User login
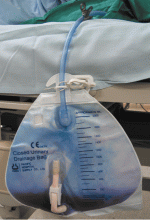
Purple urine in a woman with chronic kidney disease
On examination, her blood pressure is 138/70 mm Hg, respiratory rate 18 breaths/minute, and heart rate 80 beats/minute. Her abdomen is soft. She has never undergone abdominal surgery. She is not taking any medications that may have caused urine discoloration.
Plain radiography of the abdomen reveals no abnormal gas. Laboratory test results are as follows:
- White blood cell count 15.1 × 109/L (reference range 4–11), with 85% neutrophils (reference range 39.5%–74%)
- C-reactive protein 6.27 mg/dL (reference range 0.0–1.0)
- Blood urea nitrogen 54 mg/dL (reference range 8–25)
- Serum creatinine 2.5 mg/dL (0.70–1.40)
- Estimated glomerular filtration rate 20 mL/min/1.73 m2 (< 60 is sufficient for the diagnosis of chronic kidney disease)
- Liver function tests are normal
- Urine pH 8.0 (4.80–7.80); urine is positive for nitrates and for marked pyuria and bacteriuria.
Urine culture yields more than 100,000 colony-forming units of Pseudomonas aeruginosa, Morganella morganii, and Proteus vulgaris. These results and the patient’s presentation point to a diagnosis of purple urine bag syndrome. After placement of a new urinary catheter and 7 days of intravenous ciprofloxacin (Cipro) 250 mg every 12 hours, the color of her urine returns to normal.
PURPLE URINE BAG SYNDROME
Purple urine bag syndrome is rare, and catheter-associated urinary tract infection is the main cause.1 However, it has also been associated with intestinal intussusception.2 In our patient, the examination and radiography ruled out intussusception.
Factors reported to be involved in the development of this syndrome include older age, female sex, chronic constipation, chronic urinary catheterization, alkaline (common) or acidic (uncommon) urine, and a higher bacterial load in the urine.3,4
The pathogenesis of purple-colored urine4,5 is thought to start with the metabolism of dietary tryptophan by intestinal bacteria to indole. Indole is then absorbed into the portal circulation and is converted to indoxyl sulfate, which is excreted into the urine. In vitro experiments4,5 have shown that certain bacteria in the urine produce indoxyl sulfatase and indoxyl phosphatase, which break down indoxyl sulfate to indoxyl. Indoxyl can then be converted to indigo or indirubin in alkaline5 or acidic4 urine. When blue indigo and red indirubin mix together, the result is purple.4,5
Bacteria that possess indoxyl sulfatase or indoxyl phosphatase include P aeruginosa, M morganii, P vulgaris, Escherichia coli, and Providencia species.5,6 However, not all bacteria of the same species produce the enzymes required for the formation of purple urine.5 This may explain the rarity of this syndrome despite the common occurrence of urinary tract infection in patients with risk factors for purple urine bag syndrome.
CHRONIC KIDNEY DISEASE: A POTENTIAL RISK FACTOR
Chronic kidney disease was shown to be a risk factor for purple urine bag syndrome in a small cohort study of Taiwanese patients.7 The serum and urine levels of indoxyl sulfate increased markedly in patients who had chronic kidney disease or who were undergoing dialysis because of impaired renal clearance.6 Furthermore, indoxyl sulfate, which plays an important role in this syndrome, is also cytotoxic and may increase the rate of renal failure in uremic rats.4
Although purple urine itself is usually considered benign,3 it should prompt an evaluation for urinary tract infection, especially in patients with kidney disease. Failure to treat the underlying infection can lead to septicemia or Fourier gangrene.1,3
- Tasi YM, Huang MS, Yang CJ, Yeh SM, Liu CC. Purple urine bag syndrome, not always a benign process. Am J Emerg Med 2009; 27:895–897.
- Pillai RN, Clavijo J, Narayanan M, Zaman K. An association of purple urine bag syndrome with intussusception. Urology 2007; 70:812.e1–812.e2.
- Pillai BP, Chong VH, Yong AM. Purple urine bag syndrome. Singapore Med J 2009; 50:e193–e194.
- Bar-Or D, Rael LT, Bar-Or R, Craun ML, Statz J, Garrett RE. Mass spectrometry analysis of urine and catheter of a patient with purple urinary bag syndrome. Clin Chim Acta 2007; 378:216–218.
- Dealler SF, Hawkey PM, Millar MR. Enzymatic degradation of urinary indoxyl sulfate by Providencia stuartii and Klebsiella pneumoniae causes the purple urine bag syndrome. J Clin Microbiol 1988; 26:2152–2156.
- Wang IK, Ho DR, Chang HY, Lin CL, Chuang FR. Purple urine bag syndrome in a hemodialysis patient. Intern Med 2005; 44:859–861.
- Yang CJ, Lu PL, Chen TC, et al. Chronic kidney disease is a potential risk factor for the development of purple urine bag syndrome. J Am Geriatr Soc 2009; 57:1937–1938.
On examination, her blood pressure is 138/70 mm Hg, respiratory rate 18 breaths/minute, and heart rate 80 beats/minute. Her abdomen is soft. She has never undergone abdominal surgery. She is not taking any medications that may have caused urine discoloration.
Plain radiography of the abdomen reveals no abnormal gas. Laboratory test results are as follows:
- White blood cell count 15.1 × 109/L (reference range 4–11), with 85% neutrophils (reference range 39.5%–74%)
- C-reactive protein 6.27 mg/dL (reference range 0.0–1.0)
- Blood urea nitrogen 54 mg/dL (reference range 8–25)
- Serum creatinine 2.5 mg/dL (0.70–1.40)
- Estimated glomerular filtration rate 20 mL/min/1.73 m2 (< 60 is sufficient for the diagnosis of chronic kidney disease)
- Liver function tests are normal
- Urine pH 8.0 (4.80–7.80); urine is positive for nitrates and for marked pyuria and bacteriuria.
Urine culture yields more than 100,000 colony-forming units of Pseudomonas aeruginosa, Morganella morganii, and Proteus vulgaris. These results and the patient’s presentation point to a diagnosis of purple urine bag syndrome. After placement of a new urinary catheter and 7 days of intravenous ciprofloxacin (Cipro) 250 mg every 12 hours, the color of her urine returns to normal.
PURPLE URINE BAG SYNDROME
Purple urine bag syndrome is rare, and catheter-associated urinary tract infection is the main cause.1 However, it has also been associated with intestinal intussusception.2 In our patient, the examination and radiography ruled out intussusception.
Factors reported to be involved in the development of this syndrome include older age, female sex, chronic constipation, chronic urinary catheterization, alkaline (common) or acidic (uncommon) urine, and a higher bacterial load in the urine.3,4
The pathogenesis of purple-colored urine4,5 is thought to start with the metabolism of dietary tryptophan by intestinal bacteria to indole. Indole is then absorbed into the portal circulation and is converted to indoxyl sulfate, which is excreted into the urine. In vitro experiments4,5 have shown that certain bacteria in the urine produce indoxyl sulfatase and indoxyl phosphatase, which break down indoxyl sulfate to indoxyl. Indoxyl can then be converted to indigo or indirubin in alkaline5 or acidic4 urine. When blue indigo and red indirubin mix together, the result is purple.4,5
Bacteria that possess indoxyl sulfatase or indoxyl phosphatase include P aeruginosa, M morganii, P vulgaris, Escherichia coli, and Providencia species.5,6 However, not all bacteria of the same species produce the enzymes required for the formation of purple urine.5 This may explain the rarity of this syndrome despite the common occurrence of urinary tract infection in patients with risk factors for purple urine bag syndrome.
CHRONIC KIDNEY DISEASE: A POTENTIAL RISK FACTOR
Chronic kidney disease was shown to be a risk factor for purple urine bag syndrome in a small cohort study of Taiwanese patients.7 The serum and urine levels of indoxyl sulfate increased markedly in patients who had chronic kidney disease or who were undergoing dialysis because of impaired renal clearance.6 Furthermore, indoxyl sulfate, which plays an important role in this syndrome, is also cytotoxic and may increase the rate of renal failure in uremic rats.4
Although purple urine itself is usually considered benign,3 it should prompt an evaluation for urinary tract infection, especially in patients with kidney disease. Failure to treat the underlying infection can lead to septicemia or Fourier gangrene.1,3
On examination, her blood pressure is 138/70 mm Hg, respiratory rate 18 breaths/minute, and heart rate 80 beats/minute. Her abdomen is soft. She has never undergone abdominal surgery. She is not taking any medications that may have caused urine discoloration.
Plain radiography of the abdomen reveals no abnormal gas. Laboratory test results are as follows:
- White blood cell count 15.1 × 109/L (reference range 4–11), with 85% neutrophils (reference range 39.5%–74%)
- C-reactive protein 6.27 mg/dL (reference range 0.0–1.0)
- Blood urea nitrogen 54 mg/dL (reference range 8–25)
- Serum creatinine 2.5 mg/dL (0.70–1.40)
- Estimated glomerular filtration rate 20 mL/min/1.73 m2 (< 60 is sufficient for the diagnosis of chronic kidney disease)
- Liver function tests are normal
- Urine pH 8.0 (4.80–7.80); urine is positive for nitrates and for marked pyuria and bacteriuria.
Urine culture yields more than 100,000 colony-forming units of Pseudomonas aeruginosa, Morganella morganii, and Proteus vulgaris. These results and the patient’s presentation point to a diagnosis of purple urine bag syndrome. After placement of a new urinary catheter and 7 days of intravenous ciprofloxacin (Cipro) 250 mg every 12 hours, the color of her urine returns to normal.
PURPLE URINE BAG SYNDROME
Purple urine bag syndrome is rare, and catheter-associated urinary tract infection is the main cause.1 However, it has also been associated with intestinal intussusception.2 In our patient, the examination and radiography ruled out intussusception.
Factors reported to be involved in the development of this syndrome include older age, female sex, chronic constipation, chronic urinary catheterization, alkaline (common) or acidic (uncommon) urine, and a higher bacterial load in the urine.3,4
The pathogenesis of purple-colored urine4,5 is thought to start with the metabolism of dietary tryptophan by intestinal bacteria to indole. Indole is then absorbed into the portal circulation and is converted to indoxyl sulfate, which is excreted into the urine. In vitro experiments4,5 have shown that certain bacteria in the urine produce indoxyl sulfatase and indoxyl phosphatase, which break down indoxyl sulfate to indoxyl. Indoxyl can then be converted to indigo or indirubin in alkaline5 or acidic4 urine. When blue indigo and red indirubin mix together, the result is purple.4,5
Bacteria that possess indoxyl sulfatase or indoxyl phosphatase include P aeruginosa, M morganii, P vulgaris, Escherichia coli, and Providencia species.5,6 However, not all bacteria of the same species produce the enzymes required for the formation of purple urine.5 This may explain the rarity of this syndrome despite the common occurrence of urinary tract infection in patients with risk factors for purple urine bag syndrome.
CHRONIC KIDNEY DISEASE: A POTENTIAL RISK FACTOR
Chronic kidney disease was shown to be a risk factor for purple urine bag syndrome in a small cohort study of Taiwanese patients.7 The serum and urine levels of indoxyl sulfate increased markedly in patients who had chronic kidney disease or who were undergoing dialysis because of impaired renal clearance.6 Furthermore, indoxyl sulfate, which plays an important role in this syndrome, is also cytotoxic and may increase the rate of renal failure in uremic rats.4
Although purple urine itself is usually considered benign,3 it should prompt an evaluation for urinary tract infection, especially in patients with kidney disease. Failure to treat the underlying infection can lead to septicemia or Fourier gangrene.1,3
- Tasi YM, Huang MS, Yang CJ, Yeh SM, Liu CC. Purple urine bag syndrome, not always a benign process. Am J Emerg Med 2009; 27:895–897.
- Pillai RN, Clavijo J, Narayanan M, Zaman K. An association of purple urine bag syndrome with intussusception. Urology 2007; 70:812.e1–812.e2.
- Pillai BP, Chong VH, Yong AM. Purple urine bag syndrome. Singapore Med J 2009; 50:e193–e194.
- Bar-Or D, Rael LT, Bar-Or R, Craun ML, Statz J, Garrett RE. Mass spectrometry analysis of urine and catheter of a patient with purple urinary bag syndrome. Clin Chim Acta 2007; 378:216–218.
- Dealler SF, Hawkey PM, Millar MR. Enzymatic degradation of urinary indoxyl sulfate by Providencia stuartii and Klebsiella pneumoniae causes the purple urine bag syndrome. J Clin Microbiol 1988; 26:2152–2156.
- Wang IK, Ho DR, Chang HY, Lin CL, Chuang FR. Purple urine bag syndrome in a hemodialysis patient. Intern Med 2005; 44:859–861.
- Yang CJ, Lu PL, Chen TC, et al. Chronic kidney disease is a potential risk factor for the development of purple urine bag syndrome. J Am Geriatr Soc 2009; 57:1937–1938.
- Tasi YM, Huang MS, Yang CJ, Yeh SM, Liu CC. Purple urine bag syndrome, not always a benign process. Am J Emerg Med 2009; 27:895–897.
- Pillai RN, Clavijo J, Narayanan M, Zaman K. An association of purple urine bag syndrome with intussusception. Urology 2007; 70:812.e1–812.e2.
- Pillai BP, Chong VH, Yong AM. Purple urine bag syndrome. Singapore Med J 2009; 50:e193–e194.
- Bar-Or D, Rael LT, Bar-Or R, Craun ML, Statz J, Garrett RE. Mass spectrometry analysis of urine and catheter of a patient with purple urinary bag syndrome. Clin Chim Acta 2007; 378:216–218.
- Dealler SF, Hawkey PM, Millar MR. Enzymatic degradation of urinary indoxyl sulfate by Providencia stuartii and Klebsiella pneumoniae causes the purple urine bag syndrome. J Clin Microbiol 1988; 26:2152–2156.
- Wang IK, Ho DR, Chang HY, Lin CL, Chuang FR. Purple urine bag syndrome in a hemodialysis patient. Intern Med 2005; 44:859–861.
- Yang CJ, Lu PL, Chen TC, et al. Chronic kidney disease is a potential risk factor for the development of purple urine bag syndrome. J Am Geriatr Soc 2009; 57:1937–1938.
Bedside visit comes too late . . . Unrecognized spinal infection leads to paralysis . . .
Bedside visit comes too late
A 22-YEAR-OLD MAN underwent a liver biopsy after being admitted to the hospital a week earlier with fever, chills, diarrhea, and general malaise. A number of specialists had seen him in the hospital because of abnormal laboratory studies, increasing fever, and a maculopapular rash over his trunk and face.
After the biopsy, the patient was dizzy and diaphoretic. His attending physician ordered hemoglobin and hematocrit levels, which were lower than earlier that day. Repeat testing showed a further decrease, prompting the physician to order 2 units of red blood cells.
Typing and cross-matching delayed the transfusion for several hours. Before it could be started, the patient was found unresponsive. When the attending physician came to the bedside, the patient had no palpable pulse. A code was called, but resuscitation efforts failed.
An autopsy found a small hole in the liver and 3500 mL of blood in the peritoneal cavity, as well as hepatitis with zonal and submassive necrosis, hemoperitoneum, and hypertrophy of the heart. An HIV test performed before the biopsy eventually came back positive.
PLAINTIFF’S CLAIM The attending physician and nurses were negligent in failing to respond to signs and symptoms of internal bleeding, including falling hematocrit and hemoglobin levels. The attending physician, who was at the hospital when the patient’s condition deteriorated, should have gone to the bedside and taken steps to prevent his death.
THE DEFENSE The patient had been stable overnight; a bedside exam was unnecessary.
VERDICT $1,815,658 Texas verdict.
COMMENT Considering the many demands on clinicians’ time, it’s easy to postpone a face-to-face evaluation of a patient after a procedure. In this case, such a delay cost more than $1.8 million. A laboratory test or nurses’ notes are sometimes inadequate substitutes for a physician’s evaluation.
Failure to investigate suspicious symptoms ends badly
A MAN WITH SIGNS AND SYMPTOMS SUGGESTIVE OF AORTIC ANEURYSM/DISSECTION—including chest pain, pericardial effusion, aortic regurgitation, and aortic dilatation—saw his physician, but the doctor didn’t order any tests, such as computed tomography (CT) with contrast, magnetic resonance imaging (MRI), or transesophageal echocardiogram (TEE).
Two weeks later, the 43-year-old patient returned to the physician, who noted left ventricular hypertrophy with pericardial effusion and mild aortic loop dilatation. Once again, the doctor didn’t order tests to rule out aneurysm/dissection.
Three weeks after the second office visit, the patient collapsed and was taken by ambulance to a hospital, where he was pronounced dead. An autopsy indicated that the cause of death was cardiac tamponade resulting from an undiagnosed aortic dissection.
PLAINTIFF’S CLAIM The physician should have ordered a CT scan with contrast, an MRI, or a TEE, any of which would have confirmed an aortic aneurysm/dissection, mandating immediate admission to a hospital for surgery.
THE DEFENSE No information about the defense is available.
VERDICT $1 million Maryland settlement.
COMMENT Although many common conditions will resolve spontaneously, it’s hard to imagine temporizing in a patient with chest pain and presumed aortic dissection.
Unrecognized spinal infection leads to paralysis
A 355-LB MAN WITH DIABETES AND SPINAL DISC DISEASE experienced a sharp pain between his shoulder blades after playing golf, followed by constant back pain radiating to his chest. He went to the emergency department (ED) the next day and was admitted to the hospital to rule out a heart attack.
During a week in the hospital, the patient was seen by several doctors and diagnosed with pneumonia and excessive myoglobin levels. A computed tomography (CT) scan of the thorax and abdomen showing fluid buildup in the lining around the lungs led to the pneumonia diagnosis. No definitive spinal view was available, however, because of a mixup between a secretary and a radiology technician.
When the patient saw the hospital attending physician (at the family practice group where she was a partner) after discharge from the hospital, he complained of shooting pain down his spine. The doctor prescribed muscle relaxants. Soon afterward, the patient developed difficulty walking and reported no bowel movements for 13 days.
Almost 2 weeks after discharge from the hospital, the patient broke his ankle. He told the paramedics who responded that he felt numb from his nipples to his feet. He was taken to a community hospital, where a doctor ordered another CT scan. The radiologist who read the scan failed to identify the serious spinal infection it indicated.
The patient was transferred back to the original hospital. No doctor saw him for 8 hours after transfer, by which time he was paralyzed from the chest down.
PLAINTIFF’S CLAIM The fluid buildup on the first CT scan was caused not by pneumonia but by an infection in the spinal discs that had spread to the vertebrae and surrounding tissue.
THE DEFENSE The attending physician denied at trial that the patient had told her about the shooting pains down his spine during the posthospitalization visit.
VERDICT $4.75 million Illinois verdict, preceded by more than $2.7 million in settlements with some of the doctors involved and the community hospital.
COMMENT Careful follow-up of ED visits and coordinated care are essential to avoid large verdicts such as this one.
Bedside visit comes too late
A 22-YEAR-OLD MAN underwent a liver biopsy after being admitted to the hospital a week earlier with fever, chills, diarrhea, and general malaise. A number of specialists had seen him in the hospital because of abnormal laboratory studies, increasing fever, and a maculopapular rash over his trunk and face.
After the biopsy, the patient was dizzy and diaphoretic. His attending physician ordered hemoglobin and hematocrit levels, which were lower than earlier that day. Repeat testing showed a further decrease, prompting the physician to order 2 units of red blood cells.
Typing and cross-matching delayed the transfusion for several hours. Before it could be started, the patient was found unresponsive. When the attending physician came to the bedside, the patient had no palpable pulse. A code was called, but resuscitation efforts failed.
An autopsy found a small hole in the liver and 3500 mL of blood in the peritoneal cavity, as well as hepatitis with zonal and submassive necrosis, hemoperitoneum, and hypertrophy of the heart. An HIV test performed before the biopsy eventually came back positive.
PLAINTIFF’S CLAIM The attending physician and nurses were negligent in failing to respond to signs and symptoms of internal bleeding, including falling hematocrit and hemoglobin levels. The attending physician, who was at the hospital when the patient’s condition deteriorated, should have gone to the bedside and taken steps to prevent his death.
THE DEFENSE The patient had been stable overnight; a bedside exam was unnecessary.
VERDICT $1,815,658 Texas verdict.
COMMENT Considering the many demands on clinicians’ time, it’s easy to postpone a face-to-face evaluation of a patient after a procedure. In this case, such a delay cost more than $1.8 million. A laboratory test or nurses’ notes are sometimes inadequate substitutes for a physician’s evaluation.
Failure to investigate suspicious symptoms ends badly
A MAN WITH SIGNS AND SYMPTOMS SUGGESTIVE OF AORTIC ANEURYSM/DISSECTION—including chest pain, pericardial effusion, aortic regurgitation, and aortic dilatation—saw his physician, but the doctor didn’t order any tests, such as computed tomography (CT) with contrast, magnetic resonance imaging (MRI), or transesophageal echocardiogram (TEE).
Two weeks later, the 43-year-old patient returned to the physician, who noted left ventricular hypertrophy with pericardial effusion and mild aortic loop dilatation. Once again, the doctor didn’t order tests to rule out aneurysm/dissection.
Three weeks after the second office visit, the patient collapsed and was taken by ambulance to a hospital, where he was pronounced dead. An autopsy indicated that the cause of death was cardiac tamponade resulting from an undiagnosed aortic dissection.
PLAINTIFF’S CLAIM The physician should have ordered a CT scan with contrast, an MRI, or a TEE, any of which would have confirmed an aortic aneurysm/dissection, mandating immediate admission to a hospital for surgery.
THE DEFENSE No information about the defense is available.
VERDICT $1 million Maryland settlement.
COMMENT Although many common conditions will resolve spontaneously, it’s hard to imagine temporizing in a patient with chest pain and presumed aortic dissection.
Unrecognized spinal infection leads to paralysis
A 355-LB MAN WITH DIABETES AND SPINAL DISC DISEASE experienced a sharp pain between his shoulder blades after playing golf, followed by constant back pain radiating to his chest. He went to the emergency department (ED) the next day and was admitted to the hospital to rule out a heart attack.
During a week in the hospital, the patient was seen by several doctors and diagnosed with pneumonia and excessive myoglobin levels. A computed tomography (CT) scan of the thorax and abdomen showing fluid buildup in the lining around the lungs led to the pneumonia diagnosis. No definitive spinal view was available, however, because of a mixup between a secretary and a radiology technician.
When the patient saw the hospital attending physician (at the family practice group where she was a partner) after discharge from the hospital, he complained of shooting pain down his spine. The doctor prescribed muscle relaxants. Soon afterward, the patient developed difficulty walking and reported no bowel movements for 13 days.
Almost 2 weeks after discharge from the hospital, the patient broke his ankle. He told the paramedics who responded that he felt numb from his nipples to his feet. He was taken to a community hospital, where a doctor ordered another CT scan. The radiologist who read the scan failed to identify the serious spinal infection it indicated.
The patient was transferred back to the original hospital. No doctor saw him for 8 hours after transfer, by which time he was paralyzed from the chest down.
PLAINTIFF’S CLAIM The fluid buildup on the first CT scan was caused not by pneumonia but by an infection in the spinal discs that had spread to the vertebrae and surrounding tissue.
THE DEFENSE The attending physician denied at trial that the patient had told her about the shooting pains down his spine during the posthospitalization visit.
VERDICT $4.75 million Illinois verdict, preceded by more than $2.7 million in settlements with some of the doctors involved and the community hospital.
COMMENT Careful follow-up of ED visits and coordinated care are essential to avoid large verdicts such as this one.
Bedside visit comes too late
A 22-YEAR-OLD MAN underwent a liver biopsy after being admitted to the hospital a week earlier with fever, chills, diarrhea, and general malaise. A number of specialists had seen him in the hospital because of abnormal laboratory studies, increasing fever, and a maculopapular rash over his trunk and face.
After the biopsy, the patient was dizzy and diaphoretic. His attending physician ordered hemoglobin and hematocrit levels, which were lower than earlier that day. Repeat testing showed a further decrease, prompting the physician to order 2 units of red blood cells.
Typing and cross-matching delayed the transfusion for several hours. Before it could be started, the patient was found unresponsive. When the attending physician came to the bedside, the patient had no palpable pulse. A code was called, but resuscitation efforts failed.
An autopsy found a small hole in the liver and 3500 mL of blood in the peritoneal cavity, as well as hepatitis with zonal and submassive necrosis, hemoperitoneum, and hypertrophy of the heart. An HIV test performed before the biopsy eventually came back positive.
PLAINTIFF’S CLAIM The attending physician and nurses were negligent in failing to respond to signs and symptoms of internal bleeding, including falling hematocrit and hemoglobin levels. The attending physician, who was at the hospital when the patient’s condition deteriorated, should have gone to the bedside and taken steps to prevent his death.
THE DEFENSE The patient had been stable overnight; a bedside exam was unnecessary.
VERDICT $1,815,658 Texas verdict.
COMMENT Considering the many demands on clinicians’ time, it’s easy to postpone a face-to-face evaluation of a patient after a procedure. In this case, such a delay cost more than $1.8 million. A laboratory test or nurses’ notes are sometimes inadequate substitutes for a physician’s evaluation.
Failure to investigate suspicious symptoms ends badly
A MAN WITH SIGNS AND SYMPTOMS SUGGESTIVE OF AORTIC ANEURYSM/DISSECTION—including chest pain, pericardial effusion, aortic regurgitation, and aortic dilatation—saw his physician, but the doctor didn’t order any tests, such as computed tomography (CT) with contrast, magnetic resonance imaging (MRI), or transesophageal echocardiogram (TEE).
Two weeks later, the 43-year-old patient returned to the physician, who noted left ventricular hypertrophy with pericardial effusion and mild aortic loop dilatation. Once again, the doctor didn’t order tests to rule out aneurysm/dissection.
Three weeks after the second office visit, the patient collapsed and was taken by ambulance to a hospital, where he was pronounced dead. An autopsy indicated that the cause of death was cardiac tamponade resulting from an undiagnosed aortic dissection.
PLAINTIFF’S CLAIM The physician should have ordered a CT scan with contrast, an MRI, or a TEE, any of which would have confirmed an aortic aneurysm/dissection, mandating immediate admission to a hospital for surgery.
THE DEFENSE No information about the defense is available.
VERDICT $1 million Maryland settlement.
COMMENT Although many common conditions will resolve spontaneously, it’s hard to imagine temporizing in a patient with chest pain and presumed aortic dissection.
Unrecognized spinal infection leads to paralysis
A 355-LB MAN WITH DIABETES AND SPINAL DISC DISEASE experienced a sharp pain between his shoulder blades after playing golf, followed by constant back pain radiating to his chest. He went to the emergency department (ED) the next day and was admitted to the hospital to rule out a heart attack.
During a week in the hospital, the patient was seen by several doctors and diagnosed with pneumonia and excessive myoglobin levels. A computed tomography (CT) scan of the thorax and abdomen showing fluid buildup in the lining around the lungs led to the pneumonia diagnosis. No definitive spinal view was available, however, because of a mixup between a secretary and a radiology technician.
When the patient saw the hospital attending physician (at the family practice group where she was a partner) after discharge from the hospital, he complained of shooting pain down his spine. The doctor prescribed muscle relaxants. Soon afterward, the patient developed difficulty walking and reported no bowel movements for 13 days.
Almost 2 weeks after discharge from the hospital, the patient broke his ankle. He told the paramedics who responded that he felt numb from his nipples to his feet. He was taken to a community hospital, where a doctor ordered another CT scan. The radiologist who read the scan failed to identify the serious spinal infection it indicated.
The patient was transferred back to the original hospital. No doctor saw him for 8 hours after transfer, by which time he was paralyzed from the chest down.
PLAINTIFF’S CLAIM The fluid buildup on the first CT scan was caused not by pneumonia but by an infection in the spinal discs that had spread to the vertebrae and surrounding tissue.
THE DEFENSE The attending physician denied at trial that the patient had told her about the shooting pains down his spine during the posthospitalization visit.
VERDICT $4.75 million Illinois verdict, preceded by more than $2.7 million in settlements with some of the doctors involved and the community hospital.
COMMENT Careful follow-up of ED visits and coordinated care are essential to avoid large verdicts such as this one.
Long-Acting ESA Approved to Treat Anemia in Dialysis Patients
Peginesatide, a long-acting erythropoiesis-stimulating agent, has been approved as a treatment for anemia caused by chronic kidney disease in adults who are on dialysis, the Food and Drug Administration announced March 27.
This is the first erythropoiesis-stimulating agent (ESA) approved for this indication since 2001, Dr. Richard Pazdur, director of the office of hematology and oncology products in the FDA’s Center for Drug Evaluation and Research, said in a statement. The product "offers patients and health care providers the convenience of receiving ESA therapy just once per month instead of more frequent injections," he noted.
The FDA approved the drug with a Risk Evaluation and Mitigation Strategy (REMS) that consists of educational elements for health care professionals and a requirement to assess data on how the drug is used, the statement said. The FDA can require a REMS for a product when the agency determines certain measures are needed to ensure that a drug’s benefits outweigh its risks.
Peginesatide is not approved for treating anemia in patients with chronic kidney disease (CKD) who are not on dialysis, for patients who are being treated for cancer and whose anemia is not caused by CKD, or for use instead of a red blood cell transfusion in patients who require immediate correction of anemia – which are approved indications for other ESAs. The label also states that treatment with peginesatide has not been shown to "improve symptoms, physical functioning, or health-related quality of life."
Peginesatide, which will be marketed as Omontys by Affymax Inc., is administered once a month, intravenously or subcutaneously. Treatment is started when the hemoglobin level is below 10 g/dL, according to the prescribing information.
Approval was based on two randomized open-label studies of 1,608 patients with CKD who were on dialysis, who, after their hemoglobin levels were stabilized with epoetin, were randomized to continue treatment with epoetin or peginesatide. Treatment with peginesatide was "as safe and effective as epoetin in maintaining hemoglobin levels within the studies’ prespecified range of 10 to 12 g/dL," according to the FDA statement. Diarrhea, vomiting, hypertension, and arthralgias were among the most common adverse effects reported in 10% or more of the patients on dialysis treated with peginesatide.
At a meeting in December 2011, the majority of an FDA advisory panel voted that peginesatide had a favorable risk-benefit profile for the approved indication. At that meeting, panelists were concerned about the higher rate of serious adverse events in patients treated with peginesatide, compared with those treated with darbepoetin, in studies of patients with anemia from CKD who were not on dialysis. But in the two studies of patients on dialysis, panelists agreed that the safety and efficacy of peginesatide were comparable with epoetin and cited the convenience of once-a-month dosing for patients.
Darbepoetin alfa, marketed as Aranesp, and epoetin alfa, marketed as Epogen and Procrit, are the ESAs approved in the United States; they are administered as often as one to three times per week. A pegylated epoetin beta (Mircera) is not available in the United States.
Like the other ESAs, the peginesatide prescribing information includes a boxed warning about the increased risks of deaths and serious cardiovascular events when higher hemoglobin levels are targeted, and the recommendation to use the lowest dose needed to reduce the need for transfusions.
Peginesatide, a long-acting erythropoiesis-stimulating agent, has been approved as a treatment for anemia caused by chronic kidney disease in adults who are on dialysis, the Food and Drug Administration announced March 27.
This is the first erythropoiesis-stimulating agent (ESA) approved for this indication since 2001, Dr. Richard Pazdur, director of the office of hematology and oncology products in the FDA’s Center for Drug Evaluation and Research, said in a statement. The product "offers patients and health care providers the convenience of receiving ESA therapy just once per month instead of more frequent injections," he noted.
The FDA approved the drug with a Risk Evaluation and Mitigation Strategy (REMS) that consists of educational elements for health care professionals and a requirement to assess data on how the drug is used, the statement said. The FDA can require a REMS for a product when the agency determines certain measures are needed to ensure that a drug’s benefits outweigh its risks.
Peginesatide is not approved for treating anemia in patients with chronic kidney disease (CKD) who are not on dialysis, for patients who are being treated for cancer and whose anemia is not caused by CKD, or for use instead of a red blood cell transfusion in patients who require immediate correction of anemia – which are approved indications for other ESAs. The label also states that treatment with peginesatide has not been shown to "improve symptoms, physical functioning, or health-related quality of life."
Peginesatide, which will be marketed as Omontys by Affymax Inc., is administered once a month, intravenously or subcutaneously. Treatment is started when the hemoglobin level is below 10 g/dL, according to the prescribing information.
Approval was based on two randomized open-label studies of 1,608 patients with CKD who were on dialysis, who, after their hemoglobin levels were stabilized with epoetin, were randomized to continue treatment with epoetin or peginesatide. Treatment with peginesatide was "as safe and effective as epoetin in maintaining hemoglobin levels within the studies’ prespecified range of 10 to 12 g/dL," according to the FDA statement. Diarrhea, vomiting, hypertension, and arthralgias were among the most common adverse effects reported in 10% or more of the patients on dialysis treated with peginesatide.
At a meeting in December 2011, the majority of an FDA advisory panel voted that peginesatide had a favorable risk-benefit profile for the approved indication. At that meeting, panelists were concerned about the higher rate of serious adverse events in patients treated with peginesatide, compared with those treated with darbepoetin, in studies of patients with anemia from CKD who were not on dialysis. But in the two studies of patients on dialysis, panelists agreed that the safety and efficacy of peginesatide were comparable with epoetin and cited the convenience of once-a-month dosing for patients.
Darbepoetin alfa, marketed as Aranesp, and epoetin alfa, marketed as Epogen and Procrit, are the ESAs approved in the United States; they are administered as often as one to three times per week. A pegylated epoetin beta (Mircera) is not available in the United States.
Like the other ESAs, the peginesatide prescribing information includes a boxed warning about the increased risks of deaths and serious cardiovascular events when higher hemoglobin levels are targeted, and the recommendation to use the lowest dose needed to reduce the need for transfusions.
Peginesatide, a long-acting erythropoiesis-stimulating agent, has been approved as a treatment for anemia caused by chronic kidney disease in adults who are on dialysis, the Food and Drug Administration announced March 27.
This is the first erythropoiesis-stimulating agent (ESA) approved for this indication since 2001, Dr. Richard Pazdur, director of the office of hematology and oncology products in the FDA’s Center for Drug Evaluation and Research, said in a statement. The product "offers patients and health care providers the convenience of receiving ESA therapy just once per month instead of more frequent injections," he noted.
The FDA approved the drug with a Risk Evaluation and Mitigation Strategy (REMS) that consists of educational elements for health care professionals and a requirement to assess data on how the drug is used, the statement said. The FDA can require a REMS for a product when the agency determines certain measures are needed to ensure that a drug’s benefits outweigh its risks.
Peginesatide is not approved for treating anemia in patients with chronic kidney disease (CKD) who are not on dialysis, for patients who are being treated for cancer and whose anemia is not caused by CKD, or for use instead of a red blood cell transfusion in patients who require immediate correction of anemia – which are approved indications for other ESAs. The label also states that treatment with peginesatide has not been shown to "improve symptoms, physical functioning, or health-related quality of life."
Peginesatide, which will be marketed as Omontys by Affymax Inc., is administered once a month, intravenously or subcutaneously. Treatment is started when the hemoglobin level is below 10 g/dL, according to the prescribing information.
Approval was based on two randomized open-label studies of 1,608 patients with CKD who were on dialysis, who, after their hemoglobin levels were stabilized with epoetin, were randomized to continue treatment with epoetin or peginesatide. Treatment with peginesatide was "as safe and effective as epoetin in maintaining hemoglobin levels within the studies’ prespecified range of 10 to 12 g/dL," according to the FDA statement. Diarrhea, vomiting, hypertension, and arthralgias were among the most common adverse effects reported in 10% or more of the patients on dialysis treated with peginesatide.
At a meeting in December 2011, the majority of an FDA advisory panel voted that peginesatide had a favorable risk-benefit profile for the approved indication. At that meeting, panelists were concerned about the higher rate of serious adverse events in patients treated with peginesatide, compared with those treated with darbepoetin, in studies of patients with anemia from CKD who were not on dialysis. But in the two studies of patients on dialysis, panelists agreed that the safety and efficacy of peginesatide were comparable with epoetin and cited the convenience of once-a-month dosing for patients.
Darbepoetin alfa, marketed as Aranesp, and epoetin alfa, marketed as Epogen and Procrit, are the ESAs approved in the United States; they are administered as often as one to three times per week. A pegylated epoetin beta (Mircera) is not available in the United States.
Like the other ESAs, the peginesatide prescribing information includes a boxed warning about the increased risks of deaths and serious cardiovascular events when higher hemoglobin levels are targeted, and the recommendation to use the lowest dose needed to reduce the need for transfusions.
Azithromycin, Doxycycline Quell Nongonococcal Urethritis in Men
MINNEAPOLIS – Azithromycin and doxycycline appear to be equally effective for men with nongonococcal urethritis.
In a randomized placebo-controlled trial, both drugs had good overall effectiveness, with an 84% clinical cure rate for azithromycin and a 78% rate for doxycycline. But some pathogens were more resistant to the drugs than others, Lisa Manhart, Ph.D., said at a conference on STD prevention sponsored by the Centers for Disease Control and Prevention.
The Mycoplasma Genitalium Antibiotic Susceptibility and Treatment (MEGA) trial enrolled 606 men with nongonococcal urethritis from 2007 to 2011. The main end points of the trial were clinical and microbiological cure of urethritis associated with M. genitalium infection, when treated with the standard therapy recommended by the Centers for Disease Control and Prevention: a single dose of 1 g of azithromycin, or a 7-day regimen of 100 mg of doxycycline taken twice a day.
Several pathogens cause nongonococcal urethritis, said Dr. Manhart, an epidemiologist at the University of Washington, Seattle. Among them are Chlamydia trachomatis, M. genitalium, the recently differentiated Ureaplasma urealyticum, and Trichomonas vaginalis. In some infections, no pathogen can be identified. In addition to presenting the end points for M. genitalium infections, Dr. Manhart broke the results out by other pathogens.
The mean age of the men in the trial was 34 years. Most (67%) were heterosexual, 28% were homosexual, and about 5% were bisexual. They reported a mean of six sexual partners in the past 12 months.
The most common infectious pathogen was chlamydia (26%), followed by U. urealyticum (24%). M. genitalium was present in 14% of the study group, and T. vaginalis in 2%. The remainder of the group had an idiopathic urethritis.
Treatment consisted of either a placebo azithromycin and active doxycycline or active azithromycin and placebo doxycycline. If men still showed symptoms or microbiological failure after treatment, they were offered reverse therapy (i.e., a treatment pack with the opposite active and placebo drugs), and returned for a follow-up visit in 3 weeks. If they were still not clinically or microbiologically cured, they received a course of moxifloxacin.
Overall, there was no statistically significant difference in cure rates between the two drugs. Nor were there significant differences among men with chlamydia infections, with a clinical cure rate of 87% for azithromycin and 78% for doxycycline. For microbiological cure, the rates were 86% and 90%, respectively.
Both clinical and microbiological cures rates were much lower in men infected with M. genitalium, but the rates were not significantly different between the two drugs. Clinical cure rates were 68% for azithromycin and 48% for doxycycline. For microbiological cure, the rates were 39% and 30%, respectively.
Men with U. urealyticum infections fared a little better, but again, the cure rates were not significantly different between the drugs. A clinical cure occurred in 85% of the azithromycin group and 75% of the doxycycline group. Microbiological cure rates were 75% and 69%, respectively.
For those with idiopathic urethritis, the cure rate for both drugs was identical: 87%.
Reverse therapy was still not effective in some men with U. urealyticum or M. genitalium infections. Three weeks after that treatment, 51% of those with M. genitalium and 57% of those with U. urealyticum were still positive for the pathogen. These men received a course of moxifloxacin.
"Moxifloxacin was quite effective, but at the end of the trial, we still had two men with M. genitalium and four with U. urealyticum who did not clear their infections," Dr. Manhart said.
Moxifloxacin, although highly effective, is too expensive to be used as first-time therapy in a public health department setting, she noted. "Its preinsurance cost runs up to $150, so we are still faced with a conundrum when treating these infections."
Dr. Manhart had no financial declarations.
MINNEAPOLIS – Azithromycin and doxycycline appear to be equally effective for men with nongonococcal urethritis.
In a randomized placebo-controlled trial, both drugs had good overall effectiveness, with an 84% clinical cure rate for azithromycin and a 78% rate for doxycycline. But some pathogens were more resistant to the drugs than others, Lisa Manhart, Ph.D., said at a conference on STD prevention sponsored by the Centers for Disease Control and Prevention.
The Mycoplasma Genitalium Antibiotic Susceptibility and Treatment (MEGA) trial enrolled 606 men with nongonococcal urethritis from 2007 to 2011. The main end points of the trial were clinical and microbiological cure of urethritis associated with M. genitalium infection, when treated with the standard therapy recommended by the Centers for Disease Control and Prevention: a single dose of 1 g of azithromycin, or a 7-day regimen of 100 mg of doxycycline taken twice a day.
Several pathogens cause nongonococcal urethritis, said Dr. Manhart, an epidemiologist at the University of Washington, Seattle. Among them are Chlamydia trachomatis, M. genitalium, the recently differentiated Ureaplasma urealyticum, and Trichomonas vaginalis. In some infections, no pathogen can be identified. In addition to presenting the end points for M. genitalium infections, Dr. Manhart broke the results out by other pathogens.
The mean age of the men in the trial was 34 years. Most (67%) were heterosexual, 28% were homosexual, and about 5% were bisexual. They reported a mean of six sexual partners in the past 12 months.
The most common infectious pathogen was chlamydia (26%), followed by U. urealyticum (24%). M. genitalium was present in 14% of the study group, and T. vaginalis in 2%. The remainder of the group had an idiopathic urethritis.
Treatment consisted of either a placebo azithromycin and active doxycycline or active azithromycin and placebo doxycycline. If men still showed symptoms or microbiological failure after treatment, they were offered reverse therapy (i.e., a treatment pack with the opposite active and placebo drugs), and returned for a follow-up visit in 3 weeks. If they were still not clinically or microbiologically cured, they received a course of moxifloxacin.
Overall, there was no statistically significant difference in cure rates between the two drugs. Nor were there significant differences among men with chlamydia infections, with a clinical cure rate of 87% for azithromycin and 78% for doxycycline. For microbiological cure, the rates were 86% and 90%, respectively.
Both clinical and microbiological cures rates were much lower in men infected with M. genitalium, but the rates were not significantly different between the two drugs. Clinical cure rates were 68% for azithromycin and 48% for doxycycline. For microbiological cure, the rates were 39% and 30%, respectively.
Men with U. urealyticum infections fared a little better, but again, the cure rates were not significantly different between the drugs. A clinical cure occurred in 85% of the azithromycin group and 75% of the doxycycline group. Microbiological cure rates were 75% and 69%, respectively.
For those with idiopathic urethritis, the cure rate for both drugs was identical: 87%.
Reverse therapy was still not effective in some men with U. urealyticum or M. genitalium infections. Three weeks after that treatment, 51% of those with M. genitalium and 57% of those with U. urealyticum were still positive for the pathogen. These men received a course of moxifloxacin.
"Moxifloxacin was quite effective, but at the end of the trial, we still had two men with M. genitalium and four with U. urealyticum who did not clear their infections," Dr. Manhart said.
Moxifloxacin, although highly effective, is too expensive to be used as first-time therapy in a public health department setting, she noted. "Its preinsurance cost runs up to $150, so we are still faced with a conundrum when treating these infections."
Dr. Manhart had no financial declarations.
MINNEAPOLIS – Azithromycin and doxycycline appear to be equally effective for men with nongonococcal urethritis.
In a randomized placebo-controlled trial, both drugs had good overall effectiveness, with an 84% clinical cure rate for azithromycin and a 78% rate for doxycycline. But some pathogens were more resistant to the drugs than others, Lisa Manhart, Ph.D., said at a conference on STD prevention sponsored by the Centers for Disease Control and Prevention.
The Mycoplasma Genitalium Antibiotic Susceptibility and Treatment (MEGA) trial enrolled 606 men with nongonococcal urethritis from 2007 to 2011. The main end points of the trial were clinical and microbiological cure of urethritis associated with M. genitalium infection, when treated with the standard therapy recommended by the Centers for Disease Control and Prevention: a single dose of 1 g of azithromycin, or a 7-day regimen of 100 mg of doxycycline taken twice a day.
Several pathogens cause nongonococcal urethritis, said Dr. Manhart, an epidemiologist at the University of Washington, Seattle. Among them are Chlamydia trachomatis, M. genitalium, the recently differentiated Ureaplasma urealyticum, and Trichomonas vaginalis. In some infections, no pathogen can be identified. In addition to presenting the end points for M. genitalium infections, Dr. Manhart broke the results out by other pathogens.
The mean age of the men in the trial was 34 years. Most (67%) were heterosexual, 28% were homosexual, and about 5% were bisexual. They reported a mean of six sexual partners in the past 12 months.
The most common infectious pathogen was chlamydia (26%), followed by U. urealyticum (24%). M. genitalium was present in 14% of the study group, and T. vaginalis in 2%. The remainder of the group had an idiopathic urethritis.
Treatment consisted of either a placebo azithromycin and active doxycycline or active azithromycin and placebo doxycycline. If men still showed symptoms or microbiological failure after treatment, they were offered reverse therapy (i.e., a treatment pack with the opposite active and placebo drugs), and returned for a follow-up visit in 3 weeks. If they were still not clinically or microbiologically cured, they received a course of moxifloxacin.
Overall, there was no statistically significant difference in cure rates between the two drugs. Nor were there significant differences among men with chlamydia infections, with a clinical cure rate of 87% for azithromycin and 78% for doxycycline. For microbiological cure, the rates were 86% and 90%, respectively.
Both clinical and microbiological cures rates were much lower in men infected with M. genitalium, but the rates were not significantly different between the two drugs. Clinical cure rates were 68% for azithromycin and 48% for doxycycline. For microbiological cure, the rates were 39% and 30%, respectively.
Men with U. urealyticum infections fared a little better, but again, the cure rates were not significantly different between the drugs. A clinical cure occurred in 85% of the azithromycin group and 75% of the doxycycline group. Microbiological cure rates were 75% and 69%, respectively.
For those with idiopathic urethritis, the cure rate for both drugs was identical: 87%.
Reverse therapy was still not effective in some men with U. urealyticum or M. genitalium infections. Three weeks after that treatment, 51% of those with M. genitalium and 57% of those with U. urealyticum were still positive for the pathogen. These men received a course of moxifloxacin.
"Moxifloxacin was quite effective, but at the end of the trial, we still had two men with M. genitalium and four with U. urealyticum who did not clear their infections," Dr. Manhart said.
Moxifloxacin, although highly effective, is too expensive to be used as first-time therapy in a public health department setting, she noted. "Its preinsurance cost runs up to $150, so we are still faced with a conundrum when treating these infections."
Dr. Manhart had no financial declarations.
FROM A CONFERENCE ON STD PREVENTION SPONSORED BY THE CENTERS FOR DISEASE CONTROL AND PREVENTION
Circumcision May Lower Prostate Cancer Risk
Men who are circumcised before their first sexual intercourse have a significantly lower risk for prostate cancer than do uncircumcised men or those who are circumcised after their first sexual encounter, according to an analysis of data on 3,399 men.
The finding of a 15% reduction in relative risk points to sexually transmitted infections and inflammation as possible risk factors for prostate cancer, the authors suggested in a study published online in the journal Cancer.
"These findings are consistent with research showing that an infectious/inflammatory pathway may be involved in prostate carcinogenesis," wrote Dr. Jonathan L. Wright and colleagues at the University of Washington and the Fred Hutchinson Cancer Research Center, both in Seattle (Cancer 2012 March 12 [doi:10.1002/cncr.26653]).
The authors had hypothesized that circumcision reduces the risk for transmission of a sexually transmitted infection (STI), "and that if a [prostate cancer] develops as a result of the STI, only circumcision prior to first sexual intercourse can potentially alter risk" of prostate cancer.
They borrowed data from two population-based, case-control studies of men with prostate cancer, identifying a total of 1,754 cases in the Seattle–Puget Sound SEER (Surveillance, Epidemiology, and End Results) cancer registry. For the 1,645 controls, they used random-digit dialing to identify men from the same area with no prostate cancer, age-matched in 5-year groups.
In all, 68.8% of cases and 71.5% of controls were circumcised. Among all men who reported being circumcised, 91% had the procedure shortly after birth. Circumcision was performed after first sexual intercourse in 3.9% of cases and 2.5% of controls. Circumcision was also more commonly reported in white men (69%) than in black men (43%).
In multivariate regression analysis, the investigators controlled for age, race, prostate-specific antigen (PSA) test within 5 years of diagnosis or reference data, family history of prostate cancer, history of STIs, prior prostatitis, and number of female and male sexual partners. They found that the overall odds ratio for prostate cancer among men who were circumcised before their first sexual intercourse was 0.88 (95% confidence interval, 0.73-0.99).
The researchers also looked at prostate cancer and circumcision status by aggressiveness of disease, with "aggressive" defined as a composite of Gleason score of 7 (4 + 3) or greater; nonlocalized stage; or PSA level greater than 20 ng/mL at the time of diagnosis.
They saw that preintercourse circumcision had borderline associations with lower risk for both less-aggressive (OR, 0.88; 95% CI, 0.74-1.04) and more-aggressive cancers (OR, 0.82; 95% CI, 0.66-1.00).
The reduction in prostate cancer risk with circumcision before first intercourse was observed in whites (OR, 0.87; 95% CI, 0.73-1.02); and blacks (OR, 0.64; 95% CI, 0.39-1.08).
Circumcision has been shown in other studies to be linked to lower rates of STIs, including HIV/AIDS, herpes simplex, syphilis, and chancroid, the authors noted. They suggested that the mucosal lining of the prepuce, which is thin, may develop small tears that provide pathogens with a route to the bloodstream.
"Circumcision may reduce these injuries due to the significant keratinization that occurs following the procedure," they speculated. "In addition, the moist environment under the preputial skin may help pathogens survive for extended periods prior to direct infection. Circumcision removes this protective environment."
The authors pointed to other studies linking circumcision to reduced prostate cancer risk, including a 1987 case-control study in which circumcision was associated with a 50% reduction in relative risk among whites, and a near 40% reduction in blacks (J Natl Cancer Inst. 1987;78:869-74).
The study was supported by National Institute of Health grants and the Fred Hutchinson Cancer Research Center. The authors reported no conflicts of interest. Dr. Freedland said that he had no conflicts of interest.
Viral, bacterial, and parasitic infections have been fingered as contributing factors to various cancers, including HIV (Kaposi’s sarcoma), hepatitis B and C viruses (liver carcinoma), and Schistosomiasis haematobium (bladder cancer), noted Dr. Stephen J. Freedland, a prostate cancer expert who was not involved in the study.
The evidence linking prostate cancer to infections is, however, circumstantial, according to Dr. Freedland. "There are multiple lines of evidence that suggest [that] inflammation may be involved in prostate cancer, but inflammation is not the same as infection," he said in an interview.
Sexually transmitted infections may be one source of inflammation contributing to prostate cancer, but the theory of an infectious etiology of prostate cancer is "just one piece of a very complicated puzzle," Dr. Freedland commented.
"This study provides modest evidence: a 15% reduction. It’s not as if we should recommend that every baby be circumcised because it can reduce prostate cancer by 15%. This is more of a research finding," he said.
Dr. Freedland is an associate professor of urology and of pathology at Duke University in Durham, N.C. He said that he had no conflicts of interest.
Viral, bacterial, and parasitic infections have been fingered as contributing factors to various cancers, including HIV (Kaposi’s sarcoma), hepatitis B and C viruses (liver carcinoma), and Schistosomiasis haematobium (bladder cancer), noted Dr. Stephen J. Freedland, a prostate cancer expert who was not involved in the study.
The evidence linking prostate cancer to infections is, however, circumstantial, according to Dr. Freedland. "There are multiple lines of evidence that suggest [that] inflammation may be involved in prostate cancer, but inflammation is not the same as infection," he said in an interview.
Sexually transmitted infections may be one source of inflammation contributing to prostate cancer, but the theory of an infectious etiology of prostate cancer is "just one piece of a very complicated puzzle," Dr. Freedland commented.
"This study provides modest evidence: a 15% reduction. It’s not as if we should recommend that every baby be circumcised because it can reduce prostate cancer by 15%. This is more of a research finding," he said.
Dr. Freedland is an associate professor of urology and of pathology at Duke University in Durham, N.C. He said that he had no conflicts of interest.
Viral, bacterial, and parasitic infections have been fingered as contributing factors to various cancers, including HIV (Kaposi’s sarcoma), hepatitis B and C viruses (liver carcinoma), and Schistosomiasis haematobium (bladder cancer), noted Dr. Stephen J. Freedland, a prostate cancer expert who was not involved in the study.
The evidence linking prostate cancer to infections is, however, circumstantial, according to Dr. Freedland. "There are multiple lines of evidence that suggest [that] inflammation may be involved in prostate cancer, but inflammation is not the same as infection," he said in an interview.
Sexually transmitted infections may be one source of inflammation contributing to prostate cancer, but the theory of an infectious etiology of prostate cancer is "just one piece of a very complicated puzzle," Dr. Freedland commented.
"This study provides modest evidence: a 15% reduction. It’s not as if we should recommend that every baby be circumcised because it can reduce prostate cancer by 15%. This is more of a research finding," he said.
Dr. Freedland is an associate professor of urology and of pathology at Duke University in Durham, N.C. He said that he had no conflicts of interest.
Men who are circumcised before their first sexual intercourse have a significantly lower risk for prostate cancer than do uncircumcised men or those who are circumcised after their first sexual encounter, according to an analysis of data on 3,399 men.
The finding of a 15% reduction in relative risk points to sexually transmitted infections and inflammation as possible risk factors for prostate cancer, the authors suggested in a study published online in the journal Cancer.
"These findings are consistent with research showing that an infectious/inflammatory pathway may be involved in prostate carcinogenesis," wrote Dr. Jonathan L. Wright and colleagues at the University of Washington and the Fred Hutchinson Cancer Research Center, both in Seattle (Cancer 2012 March 12 [doi:10.1002/cncr.26653]).
The authors had hypothesized that circumcision reduces the risk for transmission of a sexually transmitted infection (STI), "and that if a [prostate cancer] develops as a result of the STI, only circumcision prior to first sexual intercourse can potentially alter risk" of prostate cancer.
They borrowed data from two population-based, case-control studies of men with prostate cancer, identifying a total of 1,754 cases in the Seattle–Puget Sound SEER (Surveillance, Epidemiology, and End Results) cancer registry. For the 1,645 controls, they used random-digit dialing to identify men from the same area with no prostate cancer, age-matched in 5-year groups.
In all, 68.8% of cases and 71.5% of controls were circumcised. Among all men who reported being circumcised, 91% had the procedure shortly after birth. Circumcision was performed after first sexual intercourse in 3.9% of cases and 2.5% of controls. Circumcision was also more commonly reported in white men (69%) than in black men (43%).
In multivariate regression analysis, the investigators controlled for age, race, prostate-specific antigen (PSA) test within 5 years of diagnosis or reference data, family history of prostate cancer, history of STIs, prior prostatitis, and number of female and male sexual partners. They found that the overall odds ratio for prostate cancer among men who were circumcised before their first sexual intercourse was 0.88 (95% confidence interval, 0.73-0.99).
The researchers also looked at prostate cancer and circumcision status by aggressiveness of disease, with "aggressive" defined as a composite of Gleason score of 7 (4 + 3) or greater; nonlocalized stage; or PSA level greater than 20 ng/mL at the time of diagnosis.
They saw that preintercourse circumcision had borderline associations with lower risk for both less-aggressive (OR, 0.88; 95% CI, 0.74-1.04) and more-aggressive cancers (OR, 0.82; 95% CI, 0.66-1.00).
The reduction in prostate cancer risk with circumcision before first intercourse was observed in whites (OR, 0.87; 95% CI, 0.73-1.02); and blacks (OR, 0.64; 95% CI, 0.39-1.08).
Circumcision has been shown in other studies to be linked to lower rates of STIs, including HIV/AIDS, herpes simplex, syphilis, and chancroid, the authors noted. They suggested that the mucosal lining of the prepuce, which is thin, may develop small tears that provide pathogens with a route to the bloodstream.
"Circumcision may reduce these injuries due to the significant keratinization that occurs following the procedure," they speculated. "In addition, the moist environment under the preputial skin may help pathogens survive for extended periods prior to direct infection. Circumcision removes this protective environment."
The authors pointed to other studies linking circumcision to reduced prostate cancer risk, including a 1987 case-control study in which circumcision was associated with a 50% reduction in relative risk among whites, and a near 40% reduction in blacks (J Natl Cancer Inst. 1987;78:869-74).
The study was supported by National Institute of Health grants and the Fred Hutchinson Cancer Research Center. The authors reported no conflicts of interest. Dr. Freedland said that he had no conflicts of interest.
Men who are circumcised before their first sexual intercourse have a significantly lower risk for prostate cancer than do uncircumcised men or those who are circumcised after their first sexual encounter, according to an analysis of data on 3,399 men.
The finding of a 15% reduction in relative risk points to sexually transmitted infections and inflammation as possible risk factors for prostate cancer, the authors suggested in a study published online in the journal Cancer.
"These findings are consistent with research showing that an infectious/inflammatory pathway may be involved in prostate carcinogenesis," wrote Dr. Jonathan L. Wright and colleagues at the University of Washington and the Fred Hutchinson Cancer Research Center, both in Seattle (Cancer 2012 March 12 [doi:10.1002/cncr.26653]).
The authors had hypothesized that circumcision reduces the risk for transmission of a sexually transmitted infection (STI), "and that if a [prostate cancer] develops as a result of the STI, only circumcision prior to first sexual intercourse can potentially alter risk" of prostate cancer.
They borrowed data from two population-based, case-control studies of men with prostate cancer, identifying a total of 1,754 cases in the Seattle–Puget Sound SEER (Surveillance, Epidemiology, and End Results) cancer registry. For the 1,645 controls, they used random-digit dialing to identify men from the same area with no prostate cancer, age-matched in 5-year groups.
In all, 68.8% of cases and 71.5% of controls were circumcised. Among all men who reported being circumcised, 91% had the procedure shortly after birth. Circumcision was performed after first sexual intercourse in 3.9% of cases and 2.5% of controls. Circumcision was also more commonly reported in white men (69%) than in black men (43%).
In multivariate regression analysis, the investigators controlled for age, race, prostate-specific antigen (PSA) test within 5 years of diagnosis or reference data, family history of prostate cancer, history of STIs, prior prostatitis, and number of female and male sexual partners. They found that the overall odds ratio for prostate cancer among men who were circumcised before their first sexual intercourse was 0.88 (95% confidence interval, 0.73-0.99).
The researchers also looked at prostate cancer and circumcision status by aggressiveness of disease, with "aggressive" defined as a composite of Gleason score of 7 (4 + 3) or greater; nonlocalized stage; or PSA level greater than 20 ng/mL at the time of diagnosis.
They saw that preintercourse circumcision had borderline associations with lower risk for both less-aggressive (OR, 0.88; 95% CI, 0.74-1.04) and more-aggressive cancers (OR, 0.82; 95% CI, 0.66-1.00).
The reduction in prostate cancer risk with circumcision before first intercourse was observed in whites (OR, 0.87; 95% CI, 0.73-1.02); and blacks (OR, 0.64; 95% CI, 0.39-1.08).
Circumcision has been shown in other studies to be linked to lower rates of STIs, including HIV/AIDS, herpes simplex, syphilis, and chancroid, the authors noted. They suggested that the mucosal lining of the prepuce, which is thin, may develop small tears that provide pathogens with a route to the bloodstream.
"Circumcision may reduce these injuries due to the significant keratinization that occurs following the procedure," they speculated. "In addition, the moist environment under the preputial skin may help pathogens survive for extended periods prior to direct infection. Circumcision removes this protective environment."
The authors pointed to other studies linking circumcision to reduced prostate cancer risk, including a 1987 case-control study in which circumcision was associated with a 50% reduction in relative risk among whites, and a near 40% reduction in blacks (J Natl Cancer Inst. 1987;78:869-74).
The study was supported by National Institute of Health grants and the Fred Hutchinson Cancer Research Center. The authors reported no conflicts of interest. Dr. Freedland said that he had no conflicts of interest.
FROM CANCER
Major Finding: Men who were circumcised before their first sexual intercourse had a 15% reduction in relative risk, compared with men who were never circumcised or were circumcised after their first intercourse.
Data Source: The analysis is based on 3,399 men in a population-based, case-control study using SEER data.
Disclosures: The study was supported by NIH grants and the Fred Hutchinson Cancer Research Center. The authors reported no conflicts of interest.
High creatinine 6 months after renal transplant
A 42-year-old man presented with acute renal failure with a serum creatinine of 6.07 mg/dL (baseline 2.0 mg/dL) 6 months after receiving a kidney transplant from a deceased donor. He was asymptomatic, had no previous symptoms of transplant rejection, and was compliant with his immunosuppressive regimen. The physical examination and the rest of the laboratory workup were normal.
Q: Which is the most likely diagnosis?
- A lymphocele
- A hematoma
- A urinoma
- A perirenal abscess
- A simple renal cyst
A: A lymphocele is the most likely diagnosis. A lymphocele— a collection of lymph without an epithelial lining—develops in as many as 20% of kidney transplant recipients.1 Many causative factors have been proposed, including leakage of lymph from recipient lymphatic channels,2 use of diuretics,2 obesity,3 kidney biopsy,4 acute rejection,3 and the use of sirolimus5 (Rapamune) and high-dose corticosteroids.6 Some believe that lymphoceles may also arise from severed lymphatic vessels of the donor-kidney allograft.7
Ultrasonography can usually distinguish a lymphocele from other fluid collections on the basis of fluid appearance, shape, and position. In most cases, the lymphocele is adjacent to the lower pole and medial to the allograft, and appears anechoic on ultrasonography, with a thin, distinct wall. The typical features on analysis of aspirated fluid—ie, a creatinine level approximately the same as in the serum, a low protein value, and a high lymphocyte count compared with serum values—confirm the diagnosis of lymphocele.
A hematoma can occur in any location and have a heterogeneous appearance, as it contains both clotted (echogenic) and unclotted (anechoic) blood. They are usually seen within the first 1 to 2 weeks after surgery and may also develop after trauma or renal biopsy.
A urinoma is a collection of urine outside the bladder, resulting from a ureteral leak. They are predominantly anechoic, with an often indistinct wall. If there is a clinical suspicion, the diagnosis can be confirmed on aspiration by a high creatinine level in the fluid compared with the serum value.
A perirenal abscess commonly presents with pain, fever, and a complex fluid collection on ultrasonography, sometimes with an air fluid level. Aspiration of purulent fluid confirms the diagnosis.
A simple renal cyst appears within or protruding from the renal parenchyma as a spherical or eggshaped fluid-filled sac with an anechoic lumen and no measurable wall thickness.
SYMPTOMS AND MANAGEMENT
Lymphoceles are mostly inconsequential but can cause renal failure by compressing the ureter, renal vessels, or renal allograft. Other manifestations may include pain and swelling at the kidney allograft site, wound drainage, unilateral lower-extremity edema, deep vein thrombosis due to compression of iliac veins,8 urinary urgency or frequency due to extrinsic bladder compression, and urinary retention.9
If the lymphocele is clinically significant, percutaneous drainage guided by ultrasonography is recommended as the initial curative procedure.10 Sclerotherapy with different chemical agents is effective, but success depends on the size of the lymphocele cavity.11
If these conservative therapies fail, lymphocele unroofing into the peritoneal cavity is needed. This is accomplished by laparoscopy12,13 or open surgery. Although laparoscopic drainage is considered the procedure of choice, open surgery may be required for multiloculated lymphoceles and those adjacent to vital structures.14,15
Kidneys are the most commonly transplanted solid organs. Every year, about 16,000 kidney transplantations are performed in the United States. It is common for the primary care physician to initially see these patients in cases of associated complications. Internists must be aware of the common causes of acute renal failure in this population, eg, acute rejection, drug toxicity, and obstruction. Lymphoceles are an important cause of renal failure due to obstruction. Early recognition and appropriate treatment of this complication can improve the outcome of the allograft.
- O’neill WC, Baumgarten DA. Ultrasonography in renal transplantation. Am J Kidney Dis 2002; 39:663–678.
- Braun WE, Banowsky LH, Straffon RA, et al. Lymphocytes associated with renal transplantation. Report of 15 cases and review of the literature. Am J Med 1974; 57:714–729.
- Goel M, Flechner SM, Zhou L, et al. The influence of various maintenance immunosuppressive drugs on lymphocele formation and treatment after kidney transplantation. J Urol 2004; 171:1788–1792.
- Mundy AR, Podesta ML, Bewick M, Rudge CJ, Ellis FG. The urological complications of 1000 renal transplants. Br J Urol 1981; 53:397–402.
- Giessing M, Fischer TJ, Deger S, et al. Increased frequency of lymphoceles under treatment with sirolimus following renal transplantation: a single center experience. Transplant Proc 2002; 34:1815–1816.
- Amante AJ, Kahan BD. Technical complications of renal transplantation. Surg Clin North Am 1994; 74:1117–1131.
- Saidi RF, Wertheim JA, Ko DS, et al. Impact of donor kidney recovery method on lymphatic complications in kidney transplantation. Transplant Proc 2008; 40:1054–1055.
- Iwan-Zietek I, Zietek Z, Sulikowski T, et al. Minimally invasive methods for the treatment of lymphocele after kidney transplantation. Transplant Proc 2009; 41:3073–3076.
- Hwang EC, Kang TW, Koh YS, et al. Post-transplant lymphocele: an unusual cause of acute urinary retention mimicking urethral injury. Int J Urol 2006; 13:468–470.
- Zietek Z, Sulikowski T, Tejchman K, et al. Lymphocele after kidney transplantation. Transplant Proc 2007; 39:2744–2747.
- Mahrer A, Ramchandani P, Trerotola SO, Shlansky-Goldberg RD, Itkin M. Sclerotherapy in the management of postoperative lymphocele. J Vasc Interv Radiol 2010; 21:1050–1053.
- Risaliti A, Corno V, Donini A, et al. Laparoscopic treatment of symptomatic lymphoceles after kidney transplantation. Surg Endosc 2000; 14:293–295.
- Ostrowski M, Lubikowski J, Kowalczyk M, Power J. Laparoscopic lymphocele drainage after renal transplantation. Ann Transplant 2000; 5:25–27.
- Fuller TF, Kang SM, Hirose R, Feng S, Stock PG, Freise CE. Management of lymphoceles after renal transplantation: laparoscopic versus open drainage. J Urol 2003; 169:2022–2025.
- Hsu TH, Gill IS, Grune MT, et al. Laparoscopic lymphocelectomy: a multi-institutional analysis. J Urol 2000; 163:1096–1098.
A 42-year-old man presented with acute renal failure with a serum creatinine of 6.07 mg/dL (baseline 2.0 mg/dL) 6 months after receiving a kidney transplant from a deceased donor. He was asymptomatic, had no previous symptoms of transplant rejection, and was compliant with his immunosuppressive regimen. The physical examination and the rest of the laboratory workup were normal.
Q: Which is the most likely diagnosis?
- A lymphocele
- A hematoma
- A urinoma
- A perirenal abscess
- A simple renal cyst
A: A lymphocele is the most likely diagnosis. A lymphocele— a collection of lymph without an epithelial lining—develops in as many as 20% of kidney transplant recipients.1 Many causative factors have been proposed, including leakage of lymph from recipient lymphatic channels,2 use of diuretics,2 obesity,3 kidney biopsy,4 acute rejection,3 and the use of sirolimus5 (Rapamune) and high-dose corticosteroids.6 Some believe that lymphoceles may also arise from severed lymphatic vessels of the donor-kidney allograft.7
Ultrasonography can usually distinguish a lymphocele from other fluid collections on the basis of fluid appearance, shape, and position. In most cases, the lymphocele is adjacent to the lower pole and medial to the allograft, and appears anechoic on ultrasonography, with a thin, distinct wall. The typical features on analysis of aspirated fluid—ie, a creatinine level approximately the same as in the serum, a low protein value, and a high lymphocyte count compared with serum values—confirm the diagnosis of lymphocele.
A hematoma can occur in any location and have a heterogeneous appearance, as it contains both clotted (echogenic) and unclotted (anechoic) blood. They are usually seen within the first 1 to 2 weeks after surgery and may also develop after trauma or renal biopsy.
A urinoma is a collection of urine outside the bladder, resulting from a ureteral leak. They are predominantly anechoic, with an often indistinct wall. If there is a clinical suspicion, the diagnosis can be confirmed on aspiration by a high creatinine level in the fluid compared with the serum value.
A perirenal abscess commonly presents with pain, fever, and a complex fluid collection on ultrasonography, sometimes with an air fluid level. Aspiration of purulent fluid confirms the diagnosis.
A simple renal cyst appears within or protruding from the renal parenchyma as a spherical or eggshaped fluid-filled sac with an anechoic lumen and no measurable wall thickness.
SYMPTOMS AND MANAGEMENT
Lymphoceles are mostly inconsequential but can cause renal failure by compressing the ureter, renal vessels, or renal allograft. Other manifestations may include pain and swelling at the kidney allograft site, wound drainage, unilateral lower-extremity edema, deep vein thrombosis due to compression of iliac veins,8 urinary urgency or frequency due to extrinsic bladder compression, and urinary retention.9
If the lymphocele is clinically significant, percutaneous drainage guided by ultrasonography is recommended as the initial curative procedure.10 Sclerotherapy with different chemical agents is effective, but success depends on the size of the lymphocele cavity.11
If these conservative therapies fail, lymphocele unroofing into the peritoneal cavity is needed. This is accomplished by laparoscopy12,13 or open surgery. Although laparoscopic drainage is considered the procedure of choice, open surgery may be required for multiloculated lymphoceles and those adjacent to vital structures.14,15
Kidneys are the most commonly transplanted solid organs. Every year, about 16,000 kidney transplantations are performed in the United States. It is common for the primary care physician to initially see these patients in cases of associated complications. Internists must be aware of the common causes of acute renal failure in this population, eg, acute rejection, drug toxicity, and obstruction. Lymphoceles are an important cause of renal failure due to obstruction. Early recognition and appropriate treatment of this complication can improve the outcome of the allograft.
A 42-year-old man presented with acute renal failure with a serum creatinine of 6.07 mg/dL (baseline 2.0 mg/dL) 6 months after receiving a kidney transplant from a deceased donor. He was asymptomatic, had no previous symptoms of transplant rejection, and was compliant with his immunosuppressive regimen. The physical examination and the rest of the laboratory workup were normal.
Q: Which is the most likely diagnosis?
- A lymphocele
- A hematoma
- A urinoma
- A perirenal abscess
- A simple renal cyst
A: A lymphocele is the most likely diagnosis. A lymphocele— a collection of lymph without an epithelial lining—develops in as many as 20% of kidney transplant recipients.1 Many causative factors have been proposed, including leakage of lymph from recipient lymphatic channels,2 use of diuretics,2 obesity,3 kidney biopsy,4 acute rejection,3 and the use of sirolimus5 (Rapamune) and high-dose corticosteroids.6 Some believe that lymphoceles may also arise from severed lymphatic vessels of the donor-kidney allograft.7
Ultrasonography can usually distinguish a lymphocele from other fluid collections on the basis of fluid appearance, shape, and position. In most cases, the lymphocele is adjacent to the lower pole and medial to the allograft, and appears anechoic on ultrasonography, with a thin, distinct wall. The typical features on analysis of aspirated fluid—ie, a creatinine level approximately the same as in the serum, a low protein value, and a high lymphocyte count compared with serum values—confirm the diagnosis of lymphocele.
A hematoma can occur in any location and have a heterogeneous appearance, as it contains both clotted (echogenic) and unclotted (anechoic) blood. They are usually seen within the first 1 to 2 weeks after surgery and may also develop after trauma or renal biopsy.
A urinoma is a collection of urine outside the bladder, resulting from a ureteral leak. They are predominantly anechoic, with an often indistinct wall. If there is a clinical suspicion, the diagnosis can be confirmed on aspiration by a high creatinine level in the fluid compared with the serum value.
A perirenal abscess commonly presents with pain, fever, and a complex fluid collection on ultrasonography, sometimes with an air fluid level. Aspiration of purulent fluid confirms the diagnosis.
A simple renal cyst appears within or protruding from the renal parenchyma as a spherical or eggshaped fluid-filled sac with an anechoic lumen and no measurable wall thickness.
SYMPTOMS AND MANAGEMENT
Lymphoceles are mostly inconsequential but can cause renal failure by compressing the ureter, renal vessels, or renal allograft. Other manifestations may include pain and swelling at the kidney allograft site, wound drainage, unilateral lower-extremity edema, deep vein thrombosis due to compression of iliac veins,8 urinary urgency or frequency due to extrinsic bladder compression, and urinary retention.9
If the lymphocele is clinically significant, percutaneous drainage guided by ultrasonography is recommended as the initial curative procedure.10 Sclerotherapy with different chemical agents is effective, but success depends on the size of the lymphocele cavity.11
If these conservative therapies fail, lymphocele unroofing into the peritoneal cavity is needed. This is accomplished by laparoscopy12,13 or open surgery. Although laparoscopic drainage is considered the procedure of choice, open surgery may be required for multiloculated lymphoceles and those adjacent to vital structures.14,15
Kidneys are the most commonly transplanted solid organs. Every year, about 16,000 kidney transplantations are performed in the United States. It is common for the primary care physician to initially see these patients in cases of associated complications. Internists must be aware of the common causes of acute renal failure in this population, eg, acute rejection, drug toxicity, and obstruction. Lymphoceles are an important cause of renal failure due to obstruction. Early recognition and appropriate treatment of this complication can improve the outcome of the allograft.
- O’neill WC, Baumgarten DA. Ultrasonography in renal transplantation. Am J Kidney Dis 2002; 39:663–678.
- Braun WE, Banowsky LH, Straffon RA, et al. Lymphocytes associated with renal transplantation. Report of 15 cases and review of the literature. Am J Med 1974; 57:714–729.
- Goel M, Flechner SM, Zhou L, et al. The influence of various maintenance immunosuppressive drugs on lymphocele formation and treatment after kidney transplantation. J Urol 2004; 171:1788–1792.
- Mundy AR, Podesta ML, Bewick M, Rudge CJ, Ellis FG. The urological complications of 1000 renal transplants. Br J Urol 1981; 53:397–402.
- Giessing M, Fischer TJ, Deger S, et al. Increased frequency of lymphoceles under treatment with sirolimus following renal transplantation: a single center experience. Transplant Proc 2002; 34:1815–1816.
- Amante AJ, Kahan BD. Technical complications of renal transplantation. Surg Clin North Am 1994; 74:1117–1131.
- Saidi RF, Wertheim JA, Ko DS, et al. Impact of donor kidney recovery method on lymphatic complications in kidney transplantation. Transplant Proc 2008; 40:1054–1055.
- Iwan-Zietek I, Zietek Z, Sulikowski T, et al. Minimally invasive methods for the treatment of lymphocele after kidney transplantation. Transplant Proc 2009; 41:3073–3076.
- Hwang EC, Kang TW, Koh YS, et al. Post-transplant lymphocele: an unusual cause of acute urinary retention mimicking urethral injury. Int J Urol 2006; 13:468–470.
- Zietek Z, Sulikowski T, Tejchman K, et al. Lymphocele after kidney transplantation. Transplant Proc 2007; 39:2744–2747.
- Mahrer A, Ramchandani P, Trerotola SO, Shlansky-Goldberg RD, Itkin M. Sclerotherapy in the management of postoperative lymphocele. J Vasc Interv Radiol 2010; 21:1050–1053.
- Risaliti A, Corno V, Donini A, et al. Laparoscopic treatment of symptomatic lymphoceles after kidney transplantation. Surg Endosc 2000; 14:293–295.
- Ostrowski M, Lubikowski J, Kowalczyk M, Power J. Laparoscopic lymphocele drainage after renal transplantation. Ann Transplant 2000; 5:25–27.
- Fuller TF, Kang SM, Hirose R, Feng S, Stock PG, Freise CE. Management of lymphoceles after renal transplantation: laparoscopic versus open drainage. J Urol 2003; 169:2022–2025.
- Hsu TH, Gill IS, Grune MT, et al. Laparoscopic lymphocelectomy: a multi-institutional analysis. J Urol 2000; 163:1096–1098.
- O’neill WC, Baumgarten DA. Ultrasonography in renal transplantation. Am J Kidney Dis 2002; 39:663–678.
- Braun WE, Banowsky LH, Straffon RA, et al. Lymphocytes associated with renal transplantation. Report of 15 cases and review of the literature. Am J Med 1974; 57:714–729.
- Goel M, Flechner SM, Zhou L, et al. The influence of various maintenance immunosuppressive drugs on lymphocele formation and treatment after kidney transplantation. J Urol 2004; 171:1788–1792.
- Mundy AR, Podesta ML, Bewick M, Rudge CJ, Ellis FG. The urological complications of 1000 renal transplants. Br J Urol 1981; 53:397–402.
- Giessing M, Fischer TJ, Deger S, et al. Increased frequency of lymphoceles under treatment with sirolimus following renal transplantation: a single center experience. Transplant Proc 2002; 34:1815–1816.
- Amante AJ, Kahan BD. Technical complications of renal transplantation. Surg Clin North Am 1994; 74:1117–1131.
- Saidi RF, Wertheim JA, Ko DS, et al. Impact of donor kidney recovery method on lymphatic complications in kidney transplantation. Transplant Proc 2008; 40:1054–1055.
- Iwan-Zietek I, Zietek Z, Sulikowski T, et al. Minimally invasive methods for the treatment of lymphocele after kidney transplantation. Transplant Proc 2009; 41:3073–3076.
- Hwang EC, Kang TW, Koh YS, et al. Post-transplant lymphocele: an unusual cause of acute urinary retention mimicking urethral injury. Int J Urol 2006; 13:468–470.
- Zietek Z, Sulikowski T, Tejchman K, et al. Lymphocele after kidney transplantation. Transplant Proc 2007; 39:2744–2747.
- Mahrer A, Ramchandani P, Trerotola SO, Shlansky-Goldberg RD, Itkin M. Sclerotherapy in the management of postoperative lymphocele. J Vasc Interv Radiol 2010; 21:1050–1053.
- Risaliti A, Corno V, Donini A, et al. Laparoscopic treatment of symptomatic lymphoceles after kidney transplantation. Surg Endosc 2000; 14:293–295.
- Ostrowski M, Lubikowski J, Kowalczyk M, Power J. Laparoscopic lymphocele drainage after renal transplantation. Ann Transplant 2000; 5:25–27.
- Fuller TF, Kang SM, Hirose R, Feng S, Stock PG, Freise CE. Management of lymphoceles after renal transplantation: laparoscopic versus open drainage. J Urol 2003; 169:2022–2025.
- Hsu TH, Gill IS, Grune MT, et al. Laparoscopic lymphocelectomy: a multi-institutional analysis. J Urol 2000; 163:1096–1098.
Can Inflamed Gums Cause Kidney Problems?
A 37-Year-Old Man With Symptoms of Fatigue, Malaise, and Dizziness
Clinicians Are Asking: Renal Diet
Your renal practitioners/department editors have chosen three typical situations you might encounter in practice.
• Nutrition and diet help control kidney disease, but also heart disease, diabetes, and other comorbid states.
• Renal patients, like many others, often require surgeries; what specific concerns exist for surgical patients requiring dialysis?
• The Medicare education benefit has been a particular bonus for advanced practitioners, as we teach many of the classes.
We welcome your questions and comments.
Q: What is the renal diet? Should my patients with chronic kidney disease (CKD) restrict their protein consumption?
The CKD nondialysis diet aims to preserve remaining kidney function. A person living with kidney disease can continue to enjoy a variety of foods, including whole grains, fruits, and vegetables. These foods must be restricted only when phosphorus, parathyroid hormone, and/or potassium levels become elevated. However, many advanced practitioners recommend avoiding dark sodas because of their high phosphorus content. Sodium is limited to help maintain blood pressure control and decrease fluid buildup.
Fluid intake is not restricted unless fluid retention becomes an issue. Adequate caloric intake from carbohydrates and healthy fats is essential so as to spare protein for growth and repair. Aiming for a healthy weight through appropriate caloric intake and regular physical activity is important. A water-soluble vitamin B complex and a vitamin C supplement may be recommended as the diet becomes more restrictive. Supplemental vitamin D requirements and iron needs are based on findings from laboratory studies.
As is always the case when advising patients on food choices, the emphasis should be on optimizing nutrition and avoiding empty calories. A review of how to interpret a food label is often helpful to patients and their families.
Dietary protein recommendations continue to be controversial in CKD stages 1 through 4 (estimated glomerular filtration rate [eGFR] < 30 mL/min/1.73 m2). The renal diet emphasizes high-quality proteins but limits protein intake to approximately 0.6 to 0.8 g/kg/d so as to decrease the workload on the kidneys and reduce urea waste production. The National Kidney Foundation Kidney Disease Outcome Quality Initiative (NKF-K/DOQI) Clinical Practice Guidelines for nutrition in patients with CKD1 recommend that patients who have an eGFR between 25 and 55 mL/min/1.73 m2 should eat at least 0.8 g/kg/d of protein; and that those whose eGFR is less than 25 mL/min/1.73 m2 and who are not receiving dialysis consume 0.6 g/kg/d. If a patient cannot tolerate the diet or is unable to maintain an adequate caloric intake, then protein intake can be 0.75 g/kg/d.
Once a patient is undergoing dialysis, the protein requirements may change, depending on the patient’s needs and type of dialysis. Fortunately, the renal dietician, an essential member of the interdisciplinary dialysis team, offers great assistance to the advanced practitioner in addressing the patient’s nutritional needs.
However, referral to a renal dietitian is recommended before dialysis, as diet is an important part of CKD treatment. A Medicare recipient with stage 3 or 4 CKD can see a registered dietitian through the Medical Nutrition Therapy benefit.2
Individualizing nutritional therapy is essential to optimize health in people living with the complexities of CKD. It is also very important, when assessing, monitoring, and intervening to avoid or treat malnutrition in these patients, to provide care as an interprofessional team that includes a renal dietician. (To provide the best evidence available, an experienced renal dietician was asked to contribute to this response.)
Debra Hain, PhD, APRN, GNP-BC
Assistant Professor, Christine E. Lynn College of Nursing, Florida Atlantic University, Boca Raton; Nurse Practitioner, Cleveland Clinic Florida, Weston
Susan Meese-Morris, RD, LD/N
Renal Dietitian, Pine Island, Weston, and Miramar, Florida
REFERENCES
1. National Kidney Foundation Kidney Disease Outcome Quality Initiative (NKF-K/DOQI) Clinical Practice Guidelines for Nutrition in Chronic Renal Failure (2000). www.kidney.org/professionals/kdoqi/guidelines_updates/doqi_nut.html. Accessed February 16, 2012.
2. Medicare.gov. Medical nutrition therapy. www.medicare.gov/navigation/manage-your-health/preventive-services/medical-nutrition-therapy.aspx?AspxAutoDetectCookieSupport=1. Accessed February 16, 2012.
3. Soundararajan R, Golper T. Medical management of the dialysis patient undergoing surgery. www.uptodate.com/contents/medical-management-of-the-dialysis-patient-undergoing-surgery. Accessed February 16, 2012.
4. Young HN, Chan MR, Yevzlin AS, Becker BN. The rationale, implementation and effects of the Medicare CKD education benefit. Am J Kidney Dis. 2011;57(3):381-386.
5. H. R. 6331: Medicare Improvements for Patients and Providers Act of 2008. www.govtrack.us/congress/bill.xpd?bill=h110-6331. Accessed February 16, 2012.
6. §410.48. Kidney disease education services. Federal Register. 2009;74(226):62003.
7. Lazarus JM. National health care policy and its effect on renal care. Presented at: NKFI Multi-Disciplinary Conference; September 24, 2009; Chicago, IL.
8. National Kidney Foundation. MIPPA Kidney Disease Education Benefit. Your Treatment, Your Choice (2010). www.kidney.org/professionals/KLS/YTYC.cfm. Accessed February 16, 2012.
Your renal practitioners/department editors have chosen three typical situations you might encounter in practice.
• Nutrition and diet help control kidney disease, but also heart disease, diabetes, and other comorbid states.
• Renal patients, like many others, often require surgeries; what specific concerns exist for surgical patients requiring dialysis?
• The Medicare education benefit has been a particular bonus for advanced practitioners, as we teach many of the classes.
We welcome your questions and comments.
Q: What is the renal diet? Should my patients with chronic kidney disease (CKD) restrict their protein consumption?
The CKD nondialysis diet aims to preserve remaining kidney function. A person living with kidney disease can continue to enjoy a variety of foods, including whole grains, fruits, and vegetables. These foods must be restricted only when phosphorus, parathyroid hormone, and/or potassium levels become elevated. However, many advanced practitioners recommend avoiding dark sodas because of their high phosphorus content. Sodium is limited to help maintain blood pressure control and decrease fluid buildup.
Fluid intake is not restricted unless fluid retention becomes an issue. Adequate caloric intake from carbohydrates and healthy fats is essential so as to spare protein for growth and repair. Aiming for a healthy weight through appropriate caloric intake and regular physical activity is important. A water-soluble vitamin B complex and a vitamin C supplement may be recommended as the diet becomes more restrictive. Supplemental vitamin D requirements and iron needs are based on findings from laboratory studies.
As is always the case when advising patients on food choices, the emphasis should be on optimizing nutrition and avoiding empty calories. A review of how to interpret a food label is often helpful to patients and their families.
Dietary protein recommendations continue to be controversial in CKD stages 1 through 4 (estimated glomerular filtration rate [eGFR] < 30 mL/min/1.73 m2). The renal diet emphasizes high-quality proteins but limits protein intake to approximately 0.6 to 0.8 g/kg/d so as to decrease the workload on the kidneys and reduce urea waste production. The National Kidney Foundation Kidney Disease Outcome Quality Initiative (NKF-K/DOQI) Clinical Practice Guidelines for nutrition in patients with CKD1 recommend that patients who have an eGFR between 25 and 55 mL/min/1.73 m2 should eat at least 0.8 g/kg/d of protein; and that those whose eGFR is less than 25 mL/min/1.73 m2 and who are not receiving dialysis consume 0.6 g/kg/d. If a patient cannot tolerate the diet or is unable to maintain an adequate caloric intake, then protein intake can be 0.75 g/kg/d.
Once a patient is undergoing dialysis, the protein requirements may change, depending on the patient’s needs and type of dialysis. Fortunately, the renal dietician, an essential member of the interdisciplinary dialysis team, offers great assistance to the advanced practitioner in addressing the patient’s nutritional needs.
However, referral to a renal dietitian is recommended before dialysis, as diet is an important part of CKD treatment. A Medicare recipient with stage 3 or 4 CKD can see a registered dietitian through the Medical Nutrition Therapy benefit.2
Individualizing nutritional therapy is essential to optimize health in people living with the complexities of CKD. It is also very important, when assessing, monitoring, and intervening to avoid or treat malnutrition in these patients, to provide care as an interprofessional team that includes a renal dietician. (To provide the best evidence available, an experienced renal dietician was asked to contribute to this response.)
Debra Hain, PhD, APRN, GNP-BC
Assistant Professor, Christine E. Lynn College of Nursing, Florida Atlantic University, Boca Raton; Nurse Practitioner, Cleveland Clinic Florida, Weston
Susan Meese-Morris, RD, LD/N
Renal Dietitian, Pine Island, Weston, and Miramar, Florida
REFERENCES
1. National Kidney Foundation Kidney Disease Outcome Quality Initiative (NKF-K/DOQI) Clinical Practice Guidelines for Nutrition in Chronic Renal Failure (2000). www.kidney.org/professionals/kdoqi/guidelines_updates/doqi_nut.html. Accessed February 16, 2012.
2. Medicare.gov. Medical nutrition therapy. www.medicare.gov/navigation/manage-your-health/preventive-services/medical-nutrition-therapy.aspx?AspxAutoDetectCookieSupport=1. Accessed February 16, 2012.
3. Soundararajan R, Golper T. Medical management of the dialysis patient undergoing surgery. www.uptodate.com/contents/medical-management-of-the-dialysis-patient-undergoing-surgery. Accessed February 16, 2012.
4. Young HN, Chan MR, Yevzlin AS, Becker BN. The rationale, implementation and effects of the Medicare CKD education benefit. Am J Kidney Dis. 2011;57(3):381-386.
5. H. R. 6331: Medicare Improvements for Patients and Providers Act of 2008. www.govtrack.us/congress/bill.xpd?bill=h110-6331. Accessed February 16, 2012.
6. §410.48. Kidney disease education services. Federal Register. 2009;74(226):62003.
7. Lazarus JM. National health care policy and its effect on renal care. Presented at: NKFI Multi-Disciplinary Conference; September 24, 2009; Chicago, IL.
8. National Kidney Foundation. MIPPA Kidney Disease Education Benefit. Your Treatment, Your Choice (2010). www.kidney.org/professionals/KLS/YTYC.cfm. Accessed February 16, 2012.
Your renal practitioners/department editors have chosen three typical situations you might encounter in practice.
• Nutrition and diet help control kidney disease, but also heart disease, diabetes, and other comorbid states.
• Renal patients, like many others, often require surgeries; what specific concerns exist for surgical patients requiring dialysis?
• The Medicare education benefit has been a particular bonus for advanced practitioners, as we teach many of the classes.
We welcome your questions and comments.
Q: What is the renal diet? Should my patients with chronic kidney disease (CKD) restrict their protein consumption?
The CKD nondialysis diet aims to preserve remaining kidney function. A person living with kidney disease can continue to enjoy a variety of foods, including whole grains, fruits, and vegetables. These foods must be restricted only when phosphorus, parathyroid hormone, and/or potassium levels become elevated. However, many advanced practitioners recommend avoiding dark sodas because of their high phosphorus content. Sodium is limited to help maintain blood pressure control and decrease fluid buildup.
Fluid intake is not restricted unless fluid retention becomes an issue. Adequate caloric intake from carbohydrates and healthy fats is essential so as to spare protein for growth and repair. Aiming for a healthy weight through appropriate caloric intake and regular physical activity is important. A water-soluble vitamin B complex and a vitamin C supplement may be recommended as the diet becomes more restrictive. Supplemental vitamin D requirements and iron needs are based on findings from laboratory studies.
As is always the case when advising patients on food choices, the emphasis should be on optimizing nutrition and avoiding empty calories. A review of how to interpret a food label is often helpful to patients and their families.
Dietary protein recommendations continue to be controversial in CKD stages 1 through 4 (estimated glomerular filtration rate [eGFR] < 30 mL/min/1.73 m2). The renal diet emphasizes high-quality proteins but limits protein intake to approximately 0.6 to 0.8 g/kg/d so as to decrease the workload on the kidneys and reduce urea waste production. The National Kidney Foundation Kidney Disease Outcome Quality Initiative (NKF-K/DOQI) Clinical Practice Guidelines for nutrition in patients with CKD1 recommend that patients who have an eGFR between 25 and 55 mL/min/1.73 m2 should eat at least 0.8 g/kg/d of protein; and that those whose eGFR is less than 25 mL/min/1.73 m2 and who are not receiving dialysis consume 0.6 g/kg/d. If a patient cannot tolerate the diet or is unable to maintain an adequate caloric intake, then protein intake can be 0.75 g/kg/d.
Once a patient is undergoing dialysis, the protein requirements may change, depending on the patient’s needs and type of dialysis. Fortunately, the renal dietician, an essential member of the interdisciplinary dialysis team, offers great assistance to the advanced practitioner in addressing the patient’s nutritional needs.
However, referral to a renal dietitian is recommended before dialysis, as diet is an important part of CKD treatment. A Medicare recipient with stage 3 or 4 CKD can see a registered dietitian through the Medical Nutrition Therapy benefit.2
Individualizing nutritional therapy is essential to optimize health in people living with the complexities of CKD. It is also very important, when assessing, monitoring, and intervening to avoid or treat malnutrition in these patients, to provide care as an interprofessional team that includes a renal dietician. (To provide the best evidence available, an experienced renal dietician was asked to contribute to this response.)
Debra Hain, PhD, APRN, GNP-BC
Assistant Professor, Christine E. Lynn College of Nursing, Florida Atlantic University, Boca Raton; Nurse Practitioner, Cleveland Clinic Florida, Weston
Susan Meese-Morris, RD, LD/N
Renal Dietitian, Pine Island, Weston, and Miramar, Florida
REFERENCES
1. National Kidney Foundation Kidney Disease Outcome Quality Initiative (NKF-K/DOQI) Clinical Practice Guidelines for Nutrition in Chronic Renal Failure (2000). www.kidney.org/professionals/kdoqi/guidelines_updates/doqi_nut.html. Accessed February 16, 2012.
2. Medicare.gov. Medical nutrition therapy. www.medicare.gov/navigation/manage-your-health/preventive-services/medical-nutrition-therapy.aspx?AspxAutoDetectCookieSupport=1. Accessed February 16, 2012.
3. Soundararajan R, Golper T. Medical management of the dialysis patient undergoing surgery. www.uptodate.com/contents/medical-management-of-the-dialysis-patient-undergoing-surgery. Accessed February 16, 2012.
4. Young HN, Chan MR, Yevzlin AS, Becker BN. The rationale, implementation and effects of the Medicare CKD education benefit. Am J Kidney Dis. 2011;57(3):381-386.
5. H. R. 6331: Medicare Improvements for Patients and Providers Act of 2008. www.govtrack.us/congress/bill.xpd?bill=h110-6331. Accessed February 16, 2012.
6. §410.48. Kidney disease education services. Federal Register. 2009;74(226):62003.
7. Lazarus JM. National health care policy and its effect on renal care. Presented at: NKFI Multi-Disciplinary Conference; September 24, 2009; Chicago, IL.
8. National Kidney Foundation. MIPPA Kidney Disease Education Benefit. Your Treatment, Your Choice (2010). www.kidney.org/professionals/KLS/YTYC.cfm. Accessed February 16, 2012.
Clinicians Are Asking: Kidney Disease Education Classes
Your renal practitioners/department editors have chosen three typical situations you might encounter in practice.
• Nutrition and diet help control kidney disease, but also heart disease, diabetes, and other comorbid states.
• Renal patients, like many others, often require surgeries; what specific concerns exist for surgical patients requiring dialysis?
• The Medicare education benefit has been a particular bonus for advanced practitioners, as we teach many of the classes.
We welcome your questions and comments.
Q: Our practice received a flyer for kidney disease education classes offered by the local nephrology group. Can you tell me more about these classes?
Patient education in kidney disease has been shown to delay disease progression and improve patient outcomes.4 Because of this, the Medicare Improvements for Patients and Providers Act (MIPPA) of 20085 provided for classes for patients with stage 4 CKD (GFR, 15 to 29 mL/min/1.73 m2) to receive six hours of education over their lifetime.
Classes can be taught by a physician or an advanced practitioner (a PA, an NP, or a clinical nurse specialist). Four broad areas are covered: management of comorbidities that occur with CKD; prevention of complications, including an explanation of how the kidneys work and a review of medications; renal replacement modalities, including hemodialysis, peritoneal dialysis, and transplantation; and opportunities to empower the patients as active partners in their own health care.6 Classes also include information on managing anemia, hypertension, and bone mineral disease.7
Class structure is up to the provider. Most practices offer classes to all stage 4 CKD patients, regardless of Medicare status. Classes can be taught on a one-to-one basis or in a group setting.8
Some practices design their own format, while others use programs designed for CKD education. The National Kidney Foundation developed a slide set called Your Treatment, Your Choice,8 while the Cleveland Clinic, the Mayo Clinic, and the University of Alabama at Birmingham (among others, no doubt), have developed their own in-house programs. All these programs have a prepared Power Point slide deck, and most include evaluation tools.
Tricia Howard, MHS, PA-C
South University, Savannah, Georgia
REFERENCES
1. National Kidney Foundation Kidney Disease Outcome Quality Initiative (NKF-K/DOQI) Clinical Practice Guidelines for Nutrition in Chronic Renal Failure (2000). www.kidney.org/professionals/kdoqi/guidelines_updates/doqi_nut.html. Accessed February 16, 2012.
2. Medicare.gov. Medical nutrition therapy. www.medicare.gov/navigation/manage-your-health/preventive-services/medical-nutrition-therapy.aspx?AspxAutoDetectCookieSupport=1. Accessed February 16, 2012.
3. Soundararajan R, Golper T. Medical management of the dialysis patient undergoing surgery. www.uptodate.com/contents/medical-management-of-the-dialysis-patient-undergoing-surgery. Accessed February 16, 2012.
4. Young HN, Chan MR, Yevzlin AS, Becker BN. The rationale, implementation and effects of the Medicare CKD education benefit. Am J Kidney Dis. 2011;57(3):381-386.
5. H. R. 6331: Medicare Improvements for Patients and Providers Act of 2008. www.govtrack.us/congress/bill.xpd?bill=h110-6331. Accessed February 16, 2012.
6. §410.48. Kidney disease education services. Federal Register. 2009;74(226):62003.
7. Lazarus JM. National health care policy and its effect on renal care. Presented at: NKFI Multi-Disciplinary Conference; September 24, 2009; Chicago, IL.
8. National Kidney Foundation. MIPPA Kidney Disease Education Benefit. Your Treatment, Your Choice (2010). www.kidney.org/professionals/KLS/YTYC.cfm. Accessed February 16, 2012.
Your renal practitioners/department editors have chosen three typical situations you might encounter in practice.
• Nutrition and diet help control kidney disease, but also heart disease, diabetes, and other comorbid states.
• Renal patients, like many others, often require surgeries; what specific concerns exist for surgical patients requiring dialysis?
• The Medicare education benefit has been a particular bonus for advanced practitioners, as we teach many of the classes.
We welcome your questions and comments.
Q: Our practice received a flyer for kidney disease education classes offered by the local nephrology group. Can you tell me more about these classes?
Patient education in kidney disease has been shown to delay disease progression and improve patient outcomes.4 Because of this, the Medicare Improvements for Patients and Providers Act (MIPPA) of 20085 provided for classes for patients with stage 4 CKD (GFR, 15 to 29 mL/min/1.73 m2) to receive six hours of education over their lifetime.
Classes can be taught by a physician or an advanced practitioner (a PA, an NP, or a clinical nurse specialist). Four broad areas are covered: management of comorbidities that occur with CKD; prevention of complications, including an explanation of how the kidneys work and a review of medications; renal replacement modalities, including hemodialysis, peritoneal dialysis, and transplantation; and opportunities to empower the patients as active partners in their own health care.6 Classes also include information on managing anemia, hypertension, and bone mineral disease.7
Class structure is up to the provider. Most practices offer classes to all stage 4 CKD patients, regardless of Medicare status. Classes can be taught on a one-to-one basis or in a group setting.8
Some practices design their own format, while others use programs designed for CKD education. The National Kidney Foundation developed a slide set called Your Treatment, Your Choice,8 while the Cleveland Clinic, the Mayo Clinic, and the University of Alabama at Birmingham (among others, no doubt), have developed their own in-house programs. All these programs have a prepared Power Point slide deck, and most include evaluation tools.
Tricia Howard, MHS, PA-C
South University, Savannah, Georgia
REFERENCES
1. National Kidney Foundation Kidney Disease Outcome Quality Initiative (NKF-K/DOQI) Clinical Practice Guidelines for Nutrition in Chronic Renal Failure (2000). www.kidney.org/professionals/kdoqi/guidelines_updates/doqi_nut.html. Accessed February 16, 2012.
2. Medicare.gov. Medical nutrition therapy. www.medicare.gov/navigation/manage-your-health/preventive-services/medical-nutrition-therapy.aspx?AspxAutoDetectCookieSupport=1. Accessed February 16, 2012.
3. Soundararajan R, Golper T. Medical management of the dialysis patient undergoing surgery. www.uptodate.com/contents/medical-management-of-the-dialysis-patient-undergoing-surgery. Accessed February 16, 2012.
4. Young HN, Chan MR, Yevzlin AS, Becker BN. The rationale, implementation and effects of the Medicare CKD education benefit. Am J Kidney Dis. 2011;57(3):381-386.
5. H. R. 6331: Medicare Improvements for Patients and Providers Act of 2008. www.govtrack.us/congress/bill.xpd?bill=h110-6331. Accessed February 16, 2012.
6. §410.48. Kidney disease education services. Federal Register. 2009;74(226):62003.
7. Lazarus JM. National health care policy and its effect on renal care. Presented at: NKFI Multi-Disciplinary Conference; September 24, 2009; Chicago, IL.
8. National Kidney Foundation. MIPPA Kidney Disease Education Benefit. Your Treatment, Your Choice (2010). www.kidney.org/professionals/KLS/YTYC.cfm. Accessed February 16, 2012.
Your renal practitioners/department editors have chosen three typical situations you might encounter in practice.
• Nutrition and diet help control kidney disease, but also heart disease, diabetes, and other comorbid states.
• Renal patients, like many others, often require surgeries; what specific concerns exist for surgical patients requiring dialysis?
• The Medicare education benefit has been a particular bonus for advanced practitioners, as we teach many of the classes.
We welcome your questions and comments.
Q: Our practice received a flyer for kidney disease education classes offered by the local nephrology group. Can you tell me more about these classes?
Patient education in kidney disease has been shown to delay disease progression and improve patient outcomes.4 Because of this, the Medicare Improvements for Patients and Providers Act (MIPPA) of 20085 provided for classes for patients with stage 4 CKD (GFR, 15 to 29 mL/min/1.73 m2) to receive six hours of education over their lifetime.
Classes can be taught by a physician or an advanced practitioner (a PA, an NP, or a clinical nurse specialist). Four broad areas are covered: management of comorbidities that occur with CKD; prevention of complications, including an explanation of how the kidneys work and a review of medications; renal replacement modalities, including hemodialysis, peritoneal dialysis, and transplantation; and opportunities to empower the patients as active partners in their own health care.6 Classes also include information on managing anemia, hypertension, and bone mineral disease.7
Class structure is up to the provider. Most practices offer classes to all stage 4 CKD patients, regardless of Medicare status. Classes can be taught on a one-to-one basis or in a group setting.8
Some practices design their own format, while others use programs designed for CKD education. The National Kidney Foundation developed a slide set called Your Treatment, Your Choice,8 while the Cleveland Clinic, the Mayo Clinic, and the University of Alabama at Birmingham (among others, no doubt), have developed their own in-house programs. All these programs have a prepared Power Point slide deck, and most include evaluation tools.
Tricia Howard, MHS, PA-C
South University, Savannah, Georgia
REFERENCES
1. National Kidney Foundation Kidney Disease Outcome Quality Initiative (NKF-K/DOQI) Clinical Practice Guidelines for Nutrition in Chronic Renal Failure (2000). www.kidney.org/professionals/kdoqi/guidelines_updates/doqi_nut.html. Accessed February 16, 2012.
2. Medicare.gov. Medical nutrition therapy. www.medicare.gov/navigation/manage-your-health/preventive-services/medical-nutrition-therapy.aspx?AspxAutoDetectCookieSupport=1. Accessed February 16, 2012.
3. Soundararajan R, Golper T. Medical management of the dialysis patient undergoing surgery. www.uptodate.com/contents/medical-management-of-the-dialysis-patient-undergoing-surgery. Accessed February 16, 2012.
4. Young HN, Chan MR, Yevzlin AS, Becker BN. The rationale, implementation and effects of the Medicare CKD education benefit. Am J Kidney Dis. 2011;57(3):381-386.
5. H. R. 6331: Medicare Improvements for Patients and Providers Act of 2008. www.govtrack.us/congress/bill.xpd?bill=h110-6331. Accessed February 16, 2012.
6. §410.48. Kidney disease education services. Federal Register. 2009;74(226):62003.
7. Lazarus JM. National health care policy and its effect on renal care. Presented at: NKFI Multi-Disciplinary Conference; September 24, 2009; Chicago, IL.
8. National Kidney Foundation. MIPPA Kidney Disease Education Benefit. Your Treatment, Your Choice (2010). www.kidney.org/professionals/KLS/YTYC.cfm. Accessed February 16, 2012.