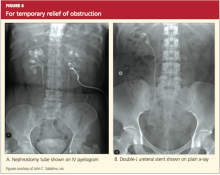
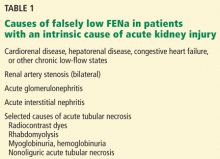
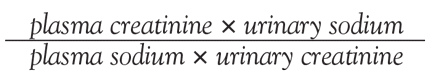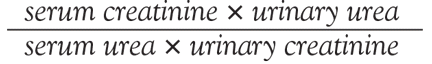User login
Clinicians Are Asking: Dialysis Patients Requiring Surgery
Your renal practitioners/department editors have chosen three typical situations you might encounter in practice.
• Nutrition and diet help control kidney disease, but also heart disease, diabetes, and other comorbid states.
• Renal patients, like many others, often require surgeries; what specific concerns exist for surgical patients requiring dialysis?
• The Medicare education benefit has been a particular bonus for advanced practitioners, as we teach many of the classes.
We welcome your questions and comments.
Q: We scheduled a total knee replacement for a patient on dialysis, and anesthesia balked because the patient had a potassium level of 5.5 mEq/L. The nephrology practice, apparently not concerned, agreed to dialyze the patient, but only because anesthesia insisted. If the practice uses our facility, where 5.3 mEq/L is the upper limit of serum potassium, how can a potassium level of 5.5 mEq/L not be of concern in a hemodialysis patient?
This is a question that occurs frequently regarding patients receiving dialysis. Hyperkalemia is a problem faced by many dialysis patients as a result of the kidneys’ inability to remove potassium with the loss of renal function. Patients’ potassium levels are monitored routinely, and low-potassium diets are a staple of any nephrology clinic or dialysis unit.
For patients in our dialysis unit, the normal potassium range is 3.5 to 6.0 mEq/L, which is 0.9 mEq/L higher than for a patient without end-stage renal disease (ESRD). Dialysis patients with ESRD often have an increased tolerance for hyperkalemia.
When potassium levels are elevated, a 12-lead ECG is used to detect any physiological cardiac changes. These are generally not seen until the serum potassium exceeds 6.0 to 6.5 mEq/L. ECG changes seen in hyperkalemia include peaked T waves, a prolonged PR interval, and absent P waves with a widened QRS complex. These changes, which can lead to ventricular tachycardia or ventricular fibrillation, are not based on numbers or values of serum potassium, but are thought to reflect the transcellular potassium gradient.3
When questioning a potassium level in a dialysis patient and considering whether presurgical dialysis is needed, it is important to consider the surgery planned. In surgeries during which potassium might be released secondary to tissue trauma, potassium levels can rise higher during surgery.3
It is important to assess hypokalemia as well. Arrhythmias such as premature atrial and ventricular beats, sinus bradycardia, paroxysmal atrial or junctional tachycardia, atrioventricular block, and ventricular tachycardia or fibrillation can occur with hypokalemia. ECG changes include depression of the ST segment, a decrease in the amplitude of the T wave, and an increase in the amplitude of U waves, which occur at the end of the T wave. U waves are often seen in the lateral precordial leads V4 to V6.3
Laura MacGregor, RN, MS, NP-C
Grand Street Medical Associates, Kingston, New York
REFERENCES
1. National Kidney Foundation Kidney Disease Outcome Quality Initiative (NKF-K/DOQI) Clinical Practice Guidelines for Nutrition in Chronic Renal Failure (2000). www.kidney.org/professionals/kdoqi/guidelines_updates/doqi_nut.html. Accessed February 16, 2012.
2. Medicare.gov. Medical nutrition therapy. www.medicare.gov/navigation/manage-your-health/preventive-services/medical-nutrition-therapy.aspx?AspxAutoDetectCookieSupport=1. Accessed February 16, 2012.
3. Soundararajan R, Golper T. Medical management of the dialysis patient undergoing surgery. www.uptodate.com/contents/medical-management-of-the-dialysis-patient-undergoing-surgery. Accessed February 16, 2012.
4. Young HN, Chan MR, Yevzlin AS, Becker BN. The rationale, implementation and effects of the Medicare CKD education benefit. Am J Kidney Dis. 2011;57(3):381-386.
5. H. R. 6331: Medicare Improvements for Patients and Providers Act of 2008. www.govtrack.us/congress/bill.xpd?bill=h110-6331. Accessed February 16, 2012.
6. §410.48. Kidney disease education services. Federal Register. 2009;74(226):62003.
7. Lazarus JM. National health care policy and its effect on renal care. Presented at: NKFI Multi-Disciplinary Conference; September 24, 2009; Chicago, IL.
8. National Kidney Foundation. MIPPA Kidney Disease Education Benefit. Your Treatment, Your Choice (2010). www.kidney.org/professionals/KLS/YTYC.cfm. Accessed February 16, 2012.
Your renal practitioners/department editors have chosen three typical situations you might encounter in practice.
• Nutrition and diet help control kidney disease, but also heart disease, diabetes, and other comorbid states.
• Renal patients, like many others, often require surgeries; what specific concerns exist for surgical patients requiring dialysis?
• The Medicare education benefit has been a particular bonus for advanced practitioners, as we teach many of the classes.
We welcome your questions and comments.
Q: We scheduled a total knee replacement for a patient on dialysis, and anesthesia balked because the patient had a potassium level of 5.5 mEq/L. The nephrology practice, apparently not concerned, agreed to dialyze the patient, but only because anesthesia insisted. If the practice uses our facility, where 5.3 mEq/L is the upper limit of serum potassium, how can a potassium level of 5.5 mEq/L not be of concern in a hemodialysis patient?
This is a question that occurs frequently regarding patients receiving dialysis. Hyperkalemia is a problem faced by many dialysis patients as a result of the kidneys’ inability to remove potassium with the loss of renal function. Patients’ potassium levels are monitored routinely, and low-potassium diets are a staple of any nephrology clinic or dialysis unit.
For patients in our dialysis unit, the normal potassium range is 3.5 to 6.0 mEq/L, which is 0.9 mEq/L higher than for a patient without end-stage renal disease (ESRD). Dialysis patients with ESRD often have an increased tolerance for hyperkalemia.
When potassium levels are elevated, a 12-lead ECG is used to detect any physiological cardiac changes. These are generally not seen until the serum potassium exceeds 6.0 to 6.5 mEq/L. ECG changes seen in hyperkalemia include peaked T waves, a prolonged PR interval, and absent P waves with a widened QRS complex. These changes, which can lead to ventricular tachycardia or ventricular fibrillation, are not based on numbers or values of serum potassium, but are thought to reflect the transcellular potassium gradient.3
When questioning a potassium level in a dialysis patient and considering whether presurgical dialysis is needed, it is important to consider the surgery planned. In surgeries during which potassium might be released secondary to tissue trauma, potassium levels can rise higher during surgery.3
It is important to assess hypokalemia as well. Arrhythmias such as premature atrial and ventricular beats, sinus bradycardia, paroxysmal atrial or junctional tachycardia, atrioventricular block, and ventricular tachycardia or fibrillation can occur with hypokalemia. ECG changes include depression of the ST segment, a decrease in the amplitude of the T wave, and an increase in the amplitude of U waves, which occur at the end of the T wave. U waves are often seen in the lateral precordial leads V4 to V6.3
Laura MacGregor, RN, MS, NP-C
Grand Street Medical Associates, Kingston, New York
REFERENCES
1. National Kidney Foundation Kidney Disease Outcome Quality Initiative (NKF-K/DOQI) Clinical Practice Guidelines for Nutrition in Chronic Renal Failure (2000). www.kidney.org/professionals/kdoqi/guidelines_updates/doqi_nut.html. Accessed February 16, 2012.
2. Medicare.gov. Medical nutrition therapy. www.medicare.gov/navigation/manage-your-health/preventive-services/medical-nutrition-therapy.aspx?AspxAutoDetectCookieSupport=1. Accessed February 16, 2012.
3. Soundararajan R, Golper T. Medical management of the dialysis patient undergoing surgery. www.uptodate.com/contents/medical-management-of-the-dialysis-patient-undergoing-surgery. Accessed February 16, 2012.
4. Young HN, Chan MR, Yevzlin AS, Becker BN. The rationale, implementation and effects of the Medicare CKD education benefit. Am J Kidney Dis. 2011;57(3):381-386.
5. H. R. 6331: Medicare Improvements for Patients and Providers Act of 2008. www.govtrack.us/congress/bill.xpd?bill=h110-6331. Accessed February 16, 2012.
6. §410.48. Kidney disease education services. Federal Register. 2009;74(226):62003.
7. Lazarus JM. National health care policy and its effect on renal care. Presented at: NKFI Multi-Disciplinary Conference; September 24, 2009; Chicago, IL.
8. National Kidney Foundation. MIPPA Kidney Disease Education Benefit. Your Treatment, Your Choice (2010). www.kidney.org/professionals/KLS/YTYC.cfm. Accessed February 16, 2012.
Your renal practitioners/department editors have chosen three typical situations you might encounter in practice.
• Nutrition and diet help control kidney disease, but also heart disease, diabetes, and other comorbid states.
• Renal patients, like many others, often require surgeries; what specific concerns exist for surgical patients requiring dialysis?
• The Medicare education benefit has been a particular bonus for advanced practitioners, as we teach many of the classes.
We welcome your questions and comments.
Q: We scheduled a total knee replacement for a patient on dialysis, and anesthesia balked because the patient had a potassium level of 5.5 mEq/L. The nephrology practice, apparently not concerned, agreed to dialyze the patient, but only because anesthesia insisted. If the practice uses our facility, where 5.3 mEq/L is the upper limit of serum potassium, how can a potassium level of 5.5 mEq/L not be of concern in a hemodialysis patient?
This is a question that occurs frequently regarding patients receiving dialysis. Hyperkalemia is a problem faced by many dialysis patients as a result of the kidneys’ inability to remove potassium with the loss of renal function. Patients’ potassium levels are monitored routinely, and low-potassium diets are a staple of any nephrology clinic or dialysis unit.
For patients in our dialysis unit, the normal potassium range is 3.5 to 6.0 mEq/L, which is 0.9 mEq/L higher than for a patient without end-stage renal disease (ESRD). Dialysis patients with ESRD often have an increased tolerance for hyperkalemia.
When potassium levels are elevated, a 12-lead ECG is used to detect any physiological cardiac changes. These are generally not seen until the serum potassium exceeds 6.0 to 6.5 mEq/L. ECG changes seen in hyperkalemia include peaked T waves, a prolonged PR interval, and absent P waves with a widened QRS complex. These changes, which can lead to ventricular tachycardia or ventricular fibrillation, are not based on numbers or values of serum potassium, but are thought to reflect the transcellular potassium gradient.3
When questioning a potassium level in a dialysis patient and considering whether presurgical dialysis is needed, it is important to consider the surgery planned. In surgeries during which potassium might be released secondary to tissue trauma, potassium levels can rise higher during surgery.3
It is important to assess hypokalemia as well. Arrhythmias such as premature atrial and ventricular beats, sinus bradycardia, paroxysmal atrial or junctional tachycardia, atrioventricular block, and ventricular tachycardia or fibrillation can occur with hypokalemia. ECG changes include depression of the ST segment, a decrease in the amplitude of the T wave, and an increase in the amplitude of U waves, which occur at the end of the T wave. U waves are often seen in the lateral precordial leads V4 to V6.3
Laura MacGregor, RN, MS, NP-C
Grand Street Medical Associates, Kingston, New York
REFERENCES
1. National Kidney Foundation Kidney Disease Outcome Quality Initiative (NKF-K/DOQI) Clinical Practice Guidelines for Nutrition in Chronic Renal Failure (2000). www.kidney.org/professionals/kdoqi/guidelines_updates/doqi_nut.html. Accessed February 16, 2012.
2. Medicare.gov. Medical nutrition therapy. www.medicare.gov/navigation/manage-your-health/preventive-services/medical-nutrition-therapy.aspx?AspxAutoDetectCookieSupport=1. Accessed February 16, 2012.
3. Soundararajan R, Golper T. Medical management of the dialysis patient undergoing surgery. www.uptodate.com/contents/medical-management-of-the-dialysis-patient-undergoing-surgery. Accessed February 16, 2012.
4. Young HN, Chan MR, Yevzlin AS, Becker BN. The rationale, implementation and effects of the Medicare CKD education benefit. Am J Kidney Dis. 2011;57(3):381-386.
5. H. R. 6331: Medicare Improvements for Patients and Providers Act of 2008. www.govtrack.us/congress/bill.xpd?bill=h110-6331. Accessed February 16, 2012.
6. §410.48. Kidney disease education services. Federal Register. 2009;74(226):62003.
7. Lazarus JM. National health care policy and its effect on renal care. Presented at: NKFI Multi-Disciplinary Conference; September 24, 2009; Chicago, IL.
8. National Kidney Foundation. MIPPA Kidney Disease Education Benefit. Your Treatment, Your Choice (2010). www.kidney.org/professionals/KLS/YTYC.cfm. Accessed February 16, 2012.
Vitamin E Supplements Linked to Increase in Prostate Cancer Risk
SAN FRANCISCO – Healthy middle-aged and older men who take vitamin E supplements have an increased risk of prostate cancer, although the risk takes some time to emerge, suggests an update of the randomized SELECT prevention trial.
At a median follow-up of 7 years – or 1.5 years after the trial had been closed early for futility and men had been told to stop taking supplements – those who had taken vitamin E had a significant 17% greater risk of prostate cancer than those who had taken a placebo. The risk had been increased, although not significantly so, at the time of trial closure.
In absolute terms, the elevated risk from taking vitamin E translated to 11 more cancers for every 1,000 men over a 7-year period, Dr. Eric A. Klein said at the Genitourinary Cancers Symposium.
The findings serve as a cautionary tale, he said. "Nutritional supplements are biologically active and may in fact be harmful, and importantly, the effect may continue after the intervention stops," Dr. Klein commented.
More than half of U.S. men over age 60 take vitamin E, with about one-quarter taking at least 400 IU daily (Ann. Intern. Med. 2005;143:116-20), the dose used in the trial. "Consumers should be skeptical about health claims for unregulated over-the-counter products in the absence of strong evidence of benefit from clinical trials," said Dr. Klein at the meeting, which was sponsored by the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Urologic Oncology.
In other updated results, men who took selenium with vitamin E and men who took selenium alone did not have any significant increase in the risk of prostate cancer. And baseline plasma levels of various tocopherols (forms of vitamin E) modified prostate cancer risk: Within the vitamin E group, for example, higher levels of alpha-tocopherol were protective, whereas higher levels of gamma-tocopherol were deleterious.
The obvious question now is, how does vitamin E increase prostate cancer risk? said Dr. Klein, who is chairman of the Glickman Urological and Kidney Institute at the Cleveland Clinic. Theories that have been advanced include the possibility that antioxidants become carcinogenic pro-oxidants at high doses; there may be interplay between the different types of tocopherols; high doses of vitamin E may inhibit absorption of other, protective fat-soluble vitamins; and genetic susceptibility may affect the actions of antioxidants. A related, as yet unanswered question is why adding selenium to vitamin E abolishes the excess risk.
"We need to sort all of this out," Dr. Klein said, pointing to the SELECT (Selenium and Vitamin E Cancer Prevention Trial) Biorepository, which contains a wealth of information about the men studied and is being offered as a resource. "I invite anybody in the scientific community to float a hypothesis. If it passes scientific muster, we will give you access to the Biorepository to help us answer [these questions]." More than a half dozen studies have already been approved, he said.
In SELECT, 35,533 healthy North American men aged 50 years or older if black or 55 years or older if of other races/ethnicities who had an average risk of prostate cancer were randomized to four groups: vitamin E (400 IU/day, all rac-alpha-tocopherol acetate), selenium (200 mcg/day from l-selenomethionine), the combination, or a placebo.
"It was recommended, but not required in this trial, unlike in the PCPT [Prostate Cancer Prevention Trial], that men be screened yearly with PSA and digital rectal exam," Dr. Klein noted. "One of the strengths of the trial is that the screening interval and the trigger for biopsy were not prescribed by the trial; they were left to local community standards at these 400-plus sites."
At the time that the trial was halted early, men in the vitamin E group had a marginally increased risk of prostate cancer compared with men in the placebo group (hazard ratio, 1.13; P = .06) (JAMA 2009;301:39-51). But with the additional follow-up, despite cessation of supplement use, the trend continued and became significant (hazard ratio, 1.17; P = .008) (JAMA 2011;306:1549-56).
"There was no difference in tumor aggressiveness across the four arms as assessed by tumor stage and grade," Dr. Klein reported. "And the increased risk of being diagnosed with prostate cancer in the vitamin E arm was not accounted for by more intense screening or an increasing rate of biopsy."
In the vitamin E group, the risk of prostate cancer generally decreased with each additional quintile of alpha-tocopherol at baseline. In contrast, within the selenium and combination groups, the risk increased with quintile, and within the placebo group, risk was unaffected.
The results were "somewhat opposite" for plasma gamma-tocopherol levels at baseline. In the vitamin E group, the risk generally increased with quintile. In contrast, within all the other groups, the risk decreased with quintile.
The findings have noteworthy implications for the design of clinical trials, said Dr. Klein.
"There were interactions between selenium and vitamin E with respect to risk, and a factorial design would not have captured that. So it’s important that these be powered to allow for these interactions to be tested for," he explained. "And maybe most importantly, for agents whose biology we don’t really understand and don’t really understand what the time line of the effect is, that postintervention follow-up is critical: If we had not continued to follow these men beyond the 5.5 years that they took the supplements, we would not have discovered [the elevated risk with vitamin E]".
Dr. Klein disclosed that he had no relevant conflicts of interest.
SAN FRANCISCO – Healthy middle-aged and older men who take vitamin E supplements have an increased risk of prostate cancer, although the risk takes some time to emerge, suggests an update of the randomized SELECT prevention trial.
At a median follow-up of 7 years – or 1.5 years after the trial had been closed early for futility and men had been told to stop taking supplements – those who had taken vitamin E had a significant 17% greater risk of prostate cancer than those who had taken a placebo. The risk had been increased, although not significantly so, at the time of trial closure.
In absolute terms, the elevated risk from taking vitamin E translated to 11 more cancers for every 1,000 men over a 7-year period, Dr. Eric A. Klein said at the Genitourinary Cancers Symposium.
The findings serve as a cautionary tale, he said. "Nutritional supplements are biologically active and may in fact be harmful, and importantly, the effect may continue after the intervention stops," Dr. Klein commented.
More than half of U.S. men over age 60 take vitamin E, with about one-quarter taking at least 400 IU daily (Ann. Intern. Med. 2005;143:116-20), the dose used in the trial. "Consumers should be skeptical about health claims for unregulated over-the-counter products in the absence of strong evidence of benefit from clinical trials," said Dr. Klein at the meeting, which was sponsored by the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Urologic Oncology.
In other updated results, men who took selenium with vitamin E and men who took selenium alone did not have any significant increase in the risk of prostate cancer. And baseline plasma levels of various tocopherols (forms of vitamin E) modified prostate cancer risk: Within the vitamin E group, for example, higher levels of alpha-tocopherol were protective, whereas higher levels of gamma-tocopherol were deleterious.
The obvious question now is, how does vitamin E increase prostate cancer risk? said Dr. Klein, who is chairman of the Glickman Urological and Kidney Institute at the Cleveland Clinic. Theories that have been advanced include the possibility that antioxidants become carcinogenic pro-oxidants at high doses; there may be interplay between the different types of tocopherols; high doses of vitamin E may inhibit absorption of other, protective fat-soluble vitamins; and genetic susceptibility may affect the actions of antioxidants. A related, as yet unanswered question is why adding selenium to vitamin E abolishes the excess risk.
"We need to sort all of this out," Dr. Klein said, pointing to the SELECT (Selenium and Vitamin E Cancer Prevention Trial) Biorepository, which contains a wealth of information about the men studied and is being offered as a resource. "I invite anybody in the scientific community to float a hypothesis. If it passes scientific muster, we will give you access to the Biorepository to help us answer [these questions]." More than a half dozen studies have already been approved, he said.
In SELECT, 35,533 healthy North American men aged 50 years or older if black or 55 years or older if of other races/ethnicities who had an average risk of prostate cancer were randomized to four groups: vitamin E (400 IU/day, all rac-alpha-tocopherol acetate), selenium (200 mcg/day from l-selenomethionine), the combination, or a placebo.
"It was recommended, but not required in this trial, unlike in the PCPT [Prostate Cancer Prevention Trial], that men be screened yearly with PSA and digital rectal exam," Dr. Klein noted. "One of the strengths of the trial is that the screening interval and the trigger for biopsy were not prescribed by the trial; they were left to local community standards at these 400-plus sites."
At the time that the trial was halted early, men in the vitamin E group had a marginally increased risk of prostate cancer compared with men in the placebo group (hazard ratio, 1.13; P = .06) (JAMA 2009;301:39-51). But with the additional follow-up, despite cessation of supplement use, the trend continued and became significant (hazard ratio, 1.17; P = .008) (JAMA 2011;306:1549-56).
"There was no difference in tumor aggressiveness across the four arms as assessed by tumor stage and grade," Dr. Klein reported. "And the increased risk of being diagnosed with prostate cancer in the vitamin E arm was not accounted for by more intense screening or an increasing rate of biopsy."
In the vitamin E group, the risk of prostate cancer generally decreased with each additional quintile of alpha-tocopherol at baseline. In contrast, within the selenium and combination groups, the risk increased with quintile, and within the placebo group, risk was unaffected.
The results were "somewhat opposite" for plasma gamma-tocopherol levels at baseline. In the vitamin E group, the risk generally increased with quintile. In contrast, within all the other groups, the risk decreased with quintile.
The findings have noteworthy implications for the design of clinical trials, said Dr. Klein.
"There were interactions between selenium and vitamin E with respect to risk, and a factorial design would not have captured that. So it’s important that these be powered to allow for these interactions to be tested for," he explained. "And maybe most importantly, for agents whose biology we don’t really understand and don’t really understand what the time line of the effect is, that postintervention follow-up is critical: If we had not continued to follow these men beyond the 5.5 years that they took the supplements, we would not have discovered [the elevated risk with vitamin E]".
Dr. Klein disclosed that he had no relevant conflicts of interest.
SAN FRANCISCO – Healthy middle-aged and older men who take vitamin E supplements have an increased risk of prostate cancer, although the risk takes some time to emerge, suggests an update of the randomized SELECT prevention trial.
At a median follow-up of 7 years – or 1.5 years after the trial had been closed early for futility and men had been told to stop taking supplements – those who had taken vitamin E had a significant 17% greater risk of prostate cancer than those who had taken a placebo. The risk had been increased, although not significantly so, at the time of trial closure.
In absolute terms, the elevated risk from taking vitamin E translated to 11 more cancers for every 1,000 men over a 7-year period, Dr. Eric A. Klein said at the Genitourinary Cancers Symposium.
The findings serve as a cautionary tale, he said. "Nutritional supplements are biologically active and may in fact be harmful, and importantly, the effect may continue after the intervention stops," Dr. Klein commented.
More than half of U.S. men over age 60 take vitamin E, with about one-quarter taking at least 400 IU daily (Ann. Intern. Med. 2005;143:116-20), the dose used in the trial. "Consumers should be skeptical about health claims for unregulated over-the-counter products in the absence of strong evidence of benefit from clinical trials," said Dr. Klein at the meeting, which was sponsored by the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Urologic Oncology.
In other updated results, men who took selenium with vitamin E and men who took selenium alone did not have any significant increase in the risk of prostate cancer. And baseline plasma levels of various tocopherols (forms of vitamin E) modified prostate cancer risk: Within the vitamin E group, for example, higher levels of alpha-tocopherol were protective, whereas higher levels of gamma-tocopherol were deleterious.
The obvious question now is, how does vitamin E increase prostate cancer risk? said Dr. Klein, who is chairman of the Glickman Urological and Kidney Institute at the Cleveland Clinic. Theories that have been advanced include the possibility that antioxidants become carcinogenic pro-oxidants at high doses; there may be interplay between the different types of tocopherols; high doses of vitamin E may inhibit absorption of other, protective fat-soluble vitamins; and genetic susceptibility may affect the actions of antioxidants. A related, as yet unanswered question is why adding selenium to vitamin E abolishes the excess risk.
"We need to sort all of this out," Dr. Klein said, pointing to the SELECT (Selenium and Vitamin E Cancer Prevention Trial) Biorepository, which contains a wealth of information about the men studied and is being offered as a resource. "I invite anybody in the scientific community to float a hypothesis. If it passes scientific muster, we will give you access to the Biorepository to help us answer [these questions]." More than a half dozen studies have already been approved, he said.
In SELECT, 35,533 healthy North American men aged 50 years or older if black or 55 years or older if of other races/ethnicities who had an average risk of prostate cancer were randomized to four groups: vitamin E (400 IU/day, all rac-alpha-tocopherol acetate), selenium (200 mcg/day from l-selenomethionine), the combination, or a placebo.
"It was recommended, but not required in this trial, unlike in the PCPT [Prostate Cancer Prevention Trial], that men be screened yearly with PSA and digital rectal exam," Dr. Klein noted. "One of the strengths of the trial is that the screening interval and the trigger for biopsy were not prescribed by the trial; they were left to local community standards at these 400-plus sites."
At the time that the trial was halted early, men in the vitamin E group had a marginally increased risk of prostate cancer compared with men in the placebo group (hazard ratio, 1.13; P = .06) (JAMA 2009;301:39-51). But with the additional follow-up, despite cessation of supplement use, the trend continued and became significant (hazard ratio, 1.17; P = .008) (JAMA 2011;306:1549-56).
"There was no difference in tumor aggressiveness across the four arms as assessed by tumor stage and grade," Dr. Klein reported. "And the increased risk of being diagnosed with prostate cancer in the vitamin E arm was not accounted for by more intense screening or an increasing rate of biopsy."
In the vitamin E group, the risk of prostate cancer generally decreased with each additional quintile of alpha-tocopherol at baseline. In contrast, within the selenium and combination groups, the risk increased with quintile, and within the placebo group, risk was unaffected.
The results were "somewhat opposite" for plasma gamma-tocopherol levels at baseline. In the vitamin E group, the risk generally increased with quintile. In contrast, within all the other groups, the risk decreased with quintile.
The findings have noteworthy implications for the design of clinical trials, said Dr. Klein.
"There were interactions between selenium and vitamin E with respect to risk, and a factorial design would not have captured that. So it’s important that these be powered to allow for these interactions to be tested for," he explained. "And maybe most importantly, for agents whose biology we don’t really understand and don’t really understand what the time line of the effect is, that postintervention follow-up is critical: If we had not continued to follow these men beyond the 5.5 years that they took the supplements, we would not have discovered [the elevated risk with vitamin E]".
Dr. Klein disclosed that he had no relevant conflicts of interest.
FROM THE GENITOURINARY CANCERS SYMPOSIUM
Major Finding: Men given vitamin E supplements had a 17% relative increase in the risk of prostate cancer compared with men given placebo; however, risk also varied with baseline plasma levels of alpha- and gamma-tocopherols.
Data Source: An update after a median 7-year follow-up of a randomized, placebo-controlled trial of vitamin E and selenium supplementation in 35,533 healthy men aged 50 years or older in the SELECT trial.
Disclosures: Dr. Klein disclosed that he had no relevant conflicts of interest.
Everolimus Shrinks Angiomyolipomas from Tuberous Sclerosis Complex
SAN FRANCISCO – Everolimus is efficacious and safe for shrinking renal angiomyolipomas and reducing the formation of new ones in patients having the genetic disorder tuberous sclerosis complex or sporadic lymphangiomyomatosis, new data suggest.
More than 40% of the 118 patients enrolled in a randomized, phase III trial met criteria for tumor response when given everolimus, an oral inhibitor of the mammalian target of rapamycin (mTOR), researchers reported at the Genitourinary Cancers Symposium. In sharp contrast, none of those given a matching placebo did.
Everolimus could potentially provide the first effective pharmacologic option for treating angiomyolipomas, the surgical management of which is often frustrating, according to lead investigator Dr. John J. Bissler, a nephrologist at the Cincinnati Children’s Hospital Medical Center.
"These lesions can be multiple and bilateral, so surgical approaches can be problematic for this genetic disease. The lesions continue to pop up. You can remove a lesion ... and then you come back and have a lesion growing there that you thought you just took out. Maybe you did take the whole lesion out, but now you have incited another to begin to grow," he explained in an interview.
"So at the end of the day, having a drug therapy is just incredibly exciting," he commented.
The trial is also important in that it adds more evidence of benefit of this class of agents in tumors having dysregulated mTOR signaling, such as subependymal giant cell astrocytomas (SEGAs), for which everolimus is already an approved treatment, Dr. Bissler said at the meeting, which was sponsored by the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Urologic Oncology.
The drug was well tolerated, too, with adverse effects that were largely expected based on prior experience. "The only thing that we saw that was a little bit different and [that] we haven’t seen in other populations as much was ... a very small increase in amenorrhea," he said. "But we know that mTOR inhibition has effects on different sex hormones, so it’s not unexpected that you would see that, and we just need to keep track of that."
Angiomyolipomas are the most common renal manifestation of tuberous sclerosis complex. They are also seen in patients having lymphangiomyomatosis, a pulmonary condition occurring both in association with tuberous sclerosis complex and sporadically.
Of relevance to targeted therapy, mutations in the tuberous sclerosis complex genes TSC1 and TSC2 in these diseases lead to constitutive up-regulation of mTOR complex 1, resulting in excessive cell growth and proliferation.
The current trial, called EXIST-2, enrolled patients at least 18 years of age who had angiomyolipomas, and randomized them 2:1 to receive oral everolimus (Afinitor) at 10 mg once daily or placebo until tumor progression or unacceptable toxicity. Those in the latter group were allowed to cross over to everolimus if their disease progressed.
The investigators used central radiology review of serial kidney CT and MRI images to assess angiomyolipoma response, which required at least a one-half reduction in the sum of the volumes of all angiomyolipomas, no new tumors measuring 1 cm or larger, no increase in kidney volume of more than 20%, and no serious angiomyolipoma-related bleeding.
Patients were enrolled at 24 centers in 11 countries. Participants were about 32 years old on average, and two-thirds were female. Nearly all had tuberous sclerosis complex. A sizable proportion (39%) had previously had surgery or an invasive procedure, such as renal embolization, for their angiomyolipomas.
Trial results, reported in a poster session at the meeting, showed that with a median follow-up of 9.5 months, the angiomyolipoma response rate was 41.8% with everolimus and 0% with placebo (P less than .0001). Benefit was similar across patient subgroups stratified by sex, age, race, and use of enzyme-inducing antiepileptic drugs.
Additionally, patients in the everolimus group had a longer median time to angiomyolipoma progression (not reached vs. 11.4 months; hazard ratio, 0.08; P less than .0001) and were more likely to have a response of skin lesions as well (26% vs. 0%; P = .0002).
Everolimus was associated with higher (although still low) rates of grade 3 or 4 stomatitis/oral mucositis and cytopenia. Amenorrhea occurred in 14% of women in the everolimus group vs. 4% of their counterparts in the placebo group.
There was a single death in the study population, occurring in the everolimus arm and resulting from status epilepticus that was thought to be unrelated to the drug.
Dr. Bissler disclosed that he is a consultant to Gambro and receives honoraria and research funding from Novartis. The trial was sponsored by Novartis.
SAN FRANCISCO – Everolimus is efficacious and safe for shrinking renal angiomyolipomas and reducing the formation of new ones in patients having the genetic disorder tuberous sclerosis complex or sporadic lymphangiomyomatosis, new data suggest.
More than 40% of the 118 patients enrolled in a randomized, phase III trial met criteria for tumor response when given everolimus, an oral inhibitor of the mammalian target of rapamycin (mTOR), researchers reported at the Genitourinary Cancers Symposium. In sharp contrast, none of those given a matching placebo did.
Everolimus could potentially provide the first effective pharmacologic option for treating angiomyolipomas, the surgical management of which is often frustrating, according to lead investigator Dr. John J. Bissler, a nephrologist at the Cincinnati Children’s Hospital Medical Center.
"These lesions can be multiple and bilateral, so surgical approaches can be problematic for this genetic disease. The lesions continue to pop up. You can remove a lesion ... and then you come back and have a lesion growing there that you thought you just took out. Maybe you did take the whole lesion out, but now you have incited another to begin to grow," he explained in an interview.
"So at the end of the day, having a drug therapy is just incredibly exciting," he commented.
The trial is also important in that it adds more evidence of benefit of this class of agents in tumors having dysregulated mTOR signaling, such as subependymal giant cell astrocytomas (SEGAs), for which everolimus is already an approved treatment, Dr. Bissler said at the meeting, which was sponsored by the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Urologic Oncology.
The drug was well tolerated, too, with adverse effects that were largely expected based on prior experience. "The only thing that we saw that was a little bit different and [that] we haven’t seen in other populations as much was ... a very small increase in amenorrhea," he said. "But we know that mTOR inhibition has effects on different sex hormones, so it’s not unexpected that you would see that, and we just need to keep track of that."
Angiomyolipomas are the most common renal manifestation of tuberous sclerosis complex. They are also seen in patients having lymphangiomyomatosis, a pulmonary condition occurring both in association with tuberous sclerosis complex and sporadically.
Of relevance to targeted therapy, mutations in the tuberous sclerosis complex genes TSC1 and TSC2 in these diseases lead to constitutive up-regulation of mTOR complex 1, resulting in excessive cell growth and proliferation.
The current trial, called EXIST-2, enrolled patients at least 18 years of age who had angiomyolipomas, and randomized them 2:1 to receive oral everolimus (Afinitor) at 10 mg once daily or placebo until tumor progression or unacceptable toxicity. Those in the latter group were allowed to cross over to everolimus if their disease progressed.
The investigators used central radiology review of serial kidney CT and MRI images to assess angiomyolipoma response, which required at least a one-half reduction in the sum of the volumes of all angiomyolipomas, no new tumors measuring 1 cm or larger, no increase in kidney volume of more than 20%, and no serious angiomyolipoma-related bleeding.
Patients were enrolled at 24 centers in 11 countries. Participants were about 32 years old on average, and two-thirds were female. Nearly all had tuberous sclerosis complex. A sizable proportion (39%) had previously had surgery or an invasive procedure, such as renal embolization, for their angiomyolipomas.
Trial results, reported in a poster session at the meeting, showed that with a median follow-up of 9.5 months, the angiomyolipoma response rate was 41.8% with everolimus and 0% with placebo (P less than .0001). Benefit was similar across patient subgroups stratified by sex, age, race, and use of enzyme-inducing antiepileptic drugs.
Additionally, patients in the everolimus group had a longer median time to angiomyolipoma progression (not reached vs. 11.4 months; hazard ratio, 0.08; P less than .0001) and were more likely to have a response of skin lesions as well (26% vs. 0%; P = .0002).
Everolimus was associated with higher (although still low) rates of grade 3 or 4 stomatitis/oral mucositis and cytopenia. Amenorrhea occurred in 14% of women in the everolimus group vs. 4% of their counterparts in the placebo group.
There was a single death in the study population, occurring in the everolimus arm and resulting from status epilepticus that was thought to be unrelated to the drug.
Dr. Bissler disclosed that he is a consultant to Gambro and receives honoraria and research funding from Novartis. The trial was sponsored by Novartis.
SAN FRANCISCO – Everolimus is efficacious and safe for shrinking renal angiomyolipomas and reducing the formation of new ones in patients having the genetic disorder tuberous sclerosis complex or sporadic lymphangiomyomatosis, new data suggest.
More than 40% of the 118 patients enrolled in a randomized, phase III trial met criteria for tumor response when given everolimus, an oral inhibitor of the mammalian target of rapamycin (mTOR), researchers reported at the Genitourinary Cancers Symposium. In sharp contrast, none of those given a matching placebo did.
Everolimus could potentially provide the first effective pharmacologic option for treating angiomyolipomas, the surgical management of which is often frustrating, according to lead investigator Dr. John J. Bissler, a nephrologist at the Cincinnati Children’s Hospital Medical Center.
"These lesions can be multiple and bilateral, so surgical approaches can be problematic for this genetic disease. The lesions continue to pop up. You can remove a lesion ... and then you come back and have a lesion growing there that you thought you just took out. Maybe you did take the whole lesion out, but now you have incited another to begin to grow," he explained in an interview.
"So at the end of the day, having a drug therapy is just incredibly exciting," he commented.
The trial is also important in that it adds more evidence of benefit of this class of agents in tumors having dysregulated mTOR signaling, such as subependymal giant cell astrocytomas (SEGAs), for which everolimus is already an approved treatment, Dr. Bissler said at the meeting, which was sponsored by the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Urologic Oncology.
The drug was well tolerated, too, with adverse effects that were largely expected based on prior experience. "The only thing that we saw that was a little bit different and [that] we haven’t seen in other populations as much was ... a very small increase in amenorrhea," he said. "But we know that mTOR inhibition has effects on different sex hormones, so it’s not unexpected that you would see that, and we just need to keep track of that."
Angiomyolipomas are the most common renal manifestation of tuberous sclerosis complex. They are also seen in patients having lymphangiomyomatosis, a pulmonary condition occurring both in association with tuberous sclerosis complex and sporadically.
Of relevance to targeted therapy, mutations in the tuberous sclerosis complex genes TSC1 and TSC2 in these diseases lead to constitutive up-regulation of mTOR complex 1, resulting in excessive cell growth and proliferation.
The current trial, called EXIST-2, enrolled patients at least 18 years of age who had angiomyolipomas, and randomized them 2:1 to receive oral everolimus (Afinitor) at 10 mg once daily or placebo until tumor progression or unacceptable toxicity. Those in the latter group were allowed to cross over to everolimus if their disease progressed.
The investigators used central radiology review of serial kidney CT and MRI images to assess angiomyolipoma response, which required at least a one-half reduction in the sum of the volumes of all angiomyolipomas, no new tumors measuring 1 cm or larger, no increase in kidney volume of more than 20%, and no serious angiomyolipoma-related bleeding.
Patients were enrolled at 24 centers in 11 countries. Participants were about 32 years old on average, and two-thirds were female. Nearly all had tuberous sclerosis complex. A sizable proportion (39%) had previously had surgery or an invasive procedure, such as renal embolization, for their angiomyolipomas.
Trial results, reported in a poster session at the meeting, showed that with a median follow-up of 9.5 months, the angiomyolipoma response rate was 41.8% with everolimus and 0% with placebo (P less than .0001). Benefit was similar across patient subgroups stratified by sex, age, race, and use of enzyme-inducing antiepileptic drugs.
Additionally, patients in the everolimus group had a longer median time to angiomyolipoma progression (not reached vs. 11.4 months; hazard ratio, 0.08; P less than .0001) and were more likely to have a response of skin lesions as well (26% vs. 0%; P = .0002).
Everolimus was associated with higher (although still low) rates of grade 3 or 4 stomatitis/oral mucositis and cytopenia. Amenorrhea occurred in 14% of women in the everolimus group vs. 4% of their counterparts in the placebo group.
There was a single death in the study population, occurring in the everolimus arm and resulting from status epilepticus that was thought to be unrelated to the drug.
Dr. Bissler disclosed that he is a consultant to Gambro and receives honoraria and research funding from Novartis. The trial was sponsored by Novartis.
FROM THE GENITOURINARY CANCERS SYMPOSIUM
Major Finding: Everolimus was superior to placebo in terms of achieving an angiomyolipoma response (41.8% vs. 0%; P less than .0001) and the time to angiomyolipoma progression (not reached vs.11.4 months; P less than .0001).
Data Source: Data came from a randomized, phase III trial of everolimus vs. placebo in 118 patients with angiomyolipomas resulting from tuberous sclerosis complex or sporadic lymphangiomyomatosis (the EXIST-2 trial).
Disclosures: Dr. Bissler disclosed that he is a consultant to Gambro and receives honoraria and research funding from Novartis. The trial was sponsored by Novartis.
Mild CKD Ups Risks of Renal, Urothelial Cancers
SAN FRANCISCO – Chronic kidney disease, even on the milder end of the spectrum, is an independent risk factor for urinary cancers and may therefore be useful for targeting screening, the results of a large observational study suggest.
In the study of nearly 1.2 million adults in the Kaiser Permanente Renal Registry, none of whom were on dialysis, the risks of urinary cancers increased in stepwise fashion with decreasing estimated glomerular filtration rate (GFR), Dr. William T. Lowrance reported at the Genitourinary Cancers Symposium.
After adjustments, patients having the lowest estimated GFRs had a more than 100% increase in the risk of renal cell cancer and a 35% increase in the risk of urothelial cancer. The risks of other types of cancers – breast, lung, prostate, and colorectal – and of cancer overall increased with decreasing estimated GFR in univariate analyses but not in multivariate analyses.
"We found an independent, graded increased risk of renal and urothelial cancer as you went to a lower estimated GFR, and this was especially true when your estimated GFR was less than 45 mL/min per 1.73 m2, in this large diverse population-based cohort," said Dr. Lowrance of the Huntsman Cancer Institute at the University of Utah, Salt Lake City.
"Estimated GFRs may play a role in identifying patients at higher risk for renal and urothelial malignancies," he added. "Certainly, prospective studies are needed to further assess any net clinical benefit of targeted cancer screening in these patients with CKD. And we also need to try and elucidate the etiology of this mechanism: Is there some underlying biological process that explains this association?"
"As far as I could determine on a literature search, this is the largest number of patients in a study to date," commented Thomas E. Hutson, D.O., Pharm.D., of the Baylor Sammons Cancer Center in Dallas, who was invited to discuss the study.
"We are used to screening patients with end-stage renal disease already, using renal ultrasounds, looking for renal tumors," he noted. This new study suggests that "GFR may play a role in identifying patients at higher risk, and therefore we may want to use that as a potential screening mechanism," a practice that should be studied prospectively, he agreed.
End-stage renal disease is a known risk factor for cancer, according to Dr. Lowrance. And previous studies have implicated CKD generally in cancer risk, "but they are somewhat limited by their size and their ability to control for important factors that may confound the association between CKD and cancer," he maintained.
The investigators studied adults aged 40 years or older who were in the Kaiser Permanente Renal Registry and had at least one outpatient, non–emergency department measurement of serum creatinine level between 2000 and 2008. Those who had cancer or a history of dialysis or renal transplantation were excluded.
Estimated GFR values within 3 months of cancer diagnosis and incident cancers in the first 2 years of follow-up were excluded from analysis to minimize the possibility of cancer affecting kidney function.
Results were based on 1.2 million patients with a median age of 55 years. A total of 76,809 cancers were diagnosed during a median follow-up of 5.3 years.
Univariate analyses showed increasing rates of various types of common cancers and of cancer overall with decreasing GFR, which was estimated with the CKD-Epi equation.
Multivariate analysis – adjusted for numerous potential confounders, such as proteinuria, comorbidities (including diabetes), smoking status, prescription medications taken, and health care use – showed that patients having an estimated GFR of 59 mL/min per 1.73 m2 or lower had a significantly increased risk of renal cell cancer, and patients having an estimated GFR of 44 mL/min per 1.73 m2 or lower had a significantly increased risk of urothelial cancer – both compared with their counterparts having an estimated GFR of 60 to 89 mL/min per 1.73 m2.
Those with the poorest renal function – an estimated GFR of less than 30 mL/min per 1.73 m2 – had a significant 2.09-fold increased risk of renal cell cancer and a significant 1.35-fold increased risk of urothelial cancer.
"A big concern [in such a study] is potential detection bias, meaning subjects with worse renal function may be followed more closely than those with normal renal function, and as a result, we are likely to diagnose more cancers in those patients," Dr. Lowrance acknowledged. However, analyses took into account numbers of outpatient visits and hospitalizations (although not specifically hematuria tests or imaging tests), reducing this possible source of bias.
The Genitourinary Cancers Symposium is sponsored by the American Society of Clinical Oncology, the American Society for Radiation Oncology, and the Society of Urologic Oncology.
Dr. Lowrance disclosed that he had no relevant conflicts of interest. Dr. Hutson disclosed that he is a consultant to and receives honoraria from Bayer, Genentech, GlaxoSmithKline, Novartis, Onyx, Pfizer, and Wyeth, and that he receives research funding from GlaxoSmithKline, Pfizer, and Wyeth.
SAN FRANCISCO – Chronic kidney disease, even on the milder end of the spectrum, is an independent risk factor for urinary cancers and may therefore be useful for targeting screening, the results of a large observational study suggest.
In the study of nearly 1.2 million adults in the Kaiser Permanente Renal Registry, none of whom were on dialysis, the risks of urinary cancers increased in stepwise fashion with decreasing estimated glomerular filtration rate (GFR), Dr. William T. Lowrance reported at the Genitourinary Cancers Symposium.
After adjustments, patients having the lowest estimated GFRs had a more than 100% increase in the risk of renal cell cancer and a 35% increase in the risk of urothelial cancer. The risks of other types of cancers – breast, lung, prostate, and colorectal – and of cancer overall increased with decreasing estimated GFR in univariate analyses but not in multivariate analyses.
"We found an independent, graded increased risk of renal and urothelial cancer as you went to a lower estimated GFR, and this was especially true when your estimated GFR was less than 45 mL/min per 1.73 m2, in this large diverse population-based cohort," said Dr. Lowrance of the Huntsman Cancer Institute at the University of Utah, Salt Lake City.
"Estimated GFRs may play a role in identifying patients at higher risk for renal and urothelial malignancies," he added. "Certainly, prospective studies are needed to further assess any net clinical benefit of targeted cancer screening in these patients with CKD. And we also need to try and elucidate the etiology of this mechanism: Is there some underlying biological process that explains this association?"
"As far as I could determine on a literature search, this is the largest number of patients in a study to date," commented Thomas E. Hutson, D.O., Pharm.D., of the Baylor Sammons Cancer Center in Dallas, who was invited to discuss the study.
"We are used to screening patients with end-stage renal disease already, using renal ultrasounds, looking for renal tumors," he noted. This new study suggests that "GFR may play a role in identifying patients at higher risk, and therefore we may want to use that as a potential screening mechanism," a practice that should be studied prospectively, he agreed.
End-stage renal disease is a known risk factor for cancer, according to Dr. Lowrance. And previous studies have implicated CKD generally in cancer risk, "but they are somewhat limited by their size and their ability to control for important factors that may confound the association between CKD and cancer," he maintained.
The investigators studied adults aged 40 years or older who were in the Kaiser Permanente Renal Registry and had at least one outpatient, non–emergency department measurement of serum creatinine level between 2000 and 2008. Those who had cancer or a history of dialysis or renal transplantation were excluded.
Estimated GFR values within 3 months of cancer diagnosis and incident cancers in the first 2 years of follow-up were excluded from analysis to minimize the possibility of cancer affecting kidney function.
Results were based on 1.2 million patients with a median age of 55 years. A total of 76,809 cancers were diagnosed during a median follow-up of 5.3 years.
Univariate analyses showed increasing rates of various types of common cancers and of cancer overall with decreasing GFR, which was estimated with the CKD-Epi equation.
Multivariate analysis – adjusted for numerous potential confounders, such as proteinuria, comorbidities (including diabetes), smoking status, prescription medications taken, and health care use – showed that patients having an estimated GFR of 59 mL/min per 1.73 m2 or lower had a significantly increased risk of renal cell cancer, and patients having an estimated GFR of 44 mL/min per 1.73 m2 or lower had a significantly increased risk of urothelial cancer – both compared with their counterparts having an estimated GFR of 60 to 89 mL/min per 1.73 m2.
Those with the poorest renal function – an estimated GFR of less than 30 mL/min per 1.73 m2 – had a significant 2.09-fold increased risk of renal cell cancer and a significant 1.35-fold increased risk of urothelial cancer.
"A big concern [in such a study] is potential detection bias, meaning subjects with worse renal function may be followed more closely than those with normal renal function, and as a result, we are likely to diagnose more cancers in those patients," Dr. Lowrance acknowledged. However, analyses took into account numbers of outpatient visits and hospitalizations (although not specifically hematuria tests or imaging tests), reducing this possible source of bias.
The Genitourinary Cancers Symposium is sponsored by the American Society of Clinical Oncology, the American Society for Radiation Oncology, and the Society of Urologic Oncology.
Dr. Lowrance disclosed that he had no relevant conflicts of interest. Dr. Hutson disclosed that he is a consultant to and receives honoraria from Bayer, Genentech, GlaxoSmithKline, Novartis, Onyx, Pfizer, and Wyeth, and that he receives research funding from GlaxoSmithKline, Pfizer, and Wyeth.
SAN FRANCISCO – Chronic kidney disease, even on the milder end of the spectrum, is an independent risk factor for urinary cancers and may therefore be useful for targeting screening, the results of a large observational study suggest.
In the study of nearly 1.2 million adults in the Kaiser Permanente Renal Registry, none of whom were on dialysis, the risks of urinary cancers increased in stepwise fashion with decreasing estimated glomerular filtration rate (GFR), Dr. William T. Lowrance reported at the Genitourinary Cancers Symposium.
After adjustments, patients having the lowest estimated GFRs had a more than 100% increase in the risk of renal cell cancer and a 35% increase in the risk of urothelial cancer. The risks of other types of cancers – breast, lung, prostate, and colorectal – and of cancer overall increased with decreasing estimated GFR in univariate analyses but not in multivariate analyses.
"We found an independent, graded increased risk of renal and urothelial cancer as you went to a lower estimated GFR, and this was especially true when your estimated GFR was less than 45 mL/min per 1.73 m2, in this large diverse population-based cohort," said Dr. Lowrance of the Huntsman Cancer Institute at the University of Utah, Salt Lake City.
"Estimated GFRs may play a role in identifying patients at higher risk for renal and urothelial malignancies," he added. "Certainly, prospective studies are needed to further assess any net clinical benefit of targeted cancer screening in these patients with CKD. And we also need to try and elucidate the etiology of this mechanism: Is there some underlying biological process that explains this association?"
"As far as I could determine on a literature search, this is the largest number of patients in a study to date," commented Thomas E. Hutson, D.O., Pharm.D., of the Baylor Sammons Cancer Center in Dallas, who was invited to discuss the study.
"We are used to screening patients with end-stage renal disease already, using renal ultrasounds, looking for renal tumors," he noted. This new study suggests that "GFR may play a role in identifying patients at higher risk, and therefore we may want to use that as a potential screening mechanism," a practice that should be studied prospectively, he agreed.
End-stage renal disease is a known risk factor for cancer, according to Dr. Lowrance. And previous studies have implicated CKD generally in cancer risk, "but they are somewhat limited by their size and their ability to control for important factors that may confound the association between CKD and cancer," he maintained.
The investigators studied adults aged 40 years or older who were in the Kaiser Permanente Renal Registry and had at least one outpatient, non–emergency department measurement of serum creatinine level between 2000 and 2008. Those who had cancer or a history of dialysis or renal transplantation were excluded.
Estimated GFR values within 3 months of cancer diagnosis and incident cancers in the first 2 years of follow-up were excluded from analysis to minimize the possibility of cancer affecting kidney function.
Results were based on 1.2 million patients with a median age of 55 years. A total of 76,809 cancers were diagnosed during a median follow-up of 5.3 years.
Univariate analyses showed increasing rates of various types of common cancers and of cancer overall with decreasing GFR, which was estimated with the CKD-Epi equation.
Multivariate analysis – adjusted for numerous potential confounders, such as proteinuria, comorbidities (including diabetes), smoking status, prescription medications taken, and health care use – showed that patients having an estimated GFR of 59 mL/min per 1.73 m2 or lower had a significantly increased risk of renal cell cancer, and patients having an estimated GFR of 44 mL/min per 1.73 m2 or lower had a significantly increased risk of urothelial cancer – both compared with their counterparts having an estimated GFR of 60 to 89 mL/min per 1.73 m2.
Those with the poorest renal function – an estimated GFR of less than 30 mL/min per 1.73 m2 – had a significant 2.09-fold increased risk of renal cell cancer and a significant 1.35-fold increased risk of urothelial cancer.
"A big concern [in such a study] is potential detection bias, meaning subjects with worse renal function may be followed more closely than those with normal renal function, and as a result, we are likely to diagnose more cancers in those patients," Dr. Lowrance acknowledged. However, analyses took into account numbers of outpatient visits and hospitalizations (although not specifically hematuria tests or imaging tests), reducing this possible source of bias.
The Genitourinary Cancers Symposium is sponsored by the American Society of Clinical Oncology, the American Society for Radiation Oncology, and the Society of Urologic Oncology.
Dr. Lowrance disclosed that he had no relevant conflicts of interest. Dr. Hutson disclosed that he is a consultant to and receives honoraria from Bayer, Genentech, GlaxoSmithKline, Novartis, Onyx, Pfizer, and Wyeth, and that he receives research funding from GlaxoSmithKline, Pfizer, and Wyeth.
FROM THE GENITOURINARY CANCERS SYMPOSIUM
Major Finding: The risks of renal cell cancer and urothelial cancer increased in a graded manner with decreasing kidney function. Patients with the poorest kidney function had 2.09-fold and 1.35-fold increases in risk, respectively.
Data Source: The observational cohort study included nearly 1.2 million adults who were not on dialysis and had not undergone renal transplantation.
Disclosures: Dr. Lowrance disclosed that he had no relevant conflicts of interest. Dr. Hutson disclosed that he is a consultant to and receives honoraria from Bayer, Genentech, GlaxoSmithKline, Novartis, Onyx, Pfizer, and Wyeth, and that he receives research funding from GlaxoSmithKline, Pfizer, and Wyeth.
MDV3100 Cuts Risk of Death in Advanced Prostate Cancer
SAN FRANCISCO – MDV3100, new oral inhibitor of androgen receptor signaling, reduces the risk of death by more than a third after a failure of docetaxel chemotherapy in men with progressive castration-resistant prostate cancer, according to results of the randomized AFFIRM trial.
Interim data for the trial, which was conducted in nearly 1,200 men, showed that those given the investigational drug lived 4.8 months longer than their counterparts who had been given a placebo, corresponding to a 37% reduced risk of death, lead investigator Dr. Howard I. Scher reported at the Genitourinary Cancers Symposium. This positive finding triggered early trial closure.
Additional analyses revealed that men treated with the drug were significantly more likely to have a soft tissue response and to have at least a halving of their prostate-specific antigen (PSA) level. At the same time, there was no increase in the rate of higher-grade adverse events; seizures (a potential concern from earlier research) occurred at low frequency.
"MDV3100 now joins the list of drugs demonstrating a survival benefit in a phase III trial post docetaxel," adding to abiraterone (Zytiga) and cabazitaxel (Jevtana), Dr. Scher maintained. "The risk-benefit ratio will likely position this as the frontline agent post docetaxel therapy."
"I’m not the [Food and Drug Administration], but I would say that when you see this kind of survival benefit and safety profile – and looking at a prior drug [investigation] that I had a privilege of leading – I would say that this should be approved relatively quickly," he commented in a press briefing.
"I have only one comment: wow! That’s very impressive," said Dr. Nicholas J. Vogelzang, the moderator of the briefing and the chair and medical director of the Developmental Therapeutics Committee of US Oncology. The median survival and dramatic rates of PSA reduction seen with MDV3100 are "unprecedented. This is going to definitely change the way we take care of patients every day in the office."
"This is a landmark study," Dr. Adam S. Kibel agreed in an interview at the meeting. "I think this is a drug that will be widely used, assuming it gets FDA approved."
Initially, MDV3100 is likely to be used in the postdocetaxel space, said, Dr. Kibel, chief of urology at Brigham and Women’s Hospital and the Dana Farber Cancer Institute and a professor at Harvard Medical School, all in Boston. "I imagine that it would probably be the first-line drug because it appears to have a little less side effect profile than abiraterone and certainly lower than cabazitaxel."
"The one tripper in there is, will insurance pay for it and how much is it going to cost?" said Dr. Kibel.
MDV3100 is also being tested in patients who have not yet received docetaxel. "I will be shocked if [those data] are not positive," he commented. And should it perform well there, "it will move prior to docetaxel, because it appears to be very well tolerated from the data presented."
The AFFIRM trial enrolled 1,199 men with castration-resistant prostate cancer who had experienced progression after receiving docetaxel – a population for whom there was no standard of care at the time the trial began, Dr. Scher noted. They were assigned in 2:1 ratio to once-daily treatment with MDV3100 (manufactured by Medivation) or placebo.
The first-in-class drug has a three-pronged mechanism of action, as well as some advantages over other antiandrogen agents, according to Dr. Scher, chief of the genitourinary oncology service and D. Wayne Calloway Chair in Urologic Oncology at the Memorial Sloan-Kettering Cancer Center in New York.
It "binds more tightly [to the androgen receptor] than the currently available agents, but is also unique in that it inhibits nuclear translocation [of the receptor] as well as the association of the receptor with DNA, inducing cell death," he explained.
The patients studied had a median age of 69 years. Most (90%) had bone metastases, and the large majority (70%) also had soft tissue metastases.
Main results showed that men given MDV3100 lived 18.4 months, whereas their counterparts given the placebo lived 13.6 months (hazard ratio, 0.63; P less than .0001). Stratified analyses showed similar benefit across most patient subgroups.
Men treated with the drug also had longer median radiographic progression free survival (8.3 vs. 2.9 months; HR, 0.40) and were more likely to have a soft tissue response on imaging (29% vs. 4%) and at least a halving of their PSA level (54% vs. 2%) (all P less than .0001).
There was no increase with MDV3100 in the rate of grade 3 or higher adverse events (45% vs. 53%) or the overall rate of treatment discontinuation due to adverse events (8% vs. 10%).
Seizures occurred in 0.6% of patients given the drug, compared with none of those given the placebo. "Obviously, these cases were studied very, very carefully. In four of the five cases, there were other potential confounders," including brain metastases and receipt of intravenous lidocaine for a biopsy, Dr. Scher noted. "This is an extremely low frequency, and considering this patient population who are symptomatic post docetaxel, for us, it’s really a nonissue."
The symposium is sponsored by the American Society of Clinical Oncology, the American Society for Radiation Oncology, and the Society of Urologic Oncology.
Medivation Inc. sponsored the trial. Dr. Scher disclosed that he is a consultant to and receives research funding from Medivation. Dr. Vogelzang disclosed relationships with numerous companies. Dr. Kibel reported that he is a consultant to Dendreon and Sanofi-Aventis.
SAN FRANCISCO – MDV3100, new oral inhibitor of androgen receptor signaling, reduces the risk of death by more than a third after a failure of docetaxel chemotherapy in men with progressive castration-resistant prostate cancer, according to results of the randomized AFFIRM trial.
Interim data for the trial, which was conducted in nearly 1,200 men, showed that those given the investigational drug lived 4.8 months longer than their counterparts who had been given a placebo, corresponding to a 37% reduced risk of death, lead investigator Dr. Howard I. Scher reported at the Genitourinary Cancers Symposium. This positive finding triggered early trial closure.
Additional analyses revealed that men treated with the drug were significantly more likely to have a soft tissue response and to have at least a halving of their prostate-specific antigen (PSA) level. At the same time, there was no increase in the rate of higher-grade adverse events; seizures (a potential concern from earlier research) occurred at low frequency.
"MDV3100 now joins the list of drugs demonstrating a survival benefit in a phase III trial post docetaxel," adding to abiraterone (Zytiga) and cabazitaxel (Jevtana), Dr. Scher maintained. "The risk-benefit ratio will likely position this as the frontline agent post docetaxel therapy."
"I’m not the [Food and Drug Administration], but I would say that when you see this kind of survival benefit and safety profile – and looking at a prior drug [investigation] that I had a privilege of leading – I would say that this should be approved relatively quickly," he commented in a press briefing.
"I have only one comment: wow! That’s very impressive," said Dr. Nicholas J. Vogelzang, the moderator of the briefing and the chair and medical director of the Developmental Therapeutics Committee of US Oncology. The median survival and dramatic rates of PSA reduction seen with MDV3100 are "unprecedented. This is going to definitely change the way we take care of patients every day in the office."
"This is a landmark study," Dr. Adam S. Kibel agreed in an interview at the meeting. "I think this is a drug that will be widely used, assuming it gets FDA approved."
Initially, MDV3100 is likely to be used in the postdocetaxel space, said, Dr. Kibel, chief of urology at Brigham and Women’s Hospital and the Dana Farber Cancer Institute and a professor at Harvard Medical School, all in Boston. "I imagine that it would probably be the first-line drug because it appears to have a little less side effect profile than abiraterone and certainly lower than cabazitaxel."
"The one tripper in there is, will insurance pay for it and how much is it going to cost?" said Dr. Kibel.
MDV3100 is also being tested in patients who have not yet received docetaxel. "I will be shocked if [those data] are not positive," he commented. And should it perform well there, "it will move prior to docetaxel, because it appears to be very well tolerated from the data presented."
The AFFIRM trial enrolled 1,199 men with castration-resistant prostate cancer who had experienced progression after receiving docetaxel – a population for whom there was no standard of care at the time the trial began, Dr. Scher noted. They were assigned in 2:1 ratio to once-daily treatment with MDV3100 (manufactured by Medivation) or placebo.
The first-in-class drug has a three-pronged mechanism of action, as well as some advantages over other antiandrogen agents, according to Dr. Scher, chief of the genitourinary oncology service and D. Wayne Calloway Chair in Urologic Oncology at the Memorial Sloan-Kettering Cancer Center in New York.
It "binds more tightly [to the androgen receptor] than the currently available agents, but is also unique in that it inhibits nuclear translocation [of the receptor] as well as the association of the receptor with DNA, inducing cell death," he explained.
The patients studied had a median age of 69 years. Most (90%) had bone metastases, and the large majority (70%) also had soft tissue metastases.
Main results showed that men given MDV3100 lived 18.4 months, whereas their counterparts given the placebo lived 13.6 months (hazard ratio, 0.63; P less than .0001). Stratified analyses showed similar benefit across most patient subgroups.
Men treated with the drug also had longer median radiographic progression free survival (8.3 vs. 2.9 months; HR, 0.40) and were more likely to have a soft tissue response on imaging (29% vs. 4%) and at least a halving of their PSA level (54% vs. 2%) (all P less than .0001).
There was no increase with MDV3100 in the rate of grade 3 or higher adverse events (45% vs. 53%) or the overall rate of treatment discontinuation due to adverse events (8% vs. 10%).
Seizures occurred in 0.6% of patients given the drug, compared with none of those given the placebo. "Obviously, these cases were studied very, very carefully. In four of the five cases, there were other potential confounders," including brain metastases and receipt of intravenous lidocaine for a biopsy, Dr. Scher noted. "This is an extremely low frequency, and considering this patient population who are symptomatic post docetaxel, for us, it’s really a nonissue."
The symposium is sponsored by the American Society of Clinical Oncology, the American Society for Radiation Oncology, and the Society of Urologic Oncology.
Medivation Inc. sponsored the trial. Dr. Scher disclosed that he is a consultant to and receives research funding from Medivation. Dr. Vogelzang disclosed relationships with numerous companies. Dr. Kibel reported that he is a consultant to Dendreon and Sanofi-Aventis.
SAN FRANCISCO – MDV3100, new oral inhibitor of androgen receptor signaling, reduces the risk of death by more than a third after a failure of docetaxel chemotherapy in men with progressive castration-resistant prostate cancer, according to results of the randomized AFFIRM trial.
Interim data for the trial, which was conducted in nearly 1,200 men, showed that those given the investigational drug lived 4.8 months longer than their counterparts who had been given a placebo, corresponding to a 37% reduced risk of death, lead investigator Dr. Howard I. Scher reported at the Genitourinary Cancers Symposium. This positive finding triggered early trial closure.
Additional analyses revealed that men treated with the drug were significantly more likely to have a soft tissue response and to have at least a halving of their prostate-specific antigen (PSA) level. At the same time, there was no increase in the rate of higher-grade adverse events; seizures (a potential concern from earlier research) occurred at low frequency.
"MDV3100 now joins the list of drugs demonstrating a survival benefit in a phase III trial post docetaxel," adding to abiraterone (Zytiga) and cabazitaxel (Jevtana), Dr. Scher maintained. "The risk-benefit ratio will likely position this as the frontline agent post docetaxel therapy."
"I’m not the [Food and Drug Administration], but I would say that when you see this kind of survival benefit and safety profile – and looking at a prior drug [investigation] that I had a privilege of leading – I would say that this should be approved relatively quickly," he commented in a press briefing.
"I have only one comment: wow! That’s very impressive," said Dr. Nicholas J. Vogelzang, the moderator of the briefing and the chair and medical director of the Developmental Therapeutics Committee of US Oncology. The median survival and dramatic rates of PSA reduction seen with MDV3100 are "unprecedented. This is going to definitely change the way we take care of patients every day in the office."
"This is a landmark study," Dr. Adam S. Kibel agreed in an interview at the meeting. "I think this is a drug that will be widely used, assuming it gets FDA approved."
Initially, MDV3100 is likely to be used in the postdocetaxel space, said, Dr. Kibel, chief of urology at Brigham and Women’s Hospital and the Dana Farber Cancer Institute and a professor at Harvard Medical School, all in Boston. "I imagine that it would probably be the first-line drug because it appears to have a little less side effect profile than abiraterone and certainly lower than cabazitaxel."
"The one tripper in there is, will insurance pay for it and how much is it going to cost?" said Dr. Kibel.
MDV3100 is also being tested in patients who have not yet received docetaxel. "I will be shocked if [those data] are not positive," he commented. And should it perform well there, "it will move prior to docetaxel, because it appears to be very well tolerated from the data presented."
The AFFIRM trial enrolled 1,199 men with castration-resistant prostate cancer who had experienced progression after receiving docetaxel – a population for whom there was no standard of care at the time the trial began, Dr. Scher noted. They were assigned in 2:1 ratio to once-daily treatment with MDV3100 (manufactured by Medivation) or placebo.
The first-in-class drug has a three-pronged mechanism of action, as well as some advantages over other antiandrogen agents, according to Dr. Scher, chief of the genitourinary oncology service and D. Wayne Calloway Chair in Urologic Oncology at the Memorial Sloan-Kettering Cancer Center in New York.
It "binds more tightly [to the androgen receptor] than the currently available agents, but is also unique in that it inhibits nuclear translocation [of the receptor] as well as the association of the receptor with DNA, inducing cell death," he explained.
The patients studied had a median age of 69 years. Most (90%) had bone metastases, and the large majority (70%) also had soft tissue metastases.
Main results showed that men given MDV3100 lived 18.4 months, whereas their counterparts given the placebo lived 13.6 months (hazard ratio, 0.63; P less than .0001). Stratified analyses showed similar benefit across most patient subgroups.
Men treated with the drug also had longer median radiographic progression free survival (8.3 vs. 2.9 months; HR, 0.40) and were more likely to have a soft tissue response on imaging (29% vs. 4%) and at least a halving of their PSA level (54% vs. 2%) (all P less than .0001).
There was no increase with MDV3100 in the rate of grade 3 or higher adverse events (45% vs. 53%) or the overall rate of treatment discontinuation due to adverse events (8% vs. 10%).
Seizures occurred in 0.6% of patients given the drug, compared with none of those given the placebo. "Obviously, these cases were studied very, very carefully. In four of the five cases, there were other potential confounders," including brain metastases and receipt of intravenous lidocaine for a biopsy, Dr. Scher noted. "This is an extremely low frequency, and considering this patient population who are symptomatic post docetaxel, for us, it’s really a nonissue."
The symposium is sponsored by the American Society of Clinical Oncology, the American Society for Radiation Oncology, and the Society of Urologic Oncology.
Medivation Inc. sponsored the trial. Dr. Scher disclosed that he is a consultant to and receives research funding from Medivation. Dr. Vogelzang disclosed relationships with numerous companies. Dr. Kibel reported that he is a consultant to Dendreon and Sanofi-Aventis.
FROM THE GENITOURINARY CANCERS SYMPOSIUM
Major Finding: Overall survival was significantly longer in the MDV3100 group than in the placebo group (18.4 vs. 13.6 months; HR, 0.63), and there was no increase in the rate of grade 3 or higher adverse events.
Data Source: The AFFIRM trial is a randomized phase III trial comparing MDV3100 vs. placebo in 1,199 patients with progressive castration-resistant prostate cancer who had experienced failure of docetaxel.
Disclosures: Medivation sponsored the trial. Dr. Scher disclosed that he is a consultant to and receives research funding from Medivation. Dr. Vogelzang disclosed relationships with numerous companies. Dr. Kibel reported that he is a consultant to Dendreon and Sanofi-Aventis.
Kidney Stones: Current Diagnosis and Management
Kidney or urinary tract stones (whose presence is referred to as nephrolithiasis) are hard, crystalline mineral concretions that form within the kidney or the urinary tract. They are a common problem, with an estimated annual incidence of 1% and a lifetime risk of 15% to 25%; this constitutes a significant health care burden, particularly for people of working age.1
Nephrolithiasis is currently more prevalent in men than in women (13% vs 7%, respectively), and it is three to four times more likely to present in white than nonwhite patients.2 However, recent epidemiologic data suggest an alarming increase in the number of women and adolescents primarily diagnosed with stone disease.3-6 The pattern of increasing incidence in women can be attributed in part to changes in diet and lifestyle.4,5 Figure 17 represents the prevalence of stone disease, specific to gender and race.2,4
Due to kidney stones’ relatively common occurrence, the diagnosis, management, and prevention of stone disease have become increasingly relevant for the primary care practitioner. In the course of stone disease management, the clinician should be aware of a vital fact: Stones have a tendency to recur.1 Indeed, evidence suggests that following an initial diagnosis of nephrolithiasis, the probability of kidney stone recurrence increases to nearly 50% after five years.8
Even more concerning, evidence from several studies suggests that patients with a history of stone disease have a higher probability of experiencing a significant reduction in renal function (ie, decrease in glomerular filtration rate) and hence end-stage renal disease, when compared with non–stone formers.9-11 This accentuates the importance of early diagnosis, treatment, and initiation of steps to prevent further recurrence of this condition.
PATHOGENESIS
Stones in the urinary tract develop under specific urinary conditions, including supersaturation of the urine with stone-forming ions (ie, calcium, oxalate, uric acid, and phosphate) and deficiency of urinary stone inhibitors (citrate, magnesium, zinc, macromolecules, and pyrophosphate). Stone formation occurs in a mucoprotein matrix that attaches to the renal epithelium. Urine becomes supersaturated as a result of increasing levels of solutes (such as the stone-forming ions) and/or decreasing free water volume. When the concentration of stone-forming ions exceeds solubility in the urine (equilibrium solubility product), these ions can combine to form crystals.12,13
Stones are typed based on the ion composition of their crystals (see Table 12,12).
Once crystals are formed, they can also aggregate with other crystals, developing into a calculus.12 Urinary pH influences ion crystallization: Alkaline urine favors formation of calcium and/or phosphate stones, whereas acidic urine favors uric acid and cystine stone formation.13
Kidney stones can be divided into four broad types: calcium-based, struvite, uric acid, and cystine stones (see Figure 2). Among these, calcium-based stones are by far the most common, with nearly 80% of stones composed of calcium compounds (usually calcium oxalate, and rarely calcium phosphate).4 The etiologies of these four types are vastly different, and prevention of stone formation must be tailored to the stone type. Once stones form, however, the appropriate treatment strategies have many similarities.
RISK FACTORS
Specific risk factors for stone formation vary widely and are unique to the type of stone. A thorough history, including a family or personal history of stone disease and dietary history, must be part of the initial work-up when a patient is being evaluated for stone disease; patients with any of these risk factors should be investigated further.
The risk factors for stone disease can be broadly categorized as either individual risk factors or dietary risk factors.
Individual Risk Factors
A positive family history increases the risk for stone formation by two- to three-fold. Other individual risk factors include congenital anatomic defects, such as medullary sponge kidney, horseshoe kidney, and ureteropelvic junction obstruction (UPJ).14-16 These can cause obstruction that leads to urinary stasis, and subsequently to stone precipitation.
Certain systemic disorders (eg, hyperparathyroidism) and situations have also been associated with stone disease and should be considered risk factors. (See Table 212,17).
In patients who undergo gastrointestinal bypass surgery, the development of hyperoxaluria, hypercalciuria, and decreased urinary volume are associated with an increased risk for stone formation,18,19 and these patients should be watched for this development. Obesity and weight gain are directly proportional to nephrolithiasis risk, especially in women.4,20
Environment plays a very important role in stone formation. Persons who live in a hot, arid climate, for example, and those who work outdoors in hot weather are at increased risk for stone formation due to excessive fluid loss from sweating.2,4,7 (In regions where the risk for kidney stone formation is high, Romero et al7 predict, nephrolithiasis incidence could rise from 40% to 56% by 2050 as a result of the effects of global warming.)
Lastly, an individual’s ability (or inability) to metabolize calcium salts plays a vital role in the pathogenesis of stone disease. Intestinal calcium absorption is a major determinant of hypercalciuria, as nearly 90% of ingested calcium is absorbed in the intestines. People can broadly be divided into high or low calcium absorbers. Hypercalciuria (mean urinary calcium excretion ≥ 300 mg/d in men and ≥ 250 mg/d in women on a 1,000-mg/d calcium diet) is detected in 20% to 40% of those with calcium stones.21-23 Hypocitraturia (mean urinary citrate excretion ≤ 320 mg/d) and hyperoxaluria (mean urinary oxalate excretion > 45 mg/d) can also increase the risk for stone formation.12,24
Dietary Risk Factors
These are primarily related to fluid intake and dietary calcium.7,17,25,26 Drinking less than 1 L of fluids daily is associated with an increased risk for forming stones; this risk is magnified when the urine volume is also decreased.7,17,27 Increased dietary intake of animal protein can elevate the risk for formation of uric acid stones as a result of elevated urinary calcium and uric acid and decreased urinary citrate.17
Low dietary calcium ingestion and high oxalate consumption, resulting in increased oxalate absorption, can also exacerbate the risk for stones.7,27 By contrast, a diet high in calcium (≥ 1,200 mg/d) reduces the risk for calcium oxalate stone recurrence,17 although the effectiveness of supplemental calcium has been questioned.26-28
Patients who are advised to make specific dietary adjustments should later undergo repeat urine chemistries to determine the effectiveness of these changes.17
CLINICAL PRESENTATION
Nephrolithiasis typically presents with colicky flank pain, often accompanied by nausea and vomiting.29 The pain radiates to the ipsilateral groin, and the patient typically has difficulty finding a comfortable position. Nephrolithiasis may also present with chronic, episodic flank pain or may even be asymptomatic.30
Physical examination may reveal signs of severe pain, such as tachycardia and hypertension. Presence of fever indicates associated urinary tract infection and possibly pyelonephritis. Some larger stones can cause urinary tract obstruction; if obstruction occurs along with a preexisting urinary tract infection, it can potentially lead to pyelonephritis, pyonephrosis, and eventually urosepsis—a potentially life-threatening condition that requires immediate surgical drainage.31
Before a diagnosis of renal stones can be confirmed, care should be exercised to rule out the differentials, including abdominal aortic aneurysm, appendicitis, bowel obstruction, cholecystitis, drug-seeking behavior (eg, painkiller addiction), gastritis, mesenteric ischemia, musculoskeletal pain, ovarian abscess, ruptured ovarian cyst, pelvic inflammatory disease, pyelonephritis, and UPJ.2,32,33 All patients with suspected nephrolithiasis should be carefully evaluated using laboratory and radiologic investigations.
LABORATORY EVALUATION
The goals in this two-step process are to confirm the diagnosis of nephrolithiasis, then to identify the composition of the stones formed and the associated risk factors.
Initial Evaluation
Tests include dipstick urine assessment, serum chemistries, and a complete blood count (CBC). Urine dipstick assessment may be positive for blood, protein, or leukocyte esterase, indicating stones or fragments of stones present in the urinary tract. While nearly 10% of patients with stone disease exhibit gross hematuria, nearly 90% of patients have microscopic hematuria.2
Urine osmolality should be reviewed to assess urine concentration. Serum chemistries should be ordered to evaluate kidney function. Elevated creatinine may indicate acute rather than chronic kidney disease. Electrolytes and carbon dioxide should be measured to evaluate the kidneys’ ability to concentrate urine and maintain an acid–base balance. The CBC may reveal mild leukocytosis in nephrolithiasis; presence of significant leukocytosis indicates infection.2
Secondary Evaluation
This step begins with a thorough review of the patient’s medical record and a detailed patient interview to ascertain all risk factors for stone formation (as summarized in Table 1). Specific studies to be considered are mentioned in Table 3.2 This evaluation is critical to prevent formation of future stones and the associated complications. In the patient with a history of stone recurrence or stone formation of identified cause, evaluation is needed for three metabolic abnormalities—hypercalciuria, hyperuricosuria, and hypocitraturia—as these conditions predispose patients to recurrent stone formation.1,25,34
The patient should also be encouraged to collect stones passed for further clinical evaluation. Infrared spectroscopy or quantitative wet analysis is used to identify the specific composition of the stone.32,35
Radiologic Evaluation
Radiologic evaluation of stones is currently performed through plain x-rays, ultrasonography, and noncontrast spiral CT.12,32,33 When a patient presents with acute signs of nephrolithiasis, a plain film x-ray of the kidneys, ureters, and bladder (KUB) is acceptable as the first imaging study, as it is inexpensive and available in most areas.33 Plain film KUB x-rays will identify calcium oxalate, calcium phosphate, struvite, and cystine stones. However, the sensitivity of plain film x-rays has been documented between 24% and 59%, and stones that overlie a bone may be missed.32,36 (See Figure 3.)
Hence, because of these limitations and the increasing availability of noncontrast spiral CT, noncontrast spiral CT is now the most commonly used and useful test in the diagnosis of kidney stones (sensitivity, 95% to 100%).32,36 Spiral CT accurately defines the size as well as the location of stones, and may additionally rule out other differential diagnoses (see Figures 4a, 4b, and 4c).
Historically, IV pyelograms and urograms were considered useful in locating urinary tract stones and diagnosing related complications,12 but these modalities carry additional risks related to IV contrast dye and radiation exposure. As a result, they have been almost completely replaced by noncontrast spiral CT because of ease of use and reduced risks.33
Stones may also be seen on renal ultrasound—particularly uric acid stones, which are radiolucent (see Figure 5). Ultrasound is appropriate for evaluation of patients whose exposure to radiation should be limited, such as children or pregnant women. In addition to plain film x-rays, renal ultrasound may also be useful for surveillance of stones.12
TREATMENT
Nephrolithiasis treatment varies between acute and chronic care. Acute care for nephrolithiasis involves management of acute pain and urinary obstruction, as well as patient stabilization. Chronic care includes prevention of recurrence and management of risks.
Acute Management
Patients who present with acute nephrolithiasis most often require fluid administration, aggressive pain management, and treatment for nausea or vomiting.31,32 Most ureteral stones measuring 5.0 mm or less will typically pass spontaneously within a few weeks,1,29 but larger stones usually require intervention—in some cases, surgery.
Patients should be hospitalized if they require IV fluids or pain management. Isotonic IV fluids should be given to increase the urine volume and facilitate passage of stones. Care must be taken to monitor fluids, as patients with kidney stones may have a limited ability to urinate (due to urinary obstruction and/or acute or chronic renal failure). Whenever possible, all urine should be strained to collect any stones for analysis.
One new strategy to assist with stone passage is medical expulsive therapy (MET), using calcium channel blockers (eg, nifedipine) or α-blockers (eg, tamsulosin).1,37 While there is conflicting evidence regarding the efficacy of calcium channel blockers for MET, one meta-analysis revealed a 29% improvement in stone passage with α-blockers.1,38
Pain management can often be accomplished with NSAIDs (eg, ketorolac, diclofenac).29 Since this class of medications can compromise renal function, however, they must be used with caution. Many patients require narcotic medications to control pain adequately.39-41 Antiemetic agents (such as the H1-receptor blocker dimenhydrinate42) should be administered to control nausea and vomiting.
Surgical and interventional management. Surgical intervention may be required if stones are too large to pass spontaneously (typically ≥ 8 mm); if they cause acute renal obstruction; or if they are located at a site with a potential for complications or can lead to persistent symptoms without evidence that they are passing.1,3 Renal obstruction should be treated aggressively to preserve renal function.
The type of intervention chosen depends on the size and location of the stone, as well as the presence or absence of obstruction. Stones that measure less than 20 mm are commonly treated with extracorporeal shockwave lithotripsy (unless they overlie the sacroiliac joint), whereas patients with larger or more complex stones may require percutaneous nephrolithotomy. Nonobstructive or uncomplicated ureteral stones may be managed medically, whereas obstructive or complicated ureteral stones require placement of a stent or a nephrostomy tube until they can be removed by endoscopic surgery.29,43
Obstruction, which may be partial or complete, is more likely when stone size exceeds 10 mm.44 Signs of obstruction include sudden-onset, excruciating flank pain that radiates to the groin, along with nausea and vomiting (renal colic). Larger obstructive stones, such as staghorn calculi (as shown in Figures 3 and 4a), can present with symptoms of a urinary tract infection, mild flank pain, or hematuria.33
Presence of signs of obstruction or infection mandates emergent treatment. Infections of the urinary tract (as serious as pyelonephritis or urosepsis) should be treated with antibiotics: initially with broad coverage, according to the appropriate guidelines for urinary tract infections, then tailored to the results of urine cultures. Obstruction can be relieved directly by nephrostomy tubes (and/or stents) or by interventions in which the stone is removed and normal urinary flow is restored.
Typically, endoscopy is used for direct removal of stones that cause obstruction.44 Nephrostomy tubes and ureteral stents (see Figures 6a and 6b) are placed to relieve obstruction temporarily and provide an alternate route for drainage of urine. The goal is to prevent renal damage until the obstruction can be relieved. Stents can remain in place for several months, but nephrostomy tubes are associated with a higher risk for infection (because they are externalized), and duration of use should be limited to only a few weeks.12,29,38
Stents are also associated with infections, but coated stents are available to reduce infection. As with any catheter material inserted into the urinary tract, ureteral stents are a prime location for development of a persistent bacterial biofilm, thus leading to infection. Recent advances in stent manufacturing have included coating stents with various biomaterials to decrease the development of this bacterial biofilm. In a preliminary study in 10 patients using a diamond-like, carbon-coated ureteral stent, Laube et al45 demonstrated a reduction in formation of this biofilm, hence lowering the probability of stent-induced infection.
Chronic Stone Management
As previously mentioned, one of the seminal characteristics of stone disease is its ability to recur. After incidental detection of kidney stones through routine diagnostic procedures, the risk for recurrence in patients who do not receive chronic medical management is 30% to 40% within five years.17,28 In treated patients, by comparison, this risk falls by approximately 50%.17,26
Patients with a history of stone recurrence must be evaluated for metabolic defects that precipitate stones, since their risk for chronic kidney disease is increased.34 All patients with a history of stone disease should be instructed to increase their fluid intake to maintain a daily urinary output of at least 2.5 L, unless contraindications exist.34
In patients with calcium-based stones who do not benefit from conservative treatment (ie, a low-sodium diet and other dietary modifications), thiazide diuretics may help reduce urinary calcium.1,46
Struvite stones can be prevented through use of long-term antibiotics to reduce the risk for urinary tract infection and by maintaining urinary pH levels below 6.0.17,27,34
For patients with uric acid stones, allopurinol may be prescribed to lower uric acid levels; moreover, the solubility of uric acid is greatly increased at higher pH, so it is beneficial to treat these patients with citrate to maintain their urinary pH above 6.0.47,34
Ensuring a high urine output (≥ 4 L/d34) and alkalinizing urine can help prevent recurrence of cystine stones.17,33 Treatment with potassium citrate has been shown to maintain a urinary pH of 6.5 to 7.0.34
CONCLUSION
The ever-increasing significance of nephrolithiasis has mandated an organized and systematic management approach. Indeed, the diagnosis and initial therapy for kidney stones have undergone considerable evolution in recent years. The basic tenets of nephrolithiasis management include early diagnosis and pertinent treatment as well as adequate prophylaxis to prevent subsequent stone recurrence.
1. Moe OW, Pearle MS, Sakhaee K. Pharmacotherapy of urolithiasis: evidence from clinical trials. Kidney Int. 2011;79(4):385-392.
2. Schade GR, Faerber GJ. Urinary tract stones. Prim Care. 2010;37(3):565-581, ix.
3. Childs M, Rangel L, Lingeman J, Krambeck A. Contemporary practice patterns in surgical management of stone disease. American Urological Association (AUA) Annual Meeting; May 2011; Washington, DC.
4. Pearle MS, Calhoun E, Curhan GC. Urolithiasis. In: Litwin MS, Saigal CS, eds; National Institute of Diabetes and Digestive and Kidney Diseases. Urologic Diseases in America (2007). 281-320. http://kidney.niddk.nih.gov/statistics/uda/Urologic_Dis eases_in_America.pdf. Accessed January 23, 2012.
5. Scales CD Jr, Curtis LH, Norris RD, et al. Changing gender prevalence of stone disease. J Urol. 2007;177(3):979-982.
6. Lieske JC, Peña de la Vega LS, Slezak JM, et al. Renal stone epidemiology in Rochester, Minnesota: an update. Kidney Int. 2006;69(4):760-764.
7. Romero V, Akpinar H, Assimos DG. Kidney stones: a global picture of prevalence, incidence, and associated risk factors. Rev Urol. 2010;12(2-3):e86-e96.
8. Sutherland JW, Parks JH, Coe FL. Recurrence after a single renal stone in a community practice. Miner Electrolyte Metab. 1985;11(4):267-269.
9. Gillen DL, Worcester EM, Coe FL. Decreased renal function among adults with a history of nephrolithiasis: a study of NHANES III. Kidney Int. 2005;67(2):685-690.
10. Stankus N, Hammes M, Gillen D, Worcester E. African American ESRD patients have a high pre-dialysis prevalence of kidney stones compared to NHANES III. Urol Res. 2007;35(2):83-87.
11. Hassan I, Juncos LA, Milliner DS, et al. Chronic renal failure secondary to oxalate nephropathy: a preventable complication after jejunoileal bypass. Mayo Clin Proc. 2001;76(7):758-760.
12. Johri N, Cooper B, Robertson W, et al. An update and practical guide to renal stone management. Nephron Clin Pract. 2010;116(3): c159-c171.
13. Wagner CA, Mohebbi N. Urinary pH and stone formation. J Nephrol. 2010;23 suppl 16: S165-S169.
14. McPhail EF, Gettman MT, Patterson DE, et al. Nephrolithiasis in medullary sponge kidney: evaluation of clinical and metabolic features. Urology. 2011 Oct 17. [Epub ahead of print]
15. Raj GV, Auge BK, Assimos D, Preminger GM. Metabolic abnormalities associated with renal calculi in patients with horseshoe kidneys.
J Endourol. 2004;18(2):157-161.
16. Soylu A, Ugras YM, Günes A, Baydinç D. Bilateral kidney stones with ureteropelvic junction obstruction. Nat Clin Pract Urol. 2005;2(7): 351-354.
17. Curhan GC. Diet and the prevention of kidney stones. Nephrology Rounds. 2004(2):4. www
.nephrologyrounds.org/crus/nephUS_0404.pdf. Accessed January 23, 2012.
18. Wu JN, Craig J, Chamie K, et al. Urolithiasis risk factors in the bariatric population undergoing gastric bypass surgery. Surg Obes Relat Dis. 2011 Sep 21. [Epub ahead of print]
19. Patel BN, Passman CM, Fernandez A, et al. Prevalence of hyperoxaluria after bariatric surgery. J Urol. 2009;181(1):161-166.
20. Taylor EN, Stampfer M, Curhan GC. Obesity, weight gain, and the risk of kidney stones. JAMA. 2005;293(4):455-462.
21. Hodgkinson A, Pyrah LN. The urinary excretion of calcium and inorganic phosphate in 344 patients with calcium stone of renal origin. Br J Surg. 1958;46(195):10-18.
22. Curhan GC, Willett WC, Speizer FE, Stampfer MJ. Twenty-four-hour urine chemistries and the risk of kidney stones among women and men. Kidney Int. 2001;59(6):2290-2298.
23. Pak CY. Citrate and renal calculi: an update. Miner Electrolyte Metab. 1994;20(6):371-377.
24. Curhan GC. Epidemiology of stone disease. Urol Clin North Am. 2007;34(3):287-293.
25. Borghi L, Schianchi T, Meschi T, et al. Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N Engl J Med. 2002;346(2):77-84.
26. Curhan G, Willett WC, Speizer FE, et al. Comparison of dietary calcium with supplemental calcium and other nutrients as factors affecting the risk for kidney stones in women. Ann Intern Med. 1997;126(7):497-504.
27. Grases F, Costa-Bauza A, Prieto RM. Renal lithiasis and nutrition. Nutr J. 2006;5:23.
28. Curhan GC, Willett WC, Knight EL, Stampfer MJ. Dietary factors and the risk of incident kidney stones in younger women. Arch Intern Med. 2004;164(8):885-891.
29. Miller NL, Lingeman JE. Management of kidney stones. BMJ. 2007;334(7591):468-472.
30. Bansal AD, Hui J, Goldfarb DS. Asymptomatic nephrolithiasis detected by ultrasound. Clin J Am Soc Nephrol. 2009;4(3):680-684.
31. Ramakrishnan K, Scheid DC. Diagnosis and management of acute pyelonephritis in adults. Am Fam Physician. 2005;71(5):933-942.
32. Portis AJ, Sundaram CP. Diagnosis and initial management of kidney stones. Am Fam Physician. 2001;63(7):1329-1338.
33. Preminger GM, Assimos DG, Lingeman JE, et al. Chapter 1: AUA guideline on management of staghorn calculi: diagnosis and treatment recommendations. J Urol. 2005;173(6):1991-2000.
34. Lipkin ME, Preminger GM. Demystifying the medical management of nephrolithiasis. Rev Urol. 2011;13(1):34-38.
35. Kourambas J, Aslan P, Teh CL, et al. Role of stone analysis in metabolic evaluation and medical treatment of nephrolithiasis. J Endourol. 2001;15(2):181-186.
36. Jackman SV, Potter SR, Regan F, Jarrett TW. Plain abdominal x-ray versus computerized tomography screening: sensitivity for stone localization after nonenhanced spiral computerized tomography. J Urol. 2000;164(2):308-310.
37. Hollingsworth JM, Rogers MA, Kaufman SR, et al. Medical therapy to facilitate urinary stone passage: a meta-analysis. Lancet. 2006;368 (9542):1171-1179.
38. Preminger GM, Tiselius HG, Assimos DG, et al. 2007 guideline for the management of ureteral calculi. J Urol. 2007;178(6):2418-2434.
39. Huerta C, Castellsague J, Varas-Lorenzo C, García Rodríguez LA. Nonsteroidal anti-inflammatory drugs and risk of ARF in the general population. Am J Kidney Dis. 2005;45(3):531-539.
40. Schneider V, Lévesque LE, Zhang B, et al. Association of selective and conventional nonsteroidal antiinflammatory drugs with acute renal failure: a population-based, nested case-control analysis. Am J Epidemiol. 2006;164(9): 881-889.
41. Davenport K, Timoney AG, Keeley FX. Conventional and alternative methods for providing analgesia in renal colic. BJU Int. 2005;95(3):297-300.
42. Yilmaz E, Batislam E, Deniz T, Yuvanc E. Histamine 1 receptor antagonist in symptomatic treatment of renal colic accompanied by nausea: two birds with one stone? Urology. 2009; 73(1):32-36.
43. Krambeck AE, LeRoy AJ, Patterson DE, Gettman MT. Long-term outcomes of percutaneous nephrolithotomy compared to shock wave lithotripsy and conservative management. J Urol. 2008;179(6):2233-2237.
44. Coll DM, Varanelli MJ, Smith RC. Relationship of spontaneous passage of ureteral calculi to stone size and location as revealed by unenhanced helical CT. AJR Am J Roentgenol. 2002; 178(1):101-103.
45. Laube N, Kleinen L, Bradenahl J, Meissner A. Diamond-like carbon coatings on ureteral stents: a new strategy for decreasing the formation of crystalline bacterial biofilms? J Urol. 2007;177 (5):1923-1927.
46. Khan SR, Glenton PA, Byer KJ. Dietary oxalate and calcium oxalate nephrolithiasis. J Urol. 2007;178(5):2191-2196.
47. Pak CY, Sakhaee K, Fuller C. Successful management of uric acid nephrolithiasis with potassium citrate. Kidney Int. 1986;30(3):422-428.
Kidney or urinary tract stones (whose presence is referred to as nephrolithiasis) are hard, crystalline mineral concretions that form within the kidney or the urinary tract. They are a common problem, with an estimated annual incidence of 1% and a lifetime risk of 15% to 25%; this constitutes a significant health care burden, particularly for people of working age.1
Nephrolithiasis is currently more prevalent in men than in women (13% vs 7%, respectively), and it is three to four times more likely to present in white than nonwhite patients.2 However, recent epidemiologic data suggest an alarming increase in the number of women and adolescents primarily diagnosed with stone disease.3-6 The pattern of increasing incidence in women can be attributed in part to changes in diet and lifestyle.4,5 Figure 17 represents the prevalence of stone disease, specific to gender and race.2,4
Due to kidney stones’ relatively common occurrence, the diagnosis, management, and prevention of stone disease have become increasingly relevant for the primary care practitioner. In the course of stone disease management, the clinician should be aware of a vital fact: Stones have a tendency to recur.1 Indeed, evidence suggests that following an initial diagnosis of nephrolithiasis, the probability of kidney stone recurrence increases to nearly 50% after five years.8
Even more concerning, evidence from several studies suggests that patients with a history of stone disease have a higher probability of experiencing a significant reduction in renal function (ie, decrease in glomerular filtration rate) and hence end-stage renal disease, when compared with non–stone formers.9-11 This accentuates the importance of early diagnosis, treatment, and initiation of steps to prevent further recurrence of this condition.
PATHOGENESIS
Stones in the urinary tract develop under specific urinary conditions, including supersaturation of the urine with stone-forming ions (ie, calcium, oxalate, uric acid, and phosphate) and deficiency of urinary stone inhibitors (citrate, magnesium, zinc, macromolecules, and pyrophosphate). Stone formation occurs in a mucoprotein matrix that attaches to the renal epithelium. Urine becomes supersaturated as a result of increasing levels of solutes (such as the stone-forming ions) and/or decreasing free water volume. When the concentration of stone-forming ions exceeds solubility in the urine (equilibrium solubility product), these ions can combine to form crystals.12,13
Stones are typed based on the ion composition of their crystals (see Table 12,12).
Once crystals are formed, they can also aggregate with other crystals, developing into a calculus.12 Urinary pH influences ion crystallization: Alkaline urine favors formation of calcium and/or phosphate stones, whereas acidic urine favors uric acid and cystine stone formation.13
Kidney stones can be divided into four broad types: calcium-based, struvite, uric acid, and cystine stones (see Figure 2). Among these, calcium-based stones are by far the most common, with nearly 80% of stones composed of calcium compounds (usually calcium oxalate, and rarely calcium phosphate).4 The etiologies of these four types are vastly different, and prevention of stone formation must be tailored to the stone type. Once stones form, however, the appropriate treatment strategies have many similarities.
RISK FACTORS
Specific risk factors for stone formation vary widely and are unique to the type of stone. A thorough history, including a family or personal history of stone disease and dietary history, must be part of the initial work-up when a patient is being evaluated for stone disease; patients with any of these risk factors should be investigated further.
The risk factors for stone disease can be broadly categorized as either individual risk factors or dietary risk factors.
Individual Risk Factors
A positive family history increases the risk for stone formation by two- to three-fold. Other individual risk factors include congenital anatomic defects, such as medullary sponge kidney, horseshoe kidney, and ureteropelvic junction obstruction (UPJ).14-16 These can cause obstruction that leads to urinary stasis, and subsequently to stone precipitation.
Certain systemic disorders (eg, hyperparathyroidism) and situations have also been associated with stone disease and should be considered risk factors. (See Table 212,17).
In patients who undergo gastrointestinal bypass surgery, the development of hyperoxaluria, hypercalciuria, and decreased urinary volume are associated with an increased risk for stone formation,18,19 and these patients should be watched for this development. Obesity and weight gain are directly proportional to nephrolithiasis risk, especially in women.4,20
Environment plays a very important role in stone formation. Persons who live in a hot, arid climate, for example, and those who work outdoors in hot weather are at increased risk for stone formation due to excessive fluid loss from sweating.2,4,7 (In regions where the risk for kidney stone formation is high, Romero et al7 predict, nephrolithiasis incidence could rise from 40% to 56% by 2050 as a result of the effects of global warming.)
Lastly, an individual’s ability (or inability) to metabolize calcium salts plays a vital role in the pathogenesis of stone disease. Intestinal calcium absorption is a major determinant of hypercalciuria, as nearly 90% of ingested calcium is absorbed in the intestines. People can broadly be divided into high or low calcium absorbers. Hypercalciuria (mean urinary calcium excretion ≥ 300 mg/d in men and ≥ 250 mg/d in women on a 1,000-mg/d calcium diet) is detected in 20% to 40% of those with calcium stones.21-23 Hypocitraturia (mean urinary citrate excretion ≤ 320 mg/d) and hyperoxaluria (mean urinary oxalate excretion > 45 mg/d) can also increase the risk for stone formation.12,24
Dietary Risk Factors
These are primarily related to fluid intake and dietary calcium.7,17,25,26 Drinking less than 1 L of fluids daily is associated with an increased risk for forming stones; this risk is magnified when the urine volume is also decreased.7,17,27 Increased dietary intake of animal protein can elevate the risk for formation of uric acid stones as a result of elevated urinary calcium and uric acid and decreased urinary citrate.17
Low dietary calcium ingestion and high oxalate consumption, resulting in increased oxalate absorption, can also exacerbate the risk for stones.7,27 By contrast, a diet high in calcium (≥ 1,200 mg/d) reduces the risk for calcium oxalate stone recurrence,17 although the effectiveness of supplemental calcium has been questioned.26-28
Patients who are advised to make specific dietary adjustments should later undergo repeat urine chemistries to determine the effectiveness of these changes.17
CLINICAL PRESENTATION
Nephrolithiasis typically presents with colicky flank pain, often accompanied by nausea and vomiting.29 The pain radiates to the ipsilateral groin, and the patient typically has difficulty finding a comfortable position. Nephrolithiasis may also present with chronic, episodic flank pain or may even be asymptomatic.30
Physical examination may reveal signs of severe pain, such as tachycardia and hypertension. Presence of fever indicates associated urinary tract infection and possibly pyelonephritis. Some larger stones can cause urinary tract obstruction; if obstruction occurs along with a preexisting urinary tract infection, it can potentially lead to pyelonephritis, pyonephrosis, and eventually urosepsis—a potentially life-threatening condition that requires immediate surgical drainage.31
Before a diagnosis of renal stones can be confirmed, care should be exercised to rule out the differentials, including abdominal aortic aneurysm, appendicitis, bowel obstruction, cholecystitis, drug-seeking behavior (eg, painkiller addiction), gastritis, mesenteric ischemia, musculoskeletal pain, ovarian abscess, ruptured ovarian cyst, pelvic inflammatory disease, pyelonephritis, and UPJ.2,32,33 All patients with suspected nephrolithiasis should be carefully evaluated using laboratory and radiologic investigations.
LABORATORY EVALUATION
The goals in this two-step process are to confirm the diagnosis of nephrolithiasis, then to identify the composition of the stones formed and the associated risk factors.
Initial Evaluation
Tests include dipstick urine assessment, serum chemistries, and a complete blood count (CBC). Urine dipstick assessment may be positive for blood, protein, or leukocyte esterase, indicating stones or fragments of stones present in the urinary tract. While nearly 10% of patients with stone disease exhibit gross hematuria, nearly 90% of patients have microscopic hematuria.2
Urine osmolality should be reviewed to assess urine concentration. Serum chemistries should be ordered to evaluate kidney function. Elevated creatinine may indicate acute rather than chronic kidney disease. Electrolytes and carbon dioxide should be measured to evaluate the kidneys’ ability to concentrate urine and maintain an acid–base balance. The CBC may reveal mild leukocytosis in nephrolithiasis; presence of significant leukocytosis indicates infection.2
Secondary Evaluation
This step begins with a thorough review of the patient’s medical record and a detailed patient interview to ascertain all risk factors for stone formation (as summarized in Table 1). Specific studies to be considered are mentioned in Table 3.2 This evaluation is critical to prevent formation of future stones and the associated complications. In the patient with a history of stone recurrence or stone formation of identified cause, evaluation is needed for three metabolic abnormalities—hypercalciuria, hyperuricosuria, and hypocitraturia—as these conditions predispose patients to recurrent stone formation.1,25,34
The patient should also be encouraged to collect stones passed for further clinical evaluation. Infrared spectroscopy or quantitative wet analysis is used to identify the specific composition of the stone.32,35
Radiologic Evaluation
Radiologic evaluation of stones is currently performed through plain x-rays, ultrasonography, and noncontrast spiral CT.12,32,33 When a patient presents with acute signs of nephrolithiasis, a plain film x-ray of the kidneys, ureters, and bladder (KUB) is acceptable as the first imaging study, as it is inexpensive and available in most areas.33 Plain film KUB x-rays will identify calcium oxalate, calcium phosphate, struvite, and cystine stones. However, the sensitivity of plain film x-rays has been documented between 24% and 59%, and stones that overlie a bone may be missed.32,36 (See Figure 3.)
Hence, because of these limitations and the increasing availability of noncontrast spiral CT, noncontrast spiral CT is now the most commonly used and useful test in the diagnosis of kidney stones (sensitivity, 95% to 100%).32,36 Spiral CT accurately defines the size as well as the location of stones, and may additionally rule out other differential diagnoses (see Figures 4a, 4b, and 4c).
Historically, IV pyelograms and urograms were considered useful in locating urinary tract stones and diagnosing related complications,12 but these modalities carry additional risks related to IV contrast dye and radiation exposure. As a result, they have been almost completely replaced by noncontrast spiral CT because of ease of use and reduced risks.33
Stones may also be seen on renal ultrasound—particularly uric acid stones, which are radiolucent (see Figure 5). Ultrasound is appropriate for evaluation of patients whose exposure to radiation should be limited, such as children or pregnant women. In addition to plain film x-rays, renal ultrasound may also be useful for surveillance of stones.12
TREATMENT
Nephrolithiasis treatment varies between acute and chronic care. Acute care for nephrolithiasis involves management of acute pain and urinary obstruction, as well as patient stabilization. Chronic care includes prevention of recurrence and management of risks.
Acute Management
Patients who present with acute nephrolithiasis most often require fluid administration, aggressive pain management, and treatment for nausea or vomiting.31,32 Most ureteral stones measuring 5.0 mm or less will typically pass spontaneously within a few weeks,1,29 but larger stones usually require intervention—in some cases, surgery.
Patients should be hospitalized if they require IV fluids or pain management. Isotonic IV fluids should be given to increase the urine volume and facilitate passage of stones. Care must be taken to monitor fluids, as patients with kidney stones may have a limited ability to urinate (due to urinary obstruction and/or acute or chronic renal failure). Whenever possible, all urine should be strained to collect any stones for analysis.
One new strategy to assist with stone passage is medical expulsive therapy (MET), using calcium channel blockers (eg, nifedipine) or α-blockers (eg, tamsulosin).1,37 While there is conflicting evidence regarding the efficacy of calcium channel blockers for MET, one meta-analysis revealed a 29% improvement in stone passage with α-blockers.1,38
Pain management can often be accomplished with NSAIDs (eg, ketorolac, diclofenac).29 Since this class of medications can compromise renal function, however, they must be used with caution. Many patients require narcotic medications to control pain adequately.39-41 Antiemetic agents (such as the H1-receptor blocker dimenhydrinate42) should be administered to control nausea and vomiting.
Surgical and interventional management. Surgical intervention may be required if stones are too large to pass spontaneously (typically ≥ 8 mm); if they cause acute renal obstruction; or if they are located at a site with a potential for complications or can lead to persistent symptoms without evidence that they are passing.1,3 Renal obstruction should be treated aggressively to preserve renal function.
The type of intervention chosen depends on the size and location of the stone, as well as the presence or absence of obstruction. Stones that measure less than 20 mm are commonly treated with extracorporeal shockwave lithotripsy (unless they overlie the sacroiliac joint), whereas patients with larger or more complex stones may require percutaneous nephrolithotomy. Nonobstructive or uncomplicated ureteral stones may be managed medically, whereas obstructive or complicated ureteral stones require placement of a stent or a nephrostomy tube until they can be removed by endoscopic surgery.29,43
Obstruction, which may be partial or complete, is more likely when stone size exceeds 10 mm.44 Signs of obstruction include sudden-onset, excruciating flank pain that radiates to the groin, along with nausea and vomiting (renal colic). Larger obstructive stones, such as staghorn calculi (as shown in Figures 3 and 4a), can present with symptoms of a urinary tract infection, mild flank pain, or hematuria.33
Presence of signs of obstruction or infection mandates emergent treatment. Infections of the urinary tract (as serious as pyelonephritis or urosepsis) should be treated with antibiotics: initially with broad coverage, according to the appropriate guidelines for urinary tract infections, then tailored to the results of urine cultures. Obstruction can be relieved directly by nephrostomy tubes (and/or stents) or by interventions in which the stone is removed and normal urinary flow is restored.
Typically, endoscopy is used for direct removal of stones that cause obstruction.44 Nephrostomy tubes and ureteral stents (see Figures 6a and 6b) are placed to relieve obstruction temporarily and provide an alternate route for drainage of urine. The goal is to prevent renal damage until the obstruction can be relieved. Stents can remain in place for several months, but nephrostomy tubes are associated with a higher risk for infection (because they are externalized), and duration of use should be limited to only a few weeks.12,29,38
Stents are also associated with infections, but coated stents are available to reduce infection. As with any catheter material inserted into the urinary tract, ureteral stents are a prime location for development of a persistent bacterial biofilm, thus leading to infection. Recent advances in stent manufacturing have included coating stents with various biomaterials to decrease the development of this bacterial biofilm. In a preliminary study in 10 patients using a diamond-like, carbon-coated ureteral stent, Laube et al45 demonstrated a reduction in formation of this biofilm, hence lowering the probability of stent-induced infection.
Chronic Stone Management
As previously mentioned, one of the seminal characteristics of stone disease is its ability to recur. After incidental detection of kidney stones through routine diagnostic procedures, the risk for recurrence in patients who do not receive chronic medical management is 30% to 40% within five years.17,28 In treated patients, by comparison, this risk falls by approximately 50%.17,26
Patients with a history of stone recurrence must be evaluated for metabolic defects that precipitate stones, since their risk for chronic kidney disease is increased.34 All patients with a history of stone disease should be instructed to increase their fluid intake to maintain a daily urinary output of at least 2.5 L, unless contraindications exist.34
In patients with calcium-based stones who do not benefit from conservative treatment (ie, a low-sodium diet and other dietary modifications), thiazide diuretics may help reduce urinary calcium.1,46
Struvite stones can be prevented through use of long-term antibiotics to reduce the risk for urinary tract infection and by maintaining urinary pH levels below 6.0.17,27,34
For patients with uric acid stones, allopurinol may be prescribed to lower uric acid levels; moreover, the solubility of uric acid is greatly increased at higher pH, so it is beneficial to treat these patients with citrate to maintain their urinary pH above 6.0.47,34
Ensuring a high urine output (≥ 4 L/d34) and alkalinizing urine can help prevent recurrence of cystine stones.17,33 Treatment with potassium citrate has been shown to maintain a urinary pH of 6.5 to 7.0.34
CONCLUSION
The ever-increasing significance of nephrolithiasis has mandated an organized and systematic management approach. Indeed, the diagnosis and initial therapy for kidney stones have undergone considerable evolution in recent years. The basic tenets of nephrolithiasis management include early diagnosis and pertinent treatment as well as adequate prophylaxis to prevent subsequent stone recurrence.
Kidney or urinary tract stones (whose presence is referred to as nephrolithiasis) are hard, crystalline mineral concretions that form within the kidney or the urinary tract. They are a common problem, with an estimated annual incidence of 1% and a lifetime risk of 15% to 25%; this constitutes a significant health care burden, particularly for people of working age.1
Nephrolithiasis is currently more prevalent in men than in women (13% vs 7%, respectively), and it is three to four times more likely to present in white than nonwhite patients.2 However, recent epidemiologic data suggest an alarming increase in the number of women and adolescents primarily diagnosed with stone disease.3-6 The pattern of increasing incidence in women can be attributed in part to changes in diet and lifestyle.4,5 Figure 17 represents the prevalence of stone disease, specific to gender and race.2,4
Due to kidney stones’ relatively common occurrence, the diagnosis, management, and prevention of stone disease have become increasingly relevant for the primary care practitioner. In the course of stone disease management, the clinician should be aware of a vital fact: Stones have a tendency to recur.1 Indeed, evidence suggests that following an initial diagnosis of nephrolithiasis, the probability of kidney stone recurrence increases to nearly 50% after five years.8
Even more concerning, evidence from several studies suggests that patients with a history of stone disease have a higher probability of experiencing a significant reduction in renal function (ie, decrease in glomerular filtration rate) and hence end-stage renal disease, when compared with non–stone formers.9-11 This accentuates the importance of early diagnosis, treatment, and initiation of steps to prevent further recurrence of this condition.
PATHOGENESIS
Stones in the urinary tract develop under specific urinary conditions, including supersaturation of the urine with stone-forming ions (ie, calcium, oxalate, uric acid, and phosphate) and deficiency of urinary stone inhibitors (citrate, magnesium, zinc, macromolecules, and pyrophosphate). Stone formation occurs in a mucoprotein matrix that attaches to the renal epithelium. Urine becomes supersaturated as a result of increasing levels of solutes (such as the stone-forming ions) and/or decreasing free water volume. When the concentration of stone-forming ions exceeds solubility in the urine (equilibrium solubility product), these ions can combine to form crystals.12,13
Stones are typed based on the ion composition of their crystals (see Table 12,12).
Once crystals are formed, they can also aggregate with other crystals, developing into a calculus.12 Urinary pH influences ion crystallization: Alkaline urine favors formation of calcium and/or phosphate stones, whereas acidic urine favors uric acid and cystine stone formation.13
Kidney stones can be divided into four broad types: calcium-based, struvite, uric acid, and cystine stones (see Figure 2). Among these, calcium-based stones are by far the most common, with nearly 80% of stones composed of calcium compounds (usually calcium oxalate, and rarely calcium phosphate).4 The etiologies of these four types are vastly different, and prevention of stone formation must be tailored to the stone type. Once stones form, however, the appropriate treatment strategies have many similarities.
RISK FACTORS
Specific risk factors for stone formation vary widely and are unique to the type of stone. A thorough history, including a family or personal history of stone disease and dietary history, must be part of the initial work-up when a patient is being evaluated for stone disease; patients with any of these risk factors should be investigated further.
The risk factors for stone disease can be broadly categorized as either individual risk factors or dietary risk factors.
Individual Risk Factors
A positive family history increases the risk for stone formation by two- to three-fold. Other individual risk factors include congenital anatomic defects, such as medullary sponge kidney, horseshoe kidney, and ureteropelvic junction obstruction (UPJ).14-16 These can cause obstruction that leads to urinary stasis, and subsequently to stone precipitation.
Certain systemic disorders (eg, hyperparathyroidism) and situations have also been associated with stone disease and should be considered risk factors. (See Table 212,17).
In patients who undergo gastrointestinal bypass surgery, the development of hyperoxaluria, hypercalciuria, and decreased urinary volume are associated with an increased risk for stone formation,18,19 and these patients should be watched for this development. Obesity and weight gain are directly proportional to nephrolithiasis risk, especially in women.4,20
Environment plays a very important role in stone formation. Persons who live in a hot, arid climate, for example, and those who work outdoors in hot weather are at increased risk for stone formation due to excessive fluid loss from sweating.2,4,7 (In regions where the risk for kidney stone formation is high, Romero et al7 predict, nephrolithiasis incidence could rise from 40% to 56% by 2050 as a result of the effects of global warming.)
Lastly, an individual’s ability (or inability) to metabolize calcium salts plays a vital role in the pathogenesis of stone disease. Intestinal calcium absorption is a major determinant of hypercalciuria, as nearly 90% of ingested calcium is absorbed in the intestines. People can broadly be divided into high or low calcium absorbers. Hypercalciuria (mean urinary calcium excretion ≥ 300 mg/d in men and ≥ 250 mg/d in women on a 1,000-mg/d calcium diet) is detected in 20% to 40% of those with calcium stones.21-23 Hypocitraturia (mean urinary citrate excretion ≤ 320 mg/d) and hyperoxaluria (mean urinary oxalate excretion > 45 mg/d) can also increase the risk for stone formation.12,24
Dietary Risk Factors
These are primarily related to fluid intake and dietary calcium.7,17,25,26 Drinking less than 1 L of fluids daily is associated with an increased risk for forming stones; this risk is magnified when the urine volume is also decreased.7,17,27 Increased dietary intake of animal protein can elevate the risk for formation of uric acid stones as a result of elevated urinary calcium and uric acid and decreased urinary citrate.17
Low dietary calcium ingestion and high oxalate consumption, resulting in increased oxalate absorption, can also exacerbate the risk for stones.7,27 By contrast, a diet high in calcium (≥ 1,200 mg/d) reduces the risk for calcium oxalate stone recurrence,17 although the effectiveness of supplemental calcium has been questioned.26-28
Patients who are advised to make specific dietary adjustments should later undergo repeat urine chemistries to determine the effectiveness of these changes.17
CLINICAL PRESENTATION
Nephrolithiasis typically presents with colicky flank pain, often accompanied by nausea and vomiting.29 The pain radiates to the ipsilateral groin, and the patient typically has difficulty finding a comfortable position. Nephrolithiasis may also present with chronic, episodic flank pain or may even be asymptomatic.30
Physical examination may reveal signs of severe pain, such as tachycardia and hypertension. Presence of fever indicates associated urinary tract infection and possibly pyelonephritis. Some larger stones can cause urinary tract obstruction; if obstruction occurs along with a preexisting urinary tract infection, it can potentially lead to pyelonephritis, pyonephrosis, and eventually urosepsis—a potentially life-threatening condition that requires immediate surgical drainage.31
Before a diagnosis of renal stones can be confirmed, care should be exercised to rule out the differentials, including abdominal aortic aneurysm, appendicitis, bowel obstruction, cholecystitis, drug-seeking behavior (eg, painkiller addiction), gastritis, mesenteric ischemia, musculoskeletal pain, ovarian abscess, ruptured ovarian cyst, pelvic inflammatory disease, pyelonephritis, and UPJ.2,32,33 All patients with suspected nephrolithiasis should be carefully evaluated using laboratory and radiologic investigations.
LABORATORY EVALUATION
The goals in this two-step process are to confirm the diagnosis of nephrolithiasis, then to identify the composition of the stones formed and the associated risk factors.
Initial Evaluation
Tests include dipstick urine assessment, serum chemistries, and a complete blood count (CBC). Urine dipstick assessment may be positive for blood, protein, or leukocyte esterase, indicating stones or fragments of stones present in the urinary tract. While nearly 10% of patients with stone disease exhibit gross hematuria, nearly 90% of patients have microscopic hematuria.2
Urine osmolality should be reviewed to assess urine concentration. Serum chemistries should be ordered to evaluate kidney function. Elevated creatinine may indicate acute rather than chronic kidney disease. Electrolytes and carbon dioxide should be measured to evaluate the kidneys’ ability to concentrate urine and maintain an acid–base balance. The CBC may reveal mild leukocytosis in nephrolithiasis; presence of significant leukocytosis indicates infection.2
Secondary Evaluation
This step begins with a thorough review of the patient’s medical record and a detailed patient interview to ascertain all risk factors for stone formation (as summarized in Table 1). Specific studies to be considered are mentioned in Table 3.2 This evaluation is critical to prevent formation of future stones and the associated complications. In the patient with a history of stone recurrence or stone formation of identified cause, evaluation is needed for three metabolic abnormalities—hypercalciuria, hyperuricosuria, and hypocitraturia—as these conditions predispose patients to recurrent stone formation.1,25,34
The patient should also be encouraged to collect stones passed for further clinical evaluation. Infrared spectroscopy or quantitative wet analysis is used to identify the specific composition of the stone.32,35
Radiologic Evaluation
Radiologic evaluation of stones is currently performed through plain x-rays, ultrasonography, and noncontrast spiral CT.12,32,33 When a patient presents with acute signs of nephrolithiasis, a plain film x-ray of the kidneys, ureters, and bladder (KUB) is acceptable as the first imaging study, as it is inexpensive and available in most areas.33 Plain film KUB x-rays will identify calcium oxalate, calcium phosphate, struvite, and cystine stones. However, the sensitivity of plain film x-rays has been documented between 24% and 59%, and stones that overlie a bone may be missed.32,36 (See Figure 3.)
Hence, because of these limitations and the increasing availability of noncontrast spiral CT, noncontrast spiral CT is now the most commonly used and useful test in the diagnosis of kidney stones (sensitivity, 95% to 100%).32,36 Spiral CT accurately defines the size as well as the location of stones, and may additionally rule out other differential diagnoses (see Figures 4a, 4b, and 4c).
Historically, IV pyelograms and urograms were considered useful in locating urinary tract stones and diagnosing related complications,12 but these modalities carry additional risks related to IV contrast dye and radiation exposure. As a result, they have been almost completely replaced by noncontrast spiral CT because of ease of use and reduced risks.33
Stones may also be seen on renal ultrasound—particularly uric acid stones, which are radiolucent (see Figure 5). Ultrasound is appropriate for evaluation of patients whose exposure to radiation should be limited, such as children or pregnant women. In addition to plain film x-rays, renal ultrasound may also be useful for surveillance of stones.12
TREATMENT
Nephrolithiasis treatment varies between acute and chronic care. Acute care for nephrolithiasis involves management of acute pain and urinary obstruction, as well as patient stabilization. Chronic care includes prevention of recurrence and management of risks.
Acute Management
Patients who present with acute nephrolithiasis most often require fluid administration, aggressive pain management, and treatment for nausea or vomiting.31,32 Most ureteral stones measuring 5.0 mm or less will typically pass spontaneously within a few weeks,1,29 but larger stones usually require intervention—in some cases, surgery.
Patients should be hospitalized if they require IV fluids or pain management. Isotonic IV fluids should be given to increase the urine volume and facilitate passage of stones. Care must be taken to monitor fluids, as patients with kidney stones may have a limited ability to urinate (due to urinary obstruction and/or acute or chronic renal failure). Whenever possible, all urine should be strained to collect any stones for analysis.
One new strategy to assist with stone passage is medical expulsive therapy (MET), using calcium channel blockers (eg, nifedipine) or α-blockers (eg, tamsulosin).1,37 While there is conflicting evidence regarding the efficacy of calcium channel blockers for MET, one meta-analysis revealed a 29% improvement in stone passage with α-blockers.1,38
Pain management can often be accomplished with NSAIDs (eg, ketorolac, diclofenac).29 Since this class of medications can compromise renal function, however, they must be used with caution. Many patients require narcotic medications to control pain adequately.39-41 Antiemetic agents (such as the H1-receptor blocker dimenhydrinate42) should be administered to control nausea and vomiting.
Surgical and interventional management. Surgical intervention may be required if stones are too large to pass spontaneously (typically ≥ 8 mm); if they cause acute renal obstruction; or if they are located at a site with a potential for complications or can lead to persistent symptoms without evidence that they are passing.1,3 Renal obstruction should be treated aggressively to preserve renal function.
The type of intervention chosen depends on the size and location of the stone, as well as the presence or absence of obstruction. Stones that measure less than 20 mm are commonly treated with extracorporeal shockwave lithotripsy (unless they overlie the sacroiliac joint), whereas patients with larger or more complex stones may require percutaneous nephrolithotomy. Nonobstructive or uncomplicated ureteral stones may be managed medically, whereas obstructive or complicated ureteral stones require placement of a stent or a nephrostomy tube until they can be removed by endoscopic surgery.29,43
Obstruction, which may be partial or complete, is more likely when stone size exceeds 10 mm.44 Signs of obstruction include sudden-onset, excruciating flank pain that radiates to the groin, along with nausea and vomiting (renal colic). Larger obstructive stones, such as staghorn calculi (as shown in Figures 3 and 4a), can present with symptoms of a urinary tract infection, mild flank pain, or hematuria.33
Presence of signs of obstruction or infection mandates emergent treatment. Infections of the urinary tract (as serious as pyelonephritis or urosepsis) should be treated with antibiotics: initially with broad coverage, according to the appropriate guidelines for urinary tract infections, then tailored to the results of urine cultures. Obstruction can be relieved directly by nephrostomy tubes (and/or stents) or by interventions in which the stone is removed and normal urinary flow is restored.
Typically, endoscopy is used for direct removal of stones that cause obstruction.44 Nephrostomy tubes and ureteral stents (see Figures 6a and 6b) are placed to relieve obstruction temporarily and provide an alternate route for drainage of urine. The goal is to prevent renal damage until the obstruction can be relieved. Stents can remain in place for several months, but nephrostomy tubes are associated with a higher risk for infection (because they are externalized), and duration of use should be limited to only a few weeks.12,29,38
Stents are also associated with infections, but coated stents are available to reduce infection. As with any catheter material inserted into the urinary tract, ureteral stents are a prime location for development of a persistent bacterial biofilm, thus leading to infection. Recent advances in stent manufacturing have included coating stents with various biomaterials to decrease the development of this bacterial biofilm. In a preliminary study in 10 patients using a diamond-like, carbon-coated ureteral stent, Laube et al45 demonstrated a reduction in formation of this biofilm, hence lowering the probability of stent-induced infection.
Chronic Stone Management
As previously mentioned, one of the seminal characteristics of stone disease is its ability to recur. After incidental detection of kidney stones through routine diagnostic procedures, the risk for recurrence in patients who do not receive chronic medical management is 30% to 40% within five years.17,28 In treated patients, by comparison, this risk falls by approximately 50%.17,26
Patients with a history of stone recurrence must be evaluated for metabolic defects that precipitate stones, since their risk for chronic kidney disease is increased.34 All patients with a history of stone disease should be instructed to increase their fluid intake to maintain a daily urinary output of at least 2.5 L, unless contraindications exist.34
In patients with calcium-based stones who do not benefit from conservative treatment (ie, a low-sodium diet and other dietary modifications), thiazide diuretics may help reduce urinary calcium.1,46
Struvite stones can be prevented through use of long-term antibiotics to reduce the risk for urinary tract infection and by maintaining urinary pH levels below 6.0.17,27,34
For patients with uric acid stones, allopurinol may be prescribed to lower uric acid levels; moreover, the solubility of uric acid is greatly increased at higher pH, so it is beneficial to treat these patients with citrate to maintain their urinary pH above 6.0.47,34
Ensuring a high urine output (≥ 4 L/d34) and alkalinizing urine can help prevent recurrence of cystine stones.17,33 Treatment with potassium citrate has been shown to maintain a urinary pH of 6.5 to 7.0.34
CONCLUSION
The ever-increasing significance of nephrolithiasis has mandated an organized and systematic management approach. Indeed, the diagnosis and initial therapy for kidney stones have undergone considerable evolution in recent years. The basic tenets of nephrolithiasis management include early diagnosis and pertinent treatment as well as adequate prophylaxis to prevent subsequent stone recurrence.
1. Moe OW, Pearle MS, Sakhaee K. Pharmacotherapy of urolithiasis: evidence from clinical trials. Kidney Int. 2011;79(4):385-392.
2. Schade GR, Faerber GJ. Urinary tract stones. Prim Care. 2010;37(3):565-581, ix.
3. Childs M, Rangel L, Lingeman J, Krambeck A. Contemporary practice patterns in surgical management of stone disease. American Urological Association (AUA) Annual Meeting; May 2011; Washington, DC.
4. Pearle MS, Calhoun E, Curhan GC. Urolithiasis. In: Litwin MS, Saigal CS, eds; National Institute of Diabetes and Digestive and Kidney Diseases. Urologic Diseases in America (2007). 281-320. http://kidney.niddk.nih.gov/statistics/uda/Urologic_Dis eases_in_America.pdf. Accessed January 23, 2012.
5. Scales CD Jr, Curtis LH, Norris RD, et al. Changing gender prevalence of stone disease. J Urol. 2007;177(3):979-982.
6. Lieske JC, Peña de la Vega LS, Slezak JM, et al. Renal stone epidemiology in Rochester, Minnesota: an update. Kidney Int. 2006;69(4):760-764.
7. Romero V, Akpinar H, Assimos DG. Kidney stones: a global picture of prevalence, incidence, and associated risk factors. Rev Urol. 2010;12(2-3):e86-e96.
8. Sutherland JW, Parks JH, Coe FL. Recurrence after a single renal stone in a community practice. Miner Electrolyte Metab. 1985;11(4):267-269.
9. Gillen DL, Worcester EM, Coe FL. Decreased renal function among adults with a history of nephrolithiasis: a study of NHANES III. Kidney Int. 2005;67(2):685-690.
10. Stankus N, Hammes M, Gillen D, Worcester E. African American ESRD patients have a high pre-dialysis prevalence of kidney stones compared to NHANES III. Urol Res. 2007;35(2):83-87.
11. Hassan I, Juncos LA, Milliner DS, et al. Chronic renal failure secondary to oxalate nephropathy: a preventable complication after jejunoileal bypass. Mayo Clin Proc. 2001;76(7):758-760.
12. Johri N, Cooper B, Robertson W, et al. An update and practical guide to renal stone management. Nephron Clin Pract. 2010;116(3): c159-c171.
13. Wagner CA, Mohebbi N. Urinary pH and stone formation. J Nephrol. 2010;23 suppl 16: S165-S169.
14. McPhail EF, Gettman MT, Patterson DE, et al. Nephrolithiasis in medullary sponge kidney: evaluation of clinical and metabolic features. Urology. 2011 Oct 17. [Epub ahead of print]
15. Raj GV, Auge BK, Assimos D, Preminger GM. Metabolic abnormalities associated with renal calculi in patients with horseshoe kidneys.
J Endourol. 2004;18(2):157-161.
16. Soylu A, Ugras YM, Günes A, Baydinç D. Bilateral kidney stones with ureteropelvic junction obstruction. Nat Clin Pract Urol. 2005;2(7): 351-354.
17. Curhan GC. Diet and the prevention of kidney stones. Nephrology Rounds. 2004(2):4. www
.nephrologyrounds.org/crus/nephUS_0404.pdf. Accessed January 23, 2012.
18. Wu JN, Craig J, Chamie K, et al. Urolithiasis risk factors in the bariatric population undergoing gastric bypass surgery. Surg Obes Relat Dis. 2011 Sep 21. [Epub ahead of print]
19. Patel BN, Passman CM, Fernandez A, et al. Prevalence of hyperoxaluria after bariatric surgery. J Urol. 2009;181(1):161-166.
20. Taylor EN, Stampfer M, Curhan GC. Obesity, weight gain, and the risk of kidney stones. JAMA. 2005;293(4):455-462.
21. Hodgkinson A, Pyrah LN. The urinary excretion of calcium and inorganic phosphate in 344 patients with calcium stone of renal origin. Br J Surg. 1958;46(195):10-18.
22. Curhan GC, Willett WC, Speizer FE, Stampfer MJ. Twenty-four-hour urine chemistries and the risk of kidney stones among women and men. Kidney Int. 2001;59(6):2290-2298.
23. Pak CY. Citrate and renal calculi: an update. Miner Electrolyte Metab. 1994;20(6):371-377.
24. Curhan GC. Epidemiology of stone disease. Urol Clin North Am. 2007;34(3):287-293.
25. Borghi L, Schianchi T, Meschi T, et al. Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N Engl J Med. 2002;346(2):77-84.
26. Curhan G, Willett WC, Speizer FE, et al. Comparison of dietary calcium with supplemental calcium and other nutrients as factors affecting the risk for kidney stones in women. Ann Intern Med. 1997;126(7):497-504.
27. Grases F, Costa-Bauza A, Prieto RM. Renal lithiasis and nutrition. Nutr J. 2006;5:23.
28. Curhan GC, Willett WC, Knight EL, Stampfer MJ. Dietary factors and the risk of incident kidney stones in younger women. Arch Intern Med. 2004;164(8):885-891.
29. Miller NL, Lingeman JE. Management of kidney stones. BMJ. 2007;334(7591):468-472.
30. Bansal AD, Hui J, Goldfarb DS. Asymptomatic nephrolithiasis detected by ultrasound. Clin J Am Soc Nephrol. 2009;4(3):680-684.
31. Ramakrishnan K, Scheid DC. Diagnosis and management of acute pyelonephritis in adults. Am Fam Physician. 2005;71(5):933-942.
32. Portis AJ, Sundaram CP. Diagnosis and initial management of kidney stones. Am Fam Physician. 2001;63(7):1329-1338.
33. Preminger GM, Assimos DG, Lingeman JE, et al. Chapter 1: AUA guideline on management of staghorn calculi: diagnosis and treatment recommendations. J Urol. 2005;173(6):1991-2000.
34. Lipkin ME, Preminger GM. Demystifying the medical management of nephrolithiasis. Rev Urol. 2011;13(1):34-38.
35. Kourambas J, Aslan P, Teh CL, et al. Role of stone analysis in metabolic evaluation and medical treatment of nephrolithiasis. J Endourol. 2001;15(2):181-186.
36. Jackman SV, Potter SR, Regan F, Jarrett TW. Plain abdominal x-ray versus computerized tomography screening: sensitivity for stone localization after nonenhanced spiral computerized tomography. J Urol. 2000;164(2):308-310.
37. Hollingsworth JM, Rogers MA, Kaufman SR, et al. Medical therapy to facilitate urinary stone passage: a meta-analysis. Lancet. 2006;368 (9542):1171-1179.
38. Preminger GM, Tiselius HG, Assimos DG, et al. 2007 guideline for the management of ureteral calculi. J Urol. 2007;178(6):2418-2434.
39. Huerta C, Castellsague J, Varas-Lorenzo C, García Rodríguez LA. Nonsteroidal anti-inflammatory drugs and risk of ARF in the general population. Am J Kidney Dis. 2005;45(3):531-539.
40. Schneider V, Lévesque LE, Zhang B, et al. Association of selective and conventional nonsteroidal antiinflammatory drugs with acute renal failure: a population-based, nested case-control analysis. Am J Epidemiol. 2006;164(9): 881-889.
41. Davenport K, Timoney AG, Keeley FX. Conventional and alternative methods for providing analgesia in renal colic. BJU Int. 2005;95(3):297-300.
42. Yilmaz E, Batislam E, Deniz T, Yuvanc E. Histamine 1 receptor antagonist in symptomatic treatment of renal colic accompanied by nausea: two birds with one stone? Urology. 2009; 73(1):32-36.
43. Krambeck AE, LeRoy AJ, Patterson DE, Gettman MT. Long-term outcomes of percutaneous nephrolithotomy compared to shock wave lithotripsy and conservative management. J Urol. 2008;179(6):2233-2237.
44. Coll DM, Varanelli MJ, Smith RC. Relationship of spontaneous passage of ureteral calculi to stone size and location as revealed by unenhanced helical CT. AJR Am J Roentgenol. 2002; 178(1):101-103.
45. Laube N, Kleinen L, Bradenahl J, Meissner A. Diamond-like carbon coatings on ureteral stents: a new strategy for decreasing the formation of crystalline bacterial biofilms? J Urol. 2007;177 (5):1923-1927.
46. Khan SR, Glenton PA, Byer KJ. Dietary oxalate and calcium oxalate nephrolithiasis. J Urol. 2007;178(5):2191-2196.
47. Pak CY, Sakhaee K, Fuller C. Successful management of uric acid nephrolithiasis with potassium citrate. Kidney Int. 1986;30(3):422-428.
1. Moe OW, Pearle MS, Sakhaee K. Pharmacotherapy of urolithiasis: evidence from clinical trials. Kidney Int. 2011;79(4):385-392.
2. Schade GR, Faerber GJ. Urinary tract stones. Prim Care. 2010;37(3):565-581, ix.
3. Childs M, Rangel L, Lingeman J, Krambeck A. Contemporary practice patterns in surgical management of stone disease. American Urological Association (AUA) Annual Meeting; May 2011; Washington, DC.
4. Pearle MS, Calhoun E, Curhan GC. Urolithiasis. In: Litwin MS, Saigal CS, eds; National Institute of Diabetes and Digestive and Kidney Diseases. Urologic Diseases in America (2007). 281-320. http://kidney.niddk.nih.gov/statistics/uda/Urologic_Dis eases_in_America.pdf. Accessed January 23, 2012.
5. Scales CD Jr, Curtis LH, Norris RD, et al. Changing gender prevalence of stone disease. J Urol. 2007;177(3):979-982.
6. Lieske JC, Peña de la Vega LS, Slezak JM, et al. Renal stone epidemiology in Rochester, Minnesota: an update. Kidney Int. 2006;69(4):760-764.
7. Romero V, Akpinar H, Assimos DG. Kidney stones: a global picture of prevalence, incidence, and associated risk factors. Rev Urol. 2010;12(2-3):e86-e96.
8. Sutherland JW, Parks JH, Coe FL. Recurrence after a single renal stone in a community practice. Miner Electrolyte Metab. 1985;11(4):267-269.
9. Gillen DL, Worcester EM, Coe FL. Decreased renal function among adults with a history of nephrolithiasis: a study of NHANES III. Kidney Int. 2005;67(2):685-690.
10. Stankus N, Hammes M, Gillen D, Worcester E. African American ESRD patients have a high pre-dialysis prevalence of kidney stones compared to NHANES III. Urol Res. 2007;35(2):83-87.
11. Hassan I, Juncos LA, Milliner DS, et al. Chronic renal failure secondary to oxalate nephropathy: a preventable complication after jejunoileal bypass. Mayo Clin Proc. 2001;76(7):758-760.
12. Johri N, Cooper B, Robertson W, et al. An update and practical guide to renal stone management. Nephron Clin Pract. 2010;116(3): c159-c171.
13. Wagner CA, Mohebbi N. Urinary pH and stone formation. J Nephrol. 2010;23 suppl 16: S165-S169.
14. McPhail EF, Gettman MT, Patterson DE, et al. Nephrolithiasis in medullary sponge kidney: evaluation of clinical and metabolic features. Urology. 2011 Oct 17. [Epub ahead of print]
15. Raj GV, Auge BK, Assimos D, Preminger GM. Metabolic abnormalities associated with renal calculi in patients with horseshoe kidneys.
J Endourol. 2004;18(2):157-161.
16. Soylu A, Ugras YM, Günes A, Baydinç D. Bilateral kidney stones with ureteropelvic junction obstruction. Nat Clin Pract Urol. 2005;2(7): 351-354.
17. Curhan GC. Diet and the prevention of kidney stones. Nephrology Rounds. 2004(2):4. www
.nephrologyrounds.org/crus/nephUS_0404.pdf. Accessed January 23, 2012.
18. Wu JN, Craig J, Chamie K, et al. Urolithiasis risk factors in the bariatric population undergoing gastric bypass surgery. Surg Obes Relat Dis. 2011 Sep 21. [Epub ahead of print]
19. Patel BN, Passman CM, Fernandez A, et al. Prevalence of hyperoxaluria after bariatric surgery. J Urol. 2009;181(1):161-166.
20. Taylor EN, Stampfer M, Curhan GC. Obesity, weight gain, and the risk of kidney stones. JAMA. 2005;293(4):455-462.
21. Hodgkinson A, Pyrah LN. The urinary excretion of calcium and inorganic phosphate in 344 patients with calcium stone of renal origin. Br J Surg. 1958;46(195):10-18.
22. Curhan GC, Willett WC, Speizer FE, Stampfer MJ. Twenty-four-hour urine chemistries and the risk of kidney stones among women and men. Kidney Int. 2001;59(6):2290-2298.
23. Pak CY. Citrate and renal calculi: an update. Miner Electrolyte Metab. 1994;20(6):371-377.
24. Curhan GC. Epidemiology of stone disease. Urol Clin North Am. 2007;34(3):287-293.
25. Borghi L, Schianchi T, Meschi T, et al. Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N Engl J Med. 2002;346(2):77-84.
26. Curhan G, Willett WC, Speizer FE, et al. Comparison of dietary calcium with supplemental calcium and other nutrients as factors affecting the risk for kidney stones in women. Ann Intern Med. 1997;126(7):497-504.
27. Grases F, Costa-Bauza A, Prieto RM. Renal lithiasis and nutrition. Nutr J. 2006;5:23.
28. Curhan GC, Willett WC, Knight EL, Stampfer MJ. Dietary factors and the risk of incident kidney stones in younger women. Arch Intern Med. 2004;164(8):885-891.
29. Miller NL, Lingeman JE. Management of kidney stones. BMJ. 2007;334(7591):468-472.
30. Bansal AD, Hui J, Goldfarb DS. Asymptomatic nephrolithiasis detected by ultrasound. Clin J Am Soc Nephrol. 2009;4(3):680-684.
31. Ramakrishnan K, Scheid DC. Diagnosis and management of acute pyelonephritis in adults. Am Fam Physician. 2005;71(5):933-942.
32. Portis AJ, Sundaram CP. Diagnosis and initial management of kidney stones. Am Fam Physician. 2001;63(7):1329-1338.
33. Preminger GM, Assimos DG, Lingeman JE, et al. Chapter 1: AUA guideline on management of staghorn calculi: diagnosis and treatment recommendations. J Urol. 2005;173(6):1991-2000.
34. Lipkin ME, Preminger GM. Demystifying the medical management of nephrolithiasis. Rev Urol. 2011;13(1):34-38.
35. Kourambas J, Aslan P, Teh CL, et al. Role of stone analysis in metabolic evaluation and medical treatment of nephrolithiasis. J Endourol. 2001;15(2):181-186.
36. Jackman SV, Potter SR, Regan F, Jarrett TW. Plain abdominal x-ray versus computerized tomography screening: sensitivity for stone localization after nonenhanced spiral computerized tomography. J Urol. 2000;164(2):308-310.
37. Hollingsworth JM, Rogers MA, Kaufman SR, et al. Medical therapy to facilitate urinary stone passage: a meta-analysis. Lancet. 2006;368 (9542):1171-1179.
38. Preminger GM, Tiselius HG, Assimos DG, et al. 2007 guideline for the management of ureteral calculi. J Urol. 2007;178(6):2418-2434.
39. Huerta C, Castellsague J, Varas-Lorenzo C, García Rodríguez LA. Nonsteroidal anti-inflammatory drugs and risk of ARF in the general population. Am J Kidney Dis. 2005;45(3):531-539.
40. Schneider V, Lévesque LE, Zhang B, et al. Association of selective and conventional nonsteroidal antiinflammatory drugs with acute renal failure: a population-based, nested case-control analysis. Am J Epidemiol. 2006;164(9): 881-889.
41. Davenport K, Timoney AG, Keeley FX. Conventional and alternative methods for providing analgesia in renal colic. BJU Int. 2005;95(3):297-300.
42. Yilmaz E, Batislam E, Deniz T, Yuvanc E. Histamine 1 receptor antagonist in symptomatic treatment of renal colic accompanied by nausea: two birds with one stone? Urology. 2009; 73(1):32-36.
43. Krambeck AE, LeRoy AJ, Patterson DE, Gettman MT. Long-term outcomes of percutaneous nephrolithotomy compared to shock wave lithotripsy and conservative management. J Urol. 2008;179(6):2233-2237.
44. Coll DM, Varanelli MJ, Smith RC. Relationship of spontaneous passage of ureteral calculi to stone size and location as revealed by unenhanced helical CT. AJR Am J Roentgenol. 2002; 178(1):101-103.
45. Laube N, Kleinen L, Bradenahl J, Meissner A. Diamond-like carbon coatings on ureteral stents: a new strategy for decreasing the formation of crystalline bacterial biofilms? J Urol. 2007;177 (5):1923-1927.
46. Khan SR, Glenton PA, Byer KJ. Dietary oxalate and calcium oxalate nephrolithiasis. J Urol. 2007;178(5):2191-2196.
47. Pak CY, Sakhaee K, Fuller C. Successful management of uric acid nephrolithiasis with potassium citrate. Kidney Int. 1986;30(3):422-428.
New and future therapies for lupus nephritis
Treatment for lupus nephritis has changed dramatically in recent years. Only 10 years ago, rheumatologists and nephrologists, whether specializing in adult or pediatric medicine, treated lupus nephritis with a similar regimen of monthly intravenous cyclophosphamide (Cytoxan) and glucocorticoids. Although the regimen is effective, side effects such as infection, hair loss, and infertility were extremely common.
Effective but very toxic therapy is common in autoimmune diseases. In the last decade, clinical trials have shown that less toxic drugs are as effective for treating lupus nephritis. This article will review new developments in therapy for lupus nephritis, which can be viewed as a prototype for other fields of medicine.
DEMOGRAPHICS ARE IMPORTANT
Although numerous factors have prognostic value in lupus nephritis (eg, serum creatinine, proteinuria, renal biopsy findings), the most important to consider when designing and interpreting studies are race and socioeconomic variables.
A retrospective study in Miami, FL,1 evaluated 213 patients with lupus nephritis, of whom 47% were Hispanic, 44% African American, and 20% white. At baseline, African Americans had higher blood pressure, higher serum creatinine levels, and lower household income. After 6 years, African Americans fared the worst in terms of doubling of serum creatinine, developing end-stage renal disease, and death; whites had the best outcomes, and Hispanics were in between. Low income was found to be a significant risk factor, independent of racial background.
In a similar retrospective study in New York City in 128 patients (43% white, 40% Hispanic, and 17% African American) with proliferative lupus nephritis,2 disease was much more likely to progress to renal failure over 10 years in patients living in a poor neighborhood, even after adjustment for race.
We need to keep in mind that racial and socioeconomic factors correlate with disease severity when we design and interpret studies of lupus nephritis. Study groups must be carefully balanced with patients of similar racial and socioeconomic profiles. Study findings must be interpreted with caution; for example, whether results from a study from China are applicable to an African American with lupus nephritis in New York City is unclear.
OLDER STANDARD THERAPY: EFFECTIVE BUT TOXIC
The last large National Institutes of Health study that involved only cyclophosphamide and a glucocorticoid was published in 2001,3 with 21 patients receiving cyclophosphamide alone and 20 patients receiving cyclophosphamide plus methylprednisolone. Although lupus nephritis improved, serious side effects occurred in one-third to one-half of patients in each group and included hypertension, hyperlipidemia, valvular heart disease, avascular necrosis, premature menopause, and major infections, including herpes zoster.
Less cyclophosphamide works just as well
The multicenter, prospective Euro-Lupus Nephritis Trial4 randomized 90 patients with proliferative lupus nephritis to receive either standard high-dose intravenous (IV) cyclophosphamide therapy (six monthly pulses and two quarterly pulses, with doses increasing according to the white blood cell count) or low-dose IV cyclophosphamide therapy (six pulses every 2 weeks at a fixed dose of 500 mg). Both regimens were followed by azathioprine (Imuran).
At 4 years, the two treatment groups were not significantly different in terms of treatment failure, remission rates, serum creatinine levels, 24-hour proteinuria, and freedom from renal flares. However, the rates of side effects were significantly different, with more patients in the low-dosage group free of severe infection.
One problem with this study is whether it is applicable to an American lupus nephritis population, since 84% of the patients were white. Since this study, others indicate that this regimen is probably also safe and effective for different racial groups in the United States.
At 10-year follow-up,5 both treatment groups still had identical excellent rates of freedom from end-stage renal disease. Serum creatinine and 24-hour proteinuria were also at excellent levels and identical in both groups. Nearly three quarters of patients still needed glucocorticoid therapy and more than half still needed immunosuppressive therapy, but the rates were not statistically significantly different between the treatment groups.
The cumulative dose of cyclophosphamide was 9.5 g in the standard-treatment group and 5.5 g in the low-dose group. This difference in exposure could make a tremendous difference to patients, not only for immediate side effects such as early menopause and infections, but for the risk of cancer in later decades.
This study showed clearly that low-dose cyclophosphamide is an option for induction therapy. Drawbacks of the study were that the population was mostly white and that patients had only moderately severe disease.
Low-dose cyclophosphamide has largely replaced the older National Institutes of Health regimen, although during the last decade drug therapy has undergone more changes.
MYCOPHENOLATE AND AZATHIOPRINE: ALTERNATIVES TO CYCLOPHOSPHAMIDE
In a Chinese study, mycophenolate was better than cyclophosphamide for induction
In a study in Hong Kong, Chan et al6 randomized 42 patients with severe lupus nephritis to receive either mycophenolate mofetil (available in the United States as CellCept; 2 g/day for 6 months, then 1 g/day for 6 months) or oral cyclophosphamide (2.5 mg/kg per day for 6 months) followed by azathioprine (1.5–2.0 mg/kg per day) for 6 months. Both groups also received prednisolone during the year.
At the end of the first year, the two groups were not significantly different in their rates of complete remission, partial remission, and relapse. The rate of infection, although not significantly different, was higher in the cyclophosphamide group (33% vs 19%). Two patients (10%) died in the cyclophosphamide group, but the difference in mortality rates was not statistically significant.
Nearly 5 years later,7 rates of chronic renal failure and relapse were still statistically the same in the two groups. Infections were fewer in the mycophenolate group (13% vs 40%, P = .013). The rate of amenorrhea was 36% in the cyclophosphamide group and only 4% in the mycophenolate group (P = .004). Four patients in the cyclophosphamide group and none in the mycophenolate group reached the composite end point of end-stage renal failure or death (P = .062).
This study appeared to offer a new option with equal efficacy and fewer side effects than standard therapy. However, its applicability to non-Chinese populations remained to be shown.
In a US study, mycophenolate or azathioprine was better than cyclophosphamide as maintenance
In a study in Miami,8 59 patients with lupus nephritis were given standard induction therapy with IV cyclophosphamide plus glucocorticoids for 6 months, then randomly assigned to one of three maintenance therapies for 1 to 3 years: IV injections of cyclophosphamide every 3 months (standard therapy), oral azathioprine, or oral mycophenolate. The population was 93% female, their average age was 33 years, and nearly half were African American, with many of the others being Hispanic. Patients tended to have severe disease, with nearly two-thirds having nephrotic syndrome.
After 6 years, there had been more deaths in the cyclophosphamide group than in the azathioprine group (P = .02) and in the mycophenolate group, although the latter difference was not statistically significant (P = .11). The combined rate of death and chronic renal failure was significantly higher with cyclophosphamide than with either of the oral agents. The cyclophosphamide group also had the highest relapse rate during the maintenance phase.
The differences in side effects were even more dramatic. Amenorrhea affected 32% of patients in the cyclophosphamide group, and only 7% and 6% in the azathioprine and mycophenolate groups, respectively. Rates of infections were 68% in the cyclophosphamide group and 28% and 21% in the azathioprine and mycophenolate groups, respectively. Patients given cyclophosphamide had 13 hospital days per patient per year, while the other groups each had only 1.
This study showed that maintenance therapy with oral azathioprine or mycophenolate was more effective and had fewer adverse effects than standard IV cyclophosphamide therapy. As a result of this study, oral agents for maintenance therapy became the new standard, but the question remained whether oral agents could safely be used for induction.
In a US study, mycophenolate was better than cyclophosphamide for induction
In a noninferiority study, Ginzler et al9 randomized 140 patients with severe lupus nephritis to receive either monthly IV cyclophosphamide or oral mycophenolate as induction therapy for 6 months. Adjunctive care with glucocorticoids was given in both groups. The study population was from 18 US academic centers and was predominantly female, and more than half were African American.
After 24 weeks, 22.5% of the mycophenolate patients were in complete remission by very strict criteria vs only 4% of those given cyclophosphamide (P = .005). The trend for partial remissions was also in favor of mycophenolate, although the difference was not statistically significant. The rate of complete and partial remissions, a prespecified end point, was significantly higher in the mycophenolate group. Although the study was trying to evaluate equivalency, it actually showed superiority for mycophenolate induction therapy.
Serum creatinine levels declined in both groups, but more in the mycophenolate group by 24 weeks. Urinary protein levels fell the same amount in both groups. At 3 years, the groups were statistically equivalent in terms of renal flares, renal failures, and deaths. However, the study groups were small, and the mycophenolate group did have a better trend for both renal failure (N = 4 vs 7) and deaths (N = 4 vs 8).
Mycophenolate also had fewer side effects, including infection, although again the numbers were too small to show statistical significance. The exception was diarrhea (N = 15 in the mycophenolate group vs 2 in the cyclophosphamide group).
A drawback of the study is that it was designed as a crossover study: a patient for whom therapy was failing after 3 months could switch to the other group, introducing potential confounding. Other problems involved the small population size and the question of whether results from patients in the United States were applicable to others worldwide.
In a worldwide study, mycophenolate was at least equivalent to cyclophosphamide for induction
The Aspreva Lupus Management Study (ALMS)10 used a similar design with 370 patients worldwide (United States, China, South America, and Europe) in one of the largest trials ever conducted in lupus nephritis. Patients were randomized to 6 months of induction therapy with either IV cyclophosphamide or oral mycophenolate but could not cross over.
At 6 months, response rates were identical between the two groups, with response defined as a combination of specific improvement in proteinuria, serum creatinine, and hematuria (50%–55%). In terms of individual renal and nonrenal variables, both groups appeared identical.
However, the side effect profiles differed between the two groups. As expected for mycophenolate, diarrhea was the most common side effect (occurring in 28% vs 12% in the cyclophosphamide group). Nausea and vomiting were more common with cyclophosphamide (45% and 37% respectively vs 14% and 13% in the mycophenolate group). Cyclophosphamide also caused hair loss in 35%, vs 10% in the mycophenolate group.
There were 14 deaths overall, which is a very low number considering the patients’ severity of illness, and it indicates the better results now achieved with therapy. The mortality rate was higher in the mycophenolate group (5% vs 3%), but the difference was not statistically significant. Six of the nine deaths with mycophenolate were from the same center in China, and none were from Europe or the United States. In summary, the study did not show that mycophenolate was superior to IV cyclophosphamide for induction therapy, but that they were equivalent in efficacy with different side effect profiles.
Membranous nephropathy: Mycophenolate vs cyclophosphamide
Less evidence is available about treatment for membranous disease, which is characterized by heavy proteinuria and the nephrotic syndrome but usually does not progress to renal failure. Radhakrishnan et al11 combined data from the trial by Ginzler et al9 and the ALMS trial10 and found 84 patients with pure membranous lupus, who were equally divided between the treatment groups receiving IV cyclophosphamide and mycophenolate. Consistent with the larger group’s data, mycophenolate and cyclophosphamide performed similarly in terms of efficacy, but there was a slightly higher rate of side effects with cyclophosphamide.
Maintenance therapy: Mycophenolate superior to azathioprine
The ALMS Maintenance Trial12 evaluated maintenance therapy in the same worldwide population that was studied for induction therapy. Of the 370 patients involved in the induction phase that compared IV cyclophosphamide and oral mycophenolate, 227 responded sufficiently to be rerandomized in a controlled, double-blinded trial of 36 months of maintenance therapy with corticosteroids and either mycophenolate (1 g twice daily) or azathioprine (2 mg/kg per day).
In intention-to-treat analysis, the time to treatment failure (ie, doubling of the serum creatinine level, progressing to renal failure, or death) was significantly shorter in the azathioprine group (P = .003). Every individual end point—end-stage renal disease, renal flares, doubling of serum creatinine, rescue immunosuppression required—was in favor of mycophenolate maintenance. At 3 years, the completion rate was 63% with mycophenolate and 49% with azathioprine. Serious adverse events and withdrawals because of adverse events were more common in the azathioprine group.
In summary, mycophenolate was superior to azathioprine in maintaining renal response and in preventing relapse in patients with active lupus nephritis who responded to induction therapy with either mycophenolate or IV cyclophosphamide. Mycophenolate was found to be superior regardless of initial induction treatment, race, or region and was confirmed by all key secondary end points.
Only one of the 227 patients died during the 3 years—from an auto accident. Again, this indicates the dramatically improved survival today compared with a decade ago.
RITUXIMAB: PROMISING BUT UNPROVEN
Rituximab (Rituxan) was originally approved to treat tumors, then rheumatoid arthritis, and most recently vasculitis. Evidence thus far is mixed regarding its use as a treatment for lupus nephritis. Although randomized clinical trials have not found it to be superior to standard regimens, there are many signs that it may be effective.
Rituximab in uncontrolled studies
Terrier et al13 analyzed prospective data from 136 patients with systemic lupus erythematosus, most of whom had renal disease, from the French Autoimmunity and Rituximab registry. Response occurred in 71% of patients using rituximab, with no difference found between patients receiving rituximab monotherapy and those concomitantly receiving immunosuppressive agents.
Melander et al14 retrospectively studied 19 women and 1 man who had been treated with rituximab for severe lupus nephritis and followed for at least 1 year. Three patients had concurrent therapy with cyclophosphamide, and 10 patients continued rituximab as maintenance therapy; 12 patients had lupus nephritis that had been refractory to standard treatment, and 6 had relapsing disease.
At a median follow-up of 22 months, 12 patients (60%) had achieved complete or partial renal remission.
Condon et al15 treated 21 patients who had severe lupus nephritis with two doses of rituximab and IV methylprednisolone 2 weeks apart, then maintenance therapy with mycophenolate without any oral steroids. At a mean follow-up of 35 months ( ± 14 months), 16 (76%) were in complete remission, with a mean time to remission of 12 months. Two (9.5%) achieved partial remission. The rate of toxicity was low.
Thus, rituximab appears promising in uncontrolled studies.
Placebo-controlled trials fail to prove rituximab effective
LUNAR trial. On the other hand, the largest placebo-controlled trial to evaluate rituximab in patients with proliferative lupus nephritis, the Lupus Nephritis Assessment With Rituximab (LUNAR) trial16 found differences in favor of rituximab, but none reached statistical significance. The trial randomized 140 patients to receive either mycophenolate plus periodic rituximab infusions or mycophenolate plus placebo infusions for 1 year. All patients received the same dosage of glucocorticoids, which was tapered over the year.
At the end of 1 year, the groups were not statistically different in terms of complete renal response and partial renal response. Rituximab appeared less likely to produce no response, but the difference was not statistically significant.
African Americans appeared to have a higher response rate to rituximab (70% in the rituximab group achieved a response vs 45% in the control group), but again, the difference did not reach statistical significance, and the total study population of African Americans was only 40.
Rituximab did have a statistically significant positive effect on two serologic markers at 1 year: levels of anti-dsDNA fell faster and complement rose faster. In addition, rates of adverse and serious adverse events were similar between the two groups, with no new or unexpected “safety signals.”
This study can be interpreted in a number of ways. The number of patients may have been too small to show significance and the follow-up may have been too short. On the other hand, it may simply not be effective to add rituximab to a full dose of mycophenolate and steroids, an already good treatment.
EXPLORER trial. Similarly, for patients with lupus without nephritis, the Exploratory Phase II/III SLE Evaluation of Rituximab (EXPLORER) trial17 also tested rituximab against a background of an effective therapeutic regimen and found no additional benefit. This study had design problems similar to those of the LUNAR trial.
Rituximab as rescue therapy
The evidence so far indicates that rituximab may have a role as rescue therapy for refractory or relapsing disease. Rituximab must be used with other therapies, but maintenance corticosteroid therapy is not necessary. Its role as a first-line agent in induction therapy for lupus nephritis remains unclear, although it may have an important role for nonwhites. In general, it has been well tolerated. Until a large randomized trial indicates otherwise, it should not be used as a first-line therapy.
The US Food and Drug Administration (FDA) sent out a warning about the danger of progressive multifocal leukoencephalopathy as an adverse effect of rituximab and of mycophenolate, but this does not appear to be a major concern for most patients and is only likely to occur in those who have been over-immunosuppressed for many years.
MULTITARGET THERAPY
The concept of using multiple drugs simultaneously—such as mycophenolate, steroids, and rituximab—is increasingly being tried. Multi-target therapy appears to offer the advantages of combining different modes of action with better results, and it offers fewer side effects because dosages of each individual drug can be lower when combined with other immunosuppressives.
Bao et al18 in China randomly assigned 40 patients with diffuse proliferative and membranous nephritis to 6 to 9 months of induction treatment with either multitarget therapy (mycophenolate, tacrolimus [Prograf], and glucocorticoids) or IV cyclophosphamide. More complete remissions occurred in the multitarget therapy group, both at 6 months (50% vs 5%) and at 9 months (65% vs 15%). Most adverse events were less frequent in the multitarget therapy group, although three patients (15%) in the multitarget therapy group developed new-onset hypertension vs none in the cyclophosphamide group.
NEW MEDICATIONS
Entirely new classes of drugs are being developed with immunomodulatory effects, including tolerance molecules, cytokine blockers, inhibitors of human B lymphocyte stimulator, and costimulatory blockers.
Belimumab offers small improvement for lupus
Belimumab (Benlysta) is a human monoclonal antibody that inhibits the biologic activity of human B lymphocyte stimulator; it has recently been approved by the FDA for lupus nephritis. In a worldwide study,19 867 patients with systemic lupus erythematosus were randomized to receive either belimumab (1 mg/kg or 10 mg/kg) or placebo.
The primary end point was the reduction of disease activity by a scoring system (SELENA-SLEDAI) that incorporated multiple features of lupus, including arthritis, vasculitis, proteinuria, rash, and others. Patients in the belimumab group had better outcomes, but the results were not dramatic. Because the drug is so expensive (about $25,000 per year) and the improvement offered is only incremental, this drug will not likely change the treatment of lupus very much.
Moreover, patients with lupus nephritis were not included in the study, but a new study is being planned to do so. Improvement is harder to demonstrate in lupus nephritis than in rheumatoid arthritis and systemic lupus erythematosus: significant changes in creatinine levels and 24-hour urinary protein must be achieved, rather than more qualitative signs and symptoms of joint pain, rash, and feeling better. Although belimumab is still unproven for lupus nephritis, it might be worth trying for patients failing other therapy.
Laquinimod: A promising experimental drug
Laquinimod is an oral immunomodulatory drug with a number of effects, including down-regulating major histocompatability complex II, chemokines, and adhesion-related molecules related to inflammation. It has been studied in more than 2,500 patients with multiple sclerosis. Pilot studies are now being done for its use for lupus nephritis. If it shows promise, a large randomized, controlled trial will be conducted.
Abatacept is in clinical trials
Abatacept (Orencia), a costimulation blocker, is undergoing clinical trials in lupus nephritis. Results should be available shortly.
INDIVIDUALIZE THERAPY
This past decade has seen such an increase in options to treat lupus nephritis that therapy can now be individualized.
Choosing IV cyclophosphamide vs mycophenolate
As a result of recent trials, doctors in the United States are increasingly using mycophenolate as the first-line drug for lupus nephritis. In Europe, however, many are choosing the shorter regimen of IV cyclophosphamide because of the results of the Euro-Lupus study.
Nowadays, I tend to use IV cyclophosphamide as the first-line drug only for patients with severe crescenteric glomerulonephritis or a very high serum creatinine level. In such cases, there is more experience with cyclophosphamide, and such severe disease does not lend itself to the luxury of trying out different therapies sequentially. If such a severely ill patient insists that a future pregnancy is very important, an alternative therapy of mycophenolate plus rituximab should be considered. I prefer mycophenolate for induction and maintenance therapy in most patients.
Dosing and formulation considerations for mycophenolate
Large dosages of mycophenolate are much better tolerated when broken up throughout the day. A patient who cannot tolerate 1 g twice daily may be able to tolerate 500 mg four times a day. The formulation can also make a difference. Some patients tolerate sustained-release mycophenolate (Myfortic) better than CellCept, and vice versa.
For patients who cannot tolerate mycophenolate, azathioprine is an acceptable alternative. In addition, for a patient who is already doing well on azathioprine, there is no need to change to mycophenolate.
Long maintenance therapy now acceptable
The ALMS Maintenance Trial12 found 3 years of maintenance therapy to be safe and effective. Such a long maintenance period is increasingly viewed as important, especially for patients in their teens and 20s, as it allows them to live a normal life, ie, to finish their education, get married, and become settled socially. Whether 5 years of maintenance therapy or even 10 years is advisable is still unknown.
Treatment during pregnancy
Neither mycophenolate nor azathioprine is recommended during pregnancy, although their effects are unknown. Because we have much more renal transplant experience with azathioprine during pregnancy, I recommend either switching from mycophenolate to azathioprine or trying to stop medication altogether if the patient has been well controlled.
- Contreras G, Lenz O, Pardo V, et al. Outcomes in African Americans and Hispanics with lupus nephritis. Kidney Int 2006; 69:1846–1851.
- Barr RG, Seliger S, Appel GB, et al. Prognosis in proliferative lupus nephritis: the role of socio-economic status and race/ethnicity. Nephrol Dial Transplant 2003; 18:2039–2046.
- Illei GG, Austin HA, Crane M, et al. Combination therapy with pulse cyclophosphamide plus pulse methylprednisolone improves long-term renal outcome without adding toxicity in patients with lupus nephritis. Ann Intern Med 2001; 135:248–257.
- Houssiau FA, Vasconcelos C, D’Cruz D, et al. Immunosuppressive therapy in lupus nephritis: the Euro-Lupus Nephritis Trial, a randomized trial of low-dose versus high-dose intravenous cyclophosphamide. Arthritis Rheum 2002; 46:2121–2131.
- Houssiau FA, Vasconcelos C, D’Cruz D, et al. The 10-year follow-up data of the Euro-Lupus Nephritis Trial comparing low-dose and high-dose intravenous cyclophosphamide. Ann Rheum Dis 2010; 69:61–64.
- Chan TM, Li FK, Tang CS, et al. Efficacy of mycophenolate mofetil in patients with diffuse proliferative lupus nephritis. Hong King-Guangzhou Nephrology Study Group. N Engl J Med 2000; 343:1156–1162.
- Chan TM, Tse KC, Tang CS, Mok MY, Li FK; Hong Kong Nephrology Study Group. Long-term study of mycophenolate mofetil as continuous induction and maintenance treatment for diffuse proliferative lupus nephritis. J Am Soc Nephrol 2005; 16:1076–1084.
- Contreras G, Pardo V, Leclercq B, et al. Sequential therapies for proliferative lupus nephritis. N Engl J Med 2004; 350:971–980.
- Ginzler EM, Dooley MA, Aranow C, et al. Mycophenolate mofetil or intravenous cyclophosphamide for lupus nephritis. N Engl J Med 2005; 353:2219–2228.
- Appel GB, Contreras G, Dooley MA, et al. Mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. J Am Soc Nephrol 2009; 20:1103–1112.
- Radhakrishnan J, Moutzouris DA, Ginzler EM, Solomons N, Siempos II, Appel GB. Mycophenolate mofetil and intravenous cyclophosphamide are similar as induction therapy for class V lupus nephritis. Kidney Int 2010; 77:152–160.
- Dooley MA, Jayne D, Ginzler EM, et al; for the ALMS Group. Mycophenolate versus azathioprine as maintenance therapy for lupus nephritis. N Engl J Med 2011; 365:1886–1895.
- Terrier B, Amoura Z, Ravaud P, et al; Club Rhumatismes et Inflammation. Safety and efficacy of rituximab in systemic lupus erythematosus: results from 136 patients from the French AutoImmunity and Rituximab registry. Arthritis Rheum 2010; 62:2458–2466.
- Melander C, Sallée M, Troillet P, et al. Rituximab in severe lupus nephritis: early B-cell depletion affects long-term renal outcome. Clin J Am Soc Nephrol 2009; 4:579–587.
- Condon MB, Griffith M, Cook HT, Levy J, Lightstone L, Cairns T. Treatment of class IV lupus nephritis with rituximab & mycophenolate mofetil (MMF) with no oral steroids is effective and safe (abstract). J Am Soc Nephrol 2010; 21(suppl):625A–626A.
- Furie RA, Looney RJ, Rovin E, et al. Efficacy and safety of rituximab in subjects with active proliferative lupus nephritis (LN): results from the randomized, double-blind phase III LUNAR study (abstract). Arthritis Rheum 2009; 60(suppl 1):S429.
- Merrill JT, Neuwelt CM, Wallace DJ, et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum 2010; 62:222–233.
- Bao H, Liu ZH, Zie HL, Hu WX, Zhang HT, Li LS. Successful treatment of class V+IV lupus nephritis with multitarget therapy. J Am Soc Nephrol 2008; 19:2001–2010.
- Navarra SV, Guzmán RM, Gallacher AE, et al; BLISS-52 Study Group. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet 2011; 377:721–731.
Treatment for lupus nephritis has changed dramatically in recent years. Only 10 years ago, rheumatologists and nephrologists, whether specializing in adult or pediatric medicine, treated lupus nephritis with a similar regimen of monthly intravenous cyclophosphamide (Cytoxan) and glucocorticoids. Although the regimen is effective, side effects such as infection, hair loss, and infertility were extremely common.
Effective but very toxic therapy is common in autoimmune diseases. In the last decade, clinical trials have shown that less toxic drugs are as effective for treating lupus nephritis. This article will review new developments in therapy for lupus nephritis, which can be viewed as a prototype for other fields of medicine.
DEMOGRAPHICS ARE IMPORTANT
Although numerous factors have prognostic value in lupus nephritis (eg, serum creatinine, proteinuria, renal biopsy findings), the most important to consider when designing and interpreting studies are race and socioeconomic variables.
A retrospective study in Miami, FL,1 evaluated 213 patients with lupus nephritis, of whom 47% were Hispanic, 44% African American, and 20% white. At baseline, African Americans had higher blood pressure, higher serum creatinine levels, and lower household income. After 6 years, African Americans fared the worst in terms of doubling of serum creatinine, developing end-stage renal disease, and death; whites had the best outcomes, and Hispanics were in between. Low income was found to be a significant risk factor, independent of racial background.
In a similar retrospective study in New York City in 128 patients (43% white, 40% Hispanic, and 17% African American) with proliferative lupus nephritis,2 disease was much more likely to progress to renal failure over 10 years in patients living in a poor neighborhood, even after adjustment for race.
We need to keep in mind that racial and socioeconomic factors correlate with disease severity when we design and interpret studies of lupus nephritis. Study groups must be carefully balanced with patients of similar racial and socioeconomic profiles. Study findings must be interpreted with caution; for example, whether results from a study from China are applicable to an African American with lupus nephritis in New York City is unclear.
OLDER STANDARD THERAPY: EFFECTIVE BUT TOXIC
The last large National Institutes of Health study that involved only cyclophosphamide and a glucocorticoid was published in 2001,3 with 21 patients receiving cyclophosphamide alone and 20 patients receiving cyclophosphamide plus methylprednisolone. Although lupus nephritis improved, serious side effects occurred in one-third to one-half of patients in each group and included hypertension, hyperlipidemia, valvular heart disease, avascular necrosis, premature menopause, and major infections, including herpes zoster.
Less cyclophosphamide works just as well
The multicenter, prospective Euro-Lupus Nephritis Trial4 randomized 90 patients with proliferative lupus nephritis to receive either standard high-dose intravenous (IV) cyclophosphamide therapy (six monthly pulses and two quarterly pulses, with doses increasing according to the white blood cell count) or low-dose IV cyclophosphamide therapy (six pulses every 2 weeks at a fixed dose of 500 mg). Both regimens were followed by azathioprine (Imuran).
At 4 years, the two treatment groups were not significantly different in terms of treatment failure, remission rates, serum creatinine levels, 24-hour proteinuria, and freedom from renal flares. However, the rates of side effects were significantly different, with more patients in the low-dosage group free of severe infection.
One problem with this study is whether it is applicable to an American lupus nephritis population, since 84% of the patients were white. Since this study, others indicate that this regimen is probably also safe and effective for different racial groups in the United States.
At 10-year follow-up,5 both treatment groups still had identical excellent rates of freedom from end-stage renal disease. Serum creatinine and 24-hour proteinuria were also at excellent levels and identical in both groups. Nearly three quarters of patients still needed glucocorticoid therapy and more than half still needed immunosuppressive therapy, but the rates were not statistically significantly different between the treatment groups.
The cumulative dose of cyclophosphamide was 9.5 g in the standard-treatment group and 5.5 g in the low-dose group. This difference in exposure could make a tremendous difference to patients, not only for immediate side effects such as early menopause and infections, but for the risk of cancer in later decades.
This study showed clearly that low-dose cyclophosphamide is an option for induction therapy. Drawbacks of the study were that the population was mostly white and that patients had only moderately severe disease.
Low-dose cyclophosphamide has largely replaced the older National Institutes of Health regimen, although during the last decade drug therapy has undergone more changes.
MYCOPHENOLATE AND AZATHIOPRINE: ALTERNATIVES TO CYCLOPHOSPHAMIDE
In a Chinese study, mycophenolate was better than cyclophosphamide for induction
In a study in Hong Kong, Chan et al6 randomized 42 patients with severe lupus nephritis to receive either mycophenolate mofetil (available in the United States as CellCept; 2 g/day for 6 months, then 1 g/day for 6 months) or oral cyclophosphamide (2.5 mg/kg per day for 6 months) followed by azathioprine (1.5–2.0 mg/kg per day) for 6 months. Both groups also received prednisolone during the year.
At the end of the first year, the two groups were not significantly different in their rates of complete remission, partial remission, and relapse. The rate of infection, although not significantly different, was higher in the cyclophosphamide group (33% vs 19%). Two patients (10%) died in the cyclophosphamide group, but the difference in mortality rates was not statistically significant.
Nearly 5 years later,7 rates of chronic renal failure and relapse were still statistically the same in the two groups. Infections were fewer in the mycophenolate group (13% vs 40%, P = .013). The rate of amenorrhea was 36% in the cyclophosphamide group and only 4% in the mycophenolate group (P = .004). Four patients in the cyclophosphamide group and none in the mycophenolate group reached the composite end point of end-stage renal failure or death (P = .062).
This study appeared to offer a new option with equal efficacy and fewer side effects than standard therapy. However, its applicability to non-Chinese populations remained to be shown.
In a US study, mycophenolate or azathioprine was better than cyclophosphamide as maintenance
In a study in Miami,8 59 patients with lupus nephritis were given standard induction therapy with IV cyclophosphamide plus glucocorticoids for 6 months, then randomly assigned to one of three maintenance therapies for 1 to 3 years: IV injections of cyclophosphamide every 3 months (standard therapy), oral azathioprine, or oral mycophenolate. The population was 93% female, their average age was 33 years, and nearly half were African American, with many of the others being Hispanic. Patients tended to have severe disease, with nearly two-thirds having nephrotic syndrome.
After 6 years, there had been more deaths in the cyclophosphamide group than in the azathioprine group (P = .02) and in the mycophenolate group, although the latter difference was not statistically significant (P = .11). The combined rate of death and chronic renal failure was significantly higher with cyclophosphamide than with either of the oral agents. The cyclophosphamide group also had the highest relapse rate during the maintenance phase.
The differences in side effects were even more dramatic. Amenorrhea affected 32% of patients in the cyclophosphamide group, and only 7% and 6% in the azathioprine and mycophenolate groups, respectively. Rates of infections were 68% in the cyclophosphamide group and 28% and 21% in the azathioprine and mycophenolate groups, respectively. Patients given cyclophosphamide had 13 hospital days per patient per year, while the other groups each had only 1.
This study showed that maintenance therapy with oral azathioprine or mycophenolate was more effective and had fewer adverse effects than standard IV cyclophosphamide therapy. As a result of this study, oral agents for maintenance therapy became the new standard, but the question remained whether oral agents could safely be used for induction.
In a US study, mycophenolate was better than cyclophosphamide for induction
In a noninferiority study, Ginzler et al9 randomized 140 patients with severe lupus nephritis to receive either monthly IV cyclophosphamide or oral mycophenolate as induction therapy for 6 months. Adjunctive care with glucocorticoids was given in both groups. The study population was from 18 US academic centers and was predominantly female, and more than half were African American.
After 24 weeks, 22.5% of the mycophenolate patients were in complete remission by very strict criteria vs only 4% of those given cyclophosphamide (P = .005). The trend for partial remissions was also in favor of mycophenolate, although the difference was not statistically significant. The rate of complete and partial remissions, a prespecified end point, was significantly higher in the mycophenolate group. Although the study was trying to evaluate equivalency, it actually showed superiority for mycophenolate induction therapy.
Serum creatinine levels declined in both groups, but more in the mycophenolate group by 24 weeks. Urinary protein levels fell the same amount in both groups. At 3 years, the groups were statistically equivalent in terms of renal flares, renal failures, and deaths. However, the study groups were small, and the mycophenolate group did have a better trend for both renal failure (N = 4 vs 7) and deaths (N = 4 vs 8).
Mycophenolate also had fewer side effects, including infection, although again the numbers were too small to show statistical significance. The exception was diarrhea (N = 15 in the mycophenolate group vs 2 in the cyclophosphamide group).
A drawback of the study is that it was designed as a crossover study: a patient for whom therapy was failing after 3 months could switch to the other group, introducing potential confounding. Other problems involved the small population size and the question of whether results from patients in the United States were applicable to others worldwide.
In a worldwide study, mycophenolate was at least equivalent to cyclophosphamide for induction
The Aspreva Lupus Management Study (ALMS)10 used a similar design with 370 patients worldwide (United States, China, South America, and Europe) in one of the largest trials ever conducted in lupus nephritis. Patients were randomized to 6 months of induction therapy with either IV cyclophosphamide or oral mycophenolate but could not cross over.
At 6 months, response rates were identical between the two groups, with response defined as a combination of specific improvement in proteinuria, serum creatinine, and hematuria (50%–55%). In terms of individual renal and nonrenal variables, both groups appeared identical.
However, the side effect profiles differed between the two groups. As expected for mycophenolate, diarrhea was the most common side effect (occurring in 28% vs 12% in the cyclophosphamide group). Nausea and vomiting were more common with cyclophosphamide (45% and 37% respectively vs 14% and 13% in the mycophenolate group). Cyclophosphamide also caused hair loss in 35%, vs 10% in the mycophenolate group.
There were 14 deaths overall, which is a very low number considering the patients’ severity of illness, and it indicates the better results now achieved with therapy. The mortality rate was higher in the mycophenolate group (5% vs 3%), but the difference was not statistically significant. Six of the nine deaths with mycophenolate were from the same center in China, and none were from Europe or the United States. In summary, the study did not show that mycophenolate was superior to IV cyclophosphamide for induction therapy, but that they were equivalent in efficacy with different side effect profiles.
Membranous nephropathy: Mycophenolate vs cyclophosphamide
Less evidence is available about treatment for membranous disease, which is characterized by heavy proteinuria and the nephrotic syndrome but usually does not progress to renal failure. Radhakrishnan et al11 combined data from the trial by Ginzler et al9 and the ALMS trial10 and found 84 patients with pure membranous lupus, who were equally divided between the treatment groups receiving IV cyclophosphamide and mycophenolate. Consistent with the larger group’s data, mycophenolate and cyclophosphamide performed similarly in terms of efficacy, but there was a slightly higher rate of side effects with cyclophosphamide.
Maintenance therapy: Mycophenolate superior to azathioprine
The ALMS Maintenance Trial12 evaluated maintenance therapy in the same worldwide population that was studied for induction therapy. Of the 370 patients involved in the induction phase that compared IV cyclophosphamide and oral mycophenolate, 227 responded sufficiently to be rerandomized in a controlled, double-blinded trial of 36 months of maintenance therapy with corticosteroids and either mycophenolate (1 g twice daily) or azathioprine (2 mg/kg per day).
In intention-to-treat analysis, the time to treatment failure (ie, doubling of the serum creatinine level, progressing to renal failure, or death) was significantly shorter in the azathioprine group (P = .003). Every individual end point—end-stage renal disease, renal flares, doubling of serum creatinine, rescue immunosuppression required—was in favor of mycophenolate maintenance. At 3 years, the completion rate was 63% with mycophenolate and 49% with azathioprine. Serious adverse events and withdrawals because of adverse events were more common in the azathioprine group.
In summary, mycophenolate was superior to azathioprine in maintaining renal response and in preventing relapse in patients with active lupus nephritis who responded to induction therapy with either mycophenolate or IV cyclophosphamide. Mycophenolate was found to be superior regardless of initial induction treatment, race, or region and was confirmed by all key secondary end points.
Only one of the 227 patients died during the 3 years—from an auto accident. Again, this indicates the dramatically improved survival today compared with a decade ago.
RITUXIMAB: PROMISING BUT UNPROVEN
Rituximab (Rituxan) was originally approved to treat tumors, then rheumatoid arthritis, and most recently vasculitis. Evidence thus far is mixed regarding its use as a treatment for lupus nephritis. Although randomized clinical trials have not found it to be superior to standard regimens, there are many signs that it may be effective.
Rituximab in uncontrolled studies
Terrier et al13 analyzed prospective data from 136 patients with systemic lupus erythematosus, most of whom had renal disease, from the French Autoimmunity and Rituximab registry. Response occurred in 71% of patients using rituximab, with no difference found between patients receiving rituximab monotherapy and those concomitantly receiving immunosuppressive agents.
Melander et al14 retrospectively studied 19 women and 1 man who had been treated with rituximab for severe lupus nephritis and followed for at least 1 year. Three patients had concurrent therapy with cyclophosphamide, and 10 patients continued rituximab as maintenance therapy; 12 patients had lupus nephritis that had been refractory to standard treatment, and 6 had relapsing disease.
At a median follow-up of 22 months, 12 patients (60%) had achieved complete or partial renal remission.
Condon et al15 treated 21 patients who had severe lupus nephritis with two doses of rituximab and IV methylprednisolone 2 weeks apart, then maintenance therapy with mycophenolate without any oral steroids. At a mean follow-up of 35 months ( ± 14 months), 16 (76%) were in complete remission, with a mean time to remission of 12 months. Two (9.5%) achieved partial remission. The rate of toxicity was low.
Thus, rituximab appears promising in uncontrolled studies.
Placebo-controlled trials fail to prove rituximab effective
LUNAR trial. On the other hand, the largest placebo-controlled trial to evaluate rituximab in patients with proliferative lupus nephritis, the Lupus Nephritis Assessment With Rituximab (LUNAR) trial16 found differences in favor of rituximab, but none reached statistical significance. The trial randomized 140 patients to receive either mycophenolate plus periodic rituximab infusions or mycophenolate plus placebo infusions for 1 year. All patients received the same dosage of glucocorticoids, which was tapered over the year.
At the end of 1 year, the groups were not statistically different in terms of complete renal response and partial renal response. Rituximab appeared less likely to produce no response, but the difference was not statistically significant.
African Americans appeared to have a higher response rate to rituximab (70% in the rituximab group achieved a response vs 45% in the control group), but again, the difference did not reach statistical significance, and the total study population of African Americans was only 40.
Rituximab did have a statistically significant positive effect on two serologic markers at 1 year: levels of anti-dsDNA fell faster and complement rose faster. In addition, rates of adverse and serious adverse events were similar between the two groups, with no new or unexpected “safety signals.”
This study can be interpreted in a number of ways. The number of patients may have been too small to show significance and the follow-up may have been too short. On the other hand, it may simply not be effective to add rituximab to a full dose of mycophenolate and steroids, an already good treatment.
EXPLORER trial. Similarly, for patients with lupus without nephritis, the Exploratory Phase II/III SLE Evaluation of Rituximab (EXPLORER) trial17 also tested rituximab against a background of an effective therapeutic regimen and found no additional benefit. This study had design problems similar to those of the LUNAR trial.
Rituximab as rescue therapy
The evidence so far indicates that rituximab may have a role as rescue therapy for refractory or relapsing disease. Rituximab must be used with other therapies, but maintenance corticosteroid therapy is not necessary. Its role as a first-line agent in induction therapy for lupus nephritis remains unclear, although it may have an important role for nonwhites. In general, it has been well tolerated. Until a large randomized trial indicates otherwise, it should not be used as a first-line therapy.
The US Food and Drug Administration (FDA) sent out a warning about the danger of progressive multifocal leukoencephalopathy as an adverse effect of rituximab and of mycophenolate, but this does not appear to be a major concern for most patients and is only likely to occur in those who have been over-immunosuppressed for many years.
MULTITARGET THERAPY
The concept of using multiple drugs simultaneously—such as mycophenolate, steroids, and rituximab—is increasingly being tried. Multi-target therapy appears to offer the advantages of combining different modes of action with better results, and it offers fewer side effects because dosages of each individual drug can be lower when combined with other immunosuppressives.
Bao et al18 in China randomly assigned 40 patients with diffuse proliferative and membranous nephritis to 6 to 9 months of induction treatment with either multitarget therapy (mycophenolate, tacrolimus [Prograf], and glucocorticoids) or IV cyclophosphamide. More complete remissions occurred in the multitarget therapy group, both at 6 months (50% vs 5%) and at 9 months (65% vs 15%). Most adverse events were less frequent in the multitarget therapy group, although three patients (15%) in the multitarget therapy group developed new-onset hypertension vs none in the cyclophosphamide group.
NEW MEDICATIONS
Entirely new classes of drugs are being developed with immunomodulatory effects, including tolerance molecules, cytokine blockers, inhibitors of human B lymphocyte stimulator, and costimulatory blockers.
Belimumab offers small improvement for lupus
Belimumab (Benlysta) is a human monoclonal antibody that inhibits the biologic activity of human B lymphocyte stimulator; it has recently been approved by the FDA for lupus nephritis. In a worldwide study,19 867 patients with systemic lupus erythematosus were randomized to receive either belimumab (1 mg/kg or 10 mg/kg) or placebo.
The primary end point was the reduction of disease activity by a scoring system (SELENA-SLEDAI) that incorporated multiple features of lupus, including arthritis, vasculitis, proteinuria, rash, and others. Patients in the belimumab group had better outcomes, but the results were not dramatic. Because the drug is so expensive (about $25,000 per year) and the improvement offered is only incremental, this drug will not likely change the treatment of lupus very much.
Moreover, patients with lupus nephritis were not included in the study, but a new study is being planned to do so. Improvement is harder to demonstrate in lupus nephritis than in rheumatoid arthritis and systemic lupus erythematosus: significant changes in creatinine levels and 24-hour urinary protein must be achieved, rather than more qualitative signs and symptoms of joint pain, rash, and feeling better. Although belimumab is still unproven for lupus nephritis, it might be worth trying for patients failing other therapy.
Laquinimod: A promising experimental drug
Laquinimod is an oral immunomodulatory drug with a number of effects, including down-regulating major histocompatability complex II, chemokines, and adhesion-related molecules related to inflammation. It has been studied in more than 2,500 patients with multiple sclerosis. Pilot studies are now being done for its use for lupus nephritis. If it shows promise, a large randomized, controlled trial will be conducted.
Abatacept is in clinical trials
Abatacept (Orencia), a costimulation blocker, is undergoing clinical trials in lupus nephritis. Results should be available shortly.
INDIVIDUALIZE THERAPY
This past decade has seen such an increase in options to treat lupus nephritis that therapy can now be individualized.
Choosing IV cyclophosphamide vs mycophenolate
As a result of recent trials, doctors in the United States are increasingly using mycophenolate as the first-line drug for lupus nephritis. In Europe, however, many are choosing the shorter regimen of IV cyclophosphamide because of the results of the Euro-Lupus study.
Nowadays, I tend to use IV cyclophosphamide as the first-line drug only for patients with severe crescenteric glomerulonephritis or a very high serum creatinine level. In such cases, there is more experience with cyclophosphamide, and such severe disease does not lend itself to the luxury of trying out different therapies sequentially. If such a severely ill patient insists that a future pregnancy is very important, an alternative therapy of mycophenolate plus rituximab should be considered. I prefer mycophenolate for induction and maintenance therapy in most patients.
Dosing and formulation considerations for mycophenolate
Large dosages of mycophenolate are much better tolerated when broken up throughout the day. A patient who cannot tolerate 1 g twice daily may be able to tolerate 500 mg four times a day. The formulation can also make a difference. Some patients tolerate sustained-release mycophenolate (Myfortic) better than CellCept, and vice versa.
For patients who cannot tolerate mycophenolate, azathioprine is an acceptable alternative. In addition, for a patient who is already doing well on azathioprine, there is no need to change to mycophenolate.
Long maintenance therapy now acceptable
The ALMS Maintenance Trial12 found 3 years of maintenance therapy to be safe and effective. Such a long maintenance period is increasingly viewed as important, especially for patients in their teens and 20s, as it allows them to live a normal life, ie, to finish their education, get married, and become settled socially. Whether 5 years of maintenance therapy or even 10 years is advisable is still unknown.
Treatment during pregnancy
Neither mycophenolate nor azathioprine is recommended during pregnancy, although their effects are unknown. Because we have much more renal transplant experience with azathioprine during pregnancy, I recommend either switching from mycophenolate to azathioprine or trying to stop medication altogether if the patient has been well controlled.
Treatment for lupus nephritis has changed dramatically in recent years. Only 10 years ago, rheumatologists and nephrologists, whether specializing in adult or pediatric medicine, treated lupus nephritis with a similar regimen of monthly intravenous cyclophosphamide (Cytoxan) and glucocorticoids. Although the regimen is effective, side effects such as infection, hair loss, and infertility were extremely common.
Effective but very toxic therapy is common in autoimmune diseases. In the last decade, clinical trials have shown that less toxic drugs are as effective for treating lupus nephritis. This article will review new developments in therapy for lupus nephritis, which can be viewed as a prototype for other fields of medicine.
DEMOGRAPHICS ARE IMPORTANT
Although numerous factors have prognostic value in lupus nephritis (eg, serum creatinine, proteinuria, renal biopsy findings), the most important to consider when designing and interpreting studies are race and socioeconomic variables.
A retrospective study in Miami, FL,1 evaluated 213 patients with lupus nephritis, of whom 47% were Hispanic, 44% African American, and 20% white. At baseline, African Americans had higher blood pressure, higher serum creatinine levels, and lower household income. After 6 years, African Americans fared the worst in terms of doubling of serum creatinine, developing end-stage renal disease, and death; whites had the best outcomes, and Hispanics were in between. Low income was found to be a significant risk factor, independent of racial background.
In a similar retrospective study in New York City in 128 patients (43% white, 40% Hispanic, and 17% African American) with proliferative lupus nephritis,2 disease was much more likely to progress to renal failure over 10 years in patients living in a poor neighborhood, even after adjustment for race.
We need to keep in mind that racial and socioeconomic factors correlate with disease severity when we design and interpret studies of lupus nephritis. Study groups must be carefully balanced with patients of similar racial and socioeconomic profiles. Study findings must be interpreted with caution; for example, whether results from a study from China are applicable to an African American with lupus nephritis in New York City is unclear.
OLDER STANDARD THERAPY: EFFECTIVE BUT TOXIC
The last large National Institutes of Health study that involved only cyclophosphamide and a glucocorticoid was published in 2001,3 with 21 patients receiving cyclophosphamide alone and 20 patients receiving cyclophosphamide plus methylprednisolone. Although lupus nephritis improved, serious side effects occurred in one-third to one-half of patients in each group and included hypertension, hyperlipidemia, valvular heart disease, avascular necrosis, premature menopause, and major infections, including herpes zoster.
Less cyclophosphamide works just as well
The multicenter, prospective Euro-Lupus Nephritis Trial4 randomized 90 patients with proliferative lupus nephritis to receive either standard high-dose intravenous (IV) cyclophosphamide therapy (six monthly pulses and two quarterly pulses, with doses increasing according to the white blood cell count) or low-dose IV cyclophosphamide therapy (six pulses every 2 weeks at a fixed dose of 500 mg). Both regimens were followed by azathioprine (Imuran).
At 4 years, the two treatment groups were not significantly different in terms of treatment failure, remission rates, serum creatinine levels, 24-hour proteinuria, and freedom from renal flares. However, the rates of side effects were significantly different, with more patients in the low-dosage group free of severe infection.
One problem with this study is whether it is applicable to an American lupus nephritis population, since 84% of the patients were white. Since this study, others indicate that this regimen is probably also safe and effective for different racial groups in the United States.
At 10-year follow-up,5 both treatment groups still had identical excellent rates of freedom from end-stage renal disease. Serum creatinine and 24-hour proteinuria were also at excellent levels and identical in both groups. Nearly three quarters of patients still needed glucocorticoid therapy and more than half still needed immunosuppressive therapy, but the rates were not statistically significantly different between the treatment groups.
The cumulative dose of cyclophosphamide was 9.5 g in the standard-treatment group and 5.5 g in the low-dose group. This difference in exposure could make a tremendous difference to patients, not only for immediate side effects such as early menopause and infections, but for the risk of cancer in later decades.
This study showed clearly that low-dose cyclophosphamide is an option for induction therapy. Drawbacks of the study were that the population was mostly white and that patients had only moderately severe disease.
Low-dose cyclophosphamide has largely replaced the older National Institutes of Health regimen, although during the last decade drug therapy has undergone more changes.
MYCOPHENOLATE AND AZATHIOPRINE: ALTERNATIVES TO CYCLOPHOSPHAMIDE
In a Chinese study, mycophenolate was better than cyclophosphamide for induction
In a study in Hong Kong, Chan et al6 randomized 42 patients with severe lupus nephritis to receive either mycophenolate mofetil (available in the United States as CellCept; 2 g/day for 6 months, then 1 g/day for 6 months) or oral cyclophosphamide (2.5 mg/kg per day for 6 months) followed by azathioprine (1.5–2.0 mg/kg per day) for 6 months. Both groups also received prednisolone during the year.
At the end of the first year, the two groups were not significantly different in their rates of complete remission, partial remission, and relapse. The rate of infection, although not significantly different, was higher in the cyclophosphamide group (33% vs 19%). Two patients (10%) died in the cyclophosphamide group, but the difference in mortality rates was not statistically significant.
Nearly 5 years later,7 rates of chronic renal failure and relapse were still statistically the same in the two groups. Infections were fewer in the mycophenolate group (13% vs 40%, P = .013). The rate of amenorrhea was 36% in the cyclophosphamide group and only 4% in the mycophenolate group (P = .004). Four patients in the cyclophosphamide group and none in the mycophenolate group reached the composite end point of end-stage renal failure or death (P = .062).
This study appeared to offer a new option with equal efficacy and fewer side effects than standard therapy. However, its applicability to non-Chinese populations remained to be shown.
In a US study, mycophenolate or azathioprine was better than cyclophosphamide as maintenance
In a study in Miami,8 59 patients with lupus nephritis were given standard induction therapy with IV cyclophosphamide plus glucocorticoids for 6 months, then randomly assigned to one of three maintenance therapies for 1 to 3 years: IV injections of cyclophosphamide every 3 months (standard therapy), oral azathioprine, or oral mycophenolate. The population was 93% female, their average age was 33 years, and nearly half were African American, with many of the others being Hispanic. Patients tended to have severe disease, with nearly two-thirds having nephrotic syndrome.
After 6 years, there had been more deaths in the cyclophosphamide group than in the azathioprine group (P = .02) and in the mycophenolate group, although the latter difference was not statistically significant (P = .11). The combined rate of death and chronic renal failure was significantly higher with cyclophosphamide than with either of the oral agents. The cyclophosphamide group also had the highest relapse rate during the maintenance phase.
The differences in side effects were even more dramatic. Amenorrhea affected 32% of patients in the cyclophosphamide group, and only 7% and 6% in the azathioprine and mycophenolate groups, respectively. Rates of infections were 68% in the cyclophosphamide group and 28% and 21% in the azathioprine and mycophenolate groups, respectively. Patients given cyclophosphamide had 13 hospital days per patient per year, while the other groups each had only 1.
This study showed that maintenance therapy with oral azathioprine or mycophenolate was more effective and had fewer adverse effects than standard IV cyclophosphamide therapy. As a result of this study, oral agents for maintenance therapy became the new standard, but the question remained whether oral agents could safely be used for induction.
In a US study, mycophenolate was better than cyclophosphamide for induction
In a noninferiority study, Ginzler et al9 randomized 140 patients with severe lupus nephritis to receive either monthly IV cyclophosphamide or oral mycophenolate as induction therapy for 6 months. Adjunctive care with glucocorticoids was given in both groups. The study population was from 18 US academic centers and was predominantly female, and more than half were African American.
After 24 weeks, 22.5% of the mycophenolate patients were in complete remission by very strict criteria vs only 4% of those given cyclophosphamide (P = .005). The trend for partial remissions was also in favor of mycophenolate, although the difference was not statistically significant. The rate of complete and partial remissions, a prespecified end point, was significantly higher in the mycophenolate group. Although the study was trying to evaluate equivalency, it actually showed superiority for mycophenolate induction therapy.
Serum creatinine levels declined in both groups, but more in the mycophenolate group by 24 weeks. Urinary protein levels fell the same amount in both groups. At 3 years, the groups were statistically equivalent in terms of renal flares, renal failures, and deaths. However, the study groups were small, and the mycophenolate group did have a better trend for both renal failure (N = 4 vs 7) and deaths (N = 4 vs 8).
Mycophenolate also had fewer side effects, including infection, although again the numbers were too small to show statistical significance. The exception was diarrhea (N = 15 in the mycophenolate group vs 2 in the cyclophosphamide group).
A drawback of the study is that it was designed as a crossover study: a patient for whom therapy was failing after 3 months could switch to the other group, introducing potential confounding. Other problems involved the small population size and the question of whether results from patients in the United States were applicable to others worldwide.
In a worldwide study, mycophenolate was at least equivalent to cyclophosphamide for induction
The Aspreva Lupus Management Study (ALMS)10 used a similar design with 370 patients worldwide (United States, China, South America, and Europe) in one of the largest trials ever conducted in lupus nephritis. Patients were randomized to 6 months of induction therapy with either IV cyclophosphamide or oral mycophenolate but could not cross over.
At 6 months, response rates were identical between the two groups, with response defined as a combination of specific improvement in proteinuria, serum creatinine, and hematuria (50%–55%). In terms of individual renal and nonrenal variables, both groups appeared identical.
However, the side effect profiles differed between the two groups. As expected for mycophenolate, diarrhea was the most common side effect (occurring in 28% vs 12% in the cyclophosphamide group). Nausea and vomiting were more common with cyclophosphamide (45% and 37% respectively vs 14% and 13% in the mycophenolate group). Cyclophosphamide also caused hair loss in 35%, vs 10% in the mycophenolate group.
There were 14 deaths overall, which is a very low number considering the patients’ severity of illness, and it indicates the better results now achieved with therapy. The mortality rate was higher in the mycophenolate group (5% vs 3%), but the difference was not statistically significant. Six of the nine deaths with mycophenolate were from the same center in China, and none were from Europe or the United States. In summary, the study did not show that mycophenolate was superior to IV cyclophosphamide for induction therapy, but that they were equivalent in efficacy with different side effect profiles.
Membranous nephropathy: Mycophenolate vs cyclophosphamide
Less evidence is available about treatment for membranous disease, which is characterized by heavy proteinuria and the nephrotic syndrome but usually does not progress to renal failure. Radhakrishnan et al11 combined data from the trial by Ginzler et al9 and the ALMS trial10 and found 84 patients with pure membranous lupus, who were equally divided between the treatment groups receiving IV cyclophosphamide and mycophenolate. Consistent with the larger group’s data, mycophenolate and cyclophosphamide performed similarly in terms of efficacy, but there was a slightly higher rate of side effects with cyclophosphamide.
Maintenance therapy: Mycophenolate superior to azathioprine
The ALMS Maintenance Trial12 evaluated maintenance therapy in the same worldwide population that was studied for induction therapy. Of the 370 patients involved in the induction phase that compared IV cyclophosphamide and oral mycophenolate, 227 responded sufficiently to be rerandomized in a controlled, double-blinded trial of 36 months of maintenance therapy with corticosteroids and either mycophenolate (1 g twice daily) or azathioprine (2 mg/kg per day).
In intention-to-treat analysis, the time to treatment failure (ie, doubling of the serum creatinine level, progressing to renal failure, or death) was significantly shorter in the azathioprine group (P = .003). Every individual end point—end-stage renal disease, renal flares, doubling of serum creatinine, rescue immunosuppression required—was in favor of mycophenolate maintenance. At 3 years, the completion rate was 63% with mycophenolate and 49% with azathioprine. Serious adverse events and withdrawals because of adverse events were more common in the azathioprine group.
In summary, mycophenolate was superior to azathioprine in maintaining renal response and in preventing relapse in patients with active lupus nephritis who responded to induction therapy with either mycophenolate or IV cyclophosphamide. Mycophenolate was found to be superior regardless of initial induction treatment, race, or region and was confirmed by all key secondary end points.
Only one of the 227 patients died during the 3 years—from an auto accident. Again, this indicates the dramatically improved survival today compared with a decade ago.
RITUXIMAB: PROMISING BUT UNPROVEN
Rituximab (Rituxan) was originally approved to treat tumors, then rheumatoid arthritis, and most recently vasculitis. Evidence thus far is mixed regarding its use as a treatment for lupus nephritis. Although randomized clinical trials have not found it to be superior to standard regimens, there are many signs that it may be effective.
Rituximab in uncontrolled studies
Terrier et al13 analyzed prospective data from 136 patients with systemic lupus erythematosus, most of whom had renal disease, from the French Autoimmunity and Rituximab registry. Response occurred in 71% of patients using rituximab, with no difference found between patients receiving rituximab monotherapy and those concomitantly receiving immunosuppressive agents.
Melander et al14 retrospectively studied 19 women and 1 man who had been treated with rituximab for severe lupus nephritis and followed for at least 1 year. Three patients had concurrent therapy with cyclophosphamide, and 10 patients continued rituximab as maintenance therapy; 12 patients had lupus nephritis that had been refractory to standard treatment, and 6 had relapsing disease.
At a median follow-up of 22 months, 12 patients (60%) had achieved complete or partial renal remission.
Condon et al15 treated 21 patients who had severe lupus nephritis with two doses of rituximab and IV methylprednisolone 2 weeks apart, then maintenance therapy with mycophenolate without any oral steroids. At a mean follow-up of 35 months ( ± 14 months), 16 (76%) were in complete remission, with a mean time to remission of 12 months. Two (9.5%) achieved partial remission. The rate of toxicity was low.
Thus, rituximab appears promising in uncontrolled studies.
Placebo-controlled trials fail to prove rituximab effective
LUNAR trial. On the other hand, the largest placebo-controlled trial to evaluate rituximab in patients with proliferative lupus nephritis, the Lupus Nephritis Assessment With Rituximab (LUNAR) trial16 found differences in favor of rituximab, but none reached statistical significance. The trial randomized 140 patients to receive either mycophenolate plus periodic rituximab infusions or mycophenolate plus placebo infusions for 1 year. All patients received the same dosage of glucocorticoids, which was tapered over the year.
At the end of 1 year, the groups were not statistically different in terms of complete renal response and partial renal response. Rituximab appeared less likely to produce no response, but the difference was not statistically significant.
African Americans appeared to have a higher response rate to rituximab (70% in the rituximab group achieved a response vs 45% in the control group), but again, the difference did not reach statistical significance, and the total study population of African Americans was only 40.
Rituximab did have a statistically significant positive effect on two serologic markers at 1 year: levels of anti-dsDNA fell faster and complement rose faster. In addition, rates of adverse and serious adverse events were similar between the two groups, with no new or unexpected “safety signals.”
This study can be interpreted in a number of ways. The number of patients may have been too small to show significance and the follow-up may have been too short. On the other hand, it may simply not be effective to add rituximab to a full dose of mycophenolate and steroids, an already good treatment.
EXPLORER trial. Similarly, for patients with lupus without nephritis, the Exploratory Phase II/III SLE Evaluation of Rituximab (EXPLORER) trial17 also tested rituximab against a background of an effective therapeutic regimen and found no additional benefit. This study had design problems similar to those of the LUNAR trial.
Rituximab as rescue therapy
The evidence so far indicates that rituximab may have a role as rescue therapy for refractory or relapsing disease. Rituximab must be used with other therapies, but maintenance corticosteroid therapy is not necessary. Its role as a first-line agent in induction therapy for lupus nephritis remains unclear, although it may have an important role for nonwhites. In general, it has been well tolerated. Until a large randomized trial indicates otherwise, it should not be used as a first-line therapy.
The US Food and Drug Administration (FDA) sent out a warning about the danger of progressive multifocal leukoencephalopathy as an adverse effect of rituximab and of mycophenolate, but this does not appear to be a major concern for most patients and is only likely to occur in those who have been over-immunosuppressed for many years.
MULTITARGET THERAPY
The concept of using multiple drugs simultaneously—such as mycophenolate, steroids, and rituximab—is increasingly being tried. Multi-target therapy appears to offer the advantages of combining different modes of action with better results, and it offers fewer side effects because dosages of each individual drug can be lower when combined with other immunosuppressives.
Bao et al18 in China randomly assigned 40 patients with diffuse proliferative and membranous nephritis to 6 to 9 months of induction treatment with either multitarget therapy (mycophenolate, tacrolimus [Prograf], and glucocorticoids) or IV cyclophosphamide. More complete remissions occurred in the multitarget therapy group, both at 6 months (50% vs 5%) and at 9 months (65% vs 15%). Most adverse events were less frequent in the multitarget therapy group, although three patients (15%) in the multitarget therapy group developed new-onset hypertension vs none in the cyclophosphamide group.
NEW MEDICATIONS
Entirely new classes of drugs are being developed with immunomodulatory effects, including tolerance molecules, cytokine blockers, inhibitors of human B lymphocyte stimulator, and costimulatory blockers.
Belimumab offers small improvement for lupus
Belimumab (Benlysta) is a human monoclonal antibody that inhibits the biologic activity of human B lymphocyte stimulator; it has recently been approved by the FDA for lupus nephritis. In a worldwide study,19 867 patients with systemic lupus erythematosus were randomized to receive either belimumab (1 mg/kg or 10 mg/kg) or placebo.
The primary end point was the reduction of disease activity by a scoring system (SELENA-SLEDAI) that incorporated multiple features of lupus, including arthritis, vasculitis, proteinuria, rash, and others. Patients in the belimumab group had better outcomes, but the results were not dramatic. Because the drug is so expensive (about $25,000 per year) and the improvement offered is only incremental, this drug will not likely change the treatment of lupus very much.
Moreover, patients with lupus nephritis were not included in the study, but a new study is being planned to do so. Improvement is harder to demonstrate in lupus nephritis than in rheumatoid arthritis and systemic lupus erythematosus: significant changes in creatinine levels and 24-hour urinary protein must be achieved, rather than more qualitative signs and symptoms of joint pain, rash, and feeling better. Although belimumab is still unproven for lupus nephritis, it might be worth trying for patients failing other therapy.
Laquinimod: A promising experimental drug
Laquinimod is an oral immunomodulatory drug with a number of effects, including down-regulating major histocompatability complex II, chemokines, and adhesion-related molecules related to inflammation. It has been studied in more than 2,500 patients with multiple sclerosis. Pilot studies are now being done for its use for lupus nephritis. If it shows promise, a large randomized, controlled trial will be conducted.
Abatacept is in clinical trials
Abatacept (Orencia), a costimulation blocker, is undergoing clinical trials in lupus nephritis. Results should be available shortly.
INDIVIDUALIZE THERAPY
This past decade has seen such an increase in options to treat lupus nephritis that therapy can now be individualized.
Choosing IV cyclophosphamide vs mycophenolate
As a result of recent trials, doctors in the United States are increasingly using mycophenolate as the first-line drug for lupus nephritis. In Europe, however, many are choosing the shorter regimen of IV cyclophosphamide because of the results of the Euro-Lupus study.
Nowadays, I tend to use IV cyclophosphamide as the first-line drug only for patients with severe crescenteric glomerulonephritis or a very high serum creatinine level. In such cases, there is more experience with cyclophosphamide, and such severe disease does not lend itself to the luxury of trying out different therapies sequentially. If such a severely ill patient insists that a future pregnancy is very important, an alternative therapy of mycophenolate plus rituximab should be considered. I prefer mycophenolate for induction and maintenance therapy in most patients.
Dosing and formulation considerations for mycophenolate
Large dosages of mycophenolate are much better tolerated when broken up throughout the day. A patient who cannot tolerate 1 g twice daily may be able to tolerate 500 mg four times a day. The formulation can also make a difference. Some patients tolerate sustained-release mycophenolate (Myfortic) better than CellCept, and vice versa.
For patients who cannot tolerate mycophenolate, azathioprine is an acceptable alternative. In addition, for a patient who is already doing well on azathioprine, there is no need to change to mycophenolate.
Long maintenance therapy now acceptable
The ALMS Maintenance Trial12 found 3 years of maintenance therapy to be safe and effective. Such a long maintenance period is increasingly viewed as important, especially for patients in their teens and 20s, as it allows them to live a normal life, ie, to finish their education, get married, and become settled socially. Whether 5 years of maintenance therapy or even 10 years is advisable is still unknown.
Treatment during pregnancy
Neither mycophenolate nor azathioprine is recommended during pregnancy, although their effects are unknown. Because we have much more renal transplant experience with azathioprine during pregnancy, I recommend either switching from mycophenolate to azathioprine or trying to stop medication altogether if the patient has been well controlled.
- Contreras G, Lenz O, Pardo V, et al. Outcomes in African Americans and Hispanics with lupus nephritis. Kidney Int 2006; 69:1846–1851.
- Barr RG, Seliger S, Appel GB, et al. Prognosis in proliferative lupus nephritis: the role of socio-economic status and race/ethnicity. Nephrol Dial Transplant 2003; 18:2039–2046.
- Illei GG, Austin HA, Crane M, et al. Combination therapy with pulse cyclophosphamide plus pulse methylprednisolone improves long-term renal outcome without adding toxicity in patients with lupus nephritis. Ann Intern Med 2001; 135:248–257.
- Houssiau FA, Vasconcelos C, D’Cruz D, et al. Immunosuppressive therapy in lupus nephritis: the Euro-Lupus Nephritis Trial, a randomized trial of low-dose versus high-dose intravenous cyclophosphamide. Arthritis Rheum 2002; 46:2121–2131.
- Houssiau FA, Vasconcelos C, D’Cruz D, et al. The 10-year follow-up data of the Euro-Lupus Nephritis Trial comparing low-dose and high-dose intravenous cyclophosphamide. Ann Rheum Dis 2010; 69:61–64.
- Chan TM, Li FK, Tang CS, et al. Efficacy of mycophenolate mofetil in patients with diffuse proliferative lupus nephritis. Hong King-Guangzhou Nephrology Study Group. N Engl J Med 2000; 343:1156–1162.
- Chan TM, Tse KC, Tang CS, Mok MY, Li FK; Hong Kong Nephrology Study Group. Long-term study of mycophenolate mofetil as continuous induction and maintenance treatment for diffuse proliferative lupus nephritis. J Am Soc Nephrol 2005; 16:1076–1084.
- Contreras G, Pardo V, Leclercq B, et al. Sequential therapies for proliferative lupus nephritis. N Engl J Med 2004; 350:971–980.
- Ginzler EM, Dooley MA, Aranow C, et al. Mycophenolate mofetil or intravenous cyclophosphamide for lupus nephritis. N Engl J Med 2005; 353:2219–2228.
- Appel GB, Contreras G, Dooley MA, et al. Mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. J Am Soc Nephrol 2009; 20:1103–1112.
- Radhakrishnan J, Moutzouris DA, Ginzler EM, Solomons N, Siempos II, Appel GB. Mycophenolate mofetil and intravenous cyclophosphamide are similar as induction therapy for class V lupus nephritis. Kidney Int 2010; 77:152–160.
- Dooley MA, Jayne D, Ginzler EM, et al; for the ALMS Group. Mycophenolate versus azathioprine as maintenance therapy for lupus nephritis. N Engl J Med 2011; 365:1886–1895.
- Terrier B, Amoura Z, Ravaud P, et al; Club Rhumatismes et Inflammation. Safety and efficacy of rituximab in systemic lupus erythematosus: results from 136 patients from the French AutoImmunity and Rituximab registry. Arthritis Rheum 2010; 62:2458–2466.
- Melander C, Sallée M, Troillet P, et al. Rituximab in severe lupus nephritis: early B-cell depletion affects long-term renal outcome. Clin J Am Soc Nephrol 2009; 4:579–587.
- Condon MB, Griffith M, Cook HT, Levy J, Lightstone L, Cairns T. Treatment of class IV lupus nephritis with rituximab & mycophenolate mofetil (MMF) with no oral steroids is effective and safe (abstract). J Am Soc Nephrol 2010; 21(suppl):625A–626A.
- Furie RA, Looney RJ, Rovin E, et al. Efficacy and safety of rituximab in subjects with active proliferative lupus nephritis (LN): results from the randomized, double-blind phase III LUNAR study (abstract). Arthritis Rheum 2009; 60(suppl 1):S429.
- Merrill JT, Neuwelt CM, Wallace DJ, et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum 2010; 62:222–233.
- Bao H, Liu ZH, Zie HL, Hu WX, Zhang HT, Li LS. Successful treatment of class V+IV lupus nephritis with multitarget therapy. J Am Soc Nephrol 2008; 19:2001–2010.
- Navarra SV, Guzmán RM, Gallacher AE, et al; BLISS-52 Study Group. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet 2011; 377:721–731.
- Contreras G, Lenz O, Pardo V, et al. Outcomes in African Americans and Hispanics with lupus nephritis. Kidney Int 2006; 69:1846–1851.
- Barr RG, Seliger S, Appel GB, et al. Prognosis in proliferative lupus nephritis: the role of socio-economic status and race/ethnicity. Nephrol Dial Transplant 2003; 18:2039–2046.
- Illei GG, Austin HA, Crane M, et al. Combination therapy with pulse cyclophosphamide plus pulse methylprednisolone improves long-term renal outcome without adding toxicity in patients with lupus nephritis. Ann Intern Med 2001; 135:248–257.
- Houssiau FA, Vasconcelos C, D’Cruz D, et al. Immunosuppressive therapy in lupus nephritis: the Euro-Lupus Nephritis Trial, a randomized trial of low-dose versus high-dose intravenous cyclophosphamide. Arthritis Rheum 2002; 46:2121–2131.
- Houssiau FA, Vasconcelos C, D’Cruz D, et al. The 10-year follow-up data of the Euro-Lupus Nephritis Trial comparing low-dose and high-dose intravenous cyclophosphamide. Ann Rheum Dis 2010; 69:61–64.
- Chan TM, Li FK, Tang CS, et al. Efficacy of mycophenolate mofetil in patients with diffuse proliferative lupus nephritis. Hong King-Guangzhou Nephrology Study Group. N Engl J Med 2000; 343:1156–1162.
- Chan TM, Tse KC, Tang CS, Mok MY, Li FK; Hong Kong Nephrology Study Group. Long-term study of mycophenolate mofetil as continuous induction and maintenance treatment for diffuse proliferative lupus nephritis. J Am Soc Nephrol 2005; 16:1076–1084.
- Contreras G, Pardo V, Leclercq B, et al. Sequential therapies for proliferative lupus nephritis. N Engl J Med 2004; 350:971–980.
- Ginzler EM, Dooley MA, Aranow C, et al. Mycophenolate mofetil or intravenous cyclophosphamide for lupus nephritis. N Engl J Med 2005; 353:2219–2228.
- Appel GB, Contreras G, Dooley MA, et al. Mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. J Am Soc Nephrol 2009; 20:1103–1112.
- Radhakrishnan J, Moutzouris DA, Ginzler EM, Solomons N, Siempos II, Appel GB. Mycophenolate mofetil and intravenous cyclophosphamide are similar as induction therapy for class V lupus nephritis. Kidney Int 2010; 77:152–160.
- Dooley MA, Jayne D, Ginzler EM, et al; for the ALMS Group. Mycophenolate versus azathioprine as maintenance therapy for lupus nephritis. N Engl J Med 2011; 365:1886–1895.
- Terrier B, Amoura Z, Ravaud P, et al; Club Rhumatismes et Inflammation. Safety and efficacy of rituximab in systemic lupus erythematosus: results from 136 patients from the French AutoImmunity and Rituximab registry. Arthritis Rheum 2010; 62:2458–2466.
- Melander C, Sallée M, Troillet P, et al. Rituximab in severe lupus nephritis: early B-cell depletion affects long-term renal outcome. Clin J Am Soc Nephrol 2009; 4:579–587.
- Condon MB, Griffith M, Cook HT, Levy J, Lightstone L, Cairns T. Treatment of class IV lupus nephritis with rituximab & mycophenolate mofetil (MMF) with no oral steroids is effective and safe (abstract). J Am Soc Nephrol 2010; 21(suppl):625A–626A.
- Furie RA, Looney RJ, Rovin E, et al. Efficacy and safety of rituximab in subjects with active proliferative lupus nephritis (LN): results from the randomized, double-blind phase III LUNAR study (abstract). Arthritis Rheum 2009; 60(suppl 1):S429.
- Merrill JT, Neuwelt CM, Wallace DJ, et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum 2010; 62:222–233.
- Bao H, Liu ZH, Zie HL, Hu WX, Zhang HT, Li LS. Successful treatment of class V+IV lupus nephritis with multitarget therapy. J Am Soc Nephrol 2008; 19:2001–2010.
- Navarra SV, Guzmán RM, Gallacher AE, et al; BLISS-52 Study Group. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet 2011; 377:721–731.
KEY POINTS
- Mycophenolate is at least equivalent to intravenous cyclophosphamide for induction and maintenance treatment of severe lupus nephritis.
- The role of rituximab is unclear, and for now it should only be used in relapsing patients or patients whose disease is resistant to standard therapy.
- Using combination therapies for induction treatment and maintenance is becoming increasingly common.
- Three-year maintenance therapy is now considered advisable in most patients.
- Entirely new drugs under study include costimulatory blockers, inhibitors of human B lymphocyte stimulator, tolerance molecules, and cytokine blockers.
Finding the cause of acute kidney injury: Which index of fractional excretion is better?
An acute kidney injury can result from a myriad of causes and pathogenic pathways. Of these, the two main categories are prerenal causes (eg, heart failure, volume depletion) and causes that are intrinsic to the kidney (eg, acute tubular necrosis). Together, these categories account for more than 70% of all cases.1–3
While early intervention improves outcomes in both of these categories, the physician in the acute care setting must quickly distinguish between them, as their treatments differ. Similar clinical presentations along with confounding laboratory values make this distinction difficult. Furthermore, prolonged prerenal azotemia can eventually lead to acute tubular necrosis.
Therefore, several methods for distinguishing prerenal from intrinsic causes of acute kidney injury have been developed, including urinalysis, response to fluid challenge, the blood urea nitrogen-to-plasma creatinine ratio, levels of various urine electrolytes and biomarkers, and, the topics of our discussion here, the fractional excretion of sodium (FENa) and the fractional excretion of urea (FEU).4 While each method offers a unique picture of renal function, the validity of each may be affected by specific clinical factors.
In light of the frequent use of diuretics in inpatients and outpatients, a review of the utility of the FEU test is warranted. We will therefore present the theory behind the use of the FENa and the FEU for distinguishing intrinsic from prerenal causes of acute kidney injury, the relevant literature comparing the utility of these investigations, and our suggestions for clinical practice.
ACUTE KIDNEY INJURY DEFINED
Acute kidney injury (formerly called acute renal failure) describes an abrupt decline in renal function. Consensus definitions of it have been published and are gaining more widespread acceptance and use.9,10 The current definition is10:
- An absolute increase in serum creatinine ≥ 0.3 mg/dL (26.4 μmol/L) in 48 hours, or
- A percentage increase in serum creatinine ≥ 50% in 48 hours, or
- Urine output < 0.5 mL/kg/hour for > 6 hours.
These clear criteria allow for earlier recognition and treatment of this condition.
Acute kidney injury is fairly common in hospitalized patients, with 172 to 620 cases per million patients per year.11–14 Furthermore, hospitalized patients with acute kidney injury continue to have high rates of morbidity and death, especially those with more severe cases, in which the mortality rate remains as high as 40%.15
FRACTIONAL EXCRETION OF SODIUM
The FENa is a measure of the extraction of sodium and water from the glomerular filtrate. It is the ratio of the rate of sodium filtration (the urinary sodium concentration times the urinary flow rate, divided by the plasma sodium concentration) to the overall glomerular filtration rate, estimated by the renal filtration of creatinine. It can be calculated as the ratio of plasma creatinine to urine creatinine divided by the ratio of plasma sodium to urine sodium:
A euvolemic person with normal renal function and moderate salt intake in a steady state will have an FENa of approximately 1%.16
In 1976, Espinel17 originally showed that the FENa could be used during the oliguric phase in patients in acute renal failure to differentiate between prerenal acute kidney injury and acute tubular necrosis. Given the kidney’s ability to reabsorb more sodium during times of volume depletion, Espinel suggested that an FENa of less than 1% reflected normal sodium retention, indicating a prerenal cause, ie, diminished effective circulating volume. A value greater than 3% likely represented tubular damage, indicating that the nephrons were unable to properly reabsorb sodium.
The clinical utility of this index was apparent, as the management of prerenal azotemia and acute tubular necrosis differ.18 While both require fluid repletion, the risk of volume overload in acute tubular necrosis is high. Furthermore, acute tubular necrosis secondary to nephrotoxins could require hemodialysis to facilitate clearance of the offending agent.
The FENa test was subsequently validated in a number of studies in different populations and is still widely used.19–21
Limitations to the use of the FENa have been noted in various clinical settings. Notably, it can be falsely depressed in a number of intrinsic renal conditions, such as contrast-induced nephropathy, rhabdomyolysis, and acute glomerulonephritis. Conversely, patients with prerenal acute kidney injury who take diuretics can have a falsely elevated value due to the pharmacologically induced renal excretion of sodium independent of volume status. This is commonly seen in patients on diuretic therapy with baseline low effective circulating volumes, such those with congestive heart failure and hepatic cirrhosis.
FRACTIONAL EXCRETION OF UREA
Urea is continuously produced in the liver as the end product of protein metabolism. It is a small, water-soluble molecule that freely passes across cell membranes and is therefore continuously filtered and excreted by the kidneys. Not merely a waste product, urea is also important in water balance and constitutes approximately half of the normal solute content of urine.22
Urea’s excretion mechanisms are well characterized.22,23 It is absorbed in the proximal tubule, the medullary loop of Henle, and the medullary collecting ducts via facilitated diffusion through specific urea transporters.24 After being absorbed in the loop of Henle, urea is resecreted, a process that creates an osmotic gradient along the medulla that ultimately regulates urea excretion and reabsorption in the medullary collecting duct. Low-volume states are associated with decreased urea excretion due to a physiologic increase in antidiuretic hormone secretion, and the reverse is true for high-volume states.
The FEU has been recognized as a clinically useful tool. The correlation between serum and urine urea concentrations was investigated as early as 1904.25 However, most studies during the ensuing century focused on the serum urea concentration or the creatinine-to-urea ratio as a measure of glomerular failure.26–28 In 1992, Kaplan and Kohn29 proposed that the FEU could be a useful measure for assessing renal dysfunction in acute kidney injury. Conceptually similar to the FENa, the FEU is calculated as:
An FEU less than 35% suggests a prerenal cause of acute kidney injury, while a value greater than 50% suggests an intrinsic one.
FRACTIONAL EXCRETION OF UREA VS FRACTIONAL EXCRETION OF SODIUM
Kaplan and Kohn (1992)
Kaplan and Kohn,29 in their 1992 study, retrospectively analyzed 87 urine samples from 40 patients with renal dysfunction (not specifically acute kidney injury) thought to be secondary to volume depletion in which the FENa was discordant with the FEU.
Findings. Thirty-nine of the 40 patients treated with diuretics had a high FENa value. However, the FEU was low in all of these patients, leading the authors to conclude that the latter may be the more useful of the two indices in evaluating patients receiving diuretics who present with symptoms that suggest prerenal azotemia.
Limitations of the study. On closer inspection, these findings were not generalizable, for several reasons. First, the time that elapsed between administration of diuretics and evaluation of urinary electrolytes varied widely. Additionally, the study was a retrospective analysis of isolated urine specimens without clear correlation to a clinical patient or context. For these reasons, prospective analyses to investigate the utility of the fractional excretion of urea needed to be conducted.
Carvounis et al (2002)
Carvounis et al30 prospectively evaluated the FENa and the FEU in 102 consecutive intensive care patients with acute kidney injury (defined as a serum creatinine concentration > 1.5 mg/dL or an increase of more than 0.5 mg/dL in less than 48 hours). Oliguria was not an inclusion criterion for the study, but patients with acute glomerulonephritis and obstructive nephropathy were excluded. The study grouped subjects into those with prerenal azotemia, prerenal azotemia plus diuretic use, or acute tubular necrosis on the basis of the clinical diagnosis of the attending nephrologist.
Findings. The FEU was more sensitive than the FENa in detecting prerenal azotemia, especially in those with prerenal azotemia who were receiving diuretics. Overall, the FEU had higher sensitivity and specificity for prerenal azotemia regardless of diuretic usage, and more importantly, the best overall positive and negative predictive value for detecting it (99% and 75% respectively).
These results indicate that, in patients given diuretics, the FENa fails to discriminate between prerenal azotemia and acute tubular necrosis. Conversely, the FEU was excellent in discriminating between all cases of prerenal azotemia and acute tubular necrosis irrespective of the use of diuretics. This has significant practical application, given the frequency of diuretic use in the hospital, particularly in intensive care patients.
Limitations of the study. While the findings supported the utility of the FEU, the study population was limited to intensive care patients. Furthermore, the authors did not report the statistical significance of their findings.30
Pépin et al (2007)
Pépin et al8 performed a similar study, investigating the diagnostic utility of the FENa and the FEU in patients with acute kidney injury, with or without diuretic therapy.
The authors prospectively studied 99 consecutive patients confirmed by an independent nephrologist to have acute kidney injury (defined as an increase in serum creatinine of more than 30% over baseline values within less than 1 week) due to either volume depletion or ischemia. They excluded patients with less common causes of acute kidney injury, such as rhabdomyolysis, obstructive nephropathy, adrenal insufficiency, acute glomerulonephritis, and nephrotoxic acute kidney injury, as well as patients with chronic kidney disease.
Patients were grouped into those with transient acute kidney injury (from decreased kidney perfusion) and persistent acute kidney injury (attributed to acute tubular necrosis), with or without diuretic therapy, according to predefined clinical criteria. They were considered to have diuretic exposure if they had received furosemide (Lasix) within 24 hours or a thiazide within 48 hours of sampling.
Findings. The FENa proved superior to the FEU in patients not taking diuretics and, contrary to the findings of Carvounis et al,30 exhibited diagnostic utility in patients taking diuretics as well. Neither index discriminated between the different etiologies exceptionally well, however.
Of note, the study population was more inclusive than in previous studies, with only 63 intensive care patients, thus making the results more generalizable to all cases of inpatient acute kidney injury. Furthermore, the study included patients with and without oliguria, and the sensitivity and specificity of both the FENa and the FEU were higher in the nonoliguric group (n = 25).
Limitations of the study. The authors admit that a long time may have elapsed between diuretic administration and urine measurements, thereby mitigating the diuretic’s natriuretic effect independent of the patient’s volume status. While this variable may account for the better performance of the FENa than in the other studies, it does not account for the poor performance of the FEU.
Additionally, few of the findings reached statistical significance.
Lastly, a high percentage (30%) of patients had sepsis. The FEU is less effective in patients with infection, as cytokines interfere with the urea transporters in the kidney and colon.31
Lim et al (2009)
Lim et al32 conducted a study similar in design to that of Pépin et al.8
Findings. The FEU was as clinically useful as the FENa at distinguishing transient from persistent acute kidney injury in patients on diuretics. Using a cutoff FEU of less than 30% and a cutoff FENa of less than 1.5% for transient acute kidney injury (based on calculated receiver operating characteristic curves), FENa was more sensitive and specific than FEU in the nondiuretic groups. In patients exposed to diuretics, FEU was more sensitive but less specific than FENa.
FRACTIONAL EXCRETION OF UREA IN OLIGURIA
Diskin et al (2010)
In 2010, Diskin et al33 published a prospective, observational study of 100 consecutive patients with oliguric azotemia referred to a nephrology service. They defined acute kidney injury as serum creatinine concentration greater than 1.9 mg/dL and urine output less than 100 mL in 24 hours. They used a higher FEU cutoff for prerenal azotemia of less than 40% to reflect the known urea secretion rate in oliguric patients (600 mL/24 hours). They used an FENa of less than 1% and greater than 3% to distinguish prerenal azotemia from acute tubular necrosis.
Findings. The FEU was more accurate than the FENa, giving the right diagnosis in 95% vs 54% of cases (P < .0001). The difference was exclusively due to the FEU’s greater utility in the 67 patients who had received diuretics (98% vs 49%, P < .0001). Both the FEU and the FENa accurately detected acute tubular necrosis. As expected, the FENa outperformed FEU in the setting of infection, in which cytokine stimulation interferes with urea excretion.
Limitations of the study. Approximately 80% of the patients had prerenal azotemia, potentially biasing the results toward a test geared toward detecting this condition. However, since prerenal causes are more common than intrinsic causes, the authors argued that their cohort more accurately reflected the population encountered in clinical practice.
Additionally, only patients with oliguria and more advanced kidney injury (serum creatinine > 1.9 mg/dL) were included in the study, potentially limiting the applicability of these results in patients with preserved urine output in the early stages of renal failure.
Table 2 summarizes the findings of the studies discussed above.8,15,30,32,33
FRACTIONAL EXCRETION OF UREA IN CHILDREN AND THE ELDERLY
The FEU has also been validated in populations at the extremes of age.
In children, Fahimi et al34 performed a cross-sectional study in 43 patients referred to a nephrology service because of acute kidney injury.
An FEU less than 35% had greater sensitivity and specificity than an FENa less than 1% for differentiating prerenal from intrinsic causes in pediatric populations. An FEU of less than 30% had an even greater power of distinguishing between the two. Interestingly, 15 of the 26 patients in the group with prerenal azotemia had an FENa greater than 1%, 8 of whom had an obvious cause (diuretic therapy in 5, salt-losing congenital adrenal hyperplasia in 2, and metabolic alkalosis in 1).
In elderly people, urinary indices are less reliable because of reduced sodium and urea reabsorption and urinary concentrating capability. Thus, the FENa and FEU are increased, making the standard cutoff values unreliable and unpredictable for distinguishing prerenal from intrinsic causes of acute kidney injury.35
WHICH TEST SHOULD BE USED?
Both the FENa and the FEU have been validated in prospective trials as useful clinical indices in identifying prerenal azotemia. Results of these studies vary as to which index is superior and when. This may be attributable to the various definitions of acute kidney injury and diagnostic criteria used in the studies as well as the heterogeneity of patients in each study.
However, the preponderance of evidence indicates that the FEU is more useful than the FENa in patients on diuretics. Since diuretics are widely used, particularly in acute care settings in which acute kidney injury is prevalent, the FEU is a useful clinical tool and should be utilized in this context accordingly. Specifically, when there is a history of recent diuretic use, the evidence supports ordering the FEU alone, or at least in conjunction with the FENa. If the two indices yield disparate results, the physician should look for circumstances that would alter each one of them, such as sepsis or an unrecognized dose of diuretic.
In managing acute kidney injury, distinguishing prerenal from intrinsic causes is a difficult task, particularly because prolonged prerenal azotemia can develop into acute tubular necrosis. Therefore, a single index, calculated at a specific time, often is insufficient to properly characterize the pathogenesis of acute kidney injury, and a combination of both of these indices may increase diagnostic sensitivity and specificity.36 Moreover, urine samples collected after acute changes in volume or osmolarity, such as blood loss, administration of intravenous fluids or parenteral nutrition, or dialysis may compromise their diagnostic utility, and care must be taken to interpret the results in the appropriate clinical context.
The clinician must be aware of both the respective applications and limitations of these indices when using them to guide management and navigate the differential diagnosis in the appropriate clinical settings.
- Nolan CR, Anderson RJ. Hospital-acquired acute renal failure. J Am Soc Nephrol 1998; 9:710–718.
- Mehta RL, Pascual MT, Soroko S, et al; Program to Improve Care in Acute Renal Disease. Spectrum of acute renal failure in the intensive care unit: the PICARD experience. Kidney Int 2004; 66:1613–1621.
- Myers BD, Miller DC, Mehigan JT, et al. Nature of the renal injury following total renal ischemia in man. J Clin Invest 1984; 73:329–341.
- Ho E, Fard A, Maisel A. Evolving use of biomarkers for kidney injury in acute care settings. Curr Opin Crit Care 2010; 16:399–407.
- Steiner RW. Low fractional excretion of sodium in myoglobinuric acute renal failure. Arch Intern Med 1982; 142:1216–1217.
- Vaz AJ. Low fractional excretion of urine sodium in acute renal failure due to sepsis. Arch Intern Med 1983; 143:738–739.
- Pru C, Kjellstrand CM. The FENa test is of no prognostic value in acute renal failure. Nephron 1984; 36:20–23.
- Pépin MN, Bouchard J, Legault L, Ethier J. Diagnostic performance of fractional excretion of urea and fractional excretion of sodium in the evaluations of patients with acute kidney injury with or without diuretic treatment. Am J Kidney Dis 2007; 50:566–573.
- Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative workgroup. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004; 8:R204–R212.
- Mehta RL, Kellum JA, Shah SV, et al; Acute Kidney Injury Network. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007; 11:R31.
- Stevens PE, Tamimi NA, Al-Hasani MK, et al. Non-specialist management of acute renal failure. QJM 2001; 94:533–540.
- Feest TG, Round A, Hamad S. Incidence of severe acute renal failure in adults: results of a community based study. BMJ 1993; 306:481–483.
- Liaño F, Pascual J. Epidemiology of acute renal failure: a prospective, multicenter, community-based study. Madrid Acute Renal Failure Study Group. Kidney Int 1996; 50:811–818.
- Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N Engl J Med 1996; 334:1448–1460.
- Bagshaw SM, George C, Bellomo R; ANZICS Database Management Committee. Changes in the incidence and outcome for early acute kidney injury in a cohort of Australian intensive care units. Crit Care 2007; 11:R68.
- Sodium homeostasis in chronic renal disease. Kidney Int 1982; 21:886–897.
- Espinel CH. The FENa test. Use in the differential diagnosis of acute renal failure. JAMA 1976; 236:579–581.
- Schrier RW, Wang W, Poole B, Mitra A. Acute renal failure: definitions, diagnosis, pathogenesis, and therapy. J Clin Invest 2004; 114:5–14.
- Miller TR, Anderson RJ, Linas SL, et al. Urinary diagnostic indices in acute renal failure: a prospective study. Ann Intern Med 1978; 89:47–50.
- Zarich S, Fang LS, Diamond JR. Fractional excretion of sodium. Exceptions to its diagnostic value. Arch Intern Med 1985; 145:108–112.
- Mandal AK, Baig M, Koutoubi Z. Management of acute renal failure in the elderly. Treatment options. Drugs Aging 1996; 9:226–250.
- Sands JM. Critical role of urea in the urine-concentrating mechanism. J Am Soc Nephrol 2007; 18:670–671.
- Goldstein MH, Lenz PR, Levitt MF. Effect of urine flow rate on urea reabsorption in man: urea as a “tubular marker”. J Appl Physiol 1969; 26:594–599.
- Fenton RA, Knepper MA. Urea and renal function in the 21st century: insights from knockout mice. J Am Soc Nephrol 2007; 18:679–688.
- Gréhant N. Physiologique des reins par le dosage de l’urée dans le sang et dans l’urine. J Physiol Pathol Gen (Paris) 1904; 6:1–8.
- Dossetor JB. Creatininemia versus uremia. The relative significance of blood urea nitrogen and serum creatinine concentrations in azotemia. Ann Intern Med 1966; 65:1287–1299.
- Kahn S, Sagel J, Eales L, Rabkin R. The significance of serum creatinine and the blood urea-serum creatinine ratio in azotaemia. S Afr Med J 1972; 46:1828–1832.
- Kerr DNS, Davison JM. The assessment of renal function. Br J Hosp Med 1975; 14:360–372.
- Kaplan AA, Kohn OF. Fractional excretion of urea as a guide to renal dysfunction. Am J Nephrol 1992; 12:49–54.
- Carvounis CP, Nisar S, Guro-Razuman S. Significance of the fractional excretion of urea in the differential diagnosis of acute renal failure. Kidney Int 2002; 62:2223–2229.
- Schmidt C, Höcherl K, Bucher M. Cytokine-mediated regulation of urea transporters during experimental endotoxemia. Am J Physiol Renal Physiol 2007; 292:F1479–F1489.
- Lim DH, Jeong JM, Oh SH, et al. Diagnostic performance of fractional excretion of urea in evaluating patients with acute kidney injury with diuretics treatment. Korean J Nephrol 2009; 28:190–198.
- Diskin CJ, Stokes TJ, Dansby LM, Radcliff L, Carter TB. The comparative benefits of the fractional excretion of urea and sodium in various azotemic oliguric states. Nephron Clin Pract 2010; 114:c145–c150.
- Fahimi D, Mohajeri S, Hajizadeh N, et al. Comparison between fractional excretions of urea and sodium in children with acute kidney injury. Pediatr Nephrol 2009; 24:2409–2412.
- Musso CG, Liakopoulos V, Ioannidis I, Eleftheriadis T, Stefanidis I. Acute renal failure in the elderly: particular characteristics. Int Urol Nephrol 2006; 38:787–793.
- Schönermarck U, Kehl K, Samtleben W. Diagnostic performance of fractional excretion of urea and sodium in acute kidney injury. Am J Kidney Dis 2008; 51:870–871.
An acute kidney injury can result from a myriad of causes and pathogenic pathways. Of these, the two main categories are prerenal causes (eg, heart failure, volume depletion) and causes that are intrinsic to the kidney (eg, acute tubular necrosis). Together, these categories account for more than 70% of all cases.1–3
While early intervention improves outcomes in both of these categories, the physician in the acute care setting must quickly distinguish between them, as their treatments differ. Similar clinical presentations along with confounding laboratory values make this distinction difficult. Furthermore, prolonged prerenal azotemia can eventually lead to acute tubular necrosis.
Therefore, several methods for distinguishing prerenal from intrinsic causes of acute kidney injury have been developed, including urinalysis, response to fluid challenge, the blood urea nitrogen-to-plasma creatinine ratio, levels of various urine electrolytes and biomarkers, and, the topics of our discussion here, the fractional excretion of sodium (FENa) and the fractional excretion of urea (FEU).4 While each method offers a unique picture of renal function, the validity of each may be affected by specific clinical factors.
In light of the frequent use of diuretics in inpatients and outpatients, a review of the utility of the FEU test is warranted. We will therefore present the theory behind the use of the FENa and the FEU for distinguishing intrinsic from prerenal causes of acute kidney injury, the relevant literature comparing the utility of these investigations, and our suggestions for clinical practice.
ACUTE KIDNEY INJURY DEFINED
Acute kidney injury (formerly called acute renal failure) describes an abrupt decline in renal function. Consensus definitions of it have been published and are gaining more widespread acceptance and use.9,10 The current definition is10:
- An absolute increase in serum creatinine ≥ 0.3 mg/dL (26.4 μmol/L) in 48 hours, or
- A percentage increase in serum creatinine ≥ 50% in 48 hours, or
- Urine output < 0.5 mL/kg/hour for > 6 hours.
These clear criteria allow for earlier recognition and treatment of this condition.
Acute kidney injury is fairly common in hospitalized patients, with 172 to 620 cases per million patients per year.11–14 Furthermore, hospitalized patients with acute kidney injury continue to have high rates of morbidity and death, especially those with more severe cases, in which the mortality rate remains as high as 40%.15
FRACTIONAL EXCRETION OF SODIUM
The FENa is a measure of the extraction of sodium and water from the glomerular filtrate. It is the ratio of the rate of sodium filtration (the urinary sodium concentration times the urinary flow rate, divided by the plasma sodium concentration) to the overall glomerular filtration rate, estimated by the renal filtration of creatinine. It can be calculated as the ratio of plasma creatinine to urine creatinine divided by the ratio of plasma sodium to urine sodium:
A euvolemic person with normal renal function and moderate salt intake in a steady state will have an FENa of approximately 1%.16
In 1976, Espinel17 originally showed that the FENa could be used during the oliguric phase in patients in acute renal failure to differentiate between prerenal acute kidney injury and acute tubular necrosis. Given the kidney’s ability to reabsorb more sodium during times of volume depletion, Espinel suggested that an FENa of less than 1% reflected normal sodium retention, indicating a prerenal cause, ie, diminished effective circulating volume. A value greater than 3% likely represented tubular damage, indicating that the nephrons were unable to properly reabsorb sodium.
The clinical utility of this index was apparent, as the management of prerenal azotemia and acute tubular necrosis differ.18 While both require fluid repletion, the risk of volume overload in acute tubular necrosis is high. Furthermore, acute tubular necrosis secondary to nephrotoxins could require hemodialysis to facilitate clearance of the offending agent.
The FENa test was subsequently validated in a number of studies in different populations and is still widely used.19–21
Limitations to the use of the FENa have been noted in various clinical settings. Notably, it can be falsely depressed in a number of intrinsic renal conditions, such as contrast-induced nephropathy, rhabdomyolysis, and acute glomerulonephritis. Conversely, patients with prerenal acute kidney injury who take diuretics can have a falsely elevated value due to the pharmacologically induced renal excretion of sodium independent of volume status. This is commonly seen in patients on diuretic therapy with baseline low effective circulating volumes, such those with congestive heart failure and hepatic cirrhosis.
FRACTIONAL EXCRETION OF UREA
Urea is continuously produced in the liver as the end product of protein metabolism. It is a small, water-soluble molecule that freely passes across cell membranes and is therefore continuously filtered and excreted by the kidneys. Not merely a waste product, urea is also important in water balance and constitutes approximately half of the normal solute content of urine.22
Urea’s excretion mechanisms are well characterized.22,23 It is absorbed in the proximal tubule, the medullary loop of Henle, and the medullary collecting ducts via facilitated diffusion through specific urea transporters.24 After being absorbed in the loop of Henle, urea is resecreted, a process that creates an osmotic gradient along the medulla that ultimately regulates urea excretion and reabsorption in the medullary collecting duct. Low-volume states are associated with decreased urea excretion due to a physiologic increase in antidiuretic hormone secretion, and the reverse is true for high-volume states.
The FEU has been recognized as a clinically useful tool. The correlation between serum and urine urea concentrations was investigated as early as 1904.25 However, most studies during the ensuing century focused on the serum urea concentration or the creatinine-to-urea ratio as a measure of glomerular failure.26–28 In 1992, Kaplan and Kohn29 proposed that the FEU could be a useful measure for assessing renal dysfunction in acute kidney injury. Conceptually similar to the FENa, the FEU is calculated as:
An FEU less than 35% suggests a prerenal cause of acute kidney injury, while a value greater than 50% suggests an intrinsic one.
FRACTIONAL EXCRETION OF UREA VS FRACTIONAL EXCRETION OF SODIUM
Kaplan and Kohn (1992)
Kaplan and Kohn,29 in their 1992 study, retrospectively analyzed 87 urine samples from 40 patients with renal dysfunction (not specifically acute kidney injury) thought to be secondary to volume depletion in which the FENa was discordant with the FEU.
Findings. Thirty-nine of the 40 patients treated with diuretics had a high FENa value. However, the FEU was low in all of these patients, leading the authors to conclude that the latter may be the more useful of the two indices in evaluating patients receiving diuretics who present with symptoms that suggest prerenal azotemia.
Limitations of the study. On closer inspection, these findings were not generalizable, for several reasons. First, the time that elapsed between administration of diuretics and evaluation of urinary electrolytes varied widely. Additionally, the study was a retrospective analysis of isolated urine specimens without clear correlation to a clinical patient or context. For these reasons, prospective analyses to investigate the utility of the fractional excretion of urea needed to be conducted.
Carvounis et al (2002)
Carvounis et al30 prospectively evaluated the FENa and the FEU in 102 consecutive intensive care patients with acute kidney injury (defined as a serum creatinine concentration > 1.5 mg/dL or an increase of more than 0.5 mg/dL in less than 48 hours). Oliguria was not an inclusion criterion for the study, but patients with acute glomerulonephritis and obstructive nephropathy were excluded. The study grouped subjects into those with prerenal azotemia, prerenal azotemia plus diuretic use, or acute tubular necrosis on the basis of the clinical diagnosis of the attending nephrologist.
Findings. The FEU was more sensitive than the FENa in detecting prerenal azotemia, especially in those with prerenal azotemia who were receiving diuretics. Overall, the FEU had higher sensitivity and specificity for prerenal azotemia regardless of diuretic usage, and more importantly, the best overall positive and negative predictive value for detecting it (99% and 75% respectively).
These results indicate that, in patients given diuretics, the FENa fails to discriminate between prerenal azotemia and acute tubular necrosis. Conversely, the FEU was excellent in discriminating between all cases of prerenal azotemia and acute tubular necrosis irrespective of the use of diuretics. This has significant practical application, given the frequency of diuretic use in the hospital, particularly in intensive care patients.
Limitations of the study. While the findings supported the utility of the FEU, the study population was limited to intensive care patients. Furthermore, the authors did not report the statistical significance of their findings.30
Pépin et al (2007)
Pépin et al8 performed a similar study, investigating the diagnostic utility of the FENa and the FEU in patients with acute kidney injury, with or without diuretic therapy.
The authors prospectively studied 99 consecutive patients confirmed by an independent nephrologist to have acute kidney injury (defined as an increase in serum creatinine of more than 30% over baseline values within less than 1 week) due to either volume depletion or ischemia. They excluded patients with less common causes of acute kidney injury, such as rhabdomyolysis, obstructive nephropathy, adrenal insufficiency, acute glomerulonephritis, and nephrotoxic acute kidney injury, as well as patients with chronic kidney disease.
Patients were grouped into those with transient acute kidney injury (from decreased kidney perfusion) and persistent acute kidney injury (attributed to acute tubular necrosis), with or without diuretic therapy, according to predefined clinical criteria. They were considered to have diuretic exposure if they had received furosemide (Lasix) within 24 hours or a thiazide within 48 hours of sampling.
Findings. The FENa proved superior to the FEU in patients not taking diuretics and, contrary to the findings of Carvounis et al,30 exhibited diagnostic utility in patients taking diuretics as well. Neither index discriminated between the different etiologies exceptionally well, however.
Of note, the study population was more inclusive than in previous studies, with only 63 intensive care patients, thus making the results more generalizable to all cases of inpatient acute kidney injury. Furthermore, the study included patients with and without oliguria, and the sensitivity and specificity of both the FENa and the FEU were higher in the nonoliguric group (n = 25).
Limitations of the study. The authors admit that a long time may have elapsed between diuretic administration and urine measurements, thereby mitigating the diuretic’s natriuretic effect independent of the patient’s volume status. While this variable may account for the better performance of the FENa than in the other studies, it does not account for the poor performance of the FEU.
Additionally, few of the findings reached statistical significance.
Lastly, a high percentage (30%) of patients had sepsis. The FEU is less effective in patients with infection, as cytokines interfere with the urea transporters in the kidney and colon.31
Lim et al (2009)
Lim et al32 conducted a study similar in design to that of Pépin et al.8
Findings. The FEU was as clinically useful as the FENa at distinguishing transient from persistent acute kidney injury in patients on diuretics. Using a cutoff FEU of less than 30% and a cutoff FENa of less than 1.5% for transient acute kidney injury (based on calculated receiver operating characteristic curves), FENa was more sensitive and specific than FEU in the nondiuretic groups. In patients exposed to diuretics, FEU was more sensitive but less specific than FENa.
FRACTIONAL EXCRETION OF UREA IN OLIGURIA
Diskin et al (2010)
In 2010, Diskin et al33 published a prospective, observational study of 100 consecutive patients with oliguric azotemia referred to a nephrology service. They defined acute kidney injury as serum creatinine concentration greater than 1.9 mg/dL and urine output less than 100 mL in 24 hours. They used a higher FEU cutoff for prerenal azotemia of less than 40% to reflect the known urea secretion rate in oliguric patients (600 mL/24 hours). They used an FENa of less than 1% and greater than 3% to distinguish prerenal azotemia from acute tubular necrosis.
Findings. The FEU was more accurate than the FENa, giving the right diagnosis in 95% vs 54% of cases (P < .0001). The difference was exclusively due to the FEU’s greater utility in the 67 patients who had received diuretics (98% vs 49%, P < .0001). Both the FEU and the FENa accurately detected acute tubular necrosis. As expected, the FENa outperformed FEU in the setting of infection, in which cytokine stimulation interferes with urea excretion.
Limitations of the study. Approximately 80% of the patients had prerenal azotemia, potentially biasing the results toward a test geared toward detecting this condition. However, since prerenal causes are more common than intrinsic causes, the authors argued that their cohort more accurately reflected the population encountered in clinical practice.
Additionally, only patients with oliguria and more advanced kidney injury (serum creatinine > 1.9 mg/dL) were included in the study, potentially limiting the applicability of these results in patients with preserved urine output in the early stages of renal failure.
Table 2 summarizes the findings of the studies discussed above.8,15,30,32,33
FRACTIONAL EXCRETION OF UREA IN CHILDREN AND THE ELDERLY
The FEU has also been validated in populations at the extremes of age.
In children, Fahimi et al34 performed a cross-sectional study in 43 patients referred to a nephrology service because of acute kidney injury.
An FEU less than 35% had greater sensitivity and specificity than an FENa less than 1% for differentiating prerenal from intrinsic causes in pediatric populations. An FEU of less than 30% had an even greater power of distinguishing between the two. Interestingly, 15 of the 26 patients in the group with prerenal azotemia had an FENa greater than 1%, 8 of whom had an obvious cause (diuretic therapy in 5, salt-losing congenital adrenal hyperplasia in 2, and metabolic alkalosis in 1).
In elderly people, urinary indices are less reliable because of reduced sodium and urea reabsorption and urinary concentrating capability. Thus, the FENa and FEU are increased, making the standard cutoff values unreliable and unpredictable for distinguishing prerenal from intrinsic causes of acute kidney injury.35
WHICH TEST SHOULD BE USED?
Both the FENa and the FEU have been validated in prospective trials as useful clinical indices in identifying prerenal azotemia. Results of these studies vary as to which index is superior and when. This may be attributable to the various definitions of acute kidney injury and diagnostic criteria used in the studies as well as the heterogeneity of patients in each study.
However, the preponderance of evidence indicates that the FEU is more useful than the FENa in patients on diuretics. Since diuretics are widely used, particularly in acute care settings in which acute kidney injury is prevalent, the FEU is a useful clinical tool and should be utilized in this context accordingly. Specifically, when there is a history of recent diuretic use, the evidence supports ordering the FEU alone, or at least in conjunction with the FENa. If the two indices yield disparate results, the physician should look for circumstances that would alter each one of them, such as sepsis or an unrecognized dose of diuretic.
In managing acute kidney injury, distinguishing prerenal from intrinsic causes is a difficult task, particularly because prolonged prerenal azotemia can develop into acute tubular necrosis. Therefore, a single index, calculated at a specific time, often is insufficient to properly characterize the pathogenesis of acute kidney injury, and a combination of both of these indices may increase diagnostic sensitivity and specificity.36 Moreover, urine samples collected after acute changes in volume or osmolarity, such as blood loss, administration of intravenous fluids or parenteral nutrition, or dialysis may compromise their diagnostic utility, and care must be taken to interpret the results in the appropriate clinical context.
The clinician must be aware of both the respective applications and limitations of these indices when using them to guide management and navigate the differential diagnosis in the appropriate clinical settings.
An acute kidney injury can result from a myriad of causes and pathogenic pathways. Of these, the two main categories are prerenal causes (eg, heart failure, volume depletion) and causes that are intrinsic to the kidney (eg, acute tubular necrosis). Together, these categories account for more than 70% of all cases.1–3
While early intervention improves outcomes in both of these categories, the physician in the acute care setting must quickly distinguish between them, as their treatments differ. Similar clinical presentations along with confounding laboratory values make this distinction difficult. Furthermore, prolonged prerenal azotemia can eventually lead to acute tubular necrosis.
Therefore, several methods for distinguishing prerenal from intrinsic causes of acute kidney injury have been developed, including urinalysis, response to fluid challenge, the blood urea nitrogen-to-plasma creatinine ratio, levels of various urine electrolytes and biomarkers, and, the topics of our discussion here, the fractional excretion of sodium (FENa) and the fractional excretion of urea (FEU).4 While each method offers a unique picture of renal function, the validity of each may be affected by specific clinical factors.
In light of the frequent use of diuretics in inpatients and outpatients, a review of the utility of the FEU test is warranted. We will therefore present the theory behind the use of the FENa and the FEU for distinguishing intrinsic from prerenal causes of acute kidney injury, the relevant literature comparing the utility of these investigations, and our suggestions for clinical practice.
ACUTE KIDNEY INJURY DEFINED
Acute kidney injury (formerly called acute renal failure) describes an abrupt decline in renal function. Consensus definitions of it have been published and are gaining more widespread acceptance and use.9,10 The current definition is10:
- An absolute increase in serum creatinine ≥ 0.3 mg/dL (26.4 μmol/L) in 48 hours, or
- A percentage increase in serum creatinine ≥ 50% in 48 hours, or
- Urine output < 0.5 mL/kg/hour for > 6 hours.
These clear criteria allow for earlier recognition and treatment of this condition.
Acute kidney injury is fairly common in hospitalized patients, with 172 to 620 cases per million patients per year.11–14 Furthermore, hospitalized patients with acute kidney injury continue to have high rates of morbidity and death, especially those with more severe cases, in which the mortality rate remains as high as 40%.15
FRACTIONAL EXCRETION OF SODIUM
The FENa is a measure of the extraction of sodium and water from the glomerular filtrate. It is the ratio of the rate of sodium filtration (the urinary sodium concentration times the urinary flow rate, divided by the plasma sodium concentration) to the overall glomerular filtration rate, estimated by the renal filtration of creatinine. It can be calculated as the ratio of plasma creatinine to urine creatinine divided by the ratio of plasma sodium to urine sodium:
A euvolemic person with normal renal function and moderate salt intake in a steady state will have an FENa of approximately 1%.16
In 1976, Espinel17 originally showed that the FENa could be used during the oliguric phase in patients in acute renal failure to differentiate between prerenal acute kidney injury and acute tubular necrosis. Given the kidney’s ability to reabsorb more sodium during times of volume depletion, Espinel suggested that an FENa of less than 1% reflected normal sodium retention, indicating a prerenal cause, ie, diminished effective circulating volume. A value greater than 3% likely represented tubular damage, indicating that the nephrons were unable to properly reabsorb sodium.
The clinical utility of this index was apparent, as the management of prerenal azotemia and acute tubular necrosis differ.18 While both require fluid repletion, the risk of volume overload in acute tubular necrosis is high. Furthermore, acute tubular necrosis secondary to nephrotoxins could require hemodialysis to facilitate clearance of the offending agent.
The FENa test was subsequently validated in a number of studies in different populations and is still widely used.19–21
Limitations to the use of the FENa have been noted in various clinical settings. Notably, it can be falsely depressed in a number of intrinsic renal conditions, such as contrast-induced nephropathy, rhabdomyolysis, and acute glomerulonephritis. Conversely, patients with prerenal acute kidney injury who take diuretics can have a falsely elevated value due to the pharmacologically induced renal excretion of sodium independent of volume status. This is commonly seen in patients on diuretic therapy with baseline low effective circulating volumes, such those with congestive heart failure and hepatic cirrhosis.
FRACTIONAL EXCRETION OF UREA
Urea is continuously produced in the liver as the end product of protein metabolism. It is a small, water-soluble molecule that freely passes across cell membranes and is therefore continuously filtered and excreted by the kidneys. Not merely a waste product, urea is also important in water balance and constitutes approximately half of the normal solute content of urine.22
Urea’s excretion mechanisms are well characterized.22,23 It is absorbed in the proximal tubule, the medullary loop of Henle, and the medullary collecting ducts via facilitated diffusion through specific urea transporters.24 After being absorbed in the loop of Henle, urea is resecreted, a process that creates an osmotic gradient along the medulla that ultimately regulates urea excretion and reabsorption in the medullary collecting duct. Low-volume states are associated with decreased urea excretion due to a physiologic increase in antidiuretic hormone secretion, and the reverse is true for high-volume states.
The FEU has been recognized as a clinically useful tool. The correlation between serum and urine urea concentrations was investigated as early as 1904.25 However, most studies during the ensuing century focused on the serum urea concentration or the creatinine-to-urea ratio as a measure of glomerular failure.26–28 In 1992, Kaplan and Kohn29 proposed that the FEU could be a useful measure for assessing renal dysfunction in acute kidney injury. Conceptually similar to the FENa, the FEU is calculated as:
An FEU less than 35% suggests a prerenal cause of acute kidney injury, while a value greater than 50% suggests an intrinsic one.
FRACTIONAL EXCRETION OF UREA VS FRACTIONAL EXCRETION OF SODIUM
Kaplan and Kohn (1992)
Kaplan and Kohn,29 in their 1992 study, retrospectively analyzed 87 urine samples from 40 patients with renal dysfunction (not specifically acute kidney injury) thought to be secondary to volume depletion in which the FENa was discordant with the FEU.
Findings. Thirty-nine of the 40 patients treated with diuretics had a high FENa value. However, the FEU was low in all of these patients, leading the authors to conclude that the latter may be the more useful of the two indices in evaluating patients receiving diuretics who present with symptoms that suggest prerenal azotemia.
Limitations of the study. On closer inspection, these findings were not generalizable, for several reasons. First, the time that elapsed between administration of diuretics and evaluation of urinary electrolytes varied widely. Additionally, the study was a retrospective analysis of isolated urine specimens without clear correlation to a clinical patient or context. For these reasons, prospective analyses to investigate the utility of the fractional excretion of urea needed to be conducted.
Carvounis et al (2002)
Carvounis et al30 prospectively evaluated the FENa and the FEU in 102 consecutive intensive care patients with acute kidney injury (defined as a serum creatinine concentration > 1.5 mg/dL or an increase of more than 0.5 mg/dL in less than 48 hours). Oliguria was not an inclusion criterion for the study, but patients with acute glomerulonephritis and obstructive nephropathy were excluded. The study grouped subjects into those with prerenal azotemia, prerenal azotemia plus diuretic use, or acute tubular necrosis on the basis of the clinical diagnosis of the attending nephrologist.
Findings. The FEU was more sensitive than the FENa in detecting prerenal azotemia, especially in those with prerenal azotemia who were receiving diuretics. Overall, the FEU had higher sensitivity and specificity for prerenal azotemia regardless of diuretic usage, and more importantly, the best overall positive and negative predictive value for detecting it (99% and 75% respectively).
These results indicate that, in patients given diuretics, the FENa fails to discriminate between prerenal azotemia and acute tubular necrosis. Conversely, the FEU was excellent in discriminating between all cases of prerenal azotemia and acute tubular necrosis irrespective of the use of diuretics. This has significant practical application, given the frequency of diuretic use in the hospital, particularly in intensive care patients.
Limitations of the study. While the findings supported the utility of the FEU, the study population was limited to intensive care patients. Furthermore, the authors did not report the statistical significance of their findings.30
Pépin et al (2007)
Pépin et al8 performed a similar study, investigating the diagnostic utility of the FENa and the FEU in patients with acute kidney injury, with or without diuretic therapy.
The authors prospectively studied 99 consecutive patients confirmed by an independent nephrologist to have acute kidney injury (defined as an increase in serum creatinine of more than 30% over baseline values within less than 1 week) due to either volume depletion or ischemia. They excluded patients with less common causes of acute kidney injury, such as rhabdomyolysis, obstructive nephropathy, adrenal insufficiency, acute glomerulonephritis, and nephrotoxic acute kidney injury, as well as patients with chronic kidney disease.
Patients were grouped into those with transient acute kidney injury (from decreased kidney perfusion) and persistent acute kidney injury (attributed to acute tubular necrosis), with or without diuretic therapy, according to predefined clinical criteria. They were considered to have diuretic exposure if they had received furosemide (Lasix) within 24 hours or a thiazide within 48 hours of sampling.
Findings. The FENa proved superior to the FEU in patients not taking diuretics and, contrary to the findings of Carvounis et al,30 exhibited diagnostic utility in patients taking diuretics as well. Neither index discriminated between the different etiologies exceptionally well, however.
Of note, the study population was more inclusive than in previous studies, with only 63 intensive care patients, thus making the results more generalizable to all cases of inpatient acute kidney injury. Furthermore, the study included patients with and without oliguria, and the sensitivity and specificity of both the FENa and the FEU were higher in the nonoliguric group (n = 25).
Limitations of the study. The authors admit that a long time may have elapsed between diuretic administration and urine measurements, thereby mitigating the diuretic’s natriuretic effect independent of the patient’s volume status. While this variable may account for the better performance of the FENa than in the other studies, it does not account for the poor performance of the FEU.
Additionally, few of the findings reached statistical significance.
Lastly, a high percentage (30%) of patients had sepsis. The FEU is less effective in patients with infection, as cytokines interfere with the urea transporters in the kidney and colon.31
Lim et al (2009)
Lim et al32 conducted a study similar in design to that of Pépin et al.8
Findings. The FEU was as clinically useful as the FENa at distinguishing transient from persistent acute kidney injury in patients on diuretics. Using a cutoff FEU of less than 30% and a cutoff FENa of less than 1.5% for transient acute kidney injury (based on calculated receiver operating characteristic curves), FENa was more sensitive and specific than FEU in the nondiuretic groups. In patients exposed to diuretics, FEU was more sensitive but less specific than FENa.
FRACTIONAL EXCRETION OF UREA IN OLIGURIA
Diskin et al (2010)
In 2010, Diskin et al33 published a prospective, observational study of 100 consecutive patients with oliguric azotemia referred to a nephrology service. They defined acute kidney injury as serum creatinine concentration greater than 1.9 mg/dL and urine output less than 100 mL in 24 hours. They used a higher FEU cutoff for prerenal azotemia of less than 40% to reflect the known urea secretion rate in oliguric patients (600 mL/24 hours). They used an FENa of less than 1% and greater than 3% to distinguish prerenal azotemia from acute tubular necrosis.
Findings. The FEU was more accurate than the FENa, giving the right diagnosis in 95% vs 54% of cases (P < .0001). The difference was exclusively due to the FEU’s greater utility in the 67 patients who had received diuretics (98% vs 49%, P < .0001). Both the FEU and the FENa accurately detected acute tubular necrosis. As expected, the FENa outperformed FEU in the setting of infection, in which cytokine stimulation interferes with urea excretion.
Limitations of the study. Approximately 80% of the patients had prerenal azotemia, potentially biasing the results toward a test geared toward detecting this condition. However, since prerenal causes are more common than intrinsic causes, the authors argued that their cohort more accurately reflected the population encountered in clinical practice.
Additionally, only patients with oliguria and more advanced kidney injury (serum creatinine > 1.9 mg/dL) were included in the study, potentially limiting the applicability of these results in patients with preserved urine output in the early stages of renal failure.
Table 2 summarizes the findings of the studies discussed above.8,15,30,32,33
FRACTIONAL EXCRETION OF UREA IN CHILDREN AND THE ELDERLY
The FEU has also been validated in populations at the extremes of age.
In children, Fahimi et al34 performed a cross-sectional study in 43 patients referred to a nephrology service because of acute kidney injury.
An FEU less than 35% had greater sensitivity and specificity than an FENa less than 1% for differentiating prerenal from intrinsic causes in pediatric populations. An FEU of less than 30% had an even greater power of distinguishing between the two. Interestingly, 15 of the 26 patients in the group with prerenal azotemia had an FENa greater than 1%, 8 of whom had an obvious cause (diuretic therapy in 5, salt-losing congenital adrenal hyperplasia in 2, and metabolic alkalosis in 1).
In elderly people, urinary indices are less reliable because of reduced sodium and urea reabsorption and urinary concentrating capability. Thus, the FENa and FEU are increased, making the standard cutoff values unreliable and unpredictable for distinguishing prerenal from intrinsic causes of acute kidney injury.35
WHICH TEST SHOULD BE USED?
Both the FENa and the FEU have been validated in prospective trials as useful clinical indices in identifying prerenal azotemia. Results of these studies vary as to which index is superior and when. This may be attributable to the various definitions of acute kidney injury and diagnostic criteria used in the studies as well as the heterogeneity of patients in each study.
However, the preponderance of evidence indicates that the FEU is more useful than the FENa in patients on diuretics. Since diuretics are widely used, particularly in acute care settings in which acute kidney injury is prevalent, the FEU is a useful clinical tool and should be utilized in this context accordingly. Specifically, when there is a history of recent diuretic use, the evidence supports ordering the FEU alone, or at least in conjunction with the FENa. If the two indices yield disparate results, the physician should look for circumstances that would alter each one of them, such as sepsis or an unrecognized dose of diuretic.
In managing acute kidney injury, distinguishing prerenal from intrinsic causes is a difficult task, particularly because prolonged prerenal azotemia can develop into acute tubular necrosis. Therefore, a single index, calculated at a specific time, often is insufficient to properly characterize the pathogenesis of acute kidney injury, and a combination of both of these indices may increase diagnostic sensitivity and specificity.36 Moreover, urine samples collected after acute changes in volume or osmolarity, such as blood loss, administration of intravenous fluids or parenteral nutrition, or dialysis may compromise their diagnostic utility, and care must be taken to interpret the results in the appropriate clinical context.
The clinician must be aware of both the respective applications and limitations of these indices when using them to guide management and navigate the differential diagnosis in the appropriate clinical settings.
- Nolan CR, Anderson RJ. Hospital-acquired acute renal failure. J Am Soc Nephrol 1998; 9:710–718.
- Mehta RL, Pascual MT, Soroko S, et al; Program to Improve Care in Acute Renal Disease. Spectrum of acute renal failure in the intensive care unit: the PICARD experience. Kidney Int 2004; 66:1613–1621.
- Myers BD, Miller DC, Mehigan JT, et al. Nature of the renal injury following total renal ischemia in man. J Clin Invest 1984; 73:329–341.
- Ho E, Fard A, Maisel A. Evolving use of biomarkers for kidney injury in acute care settings. Curr Opin Crit Care 2010; 16:399–407.
- Steiner RW. Low fractional excretion of sodium in myoglobinuric acute renal failure. Arch Intern Med 1982; 142:1216–1217.
- Vaz AJ. Low fractional excretion of urine sodium in acute renal failure due to sepsis. Arch Intern Med 1983; 143:738–739.
- Pru C, Kjellstrand CM. The FENa test is of no prognostic value in acute renal failure. Nephron 1984; 36:20–23.
- Pépin MN, Bouchard J, Legault L, Ethier J. Diagnostic performance of fractional excretion of urea and fractional excretion of sodium in the evaluations of patients with acute kidney injury with or without diuretic treatment. Am J Kidney Dis 2007; 50:566–573.
- Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative workgroup. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004; 8:R204–R212.
- Mehta RL, Kellum JA, Shah SV, et al; Acute Kidney Injury Network. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007; 11:R31.
- Stevens PE, Tamimi NA, Al-Hasani MK, et al. Non-specialist management of acute renal failure. QJM 2001; 94:533–540.
- Feest TG, Round A, Hamad S. Incidence of severe acute renal failure in adults: results of a community based study. BMJ 1993; 306:481–483.
- Liaño F, Pascual J. Epidemiology of acute renal failure: a prospective, multicenter, community-based study. Madrid Acute Renal Failure Study Group. Kidney Int 1996; 50:811–818.
- Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N Engl J Med 1996; 334:1448–1460.
- Bagshaw SM, George C, Bellomo R; ANZICS Database Management Committee. Changes in the incidence and outcome for early acute kidney injury in a cohort of Australian intensive care units. Crit Care 2007; 11:R68.
- Sodium homeostasis in chronic renal disease. Kidney Int 1982; 21:886–897.
- Espinel CH. The FENa test. Use in the differential diagnosis of acute renal failure. JAMA 1976; 236:579–581.
- Schrier RW, Wang W, Poole B, Mitra A. Acute renal failure: definitions, diagnosis, pathogenesis, and therapy. J Clin Invest 2004; 114:5–14.
- Miller TR, Anderson RJ, Linas SL, et al. Urinary diagnostic indices in acute renal failure: a prospective study. Ann Intern Med 1978; 89:47–50.
- Zarich S, Fang LS, Diamond JR. Fractional excretion of sodium. Exceptions to its diagnostic value. Arch Intern Med 1985; 145:108–112.
- Mandal AK, Baig M, Koutoubi Z. Management of acute renal failure in the elderly. Treatment options. Drugs Aging 1996; 9:226–250.
- Sands JM. Critical role of urea in the urine-concentrating mechanism. J Am Soc Nephrol 2007; 18:670–671.
- Goldstein MH, Lenz PR, Levitt MF. Effect of urine flow rate on urea reabsorption in man: urea as a “tubular marker”. J Appl Physiol 1969; 26:594–599.
- Fenton RA, Knepper MA. Urea and renal function in the 21st century: insights from knockout mice. J Am Soc Nephrol 2007; 18:679–688.
- Gréhant N. Physiologique des reins par le dosage de l’urée dans le sang et dans l’urine. J Physiol Pathol Gen (Paris) 1904; 6:1–8.
- Dossetor JB. Creatininemia versus uremia. The relative significance of blood urea nitrogen and serum creatinine concentrations in azotemia. Ann Intern Med 1966; 65:1287–1299.
- Kahn S, Sagel J, Eales L, Rabkin R. The significance of serum creatinine and the blood urea-serum creatinine ratio in azotaemia. S Afr Med J 1972; 46:1828–1832.
- Kerr DNS, Davison JM. The assessment of renal function. Br J Hosp Med 1975; 14:360–372.
- Kaplan AA, Kohn OF. Fractional excretion of urea as a guide to renal dysfunction. Am J Nephrol 1992; 12:49–54.
- Carvounis CP, Nisar S, Guro-Razuman S. Significance of the fractional excretion of urea in the differential diagnosis of acute renal failure. Kidney Int 2002; 62:2223–2229.
- Schmidt C, Höcherl K, Bucher M. Cytokine-mediated regulation of urea transporters during experimental endotoxemia. Am J Physiol Renal Physiol 2007; 292:F1479–F1489.
- Lim DH, Jeong JM, Oh SH, et al. Diagnostic performance of fractional excretion of urea in evaluating patients with acute kidney injury with diuretics treatment. Korean J Nephrol 2009; 28:190–198.
- Diskin CJ, Stokes TJ, Dansby LM, Radcliff L, Carter TB. The comparative benefits of the fractional excretion of urea and sodium in various azotemic oliguric states. Nephron Clin Pract 2010; 114:c145–c150.
- Fahimi D, Mohajeri S, Hajizadeh N, et al. Comparison between fractional excretions of urea and sodium in children with acute kidney injury. Pediatr Nephrol 2009; 24:2409–2412.
- Musso CG, Liakopoulos V, Ioannidis I, Eleftheriadis T, Stefanidis I. Acute renal failure in the elderly: particular characteristics. Int Urol Nephrol 2006; 38:787–793.
- Schönermarck U, Kehl K, Samtleben W. Diagnostic performance of fractional excretion of urea and sodium in acute kidney injury. Am J Kidney Dis 2008; 51:870–871.
- Nolan CR, Anderson RJ. Hospital-acquired acute renal failure. J Am Soc Nephrol 1998; 9:710–718.
- Mehta RL, Pascual MT, Soroko S, et al; Program to Improve Care in Acute Renal Disease. Spectrum of acute renal failure in the intensive care unit: the PICARD experience. Kidney Int 2004; 66:1613–1621.
- Myers BD, Miller DC, Mehigan JT, et al. Nature of the renal injury following total renal ischemia in man. J Clin Invest 1984; 73:329–341.
- Ho E, Fard A, Maisel A. Evolving use of biomarkers for kidney injury in acute care settings. Curr Opin Crit Care 2010; 16:399–407.
- Steiner RW. Low fractional excretion of sodium in myoglobinuric acute renal failure. Arch Intern Med 1982; 142:1216–1217.
- Vaz AJ. Low fractional excretion of urine sodium in acute renal failure due to sepsis. Arch Intern Med 1983; 143:738–739.
- Pru C, Kjellstrand CM. The FENa test is of no prognostic value in acute renal failure. Nephron 1984; 36:20–23.
- Pépin MN, Bouchard J, Legault L, Ethier J. Diagnostic performance of fractional excretion of urea and fractional excretion of sodium in the evaluations of patients with acute kidney injury with or without diuretic treatment. Am J Kidney Dis 2007; 50:566–573.
- Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative workgroup. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004; 8:R204–R212.
- Mehta RL, Kellum JA, Shah SV, et al; Acute Kidney Injury Network. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007; 11:R31.
- Stevens PE, Tamimi NA, Al-Hasani MK, et al. Non-specialist management of acute renal failure. QJM 2001; 94:533–540.
- Feest TG, Round A, Hamad S. Incidence of severe acute renal failure in adults: results of a community based study. BMJ 1993; 306:481–483.
- Liaño F, Pascual J. Epidemiology of acute renal failure: a prospective, multicenter, community-based study. Madrid Acute Renal Failure Study Group. Kidney Int 1996; 50:811–818.
- Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N Engl J Med 1996; 334:1448–1460.
- Bagshaw SM, George C, Bellomo R; ANZICS Database Management Committee. Changes in the incidence and outcome for early acute kidney injury in a cohort of Australian intensive care units. Crit Care 2007; 11:R68.
- Sodium homeostasis in chronic renal disease. Kidney Int 1982; 21:886–897.
- Espinel CH. The FENa test. Use in the differential diagnosis of acute renal failure. JAMA 1976; 236:579–581.
- Schrier RW, Wang W, Poole B, Mitra A. Acute renal failure: definitions, diagnosis, pathogenesis, and therapy. J Clin Invest 2004; 114:5–14.
- Miller TR, Anderson RJ, Linas SL, et al. Urinary diagnostic indices in acute renal failure: a prospective study. Ann Intern Med 1978; 89:47–50.
- Zarich S, Fang LS, Diamond JR. Fractional excretion of sodium. Exceptions to its diagnostic value. Arch Intern Med 1985; 145:108–112.
- Mandal AK, Baig M, Koutoubi Z. Management of acute renal failure in the elderly. Treatment options. Drugs Aging 1996; 9:226–250.
- Sands JM. Critical role of urea in the urine-concentrating mechanism. J Am Soc Nephrol 2007; 18:670–671.
- Goldstein MH, Lenz PR, Levitt MF. Effect of urine flow rate on urea reabsorption in man: urea as a “tubular marker”. J Appl Physiol 1969; 26:594–599.
- Fenton RA, Knepper MA. Urea and renal function in the 21st century: insights from knockout mice. J Am Soc Nephrol 2007; 18:679–688.
- Gréhant N. Physiologique des reins par le dosage de l’urée dans le sang et dans l’urine. J Physiol Pathol Gen (Paris) 1904; 6:1–8.
- Dossetor JB. Creatininemia versus uremia. The relative significance of blood urea nitrogen and serum creatinine concentrations in azotemia. Ann Intern Med 1966; 65:1287–1299.
- Kahn S, Sagel J, Eales L, Rabkin R. The significance of serum creatinine and the blood urea-serum creatinine ratio in azotaemia. S Afr Med J 1972; 46:1828–1832.
- Kerr DNS, Davison JM. The assessment of renal function. Br J Hosp Med 1975; 14:360–372.
- Kaplan AA, Kohn OF. Fractional excretion of urea as a guide to renal dysfunction. Am J Nephrol 1992; 12:49–54.
- Carvounis CP, Nisar S, Guro-Razuman S. Significance of the fractional excretion of urea in the differential diagnosis of acute renal failure. Kidney Int 2002; 62:2223–2229.
- Schmidt C, Höcherl K, Bucher M. Cytokine-mediated regulation of urea transporters during experimental endotoxemia. Am J Physiol Renal Physiol 2007; 292:F1479–F1489.
- Lim DH, Jeong JM, Oh SH, et al. Diagnostic performance of fractional excretion of urea in evaluating patients with acute kidney injury with diuretics treatment. Korean J Nephrol 2009; 28:190–198.
- Diskin CJ, Stokes TJ, Dansby LM, Radcliff L, Carter TB. The comparative benefits of the fractional excretion of urea and sodium in various azotemic oliguric states. Nephron Clin Pract 2010; 114:c145–c150.
- Fahimi D, Mohajeri S, Hajizadeh N, et al. Comparison between fractional excretions of urea and sodium in children with acute kidney injury. Pediatr Nephrol 2009; 24:2409–2412.
- Musso CG, Liakopoulos V, Ioannidis I, Eleftheriadis T, Stefanidis I. Acute renal failure in the elderly: particular characteristics. Int Urol Nephrol 2006; 38:787–793.
- Schönermarck U, Kehl K, Samtleben W. Diagnostic performance of fractional excretion of urea and sodium in acute kidney injury. Am J Kidney Dis 2008; 51:870–871.
KEY POINTS
- Finding the cause of acute kidney injury is important, as management strategies differ.
- Although cutoff values differ among studies, in a patient with acute kidney injury, an FENa lower than 1% suggests a prerenal cause, whereas a value higher than 3% suggests an intrinsic cause.
- Similarly, an FEU less than 35% suggests a prerenal cause of acute kidney injury, whereas a value higher than 50% suggests an intrinsic one.
- The FENa can be falsely high in patients taking a diuretic; it can be falsely low in a number of intrinsic renal conditions, such as contrast-induced nephropathy, rhabdomyolysis, and acute glomerulonephritis.
Vytorin Falls Short of New Indication for CKD Patients
The Food and Drug Administration approved a revised label for Vytorin that now includes data from a study showing a benefit of treatment in patients with moderate to severe kidney disease, but the agency did not approve a new indication that reflects these findings for Vytorin or for ezetemibe, one of the components of the combination product, according to Merck.
In a Jan. 25 press release, Merck, the manufacturer of ezetimibe (Zetia) and Vytorin – a combination of ezetimibe and simvastatin – said that the FDA had approved the new labeling that now includes data from the Study of Heart and Renal Protection (SHARP). Themultinational, randomized, double-blind placebo controlled study of almost 9,500 patients with moderate to severe chronic kidney disease who did not have a history of myocardial infarction or coronary revascularization found that the lipid-lowering treatment reduced the risk of major vascular events, compared with placebo.
However, the FDA did not approve a new indication to reflect these results, because SHARP compared the combination of the two drugs with placebo and "was not designed to assess the independent contributions of each drug to the observed effect," the Merck release said, adding: "For this reason, the FDA did not approve a new indication for Vytorin or for Zetia," and the SHARP results have not been added to the ezetimibe label.
This was despite the unanimous recommendation of the FDA’s Endocrinologic and Metabolic Drugs Advisory Committee in November to approve Vytorin for reducing major cardiovascular events in patients with CKD who are not on dialysis. The panel also voted 10-6 that the data did not support approval of the combination for the same indication in patients with end-stage renal disease who are on dialysis.
Merck had filed for approval of the claim that 10 mg of ezetimibe plus 20 mg of simvastatin (in the fixed-dose combination pill or taken separately) reduces the risk of major cardiovascular events in patients with chronic kidney disease on the basis of the SHARP results.
About two-thirds of the patients enrolled in SHARP were not on dialysis at baseline, and in these patients, there was a 23% reduction in the primary end point – the risk of a major vascular event (nonfatal MI or cardiac death, stroke, or a revascularization procedure that excluded dialysis access–related procedures) – compared with those on placebo over a mean of 5 years (Lancet 2011;377:2181-92).
Among the patients who were on dialysis at baseline, the risk reduction was less (about 6% over placebo).
The two drugs remain approved for lipid-lowering indications only: Ezetimibe, a selective inhibitor of the absorption of intestinal cholesterol and related phytosterol, was approved in 2002; Vytorin, a combination of ezetimibe and the HMG-CoA reductase inhibitor simvastatin was approved in 2004; simvastatin was approved in 1991.
In an interview, Dr. William Hiatt, one of the members of the FDA panel that reviewed the SHARP data, said that "by allowing Merck to add the information from the SHARP trial to the product label, physicians will be able to use that information to help guide their decisions to use this medication for their CKD patients." Dr. Hiatt is professor of medicine, division of cardiology, University of Colorado, Denver. Members of FDA advisory panels are cleared of potential conflicts related to the topic of the meeting.
The agency usually follows FDA panel recommendations, which are not binding. At press time, no statement was available from the FDA to explain the decision.
SHARP was funded by Merck and Schering-Plough, but was independently conducted by the Oxford (England) University Clinical Trials Service Unit.
The Food and Drug Administration approved a revised label for Vytorin that now includes data from a study showing a benefit of treatment in patients with moderate to severe kidney disease, but the agency did not approve a new indication that reflects these findings for Vytorin or for ezetemibe, one of the components of the combination product, according to Merck.
In a Jan. 25 press release, Merck, the manufacturer of ezetimibe (Zetia) and Vytorin – a combination of ezetimibe and simvastatin – said that the FDA had approved the new labeling that now includes data from the Study of Heart and Renal Protection (SHARP). Themultinational, randomized, double-blind placebo controlled study of almost 9,500 patients with moderate to severe chronic kidney disease who did not have a history of myocardial infarction or coronary revascularization found that the lipid-lowering treatment reduced the risk of major vascular events, compared with placebo.
However, the FDA did not approve a new indication to reflect these results, because SHARP compared the combination of the two drugs with placebo and "was not designed to assess the independent contributions of each drug to the observed effect," the Merck release said, adding: "For this reason, the FDA did not approve a new indication for Vytorin or for Zetia," and the SHARP results have not been added to the ezetimibe label.
This was despite the unanimous recommendation of the FDA’s Endocrinologic and Metabolic Drugs Advisory Committee in November to approve Vytorin for reducing major cardiovascular events in patients with CKD who are not on dialysis. The panel also voted 10-6 that the data did not support approval of the combination for the same indication in patients with end-stage renal disease who are on dialysis.
Merck had filed for approval of the claim that 10 mg of ezetimibe plus 20 mg of simvastatin (in the fixed-dose combination pill or taken separately) reduces the risk of major cardiovascular events in patients with chronic kidney disease on the basis of the SHARP results.
About two-thirds of the patients enrolled in SHARP were not on dialysis at baseline, and in these patients, there was a 23% reduction in the primary end point – the risk of a major vascular event (nonfatal MI or cardiac death, stroke, or a revascularization procedure that excluded dialysis access–related procedures) – compared with those on placebo over a mean of 5 years (Lancet 2011;377:2181-92).
Among the patients who were on dialysis at baseline, the risk reduction was less (about 6% over placebo).
The two drugs remain approved for lipid-lowering indications only: Ezetimibe, a selective inhibitor of the absorption of intestinal cholesterol and related phytosterol, was approved in 2002; Vytorin, a combination of ezetimibe and the HMG-CoA reductase inhibitor simvastatin was approved in 2004; simvastatin was approved in 1991.
In an interview, Dr. William Hiatt, one of the members of the FDA panel that reviewed the SHARP data, said that "by allowing Merck to add the information from the SHARP trial to the product label, physicians will be able to use that information to help guide their decisions to use this medication for their CKD patients." Dr. Hiatt is professor of medicine, division of cardiology, University of Colorado, Denver. Members of FDA advisory panels are cleared of potential conflicts related to the topic of the meeting.
The agency usually follows FDA panel recommendations, which are not binding. At press time, no statement was available from the FDA to explain the decision.
SHARP was funded by Merck and Schering-Plough, but was independently conducted by the Oxford (England) University Clinical Trials Service Unit.
The Food and Drug Administration approved a revised label for Vytorin that now includes data from a study showing a benefit of treatment in patients with moderate to severe kidney disease, but the agency did not approve a new indication that reflects these findings for Vytorin or for ezetemibe, one of the components of the combination product, according to Merck.
In a Jan. 25 press release, Merck, the manufacturer of ezetimibe (Zetia) and Vytorin – a combination of ezetimibe and simvastatin – said that the FDA had approved the new labeling that now includes data from the Study of Heart and Renal Protection (SHARP). Themultinational, randomized, double-blind placebo controlled study of almost 9,500 patients with moderate to severe chronic kidney disease who did not have a history of myocardial infarction or coronary revascularization found that the lipid-lowering treatment reduced the risk of major vascular events, compared with placebo.
However, the FDA did not approve a new indication to reflect these results, because SHARP compared the combination of the two drugs with placebo and "was not designed to assess the independent contributions of each drug to the observed effect," the Merck release said, adding: "For this reason, the FDA did not approve a new indication for Vytorin or for Zetia," and the SHARP results have not been added to the ezetimibe label.
This was despite the unanimous recommendation of the FDA’s Endocrinologic and Metabolic Drugs Advisory Committee in November to approve Vytorin for reducing major cardiovascular events in patients with CKD who are not on dialysis. The panel also voted 10-6 that the data did not support approval of the combination for the same indication in patients with end-stage renal disease who are on dialysis.
Merck had filed for approval of the claim that 10 mg of ezetimibe plus 20 mg of simvastatin (in the fixed-dose combination pill or taken separately) reduces the risk of major cardiovascular events in patients with chronic kidney disease on the basis of the SHARP results.
About two-thirds of the patients enrolled in SHARP were not on dialysis at baseline, and in these patients, there was a 23% reduction in the primary end point – the risk of a major vascular event (nonfatal MI or cardiac death, stroke, or a revascularization procedure that excluded dialysis access–related procedures) – compared with those on placebo over a mean of 5 years (Lancet 2011;377:2181-92).
Among the patients who were on dialysis at baseline, the risk reduction was less (about 6% over placebo).
The two drugs remain approved for lipid-lowering indications only: Ezetimibe, a selective inhibitor of the absorption of intestinal cholesterol and related phytosterol, was approved in 2002; Vytorin, a combination of ezetimibe and the HMG-CoA reductase inhibitor simvastatin was approved in 2004; simvastatin was approved in 1991.
In an interview, Dr. William Hiatt, one of the members of the FDA panel that reviewed the SHARP data, said that "by allowing Merck to add the information from the SHARP trial to the product label, physicians will be able to use that information to help guide their decisions to use this medication for their CKD patients." Dr. Hiatt is professor of medicine, division of cardiology, University of Colorado, Denver. Members of FDA advisory panels are cleared of potential conflicts related to the topic of the meeting.
The agency usually follows FDA panel recommendations, which are not binding. At press time, no statement was available from the FDA to explain the decision.
SHARP was funded by Merck and Schering-Plough, but was independently conducted by the Oxford (England) University Clinical Trials Service Unit.
Glucose Testing Suggested for All Inpatients
All patients admitted to the hospital in noncritical care settings should have their blood glucose tested, according to a new clinical practice guideline from the Endocrine Society.
Unlike previous guidelines based largely on data from intensive care and critical care settings, the new guideline focuses on glucose management in noncritical settings, with special emphasis on systemic issues such as patient transition between hospital units and from inpatient to outpatient settings. The guidelines also include detailed guidance for creating systems and protocols to ensure optimal patient management and safety (Diabetes Care [2009;32:1119-31]).
"Management of Hyperglycemia in Hospitalized Patients in Non-Critical Care Setting: An Endocrine Society Clinical Practice Guideline" was developed by an eight-member panel with representatives from the American Diabetes Association, American Heart Association, American Association of Diabetes Educators, European Society of Endocrinology, and the Society of Hospital Medicine. The lead author was Dr. Guillermo E. Umpierrez, professor of medicine at Emory University, and chief of diabetes and endocrinology at Grady Memorial Hospital, both in Atlanta.
The guideline has eight sections, all focused on the noncritical hospital setting: diagnosis and recognition of hyperglycemia and diabetes, monitoring glycemia, glycemic targets, management of hyperglycemia, special situations, recognition and management of hypoglycemia, implementation of a glycemic control program, and patient and professional education.
The panel’s advice was characterized as "recommended" for items with strong evidence and "suggested" for items with less evidence. In the first of the guideline’s eight sections, the panel recommended all patients be assessed on admission for a history of diabetes and suggested laboratory blood glucose testing on admission for all patients, regardless of prior diagnosis of diabetes.
"There’s abundant data to show that a very large number of people … [are admitted] with undiagnosed diabetes and people also develop stress hyperglycemia" and both conditions affect patient outcomes, Dr. Richard Hellman said in an interview. Dr. Hellman is a coauthor of the guidelines and an endocrinologist who is a clinical professor of medicine at the University of Missouri–Kansas City.
<[stk -3]>While the accuracy of point-of-care testing is not optimal, the panel recommended bedside glucose testing of capillary blood because of the need to time glucose measures to the patient’s nutritional intake and medication regimens. Personal glucose meters should not be used, and continuous glucose monitors while "promising," have not been adequately tested in acute care and therefore can’t be recommended for hospital use at this time, Dr. Umpierrez and his associates wrote.<[etk]>
As in the 2009 guideline that addressed critical care patients, the glycemic targets are less than 140 mg/dL premeal and less than 180 mg/dL random for the majority of hospitalized patients with noncritical illness. Lower targets might be considered among patients who are able to achieve them without hypoglycemia, while higher targets might be appropriate for those at high risk for hypoglycemia and those with a limited life expectancy.
Medical nutrition therapy is recommended as a component of the glycemic management program for all hospitalized patients with diabetes and hyperglycemia. Meals with consistent amounts of carbohydrate are suggested to help coordinate dosing of rapid-acting insulin.
Insulin therapy is the preferred method for achieving glycemic control in all hospitalized patients with diabetes and hyperglycemia, the panel said. At admission, they suggested, oral hypoglycemic agents should be discontinued and insulin therapy should be initiated in acutely ill patients with type 2 diabetes. Oral agents are contraindicated in hospitalized patients with decompensated heart failure, renal insufficiency, hypoperfusion, or chronic pulmonary disease, and in any patient given intravenous contrast dye, the authors noted.
<[stk -3]>For patients who are eating, the panel recommended scheduled subcutaneous basal or intermediate-acting insulin once or twice daily in combination with rapid- or short-acting insulin administered before meals. <[etk]>
Prolonged use of sliding-scale therapy should be avoided as the sole method for glycemic control, the panel wrote.
"There’s abundant data to show that a very large number of people [are admitted] with undiagnosed diabetes and people also develop stress hyperglycemia."
<[stk -3]>Two recent studies led by Dr. Umpierrez show basal-bolus insulin regimens to be superior to sliding scale insulin treatment. One of those studies was done in noncritically ill hospitalized patients with type 2 diabetes (Diabetes Care 2007;30:2181-6), and the other was done in type 2 patients undergoing general surgery (Diabetes Care 2011;34:256-61). <[etk]>
Diabetes self-management education is recommended for patients, including both short-term "survival skills" education in the hospital and referral to community sources for ongoing patient education following discharge.
At discharge, the patient’s preadmission regimen – either insulin or oral and noninsulin injectable antidiabetic drugs – can be reinstituted so long as the patient’s preadmission glycemic control was good and there are no contraindications. To assess safety and efficacy, insulin administration should be initiated at least 1 day before discharge. Patients and their caregivers should receive oral and written instructions for home glycemic management.
The guidelines also address transition from intravenous to subcutaneous insulin therapy, glycemic management of patients who are receiving enteral or parenteral nutrition, perioperative blood glucose control, and management of glucocorticoid-induced diabetes.
<[stk -3]>The panel recommended the development of protocols with specific directions for avoiding and managing hypoglycemia as well as the implementation of hospital-wide, nurse-initiated hypoglycemia treatment protocols and a system for tracking with root cause analysis the frequency of hypoglycemic events. The document lists key components of such protocols, and provides suggested nurse-initiated strategies.<[etk]>
<[stk -3]>Hospitals are advised to provide administrative support for an interdisciplinary steering committee targeting a systems approach to improve care of inpatients with hyperglycemia and diabetes. Uniform methods for collecting and evaluating point-of-care testing data and insulin use information in hospitals are recommended, as are the provision of accurate devices for glucose measurement at the bedside with ongoing staff education and competency assessments.
Dr. Hellman and Dr. Umpierrez have no financial disclosures, but three other members of the guideline panel declared relationships with manufacturers of diabetes-related products.
All patients admitted to the hospital in noncritical care settings should have their blood glucose tested, according to a new clinical practice guideline from the Endocrine Society.
Unlike previous guidelines based largely on data from intensive care and critical care settings, the new guideline focuses on glucose management in noncritical settings, with special emphasis on systemic issues such as patient transition between hospital units and from inpatient to outpatient settings. The guidelines also include detailed guidance for creating systems and protocols to ensure optimal patient management and safety (Diabetes Care [2009;32:1119-31]).
"Management of Hyperglycemia in Hospitalized Patients in Non-Critical Care Setting: An Endocrine Society Clinical Practice Guideline" was developed by an eight-member panel with representatives from the American Diabetes Association, American Heart Association, American Association of Diabetes Educators, European Society of Endocrinology, and the Society of Hospital Medicine. The lead author was Dr. Guillermo E. Umpierrez, professor of medicine at Emory University, and chief of diabetes and endocrinology at Grady Memorial Hospital, both in Atlanta.
The guideline has eight sections, all focused on the noncritical hospital setting: diagnosis and recognition of hyperglycemia and diabetes, monitoring glycemia, glycemic targets, management of hyperglycemia, special situations, recognition and management of hypoglycemia, implementation of a glycemic control program, and patient and professional education.
The panel’s advice was characterized as "recommended" for items with strong evidence and "suggested" for items with less evidence. In the first of the guideline’s eight sections, the panel recommended all patients be assessed on admission for a history of diabetes and suggested laboratory blood glucose testing on admission for all patients, regardless of prior diagnosis of diabetes.
"There’s abundant data to show that a very large number of people … [are admitted] with undiagnosed diabetes and people also develop stress hyperglycemia" and both conditions affect patient outcomes, Dr. Richard Hellman said in an interview. Dr. Hellman is a coauthor of the guidelines and an endocrinologist who is a clinical professor of medicine at the University of Missouri–Kansas City.
<[stk -3]>While the accuracy of point-of-care testing is not optimal, the panel recommended bedside glucose testing of capillary blood because of the need to time glucose measures to the patient’s nutritional intake and medication regimens. Personal glucose meters should not be used, and continuous glucose monitors while "promising," have not been adequately tested in acute care and therefore can’t be recommended for hospital use at this time, Dr. Umpierrez and his associates wrote.<[etk]>
As in the 2009 guideline that addressed critical care patients, the glycemic targets are less than 140 mg/dL premeal and less than 180 mg/dL random for the majority of hospitalized patients with noncritical illness. Lower targets might be considered among patients who are able to achieve them without hypoglycemia, while higher targets might be appropriate for those at high risk for hypoglycemia and those with a limited life expectancy.
Medical nutrition therapy is recommended as a component of the glycemic management program for all hospitalized patients with diabetes and hyperglycemia. Meals with consistent amounts of carbohydrate are suggested to help coordinate dosing of rapid-acting insulin.
Insulin therapy is the preferred method for achieving glycemic control in all hospitalized patients with diabetes and hyperglycemia, the panel said. At admission, they suggested, oral hypoglycemic agents should be discontinued and insulin therapy should be initiated in acutely ill patients with type 2 diabetes. Oral agents are contraindicated in hospitalized patients with decompensated heart failure, renal insufficiency, hypoperfusion, or chronic pulmonary disease, and in any patient given intravenous contrast dye, the authors noted.
<[stk -3]>For patients who are eating, the panel recommended scheduled subcutaneous basal or intermediate-acting insulin once or twice daily in combination with rapid- or short-acting insulin administered before meals. <[etk]>
Prolonged use of sliding-scale therapy should be avoided as the sole method for glycemic control, the panel wrote.
"There’s abundant data to show that a very large number of people [are admitted] with undiagnosed diabetes and people also develop stress hyperglycemia."
<[stk -3]>Two recent studies led by Dr. Umpierrez show basal-bolus insulin regimens to be superior to sliding scale insulin treatment. One of those studies was done in noncritically ill hospitalized patients with type 2 diabetes (Diabetes Care 2007;30:2181-6), and the other was done in type 2 patients undergoing general surgery (Diabetes Care 2011;34:256-61). <[etk]>
Diabetes self-management education is recommended for patients, including both short-term "survival skills" education in the hospital and referral to community sources for ongoing patient education following discharge.
At discharge, the patient’s preadmission regimen – either insulin or oral and noninsulin injectable antidiabetic drugs – can be reinstituted so long as the patient’s preadmission glycemic control was good and there are no contraindications. To assess safety and efficacy, insulin administration should be initiated at least 1 day before discharge. Patients and their caregivers should receive oral and written instructions for home glycemic management.
The guidelines also address transition from intravenous to subcutaneous insulin therapy, glycemic management of patients who are receiving enteral or parenteral nutrition, perioperative blood glucose control, and management of glucocorticoid-induced diabetes.
<[stk -3]>The panel recommended the development of protocols with specific directions for avoiding and managing hypoglycemia as well as the implementation of hospital-wide, nurse-initiated hypoglycemia treatment protocols and a system for tracking with root cause analysis the frequency of hypoglycemic events. The document lists key components of such protocols, and provides suggested nurse-initiated strategies.<[etk]>
<[stk -3]>Hospitals are advised to provide administrative support for an interdisciplinary steering committee targeting a systems approach to improve care of inpatients with hyperglycemia and diabetes. Uniform methods for collecting and evaluating point-of-care testing data and insulin use information in hospitals are recommended, as are the provision of accurate devices for glucose measurement at the bedside with ongoing staff education and competency assessments.
Dr. Hellman and Dr. Umpierrez have no financial disclosures, but three other members of the guideline panel declared relationships with manufacturers of diabetes-related products.
All patients admitted to the hospital in noncritical care settings should have their blood glucose tested, according to a new clinical practice guideline from the Endocrine Society.
Unlike previous guidelines based largely on data from intensive care and critical care settings, the new guideline focuses on glucose management in noncritical settings, with special emphasis on systemic issues such as patient transition between hospital units and from inpatient to outpatient settings. The guidelines also include detailed guidance for creating systems and protocols to ensure optimal patient management and safety (Diabetes Care [2009;32:1119-31]).
"Management of Hyperglycemia in Hospitalized Patients in Non-Critical Care Setting: An Endocrine Society Clinical Practice Guideline" was developed by an eight-member panel with representatives from the American Diabetes Association, American Heart Association, American Association of Diabetes Educators, European Society of Endocrinology, and the Society of Hospital Medicine. The lead author was Dr. Guillermo E. Umpierrez, professor of medicine at Emory University, and chief of diabetes and endocrinology at Grady Memorial Hospital, both in Atlanta.
The guideline has eight sections, all focused on the noncritical hospital setting: diagnosis and recognition of hyperglycemia and diabetes, monitoring glycemia, glycemic targets, management of hyperglycemia, special situations, recognition and management of hypoglycemia, implementation of a glycemic control program, and patient and professional education.
The panel’s advice was characterized as "recommended" for items with strong evidence and "suggested" for items with less evidence. In the first of the guideline’s eight sections, the panel recommended all patients be assessed on admission for a history of diabetes and suggested laboratory blood glucose testing on admission for all patients, regardless of prior diagnosis of diabetes.
"There’s abundant data to show that a very large number of people … [are admitted] with undiagnosed diabetes and people also develop stress hyperglycemia" and both conditions affect patient outcomes, Dr. Richard Hellman said in an interview. Dr. Hellman is a coauthor of the guidelines and an endocrinologist who is a clinical professor of medicine at the University of Missouri–Kansas City.
<[stk -3]>While the accuracy of point-of-care testing is not optimal, the panel recommended bedside glucose testing of capillary blood because of the need to time glucose measures to the patient’s nutritional intake and medication regimens. Personal glucose meters should not be used, and continuous glucose monitors while "promising," have not been adequately tested in acute care and therefore can’t be recommended for hospital use at this time, Dr. Umpierrez and his associates wrote.<[etk]>
As in the 2009 guideline that addressed critical care patients, the glycemic targets are less than 140 mg/dL premeal and less than 180 mg/dL random for the majority of hospitalized patients with noncritical illness. Lower targets might be considered among patients who are able to achieve them without hypoglycemia, while higher targets might be appropriate for those at high risk for hypoglycemia and those with a limited life expectancy.
Medical nutrition therapy is recommended as a component of the glycemic management program for all hospitalized patients with diabetes and hyperglycemia. Meals with consistent amounts of carbohydrate are suggested to help coordinate dosing of rapid-acting insulin.
Insulin therapy is the preferred method for achieving glycemic control in all hospitalized patients with diabetes and hyperglycemia, the panel said. At admission, they suggested, oral hypoglycemic agents should be discontinued and insulin therapy should be initiated in acutely ill patients with type 2 diabetes. Oral agents are contraindicated in hospitalized patients with decompensated heart failure, renal insufficiency, hypoperfusion, or chronic pulmonary disease, and in any patient given intravenous contrast dye, the authors noted.
<[stk -3]>For patients who are eating, the panel recommended scheduled subcutaneous basal or intermediate-acting insulin once or twice daily in combination with rapid- or short-acting insulin administered before meals. <[etk]>
Prolonged use of sliding-scale therapy should be avoided as the sole method for glycemic control, the panel wrote.
"There’s abundant data to show that a very large number of people [are admitted] with undiagnosed diabetes and people also develop stress hyperglycemia."
<[stk -3]>Two recent studies led by Dr. Umpierrez show basal-bolus insulin regimens to be superior to sliding scale insulin treatment. One of those studies was done in noncritically ill hospitalized patients with type 2 diabetes (Diabetes Care 2007;30:2181-6), and the other was done in type 2 patients undergoing general surgery (Diabetes Care 2011;34:256-61). <[etk]>
Diabetes self-management education is recommended for patients, including both short-term "survival skills" education in the hospital and referral to community sources for ongoing patient education following discharge.
At discharge, the patient’s preadmission regimen – either insulin or oral and noninsulin injectable antidiabetic drugs – can be reinstituted so long as the patient’s preadmission glycemic control was good and there are no contraindications. To assess safety and efficacy, insulin administration should be initiated at least 1 day before discharge. Patients and their caregivers should receive oral and written instructions for home glycemic management.
The guidelines also address transition from intravenous to subcutaneous insulin therapy, glycemic management of patients who are receiving enteral or parenteral nutrition, perioperative blood glucose control, and management of glucocorticoid-induced diabetes.
<[stk -3]>The panel recommended the development of protocols with specific directions for avoiding and managing hypoglycemia as well as the implementation of hospital-wide, nurse-initiated hypoglycemia treatment protocols and a system for tracking with root cause analysis the frequency of hypoglycemic events. The document lists key components of such protocols, and provides suggested nurse-initiated strategies.<[etk]>
<[stk -3]>Hospitals are advised to provide administrative support for an interdisciplinary steering committee targeting a systems approach to improve care of inpatients with hyperglycemia and diabetes. Uniform methods for collecting and evaluating point-of-care testing data and insulin use information in hospitals are recommended, as are the provision of accurate devices for glucose measurement at the bedside with ongoing staff education and competency assessments.
Dr. Hellman and Dr. Umpierrez have no financial disclosures, but three other members of the guideline panel declared relationships with manufacturers of diabetes-related products.