User login
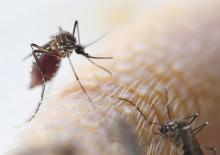
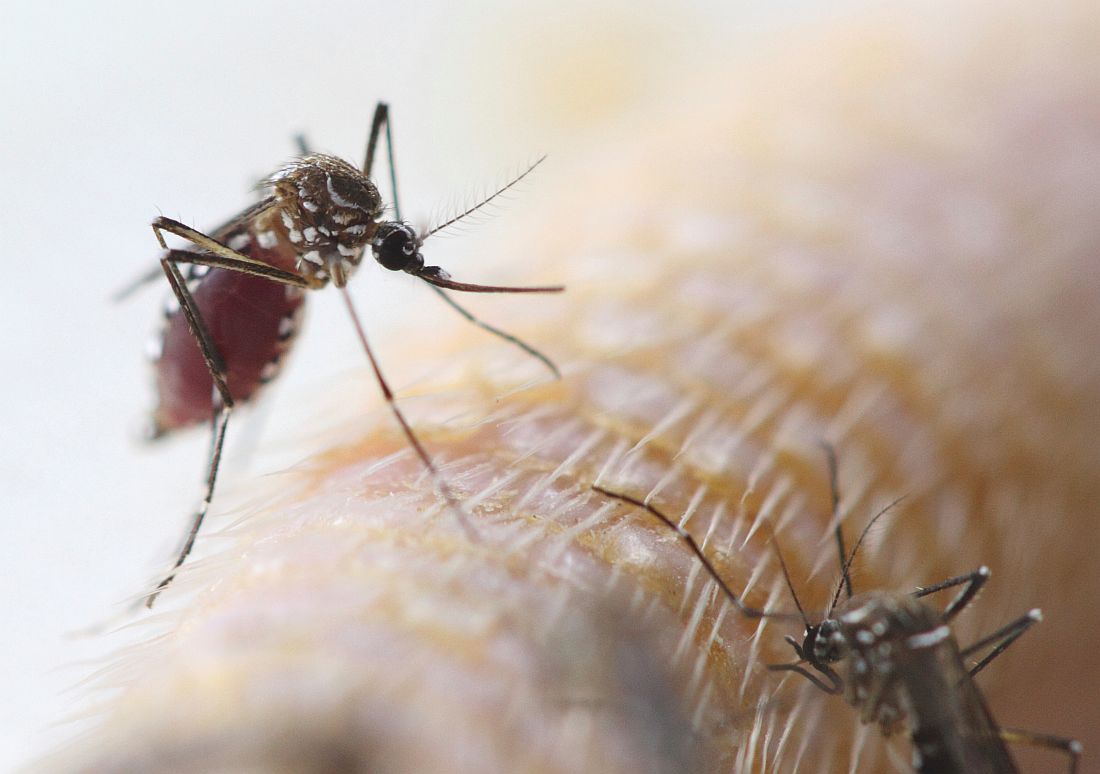
Extreme caffeine use in early pregnancy risks offspring behavioral disorders
, Susanne Hvolgaard Mikkelsen, PhD, of Aarhus (Denmark) University and her associates said.
“Caffeine metabolism is delayed during pregnancy, allowing longer opportunities for absorption. Caffeine increases dopamine levels by slowing down the rate of dopamine reabsorption, and this dysfunction of the dopaminergic system is thought to explain part of the association between caffeine consumption and offspring behavioral disorders,” the investigators said.
From 1996 to 2002, Danish physicians recruited women during their first pregnancy visit to participate in a study evaluating daily coffee and tea consumption during pregnancy, with follow-up of the children at age 11 years using the Danish National Birth Cohort. A follow-up questionnaire, including the Strength and Difficulties Questionnaire, was completed by the children, their parents, and their teachers; the results were paired with data on coffee and tea consumption by the mothers of 47,491 children. At 15 weeks’ gestation, 12% of the women had consumed more than three cups/day of coffee, and 3% ingested eight or more cups/day of coffee; 19% of the women drank more than three cups/day of tea, and 4% drank eight or more cups/day of tea. The amount of coffee and tea consumed at 30 weeks’ gestation was only slightly less.
The percentage of children predicted to have “possible/probable” ADHD was 5%, conduct-oppositional disorders was 7%, anxiety-depressive disorders was 7%, and any psychiatric disorder was 14%.
A statistically significant association was found between maternal consumption of eight or more cups of coffee at 15 weeks’ gestation and greater risk of ADHD (a risk ratio [RR] of 1.47), conduct-oppositional disorder (RR, 1.22), and any psychiatric disorder (RR, 1.23). A statistically significant link was found between maternal consumption of eight or more cups of tea at 15 weeks’ gestation and greater risk of conduct-oppositional disorder (RR, 1.21). There was a statistically significant association between maternal consumption of eight or more cups of tea at 15 weeks’ gestation and a higher risk of anxiety-depressive disorder (RR, 1.28), but not for coffee. These significant relationships were not found again at 30 weeks’ gestation.
“The period of greatest prenatal brain development is 10-26 weeks after conception,which may explain our results suggesting the fetus to be more vulnerable to caffeine in first and second trimester, compared with the third trimester,” the researchers said.
There was no information available about caffeine consumption by chocolate, energy drinks, or medication; the measure of cola consumption also was not taken into account. Confounding by genetics or socioeconomic factors may affect these findings, Dr. Mikkelsen and her associates said.
Read more at (J Pediatr. 2017 Jul 18. doi: 10.1016/j.jpeds.2017.06.051).
, Susanne Hvolgaard Mikkelsen, PhD, of Aarhus (Denmark) University and her associates said.
“Caffeine metabolism is delayed during pregnancy, allowing longer opportunities for absorption. Caffeine increases dopamine levels by slowing down the rate of dopamine reabsorption, and this dysfunction of the dopaminergic system is thought to explain part of the association between caffeine consumption and offspring behavioral disorders,” the investigators said.
From 1996 to 2002, Danish physicians recruited women during their first pregnancy visit to participate in a study evaluating daily coffee and tea consumption during pregnancy, with follow-up of the children at age 11 years using the Danish National Birth Cohort. A follow-up questionnaire, including the Strength and Difficulties Questionnaire, was completed by the children, their parents, and their teachers; the results were paired with data on coffee and tea consumption by the mothers of 47,491 children. At 15 weeks’ gestation, 12% of the women had consumed more than three cups/day of coffee, and 3% ingested eight or more cups/day of coffee; 19% of the women drank more than three cups/day of tea, and 4% drank eight or more cups/day of tea. The amount of coffee and tea consumed at 30 weeks’ gestation was only slightly less.
The percentage of children predicted to have “possible/probable” ADHD was 5%, conduct-oppositional disorders was 7%, anxiety-depressive disorders was 7%, and any psychiatric disorder was 14%.
A statistically significant association was found between maternal consumption of eight or more cups of coffee at 15 weeks’ gestation and greater risk of ADHD (a risk ratio [RR] of 1.47), conduct-oppositional disorder (RR, 1.22), and any psychiatric disorder (RR, 1.23). A statistically significant link was found between maternal consumption of eight or more cups of tea at 15 weeks’ gestation and greater risk of conduct-oppositional disorder (RR, 1.21). There was a statistically significant association between maternal consumption of eight or more cups of tea at 15 weeks’ gestation and a higher risk of anxiety-depressive disorder (RR, 1.28), but not for coffee. These significant relationships were not found again at 30 weeks’ gestation.
“The period of greatest prenatal brain development is 10-26 weeks after conception,which may explain our results suggesting the fetus to be more vulnerable to caffeine in first and second trimester, compared with the third trimester,” the researchers said.
There was no information available about caffeine consumption by chocolate, energy drinks, or medication; the measure of cola consumption also was not taken into account. Confounding by genetics or socioeconomic factors may affect these findings, Dr. Mikkelsen and her associates said.
Read more at (J Pediatr. 2017 Jul 18. doi: 10.1016/j.jpeds.2017.06.051).
, Susanne Hvolgaard Mikkelsen, PhD, of Aarhus (Denmark) University and her associates said.
“Caffeine metabolism is delayed during pregnancy, allowing longer opportunities for absorption. Caffeine increases dopamine levels by slowing down the rate of dopamine reabsorption, and this dysfunction of the dopaminergic system is thought to explain part of the association between caffeine consumption and offspring behavioral disorders,” the investigators said.
From 1996 to 2002, Danish physicians recruited women during their first pregnancy visit to participate in a study evaluating daily coffee and tea consumption during pregnancy, with follow-up of the children at age 11 years using the Danish National Birth Cohort. A follow-up questionnaire, including the Strength and Difficulties Questionnaire, was completed by the children, their parents, and their teachers; the results were paired with data on coffee and tea consumption by the mothers of 47,491 children. At 15 weeks’ gestation, 12% of the women had consumed more than three cups/day of coffee, and 3% ingested eight or more cups/day of coffee; 19% of the women drank more than three cups/day of tea, and 4% drank eight or more cups/day of tea. The amount of coffee and tea consumed at 30 weeks’ gestation was only slightly less.
The percentage of children predicted to have “possible/probable” ADHD was 5%, conduct-oppositional disorders was 7%, anxiety-depressive disorders was 7%, and any psychiatric disorder was 14%.
A statistically significant association was found between maternal consumption of eight or more cups of coffee at 15 weeks’ gestation and greater risk of ADHD (a risk ratio [RR] of 1.47), conduct-oppositional disorder (RR, 1.22), and any psychiatric disorder (RR, 1.23). A statistically significant link was found between maternal consumption of eight or more cups of tea at 15 weeks’ gestation and greater risk of conduct-oppositional disorder (RR, 1.21). There was a statistically significant association between maternal consumption of eight or more cups of tea at 15 weeks’ gestation and a higher risk of anxiety-depressive disorder (RR, 1.28), but not for coffee. These significant relationships were not found again at 30 weeks’ gestation.
“The period of greatest prenatal brain development is 10-26 weeks after conception,which may explain our results suggesting the fetus to be more vulnerable to caffeine in first and second trimester, compared with the third trimester,” the researchers said.
There was no information available about caffeine consumption by chocolate, energy drinks, or medication; the measure of cola consumption also was not taken into account. Confounding by genetics or socioeconomic factors may affect these findings, Dr. Mikkelsen and her associates said.
Read more at (J Pediatr. 2017 Jul 18. doi: 10.1016/j.jpeds.2017.06.051).
FROM THE JOURNAL OF PEDIATRICS
Prenatal SSRI exposure’s effect on development
Unfortunately, the bottom line for most of these important questions is that we really don’t know as much as we probably should.
Just when we’ve read a convincing finding from a reputable journal that establishes a link between prenatal SSRI use and an untoward outcome, we see it disputed the next month. Why is this always happening, and why can’t we really know anything with certainty? Much of the confusion can be attributed to research methods and the obvious difficulty of using randomized, controlled trials to control for potential confounding factors. While statistical techniques have become increasingly sophisticated in addressing these confounding factors, they remain imperfect. For example, one of the most difficult challenges that remains is separating any effects of a medication from any effects caused by the condition it was designed to treat. Comparing women with the same underlying condition, some of whom are treated with a medication and some of whom are not, is a step forward, but there may be important reasons that one group decides to seek treatment and the other doesn’t. One clever research design that was employed to look at congenital anomalies in the offspring of women taking SSRIs accounted for siblings of these children who were born when their mother was not taking an SSRI. This study demonstrated that these women were more likely to have children with congenital malformations even when they weren’t taking the SSRIs.1 Other factors that render this literature difficult to interpret include small sample sizes when looking at specific SSRIs (many studies cluster them all), dose effects, timing (which trimester), duration of treatment, and method of recording compliance.
The potential link between SSRIs and autism has received a fair amount of attention lately, especially after a very well-designed study in 2016 suggested a significantly increased risk.10 However, as with many of the findings, this study was quickly disputed by other high-quality, well-powered research that found no increased risk after controlling for maternal illness.11,12
ADHD generally has not been found to be related to maternal SSRI use, although one study did find a link between ADHD and tricyclic antidepressants.12,13
In terms of other neurodevelopmental outcomes, there have been many negative studies examining IQ, nonverbal communication, as well as speech and motor skills.14,15,16 However, as with so many other outcomes, some other studies contradict these negative results. According to a recent, large cohort study, there may be some concern regarding SSRI exposure prenatally and an increase in speech disorders by age 14 years, as well as lower language competence at age 3 years.17,18 Likewise, mild motor abnormalities have been observed, with maternal depression severity as an independent but contributing factor.19
Several studies demonstrate a connection between prenatal SSRI exposure and childhood internalizing symptoms, such as depression and anxiety, independent of maternal depression.12,20 These findings must be balanced with our knowledge of the serious mental health conditions in offspring that are associated with untreated maternal illness, including both internalizing and externalizing disorders.21,22
How does one come to any firm conclusions to guide a primary care clinician’s practice and recommendations? Hopefully, the evidence will become clearer over time as we adopt more sophisticated designs and accumulate observations. A larger number of observations would allow us to decrease heterogeneity by studying subgroups according to type of SSRI and duration of exposure. Enhanced understanding of the role of genetic factors also may shed some light on individual variation as the serotonin transporter gene has been suggested as a potential moderator of sensitivity.23
Dr. Guth is an assistant professor in the department of psychiatry at the University of Vermont Medical Center and the University of Vermont Robert Larner College of Medicine, both in Burlington. She works with children and adolescents as well as women in the perinatal period. She has no relevant financial disclosures.
References
1. BMJ. 2015 Apr 17;350:h1798.
2. Can J Clin Pharmacol. 2009 Winter;16(1):e66-7.
3. J Matern Fetal Neonatal Med. 2008 Oct;21(10):745-51.
4. PLoS ONE. 2014 Nov; 9(11): e111327.
5. Pediatr Res. 2017 Jun 30. doi: 10.1038/pr.2017.156. [Epub ahead of print].
6. J Clin Psychiatry. 2017 May;78(5):605-11.
7. Acta Psychiatr Scand. 2010 Jun;121(6):471-9.
8. Am J Psychiatry. 2016 Feb 1;173(2):147-57.
9. J Perinatol. 2011 Sep;31(9):615-20.
10. JAMA Pediatr. 2016 Feb;170(2):117-24.
11. JAMA. 2017 Apr 18;317(15):1544-52.
12. J Am Acad Child Adolesc Psychiatry. 2016 May;55(5):359-66.
13. Paediatr Perinat Epidemiol. 2017 Jul;31(4):363-73.
14. Acta Obstet Gynecol Scand. 2015 May;94(5):501-7.
15. J Psychopharmacol. 2017 Mar;31(3):346-55.
16. CNS Drugs. 2005;19(7):623-33.
17. JAMA Psychiatry. 2016 Nov 1;73(11):1163-70.
18. BJOG. 2014. doi: 10.1111/1471-0528.12821.
19. BJOG. 2016 Nov;123(12):1908-17.
20. Pediatr Res. 2015 Aug;78(2):174-80.
21. Neuroscience. 2017 Feb 7;342:154-66.
22. Depress Anxiety. 2014 Jan;31(1):9-18.
23. Neuroscience. 2017 Feb 7;342:212-31.
Unfortunately, the bottom line for most of these important questions is that we really don’t know as much as we probably should.
Just when we’ve read a convincing finding from a reputable journal that establishes a link between prenatal SSRI use and an untoward outcome, we see it disputed the next month. Why is this always happening, and why can’t we really know anything with certainty? Much of the confusion can be attributed to research methods and the obvious difficulty of using randomized, controlled trials to control for potential confounding factors. While statistical techniques have become increasingly sophisticated in addressing these confounding factors, they remain imperfect. For example, one of the most difficult challenges that remains is separating any effects of a medication from any effects caused by the condition it was designed to treat. Comparing women with the same underlying condition, some of whom are treated with a medication and some of whom are not, is a step forward, but there may be important reasons that one group decides to seek treatment and the other doesn’t. One clever research design that was employed to look at congenital anomalies in the offspring of women taking SSRIs accounted for siblings of these children who were born when their mother was not taking an SSRI. This study demonstrated that these women were more likely to have children with congenital malformations even when they weren’t taking the SSRIs.1 Other factors that render this literature difficult to interpret include small sample sizes when looking at specific SSRIs (many studies cluster them all), dose effects, timing (which trimester), duration of treatment, and method of recording compliance.
The potential link between SSRIs and autism has received a fair amount of attention lately, especially after a very well-designed study in 2016 suggested a significantly increased risk.10 However, as with many of the findings, this study was quickly disputed by other high-quality, well-powered research that found no increased risk after controlling for maternal illness.11,12
ADHD generally has not been found to be related to maternal SSRI use, although one study did find a link between ADHD and tricyclic antidepressants.12,13
In terms of other neurodevelopmental outcomes, there have been many negative studies examining IQ, nonverbal communication, as well as speech and motor skills.14,15,16 However, as with so many other outcomes, some other studies contradict these negative results. According to a recent, large cohort study, there may be some concern regarding SSRI exposure prenatally and an increase in speech disorders by age 14 years, as well as lower language competence at age 3 years.17,18 Likewise, mild motor abnormalities have been observed, with maternal depression severity as an independent but contributing factor.19
Several studies demonstrate a connection between prenatal SSRI exposure and childhood internalizing symptoms, such as depression and anxiety, independent of maternal depression.12,20 These findings must be balanced with our knowledge of the serious mental health conditions in offspring that are associated with untreated maternal illness, including both internalizing and externalizing disorders.21,22
How does one come to any firm conclusions to guide a primary care clinician’s practice and recommendations? Hopefully, the evidence will become clearer over time as we adopt more sophisticated designs and accumulate observations. A larger number of observations would allow us to decrease heterogeneity by studying subgroups according to type of SSRI and duration of exposure. Enhanced understanding of the role of genetic factors also may shed some light on individual variation as the serotonin transporter gene has been suggested as a potential moderator of sensitivity.23
Dr. Guth is an assistant professor in the department of psychiatry at the University of Vermont Medical Center and the University of Vermont Robert Larner College of Medicine, both in Burlington. She works with children and adolescents as well as women in the perinatal period. She has no relevant financial disclosures.
References
1. BMJ. 2015 Apr 17;350:h1798.
2. Can J Clin Pharmacol. 2009 Winter;16(1):e66-7.
3. J Matern Fetal Neonatal Med. 2008 Oct;21(10):745-51.
4. PLoS ONE. 2014 Nov; 9(11): e111327.
5. Pediatr Res. 2017 Jun 30. doi: 10.1038/pr.2017.156. [Epub ahead of print].
6. J Clin Psychiatry. 2017 May;78(5):605-11.
7. Acta Psychiatr Scand. 2010 Jun;121(6):471-9.
8. Am J Psychiatry. 2016 Feb 1;173(2):147-57.
9. J Perinatol. 2011 Sep;31(9):615-20.
10. JAMA Pediatr. 2016 Feb;170(2):117-24.
11. JAMA. 2017 Apr 18;317(15):1544-52.
12. J Am Acad Child Adolesc Psychiatry. 2016 May;55(5):359-66.
13. Paediatr Perinat Epidemiol. 2017 Jul;31(4):363-73.
14. Acta Obstet Gynecol Scand. 2015 May;94(5):501-7.
15. J Psychopharmacol. 2017 Mar;31(3):346-55.
16. CNS Drugs. 2005;19(7):623-33.
17. JAMA Psychiatry. 2016 Nov 1;73(11):1163-70.
18. BJOG. 2014. doi: 10.1111/1471-0528.12821.
19. BJOG. 2016 Nov;123(12):1908-17.
20. Pediatr Res. 2015 Aug;78(2):174-80.
21. Neuroscience. 2017 Feb 7;342:154-66.
22. Depress Anxiety. 2014 Jan;31(1):9-18.
23. Neuroscience. 2017 Feb 7;342:212-31.
Unfortunately, the bottom line for most of these important questions is that we really don’t know as much as we probably should.
Just when we’ve read a convincing finding from a reputable journal that establishes a link between prenatal SSRI use and an untoward outcome, we see it disputed the next month. Why is this always happening, and why can’t we really know anything with certainty? Much of the confusion can be attributed to research methods and the obvious difficulty of using randomized, controlled trials to control for potential confounding factors. While statistical techniques have become increasingly sophisticated in addressing these confounding factors, they remain imperfect. For example, one of the most difficult challenges that remains is separating any effects of a medication from any effects caused by the condition it was designed to treat. Comparing women with the same underlying condition, some of whom are treated with a medication and some of whom are not, is a step forward, but there may be important reasons that one group decides to seek treatment and the other doesn’t. One clever research design that was employed to look at congenital anomalies in the offspring of women taking SSRIs accounted for siblings of these children who were born when their mother was not taking an SSRI. This study demonstrated that these women were more likely to have children with congenital malformations even when they weren’t taking the SSRIs.1 Other factors that render this literature difficult to interpret include small sample sizes when looking at specific SSRIs (many studies cluster them all), dose effects, timing (which trimester), duration of treatment, and method of recording compliance.
The potential link between SSRIs and autism has received a fair amount of attention lately, especially after a very well-designed study in 2016 suggested a significantly increased risk.10 However, as with many of the findings, this study was quickly disputed by other high-quality, well-powered research that found no increased risk after controlling for maternal illness.11,12
ADHD generally has not been found to be related to maternal SSRI use, although one study did find a link between ADHD and tricyclic antidepressants.12,13
In terms of other neurodevelopmental outcomes, there have been many negative studies examining IQ, nonverbal communication, as well as speech and motor skills.14,15,16 However, as with so many other outcomes, some other studies contradict these negative results. According to a recent, large cohort study, there may be some concern regarding SSRI exposure prenatally and an increase in speech disorders by age 14 years, as well as lower language competence at age 3 years.17,18 Likewise, mild motor abnormalities have been observed, with maternal depression severity as an independent but contributing factor.19
Several studies demonstrate a connection between prenatal SSRI exposure and childhood internalizing symptoms, such as depression and anxiety, independent of maternal depression.12,20 These findings must be balanced with our knowledge of the serious mental health conditions in offspring that are associated with untreated maternal illness, including both internalizing and externalizing disorders.21,22
How does one come to any firm conclusions to guide a primary care clinician’s practice and recommendations? Hopefully, the evidence will become clearer over time as we adopt more sophisticated designs and accumulate observations. A larger number of observations would allow us to decrease heterogeneity by studying subgroups according to type of SSRI and duration of exposure. Enhanced understanding of the role of genetic factors also may shed some light on individual variation as the serotonin transporter gene has been suggested as a potential moderator of sensitivity.23
Dr. Guth is an assistant professor in the department of psychiatry at the University of Vermont Medical Center and the University of Vermont Robert Larner College of Medicine, both in Burlington. She works with children and adolescents as well as women in the perinatal period. She has no relevant financial disclosures.
References
1. BMJ. 2015 Apr 17;350:h1798.
2. Can J Clin Pharmacol. 2009 Winter;16(1):e66-7.
3. J Matern Fetal Neonatal Med. 2008 Oct;21(10):745-51.
4. PLoS ONE. 2014 Nov; 9(11): e111327.
5. Pediatr Res. 2017 Jun 30. doi: 10.1038/pr.2017.156. [Epub ahead of print].
6. J Clin Psychiatry. 2017 May;78(5):605-11.
7. Acta Psychiatr Scand. 2010 Jun;121(6):471-9.
8. Am J Psychiatry. 2016 Feb 1;173(2):147-57.
9. J Perinatol. 2011 Sep;31(9):615-20.
10. JAMA Pediatr. 2016 Feb;170(2):117-24.
11. JAMA. 2017 Apr 18;317(15):1544-52.
12. J Am Acad Child Adolesc Psychiatry. 2016 May;55(5):359-66.
13. Paediatr Perinat Epidemiol. 2017 Jul;31(4):363-73.
14. Acta Obstet Gynecol Scand. 2015 May;94(5):501-7.
15. J Psychopharmacol. 2017 Mar;31(3):346-55.
16. CNS Drugs. 2005;19(7):623-33.
17. JAMA Psychiatry. 2016 Nov 1;73(11):1163-70.
18. BJOG. 2014. doi: 10.1111/1471-0528.12821.
19. BJOG. 2016 Nov;123(12):1908-17.
20. Pediatr Res. 2015 Aug;78(2):174-80.
21. Neuroscience. 2017 Feb 7;342:154-66.
22. Depress Anxiety. 2014 Jan;31(1):9-18.
23. Neuroscience. 2017 Feb 7;342:212-31.
Early neuroimaging essential for Zika-exposed neonates
DENVER – The experience gleaned at ground zero of the Brazilian Zika virus epidemic drives home a clinical imperative: every neonate whose pregnant mother has presumed or confirmed Zika infection needs to undergo prompt neuroimaging, even if head circumference at birth is normal, Vanessa van der Linden, MD, said at the annual meeting of the Teratology Society.
Dr. van der Linden, a pediatric neurologist at the Association for Assistance of Disabled Children in Recife, Brazil, has done pioneering work in characterizing the recently recognized congenital Zika syndrome. She was the lead author of the first report of infants who had laboratory evidence of congenital Zika infection and normal head circumference at birth but who developed poor head growth and microcephaly later in infancy.
Comprehensive multispecialty medical and developmental follow-up documented that 10 of 13 infants had dysphagia, 7 had epilepsy, 3 had chorioretinal abnormalities, all 13 had hypertonia, and 12 had pyramidal and extrapyramidal signs with dystonia (MMWR. 2016 Dec 2;65[47]:1343-8).
In another recent publication, Dr. van der Linden and her coinvestigators described classic congenital Zika syndrome with microcephaly at birth as simply the tip of the Zika virus iceberg. In their retrospective review of 77 infants exposed to Zika in utero, 9 had microcephaly at birth, 7 developed microcephaly postnatally, and 3 didn’t have microcephaly at all. Those with microcephaly at birth showed the traditional neuroimaging findings of congenital Zika syndrome, including reduced brain volume, ventriculomegaly, subcortical calcifications, corpus callosum abnormalities, and an enlarged extra-axial space.
Those who subsequently developed microcephaly later in infancy showed most of the same neuroimaging abnormalities. The three infants who remained normocephalic displayed calcifications in the cortico-subcortical junction, asymmetric frontal polymicrogyria, delayed myelination, and milder ventriculomegaly than in the other two groups (AJNR Am J Neuroradiol. 2017 Jul;38[7]:1427-34).
The rehabilitation center where Dr. van der Linden and her colleagues are currently following roughly 200 children with congenital Zika syndrome is in the state of Pernambuco, which was particularly hard hit by the Zika epidemic.
She reported having no relevant financial disclosures.
DENVER – The experience gleaned at ground zero of the Brazilian Zika virus epidemic drives home a clinical imperative: every neonate whose pregnant mother has presumed or confirmed Zika infection needs to undergo prompt neuroimaging, even if head circumference at birth is normal, Vanessa van der Linden, MD, said at the annual meeting of the Teratology Society.
Dr. van der Linden, a pediatric neurologist at the Association for Assistance of Disabled Children in Recife, Brazil, has done pioneering work in characterizing the recently recognized congenital Zika syndrome. She was the lead author of the first report of infants who had laboratory evidence of congenital Zika infection and normal head circumference at birth but who developed poor head growth and microcephaly later in infancy.
Comprehensive multispecialty medical and developmental follow-up documented that 10 of 13 infants had dysphagia, 7 had epilepsy, 3 had chorioretinal abnormalities, all 13 had hypertonia, and 12 had pyramidal and extrapyramidal signs with dystonia (MMWR. 2016 Dec 2;65[47]:1343-8).
In another recent publication, Dr. van der Linden and her coinvestigators described classic congenital Zika syndrome with microcephaly at birth as simply the tip of the Zika virus iceberg. In their retrospective review of 77 infants exposed to Zika in utero, 9 had microcephaly at birth, 7 developed microcephaly postnatally, and 3 didn’t have microcephaly at all. Those with microcephaly at birth showed the traditional neuroimaging findings of congenital Zika syndrome, including reduced brain volume, ventriculomegaly, subcortical calcifications, corpus callosum abnormalities, and an enlarged extra-axial space.
Those who subsequently developed microcephaly later in infancy showed most of the same neuroimaging abnormalities. The three infants who remained normocephalic displayed calcifications in the cortico-subcortical junction, asymmetric frontal polymicrogyria, delayed myelination, and milder ventriculomegaly than in the other two groups (AJNR Am J Neuroradiol. 2017 Jul;38[7]:1427-34).
The rehabilitation center where Dr. van der Linden and her colleagues are currently following roughly 200 children with congenital Zika syndrome is in the state of Pernambuco, which was particularly hard hit by the Zika epidemic.
She reported having no relevant financial disclosures.
DENVER – The experience gleaned at ground zero of the Brazilian Zika virus epidemic drives home a clinical imperative: every neonate whose pregnant mother has presumed or confirmed Zika infection needs to undergo prompt neuroimaging, even if head circumference at birth is normal, Vanessa van der Linden, MD, said at the annual meeting of the Teratology Society.
Dr. van der Linden, a pediatric neurologist at the Association for Assistance of Disabled Children in Recife, Brazil, has done pioneering work in characterizing the recently recognized congenital Zika syndrome. She was the lead author of the first report of infants who had laboratory evidence of congenital Zika infection and normal head circumference at birth but who developed poor head growth and microcephaly later in infancy.
Comprehensive multispecialty medical and developmental follow-up documented that 10 of 13 infants had dysphagia, 7 had epilepsy, 3 had chorioretinal abnormalities, all 13 had hypertonia, and 12 had pyramidal and extrapyramidal signs with dystonia (MMWR. 2016 Dec 2;65[47]:1343-8).
In another recent publication, Dr. van der Linden and her coinvestigators described classic congenital Zika syndrome with microcephaly at birth as simply the tip of the Zika virus iceberg. In their retrospective review of 77 infants exposed to Zika in utero, 9 had microcephaly at birth, 7 developed microcephaly postnatally, and 3 didn’t have microcephaly at all. Those with microcephaly at birth showed the traditional neuroimaging findings of congenital Zika syndrome, including reduced brain volume, ventriculomegaly, subcortical calcifications, corpus callosum abnormalities, and an enlarged extra-axial space.
Those who subsequently developed microcephaly later in infancy showed most of the same neuroimaging abnormalities. The three infants who remained normocephalic displayed calcifications in the cortico-subcortical junction, asymmetric frontal polymicrogyria, delayed myelination, and milder ventriculomegaly than in the other two groups (AJNR Am J Neuroradiol. 2017 Jul;38[7]:1427-34).
The rehabilitation center where Dr. van der Linden and her colleagues are currently following roughly 200 children with congenital Zika syndrome is in the state of Pernambuco, which was particularly hard hit by the Zika epidemic.
She reported having no relevant financial disclosures.
EXPERT ANALYSIS FROM TERATOLOGY SOCIETY 2017
Antiviral shows early promise for treatment of Zika infection
DENVER – An antiviral drug that is a key player in the recent revolution in the treatment of hepatitis C infection also blocks Zika virus replication both in vitro and in a mouse model, Alysson R. Muotri, PhD, said at the annual meeting of the Teratology Society.
Sofosbuvir is a promising candidate. Not only has it demonstrated efficacy in preclinical work by Dr. Muotri and other groups of investigators, but it is also already a Food and Drug Administration–approved antiviral agent with a relatively reassuring Category B rating for use in pregnancy, meaning no evidence of teratogenicity in animal studies.
Investigators at the Scripps Clinic in La Jolla, Calif., have shown that the replication machinery in the Zika virus genome is closely similar to that of another flavivirus: hepatitis C. Both viruses express an NS2B-NS3 protease essential for generation of functional viral proteins.
“That observation led us to look for drugs that would interact with that replication pocket,” Dr. Muotri said.
The researchers first established in vitro that sofosbuvir can bind to the Zika virus NS2B-NS3 protease interface in an area known as the RNA-directed RNA polymerase domain, both in Zika virus–infected human neural progenitor cells and in cerebral organoids. Next, they demonstrated that sofosbuvir inhibited Zika virus replication in mice in dose-dependent fashion (Antiviral Res. 2017 Jul;143:218-29).
Most recently – and most importantly – the researchers showed that sofosbuvir blocked vertical transmission of Zika virus infection in the mouse model. “It’s neuroprotective for the fetus. There was no PCR [polymerase chain reaction] evidence of Zika virus in the fetal head. Those animals are born normal as far as we can tell,” he said.
The researchers are also engaged in preclinical evaluation of other antiviral agents as potential treatments for Zika infection. Some show even more potent anti-Zika activity than did sofosbuvir. But they have the disadvantage of being nonapproved investigational drugs and hence are far earlier in the developmental pipeline.
Dr. Muotri, whose research is supported by the National Institutes of Health, reported having no relevant financial disclosures.
DENVER – An antiviral drug that is a key player in the recent revolution in the treatment of hepatitis C infection also blocks Zika virus replication both in vitro and in a mouse model, Alysson R. Muotri, PhD, said at the annual meeting of the Teratology Society.
Sofosbuvir is a promising candidate. Not only has it demonstrated efficacy in preclinical work by Dr. Muotri and other groups of investigators, but it is also already a Food and Drug Administration–approved antiviral agent with a relatively reassuring Category B rating for use in pregnancy, meaning no evidence of teratogenicity in animal studies.
Investigators at the Scripps Clinic in La Jolla, Calif., have shown that the replication machinery in the Zika virus genome is closely similar to that of another flavivirus: hepatitis C. Both viruses express an NS2B-NS3 protease essential for generation of functional viral proteins.
“That observation led us to look for drugs that would interact with that replication pocket,” Dr. Muotri said.
The researchers first established in vitro that sofosbuvir can bind to the Zika virus NS2B-NS3 protease interface in an area known as the RNA-directed RNA polymerase domain, both in Zika virus–infected human neural progenitor cells and in cerebral organoids. Next, they demonstrated that sofosbuvir inhibited Zika virus replication in mice in dose-dependent fashion (Antiviral Res. 2017 Jul;143:218-29).
Most recently – and most importantly – the researchers showed that sofosbuvir blocked vertical transmission of Zika virus infection in the mouse model. “It’s neuroprotective for the fetus. There was no PCR [polymerase chain reaction] evidence of Zika virus in the fetal head. Those animals are born normal as far as we can tell,” he said.
The researchers are also engaged in preclinical evaluation of other antiviral agents as potential treatments for Zika infection. Some show even more potent anti-Zika activity than did sofosbuvir. But they have the disadvantage of being nonapproved investigational drugs and hence are far earlier in the developmental pipeline.
Dr. Muotri, whose research is supported by the National Institutes of Health, reported having no relevant financial disclosures.
DENVER – An antiviral drug that is a key player in the recent revolution in the treatment of hepatitis C infection also blocks Zika virus replication both in vitro and in a mouse model, Alysson R. Muotri, PhD, said at the annual meeting of the Teratology Society.
Sofosbuvir is a promising candidate. Not only has it demonstrated efficacy in preclinical work by Dr. Muotri and other groups of investigators, but it is also already a Food and Drug Administration–approved antiviral agent with a relatively reassuring Category B rating for use in pregnancy, meaning no evidence of teratogenicity in animal studies.
Investigators at the Scripps Clinic in La Jolla, Calif., have shown that the replication machinery in the Zika virus genome is closely similar to that of another flavivirus: hepatitis C. Both viruses express an NS2B-NS3 protease essential for generation of functional viral proteins.
“That observation led us to look for drugs that would interact with that replication pocket,” Dr. Muotri said.
The researchers first established in vitro that sofosbuvir can bind to the Zika virus NS2B-NS3 protease interface in an area known as the RNA-directed RNA polymerase domain, both in Zika virus–infected human neural progenitor cells and in cerebral organoids. Next, they demonstrated that sofosbuvir inhibited Zika virus replication in mice in dose-dependent fashion (Antiviral Res. 2017 Jul;143:218-29).
Most recently – and most importantly – the researchers showed that sofosbuvir blocked vertical transmission of Zika virus infection in the mouse model. “It’s neuroprotective for the fetus. There was no PCR [polymerase chain reaction] evidence of Zika virus in the fetal head. Those animals are born normal as far as we can tell,” he said.
The researchers are also engaged in preclinical evaluation of other antiviral agents as potential treatments for Zika infection. Some show even more potent anti-Zika activity than did sofosbuvir. But they have the disadvantage of being nonapproved investigational drugs and hence are far earlier in the developmental pipeline.
Dr. Muotri, whose research is supported by the National Institutes of Health, reported having no relevant financial disclosures.
EXPERT ANALYSIS FROM TERATOLOGY SOCIETY 2017
Preventing Zika in pregnancy: What you need to know
DENVER – Prevention of Zika virus infection in pregnancy is critical, given the lack of an effective treatment or vaccine, but it’s easier said than done.
“The particular mosquito that transmits Zika is really difficult because it tends to be indoors and live under the coffee table in your living room rather than outside. It bites in the day, as well as night, so it’s hard to prevent. Trying to consistently have repellent on [and] wear long-sleeved shirts and long pants, even when you’re indoors, in places that are typically quite warm – it’s challenging,” Margaret Honein, PhD, said at the annual meeting of the Teratology Society.
The CDC’s top Zika prevention advice is that pregnant women should not travel to any area where Zika is a risk. If they absolutely have to go, they should talk to their health care provider first, strictly follow measures to prevent mosquito bites during the trip, take steps to prevent sexual transmission – namely, use a condom – and talk with their health care provider once again upon their return.
It is now clear that Zika can be transmitted sexually. For this reason, even a pregnant woman who doesn’t visit an area with Zika risk, but whose partner has been to such a place, must also practice safe sex using a condom, even if the partner is asymptomatic.
In response to an audience question from a Washington, D.C.–area ob.gyn. who noted that there isn’t a Zika problem in his region, Dr. Honein said that her personal view is that pregnant women living in the vicinity of the nation’s capitol should also follow the CDC recommendations for travelers to, or residents of, areas where Zika is found.
“You have mosquito vectors that can transmit Zika in Washington, D.C., and a lot of other parts of the country,” she said. “There are travelers coming back from areas with risk for Zika all the time. If they get bit by a mosquito, which then bites another person and transmits the infection, there’s a problem. So, for pregnant women in areas that have a mosquito vector that can transmit the virus, I think it’s very wise advice to consistently use mosquito repellent and take the other protective measures. People should be doing everything they can to protect against Zika infection in pregnancy.”
DENVER – Prevention of Zika virus infection in pregnancy is critical, given the lack of an effective treatment or vaccine, but it’s easier said than done.
“The particular mosquito that transmits Zika is really difficult because it tends to be indoors and live under the coffee table in your living room rather than outside. It bites in the day, as well as night, so it’s hard to prevent. Trying to consistently have repellent on [and] wear long-sleeved shirts and long pants, even when you’re indoors, in places that are typically quite warm – it’s challenging,” Margaret Honein, PhD, said at the annual meeting of the Teratology Society.
The CDC’s top Zika prevention advice is that pregnant women should not travel to any area where Zika is a risk. If they absolutely have to go, they should talk to their health care provider first, strictly follow measures to prevent mosquito bites during the trip, take steps to prevent sexual transmission – namely, use a condom – and talk with their health care provider once again upon their return.
It is now clear that Zika can be transmitted sexually. For this reason, even a pregnant woman who doesn’t visit an area with Zika risk, but whose partner has been to such a place, must also practice safe sex using a condom, even if the partner is asymptomatic.
In response to an audience question from a Washington, D.C.–area ob.gyn. who noted that there isn’t a Zika problem in his region, Dr. Honein said that her personal view is that pregnant women living in the vicinity of the nation’s capitol should also follow the CDC recommendations for travelers to, or residents of, areas where Zika is found.
“You have mosquito vectors that can transmit Zika in Washington, D.C., and a lot of other parts of the country,” she said. “There are travelers coming back from areas with risk for Zika all the time. If they get bit by a mosquito, which then bites another person and transmits the infection, there’s a problem. So, for pregnant women in areas that have a mosquito vector that can transmit the virus, I think it’s very wise advice to consistently use mosquito repellent and take the other protective measures. People should be doing everything they can to protect against Zika infection in pregnancy.”
DENVER – Prevention of Zika virus infection in pregnancy is critical, given the lack of an effective treatment or vaccine, but it’s easier said than done.
“The particular mosquito that transmits Zika is really difficult because it tends to be indoors and live under the coffee table in your living room rather than outside. It bites in the day, as well as night, so it’s hard to prevent. Trying to consistently have repellent on [and] wear long-sleeved shirts and long pants, even when you’re indoors, in places that are typically quite warm – it’s challenging,” Margaret Honein, PhD, said at the annual meeting of the Teratology Society.
The CDC’s top Zika prevention advice is that pregnant women should not travel to any area where Zika is a risk. If they absolutely have to go, they should talk to their health care provider first, strictly follow measures to prevent mosquito bites during the trip, take steps to prevent sexual transmission – namely, use a condom – and talk with their health care provider once again upon their return.
It is now clear that Zika can be transmitted sexually. For this reason, even a pregnant woman who doesn’t visit an area with Zika risk, but whose partner has been to such a place, must also practice safe sex using a condom, even if the partner is asymptomatic.
In response to an audience question from a Washington, D.C.–area ob.gyn. who noted that there isn’t a Zika problem in his region, Dr. Honein said that her personal view is that pregnant women living in the vicinity of the nation’s capitol should also follow the CDC recommendations for travelers to, or residents of, areas where Zika is found.
“You have mosquito vectors that can transmit Zika in Washington, D.C., and a lot of other parts of the country,” she said. “There are travelers coming back from areas with risk for Zika all the time. If they get bit by a mosquito, which then bites another person and transmits the infection, there’s a problem. So, for pregnant women in areas that have a mosquito vector that can transmit the virus, I think it’s very wise advice to consistently use mosquito repellent and take the other protective measures. People should be doing everything they can to protect against Zika infection in pregnancy.”
EXPERT ANALYSIS FROM TERATOLOGY SOCIETY 2017
Asymptomatic maternal Zika infection doesn’t dampen birth defect risk
DENVER – One of many daunting challenges posed by the ongoing global Zika virus epidemic stems from the recent realization that the presence or absence of symptoms in women infected in pregnancy has no bearing on whether their babies will have Zika-associated birth defects, Margaret Honein, PhD, observed at the annual meeting of the Teratology Society.
This has profound clinical consequences because roughly 80% of all maternal Zika infections are asymptomatic or feature such mild symptoms that women don’t report them.
“There was, I think, some hope early on that symptoms in the mother would correlate with outcomes, but that has not been the case at all. In the U.S. population, we’ve seen about a 6% Zika-associated birth defects rate in both symptomatic and asymptomatic mothers,” said Dr. Honein, chief of the birth defects branch at the Centers for Disease Control and Prevention in Atlanta. “We have a huge challenge in identifying the women who have asymptomatic infections, yet they’re at the same risk of having an adverse outcome in their baby.”[[{"fid":"199780","view_mode":"medstat_image_flush_right","fields":
She provided conference attendees with the latest information on Zika, including preliminary results from ongoing CDC investigations. Along the way, she tackled many of the questions Zika experts hear most often from clinicians, while emphasizing that much about congenital Zika syndrome remains unknown.
Just how serious is the global threat of Zika virus infection?
On Feb. 8, 2016, the CDC activated a Level 1 emergency response to Zika. To put that into perspective, this is only the fourth time in history the agency has gone to Level 1. The other occasions were for Hurricane Katrina, the H1N1 pandemic flu, and Ebola.
“This is worse than thalidomide,” said Jan M. Friedman, MD, after listening to Dr. Honein and other speakers at the Zika update held during the conference. Dr. Friedman, a medical geneticist at the University of British Columbia, Vancouver, delivered the Robert L. Brent Lecture.
What is the level of risk for fetal/infant birth defects associated with maternal Zika virus infection?
The birth defect risk is somewhere between 5% and 10%, with the true figure probably being on the high end. Reports quoting risks on the lower end are based upon laboratory testing for maternal IgM antibodies, which couldn’t rule out cross reaction with other flaviviruses, including dengue virus, which is common in most of the same locales as Zika. Women who have been infected with dengue but not Zika are not at increased risk for birth defects. Zika is the first mosquito-borne virus to be recognized as teratogenic in humans.
The birth defects seen in conjunction with Zika infection are not unique. They can have many different causes. CDC investigators examined birth defects data from three states during the year before the Zika outbreak in the Western Hemisphere and determined that the background rate of microcephaly, neural tube defects, eye abnormalities, hearing loss, and other Zika-like birth defects was 3 per 1,000 live births. Among women with Zika virus infection in pregnancy, however, the rate is more than 20-fold higher at 50-100 per 1,000, according to Dr. Honein.
When during pregnancy does maternal Zika infection pose the highest risk to the fetus?
Studies published from Colombia and Brazil show the peak risk is when infection occurs during the first trimester or early in the second trimester. That’s consistent with the U.S. registry experience as well. Of note, the median time between development of maternal symptoms and the first notation of fetal microcephaly on ultrasound has been 18 weeks.
“This has important implications for women who’ve been infected. Just because they may have had two consecutive apparently normal monthly fetal ultrasounds doesn’t rule out by any means congenital Zika syndrome because there does appear to be a relatively long time period before these findings appear,” Dr. Honein noted.
What is the full range of potential health problems Zika infection can cause?
“What we’ve seen so far is definitely just the tip of the iceberg,” Dr. Honein cautioned. “It’s very severe, but I think we don’t yet know the full range of disabilities. There’s much more to come here.”
Most of the infants in the U.S. registry are just now reaching 1 year of age. Greater understanding of their neurodevelopment will require follow-up to age 2 or beyond.
Also, the information available to date on Zika-associated birth defects is based largely on scrutiny of infants with microcephaly along with any additional findings, such as chorioretinal scarring.
“We know relatively little about infants with only the other conditions – only hearing loss, for example, or only eye abnormalities,” Dr. Honein said. “While we know there are children who have microcephaly and they have needs, there may be a much larger number of children with lesser impairment, but who still have disabilities that are going to necessitate provision of services. Being prepared for that is very important.”
Reports from multiple countries make it clear that babies exposed to Zika in utero can have a normal-appearing head at birth but then become microcephalic later in their first year. The incidence of this phenomenon hasn’t yet been pinned down.
“We’ve learned in the last year and a-half that microcephaly is a key marker of some of the relevant underlying brain abnormalities, but microcephaly is not where our focus should be,” she said.
How long does Zika virus persist in the body?
The viremia typically lasts for anywhere from a few days up to 2 weeks. However, viral persistence for as long as 107 days has been documented in some pregnant women.
“I hesitate to put a number on it because every new publication has a longer figure,” Dr. Honein said.
It’s not yet known whether viral persistence in a woman infected prior to her pregnancy is associated with adverse fetal outcomes. The central nervous system is clearly a reservoir for persistent virus. Whole blood is now under study as possibly another. Semen poses a major challenge.
“There are case reports of Zika virus RNA being detected in semen for more than 6 months after the timing of infection, but we don’t yet know for how long it can be sexually transmitted. Is there really infectious virus present or just particles of RNA?” she said.
Resources
In partnership with the March of Dimes, the CDC has launched Zika Care Connect, a referral network of roughly 600 specialists in six high-risk states. Their ranks include specialists in maternal-fetal medicine, audiology, radiology, mental health, pediatric neurology, infectious diseases, developmental pediatrics, endocrinology, and pediatric ophthalmology. Another 10 states and at least 600 additional providers will soon be added to the referral network (www.zikacareconnect.org; 1-844-677-0447 toll-free).
Comprehensive, up-to-date Zika information is available to health care providers and the public through the CDC at http://www.cdc.gov/zika, at the Zika Pregnancy Hotline (770-488-7100), and by email at [email protected].
[email protected]
On Twitter @ObGynNews
DENVER – One of many daunting challenges posed by the ongoing global Zika virus epidemic stems from the recent realization that the presence or absence of symptoms in women infected in pregnancy has no bearing on whether their babies will have Zika-associated birth defects, Margaret Honein, PhD, observed at the annual meeting of the Teratology Society.
This has profound clinical consequences because roughly 80% of all maternal Zika infections are asymptomatic or feature such mild symptoms that women don’t report them.
“There was, I think, some hope early on that symptoms in the mother would correlate with outcomes, but that has not been the case at all. In the U.S. population, we’ve seen about a 6% Zika-associated birth defects rate in both symptomatic and asymptomatic mothers,” said Dr. Honein, chief of the birth defects branch at the Centers for Disease Control and Prevention in Atlanta. “We have a huge challenge in identifying the women who have asymptomatic infections, yet they’re at the same risk of having an adverse outcome in their baby.”[[{"fid":"199780","view_mode":"medstat_image_flush_right","fields":
She provided conference attendees with the latest information on Zika, including preliminary results from ongoing CDC investigations. Along the way, she tackled many of the questions Zika experts hear most often from clinicians, while emphasizing that much about congenital Zika syndrome remains unknown.
Just how serious is the global threat of Zika virus infection?
On Feb. 8, 2016, the CDC activated a Level 1 emergency response to Zika. To put that into perspective, this is only the fourth time in history the agency has gone to Level 1. The other occasions were for Hurricane Katrina, the H1N1 pandemic flu, and Ebola.
“This is worse than thalidomide,” said Jan M. Friedman, MD, after listening to Dr. Honein and other speakers at the Zika update held during the conference. Dr. Friedman, a medical geneticist at the University of British Columbia, Vancouver, delivered the Robert L. Brent Lecture.
What is the level of risk for fetal/infant birth defects associated with maternal Zika virus infection?
The birth defect risk is somewhere between 5% and 10%, with the true figure probably being on the high end. Reports quoting risks on the lower end are based upon laboratory testing for maternal IgM antibodies, which couldn’t rule out cross reaction with other flaviviruses, including dengue virus, which is common in most of the same locales as Zika. Women who have been infected with dengue but not Zika are not at increased risk for birth defects. Zika is the first mosquito-borne virus to be recognized as teratogenic in humans.
The birth defects seen in conjunction with Zika infection are not unique. They can have many different causes. CDC investigators examined birth defects data from three states during the year before the Zika outbreak in the Western Hemisphere and determined that the background rate of microcephaly, neural tube defects, eye abnormalities, hearing loss, and other Zika-like birth defects was 3 per 1,000 live births. Among women with Zika virus infection in pregnancy, however, the rate is more than 20-fold higher at 50-100 per 1,000, according to Dr. Honein.
When during pregnancy does maternal Zika infection pose the highest risk to the fetus?
Studies published from Colombia and Brazil show the peak risk is when infection occurs during the first trimester or early in the second trimester. That’s consistent with the U.S. registry experience as well. Of note, the median time between development of maternal symptoms and the first notation of fetal microcephaly on ultrasound has been 18 weeks.
“This has important implications for women who’ve been infected. Just because they may have had two consecutive apparently normal monthly fetal ultrasounds doesn’t rule out by any means congenital Zika syndrome because there does appear to be a relatively long time period before these findings appear,” Dr. Honein noted.
What is the full range of potential health problems Zika infection can cause?
“What we’ve seen so far is definitely just the tip of the iceberg,” Dr. Honein cautioned. “It’s very severe, but I think we don’t yet know the full range of disabilities. There’s much more to come here.”
Most of the infants in the U.S. registry are just now reaching 1 year of age. Greater understanding of their neurodevelopment will require follow-up to age 2 or beyond.
Also, the information available to date on Zika-associated birth defects is based largely on scrutiny of infants with microcephaly along with any additional findings, such as chorioretinal scarring.
“We know relatively little about infants with only the other conditions – only hearing loss, for example, or only eye abnormalities,” Dr. Honein said. “While we know there are children who have microcephaly and they have needs, there may be a much larger number of children with lesser impairment, but who still have disabilities that are going to necessitate provision of services. Being prepared for that is very important.”
Reports from multiple countries make it clear that babies exposed to Zika in utero can have a normal-appearing head at birth but then become microcephalic later in their first year. The incidence of this phenomenon hasn’t yet been pinned down.
“We’ve learned in the last year and a-half that microcephaly is a key marker of some of the relevant underlying brain abnormalities, but microcephaly is not where our focus should be,” she said.
How long does Zika virus persist in the body?
The viremia typically lasts for anywhere from a few days up to 2 weeks. However, viral persistence for as long as 107 days has been documented in some pregnant women.
“I hesitate to put a number on it because every new publication has a longer figure,” Dr. Honein said.
It’s not yet known whether viral persistence in a woman infected prior to her pregnancy is associated with adverse fetal outcomes. The central nervous system is clearly a reservoir for persistent virus. Whole blood is now under study as possibly another. Semen poses a major challenge.
“There are case reports of Zika virus RNA being detected in semen for more than 6 months after the timing of infection, but we don’t yet know for how long it can be sexually transmitted. Is there really infectious virus present or just particles of RNA?” she said.
Resources
In partnership with the March of Dimes, the CDC has launched Zika Care Connect, a referral network of roughly 600 specialists in six high-risk states. Their ranks include specialists in maternal-fetal medicine, audiology, radiology, mental health, pediatric neurology, infectious diseases, developmental pediatrics, endocrinology, and pediatric ophthalmology. Another 10 states and at least 600 additional providers will soon be added to the referral network (www.zikacareconnect.org; 1-844-677-0447 toll-free).
Comprehensive, up-to-date Zika information is available to health care providers and the public through the CDC at http://www.cdc.gov/zika, at the Zika Pregnancy Hotline (770-488-7100), and by email at [email protected].
[email protected]
On Twitter @ObGynNews
DENVER – One of many daunting challenges posed by the ongoing global Zika virus epidemic stems from the recent realization that the presence or absence of symptoms in women infected in pregnancy has no bearing on whether their babies will have Zika-associated birth defects, Margaret Honein, PhD, observed at the annual meeting of the Teratology Society.
This has profound clinical consequences because roughly 80% of all maternal Zika infections are asymptomatic or feature such mild symptoms that women don’t report them.
“There was, I think, some hope early on that symptoms in the mother would correlate with outcomes, but that has not been the case at all. In the U.S. population, we’ve seen about a 6% Zika-associated birth defects rate in both symptomatic and asymptomatic mothers,” said Dr. Honein, chief of the birth defects branch at the Centers for Disease Control and Prevention in Atlanta. “We have a huge challenge in identifying the women who have asymptomatic infections, yet they’re at the same risk of having an adverse outcome in their baby.”[[{"fid":"199780","view_mode":"medstat_image_flush_right","fields":
She provided conference attendees with the latest information on Zika, including preliminary results from ongoing CDC investigations. Along the way, she tackled many of the questions Zika experts hear most often from clinicians, while emphasizing that much about congenital Zika syndrome remains unknown.
Just how serious is the global threat of Zika virus infection?
On Feb. 8, 2016, the CDC activated a Level 1 emergency response to Zika. To put that into perspective, this is only the fourth time in history the agency has gone to Level 1. The other occasions were for Hurricane Katrina, the H1N1 pandemic flu, and Ebola.
“This is worse than thalidomide,” said Jan M. Friedman, MD, after listening to Dr. Honein and other speakers at the Zika update held during the conference. Dr. Friedman, a medical geneticist at the University of British Columbia, Vancouver, delivered the Robert L. Brent Lecture.
What is the level of risk for fetal/infant birth defects associated with maternal Zika virus infection?
The birth defect risk is somewhere between 5% and 10%, with the true figure probably being on the high end. Reports quoting risks on the lower end are based upon laboratory testing for maternal IgM antibodies, which couldn’t rule out cross reaction with other flaviviruses, including dengue virus, which is common in most of the same locales as Zika. Women who have been infected with dengue but not Zika are not at increased risk for birth defects. Zika is the first mosquito-borne virus to be recognized as teratogenic in humans.
The birth defects seen in conjunction with Zika infection are not unique. They can have many different causes. CDC investigators examined birth defects data from three states during the year before the Zika outbreak in the Western Hemisphere and determined that the background rate of microcephaly, neural tube defects, eye abnormalities, hearing loss, and other Zika-like birth defects was 3 per 1,000 live births. Among women with Zika virus infection in pregnancy, however, the rate is more than 20-fold higher at 50-100 per 1,000, according to Dr. Honein.
When during pregnancy does maternal Zika infection pose the highest risk to the fetus?
Studies published from Colombia and Brazil show the peak risk is when infection occurs during the first trimester or early in the second trimester. That’s consistent with the U.S. registry experience as well. Of note, the median time between development of maternal symptoms and the first notation of fetal microcephaly on ultrasound has been 18 weeks.
“This has important implications for women who’ve been infected. Just because they may have had two consecutive apparently normal monthly fetal ultrasounds doesn’t rule out by any means congenital Zika syndrome because there does appear to be a relatively long time period before these findings appear,” Dr. Honein noted.
What is the full range of potential health problems Zika infection can cause?
“What we’ve seen so far is definitely just the tip of the iceberg,” Dr. Honein cautioned. “It’s very severe, but I think we don’t yet know the full range of disabilities. There’s much more to come here.”
Most of the infants in the U.S. registry are just now reaching 1 year of age. Greater understanding of their neurodevelopment will require follow-up to age 2 or beyond.
Also, the information available to date on Zika-associated birth defects is based largely on scrutiny of infants with microcephaly along with any additional findings, such as chorioretinal scarring.
“We know relatively little about infants with only the other conditions – only hearing loss, for example, or only eye abnormalities,” Dr. Honein said. “While we know there are children who have microcephaly and they have needs, there may be a much larger number of children with lesser impairment, but who still have disabilities that are going to necessitate provision of services. Being prepared for that is very important.”
Reports from multiple countries make it clear that babies exposed to Zika in utero can have a normal-appearing head at birth but then become microcephalic later in their first year. The incidence of this phenomenon hasn’t yet been pinned down.
“We’ve learned in the last year and a-half that microcephaly is a key marker of some of the relevant underlying brain abnormalities, but microcephaly is not where our focus should be,” she said.
How long does Zika virus persist in the body?
The viremia typically lasts for anywhere from a few days up to 2 weeks. However, viral persistence for as long as 107 days has been documented in some pregnant women.
“I hesitate to put a number on it because every new publication has a longer figure,” Dr. Honein said.
It’s not yet known whether viral persistence in a woman infected prior to her pregnancy is associated with adverse fetal outcomes. The central nervous system is clearly a reservoir for persistent virus. Whole blood is now under study as possibly another. Semen poses a major challenge.
“There are case reports of Zika virus RNA being detected in semen for more than 6 months after the timing of infection, but we don’t yet know for how long it can be sexually transmitted. Is there really infectious virus present or just particles of RNA?” she said.
Resources
In partnership with the March of Dimes, the CDC has launched Zika Care Connect, a referral network of roughly 600 specialists in six high-risk states. Their ranks include specialists in maternal-fetal medicine, audiology, radiology, mental health, pediatric neurology, infectious diseases, developmental pediatrics, endocrinology, and pediatric ophthalmology. Another 10 states and at least 600 additional providers will soon be added to the referral network (www.zikacareconnect.org; 1-844-677-0447 toll-free).
Comprehensive, up-to-date Zika information is available to health care providers and the public through the CDC at http://www.cdc.gov/zika, at the Zika Pregnancy Hotline (770-488-7100), and by email at [email protected].
[email protected]
On Twitter @ObGynNews
EXPERT ANALYSIS FROM TERATOLOGY SOCIETY 2017
Prenatal methadone maintenance linked to poorer child neurodevelopment
SAN FRANCISCO – Infants born to mothers receiving methadone maintenance treatment show poorer-than-average neurodevelopment outcomes, a retrospective study found.
Delays or difficulties in motor abilities appeared first in these children, followed by evidence of cognitive problems in their second year of life, reported Cristina Borradori Tolsa, MD, of University Hospital, Geneva.
“Higher methadone doses during pregnancy can have a detrimental effect on neonatal characteristics and children’s psychomotor development,” Dr. Borradori Tolsa said at the Pediatric Academic Societies meeting. She noted the need for long-term follow-up of children prenatally exposed to methadone maintenance therapy to evaluate their cognitive abilities and school readiness at preschool ages.
Only 38% of the women had exclusively used methadone, while the other 62% had used a variety of substances, including cocaine, alcohol, benzodiazepine, marijuana, and antidepressants. The women had a low average socioeconomic status based on their level of education and the occupations of the children’s fathers.
The researchers drew children’s development data from their scores on the Bayley Scales of Infant Development, Second Edition (BSID-II), at 6 months and 18-24 months. The BSID-II has an average score of 100 and includes a mental development index for language and cognitive development, and a psychomotor development index to assess fine and gross motor skills.
At age 6 months, 75% of the 40 children assessed showed some level of motor skills delay, and 33% had a moderate to severe delay in psychomotor skills. A quarter had no delay at all (a score of at least 85). The average psychomotor score at 6 months was 76, and the average cognitive score was 88. Most of the children (60%) did, however, show mental development within the normal range at 6 months.
By the age of 18-24 months, half of the 36 children assessed showed no motor delays, and half showed no cognitive delays. One in five (20%) showed a moderate to severe psychomotor delay, and 14% showed a moderate to severe mental development delay. Mild delays in mental development occurred in 36% of the toddlers assessed, and 30% showed mild delays in psychomotor skills.
A dose-response effect was seen with mothers’ higher doses of methadone at birth and their children’s psychomotor scores at 6 months. No similar association existed for mental development, and the psychomotor association disappeared by 18-24 months. At this older age, however, 68% of children born to mothers taking a high dose of methadone showed cognitive delays, compared with 29% of children born to mothers on a low dose.
Although no differences were seen in newborns’ average gestational age (an average of 37.8 weeks overall) or birth weight between the high-dose and low-dose methadone groups, infants born to mothers with high doses were more likely to be small for gestational age (P = .01) and to need longer treatment duration for neonatal abstinence syndrome (NAS) (P = .03). Overall, 44% of the newborns were small for gestational age, 28% were born microcephalic, and all but three required pharmacologic treatment for NAS. NAS treatment lasted an average 54 days for the cohort, and the average hospital stay for the babies was 76 days.
The researchers did not report having any external funding or relevant financial disclosures.
SAN FRANCISCO – Infants born to mothers receiving methadone maintenance treatment show poorer-than-average neurodevelopment outcomes, a retrospective study found.
Delays or difficulties in motor abilities appeared first in these children, followed by evidence of cognitive problems in their second year of life, reported Cristina Borradori Tolsa, MD, of University Hospital, Geneva.
“Higher methadone doses during pregnancy can have a detrimental effect on neonatal characteristics and children’s psychomotor development,” Dr. Borradori Tolsa said at the Pediatric Academic Societies meeting. She noted the need for long-term follow-up of children prenatally exposed to methadone maintenance therapy to evaluate their cognitive abilities and school readiness at preschool ages.
Only 38% of the women had exclusively used methadone, while the other 62% had used a variety of substances, including cocaine, alcohol, benzodiazepine, marijuana, and antidepressants. The women had a low average socioeconomic status based on their level of education and the occupations of the children’s fathers.
The researchers drew children’s development data from their scores on the Bayley Scales of Infant Development, Second Edition (BSID-II), at 6 months and 18-24 months. The BSID-II has an average score of 100 and includes a mental development index for language and cognitive development, and a psychomotor development index to assess fine and gross motor skills.
At age 6 months, 75% of the 40 children assessed showed some level of motor skills delay, and 33% had a moderate to severe delay in psychomotor skills. A quarter had no delay at all (a score of at least 85). The average psychomotor score at 6 months was 76, and the average cognitive score was 88. Most of the children (60%) did, however, show mental development within the normal range at 6 months.
By the age of 18-24 months, half of the 36 children assessed showed no motor delays, and half showed no cognitive delays. One in five (20%) showed a moderate to severe psychomotor delay, and 14% showed a moderate to severe mental development delay. Mild delays in mental development occurred in 36% of the toddlers assessed, and 30% showed mild delays in psychomotor skills.
A dose-response effect was seen with mothers’ higher doses of methadone at birth and their children’s psychomotor scores at 6 months. No similar association existed for mental development, and the psychomotor association disappeared by 18-24 months. At this older age, however, 68% of children born to mothers taking a high dose of methadone showed cognitive delays, compared with 29% of children born to mothers on a low dose.
Although no differences were seen in newborns’ average gestational age (an average of 37.8 weeks overall) or birth weight between the high-dose and low-dose methadone groups, infants born to mothers with high doses were more likely to be small for gestational age (P = .01) and to need longer treatment duration for neonatal abstinence syndrome (NAS) (P = .03). Overall, 44% of the newborns were small for gestational age, 28% were born microcephalic, and all but three required pharmacologic treatment for NAS. NAS treatment lasted an average 54 days for the cohort, and the average hospital stay for the babies was 76 days.
The researchers did not report having any external funding or relevant financial disclosures.
SAN FRANCISCO – Infants born to mothers receiving methadone maintenance treatment show poorer-than-average neurodevelopment outcomes, a retrospective study found.
Delays or difficulties in motor abilities appeared first in these children, followed by evidence of cognitive problems in their second year of life, reported Cristina Borradori Tolsa, MD, of University Hospital, Geneva.
“Higher methadone doses during pregnancy can have a detrimental effect on neonatal characteristics and children’s psychomotor development,” Dr. Borradori Tolsa said at the Pediatric Academic Societies meeting. She noted the need for long-term follow-up of children prenatally exposed to methadone maintenance therapy to evaluate their cognitive abilities and school readiness at preschool ages.
Only 38% of the women had exclusively used methadone, while the other 62% had used a variety of substances, including cocaine, alcohol, benzodiazepine, marijuana, and antidepressants. The women had a low average socioeconomic status based on their level of education and the occupations of the children’s fathers.
The researchers drew children’s development data from their scores on the Bayley Scales of Infant Development, Second Edition (BSID-II), at 6 months and 18-24 months. The BSID-II has an average score of 100 and includes a mental development index for language and cognitive development, and a psychomotor development index to assess fine and gross motor skills.
At age 6 months, 75% of the 40 children assessed showed some level of motor skills delay, and 33% had a moderate to severe delay in psychomotor skills. A quarter had no delay at all (a score of at least 85). The average psychomotor score at 6 months was 76, and the average cognitive score was 88. Most of the children (60%) did, however, show mental development within the normal range at 6 months.
By the age of 18-24 months, half of the 36 children assessed showed no motor delays, and half showed no cognitive delays. One in five (20%) showed a moderate to severe psychomotor delay, and 14% showed a moderate to severe mental development delay. Mild delays in mental development occurred in 36% of the toddlers assessed, and 30% showed mild delays in psychomotor skills.
A dose-response effect was seen with mothers’ higher doses of methadone at birth and their children’s psychomotor scores at 6 months. No similar association existed for mental development, and the psychomotor association disappeared by 18-24 months. At this older age, however, 68% of children born to mothers taking a high dose of methadone showed cognitive delays, compared with 29% of children born to mothers on a low dose.
Although no differences were seen in newborns’ average gestational age (an average of 37.8 weeks overall) or birth weight between the high-dose and low-dose methadone groups, infants born to mothers with high doses were more likely to be small for gestational age (P = .01) and to need longer treatment duration for neonatal abstinence syndrome (NAS) (P = .03). Overall, 44% of the newborns were small for gestational age, 28% were born microcephalic, and all but three required pharmacologic treatment for NAS. NAS treatment lasted an average 54 days for the cohort, and the average hospital stay for the babies was 76 days.
The researchers did not report having any external funding or relevant financial disclosures.
AT PAS 17
Key clinical point:
Major finding: Three-fourths of methadone-exposed infants showed psychomotor delays at 6 months, and 50% showed cognitive delays and/or psychomotor delays at 18-24 months.
Data source: A retrospective analysis of neurodevelopment scores of children born to 61 mothers in Geneva who received methadone maintenance therapy during pregnancy.
Disclosures: The researchers did not report having any external funding or relevant financial disclosures.
Don’t forget about Zika
Although the Zika virus isn’t making as many news headlines as it was last summer, ob.gyns. must not forget that it could still be in our waiting rooms.
Last year, new information on Zika emerged on a weekly, sometimes daily, basis. Today, ob.gyns. remain on the front lines of counseling and treating women whose pregnancies are at risk of being affected by the Zika virus and devastating birth defects associated with it.
The American College of Obstetricians and Gynecologists prioritizes preparing ob.gyns. to comprehensively address the Zika virus with patients. To support clinicians, ACOG regularly develops, updates, and issues guidance on the risk, prevention, assessment, treatment, and outcomes of the Zika virus. This includes a regularly updated Practice Advisory, as well as information to direct ob.gyns. to critical resources from the Centers for Disease Control and Prevention, such as the U.S. Zika Pregnancy Registry.
Last month, I participated in an ad hoc meeting of international experts and professional society representatives sponsored by the Gottesfeld-Hohler Memorial Foundation. The goal of this Zika think tank was to share ongoing studies and unpublished findings and to identify ways for the groups to collaborate to fight the virus. Zika experts from endemic and risk areas, such as Brazil, Colombia, Puerto Rico, Texas, and Florida, started the meeting with on-the-ground updates. The latest epidemiologic evidence is that there appear to be waves of Zika virus infections that occur across regions/countries, spreading to virus-naive areas. Although immunity may develop eventually, Zika appears to occur in epidemics and is likely to spread further in North and South America to naive regions. Much of the United States is at risk during mosquito seasons. This collaboration with the Gottesfeld-Hohler Memorial Foundation was just the beginning, and I look forward to working more with Zika experts from all over the world.
The health reform debate in Washington also will affect our ability to respond to the ongoing Zika crisis. Current proposals allow states to opt out of essential coverage requirements, such as contraception and maternity coverage. It also would limit the federal investment in Medicaid, resulting in cuts to benefits and provider reimbursement and hamstringing our ability to treat our low-income pregnant patients with Zika exposure.
As ob.gyns., we continue to be the most important source of information for patients, and our goals of providing the most up-to-date knowledge and aiding in informed decision making are more important than ever.
Dr. Harris is a gynecologist in Gainesville, Fla., and chair of ACOG District VII. She reported having no relevant financial disclosures.
Although the Zika virus isn’t making as many news headlines as it was last summer, ob.gyns. must not forget that it could still be in our waiting rooms.
Last year, new information on Zika emerged on a weekly, sometimes daily, basis. Today, ob.gyns. remain on the front lines of counseling and treating women whose pregnancies are at risk of being affected by the Zika virus and devastating birth defects associated with it.
The American College of Obstetricians and Gynecologists prioritizes preparing ob.gyns. to comprehensively address the Zika virus with patients. To support clinicians, ACOG regularly develops, updates, and issues guidance on the risk, prevention, assessment, treatment, and outcomes of the Zika virus. This includes a regularly updated Practice Advisory, as well as information to direct ob.gyns. to critical resources from the Centers for Disease Control and Prevention, such as the U.S. Zika Pregnancy Registry.
Last month, I participated in an ad hoc meeting of international experts and professional society representatives sponsored by the Gottesfeld-Hohler Memorial Foundation. The goal of this Zika think tank was to share ongoing studies and unpublished findings and to identify ways for the groups to collaborate to fight the virus. Zika experts from endemic and risk areas, such as Brazil, Colombia, Puerto Rico, Texas, and Florida, started the meeting with on-the-ground updates. The latest epidemiologic evidence is that there appear to be waves of Zika virus infections that occur across regions/countries, spreading to virus-naive areas. Although immunity may develop eventually, Zika appears to occur in epidemics and is likely to spread further in North and South America to naive regions. Much of the United States is at risk during mosquito seasons. This collaboration with the Gottesfeld-Hohler Memorial Foundation was just the beginning, and I look forward to working more with Zika experts from all over the world.
The health reform debate in Washington also will affect our ability to respond to the ongoing Zika crisis. Current proposals allow states to opt out of essential coverage requirements, such as contraception and maternity coverage. It also would limit the federal investment in Medicaid, resulting in cuts to benefits and provider reimbursement and hamstringing our ability to treat our low-income pregnant patients with Zika exposure.
As ob.gyns., we continue to be the most important source of information for patients, and our goals of providing the most up-to-date knowledge and aiding in informed decision making are more important than ever.
Dr. Harris is a gynecologist in Gainesville, Fla., and chair of ACOG District VII. She reported having no relevant financial disclosures.
Although the Zika virus isn’t making as many news headlines as it was last summer, ob.gyns. must not forget that it could still be in our waiting rooms.
Last year, new information on Zika emerged on a weekly, sometimes daily, basis. Today, ob.gyns. remain on the front lines of counseling and treating women whose pregnancies are at risk of being affected by the Zika virus and devastating birth defects associated with it.
The American College of Obstetricians and Gynecologists prioritizes preparing ob.gyns. to comprehensively address the Zika virus with patients. To support clinicians, ACOG regularly develops, updates, and issues guidance on the risk, prevention, assessment, treatment, and outcomes of the Zika virus. This includes a regularly updated Practice Advisory, as well as information to direct ob.gyns. to critical resources from the Centers for Disease Control and Prevention, such as the U.S. Zika Pregnancy Registry.
Last month, I participated in an ad hoc meeting of international experts and professional society representatives sponsored by the Gottesfeld-Hohler Memorial Foundation. The goal of this Zika think tank was to share ongoing studies and unpublished findings and to identify ways for the groups to collaborate to fight the virus. Zika experts from endemic and risk areas, such as Brazil, Colombia, Puerto Rico, Texas, and Florida, started the meeting with on-the-ground updates. The latest epidemiologic evidence is that there appear to be waves of Zika virus infections that occur across regions/countries, spreading to virus-naive areas. Although immunity may develop eventually, Zika appears to occur in epidemics and is likely to spread further in North and South America to naive regions. Much of the United States is at risk during mosquito seasons. This collaboration with the Gottesfeld-Hohler Memorial Foundation was just the beginning, and I look forward to working more with Zika experts from all over the world.
The health reform debate in Washington also will affect our ability to respond to the ongoing Zika crisis. Current proposals allow states to opt out of essential coverage requirements, such as contraception and maternity coverage. It also would limit the federal investment in Medicaid, resulting in cuts to benefits and provider reimbursement and hamstringing our ability to treat our low-income pregnant patients with Zika exposure.
As ob.gyns., we continue to be the most important source of information for patients, and our goals of providing the most up-to-date knowledge and aiding in informed decision making are more important than ever.
Dr. Harris is a gynecologist in Gainesville, Fla., and chair of ACOG District VII. She reported having no relevant financial disclosures.
One-year cost of preeclampsia tops $2 billion
The cost of medical care for preeclampsia is estimated to be $2.18 billion for the first year surrounding delivery, split almost evenly between care for mothers and infants, according to a new report.
Preeclampsia is increasing at a more rapid rate than diabetes, ischemic heart failure, Alzheimer’s disease, obesity, and chronic kidney disease – disorders for which substantially more funding has been allocated for research and treatment, said Warren Stevens, PhD, of Precision Health Economics, Los Angeles, and his associates. Precision Health Economics provides consulting and other research services to pharmaceutical, device, government, and nongovernmental groups.
Compared with a healthy pregnancy, preeclampsia increased the probability that a mother would have at least one adverse outcome from 4.6% to 10.1%, and it increased the probability that an infant would have at least one adverse outcome from 7.9% to 14.2%. For mothers, preeclampsia was estimated to raise costs by $6,583 per birth, which translates to an increase of $1.03 billion for U.S. mothers during a single year (2012). For infants, the disorder raised costs by $1.15 billion during that year.
Infant costs varied according to the gestational age at delivery, ranging from $1,311 per birth at 36 weeks’ gestation to $150,000 per birth at 26 weeks’ gestation, Dr. Stevens and his associates reported (Am J Obstet Gynecol. 2017 Jul 11. doi: 10.1016/j.ajog.2017.04.032).
This study could not take into account longer-term adverse outcomes associated with preeclampsia. Women with a history of the disorder are at double the risk of developing ischemic heart disease or cerebrovascular disease compared with women without such a history, and at three times the risk of developing hypertension. Infants are at increased risk of developing stroke, metabolic syndrome, and chronic heart disease, the investigators noted.
The study was supported by rEVO Biologics. Five of the study authors are employees of rEVO Biologics and one author is a steering committee member for a clinical trial supported by the company; another study author is a consultant at Precision Health Economics.
Quantifying the total cost of a health problem helps to show the public, payers, and health care administrators the magnitude of the problem on a population level. By underscoring the economic burden of preeclampsia, Stevens et al. provided important information about the high costs of this condition.
However, we need to go beyond health burden and cost-of-illness studies when considering the value of interventions to prevent and better manage preeclampsia and its adverse outcomes. Cost of the intervention and the cost savings associated with preventing one case of the condition are important parameters that can be used to calculate the potential savings of interventions. In fact, if the lifetimes costs of caring for children with adverse outcomes of preeclampsia are included, the potential cost savings from effective interventions may be even greater.
Rui Li, PhD, and William M. Callaghan, MD, are in the division of reproductive health at the Centers for Disease Control and Prevention, Atlanta. Eleni Tsigas is with the Preeclampsia Foundation in Melbourne, Fla. They reported having no relevant financial disclosures. These remarks are adapted from an editorial (Am J Obstet Gynecol. 2017 Jul 11. doi: 10.1016/j.ajog.2017.06.011).
Quantifying the total cost of a health problem helps to show the public, payers, and health care administrators the magnitude of the problem on a population level. By underscoring the economic burden of preeclampsia, Stevens et al. provided important information about the high costs of this condition.
However, we need to go beyond health burden and cost-of-illness studies when considering the value of interventions to prevent and better manage preeclampsia and its adverse outcomes. Cost of the intervention and the cost savings associated with preventing one case of the condition are important parameters that can be used to calculate the potential savings of interventions. In fact, if the lifetimes costs of caring for children with adverse outcomes of preeclampsia are included, the potential cost savings from effective interventions may be even greater.
Rui Li, PhD, and William M. Callaghan, MD, are in the division of reproductive health at the Centers for Disease Control and Prevention, Atlanta. Eleni Tsigas is with the Preeclampsia Foundation in Melbourne, Fla. They reported having no relevant financial disclosures. These remarks are adapted from an editorial (Am J Obstet Gynecol. 2017 Jul 11. doi: 10.1016/j.ajog.2017.06.011).
Quantifying the total cost of a health problem helps to show the public, payers, and health care administrators the magnitude of the problem on a population level. By underscoring the economic burden of preeclampsia, Stevens et al. provided important information about the high costs of this condition.
However, we need to go beyond health burden and cost-of-illness studies when considering the value of interventions to prevent and better manage preeclampsia and its adverse outcomes. Cost of the intervention and the cost savings associated with preventing one case of the condition are important parameters that can be used to calculate the potential savings of interventions. In fact, if the lifetimes costs of caring for children with adverse outcomes of preeclampsia are included, the potential cost savings from effective interventions may be even greater.
Rui Li, PhD, and William M. Callaghan, MD, are in the division of reproductive health at the Centers for Disease Control and Prevention, Atlanta. Eleni Tsigas is with the Preeclampsia Foundation in Melbourne, Fla. They reported having no relevant financial disclosures. These remarks are adapted from an editorial (Am J Obstet Gynecol. 2017 Jul 11. doi: 10.1016/j.ajog.2017.06.011).
The cost of medical care for preeclampsia is estimated to be $2.18 billion for the first year surrounding delivery, split almost evenly between care for mothers and infants, according to a new report.
Preeclampsia is increasing at a more rapid rate than diabetes, ischemic heart failure, Alzheimer’s disease, obesity, and chronic kidney disease – disorders for which substantially more funding has been allocated for research and treatment, said Warren Stevens, PhD, of Precision Health Economics, Los Angeles, and his associates. Precision Health Economics provides consulting and other research services to pharmaceutical, device, government, and nongovernmental groups.
Compared with a healthy pregnancy, preeclampsia increased the probability that a mother would have at least one adverse outcome from 4.6% to 10.1%, and it increased the probability that an infant would have at least one adverse outcome from 7.9% to 14.2%. For mothers, preeclampsia was estimated to raise costs by $6,583 per birth, which translates to an increase of $1.03 billion for U.S. mothers during a single year (2012). For infants, the disorder raised costs by $1.15 billion during that year.
Infant costs varied according to the gestational age at delivery, ranging from $1,311 per birth at 36 weeks’ gestation to $150,000 per birth at 26 weeks’ gestation, Dr. Stevens and his associates reported (Am J Obstet Gynecol. 2017 Jul 11. doi: 10.1016/j.ajog.2017.04.032).
This study could not take into account longer-term adverse outcomes associated with preeclampsia. Women with a history of the disorder are at double the risk of developing ischemic heart disease or cerebrovascular disease compared with women without such a history, and at three times the risk of developing hypertension. Infants are at increased risk of developing stroke, metabolic syndrome, and chronic heart disease, the investigators noted.
The study was supported by rEVO Biologics. Five of the study authors are employees of rEVO Biologics and one author is a steering committee member for a clinical trial supported by the company; another study author is a consultant at Precision Health Economics.
The cost of medical care for preeclampsia is estimated to be $2.18 billion for the first year surrounding delivery, split almost evenly between care for mothers and infants, according to a new report.
Preeclampsia is increasing at a more rapid rate than diabetes, ischemic heart failure, Alzheimer’s disease, obesity, and chronic kidney disease – disorders for which substantially more funding has been allocated for research and treatment, said Warren Stevens, PhD, of Precision Health Economics, Los Angeles, and his associates. Precision Health Economics provides consulting and other research services to pharmaceutical, device, government, and nongovernmental groups.
Compared with a healthy pregnancy, preeclampsia increased the probability that a mother would have at least one adverse outcome from 4.6% to 10.1%, and it increased the probability that an infant would have at least one adverse outcome from 7.9% to 14.2%. For mothers, preeclampsia was estimated to raise costs by $6,583 per birth, which translates to an increase of $1.03 billion for U.S. mothers during a single year (2012). For infants, the disorder raised costs by $1.15 billion during that year.
Infant costs varied according to the gestational age at delivery, ranging from $1,311 per birth at 36 weeks’ gestation to $150,000 per birth at 26 weeks’ gestation, Dr. Stevens and his associates reported (Am J Obstet Gynecol. 2017 Jul 11. doi: 10.1016/j.ajog.2017.04.032).
This study could not take into account longer-term adverse outcomes associated with preeclampsia. Women with a history of the disorder are at double the risk of developing ischemic heart disease or cerebrovascular disease compared with women without such a history, and at three times the risk of developing hypertension. Infants are at increased risk of developing stroke, metabolic syndrome, and chronic heart disease, the investigators noted.
The study was supported by rEVO Biologics. Five of the study authors are employees of rEVO Biologics and one author is a steering committee member for a clinical trial supported by the company; another study author is a consultant at Precision Health Economics.
FROM THE AMERICAN JOURNAL OF OBSTETRICS & GYNECOLOGY
Key clinical point:
Major finding: Compared with healthy pregnancy, preeclampsia increased the probability that a mother would have at least one adverse outcome from 4.6% to 10.1%, and it increased the probability that an infant would have at least one adverse outcome from 7.9% to 14.2%.
Data source: A retrospective cohort study using secondary analysis of numerous data sets and extrapolating from approximately 2 million births in 2012 (1,918,498 births without preeclampsia and 69,193 with preeclampsia).
Disclosures: The study was supported by rEVO Biologics. Five of the study authors are employees of Precision Health Economics and one author is a consultant there; another author is on the steering committee for a clinical trial supported by rEVO Biologics.
FDA has the resources to answer questions about medicines and pregnancy
There are about 6 million pregnancies in the United States each year, and it’s estimated that 50% of pregnant women take at least one medicine. Physicians play an important role in helping their pregnant patients make informed health choices, especially when it comes to the safe use of medications.
- Emphasize that patients should always talk to their health care provider before taking any medicines, herbs, or vitamins. The website provides tips on how to start the conversation with patients about what medicines, herbs, or vitamins to avoid when pregnant.
- Encourage patients to check the drug label and other information that comes with their medicine to learn about possible risks for women who are pregnant or breastfeeding.
- Assist pregnant patients with changing medications as needed.
- Advise pregnant patients if they need to take more or less of their medicines.
- Advise patients about medicines that can and cannot be used when they start breastfeeding.
- Encourage patients to talk about any problems they have with their medicine.
- Report any serious problems your pregnant patients have had after taking a medicine to the FDA. It falls to physicians to report to the FDA any cases of serious side effects, problems with product quality, and product-use errors or with any of the following products: human drugs, medical devices, blood products and other biologics (except vaccines), and/or medical foods.
- Encourage patients to enroll in a Pregnancy Exposure Registry, if applicable at the FDA website, which collects information on pregnancy outcomes in women who already are taking medications. Observational studies of the patients that physicians help enroll in a pregnancy exposure registry can improve drug safety information for medicines used during pregnancy and can be used to update drug labeling. The observational studies included with the registries can also help physicians make medicine recommendations for use during pregnancy. The list includes contact information for each registry. Physicians can check online to see if there is a registry for their patients’ medicine.
The FDA website also provides information about the Pregnancy and Lactation Labeling Final Rule, which requires changes to the content and format for information presented in prescription drug labeling. The changes are implemented to help health care providers assess risk versus benefit and in subsequent counseling of pregnant women and nursing mothers.
The FDA offers free medicine safety and pregnancy resources for pregnant women, including downloadable infographics; a Medicines Record Keeper brochure in English, Spanish
Pregnancy is an exciting time for women, but they may have questions and concerns about how medicines they take will affect their babies. Our pregnancy website can help make a woman’s pregnancy happier and healthier.
Dr. Yao is the director of the division of pediatrics and maternal health, Office of Drug Evaluation IV, Center for Drug Evaluation and Research at the FDA, in Silver Spring, Md.
There are about 6 million pregnancies in the United States each year, and it’s estimated that 50% of pregnant women take at least one medicine. Physicians play an important role in helping their pregnant patients make informed health choices, especially when it comes to the safe use of medications.
- Emphasize that patients should always talk to their health care provider before taking any medicines, herbs, or vitamins. The website provides tips on how to start the conversation with patients about what medicines, herbs, or vitamins to avoid when pregnant.
- Encourage patients to check the drug label and other information that comes with their medicine to learn about possible risks for women who are pregnant or breastfeeding.
- Assist pregnant patients with changing medications as needed.
- Advise pregnant patients if they need to take more or less of their medicines.
- Advise patients about medicines that can and cannot be used when they start breastfeeding.
- Encourage patients to talk about any problems they have with their medicine.
- Report any serious problems your pregnant patients have had after taking a medicine to the FDA. It falls to physicians to report to the FDA any cases of serious side effects, problems with product quality, and product-use errors or with any of the following products: human drugs, medical devices, blood products and other biologics (except vaccines), and/or medical foods.
- Encourage patients to enroll in a Pregnancy Exposure Registry, if applicable at the FDA website, which collects information on pregnancy outcomes in women who already are taking medications. Observational studies of the patients that physicians help enroll in a pregnancy exposure registry can improve drug safety information for medicines used during pregnancy and can be used to update drug labeling. The observational studies included with the registries can also help physicians make medicine recommendations for use during pregnancy. The list includes contact information for each registry. Physicians can check online to see if there is a registry for their patients’ medicine.
The FDA website also provides information about the Pregnancy and Lactation Labeling Final Rule, which requires changes to the content and format for information presented in prescription drug labeling. The changes are implemented to help health care providers assess risk versus benefit and in subsequent counseling of pregnant women and nursing mothers.
The FDA offers free medicine safety and pregnancy resources for pregnant women, including downloadable infographics; a Medicines Record Keeper brochure in English, Spanish
Pregnancy is an exciting time for women, but they may have questions and concerns about how medicines they take will affect their babies. Our pregnancy website can help make a woman’s pregnancy happier and healthier.
Dr. Yao is the director of the division of pediatrics and maternal health, Office of Drug Evaluation IV, Center for Drug Evaluation and Research at the FDA, in Silver Spring, Md.
There are about 6 million pregnancies in the United States each year, and it’s estimated that 50% of pregnant women take at least one medicine. Physicians play an important role in helping their pregnant patients make informed health choices, especially when it comes to the safe use of medications.
- Emphasize that patients should always talk to their health care provider before taking any medicines, herbs, or vitamins. The website provides tips on how to start the conversation with patients about what medicines, herbs, or vitamins to avoid when pregnant.
- Encourage patients to check the drug label and other information that comes with their medicine to learn about possible risks for women who are pregnant or breastfeeding.
- Assist pregnant patients with changing medications as needed.
- Advise pregnant patients if they need to take more or less of their medicines.
- Advise patients about medicines that can and cannot be used when they start breastfeeding.
- Encourage patients to talk about any problems they have with their medicine.
- Report any serious problems your pregnant patients have had after taking a medicine to the FDA. It falls to physicians to report to the FDA any cases of serious side effects, problems with product quality, and product-use errors or with any of the following products: human drugs, medical devices, blood products and other biologics (except vaccines), and/or medical foods.
- Encourage patients to enroll in a Pregnancy Exposure Registry, if applicable at the FDA website, which collects information on pregnancy outcomes in women who already are taking medications. Observational studies of the patients that physicians help enroll in a pregnancy exposure registry can improve drug safety information for medicines used during pregnancy and can be used to update drug labeling. The observational studies included with the registries can also help physicians make medicine recommendations for use during pregnancy. The list includes contact information for each registry. Physicians can check online to see if there is a registry for their patients’ medicine.
The FDA website also provides information about the Pregnancy and Lactation Labeling Final Rule, which requires changes to the content and format for information presented in prescription drug labeling. The changes are implemented to help health care providers assess risk versus benefit and in subsequent counseling of pregnant women and nursing mothers.
The FDA offers free medicine safety and pregnancy resources for pregnant women, including downloadable infographics; a Medicines Record Keeper brochure in English, Spanish
Pregnancy is an exciting time for women, but they may have questions and concerns about how medicines they take will affect their babies. Our pregnancy website can help make a woman’s pregnancy happier and healthier.
Dr. Yao is the director of the division of pediatrics and maternal health, Office of Drug Evaluation IV, Center for Drug Evaluation and Research at the FDA, in Silver Spring, Md.