User login
Cardiovascular disease is implicated in link between air pollution and dementia
Virtually all of the association between air pollution and dementia seemed to occur through the presence or the development of cardiovascular disease, which suggests a need to optimize treatment of concurrent cardiovascular disease and risk-factor control in older adults at higher risk for dementia and living in polluted urban areas, said lead author Giulia Grande, MD, a researcher at the Aging Research Center, Karolinska Institutet and Stockholm University, in Solna, Sweden.
In the longitudinal, population-based cohort study, investigators studied 2,927 randomly selected residents in a district of Stockholm who were aged 60 years or older (mean, 74.1 years), lived at home or in institutions, and were free of dementia at baseline (March 2001 through August 2004).
The investigators assessed the participants’ exposure to two major air pollutants – particulate matter ≤2.5 mcm and nitrogen oxide – yearly starting in 1990, from outdoor levels at their residential addresses. Both pollutants are generated by road traffic, among other sources.
Results reported in JAMA Neurology showed that, with a mean follow-up of 6.01 years, 12.4% of the older adults received a dementia diagnosis.
Dementia risk increased with the level of air pollutants at their residential address in the past, with strongest associations seen for exposure in the preceding 5 years: The hazard ratio (HR) for dementia was 1.54 for an interquartile range difference of 0.88 mcg/m3 in particulate matter ≤2.5 mcm and 1.14 for an interquartile range difference of 8.35 mcg/m3 in nitrogen oxide during that time period.
Of note, the study cohort lived in an area having “comparatively good ambient air quality” in which restrictions on air pollution have increased in recent decades, Dr. Grande and coinvestigators noted. “Interestingly, the higher limit reported herein is not only below the current European limit for fine particulate matter but also below the US standard. In other words, we were able to establish harmful effects at levels below current standards,” they wrote.
In analyses of effect modification, the elevation of risk related to particulate matter ≤2.5 mcm exposure and nitrogen oxide exposure was significantly greater among older adults who had heart failure (HRs, 1.93 and 1.43, respectively). Risk was marginally greater among those with ischemic heart disease (HRs, 1.67 and 1.36, respectively).
Analyses of potential mediators showed that preceding stroke accounted for the largest share of all dementia cases related to particulate matter ≤2.5 mcm exposure, at 49.4%.
The stronger association for exposure in the past 5 years is noteworthy for the big picture, they added. “From a policy point of view, this result is encouraging because it might imply that reducing air pollutant levels today could yield better outcomes already in the shorter term, reinforcing the need for appropriately set air quality standards,” they said.
Dr. Grande disclosed no relevant conflicts of interest. The study was funded by the Swedish National Study on Aging and Care in Kungsholmen (SNAC-K); the Swedish Ministry of Health and Social Affairs; the participating County Councils and Municipalities; the Swedish Research Council; funding for doctoral education from the Karolinska Institutet; and the Swedish Research Council for Health, Working Life and Welfare.
SOURCE: Grande G et al. JAMA Neurol. 2020. doi:10.1001/jamaneurol.2019.4914.
Virtually all of the association between air pollution and dementia seemed to occur through the presence or the development of cardiovascular disease, which suggests a need to optimize treatment of concurrent cardiovascular disease and risk-factor control in older adults at higher risk for dementia and living in polluted urban areas, said lead author Giulia Grande, MD, a researcher at the Aging Research Center, Karolinska Institutet and Stockholm University, in Solna, Sweden.
In the longitudinal, population-based cohort study, investigators studied 2,927 randomly selected residents in a district of Stockholm who were aged 60 years or older (mean, 74.1 years), lived at home or in institutions, and were free of dementia at baseline (March 2001 through August 2004).
The investigators assessed the participants’ exposure to two major air pollutants – particulate matter ≤2.5 mcm and nitrogen oxide – yearly starting in 1990, from outdoor levels at their residential addresses. Both pollutants are generated by road traffic, among other sources.
Results reported in JAMA Neurology showed that, with a mean follow-up of 6.01 years, 12.4% of the older adults received a dementia diagnosis.
Dementia risk increased with the level of air pollutants at their residential address in the past, with strongest associations seen for exposure in the preceding 5 years: The hazard ratio (HR) for dementia was 1.54 for an interquartile range difference of 0.88 mcg/m3 in particulate matter ≤2.5 mcm and 1.14 for an interquartile range difference of 8.35 mcg/m3 in nitrogen oxide during that time period.
Of note, the study cohort lived in an area having “comparatively good ambient air quality” in which restrictions on air pollution have increased in recent decades, Dr. Grande and coinvestigators noted. “Interestingly, the higher limit reported herein is not only below the current European limit for fine particulate matter but also below the US standard. In other words, we were able to establish harmful effects at levels below current standards,” they wrote.
In analyses of effect modification, the elevation of risk related to particulate matter ≤2.5 mcm exposure and nitrogen oxide exposure was significantly greater among older adults who had heart failure (HRs, 1.93 and 1.43, respectively). Risk was marginally greater among those with ischemic heart disease (HRs, 1.67 and 1.36, respectively).
Analyses of potential mediators showed that preceding stroke accounted for the largest share of all dementia cases related to particulate matter ≤2.5 mcm exposure, at 49.4%.
The stronger association for exposure in the past 5 years is noteworthy for the big picture, they added. “From a policy point of view, this result is encouraging because it might imply that reducing air pollutant levels today could yield better outcomes already in the shorter term, reinforcing the need for appropriately set air quality standards,” they said.
Dr. Grande disclosed no relevant conflicts of interest. The study was funded by the Swedish National Study on Aging and Care in Kungsholmen (SNAC-K); the Swedish Ministry of Health and Social Affairs; the participating County Councils and Municipalities; the Swedish Research Council; funding for doctoral education from the Karolinska Institutet; and the Swedish Research Council for Health, Working Life and Welfare.
SOURCE: Grande G et al. JAMA Neurol. 2020. doi:10.1001/jamaneurol.2019.4914.
Virtually all of the association between air pollution and dementia seemed to occur through the presence or the development of cardiovascular disease, which suggests a need to optimize treatment of concurrent cardiovascular disease and risk-factor control in older adults at higher risk for dementia and living in polluted urban areas, said lead author Giulia Grande, MD, a researcher at the Aging Research Center, Karolinska Institutet and Stockholm University, in Solna, Sweden.
In the longitudinal, population-based cohort study, investigators studied 2,927 randomly selected residents in a district of Stockholm who were aged 60 years or older (mean, 74.1 years), lived at home or in institutions, and were free of dementia at baseline (March 2001 through August 2004).
The investigators assessed the participants’ exposure to two major air pollutants – particulate matter ≤2.5 mcm and nitrogen oxide – yearly starting in 1990, from outdoor levels at their residential addresses. Both pollutants are generated by road traffic, among other sources.
Results reported in JAMA Neurology showed that, with a mean follow-up of 6.01 years, 12.4% of the older adults received a dementia diagnosis.
Dementia risk increased with the level of air pollutants at their residential address in the past, with strongest associations seen for exposure in the preceding 5 years: The hazard ratio (HR) for dementia was 1.54 for an interquartile range difference of 0.88 mcg/m3 in particulate matter ≤2.5 mcm and 1.14 for an interquartile range difference of 8.35 mcg/m3 in nitrogen oxide during that time period.
Of note, the study cohort lived in an area having “comparatively good ambient air quality” in which restrictions on air pollution have increased in recent decades, Dr. Grande and coinvestigators noted. “Interestingly, the higher limit reported herein is not only below the current European limit for fine particulate matter but also below the US standard. In other words, we were able to establish harmful effects at levels below current standards,” they wrote.
In analyses of effect modification, the elevation of risk related to particulate matter ≤2.5 mcm exposure and nitrogen oxide exposure was significantly greater among older adults who had heart failure (HRs, 1.93 and 1.43, respectively). Risk was marginally greater among those with ischemic heart disease (HRs, 1.67 and 1.36, respectively).
Analyses of potential mediators showed that preceding stroke accounted for the largest share of all dementia cases related to particulate matter ≤2.5 mcm exposure, at 49.4%.
The stronger association for exposure in the past 5 years is noteworthy for the big picture, they added. “From a policy point of view, this result is encouraging because it might imply that reducing air pollutant levels today could yield better outcomes already in the shorter term, reinforcing the need for appropriately set air quality standards,” they said.
Dr. Grande disclosed no relevant conflicts of interest. The study was funded by the Swedish National Study on Aging and Care in Kungsholmen (SNAC-K); the Swedish Ministry of Health and Social Affairs; the participating County Councils and Municipalities; the Swedish Research Council; funding for doctoral education from the Karolinska Institutet; and the Swedish Research Council for Health, Working Life and Welfare.
SOURCE: Grande G et al. JAMA Neurol. 2020. doi:10.1001/jamaneurol.2019.4914.
FROM JAMA NEUROLOGY
The power and promise of person-generated health data (Part II)
In Part I of our discussion we introduced the concept of person-generated health data (PGHD), defined as wellness and/or health-related data created, recorded, or gathered by individuals.
Such rich, longitudinal information is now being used in combination with traditional clinical information to predict, diagnose, and formulate treatment plans for diseases, as well as understand the safety and effectiveness of medical interventions.
Identifying a disease early
One novel example of digital technologies being used for early identification of disease was a promising 2019 study by Eli Lilly (in collaboration with Apple and Evidation Health) called the Lilly Exploratory Digital Assessment Study.
In this study, the feasibility of using PGHD for identifying physiological and behavioral signatures of cognitive impairment was examined for the purpose of seeking new methods to detect mild cognitive impairment (MCI) in a timely and cost-effective manner. The study enrolled 31 study participants with cognitive impairment and 82 without cognitive impairment. It used consumer-grade sensor technologies (the iPhone, Apple Watch, iPad, and Beddit sleep monitor) to continuously and unobtrusively collect data. Among the information the researchers collected were interaction with the phone keyboard, accelerometer data from the Apple Watch, volume of messages sent/received, and sleep cycles.1
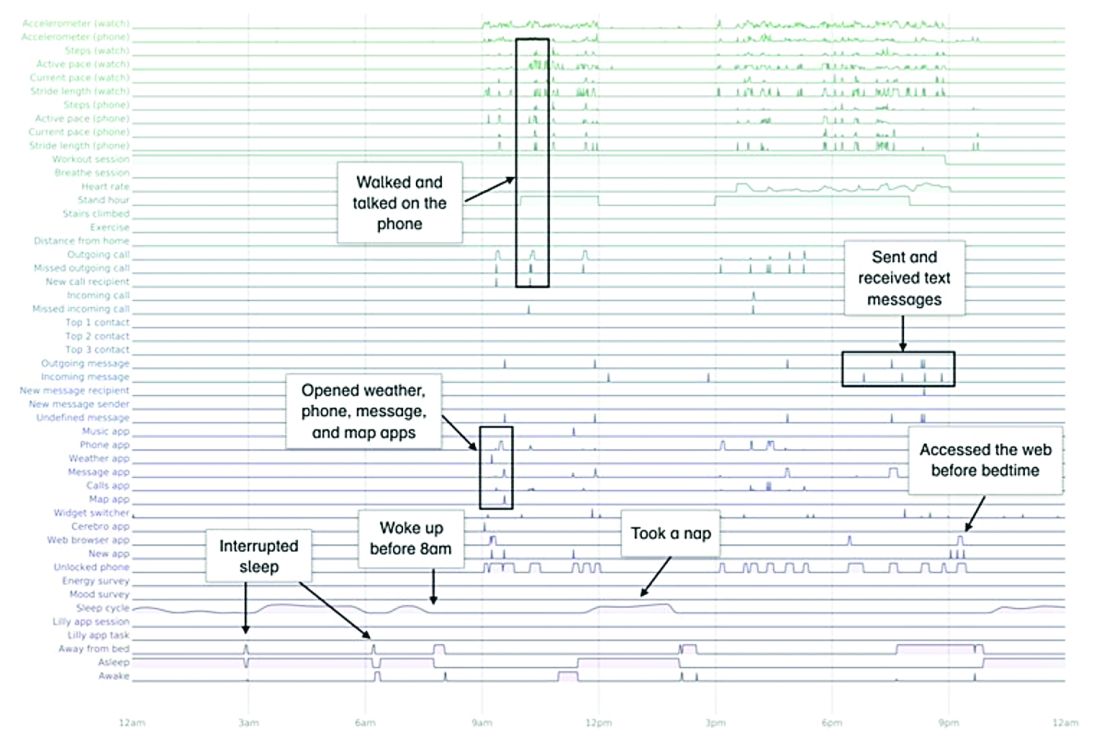
A total of 16 terabytes of data were collected over the course of 12 weeks. Data were organized into a behaviorgram (See Figure 1) that gives a holistic picture of a day in a patient’s life. A machine learning model was used to distinguish between behaviorgrams of symptomatic versus healthy controls, identifying typing speed, circadian rhythm shifts, and reliance on helper apps, among other things, as differentiating cognitively impaired from healthy controls. These behaviorgrams may someday serve as “fingerprints” of different diseases, with specific diseases displaying predictable patterns. In the near future, digital measures like the ones investigated in this study are likely to be used to help clinicians predict and diagnose disease, as well as to better understand disease progression and treatment response.
Leading to better health outcomes
The potential of PGHD to detect diseases early and lead to better health outcomes is being investigated in the Heartline study, a collaboration between Johnson & Johnson and Apple, which is supported by Evidation.2
This study aims to enroll 150,000 adults age 65 years and over to analyze the impact of Apple Watch–based early detection of irregular heart rhythms consistent with atrial fibrillation (AFib). The researchers’ hypothesis is that jointly detecting atrial fibrillation early and providing cardiovascular health programs to new AFib patients, will lead to patients being treated by a medical provider for AFib that otherwise would not have been detected. This, in turn, would lead to these AFib patients decreasing their risks of stroke and other serious cardiovascular events, including death, the study authors speculated.
Presenting new challenges
While PGHD has the potential to help people, it also presents new challenges. It is highly sensitive and personal – it can be as identifying as DNA.3
The vast amount of data that PGHD can collect from interaction with consumer wearable devices poses serious privacy risks if done improperly. To address those risks, companies like Evidation have built in protections. Evidation has an app, Achievement, that has enlisted a connected population of more than 3.5 million members who earn rewards for performing health-related actions, as tracked by wearables devices and apps. Through the Achievement app (See Figure 2.), members are provided opportunities to join research studies. As part of these studies, data collected from sensors and apps is used by permission of the member so that it is clear how their data are contributing to specific research questions or use cases.
This is a collaborative model of data collection built upon trust and permission and is substantially different than the collection of data from electronic health records (EHRs) – which is typically aggregated, deidentified, and commercialized, often without the patients’ knowledge or consent. Stringent protections, explicit permission, and transparency are absolutely imperative until privacy frameworks for data outside of HIPAA regulation catches up and protects patients from discrimination and unintended uses of their data.
Large connected cohorts can help advance our understanding of public health. In one study run on Achievement during the 2017-2018 flu season, a survey was sent to the Achievement population every week asking about symptoms of influenza-like illness and requesting permission to access historical data from their wearable around the influenza-like illness event.4 With the data, it was possible to analyze patterns of activity, sleep, and resting heart rate change around flu events. Resting heart rate, in particular, is shown to increase during fever and at the population level. In fact, through the use of PGHD, it is possible to use the fraction of people with resting heart rate above their usual baseline as a proxy to quantify the number of infected people in a region.5 This resting heart rate–informed flu surveillance method, if refined to increased accuracy, can work in near real time. This means it may be able detect influenza outbreaks days earlier than current epidemiological methods.
Health data generated by connected populations are in the early stages of development. It is clear that it will yield novel insights into health and disease. Only time will tell if it will be able to help clinicians and patients better predict, diagnose, and formulate treatment plans for disease.
Neil Skolnik, M.D. is a professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, and associate director of the Family Medicine Residency Program at Abington Jefferson Health. Luca Foschini PhD, is co-founder & chief data scientist at Evidation Health. Bray Patrick-Lake, MFS, is a patient thought leader and director of strategic partnerships at Evidation Health.
References
1. Chen R et al. Developing measures of cognitive impairment in the real world from consumer-grade multimodal sensor streams. KDD ’19. August 4–8, 2019 Aug 4-8.
2. The Heartline Study. https://www.heartline.com.
3. Foschini L. Privacy of Wearable and Sensors Data (or, the Lack Thereof?). Data Driven Investor, Medium. 2019.
4. Bradshaw B et al. Influenza surveillance using wearable mobile health devices. Online J Public Health Inform. 2019;11(1):e249.
5. Radin JM et al. Harnessing wearable device data to improve state-level real-time surveillance of influenza-like illness in the USA: a population-based study. Lancet Digital Health. 2020. doi: 10.1016/S2589-7500(19)30222-5.
In Part I of our discussion we introduced the concept of person-generated health data (PGHD), defined as wellness and/or health-related data created, recorded, or gathered by individuals.
Such rich, longitudinal information is now being used in combination with traditional clinical information to predict, diagnose, and formulate treatment plans for diseases, as well as understand the safety and effectiveness of medical interventions.
Identifying a disease early
One novel example of digital technologies being used for early identification of disease was a promising 2019 study by Eli Lilly (in collaboration with Apple and Evidation Health) called the Lilly Exploratory Digital Assessment Study.
In this study, the feasibility of using PGHD for identifying physiological and behavioral signatures of cognitive impairment was examined for the purpose of seeking new methods to detect mild cognitive impairment (MCI) in a timely and cost-effective manner. The study enrolled 31 study participants with cognitive impairment and 82 without cognitive impairment. It used consumer-grade sensor technologies (the iPhone, Apple Watch, iPad, and Beddit sleep monitor) to continuously and unobtrusively collect data. Among the information the researchers collected were interaction with the phone keyboard, accelerometer data from the Apple Watch, volume of messages sent/received, and sleep cycles.1

A total of 16 terabytes of data were collected over the course of 12 weeks. Data were organized into a behaviorgram (See Figure 1) that gives a holistic picture of a day in a patient’s life. A machine learning model was used to distinguish between behaviorgrams of symptomatic versus healthy controls, identifying typing speed, circadian rhythm shifts, and reliance on helper apps, among other things, as differentiating cognitively impaired from healthy controls. These behaviorgrams may someday serve as “fingerprints” of different diseases, with specific diseases displaying predictable patterns. In the near future, digital measures like the ones investigated in this study are likely to be used to help clinicians predict and diagnose disease, as well as to better understand disease progression and treatment response.
Leading to better health outcomes
The potential of PGHD to detect diseases early and lead to better health outcomes is being investigated in the Heartline study, a collaboration between Johnson & Johnson and Apple, which is supported by Evidation.2
This study aims to enroll 150,000 adults age 65 years and over to analyze the impact of Apple Watch–based early detection of irregular heart rhythms consistent with atrial fibrillation (AFib). The researchers’ hypothesis is that jointly detecting atrial fibrillation early and providing cardiovascular health programs to new AFib patients, will lead to patients being treated by a medical provider for AFib that otherwise would not have been detected. This, in turn, would lead to these AFib patients decreasing their risks of stroke and other serious cardiovascular events, including death, the study authors speculated.
Presenting new challenges
While PGHD has the potential to help people, it also presents new challenges. It is highly sensitive and personal – it can be as identifying as DNA.3
The vast amount of data that PGHD can collect from interaction with consumer wearable devices poses serious privacy risks if done improperly. To address those risks, companies like Evidation have built in protections. Evidation has an app, Achievement, that has enlisted a connected population of more than 3.5 million members who earn rewards for performing health-related actions, as tracked by wearables devices and apps. Through the Achievement app (See Figure 2.), members are provided opportunities to join research studies. As part of these studies, data collected from sensors and apps is used by permission of the member so that it is clear how their data are contributing to specific research questions or use cases.
This is a collaborative model of data collection built upon trust and permission and is substantially different than the collection of data from electronic health records (EHRs) – which is typically aggregated, deidentified, and commercialized, often without the patients’ knowledge or consent. Stringent protections, explicit permission, and transparency are absolutely imperative until privacy frameworks for data outside of HIPAA regulation catches up and protects patients from discrimination and unintended uses of their data.
Large connected cohorts can help advance our understanding of public health. In one study run on Achievement during the 2017-2018 flu season, a survey was sent to the Achievement population every week asking about symptoms of influenza-like illness and requesting permission to access historical data from their wearable around the influenza-like illness event.4 With the data, it was possible to analyze patterns of activity, sleep, and resting heart rate change around flu events. Resting heart rate, in particular, is shown to increase during fever and at the population level. In fact, through the use of PGHD, it is possible to use the fraction of people with resting heart rate above their usual baseline as a proxy to quantify the number of infected people in a region.5 This resting heart rate–informed flu surveillance method, if refined to increased accuracy, can work in near real time. This means it may be able detect influenza outbreaks days earlier than current epidemiological methods.
Health data generated by connected populations are in the early stages of development. It is clear that it will yield novel insights into health and disease. Only time will tell if it will be able to help clinicians and patients better predict, diagnose, and formulate treatment plans for disease.
Neil Skolnik, M.D. is a professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, and associate director of the Family Medicine Residency Program at Abington Jefferson Health. Luca Foschini PhD, is co-founder & chief data scientist at Evidation Health. Bray Patrick-Lake, MFS, is a patient thought leader and director of strategic partnerships at Evidation Health.
References
1. Chen R et al. Developing measures of cognitive impairment in the real world from consumer-grade multimodal sensor streams. KDD ’19. August 4–8, 2019 Aug 4-8.
2. The Heartline Study. https://www.heartline.com.
3. Foschini L. Privacy of Wearable and Sensors Data (or, the Lack Thereof?). Data Driven Investor, Medium. 2019.
4. Bradshaw B et al. Influenza surveillance using wearable mobile health devices. Online J Public Health Inform. 2019;11(1):e249.
5. Radin JM et al. Harnessing wearable device data to improve state-level real-time surveillance of influenza-like illness in the USA: a population-based study. Lancet Digital Health. 2020. doi: 10.1016/S2589-7500(19)30222-5.
In Part I of our discussion we introduced the concept of person-generated health data (PGHD), defined as wellness and/or health-related data created, recorded, or gathered by individuals.
Such rich, longitudinal information is now being used in combination with traditional clinical information to predict, diagnose, and formulate treatment plans for diseases, as well as understand the safety and effectiveness of medical interventions.
Identifying a disease early
One novel example of digital technologies being used for early identification of disease was a promising 2019 study by Eli Lilly (in collaboration with Apple and Evidation Health) called the Lilly Exploratory Digital Assessment Study.
In this study, the feasibility of using PGHD for identifying physiological and behavioral signatures of cognitive impairment was examined for the purpose of seeking new methods to detect mild cognitive impairment (MCI) in a timely and cost-effective manner. The study enrolled 31 study participants with cognitive impairment and 82 without cognitive impairment. It used consumer-grade sensor technologies (the iPhone, Apple Watch, iPad, and Beddit sleep monitor) to continuously and unobtrusively collect data. Among the information the researchers collected were interaction with the phone keyboard, accelerometer data from the Apple Watch, volume of messages sent/received, and sleep cycles.1

A total of 16 terabytes of data were collected over the course of 12 weeks. Data were organized into a behaviorgram (See Figure 1) that gives a holistic picture of a day in a patient’s life. A machine learning model was used to distinguish between behaviorgrams of symptomatic versus healthy controls, identifying typing speed, circadian rhythm shifts, and reliance on helper apps, among other things, as differentiating cognitively impaired from healthy controls. These behaviorgrams may someday serve as “fingerprints” of different diseases, with specific diseases displaying predictable patterns. In the near future, digital measures like the ones investigated in this study are likely to be used to help clinicians predict and diagnose disease, as well as to better understand disease progression and treatment response.
Leading to better health outcomes
The potential of PGHD to detect diseases early and lead to better health outcomes is being investigated in the Heartline study, a collaboration between Johnson & Johnson and Apple, which is supported by Evidation.2
This study aims to enroll 150,000 adults age 65 years and over to analyze the impact of Apple Watch–based early detection of irregular heart rhythms consistent with atrial fibrillation (AFib). The researchers’ hypothesis is that jointly detecting atrial fibrillation early and providing cardiovascular health programs to new AFib patients, will lead to patients being treated by a medical provider for AFib that otherwise would not have been detected. This, in turn, would lead to these AFib patients decreasing their risks of stroke and other serious cardiovascular events, including death, the study authors speculated.
Presenting new challenges
While PGHD has the potential to help people, it also presents new challenges. It is highly sensitive and personal – it can be as identifying as DNA.3
The vast amount of data that PGHD can collect from interaction with consumer wearable devices poses serious privacy risks if done improperly. To address those risks, companies like Evidation have built in protections. Evidation has an app, Achievement, that has enlisted a connected population of more than 3.5 million members who earn rewards for performing health-related actions, as tracked by wearables devices and apps. Through the Achievement app (See Figure 2.), members are provided opportunities to join research studies. As part of these studies, data collected from sensors and apps is used by permission of the member so that it is clear how their data are contributing to specific research questions or use cases.
This is a collaborative model of data collection built upon trust and permission and is substantially different than the collection of data from electronic health records (EHRs) – which is typically aggregated, deidentified, and commercialized, often without the patients’ knowledge or consent. Stringent protections, explicit permission, and transparency are absolutely imperative until privacy frameworks for data outside of HIPAA regulation catches up and protects patients from discrimination and unintended uses of their data.
Large connected cohorts can help advance our understanding of public health. In one study run on Achievement during the 2017-2018 flu season, a survey was sent to the Achievement population every week asking about symptoms of influenza-like illness and requesting permission to access historical data from their wearable around the influenza-like illness event.4 With the data, it was possible to analyze patterns of activity, sleep, and resting heart rate change around flu events. Resting heart rate, in particular, is shown to increase during fever and at the population level. In fact, through the use of PGHD, it is possible to use the fraction of people with resting heart rate above their usual baseline as a proxy to quantify the number of infected people in a region.5 This resting heart rate–informed flu surveillance method, if refined to increased accuracy, can work in near real time. This means it may be able detect influenza outbreaks days earlier than current epidemiological methods.
Health data generated by connected populations are in the early stages of development. It is clear that it will yield novel insights into health and disease. Only time will tell if it will be able to help clinicians and patients better predict, diagnose, and formulate treatment plans for disease.
Neil Skolnik, M.D. is a professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, and associate director of the Family Medicine Residency Program at Abington Jefferson Health. Luca Foschini PhD, is co-founder & chief data scientist at Evidation Health. Bray Patrick-Lake, MFS, is a patient thought leader and director of strategic partnerships at Evidation Health.
References
1. Chen R et al. Developing measures of cognitive impairment in the real world from consumer-grade multimodal sensor streams. KDD ’19. August 4–8, 2019 Aug 4-8.
2. The Heartline Study. https://www.heartline.com.
3. Foschini L. Privacy of Wearable and Sensors Data (or, the Lack Thereof?). Data Driven Investor, Medium. 2019.
4. Bradshaw B et al. Influenza surveillance using wearable mobile health devices. Online J Public Health Inform. 2019;11(1):e249.
5. Radin JM et al. Harnessing wearable device data to improve state-level real-time surveillance of influenza-like illness in the USA: a population-based study. Lancet Digital Health. 2020. doi: 10.1016/S2589-7500(19)30222-5.
Targeting gut bacteria may improve levodopa uptake
Differences in metabolism of levodopa between patients with Parkinson’s disease may be caused by variations in gut bacteria, according to investigators.
Specifically, patients with a higher abundance of Enterococcus faecalis may be converting levodopa into dopamine via decarboxylation before it can cross the blood-brain barrier, reported Emily P. Balskus, PhD, of Harvard University in Cambridge, Mass.
Although existing decarboxylase inhibitors, such as carbidopa, can reduce metabolism of levodopa by host enzymes, these drugs are unable to inhibit the enzymatic activity of E. faecalis in the gut, Dr. Balskus said at the annual Gut Microbiota for Health World Summit, sponsored by the American Gastroenterological Association and the European Society for Neurogastroenterology and Motility.
“[Carbidopa] is actually completely ineffective at inhibiting decarboxylation in human fecal suspension,” Dr. Balskus said, referring to research led by PhD student Vayu Maini Rekdal. “We think that this could indicate that patients who are taking carbidopa are not inhibiting any bacterial metabolism that they may have.”
While previous research showed that E. faecalis could decarboxylate levodopa, Dr. Balskus and colleagues linked this process with the tyrosine decarboxylase gene (TyrDC), and showed that the of abundance E. faecalis and TyrDC correlate with levodopa metabolism.
Unlike the human enzyme responsible for decarboxylation of levodopa, the E. faecalis enzyme preferentially binds with L-tyrosine. This could explain why existing decarboxylase inhibitors have little impact on decarboxylation in the gut, Dr. Balskus said.
She also noted that this unique characteristic may open doors to new therapeutics. In the lab, Dr. Balskus and colleagues tested a decarboxylase inhibitor that resembled L-tyrosine, (S)-alpha-fluoromethyltyrosine (AFMT). Indeed, AFMT completely inhibited of decarboxylation of levodopa in both E. faecalis cells and complex human microbiome samples.
“We think this is pretty exciting,” Dr. Balskus said.
Early animal studies support this enthusiasm, as germ-free mice colonized with E. faecalis maintain higher serum levels of levodopa with concurrent administration of AFMT.
“We think that this could indicate that a promising way to improve levodopa therapy for Parkinson’s patients would be to develop compounds that inhibit bacterial drug metabolism activity,” Dr. Balskus said.
Concluding her presentation, Dr. Balskus emphasized the importance of a biochemical approach to microbiome research. “Studying enzymes opens up new, exciting opportunities for microbiome manipulation. We can design or develop inhibitors of enzymes, use those inhibitors as tools to study the roles of individual metabolic activities, and potentially use them as therapeutics. We are very excited about the possibility of treating or preventing human disease not just by manipulating processes in our own cells, but by targeting activities in the microbiota.”
Dr. Balskus reported funding from HHMI, the Bill and Melinda Gates Foundation, the David and Lucile Packard Foundation, and Merck.
Differences in metabolism of levodopa between patients with Parkinson’s disease may be caused by variations in gut bacteria, according to investigators.
Specifically, patients with a higher abundance of Enterococcus faecalis may be converting levodopa into dopamine via decarboxylation before it can cross the blood-brain barrier, reported Emily P. Balskus, PhD, of Harvard University in Cambridge, Mass.
Although existing decarboxylase inhibitors, such as carbidopa, can reduce metabolism of levodopa by host enzymes, these drugs are unable to inhibit the enzymatic activity of E. faecalis in the gut, Dr. Balskus said at the annual Gut Microbiota for Health World Summit, sponsored by the American Gastroenterological Association and the European Society for Neurogastroenterology and Motility.
“[Carbidopa] is actually completely ineffective at inhibiting decarboxylation in human fecal suspension,” Dr. Balskus said, referring to research led by PhD student Vayu Maini Rekdal. “We think that this could indicate that patients who are taking carbidopa are not inhibiting any bacterial metabolism that they may have.”
While previous research showed that E. faecalis could decarboxylate levodopa, Dr. Balskus and colleagues linked this process with the tyrosine decarboxylase gene (TyrDC), and showed that the of abundance E. faecalis and TyrDC correlate with levodopa metabolism.
Unlike the human enzyme responsible for decarboxylation of levodopa, the E. faecalis enzyme preferentially binds with L-tyrosine. This could explain why existing decarboxylase inhibitors have little impact on decarboxylation in the gut, Dr. Balskus said.
She also noted that this unique characteristic may open doors to new therapeutics. In the lab, Dr. Balskus and colleagues tested a decarboxylase inhibitor that resembled L-tyrosine, (S)-alpha-fluoromethyltyrosine (AFMT). Indeed, AFMT completely inhibited of decarboxylation of levodopa in both E. faecalis cells and complex human microbiome samples.
“We think this is pretty exciting,” Dr. Balskus said.
Early animal studies support this enthusiasm, as germ-free mice colonized with E. faecalis maintain higher serum levels of levodopa with concurrent administration of AFMT.
“We think that this could indicate that a promising way to improve levodopa therapy for Parkinson’s patients would be to develop compounds that inhibit bacterial drug metabolism activity,” Dr. Balskus said.
Concluding her presentation, Dr. Balskus emphasized the importance of a biochemical approach to microbiome research. “Studying enzymes opens up new, exciting opportunities for microbiome manipulation. We can design or develop inhibitors of enzymes, use those inhibitors as tools to study the roles of individual metabolic activities, and potentially use them as therapeutics. We are very excited about the possibility of treating or preventing human disease not just by manipulating processes in our own cells, but by targeting activities in the microbiota.”
Dr. Balskus reported funding from HHMI, the Bill and Melinda Gates Foundation, the David and Lucile Packard Foundation, and Merck.
Differences in metabolism of levodopa between patients with Parkinson’s disease may be caused by variations in gut bacteria, according to investigators.
Specifically, patients with a higher abundance of Enterococcus faecalis may be converting levodopa into dopamine via decarboxylation before it can cross the blood-brain barrier, reported Emily P. Balskus, PhD, of Harvard University in Cambridge, Mass.
Although existing decarboxylase inhibitors, such as carbidopa, can reduce metabolism of levodopa by host enzymes, these drugs are unable to inhibit the enzymatic activity of E. faecalis in the gut, Dr. Balskus said at the annual Gut Microbiota for Health World Summit, sponsored by the American Gastroenterological Association and the European Society for Neurogastroenterology and Motility.
“[Carbidopa] is actually completely ineffective at inhibiting decarboxylation in human fecal suspension,” Dr. Balskus said, referring to research led by PhD student Vayu Maini Rekdal. “We think that this could indicate that patients who are taking carbidopa are not inhibiting any bacterial metabolism that they may have.”
While previous research showed that E. faecalis could decarboxylate levodopa, Dr. Balskus and colleagues linked this process with the tyrosine decarboxylase gene (TyrDC), and showed that the of abundance E. faecalis and TyrDC correlate with levodopa metabolism.
Unlike the human enzyme responsible for decarboxylation of levodopa, the E. faecalis enzyme preferentially binds with L-tyrosine. This could explain why existing decarboxylase inhibitors have little impact on decarboxylation in the gut, Dr. Balskus said.
She also noted that this unique characteristic may open doors to new therapeutics. In the lab, Dr. Balskus and colleagues tested a decarboxylase inhibitor that resembled L-tyrosine, (S)-alpha-fluoromethyltyrosine (AFMT). Indeed, AFMT completely inhibited of decarboxylation of levodopa in both E. faecalis cells and complex human microbiome samples.
“We think this is pretty exciting,” Dr. Balskus said.
Early animal studies support this enthusiasm, as germ-free mice colonized with E. faecalis maintain higher serum levels of levodopa with concurrent administration of AFMT.
“We think that this could indicate that a promising way to improve levodopa therapy for Parkinson’s patients would be to develop compounds that inhibit bacterial drug metabolism activity,” Dr. Balskus said.
Concluding her presentation, Dr. Balskus emphasized the importance of a biochemical approach to microbiome research. “Studying enzymes opens up new, exciting opportunities for microbiome manipulation. We can design or develop inhibitors of enzymes, use those inhibitors as tools to study the roles of individual metabolic activities, and potentially use them as therapeutics. We are very excited about the possibility of treating or preventing human disease not just by manipulating processes in our own cells, but by targeting activities in the microbiota.”
Dr. Balskus reported funding from HHMI, the Bill and Melinda Gates Foundation, the David and Lucile Packard Foundation, and Merck.
FROM GMFH 2020
Elderly Americans carry heavier opioid burden
according to the Agency for Healthcare Quality and Research.
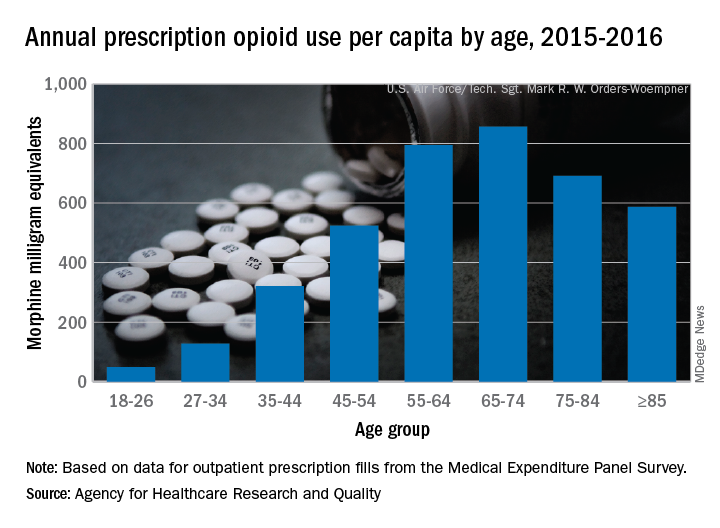
Elderly adults with chronic and acute pain obtained an average of 774 morphine milligram equivalents (MMEs) of prescription opioids annually during 2015-2016 from outpatient clinicians, compared with 376 MMEs a year for nonelderly adults, said Asako S. Moriya, PhD, and G. Edward Miller, PhD, of the AHRQ.
Narrowing the age groups shows that opioid MMEs increased with age, starting at 49 MMEs for 18- to 26-year-olds and rising to a high of 856 MMEs in the 65- to 74-year-old group, before dropping off in the oldest adults, the investigators said in a Medical Expenditure Panel Survey (MEPS) research findings report.
The analysis included “all opioid medications that are commonly used to treat pain” and excluded respiratory agents, antitussives, and drugs used for medication-assisted treatment, they noted. The MEPS data cover prescriptions purchased or obtained in outpatient settings but not those administered in inpatient settings or in clinics or physician offices.
according to the Agency for Healthcare Quality and Research.

Elderly adults with chronic and acute pain obtained an average of 774 morphine milligram equivalents (MMEs) of prescription opioids annually during 2015-2016 from outpatient clinicians, compared with 376 MMEs a year for nonelderly adults, said Asako S. Moriya, PhD, and G. Edward Miller, PhD, of the AHRQ.
Narrowing the age groups shows that opioid MMEs increased with age, starting at 49 MMEs for 18- to 26-year-olds and rising to a high of 856 MMEs in the 65- to 74-year-old group, before dropping off in the oldest adults, the investigators said in a Medical Expenditure Panel Survey (MEPS) research findings report.
The analysis included “all opioid medications that are commonly used to treat pain” and excluded respiratory agents, antitussives, and drugs used for medication-assisted treatment, they noted. The MEPS data cover prescriptions purchased or obtained in outpatient settings but not those administered in inpatient settings or in clinics or physician offices.
according to the Agency for Healthcare Quality and Research.

Elderly adults with chronic and acute pain obtained an average of 774 morphine milligram equivalents (MMEs) of prescription opioids annually during 2015-2016 from outpatient clinicians, compared with 376 MMEs a year for nonelderly adults, said Asako S. Moriya, PhD, and G. Edward Miller, PhD, of the AHRQ.
Narrowing the age groups shows that opioid MMEs increased with age, starting at 49 MMEs for 18- to 26-year-olds and rising to a high of 856 MMEs in the 65- to 74-year-old group, before dropping off in the oldest adults, the investigators said in a Medical Expenditure Panel Survey (MEPS) research findings report.
The analysis included “all opioid medications that are commonly used to treat pain” and excluded respiratory agents, antitussives, and drugs used for medication-assisted treatment, they noted. The MEPS data cover prescriptions purchased or obtained in outpatient settings but not those administered in inpatient settings or in clinics or physician offices.
FDA approves ozanimod for relapsing and secondary progressive forms of MS
The Food and Drug Administration has approved the oral medication ozanimod (Zeposia) for relapsing forms of multiple sclerosis (MS), including clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, according to a release from Bristol-Myers Squibb.
Ozanimod is a sphingosine 1-phosphate (S1P) receptor modulator that binds with high affinity to S1P receptors 1 and 5. It blocks the capacity of lymphocytes to egress from lymph nodes, reducing the number of lymphocytes in peripheral blood. Although its therapeutic mechanism of action in MS is unknown, it may involve the reduction of lymphocyte migration into the central nervous system. A genetic test is not required to start the drug, and no patient observation is required for the first dose, although up-titration of initial doses are required to reach the maintenance dose because a transient decrease in heart rate and atrioventricular conduction delays may occur, according to the company.
The approval is based on a pair of head-to-head studies that compared it with interferon beta-1a (Avonex) and together included more than 2,600 patients. It delivered better efficacy in terms of relative reduction in annualized relapse rate (48% at 1 year and 38% at 2 years). It also demonstrated better relative reduction of the number of T1-weighted gadolinium-enhanced brain lesions (63% fewer at 1 year and 53% fewer at 2 years) and number of new or enlarging T2 lesions (48% fewer at 1 year and 42% at 2 years).
Ozanimod is contraindicated in patients who, in the past 6 months, experienced a myocardial infarction, unstable angina, stroke, or other conditions. It is associated with other health risks, including infections, liver injury, additive immunosuppressive effects from prior immune-modulating therapies, and increased blood pressure. Certain assessments, such as recent complete blood count, ECG, liver function test, and current and prior medications and vaccinations, are required before initiation of treatment.
In its announcement, Bristol-Myers Squibb said that it has decided to delay the commercial launch of ozanimod during the COVID-19 pandemic until a later date.
The drug is also in development for additional immune-inflammatory indications, including ulcerative colitis and Crohn’s disease.
The full prescribing information can be found on the company’s website.
The Food and Drug Administration has approved the oral medication ozanimod (Zeposia) for relapsing forms of multiple sclerosis (MS), including clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, according to a release from Bristol-Myers Squibb.
Ozanimod is a sphingosine 1-phosphate (S1P) receptor modulator that binds with high affinity to S1P receptors 1 and 5. It blocks the capacity of lymphocytes to egress from lymph nodes, reducing the number of lymphocytes in peripheral blood. Although its therapeutic mechanism of action in MS is unknown, it may involve the reduction of lymphocyte migration into the central nervous system. A genetic test is not required to start the drug, and no patient observation is required for the first dose, although up-titration of initial doses are required to reach the maintenance dose because a transient decrease in heart rate and atrioventricular conduction delays may occur, according to the company.
The approval is based on a pair of head-to-head studies that compared it with interferon beta-1a (Avonex) and together included more than 2,600 patients. It delivered better efficacy in terms of relative reduction in annualized relapse rate (48% at 1 year and 38% at 2 years). It also demonstrated better relative reduction of the number of T1-weighted gadolinium-enhanced brain lesions (63% fewer at 1 year and 53% fewer at 2 years) and number of new or enlarging T2 lesions (48% fewer at 1 year and 42% at 2 years).
Ozanimod is contraindicated in patients who, in the past 6 months, experienced a myocardial infarction, unstable angina, stroke, or other conditions. It is associated with other health risks, including infections, liver injury, additive immunosuppressive effects from prior immune-modulating therapies, and increased blood pressure. Certain assessments, such as recent complete blood count, ECG, liver function test, and current and prior medications and vaccinations, are required before initiation of treatment.
In its announcement, Bristol-Myers Squibb said that it has decided to delay the commercial launch of ozanimod during the COVID-19 pandemic until a later date.
The drug is also in development for additional immune-inflammatory indications, including ulcerative colitis and Crohn’s disease.
The full prescribing information can be found on the company’s website.
The Food and Drug Administration has approved the oral medication ozanimod (Zeposia) for relapsing forms of multiple sclerosis (MS), including clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, according to a release from Bristol-Myers Squibb.
Ozanimod is a sphingosine 1-phosphate (S1P) receptor modulator that binds with high affinity to S1P receptors 1 and 5. It blocks the capacity of lymphocytes to egress from lymph nodes, reducing the number of lymphocytes in peripheral blood. Although its therapeutic mechanism of action in MS is unknown, it may involve the reduction of lymphocyte migration into the central nervous system. A genetic test is not required to start the drug, and no patient observation is required for the first dose, although up-titration of initial doses are required to reach the maintenance dose because a transient decrease in heart rate and atrioventricular conduction delays may occur, according to the company.
The approval is based on a pair of head-to-head studies that compared it with interferon beta-1a (Avonex) and together included more than 2,600 patients. It delivered better efficacy in terms of relative reduction in annualized relapse rate (48% at 1 year and 38% at 2 years). It also demonstrated better relative reduction of the number of T1-weighted gadolinium-enhanced brain lesions (63% fewer at 1 year and 53% fewer at 2 years) and number of new or enlarging T2 lesions (48% fewer at 1 year and 42% at 2 years).
Ozanimod is contraindicated in patients who, in the past 6 months, experienced a myocardial infarction, unstable angina, stroke, or other conditions. It is associated with other health risks, including infections, liver injury, additive immunosuppressive effects from prior immune-modulating therapies, and increased blood pressure. Certain assessments, such as recent complete blood count, ECG, liver function test, and current and prior medications and vaccinations, are required before initiation of treatment.
In its announcement, Bristol-Myers Squibb said that it has decided to delay the commercial launch of ozanimod during the COVID-19 pandemic until a later date.
The drug is also in development for additional immune-inflammatory indications, including ulcerative colitis and Crohn’s disease.
The full prescribing information can be found on the company’s website.
Due to the COVID-19 pandemic, the AAN urges feds to further expand telehealth benefits
On March 17, the Trump administration announced an expansion of telehealth benefits to help stop the spread of COVID-19 and allow more Medicare patients to receive virtual care without having to visit a healthcare center or physician office.
Under the expansion, Medicare will pay for office, hospital, and other visits furnished via telehealth across the country and including in the patient’s home, delivered by a range of providers, such as physicians, nurse practitioners, clinical psychologists, and licensed clinical social workers.
Prior to this waiver, Medicare would only pay for telehealth on a limited basis, such as when the patient receiving the service was in a designated rural area.
However, in a letter to Alex Azar, secretary of the U.S. Department of Health & Human Services (HHS), the AAN says the easing of restrictions on telehealth should be extended beyond Medicare fee-for-service to both Medicare Advantage and Medicaid patients.
Practice changing?
“It is very heartening that the government is stepping up to the plate” and lifting many telemedicine restrictions, Neil Busis, MD, member of the AAN Health Policy Subcommittee, said in an interview.
Dr. Busis, who leads the telemedicine program for the department of neurology at NYU Langone Health in New York, said the global pandemic has “heightened, focused, and sharpened” attention to the need for telehealth services, particularly for neurology.
“By definition, a lot of neurology patients have mobility problems, traveling is a burden, making it difficult to see a neurologist,” he said.
Dr. Busis hopes these waivers in telehealth, made on a temporary and emergency basis, will become permanent once the COVID-19 pandemic has passed.
“What we hope is that the usefulness of various virtual technologies tested in the crucible of this pandemic will stimulate people to think about it once the pandemic is over and not rescind these loosening of restrictions, and that this will be the beginning of a new era for telemedicine,” he said.
The COVID-19 pandemic may be a “catalyst to accelerate the incorporation of non-face-to-face care into our armamentarium,” he added.
“What we have discovered in recent years is non-face-to-face care with enabling communication technologies is as effective in many clinical situations as face-to-face care. Now is the time to really focus on making the virtual experience as good as possible and to make it as available to as many people as possible,” said Dr. Busis.
Reduce regulatory burdens
The AAN also calls on the federal government to urge states to take action to ensure access to telehealth services and allow telehealth companies to provide telehealth technology and education free of charge to providers who don’t currently use telehealth in their practices.
“The AAN notes that doing so may implicate provisions of the Anti-Kickback Statute. We believe during the current emergency that HHS should issue guidance making it clear to providers that accepting free access to telehealth platforms and education does not put them at risk of violating fraud and abuse laws,” the letter, signed by AAN President James Stevens, MD, stated.
The AAN also wants the government to reduce regulatory burdens during this public health emergency to allow physicians more time to focus on patient care. “This is especially true for providers that are self-quarantining or are in a practice that is experiencing staffing shortages due to self-quarantines,” he wrote.
Specifically, the AAN asked the Centers for Medicare & Medicaid Services to extend the March 31 deadline for physicians to submit their data for the Merit-based Incentive Payment System program for calendar year 2019 (and other compliance deadlines) by at least 30 days.
The AAN also calls on the CMS to delay implementation of the Appropriate Use Criteria program by 1 year, saying that many providers will not have the capacity to “meaningfully” participate in the current testing year for the program.
A version of this article originally appeared on Medscape.com.
On March 17, the Trump administration announced an expansion of telehealth benefits to help stop the spread of COVID-19 and allow more Medicare patients to receive virtual care without having to visit a healthcare center or physician office.
Under the expansion, Medicare will pay for office, hospital, and other visits furnished via telehealth across the country and including in the patient’s home, delivered by a range of providers, such as physicians, nurse practitioners, clinical psychologists, and licensed clinical social workers.
Prior to this waiver, Medicare would only pay for telehealth on a limited basis, such as when the patient receiving the service was in a designated rural area.
However, in a letter to Alex Azar, secretary of the U.S. Department of Health & Human Services (HHS), the AAN says the easing of restrictions on telehealth should be extended beyond Medicare fee-for-service to both Medicare Advantage and Medicaid patients.
Practice changing?
“It is very heartening that the government is stepping up to the plate” and lifting many telemedicine restrictions, Neil Busis, MD, member of the AAN Health Policy Subcommittee, said in an interview.
Dr. Busis, who leads the telemedicine program for the department of neurology at NYU Langone Health in New York, said the global pandemic has “heightened, focused, and sharpened” attention to the need for telehealth services, particularly for neurology.
“By definition, a lot of neurology patients have mobility problems, traveling is a burden, making it difficult to see a neurologist,” he said.
Dr. Busis hopes these waivers in telehealth, made on a temporary and emergency basis, will become permanent once the COVID-19 pandemic has passed.
“What we hope is that the usefulness of various virtual technologies tested in the crucible of this pandemic will stimulate people to think about it once the pandemic is over and not rescind these loosening of restrictions, and that this will be the beginning of a new era for telemedicine,” he said.
The COVID-19 pandemic may be a “catalyst to accelerate the incorporation of non-face-to-face care into our armamentarium,” he added.
“What we have discovered in recent years is non-face-to-face care with enabling communication technologies is as effective in many clinical situations as face-to-face care. Now is the time to really focus on making the virtual experience as good as possible and to make it as available to as many people as possible,” said Dr. Busis.
Reduce regulatory burdens
The AAN also calls on the federal government to urge states to take action to ensure access to telehealth services and allow telehealth companies to provide telehealth technology and education free of charge to providers who don’t currently use telehealth in their practices.
“The AAN notes that doing so may implicate provisions of the Anti-Kickback Statute. We believe during the current emergency that HHS should issue guidance making it clear to providers that accepting free access to telehealth platforms and education does not put them at risk of violating fraud and abuse laws,” the letter, signed by AAN President James Stevens, MD, stated.
The AAN also wants the government to reduce regulatory burdens during this public health emergency to allow physicians more time to focus on patient care. “This is especially true for providers that are self-quarantining or are in a practice that is experiencing staffing shortages due to self-quarantines,” he wrote.
Specifically, the AAN asked the Centers for Medicare & Medicaid Services to extend the March 31 deadline for physicians to submit their data for the Merit-based Incentive Payment System program for calendar year 2019 (and other compliance deadlines) by at least 30 days.
The AAN also calls on the CMS to delay implementation of the Appropriate Use Criteria program by 1 year, saying that many providers will not have the capacity to “meaningfully” participate in the current testing year for the program.
A version of this article originally appeared on Medscape.com.
On March 17, the Trump administration announced an expansion of telehealth benefits to help stop the spread of COVID-19 and allow more Medicare patients to receive virtual care without having to visit a healthcare center or physician office.
Under the expansion, Medicare will pay for office, hospital, and other visits furnished via telehealth across the country and including in the patient’s home, delivered by a range of providers, such as physicians, nurse practitioners, clinical psychologists, and licensed clinical social workers.
Prior to this waiver, Medicare would only pay for telehealth on a limited basis, such as when the patient receiving the service was in a designated rural area.
However, in a letter to Alex Azar, secretary of the U.S. Department of Health & Human Services (HHS), the AAN says the easing of restrictions on telehealth should be extended beyond Medicare fee-for-service to both Medicare Advantage and Medicaid patients.
Practice changing?
“It is very heartening that the government is stepping up to the plate” and lifting many telemedicine restrictions, Neil Busis, MD, member of the AAN Health Policy Subcommittee, said in an interview.
Dr. Busis, who leads the telemedicine program for the department of neurology at NYU Langone Health in New York, said the global pandemic has “heightened, focused, and sharpened” attention to the need for telehealth services, particularly for neurology.
“By definition, a lot of neurology patients have mobility problems, traveling is a burden, making it difficult to see a neurologist,” he said.
Dr. Busis hopes these waivers in telehealth, made on a temporary and emergency basis, will become permanent once the COVID-19 pandemic has passed.
“What we hope is that the usefulness of various virtual technologies tested in the crucible of this pandemic will stimulate people to think about it once the pandemic is over and not rescind these loosening of restrictions, and that this will be the beginning of a new era for telemedicine,” he said.
The COVID-19 pandemic may be a “catalyst to accelerate the incorporation of non-face-to-face care into our armamentarium,” he added.
“What we have discovered in recent years is non-face-to-face care with enabling communication technologies is as effective in many clinical situations as face-to-face care. Now is the time to really focus on making the virtual experience as good as possible and to make it as available to as many people as possible,” said Dr. Busis.
Reduce regulatory burdens
The AAN also calls on the federal government to urge states to take action to ensure access to telehealth services and allow telehealth companies to provide telehealth technology and education free of charge to providers who don’t currently use telehealth in their practices.
“The AAN notes that doing so may implicate provisions of the Anti-Kickback Statute. We believe during the current emergency that HHS should issue guidance making it clear to providers that accepting free access to telehealth platforms and education does not put them at risk of violating fraud and abuse laws,” the letter, signed by AAN President James Stevens, MD, stated.
The AAN also wants the government to reduce regulatory burdens during this public health emergency to allow physicians more time to focus on patient care. “This is especially true for providers that are self-quarantining or are in a practice that is experiencing staffing shortages due to self-quarantines,” he wrote.
Specifically, the AAN asked the Centers for Medicare & Medicaid Services to extend the March 31 deadline for physicians to submit their data for the Merit-based Incentive Payment System program for calendar year 2019 (and other compliance deadlines) by at least 30 days.
The AAN also calls on the CMS to delay implementation of the Appropriate Use Criteria program by 1 year, saying that many providers will not have the capacity to “meaningfully” participate in the current testing year for the program.
A version of this article originally appeared on Medscape.com.
Sleep-disordered breathing linked with Alzheimer’s disease biomarkers in cognitively normal older adults
investigators have found.
Among 127 adults enrolled in a randomized clinical trial of interventions to promote mental well-being in older adults, those with sleep-disordered breathing had significantly greater amyloid burden and gray-matter volume, as well as increased perfusion and metabolism in parietal-occipital regions, reported Claire André, PhD, from the French Institute of Health and Medical Research (INSERM) unit in Caen, and colleagues.
“Our findings highlight the need to treat sleep disorders in the older population, even in the absence of cognitive or behavioral manifestations,” they wrote in a study published in JAMA Neurology.
Previous studies of the possible association between sleep-disordered breathing and dementia risk have shown conflicting or inconsistent results, the authors noted.
“These discrepancies may be explained by the characteristics of patients with sleep-disordered breathing (e.g., recruited from sleep clinics versus from the community, differences in age and disease duration), the scoring criteria of respiratory events, sample sizes, or the lack of controls for possibly biasing covariates,” they wrote.
To see whether they could clear up the confusion, the investigators conducted a retrospective analysis of 127 patients who were enrolled in the Age-Well randomized, controlled trial of the Medit-Ageing European project. The participants were community-dwelling adults (mean age, 69.1 years; 63% women), who were enrolled in the trial and underwent evaluation from 2016 to 2018 at the Cyceron Cancer Center in Caen.
The participants, all of whom were cognitively unimpaired at baseline, underwent neuropsychological assessment, polysomnography, MRI, plus florbetapir- and fluorodeoxyglucose-labeled PET.
The investigators defined sleep-disordered breathing as 15 apnea-hypopnea index events per hour or higher, and compared results between those with sleep-disordered breathing and those without for each imaging modality.
Participants with sleep-disordered breathing has significantly greater amyloid burden (P = .04), gray-matter volume (P = .04), perfusion (P = .04), and metabolism (P = .001), primarily overlapping the posterior cingulate cortex and precuneus, areas known to be significantly involved in Alzheimer’s disease.
When the investigators looked for behavioral and cognitive correlates of sleep-disordered breathing severity with associated brain changes, however, they found no associations with either cognitive performance, self-reported cognitive or sleep difficulties, or symptoms of daytime sleepiness.
“Importantly, to the best of our knowledge, our results show in vivo for the first time that greater amyloid burden colocalizes with greater gray-matter volume, perfusion, and metabolism in older participants with sleep-disordered breathing who are cognitively unimpaired. We believe that these overlapping patterns reinforce the likelihood of common underlying mechanisms,” they wrote.
The Age-Well randomized clinical trial is part of the Medit-Ageing project and is funded through the European Union’s Horizon 2020 Research and Innovation Program, INSERM, and Fondation d’ Entreprise MMA des Entrepreneurs du Futur. Dr. André reported no conflicts of interest to disclose.
SOURCE: André C et al. JAMA Neurol. 2020 Mar 23. doi: 10.1001/jamaneurol.2020.0311.
investigators have found.
Among 127 adults enrolled in a randomized clinical trial of interventions to promote mental well-being in older adults, those with sleep-disordered breathing had significantly greater amyloid burden and gray-matter volume, as well as increased perfusion and metabolism in parietal-occipital regions, reported Claire André, PhD, from the French Institute of Health and Medical Research (INSERM) unit in Caen, and colleagues.
“Our findings highlight the need to treat sleep disorders in the older population, even in the absence of cognitive or behavioral manifestations,” they wrote in a study published in JAMA Neurology.
Previous studies of the possible association between sleep-disordered breathing and dementia risk have shown conflicting or inconsistent results, the authors noted.
“These discrepancies may be explained by the characteristics of patients with sleep-disordered breathing (e.g., recruited from sleep clinics versus from the community, differences in age and disease duration), the scoring criteria of respiratory events, sample sizes, or the lack of controls for possibly biasing covariates,” they wrote.
To see whether they could clear up the confusion, the investigators conducted a retrospective analysis of 127 patients who were enrolled in the Age-Well randomized, controlled trial of the Medit-Ageing European project. The participants were community-dwelling adults (mean age, 69.1 years; 63% women), who were enrolled in the trial and underwent evaluation from 2016 to 2018 at the Cyceron Cancer Center in Caen.
The participants, all of whom were cognitively unimpaired at baseline, underwent neuropsychological assessment, polysomnography, MRI, plus florbetapir- and fluorodeoxyglucose-labeled PET.
The investigators defined sleep-disordered breathing as 15 apnea-hypopnea index events per hour or higher, and compared results between those with sleep-disordered breathing and those without for each imaging modality.
Participants with sleep-disordered breathing has significantly greater amyloid burden (P = .04), gray-matter volume (P = .04), perfusion (P = .04), and metabolism (P = .001), primarily overlapping the posterior cingulate cortex and precuneus, areas known to be significantly involved in Alzheimer’s disease.
When the investigators looked for behavioral and cognitive correlates of sleep-disordered breathing severity with associated brain changes, however, they found no associations with either cognitive performance, self-reported cognitive or sleep difficulties, or symptoms of daytime sleepiness.
“Importantly, to the best of our knowledge, our results show in vivo for the first time that greater amyloid burden colocalizes with greater gray-matter volume, perfusion, and metabolism in older participants with sleep-disordered breathing who are cognitively unimpaired. We believe that these overlapping patterns reinforce the likelihood of common underlying mechanisms,” they wrote.
The Age-Well randomized clinical trial is part of the Medit-Ageing project and is funded through the European Union’s Horizon 2020 Research and Innovation Program, INSERM, and Fondation d’ Entreprise MMA des Entrepreneurs du Futur. Dr. André reported no conflicts of interest to disclose.
SOURCE: André C et al. JAMA Neurol. 2020 Mar 23. doi: 10.1001/jamaneurol.2020.0311.
investigators have found.
Among 127 adults enrolled in a randomized clinical trial of interventions to promote mental well-being in older adults, those with sleep-disordered breathing had significantly greater amyloid burden and gray-matter volume, as well as increased perfusion and metabolism in parietal-occipital regions, reported Claire André, PhD, from the French Institute of Health and Medical Research (INSERM) unit in Caen, and colleagues.
“Our findings highlight the need to treat sleep disorders in the older population, even in the absence of cognitive or behavioral manifestations,” they wrote in a study published in JAMA Neurology.
Previous studies of the possible association between sleep-disordered breathing and dementia risk have shown conflicting or inconsistent results, the authors noted.
“These discrepancies may be explained by the characteristics of patients with sleep-disordered breathing (e.g., recruited from sleep clinics versus from the community, differences in age and disease duration), the scoring criteria of respiratory events, sample sizes, or the lack of controls for possibly biasing covariates,” they wrote.
To see whether they could clear up the confusion, the investigators conducted a retrospective analysis of 127 patients who were enrolled in the Age-Well randomized, controlled trial of the Medit-Ageing European project. The participants were community-dwelling adults (mean age, 69.1 years; 63% women), who were enrolled in the trial and underwent evaluation from 2016 to 2018 at the Cyceron Cancer Center in Caen.
The participants, all of whom were cognitively unimpaired at baseline, underwent neuropsychological assessment, polysomnography, MRI, plus florbetapir- and fluorodeoxyglucose-labeled PET.
The investigators defined sleep-disordered breathing as 15 apnea-hypopnea index events per hour or higher, and compared results between those with sleep-disordered breathing and those without for each imaging modality.
Participants with sleep-disordered breathing has significantly greater amyloid burden (P = .04), gray-matter volume (P = .04), perfusion (P = .04), and metabolism (P = .001), primarily overlapping the posterior cingulate cortex and precuneus, areas known to be significantly involved in Alzheimer’s disease.
When the investigators looked for behavioral and cognitive correlates of sleep-disordered breathing severity with associated brain changes, however, they found no associations with either cognitive performance, self-reported cognitive or sleep difficulties, or symptoms of daytime sleepiness.
“Importantly, to the best of our knowledge, our results show in vivo for the first time that greater amyloid burden colocalizes with greater gray-matter volume, perfusion, and metabolism in older participants with sleep-disordered breathing who are cognitively unimpaired. We believe that these overlapping patterns reinforce the likelihood of common underlying mechanisms,” they wrote.
The Age-Well randomized clinical trial is part of the Medit-Ageing project and is funded through the European Union’s Horizon 2020 Research and Innovation Program, INSERM, and Fondation d’ Entreprise MMA des Entrepreneurs du Futur. Dr. André reported no conflicts of interest to disclose.
SOURCE: André C et al. JAMA Neurol. 2020 Mar 23. doi: 10.1001/jamaneurol.2020.0311.
FROM JAMA NEUROLOGY
New ASAM guideline released amid COVID-19 concerns
Home-based buprenorphine induction deemed safe for OUD
The American Society of Addiction Medicine has released an updated practice guideline for patients with opioid use disorder.
The guideline, called a focused update, advances ASAM’s 2015 National Practice Guidelines for the Treament of Opioid Use Disorder. “During the ongoing COVID-19 pandemic and the associated need for social distancing, it is especially important that clinicians and health care providers across the country take steps to ensure that individuals with OUD can continue to receive evidence-based care,” said Paul H. Earley, MD, president of ASAM, in a press release announcing the new guideline.
The guideline specifies that home-based buprenorphine induction is safe and effective for treatment of opioid use disorder and that no individual entering the criminal justice system should be subjected to opioid withdrawal.
“The research is clear, providing methadone or buprenorphine, even without psychosocial treatment, reduces the patient’s risk of death,” said Kyle Kampman, MD, chair of the group’s Guideline Writing Committee, in the release. “Ultimately, keeping patients with the disease of addiction alive and engaged to become ready for recovery is absolutely critical in the context of the deadly overdose epidemic that has struck communities across our country.”
The society released this focused update to reflect new medications and formulations, published evidence, and clinical guidance related to treatment of OUD. This update includes the addition of 13 new recommendations and major revisions to 35 existing recommendations. One concern the society has is how to help patients being treated for OUD who are limited in their ability to leave their homes. Because of these same concerns, the Substance Abuse and Mental Health Services Administration relaxed regulations on March 16 regarding patient eligibility for take-home medications, such as buprenorphine and methadone, which dovetails with the society’s guidance regarding home-based induction.
, continuing on to pharmacologic treatment even if the patient declines recommended psychosocial treatment, keeping naloxone kits available in correctional facilities, and more. Additional information about this update can be found on ASAM’s website.
Home-based buprenorphine induction deemed safe for OUD
Home-based buprenorphine induction deemed safe for OUD
The American Society of Addiction Medicine has released an updated practice guideline for patients with opioid use disorder.
The guideline, called a focused update, advances ASAM’s 2015 National Practice Guidelines for the Treament of Opioid Use Disorder. “During the ongoing COVID-19 pandemic and the associated need for social distancing, it is especially important that clinicians and health care providers across the country take steps to ensure that individuals with OUD can continue to receive evidence-based care,” said Paul H. Earley, MD, president of ASAM, in a press release announcing the new guideline.
The guideline specifies that home-based buprenorphine induction is safe and effective for treatment of opioid use disorder and that no individual entering the criminal justice system should be subjected to opioid withdrawal.
“The research is clear, providing methadone or buprenorphine, even without psychosocial treatment, reduces the patient’s risk of death,” said Kyle Kampman, MD, chair of the group’s Guideline Writing Committee, in the release. “Ultimately, keeping patients with the disease of addiction alive and engaged to become ready for recovery is absolutely critical in the context of the deadly overdose epidemic that has struck communities across our country.”
The society released this focused update to reflect new medications and formulations, published evidence, and clinical guidance related to treatment of OUD. This update includes the addition of 13 new recommendations and major revisions to 35 existing recommendations. One concern the society has is how to help patients being treated for OUD who are limited in their ability to leave their homes. Because of these same concerns, the Substance Abuse and Mental Health Services Administration relaxed regulations on March 16 regarding patient eligibility for take-home medications, such as buprenorphine and methadone, which dovetails with the society’s guidance regarding home-based induction.
, continuing on to pharmacologic treatment even if the patient declines recommended psychosocial treatment, keeping naloxone kits available in correctional facilities, and more. Additional information about this update can be found on ASAM’s website.
The American Society of Addiction Medicine has released an updated practice guideline for patients with opioid use disorder.
The guideline, called a focused update, advances ASAM’s 2015 National Practice Guidelines for the Treament of Opioid Use Disorder. “During the ongoing COVID-19 pandemic and the associated need for social distancing, it is especially important that clinicians and health care providers across the country take steps to ensure that individuals with OUD can continue to receive evidence-based care,” said Paul H. Earley, MD, president of ASAM, in a press release announcing the new guideline.
The guideline specifies that home-based buprenorphine induction is safe and effective for treatment of opioid use disorder and that no individual entering the criminal justice system should be subjected to opioid withdrawal.
“The research is clear, providing methadone or buprenorphine, even without psychosocial treatment, reduces the patient’s risk of death,” said Kyle Kampman, MD, chair of the group’s Guideline Writing Committee, in the release. “Ultimately, keeping patients with the disease of addiction alive and engaged to become ready for recovery is absolutely critical in the context of the deadly overdose epidemic that has struck communities across our country.”
The society released this focused update to reflect new medications and formulations, published evidence, and clinical guidance related to treatment of OUD. This update includes the addition of 13 new recommendations and major revisions to 35 existing recommendations. One concern the society has is how to help patients being treated for OUD who are limited in their ability to leave their homes. Because of these same concerns, the Substance Abuse and Mental Health Services Administration relaxed regulations on March 16 regarding patient eligibility for take-home medications, such as buprenorphine and methadone, which dovetails with the society’s guidance regarding home-based induction.
, continuing on to pharmacologic treatment even if the patient declines recommended psychosocial treatment, keeping naloxone kits available in correctional facilities, and more. Additional information about this update can be found on ASAM’s website.
Screen asymptomatic older adults for cognitive impairment? Not so fast
Reference
1. US Preventive Services Task Force. Final recommendation statement: cognitive impairment in older adults: screening. www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/cognitive-impairment-in-older-adults-screening. Published February 2020. Accessed March 19, 2020.
Reference
1. US Preventive Services Task Force. Final recommendation statement: cognitive impairment in older adults: screening. www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/cognitive-impairment-in-older-adults-screening. Published February 2020. Accessed March 19, 2020.
Reference
1. US Preventive Services Task Force. Final recommendation statement: cognitive impairment in older adults: screening. www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/cognitive-impairment-in-older-adults-screening. Published February 2020. Accessed March 19, 2020.
ICH survival lags in the community setting
LOS ANGELES – Although recent findings from circumscribed patient populations enrolled in intervention studies have shown improved survival rates in patients with a recent intracerebral hemorrhagic stroke, data from a large, observational study in the Netherlands suggested a much darker real-world picture, with a 6-month mortality of 64% identified in a total cohort of nearly 15,000 people followed prospectively starting in 1990.
In striking contrast to the survival pattern over time of patients in the same Dutch study who had a first acute ischemic stroke, which showed a statistically significant and meaningful cut in mortality for ischemic stroke patients during the 25-year period examined, survival rates for patients during the first months following a first intracerebral hemorrhage (ICH) stayed flat during 1991-2015, Reem Waziry, MD, said at the International Stroke Conference sponsored by the American Heart Association.
“The promising treatment advances [applied to patients] in the recent ICH trials may not be reflected in community-based treatment,” suggested Dr. Waziry, a research and teaching fellow in clinical epidemiology at the Harvard School of Public Health in Boston.
The data she reported came from the Rotterdam Study, which followed unselected, older people in the Rotterdam community with no stroke history, and during 25 years of monitoring identified 162 incident ICH strokes and 988 acute ischemic strokes. Concurrently with Dr. Waziry’s talk at the conference, the data she reported were published in Stroke. The data she reported also showed that, during the 25 years studied, mortality at 3 years following a first ICH stroke rose to 73% on average.
During her talk, Dr. Waziry also presented an unpublished comparison of the 64% 6-month mortality in the Rotterdam Study with the 3- to 6-month mortality reported in the control arms of four recent, randomized intervention trials, including the MISTIE III trial. Among the four randomized trials Dr. Waziry selected to make this post-hoc comparison, the study with the highest mortality among control patients was MISTIE III, which showed about 25% mortality after 6 months. In contrast, the 19% 6-month mortality among ischemic stroke patients in the Rotterdam Study was roughly similar to the mortality seem in the control arms of some recent studies of interventions for patients with acute ischemic stroke.
The Rotterdam Study receives no commercial funding. Dr. Waziry had no disclosures.
SOURCE: Waziry R et al. ISC 2020, Abstract LB14.
LOS ANGELES – Although recent findings from circumscribed patient populations enrolled in intervention studies have shown improved survival rates in patients with a recent intracerebral hemorrhagic stroke, data from a large, observational study in the Netherlands suggested a much darker real-world picture, with a 6-month mortality of 64% identified in a total cohort of nearly 15,000 people followed prospectively starting in 1990.
In striking contrast to the survival pattern over time of patients in the same Dutch study who had a first acute ischemic stroke, which showed a statistically significant and meaningful cut in mortality for ischemic stroke patients during the 25-year period examined, survival rates for patients during the first months following a first intracerebral hemorrhage (ICH) stayed flat during 1991-2015, Reem Waziry, MD, said at the International Stroke Conference sponsored by the American Heart Association.
“The promising treatment advances [applied to patients] in the recent ICH trials may not be reflected in community-based treatment,” suggested Dr. Waziry, a research and teaching fellow in clinical epidemiology at the Harvard School of Public Health in Boston.
The data she reported came from the Rotterdam Study, which followed unselected, older people in the Rotterdam community with no stroke history, and during 25 years of monitoring identified 162 incident ICH strokes and 988 acute ischemic strokes. Concurrently with Dr. Waziry’s talk at the conference, the data she reported were published in Stroke. The data she reported also showed that, during the 25 years studied, mortality at 3 years following a first ICH stroke rose to 73% on average.
During her talk, Dr. Waziry also presented an unpublished comparison of the 64% 6-month mortality in the Rotterdam Study with the 3- to 6-month mortality reported in the control arms of four recent, randomized intervention trials, including the MISTIE III trial. Among the four randomized trials Dr. Waziry selected to make this post-hoc comparison, the study with the highest mortality among control patients was MISTIE III, which showed about 25% mortality after 6 months. In contrast, the 19% 6-month mortality among ischemic stroke patients in the Rotterdam Study was roughly similar to the mortality seem in the control arms of some recent studies of interventions for patients with acute ischemic stroke.
The Rotterdam Study receives no commercial funding. Dr. Waziry had no disclosures.
SOURCE: Waziry R et al. ISC 2020, Abstract LB14.
LOS ANGELES – Although recent findings from circumscribed patient populations enrolled in intervention studies have shown improved survival rates in patients with a recent intracerebral hemorrhagic stroke, data from a large, observational study in the Netherlands suggested a much darker real-world picture, with a 6-month mortality of 64% identified in a total cohort of nearly 15,000 people followed prospectively starting in 1990.
In striking contrast to the survival pattern over time of patients in the same Dutch study who had a first acute ischemic stroke, which showed a statistically significant and meaningful cut in mortality for ischemic stroke patients during the 25-year period examined, survival rates for patients during the first months following a first intracerebral hemorrhage (ICH) stayed flat during 1991-2015, Reem Waziry, MD, said at the International Stroke Conference sponsored by the American Heart Association.
“The promising treatment advances [applied to patients] in the recent ICH trials may not be reflected in community-based treatment,” suggested Dr. Waziry, a research and teaching fellow in clinical epidemiology at the Harvard School of Public Health in Boston.
The data she reported came from the Rotterdam Study, which followed unselected, older people in the Rotterdam community with no stroke history, and during 25 years of monitoring identified 162 incident ICH strokes and 988 acute ischemic strokes. Concurrently with Dr. Waziry’s talk at the conference, the data she reported were published in Stroke. The data she reported also showed that, during the 25 years studied, mortality at 3 years following a first ICH stroke rose to 73% on average.
During her talk, Dr. Waziry also presented an unpublished comparison of the 64% 6-month mortality in the Rotterdam Study with the 3- to 6-month mortality reported in the control arms of four recent, randomized intervention trials, including the MISTIE III trial. Among the four randomized trials Dr. Waziry selected to make this post-hoc comparison, the study with the highest mortality among control patients was MISTIE III, which showed about 25% mortality after 6 months. In contrast, the 19% 6-month mortality among ischemic stroke patients in the Rotterdam Study was roughly similar to the mortality seem in the control arms of some recent studies of interventions for patients with acute ischemic stroke.
The Rotterdam Study receives no commercial funding. Dr. Waziry had no disclosures.
SOURCE: Waziry R et al. ISC 2020, Abstract LB14.
REPORTING FROM ISC 2020