User login
NIH at Forefront of New Clinical Trials Push, Director Says
WASHINGTON – Breakthroughs in human genetics combined with funding from the Affordable Care Act have poised the National Institutes of Health to make real progress in the areas of orphan human diseases, according to NIH Director Francis S. Collins.
Speaking with enthusiasm to those he addressed as his "peeps" at the annual meeting of the American Society of Human Genetics, Dr. Collins shared his excitement at the state of human genetics in the post-genomic world, in large part driven by technology that has significantly lowered the cost of DNA sequencing, in turn speeding genetic research tremendously.
This, combined with new ACA funding, has enabled NIH to fund and pursue translational research, moving laboratory results toward and into clinical trials, something that is a new way of thinking for the agency, Dr. Collins said.
Rather than relying on pharmaceutical and biotechnology companies to take charge of the translational research, Dr. Collins encouraged academic researchers to consider partnering with NIH, at least for those orphan disease conditions in which the federal government would not be seen as being in competition with private enterprise.
"There is a serious crisis underway in the way in which this pipeline for drug discovery has been floundering. ... Pharma has been investing a larger and larger amount of money – between $40 and $50 billion dollars a year – and yet in spite of that, FDA approvals of new molecular entities, that is genuinely new drug therapeutics, not ‘me-toos,’ have been dropping steadily over the last 15 years," Dr. Collins said.
The reasons for this are complex, he said, but a big part of the problem involves coming up with appropriate targets and targeting compounds. He said this is an area in which NIH is and can be very much involved.
NIH now encourages academic researchers to take their targets to the assay stage and beyond, providing high-throughput screening (HTS) assistance from the NIH Chemical Genomics Center. Subsequent medicinal chemistry assistance is also available to help to modify HTS hits to enable compounds to become more drug-like and to match current ADME (absorption, distribution, metabolism, and excretion) criteria.
With NIH assistance, more than 150 lead compounds have reached this stage over the last 4-5 years, more than half of which are "poised to go to the next step" of preclinical trials in animals, or the "Valley of Death," according to Dr. Collins, "because this is where projects often go to die."
NIH is now able to assist in this high-risk area through the Therapeutics for Rare and Neglected Diseases (TRND) program in its Office of Rare Diseases Research. The TRND was funded at $24 million in fiscal year 2009.
NIH also is positioned to assist researchers in early phase human trials of orphan diseases through its 240-bed Clinical Center, Dr. Collins said.
"And we have 50 and soon we will have 60 Clinical and Translational Science Awards scattered all across the country which will also be set up to conduct these sorts of trials for new molecular entities," he added.
This new direction in research funding has involved unprecedented cooperation with the Food and Drug Administration, Dr. Collins said, with an NIH-FDA leadership council formed to ensure that new drug candidates are most safely and efficiently moved into the clinical trials framework in ways that would best enable FDA analysis and validation, particularly for rare diseases.
Dr. Collins was particularly excited about five instances in which NIH is using this new model of helping "de-risk" the drug development process for orphan or neglected diseases through TRND. These include four rare diseases (Niemann-Pick Disease Type C, hereditary inclusion body myopathy, sickle cell disease, and chronic lymphocytic leukemia) and one neglected disease (schistosomiasis).
If and when these compounds and those for other rare diseases become ready for marketing and production, NIH would then work with private companies to achieve licensing agreement to enable their manufacture and sale, according to Dr. Collins.
Dr. Collins reported having no financial conflicts of interest with regard to his presentation.
WASHINGTON – Breakthroughs in human genetics combined with funding from the Affordable Care Act have poised the National Institutes of Health to make real progress in the areas of orphan human diseases, according to NIH Director Francis S. Collins.
Speaking with enthusiasm to those he addressed as his "peeps" at the annual meeting of the American Society of Human Genetics, Dr. Collins shared his excitement at the state of human genetics in the post-genomic world, in large part driven by technology that has significantly lowered the cost of DNA sequencing, in turn speeding genetic research tremendously.
This, combined with new ACA funding, has enabled NIH to fund and pursue translational research, moving laboratory results toward and into clinical trials, something that is a new way of thinking for the agency, Dr. Collins said.
Rather than relying on pharmaceutical and biotechnology companies to take charge of the translational research, Dr. Collins encouraged academic researchers to consider partnering with NIH, at least for those orphan disease conditions in which the federal government would not be seen as being in competition with private enterprise.
"There is a serious crisis underway in the way in which this pipeline for drug discovery has been floundering. ... Pharma has been investing a larger and larger amount of money – between $40 and $50 billion dollars a year – and yet in spite of that, FDA approvals of new molecular entities, that is genuinely new drug therapeutics, not ‘me-toos,’ have been dropping steadily over the last 15 years," Dr. Collins said.
The reasons for this are complex, he said, but a big part of the problem involves coming up with appropriate targets and targeting compounds. He said this is an area in which NIH is and can be very much involved.
NIH now encourages academic researchers to take their targets to the assay stage and beyond, providing high-throughput screening (HTS) assistance from the NIH Chemical Genomics Center. Subsequent medicinal chemistry assistance is also available to help to modify HTS hits to enable compounds to become more drug-like and to match current ADME (absorption, distribution, metabolism, and excretion) criteria.
With NIH assistance, more than 150 lead compounds have reached this stage over the last 4-5 years, more than half of which are "poised to go to the next step" of preclinical trials in animals, or the "Valley of Death," according to Dr. Collins, "because this is where projects often go to die."
NIH is now able to assist in this high-risk area through the Therapeutics for Rare and Neglected Diseases (TRND) program in its Office of Rare Diseases Research. The TRND was funded at $24 million in fiscal year 2009.
NIH also is positioned to assist researchers in early phase human trials of orphan diseases through its 240-bed Clinical Center, Dr. Collins said.
"And we have 50 and soon we will have 60 Clinical and Translational Science Awards scattered all across the country which will also be set up to conduct these sorts of trials for new molecular entities," he added.
This new direction in research funding has involved unprecedented cooperation with the Food and Drug Administration, Dr. Collins said, with an NIH-FDA leadership council formed to ensure that new drug candidates are most safely and efficiently moved into the clinical trials framework in ways that would best enable FDA analysis and validation, particularly for rare diseases.
Dr. Collins was particularly excited about five instances in which NIH is using this new model of helping "de-risk" the drug development process for orphan or neglected diseases through TRND. These include four rare diseases (Niemann-Pick Disease Type C, hereditary inclusion body myopathy, sickle cell disease, and chronic lymphocytic leukemia) and one neglected disease (schistosomiasis).
If and when these compounds and those for other rare diseases become ready for marketing and production, NIH would then work with private companies to achieve licensing agreement to enable their manufacture and sale, according to Dr. Collins.
Dr. Collins reported having no financial conflicts of interest with regard to his presentation.
WASHINGTON – Breakthroughs in human genetics combined with funding from the Affordable Care Act have poised the National Institutes of Health to make real progress in the areas of orphan human diseases, according to NIH Director Francis S. Collins.
Speaking with enthusiasm to those he addressed as his "peeps" at the annual meeting of the American Society of Human Genetics, Dr. Collins shared his excitement at the state of human genetics in the post-genomic world, in large part driven by technology that has significantly lowered the cost of DNA sequencing, in turn speeding genetic research tremendously.
This, combined with new ACA funding, has enabled NIH to fund and pursue translational research, moving laboratory results toward and into clinical trials, something that is a new way of thinking for the agency, Dr. Collins said.
Rather than relying on pharmaceutical and biotechnology companies to take charge of the translational research, Dr. Collins encouraged academic researchers to consider partnering with NIH, at least for those orphan disease conditions in which the federal government would not be seen as being in competition with private enterprise.
"There is a serious crisis underway in the way in which this pipeline for drug discovery has been floundering. ... Pharma has been investing a larger and larger amount of money – between $40 and $50 billion dollars a year – and yet in spite of that, FDA approvals of new molecular entities, that is genuinely new drug therapeutics, not ‘me-toos,’ have been dropping steadily over the last 15 years," Dr. Collins said.
The reasons for this are complex, he said, but a big part of the problem involves coming up with appropriate targets and targeting compounds. He said this is an area in which NIH is and can be very much involved.
NIH now encourages academic researchers to take their targets to the assay stage and beyond, providing high-throughput screening (HTS) assistance from the NIH Chemical Genomics Center. Subsequent medicinal chemistry assistance is also available to help to modify HTS hits to enable compounds to become more drug-like and to match current ADME (absorption, distribution, metabolism, and excretion) criteria.
With NIH assistance, more than 150 lead compounds have reached this stage over the last 4-5 years, more than half of which are "poised to go to the next step" of preclinical trials in animals, or the "Valley of Death," according to Dr. Collins, "because this is where projects often go to die."
NIH is now able to assist in this high-risk area through the Therapeutics for Rare and Neglected Diseases (TRND) program in its Office of Rare Diseases Research. The TRND was funded at $24 million in fiscal year 2009.
NIH also is positioned to assist researchers in early phase human trials of orphan diseases through its 240-bed Clinical Center, Dr. Collins said.
"And we have 50 and soon we will have 60 Clinical and Translational Science Awards scattered all across the country which will also be set up to conduct these sorts of trials for new molecular entities," he added.
This new direction in research funding has involved unprecedented cooperation with the Food and Drug Administration, Dr. Collins said, with an NIH-FDA leadership council formed to ensure that new drug candidates are most safely and efficiently moved into the clinical trials framework in ways that would best enable FDA analysis and validation, particularly for rare diseases.
Dr. Collins was particularly excited about five instances in which NIH is using this new model of helping "de-risk" the drug development process for orphan or neglected diseases through TRND. These include four rare diseases (Niemann-Pick Disease Type C, hereditary inclusion body myopathy, sickle cell disease, and chronic lymphocytic leukemia) and one neglected disease (schistosomiasis).
If and when these compounds and those for other rare diseases become ready for marketing and production, NIH would then work with private companies to achieve licensing agreement to enable their manufacture and sale, according to Dr. Collins.
Dr. Collins reported having no financial conflicts of interest with regard to his presentation.
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY OF HUMAN GENETICS
Making a Neurofibromatosis Type 1 Diagnosis: Check the Armpits!
GOTHENBURG, SWEDEN - Neurofibromatosis type 1 is a diagnosis every dermatologist should be able to make, according to Dr. Sirkku Peltonen.
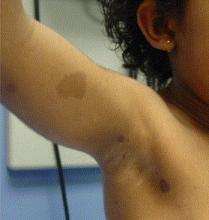
Be sure to check the armpits, she said. Examining the axillae in search of a few small freckles often makes the difference between diagnosing neurofibromatosis type 1 (NF1) and overlooking this cancer predisposition syndrome.
"The armpits are so easy to forget – and then you lose the chance to make the diagnosis," said Dr. Peltonen of the University of Turku (Finland).
Diagnosis of NF1 is not difficult, she said. Of children aged 6 years or older, 95% can be clinically diagnosed based on the presence of at least two of the following features: six or more café au lait spots, each more than 5 mm in their greatest diameter; axillary or inguinal freckling; a first-degree relative with NF1; or pseudoarthrosis of the tibia, a finding present in only about 3% of individuals with NF1. These four features, from a longer list of diagnostic criteria established by the National Institutes of Health, are the most relevant to dermatologists (JAMA 1997;278:51-7). The flat, pigmented café au lait macules usually appear by a child’s first birthday.
"If you see four or five café au lait spots, suspect NF1 and look for more," Dr. Peltonen said at the annual congress of the European Academy of Dermatology and Venereology.
Freckles under the armpit appear by 6 years of age. Prior to that, the diagnosis is typically made on the basis of the requisite café au lait spots in a child having a family history of NF1. However, half of patients with NF1 have a sporadic mutation and no family history of the disease, making early diagnosis considerably more difficult.
Laboratory analysis of NF1 is available in challenging cases, most of which involve young children. It is a laborious, expensive process because the NF1 gene, located on chromosome 17, band q11, is large, and hundreds of mutations have been identified to date, said Dr. Peltonen.
In her experience, laboratories vary widely in their ability to perform a high-quality analysis of mutations of the NF1 gene. She singled out the laboratories at the Ghent (Belgium) University and the University of Alabama at Birmingham as being particularly good when it comes to the diagnosis of NF1. Testing costs more than $1,300, she said.
The incidence of NF1, formerly known as von Recklinghausen’s disease, is roughly 1 in 3,000 live births, noted Dr. Peltonen. Inheritance is autosomal dominant with 100% penetrance. This means everyone who has the inherited form of NF1 ought to be readily diagnosable.
NF1 is a multisystem condition. Common features include cognitive impairment in 80% of patients, learning difficulties in 50%-60%, and speech abnormalities in 30%, as well as eye and skeletal abnormalities. Affected children are at risk for social exclusion because of their appearance. A dermatologist who diagnoses NF1 needs to be ready to make a referral to a physician who is familiar with the syndrome for regular follow-up, she said.
Children with NF1 are at 100-fold increased risk for brain tumors, compared with unaffected children. The lifetime risk of developing a malignant peripheral nerve sheath tumor is 10%-15%. Thirty percent or more of patients with NF1 have one or more plexiform neuromas, which are at risk for malignant transformation. The cancers can appear at any age, although most often not until after the teenage years.
Danger signs of malignancy in patients with NF1 include neurogenic pain in the extremities, a painful mass, or accelerated growth of an existing plexiform neuroma, she added.
The majority of adults with NF1 develop cutaneous neurofibromas. These were traditionally thought to have their origin in small dermal nerve twigs containing Schwann cells. In soon-to-be-published work, however, Dr. Peltonen and his coworkers have shown that the tumors actually arise from multipotent stem cells closely associated with hair follicles.
The cutaneous neurofibromas never become malignant. Nevertheless, they have a huge negative impact on quality of life, she said.
Patients consider cutaneous neurofibromas to be the greatest burden of NF1 because they are painful and unsightly. Dermatologists can do patients a great service by removing the benign cutaneous tumors, said Dr. Peltonen. The CO2 laser is effective for this purpose. Dozens of tumors can be removed under local anesthesia in one appointment, and patients are very appreciative. Dr. Peltonen has one patient who travels 9 hours each way for excision of cutaneous neurofibromas.
"I usually make a circle around the tumor and then cut in between dermis and the tumor while pulling on it at the same time," she said. "It’s quite easy to get the tumor out of the skin, and the holes heal nicely."
Numerous cancer drugs have been studied and found wanting for the treatment of plexiform neuromas and/or malignant peripheral nerve sheath tumors. Among the agents that have failed are sirolimus, sorafenib, erlotinib, sunitinib, ranibizumab, and cediranib. Imatinib has shown mixed results for the treatment of plexiform neuromas; a clinical trial is ongoing.
Disclosures: Dr. Peltonen said she had no relevant financial conflicts.
GOTHENBURG, SWEDEN - Neurofibromatosis type 1 is a diagnosis every dermatologist should be able to make, according to Dr. Sirkku Peltonen.
Be sure to check the armpits, she said. Examining the axillae in search of a few small freckles often makes the difference between diagnosing neurofibromatosis type 1 (NF1) and overlooking this cancer predisposition syndrome.
"The armpits are so easy to forget – and then you lose the chance to make the diagnosis," said Dr. Peltonen of the University of Turku (Finland).
Diagnosis of NF1 is not difficult, she said. Of children aged 6 years or older, 95% can be clinically diagnosed based on the presence of at least two of the following features: six or more café au lait spots, each more than 5 mm in their greatest diameter; axillary or inguinal freckling; a first-degree relative with NF1; or pseudoarthrosis of the tibia, a finding present in only about 3% of individuals with NF1. These four features, from a longer list of diagnostic criteria established by the National Institutes of Health, are the most relevant to dermatologists (JAMA 1997;278:51-7). The flat, pigmented café au lait macules usually appear by a child’s first birthday.
"If you see four or five café au lait spots, suspect NF1 and look for more," Dr. Peltonen said at the annual congress of the European Academy of Dermatology and Venereology.
Freckles under the armpit appear by 6 years of age. Prior to that, the diagnosis is typically made on the basis of the requisite café au lait spots in a child having a family history of NF1. However, half of patients with NF1 have a sporadic mutation and no family history of the disease, making early diagnosis considerably more difficult.
Laboratory analysis of NF1 is available in challenging cases, most of which involve young children. It is a laborious, expensive process because the NF1 gene, located on chromosome 17, band q11, is large, and hundreds of mutations have been identified to date, said Dr. Peltonen.
In her experience, laboratories vary widely in their ability to perform a high-quality analysis of mutations of the NF1 gene. She singled out the laboratories at the Ghent (Belgium) University and the University of Alabama at Birmingham as being particularly good when it comes to the diagnosis of NF1. Testing costs more than $1,300, she said.
The incidence of NF1, formerly known as von Recklinghausen’s disease, is roughly 1 in 3,000 live births, noted Dr. Peltonen. Inheritance is autosomal dominant with 100% penetrance. This means everyone who has the inherited form of NF1 ought to be readily diagnosable.
NF1 is a multisystem condition. Common features include cognitive impairment in 80% of patients, learning difficulties in 50%-60%, and speech abnormalities in 30%, as well as eye and skeletal abnormalities. Affected children are at risk for social exclusion because of their appearance. A dermatologist who diagnoses NF1 needs to be ready to make a referral to a physician who is familiar with the syndrome for regular follow-up, she said.
Children with NF1 are at 100-fold increased risk for brain tumors, compared with unaffected children. The lifetime risk of developing a malignant peripheral nerve sheath tumor is 10%-15%. Thirty percent or more of patients with NF1 have one or more plexiform neuromas, which are at risk for malignant transformation. The cancers can appear at any age, although most often not until after the teenage years.
Danger signs of malignancy in patients with NF1 include neurogenic pain in the extremities, a painful mass, or accelerated growth of an existing plexiform neuroma, she added.
The majority of adults with NF1 develop cutaneous neurofibromas. These were traditionally thought to have their origin in small dermal nerve twigs containing Schwann cells. In soon-to-be-published work, however, Dr. Peltonen and his coworkers have shown that the tumors actually arise from multipotent stem cells closely associated with hair follicles.
The cutaneous neurofibromas never become malignant. Nevertheless, they have a huge negative impact on quality of life, she said.
Patients consider cutaneous neurofibromas to be the greatest burden of NF1 because they are painful and unsightly. Dermatologists can do patients a great service by removing the benign cutaneous tumors, said Dr. Peltonen. The CO2 laser is effective for this purpose. Dozens of tumors can be removed under local anesthesia in one appointment, and patients are very appreciative. Dr. Peltonen has one patient who travels 9 hours each way for excision of cutaneous neurofibromas.
"I usually make a circle around the tumor and then cut in between dermis and the tumor while pulling on it at the same time," she said. "It’s quite easy to get the tumor out of the skin, and the holes heal nicely."
Numerous cancer drugs have been studied and found wanting for the treatment of plexiform neuromas and/or malignant peripheral nerve sheath tumors. Among the agents that have failed are sirolimus, sorafenib, erlotinib, sunitinib, ranibizumab, and cediranib. Imatinib has shown mixed results for the treatment of plexiform neuromas; a clinical trial is ongoing.
Disclosures: Dr. Peltonen said she had no relevant financial conflicts.
GOTHENBURG, SWEDEN - Neurofibromatosis type 1 is a diagnosis every dermatologist should be able to make, according to Dr. Sirkku Peltonen.
Be sure to check the armpits, she said. Examining the axillae in search of a few small freckles often makes the difference between diagnosing neurofibromatosis type 1 (NF1) and overlooking this cancer predisposition syndrome.
"The armpits are so easy to forget – and then you lose the chance to make the diagnosis," said Dr. Peltonen of the University of Turku (Finland).
Diagnosis of NF1 is not difficult, she said. Of children aged 6 years or older, 95% can be clinically diagnosed based on the presence of at least two of the following features: six or more café au lait spots, each more than 5 mm in their greatest diameter; axillary or inguinal freckling; a first-degree relative with NF1; or pseudoarthrosis of the tibia, a finding present in only about 3% of individuals with NF1. These four features, from a longer list of diagnostic criteria established by the National Institutes of Health, are the most relevant to dermatologists (JAMA 1997;278:51-7). The flat, pigmented café au lait macules usually appear by a child’s first birthday.
"If you see four or five café au lait spots, suspect NF1 and look for more," Dr. Peltonen said at the annual congress of the European Academy of Dermatology and Venereology.
Freckles under the armpit appear by 6 years of age. Prior to that, the diagnosis is typically made on the basis of the requisite café au lait spots in a child having a family history of NF1. However, half of patients with NF1 have a sporadic mutation and no family history of the disease, making early diagnosis considerably more difficult.
Laboratory analysis of NF1 is available in challenging cases, most of which involve young children. It is a laborious, expensive process because the NF1 gene, located on chromosome 17, band q11, is large, and hundreds of mutations have been identified to date, said Dr. Peltonen.
In her experience, laboratories vary widely in their ability to perform a high-quality analysis of mutations of the NF1 gene. She singled out the laboratories at the Ghent (Belgium) University and the University of Alabama at Birmingham as being particularly good when it comes to the diagnosis of NF1. Testing costs more than $1,300, she said.
The incidence of NF1, formerly known as von Recklinghausen’s disease, is roughly 1 in 3,000 live births, noted Dr. Peltonen. Inheritance is autosomal dominant with 100% penetrance. This means everyone who has the inherited form of NF1 ought to be readily diagnosable.
NF1 is a multisystem condition. Common features include cognitive impairment in 80% of patients, learning difficulties in 50%-60%, and speech abnormalities in 30%, as well as eye and skeletal abnormalities. Affected children are at risk for social exclusion because of their appearance. A dermatologist who diagnoses NF1 needs to be ready to make a referral to a physician who is familiar with the syndrome for regular follow-up, she said.
Children with NF1 are at 100-fold increased risk for brain tumors, compared with unaffected children. The lifetime risk of developing a malignant peripheral nerve sheath tumor is 10%-15%. Thirty percent or more of patients with NF1 have one or more plexiform neuromas, which are at risk for malignant transformation. The cancers can appear at any age, although most often not until after the teenage years.
Danger signs of malignancy in patients with NF1 include neurogenic pain in the extremities, a painful mass, or accelerated growth of an existing plexiform neuroma, she added.
The majority of adults with NF1 develop cutaneous neurofibromas. These were traditionally thought to have their origin in small dermal nerve twigs containing Schwann cells. In soon-to-be-published work, however, Dr. Peltonen and his coworkers have shown that the tumors actually arise from multipotent stem cells closely associated with hair follicles.
The cutaneous neurofibromas never become malignant. Nevertheless, they have a huge negative impact on quality of life, she said.
Patients consider cutaneous neurofibromas to be the greatest burden of NF1 because they are painful and unsightly. Dermatologists can do patients a great service by removing the benign cutaneous tumors, said Dr. Peltonen. The CO2 laser is effective for this purpose. Dozens of tumors can be removed under local anesthesia in one appointment, and patients are very appreciative. Dr. Peltonen has one patient who travels 9 hours each way for excision of cutaneous neurofibromas.
"I usually make a circle around the tumor and then cut in between dermis and the tumor while pulling on it at the same time," she said. "It’s quite easy to get the tumor out of the skin, and the holes heal nicely."
Numerous cancer drugs have been studied and found wanting for the treatment of plexiform neuromas and/or malignant peripheral nerve sheath tumors. Among the agents that have failed are sirolimus, sorafenib, erlotinib, sunitinib, ranibizumab, and cediranib. Imatinib has shown mixed results for the treatment of plexiform neuromas; a clinical trial is ongoing.
Disclosures: Dr. Peltonen said she had no relevant financial conflicts.
EXPERT ANALYSIS FROM THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Benefits of BRAF Inhibitor Confirmed in Metastatic Melanoma
Phase II trial data confirm that an experimental BRAF inhibitor dramatically shrinks tumors and extends time to disease progression in patients with previously treated BRAF V600 mutation–positive metastatic melanoma, according to a report presented Nov. 5 at the seventh international congress of the Society for Melanoma Research in Sydney.
Of the 132 patients in the BRIM2 trial who received RG7204, also known as PLX4032, 52% responded with tumor shrinkage of at least 30% for at least two consecutive CT scans as assessed by independent review.
In all, 82% of patients had either a response (complete response in 3 patients, partial response in 66) or stable disease (39 patients). The median duration of response was 6.8 months.
Median progression-free survival reached 6.2 months, Dr. Jeffrey Sosman said at the meeting. After a median follow-up of approximately 7 months, 38% of patients were still on treatment.
"I think the response duration being just over 6 months is a very, very significant advance," co-investigator Dr. Rene Gonzalez said in an interview. "The normal time to progression in one of these patients is 6 to 8 weeks, so this is almost a tripling of their progression-free survival."
Both investigators pointed out that the findings confirm earlier data (N. Engl. J. Med. 2010;363:809-19), in which 81% of patients with the BRAF mutation treated with RG7204 had at least 30% tumor shrinkage. No significant predictors of progression or response were identified, except for original tumor size and number.
Dr. Gonzalez said he has no doubt RG7204 will be approved; the question is whether the Food and Drug Administration will do so based on the phase II data or wait until completion of the ongoing phase III BRIM3 trial evaluating overall survival with RG7204 vs. the standard of care, dacarbazine, in patients with previously untreated BRAF V600 mutation–positive metastatic melanoma. The primary end point in that trial is overall survival.
The anti-CTLA4 antibody ipilimumab is also poised for approval after becoming the first drug to show a survival advantage in refractory melanoma in a phase III trial, but it has problems, particularly with toxicity, said Dr. Gonzalez, who worked on trials for both drugs. "I think for a community practitioner, this drug [RG7204], I have no doubt would be the first-line choice, if they had an option," he said.
In the current trial patients received RG7204 at a dose of 960 mg twice daily. Grade 3 or greater adverse events were abnormal liver function (14%), joint pain/arthritis (11%) and dysphagia/pancreatitis (10%). The most common adverse events were rash, photosensitivity, hair loss, and joint pain, reported Dr. Sosman, director of the melanoma and tumor immunotherapy program, Vanderbilt-Ingram Cancer Center, Nashville, Tenn. The secondary end point of overall survival had not yet been reached.
RG7204, which is being codeveloped by Roche Pharmaceuticals and Plexxikon, is a small molecule designed to selectively inhibit the mutated form of the BRAF protein. It is estimated that BRAF mutations are present in about half of melanomas, of which 90% are BRAF V600 mutations.
RG7204 may benefit patients with other BRAF mutations, but there are much less data in this small population, said Dr. Gonzalez, professor of medicine and director of the University of Colorado at Denver Melanoma Research Clinic.
So far, the Achilles’ heel of RG7204 appears to be resistance. "If you have a patient with a duration of 6 months, you do get resistance," Dr. Gonzalez said. Strides have been made to understand the mechanism of resistance, but one logical solution would be to combine RG7204 with an anti-CTLA4-antibody, which tends not to work as fast but has responses that seem to be more durable, he said, adding, "That buys us some time."
It might also be possible to abrogate resistance by blocking both the RAF and MEK pathways or by using pan-RAF inhibitors like RAF265 (Novartis Oncology) that block not only BRAF, but other RAF genes, he said.
Pending a decision by the FDA, plans are underway to open an expanded access program to make RG7204 available to patients with BRAF-mutation–positive advanced melanoma who have received at least one prior treatment, according to Dr. Hal Barron, head of Genentech’s global product development and chief medical officer.
"People with advanced melanoma urgently need more options for treatment and we will continue to work with global health authorities to gather the necessary data to bring this medicine to people with this type of cancer," Dr. Barron said in a statement.
BRIM2 was sponsored by Genentech and Hoffman-La Roche Ltd. Dr. Sosman has received grant support from Roche and Plexxikon.
Phase II trial data confirm that an experimental BRAF inhibitor dramatically shrinks tumors and extends time to disease progression in patients with previously treated BRAF V600 mutation–positive metastatic melanoma, according to a report presented Nov. 5 at the seventh international congress of the Society for Melanoma Research in Sydney.
Of the 132 patients in the BRIM2 trial who received RG7204, also known as PLX4032, 52% responded with tumor shrinkage of at least 30% for at least two consecutive CT scans as assessed by independent review.
In all, 82% of patients had either a response (complete response in 3 patients, partial response in 66) or stable disease (39 patients). The median duration of response was 6.8 months.
Median progression-free survival reached 6.2 months, Dr. Jeffrey Sosman said at the meeting. After a median follow-up of approximately 7 months, 38% of patients were still on treatment.
"I think the response duration being just over 6 months is a very, very significant advance," co-investigator Dr. Rene Gonzalez said in an interview. "The normal time to progression in one of these patients is 6 to 8 weeks, so this is almost a tripling of their progression-free survival."
Both investigators pointed out that the findings confirm earlier data (N. Engl. J. Med. 2010;363:809-19), in which 81% of patients with the BRAF mutation treated with RG7204 had at least 30% tumor shrinkage. No significant predictors of progression or response were identified, except for original tumor size and number.
Dr. Gonzalez said he has no doubt RG7204 will be approved; the question is whether the Food and Drug Administration will do so based on the phase II data or wait until completion of the ongoing phase III BRIM3 trial evaluating overall survival with RG7204 vs. the standard of care, dacarbazine, in patients with previously untreated BRAF V600 mutation–positive metastatic melanoma. The primary end point in that trial is overall survival.
The anti-CTLA4 antibody ipilimumab is also poised for approval after becoming the first drug to show a survival advantage in refractory melanoma in a phase III trial, but it has problems, particularly with toxicity, said Dr. Gonzalez, who worked on trials for both drugs. "I think for a community practitioner, this drug [RG7204], I have no doubt would be the first-line choice, if they had an option," he said.
In the current trial patients received RG7204 at a dose of 960 mg twice daily. Grade 3 or greater adverse events were abnormal liver function (14%), joint pain/arthritis (11%) and dysphagia/pancreatitis (10%). The most common adverse events were rash, photosensitivity, hair loss, and joint pain, reported Dr. Sosman, director of the melanoma and tumor immunotherapy program, Vanderbilt-Ingram Cancer Center, Nashville, Tenn. The secondary end point of overall survival had not yet been reached.
RG7204, which is being codeveloped by Roche Pharmaceuticals and Plexxikon, is a small molecule designed to selectively inhibit the mutated form of the BRAF protein. It is estimated that BRAF mutations are present in about half of melanomas, of which 90% are BRAF V600 mutations.
RG7204 may benefit patients with other BRAF mutations, but there are much less data in this small population, said Dr. Gonzalez, professor of medicine and director of the University of Colorado at Denver Melanoma Research Clinic.
So far, the Achilles’ heel of RG7204 appears to be resistance. "If you have a patient with a duration of 6 months, you do get resistance," Dr. Gonzalez said. Strides have been made to understand the mechanism of resistance, but one logical solution would be to combine RG7204 with an anti-CTLA4-antibody, which tends not to work as fast but has responses that seem to be more durable, he said, adding, "That buys us some time."
It might also be possible to abrogate resistance by blocking both the RAF and MEK pathways or by using pan-RAF inhibitors like RAF265 (Novartis Oncology) that block not only BRAF, but other RAF genes, he said.
Pending a decision by the FDA, plans are underway to open an expanded access program to make RG7204 available to patients with BRAF-mutation–positive advanced melanoma who have received at least one prior treatment, according to Dr. Hal Barron, head of Genentech’s global product development and chief medical officer.
"People with advanced melanoma urgently need more options for treatment and we will continue to work with global health authorities to gather the necessary data to bring this medicine to people with this type of cancer," Dr. Barron said in a statement.
BRIM2 was sponsored by Genentech and Hoffman-La Roche Ltd. Dr. Sosman has received grant support from Roche and Plexxikon.
Phase II trial data confirm that an experimental BRAF inhibitor dramatically shrinks tumors and extends time to disease progression in patients with previously treated BRAF V600 mutation–positive metastatic melanoma, according to a report presented Nov. 5 at the seventh international congress of the Society for Melanoma Research in Sydney.
Of the 132 patients in the BRIM2 trial who received RG7204, also known as PLX4032, 52% responded with tumor shrinkage of at least 30% for at least two consecutive CT scans as assessed by independent review.
In all, 82% of patients had either a response (complete response in 3 patients, partial response in 66) or stable disease (39 patients). The median duration of response was 6.8 months.
Median progression-free survival reached 6.2 months, Dr. Jeffrey Sosman said at the meeting. After a median follow-up of approximately 7 months, 38% of patients were still on treatment.
"I think the response duration being just over 6 months is a very, very significant advance," co-investigator Dr. Rene Gonzalez said in an interview. "The normal time to progression in one of these patients is 6 to 8 weeks, so this is almost a tripling of their progression-free survival."
Both investigators pointed out that the findings confirm earlier data (N. Engl. J. Med. 2010;363:809-19), in which 81% of patients with the BRAF mutation treated with RG7204 had at least 30% tumor shrinkage. No significant predictors of progression or response were identified, except for original tumor size and number.
Dr. Gonzalez said he has no doubt RG7204 will be approved; the question is whether the Food and Drug Administration will do so based on the phase II data or wait until completion of the ongoing phase III BRIM3 trial evaluating overall survival with RG7204 vs. the standard of care, dacarbazine, in patients with previously untreated BRAF V600 mutation–positive metastatic melanoma. The primary end point in that trial is overall survival.
The anti-CTLA4 antibody ipilimumab is also poised for approval after becoming the first drug to show a survival advantage in refractory melanoma in a phase III trial, but it has problems, particularly with toxicity, said Dr. Gonzalez, who worked on trials for both drugs. "I think for a community practitioner, this drug [RG7204], I have no doubt would be the first-line choice, if they had an option," he said.
In the current trial patients received RG7204 at a dose of 960 mg twice daily. Grade 3 or greater adverse events were abnormal liver function (14%), joint pain/arthritis (11%) and dysphagia/pancreatitis (10%). The most common adverse events were rash, photosensitivity, hair loss, and joint pain, reported Dr. Sosman, director of the melanoma and tumor immunotherapy program, Vanderbilt-Ingram Cancer Center, Nashville, Tenn. The secondary end point of overall survival had not yet been reached.
RG7204, which is being codeveloped by Roche Pharmaceuticals and Plexxikon, is a small molecule designed to selectively inhibit the mutated form of the BRAF protein. It is estimated that BRAF mutations are present in about half of melanomas, of which 90% are BRAF V600 mutations.
RG7204 may benefit patients with other BRAF mutations, but there are much less data in this small population, said Dr. Gonzalez, professor of medicine and director of the University of Colorado at Denver Melanoma Research Clinic.
So far, the Achilles’ heel of RG7204 appears to be resistance. "If you have a patient with a duration of 6 months, you do get resistance," Dr. Gonzalez said. Strides have been made to understand the mechanism of resistance, but one logical solution would be to combine RG7204 with an anti-CTLA4-antibody, which tends not to work as fast but has responses that seem to be more durable, he said, adding, "That buys us some time."
It might also be possible to abrogate resistance by blocking both the RAF and MEK pathways or by using pan-RAF inhibitors like RAF265 (Novartis Oncology) that block not only BRAF, but other RAF genes, he said.
Pending a decision by the FDA, plans are underway to open an expanded access program to make RG7204 available to patients with BRAF-mutation–positive advanced melanoma who have received at least one prior treatment, according to Dr. Hal Barron, head of Genentech’s global product development and chief medical officer.
"People with advanced melanoma urgently need more options for treatment and we will continue to work with global health authorities to gather the necessary data to bring this medicine to people with this type of cancer," Dr. Barron said in a statement.
BRIM2 was sponsored by Genentech and Hoffman-La Roche Ltd. Dr. Sosman has received grant support from Roche and Plexxikon.
from the seventh international congress of the Society for Melanoma Research in Sydney
Major Finding: Tumor shrinkage of at least 30% was reported in 52% of patients.
Data Source: Phase II open-label trial in 132 patients with previously treated BRAF V600 mutation-positive metastatic melanoma.
Disclosures: BRIM2 was sponsored by Genentech and Hoffman-La Roche. Dr. Sosman has received grant support from Roche and Plexxikon, and consulting fees or travel reimbursement from Roche.
FDA Postpones Ipilumimab Review
Bristol-Myers Squibb reported on Nov. 2 that the Food and Drug Administration would need more time to review ipilimumab, its biologic drug for melanoma.
The agency was due to make an approval decision by Dec. 25, but now will have until March 26, 2011, according to a statement from Bristol-Myers.
The drug maker said that it submitted additional data to the FDA at the agency’s request.
Ipilimumab, to be marketed as Yervoy, was also due to be reviewed by the FDA’s Oncologic Drugs Advisory Committee on Dec. 2. That meeting is now in doubt, said Ira Loss of "Washington Analysis," a research and analysis company, in an interview.
"I've seen this happen enough times in the past where the product is scheduled on a panel, the company submits additional data, the PDUFA [user fee] date gets pushed out, and then they dropped the meeting," said Mr. Loss.
He still expects approval for ipilimumab, in part because he believes the data are strong, and because "there hasn't been any other product approved for melanoma in years," he said.
In its statement, the drug maker said, "Bristol-Myers Squibb continues to be very encouraged by its interactions with the FDA and remains confident in the overall development program for ipilimumab."
The company noted that "ipilimumab is also currently under review with the European Medicines Agency and other health authorities worldwide."
Bristol-Myers Squibb reported on Nov. 2 that the Food and Drug Administration would need more time to review ipilimumab, its biologic drug for melanoma.
The agency was due to make an approval decision by Dec. 25, but now will have until March 26, 2011, according to a statement from Bristol-Myers.
The drug maker said that it submitted additional data to the FDA at the agency’s request.
Ipilimumab, to be marketed as Yervoy, was also due to be reviewed by the FDA’s Oncologic Drugs Advisory Committee on Dec. 2. That meeting is now in doubt, said Ira Loss of "Washington Analysis," a research and analysis company, in an interview.
"I've seen this happen enough times in the past where the product is scheduled on a panel, the company submits additional data, the PDUFA [user fee] date gets pushed out, and then they dropped the meeting," said Mr. Loss.
He still expects approval for ipilimumab, in part because he believes the data are strong, and because "there hasn't been any other product approved for melanoma in years," he said.
In its statement, the drug maker said, "Bristol-Myers Squibb continues to be very encouraged by its interactions with the FDA and remains confident in the overall development program for ipilimumab."
The company noted that "ipilimumab is also currently under review with the European Medicines Agency and other health authorities worldwide."
Bristol-Myers Squibb reported on Nov. 2 that the Food and Drug Administration would need more time to review ipilimumab, its biologic drug for melanoma.
The agency was due to make an approval decision by Dec. 25, but now will have until March 26, 2011, according to a statement from Bristol-Myers.
The drug maker said that it submitted additional data to the FDA at the agency’s request.
Ipilimumab, to be marketed as Yervoy, was also due to be reviewed by the FDA’s Oncologic Drugs Advisory Committee on Dec. 2. That meeting is now in doubt, said Ira Loss of "Washington Analysis," a research and analysis company, in an interview.
"I've seen this happen enough times in the past where the product is scheduled on a panel, the company submits additional data, the PDUFA [user fee] date gets pushed out, and then they dropped the meeting," said Mr. Loss.
He still expects approval for ipilimumab, in part because he believes the data are strong, and because "there hasn't been any other product approved for melanoma in years," he said.
In its statement, the drug maker said, "Bristol-Myers Squibb continues to be very encouraged by its interactions with the FDA and remains confident in the overall development program for ipilimumab."
The company noted that "ipilimumab is also currently under review with the European Medicines Agency and other health authorities worldwide."
Sunscreens Don't Always Live Up to Advertising Promises
Sunscreens are an important part of a dermatologist's tool kit, but they're not a panacea for UV-induced damage, according to Dr. Timothy Berger.
Although sunscreens work well in laboratory testing, almost no one applies them in a way that will provide the advertised UV protection, Dr. Berger noted at the Las Vegas Dermatology Seminar sponsored by Skin Disease Education Foundation (SDEF). And for some patients, sunscreens can exacerbate their UV-induced problems.
Sunscreens themselves, not the vehicular preservatives, are usually the culprit when a patient presents with a complaint of being allergic to sunscreens, said Dr. Berger, a professor of dermatology at the University of California, San Francisco.
"You will usually find that the dermatitis only occurs in areas touched by the sun," he said in an interview. "The problem is often an allergy to the PABA or PABA-related substances in the sunscreen they are using."
For patients like this, the best alternative is a PABA-free sunscreen that contains zinc and titanium oxide – inert compounds that are physical barriers against UV light, rather than chemical barriers.
And although SPF ratings might look great in advertisements, it is almost a sure bet that sun-seeking patients are not getting nearly the protection promised, Dr. Berger noted. "Some interesting recent studies have looked at what SPF you actually get what you put on sunscreen. The amount used in testing is actually about four times more than what people really use."
One study found that most people apply about 0.5 mg/cm2, while the lab tests use a volume of 2 mg/cm2 to achieve the advertised SPF (J. Am. Acad. Dermatol. 2010;62:218-22). "The problem is, efficacy falls off in a very sharp way," Dr. Berger said. "If you apply SPF 30 sun block [at one-quarter the recommended amount], you only end up with an actual SPF of about 3."
"We need to make sure our patients understand this. And while most people are still not going to use the necessary amount, we can stress the importance of reapplying frequently, which can help keep the protective value up somewhat," he said.
Light Therapy for Atopy
UV light therapy is a frequent treatment for eczema and other atopic dermatoses, but for about 3% of patients, it the wrong thing to offer, Dr. Berger said. "For this small population, sun exposure actually makes the condition worse. Put them in the light box and the eczema will flare."
More than a cursory skin exam should be performed on patients with atopic dermatitis, he recommended. "Have them remove their clothing at least from the waist up. Look for eczema that has a photo distribution: It might be worse on the face and side of the neck, but beneath the chin the skin could be spared. If it looks like a photo-distributed rash, and they report a history of flare with sun exposure, then prescribing aggressive sun protection might make a big difference."
A frequently missed diagnosis in young children – particularly infants – is erythropoietic protoporphyria (EPP). "The first time these infants go out into the sun, they will start screaming, because their skin is burning," Dr. Berger said. As quickly as 1 hour after sun exposure, they may develop erythema, edema, urticarial lesions, or purpura on exposed skin.
Europeans are somewhat ahead in drug research for treating EPP, Dr. Berger noted, but the United States is catching up. The University of California is one of six U.S. study sites participating in a phase II placebo-controlled trial of afamelanotide, a synthetically produced analog of human alpha-melanocytic–stimulating hormone (alpha-MSH).
"The hope is that the drug will stimulate the production of melanin, which actually has two benefits," Dr. Berger said. "First, it's a photo protectant, but it's also an antioxidant."
When porphyrins absorb UV rays, they release free radicals that lead to the acute and chronic damage that occurs on EPP patient skin. The porphyrins can accumulate and damage other tissues – including the liver, which causes problems for about 20% of EPP patients. The extra melanin can help neutralize these highly reactive oxygen species and provide protection from the acute symptoms patients suffer when skin is exposed.
While afamelanotide can never cure EPP, Dr. Berger said, it might afford patients the opportunity to live a more normal life. "While they will never be going out looking for a tan, at least they may be able to enjoy being outdoors without having to be completely encased in clothing."
Dr. Berger disclosed serving as a consultant for Prescription Solutions. SDEF and this news organization are owned by Elsevier.
Sunscreens are an important part of a dermatologist's tool kit, but they're not a panacea for UV-induced damage, according to Dr. Timothy Berger.
Although sunscreens work well in laboratory testing, almost no one applies them in a way that will provide the advertised UV protection, Dr. Berger noted at the Las Vegas Dermatology Seminar sponsored by Skin Disease Education Foundation (SDEF). And for some patients, sunscreens can exacerbate their UV-induced problems.
Sunscreens themselves, not the vehicular preservatives, are usually the culprit when a patient presents with a complaint of being allergic to sunscreens, said Dr. Berger, a professor of dermatology at the University of California, San Francisco.
"You will usually find that the dermatitis only occurs in areas touched by the sun," he said in an interview. "The problem is often an allergy to the PABA or PABA-related substances in the sunscreen they are using."
For patients like this, the best alternative is a PABA-free sunscreen that contains zinc and titanium oxide – inert compounds that are physical barriers against UV light, rather than chemical barriers.
And although SPF ratings might look great in advertisements, it is almost a sure bet that sun-seeking patients are not getting nearly the protection promised, Dr. Berger noted. "Some interesting recent studies have looked at what SPF you actually get what you put on sunscreen. The amount used in testing is actually about four times more than what people really use."
One study found that most people apply about 0.5 mg/cm2, while the lab tests use a volume of 2 mg/cm2 to achieve the advertised SPF (J. Am. Acad. Dermatol. 2010;62:218-22). "The problem is, efficacy falls off in a very sharp way," Dr. Berger said. "If you apply SPF 30 sun block [at one-quarter the recommended amount], you only end up with an actual SPF of about 3."
"We need to make sure our patients understand this. And while most people are still not going to use the necessary amount, we can stress the importance of reapplying frequently, which can help keep the protective value up somewhat," he said.
Light Therapy for Atopy
UV light therapy is a frequent treatment for eczema and other atopic dermatoses, but for about 3% of patients, it the wrong thing to offer, Dr. Berger said. "For this small population, sun exposure actually makes the condition worse. Put them in the light box and the eczema will flare."
More than a cursory skin exam should be performed on patients with atopic dermatitis, he recommended. "Have them remove their clothing at least from the waist up. Look for eczema that has a photo distribution: It might be worse on the face and side of the neck, but beneath the chin the skin could be spared. If it looks like a photo-distributed rash, and they report a history of flare with sun exposure, then prescribing aggressive sun protection might make a big difference."
A frequently missed diagnosis in young children – particularly infants – is erythropoietic protoporphyria (EPP). "The first time these infants go out into the sun, they will start screaming, because their skin is burning," Dr. Berger said. As quickly as 1 hour after sun exposure, they may develop erythema, edema, urticarial lesions, or purpura on exposed skin.
Europeans are somewhat ahead in drug research for treating EPP, Dr. Berger noted, but the United States is catching up. The University of California is one of six U.S. study sites participating in a phase II placebo-controlled trial of afamelanotide, a synthetically produced analog of human alpha-melanocytic–stimulating hormone (alpha-MSH).
"The hope is that the drug will stimulate the production of melanin, which actually has two benefits," Dr. Berger said. "First, it's a photo protectant, but it's also an antioxidant."
When porphyrins absorb UV rays, they release free radicals that lead to the acute and chronic damage that occurs on EPP patient skin. The porphyrins can accumulate and damage other tissues – including the liver, which causes problems for about 20% of EPP patients. The extra melanin can help neutralize these highly reactive oxygen species and provide protection from the acute symptoms patients suffer when skin is exposed.
While afamelanotide can never cure EPP, Dr. Berger said, it might afford patients the opportunity to live a more normal life. "While they will never be going out looking for a tan, at least they may be able to enjoy being outdoors without having to be completely encased in clothing."
Dr. Berger disclosed serving as a consultant for Prescription Solutions. SDEF and this news organization are owned by Elsevier.
Sunscreens are an important part of a dermatologist's tool kit, but they're not a panacea for UV-induced damage, according to Dr. Timothy Berger.
Although sunscreens work well in laboratory testing, almost no one applies them in a way that will provide the advertised UV protection, Dr. Berger noted at the Las Vegas Dermatology Seminar sponsored by Skin Disease Education Foundation (SDEF). And for some patients, sunscreens can exacerbate their UV-induced problems.
Sunscreens themselves, not the vehicular preservatives, are usually the culprit when a patient presents with a complaint of being allergic to sunscreens, said Dr. Berger, a professor of dermatology at the University of California, San Francisco.
"You will usually find that the dermatitis only occurs in areas touched by the sun," he said in an interview. "The problem is often an allergy to the PABA or PABA-related substances in the sunscreen they are using."
For patients like this, the best alternative is a PABA-free sunscreen that contains zinc and titanium oxide – inert compounds that are physical barriers against UV light, rather than chemical barriers.
And although SPF ratings might look great in advertisements, it is almost a sure bet that sun-seeking patients are not getting nearly the protection promised, Dr. Berger noted. "Some interesting recent studies have looked at what SPF you actually get what you put on sunscreen. The amount used in testing is actually about four times more than what people really use."
One study found that most people apply about 0.5 mg/cm2, while the lab tests use a volume of 2 mg/cm2 to achieve the advertised SPF (J. Am. Acad. Dermatol. 2010;62:218-22). "The problem is, efficacy falls off in a very sharp way," Dr. Berger said. "If you apply SPF 30 sun block [at one-quarter the recommended amount], you only end up with an actual SPF of about 3."
"We need to make sure our patients understand this. And while most people are still not going to use the necessary amount, we can stress the importance of reapplying frequently, which can help keep the protective value up somewhat," he said.
Light Therapy for Atopy
UV light therapy is a frequent treatment for eczema and other atopic dermatoses, but for about 3% of patients, it the wrong thing to offer, Dr. Berger said. "For this small population, sun exposure actually makes the condition worse. Put them in the light box and the eczema will flare."
More than a cursory skin exam should be performed on patients with atopic dermatitis, he recommended. "Have them remove their clothing at least from the waist up. Look for eczema that has a photo distribution: It might be worse on the face and side of the neck, but beneath the chin the skin could be spared. If it looks like a photo-distributed rash, and they report a history of flare with sun exposure, then prescribing aggressive sun protection might make a big difference."
A frequently missed diagnosis in young children – particularly infants – is erythropoietic protoporphyria (EPP). "The first time these infants go out into the sun, they will start screaming, because their skin is burning," Dr. Berger said. As quickly as 1 hour after sun exposure, they may develop erythema, edema, urticarial lesions, or purpura on exposed skin.
Europeans are somewhat ahead in drug research for treating EPP, Dr. Berger noted, but the United States is catching up. The University of California is one of six U.S. study sites participating in a phase II placebo-controlled trial of afamelanotide, a synthetically produced analog of human alpha-melanocytic–stimulating hormone (alpha-MSH).
"The hope is that the drug will stimulate the production of melanin, which actually has two benefits," Dr. Berger said. "First, it's a photo protectant, but it's also an antioxidant."
When porphyrins absorb UV rays, they release free radicals that lead to the acute and chronic damage that occurs on EPP patient skin. The porphyrins can accumulate and damage other tissues – including the liver, which causes problems for about 20% of EPP patients. The extra melanin can help neutralize these highly reactive oxygen species and provide protection from the acute symptoms patients suffer when skin is exposed.
While afamelanotide can never cure EPP, Dr. Berger said, it might afford patients the opportunity to live a more normal life. "While they will never be going out looking for a tan, at least they may be able to enjoy being outdoors without having to be completely encased in clothing."
Dr. Berger disclosed serving as a consultant for Prescription Solutions. SDEF and this news organization are owned by Elsevier.
EXPERT ANALYSIS FROM THE SDEF LAS VEGAS DERMATOLOGY SEMINAR
Imiquimod Effective for Many Skin Cancers, Expert Says
SANTA BARBARA, CALIF. - Imiquimod should be considered a possible treatment option for most skin cancer patients, according to Dr. Craig Kraffert.
Currently, imiquimod is approved only for the treatment actinic keratoses and basal cell carcinoma (BCC), but its greatest therapeutic benefit may occur with off-label use, Dr. Kraffert said at the annual meeting of the California Society of Dermatology and Dermatologic Surgery.
"It’s reasonable to consider for all basal cell carcinoma subtypes, but it’s not for aggressive or large squamous cell carcinoma, and it’s certainly not for invasive melanoma," said Dr. Kraffert, who practices dermatology in Redding, Calif.
Advantages to using imiquimod, he said, include the fact it doesn’t require surgery, it has a good cure rate, noncures are identifiable, and it causes minimal scarring "Disadvantages are that it’s time consuming, there’s transient morbidity, and it requires a coherent, compliant patient as well as provider oversight," he said. "And it’s a bit expensive."
In his practice, he uses imiquimod for patients with all types of BCC, for those with in situ and superficially invasive squamous cell carcinoma (SCC), and for those with melanoma in situ. "It’s also good for premalignant epithelial targets such as actinic keratoses, actinic cheilitis, vulvar intraepithelial neoplasia, vaginal intraepithelial neoplasia, and oral leukoplakia," he said. "Imiquimod therapeutic considerations for malignant and premalignant processes can be considered essentially equivalent."
He presented clinical data from patients in his practice who have been treated with imiquimod to date. Of 50 patients with SCC in situ, 70% had success with combination therapy, 28% had success with monotherapy, and 2% discontinued therapy.
Of 328 patients with BCC, 53% had success with monotherapy, 34% had success with combination therapy, and 13% discontinued therapy.
Of 25 patients with melanoma in situ, 20% had success with monotherapy, 56% had success with combination therapy, and 24% discontinued therapy.
The drug’s mechanism of action is not entirely understood, but it appears to activate both innate immunity with a cytokine cascade, as well as adaptive immunity with Langerhans cells. "It also causes a direct apoptotic and antineoplastic effect," he said.
In his opinion, high-utility niches include any patient seeking minimized scarring and who doesn’t want surgery, electrodesiccation and curettage (ED & C) failure, partially infiltrative or partially superficial BCC, multifocal temporal BCC, and large and/or ill-defined facial SCC in situ.
He finds that using imiquimod after ED & C in challenging tumors or in tumors with an infiltrative component increases the cure rate and decreases scarring. "I contend that imiquimod after ED & C generally gives you a better scar than ED & C alone," he said. "I don’t know why, but it does. Brisk reaction can be challenging, and close monitoring is required."
Using imiquimod prior to excision "may shrink tumors, leading to a smaller defect, better cosmesis, possible lower recurrence rate, and potential for complete nonsurgical response," he added.
He also finds the drug helpful for infiltrative BCCs in cosmetically vulnerable areas and for edge persistence after Mohs surgery. "Part of this is because imiquimod seems to work better on infiltrative basal cell carcinomas than it does on nodular basal cell carcinomas," he explained. "I think that’s because of access of the immune system to the thin threads of tumor. It’s also useful for melanoma in situ, particularly in cosmetically vulnerable areas. With melanoma in situ, melanin is a visible marker that serves as a clinical treatment site monitor. I don’t know of any reports of amelanotic transformation from imiquimod. Scarring is often minimal or absent, and there is tumor margin auto detection. It does have curative potential for melanoma in situ."
He emphasized that imiquimod monotherapy in patients with melanoma in situ is difficult, with varied response. "That’s where 5-FU [fluorouracil] in addition to imiquimod is often extremely useful," he said.
Dr. Kraffert described imiquimod as "a thinking practitioner’s medication" that requires thought and oversight. "It may provide excellent outcomes when least expected, so it’s important to keep an open mind regarding the clinical utility of this medication with skin cancer," he said.
Patients must be able to follow instructions, comply with treatment, return for monitoring and surveillance, and call or return to the clinic when appropriate. "Believe it or not, these limited requirements exclude many potential candidates," he remarked.
Patients typically use the drug for 6 weeks, beginning with twice daily dosing, "but it’s variable," he said. "For optimal success treatment must be strong enough for long enough."
At the initial consultation Dr. Kraffert educates his patient about the concept of delayed gratification, the reasons why the medication was suggested, and reaffirms that he will be there to oversee treatment.
He instructs them on how to use the medication, explaining they need to rub it into the lesion twice a day very well. "A pinhead amount is all that’s needed," he said, only a small portion of the 0.25 g included in each packet. His patients typically get a few to several applications from each packet.
He also educates patients about possible side effects from imiquimod, including redness, crusting, oozing, and some tenderness. "Nausea, malaise, pain, and secondary infection are rare," he said. "Patients are advised to call the office with any significant treatment related concerns and are generally offered the option of a same day add-on appointment."
At the 2-week follow-up visit he gauges the response as very brisk, brisk, moderate, mild, or none. If the response is very brisk, he halts therapy for 3-7 days, re-initiates treatment once daily for 4 weeks, and schedules a follow-up visit after 2 more weeks of treatment.
If the response is brisk, he halts therapy for 1-3 days, and then continues therapy once daily for 4 weeks.
If the response is moderate he continues therapy once daily for 4 weeks, and if the response is mild he continues therapy twice daily for 4 weeks.
"If there is no response, consider further imiquimod treatment with 2-4-week re-evaluation, or consider application of a small (less than a pea-sized) amount of 5% 5-FU after imiquimod twice daily," Dr. Kraffert said. "Or, you could consider nonimiquimod management of the problem. Sometimes imiquimod just doesn’t work."
After the 2-week follow-up, further visits are discretionary, he said, but some patients require closer follow-up, including nonresponders and patients with severe reactions, systemic complaints, and large or challenging tumors.
"Scheduling a follow-up visit for 3-6 months is best because the reaction takes a month or 2 to diminish," he said. "Taking photos pretherapy and at each visit thereafter improves surveillance."
"There are many approaches to skin cancer," Dr. Kraffert concluded. "Excision or destruction is often the best, but having more options is a plus. Imiquimod doesn’t replace what we have, it just adds to it."
Dr. Kraffert said he had no relevant financial conflicts.
SANTA BARBARA, CALIF. - Imiquimod should be considered a possible treatment option for most skin cancer patients, according to Dr. Craig Kraffert.
Currently, imiquimod is approved only for the treatment actinic keratoses and basal cell carcinoma (BCC), but its greatest therapeutic benefit may occur with off-label use, Dr. Kraffert said at the annual meeting of the California Society of Dermatology and Dermatologic Surgery.
"It’s reasonable to consider for all basal cell carcinoma subtypes, but it’s not for aggressive or large squamous cell carcinoma, and it’s certainly not for invasive melanoma," said Dr. Kraffert, who practices dermatology in Redding, Calif.
Advantages to using imiquimod, he said, include the fact it doesn’t require surgery, it has a good cure rate, noncures are identifiable, and it causes minimal scarring "Disadvantages are that it’s time consuming, there’s transient morbidity, and it requires a coherent, compliant patient as well as provider oversight," he said. "And it’s a bit expensive."
In his practice, he uses imiquimod for patients with all types of BCC, for those with in situ and superficially invasive squamous cell carcinoma (SCC), and for those with melanoma in situ. "It’s also good for premalignant epithelial targets such as actinic keratoses, actinic cheilitis, vulvar intraepithelial neoplasia, vaginal intraepithelial neoplasia, and oral leukoplakia," he said. "Imiquimod therapeutic considerations for malignant and premalignant processes can be considered essentially equivalent."
He presented clinical data from patients in his practice who have been treated with imiquimod to date. Of 50 patients with SCC in situ, 70% had success with combination therapy, 28% had success with monotherapy, and 2% discontinued therapy.
Of 328 patients with BCC, 53% had success with monotherapy, 34% had success with combination therapy, and 13% discontinued therapy.
Of 25 patients with melanoma in situ, 20% had success with monotherapy, 56% had success with combination therapy, and 24% discontinued therapy.
The drug’s mechanism of action is not entirely understood, but it appears to activate both innate immunity with a cytokine cascade, as well as adaptive immunity with Langerhans cells. "It also causes a direct apoptotic and antineoplastic effect," he said.
In his opinion, high-utility niches include any patient seeking minimized scarring and who doesn’t want surgery, electrodesiccation and curettage (ED & C) failure, partially infiltrative or partially superficial BCC, multifocal temporal BCC, and large and/or ill-defined facial SCC in situ.
He finds that using imiquimod after ED & C in challenging tumors or in tumors with an infiltrative component increases the cure rate and decreases scarring. "I contend that imiquimod after ED & C generally gives you a better scar than ED & C alone," he said. "I don’t know why, but it does. Brisk reaction can be challenging, and close monitoring is required."
Using imiquimod prior to excision "may shrink tumors, leading to a smaller defect, better cosmesis, possible lower recurrence rate, and potential for complete nonsurgical response," he added.
He also finds the drug helpful for infiltrative BCCs in cosmetically vulnerable areas and for edge persistence after Mohs surgery. "Part of this is because imiquimod seems to work better on infiltrative basal cell carcinomas than it does on nodular basal cell carcinomas," he explained. "I think that’s because of access of the immune system to the thin threads of tumor. It’s also useful for melanoma in situ, particularly in cosmetically vulnerable areas. With melanoma in situ, melanin is a visible marker that serves as a clinical treatment site monitor. I don’t know of any reports of amelanotic transformation from imiquimod. Scarring is often minimal or absent, and there is tumor margin auto detection. It does have curative potential for melanoma in situ."
He emphasized that imiquimod monotherapy in patients with melanoma in situ is difficult, with varied response. "That’s where 5-FU [fluorouracil] in addition to imiquimod is often extremely useful," he said.
Dr. Kraffert described imiquimod as "a thinking practitioner’s medication" that requires thought and oversight. "It may provide excellent outcomes when least expected, so it’s important to keep an open mind regarding the clinical utility of this medication with skin cancer," he said.
Patients must be able to follow instructions, comply with treatment, return for monitoring and surveillance, and call or return to the clinic when appropriate. "Believe it or not, these limited requirements exclude many potential candidates," he remarked.
Patients typically use the drug for 6 weeks, beginning with twice daily dosing, "but it’s variable," he said. "For optimal success treatment must be strong enough for long enough."
At the initial consultation Dr. Kraffert educates his patient about the concept of delayed gratification, the reasons why the medication was suggested, and reaffirms that he will be there to oversee treatment.
He instructs them on how to use the medication, explaining they need to rub it into the lesion twice a day very well. "A pinhead amount is all that’s needed," he said, only a small portion of the 0.25 g included in each packet. His patients typically get a few to several applications from each packet.
He also educates patients about possible side effects from imiquimod, including redness, crusting, oozing, and some tenderness. "Nausea, malaise, pain, and secondary infection are rare," he said. "Patients are advised to call the office with any significant treatment related concerns and are generally offered the option of a same day add-on appointment."
At the 2-week follow-up visit he gauges the response as very brisk, brisk, moderate, mild, or none. If the response is very brisk, he halts therapy for 3-7 days, re-initiates treatment once daily for 4 weeks, and schedules a follow-up visit after 2 more weeks of treatment.
If the response is brisk, he halts therapy for 1-3 days, and then continues therapy once daily for 4 weeks.
If the response is moderate he continues therapy once daily for 4 weeks, and if the response is mild he continues therapy twice daily for 4 weeks.
"If there is no response, consider further imiquimod treatment with 2-4-week re-evaluation, or consider application of a small (less than a pea-sized) amount of 5% 5-FU after imiquimod twice daily," Dr. Kraffert said. "Or, you could consider nonimiquimod management of the problem. Sometimes imiquimod just doesn’t work."
After the 2-week follow-up, further visits are discretionary, he said, but some patients require closer follow-up, including nonresponders and patients with severe reactions, systemic complaints, and large or challenging tumors.
"Scheduling a follow-up visit for 3-6 months is best because the reaction takes a month or 2 to diminish," he said. "Taking photos pretherapy and at each visit thereafter improves surveillance."
"There are many approaches to skin cancer," Dr. Kraffert concluded. "Excision or destruction is often the best, but having more options is a plus. Imiquimod doesn’t replace what we have, it just adds to it."
Dr. Kraffert said he had no relevant financial conflicts.
SANTA BARBARA, CALIF. - Imiquimod should be considered a possible treatment option for most skin cancer patients, according to Dr. Craig Kraffert.
Currently, imiquimod is approved only for the treatment actinic keratoses and basal cell carcinoma (BCC), but its greatest therapeutic benefit may occur with off-label use, Dr. Kraffert said at the annual meeting of the California Society of Dermatology and Dermatologic Surgery.
"It’s reasonable to consider for all basal cell carcinoma subtypes, but it’s not for aggressive or large squamous cell carcinoma, and it’s certainly not for invasive melanoma," said Dr. Kraffert, who practices dermatology in Redding, Calif.
Advantages to using imiquimod, he said, include the fact it doesn’t require surgery, it has a good cure rate, noncures are identifiable, and it causes minimal scarring "Disadvantages are that it’s time consuming, there’s transient morbidity, and it requires a coherent, compliant patient as well as provider oversight," he said. "And it’s a bit expensive."
In his practice, he uses imiquimod for patients with all types of BCC, for those with in situ and superficially invasive squamous cell carcinoma (SCC), and for those with melanoma in situ. "It’s also good for premalignant epithelial targets such as actinic keratoses, actinic cheilitis, vulvar intraepithelial neoplasia, vaginal intraepithelial neoplasia, and oral leukoplakia," he said. "Imiquimod therapeutic considerations for malignant and premalignant processes can be considered essentially equivalent."
He presented clinical data from patients in his practice who have been treated with imiquimod to date. Of 50 patients with SCC in situ, 70% had success with combination therapy, 28% had success with monotherapy, and 2% discontinued therapy.
Of 328 patients with BCC, 53% had success with monotherapy, 34% had success with combination therapy, and 13% discontinued therapy.
Of 25 patients with melanoma in situ, 20% had success with monotherapy, 56% had success with combination therapy, and 24% discontinued therapy.
The drug’s mechanism of action is not entirely understood, but it appears to activate both innate immunity with a cytokine cascade, as well as adaptive immunity with Langerhans cells. "It also causes a direct apoptotic and antineoplastic effect," he said.
In his opinion, high-utility niches include any patient seeking minimized scarring and who doesn’t want surgery, electrodesiccation and curettage (ED & C) failure, partially infiltrative or partially superficial BCC, multifocal temporal BCC, and large and/or ill-defined facial SCC in situ.
He finds that using imiquimod after ED & C in challenging tumors or in tumors with an infiltrative component increases the cure rate and decreases scarring. "I contend that imiquimod after ED & C generally gives you a better scar than ED & C alone," he said. "I don’t know why, but it does. Brisk reaction can be challenging, and close monitoring is required."
Using imiquimod prior to excision "may shrink tumors, leading to a smaller defect, better cosmesis, possible lower recurrence rate, and potential for complete nonsurgical response," he added.
He also finds the drug helpful for infiltrative BCCs in cosmetically vulnerable areas and for edge persistence after Mohs surgery. "Part of this is because imiquimod seems to work better on infiltrative basal cell carcinomas than it does on nodular basal cell carcinomas," he explained. "I think that’s because of access of the immune system to the thin threads of tumor. It’s also useful for melanoma in situ, particularly in cosmetically vulnerable areas. With melanoma in situ, melanin is a visible marker that serves as a clinical treatment site monitor. I don’t know of any reports of amelanotic transformation from imiquimod. Scarring is often minimal or absent, and there is tumor margin auto detection. It does have curative potential for melanoma in situ."
He emphasized that imiquimod monotherapy in patients with melanoma in situ is difficult, with varied response. "That’s where 5-FU [fluorouracil] in addition to imiquimod is often extremely useful," he said.
Dr. Kraffert described imiquimod as "a thinking practitioner’s medication" that requires thought and oversight. "It may provide excellent outcomes when least expected, so it’s important to keep an open mind regarding the clinical utility of this medication with skin cancer," he said.
Patients must be able to follow instructions, comply with treatment, return for monitoring and surveillance, and call or return to the clinic when appropriate. "Believe it or not, these limited requirements exclude many potential candidates," he remarked.
Patients typically use the drug for 6 weeks, beginning with twice daily dosing, "but it’s variable," he said. "For optimal success treatment must be strong enough for long enough."
At the initial consultation Dr. Kraffert educates his patient about the concept of delayed gratification, the reasons why the medication was suggested, and reaffirms that he will be there to oversee treatment.
He instructs them on how to use the medication, explaining they need to rub it into the lesion twice a day very well. "A pinhead amount is all that’s needed," he said, only a small portion of the 0.25 g included in each packet. His patients typically get a few to several applications from each packet.
He also educates patients about possible side effects from imiquimod, including redness, crusting, oozing, and some tenderness. "Nausea, malaise, pain, and secondary infection are rare," he said. "Patients are advised to call the office with any significant treatment related concerns and are generally offered the option of a same day add-on appointment."
At the 2-week follow-up visit he gauges the response as very brisk, brisk, moderate, mild, or none. If the response is very brisk, he halts therapy for 3-7 days, re-initiates treatment once daily for 4 weeks, and schedules a follow-up visit after 2 more weeks of treatment.
If the response is brisk, he halts therapy for 1-3 days, and then continues therapy once daily for 4 weeks.
If the response is moderate he continues therapy once daily for 4 weeks, and if the response is mild he continues therapy twice daily for 4 weeks.
"If there is no response, consider further imiquimod treatment with 2-4-week re-evaluation, or consider application of a small (less than a pea-sized) amount of 5% 5-FU after imiquimod twice daily," Dr. Kraffert said. "Or, you could consider nonimiquimod management of the problem. Sometimes imiquimod just doesn’t work."
After the 2-week follow-up, further visits are discretionary, he said, but some patients require closer follow-up, including nonresponders and patients with severe reactions, systemic complaints, and large or challenging tumors.
"Scheduling a follow-up visit for 3-6 months is best because the reaction takes a month or 2 to diminish," he said. "Taking photos pretherapy and at each visit thereafter improves surveillance."
"There are many approaches to skin cancer," Dr. Kraffert concluded. "Excision or destruction is often the best, but having more options is a plus. Imiquimod doesn’t replace what we have, it just adds to it."
Dr. Kraffert said he had no relevant financial conflicts.
EXPERT ANALYSIS FROM THE ANNUAL MEETING OF THE CALIFORNIA SOCIETY OF DERMATOLOGY AND DERMATOLOGIC SURGERY
Photodamage, Part 2: Management of Photoaging
EADV: Legius Syndrome Easily Misdiagnosed as Neurofibromatosis Type 1
GOTHENBURG, SWEDEN - Legius syndrome, first described only 3 years ago, can be easily misdiagnosed as neurofibromatosis type 1.
The diagnostic confusion has important consequences for the peace of mind of patients and their families. While Legius syndrome and neurofibromatosis type 1 (NF1) share several phenotypic features, Legius syndrome, unlike NF1, does not carry an increased cancer risk. Nor do patients with Legius syndrome develop clusters of benign cutaneous neurofibromas, Dr. Sirkku Peltonen said at the annual congress of the European Academy of Dermatology and Venereology.
As first described by Dr. Eric Legius and coworkers (Nat. Genet. 2007;39:1120-6) at the Catholic University of Leuven (Belgium), the hallmarks of Legius syndrome include multiple café au lait macules, axillary freckling, and autosomal dominant transmission, all of which are also among the NF1 diagnostic criteria established by the National Institutes of Health (JAMA 1997;278:51-7). But patients with Legius syndrome do not develop any other characteristic findings of NF1, such as bone lesions, plexiform or cutaneous neurofibromas, Lisch nodules in the iris, and nervous system tumors.
Legius syndrome is caused by mutations in the SPRED1 gene, while NF1 is caused by mutations in the gene encoding neurofibromin. Consider the possibility of Legius syndrome, which can be confirmed by molecular genetic analysis, in patients with six or more café au lait macules and axillary freckling but none of the other classic features of NF1, urged Dr. Peltonen of the University of Turku (Finland).
How often is Legius syndrome mistaken for NF1? A report by Dr. Legius and coworkers concluded half a cohort of 40 patients with Legius syndrome confirmed by genetic analysis fulfilled the NIH diagnostic criteria for NF1 based upon the presence of the requisite number of café au lait spots, axillary freckling, and/or a family history compatible with NF1. In the same report, the international group of investigators determined that 1.9% of a series of 1,318 patients with the clinical diagnosis of NF1 based upon the NIH criteria actually had Legius syndrome (JAMA 2009;302:2111-8).
Dr. Peltonen declared having no relevant financial interests.
GOTHENBURG, SWEDEN - Legius syndrome, first described only 3 years ago, can be easily misdiagnosed as neurofibromatosis type 1.
The diagnostic confusion has important consequences for the peace of mind of patients and their families. While Legius syndrome and neurofibromatosis type 1 (NF1) share several phenotypic features, Legius syndrome, unlike NF1, does not carry an increased cancer risk. Nor do patients with Legius syndrome develop clusters of benign cutaneous neurofibromas, Dr. Sirkku Peltonen said at the annual congress of the European Academy of Dermatology and Venereology.
As first described by Dr. Eric Legius and coworkers (Nat. Genet. 2007;39:1120-6) at the Catholic University of Leuven (Belgium), the hallmarks of Legius syndrome include multiple café au lait macules, axillary freckling, and autosomal dominant transmission, all of which are also among the NF1 diagnostic criteria established by the National Institutes of Health (JAMA 1997;278:51-7). But patients with Legius syndrome do not develop any other characteristic findings of NF1, such as bone lesions, plexiform or cutaneous neurofibromas, Lisch nodules in the iris, and nervous system tumors.
Legius syndrome is caused by mutations in the SPRED1 gene, while NF1 is caused by mutations in the gene encoding neurofibromin. Consider the possibility of Legius syndrome, which can be confirmed by molecular genetic analysis, in patients with six or more café au lait macules and axillary freckling but none of the other classic features of NF1, urged Dr. Peltonen of the University of Turku (Finland).
How often is Legius syndrome mistaken for NF1? A report by Dr. Legius and coworkers concluded half a cohort of 40 patients with Legius syndrome confirmed by genetic analysis fulfilled the NIH diagnostic criteria for NF1 based upon the presence of the requisite number of café au lait spots, axillary freckling, and/or a family history compatible with NF1. In the same report, the international group of investigators determined that 1.9% of a series of 1,318 patients with the clinical diagnosis of NF1 based upon the NIH criteria actually had Legius syndrome (JAMA 2009;302:2111-8).
Dr. Peltonen declared having no relevant financial interests.
GOTHENBURG, SWEDEN - Legius syndrome, first described only 3 years ago, can be easily misdiagnosed as neurofibromatosis type 1.
The diagnostic confusion has important consequences for the peace of mind of patients and their families. While Legius syndrome and neurofibromatosis type 1 (NF1) share several phenotypic features, Legius syndrome, unlike NF1, does not carry an increased cancer risk. Nor do patients with Legius syndrome develop clusters of benign cutaneous neurofibromas, Dr. Sirkku Peltonen said at the annual congress of the European Academy of Dermatology and Venereology.
As first described by Dr. Eric Legius and coworkers (Nat. Genet. 2007;39:1120-6) at the Catholic University of Leuven (Belgium), the hallmarks of Legius syndrome include multiple café au lait macules, axillary freckling, and autosomal dominant transmission, all of which are also among the NF1 diagnostic criteria established by the National Institutes of Health (JAMA 1997;278:51-7). But patients with Legius syndrome do not develop any other characteristic findings of NF1, such as bone lesions, plexiform or cutaneous neurofibromas, Lisch nodules in the iris, and nervous system tumors.
Legius syndrome is caused by mutations in the SPRED1 gene, while NF1 is caused by mutations in the gene encoding neurofibromin. Consider the possibility of Legius syndrome, which can be confirmed by molecular genetic analysis, in patients with six or more café au lait macules and axillary freckling but none of the other classic features of NF1, urged Dr. Peltonen of the University of Turku (Finland).
How often is Legius syndrome mistaken for NF1? A report by Dr. Legius and coworkers concluded half a cohort of 40 patients with Legius syndrome confirmed by genetic analysis fulfilled the NIH diagnostic criteria for NF1 based upon the presence of the requisite number of café au lait spots, axillary freckling, and/or a family history compatible with NF1. In the same report, the international group of investigators determined that 1.9% of a series of 1,318 patients with the clinical diagnosis of NF1 based upon the NIH criteria actually had Legius syndrome (JAMA 2009;302:2111-8).
Dr. Peltonen declared having no relevant financial interests.
EXPERT ANALYSIS FROM THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
EADV: Diclofenac Gel Clears Actinic Keratoses in Transplant Recipients
GOTHENBURG, SWEDEN – Topical diclofenac 3% gel proved effective for the treatment of actinic keratoses in organ transplant recipients, according to the results of a new study.
Sixteen weeks of twice-daily therapy with 3% diclofenac in 2.5% hyaluronic acid (Solaraze) not only proved effective and well tolerated for actinic keratoses (AK) clearance in a randomized, placebo-controlled trial, it also prevented invasive squamous cell carcinomas (SCCs) in organ transplant recipients, Dr. Eggert Stockfleth reported at the annual congress of the European Academy of Dermatology and Venereology.
Based upon an earlier favorable but uncontrolled six-patient series (B. J. Dermatol. 2007;156 Suppl. 3:40-2), Dr. Stockfleth and his coworkers conducted a follow-up randomized trial of topical diclofenace 3% gel in 32 organ transplant recipients.
Organ transplant recipients' immunocompromised status renders them highly vulnerable to multiple invasive SCCs, he said. As graft survival has improved over the years, the high incidence of aggressive cutaneous malignancies in organ transplant recipients has come to the fore as a major concern.
In Australia and New Zealand, cancer is now the No. 1 cause of death in organ transplant recipients, not heart disease, graft rejection, or infection. And the risk of aggressive skin cancers in these patients is far higher than the risk of other malignancies, according to Dr. Stockfleth of the director of the skin cancer center at Charité University Hospital, Berlin.
The 32 study participants were randomized 3-to-1 to diclofenac or a vehicle control. Study eligibility required a stable graft during the previous 12 months plus three or more AKs in a 50-cm2 area on the face, hands, or scalp.
Twenty-eight patients (88%) completed the 16-week, twice-daily treatment phase and presented for final evaluation 4 weeks later. Complete clearance of AKs was achieved in 9 of 22 (41%) of the diclofenac group, compared with 0 of 6 controls.
Side effects were limited to mild erythema and mild-to-moderate swelling of treated areas. No laboratory abnormalities or effects on graft function were noted.
With further follow-up, 55% of the patients who had previously cleared developed new AKs in the treated areas an average of 9.3 months after treatment ended. None of these patients developed invasive SCC in the study area within 24 months of follow-up, suggesting that topical diclofenac gel may also prevent invasive SCCs in this high-risk population.
Other treatments that have demonstrated efficacy for treatment of AKs and/or prevention of nonmelanoma skin cancer in high-risk organ transplant recipients include regular use of a sunscreen, imiquimod 5% cream, topical 5-fluorouracil, and photodynamic therapy.
Dr. Stockfleth disclosed serving as a consultant to Graceway, Almirall, Spirig, Intendis, Meda, Abbott, and LEO.
GOTHENBURG, SWEDEN – Topical diclofenac 3% gel proved effective for the treatment of actinic keratoses in organ transplant recipients, according to the results of a new study.
Sixteen weeks of twice-daily therapy with 3% diclofenac in 2.5% hyaluronic acid (Solaraze) not only proved effective and well tolerated for actinic keratoses (AK) clearance in a randomized, placebo-controlled trial, it also prevented invasive squamous cell carcinomas (SCCs) in organ transplant recipients, Dr. Eggert Stockfleth reported at the annual congress of the European Academy of Dermatology and Venereology.
Based upon an earlier favorable but uncontrolled six-patient series (B. J. Dermatol. 2007;156 Suppl. 3:40-2), Dr. Stockfleth and his coworkers conducted a follow-up randomized trial of topical diclofenace 3% gel in 32 organ transplant recipients.
Organ transplant recipients' immunocompromised status renders them highly vulnerable to multiple invasive SCCs, he said. As graft survival has improved over the years, the high incidence of aggressive cutaneous malignancies in organ transplant recipients has come to the fore as a major concern.
In Australia and New Zealand, cancer is now the No. 1 cause of death in organ transplant recipients, not heart disease, graft rejection, or infection. And the risk of aggressive skin cancers in these patients is far higher than the risk of other malignancies, according to Dr. Stockfleth of the director of the skin cancer center at Charité University Hospital, Berlin.
The 32 study participants were randomized 3-to-1 to diclofenac or a vehicle control. Study eligibility required a stable graft during the previous 12 months plus three or more AKs in a 50-cm2 area on the face, hands, or scalp.
Twenty-eight patients (88%) completed the 16-week, twice-daily treatment phase and presented for final evaluation 4 weeks later. Complete clearance of AKs was achieved in 9 of 22 (41%) of the diclofenac group, compared with 0 of 6 controls.
Side effects were limited to mild erythema and mild-to-moderate swelling of treated areas. No laboratory abnormalities or effects on graft function were noted.
With further follow-up, 55% of the patients who had previously cleared developed new AKs in the treated areas an average of 9.3 months after treatment ended. None of these patients developed invasive SCC in the study area within 24 months of follow-up, suggesting that topical diclofenac gel may also prevent invasive SCCs in this high-risk population.
Other treatments that have demonstrated efficacy for treatment of AKs and/or prevention of nonmelanoma skin cancer in high-risk organ transplant recipients include regular use of a sunscreen, imiquimod 5% cream, topical 5-fluorouracil, and photodynamic therapy.
Dr. Stockfleth disclosed serving as a consultant to Graceway, Almirall, Spirig, Intendis, Meda, Abbott, and LEO.
GOTHENBURG, SWEDEN – Topical diclofenac 3% gel proved effective for the treatment of actinic keratoses in organ transplant recipients, according to the results of a new study.
Sixteen weeks of twice-daily therapy with 3% diclofenac in 2.5% hyaluronic acid (Solaraze) not only proved effective and well tolerated for actinic keratoses (AK) clearance in a randomized, placebo-controlled trial, it also prevented invasive squamous cell carcinomas (SCCs) in organ transplant recipients, Dr. Eggert Stockfleth reported at the annual congress of the European Academy of Dermatology and Venereology.
Based upon an earlier favorable but uncontrolled six-patient series (B. J. Dermatol. 2007;156 Suppl. 3:40-2), Dr. Stockfleth and his coworkers conducted a follow-up randomized trial of topical diclofenace 3% gel in 32 organ transplant recipients.
Organ transplant recipients' immunocompromised status renders them highly vulnerable to multiple invasive SCCs, he said. As graft survival has improved over the years, the high incidence of aggressive cutaneous malignancies in organ transplant recipients has come to the fore as a major concern.
In Australia and New Zealand, cancer is now the No. 1 cause of death in organ transplant recipients, not heart disease, graft rejection, or infection. And the risk of aggressive skin cancers in these patients is far higher than the risk of other malignancies, according to Dr. Stockfleth of the director of the skin cancer center at Charité University Hospital, Berlin.
The 32 study participants were randomized 3-to-1 to diclofenac or a vehicle control. Study eligibility required a stable graft during the previous 12 months plus three or more AKs in a 50-cm2 area on the face, hands, or scalp.
Twenty-eight patients (88%) completed the 16-week, twice-daily treatment phase and presented for final evaluation 4 weeks later. Complete clearance of AKs was achieved in 9 of 22 (41%) of the diclofenac group, compared with 0 of 6 controls.
Side effects were limited to mild erythema and mild-to-moderate swelling of treated areas. No laboratory abnormalities or effects on graft function were noted.
With further follow-up, 55% of the patients who had previously cleared developed new AKs in the treated areas an average of 9.3 months after treatment ended. None of these patients developed invasive SCC in the study area within 24 months of follow-up, suggesting that topical diclofenac gel may also prevent invasive SCCs in this high-risk population.
Other treatments that have demonstrated efficacy for treatment of AKs and/or prevention of nonmelanoma skin cancer in high-risk organ transplant recipients include regular use of a sunscreen, imiquimod 5% cream, topical 5-fluorouracil, and photodynamic therapy.
Dr. Stockfleth disclosed serving as a consultant to Graceway, Almirall, Spirig, Intendis, Meda, Abbott, and LEO.
FROM THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Major Finding: Complete clearance of actinic keratoses was achieved in 41% of patients treated wtih diclofenac 3% gel, compared with 0% in the control group.
Data Source: The 32 organ transplant patients were randomized 3-to-1 to diclofenac or a vehicle control.
Disclosures: Dr. Stockfleth disclosed serving as a consultant to Graceway, Almirall, Spirig, Itendis, Meda, Abbott, and Leo.
Decreased PTPN13 Linked to HPV-Positive Head, Neck SCC Survival
BOSTON – Decreased expression of the PTPN13 gene is associated with improved survival of patients with head and neck squamous cell carcinoma positive for the human papillomavirus, a small retrospective study suggests.
Dr. George F. Harris IV of the University of Iowa, Iowa City, and his colleagues examined the correlation of human papillomavirus (HPV) status; protein tyrosine phosphatase, nonreceptor type 13 (PTPN13) status, and survival over 10 years in 21 patients for whom tissues samples were available for analysis. The patients, identified from University of Iowa Health Care (UIHC) Oncology Registry, were diagnosed with oropharyngeal head and neck squamous cell carcinoma (HNSCC) in 1995.
All 21 patients had HPV-positive cancer – 9 with decreased PTPN13 expression and 12 with unchanged or increased expression of PTPN13. Average survival was approximately 105 months in the low-PTPN13 group vs. 61 months in patients with unchanged or increased PTPN13 expression, Dr. Harris reported at the annual meeting of the American Academy of Otolaryngology–Head and Neck Surgery.
"An average increase in survival of about 30 months was seen for those patients who had decreased expression of PTPN13 within the tumor," he stated.
Looking at demographics, the investigators found that patients in the "decrease" group were younger at diagnosis but had more advanced disease. Observed Dr. Harris, "These may be confounding variables, but the finding is consistent with other authors' experience with HPV-associated H&N cancer in general."
The investigators employed real-time polymerase chain reaction (RT-PCR) to detect HPV type and activity. Dr. Harris said they used immunohistochemistry to evaluate the status of PTPN13, which has been shown to have a physiologically significant role in regulating mitogen-activated protein (MAP) kinase signaling. The identification and activity of HPV was confirmed by increased p16 immunochemical staining – an accepted marker for HPV activity because, in oropharyngeal HNSCC, HPV integration induces an overexpression of P16, he noted.
The improved survival observed among HPV positive HNSCC patients with decreased PTPN13 expression may be related to the fact that PTPN13 degradation is associated with fewer genetic changes than stable or elevated PTPN13 expression, according to Dr. Harris. It is possible that analyzing PTPN13 status in HPV-positive HNSCC "may identify those patients that will have better survival or, conversely, those patients who may require additional treatment," he said.
Dr. Harris reported having no conflicts of interest with respect to this presentation.
BOSTON – Decreased expression of the PTPN13 gene is associated with improved survival of patients with head and neck squamous cell carcinoma positive for the human papillomavirus, a small retrospective study suggests.
Dr. George F. Harris IV of the University of Iowa, Iowa City, and his colleagues examined the correlation of human papillomavirus (HPV) status; protein tyrosine phosphatase, nonreceptor type 13 (PTPN13) status, and survival over 10 years in 21 patients for whom tissues samples were available for analysis. The patients, identified from University of Iowa Health Care (UIHC) Oncology Registry, were diagnosed with oropharyngeal head and neck squamous cell carcinoma (HNSCC) in 1995.
All 21 patients had HPV-positive cancer – 9 with decreased PTPN13 expression and 12 with unchanged or increased expression of PTPN13. Average survival was approximately 105 months in the low-PTPN13 group vs. 61 months in patients with unchanged or increased PTPN13 expression, Dr. Harris reported at the annual meeting of the American Academy of Otolaryngology–Head and Neck Surgery.
"An average increase in survival of about 30 months was seen for those patients who had decreased expression of PTPN13 within the tumor," he stated.
Looking at demographics, the investigators found that patients in the "decrease" group were younger at diagnosis but had more advanced disease. Observed Dr. Harris, "These may be confounding variables, but the finding is consistent with other authors' experience with HPV-associated H&N cancer in general."
The investigators employed real-time polymerase chain reaction (RT-PCR) to detect HPV type and activity. Dr. Harris said they used immunohistochemistry to evaluate the status of PTPN13, which has been shown to have a physiologically significant role in regulating mitogen-activated protein (MAP) kinase signaling. The identification and activity of HPV was confirmed by increased p16 immunochemical staining – an accepted marker for HPV activity because, in oropharyngeal HNSCC, HPV integration induces an overexpression of P16, he noted.
The improved survival observed among HPV positive HNSCC patients with decreased PTPN13 expression may be related to the fact that PTPN13 degradation is associated with fewer genetic changes than stable or elevated PTPN13 expression, according to Dr. Harris. It is possible that analyzing PTPN13 status in HPV-positive HNSCC "may identify those patients that will have better survival or, conversely, those patients who may require additional treatment," he said.
Dr. Harris reported having no conflicts of interest with respect to this presentation.
BOSTON – Decreased expression of the PTPN13 gene is associated with improved survival of patients with head and neck squamous cell carcinoma positive for the human papillomavirus, a small retrospective study suggests.
Dr. George F. Harris IV of the University of Iowa, Iowa City, and his colleagues examined the correlation of human papillomavirus (HPV) status; protein tyrosine phosphatase, nonreceptor type 13 (PTPN13) status, and survival over 10 years in 21 patients for whom tissues samples were available for analysis. The patients, identified from University of Iowa Health Care (UIHC) Oncology Registry, were diagnosed with oropharyngeal head and neck squamous cell carcinoma (HNSCC) in 1995.
All 21 patients had HPV-positive cancer – 9 with decreased PTPN13 expression and 12 with unchanged or increased expression of PTPN13. Average survival was approximately 105 months in the low-PTPN13 group vs. 61 months in patients with unchanged or increased PTPN13 expression, Dr. Harris reported at the annual meeting of the American Academy of Otolaryngology–Head and Neck Surgery.
"An average increase in survival of about 30 months was seen for those patients who had decreased expression of PTPN13 within the tumor," he stated.
Looking at demographics, the investigators found that patients in the "decrease" group were younger at diagnosis but had more advanced disease. Observed Dr. Harris, "These may be confounding variables, but the finding is consistent with other authors' experience with HPV-associated H&N cancer in general."
The investigators employed real-time polymerase chain reaction (RT-PCR) to detect HPV type and activity. Dr. Harris said they used immunohistochemistry to evaluate the status of PTPN13, which has been shown to have a physiologically significant role in regulating mitogen-activated protein (MAP) kinase signaling. The identification and activity of HPV was confirmed by increased p16 immunochemical staining – an accepted marker for HPV activity because, in oropharyngeal HNSCC, HPV integration induces an overexpression of P16, he noted.
The improved survival observed among HPV positive HNSCC patients with decreased PTPN13 expression may be related to the fact that PTPN13 degradation is associated with fewer genetic changes than stable or elevated PTPN13 expression, according to Dr. Harris. It is possible that analyzing PTPN13 status in HPV-positive HNSCC "may identify those patients that will have better survival or, conversely, those patients who may require additional treatment," he said.
Dr. Harris reported having no conflicts of interest with respect to this presentation.
FROM THE AMERICAN ACADEMY OF OTOLARYNGOLOGY–HEAD AND NECK SURGERY
Major Finding: Patients with decreased expression of the PTPN13 gene lived an average of 105 months vs. 61 months by patients with unchanged or elevated PTPN13 expression.
Data Source: A retrospective review of tissue samples from 21 patients with HPV-positive head and neck squamous cell carcinoma.
Disclosures: Dr. Harris said he had no conflicts of interest.