User login
Drug combo held up in real-world HCV study
A 12-week, ribavirin-free regimen achieved sustained virologic response for 85% of patients with genotype 1 hepatitis C virus (HCV) infection, researchers reported in the February issue of Gastroenterology.
“This represents one of the first applications of a highly effective HCV regimen outside clinical trials,” said Dr. Mark S. Sulkowski of John Hopkins University in Baltimore and his associates. Adding ribavirin to the simeprevir and sofosbuvir combination regimen did not improve sustained virologic response (SVR), but patients were less likely to achieve it if they had cirrhosis, current or prior hepatic decompensation, or a history of failing other protease inhibitors, the investigators said.
Novel hepatitis C therapies have yielded “substantially lower” rates of SVR and more side effects in everyday practice than in clinical trials, the investigators noted. To better understand how some of newest HCV drugs perform in the real world, they conducted an observational cohort study of the safety, tolerability, and efficacy of simeprevir plus sofosbuvir for treating genotype 1 HCV infections in academic and nonacademic settings (HCV-TARGET) (Gastroenterology 2015 doi: 10.1053/j.gastro.2015.10.013).
A total of 836 patients received once-daily simeprevir (150 mg) and sofosbuvir (400 mg), and 169 of them also received ribavirin. Most (61%) patients had genotype 1a infection and were white (76%), male (61%), and cirrhotic (59%); 13% were black. Patients usually were treatment experienced, having failed peginterferon and ribavirin either with (12%) or without (46%) telaprevir or boceprevir, the researchers said.
In all, 675 (84%) patients achieved SVR after 12 weeks of treatment (SVR12; 95% confidence interval, 81%-87%). Adding ribavirin to the combination PI regimen did not improve SVR, regardless of cirrhosis status, genetic subtype, or treatment history. However, crude SVR12 rates were only 75% for patients with hepatic decompensation and 81% for those with cirrhosis, and these patients had significantly lower adjusted odds of achieving SVR, compared with other patients. In hindsight, decompensated and cirrhotic patients might have needed 24 weeks of treatment, as the Food and Drug Administration now recommends based on the COSMOS trial results (Lancet. 2014;384[9956]:1756-65), the investigators said.
The adjusted model did not uncover a link between genotype 1 subtype and SVR, but only about 10% of patients were tested for the Q80K polymorphism, which is more common in genotype 1a infections and is associated with treatment resistance, the investigators noted. Crude SVR12 rates were 92% for patients with genotype 1b infection and 86% for those with 1a infection, they said.
Only 3% of patients stopped treatment; 2% did so because of side effects, and ribavirin did not significantly affect rates of treatment discontinuation, said the investigators. The most common side effects were fatigue, headache, nausea, rash, and insomnia. Serious adverse events affected 5% of patients and included gastrointestinal bleeding (0.5%), hepatic failure or encephalopathy (1.2%), and infections (1.1%).
Taken together, these results show that simeprevir and sofosbuvir effectively translate from the clinical trial setting into clinical practice, said the researchers. “Additional research is needed to understand which patients may benefit from different treatment regimens or longer treatment durations,” they emphasized.
The study was supported by the University of Florida at Gainesville, the University of North Carolina at Chapel Hill, AbbVie, Bristol-Myers Squibb, Gilead, Janssen, Kadmon, Merck, Vertex, and the National Institutes of Health. Dr. Sulkowski reported grants and personal fees from Gilead, Janssen, Achillion, Abbvie, Merck, and Bristol-Myers Squibb. Of 14 coinvestigators, 13 reported financial relationships with a number of pharmaceutical companies.
A 12-week, ribavirin-free regimen achieved sustained virologic response for 85% of patients with genotype 1 hepatitis C virus (HCV) infection, researchers reported in the February issue of Gastroenterology.
“This represents one of the first applications of a highly effective HCV regimen outside clinical trials,” said Dr. Mark S. Sulkowski of John Hopkins University in Baltimore and his associates. Adding ribavirin to the simeprevir and sofosbuvir combination regimen did not improve sustained virologic response (SVR), but patients were less likely to achieve it if they had cirrhosis, current or prior hepatic decompensation, or a history of failing other protease inhibitors, the investigators said.
Novel hepatitis C therapies have yielded “substantially lower” rates of SVR and more side effects in everyday practice than in clinical trials, the investigators noted. To better understand how some of newest HCV drugs perform in the real world, they conducted an observational cohort study of the safety, tolerability, and efficacy of simeprevir plus sofosbuvir for treating genotype 1 HCV infections in academic and nonacademic settings (HCV-TARGET) (Gastroenterology 2015 doi: 10.1053/j.gastro.2015.10.013).
A total of 836 patients received once-daily simeprevir (150 mg) and sofosbuvir (400 mg), and 169 of them also received ribavirin. Most (61%) patients had genotype 1a infection and were white (76%), male (61%), and cirrhotic (59%); 13% were black. Patients usually were treatment experienced, having failed peginterferon and ribavirin either with (12%) or without (46%) telaprevir or boceprevir, the researchers said.
In all, 675 (84%) patients achieved SVR after 12 weeks of treatment (SVR12; 95% confidence interval, 81%-87%). Adding ribavirin to the combination PI regimen did not improve SVR, regardless of cirrhosis status, genetic subtype, or treatment history. However, crude SVR12 rates were only 75% for patients with hepatic decompensation and 81% for those with cirrhosis, and these patients had significantly lower adjusted odds of achieving SVR, compared with other patients. In hindsight, decompensated and cirrhotic patients might have needed 24 weeks of treatment, as the Food and Drug Administration now recommends based on the COSMOS trial results (Lancet. 2014;384[9956]:1756-65), the investigators said.
The adjusted model did not uncover a link between genotype 1 subtype and SVR, but only about 10% of patients were tested for the Q80K polymorphism, which is more common in genotype 1a infections and is associated with treatment resistance, the investigators noted. Crude SVR12 rates were 92% for patients with genotype 1b infection and 86% for those with 1a infection, they said.
Only 3% of patients stopped treatment; 2% did so because of side effects, and ribavirin did not significantly affect rates of treatment discontinuation, said the investigators. The most common side effects were fatigue, headache, nausea, rash, and insomnia. Serious adverse events affected 5% of patients and included gastrointestinal bleeding (0.5%), hepatic failure or encephalopathy (1.2%), and infections (1.1%).
Taken together, these results show that simeprevir and sofosbuvir effectively translate from the clinical trial setting into clinical practice, said the researchers. “Additional research is needed to understand which patients may benefit from different treatment regimens or longer treatment durations,” they emphasized.
The study was supported by the University of Florida at Gainesville, the University of North Carolina at Chapel Hill, AbbVie, Bristol-Myers Squibb, Gilead, Janssen, Kadmon, Merck, Vertex, and the National Institutes of Health. Dr. Sulkowski reported grants and personal fees from Gilead, Janssen, Achillion, Abbvie, Merck, and Bristol-Myers Squibb. Of 14 coinvestigators, 13 reported financial relationships with a number of pharmaceutical companies.
A 12-week, ribavirin-free regimen achieved sustained virologic response for 85% of patients with genotype 1 hepatitis C virus (HCV) infection, researchers reported in the February issue of Gastroenterology.
“This represents one of the first applications of a highly effective HCV regimen outside clinical trials,” said Dr. Mark S. Sulkowski of John Hopkins University in Baltimore and his associates. Adding ribavirin to the simeprevir and sofosbuvir combination regimen did not improve sustained virologic response (SVR), but patients were less likely to achieve it if they had cirrhosis, current or prior hepatic decompensation, or a history of failing other protease inhibitors, the investigators said.
Novel hepatitis C therapies have yielded “substantially lower” rates of SVR and more side effects in everyday practice than in clinical trials, the investigators noted. To better understand how some of newest HCV drugs perform in the real world, they conducted an observational cohort study of the safety, tolerability, and efficacy of simeprevir plus sofosbuvir for treating genotype 1 HCV infections in academic and nonacademic settings (HCV-TARGET) (Gastroenterology 2015 doi: 10.1053/j.gastro.2015.10.013).
A total of 836 patients received once-daily simeprevir (150 mg) and sofosbuvir (400 mg), and 169 of them also received ribavirin. Most (61%) patients had genotype 1a infection and were white (76%), male (61%), and cirrhotic (59%); 13% were black. Patients usually were treatment experienced, having failed peginterferon and ribavirin either with (12%) or without (46%) telaprevir or boceprevir, the researchers said.
In all, 675 (84%) patients achieved SVR after 12 weeks of treatment (SVR12; 95% confidence interval, 81%-87%). Adding ribavirin to the combination PI regimen did not improve SVR, regardless of cirrhosis status, genetic subtype, or treatment history. However, crude SVR12 rates were only 75% for patients with hepatic decompensation and 81% for those with cirrhosis, and these patients had significantly lower adjusted odds of achieving SVR, compared with other patients. In hindsight, decompensated and cirrhotic patients might have needed 24 weeks of treatment, as the Food and Drug Administration now recommends based on the COSMOS trial results (Lancet. 2014;384[9956]:1756-65), the investigators said.
The adjusted model did not uncover a link between genotype 1 subtype and SVR, but only about 10% of patients were tested for the Q80K polymorphism, which is more common in genotype 1a infections and is associated with treatment resistance, the investigators noted. Crude SVR12 rates were 92% for patients with genotype 1b infection and 86% for those with 1a infection, they said.
Only 3% of patients stopped treatment; 2% did so because of side effects, and ribavirin did not significantly affect rates of treatment discontinuation, said the investigators. The most common side effects were fatigue, headache, nausea, rash, and insomnia. Serious adverse events affected 5% of patients and included gastrointestinal bleeding (0.5%), hepatic failure or encephalopathy (1.2%), and infections (1.1%).
Taken together, these results show that simeprevir and sofosbuvir effectively translate from the clinical trial setting into clinical practice, said the researchers. “Additional research is needed to understand which patients may benefit from different treatment regimens or longer treatment durations,” they emphasized.
The study was supported by the University of Florida at Gainesville, the University of North Carolina at Chapel Hill, AbbVie, Bristol-Myers Squibb, Gilead, Janssen, Kadmon, Merck, Vertex, and the National Institutes of Health. Dr. Sulkowski reported grants and personal fees from Gilead, Janssen, Achillion, Abbvie, Merck, and Bristol-Myers Squibb. Of 14 coinvestigators, 13 reported financial relationships with a number of pharmaceutical companies.
FROM GASTROENTEROLOGY
Key clinical point: Twelve weeks of simeprevir and sofosbuvir cured about 85% of real-world patients with genotype 1 hepatitis C virus infection.
Major finding: The unadjusted rate of SVR12 was 85% (95% CI, 82%-88%).
Data source: An analysis of an observational cohort study of protease inhibitor combination regimen with or without ribavirin for 836 patients (HCV-TARGET).
Disclosures: The study was supported by the University of Florida at Gainesville, the University of North Carolina at Chapel Hill, AbbVie, Bristol-Myers Squibb, Gilead, Janssen, Kadmon, Merck, Vertex, and the National Institutes of Health. Dr. Sulkowski reported grants and personal fees from Gilead, Janssen, Achillion, Abbvie, Merck, and Bristol-Myers Squibb. Of 14 coinvestigators, 13 reported financial relationships with a number of pharmaceutical companies.
FDA approves new treatment for chronic HCV genotypes 1 and 4
The U.S. Food and Drug Administration has approved Zepatier (elbasvir and grazoprevir) with or without ribavirin for the treatment of chronic hepatitis C virus genotypes 1 and 4 infections in adults.
Zepatier, marketed by Merck, was granted breakthrough therapy designation for the treatment of chronic HCV genotype 1 infection in patients with end stage renal disease on hemodialysis and for the treatment of chronic HCV genotype 4 infection. Breakthrough therapy designation is a program designed to expedite the development and review of drugs that are intended to treat a serious condition and preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over available therapy on a clinically significant endpoint.
“Today’s approval provides another oral treatment option for patients with genotypes 1 and 4 HCV infections without requiring use of interferon,” said Dr. Edward Cox, director of the Office of Antimicrobial Products in the FDA’s Center for Drug Evaluation and Research in a statement.
The safety and efficacy of Zepatier with or without ribavirin was evaluated in clinical trials of 1,373 participants with chronic HCV genotype 1 or 4 infections with and without cirrhosis. The participants received Zepatier with or without ribavirin once daily for 12 or 16 weeks. The studies were designed to measure whether a participant’s hepatitis C virus was no longer detected in the blood 12 weeks after finishing treatment (sustained virologic response), suggesting a participant’s infection had been cured.
The overall sustained virologic response rates ranged from 94% to 97% in genotype 1–infected subjects and from 97% to 100% in genotype 4–infected subjects across trials for the approved treatment regimens.
The FDA recommends clinicians screen genotype 1a–infected patients for certain viral genetic variations prior to starting treatment with Zepatier to determine dosage regimen and duration. Zepatier carries a warning alerting patients and health care providers that elevations of liver enzymes to greater than five times the upper limit of normal occurred in approximately 1% of clinical trial participants, generally at or after treatment week 8. The FDA says liver-related blood tests should be performed prior to starting therapy and at certain times during treatment.
Zepatier is not intended for patients with moderate or severe liver impairment.
On Twitter @richpizzi
The U.S. Food and Drug Administration has approved Zepatier (elbasvir and grazoprevir) with or without ribavirin for the treatment of chronic hepatitis C virus genotypes 1 and 4 infections in adults.
Zepatier, marketed by Merck, was granted breakthrough therapy designation for the treatment of chronic HCV genotype 1 infection in patients with end stage renal disease on hemodialysis and for the treatment of chronic HCV genotype 4 infection. Breakthrough therapy designation is a program designed to expedite the development and review of drugs that are intended to treat a serious condition and preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over available therapy on a clinically significant endpoint.
“Today’s approval provides another oral treatment option for patients with genotypes 1 and 4 HCV infections without requiring use of interferon,” said Dr. Edward Cox, director of the Office of Antimicrobial Products in the FDA’s Center for Drug Evaluation and Research in a statement.
The safety and efficacy of Zepatier with or without ribavirin was evaluated in clinical trials of 1,373 participants with chronic HCV genotype 1 or 4 infections with and without cirrhosis. The participants received Zepatier with or without ribavirin once daily for 12 or 16 weeks. The studies were designed to measure whether a participant’s hepatitis C virus was no longer detected in the blood 12 weeks after finishing treatment (sustained virologic response), suggesting a participant’s infection had been cured.
The overall sustained virologic response rates ranged from 94% to 97% in genotype 1–infected subjects and from 97% to 100% in genotype 4–infected subjects across trials for the approved treatment regimens.
The FDA recommends clinicians screen genotype 1a–infected patients for certain viral genetic variations prior to starting treatment with Zepatier to determine dosage regimen and duration. Zepatier carries a warning alerting patients and health care providers that elevations of liver enzymes to greater than five times the upper limit of normal occurred in approximately 1% of clinical trial participants, generally at or after treatment week 8. The FDA says liver-related blood tests should be performed prior to starting therapy and at certain times during treatment.
Zepatier is not intended for patients with moderate or severe liver impairment.
On Twitter @richpizzi
The U.S. Food and Drug Administration has approved Zepatier (elbasvir and grazoprevir) with or without ribavirin for the treatment of chronic hepatitis C virus genotypes 1 and 4 infections in adults.
Zepatier, marketed by Merck, was granted breakthrough therapy designation for the treatment of chronic HCV genotype 1 infection in patients with end stage renal disease on hemodialysis and for the treatment of chronic HCV genotype 4 infection. Breakthrough therapy designation is a program designed to expedite the development and review of drugs that are intended to treat a serious condition and preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over available therapy on a clinically significant endpoint.
“Today’s approval provides another oral treatment option for patients with genotypes 1 and 4 HCV infections without requiring use of interferon,” said Dr. Edward Cox, director of the Office of Antimicrobial Products in the FDA’s Center for Drug Evaluation and Research in a statement.
The safety and efficacy of Zepatier with or without ribavirin was evaluated in clinical trials of 1,373 participants with chronic HCV genotype 1 or 4 infections with and without cirrhosis. The participants received Zepatier with or without ribavirin once daily for 12 or 16 weeks. The studies were designed to measure whether a participant’s hepatitis C virus was no longer detected in the blood 12 weeks after finishing treatment (sustained virologic response), suggesting a participant’s infection had been cured.
The overall sustained virologic response rates ranged from 94% to 97% in genotype 1–infected subjects and from 97% to 100% in genotype 4–infected subjects across trials for the approved treatment regimens.
The FDA recommends clinicians screen genotype 1a–infected patients for certain viral genetic variations prior to starting treatment with Zepatier to determine dosage regimen and duration. Zepatier carries a warning alerting patients and health care providers that elevations of liver enzymes to greater than five times the upper limit of normal occurred in approximately 1% of clinical trial participants, generally at or after treatment week 8. The FDA says liver-related blood tests should be performed prior to starting therapy and at certain times during treatment.
Zepatier is not intended for patients with moderate or severe liver impairment.
On Twitter @richpizzi
Acute HBV infection more than doubles in Appalachian states
Hepatitis B infection more than doubled in three Appalachian states from 2006-2013, with significant increases among those aged 30-39, injection drug users, and non-Hispanic whites, according to a study published Jan. 28 in MMWR.
“A hepatitis B epidemic is emerging in Kentucky, Tennessee, and West Virginia,” according to Dr. Aaron Harris of the division of viral hepatitis at the Centers for Disease Control and Prevention and his coauthors.
A total of 3,305 cases of acute HBV infection in the three states were reported to the National Notifiable Diseases Surveillance System (NNDSS) between 2009 and 2013. The proportion of those with HBV who reported injection drug use was significantly higher during the period from 2010-2013 than during the 2005-2009 time frame (75% vs. 53%, P less than .001).
Characteristics that significantly increased the likelihood of an individual having HBV infection included being 30-39 years, being non-Hispanic white, and being an injection drug user (all P less than .001). Sex was not a significant differentiator, and full demographic and drug use data were not available for all reported cases (MMWR Morb Mortal Wkly Rep. 2016:65;47-50).
The rise in HBV infections was statistically significant in non-urban counties, but not in urban counties (P less than .001 for trend).
This large regional increase in acute HBV infection is in contrast to stable HBV infection rates in the U.S., with an overall rate of 1 case in 100,000 in 2013. Though the vaccination is effective at preventing HBV infection, two-thirds of Americans aged 19-49 years are not fully vaccinated.
Hepatitis B vaccination has been part of the childhood immunization series since 1991, and has been recommended for children aged 18 and younger since 1999, so adults aged 33 years and older at the end of the study period would not have received HBV immunization routinely.
“The concurrent increase in reports of acute HBV and HCV infections, as well as an increase in injection drug use reported among this population is concerning,” wrote Dr. Harris and his coauthors, who added that hospital admissions for prescription opioid abuse increased by 17.1% and for heroin use by 7.4% in the three states during the study period.
The authors noted several study limitations, many of which may have resulted in under-reporting of the true incidence of HBV. The study data come from a passive surveillance system, and the case definition only includes those whose disease is symptomatic. Additionally, individuals who are high risk and underserved by the health care system may be missed.
Kentucky, West Virginia, and Tennessee are using a variety of strategies to target at-risk populations, according to the report, including partnering with jails and prisons to increase vaccination rates among incarcerated individuals, reaching out to those who run rehabilitation and harm reduction services, and mandating reporting of HBV infection in pregnant women and young children.
“Evidence-based prevention strategies, including increasing hepatitis B vaccination coverage, testing and linkage to care, and implementing education campaigns that target persons who inject drugs are urgently needed,” Dr. Harris and his coauthors wrote.
On Twitter @karioakes
Hepatitis B infection more than doubled in three Appalachian states from 2006-2013, with significant increases among those aged 30-39, injection drug users, and non-Hispanic whites, according to a study published Jan. 28 in MMWR.
“A hepatitis B epidemic is emerging in Kentucky, Tennessee, and West Virginia,” according to Dr. Aaron Harris of the division of viral hepatitis at the Centers for Disease Control and Prevention and his coauthors.
A total of 3,305 cases of acute HBV infection in the three states were reported to the National Notifiable Diseases Surveillance System (NNDSS) between 2009 and 2013. The proportion of those with HBV who reported injection drug use was significantly higher during the period from 2010-2013 than during the 2005-2009 time frame (75% vs. 53%, P less than .001).
Characteristics that significantly increased the likelihood of an individual having HBV infection included being 30-39 years, being non-Hispanic white, and being an injection drug user (all P less than .001). Sex was not a significant differentiator, and full demographic and drug use data were not available for all reported cases (MMWR Morb Mortal Wkly Rep. 2016:65;47-50).
The rise in HBV infections was statistically significant in non-urban counties, but not in urban counties (P less than .001 for trend).
This large regional increase in acute HBV infection is in contrast to stable HBV infection rates in the U.S., with an overall rate of 1 case in 100,000 in 2013. Though the vaccination is effective at preventing HBV infection, two-thirds of Americans aged 19-49 years are not fully vaccinated.
Hepatitis B vaccination has been part of the childhood immunization series since 1991, and has been recommended for children aged 18 and younger since 1999, so adults aged 33 years and older at the end of the study period would not have received HBV immunization routinely.
“The concurrent increase in reports of acute HBV and HCV infections, as well as an increase in injection drug use reported among this population is concerning,” wrote Dr. Harris and his coauthors, who added that hospital admissions for prescription opioid abuse increased by 17.1% and for heroin use by 7.4% in the three states during the study period.
The authors noted several study limitations, many of which may have resulted in under-reporting of the true incidence of HBV. The study data come from a passive surveillance system, and the case definition only includes those whose disease is symptomatic. Additionally, individuals who are high risk and underserved by the health care system may be missed.
Kentucky, West Virginia, and Tennessee are using a variety of strategies to target at-risk populations, according to the report, including partnering with jails and prisons to increase vaccination rates among incarcerated individuals, reaching out to those who run rehabilitation and harm reduction services, and mandating reporting of HBV infection in pregnant women and young children.
“Evidence-based prevention strategies, including increasing hepatitis B vaccination coverage, testing and linkage to care, and implementing education campaigns that target persons who inject drugs are urgently needed,” Dr. Harris and his coauthors wrote.
On Twitter @karioakes
Hepatitis B infection more than doubled in three Appalachian states from 2006-2013, with significant increases among those aged 30-39, injection drug users, and non-Hispanic whites, according to a study published Jan. 28 in MMWR.
“A hepatitis B epidemic is emerging in Kentucky, Tennessee, and West Virginia,” according to Dr. Aaron Harris of the division of viral hepatitis at the Centers for Disease Control and Prevention and his coauthors.
A total of 3,305 cases of acute HBV infection in the three states were reported to the National Notifiable Diseases Surveillance System (NNDSS) between 2009 and 2013. The proportion of those with HBV who reported injection drug use was significantly higher during the period from 2010-2013 than during the 2005-2009 time frame (75% vs. 53%, P less than .001).
Characteristics that significantly increased the likelihood of an individual having HBV infection included being 30-39 years, being non-Hispanic white, and being an injection drug user (all P less than .001). Sex was not a significant differentiator, and full demographic and drug use data were not available for all reported cases (MMWR Morb Mortal Wkly Rep. 2016:65;47-50).
The rise in HBV infections was statistically significant in non-urban counties, but not in urban counties (P less than .001 for trend).
This large regional increase in acute HBV infection is in contrast to stable HBV infection rates in the U.S., with an overall rate of 1 case in 100,000 in 2013. Though the vaccination is effective at preventing HBV infection, two-thirds of Americans aged 19-49 years are not fully vaccinated.
Hepatitis B vaccination has been part of the childhood immunization series since 1991, and has been recommended for children aged 18 and younger since 1999, so adults aged 33 years and older at the end of the study period would not have received HBV immunization routinely.
“The concurrent increase in reports of acute HBV and HCV infections, as well as an increase in injection drug use reported among this population is concerning,” wrote Dr. Harris and his coauthors, who added that hospital admissions for prescription opioid abuse increased by 17.1% and for heroin use by 7.4% in the three states during the study period.
The authors noted several study limitations, many of which may have resulted in under-reporting of the true incidence of HBV. The study data come from a passive surveillance system, and the case definition only includes those whose disease is symptomatic. Additionally, individuals who are high risk and underserved by the health care system may be missed.
Kentucky, West Virginia, and Tennessee are using a variety of strategies to target at-risk populations, according to the report, including partnering with jails and prisons to increase vaccination rates among incarcerated individuals, reaching out to those who run rehabilitation and harm reduction services, and mandating reporting of HBV infection in pregnant women and young children.
“Evidence-based prevention strategies, including increasing hepatitis B vaccination coverage, testing and linkage to care, and implementing education campaigns that target persons who inject drugs are urgently needed,” Dr. Harris and his coauthors wrote.
On Twitter @karioakes
FROM MMWR
Key clinical point: Acute hepatitis B virus (HBV) infections rose 114% from 2006-2013 in three Appalachian states.
Major finding: The incidence of acute HBV infections was significantly higher in non-urban areas, and among young, white injection drug users.
Data source: National Notifiable Diseases Surveillance System (NNDSS).
Disclosures: The study was conducted by the Centers for Disease Control, where Dr. Harris is employed. The study’s coauthors are employed by the public health departments of the states of Kentucky, Tennessee, and West Virginia.
Hepatitis C incidence rising in hemodialysis patients
Incidence of newly acquired hepatitis C virus has increased recently in patients undergoing hemodialysis, according to a health advisory from the Centers for Disease Control and Prevention.
In 2014 and 2015, 36 cases of HCV infection were reported to the CDC from 19 clinics in eight states. While investigation is ongoing, HCV transmission between patients has been confirmed in at least nine facilities, and in several facilities, lapses in infection control were also identified. Better screening and awareness of HCV infection potential may also play a role in the increased disease incidence.
The CDC recommends that dialysis facilities assess current infection control practices, environmental cleaning, and disinfection practices to evaluate adherence to standards, address any gaps, screen patients for HCV, and to report all HCV infections to the CDC promptly.
“Dialysis facilities should actively assess and continuously improve their infection control, environmental cleaning and disinfection, and HCV screening practices, whether or not they are aware of infections in their clinic. Any case of new HCV infection in a patient undergoing hemodialysis is likely to be a health care–associated infection and should be reported to public health authorities in a timely manner,” the CDC said
Find the full health advisory on the CDC website.
AGA Resource AGA offers an HCV Clinical Service line to provide GIs with the tools needed to be more efficient, understand quality standards, receive appropriate reimbursements, and improve the process of care for their patients with HCV at http://www.gastro.org/patient-care/conditions-diseases/hepatitis-c
Incidence of newly acquired hepatitis C virus has increased recently in patients undergoing hemodialysis, according to a health advisory from the Centers for Disease Control and Prevention.
In 2014 and 2015, 36 cases of HCV infection were reported to the CDC from 19 clinics in eight states. While investigation is ongoing, HCV transmission between patients has been confirmed in at least nine facilities, and in several facilities, lapses in infection control were also identified. Better screening and awareness of HCV infection potential may also play a role in the increased disease incidence.
The CDC recommends that dialysis facilities assess current infection control practices, environmental cleaning, and disinfection practices to evaluate adherence to standards, address any gaps, screen patients for HCV, and to report all HCV infections to the CDC promptly.
“Dialysis facilities should actively assess and continuously improve their infection control, environmental cleaning and disinfection, and HCV screening practices, whether or not they are aware of infections in their clinic. Any case of new HCV infection in a patient undergoing hemodialysis is likely to be a health care–associated infection and should be reported to public health authorities in a timely manner,” the CDC said
Find the full health advisory on the CDC website.
AGA Resource AGA offers an HCV Clinical Service line to provide GIs with the tools needed to be more efficient, understand quality standards, receive appropriate reimbursements, and improve the process of care for their patients with HCV at http://www.gastro.org/patient-care/conditions-diseases/hepatitis-c
Incidence of newly acquired hepatitis C virus has increased recently in patients undergoing hemodialysis, according to a health advisory from the Centers for Disease Control and Prevention.
In 2014 and 2015, 36 cases of HCV infection were reported to the CDC from 19 clinics in eight states. While investigation is ongoing, HCV transmission between patients has been confirmed in at least nine facilities, and in several facilities, lapses in infection control were also identified. Better screening and awareness of HCV infection potential may also play a role in the increased disease incidence.
The CDC recommends that dialysis facilities assess current infection control practices, environmental cleaning, and disinfection practices to evaluate adherence to standards, address any gaps, screen patients for HCV, and to report all HCV infections to the CDC promptly.
“Dialysis facilities should actively assess and continuously improve their infection control, environmental cleaning and disinfection, and HCV screening practices, whether or not they are aware of infections in their clinic. Any case of new HCV infection in a patient undergoing hemodialysis is likely to be a health care–associated infection and should be reported to public health authorities in a timely manner,” the CDC said
Find the full health advisory on the CDC website.
AGA Resource AGA offers an HCV Clinical Service line to provide GIs with the tools needed to be more efficient, understand quality standards, receive appropriate reimbursements, and improve the process of care for their patients with HCV at http://www.gastro.org/patient-care/conditions-diseases/hepatitis-c
New antivirals, more screening could slash hepatitis C cases
Hepatitis C could be reduced by 90% or more in the United States by the year 2040 with the use of direct-acting antivirals, near-universal screening, and enhanced treatment capacity, according to a study in Clinical Infectious Diseases.
A research team led by Jeffrey Townsend, Ph.D., of Yale School of Public Health, New Haven, Conn., said that new direct-acting antivirals (DAAs) alone could reduce the prevalence of hepatitis C virus (HCV) by 80% by the year 2040. When near-universal screening and enhanced treatment capacity are added to the equation, HCV could be eliminated in the United States, though cost and reimbursement issues may impede implementation.
“The key finding is that a fourfold increase to the number of patients treated each year could virtually eliminate HCV from the noninjecting population within a decade,” said Dr. Townsend, senior author of the study, in a statement accompanying the study [Clin Infect Dis. 2015. doi: 10.1093/cid/civ894].
First author David Durham, Ph.D., also of the Yale School of Public Health, and his collaborators analyzed currently available data and constructed a sophisticated model to generate projections of how future DAA treatment will change HCV prevalence. Dr. Durham and his colleagues also built models to account for varying levels of screening in the general population and for people who inject drugs (PWIDs).
More than 5 million people with chronic hepatitis C infection are thought to live in the United States, and just about 100,000 of those currently receive treatment each year. The treatment burden and relatively low cure rate of interferon-based therapies have historically been significant impediments for many patients. New DAAs promise a sustained virologic response (SVR) in up to 95% of patients with a well-tolerated, once-daily, one-pill several-week regimen.
Without enhanced screening or treatment rates, the model predicted an 80% decline in U.S. HCV prevalence by the year 2040. The 80% benchmark would be reached by the year 2025 if the annual treatment rate increased to 400,000, with 256,315 fewer HCV-related deaths through 2040. Just doubling the treatment rate to 200,000 patients per year would result in an 80% decrease in HCV prevalence by 2031, with 143,055 fewer deaths by 2040.
However, “more than half of those with chronic HCV are unaware of their status, including up to two-thirds of people who inject drugs,” wrote Dr. Durham and his coauthors. Therefore, enhanced HCV screening, especially among PWIDs, could provide a significant further reduction in morbidity and mortality from HCV.
Targeted screening of PWIDs to achieve a 20% screening rate (compared with the current 4.1%), combined with a treatment rate of at least 30%, would yield a 90% reduction of prevalence in HCV by 2040. Universal screening, combined with a treatment rate of at least 20%, would achieve a 90% reduction in the incidence of new infections by 2040, according to the model.
The drop in prevalence in the models took into account not only an increased SVR rate, but also the reduced transmission rate when more persons with HCV achieve an SVR. This dynamic transmission analysis “captures the impact of treatment on both chronic disease and transmission dynamics, distinguishes screening and treatment as separate but related public health objectives, and accounts for underreporting … of national HCV prevalence,” according to Dr. Durham and his coauthors.
Morbidity and mortality were accounted for by projecting the number of HCV patients in each treatment and screening condition who would spontaneously clear disease, become chronically infected, develop increasing levels of liver disease and cirrhosis, require transplant, become reinfected, or die.
As always, real world considerations temper what’s achievable. For DAAs, a chief factor is the cost of the regimens, which currently hovers around $90,000. “The improvement of health outcomes expected from greater acceptance might be tempered by the high costs of treatment and by limited insurance coverage and willingness to treat PWIDs,” Dr. Durham and his coauthors wrote.
Study limitations included some “simplifying assumptions” in the modeling, which did not account for the varying incidence of fibrosis and transmissibility among different HCV genotypes. Still, wrote Dr. Durham and his collaborators, “our analysis provides a forecast for the potential impact of DAAs in reducing HCV-associated liver disease, demonstrating that achievable expansion of HCV treatment at current screening rates can substantially reduce morbidity and mortality.”
The study was funded by the Notsew Orm Sands Foundation and Merck. Dr. Durham, Dr. Galvani, and Dr. Townsend have consulted for and received research funding from Sanofi Pasteur and Merck. Dr. Elbasha is employed by Merck and holds stock and stock options in the company. The other authors reported no conflicts of interest.
AGA Resource
Through the AGA Roadmap to the Future of Practice, AGA offers a Hepatitis C Clinical Service line to support high-quality patient care, which is available at http://www.gastro.org/patient-care/conditions-diseases/hepatitis-c.
On Twitter @karioakes
Hepatitis C could be reduced by 90% or more in the United States by the year 2040 with the use of direct-acting antivirals, near-universal screening, and enhanced treatment capacity, according to a study in Clinical Infectious Diseases.
A research team led by Jeffrey Townsend, Ph.D., of Yale School of Public Health, New Haven, Conn., said that new direct-acting antivirals (DAAs) alone could reduce the prevalence of hepatitis C virus (HCV) by 80% by the year 2040. When near-universal screening and enhanced treatment capacity are added to the equation, HCV could be eliminated in the United States, though cost and reimbursement issues may impede implementation.
“The key finding is that a fourfold increase to the number of patients treated each year could virtually eliminate HCV from the noninjecting population within a decade,” said Dr. Townsend, senior author of the study, in a statement accompanying the study [Clin Infect Dis. 2015. doi: 10.1093/cid/civ894].
First author David Durham, Ph.D., also of the Yale School of Public Health, and his collaborators analyzed currently available data and constructed a sophisticated model to generate projections of how future DAA treatment will change HCV prevalence. Dr. Durham and his colleagues also built models to account for varying levels of screening in the general population and for people who inject drugs (PWIDs).
More than 5 million people with chronic hepatitis C infection are thought to live in the United States, and just about 100,000 of those currently receive treatment each year. The treatment burden and relatively low cure rate of interferon-based therapies have historically been significant impediments for many patients. New DAAs promise a sustained virologic response (SVR) in up to 95% of patients with a well-tolerated, once-daily, one-pill several-week regimen.
Without enhanced screening or treatment rates, the model predicted an 80% decline in U.S. HCV prevalence by the year 2040. The 80% benchmark would be reached by the year 2025 if the annual treatment rate increased to 400,000, with 256,315 fewer HCV-related deaths through 2040. Just doubling the treatment rate to 200,000 patients per year would result in an 80% decrease in HCV prevalence by 2031, with 143,055 fewer deaths by 2040.
However, “more than half of those with chronic HCV are unaware of their status, including up to two-thirds of people who inject drugs,” wrote Dr. Durham and his coauthors. Therefore, enhanced HCV screening, especially among PWIDs, could provide a significant further reduction in morbidity and mortality from HCV.
Targeted screening of PWIDs to achieve a 20% screening rate (compared with the current 4.1%), combined with a treatment rate of at least 30%, would yield a 90% reduction of prevalence in HCV by 2040. Universal screening, combined with a treatment rate of at least 20%, would achieve a 90% reduction in the incidence of new infections by 2040, according to the model.
The drop in prevalence in the models took into account not only an increased SVR rate, but also the reduced transmission rate when more persons with HCV achieve an SVR. This dynamic transmission analysis “captures the impact of treatment on both chronic disease and transmission dynamics, distinguishes screening and treatment as separate but related public health objectives, and accounts for underreporting … of national HCV prevalence,” according to Dr. Durham and his coauthors.
Morbidity and mortality were accounted for by projecting the number of HCV patients in each treatment and screening condition who would spontaneously clear disease, become chronically infected, develop increasing levels of liver disease and cirrhosis, require transplant, become reinfected, or die.
As always, real world considerations temper what’s achievable. For DAAs, a chief factor is the cost of the regimens, which currently hovers around $90,000. “The improvement of health outcomes expected from greater acceptance might be tempered by the high costs of treatment and by limited insurance coverage and willingness to treat PWIDs,” Dr. Durham and his coauthors wrote.
Study limitations included some “simplifying assumptions” in the modeling, which did not account for the varying incidence of fibrosis and transmissibility among different HCV genotypes. Still, wrote Dr. Durham and his collaborators, “our analysis provides a forecast for the potential impact of DAAs in reducing HCV-associated liver disease, demonstrating that achievable expansion of HCV treatment at current screening rates can substantially reduce morbidity and mortality.”
The study was funded by the Notsew Orm Sands Foundation and Merck. Dr. Durham, Dr. Galvani, and Dr. Townsend have consulted for and received research funding from Sanofi Pasteur and Merck. Dr. Elbasha is employed by Merck and holds stock and stock options in the company. The other authors reported no conflicts of interest.
AGA Resource
Through the AGA Roadmap to the Future of Practice, AGA offers a Hepatitis C Clinical Service line to support high-quality patient care, which is available at http://www.gastro.org/patient-care/conditions-diseases/hepatitis-c.
On Twitter @karioakes
Hepatitis C could be reduced by 90% or more in the United States by the year 2040 with the use of direct-acting antivirals, near-universal screening, and enhanced treatment capacity, according to a study in Clinical Infectious Diseases.
A research team led by Jeffrey Townsend, Ph.D., of Yale School of Public Health, New Haven, Conn., said that new direct-acting antivirals (DAAs) alone could reduce the prevalence of hepatitis C virus (HCV) by 80% by the year 2040. When near-universal screening and enhanced treatment capacity are added to the equation, HCV could be eliminated in the United States, though cost and reimbursement issues may impede implementation.
“The key finding is that a fourfold increase to the number of patients treated each year could virtually eliminate HCV from the noninjecting population within a decade,” said Dr. Townsend, senior author of the study, in a statement accompanying the study [Clin Infect Dis. 2015. doi: 10.1093/cid/civ894].
First author David Durham, Ph.D., also of the Yale School of Public Health, and his collaborators analyzed currently available data and constructed a sophisticated model to generate projections of how future DAA treatment will change HCV prevalence. Dr. Durham and his colleagues also built models to account for varying levels of screening in the general population and for people who inject drugs (PWIDs).
More than 5 million people with chronic hepatitis C infection are thought to live in the United States, and just about 100,000 of those currently receive treatment each year. The treatment burden and relatively low cure rate of interferon-based therapies have historically been significant impediments for many patients. New DAAs promise a sustained virologic response (SVR) in up to 95% of patients with a well-tolerated, once-daily, one-pill several-week regimen.
Without enhanced screening or treatment rates, the model predicted an 80% decline in U.S. HCV prevalence by the year 2040. The 80% benchmark would be reached by the year 2025 if the annual treatment rate increased to 400,000, with 256,315 fewer HCV-related deaths through 2040. Just doubling the treatment rate to 200,000 patients per year would result in an 80% decrease in HCV prevalence by 2031, with 143,055 fewer deaths by 2040.
However, “more than half of those with chronic HCV are unaware of their status, including up to two-thirds of people who inject drugs,” wrote Dr. Durham and his coauthors. Therefore, enhanced HCV screening, especially among PWIDs, could provide a significant further reduction in morbidity and mortality from HCV.
Targeted screening of PWIDs to achieve a 20% screening rate (compared with the current 4.1%), combined with a treatment rate of at least 30%, would yield a 90% reduction of prevalence in HCV by 2040. Universal screening, combined with a treatment rate of at least 20%, would achieve a 90% reduction in the incidence of new infections by 2040, according to the model.
The drop in prevalence in the models took into account not only an increased SVR rate, but also the reduced transmission rate when more persons with HCV achieve an SVR. This dynamic transmission analysis “captures the impact of treatment on both chronic disease and transmission dynamics, distinguishes screening and treatment as separate but related public health objectives, and accounts for underreporting … of national HCV prevalence,” according to Dr. Durham and his coauthors.
Morbidity and mortality were accounted for by projecting the number of HCV patients in each treatment and screening condition who would spontaneously clear disease, become chronically infected, develop increasing levels of liver disease and cirrhosis, require transplant, become reinfected, or die.
As always, real world considerations temper what’s achievable. For DAAs, a chief factor is the cost of the regimens, which currently hovers around $90,000. “The improvement of health outcomes expected from greater acceptance might be tempered by the high costs of treatment and by limited insurance coverage and willingness to treat PWIDs,” Dr. Durham and his coauthors wrote.
Study limitations included some “simplifying assumptions” in the modeling, which did not account for the varying incidence of fibrosis and transmissibility among different HCV genotypes. Still, wrote Dr. Durham and his collaborators, “our analysis provides a forecast for the potential impact of DAAs in reducing HCV-associated liver disease, demonstrating that achievable expansion of HCV treatment at current screening rates can substantially reduce morbidity and mortality.”
The study was funded by the Notsew Orm Sands Foundation and Merck. Dr. Durham, Dr. Galvani, and Dr. Townsend have consulted for and received research funding from Sanofi Pasteur and Merck. Dr. Elbasha is employed by Merck and holds stock and stock options in the company. The other authors reported no conflicts of interest.
AGA Resource
Through the AGA Roadmap to the Future of Practice, AGA offers a Hepatitis C Clinical Service line to support high-quality patient care, which is available at http://www.gastro.org/patient-care/conditions-diseases/hepatitis-c.
On Twitter @karioakes
FROM CLINICAL INFECTIOUS DISEASES
Key clinical point: Hepatitis C could be reduced by 90% or more in the United States by the year 2040 with the use of direct-acting antivirals, near-universal screening, and enhanced treatment capacity.
Major finding: Targeted screening of 20% of persons who inject drugs, combined with a 30% treatment rate, would reduce HCV prevalence by 90% by the year 2040.
Data source: Dynamic modeling of HCV prevalence, screening, and treatment, using currently available data to model public health scenarios to the year 2040.
Disclosures: The study was funded by the Notsew Orm Sands Foundation and Merck. Dr. Durham, Dr. Galvani, and Dr. Townsend have consulted for and received research funding from Sanofi Pasteur and Merck. Dr. Elbasha is employed by Merck and holds stock and stock options in the company. The other authors reported no conflicts of interest.
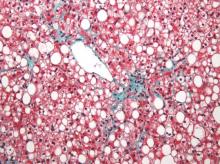
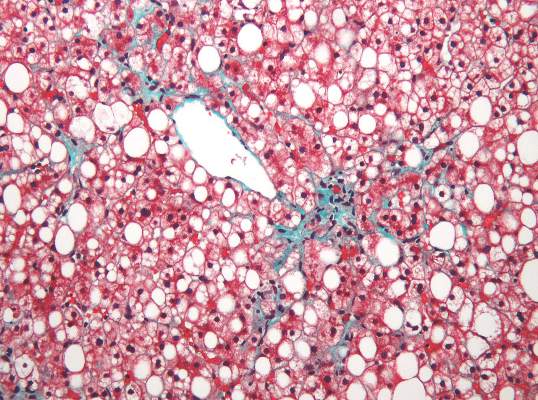
Nonalcoholic fatty liver disease will keep rising ‘in near term’
Nonalcoholic fatty liver disease (NAFLD) almost tripled among United States veterans in a recent 9-year period, investigators reported in the February issue of Clinical Gastroenterology and Hepatology.
The trend “was evident in all racial groups, across all age groups, and in both genders,” said Dr. Fasiha Kanwal of the Michael E. DeBakey Veterans Affairs Medical Center and Baylor College of Medicine, both in Houston, and her associates. The increasing prevalence of NAFLD “is likely generalizable to nonveterans,” and will probably persist because of a “fairly steady” 2%-3% overall annual incidence and a steeper rise among younger individuals, they added. “Nonalcoholic fatty liver disease will continue to remain a major public health problem in the United States, at least in the near and intermediate future.”
Although NAFLD is the leading cause of chronic liver failure in the United States, few studies have examined its incidence or prevalence over time, which are key to predicting future disease burden. Therefore, the investigators analyzed data for more than 9.78 million patients who visited the VA at least once between 2003 and 2011. They defined NAFLD as at least two elevated alanine aminotransferase (ALT) values (greater than 40 IU/mL) separated by at least 6 months, with no history of positive serology for hepatitis B surface antigen or hepatitis C virus RNA, and no alcohol-related ICD-9 codes or positive AUDIT-C scores within a year of elevated ALT levels (Clin Gastroenterol Hepatol. 2015 Aug 7. doi: 10.1016/j.cgh.2015.08.010).
During the study period, more than 1.3 million patients, or 13.6%, met the definition of NAFLD, said the researchers. Age-adjusted incidence rates dropped slightly from 3.16% in 2003 to 2.5% in 2011, ranging between 2.3% and 2.7% in most years. Prevalence, however, rose from 6.3% in 2003 (95% confidence interval, 6.26%-6.3%) to 17.6% in 2011 (95% CI, 17.58%-17.65%), a 2.8-fold increase. Moreover, about one in five patients with NAFLD who visited the VA in 2011 was at risk for advanced fibrosis.
Among individuals who were younger than 45 years, the incidence of NAFLD rose from 2.3 to 4.3 cases per 100 persons (annual percentage change, 7.4%; 95% CI, 5.7% to 9.2%), the researchers also found. “Although recent studies show that the rate of increase in both obesity and diabetes, which are both major risk factors for NAFLD, may be slowing down in the U.S., this may not be the case in the VA, where the prevalence of obesity and diabetes is in fact higher than in the U.S. population,” they said.
In general, the findings mirror a recent analysis of the National Health and Nutrition Examination Survey (Aliment Pharmacol Ther. 2015 Jan;41[1]:65-76), according to the investigators. “The VA is the largest integrated health care system in the United States,” they added. “We believe that the sheer size of the veteran cohort, combined with a complete dearth of information regarding the burden of NAFLD in the VA, renders our findings highly significant. Furthermore, the VA is in a unique position to test and implement systemic changes in medical care delivery to improve the health care of NAFLD patients.”
The study was partially supported by the Michael E. DeBakey Veterans Affairs Medical Center. The researchers had no disclosures.
Kanwal and colleagues present an interesting study assessing the trends in the incidence and prevalence of NAFLD in the United States. Findings suggest that the annual incidence of NAFLD has generally been stable (2.2%-3.2%), while the prevalence of NAFLD has increased by 2.8-fold (6.3%-17.6%). These findings are consistent with the literature and provide additional evidence supporting the increasing burden of NAFLD. Although an important study, there are some limitations to the study design. First, the diagnosis of NAFLD was solely based on elevated liver enzymes, which can underestimate the true incidence and prevalence of NAFLD. In fact, in a recent meta-analysis, NAFLD prevalence based on liver enzymes was 13%, while NAFLD prevalence based on radiologic diagnosis was 25% (Hepatology. 2015 Dec 28. doi: 10.1002/hep.28431. [Epub ahead of print]). Second, the study subjects came from the VA system, which may not be representative of the U.S. population (Patrick AFB, FL: Defense Equal Opportunity Management Institute, 2010). This is important because sex-specific differences in the prevalence of NAFLD have been reported (Hepatology. 2015 Dec 28. doi: 10.1002/hep.28431. [Epub ahead of print]). Nevertheless, these limitations do not minimize the important contribution of this study. There appears to be an alarming increase in the burden of NAFLD within all the racial and age groups in the U.S. Further, this increase in the incidence and prevalence of NAFLD is especially significant among the younger age groups (less than 45 years). This finding is in contrast to others who have reported a higher prevalence in older subjects (Presented at AASLD 2015. San Francisco. Abstract #534). If confirmed, this younger cohort of patients with NAFLD can fuel the future burden of liver disease for the next few decades (JAMA. 2012;307:491-7). Given the current lack of an effective treatment for NAFLD, a national strategy to deal with this important and rising cause of chronic liver disease is urgently needed.
Dr. Zobair M. Younossi, MPH, FACG, AGAF, FAASLD, is chairman, department of medicine, Inova Fairfax Hospital; vice president for research, Inova Health System; professor of medicine, VCU-Inova Campus and Beatty Center for Integrated Research, Falls Church, Va. He has consulted for Gilead, AbbVie, Intercept, BMS, and GSK.
Kanwal and colleagues present an interesting study assessing the trends in the incidence and prevalence of NAFLD in the United States. Findings suggest that the annual incidence of NAFLD has generally been stable (2.2%-3.2%), while the prevalence of NAFLD has increased by 2.8-fold (6.3%-17.6%). These findings are consistent with the literature and provide additional evidence supporting the increasing burden of NAFLD. Although an important study, there are some limitations to the study design. First, the diagnosis of NAFLD was solely based on elevated liver enzymes, which can underestimate the true incidence and prevalence of NAFLD. In fact, in a recent meta-analysis, NAFLD prevalence based on liver enzymes was 13%, while NAFLD prevalence based on radiologic diagnosis was 25% (Hepatology. 2015 Dec 28. doi: 10.1002/hep.28431. [Epub ahead of print]). Second, the study subjects came from the VA system, which may not be representative of the U.S. population (Patrick AFB, FL: Defense Equal Opportunity Management Institute, 2010). This is important because sex-specific differences in the prevalence of NAFLD have been reported (Hepatology. 2015 Dec 28. doi: 10.1002/hep.28431. [Epub ahead of print]). Nevertheless, these limitations do not minimize the important contribution of this study. There appears to be an alarming increase in the burden of NAFLD within all the racial and age groups in the U.S. Further, this increase in the incidence and prevalence of NAFLD is especially significant among the younger age groups (less than 45 years). This finding is in contrast to others who have reported a higher prevalence in older subjects (Presented at AASLD 2015. San Francisco. Abstract #534). If confirmed, this younger cohort of patients with NAFLD can fuel the future burden of liver disease for the next few decades (JAMA. 2012;307:491-7). Given the current lack of an effective treatment for NAFLD, a national strategy to deal with this important and rising cause of chronic liver disease is urgently needed.
Dr. Zobair M. Younossi, MPH, FACG, AGAF, FAASLD, is chairman, department of medicine, Inova Fairfax Hospital; vice president for research, Inova Health System; professor of medicine, VCU-Inova Campus and Beatty Center for Integrated Research, Falls Church, Va. He has consulted for Gilead, AbbVie, Intercept, BMS, and GSK.
Kanwal and colleagues present an interesting study assessing the trends in the incidence and prevalence of NAFLD in the United States. Findings suggest that the annual incidence of NAFLD has generally been stable (2.2%-3.2%), while the prevalence of NAFLD has increased by 2.8-fold (6.3%-17.6%). These findings are consistent with the literature and provide additional evidence supporting the increasing burden of NAFLD. Although an important study, there are some limitations to the study design. First, the diagnosis of NAFLD was solely based on elevated liver enzymes, which can underestimate the true incidence and prevalence of NAFLD. In fact, in a recent meta-analysis, NAFLD prevalence based on liver enzymes was 13%, while NAFLD prevalence based on radiologic diagnosis was 25% (Hepatology. 2015 Dec 28. doi: 10.1002/hep.28431. [Epub ahead of print]). Second, the study subjects came from the VA system, which may not be representative of the U.S. population (Patrick AFB, FL: Defense Equal Opportunity Management Institute, 2010). This is important because sex-specific differences in the prevalence of NAFLD have been reported (Hepatology. 2015 Dec 28. doi: 10.1002/hep.28431. [Epub ahead of print]). Nevertheless, these limitations do not minimize the important contribution of this study. There appears to be an alarming increase in the burden of NAFLD within all the racial and age groups in the U.S. Further, this increase in the incidence and prevalence of NAFLD is especially significant among the younger age groups (less than 45 years). This finding is in contrast to others who have reported a higher prevalence in older subjects (Presented at AASLD 2015. San Francisco. Abstract #534). If confirmed, this younger cohort of patients with NAFLD can fuel the future burden of liver disease for the next few decades (JAMA. 2012;307:491-7). Given the current lack of an effective treatment for NAFLD, a national strategy to deal with this important and rising cause of chronic liver disease is urgently needed.
Dr. Zobair M. Younossi, MPH, FACG, AGAF, FAASLD, is chairman, department of medicine, Inova Fairfax Hospital; vice president for research, Inova Health System; professor of medicine, VCU-Inova Campus and Beatty Center for Integrated Research, Falls Church, Va. He has consulted for Gilead, AbbVie, Intercept, BMS, and GSK.
Nonalcoholic fatty liver disease (NAFLD) almost tripled among United States veterans in a recent 9-year period, investigators reported in the February issue of Clinical Gastroenterology and Hepatology.
The trend “was evident in all racial groups, across all age groups, and in both genders,” said Dr. Fasiha Kanwal of the Michael E. DeBakey Veterans Affairs Medical Center and Baylor College of Medicine, both in Houston, and her associates. The increasing prevalence of NAFLD “is likely generalizable to nonveterans,” and will probably persist because of a “fairly steady” 2%-3% overall annual incidence and a steeper rise among younger individuals, they added. “Nonalcoholic fatty liver disease will continue to remain a major public health problem in the United States, at least in the near and intermediate future.”
Although NAFLD is the leading cause of chronic liver failure in the United States, few studies have examined its incidence or prevalence over time, which are key to predicting future disease burden. Therefore, the investigators analyzed data for more than 9.78 million patients who visited the VA at least once between 2003 and 2011. They defined NAFLD as at least two elevated alanine aminotransferase (ALT) values (greater than 40 IU/mL) separated by at least 6 months, with no history of positive serology for hepatitis B surface antigen or hepatitis C virus RNA, and no alcohol-related ICD-9 codes or positive AUDIT-C scores within a year of elevated ALT levels (Clin Gastroenterol Hepatol. 2015 Aug 7. doi: 10.1016/j.cgh.2015.08.010).
During the study period, more than 1.3 million patients, or 13.6%, met the definition of NAFLD, said the researchers. Age-adjusted incidence rates dropped slightly from 3.16% in 2003 to 2.5% in 2011, ranging between 2.3% and 2.7% in most years. Prevalence, however, rose from 6.3% in 2003 (95% confidence interval, 6.26%-6.3%) to 17.6% in 2011 (95% CI, 17.58%-17.65%), a 2.8-fold increase. Moreover, about one in five patients with NAFLD who visited the VA in 2011 was at risk for advanced fibrosis.
Among individuals who were younger than 45 years, the incidence of NAFLD rose from 2.3 to 4.3 cases per 100 persons (annual percentage change, 7.4%; 95% CI, 5.7% to 9.2%), the researchers also found. “Although recent studies show that the rate of increase in both obesity and diabetes, which are both major risk factors for NAFLD, may be slowing down in the U.S., this may not be the case in the VA, where the prevalence of obesity and diabetes is in fact higher than in the U.S. population,” they said.
In general, the findings mirror a recent analysis of the National Health and Nutrition Examination Survey (Aliment Pharmacol Ther. 2015 Jan;41[1]:65-76), according to the investigators. “The VA is the largest integrated health care system in the United States,” they added. “We believe that the sheer size of the veteran cohort, combined with a complete dearth of information regarding the burden of NAFLD in the VA, renders our findings highly significant. Furthermore, the VA is in a unique position to test and implement systemic changes in medical care delivery to improve the health care of NAFLD patients.”
The study was partially supported by the Michael E. DeBakey Veterans Affairs Medical Center. The researchers had no disclosures.
Nonalcoholic fatty liver disease (NAFLD) almost tripled among United States veterans in a recent 9-year period, investigators reported in the February issue of Clinical Gastroenterology and Hepatology.
The trend “was evident in all racial groups, across all age groups, and in both genders,” said Dr. Fasiha Kanwal of the Michael E. DeBakey Veterans Affairs Medical Center and Baylor College of Medicine, both in Houston, and her associates. The increasing prevalence of NAFLD “is likely generalizable to nonveterans,” and will probably persist because of a “fairly steady” 2%-3% overall annual incidence and a steeper rise among younger individuals, they added. “Nonalcoholic fatty liver disease will continue to remain a major public health problem in the United States, at least in the near and intermediate future.”
Although NAFLD is the leading cause of chronic liver failure in the United States, few studies have examined its incidence or prevalence over time, which are key to predicting future disease burden. Therefore, the investigators analyzed data for more than 9.78 million patients who visited the VA at least once between 2003 and 2011. They defined NAFLD as at least two elevated alanine aminotransferase (ALT) values (greater than 40 IU/mL) separated by at least 6 months, with no history of positive serology for hepatitis B surface antigen or hepatitis C virus RNA, and no alcohol-related ICD-9 codes or positive AUDIT-C scores within a year of elevated ALT levels (Clin Gastroenterol Hepatol. 2015 Aug 7. doi: 10.1016/j.cgh.2015.08.010).
During the study period, more than 1.3 million patients, or 13.6%, met the definition of NAFLD, said the researchers. Age-adjusted incidence rates dropped slightly from 3.16% in 2003 to 2.5% in 2011, ranging between 2.3% and 2.7% in most years. Prevalence, however, rose from 6.3% in 2003 (95% confidence interval, 6.26%-6.3%) to 17.6% in 2011 (95% CI, 17.58%-17.65%), a 2.8-fold increase. Moreover, about one in five patients with NAFLD who visited the VA in 2011 was at risk for advanced fibrosis.
Among individuals who were younger than 45 years, the incidence of NAFLD rose from 2.3 to 4.3 cases per 100 persons (annual percentage change, 7.4%; 95% CI, 5.7% to 9.2%), the researchers also found. “Although recent studies show that the rate of increase in both obesity and diabetes, which are both major risk factors for NAFLD, may be slowing down in the U.S., this may not be the case in the VA, where the prevalence of obesity and diabetes is in fact higher than in the U.S. population,” they said.
In general, the findings mirror a recent analysis of the National Health and Nutrition Examination Survey (Aliment Pharmacol Ther. 2015 Jan;41[1]:65-76), according to the investigators. “The VA is the largest integrated health care system in the United States,” they added. “We believe that the sheer size of the veteran cohort, combined with a complete dearth of information regarding the burden of NAFLD in the VA, renders our findings highly significant. Furthermore, the VA is in a unique position to test and implement systemic changes in medical care delivery to improve the health care of NAFLD patients.”
The study was partially supported by the Michael E. DeBakey Veterans Affairs Medical Center. The researchers had no disclosures.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: The prevalence of nonalcoholic fatty liver disease has risen substantially since 2003, and will probably keep increasing in the near term.
Major finding: Prevalence among veterans rose about 2.8 times between 2003 and 2011, mirroring trends reported in the general population.
Data source: An analysis of data from 9.78 million Veterans Affairs patients.
Disclosures: The study was partially supported by the Michael E. DeBakey Veterans Affairs Medical Center. The researchers had no disclosures.
Hepatitis C virus infection linked to cardiovascular death, disease, and stroke
Patients with hepatitis C virus (HCV) infection face a significantly increased risk of cardiovascular death, subclinical carotid thickening and atherosclerosis, and cerebrocardiovascular events, especially when they also have diabetes and hypertension, according to a systematic review and meta-analysis of 22 studies published in the January issue of Gastroenterology.
“To our knowledge, our meta-analysis clearly highlights, for the first time, that HCV infection increases the risk of cardiovascular disease-related mortality,” wrote Dr. Salvatore Petta and his associates at the University of Palermo, Italy. “We [also] found a twofold higher risk of subclinical carotid plaques among HCV-infected individuals compared to uninfected controls, without significant heterogeneity among studies, as well as an increased risk of carotid thickening. We observed a slightly significant increase in cerebrocardiovascular events among HCV-infected patients, despite the high heterogeneity among studies that was mostly related to the prevalence of diabetes mellitus and hypertension.”
A number of observational studies have reported cardiovascular outcomes in HCV-infected patients, but results have been “ambiguous,” Dr. Petta and his colleagues said. For their meta-analysis, they searched PubMed, Medline, EMBASE, the Cochrane Library, and reference lists of articles to identify studies published through July 2015 that either compared cardiovascular disease between HCV-infected and uninfected patients, or evaluated the prevalence of HCV infection among patients with cardiovascular disease. This literature search identified 12 case-control studies and 10 cohort studies. Outcome measures included carotid atherosclerosis (nine studies), intima media thickness (eight studies), coronary artery disease (seven studies), stroke (six studies), and cardiovascular mortality (three studies) (Gastroenterology. 2015 Sep 18. doi: 10.1053/j.gastro.2015.09.00).In the pooled analysis, the odds of cardiovascular death were 65% higher in HCV-infected patients, compared with uninfected individuals (95% confidence interval for this increase, 1.07%-2.56%). Compared with controls, HCV-infected patients also were at higher risk of carotid plaques (odds ratio, 2.27; 95% CI, 1.76-2.94), especially when they were smokers (P = .02). HCV infection also significantly increased the odds of carotid artery intima-media thickening (OR, 1.20; 95% CI, 1.03-1.40), and cerebrocardiovascular events (OR, 1.30; 95% CI, 1.10-1.55). However, subgroup analyses showed that HCV infection only increased the likelihood of cerebrocardiovascular events in populations with a more than 10% prevalence of diabetes or a more than 20% prevalence of hypertension (OR, 1.71; P less than .001 for both subgroup analyses).
Because the studies of cerebrocardiovascular events were heterogeneous, the researchers also stratified them by study design and by the average age of patients. Pooled odds ratios for the link between HCV infection and cerebrocardiovascular events remained significant at 1.21 for the cohort studies, 2.01 for the case-control studies, 2.46 among patients who averaged more than 50 years of age, and 1.35 among younger patients.
The Egger test for publication bias showed that the literature search was unlikely to have overlooked studies in terms of any of the outcome measures, the investigators noted. “From a clinical standpoint, the results of our meta-analysis suggest that HCV infection increases cardiovascular risk, particularly for individuals who already have cardiovascular risk factors, such as diabetes and hypertension,” they concluded. “Although effective and safe oral antiviral regimens are available, more information is needed to confirm whether anti-HCV medications will decrease cardiovascular risk, as suggested in some studies.”
The researchers reported having no funding sources or conflicts of interest.
Source: American Gastroenterological Association
Patients with hepatitis C virus (HCV) infection face a significantly increased risk of cardiovascular death, subclinical carotid thickening and atherosclerosis, and cerebrocardiovascular events, especially when they also have diabetes and hypertension, according to a systematic review and meta-analysis of 22 studies published in the January issue of Gastroenterology.
“To our knowledge, our meta-analysis clearly highlights, for the first time, that HCV infection increases the risk of cardiovascular disease-related mortality,” wrote Dr. Salvatore Petta and his associates at the University of Palermo, Italy. “We [also] found a twofold higher risk of subclinical carotid plaques among HCV-infected individuals compared to uninfected controls, without significant heterogeneity among studies, as well as an increased risk of carotid thickening. We observed a slightly significant increase in cerebrocardiovascular events among HCV-infected patients, despite the high heterogeneity among studies that was mostly related to the prevalence of diabetes mellitus and hypertension.”
A number of observational studies have reported cardiovascular outcomes in HCV-infected patients, but results have been “ambiguous,” Dr. Petta and his colleagues said. For their meta-analysis, they searched PubMed, Medline, EMBASE, the Cochrane Library, and reference lists of articles to identify studies published through July 2015 that either compared cardiovascular disease between HCV-infected and uninfected patients, or evaluated the prevalence of HCV infection among patients with cardiovascular disease. This literature search identified 12 case-control studies and 10 cohort studies. Outcome measures included carotid atherosclerosis (nine studies), intima media thickness (eight studies), coronary artery disease (seven studies), stroke (six studies), and cardiovascular mortality (three studies) (Gastroenterology. 2015 Sep 18. doi: 10.1053/j.gastro.2015.09.00).In the pooled analysis, the odds of cardiovascular death were 65% higher in HCV-infected patients, compared with uninfected individuals (95% confidence interval for this increase, 1.07%-2.56%). Compared with controls, HCV-infected patients also were at higher risk of carotid plaques (odds ratio, 2.27; 95% CI, 1.76-2.94), especially when they were smokers (P = .02). HCV infection also significantly increased the odds of carotid artery intima-media thickening (OR, 1.20; 95% CI, 1.03-1.40), and cerebrocardiovascular events (OR, 1.30; 95% CI, 1.10-1.55). However, subgroup analyses showed that HCV infection only increased the likelihood of cerebrocardiovascular events in populations with a more than 10% prevalence of diabetes or a more than 20% prevalence of hypertension (OR, 1.71; P less than .001 for both subgroup analyses).
Because the studies of cerebrocardiovascular events were heterogeneous, the researchers also stratified them by study design and by the average age of patients. Pooled odds ratios for the link between HCV infection and cerebrocardiovascular events remained significant at 1.21 for the cohort studies, 2.01 for the case-control studies, 2.46 among patients who averaged more than 50 years of age, and 1.35 among younger patients.
The Egger test for publication bias showed that the literature search was unlikely to have overlooked studies in terms of any of the outcome measures, the investigators noted. “From a clinical standpoint, the results of our meta-analysis suggest that HCV infection increases cardiovascular risk, particularly for individuals who already have cardiovascular risk factors, such as diabetes and hypertension,” they concluded. “Although effective and safe oral antiviral regimens are available, more information is needed to confirm whether anti-HCV medications will decrease cardiovascular risk, as suggested in some studies.”
The researchers reported having no funding sources or conflicts of interest.
Source: American Gastroenterological Association
Patients with hepatitis C virus (HCV) infection face a significantly increased risk of cardiovascular death, subclinical carotid thickening and atherosclerosis, and cerebrocardiovascular events, especially when they also have diabetes and hypertension, according to a systematic review and meta-analysis of 22 studies published in the January issue of Gastroenterology.
“To our knowledge, our meta-analysis clearly highlights, for the first time, that HCV infection increases the risk of cardiovascular disease-related mortality,” wrote Dr. Salvatore Petta and his associates at the University of Palermo, Italy. “We [also] found a twofold higher risk of subclinical carotid plaques among HCV-infected individuals compared to uninfected controls, without significant heterogeneity among studies, as well as an increased risk of carotid thickening. We observed a slightly significant increase in cerebrocardiovascular events among HCV-infected patients, despite the high heterogeneity among studies that was mostly related to the prevalence of diabetes mellitus and hypertension.”
A number of observational studies have reported cardiovascular outcomes in HCV-infected patients, but results have been “ambiguous,” Dr. Petta and his colleagues said. For their meta-analysis, they searched PubMed, Medline, EMBASE, the Cochrane Library, and reference lists of articles to identify studies published through July 2015 that either compared cardiovascular disease between HCV-infected and uninfected patients, or evaluated the prevalence of HCV infection among patients with cardiovascular disease. This literature search identified 12 case-control studies and 10 cohort studies. Outcome measures included carotid atherosclerosis (nine studies), intima media thickness (eight studies), coronary artery disease (seven studies), stroke (six studies), and cardiovascular mortality (three studies) (Gastroenterology. 2015 Sep 18. doi: 10.1053/j.gastro.2015.09.00).In the pooled analysis, the odds of cardiovascular death were 65% higher in HCV-infected patients, compared with uninfected individuals (95% confidence interval for this increase, 1.07%-2.56%). Compared with controls, HCV-infected patients also were at higher risk of carotid plaques (odds ratio, 2.27; 95% CI, 1.76-2.94), especially when they were smokers (P = .02). HCV infection also significantly increased the odds of carotid artery intima-media thickening (OR, 1.20; 95% CI, 1.03-1.40), and cerebrocardiovascular events (OR, 1.30; 95% CI, 1.10-1.55). However, subgroup analyses showed that HCV infection only increased the likelihood of cerebrocardiovascular events in populations with a more than 10% prevalence of diabetes or a more than 20% prevalence of hypertension (OR, 1.71; P less than .001 for both subgroup analyses).
Because the studies of cerebrocardiovascular events were heterogeneous, the researchers also stratified them by study design and by the average age of patients. Pooled odds ratios for the link between HCV infection and cerebrocardiovascular events remained significant at 1.21 for the cohort studies, 2.01 for the case-control studies, 2.46 among patients who averaged more than 50 years of age, and 1.35 among younger patients.
The Egger test for publication bias showed that the literature search was unlikely to have overlooked studies in terms of any of the outcome measures, the investigators noted. “From a clinical standpoint, the results of our meta-analysis suggest that HCV infection increases cardiovascular risk, particularly for individuals who already have cardiovascular risk factors, such as diabetes and hypertension,” they concluded. “Although effective and safe oral antiviral regimens are available, more information is needed to confirm whether anti-HCV medications will decrease cardiovascular risk, as suggested in some studies.”
The researchers reported having no funding sources or conflicts of interest.
Source: American Gastroenterological Association
FROM GASTROENTEROLOGY
Key clinical point: Patients with HCV infection are at increased risk of cardiovascular death, stroke, and subclinical carotid atherosclerosis and thickening.
Major finding: The pooled odds of cardiovascular death were 65% higher among infected, compared with uninfected individuals.
Data source: A systematic review and meta-analysis of 22 observational studies.
Disclosures: The researchers reported having no funding sources or conflicts of interest.
Nonalcoholic fatty liver disease linked to liver cancer without cirrhosis
About 13% of U.S. veterans with hepatocellular carcinoma had no evidence of preexisting cirrhosis, according to a report published in the January issue of Clinical Gastroenterology and Hepatology.
“The main risk factors for this entity were nonalcoholic fatty liver disease [NAFLD] or metabolic syndrome” – not hepatitis C virus infection [HCV], HBV [hepatitis B virus] infection, or alcohol abuse, said Dr. Sahil Mittal of the Michael E. DeBakey Veterans Affairs Medical Center and Baylor College of Medicine in Houston. Screening all patients with NAFLD for hepatocellular carcinoma [HCC] is impractical, so studies should seek “actionable risk factors” or biomarkers that reliably identify NAFLD patients who are at particular risk of HCC, wrote Dr. Mittal and his coinvestigators.
Researchers have debated whether chronic HCV infection or alcohol abuse can lead to HCC in the absence of cirrhosis, while at least one study has shown that NAFLD can predispose patients to this disease entity (Arch Pathol Lab Med. 2008;132:1761-6).
But few studies have systematically examined risk factors for HCC without cirrhosis in the general population, the investigators said. Therefore, they randomly selected 1,500 patients from the U.S. Veterans Affairs system who were diagnosed with HCC between 2005 and 2010 on the basis of histopathology or established imaging criteria (Hepatology 2005;42:1208-36).
They reviewed complete medical records for these patients, and classified those who did not have cirrhosis according to the quality of supporting histology, laboratory, and imaging data (Clin Gastroenterol Hepatol. 2015. doi: 0.1016/j.cgh.2015.07.019).
In all, 3% of the cohort had level 1 (“highest-quality”) evidence for not having cirrhosis, while another 10% had level 2 evidence for no cirrhosis, the investigators said. “Compared with HCC in the presence of cirrhosis, these patients were more likely to have metabolic syndrome or NAFLD or no identifiable risk factor, and less likely to have alcohol abuse or HCV infection,” they added. Only two-thirds of NAFLD patients with HCC had cirrhosis, compared with 91% of patients with chronic HCV infection, 92% of HCV-infected patients, and 88% of patients with an alcohol use disorder. Notably, the odds of HCC in the absence of cirrhosis were more than five times higher when patients had NAFLD (odds ratio [OR], 5.4; 95% confidence interval [CI], 3.4-8.5) or metabolic syndrome (OR, 5.0; 95% CI, 3.1-7.8) compared with HCV infection.
Patients with cirrhosis often go unscreened for HCC even though they are at greatest risk of this cancer. Therefore, trying to screen all patients with NAFLD for HCC would be “logistically impractical,” particularly when the absolute risk of HCC in noncirrhotic patients is unknown and no one has examined the best ways to screen this population, the investigators said. Instead, clinicians could prioritize screening and treating NAFLD patients for diabetes mellitus and obesity, both of which are associated with HCC. “There is evidence to suggest that metformin reduces the risk of HCC among diabetics,” they added. “Studies of these and other risk factors of HCC among NAFLD patients with and without cirrhosis are needed.”
Most patients in the study were male, potentially limiting the generalizability of the findings, the researchers noted.
The National Cancer Institute, the Houston Veterans Affairs Health Services Research and Development Center of Excellence, the Michael E. DeBakey Veterans Affairs Medical Center, and the Dan Duncan Cancer Center funded the study. The researchers had no disclosures.
Source: American Gastroenterological Association
About 13% of U.S. veterans with hepatocellular carcinoma had no evidence of preexisting cirrhosis, according to a report published in the January issue of Clinical Gastroenterology and Hepatology.
“The main risk factors for this entity were nonalcoholic fatty liver disease [NAFLD] or metabolic syndrome” – not hepatitis C virus infection [HCV], HBV [hepatitis B virus] infection, or alcohol abuse, said Dr. Sahil Mittal of the Michael E. DeBakey Veterans Affairs Medical Center and Baylor College of Medicine in Houston. Screening all patients with NAFLD for hepatocellular carcinoma [HCC] is impractical, so studies should seek “actionable risk factors” or biomarkers that reliably identify NAFLD patients who are at particular risk of HCC, wrote Dr. Mittal and his coinvestigators.
Researchers have debated whether chronic HCV infection or alcohol abuse can lead to HCC in the absence of cirrhosis, while at least one study has shown that NAFLD can predispose patients to this disease entity (Arch Pathol Lab Med. 2008;132:1761-6).
But few studies have systematically examined risk factors for HCC without cirrhosis in the general population, the investigators said. Therefore, they randomly selected 1,500 patients from the U.S. Veterans Affairs system who were diagnosed with HCC between 2005 and 2010 on the basis of histopathology or established imaging criteria (Hepatology 2005;42:1208-36).
They reviewed complete medical records for these patients, and classified those who did not have cirrhosis according to the quality of supporting histology, laboratory, and imaging data (Clin Gastroenterol Hepatol. 2015. doi: 0.1016/j.cgh.2015.07.019).
In all, 3% of the cohort had level 1 (“highest-quality”) evidence for not having cirrhosis, while another 10% had level 2 evidence for no cirrhosis, the investigators said. “Compared with HCC in the presence of cirrhosis, these patients were more likely to have metabolic syndrome or NAFLD or no identifiable risk factor, and less likely to have alcohol abuse or HCV infection,” they added. Only two-thirds of NAFLD patients with HCC had cirrhosis, compared with 91% of patients with chronic HCV infection, 92% of HCV-infected patients, and 88% of patients with an alcohol use disorder. Notably, the odds of HCC in the absence of cirrhosis were more than five times higher when patients had NAFLD (odds ratio [OR], 5.4; 95% confidence interval [CI], 3.4-8.5) or metabolic syndrome (OR, 5.0; 95% CI, 3.1-7.8) compared with HCV infection.
Patients with cirrhosis often go unscreened for HCC even though they are at greatest risk of this cancer. Therefore, trying to screen all patients with NAFLD for HCC would be “logistically impractical,” particularly when the absolute risk of HCC in noncirrhotic patients is unknown and no one has examined the best ways to screen this population, the investigators said. Instead, clinicians could prioritize screening and treating NAFLD patients for diabetes mellitus and obesity, both of which are associated with HCC. “There is evidence to suggest that metformin reduces the risk of HCC among diabetics,” they added. “Studies of these and other risk factors of HCC among NAFLD patients with and without cirrhosis are needed.”
Most patients in the study were male, potentially limiting the generalizability of the findings, the researchers noted.
The National Cancer Institute, the Houston Veterans Affairs Health Services Research and Development Center of Excellence, the Michael E. DeBakey Veterans Affairs Medical Center, and the Dan Duncan Cancer Center funded the study. The researchers had no disclosures.
Source: American Gastroenterological Association
About 13% of U.S. veterans with hepatocellular carcinoma had no evidence of preexisting cirrhosis, according to a report published in the January issue of Clinical Gastroenterology and Hepatology.
“The main risk factors for this entity were nonalcoholic fatty liver disease [NAFLD] or metabolic syndrome” – not hepatitis C virus infection [HCV], HBV [hepatitis B virus] infection, or alcohol abuse, said Dr. Sahil Mittal of the Michael E. DeBakey Veterans Affairs Medical Center and Baylor College of Medicine in Houston. Screening all patients with NAFLD for hepatocellular carcinoma [HCC] is impractical, so studies should seek “actionable risk factors” or biomarkers that reliably identify NAFLD patients who are at particular risk of HCC, wrote Dr. Mittal and his coinvestigators.
Researchers have debated whether chronic HCV infection or alcohol abuse can lead to HCC in the absence of cirrhosis, while at least one study has shown that NAFLD can predispose patients to this disease entity (Arch Pathol Lab Med. 2008;132:1761-6).
But few studies have systematically examined risk factors for HCC without cirrhosis in the general population, the investigators said. Therefore, they randomly selected 1,500 patients from the U.S. Veterans Affairs system who were diagnosed with HCC between 2005 and 2010 on the basis of histopathology or established imaging criteria (Hepatology 2005;42:1208-36).
They reviewed complete medical records for these patients, and classified those who did not have cirrhosis according to the quality of supporting histology, laboratory, and imaging data (Clin Gastroenterol Hepatol. 2015. doi: 0.1016/j.cgh.2015.07.019).
In all, 3% of the cohort had level 1 (“highest-quality”) evidence for not having cirrhosis, while another 10% had level 2 evidence for no cirrhosis, the investigators said. “Compared with HCC in the presence of cirrhosis, these patients were more likely to have metabolic syndrome or NAFLD or no identifiable risk factor, and less likely to have alcohol abuse or HCV infection,” they added. Only two-thirds of NAFLD patients with HCC had cirrhosis, compared with 91% of patients with chronic HCV infection, 92% of HCV-infected patients, and 88% of patients with an alcohol use disorder. Notably, the odds of HCC in the absence of cirrhosis were more than five times higher when patients had NAFLD (odds ratio [OR], 5.4; 95% confidence interval [CI], 3.4-8.5) or metabolic syndrome (OR, 5.0; 95% CI, 3.1-7.8) compared with HCV infection.
Patients with cirrhosis often go unscreened for HCC even though they are at greatest risk of this cancer. Therefore, trying to screen all patients with NAFLD for HCC would be “logistically impractical,” particularly when the absolute risk of HCC in noncirrhotic patients is unknown and no one has examined the best ways to screen this population, the investigators said. Instead, clinicians could prioritize screening and treating NAFLD patients for diabetes mellitus and obesity, both of which are associated with HCC. “There is evidence to suggest that metformin reduces the risk of HCC among diabetics,” they added. “Studies of these and other risk factors of HCC among NAFLD patients with and without cirrhosis are needed.”
Most patients in the study were male, potentially limiting the generalizability of the findings, the researchers noted.
The National Cancer Institute, the Houston Veterans Affairs Health Services Research and Development Center of Excellence, the Michael E. DeBakey Veterans Affairs Medical Center, and the Dan Duncan Cancer Center funded the study. The researchers had no disclosures.
Source: American Gastroenterological Association
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Nonalcoholic fatty liver disease and metabolic syndrome are risk factors for hepatocellular carcinoma in the absence of cirrhosis.
Major finding: Patients with these diseases were more than five times as likely to develop noncirrhotic HCC compared with patients with chronic hepatitis C virus infection.
Data source: A study of 1,500 randomly selected U.S. veterans diagnosed with HCC between 2005 and 2010.
Disclosures: The National Cancer Institute, the Houston Veterans Affairs Health Services Research and Development Center of Excellence, the Michael E. DeBakey Veterans Affairs Medical Center, and the Dan Duncan Cancer Center funded the study. The researchers had no disclosures.
Senate report underscores frustration about hepatits C drug prices
Physicians who treat hepatitis C patients were hardly surprised by a recent Senate investigation that found the maker of a hepatitis C drug allegedly put profits ahead of accessibility in pricing its medication. The final wholesale price – $1,000 per pill, or $84,000 for a single course of treatment – led to $1.3 billion in spending by state Medicaid programs, but few low-income patients actually received the drug.
“Even if sofosbuvir [Sovaldi] and Harvoni were priced 50% lower, the reality is that the burden to the health care system would still be tremendous, and it would still not be possible to treat all patients with hepatitis C,” said Dr. Sean W.P. Koppe, director of hepatology at the University of Illinois Hospital & Health Sciences System, Chicago. “Large organizations, such as [Veterans Affairs], can leverage their purchasing power to force some deeper discounting of the drug, [but] until some sort of mechanism is in place to harness the purchasing power of Medicare and Medicaid to negotiate deeper discounts, this scenario is bound to repeat itself with other drugs.”
After an 18-month analysis, investigators with the Senate Committee on Finance found that Gilead Sciences pursued a marketing strategy and wholesale price of Sovaldi that would maximize its revenue with little regard to affordable access, according to a report published Dec. 1. Building on Sovaldi’s price, Harvoni was later introduced at $94,500 per treatment course under the same marketing scheme. The investigation found no evidence that research costs or the multibillion dollar acquisition of Pharmasset, the drug’s first developer, factored into how Gilead set its prices. Investigators drew their conclusions from 20,000 pages of internal company documents, interviews with health care experts, and a trove of data from Medicaid programs in 50 states and the District of Columbia.
“Gilead pursued a calculated scheme for pricing and marketing its hepatitis C drug, based on one primary goal – maximizing revenue – regardless of the human consequences,” Sen. Ron Wyden (D-Ore.), Senate Finance Committee ranking member, said in a statement. “Gilead knew these prices would put treatment out of the reach of millions and cause extraordinary problems for Medicare and Medicaid, but still the company went ahead. If Gilead’s approach to pricing is the future of how blockbuster drugs are launched, it will cost billions and billions of dollars to treat just a fraction of patients.”
In a statement, a Gilead representative said the company respectfully disagreed with the report’s conclusions.
“We stand behind the pricing of our therapies because of the benefit they bring to patients and the significant value they represent to payers, providers, and our entire health care system by reducing the long-term costs associated with managing chronic HCV [hepatitis C virus],” Gilead spokeswoman Michele Rest said in an emailed statement. “Enabling patient access to Sovaldi and Harvoni is a top priority for Gilead. We have programs in place in the U.S. to help uninsured individuals and those who need financial assistance to access our therapies, and we will continue our efforts to make the medications available to people in need in the United States and around the world.”
Medicare, Medicaid, and the Bureau of Prisons were hit hardest by Sovaldi’s price tag, according to the report. In the 18 months following Sovaldi’s approval, Medicare has spent nearly $8.2 billion before rebates on Sovaldi and Harvoni, a sixfold monthly spending increase on hepatitis C treatments for the agency. In 2014 alone, Medicare and Medicaid spent a combined $5 billion on Sovaldi and Harvoni before rebates, a number projected to climb in 2015. The cost of Sovaldi meant many states were forced to effectively ration the drugs, providing them to only the sickest patients and requiring that patients be under the care of hepatologists or other specialists prior to receiving the drugs, according to the Senate report. Data show that state Medicaid programs nationwide spent $1.3 billion before rebates on Sovaldi in 2014, but that less than 2.4% of Medicaid enrollees with hepatitis C were treated with the drug. Sovaldi was the top drug spending item for 14 state Medicaid agencies in 2014, and the second-highest spending item for 15 more states.
“What we face is not a drug cost problem, it is a drug price problem,” Lynne Saxton, director of the Oregon Health Authority, wrote in a letter to Sen. Wyden. “State Medicaid programs are limited in [their] ability to control pharmacy benefit expenditure, particularly as federal law requires us to provide a pathway to coverage for all FDA-approved drugs, no matter how minimal the likely benefit per dollar spent. While federally mandated rebates help, they provide limited relief.”
Financial records show Gilead generated $14.3 billion in net product sales from its HCV drugs through the first 9 months of 2015, bringing its 21-month total for its HCV drugs to $26.6 billion, $20.6 billion of which is from sales to U.S. patients. Gilead justified Sovaldi’s high price point based on price-per-cure, according to the report. Documents acquired during the investigation illustrate that Gilead officials knew they were in a position to create clear savings for payers but chose to pursue a “regimen neutral” price justified by “cost-per-cure” calculations that resulted in greater revenue per treatment than previous direct-acting antivirals.
The Senate report was eye-opening and provides a likely snapshot of how future medications will be priced by drug companies, said Dr. Rajat Chander, an internist and gastroenterologist in private practice in Raleigh, N.C. “While newer drugs for hep C will ultimately drive the price lower, this Senate investigation is enlightening as to ‘price-per-cure’ for future drugs for other diseases,” he said in an interview. “As physicians, we often find ourselves on the front lines, scrambling to find discounts and rebates for individual patients. However, we need just this kind of bipartisan Senate and government regulatory oversight to balance innovation with affordability. Drug companies like Gilead perform miracles by curing hepatitis C, but we have to make sure these new drugs are affordable.”
The report underscores the frustration felt by physicians who see many hepatitis C patients go without treatment because of high drug prices, Dr. Koppe adds.
“We would all prefer to treat every single compliant patient with hepatitis C who comes through our office,” Dr. Koppe said in an interview. “We now have to spend time explaining the economics of the situation to patients and why they are denied treatment, rather than simply focusing on their medical care.”
Senate investigators note that while competition has now entered the market, concerns remain about the cost of treatment for hepatitis C patients and future medication prices.
“Even as competition lowered prices for therapies, this report documents that concerns remain, particularly in the public payer community, about high costs for treating millions of people in the U.S. infected with hepatitis C, as well as the budgetary effects of a future single-source innovator that might not face competition as quickly,” the report stated.
On Twitter @legal_med
Physicians who treat hepatitis C patients were hardly surprised by a recent Senate investigation that found the maker of a hepatitis C drug allegedly put profits ahead of accessibility in pricing its medication. The final wholesale price – $1,000 per pill, or $84,000 for a single course of treatment – led to $1.3 billion in spending by state Medicaid programs, but few low-income patients actually received the drug.
“Even if sofosbuvir [Sovaldi] and Harvoni were priced 50% lower, the reality is that the burden to the health care system would still be tremendous, and it would still not be possible to treat all patients with hepatitis C,” said Dr. Sean W.P. Koppe, director of hepatology at the University of Illinois Hospital & Health Sciences System, Chicago. “Large organizations, such as [Veterans Affairs], can leverage their purchasing power to force some deeper discounting of the drug, [but] until some sort of mechanism is in place to harness the purchasing power of Medicare and Medicaid to negotiate deeper discounts, this scenario is bound to repeat itself with other drugs.”
After an 18-month analysis, investigators with the Senate Committee on Finance found that Gilead Sciences pursued a marketing strategy and wholesale price of Sovaldi that would maximize its revenue with little regard to affordable access, according to a report published Dec. 1. Building on Sovaldi’s price, Harvoni was later introduced at $94,500 per treatment course under the same marketing scheme. The investigation found no evidence that research costs or the multibillion dollar acquisition of Pharmasset, the drug’s first developer, factored into how Gilead set its prices. Investigators drew their conclusions from 20,000 pages of internal company documents, interviews with health care experts, and a trove of data from Medicaid programs in 50 states and the District of Columbia.
“Gilead pursued a calculated scheme for pricing and marketing its hepatitis C drug, based on one primary goal – maximizing revenue – regardless of the human consequences,” Sen. Ron Wyden (D-Ore.), Senate Finance Committee ranking member, said in a statement. “Gilead knew these prices would put treatment out of the reach of millions and cause extraordinary problems for Medicare and Medicaid, but still the company went ahead. If Gilead’s approach to pricing is the future of how blockbuster drugs are launched, it will cost billions and billions of dollars to treat just a fraction of patients.”
In a statement, a Gilead representative said the company respectfully disagreed with the report’s conclusions.
“We stand behind the pricing of our therapies because of the benefit they bring to patients and the significant value they represent to payers, providers, and our entire health care system by reducing the long-term costs associated with managing chronic HCV [hepatitis C virus],” Gilead spokeswoman Michele Rest said in an emailed statement. “Enabling patient access to Sovaldi and Harvoni is a top priority for Gilead. We have programs in place in the U.S. to help uninsured individuals and those who need financial assistance to access our therapies, and we will continue our efforts to make the medications available to people in need in the United States and around the world.”
Medicare, Medicaid, and the Bureau of Prisons were hit hardest by Sovaldi’s price tag, according to the report. In the 18 months following Sovaldi’s approval, Medicare has spent nearly $8.2 billion before rebates on Sovaldi and Harvoni, a sixfold monthly spending increase on hepatitis C treatments for the agency. In 2014 alone, Medicare and Medicaid spent a combined $5 billion on Sovaldi and Harvoni before rebates, a number projected to climb in 2015. The cost of Sovaldi meant many states were forced to effectively ration the drugs, providing them to only the sickest patients and requiring that patients be under the care of hepatologists or other specialists prior to receiving the drugs, according to the Senate report. Data show that state Medicaid programs nationwide spent $1.3 billion before rebates on Sovaldi in 2014, but that less than 2.4% of Medicaid enrollees with hepatitis C were treated with the drug. Sovaldi was the top drug spending item for 14 state Medicaid agencies in 2014, and the second-highest spending item for 15 more states.
“What we face is not a drug cost problem, it is a drug price problem,” Lynne Saxton, director of the Oregon Health Authority, wrote in a letter to Sen. Wyden. “State Medicaid programs are limited in [their] ability to control pharmacy benefit expenditure, particularly as federal law requires us to provide a pathway to coverage for all FDA-approved drugs, no matter how minimal the likely benefit per dollar spent. While federally mandated rebates help, they provide limited relief.”
Financial records show Gilead generated $14.3 billion in net product sales from its HCV drugs through the first 9 months of 2015, bringing its 21-month total for its HCV drugs to $26.6 billion, $20.6 billion of which is from sales to U.S. patients. Gilead justified Sovaldi’s high price point based on price-per-cure, according to the report. Documents acquired during the investigation illustrate that Gilead officials knew they were in a position to create clear savings for payers but chose to pursue a “regimen neutral” price justified by “cost-per-cure” calculations that resulted in greater revenue per treatment than previous direct-acting antivirals.
The Senate report was eye-opening and provides a likely snapshot of how future medications will be priced by drug companies, said Dr. Rajat Chander, an internist and gastroenterologist in private practice in Raleigh, N.C. “While newer drugs for hep C will ultimately drive the price lower, this Senate investigation is enlightening as to ‘price-per-cure’ for future drugs for other diseases,” he said in an interview. “As physicians, we often find ourselves on the front lines, scrambling to find discounts and rebates for individual patients. However, we need just this kind of bipartisan Senate and government regulatory oversight to balance innovation with affordability. Drug companies like Gilead perform miracles by curing hepatitis C, but we have to make sure these new drugs are affordable.”
The report underscores the frustration felt by physicians who see many hepatitis C patients go without treatment because of high drug prices, Dr. Koppe adds.
“We would all prefer to treat every single compliant patient with hepatitis C who comes through our office,” Dr. Koppe said in an interview. “We now have to spend time explaining the economics of the situation to patients and why they are denied treatment, rather than simply focusing on their medical care.”
Senate investigators note that while competition has now entered the market, concerns remain about the cost of treatment for hepatitis C patients and future medication prices.
“Even as competition lowered prices for therapies, this report documents that concerns remain, particularly in the public payer community, about high costs for treating millions of people in the U.S. infected with hepatitis C, as well as the budgetary effects of a future single-source innovator that might not face competition as quickly,” the report stated.
On Twitter @legal_med
Physicians who treat hepatitis C patients were hardly surprised by a recent Senate investigation that found the maker of a hepatitis C drug allegedly put profits ahead of accessibility in pricing its medication. The final wholesale price – $1,000 per pill, or $84,000 for a single course of treatment – led to $1.3 billion in spending by state Medicaid programs, but few low-income patients actually received the drug.
“Even if sofosbuvir [Sovaldi] and Harvoni were priced 50% lower, the reality is that the burden to the health care system would still be tremendous, and it would still not be possible to treat all patients with hepatitis C,” said Dr. Sean W.P. Koppe, director of hepatology at the University of Illinois Hospital & Health Sciences System, Chicago. “Large organizations, such as [Veterans Affairs], can leverage their purchasing power to force some deeper discounting of the drug, [but] until some sort of mechanism is in place to harness the purchasing power of Medicare and Medicaid to negotiate deeper discounts, this scenario is bound to repeat itself with other drugs.”
After an 18-month analysis, investigators with the Senate Committee on Finance found that Gilead Sciences pursued a marketing strategy and wholesale price of Sovaldi that would maximize its revenue with little regard to affordable access, according to a report published Dec. 1. Building on Sovaldi’s price, Harvoni was later introduced at $94,500 per treatment course under the same marketing scheme. The investigation found no evidence that research costs or the multibillion dollar acquisition of Pharmasset, the drug’s first developer, factored into how Gilead set its prices. Investigators drew their conclusions from 20,000 pages of internal company documents, interviews with health care experts, and a trove of data from Medicaid programs in 50 states and the District of Columbia.
“Gilead pursued a calculated scheme for pricing and marketing its hepatitis C drug, based on one primary goal – maximizing revenue – regardless of the human consequences,” Sen. Ron Wyden (D-Ore.), Senate Finance Committee ranking member, said in a statement. “Gilead knew these prices would put treatment out of the reach of millions and cause extraordinary problems for Medicare and Medicaid, but still the company went ahead. If Gilead’s approach to pricing is the future of how blockbuster drugs are launched, it will cost billions and billions of dollars to treat just a fraction of patients.”
In a statement, a Gilead representative said the company respectfully disagreed with the report’s conclusions.
“We stand behind the pricing of our therapies because of the benefit they bring to patients and the significant value they represent to payers, providers, and our entire health care system by reducing the long-term costs associated with managing chronic HCV [hepatitis C virus],” Gilead spokeswoman Michele Rest said in an emailed statement. “Enabling patient access to Sovaldi and Harvoni is a top priority for Gilead. We have programs in place in the U.S. to help uninsured individuals and those who need financial assistance to access our therapies, and we will continue our efforts to make the medications available to people in need in the United States and around the world.”
Medicare, Medicaid, and the Bureau of Prisons were hit hardest by Sovaldi’s price tag, according to the report. In the 18 months following Sovaldi’s approval, Medicare has spent nearly $8.2 billion before rebates on Sovaldi and Harvoni, a sixfold monthly spending increase on hepatitis C treatments for the agency. In 2014 alone, Medicare and Medicaid spent a combined $5 billion on Sovaldi and Harvoni before rebates, a number projected to climb in 2015. The cost of Sovaldi meant many states were forced to effectively ration the drugs, providing them to only the sickest patients and requiring that patients be under the care of hepatologists or other specialists prior to receiving the drugs, according to the Senate report. Data show that state Medicaid programs nationwide spent $1.3 billion before rebates on Sovaldi in 2014, but that less than 2.4% of Medicaid enrollees with hepatitis C were treated with the drug. Sovaldi was the top drug spending item for 14 state Medicaid agencies in 2014, and the second-highest spending item for 15 more states.
“What we face is not a drug cost problem, it is a drug price problem,” Lynne Saxton, director of the Oregon Health Authority, wrote in a letter to Sen. Wyden. “State Medicaid programs are limited in [their] ability to control pharmacy benefit expenditure, particularly as federal law requires us to provide a pathway to coverage for all FDA-approved drugs, no matter how minimal the likely benefit per dollar spent. While federally mandated rebates help, they provide limited relief.”
Financial records show Gilead generated $14.3 billion in net product sales from its HCV drugs through the first 9 months of 2015, bringing its 21-month total for its HCV drugs to $26.6 billion, $20.6 billion of which is from sales to U.S. patients. Gilead justified Sovaldi’s high price point based on price-per-cure, according to the report. Documents acquired during the investigation illustrate that Gilead officials knew they were in a position to create clear savings for payers but chose to pursue a “regimen neutral” price justified by “cost-per-cure” calculations that resulted in greater revenue per treatment than previous direct-acting antivirals.
The Senate report was eye-opening and provides a likely snapshot of how future medications will be priced by drug companies, said Dr. Rajat Chander, an internist and gastroenterologist in private practice in Raleigh, N.C. “While newer drugs for hep C will ultimately drive the price lower, this Senate investigation is enlightening as to ‘price-per-cure’ for future drugs for other diseases,” he said in an interview. “As physicians, we often find ourselves on the front lines, scrambling to find discounts and rebates for individual patients. However, we need just this kind of bipartisan Senate and government regulatory oversight to balance innovation with affordability. Drug companies like Gilead perform miracles by curing hepatitis C, but we have to make sure these new drugs are affordable.”
The report underscores the frustration felt by physicians who see many hepatitis C patients go without treatment because of high drug prices, Dr. Koppe adds.
“We would all prefer to treat every single compliant patient with hepatitis C who comes through our office,” Dr. Koppe said in an interview. “We now have to spend time explaining the economics of the situation to patients and why they are denied treatment, rather than simply focusing on their medical care.”
Senate investigators note that while competition has now entered the market, concerns remain about the cost of treatment for hepatitis C patients and future medication prices.
“Even as competition lowered prices for therapies, this report documents that concerns remain, particularly in the public payer community, about high costs for treating millions of people in the U.S. infected with hepatitis C, as well as the budgetary effects of a future single-source innovator that might not face competition as quickly,” the report stated.
On Twitter @legal_med
FDA warns of serious liver injury from some HCV drugs
The Food and Drug Administration has issued a safety alert warning of possible serious liver injury as a result of two hepatitis C drugs, the agency announced Oct. 22.
A review of adverse events reported to the manufacturer and to the FDA MedWatch program identified serious side effects in patients with underlying liver cirrhosis, including hepatic decompensation and liver failure, the FDA said in a statement. Some of these cases resulted in liver transplantation or death, they said.
The two drugs are Viekira Pak and Technivie, manufactured by AbbVie.
“These serious outcomes were reported mostly in patients taking Viekira Pak who had evidence of advanced cirrhosis even before starting treatment with it,” the statement said.
At least 26 cases submitted to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program are believed to be linked to Viekira Pak or Technivie, with most liver injuries occurring within 1-4 weeks of starting treatment, the FDA said.
Additionally, some cases “occurred in patients for whom these medicines were contraindicated or not recommended,” they said.
Viekira Pak, approved in December 2014, is a fixed-dose combination of dasabuvir, ombitasvir, paritaprevir, and ritonavir taken with or without ribavirin. Technivie, approved in July 2015, is a fixed-dose combination of ombitasvir, paritaprevir, and ritonavir, used in combination with ribavirin.
The FDA recommends that clinicians closely monitor patients for signs of serious liver disease such as ascites, hepatic encephalopathy, variceal hemorrhage, and increases in direct bilirubin in the blood. Additionally, it recommends that patients contact their provider immediately if they develop symptoms of liver injury such as fatigue, weakness, loss of appetite, nausea, vomiting, yellow eyes or skin, and light-colored stools.
Patients and providers can report adverse events to the FDA’s MedWatch program.
The Food and Drug Administration has issued a safety alert warning of possible serious liver injury as a result of two hepatitis C drugs, the agency announced Oct. 22.
A review of adverse events reported to the manufacturer and to the FDA MedWatch program identified serious side effects in patients with underlying liver cirrhosis, including hepatic decompensation and liver failure, the FDA said in a statement. Some of these cases resulted in liver transplantation or death, they said.
The two drugs are Viekira Pak and Technivie, manufactured by AbbVie.
“These serious outcomes were reported mostly in patients taking Viekira Pak who had evidence of advanced cirrhosis even before starting treatment with it,” the statement said.
At least 26 cases submitted to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program are believed to be linked to Viekira Pak or Technivie, with most liver injuries occurring within 1-4 weeks of starting treatment, the FDA said.
Additionally, some cases “occurred in patients for whom these medicines were contraindicated or not recommended,” they said.
Viekira Pak, approved in December 2014, is a fixed-dose combination of dasabuvir, ombitasvir, paritaprevir, and ritonavir taken with or without ribavirin. Technivie, approved in July 2015, is a fixed-dose combination of ombitasvir, paritaprevir, and ritonavir, used in combination with ribavirin.
The FDA recommends that clinicians closely monitor patients for signs of serious liver disease such as ascites, hepatic encephalopathy, variceal hemorrhage, and increases in direct bilirubin in the blood. Additionally, it recommends that patients contact their provider immediately if they develop symptoms of liver injury such as fatigue, weakness, loss of appetite, nausea, vomiting, yellow eyes or skin, and light-colored stools.
Patients and providers can report adverse events to the FDA’s MedWatch program.
The Food and Drug Administration has issued a safety alert warning of possible serious liver injury as a result of two hepatitis C drugs, the agency announced Oct. 22.
A review of adverse events reported to the manufacturer and to the FDA MedWatch program identified serious side effects in patients with underlying liver cirrhosis, including hepatic decompensation and liver failure, the FDA said in a statement. Some of these cases resulted in liver transplantation or death, they said.
The two drugs are Viekira Pak and Technivie, manufactured by AbbVie.
“These serious outcomes were reported mostly in patients taking Viekira Pak who had evidence of advanced cirrhosis even before starting treatment with it,” the statement said.
At least 26 cases submitted to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program are believed to be linked to Viekira Pak or Technivie, with most liver injuries occurring within 1-4 weeks of starting treatment, the FDA said.
Additionally, some cases “occurred in patients for whom these medicines were contraindicated or not recommended,” they said.
Viekira Pak, approved in December 2014, is a fixed-dose combination of dasabuvir, ombitasvir, paritaprevir, and ritonavir taken with or without ribavirin. Technivie, approved in July 2015, is a fixed-dose combination of ombitasvir, paritaprevir, and ritonavir, used in combination with ribavirin.
The FDA recommends that clinicians closely monitor patients for signs of serious liver disease such as ascites, hepatic encephalopathy, variceal hemorrhage, and increases in direct bilirubin in the blood. Additionally, it recommends that patients contact their provider immediately if they develop symptoms of liver injury such as fatigue, weakness, loss of appetite, nausea, vomiting, yellow eyes or skin, and light-colored stools.
Patients and providers can report adverse events to the FDA’s MedWatch program.