User login
World’s dialysis burden has grown 165% since 1990
ATLANTA – The global prevalence of maintenance dialysis for end-stage renal disease has increased 165% over the past 2 decades – a rate that has far outpaced that of population growth in most regions of the world, according to Dr. Bernadette A. Thomas.
The findings – the first to quantify the global burden of end-stage renal disease – underscore a need for improved detection of early chronic kidney disease and for treatment aimed at preventing end-stage renal disease (ESRD), because a continued rise in the prevalence of maintenance dialysis may not be sustainable, Dr. Thomas of the University of Washington, Seattle, said at Kidney Week 2013.
From 1990 to 2010, the global prevalence of maintenance dialysis in areas with universal dialysis access increased 134%, after adjustment for population growth and aging. The increase was 145% among women and 123% among men. Even in countries with a lack of universal access, the adjusted prevalence increased by 102% (116% for women and 90% for men).
The regions that did not experience an increase in dialysis prevalence included Oceania, South Asia, Central Sub-Saharan Africa, Eastern Europe, and tropical Latin America, Dr. Thomas said at the conference, which was sponsored by the American Society of Nephrology.
The findings are based on data extracted from the Global Burden of Disease database, the largest existing database for global causes of morbidity and mortality. Prevalence estimates were based on national and regional ESRD registries and a structured literature review. Data from 23 countries with universal dialysis access and from 138 countries with partial access were included; data from 26 countries that lack routine access were excluded.
"The statistics are appalling," American Society of Nephrology President Bruce A. Molitoris of Indiana University, Indianapolis, noted during a press briefing. Dr. Molitoris called for stepped-up efforts to educate the public about the importance of kidney health.
"We have targeted the patients in the past [via] patient education, and we have not presented, I think, a fair outlook to the public of the potential importance of kidney disease," he said.
Up to one-third of U.S. residents will, at some point in their lives, have severe kidney disease, Dr. Molitoris said, and 8% of African Americans and 3.6% of the total population will go on dialysis or need a transplant.
"And yet, people think they are immune to kidney disease," he cautioned. "The field is in need of innovation; the field is in need of individualization of care. And to do that, we have to have public awareness."
Kidney disease ranks near the bottom when it comes to research funding, and public awareness will go a long way toward changing that, he added.
Dr. Molitoris said he is optimistic that as the pharmaceutical industry continues taking an interest in kidney disease, and as public awareness increases, funding – and therefore innovation – will also increase. He said he envisions a time when the public will be as aware of their kidney function level as they are of their cholesterol level.
"With what is going on in science and technology and biology, it’s not a far leap for us to make major strides," he said.
The study by Dr. Thomas was supported by a private foundation. Dr. Thomas reported having no relevant financial disclosures. Dr. Molitoris reported consultancy, ownership interest, research funding, honoraria, and/or a scientific advisory or membership role for numerous companies. He also reported a patent/invention with FAST Diagnostics, Indiana University.
ATLANTA – The global prevalence of maintenance dialysis for end-stage renal disease has increased 165% over the past 2 decades – a rate that has far outpaced that of population growth in most regions of the world, according to Dr. Bernadette A. Thomas.
The findings – the first to quantify the global burden of end-stage renal disease – underscore a need for improved detection of early chronic kidney disease and for treatment aimed at preventing end-stage renal disease (ESRD), because a continued rise in the prevalence of maintenance dialysis may not be sustainable, Dr. Thomas of the University of Washington, Seattle, said at Kidney Week 2013.
From 1990 to 2010, the global prevalence of maintenance dialysis in areas with universal dialysis access increased 134%, after adjustment for population growth and aging. The increase was 145% among women and 123% among men. Even in countries with a lack of universal access, the adjusted prevalence increased by 102% (116% for women and 90% for men).
The regions that did not experience an increase in dialysis prevalence included Oceania, South Asia, Central Sub-Saharan Africa, Eastern Europe, and tropical Latin America, Dr. Thomas said at the conference, which was sponsored by the American Society of Nephrology.
The findings are based on data extracted from the Global Burden of Disease database, the largest existing database for global causes of morbidity and mortality. Prevalence estimates were based on national and regional ESRD registries and a structured literature review. Data from 23 countries with universal dialysis access and from 138 countries with partial access were included; data from 26 countries that lack routine access were excluded.
"The statistics are appalling," American Society of Nephrology President Bruce A. Molitoris of Indiana University, Indianapolis, noted during a press briefing. Dr. Molitoris called for stepped-up efforts to educate the public about the importance of kidney health.
"We have targeted the patients in the past [via] patient education, and we have not presented, I think, a fair outlook to the public of the potential importance of kidney disease," he said.
Up to one-third of U.S. residents will, at some point in their lives, have severe kidney disease, Dr. Molitoris said, and 8% of African Americans and 3.6% of the total population will go on dialysis or need a transplant.
"And yet, people think they are immune to kidney disease," he cautioned. "The field is in need of innovation; the field is in need of individualization of care. And to do that, we have to have public awareness."
Kidney disease ranks near the bottom when it comes to research funding, and public awareness will go a long way toward changing that, he added.
Dr. Molitoris said he is optimistic that as the pharmaceutical industry continues taking an interest in kidney disease, and as public awareness increases, funding – and therefore innovation – will also increase. He said he envisions a time when the public will be as aware of their kidney function level as they are of their cholesterol level.
"With what is going on in science and technology and biology, it’s not a far leap for us to make major strides," he said.
The study by Dr. Thomas was supported by a private foundation. Dr. Thomas reported having no relevant financial disclosures. Dr. Molitoris reported consultancy, ownership interest, research funding, honoraria, and/or a scientific advisory or membership role for numerous companies. He also reported a patent/invention with FAST Diagnostics, Indiana University.
ATLANTA – The global prevalence of maintenance dialysis for end-stage renal disease has increased 165% over the past 2 decades – a rate that has far outpaced that of population growth in most regions of the world, according to Dr. Bernadette A. Thomas.
The findings – the first to quantify the global burden of end-stage renal disease – underscore a need for improved detection of early chronic kidney disease and for treatment aimed at preventing end-stage renal disease (ESRD), because a continued rise in the prevalence of maintenance dialysis may not be sustainable, Dr. Thomas of the University of Washington, Seattle, said at Kidney Week 2013.
From 1990 to 2010, the global prevalence of maintenance dialysis in areas with universal dialysis access increased 134%, after adjustment for population growth and aging. The increase was 145% among women and 123% among men. Even in countries with a lack of universal access, the adjusted prevalence increased by 102% (116% for women and 90% for men).
The regions that did not experience an increase in dialysis prevalence included Oceania, South Asia, Central Sub-Saharan Africa, Eastern Europe, and tropical Latin America, Dr. Thomas said at the conference, which was sponsored by the American Society of Nephrology.
The findings are based on data extracted from the Global Burden of Disease database, the largest existing database for global causes of morbidity and mortality. Prevalence estimates were based on national and regional ESRD registries and a structured literature review. Data from 23 countries with universal dialysis access and from 138 countries with partial access were included; data from 26 countries that lack routine access were excluded.
"The statistics are appalling," American Society of Nephrology President Bruce A. Molitoris of Indiana University, Indianapolis, noted during a press briefing. Dr. Molitoris called for stepped-up efforts to educate the public about the importance of kidney health.
"We have targeted the patients in the past [via] patient education, and we have not presented, I think, a fair outlook to the public of the potential importance of kidney disease," he said.
Up to one-third of U.S. residents will, at some point in their lives, have severe kidney disease, Dr. Molitoris said, and 8% of African Americans and 3.6% of the total population will go on dialysis or need a transplant.
"And yet, people think they are immune to kidney disease," he cautioned. "The field is in need of innovation; the field is in need of individualization of care. And to do that, we have to have public awareness."
Kidney disease ranks near the bottom when it comes to research funding, and public awareness will go a long way toward changing that, he added.
Dr. Molitoris said he is optimistic that as the pharmaceutical industry continues taking an interest in kidney disease, and as public awareness increases, funding – and therefore innovation – will also increase. He said he envisions a time when the public will be as aware of their kidney function level as they are of their cholesterol level.
"With what is going on in science and technology and biology, it’s not a far leap for us to make major strides," he said.
The study by Dr. Thomas was supported by a private foundation. Dr. Thomas reported having no relevant financial disclosures. Dr. Molitoris reported consultancy, ownership interest, research funding, honoraria, and/or a scientific advisory or membership role for numerous companies. He also reported a patent/invention with FAST Diagnostics, Indiana University.
AT KIDNEY WEEK 2013
Major finding: The global prevalence of maintenance dialysis for end-stage renal disease increased 165% from 1990 to 2010.
Data source: A database data extraction and literature review.
Disclosures: The study by Dr. Thomas was supported by a private foundation. Dr. Thomas reported having no relevant financial disclosures. Dr. Molitoris reported consultancy, ownership interest, research funding, honoraria, and/or a scientific advisory or membership role for numerous companies. He also reported a patent/invention with FAST Diagnostics, Indiana University.
Combined angiotensin inhibition raises hyperkalemia, acute kidney injury risks
ATLANTA – The combined use of an angiotensin-converting enzyme inhibitor and an angiotensin receptor blocker was associated with an increased risk of hyperkalemia and acute kidney injury in patients with diabetic nephropathy in a randomized, controlled trial that was halted early because of these safety concerns.
"The risk-benefit ratio does not support the use of these medications – they’re not safe," noted Dr. Linda F. Fried, speaking at Kidney Week 2013.
The VA NEPHRON-D (Veterans Affairs Nephropathy in Diabetes) study involved 1,448 patients with type 2 diabetes and moderate diabetic nephropathy who were treated for at least 30 days with 100 mg daily of the angiotensin-receptor blocker (ARB) losartan, as is standard practice in patients with diabetes. The patients were randomized to also receive 10-40 mg daily of the angiotensin-converting enzyme (ACE) inhibitor lisinopril or placebo.
At a median follow-up of 2.2 years as of the time the study was stopped, 152 patients in the combination-therapy group and 132 in the placebo group (21% vs. 18.2%) experienced the composite primary endpoint of first occurrence of a predefined change in the estimated glomerular filtration rate (eGFR), end-stage renal disease (ESRD), or death (hazard ratio with combination therapy, 0.88). The difference between the groups was not statistically significant, said Dr. Fried, professor of medicine, epidemiology, and clinical and translational science at the University of Pittsburgh and chief of peritoneal dialysis at Veterans Affairs Pittsburgh Healthcare System.
Despite an early trend toward benefit, this trend diminished over time so that no significant difference was seen between the groups in the secondary renal end point of a first occurrence of a decline in the eGFR or ESRD (hazard ratio, 0.78). Also, no difference was seen between the groups with respect to the safety outcome of mortality (HR for death, 1.04). The rate of cardiovascular events in the groups was also similar.
However, there was a greater than twofold increase in the risk of hyperkalemia in the combination therapy group, compared with the placebo group (6.3 vs. 2.6 events/100 person-years), and a nearly twofold increase in the risk of acute kidney injury in the combination therapy group (12.2 vs. 6.7 event/100 person-years), Dr. Fried said at the conference, which was sponsored by the American Society of Nephrology.
The findings were published concurrently in the New England Journal of Medicine (2013 Nov. 9 [doi10.1056/NEJMoa1303154]).
Patients in the study had type 2 diabetes, a urinary albumin-to-creatinine ratio of at least 300, and an eGFR of 30.0 to 89.9 mL/minute per 1.73 m2 or body-surface area. Change in eGFR as specified for the composite primary endpoint of the study was a decline of at least 30 mL/minute per 1.73 m2 in those with an initial eGFR of at least 60 mL/minute per 1.73 m2, or a decline of at least 50% if the initial eGFR was less than 60 mL/minute per 1.73 m2.
Treatment with medications that block angiotensin are known to slow loss of kidney function in individuals with diabetes and proteinuria. This is true for both ACE inhibitors and ARBs, which have different mechanisms of action, but the percentage of patients who progress to dialysis despite treatment with one of these medications remains high, Dr. Fried said.
"So we need more treatments. A question that comes up is, ‘Would more intensive blockade of angiotensin – using two (drugs) together – slow decline?’ ... The thought was that treatments that lowered proteinuria should lower progression. We did see the lower proteinuria but without the benefit on the endpoints," she said during a press briefing in advance of the presentation of the late-breaking abstract.
Instead, it became clear that the treatment was associated with significantly more hyperkalemia and kidney injury, she said, noting that significantly more patients in the combination therapy group experienced acute kidney injury requiring hospitalization, indicating the problem was not just one of "lab abnormalities."
In fact, for every 100 persons followed for 1 year, there were 17 more admissions to the hospital, Dr. Fried said, noting that "these were not small differences in safety outcomes."
"The risk-benefit ratio does not support the use of these medications – they’re not safe." she concluded.
The VA-Nephron-D study was supported by the cooperative studies program of the Department of Veterans Affairs Office of Research and Development. The Investigator-Initiated Studies Program of Merck provided the study drugs. Dr. Fried reported receiving support from Reata Pharmaceuticals as a site investigator for a study. Other studies authors reported receiving lecture or consulting fees from Merck, Sanofi-Aventis, Complex, and/or CytoPherx.
The VA Nephron-D study adds to existing evidence suggesting that dual renin-angiotensin-aldosterone system (RAAS) blockade does not decrease cardiovascular and renal morbidity, and actually carries increased risks, Dr. Dick de Zeeuw wrote in an editorial that accompanied the study by Fried, et al., in the New England Journal of Medicine.
"The effect of these ‘failed’ trials goes beyond the decision about whether we should use dual RAAS blockade in order to better protect our patients with diabetes. The results suggest that improvement in surrogate markers – lower blood pressure or less albuminuria – does not translate into risk reduction," he said (N. Engl. J. Med. 2013 Nov. 9 [doi:10.1056/NEJMe1312412]).
The findings make it clear that dual RAAS blockade for the treatment of patients with diabetes cannot currently be recommended, he said, adding that "we need to find ways to design trials that ultimately provide a better balance between efficacy and safety.
"An enrichment design, selecting patients with a defined albuminuria response without adverse events, is a first step and is currently being tested," he said.
While future studies will likely "measure and integrate multiple drug responses to predict the chance of obtaining positive hard outcomes," for now, dual RAAS blockade can only be resuscitated if it can be shown that renal and cardiovascular protection is possible in a defined group of patients in whom the desired effects (decreased blood pressure and/or albuminuria) can be achieved without increasing the risk of hyperkalemia and other side effects, he concluded.
Dr. de Zeeuw reported serving as an adviser or steering committee chair for AbbVie, Astellas, AstraZeneca, Bristol-Myers Squibb, ChemoCentryx, Johnson & Johnson, and Novartis, all of which have paid fees to his institution.
Dr. de Zeeuw is with the department of clinical pharmacology, University of Groningen, and University Medical Center Groningen, in the Netherlands.
The VA Nephron-D study adds to existing evidence suggesting that dual renin-angiotensin-aldosterone system (RAAS) blockade does not decrease cardiovascular and renal morbidity, and actually carries increased risks, Dr. Dick de Zeeuw wrote in an editorial that accompanied the study by Fried, et al., in the New England Journal of Medicine.
"The effect of these ‘failed’ trials goes beyond the decision about whether we should use dual RAAS blockade in order to better protect our patients with diabetes. The results suggest that improvement in surrogate markers – lower blood pressure or less albuminuria – does not translate into risk reduction," he said (N. Engl. J. Med. 2013 Nov. 9 [doi:10.1056/NEJMe1312412]).
The findings make it clear that dual RAAS blockade for the treatment of patients with diabetes cannot currently be recommended, he said, adding that "we need to find ways to design trials that ultimately provide a better balance between efficacy and safety.
"An enrichment design, selecting patients with a defined albuminuria response without adverse events, is a first step and is currently being tested," he said.
While future studies will likely "measure and integrate multiple drug responses to predict the chance of obtaining positive hard outcomes," for now, dual RAAS blockade can only be resuscitated if it can be shown that renal and cardiovascular protection is possible in a defined group of patients in whom the desired effects (decreased blood pressure and/or albuminuria) can be achieved without increasing the risk of hyperkalemia and other side effects, he concluded.
Dr. de Zeeuw reported serving as an adviser or steering committee chair for AbbVie, Astellas, AstraZeneca, Bristol-Myers Squibb, ChemoCentryx, Johnson & Johnson, and Novartis, all of which have paid fees to his institution.
Dr. de Zeeuw is with the department of clinical pharmacology, University of Groningen, and University Medical Center Groningen, in the Netherlands.
The VA Nephron-D study adds to existing evidence suggesting that dual renin-angiotensin-aldosterone system (RAAS) blockade does not decrease cardiovascular and renal morbidity, and actually carries increased risks, Dr. Dick de Zeeuw wrote in an editorial that accompanied the study by Fried, et al., in the New England Journal of Medicine.
"The effect of these ‘failed’ trials goes beyond the decision about whether we should use dual RAAS blockade in order to better protect our patients with diabetes. The results suggest that improvement in surrogate markers – lower blood pressure or less albuminuria – does not translate into risk reduction," he said (N. Engl. J. Med. 2013 Nov. 9 [doi:10.1056/NEJMe1312412]).
The findings make it clear that dual RAAS blockade for the treatment of patients with diabetes cannot currently be recommended, he said, adding that "we need to find ways to design trials that ultimately provide a better balance between efficacy and safety.
"An enrichment design, selecting patients with a defined albuminuria response without adverse events, is a first step and is currently being tested," he said.
While future studies will likely "measure and integrate multiple drug responses to predict the chance of obtaining positive hard outcomes," for now, dual RAAS blockade can only be resuscitated if it can be shown that renal and cardiovascular protection is possible in a defined group of patients in whom the desired effects (decreased blood pressure and/or albuminuria) can be achieved without increasing the risk of hyperkalemia and other side effects, he concluded.
Dr. de Zeeuw reported serving as an adviser or steering committee chair for AbbVie, Astellas, AstraZeneca, Bristol-Myers Squibb, ChemoCentryx, Johnson & Johnson, and Novartis, all of which have paid fees to his institution.
Dr. de Zeeuw is with the department of clinical pharmacology, University of Groningen, and University Medical Center Groningen, in the Netherlands.
ATLANTA – The combined use of an angiotensin-converting enzyme inhibitor and an angiotensin receptor blocker was associated with an increased risk of hyperkalemia and acute kidney injury in patients with diabetic nephropathy in a randomized, controlled trial that was halted early because of these safety concerns.
"The risk-benefit ratio does not support the use of these medications – they’re not safe," noted Dr. Linda F. Fried, speaking at Kidney Week 2013.
The VA NEPHRON-D (Veterans Affairs Nephropathy in Diabetes) study involved 1,448 patients with type 2 diabetes and moderate diabetic nephropathy who were treated for at least 30 days with 100 mg daily of the angiotensin-receptor blocker (ARB) losartan, as is standard practice in patients with diabetes. The patients were randomized to also receive 10-40 mg daily of the angiotensin-converting enzyme (ACE) inhibitor lisinopril or placebo.
At a median follow-up of 2.2 years as of the time the study was stopped, 152 patients in the combination-therapy group and 132 in the placebo group (21% vs. 18.2%) experienced the composite primary endpoint of first occurrence of a predefined change in the estimated glomerular filtration rate (eGFR), end-stage renal disease (ESRD), or death (hazard ratio with combination therapy, 0.88). The difference between the groups was not statistically significant, said Dr. Fried, professor of medicine, epidemiology, and clinical and translational science at the University of Pittsburgh and chief of peritoneal dialysis at Veterans Affairs Pittsburgh Healthcare System.
Despite an early trend toward benefit, this trend diminished over time so that no significant difference was seen between the groups in the secondary renal end point of a first occurrence of a decline in the eGFR or ESRD (hazard ratio, 0.78). Also, no difference was seen between the groups with respect to the safety outcome of mortality (HR for death, 1.04). The rate of cardiovascular events in the groups was also similar.
However, there was a greater than twofold increase in the risk of hyperkalemia in the combination therapy group, compared with the placebo group (6.3 vs. 2.6 events/100 person-years), and a nearly twofold increase in the risk of acute kidney injury in the combination therapy group (12.2 vs. 6.7 event/100 person-years), Dr. Fried said at the conference, which was sponsored by the American Society of Nephrology.
The findings were published concurrently in the New England Journal of Medicine (2013 Nov. 9 [doi10.1056/NEJMoa1303154]).
Patients in the study had type 2 diabetes, a urinary albumin-to-creatinine ratio of at least 300, and an eGFR of 30.0 to 89.9 mL/minute per 1.73 m2 or body-surface area. Change in eGFR as specified for the composite primary endpoint of the study was a decline of at least 30 mL/minute per 1.73 m2 in those with an initial eGFR of at least 60 mL/minute per 1.73 m2, or a decline of at least 50% if the initial eGFR was less than 60 mL/minute per 1.73 m2.
Treatment with medications that block angiotensin are known to slow loss of kidney function in individuals with diabetes and proteinuria. This is true for both ACE inhibitors and ARBs, which have different mechanisms of action, but the percentage of patients who progress to dialysis despite treatment with one of these medications remains high, Dr. Fried said.
"So we need more treatments. A question that comes up is, ‘Would more intensive blockade of angiotensin – using two (drugs) together – slow decline?’ ... The thought was that treatments that lowered proteinuria should lower progression. We did see the lower proteinuria but without the benefit on the endpoints," she said during a press briefing in advance of the presentation of the late-breaking abstract.
Instead, it became clear that the treatment was associated with significantly more hyperkalemia and kidney injury, she said, noting that significantly more patients in the combination therapy group experienced acute kidney injury requiring hospitalization, indicating the problem was not just one of "lab abnormalities."
In fact, for every 100 persons followed for 1 year, there were 17 more admissions to the hospital, Dr. Fried said, noting that "these were not small differences in safety outcomes."
"The risk-benefit ratio does not support the use of these medications – they’re not safe." she concluded.
The VA-Nephron-D study was supported by the cooperative studies program of the Department of Veterans Affairs Office of Research and Development. The Investigator-Initiated Studies Program of Merck provided the study drugs. Dr. Fried reported receiving support from Reata Pharmaceuticals as a site investigator for a study. Other studies authors reported receiving lecture or consulting fees from Merck, Sanofi-Aventis, Complex, and/or CytoPherx.
ATLANTA – The combined use of an angiotensin-converting enzyme inhibitor and an angiotensin receptor blocker was associated with an increased risk of hyperkalemia and acute kidney injury in patients with diabetic nephropathy in a randomized, controlled trial that was halted early because of these safety concerns.
"The risk-benefit ratio does not support the use of these medications – they’re not safe," noted Dr. Linda F. Fried, speaking at Kidney Week 2013.
The VA NEPHRON-D (Veterans Affairs Nephropathy in Diabetes) study involved 1,448 patients with type 2 diabetes and moderate diabetic nephropathy who were treated for at least 30 days with 100 mg daily of the angiotensin-receptor blocker (ARB) losartan, as is standard practice in patients with diabetes. The patients were randomized to also receive 10-40 mg daily of the angiotensin-converting enzyme (ACE) inhibitor lisinopril or placebo.
At a median follow-up of 2.2 years as of the time the study was stopped, 152 patients in the combination-therapy group and 132 in the placebo group (21% vs. 18.2%) experienced the composite primary endpoint of first occurrence of a predefined change in the estimated glomerular filtration rate (eGFR), end-stage renal disease (ESRD), or death (hazard ratio with combination therapy, 0.88). The difference between the groups was not statistically significant, said Dr. Fried, professor of medicine, epidemiology, and clinical and translational science at the University of Pittsburgh and chief of peritoneal dialysis at Veterans Affairs Pittsburgh Healthcare System.
Despite an early trend toward benefit, this trend diminished over time so that no significant difference was seen between the groups in the secondary renal end point of a first occurrence of a decline in the eGFR or ESRD (hazard ratio, 0.78). Also, no difference was seen between the groups with respect to the safety outcome of mortality (HR for death, 1.04). The rate of cardiovascular events in the groups was also similar.
However, there was a greater than twofold increase in the risk of hyperkalemia in the combination therapy group, compared with the placebo group (6.3 vs. 2.6 events/100 person-years), and a nearly twofold increase in the risk of acute kidney injury in the combination therapy group (12.2 vs. 6.7 event/100 person-years), Dr. Fried said at the conference, which was sponsored by the American Society of Nephrology.
The findings were published concurrently in the New England Journal of Medicine (2013 Nov. 9 [doi10.1056/NEJMoa1303154]).
Patients in the study had type 2 diabetes, a urinary albumin-to-creatinine ratio of at least 300, and an eGFR of 30.0 to 89.9 mL/minute per 1.73 m2 or body-surface area. Change in eGFR as specified for the composite primary endpoint of the study was a decline of at least 30 mL/minute per 1.73 m2 in those with an initial eGFR of at least 60 mL/minute per 1.73 m2, or a decline of at least 50% if the initial eGFR was less than 60 mL/minute per 1.73 m2.
Treatment with medications that block angiotensin are known to slow loss of kidney function in individuals with diabetes and proteinuria. This is true for both ACE inhibitors and ARBs, which have different mechanisms of action, but the percentage of patients who progress to dialysis despite treatment with one of these medications remains high, Dr. Fried said.
"So we need more treatments. A question that comes up is, ‘Would more intensive blockade of angiotensin – using two (drugs) together – slow decline?’ ... The thought was that treatments that lowered proteinuria should lower progression. We did see the lower proteinuria but without the benefit on the endpoints," she said during a press briefing in advance of the presentation of the late-breaking abstract.
Instead, it became clear that the treatment was associated with significantly more hyperkalemia and kidney injury, she said, noting that significantly more patients in the combination therapy group experienced acute kidney injury requiring hospitalization, indicating the problem was not just one of "lab abnormalities."
In fact, for every 100 persons followed for 1 year, there were 17 more admissions to the hospital, Dr. Fried said, noting that "these were not small differences in safety outcomes."
"The risk-benefit ratio does not support the use of these medications – they’re not safe." she concluded.
The VA-Nephron-D study was supported by the cooperative studies program of the Department of Veterans Affairs Office of Research and Development. The Investigator-Initiated Studies Program of Merck provided the study drugs. Dr. Fried reported receiving support from Reata Pharmaceuticals as a site investigator for a study. Other studies authors reported receiving lecture or consulting fees from Merck, Sanofi-Aventis, Complex, and/or CytoPherx.
AT KIDNEY WEEK 2013
Major finding: There was an increased risk of hyperkalemia (6.3 vs. 2.6 events/100 person-years) and acute kidney injury (12.2 vs. 6.7 events/100 person-years) with combination ACE inhibitor/ARB therapy vs. monotherapy with losartan.
Data source: A multicenter, double-blind, randomized, controlled study of 1,448 patients
Disclosures: The VA-Nephron-D study was supported by the cooperative studies program of the Department of Veterans Affairs Office of Research and Development. The Investigator-Initiated Studies Program of Merck provided the study drugs. Dr. Fried reported receiving support from Reata Pharmaceuticals as a site investigator for a study. Other studies authors reported receiving lecture or consulting fees from Merck, Sanofi-Aventis, Complex, and/or CytoPherx.
CV events scuttle bardoxolone for diabetic kidney disease
Bardoxolone methyl may have reduced the risk of end-stage renal disease in patients with type 2 diabetes and stage 4 chronic kidney disease in a phase III clinical trial, but researchers stopped the study early after patients taking the drug had an increased rate of cardiovascular deaths, heart failure events, nonfatal myocardial infarction, and nonfatal stroke.
The BEACON trial had only an estimated 40% statistical power to determine bardoxolone methyl’s true effects, given its termination because of safety concerns, according to data reported at the annual Kidney Week meeting sponsored by the American Society of Nephrology.
The findings were simultaneously reported online Nov. 9 in the New England Journal of Medicine (doi:10.1056/NEJMoa1306033).
Bardoxolone methyl, the most potent known activator of a transcription factor that regulates antioxidant genes, was previously shown to raise the estimated glomerular filtration rate (eGFR) in patients with diabetes-related kidney disease. However, it also increased the incidence of albuminuria and induced unintended weight loss.
To determine whether longer-term treatment with bardoxolone methyl might translate that eGFR benefit into a slower progression to end-stage renal disease (ESRD), Dr. Dick de Zeeuw of the University of Groningen (the Netherlands) and his associates performed the double-blind BEACON trial (Bardoxolone Methyl Evaluation in Patients With Chronic Kidney Disease and Type 2 Diabetes Mellitus: The Occurrence of Renal Events).
BEACON was sponsored by Reata Pharmaceuticals and included patients from the United States, Europe, Australia, Canada, Israel, and Mexico, resulting in a diverse patient population with regard to age, race/ethnicity, and area of residence. Both diabetic retinopathy and neuropathy were common comorbidities, as was cardiovascular disease.
The 2,185 study participants had type 2 diabetes and moderate to severe chronic kidney diseases, with a baseline eGFR of 15 to less than 30 mL/min per 1.73 m2 of body surface area. They were randomly assigned to receive either once-daily bardoxolone methyl 20 mg (1,088 patients) or a matching placebo (1,097 patients), along with conventional background therapies given at the discretion of their treating physicians. Those included inhibitors of the renin-angiotensin-aldosterone system, insulin or other hypoglycemic agents, and appropriate cardiovascular medications.
The median duration of exposure was 7 months for bardoxolone methyl and 8 months for placebo, and the median follow-up for both was 9 months.
Bardoxolone significantly improved eGFR, compared with placebo, and fewer patients who took the drug progressed to ESRD. But BEACON was terminated early because of an excess of CV events among patients receiving bardoxolone methyl. That "truncated" study duration limited the trial’s statistical power.
The primary endpoint was a composite of progression to ESRD or cardiovascular death, and it occurred in 6% of both study groups. However, deaths from CV causes were significantly more frequent in the active treatment group (27 patients) than in the placebo group (19 patients), with a hazard ratio of 1.44, Dr. de Zeeuw reported.
In particular, 96 patients in the bardoxolone group had heart failure (HF) events, compared with only 55 patients in the placebo group. Moreover, significantly more of the HF events in the active treatment group were severe enough to require hospitalization or cause death.
Similarly, significantly more patients taking bardoxolone methyl had a composite outcome of nonfatal myocardial infarction, nonfatal stroke, HF hospitalization, or CV death. And the number of deaths from any cause was greater – though not significantly – with bardoxolone methyl (44 deaths) than with placebo (31 deaths) (HR, 1.47; P = .10).
Compared with placebo, bardoxolone methyl also raised blood pressure and heart rate, increased levels of B-type natriuretic peptide, raised the rate of albuminuria, and caused substantial unintended weight loss. The investigators were unable to determine whether there was a loss of body fat, intracellular (that is, skeletal muscle) water, or extracellular (interstitial) water. There was a concomitant fall in serum albumin and hemoglobin levels, which may reflect hemodilution caused by fluid retention, the investigators found.
The increases in blood pressure and heart rate "constitute a potentially potent combination of factors that are likely to precipitate HF in an at-risk population." and the increase in B-type natriuretic peptide "is consistent with an increase in left ventricular wall stress," Dr. de Zeeuw said.
The investigators attempted to identify patient characteristics that might be associated with the development of HF in the bardoxolone methyl recipients, but were unable to do so.
Reata Pharmaceuticals funded the BEACON trial. Dr. de Zeeuw reported ties to AbbVie, Astellas, Chemocentryx, Johnson & Johnson, and Reata, and his associates reported ties to numerous industry sources.
*This article was updated November 11, 2013.
The adverse events linked to bardoxolone methyl in this study included excess HF and cardiovascular events, as well as increased rates of high blood pressure, high heart rate, albuminuria, GI symptoms, and muscle-related symptoms, said Dr. Jonathan Himmelfarb and Dr. Katherine R. Tuttle.
"The authors speculate that fluid retention, increased afterload, and higher heart rate contributed to heart failure," but it also is possible that bardoxolone methyl may exert direct toxic effects on the heart, Dr. Himmelfarb and Dr. Tuttle said.
In any case, "caution should be exercised whenever any drug for diabetic kidney disease increases, rather than decreases, albuminuria," they noted.
Dr. Himmelfarb and Dr. Tuttle are at the Kidney Research Institute and the division of nephrology at the University of Washington, Seattle. Dr. Tuttle also is at Providence Sacred Heart Medical Center and Children’s Hospital, Spokane. Dr. Himmelfarb reported ties to Abbott Laboratories, and Dr. Tuttle reported ties to Eli Lilly. These remarks were taken from their editorial accompanying Dr. de Zeeuw’s report (N. Engl. J. Med. 2013 Nov. 9 [doi:10.1056/NEJMe1313104]).
The adverse events linked to bardoxolone methyl in this study included excess HF and cardiovascular events, as well as increased rates of high blood pressure, high heart rate, albuminuria, GI symptoms, and muscle-related symptoms, said Dr. Jonathan Himmelfarb and Dr. Katherine R. Tuttle.
"The authors speculate that fluid retention, increased afterload, and higher heart rate contributed to heart failure," but it also is possible that bardoxolone methyl may exert direct toxic effects on the heart, Dr. Himmelfarb and Dr. Tuttle said.
In any case, "caution should be exercised whenever any drug for diabetic kidney disease increases, rather than decreases, albuminuria," they noted.
Dr. Himmelfarb and Dr. Tuttle are at the Kidney Research Institute and the division of nephrology at the University of Washington, Seattle. Dr. Tuttle also is at Providence Sacred Heart Medical Center and Children’s Hospital, Spokane. Dr. Himmelfarb reported ties to Abbott Laboratories, and Dr. Tuttle reported ties to Eli Lilly. These remarks were taken from their editorial accompanying Dr. de Zeeuw’s report (N. Engl. J. Med. 2013 Nov. 9 [doi:10.1056/NEJMe1313104]).
The adverse events linked to bardoxolone methyl in this study included excess HF and cardiovascular events, as well as increased rates of high blood pressure, high heart rate, albuminuria, GI symptoms, and muscle-related symptoms, said Dr. Jonathan Himmelfarb and Dr. Katherine R. Tuttle.
"The authors speculate that fluid retention, increased afterload, and higher heart rate contributed to heart failure," but it also is possible that bardoxolone methyl may exert direct toxic effects on the heart, Dr. Himmelfarb and Dr. Tuttle said.
In any case, "caution should be exercised whenever any drug for diabetic kidney disease increases, rather than decreases, albuminuria," they noted.
Dr. Himmelfarb and Dr. Tuttle are at the Kidney Research Institute and the division of nephrology at the University of Washington, Seattle. Dr. Tuttle also is at Providence Sacred Heart Medical Center and Children’s Hospital, Spokane. Dr. Himmelfarb reported ties to Abbott Laboratories, and Dr. Tuttle reported ties to Eli Lilly. These remarks were taken from their editorial accompanying Dr. de Zeeuw’s report (N. Engl. J. Med. 2013 Nov. 9 [doi:10.1056/NEJMe1313104]).
Bardoxolone methyl may have reduced the risk of end-stage renal disease in patients with type 2 diabetes and stage 4 chronic kidney disease in a phase III clinical trial, but researchers stopped the study early after patients taking the drug had an increased rate of cardiovascular deaths, heart failure events, nonfatal myocardial infarction, and nonfatal stroke.
The BEACON trial had only an estimated 40% statistical power to determine bardoxolone methyl’s true effects, given its termination because of safety concerns, according to data reported at the annual Kidney Week meeting sponsored by the American Society of Nephrology.
The findings were simultaneously reported online Nov. 9 in the New England Journal of Medicine (doi:10.1056/NEJMoa1306033).
Bardoxolone methyl, the most potent known activator of a transcription factor that regulates antioxidant genes, was previously shown to raise the estimated glomerular filtration rate (eGFR) in patients with diabetes-related kidney disease. However, it also increased the incidence of albuminuria and induced unintended weight loss.
To determine whether longer-term treatment with bardoxolone methyl might translate that eGFR benefit into a slower progression to end-stage renal disease (ESRD), Dr. Dick de Zeeuw of the University of Groningen (the Netherlands) and his associates performed the double-blind BEACON trial (Bardoxolone Methyl Evaluation in Patients With Chronic Kidney Disease and Type 2 Diabetes Mellitus: The Occurrence of Renal Events).
BEACON was sponsored by Reata Pharmaceuticals and included patients from the United States, Europe, Australia, Canada, Israel, and Mexico, resulting in a diverse patient population with regard to age, race/ethnicity, and area of residence. Both diabetic retinopathy and neuropathy were common comorbidities, as was cardiovascular disease.
The 2,185 study participants had type 2 diabetes and moderate to severe chronic kidney diseases, with a baseline eGFR of 15 to less than 30 mL/min per 1.73 m2 of body surface area. They were randomly assigned to receive either once-daily bardoxolone methyl 20 mg (1,088 patients) or a matching placebo (1,097 patients), along with conventional background therapies given at the discretion of their treating physicians. Those included inhibitors of the renin-angiotensin-aldosterone system, insulin or other hypoglycemic agents, and appropriate cardiovascular medications.
The median duration of exposure was 7 months for bardoxolone methyl and 8 months for placebo, and the median follow-up for both was 9 months.
Bardoxolone significantly improved eGFR, compared with placebo, and fewer patients who took the drug progressed to ESRD. But BEACON was terminated early because of an excess of CV events among patients receiving bardoxolone methyl. That "truncated" study duration limited the trial’s statistical power.
The primary endpoint was a composite of progression to ESRD or cardiovascular death, and it occurred in 6% of both study groups. However, deaths from CV causes were significantly more frequent in the active treatment group (27 patients) than in the placebo group (19 patients), with a hazard ratio of 1.44, Dr. de Zeeuw reported.
In particular, 96 patients in the bardoxolone group had heart failure (HF) events, compared with only 55 patients in the placebo group. Moreover, significantly more of the HF events in the active treatment group were severe enough to require hospitalization or cause death.
Similarly, significantly more patients taking bardoxolone methyl had a composite outcome of nonfatal myocardial infarction, nonfatal stroke, HF hospitalization, or CV death. And the number of deaths from any cause was greater – though not significantly – with bardoxolone methyl (44 deaths) than with placebo (31 deaths) (HR, 1.47; P = .10).
Compared with placebo, bardoxolone methyl also raised blood pressure and heart rate, increased levels of B-type natriuretic peptide, raised the rate of albuminuria, and caused substantial unintended weight loss. The investigators were unable to determine whether there was a loss of body fat, intracellular (that is, skeletal muscle) water, or extracellular (interstitial) water. There was a concomitant fall in serum albumin and hemoglobin levels, which may reflect hemodilution caused by fluid retention, the investigators found.
The increases in blood pressure and heart rate "constitute a potentially potent combination of factors that are likely to precipitate HF in an at-risk population." and the increase in B-type natriuretic peptide "is consistent with an increase in left ventricular wall stress," Dr. de Zeeuw said.
The investigators attempted to identify patient characteristics that might be associated with the development of HF in the bardoxolone methyl recipients, but were unable to do so.
Reata Pharmaceuticals funded the BEACON trial. Dr. de Zeeuw reported ties to AbbVie, Astellas, Chemocentryx, Johnson & Johnson, and Reata, and his associates reported ties to numerous industry sources.
*This article was updated November 11, 2013.
Bardoxolone methyl may have reduced the risk of end-stage renal disease in patients with type 2 diabetes and stage 4 chronic kidney disease in a phase III clinical trial, but researchers stopped the study early after patients taking the drug had an increased rate of cardiovascular deaths, heart failure events, nonfatal myocardial infarction, and nonfatal stroke.
The BEACON trial had only an estimated 40% statistical power to determine bardoxolone methyl’s true effects, given its termination because of safety concerns, according to data reported at the annual Kidney Week meeting sponsored by the American Society of Nephrology.
The findings were simultaneously reported online Nov. 9 in the New England Journal of Medicine (doi:10.1056/NEJMoa1306033).
Bardoxolone methyl, the most potent known activator of a transcription factor that regulates antioxidant genes, was previously shown to raise the estimated glomerular filtration rate (eGFR) in patients with diabetes-related kidney disease. However, it also increased the incidence of albuminuria and induced unintended weight loss.
To determine whether longer-term treatment with bardoxolone methyl might translate that eGFR benefit into a slower progression to end-stage renal disease (ESRD), Dr. Dick de Zeeuw of the University of Groningen (the Netherlands) and his associates performed the double-blind BEACON trial (Bardoxolone Methyl Evaluation in Patients With Chronic Kidney Disease and Type 2 Diabetes Mellitus: The Occurrence of Renal Events).
BEACON was sponsored by Reata Pharmaceuticals and included patients from the United States, Europe, Australia, Canada, Israel, and Mexico, resulting in a diverse patient population with regard to age, race/ethnicity, and area of residence. Both diabetic retinopathy and neuropathy were common comorbidities, as was cardiovascular disease.
The 2,185 study participants had type 2 diabetes and moderate to severe chronic kidney diseases, with a baseline eGFR of 15 to less than 30 mL/min per 1.73 m2 of body surface area. They were randomly assigned to receive either once-daily bardoxolone methyl 20 mg (1,088 patients) or a matching placebo (1,097 patients), along with conventional background therapies given at the discretion of their treating physicians. Those included inhibitors of the renin-angiotensin-aldosterone system, insulin or other hypoglycemic agents, and appropriate cardiovascular medications.
The median duration of exposure was 7 months for bardoxolone methyl and 8 months for placebo, and the median follow-up for both was 9 months.
Bardoxolone significantly improved eGFR, compared with placebo, and fewer patients who took the drug progressed to ESRD. But BEACON was terminated early because of an excess of CV events among patients receiving bardoxolone methyl. That "truncated" study duration limited the trial’s statistical power.
The primary endpoint was a composite of progression to ESRD or cardiovascular death, and it occurred in 6% of both study groups. However, deaths from CV causes were significantly more frequent in the active treatment group (27 patients) than in the placebo group (19 patients), with a hazard ratio of 1.44, Dr. de Zeeuw reported.
In particular, 96 patients in the bardoxolone group had heart failure (HF) events, compared with only 55 patients in the placebo group. Moreover, significantly more of the HF events in the active treatment group were severe enough to require hospitalization or cause death.
Similarly, significantly more patients taking bardoxolone methyl had a composite outcome of nonfatal myocardial infarction, nonfatal stroke, HF hospitalization, or CV death. And the number of deaths from any cause was greater – though not significantly – with bardoxolone methyl (44 deaths) than with placebo (31 deaths) (HR, 1.47; P = .10).
Compared with placebo, bardoxolone methyl also raised blood pressure and heart rate, increased levels of B-type natriuretic peptide, raised the rate of albuminuria, and caused substantial unintended weight loss. The investigators were unable to determine whether there was a loss of body fat, intracellular (that is, skeletal muscle) water, or extracellular (interstitial) water. There was a concomitant fall in serum albumin and hemoglobin levels, which may reflect hemodilution caused by fluid retention, the investigators found.
The increases in blood pressure and heart rate "constitute a potentially potent combination of factors that are likely to precipitate HF in an at-risk population." and the increase in B-type natriuretic peptide "is consistent with an increase in left ventricular wall stress," Dr. de Zeeuw said.
The investigators attempted to identify patient characteristics that might be associated with the development of HF in the bardoxolone methyl recipients, but were unable to do so.
Reata Pharmaceuticals funded the BEACON trial. Dr. de Zeeuw reported ties to AbbVie, Astellas, Chemocentryx, Johnson & Johnson, and Reata, and his associates reported ties to numerous industry sources.
*This article was updated November 11, 2013.
FROM KIDNEY WEEK
Major Finding: Bardoxolone methyl improved eGFR and may have delayed progression to ESRD, but that result was inconclusive because the trial was terminated early as a result of an excess of cardiovascular deaths, heart failure events, nonfatal myocardial infarction, and nonfatal stroke in patients given the drug.
Data Source: An international phase III double-blind trial involving 2,185 patients with type 2 diabetes and stage 4 chronic kidney disease who were randomly assigned to receive daily oral bardoxolone methyl or placebo and were followed for a median of 9 months.
Disclosures: Reata Pharmaceuticals funded the BEACON trial. Dr. de Zeeuw reported ties to AbbVie, Astellas, Chemocentryx, Johnson & Johnson, and Reata, and his associates reported ties to numerous industry sources.
Mediterranean diet may lower CKD risk
ATLANTA – Following a Mediterranean-style diet may reduce the risk of chronic kidney disease, findings from the prospective Northern Manhattan Study suggest.
Among 900 subjects from the large community-based, multiethnic cohort who had requisite data available, 14% developed new chronic kidney disease (CKD) during a mean follow-up of 6.9 years. After adjustment for several potential confounders, including demographics, medication use, laboratory values, and medical history, following a Mediterranean-style diet was associated with a 50% reduction in the risk of incident stage 3 CKD, the primary outcome measure of the study (odds ratio, 0.50), Dr. Minesh Khatri of Columbia University, New York, reported at Kidney Week 2013.
Furthermore, each 1-unit increase in a previously developed 9-point score that measured the degree to which a subject followed a Mediterranean-style diet (the MeDi score) was associated with a 17% reduction in CKD risk (OR, 0.83), Dr. Khatri said.
A MeDi score of 5 or higher also was associated with a 42% reduction in the risk of rapid kidney function decline (OR, 0.58), and each 1-point increase in the score was associated with a 12% reduction in risk (OR, 0.88).
Continuous absolute change in estimated glomerular filtration rate (eGFR) was not significantly affected by the MeDi score, but a higher score was associated with a trend toward improvement.
Study participants were adults who were stroke free and had a mean age of 64 years at baseline. They underwent measurement of serum creatinine at baseline and at follow-up, as well as brain magnetic resonance imaging at follow-up. They also completed a dietary questionnaire at baseline, from which the MeDi score was derived.
Most (65%) were Hispanic, and 59% were women. Mean eGFR was 83.1 mL/min, with a mean annualized decline of 1.1 mL/min.
Incident CKD in this study was defined as a follow-up Modification of Diet in Renal Disease eGFR of less than 60 mL/min among subjects with eGFR greater than 60 mL/min at baseline.
"CKD is highly prevalent. The lifetime risk of stage 3 CKD in the United States may be as high as 59% ... [and] the consequences of CKD are of equal magnitude. CKD increases the risk of morbidity and mortality, especially from cardiovascular disease," Dr. Khatri said. Data show that the worse the kidney function, the greater the cardiovascular disease risk and the greater the likelihood of cardiovascular events, he noted.
The financial toll is also extensive, he said during a press briefing at the conference, which was sponsored by the American Society of Nephrology.
"On top of this, the therapeutic armamentarium for CKD is actually relatively limited. We’ve made tremendous strides in treating traditional risk factors such as diabetes, hypertension, and protein in the urine, but most patients with CKD still have progressive kidney function decline over time. This therefore means we need novel approaches to prevent and ameliorate progression of CKD," he said.
Diet may be one such approach. Some diets have been studied in the context of improving CKD, but most studies have focused on protein restriction – an approach that may be harmful in some patients, such as the frail elderly. Few studies have looked at diet in the context of preventing CKD.
The Mediterranean diet – which generally includes high intake of fruits, vegetables, legumes, cereals, fish, and heart-healthy monounsaturated fats; lower intake of dairy, meats, and saturated fats; as well as moderate alcohol intake, has received a great deal of attention with respect to potential cardiovascular benefits. Studies have shown it has important benefits, including improvements in blood pressure, endothelial function, cholesterol, inflammation, and overall cardiovascular risk, Dr. Khatri said.
In fact, results from the randomized controlled PREDIMED study, published in April, demonstrated a 30% reduction in cardiovascular risk among those following a Mediterranean diet, compared with those following a standard low-fat diet (N. Engl. J. Med. 2013;368:1279-90), he noted.
The current study shows that a Mediterranean-style diet may have similarly beneficial effects for reducing CKD risk, which makes sense given the shared risk factors between CKD and cardiovascular disease, he said.
Larger observational trials and randomized controlled trials are needed to confirm these findings and to elucidate the mechanisms by which a Mediterranean-style diet may protect against kidney disease, he said.
This study was funded by the National Institutes of Health. Dr. Khatri reported having no disclosures.
ATLANTA – Following a Mediterranean-style diet may reduce the risk of chronic kidney disease, findings from the prospective Northern Manhattan Study suggest.
Among 900 subjects from the large community-based, multiethnic cohort who had requisite data available, 14% developed new chronic kidney disease (CKD) during a mean follow-up of 6.9 years. After adjustment for several potential confounders, including demographics, medication use, laboratory values, and medical history, following a Mediterranean-style diet was associated with a 50% reduction in the risk of incident stage 3 CKD, the primary outcome measure of the study (odds ratio, 0.50), Dr. Minesh Khatri of Columbia University, New York, reported at Kidney Week 2013.
Furthermore, each 1-unit increase in a previously developed 9-point score that measured the degree to which a subject followed a Mediterranean-style diet (the MeDi score) was associated with a 17% reduction in CKD risk (OR, 0.83), Dr. Khatri said.
A MeDi score of 5 or higher also was associated with a 42% reduction in the risk of rapid kidney function decline (OR, 0.58), and each 1-point increase in the score was associated with a 12% reduction in risk (OR, 0.88).
Continuous absolute change in estimated glomerular filtration rate (eGFR) was not significantly affected by the MeDi score, but a higher score was associated with a trend toward improvement.
Study participants were adults who were stroke free and had a mean age of 64 years at baseline. They underwent measurement of serum creatinine at baseline and at follow-up, as well as brain magnetic resonance imaging at follow-up. They also completed a dietary questionnaire at baseline, from which the MeDi score was derived.
Most (65%) were Hispanic, and 59% were women. Mean eGFR was 83.1 mL/min, with a mean annualized decline of 1.1 mL/min.
Incident CKD in this study was defined as a follow-up Modification of Diet in Renal Disease eGFR of less than 60 mL/min among subjects with eGFR greater than 60 mL/min at baseline.
"CKD is highly prevalent. The lifetime risk of stage 3 CKD in the United States may be as high as 59% ... [and] the consequences of CKD are of equal magnitude. CKD increases the risk of morbidity and mortality, especially from cardiovascular disease," Dr. Khatri said. Data show that the worse the kidney function, the greater the cardiovascular disease risk and the greater the likelihood of cardiovascular events, he noted.
The financial toll is also extensive, he said during a press briefing at the conference, which was sponsored by the American Society of Nephrology.
"On top of this, the therapeutic armamentarium for CKD is actually relatively limited. We’ve made tremendous strides in treating traditional risk factors such as diabetes, hypertension, and protein in the urine, but most patients with CKD still have progressive kidney function decline over time. This therefore means we need novel approaches to prevent and ameliorate progression of CKD," he said.
Diet may be one such approach. Some diets have been studied in the context of improving CKD, but most studies have focused on protein restriction – an approach that may be harmful in some patients, such as the frail elderly. Few studies have looked at diet in the context of preventing CKD.
The Mediterranean diet – which generally includes high intake of fruits, vegetables, legumes, cereals, fish, and heart-healthy monounsaturated fats; lower intake of dairy, meats, and saturated fats; as well as moderate alcohol intake, has received a great deal of attention with respect to potential cardiovascular benefits. Studies have shown it has important benefits, including improvements in blood pressure, endothelial function, cholesterol, inflammation, and overall cardiovascular risk, Dr. Khatri said.
In fact, results from the randomized controlled PREDIMED study, published in April, demonstrated a 30% reduction in cardiovascular risk among those following a Mediterranean diet, compared with those following a standard low-fat diet (N. Engl. J. Med. 2013;368:1279-90), he noted.
The current study shows that a Mediterranean-style diet may have similarly beneficial effects for reducing CKD risk, which makes sense given the shared risk factors between CKD and cardiovascular disease, he said.
Larger observational trials and randomized controlled trials are needed to confirm these findings and to elucidate the mechanisms by which a Mediterranean-style diet may protect against kidney disease, he said.
This study was funded by the National Institutes of Health. Dr. Khatri reported having no disclosures.
ATLANTA – Following a Mediterranean-style diet may reduce the risk of chronic kidney disease, findings from the prospective Northern Manhattan Study suggest.
Among 900 subjects from the large community-based, multiethnic cohort who had requisite data available, 14% developed new chronic kidney disease (CKD) during a mean follow-up of 6.9 years. After adjustment for several potential confounders, including demographics, medication use, laboratory values, and medical history, following a Mediterranean-style diet was associated with a 50% reduction in the risk of incident stage 3 CKD, the primary outcome measure of the study (odds ratio, 0.50), Dr. Minesh Khatri of Columbia University, New York, reported at Kidney Week 2013.
Furthermore, each 1-unit increase in a previously developed 9-point score that measured the degree to which a subject followed a Mediterranean-style diet (the MeDi score) was associated with a 17% reduction in CKD risk (OR, 0.83), Dr. Khatri said.
A MeDi score of 5 or higher also was associated with a 42% reduction in the risk of rapid kidney function decline (OR, 0.58), and each 1-point increase in the score was associated with a 12% reduction in risk (OR, 0.88).
Continuous absolute change in estimated glomerular filtration rate (eGFR) was not significantly affected by the MeDi score, but a higher score was associated with a trend toward improvement.
Study participants were adults who were stroke free and had a mean age of 64 years at baseline. They underwent measurement of serum creatinine at baseline and at follow-up, as well as brain magnetic resonance imaging at follow-up. They also completed a dietary questionnaire at baseline, from which the MeDi score was derived.
Most (65%) were Hispanic, and 59% were women. Mean eGFR was 83.1 mL/min, with a mean annualized decline of 1.1 mL/min.
Incident CKD in this study was defined as a follow-up Modification of Diet in Renal Disease eGFR of less than 60 mL/min among subjects with eGFR greater than 60 mL/min at baseline.
"CKD is highly prevalent. The lifetime risk of stage 3 CKD in the United States may be as high as 59% ... [and] the consequences of CKD are of equal magnitude. CKD increases the risk of morbidity and mortality, especially from cardiovascular disease," Dr. Khatri said. Data show that the worse the kidney function, the greater the cardiovascular disease risk and the greater the likelihood of cardiovascular events, he noted.
The financial toll is also extensive, he said during a press briefing at the conference, which was sponsored by the American Society of Nephrology.
"On top of this, the therapeutic armamentarium for CKD is actually relatively limited. We’ve made tremendous strides in treating traditional risk factors such as diabetes, hypertension, and protein in the urine, but most patients with CKD still have progressive kidney function decline over time. This therefore means we need novel approaches to prevent and ameliorate progression of CKD," he said.
Diet may be one such approach. Some diets have been studied in the context of improving CKD, but most studies have focused on protein restriction – an approach that may be harmful in some patients, such as the frail elderly. Few studies have looked at diet in the context of preventing CKD.
The Mediterranean diet – which generally includes high intake of fruits, vegetables, legumes, cereals, fish, and heart-healthy monounsaturated fats; lower intake of dairy, meats, and saturated fats; as well as moderate alcohol intake, has received a great deal of attention with respect to potential cardiovascular benefits. Studies have shown it has important benefits, including improvements in blood pressure, endothelial function, cholesterol, inflammation, and overall cardiovascular risk, Dr. Khatri said.
In fact, results from the randomized controlled PREDIMED study, published in April, demonstrated a 30% reduction in cardiovascular risk among those following a Mediterranean diet, compared with those following a standard low-fat diet (N. Engl. J. Med. 2013;368:1279-90), he noted.
The current study shows that a Mediterranean-style diet may have similarly beneficial effects for reducing CKD risk, which makes sense given the shared risk factors between CKD and cardiovascular disease, he said.
Larger observational trials and randomized controlled trials are needed to confirm these findings and to elucidate the mechanisms by which a Mediterranean-style diet may protect against kidney disease, he said.
This study was funded by the National Institutes of Health. Dr. Khatri reported having no disclosures.
AT KIDNEY WEEK 2013
Major finding: A Mediterranean-style diet was associated with a 50% reduction in incident CKD.
Data source: A prospective cohort study involving 900 subjects.
Disclosures: This study was funded by the National Institutes of Health. Dr. Khatri reported having no disclosures.
Surgical treatments for failed midurethral sling compared
LAS VEGAS – In a study of patients who experienced a failed midurethral sling, urethral bulking injection was associated with a greater than threefold increased risk of failure compared with a repeat midurethral sling procedure, a retrospective analysis showed.
In addition, the diagnosis of intrinsic sphincter deficiency conferred a greater than fourfold risk of failure compared with patients without the diagnosis, regardless of which procedure was performed.
Those are findings from the largest cohort study to date evaluating failure of midurethral sling (MUS), and the only one to include both repeat MUS procedures and urethral bulking injections.
"This study provides important baseline data for surgeons when faced with MUS failure," Dr. Anthony Gaddi said at the annual meeting of the American Urogynecologic Society. "Prospective, randomized data with validated subjective and objective outcomes is warranted."
In an effort to compare the efficacy and safety of a repeat MUS procedure with urethral bulking injection after failed primary MUS, Dr. Gaddi and his associates performed an electronic chart review of patients from the Southern California Permanente Medical Group who underwent MUS for stress urinary incontinence (SUI) between 2008 and 2011.
The primary outcome was a measure of subjective failure, defined as a complaint of SUI, or objective failure, defined as documentation of a positive cough stress test, urodynamic stress incontinence, or reoperation for SUI, said Dr. Gaddi of the department of obstetrics and gynecology at the University of California, Irvine. Secondary outcomes included perioperative complications and adverse events.
For the 7,412 MUS procedures performed between 2008 and 2011, there were 165 repeat procedures for sling failure. Of these, 98 were repeat MUS procedures and 67 were urethral bulking injections. The mean age of patients was 58 years, their mean body mass index was 29.3 kg/m2, 65% were menopausal, and 59% were white.
Dr. Gaddi reported that there were 11 failures in the MUS group (11.2%), compared with 26 failures in the bulking group (38.8%), a difference that reached significance (P less than .01).
In multivariable logistic regression analysis, patients who underwent urethral bulking injections experienced a 3.7-fold increased risk of failure compared with those in the repeat MUS group. In addition, patients with a preoperative diagnosis of intrinsic sphincter deficiency experienced a 4.45-fold higher risk of failure compared with those who had no such deficiency, regardless of which procedure was performed.
Perioperative complications were similar between the two groups, "suggesting that both are safe options in this cohort," Dr. Gaddi said.
He acknowledged certain limitations of the study, including its retrospective design, and "difficulty standardizing our definition of failure. The low number of complications among our repeat procedures limits conclusions that can be made about safety."
Dr. Gaddi said that he had no relevant financial conflicts to disclose.
LAS VEGAS – In a study of patients who experienced a failed midurethral sling, urethral bulking injection was associated with a greater than threefold increased risk of failure compared with a repeat midurethral sling procedure, a retrospective analysis showed.
In addition, the diagnosis of intrinsic sphincter deficiency conferred a greater than fourfold risk of failure compared with patients without the diagnosis, regardless of which procedure was performed.
Those are findings from the largest cohort study to date evaluating failure of midurethral sling (MUS), and the only one to include both repeat MUS procedures and urethral bulking injections.
"This study provides important baseline data for surgeons when faced with MUS failure," Dr. Anthony Gaddi said at the annual meeting of the American Urogynecologic Society. "Prospective, randomized data with validated subjective and objective outcomes is warranted."
In an effort to compare the efficacy and safety of a repeat MUS procedure with urethral bulking injection after failed primary MUS, Dr. Gaddi and his associates performed an electronic chart review of patients from the Southern California Permanente Medical Group who underwent MUS for stress urinary incontinence (SUI) between 2008 and 2011.
The primary outcome was a measure of subjective failure, defined as a complaint of SUI, or objective failure, defined as documentation of a positive cough stress test, urodynamic stress incontinence, or reoperation for SUI, said Dr. Gaddi of the department of obstetrics and gynecology at the University of California, Irvine. Secondary outcomes included perioperative complications and adverse events.
For the 7,412 MUS procedures performed between 2008 and 2011, there were 165 repeat procedures for sling failure. Of these, 98 were repeat MUS procedures and 67 were urethral bulking injections. The mean age of patients was 58 years, their mean body mass index was 29.3 kg/m2, 65% were menopausal, and 59% were white.
Dr. Gaddi reported that there were 11 failures in the MUS group (11.2%), compared with 26 failures in the bulking group (38.8%), a difference that reached significance (P less than .01).
In multivariable logistic regression analysis, patients who underwent urethral bulking injections experienced a 3.7-fold increased risk of failure compared with those in the repeat MUS group. In addition, patients with a preoperative diagnosis of intrinsic sphincter deficiency experienced a 4.45-fold higher risk of failure compared with those who had no such deficiency, regardless of which procedure was performed.
Perioperative complications were similar between the two groups, "suggesting that both are safe options in this cohort," Dr. Gaddi said.
He acknowledged certain limitations of the study, including its retrospective design, and "difficulty standardizing our definition of failure. The low number of complications among our repeat procedures limits conclusions that can be made about safety."
Dr. Gaddi said that he had no relevant financial conflicts to disclose.
LAS VEGAS – In a study of patients who experienced a failed midurethral sling, urethral bulking injection was associated with a greater than threefold increased risk of failure compared with a repeat midurethral sling procedure, a retrospective analysis showed.
In addition, the diagnosis of intrinsic sphincter deficiency conferred a greater than fourfold risk of failure compared with patients without the diagnosis, regardless of which procedure was performed.
Those are findings from the largest cohort study to date evaluating failure of midurethral sling (MUS), and the only one to include both repeat MUS procedures and urethral bulking injections.
"This study provides important baseline data for surgeons when faced with MUS failure," Dr. Anthony Gaddi said at the annual meeting of the American Urogynecologic Society. "Prospective, randomized data with validated subjective and objective outcomes is warranted."
In an effort to compare the efficacy and safety of a repeat MUS procedure with urethral bulking injection after failed primary MUS, Dr. Gaddi and his associates performed an electronic chart review of patients from the Southern California Permanente Medical Group who underwent MUS for stress urinary incontinence (SUI) between 2008 and 2011.
The primary outcome was a measure of subjective failure, defined as a complaint of SUI, or objective failure, defined as documentation of a positive cough stress test, urodynamic stress incontinence, or reoperation for SUI, said Dr. Gaddi of the department of obstetrics and gynecology at the University of California, Irvine. Secondary outcomes included perioperative complications and adverse events.
For the 7,412 MUS procedures performed between 2008 and 2011, there were 165 repeat procedures for sling failure. Of these, 98 were repeat MUS procedures and 67 were urethral bulking injections. The mean age of patients was 58 years, their mean body mass index was 29.3 kg/m2, 65% were menopausal, and 59% were white.
Dr. Gaddi reported that there were 11 failures in the MUS group (11.2%), compared with 26 failures in the bulking group (38.8%), a difference that reached significance (P less than .01).
In multivariable logistic regression analysis, patients who underwent urethral bulking injections experienced a 3.7-fold increased risk of failure compared with those in the repeat MUS group. In addition, patients with a preoperative diagnosis of intrinsic sphincter deficiency experienced a 4.45-fold higher risk of failure compared with those who had no such deficiency, regardless of which procedure was performed.
Perioperative complications were similar between the two groups, "suggesting that both are safe options in this cohort," Dr. Gaddi said.
He acknowledged certain limitations of the study, including its retrospective design, and "difficulty standardizing our definition of failure. The low number of complications among our repeat procedures limits conclusions that can be made about safety."
Dr. Gaddi said that he had no relevant financial conflicts to disclose.
AT THE AUGS ANNUAL MEETING
Major finding: In multivariable logistic regression analysis, patients who underwent urethral bulking injections for a failed midurethral sling experienced a 3.7-fold increased risk of failure, compared with those who underwent a repeat midurethral sling procedure.
Data source: A single-center study of 165 repeat procedures for midurethral sling failure performed between 2008 and 2011.
Disclosures: Dr. Gaddi said that he had no relevant financial conflicts to disclose.
Cost of Botox vs. anticholinergics for urge urinary incontinence
LAS VEGAS – Onabotulinum toxin A and anticholinergic medications have similar cost effectiveness in the first 6 months of treatment for urge urinary incontinence, results from a multicenter randomized trial showed.
However, if costs and outcomes are considered through 9 months, Botox has significantly lower costs than anticholinergics but similar urge urinary incontinence (UUI) control, Dr. Anthony G. Visco reported at the annual meeting of the American Urogynecologic Society.
On behalf of the National Institute of Child Health and Human Development–funded Pelvic Floor Disorders Network, Dr. Visco presented findings from an analysis of 231 women who participated in the ABC trial, which directly compared the safety and efficacy of a 6-month regimen of anticholinergic medications (solifenacin or trospium) to a single 100-U injection of Botox (N. Engl. J. Med. 2012;367:1803-13).
The current study sought to compare the cost effectiveness of anticholinergic medications and Botox for the management of urge urinary incontinence (UUI). The researchers adopted a societal cost perspective and included both direct costs such as physician visits/procedures and medication costs, as well as indirect costs such as incontinence pads, laundry use, and time lost from work.
Patients were randomized to one of the two groups and were followed for 6 months. At each month the researchers obtained bladder diaries, including the Overactive Bladder Questionnaire (OABq) and the Patient Global Symptom Control instrument (PGSC). At 6 months, all anticholinergic pills were stopped. Dr. Visco and his associates followed the patients up to an additional 6 months "to look at the effect of Botox using the PGSC scores to determine the duration of adequate symptom control," he said.
The researchers adopted two different measures of efficacy. In the first 6 months, efficacy outcome was the average reduction in UUI episodes, and utilities were obtained from the OABq completed at baseline through 6 months; quality-adjusted life-years were calculated and annualized from 6 months to a full year.
Between 6 and 9 months, the efficacy outcome was adequate symptom control as measured by the PGSC. "We chose 9 months because that was a period of time when half the participants in the Botox group still had adequate symptom control," explained Dr. Visco, chief of urogynecology and female pelvic medicine and reconstructive surgery at Duke University, Durham, N.C.
Cost analyses assumed that Botox patients incurred no additional costs between months 6 and 9 while those on anticholinergics incurred an additional 3 months of medication costs.
At 6 months the direct medical costs were similar between the anticholinergic and Botox groups ($1,339 vs. $1,266), as were the indirect costs ($150 vs. $106). Both treatments decreased the amount of UUI episodes by 3.3 per day. Both groups also had improvements in total quality-adjusted life-years (0.702 vs. 0.707) and in the annualized cost per quality-adjusted life-year ($57,890 vs. $55,869).
Dr. Visco noted that 74% of study participants who received Botox had adequate symptom control at 6 months. That decreased to 55% at the 9-month mark.
The cost-effectiveness analysis through 9 months revealed that anticholinergics cost $1,942 while Botox cost $1,266. Months of symptom control also were similar between the groups (a mean of 6.36 vs. 6.13, respectively), but in the analysis of cost per month of adequate symptom control, anticholinergics were significantly more expensive: $305 vs. $207 for Botox (P value less than .0001).
Dr. Visco acknowledged certain limitations of the study, including the fact that true cost data were not available beyond 6 months and that the researchers evaluated only solifenacin or trospium. In addition, "quality-adjusted life-year data were limited to the first 6 months, and we are unable to comment on the cost effectiveness of multiple injections," he said. "Anticholinergic assumptions were also made between 6 and 9 months."
Dr. Visco said he had no relevant financial disclosures.
LAS VEGAS – Onabotulinum toxin A and anticholinergic medications have similar cost effectiveness in the first 6 months of treatment for urge urinary incontinence, results from a multicenter randomized trial showed.
However, if costs and outcomes are considered through 9 months, Botox has significantly lower costs than anticholinergics but similar urge urinary incontinence (UUI) control, Dr. Anthony G. Visco reported at the annual meeting of the American Urogynecologic Society.
On behalf of the National Institute of Child Health and Human Development–funded Pelvic Floor Disorders Network, Dr. Visco presented findings from an analysis of 231 women who participated in the ABC trial, which directly compared the safety and efficacy of a 6-month regimen of anticholinergic medications (solifenacin or trospium) to a single 100-U injection of Botox (N. Engl. J. Med. 2012;367:1803-13).
The current study sought to compare the cost effectiveness of anticholinergic medications and Botox for the management of urge urinary incontinence (UUI). The researchers adopted a societal cost perspective and included both direct costs such as physician visits/procedures and medication costs, as well as indirect costs such as incontinence pads, laundry use, and time lost from work.
Patients were randomized to one of the two groups and were followed for 6 months. At each month the researchers obtained bladder diaries, including the Overactive Bladder Questionnaire (OABq) and the Patient Global Symptom Control instrument (PGSC). At 6 months, all anticholinergic pills were stopped. Dr. Visco and his associates followed the patients up to an additional 6 months "to look at the effect of Botox using the PGSC scores to determine the duration of adequate symptom control," he said.
The researchers adopted two different measures of efficacy. In the first 6 months, efficacy outcome was the average reduction in UUI episodes, and utilities were obtained from the OABq completed at baseline through 6 months; quality-adjusted life-years were calculated and annualized from 6 months to a full year.
Between 6 and 9 months, the efficacy outcome was adequate symptom control as measured by the PGSC. "We chose 9 months because that was a period of time when half the participants in the Botox group still had adequate symptom control," explained Dr. Visco, chief of urogynecology and female pelvic medicine and reconstructive surgery at Duke University, Durham, N.C.
Cost analyses assumed that Botox patients incurred no additional costs between months 6 and 9 while those on anticholinergics incurred an additional 3 months of medication costs.
At 6 months the direct medical costs were similar between the anticholinergic and Botox groups ($1,339 vs. $1,266), as were the indirect costs ($150 vs. $106). Both treatments decreased the amount of UUI episodes by 3.3 per day. Both groups also had improvements in total quality-adjusted life-years (0.702 vs. 0.707) and in the annualized cost per quality-adjusted life-year ($57,890 vs. $55,869).
Dr. Visco noted that 74% of study participants who received Botox had adequate symptom control at 6 months. That decreased to 55% at the 9-month mark.
The cost-effectiveness analysis through 9 months revealed that anticholinergics cost $1,942 while Botox cost $1,266. Months of symptom control also were similar between the groups (a mean of 6.36 vs. 6.13, respectively), but in the analysis of cost per month of adequate symptom control, anticholinergics were significantly more expensive: $305 vs. $207 for Botox (P value less than .0001).
Dr. Visco acknowledged certain limitations of the study, including the fact that true cost data were not available beyond 6 months and that the researchers evaluated only solifenacin or trospium. In addition, "quality-adjusted life-year data were limited to the first 6 months, and we are unable to comment on the cost effectiveness of multiple injections," he said. "Anticholinergic assumptions were also made between 6 and 9 months."
Dr. Visco said he had no relevant financial disclosures.
LAS VEGAS – Onabotulinum toxin A and anticholinergic medications have similar cost effectiveness in the first 6 months of treatment for urge urinary incontinence, results from a multicenter randomized trial showed.
However, if costs and outcomes are considered through 9 months, Botox has significantly lower costs than anticholinergics but similar urge urinary incontinence (UUI) control, Dr. Anthony G. Visco reported at the annual meeting of the American Urogynecologic Society.
On behalf of the National Institute of Child Health and Human Development–funded Pelvic Floor Disorders Network, Dr. Visco presented findings from an analysis of 231 women who participated in the ABC trial, which directly compared the safety and efficacy of a 6-month regimen of anticholinergic medications (solifenacin or trospium) to a single 100-U injection of Botox (N. Engl. J. Med. 2012;367:1803-13).
The current study sought to compare the cost effectiveness of anticholinergic medications and Botox for the management of urge urinary incontinence (UUI). The researchers adopted a societal cost perspective and included both direct costs such as physician visits/procedures and medication costs, as well as indirect costs such as incontinence pads, laundry use, and time lost from work.
Patients were randomized to one of the two groups and were followed for 6 months. At each month the researchers obtained bladder diaries, including the Overactive Bladder Questionnaire (OABq) and the Patient Global Symptom Control instrument (PGSC). At 6 months, all anticholinergic pills were stopped. Dr. Visco and his associates followed the patients up to an additional 6 months "to look at the effect of Botox using the PGSC scores to determine the duration of adequate symptom control," he said.
The researchers adopted two different measures of efficacy. In the first 6 months, efficacy outcome was the average reduction in UUI episodes, and utilities were obtained from the OABq completed at baseline through 6 months; quality-adjusted life-years were calculated and annualized from 6 months to a full year.
Between 6 and 9 months, the efficacy outcome was adequate symptom control as measured by the PGSC. "We chose 9 months because that was a period of time when half the participants in the Botox group still had adequate symptom control," explained Dr. Visco, chief of urogynecology and female pelvic medicine and reconstructive surgery at Duke University, Durham, N.C.
Cost analyses assumed that Botox patients incurred no additional costs between months 6 and 9 while those on anticholinergics incurred an additional 3 months of medication costs.
At 6 months the direct medical costs were similar between the anticholinergic and Botox groups ($1,339 vs. $1,266), as were the indirect costs ($150 vs. $106). Both treatments decreased the amount of UUI episodes by 3.3 per day. Both groups also had improvements in total quality-adjusted life-years (0.702 vs. 0.707) and in the annualized cost per quality-adjusted life-year ($57,890 vs. $55,869).
Dr. Visco noted that 74% of study participants who received Botox had adequate symptom control at 6 months. That decreased to 55% at the 9-month mark.
The cost-effectiveness analysis through 9 months revealed that anticholinergics cost $1,942 while Botox cost $1,266. Months of symptom control also were similar between the groups (a mean of 6.36 vs. 6.13, respectively), but in the analysis of cost per month of adequate symptom control, anticholinergics were significantly more expensive: $305 vs. $207 for Botox (P value less than .0001).
Dr. Visco acknowledged certain limitations of the study, including the fact that true cost data were not available beyond 6 months and that the researchers evaluated only solifenacin or trospium. In addition, "quality-adjusted life-year data were limited to the first 6 months, and we are unable to comment on the cost effectiveness of multiple injections," he said. "Anticholinergic assumptions were also made between 6 and 9 months."
Dr. Visco said he had no relevant financial disclosures.
AT THE AUGS ANNUAL MEETING
Major finding: In an analysis of cost per month of adequate symptom control for urge urinary incontinence through 9 months of treatment, anticholinergics were significantly more expensive than Botox ($305 vs. $207; P value less than .0001).
Data source: A study of 231 women who participated in the ABC trial, which directly compared the safety and efficacy of a 6-month regimen of anticholinergic medications (solifenacin or trospium) to a single 100-U injection of Botox.
Disclosures: Dr. Visco said he had no relevant financial disclosures.
CVD more prevalent in chronic kidney disease patients
Cardiovascular disease burden is much greater among elderly patients with chronic kidney disease than in those without, the U.S. Renal Disease System reported.
Among Medicare fee-for-service enrollees aged 66 years and older, the prevalence of heart failure in 2011 was 43% for those with CKD and 19% for those without CKD. The prevalence of stroke and transient ischemic attack was 27% among CKD patients and 20% in those with no CKD. Acute MI prevalence in 2011 was 15% in CKD patients and 6% among enrollees without CKD, according to the USRDS.
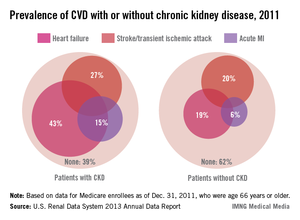
Cardiovascular disease burden is much greater among elderly patients with chronic kidney disease than in those without, the U.S. Renal Disease System reported.
Among Medicare fee-for-service enrollees aged 66 years and older, the prevalence of heart failure in 2011 was 43% for those with CKD and 19% for those without CKD. The prevalence of stroke and transient ischemic attack was 27% among CKD patients and 20% in those with no CKD. Acute MI prevalence in 2011 was 15% in CKD patients and 6% among enrollees without CKD, according to the USRDS.

Cardiovascular disease burden is much greater among elderly patients with chronic kidney disease than in those without, the U.S. Renal Disease System reported.
Among Medicare fee-for-service enrollees aged 66 years and older, the prevalence of heart failure in 2011 was 43% for those with CKD and 19% for those without CKD. The prevalence of stroke and transient ischemic attack was 27% among CKD patients and 20% in those with no CKD. Acute MI prevalence in 2011 was 15% in CKD patients and 6% among enrollees without CKD, according to the USRDS.

Renal risk stratification with the new oral anticoagulants
To the Editor: I read with interest the review of the new oral anticoagulants by Fawole et al1 and agree with their comments on the prevention of bleeding and the importance of monitoring renal function in managing patients on the new classes of oral anticoagulants. However, no specifics were given on how to proceed. Thus, I recommend that renal risk stratification be done before and 1 week after starting these new drugs.
Originally, the US Food and Drug Administration approved dabigatran (Pradaxa) at a dose of 150 mg orally twice daily in patients with a creatinine clearance of 15 to 30 mL/min/1.73 m2. This dosing corresponded to the estimated glomerular filtration rate (eGFR) in patients with stage 4 chronic kidney disease, but this dosing is contraindicated in other guidelines worldwide (Canada, Europe, the United Kingdom, Japan, Australia, and New Zealand).2 Not unexpectedly, 3,781 serious adverse effects were noted in the 2011 US postmarketing experience with dabigatran. These included death (542 cases), hemorrhage (2,367 cases), acute renal failure (291 cases), stroke (644 cases), and suspected liver failure (15 cases).3 Thirteen months after dabigatran’s approval in the United States, Boehringer Ingelheim changed the dosage and product guidelines.2–4 The new dosage4 is 75 mg twice daily for patients with a creatinine clearance of 15 to 30 mL/min/1.73 m2.
Therefore, I suggest a nephrologic “way out”5 when using the new oral anticoagulants to avoid the problems with dabigatran noted above.
First, if these drugs are to be used in nonvalvular atrial fibrillation, risk factors should be determined using the CHADS2 or the CHADS2-VASc score. Special attention should be given to patients age 75 and older, women, and patients with a history of stroke, transient ischemic attack, or systemic embolism. All of these have been noted to be major risk factors.6,7
Second, renal risk stratification8 should be done using a comprehensive metabolic panel before and 1 week after starting new oral anticoagulants, or if there is a change in the patient’s clinical condition. Most US laboratories now provide an eGFR and the stage of chronic kidney disease.3,5 For example (Table 1), if dabigatran is used, one should follow current dosing guidelines for chronic kidney disease stages 1 through 3, ie, 150 mg twice daily. If stage 4 chronic kidney disease is detected (creatinine clearance 15–29 mL/min/1.73 m2), the updated recommended dosage is 75 mg twice daily. If stage 5 is noted (eGFR ≤ 15 mL/min/1.73 m2), dabigatran is not indicated. Similar steps can be done using current guidelines for the other new oral anticoagulants.
This simple renal risk stratification guideline should help avoid some of the problems noted in the dabigatran postmarketing experience, which were aggravated by the lack of approval of a 110-mg dose and by misleading advertising, claiming that no blood monitoring was required.2–5 Thus, the new oral anticoagulants should be a welcome addition to our armamentarium in patients who need them, and we hope to avoid the risks, morbidity, mortality, and expense of trying to reverse adverse effects.
- Fawole A, Daw HA, Crowther MA. Practical management of bleeding due to the anticoagulants, dabigatran, rivaroxaban, and apixaban. Cleve Clin J Med 2013, 80:443–451.
- Pazmiño PA. Dabigatran associated acute renal failure (DAARF). El Paso Physician 2011; 34:7–9.
- Pazmiño PA. Adverse effects of dabigatran (Letter). Ann Intern Med 2012; 157:916.
- Pradaxa (prescribing information). Ridgefield, CT. Boehringer Ingelheim Pharmaceuticals 2011.
- Pazmiño PA. Dabigatran: a nephrological way out. Am J Med 2013; 126;e21–e22.
- Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach. The Euro Heart Survey on Atrial Fibrillation. Chest 2010; 137:263–272.
- Reinecke H, Brand E, Mesters R, et al. Dilemmas in the management of atrial fibrillation in chronic kidney disease. J Am Soc Nephrol 2009; 20:705–711.
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic disease: evaluation, classification and stratification. Am J Kidney Dis 2002; 39(suppl 1):S1–S266.
To the Editor: I read with interest the review of the new oral anticoagulants by Fawole et al1 and agree with their comments on the prevention of bleeding and the importance of monitoring renal function in managing patients on the new classes of oral anticoagulants. However, no specifics were given on how to proceed. Thus, I recommend that renal risk stratification be done before and 1 week after starting these new drugs.
Originally, the US Food and Drug Administration approved dabigatran (Pradaxa) at a dose of 150 mg orally twice daily in patients with a creatinine clearance of 15 to 30 mL/min/1.73 m2. This dosing corresponded to the estimated glomerular filtration rate (eGFR) in patients with stage 4 chronic kidney disease, but this dosing is contraindicated in other guidelines worldwide (Canada, Europe, the United Kingdom, Japan, Australia, and New Zealand).2 Not unexpectedly, 3,781 serious adverse effects were noted in the 2011 US postmarketing experience with dabigatran. These included death (542 cases), hemorrhage (2,367 cases), acute renal failure (291 cases), stroke (644 cases), and suspected liver failure (15 cases).3 Thirteen months after dabigatran’s approval in the United States, Boehringer Ingelheim changed the dosage and product guidelines.2–4 The new dosage4 is 75 mg twice daily for patients with a creatinine clearance of 15 to 30 mL/min/1.73 m2.
Therefore, I suggest a nephrologic “way out”5 when using the new oral anticoagulants to avoid the problems with dabigatran noted above.
First, if these drugs are to be used in nonvalvular atrial fibrillation, risk factors should be determined using the CHADS2 or the CHADS2-VASc score. Special attention should be given to patients age 75 and older, women, and patients with a history of stroke, transient ischemic attack, or systemic embolism. All of these have been noted to be major risk factors.6,7
Second, renal risk stratification8 should be done using a comprehensive metabolic panel before and 1 week after starting new oral anticoagulants, or if there is a change in the patient’s clinical condition. Most US laboratories now provide an eGFR and the stage of chronic kidney disease.3,5 For example (Table 1), if dabigatran is used, one should follow current dosing guidelines for chronic kidney disease stages 1 through 3, ie, 150 mg twice daily. If stage 4 chronic kidney disease is detected (creatinine clearance 15–29 mL/min/1.73 m2), the updated recommended dosage is 75 mg twice daily. If stage 5 is noted (eGFR ≤ 15 mL/min/1.73 m2), dabigatran is not indicated. Similar steps can be done using current guidelines for the other new oral anticoagulants.
This simple renal risk stratification guideline should help avoid some of the problems noted in the dabigatran postmarketing experience, which were aggravated by the lack of approval of a 110-mg dose and by misleading advertising, claiming that no blood monitoring was required.2–5 Thus, the new oral anticoagulants should be a welcome addition to our armamentarium in patients who need them, and we hope to avoid the risks, morbidity, mortality, and expense of trying to reverse adverse effects.
To the Editor: I read with interest the review of the new oral anticoagulants by Fawole et al1 and agree with their comments on the prevention of bleeding and the importance of monitoring renal function in managing patients on the new classes of oral anticoagulants. However, no specifics were given on how to proceed. Thus, I recommend that renal risk stratification be done before and 1 week after starting these new drugs.
Originally, the US Food and Drug Administration approved dabigatran (Pradaxa) at a dose of 150 mg orally twice daily in patients with a creatinine clearance of 15 to 30 mL/min/1.73 m2. This dosing corresponded to the estimated glomerular filtration rate (eGFR) in patients with stage 4 chronic kidney disease, but this dosing is contraindicated in other guidelines worldwide (Canada, Europe, the United Kingdom, Japan, Australia, and New Zealand).2 Not unexpectedly, 3,781 serious adverse effects were noted in the 2011 US postmarketing experience with dabigatran. These included death (542 cases), hemorrhage (2,367 cases), acute renal failure (291 cases), stroke (644 cases), and suspected liver failure (15 cases).3 Thirteen months after dabigatran’s approval in the United States, Boehringer Ingelheim changed the dosage and product guidelines.2–4 The new dosage4 is 75 mg twice daily for patients with a creatinine clearance of 15 to 30 mL/min/1.73 m2.
Therefore, I suggest a nephrologic “way out”5 when using the new oral anticoagulants to avoid the problems with dabigatran noted above.
First, if these drugs are to be used in nonvalvular atrial fibrillation, risk factors should be determined using the CHADS2 or the CHADS2-VASc score. Special attention should be given to patients age 75 and older, women, and patients with a history of stroke, transient ischemic attack, or systemic embolism. All of these have been noted to be major risk factors.6,7
Second, renal risk stratification8 should be done using a comprehensive metabolic panel before and 1 week after starting new oral anticoagulants, or if there is a change in the patient’s clinical condition. Most US laboratories now provide an eGFR and the stage of chronic kidney disease.3,5 For example (Table 1), if dabigatran is used, one should follow current dosing guidelines for chronic kidney disease stages 1 through 3, ie, 150 mg twice daily. If stage 4 chronic kidney disease is detected (creatinine clearance 15–29 mL/min/1.73 m2), the updated recommended dosage is 75 mg twice daily. If stage 5 is noted (eGFR ≤ 15 mL/min/1.73 m2), dabigatran is not indicated. Similar steps can be done using current guidelines for the other new oral anticoagulants.
This simple renal risk stratification guideline should help avoid some of the problems noted in the dabigatran postmarketing experience, which were aggravated by the lack of approval of a 110-mg dose and by misleading advertising, claiming that no blood monitoring was required.2–5 Thus, the new oral anticoagulants should be a welcome addition to our armamentarium in patients who need them, and we hope to avoid the risks, morbidity, mortality, and expense of trying to reverse adverse effects.
- Fawole A, Daw HA, Crowther MA. Practical management of bleeding due to the anticoagulants, dabigatran, rivaroxaban, and apixaban. Cleve Clin J Med 2013, 80:443–451.
- Pazmiño PA. Dabigatran associated acute renal failure (DAARF). El Paso Physician 2011; 34:7–9.
- Pazmiño PA. Adverse effects of dabigatran (Letter). Ann Intern Med 2012; 157:916.
- Pradaxa (prescribing information). Ridgefield, CT. Boehringer Ingelheim Pharmaceuticals 2011.
- Pazmiño PA. Dabigatran: a nephrological way out. Am J Med 2013; 126;e21–e22.
- Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach. The Euro Heart Survey on Atrial Fibrillation. Chest 2010; 137:263–272.
- Reinecke H, Brand E, Mesters R, et al. Dilemmas in the management of atrial fibrillation in chronic kidney disease. J Am Soc Nephrol 2009; 20:705–711.
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic disease: evaluation, classification and stratification. Am J Kidney Dis 2002; 39(suppl 1):S1–S266.
- Fawole A, Daw HA, Crowther MA. Practical management of bleeding due to the anticoagulants, dabigatran, rivaroxaban, and apixaban. Cleve Clin J Med 2013, 80:443–451.
- Pazmiño PA. Dabigatran associated acute renal failure (DAARF). El Paso Physician 2011; 34:7–9.
- Pazmiño PA. Adverse effects of dabigatran (Letter). Ann Intern Med 2012; 157:916.
- Pradaxa (prescribing information). Ridgefield, CT. Boehringer Ingelheim Pharmaceuticals 2011.
- Pazmiño PA. Dabigatran: a nephrological way out. Am J Med 2013; 126;e21–e22.
- Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach. The Euro Heart Survey on Atrial Fibrillation. Chest 2010; 137:263–272.
- Reinecke H, Brand E, Mesters R, et al. Dilemmas in the management of atrial fibrillation in chronic kidney disease. J Am Soc Nephrol 2009; 20:705–711.
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic disease: evaluation, classification and stratification. Am J Kidney Dis 2002; 39(suppl 1):S1–S266.
In reply: Renal risk stratification with the new oral anticoagulants
In Reply: We agree with the comments of Dr. Pazmiño regarding specifics of renal risk stratification in patients taking the new oral anticoagulants. In order to reduce the bleeding risks associated with these agents, they should be prescribed on the basis of the individual patient’s clinical characteristics. We did not discuss this since the focus of our article was management of bleeding that resulted from use of these drugs. We appreciate the recommendations of Dr. Pazmiño.
In Reply: We agree with the comments of Dr. Pazmiño regarding specifics of renal risk stratification in patients taking the new oral anticoagulants. In order to reduce the bleeding risks associated with these agents, they should be prescribed on the basis of the individual patient’s clinical characteristics. We did not discuss this since the focus of our article was management of bleeding that resulted from use of these drugs. We appreciate the recommendations of Dr. Pazmiño.
In Reply: We agree with the comments of Dr. Pazmiño regarding specifics of renal risk stratification in patients taking the new oral anticoagulants. In order to reduce the bleeding risks associated with these agents, they should be prescribed on the basis of the individual patient’s clinical characteristics. We did not discuss this since the focus of our article was management of bleeding that resulted from use of these drugs. We appreciate the recommendations of Dr. Pazmiño.
Pregnancy: Hypertension and Risk for Kidney Disease
Q If a pregnant woman has mild hypertension (eg, 140/90 mm Hg) but no albuminuria, is she at higher risk for kidney disease as she ages?
Gestational hypertension and preeclampsia are two types of hypertension that occur during pregnancy. Both occur after 20 weeks’ gestation and include a blood pressure reading greater than 140/90 mm Hg and no maternal history of hypertension. Proteinuria does not occur in gestational hypertension as it does in preeclampsia (proteinuria ≥ 300 mg in a 24-h urine collection).
One study evaluated more than 26,000 Taiwanese women with hypertension during pregnancy and compared them to more than 200,000 women without hypertension during pregnancy. It was found that hypertension during pregnancy increased the risk for chronic kidney disease (CKD) and end-stage renal disease (ESRD) later in life. Preeclampsia and eclampsia were even more likely to increase the risk for ESRD, compared with gestational hypertension.1
Preeclampsia occurs after 20 weeks’ gestation and includes elevated blood pressure (≥ 140 mm Hg systolic or ≥ 90 mm Hg diastolic) and proteinuria (≥ 0.3 g in 24 h). Severe preeclampsia develops with the addition of worsening hypertension and proteinuria, oliguria less than 500 mL in 24 h, thrombocytopenia, hemolysis, elevated liver enzymes, low platelets, pulmonary edema, and fetal growth restriction. Eclampsia is when seizures occur in addition to preeclampsia.2 Gestational hypertension has also been linked with a higher risk for ischemic heart disease, myocardial infarcts and death, heart failure, ischem-
ic stroke, kidney disease, and diabetes.3
Because of the association between hypertension during pregnancy and subsequent development of CKD, it is very important that mothers who have hypertension during pregnancy continue to have their renal function monitored after delivery.
Mandy Trolinger, MS, RD, PA-C
Denver Nephrology
Denver, CO
Personal Note: Mandy is a two-time kidney transplant recipient. She delivered a healthy baby boy in 2012.
REFERENCES
1. Wang I-K, Muo C-H, Liang C-C, et al. Association between hypertensive disorders during pregnancy and end-stage renal disease: a population-based study. CMAJ. 2013;85: 207-213.
2. McPhee SJ, Papadakis MA, Tierney LM, et al. Current Medical Diagnosis and Treatment. 47th ed. New York, NY: McGraw-Hill/Lange; 2008.
3. Männistö T, Mendola P, Vääräsmäki M, et al. Elevated blood pressure in pregnancy and subsequent chronic disease risk. Circulation. 2013;127:681-690.
4. Nevis IF, Reitsma A, Dominic A, et al. Pregnancy outcomes in women with chronic kidney disease: a systematic review. Clin J Am Soc Nephrol. 2011;6:2587-2598.
5. Hou S. Pregnancy in chronic renal insufficiency and end-stage renal disease. Am J Kidney Dis. 1999;33:235-252.
6. Davison JM. Dialysis, transplantation, and pregnancy. Am J Kidney Dis. 1991;17:127-132.
7. Asamiya Y, Otsubo S, Matsuda Y, et al. The importance of low blood urea nitrogen levels in pregnant patients undergoing hemodialysis to optimize birth weight and gestational age. Kidney Int. 2009;75:1217-1222.
8. Giatras I, Levy DP, Malone FD, et al. Pregnancy during dialysis: case report and management guidelines. Nephrol Dial Transplant. 1998;13:3266-3272.
9. McKay DB, Josephson MA. Pregnancy in recipients of solid organs—effects on mother and child. N Engl J Med. 2006;354:1281-1293.
10. Sifontis NM, Coscia LA, Constantinescu S, et al. Pregnancy outcomes in solid organ transplant recipients with exposure to mycophenolate mofetil or sirolimus. Transplantation. 2006;82:1698-1702.
11. Kainz A, Harabacz I, Cowlrick IS, et al. Review of the course and outcome of 100 pregnancies in 84 women treated with tacrolimus. Transplantation. 2000;70:1718-1721.
12. Josephson MA. Pregnancy in renal transplant recipients: more questions answered, still more asked. Clin J Am Soc Nephrol. 2013;8: 182-183.
Q If a pregnant woman has mild hypertension (eg, 140/90 mm Hg) but no albuminuria, is she at higher risk for kidney disease as she ages?
Gestational hypertension and preeclampsia are two types of hypertension that occur during pregnancy. Both occur after 20 weeks’ gestation and include a blood pressure reading greater than 140/90 mm Hg and no maternal history of hypertension. Proteinuria does not occur in gestational hypertension as it does in preeclampsia (proteinuria ≥ 300 mg in a 24-h urine collection).
One study evaluated more than 26,000 Taiwanese women with hypertension during pregnancy and compared them to more than 200,000 women without hypertension during pregnancy. It was found that hypertension during pregnancy increased the risk for chronic kidney disease (CKD) and end-stage renal disease (ESRD) later in life. Preeclampsia and eclampsia were even more likely to increase the risk for ESRD, compared with gestational hypertension.1
Preeclampsia occurs after 20 weeks’ gestation and includes elevated blood pressure (≥ 140 mm Hg systolic or ≥ 90 mm Hg diastolic) and proteinuria (≥ 0.3 g in 24 h). Severe preeclampsia develops with the addition of worsening hypertension and proteinuria, oliguria less than 500 mL in 24 h, thrombocytopenia, hemolysis, elevated liver enzymes, low platelets, pulmonary edema, and fetal growth restriction. Eclampsia is when seizures occur in addition to preeclampsia.2 Gestational hypertension has also been linked with a higher risk for ischemic heart disease, myocardial infarcts and death, heart failure, ischem-
ic stroke, kidney disease, and diabetes.3
Because of the association between hypertension during pregnancy and subsequent development of CKD, it is very important that mothers who have hypertension during pregnancy continue to have their renal function monitored after delivery.
Mandy Trolinger, MS, RD, PA-C
Denver Nephrology
Denver, CO
Personal Note: Mandy is a two-time kidney transplant recipient. She delivered a healthy baby boy in 2012.
REFERENCES
1. Wang I-K, Muo C-H, Liang C-C, et al. Association between hypertensive disorders during pregnancy and end-stage renal disease: a population-based study. CMAJ. 2013;85: 207-213.
2. McPhee SJ, Papadakis MA, Tierney LM, et al. Current Medical Diagnosis and Treatment. 47th ed. New York, NY: McGraw-Hill/Lange; 2008.
3. Männistö T, Mendola P, Vääräsmäki M, et al. Elevated blood pressure in pregnancy and subsequent chronic disease risk. Circulation. 2013;127:681-690.
4. Nevis IF, Reitsma A, Dominic A, et al. Pregnancy outcomes in women with chronic kidney disease: a systematic review. Clin J Am Soc Nephrol. 2011;6:2587-2598.
5. Hou S. Pregnancy in chronic renal insufficiency and end-stage renal disease. Am J Kidney Dis. 1999;33:235-252.
6. Davison JM. Dialysis, transplantation, and pregnancy. Am J Kidney Dis. 1991;17:127-132.
7. Asamiya Y, Otsubo S, Matsuda Y, et al. The importance of low blood urea nitrogen levels in pregnant patients undergoing hemodialysis to optimize birth weight and gestational age. Kidney Int. 2009;75:1217-1222.
8. Giatras I, Levy DP, Malone FD, et al. Pregnancy during dialysis: case report and management guidelines. Nephrol Dial Transplant. 1998;13:3266-3272.
9. McKay DB, Josephson MA. Pregnancy in recipients of solid organs—effects on mother and child. N Engl J Med. 2006;354:1281-1293.
10. Sifontis NM, Coscia LA, Constantinescu S, et al. Pregnancy outcomes in solid organ transplant recipients with exposure to mycophenolate mofetil or sirolimus. Transplantation. 2006;82:1698-1702.
11. Kainz A, Harabacz I, Cowlrick IS, et al. Review of the course and outcome of 100 pregnancies in 84 women treated with tacrolimus. Transplantation. 2000;70:1718-1721.
12. Josephson MA. Pregnancy in renal transplant recipients: more questions answered, still more asked. Clin J Am Soc Nephrol. 2013;8: 182-183.
Q If a pregnant woman has mild hypertension (eg, 140/90 mm Hg) but no albuminuria, is she at higher risk for kidney disease as she ages?
Gestational hypertension and preeclampsia are two types of hypertension that occur during pregnancy. Both occur after 20 weeks’ gestation and include a blood pressure reading greater than 140/90 mm Hg and no maternal history of hypertension. Proteinuria does not occur in gestational hypertension as it does in preeclampsia (proteinuria ≥ 300 mg in a 24-h urine collection).
One study evaluated more than 26,000 Taiwanese women with hypertension during pregnancy and compared them to more than 200,000 women without hypertension during pregnancy. It was found that hypertension during pregnancy increased the risk for chronic kidney disease (CKD) and end-stage renal disease (ESRD) later in life. Preeclampsia and eclampsia were even more likely to increase the risk for ESRD, compared with gestational hypertension.1
Preeclampsia occurs after 20 weeks’ gestation and includes elevated blood pressure (≥ 140 mm Hg systolic or ≥ 90 mm Hg diastolic) and proteinuria (≥ 0.3 g in 24 h). Severe preeclampsia develops with the addition of worsening hypertension and proteinuria, oliguria less than 500 mL in 24 h, thrombocytopenia, hemolysis, elevated liver enzymes, low platelets, pulmonary edema, and fetal growth restriction. Eclampsia is when seizures occur in addition to preeclampsia.2 Gestational hypertension has also been linked with a higher risk for ischemic heart disease, myocardial infarcts and death, heart failure, ischem-
ic stroke, kidney disease, and diabetes.3
Because of the association between hypertension during pregnancy and subsequent development of CKD, it is very important that mothers who have hypertension during pregnancy continue to have their renal function monitored after delivery.
Mandy Trolinger, MS, RD, PA-C
Denver Nephrology
Denver, CO
Personal Note: Mandy is a two-time kidney transplant recipient. She delivered a healthy baby boy in 2012.
REFERENCES
1. Wang I-K, Muo C-H, Liang C-C, et al. Association between hypertensive disorders during pregnancy and end-stage renal disease: a population-based study. CMAJ. 2013;85: 207-213.
2. McPhee SJ, Papadakis MA, Tierney LM, et al. Current Medical Diagnosis and Treatment. 47th ed. New York, NY: McGraw-Hill/Lange; 2008.
3. Männistö T, Mendola P, Vääräsmäki M, et al. Elevated blood pressure in pregnancy and subsequent chronic disease risk. Circulation. 2013;127:681-690.
4. Nevis IF, Reitsma A, Dominic A, et al. Pregnancy outcomes in women with chronic kidney disease: a systematic review. Clin J Am Soc Nephrol. 2011;6:2587-2598.
5. Hou S. Pregnancy in chronic renal insufficiency and end-stage renal disease. Am J Kidney Dis. 1999;33:235-252.
6. Davison JM. Dialysis, transplantation, and pregnancy. Am J Kidney Dis. 1991;17:127-132.
7. Asamiya Y, Otsubo S, Matsuda Y, et al. The importance of low blood urea nitrogen levels in pregnant patients undergoing hemodialysis to optimize birth weight and gestational age. Kidney Int. 2009;75:1217-1222.
8. Giatras I, Levy DP, Malone FD, et al. Pregnancy during dialysis: case report and management guidelines. Nephrol Dial Transplant. 1998;13:3266-3272.
9. McKay DB, Josephson MA. Pregnancy in recipients of solid organs—effects on mother and child. N Engl J Med. 2006;354:1281-1293.
10. Sifontis NM, Coscia LA, Constantinescu S, et al. Pregnancy outcomes in solid organ transplant recipients with exposure to mycophenolate mofetil or sirolimus. Transplantation. 2006;82:1698-1702.
11. Kainz A, Harabacz I, Cowlrick IS, et al. Review of the course and outcome of 100 pregnancies in 84 women treated with tacrolimus. Transplantation. 2000;70:1718-1721.
12. Josephson MA. Pregnancy in renal transplant recipients: more questions answered, still more asked. Clin J Am Soc Nephrol. 2013;8: 182-183.