified mutations in 11 genes that were called in 1% or more of patients in this dataset but had not been observed in previous AML sequencing studies. The genes were:
- CUB and Sushi multiple domains 2 (CSMD2)
- NAC alpha domain containing (NACAD)
- Teneurin transmembrane protein 2 (TENM2)
- Aggrecan (ACAN)
- ADAM metallopeptidase with thrombospondin type 1 motif 7 (ADAMTS7)
- Immunoglobulin-like and fibronectin type III domain containing 1 (IGFN1)
- Neurobeachin-like 2 (NBEAL2)
- Poly(U) binding splicing factor 60 (PUF60)
- Zinc-finger protein 687 (ZNF687)
- Cadherin EGF LAG sevenpass G-type receptor 2 (CELSR2)
- Glutamate ionotropic receptor NMDA type subunit 2B (GRIN2B).
Testing therapies
The researchers also assessed how AML cells from 409 of the patient samples responded to each of 122 targeted therapies.
The team found that mutations in TP53, ASXL1, NRAS, and KRAS caused “a broad pattern of drug resistance.”
However, cases with TP53 mutations were sensitive to elesclomol (a drug that targets cancer cell metabolism), cases with ASXL1 mutations were sensitive to the HDAC inhibitor panobinostat, and cases with KRAS/NRAS mutations were sensitive to MAPK inhibitors (with NRAS-mutated cases demonstrating greater sensitivity).
The researchers also found that IDH2 mutations “conferred sensitivity to a broad spectrum of drugs,” but IDH1 mutations were associated with resistance to most drugs.
As previously mentioned, the researchers found a significant association between mutations in FLT3, NPM1, and DNMT3A and sensitivity to ibrutinib. However, the team found that cases with DNMT3A mutations alone or mutations in DNMT3A and FLT3 were not significantly different from cases with wild-type genes.
On the other hand, cases with FLT3-ITD alone or any combination with a mutation in NPM1 (including mutations in all three genes) were significantly more sensitive to ibrutinib than cases with wild-type genes.
Cases with FLT3-ITD and mutations in NPM1 were sensitive to another kinase inhibitor, entospletinib, as well.
The researchers also found that mutations in both BCOR and RUNX1 correlated with increased sensitivity to four JAK inhibitors—momelotinib, ruxolitinib, tofacitinib, and JAK inhibitor I.
However, cases with BCOR mutations alone or mutations in BCOR and DNMT3A or SRSF2 showed no difference in sensitivity to the JAK inhibitors from cases with wild-type genes.
Next steps
“We’re just starting to scratch the surface of what we can do when we analyze the data,” Dr. Druker said. “The real power comes when you start to integrate all that data. You can analyze what drug worked and why it worked.”
In fact, the researchers are already developing and initiating clinical trials to test hypotheses generated by this study.
“You can start to sense some momentum building with new, better therapeutics for AML patients, and, hopefully, this dataset will help fuel that momentum even further,” said study author Jeff Tyner, PhD, of the OHSU School of Medicine.
“We want to parlay this information into clinical trials as much as we can, and we also want the broader community to use this dataset to accelerate their own work.”
Funding for the current study was provided by grants from The Leukemia & Lymphoma Society, the National Cancer Institute, the National Library of Medicine, and other groups.