User login
Office Management of Melanoma Patients
Marc D. Brown, MD
As the incidence of melanoma continues to increase, so does the role of the dermatologist as both medical and surgical oncologist for these patients. The dermatologist holds a key role in all phases of care, including prevention, diagnosis, treatment, and follow-up. The dermatologist is best trained to complete a full and thorough skin examination and is best able to recognize a melanoma in its early stages of growth. Dermatologists have a unique opportunity to prevent melanoma through appropriate patient education concerning sun protection, self skin examinations, and the ABCDEs of melanoma recognition (ie, asymmetry, border irregularity, color variations, dimension and evolution). The dermatologist is well trained to obtain an appropriate full-thickness skin biopsy and is knowledgeable to interpret the pathologist report and understand the significance of the various histologic prognostic indexes. Most patients present with localized disease and with thinner Breslow depth and thus can be skillfully treated in an outpatient setting under local anesthesia by a dermatologist.
*For a PDF of the full article, click on the link to the left of this introduction.
Marc D. Brown, MD
As the incidence of melanoma continues to increase, so does the role of the dermatologist as both medical and surgical oncologist for these patients. The dermatologist holds a key role in all phases of care, including prevention, diagnosis, treatment, and follow-up. The dermatologist is best trained to complete a full and thorough skin examination and is best able to recognize a melanoma in its early stages of growth. Dermatologists have a unique opportunity to prevent melanoma through appropriate patient education concerning sun protection, self skin examinations, and the ABCDEs of melanoma recognition (ie, asymmetry, border irregularity, color variations, dimension and evolution). The dermatologist is well trained to obtain an appropriate full-thickness skin biopsy and is knowledgeable to interpret the pathologist report and understand the significance of the various histologic prognostic indexes. Most patients present with localized disease and with thinner Breslow depth and thus can be skillfully treated in an outpatient setting under local anesthesia by a dermatologist.
*For a PDF of the full article, click on the link to the left of this introduction.
Marc D. Brown, MD
As the incidence of melanoma continues to increase, so does the role of the dermatologist as both medical and surgical oncologist for these patients. The dermatologist holds a key role in all phases of care, including prevention, diagnosis, treatment, and follow-up. The dermatologist is best trained to complete a full and thorough skin examination and is best able to recognize a melanoma in its early stages of growth. Dermatologists have a unique opportunity to prevent melanoma through appropriate patient education concerning sun protection, self skin examinations, and the ABCDEs of melanoma recognition (ie, asymmetry, border irregularity, color variations, dimension and evolution). The dermatologist is well trained to obtain an appropriate full-thickness skin biopsy and is knowledgeable to interpret the pathologist report and understand the significance of the various histologic prognostic indexes. Most patients present with localized disease and with thinner Breslow depth and thus can be skillfully treated in an outpatient setting under local anesthesia by a dermatologist.
*For a PDF of the full article, click on the link to the left of this introduction.
Sentinel Node Biopsy for Melanoma: An Update After Two Decades of Experience
Merrick I. Ross, MD
When detected and treated early, melanoma has an excellent prognosis. Unfortunately, as the tumor invades deeper into tissue the risk of metastatic spread to regional lymph nodes and beyond increases and the prognosis worsens significantly. Therefore, accurately detecting any regional lymphatic metastasis would significantly aid in determining a patient’s prognosis and help guide his or her treatment plan. In 1991, Don Morton and colleagues presented new paradigm in diagnosing regional lymphatic involvement of tumors termed sentinel lymph node biopsy (SLNB). By mapping the regional lymph system around a tumor and tracing the lymphatic flow, a determination of the most likely lymph node or nodes the cancer will spread to first is made. Then, a limited biopsy of the most likely nodes is performed rather than a more-invasive removal of the entire local lymphatic chain. In 20 years that have followed, a great deal of information has been gained as to its accuracy, prognostic value, appropriate candidates, and its impact on regional disease control and survival. The SLNB has been shown to accurately stage regional lymph node basins in stage I and II melanoma patients with minimal morbidity. More sensitive histologic techniques are now being applied that may allow even greater accuracy in the staging of melanoma patients. Although specific percent risk thresholds are still in question, recommendation for SLNB when melanomas are 1 mm or thicker has gained wide acceptance. SLNB may also be appropriate for patients with melanomas that are between 0.76 and 1 mm thick and have ulceration, high mitotic rates, or reach a Clark level IV. Therefore, melanomas with IB or greater staging should be considered for SLNB.
*For a PDF of the full article, click on the link to the left of this introduction.
Merrick I. Ross, MD
When detected and treated early, melanoma has an excellent prognosis. Unfortunately, as the tumor invades deeper into tissue the risk of metastatic spread to regional lymph nodes and beyond increases and the prognosis worsens significantly. Therefore, accurately detecting any regional lymphatic metastasis would significantly aid in determining a patient’s prognosis and help guide his or her treatment plan. In 1991, Don Morton and colleagues presented new paradigm in diagnosing regional lymphatic involvement of tumors termed sentinel lymph node biopsy (SLNB). By mapping the regional lymph system around a tumor and tracing the lymphatic flow, a determination of the most likely lymph node or nodes the cancer will spread to first is made. Then, a limited biopsy of the most likely nodes is performed rather than a more-invasive removal of the entire local lymphatic chain. In 20 years that have followed, a great deal of information has been gained as to its accuracy, prognostic value, appropriate candidates, and its impact on regional disease control and survival. The SLNB has been shown to accurately stage regional lymph node basins in stage I and II melanoma patients with minimal morbidity. More sensitive histologic techniques are now being applied that may allow even greater accuracy in the staging of melanoma patients. Although specific percent risk thresholds are still in question, recommendation for SLNB when melanomas are 1 mm or thicker has gained wide acceptance. SLNB may also be appropriate for patients with melanomas that are between 0.76 and 1 mm thick and have ulceration, high mitotic rates, or reach a Clark level IV. Therefore, melanomas with IB or greater staging should be considered for SLNB.
*For a PDF of the full article, click on the link to the left of this introduction.
Merrick I. Ross, MD
When detected and treated early, melanoma has an excellent prognosis. Unfortunately, as the tumor invades deeper into tissue the risk of metastatic spread to regional lymph nodes and beyond increases and the prognosis worsens significantly. Therefore, accurately detecting any regional lymphatic metastasis would significantly aid in determining a patient’s prognosis and help guide his or her treatment plan. In 1991, Don Morton and colleagues presented new paradigm in diagnosing regional lymphatic involvement of tumors termed sentinel lymph node biopsy (SLNB). By mapping the regional lymph system around a tumor and tracing the lymphatic flow, a determination of the most likely lymph node or nodes the cancer will spread to first is made. Then, a limited biopsy of the most likely nodes is performed rather than a more-invasive removal of the entire local lymphatic chain. In 20 years that have followed, a great deal of information has been gained as to its accuracy, prognostic value, appropriate candidates, and its impact on regional disease control and survival. The SLNB has been shown to accurately stage regional lymph node basins in stage I and II melanoma patients with minimal morbidity. More sensitive histologic techniques are now being applied that may allow even greater accuracy in the staging of melanoma patients. Although specific percent risk thresholds are still in question, recommendation for SLNB when melanomas are 1 mm or thicker has gained wide acceptance. SLNB may also be appropriate for patients with melanomas that are between 0.76 and 1 mm thick and have ulceration, high mitotic rates, or reach a Clark level IV. Therefore, melanomas with IB or greater staging should be considered for SLNB.
*For a PDF of the full article, click on the link to the left of this introduction.
New Therapeutic Options in the Medical Management of Advanced Melanoma
Jose Lutzky, MD, FACP
During the past 3 decades, the incidence, morbidity, and mortality of malignant melanoma have increased dramatically. Advanced melanoma has remained a disease that is for the most part incurable and has challenged all therapeutic efforts to make a dent in its natural history. Recent advances in the understanding of the molecular alterations in melanoma and in the immunologic mechanisms playing a role in this malignancy have brought hope that significant progress can be achieved, as evidenced by early encouraging clinical data. This review will summarize these recent developments and their impact on current clinical practice.
*For a PDF of the full article, click on the link to the left of this introduction.
Jose Lutzky, MD, FACP
During the past 3 decades, the incidence, morbidity, and mortality of malignant melanoma have increased dramatically. Advanced melanoma has remained a disease that is for the most part incurable and has challenged all therapeutic efforts to make a dent in its natural history. Recent advances in the understanding of the molecular alterations in melanoma and in the immunologic mechanisms playing a role in this malignancy have brought hope that significant progress can be achieved, as evidenced by early encouraging clinical data. This review will summarize these recent developments and their impact on current clinical practice.
*For a PDF of the full article, click on the link to the left of this introduction.
Jose Lutzky, MD, FACP
During the past 3 decades, the incidence, morbidity, and mortality of malignant melanoma have increased dramatically. Advanced melanoma has remained a disease that is for the most part incurable and has challenged all therapeutic efforts to make a dent in its natural history. Recent advances in the understanding of the molecular alterations in melanoma and in the immunologic mechanisms playing a role in this malignancy have brought hope that significant progress can be achieved, as evidenced by early encouraging clinical data. This review will summarize these recent developments and their impact on current clinical practice.
*For a PDF of the full article, click on the link to the left of this introduction.
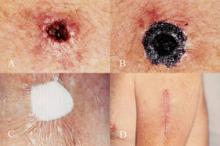
Managing Melanoma In Situ
Kristen L. Toren, MD, and Eric C. Parlette, MD
Melanoma is a highly aggressive skin cancer with an increasing incidence. Melanoma in situ is an early, non-invasive form in which the tumor is confined to the epidermis. Treatment of melanoma in situ is challenging due to the frequent subclinical microscopic spread and to the presentation on the head and neck in cosmetically sensitive areas with chronic sun damage. Optimizing tumor eradication is imperative to reduce the potential progression into invasive disease and metastasis, all while maintaining cosmesis. Multiple treatment regimens have been implemented for managing difficult melanoma in situ tumors. We provide a thorough review of surgical, and non-surgical, management of melanoma in situ which can pose therapeutic dilemmas due to size, anatomic location, and subclinical spread.
*For a PDF of the full article, click on the link to the left of this introduction.
Kristen L. Toren, MD, and Eric C. Parlette, MD
Melanoma is a highly aggressive skin cancer with an increasing incidence. Melanoma in situ is an early, non-invasive form in which the tumor is confined to the epidermis. Treatment of melanoma in situ is challenging due to the frequent subclinical microscopic spread and to the presentation on the head and neck in cosmetically sensitive areas with chronic sun damage. Optimizing tumor eradication is imperative to reduce the potential progression into invasive disease and metastasis, all while maintaining cosmesis. Multiple treatment regimens have been implemented for managing difficult melanoma in situ tumors. We provide a thorough review of surgical, and non-surgical, management of melanoma in situ which can pose therapeutic dilemmas due to size, anatomic location, and subclinical spread.
*For a PDF of the full article, click on the link to the left of this introduction.
Kristen L. Toren, MD, and Eric C. Parlette, MD
Melanoma is a highly aggressive skin cancer with an increasing incidence. Melanoma in situ is an early, non-invasive form in which the tumor is confined to the epidermis. Treatment of melanoma in situ is challenging due to the frequent subclinical microscopic spread and to the presentation on the head and neck in cosmetically sensitive areas with chronic sun damage. Optimizing tumor eradication is imperative to reduce the potential progression into invasive disease and metastasis, all while maintaining cosmesis. Multiple treatment regimens have been implemented for managing difficult melanoma in situ tumors. We provide a thorough review of surgical, and non-surgical, management of melanoma in situ which can pose therapeutic dilemmas due to size, anatomic location, and subclinical spread.
*For a PDF of the full article, click on the link to the left of this introduction.
Mohs Paste for Treating Melanoma: A Revival
SAN DIEGO - A remedy that has been used for nearly 2 centuries is still a valuable adjuvant therapy for melanoma, according to Dr. Norman Brooks.
Zinc chloride paste not only penetrates and kills even thick melanomas, it appears to stimulate a strong immune reaction that decreases the likelihood of metastasis and recurrence, Dr. Brooks said at the meeting sponsored by the American Society for Mohs Surgery.
"I believe chemosurgery with zinc chloride paste, followed by wide excision, is a better way to remove a melanoma than fresh tissue surgery," said Dr. Brooks, a dermatologist in Encino, Calif., who specializes in skin cancers. "It not only kills the tumor locally but stimulates total body resistance and immunity, supporting the idea that surgery actually enhances melanoma tumor growth and metastatic spread. That’s why I believe it's so important to use zinc chloride when treating melanoma."
History of Zinc Chloride
Zinc chloride was first used as a cancer treatment in 1815. In 1858, a U.S. physician added a powdered form of the bloodroot plant (Sanguinaria canadensis), which contains the anticancer alkaloid sanguinarine; the compound was used to treat breast cancer. The current paste, formulated by Dr. Frederic E. Mohs, is a compound of naturally occurring ingredients: the mineral stibnite, which forms the inert paste-like vehicle; powered root of the bloodroot plant, which has been shown to induce apoptosis in several types of cancer; and a saturated zinc chloride solution, which destructively penetrates tissue.
A 1997 study explored the idea that surgery can liberate atypical melanocytes and predispose a patient to metastatic disease, Dr. Brooks said (Dermatol. Surg. 1997;23:1043-6). The epidemiologic study of 1,224 primary melanomas concluded that surgery of primary melanoma may enhance tumor growth at metastatic sites.
Chemosurgery with zinc chloride paste, therefore, helps avoid this phenomenon, Dr. Brooks said. The paste not only destroys tissue where it is applied, it also fixes the tissue into a firm mass. A delayed excision allows the specimen to be removed en bloc and examined histopathologically to determine margins. However, leaving a portion of the fixed tissue in place for several more days causes a strong vaccine-like reaction on the skin surface, indicating, according to Dr. Brooks, the beginning of a systemic immunologic response.
The Evidence
According to Dr. Brooks, he and his colleagues published the only piece of objective evidence on this phenomenon in 1998 (Dermatol. Surg. 1998;24:1021-5).
The study involved mice that were injected with two different melanoma types: a poorly immunogenic (B16) type and a more immunogenic type (K1735p). The resultant tumors were freshly excised in some mice, or excised 24 hours after the application of zinc chloride paste. A week later, all the mice received a second injection of melanoma cells at a different site. K1735p tumors developed at the challenge sites in significantly fewer of the mice pretreated with zinc chloride (32% vs. 69%, respectively). The paste did not alter the development of B16 melanomas. The authors concluded: "Zinc chloride fixation of the more immunogenic K1735p melanoma increased resistance to subsequent tumor challenge, suggesting that zinc chloride fixative paste acts as an immune adjuvant."
Treatment Application
At the meeting, Dr. Brooks presented his most recent article describing a simplified method for using the paste (Dermatol. Surg. 2010;36:237-40).
His process begins with a fresh tissue biopsy to confirm melanoma. He recommended a shaved saucerized excision with a margin of 1-2 mm beyond the suspected tumor edge carried at least through the mid-dermis, or below the suspected thickness of the lesion.
After melanoma is histopathologically confirmed, he applies the Mohs paste. Since the compound will not penetrate an intact keratin layer, it must only be applied to the excised area. "If the biopsied area has developed a crust or eschar, this must also be removed," Dr. Brooks said.
He applies a 1-mm thick layer of paste with a cotton-tipped swab. More can be applied for thicker lesions, but less if the lesion overlies an important anatomic structure. If the biopsy is a small excisional one, a swab of 50% bi- or trichloracetic acid can remove the outer keratin layer and allow the paste to penetrate.
The next step is to apply a sterile cotton ball and cover the dressing with bio-occlusive tape for 24 hours – the time it takes the paste to achieve full penetration. After 24 hours, the patient can return for a conventional, wide excision with standard vertical or bevelled Mohs margins, followed by closure.
However, to take advantage of the paste's immunologic properties, the block of tissue should be left in place for a week or longer, or the main block can be removed, leaving a rim of the fixed tissue in place to stimulate the immune response. Dr. Brooks said the fixed tissue can then be histologically examined to confirm negative margins. For patients with raised lesions, he recommended debulking to create a flat tissue plane and they applying the paste as described.
He also stressed that the Mohs paste does not interfere in any way with conventional melanoma treatment. "About half of my patients end up going to [a cancer center] for excision, sentinel nodes, or other treatment," including interferon, he said. But some patients, after reviewing the comparatively small survival benefit interferon offers and its significant side effects, elect to have the fixed tissue excised and then continue with the practice of node palpation and regular ultrasounds to identify any early disease spread.
Dr. Brooks reported having no relevant financial disclosures.
"Mohs paste is a tried and true compound that does what it's purported to do," said Dr. Kenneth Gross.
"The first patient I [used the paste on] developed a sentinel node that was swollen and large, and I thought it was a metastatic, but it turned out to be clearly an immune response. On the flip side – I don't paste everyone. But if I have a high-risk patient who is well balanced and capable of understanding the implications, I do offer to paste them."
It is important to get informed consent and explain that zinc chloride is not an approved therapy; however, assure the patient it will not interfere with any other therapy they might want to pursue. Consider reserving it for high-risk patients, many of whom have melanoma that extends to the bone. "If you do your Mohs down to the periosteum and want to know if the bone is clear, you can paste it and a day or so later the bone will decalcify and a little chip will come out and you can examine that."
Mohs paste has fallen out of use over the years as fresh-tissue surgery has gained popularity. "Fresh-tissue replaced it because it allows immediate closure, while the paste causes a lot of inflammation and, the way Dr. Mohs did it, it left a wound that healed by secondary intention. It heals well but people won’t stand for that these days." Younger physicians, he said, "want to use what’s new, and what they were taught to use."
For many years, Dr. Mohs got the paste from just one pharmacist, who retired long ago. "Then there was just no reliable place to get the stuff. But recently we've been able to work with Delasco, [a medical supply company] that can compound it very reliably. It's cheap and a small amount will last virtually forever at room temperature."
Because Mohs paste is composed of natural ingredients and has been in use for so long, it is an unpatentable therapy. Therefore, it will probably always remain on the fringes of medicine.
"You will never find any large head-to-head studies with other therapies, and no pharmaceutical company is ever going to research it. It would cost millions of dollars to do the kind of studies you’d need to get approval and then what would you have at the end? Nothing you could make money off of. So it won't happen."
Aside from some slight discomfort and the inflammatory reaction, however, there are few down sides to using the paste. "If you look at it from a perspective of what can I do that won't hurt my patients, probably helps them, and won’t interfere with definitive, modern therapy, I would say Mohs paste is a shining example."
Kenneth Gross, M.D., was a course
director at the meeting, and is a surgical dermatologist in San Diego.
He reported having no relevant conflicts of interest.
"Mohs paste is a tried and true compound that does what it's purported to do," said Dr. Kenneth Gross.
"The first patient I [used the paste on] developed a sentinel node that was swollen and large, and I thought it was a metastatic, but it turned out to be clearly an immune response. On the flip side – I don't paste everyone. But if I have a high-risk patient who is well balanced and capable of understanding the implications, I do offer to paste them."
It is important to get informed consent and explain that zinc chloride is not an approved therapy; however, assure the patient it will not interfere with any other therapy they might want to pursue. Consider reserving it for high-risk patients, many of whom have melanoma that extends to the bone. "If you do your Mohs down to the periosteum and want to know if the bone is clear, you can paste it and a day or so later the bone will decalcify and a little chip will come out and you can examine that."
Mohs paste has fallen out of use over the years as fresh-tissue surgery has gained popularity. "Fresh-tissue replaced it because it allows immediate closure, while the paste causes a lot of inflammation and, the way Dr. Mohs did it, it left a wound that healed by secondary intention. It heals well but people won’t stand for that these days." Younger physicians, he said, "want to use what’s new, and what they were taught to use."
For many years, Dr. Mohs got the paste from just one pharmacist, who retired long ago. "Then there was just no reliable place to get the stuff. But recently we've been able to work with Delasco, [a medical supply company] that can compound it very reliably. It's cheap and a small amount will last virtually forever at room temperature."
Because Mohs paste is composed of natural ingredients and has been in use for so long, it is an unpatentable therapy. Therefore, it will probably always remain on the fringes of medicine.
"You will never find any large head-to-head studies with other therapies, and no pharmaceutical company is ever going to research it. It would cost millions of dollars to do the kind of studies you’d need to get approval and then what would you have at the end? Nothing you could make money off of. So it won't happen."
Aside from some slight discomfort and the inflammatory reaction, however, there are few down sides to using the paste. "If you look at it from a perspective of what can I do that won't hurt my patients, probably helps them, and won’t interfere with definitive, modern therapy, I would say Mohs paste is a shining example."
Kenneth Gross, M.D., was a course
director at the meeting, and is a surgical dermatologist in San Diego.
He reported having no relevant conflicts of interest.
"Mohs paste is a tried and true compound that does what it's purported to do," said Dr. Kenneth Gross.
"The first patient I [used the paste on] developed a sentinel node that was swollen and large, and I thought it was a metastatic, but it turned out to be clearly an immune response. On the flip side – I don't paste everyone. But if I have a high-risk patient who is well balanced and capable of understanding the implications, I do offer to paste them."
It is important to get informed consent and explain that zinc chloride is not an approved therapy; however, assure the patient it will not interfere with any other therapy they might want to pursue. Consider reserving it for high-risk patients, many of whom have melanoma that extends to the bone. "If you do your Mohs down to the periosteum and want to know if the bone is clear, you can paste it and a day or so later the bone will decalcify and a little chip will come out and you can examine that."
Mohs paste has fallen out of use over the years as fresh-tissue surgery has gained popularity. "Fresh-tissue replaced it because it allows immediate closure, while the paste causes a lot of inflammation and, the way Dr. Mohs did it, it left a wound that healed by secondary intention. It heals well but people won’t stand for that these days." Younger physicians, he said, "want to use what’s new, and what they were taught to use."
For many years, Dr. Mohs got the paste from just one pharmacist, who retired long ago. "Then there was just no reliable place to get the stuff. But recently we've been able to work with Delasco, [a medical supply company] that can compound it very reliably. It's cheap and a small amount will last virtually forever at room temperature."
Because Mohs paste is composed of natural ingredients and has been in use for so long, it is an unpatentable therapy. Therefore, it will probably always remain on the fringes of medicine.
"You will never find any large head-to-head studies with other therapies, and no pharmaceutical company is ever going to research it. It would cost millions of dollars to do the kind of studies you’d need to get approval and then what would you have at the end? Nothing you could make money off of. So it won't happen."
Aside from some slight discomfort and the inflammatory reaction, however, there are few down sides to using the paste. "If you look at it from a perspective of what can I do that won't hurt my patients, probably helps them, and won’t interfere with definitive, modern therapy, I would say Mohs paste is a shining example."
Kenneth Gross, M.D., was a course
director at the meeting, and is a surgical dermatologist in San Diego.
He reported having no relevant conflicts of interest.
SAN DIEGO - A remedy that has been used for nearly 2 centuries is still a valuable adjuvant therapy for melanoma, according to Dr. Norman Brooks.
Zinc chloride paste not only penetrates and kills even thick melanomas, it appears to stimulate a strong immune reaction that decreases the likelihood of metastasis and recurrence, Dr. Brooks said at the meeting sponsored by the American Society for Mohs Surgery.
"I believe chemosurgery with zinc chloride paste, followed by wide excision, is a better way to remove a melanoma than fresh tissue surgery," said Dr. Brooks, a dermatologist in Encino, Calif., who specializes in skin cancers. "It not only kills the tumor locally but stimulates total body resistance and immunity, supporting the idea that surgery actually enhances melanoma tumor growth and metastatic spread. That’s why I believe it's so important to use zinc chloride when treating melanoma."
History of Zinc Chloride
Zinc chloride was first used as a cancer treatment in 1815. In 1858, a U.S. physician added a powdered form of the bloodroot plant (Sanguinaria canadensis), which contains the anticancer alkaloid sanguinarine; the compound was used to treat breast cancer. The current paste, formulated by Dr. Frederic E. Mohs, is a compound of naturally occurring ingredients: the mineral stibnite, which forms the inert paste-like vehicle; powered root of the bloodroot plant, which has been shown to induce apoptosis in several types of cancer; and a saturated zinc chloride solution, which destructively penetrates tissue.
A 1997 study explored the idea that surgery can liberate atypical melanocytes and predispose a patient to metastatic disease, Dr. Brooks said (Dermatol. Surg. 1997;23:1043-6). The epidemiologic study of 1,224 primary melanomas concluded that surgery of primary melanoma may enhance tumor growth at metastatic sites.
Chemosurgery with zinc chloride paste, therefore, helps avoid this phenomenon, Dr. Brooks said. The paste not only destroys tissue where it is applied, it also fixes the tissue into a firm mass. A delayed excision allows the specimen to be removed en bloc and examined histopathologically to determine margins. However, leaving a portion of the fixed tissue in place for several more days causes a strong vaccine-like reaction on the skin surface, indicating, according to Dr. Brooks, the beginning of a systemic immunologic response.
The Evidence
According to Dr. Brooks, he and his colleagues published the only piece of objective evidence on this phenomenon in 1998 (Dermatol. Surg. 1998;24:1021-5).
The study involved mice that were injected with two different melanoma types: a poorly immunogenic (B16) type and a more immunogenic type (K1735p). The resultant tumors were freshly excised in some mice, or excised 24 hours after the application of zinc chloride paste. A week later, all the mice received a second injection of melanoma cells at a different site. K1735p tumors developed at the challenge sites in significantly fewer of the mice pretreated with zinc chloride (32% vs. 69%, respectively). The paste did not alter the development of B16 melanomas. The authors concluded: "Zinc chloride fixation of the more immunogenic K1735p melanoma increased resistance to subsequent tumor challenge, suggesting that zinc chloride fixative paste acts as an immune adjuvant."
Treatment Application
At the meeting, Dr. Brooks presented his most recent article describing a simplified method for using the paste (Dermatol. Surg. 2010;36:237-40).
His process begins with a fresh tissue biopsy to confirm melanoma. He recommended a shaved saucerized excision with a margin of 1-2 mm beyond the suspected tumor edge carried at least through the mid-dermis, or below the suspected thickness of the lesion.
After melanoma is histopathologically confirmed, he applies the Mohs paste. Since the compound will not penetrate an intact keratin layer, it must only be applied to the excised area. "If the biopsied area has developed a crust or eschar, this must also be removed," Dr. Brooks said.
He applies a 1-mm thick layer of paste with a cotton-tipped swab. More can be applied for thicker lesions, but less if the lesion overlies an important anatomic structure. If the biopsy is a small excisional one, a swab of 50% bi- or trichloracetic acid can remove the outer keratin layer and allow the paste to penetrate.
The next step is to apply a sterile cotton ball and cover the dressing with bio-occlusive tape for 24 hours – the time it takes the paste to achieve full penetration. After 24 hours, the patient can return for a conventional, wide excision with standard vertical or bevelled Mohs margins, followed by closure.
However, to take advantage of the paste's immunologic properties, the block of tissue should be left in place for a week or longer, or the main block can be removed, leaving a rim of the fixed tissue in place to stimulate the immune response. Dr. Brooks said the fixed tissue can then be histologically examined to confirm negative margins. For patients with raised lesions, he recommended debulking to create a flat tissue plane and they applying the paste as described.
He also stressed that the Mohs paste does not interfere in any way with conventional melanoma treatment. "About half of my patients end up going to [a cancer center] for excision, sentinel nodes, or other treatment," including interferon, he said. But some patients, after reviewing the comparatively small survival benefit interferon offers and its significant side effects, elect to have the fixed tissue excised and then continue with the practice of node palpation and regular ultrasounds to identify any early disease spread.
Dr. Brooks reported having no relevant financial disclosures.
SAN DIEGO - A remedy that has been used for nearly 2 centuries is still a valuable adjuvant therapy for melanoma, according to Dr. Norman Brooks.
Zinc chloride paste not only penetrates and kills even thick melanomas, it appears to stimulate a strong immune reaction that decreases the likelihood of metastasis and recurrence, Dr. Brooks said at the meeting sponsored by the American Society for Mohs Surgery.
"I believe chemosurgery with zinc chloride paste, followed by wide excision, is a better way to remove a melanoma than fresh tissue surgery," said Dr. Brooks, a dermatologist in Encino, Calif., who specializes in skin cancers. "It not only kills the tumor locally but stimulates total body resistance and immunity, supporting the idea that surgery actually enhances melanoma tumor growth and metastatic spread. That’s why I believe it's so important to use zinc chloride when treating melanoma."
History of Zinc Chloride
Zinc chloride was first used as a cancer treatment in 1815. In 1858, a U.S. physician added a powdered form of the bloodroot plant (Sanguinaria canadensis), which contains the anticancer alkaloid sanguinarine; the compound was used to treat breast cancer. The current paste, formulated by Dr. Frederic E. Mohs, is a compound of naturally occurring ingredients: the mineral stibnite, which forms the inert paste-like vehicle; powered root of the bloodroot plant, which has been shown to induce apoptosis in several types of cancer; and a saturated zinc chloride solution, which destructively penetrates tissue.
A 1997 study explored the idea that surgery can liberate atypical melanocytes and predispose a patient to metastatic disease, Dr. Brooks said (Dermatol. Surg. 1997;23:1043-6). The epidemiologic study of 1,224 primary melanomas concluded that surgery of primary melanoma may enhance tumor growth at metastatic sites.
Chemosurgery with zinc chloride paste, therefore, helps avoid this phenomenon, Dr. Brooks said. The paste not only destroys tissue where it is applied, it also fixes the tissue into a firm mass. A delayed excision allows the specimen to be removed en bloc and examined histopathologically to determine margins. However, leaving a portion of the fixed tissue in place for several more days causes a strong vaccine-like reaction on the skin surface, indicating, according to Dr. Brooks, the beginning of a systemic immunologic response.
The Evidence
According to Dr. Brooks, he and his colleagues published the only piece of objective evidence on this phenomenon in 1998 (Dermatol. Surg. 1998;24:1021-5).
The study involved mice that were injected with two different melanoma types: a poorly immunogenic (B16) type and a more immunogenic type (K1735p). The resultant tumors were freshly excised in some mice, or excised 24 hours after the application of zinc chloride paste. A week later, all the mice received a second injection of melanoma cells at a different site. K1735p tumors developed at the challenge sites in significantly fewer of the mice pretreated with zinc chloride (32% vs. 69%, respectively). The paste did not alter the development of B16 melanomas. The authors concluded: "Zinc chloride fixation of the more immunogenic K1735p melanoma increased resistance to subsequent tumor challenge, suggesting that zinc chloride fixative paste acts as an immune adjuvant."
Treatment Application
At the meeting, Dr. Brooks presented his most recent article describing a simplified method for using the paste (Dermatol. Surg. 2010;36:237-40).
His process begins with a fresh tissue biopsy to confirm melanoma. He recommended a shaved saucerized excision with a margin of 1-2 mm beyond the suspected tumor edge carried at least through the mid-dermis, or below the suspected thickness of the lesion.
After melanoma is histopathologically confirmed, he applies the Mohs paste. Since the compound will not penetrate an intact keratin layer, it must only be applied to the excised area. "If the biopsied area has developed a crust or eschar, this must also be removed," Dr. Brooks said.
He applies a 1-mm thick layer of paste with a cotton-tipped swab. More can be applied for thicker lesions, but less if the lesion overlies an important anatomic structure. If the biopsy is a small excisional one, a swab of 50% bi- or trichloracetic acid can remove the outer keratin layer and allow the paste to penetrate.
The next step is to apply a sterile cotton ball and cover the dressing with bio-occlusive tape for 24 hours – the time it takes the paste to achieve full penetration. After 24 hours, the patient can return for a conventional, wide excision with standard vertical or bevelled Mohs margins, followed by closure.
However, to take advantage of the paste's immunologic properties, the block of tissue should be left in place for a week or longer, or the main block can be removed, leaving a rim of the fixed tissue in place to stimulate the immune response. Dr. Brooks said the fixed tissue can then be histologically examined to confirm negative margins. For patients with raised lesions, he recommended debulking to create a flat tissue plane and they applying the paste as described.
He also stressed that the Mohs paste does not interfere in any way with conventional melanoma treatment. "About half of my patients end up going to [a cancer center] for excision, sentinel nodes, or other treatment," including interferon, he said. But some patients, after reviewing the comparatively small survival benefit interferon offers and its significant side effects, elect to have the fixed tissue excised and then continue with the practice of node palpation and regular ultrasounds to identify any early disease spread.
Dr. Brooks reported having no relevant financial disclosures.
EXPERT ANALYSIS FROM A MEETING SPONSORED BY THE AMERICAN SOCIETY FOR MOHS SURGERY
PV-10 Melanoma Drug Trial Enrolls Patients in Compassionate Use Program
Provectus Pharmaceuticals announced on Nov. 23 that it has enrolled at least 40 patients in its compassionate use program for PV-10, an experimental drug being studied primarily for melanoma.
PV-10 is an injectable form of Rose Bengal, a small molecule staining agent used to assess eye damage and liver ailments. Provectus determined that the drug selectively kills cancer cells and has been studying it in nonvisceral cancers.
Under the compassionate use program, patients who are not eligible for clinical trials and have certain breast cancers, basal cell carcinoma, squamous cell carcinoma, certain head and neck cancers, and melanoma can receive PV-10, the company announced.
Phase II studies of PV-10 in metastatic melanoma have just been completed, and 10 of the patients from the study joined the compassionate use program.
Patients in the program will have more frequent and extensive treatment over a longer duration than did those who received the drug in the phase II studies. The company hopes that the compassionate use program might help pinpoint a dosing regimen that can be used in a phase III trial in metastatic melanoma.
Provectus will also pursue the study of PV-10 for liver cancer.
Provectus Pharmaceuticals announced on Nov. 23 that it has enrolled at least 40 patients in its compassionate use program for PV-10, an experimental drug being studied primarily for melanoma.
PV-10 is an injectable form of Rose Bengal, a small molecule staining agent used to assess eye damage and liver ailments. Provectus determined that the drug selectively kills cancer cells and has been studying it in nonvisceral cancers.
Under the compassionate use program, patients who are not eligible for clinical trials and have certain breast cancers, basal cell carcinoma, squamous cell carcinoma, certain head and neck cancers, and melanoma can receive PV-10, the company announced.
Phase II studies of PV-10 in metastatic melanoma have just been completed, and 10 of the patients from the study joined the compassionate use program.
Patients in the program will have more frequent and extensive treatment over a longer duration than did those who received the drug in the phase II studies. The company hopes that the compassionate use program might help pinpoint a dosing regimen that can be used in a phase III trial in metastatic melanoma.
Provectus will also pursue the study of PV-10 for liver cancer.
Provectus Pharmaceuticals announced on Nov. 23 that it has enrolled at least 40 patients in its compassionate use program for PV-10, an experimental drug being studied primarily for melanoma.
PV-10 is an injectable form of Rose Bengal, a small molecule staining agent used to assess eye damage and liver ailments. Provectus determined that the drug selectively kills cancer cells and has been studying it in nonvisceral cancers.
Under the compassionate use program, patients who are not eligible for clinical trials and have certain breast cancers, basal cell carcinoma, squamous cell carcinoma, certain head and neck cancers, and melanoma can receive PV-10, the company announced.
Phase II studies of PV-10 in metastatic melanoma have just been completed, and 10 of the patients from the study joined the compassionate use program.
Patients in the program will have more frequent and extensive treatment over a longer duration than did those who received the drug in the phase II studies. The company hopes that the compassionate use program might help pinpoint a dosing regimen that can be used in a phase III trial in metastatic melanoma.
Provectus will also pursue the study of PV-10 for liver cancer.
FDA Panel Divided on MelaFind Device
Updated: 11/22/2010
COLLEGE PARK, MD. - A Food and Drug Administration advisory panel on Nov. 18 split on whether to support approval of a noninvasive device intended to help dermatologists and other physicians detect early melanomas.
At the meeting, the FDA's General and Plastic Surgery Devices Panel voted 8 to 7 with 1 abstention that the data available supported approval of the MelaFind device to evaluate clinically atypical cutaneous pigmented lesions that have one or more characteristics of melanoma, "when a physician chooses to obtain additional information before making a final decision to biopsy to rule out melanoma." That is the indication proposed for approval by the manufacturer, Mela Sciences Inc.
The first device of this kind, MelaFind is a multispectral computer vision system with a handheld component that captures the image of a lesion with a dermoscope through a thin layer of alcohol applied to the skin. The device has software that uses algorithms to analyze the image, indicating within 2 minutes whether a biopsy should be done, according to Mela Sciences. It is not intended to be used as a screening tool or in the evaluation of nonpigmented lesions; banal pigmented lesions; lesions considered definite melanomas; or mucosal, subungual and other lesions at different anatomic sites.
The device was used by dermatologists in a pivotal study of 1,257 patients (mean age 47 years) on 1,632 pigmented skin lesions that had at least one characteristic of melanoma, which were also biopsied. Of the 114 lesions that were positive for melanoma on biopsy, 112 were tagged as positive by MelaFind, for a sensitivity of 98.3%, which the company compared to the sensitivity of 70%-80% for dermatologists that has been reported in the literature. (Sensitivity for the dermatologists could not be determined because the decision to perform a biopsy had already been made by the clinician before referring to MelaFind.) Specificity was 9.5%.
Panelists voting for and against approval were concerned about the use of this device in the hands of nondermatologists, who, they said, might miss melanomas – a concern also raised by the FDA. But those voting in favor of approval said they believed with appropriate training, clinicians could learn to use the device effectively and that it would be a valuable tool. Concerns among those voting against approval included the need for more data, the risk of false negatives with the device, and issues with the data. It remained unclear how the results would guide decision-making.
Dermatologists on the panel voted on both sides of the approval question. "I would not mind having this in my practice," said Dr. Wilma Bergfeld, senior dermatologist and co-director of dermatopathology at the Cleveland Clinic, Cleveland, who voted in favor of approval. While clinical trial data and protocol were confusing, she said, "I believe that with the appropriate training and use, we might be able to use this instrument very effectively in the clinic to diagnose these equivocal clinical lesions."
Dr. Lynn Drake, lecturer in dermatology, Harvard Medical School, who voted against approval, said that while there is a need for such a device, "this falls short right now." Among her concerns were the need for more data, the high false-positive rate, and possible widespread use by clinicians not adequately trained once it becomes available. "We have to be very careful about approving something that might replace clinical judgement," she said.
FDA reviewers raised several concerns about the study, including possible selection bias, and concluded that the available data were inadequate to determine whether the use of MelaFind would add any true value to evaluating clinically atypical lesions – and that a prospective study was needed before approval.
The FDA usually follows the recommendations of its advisory panels. Panel members have been cleared of potential conflicts of interest prior to the meeting.
Updated: 11/22/2010
COLLEGE PARK, MD. - A Food and Drug Administration advisory panel on Nov. 18 split on whether to support approval of a noninvasive device intended to help dermatologists and other physicians detect early melanomas.
At the meeting, the FDA's General and Plastic Surgery Devices Panel voted 8 to 7 with 1 abstention that the data available supported approval of the MelaFind device to evaluate clinically atypical cutaneous pigmented lesions that have one or more characteristics of melanoma, "when a physician chooses to obtain additional information before making a final decision to biopsy to rule out melanoma." That is the indication proposed for approval by the manufacturer, Mela Sciences Inc.
The first device of this kind, MelaFind is a multispectral computer vision system with a handheld component that captures the image of a lesion with a dermoscope through a thin layer of alcohol applied to the skin. The device has software that uses algorithms to analyze the image, indicating within 2 minutes whether a biopsy should be done, according to Mela Sciences. It is not intended to be used as a screening tool or in the evaluation of nonpigmented lesions; banal pigmented lesions; lesions considered definite melanomas; or mucosal, subungual and other lesions at different anatomic sites.
The device was used by dermatologists in a pivotal study of 1,257 patients (mean age 47 years) on 1,632 pigmented skin lesions that had at least one characteristic of melanoma, which were also biopsied. Of the 114 lesions that were positive for melanoma on biopsy, 112 were tagged as positive by MelaFind, for a sensitivity of 98.3%, which the company compared to the sensitivity of 70%-80% for dermatologists that has been reported in the literature. (Sensitivity for the dermatologists could not be determined because the decision to perform a biopsy had already been made by the clinician before referring to MelaFind.) Specificity was 9.5%.
Panelists voting for and against approval were concerned about the use of this device in the hands of nondermatologists, who, they said, might miss melanomas – a concern also raised by the FDA. But those voting in favor of approval said they believed with appropriate training, clinicians could learn to use the device effectively and that it would be a valuable tool. Concerns among those voting against approval included the need for more data, the risk of false negatives with the device, and issues with the data. It remained unclear how the results would guide decision-making.
Dermatologists on the panel voted on both sides of the approval question. "I would not mind having this in my practice," said Dr. Wilma Bergfeld, senior dermatologist and co-director of dermatopathology at the Cleveland Clinic, Cleveland, who voted in favor of approval. While clinical trial data and protocol were confusing, she said, "I believe that with the appropriate training and use, we might be able to use this instrument very effectively in the clinic to diagnose these equivocal clinical lesions."
Dr. Lynn Drake, lecturer in dermatology, Harvard Medical School, who voted against approval, said that while there is a need for such a device, "this falls short right now." Among her concerns were the need for more data, the high false-positive rate, and possible widespread use by clinicians not adequately trained once it becomes available. "We have to be very careful about approving something that might replace clinical judgement," she said.
FDA reviewers raised several concerns about the study, including possible selection bias, and concluded that the available data were inadequate to determine whether the use of MelaFind would add any true value to evaluating clinically atypical lesions – and that a prospective study was needed before approval.
The FDA usually follows the recommendations of its advisory panels. Panel members have been cleared of potential conflicts of interest prior to the meeting.
Updated: 11/22/2010
COLLEGE PARK, MD. - A Food and Drug Administration advisory panel on Nov. 18 split on whether to support approval of a noninvasive device intended to help dermatologists and other physicians detect early melanomas.
At the meeting, the FDA's General and Plastic Surgery Devices Panel voted 8 to 7 with 1 abstention that the data available supported approval of the MelaFind device to evaluate clinically atypical cutaneous pigmented lesions that have one or more characteristics of melanoma, "when a physician chooses to obtain additional information before making a final decision to biopsy to rule out melanoma." That is the indication proposed for approval by the manufacturer, Mela Sciences Inc.
The first device of this kind, MelaFind is a multispectral computer vision system with a handheld component that captures the image of a lesion with a dermoscope through a thin layer of alcohol applied to the skin. The device has software that uses algorithms to analyze the image, indicating within 2 minutes whether a biopsy should be done, according to Mela Sciences. It is not intended to be used as a screening tool or in the evaluation of nonpigmented lesions; banal pigmented lesions; lesions considered definite melanomas; or mucosal, subungual and other lesions at different anatomic sites.
The device was used by dermatologists in a pivotal study of 1,257 patients (mean age 47 years) on 1,632 pigmented skin lesions that had at least one characteristic of melanoma, which were also biopsied. Of the 114 lesions that were positive for melanoma on biopsy, 112 were tagged as positive by MelaFind, for a sensitivity of 98.3%, which the company compared to the sensitivity of 70%-80% for dermatologists that has been reported in the literature. (Sensitivity for the dermatologists could not be determined because the decision to perform a biopsy had already been made by the clinician before referring to MelaFind.) Specificity was 9.5%.
Panelists voting for and against approval were concerned about the use of this device in the hands of nondermatologists, who, they said, might miss melanomas – a concern also raised by the FDA. But those voting in favor of approval said they believed with appropriate training, clinicians could learn to use the device effectively and that it would be a valuable tool. Concerns among those voting against approval included the need for more data, the risk of false negatives with the device, and issues with the data. It remained unclear how the results would guide decision-making.
Dermatologists on the panel voted on both sides of the approval question. "I would not mind having this in my practice," said Dr. Wilma Bergfeld, senior dermatologist and co-director of dermatopathology at the Cleveland Clinic, Cleveland, who voted in favor of approval. While clinical trial data and protocol were confusing, she said, "I believe that with the appropriate training and use, we might be able to use this instrument very effectively in the clinic to diagnose these equivocal clinical lesions."
Dr. Lynn Drake, lecturer in dermatology, Harvard Medical School, who voted against approval, said that while there is a need for such a device, "this falls short right now." Among her concerns were the need for more data, the high false-positive rate, and possible widespread use by clinicians not adequately trained once it becomes available. "We have to be very careful about approving something that might replace clinical judgement," she said.
FDA reviewers raised several concerns about the study, including possible selection bias, and concluded that the available data were inadequate to determine whether the use of MelaFind would add any true value to evaluating clinically atypical lesions – and that a prospective study was needed before approval.
The FDA usually follows the recommendations of its advisory panels. Panel members have been cleared of potential conflicts of interest prior to the meeting.
FROM A MEETING OF THE FDA'S GENERAL AND PLASTIC SURGERY DEVICES PANEL
FDA Committee Considers Gardasil for Anal Cancer Prevention
SILVER SPRING, Md. - The human papillomavirus vaccine Gardasil moved closer to an indication for anal cancer prevention after a meeting of the Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee on Nov. 17.
The vaccine, manufactured by Merck & Co. Inc., is approved for the prevention of cervical, vulvar, and vaginal cancers and precancerous lesions in females aged 9-26 years, and for the prevention of genital warts in males and females aged 9-26 years. The company is seeking an indication for the prevention of anal cancer in males and females aged 9-26 years.
Previous studies have shown that anal cancer and cervical cancer are biologically similar, and both are associated with HPV infections, Dr. Joel Palefsky, professor of medicine at the University of California, San Francisco, said at the meeting.
The primary data supporting an anal cancer indication for Gardasil came from a randomized, controlled trial of 602 men who have sex with men (MSM), who were part of the larger study that led to the indication for preventing genital warts in boys and men. The participants received three doses of vaccine or a placebo.
The vaccine showed 78% effectiveness, compared with placebo in preventing anal intraepithelial neoplasms related to human papillomavirus types 6, 11, 16, and 18. These lesions are considered precursors to anal cancer, Dr. Palefsky said.
Although MSM are at increased risk for HPV-associated anal cancer, they are not the only population at risk. Data from the National Cancer Institute presented at the meeting showed that anal cancer incidence in the United States is increasing by a rate of approximately 2% per year, and that approximately 60% of cases and deaths occur in women.
A majority of the committee members expressed opinions in favor of the indication, but no formal vote was taken. The FDA will consider the committee’s recommendations. If the indication is approved before the February 2011 meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices, the ACIP might revisit its previous recommendation against routine HPV vaccination for boys and men for genital wart prevention. ACIP has given a permissive recommendation for HPV vaccination for boys and men aged 9-26 years at the discretion of the physician.
Dr. Palefsky has served as a consultant and clinical investigator for Merck & Co. Inc.
Body text goes here
Doctor’s Bio
Body text goes here
Doctor’s Bio
Body text goes here
Doctor’s Bio
SILVER SPRING, Md. - The human papillomavirus vaccine Gardasil moved closer to an indication for anal cancer prevention after a meeting of the Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee on Nov. 17.
The vaccine, manufactured by Merck & Co. Inc., is approved for the prevention of cervical, vulvar, and vaginal cancers and precancerous lesions in females aged 9-26 years, and for the prevention of genital warts in males and females aged 9-26 years. The company is seeking an indication for the prevention of anal cancer in males and females aged 9-26 years.
Previous studies have shown that anal cancer and cervical cancer are biologically similar, and both are associated with HPV infections, Dr. Joel Palefsky, professor of medicine at the University of California, San Francisco, said at the meeting.
The primary data supporting an anal cancer indication for Gardasil came from a randomized, controlled trial of 602 men who have sex with men (MSM), who were part of the larger study that led to the indication for preventing genital warts in boys and men. The participants received three doses of vaccine or a placebo.
The vaccine showed 78% effectiveness, compared with placebo in preventing anal intraepithelial neoplasms related to human papillomavirus types 6, 11, 16, and 18. These lesions are considered precursors to anal cancer, Dr. Palefsky said.
Although MSM are at increased risk for HPV-associated anal cancer, they are not the only population at risk. Data from the National Cancer Institute presented at the meeting showed that anal cancer incidence in the United States is increasing by a rate of approximately 2% per year, and that approximately 60% of cases and deaths occur in women.
A majority of the committee members expressed opinions in favor of the indication, but no formal vote was taken. The FDA will consider the committee’s recommendations. If the indication is approved before the February 2011 meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices, the ACIP might revisit its previous recommendation against routine HPV vaccination for boys and men for genital wart prevention. ACIP has given a permissive recommendation for HPV vaccination for boys and men aged 9-26 years at the discretion of the physician.
Dr. Palefsky has served as a consultant and clinical investigator for Merck & Co. Inc.
SILVER SPRING, Md. - The human papillomavirus vaccine Gardasil moved closer to an indication for anal cancer prevention after a meeting of the Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee on Nov. 17.
The vaccine, manufactured by Merck & Co. Inc., is approved for the prevention of cervical, vulvar, and vaginal cancers and precancerous lesions in females aged 9-26 years, and for the prevention of genital warts in males and females aged 9-26 years. The company is seeking an indication for the prevention of anal cancer in males and females aged 9-26 years.
Previous studies have shown that anal cancer and cervical cancer are biologically similar, and both are associated with HPV infections, Dr. Joel Palefsky, professor of medicine at the University of California, San Francisco, said at the meeting.
The primary data supporting an anal cancer indication for Gardasil came from a randomized, controlled trial of 602 men who have sex with men (MSM), who were part of the larger study that led to the indication for preventing genital warts in boys and men. The participants received three doses of vaccine or a placebo.
The vaccine showed 78% effectiveness, compared with placebo in preventing anal intraepithelial neoplasms related to human papillomavirus types 6, 11, 16, and 18. These lesions are considered precursors to anal cancer, Dr. Palefsky said.
Although MSM are at increased risk for HPV-associated anal cancer, they are not the only population at risk. Data from the National Cancer Institute presented at the meeting showed that anal cancer incidence in the United States is increasing by a rate of approximately 2% per year, and that approximately 60% of cases and deaths occur in women.
A majority of the committee members expressed opinions in favor of the indication, but no formal vote was taken. The FDA will consider the committee’s recommendations. If the indication is approved before the February 2011 meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices, the ACIP might revisit its previous recommendation against routine HPV vaccination for boys and men for genital wart prevention. ACIP has given a permissive recommendation for HPV vaccination for boys and men aged 9-26 years at the discretion of the physician.
Dr. Palefsky has served as a consultant and clinical investigator for Merck & Co. Inc.
FROM A MEETING OF THE FOOD AND DRUG ADMINISTRATION’S VACCINE AND RELATED BIOLOGICAL PRODUCTS ADVISORY COMMITTEE
Can Bundled Cancer-Care Payments Save a Bundle?
A major insurance company is betting that oncology practices can handle cancer-care payments as if they were like the household grocery budget or a weekly allowance: Be frugal and you’ll have some left over for next time. Spend too much, and you’ll have to borrow from the future.
The idea of its pilot study, says UnitedHealthcare, based in Minnetonka, Minn., is to separate drug costs from treatment costs by paying oncologists an up-front, per-patient fee. (In insurance-speak, this is called a "bundled" or "episode" payment.) The payments will be based on best medical practices for three common malignancies: breast cancer, colon cancer, and lung cancer. Decisions about optimal treatment for each disease state will be determined by consensus among clinicians, and not by the insurer, the company said in a press release.
"By paying medical oncologists for a patient’s total cycle of treatment, rather than the number of visits and the amount of chemotherapy drugs given, this program promotes better, more patient-centric, evidence-based care with no loss of revenue for the physician," said Dr. Lee N. Newcomer, UnitedHealthcare’s senior vice president for oncology. "Everyone wins. As oncologists share best practices from the program about which treatment regimens are most effective, we expect to see consistently improved patient outcomes."
The company is working with five small- to mid-size oncology practices based in Dayton, Ohio; Fort Worth, Tex.; Kansas City, Mo; Marietta, Ga.; and Memphis. Analysis is to begin after a year’s data are collected.
"Over the course of the pilot, the various treatment regimens selected by the medical groups will be evaluated to identify which are the most effective for a range of clinical presentations [such as physical signs and symptoms and diagnoses]. UnitedHealthcare will play no role in determining which treatment plan the oncologists choose, but the intent of the pilot is to identify and reduce unnecessary drug administration that does not improve the patient’s health outcomes," the company stated.
Under this payment system, the insurer will determine its cancer-care payments based on the difference between drug costs and the current fee schedule of the oncology group, with payments made on the first day on which an insured patient receives care from that practice. The payments will include case-management fees to cover patient management costs.
Thus, if during the course of an individual patient’s care clinicians decide to switch to a more expensive drug, there would be no additional payments to compensate for the difference.
Each of the agreed-upon regimens in the pilot will be evaluated based on health outcomes and the frequency of adverse events, emergency department visits, and other consequences that can drive up costs without additional treatment benefit, the insurer says.
Worth a Look
The proposed payment model is an interesting approach to the goal of containing medical costs while fairly compensating clinicians and protecting high-quality patient care. But it also raises many questions about its applicability across a broad range of clinical and financial situations, say medical economists and oncologists who were interviewed for this article.
"We are pleased to see experimentation taking place around how oncology care is paid for," said Dr. Allen S. Lichter, CEO of the American Society of Clinical Oncology. "The oncology community needs to do these pilot projects, and understand whether moving away from the current models based on fee-for-service and some percentage of margin off the chemotherapy agents can be replaced by something else – and if so, what should that something else look like and how does it work?"
"I think it's an interesting experiment and an important one, one that Dr. Newcomer and United can run because of their large claims-based database, and we’re all interested to see how it works," agreed Dr. Samuel M. Silver, chair of the subcommittee on reimbursement for the American Society of Hematology and a professor of internal medicine at the University of Michigan in Ann Arbor.
Jack Hoadley, Ph.D., a health policy analyst and research professor at the Health Policy Institute at Georgetown University in Washington, D.C., noted that "Medicare has tried a number of experiments with various types of bundled payments, and it seems [as if] Medicare could either watch what’s going on in this private-sector pilot and use that as a way to learn, or – sooner than that – try bundled-payment structures to see how they work."
Outliers Could Be a Problem
One of the many questions that remain to be answered about the pilot project, however, is the problem of outliers (patients whose care doesn’t fit neatly within the prescribed protocol, such as those with significant comorbidities or adverse drug reactions that require a change in the treatment regimen).
"When one starts to deal with individual practices who have just a couple of these patients, one outlier can make it financially very difficult, so the question is, how often is that going to happen?" Dr. Silver said.
Dr. Hoadley said that the system needs to have flexibility to account for differences in the patient population. "Obviously, you’ve got to get that bundled amount right and appropriately adjusted for the average, across the kind of patients the practice is going to see, with some particular adjustments for patient severity," and other factors, he commented.
Where there are outliers, there are also inliers (patients who, for medical or other reasons, don’t undergo a full course of prescribed therapy), and in these cases, bundled payments would result in additional income for a practice, Dr. Lichter said. Over time, the outliers and inliers tend to balance out, and in the case of the extraordinary outlier – the patient who is admitted for a planned 2-day stay but ends up being hospitalized for 6 months – some sort of contingency payment would be made, he added.
"In talking to United Healthcare, while they didn’t sit down and write rules for every possible situation, I know that if a case is so far beyond the norm, they will sit down and look at it and agree to some type of remedial payment for it," he said.
Rare Cancers and Cherry Picking
A related issue of concern is how bundled payment systems would handle rare cancers, or clinical situations for which there is little or no consensus on optimal therapies, such as the use of chemotherapy for some soft-tissue sarcomas.
As vice-chair of the board of the National Comprehensive Cancer Network, Dr. Silver is an advocate of evidence-based guidelines, but said he’s aware that many patients have variations that don’t fit neatly into the standard chemotherapy guidelines that are acceptable under a bundled-payment system.
"Which brings us to another issue: Would there be cherry picking?" he asked. "Because it would be the patients who are young and healthy and can go through those therapies, and [who] don't have comorbidities and variations on their disease that would best fit into these bundled programs. So what happens to the others?"
In such cases, the burden of care for the more severely ill patients would fall on teaching hospitals, and it’s unclear whether they would be adequately compensated under a bundled-payment system, he said.
Stifle Drug Development?
Matt Farber, director of provider economics and public policy for the Association of Community Cancer Centers, said that bundled-payment systems could have a dampening effect on drug development. He points to sipuleucel-T (Provenge), the recently approved autologous immunotherapy vaccine for advanced prostate cancer that uses antigen-presenting cells unique to each patient.
"Would payment systems like this stop those drugs from being developed? Because if it’s a personalized treatment, would it therefore not be included in whatever benchmark is deemed the most appropriate or most effective care for most people?" Mr. Farber asked.
And what happens when novel drugs or new versions of conventional chemotherapeutic agents (such as palifosfamide, an active metabolite of ifosfamide) come on the market?
"They’re working right now with these five practices to determine what are the best courses of treatment currently for the disease states that they’re looking at. So when a new drug comes down the line, what’s the process and how quickly do they update?" Mr. Farber said.
Similarly, said Dr. Silver, if patients are being treated and oncologists are being paid according to best medical practice, there would be fewer incentives for patients to enroll in clinical trials, which are the principal means whereby the science of medicine advances.
Define 'Costs'
It’s also unclear just how drug costs would be defined under the proposed system, Georgetown’s, Dr. Hoadley said.
"The company says, 'Chemotherapy drugs will be reimbursed at manufacturer’s cost.' But as Medicare has learned, that’s an ambiguous term. Medicare has gone from reimbursing based on average wholesale price to now the average sales price, and has come up with new mechanisms to define fairly what the price is for the practices that are paying to get those drugs. So the details need to be filled in about how they’re going to go about doing that," he said.
"The good news, potentially, is that you can get away from this old system that has existed for much of the past, where the practices could make money on drug reimbursements (which is how they made money to finance other things), but it would create potentially distorted incentives. You've still got to get it right, though," he added.
Although it’s still early, the pilot program is a good place to start, according to Dr. Lichter.
"One wants to start a pilot project in situations where there’s a lot of grounding in the types of therapies that are appropriate, so the choices are relatively limited and you can draw a box around the universe of care. If you find out that this works – if the patients are satisfied, the payers are satisfied, the system seems to be working well, and you’ve done the appropriate tweaks and nips and tucks to make it better – then you have to begin to expand it," he said.
A major insurance company is betting that oncology practices can handle cancer-care payments as if they were like the household grocery budget or a weekly allowance: Be frugal and you’ll have some left over for next time. Spend too much, and you’ll have to borrow from the future.
The idea of its pilot study, says UnitedHealthcare, based in Minnetonka, Minn., is to separate drug costs from treatment costs by paying oncologists an up-front, per-patient fee. (In insurance-speak, this is called a "bundled" or "episode" payment.) The payments will be based on best medical practices for three common malignancies: breast cancer, colon cancer, and lung cancer. Decisions about optimal treatment for each disease state will be determined by consensus among clinicians, and not by the insurer, the company said in a press release.
"By paying medical oncologists for a patient’s total cycle of treatment, rather than the number of visits and the amount of chemotherapy drugs given, this program promotes better, more patient-centric, evidence-based care with no loss of revenue for the physician," said Dr. Lee N. Newcomer, UnitedHealthcare’s senior vice president for oncology. "Everyone wins. As oncologists share best practices from the program about which treatment regimens are most effective, we expect to see consistently improved patient outcomes."
The company is working with five small- to mid-size oncology practices based in Dayton, Ohio; Fort Worth, Tex.; Kansas City, Mo; Marietta, Ga.; and Memphis. Analysis is to begin after a year’s data are collected.
"Over the course of the pilot, the various treatment regimens selected by the medical groups will be evaluated to identify which are the most effective for a range of clinical presentations [such as physical signs and symptoms and diagnoses]. UnitedHealthcare will play no role in determining which treatment plan the oncologists choose, but the intent of the pilot is to identify and reduce unnecessary drug administration that does not improve the patient’s health outcomes," the company stated.
Under this payment system, the insurer will determine its cancer-care payments based on the difference between drug costs and the current fee schedule of the oncology group, with payments made on the first day on which an insured patient receives care from that practice. The payments will include case-management fees to cover patient management costs.
Thus, if during the course of an individual patient’s care clinicians decide to switch to a more expensive drug, there would be no additional payments to compensate for the difference.
Each of the agreed-upon regimens in the pilot will be evaluated based on health outcomes and the frequency of adverse events, emergency department visits, and other consequences that can drive up costs without additional treatment benefit, the insurer says.
Worth a Look
The proposed payment model is an interesting approach to the goal of containing medical costs while fairly compensating clinicians and protecting high-quality patient care. But it also raises many questions about its applicability across a broad range of clinical and financial situations, say medical economists and oncologists who were interviewed for this article.
"We are pleased to see experimentation taking place around how oncology care is paid for," said Dr. Allen S. Lichter, CEO of the American Society of Clinical Oncology. "The oncology community needs to do these pilot projects, and understand whether moving away from the current models based on fee-for-service and some percentage of margin off the chemotherapy agents can be replaced by something else – and if so, what should that something else look like and how does it work?"
"I think it's an interesting experiment and an important one, one that Dr. Newcomer and United can run because of their large claims-based database, and we’re all interested to see how it works," agreed Dr. Samuel M. Silver, chair of the subcommittee on reimbursement for the American Society of Hematology and a professor of internal medicine at the University of Michigan in Ann Arbor.
Jack Hoadley, Ph.D., a health policy analyst and research professor at the Health Policy Institute at Georgetown University in Washington, D.C., noted that "Medicare has tried a number of experiments with various types of bundled payments, and it seems [as if] Medicare could either watch what’s going on in this private-sector pilot and use that as a way to learn, or – sooner than that – try bundled-payment structures to see how they work."
Outliers Could Be a Problem
One of the many questions that remain to be answered about the pilot project, however, is the problem of outliers (patients whose care doesn’t fit neatly within the prescribed protocol, such as those with significant comorbidities or adverse drug reactions that require a change in the treatment regimen).
"When one starts to deal with individual practices who have just a couple of these patients, one outlier can make it financially very difficult, so the question is, how often is that going to happen?" Dr. Silver said.
Dr. Hoadley said that the system needs to have flexibility to account for differences in the patient population. "Obviously, you’ve got to get that bundled amount right and appropriately adjusted for the average, across the kind of patients the practice is going to see, with some particular adjustments for patient severity," and other factors, he commented.
Where there are outliers, there are also inliers (patients who, for medical or other reasons, don’t undergo a full course of prescribed therapy), and in these cases, bundled payments would result in additional income for a practice, Dr. Lichter said. Over time, the outliers and inliers tend to balance out, and in the case of the extraordinary outlier – the patient who is admitted for a planned 2-day stay but ends up being hospitalized for 6 months – some sort of contingency payment would be made, he added.
"In talking to United Healthcare, while they didn’t sit down and write rules for every possible situation, I know that if a case is so far beyond the norm, they will sit down and look at it and agree to some type of remedial payment for it," he said.
Rare Cancers and Cherry Picking
A related issue of concern is how bundled payment systems would handle rare cancers, or clinical situations for which there is little or no consensus on optimal therapies, such as the use of chemotherapy for some soft-tissue sarcomas.
As vice-chair of the board of the National Comprehensive Cancer Network, Dr. Silver is an advocate of evidence-based guidelines, but said he’s aware that many patients have variations that don’t fit neatly into the standard chemotherapy guidelines that are acceptable under a bundled-payment system.
"Which brings us to another issue: Would there be cherry picking?" he asked. "Because it would be the patients who are young and healthy and can go through those therapies, and [who] don't have comorbidities and variations on their disease that would best fit into these bundled programs. So what happens to the others?"
In such cases, the burden of care for the more severely ill patients would fall on teaching hospitals, and it’s unclear whether they would be adequately compensated under a bundled-payment system, he said.
Stifle Drug Development?
Matt Farber, director of provider economics and public policy for the Association of Community Cancer Centers, said that bundled-payment systems could have a dampening effect on drug development. He points to sipuleucel-T (Provenge), the recently approved autologous immunotherapy vaccine for advanced prostate cancer that uses antigen-presenting cells unique to each patient.
"Would payment systems like this stop those drugs from being developed? Because if it’s a personalized treatment, would it therefore not be included in whatever benchmark is deemed the most appropriate or most effective care for most people?" Mr. Farber asked.
And what happens when novel drugs or new versions of conventional chemotherapeutic agents (such as palifosfamide, an active metabolite of ifosfamide) come on the market?
"They’re working right now with these five practices to determine what are the best courses of treatment currently for the disease states that they’re looking at. So when a new drug comes down the line, what’s the process and how quickly do they update?" Mr. Farber said.
Similarly, said Dr. Silver, if patients are being treated and oncologists are being paid according to best medical practice, there would be fewer incentives for patients to enroll in clinical trials, which are the principal means whereby the science of medicine advances.
Define 'Costs'
It’s also unclear just how drug costs would be defined under the proposed system, Georgetown’s, Dr. Hoadley said.
"The company says, 'Chemotherapy drugs will be reimbursed at manufacturer’s cost.' But as Medicare has learned, that’s an ambiguous term. Medicare has gone from reimbursing based on average wholesale price to now the average sales price, and has come up with new mechanisms to define fairly what the price is for the practices that are paying to get those drugs. So the details need to be filled in about how they’re going to go about doing that," he said.
"The good news, potentially, is that you can get away from this old system that has existed for much of the past, where the practices could make money on drug reimbursements (which is how they made money to finance other things), but it would create potentially distorted incentives. You've still got to get it right, though," he added.
Although it’s still early, the pilot program is a good place to start, according to Dr. Lichter.
"One wants to start a pilot project in situations where there’s a lot of grounding in the types of therapies that are appropriate, so the choices are relatively limited and you can draw a box around the universe of care. If you find out that this works – if the patients are satisfied, the payers are satisfied, the system seems to be working well, and you’ve done the appropriate tweaks and nips and tucks to make it better – then you have to begin to expand it," he said.
A major insurance company is betting that oncology practices can handle cancer-care payments as if they were like the household grocery budget or a weekly allowance: Be frugal and you’ll have some left over for next time. Spend too much, and you’ll have to borrow from the future.
The idea of its pilot study, says UnitedHealthcare, based in Minnetonka, Minn., is to separate drug costs from treatment costs by paying oncologists an up-front, per-patient fee. (In insurance-speak, this is called a "bundled" or "episode" payment.) The payments will be based on best medical practices for three common malignancies: breast cancer, colon cancer, and lung cancer. Decisions about optimal treatment for each disease state will be determined by consensus among clinicians, and not by the insurer, the company said in a press release.
"By paying medical oncologists for a patient’s total cycle of treatment, rather than the number of visits and the amount of chemotherapy drugs given, this program promotes better, more patient-centric, evidence-based care with no loss of revenue for the physician," said Dr. Lee N. Newcomer, UnitedHealthcare’s senior vice president for oncology. "Everyone wins. As oncologists share best practices from the program about which treatment regimens are most effective, we expect to see consistently improved patient outcomes."
The company is working with five small- to mid-size oncology practices based in Dayton, Ohio; Fort Worth, Tex.; Kansas City, Mo; Marietta, Ga.; and Memphis. Analysis is to begin after a year’s data are collected.
"Over the course of the pilot, the various treatment regimens selected by the medical groups will be evaluated to identify which are the most effective for a range of clinical presentations [such as physical signs and symptoms and diagnoses]. UnitedHealthcare will play no role in determining which treatment plan the oncologists choose, but the intent of the pilot is to identify and reduce unnecessary drug administration that does not improve the patient’s health outcomes," the company stated.
Under this payment system, the insurer will determine its cancer-care payments based on the difference between drug costs and the current fee schedule of the oncology group, with payments made on the first day on which an insured patient receives care from that practice. The payments will include case-management fees to cover patient management costs.
Thus, if during the course of an individual patient’s care clinicians decide to switch to a more expensive drug, there would be no additional payments to compensate for the difference.
Each of the agreed-upon regimens in the pilot will be evaluated based on health outcomes and the frequency of adverse events, emergency department visits, and other consequences that can drive up costs without additional treatment benefit, the insurer says.
Worth a Look
The proposed payment model is an interesting approach to the goal of containing medical costs while fairly compensating clinicians and protecting high-quality patient care. But it also raises many questions about its applicability across a broad range of clinical and financial situations, say medical economists and oncologists who were interviewed for this article.
"We are pleased to see experimentation taking place around how oncology care is paid for," said Dr. Allen S. Lichter, CEO of the American Society of Clinical Oncology. "The oncology community needs to do these pilot projects, and understand whether moving away from the current models based on fee-for-service and some percentage of margin off the chemotherapy agents can be replaced by something else – and if so, what should that something else look like and how does it work?"
"I think it's an interesting experiment and an important one, one that Dr. Newcomer and United can run because of their large claims-based database, and we’re all interested to see how it works," agreed Dr. Samuel M. Silver, chair of the subcommittee on reimbursement for the American Society of Hematology and a professor of internal medicine at the University of Michigan in Ann Arbor.
Jack Hoadley, Ph.D., a health policy analyst and research professor at the Health Policy Institute at Georgetown University in Washington, D.C., noted that "Medicare has tried a number of experiments with various types of bundled payments, and it seems [as if] Medicare could either watch what’s going on in this private-sector pilot and use that as a way to learn, or – sooner than that – try bundled-payment structures to see how they work."
Outliers Could Be a Problem
One of the many questions that remain to be answered about the pilot project, however, is the problem of outliers (patients whose care doesn’t fit neatly within the prescribed protocol, such as those with significant comorbidities or adverse drug reactions that require a change in the treatment regimen).
"When one starts to deal with individual practices who have just a couple of these patients, one outlier can make it financially very difficult, so the question is, how often is that going to happen?" Dr. Silver said.
Dr. Hoadley said that the system needs to have flexibility to account for differences in the patient population. "Obviously, you’ve got to get that bundled amount right and appropriately adjusted for the average, across the kind of patients the practice is going to see, with some particular adjustments for patient severity," and other factors, he commented.
Where there are outliers, there are also inliers (patients who, for medical or other reasons, don’t undergo a full course of prescribed therapy), and in these cases, bundled payments would result in additional income for a practice, Dr. Lichter said. Over time, the outliers and inliers tend to balance out, and in the case of the extraordinary outlier – the patient who is admitted for a planned 2-day stay but ends up being hospitalized for 6 months – some sort of contingency payment would be made, he added.
"In talking to United Healthcare, while they didn’t sit down and write rules for every possible situation, I know that if a case is so far beyond the norm, they will sit down and look at it and agree to some type of remedial payment for it," he said.
Rare Cancers and Cherry Picking
A related issue of concern is how bundled payment systems would handle rare cancers, or clinical situations for which there is little or no consensus on optimal therapies, such as the use of chemotherapy for some soft-tissue sarcomas.
As vice-chair of the board of the National Comprehensive Cancer Network, Dr. Silver is an advocate of evidence-based guidelines, but said he’s aware that many patients have variations that don’t fit neatly into the standard chemotherapy guidelines that are acceptable under a bundled-payment system.
"Which brings us to another issue: Would there be cherry picking?" he asked. "Because it would be the patients who are young and healthy and can go through those therapies, and [who] don't have comorbidities and variations on their disease that would best fit into these bundled programs. So what happens to the others?"
In such cases, the burden of care for the more severely ill patients would fall on teaching hospitals, and it’s unclear whether they would be adequately compensated under a bundled-payment system, he said.
Stifle Drug Development?
Matt Farber, director of provider economics and public policy for the Association of Community Cancer Centers, said that bundled-payment systems could have a dampening effect on drug development. He points to sipuleucel-T (Provenge), the recently approved autologous immunotherapy vaccine for advanced prostate cancer that uses antigen-presenting cells unique to each patient.
"Would payment systems like this stop those drugs from being developed? Because if it’s a personalized treatment, would it therefore not be included in whatever benchmark is deemed the most appropriate or most effective care for most people?" Mr. Farber asked.
And what happens when novel drugs or new versions of conventional chemotherapeutic agents (such as palifosfamide, an active metabolite of ifosfamide) come on the market?
"They’re working right now with these five practices to determine what are the best courses of treatment currently for the disease states that they’re looking at. So when a new drug comes down the line, what’s the process and how quickly do they update?" Mr. Farber said.
Similarly, said Dr. Silver, if patients are being treated and oncologists are being paid according to best medical practice, there would be fewer incentives for patients to enroll in clinical trials, which are the principal means whereby the science of medicine advances.
Define 'Costs'
It’s also unclear just how drug costs would be defined under the proposed system, Georgetown’s, Dr. Hoadley said.
"The company says, 'Chemotherapy drugs will be reimbursed at manufacturer’s cost.' But as Medicare has learned, that’s an ambiguous term. Medicare has gone from reimbursing based on average wholesale price to now the average sales price, and has come up with new mechanisms to define fairly what the price is for the practices that are paying to get those drugs. So the details need to be filled in about how they’re going to go about doing that," he said.
"The good news, potentially, is that you can get away from this old system that has existed for much of the past, where the practices could make money on drug reimbursements (which is how they made money to finance other things), but it would create potentially distorted incentives. You've still got to get it right, though," he added.
Although it’s still early, the pilot program is a good place to start, according to Dr. Lichter.
"One wants to start a pilot project in situations where there’s a lot of grounding in the types of therapies that are appropriate, so the choices are relatively limited and you can draw a box around the universe of care. If you find out that this works – if the patients are satisfied, the payers are satisfied, the system seems to be working well, and you’ve done the appropriate tweaks and nips and tucks to make it better – then you have to begin to expand it," he said.
Primary Care Physicians Performing More Skin Cancer Screenings
CHICAGO – The rate of skin cancer screening by primary care physicians has increased, compared with relatively stable rates for breast, pelvic, and rectal examinations in this setting, according to a study.
"It is very encouraging that more primary care physicians are looking at patients' skin to hopefully detect skin cancer earlier," Dr. Jeremy S. Bordeaux said . "Hopefully the outcome will be that when patients are diagnosed with melanoma, it will be at an earlier stage, and they will be more likely to live."
There are insufficient numbers of dermatologists to perform total body examinations for all patients in the United States. In addition, many patients at risk are more likely to see a primary care doctor, said Dr. Bordeaux, director of Mohs Micrographic and Dermatologic Surgery at University Hospitals and Case Western Reserve University in Cleveland.
Dr. Bordeaux sought to quantify primary-care physician skin cancer screening and counseling. He also wanted to identify factors associated with a higher likelihood for screening and counseling.
He assessed outpatient, nonurgent visits to office-based physicians in the years 1997-2000, and then compared those numbers with 2005 and 2006 using data from the National Ambulatory Medical Care Survey (NAMCS). He looked at prevalence in aggregate for skin cancer and breast cancer screening, pelvic and rectal examinations, and counseling about skin cancer prevention and tobacco cessation.
Primary care physicians screened 19% of white patients for skin cancer in the first time period, compared with 26% in 2005-2006, Dr. Bordeaux said at the meeting, which was held jointly with the American Society of Cosmetic Dermatology and Aesthetic Surgery.
In contrast, "the screening rates for everything else stayed pretty flat," Dr. Bordeaux said. For example, prevalence of screening for breast cancer increased only modestly to 36% (2005-2006) from 31% (1997-2000). For pelvic exams, the increase was 35% vs. 32%, respectively, and for rectal exams, 24% vs. 20%. Data were incomplete to compare percentages for skin cancer counseling between the two time periods.
Interestingly, Dr. Bordeaux said, "With nonwhite patients, there were the same trends, including the increase in skin cancer screening [26% vs. 19%]." In addition, nonwhite patient screening for breast cancer increased at a similarly modest rate between the two time periods: 34% vs. 30%, respectively. For pelvic exams, the increase was 34% vs. 31%, and for rectal exams, 22% vs. 19%. Tobacco cessation counseling rates increased 7% in 2005-2006 vs. 5% in the 1997-2000 time periods.
Certain factors were significantly associated with increased likelihood of skin screening by primary care physicians. "You are more likely to get screened if you are female, are seen by a physician – versus a physician extender and if you have private insurance," Dr. Bordeaux said.
Specifically, males were less likely to be screened (odds ratio, 0.82), compared with females. Patients who saw a doctor (versus a nonphysician) were more likely to be screened (OR, 3.02), as were those with private insurance (OR, 1.54), compared with Medicare or Medicaid patients.
Age made a significant difference in terms of skin cancer prevention counseling. Compared with younger patients, the study revealed that primary care physicians were less likely to counsel those aged 40 and older about sun-protective behaviors (OR, 0.64).
A second, prospective study is now underway, Dr. Bordeaux said. He plans to correlate the depth/severity of melanomas with history of complete skin examination screening by a dermatologist or primary care physician.
The American Society for Dermatologic Surgery provided a Cutting Edge Research Grant to fund the study. Dr. Bordeaux had no relevant financial disclosures.
CHICAGO – The rate of skin cancer screening by primary care physicians has increased, compared with relatively stable rates for breast, pelvic, and rectal examinations in this setting, according to a study.
"It is very encouraging that more primary care physicians are looking at patients' skin to hopefully detect skin cancer earlier," Dr. Jeremy S. Bordeaux said . "Hopefully the outcome will be that when patients are diagnosed with melanoma, it will be at an earlier stage, and they will be more likely to live."
There are insufficient numbers of dermatologists to perform total body examinations for all patients in the United States. In addition, many patients at risk are more likely to see a primary care doctor, said Dr. Bordeaux, director of Mohs Micrographic and Dermatologic Surgery at University Hospitals and Case Western Reserve University in Cleveland.
Dr. Bordeaux sought to quantify primary-care physician skin cancer screening and counseling. He also wanted to identify factors associated with a higher likelihood for screening and counseling.
He assessed outpatient, nonurgent visits to office-based physicians in the years 1997-2000, and then compared those numbers with 2005 and 2006 using data from the National Ambulatory Medical Care Survey (NAMCS). He looked at prevalence in aggregate for skin cancer and breast cancer screening, pelvic and rectal examinations, and counseling about skin cancer prevention and tobacco cessation.
Primary care physicians screened 19% of white patients for skin cancer in the first time period, compared with 26% in 2005-2006, Dr. Bordeaux said at the meeting, which was held jointly with the American Society of Cosmetic Dermatology and Aesthetic Surgery.
In contrast, "the screening rates for everything else stayed pretty flat," Dr. Bordeaux said. For example, prevalence of screening for breast cancer increased only modestly to 36% (2005-2006) from 31% (1997-2000). For pelvic exams, the increase was 35% vs. 32%, respectively, and for rectal exams, 24% vs. 20%. Data were incomplete to compare percentages for skin cancer counseling between the two time periods.
Interestingly, Dr. Bordeaux said, "With nonwhite patients, there were the same trends, including the increase in skin cancer screening [26% vs. 19%]." In addition, nonwhite patient screening for breast cancer increased at a similarly modest rate between the two time periods: 34% vs. 30%, respectively. For pelvic exams, the increase was 34% vs. 31%, and for rectal exams, 22% vs. 19%. Tobacco cessation counseling rates increased 7% in 2005-2006 vs. 5% in the 1997-2000 time periods.
Certain factors were significantly associated with increased likelihood of skin screening by primary care physicians. "You are more likely to get screened if you are female, are seen by a physician – versus a physician extender and if you have private insurance," Dr. Bordeaux said.
Specifically, males were less likely to be screened (odds ratio, 0.82), compared with females. Patients who saw a doctor (versus a nonphysician) were more likely to be screened (OR, 3.02), as were those with private insurance (OR, 1.54), compared with Medicare or Medicaid patients.
Age made a significant difference in terms of skin cancer prevention counseling. Compared with younger patients, the study revealed that primary care physicians were less likely to counsel those aged 40 and older about sun-protective behaviors (OR, 0.64).
A second, prospective study is now underway, Dr. Bordeaux said. He plans to correlate the depth/severity of melanomas with history of complete skin examination screening by a dermatologist or primary care physician.
The American Society for Dermatologic Surgery provided a Cutting Edge Research Grant to fund the study. Dr. Bordeaux had no relevant financial disclosures.
CHICAGO – The rate of skin cancer screening by primary care physicians has increased, compared with relatively stable rates for breast, pelvic, and rectal examinations in this setting, according to a study.
"It is very encouraging that more primary care physicians are looking at patients' skin to hopefully detect skin cancer earlier," Dr. Jeremy S. Bordeaux said . "Hopefully the outcome will be that when patients are diagnosed with melanoma, it will be at an earlier stage, and they will be more likely to live."
There are insufficient numbers of dermatologists to perform total body examinations for all patients in the United States. In addition, many patients at risk are more likely to see a primary care doctor, said Dr. Bordeaux, director of Mohs Micrographic and Dermatologic Surgery at University Hospitals and Case Western Reserve University in Cleveland.
Dr. Bordeaux sought to quantify primary-care physician skin cancer screening and counseling. He also wanted to identify factors associated with a higher likelihood for screening and counseling.
He assessed outpatient, nonurgent visits to office-based physicians in the years 1997-2000, and then compared those numbers with 2005 and 2006 using data from the National Ambulatory Medical Care Survey (NAMCS). He looked at prevalence in aggregate for skin cancer and breast cancer screening, pelvic and rectal examinations, and counseling about skin cancer prevention and tobacco cessation.
Primary care physicians screened 19% of white patients for skin cancer in the first time period, compared with 26% in 2005-2006, Dr. Bordeaux said at the meeting, which was held jointly with the American Society of Cosmetic Dermatology and Aesthetic Surgery.
In contrast, "the screening rates for everything else stayed pretty flat," Dr. Bordeaux said. For example, prevalence of screening for breast cancer increased only modestly to 36% (2005-2006) from 31% (1997-2000). For pelvic exams, the increase was 35% vs. 32%, respectively, and for rectal exams, 24% vs. 20%. Data were incomplete to compare percentages for skin cancer counseling between the two time periods.
Interestingly, Dr. Bordeaux said, "With nonwhite patients, there were the same trends, including the increase in skin cancer screening [26% vs. 19%]." In addition, nonwhite patient screening for breast cancer increased at a similarly modest rate between the two time periods: 34% vs. 30%, respectively. For pelvic exams, the increase was 34% vs. 31%, and for rectal exams, 22% vs. 19%. Tobacco cessation counseling rates increased 7% in 2005-2006 vs. 5% in the 1997-2000 time periods.
Certain factors were significantly associated with increased likelihood of skin screening by primary care physicians. "You are more likely to get screened if you are female, are seen by a physician – versus a physician extender and if you have private insurance," Dr. Bordeaux said.
Specifically, males were less likely to be screened (odds ratio, 0.82), compared with females. Patients who saw a doctor (versus a nonphysician) were more likely to be screened (OR, 3.02), as were those with private insurance (OR, 1.54), compared with Medicare or Medicaid patients.
Age made a significant difference in terms of skin cancer prevention counseling. Compared with younger patients, the study revealed that primary care physicians were less likely to counsel those aged 40 and older about sun-protective behaviors (OR, 0.64).
A second, prospective study is now underway, Dr. Bordeaux said. He plans to correlate the depth/severity of melanomas with history of complete skin examination screening by a dermatologist or primary care physician.
The American Society for Dermatologic Surgery provided a Cutting Edge Research Grant to fund the study. Dr. Bordeaux had no relevant financial disclosures.
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY FOR DERMATOLOGIC SURGERY
Major Finding: Primary care physicians screened 19% of white patients for skin
cancer from 1997-2000, compared with 26% in 2005-2006.
Data Source: Outpatient, nonurgent visits to office-based physicians and data from NAMCS.
Disclosures: The American Society for Dermatologic Surgery provided a Cutting Edge
Research Grant to fund the study. Dr. Bordeaux had no relevant financial
disclosures.