User login
Hepatitis Outlook: March 2016
If you work on the front lines of medical care treating patients with hepatitis, you may not have time to review all the hepatitis research that enters the medical literature every month. Here’s a quick look at some notable news items and journal articles published over the past month, covering a variety of the major hepatitis viruses.
In the United States, hepatitis C virus (HCV)-associated mortality is increasing. From 2003-2013, the number of deaths associated with HCV has now surpassed 60 other nationally notifiable infectious conditions combined.
Chronic hepatitis B infection increased mortality and complexity among a cohort of HIV-coinfected patients in South Africa, according to a study in HIV Medicine. Researchers found that mortality was increased for chronic hepatitis B patients with hepatitis B virus DNA levels greater than 10,000 copies/mL, compared with non-coinfected patients.
A study in the Journal of Infectious Diseases found that Interferon Lambda (IFNL) genotypes were individually linked to higher rates of fibrosis in HIV–hepatitis C co-infection. Investigators said IFNL genotypes may be useful to target hepatitis C virus treatments to those who are at higher risk of liver disease.
A phase I study of a new NS3/4A protease inhibitor for treatment of chronic hepatitis C virus genotype 1-4 infection yielded positive tolerability, efficacy, and pharmacokinetic results, indicating further evaluation is warranted. The drug, GS-9857, produced by Gilead Sciences, achieved mean and median maximum reductions in HCV RNA of greater than or equal to 3 log10 IU/mL following administration of a 100-mg dose in patients with HCV genotype 1a, 1b, 2, 3, or 4 infection.
High baseline bilirubin and low albumin predict liver decompensation and serious adverse events in hepatitis C-infected patients treated with sofosbuvir-containing regimens, according to a study in the Journal of Viral Hepatitis. Among 499 previously stable patients in the cohort, the incidence of decompensation/events was 4.5%, and the mortality rate was 0.6%.
A meta-analysis of national-level hepatitis C virus prevalence in the Arabian Gulf region found that it is comparable to global levels, although higher HCV prevalence is found in specific expatriate populations reflecting the prevalence in their countries of origin.
A resistance analysis of the drug GS-9190, a NS5B non-nucleoside analogue for the treatment of hepatitis C virus infection, found that the Y448H mutation was rapidly selected in the majority of patients receiving multiple doses of GS-9190 as monotherapy, despite undetectable levels in pretreatment samples. Researchers concluded that Y448H confers reduced susceptibility to GS-9190 and other non-nucleoside inhibitors and persisted in most patients for months post-treatment.
A study published in the International Journal of Infectious Diseases found that hepatitis delta virus (HDV) patients in the Amazon region can be treated with a combination of Pegylated Interferon Alpha and Entecavir for 48 weeks, with good chances of negative HDV RNA at week 24. The results suggest that HDV-3 in the native population may be an “easy to treat” variant compared to HDV-1.
Development of acute hepatitis B virus disease in successfully vaccinated individuals is a rare event, affirmed a study of the Italian Surveillance System for Acute Viral Hepatitis (SEIEVA) from 1993 to 2014. Only 3.2% of acute hepatitis B cases had been vaccinated. Investigators said further efforts are needed to enhance the vaccine coverage rate in people at increased risk of infection, as hepatitis B is a vaccine-preventable disease.
Lab-made hepatocyte transplantation has therapeutic potential as a bridge or even alternative to whole organ liver transplantation, but researchers say deficiencies and uncertainties must be addressed in future studies aimed at developing liver cell therapies with such hepatocytes.
A major concern in potential liver transplant candidates is of unintended harm by achieving sustained viral response rates to direct-acting antiviral treatment, but without improvement in hepatic function to an extent where the patients might function well. A review essay in the Journal of Viral Hepatitis says there is a growing sentiment in some transplant quarters that those with decompensated liver disease awaiting liver transplant be treated for HCV after liver transplant instead of pre-transplant. The authors say it is essential that to develop robust predictors of improvement in liver function so patients can be carefully selected for therapy in the context of liver transplantation.
Treating mild-stage hepatitis C infection in people who inject drugs had virtually no impact on HCV-related end-stage liver disease/hepatocellular carcinoma (ESLD/HCC) within 15 years, a recent study found, but the long timescale of liver disease means relatively few people who inject drugs reach cirrhosis before cessation of injecting. Investigators said strategies focusing on treating advanced disease have the potential for dramatic reductions in severe morbidity, but virtually no preventative impact.
AGA Resource
AGA offers a Hepatitis C Clinical Service Line to provide gastroenterologists with tools to support high-quality patient care.
On Twitter @richpizzi
If you work on the front lines of medical care treating patients with hepatitis, you may not have time to review all the hepatitis research that enters the medical literature every month. Here’s a quick look at some notable news items and journal articles published over the past month, covering a variety of the major hepatitis viruses.
In the United States, hepatitis C virus (HCV)-associated mortality is increasing. From 2003-2013, the number of deaths associated with HCV has now surpassed 60 other nationally notifiable infectious conditions combined.
Chronic hepatitis B infection increased mortality and complexity among a cohort of HIV-coinfected patients in South Africa, according to a study in HIV Medicine. Researchers found that mortality was increased for chronic hepatitis B patients with hepatitis B virus DNA levels greater than 10,000 copies/mL, compared with non-coinfected patients.
A study in the Journal of Infectious Diseases found that Interferon Lambda (IFNL) genotypes were individually linked to higher rates of fibrosis in HIV–hepatitis C co-infection. Investigators said IFNL genotypes may be useful to target hepatitis C virus treatments to those who are at higher risk of liver disease.
A phase I study of a new NS3/4A protease inhibitor for treatment of chronic hepatitis C virus genotype 1-4 infection yielded positive tolerability, efficacy, and pharmacokinetic results, indicating further evaluation is warranted. The drug, GS-9857, produced by Gilead Sciences, achieved mean and median maximum reductions in HCV RNA of greater than or equal to 3 log10 IU/mL following administration of a 100-mg dose in patients with HCV genotype 1a, 1b, 2, 3, or 4 infection.
High baseline bilirubin and low albumin predict liver decompensation and serious adverse events in hepatitis C-infected patients treated with sofosbuvir-containing regimens, according to a study in the Journal of Viral Hepatitis. Among 499 previously stable patients in the cohort, the incidence of decompensation/events was 4.5%, and the mortality rate was 0.6%.
A meta-analysis of national-level hepatitis C virus prevalence in the Arabian Gulf region found that it is comparable to global levels, although higher HCV prevalence is found in specific expatriate populations reflecting the prevalence in their countries of origin.
A resistance analysis of the drug GS-9190, a NS5B non-nucleoside analogue for the treatment of hepatitis C virus infection, found that the Y448H mutation was rapidly selected in the majority of patients receiving multiple doses of GS-9190 as monotherapy, despite undetectable levels in pretreatment samples. Researchers concluded that Y448H confers reduced susceptibility to GS-9190 and other non-nucleoside inhibitors and persisted in most patients for months post-treatment.
A study published in the International Journal of Infectious Diseases found that hepatitis delta virus (HDV) patients in the Amazon region can be treated with a combination of Pegylated Interferon Alpha and Entecavir for 48 weeks, with good chances of negative HDV RNA at week 24. The results suggest that HDV-3 in the native population may be an “easy to treat” variant compared to HDV-1.
Development of acute hepatitis B virus disease in successfully vaccinated individuals is a rare event, affirmed a study of the Italian Surveillance System for Acute Viral Hepatitis (SEIEVA) from 1993 to 2014. Only 3.2% of acute hepatitis B cases had been vaccinated. Investigators said further efforts are needed to enhance the vaccine coverage rate in people at increased risk of infection, as hepatitis B is a vaccine-preventable disease.
Lab-made hepatocyte transplantation has therapeutic potential as a bridge or even alternative to whole organ liver transplantation, but researchers say deficiencies and uncertainties must be addressed in future studies aimed at developing liver cell therapies with such hepatocytes.
A major concern in potential liver transplant candidates is of unintended harm by achieving sustained viral response rates to direct-acting antiviral treatment, but without improvement in hepatic function to an extent where the patients might function well. A review essay in the Journal of Viral Hepatitis says there is a growing sentiment in some transplant quarters that those with decompensated liver disease awaiting liver transplant be treated for HCV after liver transplant instead of pre-transplant. The authors say it is essential that to develop robust predictors of improvement in liver function so patients can be carefully selected for therapy in the context of liver transplantation.
Treating mild-stage hepatitis C infection in people who inject drugs had virtually no impact on HCV-related end-stage liver disease/hepatocellular carcinoma (ESLD/HCC) within 15 years, a recent study found, but the long timescale of liver disease means relatively few people who inject drugs reach cirrhosis before cessation of injecting. Investigators said strategies focusing on treating advanced disease have the potential for dramatic reductions in severe morbidity, but virtually no preventative impact.
AGA Resource
AGA offers a Hepatitis C Clinical Service Line to provide gastroenterologists with tools to support high-quality patient care.
On Twitter @richpizzi
If you work on the front lines of medical care treating patients with hepatitis, you may not have time to review all the hepatitis research that enters the medical literature every month. Here’s a quick look at some notable news items and journal articles published over the past month, covering a variety of the major hepatitis viruses.
In the United States, hepatitis C virus (HCV)-associated mortality is increasing. From 2003-2013, the number of deaths associated with HCV has now surpassed 60 other nationally notifiable infectious conditions combined.
Chronic hepatitis B infection increased mortality and complexity among a cohort of HIV-coinfected patients in South Africa, according to a study in HIV Medicine. Researchers found that mortality was increased for chronic hepatitis B patients with hepatitis B virus DNA levels greater than 10,000 copies/mL, compared with non-coinfected patients.
A study in the Journal of Infectious Diseases found that Interferon Lambda (IFNL) genotypes were individually linked to higher rates of fibrosis in HIV–hepatitis C co-infection. Investigators said IFNL genotypes may be useful to target hepatitis C virus treatments to those who are at higher risk of liver disease.
A phase I study of a new NS3/4A protease inhibitor for treatment of chronic hepatitis C virus genotype 1-4 infection yielded positive tolerability, efficacy, and pharmacokinetic results, indicating further evaluation is warranted. The drug, GS-9857, produced by Gilead Sciences, achieved mean and median maximum reductions in HCV RNA of greater than or equal to 3 log10 IU/mL following administration of a 100-mg dose in patients with HCV genotype 1a, 1b, 2, 3, or 4 infection.
High baseline bilirubin and low albumin predict liver decompensation and serious adverse events in hepatitis C-infected patients treated with sofosbuvir-containing regimens, according to a study in the Journal of Viral Hepatitis. Among 499 previously stable patients in the cohort, the incidence of decompensation/events was 4.5%, and the mortality rate was 0.6%.
A meta-analysis of national-level hepatitis C virus prevalence in the Arabian Gulf region found that it is comparable to global levels, although higher HCV prevalence is found in specific expatriate populations reflecting the prevalence in their countries of origin.
A resistance analysis of the drug GS-9190, a NS5B non-nucleoside analogue for the treatment of hepatitis C virus infection, found that the Y448H mutation was rapidly selected in the majority of patients receiving multiple doses of GS-9190 as monotherapy, despite undetectable levels in pretreatment samples. Researchers concluded that Y448H confers reduced susceptibility to GS-9190 and other non-nucleoside inhibitors and persisted in most patients for months post-treatment.
A study published in the International Journal of Infectious Diseases found that hepatitis delta virus (HDV) patients in the Amazon region can be treated with a combination of Pegylated Interferon Alpha and Entecavir for 48 weeks, with good chances of negative HDV RNA at week 24. The results suggest that HDV-3 in the native population may be an “easy to treat” variant compared to HDV-1.
Development of acute hepatitis B virus disease in successfully vaccinated individuals is a rare event, affirmed a study of the Italian Surveillance System for Acute Viral Hepatitis (SEIEVA) from 1993 to 2014. Only 3.2% of acute hepatitis B cases had been vaccinated. Investigators said further efforts are needed to enhance the vaccine coverage rate in people at increased risk of infection, as hepatitis B is a vaccine-preventable disease.
Lab-made hepatocyte transplantation has therapeutic potential as a bridge or even alternative to whole organ liver transplantation, but researchers say deficiencies and uncertainties must be addressed in future studies aimed at developing liver cell therapies with such hepatocytes.
A major concern in potential liver transplant candidates is of unintended harm by achieving sustained viral response rates to direct-acting antiviral treatment, but without improvement in hepatic function to an extent where the patients might function well. A review essay in the Journal of Viral Hepatitis says there is a growing sentiment in some transplant quarters that those with decompensated liver disease awaiting liver transplant be treated for HCV after liver transplant instead of pre-transplant. The authors say it is essential that to develop robust predictors of improvement in liver function so patients can be carefully selected for therapy in the context of liver transplantation.
Treating mild-stage hepatitis C infection in people who inject drugs had virtually no impact on HCV-related end-stage liver disease/hepatocellular carcinoma (ESLD/HCC) within 15 years, a recent study found, but the long timescale of liver disease means relatively few people who inject drugs reach cirrhosis before cessation of injecting. Investigators said strategies focusing on treating advanced disease have the potential for dramatic reductions in severe morbidity, but virtually no preventative impact.
AGA Resource
AGA offers a Hepatitis C Clinical Service Line to provide gastroenterologists with tools to support high-quality patient care.
On Twitter @richpizzi
VIDEO: How to recognize and treat nonalcoholic fatty liver disease
PHILADELPHIA – Nonalcoholic fatty liver disease is on the rise worldwide, but many clinicians are unaware of how to recognize this potentially fatal condition, in part because it can be difficult to separate from common comorbidities such as obesity and diabetes.
In an interview at the Digestive Diseases: New Advances 2016 meeting, held by Global Academy for Medical Education and Rutgers, the State University of New Jersey, Dr. Zobair M. Younossi, chairman of the department of medicine at Inova Fairfax (Va.) Hospital, discussed what clinicians should know in order to screen for and treat this disease.
Dr. Younossi said he is a consultant to Conatus Pharmaceuticals, Enterome Bioscience, and Gilead, and on the advisory boards of Janssen, Salix Pharmaceuticals, and Vertex Pharmaceuticals.
Global Academy and this news organization are owned by the same company.
On Twitter @whitneymcknight
PHILADELPHIA – Nonalcoholic fatty liver disease is on the rise worldwide, but many clinicians are unaware of how to recognize this potentially fatal condition, in part because it can be difficult to separate from common comorbidities such as obesity and diabetes.
In an interview at the Digestive Diseases: New Advances 2016 meeting, held by Global Academy for Medical Education and Rutgers, the State University of New Jersey, Dr. Zobair M. Younossi, chairman of the department of medicine at Inova Fairfax (Va.) Hospital, discussed what clinicians should know in order to screen for and treat this disease.
Dr. Younossi said he is a consultant to Conatus Pharmaceuticals, Enterome Bioscience, and Gilead, and on the advisory boards of Janssen, Salix Pharmaceuticals, and Vertex Pharmaceuticals.
Global Academy and this news organization are owned by the same company.
On Twitter @whitneymcknight
PHILADELPHIA – Nonalcoholic fatty liver disease is on the rise worldwide, but many clinicians are unaware of how to recognize this potentially fatal condition, in part because it can be difficult to separate from common comorbidities such as obesity and diabetes.
In an interview at the Digestive Diseases: New Advances 2016 meeting, held by Global Academy for Medical Education and Rutgers, the State University of New Jersey, Dr. Zobair M. Younossi, chairman of the department of medicine at Inova Fairfax (Va.) Hospital, discussed what clinicians should know in order to screen for and treat this disease.
Dr. Younossi said he is a consultant to Conatus Pharmaceuticals, Enterome Bioscience, and Gilead, and on the advisory boards of Janssen, Salix Pharmaceuticals, and Vertex Pharmaceuticals.
Global Academy and this news organization are owned by the same company.
On Twitter @whitneymcknight
AT DIGESTIVE DISEASES: NEW ADVANCES 2016
Grim projections for hepatitis C disease burden in the U.S.
Although highly effective oral direct-acting antivirals (DAAs) provide clinicians with the opportunity to reduce the substantial disease burden associated with hepatitis C virus (HCV) infection in the United States, the promise of these agents cannot be realized without the expansion of HCV screening and treatment capacity, according to a report published online in Hepatology.
Working with his colleagues, Dr. Jagpreet Chhatwal of the Massachusetts General Hospital Institute for Technology Assessment and of the department of radiology at Harvard Medical School, both in Boston, utilized a validated projection model previously developed by this research team to estimate the numbers of people in the United States who will die, develop hepatocellular carcinoma, and develop decompensated cirrhosis over the next 35 years (Hepatology. 2016 Mar 25. doi: 10.1002/hep.28571).
The results of the model provided an estimate of 320,000 for the cumulative number of HCV-associated deaths in individuals treated with oral DAAs from 2015 to 2050. In addition, the projected cumulative incidence of hepatocellular carcinoma was 157,000, and the projected cumulative incidence of decompensated cirrhosis was 203,000 in individuals treated with oral DAAs from 2015 to 2050. Furthermore, the projected number of liver transplants for those on DAAs between 2015 and 2050 was 32,000.
When assessing the variables that most heavily influenced the projections, the authors said that most of the ongoing burden of HCV is related to the proportion of infected individuals who remain unaware of their infection status.
Despite such grim predictions, the research suggests hope remains, the authors said. With the same model, changing the rate of treatment from 150,000 patients per year in 2014 to 280,000 patients per year from 2015 onward would result in large reductions in the projected disease burden. For example, 8,600 cases of decompensated cirrhosis, 5,400 cases of hepatocellular carcinoma, 9,700 liver-related deaths, and 900 liver transplants would be prevented. These numbers would increase further if the annual treatment rate was increased to 500,000 patients per year from 2015 onward, preventing 12,000 cases of decompensated cirrhosis, 7,400 cases of hepatocellular carcinoma, 13,500 liver-related deaths, and 1,400 liver transplants. These model-based results emphasize the importance of expanding treatment capacity, as well as HCV screening efforts, the investigators said.
The results are important for the planning and distribution of health care resources and personnel in order to ensure that they match both current and future treatment demands, the investigators added. As an example, they highlighted their projection indicating that the number of clinicians and facilities offering HCV treatment would need to increase substantially over the next 3-4 years. Toward this end, they suggested that primary care physicians or infectious disease specialists be incorporated into HCV treatment capacity expansion.
This project was funded in part by the National Institutes of Health and Gilead Sciences. No conflicts of interest were declared.
Although highly effective oral direct-acting antivirals (DAAs) provide clinicians with the opportunity to reduce the substantial disease burden associated with hepatitis C virus (HCV) infection in the United States, the promise of these agents cannot be realized without the expansion of HCV screening and treatment capacity, according to a report published online in Hepatology.
Working with his colleagues, Dr. Jagpreet Chhatwal of the Massachusetts General Hospital Institute for Technology Assessment and of the department of radiology at Harvard Medical School, both in Boston, utilized a validated projection model previously developed by this research team to estimate the numbers of people in the United States who will die, develop hepatocellular carcinoma, and develop decompensated cirrhosis over the next 35 years (Hepatology. 2016 Mar 25. doi: 10.1002/hep.28571).
The results of the model provided an estimate of 320,000 for the cumulative number of HCV-associated deaths in individuals treated with oral DAAs from 2015 to 2050. In addition, the projected cumulative incidence of hepatocellular carcinoma was 157,000, and the projected cumulative incidence of decompensated cirrhosis was 203,000 in individuals treated with oral DAAs from 2015 to 2050. Furthermore, the projected number of liver transplants for those on DAAs between 2015 and 2050 was 32,000.
When assessing the variables that most heavily influenced the projections, the authors said that most of the ongoing burden of HCV is related to the proportion of infected individuals who remain unaware of their infection status.
Despite such grim predictions, the research suggests hope remains, the authors said. With the same model, changing the rate of treatment from 150,000 patients per year in 2014 to 280,000 patients per year from 2015 onward would result in large reductions in the projected disease burden. For example, 8,600 cases of decompensated cirrhosis, 5,400 cases of hepatocellular carcinoma, 9,700 liver-related deaths, and 900 liver transplants would be prevented. These numbers would increase further if the annual treatment rate was increased to 500,000 patients per year from 2015 onward, preventing 12,000 cases of decompensated cirrhosis, 7,400 cases of hepatocellular carcinoma, 13,500 liver-related deaths, and 1,400 liver transplants. These model-based results emphasize the importance of expanding treatment capacity, as well as HCV screening efforts, the investigators said.
The results are important for the planning and distribution of health care resources and personnel in order to ensure that they match both current and future treatment demands, the investigators added. As an example, they highlighted their projection indicating that the number of clinicians and facilities offering HCV treatment would need to increase substantially over the next 3-4 years. Toward this end, they suggested that primary care physicians or infectious disease specialists be incorporated into HCV treatment capacity expansion.
This project was funded in part by the National Institutes of Health and Gilead Sciences. No conflicts of interest were declared.
Although highly effective oral direct-acting antivirals (DAAs) provide clinicians with the opportunity to reduce the substantial disease burden associated with hepatitis C virus (HCV) infection in the United States, the promise of these agents cannot be realized without the expansion of HCV screening and treatment capacity, according to a report published online in Hepatology.
Working with his colleagues, Dr. Jagpreet Chhatwal of the Massachusetts General Hospital Institute for Technology Assessment and of the department of radiology at Harvard Medical School, both in Boston, utilized a validated projection model previously developed by this research team to estimate the numbers of people in the United States who will die, develop hepatocellular carcinoma, and develop decompensated cirrhosis over the next 35 years (Hepatology. 2016 Mar 25. doi: 10.1002/hep.28571).
The results of the model provided an estimate of 320,000 for the cumulative number of HCV-associated deaths in individuals treated with oral DAAs from 2015 to 2050. In addition, the projected cumulative incidence of hepatocellular carcinoma was 157,000, and the projected cumulative incidence of decompensated cirrhosis was 203,000 in individuals treated with oral DAAs from 2015 to 2050. Furthermore, the projected number of liver transplants for those on DAAs between 2015 and 2050 was 32,000.
When assessing the variables that most heavily influenced the projections, the authors said that most of the ongoing burden of HCV is related to the proportion of infected individuals who remain unaware of their infection status.
Despite such grim predictions, the research suggests hope remains, the authors said. With the same model, changing the rate of treatment from 150,000 patients per year in 2014 to 280,000 patients per year from 2015 onward would result in large reductions in the projected disease burden. For example, 8,600 cases of decompensated cirrhosis, 5,400 cases of hepatocellular carcinoma, 9,700 liver-related deaths, and 900 liver transplants would be prevented. These numbers would increase further if the annual treatment rate was increased to 500,000 patients per year from 2015 onward, preventing 12,000 cases of decompensated cirrhosis, 7,400 cases of hepatocellular carcinoma, 13,500 liver-related deaths, and 1,400 liver transplants. These model-based results emphasize the importance of expanding treatment capacity, as well as HCV screening efforts, the investigators said.
The results are important for the planning and distribution of health care resources and personnel in order to ensure that they match both current and future treatment demands, the investigators added. As an example, they highlighted their projection indicating that the number of clinicians and facilities offering HCV treatment would need to increase substantially over the next 3-4 years. Toward this end, they suggested that primary care physicians or infectious disease specialists be incorporated into HCV treatment capacity expansion.
This project was funded in part by the National Institutes of Health and Gilead Sciences. No conflicts of interest were declared.
FROM HEPATOLOGY
Key clinical point: Unless screening and treatment capacity for hepatitis C virus infection are expanded, associated disease burdens are projected to remain high, despite the availability of highly efficacious direct-acting antiviral agents.
Major Finding: Model-based projections suggest that hundreds of thousands of people will die, develop hepatocellular carcinoma, and develop decompensated cirrhosis in the United States by 2050.
Data Source: A validated hepatitis C disease burden simulation model previously developed and used to project the changing prevalence of hepatitis C virus in the United States.
Disclosures: This project was funded in part by the National Institutes of Health and Gilead Sciences. No conflicts of interest were declared.
Birth-cohort HCV testing misses one-quarter of infections
Birth-cohort screening for hepatitis C virus according to U.S. Centers for Disease Control and Prevention guidelines may miss around one-quarter of infections, researchers said.
An 8-week seroprevalence survey in an urban emergency department tested excess blood samples from 4,713 patients for hepatitis C virus, finding an overall prevalence of 13.8%, of which 31.3% was undocumented infection.
According to a paper published in Clinical Infectious Diseases, among the 204 patients with undocumented HCV infection, 48.5% were born between 1945 and 1965 and therefore would have been included in birth-cohort testing, and 26.5% would have been picked up for risk-based testing.
But 25% of the patients found to be infected with HCV in the study would not have been tested based on birth cohort or risk (Clin Infect Dis. 2016 Feb 21. doi: 10.1093/cid/ciw074).
The CDC added the recommendation for one-time testing of individuals born between 1945 and 1965 to its existing advice on risk-based screening in 2012, and this was backed by the U.S. Preventive Services Task Force in 2013.
“Since the CDC’s revised HIV testing recommendations for the health care settings were released, many EDs have had great success in implementing routine HIV testing to the population they serve over the past decade,” wrote Dr. Yu-Hsiang Hsieh of Johns Hopkins University, Baltimore, and coauthors. “This coupled with the availability of effective therapeutics makes EDs a key and strategic component of the national plan to expand HCV testing.”
At the same time, a second study, also in an urban emergency department, tested samples from 924 individuals enrolled in an HIV prevalence survey.
In this study, published in the same issue of Clinical Infectious Diseases, researchers found HCV antibodies in samples from 128 patients (14%); 34% of whom self-reported a history of HCV or hepatitis and 81% of whom were RNA positive.
The researchers noted, however, that, had they only implemented birth-cohort or risk-based screening, they would have missed 28% of individuals with antibodies and 25% of individuals with replicative HCV.
In this study, individuals with HCV infection were more likely to report injection drug use and high-risk sexual behavior, even among individuals reporting neither of these risk factors, but the prevalence of HCV infection was 7% (Clin Infect Dis. 2016 Feb 21. doi: 10.1093/cid/ciw073).
“We also cannot compare our results with the epidemiology of the surrounding population not using the ED, but suggest that as is the case with HIV, EDs are likely to provide a uniquely high level of access to populations with undiagnosed HCV who are in need of treatment,” wrote Dr. Michael S. Lyons and colleagues from the University of Cincinnati.
The authors, however, suggested that their survey may have underestimated the current prevalence of HCV because of an increase in heroin use in the area in more recent years.
Dr. Hsieh and colleagues suggested there was a need to revise the CDC recommendations and expand the age cut-off to all individuals aged 18 years or over.
The first study was supported by the National Institutes of Health and the authors declared no conflicts of interest. The second study was partly supported by Gilead Sciences, the National Institutes of Health, and Bristol-Myers Squibb. Four of the seven authors reported support, research grants, consultancies, or advisory board positions with pharmaceutical companies including Gilead and Bristol-Myers Squibb.
Birth-cohort screening for hepatitis C virus according to U.S. Centers for Disease Control and Prevention guidelines may miss around one-quarter of infections, researchers said.
An 8-week seroprevalence survey in an urban emergency department tested excess blood samples from 4,713 patients for hepatitis C virus, finding an overall prevalence of 13.8%, of which 31.3% was undocumented infection.
According to a paper published in Clinical Infectious Diseases, among the 204 patients with undocumented HCV infection, 48.5% were born between 1945 and 1965 and therefore would have been included in birth-cohort testing, and 26.5% would have been picked up for risk-based testing.
But 25% of the patients found to be infected with HCV in the study would not have been tested based on birth cohort or risk (Clin Infect Dis. 2016 Feb 21. doi: 10.1093/cid/ciw074).
The CDC added the recommendation for one-time testing of individuals born between 1945 and 1965 to its existing advice on risk-based screening in 2012, and this was backed by the U.S. Preventive Services Task Force in 2013.
“Since the CDC’s revised HIV testing recommendations for the health care settings were released, many EDs have had great success in implementing routine HIV testing to the population they serve over the past decade,” wrote Dr. Yu-Hsiang Hsieh of Johns Hopkins University, Baltimore, and coauthors. “This coupled with the availability of effective therapeutics makes EDs a key and strategic component of the national plan to expand HCV testing.”
At the same time, a second study, also in an urban emergency department, tested samples from 924 individuals enrolled in an HIV prevalence survey.
In this study, published in the same issue of Clinical Infectious Diseases, researchers found HCV antibodies in samples from 128 patients (14%); 34% of whom self-reported a history of HCV or hepatitis and 81% of whom were RNA positive.
The researchers noted, however, that, had they only implemented birth-cohort or risk-based screening, they would have missed 28% of individuals with antibodies and 25% of individuals with replicative HCV.
In this study, individuals with HCV infection were more likely to report injection drug use and high-risk sexual behavior, even among individuals reporting neither of these risk factors, but the prevalence of HCV infection was 7% (Clin Infect Dis. 2016 Feb 21. doi: 10.1093/cid/ciw073).
“We also cannot compare our results with the epidemiology of the surrounding population not using the ED, but suggest that as is the case with HIV, EDs are likely to provide a uniquely high level of access to populations with undiagnosed HCV who are in need of treatment,” wrote Dr. Michael S. Lyons and colleagues from the University of Cincinnati.
The authors, however, suggested that their survey may have underestimated the current prevalence of HCV because of an increase in heroin use in the area in more recent years.
Dr. Hsieh and colleagues suggested there was a need to revise the CDC recommendations and expand the age cut-off to all individuals aged 18 years or over.
The first study was supported by the National Institutes of Health and the authors declared no conflicts of interest. The second study was partly supported by Gilead Sciences, the National Institutes of Health, and Bristol-Myers Squibb. Four of the seven authors reported support, research grants, consultancies, or advisory board positions with pharmaceutical companies including Gilead and Bristol-Myers Squibb.
Birth-cohort screening for hepatitis C virus according to U.S. Centers for Disease Control and Prevention guidelines may miss around one-quarter of infections, researchers said.
An 8-week seroprevalence survey in an urban emergency department tested excess blood samples from 4,713 patients for hepatitis C virus, finding an overall prevalence of 13.8%, of which 31.3% was undocumented infection.
According to a paper published in Clinical Infectious Diseases, among the 204 patients with undocumented HCV infection, 48.5% were born between 1945 and 1965 and therefore would have been included in birth-cohort testing, and 26.5% would have been picked up for risk-based testing.
But 25% of the patients found to be infected with HCV in the study would not have been tested based on birth cohort or risk (Clin Infect Dis. 2016 Feb 21. doi: 10.1093/cid/ciw074).
The CDC added the recommendation for one-time testing of individuals born between 1945 and 1965 to its existing advice on risk-based screening in 2012, and this was backed by the U.S. Preventive Services Task Force in 2013.
“Since the CDC’s revised HIV testing recommendations for the health care settings were released, many EDs have had great success in implementing routine HIV testing to the population they serve over the past decade,” wrote Dr. Yu-Hsiang Hsieh of Johns Hopkins University, Baltimore, and coauthors. “This coupled with the availability of effective therapeutics makes EDs a key and strategic component of the national plan to expand HCV testing.”
At the same time, a second study, also in an urban emergency department, tested samples from 924 individuals enrolled in an HIV prevalence survey.
In this study, published in the same issue of Clinical Infectious Diseases, researchers found HCV antibodies in samples from 128 patients (14%); 34% of whom self-reported a history of HCV or hepatitis and 81% of whom were RNA positive.
The researchers noted, however, that, had they only implemented birth-cohort or risk-based screening, they would have missed 28% of individuals with antibodies and 25% of individuals with replicative HCV.
In this study, individuals with HCV infection were more likely to report injection drug use and high-risk sexual behavior, even among individuals reporting neither of these risk factors, but the prevalence of HCV infection was 7% (Clin Infect Dis. 2016 Feb 21. doi: 10.1093/cid/ciw073).
“We also cannot compare our results with the epidemiology of the surrounding population not using the ED, but suggest that as is the case with HIV, EDs are likely to provide a uniquely high level of access to populations with undiagnosed HCV who are in need of treatment,” wrote Dr. Michael S. Lyons and colleagues from the University of Cincinnati.
The authors, however, suggested that their survey may have underestimated the current prevalence of HCV because of an increase in heroin use in the area in more recent years.
Dr. Hsieh and colleagues suggested there was a need to revise the CDC recommendations and expand the age cut-off to all individuals aged 18 years or over.
The first study was supported by the National Institutes of Health and the authors declared no conflicts of interest. The second study was partly supported by Gilead Sciences, the National Institutes of Health, and Bristol-Myers Squibb. Four of the seven authors reported support, research grants, consultancies, or advisory board positions with pharmaceutical companies including Gilead and Bristol-Myers Squibb.
FROM CLINICAL INFECTIOUS DISEASES
Key clinical point: CDC recommendations for birth-cohort and high-risk screening for hepatitis C infection may miss one-quarter of infections.
Major finding: One in four patients with undocumented HCV infection would not otherwise have been tested based on birth cohort or risk.
Data source: A cross-sectional prevalence study involving 924 individuals and a seroprevalence study of 4,713 individuals.
Disclosures: The first study was supported by the National Institutes of Health and the authors declared no conflicts of interest. The second study was partly supported by Gilead Sciences, the National Institutes of Health, and Bristol-Myers Squibb. Four of the seven authors reported support, research grants, consultancies, or advisory board positions with pharmaceutical companies including Gilead and Bristol-Myers Squibb.
MRI topped transient elastography for staging nonalcoholic fatty liver disease
Two magnetic resonance imaging (MRI) techniques topped transient elastography (TE) for diagnosing hepatic fibrosis and steatosis in patients with nonalcoholic fatty liver disease (NAFLD), according to a first-in-kind study.
Magnetic resonance elastography surpassed all other methods for staging fibrosis, while MRI-based measurement of proton density fat fraction (PDFF) was superior for grading steatosis, with liver biopsy used as the comparative gold standard, said Dr. Kento Imajo at Yokohama (Japan) City University Graduate School of Medicine and his associates. “Magnetic resonance imaging–based noninvasive assessment of liver fibrosis and steatosis is a potential alternative to liver biopsy in clinical practice,” the investigators wrote in the March issue of Gastroenterology.
Assessing liver fibrosis and steatosis is important for staging NAFLD. Although “useful” overall, transient elastography can be unreliable in morbidly obese NAFLD patients or those with ascites because of low-frequency vibrations created by the probe, the researchers noted. To compare TE with MRI-based magnetic resonance elastography and PDFF, they evaluated 142 patients with biopsy-confirmed NAFLD and 10 controls, all of whom they also assessed with five clinical scoring systems for fibrosis – the FIB4 index, the NAFLD fibrosis score, the aspartate aminotransferase (AST) to platelet ratio, the AST-to-alanine transaminase (ALT) ratio, and the BARD score (Gastroenterology. 2015 Dec 8. doi: 10.1053/j.gastro.2015.11.048).
Magnetic resonance elastography detected stage 2 or higher hepatic fibrosis with an area under the receiver operating characteristic (AUROC) curve value of 0.91 (95% confidence interval, 0.86-0.96), compared with 0.82 (0.74-0.89) for transient elastography (P = .001), the investigators reported. The AUROC for MRE also significantly exceeded the AUROCs for all five clinical indexes of fibrosis severity. Furthermore, MRI-based measurement of PDFF identified hepatic steatosis of grade 2 or higher with an AUROC curve value of 0.90 (95% CI, 0.82-0.97), which was significantly greater than the AUROC obtained by using TE to measure the controlled attenuation parameter (0.73; 95% CI, 0.64-0.81; P less than .001).
Adding a measure for serum keratin 18 fragments or ALT did not significantly improve the detection of nonalcoholic steatohepatitis or macrovesicular steatosis affecting at least 5% of hepatocytes by either MRI or TE, the researchers noted. While liver biopsy remains the gold standard for assessing NAFLD, it is associated with sampling errors and intra- and interobserver variability, and these errors could have affected their study results, they acknowledged. The study also did not account for hepatic perfusion, which can elevate liver stiffness measurement independently from liver disease.
Both the magnetic resonance elastography and PDFF techniques require specialized hardware and software that are available from several commercial suppliers, the researchers also noted.
The study was partially supported by the Japanese Ministry of Health, Labor, and Welfare, the Japanese Science and Technology Agency, and Kiban-B, Shingakujuturyouiki. The investigators had no disclosures.
Two magnetic resonance imaging (MRI) techniques topped transient elastography (TE) for diagnosing hepatic fibrosis and steatosis in patients with nonalcoholic fatty liver disease (NAFLD), according to a first-in-kind study.
Magnetic resonance elastography surpassed all other methods for staging fibrosis, while MRI-based measurement of proton density fat fraction (PDFF) was superior for grading steatosis, with liver biopsy used as the comparative gold standard, said Dr. Kento Imajo at Yokohama (Japan) City University Graduate School of Medicine and his associates. “Magnetic resonance imaging–based noninvasive assessment of liver fibrosis and steatosis is a potential alternative to liver biopsy in clinical practice,” the investigators wrote in the March issue of Gastroenterology.
Assessing liver fibrosis and steatosis is important for staging NAFLD. Although “useful” overall, transient elastography can be unreliable in morbidly obese NAFLD patients or those with ascites because of low-frequency vibrations created by the probe, the researchers noted. To compare TE with MRI-based magnetic resonance elastography and PDFF, they evaluated 142 patients with biopsy-confirmed NAFLD and 10 controls, all of whom they also assessed with five clinical scoring systems for fibrosis – the FIB4 index, the NAFLD fibrosis score, the aspartate aminotransferase (AST) to platelet ratio, the AST-to-alanine transaminase (ALT) ratio, and the BARD score (Gastroenterology. 2015 Dec 8. doi: 10.1053/j.gastro.2015.11.048).
Magnetic resonance elastography detected stage 2 or higher hepatic fibrosis with an area under the receiver operating characteristic (AUROC) curve value of 0.91 (95% confidence interval, 0.86-0.96), compared with 0.82 (0.74-0.89) for transient elastography (P = .001), the investigators reported. The AUROC for MRE also significantly exceeded the AUROCs for all five clinical indexes of fibrosis severity. Furthermore, MRI-based measurement of PDFF identified hepatic steatosis of grade 2 or higher with an AUROC curve value of 0.90 (95% CI, 0.82-0.97), which was significantly greater than the AUROC obtained by using TE to measure the controlled attenuation parameter (0.73; 95% CI, 0.64-0.81; P less than .001).
Adding a measure for serum keratin 18 fragments or ALT did not significantly improve the detection of nonalcoholic steatohepatitis or macrovesicular steatosis affecting at least 5% of hepatocytes by either MRI or TE, the researchers noted. While liver biopsy remains the gold standard for assessing NAFLD, it is associated with sampling errors and intra- and interobserver variability, and these errors could have affected their study results, they acknowledged. The study also did not account for hepatic perfusion, which can elevate liver stiffness measurement independently from liver disease.
Both the magnetic resonance elastography and PDFF techniques require specialized hardware and software that are available from several commercial suppliers, the researchers also noted.
The study was partially supported by the Japanese Ministry of Health, Labor, and Welfare, the Japanese Science and Technology Agency, and Kiban-B, Shingakujuturyouiki. The investigators had no disclosures.
Two magnetic resonance imaging (MRI) techniques topped transient elastography (TE) for diagnosing hepatic fibrosis and steatosis in patients with nonalcoholic fatty liver disease (NAFLD), according to a first-in-kind study.
Magnetic resonance elastography surpassed all other methods for staging fibrosis, while MRI-based measurement of proton density fat fraction (PDFF) was superior for grading steatosis, with liver biopsy used as the comparative gold standard, said Dr. Kento Imajo at Yokohama (Japan) City University Graduate School of Medicine and his associates. “Magnetic resonance imaging–based noninvasive assessment of liver fibrosis and steatosis is a potential alternative to liver biopsy in clinical practice,” the investigators wrote in the March issue of Gastroenterology.
Assessing liver fibrosis and steatosis is important for staging NAFLD. Although “useful” overall, transient elastography can be unreliable in morbidly obese NAFLD patients or those with ascites because of low-frequency vibrations created by the probe, the researchers noted. To compare TE with MRI-based magnetic resonance elastography and PDFF, they evaluated 142 patients with biopsy-confirmed NAFLD and 10 controls, all of whom they also assessed with five clinical scoring systems for fibrosis – the FIB4 index, the NAFLD fibrosis score, the aspartate aminotransferase (AST) to platelet ratio, the AST-to-alanine transaminase (ALT) ratio, and the BARD score (Gastroenterology. 2015 Dec 8. doi: 10.1053/j.gastro.2015.11.048).
Magnetic resonance elastography detected stage 2 or higher hepatic fibrosis with an area under the receiver operating characteristic (AUROC) curve value of 0.91 (95% confidence interval, 0.86-0.96), compared with 0.82 (0.74-0.89) for transient elastography (P = .001), the investigators reported. The AUROC for MRE also significantly exceeded the AUROCs for all five clinical indexes of fibrosis severity. Furthermore, MRI-based measurement of PDFF identified hepatic steatosis of grade 2 or higher with an AUROC curve value of 0.90 (95% CI, 0.82-0.97), which was significantly greater than the AUROC obtained by using TE to measure the controlled attenuation parameter (0.73; 95% CI, 0.64-0.81; P less than .001).
Adding a measure for serum keratin 18 fragments or ALT did not significantly improve the detection of nonalcoholic steatohepatitis or macrovesicular steatosis affecting at least 5% of hepatocytes by either MRI or TE, the researchers noted. While liver biopsy remains the gold standard for assessing NAFLD, it is associated with sampling errors and intra- and interobserver variability, and these errors could have affected their study results, they acknowledged. The study also did not account for hepatic perfusion, which can elevate liver stiffness measurement independently from liver disease.
Both the magnetic resonance elastography and PDFF techniques require specialized hardware and software that are available from several commercial suppliers, the researchers also noted.
The study was partially supported by the Japanese Ministry of Health, Labor, and Welfare, the Japanese Science and Technology Agency, and Kiban-B, Shingakujuturyouiki. The investigators had no disclosures.
FROM GASTROENTEROLOGY
Key clinical point: Two specialized MRI techniques surpassed transient elastography for staging fibrosis and steatosis in nonalcoholic fatty liver disease.
Major finding: The areas under the curve for magnetic resonance elastography and the proton density fat fraction measure were significantly greater than those for transient elastography and the TE-based controlled attenuation parameter (P is less than .001 for both comparisons).
Data source: A cross-sectional study of 142 patients with nonalcoholic fatty liver disease and 10 controls.
Disclosures: The study was partially supported by the Japanese Ministry of Health, Labor, and Welfare, the Japanese Science and Technology Agency, and Kiban-B, Shingakujuturyouiki. The investigators had no disclosures.
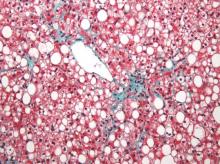
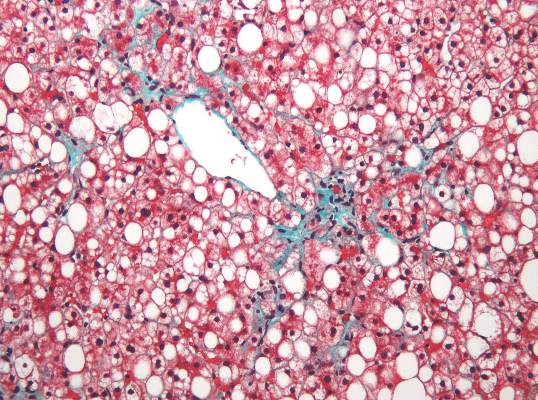
Portal inflammation in pediatric NAFLD linked to fibrosis
Portal inflammation in children with nonalcoholic fatty liver disease is associated with more than a threefold greater risk of more advanced fibrosis, according to a paper published in Hepatology.
A cross-sectional study in 430 children with nonalcoholic fatty liver disease – 12% with type 1 disease, 22% with type 2, and 66% with an overlap of both – found that the presence of portal inflammation was associated with a significant independent association with stage 2-4 fibrosis (odds ratio, 3.70; 95% confidence interval, 1.40-5.21; P = .003), after adjustment for age and sex.
Stage 2-4 fibrosis was associated with a greater incidence of steatosis (OR, 1.81; P less than .0001), lobular inflammation (OR, 1.40; P less than .0001) and a twofold greater incidence of ballooning (P less than .0001).
While children with type 2 or overlap nonalcoholic fatty liver disease typically had lower alanine aminotransferase, aspartate aminotransferase, and bilirubin scores than those with type 1, they had a higher adjusted body mass index and waist circumference, lower HDL cholesterol and higher triglycerides (Hepatology. March 2016;63:745-53).
“Our data highlight that patients with type 2 and overlap NAFLD, in particular portal inflammation and high BMI or waist circumference, may be at increased risk of hepatic or metabolic complications,” wrote Dr. Jake P. Mann from the University of Cambridge, England, and his coauthors, suggesting that an elevated waist circumference could serve as a noninvasive indicator of risk of portal inflammation and fibrosis.
No conflicts of interest were declared.
Portal inflammation in children with nonalcoholic fatty liver disease is associated with more than a threefold greater risk of more advanced fibrosis, according to a paper published in Hepatology.
A cross-sectional study in 430 children with nonalcoholic fatty liver disease – 12% with type 1 disease, 22% with type 2, and 66% with an overlap of both – found that the presence of portal inflammation was associated with a significant independent association with stage 2-4 fibrosis (odds ratio, 3.70; 95% confidence interval, 1.40-5.21; P = .003), after adjustment for age and sex.
Stage 2-4 fibrosis was associated with a greater incidence of steatosis (OR, 1.81; P less than .0001), lobular inflammation (OR, 1.40; P less than .0001) and a twofold greater incidence of ballooning (P less than .0001).
While children with type 2 or overlap nonalcoholic fatty liver disease typically had lower alanine aminotransferase, aspartate aminotransferase, and bilirubin scores than those with type 1, they had a higher adjusted body mass index and waist circumference, lower HDL cholesterol and higher triglycerides (Hepatology. March 2016;63:745-53).
“Our data highlight that patients with type 2 and overlap NAFLD, in particular portal inflammation and high BMI or waist circumference, may be at increased risk of hepatic or metabolic complications,” wrote Dr. Jake P. Mann from the University of Cambridge, England, and his coauthors, suggesting that an elevated waist circumference could serve as a noninvasive indicator of risk of portal inflammation and fibrosis.
No conflicts of interest were declared.
Portal inflammation in children with nonalcoholic fatty liver disease is associated with more than a threefold greater risk of more advanced fibrosis, according to a paper published in Hepatology.
A cross-sectional study in 430 children with nonalcoholic fatty liver disease – 12% with type 1 disease, 22% with type 2, and 66% with an overlap of both – found that the presence of portal inflammation was associated with a significant independent association with stage 2-4 fibrosis (odds ratio, 3.70; 95% confidence interval, 1.40-5.21; P = .003), after adjustment for age and sex.
Stage 2-4 fibrosis was associated with a greater incidence of steatosis (OR, 1.81; P less than .0001), lobular inflammation (OR, 1.40; P less than .0001) and a twofold greater incidence of ballooning (P less than .0001).
While children with type 2 or overlap nonalcoholic fatty liver disease typically had lower alanine aminotransferase, aspartate aminotransferase, and bilirubin scores than those with type 1, they had a higher adjusted body mass index and waist circumference, lower HDL cholesterol and higher triglycerides (Hepatology. March 2016;63:745-53).
“Our data highlight that patients with type 2 and overlap NAFLD, in particular portal inflammation and high BMI or waist circumference, may be at increased risk of hepatic or metabolic complications,” wrote Dr. Jake P. Mann from the University of Cambridge, England, and his coauthors, suggesting that an elevated waist circumference could serve as a noninvasive indicator of risk of portal inflammation and fibrosis.
No conflicts of interest were declared.
FROM HEPATOLOGY
Key clinical point: Portal inflammation in children with nonalcoholic fatty liver disease is associated with more advanced fibrosis and a worse metabolic phenotype.
Major finding: Portal inflammation in children with NAFLD is associated with a more than threefold greater risk of more advanced fibrosis.
Data source: A cross-sectional study in 430 children with NAFLD.
Disclosures: No conflicts of interest were declared.
Thrombocytopenia signals multiorgan system failure in acute liver failure
In patients with acute liver failure (ALF), decreasing platelet counts after hospital admission signaled systemic inflammation and a greater likelihood of systemic complications, such as high-grade hepatic encephalopathy, cardiovascular collapse, the need for liver transplant, and death, according to researchers.
Patients with systemic inflammatory response syndrome (SIRS) had significantly lower platelet counts at admission, compared with those without SIRS, and their platelet counts decreased dramatically (from 182 plus or minus 27 times 109/L on admission to 103 plus or minus 3.20 times 109/L on day 6) compared with stable platelet counts in patients without SIRS. For days 2-7 postadmission, lower platelet counts were associated with high-grade hepatic encephalopathy, as well as the need for vasopressor support and renal replacement therapy (P less than or equal to .001 for all).
“We hypothesize that the decrease in platelet count represents an integral event in the pathogenesis of the ALF syndrome rather than a nonspecific marker of suppressed bone marrow production. Further studies will be needed to prove the pivotal role of platelets in mediating the proinflammatory and prothrombotic features of the ALF syndrome,” wrote Dr. R. Todd Stravitz, professor of medicine, section of hepatology, Virginia Commonwealth University, Richmond, and his colleagues (Clin Gastroenterol Hepatol. 2016 Feb 25. doi: 10.1016/j.cgh.2015.09.029).
Results showed that SIRS and multiorgan system failure (MOSF) were more closely linked to a decrease in platelet counts than to an increase in the international normalized ratio (INR), a laboratory marker for liver injury. The INR was similar in patients with and without SIRS, in high-grade and low-grade hepatic encephalopathy, and in patients with and without requirements for vasopressor support and renal replacement therapy (indicators of MOSF). Given that both platelet counts and INR were associated with prognosis, the investigators suggest that these laboratory parameters may reflect different types of injury, with INR signaling primary liver injury and platelets reflecting the severity of systemic inflammation secondary to liver injury.
Platelet counts varied according to outcome on day 21. Spontaneous survivors had higher mean platelet counts than patients who underwent liver transplant, and patients who died had the lowest platelet counts. Platelet counts in spontaneous survivors decreased from day 1 to 2, then subsequently recovered; platelets decreased progressively in patients who underwent liver transplant or died. The INR trend over time according to 21-day outcome was similar to that of platelet counts.
The retrospective study evaluated data from 1598 patients who enrolled in the ALF Study Group from 1998 to 2012. The mean age was 41 years, 76% were Caucasian, and 70% were female. Nearly one-half of participants (47%) had ALF due to acetaminophen overdose, 85% had at least one positive element of SIRS on admission, 32% required vasopressors, 33% required renal replacement therapy, and 50% developed high-grade (3 or 4) hepatic encephalopathy. In total, 47% of patients recovered without liver transplant, 24% underwent liver transplant, and 32% died.
The mechanism underlying development of thrombocytopenia in patients with ALF is poorly understood. Previous findings by the investigators showed that platelet-derived, prothrombotic microparticles increased in proportion to SIRS severity, and concentration of microparticles increased in parallel with laboratory markers of poor outcome. Current results suggest the converse to be true as well: platelet counts decreased in proportion to the severity of SIRS, the development of MOSF and poor outcome at 21 days.
The researchers proposed that deficiencies in the number of platelets or in liver-derived, prohemostatic coagulation factors may be compensated by systemic inflammation. Increased levels of platelet-derived microparticles in patients with ALF may overcompensate for thrombocytopenia due to a nearly 40-fold higher prothrombotic potential in tissue factor–dependent assays, compared with healthy control populations.
The findings point to the importance of platelet count in signaling impending complications in patients with ALF.
Dr. Stravitz and his coauthors reported having no disclosures.
In patients with acute liver failure (ALF), decreasing platelet counts after hospital admission signaled systemic inflammation and a greater likelihood of systemic complications, such as high-grade hepatic encephalopathy, cardiovascular collapse, the need for liver transplant, and death, according to researchers.
Patients with systemic inflammatory response syndrome (SIRS) had significantly lower platelet counts at admission, compared with those without SIRS, and their platelet counts decreased dramatically (from 182 plus or minus 27 times 109/L on admission to 103 plus or minus 3.20 times 109/L on day 6) compared with stable platelet counts in patients without SIRS. For days 2-7 postadmission, lower platelet counts were associated with high-grade hepatic encephalopathy, as well as the need for vasopressor support and renal replacement therapy (P less than or equal to .001 for all).
“We hypothesize that the decrease in platelet count represents an integral event in the pathogenesis of the ALF syndrome rather than a nonspecific marker of suppressed bone marrow production. Further studies will be needed to prove the pivotal role of platelets in mediating the proinflammatory and prothrombotic features of the ALF syndrome,” wrote Dr. R. Todd Stravitz, professor of medicine, section of hepatology, Virginia Commonwealth University, Richmond, and his colleagues (Clin Gastroenterol Hepatol. 2016 Feb 25. doi: 10.1016/j.cgh.2015.09.029).
Results showed that SIRS and multiorgan system failure (MOSF) were more closely linked to a decrease in platelet counts than to an increase in the international normalized ratio (INR), a laboratory marker for liver injury. The INR was similar in patients with and without SIRS, in high-grade and low-grade hepatic encephalopathy, and in patients with and without requirements for vasopressor support and renal replacement therapy (indicators of MOSF). Given that both platelet counts and INR were associated with prognosis, the investigators suggest that these laboratory parameters may reflect different types of injury, with INR signaling primary liver injury and platelets reflecting the severity of systemic inflammation secondary to liver injury.
Platelet counts varied according to outcome on day 21. Spontaneous survivors had higher mean platelet counts than patients who underwent liver transplant, and patients who died had the lowest platelet counts. Platelet counts in spontaneous survivors decreased from day 1 to 2, then subsequently recovered; platelets decreased progressively in patients who underwent liver transplant or died. The INR trend over time according to 21-day outcome was similar to that of platelet counts.
The retrospective study evaluated data from 1598 patients who enrolled in the ALF Study Group from 1998 to 2012. The mean age was 41 years, 76% were Caucasian, and 70% were female. Nearly one-half of participants (47%) had ALF due to acetaminophen overdose, 85% had at least one positive element of SIRS on admission, 32% required vasopressors, 33% required renal replacement therapy, and 50% developed high-grade (3 or 4) hepatic encephalopathy. In total, 47% of patients recovered without liver transplant, 24% underwent liver transplant, and 32% died.
The mechanism underlying development of thrombocytopenia in patients with ALF is poorly understood. Previous findings by the investigators showed that platelet-derived, prothrombotic microparticles increased in proportion to SIRS severity, and concentration of microparticles increased in parallel with laboratory markers of poor outcome. Current results suggest the converse to be true as well: platelet counts decreased in proportion to the severity of SIRS, the development of MOSF and poor outcome at 21 days.
The researchers proposed that deficiencies in the number of platelets or in liver-derived, prohemostatic coagulation factors may be compensated by systemic inflammation. Increased levels of platelet-derived microparticles in patients with ALF may overcompensate for thrombocytopenia due to a nearly 40-fold higher prothrombotic potential in tissue factor–dependent assays, compared with healthy control populations.
The findings point to the importance of platelet count in signaling impending complications in patients with ALF.
Dr. Stravitz and his coauthors reported having no disclosures.
In patients with acute liver failure (ALF), decreasing platelet counts after hospital admission signaled systemic inflammation and a greater likelihood of systemic complications, such as high-grade hepatic encephalopathy, cardiovascular collapse, the need for liver transplant, and death, according to researchers.
Patients with systemic inflammatory response syndrome (SIRS) had significantly lower platelet counts at admission, compared with those without SIRS, and their platelet counts decreased dramatically (from 182 plus or minus 27 times 109/L on admission to 103 plus or minus 3.20 times 109/L on day 6) compared with stable platelet counts in patients without SIRS. For days 2-7 postadmission, lower platelet counts were associated with high-grade hepatic encephalopathy, as well as the need for vasopressor support and renal replacement therapy (P less than or equal to .001 for all).
“We hypothesize that the decrease in platelet count represents an integral event in the pathogenesis of the ALF syndrome rather than a nonspecific marker of suppressed bone marrow production. Further studies will be needed to prove the pivotal role of platelets in mediating the proinflammatory and prothrombotic features of the ALF syndrome,” wrote Dr. R. Todd Stravitz, professor of medicine, section of hepatology, Virginia Commonwealth University, Richmond, and his colleagues (Clin Gastroenterol Hepatol. 2016 Feb 25. doi: 10.1016/j.cgh.2015.09.029).
Results showed that SIRS and multiorgan system failure (MOSF) were more closely linked to a decrease in platelet counts than to an increase in the international normalized ratio (INR), a laboratory marker for liver injury. The INR was similar in patients with and without SIRS, in high-grade and low-grade hepatic encephalopathy, and in patients with and without requirements for vasopressor support and renal replacement therapy (indicators of MOSF). Given that both platelet counts and INR were associated with prognosis, the investigators suggest that these laboratory parameters may reflect different types of injury, with INR signaling primary liver injury and platelets reflecting the severity of systemic inflammation secondary to liver injury.
Platelet counts varied according to outcome on day 21. Spontaneous survivors had higher mean platelet counts than patients who underwent liver transplant, and patients who died had the lowest platelet counts. Platelet counts in spontaneous survivors decreased from day 1 to 2, then subsequently recovered; platelets decreased progressively in patients who underwent liver transplant or died. The INR trend over time according to 21-day outcome was similar to that of platelet counts.
The retrospective study evaluated data from 1598 patients who enrolled in the ALF Study Group from 1998 to 2012. The mean age was 41 years, 76% were Caucasian, and 70% were female. Nearly one-half of participants (47%) had ALF due to acetaminophen overdose, 85% had at least one positive element of SIRS on admission, 32% required vasopressors, 33% required renal replacement therapy, and 50% developed high-grade (3 or 4) hepatic encephalopathy. In total, 47% of patients recovered without liver transplant, 24% underwent liver transplant, and 32% died.
The mechanism underlying development of thrombocytopenia in patients with ALF is poorly understood. Previous findings by the investigators showed that platelet-derived, prothrombotic microparticles increased in proportion to SIRS severity, and concentration of microparticles increased in parallel with laboratory markers of poor outcome. Current results suggest the converse to be true as well: platelet counts decreased in proportion to the severity of SIRS, the development of MOSF and poor outcome at 21 days.
The researchers proposed that deficiencies in the number of platelets or in liver-derived, prohemostatic coagulation factors may be compensated by systemic inflammation. Increased levels of platelet-derived microparticles in patients with ALF may overcompensate for thrombocytopenia due to a nearly 40-fold higher prothrombotic potential in tissue factor–dependent assays, compared with healthy control populations.
The findings point to the importance of platelet count in signaling impending complications in patients with ALF.
Dr. Stravitz and his coauthors reported having no disclosures.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: In patients with acute liver failure (ALF), decreasing platelet counts were associated with systemic inflammation and greater likelihood of serious systemic complications.
Major finding: Platelet counts decreased dramatically in patients with systemic inflammatory response syndrome (SIRS) (182 plus or minus 27 times 109/L on admission to 103 plus or minus 3.20 times 109/L on day 6) but remained stable in patients without SIRS; lower platelet counts were associated with high-grade hepatic encephalopathy, as well as the need for vasopressor support and renal replacement therapy (P less than or equal to .001 for all).
Data source: From 1998 to 2012 the ALF Study Group included 1,598 patients of mean age 41 years; 76% were Caucasian and 70% were female.
Disclosures: Dr. Stravitz and his coauthors reported having no disclosures.
Lysolipid antigens prominent in MGUS and myeloma
Clonal immunoglobulin reactive to lyso-glucosylceramide (LGL1), which is elevated in Gaucher disease, was found in patients with Gaucher disease as well as in one third of patients with sporadic monoclonal gammopathies.
Antigen-specific immunoblots showed that 17 of 20 patients with Gaucher disease had clonal immunoglobulin against LGL1, researchers reported (N Engl J Med. 2016 Feb 10).
Analysis of immunoglobulin gene mutations has provided evidence for antigen-driven clonal expansion of plasma cells in multiple myeloma and monoclonal gammopathy of undetermined significance (MGUS), but the underlying antigens remained unknown. Myeloma risk is increased with lipid disorders such as Gaucher disease and obesity, suggesting lipid involvement in pathogenesis.
“These studies set the stage for newer approaches to lower the levels of these lipids in patients with Gaucher disease and others with precursors for myeloma. Potentially, this could be achieved with drugs or lifestyle changes to reduce the levels of lipids to lower the risk of cancer,” senior author Dr. Madhav Dhodapkar, chief of hematology at Yale Cancer Center, New Haven, Conn., said in a press release.
All six mouse models with Gaucher-like disease had clonal immunoglobulin against LGL1. Gaucher disease–associated gammopathy in mouse models can be targeted by the reduction of the underlying antigen. Feeding eliglustat to the mice with clonal immunoglobulins reduced anti-LGL1 antibodies detected by immunoblot and reduced clonal immunoglobulin in vivo.
Dysregulated lipids are sometimes present in sporadic myeloma, and M spikes on LGL1-specific blotting were LGL1-reactive in 22 of 66 patients (33%). These clonal immunoglobulins also were cross reactive to lysophosphatidylcholine. Patients with polyclonal gammopathies not associated with Gaucher disease did not react to the lysolipids.
“Understanding the antigenic reactivity of clonal immunoglobulin not only has direct implications for antigenic origins of myeloma but also may lead to new strategies to prevent or treat clinical cancer by targeting the underlying antigen,” wrote Shiny Nair, Ph.D., of Yale University and colleagues.
Clonal immunoglobulin reactive to lyso-glucosylceramide (LGL1), which is elevated in Gaucher disease, was found in patients with Gaucher disease as well as in one third of patients with sporadic monoclonal gammopathies.
Antigen-specific immunoblots showed that 17 of 20 patients with Gaucher disease had clonal immunoglobulin against LGL1, researchers reported (N Engl J Med. 2016 Feb 10).
Analysis of immunoglobulin gene mutations has provided evidence for antigen-driven clonal expansion of plasma cells in multiple myeloma and monoclonal gammopathy of undetermined significance (MGUS), but the underlying antigens remained unknown. Myeloma risk is increased with lipid disorders such as Gaucher disease and obesity, suggesting lipid involvement in pathogenesis.
“These studies set the stage for newer approaches to lower the levels of these lipids in patients with Gaucher disease and others with precursors for myeloma. Potentially, this could be achieved with drugs or lifestyle changes to reduce the levels of lipids to lower the risk of cancer,” senior author Dr. Madhav Dhodapkar, chief of hematology at Yale Cancer Center, New Haven, Conn., said in a press release.
All six mouse models with Gaucher-like disease had clonal immunoglobulin against LGL1. Gaucher disease–associated gammopathy in mouse models can be targeted by the reduction of the underlying antigen. Feeding eliglustat to the mice with clonal immunoglobulins reduced anti-LGL1 antibodies detected by immunoblot and reduced clonal immunoglobulin in vivo.
Dysregulated lipids are sometimes present in sporadic myeloma, and M spikes on LGL1-specific blotting were LGL1-reactive in 22 of 66 patients (33%). These clonal immunoglobulins also were cross reactive to lysophosphatidylcholine. Patients with polyclonal gammopathies not associated with Gaucher disease did not react to the lysolipids.
“Understanding the antigenic reactivity of clonal immunoglobulin not only has direct implications for antigenic origins of myeloma but also may lead to new strategies to prevent or treat clinical cancer by targeting the underlying antigen,” wrote Shiny Nair, Ph.D., of Yale University and colleagues.
Clonal immunoglobulin reactive to lyso-glucosylceramide (LGL1), which is elevated in Gaucher disease, was found in patients with Gaucher disease as well as in one third of patients with sporadic monoclonal gammopathies.
Antigen-specific immunoblots showed that 17 of 20 patients with Gaucher disease had clonal immunoglobulin against LGL1, researchers reported (N Engl J Med. 2016 Feb 10).
Analysis of immunoglobulin gene mutations has provided evidence for antigen-driven clonal expansion of plasma cells in multiple myeloma and monoclonal gammopathy of undetermined significance (MGUS), but the underlying antigens remained unknown. Myeloma risk is increased with lipid disorders such as Gaucher disease and obesity, suggesting lipid involvement in pathogenesis.
“These studies set the stage for newer approaches to lower the levels of these lipids in patients with Gaucher disease and others with precursors for myeloma. Potentially, this could be achieved with drugs or lifestyle changes to reduce the levels of lipids to lower the risk of cancer,” senior author Dr. Madhav Dhodapkar, chief of hematology at Yale Cancer Center, New Haven, Conn., said in a press release.
All six mouse models with Gaucher-like disease had clonal immunoglobulin against LGL1. Gaucher disease–associated gammopathy in mouse models can be targeted by the reduction of the underlying antigen. Feeding eliglustat to the mice with clonal immunoglobulins reduced anti-LGL1 antibodies detected by immunoblot and reduced clonal immunoglobulin in vivo.
Dysregulated lipids are sometimes present in sporadic myeloma, and M spikes on LGL1-specific blotting were LGL1-reactive in 22 of 66 patients (33%). These clonal immunoglobulins also were cross reactive to lysophosphatidylcholine. Patients with polyclonal gammopathies not associated with Gaucher disease did not react to the lysolipids.
“Understanding the antigenic reactivity of clonal immunoglobulin not only has direct implications for antigenic origins of myeloma but also may lead to new strategies to prevent or treat clinical cancer by targeting the underlying antigen,” wrote Shiny Nair, Ph.D., of Yale University and colleagues.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: In patients with Gaucher disease and in nearly one-third of patients with MGUS or myeloma, clonal immunoglobulins reacted with lysolipid antigens.
Major finding: Clonal immunoglobulin from 17 of 20 patients with Gaucher disease and from 22 of 66 patients with sporadic myeloma were reactive to lyso-glucosylceramide.
Data source: Peripheral blood or bone marrow samples from 25 healthy donors, 20 patients with Gaucher disease, and 66 patients with sporadic monoclonal gammopathy.
Disclosures: Dr. Nair reported having no disclosures. One of her coauthors reported ties to industry.
Hepatitis B vaccine protection lasts 30 years
Ninety percent of patients in a 1981 hepatitis B vaccine trial still had evidence of immune protection 30 years later, according to a study in the Journal of Infectious Diseases.
The duration of protection after vaccination with hepatitis B virus vaccine is not well understood, although HBV vaccines have been effective at preventing infection, said authors of a new study led by Dr. Michael Bruce of the division of preparedness and emerging infections at the Centers for Disease Control and Prevention. To better assess the duration of protection offered by the plasma-derived hepatitis B vaccine, investigators performed a follow-up study of 1,578 Alaska Native adults and children aged at least 6 months who had been vaccinated in 1981 with three doses of the HBV vaccine.
Dr. Bruce and his colleagues recruited 243 members of the original cohort who responded to the original primary vaccine series but received no subsequent doses during the 30-year period. The researchers tested cohort members for antibody to hepatitis B surface antigen (anti-HBs) levels. Those with levels less than 10 mIU/mL received one booster dose of recombinant hepatitis B vaccine 2-4 weeks later and were then evaluated on the basis of anti-HBs measurements 30 days after the booster.
Of the patients tested, 125 (51%) had anti-HBs levels greater than or equal to 10 mIU/mL. Among participants with anti-HBs levels below 10 mIU/mL who were available for follow-up, 75 of 85 (88%) responded to a booster dose with an anti-HBs level greater than or equal to 10 mIU/mL at 30 days.
Dr. Bruce and his colleagues concluded that, based on anti-HBs level greater than or equal to 10 mIU/mL at 30 years and an 88% booster dose response, at least 90% of participants had evidence of protection 30 years later, and thus HBV vaccine booster doses are not needed for persons 30 years out from a primary HBV vaccine series.
Read the full study in the Journal of Infectious Diseases (J Infect Dis. 2016 Jan 21. doi: 10.1093/infdis/jiv748).
On Twitter @richpizzi
Ninety percent of patients in a 1981 hepatitis B vaccine trial still had evidence of immune protection 30 years later, according to a study in the Journal of Infectious Diseases.
The duration of protection after vaccination with hepatitis B virus vaccine is not well understood, although HBV vaccines have been effective at preventing infection, said authors of a new study led by Dr. Michael Bruce of the division of preparedness and emerging infections at the Centers for Disease Control and Prevention. To better assess the duration of protection offered by the plasma-derived hepatitis B vaccine, investigators performed a follow-up study of 1,578 Alaska Native adults and children aged at least 6 months who had been vaccinated in 1981 with three doses of the HBV vaccine.
Dr. Bruce and his colleagues recruited 243 members of the original cohort who responded to the original primary vaccine series but received no subsequent doses during the 30-year period. The researchers tested cohort members for antibody to hepatitis B surface antigen (anti-HBs) levels. Those with levels less than 10 mIU/mL received one booster dose of recombinant hepatitis B vaccine 2-4 weeks later and were then evaluated on the basis of anti-HBs measurements 30 days after the booster.
Of the patients tested, 125 (51%) had anti-HBs levels greater than or equal to 10 mIU/mL. Among participants with anti-HBs levels below 10 mIU/mL who were available for follow-up, 75 of 85 (88%) responded to a booster dose with an anti-HBs level greater than or equal to 10 mIU/mL at 30 days.
Dr. Bruce and his colleagues concluded that, based on anti-HBs level greater than or equal to 10 mIU/mL at 30 years and an 88% booster dose response, at least 90% of participants had evidence of protection 30 years later, and thus HBV vaccine booster doses are not needed for persons 30 years out from a primary HBV vaccine series.
Read the full study in the Journal of Infectious Diseases (J Infect Dis. 2016 Jan 21. doi: 10.1093/infdis/jiv748).
On Twitter @richpizzi
Ninety percent of patients in a 1981 hepatitis B vaccine trial still had evidence of immune protection 30 years later, according to a study in the Journal of Infectious Diseases.
The duration of protection after vaccination with hepatitis B virus vaccine is not well understood, although HBV vaccines have been effective at preventing infection, said authors of a new study led by Dr. Michael Bruce of the division of preparedness and emerging infections at the Centers for Disease Control and Prevention. To better assess the duration of protection offered by the plasma-derived hepatitis B vaccine, investigators performed a follow-up study of 1,578 Alaska Native adults and children aged at least 6 months who had been vaccinated in 1981 with three doses of the HBV vaccine.
Dr. Bruce and his colleagues recruited 243 members of the original cohort who responded to the original primary vaccine series but received no subsequent doses during the 30-year period. The researchers tested cohort members for antibody to hepatitis B surface antigen (anti-HBs) levels. Those with levels less than 10 mIU/mL received one booster dose of recombinant hepatitis B vaccine 2-4 weeks later and were then evaluated on the basis of anti-HBs measurements 30 days after the booster.
Of the patients tested, 125 (51%) had anti-HBs levels greater than or equal to 10 mIU/mL. Among participants with anti-HBs levels below 10 mIU/mL who were available for follow-up, 75 of 85 (88%) responded to a booster dose with an anti-HBs level greater than or equal to 10 mIU/mL at 30 days.
Dr. Bruce and his colleagues concluded that, based on anti-HBs level greater than or equal to 10 mIU/mL at 30 years and an 88% booster dose response, at least 90% of participants had evidence of protection 30 years later, and thus HBV vaccine booster doses are not needed for persons 30 years out from a primary HBV vaccine series.
Read the full study in the Journal of Infectious Diseases (J Infect Dis. 2016 Jan 21. doi: 10.1093/infdis/jiv748).
On Twitter @richpizzi
FROM THE JOURNAL OF INFECTIOUS DISEASES
Sovaldi topped Medicare part D spending in 2014
In its first year on the market, the hepatitis C virus drug Sovaldi soared to the top of the Medicare part D spending list, the Centers for Medicare & Medicaid Services reported.
With innovative oral HCV drugs getting at least partial credit for a big jump in U.S. health expenditures in 2014, total part D spending for Sovaldi (sofosbuvir) was more than $3.1 billion, close to half a billion more than second-place Nexium (esomeprazole), which had almost $2.7 billion in spending for the year. Next on the list was Crestor (rosuvastatin) at $2.54 billion, followed by Abilify (aripiprazole) at $2.53 billion and Advair Diskus (fluticasone/salmeterol) at $2.3 billion, according to the Medicare drug spending dashboard.
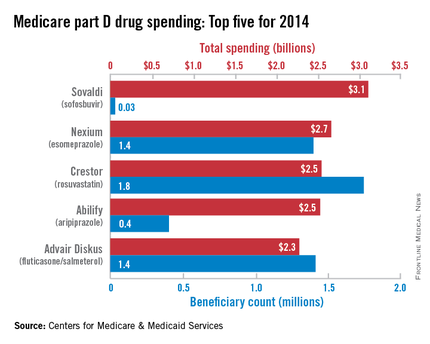
Sovaldi’s spot at the top, however, was not a result of its popularity. Compared with the other top five drugs, it was used by the fewest people (33,000) and had the highest average per-unit cost ($1,017). Crestor was most commonly prescribed among the five, with 1.7 million users in 2014. Advair Diskus was used by 1.42 million part D beneficiaries, putting it just ahead of Nexium, which had 1.4 million users. Abilify was well behind those three with 405,000 users, but it did have the second-highest per-unit cost, $28.65. The per-unit costs were similar for Crestor ($6.07), Advair Diskus ($4.94), and Nexium ($7.82), the CMS data show.
In 2014 overall, 3,761 different prescription drug products were covered by Medicare part D, with total program spending of $121.5 billion. There were 115 drugs with spending over $250 million for the year, and combined spending for those 115 drugs was $76.7 billion, or 63% of the part D total, the CMS said.
In its first year on the market, the hepatitis C virus drug Sovaldi soared to the top of the Medicare part D spending list, the Centers for Medicare & Medicaid Services reported.
With innovative oral HCV drugs getting at least partial credit for a big jump in U.S. health expenditures in 2014, total part D spending for Sovaldi (sofosbuvir) was more than $3.1 billion, close to half a billion more than second-place Nexium (esomeprazole), which had almost $2.7 billion in spending for the year. Next on the list was Crestor (rosuvastatin) at $2.54 billion, followed by Abilify (aripiprazole) at $2.53 billion and Advair Diskus (fluticasone/salmeterol) at $2.3 billion, according to the Medicare drug spending dashboard.

Sovaldi’s spot at the top, however, was not a result of its popularity. Compared with the other top five drugs, it was used by the fewest people (33,000) and had the highest average per-unit cost ($1,017). Crestor was most commonly prescribed among the five, with 1.7 million users in 2014. Advair Diskus was used by 1.42 million part D beneficiaries, putting it just ahead of Nexium, which had 1.4 million users. Abilify was well behind those three with 405,000 users, but it did have the second-highest per-unit cost, $28.65. The per-unit costs were similar for Crestor ($6.07), Advair Diskus ($4.94), and Nexium ($7.82), the CMS data show.
In 2014 overall, 3,761 different prescription drug products were covered by Medicare part D, with total program spending of $121.5 billion. There were 115 drugs with spending over $250 million for the year, and combined spending for those 115 drugs was $76.7 billion, or 63% of the part D total, the CMS said.
In its first year on the market, the hepatitis C virus drug Sovaldi soared to the top of the Medicare part D spending list, the Centers for Medicare & Medicaid Services reported.
With innovative oral HCV drugs getting at least partial credit for a big jump in U.S. health expenditures in 2014, total part D spending for Sovaldi (sofosbuvir) was more than $3.1 billion, close to half a billion more than second-place Nexium (esomeprazole), which had almost $2.7 billion in spending for the year. Next on the list was Crestor (rosuvastatin) at $2.54 billion, followed by Abilify (aripiprazole) at $2.53 billion and Advair Diskus (fluticasone/salmeterol) at $2.3 billion, according to the Medicare drug spending dashboard.

Sovaldi’s spot at the top, however, was not a result of its popularity. Compared with the other top five drugs, it was used by the fewest people (33,000) and had the highest average per-unit cost ($1,017). Crestor was most commonly prescribed among the five, with 1.7 million users in 2014. Advair Diskus was used by 1.42 million part D beneficiaries, putting it just ahead of Nexium, which had 1.4 million users. Abilify was well behind those three with 405,000 users, but it did have the second-highest per-unit cost, $28.65. The per-unit costs were similar for Crestor ($6.07), Advair Diskus ($4.94), and Nexium ($7.82), the CMS data show.
In 2014 overall, 3,761 different prescription drug products were covered by Medicare part D, with total program spending of $121.5 billion. There were 115 drugs with spending over $250 million for the year, and combined spending for those 115 drugs was $76.7 billion, or 63% of the part D total, the CMS said.