User login
Sunscreen Controversy Heating Up: Ingredients to Get Second Look From Cosmetics Panel
A cosmetics industry panel, prompted by recent criticism of sunscreen ingredients vitamin A and oxybenzone, plans to consider new safety data for the substances in 2011.
The Cosmetic Ingredient Review (CIR) Expert Panel added the ingredients to its re-review list at the group's Aug. 31 meeting.
The CIR team plans to re-review retinyl palmitate after the National Toxicology Program (NTP) releases a report on the vitamin A compound, expected in 2011. CIR Director Alan Andersen said the reviewers will use "caution that we do not proceed beyond the new data."
In its 2010 sunscreen report released in May, the Environmental Working Group offered its read on preliminary NTP data, suggesting that findings from rodent studies show retinyl palmitate is photocarcinogenic.
The organization advised consumers to avoid sunscreens containing both retinyl palmitate and oxybenzone, which it suggests is a potential hormone disrupter.
Looking to "dismiss the misinformation that sunscreens are not safe" and reinforce the idea they protect against sun damage and cancer, the American Academy of Dermatology issued a report, maintaining there is no evidence that retinyl palmitate in sunscreens causes cancer.
Dr. Steven Q. Wang of Memorial Sloan-Kettering Cancer Center, New York, and his colleagues evaluated the compound from several points of view (J. Amer. Acad. Dermatol. 2010 [doi:10.1016/j.jaad.2010.07.015]), and concluded that "there is no convincing evidence" that retinyl palmitate, a form of vitamin A, is carcinogenic in sunscreens" (see "Editorial Refutes EWG's Sunscreen Finding").
The council, with support from FDA and the Consumer Federation of America, established CIR in 1976 to review and assesses the safety of ingredients used in cosmetics in an open, unbiased, and expert manner, and publishes the results in peer-reviewed scientific literature, according to CIR's website.
The CIR panel will re-review seven other ingredient groups in 2011. Typically the group considers rereviewing ingredients every 15 years, as well as any substances that it feels should be re-examined on account of emerging science or public debate.
Hair colorants HC Red No. 1 and 4-chlororesorcinol both will receive another look next year, as well as foaming agent cocomide DEA and preservatives glutaral and methyldibromo glutaronitrile.
Ingredients reviewed in 1996 that the panel will not review in 2011 include butoxyethanol, dibutyl adipate, di-t-butylhydroquinone, disperse yellow 3 and sodium m-nitrobenzenesulfonate.
Not all ingredients on priority lists are reviewed by the panel. Ingredients from the 2009 and 2010 lists that are not being reviewed actively include amino acids, chamomile and talc. Amino acids have been "reprioritized," while work on chamomile and talc has been tabled, CIR says.
FDA currently is reviewing talc and talc-containing cosmetic products in the U.S. for asbestos contamination following reports of the carcinogen in cosmetics overseas. Its survey is expected to continue through 2010.
Disclosures: Skin & Allergy News Digital Network and "The Pink Sheet" are published by Elsevier.
A cosmetics industry panel, prompted by recent criticism of sunscreen ingredients vitamin A and oxybenzone, plans to consider new safety data for the substances in 2011.
The Cosmetic Ingredient Review (CIR) Expert Panel added the ingredients to its re-review list at the group's Aug. 31 meeting.
The CIR team plans to re-review retinyl palmitate after the National Toxicology Program (NTP) releases a report on the vitamin A compound, expected in 2011. CIR Director Alan Andersen said the reviewers will use "caution that we do not proceed beyond the new data."
In its 2010 sunscreen report released in May, the Environmental Working Group offered its read on preliminary NTP data, suggesting that findings from rodent studies show retinyl palmitate is photocarcinogenic.
The organization advised consumers to avoid sunscreens containing both retinyl palmitate and oxybenzone, which it suggests is a potential hormone disrupter.
Looking to "dismiss the misinformation that sunscreens are not safe" and reinforce the idea they protect against sun damage and cancer, the American Academy of Dermatology issued a report, maintaining there is no evidence that retinyl palmitate in sunscreens causes cancer.
Dr. Steven Q. Wang of Memorial Sloan-Kettering Cancer Center, New York, and his colleagues evaluated the compound from several points of view (J. Amer. Acad. Dermatol. 2010 [doi:10.1016/j.jaad.2010.07.015]), and concluded that "there is no convincing evidence" that retinyl palmitate, a form of vitamin A, is carcinogenic in sunscreens" (see "Editorial Refutes EWG's Sunscreen Finding").
The council, with support from FDA and the Consumer Federation of America, established CIR in 1976 to review and assesses the safety of ingredients used in cosmetics in an open, unbiased, and expert manner, and publishes the results in peer-reviewed scientific literature, according to CIR's website.
The CIR panel will re-review seven other ingredient groups in 2011. Typically the group considers rereviewing ingredients every 15 years, as well as any substances that it feels should be re-examined on account of emerging science or public debate.
Hair colorants HC Red No. 1 and 4-chlororesorcinol both will receive another look next year, as well as foaming agent cocomide DEA and preservatives glutaral and methyldibromo glutaronitrile.
Ingredients reviewed in 1996 that the panel will not review in 2011 include butoxyethanol, dibutyl adipate, di-t-butylhydroquinone, disperse yellow 3 and sodium m-nitrobenzenesulfonate.
Not all ingredients on priority lists are reviewed by the panel. Ingredients from the 2009 and 2010 lists that are not being reviewed actively include amino acids, chamomile and talc. Amino acids have been "reprioritized," while work on chamomile and talc has been tabled, CIR says.
FDA currently is reviewing talc and talc-containing cosmetic products in the U.S. for asbestos contamination following reports of the carcinogen in cosmetics overseas. Its survey is expected to continue through 2010.
Disclosures: Skin & Allergy News Digital Network and "The Pink Sheet" are published by Elsevier.
A cosmetics industry panel, prompted by recent criticism of sunscreen ingredients vitamin A and oxybenzone, plans to consider new safety data for the substances in 2011.
The Cosmetic Ingredient Review (CIR) Expert Panel added the ingredients to its re-review list at the group's Aug. 31 meeting.
The CIR team plans to re-review retinyl palmitate after the National Toxicology Program (NTP) releases a report on the vitamin A compound, expected in 2011. CIR Director Alan Andersen said the reviewers will use "caution that we do not proceed beyond the new data."
In its 2010 sunscreen report released in May, the Environmental Working Group offered its read on preliminary NTP data, suggesting that findings from rodent studies show retinyl palmitate is photocarcinogenic.
The organization advised consumers to avoid sunscreens containing both retinyl palmitate and oxybenzone, which it suggests is a potential hormone disrupter.
Looking to "dismiss the misinformation that sunscreens are not safe" and reinforce the idea they protect against sun damage and cancer, the American Academy of Dermatology issued a report, maintaining there is no evidence that retinyl palmitate in sunscreens causes cancer.
Dr. Steven Q. Wang of Memorial Sloan-Kettering Cancer Center, New York, and his colleagues evaluated the compound from several points of view (J. Amer. Acad. Dermatol. 2010 [doi:10.1016/j.jaad.2010.07.015]), and concluded that "there is no convincing evidence" that retinyl palmitate, a form of vitamin A, is carcinogenic in sunscreens" (see "Editorial Refutes EWG's Sunscreen Finding").
The council, with support from FDA and the Consumer Federation of America, established CIR in 1976 to review and assesses the safety of ingredients used in cosmetics in an open, unbiased, and expert manner, and publishes the results in peer-reviewed scientific literature, according to CIR's website.
The CIR panel will re-review seven other ingredient groups in 2011. Typically the group considers rereviewing ingredients every 15 years, as well as any substances that it feels should be re-examined on account of emerging science or public debate.
Hair colorants HC Red No. 1 and 4-chlororesorcinol both will receive another look next year, as well as foaming agent cocomide DEA and preservatives glutaral and methyldibromo glutaronitrile.
Ingredients reviewed in 1996 that the panel will not review in 2011 include butoxyethanol, dibutyl adipate, di-t-butylhydroquinone, disperse yellow 3 and sodium m-nitrobenzenesulfonate.
Not all ingredients on priority lists are reviewed by the panel. Ingredients from the 2009 and 2010 lists that are not being reviewed actively include amino acids, chamomile and talc. Amino acids have been "reprioritized," while work on chamomile and talc has been tabled, CIR says.
FDA currently is reviewing talc and talc-containing cosmetic products in the U.S. for asbestos contamination following reports of the carcinogen in cosmetics overseas. Its survey is expected to continue through 2010.
Disclosures: Skin & Allergy News Digital Network and "The Pink Sheet" are published by Elsevier.
FROM THE PINK SHEET
Sunless Tanning Intervention Cuts Women's UV Exposure
An intervention promoting the use of a “sunless” tanning product among women at a beach reduced their sunbathing behavior and sunburns acquired for the remainder of the summer, according to a report in the September issue of the Archives of Dermatology.
The intervention also had a long-term effect, as many of the women reported that they decreased their sunbathing and used the fake tanning product again the following summer, said Sherry L. Pagoto, Ph.D., of the University of Massachusetts Medical School, Worcester, and her associates
The researchers assessed the intervention in women who visited two public beaches in Massachusetts in June and July of 2006 and agreed to participate in a study of sunbathing. A total of 125 subjects at one beach were assigned to the intervention and 125 at the other beach served as a control group. The mean age was 31 years.
The intervention included completing a brief questionnaire on sunbathing behavior, receiving written and verbal instructions on the use of a sunless tanning product and free samples of the product, receiving a pamphlet about skin cancer, having their photograph taken with an instant UV-filtered camera to demonstrate sun damage on their skin, receiving free samples of sunscreen, and being strongly encouraged to use sunless tanning rather than sunbathing. The control subjects completed the questionnaire, had their picture taken with a regular instant camera, and were given free cosmetic samples unrelated to skin health.
All study subjects had a 2-month follow-up for assessment of the short-term effects of the intervention, as well as at 1 year for long-term assessment.
At short-term follow-up, women in the intervention group reported that they had reduced their sunbathing by 33%, compared with a 10% decrease reported by the control subjects. The intervention group also said they had acquired 73% fewer sunburns, compared with 37% fewer reported by the control subjects. In addition, the intervention group said they had increased their use of protective clothing by 32%, while the control group said they had done so by only 2%. All of these between-group differences were statistically significant.
At long-term follow-up, the intervention group still reported a significant decrease in sunbathing behavior and showed a significant increase in the use of sunless tanning products, compared with the control group. The differences in the number of sunburns and the use of protective clothing did not persist.
The use of sunscreen increased slightly in both groups at short-term follow-up, but the difference between groups was not significant. And the increased use of sunscreen did not persist the following year.
These findings suggest that “promoting sunless tanning to sunbathers in the context of a skin cancer prevention public health message may be helpful in reducing sunbathing and sunburns and in promoting the use of protective clothing,” Dr. Pagoto and her colleagues wrote (Arch. Dermatol. 2010;146:979-84).
“Physicians might be reluctant to recommend sunless tanning due to concerns that it might inadvertently reinforce the patient’s desire to be tan,” they noted. However, these results suggest that instead, physicians should encourage patients who sunbathe to consider safer alternatives such as sunless tanning.
This study was limited in that nearly half of the women who were approached to participate in the study refused to do so, which could have contributed to selection bias. Also, the outcome measures relied on self-report, which may have contributed to social desirability bias. Finally, studies on the safety of the long-term use of sunless tanning products are lacking, and the long-term effects on the epidermis of dihydroxyacetone, the principal component of such products, remain unknown, the investigators noted.
An intervention promoting the use of a “sunless” tanning product among women at a beach reduced their sunbathing behavior and sunburns acquired for the remainder of the summer, according to a report in the September issue of the Archives of Dermatology.
The intervention also had a long-term effect, as many of the women reported that they decreased their sunbathing and used the fake tanning product again the following summer, said Sherry L. Pagoto, Ph.D., of the University of Massachusetts Medical School, Worcester, and her associates
The researchers assessed the intervention in women who visited two public beaches in Massachusetts in June and July of 2006 and agreed to participate in a study of sunbathing. A total of 125 subjects at one beach were assigned to the intervention and 125 at the other beach served as a control group. The mean age was 31 years.
The intervention included completing a brief questionnaire on sunbathing behavior, receiving written and verbal instructions on the use of a sunless tanning product and free samples of the product, receiving a pamphlet about skin cancer, having their photograph taken with an instant UV-filtered camera to demonstrate sun damage on their skin, receiving free samples of sunscreen, and being strongly encouraged to use sunless tanning rather than sunbathing. The control subjects completed the questionnaire, had their picture taken with a regular instant camera, and were given free cosmetic samples unrelated to skin health.
All study subjects had a 2-month follow-up for assessment of the short-term effects of the intervention, as well as at 1 year for long-term assessment.
At short-term follow-up, women in the intervention group reported that they had reduced their sunbathing by 33%, compared with a 10% decrease reported by the control subjects. The intervention group also said they had acquired 73% fewer sunburns, compared with 37% fewer reported by the control subjects. In addition, the intervention group said they had increased their use of protective clothing by 32%, while the control group said they had done so by only 2%. All of these between-group differences were statistically significant.
At long-term follow-up, the intervention group still reported a significant decrease in sunbathing behavior and showed a significant increase in the use of sunless tanning products, compared with the control group. The differences in the number of sunburns and the use of protective clothing did not persist.
The use of sunscreen increased slightly in both groups at short-term follow-up, but the difference between groups was not significant. And the increased use of sunscreen did not persist the following year.
These findings suggest that “promoting sunless tanning to sunbathers in the context of a skin cancer prevention public health message may be helpful in reducing sunbathing and sunburns and in promoting the use of protective clothing,” Dr. Pagoto and her colleagues wrote (Arch. Dermatol. 2010;146:979-84).
“Physicians might be reluctant to recommend sunless tanning due to concerns that it might inadvertently reinforce the patient’s desire to be tan,” they noted. However, these results suggest that instead, physicians should encourage patients who sunbathe to consider safer alternatives such as sunless tanning.
This study was limited in that nearly half of the women who were approached to participate in the study refused to do so, which could have contributed to selection bias. Also, the outcome measures relied on self-report, which may have contributed to social desirability bias. Finally, studies on the safety of the long-term use of sunless tanning products are lacking, and the long-term effects on the epidermis of dihydroxyacetone, the principal component of such products, remain unknown, the investigators noted.
An intervention promoting the use of a “sunless” tanning product among women at a beach reduced their sunbathing behavior and sunburns acquired for the remainder of the summer, according to a report in the September issue of the Archives of Dermatology.
The intervention also had a long-term effect, as many of the women reported that they decreased their sunbathing and used the fake tanning product again the following summer, said Sherry L. Pagoto, Ph.D., of the University of Massachusetts Medical School, Worcester, and her associates
The researchers assessed the intervention in women who visited two public beaches in Massachusetts in June and July of 2006 and agreed to participate in a study of sunbathing. A total of 125 subjects at one beach were assigned to the intervention and 125 at the other beach served as a control group. The mean age was 31 years.
The intervention included completing a brief questionnaire on sunbathing behavior, receiving written and verbal instructions on the use of a sunless tanning product and free samples of the product, receiving a pamphlet about skin cancer, having their photograph taken with an instant UV-filtered camera to demonstrate sun damage on their skin, receiving free samples of sunscreen, and being strongly encouraged to use sunless tanning rather than sunbathing. The control subjects completed the questionnaire, had their picture taken with a regular instant camera, and were given free cosmetic samples unrelated to skin health.
All study subjects had a 2-month follow-up for assessment of the short-term effects of the intervention, as well as at 1 year for long-term assessment.
At short-term follow-up, women in the intervention group reported that they had reduced their sunbathing by 33%, compared with a 10% decrease reported by the control subjects. The intervention group also said they had acquired 73% fewer sunburns, compared with 37% fewer reported by the control subjects. In addition, the intervention group said they had increased their use of protective clothing by 32%, while the control group said they had done so by only 2%. All of these between-group differences were statistically significant.
At long-term follow-up, the intervention group still reported a significant decrease in sunbathing behavior and showed a significant increase in the use of sunless tanning products, compared with the control group. The differences in the number of sunburns and the use of protective clothing did not persist.
The use of sunscreen increased slightly in both groups at short-term follow-up, but the difference between groups was not significant. And the increased use of sunscreen did not persist the following year.
These findings suggest that “promoting sunless tanning to sunbathers in the context of a skin cancer prevention public health message may be helpful in reducing sunbathing and sunburns and in promoting the use of protective clothing,” Dr. Pagoto and her colleagues wrote (Arch. Dermatol. 2010;146:979-84).
“Physicians might be reluctant to recommend sunless tanning due to concerns that it might inadvertently reinforce the patient’s desire to be tan,” they noted. However, these results suggest that instead, physicians should encourage patients who sunbathe to consider safer alternatives such as sunless tanning.
This study was limited in that nearly half of the women who were approached to participate in the study refused to do so, which could have contributed to selection bias. Also, the outcome measures relied on self-report, which may have contributed to social desirability bias. Finally, studies on the safety of the long-term use of sunless tanning products are lacking, and the long-term effects on the epidermis of dihydroxyacetone, the principal component of such products, remain unknown, the investigators noted.
Major Finding: At short-term follow-up, women in the intervention group reported that they had reduced their sunbathing by 33%, compared with a 10% decrease reported by the control subjects.
Data Source: A randomized controlled trial involving 250 women followed-up at 2 months and 1 year after the intervention.
Disclosures: This study was supported in part by the National Cancer Institute. Dr. Pagoto and her associates reported no financial conflicts of interest.
Toxic Erythema of Chemotherapy Called By Many Names
PASADENA, Calif. - Dermatologists tend to use too many different names for a single entity with a defined clinical spectrum - toxic erythema of chemotherapy.
“We have too many names for one disease,” and this complicates clinical communication and decisions about management, Dr. Jean Bolognia said at the annual meeting of the Pacific Dermatologic Association.
If dermatologists used the term “toxic erythema of chemotherapy” (TEC), they would improve discussions with hematologists, oncologists, and internists, said Dr. Bolognia, professor of dermatology at Yale University, New Haven, Conn. Other terms sometimes used in place of TEC include eccrine squamous syringometaplasia and epidermal dysmaturation. While some alternative terms may be histologically accurate, they can be confusing to nondermatologists as diagnoses.
The term TEC indicates that the patient is not having an allergic reaction or an infectious process. It tells clinicians that the dermatologic problem will resolve spontaneously but can recur if the patient again uses a similar or higher dosage of the drug that caused it, noted Dr. Bolognia.
Cytarabine (Ara C) and anthracyclines are “at the top of the list” of drugs associated with TEC, so patients with acute myelogenous leukemia commonly develop TEC. The liposomal form of doxorubicin is “kinder and gentler when it comes to your bone marrow and your hair, but is worse when it comes to producing TEC,” she said.
Other drugs most commonly associated with TEC include taxanes (often used to treat breast cancer), methotrexate, multikinase inhibitors, gemcitabine, clofarabine, pralatrexate, 5-fluorouracil (5-FU), and prodrugs that turn into 5-FU in the body (like capecitabine).
Busulfan, until recently, was an oral chemotherapeutic drug that caused nausea and vomiting, which lead to underdosing. Now that it is administered intravenously, it is more likely to give rise to TEC, she said. If called upon to evaluate a possible case of erythema multiforme, Stevens-Johnson syndrome, or toxic epidermal necrolysis in a patient who has received IV busulfan in the past several weeks, consider TEC.
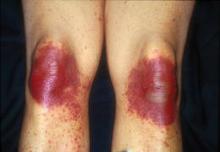
Clinically, signs and symptoms of TEC can occur a month or so after the drug is given. TEC appears as erythematous or violaceous patches or edematous plaques. “They’re going to appear on hands and feet, elbows and knees, axillae and groin, including the scrotum, and occasionally on the ears,” she said. When it’s severe, a good portion of the skin surface can have the appearance of a sunburn.
Once you know the distribution pattern for TEC bullae “you can make the diagnosis at the bedside,” she added.
The lesions are associated with pain and burning more than with pruritus, suggesting a toxic reaction. Their dusky hue can contribute to misdiagnoses, such as toxic erythema multiforme, Stevens-Johnson syndrome, or toxic epidermal necrolysis.
The lesions may become purpuric, especially in the setting of thrombocytopenia, leading to the misdiagnosis of vasculitis. Sterile bullae followed by erosions also develop within the plaques, followed by desquamation and spontaneous resolution regardless of the therapy chosen. Of note, the desquamation is dry on the palms and soles or elbows and knees, but is moist in the major body folds.
Dr. Bolognia said she would especially like to see dermatologists stop using the term “acral erythrodysesthesia,” or “hand-foot syndrome,” because patients often have additional sites of involvement and trying to explain the cutaneous findings in these areas can lead to erroneous diagnoses. For example, the lesions on the elbows and knees may be misdiagnosed as dermatitis or involvement of the axillae and groin as cutaneous candidiasis, even though the patient is already receiving voriconazole.
“By simply saying you have TEC, you have a unifying diagnosis,” she said. “Histologic findings include atypia and apoptosis of keratinocytes, as well as some loss of polarity of epidermal cells and crowding of keratinocytes. There is vacuolar degeneration of the basal layer, which may lead the dermatopathologist to raise the possibility of erythema multiforme, Stevens-Johnson syndrome, or graft-versus-host disease.”
Additional findings include eccrine squamous syringometaplasia and eccrine hidradenitis. Distinguishing TEC from graft-versus-host disease or erythema multiforme/Stevens-Johnson syndrome superimposed upon chemotherapy-induced changes may be difficult histologically, but the dermatologist can do the clinicopathologic correlation and arrive at the diagnosis, she said. For example, on the palmar surface of the hands, a clinical clue to the diagnosis of TEC is accentuation of erythema and bullae within the creases of the digits, a finding not seen in the differential diagnoses.
Pseudocellulitis (or erysipeloid reaction) also falls within the spectrum of TEC. Large patches or plaques of burning erythema appear and tense bullae may develop. The bullae do not spontaneously slough, and there is no Nikolsky’s sign. Pseudocellulitis is seen primarily following exposure to gemcitabine, but also to clofarabine, a newer drug being used as a second- or third-line agent for leukemia.
Lastly, chemotherapy-induced eccrine hidradenitis clearly falls within the histologic spectrum of TEC, she said, but clinically there are some patients who have a Sweet’s syndrome–like presentation, and these could be categorized separately.
TEC can be misdiagnosed as several other entities including cellulitis, herpetic viral infections, vasculitis, graft-versus-host disease (especially when the patient also has diarrhea), or a hypersensitivity drug reaction. It is important to note that TEC can be a skin sign of systemic disease in that severe cutaneous disease can be associated with severe cytotoxicity of the bowel, leading to sepsis caused by GI flora, she said. The team caring for the patient should be made aware of this possibility.
Disclosures: Dr. Bolognia said she has no pertinent conflicts of interest.
PASADENA, Calif. - Dermatologists tend to use too many different names for a single entity with a defined clinical spectrum - toxic erythema of chemotherapy.
“We have too many names for one disease,” and this complicates clinical communication and decisions about management, Dr. Jean Bolognia said at the annual meeting of the Pacific Dermatologic Association.
If dermatologists used the term “toxic erythema of chemotherapy” (TEC), they would improve discussions with hematologists, oncologists, and internists, said Dr. Bolognia, professor of dermatology at Yale University, New Haven, Conn. Other terms sometimes used in place of TEC include eccrine squamous syringometaplasia and epidermal dysmaturation. While some alternative terms may be histologically accurate, they can be confusing to nondermatologists as diagnoses.
The term TEC indicates that the patient is not having an allergic reaction or an infectious process. It tells clinicians that the dermatologic problem will resolve spontaneously but can recur if the patient again uses a similar or higher dosage of the drug that caused it, noted Dr. Bolognia.
Cytarabine (Ara C) and anthracyclines are “at the top of the list” of drugs associated with TEC, so patients with acute myelogenous leukemia commonly develop TEC. The liposomal form of doxorubicin is “kinder and gentler when it comes to your bone marrow and your hair, but is worse when it comes to producing TEC,” she said.
Other drugs most commonly associated with TEC include taxanes (often used to treat breast cancer), methotrexate, multikinase inhibitors, gemcitabine, clofarabine, pralatrexate, 5-fluorouracil (5-FU), and prodrugs that turn into 5-FU in the body (like capecitabine).
Busulfan, until recently, was an oral chemotherapeutic drug that caused nausea and vomiting, which lead to underdosing. Now that it is administered intravenously, it is more likely to give rise to TEC, she said. If called upon to evaluate a possible case of erythema multiforme, Stevens-Johnson syndrome, or toxic epidermal necrolysis in a patient who has received IV busulfan in the past several weeks, consider TEC.
Clinically, signs and symptoms of TEC can occur a month or so after the drug is given. TEC appears as erythematous or violaceous patches or edematous plaques. “They’re going to appear on hands and feet, elbows and knees, axillae and groin, including the scrotum, and occasionally on the ears,” she said. When it’s severe, a good portion of the skin surface can have the appearance of a sunburn.
Once you know the distribution pattern for TEC bullae “you can make the diagnosis at the bedside,” she added.
The lesions are associated with pain and burning more than with pruritus, suggesting a toxic reaction. Their dusky hue can contribute to misdiagnoses, such as toxic erythema multiforme, Stevens-Johnson syndrome, or toxic epidermal necrolysis.
The lesions may become purpuric, especially in the setting of thrombocytopenia, leading to the misdiagnosis of vasculitis. Sterile bullae followed by erosions also develop within the plaques, followed by desquamation and spontaneous resolution regardless of the therapy chosen. Of note, the desquamation is dry on the palms and soles or elbows and knees, but is moist in the major body folds.
Dr. Bolognia said she would especially like to see dermatologists stop using the term “acral erythrodysesthesia,” or “hand-foot syndrome,” because patients often have additional sites of involvement and trying to explain the cutaneous findings in these areas can lead to erroneous diagnoses. For example, the lesions on the elbows and knees may be misdiagnosed as dermatitis or involvement of the axillae and groin as cutaneous candidiasis, even though the patient is already receiving voriconazole.
“By simply saying you have TEC, you have a unifying diagnosis,” she said. “Histologic findings include atypia and apoptosis of keratinocytes, as well as some loss of polarity of epidermal cells and crowding of keratinocytes. There is vacuolar degeneration of the basal layer, which may lead the dermatopathologist to raise the possibility of erythema multiforme, Stevens-Johnson syndrome, or graft-versus-host disease.”
Additional findings include eccrine squamous syringometaplasia and eccrine hidradenitis. Distinguishing TEC from graft-versus-host disease or erythema multiforme/Stevens-Johnson syndrome superimposed upon chemotherapy-induced changes may be difficult histologically, but the dermatologist can do the clinicopathologic correlation and arrive at the diagnosis, she said. For example, on the palmar surface of the hands, a clinical clue to the diagnosis of TEC is accentuation of erythema and bullae within the creases of the digits, a finding not seen in the differential diagnoses.
Pseudocellulitis (or erysipeloid reaction) also falls within the spectrum of TEC. Large patches or plaques of burning erythema appear and tense bullae may develop. The bullae do not spontaneously slough, and there is no Nikolsky’s sign. Pseudocellulitis is seen primarily following exposure to gemcitabine, but also to clofarabine, a newer drug being used as a second- or third-line agent for leukemia.
Lastly, chemotherapy-induced eccrine hidradenitis clearly falls within the histologic spectrum of TEC, she said, but clinically there are some patients who have a Sweet’s syndrome–like presentation, and these could be categorized separately.
TEC can be misdiagnosed as several other entities including cellulitis, herpetic viral infections, vasculitis, graft-versus-host disease (especially when the patient also has diarrhea), or a hypersensitivity drug reaction. It is important to note that TEC can be a skin sign of systemic disease in that severe cutaneous disease can be associated with severe cytotoxicity of the bowel, leading to sepsis caused by GI flora, she said. The team caring for the patient should be made aware of this possibility.
Disclosures: Dr. Bolognia said she has no pertinent conflicts of interest.
PASADENA, Calif. - Dermatologists tend to use too many different names for a single entity with a defined clinical spectrum - toxic erythema of chemotherapy.
“We have too many names for one disease,” and this complicates clinical communication and decisions about management, Dr. Jean Bolognia said at the annual meeting of the Pacific Dermatologic Association.
If dermatologists used the term “toxic erythema of chemotherapy” (TEC), they would improve discussions with hematologists, oncologists, and internists, said Dr. Bolognia, professor of dermatology at Yale University, New Haven, Conn. Other terms sometimes used in place of TEC include eccrine squamous syringometaplasia and epidermal dysmaturation. While some alternative terms may be histologically accurate, they can be confusing to nondermatologists as diagnoses.
The term TEC indicates that the patient is not having an allergic reaction or an infectious process. It tells clinicians that the dermatologic problem will resolve spontaneously but can recur if the patient again uses a similar or higher dosage of the drug that caused it, noted Dr. Bolognia.
Cytarabine (Ara C) and anthracyclines are “at the top of the list” of drugs associated with TEC, so patients with acute myelogenous leukemia commonly develop TEC. The liposomal form of doxorubicin is “kinder and gentler when it comes to your bone marrow and your hair, but is worse when it comes to producing TEC,” she said.
Other drugs most commonly associated with TEC include taxanes (often used to treat breast cancer), methotrexate, multikinase inhibitors, gemcitabine, clofarabine, pralatrexate, 5-fluorouracil (5-FU), and prodrugs that turn into 5-FU in the body (like capecitabine).
Busulfan, until recently, was an oral chemotherapeutic drug that caused nausea and vomiting, which lead to underdosing. Now that it is administered intravenously, it is more likely to give rise to TEC, she said. If called upon to evaluate a possible case of erythema multiforme, Stevens-Johnson syndrome, or toxic epidermal necrolysis in a patient who has received IV busulfan in the past several weeks, consider TEC.
Clinically, signs and symptoms of TEC can occur a month or so after the drug is given. TEC appears as erythematous or violaceous patches or edematous plaques. “They’re going to appear on hands and feet, elbows and knees, axillae and groin, including the scrotum, and occasionally on the ears,” she said. When it’s severe, a good portion of the skin surface can have the appearance of a sunburn.
Once you know the distribution pattern for TEC bullae “you can make the diagnosis at the bedside,” she added.
The lesions are associated with pain and burning more than with pruritus, suggesting a toxic reaction. Their dusky hue can contribute to misdiagnoses, such as toxic erythema multiforme, Stevens-Johnson syndrome, or toxic epidermal necrolysis.
The lesions may become purpuric, especially in the setting of thrombocytopenia, leading to the misdiagnosis of vasculitis. Sterile bullae followed by erosions also develop within the plaques, followed by desquamation and spontaneous resolution regardless of the therapy chosen. Of note, the desquamation is dry on the palms and soles or elbows and knees, but is moist in the major body folds.
Dr. Bolognia said she would especially like to see dermatologists stop using the term “acral erythrodysesthesia,” or “hand-foot syndrome,” because patients often have additional sites of involvement and trying to explain the cutaneous findings in these areas can lead to erroneous diagnoses. For example, the lesions on the elbows and knees may be misdiagnosed as dermatitis or involvement of the axillae and groin as cutaneous candidiasis, even though the patient is already receiving voriconazole.
“By simply saying you have TEC, you have a unifying diagnosis,” she said. “Histologic findings include atypia and apoptosis of keratinocytes, as well as some loss of polarity of epidermal cells and crowding of keratinocytes. There is vacuolar degeneration of the basal layer, which may lead the dermatopathologist to raise the possibility of erythema multiforme, Stevens-Johnson syndrome, or graft-versus-host disease.”
Additional findings include eccrine squamous syringometaplasia and eccrine hidradenitis. Distinguishing TEC from graft-versus-host disease or erythema multiforme/Stevens-Johnson syndrome superimposed upon chemotherapy-induced changes may be difficult histologically, but the dermatologist can do the clinicopathologic correlation and arrive at the diagnosis, she said. For example, on the palmar surface of the hands, a clinical clue to the diagnosis of TEC is accentuation of erythema and bullae within the creases of the digits, a finding not seen in the differential diagnoses.
Pseudocellulitis (or erysipeloid reaction) also falls within the spectrum of TEC. Large patches or plaques of burning erythema appear and tense bullae may develop. The bullae do not spontaneously slough, and there is no Nikolsky’s sign. Pseudocellulitis is seen primarily following exposure to gemcitabine, but also to clofarabine, a newer drug being used as a second- or third-line agent for leukemia.
Lastly, chemotherapy-induced eccrine hidradenitis clearly falls within the histologic spectrum of TEC, she said, but clinically there are some patients who have a Sweet’s syndrome–like presentation, and these could be categorized separately.
TEC can be misdiagnosed as several other entities including cellulitis, herpetic viral infections, vasculitis, graft-versus-host disease (especially when the patient also has diarrhea), or a hypersensitivity drug reaction. It is important to note that TEC can be a skin sign of systemic disease in that severe cutaneous disease can be associated with severe cytotoxicity of the bowel, leading to sepsis caused by GI flora, she said. The team caring for the patient should be made aware of this possibility.
Disclosures: Dr. Bolognia said she has no pertinent conflicts of interest.
GDC-0449 and Itraconazole Look Promising for Basal Cell Carcinomas
PASADENA, Calif. - Two drugs - one old, one new - are showing promise in early clinical trials for the treatment of basal cell carcinomas.
If they prove to be useful, clinicians can thank scientists whose years of research successfully identified abnormalities in the hedgehog signaling pathway as the mechanism of disease for basal cell carcinoma (BCC), Dr. Jean Tang said at the annual meeting of the Pacific Dermatologic Association.
“Every major pharmaceutical company is now interested in the hedgehog pathway, so we may see a lot of treatments in the near future” for BCC and other cancers that have aberrations in this pathway, said Dr. Tang of Stanford (Calif.) University.
She and her associates are conducting a phase II trial of the experimental Genentech drug GDC-0449 in patients with many BCCs from basal cell nevus syndrome (Gorlin’s syndrome), and a separate proof-of-concept study of the antifungal drug itraconazole in patients with small numbers of BCCs who do not have basal cell nevus syndrome. Dr. Tang said she is pleased by the early results with both drugs so far.
For GDC-0449, a previous phase I study in 33 patients with locally advanced or metastatic BCC who took the drug for a median of 10 months found that 50%-60% had partial or complete responses and the drug was generally well tolerated (N. Engl. J. Med. 2009;361:1,164-72).
Of the 41 patients with basal cell nevus syndrome recruited thus far for the phase II trial - and randomized to 18 months of treatment with GDC-0449 or placebo - 23 patients (with 953 BCC tumors) have more than 1 month of data.
Although the study is still blinded, 15 patients in group A have developed a median of one new BCC during that month, compared with a median of 10 new BCCs in each of the 8 patients in group B, a significant difference that suggests group A may have received the drug and it may be working, Dr. Tang suggested.
In group A, patients started with a median of 252 BCCs and had 127 at the most recent follow-up, a 50% decline. In group B, patients started with 217 BCCs and had 267 at follow-up, a 23% increase in BCCs.
The difference between groups is significant, she said. The existing BCCs in group A also seem to have decreased in size.
“In the patients who have responded, it has changed their lives,” Dr. Tang said. “We are really excited about it.”
Patients in group A, however, were significantly more likely to develop dysgeusia (decreased taste sensation). The condition was seen in 14 of 15 patients, compared with none in group B. Seven patients in group A and none in group B reported some hair loss, though this difference was not statistically significant. Two patients in group A stopped treatment due to dysgeusia and hair loss, compared with no patient cessation in group A. Myalgia was also reported in group A but not group B.
These side effects may be acceptable to patients with basal cell nevus syndrome but probably not to patients with only one or two BCCs, Dr. Tang noted.
For the latter group, a proof-of-concept trial has randomized eight patients thus far to 1 month of treatment with 400 mg/day of oral itraconazole, a drug that has been on the market for approximately 2 decades and is considered relatively safe, she said. Patients are followed clinically for changes in their BCCs, and they undergo tumor biopsies before and after the 1-month therapy to measure the treatment’s effects on the hedgehog signaling pathway.
Preliminary data are “somewhat promising” in reducing BCC size, Dr. Tang said. The effect has not been as dramatic as with the GDC-0449 study, but neither have the side effects.
“This is very preliminary data but I wanted to share it with the other dermatologists in case some of them would like to put some patients on itraconazole, and, collectively, we can figure out whether or not it has a true effect” on BCC, she said.
In addition to the studies on itraconazole and GDC-0449, studies are underway by other investigators on experimental oral or topical agents by Novartis, Exelixis, and Infinity Pharmaceuticals that address aberrations in the hedgehog signaling pathway for the treatment of BCC.
PASADENA, Calif. - Two drugs - one old, one new - are showing promise in early clinical trials for the treatment of basal cell carcinomas.
If they prove to be useful, clinicians can thank scientists whose years of research successfully identified abnormalities in the hedgehog signaling pathway as the mechanism of disease for basal cell carcinoma (BCC), Dr. Jean Tang said at the annual meeting of the Pacific Dermatologic Association.
“Every major pharmaceutical company is now interested in the hedgehog pathway, so we may see a lot of treatments in the near future” for BCC and other cancers that have aberrations in this pathway, said Dr. Tang of Stanford (Calif.) University.
She and her associates are conducting a phase II trial of the experimental Genentech drug GDC-0449 in patients with many BCCs from basal cell nevus syndrome (Gorlin’s syndrome), and a separate proof-of-concept study of the antifungal drug itraconazole in patients with small numbers of BCCs who do not have basal cell nevus syndrome. Dr. Tang said she is pleased by the early results with both drugs so far.
For GDC-0449, a previous phase I study in 33 patients with locally advanced or metastatic BCC who took the drug for a median of 10 months found that 50%-60% had partial or complete responses and the drug was generally well tolerated (N. Engl. J. Med. 2009;361:1,164-72).
Of the 41 patients with basal cell nevus syndrome recruited thus far for the phase II trial - and randomized to 18 months of treatment with GDC-0449 or placebo - 23 patients (with 953 BCC tumors) have more than 1 month of data.
Although the study is still blinded, 15 patients in group A have developed a median of one new BCC during that month, compared with a median of 10 new BCCs in each of the 8 patients in group B, a significant difference that suggests group A may have received the drug and it may be working, Dr. Tang suggested.
In group A, patients started with a median of 252 BCCs and had 127 at the most recent follow-up, a 50% decline. In group B, patients started with 217 BCCs and had 267 at follow-up, a 23% increase in BCCs.
The difference between groups is significant, she said. The existing BCCs in group A also seem to have decreased in size.
“In the patients who have responded, it has changed their lives,” Dr. Tang said. “We are really excited about it.”
Patients in group A, however, were significantly more likely to develop dysgeusia (decreased taste sensation). The condition was seen in 14 of 15 patients, compared with none in group B. Seven patients in group A and none in group B reported some hair loss, though this difference was not statistically significant. Two patients in group A stopped treatment due to dysgeusia and hair loss, compared with no patient cessation in group A. Myalgia was also reported in group A but not group B.
These side effects may be acceptable to patients with basal cell nevus syndrome but probably not to patients with only one or two BCCs, Dr. Tang noted.
For the latter group, a proof-of-concept trial has randomized eight patients thus far to 1 month of treatment with 400 mg/day of oral itraconazole, a drug that has been on the market for approximately 2 decades and is considered relatively safe, she said. Patients are followed clinically for changes in their BCCs, and they undergo tumor biopsies before and after the 1-month therapy to measure the treatment’s effects on the hedgehog signaling pathway.
Preliminary data are “somewhat promising” in reducing BCC size, Dr. Tang said. The effect has not been as dramatic as with the GDC-0449 study, but neither have the side effects.
“This is very preliminary data but I wanted to share it with the other dermatologists in case some of them would like to put some patients on itraconazole, and, collectively, we can figure out whether or not it has a true effect” on BCC, she said.
In addition to the studies on itraconazole and GDC-0449, studies are underway by other investigators on experimental oral or topical agents by Novartis, Exelixis, and Infinity Pharmaceuticals that address aberrations in the hedgehog signaling pathway for the treatment of BCC.
PASADENA, Calif. - Two drugs - one old, one new - are showing promise in early clinical trials for the treatment of basal cell carcinomas.
If they prove to be useful, clinicians can thank scientists whose years of research successfully identified abnormalities in the hedgehog signaling pathway as the mechanism of disease for basal cell carcinoma (BCC), Dr. Jean Tang said at the annual meeting of the Pacific Dermatologic Association.
“Every major pharmaceutical company is now interested in the hedgehog pathway, so we may see a lot of treatments in the near future” for BCC and other cancers that have aberrations in this pathway, said Dr. Tang of Stanford (Calif.) University.
She and her associates are conducting a phase II trial of the experimental Genentech drug GDC-0449 in patients with many BCCs from basal cell nevus syndrome (Gorlin’s syndrome), and a separate proof-of-concept study of the antifungal drug itraconazole in patients with small numbers of BCCs who do not have basal cell nevus syndrome. Dr. Tang said she is pleased by the early results with both drugs so far.
For GDC-0449, a previous phase I study in 33 patients with locally advanced or metastatic BCC who took the drug for a median of 10 months found that 50%-60% had partial or complete responses and the drug was generally well tolerated (N. Engl. J. Med. 2009;361:1,164-72).
Of the 41 patients with basal cell nevus syndrome recruited thus far for the phase II trial - and randomized to 18 months of treatment with GDC-0449 or placebo - 23 patients (with 953 BCC tumors) have more than 1 month of data.
Although the study is still blinded, 15 patients in group A have developed a median of one new BCC during that month, compared with a median of 10 new BCCs in each of the 8 patients in group B, a significant difference that suggests group A may have received the drug and it may be working, Dr. Tang suggested.
In group A, patients started with a median of 252 BCCs and had 127 at the most recent follow-up, a 50% decline. In group B, patients started with 217 BCCs and had 267 at follow-up, a 23% increase in BCCs.
The difference between groups is significant, she said. The existing BCCs in group A also seem to have decreased in size.
“In the patients who have responded, it has changed their lives,” Dr. Tang said. “We are really excited about it.”
Patients in group A, however, were significantly more likely to develop dysgeusia (decreased taste sensation). The condition was seen in 14 of 15 patients, compared with none in group B. Seven patients in group A and none in group B reported some hair loss, though this difference was not statistically significant. Two patients in group A stopped treatment due to dysgeusia and hair loss, compared with no patient cessation in group A. Myalgia was also reported in group A but not group B.
These side effects may be acceptable to patients with basal cell nevus syndrome but probably not to patients with only one or two BCCs, Dr. Tang noted.
For the latter group, a proof-of-concept trial has randomized eight patients thus far to 1 month of treatment with 400 mg/day of oral itraconazole, a drug that has been on the market for approximately 2 decades and is considered relatively safe, she said. Patients are followed clinically for changes in their BCCs, and they undergo tumor biopsies before and after the 1-month therapy to measure the treatment’s effects on the hedgehog signaling pathway.
Preliminary data are “somewhat promising” in reducing BCC size, Dr. Tang said. The effect has not been as dramatic as with the GDC-0449 study, but neither have the side effects.
“This is very preliminary data but I wanted to share it with the other dermatologists in case some of them would like to put some patients on itraconazole, and, collectively, we can figure out whether or not it has a true effect” on BCC, she said.
In addition to the studies on itraconazole and GDC-0449, studies are underway by other investigators on experimental oral or topical agents by Novartis, Exelixis, and Infinity Pharmaceuticals that address aberrations in the hedgehog signaling pathway for the treatment of BCC.
Major Finding: Group A patients started with a median of 252 BCCs and had 127 at the most recent follow-up.
Data Source: Phase II trial of 41 patients recruited thus far with basal cell nevus syndrome.
Disclosures: Genentech is sponsoring the GDC-0449 trial and provided free medication and travel funds for patients. The investigators have no other conflicts of interest, Dr. Tang said.
Vitamin D - Myths or Truths?
CHICAGO – Dr. Richard L. Gallo put on his Myth Busters hat at a recent dermatology meeting to debunk – and in some cases uphold – some of the most popular ideas about vitamin D.
"This subject is nothing new," noted Dr. Gallo, recounting a bit of vitamin D yore. "In 1936, Schlitz beer urged customers to drink the beverage because it contained 100 units of vitamin D, and could ward off colds and flu. So even back then, they were on to something."
But, Dr. Gallo questioned, is the idea that vitamin D can strengthen the immune system a reality – or a myth? And how about other claims touted in the public press, that sunlight is the best source of vitamin D, that the vitamin strengthen bones and protects against cancer.
"Unfortunately, vitamin D information has become something of a shell game, with positions that overstate the strength of the evidence. As dermatologists, for example, we know the carcinogenic potential of sunlight, but there are now opposing groups that advocate health by increasing vitamin D through sun exposure."
Myth No. 1: Fifteen minutes per day of sunlight provides adequate amounts of vitamin D.
"In a test tube, ultraviolet B is the optimal spectrum for converting 25-hydroxy D into vitamin D in the human body," Dr. Gallo said. "But randomized studies on this vary in results."
One frequently cited study examined the issue in Denmark. "Northern latitudes are very useful for studies like this because of the high intensity of the sun during the summer, and the low intensity in winter," said Dr. Gallo, chief of dermatology and professor of medicine and pediatrics at the University of California San Diego. "In this study, the 25-hydroxy D in the population varied dramatically with change in sunlight exposure, and tended to lag about 1 month behind the sunlight levels."
But the study also found that 53% of the subjects who sought sun exposure were still suboptimal in their vitamin D levels. "So sun-seeking behavior in one of the most intense sun-exposed areas of the world is not sufficient to cover optimal vitamin D in a population." (Photochem. Photobiol. 2009;85:1,480-4).
A 2009 study looked at sunlight exposure and vitamin D in twins (PLoS One 2010 5(7):e11555 [doi: 10.1371/journal.pone.0011555]). More than 200 twins were evaluated for the seasonal impact of genetic factors on serum 25-hydroxy vitamin D concentrations. "This showed very wide distributions in levels during the different seasons, and concluded that more than 50% of the variation in summer levels was not due to sun or diet, but to genetic influences independent of skin pigment," Dr. Gallo said.
"So, Myth No. 1 – busted," he concluded.
Myth No. 2: Vitamin D improves bone health.
Prospective cohort studies such as the National Health and Nutrition Examination Survey show that hip fracture is reduced by more than one-third in patients with adequate vitamin D levels (more than 60 nmol/L). "However, we still have a lot to learn. Data from a recent 3-year study of 2,000 perimenopasual women concluded that if vitamin D were given as a single annual dose of 500,000 IU, the women had a 15% increased risk of falls and a 26% increase in the risk of fractures." (JAMA 2010;303:1815-22).
"As far as Myth No. 2 goes, I'd say it's true, but we don't understand everything yet."
Myth No. 3: Vitamin D protects against cancer.
"This has been quite a popular theme in the press for years now, but there are no great mechanistic explanations as to why it may be true," Dr Gallo said. "There are a number of randomized controlled trials, but the data are inconsistent."
A 2009 Agency for Healthcare Research and Quality review examined more than 170 studies and reviews for several health outcomes and vitamin D. "Only one study really showed a level of significance [for cancer reduction]. The others showed inconsistent data on cancer and some showed a slight trend toward an increased risk for colon cancer." (Evid. Rep. Technol. Assess. (Full Rep.) 2009;183:1-420).
"Myth No. 3 is still a plausible possibility, but no benefit has been clearly demonstrated."
Myth No. 4: Vitamin D improves immune function.
"We have excellent mechanistic data to support this claim, including a number of observational studies and a few randomized controlled trials," Dr. Gallo said.
He coauthored a 2009 study concluding that vitamin D activates an enzyme on the surface of monocytes and keratinocytes, increasing the cells' pattern recognition and boosting their antimicrobial effect. "This enhances the immune barrier in injured skin," Dr. Gallo said (J. Clin. Invest. 2007;117:803-11).
Animal models also "show quite clearly that the extent of infection can be limited in an animal supplemented with vitamin D compared to a deficient one," he added. "There also seems to be a relative association between viral infections and upper respiratory infections, with the highest incidence occurring at the lowest levels of vitamin D on a seasonal basis. So maybe Schlitz did have an idea there. Therefore I’d say Myth No. 4 is plausible, but not yet clearly defined."
Dr. Gallo did not have any relevant financial disclosures. However, he is a member of the Institute of Medicine’s committee on Dietary Reference Intakes for Vitamin D and Calcium. The committee will release new recommendations for national daily requirements of vitamin D and calcium later this year.
CHICAGO – Dr. Richard L. Gallo put on his Myth Busters hat at a recent dermatology meeting to debunk – and in some cases uphold – some of the most popular ideas about vitamin D.
"This subject is nothing new," noted Dr. Gallo, recounting a bit of vitamin D yore. "In 1936, Schlitz beer urged customers to drink the beverage because it contained 100 units of vitamin D, and could ward off colds and flu. So even back then, they were on to something."
But, Dr. Gallo questioned, is the idea that vitamin D can strengthen the immune system a reality – or a myth? And how about other claims touted in the public press, that sunlight is the best source of vitamin D, that the vitamin strengthen bones and protects against cancer.
"Unfortunately, vitamin D information has become something of a shell game, with positions that overstate the strength of the evidence. As dermatologists, for example, we know the carcinogenic potential of sunlight, but there are now opposing groups that advocate health by increasing vitamin D through sun exposure."
Myth No. 1: Fifteen minutes per day of sunlight provides adequate amounts of vitamin D.
"In a test tube, ultraviolet B is the optimal spectrum for converting 25-hydroxy D into vitamin D in the human body," Dr. Gallo said. "But randomized studies on this vary in results."
One frequently cited study examined the issue in Denmark. "Northern latitudes are very useful for studies like this because of the high intensity of the sun during the summer, and the low intensity in winter," said Dr. Gallo, chief of dermatology and professor of medicine and pediatrics at the University of California San Diego. "In this study, the 25-hydroxy D in the population varied dramatically with change in sunlight exposure, and tended to lag about 1 month behind the sunlight levels."
But the study also found that 53% of the subjects who sought sun exposure were still suboptimal in their vitamin D levels. "So sun-seeking behavior in one of the most intense sun-exposed areas of the world is not sufficient to cover optimal vitamin D in a population." (Photochem. Photobiol. 2009;85:1,480-4).
A 2009 study looked at sunlight exposure and vitamin D in twins (PLoS One 2010 5(7):e11555 [doi: 10.1371/journal.pone.0011555]). More than 200 twins were evaluated for the seasonal impact of genetic factors on serum 25-hydroxy vitamin D concentrations. "This showed very wide distributions in levels during the different seasons, and concluded that more than 50% of the variation in summer levels was not due to sun or diet, but to genetic influences independent of skin pigment," Dr. Gallo said.
"So, Myth No. 1 – busted," he concluded.
Myth No. 2: Vitamin D improves bone health.
Prospective cohort studies such as the National Health and Nutrition Examination Survey show that hip fracture is reduced by more than one-third in patients with adequate vitamin D levels (more than 60 nmol/L). "However, we still have a lot to learn. Data from a recent 3-year study of 2,000 perimenopasual women concluded that if vitamin D were given as a single annual dose of 500,000 IU, the women had a 15% increased risk of falls and a 26% increase in the risk of fractures." (JAMA 2010;303:1815-22).
"As far as Myth No. 2 goes, I'd say it's true, but we don't understand everything yet."
Myth No. 3: Vitamin D protects against cancer.
"This has been quite a popular theme in the press for years now, but there are no great mechanistic explanations as to why it may be true," Dr Gallo said. "There are a number of randomized controlled trials, but the data are inconsistent."
A 2009 Agency for Healthcare Research and Quality review examined more than 170 studies and reviews for several health outcomes and vitamin D. "Only one study really showed a level of significance [for cancer reduction]. The others showed inconsistent data on cancer and some showed a slight trend toward an increased risk for colon cancer." (Evid. Rep. Technol. Assess. (Full Rep.) 2009;183:1-420).
"Myth No. 3 is still a plausible possibility, but no benefit has been clearly demonstrated."
Myth No. 4: Vitamin D improves immune function.
"We have excellent mechanistic data to support this claim, including a number of observational studies and a few randomized controlled trials," Dr. Gallo said.
He coauthored a 2009 study concluding that vitamin D activates an enzyme on the surface of monocytes and keratinocytes, increasing the cells' pattern recognition and boosting their antimicrobial effect. "This enhances the immune barrier in injured skin," Dr. Gallo said (J. Clin. Invest. 2007;117:803-11).
Animal models also "show quite clearly that the extent of infection can be limited in an animal supplemented with vitamin D compared to a deficient one," he added. "There also seems to be a relative association between viral infections and upper respiratory infections, with the highest incidence occurring at the lowest levels of vitamin D on a seasonal basis. So maybe Schlitz did have an idea there. Therefore I’d say Myth No. 4 is plausible, but not yet clearly defined."
Dr. Gallo did not have any relevant financial disclosures. However, he is a member of the Institute of Medicine’s committee on Dietary Reference Intakes for Vitamin D and Calcium. The committee will release new recommendations for national daily requirements of vitamin D and calcium later this year.
CHICAGO – Dr. Richard L. Gallo put on his Myth Busters hat at a recent dermatology meeting to debunk – and in some cases uphold – some of the most popular ideas about vitamin D.
"This subject is nothing new," noted Dr. Gallo, recounting a bit of vitamin D yore. "In 1936, Schlitz beer urged customers to drink the beverage because it contained 100 units of vitamin D, and could ward off colds and flu. So even back then, they were on to something."
But, Dr. Gallo questioned, is the idea that vitamin D can strengthen the immune system a reality – or a myth? And how about other claims touted in the public press, that sunlight is the best source of vitamin D, that the vitamin strengthen bones and protects against cancer.
"Unfortunately, vitamin D information has become something of a shell game, with positions that overstate the strength of the evidence. As dermatologists, for example, we know the carcinogenic potential of sunlight, but there are now opposing groups that advocate health by increasing vitamin D through sun exposure."
Myth No. 1: Fifteen minutes per day of sunlight provides adequate amounts of vitamin D.
"In a test tube, ultraviolet B is the optimal spectrum for converting 25-hydroxy D into vitamin D in the human body," Dr. Gallo said. "But randomized studies on this vary in results."
One frequently cited study examined the issue in Denmark. "Northern latitudes are very useful for studies like this because of the high intensity of the sun during the summer, and the low intensity in winter," said Dr. Gallo, chief of dermatology and professor of medicine and pediatrics at the University of California San Diego. "In this study, the 25-hydroxy D in the population varied dramatically with change in sunlight exposure, and tended to lag about 1 month behind the sunlight levels."
But the study also found that 53% of the subjects who sought sun exposure were still suboptimal in their vitamin D levels. "So sun-seeking behavior in one of the most intense sun-exposed areas of the world is not sufficient to cover optimal vitamin D in a population." (Photochem. Photobiol. 2009;85:1,480-4).
A 2009 study looked at sunlight exposure and vitamin D in twins (PLoS One 2010 5(7):e11555 [doi: 10.1371/journal.pone.0011555]). More than 200 twins were evaluated for the seasonal impact of genetic factors on serum 25-hydroxy vitamin D concentrations. "This showed very wide distributions in levels during the different seasons, and concluded that more than 50% of the variation in summer levels was not due to sun or diet, but to genetic influences independent of skin pigment," Dr. Gallo said.
"So, Myth No. 1 – busted," he concluded.
Myth No. 2: Vitamin D improves bone health.
Prospective cohort studies such as the National Health and Nutrition Examination Survey show that hip fracture is reduced by more than one-third in patients with adequate vitamin D levels (more than 60 nmol/L). "However, we still have a lot to learn. Data from a recent 3-year study of 2,000 perimenopasual women concluded that if vitamin D were given as a single annual dose of 500,000 IU, the women had a 15% increased risk of falls and a 26% increase in the risk of fractures." (JAMA 2010;303:1815-22).
"As far as Myth No. 2 goes, I'd say it's true, but we don't understand everything yet."
Myth No. 3: Vitamin D protects against cancer.
"This has been quite a popular theme in the press for years now, but there are no great mechanistic explanations as to why it may be true," Dr Gallo said. "There are a number of randomized controlled trials, but the data are inconsistent."
A 2009 Agency for Healthcare Research and Quality review examined more than 170 studies and reviews for several health outcomes and vitamin D. "Only one study really showed a level of significance [for cancer reduction]. The others showed inconsistent data on cancer and some showed a slight trend toward an increased risk for colon cancer." (Evid. Rep. Technol. Assess. (Full Rep.) 2009;183:1-420).
"Myth No. 3 is still a plausible possibility, but no benefit has been clearly demonstrated."
Myth No. 4: Vitamin D improves immune function.
"We have excellent mechanistic data to support this claim, including a number of observational studies and a few randomized controlled trials," Dr. Gallo said.
He coauthored a 2009 study concluding that vitamin D activates an enzyme on the surface of monocytes and keratinocytes, increasing the cells' pattern recognition and boosting their antimicrobial effect. "This enhances the immune barrier in injured skin," Dr. Gallo said (J. Clin. Invest. 2007;117:803-11).
Animal models also "show quite clearly that the extent of infection can be limited in an animal supplemented with vitamin D compared to a deficient one," he added. "There also seems to be a relative association between viral infections and upper respiratory infections, with the highest incidence occurring at the lowest levels of vitamin D on a seasonal basis. So maybe Schlitz did have an idea there. Therefore I’d say Myth No. 4 is plausible, but not yet clearly defined."
Dr. Gallo did not have any relevant financial disclosures. However, he is a member of the Institute of Medicine’s committee on Dietary Reference Intakes for Vitamin D and Calcium. The committee will release new recommendations for national daily requirements of vitamin D and calcium later this year.
PIGMENTED LESIONS
141 Introduction
Michael E. Ming
142 Implications of the 2009 American Joint Committee on Cancer Melanoma Staging and Classification on Dermatologists and Their Patients
Mary Alice Nading, Charles M. Balch, and Arthur J. Sober
148 Pigmented Lesions of the Nail Unit: Clinical and Histopathologic Features
Beth S. Ruben
159 The Risk of Melanoma and Neurocutaneous Melanosis Associated with Congenital Melanocytic Nevi
Kara N. Shah
165 Spitz Nevus and Atypical Spitzoid Neoplasm
Maria Miteva and Rossitza Lazova
174 Noninvasive Imaging Technologies in the Diagnosis of Melanoma
Steven Q. Wang and Pantea Hashemi
185 Vitamin D Levels, Dietary Intake, and Photoprotective Behaviors Among Patients With Skin Cancer
Laura K. DeLong, Sarah Wetherington, Nikki Hill, Meena Kumari, Bryan Gammon, Scott Dunbar, Vin Tangpricha, and Suephy C. Chen
190 Genetic Determinants of Cutaneous Melanoma Predisposition
Durga Udayakumar, Bisundev Mahato, Michele Gabree, and Hensin Tsao
196 Targeted Molecular Therapy in Melanoma
Igor Puzanov and Keith T. Flaherty
141 Introduction
Michael E. Ming
142 Implications of the 2009 American Joint Committee on Cancer Melanoma Staging and Classification on Dermatologists and Their Patients
Mary Alice Nading, Charles M. Balch, and Arthur J. Sober
148 Pigmented Lesions of the Nail Unit: Clinical and Histopathologic Features
Beth S. Ruben
159 The Risk of Melanoma and Neurocutaneous Melanosis Associated with Congenital Melanocytic Nevi
Kara N. Shah
165 Spitz Nevus and Atypical Spitzoid Neoplasm
Maria Miteva and Rossitza Lazova
174 Noninvasive Imaging Technologies in the Diagnosis of Melanoma
Steven Q. Wang and Pantea Hashemi
185 Vitamin D Levels, Dietary Intake, and Photoprotective Behaviors Among Patients With Skin Cancer
Laura K. DeLong, Sarah Wetherington, Nikki Hill, Meena Kumari, Bryan Gammon, Scott Dunbar, Vin Tangpricha, and Suephy C. Chen
190 Genetic Determinants of Cutaneous Melanoma Predisposition
Durga Udayakumar, Bisundev Mahato, Michele Gabree, and Hensin Tsao
196 Targeted Molecular Therapy in Melanoma
Igor Puzanov and Keith T. Flaherty
141 Introduction
Michael E. Ming
142 Implications of the 2009 American Joint Committee on Cancer Melanoma Staging and Classification on Dermatologists and Their Patients
Mary Alice Nading, Charles M. Balch, and Arthur J. Sober
148 Pigmented Lesions of the Nail Unit: Clinical and Histopathologic Features
Beth S. Ruben
159 The Risk of Melanoma and Neurocutaneous Melanosis Associated with Congenital Melanocytic Nevi
Kara N. Shah
165 Spitz Nevus and Atypical Spitzoid Neoplasm
Maria Miteva and Rossitza Lazova
174 Noninvasive Imaging Technologies in the Diagnosis of Melanoma
Steven Q. Wang and Pantea Hashemi
185 Vitamin D Levels, Dietary Intake, and Photoprotective Behaviors Among Patients With Skin Cancer
Laura K. DeLong, Sarah Wetherington, Nikki Hill, Meena Kumari, Bryan Gammon, Scott Dunbar, Vin Tangpricha, and Suephy C. Chen
190 Genetic Determinants of Cutaneous Melanoma Predisposition
Durga Udayakumar, Bisundev Mahato, Michele Gabree, and Hensin Tsao
196 Targeted Molecular Therapy in Melanoma
Igor Puzanov and Keith T. Flaherty
INTRODUCTION
The proper diagnosis and management of pigmented lesions and melanoma can be extremely challenging, but significant strides have been made on multiple fronts in our understanding of the best approaches to these lesions. In this issue, Arthur Sober and his colleagues describe the new changes to the AJCC staging system, Beth Ruben discusses the issues surrounding pigmented lesions of the nail, Kara Shah examines the risk of melanoma and neurocutaneous melanosis in congenital nevi, and Rossitza Lazova and Maria Miteva review the difficulties associated with management of Spitz nevi and the “atypical Spitzoid neoplasm.” Steven Wang and Pantea Hashemi review different imaging modalities, and Suephy Chen and her colleagues discuss the literature surrounding photoprotection measures and serum vitamin D levels. Hensin Tsao and his colleagues describe our current knowledge of the genetic determinants of melanoma predisposition, and the issue concludes with Keith Flaherty and Igor Puzanov’s review of the latest information regarding targeted therapy for metastatic melanoma. I hope that the reader will find the authors’ insights to be helpful when faced with similar situations in daily practice, and I thank all the authors for sharing their expertise.
This issue is dedicated to Marie-France Demierre, who was to have been a contributor to this issue before her untimely passing. She was not only an excellent clinician, researcher, and teacher, but also a wonderful colleague and friend, and she is missed.
Michael E. Ming, MD, MSCE
Guest Editor
University of Pennsylvania School of Medicine,
Philadelphia, PA
The proper diagnosis and management of pigmented lesions and melanoma can be extremely challenging, but significant strides have been made on multiple fronts in our understanding of the best approaches to these lesions. In this issue, Arthur Sober and his colleagues describe the new changes to the AJCC staging system, Beth Ruben discusses the issues surrounding pigmented lesions of the nail, Kara Shah examines the risk of melanoma and neurocutaneous melanosis in congenital nevi, and Rossitza Lazova and Maria Miteva review the difficulties associated with management of Spitz nevi and the “atypical Spitzoid neoplasm.” Steven Wang and Pantea Hashemi review different imaging modalities, and Suephy Chen and her colleagues discuss the literature surrounding photoprotection measures and serum vitamin D levels. Hensin Tsao and his colleagues describe our current knowledge of the genetic determinants of melanoma predisposition, and the issue concludes with Keith Flaherty and Igor Puzanov’s review of the latest information regarding targeted therapy for metastatic melanoma. I hope that the reader will find the authors’ insights to be helpful when faced with similar situations in daily practice, and I thank all the authors for sharing their expertise.
This issue is dedicated to Marie-France Demierre, who was to have been a contributor to this issue before her untimely passing. She was not only an excellent clinician, researcher, and teacher, but also a wonderful colleague and friend, and she is missed.
Michael E. Ming, MD, MSCE
Guest Editor
University of Pennsylvania School of Medicine,
Philadelphia, PA
The proper diagnosis and management of pigmented lesions and melanoma can be extremely challenging, but significant strides have been made on multiple fronts in our understanding of the best approaches to these lesions. In this issue, Arthur Sober and his colleagues describe the new changes to the AJCC staging system, Beth Ruben discusses the issues surrounding pigmented lesions of the nail, Kara Shah examines the risk of melanoma and neurocutaneous melanosis in congenital nevi, and Rossitza Lazova and Maria Miteva review the difficulties associated with management of Spitz nevi and the “atypical Spitzoid neoplasm.” Steven Wang and Pantea Hashemi review different imaging modalities, and Suephy Chen and her colleagues discuss the literature surrounding photoprotection measures and serum vitamin D levels. Hensin Tsao and his colleagues describe our current knowledge of the genetic determinants of melanoma predisposition, and the issue concludes with Keith Flaherty and Igor Puzanov’s review of the latest information regarding targeted therapy for metastatic melanoma. I hope that the reader will find the authors’ insights to be helpful when faced with similar situations in daily practice, and I thank all the authors for sharing their expertise.
This issue is dedicated to Marie-France Demierre, who was to have been a contributor to this issue before her untimely passing. She was not only an excellent clinician, researcher, and teacher, but also a wonderful colleague and friend, and she is missed.
Michael E. Ming, MD, MSCE
Guest Editor
University of Pennsylvania School of Medicine,
Philadelphia, PA
Implications of the 2009 American Joint Committee on Cancer Melanoma Staging and Classification on Dermatologists and Their Patients
Mary Alice Nading, MD, Charles M. Balch, MD, and Arthur J. Sober, MD
The Melanoma Staging and Classification system was recently revised by the American Joint Committee on Cancer (AJCC) and implemented effective January 2010 with changes reflecting new prognostic data gleaned by the significantly larger patient population studied for the 7th edition. This newest analysis yields important long-term outcome data as many of the patients were followed for nearly 2 decades. Additions to edition 7 of the AJCC Melanoma Staging classification highlight several important prognostic factors, particularly the addition of mitotic rate for classifying thin melanomas, the presence of microtumor burden in lymph nodes for stage III disease, and elevated lactate dehydrogenase levels in patients with distant metastatic disease. Although the basic tumor-nodes-metastases (ie, TNM) cancer classification model remains unchanged in this newest edition, the current AJCC Melanoma Staging System has incorporated the latest prognostic data to accurately stratify patients into staging categories. It is important for clinicians and dermatopathologists to familiarize themselves with these changes so that patients are suitably managed and referred to medical and surgical oncologists when appropriate.
*For a PDF of the full article, click on the link to the left of this introduction.
Mary Alice Nading, MD, Charles M. Balch, MD, and Arthur J. Sober, MD
The Melanoma Staging and Classification system was recently revised by the American Joint Committee on Cancer (AJCC) and implemented effective January 2010 with changes reflecting new prognostic data gleaned by the significantly larger patient population studied for the 7th edition. This newest analysis yields important long-term outcome data as many of the patients were followed for nearly 2 decades. Additions to edition 7 of the AJCC Melanoma Staging classification highlight several important prognostic factors, particularly the addition of mitotic rate for classifying thin melanomas, the presence of microtumor burden in lymph nodes for stage III disease, and elevated lactate dehydrogenase levels in patients with distant metastatic disease. Although the basic tumor-nodes-metastases (ie, TNM) cancer classification model remains unchanged in this newest edition, the current AJCC Melanoma Staging System has incorporated the latest prognostic data to accurately stratify patients into staging categories. It is important for clinicians and dermatopathologists to familiarize themselves with these changes so that patients are suitably managed and referred to medical and surgical oncologists when appropriate.
*For a PDF of the full article, click on the link to the left of this introduction.
Mary Alice Nading, MD, Charles M. Balch, MD, and Arthur J. Sober, MD
The Melanoma Staging and Classification system was recently revised by the American Joint Committee on Cancer (AJCC) and implemented effective January 2010 with changes reflecting new prognostic data gleaned by the significantly larger patient population studied for the 7th edition. This newest analysis yields important long-term outcome data as many of the patients were followed for nearly 2 decades. Additions to edition 7 of the AJCC Melanoma Staging classification highlight several important prognostic factors, particularly the addition of mitotic rate for classifying thin melanomas, the presence of microtumor burden in lymph nodes for stage III disease, and elevated lactate dehydrogenase levels in patients with distant metastatic disease. Although the basic tumor-nodes-metastases (ie, TNM) cancer classification model remains unchanged in this newest edition, the current AJCC Melanoma Staging System has incorporated the latest prognostic data to accurately stratify patients into staging categories. It is important for clinicians and dermatopathologists to familiarize themselves with these changes so that patients are suitably managed and referred to medical and surgical oncologists when appropriate.
*For a PDF of the full article, click on the link to the left of this introduction.
Pigmented Lesions of the Nail Unit: Clinical and Histopathologic Features
Beth S. Ruben, MD
Probably the most common reason to perform biopsy of the nail unit is for the evaluation of irregular pigmentation, especially longitudinal melanonychia or pigmented bands. When narrow and solitary, these are usually the product of melanocytic activation/hypermelanosis, lentigines, or melanocytic nevi. Multiple pigmented bands are generally a benign finding, the result of melanocytic activation, as seen in racial pigmentation in darker-skinned patients, for example. In the context of an irregular, broad, heterogeneous or “streaky” band, the chief concern is the exclusion of subungual melanoma. Before assessing the histologic features of any such entities, it is important to understand the normal nail anatomy and melanocytic density of nail unit epithelium, as well as the type of specimen submitted, and whether it is adequate to undertake a proper histologic evaluation. The criteria for diagnosis and prognosis of melanoma of the nail unit are still evolving, and a variety of factors must be weighed in the balance to make a correct diagnosis. The importance of the clinical context cannot be overemphasized. There are also nonmelanocytic conditions to be considered that may produce worrisome nail discoloration, such as subungual hemorrhage, squamous cell carcinoma, and pigmented onychomycosis.
*For a PDF of the full article, click on the link to the left of this introduction.
Beth S. Ruben, MD
Probably the most common reason to perform biopsy of the nail unit is for the evaluation of irregular pigmentation, especially longitudinal melanonychia or pigmented bands. When narrow and solitary, these are usually the product of melanocytic activation/hypermelanosis, lentigines, or melanocytic nevi. Multiple pigmented bands are generally a benign finding, the result of melanocytic activation, as seen in racial pigmentation in darker-skinned patients, for example. In the context of an irregular, broad, heterogeneous or “streaky” band, the chief concern is the exclusion of subungual melanoma. Before assessing the histologic features of any such entities, it is important to understand the normal nail anatomy and melanocytic density of nail unit epithelium, as well as the type of specimen submitted, and whether it is adequate to undertake a proper histologic evaluation. The criteria for diagnosis and prognosis of melanoma of the nail unit are still evolving, and a variety of factors must be weighed in the balance to make a correct diagnosis. The importance of the clinical context cannot be overemphasized. There are also nonmelanocytic conditions to be considered that may produce worrisome nail discoloration, such as subungual hemorrhage, squamous cell carcinoma, and pigmented onychomycosis.
*For a PDF of the full article, click on the link to the left of this introduction.
Beth S. Ruben, MD
Probably the most common reason to perform biopsy of the nail unit is for the evaluation of irregular pigmentation, especially longitudinal melanonychia or pigmented bands. When narrow and solitary, these are usually the product of melanocytic activation/hypermelanosis, lentigines, or melanocytic nevi. Multiple pigmented bands are generally a benign finding, the result of melanocytic activation, as seen in racial pigmentation in darker-skinned patients, for example. In the context of an irregular, broad, heterogeneous or “streaky” band, the chief concern is the exclusion of subungual melanoma. Before assessing the histologic features of any such entities, it is important to understand the normal nail anatomy and melanocytic density of nail unit epithelium, as well as the type of specimen submitted, and whether it is adequate to undertake a proper histologic evaluation. The criteria for diagnosis and prognosis of melanoma of the nail unit are still evolving, and a variety of factors must be weighed in the balance to make a correct diagnosis. The importance of the clinical context cannot be overemphasized. There are also nonmelanocytic conditions to be considered that may produce worrisome nail discoloration, such as subungual hemorrhage, squamous cell carcinoma, and pigmented onychomycosis.
*For a PDF of the full article, click on the link to the left of this introduction.
The Risk of Melanoma and Neurocutaneous Melanosis Associated with Congenital Melanocytic Nevi
Kara N. Shah, MD, PhD
Congenital melanocytic nevi are commonly encountered in clinical practice. Although the development of malignant melanoma arising in small and intermediate congenital melanocytic nevi is rare, there is a significant risk of malignant degeneration associated with large congenital melanocytic nevi, in particular those that arise on the torso in the so-called “bathing trunk” distribution, where the risk is estimated to be about 2.5% to 5%. The risk of malignant melanoma arising within a large congenital melanocytic nevus is highest in the first 5 to 10 years of life and carries a significant mortality. Large congenital melanocytic nevi, in particular those overlying the posterior axis and occurring in the context of multiple satellite melanocytic nevi, are also associated with the development of neurocutaneous melanosis, which may result in neurologic and neurodevelopmental sequelae and is associated with a significant risk of primary central nervous system melanoma and death.
*For a PDF of the full article, click on the link to the left of this introduction.
Kara N. Shah, MD, PhD
Congenital melanocytic nevi are commonly encountered in clinical practice. Although the development of malignant melanoma arising in small and intermediate congenital melanocytic nevi is rare, there is a significant risk of malignant degeneration associated with large congenital melanocytic nevi, in particular those that arise on the torso in the so-called “bathing trunk” distribution, where the risk is estimated to be about 2.5% to 5%. The risk of malignant melanoma arising within a large congenital melanocytic nevus is highest in the first 5 to 10 years of life and carries a significant mortality. Large congenital melanocytic nevi, in particular those overlying the posterior axis and occurring in the context of multiple satellite melanocytic nevi, are also associated with the development of neurocutaneous melanosis, which may result in neurologic and neurodevelopmental sequelae and is associated with a significant risk of primary central nervous system melanoma and death.
*For a PDF of the full article, click on the link to the left of this introduction.
Kara N. Shah, MD, PhD
Congenital melanocytic nevi are commonly encountered in clinical practice. Although the development of malignant melanoma arising in small and intermediate congenital melanocytic nevi is rare, there is a significant risk of malignant degeneration associated with large congenital melanocytic nevi, in particular those that arise on the torso in the so-called “bathing trunk” distribution, where the risk is estimated to be about 2.5% to 5%. The risk of malignant melanoma arising within a large congenital melanocytic nevus is highest in the first 5 to 10 years of life and carries a significant mortality. Large congenital melanocytic nevi, in particular those overlying the posterior axis and occurring in the context of multiple satellite melanocytic nevi, are also associated with the development of neurocutaneous melanosis, which may result in neurologic and neurodevelopmental sequelae and is associated with a significant risk of primary central nervous system melanoma and death.
*For a PDF of the full article, click on the link to the left of this introduction.
Large congenital melanocytic nevi, in particular those overlying the posterior axis and occurring in the context of multiple satellite melanocytic nevi, are also associated with the development of neurocutaneous melanosis, which may result in neurologic and neurodevelopmental sequelae and is associated with a significant risk of primary central nervous system melanoma and death.