User login
Diagnosing placenta accreta spectrum with prenatal ultrasound
Placenta accreta spectrum (PAS) describes abnormal invasion of placental tissue into or through the myometrium, comprising 3 distinct conditions: placenta accreta, placenta increta, and placenta percreta. This complication is relatively new to obstetrics, first described in 1937.1
The overall incidence of PAS has been increasing over several decades, in parallel to an increasing rate of cesarean delivery (CD), with an incidence from 1982 through 2002 of 1 in 533 pregnancies, representing a 5-fold increase since the 1980s.2 PAS is associated with significant morbidity and mortality, including fetal growth restriction, preterm delivery, placental abruption antenatally, and hemorrhage during delivery or postpartum.
Prenatal diagnosis of PAS and planned delivery at an experienced center are associated with significant reduction in maternal and fetal morbidity.3 In an era of advanced imaging modalities, prenatal detection of PAS regrettably remains variable and largely subjective: As many as 20% to 50% of cases of PAS escape prenatal diagnosis.3,4
In this article, we review the sonographic markers of PAS, including diagnostic accuracy, and propose a standardized approach to prenatal diagnosis. Throughout our discussion, we describe protocols for detection of PAS practiced at our Maternal-Fetal Medicine Program in the Department of Obstetrics and Gynecology, Eastern Virginia Medical School (also see “US evaluation of PAS risk: The authors’ recommended approach”).
Numerous risk factors
There are many risk factors for PAS, including prior uterine surgery or instrumentation, such as CD, uterine curettage, myomectomy, pelvic radiation, and endometrial ablation. Other risk factors include smoking, in vitro fertilization, advanced maternal age, multiparity, and a brief interval between prior CD and subsequent pregnancy.5 Of major significance is the increased risk of PAS in the presence of placenta previa with prior CD.6 Knowledge of clinical risk factors by the interpreting physician appears to be associated with improved detection of PAS on ultrasonography (US).4
Ultrasonographic markers of PAS
First-trimester markers
Sonographic markers of PAS in the first trimester include:
- a gestational sac implanted in the lower uterine segment or in a CD scar
- multiple hypoechoic spaces within the placenta (lacunae).7
Lower uterine-segment implantation has been defined by Ballas and colleagues as 1) a gestational sac implanted in the lower one-third of the uterus between 8 and 10 weeks’ gestation or 2) a gestational sac occupying primarily the lower uterine segment from 10 weeks’ gestation onward (FIGURE 1).8 Our experience is that it is difficult to accurately assess lower uterine-segment implantation beyond 13 weeks of gestation because the sac typically expands to fill the upper uterine cavity.

Continue to: Color Doppler US...
Color Doppler US can help differentiate lower uterine-segment implantation from a gestational sac of a failed pregnancy in the process of expulsion by demonstrating loss of circumferential blood flow in the failed pregnancy. Furthermore, applying pressure to the anterior surface of the uterus will result in downward movement of the gestational sac of a failed pregnancy.9
Not all gestational sacs that implant in the lower uterine segment lead to PAS: Subsequent normal pregnancies have been reported in this circumstance. In such cases, a normal thick myometrium is noted anterior to the gestational sac.7 A patient with lower uterine-segment implantation without evidence of anterior myometrial thinning remains at risk for third-trimester placenta previa.7
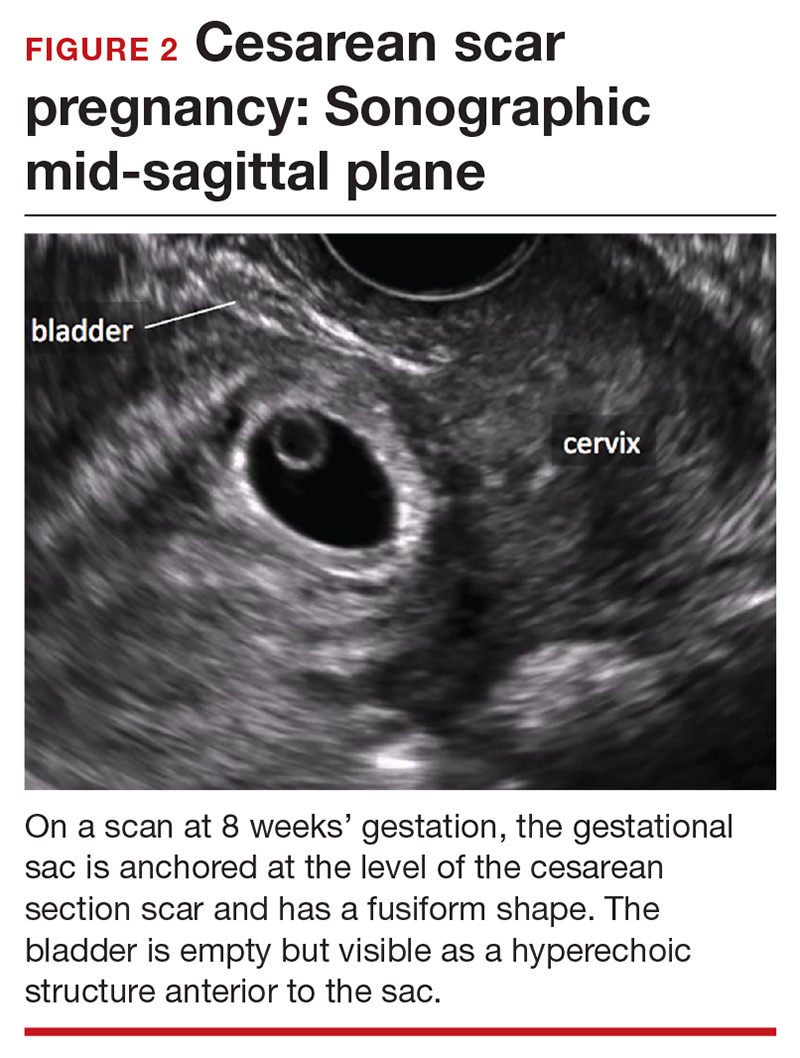
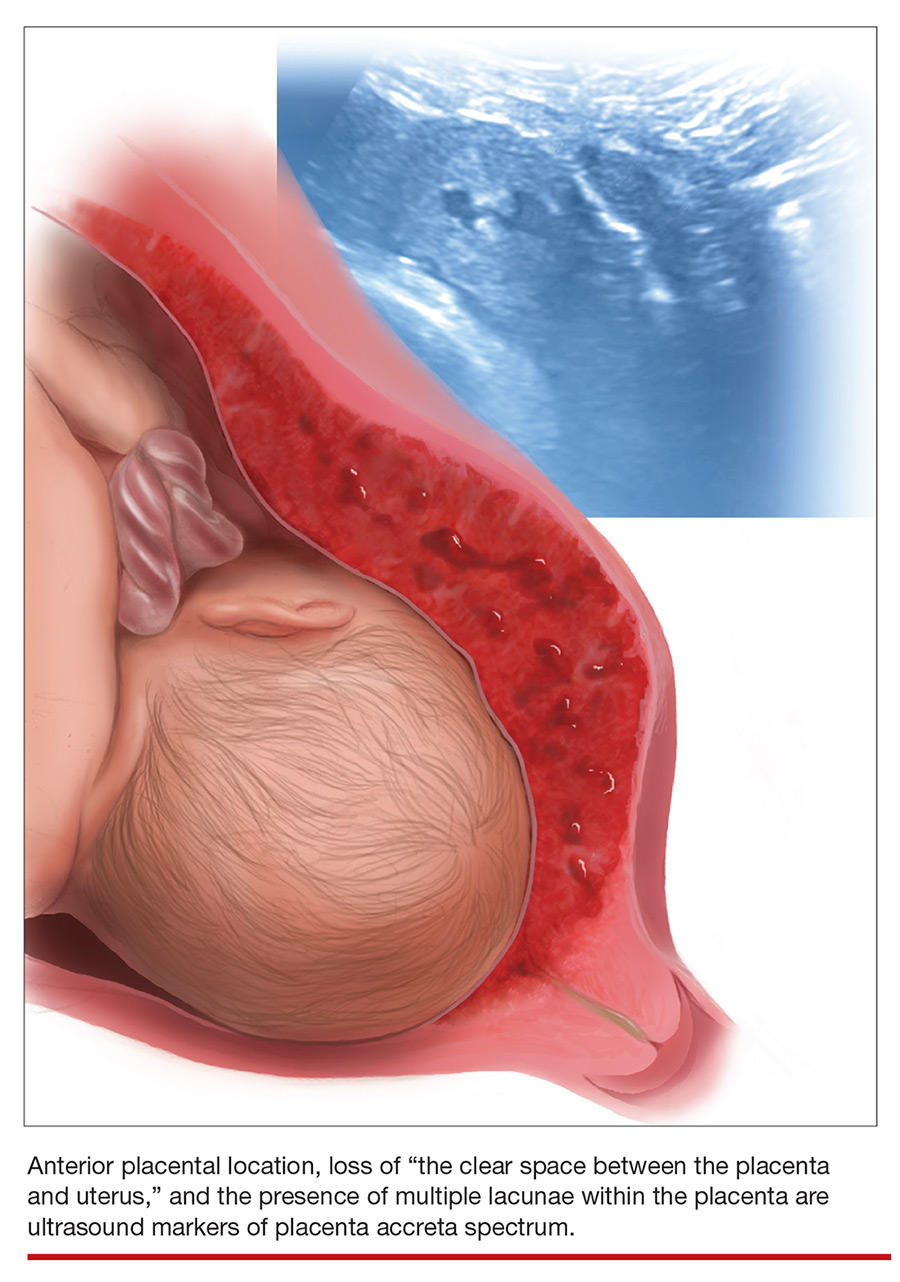
Cesarean scar pregnancy carries significant risk of PAS. In these cases, the gestational sac is typically implanted within the scar, resulting in a thin anterior myometrium and significantly increased vascularity of the placental–myometrial and bladder–uterine wall interfaces (FIGURE 2).9 Differentiating cesarean scar pregnancy from a lower uterine-segment implantation is easier to perform before the eighth week of gestation but becomes more difficult as pregnancy advances. Although it might be useful to distinguish between true cesarean scar pregnancy and lower uterine-segment implantation adjacent to or involving the scar, both carry considerable risk of PAS and excessive hemorrhage, and the approach to treating both conditions is quite similar.
Lacunae, with or without documented blood flow on color Doppler US, are the third marker of PAS in the first trimester.8 Although some retrospective series and case reports describe the finding of lacunae in the first trimester of patients with diagnosed PAS, more recent literature suggests that these spaces are seen infrequently and at a similar frequency in women with and without PAS at delivery.7
Second- and third-trimester markers
Multiple diagnostic sonographic markers of PAS have been described in the second and third trimesters.
Placental location is a significant risk factor for PAS. Placenta previa in the setting of prior CD carries the highest risk of PAS—as high as 61% in women with both placenta previa and a history of 3 CDs.10 An anterior placenta appears to be a stronger risk factor for PAS than a posterior placenta in women with prior CD; the location of the placenta should therefore be evaluated in all women in the second trimester.
Continue to: Lacunae
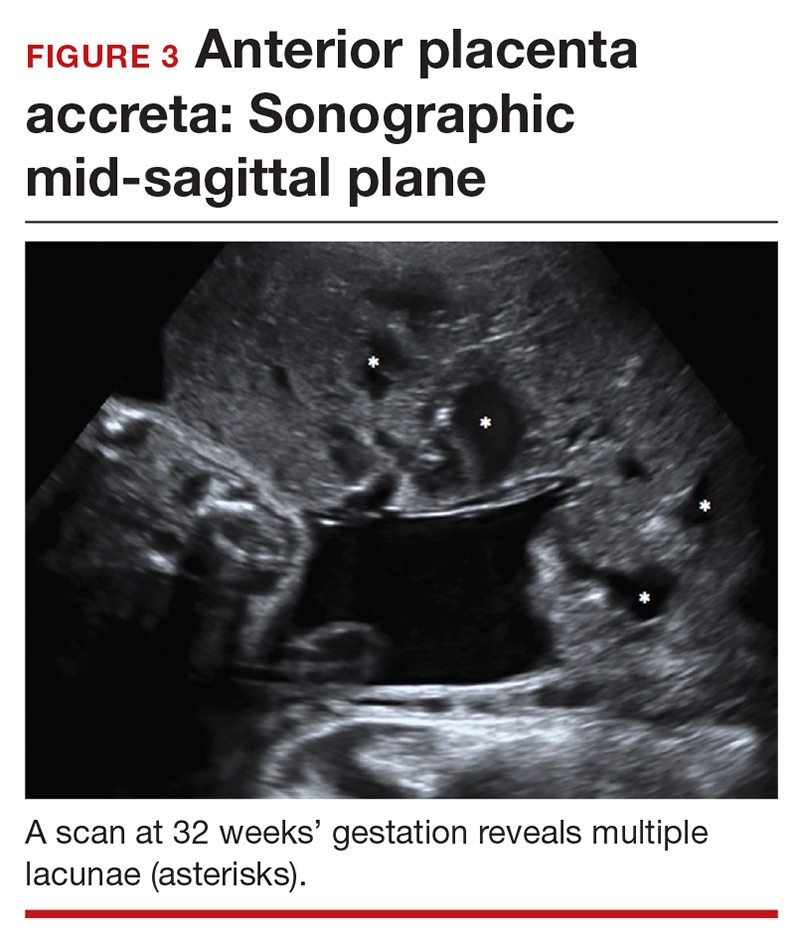
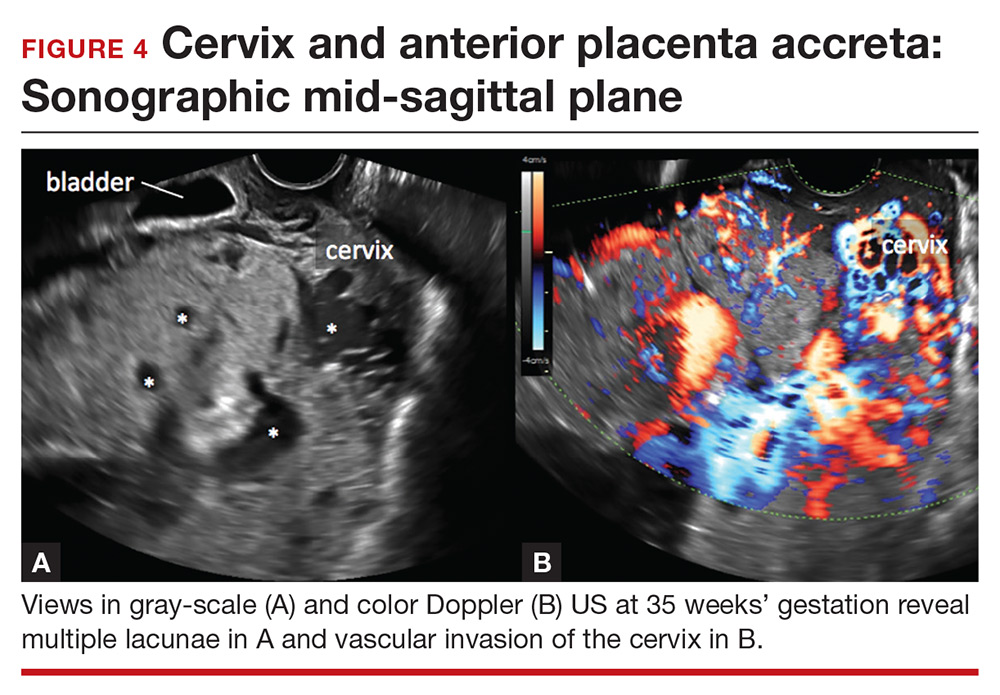
Lacunae. The finding of multiple hypoechoic vascular spaces within the placental parenchyma has been associated with PAS (FIGURES 3 and 4). The pathogenesis of this finding is probably related to alterations in placental tissue resulting from long-term exposure to pulsatile blood flow.11
Finberg and colleagues introduced a grading system for placental lacunae in 1992 that is still used:
- Grade 0: no lacunae seen
- Grade 1: 1 to 3 lacunae seen
- Grade 2: 4 to 6 lacunae seen
- Grade 3: multiple lacunae seen throughout the placenta.12
The sensitivity and specificity of lacunae as an independent marker for PAS have been reported to be 77% and 95%, respectively.13 Despite these findings, several studies report a range of sensitivity (73% to 100%) and negative predictive value (88% to 100%).14 Even in Finberg’s original work, 27% of cases of confirmed PAS had Grade 0 or Grade 1 placental lacunae and 11% of cases of placenta previa, without PAS, demonstrated Grade 2 lacunae.12 There is agreement, however, that, the more lacunae, the higher the risk of PAS.
Continue to: Other US markers for PAS
Other US markers of PAS

Retroplacental–myometrial interface

Loss of the normal hypoechoic (clear) retroplacental zone, also referred to as loss of the clear space between placenta and uterus, is another marker of PAS (FIGURE 5). This finding corresponds to pathologic loss of the decidua basalis as trophoblastic tissue invades directly through the myometrium.15 This sonographic finding has been reported to have a detection rate of approximately 93%, with sensitivity of 52% and specificity of 57%, for PAS; the false-positive rate, however, has been in the range of 21% or higher. This marker should not be used alone because it is angle-dependent and can be found (as an absent clear zone) in normal anterior placentas.16
The strength of this US marker is in its negative predictive value, which ranges from 96% to 100%. The presence of a hypoechoic retroplacental clear space that extends the length of the placenta makes PAS unlikely.17 Of note, the clear zone may appear falsely absent as a result of increased pressure from the US probe.
Retroplacental myometrial thickness
Retroplacental myometrial thickness is difficult to assess because the lower uterine-segment myometrium thins in normal pregnancy as term approaches. This measurement also can be influenced by direct pressure of the US probe and fullness of the maternal bladder.18 In patients who have had a CD but who do not have PAS, the median myometrial thickness of the lower uterine segment in the third trimester is 2.4 mm.19
Thinning of the myometrium in the upper uterine segment always should be of concern. Studies of this marker have reported sensitivity of US ranging from 22% to 100% and specificity from 72% to 100%.9,20 Given such variability, it is important to standardize the gestational age and sonographic approach for this marker.
Continue to: Uterovesical interface
Uterovesical interface
Studies also have reported that abnormalities of the uterovesical interface are predictive of PAS. The uterovesical interface is best evaluated in a sagittal plane containing the lower uterine segment and a partially full bladder in gray-scale and color Doppler US.15 The normal uterovesical interface appears as a smooth line, without irregularities or increased vascularity on sagittal imaging.
Abnormalities include focal interruption of the hyperechoic bladder wall, bulging of the bladder wall, and increased vascularity, such as varicosities (FIGURES 5, 6, and 7).15 These findings may be seen as early as the first trimester but are more commonly noted in the second and third trimesters.7 The authors of a recent meta-analysis concluded that irregularity of the uterovesical interface is the most specific marker for invasive placentation (99.75% confidence interval; range, 99.5% to 99.9%).13
Other US markers and modalities
Three-dimensional US. Studies have evaluated the role of 3-dimensional (3D) US for predicting PAS. Application of 3D US in vascular mode has shown promise because it allows for semiquantitative assessment of placental vasculature.22 Using 3D US to screen for PAS presents drawbacks, however: The technology is not well-standardized and requires significant operator expertise for volume acquisition and manipulation. Prospective studies are needed before 3D US can be applied routinely to screen for and diagnose PAS.
Color Doppler US. As an adjunct to gray-scale US, color Doppler US can be used for making a diagnosis of PAS. Color Doppler US helps differentiate a normal subplacental venous complex with nonpulsatile, low-velocity venous blood flow waveforms from markedly dilated peripheral subplacental vascular channels with pulsatile venous-type flow, which suggests PAS. These vascular channels are often located directly over the cervix. In addition, the observation of bridging vessels linking the placenta and bladder with high diastolic arterial blood flow also suggests invasion.21 In a meta-analysis, overall sensitivity of color Doppler US for the diagnosis of PAS was 91%, with specificity of 87%.13
The value of utilizing multiple markers
The accuracy of US diagnosis of PAS is likely improved by using more than 1 sonographic marker. Pilloni and colleagues,20 in a prospective analysis, found that 81% of cases of confirmed PAS had ≥2 markers and 51% of cases had ≥3 markers.
Several scoring systems have been proposed for making the diagnosis of PAS using combinations of sonographic markers, placental location, and clinical history.19,24,25 In 2016, Tovbin and colleagues,25 in a prospective study, evaluated a scoring system that included:
- number of previous CDs
- number of, maximum dimension of, and presence of blood flow in lacunae
- loss of uteroplacental clear zone
- placental location
- hypervascularity of the uterovesical or uteroplacental interface.
Tovbin assigned 1 or 2 points to each criterion. Each sonographic marker was found to be significantly associated with PAS when compared to a high-risk control group. A score of ≥8 was considered “at high risk” and predicted 69% of PAS cases.
Regrettably, no combination of US markers reliably predicts the depth of invasion of the placenta.26
Continue to: A standardized approach is needed
A standardized approach is needed
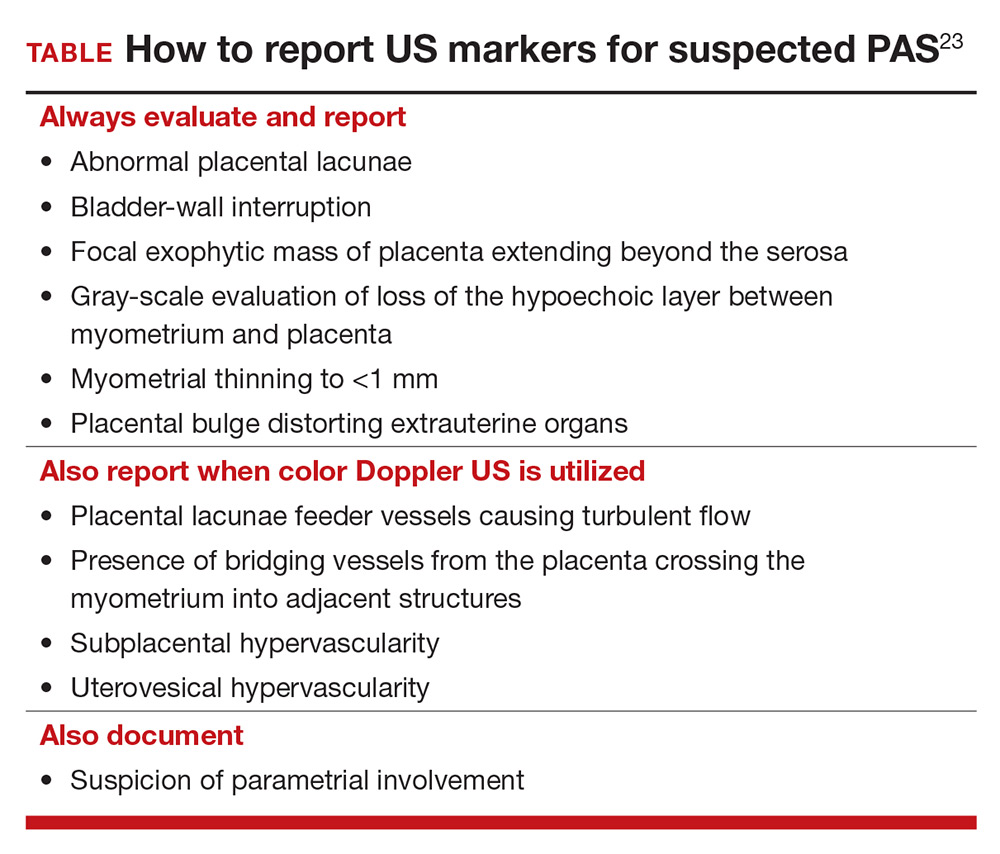
To decrease variability and improve the US diagnosis of PAS, it is important to define and standardize the diagnosis of each sonographic marker for PAS.4 In 2016, the European Working Group on Abnormally Invasive Placenta (EW-AIP) proposed a set of US markers that always should be reported when performing an US examination for suspected abnormal placentation (TABLE).23 Despite this effort by the EW-AIP, ambiguity remains over sonographic definitions of several PAS markers. For example, what determines a placental lacuna on US? And what constitutes an abnormal uterovesical interface? There is a need for a more objective definition of US markers of PAS and a standardized approach to the US examination in at-risk pregnancies.
The Society for Maternal-Fetal Medicine is coordinating a multi-society task force to address the need to define and standardize the US diagnosis of PAS.
Observations on other PAS diagnostic modalities
Magnetic resonance imaging
Adjunctive role. Magnetic resonance imaging (MRI) is often used as an adjunctive diagnostic modality in cases of suspected PAS. Several markers for PAS have been described on MRI, including15:
- intraplacental T2-weighted dark bands
- abnormal intraplacental vascularity
- heterogeneous intraplacental signal intensity
- focal interruption of the myometrium by the placenta
- uterine bulging.
- Assess a priori risk for the patient before initiating the US exam
- In the presence of a placenta previa, or low-lying placenta, we strongly recommend a transvaginal, in addition to transabdominal, US to further assess for the presence of placenta accreta spectrum (PAS) markers
- Until prospective studies clearly define the diagnostic accuracy of PAS sonographic markers and their performance in high-risk and low-risk pregnancies, we recommend that US findings be reported as a risk profile—that is, high, moderate, and low risk of PAS
- Be especially cautious with patients who are at substantially increased risk for PAS, such as those with placenta previa and prior multiple CDs. In this setting, a low-risk report for PAS only should be provided when none of the PAS markers are seen on transabdominal and transvaginal US examinations
- While awaiting national guidelines that 1) standardize the approach to the US examination and 2) define PAS US markers, we encourage US laboratories to develop local protocols to standardize the sonographic evaluation of the placenta and ensure uniform and complete placental assessment
Based on a recent meta-analysis, overall sensitivity of MRI for detecting PAS is 86% to 95%, with specificity of 80% to 95%. Although this is comparable to the sensitivity and specificity of US,27 studies of MRI in PAS are smaller and more prone to bias than in studies of US, because MRI typically is used only in patients at highest risk for PAS. Few studies comparing US to MRI for PAS have been performed; all are small and lack statistical power.
Complementary role. MRI can be complementary to US in cases in which the placenta is posterior or located laterally28 but, importantly, rarely changes decisions about surgical management when used in conjunction with US to assess patients for the diagnosis of PAS. (An exception might lie in the ability of MRI to assess the degree or depth of invasion of the placenta and discerning placenta percreta from placenta accreta.15)
Enhancement with contrast. Addition of gadolinium-based contrast might improve the ability of MRI to make a diagnosis of PAS, but gadolinium crosses the placenta barrier. Although fetal effects of gadolinium have not been observed, American College of Radiology guidelines recommend avoiding this contrast agent during pregnancy unless absolutely essential.29
Specific indications. MRI without contrast should be considered 1) when US is inconclusive and 2) to further evaluate a posterior placenta suspicious for invasion, to define the precise topography of extrauterine placental invasion. The additional information offered by MRI might alter surgical planning.15
Overall, based on current literature, gray-scale US appears to be an excellent tool for prenatal diagnosis of PAS in women at risk: Sensitivity has been reported in the range of 80% to 90%; specificity, 91% to 98%; positive predictive value, 65% to 93%; and negative predictive value, 98%.5,6
However, these values might overestimate the true ability of prenatal US to predict PAS. Why? Early studies that assessed the accuracy of US prediction of PAS might have been biased by inclusion of single-expert observations, high suspicion of placenta accreta, and prior knowledge of patients’ risk factors. In addition, small sample size, retrospective design, and wide variability in the definition of PAS and inclusion criteria led to inconsistency in performance and skewed sensitivity.7
In fact, when experienced providers, reviewing the same US images, were blinded to patients’ clinical history, the accuracy of US diagnosis of PAS decreased in regard to sensitivity (to 54%), specificity (88%), positive (82%) and negative (65%) predictive value, and accuracy (65%).4 Investigators also found wide inter-observer variability in the interpretation of markers of PAS.4 Furthermore, there is evidence that several PAS US markers are commonly seen in low-risk normal pregnancy.
Although studies have yielded variable findings of the precise sensitivity and positive predictive value of US in the diagnosis of PAS, there is a general agreement that US should be the primary imaging modality for this purpose, and can be used exclusively in most cases.
References
- Comstock CH, Bronsteen RA. The antenatal diagnosis of placenta accreta. BJOG. 2014;121:171-181.
- D’Antonio F, Iacovella C, Bhide A. Prenatal identification of invasive placentation using ultrasound: systematic review and metaanalysis. Ultrasound Obstet Gynecol. 2013;42:509-517.
- Comstock CH, Love JJ Jr, Bronsteen RA, et al. Sonographic detection of placenta accreta in the second and third trimesters of pregnancy. Am J Obstet Gynecol. 2004;190:1135-1140.
- Bowman ZS, Eller AG, Kennedy AM, et al. Interobserver variability of sonography for prediction of placenta accreta. J Ultrasound Med. 2014;33:2153-2158.
Biomarkers
Multiple serum biomarkers have been proposed to predict PAS in high-risk women. PAS might be associated with increased levels of first-trimester pregnancy-associated plasma protein A, second-trimester maternal serum alpha fetoprotein, and human chorionic gonadotropin, but studies of the utility of these biomarkers have yielded contradictory results.30,31 Biomarkers are of interest and have significant clinical applicability, but none of the ones identified to date have high sensitivity or specificity for predicting PAS prenatally. Research is ongoing to identify markers of PAS that have sufficient predictive power.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Irving FC, Hertig AT. A study of placenta accreta. Surg Gynec Obstet. 1937:64:178–200.
- Wu S, Kocherginsky M, Hibbard JU. Abnormal placentation: twenty-year analysis. Am J Obstet Gynecol. 2005;192:1458–1461.
- Hall T, Wax JR, Lucas FL, et al. Prenatal sonographic diagnosis of placenta accreta—impact on maternal and neonatal outcomes. J Clin Ultrasound. 2014;42:449–455.
- Bowman ZS, Eller AG, Kennedy AM, et al. Interobserver variability of sonography for prediction of placenta accreta. J Ultrasound Med. 2014;33:2153–2158.
- Silver RM. Abnormal placentation: placenta previa, vasa previa, and placenta accreta. Obstet Gynecol. 2015;126:654–668.
- Silver RM, Landon MB, Rouse DJ, et al; National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Maternal morbidity associated with multiple repeat cesarean deliveries. Obstet Gynecol. 2006;107:1226–1232.
- Rac MW, Moschos E, Wells CE, et al. Sonographic findings of morbidly adherent placenta in the first trimester. J Ultrasound Med. 2016;35:263–269.
- Ballas J, Pretorius D, Hull AD, et al. Identifying sonographic markers for placenta accreta in the first trimester. J Ultrasound Med. 2012;31:1835–1841.
- Comstock CH, Bronsteen RA. The antenatal diagnosis of placenta accreta. BJOG. 2014;121:171–181.
- Marshall NE, Fu R, Guise JM. Impact of multiple cesarean deliveries on maternal morbidity: a systematic review. Am J Obstet Gynecol. 2011;205:262.e1–e8.
- Baughman WC, Corteville JE, Shah RR. Placenta accreta: spectrum of US and MR imaging findings. Radiographics. 2008;28:1905–1916.
- Finberg HJ, Williams JW. Placenta accreta: prospective sonographic diagnosis in patients with placenta previa and prior cesarean section. J Ultrasound Med. 1992;11:333–343.
- D’Antonio F, Iacovella C, Bhide A. Prenatal identification of invasive placentation using ultrasound: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2013;42:509–517.
- Comstock CH, Love JJ Jr, Bronsteen RA, et al. Sonographic detection of placenta accreta in the second and third trimesters of pregnancy. Am J Obstet Gynecol. 2004;190:1135–1140.
- D’Antonio F, Palacios-Jaraquemada J, Lim PS, et al. Counseling in fetal medicine: evidence-based answers to clinical questions on morbidly adherent placenta. Ultrasound Obstet Gynecol. 2016;47:290–301.
- Hudon L, Belfort MA, Broome DR. Diagnosis and management of placenta percreta: a review. Obstet Gynecol Surv. 1998;53:509–517.
- Wong HS, Cheung YK, Zuccollo J, et al. Evaluation of sonographic diagnostic criteria for placenta accreta. J Clin Ultrasound. 2008;36:551–559.
- Jauniaux E, Collins S, Burton GJ. Placenta accreta spectrum: pathophysiology and evidence-based anatomy for prenatal ultrasound imaging. Am J Obstet Gynecol. 2018;218:75–87.
- Rac MW, Dashe JS, Wells CE, et al. Ultrasound predictors of placental invasion: the Placenta Accreta Index. Am J Obstet Gynecol. 2015;212:343.e1–e7.
- Pilloni E, Alemanno MG, Gaglioti P, et al. Accuracy of ultrasound in antenatal diagnosis of placental attachment disorders. Ultrasound Obstet Gynecol. 2016;47:302–307.
- Comstock CH. Antenatal diagnosis of placenta accreta: a review. Ultrasound Obstet Gynecol. 2005;26:89–96.
- Collins SL, Stevenson GN, Al-Khan A, et al. Three-dimensional power Doppler ultrasonography for diagnosing abnormally invasive placenta and quantifying the risk. Obstet Gynecol. 2015;126:645–653.
- Collins SL, Ashcroft A, Braun T, et al; European Working Group on Abnormally Invasive Placenta (EW-AIP). Proposal for standardized ultrasound descriptors of abnormally invasive placenta (AIP). Ultrasound Obstet Gynecol. 2016;47:271–275.
- Gilboa Y, Spira M, Mazaki-Tovi S, et al. A novel sonographic scoring system for antenatal risk assessment of obstetric complications in suspected morbidly adherent placenta. J Ultrasound Med. 2015;34:561–567.
- Tovbin J, Melcer Y, Shor S, et al. Prediction of morbidly adherent placenta using a scoring system. Ultrasound Obstet Gynecol. 2016;48:504–510.
- Jauniaux E, Collins SL, Jurkovic D, Burton GJ. Accreta placentation: a systematic review of prenatal ultrasound imaging and grading of villous invasiveness. Am J Obstet Gynecol. 2016:215:712–721.
- Familiari A, Liberati M, Lim P, et al. Diagnostic accuracy of magnetic resonance imaging in detecting the severity of abnormal invasive placenta: a systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2018;97:507–520.
- Rezk MA, Shawky M. Grey-scale and colour Doppler ultrasound versus magnetic resonance imaging for the prenatal diagnosis of placenta accreta. J Matern Fetal Neonatal Med. 2016;29:218–223.
- Expert Panel on MR Safety; Kanal E, Barkovich AJ, Bell C, et al. ACR guidance document on MR safe practices: 2013. J Magn Reson Imaging. 2013;37:501–530.
- Pekar-Zlotin M, Melcer Y, Maymon R, Jauniaux E. Secondtrimester levels of fetoplacental hormones among women with placenta accreta spectrum disorders. Int J Gynaecol Obstet. 2018;140:377–378.
- Lyell DJ, Faucett AM, Baer RJ, et al. Maternal serum markers, characteristics and morbidly adherent placenta in women with previa. J Perinatol. 2015;35:570–574.
Placenta accreta spectrum (PAS) describes abnormal invasion of placental tissue into or through the myometrium, comprising 3 distinct conditions: placenta accreta, placenta increta, and placenta percreta. This complication is relatively new to obstetrics, first described in 1937.1
The overall incidence of PAS has been increasing over several decades, in parallel to an increasing rate of cesarean delivery (CD), with an incidence from 1982 through 2002 of 1 in 533 pregnancies, representing a 5-fold increase since the 1980s.2 PAS is associated with significant morbidity and mortality, including fetal growth restriction, preterm delivery, placental abruption antenatally, and hemorrhage during delivery or postpartum.
Prenatal diagnosis of PAS and planned delivery at an experienced center are associated with significant reduction in maternal and fetal morbidity.3 In an era of advanced imaging modalities, prenatal detection of PAS regrettably remains variable and largely subjective: As many as 20% to 50% of cases of PAS escape prenatal diagnosis.3,4
In this article, we review the sonographic markers of PAS, including diagnostic accuracy, and propose a standardized approach to prenatal diagnosis. Throughout our discussion, we describe protocols for detection of PAS practiced at our Maternal-Fetal Medicine Program in the Department of Obstetrics and Gynecology, Eastern Virginia Medical School (also see “US evaluation of PAS risk: The authors’ recommended approach”).

Numerous risk factors
There are many risk factors for PAS, including prior uterine surgery or instrumentation, such as CD, uterine curettage, myomectomy, pelvic radiation, and endometrial ablation. Other risk factors include smoking, in vitro fertilization, advanced maternal age, multiparity, and a brief interval between prior CD and subsequent pregnancy.5 Of major significance is the increased risk of PAS in the presence of placenta previa with prior CD.6 Knowledge of clinical risk factors by the interpreting physician appears to be associated with improved detection of PAS on ultrasonography (US).4
Ultrasonographic markers of PAS
First-trimester markers
Sonographic markers of PAS in the first trimester include:
- a gestational sac implanted in the lower uterine segment or in a CD scar
- multiple hypoechoic spaces within the placenta (lacunae).7
Lower uterine-segment implantation has been defined by Ballas and colleagues as 1) a gestational sac implanted in the lower one-third of the uterus between 8 and 10 weeks’ gestation or 2) a gestational sac occupying primarily the lower uterine segment from 10 weeks’ gestation onward (FIGURE 1).8 Our experience is that it is difficult to accurately assess lower uterine-segment implantation beyond 13 weeks of gestation because the sac typically expands to fill the upper uterine cavity.

Continue to: Color Doppler US...
Color Doppler US can help differentiate lower uterine-segment implantation from a gestational sac of a failed pregnancy in the process of expulsion by demonstrating loss of circumferential blood flow in the failed pregnancy. Furthermore, applying pressure to the anterior surface of the uterus will result in downward movement of the gestational sac of a failed pregnancy.9
Not all gestational sacs that implant in the lower uterine segment lead to PAS: Subsequent normal pregnancies have been reported in this circumstance. In such cases, a normal thick myometrium is noted anterior to the gestational sac.7 A patient with lower uterine-segment implantation without evidence of anterior myometrial thinning remains at risk for third-trimester placenta previa.7

Cesarean scar pregnancy carries significant risk of PAS. In these cases, the gestational sac is typically implanted within the scar, resulting in a thin anterior myometrium and significantly increased vascularity of the placental–myometrial and bladder–uterine wall interfaces (FIGURE 2).9 Differentiating cesarean scar pregnancy from a lower uterine-segment implantation is easier to perform before the eighth week of gestation but becomes more difficult as pregnancy advances. Although it might be useful to distinguish between true cesarean scar pregnancy and lower uterine-segment implantation adjacent to or involving the scar, both carry considerable risk of PAS and excessive hemorrhage, and the approach to treating both conditions is quite similar.
Lacunae, with or without documented blood flow on color Doppler US, are the third marker of PAS in the first trimester.8 Although some retrospective series and case reports describe the finding of lacunae in the first trimester of patients with diagnosed PAS, more recent literature suggests that these spaces are seen infrequently and at a similar frequency in women with and without PAS at delivery.7
Second- and third-trimester markers
Multiple diagnostic sonographic markers of PAS have been described in the second and third trimesters.
Placental location is a significant risk factor for PAS. Placenta previa in the setting of prior CD carries the highest risk of PAS—as high as 61% in women with both placenta previa and a history of 3 CDs.10 An anterior placenta appears to be a stronger risk factor for PAS than a posterior placenta in women with prior CD; the location of the placenta should therefore be evaluated in all women in the second trimester.
Continue to: Lacunae
Lacunae. The finding of multiple hypoechoic vascular spaces within the placental parenchyma has been associated with PAS (FIGURES 3 and 4). The pathogenesis of this finding is probably related to alterations in placental tissue resulting from long-term exposure to pulsatile blood flow.11

Finberg and colleagues introduced a grading system for placental lacunae in 1992 that is still used:
- Grade 0: no lacunae seen
- Grade 1: 1 to 3 lacunae seen
- Grade 2: 4 to 6 lacunae seen
- Grade 3: multiple lacunae seen throughout the placenta.12
The sensitivity and specificity of lacunae as an independent marker for PAS have been reported to be 77% and 95%, respectively.13 Despite these findings, several studies report a range of sensitivity (73% to 100%) and negative predictive value (88% to 100%).14 Even in Finberg’s original work, 27% of cases of confirmed PAS had Grade 0 or Grade 1 placental lacunae and 11% of cases of placenta previa, without PAS, demonstrated Grade 2 lacunae.12 There is agreement, however, that, the more lacunae, the higher the risk of PAS.

Continue to: Other US markers for PAS
Other US markers of PAS

Retroplacental–myometrial interface

Loss of the normal hypoechoic (clear) retroplacental zone, also referred to as loss of the clear space between placenta and uterus, is another marker of PAS (FIGURE 5). This finding corresponds to pathologic loss of the decidua basalis as trophoblastic tissue invades directly through the myometrium.15 This sonographic finding has been reported to have a detection rate of approximately 93%, with sensitivity of 52% and specificity of 57%, for PAS; the false-positive rate, however, has been in the range of 21% or higher. This marker should not be used alone because it is angle-dependent and can be found (as an absent clear zone) in normal anterior placentas.16
The strength of this US marker is in its negative predictive value, which ranges from 96% to 100%. The presence of a hypoechoic retroplacental clear space that extends the length of the placenta makes PAS unlikely.17 Of note, the clear zone may appear falsely absent as a result of increased pressure from the US probe.
Retroplacental myometrial thickness

Retroplacental myometrial thickness is difficult to assess because the lower uterine-segment myometrium thins in normal pregnancy as term approaches. This measurement also can be influenced by direct pressure of the US probe and fullness of the maternal bladder.18 In patients who have had a CD but who do not have PAS, the median myometrial thickness of the lower uterine segment in the third trimester is 2.4 mm.19
Thinning of the myometrium in the upper uterine segment always should be of concern. Studies of this marker have reported sensitivity of US ranging from 22% to 100% and specificity from 72% to 100%.9,20 Given such variability, it is important to standardize the gestational age and sonographic approach for this marker.
Continue to: Uterovesical interface
Uterovesical interface
Studies also have reported that abnormalities of the uterovesical interface are predictive of PAS. The uterovesical interface is best evaluated in a sagittal plane containing the lower uterine segment and a partially full bladder in gray-scale and color Doppler US.15 The normal uterovesical interface appears as a smooth line, without irregularities or increased vascularity on sagittal imaging.
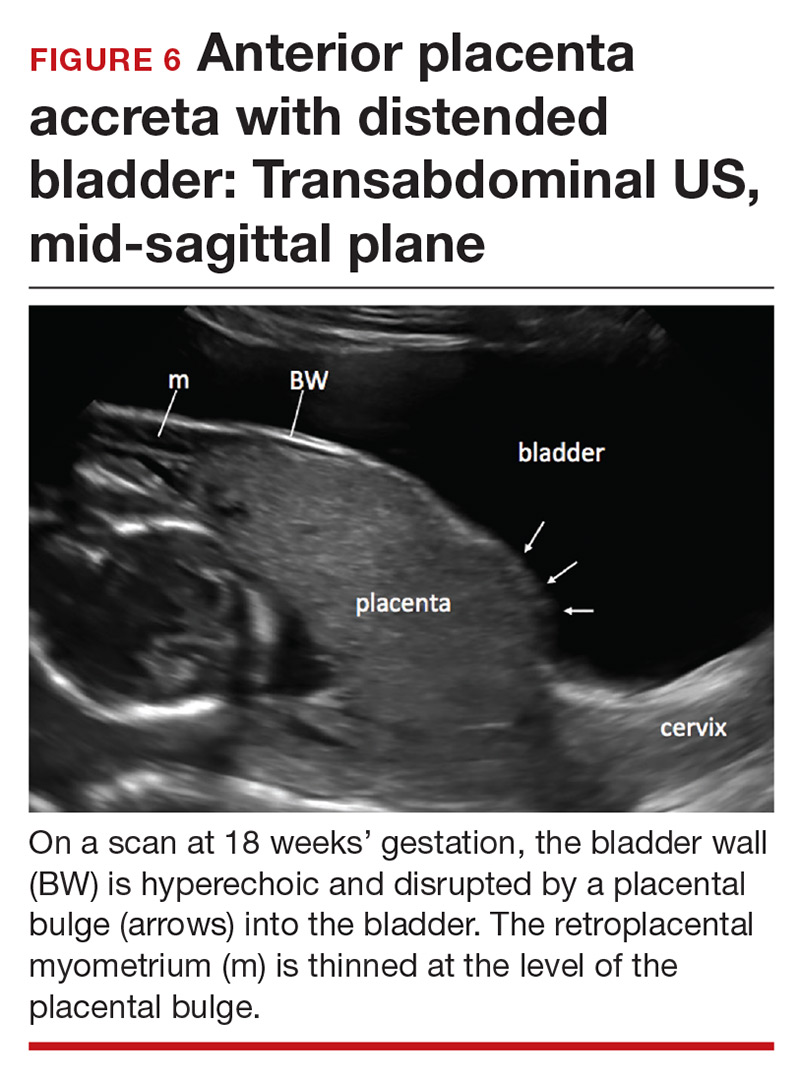
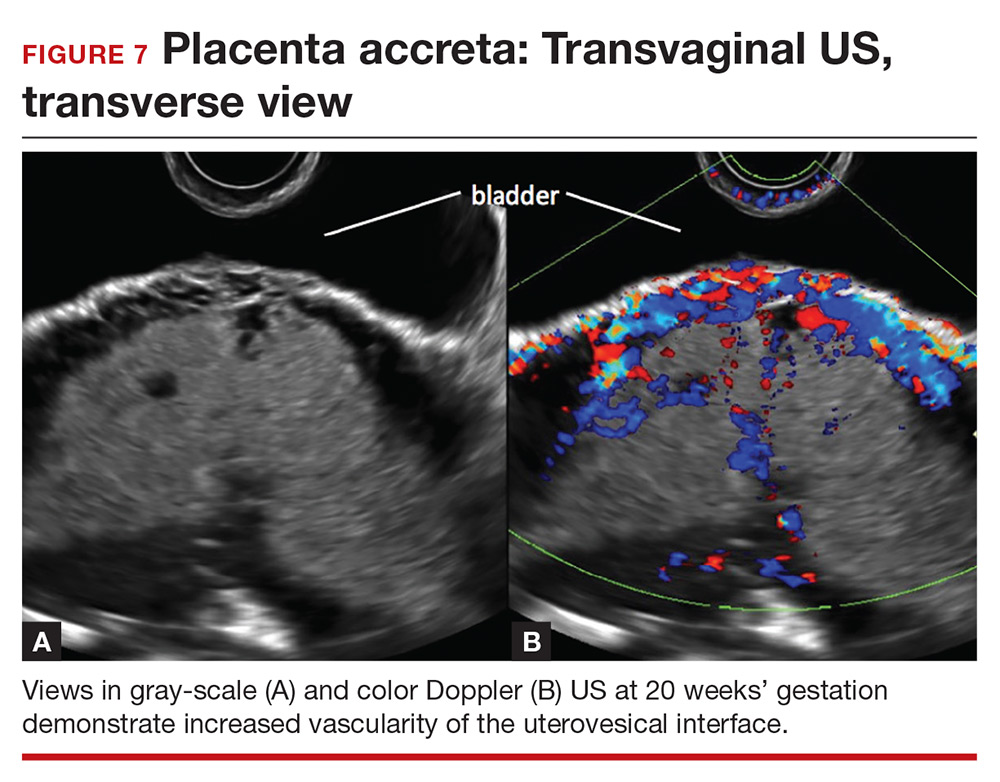
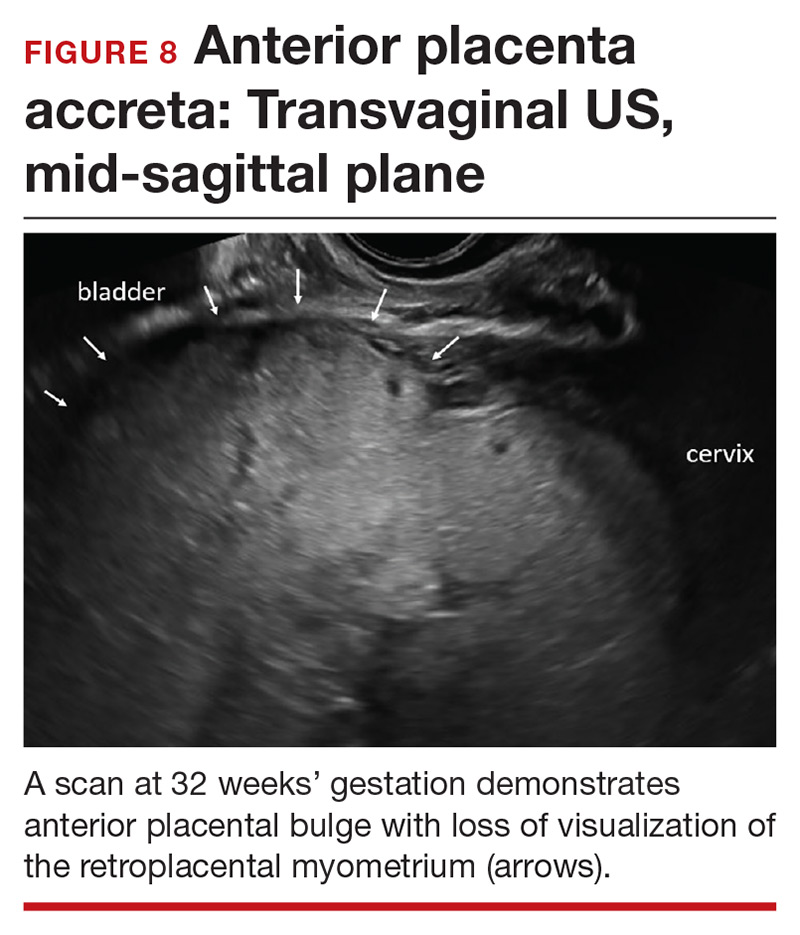
Abnormalities include focal interruption of the hyperechoic bladder wall, bulging of the bladder wall, and increased vascularity, such as varicosities (FIGURES 5, 6, and 7).15 These findings may be seen as early as the first trimester but are more commonly noted in the second and third trimesters.7 The authors of a recent meta-analysis concluded that irregularity of the uterovesical interface is the most specific marker for invasive placentation (99.75% confidence interval; range, 99.5% to 99.9%).13

Other US markers and modalities

Three-dimensional US. Studies have evaluated the role of 3-dimensional (3D) US for predicting PAS. Application of 3D US in vascular mode has shown promise because it allows for semiquantitative assessment of placental vasculature.22 Using 3D US to screen for PAS presents drawbacks, however: The technology is not well-standardized and requires significant operator expertise for volume acquisition and manipulation. Prospective studies are needed before 3D US can be applied routinely to screen for and diagnose PAS.
Color Doppler US. As an adjunct to gray-scale US, color Doppler US can be used for making a diagnosis of PAS. Color Doppler US helps differentiate a normal subplacental venous complex with nonpulsatile, low-velocity venous blood flow waveforms from markedly dilated peripheral subplacental vascular channels with pulsatile venous-type flow, which suggests PAS. These vascular channels are often located directly over the cervix. In addition, the observation of bridging vessels linking the placenta and bladder with high diastolic arterial blood flow also suggests invasion.21 In a meta-analysis, overall sensitivity of color Doppler US for the diagnosis of PAS was 91%, with specificity of 87%.13
The value of utilizing multiple markers
The accuracy of US diagnosis of PAS is likely improved by using more than 1 sonographic marker. Pilloni and colleagues,20 in a prospective analysis, found that 81% of cases of confirmed PAS had ≥2 markers and 51% of cases had ≥3 markers.
Several scoring systems have been proposed for making the diagnosis of PAS using combinations of sonographic markers, placental location, and clinical history.19,24,25 In 2016, Tovbin and colleagues,25 in a prospective study, evaluated a scoring system that included:
- number of previous CDs
- number of, maximum dimension of, and presence of blood flow in lacunae
- loss of uteroplacental clear zone
- placental location
- hypervascularity of the uterovesical or uteroplacental interface.
Tovbin assigned 1 or 2 points to each criterion. Each sonographic marker was found to be significantly associated with PAS when compared to a high-risk control group. A score of ≥8 was considered “at high risk” and predicted 69% of PAS cases.
Regrettably, no combination of US markers reliably predicts the depth of invasion of the placenta.26
Continue to: A standardized approach is needed
A standardized approach is needed
To decrease variability and improve the US diagnosis of PAS, it is important to define and standardize the diagnosis of each sonographic marker for PAS.4 In 2016, the European Working Group on Abnormally Invasive Placenta (EW-AIP) proposed a set of US markers that always should be reported when performing an US examination for suspected abnormal placentation (TABLE).23 Despite this effort by the EW-AIP, ambiguity remains over sonographic definitions of several PAS markers. For example, what determines a placental lacuna on US? And what constitutes an abnormal uterovesical interface? There is a need for a more objective definition of US markers of PAS and a standardized approach to the US examination in at-risk pregnancies.

The Society for Maternal-Fetal Medicine is coordinating a multi-society task force to address the need to define and standardize the US diagnosis of PAS.
Observations on other PAS diagnostic modalities
Magnetic resonance imaging
Adjunctive role. Magnetic resonance imaging (MRI) is often used as an adjunctive diagnostic modality in cases of suspected PAS. Several markers for PAS have been described on MRI, including15:
- intraplacental T2-weighted dark bands
- abnormal intraplacental vascularity
- heterogeneous intraplacental signal intensity
- focal interruption of the myometrium by the placenta
- uterine bulging.
- Assess a priori risk for the patient before initiating the US exam
- In the presence of a placenta previa, or low-lying placenta, we strongly recommend a transvaginal, in addition to transabdominal, US to further assess for the presence of placenta accreta spectrum (PAS) markers
- Until prospective studies clearly define the diagnostic accuracy of PAS sonographic markers and their performance in high-risk and low-risk pregnancies, we recommend that US findings be reported as a risk profile—that is, high, moderate, and low risk of PAS
- Be especially cautious with patients who are at substantially increased risk for PAS, such as those with placenta previa and prior multiple CDs. In this setting, a low-risk report for PAS only should be provided when none of the PAS markers are seen on transabdominal and transvaginal US examinations
- While awaiting national guidelines that 1) standardize the approach to the US examination and 2) define PAS US markers, we encourage US laboratories to develop local protocols to standardize the sonographic evaluation of the placenta and ensure uniform and complete placental assessment
Based on a recent meta-analysis, overall sensitivity of MRI for detecting PAS is 86% to 95%, with specificity of 80% to 95%. Although this is comparable to the sensitivity and specificity of US,27 studies of MRI in PAS are smaller and more prone to bias than in studies of US, because MRI typically is used only in patients at highest risk for PAS. Few studies comparing US to MRI for PAS have been performed; all are small and lack statistical power.
Complementary role. MRI can be complementary to US in cases in which the placenta is posterior or located laterally28 but, importantly, rarely changes decisions about surgical management when used in conjunction with US to assess patients for the diagnosis of PAS. (An exception might lie in the ability of MRI to assess the degree or depth of invasion of the placenta and discerning placenta percreta from placenta accreta.15)
Enhancement with contrast. Addition of gadolinium-based contrast might improve the ability of MRI to make a diagnosis of PAS, but gadolinium crosses the placenta barrier. Although fetal effects of gadolinium have not been observed, American College of Radiology guidelines recommend avoiding this contrast agent during pregnancy unless absolutely essential.29
Specific indications. MRI without contrast should be considered 1) when US is inconclusive and 2) to further evaluate a posterior placenta suspicious for invasion, to define the precise topography of extrauterine placental invasion. The additional information offered by MRI might alter surgical planning.15
Overall, based on current literature, gray-scale US appears to be an excellent tool for prenatal diagnosis of PAS in women at risk: Sensitivity has been reported in the range of 80% to 90%; specificity, 91% to 98%; positive predictive value, 65% to 93%; and negative predictive value, 98%.5,6
However, these values might overestimate the true ability of prenatal US to predict PAS. Why? Early studies that assessed the accuracy of US prediction of PAS might have been biased by inclusion of single-expert observations, high suspicion of placenta accreta, and prior knowledge of patients’ risk factors. In addition, small sample size, retrospective design, and wide variability in the definition of PAS and inclusion criteria led to inconsistency in performance and skewed sensitivity.7
In fact, when experienced providers, reviewing the same US images, were blinded to patients’ clinical history, the accuracy of US diagnosis of PAS decreased in regard to sensitivity (to 54%), specificity (88%), positive (82%) and negative (65%) predictive value, and accuracy (65%).4 Investigators also found wide inter-observer variability in the interpretation of markers of PAS.4 Furthermore, there is evidence that several PAS US markers are commonly seen in low-risk normal pregnancy.
Although studies have yielded variable findings of the precise sensitivity and positive predictive value of US in the diagnosis of PAS, there is a general agreement that US should be the primary imaging modality for this purpose, and can be used exclusively in most cases.
References
- Comstock CH, Bronsteen RA. The antenatal diagnosis of placenta accreta. BJOG. 2014;121:171-181.
- D’Antonio F, Iacovella C, Bhide A. Prenatal identification of invasive placentation using ultrasound: systematic review and metaanalysis. Ultrasound Obstet Gynecol. 2013;42:509-517.
- Comstock CH, Love JJ Jr, Bronsteen RA, et al. Sonographic detection of placenta accreta in the second and third trimesters of pregnancy. Am J Obstet Gynecol. 2004;190:1135-1140.
- Bowman ZS, Eller AG, Kennedy AM, et al. Interobserver variability of sonography for prediction of placenta accreta. J Ultrasound Med. 2014;33:2153-2158.
Biomarkers
Multiple serum biomarkers have been proposed to predict PAS in high-risk women. PAS might be associated with increased levels of first-trimester pregnancy-associated plasma protein A, second-trimester maternal serum alpha fetoprotein, and human chorionic gonadotropin, but studies of the utility of these biomarkers have yielded contradictory results.30,31 Biomarkers are of interest and have significant clinical applicability, but none of the ones identified to date have high sensitivity or specificity for predicting PAS prenatally. Research is ongoing to identify markers of PAS that have sufficient predictive power.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Placenta accreta spectrum (PAS) describes abnormal invasion of placental tissue into or through the myometrium, comprising 3 distinct conditions: placenta accreta, placenta increta, and placenta percreta. This complication is relatively new to obstetrics, first described in 1937.1
The overall incidence of PAS has been increasing over several decades, in parallel to an increasing rate of cesarean delivery (CD), with an incidence from 1982 through 2002 of 1 in 533 pregnancies, representing a 5-fold increase since the 1980s.2 PAS is associated with significant morbidity and mortality, including fetal growth restriction, preterm delivery, placental abruption antenatally, and hemorrhage during delivery or postpartum.
Prenatal diagnosis of PAS and planned delivery at an experienced center are associated with significant reduction in maternal and fetal morbidity.3 In an era of advanced imaging modalities, prenatal detection of PAS regrettably remains variable and largely subjective: As many as 20% to 50% of cases of PAS escape prenatal diagnosis.3,4
In this article, we review the sonographic markers of PAS, including diagnostic accuracy, and propose a standardized approach to prenatal diagnosis. Throughout our discussion, we describe protocols for detection of PAS practiced at our Maternal-Fetal Medicine Program in the Department of Obstetrics and Gynecology, Eastern Virginia Medical School (also see “US evaluation of PAS risk: The authors’ recommended approach”).

Numerous risk factors
There are many risk factors for PAS, including prior uterine surgery or instrumentation, such as CD, uterine curettage, myomectomy, pelvic radiation, and endometrial ablation. Other risk factors include smoking, in vitro fertilization, advanced maternal age, multiparity, and a brief interval between prior CD and subsequent pregnancy.5 Of major significance is the increased risk of PAS in the presence of placenta previa with prior CD.6 Knowledge of clinical risk factors by the interpreting physician appears to be associated with improved detection of PAS on ultrasonography (US).4
Ultrasonographic markers of PAS
First-trimester markers
Sonographic markers of PAS in the first trimester include:
- a gestational sac implanted in the lower uterine segment or in a CD scar
- multiple hypoechoic spaces within the placenta (lacunae).7
Lower uterine-segment implantation has been defined by Ballas and colleagues as 1) a gestational sac implanted in the lower one-third of the uterus between 8 and 10 weeks’ gestation or 2) a gestational sac occupying primarily the lower uterine segment from 10 weeks’ gestation onward (FIGURE 1).8 Our experience is that it is difficult to accurately assess lower uterine-segment implantation beyond 13 weeks of gestation because the sac typically expands to fill the upper uterine cavity.

Continue to: Color Doppler US...
Color Doppler US can help differentiate lower uterine-segment implantation from a gestational sac of a failed pregnancy in the process of expulsion by demonstrating loss of circumferential blood flow in the failed pregnancy. Furthermore, applying pressure to the anterior surface of the uterus will result in downward movement of the gestational sac of a failed pregnancy.9
Not all gestational sacs that implant in the lower uterine segment lead to PAS: Subsequent normal pregnancies have been reported in this circumstance. In such cases, a normal thick myometrium is noted anterior to the gestational sac.7 A patient with lower uterine-segment implantation without evidence of anterior myometrial thinning remains at risk for third-trimester placenta previa.7

Cesarean scar pregnancy carries significant risk of PAS. In these cases, the gestational sac is typically implanted within the scar, resulting in a thin anterior myometrium and significantly increased vascularity of the placental–myometrial and bladder–uterine wall interfaces (FIGURE 2).9 Differentiating cesarean scar pregnancy from a lower uterine-segment implantation is easier to perform before the eighth week of gestation but becomes more difficult as pregnancy advances. Although it might be useful to distinguish between true cesarean scar pregnancy and lower uterine-segment implantation adjacent to or involving the scar, both carry considerable risk of PAS and excessive hemorrhage, and the approach to treating both conditions is quite similar.
Lacunae, with or without documented blood flow on color Doppler US, are the third marker of PAS in the first trimester.8 Although some retrospective series and case reports describe the finding of lacunae in the first trimester of patients with diagnosed PAS, more recent literature suggests that these spaces are seen infrequently and at a similar frequency in women with and without PAS at delivery.7
Second- and third-trimester markers
Multiple diagnostic sonographic markers of PAS have been described in the second and third trimesters.
Placental location is a significant risk factor for PAS. Placenta previa in the setting of prior CD carries the highest risk of PAS—as high as 61% in women with both placenta previa and a history of 3 CDs.10 An anterior placenta appears to be a stronger risk factor for PAS than a posterior placenta in women with prior CD; the location of the placenta should therefore be evaluated in all women in the second trimester.
Continue to: Lacunae
Lacunae. The finding of multiple hypoechoic vascular spaces within the placental parenchyma has been associated with PAS (FIGURES 3 and 4). The pathogenesis of this finding is probably related to alterations in placental tissue resulting from long-term exposure to pulsatile blood flow.11

Finberg and colleagues introduced a grading system for placental lacunae in 1992 that is still used:
- Grade 0: no lacunae seen
- Grade 1: 1 to 3 lacunae seen
- Grade 2: 4 to 6 lacunae seen
- Grade 3: multiple lacunae seen throughout the placenta.12
The sensitivity and specificity of lacunae as an independent marker for PAS have been reported to be 77% and 95%, respectively.13 Despite these findings, several studies report a range of sensitivity (73% to 100%) and negative predictive value (88% to 100%).14 Even in Finberg’s original work, 27% of cases of confirmed PAS had Grade 0 or Grade 1 placental lacunae and 11% of cases of placenta previa, without PAS, demonstrated Grade 2 lacunae.12 There is agreement, however, that, the more lacunae, the higher the risk of PAS.

Continue to: Other US markers for PAS
Other US markers of PAS

Retroplacental–myometrial interface

Loss of the normal hypoechoic (clear) retroplacental zone, also referred to as loss of the clear space between placenta and uterus, is another marker of PAS (FIGURE 5). This finding corresponds to pathologic loss of the decidua basalis as trophoblastic tissue invades directly through the myometrium.15 This sonographic finding has been reported to have a detection rate of approximately 93%, with sensitivity of 52% and specificity of 57%, for PAS; the false-positive rate, however, has been in the range of 21% or higher. This marker should not be used alone because it is angle-dependent and can be found (as an absent clear zone) in normal anterior placentas.16
The strength of this US marker is in its negative predictive value, which ranges from 96% to 100%. The presence of a hypoechoic retroplacental clear space that extends the length of the placenta makes PAS unlikely.17 Of note, the clear zone may appear falsely absent as a result of increased pressure from the US probe.
Retroplacental myometrial thickness

Retroplacental myometrial thickness is difficult to assess because the lower uterine-segment myometrium thins in normal pregnancy as term approaches. This measurement also can be influenced by direct pressure of the US probe and fullness of the maternal bladder.18 In patients who have had a CD but who do not have PAS, the median myometrial thickness of the lower uterine segment in the third trimester is 2.4 mm.19
Thinning of the myometrium in the upper uterine segment always should be of concern. Studies of this marker have reported sensitivity of US ranging from 22% to 100% and specificity from 72% to 100%.9,20 Given such variability, it is important to standardize the gestational age and sonographic approach for this marker.
Continue to: Uterovesical interface
Uterovesical interface
Studies also have reported that abnormalities of the uterovesical interface are predictive of PAS. The uterovesical interface is best evaluated in a sagittal plane containing the lower uterine segment and a partially full bladder in gray-scale and color Doppler US.15 The normal uterovesical interface appears as a smooth line, without irregularities or increased vascularity on sagittal imaging.
Abnormalities include focal interruption of the hyperechoic bladder wall, bulging of the bladder wall, and increased vascularity, such as varicosities (FIGURES 5, 6, and 7).15 These findings may be seen as early as the first trimester but are more commonly noted in the second and third trimesters.7 The authors of a recent meta-analysis concluded that irregularity of the uterovesical interface is the most specific marker for invasive placentation (99.75% confidence interval; range, 99.5% to 99.9%).13

Other US markers and modalities

Three-dimensional US. Studies have evaluated the role of 3-dimensional (3D) US for predicting PAS. Application of 3D US in vascular mode has shown promise because it allows for semiquantitative assessment of placental vasculature.22 Using 3D US to screen for PAS presents drawbacks, however: The technology is not well-standardized and requires significant operator expertise for volume acquisition and manipulation. Prospective studies are needed before 3D US can be applied routinely to screen for and diagnose PAS.
Color Doppler US. As an adjunct to gray-scale US, color Doppler US can be used for making a diagnosis of PAS. Color Doppler US helps differentiate a normal subplacental venous complex with nonpulsatile, low-velocity venous blood flow waveforms from markedly dilated peripheral subplacental vascular channels with pulsatile venous-type flow, which suggests PAS. These vascular channels are often located directly over the cervix. In addition, the observation of bridging vessels linking the placenta and bladder with high diastolic arterial blood flow also suggests invasion.21 In a meta-analysis, overall sensitivity of color Doppler US for the diagnosis of PAS was 91%, with specificity of 87%.13
The value of utilizing multiple markers
The accuracy of US diagnosis of PAS is likely improved by using more than 1 sonographic marker. Pilloni and colleagues,20 in a prospective analysis, found that 81% of cases of confirmed PAS had ≥2 markers and 51% of cases had ≥3 markers.
Several scoring systems have been proposed for making the diagnosis of PAS using combinations of sonographic markers, placental location, and clinical history.19,24,25 In 2016, Tovbin and colleagues,25 in a prospective study, evaluated a scoring system that included:
- number of previous CDs
- number of, maximum dimension of, and presence of blood flow in lacunae
- loss of uteroplacental clear zone
- placental location
- hypervascularity of the uterovesical or uteroplacental interface.
Tovbin assigned 1 or 2 points to each criterion. Each sonographic marker was found to be significantly associated with PAS when compared to a high-risk control group. A score of ≥8 was considered “at high risk” and predicted 69% of PAS cases.
Regrettably, no combination of US markers reliably predicts the depth of invasion of the placenta.26
Continue to: A standardized approach is needed
A standardized approach is needed
To decrease variability and improve the US diagnosis of PAS, it is important to define and standardize the diagnosis of each sonographic marker for PAS.4 In 2016, the European Working Group on Abnormally Invasive Placenta (EW-AIP) proposed a set of US markers that always should be reported when performing an US examination for suspected abnormal placentation (TABLE).23 Despite this effort by the EW-AIP, ambiguity remains over sonographic definitions of several PAS markers. For example, what determines a placental lacuna on US? And what constitutes an abnormal uterovesical interface? There is a need for a more objective definition of US markers of PAS and a standardized approach to the US examination in at-risk pregnancies.

The Society for Maternal-Fetal Medicine is coordinating a multi-society task force to address the need to define and standardize the US diagnosis of PAS.
Observations on other PAS diagnostic modalities
Magnetic resonance imaging
Adjunctive role. Magnetic resonance imaging (MRI) is often used as an adjunctive diagnostic modality in cases of suspected PAS. Several markers for PAS have been described on MRI, including15:
- intraplacental T2-weighted dark bands
- abnormal intraplacental vascularity
- heterogeneous intraplacental signal intensity
- focal interruption of the myometrium by the placenta
- uterine bulging.
- Assess a priori risk for the patient before initiating the US exam
- In the presence of a placenta previa, or low-lying placenta, we strongly recommend a transvaginal, in addition to transabdominal, US to further assess for the presence of placenta accreta spectrum (PAS) markers
- Until prospective studies clearly define the diagnostic accuracy of PAS sonographic markers and their performance in high-risk and low-risk pregnancies, we recommend that US findings be reported as a risk profile—that is, high, moderate, and low risk of PAS
- Be especially cautious with patients who are at substantially increased risk for PAS, such as those with placenta previa and prior multiple CDs. In this setting, a low-risk report for PAS only should be provided when none of the PAS markers are seen on transabdominal and transvaginal US examinations
- While awaiting national guidelines that 1) standardize the approach to the US examination and 2) define PAS US markers, we encourage US laboratories to develop local protocols to standardize the sonographic evaluation of the placenta and ensure uniform and complete placental assessment
Based on a recent meta-analysis, overall sensitivity of MRI for detecting PAS is 86% to 95%, with specificity of 80% to 95%. Although this is comparable to the sensitivity and specificity of US,27 studies of MRI in PAS are smaller and more prone to bias than in studies of US, because MRI typically is used only in patients at highest risk for PAS. Few studies comparing US to MRI for PAS have been performed; all are small and lack statistical power.
Complementary role. MRI can be complementary to US in cases in which the placenta is posterior or located laterally28 but, importantly, rarely changes decisions about surgical management when used in conjunction with US to assess patients for the diagnosis of PAS. (An exception might lie in the ability of MRI to assess the degree or depth of invasion of the placenta and discerning placenta percreta from placenta accreta.15)
Enhancement with contrast. Addition of gadolinium-based contrast might improve the ability of MRI to make a diagnosis of PAS, but gadolinium crosses the placenta barrier. Although fetal effects of gadolinium have not been observed, American College of Radiology guidelines recommend avoiding this contrast agent during pregnancy unless absolutely essential.29
Specific indications. MRI without contrast should be considered 1) when US is inconclusive and 2) to further evaluate a posterior placenta suspicious for invasion, to define the precise topography of extrauterine placental invasion. The additional information offered by MRI might alter surgical planning.15
Overall, based on current literature, gray-scale US appears to be an excellent tool for prenatal diagnosis of PAS in women at risk: Sensitivity has been reported in the range of 80% to 90%; specificity, 91% to 98%; positive predictive value, 65% to 93%; and negative predictive value, 98%.5,6
However, these values might overestimate the true ability of prenatal US to predict PAS. Why? Early studies that assessed the accuracy of US prediction of PAS might have been biased by inclusion of single-expert observations, high suspicion of placenta accreta, and prior knowledge of patients’ risk factors. In addition, small sample size, retrospective design, and wide variability in the definition of PAS and inclusion criteria led to inconsistency in performance and skewed sensitivity.7
In fact, when experienced providers, reviewing the same US images, were blinded to patients’ clinical history, the accuracy of US diagnosis of PAS decreased in regard to sensitivity (to 54%), specificity (88%), positive (82%) and negative (65%) predictive value, and accuracy (65%).4 Investigators also found wide inter-observer variability in the interpretation of markers of PAS.4 Furthermore, there is evidence that several PAS US markers are commonly seen in low-risk normal pregnancy.
Although studies have yielded variable findings of the precise sensitivity and positive predictive value of US in the diagnosis of PAS, there is a general agreement that US should be the primary imaging modality for this purpose, and can be used exclusively in most cases.
References
- Comstock CH, Bronsteen RA. The antenatal diagnosis of placenta accreta. BJOG. 2014;121:171-181.
- D’Antonio F, Iacovella C, Bhide A. Prenatal identification of invasive placentation using ultrasound: systematic review and metaanalysis. Ultrasound Obstet Gynecol. 2013;42:509-517.
- Comstock CH, Love JJ Jr, Bronsteen RA, et al. Sonographic detection of placenta accreta in the second and third trimesters of pregnancy. Am J Obstet Gynecol. 2004;190:1135-1140.
- Bowman ZS, Eller AG, Kennedy AM, et al. Interobserver variability of sonography for prediction of placenta accreta. J Ultrasound Med. 2014;33:2153-2158.
Biomarkers
Multiple serum biomarkers have been proposed to predict PAS in high-risk women. PAS might be associated with increased levels of first-trimester pregnancy-associated plasma protein A, second-trimester maternal serum alpha fetoprotein, and human chorionic gonadotropin, but studies of the utility of these biomarkers have yielded contradictory results.30,31 Biomarkers are of interest and have significant clinical applicability, but none of the ones identified to date have high sensitivity or specificity for predicting PAS prenatally. Research is ongoing to identify markers of PAS that have sufficient predictive power.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Irving FC, Hertig AT. A study of placenta accreta. Surg Gynec Obstet. 1937:64:178–200.
- Wu S, Kocherginsky M, Hibbard JU. Abnormal placentation: twenty-year analysis. Am J Obstet Gynecol. 2005;192:1458–1461.
- Hall T, Wax JR, Lucas FL, et al. Prenatal sonographic diagnosis of placenta accreta—impact on maternal and neonatal outcomes. J Clin Ultrasound. 2014;42:449–455.
- Bowman ZS, Eller AG, Kennedy AM, et al. Interobserver variability of sonography for prediction of placenta accreta. J Ultrasound Med. 2014;33:2153–2158.
- Silver RM. Abnormal placentation: placenta previa, vasa previa, and placenta accreta. Obstet Gynecol. 2015;126:654–668.
- Silver RM, Landon MB, Rouse DJ, et al; National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Maternal morbidity associated with multiple repeat cesarean deliveries. Obstet Gynecol. 2006;107:1226–1232.
- Rac MW, Moschos E, Wells CE, et al. Sonographic findings of morbidly adherent placenta in the first trimester. J Ultrasound Med. 2016;35:263–269.
- Ballas J, Pretorius D, Hull AD, et al. Identifying sonographic markers for placenta accreta in the first trimester. J Ultrasound Med. 2012;31:1835–1841.
- Comstock CH, Bronsteen RA. The antenatal diagnosis of placenta accreta. BJOG. 2014;121:171–181.
- Marshall NE, Fu R, Guise JM. Impact of multiple cesarean deliveries on maternal morbidity: a systematic review. Am J Obstet Gynecol. 2011;205:262.e1–e8.
- Baughman WC, Corteville JE, Shah RR. Placenta accreta: spectrum of US and MR imaging findings. Radiographics. 2008;28:1905–1916.
- Finberg HJ, Williams JW. Placenta accreta: prospective sonographic diagnosis in patients with placenta previa and prior cesarean section. J Ultrasound Med. 1992;11:333–343.
- D’Antonio F, Iacovella C, Bhide A. Prenatal identification of invasive placentation using ultrasound: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2013;42:509–517.
- Comstock CH, Love JJ Jr, Bronsteen RA, et al. Sonographic detection of placenta accreta in the second and third trimesters of pregnancy. Am J Obstet Gynecol. 2004;190:1135–1140.
- D’Antonio F, Palacios-Jaraquemada J, Lim PS, et al. Counseling in fetal medicine: evidence-based answers to clinical questions on morbidly adherent placenta. Ultrasound Obstet Gynecol. 2016;47:290–301.
- Hudon L, Belfort MA, Broome DR. Diagnosis and management of placenta percreta: a review. Obstet Gynecol Surv. 1998;53:509–517.
- Wong HS, Cheung YK, Zuccollo J, et al. Evaluation of sonographic diagnostic criteria for placenta accreta. J Clin Ultrasound. 2008;36:551–559.
- Jauniaux E, Collins S, Burton GJ. Placenta accreta spectrum: pathophysiology and evidence-based anatomy for prenatal ultrasound imaging. Am J Obstet Gynecol. 2018;218:75–87.
- Rac MW, Dashe JS, Wells CE, et al. Ultrasound predictors of placental invasion: the Placenta Accreta Index. Am J Obstet Gynecol. 2015;212:343.e1–e7.
- Pilloni E, Alemanno MG, Gaglioti P, et al. Accuracy of ultrasound in antenatal diagnosis of placental attachment disorders. Ultrasound Obstet Gynecol. 2016;47:302–307.
- Comstock CH. Antenatal diagnosis of placenta accreta: a review. Ultrasound Obstet Gynecol. 2005;26:89–96.
- Collins SL, Stevenson GN, Al-Khan A, et al. Three-dimensional power Doppler ultrasonography for diagnosing abnormally invasive placenta and quantifying the risk. Obstet Gynecol. 2015;126:645–653.
- Collins SL, Ashcroft A, Braun T, et al; European Working Group on Abnormally Invasive Placenta (EW-AIP). Proposal for standardized ultrasound descriptors of abnormally invasive placenta (AIP). Ultrasound Obstet Gynecol. 2016;47:271–275.
- Gilboa Y, Spira M, Mazaki-Tovi S, et al. A novel sonographic scoring system for antenatal risk assessment of obstetric complications in suspected morbidly adherent placenta. J Ultrasound Med. 2015;34:561–567.
- Tovbin J, Melcer Y, Shor S, et al. Prediction of morbidly adherent placenta using a scoring system. Ultrasound Obstet Gynecol. 2016;48:504–510.
- Jauniaux E, Collins SL, Jurkovic D, Burton GJ. Accreta placentation: a systematic review of prenatal ultrasound imaging and grading of villous invasiveness. Am J Obstet Gynecol. 2016:215:712–721.
- Familiari A, Liberati M, Lim P, et al. Diagnostic accuracy of magnetic resonance imaging in detecting the severity of abnormal invasive placenta: a systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2018;97:507–520.
- Rezk MA, Shawky M. Grey-scale and colour Doppler ultrasound versus magnetic resonance imaging for the prenatal diagnosis of placenta accreta. J Matern Fetal Neonatal Med. 2016;29:218–223.
- Expert Panel on MR Safety; Kanal E, Barkovich AJ, Bell C, et al. ACR guidance document on MR safe practices: 2013. J Magn Reson Imaging. 2013;37:501–530.
- Pekar-Zlotin M, Melcer Y, Maymon R, Jauniaux E. Secondtrimester levels of fetoplacental hormones among women with placenta accreta spectrum disorders. Int J Gynaecol Obstet. 2018;140:377–378.
- Lyell DJ, Faucett AM, Baer RJ, et al. Maternal serum markers, characteristics and morbidly adherent placenta in women with previa. J Perinatol. 2015;35:570–574.
- Irving FC, Hertig AT. A study of placenta accreta. Surg Gynec Obstet. 1937:64:178–200.
- Wu S, Kocherginsky M, Hibbard JU. Abnormal placentation: twenty-year analysis. Am J Obstet Gynecol. 2005;192:1458–1461.
- Hall T, Wax JR, Lucas FL, et al. Prenatal sonographic diagnosis of placenta accreta—impact on maternal and neonatal outcomes. J Clin Ultrasound. 2014;42:449–455.
- Bowman ZS, Eller AG, Kennedy AM, et al. Interobserver variability of sonography for prediction of placenta accreta. J Ultrasound Med. 2014;33:2153–2158.
- Silver RM. Abnormal placentation: placenta previa, vasa previa, and placenta accreta. Obstet Gynecol. 2015;126:654–668.
- Silver RM, Landon MB, Rouse DJ, et al; National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Maternal morbidity associated with multiple repeat cesarean deliveries. Obstet Gynecol. 2006;107:1226–1232.
- Rac MW, Moschos E, Wells CE, et al. Sonographic findings of morbidly adherent placenta in the first trimester. J Ultrasound Med. 2016;35:263–269.
- Ballas J, Pretorius D, Hull AD, et al. Identifying sonographic markers for placenta accreta in the first trimester. J Ultrasound Med. 2012;31:1835–1841.
- Comstock CH, Bronsteen RA. The antenatal diagnosis of placenta accreta. BJOG. 2014;121:171–181.
- Marshall NE, Fu R, Guise JM. Impact of multiple cesarean deliveries on maternal morbidity: a systematic review. Am J Obstet Gynecol. 2011;205:262.e1–e8.
- Baughman WC, Corteville JE, Shah RR. Placenta accreta: spectrum of US and MR imaging findings. Radiographics. 2008;28:1905–1916.
- Finberg HJ, Williams JW. Placenta accreta: prospective sonographic diagnosis in patients with placenta previa and prior cesarean section. J Ultrasound Med. 1992;11:333–343.
- D’Antonio F, Iacovella C, Bhide A. Prenatal identification of invasive placentation using ultrasound: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2013;42:509–517.
- Comstock CH, Love JJ Jr, Bronsteen RA, et al. Sonographic detection of placenta accreta in the second and third trimesters of pregnancy. Am J Obstet Gynecol. 2004;190:1135–1140.
- D’Antonio F, Palacios-Jaraquemada J, Lim PS, et al. Counseling in fetal medicine: evidence-based answers to clinical questions on morbidly adherent placenta. Ultrasound Obstet Gynecol. 2016;47:290–301.
- Hudon L, Belfort MA, Broome DR. Diagnosis and management of placenta percreta: a review. Obstet Gynecol Surv. 1998;53:509–517.
- Wong HS, Cheung YK, Zuccollo J, et al. Evaluation of sonographic diagnostic criteria for placenta accreta. J Clin Ultrasound. 2008;36:551–559.
- Jauniaux E, Collins S, Burton GJ. Placenta accreta spectrum: pathophysiology and evidence-based anatomy for prenatal ultrasound imaging. Am J Obstet Gynecol. 2018;218:75–87.
- Rac MW, Dashe JS, Wells CE, et al. Ultrasound predictors of placental invasion: the Placenta Accreta Index. Am J Obstet Gynecol. 2015;212:343.e1–e7.
- Pilloni E, Alemanno MG, Gaglioti P, et al. Accuracy of ultrasound in antenatal diagnosis of placental attachment disorders. Ultrasound Obstet Gynecol. 2016;47:302–307.
- Comstock CH. Antenatal diagnosis of placenta accreta: a review. Ultrasound Obstet Gynecol. 2005;26:89–96.
- Collins SL, Stevenson GN, Al-Khan A, et al. Three-dimensional power Doppler ultrasonography for diagnosing abnormally invasive placenta and quantifying the risk. Obstet Gynecol. 2015;126:645–653.
- Collins SL, Ashcroft A, Braun T, et al; European Working Group on Abnormally Invasive Placenta (EW-AIP). Proposal for standardized ultrasound descriptors of abnormally invasive placenta (AIP). Ultrasound Obstet Gynecol. 2016;47:271–275.
- Gilboa Y, Spira M, Mazaki-Tovi S, et al. A novel sonographic scoring system for antenatal risk assessment of obstetric complications in suspected morbidly adherent placenta. J Ultrasound Med. 2015;34:561–567.
- Tovbin J, Melcer Y, Shor S, et al. Prediction of morbidly adherent placenta using a scoring system. Ultrasound Obstet Gynecol. 2016;48:504–510.
- Jauniaux E, Collins SL, Jurkovic D, Burton GJ. Accreta placentation: a systematic review of prenatal ultrasound imaging and grading of villous invasiveness. Am J Obstet Gynecol. 2016:215:712–721.
- Familiari A, Liberati M, Lim P, et al. Diagnostic accuracy of magnetic resonance imaging in detecting the severity of abnormal invasive placenta: a systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2018;97:507–520.
- Rezk MA, Shawky M. Grey-scale and colour Doppler ultrasound versus magnetic resonance imaging for the prenatal diagnosis of placenta accreta. J Matern Fetal Neonatal Med. 2016;29:218–223.
- Expert Panel on MR Safety; Kanal E, Barkovich AJ, Bell C, et al. ACR guidance document on MR safe practices: 2013. J Magn Reson Imaging. 2013;37:501–530.
- Pekar-Zlotin M, Melcer Y, Maymon R, Jauniaux E. Secondtrimester levels of fetoplacental hormones among women with placenta accreta spectrum disorders. Int J Gynaecol Obstet. 2018;140:377–378.
- Lyell DJ, Faucett AM, Baer RJ, et al. Maternal serum markers, characteristics and morbidly adherent placenta in women with previa. J Perinatol. 2015;35:570–574.
Anti-TNF agents preferred for severe psoriasis in pregnancy
CHICAGO – according to Kenneth B. Gordon, MD, professor and chair of dermatology at Medical College of Wisconsin in Milwaukee.
“We always had this concept that psoriasis gets better during pregnancy, that you might have 20% or 30% of patients who might have a little bit of a flare or maintain, but most keep on getting better,” Dr. Gordon told attendees at the American Academy of Dermatology summer meeting.
But the majority doesn’t mean everyone. He shared the case of one pregnant woman who came to him with severe psoriasis, covering the whole of her inner thigh, to underscore that severe cases do happen in pregnancy.
“These are real situations, and when you talk about maternal health, this woman is uncomfortable, she can’t sleep, and she’s having huge stressors that are not only going to impact her and her pregnancy but also that impact her child,” Dr. Gordon said.
Dr. Gordon clarified that he is not referring to patients with limited psoriasis or those who respond to topicals or phototherapy. But because methotrexate or acitretin are “hands-off during pregnancy,” he said, the only systemic therapy available for serious cases besides biologics is cyclosporine, which has its own risks. “We know that [cyclosporine] is associated with preterm labor and preterm birth and significant low birth weight, so even in the best scenario, when we have someone with persistent severe psoriasis in pregnancy, our best agent has a lot of downsides.”
Too few data exist on anti–interleukin (IL)-17 or anti-IL-23 therapies to draw conclusions about their use, he said, and but gastroenterology and rheumatology have a fair amount of evidence on anti–tumor necrosis factor (TNF) therapies during pregnancy because it’s usually too risky to stop treating conditions such as Crohn’s with these drugs. Still, Dr. Gordon cautioned, much of the data on biologics in pregnancy are conflicting.
The question of what medications to use, and in whom, centers on balancing risks to the fetus from the medication versus risks from the condition.
“There are impacts on the fetus of having severe psoriasis, and it varies with severity of disease,” Dr. Gordon said. For example, data suggest an increased likelihood of low birth weight in children born to mothers with severe psoriasis, and that risk may extend to preterm birth as well, although “we don’t know exactly the magnitude of that effect.”
Meanwhile, the consensus from the literature throughout dermatology, rheumatology, and gastroenterology is that anti-TNF agents do not cause birth defects or affect risk of preterm birth or low birth weight.
“The bigger question is what’s the impact on the immune system of the child,” Dr. Gordon said. Data from a small Scandinavian study suggested no increased risk of allergies, infections, or similar immunologic outcomes, but evidence remains limited.
Research has shown that infants’ exposure to anti-TNF medications persists for 3-6 months after delivery, and the American Academy of Pediatrics recommends delaying immunization in children exposed to anti-TNF agents in pregnancy. But actual evidence on immunization outcomes shows no reduced immunogenicity in such children.
“Clearly there is persistence of drug in the child, but in fact you have normal responses to immunization,” Dr. Gordon said. “The pediatricians’ argument is not based on data of what actually happens in immunization; it’s based on the fact that the drug is there.”
So what’s the bottom line?
The National Psoriasis Foundation recommends moisturizers and topical corticosteroids as first-line therapy in pregnant women with psoriasis, followed by phototherapy for second-line treatment.
But some patients will need systemic therapy during pregnancy, although it’s “best not to introduce more medications than needed in pregnancy,” Dr. Gordon said. For women with a significant flare-up or very persistent volatile disease, NPF first recommends cyclosporine, but Dr. Gordon disagrees and would go with anti-TNF agents before cyclosporine.
Data show that certolizumab is not actively transported across the placenta therefore reducing fetal exposure, so Dr. Gordon would specifically use certolizumab first, all other things being equal.
“But if the patient has been on another anti-TNF that’s been working, I don’t really have an issue with staying with it,” he added.
Existing evidence so far shows no impact in terms of genetic abnormalities, birth weight, premature birth, or even infant immunizations from anti-TNF agents. But beyond those, “there is simply not enough information on pregnancy with other forms of biologic therapy to draw conclusions.” Dr. Gordon said.
Dr. Gordon disclosed that he has received grant support and/or honoraria from Abbvie, Amgen, Almirall, and Boehringer Ingelheim.
CHICAGO – according to Kenneth B. Gordon, MD, professor and chair of dermatology at Medical College of Wisconsin in Milwaukee.
“We always had this concept that psoriasis gets better during pregnancy, that you might have 20% or 30% of patients who might have a little bit of a flare or maintain, but most keep on getting better,” Dr. Gordon told attendees at the American Academy of Dermatology summer meeting.
But the majority doesn’t mean everyone. He shared the case of one pregnant woman who came to him with severe psoriasis, covering the whole of her inner thigh, to underscore that severe cases do happen in pregnancy.
“These are real situations, and when you talk about maternal health, this woman is uncomfortable, she can’t sleep, and she’s having huge stressors that are not only going to impact her and her pregnancy but also that impact her child,” Dr. Gordon said.
Dr. Gordon clarified that he is not referring to patients with limited psoriasis or those who respond to topicals or phototherapy. But because methotrexate or acitretin are “hands-off during pregnancy,” he said, the only systemic therapy available for serious cases besides biologics is cyclosporine, which has its own risks. “We know that [cyclosporine] is associated with preterm labor and preterm birth and significant low birth weight, so even in the best scenario, when we have someone with persistent severe psoriasis in pregnancy, our best agent has a lot of downsides.”
Too few data exist on anti–interleukin (IL)-17 or anti-IL-23 therapies to draw conclusions about their use, he said, and but gastroenterology and rheumatology have a fair amount of evidence on anti–tumor necrosis factor (TNF) therapies during pregnancy because it’s usually too risky to stop treating conditions such as Crohn’s with these drugs. Still, Dr. Gordon cautioned, much of the data on biologics in pregnancy are conflicting.
The question of what medications to use, and in whom, centers on balancing risks to the fetus from the medication versus risks from the condition.
“There are impacts on the fetus of having severe psoriasis, and it varies with severity of disease,” Dr. Gordon said. For example, data suggest an increased likelihood of low birth weight in children born to mothers with severe psoriasis, and that risk may extend to preterm birth as well, although “we don’t know exactly the magnitude of that effect.”
Meanwhile, the consensus from the literature throughout dermatology, rheumatology, and gastroenterology is that anti-TNF agents do not cause birth defects or affect risk of preterm birth or low birth weight.
“The bigger question is what’s the impact on the immune system of the child,” Dr. Gordon said. Data from a small Scandinavian study suggested no increased risk of allergies, infections, or similar immunologic outcomes, but evidence remains limited.
Research has shown that infants’ exposure to anti-TNF medications persists for 3-6 months after delivery, and the American Academy of Pediatrics recommends delaying immunization in children exposed to anti-TNF agents in pregnancy. But actual evidence on immunization outcomes shows no reduced immunogenicity in such children.
“Clearly there is persistence of drug in the child, but in fact you have normal responses to immunization,” Dr. Gordon said. “The pediatricians’ argument is not based on data of what actually happens in immunization; it’s based on the fact that the drug is there.”
So what’s the bottom line?
The National Psoriasis Foundation recommends moisturizers and topical corticosteroids as first-line therapy in pregnant women with psoriasis, followed by phototherapy for second-line treatment.
But some patients will need systemic therapy during pregnancy, although it’s “best not to introduce more medications than needed in pregnancy,” Dr. Gordon said. For women with a significant flare-up or very persistent volatile disease, NPF first recommends cyclosporine, but Dr. Gordon disagrees and would go with anti-TNF agents before cyclosporine.
Data show that certolizumab is not actively transported across the placenta therefore reducing fetal exposure, so Dr. Gordon would specifically use certolizumab first, all other things being equal.
“But if the patient has been on another anti-TNF that’s been working, I don’t really have an issue with staying with it,” he added.
Existing evidence so far shows no impact in terms of genetic abnormalities, birth weight, premature birth, or even infant immunizations from anti-TNF agents. But beyond those, “there is simply not enough information on pregnancy with other forms of biologic therapy to draw conclusions.” Dr. Gordon said.
Dr. Gordon disclosed that he has received grant support and/or honoraria from Abbvie, Amgen, Almirall, and Boehringer Ingelheim.
CHICAGO – according to Kenneth B. Gordon, MD, professor and chair of dermatology at Medical College of Wisconsin in Milwaukee.
“We always had this concept that psoriasis gets better during pregnancy, that you might have 20% or 30% of patients who might have a little bit of a flare or maintain, but most keep on getting better,” Dr. Gordon told attendees at the American Academy of Dermatology summer meeting.
But the majority doesn’t mean everyone. He shared the case of one pregnant woman who came to him with severe psoriasis, covering the whole of her inner thigh, to underscore that severe cases do happen in pregnancy.
“These are real situations, and when you talk about maternal health, this woman is uncomfortable, she can’t sleep, and she’s having huge stressors that are not only going to impact her and her pregnancy but also that impact her child,” Dr. Gordon said.
Dr. Gordon clarified that he is not referring to patients with limited psoriasis or those who respond to topicals or phototherapy. But because methotrexate or acitretin are “hands-off during pregnancy,” he said, the only systemic therapy available for serious cases besides biologics is cyclosporine, which has its own risks. “We know that [cyclosporine] is associated with preterm labor and preterm birth and significant low birth weight, so even in the best scenario, when we have someone with persistent severe psoriasis in pregnancy, our best agent has a lot of downsides.”
Too few data exist on anti–interleukin (IL)-17 or anti-IL-23 therapies to draw conclusions about their use, he said, and but gastroenterology and rheumatology have a fair amount of evidence on anti–tumor necrosis factor (TNF) therapies during pregnancy because it’s usually too risky to stop treating conditions such as Crohn’s with these drugs. Still, Dr. Gordon cautioned, much of the data on biologics in pregnancy are conflicting.
The question of what medications to use, and in whom, centers on balancing risks to the fetus from the medication versus risks from the condition.
“There are impacts on the fetus of having severe psoriasis, and it varies with severity of disease,” Dr. Gordon said. For example, data suggest an increased likelihood of low birth weight in children born to mothers with severe psoriasis, and that risk may extend to preterm birth as well, although “we don’t know exactly the magnitude of that effect.”
Meanwhile, the consensus from the literature throughout dermatology, rheumatology, and gastroenterology is that anti-TNF agents do not cause birth defects or affect risk of preterm birth or low birth weight.
“The bigger question is what’s the impact on the immune system of the child,” Dr. Gordon said. Data from a small Scandinavian study suggested no increased risk of allergies, infections, or similar immunologic outcomes, but evidence remains limited.
Research has shown that infants’ exposure to anti-TNF medications persists for 3-6 months after delivery, and the American Academy of Pediatrics recommends delaying immunization in children exposed to anti-TNF agents in pregnancy. But actual evidence on immunization outcomes shows no reduced immunogenicity in such children.
“Clearly there is persistence of drug in the child, but in fact you have normal responses to immunization,” Dr. Gordon said. “The pediatricians’ argument is not based on data of what actually happens in immunization; it’s based on the fact that the drug is there.”
So what’s the bottom line?
The National Psoriasis Foundation recommends moisturizers and topical corticosteroids as first-line therapy in pregnant women with psoriasis, followed by phototherapy for second-line treatment.
But some patients will need systemic therapy during pregnancy, although it’s “best not to introduce more medications than needed in pregnancy,” Dr. Gordon said. For women with a significant flare-up or very persistent volatile disease, NPF first recommends cyclosporine, but Dr. Gordon disagrees and would go with anti-TNF agents before cyclosporine.
Data show that certolizumab is not actively transported across the placenta therefore reducing fetal exposure, so Dr. Gordon would specifically use certolizumab first, all other things being equal.
“But if the patient has been on another anti-TNF that’s been working, I don’t really have an issue with staying with it,” he added.
Existing evidence so far shows no impact in terms of genetic abnormalities, birth weight, premature birth, or even infant immunizations from anti-TNF agents. But beyond those, “there is simply not enough information on pregnancy with other forms of biologic therapy to draw conclusions.” Dr. Gordon said.
Dr. Gordon disclosed that he has received grant support and/or honoraria from Abbvie, Amgen, Almirall, and Boehringer Ingelheim.
EXPERT ANALYSIS FROM SUMMER AAD 2018
Now is the time to be heard: October is Advocacy Month!
The American College of Obstetricians and Gynecologists (ACOG) and specifically the Junior Fellow College Advisory Council (JFCAC) are rolling out steps to help you make your voice heard. Starting October 1, head to acog.org/advocacy to check out the ACOG Physician Advocacy video to get inspired. (Or watch it here!) Whether you are a seasoned advocate or just getting started, ACOG and women across the country are counting on you!
Week 1 (October 1–7): Why I advocate
The focus of this week is on delving into topics that interest you, learning why advocacy is critically important, and developing your own message to advocate for women’s health.
- View advocacy videos here to understand what advocacy is and why it is so important.
- See ACOG’s 2018 list of legislative priorities here to find topics that inspire you.
Week 2 (October 8–14): Use your voice
Explore the multitude of platforms available today for amplifying your message. Learn to use social media smartly, get advice for how to write op-eds for local outlets, add your name to support current legislative efforts, and find out who your representatives are to schedule sit-down meetings.
- For tips on communicating with elected officials, click here.
- Connect with ACOG and your district on social media, and remember to use social media responsibly to advocate effectively. See this link for more information!
- Don’t forget to include #JFadvoMonth in your posts while highlighting your advocacy work on social media!
Continued to: Week 3 (October 15–19): Empower your patients
Week 3 (October 15–21): Empower your patients
As a physician, advocating for your patient extends into the clinic itself. Access toolkits, patient websites, handouts, and resources available through ACOG.
- Familiarize yourself with the Patient Page for videos, infographics, and FAQs that are useful resources for your patients.
- Toolkits for providers are available here—use these to enhance your practice and empower your patients!
Week 4 (October 22–28): Take it forward
Advocacy happens year-round. Be sure you are actively involved in ACOG’s efforts. Participate in calls to action and remember on November 6 to GET OUT THE VOTE!
- Participate in the annual Congressional Leadership Conference (March 10–12, 2019) in Washington, DC. Descend on Washington with hundreds of fellow ObGyns to advocate to Congress on important issues. For more information, click here.
- Donate to the Ob-GynPAC, ACOG’s political action committee dedicated to electing officials who support our specialty.
- Run for office! ACOG has resources to support you. Be on the lookout for opportunities to attend candidate workshops sponsored by the Ob-GynPAC!
Get active now!
We are at a critical moment for women’s health and the future of our specialty. Key issues nationally include advocating to Congress to move forward with bills in the Senate (S 1112) and House (HR 1318) to support efforts to reduce maternal mortality. (See this article for background information on these bills.)
To find your elected officials and take action now, click here and tell Congress to help prevent maternal mortality, defend patient protections, and improve access and quality of maternity care.
You can be an advocate for your patients and your profession. Your voice matters. Now is the time to be heard.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The American College of Obstetricians and Gynecologists (ACOG) and specifically the Junior Fellow College Advisory Council (JFCAC) are rolling out steps to help you make your voice heard. Starting October 1, head to acog.org/advocacy to check out the ACOG Physician Advocacy video to get inspired. (Or watch it here!) Whether you are a seasoned advocate or just getting started, ACOG and women across the country are counting on you!
Week 1 (October 1–7): Why I advocate
The focus of this week is on delving into topics that interest you, learning why advocacy is critically important, and developing your own message to advocate for women’s health.
- View advocacy videos here to understand what advocacy is and why it is so important.
- See ACOG’s 2018 list of legislative priorities here to find topics that inspire you.
Week 2 (October 8–14): Use your voice
Explore the multitude of platforms available today for amplifying your message. Learn to use social media smartly, get advice for how to write op-eds for local outlets, add your name to support current legislative efforts, and find out who your representatives are to schedule sit-down meetings.
- For tips on communicating with elected officials, click here.
- Connect with ACOG and your district on social media, and remember to use social media responsibly to advocate effectively. See this link for more information!
- Don’t forget to include #JFadvoMonth in your posts while highlighting your advocacy work on social media!
Continued to: Week 3 (October 15–19): Empower your patients
Week 3 (October 15–21): Empower your patients
As a physician, advocating for your patient extends into the clinic itself. Access toolkits, patient websites, handouts, and resources available through ACOG.
- Familiarize yourself with the Patient Page for videos, infographics, and FAQs that are useful resources for your patients.
- Toolkits for providers are available here—use these to enhance your practice and empower your patients!
Week 4 (October 22–28): Take it forward
Advocacy happens year-round. Be sure you are actively involved in ACOG’s efforts. Participate in calls to action and remember on November 6 to GET OUT THE VOTE!
- Participate in the annual Congressional Leadership Conference (March 10–12, 2019) in Washington, DC. Descend on Washington with hundreds of fellow ObGyns to advocate to Congress on important issues. For more information, click here.
- Donate to the Ob-GynPAC, ACOG’s political action committee dedicated to electing officials who support our specialty.
- Run for office! ACOG has resources to support you. Be on the lookout for opportunities to attend candidate workshops sponsored by the Ob-GynPAC!
Get active now!
We are at a critical moment for women’s health and the future of our specialty. Key issues nationally include advocating to Congress to move forward with bills in the Senate (S 1112) and House (HR 1318) to support efforts to reduce maternal mortality. (See this article for background information on these bills.)
To find your elected officials and take action now, click here and tell Congress to help prevent maternal mortality, defend patient protections, and improve access and quality of maternity care.
You can be an advocate for your patients and your profession. Your voice matters. Now is the time to be heard.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The American College of Obstetricians and Gynecologists (ACOG) and specifically the Junior Fellow College Advisory Council (JFCAC) are rolling out steps to help you make your voice heard. Starting October 1, head to acog.org/advocacy to check out the ACOG Physician Advocacy video to get inspired. (Or watch it here!) Whether you are a seasoned advocate or just getting started, ACOG and women across the country are counting on you!
Week 1 (October 1–7): Why I advocate
The focus of this week is on delving into topics that interest you, learning why advocacy is critically important, and developing your own message to advocate for women’s health.
- View advocacy videos here to understand what advocacy is and why it is so important.
- See ACOG’s 2018 list of legislative priorities here to find topics that inspire you.
Week 2 (October 8–14): Use your voice
Explore the multitude of platforms available today for amplifying your message. Learn to use social media smartly, get advice for how to write op-eds for local outlets, add your name to support current legislative efforts, and find out who your representatives are to schedule sit-down meetings.
- For tips on communicating with elected officials, click here.
- Connect with ACOG and your district on social media, and remember to use social media responsibly to advocate effectively. See this link for more information!
- Don’t forget to include #JFadvoMonth in your posts while highlighting your advocacy work on social media!
Continued to: Week 3 (October 15–19): Empower your patients
Week 3 (October 15–21): Empower your patients
As a physician, advocating for your patient extends into the clinic itself. Access toolkits, patient websites, handouts, and resources available through ACOG.
- Familiarize yourself with the Patient Page for videos, infographics, and FAQs that are useful resources for your patients.
- Toolkits for providers are available here—use these to enhance your practice and empower your patients!
Week 4 (October 22–28): Take it forward
Advocacy happens year-round. Be sure you are actively involved in ACOG’s efforts. Participate in calls to action and remember on November 6 to GET OUT THE VOTE!
- Participate in the annual Congressional Leadership Conference (March 10–12, 2019) in Washington, DC. Descend on Washington with hundreds of fellow ObGyns to advocate to Congress on important issues. For more information, click here.
- Donate to the Ob-GynPAC, ACOG’s political action committee dedicated to electing officials who support our specialty.
- Run for office! ACOG has resources to support you. Be on the lookout for opportunities to attend candidate workshops sponsored by the Ob-GynPAC!
Get active now!
We are at a critical moment for women’s health and the future of our specialty. Key issues nationally include advocating to Congress to move forward with bills in the Senate (S 1112) and House (HR 1318) to support efforts to reduce maternal mortality. (See this article for background information on these bills.)
To find your elected officials and take action now, click here and tell Congress to help prevent maternal mortality, defend patient protections, and improve access and quality of maternity care.
You can be an advocate for your patients and your profession. Your voice matters. Now is the time to be heard.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
ACOG lends support to bill promoting maternal mortality review committees
The Preventing Maternal Deaths Act of 2018 (H.R. 1318) was the subject of a Sept. 27 hearing of the House Energy and Commerce Health Subcommittee. The bill comes at a time when 700 women a year die as a result of pregnancy or pregnancy-related complications with a rate that is increasing, while 157 of 183 countries around the world are reporting decreasing rates of maternal mortality, according to ACOG.
The bill, authored by Rep. Jamie Herrera Beutler (R-Wash.) and Diana DeGette (D-Colo.) would allocate $58 million for each fiscal year from 2019 through 2023 to support the 33 existing states with maternal mortality review committees (MMRCs) and help the remaining 17 states develop them, as well as to standardize data collection across the nation.
The goal of having these committees in place is to “improve data collection and reporting around maternal mortality, and to develop or support surveillance systems at the local, state, and national level in order to better understand the burden of maternal complications,” a background memo on the hearing noted. “These surveillance efforts include identifying groups of women with disproportionately high rates of maternal mortality and identifying the determinants of disparities in maternal care, health risks, and health outcomes.”
Necessitating this legislation was a data point that was reiterated throughout the course of the hearing – that maternal mortality rates in the United States were on the rise.
“What’s both surprising and devastating is that, despite massive innovation and advances in health care and technology, we’ve experienced recent reports that have indicated that the number of women dying due to pregnancy complications is actually increasing,” Full Committee Chairman Greg Walden (R-Ore.) said in his opening remarks at the hearing. “According to the Centers for Disease Control and Prevention, maternal mortality rates in America have more than doubled since 1987. I think we are asking, how can that be? This is not a statistic any of us wants to hear.”
Chairman Walden acknowledged that there are questions as to whether the increase was a function of better data collection or whether it was an issue with the delivery of health care.
“The bill before us today will help us answer these really important questions and hopefully ensure that expectant newborn mothers receive even better care,” he said.
Lynne M. Coslett-Charlton, MD, ACOG Pennsylvania District legislative chair, offered the organization’s support for the bill.
MMRCs “are multidisciplinary groups of local experts in maternal and public health, as well as patient and community advocates, that closely examine maternal death cases and identify locally relevant ways to prevent future deaths,” she testified before the committee. “While traditional public health surveillance using vital statistics can tell us about trends and disparities, MMRCs are best positioned to comprehensively assess maternal deaths and identify opportunities for prevention.”
Dr. Coslett-Charlton added that to clearly understand why women are dying from preventable maternal complications, which she noted that 60% of maternal deaths are, “every state must have a robust MMRC. The Preventing Maternal Deaths Act will help us reach that goal, and ultimately improve maternal health across this nation,” as these committees review every maternal death and can make a determination as to whether they could have been preventable.
Additionally, the fact that black women face a significantly higher rate of maternal mortality was another data point highlighted during the hearing, further adding to the need for this bill that has bipartisan support and more than 170 cosponsors.
Rep. DeGette called it “one of the most striking aspects” that black women “are nearly four times as likely to experience a pregnancy-related death.”
Stacey D. Stewart, president of the March of Dimes, in her written testimony praised the inclusion in H.R. 1318 of a “demonstration project to determine how best to address disparities in maternal health outcomes.”
The Preventing Maternal Deaths Act of 2018 (H.R. 1318) was the subject of a Sept. 27 hearing of the House Energy and Commerce Health Subcommittee. The bill comes at a time when 700 women a year die as a result of pregnancy or pregnancy-related complications with a rate that is increasing, while 157 of 183 countries around the world are reporting decreasing rates of maternal mortality, according to ACOG.
The bill, authored by Rep. Jamie Herrera Beutler (R-Wash.) and Diana DeGette (D-Colo.) would allocate $58 million for each fiscal year from 2019 through 2023 to support the 33 existing states with maternal mortality review committees (MMRCs) and help the remaining 17 states develop them, as well as to standardize data collection across the nation.
The goal of having these committees in place is to “improve data collection and reporting around maternal mortality, and to develop or support surveillance systems at the local, state, and national level in order to better understand the burden of maternal complications,” a background memo on the hearing noted. “These surveillance efforts include identifying groups of women with disproportionately high rates of maternal mortality and identifying the determinants of disparities in maternal care, health risks, and health outcomes.”
Necessitating this legislation was a data point that was reiterated throughout the course of the hearing – that maternal mortality rates in the United States were on the rise.
“What’s both surprising and devastating is that, despite massive innovation and advances in health care and technology, we’ve experienced recent reports that have indicated that the number of women dying due to pregnancy complications is actually increasing,” Full Committee Chairman Greg Walden (R-Ore.) said in his opening remarks at the hearing. “According to the Centers for Disease Control and Prevention, maternal mortality rates in America have more than doubled since 1987. I think we are asking, how can that be? This is not a statistic any of us wants to hear.”
Chairman Walden acknowledged that there are questions as to whether the increase was a function of better data collection or whether it was an issue with the delivery of health care.
“The bill before us today will help us answer these really important questions and hopefully ensure that expectant newborn mothers receive even better care,” he said.
Lynne M. Coslett-Charlton, MD, ACOG Pennsylvania District legislative chair, offered the organization’s support for the bill.
MMRCs “are multidisciplinary groups of local experts in maternal and public health, as well as patient and community advocates, that closely examine maternal death cases and identify locally relevant ways to prevent future deaths,” she testified before the committee. “While traditional public health surveillance using vital statistics can tell us about trends and disparities, MMRCs are best positioned to comprehensively assess maternal deaths and identify opportunities for prevention.”
Dr. Coslett-Charlton added that to clearly understand why women are dying from preventable maternal complications, which she noted that 60% of maternal deaths are, “every state must have a robust MMRC. The Preventing Maternal Deaths Act will help us reach that goal, and ultimately improve maternal health across this nation,” as these committees review every maternal death and can make a determination as to whether they could have been preventable.
Additionally, the fact that black women face a significantly higher rate of maternal mortality was another data point highlighted during the hearing, further adding to the need for this bill that has bipartisan support and more than 170 cosponsors.
Rep. DeGette called it “one of the most striking aspects” that black women “are nearly four times as likely to experience a pregnancy-related death.”
Stacey D. Stewart, president of the March of Dimes, in her written testimony praised the inclusion in H.R. 1318 of a “demonstration project to determine how best to address disparities in maternal health outcomes.”
The Preventing Maternal Deaths Act of 2018 (H.R. 1318) was the subject of a Sept. 27 hearing of the House Energy and Commerce Health Subcommittee. The bill comes at a time when 700 women a year die as a result of pregnancy or pregnancy-related complications with a rate that is increasing, while 157 of 183 countries around the world are reporting decreasing rates of maternal mortality, according to ACOG.
The bill, authored by Rep. Jamie Herrera Beutler (R-Wash.) and Diana DeGette (D-Colo.) would allocate $58 million for each fiscal year from 2019 through 2023 to support the 33 existing states with maternal mortality review committees (MMRCs) and help the remaining 17 states develop them, as well as to standardize data collection across the nation.
The goal of having these committees in place is to “improve data collection and reporting around maternal mortality, and to develop or support surveillance systems at the local, state, and national level in order to better understand the burden of maternal complications,” a background memo on the hearing noted. “These surveillance efforts include identifying groups of women with disproportionately high rates of maternal mortality and identifying the determinants of disparities in maternal care, health risks, and health outcomes.”
Necessitating this legislation was a data point that was reiterated throughout the course of the hearing – that maternal mortality rates in the United States were on the rise.
“What’s both surprising and devastating is that, despite massive innovation and advances in health care and technology, we’ve experienced recent reports that have indicated that the number of women dying due to pregnancy complications is actually increasing,” Full Committee Chairman Greg Walden (R-Ore.) said in his opening remarks at the hearing. “According to the Centers for Disease Control and Prevention, maternal mortality rates in America have more than doubled since 1987. I think we are asking, how can that be? This is not a statistic any of us wants to hear.”
Chairman Walden acknowledged that there are questions as to whether the increase was a function of better data collection or whether it was an issue with the delivery of health care.
“The bill before us today will help us answer these really important questions and hopefully ensure that expectant newborn mothers receive even better care,” he said.
Lynne M. Coslett-Charlton, MD, ACOG Pennsylvania District legislative chair, offered the organization’s support for the bill.
MMRCs “are multidisciplinary groups of local experts in maternal and public health, as well as patient and community advocates, that closely examine maternal death cases and identify locally relevant ways to prevent future deaths,” she testified before the committee. “While traditional public health surveillance using vital statistics can tell us about trends and disparities, MMRCs are best positioned to comprehensively assess maternal deaths and identify opportunities for prevention.”
Dr. Coslett-Charlton added that to clearly understand why women are dying from preventable maternal complications, which she noted that 60% of maternal deaths are, “every state must have a robust MMRC. The Preventing Maternal Deaths Act will help us reach that goal, and ultimately improve maternal health across this nation,” as these committees review every maternal death and can make a determination as to whether they could have been preventable.
Additionally, the fact that black women face a significantly higher rate of maternal mortality was another data point highlighted during the hearing, further adding to the need for this bill that has bipartisan support and more than 170 cosponsors.
Rep. DeGette called it “one of the most striking aspects” that black women “are nearly four times as likely to experience a pregnancy-related death.”
Stacey D. Stewart, president of the March of Dimes, in her written testimony praised the inclusion in H.R. 1318 of a “demonstration project to determine how best to address disparities in maternal health outcomes.”
REPORTING FROM A HOUSE ENERGY AND COMMERCE HEALTH SUBCOMMITTEE HEARING
Abortion, the travel ban, and other top Supreme Court rulings affecting your practice
The 2017−2018 term of the Supreme Court of the United States (SCOTUS) was momentous. Justice Anthony Kennedy, who had been the deciding vote in most of the 5 to 4 cases for a generation, announced his retirement as of July 31, 2018. In addition, the Court decided a number of cases of interest to ObGyns. In this article we review some of those cases, as well as consider the future of the Court without Justice Kennedy. In selecting cases, we have given special attention to those in which national medical organizations filed amicus briefs. These “amicus curiae” or “friend of the court” briefs are filed by an entity who is not party to a case but wants to provide information or views to the court.

1. Abortion rulings

The Court decided 2 abortion cases and rejected a request to hear a third.
National Institute of Family and Life Advocates v Becerra
In this case,1 the Court struck down a California law that required pregnancy crisis centers not offering abortions (generally operated by pro-life groups) to provide special notices to clients.2
At stake. These notices would inform clients that California provides free or low-cost services, including abortions, and provide a phone number to call for those services.
There were many amicus briefs filed in this case, including those by the American College of Obstetricians and Gynecologists (ACOG) and other specialty boards,3 as well as the American Association of Pro-Life Obstetricians and Gynecologists and other pro-life organizations.4 ACOG’s brief argued that the California-required notice facilitates the goal of allowing women to receive medical services without harmful delay.
Final ruling. The Court held that the law required clinics to engage in speech with which the clinics disagreed (known as “compelled speech”). It also noted that California disclosure requirements were “wildly underinclusive” because they apply only to some clinics. The majority felt that there was no strong state interest in compelling this speech because there were other alternatives for the state to provide information about the availability of abortion and other services. The Court found that the clinics were likely to succeed on the merits of their claims of a First Amendment (free speech) violation.
Right to abortion for illegal immigrants in custody
A very unusual abortion case involved “Jane Doe,” a minor who was at 8 weeks’ gestation when she illegally crossed the border into the United States.5 She was placed in a federally-funded shelter where she requested an abortion. The facility denied that request.
At stake. Legal argument ensued about releasing her to another facility for an abortion, as the argument was made that pregnant minors who are apprehended crossing into the United States illegally and placed into the custody of federal officials should have abortion access. A lower Court of Appeals ruled against the Trump Administration’s policy of denying abortions to undocumented minors in federal custody. During the process of the federal government taking the case to the Supreme Court, the attorneys for Doe moved appointments around and, without notice, the abortion was performed. Government attorneys said that Doe’s attorneys made “what appear to be material misrepresentations and omissions” designed to “thwart [the Supreme Court’s] review” of the case.5 The government requested that the Court vacate the order of the Court of Appeals so that it could not be used as precedent.
Final ruling. The Court granted the governments request to vacate the lower court’s order because the minor was no longer pregnant and the order was therefore moot. The basic issue in this case (the right of in-custody minors to access abortions) remains unresolved. It is likely to appear before the Court in the future.
Continue to: Access to medical abortions
Access to medical abortions
An Arkansas law requires that a physician administering medical abortions contract with a physician who has admitting privileges at a hospital (a “contracted physician”).
At stake. Planned Parenthood filed suit challenging the requirement as unnecessary and harmful because it would result in the closure of 2 of the 3 abortion providers in Arkansas. ACOG filed an amicus brief urging the Supreme Court to consider the case.6 (Technically this was a petition for a Writ of Certiorari, the procedure by which the Court accepts cases. It accepts only about 1% of applications.) ACOG argued that there was no medical reason for the contracted physician requirement, and noted the harm it would do to women who would not have access to abortions.
Final ruling. On May 29, 2018, the Court declined to hear the case. This case is still active in the lower courts and may eventually return to the Supreme Court.

2. The patent system

The medical profession depends on the patent system to encourage the discovery of new patents efficiently and effectively. In 2012, Congress passed the America Invents Act7 that authorizes a petition by anyone other than the patent holder to the Patent and Trademark Office (PTO) for an “inter partes review” to assess a challenge to the patent’s legitimacy. If the PTO determines that there may be merit to the claim, the Patent Trial and Appeal Board undertakes a trial-like review process that may validate, invalidate, or amend the patent. The Board’s decision is subject to appellate court review.
At stake. This term, the inter partes review was challenged as unconstitutional on technical bases.8
Final ruling. The Court rejected this claim and approved the current administrative inter partes review process. The Court determined that once the Patent Office takes a petition challenging a patent, it must decide all of the claims against the patent, not pick and choose which elements of the challenge to evaluate.9 The Court’s decision upheld patent-review reform, but will require the Patent Office to tweak its procedures.

3. The travel ban

ACOG, the American Medical Association (AMA), the Association of American Medical Colleges, and more than 30 other health care and specialty associations filed an amicus brief regarding one of the most anticipated cases of the term—the “travel ban.”10
At stake. The essential argument of these organizations was that the US health care system depends on professionals from other countries. An efficient and fair immigration program is, therefore, important to advance the nation’s “health security.” During the 2016−2017 term, the Court considered but then removed the issue from its calendar when the Trump Administration issued a revised travel ban.11
In September 2017, President Trump’s proclamation imposed a range of entry restrictions on the citizens of 8 countries, most (but not all) of which are predominantly Muslim. The government indicated that, in a study by Homeland Security and the State Department, these countries were identified as having especially deficient information-sharing practices and presented national security concerns. Trump v Hawaii12 challenged this proclamation.
Final ruling. The majority of the Court upheld the travel ban. For the 5-Justice majority led by Chief Justice Roberts, the case came down to 3 things:
- The Constitution and the laws passed by Congress of necessity give the President great authority to engage in foreign policy, including policies regarding entry into the country.
- The courts are very reluctant to get into the substance of foreign affairs—they are not equipped to know in detail what the facts are, and things change very fast.
- If courts start tinkering with foreign policy and things turn bad, it will appear that the courts are to blame and were interfering in an area about which they are not competent.
Continue to: 4. Did a credit card case add risk to health insurance markets?

It was just a credit card case, but one in which the AMA saw a real risk to regulation of the health insurance markets.
At stake. Technically, Ohio v American Express concerned a claim that American Express (AmEx) violated antitrust laws when it prohibited merchants taking its credit card from “steering” customers to cards with lower fees.13 AmEx maintained that, because credit cards were a special kind of “2-sided” market (connecting merchants on one side and customers on the other), antitrust laws should not be strictly enforced.
The AMA noticed that special rules regarding 2-sided markets might apply to health insurance, and it submitted an amicus brief14 that noted: “dominant health insurance networks … have imposed and could further impose rules or effectively erect barriers that prohibit physicians from referring patients to certain specialists, particularly out-of-network specialists, for innovative and even necessary medical tests.”14 It concluded that the antitrust rule AmEx was suggesting would make it nearly impossible to challenge these unfair provisions in health insurance arrangements.
Final ruling. The Court, however, accepted the AmEx position, making it very difficult to develop an antitrust case against 2-sided markets. It remains to be seen the degree to which the AMA concern about health insurance markets will be realized.

5. Gay wedding and a bakeshop

At stake. In Masterpiece Cakeshop v Colorado, a cakemaker declined to design a cake for a gay wedding and had been disciplined under Colorado law for discriminating against the couple based on sexual orientation.15
Final ruling. The Court, however, found that the Colorado regulators had, ironically, shown such religious animus in the way they treated the baker that the regulators themselves had discriminated on the basis of religion. As a result, the Court reversed the sanctions against the baker.
This decision was fairly narrow. It does not, for example, stand for the proposition that there may be a general religious exception to antidiscrimination laws. The question of broader religious or free-speech objections to antidiscrimination laws remains for another time.
Amicus brief. It was interesting that the American College of Pediatricians, American Association of Pro-Life Obstetricians and Gynecologists, and others, filed an amicus brief to report with concern the “demands that individual medical professionals must perform, assist with, or facilitate abortions, without regard to the teachings of their own faiths, consciences, and convictions.”16 The brief also noted that “issues in the present case implicate the fundamental rights of health care professionals, and to respectfully urge that the Court should by no means permit any weakening or qualification of well-established protections against compelled speech, and of free exercise” of religion.16
Arbitration. The Court upheld, as it has in most recent terms, another arbitration agreement.1 This case concerned an employment agreement in which employees consented to submit to arbitration rather than file lawsuits and not use class action claims.
Search of cell-phone location. Cell phones, whenever turned on, connect with cell towers that record the phone’s location several times a minute. Cell companies store this information, creating a virtual map of where the owner is at all times. The Federal Bureau of Investigation asked a cell company for location information for several people during a 127-day period in which robberies were committed.2 The Court held that the search was illegal in the absence of a warrant.
Public employee unions. The Court held that agency (fair share) fees, in which public employees who are not union members can be required to pay dues for the bargaining and grievance activities (from which they generally benefit), violate the First Amendment. The majority held that forcing public employees to pay fees to unions requires the employees, through those fees, to engage in political activities with which they disagree.3 This is a form of compelled speech, which the Court found violates the First Amendment. Health care professionals who are public employees in positions that have union representation will probably have the opportunity to opt out of agency agreements.
Internet sales tax. The Court permitted states to charge sales tax on out-of-state Internet purchases.4 In doing so, a state may require out-of-state companies to collect taxes on sales to its residents.
References
- Epic Systems Corp. v Lewis, 584 US 16 285 (2018).
- Carpenter v United States, 585 US 16 402 (2018).
- Janus v State, County, and Municipal Employees, 585 US 16 1466 (2018).
- South Dakota v Wayfair, Inc, 585 US 17 494 (2018).
Clues to the future
During the term that ran from October 2, 2017, through June 27, 2018, the Court issued 72 decisions. An unusually high proportion of cases (26%; 19 cases) were decided on a 5 to 4 vote. Last term, the rate of 5 to 4 decisions was 10%; the 6-year average was 18%. The unanimous decision rate was 39% this term, compared with 59% last term, and 50% on average.
The rate of 5 to 4 cases provides a clue about the Court’s general direction. The number of times each Justice was in the majority in those nineteen 5 to 4 decisions included: Chief Justice Roberts, 17; and Justices Kennedy, 16; Gorsuch, 16; Thomas, 15; and Alito, 15; compared with Justices Ginsburg, 5; Breyer, 4; Sotomayor, 4; and Kagan, 3.
The Court convened on October 1, 2018. At this writing, whether the new term starts with 8 or 9 justices remains a question. President Trump nominated Brett Kavanaugh, JD, to take Justice Kennedy’s place on the Court. His professional qualifications and experience appear to make him qualified for a position on the Court, but as we have seen, there are many other elements that go into confirming a Justice’s nomination.
Justice Anthony Kennedy was the deciding vote in the overwhelming majority of the 5 to 4 decisions in 20 of his 30 years on the Court. The areas in which he had an especially important impact include1:
- Gay rights. Justice Kennedy wrote the opinions (usually 5 to 4 decisions) in a number of groundbreaking gay-rights cases, including decriminalizing homosexual conduct, striking down the Defense of Marriage Act, and finding that the Constitution requires states to recognize gay marriage.
- The death penalty. Justice Kennedy wrote decisions that prohibited states from imposing the death penalty for any crime other than murder, for defendants who were under 18 when they committed the crime, and for defendants with serious developmental disabilities. He expressed reservations about long-term solitary confinement, but did not have a case that allowed him to decide its constitutionality.
- The First Amendment. Early in his service on the Court, he held that the First Amendment protected flag burning as a form of speech. He decided many important freespeech and freedom-of-religion cases that have set a standard for protecting those fundamental freedoms.
- Use of health and social science data. Justice Kennedy was more open to mental health information and cited it more often than most other Justices.
- Abortion rights? Many commentators would add protecting the right to choose to have an abortion to the above list. Justice Kennedy was a central figure in one case that declined to back away from Roe v Wade, and joined a more recent decision that struck down a Texas law that created an undue burden on women seeking abortion. Plus, he also voted to uphold abortion restrictions, such as “partial-birth-abortion laws.” So there is a good argument for including abortion rights on the list, although he did not break new ground.
Justice Kennedy as a person
Outside the courtroom, Justice Kennedy is a person of great warmth and compassion. He is a natural teacher and spends a great deal of time with students. When asked how he would like to be remembered, Justice Kennedy once replied, “Somebody who’s decent, and honest, and fair, and who’s absolutely committed to the proposition that freedom is America’s gift to the rest of the world.” I agree with that assessment.
STEVEN R. SMITH, MS, JD
Reference
- South Dakota v Wayfair, Inc, 585 US (2018)
Next term, the Court is scheduled to hear cases regarding pharmaceutical liability, double jeopardy, sex-offender registration, expert witnesses, Social Security disability benefits, and the Age Discrimination in Employment Act. There will be at least 3 arbitration cases. Health care and reproductive rights will continue to be an important part of the Court’s docket.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- National Institute of Family and Life Advocates v Becerra, 585 US 16 1140 (2018).
- California Reproductive Freedom, Accountability, Comprehensive Care, and Transparency Act (FACT Act), Cal. Health & Safety Code Ann. §123470 et seq. (West 2018).
- Brief amici curiae of American Academy of Pediatrics, et al. in National Institute of Family and Life Advocates v Becerra, February 27, 2018.
- Brief amici curiae of American Association of Pro-Life Obstetricians & Gynecologists, et al. in National Institute of Family and Life Advocates v Becerra, January 16, 2018.
- Azar v Garza, 584 US 17 654 (2018).
- Brief amici curiae of American College of Obstetricians and Gynecologists and American Public Health Association in Planned Parenthood of Arkansas and Eastern Oklahoma v Jegley, February 1, 2018.
- Chapter 31, Inter Partes Review. United States Code. Title 35: Patents. Part III, Patents and protection of patents. 2012 Ed. 35 USC 311–319.
- Oil States Energy Services, LLC v Greene’s Energy Group, LLC, 584 US 16 712 (2018).
- SAS Institute Inc. v Iancu, 584 US 16 969 (2018).
- Brief for Association of American Medical Colleges and Others as Amici Curiae Supporting Respondents, Trump v Hawaii. https://www.supremecourt.gov/Docket PDF/17/ 17-965/40128/20180327105855912_17-965%20Amicus%20Br.%20Proclamation.pdf. Accessed September 21, 2018.
- Smith SR, Sanfilippo JS. Supreme Court decisions in 2017 that affected your practice. OBG Manag. 2017;29(12)44–47.
- Trump v Hawaii, 585 US 17 965 (2018).
- Ohio v American Express Co, 585 US 16 1454 (2018).
- Brief amici curiae of American Medical Association and Ohio State Medical Association in Ohio v American Express, December 24, 2017.
- Masterpiece Cakeshop, Ltd. v Colorado Civil Rights Commission, 584 US 16 111 (2018).
- Brief amici curiae of American College of Pediatricians, et al. in Masterpiece Cakeshop v Colorado Civil Rights Commission, September 7, 2017.
The 2017−2018 term of the Supreme Court of the United States (SCOTUS) was momentous. Justice Anthony Kennedy, who had been the deciding vote in most of the 5 to 4 cases for a generation, announced his retirement as of July 31, 2018. In addition, the Court decided a number of cases of interest to ObGyns. In this article we review some of those cases, as well as consider the future of the Court without Justice Kennedy. In selecting cases, we have given special attention to those in which national medical organizations filed amicus briefs. These “amicus curiae” or “friend of the court” briefs are filed by an entity who is not party to a case but wants to provide information or views to the court.

1. Abortion rulings

The Court decided 2 abortion cases and rejected a request to hear a third.
National Institute of Family and Life Advocates v Becerra
In this case,1 the Court struck down a California law that required pregnancy crisis centers not offering abortions (generally operated by pro-life groups) to provide special notices to clients.2
At stake. These notices would inform clients that California provides free or low-cost services, including abortions, and provide a phone number to call for those services.
There were many amicus briefs filed in this case, including those by the American College of Obstetricians and Gynecologists (ACOG) and other specialty boards,3 as well as the American Association of Pro-Life Obstetricians and Gynecologists and other pro-life organizations.4 ACOG’s brief argued that the California-required notice facilitates the goal of allowing women to receive medical services without harmful delay.
Final ruling. The Court held that the law required clinics to engage in speech with which the clinics disagreed (known as “compelled speech”). It also noted that California disclosure requirements were “wildly underinclusive” because they apply only to some clinics. The majority felt that there was no strong state interest in compelling this speech because there were other alternatives for the state to provide information about the availability of abortion and other services. The Court found that the clinics were likely to succeed on the merits of their claims of a First Amendment (free speech) violation.
Right to abortion for illegal immigrants in custody
A very unusual abortion case involved “Jane Doe,” a minor who was at 8 weeks’ gestation when she illegally crossed the border into the United States.5 She was placed in a federally-funded shelter where she requested an abortion. The facility denied that request.
At stake. Legal argument ensued about releasing her to another facility for an abortion, as the argument was made that pregnant minors who are apprehended crossing into the United States illegally and placed into the custody of federal officials should have abortion access. A lower Court of Appeals ruled against the Trump Administration’s policy of denying abortions to undocumented minors in federal custody. During the process of the federal government taking the case to the Supreme Court, the attorneys for Doe moved appointments around and, without notice, the abortion was performed. Government attorneys said that Doe’s attorneys made “what appear to be material misrepresentations and omissions” designed to “thwart [the Supreme Court’s] review” of the case.5 The government requested that the Court vacate the order of the Court of Appeals so that it could not be used as precedent.
Final ruling. The Court granted the governments request to vacate the lower court’s order because the minor was no longer pregnant and the order was therefore moot. The basic issue in this case (the right of in-custody minors to access abortions) remains unresolved. It is likely to appear before the Court in the future.
Continue to: Access to medical abortions
Access to medical abortions
An Arkansas law requires that a physician administering medical abortions contract with a physician who has admitting privileges at a hospital (a “contracted physician”).
At stake. Planned Parenthood filed suit challenging the requirement as unnecessary and harmful because it would result in the closure of 2 of the 3 abortion providers in Arkansas. ACOG filed an amicus brief urging the Supreme Court to consider the case.6 (Technically this was a petition for a Writ of Certiorari, the procedure by which the Court accepts cases. It accepts only about 1% of applications.) ACOG argued that there was no medical reason for the contracted physician requirement, and noted the harm it would do to women who would not have access to abortions.
Final ruling. On May 29, 2018, the Court declined to hear the case. This case is still active in the lower courts and may eventually return to the Supreme Court.

2. The patent system

The medical profession depends on the patent system to encourage the discovery of new patents efficiently and effectively. In 2012, Congress passed the America Invents Act7 that authorizes a petition by anyone other than the patent holder to the Patent and Trademark Office (PTO) for an “inter partes review” to assess a challenge to the patent’s legitimacy. If the PTO determines that there may be merit to the claim, the Patent Trial and Appeal Board undertakes a trial-like review process that may validate, invalidate, or amend the patent. The Board’s decision is subject to appellate court review.
At stake. This term, the inter partes review was challenged as unconstitutional on technical bases.8
Final ruling. The Court rejected this claim and approved the current administrative inter partes review process. The Court determined that once the Patent Office takes a petition challenging a patent, it must decide all of the claims against the patent, not pick and choose which elements of the challenge to evaluate.9 The Court’s decision upheld patent-review reform, but will require the Patent Office to tweak its procedures.

3. The travel ban

ACOG, the American Medical Association (AMA), the Association of American Medical Colleges, and more than 30 other health care and specialty associations filed an amicus brief regarding one of the most anticipated cases of the term—the “travel ban.”10
At stake. The essential argument of these organizations was that the US health care system depends on professionals from other countries. An efficient and fair immigration program is, therefore, important to advance the nation’s “health security.” During the 2016−2017 term, the Court considered but then removed the issue from its calendar when the Trump Administration issued a revised travel ban.11
In September 2017, President Trump’s proclamation imposed a range of entry restrictions on the citizens of 8 countries, most (but not all) of which are predominantly Muslim. The government indicated that, in a study by Homeland Security and the State Department, these countries were identified as having especially deficient information-sharing practices and presented national security concerns. Trump v Hawaii12 challenged this proclamation.
Final ruling. The majority of the Court upheld the travel ban. For the 5-Justice majority led by Chief Justice Roberts, the case came down to 3 things:
- The Constitution and the laws passed by Congress of necessity give the President great authority to engage in foreign policy, including policies regarding entry into the country.
- The courts are very reluctant to get into the substance of foreign affairs—they are not equipped to know in detail what the facts are, and things change very fast.
- If courts start tinkering with foreign policy and things turn bad, it will appear that the courts are to blame and were interfering in an area about which they are not competent.
Continue to: 4. Did a credit card case add risk to health insurance markets?

It was just a credit card case, but one in which the AMA saw a real risk to regulation of the health insurance markets.
At stake. Technically, Ohio v American Express concerned a claim that American Express (AmEx) violated antitrust laws when it prohibited merchants taking its credit card from “steering” customers to cards with lower fees.13 AmEx maintained that, because credit cards were a special kind of “2-sided” market (connecting merchants on one side and customers on the other), antitrust laws should not be strictly enforced.
The AMA noticed that special rules regarding 2-sided markets might apply to health insurance, and it submitted an amicus brief14 that noted: “dominant health insurance networks … have imposed and could further impose rules or effectively erect barriers that prohibit physicians from referring patients to certain specialists, particularly out-of-network specialists, for innovative and even necessary medical tests.”14 It concluded that the antitrust rule AmEx was suggesting would make it nearly impossible to challenge these unfair provisions in health insurance arrangements.
Final ruling. The Court, however, accepted the AmEx position, making it very difficult to develop an antitrust case against 2-sided markets. It remains to be seen the degree to which the AMA concern about health insurance markets will be realized.

5. Gay wedding and a bakeshop

At stake. In Masterpiece Cakeshop v Colorado, a cakemaker declined to design a cake for a gay wedding and had been disciplined under Colorado law for discriminating against the couple based on sexual orientation.15
Final ruling. The Court, however, found that the Colorado regulators had, ironically, shown such religious animus in the way they treated the baker that the regulators themselves had discriminated on the basis of religion. As a result, the Court reversed the sanctions against the baker.
This decision was fairly narrow. It does not, for example, stand for the proposition that there may be a general religious exception to antidiscrimination laws. The question of broader religious or free-speech objections to antidiscrimination laws remains for another time.
Amicus brief. It was interesting that the American College of Pediatricians, American Association of Pro-Life Obstetricians and Gynecologists, and others, filed an amicus brief to report with concern the “demands that individual medical professionals must perform, assist with, or facilitate abortions, without regard to the teachings of their own faiths, consciences, and convictions.”16 The brief also noted that “issues in the present case implicate the fundamental rights of health care professionals, and to respectfully urge that the Court should by no means permit any weakening or qualification of well-established protections against compelled speech, and of free exercise” of religion.16
Arbitration. The Court upheld, as it has in most recent terms, another arbitration agreement.1 This case concerned an employment agreement in which employees consented to submit to arbitration rather than file lawsuits and not use class action claims.
Search of cell-phone location. Cell phones, whenever turned on, connect with cell towers that record the phone’s location several times a minute. Cell companies store this information, creating a virtual map of where the owner is at all times. The Federal Bureau of Investigation asked a cell company for location information for several people during a 127-day period in which robberies were committed.2 The Court held that the search was illegal in the absence of a warrant.
Public employee unions. The Court held that agency (fair share) fees, in which public employees who are not union members can be required to pay dues for the bargaining and grievance activities (from which they generally benefit), violate the First Amendment. The majority held that forcing public employees to pay fees to unions requires the employees, through those fees, to engage in political activities with which they disagree.3 This is a form of compelled speech, which the Court found violates the First Amendment. Health care professionals who are public employees in positions that have union representation will probably have the opportunity to opt out of agency agreements.
Internet sales tax. The Court permitted states to charge sales tax on out-of-state Internet purchases.4 In doing so, a state may require out-of-state companies to collect taxes on sales to its residents.
References
- Epic Systems Corp. v Lewis, 584 US 16 285 (2018).
- Carpenter v United States, 585 US 16 402 (2018).
- Janus v State, County, and Municipal Employees, 585 US 16 1466 (2018).
- South Dakota v Wayfair, Inc, 585 US 17 494 (2018).
Clues to the future
During the term that ran from October 2, 2017, through June 27, 2018, the Court issued 72 decisions. An unusually high proportion of cases (26%; 19 cases) were decided on a 5 to 4 vote. Last term, the rate of 5 to 4 decisions was 10%; the 6-year average was 18%. The unanimous decision rate was 39% this term, compared with 59% last term, and 50% on average.
The rate of 5 to 4 cases provides a clue about the Court’s general direction. The number of times each Justice was in the majority in those nineteen 5 to 4 decisions included: Chief Justice Roberts, 17; and Justices Kennedy, 16; Gorsuch, 16; Thomas, 15; and Alito, 15; compared with Justices Ginsburg, 5; Breyer, 4; Sotomayor, 4; and Kagan, 3.
The Court convened on October 1, 2018. At this writing, whether the new term starts with 8 or 9 justices remains a question. President Trump nominated Brett Kavanaugh, JD, to take Justice Kennedy’s place on the Court. His professional qualifications and experience appear to make him qualified for a position on the Court, but as we have seen, there are many other elements that go into confirming a Justice’s nomination.
Justice Anthony Kennedy was the deciding vote in the overwhelming majority of the 5 to 4 decisions in 20 of his 30 years on the Court. The areas in which he had an especially important impact include1:
- Gay rights. Justice Kennedy wrote the opinions (usually 5 to 4 decisions) in a number of groundbreaking gay-rights cases, including decriminalizing homosexual conduct, striking down the Defense of Marriage Act, and finding that the Constitution requires states to recognize gay marriage.
- The death penalty. Justice Kennedy wrote decisions that prohibited states from imposing the death penalty for any crime other than murder, for defendants who were under 18 when they committed the crime, and for defendants with serious developmental disabilities. He expressed reservations about long-term solitary confinement, but did not have a case that allowed him to decide its constitutionality.
- The First Amendment. Early in his service on the Court, he held that the First Amendment protected flag burning as a form of speech. He decided many important freespeech and freedom-of-religion cases that have set a standard for protecting those fundamental freedoms.
- Use of health and social science data. Justice Kennedy was more open to mental health information and cited it more often than most other Justices.
- Abortion rights? Many commentators would add protecting the right to choose to have an abortion to the above list. Justice Kennedy was a central figure in one case that declined to back away from Roe v Wade, and joined a more recent decision that struck down a Texas law that created an undue burden on women seeking abortion. Plus, he also voted to uphold abortion restrictions, such as “partial-birth-abortion laws.” So there is a good argument for including abortion rights on the list, although he did not break new ground.
Justice Kennedy as a person
Outside the courtroom, Justice Kennedy is a person of great warmth and compassion. He is a natural teacher and spends a great deal of time with students. When asked how he would like to be remembered, Justice Kennedy once replied, “Somebody who’s decent, and honest, and fair, and who’s absolutely committed to the proposition that freedom is America’s gift to the rest of the world.” I agree with that assessment.
STEVEN R. SMITH, MS, JD
Reference
- South Dakota v Wayfair, Inc, 585 US (2018)
Next term, the Court is scheduled to hear cases regarding pharmaceutical liability, double jeopardy, sex-offender registration, expert witnesses, Social Security disability benefits, and the Age Discrimination in Employment Act. There will be at least 3 arbitration cases. Health care and reproductive rights will continue to be an important part of the Court’s docket.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The 2017−2018 term of the Supreme Court of the United States (SCOTUS) was momentous. Justice Anthony Kennedy, who had been the deciding vote in most of the 5 to 4 cases for a generation, announced his retirement as of July 31, 2018. In addition, the Court decided a number of cases of interest to ObGyns. In this article we review some of those cases, as well as consider the future of the Court without Justice Kennedy. In selecting cases, we have given special attention to those in which national medical organizations filed amicus briefs. These “amicus curiae” or “friend of the court” briefs are filed by an entity who is not party to a case but wants to provide information or views to the court.

1. Abortion rulings

The Court decided 2 abortion cases and rejected a request to hear a third.
National Institute of Family and Life Advocates v Becerra
In this case,1 the Court struck down a California law that required pregnancy crisis centers not offering abortions (generally operated by pro-life groups) to provide special notices to clients.2
At stake. These notices would inform clients that California provides free or low-cost services, including abortions, and provide a phone number to call for those services.
There were many amicus briefs filed in this case, including those by the American College of Obstetricians and Gynecologists (ACOG) and other specialty boards,3 as well as the American Association of Pro-Life Obstetricians and Gynecologists and other pro-life organizations.4 ACOG’s brief argued that the California-required notice facilitates the goal of allowing women to receive medical services without harmful delay.
Final ruling. The Court held that the law required clinics to engage in speech with which the clinics disagreed (known as “compelled speech”). It also noted that California disclosure requirements were “wildly underinclusive” because they apply only to some clinics. The majority felt that there was no strong state interest in compelling this speech because there were other alternatives for the state to provide information about the availability of abortion and other services. The Court found that the clinics were likely to succeed on the merits of their claims of a First Amendment (free speech) violation.
Right to abortion for illegal immigrants in custody
A very unusual abortion case involved “Jane Doe,” a minor who was at 8 weeks’ gestation when she illegally crossed the border into the United States.5 She was placed in a federally-funded shelter where she requested an abortion. The facility denied that request.
At stake. Legal argument ensued about releasing her to another facility for an abortion, as the argument was made that pregnant minors who are apprehended crossing into the United States illegally and placed into the custody of federal officials should have abortion access. A lower Court of Appeals ruled against the Trump Administration’s policy of denying abortions to undocumented minors in federal custody. During the process of the federal government taking the case to the Supreme Court, the attorneys for Doe moved appointments around and, without notice, the abortion was performed. Government attorneys said that Doe’s attorneys made “what appear to be material misrepresentations and omissions” designed to “thwart [the Supreme Court’s] review” of the case.5 The government requested that the Court vacate the order of the Court of Appeals so that it could not be used as precedent.
Final ruling. The Court granted the governments request to vacate the lower court’s order because the minor was no longer pregnant and the order was therefore moot. The basic issue in this case (the right of in-custody minors to access abortions) remains unresolved. It is likely to appear before the Court in the future.
Continue to: Access to medical abortions
Access to medical abortions
An Arkansas law requires that a physician administering medical abortions contract with a physician who has admitting privileges at a hospital (a “contracted physician”).
At stake. Planned Parenthood filed suit challenging the requirement as unnecessary and harmful because it would result in the closure of 2 of the 3 abortion providers in Arkansas. ACOG filed an amicus brief urging the Supreme Court to consider the case.6 (Technically this was a petition for a Writ of Certiorari, the procedure by which the Court accepts cases. It accepts only about 1% of applications.) ACOG argued that there was no medical reason for the contracted physician requirement, and noted the harm it would do to women who would not have access to abortions.
Final ruling. On May 29, 2018, the Court declined to hear the case. This case is still active in the lower courts and may eventually return to the Supreme Court.

2. The patent system

The medical profession depends on the patent system to encourage the discovery of new patents efficiently and effectively. In 2012, Congress passed the America Invents Act7 that authorizes a petition by anyone other than the patent holder to the Patent and Trademark Office (PTO) for an “inter partes review” to assess a challenge to the patent’s legitimacy. If the PTO determines that there may be merit to the claim, the Patent Trial and Appeal Board undertakes a trial-like review process that may validate, invalidate, or amend the patent. The Board’s decision is subject to appellate court review.
At stake. This term, the inter partes review was challenged as unconstitutional on technical bases.8
Final ruling. The Court rejected this claim and approved the current administrative inter partes review process. The Court determined that once the Patent Office takes a petition challenging a patent, it must decide all of the claims against the patent, not pick and choose which elements of the challenge to evaluate.9 The Court’s decision upheld patent-review reform, but will require the Patent Office to tweak its procedures.

3. The travel ban

ACOG, the American Medical Association (AMA), the Association of American Medical Colleges, and more than 30 other health care and specialty associations filed an amicus brief regarding one of the most anticipated cases of the term—the “travel ban.”10
At stake. The essential argument of these organizations was that the US health care system depends on professionals from other countries. An efficient and fair immigration program is, therefore, important to advance the nation’s “health security.” During the 2016−2017 term, the Court considered but then removed the issue from its calendar when the Trump Administration issued a revised travel ban.11
In September 2017, President Trump’s proclamation imposed a range of entry restrictions on the citizens of 8 countries, most (but not all) of which are predominantly Muslim. The government indicated that, in a study by Homeland Security and the State Department, these countries were identified as having especially deficient information-sharing practices and presented national security concerns. Trump v Hawaii12 challenged this proclamation.
Final ruling. The majority of the Court upheld the travel ban. For the 5-Justice majority led by Chief Justice Roberts, the case came down to 3 things:
- The Constitution and the laws passed by Congress of necessity give the President great authority to engage in foreign policy, including policies regarding entry into the country.
- The courts are very reluctant to get into the substance of foreign affairs—they are not equipped to know in detail what the facts are, and things change very fast.
- If courts start tinkering with foreign policy and things turn bad, it will appear that the courts are to blame and were interfering in an area about which they are not competent.
Continue to: 4. Did a credit card case add risk to health insurance markets?

It was just a credit card case, but one in which the AMA saw a real risk to regulation of the health insurance markets.
At stake. Technically, Ohio v American Express concerned a claim that American Express (AmEx) violated antitrust laws when it prohibited merchants taking its credit card from “steering” customers to cards with lower fees.13 AmEx maintained that, because credit cards were a special kind of “2-sided” market (connecting merchants on one side and customers on the other), antitrust laws should not be strictly enforced.
The AMA noticed that special rules regarding 2-sided markets might apply to health insurance, and it submitted an amicus brief14 that noted: “dominant health insurance networks … have imposed and could further impose rules or effectively erect barriers that prohibit physicians from referring patients to certain specialists, particularly out-of-network specialists, for innovative and even necessary medical tests.”14 It concluded that the antitrust rule AmEx was suggesting would make it nearly impossible to challenge these unfair provisions in health insurance arrangements.
Final ruling. The Court, however, accepted the AmEx position, making it very difficult to develop an antitrust case against 2-sided markets. It remains to be seen the degree to which the AMA concern about health insurance markets will be realized.

5. Gay wedding and a bakeshop

At stake. In Masterpiece Cakeshop v Colorado, a cakemaker declined to design a cake for a gay wedding and had been disciplined under Colorado law for discriminating against the couple based on sexual orientation.15
Final ruling. The Court, however, found that the Colorado regulators had, ironically, shown such religious animus in the way they treated the baker that the regulators themselves had discriminated on the basis of religion. As a result, the Court reversed the sanctions against the baker.
This decision was fairly narrow. It does not, for example, stand for the proposition that there may be a general religious exception to antidiscrimination laws. The question of broader religious or free-speech objections to antidiscrimination laws remains for another time.
Amicus brief. It was interesting that the American College of Pediatricians, American Association of Pro-Life Obstetricians and Gynecologists, and others, filed an amicus brief to report with concern the “demands that individual medical professionals must perform, assist with, or facilitate abortions, without regard to the teachings of their own faiths, consciences, and convictions.”16 The brief also noted that “issues in the present case implicate the fundamental rights of health care professionals, and to respectfully urge that the Court should by no means permit any weakening or qualification of well-established protections against compelled speech, and of free exercise” of religion.16
Arbitration. The Court upheld, as it has in most recent terms, another arbitration agreement.1 This case concerned an employment agreement in which employees consented to submit to arbitration rather than file lawsuits and not use class action claims.
Search of cell-phone location. Cell phones, whenever turned on, connect with cell towers that record the phone’s location several times a minute. Cell companies store this information, creating a virtual map of where the owner is at all times. The Federal Bureau of Investigation asked a cell company for location information for several people during a 127-day period in which robberies were committed.2 The Court held that the search was illegal in the absence of a warrant.
Public employee unions. The Court held that agency (fair share) fees, in which public employees who are not union members can be required to pay dues for the bargaining and grievance activities (from which they generally benefit), violate the First Amendment. The majority held that forcing public employees to pay fees to unions requires the employees, through those fees, to engage in political activities with which they disagree.3 This is a form of compelled speech, which the Court found violates the First Amendment. Health care professionals who are public employees in positions that have union representation will probably have the opportunity to opt out of agency agreements.
Internet sales tax. The Court permitted states to charge sales tax on out-of-state Internet purchases.4 In doing so, a state may require out-of-state companies to collect taxes on sales to its residents.
References
- Epic Systems Corp. v Lewis, 584 US 16 285 (2018).
- Carpenter v United States, 585 US 16 402 (2018).
- Janus v State, County, and Municipal Employees, 585 US 16 1466 (2018).
- South Dakota v Wayfair, Inc, 585 US 17 494 (2018).
Clues to the future
During the term that ran from October 2, 2017, through June 27, 2018, the Court issued 72 decisions. An unusually high proportion of cases (26%; 19 cases) were decided on a 5 to 4 vote. Last term, the rate of 5 to 4 decisions was 10%; the 6-year average was 18%. The unanimous decision rate was 39% this term, compared with 59% last term, and 50% on average.
The rate of 5 to 4 cases provides a clue about the Court’s general direction. The number of times each Justice was in the majority in those nineteen 5 to 4 decisions included: Chief Justice Roberts, 17; and Justices Kennedy, 16; Gorsuch, 16; Thomas, 15; and Alito, 15; compared with Justices Ginsburg, 5; Breyer, 4; Sotomayor, 4; and Kagan, 3.
The Court convened on October 1, 2018. At this writing, whether the new term starts with 8 or 9 justices remains a question. President Trump nominated Brett Kavanaugh, JD, to take Justice Kennedy’s place on the Court. His professional qualifications and experience appear to make him qualified for a position on the Court, but as we have seen, there are many other elements that go into confirming a Justice’s nomination.
Justice Anthony Kennedy was the deciding vote in the overwhelming majority of the 5 to 4 decisions in 20 of his 30 years on the Court. The areas in which he had an especially important impact include1:
- Gay rights. Justice Kennedy wrote the opinions (usually 5 to 4 decisions) in a number of groundbreaking gay-rights cases, including decriminalizing homosexual conduct, striking down the Defense of Marriage Act, and finding that the Constitution requires states to recognize gay marriage.
- The death penalty. Justice Kennedy wrote decisions that prohibited states from imposing the death penalty for any crime other than murder, for defendants who were under 18 when they committed the crime, and for defendants with serious developmental disabilities. He expressed reservations about long-term solitary confinement, but did not have a case that allowed him to decide its constitutionality.
- The First Amendment. Early in his service on the Court, he held that the First Amendment protected flag burning as a form of speech. He decided many important freespeech and freedom-of-religion cases that have set a standard for protecting those fundamental freedoms.
- Use of health and social science data. Justice Kennedy was more open to mental health information and cited it more often than most other Justices.
- Abortion rights? Many commentators would add protecting the right to choose to have an abortion to the above list. Justice Kennedy was a central figure in one case that declined to back away from Roe v Wade, and joined a more recent decision that struck down a Texas law that created an undue burden on women seeking abortion. Plus, he also voted to uphold abortion restrictions, such as “partial-birth-abortion laws.” So there is a good argument for including abortion rights on the list, although he did not break new ground.
Justice Kennedy as a person
Outside the courtroom, Justice Kennedy is a person of great warmth and compassion. He is a natural teacher and spends a great deal of time with students. When asked how he would like to be remembered, Justice Kennedy once replied, “Somebody who’s decent, and honest, and fair, and who’s absolutely committed to the proposition that freedom is America’s gift to the rest of the world.” I agree with that assessment.
STEVEN R. SMITH, MS, JD
Reference
- South Dakota v Wayfair, Inc, 585 US (2018)
Next term, the Court is scheduled to hear cases regarding pharmaceutical liability, double jeopardy, sex-offender registration, expert witnesses, Social Security disability benefits, and the Age Discrimination in Employment Act. There will be at least 3 arbitration cases. Health care and reproductive rights will continue to be an important part of the Court’s docket.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- National Institute of Family and Life Advocates v Becerra, 585 US 16 1140 (2018).
- California Reproductive Freedom, Accountability, Comprehensive Care, and Transparency Act (FACT Act), Cal. Health & Safety Code Ann. §123470 et seq. (West 2018).
- Brief amici curiae of American Academy of Pediatrics, et al. in National Institute of Family and Life Advocates v Becerra, February 27, 2018.
- Brief amici curiae of American Association of Pro-Life Obstetricians & Gynecologists, et al. in National Institute of Family and Life Advocates v Becerra, January 16, 2018.
- Azar v Garza, 584 US 17 654 (2018).
- Brief amici curiae of American College of Obstetricians and Gynecologists and American Public Health Association in Planned Parenthood of Arkansas and Eastern Oklahoma v Jegley, February 1, 2018.
- Chapter 31, Inter Partes Review. United States Code. Title 35: Patents. Part III, Patents and protection of patents. 2012 Ed. 35 USC 311–319.
- Oil States Energy Services, LLC v Greene’s Energy Group, LLC, 584 US 16 712 (2018).
- SAS Institute Inc. v Iancu, 584 US 16 969 (2018).
- Brief for Association of American Medical Colleges and Others as Amici Curiae Supporting Respondents, Trump v Hawaii. https://www.supremecourt.gov/Docket PDF/17/ 17-965/40128/20180327105855912_17-965%20Amicus%20Br.%20Proclamation.pdf. Accessed September 21, 2018.
- Smith SR, Sanfilippo JS. Supreme Court decisions in 2017 that affected your practice. OBG Manag. 2017;29(12)44–47.
- Trump v Hawaii, 585 US 17 965 (2018).
- Ohio v American Express Co, 585 US 16 1454 (2018).
- Brief amici curiae of American Medical Association and Ohio State Medical Association in Ohio v American Express, December 24, 2017.
- Masterpiece Cakeshop, Ltd. v Colorado Civil Rights Commission, 584 US 16 111 (2018).
- Brief amici curiae of American College of Pediatricians, et al. in Masterpiece Cakeshop v Colorado Civil Rights Commission, September 7, 2017.
- National Institute of Family and Life Advocates v Becerra, 585 US 16 1140 (2018).
- California Reproductive Freedom, Accountability, Comprehensive Care, and Transparency Act (FACT Act), Cal. Health & Safety Code Ann. §123470 et seq. (West 2018).
- Brief amici curiae of American Academy of Pediatrics, et al. in National Institute of Family and Life Advocates v Becerra, February 27, 2018.
- Brief amici curiae of American Association of Pro-Life Obstetricians & Gynecologists, et al. in National Institute of Family and Life Advocates v Becerra, January 16, 2018.
- Azar v Garza, 584 US 17 654 (2018).
- Brief amici curiae of American College of Obstetricians and Gynecologists and American Public Health Association in Planned Parenthood of Arkansas and Eastern Oklahoma v Jegley, February 1, 2018.
- Chapter 31, Inter Partes Review. United States Code. Title 35: Patents. Part III, Patents and protection of patents. 2012 Ed. 35 USC 311–319.
- Oil States Energy Services, LLC v Greene’s Energy Group, LLC, 584 US 16 712 (2018).
- SAS Institute Inc. v Iancu, 584 US 16 969 (2018).
- Brief for Association of American Medical Colleges and Others as Amici Curiae Supporting Respondents, Trump v Hawaii. https://www.supremecourt.gov/Docket PDF/17/ 17-965/40128/20180327105855912_17-965%20Amicus%20Br.%20Proclamation.pdf. Accessed September 21, 2018.
- Smith SR, Sanfilippo JS. Supreme Court decisions in 2017 that affected your practice. OBG Manag. 2017;29(12)44–47.
- Trump v Hawaii, 585 US 17 965 (2018).
- Ohio v American Express Co, 585 US 16 1454 (2018).
- Brief amici curiae of American Medical Association and Ohio State Medical Association in Ohio v American Express, December 24, 2017.
- Masterpiece Cakeshop, Ltd. v Colorado Civil Rights Commission, 584 US 16 111 (2018).
- Brief amici curiae of American College of Pediatricians, et al. in Masterpiece Cakeshop v Colorado Civil Rights Commission, September 7, 2017.
Postpartum hemorrhage: Aortic compression to reduce pelvic bleeding
You are performing a repeat cesarean delivery on a 37-year-old G3P2 woman with placenta previa. Immediately after delivery, a postpartum hemorrhage occurs. You order additional uterotonic medications and blood products and prepare for standard surgical interventions including uterine devascularization, uterine compression sutures, and intrauterine balloon tamponade. As the hemorrhage continues, you begin to consider the need to perform a hysterectomy.
Suddenly the anesthesiologist reports that the patient’s blood pressure and heart rate have decreased. She asks you to initiate aortic compression to slow the pelvic bleeding and permit initiation of interventions to restore intravascular volume and optimize cardiovascular status. You have not previously performed this maneuver, and you wonder how to respond to her request.
Preoperative preparation
Anticipating possible adverse outcomes is a key task for every clinician. In the above case, in the setting of a repeat cesarean delivery in a woman with placenta previa, there is an increased risk of postpartum hemorrhage. Therefore, appropriate blood products and equipment should be made available before the operation is initiated. It also may be helpful to review the sequential steps you have found most useful in managing a postpartum hemorrhage prior to starting the procedure.
Rapid response to obstetric hemorrhage
When postpartum hemorrhage occurs during a cesarean delivery, there are many interventions that may successfully control the excessive blood loss, including uterotonics, massive transfusion of blood products, uterine massage, tranexamic acid, uterine devascularization, uterine compression sutures, intrauterine balloon tamponade, uterine artery embolization, uterine tourniquet, internal iliac artery ligation, hysterectomy, and pelvic packing.1 Rapid response to obstetric hemorrhage is important to avoid depletion of coagulation factors and subsequent development of a coagulation disorder. Once a coagulation disorder occurs, it can be very difficult to resolve the problem and complete the surgery.
Abdominal compression
The potentially benefial role of abdominal compression to help reduce blood loss caused by trauma or obstetric hemorrhage has been studied extensively in healthy volunteers. The theory is that abdominal compression will decrease blood flow in the distal aorta, helping to control bleeding in the pelvis and extremities. In one report, 80 to 140 lb of pressure applied to the epigastrium in 9 healthy male participants in a supine position on a rigid surface resulted in decreased blood flow in the common femoral artery as determined by pulsed-wave Doppler ultrasound.2 Abdominal pressure applied above the umbilicus also has been reported to reduce blood pressure in the legs.3 Abdominal compression and tourniquets used on the extremities are not meant to be definitive treatments for traumatic hemorrhages but rather are used to stabilize severely injured patients during transport to emergency surgical care facilities.4
One approach to performing manual abdominal aortic compression involves first gaining a mechanical advantage by positioning yourself above the epigastric area with arms extended. Using one closed fist with the opposite hand providing additional pressure, the equivalent of 80 to 140 lb can be applied to the patient’s upper abdomen.4 To estimate the pressure you can achieve using this method, cover a scale with a towel and use your arms to exert maximum pressure on the scale. What equivalent weight can you reach when applying maximum pressure? What weight can you sustain for a few minutes? Using manual compression, it is difficult for a clinician to exert the equivalent of 140 lb on the epigastrium for the extended period of time needed to transport an injured person to an emergency facility.5 Therefore, mechanical devices such as the abdominal aortic tourniquet (AAT) and the nonpneumatic antishock garment (NASG) have been developed to aid in providing continuous abdominal compression.
Continue to: Abdominal aortic tourniquet
Abdominal aortic tourniquet. The AAT is a corset-like device with an interior pneumatic bladder that is designed to provide sustained compression over the abdomen, therefore compressing the abdominal aorta and reducing blood flow to the pelvis and extremities. In one study with human volunteers, a median pressure of 180 mm Hg (range, 150–230 mm Hg) was associated with cessation of blood flow in the common femoral artery in 7 of 9 volunteers and a decrease in blood flow in all participants as determined by pulsed-wave Doppler ultrasound.6 Participants reported moderate to severe discomfort when the AAT was inflated to a pressure sufficient to stop blood flow in the femoral artery. The AAT device may not be as effective in individuals with an elevated body mass index and excessive abdominal girth.7 In obstetric postpartum hemorrhage, abdominal pressure also has been reported to reduce hemorrhage and femoral artery blood flow. Using a corset-like abdominal binder with an internal spring to provide continuous pressure over the anterior abdomen, Soltan and Sadekreported a beneficial effect of abdominal pressure in the management of severe postpartum hemorrhage in a large observational study in Egypt.8,9
Nonpneumatic antishock garment. The NASG has been studied extensively as a method to help safely transport a woman with severe postpartum hemorrhage to an emergency facility. The NASG is a neoprene and Velcro device with panels for the lower extremities, pelvis, and abdomen (FIGURE 1). The device also has an abdominal segment that includes a compression ball to provide continuous abdominal pressure. When the panels are closed, blood flow to the extremities and pelvis is reduced. In a study of 10 postpartum volunteers, application of the NASG caused decreased blood flow in the internal iliac artery as measured by Doppler ultrasound, but blood flow did not stop completely.10 In an observational study of women with postpartum hemorrhage, use of the NASG device in combination with usual interventions resulted in a decrease in blood loss.11
In a cluster randomized trial, 38 birth centers in Africa were randomly assigned to standard management of obstetric hemorrhage or the same protocol plus use of the NASG prior to transport to a regional emergency surgical center. Compared with the group receiving standard management alone, the women who received standard management plus the NASG device had a nonsignificant reduction in maternal mortality (odds ratio, 0.54; 95% confidence interval [CI], 0.14–2.05; P = .37) and a significantly more rapid recovery from hypovolemic shock (hazard ratio, 1.25; 95% CI, 1.02–1.52; P = .03).12 The International Federation of Gynecology and Obstetrics has issued a guideline supporting the use of the device in the management of obstetric hemorrhage in appropriate settings.13
Aortic compression in the setting of an open abdominal incision
During cesarean delivery, the surgeon has access to the abdominal aorta via the open abdominal incision and can directly apply pressure to the aorta at sites ranging from above the sacral promontory to the subdiaphragmatic aorta. Although aortic compression is occasionally noted as a potential intervention to help with the management of postpartum hemorrhage, there is very little literature on this intervention.1 In one case report of an emergency laparotomy in a Jehovah’s Witness patient with a placenta previa, uterine rupture, massive hemorrhage (hematocrit nadir of 6%), and hypovolemic shock, direct pressure applied to the infradiaphragmatic aorta and pelvic organs permitted the anesthesiologist to stabilize the patient’s cardiovascular status, facilitating the patient’s recovery from shock.14 The authors of the case concluded that compression of the aorta and pelvic organs can be lifesaving and is underutilized in the management of uncontrolled obstetric hemorrhage. Other case reports also recommend considering the use of aortic compression to permit the anesthesia team to resuscitate a bleeding patient.15
There is very little published guidance on how to perform aortic compression at cesarean delivery. Techniques for aortic compression include using a closed fist or the heel of the hand to compress the aorta against the lumbosacral spine. Alternatively, use a moist rolled-up surgical towel or laparotomy sponge to compress the aorta against the lumbosacral spine. With a low transverse abdominal incision, the aorta just above the lumbosacral promontory is closest to the surgeon (aorta zone III) (FIGURE 2). If a vertical abdominal incision has been made, the subdiaphragmatic aorta may be within reach of the surgeon (aorta zone II). If an anesthesiologist asks you to apply aortic compression, it is likely that the patient is hypotensive. In this setting, reducing blood flow through the aorta can be achieved with less pressure than required for successful aortic compression in a healthy volunteer.
Prolonged aortic compression that completely obstructs blood flow may result in downstream ischemia. This is illustrated by leg ischemia and amputation that have occurred following the use of the resuscitative endovascular balloon occlusion of the aorta (REBOA) occlusion device.16 Another strategy that has been used in the management of massive hemorrhage, when immediate replacement of clotting factors is not possible, is damage control surgery, a technique in which capillary and venous bleeding is controlled by placing pelvic packs or a pelvic umbrella pressure pack and sending the patient to the intensive care unit for resuscitation.17 With damage control surgery, a second procedure is planned to remove the packs after the patient has been stabilized.
With knowledge and practice comes preparedness
Hopefully you will never be asked by an anesthesiologist to stop operating and initiate aortic compression. With effective preprocedure preparation and rapid institution of standard postpartum hemorrhage techniques, it is unlikely aortic compression ever will be needed. If an unusually difficult case triggers a request for aortic compression, you have the knowledge and skills to provide that service.
- Hofmeyr GJ, Qureshi Z. Preventing deaths due to haemorrhage. Best Pract Res Clin Obstet Gynaecol. 2016;36:68–82.
- Blaivas M, Shiver S, Lyon M, et al. Control of hemorrhage in critical femoral or inguinal penetrating wounds—an ultrasound evaluation. Prehosp Disast Med. 2006;21(6):379–382.
- Riley DP, Burgess RW. External abdominal aortic compression: a study of a resuscitation manoeuvre for postpartum hemorrhage. Anaesth Intensive Care. 1994;22(5):571–575.
- Douma M, Smith KE, Brindley PG. Temporization of penetrating abdominal-pelvic trauma with manual external aortic compression: a novel case report. Ann Emerg Med. 2014;64(1):79–81.
- Douma M, Brindley PG. Abdominal aortic and iliac artery compression following penetrating trauma: a study of feasibility. Prehosp Disaster Med. 2014;29:299–302.
- Lyon M, Shiver SA, Greenfield EM, et al. Use of a novel abdominal aortic tourniquet to reduce or eliminate flow in the common femoral artery in human subjects. J Trauma Acute Care Surg. 2012;73(2 suppl 1):S103–S105.
- Taylor DM, Coleman M, Parker PJ. The evaluation of an abdominal aortic tourniquet for the control of pelvic and lower limb hemorrhage. Mil Med. 2013;178(11):1196–1201.
- Soltan MH, Sadek RR. Experience managing postpartum hemorrhage at Minia University Maternity Hospital, Egypt: no mortality using aortic compression. J Obstet Gynaecol Res. 2011;37(11):1557–1563.
- Soltan MH, Faragallah MF, Mosabah MH, et al. External aortic compression device: the first aid for postpartum hemorrhage control. J Obstet Gynaecol Res. 2009;35(3):453–458.
- Lester F, Stenson A, Meyer C, et al. Impact of the non-pneumatic antishock garment on pelvic blood flow in healthy postpartum women. Am J Obstet Gynecol. 2011; 204(5): 409.e1–5.
- Miller S, Hamza S, Bray EH, et al. First aid for obstetric haemorrhage: the pilot study of the non-pneumatic anti-shock garment in Egypt. BJOG. 2006;113(4):424–429.
- Miller S, Bergel EF, El Ayadi AM, et al. Non-pneumatic anti-shock garment (NASG), a first-aid device to decrease maternal mortality from obstetric hemorrhage: a cluster randomized trial. PLoS One. 2013;8(10):e76477.
- FIGO Safe Motherhoood and Newborn Health Committee; International Federation of Gynecology and Obstetrics. Non-pneumatic anti-shock garment to stabilize women with hypovolemic shock secondary to obstetric hemorrhage. Int J Gynaecol Obstet. 2015;128(3):194–195.
- Belfort M, Kofford S, Varner M. Massive obstetric hemorrhage in a Jehovah’s Witness: intraoperative strategies and high-dose erythropoietin use. Am J Perinatol. 2011;28(3):207–210.
- Keogh J, Tsokos N. Aortic compression in massive postpartum hemorrhage—an old but lifesaving technique. Aust N Z J Obstet Gyencol. 1997;37(2):237–238.
- Ribeiro MAF, Feng CYD, Nguyen ATM, et al. The complications associated with resuscitative endovascular balloon occlusion of the aorta (REBOA). World J Emerg Surg. 2018;13:20.
- Pacheco LD, Lozada MJ, Saade GR, et al. Damage-control surgery for obstetric hemorrhage. Obstet Gynecol 2018;132(2):423–427.
You are performing a repeat cesarean delivery on a 37-year-old G3P2 woman with placenta previa. Immediately after delivery, a postpartum hemorrhage occurs. You order additional uterotonic medications and blood products and prepare for standard surgical interventions including uterine devascularization, uterine compression sutures, and intrauterine balloon tamponade. As the hemorrhage continues, you begin to consider the need to perform a hysterectomy.
Suddenly the anesthesiologist reports that the patient’s blood pressure and heart rate have decreased. She asks you to initiate aortic compression to slow the pelvic bleeding and permit initiation of interventions to restore intravascular volume and optimize cardiovascular status. You have not previously performed this maneuver, and you wonder how to respond to her request.
Preoperative preparation
Anticipating possible adverse outcomes is a key task for every clinician. In the above case, in the setting of a repeat cesarean delivery in a woman with placenta previa, there is an increased risk of postpartum hemorrhage. Therefore, appropriate blood products and equipment should be made available before the operation is initiated. It also may be helpful to review the sequential steps you have found most useful in managing a postpartum hemorrhage prior to starting the procedure.
Rapid response to obstetric hemorrhage
When postpartum hemorrhage occurs during a cesarean delivery, there are many interventions that may successfully control the excessive blood loss, including uterotonics, massive transfusion of blood products, uterine massage, tranexamic acid, uterine devascularization, uterine compression sutures, intrauterine balloon tamponade, uterine artery embolization, uterine tourniquet, internal iliac artery ligation, hysterectomy, and pelvic packing.1 Rapid response to obstetric hemorrhage is important to avoid depletion of coagulation factors and subsequent development of a coagulation disorder. Once a coagulation disorder occurs, it can be very difficult to resolve the problem and complete the surgery.
Abdominal compression
The potentially benefial role of abdominal compression to help reduce blood loss caused by trauma or obstetric hemorrhage has been studied extensively in healthy volunteers. The theory is that abdominal compression will decrease blood flow in the distal aorta, helping to control bleeding in the pelvis and extremities. In one report, 80 to 140 lb of pressure applied to the epigastrium in 9 healthy male participants in a supine position on a rigid surface resulted in decreased blood flow in the common femoral artery as determined by pulsed-wave Doppler ultrasound.2 Abdominal pressure applied above the umbilicus also has been reported to reduce blood pressure in the legs.3 Abdominal compression and tourniquets used on the extremities are not meant to be definitive treatments for traumatic hemorrhages but rather are used to stabilize severely injured patients during transport to emergency surgical care facilities.4
One approach to performing manual abdominal aortic compression involves first gaining a mechanical advantage by positioning yourself above the epigastric area with arms extended. Using one closed fist with the opposite hand providing additional pressure, the equivalent of 80 to 140 lb can be applied to the patient’s upper abdomen.4 To estimate the pressure you can achieve using this method, cover a scale with a towel and use your arms to exert maximum pressure on the scale. What equivalent weight can you reach when applying maximum pressure? What weight can you sustain for a few minutes? Using manual compression, it is difficult for a clinician to exert the equivalent of 140 lb on the epigastrium for the extended period of time needed to transport an injured person to an emergency facility.5 Therefore, mechanical devices such as the abdominal aortic tourniquet (AAT) and the nonpneumatic antishock garment (NASG) have been developed to aid in providing continuous abdominal compression.
Continue to: Abdominal aortic tourniquet
Abdominal aortic tourniquet. The AAT is a corset-like device with an interior pneumatic bladder that is designed to provide sustained compression over the abdomen, therefore compressing the abdominal aorta and reducing blood flow to the pelvis and extremities. In one study with human volunteers, a median pressure of 180 mm Hg (range, 150–230 mm Hg) was associated with cessation of blood flow in the common femoral artery in 7 of 9 volunteers and a decrease in blood flow in all participants as determined by pulsed-wave Doppler ultrasound.6 Participants reported moderate to severe discomfort when the AAT was inflated to a pressure sufficient to stop blood flow in the femoral artery. The AAT device may not be as effective in individuals with an elevated body mass index and excessive abdominal girth.7 In obstetric postpartum hemorrhage, abdominal pressure also has been reported to reduce hemorrhage and femoral artery blood flow. Using a corset-like abdominal binder with an internal spring to provide continuous pressure over the anterior abdomen, Soltan and Sadekreported a beneficial effect of abdominal pressure in the management of severe postpartum hemorrhage in a large observational study in Egypt.8,9
Nonpneumatic antishock garment. The NASG has been studied extensively as a method to help safely transport a woman with severe postpartum hemorrhage to an emergency facility. The NASG is a neoprene and Velcro device with panels for the lower extremities, pelvis, and abdomen (FIGURE 1). The device also has an abdominal segment that includes a compression ball to provide continuous abdominal pressure. When the panels are closed, blood flow to the extremities and pelvis is reduced. In a study of 10 postpartum volunteers, application of the NASG caused decreased blood flow in the internal iliac artery as measured by Doppler ultrasound, but blood flow did not stop completely.10 In an observational study of women with postpartum hemorrhage, use of the NASG device in combination with usual interventions resulted in a decrease in blood loss.11

In a cluster randomized trial, 38 birth centers in Africa were randomly assigned to standard management of obstetric hemorrhage or the same protocol plus use of the NASG prior to transport to a regional emergency surgical center. Compared with the group receiving standard management alone, the women who received standard management plus the NASG device had a nonsignificant reduction in maternal mortality (odds ratio, 0.54; 95% confidence interval [CI], 0.14–2.05; P = .37) and a significantly more rapid recovery from hypovolemic shock (hazard ratio, 1.25; 95% CI, 1.02–1.52; P = .03).12 The International Federation of Gynecology and Obstetrics has issued a guideline supporting the use of the device in the management of obstetric hemorrhage in appropriate settings.13
Aortic compression in the setting of an open abdominal incision
During cesarean delivery, the surgeon has access to the abdominal aorta via the open abdominal incision and can directly apply pressure to the aorta at sites ranging from above the sacral promontory to the subdiaphragmatic aorta. Although aortic compression is occasionally noted as a potential intervention to help with the management of postpartum hemorrhage, there is very little literature on this intervention.1 In one case report of an emergency laparotomy in a Jehovah’s Witness patient with a placenta previa, uterine rupture, massive hemorrhage (hematocrit nadir of 6%), and hypovolemic shock, direct pressure applied to the infradiaphragmatic aorta and pelvic organs permitted the anesthesiologist to stabilize the patient’s cardiovascular status, facilitating the patient’s recovery from shock.14 The authors of the case concluded that compression of the aorta and pelvic organs can be lifesaving and is underutilized in the management of uncontrolled obstetric hemorrhage. Other case reports also recommend considering the use of aortic compression to permit the anesthesia team to resuscitate a bleeding patient.15
There is very little published guidance on how to perform aortic compression at cesarean delivery. Techniques for aortic compression include using a closed fist or the heel of the hand to compress the aorta against the lumbosacral spine. Alternatively, use a moist rolled-up surgical towel or laparotomy sponge to compress the aorta against the lumbosacral spine. With a low transverse abdominal incision, the aorta just above the lumbosacral promontory is closest to the surgeon (aorta zone III) (FIGURE 2). If a vertical abdominal incision has been made, the subdiaphragmatic aorta may be within reach of the surgeon (aorta zone II). If an anesthesiologist asks you to apply aortic compression, it is likely that the patient is hypotensive. In this setting, reducing blood flow through the aorta can be achieved with less pressure than required for successful aortic compression in a healthy volunteer.

Prolonged aortic compression that completely obstructs blood flow may result in downstream ischemia. This is illustrated by leg ischemia and amputation that have occurred following the use of the resuscitative endovascular balloon occlusion of the aorta (REBOA) occlusion device.16 Another strategy that has been used in the management of massive hemorrhage, when immediate replacement of clotting factors is not possible, is damage control surgery, a technique in which capillary and venous bleeding is controlled by placing pelvic packs or a pelvic umbrella pressure pack and sending the patient to the intensive care unit for resuscitation.17 With damage control surgery, a second procedure is planned to remove the packs after the patient has been stabilized.
With knowledge and practice comes preparedness
Hopefully you will never be asked by an anesthesiologist to stop operating and initiate aortic compression. With effective preprocedure preparation and rapid institution of standard postpartum hemorrhage techniques, it is unlikely aortic compression ever will be needed. If an unusually difficult case triggers a request for aortic compression, you have the knowledge and skills to provide that service.
You are performing a repeat cesarean delivery on a 37-year-old G3P2 woman with placenta previa. Immediately after delivery, a postpartum hemorrhage occurs. You order additional uterotonic medications and blood products and prepare for standard surgical interventions including uterine devascularization, uterine compression sutures, and intrauterine balloon tamponade. As the hemorrhage continues, you begin to consider the need to perform a hysterectomy.
Suddenly the anesthesiologist reports that the patient’s blood pressure and heart rate have decreased. She asks you to initiate aortic compression to slow the pelvic bleeding and permit initiation of interventions to restore intravascular volume and optimize cardiovascular status. You have not previously performed this maneuver, and you wonder how to respond to her request.
Preoperative preparation
Anticipating possible adverse outcomes is a key task for every clinician. In the above case, in the setting of a repeat cesarean delivery in a woman with placenta previa, there is an increased risk of postpartum hemorrhage. Therefore, appropriate blood products and equipment should be made available before the operation is initiated. It also may be helpful to review the sequential steps you have found most useful in managing a postpartum hemorrhage prior to starting the procedure.
Rapid response to obstetric hemorrhage
When postpartum hemorrhage occurs during a cesarean delivery, there are many interventions that may successfully control the excessive blood loss, including uterotonics, massive transfusion of blood products, uterine massage, tranexamic acid, uterine devascularization, uterine compression sutures, intrauterine balloon tamponade, uterine artery embolization, uterine tourniquet, internal iliac artery ligation, hysterectomy, and pelvic packing.1 Rapid response to obstetric hemorrhage is important to avoid depletion of coagulation factors and subsequent development of a coagulation disorder. Once a coagulation disorder occurs, it can be very difficult to resolve the problem and complete the surgery.
Abdominal compression
The potentially benefial role of abdominal compression to help reduce blood loss caused by trauma or obstetric hemorrhage has been studied extensively in healthy volunteers. The theory is that abdominal compression will decrease blood flow in the distal aorta, helping to control bleeding in the pelvis and extremities. In one report, 80 to 140 lb of pressure applied to the epigastrium in 9 healthy male participants in a supine position on a rigid surface resulted in decreased blood flow in the common femoral artery as determined by pulsed-wave Doppler ultrasound.2 Abdominal pressure applied above the umbilicus also has been reported to reduce blood pressure in the legs.3 Abdominal compression and tourniquets used on the extremities are not meant to be definitive treatments for traumatic hemorrhages but rather are used to stabilize severely injured patients during transport to emergency surgical care facilities.4
One approach to performing manual abdominal aortic compression involves first gaining a mechanical advantage by positioning yourself above the epigastric area with arms extended. Using one closed fist with the opposite hand providing additional pressure, the equivalent of 80 to 140 lb can be applied to the patient’s upper abdomen.4 To estimate the pressure you can achieve using this method, cover a scale with a towel and use your arms to exert maximum pressure on the scale. What equivalent weight can you reach when applying maximum pressure? What weight can you sustain for a few minutes? Using manual compression, it is difficult for a clinician to exert the equivalent of 140 lb on the epigastrium for the extended period of time needed to transport an injured person to an emergency facility.5 Therefore, mechanical devices such as the abdominal aortic tourniquet (AAT) and the nonpneumatic antishock garment (NASG) have been developed to aid in providing continuous abdominal compression.
Continue to: Abdominal aortic tourniquet
Abdominal aortic tourniquet. The AAT is a corset-like device with an interior pneumatic bladder that is designed to provide sustained compression over the abdomen, therefore compressing the abdominal aorta and reducing blood flow to the pelvis and extremities. In one study with human volunteers, a median pressure of 180 mm Hg (range, 150–230 mm Hg) was associated with cessation of blood flow in the common femoral artery in 7 of 9 volunteers and a decrease in blood flow in all participants as determined by pulsed-wave Doppler ultrasound.6 Participants reported moderate to severe discomfort when the AAT was inflated to a pressure sufficient to stop blood flow in the femoral artery. The AAT device may not be as effective in individuals with an elevated body mass index and excessive abdominal girth.7 In obstetric postpartum hemorrhage, abdominal pressure also has been reported to reduce hemorrhage and femoral artery blood flow. Using a corset-like abdominal binder with an internal spring to provide continuous pressure over the anterior abdomen, Soltan and Sadekreported a beneficial effect of abdominal pressure in the management of severe postpartum hemorrhage in a large observational study in Egypt.8,9
Nonpneumatic antishock garment. The NASG has been studied extensively as a method to help safely transport a woman with severe postpartum hemorrhage to an emergency facility. The NASG is a neoprene and Velcro device with panels for the lower extremities, pelvis, and abdomen (FIGURE 1). The device also has an abdominal segment that includes a compression ball to provide continuous abdominal pressure. When the panels are closed, blood flow to the extremities and pelvis is reduced. In a study of 10 postpartum volunteers, application of the NASG caused decreased blood flow in the internal iliac artery as measured by Doppler ultrasound, but blood flow did not stop completely.10 In an observational study of women with postpartum hemorrhage, use of the NASG device in combination with usual interventions resulted in a decrease in blood loss.11

In a cluster randomized trial, 38 birth centers in Africa were randomly assigned to standard management of obstetric hemorrhage or the same protocol plus use of the NASG prior to transport to a regional emergency surgical center. Compared with the group receiving standard management alone, the women who received standard management plus the NASG device had a nonsignificant reduction in maternal mortality (odds ratio, 0.54; 95% confidence interval [CI], 0.14–2.05; P = .37) and a significantly more rapid recovery from hypovolemic shock (hazard ratio, 1.25; 95% CI, 1.02–1.52; P = .03).12 The International Federation of Gynecology and Obstetrics has issued a guideline supporting the use of the device in the management of obstetric hemorrhage in appropriate settings.13
Aortic compression in the setting of an open abdominal incision
During cesarean delivery, the surgeon has access to the abdominal aorta via the open abdominal incision and can directly apply pressure to the aorta at sites ranging from above the sacral promontory to the subdiaphragmatic aorta. Although aortic compression is occasionally noted as a potential intervention to help with the management of postpartum hemorrhage, there is very little literature on this intervention.1 In one case report of an emergency laparotomy in a Jehovah’s Witness patient with a placenta previa, uterine rupture, massive hemorrhage (hematocrit nadir of 6%), and hypovolemic shock, direct pressure applied to the infradiaphragmatic aorta and pelvic organs permitted the anesthesiologist to stabilize the patient’s cardiovascular status, facilitating the patient’s recovery from shock.14 The authors of the case concluded that compression of the aorta and pelvic organs can be lifesaving and is underutilized in the management of uncontrolled obstetric hemorrhage. Other case reports also recommend considering the use of aortic compression to permit the anesthesia team to resuscitate a bleeding patient.15
There is very little published guidance on how to perform aortic compression at cesarean delivery. Techniques for aortic compression include using a closed fist or the heel of the hand to compress the aorta against the lumbosacral spine. Alternatively, use a moist rolled-up surgical towel or laparotomy sponge to compress the aorta against the lumbosacral spine. With a low transverse abdominal incision, the aorta just above the lumbosacral promontory is closest to the surgeon (aorta zone III) (FIGURE 2). If a vertical abdominal incision has been made, the subdiaphragmatic aorta may be within reach of the surgeon (aorta zone II). If an anesthesiologist asks you to apply aortic compression, it is likely that the patient is hypotensive. In this setting, reducing blood flow through the aorta can be achieved with less pressure than required for successful aortic compression in a healthy volunteer.

Prolonged aortic compression that completely obstructs blood flow may result in downstream ischemia. This is illustrated by leg ischemia and amputation that have occurred following the use of the resuscitative endovascular balloon occlusion of the aorta (REBOA) occlusion device.16 Another strategy that has been used in the management of massive hemorrhage, when immediate replacement of clotting factors is not possible, is damage control surgery, a technique in which capillary and venous bleeding is controlled by placing pelvic packs or a pelvic umbrella pressure pack and sending the patient to the intensive care unit for resuscitation.17 With damage control surgery, a second procedure is planned to remove the packs after the patient has been stabilized.
With knowledge and practice comes preparedness
Hopefully you will never be asked by an anesthesiologist to stop operating and initiate aortic compression. With effective preprocedure preparation and rapid institution of standard postpartum hemorrhage techniques, it is unlikely aortic compression ever will be needed. If an unusually difficult case triggers a request for aortic compression, you have the knowledge and skills to provide that service.
- Hofmeyr GJ, Qureshi Z. Preventing deaths due to haemorrhage. Best Pract Res Clin Obstet Gynaecol. 2016;36:68–82.
- Blaivas M, Shiver S, Lyon M, et al. Control of hemorrhage in critical femoral or inguinal penetrating wounds—an ultrasound evaluation. Prehosp Disast Med. 2006;21(6):379–382.
- Riley DP, Burgess RW. External abdominal aortic compression: a study of a resuscitation manoeuvre for postpartum hemorrhage. Anaesth Intensive Care. 1994;22(5):571–575.
- Douma M, Smith KE, Brindley PG. Temporization of penetrating abdominal-pelvic trauma with manual external aortic compression: a novel case report. Ann Emerg Med. 2014;64(1):79–81.
- Douma M, Brindley PG. Abdominal aortic and iliac artery compression following penetrating trauma: a study of feasibility. Prehosp Disaster Med. 2014;29:299–302.
- Lyon M, Shiver SA, Greenfield EM, et al. Use of a novel abdominal aortic tourniquet to reduce or eliminate flow in the common femoral artery in human subjects. J Trauma Acute Care Surg. 2012;73(2 suppl 1):S103–S105.
- Taylor DM, Coleman M, Parker PJ. The evaluation of an abdominal aortic tourniquet for the control of pelvic and lower limb hemorrhage. Mil Med. 2013;178(11):1196–1201.
- Soltan MH, Sadek RR. Experience managing postpartum hemorrhage at Minia University Maternity Hospital, Egypt: no mortality using aortic compression. J Obstet Gynaecol Res. 2011;37(11):1557–1563.
- Soltan MH, Faragallah MF, Mosabah MH, et al. External aortic compression device: the first aid for postpartum hemorrhage control. J Obstet Gynaecol Res. 2009;35(3):453–458.
- Lester F, Stenson A, Meyer C, et al. Impact of the non-pneumatic antishock garment on pelvic blood flow in healthy postpartum women. Am J Obstet Gynecol. 2011; 204(5): 409.e1–5.
- Miller S, Hamza S, Bray EH, et al. First aid for obstetric haemorrhage: the pilot study of the non-pneumatic anti-shock garment in Egypt. BJOG. 2006;113(4):424–429.
- Miller S, Bergel EF, El Ayadi AM, et al. Non-pneumatic anti-shock garment (NASG), a first-aid device to decrease maternal mortality from obstetric hemorrhage: a cluster randomized trial. PLoS One. 2013;8(10):e76477.
- FIGO Safe Motherhoood and Newborn Health Committee; International Federation of Gynecology and Obstetrics. Non-pneumatic anti-shock garment to stabilize women with hypovolemic shock secondary to obstetric hemorrhage. Int J Gynaecol Obstet. 2015;128(3):194–195.
- Belfort M, Kofford S, Varner M. Massive obstetric hemorrhage in a Jehovah’s Witness: intraoperative strategies and high-dose erythropoietin use. Am J Perinatol. 2011;28(3):207–210.
- Keogh J, Tsokos N. Aortic compression in massive postpartum hemorrhage—an old but lifesaving technique. Aust N Z J Obstet Gyencol. 1997;37(2):237–238.
- Ribeiro MAF, Feng CYD, Nguyen ATM, et al. The complications associated with resuscitative endovascular balloon occlusion of the aorta (REBOA). World J Emerg Surg. 2018;13:20.
- Pacheco LD, Lozada MJ, Saade GR, et al. Damage-control surgery for obstetric hemorrhage. Obstet Gynecol 2018;132(2):423–427.
- Hofmeyr GJ, Qureshi Z. Preventing deaths due to haemorrhage. Best Pract Res Clin Obstet Gynaecol. 2016;36:68–82.
- Blaivas M, Shiver S, Lyon M, et al. Control of hemorrhage in critical femoral or inguinal penetrating wounds—an ultrasound evaluation. Prehosp Disast Med. 2006;21(6):379–382.
- Riley DP, Burgess RW. External abdominal aortic compression: a study of a resuscitation manoeuvre for postpartum hemorrhage. Anaesth Intensive Care. 1994;22(5):571–575.
- Douma M, Smith KE, Brindley PG. Temporization of penetrating abdominal-pelvic trauma with manual external aortic compression: a novel case report. Ann Emerg Med. 2014;64(1):79–81.
- Douma M, Brindley PG. Abdominal aortic and iliac artery compression following penetrating trauma: a study of feasibility. Prehosp Disaster Med. 2014;29:299–302.
- Lyon M, Shiver SA, Greenfield EM, et al. Use of a novel abdominal aortic tourniquet to reduce or eliminate flow in the common femoral artery in human subjects. J Trauma Acute Care Surg. 2012;73(2 suppl 1):S103–S105.
- Taylor DM, Coleman M, Parker PJ. The evaluation of an abdominal aortic tourniquet for the control of pelvic and lower limb hemorrhage. Mil Med. 2013;178(11):1196–1201.
- Soltan MH, Sadek RR. Experience managing postpartum hemorrhage at Minia University Maternity Hospital, Egypt: no mortality using aortic compression. J Obstet Gynaecol Res. 2011;37(11):1557–1563.
- Soltan MH, Faragallah MF, Mosabah MH, et al. External aortic compression device: the first aid for postpartum hemorrhage control. J Obstet Gynaecol Res. 2009;35(3):453–458.
- Lester F, Stenson A, Meyer C, et al. Impact of the non-pneumatic antishock garment on pelvic blood flow in healthy postpartum women. Am J Obstet Gynecol. 2011; 204(5): 409.e1–5.
- Miller S, Hamza S, Bray EH, et al. First aid for obstetric haemorrhage: the pilot study of the non-pneumatic anti-shock garment in Egypt. BJOG. 2006;113(4):424–429.
- Miller S, Bergel EF, El Ayadi AM, et al. Non-pneumatic anti-shock garment (NASG), a first-aid device to decrease maternal mortality from obstetric hemorrhage: a cluster randomized trial. PLoS One. 2013;8(10):e76477.
- FIGO Safe Motherhoood and Newborn Health Committee; International Federation of Gynecology and Obstetrics. Non-pneumatic anti-shock garment to stabilize women with hypovolemic shock secondary to obstetric hemorrhage. Int J Gynaecol Obstet. 2015;128(3):194–195.
- Belfort M, Kofford S, Varner M. Massive obstetric hemorrhage in a Jehovah’s Witness: intraoperative strategies and high-dose erythropoietin use. Am J Perinatol. 2011;28(3):207–210.
- Keogh J, Tsokos N. Aortic compression in massive postpartum hemorrhage—an old but lifesaving technique. Aust N Z J Obstet Gyencol. 1997;37(2):237–238.
- Ribeiro MAF, Feng CYD, Nguyen ATM, et al. The complications associated with resuscitative endovascular balloon occlusion of the aorta (REBOA). World J Emerg Surg. 2018;13:20.
- Pacheco LD, Lozada MJ, Saade GR, et al. Damage-control surgery for obstetric hemorrhage. Obstet Gynecol 2018;132(2):423–427.
Use metrics for populations, not individuals
Use metrics for populations, not individuals
Dr. Kanofsky’s commentary on CD metrics is 100% correct. As an ethical question for physicians and society alike, I would ask, is applying metrics to physicians even moral?
As an ObGyn for most of 4 decades, my approach to obstetrics has not changed. In some years, my CD rate was very low, and in others my rate was average. Women must be treated as individuals. Although the industrial revolution increased quality and decreased costs in manufacturing, I do not believe that we can or should apply those principles to our patients.
Government regulators, insurance companies, and many physician leaders have lost sight of the Oath of Maimonides, which states, “May the love of my art actuate me at all times; may neither avarice nor miserliness…engage my mind,”1 as well as Hippocrates’ ancient observation, “Whatsoever house I may enter, my visit shall be for the convenience and advantage of the patient.”2 In addition, in the modern version of the Hippocratic Oath that most schools use today, physicians swear to “apply, for the benefit of the sick, all measures [that] are required...”3—not to the benefit of the government, the federal budget, or an accountable care organization (ACO).
Clearly, the informed consent of a 42-year-old who had in vitro fertilization and has a floating presentation with a low Bishop score and an estimated fetal weight of 4,000 at 40 6/7 weeks must include not only the risks of primary CD but also the risks of a long labor that may result in a CD, the occasional risk of shoulder dystocia, or third- or fourth-degree extension. Not having had a case of shoulder dystocia or a third- or fourth-degree in more than a decade clearly justifies my rationale.
The morbidity of a multiple repeat CD or even a primary CD in an obese woman is significantly more risky than a non-labored elective CD in a woman of normal weight who plans to have only 1 or 2 children. We must individualize our care. Metrics are for populations, not individuals.
Health economists who aggressively advocate lower cesarean rates accept stillbirths and babies with hypoxic ischemic encephalopathy, cerebral palsy, or Erb’s palsy as long as governmental expenditures are lowered. Do the parents of these children get a vote? The majority of practicing physicians like myself feel more aligned with the Hippocratic Oath and the Oath of Maimonides. We believe that we have a moral, ethical, and medical responsibility to the individual patient and not to an ACO or government bean counter.
I would suggest an overarching theme: choice—the freedom to make our own intelligent decisions based on reasonable data and interpretation of the medical literature.
One size does not fit all. So why do those pushing comparative metrics tell us there is only one way to practice obstetrics?
Howard C. Mandel, MD
Los Angeles, California
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Tan SY, Yeow ME. Moses Maimonides (1135–1204): rabbi, philosopher, physician. Singapore Med J. 2002;43(11):551–553.
- Copland J, ed. The Hippocratic Oath. In: The London Medical Repository, Monthly Journal, and Review, Volume III. 1825;23:258.
- Tyson P. The Hippocratic Oath today. Nova. March 27, 2001. http://www.pbs.org/wgbh/nova/body/hippocratic-oath-today.html. Accessed September 21, 2018.
Use metrics for populations, not individuals
Dr. Kanofsky’s commentary on CD metrics is 100% correct. As an ethical question for physicians and society alike, I would ask, is applying metrics to physicians even moral?
As an ObGyn for most of 4 decades, my approach to obstetrics has not changed. In some years, my CD rate was very low, and in others my rate was average. Women must be treated as individuals. Although the industrial revolution increased quality and decreased costs in manufacturing, I do not believe that we can or should apply those principles to our patients.
Government regulators, insurance companies, and many physician leaders have lost sight of the Oath of Maimonides, which states, “May the love of my art actuate me at all times; may neither avarice nor miserliness…engage my mind,”1 as well as Hippocrates’ ancient observation, “Whatsoever house I may enter, my visit shall be for the convenience and advantage of the patient.”2 In addition, in the modern version of the Hippocratic Oath that most schools use today, physicians swear to “apply, for the benefit of the sick, all measures [that] are required...”3—not to the benefit of the government, the federal budget, or an accountable care organization (ACO).
Clearly, the informed consent of a 42-year-old who had in vitro fertilization and has a floating presentation with a low Bishop score and an estimated fetal weight of 4,000 at 40 6/7 weeks must include not only the risks of primary CD but also the risks of a long labor that may result in a CD, the occasional risk of shoulder dystocia, or third- or fourth-degree extension. Not having had a case of shoulder dystocia or a third- or fourth-degree in more than a decade clearly justifies my rationale.
The morbidity of a multiple repeat CD or even a primary CD in an obese woman is significantly more risky than a non-labored elective CD in a woman of normal weight who plans to have only 1 or 2 children. We must individualize our care. Metrics are for populations, not individuals.
Health economists who aggressively advocate lower cesarean rates accept stillbirths and babies with hypoxic ischemic encephalopathy, cerebral palsy, or Erb’s palsy as long as governmental expenditures are lowered. Do the parents of these children get a vote? The majority of practicing physicians like myself feel more aligned with the Hippocratic Oath and the Oath of Maimonides. We believe that we have a moral, ethical, and medical responsibility to the individual patient and not to an ACO or government bean counter.
I would suggest an overarching theme: choice—the freedom to make our own intelligent decisions based on reasonable data and interpretation of the medical literature.
One size does not fit all. So why do those pushing comparative metrics tell us there is only one way to practice obstetrics?
Howard C. Mandel, MD
Los Angeles, California
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Use metrics for populations, not individuals
Dr. Kanofsky’s commentary on CD metrics is 100% correct. As an ethical question for physicians and society alike, I would ask, is applying metrics to physicians even moral?
As an ObGyn for most of 4 decades, my approach to obstetrics has not changed. In some years, my CD rate was very low, and in others my rate was average. Women must be treated as individuals. Although the industrial revolution increased quality and decreased costs in manufacturing, I do not believe that we can or should apply those principles to our patients.
Government regulators, insurance companies, and many physician leaders have lost sight of the Oath of Maimonides, which states, “May the love of my art actuate me at all times; may neither avarice nor miserliness…engage my mind,”1 as well as Hippocrates’ ancient observation, “Whatsoever house I may enter, my visit shall be for the convenience and advantage of the patient.”2 In addition, in the modern version of the Hippocratic Oath that most schools use today, physicians swear to “apply, for the benefit of the sick, all measures [that] are required...”3—not to the benefit of the government, the federal budget, or an accountable care organization (ACO).
Clearly, the informed consent of a 42-year-old who had in vitro fertilization and has a floating presentation with a low Bishop score and an estimated fetal weight of 4,000 at 40 6/7 weeks must include not only the risks of primary CD but also the risks of a long labor that may result in a CD, the occasional risk of shoulder dystocia, or third- or fourth-degree extension. Not having had a case of shoulder dystocia or a third- or fourth-degree in more than a decade clearly justifies my rationale.
The morbidity of a multiple repeat CD or even a primary CD in an obese woman is significantly more risky than a non-labored elective CD in a woman of normal weight who plans to have only 1 or 2 children. We must individualize our care. Metrics are for populations, not individuals.
Health economists who aggressively advocate lower cesarean rates accept stillbirths and babies with hypoxic ischemic encephalopathy, cerebral palsy, or Erb’s palsy as long as governmental expenditures are lowered. Do the parents of these children get a vote? The majority of practicing physicians like myself feel more aligned with the Hippocratic Oath and the Oath of Maimonides. We believe that we have a moral, ethical, and medical responsibility to the individual patient and not to an ACO or government bean counter.
I would suggest an overarching theme: choice—the freedom to make our own intelligent decisions based on reasonable data and interpretation of the medical literature.
One size does not fit all. So why do those pushing comparative metrics tell us there is only one way to practice obstetrics?
Howard C. Mandel, MD
Los Angeles, California
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Tan SY, Yeow ME. Moses Maimonides (1135–1204): rabbi, philosopher, physician. Singapore Med J. 2002;43(11):551–553.
- Copland J, ed. The Hippocratic Oath. In: The London Medical Repository, Monthly Journal, and Review, Volume III. 1825;23:258.
- Tyson P. The Hippocratic Oath today. Nova. March 27, 2001. http://www.pbs.org/wgbh/nova/body/hippocratic-oath-today.html. Accessed September 21, 2018.
- Tan SY, Yeow ME. Moses Maimonides (1135–1204): rabbi, philosopher, physician. Singapore Med J. 2002;43(11):551–553.
- Copland J, ed. The Hippocratic Oath. In: The London Medical Repository, Monthly Journal, and Review, Volume III. 1825;23:258.
- Tyson P. The Hippocratic Oath today. Nova. March 27, 2001. http://www.pbs.org/wgbh/nova/body/hippocratic-oath-today.html. Accessed September 21, 2018.
Under new ACC-AHA criteria, more pregnant women at risk of hypertension, adverse outcomes
A total of 164 new diagnoses (5.6%) of lower range stage 1 hypertension were made when the revised American Heart Association and the American College of Cardiology (ACC-AHA) Task Force on Clinical Practice Guidelines for chronic hypertension in adults were applied to a population of 2,947 pregnant women.
These patients had a significantly increased risk of preeclampsia as well as an increased risk of gestational diabetes mellitus and preterm birth, compared with normotensive patients, according to a study published in Obstetrics & Gynecology.
Elizabeth F. Sutton, PhD, and her associates at Magee-Womens Research Institute in Pittsburgh, used data from a Eunice Kennedy Shriver National Institute of Child Health and Human Development study. It included 2,947 pregnant women with singleton pregnancies where 94% of women were normotensive prior to 25 weeks of gestation. The researchers identified 164 new cases (6%) of lower range stage 1 hypertension under the ACC-AHA guidelines. Patients were then randomized to receive 60 mg of aspirin (1,399 normotensive patients, 66 stage 1 hypertension patients) or placebo (1,384 normotensive patients, 98 stage 1 hypertension patients).
The women had their blood pressure (BP) measured at 25 weeks of gestation or prior, and those patients with a systolic BP between 130 mm Hg and 139 mm Hg or diastolic BP between 80 mm and 89 mm were reclassified as having stage 1 hypertension under the new ACC-AHA guidelines; outcomes were compared with normotensive women with a systolic BP of 130 mm Hg and diastolic BP of 80 mm Hg. The researchers also performed a sensitivity analysis that restricted enrollment to 1,661 women who were at 20 weeks of gestation or prior, they said.
“Of important note, as a result of eligibility criterion excluding women with chronic hypertension (greater than 135/85 mm Hg) at enrollment, the enrollment BP range within the stage 1 hypertension group was limited to lower range stage 1 hypertension, that is, 130-135, 80-85 mm Hg, or both,” Dr. Sutton and her colleagues wrote.
The researchers found a significantly increased risk of preeclampsia among hypertensive pregnant women in the placebo group (15%) compared with normotensive (5%) women (relative risk, 2.66; 95% confidence interval, 1.56-4.54; P less than .01). These patients also had an increased risk of gestational diabetes mellitus (6%) compared with normotensive (2.5%; P = .03) pregnant women as well as an increased risk of preterm birth (4% vs. 1%; P = .01). Among women with stage 1 hypertension who received low-dose aspirin, there were no significant differences regarding the rate of preeclampsia, gestational diabetes mellitus, or preterm birth risk.
“Although prepregnancy BPs would be ideal for diagnosis, prior studies have shown that women do not consistently seek primary care outside of pregnancy,” Dr. Sutton and her colleagues wrote. “In the United States, preconception care engagement rates are between 18% and 45% in reproductive-aged women; thus, early pregnancy BPs may be all that is available for the obstetrician.”
Limitations to the secondary analysis included the original study’s exclusion of BPs higher than 135/85 mm Hg and the small sample size of patients who met the new criteria for stage 1 hypertension. The researchers also noted an increased risk of preeclampsia among patients with intake systolic BP between 120 mm Hg and 129 mm Hg, “with a smaller magnitude of risk compared with stage 1 hypertension.
“These findings suggest preliminarily that when considering preeclampsia risk based on early pregnancy BP, perhaps BP could be considered as a continuous variable rather than categorical,” Dr. Sutton and her colleagues noted.
This study was supported in part by the American Heart Association and the Eunice Kennedy Shriver NICHD. The authors reported no relevant financial conflicts of interest.
SOURCE: Sutton EF et al. Obstet Gynecol. 2018 Oct doi: 10.1097/AOG.0000000000002870.
I have significant concerns that, were ob.gyns. to adopt the ACC-AHA guidelines for chronic hypertension in adults and apply them to pregnant women, it would have great implications, as about 25% of all pregnant women have these findings.
Under the new criteria, there would be an additional 1 million pregnant women diagnosed with prehypertension or stage 1 hypertension. The new criteria will not only overdiagnose prehypertension in these women, it will cause more women to receive antihypertension medications.
The ACC-AHA criteria apply only to patients who have their blood pressure (BP) recorded prior to pregnancy or prior to 20 weeks of gestation. This study included women at up to 25 weeks of gestation, which means some already had been developing the condition. The Sutton et al. data also do not follow the new ACC-AHA criteria because the criteria require two BP readings, and the current study shows only one elevation which is, in my opinion, more dangerous.
Any time new criteria and screening are introduced, there always should be preparations for what happens next. There is no doubt that the ACC-AHA criteria, performed using two BP readings, is associated with hypertension, but what should clinicians do with that information? Prospective studies aimed at evaluating whether a woman with hypertension prior to or early in pregnancy may benefit from more intensive screening and other interventions to prevent hypertensive disorders and gestational diabetes in pregnancy are needed.
Baha Sibai, MD, is a visiting professor in the department of obstetrics, gynecology, and reproductive sciences at The University of Texas Health Sciences Center at Houston. He was asked to comment on the study by Sutton et al. He reported no relevant financial conflicts of interest.
I have significant concerns that, were ob.gyns. to adopt the ACC-AHA guidelines for chronic hypertension in adults and apply them to pregnant women, it would have great implications, as about 25% of all pregnant women have these findings.
Under the new criteria, there would be an additional 1 million pregnant women diagnosed with prehypertension or stage 1 hypertension. The new criteria will not only overdiagnose prehypertension in these women, it will cause more women to receive antihypertension medications.
The ACC-AHA criteria apply only to patients who have their blood pressure (BP) recorded prior to pregnancy or prior to 20 weeks of gestation. This study included women at up to 25 weeks of gestation, which means some already had been developing the condition. The Sutton et al. data also do not follow the new ACC-AHA criteria because the criteria require two BP readings, and the current study shows only one elevation which is, in my opinion, more dangerous.
Any time new criteria and screening are introduced, there always should be preparations for what happens next. There is no doubt that the ACC-AHA criteria, performed using two BP readings, is associated with hypertension, but what should clinicians do with that information? Prospective studies aimed at evaluating whether a woman with hypertension prior to or early in pregnancy may benefit from more intensive screening and other interventions to prevent hypertensive disorders and gestational diabetes in pregnancy are needed.
Baha Sibai, MD, is a visiting professor in the department of obstetrics, gynecology, and reproductive sciences at The University of Texas Health Sciences Center at Houston. He was asked to comment on the study by Sutton et al. He reported no relevant financial conflicts of interest.
I have significant concerns that, were ob.gyns. to adopt the ACC-AHA guidelines for chronic hypertension in adults and apply them to pregnant women, it would have great implications, as about 25% of all pregnant women have these findings.
Under the new criteria, there would be an additional 1 million pregnant women diagnosed with prehypertension or stage 1 hypertension. The new criteria will not only overdiagnose prehypertension in these women, it will cause more women to receive antihypertension medications.
The ACC-AHA criteria apply only to patients who have their blood pressure (BP) recorded prior to pregnancy or prior to 20 weeks of gestation. This study included women at up to 25 weeks of gestation, which means some already had been developing the condition. The Sutton et al. data also do not follow the new ACC-AHA criteria because the criteria require two BP readings, and the current study shows only one elevation which is, in my opinion, more dangerous.
Any time new criteria and screening are introduced, there always should be preparations for what happens next. There is no doubt that the ACC-AHA criteria, performed using two BP readings, is associated with hypertension, but what should clinicians do with that information? Prospective studies aimed at evaluating whether a woman with hypertension prior to or early in pregnancy may benefit from more intensive screening and other interventions to prevent hypertensive disorders and gestational diabetes in pregnancy are needed.
Baha Sibai, MD, is a visiting professor in the department of obstetrics, gynecology, and reproductive sciences at The University of Texas Health Sciences Center at Houston. He was asked to comment on the study by Sutton et al. He reported no relevant financial conflicts of interest.
A total of 164 new diagnoses (5.6%) of lower range stage 1 hypertension were made when the revised American Heart Association and the American College of Cardiology (ACC-AHA) Task Force on Clinical Practice Guidelines for chronic hypertension in adults were applied to a population of 2,947 pregnant women.
These patients had a significantly increased risk of preeclampsia as well as an increased risk of gestational diabetes mellitus and preterm birth, compared with normotensive patients, according to a study published in Obstetrics & Gynecology.
Elizabeth F. Sutton, PhD, and her associates at Magee-Womens Research Institute in Pittsburgh, used data from a Eunice Kennedy Shriver National Institute of Child Health and Human Development study. It included 2,947 pregnant women with singleton pregnancies where 94% of women were normotensive prior to 25 weeks of gestation. The researchers identified 164 new cases (6%) of lower range stage 1 hypertension under the ACC-AHA guidelines. Patients were then randomized to receive 60 mg of aspirin (1,399 normotensive patients, 66 stage 1 hypertension patients) or placebo (1,384 normotensive patients, 98 stage 1 hypertension patients).
The women had their blood pressure (BP) measured at 25 weeks of gestation or prior, and those patients with a systolic BP between 130 mm Hg and 139 mm Hg or diastolic BP between 80 mm and 89 mm were reclassified as having stage 1 hypertension under the new ACC-AHA guidelines; outcomes were compared with normotensive women with a systolic BP of 130 mm Hg and diastolic BP of 80 mm Hg. The researchers also performed a sensitivity analysis that restricted enrollment to 1,661 women who were at 20 weeks of gestation or prior, they said.
“Of important note, as a result of eligibility criterion excluding women with chronic hypertension (greater than 135/85 mm Hg) at enrollment, the enrollment BP range within the stage 1 hypertension group was limited to lower range stage 1 hypertension, that is, 130-135, 80-85 mm Hg, or both,” Dr. Sutton and her colleagues wrote.
The researchers found a significantly increased risk of preeclampsia among hypertensive pregnant women in the placebo group (15%) compared with normotensive (5%) women (relative risk, 2.66; 95% confidence interval, 1.56-4.54; P less than .01). These patients also had an increased risk of gestational diabetes mellitus (6%) compared with normotensive (2.5%; P = .03) pregnant women as well as an increased risk of preterm birth (4% vs. 1%; P = .01). Among women with stage 1 hypertension who received low-dose aspirin, there were no significant differences regarding the rate of preeclampsia, gestational diabetes mellitus, or preterm birth risk.
“Although prepregnancy BPs would be ideal for diagnosis, prior studies have shown that women do not consistently seek primary care outside of pregnancy,” Dr. Sutton and her colleagues wrote. “In the United States, preconception care engagement rates are between 18% and 45% in reproductive-aged women; thus, early pregnancy BPs may be all that is available for the obstetrician.”
Limitations to the secondary analysis included the original study’s exclusion of BPs higher than 135/85 mm Hg and the small sample size of patients who met the new criteria for stage 1 hypertension. The researchers also noted an increased risk of preeclampsia among patients with intake systolic BP between 120 mm Hg and 129 mm Hg, “with a smaller magnitude of risk compared with stage 1 hypertension.
“These findings suggest preliminarily that when considering preeclampsia risk based on early pregnancy BP, perhaps BP could be considered as a continuous variable rather than categorical,” Dr. Sutton and her colleagues noted.
This study was supported in part by the American Heart Association and the Eunice Kennedy Shriver NICHD. The authors reported no relevant financial conflicts of interest.
SOURCE: Sutton EF et al. Obstet Gynecol. 2018 Oct doi: 10.1097/AOG.0000000000002870.
A total of 164 new diagnoses (5.6%) of lower range stage 1 hypertension were made when the revised American Heart Association and the American College of Cardiology (ACC-AHA) Task Force on Clinical Practice Guidelines for chronic hypertension in adults were applied to a population of 2,947 pregnant women.
These patients had a significantly increased risk of preeclampsia as well as an increased risk of gestational diabetes mellitus and preterm birth, compared with normotensive patients, according to a study published in Obstetrics & Gynecology.
Elizabeth F. Sutton, PhD, and her associates at Magee-Womens Research Institute in Pittsburgh, used data from a Eunice Kennedy Shriver National Institute of Child Health and Human Development study. It included 2,947 pregnant women with singleton pregnancies where 94% of women were normotensive prior to 25 weeks of gestation. The researchers identified 164 new cases (6%) of lower range stage 1 hypertension under the ACC-AHA guidelines. Patients were then randomized to receive 60 mg of aspirin (1,399 normotensive patients, 66 stage 1 hypertension patients) or placebo (1,384 normotensive patients, 98 stage 1 hypertension patients).
The women had their blood pressure (BP) measured at 25 weeks of gestation or prior, and those patients with a systolic BP between 130 mm Hg and 139 mm Hg or diastolic BP between 80 mm and 89 mm were reclassified as having stage 1 hypertension under the new ACC-AHA guidelines; outcomes were compared with normotensive women with a systolic BP of 130 mm Hg and diastolic BP of 80 mm Hg. The researchers also performed a sensitivity analysis that restricted enrollment to 1,661 women who were at 20 weeks of gestation or prior, they said.
“Of important note, as a result of eligibility criterion excluding women with chronic hypertension (greater than 135/85 mm Hg) at enrollment, the enrollment BP range within the stage 1 hypertension group was limited to lower range stage 1 hypertension, that is, 130-135, 80-85 mm Hg, or both,” Dr. Sutton and her colleagues wrote.
The researchers found a significantly increased risk of preeclampsia among hypertensive pregnant women in the placebo group (15%) compared with normotensive (5%) women (relative risk, 2.66; 95% confidence interval, 1.56-4.54; P less than .01). These patients also had an increased risk of gestational diabetes mellitus (6%) compared with normotensive (2.5%; P = .03) pregnant women as well as an increased risk of preterm birth (4% vs. 1%; P = .01). Among women with stage 1 hypertension who received low-dose aspirin, there were no significant differences regarding the rate of preeclampsia, gestational diabetes mellitus, or preterm birth risk.
“Although prepregnancy BPs would be ideal for diagnosis, prior studies have shown that women do not consistently seek primary care outside of pregnancy,” Dr. Sutton and her colleagues wrote. “In the United States, preconception care engagement rates are between 18% and 45% in reproductive-aged women; thus, early pregnancy BPs may be all that is available for the obstetrician.”
Limitations to the secondary analysis included the original study’s exclusion of BPs higher than 135/85 mm Hg and the small sample size of patients who met the new criteria for stage 1 hypertension. The researchers also noted an increased risk of preeclampsia among patients with intake systolic BP between 120 mm Hg and 129 mm Hg, “with a smaller magnitude of risk compared with stage 1 hypertension.
“These findings suggest preliminarily that when considering preeclampsia risk based on early pregnancy BP, perhaps BP could be considered as a continuous variable rather than categorical,” Dr. Sutton and her colleagues noted.
This study was supported in part by the American Heart Association and the Eunice Kennedy Shriver NICHD. The authors reported no relevant financial conflicts of interest.
SOURCE: Sutton EF et al. Obstet Gynecol. 2018 Oct doi: 10.1097/AOG.0000000000002870.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point: gestational diabetes, and preterm birth.
Major finding: Pregnant women with newly diagnosed stage 1 hypertension not randomized to receive aspirin had a significantly higher risk of preeclampsia (15%) and a higher risk of gestational diabetes (6%) and preterm birth (4%), compared with normotensive pregnant women.
Study details: A secondary analysis of 2,947 women who were originally enrolled in a study by the Eunice Kennedy Shriver NICHD between 1989 and 1992.
Disclosures: This study was supported in part by the American Heart Association and the Eunice Kennedy Shriver NICHD. The authors reported no relevant financial conflicts of interest.
Source: Sutton EF et al. Obstet Gynecol. 2018 Oct doi:10.1097/AOG.0000000000002870.
Gestational weight outside guidelines adversely affects mothers, babies
Gestational weight gain above or below the level recommended by the Institute of Medicine (IOM) guidelines resulted in significantly worse outcomes for mothers and babies, according to data from nearly 30,000 women.
Previous studies of the relationship between gestational weight gain and maternal and neonatal outcomes have been limited by “small sample sizes, single sites, restricted reporting of outcomes, and a lack of racial-ethnic diversity,” Michelle A. Kominiarek, MD, of Northwestern University in Chicago and her colleagues wrote . To determine the effects of gestational weight gain on a large and more diverse population, the researchers conducted a secondary analysis of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network’s Assessment of Perinatal Excellence study. The findings were published in Obstetrics & Gynecology.
Gestational weight gain above the amount recommended by IOM guidelines was significantly associated with adverse outcomes in neonates, including macrosomia (adjusted odds ratio, 2.66), shoulder dystocia (aOR, 1.74), and neonatal hypoglycemia (aOR, 1.60).
In further multivariate analysis, adverse maternal outcomes associated with gestational weight gain above that recommended by the guidelines included hypertensive diseases of pregnancy for any parity (aOR, 1.84) and increased risk of cesarean delivery in nulliparous and multiparous women (aORs, 1.44 and 1.26, respectively).
Gestational weight gain below the recommended amount was associated with both spontaneous (aOR, 1.50) and indicated (aOR, 1.34) preterm birth. Weight gain above the guidelines was associated with a greater risk of indicated preterm birth only (aOR, 1.24).
The study population included 29,861 women at 25 hospitals over a 3-year period. Of these, 51% had gestational weight gains above the amount recommended by the IOM guidelines and 21% had gestational weight gains below it. The researchers calculated gestational weight gain by subtracting prepregnancy weight from delivery weight or, if prepregnancy weight was not available, by subtracting weight at the first prenatal visit at 13 weeks of gestation or earlier from delivery weight.
The study findings were limited by the use of self-reported prepregnancy weight and the possible effects of changes to the guidelines with respect to obese patients, the researchers said. However, the results support those from previous studies, and the “noted strengths include analysis of 29,861 women representative of the United States with rigorous ascertainment of outcomes and calculation of gestational weight gain to account for the wide range of gestational ages at delivery,” Dr. Kominiarek and her associates wrote.
Overall, the data support efforts to educate women on health behaviors and how gestational weight gain affects them and their infants, and additional research is needed to help women meet their goals for appropriate gestational weight, the researchers concluded.
SOURCE: Kominiarek MA et al. Obstet Gynecol. 2018 Oct;132(4):875-81.
“We are struggling with an obesity epidemic in this country, and pregnancy accounts for a risk time for women to gain excessive weight,” Martina L. Badell, MD, said in an interview. “This is a very well-designed large study which attempted to systematically evaluate the adverse perinatal outcomes associated with inappropriate weight gain in pregnancy across a diverse group of women.”
She emphasized that “the take home message is the importance of counseling regarding weight gain in pregnancy and monitoring it closely in real time as the associated risks are significant and potentially avoidable. The first step to solving a problem is adequately quantifying it, and this study does just that. The next step is giving this information to pregnant women along with making weight gain a part of the discussion prior to pregnancy and at every prenatal visit.”
Dr. Badell added, “Ideally, the weight gain for an individual pregnant women would be tracked and discussed with her during each prenatal visit. If she is below or above the recommendations, the risks associated with this could be discussed along with strategies to get/stay on track. In an ideal world, women struggling with weight gain goals in pregnancy would have access to a dietitian. However, in reality, ob.gyn. offices will likely need to come up with patient education handouts or staff education.”
Another useful avenue for research would be assessing the effects of patient education, Dr. Badell said. “The next best step would be implementing a study to assess if education of women during pregnancy about their individual weight gain at each visit and discussion regarding perinatal risks affects ultimate weight gain and reduces risks. Additionally, education could begin in the preconception phase as this knowledge is likely important even prior to pregnancy. Finally, studies are needed on interventions such as working with dietitians or patient education classes once a woman has been identified as not being within weight gain goals to evaluate if these can alter weight gain and improve outcomes.”
Dr. Badell is a maternal-fetal medicine specialist in the department of gynecology and obstetrics at Emory University, Atlanta. She was asked to comment on the findings of Kominiarek MA et al. Dr. Badell had no relevant financial conflicts to disclose.
“We are struggling with an obesity epidemic in this country, and pregnancy accounts for a risk time for women to gain excessive weight,” Martina L. Badell, MD, said in an interview. “This is a very well-designed large study which attempted to systematically evaluate the adverse perinatal outcomes associated with inappropriate weight gain in pregnancy across a diverse group of women.”
She emphasized that “the take home message is the importance of counseling regarding weight gain in pregnancy and monitoring it closely in real time as the associated risks are significant and potentially avoidable. The first step to solving a problem is adequately quantifying it, and this study does just that. The next step is giving this information to pregnant women along with making weight gain a part of the discussion prior to pregnancy and at every prenatal visit.”
Dr. Badell added, “Ideally, the weight gain for an individual pregnant women would be tracked and discussed with her during each prenatal visit. If she is below or above the recommendations, the risks associated with this could be discussed along with strategies to get/stay on track. In an ideal world, women struggling with weight gain goals in pregnancy would have access to a dietitian. However, in reality, ob.gyn. offices will likely need to come up with patient education handouts or staff education.”
Another useful avenue for research would be assessing the effects of patient education, Dr. Badell said. “The next best step would be implementing a study to assess if education of women during pregnancy about their individual weight gain at each visit and discussion regarding perinatal risks affects ultimate weight gain and reduces risks. Additionally, education could begin in the preconception phase as this knowledge is likely important even prior to pregnancy. Finally, studies are needed on interventions such as working with dietitians or patient education classes once a woman has been identified as not being within weight gain goals to evaluate if these can alter weight gain and improve outcomes.”
Dr. Badell is a maternal-fetal medicine specialist in the department of gynecology and obstetrics at Emory University, Atlanta. She was asked to comment on the findings of Kominiarek MA et al. Dr. Badell had no relevant financial conflicts to disclose.
“We are struggling with an obesity epidemic in this country, and pregnancy accounts for a risk time for women to gain excessive weight,” Martina L. Badell, MD, said in an interview. “This is a very well-designed large study which attempted to systematically evaluate the adverse perinatal outcomes associated with inappropriate weight gain in pregnancy across a diverse group of women.”
She emphasized that “the take home message is the importance of counseling regarding weight gain in pregnancy and monitoring it closely in real time as the associated risks are significant and potentially avoidable. The first step to solving a problem is adequately quantifying it, and this study does just that. The next step is giving this information to pregnant women along with making weight gain a part of the discussion prior to pregnancy and at every prenatal visit.”
Dr. Badell added, “Ideally, the weight gain for an individual pregnant women would be tracked and discussed with her during each prenatal visit. If she is below or above the recommendations, the risks associated with this could be discussed along with strategies to get/stay on track. In an ideal world, women struggling with weight gain goals in pregnancy would have access to a dietitian. However, in reality, ob.gyn. offices will likely need to come up with patient education handouts or staff education.”
Another useful avenue for research would be assessing the effects of patient education, Dr. Badell said. “The next best step would be implementing a study to assess if education of women during pregnancy about their individual weight gain at each visit and discussion regarding perinatal risks affects ultimate weight gain and reduces risks. Additionally, education could begin in the preconception phase as this knowledge is likely important even prior to pregnancy. Finally, studies are needed on interventions such as working with dietitians or patient education classes once a woman has been identified as not being within weight gain goals to evaluate if these can alter weight gain and improve outcomes.”
Dr. Badell is a maternal-fetal medicine specialist in the department of gynecology and obstetrics at Emory University, Atlanta. She was asked to comment on the findings of Kominiarek MA et al. Dr. Badell had no relevant financial conflicts to disclose.
Gestational weight gain above or below the level recommended by the Institute of Medicine (IOM) guidelines resulted in significantly worse outcomes for mothers and babies, according to data from nearly 30,000 women.
Previous studies of the relationship between gestational weight gain and maternal and neonatal outcomes have been limited by “small sample sizes, single sites, restricted reporting of outcomes, and a lack of racial-ethnic diversity,” Michelle A. Kominiarek, MD, of Northwestern University in Chicago and her colleagues wrote . To determine the effects of gestational weight gain on a large and more diverse population, the researchers conducted a secondary analysis of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network’s Assessment of Perinatal Excellence study. The findings were published in Obstetrics & Gynecology.
Gestational weight gain above the amount recommended by IOM guidelines was significantly associated with adverse outcomes in neonates, including macrosomia (adjusted odds ratio, 2.66), shoulder dystocia (aOR, 1.74), and neonatal hypoglycemia (aOR, 1.60).
In further multivariate analysis, adverse maternal outcomes associated with gestational weight gain above that recommended by the guidelines included hypertensive diseases of pregnancy for any parity (aOR, 1.84) and increased risk of cesarean delivery in nulliparous and multiparous women (aORs, 1.44 and 1.26, respectively).
Gestational weight gain below the recommended amount was associated with both spontaneous (aOR, 1.50) and indicated (aOR, 1.34) preterm birth. Weight gain above the guidelines was associated with a greater risk of indicated preterm birth only (aOR, 1.24).
The study population included 29,861 women at 25 hospitals over a 3-year period. Of these, 51% had gestational weight gains above the amount recommended by the IOM guidelines and 21% had gestational weight gains below it. The researchers calculated gestational weight gain by subtracting prepregnancy weight from delivery weight or, if prepregnancy weight was not available, by subtracting weight at the first prenatal visit at 13 weeks of gestation or earlier from delivery weight.
The study findings were limited by the use of self-reported prepregnancy weight and the possible effects of changes to the guidelines with respect to obese patients, the researchers said. However, the results support those from previous studies, and the “noted strengths include analysis of 29,861 women representative of the United States with rigorous ascertainment of outcomes and calculation of gestational weight gain to account for the wide range of gestational ages at delivery,” Dr. Kominiarek and her associates wrote.
Overall, the data support efforts to educate women on health behaviors and how gestational weight gain affects them and their infants, and additional research is needed to help women meet their goals for appropriate gestational weight, the researchers concluded.
SOURCE: Kominiarek MA et al. Obstet Gynecol. 2018 Oct;132(4):875-81.
Gestational weight gain above or below the level recommended by the Institute of Medicine (IOM) guidelines resulted in significantly worse outcomes for mothers and babies, according to data from nearly 30,000 women.
Previous studies of the relationship between gestational weight gain and maternal and neonatal outcomes have been limited by “small sample sizes, single sites, restricted reporting of outcomes, and a lack of racial-ethnic diversity,” Michelle A. Kominiarek, MD, of Northwestern University in Chicago and her colleagues wrote . To determine the effects of gestational weight gain on a large and more diverse population, the researchers conducted a secondary analysis of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network’s Assessment of Perinatal Excellence study. The findings were published in Obstetrics & Gynecology.
Gestational weight gain above the amount recommended by IOM guidelines was significantly associated with adverse outcomes in neonates, including macrosomia (adjusted odds ratio, 2.66), shoulder dystocia (aOR, 1.74), and neonatal hypoglycemia (aOR, 1.60).
In further multivariate analysis, adverse maternal outcomes associated with gestational weight gain above that recommended by the guidelines included hypertensive diseases of pregnancy for any parity (aOR, 1.84) and increased risk of cesarean delivery in nulliparous and multiparous women (aORs, 1.44 and 1.26, respectively).
Gestational weight gain below the recommended amount was associated with both spontaneous (aOR, 1.50) and indicated (aOR, 1.34) preterm birth. Weight gain above the guidelines was associated with a greater risk of indicated preterm birth only (aOR, 1.24).
The study population included 29,861 women at 25 hospitals over a 3-year period. Of these, 51% had gestational weight gains above the amount recommended by the IOM guidelines and 21% had gestational weight gains below it. The researchers calculated gestational weight gain by subtracting prepregnancy weight from delivery weight or, if prepregnancy weight was not available, by subtracting weight at the first prenatal visit at 13 weeks of gestation or earlier from delivery weight.
The study findings were limited by the use of self-reported prepregnancy weight and the possible effects of changes to the guidelines with respect to obese patients, the researchers said. However, the results support those from previous studies, and the “noted strengths include analysis of 29,861 women representative of the United States with rigorous ascertainment of outcomes and calculation of gestational weight gain to account for the wide range of gestational ages at delivery,” Dr. Kominiarek and her associates wrote.
Overall, the data support efforts to educate women on health behaviors and how gestational weight gain affects them and their infants, and additional research is needed to help women meet their goals for appropriate gestational weight, the researchers concluded.
SOURCE: Kominiarek MA et al. Obstet Gynecol. 2018 Oct;132(4):875-81.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point: Gestational weight gain or loss is a significant risk factor for adverse maternal and neonatal outcomes.
Major finding: Gestational weight gain above the recommended amount was significantly associated with adverse outcomes in neonates, including macrosomia (adjusted odds ratio, 2.66), shoulder dystocia (aOR, 1.74), and neonatal hypoglycemia (aOR, 1.60).
Study details: The data came from 29,861 women who delivered at 25 hospitals across the United States on randomly selected days during a 3-year period.
Disclosures: The researchers had no financial conflicts to disclose. The study was supported in part by various grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Center for Research Resources.
Source: Kominiarek MA et al. Obstet Gynecol. 2018 Oct;132(4):875-81.
Congenital syphilis rates continue skyrocketing alongside other STDs
Rapidly increasing cases of newborn syphilis have reached their highest prevalence in 2 decades, according to a new report by the Centers for Disease Control and Prevention on sexually transmitted disease surveillance in 2017.
Newborn syphilis incidence has more than doubled, from 362 cases in 2013 to 918 cases in 2017, resulting in 64 syphilitic stillbirths and 13 infant deaths that year, according to data published in Sexually Transmitted Disease Surveillance 2017.
At least one case was reported in 37 states last year, and the greatest burden of cases occurred in California, Arizona, Texas, Louisiana, and Florida, together accounting for 70% of all 2017 cases.
“The resurgence of syphilis, and particularly congenital syphilis, is not an arbitrary event, but rather a symptom of a deteriorating public health infrastructure and lack of access to health care,” wrote Gail Bolan, MD, director of the Division of STD Prevention at the CDC’s National Center for HIV/AIDS, Viral Hepatitis, STD and TB Prevention. “It is exposing hidden, fragile populations in need that are not getting the health care and preventive services they deserve.”
Dr. Bolan recommends modernizing surveillance to capture more of the cases in populations without ready access to diagnosis and treatment and in those choosing not to access care.
“It is imperative that federal, state, and local programs employ strategies that maximize long-term population impact by reducing STD incidence and promoting sexual, reproductive, maternal, and infant health,” she wrote. “Further, it will be important for us to measure and monitor the adverse health consequences of STDs, such as ocular and neurosyphilis, pelvic inflammatory disease, ectopic pregnancy, infertility, HIV, congenital syphilis, and neonatal herpes.”
Multiple sources contributed data to the report: state and local STD programs’ notifiable disease reporting, private and federal national surveys, and specific projects that collect STD prevalence data, including the National Job Training Program, the STD Surveillance Network and the Gonococcal Isolate Surveillance Project.
The four nationally notifiable STDs are chlamydia, gonorrhea, syphilis, and chancroid.
The rise in newborn syphilis cases, currently at 23.3 cases per 100,000 live births, mirrors the increased U.S. prevalence of both primary and secondary syphilis in 2017, with 9.5 cases per 100,000 people. Syphilis has increased every year since 2000-2001, when prevalence was at a record low.
Chlamydia and gonorrhea rates climb too
The report also noted increases in the prevalence of other STDs. Chlamydia, the most common STD, increased 6.9% as compared to 2016, with 528.8 cases per 100,000 people. This increase occurred in all U.S. regions and independently of sex, race, or ethnicity, though rates were highest in teens and young adults. Nearly two-thirds of chlamydia cases in 2017 occurred in people ages 15-24 years old.
Reported rates were higher in women than in men, likely due to women’s increased likelihood of undergoing screening, the report suggested. Better surveillance may also partly explain the climb in men’s cases.
“Increases in rates among men may reflect an increased number of men, including gay, bisexual and other men who have sex with men (collectively referred to as MSM) being tested and diagnosed with a chlamydial infection due to increased availability of urine testing and extragenital screening,” according to the report.
The CDC received reports of more than a half million gonorrhea infections in 2017 (555,608 cases), an increase of 18.6% since the previous year, including a 19.3% increase among men and a 17.8% increase among women.
“The magnitude of the increase among men suggests either increased transmission, increased case ascertainment (e.g., through increased extra-genital screening among MSM), or both,” the authors wrote. “The concurrent increase in cases reported among women suggests parallel increases in heterosexual transmission, increased screening among women, or both.”
Overall, gonorrhea cases have skyrocketed 75.2% since their historic low in 2009, compounding the problem of antibiotic resistance that has limited CDC-recommended treatment to just ceftriaxone and azithromycin.
The report was supported by the Centers for Disease Control and Prevention. The authors did not report having any disclosures.
SOURCE: Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2017; https://www.cdc.gov/std/stats
Rapidly increasing cases of newborn syphilis have reached their highest prevalence in 2 decades, according to a new report by the Centers for Disease Control and Prevention on sexually transmitted disease surveillance in 2017.
Newborn syphilis incidence has more than doubled, from 362 cases in 2013 to 918 cases in 2017, resulting in 64 syphilitic stillbirths and 13 infant deaths that year, according to data published in Sexually Transmitted Disease Surveillance 2017.
At least one case was reported in 37 states last year, and the greatest burden of cases occurred in California, Arizona, Texas, Louisiana, and Florida, together accounting for 70% of all 2017 cases.
“The resurgence of syphilis, and particularly congenital syphilis, is not an arbitrary event, but rather a symptom of a deteriorating public health infrastructure and lack of access to health care,” wrote Gail Bolan, MD, director of the Division of STD Prevention at the CDC’s National Center for HIV/AIDS, Viral Hepatitis, STD and TB Prevention. “It is exposing hidden, fragile populations in need that are not getting the health care and preventive services they deserve.”
Dr. Bolan recommends modernizing surveillance to capture more of the cases in populations without ready access to diagnosis and treatment and in those choosing not to access care.
“It is imperative that federal, state, and local programs employ strategies that maximize long-term population impact by reducing STD incidence and promoting sexual, reproductive, maternal, and infant health,” she wrote. “Further, it will be important for us to measure and monitor the adverse health consequences of STDs, such as ocular and neurosyphilis, pelvic inflammatory disease, ectopic pregnancy, infertility, HIV, congenital syphilis, and neonatal herpes.”
Multiple sources contributed data to the report: state and local STD programs’ notifiable disease reporting, private and federal national surveys, and specific projects that collect STD prevalence data, including the National Job Training Program, the STD Surveillance Network and the Gonococcal Isolate Surveillance Project.
The four nationally notifiable STDs are chlamydia, gonorrhea, syphilis, and chancroid.
The rise in newborn syphilis cases, currently at 23.3 cases per 100,000 live births, mirrors the increased U.S. prevalence of both primary and secondary syphilis in 2017, with 9.5 cases per 100,000 people. Syphilis has increased every year since 2000-2001, when prevalence was at a record low.
Chlamydia and gonorrhea rates climb too
The report also noted increases in the prevalence of other STDs. Chlamydia, the most common STD, increased 6.9% as compared to 2016, with 528.8 cases per 100,000 people. This increase occurred in all U.S. regions and independently of sex, race, or ethnicity, though rates were highest in teens and young adults. Nearly two-thirds of chlamydia cases in 2017 occurred in people ages 15-24 years old.
Reported rates were higher in women than in men, likely due to women’s increased likelihood of undergoing screening, the report suggested. Better surveillance may also partly explain the climb in men’s cases.
“Increases in rates among men may reflect an increased number of men, including gay, bisexual and other men who have sex with men (collectively referred to as MSM) being tested and diagnosed with a chlamydial infection due to increased availability of urine testing and extragenital screening,” according to the report.
The CDC received reports of more than a half million gonorrhea infections in 2017 (555,608 cases), an increase of 18.6% since the previous year, including a 19.3% increase among men and a 17.8% increase among women.
“The magnitude of the increase among men suggests either increased transmission, increased case ascertainment (e.g., through increased extra-genital screening among MSM), or both,” the authors wrote. “The concurrent increase in cases reported among women suggests parallel increases in heterosexual transmission, increased screening among women, or both.”
Overall, gonorrhea cases have skyrocketed 75.2% since their historic low in 2009, compounding the problem of antibiotic resistance that has limited CDC-recommended treatment to just ceftriaxone and azithromycin.
The report was supported by the Centers for Disease Control and Prevention. The authors did not report having any disclosures.
SOURCE: Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2017; https://www.cdc.gov/std/stats
Rapidly increasing cases of newborn syphilis have reached their highest prevalence in 2 decades, according to a new report by the Centers for Disease Control and Prevention on sexually transmitted disease surveillance in 2017.
Newborn syphilis incidence has more than doubled, from 362 cases in 2013 to 918 cases in 2017, resulting in 64 syphilitic stillbirths and 13 infant deaths that year, according to data published in Sexually Transmitted Disease Surveillance 2017.
At least one case was reported in 37 states last year, and the greatest burden of cases occurred in California, Arizona, Texas, Louisiana, and Florida, together accounting for 70% of all 2017 cases.
“The resurgence of syphilis, and particularly congenital syphilis, is not an arbitrary event, but rather a symptom of a deteriorating public health infrastructure and lack of access to health care,” wrote Gail Bolan, MD, director of the Division of STD Prevention at the CDC’s National Center for HIV/AIDS, Viral Hepatitis, STD and TB Prevention. “It is exposing hidden, fragile populations in need that are not getting the health care and preventive services they deserve.”
Dr. Bolan recommends modernizing surveillance to capture more of the cases in populations without ready access to diagnosis and treatment and in those choosing not to access care.
“It is imperative that federal, state, and local programs employ strategies that maximize long-term population impact by reducing STD incidence and promoting sexual, reproductive, maternal, and infant health,” she wrote. “Further, it will be important for us to measure and monitor the adverse health consequences of STDs, such as ocular and neurosyphilis, pelvic inflammatory disease, ectopic pregnancy, infertility, HIV, congenital syphilis, and neonatal herpes.”
Multiple sources contributed data to the report: state and local STD programs’ notifiable disease reporting, private and federal national surveys, and specific projects that collect STD prevalence data, including the National Job Training Program, the STD Surveillance Network and the Gonococcal Isolate Surveillance Project.
The four nationally notifiable STDs are chlamydia, gonorrhea, syphilis, and chancroid.
The rise in newborn syphilis cases, currently at 23.3 cases per 100,000 live births, mirrors the increased U.S. prevalence of both primary and secondary syphilis in 2017, with 9.5 cases per 100,000 people. Syphilis has increased every year since 2000-2001, when prevalence was at a record low.
Chlamydia and gonorrhea rates climb too
The report also noted increases in the prevalence of other STDs. Chlamydia, the most common STD, increased 6.9% as compared to 2016, with 528.8 cases per 100,000 people. This increase occurred in all U.S. regions and independently of sex, race, or ethnicity, though rates were highest in teens and young adults. Nearly two-thirds of chlamydia cases in 2017 occurred in people ages 15-24 years old.
Reported rates were higher in women than in men, likely due to women’s increased likelihood of undergoing screening, the report suggested. Better surveillance may also partly explain the climb in men’s cases.
“Increases in rates among men may reflect an increased number of men, including gay, bisexual and other men who have sex with men (collectively referred to as MSM) being tested and diagnosed with a chlamydial infection due to increased availability of urine testing and extragenital screening,” according to the report.
The CDC received reports of more than a half million gonorrhea infections in 2017 (555,608 cases), an increase of 18.6% since the previous year, including a 19.3% increase among men and a 17.8% increase among women.
“The magnitude of the increase among men suggests either increased transmission, increased case ascertainment (e.g., through increased extra-genital screening among MSM), or both,” the authors wrote. “The concurrent increase in cases reported among women suggests parallel increases in heterosexual transmission, increased screening among women, or both.”
Overall, gonorrhea cases have skyrocketed 75.2% since their historic low in 2009, compounding the problem of antibiotic resistance that has limited CDC-recommended treatment to just ceftriaxone and azithromycin.
The report was supported by the Centers for Disease Control and Prevention. The authors did not report having any disclosures.
SOURCE: Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2017; https://www.cdc.gov/std/stats
Key clinical point: Newborn syphilis cases have more than doubled in 5 years along with substantial increases in chlamydia, gonorrhea, and syphilis.
Major finding: 918 cases of newborn syphilis were reported in 37 states in 2017.
Study details: The findings are based on data from public health notifiable disease reports and multiple federal and private surveillance projects.
Disclosures: The report was supported by the Centers for Disease Control and Prevention. The authors did not report having any disclosures.
Source: Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2017.