User login
Amantadine Speeds Return to Consciousness After Brain Injury
A 4-week course of amantadine sped the rate of functional recovery in an international, randomized, double-blind, placebo-controlled trial of patients who had disorders of consciousness following traumatic brain injury.
Both patients given amantadine and those given placebo improved, but the rate of recovery was more rapid with the active drug. It affected "functionally meaningful behaviors such as consistent responses to commands, intelligible speech, reliable yes-or-no communication, and functional-object use," Joseph T. Giacino, Ph.D., of the JFK Johnson Rehabilitation Institute, Edison, N.J., and his associates reported in the March 1 issue of the New England Journal of Medicine.
The rate of recovery attenuated when treatment stopped, and scores on the Disability Rating Scale (DRS) were virtually indistinguishable between the treatment group and the control group 6 weeks later, indicating that the response was drug dependent.
"Practical and ethical constraints required the use of a brief treatment interval and a short-term assessment of the outcome [in this study], because we anticipated that caregivers would withdraw patients who were not making gains in order to try other treatments. Thus, our findings do not address the effects of prolonged treatment on long-term outcomes," the investigators noted.
"Whether treatment with amantadine, as compared with placebo, improves the long-term outcome or simply accelerates recovery en route to an equivalent level of function remains unknown."
The researchers conducted the trial after a pilot study indicated that amantadine improved functional outcomes after traumatic brain injury. They enrolled 184 patients aged 16-65 years who had sustained a nonpenetrating TBI 4-16 weeks previously and who were receiving usual inpatient rehabilitation at 11 medical centers in three countries.
Patients were diagnosed as being in a vegetative state, in which there was wakefulness without behavioral evidence of conscious awareness, or in a minimally conscious state, in which there was at least one clearly discernible behavioral sign of consciousness. All had scores above 11 on the DRS, which ranges from 0 (best) to 29 (worst); none were able to follow commands consistently or to engage in functional communication as assessed using the Coma Recovery Scale-Revised (CRS-R).
A total of 87 patients were randomly assigned to receive amantadine and 97 to receive a visually identical placebo for 4 weeks. They began at a dose of 100 mg twice daily, with escalations to 150 mg twice daily at week 3 and to 200 mg twice daily at week 4 if the DRS score had not improved by at least 2 points from baseline. Then the drug or placebo was tapered, and patients were assessed for another 6 weeks, according to Dr. Giacino and his associates. Dr. Giacino is also director of rehabilitation neuropsychology at the Spaulding Rehabilitation Hospital and is an associate professor in the department of physical medicine and rehabilitation at Harvard Medical School, both in Boston.
The primary outcome measure was the rate of improvement in the DRS score during treatment. DRS scores were assessed weekly by an interdisciplinary treatment team that was blinded to treatment assignment. Study personnel assessed clinically relevant behavioral benchmarks using the CRS-R.
Both study groups had significantly improved DRS scores at the end of the 4 weeks of treatment, but the amantadine group showed significantly faster improvement (–0.24 points/week more). In addition, at the final follow-up assessment, more patients in the amantadine group showed favorable outcomes on the DRS, fewer remained in a vegetative state, and more showed recovery of key behavioral benchmarks, Dr. Giacino and his colleagues said (N. Engl. J. Med. 2012;366:819-26). These benefits were consistent across all subgroups of patients. They were seen both in subjects in a minimally conscious state and in those in a vegetative state. And they were noted regardless of how long after the TBI injury the patients began treatment.
However, the treatment effect declined when drug therapy stopped, until there were no significant differences at the final follow-up assessment between patients who received the active drug and those who received placebo.
In particular, the proportion of patients who were able to engage in each of six clinically relevant behaviors was higher with amantadine during the month of therapy, but the difference between the two study groups was negligible at the final follow-up. These six behaviors were consistent command-following, object recognition, functional use of objects, intelligible verbalization, reliable yes-or-no communication, and sustained attention.
Amantadine "did not increase the risk of adverse medical, neurologic, or behavioral events, including those of greatest concern in this population (e.g., seizure)," the researchers noted.
The drug’s mechanism of action in these patients is uncertain. Amantadine is thought to promote dopaminergic activity "by facilitating presynaptic release and blocking reuptake postsynaptically." It may also enhance neurotransmission "in the dopamine-dependent nigrostriatal, mesolimbic, and frontostriatal circuits that are responsible for mediating arousal, drive, and attentional functions," they added.
"We conclude that amantadine is effective in accelerating the pace of recovery during acute rehabilitation in patients with prolonged post-traumatic disturbances in consciousness. Exposure to amantadine is associated with more rapid emergence of cognitively mediated behaviors that serve as the foundation for functional independence," Dr. Giacino and his associates wrote.
The study was supported by the U.S. National Institute on Disability and Rehabilitation Research. Dr. Giacino reported no relevant financial conflicts of interest, but a coauthor reported ties to Allergan, Merz, Ipsen, and Medtronic. Several other coauthors disclosed providing expert testimony on patients with TBI and disorders of consciousness.
A 4-week course of amantadine sped the rate of functional recovery in an international, randomized, double-blind, placebo-controlled trial of patients who had disorders of consciousness following traumatic brain injury.
Both patients given amantadine and those given placebo improved, but the rate of recovery was more rapid with the active drug. It affected "functionally meaningful behaviors such as consistent responses to commands, intelligible speech, reliable yes-or-no communication, and functional-object use," Joseph T. Giacino, Ph.D., of the JFK Johnson Rehabilitation Institute, Edison, N.J., and his associates reported in the March 1 issue of the New England Journal of Medicine.
The rate of recovery attenuated when treatment stopped, and scores on the Disability Rating Scale (DRS) were virtually indistinguishable between the treatment group and the control group 6 weeks later, indicating that the response was drug dependent.
"Practical and ethical constraints required the use of a brief treatment interval and a short-term assessment of the outcome [in this study], because we anticipated that caregivers would withdraw patients who were not making gains in order to try other treatments. Thus, our findings do not address the effects of prolonged treatment on long-term outcomes," the investigators noted.
"Whether treatment with amantadine, as compared with placebo, improves the long-term outcome or simply accelerates recovery en route to an equivalent level of function remains unknown."
The researchers conducted the trial after a pilot study indicated that amantadine improved functional outcomes after traumatic brain injury. They enrolled 184 patients aged 16-65 years who had sustained a nonpenetrating TBI 4-16 weeks previously and who were receiving usual inpatient rehabilitation at 11 medical centers in three countries.
Patients were diagnosed as being in a vegetative state, in which there was wakefulness without behavioral evidence of conscious awareness, or in a minimally conscious state, in which there was at least one clearly discernible behavioral sign of consciousness. All had scores above 11 on the DRS, which ranges from 0 (best) to 29 (worst); none were able to follow commands consistently or to engage in functional communication as assessed using the Coma Recovery Scale-Revised (CRS-R).
A total of 87 patients were randomly assigned to receive amantadine and 97 to receive a visually identical placebo for 4 weeks. They began at a dose of 100 mg twice daily, with escalations to 150 mg twice daily at week 3 and to 200 mg twice daily at week 4 if the DRS score had not improved by at least 2 points from baseline. Then the drug or placebo was tapered, and patients were assessed for another 6 weeks, according to Dr. Giacino and his associates. Dr. Giacino is also director of rehabilitation neuropsychology at the Spaulding Rehabilitation Hospital and is an associate professor in the department of physical medicine and rehabilitation at Harvard Medical School, both in Boston.
The primary outcome measure was the rate of improvement in the DRS score during treatment. DRS scores were assessed weekly by an interdisciplinary treatment team that was blinded to treatment assignment. Study personnel assessed clinically relevant behavioral benchmarks using the CRS-R.
Both study groups had significantly improved DRS scores at the end of the 4 weeks of treatment, but the amantadine group showed significantly faster improvement (–0.24 points/week more). In addition, at the final follow-up assessment, more patients in the amantadine group showed favorable outcomes on the DRS, fewer remained in a vegetative state, and more showed recovery of key behavioral benchmarks, Dr. Giacino and his colleagues said (N. Engl. J. Med. 2012;366:819-26). These benefits were consistent across all subgroups of patients. They were seen both in subjects in a minimally conscious state and in those in a vegetative state. And they were noted regardless of how long after the TBI injury the patients began treatment.
However, the treatment effect declined when drug therapy stopped, until there were no significant differences at the final follow-up assessment between patients who received the active drug and those who received placebo.
In particular, the proportion of patients who were able to engage in each of six clinically relevant behaviors was higher with amantadine during the month of therapy, but the difference between the two study groups was negligible at the final follow-up. These six behaviors were consistent command-following, object recognition, functional use of objects, intelligible verbalization, reliable yes-or-no communication, and sustained attention.
Amantadine "did not increase the risk of adverse medical, neurologic, or behavioral events, including those of greatest concern in this population (e.g., seizure)," the researchers noted.
The drug’s mechanism of action in these patients is uncertain. Amantadine is thought to promote dopaminergic activity "by facilitating presynaptic release and blocking reuptake postsynaptically." It may also enhance neurotransmission "in the dopamine-dependent nigrostriatal, mesolimbic, and frontostriatal circuits that are responsible for mediating arousal, drive, and attentional functions," they added.
"We conclude that amantadine is effective in accelerating the pace of recovery during acute rehabilitation in patients with prolonged post-traumatic disturbances in consciousness. Exposure to amantadine is associated with more rapid emergence of cognitively mediated behaviors that serve as the foundation for functional independence," Dr. Giacino and his associates wrote.
The study was supported by the U.S. National Institute on Disability and Rehabilitation Research. Dr. Giacino reported no relevant financial conflicts of interest, but a coauthor reported ties to Allergan, Merz, Ipsen, and Medtronic. Several other coauthors disclosed providing expert testimony on patients with TBI and disorders of consciousness.
A 4-week course of amantadine sped the rate of functional recovery in an international, randomized, double-blind, placebo-controlled trial of patients who had disorders of consciousness following traumatic brain injury.
Both patients given amantadine and those given placebo improved, but the rate of recovery was more rapid with the active drug. It affected "functionally meaningful behaviors such as consistent responses to commands, intelligible speech, reliable yes-or-no communication, and functional-object use," Joseph T. Giacino, Ph.D., of the JFK Johnson Rehabilitation Institute, Edison, N.J., and his associates reported in the March 1 issue of the New England Journal of Medicine.
The rate of recovery attenuated when treatment stopped, and scores on the Disability Rating Scale (DRS) were virtually indistinguishable between the treatment group and the control group 6 weeks later, indicating that the response was drug dependent.
"Practical and ethical constraints required the use of a brief treatment interval and a short-term assessment of the outcome [in this study], because we anticipated that caregivers would withdraw patients who were not making gains in order to try other treatments. Thus, our findings do not address the effects of prolonged treatment on long-term outcomes," the investigators noted.
"Whether treatment with amantadine, as compared with placebo, improves the long-term outcome or simply accelerates recovery en route to an equivalent level of function remains unknown."
The researchers conducted the trial after a pilot study indicated that amantadine improved functional outcomes after traumatic brain injury. They enrolled 184 patients aged 16-65 years who had sustained a nonpenetrating TBI 4-16 weeks previously and who were receiving usual inpatient rehabilitation at 11 medical centers in three countries.
Patients were diagnosed as being in a vegetative state, in which there was wakefulness without behavioral evidence of conscious awareness, or in a minimally conscious state, in which there was at least one clearly discernible behavioral sign of consciousness. All had scores above 11 on the DRS, which ranges from 0 (best) to 29 (worst); none were able to follow commands consistently or to engage in functional communication as assessed using the Coma Recovery Scale-Revised (CRS-R).
A total of 87 patients were randomly assigned to receive amantadine and 97 to receive a visually identical placebo for 4 weeks. They began at a dose of 100 mg twice daily, with escalations to 150 mg twice daily at week 3 and to 200 mg twice daily at week 4 if the DRS score had not improved by at least 2 points from baseline. Then the drug or placebo was tapered, and patients were assessed for another 6 weeks, according to Dr. Giacino and his associates. Dr. Giacino is also director of rehabilitation neuropsychology at the Spaulding Rehabilitation Hospital and is an associate professor in the department of physical medicine and rehabilitation at Harvard Medical School, both in Boston.
The primary outcome measure was the rate of improvement in the DRS score during treatment. DRS scores were assessed weekly by an interdisciplinary treatment team that was blinded to treatment assignment. Study personnel assessed clinically relevant behavioral benchmarks using the CRS-R.
Both study groups had significantly improved DRS scores at the end of the 4 weeks of treatment, but the amantadine group showed significantly faster improvement (–0.24 points/week more). In addition, at the final follow-up assessment, more patients in the amantadine group showed favorable outcomes on the DRS, fewer remained in a vegetative state, and more showed recovery of key behavioral benchmarks, Dr. Giacino and his colleagues said (N. Engl. J. Med. 2012;366:819-26). These benefits were consistent across all subgroups of patients. They were seen both in subjects in a minimally conscious state and in those in a vegetative state. And they were noted regardless of how long after the TBI injury the patients began treatment.
However, the treatment effect declined when drug therapy stopped, until there were no significant differences at the final follow-up assessment between patients who received the active drug and those who received placebo.
In particular, the proportion of patients who were able to engage in each of six clinically relevant behaviors was higher with amantadine during the month of therapy, but the difference between the two study groups was negligible at the final follow-up. These six behaviors were consistent command-following, object recognition, functional use of objects, intelligible verbalization, reliable yes-or-no communication, and sustained attention.
Amantadine "did not increase the risk of adverse medical, neurologic, or behavioral events, including those of greatest concern in this population (e.g., seizure)," the researchers noted.
The drug’s mechanism of action in these patients is uncertain. Amantadine is thought to promote dopaminergic activity "by facilitating presynaptic release and blocking reuptake postsynaptically." It may also enhance neurotransmission "in the dopamine-dependent nigrostriatal, mesolimbic, and frontostriatal circuits that are responsible for mediating arousal, drive, and attentional functions," they added.
"We conclude that amantadine is effective in accelerating the pace of recovery during acute rehabilitation in patients with prolonged post-traumatic disturbances in consciousness. Exposure to amantadine is associated with more rapid emergence of cognitively mediated behaviors that serve as the foundation for functional independence," Dr. Giacino and his associates wrote.
The study was supported by the U.S. National Institute on Disability and Rehabilitation Research. Dr. Giacino reported no relevant financial conflicts of interest, but a coauthor reported ties to Allergan, Merz, Ipsen, and Medtronic. Several other coauthors disclosed providing expert testimony on patients with TBI and disorders of consciousness.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Her Chief Complaint Is ... And by the Way She’s Also Pregnant
We emergency physicians are generally a confident bunch. But in the time it takes to slip on a peel and hit the pavement (a bananosecond), some of us ratchet up adrenaline output when we pick up a chart and notice a history like 22 yo F, minor MVC, c/o headache and back pain, 32 weeks pregnant.
From whence comes this anxiety? A bit may stem from reading about those seven-figure lawsuit verdicts for pregnancy-related malpractice cases. However, tied to this are those questions and comments I often hear from residents seeking assurance, even when they know the answers.
Can I get this x-ray?
Is it OK to give her morphine IV? Should I start with 1 mg? (Sure, if it’s in the right acupuncture point.)
Wow, I’m so used to not treating asymptomatic elevated BP that I almost forgot to address it for this pregnant patient.
Getting answers from specialists can often be frustrating. The OB doc may be uncomfortable with the non-OB aspects of the case, while the other consulting specialists may be uncomfortable applying their expertise in the context of pregnancy.
I recall asking a surgeon to look at a third-trimester patient with likely appendicitis and an equivocal ultrasound. His plan related to me was, "We’ll sit on it overnight." After making some remark about his own application of procto-tocin, I suggested an MRI. He was a bit leery, but with some education and pressure on our radiologist to do our hospital’s first MRI to rule out appendicitis (accomplished without procedural sedation on that radiologist), we identified an acute appy.
As with many aspects of EM, it may be up to the EP to coordinate optimal care in these situations. In 1981, Dr. Arnold Greensher and I developed a system called Prenatal Care – A Systems Approach to help OBs and primary care physicians integrate prenatal care within a comprehensive risk management system. It includes frequently updated information on managing nonobstetric illness and injury in this population. The system’s development was coordinated with a panel of well-regarded academic specialists, including a group of perinatologists.
The track record for the system has been quite surprising to us, as well as to the medical malpractice insurers who purchased the system for their docs: There were more than 1.5 million deliveries during this time period with only 8 malpractice claims. The expected number of claims would be 400-700. For a large number of users, premium rates went down dramatically during a time when national rates were going in the opposite direction.
Over the past year, I’ve contributed two well-received articles for the Focus On series in ACEP News: Trauma in the Obstetric Patient in July 2010 and Perinatal Disaster Management in September 2011 (both can be found at www.acep.org/focuson). I was honored to be invited by the publication’s editorial panel to provide a quarterly column that focuses on unique aspects of emergency care of the pregnant patient. The goal of this column will be to provide practical recommendations for the EP on common presenting problems in this population. I will often have coauthors, including specialists in that topic, as well as perinatologist input. One of our residents will be an integral part of this group. Our column is not intended to be a standard of care, but rather a sound, easy-to-use package of recommendations that would be considered one avenue for providing optimal care.
Each article will have a clinical tool – a summary that can stand alone for easy reference. In fact, our Trauma Table is posted in a number of EDs that I have visited. As ACEP News technology progresses, we hope to have these as a library with the tables hyperlinked to the specific didactic parts of the articles.
In this issue, we debut our first article, Stroke in Pregnancy (pp. XX-XX). This will provide a nice supplement to any stroke protocols at your hospital. Later in 2012, we plan to have one on sepsis and another on cardiac emergencies, including acute coronary syndromes.
I look forward to sharing this column with you.
Dr. Roemer is an Associate Professor in the Department of Emergency Medicine, Oklahoma University School of Community Medicine, Tulsa.
We emergency physicians are generally a confident bunch. But in the time it takes to slip on a peel and hit the pavement (a bananosecond), some of us ratchet up adrenaline output when we pick up a chart and notice a history like 22 yo F, minor MVC, c/o headache and back pain, 32 weeks pregnant.
From whence comes this anxiety? A bit may stem from reading about those seven-figure lawsuit verdicts for pregnancy-related malpractice cases. However, tied to this are those questions and comments I often hear from residents seeking assurance, even when they know the answers.
Can I get this x-ray?
Is it OK to give her morphine IV? Should I start with 1 mg? (Sure, if it’s in the right acupuncture point.)
Wow, I’m so used to not treating asymptomatic elevated BP that I almost forgot to address it for this pregnant patient.
Getting answers from specialists can often be frustrating. The OB doc may be uncomfortable with the non-OB aspects of the case, while the other consulting specialists may be uncomfortable applying their expertise in the context of pregnancy.
I recall asking a surgeon to look at a third-trimester patient with likely appendicitis and an equivocal ultrasound. His plan related to me was, "We’ll sit on it overnight." After making some remark about his own application of procto-tocin, I suggested an MRI. He was a bit leery, but with some education and pressure on our radiologist to do our hospital’s first MRI to rule out appendicitis (accomplished without procedural sedation on that radiologist), we identified an acute appy.
As with many aspects of EM, it may be up to the EP to coordinate optimal care in these situations. In 1981, Dr. Arnold Greensher and I developed a system called Prenatal Care – A Systems Approach to help OBs and primary care physicians integrate prenatal care within a comprehensive risk management system. It includes frequently updated information on managing nonobstetric illness and injury in this population. The system’s development was coordinated with a panel of well-regarded academic specialists, including a group of perinatologists.
The track record for the system has been quite surprising to us, as well as to the medical malpractice insurers who purchased the system for their docs: There were more than 1.5 million deliveries during this time period with only 8 malpractice claims. The expected number of claims would be 400-700. For a large number of users, premium rates went down dramatically during a time when national rates were going in the opposite direction.
Over the past year, I’ve contributed two well-received articles for the Focus On series in ACEP News: Trauma in the Obstetric Patient in July 2010 and Perinatal Disaster Management in September 2011 (both can be found at www.acep.org/focuson). I was honored to be invited by the publication’s editorial panel to provide a quarterly column that focuses on unique aspects of emergency care of the pregnant patient. The goal of this column will be to provide practical recommendations for the EP on common presenting problems in this population. I will often have coauthors, including specialists in that topic, as well as perinatologist input. One of our residents will be an integral part of this group. Our column is not intended to be a standard of care, but rather a sound, easy-to-use package of recommendations that would be considered one avenue for providing optimal care.
Each article will have a clinical tool – a summary that can stand alone for easy reference. In fact, our Trauma Table is posted in a number of EDs that I have visited. As ACEP News technology progresses, we hope to have these as a library with the tables hyperlinked to the specific didactic parts of the articles.
In this issue, we debut our first article, Stroke in Pregnancy (pp. XX-XX). This will provide a nice supplement to any stroke protocols at your hospital. Later in 2012, we plan to have one on sepsis and another on cardiac emergencies, including acute coronary syndromes.
I look forward to sharing this column with you.
Dr. Roemer is an Associate Professor in the Department of Emergency Medicine, Oklahoma University School of Community Medicine, Tulsa.
We emergency physicians are generally a confident bunch. But in the time it takes to slip on a peel and hit the pavement (a bananosecond), some of us ratchet up adrenaline output when we pick up a chart and notice a history like 22 yo F, minor MVC, c/o headache and back pain, 32 weeks pregnant.
From whence comes this anxiety? A bit may stem from reading about those seven-figure lawsuit verdicts for pregnancy-related malpractice cases. However, tied to this are those questions and comments I often hear from residents seeking assurance, even when they know the answers.
Can I get this x-ray?
Is it OK to give her morphine IV? Should I start with 1 mg? (Sure, if it’s in the right acupuncture point.)
Wow, I’m so used to not treating asymptomatic elevated BP that I almost forgot to address it for this pregnant patient.
Getting answers from specialists can often be frustrating. The OB doc may be uncomfortable with the non-OB aspects of the case, while the other consulting specialists may be uncomfortable applying their expertise in the context of pregnancy.
I recall asking a surgeon to look at a third-trimester patient with likely appendicitis and an equivocal ultrasound. His plan related to me was, "We’ll sit on it overnight." After making some remark about his own application of procto-tocin, I suggested an MRI. He was a bit leery, but with some education and pressure on our radiologist to do our hospital’s first MRI to rule out appendicitis (accomplished without procedural sedation on that radiologist), we identified an acute appy.
As with many aspects of EM, it may be up to the EP to coordinate optimal care in these situations. In 1981, Dr. Arnold Greensher and I developed a system called Prenatal Care – A Systems Approach to help OBs and primary care physicians integrate prenatal care within a comprehensive risk management system. It includes frequently updated information on managing nonobstetric illness and injury in this population. The system’s development was coordinated with a panel of well-regarded academic specialists, including a group of perinatologists.
The track record for the system has been quite surprising to us, as well as to the medical malpractice insurers who purchased the system for their docs: There were more than 1.5 million deliveries during this time period with only 8 malpractice claims. The expected number of claims would be 400-700. For a large number of users, premium rates went down dramatically during a time when national rates were going in the opposite direction.
Over the past year, I’ve contributed two well-received articles for the Focus On series in ACEP News: Trauma in the Obstetric Patient in July 2010 and Perinatal Disaster Management in September 2011 (both can be found at www.acep.org/focuson). I was honored to be invited by the publication’s editorial panel to provide a quarterly column that focuses on unique aspects of emergency care of the pregnant patient. The goal of this column will be to provide practical recommendations for the EP on common presenting problems in this population. I will often have coauthors, including specialists in that topic, as well as perinatologist input. One of our residents will be an integral part of this group. Our column is not intended to be a standard of care, but rather a sound, easy-to-use package of recommendations that would be considered one avenue for providing optimal care.
Each article will have a clinical tool – a summary that can stand alone for easy reference. In fact, our Trauma Table is posted in a number of EDs that I have visited. As ACEP News technology progresses, we hope to have these as a library with the tables hyperlinked to the specific didactic parts of the articles.
In this issue, we debut our first article, Stroke in Pregnancy (pp. XX-XX). This will provide a nice supplement to any stroke protocols at your hospital. Later in 2012, we plan to have one on sepsis and another on cardiac emergencies, including acute coronary syndromes.
I look forward to sharing this column with you.
Dr. Roemer is an Associate Professor in the Department of Emergency Medicine, Oklahoma University School of Community Medicine, Tulsa.
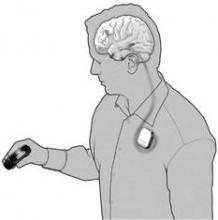
Implanted EEG Device Predicts Seizures in Early Study
BALTIMORE – A novel implantable device has demonstrated potential for predicting seizure onset in a preliminary analysis of 15 adult patients with medically refractory complex partial seizures.
The ambulatory intracranial EEG (iEEG) device, NeuroVista’s Seizure Advisory System (SAS), consists of electrodes that are implanted between the skull and the brain surface that continuously record electrical activity. The electrodes are connected by wires to a data storage device implanted in the chest. Signals are transmitted wirelessly to an external handheld device that processes the data and transmits visual and audible signals to the patient. A blue light signifies a low likelihood of seizures, white indicates medium susceptibility, and red alerts to a high likelihood of impending seizure.
"This is something we’ve never been able to do before, to predict when a seizure might happen, which potentially gives the opportunity for patients to make themselves safe, or possibly even take an acute-acting medication long-term. The uncertainty of when a seizure might occur is the most disabling part of seizures for most people. So to be able to have this sort of warning, to be able to structure day-to-day activities around [seizures] potentially, will mean a lot to people being able to control their lives," said Dr. Mark Cook, chair of medicine and director of neurosciences at St. Vincent’s Hospital, Melbourne. Dr. Cook reported the results of the study at the annual meeting of the American Epilepsy Society.
The 15 study subjects were all 18 years and older, had disabling partial and/or secondarily generalized partial seizures, and had failed therapeutic treatment with a minimum of two antiepileptic drugs. They were implanted at one of three clinical centers in Australia: Austin Health, The Royal Melbourne Hospital, and St. Vincent’s Hospital. At baseline, the patients reported experiencing 2-12 disabling partial onset seizures per month. Following intracranial implantation of the device, iEEG was collected to configure a patient-specific algorithm that identified periods of low, moderate, and high seizure likelihood.
In the data collection phase of the study, the estimated performance for the high and low likelihood advisories had to be statistically superior to a time-matched chance predictor with P value of .05 or less, and the high likelihood advisory sensitivity could not be statistically inferior to 65%. Patients who met those criteria entered an Advisory Phase where their system was configured to provide visual and audible advisories that indicate seizure likelihood.
In some instances, seizure incidence as recorded by the device was dramatically different from what the patient had reported. In one patient who initially reported having 7 seizures per month, the SAS recorded 104 per month, based on data covering 70 days. Another patient who reported 6 seizures per month actually had 80 per month, based on 126 days of data. Other patients overestimated their seizures, with one patient who reported having 3 seizures per month experiencing just 0.6 seizures per month, based on 209 days of data.
"Some patients overestimate the number of their seizures they’re having, but the amount by which [others] underestimate their seizures is very dramatic, sometimes by a factor of 10," Dr. Cook commented.
In the data collection (training) phase of the study, the sensitivity of the red advisory among 11 patients who completed the phase ranged from 0.65 to 1.00, with 10 of those patients meeting the performance criteria for the seizure advisories. Among 6 patients who have completed the subsequent 4-month advisory (prospective) study phase, red advisory sensitivity ranged from 0.56 to 1.00. In both study phases, there were no seizures during the low advisory (100% negative predictive value). The other four patients are still in the data collection phase.
The safety profile is consistent with published literature for strip electrodes and subclavicular implants, he said.
NeuroVista is continuing to study the clinical utility of the SAS, a company spokesman said.
Dr. Cook stated that he had no financial disclosures. The study was funded by NeuroVista, and four of the coinvestigators are company employees.
BALTIMORE – A novel implantable device has demonstrated potential for predicting seizure onset in a preliminary analysis of 15 adult patients with medically refractory complex partial seizures.
The ambulatory intracranial EEG (iEEG) device, NeuroVista’s Seizure Advisory System (SAS), consists of electrodes that are implanted between the skull and the brain surface that continuously record electrical activity. The electrodes are connected by wires to a data storage device implanted in the chest. Signals are transmitted wirelessly to an external handheld device that processes the data and transmits visual and audible signals to the patient. A blue light signifies a low likelihood of seizures, white indicates medium susceptibility, and red alerts to a high likelihood of impending seizure.
"This is something we’ve never been able to do before, to predict when a seizure might happen, which potentially gives the opportunity for patients to make themselves safe, or possibly even take an acute-acting medication long-term. The uncertainty of when a seizure might occur is the most disabling part of seizures for most people. So to be able to have this sort of warning, to be able to structure day-to-day activities around [seizures] potentially, will mean a lot to people being able to control their lives," said Dr. Mark Cook, chair of medicine and director of neurosciences at St. Vincent’s Hospital, Melbourne. Dr. Cook reported the results of the study at the annual meeting of the American Epilepsy Society.
The 15 study subjects were all 18 years and older, had disabling partial and/or secondarily generalized partial seizures, and had failed therapeutic treatment with a minimum of two antiepileptic drugs. They were implanted at one of three clinical centers in Australia: Austin Health, The Royal Melbourne Hospital, and St. Vincent’s Hospital. At baseline, the patients reported experiencing 2-12 disabling partial onset seizures per month. Following intracranial implantation of the device, iEEG was collected to configure a patient-specific algorithm that identified periods of low, moderate, and high seizure likelihood.
In the data collection phase of the study, the estimated performance for the high and low likelihood advisories had to be statistically superior to a time-matched chance predictor with P value of .05 or less, and the high likelihood advisory sensitivity could not be statistically inferior to 65%. Patients who met those criteria entered an Advisory Phase where their system was configured to provide visual and audible advisories that indicate seizure likelihood.
In some instances, seizure incidence as recorded by the device was dramatically different from what the patient had reported. In one patient who initially reported having 7 seizures per month, the SAS recorded 104 per month, based on data covering 70 days. Another patient who reported 6 seizures per month actually had 80 per month, based on 126 days of data. Other patients overestimated their seizures, with one patient who reported having 3 seizures per month experiencing just 0.6 seizures per month, based on 209 days of data.
"Some patients overestimate the number of their seizures they’re having, but the amount by which [others] underestimate their seizures is very dramatic, sometimes by a factor of 10," Dr. Cook commented.
In the data collection (training) phase of the study, the sensitivity of the red advisory among 11 patients who completed the phase ranged from 0.65 to 1.00, with 10 of those patients meeting the performance criteria for the seizure advisories. Among 6 patients who have completed the subsequent 4-month advisory (prospective) study phase, red advisory sensitivity ranged from 0.56 to 1.00. In both study phases, there were no seizures during the low advisory (100% negative predictive value). The other four patients are still in the data collection phase.
The safety profile is consistent with published literature for strip electrodes and subclavicular implants, he said.
NeuroVista is continuing to study the clinical utility of the SAS, a company spokesman said.
Dr. Cook stated that he had no financial disclosures. The study was funded by NeuroVista, and four of the coinvestigators are company employees.
BALTIMORE – A novel implantable device has demonstrated potential for predicting seizure onset in a preliminary analysis of 15 adult patients with medically refractory complex partial seizures.
The ambulatory intracranial EEG (iEEG) device, NeuroVista’s Seizure Advisory System (SAS), consists of electrodes that are implanted between the skull and the brain surface that continuously record electrical activity. The electrodes are connected by wires to a data storage device implanted in the chest. Signals are transmitted wirelessly to an external handheld device that processes the data and transmits visual and audible signals to the patient. A blue light signifies a low likelihood of seizures, white indicates medium susceptibility, and red alerts to a high likelihood of impending seizure.
"This is something we’ve never been able to do before, to predict when a seizure might happen, which potentially gives the opportunity for patients to make themselves safe, or possibly even take an acute-acting medication long-term. The uncertainty of when a seizure might occur is the most disabling part of seizures for most people. So to be able to have this sort of warning, to be able to structure day-to-day activities around [seizures] potentially, will mean a lot to people being able to control their lives," said Dr. Mark Cook, chair of medicine and director of neurosciences at St. Vincent’s Hospital, Melbourne. Dr. Cook reported the results of the study at the annual meeting of the American Epilepsy Society.
The 15 study subjects were all 18 years and older, had disabling partial and/or secondarily generalized partial seizures, and had failed therapeutic treatment with a minimum of two antiepileptic drugs. They were implanted at one of three clinical centers in Australia: Austin Health, The Royal Melbourne Hospital, and St. Vincent’s Hospital. At baseline, the patients reported experiencing 2-12 disabling partial onset seizures per month. Following intracranial implantation of the device, iEEG was collected to configure a patient-specific algorithm that identified periods of low, moderate, and high seizure likelihood.
In the data collection phase of the study, the estimated performance for the high and low likelihood advisories had to be statistically superior to a time-matched chance predictor with P value of .05 or less, and the high likelihood advisory sensitivity could not be statistically inferior to 65%. Patients who met those criteria entered an Advisory Phase where their system was configured to provide visual and audible advisories that indicate seizure likelihood.
In some instances, seizure incidence as recorded by the device was dramatically different from what the patient had reported. In one patient who initially reported having 7 seizures per month, the SAS recorded 104 per month, based on data covering 70 days. Another patient who reported 6 seizures per month actually had 80 per month, based on 126 days of data. Other patients overestimated their seizures, with one patient who reported having 3 seizures per month experiencing just 0.6 seizures per month, based on 209 days of data.
"Some patients overestimate the number of their seizures they’re having, but the amount by which [others] underestimate their seizures is very dramatic, sometimes by a factor of 10," Dr. Cook commented.
In the data collection (training) phase of the study, the sensitivity of the red advisory among 11 patients who completed the phase ranged from 0.65 to 1.00, with 10 of those patients meeting the performance criteria for the seizure advisories. Among 6 patients who have completed the subsequent 4-month advisory (prospective) study phase, red advisory sensitivity ranged from 0.56 to 1.00. In both study phases, there were no seizures during the low advisory (100% negative predictive value). The other four patients are still in the data collection phase.
The safety profile is consistent with published literature for strip electrodes and subclavicular implants, he said.
NeuroVista is continuing to study the clinical utility of the SAS, a company spokesman said.
Dr. Cook stated that he had no financial disclosures. The study was funded by NeuroVista, and four of the coinvestigators are company employees.
FROM THE ANNUAL MEETING OF THE AMERICAN EPILEPSY SOCIETY
Major Finding: The sensitivity of the device for time periods deemed to have a high likelihood of impending seizure ranged from 0.56 to 1.00 in six patients who completed a 4-month prospective phase of the study.
Data Source: A preliminary study of 15 patients implanted with NeuroVista’s Seizure Advisory System.
Disclosures: Dr. Cook stated that he had no financial disclosures. The study was funded by NeuroVista, and four of the coinvestigators are company employees.
Despite Potential Gains, Patients Balk at Epilepsy Surgery
BALTIMORE – Despite the very real chance of living a seizure-free life, many epilepsy patients with an excellent surgical prognosis continue to walk away from the procedures.
Researchers at the annual meeting of the American Epilepsy Society agreed: It’s not always easy to convince a patient with refractory seizures that removing part of his or her brain could be the best treatment option.
"Many times, you bring up the idea of surgery, and see a look of shock and horror," Dr. Chad Carlson said in a press briefing. "Some are intrigued by the idea that their seizures could be reduced or even eliminated, but there is a real population who are either apprehensive or who flatly say: ‘You are not taking out a piece of my brain.’ "
Dr. Carlson and his colleagues categorized 445 patients with intractable seizures into three groups, using a set of clinical characteristics predictive of surgical outcome. Grade 1 patients (110) had the highest likelihood of becoming seizure free.
"What surprised us was that only 43 of these patients went on to have surgery" at the center, he said. The attrition rate for epilepsy surgery is frustrating, especially in light of the outcomes for those who did have it. "At 18 months, 89% were completely free of seizures," Dr. Carlson said.
In a second study examining why patients refuse surgery, Dr. Christopher T. Anderson, director of the epilepsy monitoring unit at the University of Pennsylvania, Philadelphia, discussed a cohort of 32 patients, all of whom were good surgical candidates and who underwent an intensive, year-long presurgical evaluation.
The process is time consuming, expensive, and not without risk, since some of the tests are invasive – electroencephalograms, high-resolution MRIs, positron emission tomography, the intracarotid sodium amobarbital procedure to localize the brain’s language center, and a comprehensive neuropsychological test battery. The evaluation costs up to $10,000, he added.
After completing the process, 9 of the 23 surgical candidates refused to go forward with the procedure. The review identified several characteristics that predicted both acceptance and rejection of surgery.
The patients were an average of 48 years; their epilepsy began at a median age of 22 years. Despite having tried a median of six drugs, the patients continued to have up to 10 or more seizures each month.
There were some significant between-group differences, which Dr. Anderson said could be used to predict which patients eventually would accept or refuse surgery. Easily treatable psychiatric disorders were some of the most striking. Nearly half (44%) of the refusers had anxiety and 11% had depression, compared with 4% for each disorder among the surgery group. In fact, Dr. Anderson said, most of those who accepted surgery (83%) had no psychiatric disorder.
"These problems are ones that are easily treatable, if not completely solvable," he said.
Factors associated with the seizures themselves also influenced decision-making. Patients who had more seizures each month (average, 12) were more likely to accept the procedure than those with fewer seizures (average, 3).
Patients who perceived that their seizures seriously impeded their life also were more likely to accept surgery. "Those who thought of their seizures as very disabling or as a stigma, embarrassing, or dangerous were much more likely to opt for surgery," Dr. Anderson noted.
Patients who deferred surgery were significantly more likely to have a general fear of surgery and surgical complications. "They cited a lack of comfort with surgery, complications with the surgery and anesthesia, and other health conditions that might affect surgery, like diabetes, hypertension, even though these are all easily managed in the operating room," he said.
Some patients perceived the surgery as experimental and expressed worry about being a "guinea pig."
"I think we need to try a lot more to educate patients on the safety of epilepsy surgery," he said. "In no way is this experimental."
The study drives home the point that some perceived barriers could be overcome with education and open communication. "We might want to look at interventions to help patients understand the surgery. Even a program of desensitizing patients to the operating room might help," Dr. Anderson said.
Dr. Carlson, director of the Comprehensive Epilepsy Center’s video EEG lab at NYU Langone Medical Center, New York, faced a similar issue. The 39% of his cohort (43) who did have resection had excellent outcomes, but the rest of the patients were not ready to make the decision.
"Many of them did not even progress to our multidisciplinary conference, even though they were admitted for presurgical evaluation. At some point, 37% of the cohort (41) voted with their feet. They left and never followed up with us."
Among this group were 25 who had become seizure-free during the observation time. Although the data say that this probably wouldn’t continue, they still decided not to pursue the surgery, Dr. Carlson said.
A small group (8) was not seizure free but decided that their seizure control was "good enough," he said. "It wasn’t what an epileptologist would consider good control, but it wasn’t serious enough for those patients to have the surgery."
The remaining patients were lost to follow-up or had no record of a specific reason for refusing surgery. Insurance denials only affected two patients who wanted surgery.
Some of those lost who were to follow-up probably eventually had surgery at another center. Patients seek multiple opinions "until they find one that they agree with" or a provider "clicks" with them, Dr. Carlson said.
Both researchers said that primary neurologists and other providers could help by getting the topic of surgery on the table earlier. "It’s something that should be done at multiple time points," Dr. Anderson said. "Mention that they might be a candidate for brain surgery since medical therapy isn’t working well. Explain this means removal of part of the brain and ask what they feel about that – would they consider it if it would get rid of their seizures?
"If they hear this multiple times, then you are introducing this concept and revisiting it with more detailed information each time. Then the patient might be more willing to take that leap."
Neither Dr. Anderson nor Dr. Carlson had any financial declarations.
BALTIMORE – Despite the very real chance of living a seizure-free life, many epilepsy patients with an excellent surgical prognosis continue to walk away from the procedures.
Researchers at the annual meeting of the American Epilepsy Society agreed: It’s not always easy to convince a patient with refractory seizures that removing part of his or her brain could be the best treatment option.
"Many times, you bring up the idea of surgery, and see a look of shock and horror," Dr. Chad Carlson said in a press briefing. "Some are intrigued by the idea that their seizures could be reduced or even eliminated, but there is a real population who are either apprehensive or who flatly say: ‘You are not taking out a piece of my brain.’ "
Dr. Carlson and his colleagues categorized 445 patients with intractable seizures into three groups, using a set of clinical characteristics predictive of surgical outcome. Grade 1 patients (110) had the highest likelihood of becoming seizure free.
"What surprised us was that only 43 of these patients went on to have surgery" at the center, he said. The attrition rate for epilepsy surgery is frustrating, especially in light of the outcomes for those who did have it. "At 18 months, 89% were completely free of seizures," Dr. Carlson said.
In a second study examining why patients refuse surgery, Dr. Christopher T. Anderson, director of the epilepsy monitoring unit at the University of Pennsylvania, Philadelphia, discussed a cohort of 32 patients, all of whom were good surgical candidates and who underwent an intensive, year-long presurgical evaluation.
The process is time consuming, expensive, and not without risk, since some of the tests are invasive – electroencephalograms, high-resolution MRIs, positron emission tomography, the intracarotid sodium amobarbital procedure to localize the brain’s language center, and a comprehensive neuropsychological test battery. The evaluation costs up to $10,000, he added.
After completing the process, 9 of the 23 surgical candidates refused to go forward with the procedure. The review identified several characteristics that predicted both acceptance and rejection of surgery.
The patients were an average of 48 years; their epilepsy began at a median age of 22 years. Despite having tried a median of six drugs, the patients continued to have up to 10 or more seizures each month.
There were some significant between-group differences, which Dr. Anderson said could be used to predict which patients eventually would accept or refuse surgery. Easily treatable psychiatric disorders were some of the most striking. Nearly half (44%) of the refusers had anxiety and 11% had depression, compared with 4% for each disorder among the surgery group. In fact, Dr. Anderson said, most of those who accepted surgery (83%) had no psychiatric disorder.
"These problems are ones that are easily treatable, if not completely solvable," he said.
Factors associated with the seizures themselves also influenced decision-making. Patients who had more seizures each month (average, 12) were more likely to accept the procedure than those with fewer seizures (average, 3).
Patients who perceived that their seizures seriously impeded their life also were more likely to accept surgery. "Those who thought of their seizures as very disabling or as a stigma, embarrassing, or dangerous were much more likely to opt for surgery," Dr. Anderson noted.
Patients who deferred surgery were significantly more likely to have a general fear of surgery and surgical complications. "They cited a lack of comfort with surgery, complications with the surgery and anesthesia, and other health conditions that might affect surgery, like diabetes, hypertension, even though these are all easily managed in the operating room," he said.
Some patients perceived the surgery as experimental and expressed worry about being a "guinea pig."
"I think we need to try a lot more to educate patients on the safety of epilepsy surgery," he said. "In no way is this experimental."
The study drives home the point that some perceived barriers could be overcome with education and open communication. "We might want to look at interventions to help patients understand the surgery. Even a program of desensitizing patients to the operating room might help," Dr. Anderson said.
Dr. Carlson, director of the Comprehensive Epilepsy Center’s video EEG lab at NYU Langone Medical Center, New York, faced a similar issue. The 39% of his cohort (43) who did have resection had excellent outcomes, but the rest of the patients were not ready to make the decision.
"Many of them did not even progress to our multidisciplinary conference, even though they were admitted for presurgical evaluation. At some point, 37% of the cohort (41) voted with their feet. They left and never followed up with us."
Among this group were 25 who had become seizure-free during the observation time. Although the data say that this probably wouldn’t continue, they still decided not to pursue the surgery, Dr. Carlson said.
A small group (8) was not seizure free but decided that their seizure control was "good enough," he said. "It wasn’t what an epileptologist would consider good control, but it wasn’t serious enough for those patients to have the surgery."
The remaining patients were lost to follow-up or had no record of a specific reason for refusing surgery. Insurance denials only affected two patients who wanted surgery.
Some of those lost who were to follow-up probably eventually had surgery at another center. Patients seek multiple opinions "until they find one that they agree with" or a provider "clicks" with them, Dr. Carlson said.
Both researchers said that primary neurologists and other providers could help by getting the topic of surgery on the table earlier. "It’s something that should be done at multiple time points," Dr. Anderson said. "Mention that they might be a candidate for brain surgery since medical therapy isn’t working well. Explain this means removal of part of the brain and ask what they feel about that – would they consider it if it would get rid of their seizures?
"If they hear this multiple times, then you are introducing this concept and revisiting it with more detailed information each time. Then the patient might be more willing to take that leap."
Neither Dr. Anderson nor Dr. Carlson had any financial declarations.
BALTIMORE – Despite the very real chance of living a seizure-free life, many epilepsy patients with an excellent surgical prognosis continue to walk away from the procedures.
Researchers at the annual meeting of the American Epilepsy Society agreed: It’s not always easy to convince a patient with refractory seizures that removing part of his or her brain could be the best treatment option.
"Many times, you bring up the idea of surgery, and see a look of shock and horror," Dr. Chad Carlson said in a press briefing. "Some are intrigued by the idea that their seizures could be reduced or even eliminated, but there is a real population who are either apprehensive or who flatly say: ‘You are not taking out a piece of my brain.’ "
Dr. Carlson and his colleagues categorized 445 patients with intractable seizures into three groups, using a set of clinical characteristics predictive of surgical outcome. Grade 1 patients (110) had the highest likelihood of becoming seizure free.
"What surprised us was that only 43 of these patients went on to have surgery" at the center, he said. The attrition rate for epilepsy surgery is frustrating, especially in light of the outcomes for those who did have it. "At 18 months, 89% were completely free of seizures," Dr. Carlson said.
In a second study examining why patients refuse surgery, Dr. Christopher T. Anderson, director of the epilepsy monitoring unit at the University of Pennsylvania, Philadelphia, discussed a cohort of 32 patients, all of whom were good surgical candidates and who underwent an intensive, year-long presurgical evaluation.
The process is time consuming, expensive, and not without risk, since some of the tests are invasive – electroencephalograms, high-resolution MRIs, positron emission tomography, the intracarotid sodium amobarbital procedure to localize the brain’s language center, and a comprehensive neuropsychological test battery. The evaluation costs up to $10,000, he added.
After completing the process, 9 of the 23 surgical candidates refused to go forward with the procedure. The review identified several characteristics that predicted both acceptance and rejection of surgery.
The patients were an average of 48 years; their epilepsy began at a median age of 22 years. Despite having tried a median of six drugs, the patients continued to have up to 10 or more seizures each month.
There were some significant between-group differences, which Dr. Anderson said could be used to predict which patients eventually would accept or refuse surgery. Easily treatable psychiatric disorders were some of the most striking. Nearly half (44%) of the refusers had anxiety and 11% had depression, compared with 4% for each disorder among the surgery group. In fact, Dr. Anderson said, most of those who accepted surgery (83%) had no psychiatric disorder.
"These problems are ones that are easily treatable, if not completely solvable," he said.
Factors associated with the seizures themselves also influenced decision-making. Patients who had more seizures each month (average, 12) were more likely to accept the procedure than those with fewer seizures (average, 3).
Patients who perceived that their seizures seriously impeded their life also were more likely to accept surgery. "Those who thought of their seizures as very disabling or as a stigma, embarrassing, or dangerous were much more likely to opt for surgery," Dr. Anderson noted.
Patients who deferred surgery were significantly more likely to have a general fear of surgery and surgical complications. "They cited a lack of comfort with surgery, complications with the surgery and anesthesia, and other health conditions that might affect surgery, like diabetes, hypertension, even though these are all easily managed in the operating room," he said.
Some patients perceived the surgery as experimental and expressed worry about being a "guinea pig."
"I think we need to try a lot more to educate patients on the safety of epilepsy surgery," he said. "In no way is this experimental."
The study drives home the point that some perceived barriers could be overcome with education and open communication. "We might want to look at interventions to help patients understand the surgery. Even a program of desensitizing patients to the operating room might help," Dr. Anderson said.
Dr. Carlson, director of the Comprehensive Epilepsy Center’s video EEG lab at NYU Langone Medical Center, New York, faced a similar issue. The 39% of his cohort (43) who did have resection had excellent outcomes, but the rest of the patients were not ready to make the decision.
"Many of them did not even progress to our multidisciplinary conference, even though they were admitted for presurgical evaluation. At some point, 37% of the cohort (41) voted with their feet. They left and never followed up with us."
Among this group were 25 who had become seizure-free during the observation time. Although the data say that this probably wouldn’t continue, they still decided not to pursue the surgery, Dr. Carlson said.
A small group (8) was not seizure free but decided that their seizure control was "good enough," he said. "It wasn’t what an epileptologist would consider good control, but it wasn’t serious enough for those patients to have the surgery."
The remaining patients were lost to follow-up or had no record of a specific reason for refusing surgery. Insurance denials only affected two patients who wanted surgery.
Some of those lost who were to follow-up probably eventually had surgery at another center. Patients seek multiple opinions "until they find one that they agree with" or a provider "clicks" with them, Dr. Carlson said.
Both researchers said that primary neurologists and other providers could help by getting the topic of surgery on the table earlier. "It’s something that should be done at multiple time points," Dr. Anderson said. "Mention that they might be a candidate for brain surgery since medical therapy isn’t working well. Explain this means removal of part of the brain and ask what they feel about that – would they consider it if it would get rid of their seizures?
"If they hear this multiple times, then you are introducing this concept and revisiting it with more detailed information each time. Then the patient might be more willing to take that leap."
Neither Dr. Anderson nor Dr. Carlson had any financial declarations.
FROM THE ANNUAL MEETING OF THE AMERICAN EPILEPSY SOCIETY
Major Finding: Among 110 epilepsy patients with the best surgical prognosis, only 45 elected to have a surgical procedure. Of these, most (89%) became completely seizure-free.
Data Source: A retrospective review of patients with intractable seizures.
Disclosures: Neither Dr. Anderson nor Dr. Carlson had any financial disclosures.
Imaging Sheds Light on Lasting Effects of TBI
Major Finding: Various functional and structural imaging techniques can provide a prognosis for TBI patients and objective evidence to insurers to cover rehabilitative services.
Data Source: A review of recent imaging studies of traumatic brain injury.
Disclosures: None
BANGKOK, THAILAND — New imaging techniques may help to explain the disabling symptoms that can plague patients with traumatic brain injury long after their acute problems have resolved, and eventually guide the best choice for medical therapy.
Survivors of traumatic brain injury who complain of depression, irritability, and memory or cognitive problems are often written off as psychiatric cases or malingerers, Dr. Ramon Diaz-Arrastia said at the World Congress of Neurology. “This is the frustrating thing about TBI. The patients might look OK—they don't have paralysis; they are walking around. But their cognitive and behavioral problems are real.”
Imaging techniques that are now well established in other areas of neurology—such as diffusion-weighted and susceptibility-weighted magnetic resonance imaging—are now being used to show that brain injuries leave permanent, life-altering marks behind after the contusions and hematomas have healed.
These findings may have both immediate and long-range benefits, said Dr. Diaz-Arrastia, a professor of neurology at the University of Texas Southwestern Medical Center, Dallas. “Right now, imaging these patients has the primary value of providing a prognosis and perhaps helping them obtain the care that they need. In terms of reimbursement, it's useful to have an objective documentation of the injury when trying to convince insurers to cover rehabilitative service.”
In the future, imaging the post-TBI brain may help guide medical treatment choices and monitor drugs' effectiveness.
So far, nearly 30 drugs have provided effective neuroprotection in animal models of TBI, he said. However, none that has undergone testing in well-designed phase III trials has proven beneficial to humans.
Part of the problem may be the heterogeneity of human brain injury, Dr. Diaz-Arrastia said. There are many subtypes of TBI, yet “from the point of view of the clinical trials, all patients who present in a coma [after a brain injury] are treated the same way, even though the injuries can be very different, with very different prognoses.”
Susceptibility-weighted imaging (SWI) is one technique being studied in TBI patients. It measures the paramagnetic shift of intravascular deoxyhemoglobin and methemoglobin, amplifying the appearance of microhemorrhages and making them much easier to identify. “SWI picks up 640% more lesions and 200% more lesion volume than does gradient-recall echo,” Dr. Diaz-Arrastia said, referring to work by Dr. Karen Tong from Loma Linda (California) University.
SWI is very good at identifying diffuse microvascular injury, a marker for diffuse axonal injury that is usually invisible on computed axial tomography. “The only problem is that SWI may be overly sensitive,” he said. “One patient with a lot of microhemorrhages might be complaining only of headache and dizziness, whereas another with a similar volume might have a lot more problems.”
Diffusion-weighted imaging (DWI), which is well established in the stroke world, is understudied in TBI, probably because it's a challenge to perform magnetic resonance imaging on these acutely ill patients. But this technique provides detailed information about the makeup of lesions, showing vasogenic and cytotoxic edema, as well as location in the superficial or deep structures in both gray and white matter.
Dr. Diaz-Arrastia and his colleagues performed DWI on 99 patients with TBI. Of these, the study identified corpus callosum lesions in 84%. It was able to differentiate between patients with primarily cytotoxic lesions (54%) and those with vasogenic lesions (46%). “We also found that the volume of these brain lesions, irrespective of location, explained about 28% of the variance in outcome among these patients,” he said. “It's a relatively modest correlation with outcome, but it shows that what we are measuring is something that is functionally important and affects outcome.”
Diffusion tensor imaging [DTI] shows how water tracks along the axons, giving a good view of white matter lesions. Follow-up scans on TBI patients have shown tantalizing clues to the possible causes of their long-term problems.
“When we scan patients within a day or two of injury, we may see subtle changes in the parameters. But if we come back 6 months later and rescan, we see much greater dropout of axons. Initially, the patient may be in a coma, and when they are scanned later they are usually much improved and walking around, albeit with problems with memory or executive function. So this tells us that something is happening weeks or months after the injury that results in white matter dropout.”
Another technique moving into trauma field is quantitative volumetric assessment of the cortical field. This technique measures the thickness and volume of different cortical and subcortical regions, Dr. Diaz-Arrastia said. “In our patients, we have found that the brain shrinks overall after a severe traumatic injury, but that not all structures shrink at the same rate. Some cortical regions shrink very little, while others, like the hippocampus, appear particularly sensitive to injury.” This finding makes sense given the cognitive and mood issues that TBI patients can experience, he said.
On average, whole brain volume and both grey and white matter volumes decreased by 3%–10% in the first few months after severe TBI. In comparison, Dr. Diaz-Arrastia said, the rate of atrophy for patients with Alzheimer's disease is about 1%–2% a year.
Functional MRIs also provide some clues that the injured brain sustains long-term problems. In the resting state, the blood oxygen level dependent signal typically shows seemingly random fluctuations when the brain is at rest. But recent studies have shown that these fluctuations actually represent the communication between brain regions that work together.
“In our patients, we found a very high indication that the functional connectivity between the hippocampi was greatly reduced,” compared with controls, he said.
Imaging provides objective documentation to help convince insurers to cover rehabilitative services.
Source DR. DIAZ-ARRASTIA
Major Finding: Various functional and structural imaging techniques can provide a prognosis for TBI patients and objective evidence to insurers to cover rehabilitative services.
Data Source: A review of recent imaging studies of traumatic brain injury.
Disclosures: None
BANGKOK, THAILAND — New imaging techniques may help to explain the disabling symptoms that can plague patients with traumatic brain injury long after their acute problems have resolved, and eventually guide the best choice for medical therapy.
Survivors of traumatic brain injury who complain of depression, irritability, and memory or cognitive problems are often written off as psychiatric cases or malingerers, Dr. Ramon Diaz-Arrastia said at the World Congress of Neurology. “This is the frustrating thing about TBI. The patients might look OK—they don't have paralysis; they are walking around. But their cognitive and behavioral problems are real.”
Imaging techniques that are now well established in other areas of neurology—such as diffusion-weighted and susceptibility-weighted magnetic resonance imaging—are now being used to show that brain injuries leave permanent, life-altering marks behind after the contusions and hematomas have healed.
These findings may have both immediate and long-range benefits, said Dr. Diaz-Arrastia, a professor of neurology at the University of Texas Southwestern Medical Center, Dallas. “Right now, imaging these patients has the primary value of providing a prognosis and perhaps helping them obtain the care that they need. In terms of reimbursement, it's useful to have an objective documentation of the injury when trying to convince insurers to cover rehabilitative service.”
In the future, imaging the post-TBI brain may help guide medical treatment choices and monitor drugs' effectiveness.
So far, nearly 30 drugs have provided effective neuroprotection in animal models of TBI, he said. However, none that has undergone testing in well-designed phase III trials has proven beneficial to humans.
Part of the problem may be the heterogeneity of human brain injury, Dr. Diaz-Arrastia said. There are many subtypes of TBI, yet “from the point of view of the clinical trials, all patients who present in a coma [after a brain injury] are treated the same way, even though the injuries can be very different, with very different prognoses.”
Susceptibility-weighted imaging (SWI) is one technique being studied in TBI patients. It measures the paramagnetic shift of intravascular deoxyhemoglobin and methemoglobin, amplifying the appearance of microhemorrhages and making them much easier to identify. “SWI picks up 640% more lesions and 200% more lesion volume than does gradient-recall echo,” Dr. Diaz-Arrastia said, referring to work by Dr. Karen Tong from Loma Linda (California) University.
SWI is very good at identifying diffuse microvascular injury, a marker for diffuse axonal injury that is usually invisible on computed axial tomography. “The only problem is that SWI may be overly sensitive,” he said. “One patient with a lot of microhemorrhages might be complaining only of headache and dizziness, whereas another with a similar volume might have a lot more problems.”
Diffusion-weighted imaging (DWI), which is well established in the stroke world, is understudied in TBI, probably because it's a challenge to perform magnetic resonance imaging on these acutely ill patients. But this technique provides detailed information about the makeup of lesions, showing vasogenic and cytotoxic edema, as well as location in the superficial or deep structures in both gray and white matter.
Dr. Diaz-Arrastia and his colleagues performed DWI on 99 patients with TBI. Of these, the study identified corpus callosum lesions in 84%. It was able to differentiate between patients with primarily cytotoxic lesions (54%) and those with vasogenic lesions (46%). “We also found that the volume of these brain lesions, irrespective of location, explained about 28% of the variance in outcome among these patients,” he said. “It's a relatively modest correlation with outcome, but it shows that what we are measuring is something that is functionally important and affects outcome.”
Diffusion tensor imaging [DTI] shows how water tracks along the axons, giving a good view of white matter lesions. Follow-up scans on TBI patients have shown tantalizing clues to the possible causes of their long-term problems.
“When we scan patients within a day or two of injury, we may see subtle changes in the parameters. But if we come back 6 months later and rescan, we see much greater dropout of axons. Initially, the patient may be in a coma, and when they are scanned later they are usually much improved and walking around, albeit with problems with memory or executive function. So this tells us that something is happening weeks or months after the injury that results in white matter dropout.”
Another technique moving into trauma field is quantitative volumetric assessment of the cortical field. This technique measures the thickness and volume of different cortical and subcortical regions, Dr. Diaz-Arrastia said. “In our patients, we have found that the brain shrinks overall after a severe traumatic injury, but that not all structures shrink at the same rate. Some cortical regions shrink very little, while others, like the hippocampus, appear particularly sensitive to injury.” This finding makes sense given the cognitive and mood issues that TBI patients can experience, he said.
On average, whole brain volume and both grey and white matter volumes decreased by 3%–10% in the first few months after severe TBI. In comparison, Dr. Diaz-Arrastia said, the rate of atrophy for patients with Alzheimer's disease is about 1%–2% a year.
Functional MRIs also provide some clues that the injured brain sustains long-term problems. In the resting state, the blood oxygen level dependent signal typically shows seemingly random fluctuations when the brain is at rest. But recent studies have shown that these fluctuations actually represent the communication between brain regions that work together.
“In our patients, we found a very high indication that the functional connectivity between the hippocampi was greatly reduced,” compared with controls, he said.
Imaging provides objective documentation to help convince insurers to cover rehabilitative services.
Source DR. DIAZ-ARRASTIA
Major Finding: Various functional and structural imaging techniques can provide a prognosis for TBI patients and objective evidence to insurers to cover rehabilitative services.
Data Source: A review of recent imaging studies of traumatic brain injury.
Disclosures: None
BANGKOK, THAILAND — New imaging techniques may help to explain the disabling symptoms that can plague patients with traumatic brain injury long after their acute problems have resolved, and eventually guide the best choice for medical therapy.
Survivors of traumatic brain injury who complain of depression, irritability, and memory or cognitive problems are often written off as psychiatric cases or malingerers, Dr. Ramon Diaz-Arrastia said at the World Congress of Neurology. “This is the frustrating thing about TBI. The patients might look OK—they don't have paralysis; they are walking around. But their cognitive and behavioral problems are real.”
Imaging techniques that are now well established in other areas of neurology—such as diffusion-weighted and susceptibility-weighted magnetic resonance imaging—are now being used to show that brain injuries leave permanent, life-altering marks behind after the contusions and hematomas have healed.
These findings may have both immediate and long-range benefits, said Dr. Diaz-Arrastia, a professor of neurology at the University of Texas Southwestern Medical Center, Dallas. “Right now, imaging these patients has the primary value of providing a prognosis and perhaps helping them obtain the care that they need. In terms of reimbursement, it's useful to have an objective documentation of the injury when trying to convince insurers to cover rehabilitative service.”
In the future, imaging the post-TBI brain may help guide medical treatment choices and monitor drugs' effectiveness.
So far, nearly 30 drugs have provided effective neuroprotection in animal models of TBI, he said. However, none that has undergone testing in well-designed phase III trials has proven beneficial to humans.
Part of the problem may be the heterogeneity of human brain injury, Dr. Diaz-Arrastia said. There are many subtypes of TBI, yet “from the point of view of the clinical trials, all patients who present in a coma [after a brain injury] are treated the same way, even though the injuries can be very different, with very different prognoses.”
Susceptibility-weighted imaging (SWI) is one technique being studied in TBI patients. It measures the paramagnetic shift of intravascular deoxyhemoglobin and methemoglobin, amplifying the appearance of microhemorrhages and making them much easier to identify. “SWI picks up 640% more lesions and 200% more lesion volume than does gradient-recall echo,” Dr. Diaz-Arrastia said, referring to work by Dr. Karen Tong from Loma Linda (California) University.
SWI is very good at identifying diffuse microvascular injury, a marker for diffuse axonal injury that is usually invisible on computed axial tomography. “The only problem is that SWI may be overly sensitive,” he said. “One patient with a lot of microhemorrhages might be complaining only of headache and dizziness, whereas another with a similar volume might have a lot more problems.”
Diffusion-weighted imaging (DWI), which is well established in the stroke world, is understudied in TBI, probably because it's a challenge to perform magnetic resonance imaging on these acutely ill patients. But this technique provides detailed information about the makeup of lesions, showing vasogenic and cytotoxic edema, as well as location in the superficial or deep structures in both gray and white matter.
Dr. Diaz-Arrastia and his colleagues performed DWI on 99 patients with TBI. Of these, the study identified corpus callosum lesions in 84%. It was able to differentiate between patients with primarily cytotoxic lesions (54%) and those with vasogenic lesions (46%). “We also found that the volume of these brain lesions, irrespective of location, explained about 28% of the variance in outcome among these patients,” he said. “It's a relatively modest correlation with outcome, but it shows that what we are measuring is something that is functionally important and affects outcome.”
Diffusion tensor imaging [DTI] shows how water tracks along the axons, giving a good view of white matter lesions. Follow-up scans on TBI patients have shown tantalizing clues to the possible causes of their long-term problems.
“When we scan patients within a day or two of injury, we may see subtle changes in the parameters. But if we come back 6 months later and rescan, we see much greater dropout of axons. Initially, the patient may be in a coma, and when they are scanned later they are usually much improved and walking around, albeit with problems with memory or executive function. So this tells us that something is happening weeks or months after the injury that results in white matter dropout.”
Another technique moving into trauma field is quantitative volumetric assessment of the cortical field. This technique measures the thickness and volume of different cortical and subcortical regions, Dr. Diaz-Arrastia said. “In our patients, we have found that the brain shrinks overall after a severe traumatic injury, but that not all structures shrink at the same rate. Some cortical regions shrink very little, while others, like the hippocampus, appear particularly sensitive to injury.” This finding makes sense given the cognitive and mood issues that TBI patients can experience, he said.
On average, whole brain volume and both grey and white matter volumes decreased by 3%–10% in the first few months after severe TBI. In comparison, Dr. Diaz-Arrastia said, the rate of atrophy for patients with Alzheimer's disease is about 1%–2% a year.
Functional MRIs also provide some clues that the injured brain sustains long-term problems. In the resting state, the blood oxygen level dependent signal typically shows seemingly random fluctuations when the brain is at rest. But recent studies have shown that these fluctuations actually represent the communication between brain regions that work together.
“In our patients, we found a very high indication that the functional connectivity between the hippocampi was greatly reduced,” compared with controls, he said.
Imaging provides objective documentation to help convince insurers to cover rehabilitative services.
Source DR. DIAZ-ARRASTIA
Headache Pain Persists in Veterans With TBI
Persistent headaches occurred in nearly 98% of soldiers who suffered head trauma, blast exposure, or concussion while on duty in Iraq or Afghanistan, according to results of a survey of soldiers who returned from deployment between June and October 2008.
“Our goal was to try to determine the types, the duration, the frequency, and any occupational dysfunction caused by headaches in soldiers with a history of head trauma, concussion, or blasts,” Dr. Brett J. Theeler of the Madigan Army Medical Center in Tacoma, Wash., said in an interview.
Previous research has shown that about 15% of soldiers deployed to Iraq or Afghanistan experience mild traumatic brain injuries, but the prevalence and characteristics of the headaches associated with these injuries have not been well studied, he noted.
Dr. Theeler and his colleagues conducted a study based on a 13-item headache questionnaire. The study participants included 963 men and 15 women who returned from Iraq or Afghanistan within 3 months prior to enrolling in the study. The average age of the soldiers was 27 years.
The complete study results will be presented at the annual meeting of the American Academy of Neurology in April.
Overall, 351 of the 957 soldiers (37%) who reported headaches said that they started having headaches within a week of their injuries, and 20% reported that they started having headaches 1–4 weeks after their injuries.
Of those whose headaches began within 1 week of their injuries, 60% had headaches that met three or more criteria for migraines, and 40% said their headaches interfered with their normal activities. Of all of the soldiers who reported headaches, 30% said they had at least 15 days of headaches per month.
“We were very interested in the headache types,” Dr. Theeler said.
In an earlier study on headaches in soldiers, he and his colleagues found that more of these posttraumatic headaches had migraine features, compared with headaches in the general population (CLINICAL NEUROLOGY NEWS, Sept. 2006, p. 17). The current study had similar results.
“That doesn't mean that the head trauma or concussion is directly related to migraine, but we think head trauma is one of those important factors that lead these soldiers to have a higher frequency of migraines than people in the general population,” he said.
Identifying a specific type of headache might lead to a better diagnosis and possibly better treatments for the soldiers, Dr. Theeler said.
One of the take-home points for clinicians is that soldiers who have persistent migraine-type headaches might respond to migraine treatments, although the treatment of these soldiers has not been systematically studied, Dr. Theeler added.
Additional research is needed to determine the best acute medications and the best prophylactic treatments for soldiers who have headaches with migraine features after head trauma, and Dr. Theeler and his colleagues are currently conducting studies to address these issues.
Dr. Theeler said he had no financial conflicts to disclose.
Persistent headaches occurred in nearly 98% of soldiers who suffered head trauma, blast exposure, or concussion while on duty in Iraq or Afghanistan, according to results of a survey of soldiers who returned from deployment between June and October 2008.
“Our goal was to try to determine the types, the duration, the frequency, and any occupational dysfunction caused by headaches in soldiers with a history of head trauma, concussion, or blasts,” Dr. Brett J. Theeler of the Madigan Army Medical Center in Tacoma, Wash., said in an interview.
Previous research has shown that about 15% of soldiers deployed to Iraq or Afghanistan experience mild traumatic brain injuries, but the prevalence and characteristics of the headaches associated with these injuries have not been well studied, he noted.
Dr. Theeler and his colleagues conducted a study based on a 13-item headache questionnaire. The study participants included 963 men and 15 women who returned from Iraq or Afghanistan within 3 months prior to enrolling in the study. The average age of the soldiers was 27 years.
The complete study results will be presented at the annual meeting of the American Academy of Neurology in April.
Overall, 351 of the 957 soldiers (37%) who reported headaches said that they started having headaches within a week of their injuries, and 20% reported that they started having headaches 1–4 weeks after their injuries.
Of those whose headaches began within 1 week of their injuries, 60% had headaches that met three or more criteria for migraines, and 40% said their headaches interfered with their normal activities. Of all of the soldiers who reported headaches, 30% said they had at least 15 days of headaches per month.
“We were very interested in the headache types,” Dr. Theeler said.
In an earlier study on headaches in soldiers, he and his colleagues found that more of these posttraumatic headaches had migraine features, compared with headaches in the general population (CLINICAL NEUROLOGY NEWS, Sept. 2006, p. 17). The current study had similar results.
“That doesn't mean that the head trauma or concussion is directly related to migraine, but we think head trauma is one of those important factors that lead these soldiers to have a higher frequency of migraines than people in the general population,” he said.
Identifying a specific type of headache might lead to a better diagnosis and possibly better treatments for the soldiers, Dr. Theeler said.
One of the take-home points for clinicians is that soldiers who have persistent migraine-type headaches might respond to migraine treatments, although the treatment of these soldiers has not been systematically studied, Dr. Theeler added.
Additional research is needed to determine the best acute medications and the best prophylactic treatments for soldiers who have headaches with migraine features after head trauma, and Dr. Theeler and his colleagues are currently conducting studies to address these issues.
Dr. Theeler said he had no financial conflicts to disclose.
Persistent headaches occurred in nearly 98% of soldiers who suffered head trauma, blast exposure, or concussion while on duty in Iraq or Afghanistan, according to results of a survey of soldiers who returned from deployment between June and October 2008.
“Our goal was to try to determine the types, the duration, the frequency, and any occupational dysfunction caused by headaches in soldiers with a history of head trauma, concussion, or blasts,” Dr. Brett J. Theeler of the Madigan Army Medical Center in Tacoma, Wash., said in an interview.
Previous research has shown that about 15% of soldiers deployed to Iraq or Afghanistan experience mild traumatic brain injuries, but the prevalence and characteristics of the headaches associated with these injuries have not been well studied, he noted.
Dr. Theeler and his colleagues conducted a study based on a 13-item headache questionnaire. The study participants included 963 men and 15 women who returned from Iraq or Afghanistan within 3 months prior to enrolling in the study. The average age of the soldiers was 27 years.
The complete study results will be presented at the annual meeting of the American Academy of Neurology in April.
Overall, 351 of the 957 soldiers (37%) who reported headaches said that they started having headaches within a week of their injuries, and 20% reported that they started having headaches 1–4 weeks after their injuries.
Of those whose headaches began within 1 week of their injuries, 60% had headaches that met three or more criteria for migraines, and 40% said their headaches interfered with their normal activities. Of all of the soldiers who reported headaches, 30% said they had at least 15 days of headaches per month.
“We were very interested in the headache types,” Dr. Theeler said.
In an earlier study on headaches in soldiers, he and his colleagues found that more of these posttraumatic headaches had migraine features, compared with headaches in the general population (CLINICAL NEUROLOGY NEWS, Sept. 2006, p. 17). The current study had similar results.
“That doesn't mean that the head trauma or concussion is directly related to migraine, but we think head trauma is one of those important factors that lead these soldiers to have a higher frequency of migraines than people in the general population,” he said.
Identifying a specific type of headache might lead to a better diagnosis and possibly better treatments for the soldiers, Dr. Theeler said.
One of the take-home points for clinicians is that soldiers who have persistent migraine-type headaches might respond to migraine treatments, although the treatment of these soldiers has not been systematically studied, Dr. Theeler added.
Additional research is needed to determine the best acute medications and the best prophylactic treatments for soldiers who have headaches with migraine features after head trauma, and Dr. Theeler and his colleagues are currently conducting studies to address these issues.
Dr. Theeler said he had no financial conflicts to disclose.
Corrections
There was an error in the caption for the image that appeared in a recent “Neuroscience Today, Neurology Tomorrow” column (November 2008, p. 14). The caption should have said that the neurons and dendrites were located in the dentate gyrus of the mouse's hippocampus, not in the CA1 area.
There was an error in the caption for the image that appeared in a recent “Neuroscience Today, Neurology Tomorrow” column (November 2008, p. 14). The caption should have said that the neurons and dendrites were located in the dentate gyrus of the mouse's hippocampus, not in the CA1 area.
There was an error in the caption for the image that appeared in a recent “Neuroscience Today, Neurology Tomorrow” column (November 2008, p. 14). The caption should have said that the neurons and dendrites were located in the dentate gyrus of the mouse's hippocampus, not in the CA1 area.
Corrections
There was an error in the article, “After Methotrexate, Glatiramer Acetate Improves MS Outcomes” that appeared on page 17 of the December 2008 issue of CLINICAL NEUROLOGY NEWS. The treatment described by the investigator involved mitoxantrone, not methotrexate.
There was an error in the article, “After Methotrexate, Glatiramer Acetate Improves MS Outcomes” that appeared on page 17 of the December 2008 issue of CLINICAL NEUROLOGY NEWS. The treatment described by the investigator involved mitoxantrone, not methotrexate.
There was an error in the article, “After Methotrexate, Glatiramer Acetate Improves MS Outcomes” that appeared on page 17 of the December 2008 issue of CLINICAL NEUROLOGY NEWS. The treatment described by the investigator involved mitoxantrone, not methotrexate.
New Methods Find TBI Missed by Standard Scans
WASHINGTON — Advanced imaging methods with MRI and magnetoencephalography may be able to detect mild traumatic brain injury with greater accuracy than can conventional imaging techniques, according to two prospective pilot studies.
Conventional MR and CT neuroimaging focus on the detection of bleeding, which is only indirectly related to axonal injury. These methods are not able to detect about 70%–80% of mild to moderate traumatic brain injuries (TBIs), according to Mingxiong Huang, Ph.D., of the University of California, San Diego, and his colleagues.
Dr. Huang and his coinvestigators are finding that the combination of diffusion-tensor imaging (DTI) and magnetoencephalography (MEG) can reveal axonal injury resulting from tissue shearing and stretching, which is a leading cause of persistent postconcussive symptoms in mild TBI patients.
MEG pinpoints the temporal and spatial activation of neurons in the brain based on the tiny magnetic fields created by neuronal currents in cortical gray matter. DTI measures the pattern and direction of the movement of water molecules through white matter fiber tracts, which become disturbed after a TBI.
Detecting mild TBI is clinically important, Dr. Huang said, because even though roughly 85% of patients with mild TBI will be symptom free by 6 months, the remaining 15% have lingering cognitive and behavioral problems, and have a higher risk for developing epilepsy, severe depression, and dementia.
He and his colleagues studied 18 civilian and military patients with a closed-head injury and mild to moderate symptoms of TBI, along with 17 healthy control patients. None of the patients had visible lesions on conventional MRI or CT. In the patients with TBI symptoms, the researchers found that the location of neurons generating abnormal low-frequency delta waves that were seen on MEG was significantly correlated with the deafferentation of the underlying white matter fiber tracts on DTI. These findings were consistent with the patients' symptoms and the results of neuropsychological exams, and they help to confirm the hypothesis that pathological low-frequency delta waves are caused by the shearing of white matter fiber tracts, Dr. Huang reported at the annual meeting of the Society for Neuroscience.
MEG may be a more sensitive measure for mild TBI than is DTI because in some instances MEG was able to detect pathological low-frequency delta waves when DTI signals in white matter fiber tracts were within normal range, according to Dr. Huang. He also noted that the two modalities could be used to objectively monitor the effect of an intervention and provide prognostic information.
The investigators hope to expand their research by performing a longitudinal study in children that compares their recovery from mild TBI with the recovery of adults. In military personnel, the researchers would like to know how to differentiate the signs and symptoms of mild TBI from those of posttraumatic stress disorder. Both conditions have similar signs and symptoms and coincide in a subpopulation of patients, but the treatments for them are different.
In another study presented at the meeting, Andrew Maudsley, Ph.D., of the University of Miami and his colleagues used magnetic resonance spectroscopic imaging (MRSI) to detect the changes in brain metabolism that are indicative of mild TBI in patients with postconcussive symptoms.
The researchers used a volumetric acquisition method to obtain data on the whole brain rather than on just a single area, which is beneficial in imaging diffuse brain injury, according to Dr. Maudsley.
“If you used a conventional MRS method, which is a single voxel method, you have to [focus on] one brain region. You could very clearly choose a brain region, especially with mild injury, that actually looks normal on spectroscopy,” he said in an interview.
The investigators measured levels of N-acetylaspartate, creatine, and choline in the brain. The pilot study compared the average of all measured values from 22 patients who were classified as having mild brain injury with the average values from 67 age-matched controls. MRSI scans took place a median of 21 days after the patients' injuries, which were caused by motor vehicle accidents (17), falls (2), or assault (3).
Assessments of the group averages revealed that brain injury was associated with a significantly decreased level of N-acetylaspartate (a marker of neuronal and axonal viability), as well as an increased level of choline (a marker of membrane metabolism). The ratio of choline to N-acetylaspartate was the most sensitive marker for injury.
Overall, 90% of the patients had small and well-localized lesions on normal MRI, findings that are typical for mild TBI. But on MRSI, the researchers found widespread metabolite alterations throughout the cerebrum.
The patients' scores on neuropsychological tests were significantly correlated mostly with metabolite changes in the right frontal region. In one patient who underwent follow-up scans, the concentrations of N-acetylaspartate and choline continued to change significantly at 7 and 15 months post injury.
Dr. Maudsley said that he and his team hope to obtain longitudinal assessments of metabolite levels to determine if their short-term levels can predict future outcomes of patients with mild TBI. Outcomes at 6 months in close to half of the patients have shown some correlations between metabolite levels and scores on neuropsychological tests, he said.
“It's my feeling that these metabolites really take several days, if not a couple of weeks, to change. In the one example in which we had a more severe injury, things were actually worse at 6 months than they were at 5 weeks,” he added.
The use of the 3-tesla MR scanners that Dr. Maudsley and his associates used in their study is beginning to extend beyond academic medical centers and into regular clinics, especially for brain MRI applications.
Neither Dr. Huang nor Dr. Maudsley had conflicts of interest to report.
Magnetic resonance spectroscopic imaging of the brains of 18 traumatic brain injury patients (bottom three rows) show widespread alterations in the ratio of choline to N-acetyl aspartate (light blue to green color), unlike the brains of 6 control subjects (top row). Images courtesy Dr. Andrew A. Maudsley
WASHINGTON — Advanced imaging methods with MRI and magnetoencephalography may be able to detect mild traumatic brain injury with greater accuracy than can conventional imaging techniques, according to two prospective pilot studies.
Conventional MR and CT neuroimaging focus on the detection of bleeding, which is only indirectly related to axonal injury. These methods are not able to detect about 70%–80% of mild to moderate traumatic brain injuries (TBIs), according to Mingxiong Huang, Ph.D., of the University of California, San Diego, and his colleagues.
Dr. Huang and his coinvestigators are finding that the combination of diffusion-tensor imaging (DTI) and magnetoencephalography (MEG) can reveal axonal injury resulting from tissue shearing and stretching, which is a leading cause of persistent postconcussive symptoms in mild TBI patients.
MEG pinpoints the temporal and spatial activation of neurons in the brain based on the tiny magnetic fields created by neuronal currents in cortical gray matter. DTI measures the pattern and direction of the movement of water molecules through white matter fiber tracts, which become disturbed after a TBI.
Detecting mild TBI is clinically important, Dr. Huang said, because even though roughly 85% of patients with mild TBI will be symptom free by 6 months, the remaining 15% have lingering cognitive and behavioral problems, and have a higher risk for developing epilepsy, severe depression, and dementia.
He and his colleagues studied 18 civilian and military patients with a closed-head injury and mild to moderate symptoms of TBI, along with 17 healthy control patients. None of the patients had visible lesions on conventional MRI or CT. In the patients with TBI symptoms, the researchers found that the location of neurons generating abnormal low-frequency delta waves that were seen on MEG was significantly correlated with the deafferentation of the underlying white matter fiber tracts on DTI. These findings were consistent with the patients' symptoms and the results of neuropsychological exams, and they help to confirm the hypothesis that pathological low-frequency delta waves are caused by the shearing of white matter fiber tracts, Dr. Huang reported at the annual meeting of the Society for Neuroscience.
MEG may be a more sensitive measure for mild TBI than is DTI because in some instances MEG was able to detect pathological low-frequency delta waves when DTI signals in white matter fiber tracts were within normal range, according to Dr. Huang. He also noted that the two modalities could be used to objectively monitor the effect of an intervention and provide prognostic information.
The investigators hope to expand their research by performing a longitudinal study in children that compares their recovery from mild TBI with the recovery of adults. In military personnel, the researchers would like to know how to differentiate the signs and symptoms of mild TBI from those of posttraumatic stress disorder. Both conditions have similar signs and symptoms and coincide in a subpopulation of patients, but the treatments for them are different.
In another study presented at the meeting, Andrew Maudsley, Ph.D., of the University of Miami and his colleagues used magnetic resonance spectroscopic imaging (MRSI) to detect the changes in brain metabolism that are indicative of mild TBI in patients with postconcussive symptoms.
The researchers used a volumetric acquisition method to obtain data on the whole brain rather than on just a single area, which is beneficial in imaging diffuse brain injury, according to Dr. Maudsley.
“If you used a conventional MRS method, which is a single voxel method, you have to [focus on] one brain region. You could very clearly choose a brain region, especially with mild injury, that actually looks normal on spectroscopy,” he said in an interview.
The investigators measured levels of N-acetylaspartate, creatine, and choline in the brain. The pilot study compared the average of all measured values from 22 patients who were classified as having mild brain injury with the average values from 67 age-matched controls. MRSI scans took place a median of 21 days after the patients' injuries, which were caused by motor vehicle accidents (17), falls (2), or assault (3).
Assessments of the group averages revealed that brain injury was associated with a significantly decreased level of N-acetylaspartate (a marker of neuronal and axonal viability), as well as an increased level of choline (a marker of membrane metabolism). The ratio of choline to N-acetylaspartate was the most sensitive marker for injury.
Overall, 90% of the patients had small and well-localized lesions on normal MRI, findings that are typical for mild TBI. But on MRSI, the researchers found widespread metabolite alterations throughout the cerebrum.
The patients' scores on neuropsychological tests were significantly correlated mostly with metabolite changes in the right frontal region. In one patient who underwent follow-up scans, the concentrations of N-acetylaspartate and choline continued to change significantly at 7 and 15 months post injury.
Dr. Maudsley said that he and his team hope to obtain longitudinal assessments of metabolite levels to determine if their short-term levels can predict future outcomes of patients with mild TBI. Outcomes at 6 months in close to half of the patients have shown some correlations between metabolite levels and scores on neuropsychological tests, he said.
“It's my feeling that these metabolites really take several days, if not a couple of weeks, to change. In the one example in which we had a more severe injury, things were actually worse at 6 months than they were at 5 weeks,” he added.
The use of the 3-tesla MR scanners that Dr. Maudsley and his associates used in their study is beginning to extend beyond academic medical centers and into regular clinics, especially for brain MRI applications.
Neither Dr. Huang nor Dr. Maudsley had conflicts of interest to report.
Magnetic resonance spectroscopic imaging of the brains of 18 traumatic brain injury patients (bottom three rows) show widespread alterations in the ratio of choline to N-acetyl aspartate (light blue to green color), unlike the brains of 6 control subjects (top row). Images courtesy Dr. Andrew A. Maudsley
WASHINGTON — Advanced imaging methods with MRI and magnetoencephalography may be able to detect mild traumatic brain injury with greater accuracy than can conventional imaging techniques, according to two prospective pilot studies.
Conventional MR and CT neuroimaging focus on the detection of bleeding, which is only indirectly related to axonal injury. These methods are not able to detect about 70%–80% of mild to moderate traumatic brain injuries (TBIs), according to Mingxiong Huang, Ph.D., of the University of California, San Diego, and his colleagues.
Dr. Huang and his coinvestigators are finding that the combination of diffusion-tensor imaging (DTI) and magnetoencephalography (MEG) can reveal axonal injury resulting from tissue shearing and stretching, which is a leading cause of persistent postconcussive symptoms in mild TBI patients.
MEG pinpoints the temporal and spatial activation of neurons in the brain based on the tiny magnetic fields created by neuronal currents in cortical gray matter. DTI measures the pattern and direction of the movement of water molecules through white matter fiber tracts, which become disturbed after a TBI.
Detecting mild TBI is clinically important, Dr. Huang said, because even though roughly 85% of patients with mild TBI will be symptom free by 6 months, the remaining 15% have lingering cognitive and behavioral problems, and have a higher risk for developing epilepsy, severe depression, and dementia.
He and his colleagues studied 18 civilian and military patients with a closed-head injury and mild to moderate symptoms of TBI, along with 17 healthy control patients. None of the patients had visible lesions on conventional MRI or CT. In the patients with TBI symptoms, the researchers found that the location of neurons generating abnormal low-frequency delta waves that were seen on MEG was significantly correlated with the deafferentation of the underlying white matter fiber tracts on DTI. These findings were consistent with the patients' symptoms and the results of neuropsychological exams, and they help to confirm the hypothesis that pathological low-frequency delta waves are caused by the shearing of white matter fiber tracts, Dr. Huang reported at the annual meeting of the Society for Neuroscience.
MEG may be a more sensitive measure for mild TBI than is DTI because in some instances MEG was able to detect pathological low-frequency delta waves when DTI signals in white matter fiber tracts were within normal range, according to Dr. Huang. He also noted that the two modalities could be used to objectively monitor the effect of an intervention and provide prognostic information.
The investigators hope to expand their research by performing a longitudinal study in children that compares their recovery from mild TBI with the recovery of adults. In military personnel, the researchers would like to know how to differentiate the signs and symptoms of mild TBI from those of posttraumatic stress disorder. Both conditions have similar signs and symptoms and coincide in a subpopulation of patients, but the treatments for them are different.
In another study presented at the meeting, Andrew Maudsley, Ph.D., of the University of Miami and his colleagues used magnetic resonance spectroscopic imaging (MRSI) to detect the changes in brain metabolism that are indicative of mild TBI in patients with postconcussive symptoms.
The researchers used a volumetric acquisition method to obtain data on the whole brain rather than on just a single area, which is beneficial in imaging diffuse brain injury, according to Dr. Maudsley.
“If you used a conventional MRS method, which is a single voxel method, you have to [focus on] one brain region. You could very clearly choose a brain region, especially with mild injury, that actually looks normal on spectroscopy,” he said in an interview.
The investigators measured levels of N-acetylaspartate, creatine, and choline in the brain. The pilot study compared the average of all measured values from 22 patients who were classified as having mild brain injury with the average values from 67 age-matched controls. MRSI scans took place a median of 21 days after the patients' injuries, which were caused by motor vehicle accidents (17), falls (2), or assault (3).
Assessments of the group averages revealed that brain injury was associated with a significantly decreased level of N-acetylaspartate (a marker of neuronal and axonal viability), as well as an increased level of choline (a marker of membrane metabolism). The ratio of choline to N-acetylaspartate was the most sensitive marker for injury.
Overall, 90% of the patients had small and well-localized lesions on normal MRI, findings that are typical for mild TBI. But on MRSI, the researchers found widespread metabolite alterations throughout the cerebrum.
The patients' scores on neuropsychological tests were significantly correlated mostly with metabolite changes in the right frontal region. In one patient who underwent follow-up scans, the concentrations of N-acetylaspartate and choline continued to change significantly at 7 and 15 months post injury.
Dr. Maudsley said that he and his team hope to obtain longitudinal assessments of metabolite levels to determine if their short-term levels can predict future outcomes of patients with mild TBI. Outcomes at 6 months in close to half of the patients have shown some correlations between metabolite levels and scores on neuropsychological tests, he said.
“It's my feeling that these metabolites really take several days, if not a couple of weeks, to change. In the one example in which we had a more severe injury, things were actually worse at 6 months than they were at 5 weeks,” he added.
The use of the 3-tesla MR scanners that Dr. Maudsley and his associates used in their study is beginning to extend beyond academic medical centers and into regular clinics, especially for brain MRI applications.
Neither Dr. Huang nor Dr. Maudsley had conflicts of interest to report.
Magnetic resonance spectroscopic imaging of the brains of 18 traumatic brain injury patients (bottom three rows) show widespread alterations in the ratio of choline to N-acetyl aspartate (light blue to green color), unlike the brains of 6 control subjects (top row). Images courtesy Dr. Andrew A. Maudsley
CT Is Best for Risky Cortical Spine Injuries
ALBUQUERQUE — Computed tomography imaging with coronal and sagittal reconstructions beat conventional x-rays at diagnosing cervical spine injuries in high-risk pediatric trauma patients in a study.
Dr. Gregory A. Mencio of Vanderbilt University in Nashville, Tenn., reviewed 413 consecutive charts of high-risk patients younger than 18 years at a level I trauma center. All were evaluated by CT scan and conventional five-view x-ray of the cervical spine.
CT scanning detected 71 of 74 cervical spine injuries in the patients, who had an average age of 11 years. Only 50 injuries were detected by x-ray. Combining the two brought the detection rate to 72 cases—just 1 more than diagnosed by CT, Dr. Mencio said at the annual meeting of the Pediatric Orthopaedic Society of North America.
“A lot of these kids have multiple systems injury,” Dr. Mencio said. “Do a CT of the head and neck and torso and total spine. It takes about 10 minutes to scan them from top to bottom, and you have all the information that you need.”
The researchers estimated that the radiation dose was lower with CT, but the costs were higher: $1,800 for CT, vs. $500 for x-rays. For both, Dr. Mencio noted conflicting data appear in the literature.
He offered these recommendations:
▸ CT of the cervical spine with sagittal and coronal reconstructions is the initial imaging modality of choice for high-risk pediatric trauma patients.
▸ Conventional x-ray may be used to elucidate dynamic instability and follow alignment over time.
▸ Magnetic resonance imaging is used to evaluate patients with neurologic injuries and in obtunded patients who risk prolonged immobilization.
ALBUQUERQUE — Computed tomography imaging with coronal and sagittal reconstructions beat conventional x-rays at diagnosing cervical spine injuries in high-risk pediatric trauma patients in a study.
Dr. Gregory A. Mencio of Vanderbilt University in Nashville, Tenn., reviewed 413 consecutive charts of high-risk patients younger than 18 years at a level I trauma center. All were evaluated by CT scan and conventional five-view x-ray of the cervical spine.
CT scanning detected 71 of 74 cervical spine injuries in the patients, who had an average age of 11 years. Only 50 injuries were detected by x-ray. Combining the two brought the detection rate to 72 cases—just 1 more than diagnosed by CT, Dr. Mencio said at the annual meeting of the Pediatric Orthopaedic Society of North America.
“A lot of these kids have multiple systems injury,” Dr. Mencio said. “Do a CT of the head and neck and torso and total spine. It takes about 10 minutes to scan them from top to bottom, and you have all the information that you need.”
The researchers estimated that the radiation dose was lower with CT, but the costs were higher: $1,800 for CT, vs. $500 for x-rays. For both, Dr. Mencio noted conflicting data appear in the literature.
He offered these recommendations:
▸ CT of the cervical spine with sagittal and coronal reconstructions is the initial imaging modality of choice for high-risk pediatric trauma patients.
▸ Conventional x-ray may be used to elucidate dynamic instability and follow alignment over time.
▸ Magnetic resonance imaging is used to evaluate patients with neurologic injuries and in obtunded patients who risk prolonged immobilization.
ALBUQUERQUE — Computed tomography imaging with coronal and sagittal reconstructions beat conventional x-rays at diagnosing cervical spine injuries in high-risk pediatric trauma patients in a study.
Dr. Gregory A. Mencio of Vanderbilt University in Nashville, Tenn., reviewed 413 consecutive charts of high-risk patients younger than 18 years at a level I trauma center. All were evaluated by CT scan and conventional five-view x-ray of the cervical spine.
CT scanning detected 71 of 74 cervical spine injuries in the patients, who had an average age of 11 years. Only 50 injuries were detected by x-ray. Combining the two brought the detection rate to 72 cases—just 1 more than diagnosed by CT, Dr. Mencio said at the annual meeting of the Pediatric Orthopaedic Society of North America.
“A lot of these kids have multiple systems injury,” Dr. Mencio said. “Do a CT of the head and neck and torso and total spine. It takes about 10 minutes to scan them from top to bottom, and you have all the information that you need.”
The researchers estimated that the radiation dose was lower with CT, but the costs were higher: $1,800 for CT, vs. $500 for x-rays. For both, Dr. Mencio noted conflicting data appear in the literature.
He offered these recommendations:
▸ CT of the cervical spine with sagittal and coronal reconstructions is the initial imaging modality of choice for high-risk pediatric trauma patients.
▸ Conventional x-ray may be used to elucidate dynamic instability and follow alignment over time.
▸ Magnetic resonance imaging is used to evaluate patients with neurologic injuries and in obtunded patients who risk prolonged immobilization.