User login
Stopping statins linked to death, CV events in elderly
Deprescribing may help in reducing inappropriate medication use and adverse events, but for cardiovascular care in the elderly, eliminating statins among patients taking other medications may have negative effects that far outweigh the benefits, a new study suggests.
In a large cohort study, researchers found that the withdrawal of statins from an elderly population receiving polypharmacy was associated with an increase in the risk for hospital admission for heart failure and any cardiovascular outcome, as well as death from any cause.
Statins are “lifesaving” drugs, and “according to the findings of our study, the discontinuation of this therapy has significant effects,” lead study author Federico Rea, PhD, research fellow, Laboratory of Healthcare Research and Pharmacoepidemiology, the department of statistics and quantitative methods, the University of Milano-Bicocca, said in an interview.
The article was published online June 14, 2021, in JAMA Network Open.
Negative clinical consequences, including adverse drug reactions leading to hospitalizations, are causing more physicians to consider deprescribing as a way to reduce problems associated with polypharmacy, the researchers noted.
Statins are “the most widely prescribed medication in the Western world, being a pivotal component in the primary and secondary prevention of cardiovascular (CV) diseases,” they wrote, but because randomized trials usually exclude patients with serious clinical conditions, the precise role statins play for frail patients, such as those with polypharmacy, “is still unclear.”
The population-based cohort study examined 29,047 Italian residents aged 65 years and older who were receiving uninterrupted treatment with statins as well as blood pressure–lowering, antidiabetic, and antiplatelet agents over 16 months. The follow-up period was more than 3 years.
The cohort members were followed to identify those for whom statins were discontinued. Those who continued taking other therapies during the first 6 months after stopping statins were propensity score matched in a 1:1 ratio with patients who did not discontinue taking statins or other drugs. The patient pairs were then followed for fatal and nonfatal outcomes to estimate the risk associated with statin discontinuation.
Of the overall cohort exposed to polypharmacy, 5819 (20.0%) discontinued statins while continuing to take their other medications. Of those, 4,010 were matched with a comparator.
Compared with the maintaining group, those who discontinued statins had the following outcomes: an increased risk for hospital admissions for heart failure (hazard ratio, 1.24; 95% confidence interval, 1.07-1.43), any cardiovascular outcomes (HR, 1.14; 95% CI, 1.03-1.26), death from any cause (HR, 1.15; 95% CI, 1.02-1.30), and emergency admissions for any cause (HR, 1.12; 95% CI, 1.01-1.19)
The increased risk occurred in patients with mild or severe profiles, regardless of gender and whether statins were prescribed as primary or secondary CV prevention.
“We expected that the discontinuation of statins could reduce the risk of access to the emergency department for neurological causes, considered a proxy for the onset of episodes of delirium, [but] this was not observed, suggesting that statin therapy has essential benefits on the reduction of fatal/nonfatal cardiovascular events with no harm effect,” said Dr. Rea, “at least considering major adverse events like hospital and emergency department admissions.”
Findings no surprise
Neil Stone, MD, Bonow Professor of Medicine (Cardiology) and Preventive Medicine at Northwestern University, Chicago, said the study results aren’t surprising.
“Older patients have a higher absolute risk of dying, and withdrawing proven therapy shown to reduce risk of coronary/stroke events in randomized, controlled trials would be expected to result in more cardiovascular events,” Dr. Stone said.
Although polypharmacy is a concern for the elderly and is a factor in decreased adherence, he said better solutions are needed than withdrawing proven, effective therapy. “In that sense, this study indirectly supports more research in the use of polypills to address cardiovascular risk factors,” he said. Giving a single pill that combines medications of proven value in reducing blood pressure and cholesterol might be preferable to reducing the total number of medications.
Given the complexity of polypharmacy, the study investigators say more attention is needed from all health care professionals who care for elderly patients.
“We hope that future studies can shed light on the best way to balance the undeniable benefit of [statins] and the harms, especially among the elderly exposed to polypharmacy,” said Rea.
Further research is also needed into why statins are discontinued in the first place, added Dr. Stone. “We know that statins often are stopped due to symptoms that on further scrutiny may not be related to statin use.”
The study was funded by grants from Fondo d’Ateneo per la Ricerca and Modelling Effectiveness, Cost-effectiveness, and Promoting Health Care Value in the Real World: the Motive Project from the Italian Ministry of the Education, University, and Research. One coauthor served on the advisory board of Roche and has received grants from Bristol Myers Squibb, GlaxoSmithKline, and Novartis outside the submitted work. The other authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Deprescribing may help in reducing inappropriate medication use and adverse events, but for cardiovascular care in the elderly, eliminating statins among patients taking other medications may have negative effects that far outweigh the benefits, a new study suggests.
In a large cohort study, researchers found that the withdrawal of statins from an elderly population receiving polypharmacy was associated with an increase in the risk for hospital admission for heart failure and any cardiovascular outcome, as well as death from any cause.
Statins are “lifesaving” drugs, and “according to the findings of our study, the discontinuation of this therapy has significant effects,” lead study author Federico Rea, PhD, research fellow, Laboratory of Healthcare Research and Pharmacoepidemiology, the department of statistics and quantitative methods, the University of Milano-Bicocca, said in an interview.
The article was published online June 14, 2021, in JAMA Network Open.
Negative clinical consequences, including adverse drug reactions leading to hospitalizations, are causing more physicians to consider deprescribing as a way to reduce problems associated with polypharmacy, the researchers noted.
Statins are “the most widely prescribed medication in the Western world, being a pivotal component in the primary and secondary prevention of cardiovascular (CV) diseases,” they wrote, but because randomized trials usually exclude patients with serious clinical conditions, the precise role statins play for frail patients, such as those with polypharmacy, “is still unclear.”
The population-based cohort study examined 29,047 Italian residents aged 65 years and older who were receiving uninterrupted treatment with statins as well as blood pressure–lowering, antidiabetic, and antiplatelet agents over 16 months. The follow-up period was more than 3 years.
The cohort members were followed to identify those for whom statins were discontinued. Those who continued taking other therapies during the first 6 months after stopping statins were propensity score matched in a 1:1 ratio with patients who did not discontinue taking statins or other drugs. The patient pairs were then followed for fatal and nonfatal outcomes to estimate the risk associated with statin discontinuation.
Of the overall cohort exposed to polypharmacy, 5819 (20.0%) discontinued statins while continuing to take their other medications. Of those, 4,010 were matched with a comparator.
Compared with the maintaining group, those who discontinued statins had the following outcomes: an increased risk for hospital admissions for heart failure (hazard ratio, 1.24; 95% confidence interval, 1.07-1.43), any cardiovascular outcomes (HR, 1.14; 95% CI, 1.03-1.26), death from any cause (HR, 1.15; 95% CI, 1.02-1.30), and emergency admissions for any cause (HR, 1.12; 95% CI, 1.01-1.19)
The increased risk occurred in patients with mild or severe profiles, regardless of gender and whether statins were prescribed as primary or secondary CV prevention.
“We expected that the discontinuation of statins could reduce the risk of access to the emergency department for neurological causes, considered a proxy for the onset of episodes of delirium, [but] this was not observed, suggesting that statin therapy has essential benefits on the reduction of fatal/nonfatal cardiovascular events with no harm effect,” said Dr. Rea, “at least considering major adverse events like hospital and emergency department admissions.”
Findings no surprise
Neil Stone, MD, Bonow Professor of Medicine (Cardiology) and Preventive Medicine at Northwestern University, Chicago, said the study results aren’t surprising.
“Older patients have a higher absolute risk of dying, and withdrawing proven therapy shown to reduce risk of coronary/stroke events in randomized, controlled trials would be expected to result in more cardiovascular events,” Dr. Stone said.
Although polypharmacy is a concern for the elderly and is a factor in decreased adherence, he said better solutions are needed than withdrawing proven, effective therapy. “In that sense, this study indirectly supports more research in the use of polypills to address cardiovascular risk factors,” he said. Giving a single pill that combines medications of proven value in reducing blood pressure and cholesterol might be preferable to reducing the total number of medications.
Given the complexity of polypharmacy, the study investigators say more attention is needed from all health care professionals who care for elderly patients.
“We hope that future studies can shed light on the best way to balance the undeniable benefit of [statins] and the harms, especially among the elderly exposed to polypharmacy,” said Rea.
Further research is also needed into why statins are discontinued in the first place, added Dr. Stone. “We know that statins often are stopped due to symptoms that on further scrutiny may not be related to statin use.”
The study was funded by grants from Fondo d’Ateneo per la Ricerca and Modelling Effectiveness, Cost-effectiveness, and Promoting Health Care Value in the Real World: the Motive Project from the Italian Ministry of the Education, University, and Research. One coauthor served on the advisory board of Roche and has received grants from Bristol Myers Squibb, GlaxoSmithKline, and Novartis outside the submitted work. The other authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Deprescribing may help in reducing inappropriate medication use and adverse events, but for cardiovascular care in the elderly, eliminating statins among patients taking other medications may have negative effects that far outweigh the benefits, a new study suggests.
In a large cohort study, researchers found that the withdrawal of statins from an elderly population receiving polypharmacy was associated with an increase in the risk for hospital admission for heart failure and any cardiovascular outcome, as well as death from any cause.
Statins are “lifesaving” drugs, and “according to the findings of our study, the discontinuation of this therapy has significant effects,” lead study author Federico Rea, PhD, research fellow, Laboratory of Healthcare Research and Pharmacoepidemiology, the department of statistics and quantitative methods, the University of Milano-Bicocca, said in an interview.
The article was published online June 14, 2021, in JAMA Network Open.
Negative clinical consequences, including adverse drug reactions leading to hospitalizations, are causing more physicians to consider deprescribing as a way to reduce problems associated with polypharmacy, the researchers noted.
Statins are “the most widely prescribed medication in the Western world, being a pivotal component in the primary and secondary prevention of cardiovascular (CV) diseases,” they wrote, but because randomized trials usually exclude patients with serious clinical conditions, the precise role statins play for frail patients, such as those with polypharmacy, “is still unclear.”
The population-based cohort study examined 29,047 Italian residents aged 65 years and older who were receiving uninterrupted treatment with statins as well as blood pressure–lowering, antidiabetic, and antiplatelet agents over 16 months. The follow-up period was more than 3 years.
The cohort members were followed to identify those for whom statins were discontinued. Those who continued taking other therapies during the first 6 months after stopping statins were propensity score matched in a 1:1 ratio with patients who did not discontinue taking statins or other drugs. The patient pairs were then followed for fatal and nonfatal outcomes to estimate the risk associated with statin discontinuation.
Of the overall cohort exposed to polypharmacy, 5819 (20.0%) discontinued statins while continuing to take their other medications. Of those, 4,010 were matched with a comparator.
Compared with the maintaining group, those who discontinued statins had the following outcomes: an increased risk for hospital admissions for heart failure (hazard ratio, 1.24; 95% confidence interval, 1.07-1.43), any cardiovascular outcomes (HR, 1.14; 95% CI, 1.03-1.26), death from any cause (HR, 1.15; 95% CI, 1.02-1.30), and emergency admissions for any cause (HR, 1.12; 95% CI, 1.01-1.19)
The increased risk occurred in patients with mild or severe profiles, regardless of gender and whether statins were prescribed as primary or secondary CV prevention.
“We expected that the discontinuation of statins could reduce the risk of access to the emergency department for neurological causes, considered a proxy for the onset of episodes of delirium, [but] this was not observed, suggesting that statin therapy has essential benefits on the reduction of fatal/nonfatal cardiovascular events with no harm effect,” said Dr. Rea, “at least considering major adverse events like hospital and emergency department admissions.”
Findings no surprise
Neil Stone, MD, Bonow Professor of Medicine (Cardiology) and Preventive Medicine at Northwestern University, Chicago, said the study results aren’t surprising.
“Older patients have a higher absolute risk of dying, and withdrawing proven therapy shown to reduce risk of coronary/stroke events in randomized, controlled trials would be expected to result in more cardiovascular events,” Dr. Stone said.
Although polypharmacy is a concern for the elderly and is a factor in decreased adherence, he said better solutions are needed than withdrawing proven, effective therapy. “In that sense, this study indirectly supports more research in the use of polypills to address cardiovascular risk factors,” he said. Giving a single pill that combines medications of proven value in reducing blood pressure and cholesterol might be preferable to reducing the total number of medications.
Given the complexity of polypharmacy, the study investigators say more attention is needed from all health care professionals who care for elderly patients.
“We hope that future studies can shed light on the best way to balance the undeniable benefit of [statins] and the harms, especially among the elderly exposed to polypharmacy,” said Rea.
Further research is also needed into why statins are discontinued in the first place, added Dr. Stone. “We know that statins often are stopped due to symptoms that on further scrutiny may not be related to statin use.”
The study was funded by grants from Fondo d’Ateneo per la Ricerca and Modelling Effectiveness, Cost-effectiveness, and Promoting Health Care Value in the Real World: the Motive Project from the Italian Ministry of the Education, University, and Research. One coauthor served on the advisory board of Roche and has received grants from Bristol Myers Squibb, GlaxoSmithKline, and Novartis outside the submitted work. The other authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Clinicians slow to implement lipid-lowering guidelines: GOULD registry
Among patients with atherosclerotic cardiovascular disease (ASCVD), 2 years after release of treat-to-target guidelines from the American Heart Association and the European Society of Cardiology and European Atherosclerosis Society, most patients with LDL cholesterol higher than 70 mg/dL did not receive intensification of therapy, and two-thirds continued to have LDL levels above that level, according to a prospective registry study.
Both guidelines recommend driving LDL-C levels to 50% or below of baseline levels; results from the Getting to an Improved Understanding of Low-Density Lipoprotein Cholesterol and Dyslipidemia Management (GOULD) registry suggest this is rarely achieved. “Unfortunately it’s not a total surprise, but it’s disappointing,” said Christopher Cannon, MD, the study’s lead author.
“Therapeutic inertia seems to be the rule in clinical practice,” said Jennifer G. Robinson, MD, MPH, who was asked to comment on the study. Dr. Robinson is professor epidemiology and cardiology at the University of Iowa, Iowa City.
“This is yet another disappointing reminder of how we are failing our patients. Lipid lowering is one of the safest, most effective ways to prevent cardiovascular disease, and yet we are falling short. We have the tools in our toolkit to achieve guideline-based lipid lowering goals, but we just aren’t using them,” said Ann Marie Navar, MD, PhD, associate professor of cardiology at the University of Texas, Dallas.
Patients hesitant
Changes in practice following guidelines can often be slow, but in this case may have been complicated by the fact that statins have a reputation for causing side effects, so some patients may be refusing treatment based on what they’ve seen on the Internet. Even though the study looked at all lipid-lowering agents, the misinformation around statins may be spilling over, according to Dr. Cannon. “There’s in general so much misinformation around COVID and every other topic in the world. That makes people question what is real [about] anything,” said Dr. Cannon, a cardiologist at Brigham and Women’s hospital and professor of medicine at Harvard Medical School, both in Boston.
Patient characteristics may partly explain slow uptake. “Clinicians may not think further LDL-C lowering is a high enough priority in terms of potential benefit for a given patient in light of the effort being expended to take care of all their other issues and chronic health problems. If the clinician does bring it up to the patient, there may be barriers in terms of additional medication burden, cost, or acquisition issues,” said Dr. Robinson.
The answer may be better evidence and a more personalized approach. Clinical trials that explore defined patient populations could convince patients of a benefit, and payers to reimburse, according to Dr. Robinson.
Changing guidance
Another complication is that both the guidelines and the field are rapidly changing. The 2013 AHA guidelines did not include a treatment to goal and focused instead on use of high-dose statins. But the 2018 update reversed course after randomized studies demonstrated a benefit to treating to target. The researchers found no increase in the frequency of treating to target after the release of the 2018 guidelines. “Publication and announcement of guidelines doesn’t mean that people are getting treated better. We really have to implement them,” said Dr. Cannon.
On a positive note, the GOULD researchers found high acceptance of the new proprotein convertase subtilisin/kexin type 9 serine protease (PCSK9) inhibitors, with over 90% of patients continuing those medications after 2 years. “That’s nice and high. If people do get onto the very intensive lipid-lowing therapies, they tend to stay on them,” said Dr. Cannon.
What’s next
Still, the lack of intensification is concerning, and the findings led to some consternation in Twitter exchanges, said Dr. Cannon. “People posted ‘Well, what do we do now?’ ” Dr. Cannon’s team is addressing the issue with an algorithm-based risk management program with prospective enrollment. They have conducted educational webinars and provided site-specific reports on LDL status among patients at each center compared to others, and hope that information will improve compliance. In 2020, the group published an interim analysis of the first 5,000 enrollees, and Dr. Cannon expects to finish that study by the end of the year.
Dr. Navar agreed that physicians need to do a better job of testing LDL-C levels after treatment to identify patients who require more aggressive therapy. That can be deferred in some primary prevention patients with high LDL-C but normal particle numbers as measured with ApoB. “But in those at high risk for disease and those with established CVD who are not at goal, as long as they don’t have a life-limiting condition, we should always up-titrate therapy. It’s one of the safest, most effective ways to lower cardiovascular risk,” said Dr. Navar.
Key study results
The prospective study included 5,006 patients at 119 centers with a mean age of 68 years. About 40% were women, and 86.1% were White. All had ASCVD and LDL levels of at least 70 mg/dL. After 2 years, 17% had undergone intensification of lipid-lowering therapy (LLT). Among patients with LDL-C levels ≥ 100 mg/dL, 22% underwent LLT intensification, compared with 14% of patients with LDL-C levels of 70-99 mg/dL.
The vast majority, 92%, of patients who underwent LLT via addition of PCSK9 inhibitors were still taking the drug after 2 years.
Three-quarters (3,768) had lipid level measurements at least once during follow-up, and median LDL-C levels dropped from 120 to 95 mg/dL in the ≥100-mg/dL cohort (P < .001), and from 82 to 77 mg/dL in the 70- to 99-mg/dL cohort (P <. 001). There was no significant difference in the median values in the patients on PCSK9 inhibitors.
In all, 21% of the ≥100-mg/dL cohort achieved LDL-C levels <70 mg/dL at 2 years, versus 34% in the 77- to 99-mg/dL cohort and 52% of patients taking PCSK9 inhibitors.
Patients seen at teaching hospitals were more likely to undergo LLT intensification compared to nonteaching hospitals (25% versus 17%; P < .001), as were those where lipid protocols were in place (22% versus 15%; P < .001), and those treated in cardiology (22%) compared to treatment in internal or family medicine (12%; P <.001). The study was published online June 16 in JAMA Cardiology.
Dr. Cannon, Dr. Navar, and Dr. Robinson disclosed ties with Amgen, which funded the study, and other companies.
Among patients with atherosclerotic cardiovascular disease (ASCVD), 2 years after release of treat-to-target guidelines from the American Heart Association and the European Society of Cardiology and European Atherosclerosis Society, most patients with LDL cholesterol higher than 70 mg/dL did not receive intensification of therapy, and two-thirds continued to have LDL levels above that level, according to a prospective registry study.
Both guidelines recommend driving LDL-C levels to 50% or below of baseline levels; results from the Getting to an Improved Understanding of Low-Density Lipoprotein Cholesterol and Dyslipidemia Management (GOULD) registry suggest this is rarely achieved. “Unfortunately it’s not a total surprise, but it’s disappointing,” said Christopher Cannon, MD, the study’s lead author.
“Therapeutic inertia seems to be the rule in clinical practice,” said Jennifer G. Robinson, MD, MPH, who was asked to comment on the study. Dr. Robinson is professor epidemiology and cardiology at the University of Iowa, Iowa City.
“This is yet another disappointing reminder of how we are failing our patients. Lipid lowering is one of the safest, most effective ways to prevent cardiovascular disease, and yet we are falling short. We have the tools in our toolkit to achieve guideline-based lipid lowering goals, but we just aren’t using them,” said Ann Marie Navar, MD, PhD, associate professor of cardiology at the University of Texas, Dallas.
Patients hesitant
Changes in practice following guidelines can often be slow, but in this case may have been complicated by the fact that statins have a reputation for causing side effects, so some patients may be refusing treatment based on what they’ve seen on the Internet. Even though the study looked at all lipid-lowering agents, the misinformation around statins may be spilling over, according to Dr. Cannon. “There’s in general so much misinformation around COVID and every other topic in the world. That makes people question what is real [about] anything,” said Dr. Cannon, a cardiologist at Brigham and Women’s hospital and professor of medicine at Harvard Medical School, both in Boston.
Patient characteristics may partly explain slow uptake. “Clinicians may not think further LDL-C lowering is a high enough priority in terms of potential benefit for a given patient in light of the effort being expended to take care of all their other issues and chronic health problems. If the clinician does bring it up to the patient, there may be barriers in terms of additional medication burden, cost, or acquisition issues,” said Dr. Robinson.
The answer may be better evidence and a more personalized approach. Clinical trials that explore defined patient populations could convince patients of a benefit, and payers to reimburse, according to Dr. Robinson.
Changing guidance
Another complication is that both the guidelines and the field are rapidly changing. The 2013 AHA guidelines did not include a treatment to goal and focused instead on use of high-dose statins. But the 2018 update reversed course after randomized studies demonstrated a benefit to treating to target. The researchers found no increase in the frequency of treating to target after the release of the 2018 guidelines. “Publication and announcement of guidelines doesn’t mean that people are getting treated better. We really have to implement them,” said Dr. Cannon.
On a positive note, the GOULD researchers found high acceptance of the new proprotein convertase subtilisin/kexin type 9 serine protease (PCSK9) inhibitors, with over 90% of patients continuing those medications after 2 years. “That’s nice and high. If people do get onto the very intensive lipid-lowing therapies, they tend to stay on them,” said Dr. Cannon.
What’s next
Still, the lack of intensification is concerning, and the findings led to some consternation in Twitter exchanges, said Dr. Cannon. “People posted ‘Well, what do we do now?’ ” Dr. Cannon’s team is addressing the issue with an algorithm-based risk management program with prospective enrollment. They have conducted educational webinars and provided site-specific reports on LDL status among patients at each center compared to others, and hope that information will improve compliance. In 2020, the group published an interim analysis of the first 5,000 enrollees, and Dr. Cannon expects to finish that study by the end of the year.
Dr. Navar agreed that physicians need to do a better job of testing LDL-C levels after treatment to identify patients who require more aggressive therapy. That can be deferred in some primary prevention patients with high LDL-C but normal particle numbers as measured with ApoB. “But in those at high risk for disease and those with established CVD who are not at goal, as long as they don’t have a life-limiting condition, we should always up-titrate therapy. It’s one of the safest, most effective ways to lower cardiovascular risk,” said Dr. Navar.
Key study results
The prospective study included 5,006 patients at 119 centers with a mean age of 68 years. About 40% were women, and 86.1% were White. All had ASCVD and LDL levels of at least 70 mg/dL. After 2 years, 17% had undergone intensification of lipid-lowering therapy (LLT). Among patients with LDL-C levels ≥ 100 mg/dL, 22% underwent LLT intensification, compared with 14% of patients with LDL-C levels of 70-99 mg/dL.
The vast majority, 92%, of patients who underwent LLT via addition of PCSK9 inhibitors were still taking the drug after 2 years.
Three-quarters (3,768) had lipid level measurements at least once during follow-up, and median LDL-C levels dropped from 120 to 95 mg/dL in the ≥100-mg/dL cohort (P < .001), and from 82 to 77 mg/dL in the 70- to 99-mg/dL cohort (P <. 001). There was no significant difference in the median values in the patients on PCSK9 inhibitors.
In all, 21% of the ≥100-mg/dL cohort achieved LDL-C levels <70 mg/dL at 2 years, versus 34% in the 77- to 99-mg/dL cohort and 52% of patients taking PCSK9 inhibitors.
Patients seen at teaching hospitals were more likely to undergo LLT intensification compared to nonteaching hospitals (25% versus 17%; P < .001), as were those where lipid protocols were in place (22% versus 15%; P < .001), and those treated in cardiology (22%) compared to treatment in internal or family medicine (12%; P <.001). The study was published online June 16 in JAMA Cardiology.
Dr. Cannon, Dr. Navar, and Dr. Robinson disclosed ties with Amgen, which funded the study, and other companies.
Among patients with atherosclerotic cardiovascular disease (ASCVD), 2 years after release of treat-to-target guidelines from the American Heart Association and the European Society of Cardiology and European Atherosclerosis Society, most patients with LDL cholesterol higher than 70 mg/dL did not receive intensification of therapy, and two-thirds continued to have LDL levels above that level, according to a prospective registry study.
Both guidelines recommend driving LDL-C levels to 50% or below of baseline levels; results from the Getting to an Improved Understanding of Low-Density Lipoprotein Cholesterol and Dyslipidemia Management (GOULD) registry suggest this is rarely achieved. “Unfortunately it’s not a total surprise, but it’s disappointing,” said Christopher Cannon, MD, the study’s lead author.
“Therapeutic inertia seems to be the rule in clinical practice,” said Jennifer G. Robinson, MD, MPH, who was asked to comment on the study. Dr. Robinson is professor epidemiology and cardiology at the University of Iowa, Iowa City.
“This is yet another disappointing reminder of how we are failing our patients. Lipid lowering is one of the safest, most effective ways to prevent cardiovascular disease, and yet we are falling short. We have the tools in our toolkit to achieve guideline-based lipid lowering goals, but we just aren’t using them,” said Ann Marie Navar, MD, PhD, associate professor of cardiology at the University of Texas, Dallas.
Patients hesitant
Changes in practice following guidelines can often be slow, but in this case may have been complicated by the fact that statins have a reputation for causing side effects, so some patients may be refusing treatment based on what they’ve seen on the Internet. Even though the study looked at all lipid-lowering agents, the misinformation around statins may be spilling over, according to Dr. Cannon. “There’s in general so much misinformation around COVID and every other topic in the world. That makes people question what is real [about] anything,” said Dr. Cannon, a cardiologist at Brigham and Women’s hospital and professor of medicine at Harvard Medical School, both in Boston.
Patient characteristics may partly explain slow uptake. “Clinicians may not think further LDL-C lowering is a high enough priority in terms of potential benefit for a given patient in light of the effort being expended to take care of all their other issues and chronic health problems. If the clinician does bring it up to the patient, there may be barriers in terms of additional medication burden, cost, or acquisition issues,” said Dr. Robinson.
The answer may be better evidence and a more personalized approach. Clinical trials that explore defined patient populations could convince patients of a benefit, and payers to reimburse, according to Dr. Robinson.
Changing guidance
Another complication is that both the guidelines and the field are rapidly changing. The 2013 AHA guidelines did not include a treatment to goal and focused instead on use of high-dose statins. But the 2018 update reversed course after randomized studies demonstrated a benefit to treating to target. The researchers found no increase in the frequency of treating to target after the release of the 2018 guidelines. “Publication and announcement of guidelines doesn’t mean that people are getting treated better. We really have to implement them,” said Dr. Cannon.
On a positive note, the GOULD researchers found high acceptance of the new proprotein convertase subtilisin/kexin type 9 serine protease (PCSK9) inhibitors, with over 90% of patients continuing those medications after 2 years. “That’s nice and high. If people do get onto the very intensive lipid-lowing therapies, they tend to stay on them,” said Dr. Cannon.
What’s next
Still, the lack of intensification is concerning, and the findings led to some consternation in Twitter exchanges, said Dr. Cannon. “People posted ‘Well, what do we do now?’ ” Dr. Cannon’s team is addressing the issue with an algorithm-based risk management program with prospective enrollment. They have conducted educational webinars and provided site-specific reports on LDL status among patients at each center compared to others, and hope that information will improve compliance. In 2020, the group published an interim analysis of the first 5,000 enrollees, and Dr. Cannon expects to finish that study by the end of the year.
Dr. Navar agreed that physicians need to do a better job of testing LDL-C levels after treatment to identify patients who require more aggressive therapy. That can be deferred in some primary prevention patients with high LDL-C but normal particle numbers as measured with ApoB. “But in those at high risk for disease and those with established CVD who are not at goal, as long as they don’t have a life-limiting condition, we should always up-titrate therapy. It’s one of the safest, most effective ways to lower cardiovascular risk,” said Dr. Navar.
Key study results
The prospective study included 5,006 patients at 119 centers with a mean age of 68 years. About 40% were women, and 86.1% were White. All had ASCVD and LDL levels of at least 70 mg/dL. After 2 years, 17% had undergone intensification of lipid-lowering therapy (LLT). Among patients with LDL-C levels ≥ 100 mg/dL, 22% underwent LLT intensification, compared with 14% of patients with LDL-C levels of 70-99 mg/dL.
The vast majority, 92%, of patients who underwent LLT via addition of PCSK9 inhibitors were still taking the drug after 2 years.
Three-quarters (3,768) had lipid level measurements at least once during follow-up, and median LDL-C levels dropped from 120 to 95 mg/dL in the ≥100-mg/dL cohort (P < .001), and from 82 to 77 mg/dL in the 70- to 99-mg/dL cohort (P <. 001). There was no significant difference in the median values in the patients on PCSK9 inhibitors.
In all, 21% of the ≥100-mg/dL cohort achieved LDL-C levels <70 mg/dL at 2 years, versus 34% in the 77- to 99-mg/dL cohort and 52% of patients taking PCSK9 inhibitors.
Patients seen at teaching hospitals were more likely to undergo LLT intensification compared to nonteaching hospitals (25% versus 17%; P < .001), as were those where lipid protocols were in place (22% versus 15%; P < .001), and those treated in cardiology (22%) compared to treatment in internal or family medicine (12%; P <.001). The study was published online June 16 in JAMA Cardiology.
Dr. Cannon, Dr. Navar, and Dr. Robinson disclosed ties with Amgen, which funded the study, and other companies.
FROM JAMA CARDIOLOGY
No overall statin effect seen on dementia, cognition in ASPREE analysis
Statin therapy likely didn’t lead to dementia or even mild cognitive impairment (MCI) in older patients taking the drugs for cardiovascular (CV) primary prevention in a post hoc analysis of a trial that required normal cognitive ability for entry.
Nor did statins, whether lipophilic or hydrophilic, appear to influence changes in cognition or affect separate domains of mental performance, such as memory, language ability, or executive function, over the trial’s follow-up, which averaged almost 5 years.
Although such findings aren’t novel – they are consistent with observations from a number of earlier studies – the new analysis included a possible signal for a statin association with new-onset dementia in a subgroup of more than 18,000 patients. Researchers attribute the retrospective finding, from a trial not designed to explore the issue, to confounding or chance.
Still, the adjusted risk for dementia seemed to go up by a third among statin users who at baseline placed in the lowest quartile for cognitive function, based on a composite test score, in the ASPREE trial, a test of primary-prevention low-dose aspirin in patients 65 or older. The better the baseline cognitive score by quartile, the lower the risk for dementia ( interaction P < .001).
The bottom-quartile association of statins with dementia was driven by new diagnoses of Alzheimer’s disease, as opposed to the study’s other “mixed presentation” dementia subtype, wrote the authors of analysis, published June 21, 2021, in the Journal of the American College of Cardiology), led by Zhen Zhou, PhD, Menzies Institute for Medical Research, University of Tasmania, Hobart, Australia.
“I wouldn’t overinterpret that,” said senior author Mark R. Nelson, MBBS, PhD, of the same institution. Indeed, it should be “reassuring” for physicians prescribing statins to older patients that there was no overall statin effect on cognition or new-onset dementia, he said in an interview.
“This is a post hoc analysis within a dataset, although a very-high-quality dataset, it must be said.” The patients were prospectively followed for a range of cognition domains, and the results were adjudicated, Dr. Nelson observed. Although the question of statins and dementia risk is thought to be largely settled, the analysis “was just too tempting not to do.”
On the basis of the current analysis and the bulk of preceding evidence, “lipid lowering in the short term does not appear to result in improvement or deterioration of cognition irrespective of baseline LDL cholesterol levels and medication used,” Christie M. Ballantyne, MD, and Vijay Nambi, MD, PhD, both from Baylor College of Medicine, Houston, wrote in an accompanying editorial.
The current study “provides additional information that the lipo- or hydrophilicity of the statin does not affect changes in cognition. However, the potential increased risk for Alzheimer’s disease, especially among patients with baseline cognitive impairment, requires further investigation.”
The current analysis is reassuring that the likelihood of such statin effects on cognition “is vanishingly small,” Neil J. Stone MD, Northwestern University, Chicago, said in an interview. In fact, its primary finding of no such association “best summarizes what we know in 2021 about statin therapy” after exploration of the issue in a number of prospective trials and systematic reviews, said Dr. Stone, who was not a coauthor on the report.
The observed interaction between statin use and baseline neurocognitive ability “is hypothesis raising at best. It should be explored in randomized, controlled trials that can look at this question in an unbiased manner,” he agreed.
If patients believe or suspect that a statin is causing symptoms that suggest cognitive dysfunction, “what they really need to do is to stop it for 3 weeks and check out other causes. And in rechallenging, the guidelines say, if they think that it’s causing a memory problem that occurs anecdotally, then they can be given another statin, usually, which doesn’t cause it.”
ASPREE compared daily low-dose aspirin with placebo in a community-based older population numbering about 19,000 in Australia and the United States. Patients were initially without known CV disease, dementia, or physical disabilities. It did not randomize patients by statin therapy.
Of note, entry to the trial required a score of at least 78 on the Modified Mini-Mental State Examination (3MS), corresponding to normal cognition.
Aspirin showed no significant benefit for disability-free survival, an endpoint that included death and dementia, or CV events over a median of 4.7 years. It was associated with slightly more cases of major hemorrhage, as previously reported.
A subsequent ASPREE analysis suggested that the aspirin had no effect on risks of mild cognitive impairment, cognitive decline, or dementia.
Of the 18,846 patients in the current post hoc analysis, the average age of the patients was 74 years, and 56.4% were women; 31.3% were taking statins at baseline. The incidence of dementia per 1,000 person-years for those taking statins in comparison with those not taking statins was 6.91 and 6.48, respectively. Any cognitive changes were tracked by the 3MS and three other validated tests in different domains of cognition, with results contributing to the composite score.
The corresponding incidence of dementia considered probable Alzheimer’s disease was 2.97 and 2.65 for those receiving versus not receiving statins, respectively. The incidence of dementia with mixed presentation was 3.94 and 3.84, respectively.
There were no significant differences in risk for dementia overall or for either dementia subtype in multivariate analyses. Adjustments included demographics, CV lifestyle risk factors, family medical history, including dementia, ASPREE randomization group, and individual scores on the four tests of cognition.
Results for development of MCI mirrored those for dementia, as did results stratified for baseline lipids and for use of lipophilic statins, such as atorvastatin or simvastatin versus hydrophilic statins, including pravastatin and rosuvastatin.
Significant interactions were observed between composite cognitive scores and statin therapy at baseline; as scores increased, indicating better cognitive performance, the risks for dementia and its subtypes went down. Statins were associated with incident dementia at the lowest cognitive performance quartile.
That association is probably a function of the cohort’s advanced age, Dr. Nelson said. “If you get into old age, and you’ve got high cognitive scores, you’ve probably got protective factors. That’s how I would interpret that.”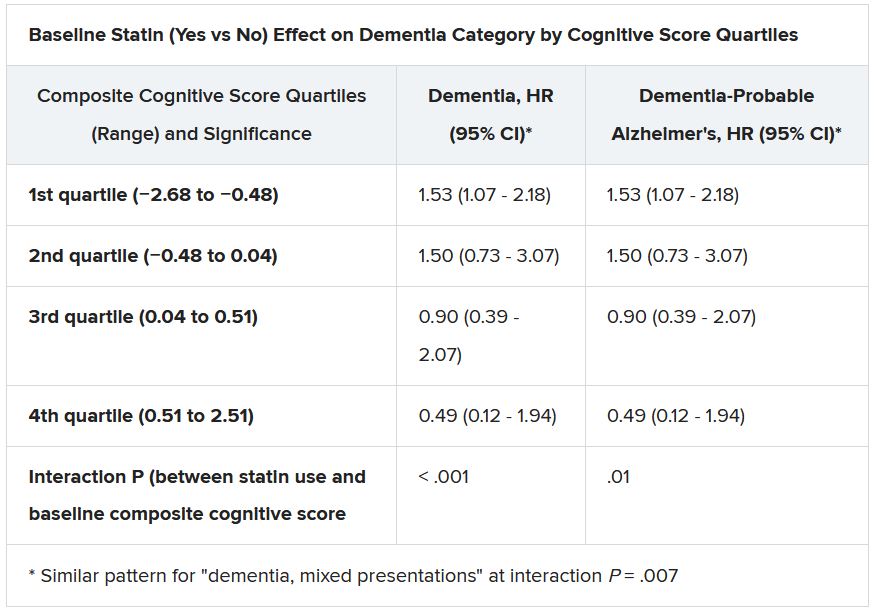
Dr. Ballantyne and Dr. Nambi also emphasized the difficulties of controlling for potential biases even with extensive covariate adjustments. The statin dosages at which patients were treated were not part of the analysis, “and achieved LDL [cholesterol levels over the study period were not known,” they wrote.
“Furthermore, patients who were treated with statins were more likely to have diabetes, hypertension, chronic kidney disease, and obesity, all of which are known to increase risk for cognitive decline, and, as might have been predicted, statin users therefore had significantly lower scores for global cognition and episodic memory.”
Dr. Nelson pointed to an ongoing prospective atorvastatin trial that includes dementia in its primary endpoint and should be “the definitive study.” STAREE (Statin Therapy for Reducing Events in the Elderly) is running throughout Australia with a projected enrollment of 18,000 and primary completion by the end of 2022. “We’ve already enrolled 8,000 patients.”
Less far along is the PREVENTABLE (Pragmatic Evaluation of Events and Benefits of Lipid-Lowering in Older Adults) trial, based in the United States and also randomizing to atorvastatin or placebo, that will have an estimated 20,000 older patients and completion in 5 years. The primary endpoint is new dementia or persistent disability.
Both trials “are powered to enable firm conclusions concerning any statin effects,” said Dr. Ballantyne and Dr. Nambi. “In the meantime, practicing clinicians can have confidence and share with their patients that short-term lipid-lowering therapy in older patients, including with statins, is unlikely to have a major impact on cognition.”
ASPREE was supported by grants from the U.S. National Institute on Aging and the National Cancer Institute and the National Health and Medical Research Council of Australia, by Monash University, and by the Victorian Cancer Agency. Dr. Nelson reported receiving honoraria from Sanofi and Amgen; support from Bayer for ASPREE; and grant support for STAREE. Disclosures for the other authors are in the report. Dr. Ballantyne disclosed grant and research support from Abbott Diagnostic, Akcea, Amgen, Esperion, Ionis, Novartis, Regeneron, and Roche Diagnostics; and consulting for Abbott Diagnostics, Althera, Amarin, Amgen, Arrowhead, AstraZeneca, Corvidia, Denka Seiken, Esperion, Genentech, Gilead, Matinas BioPharma, New Amsterdam, Novartis, Novo Nordisk, Pfizer, Regeneron, Roche Diagnostics, and Sanofi-Synthelabo. Dr. Nambi is a coinvestigator on a provisional patent along with Baylor College of Medicine and Roche on the use of biomarkers to predict heart failure, and a site principal investigator for studies sponsored by Amgen and Merck. Dr. Stone had no disclosures.
A version of this article first appeared on Medscape.com.
Statin therapy likely didn’t lead to dementia or even mild cognitive impairment (MCI) in older patients taking the drugs for cardiovascular (CV) primary prevention in a post hoc analysis of a trial that required normal cognitive ability for entry.
Nor did statins, whether lipophilic or hydrophilic, appear to influence changes in cognition or affect separate domains of mental performance, such as memory, language ability, or executive function, over the trial’s follow-up, which averaged almost 5 years.
Although such findings aren’t novel – they are consistent with observations from a number of earlier studies – the new analysis included a possible signal for a statin association with new-onset dementia in a subgroup of more than 18,000 patients. Researchers attribute the retrospective finding, from a trial not designed to explore the issue, to confounding or chance.
Still, the adjusted risk for dementia seemed to go up by a third among statin users who at baseline placed in the lowest quartile for cognitive function, based on a composite test score, in the ASPREE trial, a test of primary-prevention low-dose aspirin in patients 65 or older. The better the baseline cognitive score by quartile, the lower the risk for dementia ( interaction P < .001).
The bottom-quartile association of statins with dementia was driven by new diagnoses of Alzheimer’s disease, as opposed to the study’s other “mixed presentation” dementia subtype, wrote the authors of analysis, published June 21, 2021, in the Journal of the American College of Cardiology), led by Zhen Zhou, PhD, Menzies Institute for Medical Research, University of Tasmania, Hobart, Australia.
“I wouldn’t overinterpret that,” said senior author Mark R. Nelson, MBBS, PhD, of the same institution. Indeed, it should be “reassuring” for physicians prescribing statins to older patients that there was no overall statin effect on cognition or new-onset dementia, he said in an interview.
“This is a post hoc analysis within a dataset, although a very-high-quality dataset, it must be said.” The patients were prospectively followed for a range of cognition domains, and the results were adjudicated, Dr. Nelson observed. Although the question of statins and dementia risk is thought to be largely settled, the analysis “was just too tempting not to do.”
On the basis of the current analysis and the bulk of preceding evidence, “lipid lowering in the short term does not appear to result in improvement or deterioration of cognition irrespective of baseline LDL cholesterol levels and medication used,” Christie M. Ballantyne, MD, and Vijay Nambi, MD, PhD, both from Baylor College of Medicine, Houston, wrote in an accompanying editorial.
The current study “provides additional information that the lipo- or hydrophilicity of the statin does not affect changes in cognition. However, the potential increased risk for Alzheimer’s disease, especially among patients with baseline cognitive impairment, requires further investigation.”
The current analysis is reassuring that the likelihood of such statin effects on cognition “is vanishingly small,” Neil J. Stone MD, Northwestern University, Chicago, said in an interview. In fact, its primary finding of no such association “best summarizes what we know in 2021 about statin therapy” after exploration of the issue in a number of prospective trials and systematic reviews, said Dr. Stone, who was not a coauthor on the report.
The observed interaction between statin use and baseline neurocognitive ability “is hypothesis raising at best. It should be explored in randomized, controlled trials that can look at this question in an unbiased manner,” he agreed.
If patients believe or suspect that a statin is causing symptoms that suggest cognitive dysfunction, “what they really need to do is to stop it for 3 weeks and check out other causes. And in rechallenging, the guidelines say, if they think that it’s causing a memory problem that occurs anecdotally, then they can be given another statin, usually, which doesn’t cause it.”
ASPREE compared daily low-dose aspirin with placebo in a community-based older population numbering about 19,000 in Australia and the United States. Patients were initially without known CV disease, dementia, or physical disabilities. It did not randomize patients by statin therapy.
Of note, entry to the trial required a score of at least 78 on the Modified Mini-Mental State Examination (3MS), corresponding to normal cognition.
Aspirin showed no significant benefit for disability-free survival, an endpoint that included death and dementia, or CV events over a median of 4.7 years. It was associated with slightly more cases of major hemorrhage, as previously reported.
A subsequent ASPREE analysis suggested that the aspirin had no effect on risks of mild cognitive impairment, cognitive decline, or dementia.
Of the 18,846 patients in the current post hoc analysis, the average age of the patients was 74 years, and 56.4% were women; 31.3% were taking statins at baseline. The incidence of dementia per 1,000 person-years for those taking statins in comparison with those not taking statins was 6.91 and 6.48, respectively. Any cognitive changes were tracked by the 3MS and three other validated tests in different domains of cognition, with results contributing to the composite score.
The corresponding incidence of dementia considered probable Alzheimer’s disease was 2.97 and 2.65 for those receiving versus not receiving statins, respectively. The incidence of dementia with mixed presentation was 3.94 and 3.84, respectively.
There were no significant differences in risk for dementia overall or for either dementia subtype in multivariate analyses. Adjustments included demographics, CV lifestyle risk factors, family medical history, including dementia, ASPREE randomization group, and individual scores on the four tests of cognition.
Results for development of MCI mirrored those for dementia, as did results stratified for baseline lipids and for use of lipophilic statins, such as atorvastatin or simvastatin versus hydrophilic statins, including pravastatin and rosuvastatin.
Significant interactions were observed between composite cognitive scores and statin therapy at baseline; as scores increased, indicating better cognitive performance, the risks for dementia and its subtypes went down. Statins were associated with incident dementia at the lowest cognitive performance quartile.
That association is probably a function of the cohort’s advanced age, Dr. Nelson said. “If you get into old age, and you’ve got high cognitive scores, you’ve probably got protective factors. That’s how I would interpret that.”
Dr. Ballantyne and Dr. Nambi also emphasized the difficulties of controlling for potential biases even with extensive covariate adjustments. The statin dosages at which patients were treated were not part of the analysis, “and achieved LDL [cholesterol levels over the study period were not known,” they wrote.
“Furthermore, patients who were treated with statins were more likely to have diabetes, hypertension, chronic kidney disease, and obesity, all of which are known to increase risk for cognitive decline, and, as might have been predicted, statin users therefore had significantly lower scores for global cognition and episodic memory.”
Dr. Nelson pointed to an ongoing prospective atorvastatin trial that includes dementia in its primary endpoint and should be “the definitive study.” STAREE (Statin Therapy for Reducing Events in the Elderly) is running throughout Australia with a projected enrollment of 18,000 and primary completion by the end of 2022. “We’ve already enrolled 8,000 patients.”
Less far along is the PREVENTABLE (Pragmatic Evaluation of Events and Benefits of Lipid-Lowering in Older Adults) trial, based in the United States and also randomizing to atorvastatin or placebo, that will have an estimated 20,000 older patients and completion in 5 years. The primary endpoint is new dementia or persistent disability.
Both trials “are powered to enable firm conclusions concerning any statin effects,” said Dr. Ballantyne and Dr. Nambi. “In the meantime, practicing clinicians can have confidence and share with their patients that short-term lipid-lowering therapy in older patients, including with statins, is unlikely to have a major impact on cognition.”
ASPREE was supported by grants from the U.S. National Institute on Aging and the National Cancer Institute and the National Health and Medical Research Council of Australia, by Monash University, and by the Victorian Cancer Agency. Dr. Nelson reported receiving honoraria from Sanofi and Amgen; support from Bayer for ASPREE; and grant support for STAREE. Disclosures for the other authors are in the report. Dr. Ballantyne disclosed grant and research support from Abbott Diagnostic, Akcea, Amgen, Esperion, Ionis, Novartis, Regeneron, and Roche Diagnostics; and consulting for Abbott Diagnostics, Althera, Amarin, Amgen, Arrowhead, AstraZeneca, Corvidia, Denka Seiken, Esperion, Genentech, Gilead, Matinas BioPharma, New Amsterdam, Novartis, Novo Nordisk, Pfizer, Regeneron, Roche Diagnostics, and Sanofi-Synthelabo. Dr. Nambi is a coinvestigator on a provisional patent along with Baylor College of Medicine and Roche on the use of biomarkers to predict heart failure, and a site principal investigator for studies sponsored by Amgen and Merck. Dr. Stone had no disclosures.
A version of this article first appeared on Medscape.com.
Statin therapy likely didn’t lead to dementia or even mild cognitive impairment (MCI) in older patients taking the drugs for cardiovascular (CV) primary prevention in a post hoc analysis of a trial that required normal cognitive ability for entry.
Nor did statins, whether lipophilic or hydrophilic, appear to influence changes in cognition or affect separate domains of mental performance, such as memory, language ability, or executive function, over the trial’s follow-up, which averaged almost 5 years.
Although such findings aren’t novel – they are consistent with observations from a number of earlier studies – the new analysis included a possible signal for a statin association with new-onset dementia in a subgroup of more than 18,000 patients. Researchers attribute the retrospective finding, from a trial not designed to explore the issue, to confounding or chance.
Still, the adjusted risk for dementia seemed to go up by a third among statin users who at baseline placed in the lowest quartile for cognitive function, based on a composite test score, in the ASPREE trial, a test of primary-prevention low-dose aspirin in patients 65 or older. The better the baseline cognitive score by quartile, the lower the risk for dementia ( interaction P < .001).
The bottom-quartile association of statins with dementia was driven by new diagnoses of Alzheimer’s disease, as opposed to the study’s other “mixed presentation” dementia subtype, wrote the authors of analysis, published June 21, 2021, in the Journal of the American College of Cardiology), led by Zhen Zhou, PhD, Menzies Institute for Medical Research, University of Tasmania, Hobart, Australia.
“I wouldn’t overinterpret that,” said senior author Mark R. Nelson, MBBS, PhD, of the same institution. Indeed, it should be “reassuring” for physicians prescribing statins to older patients that there was no overall statin effect on cognition or new-onset dementia, he said in an interview.
“This is a post hoc analysis within a dataset, although a very-high-quality dataset, it must be said.” The patients were prospectively followed for a range of cognition domains, and the results were adjudicated, Dr. Nelson observed. Although the question of statins and dementia risk is thought to be largely settled, the analysis “was just too tempting not to do.”
On the basis of the current analysis and the bulk of preceding evidence, “lipid lowering in the short term does not appear to result in improvement or deterioration of cognition irrespective of baseline LDL cholesterol levels and medication used,” Christie M. Ballantyne, MD, and Vijay Nambi, MD, PhD, both from Baylor College of Medicine, Houston, wrote in an accompanying editorial.
The current study “provides additional information that the lipo- or hydrophilicity of the statin does not affect changes in cognition. However, the potential increased risk for Alzheimer’s disease, especially among patients with baseline cognitive impairment, requires further investigation.”
The current analysis is reassuring that the likelihood of such statin effects on cognition “is vanishingly small,” Neil J. Stone MD, Northwestern University, Chicago, said in an interview. In fact, its primary finding of no such association “best summarizes what we know in 2021 about statin therapy” after exploration of the issue in a number of prospective trials and systematic reviews, said Dr. Stone, who was not a coauthor on the report.
The observed interaction between statin use and baseline neurocognitive ability “is hypothesis raising at best. It should be explored in randomized, controlled trials that can look at this question in an unbiased manner,” he agreed.
If patients believe or suspect that a statin is causing symptoms that suggest cognitive dysfunction, “what they really need to do is to stop it for 3 weeks and check out other causes. And in rechallenging, the guidelines say, if they think that it’s causing a memory problem that occurs anecdotally, then they can be given another statin, usually, which doesn’t cause it.”
ASPREE compared daily low-dose aspirin with placebo in a community-based older population numbering about 19,000 in Australia and the United States. Patients were initially without known CV disease, dementia, or physical disabilities. It did not randomize patients by statin therapy.
Of note, entry to the trial required a score of at least 78 on the Modified Mini-Mental State Examination (3MS), corresponding to normal cognition.
Aspirin showed no significant benefit for disability-free survival, an endpoint that included death and dementia, or CV events over a median of 4.7 years. It was associated with slightly more cases of major hemorrhage, as previously reported.
A subsequent ASPREE analysis suggested that the aspirin had no effect on risks of mild cognitive impairment, cognitive decline, or dementia.
Of the 18,846 patients in the current post hoc analysis, the average age of the patients was 74 years, and 56.4% were women; 31.3% were taking statins at baseline. The incidence of dementia per 1,000 person-years for those taking statins in comparison with those not taking statins was 6.91 and 6.48, respectively. Any cognitive changes were tracked by the 3MS and three other validated tests in different domains of cognition, with results contributing to the composite score.
The corresponding incidence of dementia considered probable Alzheimer’s disease was 2.97 and 2.65 for those receiving versus not receiving statins, respectively. The incidence of dementia with mixed presentation was 3.94 and 3.84, respectively.
There were no significant differences in risk for dementia overall or for either dementia subtype in multivariate analyses. Adjustments included demographics, CV lifestyle risk factors, family medical history, including dementia, ASPREE randomization group, and individual scores on the four tests of cognition.
Results for development of MCI mirrored those for dementia, as did results stratified for baseline lipids and for use of lipophilic statins, such as atorvastatin or simvastatin versus hydrophilic statins, including pravastatin and rosuvastatin.
Significant interactions were observed between composite cognitive scores and statin therapy at baseline; as scores increased, indicating better cognitive performance, the risks for dementia and its subtypes went down. Statins were associated with incident dementia at the lowest cognitive performance quartile.
That association is probably a function of the cohort’s advanced age, Dr. Nelson said. “If you get into old age, and you’ve got high cognitive scores, you’ve probably got protective factors. That’s how I would interpret that.”
Dr. Ballantyne and Dr. Nambi also emphasized the difficulties of controlling for potential biases even with extensive covariate adjustments. The statin dosages at which patients were treated were not part of the analysis, “and achieved LDL [cholesterol levels over the study period were not known,” they wrote.
“Furthermore, patients who were treated with statins were more likely to have diabetes, hypertension, chronic kidney disease, and obesity, all of which are known to increase risk for cognitive decline, and, as might have been predicted, statin users therefore had significantly lower scores for global cognition and episodic memory.”
Dr. Nelson pointed to an ongoing prospective atorvastatin trial that includes dementia in its primary endpoint and should be “the definitive study.” STAREE (Statin Therapy for Reducing Events in the Elderly) is running throughout Australia with a projected enrollment of 18,000 and primary completion by the end of 2022. “We’ve already enrolled 8,000 patients.”
Less far along is the PREVENTABLE (Pragmatic Evaluation of Events and Benefits of Lipid-Lowering in Older Adults) trial, based in the United States and also randomizing to atorvastatin or placebo, that will have an estimated 20,000 older patients and completion in 5 years. The primary endpoint is new dementia or persistent disability.
Both trials “are powered to enable firm conclusions concerning any statin effects,” said Dr. Ballantyne and Dr. Nambi. “In the meantime, practicing clinicians can have confidence and share with their patients that short-term lipid-lowering therapy in older patients, including with statins, is unlikely to have a major impact on cognition.”
ASPREE was supported by grants from the U.S. National Institute on Aging and the National Cancer Institute and the National Health and Medical Research Council of Australia, by Monash University, and by the Victorian Cancer Agency. Dr. Nelson reported receiving honoraria from Sanofi and Amgen; support from Bayer for ASPREE; and grant support for STAREE. Disclosures for the other authors are in the report. Dr. Ballantyne disclosed grant and research support from Abbott Diagnostic, Akcea, Amgen, Esperion, Ionis, Novartis, Regeneron, and Roche Diagnostics; and consulting for Abbott Diagnostics, Althera, Amarin, Amgen, Arrowhead, AstraZeneca, Corvidia, Denka Seiken, Esperion, Genentech, Gilead, Matinas BioPharma, New Amsterdam, Novartis, Novo Nordisk, Pfizer, Regeneron, Roche Diagnostics, and Sanofi-Synthelabo. Dr. Nambi is a coinvestigator on a provisional patent along with Baylor College of Medicine and Roche on the use of biomarkers to predict heart failure, and a site principal investigator for studies sponsored by Amgen and Merck. Dr. Stone had no disclosures.
A version of this article first appeared on Medscape.com.
Bariatric surgery tied to 22% lower 5-year stroke risk
Patients with obesity who underwent bariatric surgery had 46% lower odds of stroke 1 year later, similar odds of stroke 3 years later, and 22% lower odds of stroke 5 years later, compared with matched control patients, in new research.
Michael D. Williams, MD, presented the study findings (abstract A002) at the annual meeting of the American Society for Metabolic & Bariatric Surgery.
The findings are “very good news,” even though the protection against stroke declined further out from the surgery, John D. Scott, MD, scientific program chair of the ASMBS meeting, told this news organization.
The investigators matched more than 56,000 patients with obesity who had bariatric surgery with an equal number of similar patients who did not have this surgery, from a large national insurance database, in what they believe is the largest study of this to date.
“Any intervention that decreases your risk of [cardiovascular] events is good news,” said Dr. Scott, a clinical professor of surgery at the University of South Carolina, Greenville, and metabolic and bariatric surgery director at Prisma Health in Greenville, S.C. “And having a 22%-45% chance of reduction in stroke risk is a very worthwhile intervention.”
Asked how this would change the way clinicians inform patients of what to expect from bariatric surgery, he said: “I would advise patients that studies like this show that surgery would not increase your risk of having a stroke.
“This is consistent with many studies that show that the risks of all macrovascular events decrease after the comorbidity reductions seen after surgery.”
According to Dr. Scott, “the next steps might include a prospective randomized trial of medical treatment versus surgery alone for [cardiovascular]/stroke outcomes, but this is unlikely.”
Similarly, Dr. Williams told this news organization that “I would tell [patients] that surgery is an effective and durable method for weight loss. It also can improve comorbid conditions, particularly diabetes and hypertension.”
Even with this study, “I’m not sure it’s appropriate to say that bariatric surgery will reduce the risk of stroke,” he cautioned.
“However, as we continue to investigate the effects of bariatric surgery, this study contributes to the greater body of knowledge that suggests that reduction in ischemic stroke risk is yet another benefit of bariatric surgery.”
The assigned discussant, Corrigan L. McBride, MD, MBA wanted to know if the lower odds ratio at 1 year might be because preoperative patient selection might eliminate patients at high risk of poor cardiovascular outcomes.
Dr. Williams, a resident at Rush Medical College, Chicago, replied that it is difficult to eliminate potential selection bias, despite best efforts, but this study shows that he can tell patients: “Having surgery is not going to increases your risk of stroke.”
“This is an important study,” Dr. McBride, professor and chief of minimally invasive surgery and bariatric surgery, University of Nebraska Medical Center, Omaha, told this news organization.
“It is the first large study to show a decreased [or no increased] risk of stroke 1, 3, and 5 years after bariatric surgery compared to matched patients, and it had enough data to look at stroke as a standalone endpoint,” Dr. McBride said. “It is important too, for patients and their physicians to understand that there is a lower chance of them having a stroke if they have surgery than if they do not.”
‘Important,’ ‘good news’ for stroke risk after bariatric surgery
The impact of bariatric surgery on remission of type 2 diabetes is well known, Dr. Williams noted, and other studies have reported how bariatric surgery affects the risk of major adverse cardiovascular events – a composite of stroke, myocardial infarction, coronary artery disease, and all-cause death – including a study presented in the same meeting session.
However, a very large sample size is needed to be able to demonstrate the effect of bariatric surgery on stroke, since stroke is a rare event.
The researchers analyzed data from the Mariner (PearlDiver.) all-payer insurance national claims database of patients in the United States.
They matched 56,514 patients with a body mass index over 35 kg/m2 and comorbidities or a BMI of more than 40 who underwent sleeve gastrectomy or Roux-en-Y gastric bypass during 2010-2019 with 56,514 control patients who did not undergo bariatric surgery.
A year after bariatric surgery, patients in that group had a lower stroke rate than patients in the control group (0.6% vs. 1.2%), and they had close to 50% lower odds of having a stroke (odds ratio, 0.54; 95% CI, 0.47-0.61).
Three years after bariatric surgery, there were 44,948 patients in each group; the rate of stroke was 2.1% in the surgery group and 2.2% in the control group, and there was no significant difference in the odds of having a stroke (OR, 0.96; 95% CI, 0.91-1.00).
Five years after bariatric surgery, there were 27,619 patients in each group; the stroke rate was lower in the bariatric surgery group than in the control group (2.8% vs 3.6%), but reduced odds of stroke was not as great as after 1 year (OR, 0.78; 95% CI, 0.65-0.90).
Dr. Williams has no relevant financial disclosures. Dr. McBride and Dr. Scott disclosed that they are speakers/trainers/faculty advisers for Gore. Dr. Scott is also a consultant for C-SATS (part of Johnson & Johnson).
Patients with obesity who underwent bariatric surgery had 46% lower odds of stroke 1 year later, similar odds of stroke 3 years later, and 22% lower odds of stroke 5 years later, compared with matched control patients, in new research.
Michael D. Williams, MD, presented the study findings (abstract A002) at the annual meeting of the American Society for Metabolic & Bariatric Surgery.
The findings are “very good news,” even though the protection against stroke declined further out from the surgery, John D. Scott, MD, scientific program chair of the ASMBS meeting, told this news organization.
The investigators matched more than 56,000 patients with obesity who had bariatric surgery with an equal number of similar patients who did not have this surgery, from a large national insurance database, in what they believe is the largest study of this to date.
“Any intervention that decreases your risk of [cardiovascular] events is good news,” said Dr. Scott, a clinical professor of surgery at the University of South Carolina, Greenville, and metabolic and bariatric surgery director at Prisma Health in Greenville, S.C. “And having a 22%-45% chance of reduction in stroke risk is a very worthwhile intervention.”
Asked how this would change the way clinicians inform patients of what to expect from bariatric surgery, he said: “I would advise patients that studies like this show that surgery would not increase your risk of having a stroke.
“This is consistent with many studies that show that the risks of all macrovascular events decrease after the comorbidity reductions seen after surgery.”
According to Dr. Scott, “the next steps might include a prospective randomized trial of medical treatment versus surgery alone for [cardiovascular]/stroke outcomes, but this is unlikely.”
Similarly, Dr. Williams told this news organization that “I would tell [patients] that surgery is an effective and durable method for weight loss. It also can improve comorbid conditions, particularly diabetes and hypertension.”
Even with this study, “I’m not sure it’s appropriate to say that bariatric surgery will reduce the risk of stroke,” he cautioned.
“However, as we continue to investigate the effects of bariatric surgery, this study contributes to the greater body of knowledge that suggests that reduction in ischemic stroke risk is yet another benefit of bariatric surgery.”
The assigned discussant, Corrigan L. McBride, MD, MBA wanted to know if the lower odds ratio at 1 year might be because preoperative patient selection might eliminate patients at high risk of poor cardiovascular outcomes.
Dr. Williams, a resident at Rush Medical College, Chicago, replied that it is difficult to eliminate potential selection bias, despite best efforts, but this study shows that he can tell patients: “Having surgery is not going to increases your risk of stroke.”
“This is an important study,” Dr. McBride, professor and chief of minimally invasive surgery and bariatric surgery, University of Nebraska Medical Center, Omaha, told this news organization.
“It is the first large study to show a decreased [or no increased] risk of stroke 1, 3, and 5 years after bariatric surgery compared to matched patients, and it had enough data to look at stroke as a standalone endpoint,” Dr. McBride said. “It is important too, for patients and their physicians to understand that there is a lower chance of them having a stroke if they have surgery than if they do not.”
‘Important,’ ‘good news’ for stroke risk after bariatric surgery
The impact of bariatric surgery on remission of type 2 diabetes is well known, Dr. Williams noted, and other studies have reported how bariatric surgery affects the risk of major adverse cardiovascular events – a composite of stroke, myocardial infarction, coronary artery disease, and all-cause death – including a study presented in the same meeting session.
However, a very large sample size is needed to be able to demonstrate the effect of bariatric surgery on stroke, since stroke is a rare event.
The researchers analyzed data from the Mariner (PearlDiver.) all-payer insurance national claims database of patients in the United States.
They matched 56,514 patients with a body mass index over 35 kg/m2 and comorbidities or a BMI of more than 40 who underwent sleeve gastrectomy or Roux-en-Y gastric bypass during 2010-2019 with 56,514 control patients who did not undergo bariatric surgery.
A year after bariatric surgery, patients in that group had a lower stroke rate than patients in the control group (0.6% vs. 1.2%), and they had close to 50% lower odds of having a stroke (odds ratio, 0.54; 95% CI, 0.47-0.61).
Three years after bariatric surgery, there were 44,948 patients in each group; the rate of stroke was 2.1% in the surgery group and 2.2% in the control group, and there was no significant difference in the odds of having a stroke (OR, 0.96; 95% CI, 0.91-1.00).
Five years after bariatric surgery, there were 27,619 patients in each group; the stroke rate was lower in the bariatric surgery group than in the control group (2.8% vs 3.6%), but reduced odds of stroke was not as great as after 1 year (OR, 0.78; 95% CI, 0.65-0.90).
Dr. Williams has no relevant financial disclosures. Dr. McBride and Dr. Scott disclosed that they are speakers/trainers/faculty advisers for Gore. Dr. Scott is also a consultant for C-SATS (part of Johnson & Johnson).
Patients with obesity who underwent bariatric surgery had 46% lower odds of stroke 1 year later, similar odds of stroke 3 years later, and 22% lower odds of stroke 5 years later, compared with matched control patients, in new research.
Michael D. Williams, MD, presented the study findings (abstract A002) at the annual meeting of the American Society for Metabolic & Bariatric Surgery.
The findings are “very good news,” even though the protection against stroke declined further out from the surgery, John D. Scott, MD, scientific program chair of the ASMBS meeting, told this news organization.
The investigators matched more than 56,000 patients with obesity who had bariatric surgery with an equal number of similar patients who did not have this surgery, from a large national insurance database, in what they believe is the largest study of this to date.
“Any intervention that decreases your risk of [cardiovascular] events is good news,” said Dr. Scott, a clinical professor of surgery at the University of South Carolina, Greenville, and metabolic and bariatric surgery director at Prisma Health in Greenville, S.C. “And having a 22%-45% chance of reduction in stroke risk is a very worthwhile intervention.”
Asked how this would change the way clinicians inform patients of what to expect from bariatric surgery, he said: “I would advise patients that studies like this show that surgery would not increase your risk of having a stroke.
“This is consistent with many studies that show that the risks of all macrovascular events decrease after the comorbidity reductions seen after surgery.”
According to Dr. Scott, “the next steps might include a prospective randomized trial of medical treatment versus surgery alone for [cardiovascular]/stroke outcomes, but this is unlikely.”
Similarly, Dr. Williams told this news organization that “I would tell [patients] that surgery is an effective and durable method for weight loss. It also can improve comorbid conditions, particularly diabetes and hypertension.”
Even with this study, “I’m not sure it’s appropriate to say that bariatric surgery will reduce the risk of stroke,” he cautioned.
“However, as we continue to investigate the effects of bariatric surgery, this study contributes to the greater body of knowledge that suggests that reduction in ischemic stroke risk is yet another benefit of bariatric surgery.”
The assigned discussant, Corrigan L. McBride, MD, MBA wanted to know if the lower odds ratio at 1 year might be because preoperative patient selection might eliminate patients at high risk of poor cardiovascular outcomes.
Dr. Williams, a resident at Rush Medical College, Chicago, replied that it is difficult to eliminate potential selection bias, despite best efforts, but this study shows that he can tell patients: “Having surgery is not going to increases your risk of stroke.”
“This is an important study,” Dr. McBride, professor and chief of minimally invasive surgery and bariatric surgery, University of Nebraska Medical Center, Omaha, told this news organization.
“It is the first large study to show a decreased [or no increased] risk of stroke 1, 3, and 5 years after bariatric surgery compared to matched patients, and it had enough data to look at stroke as a standalone endpoint,” Dr. McBride said. “It is important too, for patients and their physicians to understand that there is a lower chance of them having a stroke if they have surgery than if they do not.”
‘Important,’ ‘good news’ for stroke risk after bariatric surgery
The impact of bariatric surgery on remission of type 2 diabetes is well known, Dr. Williams noted, and other studies have reported how bariatric surgery affects the risk of major adverse cardiovascular events – a composite of stroke, myocardial infarction, coronary artery disease, and all-cause death – including a study presented in the same meeting session.
However, a very large sample size is needed to be able to demonstrate the effect of bariatric surgery on stroke, since stroke is a rare event.
The researchers analyzed data from the Mariner (PearlDiver.) all-payer insurance national claims database of patients in the United States.
They matched 56,514 patients with a body mass index over 35 kg/m2 and comorbidities or a BMI of more than 40 who underwent sleeve gastrectomy or Roux-en-Y gastric bypass during 2010-2019 with 56,514 control patients who did not undergo bariatric surgery.
A year after bariatric surgery, patients in that group had a lower stroke rate than patients in the control group (0.6% vs. 1.2%), and they had close to 50% lower odds of having a stroke (odds ratio, 0.54; 95% CI, 0.47-0.61).
Three years after bariatric surgery, there were 44,948 patients in each group; the rate of stroke was 2.1% in the surgery group and 2.2% in the control group, and there was no significant difference in the odds of having a stroke (OR, 0.96; 95% CI, 0.91-1.00).
Five years after bariatric surgery, there were 27,619 patients in each group; the stroke rate was lower in the bariatric surgery group than in the control group (2.8% vs 3.6%), but reduced odds of stroke was not as great as after 1 year (OR, 0.78; 95% CI, 0.65-0.90).
Dr. Williams has no relevant financial disclosures. Dr. McBride and Dr. Scott disclosed that they are speakers/trainers/faculty advisers for Gore. Dr. Scott is also a consultant for C-SATS (part of Johnson & Johnson).
FROM ASMBS 2021
Medically suspect criterion can determine bariatric surgery coverage
A delaying tactic used by some U.S. health insurers to limit coverage of bariatric surgery does not jibe with the clinical experience at one U.S. center with 461 patients who underwent primary or revisional bariatric surgery.
The tactic applies to patients with a baseline body mass index (BMI) of 35-39 kg/m2 who usually also need at least one comorbidity to qualify for insurance coverage for bariatric surgery, and specifically to the subgroup for whom hypertension is the qualifying comorbidity.
Some insurers limit surgery coverage to patients with hypertension who fail to reach their goal blood pressure on agents from three different drug classes, a policy that is “extremely frustrating and dangerous,” said Yannis Raftopoulos, MD, PhD, in his presentation at the annual meeting of the American Society for Metabolic and Bariatric Surgery.
Using number of antihypertensive drugs ‘is not correct’
“Using the number of antihypertensive medications to justify surgery is not correct because blood pressure control is not [always] better when patients take two or three medications, compared with when they are taking one. This harms patients because the more severe their hypertension, the worse their control,” said Dr. Raftopoulos, director of the weight management program at Holyoke (Mass.) Medical Center.
He presented findings from a retrospective study of 461 patients who underwent either sleeve gastrectomy or laparoscopic Roux-en-Y gastric bypass at his center, including 213 (46%) diagnosed with hypertension at the time of their surgery. Within this group were 68 patients with a BMI of 35-39, which meant that they could get insurance coverage for bariatric surgery only if they also had a relevant comorbidity such as hypertension, diabetes, or severe sleep apnea.
Among these patients, 36 (17% of those with hypertension) had only hypertension as their relevant comorbidity and would not have qualified for bariatric surgery under the strictest criteria applied by some insurers that require patients to remain hypertensive despite treatment with at least three different antihypertensive medications. (These 36 patients underwent bariatric surgery because their insurance coverage did not have this restriction.)
The analyses Dr. Raftopoulos presented also documented the rate of hypertension resolution among patients in the series who had hypertension at baseline and 1-year follow-up results. Among 65 patients on one antihypertensive drug at baseline, 43 (66%) had complete resolution of their hypertension after 1 year, defined as blood pressure of less than 130/90 mm Hg while completely off antihypertensive treatment. In contrast, among 55 patients on two antihypertensive medications at baseline, 28 (51%) had complete resolution after 1 year, and among 24 patients on three or more antihypertensive medications at baseline, 3 (13%) had complete resolution 1 year after bariatric surgery, he reported.
“Patients who were treated with one oral antihypertensive medication preoperatively had a higher likelihood of postoperative hypertension resolution,” concluded Dr. Raftopoulos.
Restricting access to bariatric surgery to patients with a BMI of less than 40 based on the preoperative intensity of their antihypertensive treatment “is not supported by our data, and can be potentially harmful,” he declared.
“This study was the result of discussions about this problem with multiple insurers in my area,” he added. “This affects a good number of patients.”
Waiting for hypertension to become less treatable
The results Dr. Raftopoulos presented “are not surprising, because they confirm the hypothesis that earlier intervention in the course of a disease like hypertension is more likely to be successful,” commented Bruce D. Schirmer, MD, a professor of surgery at the University of Virginia, Charlottesville, and designated discussant for the report.
The policy followed by some health insurers to delay coverage for bariatric surgery until patients fail three medications “forces patients with more treatable hypertension to wait until their disease worsens and becomes less treatable before they can receive appropriate treatment,” he said.
Dr. Schirmer attributed the motivation for this approach to a “despicable” and “reprehensible” reason: “Actuarial calculations that show paying for curative therapy is not cost effective in the short term. The duration of a patient’s policy may not be long enough to yield a positive financial outcome, so it becomes more appropriate to deny optimal care and have patients become sicker from their disease.”
“I applaud the authors for accumulating the data that point out this unfortunate rule of some insurance companies,” Dr. Schirmer added.
The practice is comparable with an insurer requiring that a patient’s cancer must be metastatic before allowing coverage for treatment, commented Ann M. Rogers, MD, professor and director of the Penn State University surgical weight loss program in Hershey, Penn., and a moderator of the session.
Dr. Raftopoulos, Dr. Schirmer, and Dr. Rogers had no disclosures.
A delaying tactic used by some U.S. health insurers to limit coverage of bariatric surgery does not jibe with the clinical experience at one U.S. center with 461 patients who underwent primary or revisional bariatric surgery.
The tactic applies to patients with a baseline body mass index (BMI) of 35-39 kg/m2 who usually also need at least one comorbidity to qualify for insurance coverage for bariatric surgery, and specifically to the subgroup for whom hypertension is the qualifying comorbidity.
Some insurers limit surgery coverage to patients with hypertension who fail to reach their goal blood pressure on agents from three different drug classes, a policy that is “extremely frustrating and dangerous,” said Yannis Raftopoulos, MD, PhD, in his presentation at the annual meeting of the American Society for Metabolic and Bariatric Surgery.
Using number of antihypertensive drugs ‘is not correct’
“Using the number of antihypertensive medications to justify surgery is not correct because blood pressure control is not [always] better when patients take two or three medications, compared with when they are taking one. This harms patients because the more severe their hypertension, the worse their control,” said Dr. Raftopoulos, director of the weight management program at Holyoke (Mass.) Medical Center.
He presented findings from a retrospective study of 461 patients who underwent either sleeve gastrectomy or laparoscopic Roux-en-Y gastric bypass at his center, including 213 (46%) diagnosed with hypertension at the time of their surgery. Within this group were 68 patients with a BMI of 35-39, which meant that they could get insurance coverage for bariatric surgery only if they also had a relevant comorbidity such as hypertension, diabetes, or severe sleep apnea.
Among these patients, 36 (17% of those with hypertension) had only hypertension as their relevant comorbidity and would not have qualified for bariatric surgery under the strictest criteria applied by some insurers that require patients to remain hypertensive despite treatment with at least three different antihypertensive medications. (These 36 patients underwent bariatric surgery because their insurance coverage did not have this restriction.)
The analyses Dr. Raftopoulos presented also documented the rate of hypertension resolution among patients in the series who had hypertension at baseline and 1-year follow-up results. Among 65 patients on one antihypertensive drug at baseline, 43 (66%) had complete resolution of their hypertension after 1 year, defined as blood pressure of less than 130/90 mm Hg while completely off antihypertensive treatment. In contrast, among 55 patients on two antihypertensive medications at baseline, 28 (51%) had complete resolution after 1 year, and among 24 patients on three or more antihypertensive medications at baseline, 3 (13%) had complete resolution 1 year after bariatric surgery, he reported.
“Patients who were treated with one oral antihypertensive medication preoperatively had a higher likelihood of postoperative hypertension resolution,” concluded Dr. Raftopoulos.
Restricting access to bariatric surgery to patients with a BMI of less than 40 based on the preoperative intensity of their antihypertensive treatment “is not supported by our data, and can be potentially harmful,” he declared.
“This study was the result of discussions about this problem with multiple insurers in my area,” he added. “This affects a good number of patients.”
Waiting for hypertension to become less treatable
The results Dr. Raftopoulos presented “are not surprising, because they confirm the hypothesis that earlier intervention in the course of a disease like hypertension is more likely to be successful,” commented Bruce D. Schirmer, MD, a professor of surgery at the University of Virginia, Charlottesville, and designated discussant for the report.
The policy followed by some health insurers to delay coverage for bariatric surgery until patients fail three medications “forces patients with more treatable hypertension to wait until their disease worsens and becomes less treatable before they can receive appropriate treatment,” he said.
Dr. Schirmer attributed the motivation for this approach to a “despicable” and “reprehensible” reason: “Actuarial calculations that show paying for curative therapy is not cost effective in the short term. The duration of a patient’s policy may not be long enough to yield a positive financial outcome, so it becomes more appropriate to deny optimal care and have patients become sicker from their disease.”
“I applaud the authors for accumulating the data that point out this unfortunate rule of some insurance companies,” Dr. Schirmer added.
The practice is comparable with an insurer requiring that a patient’s cancer must be metastatic before allowing coverage for treatment, commented Ann M. Rogers, MD, professor and director of the Penn State University surgical weight loss program in Hershey, Penn., and a moderator of the session.
Dr. Raftopoulos, Dr. Schirmer, and Dr. Rogers had no disclosures.
A delaying tactic used by some U.S. health insurers to limit coverage of bariatric surgery does not jibe with the clinical experience at one U.S. center with 461 patients who underwent primary or revisional bariatric surgery.
The tactic applies to patients with a baseline body mass index (BMI) of 35-39 kg/m2 who usually also need at least one comorbidity to qualify for insurance coverage for bariatric surgery, and specifically to the subgroup for whom hypertension is the qualifying comorbidity.
Some insurers limit surgery coverage to patients with hypertension who fail to reach their goal blood pressure on agents from three different drug classes, a policy that is “extremely frustrating and dangerous,” said Yannis Raftopoulos, MD, PhD, in his presentation at the annual meeting of the American Society for Metabolic and Bariatric Surgery.
Using number of antihypertensive drugs ‘is not correct’
“Using the number of antihypertensive medications to justify surgery is not correct because blood pressure control is not [always] better when patients take two or three medications, compared with when they are taking one. This harms patients because the more severe their hypertension, the worse their control,” said Dr. Raftopoulos, director of the weight management program at Holyoke (Mass.) Medical Center.
He presented findings from a retrospective study of 461 patients who underwent either sleeve gastrectomy or laparoscopic Roux-en-Y gastric bypass at his center, including 213 (46%) diagnosed with hypertension at the time of their surgery. Within this group were 68 patients with a BMI of 35-39, which meant that they could get insurance coverage for bariatric surgery only if they also had a relevant comorbidity such as hypertension, diabetes, or severe sleep apnea.
Among these patients, 36 (17% of those with hypertension) had only hypertension as their relevant comorbidity and would not have qualified for bariatric surgery under the strictest criteria applied by some insurers that require patients to remain hypertensive despite treatment with at least three different antihypertensive medications. (These 36 patients underwent bariatric surgery because their insurance coverage did not have this restriction.)
The analyses Dr. Raftopoulos presented also documented the rate of hypertension resolution among patients in the series who had hypertension at baseline and 1-year follow-up results. Among 65 patients on one antihypertensive drug at baseline, 43 (66%) had complete resolution of their hypertension after 1 year, defined as blood pressure of less than 130/90 mm Hg while completely off antihypertensive treatment. In contrast, among 55 patients on two antihypertensive medications at baseline, 28 (51%) had complete resolution after 1 year, and among 24 patients on three or more antihypertensive medications at baseline, 3 (13%) had complete resolution 1 year after bariatric surgery, he reported.
“Patients who were treated with one oral antihypertensive medication preoperatively had a higher likelihood of postoperative hypertension resolution,” concluded Dr. Raftopoulos.
Restricting access to bariatric surgery to patients with a BMI of less than 40 based on the preoperative intensity of their antihypertensive treatment “is not supported by our data, and can be potentially harmful,” he declared.
“This study was the result of discussions about this problem with multiple insurers in my area,” he added. “This affects a good number of patients.”
Waiting for hypertension to become less treatable
The results Dr. Raftopoulos presented “are not surprising, because they confirm the hypothesis that earlier intervention in the course of a disease like hypertension is more likely to be successful,” commented Bruce D. Schirmer, MD, a professor of surgery at the University of Virginia, Charlottesville, and designated discussant for the report.
The policy followed by some health insurers to delay coverage for bariatric surgery until patients fail three medications “forces patients with more treatable hypertension to wait until their disease worsens and becomes less treatable before they can receive appropriate treatment,” he said.
Dr. Schirmer attributed the motivation for this approach to a “despicable” and “reprehensible” reason: “Actuarial calculations that show paying for curative therapy is not cost effective in the short term. The duration of a patient’s policy may not be long enough to yield a positive financial outcome, so it becomes more appropriate to deny optimal care and have patients become sicker from their disease.”
“I applaud the authors for accumulating the data that point out this unfortunate rule of some insurance companies,” Dr. Schirmer added.
The practice is comparable with an insurer requiring that a patient’s cancer must be metastatic before allowing coverage for treatment, commented Ann M. Rogers, MD, professor and director of the Penn State University surgical weight loss program in Hershey, Penn., and a moderator of the session.
Dr. Raftopoulos, Dr. Schirmer, and Dr. Rogers had no disclosures.
FROM ASMBS 2021
Bariatric surgery cuts insulin needs in type 1 diabetes with severe obesity
While bariatric surgery does nothing to directly improve the disease of patients with type 1 diabetes, it can work indirectly by moderating severe obesity and improving insulin sensitivity to cut the total insulin needs of patients with type 1 diabetes and obesity, based on a single-center, retrospective chart review of 38 U.S. patients.
Two years following their bariatric surgery, these 38 patients with confirmed type 1 diabetes and an average body mass index of 43 kg/m2 before surgery saw their average daily insulin requirement nearly halved, dropping from 118 units/day to 60 units/day, a significant decrease, Brian J. Dessify, DO, said in a presentation at the annual meeting of the American Society for Metabolic and Bariatric Surgery.
Another measure of this effect showed that the percentage of patients who required more than one drug for treating their hyperglycemia fell from 66% before surgery to 52% 2 years after surgery, a change that was not statistically significant, said Dr. Dessify, a bariatric surgeon at Geisinger Medical Center in Danville, Pa.
Appropriate for patients with ‘double diabetes’
These results “provide good evidence for [using] bariatric surgery” in people with both obesity and type 1 diabetes,” he concluded. This includes people with what Dr. Dessify called “double diabetes,” meaning that they do not make endogenous insulin, and are also resistant to the effects of exogenous insulin and hence have features of both type 2 and type 1 diabetes.
“This is a really important study,” commented Ali Aminian, MD, director of the Bariatric and Metabolic Institute of the Cleveland Clinic. “For patients with type 1 diabetes, the primary goal of bariatric surgery is weight loss and improvement of obesity-related comorbidities. Patients with type 2 diabetes can be a candidate for bariatric surgery regardless of their weight,” Dr. Aminian said as designated discussant for the report.
“The goal of bariatric surgery in patients with type 1 diabetes is to promote sensitivity to the exogenous insulin they receive,” agreed Julie Kim, MD, a bariatric surgeon at Mount Auburn Hospital in Waltham, Mass., and a second discussant for the report. Patients with double diabetes “are probably a subclass of patients [with type 1 diabetes] who might benefit even more from bariatric surgery.”
Using gastric sleeves to avoid diabetic ketoacidosis
Dr. Aminian also noted that “at the Cleveland Clinic we consider a sleeve gastrectomy the procedure of choice” for patients with type 1 diabetes or type 2 diabetes with insulin insufficiency “unless the patient has an absolute contraindication” because of the increased risk for diabetic ketoacidosis in these patients “undergoing any surgery, including bariatric surgery.” Patients with insulin insufficiency “require intensive diabetes and insulin management preoperatively to reduce their risk for developing diabetic ketoacidosis,” and using a sleeve rather than bypass generally results in “more reliable absorption of carbohydrates and nutrients” while also reducing the risk for hypoglycemia, Dr. Aminian said.
In the series reported by Dr. Dessify, 33 patients underwent gastric bypass and 5 had sleeve gastrectomy. The decision to use bypass usually stemmed from its “marginal” improvement in weight loss, compared with a sleeve procedure, and an overall preference at Geisinger for bypass procedures. Dr. Dessify added that he had not yet run a comprehensive assessment of diabetic ketoacidosis complications among patients in his reported series.
Those 38 patients underwent their bariatric procedure during 2002-2019, constituting fewer than 1% of the 4,549 total bariatric surgeries done at Geisinger during that period. The 38 patients with type 1 diabetes averaged 41 years of age, 33 (87%) were women, and 37 (97%) were White. Dr. Dessify and associates undertook this review “to help provide supporting evidence for using bariatric surgery in people with obesity and type 1 diabetes,” he noted.
Dr. Dessify, Dr. Aminian, and Dr. Kim had no disclosures.
While bariatric surgery does nothing to directly improve the disease of patients with type 1 diabetes, it can work indirectly by moderating severe obesity and improving insulin sensitivity to cut the total insulin needs of patients with type 1 diabetes and obesity, based on a single-center, retrospective chart review of 38 U.S. patients.
Two years following their bariatric surgery, these 38 patients with confirmed type 1 diabetes and an average body mass index of 43 kg/m2 before surgery saw their average daily insulin requirement nearly halved, dropping from 118 units/day to 60 units/day, a significant decrease, Brian J. Dessify, DO, said in a presentation at the annual meeting of the American Society for Metabolic and Bariatric Surgery.
Another measure of this effect showed that the percentage of patients who required more than one drug for treating their hyperglycemia fell from 66% before surgery to 52% 2 years after surgery, a change that was not statistically significant, said Dr. Dessify, a bariatric surgeon at Geisinger Medical Center in Danville, Pa.
Appropriate for patients with ‘double diabetes’
These results “provide good evidence for [using] bariatric surgery” in people with both obesity and type 1 diabetes,” he concluded. This includes people with what Dr. Dessify called “double diabetes,” meaning that they do not make endogenous insulin, and are also resistant to the effects of exogenous insulin and hence have features of both type 2 and type 1 diabetes.
“This is a really important study,” commented Ali Aminian, MD, director of the Bariatric and Metabolic Institute of the Cleveland Clinic. “For patients with type 1 diabetes, the primary goal of bariatric surgery is weight loss and improvement of obesity-related comorbidities. Patients with type 2 diabetes can be a candidate for bariatric surgery regardless of their weight,” Dr. Aminian said as designated discussant for the report.
“The goal of bariatric surgery in patients with type 1 diabetes is to promote sensitivity to the exogenous insulin they receive,” agreed Julie Kim, MD, a bariatric surgeon at Mount Auburn Hospital in Waltham, Mass., and a second discussant for the report. Patients with double diabetes “are probably a subclass of patients [with type 1 diabetes] who might benefit even more from bariatric surgery.”
Using gastric sleeves to avoid diabetic ketoacidosis
Dr. Aminian also noted that “at the Cleveland Clinic we consider a sleeve gastrectomy the procedure of choice” for patients with type 1 diabetes or type 2 diabetes with insulin insufficiency “unless the patient has an absolute contraindication” because of the increased risk for diabetic ketoacidosis in these patients “undergoing any surgery, including bariatric surgery.” Patients with insulin insufficiency “require intensive diabetes and insulin management preoperatively to reduce their risk for developing diabetic ketoacidosis,” and using a sleeve rather than bypass generally results in “more reliable absorption of carbohydrates and nutrients” while also reducing the risk for hypoglycemia, Dr. Aminian said.
In the series reported by Dr. Dessify, 33 patients underwent gastric bypass and 5 had sleeve gastrectomy. The decision to use bypass usually stemmed from its “marginal” improvement in weight loss, compared with a sleeve procedure, and an overall preference at Geisinger for bypass procedures. Dr. Dessify added that he had not yet run a comprehensive assessment of diabetic ketoacidosis complications among patients in his reported series.
Those 38 patients underwent their bariatric procedure during 2002-2019, constituting fewer than 1% of the 4,549 total bariatric surgeries done at Geisinger during that period. The 38 patients with type 1 diabetes averaged 41 years of age, 33 (87%) were women, and 37 (97%) were White. Dr. Dessify and associates undertook this review “to help provide supporting evidence for using bariatric surgery in people with obesity and type 1 diabetes,” he noted.
Dr. Dessify, Dr. Aminian, and Dr. Kim had no disclosures.
While bariatric surgery does nothing to directly improve the disease of patients with type 1 diabetes, it can work indirectly by moderating severe obesity and improving insulin sensitivity to cut the total insulin needs of patients with type 1 diabetes and obesity, based on a single-center, retrospective chart review of 38 U.S. patients.
Two years following their bariatric surgery, these 38 patients with confirmed type 1 diabetes and an average body mass index of 43 kg/m2 before surgery saw their average daily insulin requirement nearly halved, dropping from 118 units/day to 60 units/day, a significant decrease, Brian J. Dessify, DO, said in a presentation at the annual meeting of the American Society for Metabolic and Bariatric Surgery.
Another measure of this effect showed that the percentage of patients who required more than one drug for treating their hyperglycemia fell from 66% before surgery to 52% 2 years after surgery, a change that was not statistically significant, said Dr. Dessify, a bariatric surgeon at Geisinger Medical Center in Danville, Pa.
Appropriate for patients with ‘double diabetes’
These results “provide good evidence for [using] bariatric surgery” in people with both obesity and type 1 diabetes,” he concluded. This includes people with what Dr. Dessify called “double diabetes,” meaning that they do not make endogenous insulin, and are also resistant to the effects of exogenous insulin and hence have features of both type 2 and type 1 diabetes.
“This is a really important study,” commented Ali Aminian, MD, director of the Bariatric and Metabolic Institute of the Cleveland Clinic. “For patients with type 1 diabetes, the primary goal of bariatric surgery is weight loss and improvement of obesity-related comorbidities. Patients with type 2 diabetes can be a candidate for bariatric surgery regardless of their weight,” Dr. Aminian said as designated discussant for the report.
“The goal of bariatric surgery in patients with type 1 diabetes is to promote sensitivity to the exogenous insulin they receive,” agreed Julie Kim, MD, a bariatric surgeon at Mount Auburn Hospital in Waltham, Mass., and a second discussant for the report. Patients with double diabetes “are probably a subclass of patients [with type 1 diabetes] who might benefit even more from bariatric surgery.”
Using gastric sleeves to avoid diabetic ketoacidosis
Dr. Aminian also noted that “at the Cleveland Clinic we consider a sleeve gastrectomy the procedure of choice” for patients with type 1 diabetes or type 2 diabetes with insulin insufficiency “unless the patient has an absolute contraindication” because of the increased risk for diabetic ketoacidosis in these patients “undergoing any surgery, including bariatric surgery.” Patients with insulin insufficiency “require intensive diabetes and insulin management preoperatively to reduce their risk for developing diabetic ketoacidosis,” and using a sleeve rather than bypass generally results in “more reliable absorption of carbohydrates and nutrients” while also reducing the risk for hypoglycemia, Dr. Aminian said.
In the series reported by Dr. Dessify, 33 patients underwent gastric bypass and 5 had sleeve gastrectomy. The decision to use bypass usually stemmed from its “marginal” improvement in weight loss, compared with a sleeve procedure, and an overall preference at Geisinger for bypass procedures. Dr. Dessify added that he had not yet run a comprehensive assessment of diabetic ketoacidosis complications among patients in his reported series.
Those 38 patients underwent their bariatric procedure during 2002-2019, constituting fewer than 1% of the 4,549 total bariatric surgeries done at Geisinger during that period. The 38 patients with type 1 diabetes averaged 41 years of age, 33 (87%) were women, and 37 (97%) were White. Dr. Dessify and associates undertook this review “to help provide supporting evidence for using bariatric surgery in people with obesity and type 1 diabetes,” he noted.
Dr. Dessify, Dr. Aminian, and Dr. Kim had no disclosures.
FROM ASMBS 2021
Bariatric surgery’s cardiovascular benefit extends to 7 years
Patients with obesity who had bariatric surgery had a lower risk of having a major adverse cardiovascular event (MACE) or dying from all causes during a median 7-year follow-up, compared with similar patients who did not undergo surgery.
These findings, from a province-wide retrospective cohort study from Quebec, follow two recent, slightly shorter similar trials.
Now we need a large randomized clinical trial (RCT), experts say, to definitively establish cardiovascular and mortality benefits in people with obesity who have metabolic/bariatric surgery. And such a trial is just beginning.
Philippe Bouchard, MD, a general surgery resident from McGill University in Montreal presented the Quebec study in a top papers session at the annual meeting of the American Society for Metabolic & Bariatric Surgery.
The findings showed that, among obese patients with metabolic syndrome, bariatric/metabolic surgery is associated with a sustained decrease in the incidence of MACE and all-cause mortality of at least 5 years, Dr. Bouchard said.
“The results of this population-based observational study should be validated in randomized controlled trials,” he concluded.
In the meantime, “we believe our study adds to the body of evidence in mainly two ways,” Dr. Bouchard told this news organization in an email.
It has a longer follow-up than recent observational studies, “a median of 7 years, compared to 3.9 years in a study from the Cleveland Clinic, and 4.6 years in one from Ontario, he said.
“This allows us to [estimate] an absolute risk reduction of MACE of 5.11% at 10 years,” he added. This is a smaller risk reduction than the roughly 40% risk reduction seen in the other two studies, possibly because of selection bias, Dr. Bouchard speculated.
“Second, most of the larger cohorts are heavily weighted on Roux-en-Y gastric bypass,” he continued. In contrast, their study included diverse procedures, including sleeve gastrectomy, duodenal switch, and adjustable gastric banding.
“Given the rise in popularity of a derivative of the duodenal switch – the single-anastomosis duodenal-ileal bypass with sleeve gastrectomy (SADi-S) – we believe this information is timely and relevant to clinicians,” Dr. Bouchard said.
RCT on the subject is coming
“I totally agree that we need a large randomized controlled trial of bariatric surgery versus optimal medical therapy to conclusively establish” the impact of bariatric surgery on cardiovascular outcomes, said the assigned discussant, Mehran Anvari, MD. And their research group is just about to begin one.
In the absence of RCT data, clinicians “may currently not refer [eligible] patients for bariatric surgery because of the high risk they pose,” said Dr. Anvari, professor and director of the Centre for Minimal Access Surgery of McMaster University, Hamilton, Ont., and senior author in the Ontario study.
Furthermore, an important point is that the current trial extended the follow-up to 7 years, he told this news organization in an email.
That study included patients with diabetes and hypertension, he added, whereas his group included patients with a history of cardiovascular disease and/or heart failure.
“We hope these studies encourage general practitioners and cardiologists to consider bariatric surgery as a viable treatment option to prevent and reduce the risk of MACE in the obese patients [body mass index >35 kg/m2] with significant cardiovascular disease,” he said.
“We have embarked on a pilot RCT among bariatric centers of excellence in Ontario,” Dr. Anvari added, which showed the feasibility and safety of such a study.
He estimates that the RCT will need to recruit 2,000 patients to demonstrate the safety and effectiveness of bariatric surgery in reducing MACE and cardiac and all-cause mortality among patients with existing cardiovascular disease.
This “will require international collaboration,” he added, “and our group is currently establishing collaboration with sites in North America, Europe, and Australia to conduct such a study.”
Patients matched for age, sex, number of comorbidities
Quebec has a single public health care system that covers the cost of bariatric surgery for eligible patients; that is, those with a BMI greater than 35 kg/m2 and comorbidities or a BMI greater than 40 kg/m2.
Using this provincial health care database, which covers over 97% of the population, the researchers identified 3,637 patients with diabetes and/or hypertension who had bariatric surgery during 2007-2012.
They matched the surgery patients with 5,420 control patients with obesity who lived in the same geographic region and had a similar age, sex, and number of Charlson Comorbidity Index comorbidities, but did not undergo bariatric surgery.
The patients had a mean age of 50 and 64% were women.
Half had zero to one comorbidities, a quarter had two comorbidities, and another quarter had at least three comorbidities.
Most patients in the surgery group had type 2 diabetes (70%) and 50% had hypertension, whereas in the control group, most patients had hypertension (82%) and 41% had diabetes.
The most common type of bariatric surgery was adjustable gastric banding (42% of patients), followed by duodenal switch (24%), sleeve gastrectomy (23%), and Roux-en-Y gastric bypass (11%).
The primary outcome was the incidence of MACE, defined as coronary artery events (including myocardial infarction, percutaneous coronary intervention, and coronary artery bypass graft), stroke, heart failure, and all-cause mortality,
After a median follow-up of 7-11 years, fewer patients in the surgical group than in the control group had MACE (20% vs. 25%) or died from all causes (4.1% vs. 6.3%, both statistically significant at P < .01)
Similarly, significantly fewer patients in the surgical group than in the control group had a coronary artery event or heart failure (each P < .01).
However, there were no significant between-group difference in the rate of stroke, possibly because of the small number of strokes.
The risk of MACE was 17% lower in the group that had bariatric surgery than in the control group (adjusted hazard ratio, 0.83; 95% confidence interval, 0.78-0.89), after adjusting for age, sex, and number of comorbidities.
In subgroup analysis, patients who had adjustable gastric banding, Roux-en-Y gastric bypass, or duodenal switch had a significantly lower risk of MACE than control patients.
The risk of MACE was similar in patients who had sleeve gastrectomy and in control patients.
However, these subgroup results need to be interpreted with caution since the surgery and control patients in each surgery type subgroup were not matched for age, sex, and comorbidities, said Dr. Bouchard.
He acknowledged that study limitations include a lack of information about the patients’ BMI, weight, medications, and glycemic control (hemoglobin A1c).
Dr. Bouchard and Dr. Anvari have no relevant financial disclosures.
Patients with obesity who had bariatric surgery had a lower risk of having a major adverse cardiovascular event (MACE) or dying from all causes during a median 7-year follow-up, compared with similar patients who did not undergo surgery.
These findings, from a province-wide retrospective cohort study from Quebec, follow two recent, slightly shorter similar trials.
Now we need a large randomized clinical trial (RCT), experts say, to definitively establish cardiovascular and mortality benefits in people with obesity who have metabolic/bariatric surgery. And such a trial is just beginning.
Philippe Bouchard, MD, a general surgery resident from McGill University in Montreal presented the Quebec study in a top papers session at the annual meeting of the American Society for Metabolic & Bariatric Surgery.
The findings showed that, among obese patients with metabolic syndrome, bariatric/metabolic surgery is associated with a sustained decrease in the incidence of MACE and all-cause mortality of at least 5 years, Dr. Bouchard said.
“The results of this population-based observational study should be validated in randomized controlled trials,” he concluded.
In the meantime, “we believe our study adds to the body of evidence in mainly two ways,” Dr. Bouchard told this news organization in an email.
It has a longer follow-up than recent observational studies, “a median of 7 years, compared to 3.9 years in a study from the Cleveland Clinic, and 4.6 years in one from Ontario, he said.
“This allows us to [estimate] an absolute risk reduction of MACE of 5.11% at 10 years,” he added. This is a smaller risk reduction than the roughly 40% risk reduction seen in the other two studies, possibly because of selection bias, Dr. Bouchard speculated.
“Second, most of the larger cohorts are heavily weighted on Roux-en-Y gastric bypass,” he continued. In contrast, their study included diverse procedures, including sleeve gastrectomy, duodenal switch, and adjustable gastric banding.
“Given the rise in popularity of a derivative of the duodenal switch – the single-anastomosis duodenal-ileal bypass with sleeve gastrectomy (SADi-S) – we believe this information is timely and relevant to clinicians,” Dr. Bouchard said.
RCT on the subject is coming
“I totally agree that we need a large randomized controlled trial of bariatric surgery versus optimal medical therapy to conclusively establish” the impact of bariatric surgery on cardiovascular outcomes, said the assigned discussant, Mehran Anvari, MD. And their research group is just about to begin one.
In the absence of RCT data, clinicians “may currently not refer [eligible] patients for bariatric surgery because of the high risk they pose,” said Dr. Anvari, professor and director of the Centre for Minimal Access Surgery of McMaster University, Hamilton, Ont., and senior author in the Ontario study.
Furthermore, an important point is that the current trial extended the follow-up to 7 years, he told this news organization in an email.
That study included patients with diabetes and hypertension, he added, whereas his group included patients with a history of cardiovascular disease and/or heart failure.
“We hope these studies encourage general practitioners and cardiologists to consider bariatric surgery as a viable treatment option to prevent and reduce the risk of MACE in the obese patients [body mass index >35 kg/m2] with significant cardiovascular disease,” he said.
“We have embarked on a pilot RCT among bariatric centers of excellence in Ontario,” Dr. Anvari added, which showed the feasibility and safety of such a study.
He estimates that the RCT will need to recruit 2,000 patients to demonstrate the safety and effectiveness of bariatric surgery in reducing MACE and cardiac and all-cause mortality among patients with existing cardiovascular disease.
This “will require international collaboration,” he added, “and our group is currently establishing collaboration with sites in North America, Europe, and Australia to conduct such a study.”
Patients matched for age, sex, number of comorbidities
Quebec has a single public health care system that covers the cost of bariatric surgery for eligible patients; that is, those with a BMI greater than 35 kg/m2 and comorbidities or a BMI greater than 40 kg/m2.
Using this provincial health care database, which covers over 97% of the population, the researchers identified 3,637 patients with diabetes and/or hypertension who had bariatric surgery during 2007-2012.
They matched the surgery patients with 5,420 control patients with obesity who lived in the same geographic region and had a similar age, sex, and number of Charlson Comorbidity Index comorbidities, but did not undergo bariatric surgery.
The patients had a mean age of 50 and 64% were women.
Half had zero to one comorbidities, a quarter had two comorbidities, and another quarter had at least three comorbidities.
Most patients in the surgery group had type 2 diabetes (70%) and 50% had hypertension, whereas in the control group, most patients had hypertension (82%) and 41% had diabetes.
The most common type of bariatric surgery was adjustable gastric banding (42% of patients), followed by duodenal switch (24%), sleeve gastrectomy (23%), and Roux-en-Y gastric bypass (11%).
The primary outcome was the incidence of MACE, defined as coronary artery events (including myocardial infarction, percutaneous coronary intervention, and coronary artery bypass graft), stroke, heart failure, and all-cause mortality,
After a median follow-up of 7-11 years, fewer patients in the surgical group than in the control group had MACE (20% vs. 25%) or died from all causes (4.1% vs. 6.3%, both statistically significant at P < .01)
Similarly, significantly fewer patients in the surgical group than in the control group had a coronary artery event or heart failure (each P < .01).
However, there were no significant between-group difference in the rate of stroke, possibly because of the small number of strokes.
The risk of MACE was 17% lower in the group that had bariatric surgery than in the control group (adjusted hazard ratio, 0.83; 95% confidence interval, 0.78-0.89), after adjusting for age, sex, and number of comorbidities.
In subgroup analysis, patients who had adjustable gastric banding, Roux-en-Y gastric bypass, or duodenal switch had a significantly lower risk of MACE than control patients.
The risk of MACE was similar in patients who had sleeve gastrectomy and in control patients.
However, these subgroup results need to be interpreted with caution since the surgery and control patients in each surgery type subgroup were not matched for age, sex, and comorbidities, said Dr. Bouchard.
He acknowledged that study limitations include a lack of information about the patients’ BMI, weight, medications, and glycemic control (hemoglobin A1c).
Dr. Bouchard and Dr. Anvari have no relevant financial disclosures.
Patients with obesity who had bariatric surgery had a lower risk of having a major adverse cardiovascular event (MACE) or dying from all causes during a median 7-year follow-up, compared with similar patients who did not undergo surgery.
These findings, from a province-wide retrospective cohort study from Quebec, follow two recent, slightly shorter similar trials.
Now we need a large randomized clinical trial (RCT), experts say, to definitively establish cardiovascular and mortality benefits in people with obesity who have metabolic/bariatric surgery. And such a trial is just beginning.
Philippe Bouchard, MD, a general surgery resident from McGill University in Montreal presented the Quebec study in a top papers session at the annual meeting of the American Society for Metabolic & Bariatric Surgery.
The findings showed that, among obese patients with metabolic syndrome, bariatric/metabolic surgery is associated with a sustained decrease in the incidence of MACE and all-cause mortality of at least 5 years, Dr. Bouchard said.
“The results of this population-based observational study should be validated in randomized controlled trials,” he concluded.
In the meantime, “we believe our study adds to the body of evidence in mainly two ways,” Dr. Bouchard told this news organization in an email.
It has a longer follow-up than recent observational studies, “a median of 7 years, compared to 3.9 years in a study from the Cleveland Clinic, and 4.6 years in one from Ontario, he said.
“This allows us to [estimate] an absolute risk reduction of MACE of 5.11% at 10 years,” he added. This is a smaller risk reduction than the roughly 40% risk reduction seen in the other two studies, possibly because of selection bias, Dr. Bouchard speculated.
“Second, most of the larger cohorts are heavily weighted on Roux-en-Y gastric bypass,” he continued. In contrast, their study included diverse procedures, including sleeve gastrectomy, duodenal switch, and adjustable gastric banding.
“Given the rise in popularity of a derivative of the duodenal switch – the single-anastomosis duodenal-ileal bypass with sleeve gastrectomy (SADi-S) – we believe this information is timely and relevant to clinicians,” Dr. Bouchard said.
RCT on the subject is coming
“I totally agree that we need a large randomized controlled trial of bariatric surgery versus optimal medical therapy to conclusively establish” the impact of bariatric surgery on cardiovascular outcomes, said the assigned discussant, Mehran Anvari, MD. And their research group is just about to begin one.
In the absence of RCT data, clinicians “may currently not refer [eligible] patients for bariatric surgery because of the high risk they pose,” said Dr. Anvari, professor and director of the Centre for Minimal Access Surgery of McMaster University, Hamilton, Ont., and senior author in the Ontario study.
Furthermore, an important point is that the current trial extended the follow-up to 7 years, he told this news organization in an email.
That study included patients with diabetes and hypertension, he added, whereas his group included patients with a history of cardiovascular disease and/or heart failure.
“We hope these studies encourage general practitioners and cardiologists to consider bariatric surgery as a viable treatment option to prevent and reduce the risk of MACE in the obese patients [body mass index >35 kg/m2] with significant cardiovascular disease,” he said.
“We have embarked on a pilot RCT among bariatric centers of excellence in Ontario,” Dr. Anvari added, which showed the feasibility and safety of such a study.
He estimates that the RCT will need to recruit 2,000 patients to demonstrate the safety and effectiveness of bariatric surgery in reducing MACE and cardiac and all-cause mortality among patients with existing cardiovascular disease.
This “will require international collaboration,” he added, “and our group is currently establishing collaboration with sites in North America, Europe, and Australia to conduct such a study.”
Patients matched for age, sex, number of comorbidities
Quebec has a single public health care system that covers the cost of bariatric surgery for eligible patients; that is, those with a BMI greater than 35 kg/m2 and comorbidities or a BMI greater than 40 kg/m2.
Using this provincial health care database, which covers over 97% of the population, the researchers identified 3,637 patients with diabetes and/or hypertension who had bariatric surgery during 2007-2012.
They matched the surgery patients with 5,420 control patients with obesity who lived in the same geographic region and had a similar age, sex, and number of Charlson Comorbidity Index comorbidities, but did not undergo bariatric surgery.
The patients had a mean age of 50 and 64% were women.
Half had zero to one comorbidities, a quarter had two comorbidities, and another quarter had at least three comorbidities.
Most patients in the surgery group had type 2 diabetes (70%) and 50% had hypertension, whereas in the control group, most patients had hypertension (82%) and 41% had diabetes.
The most common type of bariatric surgery was adjustable gastric banding (42% of patients), followed by duodenal switch (24%), sleeve gastrectomy (23%), and Roux-en-Y gastric bypass (11%).
The primary outcome was the incidence of MACE, defined as coronary artery events (including myocardial infarction, percutaneous coronary intervention, and coronary artery bypass graft), stroke, heart failure, and all-cause mortality,
After a median follow-up of 7-11 years, fewer patients in the surgical group than in the control group had MACE (20% vs. 25%) or died from all causes (4.1% vs. 6.3%, both statistically significant at P < .01)
Similarly, significantly fewer patients in the surgical group than in the control group had a coronary artery event or heart failure (each P < .01).
However, there were no significant between-group difference in the rate of stroke, possibly because of the small number of strokes.
The risk of MACE was 17% lower in the group that had bariatric surgery than in the control group (adjusted hazard ratio, 0.83; 95% confidence interval, 0.78-0.89), after adjusting for age, sex, and number of comorbidities.
In subgroup analysis, patients who had adjustable gastric banding, Roux-en-Y gastric bypass, or duodenal switch had a significantly lower risk of MACE than control patients.
The risk of MACE was similar in patients who had sleeve gastrectomy and in control patients.
However, these subgroup results need to be interpreted with caution since the surgery and control patients in each surgery type subgroup were not matched for age, sex, and comorbidities, said Dr. Bouchard.
He acknowledged that study limitations include a lack of information about the patients’ BMI, weight, medications, and glycemic control (hemoglobin A1c).
Dr. Bouchard and Dr. Anvari have no relevant financial disclosures.
FROM ASMBS 2021
Healthy with obesity? The latest study casts doubt
compared with people without obesity and or adverse metabolic profiles, new research suggests.
The latest data on this controversial subject come from an analysis of nearly 400,000 people in the U.K. Biobank. Although the data also showed that metabolically healthy obesity poses less risk than “metabolically unhealthy” obesity, the risk of progression from healthy to unhealthy within 3-5 years was high.
“People with metabolically healthy obesity are not ‘healthy’ as they are at higher risk of atherosclerotic cardiovascular disease [ASCVD], heart failure, and respiratory diseases, compared with nonobese people with a normal metabolic profile. As such, weight management could be beneficial to all people with obesity irrespective of metabolic profile,” Ziyi Zhou and colleagues wrote in their report, published June 10, 2021, in Diabetologia.
Moreover, they advised avoiding the term metabolically healthy obesity entirely in clinical medicine “as it is misleading, and different strategies for risk stratification should be explored.”
In interviews, two experts provided somewhat different takes on the study and the overall subject.
‘Lifestyle should be explored with every single patient regardless of their weight’
Yoni Freedhoff, MD, medical director of the Bariatric Medical Institute, Ottawa, said “clinicians and patients need to be aware that obesity increases a person’s risk of various medical problems, and in turn this might lead to more frequent screening. This increased screening might be analogous to that of a person with a strong familial history of cancer who of course we would never describe as being ‘unhealthy’ as a consequence of their increased risk.”
In addition to screening, “lifestyle should be explored with every single patient regardless of their weight, and if a person’s weight is not affecting their health or their quality of life, a clinician need only let the patient know that, were they to want to discuss weight management options in the future, that they’d be there for them,” said Dr. Freedhoff.
‘Metabolically healthy obesity’ has had many definitions
Matthias Schulze, DrPH, head of the molecular epidemiology at the German Institute of Human Nutrition, Potsdam, and professor at the University of Potsdam, pointed out that the way metabolically healthy obesity is defined and the outcomes assessed make a difference.
In the current study, the term is defined as having a body mass index of at least 30 kg/m2 and at least four of six metabolically healthy criteria: blood pressure, C-reactive protein, triacylglycerols, LDL cholesterol, HDL cholesterol, and hemoglobin A1c.
In May 2021, Dr. Schulze and associates reported in JAMA Network Open on a different definition that they found to identify individuals who do not have an increased risk of cardiovascular disease death and total mortality. Interestingly, they also used the U.K. Biobank as their validation cohort.
“We derived a new definition of metabolic health ... that is different from those used in [the current] article. Importantly, we included a measure of body fat distribution, waist-to-hip ratio. On the other side, we investigated only mortality outcomes and we can therefore not exclude the possibility that other outcomes may still be related. [For example], a higher diabetes risk may still be present among those we have defined as having metabolically healthy obesity.”
Dr. Schulze also said that several previous studies and meta-analyses have suggested that “previous common definitions of metabolically healthy obesity do not identify a subgroup without risk, or being at risk comparable to normal-weight metabolically healthy. Thus, this study confirms this conclusion. [But] this doesn’t rule out that there are better ways of defining subgroups.”
Clinically, he said “given that we investigated only mortality, we cannot conclude that our ‘metabolically healthy obesity’ group doesn’t require intervention.”
Higher rates of diabetes, ASCVD, heart failure, death
The current population-based study included 381,363 U.K. Biobank participants who were followed up for a median 11.2 years. Overall, about 55% did not have obesity or metabolic abnormalities, 9% had metabolically healthy obesity, 20% were metabolically unhealthy but did not have obesity, and 16% had metabolically unhealthy obesity as defined by the investigators.
The investigators adjusted the data for several potential confounders, including age, sex, ethnicity, education, socioeconomic status, smoking status, physical activity, and dietary factors.
Compared with individuals without obesity or metabolic abnormalities, those with metabolically healthy obesity had significantly higher rates of incident diabetes (hazard ratio, 4.32), ASCVD (HR, 1.18), myocardial infarction (HR, 1.23), stroke (HR, 1.10), heart failure (HR, 1.76), respiratory diseases (HR, 1.28), and chronic obstructive pulmonary disease (HR, 1.19).
In general, rates of cardiovascular and respiratory outcomes were highest in metabolically unhealthy obesity, followed by those without obesity but with metabolic abnormalities and those with metabolically healthy obesity. However, for incident and fatal heart failure and incident respiratory diseases, those with metabolically healthy obesity had higher rates than did those without obesity but with metabolic abnormalities.
Compared with those without obesity or metabolic abnormalities, those with metabolically healthy obesity had significantly higher all-cause mortality rates (HR, 1.22). And, compared with those without obesity (regardless of metabolic status) at baseline, those with metabolically healthy obesity were significantly more likely to have diabetes (HR, 2.06), heart failure (HR, 1.6), and respiratory diseases (HR, 1.2), but not ASCVD. The association was also significant for all-cause and heart failure mortality (HR, 1.12 and 1.44, respectively), but not for other causes of death.
Progression from metabolically healthy to unhealthy is common
Among 8,512 participants for whom longitudinal data were available for a median of 4.4 years, half of those with metabolically healthy obesity remained in that category, 20% no longer had obesity, and more than a quarter transitioned to metabolically unhealthy obesity. Compared with those without obesity or metabolic abnormalities throughout, those who transitioned from metabolically healthy to metabolically unhealthy had significantly higher rates of incident ASCVD (HR, 2.46) and all-cause mortality (HR, 3.07).
But those who remained in the metabolically healthy obesity category throughout did not have significantly increased risks for the adverse outcomes measured.
Ms. Zhou and colleagues noted that the data demonstrate heterogeneity among people with obesity, which offers the potential to stratify risk based on prognosis. For example, “people with [metabolically unhealthy obesity] were at a higher risk of mortality and morbidity than everyone else, and thus they should be prioritized for intervention.”
However, they add, “Obesity is associated with a wide range of diseases, and using a single label or categorical risk algorithm is unlikely to be effective compared with prediction algorithms based on disease-specific and continuous risk markers.”
Ms. Zhou has no disclosures. One coauthor has relationships with numerous pharmaceutical companies; the rest have none. Dr. Freedhoff has served as a director, officer, partner, employee, adviser, consultant, or trustee for the Bariatric Medical Institute and Constant Health. He is a speaker or a member of a speakers bureau for Obesity Canada and Novo Nordisk, received research grant from Novo Nordisk, and received income of at least $250 from WebMD, CTV, and Random House. Dr/ Schulze has received grants from German Federal Ministry of Education and Research.
compared with people without obesity and or adverse metabolic profiles, new research suggests.
The latest data on this controversial subject come from an analysis of nearly 400,000 people in the U.K. Biobank. Although the data also showed that metabolically healthy obesity poses less risk than “metabolically unhealthy” obesity, the risk of progression from healthy to unhealthy within 3-5 years was high.
“People with metabolically healthy obesity are not ‘healthy’ as they are at higher risk of atherosclerotic cardiovascular disease [ASCVD], heart failure, and respiratory diseases, compared with nonobese people with a normal metabolic profile. As such, weight management could be beneficial to all people with obesity irrespective of metabolic profile,” Ziyi Zhou and colleagues wrote in their report, published June 10, 2021, in Diabetologia.
Moreover, they advised avoiding the term metabolically healthy obesity entirely in clinical medicine “as it is misleading, and different strategies for risk stratification should be explored.”
In interviews, two experts provided somewhat different takes on the study and the overall subject.
‘Lifestyle should be explored with every single patient regardless of their weight’
Yoni Freedhoff, MD, medical director of the Bariatric Medical Institute, Ottawa, said “clinicians and patients need to be aware that obesity increases a person’s risk of various medical problems, and in turn this might lead to more frequent screening. This increased screening might be analogous to that of a person with a strong familial history of cancer who of course we would never describe as being ‘unhealthy’ as a consequence of their increased risk.”
In addition to screening, “lifestyle should be explored with every single patient regardless of their weight, and if a person’s weight is not affecting their health or their quality of life, a clinician need only let the patient know that, were they to want to discuss weight management options in the future, that they’d be there for them,” said Dr. Freedhoff.
‘Metabolically healthy obesity’ has had many definitions
Matthias Schulze, DrPH, head of the molecular epidemiology at the German Institute of Human Nutrition, Potsdam, and professor at the University of Potsdam, pointed out that the way metabolically healthy obesity is defined and the outcomes assessed make a difference.
In the current study, the term is defined as having a body mass index of at least 30 kg/m2 and at least four of six metabolically healthy criteria: blood pressure, C-reactive protein, triacylglycerols, LDL cholesterol, HDL cholesterol, and hemoglobin A1c.
In May 2021, Dr. Schulze and associates reported in JAMA Network Open on a different definition that they found to identify individuals who do not have an increased risk of cardiovascular disease death and total mortality. Interestingly, they also used the U.K. Biobank as their validation cohort.
“We derived a new definition of metabolic health ... that is different from those used in [the current] article. Importantly, we included a measure of body fat distribution, waist-to-hip ratio. On the other side, we investigated only mortality outcomes and we can therefore not exclude the possibility that other outcomes may still be related. [For example], a higher diabetes risk may still be present among those we have defined as having metabolically healthy obesity.”
Dr. Schulze also said that several previous studies and meta-analyses have suggested that “previous common definitions of metabolically healthy obesity do not identify a subgroup without risk, or being at risk comparable to normal-weight metabolically healthy. Thus, this study confirms this conclusion. [But] this doesn’t rule out that there are better ways of defining subgroups.”
Clinically, he said “given that we investigated only mortality, we cannot conclude that our ‘metabolically healthy obesity’ group doesn’t require intervention.”
Higher rates of diabetes, ASCVD, heart failure, death
The current population-based study included 381,363 U.K. Biobank participants who were followed up for a median 11.2 years. Overall, about 55% did not have obesity or metabolic abnormalities, 9% had metabolically healthy obesity, 20% were metabolically unhealthy but did not have obesity, and 16% had metabolically unhealthy obesity as defined by the investigators.
The investigators adjusted the data for several potential confounders, including age, sex, ethnicity, education, socioeconomic status, smoking status, physical activity, and dietary factors.
Compared with individuals without obesity or metabolic abnormalities, those with metabolically healthy obesity had significantly higher rates of incident diabetes (hazard ratio, 4.32), ASCVD (HR, 1.18), myocardial infarction (HR, 1.23), stroke (HR, 1.10), heart failure (HR, 1.76), respiratory diseases (HR, 1.28), and chronic obstructive pulmonary disease (HR, 1.19).
In general, rates of cardiovascular and respiratory outcomes were highest in metabolically unhealthy obesity, followed by those without obesity but with metabolic abnormalities and those with metabolically healthy obesity. However, for incident and fatal heart failure and incident respiratory diseases, those with metabolically healthy obesity had higher rates than did those without obesity but with metabolic abnormalities.
Compared with those without obesity or metabolic abnormalities, those with metabolically healthy obesity had significantly higher all-cause mortality rates (HR, 1.22). And, compared with those without obesity (regardless of metabolic status) at baseline, those with metabolically healthy obesity were significantly more likely to have diabetes (HR, 2.06), heart failure (HR, 1.6), and respiratory diseases (HR, 1.2), but not ASCVD. The association was also significant for all-cause and heart failure mortality (HR, 1.12 and 1.44, respectively), but not for other causes of death.
Progression from metabolically healthy to unhealthy is common
Among 8,512 participants for whom longitudinal data were available for a median of 4.4 years, half of those with metabolically healthy obesity remained in that category, 20% no longer had obesity, and more than a quarter transitioned to metabolically unhealthy obesity. Compared with those without obesity or metabolic abnormalities throughout, those who transitioned from metabolically healthy to metabolically unhealthy had significantly higher rates of incident ASCVD (HR, 2.46) and all-cause mortality (HR, 3.07).
But those who remained in the metabolically healthy obesity category throughout did not have significantly increased risks for the adverse outcomes measured.
Ms. Zhou and colleagues noted that the data demonstrate heterogeneity among people with obesity, which offers the potential to stratify risk based on prognosis. For example, “people with [metabolically unhealthy obesity] were at a higher risk of mortality and morbidity than everyone else, and thus they should be prioritized for intervention.”
However, they add, “Obesity is associated with a wide range of diseases, and using a single label or categorical risk algorithm is unlikely to be effective compared with prediction algorithms based on disease-specific and continuous risk markers.”
Ms. Zhou has no disclosures. One coauthor has relationships with numerous pharmaceutical companies; the rest have none. Dr. Freedhoff has served as a director, officer, partner, employee, adviser, consultant, or trustee for the Bariatric Medical Institute and Constant Health. He is a speaker or a member of a speakers bureau for Obesity Canada and Novo Nordisk, received research grant from Novo Nordisk, and received income of at least $250 from WebMD, CTV, and Random House. Dr/ Schulze has received grants from German Federal Ministry of Education and Research.
compared with people without obesity and or adverse metabolic profiles, new research suggests.
The latest data on this controversial subject come from an analysis of nearly 400,000 people in the U.K. Biobank. Although the data also showed that metabolically healthy obesity poses less risk than “metabolically unhealthy” obesity, the risk of progression from healthy to unhealthy within 3-5 years was high.
“People with metabolically healthy obesity are not ‘healthy’ as they are at higher risk of atherosclerotic cardiovascular disease [ASCVD], heart failure, and respiratory diseases, compared with nonobese people with a normal metabolic profile. As such, weight management could be beneficial to all people with obesity irrespective of metabolic profile,” Ziyi Zhou and colleagues wrote in their report, published June 10, 2021, in Diabetologia.
Moreover, they advised avoiding the term metabolically healthy obesity entirely in clinical medicine “as it is misleading, and different strategies for risk stratification should be explored.”
In interviews, two experts provided somewhat different takes on the study and the overall subject.
‘Lifestyle should be explored with every single patient regardless of their weight’
Yoni Freedhoff, MD, medical director of the Bariatric Medical Institute, Ottawa, said “clinicians and patients need to be aware that obesity increases a person’s risk of various medical problems, and in turn this might lead to more frequent screening. This increased screening might be analogous to that of a person with a strong familial history of cancer who of course we would never describe as being ‘unhealthy’ as a consequence of their increased risk.”
In addition to screening, “lifestyle should be explored with every single patient regardless of their weight, and if a person’s weight is not affecting their health or their quality of life, a clinician need only let the patient know that, were they to want to discuss weight management options in the future, that they’d be there for them,” said Dr. Freedhoff.
‘Metabolically healthy obesity’ has had many definitions
Matthias Schulze, DrPH, head of the molecular epidemiology at the German Institute of Human Nutrition, Potsdam, and professor at the University of Potsdam, pointed out that the way metabolically healthy obesity is defined and the outcomes assessed make a difference.
In the current study, the term is defined as having a body mass index of at least 30 kg/m2 and at least four of six metabolically healthy criteria: blood pressure, C-reactive protein, triacylglycerols, LDL cholesterol, HDL cholesterol, and hemoglobin A1c.
In May 2021, Dr. Schulze and associates reported in JAMA Network Open on a different definition that they found to identify individuals who do not have an increased risk of cardiovascular disease death and total mortality. Interestingly, they also used the U.K. Biobank as their validation cohort.
“We derived a new definition of metabolic health ... that is different from those used in [the current] article. Importantly, we included a measure of body fat distribution, waist-to-hip ratio. On the other side, we investigated only mortality outcomes and we can therefore not exclude the possibility that other outcomes may still be related. [For example], a higher diabetes risk may still be present among those we have defined as having metabolically healthy obesity.”
Dr. Schulze also said that several previous studies and meta-analyses have suggested that “previous common definitions of metabolically healthy obesity do not identify a subgroup without risk, or being at risk comparable to normal-weight metabolically healthy. Thus, this study confirms this conclusion. [But] this doesn’t rule out that there are better ways of defining subgroups.”
Clinically, he said “given that we investigated only mortality, we cannot conclude that our ‘metabolically healthy obesity’ group doesn’t require intervention.”
Higher rates of diabetes, ASCVD, heart failure, death
The current population-based study included 381,363 U.K. Biobank participants who were followed up for a median 11.2 years. Overall, about 55% did not have obesity or metabolic abnormalities, 9% had metabolically healthy obesity, 20% were metabolically unhealthy but did not have obesity, and 16% had metabolically unhealthy obesity as defined by the investigators.
The investigators adjusted the data for several potential confounders, including age, sex, ethnicity, education, socioeconomic status, smoking status, physical activity, and dietary factors.
Compared with individuals without obesity or metabolic abnormalities, those with metabolically healthy obesity had significantly higher rates of incident diabetes (hazard ratio, 4.32), ASCVD (HR, 1.18), myocardial infarction (HR, 1.23), stroke (HR, 1.10), heart failure (HR, 1.76), respiratory diseases (HR, 1.28), and chronic obstructive pulmonary disease (HR, 1.19).
In general, rates of cardiovascular and respiratory outcomes were highest in metabolically unhealthy obesity, followed by those without obesity but with metabolic abnormalities and those with metabolically healthy obesity. However, for incident and fatal heart failure and incident respiratory diseases, those with metabolically healthy obesity had higher rates than did those without obesity but with metabolic abnormalities.
Compared with those without obesity or metabolic abnormalities, those with metabolically healthy obesity had significantly higher all-cause mortality rates (HR, 1.22). And, compared with those without obesity (regardless of metabolic status) at baseline, those with metabolically healthy obesity were significantly more likely to have diabetes (HR, 2.06), heart failure (HR, 1.6), and respiratory diseases (HR, 1.2), but not ASCVD. The association was also significant for all-cause and heart failure mortality (HR, 1.12 and 1.44, respectively), but not for other causes of death.
Progression from metabolically healthy to unhealthy is common
Among 8,512 participants for whom longitudinal data were available for a median of 4.4 years, half of those with metabolically healthy obesity remained in that category, 20% no longer had obesity, and more than a quarter transitioned to metabolically unhealthy obesity. Compared with those without obesity or metabolic abnormalities throughout, those who transitioned from metabolically healthy to metabolically unhealthy had significantly higher rates of incident ASCVD (HR, 2.46) and all-cause mortality (HR, 3.07).
But those who remained in the metabolically healthy obesity category throughout did not have significantly increased risks for the adverse outcomes measured.
Ms. Zhou and colleagues noted that the data demonstrate heterogeneity among people with obesity, which offers the potential to stratify risk based on prognosis. For example, “people with [metabolically unhealthy obesity] were at a higher risk of mortality and morbidity than everyone else, and thus they should be prioritized for intervention.”
However, they add, “Obesity is associated with a wide range of diseases, and using a single label or categorical risk algorithm is unlikely to be effective compared with prediction algorithms based on disease-specific and continuous risk markers.”
Ms. Zhou has no disclosures. One coauthor has relationships with numerous pharmaceutical companies; the rest have none. Dr. Freedhoff has served as a director, officer, partner, employee, adviser, consultant, or trustee for the Bariatric Medical Institute and Constant Health. He is a speaker or a member of a speakers bureau for Obesity Canada and Novo Nordisk, received research grant from Novo Nordisk, and received income of at least $250 from WebMD, CTV, and Random House. Dr/ Schulze has received grants from German Federal Ministry of Education and Research.
FROM DIABETOLOGIA
Bariatric surgery tied to fewer HFpEF hospitalizations
Patients who underwent metabolic and bariatric surgery had fewer than half the number of hospitalizations for both acute and chronic episodes of heart failure with preserved ejection fraction (HFpEF) in a retrospective analysis of more than 2 million Americans collected in a national database.
In a multivariate analysis that adjusted for several variables patients without a history of bariatric surgery had three- to fivefold more hospitalizations for acute events involving HFpEF, and more than double the rate of hospitalizations for chronic HFpEF events, David R. Funes, MD, said at the annual meeting of the American Society for Metabolic and Bariatric Surgery.
While this analysis has the limitations of being retrospective, observational, and entirely reliant on procedure codes to define medical histories and outcomes, it had the advantage of using a large database designed to represent the U.S. adult population, said Dr. Funes, a bariatric surgeon at the Cleveland Clinic in Weston, Fla.
HFpEF effects could ‘extend’ surgery’s use
The report “adds an important article to the literature where there is a true void in trying to discern the effect of bariatric surgery on HFpEF,” commented Tammy L. Kindel, MD, PhD, director of the bariatric surgery program at the Medical College of Wisconsin, Milwaukee, and designated discussant for the report. “Minimal studies [up to now] demonstrate that weight loss in any form can modify diastolic dysfunction in patients with HFpEF. Studies that investigate the impact of bariatric surgery on clinical outcomes in patients with HFpEF are probably the most important for extending use of metabolic surgery,” Dr. Kindel said.
She added that “one of the most difficult parts of studying HFpEF” is making a firm diagnosis that often involves excluding other potential causes. She also questioned Dr. Funes about his confidence that his analysis correctly identified patients only with HFpEF. Dr. Funes replied that the diagnostic codes his team used allowed for a clear distinction between patients identified with HFpEF and those with heart failure with reduced ejection fraction, but he also admitted that his study’s complete reliance on these codes introduced a limitation to the analysis.
Including patients with diastolic dysfunction as well as HFpEF
The study used data collected during 2010-2015 by the National Inpatient Sample, run by the U.S. Department of Health & Human Services in a case-control analysis that included 296,041 patients who had undergone some form of bariatric surgery and 2,004,804 people with no history of bariatric surgery selected as controls on the basis of their obesity.
The absolute numbers showed that, during the observation period, the incidence of acute HFpEF hospitalizations was 0.19% among those with prior bariatric surgery and 0.86% among those with no surgery, and the incidence of chronic heart failure hospitalizations was 0.01% among people with prior bariatric surgery and 0.05% among those without prior surgery. Dr. Funes said. He noted that, during the period studied patients, with HFpEF were usually identified as having diastolic heart failure, an older name for the same disease.
In multivariate analyses that adjusted for age, sex, race, hypertension, diabetes, smoking, and coronary artery disease, people without prior bariatric surgery and with hypertension had a 2.8-fold increased rate of acute hospitalizations for HFpEF, while those without hypertension or prior bariatric surgery had a 5.2-fold increased rate. In addition, control patients, regardless of hypertension status, had a 2.9-fold increased rate of hospitalizations for chronic HFpEF events. All these differences were statistically significant.
Dr. Funes also reported results from additional analyses that focused on a roughly 68,000-patient subgroup of those included in the study who had a history of coronary artery disease, including about 62,000 with no prior bariatric surgery and nearly 6,000 people with prior bariatric surgery. In a multivariate analysis of this subgroup, people without prior bariatric surgery had a 2.65-fold increased rate of hospitalization for a HFpEF event (either acute or chronic), compared with those who had undergone bariatric surgery.
Dr. Funes and associates and Dr. Kindel had no relevant disclosures.
Patients who underwent metabolic and bariatric surgery had fewer than half the number of hospitalizations for both acute and chronic episodes of heart failure with preserved ejection fraction (HFpEF) in a retrospective analysis of more than 2 million Americans collected in a national database.
In a multivariate analysis that adjusted for several variables patients without a history of bariatric surgery had three- to fivefold more hospitalizations for acute events involving HFpEF, and more than double the rate of hospitalizations for chronic HFpEF events, David R. Funes, MD, said at the annual meeting of the American Society for Metabolic and Bariatric Surgery.
While this analysis has the limitations of being retrospective, observational, and entirely reliant on procedure codes to define medical histories and outcomes, it had the advantage of using a large database designed to represent the U.S. adult population, said Dr. Funes, a bariatric surgeon at the Cleveland Clinic in Weston, Fla.
HFpEF effects could ‘extend’ surgery’s use
The report “adds an important article to the literature where there is a true void in trying to discern the effect of bariatric surgery on HFpEF,” commented Tammy L. Kindel, MD, PhD, director of the bariatric surgery program at the Medical College of Wisconsin, Milwaukee, and designated discussant for the report. “Minimal studies [up to now] demonstrate that weight loss in any form can modify diastolic dysfunction in patients with HFpEF. Studies that investigate the impact of bariatric surgery on clinical outcomes in patients with HFpEF are probably the most important for extending use of metabolic surgery,” Dr. Kindel said.
She added that “one of the most difficult parts of studying HFpEF” is making a firm diagnosis that often involves excluding other potential causes. She also questioned Dr. Funes about his confidence that his analysis correctly identified patients only with HFpEF. Dr. Funes replied that the diagnostic codes his team used allowed for a clear distinction between patients identified with HFpEF and those with heart failure with reduced ejection fraction, but he also admitted that his study’s complete reliance on these codes introduced a limitation to the analysis.
Including patients with diastolic dysfunction as well as HFpEF
The study used data collected during 2010-2015 by the National Inpatient Sample, run by the U.S. Department of Health & Human Services in a case-control analysis that included 296,041 patients who had undergone some form of bariatric surgery and 2,004,804 people with no history of bariatric surgery selected as controls on the basis of their obesity.
The absolute numbers showed that, during the observation period, the incidence of acute HFpEF hospitalizations was 0.19% among those with prior bariatric surgery and 0.86% among those with no surgery, and the incidence of chronic heart failure hospitalizations was 0.01% among people with prior bariatric surgery and 0.05% among those without prior surgery. Dr. Funes said. He noted that, during the period studied patients, with HFpEF were usually identified as having diastolic heart failure, an older name for the same disease.
In multivariate analyses that adjusted for age, sex, race, hypertension, diabetes, smoking, and coronary artery disease, people without prior bariatric surgery and with hypertension had a 2.8-fold increased rate of acute hospitalizations for HFpEF, while those without hypertension or prior bariatric surgery had a 5.2-fold increased rate. In addition, control patients, regardless of hypertension status, had a 2.9-fold increased rate of hospitalizations for chronic HFpEF events. All these differences were statistically significant.
Dr. Funes also reported results from additional analyses that focused on a roughly 68,000-patient subgroup of those included in the study who had a history of coronary artery disease, including about 62,000 with no prior bariatric surgery and nearly 6,000 people with prior bariatric surgery. In a multivariate analysis of this subgroup, people without prior bariatric surgery had a 2.65-fold increased rate of hospitalization for a HFpEF event (either acute or chronic), compared with those who had undergone bariatric surgery.
Dr. Funes and associates and Dr. Kindel had no relevant disclosures.
Patients who underwent metabolic and bariatric surgery had fewer than half the number of hospitalizations for both acute and chronic episodes of heart failure with preserved ejection fraction (HFpEF) in a retrospective analysis of more than 2 million Americans collected in a national database.
In a multivariate analysis that adjusted for several variables patients without a history of bariatric surgery had three- to fivefold more hospitalizations for acute events involving HFpEF, and more than double the rate of hospitalizations for chronic HFpEF events, David R. Funes, MD, said at the annual meeting of the American Society for Metabolic and Bariatric Surgery.
While this analysis has the limitations of being retrospective, observational, and entirely reliant on procedure codes to define medical histories and outcomes, it had the advantage of using a large database designed to represent the U.S. adult population, said Dr. Funes, a bariatric surgeon at the Cleveland Clinic in Weston, Fla.
HFpEF effects could ‘extend’ surgery’s use
The report “adds an important article to the literature where there is a true void in trying to discern the effect of bariatric surgery on HFpEF,” commented Tammy L. Kindel, MD, PhD, director of the bariatric surgery program at the Medical College of Wisconsin, Milwaukee, and designated discussant for the report. “Minimal studies [up to now] demonstrate that weight loss in any form can modify diastolic dysfunction in patients with HFpEF. Studies that investigate the impact of bariatric surgery on clinical outcomes in patients with HFpEF are probably the most important for extending use of metabolic surgery,” Dr. Kindel said.
She added that “one of the most difficult parts of studying HFpEF” is making a firm diagnosis that often involves excluding other potential causes. She also questioned Dr. Funes about his confidence that his analysis correctly identified patients only with HFpEF. Dr. Funes replied that the diagnostic codes his team used allowed for a clear distinction between patients identified with HFpEF and those with heart failure with reduced ejection fraction, but he also admitted that his study’s complete reliance on these codes introduced a limitation to the analysis.
Including patients with diastolic dysfunction as well as HFpEF
The study used data collected during 2010-2015 by the National Inpatient Sample, run by the U.S. Department of Health & Human Services in a case-control analysis that included 296,041 patients who had undergone some form of bariatric surgery and 2,004,804 people with no history of bariatric surgery selected as controls on the basis of their obesity.
The absolute numbers showed that, during the observation period, the incidence of acute HFpEF hospitalizations was 0.19% among those with prior bariatric surgery and 0.86% among those with no surgery, and the incidence of chronic heart failure hospitalizations was 0.01% among people with prior bariatric surgery and 0.05% among those without prior surgery. Dr. Funes said. He noted that, during the period studied patients, with HFpEF were usually identified as having diastolic heart failure, an older name for the same disease.
In multivariate analyses that adjusted for age, sex, race, hypertension, diabetes, smoking, and coronary artery disease, people without prior bariatric surgery and with hypertension had a 2.8-fold increased rate of acute hospitalizations for HFpEF, while those without hypertension or prior bariatric surgery had a 5.2-fold increased rate. In addition, control patients, regardless of hypertension status, had a 2.9-fold increased rate of hospitalizations for chronic HFpEF events. All these differences were statistically significant.
Dr. Funes also reported results from additional analyses that focused on a roughly 68,000-patient subgroup of those included in the study who had a history of coronary artery disease, including about 62,000 with no prior bariatric surgery and nearly 6,000 people with prior bariatric surgery. In a multivariate analysis of this subgroup, people without prior bariatric surgery had a 2.65-fold increased rate of hospitalization for a HFpEF event (either acute or chronic), compared with those who had undergone bariatric surgery.
Dr. Funes and associates and Dr. Kindel had no relevant disclosures.
FROM ASMBS 2021
Eat two fruits a day, ward off diabetes?
A new study supports the recommendation of eating two servings of fruit a day for health benefits – in this case a lower risk of diabetes.
Adults who ate two servings of fruit a day had 36% lower odds of developing diabetes within 5 years compared to those who ate less than a half serving of fruit a day, after adjusting for confounders, in a population-based Australian study.
The findings by Nicola P. Bondonno, PhD, and colleagues, based on data from the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab), were published online June 2 in the Journal of Clinical Endocrinology & Metabolism.
The study also showed that a higher fruit intake was associated with higher insulin sensitivity and lower pancreatic beta-cell function in a dose-response manner.
And a higher intake of apples – but not citrus fruit or bananas, the two other fruits studied – was associated with lower post-load serum insulin levels.
“This indicates that people who consumed more fruit [especially apples] had to produce less insulin to lower their blood glucose levels,” Dr. Bondonno, from the Institute for Nutrition Research, Edith Cowan University, Perth, Australia, explained in a statement from the Endocrine Society.
“This is important since high levels of circulating insulin (hyperinsulinemia) can damage blood vessels” and this is “related not only to diabetes, but also to high blood pressure, obesity, and heart disease,” she observed.
Fruit juice doesn’t have same effect
The study supports the recommendation of the Australian Dietary Guidelines – 2 servings of fruit a day, where one serving is 150 grams, which corresponds to a medium-sized apple, orange, or banana – Dr. Bondonno clarified in an email.
However, fruit juice was not associated with better glucose or insulin levels, or lower risk of diabetes, possibly because of its relatively high glycemic load and fewer beneficial fibers, the researchers speculate; added data suggest that even juice with added fiber does not trigger satiety.
The study findings “support encouragement of the consumption of whole fruits, but not fruit juice, to preserve insulin sensitivity and mitigate [type 2 diabetes] risk,” Dr. Bondonno and colleagues summarize.
“Promoting a healthy diet and lifestyle which includes the consumption of popular fruits such as apples, bananas, and oranges, with widespread geographical availability, may lower [type 2 diabetes] incidence,” they conclude.
Lower 5-year odds of diabetes
It is not clear how eating fruit may confer protection against developing diabetes, the researchers write.
They aimed to examine how consumption of total fruit, individual fruit, and fruit juice is related to glucose tolerance, insulin sensitivity, and incident diabetes at 5 years and 12 years in participants in the nationally representative AusDiab study.
They identified 7,675 adults aged 25 and older without diabetes who had undergone blood tests and completed a food frequency questionnaire in 1999-2000.
Participants had indicated how often they ate 10 different types of fruit, any type of fruit juice, and other foods on a scale of 0 (never) to 10 (three or more times/day).
Researchers divided participants into quartiles based on their median fruit consumption: 62 (range 0-95) g/day, 122 (95-162) g/day, 230 (162-283) g/day, and 372 (283-961) g/day.
The most commonly consumed fruit was apples (23% of total fruit intake), followed by bananas (20%) and citrus fruit (18%). Other fruits each accounted for less than 8% of total fruit intake, so they were not studied separately.
Participants in each quartile had a similar mean age (54 years) and body mass index (27 kg/m2).
However, compared with participants in quartile 1 (low fruit intake), those in quartiles 3 and 4 (moderate and high fruit intakes, respectively) were more likely to be female, do at least 150 minutes of physical activity a week, and less likely to smoke. They also ate more vegetables and less red meat and processed meat, but they consumed more sugar.
Of 4,674 participants who had 5-year follow-up, 179 participants developed diabetes.
Compared to participants with a low fruit intake (quartile 1), those with a moderate fruit intake (quartile 3) had a 36% lower odds of developing diabetes within 5 years (odds ratio, 0.64; 95% confidence interval, 0.44-0.92) after adjusting for age, sex, physical activity, education, socioeconomic status, income, body mass index, smoking, cardiovascular disease, parental history of diabetes, and consumption of alcohol, vegetables, red meat, processed meat, and calories.
Of the 3,518 participants with 12-year follow-up, 247 participants had diabetes, but there were no significant associations between fruit consumption and this longer-term risk of diabetes, possibly due to the small number of participants and events.
The study was supported by grants from the National Health and Medical Research Council of Australia and the National Heart Foundation of Australia. Dr. Bondonno has reported no relevant financial disclosures. Disclosures of the other authors are listed with the article.
A version of this article first appeared on Medscape.com.
A new study supports the recommendation of eating two servings of fruit a day for health benefits – in this case a lower risk of diabetes.
Adults who ate two servings of fruit a day had 36% lower odds of developing diabetes within 5 years compared to those who ate less than a half serving of fruit a day, after adjusting for confounders, in a population-based Australian study.
The findings by Nicola P. Bondonno, PhD, and colleagues, based on data from the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab), were published online June 2 in the Journal of Clinical Endocrinology & Metabolism.
The study also showed that a higher fruit intake was associated with higher insulin sensitivity and lower pancreatic beta-cell function in a dose-response manner.
And a higher intake of apples – but not citrus fruit or bananas, the two other fruits studied – was associated with lower post-load serum insulin levels.
“This indicates that people who consumed more fruit [especially apples] had to produce less insulin to lower their blood glucose levels,” Dr. Bondonno, from the Institute for Nutrition Research, Edith Cowan University, Perth, Australia, explained in a statement from the Endocrine Society.
“This is important since high levels of circulating insulin (hyperinsulinemia) can damage blood vessels” and this is “related not only to diabetes, but also to high blood pressure, obesity, and heart disease,” she observed.
Fruit juice doesn’t have same effect
The study supports the recommendation of the Australian Dietary Guidelines – 2 servings of fruit a day, where one serving is 150 grams, which corresponds to a medium-sized apple, orange, or banana – Dr. Bondonno clarified in an email.
However, fruit juice was not associated with better glucose or insulin levels, or lower risk of diabetes, possibly because of its relatively high glycemic load and fewer beneficial fibers, the researchers speculate; added data suggest that even juice with added fiber does not trigger satiety.
The study findings “support encouragement of the consumption of whole fruits, but not fruit juice, to preserve insulin sensitivity and mitigate [type 2 diabetes] risk,” Dr. Bondonno and colleagues summarize.
“Promoting a healthy diet and lifestyle which includes the consumption of popular fruits such as apples, bananas, and oranges, with widespread geographical availability, may lower [type 2 diabetes] incidence,” they conclude.
Lower 5-year odds of diabetes
It is not clear how eating fruit may confer protection against developing diabetes, the researchers write.
They aimed to examine how consumption of total fruit, individual fruit, and fruit juice is related to glucose tolerance, insulin sensitivity, and incident diabetes at 5 years and 12 years in participants in the nationally representative AusDiab study.
They identified 7,675 adults aged 25 and older without diabetes who had undergone blood tests and completed a food frequency questionnaire in 1999-2000.
Participants had indicated how often they ate 10 different types of fruit, any type of fruit juice, and other foods on a scale of 0 (never) to 10 (three or more times/day).
Researchers divided participants into quartiles based on their median fruit consumption: 62 (range 0-95) g/day, 122 (95-162) g/day, 230 (162-283) g/day, and 372 (283-961) g/day.
The most commonly consumed fruit was apples (23% of total fruit intake), followed by bananas (20%) and citrus fruit (18%). Other fruits each accounted for less than 8% of total fruit intake, so they were not studied separately.
Participants in each quartile had a similar mean age (54 years) and body mass index (27 kg/m2).
However, compared with participants in quartile 1 (low fruit intake), those in quartiles 3 and 4 (moderate and high fruit intakes, respectively) were more likely to be female, do at least 150 minutes of physical activity a week, and less likely to smoke. They also ate more vegetables and less red meat and processed meat, but they consumed more sugar.
Of 4,674 participants who had 5-year follow-up, 179 participants developed diabetes.
Compared to participants with a low fruit intake (quartile 1), those with a moderate fruit intake (quartile 3) had a 36% lower odds of developing diabetes within 5 years (odds ratio, 0.64; 95% confidence interval, 0.44-0.92) after adjusting for age, sex, physical activity, education, socioeconomic status, income, body mass index, smoking, cardiovascular disease, parental history of diabetes, and consumption of alcohol, vegetables, red meat, processed meat, and calories.
Of the 3,518 participants with 12-year follow-up, 247 participants had diabetes, but there were no significant associations between fruit consumption and this longer-term risk of diabetes, possibly due to the small number of participants and events.
The study was supported by grants from the National Health and Medical Research Council of Australia and the National Heart Foundation of Australia. Dr. Bondonno has reported no relevant financial disclosures. Disclosures of the other authors are listed with the article.
A version of this article first appeared on Medscape.com.
A new study supports the recommendation of eating two servings of fruit a day for health benefits – in this case a lower risk of diabetes.
Adults who ate two servings of fruit a day had 36% lower odds of developing diabetes within 5 years compared to those who ate less than a half serving of fruit a day, after adjusting for confounders, in a population-based Australian study.
The findings by Nicola P. Bondonno, PhD, and colleagues, based on data from the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab), were published online June 2 in the Journal of Clinical Endocrinology & Metabolism.
The study also showed that a higher fruit intake was associated with higher insulin sensitivity and lower pancreatic beta-cell function in a dose-response manner.
And a higher intake of apples – but not citrus fruit or bananas, the two other fruits studied – was associated with lower post-load serum insulin levels.
“This indicates that people who consumed more fruit [especially apples] had to produce less insulin to lower their blood glucose levels,” Dr. Bondonno, from the Institute for Nutrition Research, Edith Cowan University, Perth, Australia, explained in a statement from the Endocrine Society.
“This is important since high levels of circulating insulin (hyperinsulinemia) can damage blood vessels” and this is “related not only to diabetes, but also to high blood pressure, obesity, and heart disease,” she observed.
Fruit juice doesn’t have same effect
The study supports the recommendation of the Australian Dietary Guidelines – 2 servings of fruit a day, where one serving is 150 grams, which corresponds to a medium-sized apple, orange, or banana – Dr. Bondonno clarified in an email.
However, fruit juice was not associated with better glucose or insulin levels, or lower risk of diabetes, possibly because of its relatively high glycemic load and fewer beneficial fibers, the researchers speculate; added data suggest that even juice with added fiber does not trigger satiety.
The study findings “support encouragement of the consumption of whole fruits, but not fruit juice, to preserve insulin sensitivity and mitigate [type 2 diabetes] risk,” Dr. Bondonno and colleagues summarize.
“Promoting a healthy diet and lifestyle which includes the consumption of popular fruits such as apples, bananas, and oranges, with widespread geographical availability, may lower [type 2 diabetes] incidence,” they conclude.
Lower 5-year odds of diabetes
It is not clear how eating fruit may confer protection against developing diabetes, the researchers write.
They aimed to examine how consumption of total fruit, individual fruit, and fruit juice is related to glucose tolerance, insulin sensitivity, and incident diabetes at 5 years and 12 years in participants in the nationally representative AusDiab study.
They identified 7,675 adults aged 25 and older without diabetes who had undergone blood tests and completed a food frequency questionnaire in 1999-2000.
Participants had indicated how often they ate 10 different types of fruit, any type of fruit juice, and other foods on a scale of 0 (never) to 10 (three or more times/day).
Researchers divided participants into quartiles based on their median fruit consumption: 62 (range 0-95) g/day, 122 (95-162) g/day, 230 (162-283) g/day, and 372 (283-961) g/day.
The most commonly consumed fruit was apples (23% of total fruit intake), followed by bananas (20%) and citrus fruit (18%). Other fruits each accounted for less than 8% of total fruit intake, so they were not studied separately.
Participants in each quartile had a similar mean age (54 years) and body mass index (27 kg/m2).
However, compared with participants in quartile 1 (low fruit intake), those in quartiles 3 and 4 (moderate and high fruit intakes, respectively) were more likely to be female, do at least 150 minutes of physical activity a week, and less likely to smoke. They also ate more vegetables and less red meat and processed meat, but they consumed more sugar.
Of 4,674 participants who had 5-year follow-up, 179 participants developed diabetes.
Compared to participants with a low fruit intake (quartile 1), those with a moderate fruit intake (quartile 3) had a 36% lower odds of developing diabetes within 5 years (odds ratio, 0.64; 95% confidence interval, 0.44-0.92) after adjusting for age, sex, physical activity, education, socioeconomic status, income, body mass index, smoking, cardiovascular disease, parental history of diabetes, and consumption of alcohol, vegetables, red meat, processed meat, and calories.
Of the 3,518 participants with 12-year follow-up, 247 participants had diabetes, but there were no significant associations between fruit consumption and this longer-term risk of diabetes, possibly due to the small number of participants and events.
The study was supported by grants from the National Health and Medical Research Council of Australia and the National Heart Foundation of Australia. Dr. Bondonno has reported no relevant financial disclosures. Disclosures of the other authors are listed with the article.
A version of this article first appeared on Medscape.com.