User login
Precision medicine: A new approach to AML, other blood cancers
The emergence of precision medicine has ushered in a groundbreaking era for the treatment of myeloid malignancies, with the ability to integrate individual molecular data into patient care.
Over the past decade, insights from research focusing on the mutations driving the malignant transformation of myeloid cells have provided the basis for the development of novel targeted therapies.1 With the recent U.S. Food and Drug Administration approval of several novel therapies for different acute myeloid leukemia (AML) indications, the current treatment landscape for AML is evolving rapidly.2
In addition, there has been substantial progress in the development of novel therapeutic strategies for other myeloid neoplasms, with numerous molecularly based therapies in early clinical trials in myeloproliferative neoplasms (MPNs) and myelodysplastic syndromes (MDSs). These advancements have been translated into optimized algorithms for diagnosis, prognostication, and treatment.
AML: Historical perspective
AML comprises a heterogeneous group of blood cell malignancies that require different treatment approaches and confer different prognoses.2 These include acute promyelocytic leukemia (APL) and core binding factor (CBF) AML, both of which have high rates of remission and prolonged survival. The remaining non-APL, non-CBF types can be divided by their cytogenetic-molecular profiles, as well as fitness for intensive chemotherapy. AML can also arise secondary to other myeloid neoplasms, especially after exposure to hypomethylating agents (HMAs), chemotherapy, or irradiation as prior treatment for the primary malignancy.
Historically, anthracycline- and cytarabine-based chemotherapy with or without allogeneic hematopoietic stem-cell transplant (allo-HSCT) was the standard of care in AML treatment with curative intent.1 In the palliative setting, low-dose cytarabine or HMAs were also treatment options. Despite 5 decades of clinical use of these options, researchers have continued to evaluate different dosing schedules of cytosine arabinoside (cytarabine or ara-C) and daunorubicin – the first two agents approved for the treatment of AML – during induction and consolidation treatment phases.
However, recent discoveries have led to the clinical development of targeted agents directed at isocitrate dehydrogenase (IDH), FMS-like tyrosine kinase 3 (FLT3), and BCL2.2 These developments, and the highly anticipated combinations arising from them, continue to challenge traditional treatment approaches, raising the question of whether intensive chemotherapy should remain the optimal standard of care.
Novel therapeutics in AML
Since 2017, several new therapies have been approved for the treatment of AML, including gemtuzumab ozogamicin, two FLT3 inhibitors (gilteritinib and midostaurin), two IDH inhibitors (ivosidenib and enasidenib), a BCL2 inhibitor (venetoclax), an oral HMA agent (azacitidine), a hedgehog inhibitor (glasdegib), and a liposomal formulation of CPX351. In addition, oral decitabine/cedazuridine may be used as an alternative oral HMA in AML, but it is currently the only FDA-approved treatment for chronic myelomonocytic leukemia (CMML) and MDS.2 Because AML subsets are very heterogeneous, an open question remains about how to best integrate these new agents into frontline and salvage combination regimens.
Acute promyelocytic leukemia
APL composes 5%-10% of AML and is characterized by the cytogenetic translocation between chromosomes 15 and 17, which leads to the PML-RAR alpha fusion oncogene and its encoded oncoprotein.2 Two therapies, all-trans retinoic acid (ATRA) and arsenic trioxide, when administered in combination with chemotherapy during induction, have been shown to improve outcomes in APL. At present, the combination of idarubicin and ATRA is the standard-of-care treatment for APL. In addition, patients with high-risk disease have been shown to benefit from the addition of gemtuzumab ozogamicin or anthracyclines.
Core binding factor AML
CBF AML includes patients with the cytogenetic-molecular subsets of inversion 16. Chemotherapy combined with gemtuzumab ozogamicin results in cure rates of 75% or higher and an estimated 5-year survival of 75%. Fludarabine, high-dose cytarabine, and gemtuzumab ozogamicin during induction and consolidation, and an alternative treatment modality (for example, allo-HSCT), for persistent minimal residual disease (MRD) in patients who achieve complete response (CR) is a commonly used regimen. Patients who cannot tolerate this regimen or who have persistent MRD may be treated with an HMA (for instance, decitabine or azacitidine) in combination with venetoclax and gemtuzumab ozogamicin, with the treatment duration adjusted according to MRD status or for 12 months or longer.
Mutations, such as N/KRAS (30%-50%), KIT (25%-30%), and FLT3 (15%-20%), also occur in CBF AML. Targeted agents may also be considered in some cases (for example, dasatinib or avapritinib for KIT mutations; FLT3 inhibitors for FLT3 mutations).
Intensive chemotherapy in younger/fit AML
Several AML regimens have demonstrated better outcomes than the conventional “3 + 7 regimen” (3 days of daunorubicin plus 7 days of cytarabine). Recently, the treatment paradigm has shifted from intensive chemotherapy alone to multidrug combination regimens, including regimens that incorporate targeted therapies, such as FLT3 inhibitors in FLT3-mutated AML, and venetoclax and/or IDH inhibitors as indicated. In addition, the recent FDA approval of oral azacitidine as maintenance therapy for patients in first CR (CR duration, 4 months or less; patients unable to complete the curative intensive chemotherapy) may allow for expanded combination regimens.
Older/unfit patients with AML: Low-intensity therapy
Prior to 2000, the majority of older/unfit patients with AML were offered supportive/palliative treatment. Today, the HMAs azacitidine and decitabine are the most commonly used drugs for the treatment of older/unfit AML. Recently, the FDA approved an oral formulation of decitabine plus oral cedazuridine for the treatment of CMML and MDS. This could provide an opportunity to investigate and develop an effective oral therapy regimen for older/unfit AML, such as oral decitabine/cedazuridine in combination with venetoclax, which may ease administration and improve quality of life for patients in CR post induction in the community setting.
Other studies have shown benefit for combining an HMA with venetoclax in patients with TP53-mutated AML. In addition, triplet regimens may also improve outcomes, with combinations such as HMA plus FLT3 inhibitor (for instance, midostaurin or gilteritinib) with or without venetoclax now being investigated. However, the potential increased risk of myelosuppression also needs to be considered with use of triplet regimens. The results of these and other combinatorial trials are greatly anticipated.
Two oral IDH inhibitors, ivosidenib (IDH1 inhibitor) and enasidenib (IDH2 inhibitor) were recently FDA approved as monotherapy for the treatment of IDH-mutated AML. Combination regimens of IDH inhibitors with chemotherapy are currently being investigated in patients with IDH-mutated AML and appear promising based on preliminary data demonstrating improved response rates and event-free survival.
Other FDA-approved therapies in AML
CPX-351 is a nanoscale liposome with a fixed 5:1 molar ratio of cytarabine and daunorubicin. Results from a phase 3 trial showed that CPX-351 resulted in higher response rates and longer survival compared with 3 + 7 chemotherapy in patients with secondary AML, a subgroup of patients with a very poor prognosis. Additional studies are ongoing, combining CPX-351 with gemtuzumab ozogamicin, venetoclax, and other targeted agents.
Results from a phase 2 trial led to the FDA approval of the hedgehog inhibitor glasdegib when given with low-dose cytarabine. The combination improved survival compared with low-dose cytarabine alone in older/unfit AML and high-risk MDS. However, because of poor survival relative to venetoclax-based combinations, glasdegib is not widely used in clinical practice; other trials exploring combinations with azacitidine and with intensive chemotherapy are ongoing.
Expert perspectives: Future of AML therapy
Amir T. Fathi, MD, associate professor of medicine at Harvard Medical School, Boston, and Farhad Ravandi, MD, professor of medicine at the University of Texas MD Anderson Cancer Center, Houston, are coauthors of a recent review that summarized the current treatment landscape in AML, including areas of evolving research.1
“In the next several years, I am hopeful there will be a series of regulatory approvals of novel, effective agents for myeloid malignancies,” Dr. Fathi explained. “Even if approvals are not as numerous as we’ve seen in AML, any additional effective options would be very welcome.”
Dr. Ravandi also noted that increased understanding of the biology underlying myeloid neoplasms has helped to develop novel therapies.
“As we’ve increased our understanding of the biology of these blood cancers, particularly the mechanisms of leukemogenesis and neoplastic change, we’ve been able to develop more effective therapies in AML,” Dr. Ravandi said.
“In the future, we are likely to see a similar trend in other myeloid neoplasms, such as MDSs and MPNs, as we better understand their underlying pathogenesis,” he further explained.
They both acknowledged that the future treatment paradigm in AML will focus on maximizing the potential of new drug approvals, largely through the development of new combination regimens; however, this could be limited by timely validation and regulatory concerns as the disease has become increasingly segmented into smaller subgroups, each with access to a variety of potentially effective therapies.
Dr. Fathi reported consulting/advisory services for Agios, BMS/Celgene, Astellas, and a variety of other pharmaceutical and biotechnology companies. He also reported receiving research support from Agios, BMS/Celgene, and AbbVie. Dr. Ravandi reported no conflicts of interest.
References
1. Westermann J and Bullinger L. Cancer Biol. 2021 April;S1044-579X(21)00084-5.
2. Kantarjian HM et al. Clin Lymphoma Myeloma Leuk. 2021 Sept;21(9):580-97.
The emergence of precision medicine has ushered in a groundbreaking era for the treatment of myeloid malignancies, with the ability to integrate individual molecular data into patient care.
Over the past decade, insights from research focusing on the mutations driving the malignant transformation of myeloid cells have provided the basis for the development of novel targeted therapies.1 With the recent U.S. Food and Drug Administration approval of several novel therapies for different acute myeloid leukemia (AML) indications, the current treatment landscape for AML is evolving rapidly.2
In addition, there has been substantial progress in the development of novel therapeutic strategies for other myeloid neoplasms, with numerous molecularly based therapies in early clinical trials in myeloproliferative neoplasms (MPNs) and myelodysplastic syndromes (MDSs). These advancements have been translated into optimized algorithms for diagnosis, prognostication, and treatment.
AML: Historical perspective
AML comprises a heterogeneous group of blood cell malignancies that require different treatment approaches and confer different prognoses.2 These include acute promyelocytic leukemia (APL) and core binding factor (CBF) AML, both of which have high rates of remission and prolonged survival. The remaining non-APL, non-CBF types can be divided by their cytogenetic-molecular profiles, as well as fitness for intensive chemotherapy. AML can also arise secondary to other myeloid neoplasms, especially after exposure to hypomethylating agents (HMAs), chemotherapy, or irradiation as prior treatment for the primary malignancy.
Historically, anthracycline- and cytarabine-based chemotherapy with or without allogeneic hematopoietic stem-cell transplant (allo-HSCT) was the standard of care in AML treatment with curative intent.1 In the palliative setting, low-dose cytarabine or HMAs were also treatment options. Despite 5 decades of clinical use of these options, researchers have continued to evaluate different dosing schedules of cytosine arabinoside (cytarabine or ara-C) and daunorubicin – the first two agents approved for the treatment of AML – during induction and consolidation treatment phases.
However, recent discoveries have led to the clinical development of targeted agents directed at isocitrate dehydrogenase (IDH), FMS-like tyrosine kinase 3 (FLT3), and BCL2.2 These developments, and the highly anticipated combinations arising from them, continue to challenge traditional treatment approaches, raising the question of whether intensive chemotherapy should remain the optimal standard of care.
Novel therapeutics in AML
Since 2017, several new therapies have been approved for the treatment of AML, including gemtuzumab ozogamicin, two FLT3 inhibitors (gilteritinib and midostaurin), two IDH inhibitors (ivosidenib and enasidenib), a BCL2 inhibitor (venetoclax), an oral HMA agent (azacitidine), a hedgehog inhibitor (glasdegib), and a liposomal formulation of CPX351. In addition, oral decitabine/cedazuridine may be used as an alternative oral HMA in AML, but it is currently the only FDA-approved treatment for chronic myelomonocytic leukemia (CMML) and MDS.2 Because AML subsets are very heterogeneous, an open question remains about how to best integrate these new agents into frontline and salvage combination regimens.
Acute promyelocytic leukemia
APL composes 5%-10% of AML and is characterized by the cytogenetic translocation between chromosomes 15 and 17, which leads to the PML-RAR alpha fusion oncogene and its encoded oncoprotein.2 Two therapies, all-trans retinoic acid (ATRA) and arsenic trioxide, when administered in combination with chemotherapy during induction, have been shown to improve outcomes in APL. At present, the combination of idarubicin and ATRA is the standard-of-care treatment for APL. In addition, patients with high-risk disease have been shown to benefit from the addition of gemtuzumab ozogamicin or anthracyclines.
Core binding factor AML
CBF AML includes patients with the cytogenetic-molecular subsets of inversion 16. Chemotherapy combined with gemtuzumab ozogamicin results in cure rates of 75% or higher and an estimated 5-year survival of 75%. Fludarabine, high-dose cytarabine, and gemtuzumab ozogamicin during induction and consolidation, and an alternative treatment modality (for example, allo-HSCT), for persistent minimal residual disease (MRD) in patients who achieve complete response (CR) is a commonly used regimen. Patients who cannot tolerate this regimen or who have persistent MRD may be treated with an HMA (for instance, decitabine or azacitidine) in combination with venetoclax and gemtuzumab ozogamicin, with the treatment duration adjusted according to MRD status or for 12 months or longer.
Mutations, such as N/KRAS (30%-50%), KIT (25%-30%), and FLT3 (15%-20%), also occur in CBF AML. Targeted agents may also be considered in some cases (for example, dasatinib or avapritinib for KIT mutations; FLT3 inhibitors for FLT3 mutations).
Intensive chemotherapy in younger/fit AML
Several AML regimens have demonstrated better outcomes than the conventional “3 + 7 regimen” (3 days of daunorubicin plus 7 days of cytarabine). Recently, the treatment paradigm has shifted from intensive chemotherapy alone to multidrug combination regimens, including regimens that incorporate targeted therapies, such as FLT3 inhibitors in FLT3-mutated AML, and venetoclax and/or IDH inhibitors as indicated. In addition, the recent FDA approval of oral azacitidine as maintenance therapy for patients in first CR (CR duration, 4 months or less; patients unable to complete the curative intensive chemotherapy) may allow for expanded combination regimens.
Older/unfit patients with AML: Low-intensity therapy
Prior to 2000, the majority of older/unfit patients with AML were offered supportive/palliative treatment. Today, the HMAs azacitidine and decitabine are the most commonly used drugs for the treatment of older/unfit AML. Recently, the FDA approved an oral formulation of decitabine plus oral cedazuridine for the treatment of CMML and MDS. This could provide an opportunity to investigate and develop an effective oral therapy regimen for older/unfit AML, such as oral decitabine/cedazuridine in combination with venetoclax, which may ease administration and improve quality of life for patients in CR post induction in the community setting.
Other studies have shown benefit for combining an HMA with venetoclax in patients with TP53-mutated AML. In addition, triplet regimens may also improve outcomes, with combinations such as HMA plus FLT3 inhibitor (for instance, midostaurin or gilteritinib) with or without venetoclax now being investigated. However, the potential increased risk of myelosuppression also needs to be considered with use of triplet regimens. The results of these and other combinatorial trials are greatly anticipated.
Two oral IDH inhibitors, ivosidenib (IDH1 inhibitor) and enasidenib (IDH2 inhibitor) were recently FDA approved as monotherapy for the treatment of IDH-mutated AML. Combination regimens of IDH inhibitors with chemotherapy are currently being investigated in patients with IDH-mutated AML and appear promising based on preliminary data demonstrating improved response rates and event-free survival.
Other FDA-approved therapies in AML
CPX-351 is a nanoscale liposome with a fixed 5:1 molar ratio of cytarabine and daunorubicin. Results from a phase 3 trial showed that CPX-351 resulted in higher response rates and longer survival compared with 3 + 7 chemotherapy in patients with secondary AML, a subgroup of patients with a very poor prognosis. Additional studies are ongoing, combining CPX-351 with gemtuzumab ozogamicin, venetoclax, and other targeted agents.
Results from a phase 2 trial led to the FDA approval of the hedgehog inhibitor glasdegib when given with low-dose cytarabine. The combination improved survival compared with low-dose cytarabine alone in older/unfit AML and high-risk MDS. However, because of poor survival relative to venetoclax-based combinations, glasdegib is not widely used in clinical practice; other trials exploring combinations with azacitidine and with intensive chemotherapy are ongoing.
Expert perspectives: Future of AML therapy
Amir T. Fathi, MD, associate professor of medicine at Harvard Medical School, Boston, and Farhad Ravandi, MD, professor of medicine at the University of Texas MD Anderson Cancer Center, Houston, are coauthors of a recent review that summarized the current treatment landscape in AML, including areas of evolving research.1
“In the next several years, I am hopeful there will be a series of regulatory approvals of novel, effective agents for myeloid malignancies,” Dr. Fathi explained. “Even if approvals are not as numerous as we’ve seen in AML, any additional effective options would be very welcome.”
Dr. Ravandi also noted that increased understanding of the biology underlying myeloid neoplasms has helped to develop novel therapies.
“As we’ve increased our understanding of the biology of these blood cancers, particularly the mechanisms of leukemogenesis and neoplastic change, we’ve been able to develop more effective therapies in AML,” Dr. Ravandi said.
“In the future, we are likely to see a similar trend in other myeloid neoplasms, such as MDSs and MPNs, as we better understand their underlying pathogenesis,” he further explained.
They both acknowledged that the future treatment paradigm in AML will focus on maximizing the potential of new drug approvals, largely through the development of new combination regimens; however, this could be limited by timely validation and regulatory concerns as the disease has become increasingly segmented into smaller subgroups, each with access to a variety of potentially effective therapies.
Dr. Fathi reported consulting/advisory services for Agios, BMS/Celgene, Astellas, and a variety of other pharmaceutical and biotechnology companies. He also reported receiving research support from Agios, BMS/Celgene, and AbbVie. Dr. Ravandi reported no conflicts of interest.
References
1. Westermann J and Bullinger L. Cancer Biol. 2021 April;S1044-579X(21)00084-5.
2. Kantarjian HM et al. Clin Lymphoma Myeloma Leuk. 2021 Sept;21(9):580-97.
The emergence of precision medicine has ushered in a groundbreaking era for the treatment of myeloid malignancies, with the ability to integrate individual molecular data into patient care.
Over the past decade, insights from research focusing on the mutations driving the malignant transformation of myeloid cells have provided the basis for the development of novel targeted therapies.1 With the recent U.S. Food and Drug Administration approval of several novel therapies for different acute myeloid leukemia (AML) indications, the current treatment landscape for AML is evolving rapidly.2
In addition, there has been substantial progress in the development of novel therapeutic strategies for other myeloid neoplasms, with numerous molecularly based therapies in early clinical trials in myeloproliferative neoplasms (MPNs) and myelodysplastic syndromes (MDSs). These advancements have been translated into optimized algorithms for diagnosis, prognostication, and treatment.
AML: Historical perspective
AML comprises a heterogeneous group of blood cell malignancies that require different treatment approaches and confer different prognoses.2 These include acute promyelocytic leukemia (APL) and core binding factor (CBF) AML, both of which have high rates of remission and prolonged survival. The remaining non-APL, non-CBF types can be divided by their cytogenetic-molecular profiles, as well as fitness for intensive chemotherapy. AML can also arise secondary to other myeloid neoplasms, especially after exposure to hypomethylating agents (HMAs), chemotherapy, or irradiation as prior treatment for the primary malignancy.
Historically, anthracycline- and cytarabine-based chemotherapy with or without allogeneic hematopoietic stem-cell transplant (allo-HSCT) was the standard of care in AML treatment with curative intent.1 In the palliative setting, low-dose cytarabine or HMAs were also treatment options. Despite 5 decades of clinical use of these options, researchers have continued to evaluate different dosing schedules of cytosine arabinoside (cytarabine or ara-C) and daunorubicin – the first two agents approved for the treatment of AML – during induction and consolidation treatment phases.
However, recent discoveries have led to the clinical development of targeted agents directed at isocitrate dehydrogenase (IDH), FMS-like tyrosine kinase 3 (FLT3), and BCL2.2 These developments, and the highly anticipated combinations arising from them, continue to challenge traditional treatment approaches, raising the question of whether intensive chemotherapy should remain the optimal standard of care.
Novel therapeutics in AML
Since 2017, several new therapies have been approved for the treatment of AML, including gemtuzumab ozogamicin, two FLT3 inhibitors (gilteritinib and midostaurin), two IDH inhibitors (ivosidenib and enasidenib), a BCL2 inhibitor (venetoclax), an oral HMA agent (azacitidine), a hedgehog inhibitor (glasdegib), and a liposomal formulation of CPX351. In addition, oral decitabine/cedazuridine may be used as an alternative oral HMA in AML, but it is currently the only FDA-approved treatment for chronic myelomonocytic leukemia (CMML) and MDS.2 Because AML subsets are very heterogeneous, an open question remains about how to best integrate these new agents into frontline and salvage combination regimens.
Acute promyelocytic leukemia
APL composes 5%-10% of AML and is characterized by the cytogenetic translocation between chromosomes 15 and 17, which leads to the PML-RAR alpha fusion oncogene and its encoded oncoprotein.2 Two therapies, all-trans retinoic acid (ATRA) and arsenic trioxide, when administered in combination with chemotherapy during induction, have been shown to improve outcomes in APL. At present, the combination of idarubicin and ATRA is the standard-of-care treatment for APL. In addition, patients with high-risk disease have been shown to benefit from the addition of gemtuzumab ozogamicin or anthracyclines.
Core binding factor AML
CBF AML includes patients with the cytogenetic-molecular subsets of inversion 16. Chemotherapy combined with gemtuzumab ozogamicin results in cure rates of 75% or higher and an estimated 5-year survival of 75%. Fludarabine, high-dose cytarabine, and gemtuzumab ozogamicin during induction and consolidation, and an alternative treatment modality (for example, allo-HSCT), for persistent minimal residual disease (MRD) in patients who achieve complete response (CR) is a commonly used regimen. Patients who cannot tolerate this regimen or who have persistent MRD may be treated with an HMA (for instance, decitabine or azacitidine) in combination with venetoclax and gemtuzumab ozogamicin, with the treatment duration adjusted according to MRD status or for 12 months or longer.
Mutations, such as N/KRAS (30%-50%), KIT (25%-30%), and FLT3 (15%-20%), also occur in CBF AML. Targeted agents may also be considered in some cases (for example, dasatinib or avapritinib for KIT mutations; FLT3 inhibitors for FLT3 mutations).
Intensive chemotherapy in younger/fit AML
Several AML regimens have demonstrated better outcomes than the conventional “3 + 7 regimen” (3 days of daunorubicin plus 7 days of cytarabine). Recently, the treatment paradigm has shifted from intensive chemotherapy alone to multidrug combination regimens, including regimens that incorporate targeted therapies, such as FLT3 inhibitors in FLT3-mutated AML, and venetoclax and/or IDH inhibitors as indicated. In addition, the recent FDA approval of oral azacitidine as maintenance therapy for patients in first CR (CR duration, 4 months or less; patients unable to complete the curative intensive chemotherapy) may allow for expanded combination regimens.
Older/unfit patients with AML: Low-intensity therapy
Prior to 2000, the majority of older/unfit patients with AML were offered supportive/palliative treatment. Today, the HMAs azacitidine and decitabine are the most commonly used drugs for the treatment of older/unfit AML. Recently, the FDA approved an oral formulation of decitabine plus oral cedazuridine for the treatment of CMML and MDS. This could provide an opportunity to investigate and develop an effective oral therapy regimen for older/unfit AML, such as oral decitabine/cedazuridine in combination with venetoclax, which may ease administration and improve quality of life for patients in CR post induction in the community setting.
Other studies have shown benefit for combining an HMA with venetoclax in patients with TP53-mutated AML. In addition, triplet regimens may also improve outcomes, with combinations such as HMA plus FLT3 inhibitor (for instance, midostaurin or gilteritinib) with or without venetoclax now being investigated. However, the potential increased risk of myelosuppression also needs to be considered with use of triplet regimens. The results of these and other combinatorial trials are greatly anticipated.
Two oral IDH inhibitors, ivosidenib (IDH1 inhibitor) and enasidenib (IDH2 inhibitor) were recently FDA approved as monotherapy for the treatment of IDH-mutated AML. Combination regimens of IDH inhibitors with chemotherapy are currently being investigated in patients with IDH-mutated AML and appear promising based on preliminary data demonstrating improved response rates and event-free survival.
Other FDA-approved therapies in AML
CPX-351 is a nanoscale liposome with a fixed 5:1 molar ratio of cytarabine and daunorubicin. Results from a phase 3 trial showed that CPX-351 resulted in higher response rates and longer survival compared with 3 + 7 chemotherapy in patients with secondary AML, a subgroup of patients with a very poor prognosis. Additional studies are ongoing, combining CPX-351 with gemtuzumab ozogamicin, venetoclax, and other targeted agents.
Results from a phase 2 trial led to the FDA approval of the hedgehog inhibitor glasdegib when given with low-dose cytarabine. The combination improved survival compared with low-dose cytarabine alone in older/unfit AML and high-risk MDS. However, because of poor survival relative to venetoclax-based combinations, glasdegib is not widely used in clinical practice; other trials exploring combinations with azacitidine and with intensive chemotherapy are ongoing.
Expert perspectives: Future of AML therapy
Amir T. Fathi, MD, associate professor of medicine at Harvard Medical School, Boston, and Farhad Ravandi, MD, professor of medicine at the University of Texas MD Anderson Cancer Center, Houston, are coauthors of a recent review that summarized the current treatment landscape in AML, including areas of evolving research.1
“In the next several years, I am hopeful there will be a series of regulatory approvals of novel, effective agents for myeloid malignancies,” Dr. Fathi explained. “Even if approvals are not as numerous as we’ve seen in AML, any additional effective options would be very welcome.”
Dr. Ravandi also noted that increased understanding of the biology underlying myeloid neoplasms has helped to develop novel therapies.
“As we’ve increased our understanding of the biology of these blood cancers, particularly the mechanisms of leukemogenesis and neoplastic change, we’ve been able to develop more effective therapies in AML,” Dr. Ravandi said.
“In the future, we are likely to see a similar trend in other myeloid neoplasms, such as MDSs and MPNs, as we better understand their underlying pathogenesis,” he further explained.
They both acknowledged that the future treatment paradigm in AML will focus on maximizing the potential of new drug approvals, largely through the development of new combination regimens; however, this could be limited by timely validation and regulatory concerns as the disease has become increasingly segmented into smaller subgroups, each with access to a variety of potentially effective therapies.
Dr. Fathi reported consulting/advisory services for Agios, BMS/Celgene, Astellas, and a variety of other pharmaceutical and biotechnology companies. He also reported receiving research support from Agios, BMS/Celgene, and AbbVie. Dr. Ravandi reported no conflicts of interest.
References
1. Westermann J and Bullinger L. Cancer Biol. 2021 April;S1044-579X(21)00084-5.
2. Kantarjian HM et al. Clin Lymphoma Myeloma Leuk. 2021 Sept;21(9):580-97.
Myeloid patients respond robustly to Moderna COVID vaccine
Factors including age, gender, race, disease status, lower-intensity active treatment, baseline neutrophil and lymphocyte counts, and past history of stem cell transplant had no effects on seroconversion in the study, which, despite its small numbers, is one of the largest series to date among patients with myeloid cancers. The findings were reported at the annual meeting of the American Society of Hematology.
COVID vaccination “appears to induce a strong antibody response” in patients with acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS), unlike with B-cell malignancies. “It indicates we should be aggressive about vaccinating such patients,” said senior investigator Jeffrey Lancet, MD, a blood cancer specialist at Moffitt, when he presented the findings at the meeting.
Presentation moderator Laura Michaelis, MD, a hematologist-oncologist at the Medical College of Wisconsin, Milwaukee, agreed.
The “strong antibody response in this group,” coupled with its high risk for severe COVID, “confirm the importance of these patients getting vaccinated,” she said.
Thirty patients with AML and 16 with MDS were included in the review. Most patients were in remission at the time of vaccination, but a third were in active treatment, including six on hypomethylating agents, six on targeted therapies, two on luspatercept, and one on lenalidomide. Thirty-two patients (69.6%) were a median of 17 months past allogeneic stem cell transplant.
Overall, 69.6% of patients developed IgG against spike proteins after the first shot and 95.7% of patients after the second dose, with a large increase in titer levels from the first to the second dose, from a mean of 315 AU/mL to 3,806.5 AU/mL following the second dose.
“Lab and clinical variables did not affect the antibody positivity rate after the second dose,” but patients on steroids and other immunosuppressants seemed less likely to respond to the first shot, Dr. Lancet said.
The study, conducted in early 2021, did not include acutely ill patients or those undergoing cheomotherapy induction and other aggressive treatments, because such patients were not being vaccinated at Moffitt during the study period.
The investigators measured anti-spike IgG by ELISA at baseline, then again about a month after the first shot and a month after the second shot.
Side effects were common and typically mild, including injection site pain, fatigue, headache, and arm swelling. Two patients with AML relapsed after vaccination.
Patients were a median of 68 years old when they were vaccinated; 58.7% were men; and almost all of the subjects were White. The median time from diagnosis to the first shot was 2 years.
The next step in the project is to study the timing of vaccination and response to it among patients on aggressive treatment and to perform neutralizing antibody assays to correlate IgG response with protection from COVID.
No funding was reported for the study. Investigators had numerous industry ties, including Dr. Lancet, a consultant for Celgene/BMS, Millenium Pharma/Takeda, AbbVie, and other firms. Dr. Michaelis didn’t have any disclosures.
[email protected]
Factors including age, gender, race, disease status, lower-intensity active treatment, baseline neutrophil and lymphocyte counts, and past history of stem cell transplant had no effects on seroconversion in the study, which, despite its small numbers, is one of the largest series to date among patients with myeloid cancers. The findings were reported at the annual meeting of the American Society of Hematology.
COVID vaccination “appears to induce a strong antibody response” in patients with acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS), unlike with B-cell malignancies. “It indicates we should be aggressive about vaccinating such patients,” said senior investigator Jeffrey Lancet, MD, a blood cancer specialist at Moffitt, when he presented the findings at the meeting.
Presentation moderator Laura Michaelis, MD, a hematologist-oncologist at the Medical College of Wisconsin, Milwaukee, agreed.
The “strong antibody response in this group,” coupled with its high risk for severe COVID, “confirm the importance of these patients getting vaccinated,” she said.
Thirty patients with AML and 16 with MDS were included in the review. Most patients were in remission at the time of vaccination, but a third were in active treatment, including six on hypomethylating agents, six on targeted therapies, two on luspatercept, and one on lenalidomide. Thirty-two patients (69.6%) were a median of 17 months past allogeneic stem cell transplant.
Overall, 69.6% of patients developed IgG against spike proteins after the first shot and 95.7% of patients after the second dose, with a large increase in titer levels from the first to the second dose, from a mean of 315 AU/mL to 3,806.5 AU/mL following the second dose.
“Lab and clinical variables did not affect the antibody positivity rate after the second dose,” but patients on steroids and other immunosuppressants seemed less likely to respond to the first shot, Dr. Lancet said.
The study, conducted in early 2021, did not include acutely ill patients or those undergoing cheomotherapy induction and other aggressive treatments, because such patients were not being vaccinated at Moffitt during the study period.
The investigators measured anti-spike IgG by ELISA at baseline, then again about a month after the first shot and a month after the second shot.
Side effects were common and typically mild, including injection site pain, fatigue, headache, and arm swelling. Two patients with AML relapsed after vaccination.
Patients were a median of 68 years old when they were vaccinated; 58.7% were men; and almost all of the subjects were White. The median time from diagnosis to the first shot was 2 years.
The next step in the project is to study the timing of vaccination and response to it among patients on aggressive treatment and to perform neutralizing antibody assays to correlate IgG response with protection from COVID.
No funding was reported for the study. Investigators had numerous industry ties, including Dr. Lancet, a consultant for Celgene/BMS, Millenium Pharma/Takeda, AbbVie, and other firms. Dr. Michaelis didn’t have any disclosures.
[email protected]
Factors including age, gender, race, disease status, lower-intensity active treatment, baseline neutrophil and lymphocyte counts, and past history of stem cell transplant had no effects on seroconversion in the study, which, despite its small numbers, is one of the largest series to date among patients with myeloid cancers. The findings were reported at the annual meeting of the American Society of Hematology.
COVID vaccination “appears to induce a strong antibody response” in patients with acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS), unlike with B-cell malignancies. “It indicates we should be aggressive about vaccinating such patients,” said senior investigator Jeffrey Lancet, MD, a blood cancer specialist at Moffitt, when he presented the findings at the meeting.
Presentation moderator Laura Michaelis, MD, a hematologist-oncologist at the Medical College of Wisconsin, Milwaukee, agreed.
The “strong antibody response in this group,” coupled with its high risk for severe COVID, “confirm the importance of these patients getting vaccinated,” she said.
Thirty patients with AML and 16 with MDS were included in the review. Most patients were in remission at the time of vaccination, but a third were in active treatment, including six on hypomethylating agents, six on targeted therapies, two on luspatercept, and one on lenalidomide. Thirty-two patients (69.6%) were a median of 17 months past allogeneic stem cell transplant.
Overall, 69.6% of patients developed IgG against spike proteins after the first shot and 95.7% of patients after the second dose, with a large increase in titer levels from the first to the second dose, from a mean of 315 AU/mL to 3,806.5 AU/mL following the second dose.
“Lab and clinical variables did not affect the antibody positivity rate after the second dose,” but patients on steroids and other immunosuppressants seemed less likely to respond to the first shot, Dr. Lancet said.
The study, conducted in early 2021, did not include acutely ill patients or those undergoing cheomotherapy induction and other aggressive treatments, because such patients were not being vaccinated at Moffitt during the study period.
The investigators measured anti-spike IgG by ELISA at baseline, then again about a month after the first shot and a month after the second shot.
Side effects were common and typically mild, including injection site pain, fatigue, headache, and arm swelling. Two patients with AML relapsed after vaccination.
Patients were a median of 68 years old when they were vaccinated; 58.7% were men; and almost all of the subjects were White. The median time from diagnosis to the first shot was 2 years.
The next step in the project is to study the timing of vaccination and response to it among patients on aggressive treatment and to perform neutralizing antibody assays to correlate IgG response with protection from COVID.
No funding was reported for the study. Investigators had numerous industry ties, including Dr. Lancet, a consultant for Celgene/BMS, Millenium Pharma/Takeda, AbbVie, and other firms. Dr. Michaelis didn’t have any disclosures.
[email protected]
FROM ASH 2021
For leukemias, COVID-19 death risks tied to poor prognoses, ICU deferrals
, results of an American Society of Hematology (ASH) COVID-19 registry study suggest.
Rates of severe COVID-19 were significantly higher among patients who had active disease or neutropenia at the time of their COVID-19 diagnosis. Mortality related to COVID-19 was linked to neutropenia, primary disease prognosis of less than 6 months, and deferral of recommended ICU care, study results show.
By contrast, mortality was not associated with active primary disease or its treatment, according to researcher Pinkal Desai, MD, MPH.
Taken together, these findings provide preliminary evidence to support the use of aggressive supportive treatment of COVID-19 in patients with acute leukemias and myelodysplastic syndromes, said Dr. Desai, a hematologist-oncologist with Weill Cornell Medicine and NewYork-Presbyterian in New York.
“If desired by patients, aggressive support for hospitalized patients with COVID-19 is appropriate, regardless of remission status, given the results of our study,” Dr. Desai said in a press conference during the annual meeting of the American Society of Hematology.
In non-cancer patient populations, advanced age and cytopenias have been associated with mortality related to COVID-19, Dr. Desai said. Likewise, patients with acute leukemias and myelodysplastic syndrome are generally older and have disease- or treatment-related cytopenias, which might affect the severity of and mortality from COVID-19, she added.
With that concern in mind, Dr. Desai and co-investigators looked at predictors of severe COVID-19 disease and death among patients in the ASH Research Collaborative (ASH RC) COVID-19 Registry for Hematology.
This registry was started in the early days of the pandemic to provide real-time observational COVID-19 data to clinicians, according to an ASH news release.
The analysis by Dr. Desai and co-authors included 257 patients with COVID-19 as determined by their physician, including 135 with a primary diagnosis of acute myeloid leukemia, 82 with acute lymphocytic leukemia, and 40 with myelodysplastic syndromes. Sixty percent of the patients were hospitalized due to COVID-19.
At the time of COVID-19 diagnosis, 46% of patients were in remission, and 44% had active disease, according to the report.
Both neutropenia and active disease status at COVID-19 diagnosis were linked to severe COVID-19, defined as ICU admission due to a COVID-19-related reason, according to results of multivariable analysis. Among patients with severe COVID-19, 67% had active disease, meaning just 33% were in remission, Dr. Desai noted.
In multivariable analysis, two factors were significantly associated with mortality, she added: having an estimated pre-COVID-19 prognosis from the primary disease of less than 6 months, and deferral of ICU care when it was recommended to the patient.
Mortality was 21% overall, higher than would be expected in a non-cancer population, Dr. Desai said. For patients with COVID-19 requiring hospitalization, the mortality rate was 34% and for those patients who did go to the ICU, the mortality rate was 68%.
By contrast, there was no significant association between mortality and active disease as compared to disease in remission, Dr. Desai noted in her presentation. Likewise, mortality was not associated with active treatment at the time of COVID-19 diagnosis as compared to no treatment.
Gwen Nichols, MD, executive vice president and chief medical officer of the Leukemia & Lymphoma Society, New York, said those are reassuring data for patients with acute leukemias and myelodysplastic syndromes and their healthcare providers.
“From our point of view, it helps us say, ‘do not stop your treatment because of worries about COVID-19—it’s more important that you treat your cancer,” Dr. Nichols said in an interview. “We now know we can help people through COVID-19, and I think this is just really important data to back that up,” she added.
Dr. Desai provided disclosures related to Agios, Kura Oncology, and Bristol Myers Squibb (consultancy), and to Janssen R&D and Astex (research funding).
, results of an American Society of Hematology (ASH) COVID-19 registry study suggest.
Rates of severe COVID-19 were significantly higher among patients who had active disease or neutropenia at the time of their COVID-19 diagnosis. Mortality related to COVID-19 was linked to neutropenia, primary disease prognosis of less than 6 months, and deferral of recommended ICU care, study results show.
By contrast, mortality was not associated with active primary disease or its treatment, according to researcher Pinkal Desai, MD, MPH.
Taken together, these findings provide preliminary evidence to support the use of aggressive supportive treatment of COVID-19 in patients with acute leukemias and myelodysplastic syndromes, said Dr. Desai, a hematologist-oncologist with Weill Cornell Medicine and NewYork-Presbyterian in New York.
“If desired by patients, aggressive support for hospitalized patients with COVID-19 is appropriate, regardless of remission status, given the results of our study,” Dr. Desai said in a press conference during the annual meeting of the American Society of Hematology.
In non-cancer patient populations, advanced age and cytopenias have been associated with mortality related to COVID-19, Dr. Desai said. Likewise, patients with acute leukemias and myelodysplastic syndrome are generally older and have disease- or treatment-related cytopenias, which might affect the severity of and mortality from COVID-19, she added.
With that concern in mind, Dr. Desai and co-investigators looked at predictors of severe COVID-19 disease and death among patients in the ASH Research Collaborative (ASH RC) COVID-19 Registry for Hematology.
This registry was started in the early days of the pandemic to provide real-time observational COVID-19 data to clinicians, according to an ASH news release.
The analysis by Dr. Desai and co-authors included 257 patients with COVID-19 as determined by their physician, including 135 with a primary diagnosis of acute myeloid leukemia, 82 with acute lymphocytic leukemia, and 40 with myelodysplastic syndromes. Sixty percent of the patients were hospitalized due to COVID-19.
At the time of COVID-19 diagnosis, 46% of patients were in remission, and 44% had active disease, according to the report.
Both neutropenia and active disease status at COVID-19 diagnosis were linked to severe COVID-19, defined as ICU admission due to a COVID-19-related reason, according to results of multivariable analysis. Among patients with severe COVID-19, 67% had active disease, meaning just 33% were in remission, Dr. Desai noted.
In multivariable analysis, two factors were significantly associated with mortality, she added: having an estimated pre-COVID-19 prognosis from the primary disease of less than 6 months, and deferral of ICU care when it was recommended to the patient.
Mortality was 21% overall, higher than would be expected in a non-cancer population, Dr. Desai said. For patients with COVID-19 requiring hospitalization, the mortality rate was 34% and for those patients who did go to the ICU, the mortality rate was 68%.
By contrast, there was no significant association between mortality and active disease as compared to disease in remission, Dr. Desai noted in her presentation. Likewise, mortality was not associated with active treatment at the time of COVID-19 diagnosis as compared to no treatment.
Gwen Nichols, MD, executive vice president and chief medical officer of the Leukemia & Lymphoma Society, New York, said those are reassuring data for patients with acute leukemias and myelodysplastic syndromes and their healthcare providers.
“From our point of view, it helps us say, ‘do not stop your treatment because of worries about COVID-19—it’s more important that you treat your cancer,” Dr. Nichols said in an interview. “We now know we can help people through COVID-19, and I think this is just really important data to back that up,” she added.
Dr. Desai provided disclosures related to Agios, Kura Oncology, and Bristol Myers Squibb (consultancy), and to Janssen R&D and Astex (research funding).
, results of an American Society of Hematology (ASH) COVID-19 registry study suggest.
Rates of severe COVID-19 were significantly higher among patients who had active disease or neutropenia at the time of their COVID-19 diagnosis. Mortality related to COVID-19 was linked to neutropenia, primary disease prognosis of less than 6 months, and deferral of recommended ICU care, study results show.
By contrast, mortality was not associated with active primary disease or its treatment, according to researcher Pinkal Desai, MD, MPH.
Taken together, these findings provide preliminary evidence to support the use of aggressive supportive treatment of COVID-19 in patients with acute leukemias and myelodysplastic syndromes, said Dr. Desai, a hematologist-oncologist with Weill Cornell Medicine and NewYork-Presbyterian in New York.
“If desired by patients, aggressive support for hospitalized patients with COVID-19 is appropriate, regardless of remission status, given the results of our study,” Dr. Desai said in a press conference during the annual meeting of the American Society of Hematology.
In non-cancer patient populations, advanced age and cytopenias have been associated with mortality related to COVID-19, Dr. Desai said. Likewise, patients with acute leukemias and myelodysplastic syndrome are generally older and have disease- or treatment-related cytopenias, which might affect the severity of and mortality from COVID-19, she added.
With that concern in mind, Dr. Desai and co-investigators looked at predictors of severe COVID-19 disease and death among patients in the ASH Research Collaborative (ASH RC) COVID-19 Registry for Hematology.
This registry was started in the early days of the pandemic to provide real-time observational COVID-19 data to clinicians, according to an ASH news release.
The analysis by Dr. Desai and co-authors included 257 patients with COVID-19 as determined by their physician, including 135 with a primary diagnosis of acute myeloid leukemia, 82 with acute lymphocytic leukemia, and 40 with myelodysplastic syndromes. Sixty percent of the patients were hospitalized due to COVID-19.
At the time of COVID-19 diagnosis, 46% of patients were in remission, and 44% had active disease, according to the report.
Both neutropenia and active disease status at COVID-19 diagnosis were linked to severe COVID-19, defined as ICU admission due to a COVID-19-related reason, according to results of multivariable analysis. Among patients with severe COVID-19, 67% had active disease, meaning just 33% were in remission, Dr. Desai noted.
In multivariable analysis, two factors were significantly associated with mortality, she added: having an estimated pre-COVID-19 prognosis from the primary disease of less than 6 months, and deferral of ICU care when it was recommended to the patient.
Mortality was 21% overall, higher than would be expected in a non-cancer population, Dr. Desai said. For patients with COVID-19 requiring hospitalization, the mortality rate was 34% and for those patients who did go to the ICU, the mortality rate was 68%.
By contrast, there was no significant association between mortality and active disease as compared to disease in remission, Dr. Desai noted in her presentation. Likewise, mortality was not associated with active treatment at the time of COVID-19 diagnosis as compared to no treatment.
Gwen Nichols, MD, executive vice president and chief medical officer of the Leukemia & Lymphoma Society, New York, said those are reassuring data for patients with acute leukemias and myelodysplastic syndromes and their healthcare providers.
“From our point of view, it helps us say, ‘do not stop your treatment because of worries about COVID-19—it’s more important that you treat your cancer,” Dr. Nichols said in an interview. “We now know we can help people through COVID-19, and I think this is just really important data to back that up,” she added.
Dr. Desai provided disclosures related to Agios, Kura Oncology, and Bristol Myers Squibb (consultancy), and to Janssen R&D and Astex (research funding).
FROM ASH 2021
Genotype, need for transfusion predict death in VEXAS syndrome
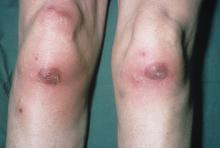
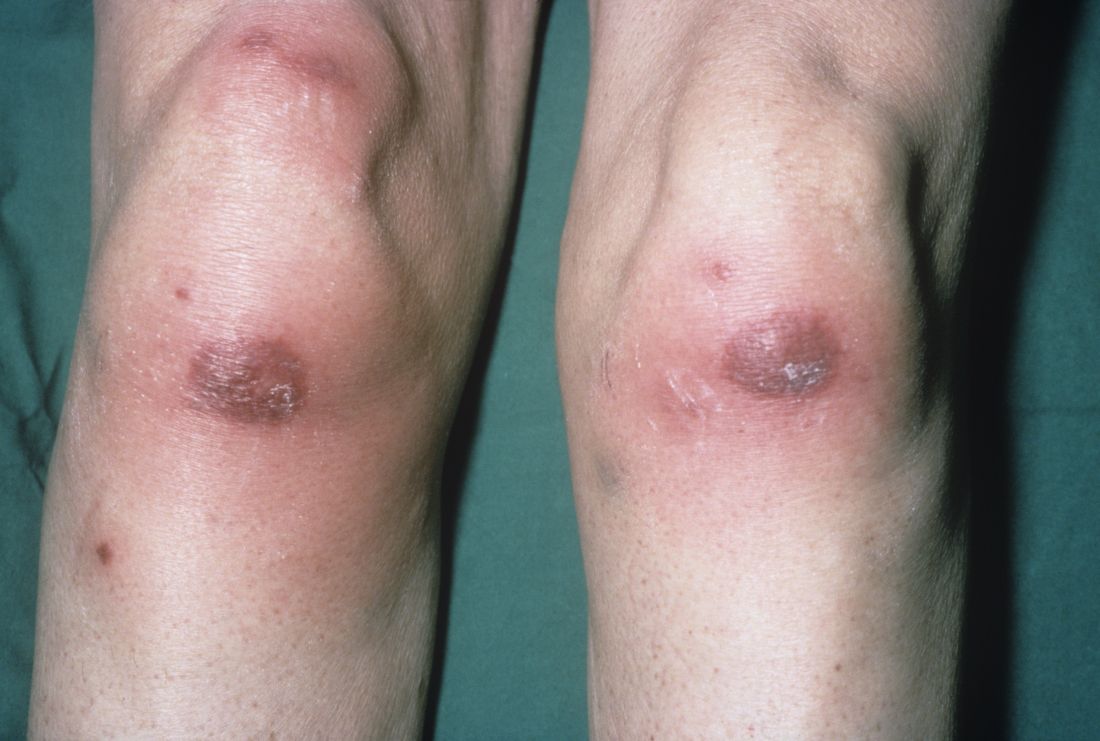
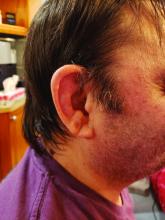
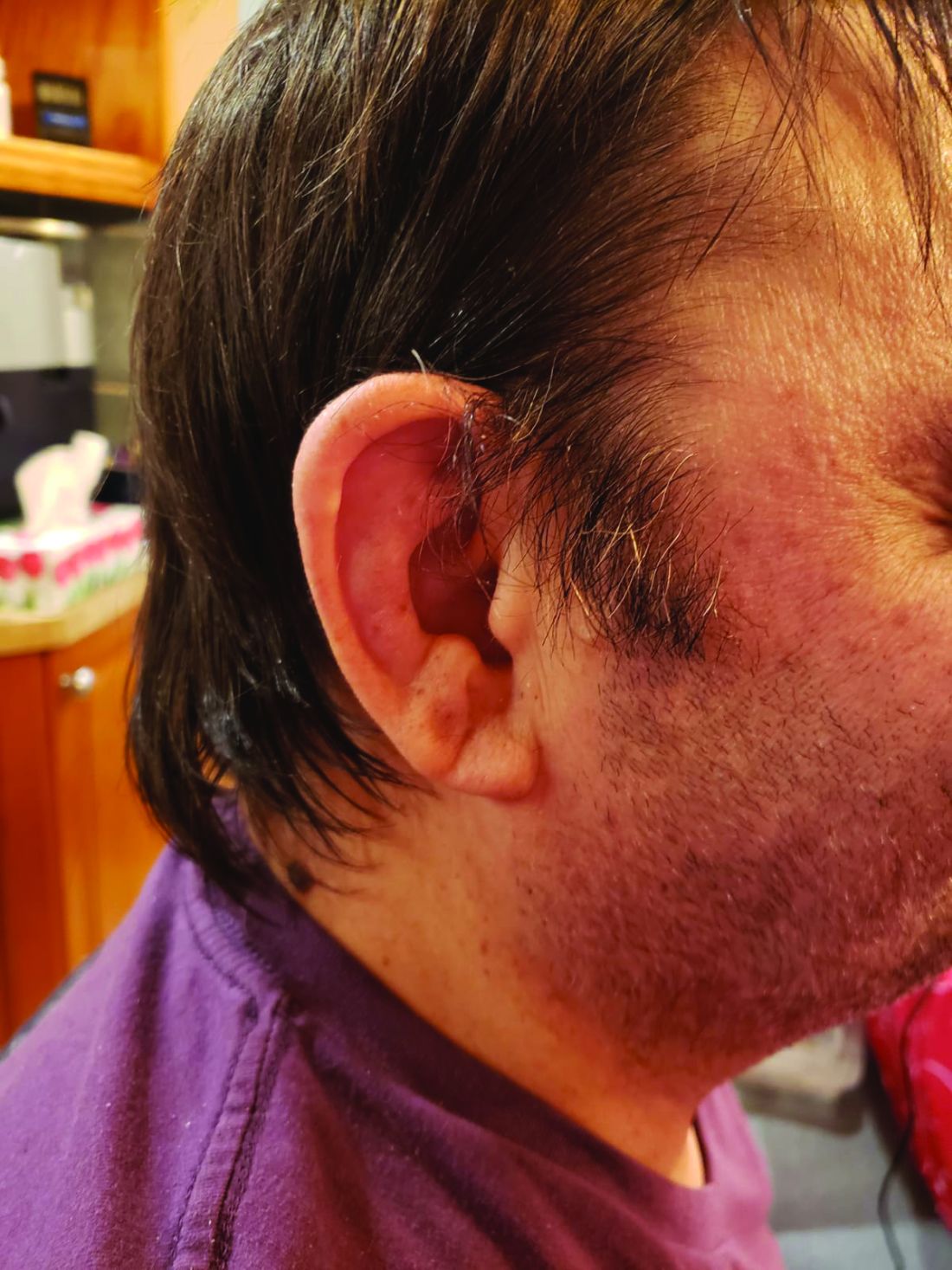
Among patients with the recently defined severe autoinflammatory syndrome VEXAS, those who are transfusion dependent or have a specific amino acid substitution are at highest risk for death, whereas those with ear chondritis are at significantly lower risk, a multinational team of investigators has found.
Their study of mortality and predictors of survival among patients with genetically confirmed VEXAS showed that patients with a VEXAS variant resulting in an amino acid substitution of a methionine for a valine had a 3.5-fold higher risk for death, compared with patients with either a methionine-to-threonine substitution or a methionine-to-leucine swap.
Transfusion dependence was an independent predictor of mortality. Patients who became dependent on transfusions after symptom onset had a nearly threefold higher risk for death, reported Marcela A. Ferrada, MD, a clinical fellow at the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
“These findings should inform risk assessment and clinical management in patients with VEXAS syndrome,” she said in an oral abstract presentation during the virtual annual meeting of the American College of Rheumatology.
“These genetic findings have proven right now to be not only diagnostic, but we have shown that they’re also prognostic, and we hope that this is going to help us identify patients who could have more aggressive treatment,” Dr. Ferrada said.
She also discussed her findings in a media briefing held 2 days prior to her plenary presentation. At that briefing, this news organization asked participating clinicians whether they had patients who they suspected may have had undiagnosed VEXAS.
“My answer to that is interesting,” replied moderator Vaneet Sandhu, MD, from Loma Linda (Calif.) University and Riverside University Health System.
“In the last couple of days, I’ve been reading about VEXAS, and actually texted one of my colleagues yesterday and said, ‘Hey, you know these patients we’ve been seeing who have these strange rashes and chondritis and have maybe a diagnosis of leukocytoclastic vasculitis or something else – are we not diagnosing these patients?’ ” she said.
“I think we are looking at every patient with chondritis and reexamining their phenotype. We had dismissed certain symptoms because they didn’t fit the archetype for relapsing polychondritis, for example, but it could be VEXAS,” said Alfred Kim, MD, PhD, of Washington University in St. Louis, who also presented data during the briefing.
Three variants
VEXAS is caused by somatic mutations in UBA1, a gene that initiates cytoplasmic ubiquitylation, a process by which misfolded proteins are tagged for degradation.
The syndrome’s name is an acronym descriptive of the major features:
- Vacuoles in bone marrow cells.
- E-1 activating enzyme that UBA1 encodes for.
- X-linked.
- Autoinflammatory.
- Somatic mutation featuring hematologic mosaicism.
VEXAS results in rheumatologic, dermatologic, and hematologic symptoms that are often misdiagnosed as being caused by treatment-refractory relapsing polychondritis, polyarteritis nodosa, Sweet syndrome, giant cell arteritis, or myelodysplastic syndrome (MDS).
VEXAS was identified as a distinct syndrome within the past year by Dr. Ferrada and other investigators at NIAMS, the National Human Genome Research Institute, and other institutions.
In the study reported at ACR 2021, Dr. Ferrada and colleagues assessed 83 men who had been referred for genetic testing for VEXAS at the National Institutes of Health, in Bethesda, Md., and at Leeds (England) Teaching Hospitals NHS Trust.
All patients were confirmed to have VEXAS-defining genetic mutations in UBA1 by Sanger sequencing of peripheral blood samples. Only those patients with mutations at codon p.Met41 were included in the investigators’ analysis. Mutations at that site account for nearly all cases of VEXAS that have been identified to date.
The most common clinical manifestation of VEXAS was skin involvement, which occurred in all but one of the 83 patients. Other common manifestations included arthritis (58 patients), pulmonary infiltrates (57 patients), and ear chondritis (54 patients).
Fifteen patients were found to have the leucine variant, 18 had the valine variant, and 50 had the threonine variant. The median age at disease onset was 66 years in the leucine and threonine variant groups and 65 in the valine variant group.
The clinical diagnosis differed according to genotype: 4 of 18 patients (22%) with the valine variant were diagnosed with relapsing polychondritis, compared with 8 of 15 (53%) with the leucine variant and 31 of 50 (62%) with the threonine variant (P = .01).
In contrast, 55% of patients with valine genotype were diagnosed with undifferentiated fever, compared with 6% of those with the leucine and 16% with the threonine genotypes (P = .001). More patients with the leucine variant (60%) were diagnosed with Sweet syndrome, compared with 11% and 14% of patients with the valine and threonine variants, respectively (P = .001).
There was no significant difference among the three genotypes in the percentage of patients diagnosed with MDS.
The follow-up period ranged from 1 to 18 years (median, 4.7 years). The median survival time from disease onset for all patients was 10 years.
Among patients with the valine variant, median survival was 9 years, which was significantly less than among patients with the other two variants (P = .01).
In univariable analysis, independent predictors of mortality were ear chondritis (hazard ratio, 0.26; P = .005), transfusion dependence, a time-dependent variable (HR, 2.59; P = .03), and the valine variant (HR, 3.5; P = .008).
The association between VEXAS genotype and phenotype could be explained by the finding that, among patients with the valine variant, there was significantly less translation of the catalytically proficient UBA1b isoform than in patients with the other two variants, Dr. Ferrada said.
Therapeutic options
Dr. Ferrada noted that to date no drugs have been shown to provide consistent therapeutic benefits for patients with VEXAS, but evidence as to the etiology of the syndrome points to possible treatment approaches.
“All of these findings I think are extremely important to help us guide management of these patients, as we know that the mutation is located in the stem cells in the bone marrow. So we suspect that doing a bone marrow transplant in these patients is going to be curative,” Dr. Ferrada said during the briefing.
Investigators are planning a phase 2 trial of allogeneic hematopoietic stem cell transplant for patients with VEXAS.
The study was supported by the National Institutes of Health. Dr. Ferrada, Dr. Sandhu, and Dr. Kim have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Among patients with the recently defined severe autoinflammatory syndrome VEXAS, those who are transfusion dependent or have a specific amino acid substitution are at highest risk for death, whereas those with ear chondritis are at significantly lower risk, a multinational team of investigators has found.
Their study of mortality and predictors of survival among patients with genetically confirmed VEXAS showed that patients with a VEXAS variant resulting in an amino acid substitution of a methionine for a valine had a 3.5-fold higher risk for death, compared with patients with either a methionine-to-threonine substitution or a methionine-to-leucine swap.
Transfusion dependence was an independent predictor of mortality. Patients who became dependent on transfusions after symptom onset had a nearly threefold higher risk for death, reported Marcela A. Ferrada, MD, a clinical fellow at the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
“These findings should inform risk assessment and clinical management in patients with VEXAS syndrome,” she said in an oral abstract presentation during the virtual annual meeting of the American College of Rheumatology.
“These genetic findings have proven right now to be not only diagnostic, but we have shown that they’re also prognostic, and we hope that this is going to help us identify patients who could have more aggressive treatment,” Dr. Ferrada said.
She also discussed her findings in a media briefing held 2 days prior to her plenary presentation. At that briefing, this news organization asked participating clinicians whether they had patients who they suspected may have had undiagnosed VEXAS.
“My answer to that is interesting,” replied moderator Vaneet Sandhu, MD, from Loma Linda (Calif.) University and Riverside University Health System.
“In the last couple of days, I’ve been reading about VEXAS, and actually texted one of my colleagues yesterday and said, ‘Hey, you know these patients we’ve been seeing who have these strange rashes and chondritis and have maybe a diagnosis of leukocytoclastic vasculitis or something else – are we not diagnosing these patients?’ ” she said.
“I think we are looking at every patient with chondritis and reexamining their phenotype. We had dismissed certain symptoms because they didn’t fit the archetype for relapsing polychondritis, for example, but it could be VEXAS,” said Alfred Kim, MD, PhD, of Washington University in St. Louis, who also presented data during the briefing.
Three variants
VEXAS is caused by somatic mutations in UBA1, a gene that initiates cytoplasmic ubiquitylation, a process by which misfolded proteins are tagged for degradation.
The syndrome’s name is an acronym descriptive of the major features:
- Vacuoles in bone marrow cells.
- E-1 activating enzyme that UBA1 encodes for.
- X-linked.
- Autoinflammatory.
- Somatic mutation featuring hematologic mosaicism.
VEXAS results in rheumatologic, dermatologic, and hematologic symptoms that are often misdiagnosed as being caused by treatment-refractory relapsing polychondritis, polyarteritis nodosa, Sweet syndrome, giant cell arteritis, or myelodysplastic syndrome (MDS).
VEXAS was identified as a distinct syndrome within the past year by Dr. Ferrada and other investigators at NIAMS, the National Human Genome Research Institute, and other institutions.
In the study reported at ACR 2021, Dr. Ferrada and colleagues assessed 83 men who had been referred for genetic testing for VEXAS at the National Institutes of Health, in Bethesda, Md., and at Leeds (England) Teaching Hospitals NHS Trust.
All patients were confirmed to have VEXAS-defining genetic mutations in UBA1 by Sanger sequencing of peripheral blood samples. Only those patients with mutations at codon p.Met41 were included in the investigators’ analysis. Mutations at that site account for nearly all cases of VEXAS that have been identified to date.
The most common clinical manifestation of VEXAS was skin involvement, which occurred in all but one of the 83 patients. Other common manifestations included arthritis (58 patients), pulmonary infiltrates (57 patients), and ear chondritis (54 patients).
Fifteen patients were found to have the leucine variant, 18 had the valine variant, and 50 had the threonine variant. The median age at disease onset was 66 years in the leucine and threonine variant groups and 65 in the valine variant group.
The clinical diagnosis differed according to genotype: 4 of 18 patients (22%) with the valine variant were diagnosed with relapsing polychondritis, compared with 8 of 15 (53%) with the leucine variant and 31 of 50 (62%) with the threonine variant (P = .01).
In contrast, 55% of patients with valine genotype were diagnosed with undifferentiated fever, compared with 6% of those with the leucine and 16% with the threonine genotypes (P = .001). More patients with the leucine variant (60%) were diagnosed with Sweet syndrome, compared with 11% and 14% of patients with the valine and threonine variants, respectively (P = .001).
There was no significant difference among the three genotypes in the percentage of patients diagnosed with MDS.
The follow-up period ranged from 1 to 18 years (median, 4.7 years). The median survival time from disease onset for all patients was 10 years.
Among patients with the valine variant, median survival was 9 years, which was significantly less than among patients with the other two variants (P = .01).
In univariable analysis, independent predictors of mortality were ear chondritis (hazard ratio, 0.26; P = .005), transfusion dependence, a time-dependent variable (HR, 2.59; P = .03), and the valine variant (HR, 3.5; P = .008).
The association between VEXAS genotype and phenotype could be explained by the finding that, among patients with the valine variant, there was significantly less translation of the catalytically proficient UBA1b isoform than in patients with the other two variants, Dr. Ferrada said.
Therapeutic options
Dr. Ferrada noted that to date no drugs have been shown to provide consistent therapeutic benefits for patients with VEXAS, but evidence as to the etiology of the syndrome points to possible treatment approaches.
“All of these findings I think are extremely important to help us guide management of these patients, as we know that the mutation is located in the stem cells in the bone marrow. So we suspect that doing a bone marrow transplant in these patients is going to be curative,” Dr. Ferrada said during the briefing.
Investigators are planning a phase 2 trial of allogeneic hematopoietic stem cell transplant for patients with VEXAS.
The study was supported by the National Institutes of Health. Dr. Ferrada, Dr. Sandhu, and Dr. Kim have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Among patients with the recently defined severe autoinflammatory syndrome VEXAS, those who are transfusion dependent or have a specific amino acid substitution are at highest risk for death, whereas those with ear chondritis are at significantly lower risk, a multinational team of investigators has found.
Their study of mortality and predictors of survival among patients with genetically confirmed VEXAS showed that patients with a VEXAS variant resulting in an amino acid substitution of a methionine for a valine had a 3.5-fold higher risk for death, compared with patients with either a methionine-to-threonine substitution or a methionine-to-leucine swap.
Transfusion dependence was an independent predictor of mortality. Patients who became dependent on transfusions after symptom onset had a nearly threefold higher risk for death, reported Marcela A. Ferrada, MD, a clinical fellow at the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
“These findings should inform risk assessment and clinical management in patients with VEXAS syndrome,” she said in an oral abstract presentation during the virtual annual meeting of the American College of Rheumatology.
“These genetic findings have proven right now to be not only diagnostic, but we have shown that they’re also prognostic, and we hope that this is going to help us identify patients who could have more aggressive treatment,” Dr. Ferrada said.
She also discussed her findings in a media briefing held 2 days prior to her plenary presentation. At that briefing, this news organization asked participating clinicians whether they had patients who they suspected may have had undiagnosed VEXAS.
“My answer to that is interesting,” replied moderator Vaneet Sandhu, MD, from Loma Linda (Calif.) University and Riverside University Health System.
“In the last couple of days, I’ve been reading about VEXAS, and actually texted one of my colleagues yesterday and said, ‘Hey, you know these patients we’ve been seeing who have these strange rashes and chondritis and have maybe a diagnosis of leukocytoclastic vasculitis or something else – are we not diagnosing these patients?’ ” she said.
“I think we are looking at every patient with chondritis and reexamining their phenotype. We had dismissed certain symptoms because they didn’t fit the archetype for relapsing polychondritis, for example, but it could be VEXAS,” said Alfred Kim, MD, PhD, of Washington University in St. Louis, who also presented data during the briefing.
Three variants
VEXAS is caused by somatic mutations in UBA1, a gene that initiates cytoplasmic ubiquitylation, a process by which misfolded proteins are tagged for degradation.
The syndrome’s name is an acronym descriptive of the major features:
- Vacuoles in bone marrow cells.
- E-1 activating enzyme that UBA1 encodes for.
- X-linked.
- Autoinflammatory.
- Somatic mutation featuring hematologic mosaicism.
VEXAS results in rheumatologic, dermatologic, and hematologic symptoms that are often misdiagnosed as being caused by treatment-refractory relapsing polychondritis, polyarteritis nodosa, Sweet syndrome, giant cell arteritis, or myelodysplastic syndrome (MDS).
VEXAS was identified as a distinct syndrome within the past year by Dr. Ferrada and other investigators at NIAMS, the National Human Genome Research Institute, and other institutions.
In the study reported at ACR 2021, Dr. Ferrada and colleagues assessed 83 men who had been referred for genetic testing for VEXAS at the National Institutes of Health, in Bethesda, Md., and at Leeds (England) Teaching Hospitals NHS Trust.
All patients were confirmed to have VEXAS-defining genetic mutations in UBA1 by Sanger sequencing of peripheral blood samples. Only those patients with mutations at codon p.Met41 were included in the investigators’ analysis. Mutations at that site account for nearly all cases of VEXAS that have been identified to date.
The most common clinical manifestation of VEXAS was skin involvement, which occurred in all but one of the 83 patients. Other common manifestations included arthritis (58 patients), pulmonary infiltrates (57 patients), and ear chondritis (54 patients).
Fifteen patients were found to have the leucine variant, 18 had the valine variant, and 50 had the threonine variant. The median age at disease onset was 66 years in the leucine and threonine variant groups and 65 in the valine variant group.
The clinical diagnosis differed according to genotype: 4 of 18 patients (22%) with the valine variant were diagnosed with relapsing polychondritis, compared with 8 of 15 (53%) with the leucine variant and 31 of 50 (62%) with the threonine variant (P = .01).
In contrast, 55% of patients with valine genotype were diagnosed with undifferentiated fever, compared with 6% of those with the leucine and 16% with the threonine genotypes (P = .001). More patients with the leucine variant (60%) were diagnosed with Sweet syndrome, compared with 11% and 14% of patients with the valine and threonine variants, respectively (P = .001).
There was no significant difference among the three genotypes in the percentage of patients diagnosed with MDS.
The follow-up period ranged from 1 to 18 years (median, 4.7 years). The median survival time from disease onset for all patients was 10 years.
Among patients with the valine variant, median survival was 9 years, which was significantly less than among patients with the other two variants (P = .01).
In univariable analysis, independent predictors of mortality were ear chondritis (hazard ratio, 0.26; P = .005), transfusion dependence, a time-dependent variable (HR, 2.59; P = .03), and the valine variant (HR, 3.5; P = .008).
The association between VEXAS genotype and phenotype could be explained by the finding that, among patients with the valine variant, there was significantly less translation of the catalytically proficient UBA1b isoform than in patients with the other two variants, Dr. Ferrada said.
Therapeutic options
Dr. Ferrada noted that to date no drugs have been shown to provide consistent therapeutic benefits for patients with VEXAS, but evidence as to the etiology of the syndrome points to possible treatment approaches.
“All of these findings I think are extremely important to help us guide management of these patients, as we know that the mutation is located in the stem cells in the bone marrow. So we suspect that doing a bone marrow transplant in these patients is going to be curative,” Dr. Ferrada said during the briefing.
Investigators are planning a phase 2 trial of allogeneic hematopoietic stem cell transplant for patients with VEXAS.
The study was supported by the National Institutes of Health. Dr. Ferrada, Dr. Sandhu, and Dr. Kim have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ACR 2021
MDS: Elevated mature monocytes in bone marrow can supplement IPSS-R as a prognostic indicator
Key clinical point: Increased percentage of mature monocyte in bone marrow (PMMBM) may assist the Revised International Prognostic Scoring System (IPSS-R) to predict poor prognosis in patients with myelodysplastic syndromes (MDS).
Major finding: Elevated (>6%) vs. normal PMMBM was associated with shorter overall survival (24 months vs. 37 months; P = .026) along with higher risk distribution in terms of IPSS-R (P = .025) and higher frequency of IDH2 mutation (P = .007).
Study details: The data come from a retrospective analysis of 216 MDS patients, categorized into elevated and normal PMMBM groups.
Disclosures: The study was supported by the Zhejiang Provincial Natural Science Foundation of China, Medical and Health Science and Technology Projects of Zhejiang Province, National Science Foundation of Ningbo, and Chinese Medicine Science and Technology Plan Project of Zhejiang Province. The authors declared no conflicts of interest.
Source: Wu A et al. BMC Cancer. 2021 May 13. doi: 10.1186/s12885-021-08303-8.
Key clinical point: Increased percentage of mature monocyte in bone marrow (PMMBM) may assist the Revised International Prognostic Scoring System (IPSS-R) to predict poor prognosis in patients with myelodysplastic syndromes (MDS).
Major finding: Elevated (>6%) vs. normal PMMBM was associated with shorter overall survival (24 months vs. 37 months; P = .026) along with higher risk distribution in terms of IPSS-R (P = .025) and higher frequency of IDH2 mutation (P = .007).
Study details: The data come from a retrospective analysis of 216 MDS patients, categorized into elevated and normal PMMBM groups.
Disclosures: The study was supported by the Zhejiang Provincial Natural Science Foundation of China, Medical and Health Science and Technology Projects of Zhejiang Province, National Science Foundation of Ningbo, and Chinese Medicine Science and Technology Plan Project of Zhejiang Province. The authors declared no conflicts of interest.
Source: Wu A et al. BMC Cancer. 2021 May 13. doi: 10.1186/s12885-021-08303-8.
Key clinical point: Increased percentage of mature monocyte in bone marrow (PMMBM) may assist the Revised International Prognostic Scoring System (IPSS-R) to predict poor prognosis in patients with myelodysplastic syndromes (MDS).
Major finding: Elevated (>6%) vs. normal PMMBM was associated with shorter overall survival (24 months vs. 37 months; P = .026) along with higher risk distribution in terms of IPSS-R (P = .025) and higher frequency of IDH2 mutation (P = .007).
Study details: The data come from a retrospective analysis of 216 MDS patients, categorized into elevated and normal PMMBM groups.
Disclosures: The study was supported by the Zhejiang Provincial Natural Science Foundation of China, Medical and Health Science and Technology Projects of Zhejiang Province, National Science Foundation of Ningbo, and Chinese Medicine Science and Technology Plan Project of Zhejiang Province. The authors declared no conflicts of interest.
Source: Wu A et al. BMC Cancer. 2021 May 13. doi: 10.1186/s12885-021-08303-8.
MDS: Antibiotics can be stopped after 3 days in patients with febrile neutropenia after chemotherapy
Key clinical point: During remission induction chemotherapy in patients with myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML), antibiotics can be safely stopped after 3 days of febrile neutropenia in the absence of infection.
Major finding: Serious medical complication (SMC) was seen in 12.5% of patients receiving the 3-day empirical broad-spectrum antibiotic therapy (EBAT) vs. 8.9% of patients receiving the prolonged regimen (P = .17). After adjustment for confounders, there was no significant difference between both strategies in the number of SMCs (hazard ratio, 1.357; P = .297).
Study details: AML or MDS patients who received chemotherapy were treated with either 3-day EBAT or a prolonged antibiotic regimen (until neutrophil recovery).
Disclosures: The study did not receive any specific funding. A Schauwvlieghe, J Maertens, and T Mercier reported relationships with various pharmaceutical companies. The remaining authors declared no conflicts of interest.
Source: Schauwvlieghe A et al. EClinicalMedicine. 2021 Apr 25. doi: 10.1016/j.eclinm.2021.100855.
Key clinical point: During remission induction chemotherapy in patients with myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML), antibiotics can be safely stopped after 3 days of febrile neutropenia in the absence of infection.
Major finding: Serious medical complication (SMC) was seen in 12.5% of patients receiving the 3-day empirical broad-spectrum antibiotic therapy (EBAT) vs. 8.9% of patients receiving the prolonged regimen (P = .17). After adjustment for confounders, there was no significant difference between both strategies in the number of SMCs (hazard ratio, 1.357; P = .297).
Study details: AML or MDS patients who received chemotherapy were treated with either 3-day EBAT or a prolonged antibiotic regimen (until neutrophil recovery).
Disclosures: The study did not receive any specific funding. A Schauwvlieghe, J Maertens, and T Mercier reported relationships with various pharmaceutical companies. The remaining authors declared no conflicts of interest.
Source: Schauwvlieghe A et al. EClinicalMedicine. 2021 Apr 25. doi: 10.1016/j.eclinm.2021.100855.
Key clinical point: During remission induction chemotherapy in patients with myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML), antibiotics can be safely stopped after 3 days of febrile neutropenia in the absence of infection.
Major finding: Serious medical complication (SMC) was seen in 12.5% of patients receiving the 3-day empirical broad-spectrum antibiotic therapy (EBAT) vs. 8.9% of patients receiving the prolonged regimen (P = .17). After adjustment for confounders, there was no significant difference between both strategies in the number of SMCs (hazard ratio, 1.357; P = .297).
Study details: AML or MDS patients who received chemotherapy were treated with either 3-day EBAT or a prolonged antibiotic regimen (until neutrophil recovery).
Disclosures: The study did not receive any specific funding. A Schauwvlieghe, J Maertens, and T Mercier reported relationships with various pharmaceutical companies. The remaining authors declared no conflicts of interest.
Source: Schauwvlieghe A et al. EClinicalMedicine. 2021 Apr 25. doi: 10.1016/j.eclinm.2021.100855.
De novo MDS: Pretransplant RBC and platelet transfusion burden tied to poor survival outcomes
Key clinical point: Higher pretransplant red blood cell (RBC) and platelet transfusion burden has an independent association with higher overall and relapse-related mortality following allogeneic hematopoietic stem cell transplantation (allo-HSCT) for de novo myelodysplastic syndrome (MDS).
Major finding: A higher pretransplant RBC transfusion burden was significantly associated with lower overall survival (OS; P less than .001) and higher relapse-related mortality (P less than .001). Similarly, a higher pretransplant platelet transfusion burden was associated with lower OS (P less than .001) and higher relapse-related mortality (P = .001).
Study details: A retrospective study examined the effects of pretransplant RBC and platelet transfusion burden on outcomes after allo-HSCT in 1,007 adults with de novo MDS.
Disclosures: This study was supported in part by the Practical Research Project for Allergic Diseases and Immunology (Research Technology of Medical Transplantation) from the Japan Agency for Medical Research and Development. The authors declared no conflicts of interest.
Source: Konuma T et al. Transplant Cell Ther. 2021 May 12. doi: 10.1016/j.jtct.2021.05.003.
Key clinical point: Higher pretransplant red blood cell (RBC) and platelet transfusion burden has an independent association with higher overall and relapse-related mortality following allogeneic hematopoietic stem cell transplantation (allo-HSCT) for de novo myelodysplastic syndrome (MDS).
Major finding: A higher pretransplant RBC transfusion burden was significantly associated with lower overall survival (OS; P less than .001) and higher relapse-related mortality (P less than .001). Similarly, a higher pretransplant platelet transfusion burden was associated with lower OS (P less than .001) and higher relapse-related mortality (P = .001).
Study details: A retrospective study examined the effects of pretransplant RBC and platelet transfusion burden on outcomes after allo-HSCT in 1,007 adults with de novo MDS.
Disclosures: This study was supported in part by the Practical Research Project for Allergic Diseases and Immunology (Research Technology of Medical Transplantation) from the Japan Agency for Medical Research and Development. The authors declared no conflicts of interest.
Source: Konuma T et al. Transplant Cell Ther. 2021 May 12. doi: 10.1016/j.jtct.2021.05.003.
Key clinical point: Higher pretransplant red blood cell (RBC) and platelet transfusion burden has an independent association with higher overall and relapse-related mortality following allogeneic hematopoietic stem cell transplantation (allo-HSCT) for de novo myelodysplastic syndrome (MDS).
Major finding: A higher pretransplant RBC transfusion burden was significantly associated with lower overall survival (OS; P less than .001) and higher relapse-related mortality (P less than .001). Similarly, a higher pretransplant platelet transfusion burden was associated with lower OS (P less than .001) and higher relapse-related mortality (P = .001).
Study details: A retrospective study examined the effects of pretransplant RBC and platelet transfusion burden on outcomes after allo-HSCT in 1,007 adults with de novo MDS.
Disclosures: This study was supported in part by the Practical Research Project for Allergic Diseases and Immunology (Research Technology of Medical Transplantation) from the Japan Agency for Medical Research and Development. The authors declared no conflicts of interest.
Source: Konuma T et al. Transplant Cell Ther. 2021 May 12. doi: 10.1016/j.jtct.2021.05.003.
Less restrictive enrollment criteria warranted for MDS clinical trials
Key clinical point: Less restrictive inclusion and exclusion criteria are warranted to improve the participation of patients with myelodysplastic syndrome (MDS) in clinical trials.
Major finding: Each trial was suitable for ~18% of patients in the cohort, whereas 34% of the patients were eligible for at least 1 of the 9 trials. Pharma-initiated trials excluded more than twice the fraction of patients vs. investigator-initiated trials (inclusion, 10% vs. 21%). Key reasons for exclusion included karyotype (average exclusion rate, 58%), comorbidities (40%), and previous therapies (55%)
Study details: A simulation exercise was performed to estimate the average proportion of MDS patients eligible for participation in a clinical trial. A total of 1,809 patients were included in the cohort.
Disclosures: This study did not receive any funding. K Nachtkamp, T Schroeder, E Schuler, J Kaivers, A Giagounidis, C Rautenberg, N Gattermann, and U Germing reported relationships with various pharmaceutical companies. The remaining authors declared no conflicts of interest.
Source: Nachtkamp K et al. Leuk Res. 2021 May 11. doi: 10.1016/j.leukres.2021.106611.
Key clinical point: Less restrictive inclusion and exclusion criteria are warranted to improve the participation of patients with myelodysplastic syndrome (MDS) in clinical trials.
Major finding: Each trial was suitable for ~18% of patients in the cohort, whereas 34% of the patients were eligible for at least 1 of the 9 trials. Pharma-initiated trials excluded more than twice the fraction of patients vs. investigator-initiated trials (inclusion, 10% vs. 21%). Key reasons for exclusion included karyotype (average exclusion rate, 58%), comorbidities (40%), and previous therapies (55%)
Study details: A simulation exercise was performed to estimate the average proportion of MDS patients eligible for participation in a clinical trial. A total of 1,809 patients were included in the cohort.
Disclosures: This study did not receive any funding. K Nachtkamp, T Schroeder, E Schuler, J Kaivers, A Giagounidis, C Rautenberg, N Gattermann, and U Germing reported relationships with various pharmaceutical companies. The remaining authors declared no conflicts of interest.
Source: Nachtkamp K et al. Leuk Res. 2021 May 11. doi: 10.1016/j.leukres.2021.106611.
Key clinical point: Less restrictive inclusion and exclusion criteria are warranted to improve the participation of patients with myelodysplastic syndrome (MDS) in clinical trials.
Major finding: Each trial was suitable for ~18% of patients in the cohort, whereas 34% of the patients were eligible for at least 1 of the 9 trials. Pharma-initiated trials excluded more than twice the fraction of patients vs. investigator-initiated trials (inclusion, 10% vs. 21%). Key reasons for exclusion included karyotype (average exclusion rate, 58%), comorbidities (40%), and previous therapies (55%)
Study details: A simulation exercise was performed to estimate the average proportion of MDS patients eligible for participation in a clinical trial. A total of 1,809 patients were included in the cohort.
Disclosures: This study did not receive any funding. K Nachtkamp, T Schroeder, E Schuler, J Kaivers, A Giagounidis, C Rautenberg, N Gattermann, and U Germing reported relationships with various pharmaceutical companies. The remaining authors declared no conflicts of interest.
Source: Nachtkamp K et al. Leuk Res. 2021 May 11. doi: 10.1016/j.leukres.2021.106611.
T-cell inhibition by PD-L1-expressing stem cells may play a role in MDS development
Key clinical point: Inhibition of T cells by programmed death-ligand 1 (PD-L1)-expressing hematopoietic stem cells could be an underlying mechanism in the development of myelodysplastic syndrome (MDS). The findings support the potential use of immune checkpoint inhibitors in the treatment of suitable MDS patients.
Major finding: Significantly increased proportions of PD-L1+CD34+ stem cells were seen in MDS patients compared with hematopoietic stem cell transplantation (HSCT) recipients in remission for both the CD38− subset (P = .0127) and CD38+ subset (P = .0336).
Study details: The study included 7 MDS and 9 acute myeloid leukemia samples. Six HSCT recipients who remained in remission for more than 6 months were considered controls.
Disclosures: The study was supported by the Düsseldorf School of Oncology (funded by the Comprehensive Cancer Center Düsseldorf/Deutsche Krebshilfe and the Medical Faculty HHU Düsseldorf). The authors declared no conflicts of interest.
Source: Moskorz W et al. Br J Haematol. 2021 May 6. doi: 10.1111/bjh.17461.
Key clinical point: Inhibition of T cells by programmed death-ligand 1 (PD-L1)-expressing hematopoietic stem cells could be an underlying mechanism in the development of myelodysplastic syndrome (MDS). The findings support the potential use of immune checkpoint inhibitors in the treatment of suitable MDS patients.
Major finding: Significantly increased proportions of PD-L1+CD34+ stem cells were seen in MDS patients compared with hematopoietic stem cell transplantation (HSCT) recipients in remission for both the CD38− subset (P = .0127) and CD38+ subset (P = .0336).
Study details: The study included 7 MDS and 9 acute myeloid leukemia samples. Six HSCT recipients who remained in remission for more than 6 months were considered controls.
Disclosures: The study was supported by the Düsseldorf School of Oncology (funded by the Comprehensive Cancer Center Düsseldorf/Deutsche Krebshilfe and the Medical Faculty HHU Düsseldorf). The authors declared no conflicts of interest.
Source: Moskorz W et al. Br J Haematol. 2021 May 6. doi: 10.1111/bjh.17461.
Key clinical point: Inhibition of T cells by programmed death-ligand 1 (PD-L1)-expressing hematopoietic stem cells could be an underlying mechanism in the development of myelodysplastic syndrome (MDS). The findings support the potential use of immune checkpoint inhibitors in the treatment of suitable MDS patients.
Major finding: Significantly increased proportions of PD-L1+CD34+ stem cells were seen in MDS patients compared with hematopoietic stem cell transplantation (HSCT) recipients in remission for both the CD38− subset (P = .0127) and CD38+ subset (P = .0336).
Study details: The study included 7 MDS and 9 acute myeloid leukemia samples. Six HSCT recipients who remained in remission for more than 6 months were considered controls.
Disclosures: The study was supported by the Düsseldorf School of Oncology (funded by the Comprehensive Cancer Center Düsseldorf/Deutsche Krebshilfe and the Medical Faculty HHU Düsseldorf). The authors declared no conflicts of interest.
Source: Moskorz W et al. Br J Haematol. 2021 May 6. doi: 10.1111/bjh.17461.
Increased cumulative exposure to melphalan in multiple myeloma patients increases MDS risk
Key clinical point: Increased cumulative exposure to the alkylating agent melphalan increases the subsequent risk for developing acute myeloid leukemia/myelodysplastic syndromes (AML/MDS) in patients with multiple myeloma (MM).
Major finding: Cumulative exposure to melphalan was significantly higher (odds ratio, 2.8; P less than .001) among patients with MM and AML/MDS (median, 988 mg) than control participants (median, 578 mg). The median time to development of AML/MDS was 3.8 years.
Study details: The study included 26,627 patients diagnosed with MM between 1985 and 2011, of which 124 (0.5%) patients developed subsequent AML/MDS. Each patient with MM and AML/MDS diagnosis was matched with a control MM patient without AML/MDS.
Disclosures: The study was supported by grants from the Asrun Einarsdottir Foundation in Iceland, University of Iceland Research Fund, Icelandic Centre for Research, Landspitali University Hospital Research Fund, Thorman’s foundation, and Sylvester Comprehensive Cancer Center NCI Core Grant. O Landgren and M Björkholm reported ties with various pharmaceutical companies. The remaining authors declared no conflicts of interest.
Source: Jonsdottir G et al. Eur J Haematol. 2021 May 9. doi: 10.1111/ejh.13650.
Key clinical point: Increased cumulative exposure to the alkylating agent melphalan increases the subsequent risk for developing acute myeloid leukemia/myelodysplastic syndromes (AML/MDS) in patients with multiple myeloma (MM).
Major finding: Cumulative exposure to melphalan was significantly higher (odds ratio, 2.8; P less than .001) among patients with MM and AML/MDS (median, 988 mg) than control participants (median, 578 mg). The median time to development of AML/MDS was 3.8 years.
Study details: The study included 26,627 patients diagnosed with MM between 1985 and 2011, of which 124 (0.5%) patients developed subsequent AML/MDS. Each patient with MM and AML/MDS diagnosis was matched with a control MM patient without AML/MDS.
Disclosures: The study was supported by grants from the Asrun Einarsdottir Foundation in Iceland, University of Iceland Research Fund, Icelandic Centre for Research, Landspitali University Hospital Research Fund, Thorman’s foundation, and Sylvester Comprehensive Cancer Center NCI Core Grant. O Landgren and M Björkholm reported ties with various pharmaceutical companies. The remaining authors declared no conflicts of interest.
Source: Jonsdottir G et al. Eur J Haematol. 2021 May 9. doi: 10.1111/ejh.13650.
Key clinical point: Increased cumulative exposure to the alkylating agent melphalan increases the subsequent risk for developing acute myeloid leukemia/myelodysplastic syndromes (AML/MDS) in patients with multiple myeloma (MM).
Major finding: Cumulative exposure to melphalan was significantly higher (odds ratio, 2.8; P less than .001) among patients with MM and AML/MDS (median, 988 mg) than control participants (median, 578 mg). The median time to development of AML/MDS was 3.8 years.
Study details: The study included 26,627 patients diagnosed with MM between 1985 and 2011, of which 124 (0.5%) patients developed subsequent AML/MDS. Each patient with MM and AML/MDS diagnosis was matched with a control MM patient without AML/MDS.
Disclosures: The study was supported by grants from the Asrun Einarsdottir Foundation in Iceland, University of Iceland Research Fund, Icelandic Centre for Research, Landspitali University Hospital Research Fund, Thorman’s foundation, and Sylvester Comprehensive Cancer Center NCI Core Grant. O Landgren and M Björkholm reported ties with various pharmaceutical companies. The remaining authors declared no conflicts of interest.
Source: Jonsdottir G et al. Eur J Haematol. 2021 May 9. doi: 10.1111/ejh.13650.