User login
Pregnancy: CKD, Dialysis, and Transplant Patients
Q) I was having a “discussion” over lunch about CKD, pregnancy, and transplant. I said that dialysis patients cannot get pregnant, but someone said I was wrong. A friend said that transplant patients should not get pregnant because of the toxicity of the immunosuppressant medications they take, but another practitioner said that was in the “olden days.” What is the current state of the CKD, dialysis, and transplant patient and pregnancy?
The first healthy baby delivered by a pregnant kidney transplant patient was born in 1958. With the advances in treatment of kidney disease, we are now seeing more pregnancies in these patients. However, they are still considered high risk and should be monitored by a transplant nephrologist and a high-risk obstetrician.4
CKD patients (not on dialysis) are at increased risk for pregnancy complications. Maternal risks include gestational hypertension, preeclampsia/eclampsia, ESRD, or death. Fetal complications include prematurity, small-for-gestational-age babies, and stillbirth. It is very important to control hypertension because it is directly linked to fetal outcome; however, not all blood pressure medications are safe in pregnancy. ACE inhibitors, for example, are teratogenic and absolutely contraindicated.4
Fertility is decreased in dialysis patients; however, pregnancy occurs in 0.3% to 1.5% of women of childbearing age on dialysis. Pregnancy should be confirmed with an ultrasound because serum β-human chorionic gonadotropin can be falsely elevated in ESRD.5
It also can be difficult to monitor pregnancy weight gain due to fluid gains between dialysis treatments. Dialysis prescriptions should be increased either in time or frequency (or both), with a goal of keeping the blood urea nitrogen concentration below 50 mg/dL to improve maternal and fetal outcomes.6,7 Other important factors to control are metabolic acidosis, hypocalcemia, and anemia (increased erythropoietin-stimulating agents may be needed). Frequent uterine and fetal monitoring is also indicated to prevent preterm labor due to the dialysis process. Preeclampsia, prematurity, low birth weight, and hypertension are the most common risks in these pregnancies.8
Renal transplantation often returns fertility to normal and allows pregnancy to occur; however, it is recommended that female patients wait until one year post-transplant if the transplanted kidney is from a living related donor (two years if from a deceased donor), have a serum creatinine level less than 1.5 mg/dL, and a urinary protein level less than 500 mg/d.9 The immunosuppression regimen usually needs adjustment because certain immunosuppressants are contraindicated in pregnancy and have been linked to teratogenic effects; however, data is still limited in this area.10,11 Pregnant transplant recipients are at higher risk for preeclampsia, gestational diabetes, preterm delivery, small-for-gestational-age babies, miscarriage, stillbirth, neonatal death, and congenital abnormalities.12
The National Transplantation Pregnancy Registry (www.ntpr.giftoflifeinstitute.org/) is an ongoing registry in the United States that reports on transplant pregnancies and their outcomes. Data collected by the registry show that there have been many healthy pregnancies without any adverse maternal or fetal outcomes among transplant recipients.
Mandy Trolinger, MS, RD, PA-C
Denver Nephrology
Denver, CO
Personal Note: Mandy is a two-time kidney transplant recipient. She delivered a healthy baby boy in 2012.
REFERENCES
1. Wang I-K, Muo C-H, Liang C-C, et al. Association between hypertensive disorders during pregnancy and end-stage renal disease: a population-based study. CMAJ. 2013;85: 207-213.
2. McPhee SJ, Papadakis MA, Tierney LM, et al. Current Medical Diagnosis and Treatment. 47th ed. New York, NY: McGraw-Hill/Lange; 2008.
3. Männistö T, Mendola P, Vääräsmäki M, et al. Elevated blood pressure in pregnancy and subsequent chronic disease risk. Circulation. 2013;127:681-690.
4. Nevis IF, Reitsma A, Dominic A, et al. Pregnancy outcomes in women with chronic kidney disease: a systematic review. Clin J Am Soc Nephrol. 2011;6:2587-2598.
5. Hou S. Pregnancy in chronic renal insufficiency and end-stage renal disease. Am J Kidney Dis. 1999;33:235-252.
6. Davison JM. Dialysis, transplantation, and pregnancy. Am J Kidney Dis. 1991;17:127-132.
7. Asamiya Y, Otsubo S, Matsuda Y, et al. The importance of low blood urea nitrogen levels in pregnant patients undergoing hemodialysis to optimize birth weight and gestational age. Kidney Int. 2009;75:1217-1222.
8. Giatras I, Levy DP, Malone FD, et al. Pregnancy during dialysis: case report and management guidelines. Nephrol Dial Transplant. 1998;13:3266-3272.
9. McKay DB, Josephson MA. Pregnancy in recipients of solid organs—effects on mother and child. N Engl J Med. 2006;354:1281-1293.
10. Sifontis NM, Coscia LA, Constantinescu S, et al. Pregnancy outcomes in solid organ transplant recipients with exposure to mycophenolate mofetil or sirolimus. Transplantation. 2006;82:1698-1702.
11. Kainz A, Harabacz I, Cowlrick IS, et al. Review of the course and outcome of 100 pregnancies in 84 women treated with tacrolimus. Transplantation. 2000;70:1718-1721.
12. Josephson MA. Pregnancy in renal transplant recipients: more questions answered, still more asked. Clin J Am Soc Nephrol. 2013;8: 182-183.
Q) I was having a “discussion” over lunch about CKD, pregnancy, and transplant. I said that dialysis patients cannot get pregnant, but someone said I was wrong. A friend said that transplant patients should not get pregnant because of the toxicity of the immunosuppressant medications they take, but another practitioner said that was in the “olden days.” What is the current state of the CKD, dialysis, and transplant patient and pregnancy?
The first healthy baby delivered by a pregnant kidney transplant patient was born in 1958. With the advances in treatment of kidney disease, we are now seeing more pregnancies in these patients. However, they are still considered high risk and should be monitored by a transplant nephrologist and a high-risk obstetrician.4
CKD patients (not on dialysis) are at increased risk for pregnancy complications. Maternal risks include gestational hypertension, preeclampsia/eclampsia, ESRD, or death. Fetal complications include prematurity, small-for-gestational-age babies, and stillbirth. It is very important to control hypertension because it is directly linked to fetal outcome; however, not all blood pressure medications are safe in pregnancy. ACE inhibitors, for example, are teratogenic and absolutely contraindicated.4
Fertility is decreased in dialysis patients; however, pregnancy occurs in 0.3% to 1.5% of women of childbearing age on dialysis. Pregnancy should be confirmed with an ultrasound because serum β-human chorionic gonadotropin can be falsely elevated in ESRD.5
It also can be difficult to monitor pregnancy weight gain due to fluid gains between dialysis treatments. Dialysis prescriptions should be increased either in time or frequency (or both), with a goal of keeping the blood urea nitrogen concentration below 50 mg/dL to improve maternal and fetal outcomes.6,7 Other important factors to control are metabolic acidosis, hypocalcemia, and anemia (increased erythropoietin-stimulating agents may be needed). Frequent uterine and fetal monitoring is also indicated to prevent preterm labor due to the dialysis process. Preeclampsia, prematurity, low birth weight, and hypertension are the most common risks in these pregnancies.8
Renal transplantation often returns fertility to normal and allows pregnancy to occur; however, it is recommended that female patients wait until one year post-transplant if the transplanted kidney is from a living related donor (two years if from a deceased donor), have a serum creatinine level less than 1.5 mg/dL, and a urinary protein level less than 500 mg/d.9 The immunosuppression regimen usually needs adjustment because certain immunosuppressants are contraindicated in pregnancy and have been linked to teratogenic effects; however, data is still limited in this area.10,11 Pregnant transplant recipients are at higher risk for preeclampsia, gestational diabetes, preterm delivery, small-for-gestational-age babies, miscarriage, stillbirth, neonatal death, and congenital abnormalities.12
The National Transplantation Pregnancy Registry (www.ntpr.giftoflifeinstitute.org/) is an ongoing registry in the United States that reports on transplant pregnancies and their outcomes. Data collected by the registry show that there have been many healthy pregnancies without any adverse maternal or fetal outcomes among transplant recipients.
Mandy Trolinger, MS, RD, PA-C
Denver Nephrology
Denver, CO
Personal Note: Mandy is a two-time kidney transplant recipient. She delivered a healthy baby boy in 2012.
REFERENCES
1. Wang I-K, Muo C-H, Liang C-C, et al. Association between hypertensive disorders during pregnancy and end-stage renal disease: a population-based study. CMAJ. 2013;85: 207-213.
2. McPhee SJ, Papadakis MA, Tierney LM, et al. Current Medical Diagnosis and Treatment. 47th ed. New York, NY: McGraw-Hill/Lange; 2008.
3. Männistö T, Mendola P, Vääräsmäki M, et al. Elevated blood pressure in pregnancy and subsequent chronic disease risk. Circulation. 2013;127:681-690.
4. Nevis IF, Reitsma A, Dominic A, et al. Pregnancy outcomes in women with chronic kidney disease: a systematic review. Clin J Am Soc Nephrol. 2011;6:2587-2598.
5. Hou S. Pregnancy in chronic renal insufficiency and end-stage renal disease. Am J Kidney Dis. 1999;33:235-252.
6. Davison JM. Dialysis, transplantation, and pregnancy. Am J Kidney Dis. 1991;17:127-132.
7. Asamiya Y, Otsubo S, Matsuda Y, et al. The importance of low blood urea nitrogen levels in pregnant patients undergoing hemodialysis to optimize birth weight and gestational age. Kidney Int. 2009;75:1217-1222.
8. Giatras I, Levy DP, Malone FD, et al. Pregnancy during dialysis: case report and management guidelines. Nephrol Dial Transplant. 1998;13:3266-3272.
9. McKay DB, Josephson MA. Pregnancy in recipients of solid organs—effects on mother and child. N Engl J Med. 2006;354:1281-1293.
10. Sifontis NM, Coscia LA, Constantinescu S, et al. Pregnancy outcomes in solid organ transplant recipients with exposure to mycophenolate mofetil or sirolimus. Transplantation. 2006;82:1698-1702.
11. Kainz A, Harabacz I, Cowlrick IS, et al. Review of the course and outcome of 100 pregnancies in 84 women treated with tacrolimus. Transplantation. 2000;70:1718-1721.
12. Josephson MA. Pregnancy in renal transplant recipients: more questions answered, still more asked. Clin J Am Soc Nephrol. 2013;8: 182-183.
Q) I was having a “discussion” over lunch about CKD, pregnancy, and transplant. I said that dialysis patients cannot get pregnant, but someone said I was wrong. A friend said that transplant patients should not get pregnant because of the toxicity of the immunosuppressant medications they take, but another practitioner said that was in the “olden days.” What is the current state of the CKD, dialysis, and transplant patient and pregnancy?
The first healthy baby delivered by a pregnant kidney transplant patient was born in 1958. With the advances in treatment of kidney disease, we are now seeing more pregnancies in these patients. However, they are still considered high risk and should be monitored by a transplant nephrologist and a high-risk obstetrician.4
CKD patients (not on dialysis) are at increased risk for pregnancy complications. Maternal risks include gestational hypertension, preeclampsia/eclampsia, ESRD, or death. Fetal complications include prematurity, small-for-gestational-age babies, and stillbirth. It is very important to control hypertension because it is directly linked to fetal outcome; however, not all blood pressure medications are safe in pregnancy. ACE inhibitors, for example, are teratogenic and absolutely contraindicated.4
Fertility is decreased in dialysis patients; however, pregnancy occurs in 0.3% to 1.5% of women of childbearing age on dialysis. Pregnancy should be confirmed with an ultrasound because serum β-human chorionic gonadotropin can be falsely elevated in ESRD.5
It also can be difficult to monitor pregnancy weight gain due to fluid gains between dialysis treatments. Dialysis prescriptions should be increased either in time or frequency (or both), with a goal of keeping the blood urea nitrogen concentration below 50 mg/dL to improve maternal and fetal outcomes.6,7 Other important factors to control are metabolic acidosis, hypocalcemia, and anemia (increased erythropoietin-stimulating agents may be needed). Frequent uterine and fetal monitoring is also indicated to prevent preterm labor due to the dialysis process. Preeclampsia, prematurity, low birth weight, and hypertension are the most common risks in these pregnancies.8
Renal transplantation often returns fertility to normal and allows pregnancy to occur; however, it is recommended that female patients wait until one year post-transplant if the transplanted kidney is from a living related donor (two years if from a deceased donor), have a serum creatinine level less than 1.5 mg/dL, and a urinary protein level less than 500 mg/d.9 The immunosuppression regimen usually needs adjustment because certain immunosuppressants are contraindicated in pregnancy and have been linked to teratogenic effects; however, data is still limited in this area.10,11 Pregnant transplant recipients are at higher risk for preeclampsia, gestational diabetes, preterm delivery, small-for-gestational-age babies, miscarriage, stillbirth, neonatal death, and congenital abnormalities.12
The National Transplantation Pregnancy Registry (www.ntpr.giftoflifeinstitute.org/) is an ongoing registry in the United States that reports on transplant pregnancies and their outcomes. Data collected by the registry show that there have been many healthy pregnancies without any adverse maternal or fetal outcomes among transplant recipients.
Mandy Trolinger, MS, RD, PA-C
Denver Nephrology
Denver, CO
Personal Note: Mandy is a two-time kidney transplant recipient. She delivered a healthy baby boy in 2012.
REFERENCES
1. Wang I-K, Muo C-H, Liang C-C, et al. Association between hypertensive disorders during pregnancy and end-stage renal disease: a population-based study. CMAJ. 2013;85: 207-213.
2. McPhee SJ, Papadakis MA, Tierney LM, et al. Current Medical Diagnosis and Treatment. 47th ed. New York, NY: McGraw-Hill/Lange; 2008.
3. Männistö T, Mendola P, Vääräsmäki M, et al. Elevated blood pressure in pregnancy and subsequent chronic disease risk. Circulation. 2013;127:681-690.
4. Nevis IF, Reitsma A, Dominic A, et al. Pregnancy outcomes in women with chronic kidney disease: a systematic review. Clin J Am Soc Nephrol. 2011;6:2587-2598.
5. Hou S. Pregnancy in chronic renal insufficiency and end-stage renal disease. Am J Kidney Dis. 1999;33:235-252.
6. Davison JM. Dialysis, transplantation, and pregnancy. Am J Kidney Dis. 1991;17:127-132.
7. Asamiya Y, Otsubo S, Matsuda Y, et al. The importance of low blood urea nitrogen levels in pregnant patients undergoing hemodialysis to optimize birth weight and gestational age. Kidney Int. 2009;75:1217-1222.
8. Giatras I, Levy DP, Malone FD, et al. Pregnancy during dialysis: case report and management guidelines. Nephrol Dial Transplant. 1998;13:3266-3272.
9. McKay DB, Josephson MA. Pregnancy in recipients of solid organs—effects on mother and child. N Engl J Med. 2006;354:1281-1293.
10. Sifontis NM, Coscia LA, Constantinescu S, et al. Pregnancy outcomes in solid organ transplant recipients with exposure to mycophenolate mofetil or sirolimus. Transplantation. 2006;82:1698-1702.
11. Kainz A, Harabacz I, Cowlrick IS, et al. Review of the course and outcome of 100 pregnancies in 84 women treated with tacrolimus. Transplantation. 2000;70:1718-1721.
12. Josephson MA. Pregnancy in renal transplant recipients: more questions answered, still more asked. Clin J Am Soc Nephrol. 2013;8: 182-183.
Protocol boosts antimicrobial dosing practices during CRRT
DENVER – Before a new protocol was implemented, antimicrobial dosing in patients receiving continuous renal replacement therapy varied and was adherent to evidence-based recommendations in about one-quarter of antimicrobial orders, results from a single-center study showed.
"For any kind of renal replacement therapy, there is always an uncertainty as to how much residual antibiotic is being removed, how much residual renal function the patient has, and how much of the antibiotic is actually staying within the patient for them to achieve therapeutic levels of the drug to combat their infection," Jamie Wagner, Pharm.D., said in an interview during a poster session at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
"With renal replacement therapy, everything is dependent on the filter, the flow rate, and how much residual renal function the patient has. We set out to try to determine how well the interdisciplinary teams were adhering with dosing recommendations, defined as use of evidence-based dose for each CRRT [continuous renal replacement therapy] modality employed for the entire duration of antibiotics used during CRRT."
Dr. Wagner, an infectious diseases pharmacy fellow at the 802-bed Henry Ford Hospital, Detroit, and her associates evaluated 246 antimicrobial orders placed for 43 patients from November 2008 to May 2012. Patients were included in the analysis if they had an order placed for a beta-lactam, vancomycin, tobramycin, gentamicin, or daptomycin; if they received the drug in the ICU; and if they were on CRRT at the time the drug was administered. Patients receiving intermittent hemodialysis or peritoneal dialysis were excluded from the study.
Using medical records, the researchers evaluated demographics, CRRT modality, dates of changes in CRRT, and antibiotic dosing information. Each antibiotic order was evaluated for adherence to evidence-based dosing recommendation, which was the primary outcome of interest.
In August 2011, the Henry Ford Health System implemented an institutional guideline for antibiotic dosing in CRRT, which contained a summary of evidence-based dosing recommendations for the most common antimicrobial agents used in the ICU.
Of the 43 patients, 14 met study inclusion criteria before implementation of the guideline (group A), while the remaining 29 met inclusion criteria after implementation of the guideline (group B). The mean ages of patients in both groups were similar (55 years in group A vs. 59 years in group B), as were other variables.
Dr. Wagner reported that no differences were observed in antibiotic use between pre- and postguideline antibiotic orders. The three most commonly prescribed agents were vancomycin (32%), cefepime (21%), and aminoglycosides (15%). Following implementation of the guideline, overall adherence with evidence-based dosing recommendations improved from 24% to 49% between groups A and B, a difference which reached significance (P less than .001).
Four CRRT modalities changed significantly between groups A and B: continuous venovenous hemofiltration (CVVH) for 8-12 hours (24% vs. 0%, respectively); sustained, low-efficiency, daily diafiltration (SLEDD) for 8-12 hours with an F8 filter (29% vs. 6%); SLEDD for 8-12 hours with an F250 filter (14% vs. 1%); and SLEDD for 24 hours (11% vs. 75%).
Changes between modalities occurred in 13% of all orders assessed. Variables found to be associated with nonadherent orders were change of CRRT mode that resulted in a new recommended dose (7%), SLEDD for 8-12 hours (15%), and the use of any aminoglycoside (15%).
"Communication is key between all patient care providers on a daily basis," Dr. Wagner concluded. "At Henry Ford Hospital, providers must submit a new order for CRRT every single day for patients requiring antibiotic dosing. There needs to be communication about this between all providers involved in that patient’s care."
She acknowledged certain limitations of the study, including increased use of 24-hour SLEDD during the postguideline period, strict definition for adherence to the guideline, and the inability to systematically evaluate clinical response or residual function.
"Understanding factors associated with nonadherent orders can provide a starting point for clinicians to improve the antimicrobial use process in CRRT," she said.
Dr. Wagner said that she had no relevant conflicts of interest to disclose.
DENVER – Before a new protocol was implemented, antimicrobial dosing in patients receiving continuous renal replacement therapy varied and was adherent to evidence-based recommendations in about one-quarter of antimicrobial orders, results from a single-center study showed.
"For any kind of renal replacement therapy, there is always an uncertainty as to how much residual antibiotic is being removed, how much residual renal function the patient has, and how much of the antibiotic is actually staying within the patient for them to achieve therapeutic levels of the drug to combat their infection," Jamie Wagner, Pharm.D., said in an interview during a poster session at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
"With renal replacement therapy, everything is dependent on the filter, the flow rate, and how much residual renal function the patient has. We set out to try to determine how well the interdisciplinary teams were adhering with dosing recommendations, defined as use of evidence-based dose for each CRRT [continuous renal replacement therapy] modality employed for the entire duration of antibiotics used during CRRT."
Dr. Wagner, an infectious diseases pharmacy fellow at the 802-bed Henry Ford Hospital, Detroit, and her associates evaluated 246 antimicrobial orders placed for 43 patients from November 2008 to May 2012. Patients were included in the analysis if they had an order placed for a beta-lactam, vancomycin, tobramycin, gentamicin, or daptomycin; if they received the drug in the ICU; and if they were on CRRT at the time the drug was administered. Patients receiving intermittent hemodialysis or peritoneal dialysis were excluded from the study.
Using medical records, the researchers evaluated demographics, CRRT modality, dates of changes in CRRT, and antibiotic dosing information. Each antibiotic order was evaluated for adherence to evidence-based dosing recommendation, which was the primary outcome of interest.
In August 2011, the Henry Ford Health System implemented an institutional guideline for antibiotic dosing in CRRT, which contained a summary of evidence-based dosing recommendations for the most common antimicrobial agents used in the ICU.
Of the 43 patients, 14 met study inclusion criteria before implementation of the guideline (group A), while the remaining 29 met inclusion criteria after implementation of the guideline (group B). The mean ages of patients in both groups were similar (55 years in group A vs. 59 years in group B), as were other variables.
Dr. Wagner reported that no differences were observed in antibiotic use between pre- and postguideline antibiotic orders. The three most commonly prescribed agents were vancomycin (32%), cefepime (21%), and aminoglycosides (15%). Following implementation of the guideline, overall adherence with evidence-based dosing recommendations improved from 24% to 49% between groups A and B, a difference which reached significance (P less than .001).
Four CRRT modalities changed significantly between groups A and B: continuous venovenous hemofiltration (CVVH) for 8-12 hours (24% vs. 0%, respectively); sustained, low-efficiency, daily diafiltration (SLEDD) for 8-12 hours with an F8 filter (29% vs. 6%); SLEDD for 8-12 hours with an F250 filter (14% vs. 1%); and SLEDD for 24 hours (11% vs. 75%).
Changes between modalities occurred in 13% of all orders assessed. Variables found to be associated with nonadherent orders were change of CRRT mode that resulted in a new recommended dose (7%), SLEDD for 8-12 hours (15%), and the use of any aminoglycoside (15%).
"Communication is key between all patient care providers on a daily basis," Dr. Wagner concluded. "At Henry Ford Hospital, providers must submit a new order for CRRT every single day for patients requiring antibiotic dosing. There needs to be communication about this between all providers involved in that patient’s care."
She acknowledged certain limitations of the study, including increased use of 24-hour SLEDD during the postguideline period, strict definition for adherence to the guideline, and the inability to systematically evaluate clinical response or residual function.
"Understanding factors associated with nonadherent orders can provide a starting point for clinicians to improve the antimicrobial use process in CRRT," she said.
Dr. Wagner said that she had no relevant conflicts of interest to disclose.
DENVER – Before a new protocol was implemented, antimicrobial dosing in patients receiving continuous renal replacement therapy varied and was adherent to evidence-based recommendations in about one-quarter of antimicrobial orders, results from a single-center study showed.
"For any kind of renal replacement therapy, there is always an uncertainty as to how much residual antibiotic is being removed, how much residual renal function the patient has, and how much of the antibiotic is actually staying within the patient for them to achieve therapeutic levels of the drug to combat their infection," Jamie Wagner, Pharm.D., said in an interview during a poster session at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
"With renal replacement therapy, everything is dependent on the filter, the flow rate, and how much residual renal function the patient has. We set out to try to determine how well the interdisciplinary teams were adhering with dosing recommendations, defined as use of evidence-based dose for each CRRT [continuous renal replacement therapy] modality employed for the entire duration of antibiotics used during CRRT."
Dr. Wagner, an infectious diseases pharmacy fellow at the 802-bed Henry Ford Hospital, Detroit, and her associates evaluated 246 antimicrobial orders placed for 43 patients from November 2008 to May 2012. Patients were included in the analysis if they had an order placed for a beta-lactam, vancomycin, tobramycin, gentamicin, or daptomycin; if they received the drug in the ICU; and if they were on CRRT at the time the drug was administered. Patients receiving intermittent hemodialysis or peritoneal dialysis were excluded from the study.
Using medical records, the researchers evaluated demographics, CRRT modality, dates of changes in CRRT, and antibiotic dosing information. Each antibiotic order was evaluated for adherence to evidence-based dosing recommendation, which was the primary outcome of interest.
In August 2011, the Henry Ford Health System implemented an institutional guideline for antibiotic dosing in CRRT, which contained a summary of evidence-based dosing recommendations for the most common antimicrobial agents used in the ICU.
Of the 43 patients, 14 met study inclusion criteria before implementation of the guideline (group A), while the remaining 29 met inclusion criteria after implementation of the guideline (group B). The mean ages of patients in both groups were similar (55 years in group A vs. 59 years in group B), as were other variables.
Dr. Wagner reported that no differences were observed in antibiotic use between pre- and postguideline antibiotic orders. The three most commonly prescribed agents were vancomycin (32%), cefepime (21%), and aminoglycosides (15%). Following implementation of the guideline, overall adherence with evidence-based dosing recommendations improved from 24% to 49% between groups A and B, a difference which reached significance (P less than .001).
Four CRRT modalities changed significantly between groups A and B: continuous venovenous hemofiltration (CVVH) for 8-12 hours (24% vs. 0%, respectively); sustained, low-efficiency, daily diafiltration (SLEDD) for 8-12 hours with an F8 filter (29% vs. 6%); SLEDD for 8-12 hours with an F250 filter (14% vs. 1%); and SLEDD for 24 hours (11% vs. 75%).
Changes between modalities occurred in 13% of all orders assessed. Variables found to be associated with nonadherent orders were change of CRRT mode that resulted in a new recommended dose (7%), SLEDD for 8-12 hours (15%), and the use of any aminoglycoside (15%).
"Communication is key between all patient care providers on a daily basis," Dr. Wagner concluded. "At Henry Ford Hospital, providers must submit a new order for CRRT every single day for patients requiring antibiotic dosing. There needs to be communication about this between all providers involved in that patient’s care."
She acknowledged certain limitations of the study, including increased use of 24-hour SLEDD during the postguideline period, strict definition for adherence to the guideline, and the inability to systematically evaluate clinical response or residual function.
"Understanding factors associated with nonadherent orders can provide a starting point for clinicians to improve the antimicrobial use process in CRRT," she said.
Dr. Wagner said that she had no relevant conflicts of interest to disclose.
AT ICAAC 2013
Four-variable score predicts acute kidney injury
DENVER – A four-variable risk score predicted acute kidney injury with high specificity in patients receiving vancomycin, results from a single-center study demonstrated.
During a poster session at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy, Joseph J. Carreno, Pharm.D., discussed findings from a study that set out to identify patients at high risk for AKI during vancomycin therapy.
"Vancomycin has been the standard therapy for infections with methicillin-resistant Staphylococcus aureus for many years," Dr. Carreno of Albany College of Pharmacy and Health Sciences and his associates wrote in their abstract. "Treatment with vancomycin can be limited by the onset of renal dysfunction, which has been associated with additional morbidity. Recently, numerous investigations have evaluated and identified multiple risk factors for acute kidney injury in patients receiving vancomycin. However, few have validated the predictive probability of only those risk factors readily available at bedside at the initiation of therapy."
In a study conducted during his infectious disease pharmacy fellowship at Henry Ford Hospital, Detroit, the researchers retrospectively evaluated the medical records of 112 adult patients who were prescribed intravenous vancomycin for any suspected or confirmed infection between January 2011 and January 2012. They excluded patients who were pregnant, had end-stage renal disease at baseline, or had an absolute neutrophil count of less than 1,000/mm3.
Four risk factors were evaluated: receiving at least 4 g of vancomycin daily or having a body weight of at least 110 kg; a history of renal dysfunction; concurrent use of intravenous vasopressors, and use of concurrent nephrotoxins.
The mean age of the 112 patients was 58 years, and more than half (54%) were male. The majority (84) had fewer than two risk factors while the remaining 28 had at least two risk factors. The most common indications for therapy were infections of the lower respiratory tract and/or skin and soft tissue (49% and 27%, respectively).
Dr. Carreno and his associates reported that the prevalence of AKI was 46%. In logistic regression analysis adjusted for the other three risk factors, the odds for the development of AKI was greatest among patients on vasopressors (odds ratio, 5.92), followed by those with a history of AKI or preexisting chronic kidney disease (OR, 2.99), those on high dose vancomycin or with a body weight of at least 110 kg (OR, 1.68), and those on nephrotoxins (OR, 1.07).
More than two-thirds of patients (68%) with at least two risk factors at baseline developed AKI, compared with 38% of those who had fewer than two risk factors at baseline. The difference was significant with a P value of less than 0.01.
The sensitivity and specificity of the four-variable prediction model were 78% and 33%, respectively, among patients with at least one risk factor, and 37% and 85% among patients with at least two risk factors.
"This is a bedside tool you can use that condenses 20 years’ worth of research into a small, four-variable score that’s clinically applicable," Dr. Carreno said in an interview at the meeting. "It takes less than 5 minutes to apply this to a patient."
He acknowledged that the study’s retrospective design was a limitation.
Dr. Carreno said he had no relevant financial disclosures.
Dr. Steven Q. Simpson, FCCP, comments: This is an interesting and easy-to-use tool that has the potential for predicting the development of acute renal failure in patients receiving vancomycin.
The results are interesting, but the retrospective study is small, and the predictive value is moderate. The risk factors in the scoring system are all known to be associated with AKI during vancomycin therapy, and there is value in quantifying the association.
Dr. Steven Q. Simpson, FCCP, is with the University of
Kansas Medical Center, Kansas City.
Dr. Steven Q. Simpson, FCCP, comments: This is an interesting and easy-to-use tool that has the potential for predicting the development of acute renal failure in patients receiving vancomycin.
The results are interesting, but the retrospective study is small, and the predictive value is moderate. The risk factors in the scoring system are all known to be associated with AKI during vancomycin therapy, and there is value in quantifying the association.
Dr. Steven Q. Simpson, FCCP, is with the University of
Kansas Medical Center, Kansas City.
Dr. Steven Q. Simpson, FCCP, comments: This is an interesting and easy-to-use tool that has the potential for predicting the development of acute renal failure in patients receiving vancomycin.
The results are interesting, but the retrospective study is small, and the predictive value is moderate. The risk factors in the scoring system are all known to be associated with AKI during vancomycin therapy, and there is value in quantifying the association.
Dr. Steven Q. Simpson, FCCP, is with the University of
Kansas Medical Center, Kansas City.
DENVER – A four-variable risk score predicted acute kidney injury with high specificity in patients receiving vancomycin, results from a single-center study demonstrated.
During a poster session at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy, Joseph J. Carreno, Pharm.D., discussed findings from a study that set out to identify patients at high risk for AKI during vancomycin therapy.
"Vancomycin has been the standard therapy for infections with methicillin-resistant Staphylococcus aureus for many years," Dr. Carreno of Albany College of Pharmacy and Health Sciences and his associates wrote in their abstract. "Treatment with vancomycin can be limited by the onset of renal dysfunction, which has been associated with additional morbidity. Recently, numerous investigations have evaluated and identified multiple risk factors for acute kidney injury in patients receiving vancomycin. However, few have validated the predictive probability of only those risk factors readily available at bedside at the initiation of therapy."
In a study conducted during his infectious disease pharmacy fellowship at Henry Ford Hospital, Detroit, the researchers retrospectively evaluated the medical records of 112 adult patients who were prescribed intravenous vancomycin for any suspected or confirmed infection between January 2011 and January 2012. They excluded patients who were pregnant, had end-stage renal disease at baseline, or had an absolute neutrophil count of less than 1,000/mm3.
Four risk factors were evaluated: receiving at least 4 g of vancomycin daily or having a body weight of at least 110 kg; a history of renal dysfunction; concurrent use of intravenous vasopressors, and use of concurrent nephrotoxins.
The mean age of the 112 patients was 58 years, and more than half (54%) were male. The majority (84) had fewer than two risk factors while the remaining 28 had at least two risk factors. The most common indications for therapy were infections of the lower respiratory tract and/or skin and soft tissue (49% and 27%, respectively).
Dr. Carreno and his associates reported that the prevalence of AKI was 46%. In logistic regression analysis adjusted for the other three risk factors, the odds for the development of AKI was greatest among patients on vasopressors (odds ratio, 5.92), followed by those with a history of AKI or preexisting chronic kidney disease (OR, 2.99), those on high dose vancomycin or with a body weight of at least 110 kg (OR, 1.68), and those on nephrotoxins (OR, 1.07).
More than two-thirds of patients (68%) with at least two risk factors at baseline developed AKI, compared with 38% of those who had fewer than two risk factors at baseline. The difference was significant with a P value of less than 0.01.
The sensitivity and specificity of the four-variable prediction model were 78% and 33%, respectively, among patients with at least one risk factor, and 37% and 85% among patients with at least two risk factors.
"This is a bedside tool you can use that condenses 20 years’ worth of research into a small, four-variable score that’s clinically applicable," Dr. Carreno said in an interview at the meeting. "It takes less than 5 minutes to apply this to a patient."
He acknowledged that the study’s retrospective design was a limitation.
Dr. Carreno said he had no relevant financial disclosures.
DENVER – A four-variable risk score predicted acute kidney injury with high specificity in patients receiving vancomycin, results from a single-center study demonstrated.
During a poster session at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy, Joseph J. Carreno, Pharm.D., discussed findings from a study that set out to identify patients at high risk for AKI during vancomycin therapy.
"Vancomycin has been the standard therapy for infections with methicillin-resistant Staphylococcus aureus for many years," Dr. Carreno of Albany College of Pharmacy and Health Sciences and his associates wrote in their abstract. "Treatment with vancomycin can be limited by the onset of renal dysfunction, which has been associated with additional morbidity. Recently, numerous investigations have evaluated and identified multiple risk factors for acute kidney injury in patients receiving vancomycin. However, few have validated the predictive probability of only those risk factors readily available at bedside at the initiation of therapy."
In a study conducted during his infectious disease pharmacy fellowship at Henry Ford Hospital, Detroit, the researchers retrospectively evaluated the medical records of 112 adult patients who were prescribed intravenous vancomycin for any suspected or confirmed infection between January 2011 and January 2012. They excluded patients who were pregnant, had end-stage renal disease at baseline, or had an absolute neutrophil count of less than 1,000/mm3.
Four risk factors were evaluated: receiving at least 4 g of vancomycin daily or having a body weight of at least 110 kg; a history of renal dysfunction; concurrent use of intravenous vasopressors, and use of concurrent nephrotoxins.
The mean age of the 112 patients was 58 years, and more than half (54%) were male. The majority (84) had fewer than two risk factors while the remaining 28 had at least two risk factors. The most common indications for therapy were infections of the lower respiratory tract and/or skin and soft tissue (49% and 27%, respectively).
Dr. Carreno and his associates reported that the prevalence of AKI was 46%. In logistic regression analysis adjusted for the other three risk factors, the odds for the development of AKI was greatest among patients on vasopressors (odds ratio, 5.92), followed by those with a history of AKI or preexisting chronic kidney disease (OR, 2.99), those on high dose vancomycin or with a body weight of at least 110 kg (OR, 1.68), and those on nephrotoxins (OR, 1.07).
More than two-thirds of patients (68%) with at least two risk factors at baseline developed AKI, compared with 38% of those who had fewer than two risk factors at baseline. The difference was significant with a P value of less than 0.01.
The sensitivity and specificity of the four-variable prediction model were 78% and 33%, respectively, among patients with at least one risk factor, and 37% and 85% among patients with at least two risk factors.
"This is a bedside tool you can use that condenses 20 years’ worth of research into a small, four-variable score that’s clinically applicable," Dr. Carreno said in an interview at the meeting. "It takes less than 5 minutes to apply this to a patient."
He acknowledged that the study’s retrospective design was a limitation.
Dr. Carreno said he had no relevant financial disclosures.
AT ICAAC 2013
Major finding: The odds for developing acute kidney injury was greatest among patients on vasopressors (OR, 5.92), followed by those with a history of AKI or preexisting chronic kidney disease (OR, 2.99), those on high-dose vancomycin or with a body weight of at least 110 kg (OR, 1.68), and those on nephrotoxins (OR, 1.07).
Data source: A retrospective study of 112 adult patients who were prescribed intravenous vancomycin for any suspected or confirmed infection between January 2011 and January 2012.
Disclosures: Dr. Carreno said he had no relevant financial conflicts.
Antibiotic doses often fall short in ICU hemodialysis patients
DENVER – Antibiotics were dosed too low about 20% of the time in ICU patients on continuous venovenous hemodialysis at the Cleveland Clinic.
Continuous venovenous hemodialysis (CVVHD) artificially improves creatinine clearance; the clinic’s guidelines call for increasing antibiotic doses to compensate.
That didn’t always happen in the 42 Cleveland Clinic patients, and doesn’t always happen elsewhere, said lead investigator Marianna Fedorenko, Pharm.D., a Cleveland Clinic pharmacy resident when the study was done but currently at Barnes-Jewish Hospital in St. Louis.
The clinic has since added an alert to the electronic medical record system to notify prescribers that patients are on CVVHD.
Poor communication was probably to blame. Amid the stress of ICU care, residents, nephrologists, internists, and others may not have known when ordering or adjusting antibiotic doses that patients were on CVVHD. "This is an [issue] that people need to look at it. There are a lot of points during dialysis that are critical for communication. These patients need a closer eye than some other intensive care unit patients," Dr. Fedorenko said.
Most of the patients had failing kidneys and were on pressors and mechanical ventilation; the majority were probably septic. The investigators assessed them at 24 hours for appropriate antibiotic dose. Vancomycin and aminoglycosides – both dosed according to blood levels – were excluded from the analysis.
The 42 patients had a total of 209 antimicrobial days; some were on more than one antibiotic. The median CVVHD flow rate was 26 mL/kg per hour; about half of the patients died during the study period. Overall, "78% [163] of our 209 study days met" CVVHD Cleveland Clinic antibiotic dosing guidelines. The rest were underdosed, Dr. Fedorenko said at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
That seemed to be a particular problem with ciprofloxacin, ampicillin/sulbactam, and meropenem. There were fewer problems with Zosyn (piperacillin/tazobactam). "We are more familiar with it," Dr. Fedorenko said.
It took a median of about 20 hours to catch and fix the problems, but some patients remained underdosed throughout CVVHD.
Mistakes were more common on weekdays. "Patients are more likely to be started on CVVHD then, so there’s more room for errors – that’s my hypothesis," she said.
Dr. Fedorenko and the other investigators said they had no financial conflicts of interest.
DENVER – Antibiotics were dosed too low about 20% of the time in ICU patients on continuous venovenous hemodialysis at the Cleveland Clinic.
Continuous venovenous hemodialysis (CVVHD) artificially improves creatinine clearance; the clinic’s guidelines call for increasing antibiotic doses to compensate.
That didn’t always happen in the 42 Cleveland Clinic patients, and doesn’t always happen elsewhere, said lead investigator Marianna Fedorenko, Pharm.D., a Cleveland Clinic pharmacy resident when the study was done but currently at Barnes-Jewish Hospital in St. Louis.
The clinic has since added an alert to the electronic medical record system to notify prescribers that patients are on CVVHD.
Poor communication was probably to blame. Amid the stress of ICU care, residents, nephrologists, internists, and others may not have known when ordering or adjusting antibiotic doses that patients were on CVVHD. "This is an [issue] that people need to look at it. There are a lot of points during dialysis that are critical for communication. These patients need a closer eye than some other intensive care unit patients," Dr. Fedorenko said.
Most of the patients had failing kidneys and were on pressors and mechanical ventilation; the majority were probably septic. The investigators assessed them at 24 hours for appropriate antibiotic dose. Vancomycin and aminoglycosides – both dosed according to blood levels – were excluded from the analysis.
The 42 patients had a total of 209 antimicrobial days; some were on more than one antibiotic. The median CVVHD flow rate was 26 mL/kg per hour; about half of the patients died during the study period. Overall, "78% [163] of our 209 study days met" CVVHD Cleveland Clinic antibiotic dosing guidelines. The rest were underdosed, Dr. Fedorenko said at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
That seemed to be a particular problem with ciprofloxacin, ampicillin/sulbactam, and meropenem. There were fewer problems with Zosyn (piperacillin/tazobactam). "We are more familiar with it," Dr. Fedorenko said.
It took a median of about 20 hours to catch and fix the problems, but some patients remained underdosed throughout CVVHD.
Mistakes were more common on weekdays. "Patients are more likely to be started on CVVHD then, so there’s more room for errors – that’s my hypothesis," she said.
Dr. Fedorenko and the other investigators said they had no financial conflicts of interest.
DENVER – Antibiotics were dosed too low about 20% of the time in ICU patients on continuous venovenous hemodialysis at the Cleveland Clinic.
Continuous venovenous hemodialysis (CVVHD) artificially improves creatinine clearance; the clinic’s guidelines call for increasing antibiotic doses to compensate.
That didn’t always happen in the 42 Cleveland Clinic patients, and doesn’t always happen elsewhere, said lead investigator Marianna Fedorenko, Pharm.D., a Cleveland Clinic pharmacy resident when the study was done but currently at Barnes-Jewish Hospital in St. Louis.
The clinic has since added an alert to the electronic medical record system to notify prescribers that patients are on CVVHD.
Poor communication was probably to blame. Amid the stress of ICU care, residents, nephrologists, internists, and others may not have known when ordering or adjusting antibiotic doses that patients were on CVVHD. "This is an [issue] that people need to look at it. There are a lot of points during dialysis that are critical for communication. These patients need a closer eye than some other intensive care unit patients," Dr. Fedorenko said.
Most of the patients had failing kidneys and were on pressors and mechanical ventilation; the majority were probably septic. The investigators assessed them at 24 hours for appropriate antibiotic dose. Vancomycin and aminoglycosides – both dosed according to blood levels – were excluded from the analysis.
The 42 patients had a total of 209 antimicrobial days; some were on more than one antibiotic. The median CVVHD flow rate was 26 mL/kg per hour; about half of the patients died during the study period. Overall, "78% [163] of our 209 study days met" CVVHD Cleveland Clinic antibiotic dosing guidelines. The rest were underdosed, Dr. Fedorenko said at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
That seemed to be a particular problem with ciprofloxacin, ampicillin/sulbactam, and meropenem. There were fewer problems with Zosyn (piperacillin/tazobactam). "We are more familiar with it," Dr. Fedorenko said.
It took a median of about 20 hours to catch and fix the problems, but some patients remained underdosed throughout CVVHD.
Mistakes were more common on weekdays. "Patients are more likely to be started on CVVHD then, so there’s more room for errors – that’s my hypothesis," she said.
Dr. Fedorenko and the other investigators said they had no financial conflicts of interest.
AT ICAAC 2013
Major finding: Antibiotics were underdosed in 46 of 209 (22%) treatment days in 42 continuous venovenous hemodialysis patients at the Cleveland Clinic in Ohio.
Data Source: Chart review.
Disclosures: The investigators said they had no disclosures.
M. genitalium demands new STI treatment strategy
VIENNA – Mycoplasma genitalium is a new bad boy of sexually transmitted infections, prompting experts to rethink how to treat nongonococcal urethritis, pelvic inflammatory disease, and other infections caused by the pathogen.
The full scope of M. genitalium in sexually transmitted infections (STI) of men and women is just now becoming clear – as are the treatment demands of M. genitalium’s susceptibility profile. Given that it’s notoriously hard to culture and that genetic-based assays are only recently available and not yet sold commercially, reliable management of M. genitalium depends on the fluoroquinolone moxifloxacin. Yet the threat of widespread resistance to that drug looms, with no good back-up agents currently available.
Because successful treatment of M. genitalium differs sharply from that of gonorrhea and Chlamydia trachomatis – the other two pathogens most common in urethritis, cervicitis, and pelvic inflammatory disease – clinicians increasingly confront infections unresponsive to or persistent despite a course of doxycycline or azithromycin (Zithromax).
Podium talks from a series of researchers in the United States and Europe at the joint meeting of the International Society for Sexually Transmitted Diseases Research and the International Union Against Sexually Transmitted Infections documented the STI niche that M. genitalium occupies and how well various antibiotics work against the pathogen.
"M. genitalium is associated with 15%-22% of nongonococcal urethritis cases, and 10%-15% of cervicitis cases, and in many settings is more common that Neisseria gonorrhoeae with treatment outcomes often far worse," said Lisa E. Manhart, Ph.D., an epidemiologist at the University of Washington, Seattle. "There is no characteristic clinical syndrome for M. genitalium infections; they look very similar to Chlamydia. Clinical judgment is the only option for treatment decisions in many settings, and no FDA-approved diagnostic test [for M. genitalium] exists."
Persistent cases of nongonococcal urethritis, cervicitis, and possibly pelvic inflammatory disease could benefit from treatment with moxifloxacin (Avelox), Dr. Manhart noted. But "it is becoming clear that resistance in M. genitalium develops rapidly."
"M. genitalium is an important STI, and guidelines should reflect this; but there is no good evidence base for optimal treatment. Optimal treatment is a moving target," said Dr. Jørgen S. Jensen, a researcher at the Statens Serum Institut in Copenhagen.
"Widespread use of azithromycin and moxifloxacin will select for multidrug-resistant strains; the time for single-drug, one-dose regimens is probably over," said Dr. Jensen, specifically referring to the common practice of treating nongonococcal urethritis with a single dose of azithromycin.
M. genitalium invades U.S.
Dr. Manhart and a second U.S. researcher, Dr. Harold C. Wiesenfeld from the University of Pittsburgh, each reported new data at the meeting showing how common M. genitalium STI infections have become among U.S. patients.
Dr. Manhart presented new data from the MEGA (Mycoplasma Genitalium Antibiotic Susceptibility and Treatment) trial, which enrolled 606 men with nongonococcal urethritis (NGU) at an STI clinic in Seattle. The study’s primary endpoint was a comparison of 100 mg doxycycline b.i.d. for 7 days and a single 1-g dose of azithromycin.
The two regimens produced similar cure rates – 76% in the doxycycline arm, and 80% in the azithromycin arm, Dr. Manhart and her associates reported earlier this year (Clin. Infec. Dis. 2013;56:934-42). The initial report also identified M. genitalium in 13% of those men – identified using an in-house polymerase chain reaction assay – compared with 24% who tested positive for Chlamydia and 23% infected with Ureaplasma urealyticum biovar.
The new analyses Dr. Manhart reported tracked the outcomes of patients infected with M. genitalium. Treatment with either of the standard doxycycline or azithromycin regimens failed about half the time, Dr. Manhart said: 29% of men with doxycycline-resistant infections who were retreated with azithromycin as part of the study’s extended protocol carried M. genitalium, and 70% of the men who failed initial azithromycin treatment who were then retreated with doxycycline had persistent infection with M. genitalium.
Results from the extended portion of the study also showed that treatment with moxifloxacin was the answer for most of the otherwise unresponsive M. genitalium infections, but it wasn’t perfect. The M. genitalium infection persisted in 12%-15% of those men after a full course of moxifloxacin.
The full results suggest that moxifloxacin is potentially effective for treating various persistent STIs, not only NGU but also cervicitis and possibly pelvic inflammatory disease (PID). But resistance to moxifloxacin develops "rapidly," meaning that surveillance for resistance is needed, as well as new drug alternatives, she said.
New suspect in acute PID?
Although Dr. Manhart hedged on the role of M. genitalium in PID, results from a different U.S. study created a strong case for a role in acute PID.
M. genitalium appeared in 28 (18%) of 157 diagnosed women with acute PID who were enrolled in a study that had primarily focused on comparing two antibiotic regimens, and in 30% of those women with histologically proven acute PID. Using an in-house transcription-mediated assay for M. genitalium, researchers at the University of Pittsburgh found that endometrial identification of M. genitalium linked independently with a fourfold increased prevalence of histologically confirmed acute PID.
Those numbers for M. genitalium put it in the same ball park in the study with the two traditional heavy hitters of acute PID, N. gonorrhoeae and C. trachomatis. By establishing a significant role for M. genitalium in acute PID, the data immediately called into question the standard empiric therapies for acute PID.
"The PID treatments we use fall short for eradicating M. genitalium," said Dr. Wiesenfeld, an ob.gyn. and infectious diseases physician at the University of Pittsburgh, who reported the results. "Whether these findings [affect] treatment guidelines for acute PID remains to be seen; but if it is truly important to treat M. genitalium, it will completely turn around our treatment regimens."
The looming dilemma is that the azithromycin or doxycycline used for gonorrhea will not stop many of the infections by M. genitalium, while the moxifloxacin that can handle most M. genitalium today does not eradicate N. gonorrhoeae.
However, it’s premature to consider routinely testing or screening for M. genitalium in patients with PID or other possible forms of M. genitalium infection, Dr. Wiesenfeld cautioned. That’s in part because of the current logistical limitations on testing, and in part because the long-term impact of M. genitalium infection on reproductive health is not yet established. Longer follow-up of women in the study should shed more light on the natural history of the patients who received treatments that did not eradicate M. genitalium.
"If M. genitalium turns out to be associated with PID, it is the single organism that is not covered by current treatment with a cephalosporin, doxycycline, and metronidazole," said Sharon L. Hillier, Ph. D., in an interview during the meeting. "We are very concerned about it because it is a fairly sizable fraction of the STIs we’ve seen in these women with acute PID," said Dr. Hillier, professor of ob.gyn. and reproductive sciences at the University of Pittsburgh and a collaborator with Dr. Wiesenfeld on his study.
Dr. Hillier agreed that the key question to address is the fertility risk to women from having PID caused by M. genitalium.
"It’s so common that it might have a huge population impact," she said. "We know fertility outcomes are bad from gonorrhea and Chlamydia." The fertility risk from M. genitalium "will be what makes us decide if we need to add another treatment."
Adding moxifloxacin to routine, empiric treatment for acute PID would be an especially tough call if it also meant dropping doxycycline, a drug that is otherwise attractive because of its low cost and broad spectrum of activity against other PID pathogens.
"It remains to be seen what we should do for empiric therapy for women who walk in with PID, what is the best way to try to preserve her fertility," Dr. Hillier said. "We simply don’t know right now, but it’s been a huge topic of conversation."
Changing the treatment strategies
While the best initial management strategy for acute PID remains unclear, the specter of M. genitalium has already changed the management strategy used by Dr. Paddy Horner to treat men with NGU, said Dr. Horner, a physician in the school of social and community medicine at the University of Bristol, U.K.
These days, his preferred approach is what he calls "infection-specific" first-line therapy: Before treatment begins, he eliminates purely empiric therapy by employing a commercially available, nucleic-acid amplification test for gonorrhea and Chlamydia at the first encounter and getting the result in 30 minutes.
That means treating men who test positive for Chlamydia with a week of doxycycline first, or starting with a 5-day course of azithromycin for men who are Chlamydia negative. However, he advised using a single, 1-g dosage of azithromycin with caution, because of the prevalence of macrolide resistance. But Dr. Horner also admitted that no evidence has proven the superiority of the 5-day alternative that starts with a 1-g dose followed by 500 mg daily for 4 more days. Men who test positive for N. gonorrhoeae should receive 1 g of azithromycin plus 500 mg ceftriaxone.
If the urethritis persists 2 weeks later, Dr. Horner recommended treating patients empirically with a combination of moxifloxacin and metronidazole to cover possible infection by either M. genitalium or U. urealyticum.
In theory, this overall approach has the potential to resolve 89% of infections after the first round of treatment and 99% after the second round, with low potential for generating resistant strains of M. genitalium, based on pathogen prevalence and susceptibility profiles that Dr. Horner sees in Bristol. Those outcomes are an improvement on the cure rates and resistance risks when initial treatment is applied completely empirically, he explained.
Infection-specific treatment would work even better once rapid, point-of-care genetic tests become available for M. genitalium and U. urealyticum, Dr. Horner said.
Dr. Manhart, Dr. Wiesenfeld, and Dr. Hillier had no disclosures. Dr. Jensen said that his institution provides diagnostic testing for M. genitalium commercially and also evaluates various new antimicrobials under contract. Dr. Horner said that he has been a consultant to or received research support from Aquarius Population Health, Cepheid, Hologic, and Siemens.
On Twitter @mitchelzoler
VIENNA – Mycoplasma genitalium is a new bad boy of sexually transmitted infections, prompting experts to rethink how to treat nongonococcal urethritis, pelvic inflammatory disease, and other infections caused by the pathogen.
The full scope of M. genitalium in sexually transmitted infections (STI) of men and women is just now becoming clear – as are the treatment demands of M. genitalium’s susceptibility profile. Given that it’s notoriously hard to culture and that genetic-based assays are only recently available and not yet sold commercially, reliable management of M. genitalium depends on the fluoroquinolone moxifloxacin. Yet the threat of widespread resistance to that drug looms, with no good back-up agents currently available.
Because successful treatment of M. genitalium differs sharply from that of gonorrhea and Chlamydia trachomatis – the other two pathogens most common in urethritis, cervicitis, and pelvic inflammatory disease – clinicians increasingly confront infections unresponsive to or persistent despite a course of doxycycline or azithromycin (Zithromax).
Podium talks from a series of researchers in the United States and Europe at the joint meeting of the International Society for Sexually Transmitted Diseases Research and the International Union Against Sexually Transmitted Infections documented the STI niche that M. genitalium occupies and how well various antibiotics work against the pathogen.
"M. genitalium is associated with 15%-22% of nongonococcal urethritis cases, and 10%-15% of cervicitis cases, and in many settings is more common that Neisseria gonorrhoeae with treatment outcomes often far worse," said Lisa E. Manhart, Ph.D., an epidemiologist at the University of Washington, Seattle. "There is no characteristic clinical syndrome for M. genitalium infections; they look very similar to Chlamydia. Clinical judgment is the only option for treatment decisions in many settings, and no FDA-approved diagnostic test [for M. genitalium] exists."
Persistent cases of nongonococcal urethritis, cervicitis, and possibly pelvic inflammatory disease could benefit from treatment with moxifloxacin (Avelox), Dr. Manhart noted. But "it is becoming clear that resistance in M. genitalium develops rapidly."
"M. genitalium is an important STI, and guidelines should reflect this; but there is no good evidence base for optimal treatment. Optimal treatment is a moving target," said Dr. Jørgen S. Jensen, a researcher at the Statens Serum Institut in Copenhagen.
"Widespread use of azithromycin and moxifloxacin will select for multidrug-resistant strains; the time for single-drug, one-dose regimens is probably over," said Dr. Jensen, specifically referring to the common practice of treating nongonococcal urethritis with a single dose of azithromycin.
M. genitalium invades U.S.
Dr. Manhart and a second U.S. researcher, Dr. Harold C. Wiesenfeld from the University of Pittsburgh, each reported new data at the meeting showing how common M. genitalium STI infections have become among U.S. patients.
Dr. Manhart presented new data from the MEGA (Mycoplasma Genitalium Antibiotic Susceptibility and Treatment) trial, which enrolled 606 men with nongonococcal urethritis (NGU) at an STI clinic in Seattle. The study’s primary endpoint was a comparison of 100 mg doxycycline b.i.d. for 7 days and a single 1-g dose of azithromycin.
The two regimens produced similar cure rates – 76% in the doxycycline arm, and 80% in the azithromycin arm, Dr. Manhart and her associates reported earlier this year (Clin. Infec. Dis. 2013;56:934-42). The initial report also identified M. genitalium in 13% of those men – identified using an in-house polymerase chain reaction assay – compared with 24% who tested positive for Chlamydia and 23% infected with Ureaplasma urealyticum biovar.
The new analyses Dr. Manhart reported tracked the outcomes of patients infected with M. genitalium. Treatment with either of the standard doxycycline or azithromycin regimens failed about half the time, Dr. Manhart said: 29% of men with doxycycline-resistant infections who were retreated with azithromycin as part of the study’s extended protocol carried M. genitalium, and 70% of the men who failed initial azithromycin treatment who were then retreated with doxycycline had persistent infection with M. genitalium.
Results from the extended portion of the study also showed that treatment with moxifloxacin was the answer for most of the otherwise unresponsive M. genitalium infections, but it wasn’t perfect. The M. genitalium infection persisted in 12%-15% of those men after a full course of moxifloxacin.
The full results suggest that moxifloxacin is potentially effective for treating various persistent STIs, not only NGU but also cervicitis and possibly pelvic inflammatory disease (PID). But resistance to moxifloxacin develops "rapidly," meaning that surveillance for resistance is needed, as well as new drug alternatives, she said.
New suspect in acute PID?
Although Dr. Manhart hedged on the role of M. genitalium in PID, results from a different U.S. study created a strong case for a role in acute PID.
M. genitalium appeared in 28 (18%) of 157 diagnosed women with acute PID who were enrolled in a study that had primarily focused on comparing two antibiotic regimens, and in 30% of those women with histologically proven acute PID. Using an in-house transcription-mediated assay for M. genitalium, researchers at the University of Pittsburgh found that endometrial identification of M. genitalium linked independently with a fourfold increased prevalence of histologically confirmed acute PID.
Those numbers for M. genitalium put it in the same ball park in the study with the two traditional heavy hitters of acute PID, N. gonorrhoeae and C. trachomatis. By establishing a significant role for M. genitalium in acute PID, the data immediately called into question the standard empiric therapies for acute PID.
"The PID treatments we use fall short for eradicating M. genitalium," said Dr. Wiesenfeld, an ob.gyn. and infectious diseases physician at the University of Pittsburgh, who reported the results. "Whether these findings [affect] treatment guidelines for acute PID remains to be seen; but if it is truly important to treat M. genitalium, it will completely turn around our treatment regimens."
The looming dilemma is that the azithromycin or doxycycline used for gonorrhea will not stop many of the infections by M. genitalium, while the moxifloxacin that can handle most M. genitalium today does not eradicate N. gonorrhoeae.
However, it’s premature to consider routinely testing or screening for M. genitalium in patients with PID or other possible forms of M. genitalium infection, Dr. Wiesenfeld cautioned. That’s in part because of the current logistical limitations on testing, and in part because the long-term impact of M. genitalium infection on reproductive health is not yet established. Longer follow-up of women in the study should shed more light on the natural history of the patients who received treatments that did not eradicate M. genitalium.
"If M. genitalium turns out to be associated with PID, it is the single organism that is not covered by current treatment with a cephalosporin, doxycycline, and metronidazole," said Sharon L. Hillier, Ph. D., in an interview during the meeting. "We are very concerned about it because it is a fairly sizable fraction of the STIs we’ve seen in these women with acute PID," said Dr. Hillier, professor of ob.gyn. and reproductive sciences at the University of Pittsburgh and a collaborator with Dr. Wiesenfeld on his study.
Dr. Hillier agreed that the key question to address is the fertility risk to women from having PID caused by M. genitalium.
"It’s so common that it might have a huge population impact," she said. "We know fertility outcomes are bad from gonorrhea and Chlamydia." The fertility risk from M. genitalium "will be what makes us decide if we need to add another treatment."
Adding moxifloxacin to routine, empiric treatment for acute PID would be an especially tough call if it also meant dropping doxycycline, a drug that is otherwise attractive because of its low cost and broad spectrum of activity against other PID pathogens.
"It remains to be seen what we should do for empiric therapy for women who walk in with PID, what is the best way to try to preserve her fertility," Dr. Hillier said. "We simply don’t know right now, but it’s been a huge topic of conversation."
Changing the treatment strategies
While the best initial management strategy for acute PID remains unclear, the specter of M. genitalium has already changed the management strategy used by Dr. Paddy Horner to treat men with NGU, said Dr. Horner, a physician in the school of social and community medicine at the University of Bristol, U.K.
These days, his preferred approach is what he calls "infection-specific" first-line therapy: Before treatment begins, he eliminates purely empiric therapy by employing a commercially available, nucleic-acid amplification test for gonorrhea and Chlamydia at the first encounter and getting the result in 30 minutes.
That means treating men who test positive for Chlamydia with a week of doxycycline first, or starting with a 5-day course of azithromycin for men who are Chlamydia negative. However, he advised using a single, 1-g dosage of azithromycin with caution, because of the prevalence of macrolide resistance. But Dr. Horner also admitted that no evidence has proven the superiority of the 5-day alternative that starts with a 1-g dose followed by 500 mg daily for 4 more days. Men who test positive for N. gonorrhoeae should receive 1 g of azithromycin plus 500 mg ceftriaxone.
If the urethritis persists 2 weeks later, Dr. Horner recommended treating patients empirically with a combination of moxifloxacin and metronidazole to cover possible infection by either M. genitalium or U. urealyticum.
In theory, this overall approach has the potential to resolve 89% of infections after the first round of treatment and 99% after the second round, with low potential for generating resistant strains of M. genitalium, based on pathogen prevalence and susceptibility profiles that Dr. Horner sees in Bristol. Those outcomes are an improvement on the cure rates and resistance risks when initial treatment is applied completely empirically, he explained.
Infection-specific treatment would work even better once rapid, point-of-care genetic tests become available for M. genitalium and U. urealyticum, Dr. Horner said.
Dr. Manhart, Dr. Wiesenfeld, and Dr. Hillier had no disclosures. Dr. Jensen said that his institution provides diagnostic testing for M. genitalium commercially and also evaluates various new antimicrobials under contract. Dr. Horner said that he has been a consultant to or received research support from Aquarius Population Health, Cepheid, Hologic, and Siemens.
On Twitter @mitchelzoler
VIENNA – Mycoplasma genitalium is a new bad boy of sexually transmitted infections, prompting experts to rethink how to treat nongonococcal urethritis, pelvic inflammatory disease, and other infections caused by the pathogen.
The full scope of M. genitalium in sexually transmitted infections (STI) of men and women is just now becoming clear – as are the treatment demands of M. genitalium’s susceptibility profile. Given that it’s notoriously hard to culture and that genetic-based assays are only recently available and not yet sold commercially, reliable management of M. genitalium depends on the fluoroquinolone moxifloxacin. Yet the threat of widespread resistance to that drug looms, with no good back-up agents currently available.
Because successful treatment of M. genitalium differs sharply from that of gonorrhea and Chlamydia trachomatis – the other two pathogens most common in urethritis, cervicitis, and pelvic inflammatory disease – clinicians increasingly confront infections unresponsive to or persistent despite a course of doxycycline or azithromycin (Zithromax).
Podium talks from a series of researchers in the United States and Europe at the joint meeting of the International Society for Sexually Transmitted Diseases Research and the International Union Against Sexually Transmitted Infections documented the STI niche that M. genitalium occupies and how well various antibiotics work against the pathogen.
"M. genitalium is associated with 15%-22% of nongonococcal urethritis cases, and 10%-15% of cervicitis cases, and in many settings is more common that Neisseria gonorrhoeae with treatment outcomes often far worse," said Lisa E. Manhart, Ph.D., an epidemiologist at the University of Washington, Seattle. "There is no characteristic clinical syndrome for M. genitalium infections; they look very similar to Chlamydia. Clinical judgment is the only option for treatment decisions in many settings, and no FDA-approved diagnostic test [for M. genitalium] exists."
Persistent cases of nongonococcal urethritis, cervicitis, and possibly pelvic inflammatory disease could benefit from treatment with moxifloxacin (Avelox), Dr. Manhart noted. But "it is becoming clear that resistance in M. genitalium develops rapidly."
"M. genitalium is an important STI, and guidelines should reflect this; but there is no good evidence base for optimal treatment. Optimal treatment is a moving target," said Dr. Jørgen S. Jensen, a researcher at the Statens Serum Institut in Copenhagen.
"Widespread use of azithromycin and moxifloxacin will select for multidrug-resistant strains; the time for single-drug, one-dose regimens is probably over," said Dr. Jensen, specifically referring to the common practice of treating nongonococcal urethritis with a single dose of azithromycin.
M. genitalium invades U.S.
Dr. Manhart and a second U.S. researcher, Dr. Harold C. Wiesenfeld from the University of Pittsburgh, each reported new data at the meeting showing how common M. genitalium STI infections have become among U.S. patients.
Dr. Manhart presented new data from the MEGA (Mycoplasma Genitalium Antibiotic Susceptibility and Treatment) trial, which enrolled 606 men with nongonococcal urethritis (NGU) at an STI clinic in Seattle. The study’s primary endpoint was a comparison of 100 mg doxycycline b.i.d. for 7 days and a single 1-g dose of azithromycin.
The two regimens produced similar cure rates – 76% in the doxycycline arm, and 80% in the azithromycin arm, Dr. Manhart and her associates reported earlier this year (Clin. Infec. Dis. 2013;56:934-42). The initial report also identified M. genitalium in 13% of those men – identified using an in-house polymerase chain reaction assay – compared with 24% who tested positive for Chlamydia and 23% infected with Ureaplasma urealyticum biovar.
The new analyses Dr. Manhart reported tracked the outcomes of patients infected with M. genitalium. Treatment with either of the standard doxycycline or azithromycin regimens failed about half the time, Dr. Manhart said: 29% of men with doxycycline-resistant infections who were retreated with azithromycin as part of the study’s extended protocol carried M. genitalium, and 70% of the men who failed initial azithromycin treatment who were then retreated with doxycycline had persistent infection with M. genitalium.
Results from the extended portion of the study also showed that treatment with moxifloxacin was the answer for most of the otherwise unresponsive M. genitalium infections, but it wasn’t perfect. The M. genitalium infection persisted in 12%-15% of those men after a full course of moxifloxacin.
The full results suggest that moxifloxacin is potentially effective for treating various persistent STIs, not only NGU but also cervicitis and possibly pelvic inflammatory disease (PID). But resistance to moxifloxacin develops "rapidly," meaning that surveillance for resistance is needed, as well as new drug alternatives, she said.
New suspect in acute PID?
Although Dr. Manhart hedged on the role of M. genitalium in PID, results from a different U.S. study created a strong case for a role in acute PID.
M. genitalium appeared in 28 (18%) of 157 diagnosed women with acute PID who were enrolled in a study that had primarily focused on comparing two antibiotic regimens, and in 30% of those women with histologically proven acute PID. Using an in-house transcription-mediated assay for M. genitalium, researchers at the University of Pittsburgh found that endometrial identification of M. genitalium linked independently with a fourfold increased prevalence of histologically confirmed acute PID.
Those numbers for M. genitalium put it in the same ball park in the study with the two traditional heavy hitters of acute PID, N. gonorrhoeae and C. trachomatis. By establishing a significant role for M. genitalium in acute PID, the data immediately called into question the standard empiric therapies for acute PID.
"The PID treatments we use fall short for eradicating M. genitalium," said Dr. Wiesenfeld, an ob.gyn. and infectious diseases physician at the University of Pittsburgh, who reported the results. "Whether these findings [affect] treatment guidelines for acute PID remains to be seen; but if it is truly important to treat M. genitalium, it will completely turn around our treatment regimens."
The looming dilemma is that the azithromycin or doxycycline used for gonorrhea will not stop many of the infections by M. genitalium, while the moxifloxacin that can handle most M. genitalium today does not eradicate N. gonorrhoeae.
However, it’s premature to consider routinely testing or screening for M. genitalium in patients with PID or other possible forms of M. genitalium infection, Dr. Wiesenfeld cautioned. That’s in part because of the current logistical limitations on testing, and in part because the long-term impact of M. genitalium infection on reproductive health is not yet established. Longer follow-up of women in the study should shed more light on the natural history of the patients who received treatments that did not eradicate M. genitalium.
"If M. genitalium turns out to be associated with PID, it is the single organism that is not covered by current treatment with a cephalosporin, doxycycline, and metronidazole," said Sharon L. Hillier, Ph. D., in an interview during the meeting. "We are very concerned about it because it is a fairly sizable fraction of the STIs we’ve seen in these women with acute PID," said Dr. Hillier, professor of ob.gyn. and reproductive sciences at the University of Pittsburgh and a collaborator with Dr. Wiesenfeld on his study.
Dr. Hillier agreed that the key question to address is the fertility risk to women from having PID caused by M. genitalium.
"It’s so common that it might have a huge population impact," she said. "We know fertility outcomes are bad from gonorrhea and Chlamydia." The fertility risk from M. genitalium "will be what makes us decide if we need to add another treatment."
Adding moxifloxacin to routine, empiric treatment for acute PID would be an especially tough call if it also meant dropping doxycycline, a drug that is otherwise attractive because of its low cost and broad spectrum of activity against other PID pathogens.
"It remains to be seen what we should do for empiric therapy for women who walk in with PID, what is the best way to try to preserve her fertility," Dr. Hillier said. "We simply don’t know right now, but it’s been a huge topic of conversation."
Changing the treatment strategies
While the best initial management strategy for acute PID remains unclear, the specter of M. genitalium has already changed the management strategy used by Dr. Paddy Horner to treat men with NGU, said Dr. Horner, a physician in the school of social and community medicine at the University of Bristol, U.K.
These days, his preferred approach is what he calls "infection-specific" first-line therapy: Before treatment begins, he eliminates purely empiric therapy by employing a commercially available, nucleic-acid amplification test for gonorrhea and Chlamydia at the first encounter and getting the result in 30 minutes.
That means treating men who test positive for Chlamydia with a week of doxycycline first, or starting with a 5-day course of azithromycin for men who are Chlamydia negative. However, he advised using a single, 1-g dosage of azithromycin with caution, because of the prevalence of macrolide resistance. But Dr. Horner also admitted that no evidence has proven the superiority of the 5-day alternative that starts with a 1-g dose followed by 500 mg daily for 4 more days. Men who test positive for N. gonorrhoeae should receive 1 g of azithromycin plus 500 mg ceftriaxone.
If the urethritis persists 2 weeks later, Dr. Horner recommended treating patients empirically with a combination of moxifloxacin and metronidazole to cover possible infection by either M. genitalium or U. urealyticum.
In theory, this overall approach has the potential to resolve 89% of infections after the first round of treatment and 99% after the second round, with low potential for generating resistant strains of M. genitalium, based on pathogen prevalence and susceptibility profiles that Dr. Horner sees in Bristol. Those outcomes are an improvement on the cure rates and resistance risks when initial treatment is applied completely empirically, he explained.
Infection-specific treatment would work even better once rapid, point-of-care genetic tests become available for M. genitalium and U. urealyticum, Dr. Horner said.
Dr. Manhart, Dr. Wiesenfeld, and Dr. Hillier had no disclosures. Dr. Jensen said that his institution provides diagnostic testing for M. genitalium commercially and also evaluates various new antimicrobials under contract. Dr. Horner said that he has been a consultant to or received research support from Aquarius Population Health, Cepheid, Hologic, and Siemens.
On Twitter @mitchelzoler
EXPERT ANALYSIS FROM THE STI & AIDS WORLD CONGRESS 2013
Elevated troponin but no CVD: What’s the prognosis?
Patients with elevated troponin levels and chronic renal disease, pulmonary hypertension, pulmonary embolism, chronic obstructive pulmonary disease, sepsis, or acute ischemic stroke have a 2- to 5-fold increased risk of death, even in the absence of known cardiovascular disease (TABLE)1-6 (strength of recommendation: B, meta-analysis, multiple prospective and retrospective observational studies.)
EVIDENCE SUMMARY
To investigate the prognostic value of troponin on overall mortality, a multicenter prospective study followed 847 patients 18 years and older (mean age 59 years) with end-stage renal disease whose troponin T levels were measured 3 months from the start of peritoneal dialysis or hemodialysis until transplantation or death.1 At enrollment, 566 patients had a troponin level of ≤0.04 ng/dL, 188 had a value between 0.05 and 0.10 ng/dL, and 93 had a level of more than 0.10 ng/dL.
Using Cox regression, patients whose troponin levels were more than 0.10 ng/dL had an increased hazard ratio (HR) for all-cause mortality of 2.2 (95% confidence interval [CI], 1.7-2.8) compared with patients who had levels ≤0.04 ng/dL. Cardiovascular mortality also was higher (HR=1.9; 95% CI, 0.9-3.7) with troponin elevations, but didn’t reach statistical significance. Investigators found no significant differences in mortality risk between patients on peritoneal or hemodialysis, patients with or without a history of acute myocardial infarction, or patients who suffered cerebrovascular accidents.
Elevated troponin raises risk of death 5-fold in pulmonary embolism patients
A meta-analysis of 20 trials with a total of 1985 patients assessed the prognostic value of troponin for short-term mortality in patients admitted with acute pulmonary embolism.2 Sixteen studies (1527 patients) were prospective trials and the remainder (458 patients) were retrospective trials. Investigators obtained troponin levels for all patients at admission. They used several different troponin assays (both I and T), but most of the studies used the assay manufacturers’ cutoff points (exceeding the 99th percentile).
High troponin levels were associated with a 5-fold increased risk of short-term death, defined as in-hospital death up to 30 days after discharge (19.7% with elevated troponin vs 3.7% with normal troponin; odds ratio [OR]=5.24; 95% CI, 3.3-8.4).
Increased risk of death among those with pulmonary hypertension, COPD A prospective single-center study of 56 patients with chronic pulmonary hypertension found that the 14% of those whose troponin T was elevated (≥0.01 ng/mL) had a lower survival rate than the other patients. Patients who either had a positive troponin on initial assessment or developed troponin elevation within the 2-year follow-up period had a cumulative 24-month survival rate of 29%, compared with 81% for their troponin T-negative counterparts (P=.001).3
Patients with elevated troponin levels and certain conditions have a 2- to 5-fold increased risk of death, even without known cardiovascular disease.
Elevated troponin I is an independent predictor of mortality in severe sepsis
A double-blind, placebo-controlled, phase 3 trial evaluated the effect of drotrecogin alfa (activated)—withdrawn from the market in 2011—on survival of patients with severe sepsis.5 Investigators used positive troponin I levels (≥0.06 ng/mL) as a prognostic indicator of mortality. Patients who were troponin-positive had a 28-day mortality rate of 32%, compared with 14% in the troponin-negative group (P<.0001).
A bias of this study is that the patients with positive troponin levels tended to be older and more critically ill. However, in a multivariate model, troponin I still remained an independent predictor of mortality.
Elevated troponin predicts increased death risk in up to 20% of stroke patients
A systematic review of 15 trials with a total of 2901 patients evaluated the relationship between troponin levels and stroke.6 Investigators assessed the prevalence of elevated troponin in acute stroke patients, the association of elevated troponin levels with electrocardiographic changes, and the overall morbidity and mortality associated with troponin levels. Thirteen of the 15 studies used a troponin T or I level obtained within 72 hours of admission and a cut-off level of 0.1 ng/mL. The remaining 2 studies used troponin I cut-off levels >0.2 and 0.4 ng/mL.
Overall, 18% of acute stroke patients had elevated troponin levels. Studies that excluded patients with known cardiac disease had a lower prevalence of elevated levels (10% vs 22%). Patients with elevated troponin levels had an associated overall increased risk of death (OR=2.9; 95% CI, 1.7-4.8) and were 3 times more likely to have ischemic changes on electrocardiogram (OR=3.0; 95% CI, 1.5-6.2). Investigators concluded that elevated troponin levels occur in as many as one in 5 patients and are associated with an increased risk of death.
Troponin elevations may be observed in congestive heart failure, chest wall trauma, cardioversion/defibrillator shocks, rhabdomyolysis, and ultra-endurance activities.7 However, this analysis didn’t address prognostic implications of elevated troponins.
RECOMMENDATIONS
No recommendation exists for biochemical testing of troponins in various medical conditions except in the presence of signs and symptoms consistent with acute coronary syndrome. The American College of Cardiology and American Heart Association recommend routine testing of cardiac troponins in patients hospitalized for worsening congestive heart failure symptoms.8
The European Society of Cardiology recommends measuring troponin levels to further stratify risk in non-high-risk patients with confirmed pulmonary embolus.9
The National Academy of Clinical Biochemistry recommends using cardiac troponins to help define mortality risk in end-stage renal disease and critically ill patients.10
1. Havekes B, van Manen J, Krediet R, et al. Serum troponin T concentration as a predictor of mortality in hemodialysis and peritoneal dialysis patients. Am J Kidney Dis. 2006;47:823-829.
2. Becattini C, Vedovati MC, Agnelli G. Prognostic value of tropo- nins in acute pulmonary embolism. Circulation. 2007;116:427- 433.
3. Torbicki A, Kurzyna M, Kuca P, et al. Detectable serum cardiac troponin T as a marker of poor prognosis among patients with chronic precapillary pulmonary hypertension. Circulation. 2003;108:844-848.
4. Brekke PH, Omland T, Holmedal SH, et al. Troponin T eleva- tion and long-term mortality after chronic obstructive pulmo- nary disease exacerbation. Eur Respir J. 2008;31:563-570.
5. John J, Woodward DB, Wang Y, et al. Troponin I as a prog- nosticator of mortality in severe sepsis patients. J Crit Care. 2010;25:270-275.
6. Kerr G, Ray G, Wu O, et al. Elevated troponin after stroke: a sys- tematic review. Cerebrovasc Dis. 2009;28:220-226.
7. Korff S, Katus HA, Giannitsis E. Differential diagnosis of el- evated troponins. Heart. 2006;92:987-993.
8. Hunt SA, Abraham WT, Chin MH, et al. 2009 Focused update incorporated into the ACC/AHA 2005 guidelines for the diag- nosis and management of heart failure in adults. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines devel- oped in collaboration with the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2009;53:e1-e90.
9. Torbicki A, Perrier A, Konstantinides S, et al. Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology. Eur Heart J. 2008;29:2276-2315.
10. Wu AH, Jaffe AS, Apple FS, et al. National Academy of Clinical Biochemistry laboratory medicine practice guidelines: use of cardiac troponin and B-type natriuretic peptide or N-terminal proB-type natriuretic peptide for etiologies other than acute coronary syndromes and heart failure. Clin Chem. 2007;53:2086-2096.
Patients with elevated troponin levels and chronic renal disease, pulmonary hypertension, pulmonary embolism, chronic obstructive pulmonary disease, sepsis, or acute ischemic stroke have a 2- to 5-fold increased risk of death, even in the absence of known cardiovascular disease (TABLE)1-6 (strength of recommendation: B, meta-analysis, multiple prospective and retrospective observational studies.)
EVIDENCE SUMMARY
To investigate the prognostic value of troponin on overall mortality, a multicenter prospective study followed 847 patients 18 years and older (mean age 59 years) with end-stage renal disease whose troponin T levels were measured 3 months from the start of peritoneal dialysis or hemodialysis until transplantation or death.1 At enrollment, 566 patients had a troponin level of ≤0.04 ng/dL, 188 had a value between 0.05 and 0.10 ng/dL, and 93 had a level of more than 0.10 ng/dL.
Using Cox regression, patients whose troponin levels were more than 0.10 ng/dL had an increased hazard ratio (HR) for all-cause mortality of 2.2 (95% confidence interval [CI], 1.7-2.8) compared with patients who had levels ≤0.04 ng/dL. Cardiovascular mortality also was higher (HR=1.9; 95% CI, 0.9-3.7) with troponin elevations, but didn’t reach statistical significance. Investigators found no significant differences in mortality risk between patients on peritoneal or hemodialysis, patients with or without a history of acute myocardial infarction, or patients who suffered cerebrovascular accidents.
Elevated troponin raises risk of death 5-fold in pulmonary embolism patients
A meta-analysis of 20 trials with a total of 1985 patients assessed the prognostic value of troponin for short-term mortality in patients admitted with acute pulmonary embolism.2 Sixteen studies (1527 patients) were prospective trials and the remainder (458 patients) were retrospective trials. Investigators obtained troponin levels for all patients at admission. They used several different troponin assays (both I and T), but most of the studies used the assay manufacturers’ cutoff points (exceeding the 99th percentile).
High troponin levels were associated with a 5-fold increased risk of short-term death, defined as in-hospital death up to 30 days after discharge (19.7% with elevated troponin vs 3.7% with normal troponin; odds ratio [OR]=5.24; 95% CI, 3.3-8.4).
Increased risk of death among those with pulmonary hypertension, COPD A prospective single-center study of 56 patients with chronic pulmonary hypertension found that the 14% of those whose troponin T was elevated (≥0.01 ng/mL) had a lower survival rate than the other patients. Patients who either had a positive troponin on initial assessment or developed troponin elevation within the 2-year follow-up period had a cumulative 24-month survival rate of 29%, compared with 81% for their troponin T-negative counterparts (P=.001).3
Patients with elevated troponin levels and certain conditions have a 2- to 5-fold increased risk of death, even without known cardiovascular disease.
Elevated troponin I is an independent predictor of mortality in severe sepsis
A double-blind, placebo-controlled, phase 3 trial evaluated the effect of drotrecogin alfa (activated)—withdrawn from the market in 2011—on survival of patients with severe sepsis.5 Investigators used positive troponin I levels (≥0.06 ng/mL) as a prognostic indicator of mortality. Patients who were troponin-positive had a 28-day mortality rate of 32%, compared with 14% in the troponin-negative group (P<.0001).
A bias of this study is that the patients with positive troponin levels tended to be older and more critically ill. However, in a multivariate model, troponin I still remained an independent predictor of mortality.
Elevated troponin predicts increased death risk in up to 20% of stroke patients
A systematic review of 15 trials with a total of 2901 patients evaluated the relationship between troponin levels and stroke.6 Investigators assessed the prevalence of elevated troponin in acute stroke patients, the association of elevated troponin levels with electrocardiographic changes, and the overall morbidity and mortality associated with troponin levels. Thirteen of the 15 studies used a troponin T or I level obtained within 72 hours of admission and a cut-off level of 0.1 ng/mL. The remaining 2 studies used troponin I cut-off levels >0.2 and 0.4 ng/mL.
Overall, 18% of acute stroke patients had elevated troponin levels. Studies that excluded patients with known cardiac disease had a lower prevalence of elevated levels (10% vs 22%). Patients with elevated troponin levels had an associated overall increased risk of death (OR=2.9; 95% CI, 1.7-4.8) and were 3 times more likely to have ischemic changes on electrocardiogram (OR=3.0; 95% CI, 1.5-6.2). Investigators concluded that elevated troponin levels occur in as many as one in 5 patients and are associated with an increased risk of death.
Troponin elevations may be observed in congestive heart failure, chest wall trauma, cardioversion/defibrillator shocks, rhabdomyolysis, and ultra-endurance activities.7 However, this analysis didn’t address prognostic implications of elevated troponins.
RECOMMENDATIONS
No recommendation exists for biochemical testing of troponins in various medical conditions except in the presence of signs and symptoms consistent with acute coronary syndrome. The American College of Cardiology and American Heart Association recommend routine testing of cardiac troponins in patients hospitalized for worsening congestive heart failure symptoms.8
The European Society of Cardiology recommends measuring troponin levels to further stratify risk in non-high-risk patients with confirmed pulmonary embolus.9
The National Academy of Clinical Biochemistry recommends using cardiac troponins to help define mortality risk in end-stage renal disease and critically ill patients.10
Patients with elevated troponin levels and chronic renal disease, pulmonary hypertension, pulmonary embolism, chronic obstructive pulmonary disease, sepsis, or acute ischemic stroke have a 2- to 5-fold increased risk of death, even in the absence of known cardiovascular disease (TABLE)1-6 (strength of recommendation: B, meta-analysis, multiple prospective and retrospective observational studies.)
EVIDENCE SUMMARY
To investigate the prognostic value of troponin on overall mortality, a multicenter prospective study followed 847 patients 18 years and older (mean age 59 years) with end-stage renal disease whose troponin T levels were measured 3 months from the start of peritoneal dialysis or hemodialysis until transplantation or death.1 At enrollment, 566 patients had a troponin level of ≤0.04 ng/dL, 188 had a value between 0.05 and 0.10 ng/dL, and 93 had a level of more than 0.10 ng/dL.
Using Cox regression, patients whose troponin levels were more than 0.10 ng/dL had an increased hazard ratio (HR) for all-cause mortality of 2.2 (95% confidence interval [CI], 1.7-2.8) compared with patients who had levels ≤0.04 ng/dL. Cardiovascular mortality also was higher (HR=1.9; 95% CI, 0.9-3.7) with troponin elevations, but didn’t reach statistical significance. Investigators found no significant differences in mortality risk between patients on peritoneal or hemodialysis, patients with or without a history of acute myocardial infarction, or patients who suffered cerebrovascular accidents.
Elevated troponin raises risk of death 5-fold in pulmonary embolism patients
A meta-analysis of 20 trials with a total of 1985 patients assessed the prognostic value of troponin for short-term mortality in patients admitted with acute pulmonary embolism.2 Sixteen studies (1527 patients) were prospective trials and the remainder (458 patients) were retrospective trials. Investigators obtained troponin levels for all patients at admission. They used several different troponin assays (both I and T), but most of the studies used the assay manufacturers’ cutoff points (exceeding the 99th percentile).
High troponin levels were associated with a 5-fold increased risk of short-term death, defined as in-hospital death up to 30 days after discharge (19.7% with elevated troponin vs 3.7% with normal troponin; odds ratio [OR]=5.24; 95% CI, 3.3-8.4).
Increased risk of death among those with pulmonary hypertension, COPD A prospective single-center study of 56 patients with chronic pulmonary hypertension found that the 14% of those whose troponin T was elevated (≥0.01 ng/mL) had a lower survival rate than the other patients. Patients who either had a positive troponin on initial assessment or developed troponin elevation within the 2-year follow-up period had a cumulative 24-month survival rate of 29%, compared with 81% for their troponin T-negative counterparts (P=.001).3
Patients with elevated troponin levels and certain conditions have a 2- to 5-fold increased risk of death, even without known cardiovascular disease.
Elevated troponin I is an independent predictor of mortality in severe sepsis
A double-blind, placebo-controlled, phase 3 trial evaluated the effect of drotrecogin alfa (activated)—withdrawn from the market in 2011—on survival of patients with severe sepsis.5 Investigators used positive troponin I levels (≥0.06 ng/mL) as a prognostic indicator of mortality. Patients who were troponin-positive had a 28-day mortality rate of 32%, compared with 14% in the troponin-negative group (P<.0001).
A bias of this study is that the patients with positive troponin levels tended to be older and more critically ill. However, in a multivariate model, troponin I still remained an independent predictor of mortality.
Elevated troponin predicts increased death risk in up to 20% of stroke patients
A systematic review of 15 trials with a total of 2901 patients evaluated the relationship between troponin levels and stroke.6 Investigators assessed the prevalence of elevated troponin in acute stroke patients, the association of elevated troponin levels with electrocardiographic changes, and the overall morbidity and mortality associated with troponin levels. Thirteen of the 15 studies used a troponin T or I level obtained within 72 hours of admission and a cut-off level of 0.1 ng/mL. The remaining 2 studies used troponin I cut-off levels >0.2 and 0.4 ng/mL.
Overall, 18% of acute stroke patients had elevated troponin levels. Studies that excluded patients with known cardiac disease had a lower prevalence of elevated levels (10% vs 22%). Patients with elevated troponin levels had an associated overall increased risk of death (OR=2.9; 95% CI, 1.7-4.8) and were 3 times more likely to have ischemic changes on electrocardiogram (OR=3.0; 95% CI, 1.5-6.2). Investigators concluded that elevated troponin levels occur in as many as one in 5 patients and are associated with an increased risk of death.
Troponin elevations may be observed in congestive heart failure, chest wall trauma, cardioversion/defibrillator shocks, rhabdomyolysis, and ultra-endurance activities.7 However, this analysis didn’t address prognostic implications of elevated troponins.
RECOMMENDATIONS
No recommendation exists for biochemical testing of troponins in various medical conditions except in the presence of signs and symptoms consistent with acute coronary syndrome. The American College of Cardiology and American Heart Association recommend routine testing of cardiac troponins in patients hospitalized for worsening congestive heart failure symptoms.8
The European Society of Cardiology recommends measuring troponin levels to further stratify risk in non-high-risk patients with confirmed pulmonary embolus.9
The National Academy of Clinical Biochemistry recommends using cardiac troponins to help define mortality risk in end-stage renal disease and critically ill patients.10
1. Havekes B, van Manen J, Krediet R, et al. Serum troponin T concentration as a predictor of mortality in hemodialysis and peritoneal dialysis patients. Am J Kidney Dis. 2006;47:823-829.
2. Becattini C, Vedovati MC, Agnelli G. Prognostic value of tropo- nins in acute pulmonary embolism. Circulation. 2007;116:427- 433.
3. Torbicki A, Kurzyna M, Kuca P, et al. Detectable serum cardiac troponin T as a marker of poor prognosis among patients with chronic precapillary pulmonary hypertension. Circulation. 2003;108:844-848.
4. Brekke PH, Omland T, Holmedal SH, et al. Troponin T eleva- tion and long-term mortality after chronic obstructive pulmo- nary disease exacerbation. Eur Respir J. 2008;31:563-570.
5. John J, Woodward DB, Wang Y, et al. Troponin I as a prog- nosticator of mortality in severe sepsis patients. J Crit Care. 2010;25:270-275.
6. Kerr G, Ray G, Wu O, et al. Elevated troponin after stroke: a sys- tematic review. Cerebrovasc Dis. 2009;28:220-226.
7. Korff S, Katus HA, Giannitsis E. Differential diagnosis of el- evated troponins. Heart. 2006;92:987-993.
8. Hunt SA, Abraham WT, Chin MH, et al. 2009 Focused update incorporated into the ACC/AHA 2005 guidelines for the diag- nosis and management of heart failure in adults. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines devel- oped in collaboration with the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2009;53:e1-e90.
9. Torbicki A, Perrier A, Konstantinides S, et al. Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology. Eur Heart J. 2008;29:2276-2315.
10. Wu AH, Jaffe AS, Apple FS, et al. National Academy of Clinical Biochemistry laboratory medicine practice guidelines: use of cardiac troponin and B-type natriuretic peptide or N-terminal proB-type natriuretic peptide for etiologies other than acute coronary syndromes and heart failure. Clin Chem. 2007;53:2086-2096.
1. Havekes B, van Manen J, Krediet R, et al. Serum troponin T concentration as a predictor of mortality in hemodialysis and peritoneal dialysis patients. Am J Kidney Dis. 2006;47:823-829.
2. Becattini C, Vedovati MC, Agnelli G. Prognostic value of tropo- nins in acute pulmonary embolism. Circulation. 2007;116:427- 433.
3. Torbicki A, Kurzyna M, Kuca P, et al. Detectable serum cardiac troponin T as a marker of poor prognosis among patients with chronic precapillary pulmonary hypertension. Circulation. 2003;108:844-848.
4. Brekke PH, Omland T, Holmedal SH, et al. Troponin T eleva- tion and long-term mortality after chronic obstructive pulmo- nary disease exacerbation. Eur Respir J. 2008;31:563-570.
5. John J, Woodward DB, Wang Y, et al. Troponin I as a prog- nosticator of mortality in severe sepsis patients. J Crit Care. 2010;25:270-275.
6. Kerr G, Ray G, Wu O, et al. Elevated troponin after stroke: a sys- tematic review. Cerebrovasc Dis. 2009;28:220-226.
7. Korff S, Katus HA, Giannitsis E. Differential diagnosis of el- evated troponins. Heart. 2006;92:987-993.
8. Hunt SA, Abraham WT, Chin MH, et al. 2009 Focused update incorporated into the ACC/AHA 2005 guidelines for the diag- nosis and management of heart failure in adults. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines devel- oped in collaboration with the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2009;53:e1-e90.
9. Torbicki A, Perrier A, Konstantinides S, et al. Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology. Eur Heart J. 2008;29:2276-2315.
10. Wu AH, Jaffe AS, Apple FS, et al. National Academy of Clinical Biochemistry laboratory medicine practice guidelines: use of cardiac troponin and B-type natriuretic peptide or N-terminal proB-type natriuretic peptide for etiologies other than acute coronary syndromes and heart failure. Clin Chem. 2007;53:2086-2096.
Evidence-based answers from the Family Physicians Inquiries Network
Just over a third of hypertensive kidney patients are at target BP
AMSTERDAM – Eighty percent of Americans with chronic kidney disease have comorbid hypertension, which is controlled in only 36% of cases, according to a large national study.
This is an area of significant unmet medical need in spite of the wide availability of antihypertensive medications, Panagiotis Mavros, Ph.D., said at the annual congress of the European Society of Cardiology.
He presented a cross-sectional study involving 159,306 American adults with stage 1-4 chronic kidney disease (CKD) identified in the GE Centricity electronic medical record database. Fifty-seven percent were in stage 3 and 29% were in stage 2 CKD.
Roughly 43% of this very large group of CKD patients carried the diagnosis of diabetes mellitus. Eighty percent of the CKD population had hypertension, including 10% of the overall study population with previously undiagnosed hypertension. Among the CKD patients with hypertension, 15% were not being treated for it, said Dr. Mavros of Merck in Whitehouse Station, N.J.
Blood pressure was controlled to the target of less than 130/80 mm Hg in 36% of patients with diagnosed hypertension.
Seventy-three percent of treated hypertensive patients with CKD were on combination therapy, typically with three different classes of antihypertensive drugs. Patients on more than three different classes of antihypertensive medications tended to have more advanced CKD. However, the proportion of treated patients with good blood pressure control did not differ according to CKD stage.
This cross-sectional study was funded by Merck. Dr. Mavros is a full-time company employee.
AMSTERDAM – Eighty percent of Americans with chronic kidney disease have comorbid hypertension, which is controlled in only 36% of cases, according to a large national study.
This is an area of significant unmet medical need in spite of the wide availability of antihypertensive medications, Panagiotis Mavros, Ph.D., said at the annual congress of the European Society of Cardiology.
He presented a cross-sectional study involving 159,306 American adults with stage 1-4 chronic kidney disease (CKD) identified in the GE Centricity electronic medical record database. Fifty-seven percent were in stage 3 and 29% were in stage 2 CKD.
Roughly 43% of this very large group of CKD patients carried the diagnosis of diabetes mellitus. Eighty percent of the CKD population had hypertension, including 10% of the overall study population with previously undiagnosed hypertension. Among the CKD patients with hypertension, 15% were not being treated for it, said Dr. Mavros of Merck in Whitehouse Station, N.J.
Blood pressure was controlled to the target of less than 130/80 mm Hg in 36% of patients with diagnosed hypertension.
Seventy-three percent of treated hypertensive patients with CKD were on combination therapy, typically with three different classes of antihypertensive drugs. Patients on more than three different classes of antihypertensive medications tended to have more advanced CKD. However, the proportion of treated patients with good blood pressure control did not differ according to CKD stage.
This cross-sectional study was funded by Merck. Dr. Mavros is a full-time company employee.
AMSTERDAM – Eighty percent of Americans with chronic kidney disease have comorbid hypertension, which is controlled in only 36% of cases, according to a large national study.
This is an area of significant unmet medical need in spite of the wide availability of antihypertensive medications, Panagiotis Mavros, Ph.D., said at the annual congress of the European Society of Cardiology.
He presented a cross-sectional study involving 159,306 American adults with stage 1-4 chronic kidney disease (CKD) identified in the GE Centricity electronic medical record database. Fifty-seven percent were in stage 3 and 29% were in stage 2 CKD.
Roughly 43% of this very large group of CKD patients carried the diagnosis of diabetes mellitus. Eighty percent of the CKD population had hypertension, including 10% of the overall study population with previously undiagnosed hypertension. Among the CKD patients with hypertension, 15% were not being treated for it, said Dr. Mavros of Merck in Whitehouse Station, N.J.
Blood pressure was controlled to the target of less than 130/80 mm Hg in 36% of patients with diagnosed hypertension.
Seventy-three percent of treated hypertensive patients with CKD were on combination therapy, typically with three different classes of antihypertensive drugs. Patients on more than three different classes of antihypertensive medications tended to have more advanced CKD. However, the proportion of treated patients with good blood pressure control did not differ according to CKD stage.
This cross-sectional study was funded by Merck. Dr. Mavros is a full-time company employee.
AT THE ESC CONGRESS 2013
Major finding: Of a large group of patients with chronic kidney disease and comorbid hypertension, 36% were at the blood pressure goal of less than 130/80 mm Hg.
Data source: A cross-sectional study involving more than 159,000 U.S. adults with stage 1-4 chronic kidney disease, 80% of whom had comorbid hypertension.
Disclosures: The study was funded by Merck. The presenter is a full-time employee of the pharmaceutical company.
When to Worry About Incidental Renal and Adrenal Masses
› Use computed tomography studies and the Bosniak classification system to
guide management of renal cystic masses. A
› Perform laboratory tests for hypercortisolism, hyperaldosteronism, and hypersecretion of catecholamines (pheochromocytoma) on any patient with an incidental adrenal mass, regardless of signs or symptoms. C
› Refer patients with adrenal masses >4 cm for surgical evaluation. Refer any individual who has a history of malignancy and an adrenal mass for oncologic evaluation. B
Strength of recommendation (SOR)
A. Good-quality patient-oriented evidence
B. Inconsistent or limited-quality patient-oriented evidence
C. Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Jane C, a 76-year-old patient, reports lower abdominal discomfort and increased bowel movements. Her left lower quadrant is tender to palpation, without signs of a surgical abdomen, and vital signs are normal. Laboratory studies are also normal, except for mild anemia and a positive fecal occult blood test. Abdominal and pelvic computed tomography (CT), with and without contrast, are negative for acute pathology, but a 1.7-cm lesion is found in the upper pole of the left kidney. What is your next step?
Renal or adrenal masses may be discovered during imaging studies for complaints unrelated to the kidneys or adrenals. Detection of incidentalomas has increased dramatically, keeping pace with the growing use of ultrasonography, CT, and magnetic resonance imaging (MRI) for abdominal, chest, and back complaints.1
Family physicians can evaluate most of these masses and determine the need for referral by using clinical judgment, appropriate imaging studies, and screening laboratory tests. In the pages that follow, we present a systematic approach for evaluating these incidentalomas and determining when consultation or referral is needed.
Incidental renal masses are common
Lesions are commonly found in normal kidneys, and the incidence increases with age. Approximately one-third of individuals age 50 and older will have at least one renal cyst on CT.2
Most incidental renal masses are benign cysts requiring no further evaluation. Other possibilities include indeterminate or malignant cysts or solid masses, which may be malignant or benign. Inflammatory renal lesions from infection, infarction, or trauma also occur, but these tend to be symptomatic and are rarely found incidentally.
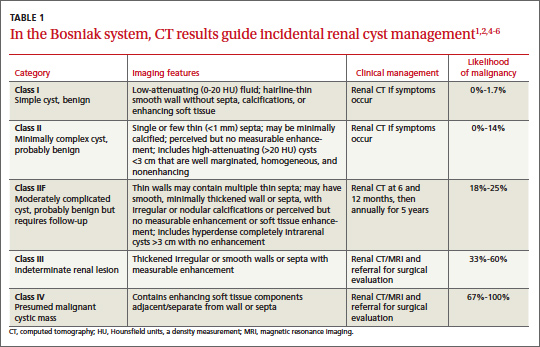
Classification of renal cysts—not based on size
Cysts are the most common adult renal masses. Typically they are unilocular and located in the renal cortex, frequently extending to the renal surface.3 Renal function is usually preserved, regardless of the cyst’s location or size. Careful examination of adjacent tissue is essential, as secondary cysts may form when solid tumors obstruct tubules of normal parenchyma. Cystic lesions containing enhancing soft tissue unattached to the wall or septa likely are malignant.4
The Bosniak classification system, with 5 classes based on CT characteristics
(TABLE 1), is a useful guide for managing renal cystic lesions.4 Size is not an important feature in the Bosniak system; small cysts may be malignant and larger ones benign. Small cysts may grow into larger benign lesions, occasionally causing flank or abdominal pain, palpable masses, or hematuria.
Simple cysts. Renal cysts that meet Bosniak class I criteria can be confidently labeled benign and need no further evaluation (FIGURE 1). Simple renal cysts on CT have homogenous low-attenuating fluid and thin nonenhancing walls without septa.4
On ultrasound, simple renal cysts show spherical or ovoid shape without internal echoes, a thin smooth wall separate from the surrounding parenchyma, and posterior wall enhancement caused by increased transmission through the water-filled cyst. The likelihood of malignancy is extremely low in a renal cyst that meets these criteria, which have a reported accuracy of 98% to 100%.3 Thus, no further evaluation is required if an obviously benign simple cyst is first noted on an adequate ultrasound. Inadequate ultrasound visualization or evidence of calcifications, septa, or multiple chambers calls for prompt renal CT.
CASE The mass on Ms. C’s left kidney is hypoattenuating and nonenhancing on CT. It meets Bosniak criteria for a benign simple cyst (class I) and requires no further evaluation or follow-up. Colonoscopy detects multiple colonic polyps that are removed, and the patient does well.
Mildly complicated cysts. Less diagnostic certainty characterizes cysts with mild abnormalities that keep them from being labeled as simple. Bosniak classes II and IIF describe mildly abnormal renal cysts. Class II cysts can be dismissed, whereas class IIF cysts require follow-up.
Class II cysts may contain a few hairline septa, fine calcium deposits in walls or septa, or an unmeasurable enhancement of the walls. A hyperattenuating but nonenhancing fluid also is described as category II. Small homogeneous cysts <3 cm, without enhancement but hyperattenuated, are reliably considered benign and need not be evaluated.2,7
Class IIF cysts may have multiple hairline-thin septa with unmeasurable enhancement or minimal smooth thickening or irregular/nodular calcifications of wall or septa without enhancing soft tissue components. Hyperattenuating cystic lesions >3 cm and intrarenal “noncortical” cysts are included in this category. Class IIF cysts require follow-up at 6 months with CT or MRI, then annually for at least 5 years.8
Obviously complicated cysts. Bosniak class III is indeterminate—neither benign nor clearly malignant. Class III cysts may have thickened borders or septa with measurable enhancement, or they may be multilocular, hemorrhagic, or infected. In 5 case series, 29 of 57 class III lesions proved to be malignant.5 MRI may characterize these lesions more definitively than CT prior to urologic referral.
Malignant cysts. Bosniak class IV renal lesions are clearly malignant, with large heterogeneous cysts or necrotic components, shaggy thickened walls, or enhancing soft tissue components separate from the wall or septa. Their unequivocal appearance results from solid tumor necrosis and liquefaction. Diagnosis is straightforward, and excision is indicated.2
A closer look at solid renal masses
Solid renal masses usually consist of enhancing tissue with little or no fluid. The goal of evaluation is to exclude malignancies, such as renal cell cancer, lymphomas, sarcomas, or metastasis. Benign solid masses include renal adenomas, angiomyolipomas, and oncocytomas, among others.
Several lesions can be diagnosed by appearance or symptoms:
Angiomyolipomas are recognized by their fat content within a noncalcified mass. Unenhanced CT usually is sufficient for diagnosis, unless the mass is very small or has atypical features.9
Vascular lesions can be identified because they enhance to the same degree as the vasculature. With the exception of inflammatory or vascular abnormalities, all enhancing lesions that do not contain fat should be presumed to be malignant.
In patients with a known extrarenal primary malignancy, 50% to 85% of incidental solid renal masses will represent metastatic disease.10 Percutaneous biopsy may be warranted to differentiate metastatic lesions from a secondary, primary (ie, renal cell carcinoma), or benign process.11
A study of 2770 solid renal mass excisions revealed that 12.8% were benign, with a direct relationship between malignancy and size. Masses <1 cm were benign 44% of the time.12 Early identification of small renal carcinomas may improve survival rates. Although renal cell carcinomas <3 cm in diameter have low metastatic potential, a solid, nonfat-containing mass should be evaluated for aggressive nephron-sparing surgery.6,13
Incidental adrenal masses occur infrequently
Adrenal incidentalomas are defined as radiographically identified masses >1 cm in diameter.14 They are much less common than their renal counterparts, with a reported prevalence of 0.35% to 5% on CT.15 Because the adrenal glands are hormonally active and receive substantial blood flow, metastatic, hormonally active, and nonfunctional causes for adrenal masses need to be considered.16
Adrenal pathology
Adrenal masses may be characterized by increased or normal adrenal function. Hyperfunctioning syndromes include hypercortisolism, hyperaldosteronism, adrenogenital hypersecretion of adrenocortical origin, and pheochromocytomas of the medulla. Symptom evaluation of these syndromes is important, but not sufficient to rule out a hyperfunctioning syndrome.
In a retrospective review of inapparent adrenal masses, ≤13% of pheochromocytomas were clinically silent.17 Therefore, laboratory testing is necessary for an incidental adrenal mass.
Nonfunctional lesions include adenomas, metastases, cysts, myelolipomas, hemorrhage, and adrenal carcinomas. These masses require evaluation for the possibility of cancer, the most common of which is metastasis. In patients with an extra-adrenal malignancy, the likelihood of malignancy in an incidental adrenal mass is at least 50%.18 An adrenal mass representing metastasis of a previously unrecognized cancer is exceedingly rare.19
Primary adrenal carcinoma is also rare, with an estimated incidence of 2 cases per one million in the general population. For patients with adrenal masses, the prevalence of carcinoma increases with lesion size (2% for tumors <4 cm, 6% for tumors 4-6 cm, and 25% for tumors >6 cm in diameter). 17 For this reason, tumors >4 cm in diameter are usually surgically resected in patients with no previous cancer history, unless radiologic criteria demonstrate clearly benign characteristics.
Although adrenal carcinomas are considered nonfunctioning, some evidence suggests they produce low levels of cortisol that may be associated with clinical features of metabolic syndrome.20
CT is first choice for adrenal mass evaluation
Dedicated adrenal CT with both unenhanced and delayed contrast-enhanced images is the most reliable study to evaluate an adrenal mass, according to the American College of Radiology. Consider another study only in patients with contrast allergy, renal compromise, or cancer history.21
Unenhanced CT can diagnose the approximately 70% of adenomas that are small, well-defined round masses with homogenous low-density lipid deposition.22 Delayed contrast enhancement can characterize most of the remaining 30%.23 Unenhanced CT with attenuation values of <10 Hounsfield units (HU) can diagnose adenomas with 71% specificity and 98% sensitivity,24 and can often diagnose simple cysts and myelolipomas, as well.
Other imaging options. MRI is an alternative to CT for patients with contraindications for contrast or radiation exposure. MRI provides less spatial resolution than CT, but chemical shift imaging can measure cytoplasmic lipid content similar to unenhanced CT. A small study found chemical shift MRI more reliable than unenhanced CT, but less reliable than CT with delayed contrast enhancement.25
Positron emission tomography (PET) is useful to noninvasively evaluate biochemical and physiologic processes. PET-CT incorporates unenhanced CT density measurements to improve PET accuracy. In a patient with a history of cancer, PET-CT has a sensitivity of 93% to 100% and a specificity of 95% in differentiating benign from malignant adrenal tumors.26
When to order a biopsy
The need for biopsy has decreased as imaging has improved, but biopsy is required whenever diagnostic imaging fails to differentiatea lesion as benign or malignant. CT guided biopsy provides diagnostic accuracy of 85% to 95%.27 Complications such as pneumothorax, hemorrhage, and bacteremia occur in 3% to 9% of biopsies. Before any adrenal biopsy, measure plasma-free metanephrines to exclude undiagnosed pheochromocytoma, which could precipitate a hypertensive crisis if untreated.22
These 3 laboratory screening tests are critical
Family physicians can perform the initial biochemical evaluation of an adrenal incidentaloma. Guidance is available from the National Institutes of Health (NIH)28 and the American Academy of Clinical Endocrinologists (AACE) (FIGURE 2).29
Regardless of signs or symptoms, perform screening laboratory tests for 3 types of adrenal hyperfunction: hypercortisolism, hyperaldosteronism, and hypersecretion of catecholamines (pheochromocytoma). Screening tests are not recommended for androgen hypersecretion, which is extremely rare and causes recognizable symptoms such as hirsutism (Table 2).29
Hypercortisolism occurs in approximately 5% of adrenal incidentalomas.30 An overnight dexamethasone suppression test (DST) is most reliable for screening, with sensitivity >95% for Cushing syndrome.31 The patient takes a 1-mg dose of oral dexamethasone at 11 pm, and a fasting plasma cortisol sample is drawn the next day at 8 am.
Dexamethasone binds to glucocorticoid receptors in the pituitary gland, suppressing adrenocorticotropic hormone secretion. Cortisol will be depressed the next morning unless the adrenal mass produces cortisol autonomously. Patients with a DST >5 mcg/dL—highly suggestive of Cushing syndrome—require further evaluation, and we suggest referral to an endocrinologist.
Hyperaldosteronism is seen in 1% to 2% of adrenal incidentalomas.32 The aldosterone- to-renin ratio (ARR) is recommended as a screening test for hyperaldosteronism, with an ARR >20 requiring further testing.33 Medications that may affect the ARR include beta-blockers, spironolactone, clonidine, diuretics, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers.29
Refer a patient with evidence of hyperaldosteronism to an endocrinologist and a surgeon with experience in managing these lesions. If the ARR test result suggests an aldosterone excess, a salt-loading test is used to verify failure of aldosterone suppression. Adrenal venous sampling is often performed prior to surgical removal to confirm that an incidentaloma is the source of hyperaldosteronism.
Pheochromocytoma. Approximately 5% of incidental adrenal lesions are pheochromocytomas.30 Many patients with these epinephrine/norepinephrine secreting tumors do not show the classic symptom triad of headache, palpitations, and diaphoresis, and approximately half have normal blood pressure.34
Identifying a pheochromocytoma is important in any patient requiring surgery or biopsy, as surgical manipulation can cause a potentially fatal intraoperative catecholamine surge. Presurgical medical management can mitigate this reaction.
A plasma-free metanephrines test, which has 95% sensitivity, is the most reliable test for pheochromocytoma.35 Medications, including tricyclic antidepressants, decongestants, amphetamines, reserpine, and phenoxybenzamine, can cause falsepositive results.29 Confirm a positive plasma-free metanephrines test with a 24-hour fractionated urine metanephrines test, and refer the patient to an endocrinologist.
Managing adrenal incidentalomas
Refer all patients with adrenal masses >4 cm for surgical evaluation because of the risk of malignancy; all patients who have a history of malignancy and an adrenal mass of any size require a referral to an oncologist. Perform the AACE-recommended 3-element biochemical workup for all masses, with the exception of definitively diagnosed cysts or myelolipomas.
Refer to an endocrinologist all patients with abnormal screening laboratory results, regardless of adrenal mass size, as well as patients with concerning clinical findings. Initiate cardiovascular, diabetes, and bone density evaluation and management for metabolic syndrome.20
Monitoring after a negative workup
Little evidence exists to guide monitoring of small adrenal incidentalomas (<4 cm) with a negative workup. The 2002 NIH report recommended annual radiologic follow-up for 5 years,28 whereas the 2009 AACE guidelines recommend radiographic follow-up at 3 to 6 months, then at one and 2 years.29
Evidence indicates that 14% of lesions will enlarge in 2 years, although the clinical significance of enlargement is unknown. Some authors argue against CT monitoring because the risk of adrenal mass progression is similar to the malignancy risk posed by 3 years of radiation exposure with CT.20
Some guidelines recommend repeat biochemical screening every 3 to 4 years.28,29 AACE guidelines quote a 47% rate of progression over 3 years, but most adrenal masses progress to subclinical Cushing syndrome— a condition of uncertain significance. Subclinical Cushing’s has not been reported to progress to the overt syndrome, and new catecholamine or aldosterone secretion is rare.
Many endocrinologists reduce the frequency of follow-up, depending on the type of adrenal mass (cyst or solid) and its size. AACE suggests CT for adenomas one to 4 cm at 12 months. AACE and NIH recommend hormonal evaluation annually for 4 years. Adrenal cysts or myelolipoma in patients without cancer need no follow-up.29
CORRESPONDENCE
James C. Higgins, DO, CAPT, MC, USN, Ret., Naval Hospital Jacksonville, Family Medicine Department, 2080 Child Street, Box 1000, Jacksonville, FL 32214;
[email protected]
1. Berland LL, Silverman SG, Gore RM, et al. Managing incidental findings on abdominal CT: white paper of the ACR incidental findings committee. J Am Coll Radiol. 2010;7:754-773.
2. Silverman S, Israel G, Herts B, et al. Management of the incidental renal mass. Radiology. 2008;249:16-31.
3. Curry NS, Bissada NK. Radiologic evaluation of small and indeterminate renal masses. Urol Clin North Am. 1997;24:493-505.
4. Bosniak MA. The current radiological approach to renal cysts. Radiology. 1986;158:1-10.
5. Harisinghani M, Maher M, Gervais D, et al. Incidence of malignancy in complex cystic renal masses (Bosniak category III): should imaging guided biopsy precede surgery? AJR Am J Roentgenol. 2003;180:755-758.
6. Remzi M, Ozsoy M, Klingler HC. Are small renal tumors harmless? Analysis of histopathological features according to tumors less than 4 cm in diameter. J Urol. 2006;176:896-899.
7. Jonisch AI, Rubinowitz A, Mutalik P, et al. Can high attenuation renal cysts be differentiated from renal cell carcinoma at unenhanced computed tomography? Radiology. 2007;243:445-450.
8. Israel GM, Bosniak MA. Follow-up CT of moderately complex cystic lesions of the kidney. AJR Am J Roentgenol. 2003;181: 627-633.
9. Bosniak MA, Megibow AJ, Hulnick DH, et al. CT diagnosis of renal angiomyolipoma: the importance of detecting small amounts of fat. AJR Am J Roentengol. 1988;151:497-501.
10. Mitnick JS, Bosniak MA, Rothberg M, et al. Metastatic neoplasm to the kidney studied by computed tomography and sonogram. J Comput Assist Tomogr. 1985;9:43-49.
11. Rybicki FJ, Shu KM, Cibas ES, et al. Percutaneous biopsy of renal masses: sensitivity and negative predictive value stratified by clinical setting and size of masses. AJR Am J Roentgenol. 2003;180:1281-1287.
12. Frank I, Blure MI, Cheville JC, et al. Solid renal tumors: an analysis of pathological features related to tumor size. J Urol. 2003;170:2217-2220.
13. Hollingsworth JM, Miller DC, Daignault S, et al. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst. 2006;98:1331-1334.
14. Geelhoed GW, Spiegel CT. “Incidental” adrenal cyst: a correctable lesion possibly associated with hypertension. South Med J. 1981;74:626-630.
15. Davenport C, Liew A, Doherty B, et al. The prevalence of adrenal incidentaloma in routine clinical practice. Endocrine. 2011;40: 80-83.
16. Cook DM, Loriaux LD. The incidental adrenal mass. Am J Med. 1996;101:88 94.
17. Mansmann G, Lau J, Balk E, et al. The clinically inapparent adrenal mass: update in diagnosis and management. Endocr Rev. 2004;25:309-340.
18. Androulakis II, Kaltsas G, Piatitis G, et al. The clinical significance of adrenal incidentalomas. Eur J Clin Invest. 2011;41: 552-560.
19. Lee JE, Evans DB, Hickey RC, et al. Unknown primary cancer presenting as an adrenal mass: frequency and implications for diagnostic evaluation of adrenal incidentalomas. Surgery. 1998;124:1115-1122.
20. Aron D, Terzolo M, Cawood TJ. Adrenal incidentalomas. Best Pract Res Clin Endocrinol Metab. 2012;26:69-82.
21. ACR appropriateness criteria: incidentally discovered adrenal mass. American College of Radiology. Available at: http://www.acr.org/~/media/ACR/Documents/AppCriteria/Diagnostic/IncidentallyDiscoveredAdrenalMass.pdf. Accessed November 20, 2012.
22. Song JH, Mayo-Smith WW. Incidentally discovered adrenal mass. Radiol Clin North Am. 2011;49:361-368.
23. Korobkin M, Brodeur FJ, Francis IR, et al. CT time-attenuation washout curves of adrenal adenomas and nonadenomas. AJR Am J Roentgenol. 1998;170:747-752.
24. Boland GW, Lee MJ, Gazelle GS, et al. Characterization of adrenal masses using unenhanced CT: an analysis of the CT literature. AJR Am J Roentgenol. 1998;171:201-204.
25. Park BK, Kim CK, Kim B, et al. Chemical shift MR imaging of hyperattenuating (>10 HU) adrenal masses: does it still have a role? Radiology. 2004;231:711-716.
26. Boland GW, Blake MA, Holakere NS, et al. PET/CT for the characterization of adrenal masses in patients with cancer: qualitative vs quantitative accuracy in 150 consecutive patients. AJR Am J Roentgenol. 2009;192:956-962.
27. Paulsen SD, Nghiem HV, Korobkin M, et al. Changing role of imaging- guided percutaneous biopsy of adrenal masses: evaluation of 50 adrenal biopsies. AJR Am J Roentgenol. 2004;182:1033-1037
28. Grumbach MM, Biller BMK, Braunstein GD, et al. Management of the clinically inapparent adrenal mass (“incidentalomas”). Ann Intern Med. 2003;138:424-429.
29. Zeiger MA, Thompson GB, Quan-Yang D, et al. American Association of Clinical Endocrinologists and American Association of Endocrine Surgeons medical guidelines for the management of adrenal incidentalomas. Endocr Pract. 2009;15(suppl 1):1-20.
30. Young WF. The incidentally discovered adrenal mass. N Engl J Med. 2007; 356:601-610.
31. Deutschbein T, Unger N, Hinrichs J, et al. Late-night and lowdose dexamethasone-suppressed cortisol in saliva and serum for the diagnosis of cortisol-secreting adrenal adenomas. Eur J Endocrinol. 2009;161:747-753.
32. Bernini G, Moretti A, Gianfranco A, et al. Primary aldosteronism in normokalemic patients with adrenal incidentalomas. Eur J Endocrinol. 2002;146:523-529.
33. Montori VM, Young WF Jr. Use of plasma aldosterone concentration-to-plasma renin activity ratio as a screening test for primary aldosteronism: a systematic review of the literature. Endocrinol Metab Clin North Am. 2002;31:619-632.
34. Motta-Ramirez GA, Remer EM, Herts BR, et al. Comparison of CT findings in symptomatic and incidentally discovered pheochromocytomas. AJR Am J Roentgenol. 2005;185:684-688.
35. Pacak K, Eisenhofer G, Grossman A. The incidentally discovered adrenal mass. N Engl J Med. 2007;356:2005.
› Use computed tomography studies and the Bosniak classification system to
guide management of renal cystic masses. A
› Perform laboratory tests for hypercortisolism, hyperaldosteronism, and hypersecretion of catecholamines (pheochromocytoma) on any patient with an incidental adrenal mass, regardless of signs or symptoms. C
› Refer patients with adrenal masses >4 cm for surgical evaluation. Refer any individual who has a history of malignancy and an adrenal mass for oncologic evaluation. B
Strength of recommendation (SOR)
A. Good-quality patient-oriented evidence
B. Inconsistent or limited-quality patient-oriented evidence
C. Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Jane C, a 76-year-old patient, reports lower abdominal discomfort and increased bowel movements. Her left lower quadrant is tender to palpation, without signs of a surgical abdomen, and vital signs are normal. Laboratory studies are also normal, except for mild anemia and a positive fecal occult blood test. Abdominal and pelvic computed tomography (CT), with and without contrast, are negative for acute pathology, but a 1.7-cm lesion is found in the upper pole of the left kidney. What is your next step?
Renal or adrenal masses may be discovered during imaging studies for complaints unrelated to the kidneys or adrenals. Detection of incidentalomas has increased dramatically, keeping pace with the growing use of ultrasonography, CT, and magnetic resonance imaging (MRI) for abdominal, chest, and back complaints.1
Family physicians can evaluate most of these masses and determine the need for referral by using clinical judgment, appropriate imaging studies, and screening laboratory tests. In the pages that follow, we present a systematic approach for evaluating these incidentalomas and determining when consultation or referral is needed.
Incidental renal masses are common
Lesions are commonly found in normal kidneys, and the incidence increases with age. Approximately one-third of individuals age 50 and older will have at least one renal cyst on CT.2
Most incidental renal masses are benign cysts requiring no further evaluation. Other possibilities include indeterminate or malignant cysts or solid masses, which may be malignant or benign. Inflammatory renal lesions from infection, infarction, or trauma also occur, but these tend to be symptomatic and are rarely found incidentally.

Classification of renal cysts—not based on size
Cysts are the most common adult renal masses. Typically they are unilocular and located in the renal cortex, frequently extending to the renal surface.3 Renal function is usually preserved, regardless of the cyst’s location or size. Careful examination of adjacent tissue is essential, as secondary cysts may form when solid tumors obstruct tubules of normal parenchyma. Cystic lesions containing enhancing soft tissue unattached to the wall or septa likely are malignant.4
The Bosniak classification system, with 5 classes based on CT characteristics
(TABLE 1), is a useful guide for managing renal cystic lesions.4 Size is not an important feature in the Bosniak system; small cysts may be malignant and larger ones benign. Small cysts may grow into larger benign lesions, occasionally causing flank or abdominal pain, palpable masses, or hematuria.
Simple cysts. Renal cysts that meet Bosniak class I criteria can be confidently labeled benign and need no further evaluation (FIGURE 1). Simple renal cysts on CT have homogenous low-attenuating fluid and thin nonenhancing walls without septa.4
On ultrasound, simple renal cysts show spherical or ovoid shape without internal echoes, a thin smooth wall separate from the surrounding parenchyma, and posterior wall enhancement caused by increased transmission through the water-filled cyst. The likelihood of malignancy is extremely low in a renal cyst that meets these criteria, which have a reported accuracy of 98% to 100%.3 Thus, no further evaluation is required if an obviously benign simple cyst is first noted on an adequate ultrasound. Inadequate ultrasound visualization or evidence of calcifications, septa, or multiple chambers calls for prompt renal CT.
CASE The mass on Ms. C’s left kidney is hypoattenuating and nonenhancing on CT. It meets Bosniak criteria for a benign simple cyst (class I) and requires no further evaluation or follow-up. Colonoscopy detects multiple colonic polyps that are removed, and the patient does well.
Mildly complicated cysts. Less diagnostic certainty characterizes cysts with mild abnormalities that keep them from being labeled as simple. Bosniak classes II and IIF describe mildly abnormal renal cysts. Class II cysts can be dismissed, whereas class IIF cysts require follow-up.
Class II cysts may contain a few hairline septa, fine calcium deposits in walls or septa, or an unmeasurable enhancement of the walls. A hyperattenuating but nonenhancing fluid also is described as category II. Small homogeneous cysts <3 cm, without enhancement but hyperattenuated, are reliably considered benign and need not be evaluated.2,7
Class IIF cysts may have multiple hairline-thin septa with unmeasurable enhancement or minimal smooth thickening or irregular/nodular calcifications of wall or septa without enhancing soft tissue components. Hyperattenuating cystic lesions >3 cm and intrarenal “noncortical” cysts are included in this category. Class IIF cysts require follow-up at 6 months with CT or MRI, then annually for at least 5 years.8
Obviously complicated cysts. Bosniak class III is indeterminate—neither benign nor clearly malignant. Class III cysts may have thickened borders or septa with measurable enhancement, or they may be multilocular, hemorrhagic, or infected. In 5 case series, 29 of 57 class III lesions proved to be malignant.5 MRI may characterize these lesions more definitively than CT prior to urologic referral.
Malignant cysts. Bosniak class IV renal lesions are clearly malignant, with large heterogeneous cysts or necrotic components, shaggy thickened walls, or enhancing soft tissue components separate from the wall or septa. Their unequivocal appearance results from solid tumor necrosis and liquefaction. Diagnosis is straightforward, and excision is indicated.2
A closer look at solid renal masses
Solid renal masses usually consist of enhancing tissue with little or no fluid. The goal of evaluation is to exclude malignancies, such as renal cell cancer, lymphomas, sarcomas, or metastasis. Benign solid masses include renal adenomas, angiomyolipomas, and oncocytomas, among others.
Several lesions can be diagnosed by appearance or symptoms:
Angiomyolipomas are recognized by their fat content within a noncalcified mass. Unenhanced CT usually is sufficient for diagnosis, unless the mass is very small or has atypical features.9
Vascular lesions can be identified because they enhance to the same degree as the vasculature. With the exception of inflammatory or vascular abnormalities, all enhancing lesions that do not contain fat should be presumed to be malignant.
In patients with a known extrarenal primary malignancy, 50% to 85% of incidental solid renal masses will represent metastatic disease.10 Percutaneous biopsy may be warranted to differentiate metastatic lesions from a secondary, primary (ie, renal cell carcinoma), or benign process.11
A study of 2770 solid renal mass excisions revealed that 12.8% were benign, with a direct relationship between malignancy and size. Masses <1 cm were benign 44% of the time.12 Early identification of small renal carcinomas may improve survival rates. Although renal cell carcinomas <3 cm in diameter have low metastatic potential, a solid, nonfat-containing mass should be evaluated for aggressive nephron-sparing surgery.6,13
Incidental adrenal masses occur infrequently
Adrenal incidentalomas are defined as radiographically identified masses >1 cm in diameter.14 They are much less common than their renal counterparts, with a reported prevalence of 0.35% to 5% on CT.15 Because the adrenal glands are hormonally active and receive substantial blood flow, metastatic, hormonally active, and nonfunctional causes for adrenal masses need to be considered.16
Adrenal pathology
Adrenal masses may be characterized by increased or normal adrenal function. Hyperfunctioning syndromes include hypercortisolism, hyperaldosteronism, adrenogenital hypersecretion of adrenocortical origin, and pheochromocytomas of the medulla. Symptom evaluation of these syndromes is important, but not sufficient to rule out a hyperfunctioning syndrome.
In a retrospective review of inapparent adrenal masses, ≤13% of pheochromocytomas were clinically silent.17 Therefore, laboratory testing is necessary for an incidental adrenal mass.
Nonfunctional lesions include adenomas, metastases, cysts, myelolipomas, hemorrhage, and adrenal carcinomas. These masses require evaluation for the possibility of cancer, the most common of which is metastasis. In patients with an extra-adrenal malignancy, the likelihood of malignancy in an incidental adrenal mass is at least 50%.18 An adrenal mass representing metastasis of a previously unrecognized cancer is exceedingly rare.19
Primary adrenal carcinoma is also rare, with an estimated incidence of 2 cases per one million in the general population. For patients with adrenal masses, the prevalence of carcinoma increases with lesion size (2% for tumors <4 cm, 6% for tumors 4-6 cm, and 25% for tumors >6 cm in diameter). 17 For this reason, tumors >4 cm in diameter are usually surgically resected in patients with no previous cancer history, unless radiologic criteria demonstrate clearly benign characteristics.
Although adrenal carcinomas are considered nonfunctioning, some evidence suggests they produce low levels of cortisol that may be associated with clinical features of metabolic syndrome.20
CT is first choice for adrenal mass evaluation
Dedicated adrenal CT with both unenhanced and delayed contrast-enhanced images is the most reliable study to evaluate an adrenal mass, according to the American College of Radiology. Consider another study only in patients with contrast allergy, renal compromise, or cancer history.21
Unenhanced CT can diagnose the approximately 70% of adenomas that are small, well-defined round masses with homogenous low-density lipid deposition.22 Delayed contrast enhancement can characterize most of the remaining 30%.23 Unenhanced CT with attenuation values of <10 Hounsfield units (HU) can diagnose adenomas with 71% specificity and 98% sensitivity,24 and can often diagnose simple cysts and myelolipomas, as well.
Other imaging options. MRI is an alternative to CT for patients with contraindications for contrast or radiation exposure. MRI provides less spatial resolution than CT, but chemical shift imaging can measure cytoplasmic lipid content similar to unenhanced CT. A small study found chemical shift MRI more reliable than unenhanced CT, but less reliable than CT with delayed contrast enhancement.25
Positron emission tomography (PET) is useful to noninvasively evaluate biochemical and physiologic processes. PET-CT incorporates unenhanced CT density measurements to improve PET accuracy. In a patient with a history of cancer, PET-CT has a sensitivity of 93% to 100% and a specificity of 95% in differentiating benign from malignant adrenal tumors.26
When to order a biopsy
The need for biopsy has decreased as imaging has improved, but biopsy is required whenever diagnostic imaging fails to differentiatea lesion as benign or malignant. CT guided biopsy provides diagnostic accuracy of 85% to 95%.27 Complications such as pneumothorax, hemorrhage, and bacteremia occur in 3% to 9% of biopsies. Before any adrenal biopsy, measure plasma-free metanephrines to exclude undiagnosed pheochromocytoma, which could precipitate a hypertensive crisis if untreated.22
These 3 laboratory screening tests are critical
Family physicians can perform the initial biochemical evaluation of an adrenal incidentaloma. Guidance is available from the National Institutes of Health (NIH)28 and the American Academy of Clinical Endocrinologists (AACE) (FIGURE 2).29
Regardless of signs or symptoms, perform screening laboratory tests for 3 types of adrenal hyperfunction: hypercortisolism, hyperaldosteronism, and hypersecretion of catecholamines (pheochromocytoma). Screening tests are not recommended for androgen hypersecretion, which is extremely rare and causes recognizable symptoms such as hirsutism (Table 2).29
Hypercortisolism occurs in approximately 5% of adrenal incidentalomas.30 An overnight dexamethasone suppression test (DST) is most reliable for screening, with sensitivity >95% for Cushing syndrome.31 The patient takes a 1-mg dose of oral dexamethasone at 11 pm, and a fasting plasma cortisol sample is drawn the next day at 8 am.
Dexamethasone binds to glucocorticoid receptors in the pituitary gland, suppressing adrenocorticotropic hormone secretion. Cortisol will be depressed the next morning unless the adrenal mass produces cortisol autonomously. Patients with a DST >5 mcg/dL—highly suggestive of Cushing syndrome—require further evaluation, and we suggest referral to an endocrinologist.
Hyperaldosteronism is seen in 1% to 2% of adrenal incidentalomas.32 The aldosterone- to-renin ratio (ARR) is recommended as a screening test for hyperaldosteronism, with an ARR >20 requiring further testing.33 Medications that may affect the ARR include beta-blockers, spironolactone, clonidine, diuretics, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers.29
Refer a patient with evidence of hyperaldosteronism to an endocrinologist and a surgeon with experience in managing these lesions. If the ARR test result suggests an aldosterone excess, a salt-loading test is used to verify failure of aldosterone suppression. Adrenal venous sampling is often performed prior to surgical removal to confirm that an incidentaloma is the source of hyperaldosteronism.
Pheochromocytoma. Approximately 5% of incidental adrenal lesions are pheochromocytomas.30 Many patients with these epinephrine/norepinephrine secreting tumors do not show the classic symptom triad of headache, palpitations, and diaphoresis, and approximately half have normal blood pressure.34
Identifying a pheochromocytoma is important in any patient requiring surgery or biopsy, as surgical manipulation can cause a potentially fatal intraoperative catecholamine surge. Presurgical medical management can mitigate this reaction.
A plasma-free metanephrines test, which has 95% sensitivity, is the most reliable test for pheochromocytoma.35 Medications, including tricyclic antidepressants, decongestants, amphetamines, reserpine, and phenoxybenzamine, can cause falsepositive results.29 Confirm a positive plasma-free metanephrines test with a 24-hour fractionated urine metanephrines test, and refer the patient to an endocrinologist.
Managing adrenal incidentalomas
Refer all patients with adrenal masses >4 cm for surgical evaluation because of the risk of malignancy; all patients who have a history of malignancy and an adrenal mass of any size require a referral to an oncologist. Perform the AACE-recommended 3-element biochemical workup for all masses, with the exception of definitively diagnosed cysts or myelolipomas.
Refer to an endocrinologist all patients with abnormal screening laboratory results, regardless of adrenal mass size, as well as patients with concerning clinical findings. Initiate cardiovascular, diabetes, and bone density evaluation and management for metabolic syndrome.20
Monitoring after a negative workup
Little evidence exists to guide monitoring of small adrenal incidentalomas (<4 cm) with a negative workup. The 2002 NIH report recommended annual radiologic follow-up for 5 years,28 whereas the 2009 AACE guidelines recommend radiographic follow-up at 3 to 6 months, then at one and 2 years.29
Evidence indicates that 14% of lesions will enlarge in 2 years, although the clinical significance of enlargement is unknown. Some authors argue against CT monitoring because the risk of adrenal mass progression is similar to the malignancy risk posed by 3 years of radiation exposure with CT.20
Some guidelines recommend repeat biochemical screening every 3 to 4 years.28,29 AACE guidelines quote a 47% rate of progression over 3 years, but most adrenal masses progress to subclinical Cushing syndrome— a condition of uncertain significance. Subclinical Cushing’s has not been reported to progress to the overt syndrome, and new catecholamine or aldosterone secretion is rare.
Many endocrinologists reduce the frequency of follow-up, depending on the type of adrenal mass (cyst or solid) and its size. AACE suggests CT for adenomas one to 4 cm at 12 months. AACE and NIH recommend hormonal evaluation annually for 4 years. Adrenal cysts or myelolipoma in patients without cancer need no follow-up.29
CORRESPONDENCE
James C. Higgins, DO, CAPT, MC, USN, Ret., Naval Hospital Jacksonville, Family Medicine Department, 2080 Child Street, Box 1000, Jacksonville, FL 32214;
[email protected]
› Use computed tomography studies and the Bosniak classification system to
guide management of renal cystic masses. A
› Perform laboratory tests for hypercortisolism, hyperaldosteronism, and hypersecretion of catecholamines (pheochromocytoma) on any patient with an incidental adrenal mass, regardless of signs or symptoms. C
› Refer patients with adrenal masses >4 cm for surgical evaluation. Refer any individual who has a history of malignancy and an adrenal mass for oncologic evaluation. B
Strength of recommendation (SOR)
A. Good-quality patient-oriented evidence
B. Inconsistent or limited-quality patient-oriented evidence
C. Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Jane C, a 76-year-old patient, reports lower abdominal discomfort and increased bowel movements. Her left lower quadrant is tender to palpation, without signs of a surgical abdomen, and vital signs are normal. Laboratory studies are also normal, except for mild anemia and a positive fecal occult blood test. Abdominal and pelvic computed tomography (CT), with and without contrast, are negative for acute pathology, but a 1.7-cm lesion is found in the upper pole of the left kidney. What is your next step?
Renal or adrenal masses may be discovered during imaging studies for complaints unrelated to the kidneys or adrenals. Detection of incidentalomas has increased dramatically, keeping pace with the growing use of ultrasonography, CT, and magnetic resonance imaging (MRI) for abdominal, chest, and back complaints.1
Family physicians can evaluate most of these masses and determine the need for referral by using clinical judgment, appropriate imaging studies, and screening laboratory tests. In the pages that follow, we present a systematic approach for evaluating these incidentalomas and determining when consultation or referral is needed.
Incidental renal masses are common
Lesions are commonly found in normal kidneys, and the incidence increases with age. Approximately one-third of individuals age 50 and older will have at least one renal cyst on CT.2
Most incidental renal masses are benign cysts requiring no further evaluation. Other possibilities include indeterminate or malignant cysts or solid masses, which may be malignant or benign. Inflammatory renal lesions from infection, infarction, or trauma also occur, but these tend to be symptomatic and are rarely found incidentally.

Classification of renal cysts—not based on size
Cysts are the most common adult renal masses. Typically they are unilocular and located in the renal cortex, frequently extending to the renal surface.3 Renal function is usually preserved, regardless of the cyst’s location or size. Careful examination of adjacent tissue is essential, as secondary cysts may form when solid tumors obstruct tubules of normal parenchyma. Cystic lesions containing enhancing soft tissue unattached to the wall or septa likely are malignant.4
The Bosniak classification system, with 5 classes based on CT characteristics
(TABLE 1), is a useful guide for managing renal cystic lesions.4 Size is not an important feature in the Bosniak system; small cysts may be malignant and larger ones benign. Small cysts may grow into larger benign lesions, occasionally causing flank or abdominal pain, palpable masses, or hematuria.
Simple cysts. Renal cysts that meet Bosniak class I criteria can be confidently labeled benign and need no further evaluation (FIGURE 1). Simple renal cysts on CT have homogenous low-attenuating fluid and thin nonenhancing walls without septa.4
On ultrasound, simple renal cysts show spherical or ovoid shape without internal echoes, a thin smooth wall separate from the surrounding parenchyma, and posterior wall enhancement caused by increased transmission through the water-filled cyst. The likelihood of malignancy is extremely low in a renal cyst that meets these criteria, which have a reported accuracy of 98% to 100%.3 Thus, no further evaluation is required if an obviously benign simple cyst is first noted on an adequate ultrasound. Inadequate ultrasound visualization or evidence of calcifications, septa, or multiple chambers calls for prompt renal CT.
CASE The mass on Ms. C’s left kidney is hypoattenuating and nonenhancing on CT. It meets Bosniak criteria for a benign simple cyst (class I) and requires no further evaluation or follow-up. Colonoscopy detects multiple colonic polyps that are removed, and the patient does well.
Mildly complicated cysts. Less diagnostic certainty characterizes cysts with mild abnormalities that keep them from being labeled as simple. Bosniak classes II and IIF describe mildly abnormal renal cysts. Class II cysts can be dismissed, whereas class IIF cysts require follow-up.
Class II cysts may contain a few hairline septa, fine calcium deposits in walls or septa, or an unmeasurable enhancement of the walls. A hyperattenuating but nonenhancing fluid also is described as category II. Small homogeneous cysts <3 cm, without enhancement but hyperattenuated, are reliably considered benign and need not be evaluated.2,7
Class IIF cysts may have multiple hairline-thin septa with unmeasurable enhancement or minimal smooth thickening or irregular/nodular calcifications of wall or septa without enhancing soft tissue components. Hyperattenuating cystic lesions >3 cm and intrarenal “noncortical” cysts are included in this category. Class IIF cysts require follow-up at 6 months with CT or MRI, then annually for at least 5 years.8
Obviously complicated cysts. Bosniak class III is indeterminate—neither benign nor clearly malignant. Class III cysts may have thickened borders or septa with measurable enhancement, or they may be multilocular, hemorrhagic, or infected. In 5 case series, 29 of 57 class III lesions proved to be malignant.5 MRI may characterize these lesions more definitively than CT prior to urologic referral.
Malignant cysts. Bosniak class IV renal lesions are clearly malignant, with large heterogeneous cysts or necrotic components, shaggy thickened walls, or enhancing soft tissue components separate from the wall or septa. Their unequivocal appearance results from solid tumor necrosis and liquefaction. Diagnosis is straightforward, and excision is indicated.2
A closer look at solid renal masses
Solid renal masses usually consist of enhancing tissue with little or no fluid. The goal of evaluation is to exclude malignancies, such as renal cell cancer, lymphomas, sarcomas, or metastasis. Benign solid masses include renal adenomas, angiomyolipomas, and oncocytomas, among others.
Several lesions can be diagnosed by appearance or symptoms:
Angiomyolipomas are recognized by their fat content within a noncalcified mass. Unenhanced CT usually is sufficient for diagnosis, unless the mass is very small or has atypical features.9
Vascular lesions can be identified because they enhance to the same degree as the vasculature. With the exception of inflammatory or vascular abnormalities, all enhancing lesions that do not contain fat should be presumed to be malignant.
In patients with a known extrarenal primary malignancy, 50% to 85% of incidental solid renal masses will represent metastatic disease.10 Percutaneous biopsy may be warranted to differentiate metastatic lesions from a secondary, primary (ie, renal cell carcinoma), or benign process.11
A study of 2770 solid renal mass excisions revealed that 12.8% were benign, with a direct relationship between malignancy and size. Masses <1 cm were benign 44% of the time.12 Early identification of small renal carcinomas may improve survival rates. Although renal cell carcinomas <3 cm in diameter have low metastatic potential, a solid, nonfat-containing mass should be evaluated for aggressive nephron-sparing surgery.6,13
Incidental adrenal masses occur infrequently
Adrenal incidentalomas are defined as radiographically identified masses >1 cm in diameter.14 They are much less common than their renal counterparts, with a reported prevalence of 0.35% to 5% on CT.15 Because the adrenal glands are hormonally active and receive substantial blood flow, metastatic, hormonally active, and nonfunctional causes for adrenal masses need to be considered.16
Adrenal pathology
Adrenal masses may be characterized by increased or normal adrenal function. Hyperfunctioning syndromes include hypercortisolism, hyperaldosteronism, adrenogenital hypersecretion of adrenocortical origin, and pheochromocytomas of the medulla. Symptom evaluation of these syndromes is important, but not sufficient to rule out a hyperfunctioning syndrome.
In a retrospective review of inapparent adrenal masses, ≤13% of pheochromocytomas were clinically silent.17 Therefore, laboratory testing is necessary for an incidental adrenal mass.
Nonfunctional lesions include adenomas, metastases, cysts, myelolipomas, hemorrhage, and adrenal carcinomas. These masses require evaluation for the possibility of cancer, the most common of which is metastasis. In patients with an extra-adrenal malignancy, the likelihood of malignancy in an incidental adrenal mass is at least 50%.18 An adrenal mass representing metastasis of a previously unrecognized cancer is exceedingly rare.19
Primary adrenal carcinoma is also rare, with an estimated incidence of 2 cases per one million in the general population. For patients with adrenal masses, the prevalence of carcinoma increases with lesion size (2% for tumors <4 cm, 6% for tumors 4-6 cm, and 25% for tumors >6 cm in diameter). 17 For this reason, tumors >4 cm in diameter are usually surgically resected in patients with no previous cancer history, unless radiologic criteria demonstrate clearly benign characteristics.
Although adrenal carcinomas are considered nonfunctioning, some evidence suggests they produce low levels of cortisol that may be associated with clinical features of metabolic syndrome.20
CT is first choice for adrenal mass evaluation
Dedicated adrenal CT with both unenhanced and delayed contrast-enhanced images is the most reliable study to evaluate an adrenal mass, according to the American College of Radiology. Consider another study only in patients with contrast allergy, renal compromise, or cancer history.21
Unenhanced CT can diagnose the approximately 70% of adenomas that are small, well-defined round masses with homogenous low-density lipid deposition.22 Delayed contrast enhancement can characterize most of the remaining 30%.23 Unenhanced CT with attenuation values of <10 Hounsfield units (HU) can diagnose adenomas with 71% specificity and 98% sensitivity,24 and can often diagnose simple cysts and myelolipomas, as well.
Other imaging options. MRI is an alternative to CT for patients with contraindications for contrast or radiation exposure. MRI provides less spatial resolution than CT, but chemical shift imaging can measure cytoplasmic lipid content similar to unenhanced CT. A small study found chemical shift MRI more reliable than unenhanced CT, but less reliable than CT with delayed contrast enhancement.25
Positron emission tomography (PET) is useful to noninvasively evaluate biochemical and physiologic processes. PET-CT incorporates unenhanced CT density measurements to improve PET accuracy. In a patient with a history of cancer, PET-CT has a sensitivity of 93% to 100% and a specificity of 95% in differentiating benign from malignant adrenal tumors.26
When to order a biopsy
The need for biopsy has decreased as imaging has improved, but biopsy is required whenever diagnostic imaging fails to differentiatea lesion as benign or malignant. CT guided biopsy provides diagnostic accuracy of 85% to 95%.27 Complications such as pneumothorax, hemorrhage, and bacteremia occur in 3% to 9% of biopsies. Before any adrenal biopsy, measure plasma-free metanephrines to exclude undiagnosed pheochromocytoma, which could precipitate a hypertensive crisis if untreated.22
These 3 laboratory screening tests are critical
Family physicians can perform the initial biochemical evaluation of an adrenal incidentaloma. Guidance is available from the National Institutes of Health (NIH)28 and the American Academy of Clinical Endocrinologists (AACE) (FIGURE 2).29
Regardless of signs or symptoms, perform screening laboratory tests for 3 types of adrenal hyperfunction: hypercortisolism, hyperaldosteronism, and hypersecretion of catecholamines (pheochromocytoma). Screening tests are not recommended for androgen hypersecretion, which is extremely rare and causes recognizable symptoms such as hirsutism (Table 2).29
Hypercortisolism occurs in approximately 5% of adrenal incidentalomas.30 An overnight dexamethasone suppression test (DST) is most reliable for screening, with sensitivity >95% for Cushing syndrome.31 The patient takes a 1-mg dose of oral dexamethasone at 11 pm, and a fasting plasma cortisol sample is drawn the next day at 8 am.
Dexamethasone binds to glucocorticoid receptors in the pituitary gland, suppressing adrenocorticotropic hormone secretion. Cortisol will be depressed the next morning unless the adrenal mass produces cortisol autonomously. Patients with a DST >5 mcg/dL—highly suggestive of Cushing syndrome—require further evaluation, and we suggest referral to an endocrinologist.
Hyperaldosteronism is seen in 1% to 2% of adrenal incidentalomas.32 The aldosterone- to-renin ratio (ARR) is recommended as a screening test for hyperaldosteronism, with an ARR >20 requiring further testing.33 Medications that may affect the ARR include beta-blockers, spironolactone, clonidine, diuretics, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers.29
Refer a patient with evidence of hyperaldosteronism to an endocrinologist and a surgeon with experience in managing these lesions. If the ARR test result suggests an aldosterone excess, a salt-loading test is used to verify failure of aldosterone suppression. Adrenal venous sampling is often performed prior to surgical removal to confirm that an incidentaloma is the source of hyperaldosteronism.
Pheochromocytoma. Approximately 5% of incidental adrenal lesions are pheochromocytomas.30 Many patients with these epinephrine/norepinephrine secreting tumors do not show the classic symptom triad of headache, palpitations, and diaphoresis, and approximately half have normal blood pressure.34
Identifying a pheochromocytoma is important in any patient requiring surgery or biopsy, as surgical manipulation can cause a potentially fatal intraoperative catecholamine surge. Presurgical medical management can mitigate this reaction.
A plasma-free metanephrines test, which has 95% sensitivity, is the most reliable test for pheochromocytoma.35 Medications, including tricyclic antidepressants, decongestants, amphetamines, reserpine, and phenoxybenzamine, can cause falsepositive results.29 Confirm a positive plasma-free metanephrines test with a 24-hour fractionated urine metanephrines test, and refer the patient to an endocrinologist.
Managing adrenal incidentalomas
Refer all patients with adrenal masses >4 cm for surgical evaluation because of the risk of malignancy; all patients who have a history of malignancy and an adrenal mass of any size require a referral to an oncologist. Perform the AACE-recommended 3-element biochemical workup for all masses, with the exception of definitively diagnosed cysts or myelolipomas.
Refer to an endocrinologist all patients with abnormal screening laboratory results, regardless of adrenal mass size, as well as patients with concerning clinical findings. Initiate cardiovascular, diabetes, and bone density evaluation and management for metabolic syndrome.20
Monitoring after a negative workup
Little evidence exists to guide monitoring of small adrenal incidentalomas (<4 cm) with a negative workup. The 2002 NIH report recommended annual radiologic follow-up for 5 years,28 whereas the 2009 AACE guidelines recommend radiographic follow-up at 3 to 6 months, then at one and 2 years.29
Evidence indicates that 14% of lesions will enlarge in 2 years, although the clinical significance of enlargement is unknown. Some authors argue against CT monitoring because the risk of adrenal mass progression is similar to the malignancy risk posed by 3 years of radiation exposure with CT.20
Some guidelines recommend repeat biochemical screening every 3 to 4 years.28,29 AACE guidelines quote a 47% rate of progression over 3 years, but most adrenal masses progress to subclinical Cushing syndrome— a condition of uncertain significance. Subclinical Cushing’s has not been reported to progress to the overt syndrome, and new catecholamine or aldosterone secretion is rare.
Many endocrinologists reduce the frequency of follow-up, depending on the type of adrenal mass (cyst or solid) and its size. AACE suggests CT for adenomas one to 4 cm at 12 months. AACE and NIH recommend hormonal evaluation annually for 4 years. Adrenal cysts or myelolipoma in patients without cancer need no follow-up.29
CORRESPONDENCE
James C. Higgins, DO, CAPT, MC, USN, Ret., Naval Hospital Jacksonville, Family Medicine Department, 2080 Child Street, Box 1000, Jacksonville, FL 32214;
[email protected]
1. Berland LL, Silverman SG, Gore RM, et al. Managing incidental findings on abdominal CT: white paper of the ACR incidental findings committee. J Am Coll Radiol. 2010;7:754-773.
2. Silverman S, Israel G, Herts B, et al. Management of the incidental renal mass. Radiology. 2008;249:16-31.
3. Curry NS, Bissada NK. Radiologic evaluation of small and indeterminate renal masses. Urol Clin North Am. 1997;24:493-505.
4. Bosniak MA. The current radiological approach to renal cysts. Radiology. 1986;158:1-10.
5. Harisinghani M, Maher M, Gervais D, et al. Incidence of malignancy in complex cystic renal masses (Bosniak category III): should imaging guided biopsy precede surgery? AJR Am J Roentgenol. 2003;180:755-758.
6. Remzi M, Ozsoy M, Klingler HC. Are small renal tumors harmless? Analysis of histopathological features according to tumors less than 4 cm in diameter. J Urol. 2006;176:896-899.
7. Jonisch AI, Rubinowitz A, Mutalik P, et al. Can high attenuation renal cysts be differentiated from renal cell carcinoma at unenhanced computed tomography? Radiology. 2007;243:445-450.
8. Israel GM, Bosniak MA. Follow-up CT of moderately complex cystic lesions of the kidney. AJR Am J Roentgenol. 2003;181: 627-633.
9. Bosniak MA, Megibow AJ, Hulnick DH, et al. CT diagnosis of renal angiomyolipoma: the importance of detecting small amounts of fat. AJR Am J Roentengol. 1988;151:497-501.
10. Mitnick JS, Bosniak MA, Rothberg M, et al. Metastatic neoplasm to the kidney studied by computed tomography and sonogram. J Comput Assist Tomogr. 1985;9:43-49.
11. Rybicki FJ, Shu KM, Cibas ES, et al. Percutaneous biopsy of renal masses: sensitivity and negative predictive value stratified by clinical setting and size of masses. AJR Am J Roentgenol. 2003;180:1281-1287.
12. Frank I, Blure MI, Cheville JC, et al. Solid renal tumors: an analysis of pathological features related to tumor size. J Urol. 2003;170:2217-2220.
13. Hollingsworth JM, Miller DC, Daignault S, et al. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst. 2006;98:1331-1334.
14. Geelhoed GW, Spiegel CT. “Incidental” adrenal cyst: a correctable lesion possibly associated with hypertension. South Med J. 1981;74:626-630.
15. Davenport C, Liew A, Doherty B, et al. The prevalence of adrenal incidentaloma in routine clinical practice. Endocrine. 2011;40: 80-83.
16. Cook DM, Loriaux LD. The incidental adrenal mass. Am J Med. 1996;101:88 94.
17. Mansmann G, Lau J, Balk E, et al. The clinically inapparent adrenal mass: update in diagnosis and management. Endocr Rev. 2004;25:309-340.
18. Androulakis II, Kaltsas G, Piatitis G, et al. The clinical significance of adrenal incidentalomas. Eur J Clin Invest. 2011;41: 552-560.
19. Lee JE, Evans DB, Hickey RC, et al. Unknown primary cancer presenting as an adrenal mass: frequency and implications for diagnostic evaluation of adrenal incidentalomas. Surgery. 1998;124:1115-1122.
20. Aron D, Terzolo M, Cawood TJ. Adrenal incidentalomas. Best Pract Res Clin Endocrinol Metab. 2012;26:69-82.
21. ACR appropriateness criteria: incidentally discovered adrenal mass. American College of Radiology. Available at: http://www.acr.org/~/media/ACR/Documents/AppCriteria/Diagnostic/IncidentallyDiscoveredAdrenalMass.pdf. Accessed November 20, 2012.
22. Song JH, Mayo-Smith WW. Incidentally discovered adrenal mass. Radiol Clin North Am. 2011;49:361-368.
23. Korobkin M, Brodeur FJ, Francis IR, et al. CT time-attenuation washout curves of adrenal adenomas and nonadenomas. AJR Am J Roentgenol. 1998;170:747-752.
24. Boland GW, Lee MJ, Gazelle GS, et al. Characterization of adrenal masses using unenhanced CT: an analysis of the CT literature. AJR Am J Roentgenol. 1998;171:201-204.
25. Park BK, Kim CK, Kim B, et al. Chemical shift MR imaging of hyperattenuating (>10 HU) adrenal masses: does it still have a role? Radiology. 2004;231:711-716.
26. Boland GW, Blake MA, Holakere NS, et al. PET/CT for the characterization of adrenal masses in patients with cancer: qualitative vs quantitative accuracy in 150 consecutive patients. AJR Am J Roentgenol. 2009;192:956-962.
27. Paulsen SD, Nghiem HV, Korobkin M, et al. Changing role of imaging- guided percutaneous biopsy of adrenal masses: evaluation of 50 adrenal biopsies. AJR Am J Roentgenol. 2004;182:1033-1037
28. Grumbach MM, Biller BMK, Braunstein GD, et al. Management of the clinically inapparent adrenal mass (“incidentalomas”). Ann Intern Med. 2003;138:424-429.
29. Zeiger MA, Thompson GB, Quan-Yang D, et al. American Association of Clinical Endocrinologists and American Association of Endocrine Surgeons medical guidelines for the management of adrenal incidentalomas. Endocr Pract. 2009;15(suppl 1):1-20.
30. Young WF. The incidentally discovered adrenal mass. N Engl J Med. 2007; 356:601-610.
31. Deutschbein T, Unger N, Hinrichs J, et al. Late-night and lowdose dexamethasone-suppressed cortisol in saliva and serum for the diagnosis of cortisol-secreting adrenal adenomas. Eur J Endocrinol. 2009;161:747-753.
32. Bernini G, Moretti A, Gianfranco A, et al. Primary aldosteronism in normokalemic patients with adrenal incidentalomas. Eur J Endocrinol. 2002;146:523-529.
33. Montori VM, Young WF Jr. Use of plasma aldosterone concentration-to-plasma renin activity ratio as a screening test for primary aldosteronism: a systematic review of the literature. Endocrinol Metab Clin North Am. 2002;31:619-632.
34. Motta-Ramirez GA, Remer EM, Herts BR, et al. Comparison of CT findings in symptomatic and incidentally discovered pheochromocytomas. AJR Am J Roentgenol. 2005;185:684-688.
35. Pacak K, Eisenhofer G, Grossman A. The incidentally discovered adrenal mass. N Engl J Med. 2007;356:2005.
1. Berland LL, Silverman SG, Gore RM, et al. Managing incidental findings on abdominal CT: white paper of the ACR incidental findings committee. J Am Coll Radiol. 2010;7:754-773.
2. Silverman S, Israel G, Herts B, et al. Management of the incidental renal mass. Radiology. 2008;249:16-31.
3. Curry NS, Bissada NK. Radiologic evaluation of small and indeterminate renal masses. Urol Clin North Am. 1997;24:493-505.
4. Bosniak MA. The current radiological approach to renal cysts. Radiology. 1986;158:1-10.
5. Harisinghani M, Maher M, Gervais D, et al. Incidence of malignancy in complex cystic renal masses (Bosniak category III): should imaging guided biopsy precede surgery? AJR Am J Roentgenol. 2003;180:755-758.
6. Remzi M, Ozsoy M, Klingler HC. Are small renal tumors harmless? Analysis of histopathological features according to tumors less than 4 cm in diameter. J Urol. 2006;176:896-899.
7. Jonisch AI, Rubinowitz A, Mutalik P, et al. Can high attenuation renal cysts be differentiated from renal cell carcinoma at unenhanced computed tomography? Radiology. 2007;243:445-450.
8. Israel GM, Bosniak MA. Follow-up CT of moderately complex cystic lesions of the kidney. AJR Am J Roentgenol. 2003;181: 627-633.
9. Bosniak MA, Megibow AJ, Hulnick DH, et al. CT diagnosis of renal angiomyolipoma: the importance of detecting small amounts of fat. AJR Am J Roentengol. 1988;151:497-501.
10. Mitnick JS, Bosniak MA, Rothberg M, et al. Metastatic neoplasm to the kidney studied by computed tomography and sonogram. J Comput Assist Tomogr. 1985;9:43-49.
11. Rybicki FJ, Shu KM, Cibas ES, et al. Percutaneous biopsy of renal masses: sensitivity and negative predictive value stratified by clinical setting and size of masses. AJR Am J Roentgenol. 2003;180:1281-1287.
12. Frank I, Blure MI, Cheville JC, et al. Solid renal tumors: an analysis of pathological features related to tumor size. J Urol. 2003;170:2217-2220.
13. Hollingsworth JM, Miller DC, Daignault S, et al. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst. 2006;98:1331-1334.
14. Geelhoed GW, Spiegel CT. “Incidental” adrenal cyst: a correctable lesion possibly associated with hypertension. South Med J. 1981;74:626-630.
15. Davenport C, Liew A, Doherty B, et al. The prevalence of adrenal incidentaloma in routine clinical practice. Endocrine. 2011;40: 80-83.
16. Cook DM, Loriaux LD. The incidental adrenal mass. Am J Med. 1996;101:88 94.
17. Mansmann G, Lau J, Balk E, et al. The clinically inapparent adrenal mass: update in diagnosis and management. Endocr Rev. 2004;25:309-340.
18. Androulakis II, Kaltsas G, Piatitis G, et al. The clinical significance of adrenal incidentalomas. Eur J Clin Invest. 2011;41: 552-560.
19. Lee JE, Evans DB, Hickey RC, et al. Unknown primary cancer presenting as an adrenal mass: frequency and implications for diagnostic evaluation of adrenal incidentalomas. Surgery. 1998;124:1115-1122.
20. Aron D, Terzolo M, Cawood TJ. Adrenal incidentalomas. Best Pract Res Clin Endocrinol Metab. 2012;26:69-82.
21. ACR appropriateness criteria: incidentally discovered adrenal mass. American College of Radiology. Available at: http://www.acr.org/~/media/ACR/Documents/AppCriteria/Diagnostic/IncidentallyDiscoveredAdrenalMass.pdf. Accessed November 20, 2012.
22. Song JH, Mayo-Smith WW. Incidentally discovered adrenal mass. Radiol Clin North Am. 2011;49:361-368.
23. Korobkin M, Brodeur FJ, Francis IR, et al. CT time-attenuation washout curves of adrenal adenomas and nonadenomas. AJR Am J Roentgenol. 1998;170:747-752.
24. Boland GW, Lee MJ, Gazelle GS, et al. Characterization of adrenal masses using unenhanced CT: an analysis of the CT literature. AJR Am J Roentgenol. 1998;171:201-204.
25. Park BK, Kim CK, Kim B, et al. Chemical shift MR imaging of hyperattenuating (>10 HU) adrenal masses: does it still have a role? Radiology. 2004;231:711-716.
26. Boland GW, Blake MA, Holakere NS, et al. PET/CT for the characterization of adrenal masses in patients with cancer: qualitative vs quantitative accuracy in 150 consecutive patients. AJR Am J Roentgenol. 2009;192:956-962.
27. Paulsen SD, Nghiem HV, Korobkin M, et al. Changing role of imaging- guided percutaneous biopsy of adrenal masses: evaluation of 50 adrenal biopsies. AJR Am J Roentgenol. 2004;182:1033-1037
28. Grumbach MM, Biller BMK, Braunstein GD, et al. Management of the clinically inapparent adrenal mass (“incidentalomas”). Ann Intern Med. 2003;138:424-429.
29. Zeiger MA, Thompson GB, Quan-Yang D, et al. American Association of Clinical Endocrinologists and American Association of Endocrine Surgeons medical guidelines for the management of adrenal incidentalomas. Endocr Pract. 2009;15(suppl 1):1-20.
30. Young WF. The incidentally discovered adrenal mass. N Engl J Med. 2007; 356:601-610.
31. Deutschbein T, Unger N, Hinrichs J, et al. Late-night and lowdose dexamethasone-suppressed cortisol in saliva and serum for the diagnosis of cortisol-secreting adrenal adenomas. Eur J Endocrinol. 2009;161:747-753.
32. Bernini G, Moretti A, Gianfranco A, et al. Primary aldosteronism in normokalemic patients with adrenal incidentalomas. Eur J Endocrinol. 2002;146:523-529.
33. Montori VM, Young WF Jr. Use of plasma aldosterone concentration-to-plasma renin activity ratio as a screening test for primary aldosteronism: a systematic review of the literature. Endocrinol Metab Clin North Am. 2002;31:619-632.
34. Motta-Ramirez GA, Remer EM, Herts BR, et al. Comparison of CT findings in symptomatic and incidentally discovered pheochromocytomas. AJR Am J Roentgenol. 2005;185:684-688.
35. Pacak K, Eisenhofer G, Grossman A. The incidentally discovered adrenal mass. N Engl J Med. 2007;356:2005.
When to worry about incidental renal and adrenal masses
› Use computed tomography studies and the Bosniak classification system to
guide management of renal cystic masses. A
› Perform laboratory tests for hypercortisolism, hyperaldosteronism, and hypersecretion of catecholamines (pheochromocytoma) on any patient with an incidental adrenal mass, regardless of signs or symptoms. C
› Refer patients with adrenal masses >4 cm for surgical evaluation. Refer any individual who has a history of malignancy and an adrenal mass for oncologic evaluation. B
Strength of recommendation (SOR)
A. Good-quality patient-oriented evidence
B. Inconsistent or limited-quality patient-oriented evidence
C. Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Jane C, a 76-year-old patient, reports lower abdominal discomfort and increased bowel movements. Her left lower quadrant is tender to palpation, without signs of a surgical abdomen, and vital signs are normal. Laboratory studies are also normal, except for mild anemia and a positive fecal occult blood test. Abdominal and pelvic computed tomography (CT), with and without contrast, are negative for acute pathology, but a 1.7-cm lesion is found in the upper pole of the left kidney. What is your next step?
Renal or adrenal masses may be discovered during imaging studies for complaints unrelated to the kidneys or adrenals. Detection of incidentalomas has increased dramatically, keeping pace with the growing use of ultrasonography, CT, and magnetic resonance imaging (MRI) for abdominal, chest, and back complaints.1
Family physicians can evaluate most of these masses and determine the need for referral by using clinical judgment, appropriate imaging studies, and screening laboratory tests. In the pages that follow, we present a systematic approach for evaluating these incidentalomas and determining when consultation or referral is needed.
Incidental renal masses are common
Lesions are commonly found in normal kidneys, and the incidence increases with age. Approximately one-third of individuals age 50 and older will have at least one renal cyst on CT.2
Most incidental renal masses are benign cysts requiring no further evaluation. Other possibilities include indeterminate or malignant cysts or solid masses, which may be malignant or benign. Inflammatory renal lesions from infection, infarction, or trauma also occur, but these tend to be symptomatic and are rarely found incidentally.
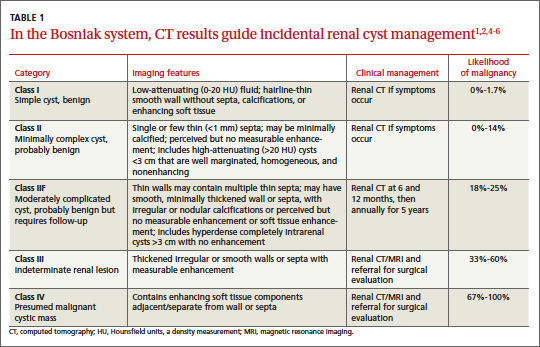
Classification of renal cysts—not based on size
Cysts are the most common adult renal masses. Typically they are unilocular and located in the renal cortex, frequently extending to the renal surface.3 Renal function is usually preserved, regardless of the cyst’s location or size. Careful examination of adjacent tissue is essential, as secondary cysts may form when solid tumors obstruct tubules of normal parenchyma. Cystic lesions containing enhancing soft tissue unattached to the wall or septa likely are malignant.4
The Bosniak classification system, with 5 classes based on CT characteristics
(TABLE 1), is a useful guide for managing renal cystic lesions.4 Size is not an important feature in the Bosniak system; small cysts may be malignant and larger ones benign. Small cysts may grow into larger benign lesions, occasionally causing flank or abdominal pain, palpable masses, or hematuria.
Simple cysts. Renal cysts that meet Bosniak class I criteria can be confidently labeled benign and need no further evaluation (FIGURE 1). Simple renal cysts on CT have homogenous low-attenuating fluid and thin nonenhancing walls without septa.4
On ultrasound, simple renal cysts show spherical or ovoid shape without internal echoes, a thin smooth wall separate from the surrounding parenchyma, and posterior wall enhancement caused by increased transmission through the water-filled cyst. The likelihood of malignancy is extremely low in a renal cyst that meets these criteria, which have a reported accuracy of 98% to 100%.3 Thus, no further evaluation is required if an obviously benign simple cyst is first noted on an adequate ultrasound. Inadequate ultrasound visualization or evidence of calcifications, septa, or multiple chambers calls for prompt renal CT.
CASE The mass on Ms. C’s left kidney is hypoattenuating and nonenhancing on CT. It meets Bosniak criteria for a benign simple cyst (class I) and requires no further evaluation or follow-up. Colonoscopy detects multiple colonic polyps that are removed, and the patient does well.
Mildly complicated cysts. Less diagnostic certainty characterizes cysts with mild abnormalities that keep them from being labeled as simple. Bosniak classes II and IIF describe mildly abnormal renal cysts. Class II cysts can be dismissed, whereas class IIF cysts require follow-up.
Class II cysts may contain a few hairline septa, fine calcium deposits in walls or septa, or an unmeasurable enhancement of the walls. A hyperattenuating but nonenhancing fluid also is described as category II. Small homogeneous cysts <3 cm, without enhancement but hyperattenuated, are reliably considered benign and need not be evaluated.2,7
Class IIF cysts may have multiple hairline-thin septa with unmeasurable enhancement or minimal smooth thickening or irregular/nodular calcifications of wall or septa without enhancing soft tissue components. Hyperattenuating cystic lesions >3 cm and intrarenal “noncortical” cysts are included in this category. Class IIF cysts require follow-up at 6 months with CT or MRI, then annually for at least 5 years.8
Obviously complicated cysts. Bosniak class III is indeterminate—neither benign nor clearly malignant. Class III cysts may have thickened borders or septa with measurable enhancement, or they may be multilocular, hemorrhagic, or infected. In 5 case series, 29 of 57 class III lesions proved to be malignant.5 MRI may characterize these lesions more definitively than CT prior to urologic referral.
Malignant cysts. Bosniak class IV renal lesions are clearly malignant, with large heterogeneous cysts or necrotic components, shaggy thickened walls, or enhancing soft tissue components separate from the wall or septa. Their unequivocal appearance results from solid tumor necrosis and liquefaction. Diagnosis is straightforward, and excision is indicated.2
A closer look at solid renal masses
Solid renal masses usually consist of enhancing tissue with little or no fluid. The goal of evaluation is to exclude malignancies, such as renal cell cancer, lymphomas, sarcomas, or metastasis. Benign solid masses include renal adenomas, angiomyolipomas, and oncocytomas, among others.
Several lesions can be diagnosed by appearance or symptoms:
Angiomyolipomas are recognized by their fat content within a noncalcified mass. Unenhanced CT usually is sufficient for diagnosis, unless the mass is very small or has atypical features.9
Vascular lesions can be identified because they enhance to the same degree as the vasculature. With the exception of inflammatory or vascular abnormalities, all enhancing lesions that do not contain fat should be presumed to be malignant.
In patients with a known extrarenal primary malignancy, 50% to 85% of incidental solid renal masses will represent metastatic disease.10 Percutaneous biopsy may be warranted to differentiate metastatic lesions from a secondary, primary (ie, renal cell carcinoma), or benign process.11
A study of 2770 solid renal mass excisions revealed that 12.8% were benign, with a direct relationship between malignancy and size. Masses <1 cm were benign 44% of the time.12 Early identification of small renal carcinomas may improve survival rates. Although renal cell carcinomas <3 cm in diameter have low metastatic potential, a solid, nonfat-containing mass should be evaluated for aggressive nephron-sparing surgery.6,13
Incidental adrenal masses occur infrequently
Adrenal incidentalomas are defined as radiographically identified masses >1 cm in diameter.14 They are much less common than their renal counterparts, with a reported prevalence of 0.35% to 5% on CT.15 Because the adrenal glands are hormonally active and receive substantial blood flow, metastatic, hormonally active, and nonfunctional causes for adrenal masses need to be considered.16
Adrenal pathology
Adrenal masses may be characterized by increased or normal adrenal function. Hyperfunctioning syndromes include hypercortisolism, hyperaldosteronism, adrenogenital hypersecretion of adrenocortical origin, and pheochromocytomas of the medulla. Symptom evaluation of these syndromes is important, but not sufficient to rule out a hyperfunctioning syndrome.
In a retrospective review of inapparent adrenal masses, ≤13% of pheochromocytomas were clinically silent.17 Therefore, laboratory testing is necessary for an incidental adrenal mass.
Nonfunctional lesions include adenomas, metastases, cysts, myelolipomas, hemorrhage, and adrenal carcinomas. These masses require evaluation for the possibility of cancer, the most common of which is metastasis. In patients with an extra-adrenal malignancy, the likelihood of malignancy in an incidental adrenal mass is at least 50%.18 An adrenal mass representing metastasis of a previously unrecognized cancer is exceedingly rare.19
Primary adrenal carcinoma is also rare, with an estimated incidence of 2 cases per one million in the general population. For patients with adrenal masses, the prevalence of carcinoma increases with lesion size (2% for tumors <4 cm, 6% for tumors 4-6 cm, and 25% for tumors >6 cm in diameter). 17 For this reason, tumors >4 cm in diameter are usually surgically resected in patients with no previous cancer history, unless radiologic criteria demonstrate clearly benign characteristics.
Although adrenal carcinomas are considered nonfunctioning, some evidence suggests they produce low levels of cortisol that may be associated with clinical features of metabolic syndrome.20
CT is first choice for adrenal mass evaluation
Dedicated adrenal CT with both unenhanced and delayed contrast-enhanced images is the most reliable study to evaluate an adrenal mass, according to the American College of Radiology. Consider another study only in patients with contrast allergy, renal compromise, or cancer history.21
Unenhanced CT can diagnose the approximately 70% of adenomas that are small, well-defined round masses with homogenous low-density lipid deposition.22 Delayed contrast enhancement can characterize most of the remaining 30%.23 Unenhanced CT with attenuation values of <10 Hounsfield units (HU) can diagnose adenomas with 71% specificity and 98% sensitivity,24 and can often diagnose simple cysts and myelolipomas, as well.
Other imaging options. MRI is an alternative to CT for patients with contraindications for contrast or radiation exposure. MRI provides less spatial resolution than CT, but chemical shift imaging can measure cytoplasmic lipid content similar to unenhanced CT. A small study found chemical shift MRI more reliable than unenhanced CT, but less reliable than CT with delayed contrast enhancement.25
Positron emission tomography (PET) is useful to noninvasively evaluate biochemical and physiologic processes. PET-CT incorporates unenhanced CT density measurements to improve PET accuracy. In a patient with a history of cancer, PET-CT has a sensitivity of 93% to 100% and a specificity of 95% in differentiating benign from malignant adrenal tumors.26
When to order a biopsy
The need for biopsy has decreased as imaging has improved, but biopsy is required whenever diagnostic imaging fails to differentiatea lesion as benign or malignant. CT guided biopsy provides diagnostic accuracy of 85% to 95%.27 Complications such as pneumothorax, hemorrhage, and bacteremia occur in 3% to 9% of biopsies. Before any adrenal biopsy, measure plasma-free metanephrines to exclude undiagnosed pheochromocytoma, which could precipitate a hypertensive crisis if untreated.22
These 3 laboratory screening tests are critical
Family physicians can perform the initial biochemical evaluation of an adrenal incidentaloma. Guidance is available from the National Institutes of Health (NIH)28 and the American Academy of Clinical Endocrinologists (AACE) (FIGURE 2).29
Regardless of signs or symptoms, perform screening laboratory tests for 3 types of adrenal hyperfunction: hypercortisolism, hyperaldosteronism, and hypersecretion of catecholamines (pheochromocytoma). Screening tests are not recommended for androgen hypersecretion, which is extremely rare and causes recognizable symptoms such as hirsutism (Table 2).29
Hypercortisolism occurs in approximately 5% of adrenal incidentalomas.30 An overnight dexamethasone suppression test (DST) is most reliable for screening, with sensitivity >95% for Cushing syndrome.31 The patient takes a 1-mg dose of oral dexamethasone at 11 pm, and a fasting plasma cortisol sample is drawn the next day at 8 am.
Dexamethasone binds to glucocorticoid receptors in the pituitary gland, suppressing adrenocorticotropic hormone secretion. Cortisol will be depressed the next morning unless the adrenal mass produces cortisol autonomously. Patients with a DST >5 mcg/dL—highly suggestive of Cushing syndrome—require further evaluation, and we suggest referral to an endocrinologist.
Hyperaldosteronism is seen in 1% to 2% of adrenal incidentalomas.32 The aldosterone- to-renin ratio (ARR) is recommended as a screening test for hyperaldosteronism, with an ARR >20 requiring further testing.33 Medications that may affect the ARR include beta-blockers, spironolactone, clonidine, diuretics, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers.29
Refer a patient with evidence of hyperaldosteronism to an endocrinologist and a surgeon with experience in managing these lesions. If the ARR test result suggests an aldosterone excess, a salt-loading test is used to verify failure of aldosterone suppression. Adrenal venous sampling is often performed prior to surgical removal to confirm that an incidentaloma is the source of hyperaldosteronism.
Pheochromocytoma. Approximately 5% of incidental adrenal lesions are pheochromocytomas.30 Many patients with these epinephrine/norepinephrine secreting tumors do not show the classic symptom triad of headache, palpitations, and diaphoresis, and approximately half have normal blood pressure.34
Identifying a pheochromocytoma is important in any patient requiring surgery or biopsy, as surgical manipulation can cause a potentially fatal intraoperative catecholamine surge. Presurgical medical management can mitigate this reaction.
A plasma-free metanephrines test, which has 95% sensitivity, is the most reliable test for pheochromocytoma.35 Medications, including tricyclic antidepressants, decongestants, amphetamines, reserpine, and phenoxybenzamine, can cause falsepositive results.29 Confirm a positive plasma-free metanephrines test with a 24-hour fractionated urine metanephrines test, and refer the patient to an endocrinologist.
Managing adrenal incidentalomas
Refer all patients with adrenal masses >4 cm for surgical evaluation because of the risk of malignancy; all patients who have a history of malignancy and an adrenal mass of any size require a referral to an oncologist. Perform the AACE-recommended 3-element biochemical workup for all masses, with the exception of definitively diagnosed cysts or myelolipomas.
Refer to an endocrinologist all patients with abnormal screening laboratory results, regardless of adrenal mass size, as well as patients with concerning clinical findings. Initiate cardiovascular, diabetes, and bone density evaluation and management for metabolic syndrome.20
Monitoring after a negative workup
Little evidence exists to guide monitoring of small adrenal incidentalomas (<4 cm) with a negative workup. The 2002 NIH report recommended annual radiologic follow-up for 5 years,28 whereas the 2009 AACE guidelines recommend radiographic follow-up at 3 to 6 months, then at one and 2 years.29
Evidence indicates that 14% of lesions will enlarge in 2 years, although the clinical significance of enlargement is unknown. Some authors argue against CT monitoring because the risk of adrenal mass progression is similar to the malignancy risk posed by 3 years of radiation exposure with CT.20
Some guidelines recommend repeat biochemical screening every 3 to 4 years.28,29 AACE guidelines quote a 47% rate of progression over 3 years, but most adrenal masses progress to subclinical Cushing syndrome— a condition of uncertain significance. Subclinical Cushing’s has not been reported to progress to the overt syndrome, and new catecholamine or aldosterone secretion is rare.
Many endocrinologists reduce the frequency of follow-up, depending on the type of adrenal mass (cyst or solid) and its size. AACE suggests CT for adenomas one to 4 cm at 12 months. AACE and NIH recommend hormonal evaluation annually for 4 years. Adrenal cysts or myelolipoma in patients without cancer need no follow-up.29
CORRESPONDENCE
James C. Higgins, DO, CAPT, MC, USN, Ret., Naval Hospital Jacksonville, Family Medicine Department, 2080 Child Street, Box 1000, Jacksonville, FL 32214;
[email protected]
1. Berland LL, Silverman SG, Gore RM, et al. Managing incidental findings on abdominal CT: white paper of the ACR incidental findings committee. J Am Coll Radiol. 2010;7:754-773.
2. Silverman S, Israel G, Herts B, et al. Management of the incidental renal mass. Radiology. 2008;249:16-31.
3. Curry NS, Bissada NK. Radiologic evaluation of small and indeterminate renal masses. Urol Clin North Am. 1997;24:493-505.
4. Bosniak MA. The current radiological approach to renal cysts. Radiology. 1986;158:1-10.
5. Harisinghani M, Maher M, Gervais D, et al. Incidence of malignancy in complex cystic renal masses (Bosniak category III): should imaging guided biopsy precede surgery? AJR Am J Roentgenol. 2003;180:755-758.
6. Remzi M, Ozsoy M, Klingler HC. Are small renal tumors harmless? Analysis of histopathological features according to tumors less than 4 cm in diameter. J Urol. 2006;176:896-899.
7. Jonisch AI, Rubinowitz A, Mutalik P, et al. Can high attenuation renal cysts be differentiated from renal cell carcinoma at unenhanced computed tomography? Radiology. 2007;243:445-450.
8. Israel GM, Bosniak MA. Follow-up CT of moderately complex cystic lesions of the kidney. AJR Am J Roentgenol. 2003;181: 627-633.
9. Bosniak MA, Megibow AJ, Hulnick DH, et al. CT diagnosis of renal angiomyolipoma: the importance of detecting small amounts of fat. AJR Am J Roentengol. 1988;151:497-501.
10. Mitnick JS, Bosniak MA, Rothberg M, et al. Metastatic neoplasm to the kidney studied by computed tomography and sonogram. J Comput Assist Tomogr. 1985;9:43-49.
11. Rybicki FJ, Shu KM, Cibas ES, et al. Percutaneous biopsy of renal masses: sensitivity and negative predictive value stratified by clinical setting and size of masses. AJR Am J Roentgenol. 2003;180:1281-1287.
12. Frank I, Blure MI, Cheville JC, et al. Solid renal tumors: an analysis of pathological features related to tumor size. J Urol. 2003;170:2217-2220.
13. Hollingsworth JM, Miller DC, Daignault S, et al. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst. 2006;98:1331-1334.
14. Geelhoed GW, Spiegel CT. “Incidental” adrenal cyst: a correctable lesion possibly associated with hypertension. South Med J. 1981;74:626-630.
15. Davenport C, Liew A, Doherty B, et al. The prevalence of adrenal incidentaloma in routine clinical practice. Endocrine. 2011;40: 80-83.
16. Cook DM, Loriaux LD. The incidental adrenal mass. Am J Med. 1996;101:88 94.
17. Mansmann G, Lau J, Balk E, et al. The clinically inapparent adrenal mass: update in diagnosis and management. Endocr Rev. 2004;25:309-340.
18. Androulakis II, Kaltsas G, Piatitis G, et al. The clinical significance of adrenal incidentalomas. Eur J Clin Invest. 2011;41: 552-560.
19. Lee JE, Evans DB, Hickey RC, et al. Unknown primary cancer presenting as an adrenal mass: frequency and implications for diagnostic evaluation of adrenal incidentalomas. Surgery. 1998;124:1115-1122.
20. Aron D, Terzolo M, Cawood TJ. Adrenal incidentalomas. Best Pract Res Clin Endocrinol Metab. 2012;26:69-82.
21. ACR appropriateness criteria: incidentally discovered adrenal mass. American College of Radiology. Available at: http://www.acr.org/~/media/ACR/Documents/AppCriteria/Diagnostic/IncidentallyDiscoveredAdrenalMass.pdf. Accessed November 20, 2012.
22. Song JH, Mayo-Smith WW. Incidentally discovered adrenal mass. Radiol Clin North Am. 2011;49:361-368.
23. Korobkin M, Brodeur FJ, Francis IR, et al. CT time-attenuation washout curves of adrenal adenomas and nonadenomas. AJR Am J Roentgenol. 1998;170:747-752.
24. Boland GW, Lee MJ, Gazelle GS, et al. Characterization of adrenal masses using unenhanced CT: an analysis of the CT literature. AJR Am J Roentgenol. 1998;171:201-204.
25. Park BK, Kim CK, Kim B, et al. Chemical shift MR imaging of hyperattenuating (>10 HU) adrenal masses: does it still have a role? Radiology. 2004;231:711-716.
26. Boland GW, Blake MA, Holakere NS, et al. PET/CT for the characterization of adrenal masses in patients with cancer: qualitative vs quantitative accuracy in 150 consecutive patients. AJR Am J Roentgenol. 2009;192:956-962.
27. Paulsen SD, Nghiem HV, Korobkin M, et al. Changing role of imaging- guided percutaneous biopsy of adrenal masses: evaluation of 50 adrenal biopsies. AJR Am J Roentgenol. 2004;182:1033-1037
28. Grumbach MM, Biller BMK, Braunstein GD, et al. Management of the clinically inapparent adrenal mass (“incidentalomas”). Ann Intern Med. 2003;138:424-429.
29. Zeiger MA, Thompson GB, Quan-Yang D, et al. American Association of Clinical Endocrinologists and American Association of Endocrine Surgeons medical guidelines for the management of adrenal incidentalomas. Endocr Pract. 2009;15(suppl 1):1-20.
30. Young WF. The incidentally discovered adrenal mass. N Engl J Med. 2007; 356:601-610.
31. Deutschbein T, Unger N, Hinrichs J, et al. Late-night and lowdose dexamethasone-suppressed cortisol in saliva and serum for the diagnosis of cortisol-secreting adrenal adenomas. Eur J Endocrinol. 2009;161:747-753.
32. Bernini G, Moretti A, Gianfranco A, et al. Primary aldosteronism in normokalemic patients with adrenal incidentalomas. Eur J Endocrinol. 2002;146:523-529.
33. Montori VM, Young WF Jr. Use of plasma aldosterone concentration-to-plasma renin activity ratio as a screening test for primary aldosteronism: a systematic review of the literature. Endocrinol Metab Clin North Am. 2002;31:619-632.
34. Motta-Ramirez GA, Remer EM, Herts BR, et al. Comparison of CT findings in symptomatic and incidentally discovered pheochromocytomas. AJR Am J Roentgenol. 2005;185:684-688.
35. Pacak K, Eisenhofer G, Grossman A. The incidentally discovered adrenal mass. N Engl J Med. 2007;356:2005.
› Use computed tomography studies and the Bosniak classification system to
guide management of renal cystic masses. A
› Perform laboratory tests for hypercortisolism, hyperaldosteronism, and hypersecretion of catecholamines (pheochromocytoma) on any patient with an incidental adrenal mass, regardless of signs or symptoms. C
› Refer patients with adrenal masses >4 cm for surgical evaluation. Refer any individual who has a history of malignancy and an adrenal mass for oncologic evaluation. B
Strength of recommendation (SOR)
A. Good-quality patient-oriented evidence
B. Inconsistent or limited-quality patient-oriented evidence
C. Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Jane C, a 76-year-old patient, reports lower abdominal discomfort and increased bowel movements. Her left lower quadrant is tender to palpation, without signs of a surgical abdomen, and vital signs are normal. Laboratory studies are also normal, except for mild anemia and a positive fecal occult blood test. Abdominal and pelvic computed tomography (CT), with and without contrast, are negative for acute pathology, but a 1.7-cm lesion is found in the upper pole of the left kidney. What is your next step?
Renal or adrenal masses may be discovered during imaging studies for complaints unrelated to the kidneys or adrenals. Detection of incidentalomas has increased dramatically, keeping pace with the growing use of ultrasonography, CT, and magnetic resonance imaging (MRI) for abdominal, chest, and back complaints.1
Family physicians can evaluate most of these masses and determine the need for referral by using clinical judgment, appropriate imaging studies, and screening laboratory tests. In the pages that follow, we present a systematic approach for evaluating these incidentalomas and determining when consultation or referral is needed.
Incidental renal masses are common
Lesions are commonly found in normal kidneys, and the incidence increases with age. Approximately one-third of individuals age 50 and older will have at least one renal cyst on CT.2
Most incidental renal masses are benign cysts requiring no further evaluation. Other possibilities include indeterminate or malignant cysts or solid masses, which may be malignant or benign. Inflammatory renal lesions from infection, infarction, or trauma also occur, but these tend to be symptomatic and are rarely found incidentally.

Classification of renal cysts—not based on size
Cysts are the most common adult renal masses. Typically they are unilocular and located in the renal cortex, frequently extending to the renal surface.3 Renal function is usually preserved, regardless of the cyst’s location or size. Careful examination of adjacent tissue is essential, as secondary cysts may form when solid tumors obstruct tubules of normal parenchyma. Cystic lesions containing enhancing soft tissue unattached to the wall or septa likely are malignant.4
The Bosniak classification system, with 5 classes based on CT characteristics
(TABLE 1), is a useful guide for managing renal cystic lesions.4 Size is not an important feature in the Bosniak system; small cysts may be malignant and larger ones benign. Small cysts may grow into larger benign lesions, occasionally causing flank or abdominal pain, palpable masses, or hematuria.
Simple cysts. Renal cysts that meet Bosniak class I criteria can be confidently labeled benign and need no further evaluation (FIGURE 1). Simple renal cysts on CT have homogenous low-attenuating fluid and thin nonenhancing walls without septa.4
On ultrasound, simple renal cysts show spherical or ovoid shape without internal echoes, a thin smooth wall separate from the surrounding parenchyma, and posterior wall enhancement caused by increased transmission through the water-filled cyst. The likelihood of malignancy is extremely low in a renal cyst that meets these criteria, which have a reported accuracy of 98% to 100%.3 Thus, no further evaluation is required if an obviously benign simple cyst is first noted on an adequate ultrasound. Inadequate ultrasound visualization or evidence of calcifications, septa, or multiple chambers calls for prompt renal CT.
CASE The mass on Ms. C’s left kidney is hypoattenuating and nonenhancing on CT. It meets Bosniak criteria for a benign simple cyst (class I) and requires no further evaluation or follow-up. Colonoscopy detects multiple colonic polyps that are removed, and the patient does well.
Mildly complicated cysts. Less diagnostic certainty characterizes cysts with mild abnormalities that keep them from being labeled as simple. Bosniak classes II and IIF describe mildly abnormal renal cysts. Class II cysts can be dismissed, whereas class IIF cysts require follow-up.
Class II cysts may contain a few hairline septa, fine calcium deposits in walls or septa, or an unmeasurable enhancement of the walls. A hyperattenuating but nonenhancing fluid also is described as category II. Small homogeneous cysts <3 cm, without enhancement but hyperattenuated, are reliably considered benign and need not be evaluated.2,7
Class IIF cysts may have multiple hairline-thin septa with unmeasurable enhancement or minimal smooth thickening or irregular/nodular calcifications of wall or septa without enhancing soft tissue components. Hyperattenuating cystic lesions >3 cm and intrarenal “noncortical” cysts are included in this category. Class IIF cysts require follow-up at 6 months with CT or MRI, then annually for at least 5 years.8
Obviously complicated cysts. Bosniak class III is indeterminate—neither benign nor clearly malignant. Class III cysts may have thickened borders or septa with measurable enhancement, or they may be multilocular, hemorrhagic, or infected. In 5 case series, 29 of 57 class III lesions proved to be malignant.5 MRI may characterize these lesions more definitively than CT prior to urologic referral.
Malignant cysts. Bosniak class IV renal lesions are clearly malignant, with large heterogeneous cysts or necrotic components, shaggy thickened walls, or enhancing soft tissue components separate from the wall or septa. Their unequivocal appearance results from solid tumor necrosis and liquefaction. Diagnosis is straightforward, and excision is indicated.2
A closer look at solid renal masses
Solid renal masses usually consist of enhancing tissue with little or no fluid. The goal of evaluation is to exclude malignancies, such as renal cell cancer, lymphomas, sarcomas, or metastasis. Benign solid masses include renal adenomas, angiomyolipomas, and oncocytomas, among others.
Several lesions can be diagnosed by appearance or symptoms:
Angiomyolipomas are recognized by their fat content within a noncalcified mass. Unenhanced CT usually is sufficient for diagnosis, unless the mass is very small or has atypical features.9
Vascular lesions can be identified because they enhance to the same degree as the vasculature. With the exception of inflammatory or vascular abnormalities, all enhancing lesions that do not contain fat should be presumed to be malignant.
In patients with a known extrarenal primary malignancy, 50% to 85% of incidental solid renal masses will represent metastatic disease.10 Percutaneous biopsy may be warranted to differentiate metastatic lesions from a secondary, primary (ie, renal cell carcinoma), or benign process.11
A study of 2770 solid renal mass excisions revealed that 12.8% were benign, with a direct relationship between malignancy and size. Masses <1 cm were benign 44% of the time.12 Early identification of small renal carcinomas may improve survival rates. Although renal cell carcinomas <3 cm in diameter have low metastatic potential, a solid, nonfat-containing mass should be evaluated for aggressive nephron-sparing surgery.6,13
Incidental adrenal masses occur infrequently
Adrenal incidentalomas are defined as radiographically identified masses >1 cm in diameter.14 They are much less common than their renal counterparts, with a reported prevalence of 0.35% to 5% on CT.15 Because the adrenal glands are hormonally active and receive substantial blood flow, metastatic, hormonally active, and nonfunctional causes for adrenal masses need to be considered.16
Adrenal pathology
Adrenal masses may be characterized by increased or normal adrenal function. Hyperfunctioning syndromes include hypercortisolism, hyperaldosteronism, adrenogenital hypersecretion of adrenocortical origin, and pheochromocytomas of the medulla. Symptom evaluation of these syndromes is important, but not sufficient to rule out a hyperfunctioning syndrome.
In a retrospective review of inapparent adrenal masses, ≤13% of pheochromocytomas were clinically silent.17 Therefore, laboratory testing is necessary for an incidental adrenal mass.
Nonfunctional lesions include adenomas, metastases, cysts, myelolipomas, hemorrhage, and adrenal carcinomas. These masses require evaluation for the possibility of cancer, the most common of which is metastasis. In patients with an extra-adrenal malignancy, the likelihood of malignancy in an incidental adrenal mass is at least 50%.18 An adrenal mass representing metastasis of a previously unrecognized cancer is exceedingly rare.19
Primary adrenal carcinoma is also rare, with an estimated incidence of 2 cases per one million in the general population. For patients with adrenal masses, the prevalence of carcinoma increases with lesion size (2% for tumors <4 cm, 6% for tumors 4-6 cm, and 25% for tumors >6 cm in diameter). 17 For this reason, tumors >4 cm in diameter are usually surgically resected in patients with no previous cancer history, unless radiologic criteria demonstrate clearly benign characteristics.
Although adrenal carcinomas are considered nonfunctioning, some evidence suggests they produce low levels of cortisol that may be associated with clinical features of metabolic syndrome.20
CT is first choice for adrenal mass evaluation
Dedicated adrenal CT with both unenhanced and delayed contrast-enhanced images is the most reliable study to evaluate an adrenal mass, according to the American College of Radiology. Consider another study only in patients with contrast allergy, renal compromise, or cancer history.21
Unenhanced CT can diagnose the approximately 70% of adenomas that are small, well-defined round masses with homogenous low-density lipid deposition.22 Delayed contrast enhancement can characterize most of the remaining 30%.23 Unenhanced CT with attenuation values of <10 Hounsfield units (HU) can diagnose adenomas with 71% specificity and 98% sensitivity,24 and can often diagnose simple cysts and myelolipomas, as well.
Other imaging options. MRI is an alternative to CT for patients with contraindications for contrast or radiation exposure. MRI provides less spatial resolution than CT, but chemical shift imaging can measure cytoplasmic lipid content similar to unenhanced CT. A small study found chemical shift MRI more reliable than unenhanced CT, but less reliable than CT with delayed contrast enhancement.25
Positron emission tomography (PET) is useful to noninvasively evaluate biochemical and physiologic processes. PET-CT incorporates unenhanced CT density measurements to improve PET accuracy. In a patient with a history of cancer, PET-CT has a sensitivity of 93% to 100% and a specificity of 95% in differentiating benign from malignant adrenal tumors.26
When to order a biopsy
The need for biopsy has decreased as imaging has improved, but biopsy is required whenever diagnostic imaging fails to differentiatea lesion as benign or malignant. CT guided biopsy provides diagnostic accuracy of 85% to 95%.27 Complications such as pneumothorax, hemorrhage, and bacteremia occur in 3% to 9% of biopsies. Before any adrenal biopsy, measure plasma-free metanephrines to exclude undiagnosed pheochromocytoma, which could precipitate a hypertensive crisis if untreated.22
These 3 laboratory screening tests are critical
Family physicians can perform the initial biochemical evaluation of an adrenal incidentaloma. Guidance is available from the National Institutes of Health (NIH)28 and the American Academy of Clinical Endocrinologists (AACE) (FIGURE 2).29
Regardless of signs or symptoms, perform screening laboratory tests for 3 types of adrenal hyperfunction: hypercortisolism, hyperaldosteronism, and hypersecretion of catecholamines (pheochromocytoma). Screening tests are not recommended for androgen hypersecretion, which is extremely rare and causes recognizable symptoms such as hirsutism (Table 2).29
Hypercortisolism occurs in approximately 5% of adrenal incidentalomas.30 An overnight dexamethasone suppression test (DST) is most reliable for screening, with sensitivity >95% for Cushing syndrome.31 The patient takes a 1-mg dose of oral dexamethasone at 11 pm, and a fasting plasma cortisol sample is drawn the next day at 8 am.
Dexamethasone binds to glucocorticoid receptors in the pituitary gland, suppressing adrenocorticotropic hormone secretion. Cortisol will be depressed the next morning unless the adrenal mass produces cortisol autonomously. Patients with a DST >5 mcg/dL—highly suggestive of Cushing syndrome—require further evaluation, and we suggest referral to an endocrinologist.
Hyperaldosteronism is seen in 1% to 2% of adrenal incidentalomas.32 The aldosterone- to-renin ratio (ARR) is recommended as a screening test for hyperaldosteronism, with an ARR >20 requiring further testing.33 Medications that may affect the ARR include beta-blockers, spironolactone, clonidine, diuretics, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers.29
Refer a patient with evidence of hyperaldosteronism to an endocrinologist and a surgeon with experience in managing these lesions. If the ARR test result suggests an aldosterone excess, a salt-loading test is used to verify failure of aldosterone suppression. Adrenal venous sampling is often performed prior to surgical removal to confirm that an incidentaloma is the source of hyperaldosteronism.
Pheochromocytoma. Approximately 5% of incidental adrenal lesions are pheochromocytomas.30 Many patients with these epinephrine/norepinephrine secreting tumors do not show the classic symptom triad of headache, palpitations, and diaphoresis, and approximately half have normal blood pressure.34
Identifying a pheochromocytoma is important in any patient requiring surgery or biopsy, as surgical manipulation can cause a potentially fatal intraoperative catecholamine surge. Presurgical medical management can mitigate this reaction.
A plasma-free metanephrines test, which has 95% sensitivity, is the most reliable test for pheochromocytoma.35 Medications, including tricyclic antidepressants, decongestants, amphetamines, reserpine, and phenoxybenzamine, can cause falsepositive results.29 Confirm a positive plasma-free metanephrines test with a 24-hour fractionated urine metanephrines test, and refer the patient to an endocrinologist.
Managing adrenal incidentalomas
Refer all patients with adrenal masses >4 cm for surgical evaluation because of the risk of malignancy; all patients who have a history of malignancy and an adrenal mass of any size require a referral to an oncologist. Perform the AACE-recommended 3-element biochemical workup for all masses, with the exception of definitively diagnosed cysts or myelolipomas.
Refer to an endocrinologist all patients with abnormal screening laboratory results, regardless of adrenal mass size, as well as patients with concerning clinical findings. Initiate cardiovascular, diabetes, and bone density evaluation and management for metabolic syndrome.20
Monitoring after a negative workup
Little evidence exists to guide monitoring of small adrenal incidentalomas (<4 cm) with a negative workup. The 2002 NIH report recommended annual radiologic follow-up for 5 years,28 whereas the 2009 AACE guidelines recommend radiographic follow-up at 3 to 6 months, then at one and 2 years.29
Evidence indicates that 14% of lesions will enlarge in 2 years, although the clinical significance of enlargement is unknown. Some authors argue against CT monitoring because the risk of adrenal mass progression is similar to the malignancy risk posed by 3 years of radiation exposure with CT.20
Some guidelines recommend repeat biochemical screening every 3 to 4 years.28,29 AACE guidelines quote a 47% rate of progression over 3 years, but most adrenal masses progress to subclinical Cushing syndrome— a condition of uncertain significance. Subclinical Cushing’s has not been reported to progress to the overt syndrome, and new catecholamine or aldosterone secretion is rare.
Many endocrinologists reduce the frequency of follow-up, depending on the type of adrenal mass (cyst or solid) and its size. AACE suggests CT for adenomas one to 4 cm at 12 months. AACE and NIH recommend hormonal evaluation annually for 4 years. Adrenal cysts or myelolipoma in patients without cancer need no follow-up.29
CORRESPONDENCE
James C. Higgins, DO, CAPT, MC, USN, Ret., Naval Hospital Jacksonville, Family Medicine Department, 2080 Child Street, Box 1000, Jacksonville, FL 32214;
[email protected]
› Use computed tomography studies and the Bosniak classification system to
guide management of renal cystic masses. A
› Perform laboratory tests for hypercortisolism, hyperaldosteronism, and hypersecretion of catecholamines (pheochromocytoma) on any patient with an incidental adrenal mass, regardless of signs or symptoms. C
› Refer patients with adrenal masses >4 cm for surgical evaluation. Refer any individual who has a history of malignancy and an adrenal mass for oncologic evaluation. B
Strength of recommendation (SOR)
A. Good-quality patient-oriented evidence
B. Inconsistent or limited-quality patient-oriented evidence
C. Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Jane C, a 76-year-old patient, reports lower abdominal discomfort and increased bowel movements. Her left lower quadrant is tender to palpation, without signs of a surgical abdomen, and vital signs are normal. Laboratory studies are also normal, except for mild anemia and a positive fecal occult blood test. Abdominal and pelvic computed tomography (CT), with and without contrast, are negative for acute pathology, but a 1.7-cm lesion is found in the upper pole of the left kidney. What is your next step?
Renal or adrenal masses may be discovered during imaging studies for complaints unrelated to the kidneys or adrenals. Detection of incidentalomas has increased dramatically, keeping pace with the growing use of ultrasonography, CT, and magnetic resonance imaging (MRI) for abdominal, chest, and back complaints.1
Family physicians can evaluate most of these masses and determine the need for referral by using clinical judgment, appropriate imaging studies, and screening laboratory tests. In the pages that follow, we present a systematic approach for evaluating these incidentalomas and determining when consultation or referral is needed.
Incidental renal masses are common
Lesions are commonly found in normal kidneys, and the incidence increases with age. Approximately one-third of individuals age 50 and older will have at least one renal cyst on CT.2
Most incidental renal masses are benign cysts requiring no further evaluation. Other possibilities include indeterminate or malignant cysts or solid masses, which may be malignant or benign. Inflammatory renal lesions from infection, infarction, or trauma also occur, but these tend to be symptomatic and are rarely found incidentally.

Classification of renal cysts—not based on size
Cysts are the most common adult renal masses. Typically they are unilocular and located in the renal cortex, frequently extending to the renal surface.3 Renal function is usually preserved, regardless of the cyst’s location or size. Careful examination of adjacent tissue is essential, as secondary cysts may form when solid tumors obstruct tubules of normal parenchyma. Cystic lesions containing enhancing soft tissue unattached to the wall or septa likely are malignant.4
The Bosniak classification system, with 5 classes based on CT characteristics
(TABLE 1), is a useful guide for managing renal cystic lesions.4 Size is not an important feature in the Bosniak system; small cysts may be malignant and larger ones benign. Small cysts may grow into larger benign lesions, occasionally causing flank or abdominal pain, palpable masses, or hematuria.
Simple cysts. Renal cysts that meet Bosniak class I criteria can be confidently labeled benign and need no further evaluation (FIGURE 1). Simple renal cysts on CT have homogenous low-attenuating fluid and thin nonenhancing walls without septa.4
On ultrasound, simple renal cysts show spherical or ovoid shape without internal echoes, a thin smooth wall separate from the surrounding parenchyma, and posterior wall enhancement caused by increased transmission through the water-filled cyst. The likelihood of malignancy is extremely low in a renal cyst that meets these criteria, which have a reported accuracy of 98% to 100%.3 Thus, no further evaluation is required if an obviously benign simple cyst is first noted on an adequate ultrasound. Inadequate ultrasound visualization or evidence of calcifications, septa, or multiple chambers calls for prompt renal CT.
CASE The mass on Ms. C’s left kidney is hypoattenuating and nonenhancing on CT. It meets Bosniak criteria for a benign simple cyst (class I) and requires no further evaluation or follow-up. Colonoscopy detects multiple colonic polyps that are removed, and the patient does well.
Mildly complicated cysts. Less diagnostic certainty characterizes cysts with mild abnormalities that keep them from being labeled as simple. Bosniak classes II and IIF describe mildly abnormal renal cysts. Class II cysts can be dismissed, whereas class IIF cysts require follow-up.
Class II cysts may contain a few hairline septa, fine calcium deposits in walls or septa, or an unmeasurable enhancement of the walls. A hyperattenuating but nonenhancing fluid also is described as category II. Small homogeneous cysts <3 cm, without enhancement but hyperattenuated, are reliably considered benign and need not be evaluated.2,7
Class IIF cysts may have multiple hairline-thin septa with unmeasurable enhancement or minimal smooth thickening or irregular/nodular calcifications of wall or septa without enhancing soft tissue components. Hyperattenuating cystic lesions >3 cm and intrarenal “noncortical” cysts are included in this category. Class IIF cysts require follow-up at 6 months with CT or MRI, then annually for at least 5 years.8
Obviously complicated cysts. Bosniak class III is indeterminate—neither benign nor clearly malignant. Class III cysts may have thickened borders or septa with measurable enhancement, or they may be multilocular, hemorrhagic, or infected. In 5 case series, 29 of 57 class III lesions proved to be malignant.5 MRI may characterize these lesions more definitively than CT prior to urologic referral.
Malignant cysts. Bosniak class IV renal lesions are clearly malignant, with large heterogeneous cysts or necrotic components, shaggy thickened walls, or enhancing soft tissue components separate from the wall or septa. Their unequivocal appearance results from solid tumor necrosis and liquefaction. Diagnosis is straightforward, and excision is indicated.2
A closer look at solid renal masses
Solid renal masses usually consist of enhancing tissue with little or no fluid. The goal of evaluation is to exclude malignancies, such as renal cell cancer, lymphomas, sarcomas, or metastasis. Benign solid masses include renal adenomas, angiomyolipomas, and oncocytomas, among others.
Several lesions can be diagnosed by appearance or symptoms:
Angiomyolipomas are recognized by their fat content within a noncalcified mass. Unenhanced CT usually is sufficient for diagnosis, unless the mass is very small or has atypical features.9
Vascular lesions can be identified because they enhance to the same degree as the vasculature. With the exception of inflammatory or vascular abnormalities, all enhancing lesions that do not contain fat should be presumed to be malignant.
In patients with a known extrarenal primary malignancy, 50% to 85% of incidental solid renal masses will represent metastatic disease.10 Percutaneous biopsy may be warranted to differentiate metastatic lesions from a secondary, primary (ie, renal cell carcinoma), or benign process.11
A study of 2770 solid renal mass excisions revealed that 12.8% were benign, with a direct relationship between malignancy and size. Masses <1 cm were benign 44% of the time.12 Early identification of small renal carcinomas may improve survival rates. Although renal cell carcinomas <3 cm in diameter have low metastatic potential, a solid, nonfat-containing mass should be evaluated for aggressive nephron-sparing surgery.6,13
Incidental adrenal masses occur infrequently
Adrenal incidentalomas are defined as radiographically identified masses >1 cm in diameter.14 They are much less common than their renal counterparts, with a reported prevalence of 0.35% to 5% on CT.15 Because the adrenal glands are hormonally active and receive substantial blood flow, metastatic, hormonally active, and nonfunctional causes for adrenal masses need to be considered.16
Adrenal pathology
Adrenal masses may be characterized by increased or normal adrenal function. Hyperfunctioning syndromes include hypercortisolism, hyperaldosteronism, adrenogenital hypersecretion of adrenocortical origin, and pheochromocytomas of the medulla. Symptom evaluation of these syndromes is important, but not sufficient to rule out a hyperfunctioning syndrome.
In a retrospective review of inapparent adrenal masses, ≤13% of pheochromocytomas were clinically silent.17 Therefore, laboratory testing is necessary for an incidental adrenal mass.
Nonfunctional lesions include adenomas, metastases, cysts, myelolipomas, hemorrhage, and adrenal carcinomas. These masses require evaluation for the possibility of cancer, the most common of which is metastasis. In patients with an extra-adrenal malignancy, the likelihood of malignancy in an incidental adrenal mass is at least 50%.18 An adrenal mass representing metastasis of a previously unrecognized cancer is exceedingly rare.19
Primary adrenal carcinoma is also rare, with an estimated incidence of 2 cases per one million in the general population. For patients with adrenal masses, the prevalence of carcinoma increases with lesion size (2% for tumors <4 cm, 6% for tumors 4-6 cm, and 25% for tumors >6 cm in diameter). 17 For this reason, tumors >4 cm in diameter are usually surgically resected in patients with no previous cancer history, unless radiologic criteria demonstrate clearly benign characteristics.
Although adrenal carcinomas are considered nonfunctioning, some evidence suggests they produce low levels of cortisol that may be associated with clinical features of metabolic syndrome.20
CT is first choice for adrenal mass evaluation
Dedicated adrenal CT with both unenhanced and delayed contrast-enhanced images is the most reliable study to evaluate an adrenal mass, according to the American College of Radiology. Consider another study only in patients with contrast allergy, renal compromise, or cancer history.21
Unenhanced CT can diagnose the approximately 70% of adenomas that are small, well-defined round masses with homogenous low-density lipid deposition.22 Delayed contrast enhancement can characterize most of the remaining 30%.23 Unenhanced CT with attenuation values of <10 Hounsfield units (HU) can diagnose adenomas with 71% specificity and 98% sensitivity,24 and can often diagnose simple cysts and myelolipomas, as well.
Other imaging options. MRI is an alternative to CT for patients with contraindications for contrast or radiation exposure. MRI provides less spatial resolution than CT, but chemical shift imaging can measure cytoplasmic lipid content similar to unenhanced CT. A small study found chemical shift MRI more reliable than unenhanced CT, but less reliable than CT with delayed contrast enhancement.25
Positron emission tomography (PET) is useful to noninvasively evaluate biochemical and physiologic processes. PET-CT incorporates unenhanced CT density measurements to improve PET accuracy. In a patient with a history of cancer, PET-CT has a sensitivity of 93% to 100% and a specificity of 95% in differentiating benign from malignant adrenal tumors.26
When to order a biopsy
The need for biopsy has decreased as imaging has improved, but biopsy is required whenever diagnostic imaging fails to differentiatea lesion as benign or malignant. CT guided biopsy provides diagnostic accuracy of 85% to 95%.27 Complications such as pneumothorax, hemorrhage, and bacteremia occur in 3% to 9% of biopsies. Before any adrenal biopsy, measure plasma-free metanephrines to exclude undiagnosed pheochromocytoma, which could precipitate a hypertensive crisis if untreated.22
These 3 laboratory screening tests are critical
Family physicians can perform the initial biochemical evaluation of an adrenal incidentaloma. Guidance is available from the National Institutes of Health (NIH)28 and the American Academy of Clinical Endocrinologists (AACE) (FIGURE 2).29
Regardless of signs or symptoms, perform screening laboratory tests for 3 types of adrenal hyperfunction: hypercortisolism, hyperaldosteronism, and hypersecretion of catecholamines (pheochromocytoma). Screening tests are not recommended for androgen hypersecretion, which is extremely rare and causes recognizable symptoms such as hirsutism (Table 2).29
Hypercortisolism occurs in approximately 5% of adrenal incidentalomas.30 An overnight dexamethasone suppression test (DST) is most reliable for screening, with sensitivity >95% for Cushing syndrome.31 The patient takes a 1-mg dose of oral dexamethasone at 11 pm, and a fasting plasma cortisol sample is drawn the next day at 8 am.
Dexamethasone binds to glucocorticoid receptors in the pituitary gland, suppressing adrenocorticotropic hormone secretion. Cortisol will be depressed the next morning unless the adrenal mass produces cortisol autonomously. Patients with a DST >5 mcg/dL—highly suggestive of Cushing syndrome—require further evaluation, and we suggest referral to an endocrinologist.
Hyperaldosteronism is seen in 1% to 2% of adrenal incidentalomas.32 The aldosterone- to-renin ratio (ARR) is recommended as a screening test for hyperaldosteronism, with an ARR >20 requiring further testing.33 Medications that may affect the ARR include beta-blockers, spironolactone, clonidine, diuretics, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers.29
Refer a patient with evidence of hyperaldosteronism to an endocrinologist and a surgeon with experience in managing these lesions. If the ARR test result suggests an aldosterone excess, a salt-loading test is used to verify failure of aldosterone suppression. Adrenal venous sampling is often performed prior to surgical removal to confirm that an incidentaloma is the source of hyperaldosteronism.
Pheochromocytoma. Approximately 5% of incidental adrenal lesions are pheochromocytomas.30 Many patients with these epinephrine/norepinephrine secreting tumors do not show the classic symptom triad of headache, palpitations, and diaphoresis, and approximately half have normal blood pressure.34
Identifying a pheochromocytoma is important in any patient requiring surgery or biopsy, as surgical manipulation can cause a potentially fatal intraoperative catecholamine surge. Presurgical medical management can mitigate this reaction.
A plasma-free metanephrines test, which has 95% sensitivity, is the most reliable test for pheochromocytoma.35 Medications, including tricyclic antidepressants, decongestants, amphetamines, reserpine, and phenoxybenzamine, can cause falsepositive results.29 Confirm a positive plasma-free metanephrines test with a 24-hour fractionated urine metanephrines test, and refer the patient to an endocrinologist.
Managing adrenal incidentalomas
Refer all patients with adrenal masses >4 cm for surgical evaluation because of the risk of malignancy; all patients who have a history of malignancy and an adrenal mass of any size require a referral to an oncologist. Perform the AACE-recommended 3-element biochemical workup for all masses, with the exception of definitively diagnosed cysts or myelolipomas.
Refer to an endocrinologist all patients with abnormal screening laboratory results, regardless of adrenal mass size, as well as patients with concerning clinical findings. Initiate cardiovascular, diabetes, and bone density evaluation and management for metabolic syndrome.20
Monitoring after a negative workup
Little evidence exists to guide monitoring of small adrenal incidentalomas (<4 cm) with a negative workup. The 2002 NIH report recommended annual radiologic follow-up for 5 years,28 whereas the 2009 AACE guidelines recommend radiographic follow-up at 3 to 6 months, then at one and 2 years.29
Evidence indicates that 14% of lesions will enlarge in 2 years, although the clinical significance of enlargement is unknown. Some authors argue against CT monitoring because the risk of adrenal mass progression is similar to the malignancy risk posed by 3 years of radiation exposure with CT.20
Some guidelines recommend repeat biochemical screening every 3 to 4 years.28,29 AACE guidelines quote a 47% rate of progression over 3 years, but most adrenal masses progress to subclinical Cushing syndrome— a condition of uncertain significance. Subclinical Cushing’s has not been reported to progress to the overt syndrome, and new catecholamine or aldosterone secretion is rare.
Many endocrinologists reduce the frequency of follow-up, depending on the type of adrenal mass (cyst or solid) and its size. AACE suggests CT for adenomas one to 4 cm at 12 months. AACE and NIH recommend hormonal evaluation annually for 4 years. Adrenal cysts or myelolipoma in patients without cancer need no follow-up.29
CORRESPONDENCE
James C. Higgins, DO, CAPT, MC, USN, Ret., Naval Hospital Jacksonville, Family Medicine Department, 2080 Child Street, Box 1000, Jacksonville, FL 32214;
[email protected]
1. Berland LL, Silverman SG, Gore RM, et al. Managing incidental findings on abdominal CT: white paper of the ACR incidental findings committee. J Am Coll Radiol. 2010;7:754-773.
2. Silverman S, Israel G, Herts B, et al. Management of the incidental renal mass. Radiology. 2008;249:16-31.
3. Curry NS, Bissada NK. Radiologic evaluation of small and indeterminate renal masses. Urol Clin North Am. 1997;24:493-505.
4. Bosniak MA. The current radiological approach to renal cysts. Radiology. 1986;158:1-10.
5. Harisinghani M, Maher M, Gervais D, et al. Incidence of malignancy in complex cystic renal masses (Bosniak category III): should imaging guided biopsy precede surgery? AJR Am J Roentgenol. 2003;180:755-758.
6. Remzi M, Ozsoy M, Klingler HC. Are small renal tumors harmless? Analysis of histopathological features according to tumors less than 4 cm in diameter. J Urol. 2006;176:896-899.
7. Jonisch AI, Rubinowitz A, Mutalik P, et al. Can high attenuation renal cysts be differentiated from renal cell carcinoma at unenhanced computed tomography? Radiology. 2007;243:445-450.
8. Israel GM, Bosniak MA. Follow-up CT of moderately complex cystic lesions of the kidney. AJR Am J Roentgenol. 2003;181: 627-633.
9. Bosniak MA, Megibow AJ, Hulnick DH, et al. CT diagnosis of renal angiomyolipoma: the importance of detecting small amounts of fat. AJR Am J Roentengol. 1988;151:497-501.
10. Mitnick JS, Bosniak MA, Rothberg M, et al. Metastatic neoplasm to the kidney studied by computed tomography and sonogram. J Comput Assist Tomogr. 1985;9:43-49.
11. Rybicki FJ, Shu KM, Cibas ES, et al. Percutaneous biopsy of renal masses: sensitivity and negative predictive value stratified by clinical setting and size of masses. AJR Am J Roentgenol. 2003;180:1281-1287.
12. Frank I, Blure MI, Cheville JC, et al. Solid renal tumors: an analysis of pathological features related to tumor size. J Urol. 2003;170:2217-2220.
13. Hollingsworth JM, Miller DC, Daignault S, et al. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst. 2006;98:1331-1334.
14. Geelhoed GW, Spiegel CT. “Incidental” adrenal cyst: a correctable lesion possibly associated with hypertension. South Med J. 1981;74:626-630.
15. Davenport C, Liew A, Doherty B, et al. The prevalence of adrenal incidentaloma in routine clinical practice. Endocrine. 2011;40: 80-83.
16. Cook DM, Loriaux LD. The incidental adrenal mass. Am J Med. 1996;101:88 94.
17. Mansmann G, Lau J, Balk E, et al. The clinically inapparent adrenal mass: update in diagnosis and management. Endocr Rev. 2004;25:309-340.
18. Androulakis II, Kaltsas G, Piatitis G, et al. The clinical significance of adrenal incidentalomas. Eur J Clin Invest. 2011;41: 552-560.
19. Lee JE, Evans DB, Hickey RC, et al. Unknown primary cancer presenting as an adrenal mass: frequency and implications for diagnostic evaluation of adrenal incidentalomas. Surgery. 1998;124:1115-1122.
20. Aron D, Terzolo M, Cawood TJ. Adrenal incidentalomas. Best Pract Res Clin Endocrinol Metab. 2012;26:69-82.
21. ACR appropriateness criteria: incidentally discovered adrenal mass. American College of Radiology. Available at: http://www.acr.org/~/media/ACR/Documents/AppCriteria/Diagnostic/IncidentallyDiscoveredAdrenalMass.pdf. Accessed November 20, 2012.
22. Song JH, Mayo-Smith WW. Incidentally discovered adrenal mass. Radiol Clin North Am. 2011;49:361-368.
23. Korobkin M, Brodeur FJ, Francis IR, et al. CT time-attenuation washout curves of adrenal adenomas and nonadenomas. AJR Am J Roentgenol. 1998;170:747-752.
24. Boland GW, Lee MJ, Gazelle GS, et al. Characterization of adrenal masses using unenhanced CT: an analysis of the CT literature. AJR Am J Roentgenol. 1998;171:201-204.
25. Park BK, Kim CK, Kim B, et al. Chemical shift MR imaging of hyperattenuating (>10 HU) adrenal masses: does it still have a role? Radiology. 2004;231:711-716.
26. Boland GW, Blake MA, Holakere NS, et al. PET/CT for the characterization of adrenal masses in patients with cancer: qualitative vs quantitative accuracy in 150 consecutive patients. AJR Am J Roentgenol. 2009;192:956-962.
27. Paulsen SD, Nghiem HV, Korobkin M, et al. Changing role of imaging- guided percutaneous biopsy of adrenal masses: evaluation of 50 adrenal biopsies. AJR Am J Roentgenol. 2004;182:1033-1037
28. Grumbach MM, Biller BMK, Braunstein GD, et al. Management of the clinically inapparent adrenal mass (“incidentalomas”). Ann Intern Med. 2003;138:424-429.
29. Zeiger MA, Thompson GB, Quan-Yang D, et al. American Association of Clinical Endocrinologists and American Association of Endocrine Surgeons medical guidelines for the management of adrenal incidentalomas. Endocr Pract. 2009;15(suppl 1):1-20.
30. Young WF. The incidentally discovered adrenal mass. N Engl J Med. 2007; 356:601-610.
31. Deutschbein T, Unger N, Hinrichs J, et al. Late-night and lowdose dexamethasone-suppressed cortisol in saliva and serum for the diagnosis of cortisol-secreting adrenal adenomas. Eur J Endocrinol. 2009;161:747-753.
32. Bernini G, Moretti A, Gianfranco A, et al. Primary aldosteronism in normokalemic patients with adrenal incidentalomas. Eur J Endocrinol. 2002;146:523-529.
33. Montori VM, Young WF Jr. Use of plasma aldosterone concentration-to-plasma renin activity ratio as a screening test for primary aldosteronism: a systematic review of the literature. Endocrinol Metab Clin North Am. 2002;31:619-632.
34. Motta-Ramirez GA, Remer EM, Herts BR, et al. Comparison of CT findings in symptomatic and incidentally discovered pheochromocytomas. AJR Am J Roentgenol. 2005;185:684-688.
35. Pacak K, Eisenhofer G, Grossman A. The incidentally discovered adrenal mass. N Engl J Med. 2007;356:2005.
1. Berland LL, Silverman SG, Gore RM, et al. Managing incidental findings on abdominal CT: white paper of the ACR incidental findings committee. J Am Coll Radiol. 2010;7:754-773.
2. Silverman S, Israel G, Herts B, et al. Management of the incidental renal mass. Radiology. 2008;249:16-31.
3. Curry NS, Bissada NK. Radiologic evaluation of small and indeterminate renal masses. Urol Clin North Am. 1997;24:493-505.
4. Bosniak MA. The current radiological approach to renal cysts. Radiology. 1986;158:1-10.
5. Harisinghani M, Maher M, Gervais D, et al. Incidence of malignancy in complex cystic renal masses (Bosniak category III): should imaging guided biopsy precede surgery? AJR Am J Roentgenol. 2003;180:755-758.
6. Remzi M, Ozsoy M, Klingler HC. Are small renal tumors harmless? Analysis of histopathological features according to tumors less than 4 cm in diameter. J Urol. 2006;176:896-899.
7. Jonisch AI, Rubinowitz A, Mutalik P, et al. Can high attenuation renal cysts be differentiated from renal cell carcinoma at unenhanced computed tomography? Radiology. 2007;243:445-450.
8. Israel GM, Bosniak MA. Follow-up CT of moderately complex cystic lesions of the kidney. AJR Am J Roentgenol. 2003;181: 627-633.
9. Bosniak MA, Megibow AJ, Hulnick DH, et al. CT diagnosis of renal angiomyolipoma: the importance of detecting small amounts of fat. AJR Am J Roentengol. 1988;151:497-501.
10. Mitnick JS, Bosniak MA, Rothberg M, et al. Metastatic neoplasm to the kidney studied by computed tomography and sonogram. J Comput Assist Tomogr. 1985;9:43-49.
11. Rybicki FJ, Shu KM, Cibas ES, et al. Percutaneous biopsy of renal masses: sensitivity and negative predictive value stratified by clinical setting and size of masses. AJR Am J Roentgenol. 2003;180:1281-1287.
12. Frank I, Blure MI, Cheville JC, et al. Solid renal tumors: an analysis of pathological features related to tumor size. J Urol. 2003;170:2217-2220.
13. Hollingsworth JM, Miller DC, Daignault S, et al. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst. 2006;98:1331-1334.
14. Geelhoed GW, Spiegel CT. “Incidental” adrenal cyst: a correctable lesion possibly associated with hypertension. South Med J. 1981;74:626-630.
15. Davenport C, Liew A, Doherty B, et al. The prevalence of adrenal incidentaloma in routine clinical practice. Endocrine. 2011;40: 80-83.
16. Cook DM, Loriaux LD. The incidental adrenal mass. Am J Med. 1996;101:88 94.
17. Mansmann G, Lau J, Balk E, et al. The clinically inapparent adrenal mass: update in diagnosis and management. Endocr Rev. 2004;25:309-340.
18. Androulakis II, Kaltsas G, Piatitis G, et al. The clinical significance of adrenal incidentalomas. Eur J Clin Invest. 2011;41: 552-560.
19. Lee JE, Evans DB, Hickey RC, et al. Unknown primary cancer presenting as an adrenal mass: frequency and implications for diagnostic evaluation of adrenal incidentalomas. Surgery. 1998;124:1115-1122.
20. Aron D, Terzolo M, Cawood TJ. Adrenal incidentalomas. Best Pract Res Clin Endocrinol Metab. 2012;26:69-82.
21. ACR appropriateness criteria: incidentally discovered adrenal mass. American College of Radiology. Available at: http://www.acr.org/~/media/ACR/Documents/AppCriteria/Diagnostic/IncidentallyDiscoveredAdrenalMass.pdf. Accessed November 20, 2012.
22. Song JH, Mayo-Smith WW. Incidentally discovered adrenal mass. Radiol Clin North Am. 2011;49:361-368.
23. Korobkin M, Brodeur FJ, Francis IR, et al. CT time-attenuation washout curves of adrenal adenomas and nonadenomas. AJR Am J Roentgenol. 1998;170:747-752.
24. Boland GW, Lee MJ, Gazelle GS, et al. Characterization of adrenal masses using unenhanced CT: an analysis of the CT literature. AJR Am J Roentgenol. 1998;171:201-204.
25. Park BK, Kim CK, Kim B, et al. Chemical shift MR imaging of hyperattenuating (>10 HU) adrenal masses: does it still have a role? Radiology. 2004;231:711-716.
26. Boland GW, Blake MA, Holakere NS, et al. PET/CT for the characterization of adrenal masses in patients with cancer: qualitative vs quantitative accuracy in 150 consecutive patients. AJR Am J Roentgenol. 2009;192:956-962.
27. Paulsen SD, Nghiem HV, Korobkin M, et al. Changing role of imaging- guided percutaneous biopsy of adrenal masses: evaluation of 50 adrenal biopsies. AJR Am J Roentgenol. 2004;182:1033-1037
28. Grumbach MM, Biller BMK, Braunstein GD, et al. Management of the clinically inapparent adrenal mass (“incidentalomas”). Ann Intern Med. 2003;138:424-429.
29. Zeiger MA, Thompson GB, Quan-Yang D, et al. American Association of Clinical Endocrinologists and American Association of Endocrine Surgeons medical guidelines for the management of adrenal incidentalomas. Endocr Pract. 2009;15(suppl 1):1-20.
30. Young WF. The incidentally discovered adrenal mass. N Engl J Med. 2007; 356:601-610.
31. Deutschbein T, Unger N, Hinrichs J, et al. Late-night and lowdose dexamethasone-suppressed cortisol in saliva and serum for the diagnosis of cortisol-secreting adrenal adenomas. Eur J Endocrinol. 2009;161:747-753.
32. Bernini G, Moretti A, Gianfranco A, et al. Primary aldosteronism in normokalemic patients with adrenal incidentalomas. Eur J Endocrinol. 2002;146:523-529.
33. Montori VM, Young WF Jr. Use of plasma aldosterone concentration-to-plasma renin activity ratio as a screening test for primary aldosteronism: a systematic review of the literature. Endocrinol Metab Clin North Am. 2002;31:619-632.
34. Motta-Ramirez GA, Remer EM, Herts BR, et al. Comparison of CT findings in symptomatic and incidentally discovered pheochromocytomas. AJR Am J Roentgenol. 2005;185:684-688.
35. Pacak K, Eisenhofer G, Grossman A. The incidentally discovered adrenal mass. N Engl J Med. 2007;356:2005.
Urinary albumin, incident heart disease linked in black adults
A higher urinary albumin-to-creatinine ratio was associated with an increased risk of incident coronary heart disease in black adults in the large, population-based REGARDS study.
No such association was seen in white adults in the prospective cohort study, suggesting that black individuals are more susceptible to vascular injury, according to Dr. Orlando M. Gutierrez of the University of Alabama at Birmingham and his colleagues, who reported the findings on behalf of the Reasons for Geographic and Racial Differences in Stroke (REGARDS) investigators.
Over a mean follow-up period of 4.5 years, 616 incident coronary heart disease events – 421 nonfatal myocardial infarctions and 195 CHD deaths – occurred in 23,273 individuals who were free of CHD at baseline. The incidence rates of CHD per 1,000 person-years of follow-up increased with increasing albumin-to-creatinine ratio (ACR) in these patients, and the increases were significantly greater for black adults, compared with white adults, the investigators reported. The study was published in the Aug. 21 issue of JAMA.
Age- and sex-adjusted incidence rates were nearly 1.5-fold greater in black adults than in white adults in the two highest categories of ACR: For black vs. white participants with ACR of 30-300 mg/g, the incidence rates per 1,000 person years were 11.2 and 8.0, respectively, and for those with ACR greater than 300 mg/g, the rates were 20.6 and 13.6, respectively, both significant differences.
After adjustment for traditional cardiovascular risk factors and medications, higher baseline urinary ACR (greater than 300 mg/g vs. less than 10 mg/g) was associated with greater risk of incident CHD among blacks, but not whites (hazard ratios of 3.21 and 1.49, respectively), they said.
A similar association was not seen for recurrent CHD. Over 4.4 years of follow-up, 468 recurrent CHD events – 279 nonfatal MIs and 189 CHD deaths – occurred in 4,934 individuals who had CHD at baseline. No differences were seen between black and white adults in this group with respect to baseline urinary ACR and first recurrent CHD event (JAMA 2013; 310:706-13).
The REGARDS study, a population-based investigation of stroke incidence in black and white adults in the United States, comprised individuals aged 45 years and older at baseline between 2003 and 2007, and oversampled those who self-reported as black and those living in the U.S. stroke belt.
Black individuals are known to have higher levels of urinary albumin excretion than those of white individuals – a finding that may contribute to racial disparities in cardiovascular outcomes. Previous REGARDS study findings showed that an association between urinary ACR and incident stroke differed by race, and that higher urinary ACR was independently associated with a greater risk of incident stroke in blacks, but not whites, the investigators said.
However, little is known about racial differences with respect to the association of urinary ACR and cardiovascular outcomes apart from stroke, they noted.
"These findings confirm the results of prior studies showing that urinary ACR is an important biomarker for CHD risk in the general population, even among individuals with ACR values that are less than the current threshold for defining microalbuminuria (30 mg/g). Additionally, to our knowledge, this is the first study to demonstrate that the higher risk of incident CHD associated with excess ACR differs by race," they said.
The findings contribute to increasing evidence suggesting that blacks are more susceptible than are whites to vascular injury, and suggest that this greater susceptibility may account for much of the excess risk of cardiovascular disease events, including stroke and CHD, in black individuals, they added.
This study is limited by a number of factors, including the use of a single measure of ACR, which may have led to exposure misclassification for some patients, and also by reduced power to detect significant associations due to relatively few events occurring in some ACR categories. Also, only black and white adults were included in REGARDS, which may limit the applicability of the results to other races and ethnicities, the investigators noted.
Nonetheless, the findings indicate that higher urinary ACR is a strong risk factor for incident CHD events (but not recurrent CHD events) in black vs. white individuals, they said, concluding that future studies should examine whether the addition of ACR can improve the diagnosis and management of CHD in black individuals.
This study was supported by a cooperative agreement from the National Institute of Neurological Disorders and Stroke and from the National Heart, Lung, and Blood Institute. Dr. Gutierrez was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases and from NINDS. Amgen provided funding in the form of an investigator-initiated grant-in-aid. Several study authors disclosed ties with Amgen, REATA Pharmaceuticals, Arbor Research, Sanofi-Genzyme, and/or diaDexus.
These findings from the REGARDS study highlight the complexities inherent in the relation between albuminuria and cardiovascular disease risk, and underscore the importance of urine ACR elevations, Dr. Daniel E. Weiner and Dr. Wolfgang C. Winkelmayer wrote in an editorial.
Key questions raised by the study are, why do black individuals have higher levels of albuminuria than white individuals, and what can be done to reduce associated cardiovascular disease risk in those at higher risk, they said.
The questions could only be answered in a setting of equal care access and use, and equally healthy living strategies beginning early in life, "such that genetic factors that may influence kidney disease can be distinguished from factors related to indolent chronic diseases (metabolic syndrome, hypertension, type 2 diabetes, and prediabetes)," they said, noting that such diseases are at least somewhat preventable with healthy living, are more common in black individuals and people of lower socioeconomic status, and are associated with cardiovascular disease and higher albuminuria (JAMA. 2013;310:697-8).
"Until these complex relationships are better disentangled, the study by Dr. Gutierrez and colleagues reinforces that even mild elevations in urine ACR are associated with increased CVD risk, even though this level of albuminuria will have no meaningful systemic effects," they said, adding that differentiating between low normal (less than 10 mg/g) and high normal (10-30 mg/g) urinary ACR may help with cardiovascular risk stratification, particularly in black individuals, perhaps leading to preventive efforts and improved monitoring.
Dr. Weiner is with Tufts Medical Center, Boston. He reported having no disclosures. Dr. Winkelmayer is with Stanford (Calif.) University. He reported having served as an adviser or consultant to Amgen and numerous other pharmaceutical and device manufacturers.
These findings from the REGARDS study highlight the complexities inherent in the relation between albuminuria and cardiovascular disease risk, and underscore the importance of urine ACR elevations, Dr. Daniel E. Weiner and Dr. Wolfgang C. Winkelmayer wrote in an editorial.
Key questions raised by the study are, why do black individuals have higher levels of albuminuria than white individuals, and what can be done to reduce associated cardiovascular disease risk in those at higher risk, they said.
The questions could only be answered in a setting of equal care access and use, and equally healthy living strategies beginning early in life, "such that genetic factors that may influence kidney disease can be distinguished from factors related to indolent chronic diseases (metabolic syndrome, hypertension, type 2 diabetes, and prediabetes)," they said, noting that such diseases are at least somewhat preventable with healthy living, are more common in black individuals and people of lower socioeconomic status, and are associated with cardiovascular disease and higher albuminuria (JAMA. 2013;310:697-8).
"Until these complex relationships are better disentangled, the study by Dr. Gutierrez and colleagues reinforces that even mild elevations in urine ACR are associated with increased CVD risk, even though this level of albuminuria will have no meaningful systemic effects," they said, adding that differentiating between low normal (less than 10 mg/g) and high normal (10-30 mg/g) urinary ACR may help with cardiovascular risk stratification, particularly in black individuals, perhaps leading to preventive efforts and improved monitoring.
Dr. Weiner is with Tufts Medical Center, Boston. He reported having no disclosures. Dr. Winkelmayer is with Stanford (Calif.) University. He reported having served as an adviser or consultant to Amgen and numerous other pharmaceutical and device manufacturers.
These findings from the REGARDS study highlight the complexities inherent in the relation between albuminuria and cardiovascular disease risk, and underscore the importance of urine ACR elevations, Dr. Daniel E. Weiner and Dr. Wolfgang C. Winkelmayer wrote in an editorial.
Key questions raised by the study are, why do black individuals have higher levels of albuminuria than white individuals, and what can be done to reduce associated cardiovascular disease risk in those at higher risk, they said.
The questions could only be answered in a setting of equal care access and use, and equally healthy living strategies beginning early in life, "such that genetic factors that may influence kidney disease can be distinguished from factors related to indolent chronic diseases (metabolic syndrome, hypertension, type 2 diabetes, and prediabetes)," they said, noting that such diseases are at least somewhat preventable with healthy living, are more common in black individuals and people of lower socioeconomic status, and are associated with cardiovascular disease and higher albuminuria (JAMA. 2013;310:697-8).
"Until these complex relationships are better disentangled, the study by Dr. Gutierrez and colleagues reinforces that even mild elevations in urine ACR are associated with increased CVD risk, even though this level of albuminuria will have no meaningful systemic effects," they said, adding that differentiating between low normal (less than 10 mg/g) and high normal (10-30 mg/g) urinary ACR may help with cardiovascular risk stratification, particularly in black individuals, perhaps leading to preventive efforts and improved monitoring.
Dr. Weiner is with Tufts Medical Center, Boston. He reported having no disclosures. Dr. Winkelmayer is with Stanford (Calif.) University. He reported having served as an adviser or consultant to Amgen and numerous other pharmaceutical and device manufacturers.
A higher urinary albumin-to-creatinine ratio was associated with an increased risk of incident coronary heart disease in black adults in the large, population-based REGARDS study.
No such association was seen in white adults in the prospective cohort study, suggesting that black individuals are more susceptible to vascular injury, according to Dr. Orlando M. Gutierrez of the University of Alabama at Birmingham and his colleagues, who reported the findings on behalf of the Reasons for Geographic and Racial Differences in Stroke (REGARDS) investigators.
Over a mean follow-up period of 4.5 years, 616 incident coronary heart disease events – 421 nonfatal myocardial infarctions and 195 CHD deaths – occurred in 23,273 individuals who were free of CHD at baseline. The incidence rates of CHD per 1,000 person-years of follow-up increased with increasing albumin-to-creatinine ratio (ACR) in these patients, and the increases were significantly greater for black adults, compared with white adults, the investigators reported. The study was published in the Aug. 21 issue of JAMA.
Age- and sex-adjusted incidence rates were nearly 1.5-fold greater in black adults than in white adults in the two highest categories of ACR: For black vs. white participants with ACR of 30-300 mg/g, the incidence rates per 1,000 person years were 11.2 and 8.0, respectively, and for those with ACR greater than 300 mg/g, the rates were 20.6 and 13.6, respectively, both significant differences.
After adjustment for traditional cardiovascular risk factors and medications, higher baseline urinary ACR (greater than 300 mg/g vs. less than 10 mg/g) was associated with greater risk of incident CHD among blacks, but not whites (hazard ratios of 3.21 and 1.49, respectively), they said.
A similar association was not seen for recurrent CHD. Over 4.4 years of follow-up, 468 recurrent CHD events – 279 nonfatal MIs and 189 CHD deaths – occurred in 4,934 individuals who had CHD at baseline. No differences were seen between black and white adults in this group with respect to baseline urinary ACR and first recurrent CHD event (JAMA 2013; 310:706-13).
The REGARDS study, a population-based investigation of stroke incidence in black and white adults in the United States, comprised individuals aged 45 years and older at baseline between 2003 and 2007, and oversampled those who self-reported as black and those living in the U.S. stroke belt.
Black individuals are known to have higher levels of urinary albumin excretion than those of white individuals – a finding that may contribute to racial disparities in cardiovascular outcomes. Previous REGARDS study findings showed that an association between urinary ACR and incident stroke differed by race, and that higher urinary ACR was independently associated with a greater risk of incident stroke in blacks, but not whites, the investigators said.
However, little is known about racial differences with respect to the association of urinary ACR and cardiovascular outcomes apart from stroke, they noted.
"These findings confirm the results of prior studies showing that urinary ACR is an important biomarker for CHD risk in the general population, even among individuals with ACR values that are less than the current threshold for defining microalbuminuria (30 mg/g). Additionally, to our knowledge, this is the first study to demonstrate that the higher risk of incident CHD associated with excess ACR differs by race," they said.
The findings contribute to increasing evidence suggesting that blacks are more susceptible than are whites to vascular injury, and suggest that this greater susceptibility may account for much of the excess risk of cardiovascular disease events, including stroke and CHD, in black individuals, they added.
This study is limited by a number of factors, including the use of a single measure of ACR, which may have led to exposure misclassification for some patients, and also by reduced power to detect significant associations due to relatively few events occurring in some ACR categories. Also, only black and white adults were included in REGARDS, which may limit the applicability of the results to other races and ethnicities, the investigators noted.
Nonetheless, the findings indicate that higher urinary ACR is a strong risk factor for incident CHD events (but not recurrent CHD events) in black vs. white individuals, they said, concluding that future studies should examine whether the addition of ACR can improve the diagnosis and management of CHD in black individuals.
This study was supported by a cooperative agreement from the National Institute of Neurological Disorders and Stroke and from the National Heart, Lung, and Blood Institute. Dr. Gutierrez was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases and from NINDS. Amgen provided funding in the form of an investigator-initiated grant-in-aid. Several study authors disclosed ties with Amgen, REATA Pharmaceuticals, Arbor Research, Sanofi-Genzyme, and/or diaDexus.
A higher urinary albumin-to-creatinine ratio was associated with an increased risk of incident coronary heart disease in black adults in the large, population-based REGARDS study.
No such association was seen in white adults in the prospective cohort study, suggesting that black individuals are more susceptible to vascular injury, according to Dr. Orlando M. Gutierrez of the University of Alabama at Birmingham and his colleagues, who reported the findings on behalf of the Reasons for Geographic and Racial Differences in Stroke (REGARDS) investigators.
Over a mean follow-up period of 4.5 years, 616 incident coronary heart disease events – 421 nonfatal myocardial infarctions and 195 CHD deaths – occurred in 23,273 individuals who were free of CHD at baseline. The incidence rates of CHD per 1,000 person-years of follow-up increased with increasing albumin-to-creatinine ratio (ACR) in these patients, and the increases were significantly greater for black adults, compared with white adults, the investigators reported. The study was published in the Aug. 21 issue of JAMA.
Age- and sex-adjusted incidence rates were nearly 1.5-fold greater in black adults than in white adults in the two highest categories of ACR: For black vs. white participants with ACR of 30-300 mg/g, the incidence rates per 1,000 person years were 11.2 and 8.0, respectively, and for those with ACR greater than 300 mg/g, the rates were 20.6 and 13.6, respectively, both significant differences.
After adjustment for traditional cardiovascular risk factors and medications, higher baseline urinary ACR (greater than 300 mg/g vs. less than 10 mg/g) was associated with greater risk of incident CHD among blacks, but not whites (hazard ratios of 3.21 and 1.49, respectively), they said.
A similar association was not seen for recurrent CHD. Over 4.4 years of follow-up, 468 recurrent CHD events – 279 nonfatal MIs and 189 CHD deaths – occurred in 4,934 individuals who had CHD at baseline. No differences were seen between black and white adults in this group with respect to baseline urinary ACR and first recurrent CHD event (JAMA 2013; 310:706-13).
The REGARDS study, a population-based investigation of stroke incidence in black and white adults in the United States, comprised individuals aged 45 years and older at baseline between 2003 and 2007, and oversampled those who self-reported as black and those living in the U.S. stroke belt.
Black individuals are known to have higher levels of urinary albumin excretion than those of white individuals – a finding that may contribute to racial disparities in cardiovascular outcomes. Previous REGARDS study findings showed that an association between urinary ACR and incident stroke differed by race, and that higher urinary ACR was independently associated with a greater risk of incident stroke in blacks, but not whites, the investigators said.
However, little is known about racial differences with respect to the association of urinary ACR and cardiovascular outcomes apart from stroke, they noted.
"These findings confirm the results of prior studies showing that urinary ACR is an important biomarker for CHD risk in the general population, even among individuals with ACR values that are less than the current threshold for defining microalbuminuria (30 mg/g). Additionally, to our knowledge, this is the first study to demonstrate that the higher risk of incident CHD associated with excess ACR differs by race," they said.
The findings contribute to increasing evidence suggesting that blacks are more susceptible than are whites to vascular injury, and suggest that this greater susceptibility may account for much of the excess risk of cardiovascular disease events, including stroke and CHD, in black individuals, they added.
This study is limited by a number of factors, including the use of a single measure of ACR, which may have led to exposure misclassification for some patients, and also by reduced power to detect significant associations due to relatively few events occurring in some ACR categories. Also, only black and white adults were included in REGARDS, which may limit the applicability of the results to other races and ethnicities, the investigators noted.
Nonetheless, the findings indicate that higher urinary ACR is a strong risk factor for incident CHD events (but not recurrent CHD events) in black vs. white individuals, they said, concluding that future studies should examine whether the addition of ACR can improve the diagnosis and management of CHD in black individuals.
This study was supported by a cooperative agreement from the National Institute of Neurological Disorders and Stroke and from the National Heart, Lung, and Blood Institute. Dr. Gutierrez was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases and from NINDS. Amgen provided funding in the form of an investigator-initiated grant-in-aid. Several study authors disclosed ties with Amgen, REATA Pharmaceuticals, Arbor Research, Sanofi-Genzyme, and/or diaDexus.
FROM JAMA
Major finding: Adjusted hazard ratios for incident CHD in black vs. white adults with high ACR: 3.21 and 1.49, respectively.
Data source: A prospective cohort study involving 28,207 adults.
Disclosures: This study was supported by a cooperative agreement from the National Institute of Neurological Disorders and Stroke and from the National Heart, Lung, and Blood Institute. Dr. Gutierrez was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases and from NINDS. Amgen provided funding in the form of an investigator-initiated grant-in-aid. Several study authors disclosed ties with Amgen, REATA Pharmaceuticals, Arbor Research, Sanofi-Genzyme, and/or diaDexus.