User login
BRAF Kinase Inhibitor Shrinks Melanoma Brain Metastases
MILAN – Hope for patients with melanoma who develop brain metastases may be on the horizon with the finding that a novel targeted agent reduces the size of multiple brain lesions.
Data from a phase I/II study in 10 patients show that all but one of the participants treated with the oral agent GSK2118436 experienced a partial or complete response, with overall reductions in the size of brain metastases ranging from -20% to -100%.
"These are extremely promising results," said Dr. Caroline Robert of Institut Gustave-Roussy, Villejuif, France, who was asked to comment on the study at the annual congress of the European Society for Medical Oncology.
GSK2118436 inhibits a specific mutation (V600) in the BRAF gene that is present in around 50% of melanomas and "locks BRAF into its active conformation," study investigator Dr. Georgina V. Long explained. The researcher, from Melanoma Institute Australia and Westmead Hospital in Sydney, added that the mutation enhances cancer cells' ability to proliferate, grow, and survive and that GSK2118436 disrupts this unwanted activity.
The positive effect of the novel agent in treating brain metastases was first seen about a year ago, Dr. Long said in an interview: "We had an inkling at the end of 2009, and that's when we really pushed to do a formal, prospective study of the brain cohort."
The melanoma brain cohort consisted of 10 patients with one or more brain lesions of at least 3 mm in size who were asymptomatic and who had not received any prior treatment specifically for their brain lesions. The median age of recruited patients was 59 years, 90% had the V600E mutation in the BRAF gene, and 30% had more than three brain metastases.
Gadolinium-enhanced and T1-weighted magnetic resonance imaging showed that twice-daily oral dosing (150 mg) of GSK2118436 resulted in reductions in brain lesions that correlated to extracranial tumor responses.
The tolerability was very similar to that presented recently at the American Society of Clinical Oncology (J. Clin. Oncol. 2010;28(15s):Abstr 8503). Grade 3/4 adverse events reported in the brain cohort included pyrexia/chills, fatigue, dehydration, nausea, in one patient each, and anemia in two patients.
Patients remained on treatment for a relatively short period of time, however, and it is too early to tell if GSK2118436 is likely to have any effect on the very poor overall survival of patients who develop brain metastases, which is currently around 16 weeks from the time that brain involvement in diagnosed.
"We need to do the phase II study in more patients to get better data," Dr. Long said. A phase II trial has already been started in patients who do not have brain metastases, now it is time to set up a similar trial in those that do, she added.
The phase II brain cohort trial should start recruitment early in 2011 and accruing enough patients should not be an issue. "In melanoma, brain metastases is a major problem; 15%-20% of patients have them at baseline, and nearly three-quarters develop them eventually. It is the cancer where brain metastases are a major problem, more so than any other cancer," commented Dr. Long.
Dr. Long's travel expenses to present the study data were paid for by GlaxoSmithKline, the study's sponsor. She also participated in a phase I advisory board in 2009. Dr. Long's coauthors have received consultancy fees and research report from the company, and three were current employees of GSK with stock ownership. Dr. Robert had no relevant conflicts of interest.
MILAN – Hope for patients with melanoma who develop brain metastases may be on the horizon with the finding that a novel targeted agent reduces the size of multiple brain lesions.
Data from a phase I/II study in 10 patients show that all but one of the participants treated with the oral agent GSK2118436 experienced a partial or complete response, with overall reductions in the size of brain metastases ranging from -20% to -100%.
"These are extremely promising results," said Dr. Caroline Robert of Institut Gustave-Roussy, Villejuif, France, who was asked to comment on the study at the annual congress of the European Society for Medical Oncology.
GSK2118436 inhibits a specific mutation (V600) in the BRAF gene that is present in around 50% of melanomas and "locks BRAF into its active conformation," study investigator Dr. Georgina V. Long explained. The researcher, from Melanoma Institute Australia and Westmead Hospital in Sydney, added that the mutation enhances cancer cells' ability to proliferate, grow, and survive and that GSK2118436 disrupts this unwanted activity.
The positive effect of the novel agent in treating brain metastases was first seen about a year ago, Dr. Long said in an interview: "We had an inkling at the end of 2009, and that's when we really pushed to do a formal, prospective study of the brain cohort."
The melanoma brain cohort consisted of 10 patients with one or more brain lesions of at least 3 mm in size who were asymptomatic and who had not received any prior treatment specifically for their brain lesions. The median age of recruited patients was 59 years, 90% had the V600E mutation in the BRAF gene, and 30% had more than three brain metastases.
Gadolinium-enhanced and T1-weighted magnetic resonance imaging showed that twice-daily oral dosing (150 mg) of GSK2118436 resulted in reductions in brain lesions that correlated to extracranial tumor responses.
The tolerability was very similar to that presented recently at the American Society of Clinical Oncology (J. Clin. Oncol. 2010;28(15s):Abstr 8503). Grade 3/4 adverse events reported in the brain cohort included pyrexia/chills, fatigue, dehydration, nausea, in one patient each, and anemia in two patients.
Patients remained on treatment for a relatively short period of time, however, and it is too early to tell if GSK2118436 is likely to have any effect on the very poor overall survival of patients who develop brain metastases, which is currently around 16 weeks from the time that brain involvement in diagnosed.
"We need to do the phase II study in more patients to get better data," Dr. Long said. A phase II trial has already been started in patients who do not have brain metastases, now it is time to set up a similar trial in those that do, she added.
The phase II brain cohort trial should start recruitment early in 2011 and accruing enough patients should not be an issue. "In melanoma, brain metastases is a major problem; 15%-20% of patients have them at baseline, and nearly three-quarters develop them eventually. It is the cancer where brain metastases are a major problem, more so than any other cancer," commented Dr. Long.
Dr. Long's travel expenses to present the study data were paid for by GlaxoSmithKline, the study's sponsor. She also participated in a phase I advisory board in 2009. Dr. Long's coauthors have received consultancy fees and research report from the company, and three were current employees of GSK with stock ownership. Dr. Robert had no relevant conflicts of interest.
MILAN – Hope for patients with melanoma who develop brain metastases may be on the horizon with the finding that a novel targeted agent reduces the size of multiple brain lesions.
Data from a phase I/II study in 10 patients show that all but one of the participants treated with the oral agent GSK2118436 experienced a partial or complete response, with overall reductions in the size of brain metastases ranging from -20% to -100%.
"These are extremely promising results," said Dr. Caroline Robert of Institut Gustave-Roussy, Villejuif, France, who was asked to comment on the study at the annual congress of the European Society for Medical Oncology.
GSK2118436 inhibits a specific mutation (V600) in the BRAF gene that is present in around 50% of melanomas and "locks BRAF into its active conformation," study investigator Dr. Georgina V. Long explained. The researcher, from Melanoma Institute Australia and Westmead Hospital in Sydney, added that the mutation enhances cancer cells' ability to proliferate, grow, and survive and that GSK2118436 disrupts this unwanted activity.
The positive effect of the novel agent in treating brain metastases was first seen about a year ago, Dr. Long said in an interview: "We had an inkling at the end of 2009, and that's when we really pushed to do a formal, prospective study of the brain cohort."
The melanoma brain cohort consisted of 10 patients with one or more brain lesions of at least 3 mm in size who were asymptomatic and who had not received any prior treatment specifically for their brain lesions. The median age of recruited patients was 59 years, 90% had the V600E mutation in the BRAF gene, and 30% had more than three brain metastases.
Gadolinium-enhanced and T1-weighted magnetic resonance imaging showed that twice-daily oral dosing (150 mg) of GSK2118436 resulted in reductions in brain lesions that correlated to extracranial tumor responses.
The tolerability was very similar to that presented recently at the American Society of Clinical Oncology (J. Clin. Oncol. 2010;28(15s):Abstr 8503). Grade 3/4 adverse events reported in the brain cohort included pyrexia/chills, fatigue, dehydration, nausea, in one patient each, and anemia in two patients.
Patients remained on treatment for a relatively short period of time, however, and it is too early to tell if GSK2118436 is likely to have any effect on the very poor overall survival of patients who develop brain metastases, which is currently around 16 weeks from the time that brain involvement in diagnosed.
"We need to do the phase II study in more patients to get better data," Dr. Long said. A phase II trial has already been started in patients who do not have brain metastases, now it is time to set up a similar trial in those that do, she added.
The phase II brain cohort trial should start recruitment early in 2011 and accruing enough patients should not be an issue. "In melanoma, brain metastases is a major problem; 15%-20% of patients have them at baseline, and nearly three-quarters develop them eventually. It is the cancer where brain metastases are a major problem, more so than any other cancer," commented Dr. Long.
Dr. Long's travel expenses to present the study data were paid for by GlaxoSmithKline, the study's sponsor. She also participated in a phase I advisory board in 2009. Dr. Long's coauthors have received consultancy fees and research report from the company, and three were current employees of GSK with stock ownership. Dr. Robert had no relevant conflicts of interest.
FROM THE ANNUAL CONGRESS OF THE EUROPEAN SOCIETY FOR MEDICAL ONCOLOGY
Major Finding: Overall reductions in the size of brain metastases after treatment with the BRAF kinase inhibitor ranged from –20% to –100%.
Data Source: Open-label, phase I/II study of GSK2118436 in 10 patients with melanoma brain metastases.
Disclosures: Dr. Long's travel expenses to present the study data were paid for by GlaxoSmithKline, the study’s sponsor. She also participated in a phase I advisory board in 2009. Dr. Long’s coauthors have also received advisory fees and research support from the company, and three were current employees of GSK with stock ownership. Dr. Robert had no relevant conflicts of interest.
MelaFind Device Surpassed Dermatologists in Identifying Melanoma
A hand-held, computerized imaging device identified 98% of melanomas in a set of suspicious pigmented skin lesions, according to results published online in the Archives of Dermatology.
The study findings were also published online on Skin & Allergy News Digital Network on May 6, 2010 (AAD: Digital Dermoscopy Device Detects Melanoma).
Results of the automated device – MelaFind from MELA Sciences Inc. – were compared with dermatologists who examined a subset of lesions from the same group, for a 78% identification rate. On lesions that had been previously biopsied to rule out melanoma, MelaFind's average specificity was 10%, significantly better than the 3.7% rate of dermatologists.
The device's 98% sensitivity rating for malignant melanoma was significantly better than that of dermatologists, who showed a wide range of variability about which lesions would have been recommended for biopsy and which relegated to observation, Dr. Gary Monheit and his colleagues reported (Arch. Dermatol. 2010 [doi:10.1001/archdermatol.2010.302]).
The study compared MelaFind's performance on a group of 1,632 lesions with a reading study of 50 lesions randomly extracted from the same set. All of the lesions had been biopsied by the patients’ attending dermatologists.
Lesion data, including the clinical overview and close-up and dermatoscopy images, were provided to 39 dermatologists, who were blinded to the biopsy results. The dermatologists evaluated which lesions they believed should be biopsied as probable melanoma and which should be followed clinically, wrote Dr. Monheit, a dermatologist in private practice in Birmingham, Ala., and his coauthors. The lesion samples came from a group of 1,257 patients whose mean age was 46 years. Most (98%) were white.
The MelaFind study's primary end point was the device’s sensitivity and specificity for lesions classified before biopsy as "melanoma cannot be ruled out" or as "not melanoma." After reviewing each lesion, MelaFind generated one of two conclusions: positive (biopsy) or negative (follow clinically).
Pigmented nevi made up the bulk of the lesion group (77%; 1,258), with 61% being low-grade dysplasia. Another 15% of the lesions were nonmelanocytic. Melanomas accounted for 8% of the group (127), with 57 in situ and 70 invasive; however, the invasive lesions were mostly thin, with a median thickness of 0.36 mm. Only two were relatively thick (1.0 mm and 1.2 mm). "Thus," the investigators noted, "almost all melanomas in this trial were early lesions that are difficult to differentiate from benign simulants."
Other components of the group included keratoses (119), lentigos (76), pigmented basal and squamous cell carcinomas (33), and other lesions (14).
In the reader study, the average biopsy sensitivity of the 39 dermatologists was 78%. However, the authors noted, the inter-reader variability was high. "Only 5 of the 25 melanomas would have been biopsied by all readers, and different readers missed different melanomas." Since all of the lesions had previously been biopsied, the paper did not compare clinician and MelaFind biopsy ratios. However, the authors noted, historical biopsy ratios among dermatologists are about 8:1 for the general population and up to 47:1 for high-risk patients.
The authors concluded that the device could be a valuable tool in accurately assessing pigmented lesions – an area that is largely dependent on clinicians’ individual observations and conclusions.
In a statement from MELA Sciences, coauthor Dr. Kenneth Gross stressed the need for an objective tool in the biopsy decision-making process.
"The pilot reader study found that dermatologists do miss early melanomas," said Dr. Gross, who practices surgical dermatology in San Diego. "Only 20% of the melanomas in the pilot reader study would have been biopsied by all readers and different readers missed different melanomas. Thus, even though all lesions in the clinical trial were biopsied by the examining dermatologists, many of the melanomas would not have been biopsied by other dermatologists, underscoring the need for an objective tool to aid in the decision to biopsy. We believe that the results of the pivotal trial and the pilot reader study underscore the clinical utility of MelaFind as an objective tool to aid clinicians in the detection of early melanoma."
The Food and Drug Administration will consider MelaFind on Nov. 18, during the General and Plastic Surgery Devices Panel of the Medical Devices Advisory Committee meeting. The committee will make recommendations and vote on data related to the device's premarket approval.
According to the company Web site, MelaFind is a hand-held device that acquires and displays multispectral digital images from blue to near-infrared, for pigmented skin lesions. The computerized device uses automatic image analysis and statistical pattern recognition to help identify lesions that should be considered for biopsy to rule out melanoma. The device consists of an illuminator that produces light in 10 wavelengths, a lens system that creates the images, a light sensor, and an image processor that identifies discrete characteristics of each picture.
MELA Sciences sponsored the study. Dr. Monheit and 7 of the other 15 investigators are on the MELA Sciences Advisory Committee. Dr. Gross did not disclose any financial relationship with the company.
A hand-held, computerized imaging device identified 98% of melanomas in a set of suspicious pigmented skin lesions, according to results published online in the Archives of Dermatology.
The study findings were also published online on Skin & Allergy News Digital Network on May 6, 2010 (AAD: Digital Dermoscopy Device Detects Melanoma).
Results of the automated device – MelaFind from MELA Sciences Inc. – were compared with dermatologists who examined a subset of lesions from the same group, for a 78% identification rate. On lesions that had been previously biopsied to rule out melanoma, MelaFind's average specificity was 10%, significantly better than the 3.7% rate of dermatologists.
The device's 98% sensitivity rating for malignant melanoma was significantly better than that of dermatologists, who showed a wide range of variability about which lesions would have been recommended for biopsy and which relegated to observation, Dr. Gary Monheit and his colleagues reported (Arch. Dermatol. 2010 [doi:10.1001/archdermatol.2010.302]).
The study compared MelaFind's performance on a group of 1,632 lesions with a reading study of 50 lesions randomly extracted from the same set. All of the lesions had been biopsied by the patients’ attending dermatologists.
Lesion data, including the clinical overview and close-up and dermatoscopy images, were provided to 39 dermatologists, who were blinded to the biopsy results. The dermatologists evaluated which lesions they believed should be biopsied as probable melanoma and which should be followed clinically, wrote Dr. Monheit, a dermatologist in private practice in Birmingham, Ala., and his coauthors. The lesion samples came from a group of 1,257 patients whose mean age was 46 years. Most (98%) were white.
The MelaFind study's primary end point was the device’s sensitivity and specificity for lesions classified before biopsy as "melanoma cannot be ruled out" or as "not melanoma." After reviewing each lesion, MelaFind generated one of two conclusions: positive (biopsy) or negative (follow clinically).
Pigmented nevi made up the bulk of the lesion group (77%; 1,258), with 61% being low-grade dysplasia. Another 15% of the lesions were nonmelanocytic. Melanomas accounted for 8% of the group (127), with 57 in situ and 70 invasive; however, the invasive lesions were mostly thin, with a median thickness of 0.36 mm. Only two were relatively thick (1.0 mm and 1.2 mm). "Thus," the investigators noted, "almost all melanomas in this trial were early lesions that are difficult to differentiate from benign simulants."
Other components of the group included keratoses (119), lentigos (76), pigmented basal and squamous cell carcinomas (33), and other lesions (14).
In the reader study, the average biopsy sensitivity of the 39 dermatologists was 78%. However, the authors noted, the inter-reader variability was high. "Only 5 of the 25 melanomas would have been biopsied by all readers, and different readers missed different melanomas." Since all of the lesions had previously been biopsied, the paper did not compare clinician and MelaFind biopsy ratios. However, the authors noted, historical biopsy ratios among dermatologists are about 8:1 for the general population and up to 47:1 for high-risk patients.
The authors concluded that the device could be a valuable tool in accurately assessing pigmented lesions – an area that is largely dependent on clinicians’ individual observations and conclusions.
In a statement from MELA Sciences, coauthor Dr. Kenneth Gross stressed the need for an objective tool in the biopsy decision-making process.
"The pilot reader study found that dermatologists do miss early melanomas," said Dr. Gross, who practices surgical dermatology in San Diego. "Only 20% of the melanomas in the pilot reader study would have been biopsied by all readers and different readers missed different melanomas. Thus, even though all lesions in the clinical trial were biopsied by the examining dermatologists, many of the melanomas would not have been biopsied by other dermatologists, underscoring the need for an objective tool to aid in the decision to biopsy. We believe that the results of the pivotal trial and the pilot reader study underscore the clinical utility of MelaFind as an objective tool to aid clinicians in the detection of early melanoma."
The Food and Drug Administration will consider MelaFind on Nov. 18, during the General and Plastic Surgery Devices Panel of the Medical Devices Advisory Committee meeting. The committee will make recommendations and vote on data related to the device's premarket approval.
According to the company Web site, MelaFind is a hand-held device that acquires and displays multispectral digital images from blue to near-infrared, for pigmented skin lesions. The computerized device uses automatic image analysis and statistical pattern recognition to help identify lesions that should be considered for biopsy to rule out melanoma. The device consists of an illuminator that produces light in 10 wavelengths, a lens system that creates the images, a light sensor, and an image processor that identifies discrete characteristics of each picture.
MELA Sciences sponsored the study. Dr. Monheit and 7 of the other 15 investigators are on the MELA Sciences Advisory Committee. Dr. Gross did not disclose any financial relationship with the company.
A hand-held, computerized imaging device identified 98% of melanomas in a set of suspicious pigmented skin lesions, according to results published online in the Archives of Dermatology.
The study findings were also published online on Skin & Allergy News Digital Network on May 6, 2010 (AAD: Digital Dermoscopy Device Detects Melanoma).
Results of the automated device – MelaFind from MELA Sciences Inc. – were compared with dermatologists who examined a subset of lesions from the same group, for a 78% identification rate. On lesions that had been previously biopsied to rule out melanoma, MelaFind's average specificity was 10%, significantly better than the 3.7% rate of dermatologists.
The device's 98% sensitivity rating for malignant melanoma was significantly better than that of dermatologists, who showed a wide range of variability about which lesions would have been recommended for biopsy and which relegated to observation, Dr. Gary Monheit and his colleagues reported (Arch. Dermatol. 2010 [doi:10.1001/archdermatol.2010.302]).
The study compared MelaFind's performance on a group of 1,632 lesions with a reading study of 50 lesions randomly extracted from the same set. All of the lesions had been biopsied by the patients’ attending dermatologists.
Lesion data, including the clinical overview and close-up and dermatoscopy images, were provided to 39 dermatologists, who were blinded to the biopsy results. The dermatologists evaluated which lesions they believed should be biopsied as probable melanoma and which should be followed clinically, wrote Dr. Monheit, a dermatologist in private practice in Birmingham, Ala., and his coauthors. The lesion samples came from a group of 1,257 patients whose mean age was 46 years. Most (98%) were white.
The MelaFind study's primary end point was the device’s sensitivity and specificity for lesions classified before biopsy as "melanoma cannot be ruled out" or as "not melanoma." After reviewing each lesion, MelaFind generated one of two conclusions: positive (biopsy) or negative (follow clinically).
Pigmented nevi made up the bulk of the lesion group (77%; 1,258), with 61% being low-grade dysplasia. Another 15% of the lesions were nonmelanocytic. Melanomas accounted for 8% of the group (127), with 57 in situ and 70 invasive; however, the invasive lesions were mostly thin, with a median thickness of 0.36 mm. Only two were relatively thick (1.0 mm and 1.2 mm). "Thus," the investigators noted, "almost all melanomas in this trial were early lesions that are difficult to differentiate from benign simulants."
Other components of the group included keratoses (119), lentigos (76), pigmented basal and squamous cell carcinomas (33), and other lesions (14).
In the reader study, the average biopsy sensitivity of the 39 dermatologists was 78%. However, the authors noted, the inter-reader variability was high. "Only 5 of the 25 melanomas would have been biopsied by all readers, and different readers missed different melanomas." Since all of the lesions had previously been biopsied, the paper did not compare clinician and MelaFind biopsy ratios. However, the authors noted, historical biopsy ratios among dermatologists are about 8:1 for the general population and up to 47:1 for high-risk patients.
The authors concluded that the device could be a valuable tool in accurately assessing pigmented lesions – an area that is largely dependent on clinicians’ individual observations and conclusions.
In a statement from MELA Sciences, coauthor Dr. Kenneth Gross stressed the need for an objective tool in the biopsy decision-making process.
"The pilot reader study found that dermatologists do miss early melanomas," said Dr. Gross, who practices surgical dermatology in San Diego. "Only 20% of the melanomas in the pilot reader study would have been biopsied by all readers and different readers missed different melanomas. Thus, even though all lesions in the clinical trial were biopsied by the examining dermatologists, many of the melanomas would not have been biopsied by other dermatologists, underscoring the need for an objective tool to aid in the decision to biopsy. We believe that the results of the pivotal trial and the pilot reader study underscore the clinical utility of MelaFind as an objective tool to aid clinicians in the detection of early melanoma."
The Food and Drug Administration will consider MelaFind on Nov. 18, during the General and Plastic Surgery Devices Panel of the Medical Devices Advisory Committee meeting. The committee will make recommendations and vote on data related to the device's premarket approval.
According to the company Web site, MelaFind is a hand-held device that acquires and displays multispectral digital images from blue to near-infrared, for pigmented skin lesions. The computerized device uses automatic image analysis and statistical pattern recognition to help identify lesions that should be considered for biopsy to rule out melanoma. The device consists of an illuminator that produces light in 10 wavelengths, a lens system that creates the images, a light sensor, and an image processor that identifies discrete characteristics of each picture.
MELA Sciences sponsored the study. Dr. Monheit and 7 of the other 15 investigators are on the MELA Sciences Advisory Committee. Dr. Gross did not disclose any financial relationship with the company.
FROM THE ARCHIVES OF DERMATOLOGY
Major Finding: A computerized image evaluator identified 98% of melanomas in a blinded testing set, compared with a 78% rate for dermatologists looking at some of the same lesions.
Data Source: The 1,632 lesion samples came from a group of 1,257 patients whose mean age was 46 years. Most (98%) were white.
Disclosures: MELA Sciences Inc. sponsored the study. Dr. Monheit and 7 of the other 15 investigators are on the MELA Sciences Advisory Committee. Dr. Gross did not disclose any financial relationship with the company.
EADV: Leukemia Patients Predisposed to Aggressive Melanoma
GOTHENBURG, Sweden - Patients with a history of chronic lymphocytic leukemia have an elevated risk of developing malignant melanoma of a particularly aggressive nature.
A new analysis of data from the National Cancer Institute's Surveillance, Epidemiology, and End Results registry demonstrates that individuals with a history of chronic lymphocytic leukemia (CLL) are at 2.5-fold increased risk of subsequently developing melanoma. And once they do, these patients have an adjusted 2.7-fold greater 10-year all-cause mortality and a 2.8-fold increased mortality caused by melanoma, compared with melanoma patients without prior CLL, Dr. Jerry D. Brewer reported at the annual congress of the European Academy of Dermatology and Venereology .
"Melanoma and CLL is a dangerously common association with bad outcomes," said Dr. Brewer, a dermatologic surgeon at the Mayo Clinic, Rochester, Minn.
The pattern of worse outcomes in melanoma patients with a history of CLL was significant across all categories of Breslow tumor depth and Clark's level, but it was most striking in patients with thicker lesions. For example, melanoma patients with a Breslow depth greater than 4.0 mm had a 2-year overall survival rate of just 36% if they had a history of prior CLL, compared with 71% if they did not. Their 2-year melanoma-specific survival was 50% with prior CLL and 82% without such a history.
These findings have important implications for clinical practice, according to the dermatologist. For example, patients with CLL have to get serious about daily sun protection, and they need to learn how to do regular skin self-examinations.
"We also need to educate our professional colleagues, specifically our hematologists/oncologists, that if their patients with CLL have a lot of moles or a lot of risk factors for melanoma, they should consider referring those patients to a dermatologist sooner, to catch melanomas earlier," he continued.
The population-based study included 212,245 melanoma patients in the SEER database in the years 1990-2006. Among them were 1,246 patients with a prior diagnosis of lymphoma, of which 31% were CLL. Those diagnosed with melanoma after CLL were on average 12 years older than patients diagnosed with melanoma without prior CLL, a difference adjusted for in determining standardized mortality ratios.
The 10-year overall survival in patients diagnosed with melanoma preceded by CLL was 19%, compared with an expected 55% if they had no history of prior CLL. The 10-year melanoma-specific survival rates were 62% with a prior diagnosis of CLL and 84% without.
Other investigators have previously reported higher rates of metastasis and worse survival in patients with squamous cell carcinoma or Merkel cell carcinoma preceded by CLL. "Now we know that’s true for melanoma, too," Dr. Brewer said.
The impetus for the SEER study was an earlier small study he and his coinvestigators conducted involving 69 Mayo Clinic patients with CLL and melanoma. They found worse outcomes in patients who had CLL prior to melanoma than in those diagnosed with melanoma prior to CLL (Dermatol. Surg. 2010;36:368-76).
One case in that series that particularly impressed Dr. Brewer involved a documented metastasis in a patient with a history of CLL prior to diagnosis of melanoma in situ. This was a melanoma in situ without an inflammatory infiltrate, so there was no confusion about the lesion's true depth. It was unmistakably a melanoma in situ, yet after standard therapy it recurred and metastasized.
Like patients with a history of CLL, organ transplant recipients are also at increased risk of aggressive skin cancers. Theories abound as to why immunosuppression, whether caused by lymphoma or organ transplantation, should have this effect. Among the proposed explanations are decreased immune surveillance, direct carcinogenesis caused by chemotherapeutic agents or antitransplant-rejection drugs, and an increased rate of infections with human papillomavirus.
However, Dr. Brewer thinks the most likely explanation involves a shared underlying genetic predisposition. Patients with CLL are rife with genetic aberrations. For example, 7%-10% of patients with CLL have a deletion mutation at 17 p.
"That's where the p53 gene is, which is the strongest predictor of poor survival in patients with CLL, with a median survival of only 32 months. Maybe these patients also have a higher risk of developing aggressive skin cancer; that’s something we just don't know yet," he noted.
Another genetic aberration worthy of further study involves the proto-oncogene B-cell lymphoma 2 (Bcl-2), which suppresses apoptosis. Bcl-2 expression is elevated in 95% of patients with CLL and in 90% of melanoma patients. Intriguingly, the antisense oligonucleotide oblimersen, which is targeted at Bcl-2, has shown encouraging results in combination with dacarbazine in patients with advanced melanoma (Eur. J. Cancer 2009;45:1807-14).
"Maybe that’s something we should consider using in patients with CLL," Dr. Brewer observed.
He argued that a high degree of suspicion is warranted regarding potential tumor recurrence in melanoma patients with a history of CLL. This may warrant more frequent follow-up, a low threshold for biopsy of suspicious lesions, and perhaps a lower bar for adjuvant chemotherapy.
"Perhaps we should consider doing a sentinel lymph node biopsy more often in thinner melanomas in patients with prior CLL. If they have a higher chance of metastasis, then maybe they have a higher chance of sentinel node involvement with thinner melanomas. There is [a lot] of speculation at this point, a lot more questions than answers," he said.
He declared having no financial conflicts, noting that his research on lymphoma-related skin cancer has been funded mainly by the Dermatology Foundation.
GOTHENBURG, Sweden - Patients with a history of chronic lymphocytic leukemia have an elevated risk of developing malignant melanoma of a particularly aggressive nature.
A new analysis of data from the National Cancer Institute's Surveillance, Epidemiology, and End Results registry demonstrates that individuals with a history of chronic lymphocytic leukemia (CLL) are at 2.5-fold increased risk of subsequently developing melanoma. And once they do, these patients have an adjusted 2.7-fold greater 10-year all-cause mortality and a 2.8-fold increased mortality caused by melanoma, compared with melanoma patients without prior CLL, Dr. Jerry D. Brewer reported at the annual congress of the European Academy of Dermatology and Venereology .
"Melanoma and CLL is a dangerously common association with bad outcomes," said Dr. Brewer, a dermatologic surgeon at the Mayo Clinic, Rochester, Minn.
The pattern of worse outcomes in melanoma patients with a history of CLL was significant across all categories of Breslow tumor depth and Clark's level, but it was most striking in patients with thicker lesions. For example, melanoma patients with a Breslow depth greater than 4.0 mm had a 2-year overall survival rate of just 36% if they had a history of prior CLL, compared with 71% if they did not. Their 2-year melanoma-specific survival was 50% with prior CLL and 82% without such a history.
These findings have important implications for clinical practice, according to the dermatologist. For example, patients with CLL have to get serious about daily sun protection, and they need to learn how to do regular skin self-examinations.
"We also need to educate our professional colleagues, specifically our hematologists/oncologists, that if their patients with CLL have a lot of moles or a lot of risk factors for melanoma, they should consider referring those patients to a dermatologist sooner, to catch melanomas earlier," he continued.
The population-based study included 212,245 melanoma patients in the SEER database in the years 1990-2006. Among them were 1,246 patients with a prior diagnosis of lymphoma, of which 31% were CLL. Those diagnosed with melanoma after CLL were on average 12 years older than patients diagnosed with melanoma without prior CLL, a difference adjusted for in determining standardized mortality ratios.
The 10-year overall survival in patients diagnosed with melanoma preceded by CLL was 19%, compared with an expected 55% if they had no history of prior CLL. The 10-year melanoma-specific survival rates were 62% with a prior diagnosis of CLL and 84% without.
Other investigators have previously reported higher rates of metastasis and worse survival in patients with squamous cell carcinoma or Merkel cell carcinoma preceded by CLL. "Now we know that’s true for melanoma, too," Dr. Brewer said.
The impetus for the SEER study was an earlier small study he and his coinvestigators conducted involving 69 Mayo Clinic patients with CLL and melanoma. They found worse outcomes in patients who had CLL prior to melanoma than in those diagnosed with melanoma prior to CLL (Dermatol. Surg. 2010;36:368-76).
One case in that series that particularly impressed Dr. Brewer involved a documented metastasis in a patient with a history of CLL prior to diagnosis of melanoma in situ. This was a melanoma in situ without an inflammatory infiltrate, so there was no confusion about the lesion's true depth. It was unmistakably a melanoma in situ, yet after standard therapy it recurred and metastasized.
Like patients with a history of CLL, organ transplant recipients are also at increased risk of aggressive skin cancers. Theories abound as to why immunosuppression, whether caused by lymphoma or organ transplantation, should have this effect. Among the proposed explanations are decreased immune surveillance, direct carcinogenesis caused by chemotherapeutic agents or antitransplant-rejection drugs, and an increased rate of infections with human papillomavirus.
However, Dr. Brewer thinks the most likely explanation involves a shared underlying genetic predisposition. Patients with CLL are rife with genetic aberrations. For example, 7%-10% of patients with CLL have a deletion mutation at 17 p.
"That's where the p53 gene is, which is the strongest predictor of poor survival in patients with CLL, with a median survival of only 32 months. Maybe these patients also have a higher risk of developing aggressive skin cancer; that’s something we just don't know yet," he noted.
Another genetic aberration worthy of further study involves the proto-oncogene B-cell lymphoma 2 (Bcl-2), which suppresses apoptosis. Bcl-2 expression is elevated in 95% of patients with CLL and in 90% of melanoma patients. Intriguingly, the antisense oligonucleotide oblimersen, which is targeted at Bcl-2, has shown encouraging results in combination with dacarbazine in patients with advanced melanoma (Eur. J. Cancer 2009;45:1807-14).
"Maybe that’s something we should consider using in patients with CLL," Dr. Brewer observed.
He argued that a high degree of suspicion is warranted regarding potential tumor recurrence in melanoma patients with a history of CLL. This may warrant more frequent follow-up, a low threshold for biopsy of suspicious lesions, and perhaps a lower bar for adjuvant chemotherapy.
"Perhaps we should consider doing a sentinel lymph node biopsy more often in thinner melanomas in patients with prior CLL. If they have a higher chance of metastasis, then maybe they have a higher chance of sentinel node involvement with thinner melanomas. There is [a lot] of speculation at this point, a lot more questions than answers," he said.
He declared having no financial conflicts, noting that his research on lymphoma-related skin cancer has been funded mainly by the Dermatology Foundation.
GOTHENBURG, Sweden - Patients with a history of chronic lymphocytic leukemia have an elevated risk of developing malignant melanoma of a particularly aggressive nature.
A new analysis of data from the National Cancer Institute's Surveillance, Epidemiology, and End Results registry demonstrates that individuals with a history of chronic lymphocytic leukemia (CLL) are at 2.5-fold increased risk of subsequently developing melanoma. And once they do, these patients have an adjusted 2.7-fold greater 10-year all-cause mortality and a 2.8-fold increased mortality caused by melanoma, compared with melanoma patients without prior CLL, Dr. Jerry D. Brewer reported at the annual congress of the European Academy of Dermatology and Venereology .
"Melanoma and CLL is a dangerously common association with bad outcomes," said Dr. Brewer, a dermatologic surgeon at the Mayo Clinic, Rochester, Minn.
The pattern of worse outcomes in melanoma patients with a history of CLL was significant across all categories of Breslow tumor depth and Clark's level, but it was most striking in patients with thicker lesions. For example, melanoma patients with a Breslow depth greater than 4.0 mm had a 2-year overall survival rate of just 36% if they had a history of prior CLL, compared with 71% if they did not. Their 2-year melanoma-specific survival was 50% with prior CLL and 82% without such a history.
These findings have important implications for clinical practice, according to the dermatologist. For example, patients with CLL have to get serious about daily sun protection, and they need to learn how to do regular skin self-examinations.
"We also need to educate our professional colleagues, specifically our hematologists/oncologists, that if their patients with CLL have a lot of moles or a lot of risk factors for melanoma, they should consider referring those patients to a dermatologist sooner, to catch melanomas earlier," he continued.
The population-based study included 212,245 melanoma patients in the SEER database in the years 1990-2006. Among them were 1,246 patients with a prior diagnosis of lymphoma, of which 31% were CLL. Those diagnosed with melanoma after CLL were on average 12 years older than patients diagnosed with melanoma without prior CLL, a difference adjusted for in determining standardized mortality ratios.
The 10-year overall survival in patients diagnosed with melanoma preceded by CLL was 19%, compared with an expected 55% if they had no history of prior CLL. The 10-year melanoma-specific survival rates were 62% with a prior diagnosis of CLL and 84% without.
Other investigators have previously reported higher rates of metastasis and worse survival in patients with squamous cell carcinoma or Merkel cell carcinoma preceded by CLL. "Now we know that’s true for melanoma, too," Dr. Brewer said.
The impetus for the SEER study was an earlier small study he and his coinvestigators conducted involving 69 Mayo Clinic patients with CLL and melanoma. They found worse outcomes in patients who had CLL prior to melanoma than in those diagnosed with melanoma prior to CLL (Dermatol. Surg. 2010;36:368-76).
One case in that series that particularly impressed Dr. Brewer involved a documented metastasis in a patient with a history of CLL prior to diagnosis of melanoma in situ. This was a melanoma in situ without an inflammatory infiltrate, so there was no confusion about the lesion's true depth. It was unmistakably a melanoma in situ, yet after standard therapy it recurred and metastasized.
Like patients with a history of CLL, organ transplant recipients are also at increased risk of aggressive skin cancers. Theories abound as to why immunosuppression, whether caused by lymphoma or organ transplantation, should have this effect. Among the proposed explanations are decreased immune surveillance, direct carcinogenesis caused by chemotherapeutic agents or antitransplant-rejection drugs, and an increased rate of infections with human papillomavirus.
However, Dr. Brewer thinks the most likely explanation involves a shared underlying genetic predisposition. Patients with CLL are rife with genetic aberrations. For example, 7%-10% of patients with CLL have a deletion mutation at 17 p.
"That's where the p53 gene is, which is the strongest predictor of poor survival in patients with CLL, with a median survival of only 32 months. Maybe these patients also have a higher risk of developing aggressive skin cancer; that’s something we just don't know yet," he noted.
Another genetic aberration worthy of further study involves the proto-oncogene B-cell lymphoma 2 (Bcl-2), which suppresses apoptosis. Bcl-2 expression is elevated in 95% of patients with CLL and in 90% of melanoma patients. Intriguingly, the antisense oligonucleotide oblimersen, which is targeted at Bcl-2, has shown encouraging results in combination with dacarbazine in patients with advanced melanoma (Eur. J. Cancer 2009;45:1807-14).
"Maybe that’s something we should consider using in patients with CLL," Dr. Brewer observed.
He argued that a high degree of suspicion is warranted regarding potential tumor recurrence in melanoma patients with a history of CLL. This may warrant more frequent follow-up, a low threshold for biopsy of suspicious lesions, and perhaps a lower bar for adjuvant chemotherapy.
"Perhaps we should consider doing a sentinel lymph node biopsy more often in thinner melanomas in patients with prior CLL. If they have a higher chance of metastasis, then maybe they have a higher chance of sentinel node involvement with thinner melanomas. There is [a lot] of speculation at this point, a lot more questions than answers," he said.
He declared having no financial conflicts, noting that his research on lymphoma-related skin cancer has been funded mainly by the Dermatology Foundation.
FROM THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Major Finding: Individuals with a history of chronic lymphocytic leukemia are at
2.5-fold increased risk of subsequently developing melanoma.
Data Source: A population-based study that included 212,245 melanoma patients in the National Cancer Institute's Surveillance, Epidemiology, and End Results database in the years 1990-2006.
Disclosures: Dr. Brewer declared having no financial conflicts, noting that his research on
lymphoma-related skin cancer has been funded mainly by the Dermatology
Foundation.
EADV: Cutaneous Lupus Linked to Increased Skin Cancer Risk
GOTHENBURG, SWEDEN – Patients with cutaneous lupus erythematosus appear to have an elevated overall risk of cancer, especially nonmelanoma skin cancer, lung cancer, and non-Hodgkin's lymphoma.
That's the preliminary conclusion from a Swedish national cohort study involving 3,788 Swedes with cutaneous LE (CLE), each matched to three controls and followed for an average of 4.1 years, said Dr. Carina M. Grönhagen at the annual congress of the European Academy of Dermatology and Venereology.
The take-home message from this first-ever look at the cancer risk associated with CLE is that patients with this skin disease need to be followed regularly for the emergence of malignancy. And they need to receive a strong antismoking message.
"Many of these cancers are connected to smoking, and patients with CLE are known to be smokers to a higher degree than in a normal population," observed Dr. Grönhagen, a dermatology resident at Danderyd Hospital and doctoral candidate in medical epidemiology at the Karolinska Institute, Stockholm.
She and her coworkers decided to look at cancer rates in patients with CLE because CLE is an autoimmune disease, and epidemiologic studies indicate other autoimmune diseases are associated with increased cancer risk.
The overall number of cases of cancer documented in the CLE group during the study period was 188, compared with an expected 112. This 67% increased incidence rate ratio remained significant after adjustment for comorbid SLE, which dropped the ratio only to 60%.
The greatest increase in cancer risk seen in the CLE cohort was for nonmelanoma skin cancer, with a 4.3-fold relative risk, compared with controls. The other strongest risk increases were the 2.9-fold increase in lung cancer, the 2.7-fold increase in non-Hodgkin’s lymphoma, and the 2.7-fold rise in buccal cancer.
Asked if she thinks the observed increase in cancer in association with CLE is caused by the skin disease itself, or instead perhaps the immunosuppressive therapies employed in its treatment, Dr. Grönhagen replied that the well-established high rate of smoking among CLE patients is probably a significant contributor. But the immunologic derangement inherent in CLE is also likely to play a role, especially with regard to the increase in nonmelanoma skin cancer.
Dr. Grönhagen said her presentation at the congress was an interim analysis. Assigning three controls per CLE patient is insufficient to draw ironclad conclusions. She reported that with her coworkers, she is in the process of comparing cancer rates in the CLE cohort to those in the entire Swedish population in order to generate standardized incidence rates rather than incidence rate ratios.
She declared having no relevant financial relationships.
GOTHENBURG, SWEDEN – Patients with cutaneous lupus erythematosus appear to have an elevated overall risk of cancer, especially nonmelanoma skin cancer, lung cancer, and non-Hodgkin's lymphoma.
That's the preliminary conclusion from a Swedish national cohort study involving 3,788 Swedes with cutaneous LE (CLE), each matched to three controls and followed for an average of 4.1 years, said Dr. Carina M. Grönhagen at the annual congress of the European Academy of Dermatology and Venereology.
The take-home message from this first-ever look at the cancer risk associated with CLE is that patients with this skin disease need to be followed regularly for the emergence of malignancy. And they need to receive a strong antismoking message.
"Many of these cancers are connected to smoking, and patients with CLE are known to be smokers to a higher degree than in a normal population," observed Dr. Grönhagen, a dermatology resident at Danderyd Hospital and doctoral candidate in medical epidemiology at the Karolinska Institute, Stockholm.
She and her coworkers decided to look at cancer rates in patients with CLE because CLE is an autoimmune disease, and epidemiologic studies indicate other autoimmune diseases are associated with increased cancer risk.
The overall number of cases of cancer documented in the CLE group during the study period was 188, compared with an expected 112. This 67% increased incidence rate ratio remained significant after adjustment for comorbid SLE, which dropped the ratio only to 60%.
The greatest increase in cancer risk seen in the CLE cohort was for nonmelanoma skin cancer, with a 4.3-fold relative risk, compared with controls. The other strongest risk increases were the 2.9-fold increase in lung cancer, the 2.7-fold increase in non-Hodgkin’s lymphoma, and the 2.7-fold rise in buccal cancer.
Asked if she thinks the observed increase in cancer in association with CLE is caused by the skin disease itself, or instead perhaps the immunosuppressive therapies employed in its treatment, Dr. Grönhagen replied that the well-established high rate of smoking among CLE patients is probably a significant contributor. But the immunologic derangement inherent in CLE is also likely to play a role, especially with regard to the increase in nonmelanoma skin cancer.
Dr. Grönhagen said her presentation at the congress was an interim analysis. Assigning three controls per CLE patient is insufficient to draw ironclad conclusions. She reported that with her coworkers, she is in the process of comparing cancer rates in the CLE cohort to those in the entire Swedish population in order to generate standardized incidence rates rather than incidence rate ratios.
She declared having no relevant financial relationships.
GOTHENBURG, SWEDEN – Patients with cutaneous lupus erythematosus appear to have an elevated overall risk of cancer, especially nonmelanoma skin cancer, lung cancer, and non-Hodgkin's lymphoma.
That's the preliminary conclusion from a Swedish national cohort study involving 3,788 Swedes with cutaneous LE (CLE), each matched to three controls and followed for an average of 4.1 years, said Dr. Carina M. Grönhagen at the annual congress of the European Academy of Dermatology and Venereology.
The take-home message from this first-ever look at the cancer risk associated with CLE is that patients with this skin disease need to be followed regularly for the emergence of malignancy. And they need to receive a strong antismoking message.
"Many of these cancers are connected to smoking, and patients with CLE are known to be smokers to a higher degree than in a normal population," observed Dr. Grönhagen, a dermatology resident at Danderyd Hospital and doctoral candidate in medical epidemiology at the Karolinska Institute, Stockholm.
She and her coworkers decided to look at cancer rates in patients with CLE because CLE is an autoimmune disease, and epidemiologic studies indicate other autoimmune diseases are associated with increased cancer risk.
The overall number of cases of cancer documented in the CLE group during the study period was 188, compared with an expected 112. This 67% increased incidence rate ratio remained significant after adjustment for comorbid SLE, which dropped the ratio only to 60%.
The greatest increase in cancer risk seen in the CLE cohort was for nonmelanoma skin cancer, with a 4.3-fold relative risk, compared with controls. The other strongest risk increases were the 2.9-fold increase in lung cancer, the 2.7-fold increase in non-Hodgkin’s lymphoma, and the 2.7-fold rise in buccal cancer.
Asked if she thinks the observed increase in cancer in association with CLE is caused by the skin disease itself, or instead perhaps the immunosuppressive therapies employed in its treatment, Dr. Grönhagen replied that the well-established high rate of smoking among CLE patients is probably a significant contributor. But the immunologic derangement inherent in CLE is also likely to play a role, especially with regard to the increase in nonmelanoma skin cancer.
Dr. Grönhagen said her presentation at the congress was an interim analysis. Assigning three controls per CLE patient is insufficient to draw ironclad conclusions. She reported that with her coworkers, she is in the process of comparing cancer rates in the CLE cohort to those in the entire Swedish population in order to generate standardized incidence rates rather than incidence rate ratios.
She declared having no relevant financial relationships.
ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Major Finding: Among patients with cutaneous lupus erythematosus, 188 cases of cancer were documented during
the study perio, compared with an expected 112. This 67%
increased incidence rate ratio remained significant after adjustment for
comorbid systemic LE, which dropped the ratio only to 60%.
Data Source: A Swedish national cohort study involving 3,788 Swedes with cutaneous LE, each matched to three controls and followed for an average of
4.1 years,.
Disclosures: Dr. Grönhagen declared having no relevant financial relationships.
Melanonychia Striata Warrants Biopsy in Most Cases
SANTA BARBARA, Calif. - The only way to definitively rule out melanoma in a case of melanonychia striata is to perform a biopsy of the nail matrix, according to Dr. Richard K. Scher.
"The source of pigmentation is in the nail matrix, so the biopsy specimen must be taken from there," Dr. Scher said at the annual meeting of the California Society of Dermatology and Dermatologic Surgery.
"It's of no value to do a biopsy of the nail bed. There are almost never melanocytes there, but it's amazing to me how many dermatologists are still doing nail bed biopsies," Dr. Scher, professor of dermatology at the University of North Carolina, Chapel Hill.
While some clinicians prefer to use dermoscopy to inspect cases of melanonychia striata, it is not as accurate or as reliable as microscopic examination.
"At most, dermoscopy should be an aide," he commented.
There are probably too many biopsies being performed, but "I really don't know what the alternative is," Dr. Scher noted. "We need reliable clinical criteria to determine melanoma probability, but we don't have it yet. ... We also need reliable histologic criteria to determine melanoma probability, but we don't always have that, either."
Dr. Scher estimated that 90% of melanocytic bands arise from the distal matrix, which contains more melanocytes than the proximal matrix does. Melanonychia melanoma most commonly occurs in the thumb and the big toe, followed by the index finger. "About 20% of nail melanomas are amelanotic," he said. "That's a number that keeps me awake at night."
Two risk factors for developing melanonychia include older age and being African American. "In general, African Americans do not get melanomas to a great extent, but they do have a lot of benign melanonychia," Dr. Scher noted. "However, acral melanoma is more common in African Americans, compared with other populations."
Other causes of melanonychia include trauma to the nail, infection, and certain medications including antibiotics and chemotherapeutics agents.
Wider, darker nail lesions are more likely to be melanoma, compared with thinner, lighter lesions. "That's true, but I've seen very narrow bands that were melanomas, and I’ve seen very wide bands that were not," Dr. Scher said. "Uniformity of pigment is relatively more reliable than some of the other criteria. So if you see lack of uniformity, think of things like atypical nevi, dysplastic nevi, or melanoma."
In addition, if a patient presents with pigmented nails in four or five digits, "it's not likely that patient will have four or five melanomas," he said. "But if you see a patient particularly a dark-skinned individual who has normal ethnic melanonychia in many digits, but one of them is very different, then you have to become suspicious."
Evolution of the lesion is another worrisome sign. "If the band is changing if it’s getting wider or darker or more streaky then you have to think that you’re dealing with a melanoma."
He emphasized the importance of retrieving the nail plate during the nail biopsy procedure and sending it to the pathologist with the nail matrix specimen, "because sometimes it's hard for the pathologist to find the pigment," he explained. "If the pathologist sends you back a report that there’s no pigment here, the implication is that you missed the lesion. But if you have nail plate there, which has pigment in it, you didn’t miss the lesion. The pathologist did."
Some recent articles in the medical literature suggest that if cases of melanonychia striata in childhood are stable and not atypical in appearance, observation may be an option. However, Dr. Scher urged caution with this approach, saying that "every case must be evaluated individually."
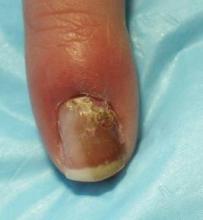
To illustrate his point he discussed the case of a 4-year-old healthy girl who presented to his office with an 8-month history of melanonychia and a 3-month history of possible nail fold pigmentation (see image above). Family history revealed a maternal second cousin with acral melanoma. Dermoscopy was consistent with a nevus diagnosis.
The parents agreed to proceed with a nail biopsy, Dr. Scher said. The pathology report read (in part): "atypical junctional melanocytic proliferation with increased numbers of single melanocytes ...; although this lesion is most probably a nevus, it is recommended that it be completely but conservatively excised."
The girl's parents sought the opinion of seven other pathologists and clinicians at a melanoma conference, and all agreed with complete excision. "In this case, there was not much alternative as to what to do," Dr. Scher said.
The entire nail unit was removed during surgery and the lesion healed with "a terrific cosmetic result," he said.
Dr. Scher said that he had no relevant conflicts to disclose.
SANTA BARBARA, Calif. - The only way to definitively rule out melanoma in a case of melanonychia striata is to perform a biopsy of the nail matrix, according to Dr. Richard K. Scher.
"The source of pigmentation is in the nail matrix, so the biopsy specimen must be taken from there," Dr. Scher said at the annual meeting of the California Society of Dermatology and Dermatologic Surgery.
"It's of no value to do a biopsy of the nail bed. There are almost never melanocytes there, but it's amazing to me how many dermatologists are still doing nail bed biopsies," Dr. Scher, professor of dermatology at the University of North Carolina, Chapel Hill.
While some clinicians prefer to use dermoscopy to inspect cases of melanonychia striata, it is not as accurate or as reliable as microscopic examination.
"At most, dermoscopy should be an aide," he commented.
There are probably too many biopsies being performed, but "I really don't know what the alternative is," Dr. Scher noted. "We need reliable clinical criteria to determine melanoma probability, but we don't have it yet. ... We also need reliable histologic criteria to determine melanoma probability, but we don't always have that, either."
Dr. Scher estimated that 90% of melanocytic bands arise from the distal matrix, which contains more melanocytes than the proximal matrix does. Melanonychia melanoma most commonly occurs in the thumb and the big toe, followed by the index finger. "About 20% of nail melanomas are amelanotic," he said. "That's a number that keeps me awake at night."
Two risk factors for developing melanonychia include older age and being African American. "In general, African Americans do not get melanomas to a great extent, but they do have a lot of benign melanonychia," Dr. Scher noted. "However, acral melanoma is more common in African Americans, compared with other populations."
Other causes of melanonychia include trauma to the nail, infection, and certain medications including antibiotics and chemotherapeutics agents.
Wider, darker nail lesions are more likely to be melanoma, compared with thinner, lighter lesions. "That's true, but I've seen very narrow bands that were melanomas, and I’ve seen very wide bands that were not," Dr. Scher said. "Uniformity of pigment is relatively more reliable than some of the other criteria. So if you see lack of uniformity, think of things like atypical nevi, dysplastic nevi, or melanoma."
In addition, if a patient presents with pigmented nails in four or five digits, "it's not likely that patient will have four or five melanomas," he said. "But if you see a patient particularly a dark-skinned individual who has normal ethnic melanonychia in many digits, but one of them is very different, then you have to become suspicious."
Evolution of the lesion is another worrisome sign. "If the band is changing if it’s getting wider or darker or more streaky then you have to think that you’re dealing with a melanoma."
He emphasized the importance of retrieving the nail plate during the nail biopsy procedure and sending it to the pathologist with the nail matrix specimen, "because sometimes it's hard for the pathologist to find the pigment," he explained. "If the pathologist sends you back a report that there’s no pigment here, the implication is that you missed the lesion. But if you have nail plate there, which has pigment in it, you didn’t miss the lesion. The pathologist did."
Some recent articles in the medical literature suggest that if cases of melanonychia striata in childhood are stable and not atypical in appearance, observation may be an option. However, Dr. Scher urged caution with this approach, saying that "every case must be evaluated individually."
To illustrate his point he discussed the case of a 4-year-old healthy girl who presented to his office with an 8-month history of melanonychia and a 3-month history of possible nail fold pigmentation (see image above). Family history revealed a maternal second cousin with acral melanoma. Dermoscopy was consistent with a nevus diagnosis.
The parents agreed to proceed with a nail biopsy, Dr. Scher said. The pathology report read (in part): "atypical junctional melanocytic proliferation with increased numbers of single melanocytes ...; although this lesion is most probably a nevus, it is recommended that it be completely but conservatively excised."
The girl's parents sought the opinion of seven other pathologists and clinicians at a melanoma conference, and all agreed with complete excision. "In this case, there was not much alternative as to what to do," Dr. Scher said.
The entire nail unit was removed during surgery and the lesion healed with "a terrific cosmetic result," he said.
Dr. Scher said that he had no relevant conflicts to disclose.
SANTA BARBARA, Calif. - The only way to definitively rule out melanoma in a case of melanonychia striata is to perform a biopsy of the nail matrix, according to Dr. Richard K. Scher.
"The source of pigmentation is in the nail matrix, so the biopsy specimen must be taken from there," Dr. Scher said at the annual meeting of the California Society of Dermatology and Dermatologic Surgery.
"It's of no value to do a biopsy of the nail bed. There are almost never melanocytes there, but it's amazing to me how many dermatologists are still doing nail bed biopsies," Dr. Scher, professor of dermatology at the University of North Carolina, Chapel Hill.
While some clinicians prefer to use dermoscopy to inspect cases of melanonychia striata, it is not as accurate or as reliable as microscopic examination.
"At most, dermoscopy should be an aide," he commented.
There are probably too many biopsies being performed, but "I really don't know what the alternative is," Dr. Scher noted. "We need reliable clinical criteria to determine melanoma probability, but we don't have it yet. ... We also need reliable histologic criteria to determine melanoma probability, but we don't always have that, either."
Dr. Scher estimated that 90% of melanocytic bands arise from the distal matrix, which contains more melanocytes than the proximal matrix does. Melanonychia melanoma most commonly occurs in the thumb and the big toe, followed by the index finger. "About 20% of nail melanomas are amelanotic," he said. "That's a number that keeps me awake at night."
Two risk factors for developing melanonychia include older age and being African American. "In general, African Americans do not get melanomas to a great extent, but they do have a lot of benign melanonychia," Dr. Scher noted. "However, acral melanoma is more common in African Americans, compared with other populations."
Other causes of melanonychia include trauma to the nail, infection, and certain medications including antibiotics and chemotherapeutics agents.
Wider, darker nail lesions are more likely to be melanoma, compared with thinner, lighter lesions. "That's true, but I've seen very narrow bands that were melanomas, and I’ve seen very wide bands that were not," Dr. Scher said. "Uniformity of pigment is relatively more reliable than some of the other criteria. So if you see lack of uniformity, think of things like atypical nevi, dysplastic nevi, or melanoma."
In addition, if a patient presents with pigmented nails in four or five digits, "it's not likely that patient will have four or five melanomas," he said. "But if you see a patient particularly a dark-skinned individual who has normal ethnic melanonychia in many digits, but one of them is very different, then you have to become suspicious."
Evolution of the lesion is another worrisome sign. "If the band is changing if it’s getting wider or darker or more streaky then you have to think that you’re dealing with a melanoma."
He emphasized the importance of retrieving the nail plate during the nail biopsy procedure and sending it to the pathologist with the nail matrix specimen, "because sometimes it's hard for the pathologist to find the pigment," he explained. "If the pathologist sends you back a report that there’s no pigment here, the implication is that you missed the lesion. But if you have nail plate there, which has pigment in it, you didn’t miss the lesion. The pathologist did."
Some recent articles in the medical literature suggest that if cases of melanonychia striata in childhood are stable and not atypical in appearance, observation may be an option. However, Dr. Scher urged caution with this approach, saying that "every case must be evaluated individually."
To illustrate his point he discussed the case of a 4-year-old healthy girl who presented to his office with an 8-month history of melanonychia and a 3-month history of possible nail fold pigmentation (see image above). Family history revealed a maternal second cousin with acral melanoma. Dermoscopy was consistent with a nevus diagnosis.
The parents agreed to proceed with a nail biopsy, Dr. Scher said. The pathology report read (in part): "atypical junctional melanocytic proliferation with increased numbers of single melanocytes ...; although this lesion is most probably a nevus, it is recommended that it be completely but conservatively excised."
The girl's parents sought the opinion of seven other pathologists and clinicians at a melanoma conference, and all agreed with complete excision. "In this case, there was not much alternative as to what to do," Dr. Scher said.
The entire nail unit was removed during surgery and the lesion healed with "a terrific cosmetic result," he said.
Dr. Scher said that he had no relevant conflicts to disclose.
annual meeting of the California Society of Dermatology and Dermatologic Surgery
EADV: Topical Smoothened Inhibitor Promising for Multiple BCCs
GOTHENBURG, Sweden - A novel, topically applied inhibitor of the hedgehog signaling pathway showed considerable promise for the treatment of nevoid basal cell carcinoma syndrome in a pilot study.
The 0.75% cream, LDE225, is a potent and selective inhibitor of the protein "smoothened," a key player in the hedgehog signaling pathway, according to Dr. Hans Skvara.
The hedgehog pathway is important in embryonic development and tissue homeostasis. Its uncontrolled overactivity is responsible for the formation of the multiple aggressive basal cell carcinomas (BCCs) characteristic of nevoid basal cell carcinoma syndrome, an autosomal dominant disorder also known as Gorlin-Goltz syndrome, Dr. Skvara said at the annual congress of the European Academy of Dermatology and Venereology.
Twice-daily application of LDE225 for 4 weeks resulted in complete clinical response in 3 of 13 treated BCCs in eight patients and partial clinical response in another 9 tumors. Only one treated BCC showed no clinical response, according to Dr. Skvara of the Medical University of Vienna.
Of the 14 vehicle-treated BCCs in the same eight participants in this randomized, double-blind, 4-week, proof-of-concept study, only one showed a partial clinical response.
Mean tumor volume decreased by 50% in the smoothened inhibitor–treated BCCs, with no significant change over time in the vehicle-treated lesions.
Tumors treated with LDE225 showed downregulation of key genes in the hedgehog pathway, including PTCH 1 and 2 and Gli 1 and 2, in six of eight patients.
Histopathology, however, revealed nests of detectable BCC cells in all treated lesions after 4 weeks of therapy. "Four weeks was probably too short a treatment," Dr. Skvara noted.
The topical smoothened inhibitor was well tolerated, without the marked irritation characteristic of current topical therapies for BCC, such as imiquimod and 5-fluorouracil, he said. If further studies show the smoothened antagonist is able to microscopically eradicate BCC tumor nests, it could become a valuable treatment modality.
"These are some very exciting results. I’m impressed," commented session chair Dr. Jean-Paul Claudel of the University of Tours (France). He asked Dr. Skvara about next steps.
Dr. Skvara replied that a study is underway comparing 6 versus 9 weeks of treatment with two different strengths of the LDE225 cream: the 0.75% formulation used in the pilot study and a slightly lower concentration.
Dr. Skvara declared that he received a research grant from Novartis, which is developing LDE225, to conduct the pilot study.
GOTHENBURG, Sweden - A novel, topically applied inhibitor of the hedgehog signaling pathway showed considerable promise for the treatment of nevoid basal cell carcinoma syndrome in a pilot study.
The 0.75% cream, LDE225, is a potent and selective inhibitor of the protein "smoothened," a key player in the hedgehog signaling pathway, according to Dr. Hans Skvara.
The hedgehog pathway is important in embryonic development and tissue homeostasis. Its uncontrolled overactivity is responsible for the formation of the multiple aggressive basal cell carcinomas (BCCs) characteristic of nevoid basal cell carcinoma syndrome, an autosomal dominant disorder also known as Gorlin-Goltz syndrome, Dr. Skvara said at the annual congress of the European Academy of Dermatology and Venereology.
Twice-daily application of LDE225 for 4 weeks resulted in complete clinical response in 3 of 13 treated BCCs in eight patients and partial clinical response in another 9 tumors. Only one treated BCC showed no clinical response, according to Dr. Skvara of the Medical University of Vienna.
Of the 14 vehicle-treated BCCs in the same eight participants in this randomized, double-blind, 4-week, proof-of-concept study, only one showed a partial clinical response.
Mean tumor volume decreased by 50% in the smoothened inhibitor–treated BCCs, with no significant change over time in the vehicle-treated lesions.
Tumors treated with LDE225 showed downregulation of key genes in the hedgehog pathway, including PTCH 1 and 2 and Gli 1 and 2, in six of eight patients.
Histopathology, however, revealed nests of detectable BCC cells in all treated lesions after 4 weeks of therapy. "Four weeks was probably too short a treatment," Dr. Skvara noted.
The topical smoothened inhibitor was well tolerated, without the marked irritation characteristic of current topical therapies for BCC, such as imiquimod and 5-fluorouracil, he said. If further studies show the smoothened antagonist is able to microscopically eradicate BCC tumor nests, it could become a valuable treatment modality.
"These are some very exciting results. I’m impressed," commented session chair Dr. Jean-Paul Claudel of the University of Tours (France). He asked Dr. Skvara about next steps.
Dr. Skvara replied that a study is underway comparing 6 versus 9 weeks of treatment with two different strengths of the LDE225 cream: the 0.75% formulation used in the pilot study and a slightly lower concentration.
Dr. Skvara declared that he received a research grant from Novartis, which is developing LDE225, to conduct the pilot study.
GOTHENBURG, Sweden - A novel, topically applied inhibitor of the hedgehog signaling pathway showed considerable promise for the treatment of nevoid basal cell carcinoma syndrome in a pilot study.
The 0.75% cream, LDE225, is a potent and selective inhibitor of the protein "smoothened," a key player in the hedgehog signaling pathway, according to Dr. Hans Skvara.
The hedgehog pathway is important in embryonic development and tissue homeostasis. Its uncontrolled overactivity is responsible for the formation of the multiple aggressive basal cell carcinomas (BCCs) characteristic of nevoid basal cell carcinoma syndrome, an autosomal dominant disorder also known as Gorlin-Goltz syndrome, Dr. Skvara said at the annual congress of the European Academy of Dermatology and Venereology.
Twice-daily application of LDE225 for 4 weeks resulted in complete clinical response in 3 of 13 treated BCCs in eight patients and partial clinical response in another 9 tumors. Only one treated BCC showed no clinical response, according to Dr. Skvara of the Medical University of Vienna.
Of the 14 vehicle-treated BCCs in the same eight participants in this randomized, double-blind, 4-week, proof-of-concept study, only one showed a partial clinical response.
Mean tumor volume decreased by 50% in the smoothened inhibitor–treated BCCs, with no significant change over time in the vehicle-treated lesions.
Tumors treated with LDE225 showed downregulation of key genes in the hedgehog pathway, including PTCH 1 and 2 and Gli 1 and 2, in six of eight patients.
Histopathology, however, revealed nests of detectable BCC cells in all treated lesions after 4 weeks of therapy. "Four weeks was probably too short a treatment," Dr. Skvara noted.
The topical smoothened inhibitor was well tolerated, without the marked irritation characteristic of current topical therapies for BCC, such as imiquimod and 5-fluorouracil, he said. If further studies show the smoothened antagonist is able to microscopically eradicate BCC tumor nests, it could become a valuable treatment modality.
"These are some very exciting results. I’m impressed," commented session chair Dr. Jean-Paul Claudel of the University of Tours (France). He asked Dr. Skvara about next steps.
Dr. Skvara replied that a study is underway comparing 6 versus 9 weeks of treatment with two different strengths of the LDE225 cream: the 0.75% formulation used in the pilot study and a slightly lower concentration.
Dr. Skvara declared that he received a research grant from Novartis, which is developing LDE225, to conduct the pilot study.
NCCN's Global Reach: Footprint Extends From U.S. Payers to Foreign Practices
When a handful of oncologists from competing institutions gathered their expertise and egos together in a joint effort to define appropriate care of patients with cancer, it wasn’t entirely clear the endeavor would work. Shared concerns about HMOs had brought them together in the mid 1990s, but they also vied for the same grants, philanthropic gifts, and patients.
Under the deft leadership of the late Dr. Roger J. Winn, the National Comprehensive Cancer Network (NCCN) has parlayed that first guideline, published in 1995, into the most widely used clinical practice guidelines in oncology. By the NCCN’s own account, the guidelines cover 97% of all patients with cancer. Last year alone, nearly 3.3 million PDF copies were downloaded, with select editions now available in 10 languages including Mandarin and Turkish.
The NCCN, which has seen its original budget increase from $2 million to a little more than $30 million, also successfully flexed its political muscle in recent months over the issue of risk evaluation and mitigation strategies (or REMs), and expanded its global reach through conferences in Beijing, Brazil, and Abu Dhabi.
Membership in the not-for-profit alliance was capped 2 years ago at 21 cancer centers in the United States. These member institutions fund the guidelines through dues and millions of dollars worth of free time, said Dr. David S. Ettinger, the Alex Grass professor of oncology at Johns Hopkins University, Baltimore, and chair of the NCCN’s non–small cell lung cancer and occult primary clinical practice panels.
“Fifteen years later, although there may still be some differences, the guidelines are as specific as you can get,” he said.
They also hold tremendous sway with public and private insurers, raising concerns over how they are being used and whether strict adherence can lead to “cookbook” medicine.
Insurers Base Coverage on Guidelines
The NCCN tracks and monitors guideline compliance for breast, colorectal, non-Hodgkin’s lymphoma, non–small cell lung cancer and, most recently, ovarian cancer, with compliance at about 90%-92% for recommendations with level 1 evidence and 85% overall, said NCCN chief executive officer William T. McGivney, Ph.D. For the remaining 15%, patient characteristics, patient preference, and physician disagreement with the guidelines will direct care. The transparency of the guidelines allows physicians to review the references, the category of evidence, and level of consensus, and decide on their own to use them or not, he said. Still, Dr. Ettinger acknowledges that treatment “decisions are made not wanting to fight insurance companies.”
The NCCN noticed early on that its guidelines were being used as the basis for setting coverage policy, prompting the creation in 2004 of the first NCCN Drugs and Biologics Compendium, Dr. McGivney said. The compendium is now used by such key stakeholders as the Centers for Medicare and Medicaid Services and UnitedHealthcare Inc., one of the nation’s largest private insurers.
“On the private side, you see payers basically saying “If it’s in the NCCN Compendium, it’s covered,’ which is critically important for clinicians and patients,” he said. “We have tremendous influence and collaborative influence I think, with payers.”
If a physician uses a drug covered by the compendium to treat any of the 20,000 patients UnitedHealthcare (UHC) has on active chemotherapy in any given year, the drug is automatically covered, said Dr. Lee N. Newcomer, senior vice president, oncology, for the Minnesota-based insurer. Cases involving drugs not covered by the compendium are reviewed by a medical director who looks at state laws and regulations and the employer’s specific requirements. If an employer requires that any chemotherapy recommended by a physician is covered, then the drug would be covered by UHC, even if it’s not in the compendium, he said.
Prior to UHC’s adopting the compendium, 15% of treatments given by its oncologists didn’t match NCCN recommendations. “I would argue that this was both waste and exposing patients to toxicity and drugs that wouldn’t help them,” said Dr. Newcomer, adding that noncompliance is now less than 1%.
Oncologists Rated on Quality of Care
Earlier this year, UHC unveiled the Oncology Care Analysis program that uses clinical and claims data from about 2,600 oncologists and 14,000 patients from across the country with breast, colon and lung cancer to gauge quality of care based on adherence to NCCN guidelines. Oncologists receive a report showing their individual results along with aggregate national results.
Dr. Newcomer dismisses concerns that the program could pressure oncologists into making treatment decisions based on their standing with UHC rather than the best choice for patients who don’t fit the guidelines because such patients are excluded from the analysis. Moreover, there are no financial incentives or penalties associated with the program. “UHC has always emphasized that the information is for quality improvement,” he said. “We let physicians decide how to act on the information.”
The first pass at the data shows that compliance with the NCCN guidelines is stronger in the treatment of breast than colon or lung cancer, but also that about one-third of breast cancer patients prescribed tamoxifen or an aromatase inhibitor aren’t renewing their prescriptions. “We can bring that information back to the physician, and they can have a discussion with their patient about why they aren’t taking that medicine,” he said.
Feedback from physicians has also identified coding problems for carcinoid tumors of the colon and disagreement over whether bevacizumab (Avastin) is truly contraindicated in patients with lung cancer and brain metastases. UHC shares these concerns, along with the national aggregate data, with the NCCN.
Dr. Eric P. Winer, director of the Breast Oncology Center at the Dana-Farber Cancer Institute in Boston and chief scientific adviser for Susan G. Komen for the Cure, said that of all the guidelines to use, the NCCN guidelines make the most sense, but that oncologists would hear and listen more closely to the message that their practice is out of line if it were delivered by a state or national accreditation program.
“The practicing physician is less likely to trust that the insurer has a clear understanding of what the optimal practice would be,” he said. “It’s just not a credible source in the way that a committee of one’s peers might be.”
Dr. Newcomer said he’d love to see other groups take on the task but has not seen similar published quality data coming out of medical groups. “We are filling a void that’s there,” he said.
Is the Fast Lane Too Broad?
Dr. Ira Klein, clinical head of oncology condition analysis for Aetna Inc., said it doesn’t rely exclusively on the NCCN for the development of guidelines and payment. Aetna has covered drugs for indications in NCCN’s Compendium with a consensus rating of 2b or greater but looks to a variety of sources including top-tier journals and outside opinions when creating guidelines and their clinical practice policy bulletins.
“Relying solely on the NCCN would be like using the Cliff Notes,” he said.
Dr. Klein went on to say that the strength of the NCCN guidelines is that they are evidence based and very good at bringing experts together for consensus-based care when the evidence isn’t particularly strong. Their weakness is that they “could be likened to a 50-lane highway” in cases where multiple treatment options would be appropriate for one disease at one stage, Dr. Klein said.
Dr. Newcomer echoed this sentiment, faulting the NCCN for not assigning a hierarchy to its decisions. He cited the array of recommended chemotherapy regimens for breast cancer, and said, “Where I’d like to see the NCCN guidelines go is to say that of these 14, these 2 are the best.”
Still, both insurers are looking to the NCCN regarding coverage of bevacizumab, following the FDA’s Oncologic Drugs Advisory Committee July vote recommending against its use for the first-line treatment of metastatic breast cancer. Dr. Newcomer said UHC will continue to cover the drug for this indication until the NCCN makes its recommendation following the FDA’s final vote. Dr. Klein said if the FDA removes breast cancer as an indication for bevacizumab, Aetna would consider dropping its existing coverage for the drug and would take the NCCN’s position into consideration in its decision.
The speed at which the NCCN can make such a revision to its guidelines is part of what makes them so unusual. “They are assiduously up to date,” Dr. McGivney said. Staff members track the literature, news of large clinical trials, and FDA filings in order to stay abreast of changes in the evidence, with some 2010 guidelines already in their third or fourth edition.
“We really are in the information age and, of all the areas of medicine, cancer care is probably the most rapidly advancing with respect to the science and research,” he said.
This point was driven home when the American Society of Clinical Oncology (ASCO) published guidelines for stage IV non–small cell lung cancer earlier this year without encompassing emerging data on the importance of epidermal growth factor receptor mutations in treatment response.
“The advantage of systematic review-based guidelines like ASCO uses is that they are more evidence-based, strictly speaking, but take longer to conduct and require methodological expertise,” said Dr. Ethan M. Basch, chair of ASCO’s clinical practice guidelines committee. “The advantage of narrative review with consensus-based guidelines such as used by NCCN is that they can be conducted more rapidly, but are less evidence-based in terms of their degree of systematic approach and hence may be more subject to the biases of panel members.”
When a handful of oncologists from competing institutions gathered their expertise and egos together in a joint effort to define appropriate care of patients with cancer, it wasn’t entirely clear the endeavor would work. Shared concerns about HMOs had brought them together in the mid 1990s, but they also vied for the same grants, philanthropic gifts, and patients.
Under the deft leadership of the late Dr. Roger J. Winn, the National Comprehensive Cancer Network (NCCN) has parlayed that first guideline, published in 1995, into the most widely used clinical practice guidelines in oncology. By the NCCN’s own account, the guidelines cover 97% of all patients with cancer. Last year alone, nearly 3.3 million PDF copies were downloaded, with select editions now available in 10 languages including Mandarin and Turkish.
The NCCN, which has seen its original budget increase from $2 million to a little more than $30 million, also successfully flexed its political muscle in recent months over the issue of risk evaluation and mitigation strategies (or REMs), and expanded its global reach through conferences in Beijing, Brazil, and Abu Dhabi.
Membership in the not-for-profit alliance was capped 2 years ago at 21 cancer centers in the United States. These member institutions fund the guidelines through dues and millions of dollars worth of free time, said Dr. David S. Ettinger, the Alex Grass professor of oncology at Johns Hopkins University, Baltimore, and chair of the NCCN’s non–small cell lung cancer and occult primary clinical practice panels.
“Fifteen years later, although there may still be some differences, the guidelines are as specific as you can get,” he said.
They also hold tremendous sway with public and private insurers, raising concerns over how they are being used and whether strict adherence can lead to “cookbook” medicine.
Insurers Base Coverage on Guidelines
The NCCN tracks and monitors guideline compliance for breast, colorectal, non-Hodgkin’s lymphoma, non–small cell lung cancer and, most recently, ovarian cancer, with compliance at about 90%-92% for recommendations with level 1 evidence and 85% overall, said NCCN chief executive officer William T. McGivney, Ph.D. For the remaining 15%, patient characteristics, patient preference, and physician disagreement with the guidelines will direct care. The transparency of the guidelines allows physicians to review the references, the category of evidence, and level of consensus, and decide on their own to use them or not, he said. Still, Dr. Ettinger acknowledges that treatment “decisions are made not wanting to fight insurance companies.”
The NCCN noticed early on that its guidelines were being used as the basis for setting coverage policy, prompting the creation in 2004 of the first NCCN Drugs and Biologics Compendium, Dr. McGivney said. The compendium is now used by such key stakeholders as the Centers for Medicare and Medicaid Services and UnitedHealthcare Inc., one of the nation’s largest private insurers.
“On the private side, you see payers basically saying “If it’s in the NCCN Compendium, it’s covered,’ which is critically important for clinicians and patients,” he said. “We have tremendous influence and collaborative influence I think, with payers.”
If a physician uses a drug covered by the compendium to treat any of the 20,000 patients UnitedHealthcare (UHC) has on active chemotherapy in any given year, the drug is automatically covered, said Dr. Lee N. Newcomer, senior vice president, oncology, for the Minnesota-based insurer. Cases involving drugs not covered by the compendium are reviewed by a medical director who looks at state laws and regulations and the employer’s specific requirements. If an employer requires that any chemotherapy recommended by a physician is covered, then the drug would be covered by UHC, even if it’s not in the compendium, he said.
Prior to UHC’s adopting the compendium, 15% of treatments given by its oncologists didn’t match NCCN recommendations. “I would argue that this was both waste and exposing patients to toxicity and drugs that wouldn’t help them,” said Dr. Newcomer, adding that noncompliance is now less than 1%.
Oncologists Rated on Quality of Care
Earlier this year, UHC unveiled the Oncology Care Analysis program that uses clinical and claims data from about 2,600 oncologists and 14,000 patients from across the country with breast, colon and lung cancer to gauge quality of care based on adherence to NCCN guidelines. Oncologists receive a report showing their individual results along with aggregate national results.
Dr. Newcomer dismisses concerns that the program could pressure oncologists into making treatment decisions based on their standing with UHC rather than the best choice for patients who don’t fit the guidelines because such patients are excluded from the analysis. Moreover, there are no financial incentives or penalties associated with the program. “UHC has always emphasized that the information is for quality improvement,” he said. “We let physicians decide how to act on the information.”
The first pass at the data shows that compliance with the NCCN guidelines is stronger in the treatment of breast than colon or lung cancer, but also that about one-third of breast cancer patients prescribed tamoxifen or an aromatase inhibitor aren’t renewing their prescriptions. “We can bring that information back to the physician, and they can have a discussion with their patient about why they aren’t taking that medicine,” he said.
Feedback from physicians has also identified coding problems for carcinoid tumors of the colon and disagreement over whether bevacizumab (Avastin) is truly contraindicated in patients with lung cancer and brain metastases. UHC shares these concerns, along with the national aggregate data, with the NCCN.
Dr. Eric P. Winer, director of the Breast Oncology Center at the Dana-Farber Cancer Institute in Boston and chief scientific adviser for Susan G. Komen for the Cure, said that of all the guidelines to use, the NCCN guidelines make the most sense, but that oncologists would hear and listen more closely to the message that their practice is out of line if it were delivered by a state or national accreditation program.
“The practicing physician is less likely to trust that the insurer has a clear understanding of what the optimal practice would be,” he said. “It’s just not a credible source in the way that a committee of one’s peers might be.”
Dr. Newcomer said he’d love to see other groups take on the task but has not seen similar published quality data coming out of medical groups. “We are filling a void that’s there,” he said.
Is the Fast Lane Too Broad?
Dr. Ira Klein, clinical head of oncology condition analysis for Aetna Inc., said it doesn’t rely exclusively on the NCCN for the development of guidelines and payment. Aetna has covered drugs for indications in NCCN’s Compendium with a consensus rating of 2b or greater but looks to a variety of sources including top-tier journals and outside opinions when creating guidelines and their clinical practice policy bulletins.
“Relying solely on the NCCN would be like using the Cliff Notes,” he said.
Dr. Klein went on to say that the strength of the NCCN guidelines is that they are evidence based and very good at bringing experts together for consensus-based care when the evidence isn’t particularly strong. Their weakness is that they “could be likened to a 50-lane highway” in cases where multiple treatment options would be appropriate for one disease at one stage, Dr. Klein said.
Dr. Newcomer echoed this sentiment, faulting the NCCN for not assigning a hierarchy to its decisions. He cited the array of recommended chemotherapy regimens for breast cancer, and said, “Where I’d like to see the NCCN guidelines go is to say that of these 14, these 2 are the best.”
Still, both insurers are looking to the NCCN regarding coverage of bevacizumab, following the FDA’s Oncologic Drugs Advisory Committee July vote recommending against its use for the first-line treatment of metastatic breast cancer. Dr. Newcomer said UHC will continue to cover the drug for this indication until the NCCN makes its recommendation following the FDA’s final vote. Dr. Klein said if the FDA removes breast cancer as an indication for bevacizumab, Aetna would consider dropping its existing coverage for the drug and would take the NCCN’s position into consideration in its decision.
The speed at which the NCCN can make such a revision to its guidelines is part of what makes them so unusual. “They are assiduously up to date,” Dr. McGivney said. Staff members track the literature, news of large clinical trials, and FDA filings in order to stay abreast of changes in the evidence, with some 2010 guidelines already in their third or fourth edition.
“We really are in the information age and, of all the areas of medicine, cancer care is probably the most rapidly advancing with respect to the science and research,” he said.
This point was driven home when the American Society of Clinical Oncology (ASCO) published guidelines for stage IV non–small cell lung cancer earlier this year without encompassing emerging data on the importance of epidermal growth factor receptor mutations in treatment response.
“The advantage of systematic review-based guidelines like ASCO uses is that they are more evidence-based, strictly speaking, but take longer to conduct and require methodological expertise,” said Dr. Ethan M. Basch, chair of ASCO’s clinical practice guidelines committee. “The advantage of narrative review with consensus-based guidelines such as used by NCCN is that they can be conducted more rapidly, but are less evidence-based in terms of their degree of systematic approach and hence may be more subject to the biases of panel members.”
When a handful of oncologists from competing institutions gathered their expertise and egos together in a joint effort to define appropriate care of patients with cancer, it wasn’t entirely clear the endeavor would work. Shared concerns about HMOs had brought them together in the mid 1990s, but they also vied for the same grants, philanthropic gifts, and patients.
Under the deft leadership of the late Dr. Roger J. Winn, the National Comprehensive Cancer Network (NCCN) has parlayed that first guideline, published in 1995, into the most widely used clinical practice guidelines in oncology. By the NCCN’s own account, the guidelines cover 97% of all patients with cancer. Last year alone, nearly 3.3 million PDF copies were downloaded, with select editions now available in 10 languages including Mandarin and Turkish.
The NCCN, which has seen its original budget increase from $2 million to a little more than $30 million, also successfully flexed its political muscle in recent months over the issue of risk evaluation and mitigation strategies (or REMs), and expanded its global reach through conferences in Beijing, Brazil, and Abu Dhabi.
Membership in the not-for-profit alliance was capped 2 years ago at 21 cancer centers in the United States. These member institutions fund the guidelines through dues and millions of dollars worth of free time, said Dr. David S. Ettinger, the Alex Grass professor of oncology at Johns Hopkins University, Baltimore, and chair of the NCCN’s non–small cell lung cancer and occult primary clinical practice panels.
“Fifteen years later, although there may still be some differences, the guidelines are as specific as you can get,” he said.
They also hold tremendous sway with public and private insurers, raising concerns over how they are being used and whether strict adherence can lead to “cookbook” medicine.
Insurers Base Coverage on Guidelines
The NCCN tracks and monitors guideline compliance for breast, colorectal, non-Hodgkin’s lymphoma, non–small cell lung cancer and, most recently, ovarian cancer, with compliance at about 90%-92% for recommendations with level 1 evidence and 85% overall, said NCCN chief executive officer William T. McGivney, Ph.D. For the remaining 15%, patient characteristics, patient preference, and physician disagreement with the guidelines will direct care. The transparency of the guidelines allows physicians to review the references, the category of evidence, and level of consensus, and decide on their own to use them or not, he said. Still, Dr. Ettinger acknowledges that treatment “decisions are made not wanting to fight insurance companies.”
The NCCN noticed early on that its guidelines were being used as the basis for setting coverage policy, prompting the creation in 2004 of the first NCCN Drugs and Biologics Compendium, Dr. McGivney said. The compendium is now used by such key stakeholders as the Centers for Medicare and Medicaid Services and UnitedHealthcare Inc., one of the nation’s largest private insurers.
“On the private side, you see payers basically saying “If it’s in the NCCN Compendium, it’s covered,’ which is critically important for clinicians and patients,” he said. “We have tremendous influence and collaborative influence I think, with payers.”
If a physician uses a drug covered by the compendium to treat any of the 20,000 patients UnitedHealthcare (UHC) has on active chemotherapy in any given year, the drug is automatically covered, said Dr. Lee N. Newcomer, senior vice president, oncology, for the Minnesota-based insurer. Cases involving drugs not covered by the compendium are reviewed by a medical director who looks at state laws and regulations and the employer’s specific requirements. If an employer requires that any chemotherapy recommended by a physician is covered, then the drug would be covered by UHC, even if it’s not in the compendium, he said.
Prior to UHC’s adopting the compendium, 15% of treatments given by its oncologists didn’t match NCCN recommendations. “I would argue that this was both waste and exposing patients to toxicity and drugs that wouldn’t help them,” said Dr. Newcomer, adding that noncompliance is now less than 1%.
Oncologists Rated on Quality of Care
Earlier this year, UHC unveiled the Oncology Care Analysis program that uses clinical and claims data from about 2,600 oncologists and 14,000 patients from across the country with breast, colon and lung cancer to gauge quality of care based on adherence to NCCN guidelines. Oncologists receive a report showing their individual results along with aggregate national results.
Dr. Newcomer dismisses concerns that the program could pressure oncologists into making treatment decisions based on their standing with UHC rather than the best choice for patients who don’t fit the guidelines because such patients are excluded from the analysis. Moreover, there are no financial incentives or penalties associated with the program. “UHC has always emphasized that the information is for quality improvement,” he said. “We let physicians decide how to act on the information.”
The first pass at the data shows that compliance with the NCCN guidelines is stronger in the treatment of breast than colon or lung cancer, but also that about one-third of breast cancer patients prescribed tamoxifen or an aromatase inhibitor aren’t renewing their prescriptions. “We can bring that information back to the physician, and they can have a discussion with their patient about why they aren’t taking that medicine,” he said.
Feedback from physicians has also identified coding problems for carcinoid tumors of the colon and disagreement over whether bevacizumab (Avastin) is truly contraindicated in patients with lung cancer and brain metastases. UHC shares these concerns, along with the national aggregate data, with the NCCN.
Dr. Eric P. Winer, director of the Breast Oncology Center at the Dana-Farber Cancer Institute in Boston and chief scientific adviser for Susan G. Komen for the Cure, said that of all the guidelines to use, the NCCN guidelines make the most sense, but that oncologists would hear and listen more closely to the message that their practice is out of line if it were delivered by a state or national accreditation program.
“The practicing physician is less likely to trust that the insurer has a clear understanding of what the optimal practice would be,” he said. “It’s just not a credible source in the way that a committee of one’s peers might be.”
Dr. Newcomer said he’d love to see other groups take on the task but has not seen similar published quality data coming out of medical groups. “We are filling a void that’s there,” he said.
Is the Fast Lane Too Broad?
Dr. Ira Klein, clinical head of oncology condition analysis for Aetna Inc., said it doesn’t rely exclusively on the NCCN for the development of guidelines and payment. Aetna has covered drugs for indications in NCCN’s Compendium with a consensus rating of 2b or greater but looks to a variety of sources including top-tier journals and outside opinions when creating guidelines and their clinical practice policy bulletins.
“Relying solely on the NCCN would be like using the Cliff Notes,” he said.
Dr. Klein went on to say that the strength of the NCCN guidelines is that they are evidence based and very good at bringing experts together for consensus-based care when the evidence isn’t particularly strong. Their weakness is that they “could be likened to a 50-lane highway” in cases where multiple treatment options would be appropriate for one disease at one stage, Dr. Klein said.
Dr. Newcomer echoed this sentiment, faulting the NCCN for not assigning a hierarchy to its decisions. He cited the array of recommended chemotherapy regimens for breast cancer, and said, “Where I’d like to see the NCCN guidelines go is to say that of these 14, these 2 are the best.”
Still, both insurers are looking to the NCCN regarding coverage of bevacizumab, following the FDA’s Oncologic Drugs Advisory Committee July vote recommending against its use for the first-line treatment of metastatic breast cancer. Dr. Newcomer said UHC will continue to cover the drug for this indication until the NCCN makes its recommendation following the FDA’s final vote. Dr. Klein said if the FDA removes breast cancer as an indication for bevacizumab, Aetna would consider dropping its existing coverage for the drug and would take the NCCN’s position into consideration in its decision.
The speed at which the NCCN can make such a revision to its guidelines is part of what makes them so unusual. “They are assiduously up to date,” Dr. McGivney said. Staff members track the literature, news of large clinical trials, and FDA filings in order to stay abreast of changes in the evidence, with some 2010 guidelines already in their third or fourth edition.
“We really are in the information age and, of all the areas of medicine, cancer care is probably the most rapidly advancing with respect to the science and research,” he said.
This point was driven home when the American Society of Clinical Oncology (ASCO) published guidelines for stage IV non–small cell lung cancer earlier this year without encompassing emerging data on the importance of epidermal growth factor receptor mutations in treatment response.
“The advantage of systematic review-based guidelines like ASCO uses is that they are more evidence-based, strictly speaking, but take longer to conduct and require methodological expertise,” said Dr. Ethan M. Basch, chair of ASCO’s clinical practice guidelines committee. “The advantage of narrative review with consensus-based guidelines such as used by NCCN is that they can be conducted more rapidly, but are less evidence-based in terms of their degree of systematic approach and hence may be more subject to the biases of panel members.”
Elevated Mitotic Rate Is Potential Game Changer in Melanoma
SANTA BARBARA, Calif. – Current American Joint Committee on Cancer melanoma staging criteria incorporate a mitotic rate of 1/mm2 or greater into the T1b classification, recognizing mitotic rate as an independent prognostic factor in patients with primary melanoma.
This change, which went into effect in January,"is going to have a profound impact in whom we consider eligible for staging with sentinel lymph node biopsy" Dr. Susan Swetter predicted at the annual meeting of the California Society of Dermatology and Dermatologic Surgery. "The reason is that only about 6%-8% of these T1 tumors (1.0 mm) are ulcerated, but it is estimated that up to 30%-40% will have an elevated mitotic rate of 1.0 or more per square millimeter. This leaves us with a conundrum: Are all of these patients going to be eligible for sentinel lymph node biopsy?"
Current AJCC melanoma staging criteria are based on data analysis from the 2008 AJCC collaborative melanoma staging database from 14 cancer centers and cooperative oncology organizations worldwide, said Dr. Swetter, professor of dermatology and director of the pigmented lesion and melanoma program at Stanford University Medical Center/VA Palo Alto Health Care System, Calif.
The criteria are based on prognostic factor analysis of nearly 60,000 patients to validate staging criteria and groupings for the 7th edition of the AJCC Cancer Staging Manual (Springer, New York), which was published in the fall of 2009 and became active in January 2010.
"The concept is that each stage grouping has a uniform risk for survival, and there are a wealth of patients per tumor node metastasis (TNM) categories, with more than 27,000 with stage I and II disease, more than 3,400 with stage III disease, and more than 7,600 with stage IV disease," Dr. Swetter said.
The newest revision of the AJCC staging for melanoma involves no major changes for TNM and stage grouping criteria, with the exception of mitotic rate.
Other changes involve more advanced disease. For example, "immunohistochemical detection of nodal metastases is now acceptable, whereas only routine histology was used previously," Dr. Swetter said. "Also, there is no longer a lower limit to designate node-positive disease. The size of the isolated tumor cells is no longer used, although that is quite controversial."
Thickness of tumors is potentially a marker of duration of growth, with increasing tumor thickness correlating adversely with survival. According to the 2008 AJCC Melanoma Database, patients with tumor thickness of 0.01-0.55 mm have a 10-year survival rate of 95%, while those with a tumor thickness of 4.01-6.0 mm have a 10-year survival rate of 54%.
The most relevant correlate of a mitotic rate increase and its effect on prognosis appears to be in thin tumors, Dr. Swetter said. "This is why the new AJCC guidelines incorporate mitotic rate in tumors that are less than or equal to 1.0 mm thick."
Survival data from the 2008 AJCC Melanoma Database suggest there is little to no value in promoting sentinel lymph node biopsy in patients who have tumors up to 0.50 mm in depth, regardless of mitotic rate, because the survival rate in these patients is excellent. Currently, the T1b designation is used for staging in terms of survival. "It is not a criterion in itself to perform sentinel lymph node biopsy," Dr. Swetter emphasized. "There is some evolving data suggesting that mitotic rate as a continuous variable may be predictive of occult regional nodal disease."
One published study suggests that sentinel lymph node biopsy is appropriate for patients with T1b melanomas, including those defined by mitotic rate (J. Natl. Compr. Canc. Netw. 2009;7:308-17). "We are now awaiting publication of a larger analysis of patients with thin melanoma," Dr. Swetter said. "Both the National Comprehensive Cancer Network and the AAD [American Academy of Dermatology] melanoma panels are weighing in to establish an appropriate threshold in these T1b patients."
Dr. Swetter, who serves on both of the panels, noted that there is currently "very little enthusiasm from a surgical perspective to be pursuing sentinel lymph node biopsy on every patient who is T1b by virtue of mitotic rate."
The National Comprehensive Cancer Network recommends that clinicians "discuss and offer" sentinel lymph node biopsy for patients with stage IB and stage II cutaneous melanoma, "recognizing that the sentinel node biopsy is an important staging tool, but its impact on overall survival is unclear," Dr. Swetter said.
The procedure should also be considered for stage IA melanomas with adverse features including positive deep margins, lymphovascular invasion, thickness of 0.75 mm or greater, or younger age.
The decision not to perform sentinel lymph node biopsy can be based on significant patient comorbidities, patient preference, or other factors.
An unresolved question is whether or not sentinel lymph node biopsy is a valid staging technique in older patients. "Older age is associated with worse prognosis, but lower rates of sentinel lymph node positivity," Dr. Swetter said. "The question is, why? One issue is whether there is different biology of melanoma in the elderly, or whether host immune factors come into play. Another [factor] could be delayed lymphatic spread, or it might be that tumors are more likely to disseminate hematogenously in older patients, compared with younger patients."
In the future, Dr. Swetter predicted the emergence of individualized prognoses based on novel weighted mathematical equations using AJCC staging and other factors. A Web-based predictive tool for prognosis developed by the AJCC Melanoma Database is currently available at www.melanomaprognosis.org.
Dr. Swetter stated having no relevant financial conflicts to disclose.
SANTA BARBARA, Calif. – Current American Joint Committee on Cancer melanoma staging criteria incorporate a mitotic rate of 1/mm2 or greater into the T1b classification, recognizing mitotic rate as an independent prognostic factor in patients with primary melanoma.
This change, which went into effect in January,"is going to have a profound impact in whom we consider eligible for staging with sentinel lymph node biopsy" Dr. Susan Swetter predicted at the annual meeting of the California Society of Dermatology and Dermatologic Surgery. "The reason is that only about 6%-8% of these T1 tumors (1.0 mm) are ulcerated, but it is estimated that up to 30%-40% will have an elevated mitotic rate of 1.0 or more per square millimeter. This leaves us with a conundrum: Are all of these patients going to be eligible for sentinel lymph node biopsy?"
Current AJCC melanoma staging criteria are based on data analysis from the 2008 AJCC collaborative melanoma staging database from 14 cancer centers and cooperative oncology organizations worldwide, said Dr. Swetter, professor of dermatology and director of the pigmented lesion and melanoma program at Stanford University Medical Center/VA Palo Alto Health Care System, Calif.
The criteria are based on prognostic factor analysis of nearly 60,000 patients to validate staging criteria and groupings for the 7th edition of the AJCC Cancer Staging Manual (Springer, New York), which was published in the fall of 2009 and became active in January 2010.
"The concept is that each stage grouping has a uniform risk for survival, and there are a wealth of patients per tumor node metastasis (TNM) categories, with more than 27,000 with stage I and II disease, more than 3,400 with stage III disease, and more than 7,600 with stage IV disease," Dr. Swetter said.
The newest revision of the AJCC staging for melanoma involves no major changes for TNM and stage grouping criteria, with the exception of mitotic rate.
Other changes involve more advanced disease. For example, "immunohistochemical detection of nodal metastases is now acceptable, whereas only routine histology was used previously," Dr. Swetter said. "Also, there is no longer a lower limit to designate node-positive disease. The size of the isolated tumor cells is no longer used, although that is quite controversial."
Thickness of tumors is potentially a marker of duration of growth, with increasing tumor thickness correlating adversely with survival. According to the 2008 AJCC Melanoma Database, patients with tumor thickness of 0.01-0.55 mm have a 10-year survival rate of 95%, while those with a tumor thickness of 4.01-6.0 mm have a 10-year survival rate of 54%.
The most relevant correlate of a mitotic rate increase and its effect on prognosis appears to be in thin tumors, Dr. Swetter said. "This is why the new AJCC guidelines incorporate mitotic rate in tumors that are less than or equal to 1.0 mm thick."
Survival data from the 2008 AJCC Melanoma Database suggest there is little to no value in promoting sentinel lymph node biopsy in patients who have tumors up to 0.50 mm in depth, regardless of mitotic rate, because the survival rate in these patients is excellent. Currently, the T1b designation is used for staging in terms of survival. "It is not a criterion in itself to perform sentinel lymph node biopsy," Dr. Swetter emphasized. "There is some evolving data suggesting that mitotic rate as a continuous variable may be predictive of occult regional nodal disease."
One published study suggests that sentinel lymph node biopsy is appropriate for patients with T1b melanomas, including those defined by mitotic rate (J. Natl. Compr. Canc. Netw. 2009;7:308-17). "We are now awaiting publication of a larger analysis of patients with thin melanoma," Dr. Swetter said. "Both the National Comprehensive Cancer Network and the AAD [American Academy of Dermatology] melanoma panels are weighing in to establish an appropriate threshold in these T1b patients."
Dr. Swetter, who serves on both of the panels, noted that there is currently "very little enthusiasm from a surgical perspective to be pursuing sentinel lymph node biopsy on every patient who is T1b by virtue of mitotic rate."
The National Comprehensive Cancer Network recommends that clinicians "discuss and offer" sentinel lymph node biopsy for patients with stage IB and stage II cutaneous melanoma, "recognizing that the sentinel node biopsy is an important staging tool, but its impact on overall survival is unclear," Dr. Swetter said.
The procedure should also be considered for stage IA melanomas with adverse features including positive deep margins, lymphovascular invasion, thickness of 0.75 mm or greater, or younger age.
The decision not to perform sentinel lymph node biopsy can be based on significant patient comorbidities, patient preference, or other factors.
An unresolved question is whether or not sentinel lymph node biopsy is a valid staging technique in older patients. "Older age is associated with worse prognosis, but lower rates of sentinel lymph node positivity," Dr. Swetter said. "The question is, why? One issue is whether there is different biology of melanoma in the elderly, or whether host immune factors come into play. Another [factor] could be delayed lymphatic spread, or it might be that tumors are more likely to disseminate hematogenously in older patients, compared with younger patients."
In the future, Dr. Swetter predicted the emergence of individualized prognoses based on novel weighted mathematical equations using AJCC staging and other factors. A Web-based predictive tool for prognosis developed by the AJCC Melanoma Database is currently available at www.melanomaprognosis.org.
Dr. Swetter stated having no relevant financial conflicts to disclose.
SANTA BARBARA, Calif. – Current American Joint Committee on Cancer melanoma staging criteria incorporate a mitotic rate of 1/mm2 or greater into the T1b classification, recognizing mitotic rate as an independent prognostic factor in patients with primary melanoma.
This change, which went into effect in January,"is going to have a profound impact in whom we consider eligible for staging with sentinel lymph node biopsy" Dr. Susan Swetter predicted at the annual meeting of the California Society of Dermatology and Dermatologic Surgery. "The reason is that only about 6%-8% of these T1 tumors (1.0 mm) are ulcerated, but it is estimated that up to 30%-40% will have an elevated mitotic rate of 1.0 or more per square millimeter. This leaves us with a conundrum: Are all of these patients going to be eligible for sentinel lymph node biopsy?"
Current AJCC melanoma staging criteria are based on data analysis from the 2008 AJCC collaborative melanoma staging database from 14 cancer centers and cooperative oncology organizations worldwide, said Dr. Swetter, professor of dermatology and director of the pigmented lesion and melanoma program at Stanford University Medical Center/VA Palo Alto Health Care System, Calif.
The criteria are based on prognostic factor analysis of nearly 60,000 patients to validate staging criteria and groupings for the 7th edition of the AJCC Cancer Staging Manual (Springer, New York), which was published in the fall of 2009 and became active in January 2010.
"The concept is that each stage grouping has a uniform risk for survival, and there are a wealth of patients per tumor node metastasis (TNM) categories, with more than 27,000 with stage I and II disease, more than 3,400 with stage III disease, and more than 7,600 with stage IV disease," Dr. Swetter said.
The newest revision of the AJCC staging for melanoma involves no major changes for TNM and stage grouping criteria, with the exception of mitotic rate.
Other changes involve more advanced disease. For example, "immunohistochemical detection of nodal metastases is now acceptable, whereas only routine histology was used previously," Dr. Swetter said. "Also, there is no longer a lower limit to designate node-positive disease. The size of the isolated tumor cells is no longer used, although that is quite controversial."
Thickness of tumors is potentially a marker of duration of growth, with increasing tumor thickness correlating adversely with survival. According to the 2008 AJCC Melanoma Database, patients with tumor thickness of 0.01-0.55 mm have a 10-year survival rate of 95%, while those with a tumor thickness of 4.01-6.0 mm have a 10-year survival rate of 54%.
The most relevant correlate of a mitotic rate increase and its effect on prognosis appears to be in thin tumors, Dr. Swetter said. "This is why the new AJCC guidelines incorporate mitotic rate in tumors that are less than or equal to 1.0 mm thick."
Survival data from the 2008 AJCC Melanoma Database suggest there is little to no value in promoting sentinel lymph node biopsy in patients who have tumors up to 0.50 mm in depth, regardless of mitotic rate, because the survival rate in these patients is excellent. Currently, the T1b designation is used for staging in terms of survival. "It is not a criterion in itself to perform sentinel lymph node biopsy," Dr. Swetter emphasized. "There is some evolving data suggesting that mitotic rate as a continuous variable may be predictive of occult regional nodal disease."
One published study suggests that sentinel lymph node biopsy is appropriate for patients with T1b melanomas, including those defined by mitotic rate (J. Natl. Compr. Canc. Netw. 2009;7:308-17). "We are now awaiting publication of a larger analysis of patients with thin melanoma," Dr. Swetter said. "Both the National Comprehensive Cancer Network and the AAD [American Academy of Dermatology] melanoma panels are weighing in to establish an appropriate threshold in these T1b patients."
Dr. Swetter, who serves on both of the panels, noted that there is currently "very little enthusiasm from a surgical perspective to be pursuing sentinel lymph node biopsy on every patient who is T1b by virtue of mitotic rate."
The National Comprehensive Cancer Network recommends that clinicians "discuss and offer" sentinel lymph node biopsy for patients with stage IB and stage II cutaneous melanoma, "recognizing that the sentinel node biopsy is an important staging tool, but its impact on overall survival is unclear," Dr. Swetter said.
The procedure should also be considered for stage IA melanomas with adverse features including positive deep margins, lymphovascular invasion, thickness of 0.75 mm or greater, or younger age.
The decision not to perform sentinel lymph node biopsy can be based on significant patient comorbidities, patient preference, or other factors.
An unresolved question is whether or not sentinel lymph node biopsy is a valid staging technique in older patients. "Older age is associated with worse prognosis, but lower rates of sentinel lymph node positivity," Dr. Swetter said. "The question is, why? One issue is whether there is different biology of melanoma in the elderly, or whether host immune factors come into play. Another [factor] could be delayed lymphatic spread, or it might be that tumors are more likely to disseminate hematogenously in older patients, compared with younger patients."
In the future, Dr. Swetter predicted the emergence of individualized prognoses based on novel weighted mathematical equations using AJCC staging and other factors. A Web-based predictive tool for prognosis developed by the AJCC Melanoma Database is currently available at www.melanomaprognosis.org.
Dr. Swetter stated having no relevant financial conflicts to disclose.
EXPERT ANALYSIS FROM THE ANNUAL MEETING OF THE CALIFORNIA SOCIETY OF DERMATOLOGY AND DERMATOLOGIC SURGERY
Pediatric Melanoma Research Continues
Melanocytic nevi – spitz, congenital, and acquired – can pose diagnostic challenges for the pediatric dermatologist.
Dr. Jonathan A. Dyer offered a review of the latest literature on these pediatric melanomas at a seminar on women's and pediatric dermatology sponsored by Skin Disease Education Foundation.
Atypical Spitzoid Melanocytic Tumors (ASMT)
Data suggest that a positive sentinel lymph node biopsy (SLNB) in ASMT does not necessarily mean poor prognosis, according to Dr. Dyer, who is an assistant professor of clinical dermatology at the University of Missouri in Columbia.
Positive SLNB is not uncommon in ASMT, occurring in 28%-50% of cases. Nodal lesion morphology appears to be what matters – typical-appearing subcapsular cells versus ugly cells that replace a significant portion of the node (Am. J. Surg. Pathol. 2009;33:1386-95).
In a series of six published studies with more than 100 patients, no patient with ASMT and positive SLNB died of disease, he reported. However, follow-up was limited in many reports (median 10 months). Most malignant melanomas recur within 36 months.
In the Multicenter Selective Lymphadenectomy Trial (MSLT), the 5-year survival rate for patients younger than 19 years with malignant melanoma with a positive SLN was 68%, compared with 59% for adults.
Congenital Melanocytic Nevi (CMN)
A 19-year prospective study at Great Ormond Street Hospital in London provided a plethora of information on CMN (Br. J. Dermatol. 2009;160:143-50). The study included 349 families with CMN and 79 control families. Poor maternal health (threatened miscarriage, maternal hypertension, or severe nausea/vomiting) was associated with an increased incidence of CMN.
The researchers also found a possible positive association between maternal smoking and projected adult size and overall CMN incidence. CMN was more likely in females, while maternal freckling was independently associated with smaller projected adult size. A quarter of those with CMN had a second-degree relative with CMN, noted Dr. Dyer.
Furthermore, it is estimated that 5%-30% of patients with large CMN have asymptomatic neurocutaneous melanosis (NCM). NCM symptoms – increased intracranial pressure or seizures – typically occur in the first 2 years of life, he reported. It is possible for patients to convert from asymptomatic NCM to symptomatic but it is unclear how common it is (J. Clin. Oncol. 2009;27:e136).
Occasionally NCM symptoms are temporary or controllable; however, prognosis of symptomatic NCM is guarded. MRI is sensitive for NCM in the first 4-6 months but small deposits can be missed.
In terms of risk, satellites appear to be a major risk factor for NCM in patients with CMN. If there are no satellites, the risk of NCM is very low, summarized Dr. Dyer. In addition, CMN size appears to matter more than location. Based on several studies, the risk of NCM tends to be about 20%.
It has been suggested that up to two-thirds of pediatric patients with NCM go on to develop leptomeningeal tumors. Some patients may develop symptomatic NCM as a result, reported Dr. Dyer. An estimated 50%-90% of these patients die within 3 years of symptom onset. It is also estimated that 50% develop malignant melanoma of the central nervous system (Clin. Dermatol. 2009;27:529-36) .
Many CMN improve with time, he noted. In the Great Ormond Street study, for example, the majority of untreated lesions lightened during the follow-up period (Br. J. Dermatol. 2009;160:387-92). Surgery and the presence of satellites at birth were independently associated with darkening.
Satisfaction with surgery was high for patients with CMN of less than 20 cm and for those with facial lesions. However, satisfaction was reduced with increasing projected adult size. Girls were more likely to have surgery.
Treatment seemed to increase the darkening of remaining or later-developing nevi, independent of type, timing, or nevus. Tissue expansion appeared to be associated with increased satellite development, noted Dr. Dyer. Projected adult size was associated with satellite number but not treatment type.
Childhood Melanoma
Melanoma accounts for 1%-3% of childhood malignancies. The incidence increased by 2.9% per year from 1973 to 2001 (Clin. Dermatol. 2009;27:529-36). Melanoma is seven times more common in the second decade than in the first, said Dr. Dyer. It is estimated that teens aged 15-19 years account for 73%-79% of cases.
Interestingly, there is a slight male predominance in young children that changes to a slight female predominance in older groups. Adolescent white girls with a history of ultraviolet exposure have the greatest melanoma risk, particularly on the trunk and lower extremities, he pointed out.
Approximately 30% of childhood melanomas arise with giant CMN. Lifetime risk appears to be bimodal, with the first peak in the first decade of life (usually in the first 5 years). It is estimated that 50%-70% arise before puberty. Head and neck melanomas are more common at age 1-4 years, while melanomas on the trunk are more common with increasing age. Early-life melanomas tend to be dermal with poorer outcomes. The second peak occurs in adulthood, reported Dr. Dyer.
Approximately 20% of childhood melanomas occur with other melanocytic lesions. Malignant melanomas from small CMN typically occur after puberty. These are more similar to adult malignant melanomas.
Childhood melanomas are associated with an increased incidence of amelanotic and nodular lesions. Risk factors include the following: intermittent intense sun exposure, tendency to sunburn, tendency to freckle, fair skin, blue/green eyes, blonde/red hair, xeroderma pigmentosum, giant CMN, dysplastic nevus syndrome, atypical nevi, a family history of malignant melanoma, and immunosuppression.
The risk of transformation for CMN is associated with size. Small to medium CMN (less than 20 cm) have a 1%-5% risk of transformation, while large/giant CMN (greater than 20 cm) have at least a 5%-10% risk; however, the risk may be as great as 20%.
Some studies suggest that 30%-75% of pediatric malignant melanoma originated in giant CMN, and one-third are fatal, reported Dr. Dyer. Excision does not completely eliminate the risk. In one study, 8% of patients developed extracutaneous malignant melanoma after CMN excision.
He noted that the risk of childhood melanoma is associated with immunodeficiency. The risk is six times greater if immunodeficiency is genetic in origin; the risk is four times greater if immunodeficiency is acquired.
The indications for sentinel lymph node biopsy in children are the same as in adults, he noted. Children have a higher incidence positive sentinel lymph node. However, this does not predict likelihood of recurrence or prognosis in children.
Surgical excision should use the same margins as in adults whenever possible. Thickness, ulceration, and stage at diagnosis are all prognostic factors.
Disclosures: Dr. Dyer reported having no conflicts of interest. SDEF and this news organization are owned by Elsevier.
Melanocytic nevi – spitz, congenital, and acquired – can pose diagnostic challenges for the pediatric dermatologist.
Dr. Jonathan A. Dyer offered a review of the latest literature on these pediatric melanomas at a seminar on women's and pediatric dermatology sponsored by Skin Disease Education Foundation.
Atypical Spitzoid Melanocytic Tumors (ASMT)
Data suggest that a positive sentinel lymph node biopsy (SLNB) in ASMT does not necessarily mean poor prognosis, according to Dr. Dyer, who is an assistant professor of clinical dermatology at the University of Missouri in Columbia.
Positive SLNB is not uncommon in ASMT, occurring in 28%-50% of cases. Nodal lesion morphology appears to be what matters – typical-appearing subcapsular cells versus ugly cells that replace a significant portion of the node (Am. J. Surg. Pathol. 2009;33:1386-95).
In a series of six published studies with more than 100 patients, no patient with ASMT and positive SLNB died of disease, he reported. However, follow-up was limited in many reports (median 10 months). Most malignant melanomas recur within 36 months.
In the Multicenter Selective Lymphadenectomy Trial (MSLT), the 5-year survival rate for patients younger than 19 years with malignant melanoma with a positive SLN was 68%, compared with 59% for adults.
Congenital Melanocytic Nevi (CMN)
A 19-year prospective study at Great Ormond Street Hospital in London provided a plethora of information on CMN (Br. J. Dermatol. 2009;160:143-50). The study included 349 families with CMN and 79 control families. Poor maternal health (threatened miscarriage, maternal hypertension, or severe nausea/vomiting) was associated with an increased incidence of CMN.
The researchers also found a possible positive association between maternal smoking and projected adult size and overall CMN incidence. CMN was more likely in females, while maternal freckling was independently associated with smaller projected adult size. A quarter of those with CMN had a second-degree relative with CMN, noted Dr. Dyer.
Furthermore, it is estimated that 5%-30% of patients with large CMN have asymptomatic neurocutaneous melanosis (NCM). NCM symptoms – increased intracranial pressure or seizures – typically occur in the first 2 years of life, he reported. It is possible for patients to convert from asymptomatic NCM to symptomatic but it is unclear how common it is (J. Clin. Oncol. 2009;27:e136).
Occasionally NCM symptoms are temporary or controllable; however, prognosis of symptomatic NCM is guarded. MRI is sensitive for NCM in the first 4-6 months but small deposits can be missed.
In terms of risk, satellites appear to be a major risk factor for NCM in patients with CMN. If there are no satellites, the risk of NCM is very low, summarized Dr. Dyer. In addition, CMN size appears to matter more than location. Based on several studies, the risk of NCM tends to be about 20%.
It has been suggested that up to two-thirds of pediatric patients with NCM go on to develop leptomeningeal tumors. Some patients may develop symptomatic NCM as a result, reported Dr. Dyer. An estimated 50%-90% of these patients die within 3 years of symptom onset. It is also estimated that 50% develop malignant melanoma of the central nervous system (Clin. Dermatol. 2009;27:529-36) .
Many CMN improve with time, he noted. In the Great Ormond Street study, for example, the majority of untreated lesions lightened during the follow-up period (Br. J. Dermatol. 2009;160:387-92). Surgery and the presence of satellites at birth were independently associated with darkening.
Satisfaction with surgery was high for patients with CMN of less than 20 cm and for those with facial lesions. However, satisfaction was reduced with increasing projected adult size. Girls were more likely to have surgery.
Treatment seemed to increase the darkening of remaining or later-developing nevi, independent of type, timing, or nevus. Tissue expansion appeared to be associated with increased satellite development, noted Dr. Dyer. Projected adult size was associated with satellite number but not treatment type.
Childhood Melanoma
Melanoma accounts for 1%-3% of childhood malignancies. The incidence increased by 2.9% per year from 1973 to 2001 (Clin. Dermatol. 2009;27:529-36). Melanoma is seven times more common in the second decade than in the first, said Dr. Dyer. It is estimated that teens aged 15-19 years account for 73%-79% of cases.
Interestingly, there is a slight male predominance in young children that changes to a slight female predominance in older groups. Adolescent white girls with a history of ultraviolet exposure have the greatest melanoma risk, particularly on the trunk and lower extremities, he pointed out.
Approximately 30% of childhood melanomas arise with giant CMN. Lifetime risk appears to be bimodal, with the first peak in the first decade of life (usually in the first 5 years). It is estimated that 50%-70% arise before puberty. Head and neck melanomas are more common at age 1-4 years, while melanomas on the trunk are more common with increasing age. Early-life melanomas tend to be dermal with poorer outcomes. The second peak occurs in adulthood, reported Dr. Dyer.
Approximately 20% of childhood melanomas occur with other melanocytic lesions. Malignant melanomas from small CMN typically occur after puberty. These are more similar to adult malignant melanomas.
Childhood melanomas are associated with an increased incidence of amelanotic and nodular lesions. Risk factors include the following: intermittent intense sun exposure, tendency to sunburn, tendency to freckle, fair skin, blue/green eyes, blonde/red hair, xeroderma pigmentosum, giant CMN, dysplastic nevus syndrome, atypical nevi, a family history of malignant melanoma, and immunosuppression.
The risk of transformation for CMN is associated with size. Small to medium CMN (less than 20 cm) have a 1%-5% risk of transformation, while large/giant CMN (greater than 20 cm) have at least a 5%-10% risk; however, the risk may be as great as 20%.
Some studies suggest that 30%-75% of pediatric malignant melanoma originated in giant CMN, and one-third are fatal, reported Dr. Dyer. Excision does not completely eliminate the risk. In one study, 8% of patients developed extracutaneous malignant melanoma after CMN excision.
He noted that the risk of childhood melanoma is associated with immunodeficiency. The risk is six times greater if immunodeficiency is genetic in origin; the risk is four times greater if immunodeficiency is acquired.
The indications for sentinel lymph node biopsy in children are the same as in adults, he noted. Children have a higher incidence positive sentinel lymph node. However, this does not predict likelihood of recurrence or prognosis in children.
Surgical excision should use the same margins as in adults whenever possible. Thickness, ulceration, and stage at diagnosis are all prognostic factors.
Disclosures: Dr. Dyer reported having no conflicts of interest. SDEF and this news organization are owned by Elsevier.
Melanocytic nevi – spitz, congenital, and acquired – can pose diagnostic challenges for the pediatric dermatologist.
Dr. Jonathan A. Dyer offered a review of the latest literature on these pediatric melanomas at a seminar on women's and pediatric dermatology sponsored by Skin Disease Education Foundation.
Atypical Spitzoid Melanocytic Tumors (ASMT)
Data suggest that a positive sentinel lymph node biopsy (SLNB) in ASMT does not necessarily mean poor prognosis, according to Dr. Dyer, who is an assistant professor of clinical dermatology at the University of Missouri in Columbia.
Positive SLNB is not uncommon in ASMT, occurring in 28%-50% of cases. Nodal lesion morphology appears to be what matters – typical-appearing subcapsular cells versus ugly cells that replace a significant portion of the node (Am. J. Surg. Pathol. 2009;33:1386-95).
In a series of six published studies with more than 100 patients, no patient with ASMT and positive SLNB died of disease, he reported. However, follow-up was limited in many reports (median 10 months). Most malignant melanomas recur within 36 months.
In the Multicenter Selective Lymphadenectomy Trial (MSLT), the 5-year survival rate for patients younger than 19 years with malignant melanoma with a positive SLN was 68%, compared with 59% for adults.
Congenital Melanocytic Nevi (CMN)
A 19-year prospective study at Great Ormond Street Hospital in London provided a plethora of information on CMN (Br. J. Dermatol. 2009;160:143-50). The study included 349 families with CMN and 79 control families. Poor maternal health (threatened miscarriage, maternal hypertension, or severe nausea/vomiting) was associated with an increased incidence of CMN.
The researchers also found a possible positive association between maternal smoking and projected adult size and overall CMN incidence. CMN was more likely in females, while maternal freckling was independently associated with smaller projected adult size. A quarter of those with CMN had a second-degree relative with CMN, noted Dr. Dyer.
Furthermore, it is estimated that 5%-30% of patients with large CMN have asymptomatic neurocutaneous melanosis (NCM). NCM symptoms – increased intracranial pressure or seizures – typically occur in the first 2 years of life, he reported. It is possible for patients to convert from asymptomatic NCM to symptomatic but it is unclear how common it is (J. Clin. Oncol. 2009;27:e136).
Occasionally NCM symptoms are temporary or controllable; however, prognosis of symptomatic NCM is guarded. MRI is sensitive for NCM in the first 4-6 months but small deposits can be missed.
In terms of risk, satellites appear to be a major risk factor for NCM in patients with CMN. If there are no satellites, the risk of NCM is very low, summarized Dr. Dyer. In addition, CMN size appears to matter more than location. Based on several studies, the risk of NCM tends to be about 20%.
It has been suggested that up to two-thirds of pediatric patients with NCM go on to develop leptomeningeal tumors. Some patients may develop symptomatic NCM as a result, reported Dr. Dyer. An estimated 50%-90% of these patients die within 3 years of symptom onset. It is also estimated that 50% develop malignant melanoma of the central nervous system (Clin. Dermatol. 2009;27:529-36) .
Many CMN improve with time, he noted. In the Great Ormond Street study, for example, the majority of untreated lesions lightened during the follow-up period (Br. J. Dermatol. 2009;160:387-92). Surgery and the presence of satellites at birth were independently associated with darkening.
Satisfaction with surgery was high for patients with CMN of less than 20 cm and for those with facial lesions. However, satisfaction was reduced with increasing projected adult size. Girls were more likely to have surgery.
Treatment seemed to increase the darkening of remaining or later-developing nevi, independent of type, timing, or nevus. Tissue expansion appeared to be associated with increased satellite development, noted Dr. Dyer. Projected adult size was associated with satellite number but not treatment type.
Childhood Melanoma
Melanoma accounts for 1%-3% of childhood malignancies. The incidence increased by 2.9% per year from 1973 to 2001 (Clin. Dermatol. 2009;27:529-36). Melanoma is seven times more common in the second decade than in the first, said Dr. Dyer. It is estimated that teens aged 15-19 years account for 73%-79% of cases.
Interestingly, there is a slight male predominance in young children that changes to a slight female predominance in older groups. Adolescent white girls with a history of ultraviolet exposure have the greatest melanoma risk, particularly on the trunk and lower extremities, he pointed out.
Approximately 30% of childhood melanomas arise with giant CMN. Lifetime risk appears to be bimodal, with the first peak in the first decade of life (usually in the first 5 years). It is estimated that 50%-70% arise before puberty. Head and neck melanomas are more common at age 1-4 years, while melanomas on the trunk are more common with increasing age. Early-life melanomas tend to be dermal with poorer outcomes. The second peak occurs in adulthood, reported Dr. Dyer.
Approximately 20% of childhood melanomas occur with other melanocytic lesions. Malignant melanomas from small CMN typically occur after puberty. These are more similar to adult malignant melanomas.
Childhood melanomas are associated with an increased incidence of amelanotic and nodular lesions. Risk factors include the following: intermittent intense sun exposure, tendency to sunburn, tendency to freckle, fair skin, blue/green eyes, blonde/red hair, xeroderma pigmentosum, giant CMN, dysplastic nevus syndrome, atypical nevi, a family history of malignant melanoma, and immunosuppression.
The risk of transformation for CMN is associated with size. Small to medium CMN (less than 20 cm) have a 1%-5% risk of transformation, while large/giant CMN (greater than 20 cm) have at least a 5%-10% risk; however, the risk may be as great as 20%.
Some studies suggest that 30%-75% of pediatric malignant melanoma originated in giant CMN, and one-third are fatal, reported Dr. Dyer. Excision does not completely eliminate the risk. In one study, 8% of patients developed extracutaneous malignant melanoma after CMN excision.
He noted that the risk of childhood melanoma is associated with immunodeficiency. The risk is six times greater if immunodeficiency is genetic in origin; the risk is four times greater if immunodeficiency is acquired.
The indications for sentinel lymph node biopsy in children are the same as in adults, he noted. Children have a higher incidence positive sentinel lymph node. However, this does not predict likelihood of recurrence or prognosis in children.
Surgical excision should use the same margins as in adults whenever possible. Thickness, ulceration, and stage at diagnosis are all prognostic factors.
Disclosures: Dr. Dyer reported having no conflicts of interest. SDEF and this news organization are owned by Elsevier.
EXPERT ANALYSIS FROM A SEMINAR ON WOMEN'S AND PEDIATRIC DERMATOLOGY
Dermoscopy Underutilized by Dermatologists
While dermoscopy can enhance the diagnosis of a number of skin conditions, it is especially useful in determining whether neoplasms should undergo biopsy.
"Patients are becoming aware of the technique and more and more are expecting their dermatologists to be skilled in its application," Dr. David L. Swanson said in an interview.
However, fewer than half of academic dermatologists and less than a quarter of practicing dermatologists use dermoscopy in the United States, according to Dr. Swanson, chief of medical dermatology at the Mayo Clinic in Scottsdale, Ariz. In comparison, dermoscopy is taught to primary care physicians in Europe and Australia.
He discussed methods for assessing whether a lesion should be biopsied.
With the two-step pattern recognition method, the dermoscopist must first determine whether a neoplasm is a melanocytic proliferative one, such as a nevus or melanoma. "The reason that the first step is to consider nevi or melanomas is the importance of identifying the latter – especially melanomas that are not obvious with the naked eye," said Dr. Swanson at a seminar on women's and pediatric dermatology sponsored by Skin Disease Education Foundation.
If the lesion is melanocytic proliferative, the dermoscopist then applies the methods of interpretation for nevi or melanoma – such as pattern recognition or an algorithm. "If it isn't, then there are other diagnostic features that are used to lead to a clinical impression," he said.
The dermoscopy three-point rule has a high sensitivity for melanoma, he said. If a pigmented lesion has any two of the three criteria – atypical asymmetry, atypical pigment network, or blue-white structures – there is a likelihood of melanoma, and the lesion should be biopsied.
"The three-point rule is easier to learn and apply but as with all algorithms, there are a lot of exceptions to the rules. So one has to be willing to learn those exceptions and step out of the algorithm if they aren't certain," said Dr. Swanson. "One specific problem with the three-point method is that it doesn't take into account an atypical vasculature. Any dermoscopist using the three-point rule has to keep that in mind as an additional feature."
The seven-point algorithm – developed by Dr. Giuseppe Argenziano and others – is aimed at helping the novice dermoscopist. "It is a fairly reproducible method with very good specificity and sensitivity for making a decision to biopsy a melanocytic lesion. The three-point algorithm actually was derived from it as a method to simplify," said Dr. Swanson. "Most experienced dermoscopists only use algorithms occasionally, because with experience they become comfortable with pattern recognition."
The seven-point algorithm includes the criteria of the three-point rule, plus four minor criteria: streaks, regression pattern, irregular diffuse pigmentation, and irregular dot and globules.
Disclosures: Dr. Swanson reported having none. SDEF and this news organization are owned by Elsevier.
While dermoscopy can enhance the diagnosis of a number of skin conditions, it is especially useful in determining whether neoplasms should undergo biopsy.
"Patients are becoming aware of the technique and more and more are expecting their dermatologists to be skilled in its application," Dr. David L. Swanson said in an interview.
However, fewer than half of academic dermatologists and less than a quarter of practicing dermatologists use dermoscopy in the United States, according to Dr. Swanson, chief of medical dermatology at the Mayo Clinic in Scottsdale, Ariz. In comparison, dermoscopy is taught to primary care physicians in Europe and Australia.
He discussed methods for assessing whether a lesion should be biopsied.
With the two-step pattern recognition method, the dermoscopist must first determine whether a neoplasm is a melanocytic proliferative one, such as a nevus or melanoma. "The reason that the first step is to consider nevi or melanomas is the importance of identifying the latter – especially melanomas that are not obvious with the naked eye," said Dr. Swanson at a seminar on women's and pediatric dermatology sponsored by Skin Disease Education Foundation.
If the lesion is melanocytic proliferative, the dermoscopist then applies the methods of interpretation for nevi or melanoma – such as pattern recognition or an algorithm. "If it isn't, then there are other diagnostic features that are used to lead to a clinical impression," he said.
The dermoscopy three-point rule has a high sensitivity for melanoma, he said. If a pigmented lesion has any two of the three criteria – atypical asymmetry, atypical pigment network, or blue-white structures – there is a likelihood of melanoma, and the lesion should be biopsied.
"The three-point rule is easier to learn and apply but as with all algorithms, there are a lot of exceptions to the rules. So one has to be willing to learn those exceptions and step out of the algorithm if they aren't certain," said Dr. Swanson. "One specific problem with the three-point method is that it doesn't take into account an atypical vasculature. Any dermoscopist using the three-point rule has to keep that in mind as an additional feature."
The seven-point algorithm – developed by Dr. Giuseppe Argenziano and others – is aimed at helping the novice dermoscopist. "It is a fairly reproducible method with very good specificity and sensitivity for making a decision to biopsy a melanocytic lesion. The three-point algorithm actually was derived from it as a method to simplify," said Dr. Swanson. "Most experienced dermoscopists only use algorithms occasionally, because with experience they become comfortable with pattern recognition."
The seven-point algorithm includes the criteria of the three-point rule, plus four minor criteria: streaks, regression pattern, irregular diffuse pigmentation, and irregular dot and globules.
Disclosures: Dr. Swanson reported having none. SDEF and this news organization are owned by Elsevier.
While dermoscopy can enhance the diagnosis of a number of skin conditions, it is especially useful in determining whether neoplasms should undergo biopsy.
"Patients are becoming aware of the technique and more and more are expecting their dermatologists to be skilled in its application," Dr. David L. Swanson said in an interview.
However, fewer than half of academic dermatologists and less than a quarter of practicing dermatologists use dermoscopy in the United States, according to Dr. Swanson, chief of medical dermatology at the Mayo Clinic in Scottsdale, Ariz. In comparison, dermoscopy is taught to primary care physicians in Europe and Australia.
He discussed methods for assessing whether a lesion should be biopsied.
With the two-step pattern recognition method, the dermoscopist must first determine whether a neoplasm is a melanocytic proliferative one, such as a nevus or melanoma. "The reason that the first step is to consider nevi or melanomas is the importance of identifying the latter – especially melanomas that are not obvious with the naked eye," said Dr. Swanson at a seminar on women's and pediatric dermatology sponsored by Skin Disease Education Foundation.
If the lesion is melanocytic proliferative, the dermoscopist then applies the methods of interpretation for nevi or melanoma – such as pattern recognition or an algorithm. "If it isn't, then there are other diagnostic features that are used to lead to a clinical impression," he said.
The dermoscopy three-point rule has a high sensitivity for melanoma, he said. If a pigmented lesion has any two of the three criteria – atypical asymmetry, atypical pigment network, or blue-white structures – there is a likelihood of melanoma, and the lesion should be biopsied.
"The three-point rule is easier to learn and apply but as with all algorithms, there are a lot of exceptions to the rules. So one has to be willing to learn those exceptions and step out of the algorithm if they aren't certain," said Dr. Swanson. "One specific problem with the three-point method is that it doesn't take into account an atypical vasculature. Any dermoscopist using the three-point rule has to keep that in mind as an additional feature."
The seven-point algorithm – developed by Dr. Giuseppe Argenziano and others – is aimed at helping the novice dermoscopist. "It is a fairly reproducible method with very good specificity and sensitivity for making a decision to biopsy a melanocytic lesion. The three-point algorithm actually was derived from it as a method to simplify," said Dr. Swanson. "Most experienced dermoscopists only use algorithms occasionally, because with experience they become comfortable with pattern recognition."
The seven-point algorithm includes the criteria of the three-point rule, plus four minor criteria: streaks, regression pattern, irregular diffuse pigmentation, and irregular dot and globules.
Disclosures: Dr. Swanson reported having none. SDEF and this news organization are owned by Elsevier.
EXPERT ANALYSIS FROM A SEMINAR ON WOMEN'S AND PEDIATRIC DERMATOLOGY