User login
Low blood pressure may harm rather than help patients with chronic kidney disease
In a large, national cohort study of U.S. veterans with non–dialysis dependent chronic kidney disease, lower systolic and diastolic blood pressures were associated with lower mortality rates – but only when the diastolic value was higher than about 70 mm Hg.
In addition, mortality rates were significantly increased among those patients with "ideal" blood pressure values (less than 130/80 mm Hg), "because of the inclusion of patients with low SBP and DBP," reported Dr. Csaba P. Kovesdy, chief of nephrology at the Memphis Veterans Affairs Medical Center, and his associates. The study was published in the Annals of Internal Medicine on Aug. 19 (Ann. Intern. Med. 2013;159:233-42).
The results indicate that current guidelines for patients with chronic kidney disease (CKD), which recommend a systolic blood pressure (SBP) of 130 mm Hg or lower "at the expense of lowering DBP [diastolic blood pressure] to less than approximately 70 mm Hg," may be harmful, they concluded. However, one of the limitations of the study was that it was an observational study and cannot establish a causal association, so "clinical trials are needed to inform us about the ideal BP target for antihypertensive therapy in patients with CKD," they added.
Using more than 18 million BP readings, the study evaluated the association of SBP and DBP values separately and SBP/DBP combinations on all-cause mortality in almost 652,000 U.S. veterans with CKD, who were not dependent on dialysis, between 2005 and 2012. Their mean age was 74 years, most were male (97%), 88% were white, 9% were black, 43% had coronary artery disease, and 43% had diabetes. The mean SBP values at baseline were 135 mm Hg while the mean DBP was 72 mm Hg; the mean glomerular filtration rate (GFR) was 50.4 mL/min per 1.73 m2. The study looked at 96 different SBP/DBP combinations. During the time period of the study, 238,640 patients died.
They identified a U-shaped curve when analyzing mortality with SBP and DBP separately, "with both lower and higher levels showing a substantial and statistically significant association" with mortality risk. Based on the adjusted hazard ratios for the combinations of SBP and DBP, the lowest mortality rates were associated with blood pressures of 130-139/90-99 mm Hg, and 130-159/70-89 mm Hg, adjusted for factors that included age, sex, race, diabetes, and cardiovascular and cerebrovascular disease, age and medication use).
But combinations of lower SBP and DBP values "were associated with relatively lower mortality rates only if the lower DBP component was greater than approximately 70 mm Hg," they said.
When evaluating risk based on JNC 7 (Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure) categories,they found that that those with stage 1 hypertension (SBP of 140-159 mm Hg or DBP of 90-99 mm Hg) were associated with the lowest mortality rates, while those in the normal category (an SBP lower than 120 and a DBP below 80) had the highest mortality rates," results that were independent of confounding factors and were statistically significant.
The authors described an elevated SBP combined with a low DBP, which is common in CKD patients, as "an especially problematic BP pattern," they said, pointing out that 33% of the patients had an SBP greater than 140 mm Hg and a DBP less than 70 mm Hg at some point during the study period.
The study strengths included the large size and the representation of the U.S. veterans’ population, but the limitations included the mostly male population and the observational design of the study, so more studies are needed, the authors said. "Until such trials become available, low BP should be regarded as potentially deleterious in this patient population, and we suggest caution in lowering BP to less than what has been demonstrated as beneficial in randomized controlled trials," they concluded.
Dr. Kovesdy, professor of medicine at University of Tennessee Health Science Center, Memphis, disclosed having received grants from the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, and nonfinancial support from the Department of Veterans Affairs while the study was conducted. Four authors had no disclosures, and one author disclosed having received NIH grants during the study. The remaining two authors disclosed having received research grants or personal fees from different pharmaceutical companies.
While the study results raise questions about the optimal BP targets in patients with CKD, "these are observational data with attendant limitations," Dr. Dena Rifkin and Dr. Mark Sarnak wrote in an accompanying editorial,
"A seemingly acceptable SBP combined with a low DBP may be a cause for concern, especially in older patients with CKD and comorbid conditions." However, "lower [systolic blood pressure] and [diastolic blood pressure] may be markers of the severity of chronic illness or vascular disease," they wrote, adding that the results "may not generalize beyond older white men with stage 3A CKD, and the fact that only a small percentage of persons had proteinuria measurements makes it difficult to draw any conclusions regarding applicability to proteinuric CKD." (Ann. Intern. Med. 2013;159:302-3).
Dr. Rifkin is a nephrologist and epidemiologist at the University of California, San Diego, and the Veterans Affairs Healthcare System, San Diego; and Dr. Sarnak is with the division of nephrology, Tufts Medical Center, Boston. Dr. Rifkin had no disclosures. Dr. Sarnak disclosed having been a member of the KDIGO (Kidney disease improving global outcomes) Clinical Practice Guideline for the Management of Blood Pressure in CKD workgroup.
While the study results raise questions about the optimal BP targets in patients with CKD, "these are observational data with attendant limitations," Dr. Dena Rifkin and Dr. Mark Sarnak wrote in an accompanying editorial,
"A seemingly acceptable SBP combined with a low DBP may be a cause for concern, especially in older patients with CKD and comorbid conditions." However, "lower [systolic blood pressure] and [diastolic blood pressure] may be markers of the severity of chronic illness or vascular disease," they wrote, adding that the results "may not generalize beyond older white men with stage 3A CKD, and the fact that only a small percentage of persons had proteinuria measurements makes it difficult to draw any conclusions regarding applicability to proteinuric CKD." (Ann. Intern. Med. 2013;159:302-3).
Dr. Rifkin is a nephrologist and epidemiologist at the University of California, San Diego, and the Veterans Affairs Healthcare System, San Diego; and Dr. Sarnak is with the division of nephrology, Tufts Medical Center, Boston. Dr. Rifkin had no disclosures. Dr. Sarnak disclosed having been a member of the KDIGO (Kidney disease improving global outcomes) Clinical Practice Guideline for the Management of Blood Pressure in CKD workgroup.
While the study results raise questions about the optimal BP targets in patients with CKD, "these are observational data with attendant limitations," Dr. Dena Rifkin and Dr. Mark Sarnak wrote in an accompanying editorial,
"A seemingly acceptable SBP combined with a low DBP may be a cause for concern, especially in older patients with CKD and comorbid conditions." However, "lower [systolic blood pressure] and [diastolic blood pressure] may be markers of the severity of chronic illness or vascular disease," they wrote, adding that the results "may not generalize beyond older white men with stage 3A CKD, and the fact that only a small percentage of persons had proteinuria measurements makes it difficult to draw any conclusions regarding applicability to proteinuric CKD." (Ann. Intern. Med. 2013;159:302-3).
Dr. Rifkin is a nephrologist and epidemiologist at the University of California, San Diego, and the Veterans Affairs Healthcare System, San Diego; and Dr. Sarnak is with the division of nephrology, Tufts Medical Center, Boston. Dr. Rifkin had no disclosures. Dr. Sarnak disclosed having been a member of the KDIGO (Kidney disease improving global outcomes) Clinical Practice Guideline for the Management of Blood Pressure in CKD workgroup.
In a large, national cohort study of U.S. veterans with non–dialysis dependent chronic kidney disease, lower systolic and diastolic blood pressures were associated with lower mortality rates – but only when the diastolic value was higher than about 70 mm Hg.
In addition, mortality rates were significantly increased among those patients with "ideal" blood pressure values (less than 130/80 mm Hg), "because of the inclusion of patients with low SBP and DBP," reported Dr. Csaba P. Kovesdy, chief of nephrology at the Memphis Veterans Affairs Medical Center, and his associates. The study was published in the Annals of Internal Medicine on Aug. 19 (Ann. Intern. Med. 2013;159:233-42).
The results indicate that current guidelines for patients with chronic kidney disease (CKD), which recommend a systolic blood pressure (SBP) of 130 mm Hg or lower "at the expense of lowering DBP [diastolic blood pressure] to less than approximately 70 mm Hg," may be harmful, they concluded. However, one of the limitations of the study was that it was an observational study and cannot establish a causal association, so "clinical trials are needed to inform us about the ideal BP target for antihypertensive therapy in patients with CKD," they added.
Using more than 18 million BP readings, the study evaluated the association of SBP and DBP values separately and SBP/DBP combinations on all-cause mortality in almost 652,000 U.S. veterans with CKD, who were not dependent on dialysis, between 2005 and 2012. Their mean age was 74 years, most were male (97%), 88% were white, 9% were black, 43% had coronary artery disease, and 43% had diabetes. The mean SBP values at baseline were 135 mm Hg while the mean DBP was 72 mm Hg; the mean glomerular filtration rate (GFR) was 50.4 mL/min per 1.73 m2. The study looked at 96 different SBP/DBP combinations. During the time period of the study, 238,640 patients died.
They identified a U-shaped curve when analyzing mortality with SBP and DBP separately, "with both lower and higher levels showing a substantial and statistically significant association" with mortality risk. Based on the adjusted hazard ratios for the combinations of SBP and DBP, the lowest mortality rates were associated with blood pressures of 130-139/90-99 mm Hg, and 130-159/70-89 mm Hg, adjusted for factors that included age, sex, race, diabetes, and cardiovascular and cerebrovascular disease, age and medication use).
But combinations of lower SBP and DBP values "were associated with relatively lower mortality rates only if the lower DBP component was greater than approximately 70 mm Hg," they said.
When evaluating risk based on JNC 7 (Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure) categories,they found that that those with stage 1 hypertension (SBP of 140-159 mm Hg or DBP of 90-99 mm Hg) were associated with the lowest mortality rates, while those in the normal category (an SBP lower than 120 and a DBP below 80) had the highest mortality rates," results that were independent of confounding factors and were statistically significant.
The authors described an elevated SBP combined with a low DBP, which is common in CKD patients, as "an especially problematic BP pattern," they said, pointing out that 33% of the patients had an SBP greater than 140 mm Hg and a DBP less than 70 mm Hg at some point during the study period.
The study strengths included the large size and the representation of the U.S. veterans’ population, but the limitations included the mostly male population and the observational design of the study, so more studies are needed, the authors said. "Until such trials become available, low BP should be regarded as potentially deleterious in this patient population, and we suggest caution in lowering BP to less than what has been demonstrated as beneficial in randomized controlled trials," they concluded.
Dr. Kovesdy, professor of medicine at University of Tennessee Health Science Center, Memphis, disclosed having received grants from the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, and nonfinancial support from the Department of Veterans Affairs while the study was conducted. Four authors had no disclosures, and one author disclosed having received NIH grants during the study. The remaining two authors disclosed having received research grants or personal fees from different pharmaceutical companies.
In a large, national cohort study of U.S. veterans with non–dialysis dependent chronic kidney disease, lower systolic and diastolic blood pressures were associated with lower mortality rates – but only when the diastolic value was higher than about 70 mm Hg.
In addition, mortality rates were significantly increased among those patients with "ideal" blood pressure values (less than 130/80 mm Hg), "because of the inclusion of patients with low SBP and DBP," reported Dr. Csaba P. Kovesdy, chief of nephrology at the Memphis Veterans Affairs Medical Center, and his associates. The study was published in the Annals of Internal Medicine on Aug. 19 (Ann. Intern. Med. 2013;159:233-42).
The results indicate that current guidelines for patients with chronic kidney disease (CKD), which recommend a systolic blood pressure (SBP) of 130 mm Hg or lower "at the expense of lowering DBP [diastolic blood pressure] to less than approximately 70 mm Hg," may be harmful, they concluded. However, one of the limitations of the study was that it was an observational study and cannot establish a causal association, so "clinical trials are needed to inform us about the ideal BP target for antihypertensive therapy in patients with CKD," they added.
Using more than 18 million BP readings, the study evaluated the association of SBP and DBP values separately and SBP/DBP combinations on all-cause mortality in almost 652,000 U.S. veterans with CKD, who were not dependent on dialysis, between 2005 and 2012. Their mean age was 74 years, most were male (97%), 88% were white, 9% were black, 43% had coronary artery disease, and 43% had diabetes. The mean SBP values at baseline were 135 mm Hg while the mean DBP was 72 mm Hg; the mean glomerular filtration rate (GFR) was 50.4 mL/min per 1.73 m2. The study looked at 96 different SBP/DBP combinations. During the time period of the study, 238,640 patients died.
They identified a U-shaped curve when analyzing mortality with SBP and DBP separately, "with both lower and higher levels showing a substantial and statistically significant association" with mortality risk. Based on the adjusted hazard ratios for the combinations of SBP and DBP, the lowest mortality rates were associated with blood pressures of 130-139/90-99 mm Hg, and 130-159/70-89 mm Hg, adjusted for factors that included age, sex, race, diabetes, and cardiovascular and cerebrovascular disease, age and medication use).
But combinations of lower SBP and DBP values "were associated with relatively lower mortality rates only if the lower DBP component was greater than approximately 70 mm Hg," they said.
When evaluating risk based on JNC 7 (Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure) categories,they found that that those with stage 1 hypertension (SBP of 140-159 mm Hg or DBP of 90-99 mm Hg) were associated with the lowest mortality rates, while those in the normal category (an SBP lower than 120 and a DBP below 80) had the highest mortality rates," results that were independent of confounding factors and were statistically significant.
The authors described an elevated SBP combined with a low DBP, which is common in CKD patients, as "an especially problematic BP pattern," they said, pointing out that 33% of the patients had an SBP greater than 140 mm Hg and a DBP less than 70 mm Hg at some point during the study period.
The study strengths included the large size and the representation of the U.S. veterans’ population, but the limitations included the mostly male population and the observational design of the study, so more studies are needed, the authors said. "Until such trials become available, low BP should be regarded as potentially deleterious in this patient population, and we suggest caution in lowering BP to less than what has been demonstrated as beneficial in randomized controlled trials," they concluded.
Dr. Kovesdy, professor of medicine at University of Tennessee Health Science Center, Memphis, disclosed having received grants from the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, and nonfinancial support from the Department of Veterans Affairs while the study was conducted. Four authors had no disclosures, and one author disclosed having received NIH grants during the study. The remaining two authors disclosed having received research grants or personal fees from different pharmaceutical companies.
FROM THE ANNALS OF INTERNAL MEDICINE
Major finding: The results – which include the finding that a diastolic blood pressure value below 70 mm Hg was associated with higher mortality in patients with chronic kidney failure who were not on dialysis – indicate that low blood pressure could be considered possibly harmful in this population.
Data source: A national cohort study of 651,749 U.S. veterans with non–dialysis dependent CKD evaluated the association of systolic and diastolic BP values, separately and combined, on mortality risk.
Disclosures: The study was funded by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, and U.S. Department of Veterans Affairs. The lead author disclosed having received grants from the NIH-NIDDK and nonfinancial support from the V.A. while the study was conducted. Four authors had no disclosures, and one author disclosed having received NIH grants during the study. The remaining two authors disclosed having received research grants or personal fees from different pharmaceutical companies.
Determining Renal Function: What Those Test Results Mean
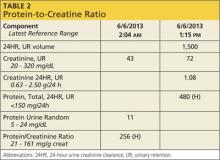
Q: Even though you suggested a random urine ACR (albumin-to-creatinine ratio), the internal medicine group ordered a 24-hour urine test for protein. As you can see from the results (see Table 2), the PCR (protein-to-creatinine ratio) is high. What does this mean? Does my patient have more severe kidney disease than I thought for her age?
Advanced age is a risk factor for CKD, and the patient has also had weight loss that can affect her serum creatinine. Because of her femur fracture, she has likely been in pain and probably has been taking nephrotoxic analgesics, such as NSAIDs or a ketorolac injection, commonly given postoperatively.
The patient’s weight does not appear to be stable, and she may have a degree of malnutrition. Both malnutrition and reduced muscle mass are known to decrease serum creatinine, which can mask worsening kidney disease. Thus she may have a lower true GFR than predicted by CG, which tends to overestimate renal function in the case of lower levels of creatinine production.6
Looking at all of these factors, it is likely that she has some degree of renal disease; however, it is important to determine if this is an acute change or a chronic issue. Looking closely at the higher-than-normal urinary protein result requires some out-of-the-box thinking.
Proteinuria has four types; each indicates a particular disorder.5 Table 3 provides examples of causative factors for each type.
Based on the data provided (Table 2), you have a high urinary protein result and are unsure if it is albumin. It is important to determine if this is albumin—and therefore pathognomonic for progressive kidney disease—or if the protein is of a nonalbumin type that will require further evaluation. What started as just an elderly female with a femur fracture and decreased GFR can turn into a diagnosis of multiple myeloma (which is more common in this age-group), kidney damage from postoperative medications, or another form of kidney disease. Only by looking at urinary protein type can one “tease out” what this might be.
In conclusion, there are many different ways to determine renal function, either by creatinine clearance or by using an estimation formula. Each one, used correctly, can offer advantages in certain populations. It is extremely important to determine whether an individual has diminished kidney function in order to be able to delay the progression of CKD.
Catherine B. York, MSN, APRN-BC
Springfield Nephrology
Associates, Springfield, MO
References
1. CDC. National chronic kidney disease fact sheet: general information and national estimates on chronic kidney disease in the United States, 2010. Atlanta, GA: US Department of Health and Human Services, CDC; 2010.
2. US Renal Data System. USRDS 2012 annual data report: atlas of end-stage renal disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2012.
3. US Renal Data System. USRDS 2012 annual data report: atlas of end-stage renal disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2011.
4. Clarkson MR, Brenner BM. Clinical assessment of the patient with kidney disease. In: Clarkson MR, Brenner BM. Pocket Companion to Brenner & Rector’s The Kidney. 7th ed. Philadelphia, PA: Elsevier Saunders; 2005: 3-19.5.
5. Hsu C. Clinical evaluation of kidney function. In: Greenberg A, Cheung A, Coffman T, et al, eds. Primer on Kidney Diseases, 5th ed. Philadelphia, PA; Saunders Elsevier; 2009:19-237.
6. Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1-150.
7. National Kidney Foundation. Guideline 5: assessment of proteinuria. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification; 2000.
8. Stevens LA, Coresh J, Greene T, et al. Assessing kidney function-measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473-2483.
Q: Even though you suggested a random urine ACR (albumin-to-creatinine ratio), the internal medicine group ordered a 24-hour urine test for protein. As you can see from the results (see Table 2), the PCR (protein-to-creatinine ratio) is high. What does this mean? Does my patient have more severe kidney disease than I thought for her age?
Advanced age is a risk factor for CKD, and the patient has also had weight loss that can affect her serum creatinine. Because of her femur fracture, she has likely been in pain and probably has been taking nephrotoxic analgesics, such as NSAIDs or a ketorolac injection, commonly given postoperatively.
The patient’s weight does not appear to be stable, and she may have a degree of malnutrition. Both malnutrition and reduced muscle mass are known to decrease serum creatinine, which can mask worsening kidney disease. Thus she may have a lower true GFR than predicted by CG, which tends to overestimate renal function in the case of lower levels of creatinine production.6
Looking at all of these factors, it is likely that she has some degree of renal disease; however, it is important to determine if this is an acute change or a chronic issue. Looking closely at the higher-than-normal urinary protein result requires some out-of-the-box thinking.
Proteinuria has four types; each indicates a particular disorder.5 Table 3 provides examples of causative factors for each type.
Based on the data provided (Table 2), you have a high urinary protein result and are unsure if it is albumin. It is important to determine if this is albumin—and therefore pathognomonic for progressive kidney disease—or if the protein is of a nonalbumin type that will require further evaluation. What started as just an elderly female with a femur fracture and decreased GFR can turn into a diagnosis of multiple myeloma (which is more common in this age-group), kidney damage from postoperative medications, or another form of kidney disease. Only by looking at urinary protein type can one “tease out” what this might be.
In conclusion, there are many different ways to determine renal function, either by creatinine clearance or by using an estimation formula. Each one, used correctly, can offer advantages in certain populations. It is extremely important to determine whether an individual has diminished kidney function in order to be able to delay the progression of CKD.
Catherine B. York, MSN, APRN-BC
Springfield Nephrology
Associates, Springfield, MO
References
1. CDC. National chronic kidney disease fact sheet: general information and national estimates on chronic kidney disease in the United States, 2010. Atlanta, GA: US Department of Health and Human Services, CDC; 2010.
2. US Renal Data System. USRDS 2012 annual data report: atlas of end-stage renal disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2012.
3. US Renal Data System. USRDS 2012 annual data report: atlas of end-stage renal disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2011.
4. Clarkson MR, Brenner BM. Clinical assessment of the patient with kidney disease. In: Clarkson MR, Brenner BM. Pocket Companion to Brenner & Rector’s The Kidney. 7th ed. Philadelphia, PA: Elsevier Saunders; 2005: 3-19.5.
5. Hsu C. Clinical evaluation of kidney function. In: Greenberg A, Cheung A, Coffman T, et al, eds. Primer on Kidney Diseases, 5th ed. Philadelphia, PA; Saunders Elsevier; 2009:19-237.
6. Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1-150.
7. National Kidney Foundation. Guideline 5: assessment of proteinuria. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification; 2000.
8. Stevens LA, Coresh J, Greene T, et al. Assessing kidney function-measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473-2483.
Q: Even though you suggested a random urine ACR (albumin-to-creatinine ratio), the internal medicine group ordered a 24-hour urine test for protein. As you can see from the results (see Table 2), the PCR (protein-to-creatinine ratio) is high. What does this mean? Does my patient have more severe kidney disease than I thought for her age?
Advanced age is a risk factor for CKD, and the patient has also had weight loss that can affect her serum creatinine. Because of her femur fracture, she has likely been in pain and probably has been taking nephrotoxic analgesics, such as NSAIDs or a ketorolac injection, commonly given postoperatively.
The patient’s weight does not appear to be stable, and she may have a degree of malnutrition. Both malnutrition and reduced muscle mass are known to decrease serum creatinine, which can mask worsening kidney disease. Thus she may have a lower true GFR than predicted by CG, which tends to overestimate renal function in the case of lower levels of creatinine production.6
Looking at all of these factors, it is likely that she has some degree of renal disease; however, it is important to determine if this is an acute change or a chronic issue. Looking closely at the higher-than-normal urinary protein result requires some out-of-the-box thinking.
Proteinuria has four types; each indicates a particular disorder.5 Table 3 provides examples of causative factors for each type.
Based on the data provided (Table 2), you have a high urinary protein result and are unsure if it is albumin. It is important to determine if this is albumin—and therefore pathognomonic for progressive kidney disease—or if the protein is of a nonalbumin type that will require further evaluation. What started as just an elderly female with a femur fracture and decreased GFR can turn into a diagnosis of multiple myeloma (which is more common in this age-group), kidney damage from postoperative medications, or another form of kidney disease. Only by looking at urinary protein type can one “tease out” what this might be.
In conclusion, there are many different ways to determine renal function, either by creatinine clearance or by using an estimation formula. Each one, used correctly, can offer advantages in certain populations. It is extremely important to determine whether an individual has diminished kidney function in order to be able to delay the progression of CKD.
Catherine B. York, MSN, APRN-BC
Springfield Nephrology
Associates, Springfield, MO
References
1. CDC. National chronic kidney disease fact sheet: general information and national estimates on chronic kidney disease in the United States, 2010. Atlanta, GA: US Department of Health and Human Services, CDC; 2010.
2. US Renal Data System. USRDS 2012 annual data report: atlas of end-stage renal disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2012.
3. US Renal Data System. USRDS 2012 annual data report: atlas of end-stage renal disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2011.
4. Clarkson MR, Brenner BM. Clinical assessment of the patient with kidney disease. In: Clarkson MR, Brenner BM. Pocket Companion to Brenner & Rector’s The Kidney. 7th ed. Philadelphia, PA: Elsevier Saunders; 2005: 3-19.5.
5. Hsu C. Clinical evaluation of kidney function. In: Greenberg A, Cheung A, Coffman T, et al, eds. Primer on Kidney Diseases, 5th ed. Philadelphia, PA; Saunders Elsevier; 2009:19-237.
6. Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1-150.
7. National Kidney Foundation. Guideline 5: assessment of proteinuria. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification; 2000.
8. Stevens LA, Coresh J, Greene T, et al. Assessing kidney function-measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473-2483.
Determining Renal Function: What’s the Best Way to Evaluate?
Q: One of my patients is a 72-year-old woman who weighs 59 kg. Her creatinine clearance by Cockcroft-Gault (CG) came back low (49 mL/min). Is this due to her age, gender, and weight loss during the past five months (subsequent to a femur fracture), or does she have underlying kidney disease? Would a 24-hour urine creatinine test be the best way to determine her level of kidney function—and would it be appropriate for someone her age? Is there a better way to evaluate her kidney function?
Accurate measurement of renal function is vital for any patient suspected of having chronic kidney disease (CKD). More than 20 million adults in the United States, or more than 10% of the adult population, have CKD.1 The 2012 US Renal Data System (USRDS) Annual Data Report states that the prevalence of chronic kidney disease in the Medicare population alone rose more than three-fold between 2000 and 2010, from 2.7% to 9.2%.2
CKD consumes a large proportion of Medicare dollars: more than $23,000 per person per year (PPPY) annually. For end-stage renal disease (ESRD) patients on hemodialysis, the cost is an astounding $88,000 PPPY.2 The cost of treating 871,000 ESRD patients was more than $40 billion in both public and private funds in 2009.3
Risk factors for CKD include but are not limited to: advancing age, male sex, race, hypertension, diabetes mellitus, smoking, family history of kidney disease, proteinuria, exposure to nephrotoxins, and atherosclerosis.4
In the US, the most common methods used to estimate renal function are the CG (Cockcroft-Gault) equation, Modification of Diet in Renal Disease (MDRD) study equations, and the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation. It is often difficult to determine which test is best suited for a patient, because there are pros and cons to each formula and no one test is perfectly suited for every clinical application.4
Since we know this patient’s renal function is low via CG (49 mL/min), the next important question to ask is, “Is it progressive?” I would recommend obtaining a urinalysis to look for hematuria and albuminuria. Proteinuria is an all-encompassing term. Albumin is only one type of protein and is the single most predictive risk factor for kidney disease progression. Persistent albuminuria alone is diagnostic of renal disease.5 The recommended test is a random urine albumin-to-creatinine ratio (ACR; see Table 1).6
You asked if a 24-hour urine creatinine clearance might evaluate her renal function better. Creatinine clearance can be determined by a 24-hour urine test and a serum blood sample in a steady state. However, this test should be interpreted with caution due to both collection errors and the fact that creatinine clearance overestimates true glomerular filtration rate (GFR) due to tubular secretion of creatinine.7,8 Thus, this test is no longer routinely recommended to determine kidney function.8
Catherine B. York, MSN, APRN-BC
Springfield Nephrology
Associates, Springfield, MO
References
1. CDC. National chronic kidney disease fact sheet: general information and national estimates on chronic kidney disease in the United States, 2010. Atlanta, GA: US Department of Health and Human Services, CDC; 2010.
2. US Renal Data System. USRDS 2012 annual data report: atlas of end-stage renal disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2012.
3. US Renal Data System. USRDS 2012 annual data report: atlas of end-stage renal disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2011.
4. Clarkson MR, Brenner BM. Clinical assessment of the patient with kidney disease. In: Clarkson MR, Brenner BM. Pocket Companion to Brenner & Rector’s The Kidney. 7th ed. Philadelphia, PA: Elsevier Saunders; 2005: 3-19.5.
5. Hsu C. Clinical evaluation of kidney function. In: Greenberg A, Cheung A, Coffman T, et al, eds. Primer on Kidney Diseases, 5th ed. Philadelphia, PA; Saunders Elsevier; 2009:19-237.
6. Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1-150.
7. National Kidney Foundation. Guideline 5: assessment of proteinuria. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification; 2000.
8. Stevens LA, Coresh J, Greene T, et al. Assessing kidney function-measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473-2483.
Q: One of my patients is a 72-year-old woman who weighs 59 kg. Her creatinine clearance by Cockcroft-Gault (CG) came back low (49 mL/min). Is this due to her age, gender, and weight loss during the past five months (subsequent to a femur fracture), or does she have underlying kidney disease? Would a 24-hour urine creatinine test be the best way to determine her level of kidney function—and would it be appropriate for someone her age? Is there a better way to evaluate her kidney function?
Accurate measurement of renal function is vital for any patient suspected of having chronic kidney disease (CKD). More than 20 million adults in the United States, or more than 10% of the adult population, have CKD.1 The 2012 US Renal Data System (USRDS) Annual Data Report states that the prevalence of chronic kidney disease in the Medicare population alone rose more than three-fold between 2000 and 2010, from 2.7% to 9.2%.2
CKD consumes a large proportion of Medicare dollars: more than $23,000 per person per year (PPPY) annually. For end-stage renal disease (ESRD) patients on hemodialysis, the cost is an astounding $88,000 PPPY.2 The cost of treating 871,000 ESRD patients was more than $40 billion in both public and private funds in 2009.3
Risk factors for CKD include but are not limited to: advancing age, male sex, race, hypertension, diabetes mellitus, smoking, family history of kidney disease, proteinuria, exposure to nephrotoxins, and atherosclerosis.4
In the US, the most common methods used to estimate renal function are the CG (Cockcroft-Gault) equation, Modification of Diet in Renal Disease (MDRD) study equations, and the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation. It is often difficult to determine which test is best suited for a patient, because there are pros and cons to each formula and no one test is perfectly suited for every clinical application.4
Since we know this patient’s renal function is low via CG (49 mL/min), the next important question to ask is, “Is it progressive?” I would recommend obtaining a urinalysis to look for hematuria and albuminuria. Proteinuria is an all-encompassing term. Albumin is only one type of protein and is the single most predictive risk factor for kidney disease progression. Persistent albuminuria alone is diagnostic of renal disease.5 The recommended test is a random urine albumin-to-creatinine ratio (ACR; see Table 1).6
You asked if a 24-hour urine creatinine clearance might evaluate her renal function better. Creatinine clearance can be determined by a 24-hour urine test and a serum blood sample in a steady state. However, this test should be interpreted with caution due to both collection errors and the fact that creatinine clearance overestimates true glomerular filtration rate (GFR) due to tubular secretion of creatinine.7,8 Thus, this test is no longer routinely recommended to determine kidney function.8
Catherine B. York, MSN, APRN-BC
Springfield Nephrology
Associates, Springfield, MO
References
1. CDC. National chronic kidney disease fact sheet: general information and national estimates on chronic kidney disease in the United States, 2010. Atlanta, GA: US Department of Health and Human Services, CDC; 2010.
2. US Renal Data System. USRDS 2012 annual data report: atlas of end-stage renal disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2012.
3. US Renal Data System. USRDS 2012 annual data report: atlas of end-stage renal disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2011.
4. Clarkson MR, Brenner BM. Clinical assessment of the patient with kidney disease. In: Clarkson MR, Brenner BM. Pocket Companion to Brenner & Rector’s The Kidney. 7th ed. Philadelphia, PA: Elsevier Saunders; 2005: 3-19.5.
5. Hsu C. Clinical evaluation of kidney function. In: Greenberg A, Cheung A, Coffman T, et al, eds. Primer on Kidney Diseases, 5th ed. Philadelphia, PA; Saunders Elsevier; 2009:19-237.
6. Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1-150.
7. National Kidney Foundation. Guideline 5: assessment of proteinuria. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification; 2000.
8. Stevens LA, Coresh J, Greene T, et al. Assessing kidney function-measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473-2483.
Q: One of my patients is a 72-year-old woman who weighs 59 kg. Her creatinine clearance by Cockcroft-Gault (CG) came back low (49 mL/min). Is this due to her age, gender, and weight loss during the past five months (subsequent to a femur fracture), or does she have underlying kidney disease? Would a 24-hour urine creatinine test be the best way to determine her level of kidney function—and would it be appropriate for someone her age? Is there a better way to evaluate her kidney function?
Accurate measurement of renal function is vital for any patient suspected of having chronic kidney disease (CKD). More than 20 million adults in the United States, or more than 10% of the adult population, have CKD.1 The 2012 US Renal Data System (USRDS) Annual Data Report states that the prevalence of chronic kidney disease in the Medicare population alone rose more than three-fold between 2000 and 2010, from 2.7% to 9.2%.2
CKD consumes a large proportion of Medicare dollars: more than $23,000 per person per year (PPPY) annually. For end-stage renal disease (ESRD) patients on hemodialysis, the cost is an astounding $88,000 PPPY.2 The cost of treating 871,000 ESRD patients was more than $40 billion in both public and private funds in 2009.3
Risk factors for CKD include but are not limited to: advancing age, male sex, race, hypertension, diabetes mellitus, smoking, family history of kidney disease, proteinuria, exposure to nephrotoxins, and atherosclerosis.4
In the US, the most common methods used to estimate renal function are the CG (Cockcroft-Gault) equation, Modification of Diet in Renal Disease (MDRD) study equations, and the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation. It is often difficult to determine which test is best suited for a patient, because there are pros and cons to each formula and no one test is perfectly suited for every clinical application.4
Since we know this patient’s renal function is low via CG (49 mL/min), the next important question to ask is, “Is it progressive?” I would recommend obtaining a urinalysis to look for hematuria and albuminuria. Proteinuria is an all-encompassing term. Albumin is only one type of protein and is the single most predictive risk factor for kidney disease progression. Persistent albuminuria alone is diagnostic of renal disease.5 The recommended test is a random urine albumin-to-creatinine ratio (ACR; see Table 1).6
You asked if a 24-hour urine creatinine clearance might evaluate her renal function better. Creatinine clearance can be determined by a 24-hour urine test and a serum blood sample in a steady state. However, this test should be interpreted with caution due to both collection errors and the fact that creatinine clearance overestimates true glomerular filtration rate (GFR) due to tubular secretion of creatinine.7,8 Thus, this test is no longer routinely recommended to determine kidney function.8
Catherine B. York, MSN, APRN-BC
Springfield Nephrology
Associates, Springfield, MO
References
1. CDC. National chronic kidney disease fact sheet: general information and national estimates on chronic kidney disease in the United States, 2010. Atlanta, GA: US Department of Health and Human Services, CDC; 2010.
2. US Renal Data System. USRDS 2012 annual data report: atlas of end-stage renal disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2012.
3. US Renal Data System. USRDS 2012 annual data report: atlas of end-stage renal disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2011.
4. Clarkson MR, Brenner BM. Clinical assessment of the patient with kidney disease. In: Clarkson MR, Brenner BM. Pocket Companion to Brenner & Rector’s The Kidney. 7th ed. Philadelphia, PA: Elsevier Saunders; 2005: 3-19.5.
5. Hsu C. Clinical evaluation of kidney function. In: Greenberg A, Cheung A, Coffman T, et al, eds. Primer on Kidney Diseases, 5th ed. Philadelphia, PA; Saunders Elsevier; 2009:19-237.
6. Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1-150.
7. National Kidney Foundation. Guideline 5: assessment of proteinuria. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification; 2000.
8. Stevens LA, Coresh J, Greene T, et al. Assessing kidney function-measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473-2483.
Fracture risk varied by renal function equations
SAN FRANCISCO – Assessing renal health using a modified Cockroft-Gault equation to measure creatinine clearance was more sensitive than using the Modification of Diet in Renal Disease equation to estimate glomerular filtration rate when estimating risk for osteoporosis and fracture, an award-winning study of 400 postmenopausal Puerto Rican women showed.
The study found a high prevalence of mild renal dysfunction (stage 2 chronic kidney disease) in 54%-59% of women, depending on the equation used. With the Cockroft-Gault equation adjusted for body surface area, a determination of mild renal dysfunction was associated with significantly decreased bone mineral density and with a doubling in risk for vertebral or nonvertebral fractures. When the Modification of Diet in Renal Disease (MDRD) equation was used, however, no significant associations were found between mild renal dysfunction and fracture risk, Dr. Loida A. González-Rodriguez reported.
Previous data have shown that severe renal dysfunction is associated with reduced bone mineral density and fractures, and that a creatinine clearance below 65 mL/min per 1.73 m2 is associated with a higher risk for falls and hip fractures in elderly people. Less is known about the effects of mild renal dysfunction on bone mineral density.
"We are postulating that this Cockroft-Gault equation is better to estimate bone," because it includes factors such as weight and age, and is adjusted for body surface area, Dr. González-Rodriguez said in an interview at the annual meeting of the Endocrine Society. She received an award at the meeting for her retrospective secondary analysis of data from the Latin American Vertebral Osteoporosis Study, the first population-based study of vertebral fractures in Latin America.
Many clinicians use the MDRD equation to estimate renal function. Dr. González-Rodriguez of the University of Puerto Rico, San Juan, said she has switched to using the Cockroft-Gault equation, and is trying to get colleagues at her institution to do the same. The MDRD equation will miss some patients who are at risk for osteopenia, osteoporosis, and fracture, she said.
Seventeen percent of patients in the study had normal bone mineral density, 43% had osteopenia, and 41% had osteoporosis. (Percentages were rounded and so exceed 100%.)
When the Cockroft-Gault equation was used to categorize renal function, 9% of patients had stage 1 chronic kidney disease, 54% had stage 2, 35% had stage 3, and 2% had stage 4. When the MDRD equation was used, 2% of patients had stage 1 chronic kidney disease, 59% had stage 2, 38% had stage 3, and 1% had stage 4.
Among patients with stage 2 chronic kidney disease as assessed by the Cockroft-Gault equation, 19% had normal bone mineral density, 49% had osteopenia, and 32% had osteoporosis. Among patients with stage 2 disease assessed using the MDRD equation, 4% had normal bone mineral density, 35% had osteopenia, and 60% had osteoporosis.
Vertebral fractures occurred in 9% and nonvertebral fractures occurred in 18% of patients with stage 2 disease assessed with the Cockroft-Gault equation. When the MDRD equation was used, 9% of patients with stage 2 disease developed vertebral fractures and 24% developed nonvertebral fractures.
Among patients with stage 3 chronic kidney disease assessed using the Cockroft-Gault equation, 18% developed vertebral fractures and 31% developed nonvertebral fractures, compared with vertebral fractures in 16% and nonvertebral fractures in 22% of patients with stage 3 disease assessed using the MDRD equation.
"One of the most important risk factors for vertebral and nonvertebral fractures is osteoporosis," Dr. González-Rodriguez noted. "So, if we can identify earlier the patients that have mild renal dysfunction" using the Cockroft-Gault equation and manage the osteoporosis risk, some fractures may be prevented.
The findings are limited by the retrospective design of the study, a lack of blood pressure measurements to assess arterial hypertension, self-reported nonvertebral fractures, and a lack of measurements of intact parathyroid hormone, 25-hydroxyvitamin D, and microalbuminuria.
Dr. González-Rodriguez reported having no relevant financial disclosures. The National Center for Research Resources and the National Institute on Minority Health and Health Disparities funded the study.
On Twitter @sherryboschert
SAN FRANCISCO – Assessing renal health using a modified Cockroft-Gault equation to measure creatinine clearance was more sensitive than using the Modification of Diet in Renal Disease equation to estimate glomerular filtration rate when estimating risk for osteoporosis and fracture, an award-winning study of 400 postmenopausal Puerto Rican women showed.
The study found a high prevalence of mild renal dysfunction (stage 2 chronic kidney disease) in 54%-59% of women, depending on the equation used. With the Cockroft-Gault equation adjusted for body surface area, a determination of mild renal dysfunction was associated with significantly decreased bone mineral density and with a doubling in risk for vertebral or nonvertebral fractures. When the Modification of Diet in Renal Disease (MDRD) equation was used, however, no significant associations were found between mild renal dysfunction and fracture risk, Dr. Loida A. González-Rodriguez reported.
Previous data have shown that severe renal dysfunction is associated with reduced bone mineral density and fractures, and that a creatinine clearance below 65 mL/min per 1.73 m2 is associated with a higher risk for falls and hip fractures in elderly people. Less is known about the effects of mild renal dysfunction on bone mineral density.
"We are postulating that this Cockroft-Gault equation is better to estimate bone," because it includes factors such as weight and age, and is adjusted for body surface area, Dr. González-Rodriguez said in an interview at the annual meeting of the Endocrine Society. She received an award at the meeting for her retrospective secondary analysis of data from the Latin American Vertebral Osteoporosis Study, the first population-based study of vertebral fractures in Latin America.
Many clinicians use the MDRD equation to estimate renal function. Dr. González-Rodriguez of the University of Puerto Rico, San Juan, said she has switched to using the Cockroft-Gault equation, and is trying to get colleagues at her institution to do the same. The MDRD equation will miss some patients who are at risk for osteopenia, osteoporosis, and fracture, she said.
Seventeen percent of patients in the study had normal bone mineral density, 43% had osteopenia, and 41% had osteoporosis. (Percentages were rounded and so exceed 100%.)
When the Cockroft-Gault equation was used to categorize renal function, 9% of patients had stage 1 chronic kidney disease, 54% had stage 2, 35% had stage 3, and 2% had stage 4. When the MDRD equation was used, 2% of patients had stage 1 chronic kidney disease, 59% had stage 2, 38% had stage 3, and 1% had stage 4.
Among patients with stage 2 chronic kidney disease as assessed by the Cockroft-Gault equation, 19% had normal bone mineral density, 49% had osteopenia, and 32% had osteoporosis. Among patients with stage 2 disease assessed using the MDRD equation, 4% had normal bone mineral density, 35% had osteopenia, and 60% had osteoporosis.
Vertebral fractures occurred in 9% and nonvertebral fractures occurred in 18% of patients with stage 2 disease assessed with the Cockroft-Gault equation. When the MDRD equation was used, 9% of patients with stage 2 disease developed vertebral fractures and 24% developed nonvertebral fractures.
Among patients with stage 3 chronic kidney disease assessed using the Cockroft-Gault equation, 18% developed vertebral fractures and 31% developed nonvertebral fractures, compared with vertebral fractures in 16% and nonvertebral fractures in 22% of patients with stage 3 disease assessed using the MDRD equation.
"One of the most important risk factors for vertebral and nonvertebral fractures is osteoporosis," Dr. González-Rodriguez noted. "So, if we can identify earlier the patients that have mild renal dysfunction" using the Cockroft-Gault equation and manage the osteoporosis risk, some fractures may be prevented.
The findings are limited by the retrospective design of the study, a lack of blood pressure measurements to assess arterial hypertension, self-reported nonvertebral fractures, and a lack of measurements of intact parathyroid hormone, 25-hydroxyvitamin D, and microalbuminuria.
Dr. González-Rodriguez reported having no relevant financial disclosures. The National Center for Research Resources and the National Institute on Minority Health and Health Disparities funded the study.
On Twitter @sherryboschert
SAN FRANCISCO – Assessing renal health using a modified Cockroft-Gault equation to measure creatinine clearance was more sensitive than using the Modification of Diet in Renal Disease equation to estimate glomerular filtration rate when estimating risk for osteoporosis and fracture, an award-winning study of 400 postmenopausal Puerto Rican women showed.
The study found a high prevalence of mild renal dysfunction (stage 2 chronic kidney disease) in 54%-59% of women, depending on the equation used. With the Cockroft-Gault equation adjusted for body surface area, a determination of mild renal dysfunction was associated with significantly decreased bone mineral density and with a doubling in risk for vertebral or nonvertebral fractures. When the Modification of Diet in Renal Disease (MDRD) equation was used, however, no significant associations were found between mild renal dysfunction and fracture risk, Dr. Loida A. González-Rodriguez reported.
Previous data have shown that severe renal dysfunction is associated with reduced bone mineral density and fractures, and that a creatinine clearance below 65 mL/min per 1.73 m2 is associated with a higher risk for falls and hip fractures in elderly people. Less is known about the effects of mild renal dysfunction on bone mineral density.
"We are postulating that this Cockroft-Gault equation is better to estimate bone," because it includes factors such as weight and age, and is adjusted for body surface area, Dr. González-Rodriguez said in an interview at the annual meeting of the Endocrine Society. She received an award at the meeting for her retrospective secondary analysis of data from the Latin American Vertebral Osteoporosis Study, the first population-based study of vertebral fractures in Latin America.
Many clinicians use the MDRD equation to estimate renal function. Dr. González-Rodriguez of the University of Puerto Rico, San Juan, said she has switched to using the Cockroft-Gault equation, and is trying to get colleagues at her institution to do the same. The MDRD equation will miss some patients who are at risk for osteopenia, osteoporosis, and fracture, she said.
Seventeen percent of patients in the study had normal bone mineral density, 43% had osteopenia, and 41% had osteoporosis. (Percentages were rounded and so exceed 100%.)
When the Cockroft-Gault equation was used to categorize renal function, 9% of patients had stage 1 chronic kidney disease, 54% had stage 2, 35% had stage 3, and 2% had stage 4. When the MDRD equation was used, 2% of patients had stage 1 chronic kidney disease, 59% had stage 2, 38% had stage 3, and 1% had stage 4.
Among patients with stage 2 chronic kidney disease as assessed by the Cockroft-Gault equation, 19% had normal bone mineral density, 49% had osteopenia, and 32% had osteoporosis. Among patients with stage 2 disease assessed using the MDRD equation, 4% had normal bone mineral density, 35% had osteopenia, and 60% had osteoporosis.
Vertebral fractures occurred in 9% and nonvertebral fractures occurred in 18% of patients with stage 2 disease assessed with the Cockroft-Gault equation. When the MDRD equation was used, 9% of patients with stage 2 disease developed vertebral fractures and 24% developed nonvertebral fractures.
Among patients with stage 3 chronic kidney disease assessed using the Cockroft-Gault equation, 18% developed vertebral fractures and 31% developed nonvertebral fractures, compared with vertebral fractures in 16% and nonvertebral fractures in 22% of patients with stage 3 disease assessed using the MDRD equation.
"One of the most important risk factors for vertebral and nonvertebral fractures is osteoporosis," Dr. González-Rodriguez noted. "So, if we can identify earlier the patients that have mild renal dysfunction" using the Cockroft-Gault equation and manage the osteoporosis risk, some fractures may be prevented.
The findings are limited by the retrospective design of the study, a lack of blood pressure measurements to assess arterial hypertension, self-reported nonvertebral fractures, and a lack of measurements of intact parathyroid hormone, 25-hydroxyvitamin D, and microalbuminuria.
Dr. González-Rodriguez reported having no relevant financial disclosures. The National Center for Research Resources and the National Institute on Minority Health and Health Disparities funded the study.
On Twitter @sherryboschert
AT ENDO 2013
Major finding: Fracture risk doubled in women with mild renal dysfunction as assessed by the Cockroft-Gault equation but not when renal function was assessed using the Modification of Diet in Renal Disease equation.
Data source: A retrospective secondary analysis of data on 400 postmenopausal Puerto Rican women.
Disclosures: Dr. Loida A. González-Rodriguez reported having no relevant financial disclosures. The National Center for Research Resources and the National Institute on Minority Health and Health Disparities funded the study.
Vascular testing appropriate use criteria cover 116 scenarios
Venous duplex ultrasound is rarely appropriate as a screening tool for upper or lower extremity deep vein thrombosis in the absence of pain or swelling, according to new appropriate use criteria for noninvasive vascular laboratory testing issued by the American College of Cardiology.
The clinical scenarios involving venous duplex ultrasound for DVT screening that were deemed rarely appropriate – such as screening in those with a prolonged ICU stay and those with high DVT risk – represent just a few of the 116 scenarios included in the report, which was developed in collaboration with 10 other leading professional societies to promote the most effective and most efficient use of peripheral vascular ultrasound and physiological testing in clinical practice.
The report, published online on July 19 in the Journal of the American College of Cardiology, is the second in a two-part series evaluating noninvasive testing for peripheral vascular disorders. Part I, published last year (J. Am. Coll. Cardiol. 2012;60:242-76), addressed peripheral arterial disorders, and Part II (J. Am. Coll. Cardiol. 2013 July 19 [doi:10.1016/j.jacc.2013.05.001]) addresses venous disease and evaluation of hemodialysis access, according to Dr. Heather Gornik, chair of the Part II writing committee.
"Vascular laboratory tests really play a central role in evaluating patients with peripheral vascular disorders. They are noninvasive, they have good accuracy data, and they don’t require radiation or dye. But we want to make sure the right tests are being ordered for the right reasons," Dr. Gornik, a cardiologist and vascular medicine specialist at the Cleveland Clinic, said in an interview.
Because these tests are low risk and easily accessible, there is concern that they are sometimes used excessively, she explained – specifically mentioning the use of duplex ultrasound for DVT screening as a commonly overused procedure.
"There is very little evidence, if any, to support broad screening for blood clots in someone who has no symptoms," she said.
The goal of the ACC Foundation Appropriate Use Criteria Task Force responsible for developing the criteria was to help clinicians minimize unnecessary testing, and maximize the most effective and efficient testing, she added.
Each of the clinical scenarios that were developed by the writing committee were rated by a technical panel as to whether they represent an "appropriate use," or whether they are "maybe appropriate" or "rarely appropriate."
The various scenarios are listed, along with their rating, in eight "at-a-glance" tables that address the following more general categories: venous duplex of the upper extremities for assessing patency and thrombosis; venous duplex of the lower extremities for assessing patency and thrombosis; duplex evaluation for venous incompetency; venous physiological testing with provocative maneuvers to assess for patency and/or incompetency; duplex of the inferior vena cava and iliac veins for patency and thrombosis; duplex of the hepatoportal system for patency, thrombosis, and flow direction; duplex of the renal vein for patency and thrombosis; and preoperative planning and postoperative assessment of a vascular access site.
Considering venous duplex ultrasound in a patient with acute unilateral limb swelling? Table 1 lists this as an appropriate use. How about duplex evaluation for venous incompetency in a patient with asymptomatic varicose veins? Table 3 says this may be appropriate, but notes that it is rarely appropriate in a patient with spider veins.
The report also covers indications for vascular testing prior to or after placement of hemodialysis access, because "evaluation of the superficial, deep, and central veins of the upper extremity constitutes a large component of these examinations," the report states.
In general, vascular studies were deemed appropriate in the presence of clinical signs and symptoms. The report also shows that the vascular laboratory plays a central role in the evaluation of patients with chronic venous insufficiency, and that preoperative vascular testing for preparing a dialysis access site is appropriate within three months of the procedure – but not for general surveillance of a functional dialysis fistula or graft in the absence of an indication of a problem, such as a palpable mass or swelling in the arm.
The report is not intended to be comprehensive, but rather is an attempt to address common and important clinical scenarios encountered in the patient with manifestations of peripheral vascular disease, the authors noted.
"The beauty of this report is that it spans many disciplines," Dr. Gornik said, noting that numerous parties have an interest in peripheral vascular disease, and that many specialties order vascular laboratory tests.
A number of them were represented in the development of these appropriate use criteria. Collaborating organizations included the American College of Radiology, the American Institute of Ultrasound in Medicine, the American Society of Echocardiography, the American Society of Nephrology, Intersocietal Accreditation Commission, Society for Cardiovascular Angiography and Interventions, the Society of Cardiovascular Computed Tomography, the Society for Interventional Radiology, the Society for Vascular Medicine, and the Society for Vascular Surgery.
While other organizations have developed appropriate use criteria for other modalities, such as cardiac testing, few have specifically addressed vascular testing.
"I hope that these criteria will allow clinicians and vascular laboratories to really focus on doing the highest quality work, and to evaluate their use of vascular testing, maximize the use of the vascular lab, and assure that the right test is done for the right indication and that tests that are not needed are not performed just because they are readily available," she said.
Dr. Gornik disclosed financial or other relationships with Zin Medical, Summit Doppler Systems Inc., the Fibromuscular Dysplasia Society of America, and the Intersocietal Accreditation Commission. A detailed list of disclosures for all Appropriate Use Criteria Task Force Members is included with the full text of the report.
Venous duplex ultrasound is rarely appropriate as a screening tool for upper or lower extremity deep vein thrombosis in the absence of pain or swelling, according to new appropriate use criteria for noninvasive vascular laboratory testing issued by the American College of Cardiology.
The clinical scenarios involving venous duplex ultrasound for DVT screening that were deemed rarely appropriate – such as screening in those with a prolonged ICU stay and those with high DVT risk – represent just a few of the 116 scenarios included in the report, which was developed in collaboration with 10 other leading professional societies to promote the most effective and most efficient use of peripheral vascular ultrasound and physiological testing in clinical practice.
The report, published online on July 19 in the Journal of the American College of Cardiology, is the second in a two-part series evaluating noninvasive testing for peripheral vascular disorders. Part I, published last year (J. Am. Coll. Cardiol. 2012;60:242-76), addressed peripheral arterial disorders, and Part II (J. Am. Coll. Cardiol. 2013 July 19 [doi:10.1016/j.jacc.2013.05.001]) addresses venous disease and evaluation of hemodialysis access, according to Dr. Heather Gornik, chair of the Part II writing committee.
"Vascular laboratory tests really play a central role in evaluating patients with peripheral vascular disorders. They are noninvasive, they have good accuracy data, and they don’t require radiation or dye. But we want to make sure the right tests are being ordered for the right reasons," Dr. Gornik, a cardiologist and vascular medicine specialist at the Cleveland Clinic, said in an interview.
Because these tests are low risk and easily accessible, there is concern that they are sometimes used excessively, she explained – specifically mentioning the use of duplex ultrasound for DVT screening as a commonly overused procedure.
"There is very little evidence, if any, to support broad screening for blood clots in someone who has no symptoms," she said.
The goal of the ACC Foundation Appropriate Use Criteria Task Force responsible for developing the criteria was to help clinicians minimize unnecessary testing, and maximize the most effective and efficient testing, she added.
Each of the clinical scenarios that were developed by the writing committee were rated by a technical panel as to whether they represent an "appropriate use," or whether they are "maybe appropriate" or "rarely appropriate."
The various scenarios are listed, along with their rating, in eight "at-a-glance" tables that address the following more general categories: venous duplex of the upper extremities for assessing patency and thrombosis; venous duplex of the lower extremities for assessing patency and thrombosis; duplex evaluation for venous incompetency; venous physiological testing with provocative maneuvers to assess for patency and/or incompetency; duplex of the inferior vena cava and iliac veins for patency and thrombosis; duplex of the hepatoportal system for patency, thrombosis, and flow direction; duplex of the renal vein for patency and thrombosis; and preoperative planning and postoperative assessment of a vascular access site.
Considering venous duplex ultrasound in a patient with acute unilateral limb swelling? Table 1 lists this as an appropriate use. How about duplex evaluation for venous incompetency in a patient with asymptomatic varicose veins? Table 3 says this may be appropriate, but notes that it is rarely appropriate in a patient with spider veins.
The report also covers indications for vascular testing prior to or after placement of hemodialysis access, because "evaluation of the superficial, deep, and central veins of the upper extremity constitutes a large component of these examinations," the report states.
In general, vascular studies were deemed appropriate in the presence of clinical signs and symptoms. The report also shows that the vascular laboratory plays a central role in the evaluation of patients with chronic venous insufficiency, and that preoperative vascular testing for preparing a dialysis access site is appropriate within three months of the procedure – but not for general surveillance of a functional dialysis fistula or graft in the absence of an indication of a problem, such as a palpable mass or swelling in the arm.
The report is not intended to be comprehensive, but rather is an attempt to address common and important clinical scenarios encountered in the patient with manifestations of peripheral vascular disease, the authors noted.
"The beauty of this report is that it spans many disciplines," Dr. Gornik said, noting that numerous parties have an interest in peripheral vascular disease, and that many specialties order vascular laboratory tests.
A number of them were represented in the development of these appropriate use criteria. Collaborating organizations included the American College of Radiology, the American Institute of Ultrasound in Medicine, the American Society of Echocardiography, the American Society of Nephrology, Intersocietal Accreditation Commission, Society for Cardiovascular Angiography and Interventions, the Society of Cardiovascular Computed Tomography, the Society for Interventional Radiology, the Society for Vascular Medicine, and the Society for Vascular Surgery.
While other organizations have developed appropriate use criteria for other modalities, such as cardiac testing, few have specifically addressed vascular testing.
"I hope that these criteria will allow clinicians and vascular laboratories to really focus on doing the highest quality work, and to evaluate their use of vascular testing, maximize the use of the vascular lab, and assure that the right test is done for the right indication and that tests that are not needed are not performed just because they are readily available," she said.
Dr. Gornik disclosed financial or other relationships with Zin Medical, Summit Doppler Systems Inc., the Fibromuscular Dysplasia Society of America, and the Intersocietal Accreditation Commission. A detailed list of disclosures for all Appropriate Use Criteria Task Force Members is included with the full text of the report.
Venous duplex ultrasound is rarely appropriate as a screening tool for upper or lower extremity deep vein thrombosis in the absence of pain or swelling, according to new appropriate use criteria for noninvasive vascular laboratory testing issued by the American College of Cardiology.
The clinical scenarios involving venous duplex ultrasound for DVT screening that were deemed rarely appropriate – such as screening in those with a prolonged ICU stay and those with high DVT risk – represent just a few of the 116 scenarios included in the report, which was developed in collaboration with 10 other leading professional societies to promote the most effective and most efficient use of peripheral vascular ultrasound and physiological testing in clinical practice.
The report, published online on July 19 in the Journal of the American College of Cardiology, is the second in a two-part series evaluating noninvasive testing for peripheral vascular disorders. Part I, published last year (J. Am. Coll. Cardiol. 2012;60:242-76), addressed peripheral arterial disorders, and Part II (J. Am. Coll. Cardiol. 2013 July 19 [doi:10.1016/j.jacc.2013.05.001]) addresses venous disease and evaluation of hemodialysis access, according to Dr. Heather Gornik, chair of the Part II writing committee.
"Vascular laboratory tests really play a central role in evaluating patients with peripheral vascular disorders. They are noninvasive, they have good accuracy data, and they don’t require radiation or dye. But we want to make sure the right tests are being ordered for the right reasons," Dr. Gornik, a cardiologist and vascular medicine specialist at the Cleveland Clinic, said in an interview.
Because these tests are low risk and easily accessible, there is concern that they are sometimes used excessively, she explained – specifically mentioning the use of duplex ultrasound for DVT screening as a commonly overused procedure.
"There is very little evidence, if any, to support broad screening for blood clots in someone who has no symptoms," she said.
The goal of the ACC Foundation Appropriate Use Criteria Task Force responsible for developing the criteria was to help clinicians minimize unnecessary testing, and maximize the most effective and efficient testing, she added.
Each of the clinical scenarios that were developed by the writing committee were rated by a technical panel as to whether they represent an "appropriate use," or whether they are "maybe appropriate" or "rarely appropriate."
The various scenarios are listed, along with their rating, in eight "at-a-glance" tables that address the following more general categories: venous duplex of the upper extremities for assessing patency and thrombosis; venous duplex of the lower extremities for assessing patency and thrombosis; duplex evaluation for venous incompetency; venous physiological testing with provocative maneuvers to assess for patency and/or incompetency; duplex of the inferior vena cava and iliac veins for patency and thrombosis; duplex of the hepatoportal system for patency, thrombosis, and flow direction; duplex of the renal vein for patency and thrombosis; and preoperative planning and postoperative assessment of a vascular access site.
Considering venous duplex ultrasound in a patient with acute unilateral limb swelling? Table 1 lists this as an appropriate use. How about duplex evaluation for venous incompetency in a patient with asymptomatic varicose veins? Table 3 says this may be appropriate, but notes that it is rarely appropriate in a patient with spider veins.
The report also covers indications for vascular testing prior to or after placement of hemodialysis access, because "evaluation of the superficial, deep, and central veins of the upper extremity constitutes a large component of these examinations," the report states.
In general, vascular studies were deemed appropriate in the presence of clinical signs and symptoms. The report also shows that the vascular laboratory plays a central role in the evaluation of patients with chronic venous insufficiency, and that preoperative vascular testing for preparing a dialysis access site is appropriate within three months of the procedure – but not for general surveillance of a functional dialysis fistula or graft in the absence of an indication of a problem, such as a palpable mass or swelling in the arm.
The report is not intended to be comprehensive, but rather is an attempt to address common and important clinical scenarios encountered in the patient with manifestations of peripheral vascular disease, the authors noted.
"The beauty of this report is that it spans many disciplines," Dr. Gornik said, noting that numerous parties have an interest in peripheral vascular disease, and that many specialties order vascular laboratory tests.
A number of them were represented in the development of these appropriate use criteria. Collaborating organizations included the American College of Radiology, the American Institute of Ultrasound in Medicine, the American Society of Echocardiography, the American Society of Nephrology, Intersocietal Accreditation Commission, Society for Cardiovascular Angiography and Interventions, the Society of Cardiovascular Computed Tomography, the Society for Interventional Radiology, the Society for Vascular Medicine, and the Society for Vascular Surgery.
While other organizations have developed appropriate use criteria for other modalities, such as cardiac testing, few have specifically addressed vascular testing.
"I hope that these criteria will allow clinicians and vascular laboratories to really focus on doing the highest quality work, and to evaluate their use of vascular testing, maximize the use of the vascular lab, and assure that the right test is done for the right indication and that tests that are not needed are not performed just because they are readily available," she said.
Dr. Gornik disclosed financial or other relationships with Zin Medical, Summit Doppler Systems Inc., the Fibromuscular Dysplasia Society of America, and the Intersocietal Accreditation Commission. A detailed list of disclosures for all Appropriate Use Criteria Task Force Members is included with the full text of the report.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Androgen deprivation therapy linked to acute kidney injury
Androgen deprivation therapy was strongly associated with an increased risk of acute kidney injury among men with nonmetastatic prostate cancer, according to a report in the July 17 issue of JAMA.
This elevation in risk varied slightly among different types of androgen deprivation agents, and was strongest with therapies that combine gonadotropin-releasing hormone agonists with oral antiandrogens. That suggests "a possible additive effect ... on both receptor antagonism and reduction of testosterone excretion," said Francesco Lapi, Pharm.D., Ph.D., of the Centre for Clinical Epidemiology, Jewish General Hospital, Montreal, and his associates (JAMA 2013;310:289-96).
The researchers discovered the risk elevation in what they described as the first population-based study to investigate the association between androgen deprivation therapy and acute kidney injury. They performed the study because even though the treatment traditionally has been reserved for advanced disease, it is now used increasingly in patients with earlier stages of prostate cancer.
In addition, the investigators were prompted to examine a possible link because of the high mortality (approximately 50%) associated with acute kidney injury.
"Although only one case report of flutamide-related acute kidney injury has been published to date, androgen deprivation therapy and its hypogonadal effect have well-known consequences consistent with our findings," they noted.
Dr. Lapi and his colleagues used two large databases in the United Kingdom, the Clinical Practice Research Datalink and the Hospital Episodes Statistics database, to identify 10,250 men newly diagnosed as having prostate cancer in 1998-2008 who were 40 years of age or older at diagnosis and were followed for a mean of 4 years. This yielded more than 42,000 person-years of follow-up.
A total of 232 cases of acute kidney injury occurred, for an overall incidence of 5.5/1,000 person-years, said Dr. Lapi and his associates.
These cases were matched for age, year of diagnosis, and duration of follow-up with 2,721 control subjects who did not develop acute kidney injury.
Compared with control subjects, men who were using androgen deprivation therapy had a significantly increased risk of acute kidney injury, with an odds ratio of 2.48. That association did not change when the data were adjusted to account for possible confounders, such as comorbidities known to impair kidney function, medications known to have renal toxicity, the severity of the underlying prostate cancer, and the intensity of other cancer treatments.
The investigators then analyzed the data according to type of androgen deprivation therapy, dividing the regimens into six mutually exclusive categories: gonadotropin-releasing hormone (GnRH) agonists (leuprolide, goserelin, triptorelin); oral antiandrogens (cyproterone acetate, flutamide, bicalutamide, nilutamide); combined androgen blockade (GnRH agonists plus oral antiandrogens); bilateral orchiectomy; estrogens; and combinations of those.
The odds ratios were highest for combined androgen blockade and also were significantly elevated for other combination therapies. Only the odd ratios for oral antiandrogens alone and for orchiectomy alone failed to reach statistical significance, although both were above 1.0, the investigators said.
The duration of androgen deprivation therapy was examined in a further analysis of the data. The risk of acute kidney injury was highest early in the course of treatment and decreased slightly, but it remained significantly elevated with longer duration of use.
Finally, in a sensitivity analysis that excluded the 54 cases and 842 controls who had abnormal creatinine levels at baseline, the results were consistent with those of the primary analysis.
The mechanism by which androgen deprivation therapy exerts an adverse effect on the kidney is not known, but the treatment is known to raise the risks of the metabolic syndrome and cardiovascular disease. "A similar rationale can be postulated for the risk of acute kidney injury," Dr. Lapi and his associates said.
The dyslipidemia and hyperglycemia of the metabolic syndrome may promote tubular atrophy and interstitial fibrosis, and may impair glomerular function by expanding and thickening the membranes of the interstitial tubules. Both dyslipidemia and hyperglycemia also raise the risk of thrombosis and induce oxidative stress, which can impact renal function.
In addition, testosterone is thought to protect the kidneys by inducing vasodilation in the renal vessels and enhancing nitric oxide production. So, antagonizing testosterone could promote damage to the glomerulus. And the hypogonadism induced by androgen deprivation can also lead to estrogen deficiency, reducing estrogen’s protective effect against ischemic renal injury, the investigators said.
The study was supported by Prostate Cancer Canada, the Canadian Institutes of Health Research, and Fonds de recherche en Sant
Androgen deprivation therapy was strongly associated with an increased risk of acute kidney injury among men with nonmetastatic prostate cancer, according to a report in the July 17 issue of JAMA.
This elevation in risk varied slightly among different types of androgen deprivation agents, and was strongest with therapies that combine gonadotropin-releasing hormone agonists with oral antiandrogens. That suggests "a possible additive effect ... on both receptor antagonism and reduction of testosterone excretion," said Francesco Lapi, Pharm.D., Ph.D., of the Centre for Clinical Epidemiology, Jewish General Hospital, Montreal, and his associates (JAMA 2013;310:289-96).
The researchers discovered the risk elevation in what they described as the first population-based study to investigate the association between androgen deprivation therapy and acute kidney injury. They performed the study because even though the treatment traditionally has been reserved for advanced disease, it is now used increasingly in patients with earlier stages of prostate cancer.
In addition, the investigators were prompted to examine a possible link because of the high mortality (approximately 50%) associated with acute kidney injury.
"Although only one case report of flutamide-related acute kidney injury has been published to date, androgen deprivation therapy and its hypogonadal effect have well-known consequences consistent with our findings," they noted.
Dr. Lapi and his colleagues used two large databases in the United Kingdom, the Clinical Practice Research Datalink and the Hospital Episodes Statistics database, to identify 10,250 men newly diagnosed as having prostate cancer in 1998-2008 who were 40 years of age or older at diagnosis and were followed for a mean of 4 years. This yielded more than 42,000 person-years of follow-up.
A total of 232 cases of acute kidney injury occurred, for an overall incidence of 5.5/1,000 person-years, said Dr. Lapi and his associates.
These cases were matched for age, year of diagnosis, and duration of follow-up with 2,721 control subjects who did not develop acute kidney injury.
Compared with control subjects, men who were using androgen deprivation therapy had a significantly increased risk of acute kidney injury, with an odds ratio of 2.48. That association did not change when the data were adjusted to account for possible confounders, such as comorbidities known to impair kidney function, medications known to have renal toxicity, the severity of the underlying prostate cancer, and the intensity of other cancer treatments.
The investigators then analyzed the data according to type of androgen deprivation therapy, dividing the regimens into six mutually exclusive categories: gonadotropin-releasing hormone (GnRH) agonists (leuprolide, goserelin, triptorelin); oral antiandrogens (cyproterone acetate, flutamide, bicalutamide, nilutamide); combined androgen blockade (GnRH agonists plus oral antiandrogens); bilateral orchiectomy; estrogens; and combinations of those.
The odds ratios were highest for combined androgen blockade and also were significantly elevated for other combination therapies. Only the odd ratios for oral antiandrogens alone and for orchiectomy alone failed to reach statistical significance, although both were above 1.0, the investigators said.
The duration of androgen deprivation therapy was examined in a further analysis of the data. The risk of acute kidney injury was highest early in the course of treatment and decreased slightly, but it remained significantly elevated with longer duration of use.
Finally, in a sensitivity analysis that excluded the 54 cases and 842 controls who had abnormal creatinine levels at baseline, the results were consistent with those of the primary analysis.
The mechanism by which androgen deprivation therapy exerts an adverse effect on the kidney is not known, but the treatment is known to raise the risks of the metabolic syndrome and cardiovascular disease. "A similar rationale can be postulated for the risk of acute kidney injury," Dr. Lapi and his associates said.
The dyslipidemia and hyperglycemia of the metabolic syndrome may promote tubular atrophy and interstitial fibrosis, and may impair glomerular function by expanding and thickening the membranes of the interstitial tubules. Both dyslipidemia and hyperglycemia also raise the risk of thrombosis and induce oxidative stress, which can impact renal function.
In addition, testosterone is thought to protect the kidneys by inducing vasodilation in the renal vessels and enhancing nitric oxide production. So, antagonizing testosterone could promote damage to the glomerulus. And the hypogonadism induced by androgen deprivation can also lead to estrogen deficiency, reducing estrogen’s protective effect against ischemic renal injury, the investigators said.
The study was supported by Prostate Cancer Canada, the Canadian Institutes of Health Research, and Fonds de recherche en Sant
Androgen deprivation therapy was strongly associated with an increased risk of acute kidney injury among men with nonmetastatic prostate cancer, according to a report in the July 17 issue of JAMA.
This elevation in risk varied slightly among different types of androgen deprivation agents, and was strongest with therapies that combine gonadotropin-releasing hormone agonists with oral antiandrogens. That suggests "a possible additive effect ... on both receptor antagonism and reduction of testosterone excretion," said Francesco Lapi, Pharm.D., Ph.D., of the Centre for Clinical Epidemiology, Jewish General Hospital, Montreal, and his associates (JAMA 2013;310:289-96).
The researchers discovered the risk elevation in what they described as the first population-based study to investigate the association between androgen deprivation therapy and acute kidney injury. They performed the study because even though the treatment traditionally has been reserved for advanced disease, it is now used increasingly in patients with earlier stages of prostate cancer.
In addition, the investigators were prompted to examine a possible link because of the high mortality (approximately 50%) associated with acute kidney injury.
"Although only one case report of flutamide-related acute kidney injury has been published to date, androgen deprivation therapy and its hypogonadal effect have well-known consequences consistent with our findings," they noted.
Dr. Lapi and his colleagues used two large databases in the United Kingdom, the Clinical Practice Research Datalink and the Hospital Episodes Statistics database, to identify 10,250 men newly diagnosed as having prostate cancer in 1998-2008 who were 40 years of age or older at diagnosis and were followed for a mean of 4 years. This yielded more than 42,000 person-years of follow-up.
A total of 232 cases of acute kidney injury occurred, for an overall incidence of 5.5/1,000 person-years, said Dr. Lapi and his associates.
These cases were matched for age, year of diagnosis, and duration of follow-up with 2,721 control subjects who did not develop acute kidney injury.
Compared with control subjects, men who were using androgen deprivation therapy had a significantly increased risk of acute kidney injury, with an odds ratio of 2.48. That association did not change when the data were adjusted to account for possible confounders, such as comorbidities known to impair kidney function, medications known to have renal toxicity, the severity of the underlying prostate cancer, and the intensity of other cancer treatments.
The investigators then analyzed the data according to type of androgen deprivation therapy, dividing the regimens into six mutually exclusive categories: gonadotropin-releasing hormone (GnRH) agonists (leuprolide, goserelin, triptorelin); oral antiandrogens (cyproterone acetate, flutamide, bicalutamide, nilutamide); combined androgen blockade (GnRH agonists plus oral antiandrogens); bilateral orchiectomy; estrogens; and combinations of those.
The odds ratios were highest for combined androgen blockade and also were significantly elevated for other combination therapies. Only the odd ratios for oral antiandrogens alone and for orchiectomy alone failed to reach statistical significance, although both were above 1.0, the investigators said.
The duration of androgen deprivation therapy was examined in a further analysis of the data. The risk of acute kidney injury was highest early in the course of treatment and decreased slightly, but it remained significantly elevated with longer duration of use.
Finally, in a sensitivity analysis that excluded the 54 cases and 842 controls who had abnormal creatinine levels at baseline, the results were consistent with those of the primary analysis.
The mechanism by which androgen deprivation therapy exerts an adverse effect on the kidney is not known, but the treatment is known to raise the risks of the metabolic syndrome and cardiovascular disease. "A similar rationale can be postulated for the risk of acute kidney injury," Dr. Lapi and his associates said.
The dyslipidemia and hyperglycemia of the metabolic syndrome may promote tubular atrophy and interstitial fibrosis, and may impair glomerular function by expanding and thickening the membranes of the interstitial tubules. Both dyslipidemia and hyperglycemia also raise the risk of thrombosis and induce oxidative stress, which can impact renal function.
In addition, testosterone is thought to protect the kidneys by inducing vasodilation in the renal vessels and enhancing nitric oxide production. So, antagonizing testosterone could promote damage to the glomerulus. And the hypogonadism induced by androgen deprivation can also lead to estrogen deficiency, reducing estrogen’s protective effect against ischemic renal injury, the investigators said.
The study was supported by Prostate Cancer Canada, the Canadian Institutes of Health Research, and Fonds de recherche en Sant
FROM JAMA
Major finding: Men with prostate cancer who used androgen deprivation therapy had a significantly increased risk of acute kidney injury, with an odds ratio of 2.48, compared with those who didn’t use the therapy.
Data Source: A population-based case-control study involving 10,250 men aged 40 years and older, newly diagnosed with nonmetastatic prostate cancer, who were followed for a mean of 4 years for the development of acute kidney injury.
Disclosures: The study was supported by Prostate Cancer Canada, the Canadian Institutes of Health Research, and Fonds de recherche en Sant
Urinary symptoms often unaddressed in MS
ORLANDO – Lower urinary tract symptoms in patients with multiple sclerosis are common, bothersome, and often undiscussed and untreated, according to a national patient survey.
The online survey was completed by a convenience sample of 1,052 MS patients recruited through the National Multiple Sclerosis Society and other patient advocacy organizations. Fully 88% of the respondents indicated they have lower urinary tract symptoms involving bladder dysfunction and urinary incontinence. The source of these common symptoms in patients with MS is increased contractile activity of the bladder’s detrusor muscle.
The most common lower urinary tract symptom reported by survey respondents was terminal dribble upon voiding, which affected 65% of patients. The next most common symptoms were urinary urgency, experienced by 62%, and incomplete emptying, cited by 61%, Kristin M. Khalaf, Pharm.D., reported at the fifth Cooperative Meeting of the Consortium of Multiple Sclerosis Centers and the Americas Committee for Treatment and Research in Multiple Sclerosis.
Fifty-three percent of patients reported having urgency urinary incontinence, and 45% complained of stress urinary incontinence.
The lower urinary tract symptom that patients found most bothersome was urgency; indeed, one-third of the overall study population indicated they were bothered "quite a bit" or "a great deal" by this problem. Twenty-nine percent of respondents stated they were bothered at least quite a bit by urgency incontinence, added Dr. Khalaf of Allergan, Irvine, Calif.
Only one-third of the 922 MS patients with lower urinary tract symptoms had discussed their symptoms with a health care provider during the past year. When they did speak with a professional, 74% of the time it was with their neurologist. Fifty-two percent spoke to their primary care physician about their problem within the last year.
Among the 42% of survey respondents who indicated they were bothered at least quite a bit by urinary incontinence, 46% hadn’t discussed the problem with a health care provider within the past year, and 35% had never received any form of treatment for it.
Among patients who had ever discussed their lower urinary tract symptoms with a physician or other health care professional, 38% reported currently treating their problem via pelvic exercises or bladder training, 23% were using an oral anticholinergic agent, 4% were taking herbal medicines for their symptoms, 3% were receiving botulinum toxin type A (onabotulinumtoxinA) injections, and 2% had a neural stimulation device.
Most patients currently receiving treatment for their lower urinary tract symptoms pronounced themselves very or somewhat satisfied with their therapy.
The survey was funded by Allergan.
ORLANDO – Lower urinary tract symptoms in patients with multiple sclerosis are common, bothersome, and often undiscussed and untreated, according to a national patient survey.
The online survey was completed by a convenience sample of 1,052 MS patients recruited through the National Multiple Sclerosis Society and other patient advocacy organizations. Fully 88% of the respondents indicated they have lower urinary tract symptoms involving bladder dysfunction and urinary incontinence. The source of these common symptoms in patients with MS is increased contractile activity of the bladder’s detrusor muscle.
The most common lower urinary tract symptom reported by survey respondents was terminal dribble upon voiding, which affected 65% of patients. The next most common symptoms were urinary urgency, experienced by 62%, and incomplete emptying, cited by 61%, Kristin M. Khalaf, Pharm.D., reported at the fifth Cooperative Meeting of the Consortium of Multiple Sclerosis Centers and the Americas Committee for Treatment and Research in Multiple Sclerosis.
Fifty-three percent of patients reported having urgency urinary incontinence, and 45% complained of stress urinary incontinence.
The lower urinary tract symptom that patients found most bothersome was urgency; indeed, one-third of the overall study population indicated they were bothered "quite a bit" or "a great deal" by this problem. Twenty-nine percent of respondents stated they were bothered at least quite a bit by urgency incontinence, added Dr. Khalaf of Allergan, Irvine, Calif.
Only one-third of the 922 MS patients with lower urinary tract symptoms had discussed their symptoms with a health care provider during the past year. When they did speak with a professional, 74% of the time it was with their neurologist. Fifty-two percent spoke to their primary care physician about their problem within the last year.
Among the 42% of survey respondents who indicated they were bothered at least quite a bit by urinary incontinence, 46% hadn’t discussed the problem with a health care provider within the past year, and 35% had never received any form of treatment for it.
Among patients who had ever discussed their lower urinary tract symptoms with a physician or other health care professional, 38% reported currently treating their problem via pelvic exercises or bladder training, 23% were using an oral anticholinergic agent, 4% were taking herbal medicines for their symptoms, 3% were receiving botulinum toxin type A (onabotulinumtoxinA) injections, and 2% had a neural stimulation device.
Most patients currently receiving treatment for their lower urinary tract symptoms pronounced themselves very or somewhat satisfied with their therapy.
The survey was funded by Allergan.
ORLANDO – Lower urinary tract symptoms in patients with multiple sclerosis are common, bothersome, and often undiscussed and untreated, according to a national patient survey.
The online survey was completed by a convenience sample of 1,052 MS patients recruited through the National Multiple Sclerosis Society and other patient advocacy organizations. Fully 88% of the respondents indicated they have lower urinary tract symptoms involving bladder dysfunction and urinary incontinence. The source of these common symptoms in patients with MS is increased contractile activity of the bladder’s detrusor muscle.
The most common lower urinary tract symptom reported by survey respondents was terminal dribble upon voiding, which affected 65% of patients. The next most common symptoms were urinary urgency, experienced by 62%, and incomplete emptying, cited by 61%, Kristin M. Khalaf, Pharm.D., reported at the fifth Cooperative Meeting of the Consortium of Multiple Sclerosis Centers and the Americas Committee for Treatment and Research in Multiple Sclerosis.
Fifty-three percent of patients reported having urgency urinary incontinence, and 45% complained of stress urinary incontinence.
The lower urinary tract symptom that patients found most bothersome was urgency; indeed, one-third of the overall study population indicated they were bothered "quite a bit" or "a great deal" by this problem. Twenty-nine percent of respondents stated they were bothered at least quite a bit by urgency incontinence, added Dr. Khalaf of Allergan, Irvine, Calif.
Only one-third of the 922 MS patients with lower urinary tract symptoms had discussed their symptoms with a health care provider during the past year. When they did speak with a professional, 74% of the time it was with their neurologist. Fifty-two percent spoke to their primary care physician about their problem within the last year.
Among the 42% of survey respondents who indicated they were bothered at least quite a bit by urinary incontinence, 46% hadn’t discussed the problem with a health care provider within the past year, and 35% had never received any form of treatment for it.
Among patients who had ever discussed their lower urinary tract symptoms with a physician or other health care professional, 38% reported currently treating their problem via pelvic exercises or bladder training, 23% were using an oral anticholinergic agent, 4% were taking herbal medicines for their symptoms, 3% were receiving botulinum toxin type A (onabotulinumtoxinA) injections, and 2% had a neural stimulation device.
Most patients currently receiving treatment for their lower urinary tract symptoms pronounced themselves very or somewhat satisfied with their therapy.
The survey was funded by Allergan.
AT THE CMSC/ACTRIMS ANNUAL MEETING
Major Finding: Eighty-eight percent of patients with multiple sclerosis who responded to an online survey indicated they experience lower urinary tract symptoms, but only one-third of those with such symptoms reported having discussed the matter with a physician or other health professional within the past year.
Data Source: The survey included 1,052 respondents, a convenience sample recruited from several patient advocacy organizations.
Disclosures: The study was sponsored by Allergan. The presenter is a company employee.
Prolaris test eyed as predictor of prostate cancer outcomes
CHICAGO – Prostate cancer outcomes were predicted by a test that measures the expression of cell cycle progression genes, according to results from a retrospective analysis of prostate tissue samples from five patient cohorts.
The Prolaris test gives each tissue sample a cell cycle progression (CCP) score based on measures of 31 CCP genes, normalized to 15 "housekeeper" genes. A unit change in the test is defined as a doubling in CCP genes. For each unit increase in the test’s score, there was a two- to threefold increase in the risk of disease progression, Dr. Jack M. Cuzick reported at the annual meeting of the American Society of Clinical Oncology.
The CCP signature of Myriad Genetics’ Prolaris test was a highly significant predictor of outcome, said Dr. Cuzick of the Wolfson Institute of Preventive Medicine, London. In all five studies, the hazard ratio per unit change in the CCP score was similar, ranging from 1.89 to 2.92. The findings indicate that the effect size for the CCP score is robust in multiple patient cohorts and diverse clinical settings.
The test provides information for differentiating aggressive and indolent disease beyond that available from clinicopathologic variables, he said. As the natural history of prostate cancer can be variable and difficult to predict, the Prolaris test could help to match treatment more appropriately to each individual’s risk of progression.
In the study that examined the test’s predictive value, five patient groups were evaluated. Formalin-fixed tissue samples were obtained from two English patient cohorts that were conservatively managed (n = 337 and 349), two U.S. patient cohorts that underwent radical prostatectomy (366 men treated at Scott & White Hospital, Temple, Tex.; and 413 men treated at the University of California, San Francisco), and one U.S. cohort that underwent external beam radiation therapy (141 men treated at the Durham, N.C., VA Medical Center).
The cohort of conservatively managed English patients was from the late 1990s and had more than 15 years of follow up. In the 337-patient cohort diagnosed via transurethral resection of the prostate (TURP) and conservatively managed, there were 57 deaths from prostate cancer. In the 349-patient cohort diagnosed via needle biopsy and conservatively managed, there were 90 deaths from prostate cancer.
For each unit increase in the CCP score, the hazard ratio for the cohort diagnosed via TURP was 2.9 and the hazard ratio for those diagnosed via needle biopsy was 2. The CCP score was the dominant variable for predicting death from prostate cancer in univariate analysis (P = 6.1 x 10–22 after diagnosis via TURP, and P = 8.6 x 10–10 after diagnosis via needle biopsy). In both studies, the CCP score remained highly significant in multivariate analysis and was a stronger predictor of disease-specific mortality than other prognostic variables, he said.
In the U.S. prostatectomy cohorts, there were 132 biochemical recurrences (BCRs) in the first cohort and 83 BCRs in the second cohort. With each unit increase in the CCP score, there was a doubling of risk for recurrence. After prostatectomy, the CCP score predicted BCR in univariate analysis (Scott & White: P = 5.6 x 10–9; University of California: P = 2.23 x 10–6) and provided additional prognostic information in multivariate analysis (Scott & White: P = 3.3 x 10–6; University of California: P = 9.5 x10–5).
After radiation therapy, the CCP score predicted BCR in univariate (P = .0017) and multivariate (P = .034) analysis. In the 141-patient cohort that was diagnosed by needle biopsy and underwent external beam radiation, there were 19 prostate cancer deaths and more than a doubling of risk with each unit increase in CCP score.
CCP scores only modestly correlated with the Gleason score and prostate-specific antigen (PSA) value. The test adds value beyond those measures, Dr. Cuzick said.
CCP scores predict patient outcome in multiple clinical settings, provide independent information beyond clinicopathological variables, and help to further differentiate aggressive from indolent prostate cancer. With low-grade Gleason 6 cancers, the results can aid in telling who is at low risk and who needs aggressive therapy, he concluded.
The study was funded by Myriad Genetics, the maker of the Prolaris test. Dr. Cuzick received honoraria and research support from Myriad.
The ability to improve clinical management by finding prostate cancer patients who would benefit from more – or less – therapy is much needed. Clinicians are concerned that many prostate cancer patients are now overtreated, but they lack reliable prognostic guides.
Cell cycle progression (CCP) scores are interesting retrospectively, but how much are they able to improve on CAPRA (Cancer of the Prostate Risk Assessment) scores for predicting prognosis?
Prostate cancer is uniquely multifocal, with most men having multiple independent foci of cancer. In the example of the conservatively managed patients, if one is looking at men with indolent disease who have low-volume disease as a single core of one or two foci, is one really going to be able to predict the biologic outcome of the cancer? Those who fail after a surveillance approach often do so early and had undersampling of their disease. So it hasn’t been proven yet that this test can predict the behavior of cancer that hasn’t been sampled.
The CCP results proved to be statistically significant, but that finding does not indicate clinical utility. It’s not known whether the novel biomarkers in this test improve on existing markers. You find yourself asking what you would do differently in a patient whose risk of progression goes from 7% to 12%.
Even if a test independently predicts outcome, that doesn’t necessarily indicate it has clinical utility. The ability to improve clinical management is key to the adoption of new prognostic tests. The real question is whether CCP results improve on the existing model. Does the test improve on CAPRA for prognosis?
Dr. Scott Tomlins is with the department of urology at the University of Michigan Health System, Ann Arbor. He was the invited discussant of the paper at the meeting. Dr. Tomlins disclosed that he is a consultant to and receives honoraria from Ventana Medical Systems/Roche. He has patents via the University of Michigan on several diagnostic genetic tests.
The ability to improve clinical management by finding prostate cancer patients who would benefit from more – or less – therapy is much needed. Clinicians are concerned that many prostate cancer patients are now overtreated, but they lack reliable prognostic guides.
Cell cycle progression (CCP) scores are interesting retrospectively, but how much are they able to improve on CAPRA (Cancer of the Prostate Risk Assessment) scores for predicting prognosis?
Prostate cancer is uniquely multifocal, with most men having multiple independent foci of cancer. In the example of the conservatively managed patients, if one is looking at men with indolent disease who have low-volume disease as a single core of one or two foci, is one really going to be able to predict the biologic outcome of the cancer? Those who fail after a surveillance approach often do so early and had undersampling of their disease. So it hasn’t been proven yet that this test can predict the behavior of cancer that hasn’t been sampled.
The CCP results proved to be statistically significant, but that finding does not indicate clinical utility. It’s not known whether the novel biomarkers in this test improve on existing markers. You find yourself asking what you would do differently in a patient whose risk of progression goes from 7% to 12%.
Even if a test independently predicts outcome, that doesn’t necessarily indicate it has clinical utility. The ability to improve clinical management is key to the adoption of new prognostic tests. The real question is whether CCP results improve on the existing model. Does the test improve on CAPRA for prognosis?
Dr. Scott Tomlins is with the department of urology at the University of Michigan Health System, Ann Arbor. He was the invited discussant of the paper at the meeting. Dr. Tomlins disclosed that he is a consultant to and receives honoraria from Ventana Medical Systems/Roche. He has patents via the University of Michigan on several diagnostic genetic tests.
The ability to improve clinical management by finding prostate cancer patients who would benefit from more – or less – therapy is much needed. Clinicians are concerned that many prostate cancer patients are now overtreated, but they lack reliable prognostic guides.
Cell cycle progression (CCP) scores are interesting retrospectively, but how much are they able to improve on CAPRA (Cancer of the Prostate Risk Assessment) scores for predicting prognosis?
Prostate cancer is uniquely multifocal, with most men having multiple independent foci of cancer. In the example of the conservatively managed patients, if one is looking at men with indolent disease who have low-volume disease as a single core of one or two foci, is one really going to be able to predict the biologic outcome of the cancer? Those who fail after a surveillance approach often do so early and had undersampling of their disease. So it hasn’t been proven yet that this test can predict the behavior of cancer that hasn’t been sampled.
The CCP results proved to be statistically significant, but that finding does not indicate clinical utility. It’s not known whether the novel biomarkers in this test improve on existing markers. You find yourself asking what you would do differently in a patient whose risk of progression goes from 7% to 12%.
Even if a test independently predicts outcome, that doesn’t necessarily indicate it has clinical utility. The ability to improve clinical management is key to the adoption of new prognostic tests. The real question is whether CCP results improve on the existing model. Does the test improve on CAPRA for prognosis?
Dr. Scott Tomlins is with the department of urology at the University of Michigan Health System, Ann Arbor. He was the invited discussant of the paper at the meeting. Dr. Tomlins disclosed that he is a consultant to and receives honoraria from Ventana Medical Systems/Roche. He has patents via the University of Michigan on several diagnostic genetic tests.
CHICAGO – Prostate cancer outcomes were predicted by a test that measures the expression of cell cycle progression genes, according to results from a retrospective analysis of prostate tissue samples from five patient cohorts.
The Prolaris test gives each tissue sample a cell cycle progression (CCP) score based on measures of 31 CCP genes, normalized to 15 "housekeeper" genes. A unit change in the test is defined as a doubling in CCP genes. For each unit increase in the test’s score, there was a two- to threefold increase in the risk of disease progression, Dr. Jack M. Cuzick reported at the annual meeting of the American Society of Clinical Oncology.
The CCP signature of Myriad Genetics’ Prolaris test was a highly significant predictor of outcome, said Dr. Cuzick of the Wolfson Institute of Preventive Medicine, London. In all five studies, the hazard ratio per unit change in the CCP score was similar, ranging from 1.89 to 2.92. The findings indicate that the effect size for the CCP score is robust in multiple patient cohorts and diverse clinical settings.
The test provides information for differentiating aggressive and indolent disease beyond that available from clinicopathologic variables, he said. As the natural history of prostate cancer can be variable and difficult to predict, the Prolaris test could help to match treatment more appropriately to each individual’s risk of progression.
In the study that examined the test’s predictive value, five patient groups were evaluated. Formalin-fixed tissue samples were obtained from two English patient cohorts that were conservatively managed (n = 337 and 349), two U.S. patient cohorts that underwent radical prostatectomy (366 men treated at Scott & White Hospital, Temple, Tex.; and 413 men treated at the University of California, San Francisco), and one U.S. cohort that underwent external beam radiation therapy (141 men treated at the Durham, N.C., VA Medical Center).
The cohort of conservatively managed English patients was from the late 1990s and had more than 15 years of follow up. In the 337-patient cohort diagnosed via transurethral resection of the prostate (TURP) and conservatively managed, there were 57 deaths from prostate cancer. In the 349-patient cohort diagnosed via needle biopsy and conservatively managed, there were 90 deaths from prostate cancer.
For each unit increase in the CCP score, the hazard ratio for the cohort diagnosed via TURP was 2.9 and the hazard ratio for those diagnosed via needle biopsy was 2. The CCP score was the dominant variable for predicting death from prostate cancer in univariate analysis (P = 6.1 x 10–22 after diagnosis via TURP, and P = 8.6 x 10–10 after diagnosis via needle biopsy). In both studies, the CCP score remained highly significant in multivariate analysis and was a stronger predictor of disease-specific mortality than other prognostic variables, he said.
In the U.S. prostatectomy cohorts, there were 132 biochemical recurrences (BCRs) in the first cohort and 83 BCRs in the second cohort. With each unit increase in the CCP score, there was a doubling of risk for recurrence. After prostatectomy, the CCP score predicted BCR in univariate analysis (Scott & White: P = 5.6 x 10–9; University of California: P = 2.23 x 10–6) and provided additional prognostic information in multivariate analysis (Scott & White: P = 3.3 x 10–6; University of California: P = 9.5 x10–5).
After radiation therapy, the CCP score predicted BCR in univariate (P = .0017) and multivariate (P = .034) analysis. In the 141-patient cohort that was diagnosed by needle biopsy and underwent external beam radiation, there were 19 prostate cancer deaths and more than a doubling of risk with each unit increase in CCP score.
CCP scores only modestly correlated with the Gleason score and prostate-specific antigen (PSA) value. The test adds value beyond those measures, Dr. Cuzick said.
CCP scores predict patient outcome in multiple clinical settings, provide independent information beyond clinicopathological variables, and help to further differentiate aggressive from indolent prostate cancer. With low-grade Gleason 6 cancers, the results can aid in telling who is at low risk and who needs aggressive therapy, he concluded.
The study was funded by Myriad Genetics, the maker of the Prolaris test. Dr. Cuzick received honoraria and research support from Myriad.
CHICAGO – Prostate cancer outcomes were predicted by a test that measures the expression of cell cycle progression genes, according to results from a retrospective analysis of prostate tissue samples from five patient cohorts.
The Prolaris test gives each tissue sample a cell cycle progression (CCP) score based on measures of 31 CCP genes, normalized to 15 "housekeeper" genes. A unit change in the test is defined as a doubling in CCP genes. For each unit increase in the test’s score, there was a two- to threefold increase in the risk of disease progression, Dr. Jack M. Cuzick reported at the annual meeting of the American Society of Clinical Oncology.
The CCP signature of Myriad Genetics’ Prolaris test was a highly significant predictor of outcome, said Dr. Cuzick of the Wolfson Institute of Preventive Medicine, London. In all five studies, the hazard ratio per unit change in the CCP score was similar, ranging from 1.89 to 2.92. The findings indicate that the effect size for the CCP score is robust in multiple patient cohorts and diverse clinical settings.
The test provides information for differentiating aggressive and indolent disease beyond that available from clinicopathologic variables, he said. As the natural history of prostate cancer can be variable and difficult to predict, the Prolaris test could help to match treatment more appropriately to each individual’s risk of progression.
In the study that examined the test’s predictive value, five patient groups were evaluated. Formalin-fixed tissue samples were obtained from two English patient cohorts that were conservatively managed (n = 337 and 349), two U.S. patient cohorts that underwent radical prostatectomy (366 men treated at Scott & White Hospital, Temple, Tex.; and 413 men treated at the University of California, San Francisco), and one U.S. cohort that underwent external beam radiation therapy (141 men treated at the Durham, N.C., VA Medical Center).
The cohort of conservatively managed English patients was from the late 1990s and had more than 15 years of follow up. In the 337-patient cohort diagnosed via transurethral resection of the prostate (TURP) and conservatively managed, there were 57 deaths from prostate cancer. In the 349-patient cohort diagnosed via needle biopsy and conservatively managed, there were 90 deaths from prostate cancer.
For each unit increase in the CCP score, the hazard ratio for the cohort diagnosed via TURP was 2.9 and the hazard ratio for those diagnosed via needle biopsy was 2. The CCP score was the dominant variable for predicting death from prostate cancer in univariate analysis (P = 6.1 x 10–22 after diagnosis via TURP, and P = 8.6 x 10–10 after diagnosis via needle biopsy). In both studies, the CCP score remained highly significant in multivariate analysis and was a stronger predictor of disease-specific mortality than other prognostic variables, he said.
In the U.S. prostatectomy cohorts, there were 132 biochemical recurrences (BCRs) in the first cohort and 83 BCRs in the second cohort. With each unit increase in the CCP score, there was a doubling of risk for recurrence. After prostatectomy, the CCP score predicted BCR in univariate analysis (Scott & White: P = 5.6 x 10–9; University of California: P = 2.23 x 10–6) and provided additional prognostic information in multivariate analysis (Scott & White: P = 3.3 x 10–6; University of California: P = 9.5 x10–5).
After radiation therapy, the CCP score predicted BCR in univariate (P = .0017) and multivariate (P = .034) analysis. In the 141-patient cohort that was diagnosed by needle biopsy and underwent external beam radiation, there were 19 prostate cancer deaths and more than a doubling of risk with each unit increase in CCP score.
CCP scores only modestly correlated with the Gleason score and prostate-specific antigen (PSA) value. The test adds value beyond those measures, Dr. Cuzick said.
CCP scores predict patient outcome in multiple clinical settings, provide independent information beyond clinicopathological variables, and help to further differentiate aggressive from indolent prostate cancer. With low-grade Gleason 6 cancers, the results can aid in telling who is at low risk and who needs aggressive therapy, he concluded.
The study was funded by Myriad Genetics, the maker of the Prolaris test. Dr. Cuzick received honoraria and research support from Myriad.
AT THE ASCO ANNUAL MEETING 2013
Major finding: In conservatively managed prostate cancer patients, the cell cycle progression score in tissue samples was the dominant variable for predicting death from prostate cancer in univariate analysis (P = 6.1 x 10–22 after diagnosis via TURP, and P = 8.6 x 10–10 after diagnosis via needle biopsy).
Data source: A retrospective study of tissue samples from more than 1,600 patients in five patient cohorts who were either managed conservatively, underwent prostatectomy, or received external beam radiotherapy.
Disclosures: The study was funded by Myriad Genetics, the maker of the Prolaris test. Dr. Cuzick received honoraria and research support from Myriad.
Why Take This Patient Off Her ACEI?
Q: I sent a 68-year-old, hypertensive, diabetic woman with stage 4 kidney disease (estimated glomerular filtration rate [eGFR], 25 mL/min/1.73 m2; serum creatinine [SCr], 2 mg/mL) to a local nephrology group. Since she was diabetic, we had had her taking an angiotensin-converting enzyme inhibitor (ACEI) for years. The first thing the nephrology group did was take her off the ACEI. Why would they do that? I thought the hypertensive drug of choice for all diabetic patients is an ACEI or an angiotensin receptor blocker (ARB). Am I wrong?
A: The renin-angiotensin-aldosterone system (RAAS) plays an important role in the regulation of blood pressure and intravascular volume through its effects on renin, angiotensin, and aldosterone production. Activation of RAAS causes an increase in blood pressure through vasoconstriction (angiotensin II effects) and the fluid retention associated with reabsorption of sodium and water (aldosterone effects). As such, the physiologic effects of RAAS have been implicated in the pathophysiology of cardiovascular diseases, such as heart failure, kidney disease, and hypertension.1
The JNC 7 guidelines2 (Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure) recommend the use of ACEIs or ARBs for the treatment of hypertension in patients with chronic kidney disease (CKD) or diabetes to slow progression of kidney disease. Given the benefits of ACEI or ARB therapy in reducing mortality in cardiovascular disease (for which patients with CKD are at increased risk3), these medications are indicated in patients with CKD.
Angiotensin II causes systemic vasoconstriction and also acts on the efferent arterioles of the glomerulus. ACEI/ARB therapy causes vasodilation of the efferent arterioles, thus lowering the intraglomerular capillary pressure.1 This mechanism accounts for the antiproteinuric effect and the subsequent decrease in GFR when these agents are initiated.
Proteinuria is a marker that may indicate nephropathy and may lead to further kidney damage.1 Slowing renal disease progression depends on controlling blood pressure and proteinuria.3
To date, a number of trials have been conducted to explore the benefit of the antiproteinuric effects of ACEI/ARB therapy. The antiproteinuric effect is more pronounced in patients with more severe proteinuria. Studies of these agents in patients with diabetic and nondiabetic nephropathy have shown that besides reducing proteinuria, they slow progression to end-stage renal disease (ESRD), thus delaying the need for renal replacement therapy.4 As such, ACEI/ARB therapy is widely used to reduce proteinuria, independent of the blood pressure–lowering effects.
Initiation of ACEI/ARB therapy is associated with an increase in SCr due to the drugs’ effects on the efferent arterioles, resulting in a decrease in intraglomerular pressure.3 However, discontinuing therapy is not warranted unless the SCr rises to more than 30% above baseline. According to findings from one meta-analysis, the degree of loss in renal function when ACEI therapy was initiated was inversely related to the rate of annual decline in renal function.5 Thus, patients with higher SCr levels at the start of therapy had poorer renal function initially, but they received the greatest benefit in long-term renal preservation.
The rise in SCr typically occurs within a few days of therapy initiation; thus, SCr should be measured within the first seven days of therapy.3 The SCr level is expected to stabilize within six to eight weeks of therapy. Patients whose SCr level continues to rise more than 30% to 35% above baseline may need to discontinue ACEI/ARB therapy; this rise may be attributed to kidney hypoperfusion. Kidney hypoperfusion can also occur when diuretics are initiated or their dosage increased, when NSAIDs are used, or in patients with bilateral renal artery stenosis or volume depletion resulting from gastroenteritis.3
Hyperkalemia due to decreased urinary excretion of potassium (K+) may present yet another reason to discontinue ACEI/ARB therapy in patients with CKD. Incidence of hyperkalemia in those with CKD stages 3 through 5 who receive either an ACEI or an ARB ranges from 5% to 50%.3 Hyperkalemia can occur when a long-acting ACEI is prescribed or when ACEI/ARB therapy is used concurrently with NSAIDs or potassium-sparing diuretics; thiazides or loop diuretics, by contrast, can reduce the risk for hyperkalemia.
Risk factors for moderate hyperkalemia (serum K+ ≥ 5.6 mmol/L) include age older than 65, congestive heart failure, SCr level greater than 1.6 mg/dL, and a blood urea nitrogen level exceeding 18 mg/dL.5
A dosing reduction or discontinuation of ACEI/ARB therapy should be considered when serum K+ levels are 5.6 mmol/L or greater.3 Patients who are older than 70 or whose serum urea nitrogen level exceeds 25 mg/dL are at increased risk for severe hyperkalemia (K+ > 6.0 mmol/L). Termination of an ACEI or an ARB may be warranted in patients with a serum K+ level exceeding 6.0 mmol/L or in those considered at increased risk for severe hyperkalemia.3
Other patients may also benefit from discontinuing ACEI/ARB therapy. Few data exist to support their use in patients with kidney function at 25% or less, or those with a GFR below 30 mL/min/1.73 m2.3 Onuigbo6 suggests that ESRD in patients with CKD can either progress gradually and steadily or rapidly as a result of acute kidney injury (AKI). Patients with CKD who are at risk for ESRD because of AKI include those currently taking an ACEI or an ARB, older patients, and those who experience unexplained decreases in GFR.
To avoid any worsening of renal function, Onuigbo6 suggests temporarily discontinuing ACEI/ARB therapy in the following patients:
• Those older than 65 who are scheduled for colonoscopy, administration of IV radiocontrast, or surgery (especially cardiovascular surgery); or
• Patients hospitalized for an acute ailment.
Discontinuing these agents may prevent progression to ESRD in such patients.
In summary, use of ACEIs or ARBs to delay progression of renal disease may be continued if a clear therapeutic benefit exists. However, discontinuation should be considered if a patient with CKD is at risk for hyperkalemia or if the K+ level remains higher than 5.6 mmol/L, if SCr levels increase more than 30% above baseline, or if patients (especially those older than 65) are at risk for AKI.
References
1. Remuzzi G, Perico N, Macia M, Ruggenenti P. The role of renin-angiotensin-aldosterone system in the progression of chronic kidney disease. Kidney Int Suppl. 2005;99:S57-S65.
2. Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560-2572.
3. Mangrum AJ, Bakris GL. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in chronic renal disease: safety issues. Semin Nephrol. 2004;24:168-175.
4. St Peter WL, Odum LE, Whaley-Connell AT. To RAS or not to RAS? The evidence for and cautions with renin-angiotensin system inhibition in patients with diabetic kidney disease. Pharmacotherapy. 2013 Apr 9.
5. Bakris GL, Weir MR. Angiotensin-converting enzyme inhibitor–associated elevations in serum creatinine: is this a cause for concern? Arch Intern Med. 2000;160:685-693.
6. Onuigbo MA. Is renoprotection with RAAS blockade a failed paradigm? Have we learnt any lessons so far? Int J Clin Pract. 2010;64:1341-1346.
Q: I sent a 68-year-old, hypertensive, diabetic woman with stage 4 kidney disease (estimated glomerular filtration rate [eGFR], 25 mL/min/1.73 m2; serum creatinine [SCr], 2 mg/mL) to a local nephrology group. Since she was diabetic, we had had her taking an angiotensin-converting enzyme inhibitor (ACEI) for years. The first thing the nephrology group did was take her off the ACEI. Why would they do that? I thought the hypertensive drug of choice for all diabetic patients is an ACEI or an angiotensin receptor blocker (ARB). Am I wrong?
A: The renin-angiotensin-aldosterone system (RAAS) plays an important role in the regulation of blood pressure and intravascular volume through its effects on renin, angiotensin, and aldosterone production. Activation of RAAS causes an increase in blood pressure through vasoconstriction (angiotensin II effects) and the fluid retention associated with reabsorption of sodium and water (aldosterone effects). As such, the physiologic effects of RAAS have been implicated in the pathophysiology of cardiovascular diseases, such as heart failure, kidney disease, and hypertension.1
The JNC 7 guidelines2 (Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure) recommend the use of ACEIs or ARBs for the treatment of hypertension in patients with chronic kidney disease (CKD) or diabetes to slow progression of kidney disease. Given the benefits of ACEI or ARB therapy in reducing mortality in cardiovascular disease (for which patients with CKD are at increased risk3), these medications are indicated in patients with CKD.
Angiotensin II causes systemic vasoconstriction and also acts on the efferent arterioles of the glomerulus. ACEI/ARB therapy causes vasodilation of the efferent arterioles, thus lowering the intraglomerular capillary pressure.1 This mechanism accounts for the antiproteinuric effect and the subsequent decrease in GFR when these agents are initiated.
Proteinuria is a marker that may indicate nephropathy and may lead to further kidney damage.1 Slowing renal disease progression depends on controlling blood pressure and proteinuria.3
To date, a number of trials have been conducted to explore the benefit of the antiproteinuric effects of ACEI/ARB therapy. The antiproteinuric effect is more pronounced in patients with more severe proteinuria. Studies of these agents in patients with diabetic and nondiabetic nephropathy have shown that besides reducing proteinuria, they slow progression to end-stage renal disease (ESRD), thus delaying the need for renal replacement therapy.4 As such, ACEI/ARB therapy is widely used to reduce proteinuria, independent of the blood pressure–lowering effects.
Initiation of ACEI/ARB therapy is associated with an increase in SCr due to the drugs’ effects on the efferent arterioles, resulting in a decrease in intraglomerular pressure.3 However, discontinuing therapy is not warranted unless the SCr rises to more than 30% above baseline. According to findings from one meta-analysis, the degree of loss in renal function when ACEI therapy was initiated was inversely related to the rate of annual decline in renal function.5 Thus, patients with higher SCr levels at the start of therapy had poorer renal function initially, but they received the greatest benefit in long-term renal preservation.
The rise in SCr typically occurs within a few days of therapy initiation; thus, SCr should be measured within the first seven days of therapy.3 The SCr level is expected to stabilize within six to eight weeks of therapy. Patients whose SCr level continues to rise more than 30% to 35% above baseline may need to discontinue ACEI/ARB therapy; this rise may be attributed to kidney hypoperfusion. Kidney hypoperfusion can also occur when diuretics are initiated or their dosage increased, when NSAIDs are used, or in patients with bilateral renal artery stenosis or volume depletion resulting from gastroenteritis.3
Hyperkalemia due to decreased urinary excretion of potassium (K+) may present yet another reason to discontinue ACEI/ARB therapy in patients with CKD. Incidence of hyperkalemia in those with CKD stages 3 through 5 who receive either an ACEI or an ARB ranges from 5% to 50%.3 Hyperkalemia can occur when a long-acting ACEI is prescribed or when ACEI/ARB therapy is used concurrently with NSAIDs or potassium-sparing diuretics; thiazides or loop diuretics, by contrast, can reduce the risk for hyperkalemia.
Risk factors for moderate hyperkalemia (serum K+ ≥ 5.6 mmol/L) include age older than 65, congestive heart failure, SCr level greater than 1.6 mg/dL, and a blood urea nitrogen level exceeding 18 mg/dL.5
A dosing reduction or discontinuation of ACEI/ARB therapy should be considered when serum K+ levels are 5.6 mmol/L or greater.3 Patients who are older than 70 or whose serum urea nitrogen level exceeds 25 mg/dL are at increased risk for severe hyperkalemia (K+ > 6.0 mmol/L). Termination of an ACEI or an ARB may be warranted in patients with a serum K+ level exceeding 6.0 mmol/L or in those considered at increased risk for severe hyperkalemia.3
Other patients may also benefit from discontinuing ACEI/ARB therapy. Few data exist to support their use in patients with kidney function at 25% or less, or those with a GFR below 30 mL/min/1.73 m2.3 Onuigbo6 suggests that ESRD in patients with CKD can either progress gradually and steadily or rapidly as a result of acute kidney injury (AKI). Patients with CKD who are at risk for ESRD because of AKI include those currently taking an ACEI or an ARB, older patients, and those who experience unexplained decreases in GFR.
To avoid any worsening of renal function, Onuigbo6 suggests temporarily discontinuing ACEI/ARB therapy in the following patients:
• Those older than 65 who are scheduled for colonoscopy, administration of IV radiocontrast, or surgery (especially cardiovascular surgery); or
• Patients hospitalized for an acute ailment.
Discontinuing these agents may prevent progression to ESRD in such patients.
In summary, use of ACEIs or ARBs to delay progression of renal disease may be continued if a clear therapeutic benefit exists. However, discontinuation should be considered if a patient with CKD is at risk for hyperkalemia or if the K+ level remains higher than 5.6 mmol/L, if SCr levels increase more than 30% above baseline, or if patients (especially those older than 65) are at risk for AKI.
References
1. Remuzzi G, Perico N, Macia M, Ruggenenti P. The role of renin-angiotensin-aldosterone system in the progression of chronic kidney disease. Kidney Int Suppl. 2005;99:S57-S65.
2. Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560-2572.
3. Mangrum AJ, Bakris GL. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in chronic renal disease: safety issues. Semin Nephrol. 2004;24:168-175.
4. St Peter WL, Odum LE, Whaley-Connell AT. To RAS or not to RAS? The evidence for and cautions with renin-angiotensin system inhibition in patients with diabetic kidney disease. Pharmacotherapy. 2013 Apr 9.
5. Bakris GL, Weir MR. Angiotensin-converting enzyme inhibitor–associated elevations in serum creatinine: is this a cause for concern? Arch Intern Med. 2000;160:685-693.
6. Onuigbo MA. Is renoprotection with RAAS blockade a failed paradigm? Have we learnt any lessons so far? Int J Clin Pract. 2010;64:1341-1346.
Q: I sent a 68-year-old, hypertensive, diabetic woman with stage 4 kidney disease (estimated glomerular filtration rate [eGFR], 25 mL/min/1.73 m2; serum creatinine [SCr], 2 mg/mL) to a local nephrology group. Since she was diabetic, we had had her taking an angiotensin-converting enzyme inhibitor (ACEI) for years. The first thing the nephrology group did was take her off the ACEI. Why would they do that? I thought the hypertensive drug of choice for all diabetic patients is an ACEI or an angiotensin receptor blocker (ARB). Am I wrong?
A: The renin-angiotensin-aldosterone system (RAAS) plays an important role in the regulation of blood pressure and intravascular volume through its effects on renin, angiotensin, and aldosterone production. Activation of RAAS causes an increase in blood pressure through vasoconstriction (angiotensin II effects) and the fluid retention associated with reabsorption of sodium and water (aldosterone effects). As such, the physiologic effects of RAAS have been implicated in the pathophysiology of cardiovascular diseases, such as heart failure, kidney disease, and hypertension.1
The JNC 7 guidelines2 (Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure) recommend the use of ACEIs or ARBs for the treatment of hypertension in patients with chronic kidney disease (CKD) or diabetes to slow progression of kidney disease. Given the benefits of ACEI or ARB therapy in reducing mortality in cardiovascular disease (for which patients with CKD are at increased risk3), these medications are indicated in patients with CKD.
Angiotensin II causes systemic vasoconstriction and also acts on the efferent arterioles of the glomerulus. ACEI/ARB therapy causes vasodilation of the efferent arterioles, thus lowering the intraglomerular capillary pressure.1 This mechanism accounts for the antiproteinuric effect and the subsequent decrease in GFR when these agents are initiated.
Proteinuria is a marker that may indicate nephropathy and may lead to further kidney damage.1 Slowing renal disease progression depends on controlling blood pressure and proteinuria.3
To date, a number of trials have been conducted to explore the benefit of the antiproteinuric effects of ACEI/ARB therapy. The antiproteinuric effect is more pronounced in patients with more severe proteinuria. Studies of these agents in patients with diabetic and nondiabetic nephropathy have shown that besides reducing proteinuria, they slow progression to end-stage renal disease (ESRD), thus delaying the need for renal replacement therapy.4 As such, ACEI/ARB therapy is widely used to reduce proteinuria, independent of the blood pressure–lowering effects.
Initiation of ACEI/ARB therapy is associated with an increase in SCr due to the drugs’ effects on the efferent arterioles, resulting in a decrease in intraglomerular pressure.3 However, discontinuing therapy is not warranted unless the SCr rises to more than 30% above baseline. According to findings from one meta-analysis, the degree of loss in renal function when ACEI therapy was initiated was inversely related to the rate of annual decline in renal function.5 Thus, patients with higher SCr levels at the start of therapy had poorer renal function initially, but they received the greatest benefit in long-term renal preservation.
The rise in SCr typically occurs within a few days of therapy initiation; thus, SCr should be measured within the first seven days of therapy.3 The SCr level is expected to stabilize within six to eight weeks of therapy. Patients whose SCr level continues to rise more than 30% to 35% above baseline may need to discontinue ACEI/ARB therapy; this rise may be attributed to kidney hypoperfusion. Kidney hypoperfusion can also occur when diuretics are initiated or their dosage increased, when NSAIDs are used, or in patients with bilateral renal artery stenosis or volume depletion resulting from gastroenteritis.3
Hyperkalemia due to decreased urinary excretion of potassium (K+) may present yet another reason to discontinue ACEI/ARB therapy in patients with CKD. Incidence of hyperkalemia in those with CKD stages 3 through 5 who receive either an ACEI or an ARB ranges from 5% to 50%.3 Hyperkalemia can occur when a long-acting ACEI is prescribed or when ACEI/ARB therapy is used concurrently with NSAIDs or potassium-sparing diuretics; thiazides or loop diuretics, by contrast, can reduce the risk for hyperkalemia.
Risk factors for moderate hyperkalemia (serum K+ ≥ 5.6 mmol/L) include age older than 65, congestive heart failure, SCr level greater than 1.6 mg/dL, and a blood urea nitrogen level exceeding 18 mg/dL.5
A dosing reduction or discontinuation of ACEI/ARB therapy should be considered when serum K+ levels are 5.6 mmol/L or greater.3 Patients who are older than 70 or whose serum urea nitrogen level exceeds 25 mg/dL are at increased risk for severe hyperkalemia (K+ > 6.0 mmol/L). Termination of an ACEI or an ARB may be warranted in patients with a serum K+ level exceeding 6.0 mmol/L or in those considered at increased risk for severe hyperkalemia.3
Other patients may also benefit from discontinuing ACEI/ARB therapy. Few data exist to support their use in patients with kidney function at 25% or less, or those with a GFR below 30 mL/min/1.73 m2.3 Onuigbo6 suggests that ESRD in patients with CKD can either progress gradually and steadily or rapidly as a result of acute kidney injury (AKI). Patients with CKD who are at risk for ESRD because of AKI include those currently taking an ACEI or an ARB, older patients, and those who experience unexplained decreases in GFR.
To avoid any worsening of renal function, Onuigbo6 suggests temporarily discontinuing ACEI/ARB therapy in the following patients:
• Those older than 65 who are scheduled for colonoscopy, administration of IV radiocontrast, or surgery (especially cardiovascular surgery); or
• Patients hospitalized for an acute ailment.
Discontinuing these agents may prevent progression to ESRD in such patients.
In summary, use of ACEIs or ARBs to delay progression of renal disease may be continued if a clear therapeutic benefit exists. However, discontinuation should be considered if a patient with CKD is at risk for hyperkalemia or if the K+ level remains higher than 5.6 mmol/L, if SCr levels increase more than 30% above baseline, or if patients (especially those older than 65) are at risk for AKI.
References
1. Remuzzi G, Perico N, Macia M, Ruggenenti P. The role of renin-angiotensin-aldosterone system in the progression of chronic kidney disease. Kidney Int Suppl. 2005;99:S57-S65.
2. Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560-2572.
3. Mangrum AJ, Bakris GL. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in chronic renal disease: safety issues. Semin Nephrol. 2004;24:168-175.
4. St Peter WL, Odum LE, Whaley-Connell AT. To RAS or not to RAS? The evidence for and cautions with renin-angiotensin system inhibition in patients with diabetic kidney disease. Pharmacotherapy. 2013 Apr 9.
5. Bakris GL, Weir MR. Angiotensin-converting enzyme inhibitor–associated elevations in serum creatinine: is this a cause for concern? Arch Intern Med. 2000;160:685-693.
6. Onuigbo MA. Is renoprotection with RAAS blockade a failed paradigm? Have we learnt any lessons so far? Int J Clin Pract. 2010;64:1341-1346.
Does Combination ACEi/ARB Therapy Benefit Patients With Proteinuria?
Q: I have a diabetic patient with microscopic proteinuria. I put her on an ACE inhibitor, but she still has the same albumin–creatinine ratio. My supervising physician suggested I add an ARB to her regimen, but I seem to remember reading that this does not work. Is that true? Or should I prescribe an ACE inhibitor/ARB combination?
This is a common question, but there is no consensus regarding the correct answer. The question should be: Are two drugs better than one when it comes to reducing proteinuria and progression to end-stage renal disease? Researchers have demonstrated a decrease in proteinuria in patients given combination ACE inhibitor/angiotension receptor blocker (ARB) therapy; however, the studies involved were found to have flaws, including small sample sizes and relatively short follow-up, once treatment was initiated.1,2
A larger study, known as Ongoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial (ONTARGET),3,4 is a multiyear study with more than 25,000 patients enrolled. ONTARGET (which excludes patients with heart failure) addresses the question of whether an ACE inhibitor in combination with an ARB, or either agent alone, is more effective in the reduction of proteinuria. In ONTARGET, combination therapy was associated with a decrease in proteinuria; however, the incidence of renal impairment was much higher.3
ONTARGET was the first trial to cast doubt on the belief that proteinuria is an accurate marker for progressive renal dysfunction. Combination treatment led to an advanced risk for increased serum creatinine and need for dialysis, despite the reduction in proteinuria. Further, combination therapy was more likely than either agent alone to cause adverse effects, including hypotension and hyperkalemia.3,4
Finally, the study also demonstrated that ACE inhibitors are not superior to ARBs. Both drugs reduce proteinuria, and each one taken alone decreases progression to end-stage renal disease. Therefore, the conclusion is that either an ACE inhibitor or an ARB alone is more efficacious than the two drugs combined.
Tricia A. Howard, MHS, PA-C, South University PA Program, Tampa, FL
References
1. Misra S, Stevermer JJ. ACE inhibitors and ARBs: one or the other—not both—for high-risk patients. J Fam Pract. 2009;58:24-26.
2. Jennings DL, Kalus JS, Coleman CI, et al. Combination therapy with an ACE inhibitor and an angiotensin receptor blocker for diabetic nephropathy: a meta-analysis. Diabet Med. 2007;24:486-493.
3. Mann JF, Schmieder RE, McQueen M, et al; ONTARGET investigators. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet. 2008;372:547-553.
4. Yusuf S, Teo KK, Pogue J, et al; ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547-1559.
Q: I have a diabetic patient with microscopic proteinuria. I put her on an ACE inhibitor, but she still has the same albumin–creatinine ratio. My supervising physician suggested I add an ARB to her regimen, but I seem to remember reading that this does not work. Is that true? Or should I prescribe an ACE inhibitor/ARB combination?
This is a common question, but there is no consensus regarding the correct answer. The question should be: Are two drugs better than one when it comes to reducing proteinuria and progression to end-stage renal disease? Researchers have demonstrated a decrease in proteinuria in patients given combination ACE inhibitor/angiotension receptor blocker (ARB) therapy; however, the studies involved were found to have flaws, including small sample sizes and relatively short follow-up, once treatment was initiated.1,2
A larger study, known as Ongoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial (ONTARGET),3,4 is a multiyear study with more than 25,000 patients enrolled. ONTARGET (which excludes patients with heart failure) addresses the question of whether an ACE inhibitor in combination with an ARB, or either agent alone, is more effective in the reduction of proteinuria. In ONTARGET, combination therapy was associated with a decrease in proteinuria; however, the incidence of renal impairment was much higher.3
ONTARGET was the first trial to cast doubt on the belief that proteinuria is an accurate marker for progressive renal dysfunction. Combination treatment led to an advanced risk for increased serum creatinine and need for dialysis, despite the reduction in proteinuria. Further, combination therapy was more likely than either agent alone to cause adverse effects, including hypotension and hyperkalemia.3,4
Finally, the study also demonstrated that ACE inhibitors are not superior to ARBs. Both drugs reduce proteinuria, and each one taken alone decreases progression to end-stage renal disease. Therefore, the conclusion is that either an ACE inhibitor or an ARB alone is more efficacious than the two drugs combined.
Tricia A. Howard, MHS, PA-C, South University PA Program, Tampa, FL
References
1. Misra S, Stevermer JJ. ACE inhibitors and ARBs: one or the other—not both—for high-risk patients. J Fam Pract. 2009;58:24-26.
2. Jennings DL, Kalus JS, Coleman CI, et al. Combination therapy with an ACE inhibitor and an angiotensin receptor blocker for diabetic nephropathy: a meta-analysis. Diabet Med. 2007;24:486-493.
3. Mann JF, Schmieder RE, McQueen M, et al; ONTARGET investigators. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet. 2008;372:547-553.
4. Yusuf S, Teo KK, Pogue J, et al; ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547-1559.
Q: I have a diabetic patient with microscopic proteinuria. I put her on an ACE inhibitor, but she still has the same albumin–creatinine ratio. My supervising physician suggested I add an ARB to her regimen, but I seem to remember reading that this does not work. Is that true? Or should I prescribe an ACE inhibitor/ARB combination?
This is a common question, but there is no consensus regarding the correct answer. The question should be: Are two drugs better than one when it comes to reducing proteinuria and progression to end-stage renal disease? Researchers have demonstrated a decrease in proteinuria in patients given combination ACE inhibitor/angiotension receptor blocker (ARB) therapy; however, the studies involved were found to have flaws, including small sample sizes and relatively short follow-up, once treatment was initiated.1,2
A larger study, known as Ongoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial (ONTARGET),3,4 is a multiyear study with more than 25,000 patients enrolled. ONTARGET (which excludes patients with heart failure) addresses the question of whether an ACE inhibitor in combination with an ARB, or either agent alone, is more effective in the reduction of proteinuria. In ONTARGET, combination therapy was associated with a decrease in proteinuria; however, the incidence of renal impairment was much higher.3
ONTARGET was the first trial to cast doubt on the belief that proteinuria is an accurate marker for progressive renal dysfunction. Combination treatment led to an advanced risk for increased serum creatinine and need for dialysis, despite the reduction in proteinuria. Further, combination therapy was more likely than either agent alone to cause adverse effects, including hypotension and hyperkalemia.3,4
Finally, the study also demonstrated that ACE inhibitors are not superior to ARBs. Both drugs reduce proteinuria, and each one taken alone decreases progression to end-stage renal disease. Therefore, the conclusion is that either an ACE inhibitor or an ARB alone is more efficacious than the two drugs combined.
Tricia A. Howard, MHS, PA-C, South University PA Program, Tampa, FL
References
1. Misra S, Stevermer JJ. ACE inhibitors and ARBs: one or the other—not both—for high-risk patients. J Fam Pract. 2009;58:24-26.
2. Jennings DL, Kalus JS, Coleman CI, et al. Combination therapy with an ACE inhibitor and an angiotensin receptor blocker for diabetic nephropathy: a meta-analysis. Diabet Med. 2007;24:486-493.
3. Mann JF, Schmieder RE, McQueen M, et al; ONTARGET investigators. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet. 2008;372:547-553.
4. Yusuf S, Teo KK, Pogue J, et al; ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547-1559.