User login
2016 Update on bone health
Prioritize bone health, as osteoporotic fracture is a major source of morbidity and mortality among women. In this article: fracture risk with OC use in perimenopause, data that inform calcium’s role in cardiovascular disease, sarcopenia management, and an emerging treatment.
Most women’s health care providers are aware of recent changes and controversies regarding cervical cancer screening, mammography frequency, and whether a pelvic bimanual exam should be part of our annual well woman evaluation.1 However, I believe one of the most important things we as clinicians can do is be frontline in promoting bone health. Osteoporotic fracture is a major source of morbidity and mortality.2,3 Thus, promoting the maintenance of bone health is a priority in my own practice. It is also one of my many academic interests.
What follows is an update on bone health. In past years, this update has been entitled, “Update on osteoporosis,” but what we are trying to accomplish is fracture reduction. Thus, priorities for bone health consist of recognition of risk, lifestyle and dietary counseling, as well as the use of pharmacologic agents when appropriate. Certain research stands out as informative for your practice:
- a recent study on the risk of fracture with oral contraceptive (OC) use in perimenopause
- 3 just-published studies that inform our understanding of calcium’s role in cardiovascular health
- a review on sarcopenia management
- new data on romosozumab.
Oral contraceptive use in perimenopause
Scholes D, LaCroix AZ, Hubbard RA, et al. Oral contraceptive use and fracture risk around the menopausal transition. Menopause. 2016;23(2):166-174.
The use of OCs in women of older reproductive age has increased ever since the cutoff age of 35 years was eliminated.4 Lower doses have continued to be utilized in these "older" women with excellent control of irregular bleeding due to ovulatory dysfunction (and reduction in psychosocial symptoms as well).5
The effect of OC use on risk of fracture remains unclear, and use during later reproductive life may be increasing. To determine the association between OC use during later reproductive life and risk of fracture across the menopausal transition, Scholes and colleagues conducted a population-based case-controlled study in a Pacific Northwest HMO, Group Health Cooperative.
Details of the study
Scholes and colleagues enrolled 1,204 case women aged 45 to 59 years with incident fractures, and 2,275 control women. Potential cases with fracture codes in automated data were adjudicated by electronic health record review. Potential control women without fracture codes were selected concurrently, sampling based on age. Participants received a structured study interview. Using logistic regression, associations between OC use and fracture risk were calculated as odds ratios (ORs) and 95% confidence intervals (CIs).
Participation was 69% for cases and 64% for controls. The study sample was 82% white; mean age was 54 years. The most common fracture site for cases was the wrist/forearm (32%). Adjusted fracture risk did not differ between cases and controls for OC use:
- in the 10 years before menopause (OR, 0.90; 95% CI, 0.74-1.11)
- after age 38 years (OR, 0.94; 95% CI, 0.78-1.14)
- over the duration, or
- for other OC exposures.
Related article:
2016 Update on female sexual dysfunction
Association between fractures and OC use near menopause
The current study does not show an association between fractures near the menopausal transition and OC use in the decade before menopause or after age 38 years. For women considering OC use at these times, fracture risk does not seem to be either reduced or increased.
These results, looking at fracture, seem to be further supported by trials conducted by Gambacciani and colleagues,6 in which researchers randomly assigned irregularly cycling perimenopausal women (aged 40-49 years) to 20 μg ethinyl estradiol OCs or calcium/placebo. Results showed that this low-dose OC use significantly increased bone density at the femoral neck, spine, and other sites relative to control women after 24 months.
In the current Scholes study, the use of OCs in the decade before menopause or after age 38 did not reduce fracture risk in the years around the time of menopause. It is reassuring that their use was not associated with any increased fracture risk.
These findings provide additional clarity and guidance to women and their clinicians at a time of increasing public health concern about fractures. For women who may choose to use OCs during late premenopause (around age 38-48 years), fracture risk around the menopausal transition will not differ from women not choosing this option.
Calcium and calcium supplements: The data continue to grow
Anderson JJ, Kruszka B, Delaney JA, et al. Calcium intake from diet and supplements and the risk of coronary artery calcification and its progression among older adults: 10-year follow-up of the Multi-Ethnic Study of Atherosclerosis (MESA) [published online ahead of print October 11, 2016]. J Am Heart Assoc. pii: e003815.
Billington EO, Bristow SM, Gamble GD, et al. Acute effects of calcium supplements on blood pressure: randomised, crossover trial in postmenopausal women [published online ahead of print August 20, 2016]. Osteoporos Int. doi:10.1007/s00198-016-3744-y.
Crandall CJ, Aragaki AK, LeBoff MS, et al. Calcium plus vitamin D supplementation and height loss: findings from the Women's Health Initiative Calcium and Vitamin D clinical trial [published online ahead of print August 1, 2016]. Menopause. doi:10.1097 /GME.0000000000000704.
In 2001, a National Institutes of Health (NIH) Consensus Development Panel on osteoporosis concluded that calcium intake is crucial to maintain bone mass and should be maintained at 1,000-1,500 mg/day in older adults. The panel acknowledged that the majority of older adults did not meet the recommended intake from dietary sources alone, and therefore would require calcium supplementation. Calcium supplements are one of the most commonly used dietary supplements, and population-based surveys have shown that they are used by the majority of older men and women in the United States.7
More recently results from large randomized controlled trials (RCTs) of calcium supplements have been reported, leading to concerns about calcium efficacy for fracture risk and safety. Bolland and colleagues8 reported that calcium supplements increased the rate of cardiovascular events in healthy older women and suggested that their role in osteoporosis management be reconsidered. More recently, the US Preventive Services Task Force recommended against calcium supplements for the primary prevention of fractures in noninstitutionalized postmenopausal women.9
The association between calcium intake and CVD events
Anderson and colleagues acknowledged that recent randomized data suggest that calcium supplements may be associated with increased risk of cardiovascular disease (CVD) events. Using a longitudinal cohort study, they assessed the association between calcium intake, from both foods and supplements, and atherosclerosis, as measured by coronary artery calcification (CAC).
Details of the study by Anderson and colleagues
The authors studied 5,448 adults free of clinically diagnosed CVD (52% female; age range, 45-84 years) from the Multi-Ethnic Study of Atherosclerosis. Baseline total calcium intake was assessed from diet (using a food frequency questionnaire) and calcium supplements (by a medication inventory) and categorized into quintiles based on overall population distribution. Baseline CAC was measured by computed tomography (CT) scan, and CAC measurements were repeated in 2,742 participants approximately 10 years later. Women had higher calcium intakes than men.
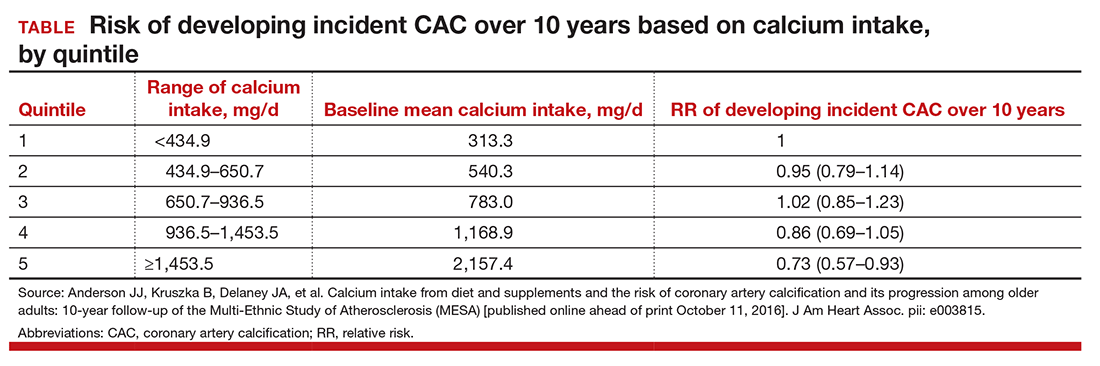
After adjustment for potential confounders, among 1,567 participants without baseline CAC, the relative risk (RR) of developing incident CAC over 10 years, by quintile 1 to 5 of calcium intake is included in the TABLE. After accounting for total calcium intake, calcium supplement use was associated with increased risk for incident CAC (RR, 1.22; 95% CI, 1.07-1.39). No relation was found between baseline calcium intake and 10-year changes in CAC among those participants with baseline CAC less than zero.
They concluded that high total calcium intake was associated with a decreased risk of incident atherosclerosis over long-term follow-up, particularly if achieved without supplement use. However, calcium supplement use may increase the risk for incident CAC.
Related article:
Does the discontinuation of menopausal hormone therapy affect a woman’s cardiovascular risk?
Calcium supplements and blood pressure
Billington and colleagues acknowledged that calcium supplements appear to increase cardiovascular risk but that the mechanism is unknown. They had previously reported that blood pressure declines over the course of the day in older women.10
Details of the study by Billington and colleagues
In this new study the investigators examined the acute effects of calcium supplements on blood pressure in a randomized controlled crossover trial in 40 healthy postmenopausal women (mean age, 71 years; body mass index [BMI], 27.2 kg/m2). Women attended on 2 occasions, with visits separated by 7 or more days. At each visit, they received either 1 g of calcium as citrate or placebo. Blood pressure and serum calcium concentrations were measured immediately before and 2, 4, and 6 hours after each intervention.
Ionized and total calcium concentrations increased after calcium (P<.0001 vs placebo). Systolic blood pressure (SBP) measurements decreased after both calcium and placebo but significantly less so after calcium (P=.02). The reduction in SBP from baseline was smaller after calcium compared with placebo by 6 mm Hg at 4 hours (P=.036) and by 9 mm Hg at 6 hours (P=.002). The reduction in diastolic blood pressure was similar after calcium and placebo.
These findings indicate that the use of calcium supplements in postmenopausal women attenuates the postbreakfast reduction in SBP by 6 to 9 mm Hg. Whether these changes in blood pressure influence cardiovascular risk requires further study.
Association between calcium, vitamin D, and height loss
Crandall and colleagues looked at the association between calcium and vitamin D supplementation and height loss in 36,282 participants of the Women's Health Initiative Calcium and Vitamin D trial.
Details of the study by Crandall and colleagues
The authors performed a post hoc analysis of data from a double-blind randomized controlled trial of 1,000 mg of elemental calcium as calcium carbonate with 400 IU of vitamin D3 daily (CaD) or placebo in postmenopausal women at 40 US clinical centers. Height was measured annually (mean follow-up, 5.9 years) with a stadiometer.
Average height loss was 1.28 mm/yr among participants assigned to CaD, versus 1.26 mm/yr for women assigned to placebo (P=.35). A strong association (P<.001) was observed between age group and height loss. The study authors concluded that, compared with placebo, calcium and vitamin D supplementation used in this trial did not prevent height loss in healthy postmenopausal women.
Adequate calcium is necessary for bone health. While calcium supplementation may not be adequate to prevent fractures, it is also not involved in the inevitable loss of overall height seen in postmenopausal women. Calcium supplementation has been implicated in an increase in CVD. These data seem to indicate that, while calcium supplementation results in higher systolic blood pressure during the day, as well as higher coronary artery calcium scores, greater dietary calcium actually may decrease the incidence of atherosclerosis.
Sarcopenia: Still important, clinical approaches to easily detect it
Beaudart C, McCloskey E, Bruyére O, et al. Sarcopenia in daily practice: assessment and management. BMC Geriatr. 2016;16(1):170.
In last year's update, I reviewed the article by He and colleagues11 on the relationship between sarcopenia and body composition with osteoporosis. Sarcopenia, which is the age-related loss of muscle mass and strength, is important to address in patients. Body composition and muscle strength are directly correlated with bone density, and this is not surprising since bone and muscle share some common hormonal, genetic, nutritional, and lifestyle determinants.12,13 Sarcopenia can be diagnosed via dual-energy x-ray absorptiometry (DXA) scan looking at lean muscle mass.
The term sarcopenia was first coined by Rosenberg and colleagues in 198914 as a progressive loss of skeletal muscle mass with advancing age. Since then, the definition has expanded to incorporate the notion of impaired muscle strength or physical performance. Sarcopenia is associated with morbidity and mortality from linked physical disability, falls, fractures, poor quality of life, depression, and hospitalization.15
Current research is focusing on nutritional exercise/activity-based and other novel interventions for improving the quality and quantity of skeletal muscle in older people. Some studies demonstrated that resistance training combined with nutritional supplements can improve muscle function.16
Details of the study
Beaudart and colleagues propose some user-friendly and inexpensive methods that can be utilized to assess sarcopenia in real life settings. They acknowledge that in research settings or even specialist clinical settings, DXA or computed tomography (CT) scans are the best assessment of muscle mass.
Anthropometric measurements. In a primary care setting, anthropometric measurement, especially calf circumference and mid-upper arm muscle circumference, correlate with overall muscle mass and reflect both health and nutritional status and predict performance, health, and survival in older people.
However, with advancing age, changes in the distribution of fat and loss of skin elasticity are such that circumference incurs a loss of accuracy and precision in older people. Some studies suggest that an adjustment of anthropometric measurements for age, sex, or BMI results in a better correlation with DXA-measured lean mass.17 Anthropometric measurements are simple clinical prediction tools that can be easily applied for sarcopenia since they offer the most portable, commonly applicable, inexpensive, and noninvasive technique for assessing size, proportions, and composition of the human body. However, their validity is limited when applied to individuals because cutoff points to identify low muscle mass still need to be defined. Still, serial measurements in a patient over time may be valuable.
Related article:
2014 Update on osteoporosis
Handgrip strength, as measured with a dynamometer, appears to be the most widely used method for the measurement of muscle strength. In general, isometric handgrip strength shows a good correlation with leg strength and also with lower extremity power, and calf cross-sectional muscle area. The measurement is easy to perform, inexpensive and does not require a specialist-trained staff.
Standardized conditions for the test include seating the patient in a standard chair with her forearms resting flat on the chair arms. Clinicians should demonstrate the use of the dynamometer and show that gripping very tightly registers the best score. Six measurements should be taken, 3 with each arm. Ideally, patients should be encouraged to squeeze as hard and tightly as possible during 3 to 5 seconds for each of the 6 trials; usually the highest reading of the 6 measurements is reported as the final result. The Jamar dynamometer, or similar hydraulic dynamometer, is the gold standard for this measurement.
Gait speed measurement. The most widely used tool in clinical practice for the assessment of physical performance is the gait speed measurement. The test is highly acceptable for participants and health professionals in clinical settings. No special equipment is required; it needs only a flat floor devoid of obstacles. In the 4-meter gait speed test, men and women with a gait speed of less than 0.8 meters/sec are described as having a poor physical performance. The average extra time added to the consultation by measuring the 4-meter gait speed was only 95 seconds (SD, 20 seconds).
Loss of muscle mass correlates with loss of bone mass as our patients age. In addition, such sarcopenia increases the risk of falls, a significant component of the rising rate of fragility fractures. Anthropometric measures, grip strength, and gait speed are easy, low-cost measures that can identify patients at increased risk.
Romosozumab: An interesting new agent to look forward to
Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375(16):1532-1543.
Romosozumab is a monoclonal antibody that binds sclerostin, increasing bone formation and decreasing bone resorption. Cosman and colleagues enrolled 7,180 postmenopausal women with a T score of -2.5 to -3.5 at the total hip or femoral neck. Participants were randomly assigned to receive subcutaneous injections of romosozumab 210 mg or placebo monthly for 12 months. Thereafter, women in each group received subcutaneous denosumab 60 mg for 12 months--administered every 6 months. The coprimary end points were the cumulative incidences of new vertebral fractures at 12 and 24 months. Secondary end points included clinical and nonvertebral fractures.
Details of the study
At 12 months, new vertebral fractures had occurred in 16 of 3,321 women (0.5%) in the romosozumab group, as compared with 59 of 3,322 (1.8%) in the placebo group (representing a 73% lower risk of fracture with romosozumab; P<.001). Clinical fractures had occurred in 58 of 3,589 women (1.6%) in the romosozumab group, as compared with 90 of 3,591 (2.5%) in the placebo group (a 36% lower fracture risk with romosozumab; P = .008). Nonvertebral fractures had occurred in 56 of 3,589 women (1.6%) in the romosozumab group and in 75 of 3,591 (2.1%) in the placebo group (P = .10).
At 24 months, the rates of vertebral fractures were significantly lower in the romosozumab group than in the placebo group after each group made the transition to denosumab (0.6% [21 of 3,325 women] in the romosozumab group vs 2.5% [84 of 3,327 women] in the placebo group, a 75% lower risk with romosozumab; P<.001). Adverse events, including cardiovascular events, osteoarthritis, and cancer, appeared to be balanced between the groups. One atypical femoral fracture and 2 cases of osteonecrosis of the jaw were observed in the romosozumab group.
Lower risk of fracture
Thus, in postmenopausal women with osteoporosis, romosozumab was associated with a lower risk of vertebral fracture than placebo at 12 months and, after the transition to denosumab, at 24 months. The lower risk of clinical fracture that was seen with romosozumab was evident at 1 year.
Of note, the effect of romosozumab on the risk of vertebral fracture was rapid, with only 2 additional vertebral fractures (of a total of 16 such fractures in the romosozumab group) occurring in the second 6 months of the first year of therapy. Because vertebral and clinical fractures are associated with increased morbidity and considerable health care costs, a treatment that would reduce this risk rapidly could offer appropriate patients an important benefit.
Romosozumab is a new agent. Though not yet available, it is extremely interesting because it not only decreases bone resorption but also increases bone formation. The results of this large prospective trial show that such an agent reduces both vertebral and clinical fracture and reduces that fracture risk quite rapidly within the first 6 months of therapy.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- MacLaughlin KL, Faubion SS, Long ME, Pruthi S, Casey PM. Should the annual pelvic examination go the way of annual cervical cytology? Womens Health (Lond). 2014;10(4):373–384.
- Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29(11):2520–2526.
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King AB, Tosterson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22(3):465–475.
- Kaunitz AM. Hormonal contraception in women of older reproductive age. N Engl J Med. 2008;358:1262–1270.
- Kaunitz AM. Oral contraceptive use in perimenopause. Am J Obstet Gynecol. 2001;185(2 suppl):S32–S37.
- Gambacciani M, Cappagli B, Lazzarini V, Ciaponi M, Fruzzetti F, Genazzani AR. Longitudinal evaluation of perimenopausal bone loss: effects of different low dose oral contraceptive preparations on bone mineral density. Maturitas. 2006;54(2):176–180.
- Bailey R, Dodd K, Goldman J, et al. Estimation of total usual calcium and vitamin D intakes in the United States. J Nutr. 2010;140(4):817–822.
- Bolland MJ, Grey A, Reid IR. Calcium supplements and cardiovascular risk: 5 years on. Ther Adv Drug Saf. 2013;4(5):199–210.
- Moyer VA; U.S. Preventive Services Task Force. Vitamin D and calcium supplementation to prevent fractures in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;158(9):691–696.
- Bristow SM, Gamble GD, Stewart A, Horne AM, Reid IR. Acute effects of calcium supplements on blood pressure and blood coagulation: secondary analysis of a randomised controlled trial in post-menopausal women. Br J Nutr. 2015;114(11):1868–1874.
- He H, Liu Y, Tian Q, Papasian CJ, Hu T, Deng HW. Relationship of sarcopenia and body composition with osteoporosis. Osteoporos Int. 2016;27(2):473–482.
- Coin A, Perissinotto E, Enzi G, et al. Predictors of low bone mineral density in the elderly: the role of dietary intake, nutritional status and sarcopenia. Eur J Clin Nutr. 2008;62(6):802–809.
- Taaffe DR, Cauley JA, Danielson M, et al. Race and sex effects on the association between muscle strength, soft tissue, and bone mineral density in healthy elders: the Health, Aging, and Body Composition Study. J Bone Miner Res. 2001;16(7):1343–1352.
- Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997;127(5 suppl):990S–991S.
- Beaudart C, Rizzoli R, Bruyere O, Reginster JY, Biver E. Sarcopenia: Burden and challenges for Public Health. Arch Public Health. 2014;72(1):45.
- Cruz-Jentoft AJ, Landi F, Schneider SM, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing. 2014;43(6):748–759.
- Kulkarni B, Kuper H, Taylor A, et al. Development and validation of anthropometric prediction equations for estimation of lean body mass and appendicular lean soft tissue in Indian men and women. J Appl Physiol. 2013;115(8):1156–1162.
Prioritize bone health, as osteoporotic fracture is a major source of morbidity and mortality among women. In this article: fracture risk with OC use in perimenopause, data that inform calcium’s role in cardiovascular disease, sarcopenia management, and an emerging treatment.
Most women’s health care providers are aware of recent changes and controversies regarding cervical cancer screening, mammography frequency, and whether a pelvic bimanual exam should be part of our annual well woman evaluation.1 However, I believe one of the most important things we as clinicians can do is be frontline in promoting bone health. Osteoporotic fracture is a major source of morbidity and mortality.2,3 Thus, promoting the maintenance of bone health is a priority in my own practice. It is also one of my many academic interests.
What follows is an update on bone health. In past years, this update has been entitled, “Update on osteoporosis,” but what we are trying to accomplish is fracture reduction. Thus, priorities for bone health consist of recognition of risk, lifestyle and dietary counseling, as well as the use of pharmacologic agents when appropriate. Certain research stands out as informative for your practice:
- a recent study on the risk of fracture with oral contraceptive (OC) use in perimenopause
- 3 just-published studies that inform our understanding of calcium’s role in cardiovascular health
- a review on sarcopenia management
- new data on romosozumab.
Oral contraceptive use in perimenopause
Scholes D, LaCroix AZ, Hubbard RA, et al. Oral contraceptive use and fracture risk around the menopausal transition. Menopause. 2016;23(2):166-174.
The use of OCs in women of older reproductive age has increased ever since the cutoff age of 35 years was eliminated.4 Lower doses have continued to be utilized in these "older" women with excellent control of irregular bleeding due to ovulatory dysfunction (and reduction in psychosocial symptoms as well).5
The effect of OC use on risk of fracture remains unclear, and use during later reproductive life may be increasing. To determine the association between OC use during later reproductive life and risk of fracture across the menopausal transition, Scholes and colleagues conducted a population-based case-controlled study in a Pacific Northwest HMO, Group Health Cooperative.
Details of the study
Scholes and colleagues enrolled 1,204 case women aged 45 to 59 years with incident fractures, and 2,275 control women. Potential cases with fracture codes in automated data were adjudicated by electronic health record review. Potential control women without fracture codes were selected concurrently, sampling based on age. Participants received a structured study interview. Using logistic regression, associations between OC use and fracture risk were calculated as odds ratios (ORs) and 95% confidence intervals (CIs).
Participation was 69% for cases and 64% for controls. The study sample was 82% white; mean age was 54 years. The most common fracture site for cases was the wrist/forearm (32%). Adjusted fracture risk did not differ between cases and controls for OC use:
- in the 10 years before menopause (OR, 0.90; 95% CI, 0.74-1.11)
- after age 38 years (OR, 0.94; 95% CI, 0.78-1.14)
- over the duration, or
- for other OC exposures.
Related article:
2016 Update on female sexual dysfunction
Association between fractures and OC use near menopause
The current study does not show an association between fractures near the menopausal transition and OC use in the decade before menopause or after age 38 years. For women considering OC use at these times, fracture risk does not seem to be either reduced or increased.
These results, looking at fracture, seem to be further supported by trials conducted by Gambacciani and colleagues,6 in which researchers randomly assigned irregularly cycling perimenopausal women (aged 40-49 years) to 20 μg ethinyl estradiol OCs or calcium/placebo. Results showed that this low-dose OC use significantly increased bone density at the femoral neck, spine, and other sites relative to control women after 24 months.
In the current Scholes study, the use of OCs in the decade before menopause or after age 38 did not reduce fracture risk in the years around the time of menopause. It is reassuring that their use was not associated with any increased fracture risk.
These findings provide additional clarity and guidance to women and their clinicians at a time of increasing public health concern about fractures. For women who may choose to use OCs during late premenopause (around age 38-48 years), fracture risk around the menopausal transition will not differ from women not choosing this option.
Calcium and calcium supplements: The data continue to grow
Anderson JJ, Kruszka B, Delaney JA, et al. Calcium intake from diet and supplements and the risk of coronary artery calcification and its progression among older adults: 10-year follow-up of the Multi-Ethnic Study of Atherosclerosis (MESA) [published online ahead of print October 11, 2016]. J Am Heart Assoc. pii: e003815.
Billington EO, Bristow SM, Gamble GD, et al. Acute effects of calcium supplements on blood pressure: randomised, crossover trial in postmenopausal women [published online ahead of print August 20, 2016]. Osteoporos Int. doi:10.1007/s00198-016-3744-y.
Crandall CJ, Aragaki AK, LeBoff MS, et al. Calcium plus vitamin D supplementation and height loss: findings from the Women's Health Initiative Calcium and Vitamin D clinical trial [published online ahead of print August 1, 2016]. Menopause. doi:10.1097 /GME.0000000000000704.
In 2001, a National Institutes of Health (NIH) Consensus Development Panel on osteoporosis concluded that calcium intake is crucial to maintain bone mass and should be maintained at 1,000-1,500 mg/day in older adults. The panel acknowledged that the majority of older adults did not meet the recommended intake from dietary sources alone, and therefore would require calcium supplementation. Calcium supplements are one of the most commonly used dietary supplements, and population-based surveys have shown that they are used by the majority of older men and women in the United States.7
More recently results from large randomized controlled trials (RCTs) of calcium supplements have been reported, leading to concerns about calcium efficacy for fracture risk and safety. Bolland and colleagues8 reported that calcium supplements increased the rate of cardiovascular events in healthy older women and suggested that their role in osteoporosis management be reconsidered. More recently, the US Preventive Services Task Force recommended against calcium supplements for the primary prevention of fractures in noninstitutionalized postmenopausal women.9
The association between calcium intake and CVD events
Anderson and colleagues acknowledged that recent randomized data suggest that calcium supplements may be associated with increased risk of cardiovascular disease (CVD) events. Using a longitudinal cohort study, they assessed the association between calcium intake, from both foods and supplements, and atherosclerosis, as measured by coronary artery calcification (CAC).
Details of the study by Anderson and colleagues
The authors studied 5,448 adults free of clinically diagnosed CVD (52% female; age range, 45-84 years) from the Multi-Ethnic Study of Atherosclerosis. Baseline total calcium intake was assessed from diet (using a food frequency questionnaire) and calcium supplements (by a medication inventory) and categorized into quintiles based on overall population distribution. Baseline CAC was measured by computed tomography (CT) scan, and CAC measurements were repeated in 2,742 participants approximately 10 years later. Women had higher calcium intakes than men.
After adjustment for potential confounders, among 1,567 participants without baseline CAC, the relative risk (RR) of developing incident CAC over 10 years, by quintile 1 to 5 of calcium intake is included in the TABLE. After accounting for total calcium intake, calcium supplement use was associated with increased risk for incident CAC (RR, 1.22; 95% CI, 1.07-1.39). No relation was found between baseline calcium intake and 10-year changes in CAC among those participants with baseline CAC less than zero.

They concluded that high total calcium intake was associated with a decreased risk of incident atherosclerosis over long-term follow-up, particularly if achieved without supplement use. However, calcium supplement use may increase the risk for incident CAC.
Related article:
Does the discontinuation of menopausal hormone therapy affect a woman’s cardiovascular risk?
Calcium supplements and blood pressure
Billington and colleagues acknowledged that calcium supplements appear to increase cardiovascular risk but that the mechanism is unknown. They had previously reported that blood pressure declines over the course of the day in older women.10
Details of the study by Billington and colleagues
In this new study the investigators examined the acute effects of calcium supplements on blood pressure in a randomized controlled crossover trial in 40 healthy postmenopausal women (mean age, 71 years; body mass index [BMI], 27.2 kg/m2). Women attended on 2 occasions, with visits separated by 7 or more days. At each visit, they received either 1 g of calcium as citrate or placebo. Blood pressure and serum calcium concentrations were measured immediately before and 2, 4, and 6 hours after each intervention.
Ionized and total calcium concentrations increased after calcium (P<.0001 vs placebo). Systolic blood pressure (SBP) measurements decreased after both calcium and placebo but significantly less so after calcium (P=.02). The reduction in SBP from baseline was smaller after calcium compared with placebo by 6 mm Hg at 4 hours (P=.036) and by 9 mm Hg at 6 hours (P=.002). The reduction in diastolic blood pressure was similar after calcium and placebo.
These findings indicate that the use of calcium supplements in postmenopausal women attenuates the postbreakfast reduction in SBP by 6 to 9 mm Hg. Whether these changes in blood pressure influence cardiovascular risk requires further study.
Association between calcium, vitamin D, and height loss
Crandall and colleagues looked at the association between calcium and vitamin D supplementation and height loss in 36,282 participants of the Women's Health Initiative Calcium and Vitamin D trial.
Details of the study by Crandall and colleagues
The authors performed a post hoc analysis of data from a double-blind randomized controlled trial of 1,000 mg of elemental calcium as calcium carbonate with 400 IU of vitamin D3 daily (CaD) or placebo in postmenopausal women at 40 US clinical centers. Height was measured annually (mean follow-up, 5.9 years) with a stadiometer.
Average height loss was 1.28 mm/yr among participants assigned to CaD, versus 1.26 mm/yr for women assigned to placebo (P=.35). A strong association (P<.001) was observed between age group and height loss. The study authors concluded that, compared with placebo, calcium and vitamin D supplementation used in this trial did not prevent height loss in healthy postmenopausal women.
Adequate calcium is necessary for bone health. While calcium supplementation may not be adequate to prevent fractures, it is also not involved in the inevitable loss of overall height seen in postmenopausal women. Calcium supplementation has been implicated in an increase in CVD. These data seem to indicate that, while calcium supplementation results in higher systolic blood pressure during the day, as well as higher coronary artery calcium scores, greater dietary calcium actually may decrease the incidence of atherosclerosis.
Sarcopenia: Still important, clinical approaches to easily detect it
Beaudart C, McCloskey E, Bruyére O, et al. Sarcopenia in daily practice: assessment and management. BMC Geriatr. 2016;16(1):170.
In last year's update, I reviewed the article by He and colleagues11 on the relationship between sarcopenia and body composition with osteoporosis. Sarcopenia, which is the age-related loss of muscle mass and strength, is important to address in patients. Body composition and muscle strength are directly correlated with bone density, and this is not surprising since bone and muscle share some common hormonal, genetic, nutritional, and lifestyle determinants.12,13 Sarcopenia can be diagnosed via dual-energy x-ray absorptiometry (DXA) scan looking at lean muscle mass.
The term sarcopenia was first coined by Rosenberg and colleagues in 198914 as a progressive loss of skeletal muscle mass with advancing age. Since then, the definition has expanded to incorporate the notion of impaired muscle strength or physical performance. Sarcopenia is associated with morbidity and mortality from linked physical disability, falls, fractures, poor quality of life, depression, and hospitalization.15
Current research is focusing on nutritional exercise/activity-based and other novel interventions for improving the quality and quantity of skeletal muscle in older people. Some studies demonstrated that resistance training combined with nutritional supplements can improve muscle function.16
Details of the study
Beaudart and colleagues propose some user-friendly and inexpensive methods that can be utilized to assess sarcopenia in real life settings. They acknowledge that in research settings or even specialist clinical settings, DXA or computed tomography (CT) scans are the best assessment of muscle mass.
Anthropometric measurements. In a primary care setting, anthropometric measurement, especially calf circumference and mid-upper arm muscle circumference, correlate with overall muscle mass and reflect both health and nutritional status and predict performance, health, and survival in older people.
However, with advancing age, changes in the distribution of fat and loss of skin elasticity are such that circumference incurs a loss of accuracy and precision in older people. Some studies suggest that an adjustment of anthropometric measurements for age, sex, or BMI results in a better correlation with DXA-measured lean mass.17 Anthropometric measurements are simple clinical prediction tools that can be easily applied for sarcopenia since they offer the most portable, commonly applicable, inexpensive, and noninvasive technique for assessing size, proportions, and composition of the human body. However, their validity is limited when applied to individuals because cutoff points to identify low muscle mass still need to be defined. Still, serial measurements in a patient over time may be valuable.
Related article:
2014 Update on osteoporosis
Handgrip strength, as measured with a dynamometer, appears to be the most widely used method for the measurement of muscle strength. In general, isometric handgrip strength shows a good correlation with leg strength and also with lower extremity power, and calf cross-sectional muscle area. The measurement is easy to perform, inexpensive and does not require a specialist-trained staff.
Standardized conditions for the test include seating the patient in a standard chair with her forearms resting flat on the chair arms. Clinicians should demonstrate the use of the dynamometer and show that gripping very tightly registers the best score. Six measurements should be taken, 3 with each arm. Ideally, patients should be encouraged to squeeze as hard and tightly as possible during 3 to 5 seconds for each of the 6 trials; usually the highest reading of the 6 measurements is reported as the final result. The Jamar dynamometer, or similar hydraulic dynamometer, is the gold standard for this measurement.
Gait speed measurement. The most widely used tool in clinical practice for the assessment of physical performance is the gait speed measurement. The test is highly acceptable for participants and health professionals in clinical settings. No special equipment is required; it needs only a flat floor devoid of obstacles. In the 4-meter gait speed test, men and women with a gait speed of less than 0.8 meters/sec are described as having a poor physical performance. The average extra time added to the consultation by measuring the 4-meter gait speed was only 95 seconds (SD, 20 seconds).
Loss of muscle mass correlates with loss of bone mass as our patients age. In addition, such sarcopenia increases the risk of falls, a significant component of the rising rate of fragility fractures. Anthropometric measures, grip strength, and gait speed are easy, low-cost measures that can identify patients at increased risk.
Romosozumab: An interesting new agent to look forward to
Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375(16):1532-1543.
Romosozumab is a monoclonal antibody that binds sclerostin, increasing bone formation and decreasing bone resorption. Cosman and colleagues enrolled 7,180 postmenopausal women with a T score of -2.5 to -3.5 at the total hip or femoral neck. Participants were randomly assigned to receive subcutaneous injections of romosozumab 210 mg or placebo monthly for 12 months. Thereafter, women in each group received subcutaneous denosumab 60 mg for 12 months--administered every 6 months. The coprimary end points were the cumulative incidences of new vertebral fractures at 12 and 24 months. Secondary end points included clinical and nonvertebral fractures.
Details of the study
At 12 months, new vertebral fractures had occurred in 16 of 3,321 women (0.5%) in the romosozumab group, as compared with 59 of 3,322 (1.8%) in the placebo group (representing a 73% lower risk of fracture with romosozumab; P<.001). Clinical fractures had occurred in 58 of 3,589 women (1.6%) in the romosozumab group, as compared with 90 of 3,591 (2.5%) in the placebo group (a 36% lower fracture risk with romosozumab; P = .008). Nonvertebral fractures had occurred in 56 of 3,589 women (1.6%) in the romosozumab group and in 75 of 3,591 (2.1%) in the placebo group (P = .10).
At 24 months, the rates of vertebral fractures were significantly lower in the romosozumab group than in the placebo group after each group made the transition to denosumab (0.6% [21 of 3,325 women] in the romosozumab group vs 2.5% [84 of 3,327 women] in the placebo group, a 75% lower risk with romosozumab; P<.001). Adverse events, including cardiovascular events, osteoarthritis, and cancer, appeared to be balanced between the groups. One atypical femoral fracture and 2 cases of osteonecrosis of the jaw were observed in the romosozumab group.
Lower risk of fracture
Thus, in postmenopausal women with osteoporosis, romosozumab was associated with a lower risk of vertebral fracture than placebo at 12 months and, after the transition to denosumab, at 24 months. The lower risk of clinical fracture that was seen with romosozumab was evident at 1 year.
Of note, the effect of romosozumab on the risk of vertebral fracture was rapid, with only 2 additional vertebral fractures (of a total of 16 such fractures in the romosozumab group) occurring in the second 6 months of the first year of therapy. Because vertebral and clinical fractures are associated with increased morbidity and considerable health care costs, a treatment that would reduce this risk rapidly could offer appropriate patients an important benefit.
Romosozumab is a new agent. Though not yet available, it is extremely interesting because it not only decreases bone resorption but also increases bone formation. The results of this large prospective trial show that such an agent reduces both vertebral and clinical fracture and reduces that fracture risk quite rapidly within the first 6 months of therapy.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Prioritize bone health, as osteoporotic fracture is a major source of morbidity and mortality among women. In this article: fracture risk with OC use in perimenopause, data that inform calcium’s role in cardiovascular disease, sarcopenia management, and an emerging treatment.
Most women’s health care providers are aware of recent changes and controversies regarding cervical cancer screening, mammography frequency, and whether a pelvic bimanual exam should be part of our annual well woman evaluation.1 However, I believe one of the most important things we as clinicians can do is be frontline in promoting bone health. Osteoporotic fracture is a major source of morbidity and mortality.2,3 Thus, promoting the maintenance of bone health is a priority in my own practice. It is also one of my many academic interests.
What follows is an update on bone health. In past years, this update has been entitled, “Update on osteoporosis,” but what we are trying to accomplish is fracture reduction. Thus, priorities for bone health consist of recognition of risk, lifestyle and dietary counseling, as well as the use of pharmacologic agents when appropriate. Certain research stands out as informative for your practice:
- a recent study on the risk of fracture with oral contraceptive (OC) use in perimenopause
- 3 just-published studies that inform our understanding of calcium’s role in cardiovascular health
- a review on sarcopenia management
- new data on romosozumab.
Oral contraceptive use in perimenopause
Scholes D, LaCroix AZ, Hubbard RA, et al. Oral contraceptive use and fracture risk around the menopausal transition. Menopause. 2016;23(2):166-174.
The use of OCs in women of older reproductive age has increased ever since the cutoff age of 35 years was eliminated.4 Lower doses have continued to be utilized in these "older" women with excellent control of irregular bleeding due to ovulatory dysfunction (and reduction in psychosocial symptoms as well).5
The effect of OC use on risk of fracture remains unclear, and use during later reproductive life may be increasing. To determine the association between OC use during later reproductive life and risk of fracture across the menopausal transition, Scholes and colleagues conducted a population-based case-controlled study in a Pacific Northwest HMO, Group Health Cooperative.
Details of the study
Scholes and colleagues enrolled 1,204 case women aged 45 to 59 years with incident fractures, and 2,275 control women. Potential cases with fracture codes in automated data were adjudicated by electronic health record review. Potential control women without fracture codes were selected concurrently, sampling based on age. Participants received a structured study interview. Using logistic regression, associations between OC use and fracture risk were calculated as odds ratios (ORs) and 95% confidence intervals (CIs).
Participation was 69% for cases and 64% for controls. The study sample was 82% white; mean age was 54 years. The most common fracture site for cases was the wrist/forearm (32%). Adjusted fracture risk did not differ between cases and controls for OC use:
- in the 10 years before menopause (OR, 0.90; 95% CI, 0.74-1.11)
- after age 38 years (OR, 0.94; 95% CI, 0.78-1.14)
- over the duration, or
- for other OC exposures.
Related article:
2016 Update on female sexual dysfunction
Association between fractures and OC use near menopause
The current study does not show an association between fractures near the menopausal transition and OC use in the decade before menopause or after age 38 years. For women considering OC use at these times, fracture risk does not seem to be either reduced or increased.
These results, looking at fracture, seem to be further supported by trials conducted by Gambacciani and colleagues,6 in which researchers randomly assigned irregularly cycling perimenopausal women (aged 40-49 years) to 20 μg ethinyl estradiol OCs or calcium/placebo. Results showed that this low-dose OC use significantly increased bone density at the femoral neck, spine, and other sites relative to control women after 24 months.
In the current Scholes study, the use of OCs in the decade before menopause or after age 38 did not reduce fracture risk in the years around the time of menopause. It is reassuring that their use was not associated with any increased fracture risk.
These findings provide additional clarity and guidance to women and their clinicians at a time of increasing public health concern about fractures. For women who may choose to use OCs during late premenopause (around age 38-48 years), fracture risk around the menopausal transition will not differ from women not choosing this option.
Calcium and calcium supplements: The data continue to grow
Anderson JJ, Kruszka B, Delaney JA, et al. Calcium intake from diet and supplements and the risk of coronary artery calcification and its progression among older adults: 10-year follow-up of the Multi-Ethnic Study of Atherosclerosis (MESA) [published online ahead of print October 11, 2016]. J Am Heart Assoc. pii: e003815.
Billington EO, Bristow SM, Gamble GD, et al. Acute effects of calcium supplements on blood pressure: randomised, crossover trial in postmenopausal women [published online ahead of print August 20, 2016]. Osteoporos Int. doi:10.1007/s00198-016-3744-y.
Crandall CJ, Aragaki AK, LeBoff MS, et al. Calcium plus vitamin D supplementation and height loss: findings from the Women's Health Initiative Calcium and Vitamin D clinical trial [published online ahead of print August 1, 2016]. Menopause. doi:10.1097 /GME.0000000000000704.
In 2001, a National Institutes of Health (NIH) Consensus Development Panel on osteoporosis concluded that calcium intake is crucial to maintain bone mass and should be maintained at 1,000-1,500 mg/day in older adults. The panel acknowledged that the majority of older adults did not meet the recommended intake from dietary sources alone, and therefore would require calcium supplementation. Calcium supplements are one of the most commonly used dietary supplements, and population-based surveys have shown that they are used by the majority of older men and women in the United States.7
More recently results from large randomized controlled trials (RCTs) of calcium supplements have been reported, leading to concerns about calcium efficacy for fracture risk and safety. Bolland and colleagues8 reported that calcium supplements increased the rate of cardiovascular events in healthy older women and suggested that their role in osteoporosis management be reconsidered. More recently, the US Preventive Services Task Force recommended against calcium supplements for the primary prevention of fractures in noninstitutionalized postmenopausal women.9
The association between calcium intake and CVD events
Anderson and colleagues acknowledged that recent randomized data suggest that calcium supplements may be associated with increased risk of cardiovascular disease (CVD) events. Using a longitudinal cohort study, they assessed the association between calcium intake, from both foods and supplements, and atherosclerosis, as measured by coronary artery calcification (CAC).
Details of the study by Anderson and colleagues
The authors studied 5,448 adults free of clinically diagnosed CVD (52% female; age range, 45-84 years) from the Multi-Ethnic Study of Atherosclerosis. Baseline total calcium intake was assessed from diet (using a food frequency questionnaire) and calcium supplements (by a medication inventory) and categorized into quintiles based on overall population distribution. Baseline CAC was measured by computed tomography (CT) scan, and CAC measurements were repeated in 2,742 participants approximately 10 years later. Women had higher calcium intakes than men.
After adjustment for potential confounders, among 1,567 participants without baseline CAC, the relative risk (RR) of developing incident CAC over 10 years, by quintile 1 to 5 of calcium intake is included in the TABLE. After accounting for total calcium intake, calcium supplement use was associated with increased risk for incident CAC (RR, 1.22; 95% CI, 1.07-1.39). No relation was found between baseline calcium intake and 10-year changes in CAC among those participants with baseline CAC less than zero.

They concluded that high total calcium intake was associated with a decreased risk of incident atherosclerosis over long-term follow-up, particularly if achieved without supplement use. However, calcium supplement use may increase the risk for incident CAC.
Related article:
Does the discontinuation of menopausal hormone therapy affect a woman’s cardiovascular risk?
Calcium supplements and blood pressure
Billington and colleagues acknowledged that calcium supplements appear to increase cardiovascular risk but that the mechanism is unknown. They had previously reported that blood pressure declines over the course of the day in older women.10
Details of the study by Billington and colleagues
In this new study the investigators examined the acute effects of calcium supplements on blood pressure in a randomized controlled crossover trial in 40 healthy postmenopausal women (mean age, 71 years; body mass index [BMI], 27.2 kg/m2). Women attended on 2 occasions, with visits separated by 7 or more days. At each visit, they received either 1 g of calcium as citrate or placebo. Blood pressure and serum calcium concentrations were measured immediately before and 2, 4, and 6 hours after each intervention.
Ionized and total calcium concentrations increased after calcium (P<.0001 vs placebo). Systolic blood pressure (SBP) measurements decreased after both calcium and placebo but significantly less so after calcium (P=.02). The reduction in SBP from baseline was smaller after calcium compared with placebo by 6 mm Hg at 4 hours (P=.036) and by 9 mm Hg at 6 hours (P=.002). The reduction in diastolic blood pressure was similar after calcium and placebo.
These findings indicate that the use of calcium supplements in postmenopausal women attenuates the postbreakfast reduction in SBP by 6 to 9 mm Hg. Whether these changes in blood pressure influence cardiovascular risk requires further study.
Association between calcium, vitamin D, and height loss
Crandall and colleagues looked at the association between calcium and vitamin D supplementation and height loss in 36,282 participants of the Women's Health Initiative Calcium and Vitamin D trial.
Details of the study by Crandall and colleagues
The authors performed a post hoc analysis of data from a double-blind randomized controlled trial of 1,000 mg of elemental calcium as calcium carbonate with 400 IU of vitamin D3 daily (CaD) or placebo in postmenopausal women at 40 US clinical centers. Height was measured annually (mean follow-up, 5.9 years) with a stadiometer.
Average height loss was 1.28 mm/yr among participants assigned to CaD, versus 1.26 mm/yr for women assigned to placebo (P=.35). A strong association (P<.001) was observed between age group and height loss. The study authors concluded that, compared with placebo, calcium and vitamin D supplementation used in this trial did not prevent height loss in healthy postmenopausal women.
Adequate calcium is necessary for bone health. While calcium supplementation may not be adequate to prevent fractures, it is also not involved in the inevitable loss of overall height seen in postmenopausal women. Calcium supplementation has been implicated in an increase in CVD. These data seem to indicate that, while calcium supplementation results in higher systolic blood pressure during the day, as well as higher coronary artery calcium scores, greater dietary calcium actually may decrease the incidence of atherosclerosis.
Sarcopenia: Still important, clinical approaches to easily detect it
Beaudart C, McCloskey E, Bruyére O, et al. Sarcopenia in daily practice: assessment and management. BMC Geriatr. 2016;16(1):170.
In last year's update, I reviewed the article by He and colleagues11 on the relationship between sarcopenia and body composition with osteoporosis. Sarcopenia, which is the age-related loss of muscle mass and strength, is important to address in patients. Body composition and muscle strength are directly correlated with bone density, and this is not surprising since bone and muscle share some common hormonal, genetic, nutritional, and lifestyle determinants.12,13 Sarcopenia can be diagnosed via dual-energy x-ray absorptiometry (DXA) scan looking at lean muscle mass.
The term sarcopenia was first coined by Rosenberg and colleagues in 198914 as a progressive loss of skeletal muscle mass with advancing age. Since then, the definition has expanded to incorporate the notion of impaired muscle strength or physical performance. Sarcopenia is associated with morbidity and mortality from linked physical disability, falls, fractures, poor quality of life, depression, and hospitalization.15
Current research is focusing on nutritional exercise/activity-based and other novel interventions for improving the quality and quantity of skeletal muscle in older people. Some studies demonstrated that resistance training combined with nutritional supplements can improve muscle function.16
Details of the study
Beaudart and colleagues propose some user-friendly and inexpensive methods that can be utilized to assess sarcopenia in real life settings. They acknowledge that in research settings or even specialist clinical settings, DXA or computed tomography (CT) scans are the best assessment of muscle mass.
Anthropometric measurements. In a primary care setting, anthropometric measurement, especially calf circumference and mid-upper arm muscle circumference, correlate with overall muscle mass and reflect both health and nutritional status and predict performance, health, and survival in older people.
However, with advancing age, changes in the distribution of fat and loss of skin elasticity are such that circumference incurs a loss of accuracy and precision in older people. Some studies suggest that an adjustment of anthropometric measurements for age, sex, or BMI results in a better correlation with DXA-measured lean mass.17 Anthropometric measurements are simple clinical prediction tools that can be easily applied for sarcopenia since they offer the most portable, commonly applicable, inexpensive, and noninvasive technique for assessing size, proportions, and composition of the human body. However, their validity is limited when applied to individuals because cutoff points to identify low muscle mass still need to be defined. Still, serial measurements in a patient over time may be valuable.
Related article:
2014 Update on osteoporosis
Handgrip strength, as measured with a dynamometer, appears to be the most widely used method for the measurement of muscle strength. In general, isometric handgrip strength shows a good correlation with leg strength and also with lower extremity power, and calf cross-sectional muscle area. The measurement is easy to perform, inexpensive and does not require a specialist-trained staff.
Standardized conditions for the test include seating the patient in a standard chair with her forearms resting flat on the chair arms. Clinicians should demonstrate the use of the dynamometer and show that gripping very tightly registers the best score. Six measurements should be taken, 3 with each arm. Ideally, patients should be encouraged to squeeze as hard and tightly as possible during 3 to 5 seconds for each of the 6 trials; usually the highest reading of the 6 measurements is reported as the final result. The Jamar dynamometer, or similar hydraulic dynamometer, is the gold standard for this measurement.
Gait speed measurement. The most widely used tool in clinical practice for the assessment of physical performance is the gait speed measurement. The test is highly acceptable for participants and health professionals in clinical settings. No special equipment is required; it needs only a flat floor devoid of obstacles. In the 4-meter gait speed test, men and women with a gait speed of less than 0.8 meters/sec are described as having a poor physical performance. The average extra time added to the consultation by measuring the 4-meter gait speed was only 95 seconds (SD, 20 seconds).
Loss of muscle mass correlates with loss of bone mass as our patients age. In addition, such sarcopenia increases the risk of falls, a significant component of the rising rate of fragility fractures. Anthropometric measures, grip strength, and gait speed are easy, low-cost measures that can identify patients at increased risk.
Romosozumab: An interesting new agent to look forward to
Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375(16):1532-1543.
Romosozumab is a monoclonal antibody that binds sclerostin, increasing bone formation and decreasing bone resorption. Cosman and colleagues enrolled 7,180 postmenopausal women with a T score of -2.5 to -3.5 at the total hip or femoral neck. Participants were randomly assigned to receive subcutaneous injections of romosozumab 210 mg or placebo monthly for 12 months. Thereafter, women in each group received subcutaneous denosumab 60 mg for 12 months--administered every 6 months. The coprimary end points were the cumulative incidences of new vertebral fractures at 12 and 24 months. Secondary end points included clinical and nonvertebral fractures.
Details of the study
At 12 months, new vertebral fractures had occurred in 16 of 3,321 women (0.5%) in the romosozumab group, as compared with 59 of 3,322 (1.8%) in the placebo group (representing a 73% lower risk of fracture with romosozumab; P<.001). Clinical fractures had occurred in 58 of 3,589 women (1.6%) in the romosozumab group, as compared with 90 of 3,591 (2.5%) in the placebo group (a 36% lower fracture risk with romosozumab; P = .008). Nonvertebral fractures had occurred in 56 of 3,589 women (1.6%) in the romosozumab group and in 75 of 3,591 (2.1%) in the placebo group (P = .10).
At 24 months, the rates of vertebral fractures were significantly lower in the romosozumab group than in the placebo group after each group made the transition to denosumab (0.6% [21 of 3,325 women] in the romosozumab group vs 2.5% [84 of 3,327 women] in the placebo group, a 75% lower risk with romosozumab; P<.001). Adverse events, including cardiovascular events, osteoarthritis, and cancer, appeared to be balanced between the groups. One atypical femoral fracture and 2 cases of osteonecrosis of the jaw were observed in the romosozumab group.
Lower risk of fracture
Thus, in postmenopausal women with osteoporosis, romosozumab was associated with a lower risk of vertebral fracture than placebo at 12 months and, after the transition to denosumab, at 24 months. The lower risk of clinical fracture that was seen with romosozumab was evident at 1 year.
Of note, the effect of romosozumab on the risk of vertebral fracture was rapid, with only 2 additional vertebral fractures (of a total of 16 such fractures in the romosozumab group) occurring in the second 6 months of the first year of therapy. Because vertebral and clinical fractures are associated with increased morbidity and considerable health care costs, a treatment that would reduce this risk rapidly could offer appropriate patients an important benefit.
Romosozumab is a new agent. Though not yet available, it is extremely interesting because it not only decreases bone resorption but also increases bone formation. The results of this large prospective trial show that such an agent reduces both vertebral and clinical fracture and reduces that fracture risk quite rapidly within the first 6 months of therapy.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- MacLaughlin KL, Faubion SS, Long ME, Pruthi S, Casey PM. Should the annual pelvic examination go the way of annual cervical cytology? Womens Health (Lond). 2014;10(4):373–384.
- Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29(11):2520–2526.
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King AB, Tosterson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22(3):465–475.
- Kaunitz AM. Hormonal contraception in women of older reproductive age. N Engl J Med. 2008;358:1262–1270.
- Kaunitz AM. Oral contraceptive use in perimenopause. Am J Obstet Gynecol. 2001;185(2 suppl):S32–S37.
- Gambacciani M, Cappagli B, Lazzarini V, Ciaponi M, Fruzzetti F, Genazzani AR. Longitudinal evaluation of perimenopausal bone loss: effects of different low dose oral contraceptive preparations on bone mineral density. Maturitas. 2006;54(2):176–180.
- Bailey R, Dodd K, Goldman J, et al. Estimation of total usual calcium and vitamin D intakes in the United States. J Nutr. 2010;140(4):817–822.
- Bolland MJ, Grey A, Reid IR. Calcium supplements and cardiovascular risk: 5 years on. Ther Adv Drug Saf. 2013;4(5):199–210.
- Moyer VA; U.S. Preventive Services Task Force. Vitamin D and calcium supplementation to prevent fractures in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;158(9):691–696.
- Bristow SM, Gamble GD, Stewart A, Horne AM, Reid IR. Acute effects of calcium supplements on blood pressure and blood coagulation: secondary analysis of a randomised controlled trial in post-menopausal women. Br J Nutr. 2015;114(11):1868–1874.
- He H, Liu Y, Tian Q, Papasian CJ, Hu T, Deng HW. Relationship of sarcopenia and body composition with osteoporosis. Osteoporos Int. 2016;27(2):473–482.
- Coin A, Perissinotto E, Enzi G, et al. Predictors of low bone mineral density in the elderly: the role of dietary intake, nutritional status and sarcopenia. Eur J Clin Nutr. 2008;62(6):802–809.
- Taaffe DR, Cauley JA, Danielson M, et al. Race and sex effects on the association between muscle strength, soft tissue, and bone mineral density in healthy elders: the Health, Aging, and Body Composition Study. J Bone Miner Res. 2001;16(7):1343–1352.
- Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997;127(5 suppl):990S–991S.
- Beaudart C, Rizzoli R, Bruyere O, Reginster JY, Biver E. Sarcopenia: Burden and challenges for Public Health. Arch Public Health. 2014;72(1):45.
- Cruz-Jentoft AJ, Landi F, Schneider SM, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing. 2014;43(6):748–759.
- Kulkarni B, Kuper H, Taylor A, et al. Development and validation of anthropometric prediction equations for estimation of lean body mass and appendicular lean soft tissue in Indian men and women. J Appl Physiol. 2013;115(8):1156–1162.
- MacLaughlin KL, Faubion SS, Long ME, Pruthi S, Casey PM. Should the annual pelvic examination go the way of annual cervical cytology? Womens Health (Lond). 2014;10(4):373–384.
- Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29(11):2520–2526.
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King AB, Tosterson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22(3):465–475.
- Kaunitz AM. Hormonal contraception in women of older reproductive age. N Engl J Med. 2008;358:1262–1270.
- Kaunitz AM. Oral contraceptive use in perimenopause. Am J Obstet Gynecol. 2001;185(2 suppl):S32–S37.
- Gambacciani M, Cappagli B, Lazzarini V, Ciaponi M, Fruzzetti F, Genazzani AR. Longitudinal evaluation of perimenopausal bone loss: effects of different low dose oral contraceptive preparations on bone mineral density. Maturitas. 2006;54(2):176–180.
- Bailey R, Dodd K, Goldman J, et al. Estimation of total usual calcium and vitamin D intakes in the United States. J Nutr. 2010;140(4):817–822.
- Bolland MJ, Grey A, Reid IR. Calcium supplements and cardiovascular risk: 5 years on. Ther Adv Drug Saf. 2013;4(5):199–210.
- Moyer VA; U.S. Preventive Services Task Force. Vitamin D and calcium supplementation to prevent fractures in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;158(9):691–696.
- Bristow SM, Gamble GD, Stewart A, Horne AM, Reid IR. Acute effects of calcium supplements on blood pressure and blood coagulation: secondary analysis of a randomised controlled trial in post-menopausal women. Br J Nutr. 2015;114(11):1868–1874.
- He H, Liu Y, Tian Q, Papasian CJ, Hu T, Deng HW. Relationship of sarcopenia and body composition with osteoporosis. Osteoporos Int. 2016;27(2):473–482.
- Coin A, Perissinotto E, Enzi G, et al. Predictors of low bone mineral density in the elderly: the role of dietary intake, nutritional status and sarcopenia. Eur J Clin Nutr. 2008;62(6):802–809.
- Taaffe DR, Cauley JA, Danielson M, et al. Race and sex effects on the association between muscle strength, soft tissue, and bone mineral density in healthy elders: the Health, Aging, and Body Composition Study. J Bone Miner Res. 2001;16(7):1343–1352.
- Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997;127(5 suppl):990S–991S.
- Beaudart C, Rizzoli R, Bruyere O, Reginster JY, Biver E. Sarcopenia: Burden and challenges for Public Health. Arch Public Health. 2014;72(1):45.
- Cruz-Jentoft AJ, Landi F, Schneider SM, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing. 2014;43(6):748–759.
- Kulkarni B, Kuper H, Taylor A, et al. Development and validation of anthropometric prediction equations for estimation of lean body mass and appendicular lean soft tissue in Indian men and women. J Appl Physiol. 2013;115(8):1156–1162.
FDA approves vaginal insert to treat dyspareunia in menopause
Prasterone (Intrarosa), a vaginal insert containing dehydroepiandrosterone (DHEA) to treat dyspareunia in menopause caused by vulvar and vaginal atrophy, has won Food and Drug Administration approval.
It’s the first FDA-approved product containing the active ingredient prasterone, known also as DHEA. It will be marketed by Endoceutics Inc., a Quebec-based pharmaceutical company focused on women’s health.
The approval is based on the results of two 12-week placebo-controlled trials of 406 healthy, postmenopausal women, ranging in age from 40 to 80 years, who identified dyspareunia as their most bothersome symptom of VVA. During the trials, prasterone reduced the severity of pain experienced during sexual intercourse, when compared with placebo. Safety of the treatment was established in four 12-week placebo-controlled trials and one 52-week open-label trial. The most common adverse events were vaginal discharge and abnormal Pap smear, according to the FDA.
[email protected]
On Twitter @maryellenny
Prasterone (Intrarosa), a vaginal insert containing dehydroepiandrosterone (DHEA) to treat dyspareunia in menopause caused by vulvar and vaginal atrophy, has won Food and Drug Administration approval.
It’s the first FDA-approved product containing the active ingredient prasterone, known also as DHEA. It will be marketed by Endoceutics Inc., a Quebec-based pharmaceutical company focused on women’s health.
The approval is based on the results of two 12-week placebo-controlled trials of 406 healthy, postmenopausal women, ranging in age from 40 to 80 years, who identified dyspareunia as their most bothersome symptom of VVA. During the trials, prasterone reduced the severity of pain experienced during sexual intercourse, when compared with placebo. Safety of the treatment was established in four 12-week placebo-controlled trials and one 52-week open-label trial. The most common adverse events were vaginal discharge and abnormal Pap smear, according to the FDA.
[email protected]
On Twitter @maryellenny
Prasterone (Intrarosa), a vaginal insert containing dehydroepiandrosterone (DHEA) to treat dyspareunia in menopause caused by vulvar and vaginal atrophy, has won Food and Drug Administration approval.
It’s the first FDA-approved product containing the active ingredient prasterone, known also as DHEA. It will be marketed by Endoceutics Inc., a Quebec-based pharmaceutical company focused on women’s health.
The approval is based on the results of two 12-week placebo-controlled trials of 406 healthy, postmenopausal women, ranging in age from 40 to 80 years, who identified dyspareunia as their most bothersome symptom of VVA. During the trials, prasterone reduced the severity of pain experienced during sexual intercourse, when compared with placebo. Safety of the treatment was established in four 12-week placebo-controlled trials and one 52-week open-label trial. The most common adverse events were vaginal discharge and abnormal Pap smear, according to the FDA.
[email protected]
On Twitter @maryellenny
It’s not easy to identify clinical depression in menopause
The notion that women in midlife are moody is so pervasive that entire Pinterest boards and Facebook pages are dedicated to menopause jokes. For physicians, however, it’s not always so easy to sort out when a patient who says she’s down, or short tempered, might really have major depressive disorder or another serious psychiatric diagnosis.
“The key point is that depressive symptoms are not the same as clinical depression,” said Hadine Joffe, MD, in an interview that recapped her recent presentation at the annual meeting of the North American Menopause Society (NAMS). “The causal factors and contributions to depressive symptoms and major depression are different.”
Practically speaking, this means two things. The first message is that treating common menopausal symptoms can have a positive impact on mood. However, it’s also true that clinicians must be educated and vigilant about more serious mood symptoms, and not expect major depression to lift when a patient starts hormone therapy, according to Dr. Joffe, a psychiatrist and director of the women’s hormone and aging research program at Brigham and Women’s Hospital, Boston.
Using a novel experimental model, Dr. Joffe and her coinvestigators recently completed work that examined the relationship among daytime and nighttime hot flashes, sleep disturbance, and mood changes. In a small study of 29 healthy premenopausal women, they tracked mood, hormone levels, and sleep fragmentation both before and after suppressing ovarian function with a gonadotropin-releasing hormone agonist. The study found independent and significant effects on mood for nighttime (but not daytime) hot flashes, as well as subjective sleep disturbance (J Clin Endocrinol Metab. 2016;101[10]:3847-55).
“We were struck by the fact that the nighttime hot flashes, separate from the sleep problems, contributed to mood symptoms,” Dr. Joffe said.
This study, though small, validates and extends the notion that multiple symptoms of the menopausal transition contribute to the mild mood symptoms that are common during this phase of a woman’s life, said Dr. Joffe. To date, most of the studies have been large epidemiologic studies, which while important, tend to assess people at one cross-sectional time point annually, and can’t always capture a nuanced and precise view of symptoms and how they relate to hormone levels, she said.
“Perimenopausal hormone changes are dynamic,” said Dr. Joffe, and have wide interindividual variation during the menopausal transition. Overall, though, she said, “clinical studies show that mood is better as ovarian activity is more normalized in perimenopausal women. Conversely, the more abnormal the hormonal profile, the worse the mood.”
Major depression is another story, said Dr. Joffe. “Menopause-specific factors have not, for the most part, been linked,” she said, noting that the best predictors for clinical depression in midlife that have been identified to date are a previous history of clinical depression or anxiety, as well as other traditional psychiatric risk factors such as low socioeconomic status and a low level of social support.
“People often present with all these symptoms all at once; they have mood disturbances, they have hot flashes, they have sleep disturbances. For the people experiencing these symptoms, it matters a lot to be able to have an explanation that’s credible, and defendable, and evidence based,” Dr. Joffe said. “And it also has implications for treatment, of course.”
This real world complexity inevitably intrudes during a clinical encounter, and it’s not an easy task, or a quick one, to tease apart the extent to which the menopausal transition is contributing to depressive symptoms when women have so many other potential stressors.
While acknowledging that it’s very difficult to unpack this in a brief clinic visit, Dr. Shifren also emphasized that it’s critically important. For patients with mild depressive symptoms, hormone therapy or other treatment to address vasomotor symptoms, along with evidence-based treatment of sleep problems, can be of real help.
“That’s very different from women who come in with real depression,” Dr. Shifren said in an interview. “It’s very important not to say, ‘Oh, that’s just a little bit of perimenopause.’ ”
“There is not enough education of general providers about the nuances of mood changes and mental health of women in midlife,” Dr. Montgomery said in an interview. “These are nicely dealt with in publications of the North American Menopause Society,” he said, also noting that the ob.gyn. residency curriculum at Drexel University includes specific modules to address the menopausal transition and menopause. “If you are going to be caring for women in transition, you probably should do a little deeper dive,” he said.
“My advice for clinicians is don’t do this alone,” Dr. Shifren said. If clinicians feel they lack expertise in diagnosing and managing mood disorders, they should establish collaborative relationships with colleagues in mental health. “We can still help with hormone management, sleep management, and keeping in touch with the patient,” she said.
One trick of the trade is to use your nursing staff to follow up with patients, Dr. Shifren said. She will ask a nurse to make a follow-up phone call 2-6 weeks after a patient visit to assess how the woman is faring with symptoms and any treatments that have been initiated or modified. The interval and intensity of follow-up can be modified based on feedback from this call, she said.
The bottom line for Dr. Montgomery is twofold: “Being menopausal does not make you depressed,” he said. “True clinical depression is a rare event, but one that should not be missed.”
Dr. Joffe reported consulting agreements with several pharmaceutical companies, and grant support from Merck for research related to vasomotor symptoms and insomnia. Dr. Montgomery reported no relevant financial disclosures. Dr. Shifren reported that she has a consulting agreement with the New England Research Institutes.
[email protected]
On Twitter @karioakes
The notion that women in midlife are moody is so pervasive that entire Pinterest boards and Facebook pages are dedicated to menopause jokes. For physicians, however, it’s not always so easy to sort out when a patient who says she’s down, or short tempered, might really have major depressive disorder or another serious psychiatric diagnosis.
“The key point is that depressive symptoms are not the same as clinical depression,” said Hadine Joffe, MD, in an interview that recapped her recent presentation at the annual meeting of the North American Menopause Society (NAMS). “The causal factors and contributions to depressive symptoms and major depression are different.”
Practically speaking, this means two things. The first message is that treating common menopausal symptoms can have a positive impact on mood. However, it’s also true that clinicians must be educated and vigilant about more serious mood symptoms, and not expect major depression to lift when a patient starts hormone therapy, according to Dr. Joffe, a psychiatrist and director of the women’s hormone and aging research program at Brigham and Women’s Hospital, Boston.
Using a novel experimental model, Dr. Joffe and her coinvestigators recently completed work that examined the relationship among daytime and nighttime hot flashes, sleep disturbance, and mood changes. In a small study of 29 healthy premenopausal women, they tracked mood, hormone levels, and sleep fragmentation both before and after suppressing ovarian function with a gonadotropin-releasing hormone agonist. The study found independent and significant effects on mood for nighttime (but not daytime) hot flashes, as well as subjective sleep disturbance (J Clin Endocrinol Metab. 2016;101[10]:3847-55).
“We were struck by the fact that the nighttime hot flashes, separate from the sleep problems, contributed to mood symptoms,” Dr. Joffe said.
This study, though small, validates and extends the notion that multiple symptoms of the menopausal transition contribute to the mild mood symptoms that are common during this phase of a woman’s life, said Dr. Joffe. To date, most of the studies have been large epidemiologic studies, which while important, tend to assess people at one cross-sectional time point annually, and can’t always capture a nuanced and precise view of symptoms and how they relate to hormone levels, she said.
“Perimenopausal hormone changes are dynamic,” said Dr. Joffe, and have wide interindividual variation during the menopausal transition. Overall, though, she said, “clinical studies show that mood is better as ovarian activity is more normalized in perimenopausal women. Conversely, the more abnormal the hormonal profile, the worse the mood.”
Major depression is another story, said Dr. Joffe. “Menopause-specific factors have not, for the most part, been linked,” she said, noting that the best predictors for clinical depression in midlife that have been identified to date are a previous history of clinical depression or anxiety, as well as other traditional psychiatric risk factors such as low socioeconomic status and a low level of social support.
“People often present with all these symptoms all at once; they have mood disturbances, they have hot flashes, they have sleep disturbances. For the people experiencing these symptoms, it matters a lot to be able to have an explanation that’s credible, and defendable, and evidence based,” Dr. Joffe said. “And it also has implications for treatment, of course.”
This real world complexity inevitably intrudes during a clinical encounter, and it’s not an easy task, or a quick one, to tease apart the extent to which the menopausal transition is contributing to depressive symptoms when women have so many other potential stressors.
While acknowledging that it’s very difficult to unpack this in a brief clinic visit, Dr. Shifren also emphasized that it’s critically important. For patients with mild depressive symptoms, hormone therapy or other treatment to address vasomotor symptoms, along with evidence-based treatment of sleep problems, can be of real help.
“That’s very different from women who come in with real depression,” Dr. Shifren said in an interview. “It’s very important not to say, ‘Oh, that’s just a little bit of perimenopause.’ ”
“There is not enough education of general providers about the nuances of mood changes and mental health of women in midlife,” Dr. Montgomery said in an interview. “These are nicely dealt with in publications of the North American Menopause Society,” he said, also noting that the ob.gyn. residency curriculum at Drexel University includes specific modules to address the menopausal transition and menopause. “If you are going to be caring for women in transition, you probably should do a little deeper dive,” he said.
“My advice for clinicians is don’t do this alone,” Dr. Shifren said. If clinicians feel they lack expertise in diagnosing and managing mood disorders, they should establish collaborative relationships with colleagues in mental health. “We can still help with hormone management, sleep management, and keeping in touch with the patient,” she said.
One trick of the trade is to use your nursing staff to follow up with patients, Dr. Shifren said. She will ask a nurse to make a follow-up phone call 2-6 weeks after a patient visit to assess how the woman is faring with symptoms and any treatments that have been initiated or modified. The interval and intensity of follow-up can be modified based on feedback from this call, she said.
The bottom line for Dr. Montgomery is twofold: “Being menopausal does not make you depressed,” he said. “True clinical depression is a rare event, but one that should not be missed.”
Dr. Joffe reported consulting agreements with several pharmaceutical companies, and grant support from Merck for research related to vasomotor symptoms and insomnia. Dr. Montgomery reported no relevant financial disclosures. Dr. Shifren reported that she has a consulting agreement with the New England Research Institutes.
[email protected]
On Twitter @karioakes
The notion that women in midlife are moody is so pervasive that entire Pinterest boards and Facebook pages are dedicated to menopause jokes. For physicians, however, it’s not always so easy to sort out when a patient who says she’s down, or short tempered, might really have major depressive disorder or another serious psychiatric diagnosis.
“The key point is that depressive symptoms are not the same as clinical depression,” said Hadine Joffe, MD, in an interview that recapped her recent presentation at the annual meeting of the North American Menopause Society (NAMS). “The causal factors and contributions to depressive symptoms and major depression are different.”
Practically speaking, this means two things. The first message is that treating common menopausal symptoms can have a positive impact on mood. However, it’s also true that clinicians must be educated and vigilant about more serious mood symptoms, and not expect major depression to lift when a patient starts hormone therapy, according to Dr. Joffe, a psychiatrist and director of the women’s hormone and aging research program at Brigham and Women’s Hospital, Boston.
Using a novel experimental model, Dr. Joffe and her coinvestigators recently completed work that examined the relationship among daytime and nighttime hot flashes, sleep disturbance, and mood changes. In a small study of 29 healthy premenopausal women, they tracked mood, hormone levels, and sleep fragmentation both before and after suppressing ovarian function with a gonadotropin-releasing hormone agonist. The study found independent and significant effects on mood for nighttime (but not daytime) hot flashes, as well as subjective sleep disturbance (J Clin Endocrinol Metab. 2016;101[10]:3847-55).
“We were struck by the fact that the nighttime hot flashes, separate from the sleep problems, contributed to mood symptoms,” Dr. Joffe said.
This study, though small, validates and extends the notion that multiple symptoms of the menopausal transition contribute to the mild mood symptoms that are common during this phase of a woman’s life, said Dr. Joffe. To date, most of the studies have been large epidemiologic studies, which while important, tend to assess people at one cross-sectional time point annually, and can’t always capture a nuanced and precise view of symptoms and how they relate to hormone levels, she said.
“Perimenopausal hormone changes are dynamic,” said Dr. Joffe, and have wide interindividual variation during the menopausal transition. Overall, though, she said, “clinical studies show that mood is better as ovarian activity is more normalized in perimenopausal women. Conversely, the more abnormal the hormonal profile, the worse the mood.”
Major depression is another story, said Dr. Joffe. “Menopause-specific factors have not, for the most part, been linked,” she said, noting that the best predictors for clinical depression in midlife that have been identified to date are a previous history of clinical depression or anxiety, as well as other traditional psychiatric risk factors such as low socioeconomic status and a low level of social support.
“People often present with all these symptoms all at once; they have mood disturbances, they have hot flashes, they have sleep disturbances. For the people experiencing these symptoms, it matters a lot to be able to have an explanation that’s credible, and defendable, and evidence based,” Dr. Joffe said. “And it also has implications for treatment, of course.”
This real world complexity inevitably intrudes during a clinical encounter, and it’s not an easy task, or a quick one, to tease apart the extent to which the menopausal transition is contributing to depressive symptoms when women have so many other potential stressors.
While acknowledging that it’s very difficult to unpack this in a brief clinic visit, Dr. Shifren also emphasized that it’s critically important. For patients with mild depressive symptoms, hormone therapy or other treatment to address vasomotor symptoms, along with evidence-based treatment of sleep problems, can be of real help.
“That’s very different from women who come in with real depression,” Dr. Shifren said in an interview. “It’s very important not to say, ‘Oh, that’s just a little bit of perimenopause.’ ”
“There is not enough education of general providers about the nuances of mood changes and mental health of women in midlife,” Dr. Montgomery said in an interview. “These are nicely dealt with in publications of the North American Menopause Society,” he said, also noting that the ob.gyn. residency curriculum at Drexel University includes specific modules to address the menopausal transition and menopause. “If you are going to be caring for women in transition, you probably should do a little deeper dive,” he said.
“My advice for clinicians is don’t do this alone,” Dr. Shifren said. If clinicians feel they lack expertise in diagnosing and managing mood disorders, they should establish collaborative relationships with colleagues in mental health. “We can still help with hormone management, sleep management, and keeping in touch with the patient,” she said.
One trick of the trade is to use your nursing staff to follow up with patients, Dr. Shifren said. She will ask a nurse to make a follow-up phone call 2-6 weeks after a patient visit to assess how the woman is faring with symptoms and any treatments that have been initiated or modified. The interval and intensity of follow-up can be modified based on feedback from this call, she said.
The bottom line for Dr. Montgomery is twofold: “Being menopausal does not make you depressed,” he said. “True clinical depression is a rare event, but one that should not be missed.”
Dr. Joffe reported consulting agreements with several pharmaceutical companies, and grant support from Merck for research related to vasomotor symptoms and insomnia. Dr. Montgomery reported no relevant financial disclosures. Dr. Shifren reported that she has a consulting agreement with the New England Research Institutes.
[email protected]
On Twitter @karioakes
NAMS hormone therapy guidelines stress individualized treatment
An update from the society’s 2012 recommendations, the new statement will also give targeted recommendations for special populations of women to help guide clinicians in individualized treatment.
Highlights from the new position statement were released at the NAMS 2016 annual meeting, and the full document is expected to be published later this year. Among the highlights is the assertion that the clearest benefit for hormone therapy (HT) for treating hot flashes and preventing bone loss is in the early postmenopausal group.
The position statement also represents something of a shift away from the old mantra of “the lowest dose for the shortest period of time,” said Dr. Pinkerton, professor of obstetrics and gynecology at the University of Virginia Health System, Charlottesville.
As a practical matter, clinicians should budget time for these individualized discussions, Cynthia Stuenkel, MD, another member of the guidelines committee, said in an interview.
Currently, HT is approved by the Food and Drug administration as first-line therapy for menopausal vasomotor symptoms (VMS) for women without contraindications. For prevention of bone loss and fractures in postmenopausal women at higher risk, HT may be considered, especially for women younger than 60 years old and less than 10 years post menopause, according to the position statement.
When the predominant symptom pattern involves genitourinary syndrome of menopause (GSM, also known as vulvovaginal atrophy), the position statement recommends starting with low-dose vaginal estrogen as first-line treatment. These are all level I recommendations.
The use of HT in early menopause both provides the most effective treatment for symptoms and the greatest skeletal benefits, according to Michael R. McClung, MD, founding director of the Oregon Osteoporosis Center in Portland. “The benefit far outweighs the risk,” he said, especially in women at risk for bone density loss without contraindication for HT.
Special populations
Several special populations are addressed in the updated position statement. These include those who have reached early menopause because of primary ovarian insufficiency or because of oophorectomy. For these women, NAMS recommends hormone therapy until at least the median age of menopause. Making a level II recommendation, the NAMS committee wrote, “Observational studies suggest that benefits appear to outweigh the risks for effects on bone, heart, cognition, GSM, sexual function, and mood.”
Other special populations for whom HT may be considered include women with a family history of breast cancer and women who are positive for the BRCA gene. Again turning to observational evidence, the NAMS committee makes a level II recommendation that “use of HT does not alter the risk for breast cancer in women with family history of breast cancer, although family history is one risk, among many, that should be assessed.”
BRCA-positive women who do not have breast cancer are at higher risk for primarily estrogen receptor–negative breast cancer. BRCA-positive women may have opted for elective oophorectomy, though, and the committee recommends considering the potential negative effects of estrogen depletion at a premenopausal age when weighing risks and benefits in surgically menopausal BRCA-positive patients. It’s appropriate to offer systemic HT until the median age of menopause in this population, if there are no contraindications, and after appropriate counseling, according to the position statement.
Individualized discussions about continuing HT beyond the median age of menopause are recommended, said Dr. Pinkerton. “We reviewed the literature and found no increased risk in observational studies of women with BRCA genes after oophorectomy who receive hormone therapy,” she said. “These decisions are best taken on an individual basis.” The recommendations for the BRCA population are also a level II recommendation.
Duration of use
Regarding extended use of HT, the NAMS statement breaks with the Beers criteria, saying that routine discontinuation of HT after the age of 65 years “is not supported by data.” These decisions, according to the new recommendations, should be individualized. This is a level III recommendation. Still, said Dr. Kaunitz, “many women grow out of their vasomotor symptoms,” and so an individualized approach might include indefinite use of low-dose vaginal estrogen therapy for GSM, he said.
The overall benefit-risk ratio for HT is also addressed in the position statement, which emphasizes an individualized approach that includes periodic reassessment of risk and benefit for particular patients. However, for patients younger than 60 years of age, or who are within 10 years of menopause, NAMS endorses an overall favorable risk-benefit profile for HT in two particular areas, barring contraindications. For this younger postmenopausal population, hormone therapy is beneficial for bothersome vasomotor symptoms, according to the position statement, and women with an increased risk of osteoporosis or fracture may also benefit from HT.
The benefit-risk profile may tip against HT for women who are starting hormone therapy more than 10 years after menopause, or when they are 60 years old or older, according to the statement. The authors cite elevated risks of coronary heart disease, stroke, venous thromboembolism, and dementia.
The recommendations embodied in the new position statement take into account the “substantial benefit” of estrogen for many women, and provide an updated view of the safety of HT, Dr. McClung said. It’s important for physicians to talk to their patients, because “that information has not made it back to the Internet,” he said.
Dr. Pinkerton, Dr. McClung, and Dr. Kaunitz all reported financial relationships with several pharmaceutical companies. Dr. Kaunitz reported receiving royalties from UpToDate. Dr. Stuenkel reported no relevant financial disclosures.
[email protected]
On Twitter @karioakes
An update from the society’s 2012 recommendations, the new statement will also give targeted recommendations for special populations of women to help guide clinicians in individualized treatment.
Highlights from the new position statement were released at the NAMS 2016 annual meeting, and the full document is expected to be published later this year. Among the highlights is the assertion that the clearest benefit for hormone therapy (HT) for treating hot flashes and preventing bone loss is in the early postmenopausal group.
The position statement also represents something of a shift away from the old mantra of “the lowest dose for the shortest period of time,” said Dr. Pinkerton, professor of obstetrics and gynecology at the University of Virginia Health System, Charlottesville.
As a practical matter, clinicians should budget time for these individualized discussions, Cynthia Stuenkel, MD, another member of the guidelines committee, said in an interview.
Currently, HT is approved by the Food and Drug administration as first-line therapy for menopausal vasomotor symptoms (VMS) for women without contraindications. For prevention of bone loss and fractures in postmenopausal women at higher risk, HT may be considered, especially for women younger than 60 years old and less than 10 years post menopause, according to the position statement.
When the predominant symptom pattern involves genitourinary syndrome of menopause (GSM, also known as vulvovaginal atrophy), the position statement recommends starting with low-dose vaginal estrogen as first-line treatment. These are all level I recommendations.
The use of HT in early menopause both provides the most effective treatment for symptoms and the greatest skeletal benefits, according to Michael R. McClung, MD, founding director of the Oregon Osteoporosis Center in Portland. “The benefit far outweighs the risk,” he said, especially in women at risk for bone density loss without contraindication for HT.
Special populations
Several special populations are addressed in the updated position statement. These include those who have reached early menopause because of primary ovarian insufficiency or because of oophorectomy. For these women, NAMS recommends hormone therapy until at least the median age of menopause. Making a level II recommendation, the NAMS committee wrote, “Observational studies suggest that benefits appear to outweigh the risks for effects on bone, heart, cognition, GSM, sexual function, and mood.”
Other special populations for whom HT may be considered include women with a family history of breast cancer and women who are positive for the BRCA gene. Again turning to observational evidence, the NAMS committee makes a level II recommendation that “use of HT does not alter the risk for breast cancer in women with family history of breast cancer, although family history is one risk, among many, that should be assessed.”
BRCA-positive women who do not have breast cancer are at higher risk for primarily estrogen receptor–negative breast cancer. BRCA-positive women may have opted for elective oophorectomy, though, and the committee recommends considering the potential negative effects of estrogen depletion at a premenopausal age when weighing risks and benefits in surgically menopausal BRCA-positive patients. It’s appropriate to offer systemic HT until the median age of menopause in this population, if there are no contraindications, and after appropriate counseling, according to the position statement.
Individualized discussions about continuing HT beyond the median age of menopause are recommended, said Dr. Pinkerton. “We reviewed the literature and found no increased risk in observational studies of women with BRCA genes after oophorectomy who receive hormone therapy,” she said. “These decisions are best taken on an individual basis.” The recommendations for the BRCA population are also a level II recommendation.
Duration of use
Regarding extended use of HT, the NAMS statement breaks with the Beers criteria, saying that routine discontinuation of HT after the age of 65 years “is not supported by data.” These decisions, according to the new recommendations, should be individualized. This is a level III recommendation. Still, said Dr. Kaunitz, “many women grow out of their vasomotor symptoms,” and so an individualized approach might include indefinite use of low-dose vaginal estrogen therapy for GSM, he said.
The overall benefit-risk ratio for HT is also addressed in the position statement, which emphasizes an individualized approach that includes periodic reassessment of risk and benefit for particular patients. However, for patients younger than 60 years of age, or who are within 10 years of menopause, NAMS endorses an overall favorable risk-benefit profile for HT in two particular areas, barring contraindications. For this younger postmenopausal population, hormone therapy is beneficial for bothersome vasomotor symptoms, according to the position statement, and women with an increased risk of osteoporosis or fracture may also benefit from HT.
The benefit-risk profile may tip against HT for women who are starting hormone therapy more than 10 years after menopause, or when they are 60 years old or older, according to the statement. The authors cite elevated risks of coronary heart disease, stroke, venous thromboembolism, and dementia.
The recommendations embodied in the new position statement take into account the “substantial benefit” of estrogen for many women, and provide an updated view of the safety of HT, Dr. McClung said. It’s important for physicians to talk to their patients, because “that information has not made it back to the Internet,” he said.
Dr. Pinkerton, Dr. McClung, and Dr. Kaunitz all reported financial relationships with several pharmaceutical companies. Dr. Kaunitz reported receiving royalties from UpToDate. Dr. Stuenkel reported no relevant financial disclosures.
[email protected]
On Twitter @karioakes
An update from the society’s 2012 recommendations, the new statement will also give targeted recommendations for special populations of women to help guide clinicians in individualized treatment.
Highlights from the new position statement were released at the NAMS 2016 annual meeting, and the full document is expected to be published later this year. Among the highlights is the assertion that the clearest benefit for hormone therapy (HT) for treating hot flashes and preventing bone loss is in the early postmenopausal group.
The position statement also represents something of a shift away from the old mantra of “the lowest dose for the shortest period of time,” said Dr. Pinkerton, professor of obstetrics and gynecology at the University of Virginia Health System, Charlottesville.
As a practical matter, clinicians should budget time for these individualized discussions, Cynthia Stuenkel, MD, another member of the guidelines committee, said in an interview.
Currently, HT is approved by the Food and Drug administration as first-line therapy for menopausal vasomotor symptoms (VMS) for women without contraindications. For prevention of bone loss and fractures in postmenopausal women at higher risk, HT may be considered, especially for women younger than 60 years old and less than 10 years post menopause, according to the position statement.
When the predominant symptom pattern involves genitourinary syndrome of menopause (GSM, also known as vulvovaginal atrophy), the position statement recommends starting with low-dose vaginal estrogen as first-line treatment. These are all level I recommendations.
The use of HT in early menopause both provides the most effective treatment for symptoms and the greatest skeletal benefits, according to Michael R. McClung, MD, founding director of the Oregon Osteoporosis Center in Portland. “The benefit far outweighs the risk,” he said, especially in women at risk for bone density loss without contraindication for HT.
Special populations
Several special populations are addressed in the updated position statement. These include those who have reached early menopause because of primary ovarian insufficiency or because of oophorectomy. For these women, NAMS recommends hormone therapy until at least the median age of menopause. Making a level II recommendation, the NAMS committee wrote, “Observational studies suggest that benefits appear to outweigh the risks for effects on bone, heart, cognition, GSM, sexual function, and mood.”
Other special populations for whom HT may be considered include women with a family history of breast cancer and women who are positive for the BRCA gene. Again turning to observational evidence, the NAMS committee makes a level II recommendation that “use of HT does not alter the risk for breast cancer in women with family history of breast cancer, although family history is one risk, among many, that should be assessed.”
BRCA-positive women who do not have breast cancer are at higher risk for primarily estrogen receptor–negative breast cancer. BRCA-positive women may have opted for elective oophorectomy, though, and the committee recommends considering the potential negative effects of estrogen depletion at a premenopausal age when weighing risks and benefits in surgically menopausal BRCA-positive patients. It’s appropriate to offer systemic HT until the median age of menopause in this population, if there are no contraindications, and after appropriate counseling, according to the position statement.
Individualized discussions about continuing HT beyond the median age of menopause are recommended, said Dr. Pinkerton. “We reviewed the literature and found no increased risk in observational studies of women with BRCA genes after oophorectomy who receive hormone therapy,” she said. “These decisions are best taken on an individual basis.” The recommendations for the BRCA population are also a level II recommendation.
Duration of use
Regarding extended use of HT, the NAMS statement breaks with the Beers criteria, saying that routine discontinuation of HT after the age of 65 years “is not supported by data.” These decisions, according to the new recommendations, should be individualized. This is a level III recommendation. Still, said Dr. Kaunitz, “many women grow out of their vasomotor symptoms,” and so an individualized approach might include indefinite use of low-dose vaginal estrogen therapy for GSM, he said.
The overall benefit-risk ratio for HT is also addressed in the position statement, which emphasizes an individualized approach that includes periodic reassessment of risk and benefit for particular patients. However, for patients younger than 60 years of age, or who are within 10 years of menopause, NAMS endorses an overall favorable risk-benefit profile for HT in two particular areas, barring contraindications. For this younger postmenopausal population, hormone therapy is beneficial for bothersome vasomotor symptoms, according to the position statement, and women with an increased risk of osteoporosis or fracture may also benefit from HT.
The benefit-risk profile may tip against HT for women who are starting hormone therapy more than 10 years after menopause, or when they are 60 years old or older, according to the statement. The authors cite elevated risks of coronary heart disease, stroke, venous thromboembolism, and dementia.
The recommendations embodied in the new position statement take into account the “substantial benefit” of estrogen for many women, and provide an updated view of the safety of HT, Dr. McClung said. It’s important for physicians to talk to their patients, because “that information has not made it back to the Internet,” he said.
Dr. Pinkerton, Dr. McClung, and Dr. Kaunitz all reported financial relationships with several pharmaceutical companies. Dr. Kaunitz reported receiving royalties from UpToDate. Dr. Stuenkel reported no relevant financial disclosures.
[email protected]
On Twitter @karioakes
Odanacatib reduced fractures but upped stroke risk
The novel oral osteoporosis drug odanacatib significantly reduced fractures in postmenopausal women but was also associated with a significantly higher risk for stroke, according to data from a 5-year extension of a large phase III clinical trial.
Based on an independent analysis and verification of the risk for stroke, the drug’s sponsor, Merck, has withdrawn odanacatib from review by the Food and Drug Administration. “We are disappointed that the overall benefit-risk profile for odanacatib does not support filing or further development,” Roger M. Perlmutter, MD, president of Merck Research Laboratories, said in a statement.
Compared with placebo, odanacatib resulted in relative risk reductions of 52% for vertebral fracture, 48% for hip fracture, 26% for nonvertebral fracture, and 67% for clinical vertebral fracture (all values, P less than .001). Lumbar spine bone density increased by a mean of 10.9%, as did total hip bone density by a mean of 10.3%, in the odanacatib group (for both, P less than .001), according to results presented at the meeting.
The Long-Term Odanacatib Fracture Trial (LOFT) was a randomized, double-blind placebo-controlled study that investigated the efficacy and safety of odanacatib, a cathepsin K inhibitor, as a treatment for osteoporosis and for fracture prevention in postmenopausal women. Odanacatib was taken as an oral, once-weekly 50-mg pill.
During the base period of the study, 16,071 postmenopausal women aged 65 and older were enrolled, and 12,290 completed the study. Women had to have total hip or femoral neck bone mineral density T scores less than or equal to –2.5, or a radiographically confirmed vertebral fracture and total hip or femoral neck T scores less than or equal to –1.5. Participants were demographically well matched between study arms; 6,092 odanacatib patients and 6,198 on placebo completed the base period of the study.
The original phase III study was stopped early because of robust efficacy data (Osteoporos Int. 2015 Feb;26[2]:699-712). Participants were eligible to continue in a preplanned 5-year double-blind, placebo-controlled extension period of the LOFT study; 3,432 odanacatib and 2,615 placebo participants completed the full 5 years.
An early, but statistically nonsignificant, signal for increased stroke and atrial fibrillation and flutter was seen at the end of the study’s base period. The trend continued and appeared to be amplified during the 5-year, double-blind, placebo-controlled extension period of the LOFT study.
In an interview, Dr. O’Donoghue said that the TIMI study group was brought in by Merck for a “second perspective,” to add rigor to an examination of events that had not been anticipated in the base period of the LOFT study. This was an important step, said Dr. O’Donoghue, because LOFT was not a dedicated cardiovascular safety trial.
The prespecified primary endpoints for TIMI’s safety analysis included new-onset atrial flutter or atrial fibrillation, as well as a composite endpoint of major adverse cardiovascular events (MACE), defined as myocardial infarction, stroke, or cardiovascular death.
Examination of the composite MACE endpoint showed a numeric, but not statistically significant, difference between the odanacatib and placebo arms of the extension study. However, in a preplanned subanalysis, when the 324 stroke events were isolated, a hazard ratio (HR) for stroke of 1.37 for odanacatib emerged (95% confidence interval [CI], 1.10-1.71; P = .005).
Atrial fibrillation and atrial flutter were more common in the odanacatib group, but the difference was not significant (HR, 1.22; 95% CI, 0.99-1.50; P = .06). Dr. O’Donoghue said that the individuals with supraventricular arrhythmias were not the same individuals who had strokes, although the strokes were almost entirely ischemic, rather than hemorrhagic, events.
Dr. O’Donoghue, a cardiovascular medicine specialist at Brigham and Women’s Hospital, noted in her presentation that “preclinical data suggested that inhibition of cathepsin K may reduce atherosclerosis progression and promote plaque stability.”
Odanacatib is a cathepsin K inhibitor, one of a member of a class of proteases. Cathepsin K targets kinins that are involved in bone resorption, but it is expressed in other tissues as well, and it may target other classes of kinins. However, exactly how odanacatib may have contributed to strokes in the study population remains a mystery.
“The mechanistic underpinnings to explain these findings is unknown and warrants investigation,” Dr. O’Donoghue said. “It’s a little bit more unsatisfying when you’re not able to provide a cause. … I share the disappointment of the endocrinologists in the community who were very hopeful for the cathepsin K class to be the next breakthrough in the management of osteoporosis.”
Dr. McClung reported financial relationships with Merck and several other pharmaceutical companies. Dr. O’Donoghue reported receiving grant support from several pharmaceutical companies. The LOFT trial and the TIMI study group analysis were sponsored by Merck.
[email protected]
On Twitter @karioakes
The novel oral osteoporosis drug odanacatib significantly reduced fractures in postmenopausal women but was also associated with a significantly higher risk for stroke, according to data from a 5-year extension of a large phase III clinical trial.
Based on an independent analysis and verification of the risk for stroke, the drug’s sponsor, Merck, has withdrawn odanacatib from review by the Food and Drug Administration. “We are disappointed that the overall benefit-risk profile for odanacatib does not support filing or further development,” Roger M. Perlmutter, MD, president of Merck Research Laboratories, said in a statement.
Compared with placebo, odanacatib resulted in relative risk reductions of 52% for vertebral fracture, 48% for hip fracture, 26% for nonvertebral fracture, and 67% for clinical vertebral fracture (all values, P less than .001). Lumbar spine bone density increased by a mean of 10.9%, as did total hip bone density by a mean of 10.3%, in the odanacatib group (for both, P less than .001), according to results presented at the meeting.
The Long-Term Odanacatib Fracture Trial (LOFT) was a randomized, double-blind placebo-controlled study that investigated the efficacy and safety of odanacatib, a cathepsin K inhibitor, as a treatment for osteoporosis and for fracture prevention in postmenopausal women. Odanacatib was taken as an oral, once-weekly 50-mg pill.
During the base period of the study, 16,071 postmenopausal women aged 65 and older were enrolled, and 12,290 completed the study. Women had to have total hip or femoral neck bone mineral density T scores less than or equal to –2.5, or a radiographically confirmed vertebral fracture and total hip or femoral neck T scores less than or equal to –1.5. Participants were demographically well matched between study arms; 6,092 odanacatib patients and 6,198 on placebo completed the base period of the study.
The original phase III study was stopped early because of robust efficacy data (Osteoporos Int. 2015 Feb;26[2]:699-712). Participants were eligible to continue in a preplanned 5-year double-blind, placebo-controlled extension period of the LOFT study; 3,432 odanacatib and 2,615 placebo participants completed the full 5 years.
An early, but statistically nonsignificant, signal for increased stroke and atrial fibrillation and flutter was seen at the end of the study’s base period. The trend continued and appeared to be amplified during the 5-year, double-blind, placebo-controlled extension period of the LOFT study.
In an interview, Dr. O’Donoghue said that the TIMI study group was brought in by Merck for a “second perspective,” to add rigor to an examination of events that had not been anticipated in the base period of the LOFT study. This was an important step, said Dr. O’Donoghue, because LOFT was not a dedicated cardiovascular safety trial.
The prespecified primary endpoints for TIMI’s safety analysis included new-onset atrial flutter or atrial fibrillation, as well as a composite endpoint of major adverse cardiovascular events (MACE), defined as myocardial infarction, stroke, or cardiovascular death.
Examination of the composite MACE endpoint showed a numeric, but not statistically significant, difference between the odanacatib and placebo arms of the extension study. However, in a preplanned subanalysis, when the 324 stroke events were isolated, a hazard ratio (HR) for stroke of 1.37 for odanacatib emerged (95% confidence interval [CI], 1.10-1.71; P = .005).
Atrial fibrillation and atrial flutter were more common in the odanacatib group, but the difference was not significant (HR, 1.22; 95% CI, 0.99-1.50; P = .06). Dr. O’Donoghue said that the individuals with supraventricular arrhythmias were not the same individuals who had strokes, although the strokes were almost entirely ischemic, rather than hemorrhagic, events.
Dr. O’Donoghue, a cardiovascular medicine specialist at Brigham and Women’s Hospital, noted in her presentation that “preclinical data suggested that inhibition of cathepsin K may reduce atherosclerosis progression and promote plaque stability.”
Odanacatib is a cathepsin K inhibitor, one of a member of a class of proteases. Cathepsin K targets kinins that are involved in bone resorption, but it is expressed in other tissues as well, and it may target other classes of kinins. However, exactly how odanacatib may have contributed to strokes in the study population remains a mystery.
“The mechanistic underpinnings to explain these findings is unknown and warrants investigation,” Dr. O’Donoghue said. “It’s a little bit more unsatisfying when you’re not able to provide a cause. … I share the disappointment of the endocrinologists in the community who were very hopeful for the cathepsin K class to be the next breakthrough in the management of osteoporosis.”
Dr. McClung reported financial relationships with Merck and several other pharmaceutical companies. Dr. O’Donoghue reported receiving grant support from several pharmaceutical companies. The LOFT trial and the TIMI study group analysis were sponsored by Merck.
[email protected]
On Twitter @karioakes
The novel oral osteoporosis drug odanacatib significantly reduced fractures in postmenopausal women but was also associated with a significantly higher risk for stroke, according to data from a 5-year extension of a large phase III clinical trial.
Based on an independent analysis and verification of the risk for stroke, the drug’s sponsor, Merck, has withdrawn odanacatib from review by the Food and Drug Administration. “We are disappointed that the overall benefit-risk profile for odanacatib does not support filing or further development,” Roger M. Perlmutter, MD, president of Merck Research Laboratories, said in a statement.
Compared with placebo, odanacatib resulted in relative risk reductions of 52% for vertebral fracture, 48% for hip fracture, 26% for nonvertebral fracture, and 67% for clinical vertebral fracture (all values, P less than .001). Lumbar spine bone density increased by a mean of 10.9%, as did total hip bone density by a mean of 10.3%, in the odanacatib group (for both, P less than .001), according to results presented at the meeting.
The Long-Term Odanacatib Fracture Trial (LOFT) was a randomized, double-blind placebo-controlled study that investigated the efficacy and safety of odanacatib, a cathepsin K inhibitor, as a treatment for osteoporosis and for fracture prevention in postmenopausal women. Odanacatib was taken as an oral, once-weekly 50-mg pill.
During the base period of the study, 16,071 postmenopausal women aged 65 and older were enrolled, and 12,290 completed the study. Women had to have total hip or femoral neck bone mineral density T scores less than or equal to –2.5, or a radiographically confirmed vertebral fracture and total hip or femoral neck T scores less than or equal to –1.5. Participants were demographically well matched between study arms; 6,092 odanacatib patients and 6,198 on placebo completed the base period of the study.
The original phase III study was stopped early because of robust efficacy data (Osteoporos Int. 2015 Feb;26[2]:699-712). Participants were eligible to continue in a preplanned 5-year double-blind, placebo-controlled extension period of the LOFT study; 3,432 odanacatib and 2,615 placebo participants completed the full 5 years.
An early, but statistically nonsignificant, signal for increased stroke and atrial fibrillation and flutter was seen at the end of the study’s base period. The trend continued and appeared to be amplified during the 5-year, double-blind, placebo-controlled extension period of the LOFT study.
In an interview, Dr. O’Donoghue said that the TIMI study group was brought in by Merck for a “second perspective,” to add rigor to an examination of events that had not been anticipated in the base period of the LOFT study. This was an important step, said Dr. O’Donoghue, because LOFT was not a dedicated cardiovascular safety trial.
The prespecified primary endpoints for TIMI’s safety analysis included new-onset atrial flutter or atrial fibrillation, as well as a composite endpoint of major adverse cardiovascular events (MACE), defined as myocardial infarction, stroke, or cardiovascular death.
Examination of the composite MACE endpoint showed a numeric, but not statistically significant, difference between the odanacatib and placebo arms of the extension study. However, in a preplanned subanalysis, when the 324 stroke events were isolated, a hazard ratio (HR) for stroke of 1.37 for odanacatib emerged (95% confidence interval [CI], 1.10-1.71; P = .005).
Atrial fibrillation and atrial flutter were more common in the odanacatib group, but the difference was not significant (HR, 1.22; 95% CI, 0.99-1.50; P = .06). Dr. O’Donoghue said that the individuals with supraventricular arrhythmias were not the same individuals who had strokes, although the strokes were almost entirely ischemic, rather than hemorrhagic, events.
Dr. O’Donoghue, a cardiovascular medicine specialist at Brigham and Women’s Hospital, noted in her presentation that “preclinical data suggested that inhibition of cathepsin K may reduce atherosclerosis progression and promote plaque stability.”
Odanacatib is a cathepsin K inhibitor, one of a member of a class of proteases. Cathepsin K targets kinins that are involved in bone resorption, but it is expressed in other tissues as well, and it may target other classes of kinins. However, exactly how odanacatib may have contributed to strokes in the study population remains a mystery.
“The mechanistic underpinnings to explain these findings is unknown and warrants investigation,” Dr. O’Donoghue said. “It’s a little bit more unsatisfying when you’re not able to provide a cause. … I share the disappointment of the endocrinologists in the community who were very hopeful for the cathepsin K class to be the next breakthrough in the management of osteoporosis.”
Dr. McClung reported financial relationships with Merck and several other pharmaceutical companies. Dr. O’Donoghue reported receiving grant support from several pharmaceutical companies. The LOFT trial and the TIMI study group analysis were sponsored by Merck.
[email protected]
On Twitter @karioakes
FROM THE NAMS 2016 ANNUAL MEETING
Early menopause a risk factor for type 2 diabetes
“What we see in our data is that indeed, early onset of menopause is associated with increased risk of type 2 diabetes, and this association is independent of potential intermediate risk factors: obesity, insulin, glucose, inflammation, but also of estradiol and other endogenous sex hormone levels,” said Taulant Muka, MD, PhD, in an interview.
Dr. Muka, a postdoctoral fellow at Erasmus Medical College, Rotterdam, the Netherlands, presented the analysis from a large, prospective cohort of menopausal women at the annual meeting of the North American Menopause Society.
In an interview, Dr. Muka noted that the study investigated whether the association between early age at natural menopause (ANM) and type 2 diabetes is independent of intermediate risk factors for type 2 diabetes, such as obesity. Finally, the study also assessed whether endogenous sex hormone levels play a role in the link between early ANM and type 2 diabetes.
Enrollment in the Rotterdam study has been continuing in waves since the 1990s, with enrollment for participants in this particular study cohort occurring in 1997 and with additional cohorts enrolled in 2000-2001 and 2006-2008.
“I think this is the first prospective study with such long follow-up data and with a broad adjustment for confounding factors. Previous studies have been mainly cross-sectional, providing conflicting results,” said Dr. Muka.
Using self-reported age at menopause, the investigators excluded from the study women whose menopausal status was not known, who were actually not menopausal, or whose menopause had not occurred naturally. The study population also excluded women with prevalent type 2 diabetes or for whom no information about diabetes follow-up could be found.
The remaining 3,210 women who were included in the study had a median age of 67 years and had reached menopause at a median of 49.9 years. Most women (82.6%) had an ANM of 45-55 years; ANM for 8.8% was 40-44 years, while just 2.3% had an ANM of under 40 years. Mean body mass index was 27.1 kg/m2.
Participants were considered to have incident diabetes based on several sources: a general practitioner’s records, hospital discharge paperwork, or glucose measurements from visits during the Rotterdam study. Participants also were classified as having diabetes if they used a hypoglycemic medication or had a fasting blood glucose level of at least 7 mmol/L (126 mg/dL) or, in the absence of a fasting blood glucose measurement, a nonfasting blood glucose of at least 11.1 mmol/L (200 mg/dL).
Dr. Muka said he and his coinvestigators identified and adjusted for a large number of variables, using a series of three Cox proportion hazard models.
The first model adjusted for age, which wave of enrollment (cohort) participants were in, hormone therapy status, age at menarche, and the number of pregnancies that reached at least 6 months’ gestation.
The second model used all of the factors in model 1, and added BMI and glucose and insulin levels. The third model used all of the factors in model 2, and also added total cholesterol level, systolic blood pressure, the use of lipid-lowering or antihypertensive medications, alcohol and tobacco use, educational level, prevalent cardiovascular disease, and C-reactive protein levels.
The association of early ANM with the risk of type 2 diabetes was statistically significant in all three models (P less than .001), with very similar hazard ratios (HRs) in all models. For the third, the most comprehensive model, the HR was 1.42 (95% confidence interval, 0.83-2.45).
Extensive sensitivity analyses were carried out, and the association held independent of physical activity level, smoking status, use of hormone therapy, and age. The investigators also used multivariable analyses to ensure that the effect was not mediated by serum levels of thyroid-stimulating hormone, dihydroepiandosterone (DHEA) and DHEA sulfate, estradiol, testosterone, sex hormone–binding globulin (SHBG), or androstenedione.
This study was the first in this population to obtain and adjust for serum sex hormone and SHBG levels, said Dr. Muka.
The prospective design of the study and the long follow-up period were study strengths, said Dr. Muka. Additionally, blood glucose readings taken at study visits, together with electronically linked pharmacy dispensing records, were used for incident diabetes diagnoses.
Study limitations, Dr. Muka said, included the possibility of survival bias, since enrollees “may represent survivors of early menopause who did not develop [type 2 diabetes] or die prior to enrollment.” However, he said, this would mean that “we have underestimated the association, so the risk would be even higher.” Also, all study participants were white, so the results cannot be extrapolated to nonwhite populations.
Dr. Muka said that the largely American audience for his presentation was interested in the fact that the association existed independent of BMI, an obesity marker, based on questions and comments following the talk. “Indeed, we stratified the analysis, and we didn’t find any difference between participants who were obese and nonobese.” Also, he said that there were queries about whether the analyses had corrected for DEXA measurements, in order to assess lean versus fat body composition more accurately. This analysis has not been done, but Dr. Muka plans to complete it on his return to Rotterdam, he said.
Up to 10% of women will reach menopause before the age of 45, said Dr. Muka, so this analysis has the potential to impact primary care for millions of women. “Women who undergo early menopause may be a target for type 2 diabetes prevention measures and might need to be screened for other cardiovascular risk factors like high blood pressure and dyslipidemia, since they are also at risk for cardiovascular disease.”
Dr. Muka reported no outside funding sources and had no relevant financial disclosures.
[email protected]
On Twitter @karioakes
“What we see in our data is that indeed, early onset of menopause is associated with increased risk of type 2 diabetes, and this association is independent of potential intermediate risk factors: obesity, insulin, glucose, inflammation, but also of estradiol and other endogenous sex hormone levels,” said Taulant Muka, MD, PhD, in an interview.
Dr. Muka, a postdoctoral fellow at Erasmus Medical College, Rotterdam, the Netherlands, presented the analysis from a large, prospective cohort of menopausal women at the annual meeting of the North American Menopause Society.
In an interview, Dr. Muka noted that the study investigated whether the association between early age at natural menopause (ANM) and type 2 diabetes is independent of intermediate risk factors for type 2 diabetes, such as obesity. Finally, the study also assessed whether endogenous sex hormone levels play a role in the link between early ANM and type 2 diabetes.
Enrollment in the Rotterdam study has been continuing in waves since the 1990s, with enrollment for participants in this particular study cohort occurring in 1997 and with additional cohorts enrolled in 2000-2001 and 2006-2008.
“I think this is the first prospective study with such long follow-up data and with a broad adjustment for confounding factors. Previous studies have been mainly cross-sectional, providing conflicting results,” said Dr. Muka.
Using self-reported age at menopause, the investigators excluded from the study women whose menopausal status was not known, who were actually not menopausal, or whose menopause had not occurred naturally. The study population also excluded women with prevalent type 2 diabetes or for whom no information about diabetes follow-up could be found.
The remaining 3,210 women who were included in the study had a median age of 67 years and had reached menopause at a median of 49.9 years. Most women (82.6%) had an ANM of 45-55 years; ANM for 8.8% was 40-44 years, while just 2.3% had an ANM of under 40 years. Mean body mass index was 27.1 kg/m2.
Participants were considered to have incident diabetes based on several sources: a general practitioner’s records, hospital discharge paperwork, or glucose measurements from visits during the Rotterdam study. Participants also were classified as having diabetes if they used a hypoglycemic medication or had a fasting blood glucose level of at least 7 mmol/L (126 mg/dL) or, in the absence of a fasting blood glucose measurement, a nonfasting blood glucose of at least 11.1 mmol/L (200 mg/dL).
Dr. Muka said he and his coinvestigators identified and adjusted for a large number of variables, using a series of three Cox proportion hazard models.
The first model adjusted for age, which wave of enrollment (cohort) participants were in, hormone therapy status, age at menarche, and the number of pregnancies that reached at least 6 months’ gestation.
The second model used all of the factors in model 1, and added BMI and glucose and insulin levels. The third model used all of the factors in model 2, and also added total cholesterol level, systolic blood pressure, the use of lipid-lowering or antihypertensive medications, alcohol and tobacco use, educational level, prevalent cardiovascular disease, and C-reactive protein levels.
The association of early ANM with the risk of type 2 diabetes was statistically significant in all three models (P less than .001), with very similar hazard ratios (HRs) in all models. For the third, the most comprehensive model, the HR was 1.42 (95% confidence interval, 0.83-2.45).
Extensive sensitivity analyses were carried out, and the association held independent of physical activity level, smoking status, use of hormone therapy, and age. The investigators also used multivariable analyses to ensure that the effect was not mediated by serum levels of thyroid-stimulating hormone, dihydroepiandosterone (DHEA) and DHEA sulfate, estradiol, testosterone, sex hormone–binding globulin (SHBG), or androstenedione.
This study was the first in this population to obtain and adjust for serum sex hormone and SHBG levels, said Dr. Muka.
The prospective design of the study and the long follow-up period were study strengths, said Dr. Muka. Additionally, blood glucose readings taken at study visits, together with electronically linked pharmacy dispensing records, were used for incident diabetes diagnoses.
Study limitations, Dr. Muka said, included the possibility of survival bias, since enrollees “may represent survivors of early menopause who did not develop [type 2 diabetes] or die prior to enrollment.” However, he said, this would mean that “we have underestimated the association, so the risk would be even higher.” Also, all study participants were white, so the results cannot be extrapolated to nonwhite populations.
Dr. Muka said that the largely American audience for his presentation was interested in the fact that the association existed independent of BMI, an obesity marker, based on questions and comments following the talk. “Indeed, we stratified the analysis, and we didn’t find any difference between participants who were obese and nonobese.” Also, he said that there were queries about whether the analyses had corrected for DEXA measurements, in order to assess lean versus fat body composition more accurately. This analysis has not been done, but Dr. Muka plans to complete it on his return to Rotterdam, he said.
Up to 10% of women will reach menopause before the age of 45, said Dr. Muka, so this analysis has the potential to impact primary care for millions of women. “Women who undergo early menopause may be a target for type 2 diabetes prevention measures and might need to be screened for other cardiovascular risk factors like high blood pressure and dyslipidemia, since they are also at risk for cardiovascular disease.”
Dr. Muka reported no outside funding sources and had no relevant financial disclosures.
[email protected]
On Twitter @karioakes
“What we see in our data is that indeed, early onset of menopause is associated with increased risk of type 2 diabetes, and this association is independent of potential intermediate risk factors: obesity, insulin, glucose, inflammation, but also of estradiol and other endogenous sex hormone levels,” said Taulant Muka, MD, PhD, in an interview.
Dr. Muka, a postdoctoral fellow at Erasmus Medical College, Rotterdam, the Netherlands, presented the analysis from a large, prospective cohort of menopausal women at the annual meeting of the North American Menopause Society.
In an interview, Dr. Muka noted that the study investigated whether the association between early age at natural menopause (ANM) and type 2 diabetes is independent of intermediate risk factors for type 2 diabetes, such as obesity. Finally, the study also assessed whether endogenous sex hormone levels play a role in the link between early ANM and type 2 diabetes.
Enrollment in the Rotterdam study has been continuing in waves since the 1990s, with enrollment for participants in this particular study cohort occurring in 1997 and with additional cohorts enrolled in 2000-2001 and 2006-2008.
“I think this is the first prospective study with such long follow-up data and with a broad adjustment for confounding factors. Previous studies have been mainly cross-sectional, providing conflicting results,” said Dr. Muka.
Using self-reported age at menopause, the investigators excluded from the study women whose menopausal status was not known, who were actually not menopausal, or whose menopause had not occurred naturally. The study population also excluded women with prevalent type 2 diabetes or for whom no information about diabetes follow-up could be found.
The remaining 3,210 women who were included in the study had a median age of 67 years and had reached menopause at a median of 49.9 years. Most women (82.6%) had an ANM of 45-55 years; ANM for 8.8% was 40-44 years, while just 2.3% had an ANM of under 40 years. Mean body mass index was 27.1 kg/m2.
Participants were considered to have incident diabetes based on several sources: a general practitioner’s records, hospital discharge paperwork, or glucose measurements from visits during the Rotterdam study. Participants also were classified as having diabetes if they used a hypoglycemic medication or had a fasting blood glucose level of at least 7 mmol/L (126 mg/dL) or, in the absence of a fasting blood glucose measurement, a nonfasting blood glucose of at least 11.1 mmol/L (200 mg/dL).
Dr. Muka said he and his coinvestigators identified and adjusted for a large number of variables, using a series of three Cox proportion hazard models.
The first model adjusted for age, which wave of enrollment (cohort) participants were in, hormone therapy status, age at menarche, and the number of pregnancies that reached at least 6 months’ gestation.
The second model used all of the factors in model 1, and added BMI and glucose and insulin levels. The third model used all of the factors in model 2, and also added total cholesterol level, systolic blood pressure, the use of lipid-lowering or antihypertensive medications, alcohol and tobacco use, educational level, prevalent cardiovascular disease, and C-reactive protein levels.
The association of early ANM with the risk of type 2 diabetes was statistically significant in all three models (P less than .001), with very similar hazard ratios (HRs) in all models. For the third, the most comprehensive model, the HR was 1.42 (95% confidence interval, 0.83-2.45).
Extensive sensitivity analyses were carried out, and the association held independent of physical activity level, smoking status, use of hormone therapy, and age. The investigators also used multivariable analyses to ensure that the effect was not mediated by serum levels of thyroid-stimulating hormone, dihydroepiandosterone (DHEA) and DHEA sulfate, estradiol, testosterone, sex hormone–binding globulin (SHBG), or androstenedione.
This study was the first in this population to obtain and adjust for serum sex hormone and SHBG levels, said Dr. Muka.
The prospective design of the study and the long follow-up period were study strengths, said Dr. Muka. Additionally, blood glucose readings taken at study visits, together with electronically linked pharmacy dispensing records, were used for incident diabetes diagnoses.
Study limitations, Dr. Muka said, included the possibility of survival bias, since enrollees “may represent survivors of early menopause who did not develop [type 2 diabetes] or die prior to enrollment.” However, he said, this would mean that “we have underestimated the association, so the risk would be even higher.” Also, all study participants were white, so the results cannot be extrapolated to nonwhite populations.
Dr. Muka said that the largely American audience for his presentation was interested in the fact that the association existed independent of BMI, an obesity marker, based on questions and comments following the talk. “Indeed, we stratified the analysis, and we didn’t find any difference between participants who were obese and nonobese.” Also, he said that there were queries about whether the analyses had corrected for DEXA measurements, in order to assess lean versus fat body composition more accurately. This analysis has not been done, but Dr. Muka plans to complete it on his return to Rotterdam, he said.
Up to 10% of women will reach menopause before the age of 45, said Dr. Muka, so this analysis has the potential to impact primary care for millions of women. “Women who undergo early menopause may be a target for type 2 diabetes prevention measures and might need to be screened for other cardiovascular risk factors like high blood pressure and dyslipidemia, since they are also at risk for cardiovascular disease.”
Dr. Muka reported no outside funding sources and had no relevant financial disclosures.
[email protected]
On Twitter @karioakes
Key clinical point: Early menopause is associated with an increased risk for type 2 diabetes.
Major finding: Age at menopause between 40 and 45 was associated with a relative risk of 1.49 for type 2 diabetes.
Data source: A prospective cohort study of an initial cohort of 3,210 menopausal women.
Disclosures: No outside funding source was reported. Dr. Muka reported having no relevant financial conflicts.
Novel fractional laser eased genitourinary syndrome of menopause
DENVER – Novel fractional laser therapy achieved significant 12-month reductions in vaginal dryness, burning, pain, and itching associated with menopause, Eric Sokol, MD, reported at Pelvic Floor Disorders Week, sponsored by the American Urogynecologic Society.
Most patients – 92% – said they were satisfied or very satisfied with the treatment, said Dr. Sokol of Stanford (Calif.) University. “Treatments take 45 seconds and are painless,” he added. “We are part of a group that is planning a larger multicenter randomized trial of this laser, as well as some histologic studies.”
The two-center pilot study included 30 women with vulvovaginal atrophy treated with a novel fractional carbon dioxide laser system called SmartXide2 V2LR (MonaLisa Touch). The system has a maximum power of 60 W and emits laser energy at a 10,600-nm wavelength, Dr. Sokol noted. Patients underwent three treatments spaced by 6 weeks, and used 10-point visual analogue scales to score baseline and subsequent levels of vaginal pain, burning, itching, dryness, dyspareunia, and dysuria.
Average scores on the Female Sexual Function Index rose from 11.3 at baseline to 21.25 at 12 months, a statistically significant improvement.
Laser therapy caused no major adverse events, but about 10% of patients developed slight vaginal discharge or minor spotting after treatment, Dr. Sokol reported. Patients were not allowed to use lubricants or estrogens during the study.
The study was supported by DEKA M.E.L.A. Srl, using an investigator-initiated protocol. Patients did not pay for treatment. Dr. Sokol reported that he has no financial disclosures related to this study. His coinvestigator is a paid consultant for Cynosure.
DENVER – Novel fractional laser therapy achieved significant 12-month reductions in vaginal dryness, burning, pain, and itching associated with menopause, Eric Sokol, MD, reported at Pelvic Floor Disorders Week, sponsored by the American Urogynecologic Society.
Most patients – 92% – said they were satisfied or very satisfied with the treatment, said Dr. Sokol of Stanford (Calif.) University. “Treatments take 45 seconds and are painless,” he added. “We are part of a group that is planning a larger multicenter randomized trial of this laser, as well as some histologic studies.”
The two-center pilot study included 30 women with vulvovaginal atrophy treated with a novel fractional carbon dioxide laser system called SmartXide2 V2LR (MonaLisa Touch). The system has a maximum power of 60 W and emits laser energy at a 10,600-nm wavelength, Dr. Sokol noted. Patients underwent three treatments spaced by 6 weeks, and used 10-point visual analogue scales to score baseline and subsequent levels of vaginal pain, burning, itching, dryness, dyspareunia, and dysuria.
Average scores on the Female Sexual Function Index rose from 11.3 at baseline to 21.25 at 12 months, a statistically significant improvement.
Laser therapy caused no major adverse events, but about 10% of patients developed slight vaginal discharge or minor spotting after treatment, Dr. Sokol reported. Patients were not allowed to use lubricants or estrogens during the study.
The study was supported by DEKA M.E.L.A. Srl, using an investigator-initiated protocol. Patients did not pay for treatment. Dr. Sokol reported that he has no financial disclosures related to this study. His coinvestigator is a paid consultant for Cynosure.
DENVER – Novel fractional laser therapy achieved significant 12-month reductions in vaginal dryness, burning, pain, and itching associated with menopause, Eric Sokol, MD, reported at Pelvic Floor Disorders Week, sponsored by the American Urogynecologic Society.
Most patients – 92% – said they were satisfied or very satisfied with the treatment, said Dr. Sokol of Stanford (Calif.) University. “Treatments take 45 seconds and are painless,” he added. “We are part of a group that is planning a larger multicenter randomized trial of this laser, as well as some histologic studies.”
The two-center pilot study included 30 women with vulvovaginal atrophy treated with a novel fractional carbon dioxide laser system called SmartXide2 V2LR (MonaLisa Touch). The system has a maximum power of 60 W and emits laser energy at a 10,600-nm wavelength, Dr. Sokol noted. Patients underwent three treatments spaced by 6 weeks, and used 10-point visual analogue scales to score baseline and subsequent levels of vaginal pain, burning, itching, dryness, dyspareunia, and dysuria.
Average scores on the Female Sexual Function Index rose from 11.3 at baseline to 21.25 at 12 months, a statistically significant improvement.
Laser therapy caused no major adverse events, but about 10% of patients developed slight vaginal discharge or minor spotting after treatment, Dr. Sokol reported. Patients were not allowed to use lubricants or estrogens during the study.
The study was supported by DEKA M.E.L.A. Srl, using an investigator-initiated protocol. Patients did not pay for treatment. Dr. Sokol reported that he has no financial disclosures related to this study. His coinvestigator is a paid consultant for Cynosure.
Key clinical point:
Major finding: Reductions in dryness and dyspareunia were especially marked, dropping by an average of about 75% and 66%, respectively.
Data source: A two-center pilot study of 30 women with genitourinary syndrome of atrophy (previously known as vaginal atrophy).
Disclosures: The study was supported by DEKA M.E.L.A. Srl, using an investigator-initiated protocol. Patients did not pay for treatment. Dr. Sokol reported that he has no financial disclosures related to this study. His coinvestigator is a paid consultant for Cynosure.
When is it time to stop hormone therapy?
Extended use of systemic hormone therapy represents one area that clinicians commonly encounter. However, because randomized trial data are not available, helping women make decisions regarding long-term use of hormone therapy is controversial. When it is finalized, the 2016 hormone therapy position statement from the North American Menopause Society will address this topic.
Here I’m referring to the patient who likely started systemic hormone therapy when she was a younger, or more recently menopausal, woman (perhaps in her early 50s), but now she’s in her 60s. In my practice, a patient in this age range would likely be on a lower-than-standard dose of hormone therapy than what she began with originally.
And now the question is, Should she continue, or should she stop, hormone therapy? The median duration of bothersome symptoms is about 10 or 11 years, from the best available data – far longer than what many physicians assume.
And risk stratification also becomes relevant. Let’s say the patient is a slender white or Asian woman with a low body mass index , or she has a parent who had a hip fracture. Continuing low-dose systemic hormone therapy in this case, particularly for osteoporosis prevention when vasomotor symptoms are no longer present, might make sense. However, if the patient is obese, and, therefore has a lower risk for osteoporosis, it may be that ongoing use of systemic hormone therapy would not be indicated, and that patient should be encouraged to discontinue at that point.
Also, if a uterus is not present – and we’re talking only about estrogen therapy, given its greater safety profile with long-term use with respect to breast cancer – clinicians and women can be more comfortable with continued use of low-dose systemic hormone therapy for osteoporosis prevention. If a uterus is present, then women making decisions about long-term use of hormone therapy need to be aware of the small, but I believe real, elevated risk of breast cancer. With each office visit and when decisions about refilling prescriptions or continuing hormone therapy are made, this is an important issue to discuss, particularly if there’s an intact uterus. These discussions also need to be documented in the record.
What about the route of estrogen? Age, BMI, and oral estrogen therapy each represent independent risk factors for venous thromboembolism. For older, as well as obese menopausal women, who are candidates for systemic hormone therapy, I prefer transdermal over oral estrogen therapy.
Finally, I counsel women that although vasomotor symptoms/hot flashes improve as women age, the same is not true for genitourinary syndrome of menopause (GSM, also known as genital atrophy). If vaginal dryness, pain with sex, or other manifestations of GSM occur in women tapering their dose of systemic hormone therapy or in women who have discontinued systemic hormone therapy, initiation and long-term use of low-dose vaginal estrogen should be considered.
References
1. Menopause. 2014 Jun;21(6):679-81.
Dr. Kaunitz is a professor and associate chair of the department of obstetrics and gynecology, University of Florida in Jacksonville. He is on the board of trustees of the North American Menopause Society. He reports being a consultant or on the advisory board or review panel of several pharmaceutical companies, and receiving grant support from several pharmaceutical companies. He receives royalties from UpToDate.
Extended use of systemic hormone therapy represents one area that clinicians commonly encounter. However, because randomized trial data are not available, helping women make decisions regarding long-term use of hormone therapy is controversial. When it is finalized, the 2016 hormone therapy position statement from the North American Menopause Society will address this topic.
Here I’m referring to the patient who likely started systemic hormone therapy when she was a younger, or more recently menopausal, woman (perhaps in her early 50s), but now she’s in her 60s. In my practice, a patient in this age range would likely be on a lower-than-standard dose of hormone therapy than what she began with originally.
And now the question is, Should she continue, or should she stop, hormone therapy? The median duration of bothersome symptoms is about 10 or 11 years, from the best available data – far longer than what many physicians assume.
And risk stratification also becomes relevant. Let’s say the patient is a slender white or Asian woman with a low body mass index , or she has a parent who had a hip fracture. Continuing low-dose systemic hormone therapy in this case, particularly for osteoporosis prevention when vasomotor symptoms are no longer present, might make sense. However, if the patient is obese, and, therefore has a lower risk for osteoporosis, it may be that ongoing use of systemic hormone therapy would not be indicated, and that patient should be encouraged to discontinue at that point.
Also, if a uterus is not present – and we’re talking only about estrogen therapy, given its greater safety profile with long-term use with respect to breast cancer – clinicians and women can be more comfortable with continued use of low-dose systemic hormone therapy for osteoporosis prevention. If a uterus is present, then women making decisions about long-term use of hormone therapy need to be aware of the small, but I believe real, elevated risk of breast cancer. With each office visit and when decisions about refilling prescriptions or continuing hormone therapy are made, this is an important issue to discuss, particularly if there’s an intact uterus. These discussions also need to be documented in the record.
What about the route of estrogen? Age, BMI, and oral estrogen therapy each represent independent risk factors for venous thromboembolism. For older, as well as obese menopausal women, who are candidates for systemic hormone therapy, I prefer transdermal over oral estrogen therapy.
Finally, I counsel women that although vasomotor symptoms/hot flashes improve as women age, the same is not true for genitourinary syndrome of menopause (GSM, also known as genital atrophy). If vaginal dryness, pain with sex, or other manifestations of GSM occur in women tapering their dose of systemic hormone therapy or in women who have discontinued systemic hormone therapy, initiation and long-term use of low-dose vaginal estrogen should be considered.
References
1. Menopause. 2014 Jun;21(6):679-81.
Dr. Kaunitz is a professor and associate chair of the department of obstetrics and gynecology, University of Florida in Jacksonville. He is on the board of trustees of the North American Menopause Society. He reports being a consultant or on the advisory board or review panel of several pharmaceutical companies, and receiving grant support from several pharmaceutical companies. He receives royalties from UpToDate.
Extended use of systemic hormone therapy represents one area that clinicians commonly encounter. However, because randomized trial data are not available, helping women make decisions regarding long-term use of hormone therapy is controversial. When it is finalized, the 2016 hormone therapy position statement from the North American Menopause Society will address this topic.
Here I’m referring to the patient who likely started systemic hormone therapy when she was a younger, or more recently menopausal, woman (perhaps in her early 50s), but now she’s in her 60s. In my practice, a patient in this age range would likely be on a lower-than-standard dose of hormone therapy than what she began with originally.
And now the question is, Should she continue, or should she stop, hormone therapy? The median duration of bothersome symptoms is about 10 or 11 years, from the best available data – far longer than what many physicians assume.
And risk stratification also becomes relevant. Let’s say the patient is a slender white or Asian woman with a low body mass index , or she has a parent who had a hip fracture. Continuing low-dose systemic hormone therapy in this case, particularly for osteoporosis prevention when vasomotor symptoms are no longer present, might make sense. However, if the patient is obese, and, therefore has a lower risk for osteoporosis, it may be that ongoing use of systemic hormone therapy would not be indicated, and that patient should be encouraged to discontinue at that point.
Also, if a uterus is not present – and we’re talking only about estrogen therapy, given its greater safety profile with long-term use with respect to breast cancer – clinicians and women can be more comfortable with continued use of low-dose systemic hormone therapy for osteoporosis prevention. If a uterus is present, then women making decisions about long-term use of hormone therapy need to be aware of the small, but I believe real, elevated risk of breast cancer. With each office visit and when decisions about refilling prescriptions or continuing hormone therapy are made, this is an important issue to discuss, particularly if there’s an intact uterus. These discussions also need to be documented in the record.
What about the route of estrogen? Age, BMI, and oral estrogen therapy each represent independent risk factors for venous thromboembolism. For older, as well as obese menopausal women, who are candidates for systemic hormone therapy, I prefer transdermal over oral estrogen therapy.
Finally, I counsel women that although vasomotor symptoms/hot flashes improve as women age, the same is not true for genitourinary syndrome of menopause (GSM, also known as genital atrophy). If vaginal dryness, pain with sex, or other manifestations of GSM occur in women tapering their dose of systemic hormone therapy or in women who have discontinued systemic hormone therapy, initiation and long-term use of low-dose vaginal estrogen should be considered.
References
1. Menopause. 2014 Jun;21(6):679-81.
Dr. Kaunitz is a professor and associate chair of the department of obstetrics and gynecology, University of Florida in Jacksonville. He is on the board of trustees of the North American Menopause Society. He reports being a consultant or on the advisory board or review panel of several pharmaceutical companies, and receiving grant support from several pharmaceutical companies. He receives royalties from UpToDate.
NAMS 2016 hormone therapy position statement
JoAnn Pinkerton, MD, Professor of Obstetrics and Gynecology at the University of Virginia, Executive Director of the North American Menopause Society (NAMS), and OBG Management Board of Editors Member, revealed the 2016 NAMS position statement on hormone therapy (HT) in Orlando, Florida, on Thursday, October 6, at the NAMS 2016 Annual Scientific Meeting.
The process of consensus among the more than 20 menopause experts who authored the 2016 statement was at times a challenge, indicated Pinkerton, given the variance in views on the significance of published clinical trial findings since the Society’s 20121 HT position statement. Over a 9-month period, the experts developed guidelines for clinicians, using levels of evidence to identify strength of the recommendations.
The clearest benefit for HT to treat hot flashes and prevent bone loss was found for women aged younger than 60 years and within 10 years of menopause onset.
According to the 2016 statement presented at NAMS:
Level I US Food and Drug Administration (FDA)-approved indications for HT include:
- as first-line therapy for women with vasomotor symptoms (VMS) of menopause without contraindications
- possible first-line therapy for prevention of bone loss and fracture in postmenopausal women at elevated risk for fracture (primarily for women aged younger than 60 years and within 10 years of menopause onset)
- low-dose vaginal estrogen as first-line treatment for women with isolated genitourinary symptoms caused by menopause (genitourinary syndrome of menopause [GSM]/vulvovaginal atrophy).
Level II FDA-approved indications for HT include:
- at least until age 52 (the median age of menopause onset) for women with early onset menopause (women with hypogonadism, primary ovarian insufficiency, or premature surgical menopause) and no HT contraindications.
Other level II indications, with observational data indicating benefit over risk, for HT include:
- at least until the median age of menopause for women with early onset menopause
- consideration among women with a family history of breast cancer, although family history is one risk among many for breast cancer that should be assessed
- benefit/risk consideration for women with a BRCA gene mutation who have undergone risk-reducing oophorectomy
- consideration of systemic use until the median age of menopause—after appropriate counseling and in the absence of HT contraindications, with longer duration of HT use individualized.
Level III indications for HT include:
- individualized decisions on use after the age of 60. (The position statement authors did not find that the current Beers criteria recommendation to routinely discontinue HT at age 65 was supported by data.)
The 2016 bottom line on HT
Overall, HT has clear benefits for the treatment of VMS and bone loss prevention, according to the presented position statement. These benefits are most favorable among women aged younger than 60 years who are within 10 years of menopause onset and have no contraindications to HT use. Women older than age 60 who initiate HT beyond 10 years of menopause onset appear to have a less favorable benefit-risk ratio because of elevated risks of coronary heart disease, stroke, venous thromboembolism, and dementia.
The risks of HT vary among women depending on the HT type, duration of use, administration route, timing of treatment initiation, and whether a progestogen is needed. (With longer HT use, estrogen therapy is more favorable than estrogen-progestin therapy.) Therefore, HT should be individualized and reevaluated periodically to maximize the benefits as well as minimize the risks of use, according to the position statement.
Nonhormonal therapies for menopausal symptoms
The Society released its position on nonhormonal management of menopause-associated VMS in 2015.2 Based on examination of 340 original research articles and 105 systematic reviews, clinical and research experts categorized therapies as recommended, recommended with caution, and not recommended at this time.
Recommended non-HT to reduce VMS include:
- cognitive-behavioral therapy
- clinical hypnosis
- low-dose salt of paroxetine (FDA approved for menopausal VMS management)
- other SSRIs/SNRIs
- gabapentinoids
- clonidine.
Recommended-with-caution non-HT for VMS include:
- weight loss
- stress reduction (mindfulness based)
- S-equol derivatives of soy isoflavones
- stellate ganglion block.
Not recommended non-HT for VMS due to negative, insufficient, or inconclusive data include:
- cooling techniques
- avoidance of triggers
- exercise
- yoga
- paced respiration
- relaxation
- over-the-counter supplements and herbs
- acupuncture
- calibration of neural oscillations
- chiropractic interventions.
Note that the NAMS 2016 Hormone Therapy Position Statement was presented at the 2016 Annual Scientific Meeting of the North American Menopause Society, but the statement is not yet published.
- North American Menopause Society. The 2012 hormone therapy position statement of: The North American Menopause Society. Menopause. 2012;19(3):257−271.
- Nonhormonal management of menopause-associated vasomotor symptoms: 2015 position statement of The North American Menopause Society. Menopause. 2015;(11):1155−1172.
JoAnn Pinkerton, MD, Professor of Obstetrics and Gynecology at the University of Virginia, Executive Director of the North American Menopause Society (NAMS), and OBG Management Board of Editors Member, revealed the 2016 NAMS position statement on hormone therapy (HT) in Orlando, Florida, on Thursday, October 6, at the NAMS 2016 Annual Scientific Meeting.
The process of consensus among the more than 20 menopause experts who authored the 2016 statement was at times a challenge, indicated Pinkerton, given the variance in views on the significance of published clinical trial findings since the Society’s 20121 HT position statement. Over a 9-month period, the experts developed guidelines for clinicians, using levels of evidence to identify strength of the recommendations.
The clearest benefit for HT to treat hot flashes and prevent bone loss was found for women aged younger than 60 years and within 10 years of menopause onset.
According to the 2016 statement presented at NAMS:
Level I US Food and Drug Administration (FDA)-approved indications for HT include:
- as first-line therapy for women with vasomotor symptoms (VMS) of menopause without contraindications
- possible first-line therapy for prevention of bone loss and fracture in postmenopausal women at elevated risk for fracture (primarily for women aged younger than 60 years and within 10 years of menopause onset)
- low-dose vaginal estrogen as first-line treatment for women with isolated genitourinary symptoms caused by menopause (genitourinary syndrome of menopause [GSM]/vulvovaginal atrophy).
Level II FDA-approved indications for HT include:
- at least until age 52 (the median age of menopause onset) for women with early onset menopause (women with hypogonadism, primary ovarian insufficiency, or premature surgical menopause) and no HT contraindications.
Other level II indications, with observational data indicating benefit over risk, for HT include:
- at least until the median age of menopause for women with early onset menopause
- consideration among women with a family history of breast cancer, although family history is one risk among many for breast cancer that should be assessed
- benefit/risk consideration for women with a BRCA gene mutation who have undergone risk-reducing oophorectomy
- consideration of systemic use until the median age of menopause—after appropriate counseling and in the absence of HT contraindications, with longer duration of HT use individualized.
Level III indications for HT include:
- individualized decisions on use after the age of 60. (The position statement authors did not find that the current Beers criteria recommendation to routinely discontinue HT at age 65 was supported by data.)
The 2016 bottom line on HT
Overall, HT has clear benefits for the treatment of VMS and bone loss prevention, according to the presented position statement. These benefits are most favorable among women aged younger than 60 years who are within 10 years of menopause onset and have no contraindications to HT use. Women older than age 60 who initiate HT beyond 10 years of menopause onset appear to have a less favorable benefit-risk ratio because of elevated risks of coronary heart disease, stroke, venous thromboembolism, and dementia.
The risks of HT vary among women depending on the HT type, duration of use, administration route, timing of treatment initiation, and whether a progestogen is needed. (With longer HT use, estrogen therapy is more favorable than estrogen-progestin therapy.) Therefore, HT should be individualized and reevaluated periodically to maximize the benefits as well as minimize the risks of use, according to the position statement.
Nonhormonal therapies for menopausal symptoms
The Society released its position on nonhormonal management of menopause-associated VMS in 2015.2 Based on examination of 340 original research articles and 105 systematic reviews, clinical and research experts categorized therapies as recommended, recommended with caution, and not recommended at this time.
Recommended non-HT to reduce VMS include:
- cognitive-behavioral therapy
- clinical hypnosis
- low-dose salt of paroxetine (FDA approved for menopausal VMS management)
- other SSRIs/SNRIs
- gabapentinoids
- clonidine.
Recommended-with-caution non-HT for VMS include:
- weight loss
- stress reduction (mindfulness based)
- S-equol derivatives of soy isoflavones
- stellate ganglion block.
Not recommended non-HT for VMS due to negative, insufficient, or inconclusive data include:
- cooling techniques
- avoidance of triggers
- exercise
- yoga
- paced respiration
- relaxation
- over-the-counter supplements and herbs
- acupuncture
- calibration of neural oscillations
- chiropractic interventions.
Note that the NAMS 2016 Hormone Therapy Position Statement was presented at the 2016 Annual Scientific Meeting of the North American Menopause Society, but the statement is not yet published.
JoAnn Pinkerton, MD, Professor of Obstetrics and Gynecology at the University of Virginia, Executive Director of the North American Menopause Society (NAMS), and OBG Management Board of Editors Member, revealed the 2016 NAMS position statement on hormone therapy (HT) in Orlando, Florida, on Thursday, October 6, at the NAMS 2016 Annual Scientific Meeting.
The process of consensus among the more than 20 menopause experts who authored the 2016 statement was at times a challenge, indicated Pinkerton, given the variance in views on the significance of published clinical trial findings since the Society’s 20121 HT position statement. Over a 9-month period, the experts developed guidelines for clinicians, using levels of evidence to identify strength of the recommendations.
The clearest benefit for HT to treat hot flashes and prevent bone loss was found for women aged younger than 60 years and within 10 years of menopause onset.
According to the 2016 statement presented at NAMS:
Level I US Food and Drug Administration (FDA)-approved indications for HT include:
- as first-line therapy for women with vasomotor symptoms (VMS) of menopause without contraindications
- possible first-line therapy for prevention of bone loss and fracture in postmenopausal women at elevated risk for fracture (primarily for women aged younger than 60 years and within 10 years of menopause onset)
- low-dose vaginal estrogen as first-line treatment for women with isolated genitourinary symptoms caused by menopause (genitourinary syndrome of menopause [GSM]/vulvovaginal atrophy).
Level II FDA-approved indications for HT include:
- at least until age 52 (the median age of menopause onset) for women with early onset menopause (women with hypogonadism, primary ovarian insufficiency, or premature surgical menopause) and no HT contraindications.
Other level II indications, with observational data indicating benefit over risk, for HT include:
- at least until the median age of menopause for women with early onset menopause
- consideration among women with a family history of breast cancer, although family history is one risk among many for breast cancer that should be assessed
- benefit/risk consideration for women with a BRCA gene mutation who have undergone risk-reducing oophorectomy
- consideration of systemic use until the median age of menopause—after appropriate counseling and in the absence of HT contraindications, with longer duration of HT use individualized.
Level III indications for HT include:
- individualized decisions on use after the age of 60. (The position statement authors did not find that the current Beers criteria recommendation to routinely discontinue HT at age 65 was supported by data.)
The 2016 bottom line on HT
Overall, HT has clear benefits for the treatment of VMS and bone loss prevention, according to the presented position statement. These benefits are most favorable among women aged younger than 60 years who are within 10 years of menopause onset and have no contraindications to HT use. Women older than age 60 who initiate HT beyond 10 years of menopause onset appear to have a less favorable benefit-risk ratio because of elevated risks of coronary heart disease, stroke, venous thromboembolism, and dementia.
The risks of HT vary among women depending on the HT type, duration of use, administration route, timing of treatment initiation, and whether a progestogen is needed. (With longer HT use, estrogen therapy is more favorable than estrogen-progestin therapy.) Therefore, HT should be individualized and reevaluated periodically to maximize the benefits as well as minimize the risks of use, according to the position statement.
Nonhormonal therapies for menopausal symptoms
The Society released its position on nonhormonal management of menopause-associated VMS in 2015.2 Based on examination of 340 original research articles and 105 systematic reviews, clinical and research experts categorized therapies as recommended, recommended with caution, and not recommended at this time.
Recommended non-HT to reduce VMS include:
- cognitive-behavioral therapy
- clinical hypnosis
- low-dose salt of paroxetine (FDA approved for menopausal VMS management)
- other SSRIs/SNRIs
- gabapentinoids
- clonidine.
Recommended-with-caution non-HT for VMS include:
- weight loss
- stress reduction (mindfulness based)
- S-equol derivatives of soy isoflavones
- stellate ganglion block.
Not recommended non-HT for VMS due to negative, insufficient, or inconclusive data include:
- cooling techniques
- avoidance of triggers
- exercise
- yoga
- paced respiration
- relaxation
- over-the-counter supplements and herbs
- acupuncture
- calibration of neural oscillations
- chiropractic interventions.
Note that the NAMS 2016 Hormone Therapy Position Statement was presented at the 2016 Annual Scientific Meeting of the North American Menopause Society, but the statement is not yet published.
- North American Menopause Society. The 2012 hormone therapy position statement of: The North American Menopause Society. Menopause. 2012;19(3):257−271.
- Nonhormonal management of menopause-associated vasomotor symptoms: 2015 position statement of The North American Menopause Society. Menopause. 2015;(11):1155−1172.
- North American Menopause Society. The 2012 hormone therapy position statement of: The North American Menopause Society. Menopause. 2012;19(3):257−271.
- Nonhormonal management of menopause-associated vasomotor symptoms: 2015 position statement of The North American Menopause Society. Menopause. 2015;(11):1155−1172.
Breast arterial calcifications predict atherosclerotic cardiovascular events
Longitudinal results of a prospective study of women with and without breast arterial calcifications showed that women with the calcifications were significantly more likely to have atherosclerotic cardiovascular events than were women without them.
In a 10-year follow-up of a cohort of women receiving screening mammograms, women who did not have cardiovascular disease at baseline and were positive for breast arterial calcifications (BACs) were three times more likely to develop atherosclerotic cardiovascular disease (ASCVD) as those who were BAC negative at baseline (9.8% vs. 3.3%; P = .001).
These results paralleled those found 5 years previously among the cohort. At that point, 6.3% of the BAC-positive group developed ASCVD, compared with 2.3% of the BAC-negative group (P = .003). At 10 years, “multiple logistic regression analysis found BAC to be strongly associated with ASCVD events, with an odds ratio of 2.29,” senior investigator Peter Schnatz, DO, said in an interview.
Based on these results, Dr. Schnatz and his coinvestigators are suggesting that BACs be routinely reported on mammograms and viewed as a marker for the development of cardiovascular disease.
Presenting the unpublished 10-year findings at the annual meeting of the North American Menopause Society, Dr. Schnatz, the society’s 2015-2016 president, said that the BAC-positive group was also more likely to develop risk factors for ASCVD (86.8% vs. 76.3; P = .01).
These risk factors were age, hypertension, hypercholesterolemia, diabetes, and menopause. The other results were significant after the investigators controlled for age, so the increased risk was not merely caused by the passage of time and the normal aging process.
The prospective study compared age-matched controls with and without BACs to determine whether BACs seen on routine mammography can predict the development of ASCVD, by tracking the presence of BACs and gathering information about ASCVD risk factors and events via patient self-report in an annual questionnaire. The events that were considered ASCVD markers were angina, myocardial infarction, an abnormal angiogram, coronary artery bypass grafting, and stroke.
The initial baseline study upon which the longitudinal prospective studies are based gathered data from 1,919 women with a median age of 56, plus or minus 12.7 years (range, 25-96), to determine whether women with BAC had an increased frequency of cardiovascular disease risk factors, and of ASCVD. Baseline findings showed an independent association of BAC with multiple risk factors and with ASCVD events, even after age was controlled for.
Dr. Schnatz, who is a professor of ob.gyn. and internal medicine at Thomas Jefferson University, Philadelphia, said in his presentation that BACs “appear to be a risk indicator of the presence of ASCVD. More importantly, in this first prospective analysis of BAC as a risk predictor, BAC appears to be a risk predictor for the future development of ASCVD.”
Patients were considered BAC positive if their mammograms showed BAC on at least one of two standard views of either or both breasts. The screening mammograms were read in a uniform fashion by 1 of 21 radiologists, who used identical and well-accepted criteria for BACs. Overall, 268 women (14%) were BAC positive, in line with the 9%-17.5% prevalence reported in the literature, Dr. Schnatz said.
BACs are diffuse calcifications of the arterial tunica media, which are common but often unreported by radiologists who find them on screening mammograms, though they are seen in up to 17.5% of mammograms.
Medial arterial calcifications, such as BACs, are fine-grained deposits seen in small- to medium-sized muscular arteries. Though they have been observed for some time, their significance has been unknown, and they are often seen as part of the normal aging process, Dr. Schnatz said. With the advent of newer mammography technology in the 2000s, much smaller calcifications could be seen, and research into the significance of BACs detected on screening mammogram was revived and refined, he said.
“If BAC has value as a marker for coronary artery disease, then mammograms could be a practical tool for detecting CAD risk in women,” Dr. Schnatz said. “This might contribute to earlier detection of vascular damage, especially important in women at high risk of CAD or with unrecognized heart disease.”
Dr. Schnatz said he plans to incorporate BAC status into validated cardiovascular risk predictors, and see how a prediction model that includes BAC fares.
Dr. Schnatz reported having no relevant financial disclosures.
[email protected]
On Twitter @karioakes
Longitudinal results of a prospective study of women with and without breast arterial calcifications showed that women with the calcifications were significantly more likely to have atherosclerotic cardiovascular events than were women without them.
In a 10-year follow-up of a cohort of women receiving screening mammograms, women who did not have cardiovascular disease at baseline and were positive for breast arterial calcifications (BACs) were three times more likely to develop atherosclerotic cardiovascular disease (ASCVD) as those who were BAC negative at baseline (9.8% vs. 3.3%; P = .001).
These results paralleled those found 5 years previously among the cohort. At that point, 6.3% of the BAC-positive group developed ASCVD, compared with 2.3% of the BAC-negative group (P = .003). At 10 years, “multiple logistic regression analysis found BAC to be strongly associated with ASCVD events, with an odds ratio of 2.29,” senior investigator Peter Schnatz, DO, said in an interview.
Based on these results, Dr. Schnatz and his coinvestigators are suggesting that BACs be routinely reported on mammograms and viewed as a marker for the development of cardiovascular disease.
Presenting the unpublished 10-year findings at the annual meeting of the North American Menopause Society, Dr. Schnatz, the society’s 2015-2016 president, said that the BAC-positive group was also more likely to develop risk factors for ASCVD (86.8% vs. 76.3; P = .01).
These risk factors were age, hypertension, hypercholesterolemia, diabetes, and menopause. The other results were significant after the investigators controlled for age, so the increased risk was not merely caused by the passage of time and the normal aging process.
The prospective study compared age-matched controls with and without BACs to determine whether BACs seen on routine mammography can predict the development of ASCVD, by tracking the presence of BACs and gathering information about ASCVD risk factors and events via patient self-report in an annual questionnaire. The events that were considered ASCVD markers were angina, myocardial infarction, an abnormal angiogram, coronary artery bypass grafting, and stroke.
The initial baseline study upon which the longitudinal prospective studies are based gathered data from 1,919 women with a median age of 56, plus or minus 12.7 years (range, 25-96), to determine whether women with BAC had an increased frequency of cardiovascular disease risk factors, and of ASCVD. Baseline findings showed an independent association of BAC with multiple risk factors and with ASCVD events, even after age was controlled for.
Dr. Schnatz, who is a professor of ob.gyn. and internal medicine at Thomas Jefferson University, Philadelphia, said in his presentation that BACs “appear to be a risk indicator of the presence of ASCVD. More importantly, in this first prospective analysis of BAC as a risk predictor, BAC appears to be a risk predictor for the future development of ASCVD.”
Patients were considered BAC positive if their mammograms showed BAC on at least one of two standard views of either or both breasts. The screening mammograms were read in a uniform fashion by 1 of 21 radiologists, who used identical and well-accepted criteria for BACs. Overall, 268 women (14%) were BAC positive, in line with the 9%-17.5% prevalence reported in the literature, Dr. Schnatz said.
BACs are diffuse calcifications of the arterial tunica media, which are common but often unreported by radiologists who find them on screening mammograms, though they are seen in up to 17.5% of mammograms.
Medial arterial calcifications, such as BACs, are fine-grained deposits seen in small- to medium-sized muscular arteries. Though they have been observed for some time, their significance has been unknown, and they are often seen as part of the normal aging process, Dr. Schnatz said. With the advent of newer mammography technology in the 2000s, much smaller calcifications could be seen, and research into the significance of BACs detected on screening mammogram was revived and refined, he said.
“If BAC has value as a marker for coronary artery disease, then mammograms could be a practical tool for detecting CAD risk in women,” Dr. Schnatz said. “This might contribute to earlier detection of vascular damage, especially important in women at high risk of CAD or with unrecognized heart disease.”
Dr. Schnatz said he plans to incorporate BAC status into validated cardiovascular risk predictors, and see how a prediction model that includes BAC fares.
Dr. Schnatz reported having no relevant financial disclosures.
[email protected]
On Twitter @karioakes
Longitudinal results of a prospective study of women with and without breast arterial calcifications showed that women with the calcifications were significantly more likely to have atherosclerotic cardiovascular events than were women without them.
In a 10-year follow-up of a cohort of women receiving screening mammograms, women who did not have cardiovascular disease at baseline and were positive for breast arterial calcifications (BACs) were three times more likely to develop atherosclerotic cardiovascular disease (ASCVD) as those who were BAC negative at baseline (9.8% vs. 3.3%; P = .001).
These results paralleled those found 5 years previously among the cohort. At that point, 6.3% of the BAC-positive group developed ASCVD, compared with 2.3% of the BAC-negative group (P = .003). At 10 years, “multiple logistic regression analysis found BAC to be strongly associated with ASCVD events, with an odds ratio of 2.29,” senior investigator Peter Schnatz, DO, said in an interview.
Based on these results, Dr. Schnatz and his coinvestigators are suggesting that BACs be routinely reported on mammograms and viewed as a marker for the development of cardiovascular disease.
Presenting the unpublished 10-year findings at the annual meeting of the North American Menopause Society, Dr. Schnatz, the society’s 2015-2016 president, said that the BAC-positive group was also more likely to develop risk factors for ASCVD (86.8% vs. 76.3; P = .01).
These risk factors were age, hypertension, hypercholesterolemia, diabetes, and menopause. The other results were significant after the investigators controlled for age, so the increased risk was not merely caused by the passage of time and the normal aging process.
The prospective study compared age-matched controls with and without BACs to determine whether BACs seen on routine mammography can predict the development of ASCVD, by tracking the presence of BACs and gathering information about ASCVD risk factors and events via patient self-report in an annual questionnaire. The events that were considered ASCVD markers were angina, myocardial infarction, an abnormal angiogram, coronary artery bypass grafting, and stroke.
The initial baseline study upon which the longitudinal prospective studies are based gathered data from 1,919 women with a median age of 56, plus or minus 12.7 years (range, 25-96), to determine whether women with BAC had an increased frequency of cardiovascular disease risk factors, and of ASCVD. Baseline findings showed an independent association of BAC with multiple risk factors and with ASCVD events, even after age was controlled for.
Dr. Schnatz, who is a professor of ob.gyn. and internal medicine at Thomas Jefferson University, Philadelphia, said in his presentation that BACs “appear to be a risk indicator of the presence of ASCVD. More importantly, in this first prospective analysis of BAC as a risk predictor, BAC appears to be a risk predictor for the future development of ASCVD.”
Patients were considered BAC positive if their mammograms showed BAC on at least one of two standard views of either or both breasts. The screening mammograms were read in a uniform fashion by 1 of 21 radiologists, who used identical and well-accepted criteria for BACs. Overall, 268 women (14%) were BAC positive, in line with the 9%-17.5% prevalence reported in the literature, Dr. Schnatz said.
BACs are diffuse calcifications of the arterial tunica media, which are common but often unreported by radiologists who find them on screening mammograms, though they are seen in up to 17.5% of mammograms.
Medial arterial calcifications, such as BACs, are fine-grained deposits seen in small- to medium-sized muscular arteries. Though they have been observed for some time, their significance has been unknown, and they are often seen as part of the normal aging process, Dr. Schnatz said. With the advent of newer mammography technology in the 2000s, much smaller calcifications could be seen, and research into the significance of BACs detected on screening mammogram was revived and refined, he said.
“If BAC has value as a marker for coronary artery disease, then mammograms could be a practical tool for detecting CAD risk in women,” Dr. Schnatz said. “This might contribute to earlier detection of vascular damage, especially important in women at high risk of CAD or with unrecognized heart disease.”
Dr. Schnatz said he plans to incorporate BAC status into validated cardiovascular risk predictors, and see how a prediction model that includes BAC fares.
Dr. Schnatz reported having no relevant financial disclosures.
[email protected]
On Twitter @karioakes
FROM THE NAMS 2016 ANNUAL MEETING
Key clinical point:
Major finding: BAC-positive women were three times more likely to develop ASCVD than were BAC-negative women (9.8% vs. 3.3%; P = .001)
Data source: A prospective longitudinal study of an initial cohort of 1,919 women receiving screening mammograms.
Disclosures: No outside funding source was reported. Dr. Schnatz reported having no relevant financial disclosures.