User login
The moving target of gestational diabetes care
With the rise of obesity and diabetes, especially type 2 diabetes, in the general population, the likelihood of encountering a patient with diabetes in pregnancy also continues to increase. Women with diabetes who are pregnant require specialized medical guidance, additional monitoring, and a health care team well versed in the possible complications that can arise during pregnancy, delivery, and after birth.
Even with strict glycemic control, women with diabetes in pregnancy are much more likely to experience complications, such as preeclampsia, babies with major congenital defects, large-for-gestational-age fetuses, and children with a higher propensity for chronic diseases later in life, compared with women without diabetes.
Therefore, it has been an incredible honor for me to have taken part in the work of the Diabetes in Pregnancy Study Group of North America (DPSG-NA) for the last 20 years. The DPSG-NA meetings have served as a forum for the dissemination of data, gathered through collaboration between researchers and clinical care teams in the United States and abroad. This year, the DPSG-NA will meet in Washington, D.C., Oct. 26-28, to discuss a range of topics under the theme of managing and preventing diabetes and obesity in pregnancy.
I am delighted that one of the speakers at the DPSG-NA meeting is this month’s Master Class guest author, Lynn Yee, MD, assistant professor of obstetrics and gynecology at Northwestern University Feinberg School of Medicine, Chicago. Dr. Yee will address the need to reduce disparities in the quality and availability of care for patients with diabetes in pregnancy, an extension of the June Master Class column that discussed the critical role that ob.gyns. can play in improving health equity for all patients.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
With the rise of obesity and diabetes, especially type 2 diabetes, in the general population, the likelihood of encountering a patient with diabetes in pregnancy also continues to increase. Women with diabetes who are pregnant require specialized medical guidance, additional monitoring, and a health care team well versed in the possible complications that can arise during pregnancy, delivery, and after birth.
Even with strict glycemic control, women with diabetes in pregnancy are much more likely to experience complications, such as preeclampsia, babies with major congenital defects, large-for-gestational-age fetuses, and children with a higher propensity for chronic diseases later in life, compared with women without diabetes.
Therefore, it has been an incredible honor for me to have taken part in the work of the Diabetes in Pregnancy Study Group of North America (DPSG-NA) for the last 20 years. The DPSG-NA meetings have served as a forum for the dissemination of data, gathered through collaboration between researchers and clinical care teams in the United States and abroad. This year, the DPSG-NA will meet in Washington, D.C., Oct. 26-28, to discuss a range of topics under the theme of managing and preventing diabetes and obesity in pregnancy.
I am delighted that one of the speakers at the DPSG-NA meeting is this month’s Master Class guest author, Lynn Yee, MD, assistant professor of obstetrics and gynecology at Northwestern University Feinberg School of Medicine, Chicago. Dr. Yee will address the need to reduce disparities in the quality and availability of care for patients with diabetes in pregnancy, an extension of the June Master Class column that discussed the critical role that ob.gyns. can play in improving health equity for all patients.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
With the rise of obesity and diabetes, especially type 2 diabetes, in the general population, the likelihood of encountering a patient with diabetes in pregnancy also continues to increase. Women with diabetes who are pregnant require specialized medical guidance, additional monitoring, and a health care team well versed in the possible complications that can arise during pregnancy, delivery, and after birth.
Even with strict glycemic control, women with diabetes in pregnancy are much more likely to experience complications, such as preeclampsia, babies with major congenital defects, large-for-gestational-age fetuses, and children with a higher propensity for chronic diseases later in life, compared with women without diabetes.
Therefore, it has been an incredible honor for me to have taken part in the work of the Diabetes in Pregnancy Study Group of North America (DPSG-NA) for the last 20 years. The DPSG-NA meetings have served as a forum for the dissemination of data, gathered through collaboration between researchers and clinical care teams in the United States and abroad. This year, the DPSG-NA will meet in Washington, D.C., Oct. 26-28, to discuss a range of topics under the theme of managing and preventing diabetes and obesity in pregnancy.
I am delighted that one of the speakers at the DPSG-NA meeting is this month’s Master Class guest author, Lynn Yee, MD, assistant professor of obstetrics and gynecology at Northwestern University Feinberg School of Medicine, Chicago. Dr. Yee will address the need to reduce disparities in the quality and availability of care for patients with diabetes in pregnancy, an extension of the June Master Class column that discussed the critical role that ob.gyns. can play in improving health equity for all patients.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
Safety of oral antidiabetic agents in pregnancy
The three most potent human teratogens, with the possible inclusion of some of the first antineoplastics, are isotretinoin, alcohol, and hyperglycemia.
As with all teratogens, the toxicity is dose related. For example, the risk of embryo-fetal harm from hyperglycemia increases markedly when the HbA1c is greater than 8%. Moreover, diabetes accounts for more than 90% of the harm caused by chronic diseases. Consequently, control of glucose levels in pregnancy is critical.
If these agents are used near term, there is a risk that they will cause hypoglycemia in the newborn. Changing from oral therapy to insulin is the safest course.
There are seven pharmacologic subclasses of oral antidiabetic agents: alpha-glucosidase inhibitors, biguanides, dipeptidyl peptidase-4 inhibitors, meglitinides, sulfonylureas, sodium-glucose cotransporter-2 inhibitors, and thiazolidinediones. Many of these drugs are available in combination with metformin. All of these agents are indicated as adjunct to diet and exercise for type 2 diabetes, but they also can be used for gestational diabetes. Although the human pregnancy data are very limited or nonexistent for most of these agents, none are known to cause structural defects in humans. Additional details of the exposures are available in the 11th edition of “Drugs in Pregnancy and Lactation” (2017: Wolters Kluwer).
Alpha-glucosidase inhibitors
The two agents is this subclass are acarbose (Precose) and miglitol (Glyset). The human pregnancy data with acarbose are limited, and no human pregnancy data have been found for miglitol. The animal data for both drugs suggest low risk.
Biguanides
There are substantial human pregnancy data for metformin in both type 2 and gestational diabetes. When combined with insulin, it is effective in significantly lowering the amount of insulin required to control hyperglycemia. It also may be effective when used alone. The risk of embryo-fetal harm with this drug appears to be very low or nonexistent. The animal data suggest low risk.
Dipeptidyl peptidase-4 inhibitors
There are four drugs in this subclass: alogliptin (Nesina), linagliptin (Tradjenta), saxagliptin (Onglyza), and sitagliptin (Januvia). No reports of the use of the first three drugs in human pregnancy have been found. However, the Merck Pregnancy Registries (2006-2009) described the outcomes of eight women who were exposed to sitagliptin or sitagliptin/metformin in the first trimester. The outcomes of these pregnancies were five healthy newborns, two spontaneous abortions, and one fetal death at 34 weeks’ gestation. In that case, the mother took sitagliptin and metformin separately during the first 5 weeks of gestation. The animal data for all four drugs suggest low risk.
Meglitinides
Nateglinide (Starlix) and repaglinide (Prandin) are the agents in this subclass. There is no human pregnancy data for nateglinide, but there is limited data (eight pregnancies) for repaglinide. No birth defects or other toxicity was noted in these cases. The animal data suggest low risk.
Sulfonylureas
Six drugs are included in this subclass: chlorpropamide, glimepiride (Amaryl), glipizide (Glucotrol), glyburide, tolazamide (Tolinase), and tolbutamide. These agents were among the first oral antidiabetic agents. As a result, they have the most human pregnancy data. Although birth defects were observed in newborns of mothers who had used one of these drugs, the defects were thought to be the result of uncontrolled diabetes. The animal data suggest low risk.
SGLT2 inhibitors
There are three drugs in this sodium-glucose cotransporter-2 inhibitor subclass: canagliflozin (Invokana), dapagliflozin (Farxiga), and empagliflozin (Jardiance). No reports describing the use of these drugs in human pregnancy have been located. The animal data suggest low risk.
Thiazolidinediones
Pioglitazone (Actos) and rosiglitazone (Avandia) form this subclass. There are limited human pregnancy data for both drugs. The animal data suggest moderate risk for embryo-fetal toxicity but not for structural defects.
Lactation
All of the above drugs will probably be excreted into breast milk, but the amounts are typically unknown. When they have been measured, the amounts were usually low. However, there is still a risk for hypoglycemia in a nursing infant. Combination products containing two antidiabetic agents are best avoided. The safest course is to use insulin, but, if this is not an option, then the lowest effective dose should be used. In addition, the infant’s blood glucose levels should be routinely monitored.
Mr. Briggs is a clinical professor of pharmacy at the University of California, San Francisco, and an adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. He coauthored “Drugs in Pregnancy and Lactation” and coedited “Diseases, Complications, and Drug Therapy in Obstetrics.” He reported having no relevant financial disclosures.
The three most potent human teratogens, with the possible inclusion of some of the first antineoplastics, are isotretinoin, alcohol, and hyperglycemia.
As with all teratogens, the toxicity is dose related. For example, the risk of embryo-fetal harm from hyperglycemia increases markedly when the HbA1c is greater than 8%. Moreover, diabetes accounts for more than 90% of the harm caused by chronic diseases. Consequently, control of glucose levels in pregnancy is critical.
If these agents are used near term, there is a risk that they will cause hypoglycemia in the newborn. Changing from oral therapy to insulin is the safest course.
There are seven pharmacologic subclasses of oral antidiabetic agents: alpha-glucosidase inhibitors, biguanides, dipeptidyl peptidase-4 inhibitors, meglitinides, sulfonylureas, sodium-glucose cotransporter-2 inhibitors, and thiazolidinediones. Many of these drugs are available in combination with metformin. All of these agents are indicated as adjunct to diet and exercise for type 2 diabetes, but they also can be used for gestational diabetes. Although the human pregnancy data are very limited or nonexistent for most of these agents, none are known to cause structural defects in humans. Additional details of the exposures are available in the 11th edition of “Drugs in Pregnancy and Lactation” (2017: Wolters Kluwer).
Alpha-glucosidase inhibitors
The two agents is this subclass are acarbose (Precose) and miglitol (Glyset). The human pregnancy data with acarbose are limited, and no human pregnancy data have been found for miglitol. The animal data for both drugs suggest low risk.
Biguanides
There are substantial human pregnancy data for metformin in both type 2 and gestational diabetes. When combined with insulin, it is effective in significantly lowering the amount of insulin required to control hyperglycemia. It also may be effective when used alone. The risk of embryo-fetal harm with this drug appears to be very low or nonexistent. The animal data suggest low risk.
Dipeptidyl peptidase-4 inhibitors
There are four drugs in this subclass: alogliptin (Nesina), linagliptin (Tradjenta), saxagliptin (Onglyza), and sitagliptin (Januvia). No reports of the use of the first three drugs in human pregnancy have been found. However, the Merck Pregnancy Registries (2006-2009) described the outcomes of eight women who were exposed to sitagliptin or sitagliptin/metformin in the first trimester. The outcomes of these pregnancies were five healthy newborns, two spontaneous abortions, and one fetal death at 34 weeks’ gestation. In that case, the mother took sitagliptin and metformin separately during the first 5 weeks of gestation. The animal data for all four drugs suggest low risk.
Meglitinides
Nateglinide (Starlix) and repaglinide (Prandin) are the agents in this subclass. There is no human pregnancy data for nateglinide, but there is limited data (eight pregnancies) for repaglinide. No birth defects or other toxicity was noted in these cases. The animal data suggest low risk.
Sulfonylureas
Six drugs are included in this subclass: chlorpropamide, glimepiride (Amaryl), glipizide (Glucotrol), glyburide, tolazamide (Tolinase), and tolbutamide. These agents were among the first oral antidiabetic agents. As a result, they have the most human pregnancy data. Although birth defects were observed in newborns of mothers who had used one of these drugs, the defects were thought to be the result of uncontrolled diabetes. The animal data suggest low risk.
SGLT2 inhibitors
There are three drugs in this sodium-glucose cotransporter-2 inhibitor subclass: canagliflozin (Invokana), dapagliflozin (Farxiga), and empagliflozin (Jardiance). No reports describing the use of these drugs in human pregnancy have been located. The animal data suggest low risk.
Thiazolidinediones
Pioglitazone (Actos) and rosiglitazone (Avandia) form this subclass. There are limited human pregnancy data for both drugs. The animal data suggest moderate risk for embryo-fetal toxicity but not for structural defects.
Lactation
All of the above drugs will probably be excreted into breast milk, but the amounts are typically unknown. When they have been measured, the amounts were usually low. However, there is still a risk for hypoglycemia in a nursing infant. Combination products containing two antidiabetic agents are best avoided. The safest course is to use insulin, but, if this is not an option, then the lowest effective dose should be used. In addition, the infant’s blood glucose levels should be routinely monitored.
Mr. Briggs is a clinical professor of pharmacy at the University of California, San Francisco, and an adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. He coauthored “Drugs in Pregnancy and Lactation” and coedited “Diseases, Complications, and Drug Therapy in Obstetrics.” He reported having no relevant financial disclosures.
The three most potent human teratogens, with the possible inclusion of some of the first antineoplastics, are isotretinoin, alcohol, and hyperglycemia.
As with all teratogens, the toxicity is dose related. For example, the risk of embryo-fetal harm from hyperglycemia increases markedly when the HbA1c is greater than 8%. Moreover, diabetes accounts for more than 90% of the harm caused by chronic diseases. Consequently, control of glucose levels in pregnancy is critical.
If these agents are used near term, there is a risk that they will cause hypoglycemia in the newborn. Changing from oral therapy to insulin is the safest course.
There are seven pharmacologic subclasses of oral antidiabetic agents: alpha-glucosidase inhibitors, biguanides, dipeptidyl peptidase-4 inhibitors, meglitinides, sulfonylureas, sodium-glucose cotransporter-2 inhibitors, and thiazolidinediones. Many of these drugs are available in combination with metformin. All of these agents are indicated as adjunct to diet and exercise for type 2 diabetes, but they also can be used for gestational diabetes. Although the human pregnancy data are very limited or nonexistent for most of these agents, none are known to cause structural defects in humans. Additional details of the exposures are available in the 11th edition of “Drugs in Pregnancy and Lactation” (2017: Wolters Kluwer).
Alpha-glucosidase inhibitors
The two agents is this subclass are acarbose (Precose) and miglitol (Glyset). The human pregnancy data with acarbose are limited, and no human pregnancy data have been found for miglitol. The animal data for both drugs suggest low risk.
Biguanides
There are substantial human pregnancy data for metformin in both type 2 and gestational diabetes. When combined with insulin, it is effective in significantly lowering the amount of insulin required to control hyperglycemia. It also may be effective when used alone. The risk of embryo-fetal harm with this drug appears to be very low or nonexistent. The animal data suggest low risk.
Dipeptidyl peptidase-4 inhibitors
There are four drugs in this subclass: alogliptin (Nesina), linagliptin (Tradjenta), saxagliptin (Onglyza), and sitagliptin (Januvia). No reports of the use of the first three drugs in human pregnancy have been found. However, the Merck Pregnancy Registries (2006-2009) described the outcomes of eight women who were exposed to sitagliptin or sitagliptin/metformin in the first trimester. The outcomes of these pregnancies were five healthy newborns, two spontaneous abortions, and one fetal death at 34 weeks’ gestation. In that case, the mother took sitagliptin and metformin separately during the first 5 weeks of gestation. The animal data for all four drugs suggest low risk.
Meglitinides
Nateglinide (Starlix) and repaglinide (Prandin) are the agents in this subclass. There is no human pregnancy data for nateglinide, but there is limited data (eight pregnancies) for repaglinide. No birth defects or other toxicity was noted in these cases. The animal data suggest low risk.
Sulfonylureas
Six drugs are included in this subclass: chlorpropamide, glimepiride (Amaryl), glipizide (Glucotrol), glyburide, tolazamide (Tolinase), and tolbutamide. These agents were among the first oral antidiabetic agents. As a result, they have the most human pregnancy data. Although birth defects were observed in newborns of mothers who had used one of these drugs, the defects were thought to be the result of uncontrolled diabetes. The animal data suggest low risk.
SGLT2 inhibitors
There are three drugs in this sodium-glucose cotransporter-2 inhibitor subclass: canagliflozin (Invokana), dapagliflozin (Farxiga), and empagliflozin (Jardiance). No reports describing the use of these drugs in human pregnancy have been located. The animal data suggest low risk.
Thiazolidinediones
Pioglitazone (Actos) and rosiglitazone (Avandia) form this subclass. There are limited human pregnancy data for both drugs. The animal data suggest moderate risk for embryo-fetal toxicity but not for structural defects.
Lactation
All of the above drugs will probably be excreted into breast milk, but the amounts are typically unknown. When they have been measured, the amounts were usually low. However, there is still a risk for hypoglycemia in a nursing infant. Combination products containing two antidiabetic agents are best avoided. The safest course is to use insulin, but, if this is not an option, then the lowest effective dose should be used. In addition, the infant’s blood glucose levels should be routinely monitored.
Mr. Briggs is a clinical professor of pharmacy at the University of California, San Francisco, and an adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. He coauthored “Drugs in Pregnancy and Lactation” and coedited “Diseases, Complications, and Drug Therapy in Obstetrics.” He reported having no relevant financial disclosures.
Thirdhand smoke shaping up as potential health hazard
DENVER – Thirdhand smoke – the persistent residue that collects on indoor surfaces where people have smoked – is “clearly” a potentially hazardous exposure, John M. Rogers, PhD, said at the annual meeting of the Teratology Society.
Everyone knows about the hazards of secondhand smoke, which have led to widespread bans on smoking in public spaces. Still, the Centers for Disease Control and Prevention estimates that 58 million nonsmokers in the United States are exposed to secondhand smoke on a regular basis. And where there is secondhand smoke, there is typically exposure to thirdhand smoke as well.
“If you walk into a hotel room you were told is a nonsmoking room and you take one breath and you know it’s not nonsmoking, that’s thirdhand smoke. Thirdhand smoke is all over the place where smokers have been,” explained Dr. Rogers, director of the toxicity assessment division at the Environmental Protection Agency in Research Triangle Park, N.C.
The main potential health risk is to young children, who ingest thirdhand smoke by the hand-to-mouth route and skin contact.
Thirdhand smoke is a much newer concept than secondhand smoke and has not yet actually been shown to pose a significant health risk. . But that is likely to change.
Thirdhand smoke has become an area of intensive research interest, with California leading the way. The Tobacco-Related Disease Research Program, a state agency funded by a tax on the sale of tobacco products, has created a research consortium on thirdhand smoke, with studies underway investigating thirdhand smoke’s precise chemical composition, cytotoxicity, genotoxicity, and true impact on public health (www.trdrp.org).
Concern regarding thirdhand smoke’s potential public health impact ramped up in response to a study in which investigators at the University of York, England, measured levels of various tobacco-specific nitrosamines, N-nitrosamines, and nicotine in house dust samples from the homes of smokers. The researchers estimated that years of early life exposure to these compounds at the levels they detected could result in one excess case of cancer per 1,000 exposed individuals (Environ Int. 2014 Oct;71:139-47).
In addition to his update on thirdhand smoke, Dr. Rogers also touched on other recent tobacco-related developments, including a determination by the Food and Drug Administration that there has been no decline in tobacco use in the last 5 years in adolescents and young adults. While cigarette smoking by young people decreased, this was offset by a large increase in the use of electronic cigarettes and a smaller rise in the use of hookah tobacco. Indeed, e-cigarette use is now about double that of cigarettes among youth.
Also of concern is evidence of a striking socioeconomic disparity in smoking prevalence: Low-education, low-income Americans have far higher tobacco use rates.
“That’s pretty alarming,” he said. “I think a lot of people in this audience probably don’t see a lot of smoking these days, but it’s still around.”
Dr. Rogers drew attention to updated evidence reviews on the reproductive and developmental effects of smoking contained in the U.S. Surgeon General’s voluminous 2014 report on the health consequences of smoking. The report concluded that there is now sufficient evidence to infer a causal relationship between maternal smoking in pregnancy, ectopic pregnancy, and orofacial clefts. The available evidence is “suggestive but not sufficient” to infer causality between maternal smoking in pregnancy and atrial septal defects, clubfoot, gastroschisis, and attention-deficit/hyperactivity disorder and other disruptive behavior disorders.
Dr. Rogers reported having no financial disclosures related to his presentation, which he noted did not necessarily reflect the views and policies of the EPA.
DENVER – Thirdhand smoke – the persistent residue that collects on indoor surfaces where people have smoked – is “clearly” a potentially hazardous exposure, John M. Rogers, PhD, said at the annual meeting of the Teratology Society.
Everyone knows about the hazards of secondhand smoke, which have led to widespread bans on smoking in public spaces. Still, the Centers for Disease Control and Prevention estimates that 58 million nonsmokers in the United States are exposed to secondhand smoke on a regular basis. And where there is secondhand smoke, there is typically exposure to thirdhand smoke as well.
“If you walk into a hotel room you were told is a nonsmoking room and you take one breath and you know it’s not nonsmoking, that’s thirdhand smoke. Thirdhand smoke is all over the place where smokers have been,” explained Dr. Rogers, director of the toxicity assessment division at the Environmental Protection Agency in Research Triangle Park, N.C.
The main potential health risk is to young children, who ingest thirdhand smoke by the hand-to-mouth route and skin contact.
Thirdhand smoke is a much newer concept than secondhand smoke and has not yet actually been shown to pose a significant health risk. . But that is likely to change.
Thirdhand smoke has become an area of intensive research interest, with California leading the way. The Tobacco-Related Disease Research Program, a state agency funded by a tax on the sale of tobacco products, has created a research consortium on thirdhand smoke, with studies underway investigating thirdhand smoke’s precise chemical composition, cytotoxicity, genotoxicity, and true impact on public health (www.trdrp.org).
Concern regarding thirdhand smoke’s potential public health impact ramped up in response to a study in which investigators at the University of York, England, measured levels of various tobacco-specific nitrosamines, N-nitrosamines, and nicotine in house dust samples from the homes of smokers. The researchers estimated that years of early life exposure to these compounds at the levels they detected could result in one excess case of cancer per 1,000 exposed individuals (Environ Int. 2014 Oct;71:139-47).
In addition to his update on thirdhand smoke, Dr. Rogers also touched on other recent tobacco-related developments, including a determination by the Food and Drug Administration that there has been no decline in tobacco use in the last 5 years in adolescents and young adults. While cigarette smoking by young people decreased, this was offset by a large increase in the use of electronic cigarettes and a smaller rise in the use of hookah tobacco. Indeed, e-cigarette use is now about double that of cigarettes among youth.
Also of concern is evidence of a striking socioeconomic disparity in smoking prevalence: Low-education, low-income Americans have far higher tobacco use rates.
“That’s pretty alarming,” he said. “I think a lot of people in this audience probably don’t see a lot of smoking these days, but it’s still around.”
Dr. Rogers drew attention to updated evidence reviews on the reproductive and developmental effects of smoking contained in the U.S. Surgeon General’s voluminous 2014 report on the health consequences of smoking. The report concluded that there is now sufficient evidence to infer a causal relationship between maternal smoking in pregnancy, ectopic pregnancy, and orofacial clefts. The available evidence is “suggestive but not sufficient” to infer causality between maternal smoking in pregnancy and atrial septal defects, clubfoot, gastroschisis, and attention-deficit/hyperactivity disorder and other disruptive behavior disorders.
Dr. Rogers reported having no financial disclosures related to his presentation, which he noted did not necessarily reflect the views and policies of the EPA.
DENVER – Thirdhand smoke – the persistent residue that collects on indoor surfaces where people have smoked – is “clearly” a potentially hazardous exposure, John M. Rogers, PhD, said at the annual meeting of the Teratology Society.
Everyone knows about the hazards of secondhand smoke, which have led to widespread bans on smoking in public spaces. Still, the Centers for Disease Control and Prevention estimates that 58 million nonsmokers in the United States are exposed to secondhand smoke on a regular basis. And where there is secondhand smoke, there is typically exposure to thirdhand smoke as well.
“If you walk into a hotel room you were told is a nonsmoking room and you take one breath and you know it’s not nonsmoking, that’s thirdhand smoke. Thirdhand smoke is all over the place where smokers have been,” explained Dr. Rogers, director of the toxicity assessment division at the Environmental Protection Agency in Research Triangle Park, N.C.
The main potential health risk is to young children, who ingest thirdhand smoke by the hand-to-mouth route and skin contact.
Thirdhand smoke is a much newer concept than secondhand smoke and has not yet actually been shown to pose a significant health risk. . But that is likely to change.
Thirdhand smoke has become an area of intensive research interest, with California leading the way. The Tobacco-Related Disease Research Program, a state agency funded by a tax on the sale of tobacco products, has created a research consortium on thirdhand smoke, with studies underway investigating thirdhand smoke’s precise chemical composition, cytotoxicity, genotoxicity, and true impact on public health (www.trdrp.org).
Concern regarding thirdhand smoke’s potential public health impact ramped up in response to a study in which investigators at the University of York, England, measured levels of various tobacco-specific nitrosamines, N-nitrosamines, and nicotine in house dust samples from the homes of smokers. The researchers estimated that years of early life exposure to these compounds at the levels they detected could result in one excess case of cancer per 1,000 exposed individuals (Environ Int. 2014 Oct;71:139-47).
In addition to his update on thirdhand smoke, Dr. Rogers also touched on other recent tobacco-related developments, including a determination by the Food and Drug Administration that there has been no decline in tobacco use in the last 5 years in adolescents and young adults. While cigarette smoking by young people decreased, this was offset by a large increase in the use of electronic cigarettes and a smaller rise in the use of hookah tobacco. Indeed, e-cigarette use is now about double that of cigarettes among youth.
Also of concern is evidence of a striking socioeconomic disparity in smoking prevalence: Low-education, low-income Americans have far higher tobacco use rates.
“That’s pretty alarming,” he said. “I think a lot of people in this audience probably don’t see a lot of smoking these days, but it’s still around.”
Dr. Rogers drew attention to updated evidence reviews on the reproductive and developmental effects of smoking contained in the U.S. Surgeon General’s voluminous 2014 report on the health consequences of smoking. The report concluded that there is now sufficient evidence to infer a causal relationship between maternal smoking in pregnancy, ectopic pregnancy, and orofacial clefts. The available evidence is “suggestive but not sufficient” to infer causality between maternal smoking in pregnancy and atrial septal defects, clubfoot, gastroschisis, and attention-deficit/hyperactivity disorder and other disruptive behavior disorders.
Dr. Rogers reported having no financial disclosures related to his presentation, which he noted did not necessarily reflect the views and policies of the EPA.
EXPERT ANALYSIS FROM TERATOLOGY SOCIETY 2017
Delay in delivery--mother and child die: $1.4M settlement
Delay in delivery--mother and child die: $1.4M settlement
ESTATE'S CLAIM:
The standard of care for placenta accreta requires delivery between 34 and 36 weeks of gestation. The mother died from a placental abruption and amniotic fluid embolism. Placenta accreta increases the risk of catastrophic hemorrhage. If delivery had occurred on January 14, both the mother and child would be alive.
DEFENDANTS' DEFENSE:
The case settled before trial.
VERDICT:
A $1.425 million Georgia settlement was reached. The settlement amount was limited by a damages cap unique to the defendant hospital.
Placental abruption not detected: $6.2M settlement
At 24 weeks of gestation, a mother presented to the hospital with premature contractions that subsided after her arrival. She was discharged from the hospital. The woman gave birth in her bathtub several hours later. The baby was 10 weeks premature. He suffered profound brain damage and has significant physical defects.
PARENT'S CLAIM:
Neither the ObGyn nor the hospital staff appreciated that the mother was experiencing placental abruption. If diagnosed, treatment could have prevented fetal injury.
DEFENDANTS' DEFENSE:
The case was settled prior to trial.
VERDICT:
A $6.2 million New York settlement was reached.
Child has brachial plexus injury: $2M award
A woman was admitted to the hospital for elective induction of labor. She gained a significant amount of weight while pregnant. During delivery, her family practitioner (FP) determined that vacuum extraction was needed but he was not qualified to use the device. An in-house ObGyn was called in to use the vacuum extractor. The FP delivered the baby's shoulders. The infant was born with a floppy right arm and later diagnosed with rupture injuries to the C-5 and C-6 vertebrae and permanent brachial plexus damage. She has limited range of motion in her right arm and shoulder.
PARENT'S CLAIM:
The FP was relatively inexperienced in labor and delivery. He should not have ordered vacuum extraction because of risk factors including the mother's small stature, her significant weight gain during pregnancy, the use of epidural anesthesia, and induction of labor. Using vacuum extraction increases the risk of shoulder dystocia.
The FP improperly applied excessive downward traction on the fetus causing the infant to sustain a brachial plexus injury.
The FP did not notify the parents of the child's injury immediately after birth; he told them about the injury just before discharge.
DEFENDANTS' DEFENSE:
There is no evidence in the medical records of a shoulder dystocia; "no shoulder dystocia" was charted shortly after delivery. No one in the delivery room testified to a delay in delivering the infant's shoulders. The mother's internal contractions caused the injury. The baby was not injured to the extent claimed.
VERDICT:
The ObGyn who used the vacuum extractor settled before the trial for $300,000. A $2 million Illinois verdict was returned against the FP.
Delay in treating infant in respiratory distress: $7.27M settlement
A child was delivered by a certified nurse midwife at a birthing center. At birth, the baby had a heart rate of 60 bpm and was in respiratory distress but there was no one at the clinic qualified to intubate the infant. Emergency personnel were called but the infant remained in respiratory distress for 8 minutes. The baby experienced birth asphyxia with hypoxic ischemic encephalopathy resulting in severe cerebral palsy.
PARENT'S CLAIM:
The birthing center was poorly staffed and unprepared to treat an emergency situation.
DEFENDANTS' DEFENSE:
The defendants denied all allegations of negligence. The case was settled during trial.
VERDICT:
A $7.27 million Pennsylvania settlement was reached.
Was the spinal block given at wrong level?
A MOTHER WENT TO THE HOSPITAL in labor. Prior to cesarean delivery, she underwent an anesthetic spinal block administered by a CRNA. Initially, the patient reported pain shortly after the injection was performed until the block worked. The baby's delivery was uneventful.
In recovery a few hours later, the patient reported intense and uncontrollable pain in her legs. Magnetic resonance imaging revealed a fluid pocket on her spinal cord at the L1-L2 level. The patient has permanent pain, numbness, and tingling in in both legs.
PATIENT'S CLAIM:
The CRNA failed to insert the spinal block needle in the proper location.
DEFENDANTS' DEFENSE:
The CRNA contended that he complied with the standard of care. He claimed that the patient had an unusual spinal cord anatomy: it was tethered down to the L3-L4 level.
VERDICT:
A $509,152 Kentucky verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Delay in delivery--mother and child die: $1.4M settlement
ESTATE'S CLAIM:
The standard of care for placenta accreta requires delivery between 34 and 36 weeks of gestation. The mother died from a placental abruption and amniotic fluid embolism. Placenta accreta increases the risk of catastrophic hemorrhage. If delivery had occurred on January 14, both the mother and child would be alive.
DEFENDANTS' DEFENSE:
The case settled before trial.
VERDICT:
A $1.425 million Georgia settlement was reached. The settlement amount was limited by a damages cap unique to the defendant hospital.
Placental abruption not detected: $6.2M settlement
At 24 weeks of gestation, a mother presented to the hospital with premature contractions that subsided after her arrival. She was discharged from the hospital. The woman gave birth in her bathtub several hours later. The baby was 10 weeks premature. He suffered profound brain damage and has significant physical defects.
PARENT'S CLAIM:
Neither the ObGyn nor the hospital staff appreciated that the mother was experiencing placental abruption. If diagnosed, treatment could have prevented fetal injury.
DEFENDANTS' DEFENSE:
The case was settled prior to trial.
VERDICT:
A $6.2 million New York settlement was reached.
Child has brachial plexus injury: $2M award
A woman was admitted to the hospital for elective induction of labor. She gained a significant amount of weight while pregnant. During delivery, her family practitioner (FP) determined that vacuum extraction was needed but he was not qualified to use the device. An in-house ObGyn was called in to use the vacuum extractor. The FP delivered the baby's shoulders. The infant was born with a floppy right arm and later diagnosed with rupture injuries to the C-5 and C-6 vertebrae and permanent brachial plexus damage. She has limited range of motion in her right arm and shoulder.
PARENT'S CLAIM:
The FP was relatively inexperienced in labor and delivery. He should not have ordered vacuum extraction because of risk factors including the mother's small stature, her significant weight gain during pregnancy, the use of epidural anesthesia, and induction of labor. Using vacuum extraction increases the risk of shoulder dystocia.
The FP improperly applied excessive downward traction on the fetus causing the infant to sustain a brachial plexus injury.
The FP did not notify the parents of the child's injury immediately after birth; he told them about the injury just before discharge.
DEFENDANTS' DEFENSE:
There is no evidence in the medical records of a shoulder dystocia; "no shoulder dystocia" was charted shortly after delivery. No one in the delivery room testified to a delay in delivering the infant's shoulders. The mother's internal contractions caused the injury. The baby was not injured to the extent claimed.
VERDICT:
The ObGyn who used the vacuum extractor settled before the trial for $300,000. A $2 million Illinois verdict was returned against the FP.
Delay in treating infant in respiratory distress: $7.27M settlement
A child was delivered by a certified nurse midwife at a birthing center. At birth, the baby had a heart rate of 60 bpm and was in respiratory distress but there was no one at the clinic qualified to intubate the infant. Emergency personnel were called but the infant remained in respiratory distress for 8 minutes. The baby experienced birth asphyxia with hypoxic ischemic encephalopathy resulting in severe cerebral palsy.
PARENT'S CLAIM:
The birthing center was poorly staffed and unprepared to treat an emergency situation.
DEFENDANTS' DEFENSE:
The defendants denied all allegations of negligence. The case was settled during trial.
VERDICT:
A $7.27 million Pennsylvania settlement was reached.
Was the spinal block given at wrong level?
A MOTHER WENT TO THE HOSPITAL in labor. Prior to cesarean delivery, she underwent an anesthetic spinal block administered by a CRNA. Initially, the patient reported pain shortly after the injection was performed until the block worked. The baby's delivery was uneventful.
In recovery a few hours later, the patient reported intense and uncontrollable pain in her legs. Magnetic resonance imaging revealed a fluid pocket on her spinal cord at the L1-L2 level. The patient has permanent pain, numbness, and tingling in in both legs.
PATIENT'S CLAIM:
The CRNA failed to insert the spinal block needle in the proper location.
DEFENDANTS' DEFENSE:
The CRNA contended that he complied with the standard of care. He claimed that the patient had an unusual spinal cord anatomy: it was tethered down to the L3-L4 level.
VERDICT:
A $509,152 Kentucky verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Delay in delivery--mother and child die: $1.4M settlement
ESTATE'S CLAIM:
The standard of care for placenta accreta requires delivery between 34 and 36 weeks of gestation. The mother died from a placental abruption and amniotic fluid embolism. Placenta accreta increases the risk of catastrophic hemorrhage. If delivery had occurred on January 14, both the mother and child would be alive.
DEFENDANTS' DEFENSE:
The case settled before trial.
VERDICT:
A $1.425 million Georgia settlement was reached. The settlement amount was limited by a damages cap unique to the defendant hospital.
Placental abruption not detected: $6.2M settlement
At 24 weeks of gestation, a mother presented to the hospital with premature contractions that subsided after her arrival. She was discharged from the hospital. The woman gave birth in her bathtub several hours later. The baby was 10 weeks premature. He suffered profound brain damage and has significant physical defects.
PARENT'S CLAIM:
Neither the ObGyn nor the hospital staff appreciated that the mother was experiencing placental abruption. If diagnosed, treatment could have prevented fetal injury.
DEFENDANTS' DEFENSE:
The case was settled prior to trial.
VERDICT:
A $6.2 million New York settlement was reached.
Child has brachial plexus injury: $2M award
A woman was admitted to the hospital for elective induction of labor. She gained a significant amount of weight while pregnant. During delivery, her family practitioner (FP) determined that vacuum extraction was needed but he was not qualified to use the device. An in-house ObGyn was called in to use the vacuum extractor. The FP delivered the baby's shoulders. The infant was born with a floppy right arm and later diagnosed with rupture injuries to the C-5 and C-6 vertebrae and permanent brachial plexus damage. She has limited range of motion in her right arm and shoulder.
PARENT'S CLAIM:
The FP was relatively inexperienced in labor and delivery. He should not have ordered vacuum extraction because of risk factors including the mother's small stature, her significant weight gain during pregnancy, the use of epidural anesthesia, and induction of labor. Using vacuum extraction increases the risk of shoulder dystocia.
The FP improperly applied excessive downward traction on the fetus causing the infant to sustain a brachial plexus injury.
The FP did not notify the parents of the child's injury immediately after birth; he told them about the injury just before discharge.
DEFENDANTS' DEFENSE:
There is no evidence in the medical records of a shoulder dystocia; "no shoulder dystocia" was charted shortly after delivery. No one in the delivery room testified to a delay in delivering the infant's shoulders. The mother's internal contractions caused the injury. The baby was not injured to the extent claimed.
VERDICT:
The ObGyn who used the vacuum extractor settled before the trial for $300,000. A $2 million Illinois verdict was returned against the FP.
Delay in treating infant in respiratory distress: $7.27M settlement
A child was delivered by a certified nurse midwife at a birthing center. At birth, the baby had a heart rate of 60 bpm and was in respiratory distress but there was no one at the clinic qualified to intubate the infant. Emergency personnel were called but the infant remained in respiratory distress for 8 minutes. The baby experienced birth asphyxia with hypoxic ischemic encephalopathy resulting in severe cerebral palsy.
PARENT'S CLAIM:
The birthing center was poorly staffed and unprepared to treat an emergency situation.
DEFENDANTS' DEFENSE:
The defendants denied all allegations of negligence. The case was settled during trial.
VERDICT:
A $7.27 million Pennsylvania settlement was reached.
Was the spinal block given at wrong level?
A MOTHER WENT TO THE HOSPITAL in labor. Prior to cesarean delivery, she underwent an anesthetic spinal block administered by a CRNA. Initially, the patient reported pain shortly after the injection was performed until the block worked. The baby's delivery was uneventful.
In recovery a few hours later, the patient reported intense and uncontrollable pain in her legs. Magnetic resonance imaging revealed a fluid pocket on her spinal cord at the L1-L2 level. The patient has permanent pain, numbness, and tingling in in both legs.
PATIENT'S CLAIM:
The CRNA failed to insert the spinal block needle in the proper location.
DEFENDANTS' DEFENSE:
The CRNA contended that he complied with the standard of care. He claimed that the patient had an unusual spinal cord anatomy: it was tethered down to the L3-L4 level.
VERDICT:
A $509,152 Kentucky verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Are women of advanced maternal age at increased risk for severe maternal morbidity?
EXPERT COMMENTARY
While numerous studies have investigated the risk of perinatal outcomes with advancing maternal age, the primary objective of a recent study by Lisonkova and colleagues was to examine the association between advancing maternal age and severe maternal morbidities and mortality.
Details of the study
The population-based retrospective cohort study compared age-specific rates of severe maternal morbidities and mortality among 828,269 pregnancies in Washington state between 2003 and 2013. Singleton births to women 15 to 60 years of age were included; out-of-hospital births were excluded. Information was obtained by linking the Birth Events Record Database (which includes information on maternal, pregnancy, and labor and delivery characteristics and birth outcomes), and the Comprehensive Hospital Abstract Reporting System database (which includes diagnostic and procedural codes for all hospitalizations in Washington state).
The primary objective was to examine the association between age and severe maternal morbidities. Maternal morbidities were divided into categories: antepartum hemorrhage, respiratory morbidity, thromboembolism, cerebrovascular morbidity, acute cardiac morbidity, severe postpartum hemorrhage, maternal sepsis, renal failure, obstetric shock, complications of anesthesia and obstetric interventions, and need for life-saving procedures. A composite outcome, comprised of severe maternal morbidities, intensive care unit admission, and maternal mortality, was also created.
Rates of severe morbidities were compared for age groups 15 to 19, 20 to 24, 25 to 29, 30 to 34, 35 to 39, 40 to 44, and ≥45 years to the referent category (25 to 29 years). Additional comparisons were also performed for ages 45 to 49 and ≥50 years for the composite and for morbidities with high incidence. Logistic regression and sensitivity analyses were used to control for demographic and prepregnancy characteristics, underlying medical conditions, assisted conception, and delivery characteristics.
Severe maternal morbidities demonstrated a J-shaped association with age: the lowest rates of morbidity were observed in women 20 to 34 years of age, and steeply increasing rates of morbidity were observed for women aged 40 and older. One notable exception was the rate of sepsis, which was increased in teen mothers compared with all other groups.
The unadjusted rate of the composite outcome of severe maternal morbidity and mortality was 2.1% in teenagers, 1.5% among women 25 to 29 years, 2.3% among those aged 40 to 44, and 3.6% among women aged 45 and older.
Although rates were somewhat attenuated after adjustment for demographic and prepregnancy characteristics, chronic medical conditions, assisted conception, and delivery characteristics, most morbidities remained significantly increased among women aged 39 years and older, including the composite outcome. Among the individual morbidities considered, increased risk was highest for renal failure, amniotic fluid embolism, cardiac morbidity, and shock, with adjusted odds ratios of 2.0 or greater for women older than 39 years.
Related article:
Reducing maternal mortality in the United States—Let’s get organized!
Study strengths and weaknesses
This study contributes substantially to the existing literature that demonstrates higher rates of pregnancy-associated morbidities in women of increasing maternal age.1,2 Prior studies in this area focused on perinatal morbidity and mortality and on obstetric outcomes such as cesarean delivery.3–5 This large-scale study examined the association between advancing maternal age and a variety of serious maternal morbidities. In another study, Callaghan and Berg found a similar pattern among mortalities, with high rates of mortality attributable to hemorrhage, embolism, and cardiomyopathy in women aged 40 years and older.1
Exclusion of multiple gestations. As in any study, we must consider the methodology, and it is notable that Lisonkova and colleagues’ study excluded multiple gestations. Given the association with advanced maternal age, assisted reproductive technology, and the incidence of multiple gestations, a high rate of multiple gestations would be expected among women of advanced maternal age. (Generally, maternal age of at least 35 years is considered “advanced,” with greater than 40 years “very advanced.”) Since multiple gestations tend to be associated with increases in morbidity, excluding these pregnancies would likely bias the study results toward the null. If multiple gestations had been included, the rates of serious maternal morbidities in older women might be even higher than those demonstrated, potentially strengthening the associations reported here.
This large, retrospective study (level II evidence) suggests that women of advancing age are at significantly increased risk of severe maternal morbidities, even after controlling for preexisting medical conditions. We therefore recommend that clinicians inform and counsel women who are considering pregnancy at an advanced age, and those considering oocyte cryopreservation as a means of extending their reproductive life span, about the increased maternal morbidities associated with pregnancy at age 40 and older.
-- Amy E. Judy, MD, MPH, and Yasser Y. El-Sayed, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Callaghan WM, Berg CJ. Pregnancy-related mortality among women aged 35 years and older, United States, 1991–1997. Obstet Gynecol. 2003;102(5 pt 1):1015–1021.
- McCall SJ, Nair M, Knight M. Factors associated with maternal mortality at advanced maternal age: a population-based case-control study. BJOG. 2017;124(8):1225–1233.
- Yogev Y, Melamed N, Bardin R, Tenenbaum-Gavish K, Ben-Shitrit G, Ben-Haroush A. Pregnancy outcome at extremely advanced maternal age. Am J Obstet Gynecol. 2010;203(6):558.e1–e7.
- Gilbert WM, Nesbitt TS, Danielsen B. Childbearing beyond age 40: pregnancy outcome in 24,032 cases. Obstet Gynecol. 1999;93(1):9–14.
- Luke B, Brown MB. Elevated risks of pregnancy complications and adverse outcomes with increasing maternal age. Hum Reprod. 2007;22(5):1264–1272.
EXPERT COMMENTARY
While numerous studies have investigated the risk of perinatal outcomes with advancing maternal age, the primary objective of a recent study by Lisonkova and colleagues was to examine the association between advancing maternal age and severe maternal morbidities and mortality.
Details of the study
The population-based retrospective cohort study compared age-specific rates of severe maternal morbidities and mortality among 828,269 pregnancies in Washington state between 2003 and 2013. Singleton births to women 15 to 60 years of age were included; out-of-hospital births were excluded. Information was obtained by linking the Birth Events Record Database (which includes information on maternal, pregnancy, and labor and delivery characteristics and birth outcomes), and the Comprehensive Hospital Abstract Reporting System database (which includes diagnostic and procedural codes for all hospitalizations in Washington state).
The primary objective was to examine the association between age and severe maternal morbidities. Maternal morbidities were divided into categories: antepartum hemorrhage, respiratory morbidity, thromboembolism, cerebrovascular morbidity, acute cardiac morbidity, severe postpartum hemorrhage, maternal sepsis, renal failure, obstetric shock, complications of anesthesia and obstetric interventions, and need for life-saving procedures. A composite outcome, comprised of severe maternal morbidities, intensive care unit admission, and maternal mortality, was also created.
Rates of severe morbidities were compared for age groups 15 to 19, 20 to 24, 25 to 29, 30 to 34, 35 to 39, 40 to 44, and ≥45 years to the referent category (25 to 29 years). Additional comparisons were also performed for ages 45 to 49 and ≥50 years for the composite and for morbidities with high incidence. Logistic regression and sensitivity analyses were used to control for demographic and prepregnancy characteristics, underlying medical conditions, assisted conception, and delivery characteristics.
Severe maternal morbidities demonstrated a J-shaped association with age: the lowest rates of morbidity were observed in women 20 to 34 years of age, and steeply increasing rates of morbidity were observed for women aged 40 and older. One notable exception was the rate of sepsis, which was increased in teen mothers compared with all other groups.
The unadjusted rate of the composite outcome of severe maternal morbidity and mortality was 2.1% in teenagers, 1.5% among women 25 to 29 years, 2.3% among those aged 40 to 44, and 3.6% among women aged 45 and older.
Although rates were somewhat attenuated after adjustment for demographic and prepregnancy characteristics, chronic medical conditions, assisted conception, and delivery characteristics, most morbidities remained significantly increased among women aged 39 years and older, including the composite outcome. Among the individual morbidities considered, increased risk was highest for renal failure, amniotic fluid embolism, cardiac morbidity, and shock, with adjusted odds ratios of 2.0 or greater for women older than 39 years.
Related article:
Reducing maternal mortality in the United States—Let’s get organized!
Study strengths and weaknesses
This study contributes substantially to the existing literature that demonstrates higher rates of pregnancy-associated morbidities in women of increasing maternal age.1,2 Prior studies in this area focused on perinatal morbidity and mortality and on obstetric outcomes such as cesarean delivery.3–5 This large-scale study examined the association between advancing maternal age and a variety of serious maternal morbidities. In another study, Callaghan and Berg found a similar pattern among mortalities, with high rates of mortality attributable to hemorrhage, embolism, and cardiomyopathy in women aged 40 years and older.1
Exclusion of multiple gestations. As in any study, we must consider the methodology, and it is notable that Lisonkova and colleagues’ study excluded multiple gestations. Given the association with advanced maternal age, assisted reproductive technology, and the incidence of multiple gestations, a high rate of multiple gestations would be expected among women of advanced maternal age. (Generally, maternal age of at least 35 years is considered “advanced,” with greater than 40 years “very advanced.”) Since multiple gestations tend to be associated with increases in morbidity, excluding these pregnancies would likely bias the study results toward the null. If multiple gestations had been included, the rates of serious maternal morbidities in older women might be even higher than those demonstrated, potentially strengthening the associations reported here.
This large, retrospective study (level II evidence) suggests that women of advancing age are at significantly increased risk of severe maternal morbidities, even after controlling for preexisting medical conditions. We therefore recommend that clinicians inform and counsel women who are considering pregnancy at an advanced age, and those considering oocyte cryopreservation as a means of extending their reproductive life span, about the increased maternal morbidities associated with pregnancy at age 40 and older.
-- Amy E. Judy, MD, MPH, and Yasser Y. El-Sayed, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
EXPERT COMMENTARY
While numerous studies have investigated the risk of perinatal outcomes with advancing maternal age, the primary objective of a recent study by Lisonkova and colleagues was to examine the association between advancing maternal age and severe maternal morbidities and mortality.
Details of the study
The population-based retrospective cohort study compared age-specific rates of severe maternal morbidities and mortality among 828,269 pregnancies in Washington state between 2003 and 2013. Singleton births to women 15 to 60 years of age were included; out-of-hospital births were excluded. Information was obtained by linking the Birth Events Record Database (which includes information on maternal, pregnancy, and labor and delivery characteristics and birth outcomes), and the Comprehensive Hospital Abstract Reporting System database (which includes diagnostic and procedural codes for all hospitalizations in Washington state).
The primary objective was to examine the association between age and severe maternal morbidities. Maternal morbidities were divided into categories: antepartum hemorrhage, respiratory morbidity, thromboembolism, cerebrovascular morbidity, acute cardiac morbidity, severe postpartum hemorrhage, maternal sepsis, renal failure, obstetric shock, complications of anesthesia and obstetric interventions, and need for life-saving procedures. A composite outcome, comprised of severe maternal morbidities, intensive care unit admission, and maternal mortality, was also created.
Rates of severe morbidities were compared for age groups 15 to 19, 20 to 24, 25 to 29, 30 to 34, 35 to 39, 40 to 44, and ≥45 years to the referent category (25 to 29 years). Additional comparisons were also performed for ages 45 to 49 and ≥50 years for the composite and for morbidities with high incidence. Logistic regression and sensitivity analyses were used to control for demographic and prepregnancy characteristics, underlying medical conditions, assisted conception, and delivery characteristics.
Severe maternal morbidities demonstrated a J-shaped association with age: the lowest rates of morbidity were observed in women 20 to 34 years of age, and steeply increasing rates of morbidity were observed for women aged 40 and older. One notable exception was the rate of sepsis, which was increased in teen mothers compared with all other groups.
The unadjusted rate of the composite outcome of severe maternal morbidity and mortality was 2.1% in teenagers, 1.5% among women 25 to 29 years, 2.3% among those aged 40 to 44, and 3.6% among women aged 45 and older.
Although rates were somewhat attenuated after adjustment for demographic and prepregnancy characteristics, chronic medical conditions, assisted conception, and delivery characteristics, most morbidities remained significantly increased among women aged 39 years and older, including the composite outcome. Among the individual morbidities considered, increased risk was highest for renal failure, amniotic fluid embolism, cardiac morbidity, and shock, with adjusted odds ratios of 2.0 or greater for women older than 39 years.
Related article:
Reducing maternal mortality in the United States—Let’s get organized!
Study strengths and weaknesses
This study contributes substantially to the existing literature that demonstrates higher rates of pregnancy-associated morbidities in women of increasing maternal age.1,2 Prior studies in this area focused on perinatal morbidity and mortality and on obstetric outcomes such as cesarean delivery.3–5 This large-scale study examined the association between advancing maternal age and a variety of serious maternal morbidities. In another study, Callaghan and Berg found a similar pattern among mortalities, with high rates of mortality attributable to hemorrhage, embolism, and cardiomyopathy in women aged 40 years and older.1
Exclusion of multiple gestations. As in any study, we must consider the methodology, and it is notable that Lisonkova and colleagues’ study excluded multiple gestations. Given the association with advanced maternal age, assisted reproductive technology, and the incidence of multiple gestations, a high rate of multiple gestations would be expected among women of advanced maternal age. (Generally, maternal age of at least 35 years is considered “advanced,” with greater than 40 years “very advanced.”) Since multiple gestations tend to be associated with increases in morbidity, excluding these pregnancies would likely bias the study results toward the null. If multiple gestations had been included, the rates of serious maternal morbidities in older women might be even higher than those demonstrated, potentially strengthening the associations reported here.
This large, retrospective study (level II evidence) suggests that women of advancing age are at significantly increased risk of severe maternal morbidities, even after controlling for preexisting medical conditions. We therefore recommend that clinicians inform and counsel women who are considering pregnancy at an advanced age, and those considering oocyte cryopreservation as a means of extending their reproductive life span, about the increased maternal morbidities associated with pregnancy at age 40 and older.
-- Amy E. Judy, MD, MPH, and Yasser Y. El-Sayed, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Callaghan WM, Berg CJ. Pregnancy-related mortality among women aged 35 years and older, United States, 1991–1997. Obstet Gynecol. 2003;102(5 pt 1):1015–1021.
- McCall SJ, Nair M, Knight M. Factors associated with maternal mortality at advanced maternal age: a population-based case-control study. BJOG. 2017;124(8):1225–1233.
- Yogev Y, Melamed N, Bardin R, Tenenbaum-Gavish K, Ben-Shitrit G, Ben-Haroush A. Pregnancy outcome at extremely advanced maternal age. Am J Obstet Gynecol. 2010;203(6):558.e1–e7.
- Gilbert WM, Nesbitt TS, Danielsen B. Childbearing beyond age 40: pregnancy outcome in 24,032 cases. Obstet Gynecol. 1999;93(1):9–14.
- Luke B, Brown MB. Elevated risks of pregnancy complications and adverse outcomes with increasing maternal age. Hum Reprod. 2007;22(5):1264–1272.
- Callaghan WM, Berg CJ. Pregnancy-related mortality among women aged 35 years and older, United States, 1991–1997. Obstet Gynecol. 2003;102(5 pt 1):1015–1021.
- McCall SJ, Nair M, Knight M. Factors associated with maternal mortality at advanced maternal age: a population-based case-control study. BJOG. 2017;124(8):1225–1233.
- Yogev Y, Melamed N, Bardin R, Tenenbaum-Gavish K, Ben-Shitrit G, Ben-Haroush A. Pregnancy outcome at extremely advanced maternal age. Am J Obstet Gynecol. 2010;203(6):558.e1–e7.
- Gilbert WM, Nesbitt TS, Danielsen B. Childbearing beyond age 40: pregnancy outcome in 24,032 cases. Obstet Gynecol. 1999;93(1):9–14.
- Luke B, Brown MB. Elevated risks of pregnancy complications and adverse outcomes with increasing maternal age. Hum Reprod. 2007;22(5):1264–1272.
Managing psychiatric illness during pregnancy and breastfeeding: Tools for decision making
Increasingly, women with psychiatric illness are undergoing pharmacologic treatment during pregnancy. In the United States, an estimated 8% of pregnant women are prescribed antidepressants, and the number of such cases has risen over the past 15 years.1 Women with a psychiatric diagnosis were once instructed either to discontinue all medication immediately on learning they were pregnant, or to forgo motherhood because their illness might have a negative effect on a child or because avoiding medication during pregnancy might lead to a relapse.
Fortunately, women with depression, anxiety, bipolar disorder, or schizophrenia no longer are being told that they cannot become mothers. For many women, however, stopping medication is not an option. Furthermore, psychiatric illness sometimes is diagnosed initially during pregnancy and requires treatment.
Pregnant women and their physicians need accurate information about when to taper off medication, when to start or continue, and which medications are safest. Even for clinicians with a solid knowledge base, counseling a woman who needs or may need psychotropic medication during pregnancy and breastfeeding is a daunting task. Some clinicians still recommend no drug treatment as the safest and best option, given the potential risks to the fetus.
In this review we offer a methodologic approach for decision making about pharmacologic treatment during pregnancy. As the scientific literature is constantly being updated, it is imperative to have the most current information on psychotropics and to know how to individualize that information when counseling a pregnant woman and her family. Using this framework for analyzing the risks and benefits for both mother and fetus, clinicians can avoid the unanswerable question of which medication is the “safest.”
A patient’s mental health care provider is a useful resource for information about a woman’s mental health history and current stability, but he or she may not be expert or comfortable in recommending treatment for a pregnant patient. During pregnancy, a woman’s obstetrician often becomes the “expert” for all treatment decisions.
Antidepressants. Previous studies may have overestimated the association between prenatal use of antidepressants and attention deficit/hyperactivity disorder (ADHD) in children because they did not control for shared family factors, according to investigators who say that their recent study findings raise the possibility that "confounding by indication" might partially explain the observed association.1
In a population-based cohort study in Hong Kong, Man and colleagues analyzed the records of 190,618 maternal-child pairs.1 A total of 1,252 children were exposed to maternal antidepressant use during pregnancy. Medications included selective serotonin reuptake inhibitors (SSRIs), non-SSRIs, and antipsychotics as monotherapy or in various combination regimens. Overall, 5,659 of the cohort children (3%) were diagnosed with or received treatment for ADHD.
When gestational medication users were compared with nongestational users, the crude hazard ratio (HR) of antidepressant use during pregnancy and ADHD was 2.26 (P<.01). After adjusting for potential confounding factors (such as maternal psychiatric disorders and use of other psychotropic drugs), this reduced to 1.39 (95% confidence interval [CI], 1.07-1.82; P = .01). Children of mothers with psychiatric disorders had a higher risk of ADHD than did children of mothers without psychiatric disorders (HR, 1.84; 95% CI, 1.54-2.18; P<.01), even if the mothers had never used antidepressants.
While acknowledging the potential for type 2 error in the study analysis, the investigators proposed that the results "further strengthen our hypothesis that confounding by indication may play a major role in the observed positive association between gestational use of antidepressants and ADHD in offspring."
Lithium. Similarly, investigators of another recently published study found that the magnitude of the association between prenatal lithium use and increased risk of cardiac malformations in infants was smaller than previously shown.2 This finding may be important clinically because lithium is a first-line treatment for many US women of reproductive age with bipolar disorder.
Most earlier data were derived from a database registry, case reports, and small studies that often had conflicting results. However, Patorno and colleagues conducted a large retrospective cohort study that involved data on 1,325,563 pregnancies in women enrolled in Medicaid.2 Exposure to lithium was defined as at least 1 filled prescription during the first trimester, and the primary reference group included women with no lithium or lamotrigine (another mood stabilizer not associated with congenital malformations) dispensing during the 3 months before the start of pregnancy or during the first trimester.
A total of 663 pregnancies (0.05%) were exposed to lithium and 1,945 (0.15%) were exposed to lamotrigine during the first trimester. The adjusted risk ratios for cardiac malformations among infants exposed to lithium were 1.65 (95% CI, 1.02-2.68) as compared with nonexposed infants and 2.25 (95% CI, 1.17-4.34) as compared with lamotrigine-exposed infants. Notably, all right ventricular outflow tract obstruction defects identified in the infants exposed to lithium occurred with a daily dose of more than 600 mg.
Although the study results suggest an increased risk of cardiac malformations--of approximately 1 additional case per 100 live births--associated with lithium use in early pregnancy, the magnitude of risk is much lower than originally proposed based on early lithium registry data.
-- Kathy Christie, Senior Editor
References
- Man KC, Chan EW, Ip P, et al. Prenatal antidepressant use and risk of attention-deficit/hyperactivity disorder in offspring: population based cohort study. BMJ. 2017;357:j2350.
- Patorno E, Huybrechts KR, Bateman BT, et al. Lithium use in pregnancy and risk of cardiac malformations. N Engl J Med. 2017;376(23):2245-2254.
Analyze risks and benefits of medication versus no medication
The US Food and Drug Administration (FDA) has not approved any psychotropic medication for use during pregnancy. While a clinical study would provide more scientifically rigorous safety data, conducting a double-blinded, placebo-controlled trial in pregnant women with a psychiatric disorder is unethical. Thus, the literature consists mostly of reports on case series, retrospective chart reviews, prospective naturalistic studies, and analyses of large registry databases. Each has benefits and limitations. It is important to understand the limitations when making treatment decisions.
In 1979, the FDA developed a 5-lettersystem (A, B, C, D, X) for classifying the relative safety of medications used during pregnancy.2 Many clinicians and pregnant women relied on this system to decide which medications were safe. Unfortunately, the information in the system was inadequate for making informed decisions. For example, although a class B medication might have appeared safer than one in class C, the studies of risk in humans might not have been adequate to permit comparisons. Drug safety classifications were seldom changed, despite the availability of additional data.
In June 2015, the FDA changed the requirements for the Pregnancy and Lactation subsections of the labeling for human prescription drugs and biologic products. Drug manufacturers must now include in each subsection a risk summary, clinical considerations supporting patient care decisions and counseling, and detailed data. These subsections provide information on available human and animal studies, known or potential maternal or fetal adverse reactions, and dose adjustments needed during pregnancy and the postpartum period. In addition, the FDA added a subsection: Females and Males of Reproductive Potential.3
These changes acknowledge there is no list of “safe” medications. The safest medication generally is the one that works for a particular patient at the lowest effective dose. As each woman’s history of illness and effective treatment is different, the best medication may differ as well, even among women with the same illness. Therefore, medication should be individualized to the patient. A risk–benefit analysis comparing psychotropic medication treatment with no medication treatment must be performed for each patient according to her personal history and the best available data.
Read about the risks of untreated illness during pregnancy
What is the risk of untreated illness during pregnancy?
During pregnancy, women are treated for many medical disorders, including psychiatric illness. One general guideline is that, if a pregnant woman does not need a medication—whether it be for an allergy, hypertension, or another disorder—she should not take it. Conversely, if a medication is required for a patient’s well-being, her physician should continue it or switch to a safer one. This general guideline is the same for women with depression, anxiety, or a psychotic disorder.
Managing hypertension during pregnancy is an example of choosing treatment when the risk of the illness to the mother and the infant outweighs the likely small risk associated with taking a medication. Blood pressure is monitored, and, when it reaches a threshold, an antihypertensive is started promptly to avoid morbidity and mortality.
Psychiatric illness carries risks for both mother and fetus as well, but no data show a clear threshold for initiating pharmacologic treatment. Therefore, in prescribing medication the most important steps are to take a complete history and perform a thorough evaluation. Important information includes the number and severity of previous episodes, prior history of hospitalization or suicidal thoughts or attempts, and any history of psychotic or manic status.
Whether to continue or discontinue medication is often decided after inquiring about other times a medication was discontinued. A patient who in the past stayed well for several years after stopping a medication may be able to taper off a medication and conceive during a window of wellness. Some women who have experienced only one episode of illness and have been stable for at least a year may be able to taper off a medication before conceiving (TABLE 1).
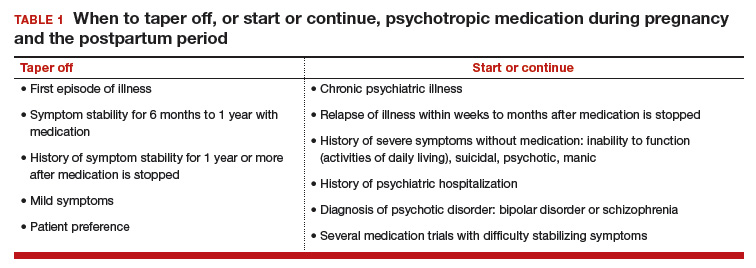
In the risk–benefit analysis, assess the need for pharmacologic treatment by considering the risk that untreated illness poses for both mother and fetus, the benefits of treatment for both, and the risk of medication exposure for the fetus.4
Mother: Risk of untreated illness versus benefit of treatment
A complete history and a current symptom evaluation are needed to assess the risk that nonpharmacologic treatment poses for the mother. Women with functional impairment, including inability to work, to perform activities of daily living, or to take care of other children, likely require treatment. Studies have found that women who discontinue treatment for a psychiatric illness around the time of conception are likely to experience a recurrence of illness during pregnancy, often in the first trimester, and must restart medication.5,6 For some diagnoses, particularly bipolar disorder, symptoms during a relapse can be more severe and more difficult to treat, and they carry a risk for both mother and fetus.7 A longitudinal study of pregnant women who stopped medication for bipolar disorder found a 71% rate of relapse.7 In cases in which there is a history of hospitalization, suicide attempt, or psychosis, discontinuing treatment is not an option; instead, the physician must determine which medication is safest for the particular patient.
Related article:
Does PTSD during pregnancy increase the likelihood of preterm birth?
Fetus: Risk of untreated illness versus benefit of treatment
Mothers with untreated psychiatric illness are at higher risk for poor prenatal care, substance abuse, and inadequate nutrition, all of which increase the risk of negative obstetric and neonatal outcomes.8 Evidence indicates that untreated maternal depression increases the risk of preterm delivery and low birth weight.9 Children born to mothers with depression have more behavioral problems, more psychiatric illness, more visits to pediatricians, lower IQ scores, and attachment issues.10 Some of the long-term negative effects of intrauterine stress, which include hypertension, coronary heart disease, and autoimmune disorders, persist into adulthood.11
Fetus: Risk of medication exposure
With any pharmacologic treatment, the timing of fetal exposure affects resultant risks and therefore must be considered in the management plan.
Before conception. Is there any effect on ovulation or fertilization?
Implantation. Does the exposure impair the blastocyst’s ability to implant in the uterine lining?
First trimester. This is the period of organogenesis. Regardless of drug exposure, there is a 2% to 4% baseline risk of a major malformation during any pregnancy. The risk of a particular malformation must be weighed against this baseline risk.
According to limited data, selective serotonin reuptake inhibitors (SSRIs) may increase the risk of early miscarriage.12 SSRIs also have been implicated in increasing the risk of cardiovascular malformations, although the data are conflicting.13,14
Antiepileptics such as valproate and carbamazepine are used as mood stabilizers in the treatment of bipolar disorder.15 Extensive data have shown an association with teratogenicity. Pregnant women who require either of these medications also should be prescribed folic acid 4 or 5 mg/day. Given the high risk of birth defects and cognitive delay, valproate no longer is recommended for women of reproductive potential.16
Lithium, one of the safest medications used in the treatment of bipolar disorder, is associated with a very small risk of Ebstein anomaly.17
Lamotrigine is used to treat bipolar depression and appears to have a good safety profile, along with a possible small increased risk of oral clefts.18,19
Atypical antipsychotics (such as aripiprazole, olanzapine, quetiapine, and risperidone) are often used first-line in the treatment of psychotic disorders and bipolar disorder in women who are not pregnant. Although the safety data on use of these drugs during pregnancy are limited, a recent analysis of pregnant Medicaid enrollees found no increased risk of birth defects after controlling for potential confounding factors.20 Common practice is to avoid these newer agents, given their limited data and the time needed for rare malformations to emerge (adequate numbers require many exposures during pregnancy).
Read additional fetal risks of medication exposure
Second trimester. This is a period of growth and neural development. A 2006 study suggested that SSRI exposure after pregnancy week 20 increases the risk of persistent pulmonary hypertension of the newborn (PPHN).21 In 2011, however, the FDA removed the PPHN warning label for SSRIs, citing inconsistent data. Whether the PPHN risk is increased with SSRI use is unclear, but the risk is presumed to be smaller than previously suggested.22 Stopping SSRIs before week 20 puts the mother at risk for relapse during pregnancy and increases her risk of developing postpartum depression. If we follow the recommendation to prescribe medication only for women who need it most, then stopping the medication at any time during pregnancy is not an option.
Third trimester. This is a period of continued growth and lung maturation.
Delivery. Is there a potential for impairment in parturition?
Neonatal adaptation. Newborns are active mainly in adapting to extrauterine life: They regulate their temperature and muscle tone and learn to coordinate sucking, swallowing, and breathing. Does medication exposure impair adaptation, or are signs or symptoms of withdrawal or toxicity present? The evidence that in utero SSRI exposure increases the risk of neonatal adaptation syndrome is consistent, but symptoms are mild and self-limited.23 Tapering off SSRIs before delivery currently is not recommended, as doing so increases the mother’s risk for postpartum depression and, according to one study, does not prevent symptoms of neonatal adaptation syndrome from developing.24
Behavioral teratogenicity. What are the long-term developmental outcomes for the child? Are there any differences in IQ, speech and language, or psychiatric illness? One study found an increased risk of autism with in utero exposure to sertraline, but the study had many methodologic flaws and its findings have not been replicated.25 Most studies have not found consistent differences in speech, IQ, or behavior between infants exposed and infants not exposed to antidepressants.26,27 By contrast, in utero exposure to anticonvulsants, particularly valproate, has led to significant developmental problems in children.28 The data on atypical antipsychotics are limited.
Related article:
Do antidepressants really cause autism?
None of the medications used to treat depression, bipolar disorder, anxiety, or schizophrenia is considered first-line or safest therapy for the pregnant woman. For any woman who is doing well on a certain medication, but particularly for a pregnant woman, there is no compelling, data-supported reason to switch to another agent. For depression, options include all of the SSRIs, with the possible exception of paroxetine (TABLE 2). In conflicting studies, paroxetine was no different from any other SSRI in not being associated with cardiovascular defects.29
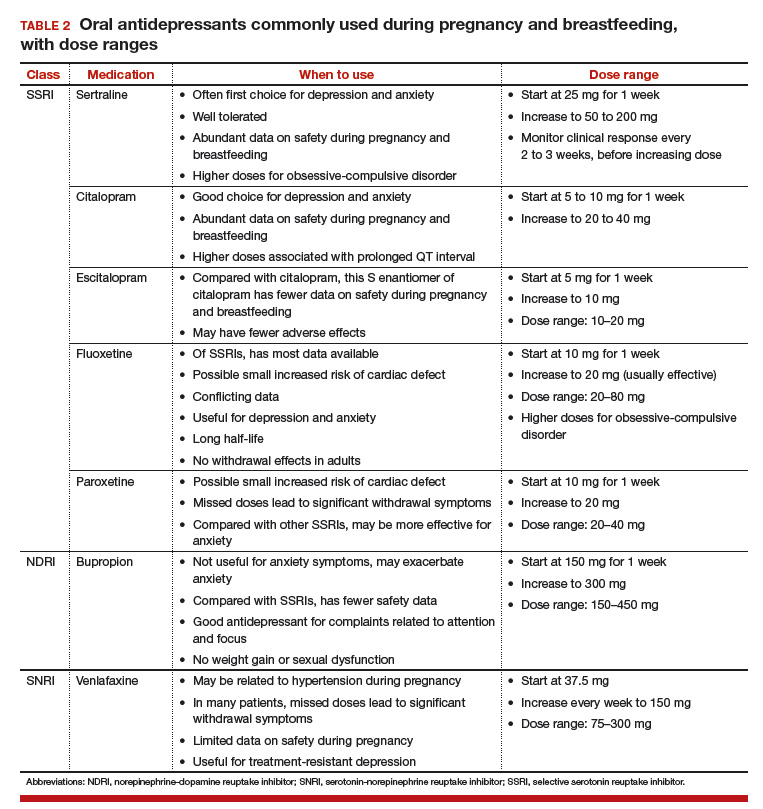
One goal in treatment is to use a medication that previously was effective in the remission of symptoms and to use it at the lowest dose possible. Treating simply to maintain a low dose of drug, however, and not to effect symptom remission, exposes the fetus to both the drug and the illness. Again, the lowest effective dose is the best choice.
Read about treatment during breastfeeding
Treatment during breastfeeding
Women are encouraged to breastfeed for physical and psychological health benefits, for both themselves and their babies. Many medications are compatible with breastfeeding.30 The amount of drug an infant receives through breast milk is considerably less than the amount received during the mother’s pregnancy. Breastfeeding generally is allowed if the calculated infant dose is less than 10% of the weight-adjusted maternal dose.31
The amount of drug transferred from maternal plasma into milk is highest for drugs with low protein binding and high lipid solubility.32 Drug clearance in infants must be considered as well. Renal clearance is decreased in newborns and does not reach adult levels until 5 or 6 months of age. In addition, liver metabolism is impaired in neonates and even more so in premature infants.33 Drugs that require extensive first-pass metabolism may have higher bioavailability, and this factor should be considered.
Some clinicians recommend pumping and discarding breast milk when the drug in it is at its peak level; although the drug is not eliminated, the infant ingests less of it.34 Most women who are anxious about breastfeeding while on medication “pump and dump” until they are more comfortable nursing and the infants are doing well. Except in cases of mother preference, most physicians with expertise in reproductive mental health generally recommend against pumping and discarding milk.
Through breast milk, infants ingest drugs in varying amounts. The amount depends on the qualities of the medication, the timing and duration of breastfeeding, and the characteristics of the infant. Few psychotropic drugs have significant effects on breastfed infants. Even lithium, previously contraindicated, is successfully used, with infant monitoring, during breastfeeding.35 Given breastfeeding’s benefits for both mother and child, many more women on psychotropic medications are choosing to breastfeed.
Related article:
USPSTF Recommendations to Support Breastfeeding
Balance the pros and cons
Deciding to use medication during pregnancy and breastfeeding involves considering the risk of untreated illness versus the benefit of treatment for both mother and fetus, and the risk of medication exposure for the fetus. Mother and fetus are inseparable, and neither can be isolated from the other in treatment decisions. Avoiding psychotropic medication during pregnancy is not always the safest option for mother or fetus. The patient and her clinician and support system must make an informed decision that is based on the best available data and that takes into account the mother’s history of illness and effective treatment. Many women with psychiatric illness no longer have to choose between mental health and starting a family, and their babies will be healthy.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Andrade SE, Raebel MA, Brown J, et al. Use of antidepressant medications during pregnancy: a multisite study. Am J Obstet Gynecol. 2008;198(2):194.e1–e5.
- Hecht A. Drug safety labeling for doctors. FDA Consum. 1979;13(8):12–13.
- Ramoz LL, Patel-Shori NM. Recent changes in pregnancy and lactation labeling: retirement of risk categories. Pharmacotherapy. 2014;34(4):389–395.
- Yonkers KA, Wisner KL, Stewart DE, et al. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Gen Hosp Psychiatry. 2009;31(5):403–413.
- Cohen LS, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295(5):499–507.
- O’Brien L, Laporte A, Koren G. Estimating the economic costs of antidepressant discontinuation during pregnancy. Can J Psychiatry. 2009;54(6):399–408.
- Viguera AC, Whitfield T, Baldessarini RJ, et al. Risk of recurrence in women with bipolar disorder during pregnancy: prospective study of mood stabilizer discontinuation. Am J Psychiatry. 2007;164(12):1817–1824.
- Bonari L, Pinto N, Ahn E, Einarson A, Steiner M, Koren G. Perinatal risks of untreated depression during pregnancy. Can J Psychiatry. 2004;49(11):726–735.
- Straub H, Adams M, Kim JJ, Silver RK. Antenatal depressive symptoms increase the likelihood of preterm birth. Am J Obstet Gynecol. 2012;207(4):329.e1–e4.
- Hayes LJ, Goodman SH, Carlson E. Maternal antenatal depression and infant disorganized attachment at 12 months. Attach Hum Dev. 2013;15(2):133–153.
- Field T. Prenatal depression effects on early development: a review. Infant Behav Dev. 2011;34(1):1–14.
- Kjaersgaard MI, Parner ET, Vestergaard M, et al. Prenatal antidepressant exposure and risk of spontaneous abortion—a population-based study. PLoS One. 2013;8(8):e72095.
- Nordeng H, van Gelder MM, Spigset O, Koren G, Einarson A, Eberhard-Gran M. Pregnancy outcome after exposure to antidepressants and the role of maternal depression: results from the Norwegian Mother and Child Cohort Study. J Clin Psychopharmacol. 2012;32(2):186–194.
- Källén BA, Otterblad Olausson P. Maternal use of selective serotonin re-uptake inhibitors in early pregnancy and infant congenital malformations. Birth Defects Res A Clin Mol Teratol. 2007;79(4):301–308.
- Tomson T, Battino D. Teratogenic effects of antiepileptic drugs. Lancet Neurol. 2012;11(9):803–813.
- Balon R, Riba M. Should women of childbearing potential be prescribed valproate? A call to action. J Clin Psychiatry. 2016;77(4):525–526.
- Giles JJ, Bannigan JG. Teratogenic and developmental effects of lithium. Curr Pharm Design. 2006;12(12):1531–1541.
- Nguyen HT, Sharma V, McIntyre RS. Teratogenesis associated with antibipolar agents. Adv Ther. 2009;26(3):281–294.
- Campbell E, Kennedy F, Irwin B, et al. Malformation risks of antiepileptic drug monotherapies in pregnancy. J Neurol Neurosurg Psychiatry. 2013;84(11):e2.
- Huybrechts KF, Hernández-Díaz S, Patorno E, et al. Antipsychotic use in pregnancy and the risk for congenital malformations. JAMA Psychiatry. 2016;73(9):938–946.
- Chambers CD, Hernández-Díaz S, Van Marter LJ, et al. Selective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. N Engl J Med. 2006;354(6):579–587.
- ‘t Jong GW, Einarson T, Koren G, Einarson A. Antidepressant use in pregnancy and persistent pulmonary hypertension of the newborn (PPHN): a systematic review. Reprod Toxicol. 2012;34(3):293–297.
- Oberlander TF, Misri S, Fitzgerald CE, Kostaras X, Rurak D, Riggs W. Pharmacologic factors associated with transient neonatal symptoms following prenatal psychotropic medication exposure. J Clin Psychiatry. 2004;65(2):230–237.
- Warburton W, Hertzman C, Oberlander TF. A register study of the impact of stopping third trimester selective serotonin reuptake inhibitor exposure on neonatal health. Acta Psychiatr Scand. 2010;121(6):471–479.
- Croen LA, Grether JK, Yoshida CK, Odouli R, Hendrick V. Antidepressant use during pregnancy and childhood autism spectrum disorders. Arch Gen Psychiatry. 2011;68(11):1104–1112.
- Batton B, Batton E, Weigler K, Aylward G, Batton D. In utero antidepressant exposure and neurodevelopment in preterm infants. Am J Perinatol. 2013;30(4):297–301.
- Austin MP, Karatas JC, Mishra P, Christl B, Kennedy D, Oei J. Infant neurodevelopment following in utero exposure to antidepressant medication. Acta Paediatr. 2013;102(11):1054–1059.
- Bromley RL, Mawer GE, Briggs M, et al. The prevalence of neurodevelopmental disorders in children prenatally exposed to antiepileptic drugs. J Neurol Neurosurg Psychiatry. 2013;84(6):637–643.
- Einarson A, Pistelli A, DeSantis M, et al. Evaluation of the risk of congenital cardiovascular defects associated with use of paroxetine during pregnancy. Am J Psychiatry. 2008;165(6):749–752.
- Davanzo R, Copertino M, De Cunto A, Minen F, Amaddeo A. Antidepressant drugs and breastfeeding: a review of the literature. Breastfeed Med. 2011;6(2):89–98.
- Ito S. Drug therapy for breast-feeding women. N Engl J Med. 2000;343(2):118–126.
- Suri RA, Altshuler LL, Burt VK, Hendrick VC. Managing psychiatric medications in the breast-feeding woman. Medscape Womens Health. 1998;3(1):1.
- Milsap RL, Jusko WJ. Pharmacokinetics in the infant. Environ Health Perspect. 1994;102(suppl 11):107–110.
- Newport DJ, Hostetter A, Arnold A, Stowe ZN. The treatment of postpartum depression: minimizing infant exposures. J Clin Psychiatry. 2002;63(suppl 7):31–44.
- Viguera AC, Newport DJ, Ritchie J, et al. Lithium in breast milk and nursing infants: clinical implications. Am J Psychiatry. 2007;164(2):342–345.
Increasingly, women with psychiatric illness are undergoing pharmacologic treatment during pregnancy. In the United States, an estimated 8% of pregnant women are prescribed antidepressants, and the number of such cases has risen over the past 15 years.1 Women with a psychiatric diagnosis were once instructed either to discontinue all medication immediately on learning they were pregnant, or to forgo motherhood because their illness might have a negative effect on a child or because avoiding medication during pregnancy might lead to a relapse.
Fortunately, women with depression, anxiety, bipolar disorder, or schizophrenia no longer are being told that they cannot become mothers. For many women, however, stopping medication is not an option. Furthermore, psychiatric illness sometimes is diagnosed initially during pregnancy and requires treatment.

Pregnant women and their physicians need accurate information about when to taper off medication, when to start or continue, and which medications are safest. Even for clinicians with a solid knowledge base, counseling a woman who needs or may need psychotropic medication during pregnancy and breastfeeding is a daunting task. Some clinicians still recommend no drug treatment as the safest and best option, given the potential risks to the fetus.
In this review we offer a methodologic approach for decision making about pharmacologic treatment during pregnancy. As the scientific literature is constantly being updated, it is imperative to have the most current information on psychotropics and to know how to individualize that information when counseling a pregnant woman and her family. Using this framework for analyzing the risks and benefits for both mother and fetus, clinicians can avoid the unanswerable question of which medication is the “safest.”
A patient’s mental health care provider is a useful resource for information about a woman’s mental health history and current stability, but he or she may not be expert or comfortable in recommending treatment for a pregnant patient. During pregnancy, a woman’s obstetrician often becomes the “expert” for all treatment decisions.
Antidepressants. Previous studies may have overestimated the association between prenatal use of antidepressants and attention deficit/hyperactivity disorder (ADHD) in children because they did not control for shared family factors, according to investigators who say that their recent study findings raise the possibility that "confounding by indication" might partially explain the observed association.1
In a population-based cohort study in Hong Kong, Man and colleagues analyzed the records of 190,618 maternal-child pairs.1 A total of 1,252 children were exposed to maternal antidepressant use during pregnancy. Medications included selective serotonin reuptake inhibitors (SSRIs), non-SSRIs, and antipsychotics as monotherapy or in various combination regimens. Overall, 5,659 of the cohort children (3%) were diagnosed with or received treatment for ADHD.
When gestational medication users were compared with nongestational users, the crude hazard ratio (HR) of antidepressant use during pregnancy and ADHD was 2.26 (P<.01). After adjusting for potential confounding factors (such as maternal psychiatric disorders and use of other psychotropic drugs), this reduced to 1.39 (95% confidence interval [CI], 1.07-1.82; P = .01). Children of mothers with psychiatric disorders had a higher risk of ADHD than did children of mothers without psychiatric disorders (HR, 1.84; 95% CI, 1.54-2.18; P<.01), even if the mothers had never used antidepressants.
While acknowledging the potential for type 2 error in the study analysis, the investigators proposed that the results "further strengthen our hypothesis that confounding by indication may play a major role in the observed positive association between gestational use of antidepressants and ADHD in offspring."
Lithium. Similarly, investigators of another recently published study found that the magnitude of the association between prenatal lithium use and increased risk of cardiac malformations in infants was smaller than previously shown.2 This finding may be important clinically because lithium is a first-line treatment for many US women of reproductive age with bipolar disorder.
Most earlier data were derived from a database registry, case reports, and small studies that often had conflicting results. However, Patorno and colleagues conducted a large retrospective cohort study that involved data on 1,325,563 pregnancies in women enrolled in Medicaid.2 Exposure to lithium was defined as at least 1 filled prescription during the first trimester, and the primary reference group included women with no lithium or lamotrigine (another mood stabilizer not associated with congenital malformations) dispensing during the 3 months before the start of pregnancy or during the first trimester.
A total of 663 pregnancies (0.05%) were exposed to lithium and 1,945 (0.15%) were exposed to lamotrigine during the first trimester. The adjusted risk ratios for cardiac malformations among infants exposed to lithium were 1.65 (95% CI, 1.02-2.68) as compared with nonexposed infants and 2.25 (95% CI, 1.17-4.34) as compared with lamotrigine-exposed infants. Notably, all right ventricular outflow tract obstruction defects identified in the infants exposed to lithium occurred with a daily dose of more than 600 mg.
Although the study results suggest an increased risk of cardiac malformations--of approximately 1 additional case per 100 live births--associated with lithium use in early pregnancy, the magnitude of risk is much lower than originally proposed based on early lithium registry data.
-- Kathy Christie, Senior Editor
References
- Man KC, Chan EW, Ip P, et al. Prenatal antidepressant use and risk of attention-deficit/hyperactivity disorder in offspring: population based cohort study. BMJ. 2017;357:j2350.
- Patorno E, Huybrechts KR, Bateman BT, et al. Lithium use in pregnancy and risk of cardiac malformations. N Engl J Med. 2017;376(23):2245-2254.
Analyze risks and benefits of medication versus no medication
The US Food and Drug Administration (FDA) has not approved any psychotropic medication for use during pregnancy. While a clinical study would provide more scientifically rigorous safety data, conducting a double-blinded, placebo-controlled trial in pregnant women with a psychiatric disorder is unethical. Thus, the literature consists mostly of reports on case series, retrospective chart reviews, prospective naturalistic studies, and analyses of large registry databases. Each has benefits and limitations. It is important to understand the limitations when making treatment decisions.
In 1979, the FDA developed a 5-lettersystem (A, B, C, D, X) for classifying the relative safety of medications used during pregnancy.2 Many clinicians and pregnant women relied on this system to decide which medications were safe. Unfortunately, the information in the system was inadequate for making informed decisions. For example, although a class B medication might have appeared safer than one in class C, the studies of risk in humans might not have been adequate to permit comparisons. Drug safety classifications were seldom changed, despite the availability of additional data.
In June 2015, the FDA changed the requirements for the Pregnancy and Lactation subsections of the labeling for human prescription drugs and biologic products. Drug manufacturers must now include in each subsection a risk summary, clinical considerations supporting patient care decisions and counseling, and detailed data. These subsections provide information on available human and animal studies, known or potential maternal or fetal adverse reactions, and dose adjustments needed during pregnancy and the postpartum period. In addition, the FDA added a subsection: Females and Males of Reproductive Potential.3
These changes acknowledge there is no list of “safe” medications. The safest medication generally is the one that works for a particular patient at the lowest effective dose. As each woman’s history of illness and effective treatment is different, the best medication may differ as well, even among women with the same illness. Therefore, medication should be individualized to the patient. A risk–benefit analysis comparing psychotropic medication treatment with no medication treatment must be performed for each patient according to her personal history and the best available data.
Read about the risks of untreated illness during pregnancy
What is the risk of untreated illness during pregnancy?
During pregnancy, women are treated for many medical disorders, including psychiatric illness. One general guideline is that, if a pregnant woman does not need a medication—whether it be for an allergy, hypertension, or another disorder—she should not take it. Conversely, if a medication is required for a patient’s well-being, her physician should continue it or switch to a safer one. This general guideline is the same for women with depression, anxiety, or a psychotic disorder.
Managing hypertension during pregnancy is an example of choosing treatment when the risk of the illness to the mother and the infant outweighs the likely small risk associated with taking a medication. Blood pressure is monitored, and, when it reaches a threshold, an antihypertensive is started promptly to avoid morbidity and mortality.
Psychiatric illness carries risks for both mother and fetus as well, but no data show a clear threshold for initiating pharmacologic treatment. Therefore, in prescribing medication the most important steps are to take a complete history and perform a thorough evaluation. Important information includes the number and severity of previous episodes, prior history of hospitalization or suicidal thoughts or attempts, and any history of psychotic or manic status.
Whether to continue or discontinue medication is often decided after inquiring about other times a medication was discontinued. A patient who in the past stayed well for several years after stopping a medication may be able to taper off a medication and conceive during a window of wellness. Some women who have experienced only one episode of illness and have been stable for at least a year may be able to taper off a medication before conceiving (TABLE 1).

In the risk–benefit analysis, assess the need for pharmacologic treatment by considering the risk that untreated illness poses for both mother and fetus, the benefits of treatment for both, and the risk of medication exposure for the fetus.4
Mother: Risk of untreated illness versus benefit of treatment
A complete history and a current symptom evaluation are needed to assess the risk that nonpharmacologic treatment poses for the mother. Women with functional impairment, including inability to work, to perform activities of daily living, or to take care of other children, likely require treatment. Studies have found that women who discontinue treatment for a psychiatric illness around the time of conception are likely to experience a recurrence of illness during pregnancy, often in the first trimester, and must restart medication.5,6 For some diagnoses, particularly bipolar disorder, symptoms during a relapse can be more severe and more difficult to treat, and they carry a risk for both mother and fetus.7 A longitudinal study of pregnant women who stopped medication for bipolar disorder found a 71% rate of relapse.7 In cases in which there is a history of hospitalization, suicide attempt, or psychosis, discontinuing treatment is not an option; instead, the physician must determine which medication is safest for the particular patient.
Related article:
Does PTSD during pregnancy increase the likelihood of preterm birth?
Fetus: Risk of untreated illness versus benefit of treatment
Mothers with untreated psychiatric illness are at higher risk for poor prenatal care, substance abuse, and inadequate nutrition, all of which increase the risk of negative obstetric and neonatal outcomes.8 Evidence indicates that untreated maternal depression increases the risk of preterm delivery and low birth weight.9 Children born to mothers with depression have more behavioral problems, more psychiatric illness, more visits to pediatricians, lower IQ scores, and attachment issues.10 Some of the long-term negative effects of intrauterine stress, which include hypertension, coronary heart disease, and autoimmune disorders, persist into adulthood.11
Fetus: Risk of medication exposure
With any pharmacologic treatment, the timing of fetal exposure affects resultant risks and therefore must be considered in the management plan.
Before conception. Is there any effect on ovulation or fertilization?
Implantation. Does the exposure impair the blastocyst’s ability to implant in the uterine lining?
First trimester. This is the period of organogenesis. Regardless of drug exposure, there is a 2% to 4% baseline risk of a major malformation during any pregnancy. The risk of a particular malformation must be weighed against this baseline risk.
According to limited data, selective serotonin reuptake inhibitors (SSRIs) may increase the risk of early miscarriage.12 SSRIs also have been implicated in increasing the risk of cardiovascular malformations, although the data are conflicting.13,14
Antiepileptics such as valproate and carbamazepine are used as mood stabilizers in the treatment of bipolar disorder.15 Extensive data have shown an association with teratogenicity. Pregnant women who require either of these medications also should be prescribed folic acid 4 or 5 mg/day. Given the high risk of birth defects and cognitive delay, valproate no longer is recommended for women of reproductive potential.16
Lithium, one of the safest medications used in the treatment of bipolar disorder, is associated with a very small risk of Ebstein anomaly.17
Lamotrigine is used to treat bipolar depression and appears to have a good safety profile, along with a possible small increased risk of oral clefts.18,19
Atypical antipsychotics (such as aripiprazole, olanzapine, quetiapine, and risperidone) are often used first-line in the treatment of psychotic disorders and bipolar disorder in women who are not pregnant. Although the safety data on use of these drugs during pregnancy are limited, a recent analysis of pregnant Medicaid enrollees found no increased risk of birth defects after controlling for potential confounding factors.20 Common practice is to avoid these newer agents, given their limited data and the time needed for rare malformations to emerge (adequate numbers require many exposures during pregnancy).
Read additional fetal risks of medication exposure
Second trimester. This is a period of growth and neural development. A 2006 study suggested that SSRI exposure after pregnancy week 20 increases the risk of persistent pulmonary hypertension of the newborn (PPHN).21 In 2011, however, the FDA removed the PPHN warning label for SSRIs, citing inconsistent data. Whether the PPHN risk is increased with SSRI use is unclear, but the risk is presumed to be smaller than previously suggested.22 Stopping SSRIs before week 20 puts the mother at risk for relapse during pregnancy and increases her risk of developing postpartum depression. If we follow the recommendation to prescribe medication only for women who need it most, then stopping the medication at any time during pregnancy is not an option.
Third trimester. This is a period of continued growth and lung maturation.
Delivery. Is there a potential for impairment in parturition?
Neonatal adaptation. Newborns are active mainly in adapting to extrauterine life: They regulate their temperature and muscle tone and learn to coordinate sucking, swallowing, and breathing. Does medication exposure impair adaptation, or are signs or symptoms of withdrawal or toxicity present? The evidence that in utero SSRI exposure increases the risk of neonatal adaptation syndrome is consistent, but symptoms are mild and self-limited.23 Tapering off SSRIs before delivery currently is not recommended, as doing so increases the mother’s risk for postpartum depression and, according to one study, does not prevent symptoms of neonatal adaptation syndrome from developing.24
Behavioral teratogenicity. What are the long-term developmental outcomes for the child? Are there any differences in IQ, speech and language, or psychiatric illness? One study found an increased risk of autism with in utero exposure to sertraline, but the study had many methodologic flaws and its findings have not been replicated.25 Most studies have not found consistent differences in speech, IQ, or behavior between infants exposed and infants not exposed to antidepressants.26,27 By contrast, in utero exposure to anticonvulsants, particularly valproate, has led to significant developmental problems in children.28 The data on atypical antipsychotics are limited.
Related article:
Do antidepressants really cause autism?
None of the medications used to treat depression, bipolar disorder, anxiety, or schizophrenia is considered first-line or safest therapy for the pregnant woman. For any woman who is doing well on a certain medication, but particularly for a pregnant woman, there is no compelling, data-supported reason to switch to another agent. For depression, options include all of the SSRIs, with the possible exception of paroxetine (TABLE 2). In conflicting studies, paroxetine was no different from any other SSRI in not being associated with cardiovascular defects.29

One goal in treatment is to use a medication that previously was effective in the remission of symptoms and to use it at the lowest dose possible. Treating simply to maintain a low dose of drug, however, and not to effect symptom remission, exposes the fetus to both the drug and the illness. Again, the lowest effective dose is the best choice.
Read about treatment during breastfeeding
Treatment during breastfeeding
Women are encouraged to breastfeed for physical and psychological health benefits, for both themselves and their babies. Many medications are compatible with breastfeeding.30 The amount of drug an infant receives through breast milk is considerably less than the amount received during the mother’s pregnancy. Breastfeeding generally is allowed if the calculated infant dose is less than 10% of the weight-adjusted maternal dose.31
The amount of drug transferred from maternal plasma into milk is highest for drugs with low protein binding and high lipid solubility.32 Drug clearance in infants must be considered as well. Renal clearance is decreased in newborns and does not reach adult levels until 5 or 6 months of age. In addition, liver metabolism is impaired in neonates and even more so in premature infants.33 Drugs that require extensive first-pass metabolism may have higher bioavailability, and this factor should be considered.
Some clinicians recommend pumping and discarding breast milk when the drug in it is at its peak level; although the drug is not eliminated, the infant ingests less of it.34 Most women who are anxious about breastfeeding while on medication “pump and dump” until they are more comfortable nursing and the infants are doing well. Except in cases of mother preference, most physicians with expertise in reproductive mental health generally recommend against pumping and discarding milk.
Through breast milk, infants ingest drugs in varying amounts. The amount depends on the qualities of the medication, the timing and duration of breastfeeding, and the characteristics of the infant. Few psychotropic drugs have significant effects on breastfed infants. Even lithium, previously contraindicated, is successfully used, with infant monitoring, during breastfeeding.35 Given breastfeeding’s benefits for both mother and child, many more women on psychotropic medications are choosing to breastfeed.
Related article:
USPSTF Recommendations to Support Breastfeeding
Balance the pros and cons
Deciding to use medication during pregnancy and breastfeeding involves considering the risk of untreated illness versus the benefit of treatment for both mother and fetus, and the risk of medication exposure for the fetus. Mother and fetus are inseparable, and neither can be isolated from the other in treatment decisions. Avoiding psychotropic medication during pregnancy is not always the safest option for mother or fetus. The patient and her clinician and support system must make an informed decision that is based on the best available data and that takes into account the mother’s history of illness and effective treatment. Many women with psychiatric illness no longer have to choose between mental health and starting a family, and their babies will be healthy.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Increasingly, women with psychiatric illness are undergoing pharmacologic treatment during pregnancy. In the United States, an estimated 8% of pregnant women are prescribed antidepressants, and the number of such cases has risen over the past 15 years.1 Women with a psychiatric diagnosis were once instructed either to discontinue all medication immediately on learning they were pregnant, or to forgo motherhood because their illness might have a negative effect on a child or because avoiding medication during pregnancy might lead to a relapse.
Fortunately, women with depression, anxiety, bipolar disorder, or schizophrenia no longer are being told that they cannot become mothers. For many women, however, stopping medication is not an option. Furthermore, psychiatric illness sometimes is diagnosed initially during pregnancy and requires treatment.

Pregnant women and their physicians need accurate information about when to taper off medication, when to start or continue, and which medications are safest. Even for clinicians with a solid knowledge base, counseling a woman who needs or may need psychotropic medication during pregnancy and breastfeeding is a daunting task. Some clinicians still recommend no drug treatment as the safest and best option, given the potential risks to the fetus.
In this review we offer a methodologic approach for decision making about pharmacologic treatment during pregnancy. As the scientific literature is constantly being updated, it is imperative to have the most current information on psychotropics and to know how to individualize that information when counseling a pregnant woman and her family. Using this framework for analyzing the risks and benefits for both mother and fetus, clinicians can avoid the unanswerable question of which medication is the “safest.”
A patient’s mental health care provider is a useful resource for information about a woman’s mental health history and current stability, but he or she may not be expert or comfortable in recommending treatment for a pregnant patient. During pregnancy, a woman’s obstetrician often becomes the “expert” for all treatment decisions.
Antidepressants. Previous studies may have overestimated the association between prenatal use of antidepressants and attention deficit/hyperactivity disorder (ADHD) in children because they did not control for shared family factors, according to investigators who say that their recent study findings raise the possibility that "confounding by indication" might partially explain the observed association.1
In a population-based cohort study in Hong Kong, Man and colleagues analyzed the records of 190,618 maternal-child pairs.1 A total of 1,252 children were exposed to maternal antidepressant use during pregnancy. Medications included selective serotonin reuptake inhibitors (SSRIs), non-SSRIs, and antipsychotics as monotherapy or in various combination regimens. Overall, 5,659 of the cohort children (3%) were diagnosed with or received treatment for ADHD.
When gestational medication users were compared with nongestational users, the crude hazard ratio (HR) of antidepressant use during pregnancy and ADHD was 2.26 (P<.01). After adjusting for potential confounding factors (such as maternal psychiatric disorders and use of other psychotropic drugs), this reduced to 1.39 (95% confidence interval [CI], 1.07-1.82; P = .01). Children of mothers with psychiatric disorders had a higher risk of ADHD than did children of mothers without psychiatric disorders (HR, 1.84; 95% CI, 1.54-2.18; P<.01), even if the mothers had never used antidepressants.
While acknowledging the potential for type 2 error in the study analysis, the investigators proposed that the results "further strengthen our hypothesis that confounding by indication may play a major role in the observed positive association between gestational use of antidepressants and ADHD in offspring."
Lithium. Similarly, investigators of another recently published study found that the magnitude of the association between prenatal lithium use and increased risk of cardiac malformations in infants was smaller than previously shown.2 This finding may be important clinically because lithium is a first-line treatment for many US women of reproductive age with bipolar disorder.
Most earlier data were derived from a database registry, case reports, and small studies that often had conflicting results. However, Patorno and colleagues conducted a large retrospective cohort study that involved data on 1,325,563 pregnancies in women enrolled in Medicaid.2 Exposure to lithium was defined as at least 1 filled prescription during the first trimester, and the primary reference group included women with no lithium or lamotrigine (another mood stabilizer not associated with congenital malformations) dispensing during the 3 months before the start of pregnancy or during the first trimester.
A total of 663 pregnancies (0.05%) were exposed to lithium and 1,945 (0.15%) were exposed to lamotrigine during the first trimester. The adjusted risk ratios for cardiac malformations among infants exposed to lithium were 1.65 (95% CI, 1.02-2.68) as compared with nonexposed infants and 2.25 (95% CI, 1.17-4.34) as compared with lamotrigine-exposed infants. Notably, all right ventricular outflow tract obstruction defects identified in the infants exposed to lithium occurred with a daily dose of more than 600 mg.
Although the study results suggest an increased risk of cardiac malformations--of approximately 1 additional case per 100 live births--associated with lithium use in early pregnancy, the magnitude of risk is much lower than originally proposed based on early lithium registry data.
-- Kathy Christie, Senior Editor
References
- Man KC, Chan EW, Ip P, et al. Prenatal antidepressant use and risk of attention-deficit/hyperactivity disorder in offspring: population based cohort study. BMJ. 2017;357:j2350.
- Patorno E, Huybrechts KR, Bateman BT, et al. Lithium use in pregnancy and risk of cardiac malformations. N Engl J Med. 2017;376(23):2245-2254.
Analyze risks and benefits of medication versus no medication
The US Food and Drug Administration (FDA) has not approved any psychotropic medication for use during pregnancy. While a clinical study would provide more scientifically rigorous safety data, conducting a double-blinded, placebo-controlled trial in pregnant women with a psychiatric disorder is unethical. Thus, the literature consists mostly of reports on case series, retrospective chart reviews, prospective naturalistic studies, and analyses of large registry databases. Each has benefits and limitations. It is important to understand the limitations when making treatment decisions.
In 1979, the FDA developed a 5-lettersystem (A, B, C, D, X) for classifying the relative safety of medications used during pregnancy.2 Many clinicians and pregnant women relied on this system to decide which medications were safe. Unfortunately, the information in the system was inadequate for making informed decisions. For example, although a class B medication might have appeared safer than one in class C, the studies of risk in humans might not have been adequate to permit comparisons. Drug safety classifications were seldom changed, despite the availability of additional data.
In June 2015, the FDA changed the requirements for the Pregnancy and Lactation subsections of the labeling for human prescription drugs and biologic products. Drug manufacturers must now include in each subsection a risk summary, clinical considerations supporting patient care decisions and counseling, and detailed data. These subsections provide information on available human and animal studies, known or potential maternal or fetal adverse reactions, and dose adjustments needed during pregnancy and the postpartum period. In addition, the FDA added a subsection: Females and Males of Reproductive Potential.3
These changes acknowledge there is no list of “safe” medications. The safest medication generally is the one that works for a particular patient at the lowest effective dose. As each woman’s history of illness and effective treatment is different, the best medication may differ as well, even among women with the same illness. Therefore, medication should be individualized to the patient. A risk–benefit analysis comparing psychotropic medication treatment with no medication treatment must be performed for each patient according to her personal history and the best available data.
Read about the risks of untreated illness during pregnancy
What is the risk of untreated illness during pregnancy?
During pregnancy, women are treated for many medical disorders, including psychiatric illness. One general guideline is that, if a pregnant woman does not need a medication—whether it be for an allergy, hypertension, or another disorder—she should not take it. Conversely, if a medication is required for a patient’s well-being, her physician should continue it or switch to a safer one. This general guideline is the same for women with depression, anxiety, or a psychotic disorder.
Managing hypertension during pregnancy is an example of choosing treatment when the risk of the illness to the mother and the infant outweighs the likely small risk associated with taking a medication. Blood pressure is monitored, and, when it reaches a threshold, an antihypertensive is started promptly to avoid morbidity and mortality.
Psychiatric illness carries risks for both mother and fetus as well, but no data show a clear threshold for initiating pharmacologic treatment. Therefore, in prescribing medication the most important steps are to take a complete history and perform a thorough evaluation. Important information includes the number and severity of previous episodes, prior history of hospitalization or suicidal thoughts or attempts, and any history of psychotic or manic status.
Whether to continue or discontinue medication is often decided after inquiring about other times a medication was discontinued. A patient who in the past stayed well for several years after stopping a medication may be able to taper off a medication and conceive during a window of wellness. Some women who have experienced only one episode of illness and have been stable for at least a year may be able to taper off a medication before conceiving (TABLE 1).

In the risk–benefit analysis, assess the need for pharmacologic treatment by considering the risk that untreated illness poses for both mother and fetus, the benefits of treatment for both, and the risk of medication exposure for the fetus.4
Mother: Risk of untreated illness versus benefit of treatment
A complete history and a current symptom evaluation are needed to assess the risk that nonpharmacologic treatment poses for the mother. Women with functional impairment, including inability to work, to perform activities of daily living, or to take care of other children, likely require treatment. Studies have found that women who discontinue treatment for a psychiatric illness around the time of conception are likely to experience a recurrence of illness during pregnancy, often in the first trimester, and must restart medication.5,6 For some diagnoses, particularly bipolar disorder, symptoms during a relapse can be more severe and more difficult to treat, and they carry a risk for both mother and fetus.7 A longitudinal study of pregnant women who stopped medication for bipolar disorder found a 71% rate of relapse.7 In cases in which there is a history of hospitalization, suicide attempt, or psychosis, discontinuing treatment is not an option; instead, the physician must determine which medication is safest for the particular patient.
Related article:
Does PTSD during pregnancy increase the likelihood of preterm birth?
Fetus: Risk of untreated illness versus benefit of treatment
Mothers with untreated psychiatric illness are at higher risk for poor prenatal care, substance abuse, and inadequate nutrition, all of which increase the risk of negative obstetric and neonatal outcomes.8 Evidence indicates that untreated maternal depression increases the risk of preterm delivery and low birth weight.9 Children born to mothers with depression have more behavioral problems, more psychiatric illness, more visits to pediatricians, lower IQ scores, and attachment issues.10 Some of the long-term negative effects of intrauterine stress, which include hypertension, coronary heart disease, and autoimmune disorders, persist into adulthood.11
Fetus: Risk of medication exposure
With any pharmacologic treatment, the timing of fetal exposure affects resultant risks and therefore must be considered in the management plan.
Before conception. Is there any effect on ovulation or fertilization?
Implantation. Does the exposure impair the blastocyst’s ability to implant in the uterine lining?
First trimester. This is the period of organogenesis. Regardless of drug exposure, there is a 2% to 4% baseline risk of a major malformation during any pregnancy. The risk of a particular malformation must be weighed against this baseline risk.
According to limited data, selective serotonin reuptake inhibitors (SSRIs) may increase the risk of early miscarriage.12 SSRIs also have been implicated in increasing the risk of cardiovascular malformations, although the data are conflicting.13,14
Antiepileptics such as valproate and carbamazepine are used as mood stabilizers in the treatment of bipolar disorder.15 Extensive data have shown an association with teratogenicity. Pregnant women who require either of these medications also should be prescribed folic acid 4 or 5 mg/day. Given the high risk of birth defects and cognitive delay, valproate no longer is recommended for women of reproductive potential.16
Lithium, one of the safest medications used in the treatment of bipolar disorder, is associated with a very small risk of Ebstein anomaly.17
Lamotrigine is used to treat bipolar depression and appears to have a good safety profile, along with a possible small increased risk of oral clefts.18,19
Atypical antipsychotics (such as aripiprazole, olanzapine, quetiapine, and risperidone) are often used first-line in the treatment of psychotic disorders and bipolar disorder in women who are not pregnant. Although the safety data on use of these drugs during pregnancy are limited, a recent analysis of pregnant Medicaid enrollees found no increased risk of birth defects after controlling for potential confounding factors.20 Common practice is to avoid these newer agents, given their limited data and the time needed for rare malformations to emerge (adequate numbers require many exposures during pregnancy).
Read additional fetal risks of medication exposure
Second trimester. This is a period of growth and neural development. A 2006 study suggested that SSRI exposure after pregnancy week 20 increases the risk of persistent pulmonary hypertension of the newborn (PPHN).21 In 2011, however, the FDA removed the PPHN warning label for SSRIs, citing inconsistent data. Whether the PPHN risk is increased with SSRI use is unclear, but the risk is presumed to be smaller than previously suggested.22 Stopping SSRIs before week 20 puts the mother at risk for relapse during pregnancy and increases her risk of developing postpartum depression. If we follow the recommendation to prescribe medication only for women who need it most, then stopping the medication at any time during pregnancy is not an option.
Third trimester. This is a period of continued growth and lung maturation.
Delivery. Is there a potential for impairment in parturition?
Neonatal adaptation. Newborns are active mainly in adapting to extrauterine life: They regulate their temperature and muscle tone and learn to coordinate sucking, swallowing, and breathing. Does medication exposure impair adaptation, or are signs or symptoms of withdrawal or toxicity present? The evidence that in utero SSRI exposure increases the risk of neonatal adaptation syndrome is consistent, but symptoms are mild and self-limited.23 Tapering off SSRIs before delivery currently is not recommended, as doing so increases the mother’s risk for postpartum depression and, according to one study, does not prevent symptoms of neonatal adaptation syndrome from developing.24
Behavioral teratogenicity. What are the long-term developmental outcomes for the child? Are there any differences in IQ, speech and language, or psychiatric illness? One study found an increased risk of autism with in utero exposure to sertraline, but the study had many methodologic flaws and its findings have not been replicated.25 Most studies have not found consistent differences in speech, IQ, or behavior between infants exposed and infants not exposed to antidepressants.26,27 By contrast, in utero exposure to anticonvulsants, particularly valproate, has led to significant developmental problems in children.28 The data on atypical antipsychotics are limited.
Related article:
Do antidepressants really cause autism?
None of the medications used to treat depression, bipolar disorder, anxiety, or schizophrenia is considered first-line or safest therapy for the pregnant woman. For any woman who is doing well on a certain medication, but particularly for a pregnant woman, there is no compelling, data-supported reason to switch to another agent. For depression, options include all of the SSRIs, with the possible exception of paroxetine (TABLE 2). In conflicting studies, paroxetine was no different from any other SSRI in not being associated with cardiovascular defects.29

One goal in treatment is to use a medication that previously was effective in the remission of symptoms and to use it at the lowest dose possible. Treating simply to maintain a low dose of drug, however, and not to effect symptom remission, exposes the fetus to both the drug and the illness. Again, the lowest effective dose is the best choice.
Read about treatment during breastfeeding
Treatment during breastfeeding
Women are encouraged to breastfeed for physical and psychological health benefits, for both themselves and their babies. Many medications are compatible with breastfeeding.30 The amount of drug an infant receives through breast milk is considerably less than the amount received during the mother’s pregnancy. Breastfeeding generally is allowed if the calculated infant dose is less than 10% of the weight-adjusted maternal dose.31
The amount of drug transferred from maternal plasma into milk is highest for drugs with low protein binding and high lipid solubility.32 Drug clearance in infants must be considered as well. Renal clearance is decreased in newborns and does not reach adult levels until 5 or 6 months of age. In addition, liver metabolism is impaired in neonates and even more so in premature infants.33 Drugs that require extensive first-pass metabolism may have higher bioavailability, and this factor should be considered.
Some clinicians recommend pumping and discarding breast milk when the drug in it is at its peak level; although the drug is not eliminated, the infant ingests less of it.34 Most women who are anxious about breastfeeding while on medication “pump and dump” until they are more comfortable nursing and the infants are doing well. Except in cases of mother preference, most physicians with expertise in reproductive mental health generally recommend against pumping and discarding milk.
Through breast milk, infants ingest drugs in varying amounts. The amount depends on the qualities of the medication, the timing and duration of breastfeeding, and the characteristics of the infant. Few psychotropic drugs have significant effects on breastfed infants. Even lithium, previously contraindicated, is successfully used, with infant monitoring, during breastfeeding.35 Given breastfeeding’s benefits for both mother and child, many more women on psychotropic medications are choosing to breastfeed.
Related article:
USPSTF Recommendations to Support Breastfeeding
Balance the pros and cons
Deciding to use medication during pregnancy and breastfeeding involves considering the risk of untreated illness versus the benefit of treatment for both mother and fetus, and the risk of medication exposure for the fetus. Mother and fetus are inseparable, and neither can be isolated from the other in treatment decisions. Avoiding psychotropic medication during pregnancy is not always the safest option for mother or fetus. The patient and her clinician and support system must make an informed decision that is based on the best available data and that takes into account the mother’s history of illness and effective treatment. Many women with psychiatric illness no longer have to choose between mental health and starting a family, and their babies will be healthy.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Andrade SE, Raebel MA, Brown J, et al. Use of antidepressant medications during pregnancy: a multisite study. Am J Obstet Gynecol. 2008;198(2):194.e1–e5.
- Hecht A. Drug safety labeling for doctors. FDA Consum. 1979;13(8):12–13.
- Ramoz LL, Patel-Shori NM. Recent changes in pregnancy and lactation labeling: retirement of risk categories. Pharmacotherapy. 2014;34(4):389–395.
- Yonkers KA, Wisner KL, Stewart DE, et al. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Gen Hosp Psychiatry. 2009;31(5):403–413.
- Cohen LS, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295(5):499–507.
- O’Brien L, Laporte A, Koren G. Estimating the economic costs of antidepressant discontinuation during pregnancy. Can J Psychiatry. 2009;54(6):399–408.
- Viguera AC, Whitfield T, Baldessarini RJ, et al. Risk of recurrence in women with bipolar disorder during pregnancy: prospective study of mood stabilizer discontinuation. Am J Psychiatry. 2007;164(12):1817–1824.
- Bonari L, Pinto N, Ahn E, Einarson A, Steiner M, Koren G. Perinatal risks of untreated depression during pregnancy. Can J Psychiatry. 2004;49(11):726–735.
- Straub H, Adams M, Kim JJ, Silver RK. Antenatal depressive symptoms increase the likelihood of preterm birth. Am J Obstet Gynecol. 2012;207(4):329.e1–e4.
- Hayes LJ, Goodman SH, Carlson E. Maternal antenatal depression and infant disorganized attachment at 12 months. Attach Hum Dev. 2013;15(2):133–153.
- Field T. Prenatal depression effects on early development: a review. Infant Behav Dev. 2011;34(1):1–14.
- Kjaersgaard MI, Parner ET, Vestergaard M, et al. Prenatal antidepressant exposure and risk of spontaneous abortion—a population-based study. PLoS One. 2013;8(8):e72095.
- Nordeng H, van Gelder MM, Spigset O, Koren G, Einarson A, Eberhard-Gran M. Pregnancy outcome after exposure to antidepressants and the role of maternal depression: results from the Norwegian Mother and Child Cohort Study. J Clin Psychopharmacol. 2012;32(2):186–194.
- Källén BA, Otterblad Olausson P. Maternal use of selective serotonin re-uptake inhibitors in early pregnancy and infant congenital malformations. Birth Defects Res A Clin Mol Teratol. 2007;79(4):301–308.
- Tomson T, Battino D. Teratogenic effects of antiepileptic drugs. Lancet Neurol. 2012;11(9):803–813.
- Balon R, Riba M. Should women of childbearing potential be prescribed valproate? A call to action. J Clin Psychiatry. 2016;77(4):525–526.
- Giles JJ, Bannigan JG. Teratogenic and developmental effects of lithium. Curr Pharm Design. 2006;12(12):1531–1541.
- Nguyen HT, Sharma V, McIntyre RS. Teratogenesis associated with antibipolar agents. Adv Ther. 2009;26(3):281–294.
- Campbell E, Kennedy F, Irwin B, et al. Malformation risks of antiepileptic drug monotherapies in pregnancy. J Neurol Neurosurg Psychiatry. 2013;84(11):e2.
- Huybrechts KF, Hernández-Díaz S, Patorno E, et al. Antipsychotic use in pregnancy and the risk for congenital malformations. JAMA Psychiatry. 2016;73(9):938–946.
- Chambers CD, Hernández-Díaz S, Van Marter LJ, et al. Selective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. N Engl J Med. 2006;354(6):579–587.
- ‘t Jong GW, Einarson T, Koren G, Einarson A. Antidepressant use in pregnancy and persistent pulmonary hypertension of the newborn (PPHN): a systematic review. Reprod Toxicol. 2012;34(3):293–297.
- Oberlander TF, Misri S, Fitzgerald CE, Kostaras X, Rurak D, Riggs W. Pharmacologic factors associated with transient neonatal symptoms following prenatal psychotropic medication exposure. J Clin Psychiatry. 2004;65(2):230–237.
- Warburton W, Hertzman C, Oberlander TF. A register study of the impact of stopping third trimester selective serotonin reuptake inhibitor exposure on neonatal health. Acta Psychiatr Scand. 2010;121(6):471–479.
- Croen LA, Grether JK, Yoshida CK, Odouli R, Hendrick V. Antidepressant use during pregnancy and childhood autism spectrum disorders. Arch Gen Psychiatry. 2011;68(11):1104–1112.
- Batton B, Batton E, Weigler K, Aylward G, Batton D. In utero antidepressant exposure and neurodevelopment in preterm infants. Am J Perinatol. 2013;30(4):297–301.
- Austin MP, Karatas JC, Mishra P, Christl B, Kennedy D, Oei J. Infant neurodevelopment following in utero exposure to antidepressant medication. Acta Paediatr. 2013;102(11):1054–1059.
- Bromley RL, Mawer GE, Briggs M, et al. The prevalence of neurodevelopmental disorders in children prenatally exposed to antiepileptic drugs. J Neurol Neurosurg Psychiatry. 2013;84(6):637–643.
- Einarson A, Pistelli A, DeSantis M, et al. Evaluation of the risk of congenital cardiovascular defects associated with use of paroxetine during pregnancy. Am J Psychiatry. 2008;165(6):749–752.
- Davanzo R, Copertino M, De Cunto A, Minen F, Amaddeo A. Antidepressant drugs and breastfeeding: a review of the literature. Breastfeed Med. 2011;6(2):89–98.
- Ito S. Drug therapy for breast-feeding women. N Engl J Med. 2000;343(2):118–126.
- Suri RA, Altshuler LL, Burt VK, Hendrick VC. Managing psychiatric medications in the breast-feeding woman. Medscape Womens Health. 1998;3(1):1.
- Milsap RL, Jusko WJ. Pharmacokinetics in the infant. Environ Health Perspect. 1994;102(suppl 11):107–110.
- Newport DJ, Hostetter A, Arnold A, Stowe ZN. The treatment of postpartum depression: minimizing infant exposures. J Clin Psychiatry. 2002;63(suppl 7):31–44.
- Viguera AC, Newport DJ, Ritchie J, et al. Lithium in breast milk and nursing infants: clinical implications. Am J Psychiatry. 2007;164(2):342–345.
- Andrade SE, Raebel MA, Brown J, et al. Use of antidepressant medications during pregnancy: a multisite study. Am J Obstet Gynecol. 2008;198(2):194.e1–e5.
- Hecht A. Drug safety labeling for doctors. FDA Consum. 1979;13(8):12–13.
- Ramoz LL, Patel-Shori NM. Recent changes in pregnancy and lactation labeling: retirement of risk categories. Pharmacotherapy. 2014;34(4):389–395.
- Yonkers KA, Wisner KL, Stewart DE, et al. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Gen Hosp Psychiatry. 2009;31(5):403–413.
- Cohen LS, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295(5):499–507.
- O’Brien L, Laporte A, Koren G. Estimating the economic costs of antidepressant discontinuation during pregnancy. Can J Psychiatry. 2009;54(6):399–408.
- Viguera AC, Whitfield T, Baldessarini RJ, et al. Risk of recurrence in women with bipolar disorder during pregnancy: prospective study of mood stabilizer discontinuation. Am J Psychiatry. 2007;164(12):1817–1824.
- Bonari L, Pinto N, Ahn E, Einarson A, Steiner M, Koren G. Perinatal risks of untreated depression during pregnancy. Can J Psychiatry. 2004;49(11):726–735.
- Straub H, Adams M, Kim JJ, Silver RK. Antenatal depressive symptoms increase the likelihood of preterm birth. Am J Obstet Gynecol. 2012;207(4):329.e1–e4.
- Hayes LJ, Goodman SH, Carlson E. Maternal antenatal depression and infant disorganized attachment at 12 months. Attach Hum Dev. 2013;15(2):133–153.
- Field T. Prenatal depression effects on early development: a review. Infant Behav Dev. 2011;34(1):1–14.
- Kjaersgaard MI, Parner ET, Vestergaard M, et al. Prenatal antidepressant exposure and risk of spontaneous abortion—a population-based study. PLoS One. 2013;8(8):e72095.
- Nordeng H, van Gelder MM, Spigset O, Koren G, Einarson A, Eberhard-Gran M. Pregnancy outcome after exposure to antidepressants and the role of maternal depression: results from the Norwegian Mother and Child Cohort Study. J Clin Psychopharmacol. 2012;32(2):186–194.
- Källén BA, Otterblad Olausson P. Maternal use of selective serotonin re-uptake inhibitors in early pregnancy and infant congenital malformations. Birth Defects Res A Clin Mol Teratol. 2007;79(4):301–308.
- Tomson T, Battino D. Teratogenic effects of antiepileptic drugs. Lancet Neurol. 2012;11(9):803–813.
- Balon R, Riba M. Should women of childbearing potential be prescribed valproate? A call to action. J Clin Psychiatry. 2016;77(4):525–526.
- Giles JJ, Bannigan JG. Teratogenic and developmental effects of lithium. Curr Pharm Design. 2006;12(12):1531–1541.
- Nguyen HT, Sharma V, McIntyre RS. Teratogenesis associated with antibipolar agents. Adv Ther. 2009;26(3):281–294.
- Campbell E, Kennedy F, Irwin B, et al. Malformation risks of antiepileptic drug monotherapies in pregnancy. J Neurol Neurosurg Psychiatry. 2013;84(11):e2.
- Huybrechts KF, Hernández-Díaz S, Patorno E, et al. Antipsychotic use in pregnancy and the risk for congenital malformations. JAMA Psychiatry. 2016;73(9):938–946.
- Chambers CD, Hernández-Díaz S, Van Marter LJ, et al. Selective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. N Engl J Med. 2006;354(6):579–587.
- ‘t Jong GW, Einarson T, Koren G, Einarson A. Antidepressant use in pregnancy and persistent pulmonary hypertension of the newborn (PPHN): a systematic review. Reprod Toxicol. 2012;34(3):293–297.
- Oberlander TF, Misri S, Fitzgerald CE, Kostaras X, Rurak D, Riggs W. Pharmacologic factors associated with transient neonatal symptoms following prenatal psychotropic medication exposure. J Clin Psychiatry. 2004;65(2):230–237.
- Warburton W, Hertzman C, Oberlander TF. A register study of the impact of stopping third trimester selective serotonin reuptake inhibitor exposure on neonatal health. Acta Psychiatr Scand. 2010;121(6):471–479.
- Croen LA, Grether JK, Yoshida CK, Odouli R, Hendrick V. Antidepressant use during pregnancy and childhood autism spectrum disorders. Arch Gen Psychiatry. 2011;68(11):1104–1112.
- Batton B, Batton E, Weigler K, Aylward G, Batton D. In utero antidepressant exposure and neurodevelopment in preterm infants. Am J Perinatol. 2013;30(4):297–301.
- Austin MP, Karatas JC, Mishra P, Christl B, Kennedy D, Oei J. Infant neurodevelopment following in utero exposure to antidepressant medication. Acta Paediatr. 2013;102(11):1054–1059.
- Bromley RL, Mawer GE, Briggs M, et al. The prevalence of neurodevelopmental disorders in children prenatally exposed to antiepileptic drugs. J Neurol Neurosurg Psychiatry. 2013;84(6):637–643.
- Einarson A, Pistelli A, DeSantis M, et al. Evaluation of the risk of congenital cardiovascular defects associated with use of paroxetine during pregnancy. Am J Psychiatry. 2008;165(6):749–752.
- Davanzo R, Copertino M, De Cunto A, Minen F, Amaddeo A. Antidepressant drugs and breastfeeding: a review of the literature. Breastfeed Med. 2011;6(2):89–98.
- Ito S. Drug therapy for breast-feeding women. N Engl J Med. 2000;343(2):118–126.
- Suri RA, Altshuler LL, Burt VK, Hendrick VC. Managing psychiatric medications in the breast-feeding woman. Medscape Womens Health. 1998;3(1):1.
- Milsap RL, Jusko WJ. Pharmacokinetics in the infant. Environ Health Perspect. 1994;102(suppl 11):107–110.
- Newport DJ, Hostetter A, Arnold A, Stowe ZN. The treatment of postpartum depression: minimizing infant exposures. J Clin Psychiatry. 2002;63(suppl 7):31–44.
- Viguera AC, Newport DJ, Ritchie J, et al. Lithium in breast milk and nursing infants: clinical implications. Am J Psychiatry. 2007;164(2):342–345.
First trimester antibiotics may increase birth defect risk
Exposure to clindamycin and doxycycline during the first trimester may be associated with an increased risk of organ-specific malformations such as ventricular/atrial septal defect, new research suggests.
In a population-based cohort study in 139,938 liveborn singletons, there was an 81% increased risk of ventricular/septal defect (adjusted odds ratio, 1.81) associated with clindamycin exposure during the first trimester, compared with no exposure. Similarly, there was a 67% greater risk of musculoskeletal system malformations (aOR, 1.67) associated with clindamycin exposure (Br J Clin Pharmacol. 2017 Jul 19. doi: 10.1111/bcp.13364).
Doxycycline exposure was associated with a greater than threefold increased risk of ventricular/atrial septal defect (aOR, 3.19), and greater than twofold increase in the risk of circulatory system malformation (aOR, 2.38) and cardiac malformations (aOR, 2.46).
The study also found a 46% higher risk of digestive system malformations associated with macrolide exposure (aOR, 1.46), while quinolone exposure was associated with an 89% higher risk of urinary system malformations (aOR, 1.89).
“There is currently a debate on a possible association between macrolide use and infantile pyloric stenosis,” the researchers wrote. “Though evidence suggested that late pregnancy and early infancy were the time windows of interest for this malformation, little attention has been paid to the first trimester of pregnancy.”
Phenoxymethylpenicillin exposure was associated with a 85% increased risk of nervous system malformations (aOR, 1.85), and erythromycin exposure doubled the risk of urinary system malformations (aOR, 2.12). Moxifloxacin exposure was associated with a fivefold increased risk of respiratory system malformations (aOR, 5.48), but the authors noted that there were just two exposed cases.
However, there was no increased risk for major congenital malformation seen with amoxicillin, cephalosporins, and nitrofurantoin.
Overall, 11% of pregnancies in the study recorded exposure to antibiotics during the first trimester, and 9.9% of the study population were diagnosed with a major congenital malformation in the first year of life.
“Though the absolute risks for specific birth defects was small, physicians should consider prescribing safer antibiotics for the treatment of maternal infections when possible until more data are available,” the researchers wrote.
The study was supported by the Réseau Québécois. One author reported being a consultant on litigation involving antidepressants and birth defects. No other conflicts of interest were declared.
Exposure to clindamycin and doxycycline during the first trimester may be associated with an increased risk of organ-specific malformations such as ventricular/atrial septal defect, new research suggests.
In a population-based cohort study in 139,938 liveborn singletons, there was an 81% increased risk of ventricular/septal defect (adjusted odds ratio, 1.81) associated with clindamycin exposure during the first trimester, compared with no exposure. Similarly, there was a 67% greater risk of musculoskeletal system malformations (aOR, 1.67) associated with clindamycin exposure (Br J Clin Pharmacol. 2017 Jul 19. doi: 10.1111/bcp.13364).
Doxycycline exposure was associated with a greater than threefold increased risk of ventricular/atrial septal defect (aOR, 3.19), and greater than twofold increase in the risk of circulatory system malformation (aOR, 2.38) and cardiac malformations (aOR, 2.46).
The study also found a 46% higher risk of digestive system malformations associated with macrolide exposure (aOR, 1.46), while quinolone exposure was associated with an 89% higher risk of urinary system malformations (aOR, 1.89).
“There is currently a debate on a possible association between macrolide use and infantile pyloric stenosis,” the researchers wrote. “Though evidence suggested that late pregnancy and early infancy were the time windows of interest for this malformation, little attention has been paid to the first trimester of pregnancy.”
Phenoxymethylpenicillin exposure was associated with a 85% increased risk of nervous system malformations (aOR, 1.85), and erythromycin exposure doubled the risk of urinary system malformations (aOR, 2.12). Moxifloxacin exposure was associated with a fivefold increased risk of respiratory system malformations (aOR, 5.48), but the authors noted that there were just two exposed cases.
However, there was no increased risk for major congenital malformation seen with amoxicillin, cephalosporins, and nitrofurantoin.
Overall, 11% of pregnancies in the study recorded exposure to antibiotics during the first trimester, and 9.9% of the study population were diagnosed with a major congenital malformation in the first year of life.
“Though the absolute risks for specific birth defects was small, physicians should consider prescribing safer antibiotics for the treatment of maternal infections when possible until more data are available,” the researchers wrote.
The study was supported by the Réseau Québécois. One author reported being a consultant on litigation involving antidepressants and birth defects. No other conflicts of interest were declared.
Exposure to clindamycin and doxycycline during the first trimester may be associated with an increased risk of organ-specific malformations such as ventricular/atrial septal defect, new research suggests.
In a population-based cohort study in 139,938 liveborn singletons, there was an 81% increased risk of ventricular/septal defect (adjusted odds ratio, 1.81) associated with clindamycin exposure during the first trimester, compared with no exposure. Similarly, there was a 67% greater risk of musculoskeletal system malformations (aOR, 1.67) associated with clindamycin exposure (Br J Clin Pharmacol. 2017 Jul 19. doi: 10.1111/bcp.13364).
Doxycycline exposure was associated with a greater than threefold increased risk of ventricular/atrial septal defect (aOR, 3.19), and greater than twofold increase in the risk of circulatory system malformation (aOR, 2.38) and cardiac malformations (aOR, 2.46).
The study also found a 46% higher risk of digestive system malformations associated with macrolide exposure (aOR, 1.46), while quinolone exposure was associated with an 89% higher risk of urinary system malformations (aOR, 1.89).
“There is currently a debate on a possible association between macrolide use and infantile pyloric stenosis,” the researchers wrote. “Though evidence suggested that late pregnancy and early infancy were the time windows of interest for this malformation, little attention has been paid to the first trimester of pregnancy.”
Phenoxymethylpenicillin exposure was associated with a 85% increased risk of nervous system malformations (aOR, 1.85), and erythromycin exposure doubled the risk of urinary system malformations (aOR, 2.12). Moxifloxacin exposure was associated with a fivefold increased risk of respiratory system malformations (aOR, 5.48), but the authors noted that there were just two exposed cases.
However, there was no increased risk for major congenital malformation seen with amoxicillin, cephalosporins, and nitrofurantoin.
Overall, 11% of pregnancies in the study recorded exposure to antibiotics during the first trimester, and 9.9% of the study population were diagnosed with a major congenital malformation in the first year of life.
“Though the absolute risks for specific birth defects was small, physicians should consider prescribing safer antibiotics for the treatment of maternal infections when possible until more data are available,” the researchers wrote.
The study was supported by the Réseau Québécois. One author reported being a consultant on litigation involving antidepressants and birth defects. No other conflicts of interest were declared.
FROM THE BRITISH JOURNAL OF CLINICAL PHARMACOLOGY
Key clinical point:
Major finding: Clindamycin exposure during the first trimester is associated with an 81% increased risk of ventricular/septal defect and 67% greater risk of musculoskeletal system malformations.
Data source: Population-based cohort study in 139,938 liveborn singletons.
Disclosures: The study was supported by the Réseau Québécois. One author reported being a consultant on litigation involving antidepressants and birth defects. No other conflicts of interest were declared.
For the management of labor, patience is a virtue
During the past 45 years, the cesarean delivery (CD) rate in the United States has increased from 5.5% in 1970 to 33% from 2009 to 2013, followed by a small decrease to 32% in 2014 and 2015.1 Many clinical problems cause clinicians and patients to decide that CD is an optimal birth route, including: abnormal labor progress, abnormal or indeterminate fetal heart rate pattern, breech presentation, multiple gestation, macrosomia, placental and cord abnormalities, preeclampsia, prior uterine surgery, and prior CD.2 Recent secular trends that contribute to the current rate of CD include an adversarial liability environment,3,4 increasing rates of maternal obesity,5 and widespread use of continuous fetal-heart monitoring during labor.6
Wide variation in CD rate has been reported among countries, states, and hospitals. The variation is due, in part, to different perspectives about balancing the harms and benefits of vaginal delivery versus CD. In Europe, in 2010 the CD rates in Sweden and Italy were 17.1% and 38%, respectively.7 In 2010, among the states, Alaska had the lowest rate of CD at 22% and Kentucky had the highest rate at 40%.8 In 2015, the highest rate was 38%, in Mississippi (FIGURE).9 In 2014, among Massachusetts hospitals with more than 2,500 births, the CD rate ranged from a low of 22% to a high of 37%.10
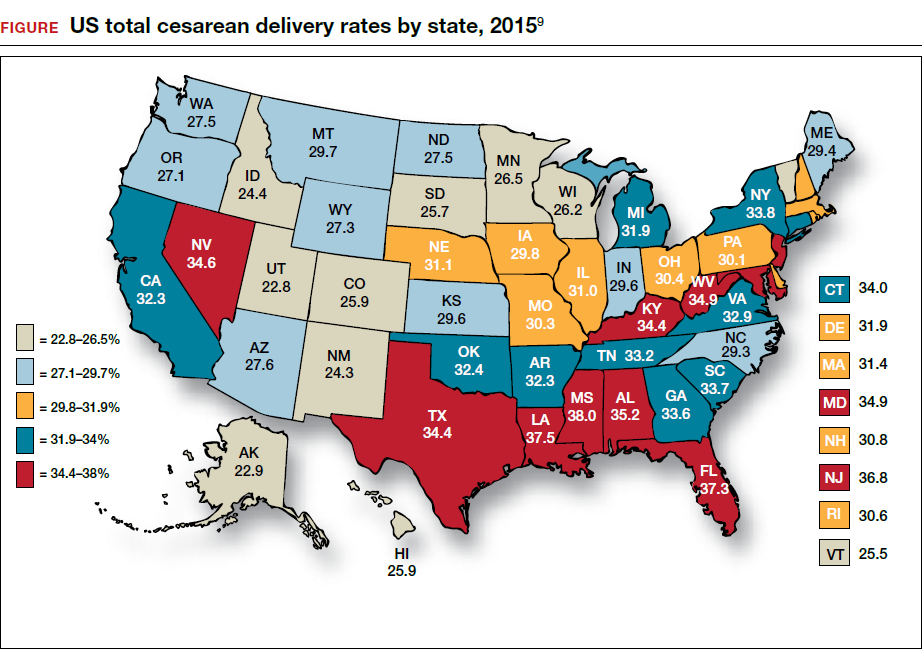
Clinicians, patients, policy experts, and the media are perplexed and troubled by the “high” US CD rate and the major variation in rate among countries, states, and hospitals. Labor management practices likely influence the rate of CD and diverse approaches to labor management likely account for the wide variation in CD rates.
A nationwide effort to standardize and continuously improve labor management might result in a decrease in the CD rate. Building on this opportunity, the American College of Obstetricians and Gynecologists (ACOG) and the Society of Maternal-Fetal Medicine (SMFM) have jointly recommended new labor management guidelines that may reduce the primary CD rate.8
The ACOG/SMFM guidelines encourage obstetricians to extend the time for labor progress in both the 1st and 2nd stages prior to recommending a CD.8 These new guidelines emphasize that for a modern obstetrician, patience is a virtue. There are 2 important caveats to this statement: to safely extend the length of time of labor requires both (1) a reassuring fetal heart rate tracing and (2) stable maternal health. If the fetus demonstrates a persistent worrisome Category II or a Category IIIheart-rate tracing, decisive intervention is necessary and permitting an extended labor would not be optimal. Similarly, if the mother has rapidly worsening preeclampsia it may not be wise to extend an induction of labor (IOL) over many days.
There are risks with extending the length of labor. An extended duration of the 1st stage of labor is associated with an increased rate of maternal chorioamnionitis and shoulder dystocia at birth.11 An extended duration of the 2nd stage of labor is associated with an increase in the rate of maternal chorioamnionitis, anal sphincter injury, uterine atony, and neonatal admission to an intensive care unit.12 Clinicians who adopt practices that permit an extended length of labor must weigh the benefits of avoiding a CD against these maternal and fetal complications.
Active phase redefined
Central to the ACOG/SMFM guidelines is a new definition of the active phase of labor. The research of Dr. Emmanuel Friedman indicated that at approximately 4 cm of cervical dilation many women in labor transition from the latent phase, a time of slow change in cervical dilation, to the active phase, a time of more rapid change in cervical dilation.13,14 However, more recent research indicates that the transition between the latent and active phase is difficult to precisely define, but more often occurs at about 6 cm of cervical dilation and not 4 cm of dilation.15 Adopting these new norms means that laboring women will spend much more time in the latent phase, a phase of labor in which patience is a virtue.
The ACOG/SMFM guidelines
Main takeaways from the ACOG/SMFM guidelines are summarized below. Interventions that address common obstetric issues and labor abnormalities are outlined below.
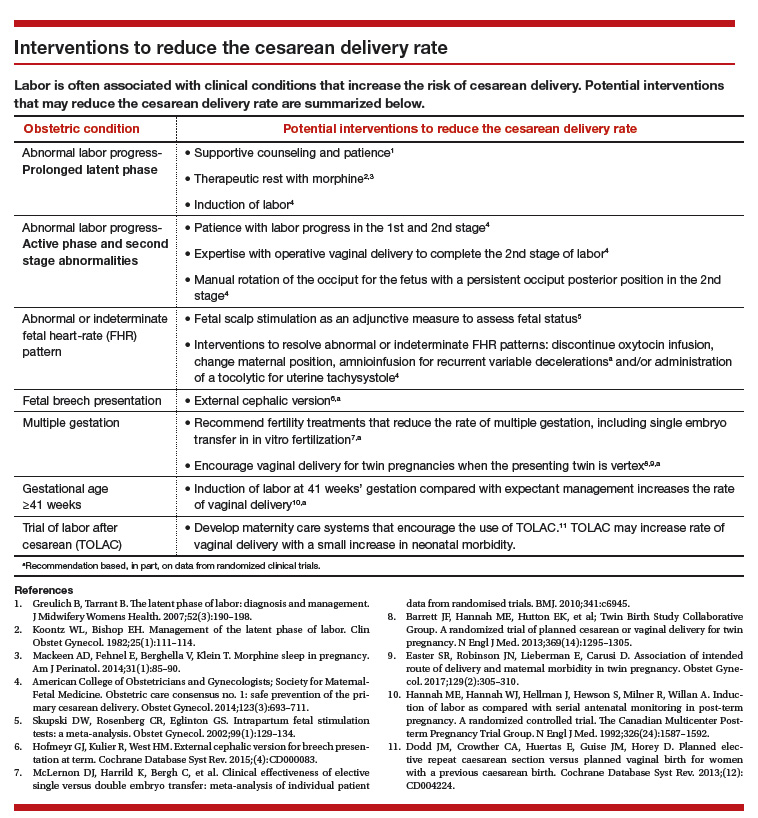
Do not perform CD for a prolonged latent phase of labor, defined as regular contractions of >20 hours duration in nulliparous women and >14 hours duration in multiparous women. Patience with a prolonged latent phase will be rewarded by the majority of women entering the active phase of labor. Alternatively, if appropriate, cervical ripening followed by oxytocin IOL and amniotomy will help the patient with a prolonged latent phase to enter the active phase of labor.16
For women with an unfavorable cervix as assessed by the Bishop score, cervical ripening should be performed prior to IOL. Use of cervical ripening prior to IOL increases the chance of achieving vaginal delivery within 24 hours and may result in a modest decrease in the rate of CD.17,18
Related article:
Should oxytocin and a Foley catheter be used concurrently for cervical ripening in induction of labor?
Failed IOL in the latent phase should only be diagnosed following 12 to 18 hours of both ruptured membranes and adequate contractions stimulated with oxytocin. The key ingredients for the successful management of the latent phase of labor are patience, oxytocin, and amniotomy.16
CD for the indication of active phase arrest requires cervical dilation ≥6 cm with ruptured membranes and no change in cervical dilation for ≥4 hours of adequate uterine activity. In the past, most obstetricians defined active phase arrest, a potential indication for CD, as the absence of cervical change for 2 or more hours in the presence of adequate uterine contractions and cervical dilation of at least 4 cm. Given the new definition of active phase arrest, slow but progressive progress in the 1st stage of labor is not an indication for CD.11,19
“A specific absolute maximum length of time spent in the 2nd stage beyond which all women should be offered an operative delivery has not been identified.”8 Diagnosis of arrest of labor in the 2nd stage may be considered after at least 2 hours of pushing in multiparous women and 3 hours of pushing in nulliparous women, especially if no fetal descent is occurring. The guidelines also state “longer durations may be appropriate on an individualized basis (eg, with use of epidural analgesia or with fetal malposition)” as long as fetal descent is observed.
Patience is a virtue, especially in the management of the 2nd stage of labor. Extending the 2nd stage up to 4 hours appears to be reasonably safe if the fetal status is reassuring and the mother is physiologically stable. In a study from San Francisco of 42,268 births with normal newborn outcomes, the 95th percentile for the length of the 2nd stage of labor for nulliparous women was 3.3 hours without an epidural and 5.6 hours with an epidural.20
In a study of 53,285 births, longer duration of pushing was associated with a small increase in the rate of neonatal adverse outcomes. In nulliparous women the rate of adverse neonatal outcomes increased from 1.3% with less than 60 minutes of pushing to 2.4% with greater than 240 minutes of pushing. Remarkably, even after 4 hours of pushing, 78% of nulliparous women who continued to push had a vaginal delivery.21 In this study, among nulliparous women the rate of anal sphincter injury increased from 5% with less than 60 minutes of pushing to 16% with greater than 240 minutes of pushing, and the rate of postpartum hemorrhage increased from 1% with less than 60 minutes of pushing to 3.3% with greater than 240 minutes of pushing.
I am not enthusiastic about patiently watching a labor extend into the 5th hour of the 2nd stage, especially if the fetus is at +2 station or lower. In a nulliparous woman, after 4 hours of managing the 2nd stage of labor, my patience is exhausted and I am inclined to identify a clear plan for delivery, either by enhanced labor coaching, operative vaginal delivery, or CD.
Operative vaginal delivery in the 2nd stage of labor is an acceptable alternative to CD. The rate of operative vaginal delivery in the United States has declined over the past 2 decades (TABLE). In Sweden in 2010 the operative vaginal delivery rate was 7.6% with a CD rate of 17.1%.7 In the United States in 2010 the operative delivery rate was 3.6%, and the CD rate was 33%.1 A renewed focus on operative vaginal delivery with ongoing training and team simulation for the procedure would increase our use of operative delivery and decrease the overall rate of CD.
Related article:
STOP using instruments to assist with delivery of the head at cesarean
Encourage the detection of persistent fetal occiput posterior position by physical examination and/or ultrasound and consider manual rotation of the fetal occiput from the posterior to anterior position in the 2nd stage. Persistent occiput posterior is the most common fetal malposition.22 This malposition is associated with an increased rate of CD.23 There are few randomized trials of manual rotation of the fetal occiput from posterior to anterior position in the 2nd stage of labor, and the evidence is insufficient to determine the efficacy of manual rotation.24 Small nonrandomized studies report that manual rotation of the occiput from posterior to anterior position may reduce the CD rate.25–27
For persistent 2nd stage fetal occiput posterior position in a woman with an adequate pelvis, where manual rotation was not successful and the fetus is at +2 station or below, operative vaginal delivery is an option. “Vacuum or forceps?” and “If forceps, to rotate or not to rotate?” those are the clinical questions. Forceps delivery is more likely to be successfulthan vacuum delivery.28 Direct forceps delivery of the occiput posterior fetus is associated with more anal sphincter injuries than forceps delivery after successful rotation, but few clinicians regularly perform rotational forceps.29 In a study of 2,351 women in the 2nd stage of labor with the fetus at +2 station or below, compared with either forceps or vacuum delivery, CD was associated with more maternal infections and fewer perineal lacerations. Neonatal composite morbidity was not significantly different among the 3 routes of operative delivery.30
Amnioinfusion for repetitive variable decelerations of the fetal heart rate may reduce the risk of CD for an indeterminate fetal heart-rate pattern.31
IOL in a well-dated pregnancy at 41 weeks will reduce the risk of CD. In a large clinical trial, 3,407 women at 41 weeks of gestation were randomly assigned to IOL or expectant management. The rate of CD was significantly lower in the women assigned to IOL compared with expectant management (21% vs 25%, respectively; P = .03).32 The rate of neonatal morbidity was similar in the 2 groups.
Women with twin gestations and the first twin in a cephalic presentation may elect vaginal delivery. In a large clinical trial, 1,398 women with a twin gestation and the first twin in a cephalic presentation were randomly assigned to planned vaginal delivery (with cesarean only if necessary) or planned CD.33 The rate of CD was 44% and 91% for the women in the planned-vaginal and planned-cesarean groups, respectively. There was no significant difference in composite fetal or neonatal death or serious morbidity. The authors concluded that, for twin pregnancy with the presenting twin in the cephalic presentation, there were no demonstrated benefits of planned CD.
Develop maternity care systems that encourage the use of trial of labor after cesarean (TOLAC). The ACOG/SMFM guidelines focus on interventions to reduce the rate of primary CD and do not address the role of TOLAC in reducing CD rates. There are little data from clinical trials to assess the benefits and harms from TOLAC versus scheduled repeat CD.34 However, our experience with TOLAC in the 1990s strongly suggests that encouraging TOLAC will decrease the rate of CD. In 1996 the US rate of vaginal birth after cesarean (VBAC) peaked at 28%, and the rate of CD achieved a recent historic nadir of 21%. Growing concerns that TOLAC occasionally results in fetal harm was followed by a decrease in the VBAC rate to 12% in 2015.1 A recent study of obstetric practices in countries with high and low VBAC rates concluded that patient and clinician commitment and comfort with prioritizing TOLAC over scheduled repeat CD greatly influenced the VBAC rate.35
Related article:
Should lower uterine segment thickness measurement be included in the TOLAC decision-making process?
Labor management is an art
During labor obstetricians must balance the unique needs of mother and fetus, which requires great clinical skill and patience. Evolving concepts of normal labor progress necessitate that we change our expectations concerning the acceptable rate of progress in the 1st and 2nd stage of labor. Consistent application of these new labor guidelines may help to reduce the rate of CD.
- Martin JA, Hamilton BE, Osterman MJ, Driscoll AK, Matthews TJ. Births: final data for 2015. Natl Vital Stat Rep. 2017;66(1):1–70. https://www.cdc.gov/nchs/data/nvsr/nvsr66/nvsr66_01.pdf. Accessed July 5, 2017.
- Barber EL, Lundsberg LS, Belanger K, Pettker CM, Funai EF, Illuzzi JL. Indications contributing to the increasing cesarean delivery rate. Obstet Gynecol. 2011;118(1):29–38.
- Localio AR, Lawthers AG, Bengtson JM, et al. Relationship between malpractice claims and cesarean delivery. JAMA. 1993;269(3):366–373.
- Cheng YW, Snowden JM, Handler SJ, Tager IB, Hubbard AE, Caughey AB. Litigation in obstetrics: does defensive medicine contribute to increases in cesarean delivery? J Matern Fetal Neonatal Med. 2014;27(16):1668–1675.
- Graham LE, Brunner Huber LR, Thompson ME, Ersek JL. Does amount of weight gain during pregnancy modify the association between obesity and cesarean section delivery? Birth. 2014;41(1):93–99.
- Alfirevic Z, Devane D, Gyte GM. Continuous cardiotocography (CTG) as a form of electronic fetal monitoring (EFM) for fetal assessment during labour. Cochrane Database Syst Rev. 2013;(5):CD006066.
- European Perinatal Health Report. Euro-Peristat website. http://www.europeristat.com/. Published 2012. Accessed July 5, 2017.
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. Obstetric care consensus no. 1: safe prevention of the primary cesarean delivery. Obstet Gynecol. 2014;123(3):693–711.
- Cesarean delivery rate by state, 2015. Centers for Disease Control and Prevention website. https://www.cdc.gov/nchs/pressroom/sosmap/cesarean_births/cesareans.htm. Updated January 9, 2017. Accessed July 18, 2017.
- Baker CD, Land T; Massachusetts Department of Public Health. Massachusetts Births 2014. Massachusetts Executive Office of Health and Human Services website. http://www.mass.gov/eohhs/gov/departments/dph/programs/admin/dmoa/repi/birth-data.html. Published September 2015. Accessed July 5, 2017.
- Henry DE, Cheng YW, Shaffer BL, Kaimal AJ, Bianco K, Caughey AB. Perinatal outcomes in the setting of active phase arrest of labor. Obstet Gynecol. 2008;112(5):1109–1115.
- Rouse DJ, Weiner SJ, Bloom SL, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Second-stage labor duration in nulliparous women: relationship to maternal and perinatal outcomes. Am J Obstet Gynecol. 2009;201(4):357.e1–e7.
- Friedman EZ. Labour: Clinical evaluation and management. Appleton-Century-Crofts: New York, NY; 1967.
- Friedman E. The graphic analysis of labor. Am J Obstet Gynecol. 1954;68(6):1568–1575.
- Zhang J, Landy HJ, Branch DW, et al; Consortium on Safe Labor. Contemporary patterns of spontaneous labor with normal neonatal outcomes. Obstet Gynecol. 2010;116(6):1281–1287.
- Wei S, Wo BL, Qi HP, et al. Early amniotomy and early oxytocin for prevention of, or therapy for, delay in first stage spontaneous labour compared with routine care. Cochrane Database Syst Rev. 2013;(8):CD006794.
- Thomas J, Fairclough A, Kavanagh J, Kelly AJ. Vaginal prostaglandin (PGE2 and PGF2a) for induction of labour at term. Cochrane Database Syst Rev. 2014;(6):CD003101.
- Alfirevic Z, Kelly AJ, Dowswell T. Intravenous oxytocin alone for cervical ripening and induction of labour. Cochrane Database Syst Rev. 2009;(4):CD003246.
- Rouse DJ, Owen J, Savage KG, Hauth JC. Active phase labor arrest: revisiting the 2-hour minimum. Obstet Gynecol. 2001;98(4):550–554.
- Cheng YW, Shaffer BL, Nicholson JM, Caughey AB. Second stage of labor and epidural use: a larger effect than previously suggested. Obstet Gynecol. 2014;123(3):527–535.
- Grobman WA, Bailit J, Lai Y, et al; Eunice Kennedy Shriver National Institute of Child and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network. Association of the duration of active pushing with obstetric outcomes. Obstet Gynecol. 2016;127(4):667–673.
- Barth WH Jr. Persistent occiput posterior. Obstet Gynecol. 2015;125(3):695–709.
- Carseldine WJ, Phipps H, Zawada SF, et al. Does occiput posterior position in the second stage of labour increase the operative delivery rate? Aust N Z J Obstet Gynaecol. 2013;53(3):265–270.
- Phipps H, de Vries B, Hyett J, Osborn DA. Prophylactic manual rotation for fetal malposition to reduce operative delivery. Cochrane Database Syst Rev. 2014;(12):CD009298.
- Shaffer BL, Cheng YW, Vargas JE, Caughey AB. Manual rotation to reduce caesarean delivery in persistent occiput posterior or transverse position. J Matern Fetal Neonatal Med. 2011;24(1):65–72.
- Le Ray C, Serres P, Schmitz T, Cabrol D, Goffinet F. Manual rotation in occiput posterior or transverse positions: risk factors and consequences on the cesarean delivery rate. Obstet Gynecol. 2007;110(4):873–879.
- Reichman O, Gdansky E, Latinsky B, Labi S, Samueloff A. Digital rotation from occipito-posterior to occipito-anterior decreases the need for cesarean section. Eur J Obstet Gynecol Repro Biol. 2008;136:25–28.
- O’Mahony F, Hofmeyr GJ, Menon V. Choice of instruments for assisted vaginal delivery. Cochrane Database Syst Rev. 2010;(11):CD005455.
- Hirsch E, Elue R, Wagner A Jr, et al. Severe perineal laceration during operative vaginal delivery: the impact of occiput posterior position. J Perinatol. 2014;34(12):898–900.
- Bailit JL, Grobman WA, Rice MM, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Evaluation of delivery options for second-stage events. Am J Obstet Gynecol. 2016;214(5):638.e1–e10.
- Hofmeyr GJ, Lawrie TA. Amnioinfusion for potential or suspected umbilical cord compression in labour. Cochrane Database Syst Rev. 2012;1:CD000013.
- Hannah ME, Hannah WJ, Hellmann J, Hewson S, Milner R, Willan A. Induction of labor as compared with serial antenatal monitoring in post-term pregnancy. A randomized controlled trial. The Canadian Multicenter Post-term Pregnancy Trial Group. N Engl J Med. 1992;326(24): 1587–1592.
- Barrett JF, Hannah ME, Hutton EK, et al; Twin Birth Study Collaborative Group. A randomized trial of planned cesarean or vaginal delivery for twin pregnancy. N Engl J Med. 2013;369(14):1295–1305.
- Dodd JM, Crowther CA, Huertas E, Guise JM, Horey D. Planned elective repeat cesarean section versus planned vaginal birth for women with a previous caesarean birth. Cochrane Database Syst Rev. 2013;(12):CD004224.
- Lundgren I, van Limbeek E, Vehvilainen-Julkunen K, Nilsson C. Clinicians’ views of factors of importance for improving the rate of VBAC (vaginal birth after caesarean section): a qualitative study from countries with high VBAC rates. BMC Pregnancy Childbirth. 2015;15:196.
During the past 45 years, the cesarean delivery (CD) rate in the United States has increased from 5.5% in 1970 to 33% from 2009 to 2013, followed by a small decrease to 32% in 2014 and 2015.1 Many clinical problems cause clinicians and patients to decide that CD is an optimal birth route, including: abnormal labor progress, abnormal or indeterminate fetal heart rate pattern, breech presentation, multiple gestation, macrosomia, placental and cord abnormalities, preeclampsia, prior uterine surgery, and prior CD.2 Recent secular trends that contribute to the current rate of CD include an adversarial liability environment,3,4 increasing rates of maternal obesity,5 and widespread use of continuous fetal-heart monitoring during labor.6
Wide variation in CD rate has been reported among countries, states, and hospitals. The variation is due, in part, to different perspectives about balancing the harms and benefits of vaginal delivery versus CD. In Europe, in 2010 the CD rates in Sweden and Italy were 17.1% and 38%, respectively.7 In 2010, among the states, Alaska had the lowest rate of CD at 22% and Kentucky had the highest rate at 40%.8 In 2015, the highest rate was 38%, in Mississippi (FIGURE).9 In 2014, among Massachusetts hospitals with more than 2,500 births, the CD rate ranged from a low of 22% to a high of 37%.10

Clinicians, patients, policy experts, and the media are perplexed and troubled by the “high” US CD rate and the major variation in rate among countries, states, and hospitals. Labor management practices likely influence the rate of CD and diverse approaches to labor management likely account for the wide variation in CD rates.
A nationwide effort to standardize and continuously improve labor management might result in a decrease in the CD rate. Building on this opportunity, the American College of Obstetricians and Gynecologists (ACOG) and the Society of Maternal-Fetal Medicine (SMFM) have jointly recommended new labor management guidelines that may reduce the primary CD rate.8
The ACOG/SMFM guidelines encourage obstetricians to extend the time for labor progress in both the 1st and 2nd stages prior to recommending a CD.8 These new guidelines emphasize that for a modern obstetrician, patience is a virtue. There are 2 important caveats to this statement: to safely extend the length of time of labor requires both (1) a reassuring fetal heart rate tracing and (2) stable maternal health. If the fetus demonstrates a persistent worrisome Category II or a Category IIIheart-rate tracing, decisive intervention is necessary and permitting an extended labor would not be optimal. Similarly, if the mother has rapidly worsening preeclampsia it may not be wise to extend an induction of labor (IOL) over many days.
There are risks with extending the length of labor. An extended duration of the 1st stage of labor is associated with an increased rate of maternal chorioamnionitis and shoulder dystocia at birth.11 An extended duration of the 2nd stage of labor is associated with an increase in the rate of maternal chorioamnionitis, anal sphincter injury, uterine atony, and neonatal admission to an intensive care unit.12 Clinicians who adopt practices that permit an extended length of labor must weigh the benefits of avoiding a CD against these maternal and fetal complications.
Active phase redefined
Central to the ACOG/SMFM guidelines is a new definition of the active phase of labor. The research of Dr. Emmanuel Friedman indicated that at approximately 4 cm of cervical dilation many women in labor transition from the latent phase, a time of slow change in cervical dilation, to the active phase, a time of more rapid change in cervical dilation.13,14 However, more recent research indicates that the transition between the latent and active phase is difficult to precisely define, but more often occurs at about 6 cm of cervical dilation and not 4 cm of dilation.15 Adopting these new norms means that laboring women will spend much more time in the latent phase, a phase of labor in which patience is a virtue.
The ACOG/SMFM guidelines
Main takeaways from the ACOG/SMFM guidelines are summarized below. Interventions that address common obstetric issues and labor abnormalities are outlined below.

Do not perform CD for a prolonged latent phase of labor, defined as regular contractions of >20 hours duration in nulliparous women and >14 hours duration in multiparous women. Patience with a prolonged latent phase will be rewarded by the majority of women entering the active phase of labor. Alternatively, if appropriate, cervical ripening followed by oxytocin IOL and amniotomy will help the patient with a prolonged latent phase to enter the active phase of labor.16
For women with an unfavorable cervix as assessed by the Bishop score, cervical ripening should be performed prior to IOL. Use of cervical ripening prior to IOL increases the chance of achieving vaginal delivery within 24 hours and may result in a modest decrease in the rate of CD.17,18
Related article:
Should oxytocin and a Foley catheter be used concurrently for cervical ripening in induction of labor?
Failed IOL in the latent phase should only be diagnosed following 12 to 18 hours of both ruptured membranes and adequate contractions stimulated with oxytocin. The key ingredients for the successful management of the latent phase of labor are patience, oxytocin, and amniotomy.16
CD for the indication of active phase arrest requires cervical dilation ≥6 cm with ruptured membranes and no change in cervical dilation for ≥4 hours of adequate uterine activity. In the past, most obstetricians defined active phase arrest, a potential indication for CD, as the absence of cervical change for 2 or more hours in the presence of adequate uterine contractions and cervical dilation of at least 4 cm. Given the new definition of active phase arrest, slow but progressive progress in the 1st stage of labor is not an indication for CD.11,19
“A specific absolute maximum length of time spent in the 2nd stage beyond which all women should be offered an operative delivery has not been identified.”8 Diagnosis of arrest of labor in the 2nd stage may be considered after at least 2 hours of pushing in multiparous women and 3 hours of pushing in nulliparous women, especially if no fetal descent is occurring. The guidelines also state “longer durations may be appropriate on an individualized basis (eg, with use of epidural analgesia or with fetal malposition)” as long as fetal descent is observed.
Patience is a virtue, especially in the management of the 2nd stage of labor. Extending the 2nd stage up to 4 hours appears to be reasonably safe if the fetal status is reassuring and the mother is physiologically stable. In a study from San Francisco of 42,268 births with normal newborn outcomes, the 95th percentile for the length of the 2nd stage of labor for nulliparous women was 3.3 hours without an epidural and 5.6 hours with an epidural.20
In a study of 53,285 births, longer duration of pushing was associated with a small increase in the rate of neonatal adverse outcomes. In nulliparous women the rate of adverse neonatal outcomes increased from 1.3% with less than 60 minutes of pushing to 2.4% with greater than 240 minutes of pushing. Remarkably, even after 4 hours of pushing, 78% of nulliparous women who continued to push had a vaginal delivery.21 In this study, among nulliparous women the rate of anal sphincter injury increased from 5% with less than 60 minutes of pushing to 16% with greater than 240 minutes of pushing, and the rate of postpartum hemorrhage increased from 1% with less than 60 minutes of pushing to 3.3% with greater than 240 minutes of pushing.
I am not enthusiastic about patiently watching a labor extend into the 5th hour of the 2nd stage, especially if the fetus is at +2 station or lower. In a nulliparous woman, after 4 hours of managing the 2nd stage of labor, my patience is exhausted and I am inclined to identify a clear plan for delivery, either by enhanced labor coaching, operative vaginal delivery, or CD.
Operative vaginal delivery in the 2nd stage of labor is an acceptable alternative to CD. The rate of operative vaginal delivery in the United States has declined over the past 2 decades (TABLE). In Sweden in 2010 the operative vaginal delivery rate was 7.6% with a CD rate of 17.1%.7 In the United States in 2010 the operative delivery rate was 3.6%, and the CD rate was 33%.1 A renewed focus on operative vaginal delivery with ongoing training and team simulation for the procedure would increase our use of operative delivery and decrease the overall rate of CD.
Related article:
STOP using instruments to assist with delivery of the head at cesarean
Encourage the detection of persistent fetal occiput posterior position by physical examination and/or ultrasound and consider manual rotation of the fetal occiput from the posterior to anterior position in the 2nd stage. Persistent occiput posterior is the most common fetal malposition.22 This malposition is associated with an increased rate of CD.23 There are few randomized trials of manual rotation of the fetal occiput from posterior to anterior position in the 2nd stage of labor, and the evidence is insufficient to determine the efficacy of manual rotation.24 Small nonrandomized studies report that manual rotation of the occiput from posterior to anterior position may reduce the CD rate.25–27
For persistent 2nd stage fetal occiput posterior position in a woman with an adequate pelvis, where manual rotation was not successful and the fetus is at +2 station or below, operative vaginal delivery is an option. “Vacuum or forceps?” and “If forceps, to rotate or not to rotate?” those are the clinical questions. Forceps delivery is more likely to be successfulthan vacuum delivery.28 Direct forceps delivery of the occiput posterior fetus is associated with more anal sphincter injuries than forceps delivery after successful rotation, but few clinicians regularly perform rotational forceps.29 In a study of 2,351 women in the 2nd stage of labor with the fetus at +2 station or below, compared with either forceps or vacuum delivery, CD was associated with more maternal infections and fewer perineal lacerations. Neonatal composite morbidity was not significantly different among the 3 routes of operative delivery.30
Amnioinfusion for repetitive variable decelerations of the fetal heart rate may reduce the risk of CD for an indeterminate fetal heart-rate pattern.31
IOL in a well-dated pregnancy at 41 weeks will reduce the risk of CD. In a large clinical trial, 3,407 women at 41 weeks of gestation were randomly assigned to IOL or expectant management. The rate of CD was significantly lower in the women assigned to IOL compared with expectant management (21% vs 25%, respectively; P = .03).32 The rate of neonatal morbidity was similar in the 2 groups.
Women with twin gestations and the first twin in a cephalic presentation may elect vaginal delivery. In a large clinical trial, 1,398 women with a twin gestation and the first twin in a cephalic presentation were randomly assigned to planned vaginal delivery (with cesarean only if necessary) or planned CD.33 The rate of CD was 44% and 91% for the women in the planned-vaginal and planned-cesarean groups, respectively. There was no significant difference in composite fetal or neonatal death or serious morbidity. The authors concluded that, for twin pregnancy with the presenting twin in the cephalic presentation, there were no demonstrated benefits of planned CD.
Develop maternity care systems that encourage the use of trial of labor after cesarean (TOLAC). The ACOG/SMFM guidelines focus on interventions to reduce the rate of primary CD and do not address the role of TOLAC in reducing CD rates. There are little data from clinical trials to assess the benefits and harms from TOLAC versus scheduled repeat CD.34 However, our experience with TOLAC in the 1990s strongly suggests that encouraging TOLAC will decrease the rate of CD. In 1996 the US rate of vaginal birth after cesarean (VBAC) peaked at 28%, and the rate of CD achieved a recent historic nadir of 21%. Growing concerns that TOLAC occasionally results in fetal harm was followed by a decrease in the VBAC rate to 12% in 2015.1 A recent study of obstetric practices in countries with high and low VBAC rates concluded that patient and clinician commitment and comfort with prioritizing TOLAC over scheduled repeat CD greatly influenced the VBAC rate.35
Related article:
Should lower uterine segment thickness measurement be included in the TOLAC decision-making process?
Labor management is an art
During labor obstetricians must balance the unique needs of mother and fetus, which requires great clinical skill and patience. Evolving concepts of normal labor progress necessitate that we change our expectations concerning the acceptable rate of progress in the 1st and 2nd stage of labor. Consistent application of these new labor guidelines may help to reduce the rate of CD.
During the past 45 years, the cesarean delivery (CD) rate in the United States has increased from 5.5% in 1970 to 33% from 2009 to 2013, followed by a small decrease to 32% in 2014 and 2015.1 Many clinical problems cause clinicians and patients to decide that CD is an optimal birth route, including: abnormal labor progress, abnormal or indeterminate fetal heart rate pattern, breech presentation, multiple gestation, macrosomia, placental and cord abnormalities, preeclampsia, prior uterine surgery, and prior CD.2 Recent secular trends that contribute to the current rate of CD include an adversarial liability environment,3,4 increasing rates of maternal obesity,5 and widespread use of continuous fetal-heart monitoring during labor.6
Wide variation in CD rate has been reported among countries, states, and hospitals. The variation is due, in part, to different perspectives about balancing the harms and benefits of vaginal delivery versus CD. In Europe, in 2010 the CD rates in Sweden and Italy were 17.1% and 38%, respectively.7 In 2010, among the states, Alaska had the lowest rate of CD at 22% and Kentucky had the highest rate at 40%.8 In 2015, the highest rate was 38%, in Mississippi (FIGURE).9 In 2014, among Massachusetts hospitals with more than 2,500 births, the CD rate ranged from a low of 22% to a high of 37%.10

Clinicians, patients, policy experts, and the media are perplexed and troubled by the “high” US CD rate and the major variation in rate among countries, states, and hospitals. Labor management practices likely influence the rate of CD and diverse approaches to labor management likely account for the wide variation in CD rates.
A nationwide effort to standardize and continuously improve labor management might result in a decrease in the CD rate. Building on this opportunity, the American College of Obstetricians and Gynecologists (ACOG) and the Society of Maternal-Fetal Medicine (SMFM) have jointly recommended new labor management guidelines that may reduce the primary CD rate.8
The ACOG/SMFM guidelines encourage obstetricians to extend the time for labor progress in both the 1st and 2nd stages prior to recommending a CD.8 These new guidelines emphasize that for a modern obstetrician, patience is a virtue. There are 2 important caveats to this statement: to safely extend the length of time of labor requires both (1) a reassuring fetal heart rate tracing and (2) stable maternal health. If the fetus demonstrates a persistent worrisome Category II or a Category IIIheart-rate tracing, decisive intervention is necessary and permitting an extended labor would not be optimal. Similarly, if the mother has rapidly worsening preeclampsia it may not be wise to extend an induction of labor (IOL) over many days.
There are risks with extending the length of labor. An extended duration of the 1st stage of labor is associated with an increased rate of maternal chorioamnionitis and shoulder dystocia at birth.11 An extended duration of the 2nd stage of labor is associated with an increase in the rate of maternal chorioamnionitis, anal sphincter injury, uterine atony, and neonatal admission to an intensive care unit.12 Clinicians who adopt practices that permit an extended length of labor must weigh the benefits of avoiding a CD against these maternal and fetal complications.
Active phase redefined
Central to the ACOG/SMFM guidelines is a new definition of the active phase of labor. The research of Dr. Emmanuel Friedman indicated that at approximately 4 cm of cervical dilation many women in labor transition from the latent phase, a time of slow change in cervical dilation, to the active phase, a time of more rapid change in cervical dilation.13,14 However, more recent research indicates that the transition between the latent and active phase is difficult to precisely define, but more often occurs at about 6 cm of cervical dilation and not 4 cm of dilation.15 Adopting these new norms means that laboring women will spend much more time in the latent phase, a phase of labor in which patience is a virtue.
The ACOG/SMFM guidelines
Main takeaways from the ACOG/SMFM guidelines are summarized below. Interventions that address common obstetric issues and labor abnormalities are outlined below.

Do not perform CD for a prolonged latent phase of labor, defined as regular contractions of >20 hours duration in nulliparous women and >14 hours duration in multiparous women. Patience with a prolonged latent phase will be rewarded by the majority of women entering the active phase of labor. Alternatively, if appropriate, cervical ripening followed by oxytocin IOL and amniotomy will help the patient with a prolonged latent phase to enter the active phase of labor.16
For women with an unfavorable cervix as assessed by the Bishop score, cervical ripening should be performed prior to IOL. Use of cervical ripening prior to IOL increases the chance of achieving vaginal delivery within 24 hours and may result in a modest decrease in the rate of CD.17,18
Related article:
Should oxytocin and a Foley catheter be used concurrently for cervical ripening in induction of labor?
Failed IOL in the latent phase should only be diagnosed following 12 to 18 hours of both ruptured membranes and adequate contractions stimulated with oxytocin. The key ingredients for the successful management of the latent phase of labor are patience, oxytocin, and amniotomy.16
CD for the indication of active phase arrest requires cervical dilation ≥6 cm with ruptured membranes and no change in cervical dilation for ≥4 hours of adequate uterine activity. In the past, most obstetricians defined active phase arrest, a potential indication for CD, as the absence of cervical change for 2 or more hours in the presence of adequate uterine contractions and cervical dilation of at least 4 cm. Given the new definition of active phase arrest, slow but progressive progress in the 1st stage of labor is not an indication for CD.11,19
“A specific absolute maximum length of time spent in the 2nd stage beyond which all women should be offered an operative delivery has not been identified.”8 Diagnosis of arrest of labor in the 2nd stage may be considered after at least 2 hours of pushing in multiparous women and 3 hours of pushing in nulliparous women, especially if no fetal descent is occurring. The guidelines also state “longer durations may be appropriate on an individualized basis (eg, with use of epidural analgesia or with fetal malposition)” as long as fetal descent is observed.
Patience is a virtue, especially in the management of the 2nd stage of labor. Extending the 2nd stage up to 4 hours appears to be reasonably safe if the fetal status is reassuring and the mother is physiologically stable. In a study from San Francisco of 42,268 births with normal newborn outcomes, the 95th percentile for the length of the 2nd stage of labor for nulliparous women was 3.3 hours without an epidural and 5.6 hours with an epidural.20
In a study of 53,285 births, longer duration of pushing was associated with a small increase in the rate of neonatal adverse outcomes. In nulliparous women the rate of adverse neonatal outcomes increased from 1.3% with less than 60 minutes of pushing to 2.4% with greater than 240 minutes of pushing. Remarkably, even after 4 hours of pushing, 78% of nulliparous women who continued to push had a vaginal delivery.21 In this study, among nulliparous women the rate of anal sphincter injury increased from 5% with less than 60 minutes of pushing to 16% with greater than 240 minutes of pushing, and the rate of postpartum hemorrhage increased from 1% with less than 60 minutes of pushing to 3.3% with greater than 240 minutes of pushing.
I am not enthusiastic about patiently watching a labor extend into the 5th hour of the 2nd stage, especially if the fetus is at +2 station or lower. In a nulliparous woman, after 4 hours of managing the 2nd stage of labor, my patience is exhausted and I am inclined to identify a clear plan for delivery, either by enhanced labor coaching, operative vaginal delivery, or CD.
Operative vaginal delivery in the 2nd stage of labor is an acceptable alternative to CD. The rate of operative vaginal delivery in the United States has declined over the past 2 decades (TABLE). In Sweden in 2010 the operative vaginal delivery rate was 7.6% with a CD rate of 17.1%.7 In the United States in 2010 the operative delivery rate was 3.6%, and the CD rate was 33%.1 A renewed focus on operative vaginal delivery with ongoing training and team simulation for the procedure would increase our use of operative delivery and decrease the overall rate of CD.
Related article:
STOP using instruments to assist with delivery of the head at cesarean
Encourage the detection of persistent fetal occiput posterior position by physical examination and/or ultrasound and consider manual rotation of the fetal occiput from the posterior to anterior position in the 2nd stage. Persistent occiput posterior is the most common fetal malposition.22 This malposition is associated with an increased rate of CD.23 There are few randomized trials of manual rotation of the fetal occiput from posterior to anterior position in the 2nd stage of labor, and the evidence is insufficient to determine the efficacy of manual rotation.24 Small nonrandomized studies report that manual rotation of the occiput from posterior to anterior position may reduce the CD rate.25–27
For persistent 2nd stage fetal occiput posterior position in a woman with an adequate pelvis, where manual rotation was not successful and the fetus is at +2 station or below, operative vaginal delivery is an option. “Vacuum or forceps?” and “If forceps, to rotate or not to rotate?” those are the clinical questions. Forceps delivery is more likely to be successfulthan vacuum delivery.28 Direct forceps delivery of the occiput posterior fetus is associated with more anal sphincter injuries than forceps delivery after successful rotation, but few clinicians regularly perform rotational forceps.29 In a study of 2,351 women in the 2nd stage of labor with the fetus at +2 station or below, compared with either forceps or vacuum delivery, CD was associated with more maternal infections and fewer perineal lacerations. Neonatal composite morbidity was not significantly different among the 3 routes of operative delivery.30
Amnioinfusion for repetitive variable decelerations of the fetal heart rate may reduce the risk of CD for an indeterminate fetal heart-rate pattern.31
IOL in a well-dated pregnancy at 41 weeks will reduce the risk of CD. In a large clinical trial, 3,407 women at 41 weeks of gestation were randomly assigned to IOL or expectant management. The rate of CD was significantly lower in the women assigned to IOL compared with expectant management (21% vs 25%, respectively; P = .03).32 The rate of neonatal morbidity was similar in the 2 groups.
Women with twin gestations and the first twin in a cephalic presentation may elect vaginal delivery. In a large clinical trial, 1,398 women with a twin gestation and the first twin in a cephalic presentation were randomly assigned to planned vaginal delivery (with cesarean only if necessary) or planned CD.33 The rate of CD was 44% and 91% for the women in the planned-vaginal and planned-cesarean groups, respectively. There was no significant difference in composite fetal or neonatal death or serious morbidity. The authors concluded that, for twin pregnancy with the presenting twin in the cephalic presentation, there were no demonstrated benefits of planned CD.
Develop maternity care systems that encourage the use of trial of labor after cesarean (TOLAC). The ACOG/SMFM guidelines focus on interventions to reduce the rate of primary CD and do not address the role of TOLAC in reducing CD rates. There are little data from clinical trials to assess the benefits and harms from TOLAC versus scheduled repeat CD.34 However, our experience with TOLAC in the 1990s strongly suggests that encouraging TOLAC will decrease the rate of CD. In 1996 the US rate of vaginal birth after cesarean (VBAC) peaked at 28%, and the rate of CD achieved a recent historic nadir of 21%. Growing concerns that TOLAC occasionally results in fetal harm was followed by a decrease in the VBAC rate to 12% in 2015.1 A recent study of obstetric practices in countries with high and low VBAC rates concluded that patient and clinician commitment and comfort with prioritizing TOLAC over scheduled repeat CD greatly influenced the VBAC rate.35
Related article:
Should lower uterine segment thickness measurement be included in the TOLAC decision-making process?
Labor management is an art
During labor obstetricians must balance the unique needs of mother and fetus, which requires great clinical skill and patience. Evolving concepts of normal labor progress necessitate that we change our expectations concerning the acceptable rate of progress in the 1st and 2nd stage of labor. Consistent application of these new labor guidelines may help to reduce the rate of CD.
- Martin JA, Hamilton BE, Osterman MJ, Driscoll AK, Matthews TJ. Births: final data for 2015. Natl Vital Stat Rep. 2017;66(1):1–70. https://www.cdc.gov/nchs/data/nvsr/nvsr66/nvsr66_01.pdf. Accessed July 5, 2017.
- Barber EL, Lundsberg LS, Belanger K, Pettker CM, Funai EF, Illuzzi JL. Indications contributing to the increasing cesarean delivery rate. Obstet Gynecol. 2011;118(1):29–38.
- Localio AR, Lawthers AG, Bengtson JM, et al. Relationship between malpractice claims and cesarean delivery. JAMA. 1993;269(3):366–373.
- Cheng YW, Snowden JM, Handler SJ, Tager IB, Hubbard AE, Caughey AB. Litigation in obstetrics: does defensive medicine contribute to increases in cesarean delivery? J Matern Fetal Neonatal Med. 2014;27(16):1668–1675.
- Graham LE, Brunner Huber LR, Thompson ME, Ersek JL. Does amount of weight gain during pregnancy modify the association between obesity and cesarean section delivery? Birth. 2014;41(1):93–99.
- Alfirevic Z, Devane D, Gyte GM. Continuous cardiotocography (CTG) as a form of electronic fetal monitoring (EFM) for fetal assessment during labour. Cochrane Database Syst Rev. 2013;(5):CD006066.
- European Perinatal Health Report. Euro-Peristat website. http://www.europeristat.com/. Published 2012. Accessed July 5, 2017.
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. Obstetric care consensus no. 1: safe prevention of the primary cesarean delivery. Obstet Gynecol. 2014;123(3):693–711.
- Cesarean delivery rate by state, 2015. Centers for Disease Control and Prevention website. https://www.cdc.gov/nchs/pressroom/sosmap/cesarean_births/cesareans.htm. Updated January 9, 2017. Accessed July 18, 2017.
- Baker CD, Land T; Massachusetts Department of Public Health. Massachusetts Births 2014. Massachusetts Executive Office of Health and Human Services website. http://www.mass.gov/eohhs/gov/departments/dph/programs/admin/dmoa/repi/birth-data.html. Published September 2015. Accessed July 5, 2017.
- Henry DE, Cheng YW, Shaffer BL, Kaimal AJ, Bianco K, Caughey AB. Perinatal outcomes in the setting of active phase arrest of labor. Obstet Gynecol. 2008;112(5):1109–1115.
- Rouse DJ, Weiner SJ, Bloom SL, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Second-stage labor duration in nulliparous women: relationship to maternal and perinatal outcomes. Am J Obstet Gynecol. 2009;201(4):357.e1–e7.
- Friedman EZ. Labour: Clinical evaluation and management. Appleton-Century-Crofts: New York, NY; 1967.
- Friedman E. The graphic analysis of labor. Am J Obstet Gynecol. 1954;68(6):1568–1575.
- Zhang J, Landy HJ, Branch DW, et al; Consortium on Safe Labor. Contemporary patterns of spontaneous labor with normal neonatal outcomes. Obstet Gynecol. 2010;116(6):1281–1287.
- Wei S, Wo BL, Qi HP, et al. Early amniotomy and early oxytocin for prevention of, or therapy for, delay in first stage spontaneous labour compared with routine care. Cochrane Database Syst Rev. 2013;(8):CD006794.
- Thomas J, Fairclough A, Kavanagh J, Kelly AJ. Vaginal prostaglandin (PGE2 and PGF2a) for induction of labour at term. Cochrane Database Syst Rev. 2014;(6):CD003101.
- Alfirevic Z, Kelly AJ, Dowswell T. Intravenous oxytocin alone for cervical ripening and induction of labour. Cochrane Database Syst Rev. 2009;(4):CD003246.
- Rouse DJ, Owen J, Savage KG, Hauth JC. Active phase labor arrest: revisiting the 2-hour minimum. Obstet Gynecol. 2001;98(4):550–554.
- Cheng YW, Shaffer BL, Nicholson JM, Caughey AB. Second stage of labor and epidural use: a larger effect than previously suggested. Obstet Gynecol. 2014;123(3):527–535.
- Grobman WA, Bailit J, Lai Y, et al; Eunice Kennedy Shriver National Institute of Child and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network. Association of the duration of active pushing with obstetric outcomes. Obstet Gynecol. 2016;127(4):667–673.
- Barth WH Jr. Persistent occiput posterior. Obstet Gynecol. 2015;125(3):695–709.
- Carseldine WJ, Phipps H, Zawada SF, et al. Does occiput posterior position in the second stage of labour increase the operative delivery rate? Aust N Z J Obstet Gynaecol. 2013;53(3):265–270.
- Phipps H, de Vries B, Hyett J, Osborn DA. Prophylactic manual rotation for fetal malposition to reduce operative delivery. Cochrane Database Syst Rev. 2014;(12):CD009298.
- Shaffer BL, Cheng YW, Vargas JE, Caughey AB. Manual rotation to reduce caesarean delivery in persistent occiput posterior or transverse position. J Matern Fetal Neonatal Med. 2011;24(1):65–72.
- Le Ray C, Serres P, Schmitz T, Cabrol D, Goffinet F. Manual rotation in occiput posterior or transverse positions: risk factors and consequences on the cesarean delivery rate. Obstet Gynecol. 2007;110(4):873–879.
- Reichman O, Gdansky E, Latinsky B, Labi S, Samueloff A. Digital rotation from occipito-posterior to occipito-anterior decreases the need for cesarean section. Eur J Obstet Gynecol Repro Biol. 2008;136:25–28.
- O’Mahony F, Hofmeyr GJ, Menon V. Choice of instruments for assisted vaginal delivery. Cochrane Database Syst Rev. 2010;(11):CD005455.
- Hirsch E, Elue R, Wagner A Jr, et al. Severe perineal laceration during operative vaginal delivery: the impact of occiput posterior position. J Perinatol. 2014;34(12):898–900.
- Bailit JL, Grobman WA, Rice MM, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Evaluation of delivery options for second-stage events. Am J Obstet Gynecol. 2016;214(5):638.e1–e10.
- Hofmeyr GJ, Lawrie TA. Amnioinfusion for potential or suspected umbilical cord compression in labour. Cochrane Database Syst Rev. 2012;1:CD000013.
- Hannah ME, Hannah WJ, Hellmann J, Hewson S, Milner R, Willan A. Induction of labor as compared with serial antenatal monitoring in post-term pregnancy. A randomized controlled trial. The Canadian Multicenter Post-term Pregnancy Trial Group. N Engl J Med. 1992;326(24): 1587–1592.
- Barrett JF, Hannah ME, Hutton EK, et al; Twin Birth Study Collaborative Group. A randomized trial of planned cesarean or vaginal delivery for twin pregnancy. N Engl J Med. 2013;369(14):1295–1305.
- Dodd JM, Crowther CA, Huertas E, Guise JM, Horey D. Planned elective repeat cesarean section versus planned vaginal birth for women with a previous caesarean birth. Cochrane Database Syst Rev. 2013;(12):CD004224.
- Lundgren I, van Limbeek E, Vehvilainen-Julkunen K, Nilsson C. Clinicians’ views of factors of importance for improving the rate of VBAC (vaginal birth after caesarean section): a qualitative study from countries with high VBAC rates. BMC Pregnancy Childbirth. 2015;15:196.
- Martin JA, Hamilton BE, Osterman MJ, Driscoll AK, Matthews TJ. Births: final data for 2015. Natl Vital Stat Rep. 2017;66(1):1–70. https://www.cdc.gov/nchs/data/nvsr/nvsr66/nvsr66_01.pdf. Accessed July 5, 2017.
- Barber EL, Lundsberg LS, Belanger K, Pettker CM, Funai EF, Illuzzi JL. Indications contributing to the increasing cesarean delivery rate. Obstet Gynecol. 2011;118(1):29–38.
- Localio AR, Lawthers AG, Bengtson JM, et al. Relationship between malpractice claims and cesarean delivery. JAMA. 1993;269(3):366–373.
- Cheng YW, Snowden JM, Handler SJ, Tager IB, Hubbard AE, Caughey AB. Litigation in obstetrics: does defensive medicine contribute to increases in cesarean delivery? J Matern Fetal Neonatal Med. 2014;27(16):1668–1675.
- Graham LE, Brunner Huber LR, Thompson ME, Ersek JL. Does amount of weight gain during pregnancy modify the association between obesity and cesarean section delivery? Birth. 2014;41(1):93–99.
- Alfirevic Z, Devane D, Gyte GM. Continuous cardiotocography (CTG) as a form of electronic fetal monitoring (EFM) for fetal assessment during labour. Cochrane Database Syst Rev. 2013;(5):CD006066.
- European Perinatal Health Report. Euro-Peristat website. http://www.europeristat.com/. Published 2012. Accessed July 5, 2017.
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. Obstetric care consensus no. 1: safe prevention of the primary cesarean delivery. Obstet Gynecol. 2014;123(3):693–711.
- Cesarean delivery rate by state, 2015. Centers for Disease Control and Prevention website. https://www.cdc.gov/nchs/pressroom/sosmap/cesarean_births/cesareans.htm. Updated January 9, 2017. Accessed July 18, 2017.
- Baker CD, Land T; Massachusetts Department of Public Health. Massachusetts Births 2014. Massachusetts Executive Office of Health and Human Services website. http://www.mass.gov/eohhs/gov/departments/dph/programs/admin/dmoa/repi/birth-data.html. Published September 2015. Accessed July 5, 2017.
- Henry DE, Cheng YW, Shaffer BL, Kaimal AJ, Bianco K, Caughey AB. Perinatal outcomes in the setting of active phase arrest of labor. Obstet Gynecol. 2008;112(5):1109–1115.
- Rouse DJ, Weiner SJ, Bloom SL, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Second-stage labor duration in nulliparous women: relationship to maternal and perinatal outcomes. Am J Obstet Gynecol. 2009;201(4):357.e1–e7.
- Friedman EZ. Labour: Clinical evaluation and management. Appleton-Century-Crofts: New York, NY; 1967.
- Friedman E. The graphic analysis of labor. Am J Obstet Gynecol. 1954;68(6):1568–1575.
- Zhang J, Landy HJ, Branch DW, et al; Consortium on Safe Labor. Contemporary patterns of spontaneous labor with normal neonatal outcomes. Obstet Gynecol. 2010;116(6):1281–1287.
- Wei S, Wo BL, Qi HP, et al. Early amniotomy and early oxytocin for prevention of, or therapy for, delay in first stage spontaneous labour compared with routine care. Cochrane Database Syst Rev. 2013;(8):CD006794.
- Thomas J, Fairclough A, Kavanagh J, Kelly AJ. Vaginal prostaglandin (PGE2 and PGF2a) for induction of labour at term. Cochrane Database Syst Rev. 2014;(6):CD003101.
- Alfirevic Z, Kelly AJ, Dowswell T. Intravenous oxytocin alone for cervical ripening and induction of labour. Cochrane Database Syst Rev. 2009;(4):CD003246.
- Rouse DJ, Owen J, Savage KG, Hauth JC. Active phase labor arrest: revisiting the 2-hour minimum. Obstet Gynecol. 2001;98(4):550–554.
- Cheng YW, Shaffer BL, Nicholson JM, Caughey AB. Second stage of labor and epidural use: a larger effect than previously suggested. Obstet Gynecol. 2014;123(3):527–535.
- Grobman WA, Bailit J, Lai Y, et al; Eunice Kennedy Shriver National Institute of Child and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network. Association of the duration of active pushing with obstetric outcomes. Obstet Gynecol. 2016;127(4):667–673.
- Barth WH Jr. Persistent occiput posterior. Obstet Gynecol. 2015;125(3):695–709.
- Carseldine WJ, Phipps H, Zawada SF, et al. Does occiput posterior position in the second stage of labour increase the operative delivery rate? Aust N Z J Obstet Gynaecol. 2013;53(3):265–270.
- Phipps H, de Vries B, Hyett J, Osborn DA. Prophylactic manual rotation for fetal malposition to reduce operative delivery. Cochrane Database Syst Rev. 2014;(12):CD009298.
- Shaffer BL, Cheng YW, Vargas JE, Caughey AB. Manual rotation to reduce caesarean delivery in persistent occiput posterior or transverse position. J Matern Fetal Neonatal Med. 2011;24(1):65–72.
- Le Ray C, Serres P, Schmitz T, Cabrol D, Goffinet F. Manual rotation in occiput posterior or transverse positions: risk factors and consequences on the cesarean delivery rate. Obstet Gynecol. 2007;110(4):873–879.
- Reichman O, Gdansky E, Latinsky B, Labi S, Samueloff A. Digital rotation from occipito-posterior to occipito-anterior decreases the need for cesarean section. Eur J Obstet Gynecol Repro Biol. 2008;136:25–28.
- O’Mahony F, Hofmeyr GJ, Menon V. Choice of instruments for assisted vaginal delivery. Cochrane Database Syst Rev. 2010;(11):CD005455.
- Hirsch E, Elue R, Wagner A Jr, et al. Severe perineal laceration during operative vaginal delivery: the impact of occiput posterior position. J Perinatol. 2014;34(12):898–900.
- Bailit JL, Grobman WA, Rice MM, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Evaluation of delivery options for second-stage events. Am J Obstet Gynecol. 2016;214(5):638.e1–e10.
- Hofmeyr GJ, Lawrie TA. Amnioinfusion for potential or suspected umbilical cord compression in labour. Cochrane Database Syst Rev. 2012;1:CD000013.
- Hannah ME, Hannah WJ, Hellmann J, Hewson S, Milner R, Willan A. Induction of labor as compared with serial antenatal monitoring in post-term pregnancy. A randomized controlled trial. The Canadian Multicenter Post-term Pregnancy Trial Group. N Engl J Med. 1992;326(24): 1587–1592.
- Barrett JF, Hannah ME, Hutton EK, et al; Twin Birth Study Collaborative Group. A randomized trial of planned cesarean or vaginal delivery for twin pregnancy. N Engl J Med. 2013;369(14):1295–1305.
- Dodd JM, Crowther CA, Huertas E, Guise JM, Horey D. Planned elective repeat cesarean section versus planned vaginal birth for women with a previous caesarean birth. Cochrane Database Syst Rev. 2013;(12):CD004224.
- Lundgren I, van Limbeek E, Vehvilainen-Julkunen K, Nilsson C. Clinicians’ views of factors of importance for improving the rate of VBAC (vaginal birth after caesarean section): a qualitative study from countries with high VBAC rates. BMC Pregnancy Childbirth. 2015;15:196.
Product Update: Hologic Aptima HSV Assay, Cianna SAVI SCOUT, Olympus Hystero-Resectoscope, and Clarius Ultrasound Scanners
DIAGNOSE HERPES SIMPLEX VIRUS
According to the CDC, infections with HSV-2 affect more than 24 million Americans. Patients with HSV-2 strain are at increased risk for contracting and transmitting HIV. Pregnant women infected with HSV-2 are at risk of transmitting the virus to their babies, with increased risk for neurologic complications in the child.
FOR MORE INFORMATION, VISIT: http://www.hologic.com/search/site/aptima%20hsv
PRECISELY TARGET TISSUE DURING LUMPECTOMY OR BIOPSY
Cianna Medical reported recent data showing that, when compared with wire localization, the SCOUT reduces breast surgery operating room (OR) delay times by 72.5%, resulted in an average 29- minute reduction in OR waiting time, and significantly improved workflow efficiency.
FOR MORE INFORMATION, VISIT: https://www.ciannamedical.com/savi-scout/
PLASMA HYSTEROSCOPIC RESECTION
During gynecologic procedures, the Olympus 8.5-mm hystero-resectoscope uses a combination of radio frequency, energy, and saline to create plasma, an electrically conductive gas cloud of vapor and charged particles. Due to its conductivity, plasma allows energy to cross into targeted tissue at lower energy levels than with more traditional approaches. This effect leads to lower operating temperatures and therefore less thermal spread.
FOR MORE INFORMATION, VISIT: http://olympusmedical.com.sg
APP-BASED HANDHELD ULTRASOUND
High-resolution images can be saved, reviewed, and managed on the secure Clarius Cloud. Built with a durable magnesium shell, each device has an IPX7 immersion rating so it can be sterilized. Power is obtained from a rechargeable battery that will last for more than 45 minutes of scanning; 2 batteries come with each Clarius device.
FOR MORE INFORMATION, VISIT: https://www.clarius.me/
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
DIAGNOSE HERPES SIMPLEX VIRUS
According to the CDC, infections with HSV-2 affect more than 24 million Americans. Patients with HSV-2 strain are at increased risk for contracting and transmitting HIV. Pregnant women infected with HSV-2 are at risk of transmitting the virus to their babies, with increased risk for neurologic complications in the child.
FOR MORE INFORMATION, VISIT: http://www.hologic.com/search/site/aptima%20hsv
PRECISELY TARGET TISSUE DURING LUMPECTOMY OR BIOPSY
Cianna Medical reported recent data showing that, when compared with wire localization, the SCOUT reduces breast surgery operating room (OR) delay times by 72.5%, resulted in an average 29- minute reduction in OR waiting time, and significantly improved workflow efficiency.
FOR MORE INFORMATION, VISIT: https://www.ciannamedical.com/savi-scout/
PLASMA HYSTEROSCOPIC RESECTION
During gynecologic procedures, the Olympus 8.5-mm hystero-resectoscope uses a combination of radio frequency, energy, and saline to create plasma, an electrically conductive gas cloud of vapor and charged particles. Due to its conductivity, plasma allows energy to cross into targeted tissue at lower energy levels than with more traditional approaches. This effect leads to lower operating temperatures and therefore less thermal spread.
FOR MORE INFORMATION, VISIT: http://olympusmedical.com.sg
APP-BASED HANDHELD ULTRASOUND
High-resolution images can be saved, reviewed, and managed on the secure Clarius Cloud. Built with a durable magnesium shell, each device has an IPX7 immersion rating so it can be sterilized. Power is obtained from a rechargeable battery that will last for more than 45 minutes of scanning; 2 batteries come with each Clarius device.
FOR MORE INFORMATION, VISIT: https://www.clarius.me/
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
DIAGNOSE HERPES SIMPLEX VIRUS
According to the CDC, infections with HSV-2 affect more than 24 million Americans. Patients with HSV-2 strain are at increased risk for contracting and transmitting HIV. Pregnant women infected with HSV-2 are at risk of transmitting the virus to their babies, with increased risk for neurologic complications in the child.
FOR MORE INFORMATION, VISIT: http://www.hologic.com/search/site/aptima%20hsv
PRECISELY TARGET TISSUE DURING LUMPECTOMY OR BIOPSY
Cianna Medical reported recent data showing that, when compared with wire localization, the SCOUT reduces breast surgery operating room (OR) delay times by 72.5%, resulted in an average 29- minute reduction in OR waiting time, and significantly improved workflow efficiency.
FOR MORE INFORMATION, VISIT: https://www.ciannamedical.com/savi-scout/
PLASMA HYSTEROSCOPIC RESECTION
During gynecologic procedures, the Olympus 8.5-mm hystero-resectoscope uses a combination of radio frequency, energy, and saline to create plasma, an electrically conductive gas cloud of vapor and charged particles. Due to its conductivity, plasma allows energy to cross into targeted tissue at lower energy levels than with more traditional approaches. This effect leads to lower operating temperatures and therefore less thermal spread.
FOR MORE INFORMATION, VISIT: http://olympusmedical.com.sg
APP-BASED HANDHELD ULTRASOUND
High-resolution images can be saved, reviewed, and managed on the secure Clarius Cloud. Built with a durable magnesium shell, each device has an IPX7 immersion rating so it can be sterilized. Power is obtained from a rechargeable battery that will last for more than 45 minutes of scanning; 2 batteries come with each Clarius device.
FOR MORE INFORMATION, VISIT: https://www.clarius.me/
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
CDC refocuses Zika testing recommendations in pregnancy
, including those who may have been exposed before pregnancy through travel or sexual contact.
In updated guidance released July 24, the Centers for Disease Control and Prevention cited a combination of factors behind the change in recommendations, including the declining prevalence of Zika virus across the Americas and a high likelihood of false positives associated with the use of a common serologic assay (MMWR Morb Mortal Wkly Rep. ePub 2017 Jul 24. doi: 10.15585/mmwr.mm6629e1).
Positive IgM results can also occur after previous exposure to other flaviviruses besides Zika, Dr. Oduyebo and her colleagues noted.
The CDC now recommends that pregnant women with likely continuing – not previous – exposure to the Zika virus and those with symptoms suggestive of Zika virus disease be tested. Those higher-risk groups should receive nucleic acid testing (NAT).
The new guidance presents two updated testing algorithms, one for each group.
Any pregnant woman with symptoms suggestive of Zika should be tested “as soon as possible through 12 weeks after symptom onset,” the CDC said, with both NAT (serum and urine) and IgM serology testing.
Women with likely ongoing exposure to Zika – such as those living in or traveling to an area of mosquito-borne Zika transmission or those whose partners are living in or traveling to such an area – should be tested up to three times during the pregnancy using NAT serum and urine tests. IgM testing is not recommended for that group.
All pregnant women should be asked about their potential Zika exposures before and during the current pregnancy, the CDC said. That discussion, which covers potential travel and partner exposures along with questions about symptoms, should be repeated at every prenatal visit.
While routine testing of asymptomatic women without ongoing exposure is not recommended, patient preferences, clinical judgment, and a “balanced assessment of risks and expected outcomes” should guide decisions about testing, according to the CDC.
, including those who may have been exposed before pregnancy through travel or sexual contact.
In updated guidance released July 24, the Centers for Disease Control and Prevention cited a combination of factors behind the change in recommendations, including the declining prevalence of Zika virus across the Americas and a high likelihood of false positives associated with the use of a common serologic assay (MMWR Morb Mortal Wkly Rep. ePub 2017 Jul 24. doi: 10.15585/mmwr.mm6629e1).
Positive IgM results can also occur after previous exposure to other flaviviruses besides Zika, Dr. Oduyebo and her colleagues noted.
The CDC now recommends that pregnant women with likely continuing – not previous – exposure to the Zika virus and those with symptoms suggestive of Zika virus disease be tested. Those higher-risk groups should receive nucleic acid testing (NAT).
The new guidance presents two updated testing algorithms, one for each group.
Any pregnant woman with symptoms suggestive of Zika should be tested “as soon as possible through 12 weeks after symptom onset,” the CDC said, with both NAT (serum and urine) and IgM serology testing.
Women with likely ongoing exposure to Zika – such as those living in or traveling to an area of mosquito-borne Zika transmission or those whose partners are living in or traveling to such an area – should be tested up to three times during the pregnancy using NAT serum and urine tests. IgM testing is not recommended for that group.
All pregnant women should be asked about their potential Zika exposures before and during the current pregnancy, the CDC said. That discussion, which covers potential travel and partner exposures along with questions about symptoms, should be repeated at every prenatal visit.
While routine testing of asymptomatic women without ongoing exposure is not recommended, patient preferences, clinical judgment, and a “balanced assessment of risks and expected outcomes” should guide decisions about testing, according to the CDC.
, including those who may have been exposed before pregnancy through travel or sexual contact.
In updated guidance released July 24, the Centers for Disease Control and Prevention cited a combination of factors behind the change in recommendations, including the declining prevalence of Zika virus across the Americas and a high likelihood of false positives associated with the use of a common serologic assay (MMWR Morb Mortal Wkly Rep. ePub 2017 Jul 24. doi: 10.15585/mmwr.mm6629e1).
Positive IgM results can also occur after previous exposure to other flaviviruses besides Zika, Dr. Oduyebo and her colleagues noted.
The CDC now recommends that pregnant women with likely continuing – not previous – exposure to the Zika virus and those with symptoms suggestive of Zika virus disease be tested. Those higher-risk groups should receive nucleic acid testing (NAT).
The new guidance presents two updated testing algorithms, one for each group.
Any pregnant woman with symptoms suggestive of Zika should be tested “as soon as possible through 12 weeks after symptom onset,” the CDC said, with both NAT (serum and urine) and IgM serology testing.
Women with likely ongoing exposure to Zika – such as those living in or traveling to an area of mosquito-borne Zika transmission or those whose partners are living in or traveling to such an area – should be tested up to three times during the pregnancy using NAT serum and urine tests. IgM testing is not recommended for that group.
All pregnant women should be asked about their potential Zika exposures before and during the current pregnancy, the CDC said. That discussion, which covers potential travel and partner exposures along with questions about symptoms, should be repeated at every prenatal visit.
While routine testing of asymptomatic women without ongoing exposure is not recommended, patient preferences, clinical judgment, and a “balanced assessment of risks and expected outcomes” should guide decisions about testing, according to the CDC.
FROM MMWR