User login
Metabolic Health Tied to Lower Prediabetes Risk
TOPLINE:
Whether they have normal weight, overweight, or obesity, individuals with metabolically healthy (MH) phenotypes show a lower frequency of impaired glucose metabolism than their unhealthy counterparts across all weight categories.
METHODOLOGY:
- The concepts of MH overweight and MH obesity refer to a subset of people who exhibit an absence of cardiometabolic risk factors despite excess body fat, but the prevalence of prediabetes has not been investigated by metabolic phenotype and body mass index (BMI).
- This study first validated the use of estimated glucose disposal rate (eGDR), an index of insulin sensitivity calculated from clinical variables, in 350 individuals without diabetes (mean age, 37 years; 219 women; mean BMI, 30.3) from the EUGENE2 project who had varying glucose tolerance values originally assessed by insulin-stimulated glucose disposal.
- Researchers then stratified 2201 participants without diabetes (mean age, 46 years; White; 1290 women; mean BMI, 31.2) from the CATAMERI study according to BMI into three groups — individuals with normal weight (BMI, 18-24.9), overweight (BMI, 25-29.9), and obesity (BMI, ≥ 30).
- The men and women in each BMI group were separated into quartiles of insulin sensitivity based on eGDR index:
- In the normal weight group, men and women were defined as MH in the top three eGDR quartiles and metabolically unhealthy (MU) in the lowest quartile.
- In the overweight and obesity groups, people were defined as MH in the top eGDR quartile and MU in the lower three quartiles.
- Impaired glucose tolerance (IGT), impaired fasting glucose (IFG), and combined IFG+IGT conditions (from an oral glucose tolerance test) were compared in individuals without diabetes based on MH or unhealthy phenotypes across normal weight, overweight, and obese categories.
TAKEAWAY:
- eGDR demonstrated good accuracy in detecting individuals with higher insulin sensitivity in the EUGENE2 cohort.
- The MH overweight and MH obesity groups showed comparable glycemic parameters as the MH normal weight group, whereas the MU overweight and MU obesity groups exhibited higher A1c levels and fasting and 2-hour post-load glucose than the MH normal weight group.
- The frequencies of IFG, IGT, and IFG+IGT conditions were similar among the MH normal weight, MH overweight, and MH obesity groups but were higher in the MU overweight and MU obesity groups than in the MU normal weight group.
- Furthermore, compared with those in the MH normal weight group, the odds of prediabetes were at least two times higher in the MU obesity (odds ratio [OR], 2.54; P < .001) and MU overweight (OR, 2.06; P < .001) groups but not significantly different in the MU normal weight, MH obesity, and MH overweight groups.
IN PRACTICE:
The authors wrote, “Overall, the results of this cross-sectional study support the notion that metabolically healthy individuals with overweight or obesity have a more favorable metabolic risk profile in comparison to metabolically unhealthy subjects with overweight or obesity.”
SOURCE:
The study was conducted by Chiara M.A. Cefalo, MD, department of clinical and molecular medicine, Sapienza University of Rome, Rome, Italy, and was published online in Diabetes, Obesity and Metabolism.
LIMITATIONS:
There was no consensus on the parameters and cutoff values for defining metabolic health status, allowing for potential variations in results. The study design suggested an association with prevalent IFG and IGT conditions but not with incident IFG and IGT conditions. All participants in this study were White, limiting the generalizability of its findings.
DISCLOSURES:
The study was supported by Sapienza University of Rome and the Italian Ministry of University. The authors declared no conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
Whether they have normal weight, overweight, or obesity, individuals with metabolically healthy (MH) phenotypes show a lower frequency of impaired glucose metabolism than their unhealthy counterparts across all weight categories.
METHODOLOGY:
- The concepts of MH overweight and MH obesity refer to a subset of people who exhibit an absence of cardiometabolic risk factors despite excess body fat, but the prevalence of prediabetes has not been investigated by metabolic phenotype and body mass index (BMI).
- This study first validated the use of estimated glucose disposal rate (eGDR), an index of insulin sensitivity calculated from clinical variables, in 350 individuals without diabetes (mean age, 37 years; 219 women; mean BMI, 30.3) from the EUGENE2 project who had varying glucose tolerance values originally assessed by insulin-stimulated glucose disposal.
- Researchers then stratified 2201 participants without diabetes (mean age, 46 years; White; 1290 women; mean BMI, 31.2) from the CATAMERI study according to BMI into three groups — individuals with normal weight (BMI, 18-24.9), overweight (BMI, 25-29.9), and obesity (BMI, ≥ 30).
- The men and women in each BMI group were separated into quartiles of insulin sensitivity based on eGDR index:
- In the normal weight group, men and women were defined as MH in the top three eGDR quartiles and metabolically unhealthy (MU) in the lowest quartile.
- In the overweight and obesity groups, people were defined as MH in the top eGDR quartile and MU in the lower three quartiles.
- Impaired glucose tolerance (IGT), impaired fasting glucose (IFG), and combined IFG+IGT conditions (from an oral glucose tolerance test) were compared in individuals without diabetes based on MH or unhealthy phenotypes across normal weight, overweight, and obese categories.
TAKEAWAY:
- eGDR demonstrated good accuracy in detecting individuals with higher insulin sensitivity in the EUGENE2 cohort.
- The MH overweight and MH obesity groups showed comparable glycemic parameters as the MH normal weight group, whereas the MU overweight and MU obesity groups exhibited higher A1c levels and fasting and 2-hour post-load glucose than the MH normal weight group.
- The frequencies of IFG, IGT, and IFG+IGT conditions were similar among the MH normal weight, MH overweight, and MH obesity groups but were higher in the MU overweight and MU obesity groups than in the MU normal weight group.
- Furthermore, compared with those in the MH normal weight group, the odds of prediabetes were at least two times higher in the MU obesity (odds ratio [OR], 2.54; P < .001) and MU overweight (OR, 2.06; P < .001) groups but not significantly different in the MU normal weight, MH obesity, and MH overweight groups.
IN PRACTICE:
The authors wrote, “Overall, the results of this cross-sectional study support the notion that metabolically healthy individuals with overweight or obesity have a more favorable metabolic risk profile in comparison to metabolically unhealthy subjects with overweight or obesity.”
SOURCE:
The study was conducted by Chiara M.A. Cefalo, MD, department of clinical and molecular medicine, Sapienza University of Rome, Rome, Italy, and was published online in Diabetes, Obesity and Metabolism.
LIMITATIONS:
There was no consensus on the parameters and cutoff values for defining metabolic health status, allowing for potential variations in results. The study design suggested an association with prevalent IFG and IGT conditions but not with incident IFG and IGT conditions. All participants in this study were White, limiting the generalizability of its findings.
DISCLOSURES:
The study was supported by Sapienza University of Rome and the Italian Ministry of University. The authors declared no conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
Whether they have normal weight, overweight, or obesity, individuals with metabolically healthy (MH) phenotypes show a lower frequency of impaired glucose metabolism than their unhealthy counterparts across all weight categories.
METHODOLOGY:
- The concepts of MH overweight and MH obesity refer to a subset of people who exhibit an absence of cardiometabolic risk factors despite excess body fat, but the prevalence of prediabetes has not been investigated by metabolic phenotype and body mass index (BMI).
- This study first validated the use of estimated glucose disposal rate (eGDR), an index of insulin sensitivity calculated from clinical variables, in 350 individuals without diabetes (mean age, 37 years; 219 women; mean BMI, 30.3) from the EUGENE2 project who had varying glucose tolerance values originally assessed by insulin-stimulated glucose disposal.
- Researchers then stratified 2201 participants without diabetes (mean age, 46 years; White; 1290 women; mean BMI, 31.2) from the CATAMERI study according to BMI into three groups — individuals with normal weight (BMI, 18-24.9), overweight (BMI, 25-29.9), and obesity (BMI, ≥ 30).
- The men and women in each BMI group were separated into quartiles of insulin sensitivity based on eGDR index:
- In the normal weight group, men and women were defined as MH in the top three eGDR quartiles and metabolically unhealthy (MU) in the lowest quartile.
- In the overweight and obesity groups, people were defined as MH in the top eGDR quartile and MU in the lower three quartiles.
- Impaired glucose tolerance (IGT), impaired fasting glucose (IFG), and combined IFG+IGT conditions (from an oral glucose tolerance test) were compared in individuals without diabetes based on MH or unhealthy phenotypes across normal weight, overweight, and obese categories.
TAKEAWAY:
- eGDR demonstrated good accuracy in detecting individuals with higher insulin sensitivity in the EUGENE2 cohort.
- The MH overweight and MH obesity groups showed comparable glycemic parameters as the MH normal weight group, whereas the MU overweight and MU obesity groups exhibited higher A1c levels and fasting and 2-hour post-load glucose than the MH normal weight group.
- The frequencies of IFG, IGT, and IFG+IGT conditions were similar among the MH normal weight, MH overweight, and MH obesity groups but were higher in the MU overweight and MU obesity groups than in the MU normal weight group.
- Furthermore, compared with those in the MH normal weight group, the odds of prediabetes were at least two times higher in the MU obesity (odds ratio [OR], 2.54; P < .001) and MU overweight (OR, 2.06; P < .001) groups but not significantly different in the MU normal weight, MH obesity, and MH overweight groups.
IN PRACTICE:
The authors wrote, “Overall, the results of this cross-sectional study support the notion that metabolically healthy individuals with overweight or obesity have a more favorable metabolic risk profile in comparison to metabolically unhealthy subjects with overweight or obesity.”
SOURCE:
The study was conducted by Chiara M.A. Cefalo, MD, department of clinical and molecular medicine, Sapienza University of Rome, Rome, Italy, and was published online in Diabetes, Obesity and Metabolism.
LIMITATIONS:
There was no consensus on the parameters and cutoff values for defining metabolic health status, allowing for potential variations in results. The study design suggested an association with prevalent IFG and IGT conditions but not with incident IFG and IGT conditions. All participants in this study were White, limiting the generalizability of its findings.
DISCLOSURES:
The study was supported by Sapienza University of Rome and the Italian Ministry of University. The authors declared no conflicts of interest.
A version of this article first appeared on Medscape.com.
Bariatric Surgery Beats GLP-1 RAs in Reducing Mortality Risk
TOPLINE:
Bariatric metabolic surgery (BMS) offers a survival advantage over glucagon-like peptide 1 receptor agonists (GLP-1 RAs) in adults with obesity and diabetes for 10 years or less, which may be explained by greater weight loss with surgery, new research shows.
METHODOLOGY:
- There is limited evidence regarding the relative effectiveness of BMS and GLP-1 RAs in reducing mortality and major adverse cardiovascular events (MACE).
- This observational, retrospective cohort study analyzed the electronic medical records of Clalit Health Services, Israel’s largest healthcare organization.
- Researchers included patients aged 24 years or older who had diabetes and obesity but no prior cardiovascular disease and who either underwent BMS or received a GLP-1 RA.
- The primary outcome was all-cause mortality, assessed by multivariate Cox proportional hazards regression models. The secondary outcome was nonfatal MACE, assessed by multivariate competing risk models.
TAKEAWAY:
- Researchers included 3035 matched pairs of patients (total, 6070; mean age, 51 years; 65% women), who were followed for a median of 6.8 years.
- Among patients with diabetes for 10 years or less, those who underwent BMS had a 62% lower risk for mortality than those treated with a GLP-1 RA (hazard ratio [HR], 0.38).
- The survival advantage associated with BMS vs GLP-1 RA may be explained by the greater relative decrease in body mass index in the surgery group (–31.4% vs –12.8%, respectively).
- Among patients with diabetes for more than 10 years, no survival advantage was observed for BMS over GLP-1 RA (HR, 0.65), which may be explained by the adverse effects of prolonged diabetes duration masking the benefit associated with weight loss.
- The risk for nonfatal MACE did not differ significantly between the treatment groups in both diabetes duration categories.
IN PRACTICE:
“This study suggests that BMS was associated with greater reduced mortality compared with GLP-1 RAs among individuals with a diabetes duration of 10 years or less, mediated via greater weight loss,” the authors wrote.
SOURCE:
The study, with first author Dror Dicker, MD, Hasharon Hospital, Rabin Medical Center, Petah Tikva, Israel, was published online in JAMA Network Open.
LIMITATIONS:
The observational design may have introduced residual confounding despite matching and multivariable adjustment. The analyses did not account for the types of BMS or GLP-1 RAs or the level of adherence to GLP-1 RA treatment. Information regarding cause of death was unavailable.
DISCLOSURES:
The study was funded by the Israel Science Foundation. Dicker reported financial relationships with Novo Nordisk, Eli Lilly, and Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
TOPLINE:
Bariatric metabolic surgery (BMS) offers a survival advantage over glucagon-like peptide 1 receptor agonists (GLP-1 RAs) in adults with obesity and diabetes for 10 years or less, which may be explained by greater weight loss with surgery, new research shows.
METHODOLOGY:
- There is limited evidence regarding the relative effectiveness of BMS and GLP-1 RAs in reducing mortality and major adverse cardiovascular events (MACE).
- This observational, retrospective cohort study analyzed the electronic medical records of Clalit Health Services, Israel’s largest healthcare organization.
- Researchers included patients aged 24 years or older who had diabetes and obesity but no prior cardiovascular disease and who either underwent BMS or received a GLP-1 RA.
- The primary outcome was all-cause mortality, assessed by multivariate Cox proportional hazards regression models. The secondary outcome was nonfatal MACE, assessed by multivariate competing risk models.
TAKEAWAY:
- Researchers included 3035 matched pairs of patients (total, 6070; mean age, 51 years; 65% women), who were followed for a median of 6.8 years.
- Among patients with diabetes for 10 years or less, those who underwent BMS had a 62% lower risk for mortality than those treated with a GLP-1 RA (hazard ratio [HR], 0.38).
- The survival advantage associated with BMS vs GLP-1 RA may be explained by the greater relative decrease in body mass index in the surgery group (–31.4% vs –12.8%, respectively).
- Among patients with diabetes for more than 10 years, no survival advantage was observed for BMS over GLP-1 RA (HR, 0.65), which may be explained by the adverse effects of prolonged diabetes duration masking the benefit associated with weight loss.
- The risk for nonfatal MACE did not differ significantly between the treatment groups in both diabetes duration categories.
IN PRACTICE:
“This study suggests that BMS was associated with greater reduced mortality compared with GLP-1 RAs among individuals with a diabetes duration of 10 years or less, mediated via greater weight loss,” the authors wrote.
SOURCE:
The study, with first author Dror Dicker, MD, Hasharon Hospital, Rabin Medical Center, Petah Tikva, Israel, was published online in JAMA Network Open.
LIMITATIONS:
The observational design may have introduced residual confounding despite matching and multivariable adjustment. The analyses did not account for the types of BMS or GLP-1 RAs or the level of adherence to GLP-1 RA treatment. Information regarding cause of death was unavailable.
DISCLOSURES:
The study was funded by the Israel Science Foundation. Dicker reported financial relationships with Novo Nordisk, Eli Lilly, and Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
TOPLINE:
Bariatric metabolic surgery (BMS) offers a survival advantage over glucagon-like peptide 1 receptor agonists (GLP-1 RAs) in adults with obesity and diabetes for 10 years or less, which may be explained by greater weight loss with surgery, new research shows.
METHODOLOGY:
- There is limited evidence regarding the relative effectiveness of BMS and GLP-1 RAs in reducing mortality and major adverse cardiovascular events (MACE).
- This observational, retrospective cohort study analyzed the electronic medical records of Clalit Health Services, Israel’s largest healthcare organization.
- Researchers included patients aged 24 years or older who had diabetes and obesity but no prior cardiovascular disease and who either underwent BMS or received a GLP-1 RA.
- The primary outcome was all-cause mortality, assessed by multivariate Cox proportional hazards regression models. The secondary outcome was nonfatal MACE, assessed by multivariate competing risk models.
TAKEAWAY:
- Researchers included 3035 matched pairs of patients (total, 6070; mean age, 51 years; 65% women), who were followed for a median of 6.8 years.
- Among patients with diabetes for 10 years or less, those who underwent BMS had a 62% lower risk for mortality than those treated with a GLP-1 RA (hazard ratio [HR], 0.38).
- The survival advantage associated with BMS vs GLP-1 RA may be explained by the greater relative decrease in body mass index in the surgery group (–31.4% vs –12.8%, respectively).
- Among patients with diabetes for more than 10 years, no survival advantage was observed for BMS over GLP-1 RA (HR, 0.65), which may be explained by the adverse effects of prolonged diabetes duration masking the benefit associated with weight loss.
- The risk for nonfatal MACE did not differ significantly between the treatment groups in both diabetes duration categories.
IN PRACTICE:
“This study suggests that BMS was associated with greater reduced mortality compared with GLP-1 RAs among individuals with a diabetes duration of 10 years or less, mediated via greater weight loss,” the authors wrote.
SOURCE:
The study, with first author Dror Dicker, MD, Hasharon Hospital, Rabin Medical Center, Petah Tikva, Israel, was published online in JAMA Network Open.
LIMITATIONS:
The observational design may have introduced residual confounding despite matching and multivariable adjustment. The analyses did not account for the types of BMS or GLP-1 RAs or the level of adherence to GLP-1 RA treatment. Information regarding cause of death was unavailable.
DISCLOSURES:
The study was funded by the Israel Science Foundation. Dicker reported financial relationships with Novo Nordisk, Eli Lilly, and Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
Knee pain on walking
Overall, persons with schizophrenia are more likely than the general population to be overweight and have cardiovascular risk factors before starting treatment with antipsychotics, and such treatment generally worsens these measures. Weight gain and associated morbidity and mortality are common side effects of antipsychotic medications. Olanzapine is associated with significant weight gain of 7% or more, higher than other second-generation antipsychotics. Olanzapine treatment is the major contributor to this patient's additional weight gain over the past 2 years. This added weight has translated to excess wear and tear on her joints, leading to evidence of osteoarthritis. Treatment with olanzapine is also independently associated with detrimental changes in cardiometabolic parameters.
Interventions to prevent or mitigate weight gain with antipsychotics are limited. In general, the American Psychiatric Association does not recommend switching antipsychotics for patients whose schizophrenia is well managed. However, there is increasing evidence that metformin may have a role in mitigating weight gain as well as beneficially modifying cardiometabolic factors in patients with schizophrenia being treated with olanzapine. A systematic review of emerging evidence with metformin in patients with schizophrenia suggests that metformin may also improve some cognitive symptoms of the illness, although further research is needed. The randomized, double-blind MELIA trial of metformin plus lifestyle intervention in antipsychotic-induced weight gain is ongoing. Starting metformin as a preventive measure at the same time as antipsychotic therapy may help to limit excess weight gain.
Research continues on the potential benefit of adding weight loss medications, including glucagon-like peptide-1 (GLP-1) receptor agonists, to antipsychotics. Daily liraglutide is most widely studied, but a published case series with weekly semaglutide also demonstrated weight loss in this setting. Liraglutide also has shown beneficial cardiometabolic effects in patients using antipsychotic medications. More studies of these drugs and of GLP-1/glucose-dependent insulinotropic polypeptide agonists are needed to elucidate the optimal use of these therapies for patients with schizophrenia.
There are few other effective ways to mitigate weight gain with olanzapine. Patients should be counseled on nutrition and lifestyle modifications. Evidence supports improvement with structured lifestyle modifications across a range of patients with less severe mental health issues, and structured programs combined with motivational interviewing were associated with reductions in antipsychotic-induced weight gain in patients with severe mental illness. As with any patient with obesity, however, the success of lifestyle modifications is heavily dependent on the individual's ability and motivation to comply with recommended interventions.
Nonpharmacologic interventions to address joint pain include heat or cold compresses, physical therapy, and strength and resistance training to improve the strength of muscles supporting the joints. If these measures are ineffective, nonsteroidal anti-inflammatory drugs (NSAIDs), including ibuprofen, naproxen, meloxicam, diclofenac, or celecoxib may be used with regular follow-up to assess cardiovascular and gastrointestinal health. Topical NSAIDs also may be useful. For more intractable joint pain, options include injecting a corticosteroid or sodium hyaluronate into the affected joints or joint replacement.
Carolyn Newberry, MD, Assistant Professor of Medicine, Director of GI Nutrition, Innovative Center for Health and Nutrition in Gastroenterology (ICHANGE), Division of Gastroenterology, Weill Cornell Medical Center, New York, NY.
Disclosure: Carolyn Newberry, MD, has disclosed the following relevant financial relationships:
Serve(d) as a speaker or a member of a speakers bureau for: Baster International; InBody.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Overall, persons with schizophrenia are more likely than the general population to be overweight and have cardiovascular risk factors before starting treatment with antipsychotics, and such treatment generally worsens these measures. Weight gain and associated morbidity and mortality are common side effects of antipsychotic medications. Olanzapine is associated with significant weight gain of 7% or more, higher than other second-generation antipsychotics. Olanzapine treatment is the major contributor to this patient's additional weight gain over the past 2 years. This added weight has translated to excess wear and tear on her joints, leading to evidence of osteoarthritis. Treatment with olanzapine is also independently associated with detrimental changes in cardiometabolic parameters.
Interventions to prevent or mitigate weight gain with antipsychotics are limited. In general, the American Psychiatric Association does not recommend switching antipsychotics for patients whose schizophrenia is well managed. However, there is increasing evidence that metformin may have a role in mitigating weight gain as well as beneficially modifying cardiometabolic factors in patients with schizophrenia being treated with olanzapine. A systematic review of emerging evidence with metformin in patients with schizophrenia suggests that metformin may also improve some cognitive symptoms of the illness, although further research is needed. The randomized, double-blind MELIA trial of metformin plus lifestyle intervention in antipsychotic-induced weight gain is ongoing. Starting metformin as a preventive measure at the same time as antipsychotic therapy may help to limit excess weight gain.
Research continues on the potential benefit of adding weight loss medications, including glucagon-like peptide-1 (GLP-1) receptor agonists, to antipsychotics. Daily liraglutide is most widely studied, but a published case series with weekly semaglutide also demonstrated weight loss in this setting. Liraglutide also has shown beneficial cardiometabolic effects in patients using antipsychotic medications. More studies of these drugs and of GLP-1/glucose-dependent insulinotropic polypeptide agonists are needed to elucidate the optimal use of these therapies for patients with schizophrenia.
There are few other effective ways to mitigate weight gain with olanzapine. Patients should be counseled on nutrition and lifestyle modifications. Evidence supports improvement with structured lifestyle modifications across a range of patients with less severe mental health issues, and structured programs combined with motivational interviewing were associated with reductions in antipsychotic-induced weight gain in patients with severe mental illness. As with any patient with obesity, however, the success of lifestyle modifications is heavily dependent on the individual's ability and motivation to comply with recommended interventions.
Nonpharmacologic interventions to address joint pain include heat or cold compresses, physical therapy, and strength and resistance training to improve the strength of muscles supporting the joints. If these measures are ineffective, nonsteroidal anti-inflammatory drugs (NSAIDs), including ibuprofen, naproxen, meloxicam, diclofenac, or celecoxib may be used with regular follow-up to assess cardiovascular and gastrointestinal health. Topical NSAIDs also may be useful. For more intractable joint pain, options include injecting a corticosteroid or sodium hyaluronate into the affected joints or joint replacement.
Carolyn Newberry, MD, Assistant Professor of Medicine, Director of GI Nutrition, Innovative Center for Health and Nutrition in Gastroenterology (ICHANGE), Division of Gastroenterology, Weill Cornell Medical Center, New York, NY.
Disclosure: Carolyn Newberry, MD, has disclosed the following relevant financial relationships:
Serve(d) as a speaker or a member of a speakers bureau for: Baster International; InBody.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Overall, persons with schizophrenia are more likely than the general population to be overweight and have cardiovascular risk factors before starting treatment with antipsychotics, and such treatment generally worsens these measures. Weight gain and associated morbidity and mortality are common side effects of antipsychotic medications. Olanzapine is associated with significant weight gain of 7% or more, higher than other second-generation antipsychotics. Olanzapine treatment is the major contributor to this patient's additional weight gain over the past 2 years. This added weight has translated to excess wear and tear on her joints, leading to evidence of osteoarthritis. Treatment with olanzapine is also independently associated with detrimental changes in cardiometabolic parameters.
Interventions to prevent or mitigate weight gain with antipsychotics are limited. In general, the American Psychiatric Association does not recommend switching antipsychotics for patients whose schizophrenia is well managed. However, there is increasing evidence that metformin may have a role in mitigating weight gain as well as beneficially modifying cardiometabolic factors in patients with schizophrenia being treated with olanzapine. A systematic review of emerging evidence with metformin in patients with schizophrenia suggests that metformin may also improve some cognitive symptoms of the illness, although further research is needed. The randomized, double-blind MELIA trial of metformin plus lifestyle intervention in antipsychotic-induced weight gain is ongoing. Starting metformin as a preventive measure at the same time as antipsychotic therapy may help to limit excess weight gain.
Research continues on the potential benefit of adding weight loss medications, including glucagon-like peptide-1 (GLP-1) receptor agonists, to antipsychotics. Daily liraglutide is most widely studied, but a published case series with weekly semaglutide also demonstrated weight loss in this setting. Liraglutide also has shown beneficial cardiometabolic effects in patients using antipsychotic medications. More studies of these drugs and of GLP-1/glucose-dependent insulinotropic polypeptide agonists are needed to elucidate the optimal use of these therapies for patients with schizophrenia.
There are few other effective ways to mitigate weight gain with olanzapine. Patients should be counseled on nutrition and lifestyle modifications. Evidence supports improvement with structured lifestyle modifications across a range of patients with less severe mental health issues, and structured programs combined with motivational interviewing were associated with reductions in antipsychotic-induced weight gain in patients with severe mental illness. As with any patient with obesity, however, the success of lifestyle modifications is heavily dependent on the individual's ability and motivation to comply with recommended interventions.
Nonpharmacologic interventions to address joint pain include heat or cold compresses, physical therapy, and strength and resistance training to improve the strength of muscles supporting the joints. If these measures are ineffective, nonsteroidal anti-inflammatory drugs (NSAIDs), including ibuprofen, naproxen, meloxicam, diclofenac, or celecoxib may be used with regular follow-up to assess cardiovascular and gastrointestinal health. Topical NSAIDs also may be useful. For more intractable joint pain, options include injecting a corticosteroid or sodium hyaluronate into the affected joints or joint replacement.
Carolyn Newberry, MD, Assistant Professor of Medicine, Director of GI Nutrition, Innovative Center for Health and Nutrition in Gastroenterology (ICHANGE), Division of Gastroenterology, Weill Cornell Medical Center, New York, NY.
Disclosure: Carolyn Newberry, MD, has disclosed the following relevant financial relationships:
Serve(d) as a speaker or a member of a speakers bureau for: Baster International; InBody.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
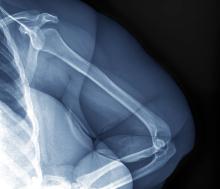
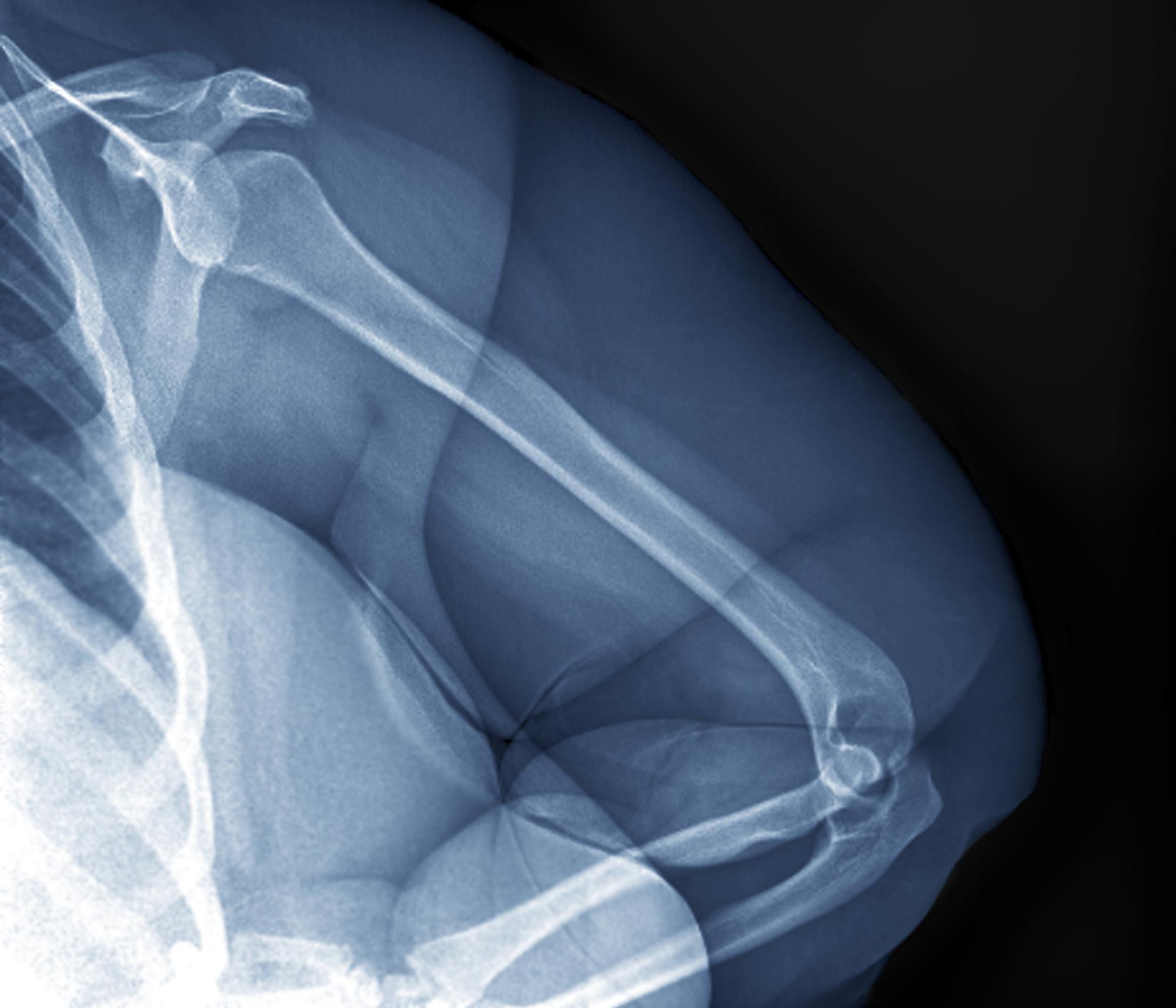
A 32-year-old woman presents with knee pain on walking and elbow pain. She is 5 ft 6 in tall and weighs 187 lb (BMI 30.2). She was diagnosed with schizophrenia 2 years ago and began treatment with olanzapine at diagnosis; her symptoms currently are controlled, and she has tolerated the medication well.
The patient says that she has been overweight since her teenage years and weighed 170 lb (BMI ~27) at age 30. However, she remained physically active until development of painful joints over the past 18 months. She works remotely full time and lives alone. She describes her long-standing diet as heavy on meat protein and light on vegetables and snacks and says it hasn't changed; she denies binge eating or other disordered eating.
Physical exam reveals tender joints at knees and elbows and central obesity (waist circumference, 42 in). Blood pressure is 135/90 mm Hg. Lab results indicate a fasting glucose level of 115 mg/dL and a triglyceride level of 170 mg/dL. She is negative for rheumatoid factor. Radiography shows premature joint erosion at the knees and elbows.
Obesity and Pregnancy
Intensive Interventions Are Needed for High-BMI Youth
The U.S. Preventive Services Task Force (USPSTF) is recommending that clinicians provide comprehensive, intensive behavioral interventions for children 6 years and older who have a high body mass index (BMI) at or above the 95th percentile (for age and sex) or refer those patients to an appropriate provider.
One in five children (19.7%) and adolescents ages 2-19 in the United States are at or above this range, based on Centers for Disease Control and Prevention growth charts from 2000, the task force wrote in its statement. The rate of BMI increase nearly doubled in this age group during the COVID pandemic, compared with prepandemic levels.
Publishing their recommendations in JAMA, the task force, with lead author Wanda K. Nicholson, MD, MPH, MBA, with the Milken Institute of Public Health, George Washington University, Washington, D.C., also noted that the prevalence of high BMI increases with age and rates are higher among children from lower-income families. Rates are also higher in Hispanic/Latino, Native American/Alaska Native and non-Hispanic Black children.
At Least 26 Hours of Interventions
It is important that children and adolescents 6 years or older with a high BMI receive intensive interventions for at least 26 contact hours for up to a year, as evidence showed that was the threshold for weight loss, the task force said.
Based on its evidence review, the USPSTF assigned this recommendation a B grade indicating “moderate certainty ... of moderate net benefit.” The task force analyzed 50 randomized clinical trials (RCTs) (n = 8,798) that examined behavioral interventions. They also analyzed eight trials that assessed pharmacotherapy interventions: liraglutide (three RCTs), semaglutide (one RCT), orlistat (two RCTs) and phentermine/topiramate (two RCTs). Five trials included behavioral counseling with the medication or placebo.
These new recommendations also reaffirm the task force’s 2010 and 2023 recommendations.
Effective interventions had multiple components. They included interventions targeting both the parent and child (separately, together or both); group sessions; information about healthy eating, information on reading food labels, and safe exercising; and interventions for encouraging behavioral changes, such as monitoring food intake and problem solving, changing physical activity behaviors, and goal setting.
These types of interventions are often delivered by multidisciplinary teams, including pediatricians, exercise physiologists or physical therapists, dietitians, psychologists, social workers, or other behavioral specialists.
Personalizing Treatment for Optimal Benefit
“The time to prevent and intervene on childhood obesity is now, and the need to start with ILT [intensive lifestyle therapy] is clear,” Roohi Y. Kharofa, MD, with the department of pediatrics, University of Cincinnati College of Medicine, Cincinnati, Ohio, and colleagues wrote in a related editorial.
However, the editorialists noted it will be important to personalize the level of interventions as ILT won’t be enough for some to prevent serious outcomes. For such patients, bariatric surgery or pharmacotherapy may need to be considered as well.
Ways to Reach the 26 Hours
Dr. Kharofa and coauthors pointed out that, while the threshold of at least 26 contact hours is associated with significant improvement in BMI (mean BMI difference, –0.8; 95% CI, –1.2 to –0.4), and while it’s important to now have an evidence-based threshold, the number may be disheartening given limits on clinicians, staff, and resources. The key may be prescribing physical activity sessions outside the health system.
For patients not interested in group sports or burdened by participation fees, collaboration with local community organizations, such as the YMCA or the Boys & Girls Club, could be arranged, the authors suggested.
“The inability to attain 26 hours should not deter patients or practitioners from participating in, referring to, or implementing obesity interventions. Rather, clinical teams and families should work together to maximize intervention dose using clinical and community programs synergistically,” they wrote.
They noted that the USPSTF in this 2024 update found “inadequate evidence on the benefits of pharmacotherapy in youth with obesity, encouraging clinicians to use ILT as the primary intervention.”
What About Medications?
New since the previous USPSTF review, several new medications have been approved for weight loss in pediatric populations, Elizabeth A. O’Connor, PhD, with The Center for Health Research, Kaiser Permanente Northwest, Portland, Oregon, and colleagues noted in their updated evidence report.
They noted that the 2023 Clinical Practice Guideline developed by the American Academy of Pediatrics states that clinicians “may offer children ages 8 through 11 years of age with obesity weight loss pharmacotherapy, according to medication indications, risks, and benefits, as an adjunct to health behavior and lifestyle treatment.”
However, Dr. O’Connor and coauthors wrote, the evidence base for each agent is limited and there is no information in the literature supporting their findings on harms of medication use beyond 17 months.
“For pharmacotherapy, when evidence was available on weight maintenance after discontinuation, weight rebounded quickly after medication use ended,” the authors wrote. “This suggests that long-term use is required for weight maintenance and underscores the need for evidence about potential harms from long-term use.”
Changes in Investment, Food, Government Priorities Are Needed
In a separate accompanying editorial, Thomas N. Robinson, MD, MPH, with Stanford University’s Center for Healthy Weight and General Pediatrics Department in Palo Alto, California, and Sarah C. Armstrong, MD, with the Duke Center for Childhood Obesity Research, Chapel Hill, North Carolina, wrote that experience to date has shown that current approaches aren’t working and, in fact, pediatric obesity rates are worsening.
“After nearly 15 years of authoritative, evidence-backed USPSTF recommendations for effective interventions for children with high BMI, it is long past time to implement them,” they wrote.
But changes will need to go far beyond clinicians’ offices and priorities must change at local, state, and federal levels, Dr. Robinson and Dr. Armstrong wrote. A shift in priorities is needed to make screening and behavioral interventions available to all children and teens with obesity.
Public policies, they wrote, must address larger issues, such as food content and availability of healthy foods, transportation innovations, and ways to make active lifestyles available equitably.
The authors said that strategies may include taxing sugary drinks, regulating marketing of unhealthful foods, crafting legislation to regulate the nutritional content of school meals, and creating policies to reduce poverty and address social drivers of health.
“A synergistic combination of effective clinical care, as recommended by the USPSTF, and public policy interventions is critically needed to turn the tide on childhood obesity,” Dr. Robinson and Dr. Armstrong wrote.
The full recommendation statement is available at the USPSTF website or the JAMA website.
One coauthor of the recommendation statement reported receiving publications and federal grand funding to his institution for the relationship between obesity and the potential effect of nutrition policy interventions on cardiovascular disease and cancer and for a meta-analysis of the effect of dietary counseling for weight loss. The authors of the evidence report had no relevant conflicts of interest. Dr. Kharofa reported receiving grants from Rhythm Pharmaceuticals outside the submitted work. Dr. Robinson has served on the scientific advisory board of WW International (through December 2022). Dr. Armstrong has served as chair of the Section on Obesity, American Academy of Pediatrics; and is a coauthor of the Clinical Practice Guidelines for the Evaluation and Treatment of Children and Adolescents with Obesity.
The U.S. Preventive Services Task Force (USPSTF) is recommending that clinicians provide comprehensive, intensive behavioral interventions for children 6 years and older who have a high body mass index (BMI) at or above the 95th percentile (for age and sex) or refer those patients to an appropriate provider.
One in five children (19.7%) and adolescents ages 2-19 in the United States are at or above this range, based on Centers for Disease Control and Prevention growth charts from 2000, the task force wrote in its statement. The rate of BMI increase nearly doubled in this age group during the COVID pandemic, compared with prepandemic levels.
Publishing their recommendations in JAMA, the task force, with lead author Wanda K. Nicholson, MD, MPH, MBA, with the Milken Institute of Public Health, George Washington University, Washington, D.C., also noted that the prevalence of high BMI increases with age and rates are higher among children from lower-income families. Rates are also higher in Hispanic/Latino, Native American/Alaska Native and non-Hispanic Black children.
At Least 26 Hours of Interventions
It is important that children and adolescents 6 years or older with a high BMI receive intensive interventions for at least 26 contact hours for up to a year, as evidence showed that was the threshold for weight loss, the task force said.
Based on its evidence review, the USPSTF assigned this recommendation a B grade indicating “moderate certainty ... of moderate net benefit.” The task force analyzed 50 randomized clinical trials (RCTs) (n = 8,798) that examined behavioral interventions. They also analyzed eight trials that assessed pharmacotherapy interventions: liraglutide (three RCTs), semaglutide (one RCT), orlistat (two RCTs) and phentermine/topiramate (two RCTs). Five trials included behavioral counseling with the medication or placebo.
These new recommendations also reaffirm the task force’s 2010 and 2023 recommendations.
Effective interventions had multiple components. They included interventions targeting both the parent and child (separately, together or both); group sessions; information about healthy eating, information on reading food labels, and safe exercising; and interventions for encouraging behavioral changes, such as monitoring food intake and problem solving, changing physical activity behaviors, and goal setting.
These types of interventions are often delivered by multidisciplinary teams, including pediatricians, exercise physiologists or physical therapists, dietitians, psychologists, social workers, or other behavioral specialists.
Personalizing Treatment for Optimal Benefit
“The time to prevent and intervene on childhood obesity is now, and the need to start with ILT [intensive lifestyle therapy] is clear,” Roohi Y. Kharofa, MD, with the department of pediatrics, University of Cincinnati College of Medicine, Cincinnati, Ohio, and colleagues wrote in a related editorial.
However, the editorialists noted it will be important to personalize the level of interventions as ILT won’t be enough for some to prevent serious outcomes. For such patients, bariatric surgery or pharmacotherapy may need to be considered as well.
Ways to Reach the 26 Hours
Dr. Kharofa and coauthors pointed out that, while the threshold of at least 26 contact hours is associated with significant improvement in BMI (mean BMI difference, –0.8; 95% CI, –1.2 to –0.4), and while it’s important to now have an evidence-based threshold, the number may be disheartening given limits on clinicians, staff, and resources. The key may be prescribing physical activity sessions outside the health system.
For patients not interested in group sports or burdened by participation fees, collaboration with local community organizations, such as the YMCA or the Boys & Girls Club, could be arranged, the authors suggested.
“The inability to attain 26 hours should not deter patients or practitioners from participating in, referring to, or implementing obesity interventions. Rather, clinical teams and families should work together to maximize intervention dose using clinical and community programs synergistically,” they wrote.
They noted that the USPSTF in this 2024 update found “inadequate evidence on the benefits of pharmacotherapy in youth with obesity, encouraging clinicians to use ILT as the primary intervention.”
What About Medications?
New since the previous USPSTF review, several new medications have been approved for weight loss in pediatric populations, Elizabeth A. O’Connor, PhD, with The Center for Health Research, Kaiser Permanente Northwest, Portland, Oregon, and colleagues noted in their updated evidence report.
They noted that the 2023 Clinical Practice Guideline developed by the American Academy of Pediatrics states that clinicians “may offer children ages 8 through 11 years of age with obesity weight loss pharmacotherapy, according to medication indications, risks, and benefits, as an adjunct to health behavior and lifestyle treatment.”
However, Dr. O’Connor and coauthors wrote, the evidence base for each agent is limited and there is no information in the literature supporting their findings on harms of medication use beyond 17 months.
“For pharmacotherapy, when evidence was available on weight maintenance after discontinuation, weight rebounded quickly after medication use ended,” the authors wrote. “This suggests that long-term use is required for weight maintenance and underscores the need for evidence about potential harms from long-term use.”
Changes in Investment, Food, Government Priorities Are Needed
In a separate accompanying editorial, Thomas N. Robinson, MD, MPH, with Stanford University’s Center for Healthy Weight and General Pediatrics Department in Palo Alto, California, and Sarah C. Armstrong, MD, with the Duke Center for Childhood Obesity Research, Chapel Hill, North Carolina, wrote that experience to date has shown that current approaches aren’t working and, in fact, pediatric obesity rates are worsening.
“After nearly 15 years of authoritative, evidence-backed USPSTF recommendations for effective interventions for children with high BMI, it is long past time to implement them,” they wrote.
But changes will need to go far beyond clinicians’ offices and priorities must change at local, state, and federal levels, Dr. Robinson and Dr. Armstrong wrote. A shift in priorities is needed to make screening and behavioral interventions available to all children and teens with obesity.
Public policies, they wrote, must address larger issues, such as food content and availability of healthy foods, transportation innovations, and ways to make active lifestyles available equitably.
The authors said that strategies may include taxing sugary drinks, regulating marketing of unhealthful foods, crafting legislation to regulate the nutritional content of school meals, and creating policies to reduce poverty and address social drivers of health.
“A synergistic combination of effective clinical care, as recommended by the USPSTF, and public policy interventions is critically needed to turn the tide on childhood obesity,” Dr. Robinson and Dr. Armstrong wrote.
The full recommendation statement is available at the USPSTF website or the JAMA website.
One coauthor of the recommendation statement reported receiving publications and federal grand funding to his institution for the relationship between obesity and the potential effect of nutrition policy interventions on cardiovascular disease and cancer and for a meta-analysis of the effect of dietary counseling for weight loss. The authors of the evidence report had no relevant conflicts of interest. Dr. Kharofa reported receiving grants from Rhythm Pharmaceuticals outside the submitted work. Dr. Robinson has served on the scientific advisory board of WW International (through December 2022). Dr. Armstrong has served as chair of the Section on Obesity, American Academy of Pediatrics; and is a coauthor of the Clinical Practice Guidelines for the Evaluation and Treatment of Children and Adolescents with Obesity.
The U.S. Preventive Services Task Force (USPSTF) is recommending that clinicians provide comprehensive, intensive behavioral interventions for children 6 years and older who have a high body mass index (BMI) at or above the 95th percentile (for age and sex) or refer those patients to an appropriate provider.
One in five children (19.7%) and adolescents ages 2-19 in the United States are at or above this range, based on Centers for Disease Control and Prevention growth charts from 2000, the task force wrote in its statement. The rate of BMI increase nearly doubled in this age group during the COVID pandemic, compared with prepandemic levels.
Publishing their recommendations in JAMA, the task force, with lead author Wanda K. Nicholson, MD, MPH, MBA, with the Milken Institute of Public Health, George Washington University, Washington, D.C., also noted that the prevalence of high BMI increases with age and rates are higher among children from lower-income families. Rates are also higher in Hispanic/Latino, Native American/Alaska Native and non-Hispanic Black children.
At Least 26 Hours of Interventions
It is important that children and adolescents 6 years or older with a high BMI receive intensive interventions for at least 26 contact hours for up to a year, as evidence showed that was the threshold for weight loss, the task force said.
Based on its evidence review, the USPSTF assigned this recommendation a B grade indicating “moderate certainty ... of moderate net benefit.” The task force analyzed 50 randomized clinical trials (RCTs) (n = 8,798) that examined behavioral interventions. They also analyzed eight trials that assessed pharmacotherapy interventions: liraglutide (three RCTs), semaglutide (one RCT), orlistat (two RCTs) and phentermine/topiramate (two RCTs). Five trials included behavioral counseling with the medication or placebo.
These new recommendations also reaffirm the task force’s 2010 and 2023 recommendations.
Effective interventions had multiple components. They included interventions targeting both the parent and child (separately, together or both); group sessions; information about healthy eating, information on reading food labels, and safe exercising; and interventions for encouraging behavioral changes, such as monitoring food intake and problem solving, changing physical activity behaviors, and goal setting.
These types of interventions are often delivered by multidisciplinary teams, including pediatricians, exercise physiologists or physical therapists, dietitians, psychologists, social workers, or other behavioral specialists.
Personalizing Treatment for Optimal Benefit
“The time to prevent and intervene on childhood obesity is now, and the need to start with ILT [intensive lifestyle therapy] is clear,” Roohi Y. Kharofa, MD, with the department of pediatrics, University of Cincinnati College of Medicine, Cincinnati, Ohio, and colleagues wrote in a related editorial.
However, the editorialists noted it will be important to personalize the level of interventions as ILT won’t be enough for some to prevent serious outcomes. For such patients, bariatric surgery or pharmacotherapy may need to be considered as well.
Ways to Reach the 26 Hours
Dr. Kharofa and coauthors pointed out that, while the threshold of at least 26 contact hours is associated with significant improvement in BMI (mean BMI difference, –0.8; 95% CI, –1.2 to –0.4), and while it’s important to now have an evidence-based threshold, the number may be disheartening given limits on clinicians, staff, and resources. The key may be prescribing physical activity sessions outside the health system.
For patients not interested in group sports or burdened by participation fees, collaboration with local community organizations, such as the YMCA or the Boys & Girls Club, could be arranged, the authors suggested.
“The inability to attain 26 hours should not deter patients or practitioners from participating in, referring to, or implementing obesity interventions. Rather, clinical teams and families should work together to maximize intervention dose using clinical and community programs synergistically,” they wrote.
They noted that the USPSTF in this 2024 update found “inadequate evidence on the benefits of pharmacotherapy in youth with obesity, encouraging clinicians to use ILT as the primary intervention.”
What About Medications?
New since the previous USPSTF review, several new medications have been approved for weight loss in pediatric populations, Elizabeth A. O’Connor, PhD, with The Center for Health Research, Kaiser Permanente Northwest, Portland, Oregon, and colleagues noted in their updated evidence report.
They noted that the 2023 Clinical Practice Guideline developed by the American Academy of Pediatrics states that clinicians “may offer children ages 8 through 11 years of age with obesity weight loss pharmacotherapy, according to medication indications, risks, and benefits, as an adjunct to health behavior and lifestyle treatment.”
However, Dr. O’Connor and coauthors wrote, the evidence base for each agent is limited and there is no information in the literature supporting their findings on harms of medication use beyond 17 months.
“For pharmacotherapy, when evidence was available on weight maintenance after discontinuation, weight rebounded quickly after medication use ended,” the authors wrote. “This suggests that long-term use is required for weight maintenance and underscores the need for evidence about potential harms from long-term use.”
Changes in Investment, Food, Government Priorities Are Needed
In a separate accompanying editorial, Thomas N. Robinson, MD, MPH, with Stanford University’s Center for Healthy Weight and General Pediatrics Department in Palo Alto, California, and Sarah C. Armstrong, MD, with the Duke Center for Childhood Obesity Research, Chapel Hill, North Carolina, wrote that experience to date has shown that current approaches aren’t working and, in fact, pediatric obesity rates are worsening.
“After nearly 15 years of authoritative, evidence-backed USPSTF recommendations for effective interventions for children with high BMI, it is long past time to implement them,” they wrote.
But changes will need to go far beyond clinicians’ offices and priorities must change at local, state, and federal levels, Dr. Robinson and Dr. Armstrong wrote. A shift in priorities is needed to make screening and behavioral interventions available to all children and teens with obesity.
Public policies, they wrote, must address larger issues, such as food content and availability of healthy foods, transportation innovations, and ways to make active lifestyles available equitably.
The authors said that strategies may include taxing sugary drinks, regulating marketing of unhealthful foods, crafting legislation to regulate the nutritional content of school meals, and creating policies to reduce poverty and address social drivers of health.
“A synergistic combination of effective clinical care, as recommended by the USPSTF, and public policy interventions is critically needed to turn the tide on childhood obesity,” Dr. Robinson and Dr. Armstrong wrote.
The full recommendation statement is available at the USPSTF website or the JAMA website.
One coauthor of the recommendation statement reported receiving publications and federal grand funding to his institution for the relationship between obesity and the potential effect of nutrition policy interventions on cardiovascular disease and cancer and for a meta-analysis of the effect of dietary counseling for weight loss. The authors of the evidence report had no relevant conflicts of interest. Dr. Kharofa reported receiving grants from Rhythm Pharmaceuticals outside the submitted work. Dr. Robinson has served on the scientific advisory board of WW International (through December 2022). Dr. Armstrong has served as chair of the Section on Obesity, American Academy of Pediatrics; and is a coauthor of the Clinical Practice Guidelines for the Evaluation and Treatment of Children and Adolescents with Obesity.
FROM JAMA
GLP-1s Reduced Secondary Stroke Risk in Patients With Diabetes, Obesity
, according to authors of a recent meta-analysis. With benefits across administration routes, dosing regimens, type 2 diabetes status, and total and nonfatal strokes, the findings could improve GLP-1 RA implementation by stroke specialists in patients with stroke history and concurrent type 2 diabetes or obesity, authors said. The study was published online in the International Journal of Stoke.
Extending Longevity
Agents including GLP-1 RAs that have been found to reduce cardiovascular events among patients with type 2 diabetes and patients who are overweight or obese also reduce risk of recurrent stroke among patients with a history of stroke who are overweight, obese, or have metabolic disease, said American Heart Association (AHA) Chief Clinical Science Officer Mitchell S. V. Elkind, MD, who was not involved with the study but was asked to comment.
“Stroke is a leading cause of mortality and the leading cause of serious long-term disability,” he added, “so medications that help to reduce that risk can play an important role in improving overall health and well-being and hopefully reducing premature mortality.”
Investigators Anastasia Adamou, MD, an internal medicine resident at AHEPA University Hospital in Thessaloniki, Greece, and colleagues searched MEDLINE and Scopus for cardiovascular outcome trials involving adults randomly assigned to GLP-1 RAs or placebo through November 2023, ultimately analyzing 11 randomized controlled trials (RCTs).
Among 60,380 participants in the nine studies that assessed total strokes, 2.5% of the GLP-1 RA group experienced strokes during follow-up, versus 3% in the placebo group (relative risk [RR] 0.85, 95% confidence interval [CI] 0.77-0.93). Regarding secondary outcomes, the GLP-1 RA group showed a significantly lower rate of nonfatal strokes versus patients on placebo (RR 0.87, 95% CI 0.79-0.95). Conversely, investigators observed no significant risk difference among the groups regarding fatal strokes, probably due to the low rate of events — 0.3% and 0.4% for treated and untreated patients, respectively.
Subgroup analyses revealed no interaction between dosing frequency and total, nonfatal, or fatal strokes. The investigators observed no difference in nonfatal strokes among participants by type 2 diabetes status and medication administration route (oral versus subcutaneous).
“The oral administration route could provide the advantage of lower local ecchymoses and allergic reactions due to subcutaneous infusions,” Dr. Adamou said in an interview. But because oral administration demands daily intake, she added, treatment adherence might be affected. “For this reason, our team performed another subgroup analysis to compare the once-a-day to the once-a-month administration. No interaction effect was again presented between the two subgroups. This outcome allows for personalization of the administration method for each patient.”
Addressing Underutilization
Despite more than 2 decades of widespread use and well-established effects on body weight, HbA1c, and cardiovascular risk, GLP-1 RAs remain underutilized, authors wrote. This is especially true in primary care, noted one study published in Clinical Diabetes.
“GLP-1 RAs have been used for many years to treat diabetic patients,” said Dr. Adamou. But because their impact on cardiovascular health regardless of diabetic status is only recently known, she said, physicians are exercising caution when prescribing this medication to patients without diabetes. “This is why more studies need to be available, especially RCTs.”
Most neurologists traditionally have left management of type 2 diabetes and other metabolic disorders to primary care doctors, said Dr. Elkind. “However, these medications are increasingly important to vascular risk reduction and should be considered part of the stroke specialist’s armamentarium.”
Vascular neurologists can play an important role in managing metabolic disease and obesity by recommending GLP-1 RAs for patients with a history of stroke, or by initiating these medications themselves, Dr. Elkind said. “These drugs are likely to become an important part of stroke patients’ medication regimens, along with antithrombotic agents, blood pressure control, and statins. Neurologists are well-positioned to educate other physicians about the important connections among brain, heart, and metabolic health.”
To that end, he said, the AHA will update guidelines for both primary and secondary stroke prevention as warranted by evidence supporting GLP-1 RAs and other medications that could impact stroke risk in type 2 diabetes and related metabolic disorders. However, no guidelines concerning use of GLP-1 RAs for secondary stroke prevention in obesity exist. Here, said Dr. Elkind, the AHA will continue building on its innovative Cardiovascular-Kidney Metabolic Health program, which includes clinical suggestions and may include more formal clinical practice guidelines as the evidence evolves.
Among the main drivers of the initiative, he said, is the recognition that cardiovascular disease — including stroke — is the major cause of death and morbidity among patients with obesity, type 2 diabetes, and metabolic disorders. “Stroke should be considered an important part of overall cardiovascular risk, and the findings that these drugs can help to reduce the risk of stroke specifically is an important additional reason for their use.”
Dr. Elkind and Dr. Adamou reported no conflicting interests. The authors received no financial support for the study.
, according to authors of a recent meta-analysis. With benefits across administration routes, dosing regimens, type 2 diabetes status, and total and nonfatal strokes, the findings could improve GLP-1 RA implementation by stroke specialists in patients with stroke history and concurrent type 2 diabetes or obesity, authors said. The study was published online in the International Journal of Stoke.
Extending Longevity
Agents including GLP-1 RAs that have been found to reduce cardiovascular events among patients with type 2 diabetes and patients who are overweight or obese also reduce risk of recurrent stroke among patients with a history of stroke who are overweight, obese, or have metabolic disease, said American Heart Association (AHA) Chief Clinical Science Officer Mitchell S. V. Elkind, MD, who was not involved with the study but was asked to comment.
“Stroke is a leading cause of mortality and the leading cause of serious long-term disability,” he added, “so medications that help to reduce that risk can play an important role in improving overall health and well-being and hopefully reducing premature mortality.”
Investigators Anastasia Adamou, MD, an internal medicine resident at AHEPA University Hospital in Thessaloniki, Greece, and colleagues searched MEDLINE and Scopus for cardiovascular outcome trials involving adults randomly assigned to GLP-1 RAs or placebo through November 2023, ultimately analyzing 11 randomized controlled trials (RCTs).
Among 60,380 participants in the nine studies that assessed total strokes, 2.5% of the GLP-1 RA group experienced strokes during follow-up, versus 3% in the placebo group (relative risk [RR] 0.85, 95% confidence interval [CI] 0.77-0.93). Regarding secondary outcomes, the GLP-1 RA group showed a significantly lower rate of nonfatal strokes versus patients on placebo (RR 0.87, 95% CI 0.79-0.95). Conversely, investigators observed no significant risk difference among the groups regarding fatal strokes, probably due to the low rate of events — 0.3% and 0.4% for treated and untreated patients, respectively.
Subgroup analyses revealed no interaction between dosing frequency and total, nonfatal, or fatal strokes. The investigators observed no difference in nonfatal strokes among participants by type 2 diabetes status and medication administration route (oral versus subcutaneous).
“The oral administration route could provide the advantage of lower local ecchymoses and allergic reactions due to subcutaneous infusions,” Dr. Adamou said in an interview. But because oral administration demands daily intake, she added, treatment adherence might be affected. “For this reason, our team performed another subgroup analysis to compare the once-a-day to the once-a-month administration. No interaction effect was again presented between the two subgroups. This outcome allows for personalization of the administration method for each patient.”
Addressing Underutilization
Despite more than 2 decades of widespread use and well-established effects on body weight, HbA1c, and cardiovascular risk, GLP-1 RAs remain underutilized, authors wrote. This is especially true in primary care, noted one study published in Clinical Diabetes.
“GLP-1 RAs have been used for many years to treat diabetic patients,” said Dr. Adamou. But because their impact on cardiovascular health regardless of diabetic status is only recently known, she said, physicians are exercising caution when prescribing this medication to patients without diabetes. “This is why more studies need to be available, especially RCTs.”
Most neurologists traditionally have left management of type 2 diabetes and other metabolic disorders to primary care doctors, said Dr. Elkind. “However, these medications are increasingly important to vascular risk reduction and should be considered part of the stroke specialist’s armamentarium.”
Vascular neurologists can play an important role in managing metabolic disease and obesity by recommending GLP-1 RAs for patients with a history of stroke, or by initiating these medications themselves, Dr. Elkind said. “These drugs are likely to become an important part of stroke patients’ medication regimens, along with antithrombotic agents, blood pressure control, and statins. Neurologists are well-positioned to educate other physicians about the important connections among brain, heart, and metabolic health.”
To that end, he said, the AHA will update guidelines for both primary and secondary stroke prevention as warranted by evidence supporting GLP-1 RAs and other medications that could impact stroke risk in type 2 diabetes and related metabolic disorders. However, no guidelines concerning use of GLP-1 RAs for secondary stroke prevention in obesity exist. Here, said Dr. Elkind, the AHA will continue building on its innovative Cardiovascular-Kidney Metabolic Health program, which includes clinical suggestions and may include more formal clinical practice guidelines as the evidence evolves.
Among the main drivers of the initiative, he said, is the recognition that cardiovascular disease — including stroke — is the major cause of death and morbidity among patients with obesity, type 2 diabetes, and metabolic disorders. “Stroke should be considered an important part of overall cardiovascular risk, and the findings that these drugs can help to reduce the risk of stroke specifically is an important additional reason for their use.”
Dr. Elkind and Dr. Adamou reported no conflicting interests. The authors received no financial support for the study.
, according to authors of a recent meta-analysis. With benefits across administration routes, dosing regimens, type 2 diabetes status, and total and nonfatal strokes, the findings could improve GLP-1 RA implementation by stroke specialists in patients with stroke history and concurrent type 2 diabetes or obesity, authors said. The study was published online in the International Journal of Stoke.
Extending Longevity
Agents including GLP-1 RAs that have been found to reduce cardiovascular events among patients with type 2 diabetes and patients who are overweight or obese also reduce risk of recurrent stroke among patients with a history of stroke who are overweight, obese, or have metabolic disease, said American Heart Association (AHA) Chief Clinical Science Officer Mitchell S. V. Elkind, MD, who was not involved with the study but was asked to comment.
“Stroke is a leading cause of mortality and the leading cause of serious long-term disability,” he added, “so medications that help to reduce that risk can play an important role in improving overall health and well-being and hopefully reducing premature mortality.”
Investigators Anastasia Adamou, MD, an internal medicine resident at AHEPA University Hospital in Thessaloniki, Greece, and colleagues searched MEDLINE and Scopus for cardiovascular outcome trials involving adults randomly assigned to GLP-1 RAs or placebo through November 2023, ultimately analyzing 11 randomized controlled trials (RCTs).
Among 60,380 participants in the nine studies that assessed total strokes, 2.5% of the GLP-1 RA group experienced strokes during follow-up, versus 3% in the placebo group (relative risk [RR] 0.85, 95% confidence interval [CI] 0.77-0.93). Regarding secondary outcomes, the GLP-1 RA group showed a significantly lower rate of nonfatal strokes versus patients on placebo (RR 0.87, 95% CI 0.79-0.95). Conversely, investigators observed no significant risk difference among the groups regarding fatal strokes, probably due to the low rate of events — 0.3% and 0.4% for treated and untreated patients, respectively.
Subgroup analyses revealed no interaction between dosing frequency and total, nonfatal, or fatal strokes. The investigators observed no difference in nonfatal strokes among participants by type 2 diabetes status and medication administration route (oral versus subcutaneous).
“The oral administration route could provide the advantage of lower local ecchymoses and allergic reactions due to subcutaneous infusions,” Dr. Adamou said in an interview. But because oral administration demands daily intake, she added, treatment adherence might be affected. “For this reason, our team performed another subgroup analysis to compare the once-a-day to the once-a-month administration. No interaction effect was again presented between the two subgroups. This outcome allows for personalization of the administration method for each patient.”
Addressing Underutilization
Despite more than 2 decades of widespread use and well-established effects on body weight, HbA1c, and cardiovascular risk, GLP-1 RAs remain underutilized, authors wrote. This is especially true in primary care, noted one study published in Clinical Diabetes.
“GLP-1 RAs have been used for many years to treat diabetic patients,” said Dr. Adamou. But because their impact on cardiovascular health regardless of diabetic status is only recently known, she said, physicians are exercising caution when prescribing this medication to patients without diabetes. “This is why more studies need to be available, especially RCTs.”
Most neurologists traditionally have left management of type 2 diabetes and other metabolic disorders to primary care doctors, said Dr. Elkind. “However, these medications are increasingly important to vascular risk reduction and should be considered part of the stroke specialist’s armamentarium.”
Vascular neurologists can play an important role in managing metabolic disease and obesity by recommending GLP-1 RAs for patients with a history of stroke, or by initiating these medications themselves, Dr. Elkind said. “These drugs are likely to become an important part of stroke patients’ medication regimens, along with antithrombotic agents, blood pressure control, and statins. Neurologists are well-positioned to educate other physicians about the important connections among brain, heart, and metabolic health.”
To that end, he said, the AHA will update guidelines for both primary and secondary stroke prevention as warranted by evidence supporting GLP-1 RAs and other medications that could impact stroke risk in type 2 diabetes and related metabolic disorders. However, no guidelines concerning use of GLP-1 RAs for secondary stroke prevention in obesity exist. Here, said Dr. Elkind, the AHA will continue building on its innovative Cardiovascular-Kidney Metabolic Health program, which includes clinical suggestions and may include more formal clinical practice guidelines as the evidence evolves.
Among the main drivers of the initiative, he said, is the recognition that cardiovascular disease — including stroke — is the major cause of death and morbidity among patients with obesity, type 2 diabetes, and metabolic disorders. “Stroke should be considered an important part of overall cardiovascular risk, and the findings that these drugs can help to reduce the risk of stroke specifically is an important additional reason for their use.”
Dr. Elkind and Dr. Adamou reported no conflicting interests. The authors received no financial support for the study.
FROM THE INTERNATIONAL JOURNAL OF STROKE
Sugar Substitute Tied to Higher Risk for Heart Attack, Stroke
High levels of xylitol, a low-calorie sweetener used in many reduced-sugar foods as well as gum and toothpaste, are linked to an increased risk of heart attacks, strokes, and death, says a new study published in the European Heart Journal.
The research team studied more than 3000 people in the US and Europe over 3 years and found that people with the highest amount of xylitol in their plasma were more likely to have a problem with their heart or blood vessels.
To show the early effects of xylitol, researchers studied platelet activity in volunteers who consumed a xylitol-sweetened drink and a glucose-sweetened drink. The xylitol levels went up by 1000 times in people after the xylitol drink but not after the glucose-sweetened drink.
Xylitol is naturally found in small amounts in fruit and vegetables, and it’s been used more as a sugar substitute over the past decade in processed foods, toothpaste, chewing gum, and other products.
“This study again shows the immediate need for investigating sugar alcohols and artificial sweeteners, especially as they continue to be recommended in combating conditions like obesity or diabetes,” Stanley Hazen, MD, chair of the Department of Cardiovascular and Metabolic Sciences at Cleveland Clinic’s Lerner Research Institute, Cleveland, Ohio, said in a news release.
“It does not mean throw out your toothpaste if it has xylitol in it, but we should be aware that consumption of a product containing high levels could increase the risk of blood clot-related events.”
A similar link between erythritol, another sugar substance, and problems with the heart and blood vessels was found last year by the same research team, the release said.
In a response to the study, the Calorie Control Council, a trade association representing the low- and reduced-calorie food and beverage industry, said xylitol has been approved for decades by government agencies. The study results may not apply to the general population because some people in the study already had a higher risk of having problems with their heart and blood vessels, it said.
A version of this article first appeared on WebMD.com.
High levels of xylitol, a low-calorie sweetener used in many reduced-sugar foods as well as gum and toothpaste, are linked to an increased risk of heart attacks, strokes, and death, says a new study published in the European Heart Journal.
The research team studied more than 3000 people in the US and Europe over 3 years and found that people with the highest amount of xylitol in their plasma were more likely to have a problem with their heart or blood vessels.
To show the early effects of xylitol, researchers studied platelet activity in volunteers who consumed a xylitol-sweetened drink and a glucose-sweetened drink. The xylitol levels went up by 1000 times in people after the xylitol drink but not after the glucose-sweetened drink.
Xylitol is naturally found in small amounts in fruit and vegetables, and it’s been used more as a sugar substitute over the past decade in processed foods, toothpaste, chewing gum, and other products.
“This study again shows the immediate need for investigating sugar alcohols and artificial sweeteners, especially as they continue to be recommended in combating conditions like obesity or diabetes,” Stanley Hazen, MD, chair of the Department of Cardiovascular and Metabolic Sciences at Cleveland Clinic’s Lerner Research Institute, Cleveland, Ohio, said in a news release.
“It does not mean throw out your toothpaste if it has xylitol in it, but we should be aware that consumption of a product containing high levels could increase the risk of blood clot-related events.”
A similar link between erythritol, another sugar substance, and problems with the heart and blood vessels was found last year by the same research team, the release said.
In a response to the study, the Calorie Control Council, a trade association representing the low- and reduced-calorie food and beverage industry, said xylitol has been approved for decades by government agencies. The study results may not apply to the general population because some people in the study already had a higher risk of having problems with their heart and blood vessels, it said.
A version of this article first appeared on WebMD.com.
High levels of xylitol, a low-calorie sweetener used in many reduced-sugar foods as well as gum and toothpaste, are linked to an increased risk of heart attacks, strokes, and death, says a new study published in the European Heart Journal.
The research team studied more than 3000 people in the US and Europe over 3 years and found that people with the highest amount of xylitol in their plasma were more likely to have a problem with their heart or blood vessels.
To show the early effects of xylitol, researchers studied platelet activity in volunteers who consumed a xylitol-sweetened drink and a glucose-sweetened drink. The xylitol levels went up by 1000 times in people after the xylitol drink but not after the glucose-sweetened drink.
Xylitol is naturally found in small amounts in fruit and vegetables, and it’s been used more as a sugar substitute over the past decade in processed foods, toothpaste, chewing gum, and other products.
“This study again shows the immediate need for investigating sugar alcohols and artificial sweeteners, especially as they continue to be recommended in combating conditions like obesity or diabetes,” Stanley Hazen, MD, chair of the Department of Cardiovascular and Metabolic Sciences at Cleveland Clinic’s Lerner Research Institute, Cleveland, Ohio, said in a news release.
“It does not mean throw out your toothpaste if it has xylitol in it, but we should be aware that consumption of a product containing high levels could increase the risk of blood clot-related events.”
A similar link between erythritol, another sugar substance, and problems with the heart and blood vessels was found last year by the same research team, the release said.
In a response to the study, the Calorie Control Council, a trade association representing the low- and reduced-calorie food and beverage industry, said xylitol has been approved for decades by government agencies. The study results may not apply to the general population because some people in the study already had a higher risk of having problems with their heart and blood vessels, it said.
A version of this article first appeared on WebMD.com.
Is Cushing Syndrome More Common in the US Than We Think?
BOSTON — The prevalence of Cushing syndrome (CS) in the United States may be considerably higher than currently appreciated, new data from a single US institution suggest.
In contrast to estimates of 1 to 3 cases per million patient-years from population-based European studies, researchers at the University of Wisconsin, Milwaukee, estimated that the incidence of CS in Wisconsin is a minimum of 7.2 cases per million patient-years. What’s more, contrary to all previous studies, they found that adrenal Cushing syndrome was more common than pituitary adrenocorticotropic hormone (ACTH)–secreting tumors (Cushing disease), and that fewer than half of individuals with adrenal Cushing syndrome had classic physical features of hypercortisolism, such as weight gain, round face, excessive hair growth, and stretch marks.
“Cases are absolutely being missed. ... Clinicians should realize that cortisol excess is not rare. It may not be common, but it needs to be considered in patients with any constellation of features that are seen in cortisol excess,” study investigator Ty B. Carroll, MD, associate professor of medicine, endocrinology and molecular medicine, and the endocrine fellowship program director at Medical College of Wisconsin in Milwaukee, told this news organization.
There are several contributing factors, he noted, “including the obesity and diabetes epidemics which make some clinical features of cortisol excess more common and less notable. Providers get used to seeing patients with some features of cortisol excess and don’t think to screen. The consequence of this is more difficult-to-control diabetes and hypertension, more advance metabolic bone disease, and likely more advanced cardiovascular disease, all resulting from extended exposure to cortisol excess,” he said.
Are Milder Cases the Ones Being Missed?
Asked to comment, session moderator Sharon L. Wardlaw, MD, professor of medicine at Columbia University College of Physicians and Surgeons, New York City, said, “When we talk about Cushing [syndrome], we usually think of pituitary ACTH as more [common], followed by adrenal adenomas, and then ectopic. But they’re seeing more adrenal adenoma ... we are probably diagnosing this a little more now.”
She also suggested that the Wisconsin group may have a lower threshold for diagnosing the milder cortisol elevation seen with adrenal Cushing syndrome. “If you screen for Cushing with a dexamethasone suppression test … [i]f you have autonomous secretion by the adrenal, you don’t suppress as much. ... When you measure 24-hour urinary cortisol, it may be normal. So you’re in this in-between [state]. ... Maybe in Wisconsin they’re diagnosing it more. Or, maybe it’s just being underdiagnosed in other places.”
She also pointed out that “you can’t diagnose it unless you think of it. I’m not so sure that with these mild cases it’s so much that it’s more common, but maybe it’s like thyroid nodules, where we didn’t know about it until everybody started getting all of these CT scans. We’re now seeing all these incidental thyroid nodules ... I don’t think we’re missing florid Cushing.”
However, Dr. Wardlaw said, it’s probably worthwhile to detect even milder hypercortisolism because it could still have long-term damaging effects, including osteoporosis, muscle weakness, glucose intolerance, and frailty. “You could do something about it and normalize it if you found it. I think that would be the reason to do it.”
Is Wisconsin Representative of Cushing Everywhere?
Dr. Carroll presented the findings at the annual meeting of the Endocrine Society. He began by noting that most of the previous CS incidence studies, with estimates of 1.2-3.2 cases per million per year, come from European data published from 1994 to 2019 and collected as far back as 1955. The method of acquisition of patients and the definitions of confirmed cases varied widely in those studies, which reported CS etiologies of ACTH-secreting neoplasms (pituitary or ectopic) in 75%-85% and adrenal-dependent cortisol excess in 15%-20%.
The current study included data from clinic records between May 1, 2017, and December 31, 2022, of Wisconsin residents newly diagnosed with and treated for CS. The CS diagnosis was established with standard guideline-supported biochemical testing and appropriate imaging. Patients with exogenous and non-neoplastic hypercortisolism and those who did not receive therapy for CS were excluded.
A total of 185 patients (73% female, 27% male) were identified from 27 of the total 72 counties in Wisconsin, representing a population of 4.5 million. On the basis of the total 5.9 million population of Wisconsin, the incidence of CS in the state works out to 7.2 cases per million population per year, Dr. Carroll said.
However, data from the Wisconsin Hospital Association show that the University of Wisconsin’s Milwaukee facility treated just about half of patients in the state who are discharged from the hospital with a diagnosis of CS during 2019-2023. “So ... that means that an actual or approximate incidence of 14-15 cases per million per year rather than the 7.2 cases that we produce,” he said.
Etiologies were 60% adrenal (111 patients), 36.8% pituitary (68 patients), and 3.2% ectopic (6 patients). Those proportions were similar between genders.
On biochemical testing, values for late-night salivary cortisol, dexamethasone suppression, and urinary free cortisol were highest for the ectopic group (3.189 µg/dL, 42.5 µg/dL, and 1514.2 µg/24 h, respectively) and lowest for the adrenal group (0.236 µg/dL, 6.5 µg/dL, and 64.2 µg/24 h, respectively). All differences between groups were highly statistically significant, at P < .0001, Dr. Carroll noted.
Classic physical features of CS were present in 91% of people with pituitary CS and 100% of those ectopic CS but just 44% of individuals with adrenal CS. “We found that adrenal-dependent disease was the most common form of Cushing syndrome. It frequently presented without classic physical features that may be due to the milder biochemical presentation,” he concluded.
Dr. Carroll reported consulting and investigator fees from Corcept Therapeutics. Dr. Wardlaw has no disclosures.
A version of this article appeared on Medscape.com.
BOSTON — The prevalence of Cushing syndrome (CS) in the United States may be considerably higher than currently appreciated, new data from a single US institution suggest.
In contrast to estimates of 1 to 3 cases per million patient-years from population-based European studies, researchers at the University of Wisconsin, Milwaukee, estimated that the incidence of CS in Wisconsin is a minimum of 7.2 cases per million patient-years. What’s more, contrary to all previous studies, they found that adrenal Cushing syndrome was more common than pituitary adrenocorticotropic hormone (ACTH)–secreting tumors (Cushing disease), and that fewer than half of individuals with adrenal Cushing syndrome had classic physical features of hypercortisolism, such as weight gain, round face, excessive hair growth, and stretch marks.
“Cases are absolutely being missed. ... Clinicians should realize that cortisol excess is not rare. It may not be common, but it needs to be considered in patients with any constellation of features that are seen in cortisol excess,” study investigator Ty B. Carroll, MD, associate professor of medicine, endocrinology and molecular medicine, and the endocrine fellowship program director at Medical College of Wisconsin in Milwaukee, told this news organization.
There are several contributing factors, he noted, “including the obesity and diabetes epidemics which make some clinical features of cortisol excess more common and less notable. Providers get used to seeing patients with some features of cortisol excess and don’t think to screen. The consequence of this is more difficult-to-control diabetes and hypertension, more advance metabolic bone disease, and likely more advanced cardiovascular disease, all resulting from extended exposure to cortisol excess,” he said.
Are Milder Cases the Ones Being Missed?
Asked to comment, session moderator Sharon L. Wardlaw, MD, professor of medicine at Columbia University College of Physicians and Surgeons, New York City, said, “When we talk about Cushing [syndrome], we usually think of pituitary ACTH as more [common], followed by adrenal adenomas, and then ectopic. But they’re seeing more adrenal adenoma ... we are probably diagnosing this a little more now.”
She also suggested that the Wisconsin group may have a lower threshold for diagnosing the milder cortisol elevation seen with adrenal Cushing syndrome. “If you screen for Cushing with a dexamethasone suppression test … [i]f you have autonomous secretion by the adrenal, you don’t suppress as much. ... When you measure 24-hour urinary cortisol, it may be normal. So you’re in this in-between [state]. ... Maybe in Wisconsin they’re diagnosing it more. Or, maybe it’s just being underdiagnosed in other places.”
She also pointed out that “you can’t diagnose it unless you think of it. I’m not so sure that with these mild cases it’s so much that it’s more common, but maybe it’s like thyroid nodules, where we didn’t know about it until everybody started getting all of these CT scans. We’re now seeing all these incidental thyroid nodules ... I don’t think we’re missing florid Cushing.”
However, Dr. Wardlaw said, it’s probably worthwhile to detect even milder hypercortisolism because it could still have long-term damaging effects, including osteoporosis, muscle weakness, glucose intolerance, and frailty. “You could do something about it and normalize it if you found it. I think that would be the reason to do it.”
Is Wisconsin Representative of Cushing Everywhere?
Dr. Carroll presented the findings at the annual meeting of the Endocrine Society. He began by noting that most of the previous CS incidence studies, with estimates of 1.2-3.2 cases per million per year, come from European data published from 1994 to 2019 and collected as far back as 1955. The method of acquisition of patients and the definitions of confirmed cases varied widely in those studies, which reported CS etiologies of ACTH-secreting neoplasms (pituitary or ectopic) in 75%-85% and adrenal-dependent cortisol excess in 15%-20%.
The current study included data from clinic records between May 1, 2017, and December 31, 2022, of Wisconsin residents newly diagnosed with and treated for CS. The CS diagnosis was established with standard guideline-supported biochemical testing and appropriate imaging. Patients with exogenous and non-neoplastic hypercortisolism and those who did not receive therapy for CS were excluded.
A total of 185 patients (73% female, 27% male) were identified from 27 of the total 72 counties in Wisconsin, representing a population of 4.5 million. On the basis of the total 5.9 million population of Wisconsin, the incidence of CS in the state works out to 7.2 cases per million population per year, Dr. Carroll said.
However, data from the Wisconsin Hospital Association show that the University of Wisconsin’s Milwaukee facility treated just about half of patients in the state who are discharged from the hospital with a diagnosis of CS during 2019-2023. “So ... that means that an actual or approximate incidence of 14-15 cases per million per year rather than the 7.2 cases that we produce,” he said.
Etiologies were 60% adrenal (111 patients), 36.8% pituitary (68 patients), and 3.2% ectopic (6 patients). Those proportions were similar between genders.
On biochemical testing, values for late-night salivary cortisol, dexamethasone suppression, and urinary free cortisol were highest for the ectopic group (3.189 µg/dL, 42.5 µg/dL, and 1514.2 µg/24 h, respectively) and lowest for the adrenal group (0.236 µg/dL, 6.5 µg/dL, and 64.2 µg/24 h, respectively). All differences between groups were highly statistically significant, at P < .0001, Dr. Carroll noted.
Classic physical features of CS were present in 91% of people with pituitary CS and 100% of those ectopic CS but just 44% of individuals with adrenal CS. “We found that adrenal-dependent disease was the most common form of Cushing syndrome. It frequently presented without classic physical features that may be due to the milder biochemical presentation,” he concluded.
Dr. Carroll reported consulting and investigator fees from Corcept Therapeutics. Dr. Wardlaw has no disclosures.
A version of this article appeared on Medscape.com.
BOSTON — The prevalence of Cushing syndrome (CS) in the United States may be considerably higher than currently appreciated, new data from a single US institution suggest.
In contrast to estimates of 1 to 3 cases per million patient-years from population-based European studies, researchers at the University of Wisconsin, Milwaukee, estimated that the incidence of CS in Wisconsin is a minimum of 7.2 cases per million patient-years. What’s more, contrary to all previous studies, they found that adrenal Cushing syndrome was more common than pituitary adrenocorticotropic hormone (ACTH)–secreting tumors (Cushing disease), and that fewer than half of individuals with adrenal Cushing syndrome had classic physical features of hypercortisolism, such as weight gain, round face, excessive hair growth, and stretch marks.
“Cases are absolutely being missed. ... Clinicians should realize that cortisol excess is not rare. It may not be common, but it needs to be considered in patients with any constellation of features that are seen in cortisol excess,” study investigator Ty B. Carroll, MD, associate professor of medicine, endocrinology and molecular medicine, and the endocrine fellowship program director at Medical College of Wisconsin in Milwaukee, told this news organization.
There are several contributing factors, he noted, “including the obesity and diabetes epidemics which make some clinical features of cortisol excess more common and less notable. Providers get used to seeing patients with some features of cortisol excess and don’t think to screen. The consequence of this is more difficult-to-control diabetes and hypertension, more advance metabolic bone disease, and likely more advanced cardiovascular disease, all resulting from extended exposure to cortisol excess,” he said.
Are Milder Cases the Ones Being Missed?
Asked to comment, session moderator Sharon L. Wardlaw, MD, professor of medicine at Columbia University College of Physicians and Surgeons, New York City, said, “When we talk about Cushing [syndrome], we usually think of pituitary ACTH as more [common], followed by adrenal adenomas, and then ectopic. But they’re seeing more adrenal adenoma ... we are probably diagnosing this a little more now.”
She also suggested that the Wisconsin group may have a lower threshold for diagnosing the milder cortisol elevation seen with adrenal Cushing syndrome. “If you screen for Cushing with a dexamethasone suppression test … [i]f you have autonomous secretion by the adrenal, you don’t suppress as much. ... When you measure 24-hour urinary cortisol, it may be normal. So you’re in this in-between [state]. ... Maybe in Wisconsin they’re diagnosing it more. Or, maybe it’s just being underdiagnosed in other places.”
She also pointed out that “you can’t diagnose it unless you think of it. I’m not so sure that with these mild cases it’s so much that it’s more common, but maybe it’s like thyroid nodules, where we didn’t know about it until everybody started getting all of these CT scans. We’re now seeing all these incidental thyroid nodules ... I don’t think we’re missing florid Cushing.”
However, Dr. Wardlaw said, it’s probably worthwhile to detect even milder hypercortisolism because it could still have long-term damaging effects, including osteoporosis, muscle weakness, glucose intolerance, and frailty. “You could do something about it and normalize it if you found it. I think that would be the reason to do it.”
Is Wisconsin Representative of Cushing Everywhere?
Dr. Carroll presented the findings at the annual meeting of the Endocrine Society. He began by noting that most of the previous CS incidence studies, with estimates of 1.2-3.2 cases per million per year, come from European data published from 1994 to 2019 and collected as far back as 1955. The method of acquisition of patients and the definitions of confirmed cases varied widely in those studies, which reported CS etiologies of ACTH-secreting neoplasms (pituitary or ectopic) in 75%-85% and adrenal-dependent cortisol excess in 15%-20%.
The current study included data from clinic records between May 1, 2017, and December 31, 2022, of Wisconsin residents newly diagnosed with and treated for CS. The CS diagnosis was established with standard guideline-supported biochemical testing and appropriate imaging. Patients with exogenous and non-neoplastic hypercortisolism and those who did not receive therapy for CS were excluded.
A total of 185 patients (73% female, 27% male) were identified from 27 of the total 72 counties in Wisconsin, representing a population of 4.5 million. On the basis of the total 5.9 million population of Wisconsin, the incidence of CS in the state works out to 7.2 cases per million population per year, Dr. Carroll said.
However, data from the Wisconsin Hospital Association show that the University of Wisconsin’s Milwaukee facility treated just about half of patients in the state who are discharged from the hospital with a diagnosis of CS during 2019-2023. “So ... that means that an actual or approximate incidence of 14-15 cases per million per year rather than the 7.2 cases that we produce,” he said.
Etiologies were 60% adrenal (111 patients), 36.8% pituitary (68 patients), and 3.2% ectopic (6 patients). Those proportions were similar between genders.
On biochemical testing, values for late-night salivary cortisol, dexamethasone suppression, and urinary free cortisol were highest for the ectopic group (3.189 µg/dL, 42.5 µg/dL, and 1514.2 µg/24 h, respectively) and lowest for the adrenal group (0.236 µg/dL, 6.5 µg/dL, and 64.2 µg/24 h, respectively). All differences between groups were highly statistically significant, at P < .0001, Dr. Carroll noted.
Classic physical features of CS were present in 91% of people with pituitary CS and 100% of those ectopic CS but just 44% of individuals with adrenal CS. “We found that adrenal-dependent disease was the most common form of Cushing syndrome. It frequently presented without classic physical features that may be due to the milder biochemical presentation,” he concluded.
Dr. Carroll reported consulting and investigator fees from Corcept Therapeutics. Dr. Wardlaw has no disclosures.
A version of this article appeared on Medscape.com.
About 20% of Breast Cancer Survivors Gain Excess Weight
BOSTON — Nearly one in five breast cancer survivors will gain more than 10% of their body weight in the 6 years following their diagnosis, according to new research presented at ENDO 2024, the annual meeting of the Endocrine Society.
Younger age and lower weight at diagnosis were the strongest predictors of this excessive weight gain over time.
“Weight gain is a common concern after breast cancer diagnosis and treatment,” said Maria Daniela Hurtado Andrade, MD, PhD, of the Mayo Clinic in Jacksonville, Florida, who led the research. “This weight gain in breast cancer survivor increases breast cancer recurrence and mortality, increases cardiovascular disease and mortality, and also increases all-cause mortality.”
Previous studies have found an association between breast cancer survivorship and weight gain, but the reported incidences of weight gain — and the amounts gained — have been highly variable, she added.
In the study, researchers used the Mayo Clinic Breast Cancer Registry to identify 4575 breast cancer survivors and tracked their weight over the course of 6 years following cancer diagnosis. These patients were age-matched to women in the general population selected from the Rochester Epidemiology Project, which contains the medical records of residents of 27 counties in Minnesota and Wisconsin. All controls had no history of cancer or bariatric surgery.
Nearly all patients and controls were White (97%); at breast cancer diagnosis, patients were on average 58 years of age and weighed 76 kg (165.5 lb). Controls had similar ages and baseline weights.
At 6 years following breast cancer diagnosis, average weight gain was modest: Breast cancer survivors gained 1.6% of their body weight, compared with 0.7% in controls (P = .004).
However, 18% of breast cancer survivors had gained at least 10% of their body weight over that time. By comparison, 8% of controls experienced this excessive weight gain during that same time frame (P < .0001). The same trend was observed for 15% and 20% weight gain.
After adjustment for confounding factors, younger age at breast cancer diagnosis and lower baseline weight were the strongest predictors of more than 10% weight gain. BRCA2 mutation and use of systemic chemotherapy treatment were also associated with excessive weight gain.
Several factors could be driving weight gain in these patients, said Zeynep Madak-Erdogan, PhD, at the University of Illinois Urbana-Champaign, who was not involved with the research. Her work focuses on how diet and nutrition affect hormone action in postmenopausal women and breast cancer survivors. Certain therapies can induce temporary or permanent menopause in patients, “and this early menopause might shift balance of estrogens and cause increased weight gain,” she said. Along the same lines, endocrine therapies can also affect estrogen production.
Stress and exhaustion from treatment — especially compounded by the two previous factors — are also likely culprits in weight gain, she continued.
“These findings highlight importance of lifestyle interventions,” added Dr. Madak-Erdogan. “In addition to changes in the diet (increased vegetable, fruit, [and] whole grain intake; reduction in saturated fats, alcohol, [and] sweetened beverage consumption), survivors should be consulted on importance of regular exercise.”
“These data clearly show we must consider weight changes in breast cancer survivors, and we must find ways of instituting strategies to mitigate these weight gains,” Dr. Hurtado Andrade said. “These women have a lot to think of when they have a breast cancer diagnosis, so we also must find ways of instituting these measures in a way that doesn’t increase the burden of their health.”
Dr. Hurtado Andrade has received research funding from the National Institutes of Health and by Phenomix Sciences. She also is a consultant for Novo Nordisk. These three organizations were not involved with this study. Dr. Madak-Erdogan had no disclosures.
A version of this article first appeared on Medscape.com.
BOSTON — Nearly one in five breast cancer survivors will gain more than 10% of their body weight in the 6 years following their diagnosis, according to new research presented at ENDO 2024, the annual meeting of the Endocrine Society.
Younger age and lower weight at diagnosis were the strongest predictors of this excessive weight gain over time.
“Weight gain is a common concern after breast cancer diagnosis and treatment,” said Maria Daniela Hurtado Andrade, MD, PhD, of the Mayo Clinic in Jacksonville, Florida, who led the research. “This weight gain in breast cancer survivor increases breast cancer recurrence and mortality, increases cardiovascular disease and mortality, and also increases all-cause mortality.”
Previous studies have found an association between breast cancer survivorship and weight gain, but the reported incidences of weight gain — and the amounts gained — have been highly variable, she added.
In the study, researchers used the Mayo Clinic Breast Cancer Registry to identify 4575 breast cancer survivors and tracked their weight over the course of 6 years following cancer diagnosis. These patients were age-matched to women in the general population selected from the Rochester Epidemiology Project, which contains the medical records of residents of 27 counties in Minnesota and Wisconsin. All controls had no history of cancer or bariatric surgery.
Nearly all patients and controls were White (97%); at breast cancer diagnosis, patients were on average 58 years of age and weighed 76 kg (165.5 lb). Controls had similar ages and baseline weights.
At 6 years following breast cancer diagnosis, average weight gain was modest: Breast cancer survivors gained 1.6% of their body weight, compared with 0.7% in controls (P = .004).
However, 18% of breast cancer survivors had gained at least 10% of their body weight over that time. By comparison, 8% of controls experienced this excessive weight gain during that same time frame (P < .0001). The same trend was observed for 15% and 20% weight gain.
After adjustment for confounding factors, younger age at breast cancer diagnosis and lower baseline weight were the strongest predictors of more than 10% weight gain. BRCA2 mutation and use of systemic chemotherapy treatment were also associated with excessive weight gain.
Several factors could be driving weight gain in these patients, said Zeynep Madak-Erdogan, PhD, at the University of Illinois Urbana-Champaign, who was not involved with the research. Her work focuses on how diet and nutrition affect hormone action in postmenopausal women and breast cancer survivors. Certain therapies can induce temporary or permanent menopause in patients, “and this early menopause might shift balance of estrogens and cause increased weight gain,” she said. Along the same lines, endocrine therapies can also affect estrogen production.
Stress and exhaustion from treatment — especially compounded by the two previous factors — are also likely culprits in weight gain, she continued.
“These findings highlight importance of lifestyle interventions,” added Dr. Madak-Erdogan. “In addition to changes in the diet (increased vegetable, fruit, [and] whole grain intake; reduction in saturated fats, alcohol, [and] sweetened beverage consumption), survivors should be consulted on importance of regular exercise.”
“These data clearly show we must consider weight changes in breast cancer survivors, and we must find ways of instituting strategies to mitigate these weight gains,” Dr. Hurtado Andrade said. “These women have a lot to think of when they have a breast cancer diagnosis, so we also must find ways of instituting these measures in a way that doesn’t increase the burden of their health.”
Dr. Hurtado Andrade has received research funding from the National Institutes of Health and by Phenomix Sciences. She also is a consultant for Novo Nordisk. These three organizations were not involved with this study. Dr. Madak-Erdogan had no disclosures.
A version of this article first appeared on Medscape.com.
BOSTON — Nearly one in five breast cancer survivors will gain more than 10% of their body weight in the 6 years following their diagnosis, according to new research presented at ENDO 2024, the annual meeting of the Endocrine Society.
Younger age and lower weight at diagnosis were the strongest predictors of this excessive weight gain over time.
“Weight gain is a common concern after breast cancer diagnosis and treatment,” said Maria Daniela Hurtado Andrade, MD, PhD, of the Mayo Clinic in Jacksonville, Florida, who led the research. “This weight gain in breast cancer survivor increases breast cancer recurrence and mortality, increases cardiovascular disease and mortality, and also increases all-cause mortality.”
Previous studies have found an association between breast cancer survivorship and weight gain, but the reported incidences of weight gain — and the amounts gained — have been highly variable, she added.
In the study, researchers used the Mayo Clinic Breast Cancer Registry to identify 4575 breast cancer survivors and tracked their weight over the course of 6 years following cancer diagnosis. These patients were age-matched to women in the general population selected from the Rochester Epidemiology Project, which contains the medical records of residents of 27 counties in Minnesota and Wisconsin. All controls had no history of cancer or bariatric surgery.
Nearly all patients and controls were White (97%); at breast cancer diagnosis, patients were on average 58 years of age and weighed 76 kg (165.5 lb). Controls had similar ages and baseline weights.
At 6 years following breast cancer diagnosis, average weight gain was modest: Breast cancer survivors gained 1.6% of their body weight, compared with 0.7% in controls (P = .004).
However, 18% of breast cancer survivors had gained at least 10% of their body weight over that time. By comparison, 8% of controls experienced this excessive weight gain during that same time frame (P < .0001). The same trend was observed for 15% and 20% weight gain.
After adjustment for confounding factors, younger age at breast cancer diagnosis and lower baseline weight were the strongest predictors of more than 10% weight gain. BRCA2 mutation and use of systemic chemotherapy treatment were also associated with excessive weight gain.
Several factors could be driving weight gain in these patients, said Zeynep Madak-Erdogan, PhD, at the University of Illinois Urbana-Champaign, who was not involved with the research. Her work focuses on how diet and nutrition affect hormone action in postmenopausal women and breast cancer survivors. Certain therapies can induce temporary or permanent menopause in patients, “and this early menopause might shift balance of estrogens and cause increased weight gain,” she said. Along the same lines, endocrine therapies can also affect estrogen production.
Stress and exhaustion from treatment — especially compounded by the two previous factors — are also likely culprits in weight gain, she continued.
“These findings highlight importance of lifestyle interventions,” added Dr. Madak-Erdogan. “In addition to changes in the diet (increased vegetable, fruit, [and] whole grain intake; reduction in saturated fats, alcohol, [and] sweetened beverage consumption), survivors should be consulted on importance of regular exercise.”
“These data clearly show we must consider weight changes in breast cancer survivors, and we must find ways of instituting strategies to mitigate these weight gains,” Dr. Hurtado Andrade said. “These women have a lot to think of when they have a breast cancer diagnosis, so we also must find ways of instituting these measures in a way that doesn’t increase the burden of their health.”
Dr. Hurtado Andrade has received research funding from the National Institutes of Health and by Phenomix Sciences. She also is a consultant for Novo Nordisk. These three organizations were not involved with this study. Dr. Madak-Erdogan had no disclosures.
A version of this article first appeared on Medscape.com.
FROM ENDO 2024
New Oral Weight Loss Drugs: Where Are We and What’s Next?
, in line with patient preference.
One particularly intriguing entry is ARD-101, in development by Aardvark Therapeutics in San Diego, California. Aardvark came out of stealth on May 9 with the announcement of $85 million in new financing. The biopharma will use the money to complete trials of ARD-101 to treat hyperphagia in Prader-Willi syndrome, both to help patients quell the unrelenting hunger that characterizes the orphan disease and as a proof of principle to demonstrate the compound’s complementary mechanism of action to the current glucagon-like peptide 1 (GLP-1) therapies for obesity.
Oral ARD-101 is a bitter taste receptor (TAS2R) that mediates hunger, whereas the GLP-1 drugs mainly influence appetite, said the company’s CEO, Tien Lee, MD.
“If you love chocolate cake, for instance, appetite is driving you to eat that. And if that chocolate cake were to turn magically into dog food, your appetite probably would go to zero. But if that dog food were your only food source, over enough time, hunger would eventually compel you to eat it. That’s how they’re differentially driven.”
He added, “Hunger and appetite approaches are not mutually exclusive. In fact, they’re complementary to each other, and they’re additive in terms of treatment effect.”
Now that the company is out of stealth, expect more published data and updates on ongoing studies, he added.
Here’s a look at other promising oral drugs on the horizon.
Oral Semaglutide
The once-daily 50 mg tablet formulation of this GLP-1 receptor agonist is among the nearest to approval. The formulation was studied for weight loss in individuals with overweight/obesity in the OASIS 1 phase 3a trial. When applying the treatment policy estimand (defined as the treatment effect regardless of adherence), people who took the pill achieved a weight loss of 15.1% over 68 weeks compared with a 2.4% reduction with placebo, and 84.9% achieved a weight loss of ≥ 5% vs 25.8% with placebo, according to the manufacturer Novo Nordisk.
A spokesperson for the company told this news organization that, contrary to earlier reports, the 50 mg pill will be submitted for regulatory approval after results from OASIS 4 are in, “so we have the full data set.” OASIS 4 is investigating the 25 mg oral dose, and results are expected this year.
“The US launch of oral semaglutide for obesity will be contingent on portfolio prioritization and manufacturing capacity,” the spokesperson said. The company can produce semaglutide as a tablet or injectable, but the oral form requires more an active pharmaceutical ingredient. Therefore, production capacities are being expanded globally for both formulations.
Oral Amycretin
Novo Nordisk’s spokesperson said that, as announced in March, results from an exploratory endpoint on body weight change in a phase 1 trial showed an average −13.1% reduction after 12 weeks of treatment with once-daily oral amycretin compared with −1.1% for placebo. The favorable safety/tolerability and pharmacokinetic profile observed in the trial allows for further development of amycretin.
“Moreover,” the spokesperson said, “we are developing the oral small molecule CB1 receptor inverse agonist monlunabant (INV-202), which has shown weight loss potential in phase 1 with a favorable safety and tolerability profile and is currently being investigated in phase 2 in diabetic kidney disease and obesity.”
APH-012
As of April 25, Aphaia Pharma completed enrollment of the first two cohorts in its randomized, double-blind, placebo-controlled proof-of-concept phase 2 trial evaluating a once-daily 12-g dose of its proprietary oral glucose formulation APHD-12 for obesity.
The company also announced that the US Food and Drug Administration (FDA) has approved an expansion of the trial›s protocol to investigate the contribution of circadian effects in weight loss treatment. The new protocol will include additional cohorts, which will be dosed with either 6 g (APHD-006) or 8 g (APHD-008) of Aphaia’s formulation or placebos twice daily. The primary endpoint of the trial is the change from baseline in percent weight compared with placebo. The study will also evaluate exploratory secondary endpoints, which are considered hallmarks of multiple metabolic diseases closely associated with obesity.
The drug candidate is “designed to be released at discrete parts of the small intestine to restore endogenous nutrient-sensing signaling pathways and stimulate the release of the broad spectrum of enteric hormones that control multiple homeostatic functions like appetite, hunger, satiety, glucose metabolism, and energy expenditure,” according to the company’s announcement. “This includes glucagon-like peptide 1, peptide tyrosine-tyrosine, glicentin, and oxyntomodulin, among others.”
Topline data from the first part of the study are expected to be released by the third quarter.
AZD5004
In November 2023, AstraZeneca entered into an exclusive licensing agreement with Eccogene to develop and commercialize ECC5004 (now AZD5004), a tablet formulation of a small molecule GLP-1 receptor agonist, both as monotherapy and in combination with AZD6234, its antiobesity agent that targets the gut hormone amylin.
“We are excited by the potential of AZD5004 as a novel oral small molecule GLP-1 receptor agonist,” a company spokesperson told this news organization. “The phase 1 study has provided us with the confidence to progress development into a phase 2 program studying patients with type 2 diabetes and in obesity. We are in the process of designing these studies and expect to start them in the second half of 2024.”
Ecnoglutide
In January, Sciwind Biosciences announced positive interim results from the first four cohorts of a phase 1 clinical trial of oral ecnoglutide (XW004). Ecnoglutide is a long-acting, cAMP signaling biased, GLP-1 analog being developed for the treatment of obesity and type 2 diabetes.
The phase 1 trial (NCT05184322) is a randomized, double-blind, placebo-controlled multiple ascending dose study that enrolled 42 healthy (cohorts 1-3) and 14 healthy obese (cohort 4) participants in Australia. In cohorts 1-3, target doses were 7 mg, 15 mg, or 30 mg XW004 once daily for 2 weeks; in cohort 4, the target dose was 30 mg XW004 once daily for 6 weeks. Treatment periods included gradual dose escalation to the target doses.
Study participants achieved a mean body weight reduction of −6.8% from baseline, compared with −0.9% for the placebo group, according to the company. Based on the positive results, the study is continuing and will evaluate additional dosing regimens, including once-weekly oral administration in participants with obesity.
The company is also developing an injectable formulation of ecnoglutide.
GSBR-1290
On May 9, Structure Therapeutics released highlights of the company›s evaluation of GSBR-1290, an oral small molecule selective GLP-1 receptor agonist. Topline data from the obesity cohort of the phase 2a study, including 12-week efficacy data for 40 participants and safety and tolerability for all 64 participants, are expected in June.
In preparation for later stage clinical trials, the company said it is conducting a formulation bridging and titration study to evaluate capsule vs tablet pharmacokinetics and explore different titration regimens of the molecule. Pharmacokinetic study results are also expected in June.
A global phase 2b obesity study is planned for the fourth quarter of 2024.
Orforglipron
Orforglipron is an oral GLP-1 receptor agonist being developed by Eli Lilly and Co. A phase 3 study of the once-daily capsule is underway, and will run until mid-2027.
Phase 2 data presented last year at the American Diabetes Association conference showed that participants with obesity had up to a 14.7% body weight reduction at 36 weeks. Nearly half of participants lost ≥ 15% of their body weight at 36 weeks.
Additionally, a meta-analysis of randomized controlled trials of the drug was recently published.
A Lilly spokesperson told this news organization that phase 3 results from the ATTAIN-1 study are “expected to be to be available beginning in 2025, and we can expect a launch possibly a year after that.”
VK2735
VK2735, a dual agonist of the GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) receptors, is being developed by Viking Therapeutics for the treatment of metabolic disorders, including obesity, in both subcutaneous and oral formulations.
In a phase 1, 28-day multiple ascending dose study, cohorts receiving oral formulation VK2735 had dose-dependent reductions in mean body weight from baseline, ranging up to 5.3%, and also demonstrated reductions in mean body weight relative to placebo, ranging up to 3.3%. For doses ≥ 10 mg, placebo-adjusted reductions in mean body weight were maintained or improved at day 34, 6 days after the last dose of VK2735 was administered, ranging up to 3.6% relative to placebo.
Based on these phase 1 results, the company plans to initiate a phase 2 trial in obesity later this year.
A version of this article appeared on Medscape.com.
, in line with patient preference.
One particularly intriguing entry is ARD-101, in development by Aardvark Therapeutics in San Diego, California. Aardvark came out of stealth on May 9 with the announcement of $85 million in new financing. The biopharma will use the money to complete trials of ARD-101 to treat hyperphagia in Prader-Willi syndrome, both to help patients quell the unrelenting hunger that characterizes the orphan disease and as a proof of principle to demonstrate the compound’s complementary mechanism of action to the current glucagon-like peptide 1 (GLP-1) therapies for obesity.
Oral ARD-101 is a bitter taste receptor (TAS2R) that mediates hunger, whereas the GLP-1 drugs mainly influence appetite, said the company’s CEO, Tien Lee, MD.
“If you love chocolate cake, for instance, appetite is driving you to eat that. And if that chocolate cake were to turn magically into dog food, your appetite probably would go to zero. But if that dog food were your only food source, over enough time, hunger would eventually compel you to eat it. That’s how they’re differentially driven.”
He added, “Hunger and appetite approaches are not mutually exclusive. In fact, they’re complementary to each other, and they’re additive in terms of treatment effect.”
Now that the company is out of stealth, expect more published data and updates on ongoing studies, he added.
Here’s a look at other promising oral drugs on the horizon.
Oral Semaglutide
The once-daily 50 mg tablet formulation of this GLP-1 receptor agonist is among the nearest to approval. The formulation was studied for weight loss in individuals with overweight/obesity in the OASIS 1 phase 3a trial. When applying the treatment policy estimand (defined as the treatment effect regardless of adherence), people who took the pill achieved a weight loss of 15.1% over 68 weeks compared with a 2.4% reduction with placebo, and 84.9% achieved a weight loss of ≥ 5% vs 25.8% with placebo, according to the manufacturer Novo Nordisk.
A spokesperson for the company told this news organization that, contrary to earlier reports, the 50 mg pill will be submitted for regulatory approval after results from OASIS 4 are in, “so we have the full data set.” OASIS 4 is investigating the 25 mg oral dose, and results are expected this year.
“The US launch of oral semaglutide for obesity will be contingent on portfolio prioritization and manufacturing capacity,” the spokesperson said. The company can produce semaglutide as a tablet or injectable, but the oral form requires more an active pharmaceutical ingredient. Therefore, production capacities are being expanded globally for both formulations.
Oral Amycretin
Novo Nordisk’s spokesperson said that, as announced in March, results from an exploratory endpoint on body weight change in a phase 1 trial showed an average −13.1% reduction after 12 weeks of treatment with once-daily oral amycretin compared with −1.1% for placebo. The favorable safety/tolerability and pharmacokinetic profile observed in the trial allows for further development of amycretin.
“Moreover,” the spokesperson said, “we are developing the oral small molecule CB1 receptor inverse agonist monlunabant (INV-202), which has shown weight loss potential in phase 1 with a favorable safety and tolerability profile and is currently being investigated in phase 2 in diabetic kidney disease and obesity.”
APH-012
As of April 25, Aphaia Pharma completed enrollment of the first two cohorts in its randomized, double-blind, placebo-controlled proof-of-concept phase 2 trial evaluating a once-daily 12-g dose of its proprietary oral glucose formulation APHD-12 for obesity.
The company also announced that the US Food and Drug Administration (FDA) has approved an expansion of the trial›s protocol to investigate the contribution of circadian effects in weight loss treatment. The new protocol will include additional cohorts, which will be dosed with either 6 g (APHD-006) or 8 g (APHD-008) of Aphaia’s formulation or placebos twice daily. The primary endpoint of the trial is the change from baseline in percent weight compared with placebo. The study will also evaluate exploratory secondary endpoints, which are considered hallmarks of multiple metabolic diseases closely associated with obesity.
The drug candidate is “designed to be released at discrete parts of the small intestine to restore endogenous nutrient-sensing signaling pathways and stimulate the release of the broad spectrum of enteric hormones that control multiple homeostatic functions like appetite, hunger, satiety, glucose metabolism, and energy expenditure,” according to the company’s announcement. “This includes glucagon-like peptide 1, peptide tyrosine-tyrosine, glicentin, and oxyntomodulin, among others.”
Topline data from the first part of the study are expected to be released by the third quarter.
AZD5004
In November 2023, AstraZeneca entered into an exclusive licensing agreement with Eccogene to develop and commercialize ECC5004 (now AZD5004), a tablet formulation of a small molecule GLP-1 receptor agonist, both as monotherapy and in combination with AZD6234, its antiobesity agent that targets the gut hormone amylin.
“We are excited by the potential of AZD5004 as a novel oral small molecule GLP-1 receptor agonist,” a company spokesperson told this news organization. “The phase 1 study has provided us with the confidence to progress development into a phase 2 program studying patients with type 2 diabetes and in obesity. We are in the process of designing these studies and expect to start them in the second half of 2024.”
Ecnoglutide
In January, Sciwind Biosciences announced positive interim results from the first four cohorts of a phase 1 clinical trial of oral ecnoglutide (XW004). Ecnoglutide is a long-acting, cAMP signaling biased, GLP-1 analog being developed for the treatment of obesity and type 2 diabetes.
The phase 1 trial (NCT05184322) is a randomized, double-blind, placebo-controlled multiple ascending dose study that enrolled 42 healthy (cohorts 1-3) and 14 healthy obese (cohort 4) participants in Australia. In cohorts 1-3, target doses were 7 mg, 15 mg, or 30 mg XW004 once daily for 2 weeks; in cohort 4, the target dose was 30 mg XW004 once daily for 6 weeks. Treatment periods included gradual dose escalation to the target doses.
Study participants achieved a mean body weight reduction of −6.8% from baseline, compared with −0.9% for the placebo group, according to the company. Based on the positive results, the study is continuing and will evaluate additional dosing regimens, including once-weekly oral administration in participants with obesity.
The company is also developing an injectable formulation of ecnoglutide.
GSBR-1290
On May 9, Structure Therapeutics released highlights of the company›s evaluation of GSBR-1290, an oral small molecule selective GLP-1 receptor agonist. Topline data from the obesity cohort of the phase 2a study, including 12-week efficacy data for 40 participants and safety and tolerability for all 64 participants, are expected in June.
In preparation for later stage clinical trials, the company said it is conducting a formulation bridging and titration study to evaluate capsule vs tablet pharmacokinetics and explore different titration regimens of the molecule. Pharmacokinetic study results are also expected in June.
A global phase 2b obesity study is planned for the fourth quarter of 2024.
Orforglipron
Orforglipron is an oral GLP-1 receptor agonist being developed by Eli Lilly and Co. A phase 3 study of the once-daily capsule is underway, and will run until mid-2027.
Phase 2 data presented last year at the American Diabetes Association conference showed that participants with obesity had up to a 14.7% body weight reduction at 36 weeks. Nearly half of participants lost ≥ 15% of their body weight at 36 weeks.
Additionally, a meta-analysis of randomized controlled trials of the drug was recently published.
A Lilly spokesperson told this news organization that phase 3 results from the ATTAIN-1 study are “expected to be to be available beginning in 2025, and we can expect a launch possibly a year after that.”
VK2735
VK2735, a dual agonist of the GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) receptors, is being developed by Viking Therapeutics for the treatment of metabolic disorders, including obesity, in both subcutaneous and oral formulations.
In a phase 1, 28-day multiple ascending dose study, cohorts receiving oral formulation VK2735 had dose-dependent reductions in mean body weight from baseline, ranging up to 5.3%, and also demonstrated reductions in mean body weight relative to placebo, ranging up to 3.3%. For doses ≥ 10 mg, placebo-adjusted reductions in mean body weight were maintained or improved at day 34, 6 days after the last dose of VK2735 was administered, ranging up to 3.6% relative to placebo.
Based on these phase 1 results, the company plans to initiate a phase 2 trial in obesity later this year.
A version of this article appeared on Medscape.com.
, in line with patient preference.
One particularly intriguing entry is ARD-101, in development by Aardvark Therapeutics in San Diego, California. Aardvark came out of stealth on May 9 with the announcement of $85 million in new financing. The biopharma will use the money to complete trials of ARD-101 to treat hyperphagia in Prader-Willi syndrome, both to help patients quell the unrelenting hunger that characterizes the orphan disease and as a proof of principle to demonstrate the compound’s complementary mechanism of action to the current glucagon-like peptide 1 (GLP-1) therapies for obesity.
Oral ARD-101 is a bitter taste receptor (TAS2R) that mediates hunger, whereas the GLP-1 drugs mainly influence appetite, said the company’s CEO, Tien Lee, MD.
“If you love chocolate cake, for instance, appetite is driving you to eat that. And if that chocolate cake were to turn magically into dog food, your appetite probably would go to zero. But if that dog food were your only food source, over enough time, hunger would eventually compel you to eat it. That’s how they’re differentially driven.”
He added, “Hunger and appetite approaches are not mutually exclusive. In fact, they’re complementary to each other, and they’re additive in terms of treatment effect.”
Now that the company is out of stealth, expect more published data and updates on ongoing studies, he added.
Here’s a look at other promising oral drugs on the horizon.
Oral Semaglutide
The once-daily 50 mg tablet formulation of this GLP-1 receptor agonist is among the nearest to approval. The formulation was studied for weight loss in individuals with overweight/obesity in the OASIS 1 phase 3a trial. When applying the treatment policy estimand (defined as the treatment effect regardless of adherence), people who took the pill achieved a weight loss of 15.1% over 68 weeks compared with a 2.4% reduction with placebo, and 84.9% achieved a weight loss of ≥ 5% vs 25.8% with placebo, according to the manufacturer Novo Nordisk.
A spokesperson for the company told this news organization that, contrary to earlier reports, the 50 mg pill will be submitted for regulatory approval after results from OASIS 4 are in, “so we have the full data set.” OASIS 4 is investigating the 25 mg oral dose, and results are expected this year.
“The US launch of oral semaglutide for obesity will be contingent on portfolio prioritization and manufacturing capacity,” the spokesperson said. The company can produce semaglutide as a tablet or injectable, but the oral form requires more an active pharmaceutical ingredient. Therefore, production capacities are being expanded globally for both formulations.
Oral Amycretin
Novo Nordisk’s spokesperson said that, as announced in March, results from an exploratory endpoint on body weight change in a phase 1 trial showed an average −13.1% reduction after 12 weeks of treatment with once-daily oral amycretin compared with −1.1% for placebo. The favorable safety/tolerability and pharmacokinetic profile observed in the trial allows for further development of amycretin.
“Moreover,” the spokesperson said, “we are developing the oral small molecule CB1 receptor inverse agonist monlunabant (INV-202), which has shown weight loss potential in phase 1 with a favorable safety and tolerability profile and is currently being investigated in phase 2 in diabetic kidney disease and obesity.”
APH-012
As of April 25, Aphaia Pharma completed enrollment of the first two cohorts in its randomized, double-blind, placebo-controlled proof-of-concept phase 2 trial evaluating a once-daily 12-g dose of its proprietary oral glucose formulation APHD-12 for obesity.
The company also announced that the US Food and Drug Administration (FDA) has approved an expansion of the trial›s protocol to investigate the contribution of circadian effects in weight loss treatment. The new protocol will include additional cohorts, which will be dosed with either 6 g (APHD-006) or 8 g (APHD-008) of Aphaia’s formulation or placebos twice daily. The primary endpoint of the trial is the change from baseline in percent weight compared with placebo. The study will also evaluate exploratory secondary endpoints, which are considered hallmarks of multiple metabolic diseases closely associated with obesity.
The drug candidate is “designed to be released at discrete parts of the small intestine to restore endogenous nutrient-sensing signaling pathways and stimulate the release of the broad spectrum of enteric hormones that control multiple homeostatic functions like appetite, hunger, satiety, glucose metabolism, and energy expenditure,” according to the company’s announcement. “This includes glucagon-like peptide 1, peptide tyrosine-tyrosine, glicentin, and oxyntomodulin, among others.”
Topline data from the first part of the study are expected to be released by the third quarter.
AZD5004
In November 2023, AstraZeneca entered into an exclusive licensing agreement with Eccogene to develop and commercialize ECC5004 (now AZD5004), a tablet formulation of a small molecule GLP-1 receptor agonist, both as monotherapy and in combination with AZD6234, its antiobesity agent that targets the gut hormone amylin.
“We are excited by the potential of AZD5004 as a novel oral small molecule GLP-1 receptor agonist,” a company spokesperson told this news organization. “The phase 1 study has provided us with the confidence to progress development into a phase 2 program studying patients with type 2 diabetes and in obesity. We are in the process of designing these studies and expect to start them in the second half of 2024.”
Ecnoglutide
In January, Sciwind Biosciences announced positive interim results from the first four cohorts of a phase 1 clinical trial of oral ecnoglutide (XW004). Ecnoglutide is a long-acting, cAMP signaling biased, GLP-1 analog being developed for the treatment of obesity and type 2 diabetes.
The phase 1 trial (NCT05184322) is a randomized, double-blind, placebo-controlled multiple ascending dose study that enrolled 42 healthy (cohorts 1-3) and 14 healthy obese (cohort 4) participants in Australia. In cohorts 1-3, target doses were 7 mg, 15 mg, or 30 mg XW004 once daily for 2 weeks; in cohort 4, the target dose was 30 mg XW004 once daily for 6 weeks. Treatment periods included gradual dose escalation to the target doses.
Study participants achieved a mean body weight reduction of −6.8% from baseline, compared with −0.9% for the placebo group, according to the company. Based on the positive results, the study is continuing and will evaluate additional dosing regimens, including once-weekly oral administration in participants with obesity.
The company is also developing an injectable formulation of ecnoglutide.
GSBR-1290
On May 9, Structure Therapeutics released highlights of the company›s evaluation of GSBR-1290, an oral small molecule selective GLP-1 receptor agonist. Topline data from the obesity cohort of the phase 2a study, including 12-week efficacy data for 40 participants and safety and tolerability for all 64 participants, are expected in June.
In preparation for later stage clinical trials, the company said it is conducting a formulation bridging and titration study to evaluate capsule vs tablet pharmacokinetics and explore different titration regimens of the molecule. Pharmacokinetic study results are also expected in June.
A global phase 2b obesity study is planned for the fourth quarter of 2024.
Orforglipron
Orforglipron is an oral GLP-1 receptor agonist being developed by Eli Lilly and Co. A phase 3 study of the once-daily capsule is underway, and will run until mid-2027.
Phase 2 data presented last year at the American Diabetes Association conference showed that participants with obesity had up to a 14.7% body weight reduction at 36 weeks. Nearly half of participants lost ≥ 15% of their body weight at 36 weeks.
Additionally, a meta-analysis of randomized controlled trials of the drug was recently published.
A Lilly spokesperson told this news organization that phase 3 results from the ATTAIN-1 study are “expected to be to be available beginning in 2025, and we can expect a launch possibly a year after that.”
VK2735
VK2735, a dual agonist of the GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) receptors, is being developed by Viking Therapeutics for the treatment of metabolic disorders, including obesity, in both subcutaneous and oral formulations.
In a phase 1, 28-day multiple ascending dose study, cohorts receiving oral formulation VK2735 had dose-dependent reductions in mean body weight from baseline, ranging up to 5.3%, and also demonstrated reductions in mean body weight relative to placebo, ranging up to 3.3%. For doses ≥ 10 mg, placebo-adjusted reductions in mean body weight were maintained or improved at day 34, 6 days after the last dose of VK2735 was administered, ranging up to 3.6% relative to placebo.
Based on these phase 1 results, the company plans to initiate a phase 2 trial in obesity later this year.
A version of this article appeared on Medscape.com.