User login
Tdap during pregnancy: No link to adverse outcomes
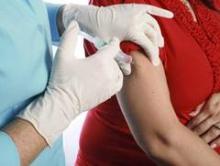
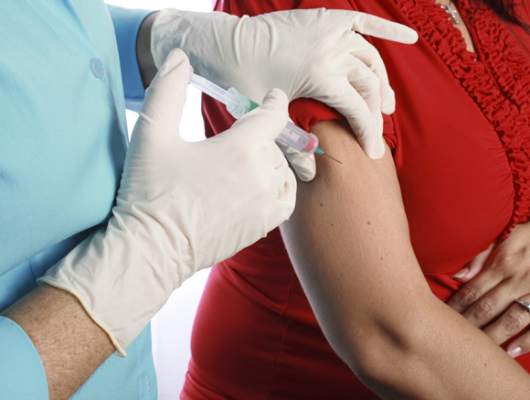
Receiving the Tdap vaccine during pregnancy did not raise the risk of maternal hypertensive disorders, preterm birth, or small-for-gestational age infants in an observational study of 123,494 California pregnancies, according to a report published online November 11 in JAMA.
In response to recent outbreaks of pertussis, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices recommends that the Tdap vaccine be given to all pregnant women, preferably at 27-36 weeks’ gestation. However, specific data regarding possible adverse effects on mothers or children are “limited,” said Dr. Elyse O. Kharbanda of HealthPartners Institute for Education and Research, Minneapolis, and her associates.
To examine the issue, the investigators analyzed information from the Vaccine Safety Datalink regarding singleton pregnancies resulting in a live birth at two California sites during a 3-year period. A total of 26,229 (21%) of mothers received the Tdap vaccine during pregnancy, and the remaining 97,265 did not, although 46% of them had received Tdap prior to pregnancy.
The rates of preterm birth were 6.3% in vaccine-exposed pregnancies and 7.8% in nonexposed pregnancies, a nonsignificant difference. Similarly, the rates of SGA infants were nearly identical between the two study groups at 8.4% and 8.3%, respectively. And the rates of maternal hypertensive disorders – including gestational hypertension, hypertension in pregnancy not otherwise specified, preeclampsia, and eclampsia – were not significantly different at 8.2% and 8.0%, respectively, Dr. Kharbanda and her associates said (JAMA 2014 [doi:10.1001/jama.2014.14825]).
“We detected an increased risk of being diagnosed with chorioamnionitis following vaccination,” but that finding should be interpreted with caution because the magnitude of the risk was small, and it didn’t translate into increased risk of preterm delivery. This weak association may have been due to residual confounding, especially since the data could not be adjusted to account for important chorioamnionitis risk factors such as prolonged rupture of membranes, prolonged labor, or the presence of pathogens in the mother’s genital tract, the investigators noted.
This study was funded by the Centers for Disease Control and Prevention. Dr. Kharbanda reported having no financial disclosures; some of her associates reported ties to GlaxoSmithKline, Sanofi Pasteur, Pfizer, Merck, Novartis, Nuron Biotech, Protein Science, and MedImmune. Two associates are CDC employees.
Receiving the Tdap vaccine during pregnancy did not raise the risk of maternal hypertensive disorders, preterm birth, or small-for-gestational age infants in an observational study of 123,494 California pregnancies, according to a report published online November 11 in JAMA.
In response to recent outbreaks of pertussis, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices recommends that the Tdap vaccine be given to all pregnant women, preferably at 27-36 weeks’ gestation. However, specific data regarding possible adverse effects on mothers or children are “limited,” said Dr. Elyse O. Kharbanda of HealthPartners Institute for Education and Research, Minneapolis, and her associates.
To examine the issue, the investigators analyzed information from the Vaccine Safety Datalink regarding singleton pregnancies resulting in a live birth at two California sites during a 3-year period. A total of 26,229 (21%) of mothers received the Tdap vaccine during pregnancy, and the remaining 97,265 did not, although 46% of them had received Tdap prior to pregnancy.
The rates of preterm birth were 6.3% in vaccine-exposed pregnancies and 7.8% in nonexposed pregnancies, a nonsignificant difference. Similarly, the rates of SGA infants were nearly identical between the two study groups at 8.4% and 8.3%, respectively. And the rates of maternal hypertensive disorders – including gestational hypertension, hypertension in pregnancy not otherwise specified, preeclampsia, and eclampsia – were not significantly different at 8.2% and 8.0%, respectively, Dr. Kharbanda and her associates said (JAMA 2014 [doi:10.1001/jama.2014.14825]).
“We detected an increased risk of being diagnosed with chorioamnionitis following vaccination,” but that finding should be interpreted with caution because the magnitude of the risk was small, and it didn’t translate into increased risk of preterm delivery. This weak association may have been due to residual confounding, especially since the data could not be adjusted to account for important chorioamnionitis risk factors such as prolonged rupture of membranes, prolonged labor, or the presence of pathogens in the mother’s genital tract, the investigators noted.
This study was funded by the Centers for Disease Control and Prevention. Dr. Kharbanda reported having no financial disclosures; some of her associates reported ties to GlaxoSmithKline, Sanofi Pasteur, Pfizer, Merck, Novartis, Nuron Biotech, Protein Science, and MedImmune. Two associates are CDC employees.
Receiving the Tdap vaccine during pregnancy did not raise the risk of maternal hypertensive disorders, preterm birth, or small-for-gestational age infants in an observational study of 123,494 California pregnancies, according to a report published online November 11 in JAMA.
In response to recent outbreaks of pertussis, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices recommends that the Tdap vaccine be given to all pregnant women, preferably at 27-36 weeks’ gestation. However, specific data regarding possible adverse effects on mothers or children are “limited,” said Dr. Elyse O. Kharbanda of HealthPartners Institute for Education and Research, Minneapolis, and her associates.
To examine the issue, the investigators analyzed information from the Vaccine Safety Datalink regarding singleton pregnancies resulting in a live birth at two California sites during a 3-year period. A total of 26,229 (21%) of mothers received the Tdap vaccine during pregnancy, and the remaining 97,265 did not, although 46% of them had received Tdap prior to pregnancy.
The rates of preterm birth were 6.3% in vaccine-exposed pregnancies and 7.8% in nonexposed pregnancies, a nonsignificant difference. Similarly, the rates of SGA infants were nearly identical between the two study groups at 8.4% and 8.3%, respectively. And the rates of maternal hypertensive disorders – including gestational hypertension, hypertension in pregnancy not otherwise specified, preeclampsia, and eclampsia – were not significantly different at 8.2% and 8.0%, respectively, Dr. Kharbanda and her associates said (JAMA 2014 [doi:10.1001/jama.2014.14825]).
“We detected an increased risk of being diagnosed with chorioamnionitis following vaccination,” but that finding should be interpreted with caution because the magnitude of the risk was small, and it didn’t translate into increased risk of preterm delivery. This weak association may have been due to residual confounding, especially since the data could not be adjusted to account for important chorioamnionitis risk factors such as prolonged rupture of membranes, prolonged labor, or the presence of pathogens in the mother’s genital tract, the investigators noted.
This study was funded by the Centers for Disease Control and Prevention. Dr. Kharbanda reported having no financial disclosures; some of her associates reported ties to GlaxoSmithKline, Sanofi Pasteur, Pfizer, Merck, Novartis, Nuron Biotech, Protein Science, and MedImmune. Two associates are CDC employees.
Key clinical point: Receipt of the pertussis vaccine during pregnancy was not associated with preterm birth, SGA birth, or hypertensive disorders of pregnancy.
Major finding: Rates of preterm birth were 6.3% in vaccine-exposed pregnancies and 7.8% in nonexposed pregnancies, rates of SGA infants were 8.4% and 8.3%, respectively, and rates of maternal hypertensive disorders were 8.2% and 8.0.
Data source: A retrospective observational cohort study involving 123,494 singleton pregnancies in California, including 26,229 (21%) in which the mother received the Tdap vaccine.
Disclosures: This study was funded by the Centers for Disease Control and Prevention. Dr. Kharbanda reported having no financial disclosures; some of her associates reported ties to GlaxoSmithKline, Sanofi Pasteur, Pfizer, Merck, Novartis, Nuron Biotech, Protein Science, and MedImmune. Two associates are CDC employees.
What are the heightened risks of pregnancy in women older than age 45?
Prompt frenotomy can improve nursing for mom, baby
SAN DIEGO – Pediatricians should perform frenotomy to release tongue-tie if an affected baby is struggling to nurse and the mother reports breast pain and trauma as a result, according to Dr. Anthony Magit.
“There are so few problems with this procedure, and it works so well that there is really no excuse for not doing it when it’s indicated,” added Dr. James Murphy, a pediatrician and certified lactation consultant based in San Diego.
About 4% of babies are born with tongue-tie (or ankyloglossia), an anatomic variation in the frenulum that restricts the tongue’s movement. The condition impedes nursing and can later cause problems with speech articulation, particularly for languages such as Spanish that require a relatively high amount of tongue movement, said Dr. Magit, professor of surgery at the University of California, San Diego.
Babies with tongue-tie may latch poorly, chomp at the breast, fuss, or fall asleep while nursing, and fail to gain weight normally, Dr. Magit added. Their mothers tend to develop painful, engorged breasts, which increases their risk for mastitis and is a reason to perform frenotomy promptly, he said. “If frenotomy is performed early – at 1 or 2 days of age – you will see more rapid improvement, whereas if it’s done at 2-3 weeks old, the mom is less likely to have problems completely resolve,” Dr. Magit emphasized at the annual meeting of the American Academy of Pediatrics.
If tongue-tie is suspected, a tongue depressor can be used to elevate the tongue and visualize the frenulum, said Dr. Magit. Tongue-tie appears as an unusually short, long, tight, or thickened frenulum (or frenum) that may be pyramidal, triangular, vertical, or even bumplike, Dr. Murphy added. The lateral edge of the tongue may form the shape of a W, V, or heart, and the baby’s lips may appear cobblestoned as a result of trauma during attempts to nurse, he said.
When Dr. Murphy suspects tongue-tie, he said he lays the baby on its back on an examining table with the shoulders slightly elevated on a blanket. Then he pulls the lower jaw gently down with both thumbs while using his palms to restrain the baby’s arms by the sides. This approach enables him to best see the frenulum and to observe the extent to which it is restricting the tongue’s movement, he added. An assistant uses the same hold technique when he performs frenotomies, Dr. Murphy added.
Frenotomy in newborns requires no anesthesia and can be performed in a nursery or office, said Dr. Magit. The infant is swaddled, a grooved retractor is used to direct the tongue toward the palate, the frenulum is clamped to create crush injury and direct the line of incision, and scissors are used to clip the frenulum within 1-2 mm of the junction of Wharton’s ducts, he said. After the procedure, the tongue is swept with a gloved finger and stretched to ensure complete release of the frenulum, Dr. Magit added. Most mothers report an immediate improvement in breastfeeding, including better latch, suction, and milk flow, he said.
Frenotomy in older infants and young children requires general anesthetic in the operating room, while children older than 5 years can undergo the procedure under local anesthetic in an office setting, Dr. Magit said. Complications after frenotomy are “extremely rare,” and include scarring or recurrent ankyloglossia and trauma to Wharton’s ducts, he added. Parents should be told that it is normal for yellow transitional tissue to develop at the wound site during healing, said Dr. Murphy.
Adults with tongue-tie also can benefit from frenotomy because the condition causes chronic tightness of muscles surrounding the tongue, said Dr. Murphy. “When you snip that fibrous band, the surrounding muscles relax, the hyoid bone goes down, and the larynx goes down,” he said. He has released frenula in adults and has had them report a dramatic improvement in sleep afterward, he noted.
Dr. Murphy and Dr. Magit declared no relevant financial conflicts.
SAN DIEGO – Pediatricians should perform frenotomy to release tongue-tie if an affected baby is struggling to nurse and the mother reports breast pain and trauma as a result, according to Dr. Anthony Magit.
“There are so few problems with this procedure, and it works so well that there is really no excuse for not doing it when it’s indicated,” added Dr. James Murphy, a pediatrician and certified lactation consultant based in San Diego.
About 4% of babies are born with tongue-tie (or ankyloglossia), an anatomic variation in the frenulum that restricts the tongue’s movement. The condition impedes nursing and can later cause problems with speech articulation, particularly for languages such as Spanish that require a relatively high amount of tongue movement, said Dr. Magit, professor of surgery at the University of California, San Diego.
Babies with tongue-tie may latch poorly, chomp at the breast, fuss, or fall asleep while nursing, and fail to gain weight normally, Dr. Magit added. Their mothers tend to develop painful, engorged breasts, which increases their risk for mastitis and is a reason to perform frenotomy promptly, he said. “If frenotomy is performed early – at 1 or 2 days of age – you will see more rapid improvement, whereas if it’s done at 2-3 weeks old, the mom is less likely to have problems completely resolve,” Dr. Magit emphasized at the annual meeting of the American Academy of Pediatrics.
If tongue-tie is suspected, a tongue depressor can be used to elevate the tongue and visualize the frenulum, said Dr. Magit. Tongue-tie appears as an unusually short, long, tight, or thickened frenulum (or frenum) that may be pyramidal, triangular, vertical, or even bumplike, Dr. Murphy added. The lateral edge of the tongue may form the shape of a W, V, or heart, and the baby’s lips may appear cobblestoned as a result of trauma during attempts to nurse, he said.
When Dr. Murphy suspects tongue-tie, he said he lays the baby on its back on an examining table with the shoulders slightly elevated on a blanket. Then he pulls the lower jaw gently down with both thumbs while using his palms to restrain the baby’s arms by the sides. This approach enables him to best see the frenulum and to observe the extent to which it is restricting the tongue’s movement, he added. An assistant uses the same hold technique when he performs frenotomies, Dr. Murphy added.
Frenotomy in newborns requires no anesthesia and can be performed in a nursery or office, said Dr. Magit. The infant is swaddled, a grooved retractor is used to direct the tongue toward the palate, the frenulum is clamped to create crush injury and direct the line of incision, and scissors are used to clip the frenulum within 1-2 mm of the junction of Wharton’s ducts, he said. After the procedure, the tongue is swept with a gloved finger and stretched to ensure complete release of the frenulum, Dr. Magit added. Most mothers report an immediate improvement in breastfeeding, including better latch, suction, and milk flow, he said.
Frenotomy in older infants and young children requires general anesthetic in the operating room, while children older than 5 years can undergo the procedure under local anesthetic in an office setting, Dr. Magit said. Complications after frenotomy are “extremely rare,” and include scarring or recurrent ankyloglossia and trauma to Wharton’s ducts, he added. Parents should be told that it is normal for yellow transitional tissue to develop at the wound site during healing, said Dr. Murphy.
Adults with tongue-tie also can benefit from frenotomy because the condition causes chronic tightness of muscles surrounding the tongue, said Dr. Murphy. “When you snip that fibrous band, the surrounding muscles relax, the hyoid bone goes down, and the larynx goes down,” he said. He has released frenula in adults and has had them report a dramatic improvement in sleep afterward, he noted.
Dr. Murphy and Dr. Magit declared no relevant financial conflicts.
SAN DIEGO – Pediatricians should perform frenotomy to release tongue-tie if an affected baby is struggling to nurse and the mother reports breast pain and trauma as a result, according to Dr. Anthony Magit.
“There are so few problems with this procedure, and it works so well that there is really no excuse for not doing it when it’s indicated,” added Dr. James Murphy, a pediatrician and certified lactation consultant based in San Diego.
About 4% of babies are born with tongue-tie (or ankyloglossia), an anatomic variation in the frenulum that restricts the tongue’s movement. The condition impedes nursing and can later cause problems with speech articulation, particularly for languages such as Spanish that require a relatively high amount of tongue movement, said Dr. Magit, professor of surgery at the University of California, San Diego.
Babies with tongue-tie may latch poorly, chomp at the breast, fuss, or fall asleep while nursing, and fail to gain weight normally, Dr. Magit added. Their mothers tend to develop painful, engorged breasts, which increases their risk for mastitis and is a reason to perform frenotomy promptly, he said. “If frenotomy is performed early – at 1 or 2 days of age – you will see more rapid improvement, whereas if it’s done at 2-3 weeks old, the mom is less likely to have problems completely resolve,” Dr. Magit emphasized at the annual meeting of the American Academy of Pediatrics.
If tongue-tie is suspected, a tongue depressor can be used to elevate the tongue and visualize the frenulum, said Dr. Magit. Tongue-tie appears as an unusually short, long, tight, or thickened frenulum (or frenum) that may be pyramidal, triangular, vertical, or even bumplike, Dr. Murphy added. The lateral edge of the tongue may form the shape of a W, V, or heart, and the baby’s lips may appear cobblestoned as a result of trauma during attempts to nurse, he said.
When Dr. Murphy suspects tongue-tie, he said he lays the baby on its back on an examining table with the shoulders slightly elevated on a blanket. Then he pulls the lower jaw gently down with both thumbs while using his palms to restrain the baby’s arms by the sides. This approach enables him to best see the frenulum and to observe the extent to which it is restricting the tongue’s movement, he added. An assistant uses the same hold technique when he performs frenotomies, Dr. Murphy added.
Frenotomy in newborns requires no anesthesia and can be performed in a nursery or office, said Dr. Magit. The infant is swaddled, a grooved retractor is used to direct the tongue toward the palate, the frenulum is clamped to create crush injury and direct the line of incision, and scissors are used to clip the frenulum within 1-2 mm of the junction of Wharton’s ducts, he said. After the procedure, the tongue is swept with a gloved finger and stretched to ensure complete release of the frenulum, Dr. Magit added. Most mothers report an immediate improvement in breastfeeding, including better latch, suction, and milk flow, he said.
Frenotomy in older infants and young children requires general anesthetic in the operating room, while children older than 5 years can undergo the procedure under local anesthetic in an office setting, Dr. Magit said. Complications after frenotomy are “extremely rare,” and include scarring or recurrent ankyloglossia and trauma to Wharton’s ducts, he added. Parents should be told that it is normal for yellow transitional tissue to develop at the wound site during healing, said Dr. Murphy.
Adults with tongue-tie also can benefit from frenotomy because the condition causes chronic tightness of muscles surrounding the tongue, said Dr. Murphy. “When you snip that fibrous band, the surrounding muscles relax, the hyoid bone goes down, and the larynx goes down,” he said. He has released frenula in adults and has had them report a dramatic improvement in sleep afterward, he noted.
Dr. Murphy and Dr. Magit declared no relevant financial conflicts.
US Preterm Birth Rate Falls to 17-Year Low
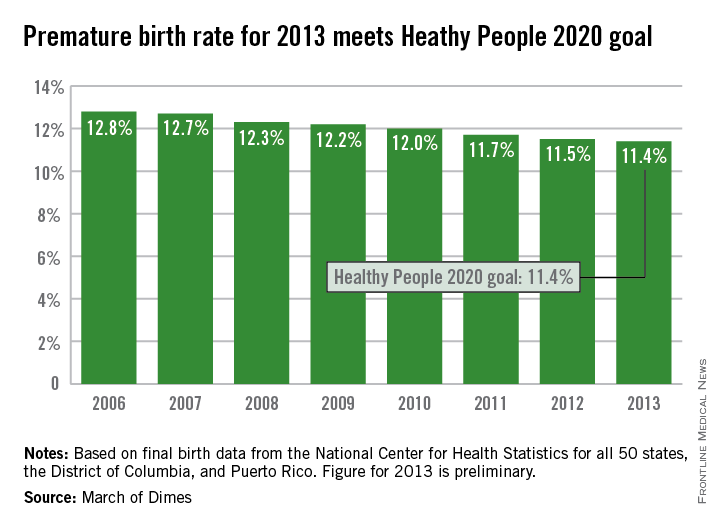
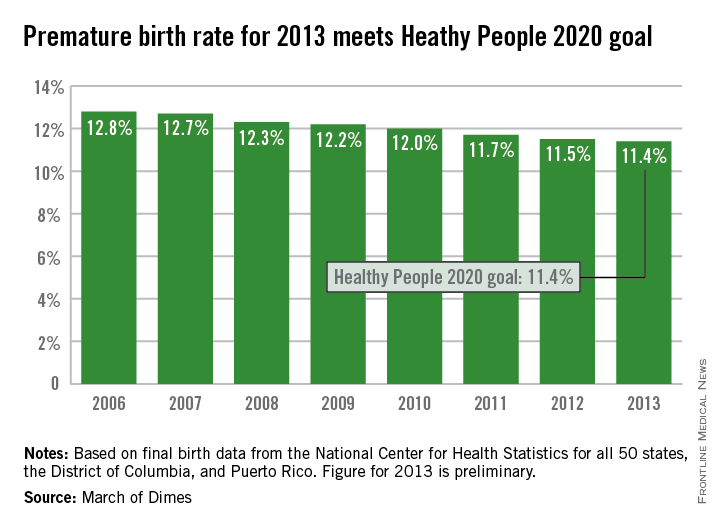
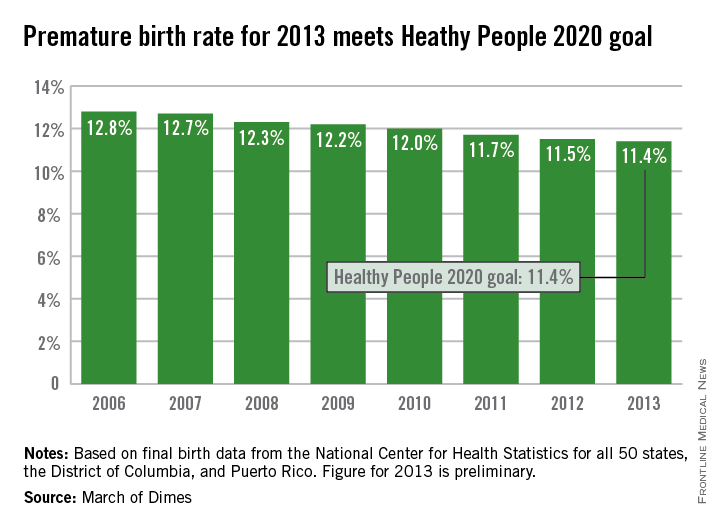
The U.S. preterm birth rate in 2013 was the lowest since 1996, reaching the Healthy People 2020 goal 7 years early, according to a report from the March of Dimes.
Although the U.S. preterm birth rate of 11.4% met the Healthy People 2020 goal, it earned only a C on the March of Dimes’ report card because it did not meet the organization’s goal of a 9.6% rate by 2020. “The U.S. still has one of the highest rates of preterm birth of any high-resource country and we must change that,” Dr. Jennifer L. Howse, March of Dimes president, said in a statement.
The preterm birth rate was at 12.8% in 2006, but since then the rate has declined slowly every year. More than 540,000 babies were born premature in 2006, but fewer than 460,000 were born in 2013. Overall, about 231,000 fewer babies were born preterm since 2006 through sustained intervention, saving $11.9 billion in health care costs, the March of Dimes noted.
The preliminary data for 2013 show that Vermont had the lowest preterm birth rate in the nation at 8.1%, followed by California at 8.8%, and New Hampshire at 9%. At 16.6%, Mississippi had the highest rate, with Alabama and Louisiana at 15.1%. The Southeast United States had the highest preterm birth rates of any region, with the five highest rates all in the Deep South and only Virginia having a rate below 12%, the March of Dimes reported, using data from the National Center for Health Statistics.
The U.S. preterm birth rate in 2013 was the lowest since 1996, reaching the Healthy People 2020 goal 7 years early, according to a report from the March of Dimes.
Although the U.S. preterm birth rate of 11.4% met the Healthy People 2020 goal, it earned only a C on the March of Dimes’ report card because it did not meet the organization’s goal of a 9.6% rate by 2020. “The U.S. still has one of the highest rates of preterm birth of any high-resource country and we must change that,” Dr. Jennifer L. Howse, March of Dimes president, said in a statement.
The preterm birth rate was at 12.8% in 2006, but since then the rate has declined slowly every year. More than 540,000 babies were born premature in 2006, but fewer than 460,000 were born in 2013. Overall, about 231,000 fewer babies were born preterm since 2006 through sustained intervention, saving $11.9 billion in health care costs, the March of Dimes noted.
The preliminary data for 2013 show that Vermont had the lowest preterm birth rate in the nation at 8.1%, followed by California at 8.8%, and New Hampshire at 9%. At 16.6%, Mississippi had the highest rate, with Alabama and Louisiana at 15.1%. The Southeast United States had the highest preterm birth rates of any region, with the five highest rates all in the Deep South and only Virginia having a rate below 12%, the March of Dimes reported, using data from the National Center for Health Statistics.

The U.S. preterm birth rate in 2013 was the lowest since 1996, reaching the Healthy People 2020 goal 7 years early, according to a report from the March of Dimes.
Although the U.S. preterm birth rate of 11.4% met the Healthy People 2020 goal, it earned only a C on the March of Dimes’ report card because it did not meet the organization’s goal of a 9.6% rate by 2020. “The U.S. still has one of the highest rates of preterm birth of any high-resource country and we must change that,” Dr. Jennifer L. Howse, March of Dimes president, said in a statement.
The preterm birth rate was at 12.8% in 2006, but since then the rate has declined slowly every year. More than 540,000 babies were born premature in 2006, but fewer than 460,000 were born in 2013. Overall, about 231,000 fewer babies were born preterm since 2006 through sustained intervention, saving $11.9 billion in health care costs, the March of Dimes noted.
The preliminary data for 2013 show that Vermont had the lowest preterm birth rate in the nation at 8.1%, followed by California at 8.8%, and New Hampshire at 9%. At 16.6%, Mississippi had the highest rate, with Alabama and Louisiana at 15.1%. The Southeast United States had the highest preterm birth rates of any region, with the five highest rates all in the Deep South and only Virginia having a rate below 12%, the March of Dimes reported, using data from the National Center for Health Statistics.

U.S. preterm birth rate falls to 17-year low
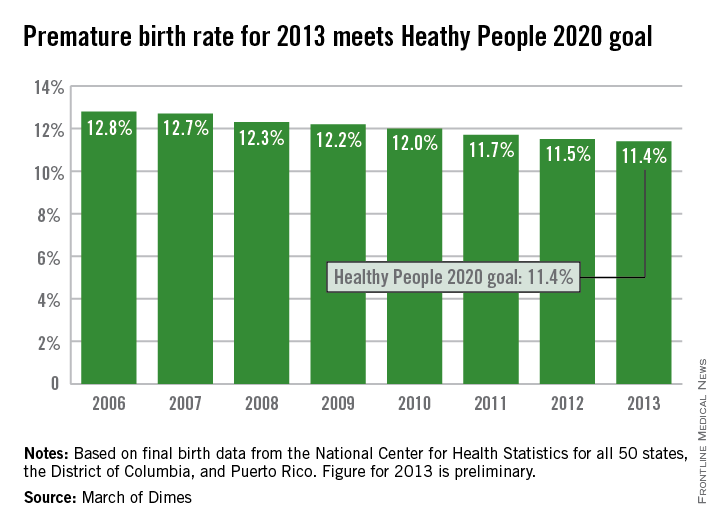
The U.S. preterm birth rate in 2013 was the lowest since 1996, reaching the Healthy People 2020 goal 7 years early, according to a report from the March of Dimes.
Although the U.S. preterm birth rate of 11.4% met the Healthy People 2020 goal, it earned only a C on the March of Dimes’ report card because it did not meet the organization’s goal of a 9.6% rate by 2020. “The U.S. still has one of the highest rates of preterm birth of any high-resource country and we must change that,” Dr. Jennifer L. Howse, March of Dimes president, said in a statement.
The preterm birth rate was at 12.8% in 2006, but since then the rate has declined slowly every year. More than 540,000 babies were born premature in 2006, but fewer than 460,000 were born in 2013. Overall, about 231,000 fewer babies were born preterm since 2006 through sustained intervention, saving $11.9 billion in health care costs, the March of Dimes noted.
The preliminary data for 2013 show that Vermont had the lowest preterm birth rate in the nation at 8.1%, followed by California at 8.8%, and New Hampshire at 9%. At 16.6%, Mississippi had the highest rate, with Alabama and Louisiana at 15.1%. The Southeast United States had the highest preterm birth rates of any region, with the five highest rates all in the Deep South and only Virginia having a rate below 12%, the March of Dimes reported, using data from the National Center for Health Statistics.
The U.S. preterm birth rate in 2013 was the lowest since 1996, reaching the Healthy People 2020 goal 7 years early, according to a report from the March of Dimes.
Although the U.S. preterm birth rate of 11.4% met the Healthy People 2020 goal, it earned only a C on the March of Dimes’ report card because it did not meet the organization’s goal of a 9.6% rate by 2020. “The U.S. still has one of the highest rates of preterm birth of any high-resource country and we must change that,” Dr. Jennifer L. Howse, March of Dimes president, said in a statement.
The preterm birth rate was at 12.8% in 2006, but since then the rate has declined slowly every year. More than 540,000 babies were born premature in 2006, but fewer than 460,000 were born in 2013. Overall, about 231,000 fewer babies were born preterm since 2006 through sustained intervention, saving $11.9 billion in health care costs, the March of Dimes noted.
The preliminary data for 2013 show that Vermont had the lowest preterm birth rate in the nation at 8.1%, followed by California at 8.8%, and New Hampshire at 9%. At 16.6%, Mississippi had the highest rate, with Alabama and Louisiana at 15.1%. The Southeast United States had the highest preterm birth rates of any region, with the five highest rates all in the Deep South and only Virginia having a rate below 12%, the March of Dimes reported, using data from the National Center for Health Statistics.

The U.S. preterm birth rate in 2013 was the lowest since 1996, reaching the Healthy People 2020 goal 7 years early, according to a report from the March of Dimes.
Although the U.S. preterm birth rate of 11.4% met the Healthy People 2020 goal, it earned only a C on the March of Dimes’ report card because it did not meet the organization’s goal of a 9.6% rate by 2020. “The U.S. still has one of the highest rates of preterm birth of any high-resource country and we must change that,” Dr. Jennifer L. Howse, March of Dimes president, said in a statement.
The preterm birth rate was at 12.8% in 2006, but since then the rate has declined slowly every year. More than 540,000 babies were born premature in 2006, but fewer than 460,000 were born in 2013. Overall, about 231,000 fewer babies were born preterm since 2006 through sustained intervention, saving $11.9 billion in health care costs, the March of Dimes noted.
The preliminary data for 2013 show that Vermont had the lowest preterm birth rate in the nation at 8.1%, followed by California at 8.8%, and New Hampshire at 9%. At 16.6%, Mississippi had the highest rate, with Alabama and Louisiana at 15.1%. The Southeast United States had the highest preterm birth rates of any region, with the five highest rates all in the Deep South and only Virginia having a rate below 12%, the March of Dimes reported, using data from the National Center for Health Statistics.

Optimal obstetric care for women aged 40 and older
CASE: Preterm labor in an older woman
G.S. is a 41-year-old G1P0 with a several-year history of infertility and a medical history of chronic hypertension. She undergoes in vitro fertilization (IVF) using her own oocytes, with transfer of two embryos. Early ultrasonography (US) confirms a diamniotic/dichorionic twin gestation. She undergoes chorionic villus sampling (CVS) during the first trimester, with normal fetal karyotypes noted.
For her chronic hypertension, the patient is treated with oral labetalol 200 mg twice daily, beginning in the first trimester. Results of a baseline comprehensive metabolic profile and complete blood count, and electrocardiogram are normal. Baseline 24-hour urine study results reveal no significant proteinuria and a normal creatinine clearance.
At 18 weeks’ gestation, US results show normal growth and amniotic fluid volume for each fetus, with no anomalies detected. Because of a gradual increase in the patient’s blood pressure, her labetalol dose is increased to 400 mg orally thrice daily. Her urine protein output remains negative on dipstick, and US every 4 weeks until 28 weeks’ gestation continues to show normal fetal growth and amniotic fluid volume.
At 33 weeks’ gestation, the patient presents with regular uterine activity. Nonstress tests for both fetuses are reactive. She is given a 1-L intravenous (IV) fluid bolus of lactated Ringers solution, as well as subcutaneous terbutaline sulfate every 15 minutes for four doses, without resolution of the uterine contractions. Her pulse has increased to 120 bpm.
How do you manage this patient’s care?
Nine times as many women aged 35 and older gave birth to their first child in 2012 than did women of the same age 40 years ago, according to the most recent data from the National Center for Health Statistics.1 The rate of first births for women aged 40 to 44 remained essentially stable during the 1970s and early 1980s but increased more than fourfold from 1985 through 2012—from 0.5 to 2.3 per 1,000 women.1 Clearly, more women are delaying childbearing to a later age by personal choice for reasons such as completion of education and career advancement.2
The path to late motherhood is not without thorns, however. Heightened risks associated with increasing maternal age include:
- fetal aneuploidy
- fetal malformation
- gestational diabetes
- chronic and gestational hypertension
- antepartum hemorrhage
- placenta previa
- prelabor rupture of membranes
- preterm labor.3,4
Women with advanced age at conception also are more likely to have a multifetal gestation because of the need for assisted reproduction and are more likely to require cesarean delivery5 as a result of abnormal placentation, fetal malpresentation, an abnormal pattern of labor, or increased use of oxytocin in labor. In addition, they are more likely to experience rupture of the sphincter, postpartum hemorrhage, and thromboembolism.3 Advanced maternal age also is associated with a higher risk of stillbirth throughout gestation, with the peak risk period reported to occur at 37 to 41 weeks.6
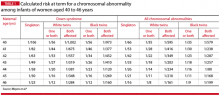
Maternal age-related risks of autosomal trisomies (especially Down syndrome) are well understood and have been quantified for singleton and twin gestations. TABLE 1 shows the risks at term for singleton and twin gestations for at least one chromosomally abnormal fetus by maternal age (40–46 years) and race.7
Preconception considerations
Aging and fertility
These combined result of aging of the ovary and uterus and an escalating risk of underlying medical comorbidities has a detrimental effect on fertility.8 Although assisted reproductive technologies are helpful, they cannot guarantee a live birth or completely compensate for an age-related decline in fertility.9
Many IVF programs refuse infertility treatment to women over age 43 or 44 who want to use their own oocytes. The reason: low pregnancy rates. The use of donor oocytes, however, increases the risks of gestational hypertension and preeclampsia. And if assisted reproductive technologies are needed, the risk for multifetal pregnancy increases.
Women of advanced maternal age are likely to have an older spouse or partner. There is no clearly accepted definition of advanced paternal age, but it is most often defined as an age of 40 years or older at the time of conception. Advanced paternal age has been associated with a higher risk for autism spectrum disorder and schizophrenia, as well as mutations in the FGFR2 and FGFR3 genes that result in skeletal dysplasias and craniosynostosis syndromes.10
Medical conditions are more common
Women of advanced maternal age have an increased rate of such prepregnancy chronic medical complications as diabetes, chronic hypertension, obesity, and renal and cardiac disease. Therefore, it is best to optimize control of these chronic illnesses prior to conception to minimize the risks of miscarriage, fetal anomalies, and gestational hypertension and preeclampsia.
Preeclampsia. Although daily low-dose (60–81 mg) aspirin has been used to reduce the risk of preeclampsia, current recommendations from the American College of Obstetricians and Gynecologists (ACOG) suggest that this therapy be reserved for women with a medical history of early-onset preeclampsia or those who have had preeclampsia in more than one pregnancy.11
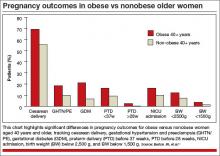
Impact of obesity. We recently examined the influence of age and obesity on pregnancy outcomes of nulliparous women aged 40 or older at delivery.12 The study included women aged 20 to 29 years (n = 52,249) and 40 or older (n = 1,231) who delivered singleton infants. Women who reported medical disorders, tobacco use, or conception with assisted reproductive technology were excluded.
In the older age group (≥40 years), obese women had significantly higher rates of cesarean delivery, gestational hypertension, preeclampsia, gestational diabetes, preterm delivery before 37 weeks’ gestation, and preterm delivery before 28 weeks, and their infants had higher rates of admission to the neonatal intensive care unit (NICU), compared with nonobese women (FIGURE).
It would appear, however, that healthy, obese women who delay pregnancy until the age of 40 or later may modify their risk for cesarean delivery, gestational diabetes mellitus, and gestational hypertension and preeclampsia by reducing their body mass index to nonobese levels prior to conception.
In addition to maternal risks for women of advanced maternal age, there are risks to the fetus and neonate, as well as a risk of placental abnormalities. These risks are summarized in TABLE 2.
Placental
- Molar or partial molar pregnancy
- Fetus or twins with a complete mole
- Placenta previa, vasa previa
Fetal/neonatal
- Aneuploidy
- Selective fetal growth restriction in twin gestation
- Twin-twin transfusion syndrome
- Preterm birth
- Perinatal death
Antepartum
- Gestational diabetes
- Insulin-dependent diabetes
- Gestational hypertension and preeclampsia
- Cholestasis of pregnancy
- Acute fatty liver of pregnancy
- Venous thromboembolism
- Preterm labor, preterm premature rupture
of membranes
Intrapartum
- Dysfunctional labor
- Malpresentation
- Cesarean delivery
Postpartum
- Venous thromboembolism
- Postpartum hemorrhage
Folic acid supplementation can reduce some risks
The potential benefit of folic acid supplementation to reduce the risk of fetal open neural tube defects is well documented. More recent data suggest that folic acid also is associated with a reduction in the risks of congenital heart defects, abdominal wall defects, cleft lip and palate, and spontaneous abortion. Supplementation should be initiated at least 3 months prior to conception and continued through the first trimester.
The first trimester
Early pregnancy loss is a risk
Women of advanced maternal age are more likely than younger women to experience early pregnancy loss. This risk is due to higher rates of fetal aneuploidy as well as declining ovarian and uterine function and a higher rate of ectopic pregnancy.
In the First and Second Trimester Evaluation of Risk (FASTER) trial, in which investigators reported pregnancy outcomes by maternal age for 36,056 pregnancies, the rate of spontaneous abortion after 10 weeks of gestation was 0.8% among women younger than 35 years, compared with 2.2% for women aged 40 or older.4
The likelihood of multiple gestation increases
The background risk of multiple births is higher in women of advanced maternal age, compared with younger women. This risk increases further with fertility treatment.
Multiple gestations at any age are associated with increased risks for preterm birth and very-low–birthweight infants. Potential maternal risks are listed in TABLE 3.
- Hypertension (2.5 times the risk of a singleton gestation)
- Abruption (3.0 times the risk)
- Anemia (2.5 times the risk)
- Urinary tract infection (1.5 times the risk)
- Preeclampsia (risk of 26%–75%) (occurs at earlier gestation) — HELLP syndrome (risk of 9%)
- Abruption (20%) (10 times the risk of a singleton gestation)
- Anemia (24%)
- Preterm premature rupture of membranes (24%)
- Gestational diabetes (14%)
- Acute fatty liver (4%) (1 in 10,000 singletons)
- Postpartum hemorrhage (9%)
To reduce the number of multiple gestations with assisted reproduction, consider elective single embryo transfer, especially if the mother has significant underlying medical complications.
Multiple gestations present difficult management issues in older women. Strategies shown to prevent preterm delivery in singleton gestations, including weekly 17-hydroxyprogesterone injections and cervical cerclage, are not effective in multiple gestations. Moreover, many of these therapies—including bed rest—increase the risk of thromboembolic events in multiple gestations, particularly when the mother is of advanced age.
Maternal adaptations in multiple gestations also may be poorly tolerated by older patients, particularly cardiac changes that markedly increase stroke volume, heart rate, cardiac output, and plasma volume.
The range of genetic screening and testing options has broadened
Options include first-trimester CVS, which provides information about the fetal chromosomal complement but not the presence of a fetal open neural tube defect. The procedure-related rate of fetal loss with CVS is quoted as 1%.
Options for genetic testing in the second trimester include transabdominal amniocentesis. A procedure-related fetal loss rate of 1 in 500 to 1 in 1,600 is quoted for midtrimester amniocentesis.
A relatively new screening option is analysis of cell-free fetal DNA in maternal blood, which can be performed after 10 weeks’ gestation in singleton and multiple gestations. This directed analysis measures the relative proportions of chromosomes. The detection rate for fetal Down syndrome using cell-free fetal DNA is greater than 98%, with a false-positive rate of less than 0.5%. However, this screening is unreliable in triplet gestations.
Other screening options include US and biochemical screening to detect fetal aneuploidy and open neural tube defects during the second trimester. These options should be included in counseling of the patient.
Second and third trimesters
Gestational hypertension and preeclampsia are significant risks
Older pregnant women have an incidence of gestational hypertension and preeclampsia 2 to 4 times as high as that of patients younger than 30 years.13 The underlying risk for preeclampsia is further increased if coexisting medical disorders such as diabetes or chronic hypertension are present. Moreover, the risk for preeclampsia increases to 10% to 20% in twin gestations and 25% to 60% in triplet gestations. Le Ray and colleagues reported that, if oocyte donation is used with IVF in women older than age 43, the risk for preeclampsia triples.14
We previously studied 379 women aged 35 and older who had mild gestational hypertension remote from term, comparing them with their younger adult counterparts in a matched cohort design.15 Outpatient management produced similar maternal outcomes in both groups, but older women had a statistically insignificant increase in the rate of stillbirth (5 vs 0; P = .063).15
Gestational diabetes risk doubles
The rates of both diabetes mellitus and gestational diabetes increase with advanced maternal age. Data from the FASTER consortium included an adjusted odds ratio of 2.4 for gestational diabetes in women aged 40 or older, compared with a younger control group.4 This increased risk may be a consequence of greater maternal habitus as well as declining insulin sensitivity.
Diabetes increases the risks of macrosomia, cesarean birth, and gestational hypertension. Among women with pregestational diabetes, the risks of congenital heart disease and fetal neural tube defects increase threefold. Because of this increased risk, perinatal screening is indicated for both anomalies in older women.
Pulmonary complications increase
Another risk facing women of advanced maternal age—particularly those carrying a multiple gestation—is pulmonary edema, owing to the increased cardiac output, heart rate, and blood volume, the decreased systemic vascular resistance, and the physiologic anemia of pregnancy. These risks rise further in women who develop preterm labor that requires therapy and in those who develop gestational hypertension and/or preeclampsia. Judicious use of IV fluids, particularly those with lower sodium concentrations, can reduce the risk of pulmonary complications.
Women who develop pulmonary edema have an increased risk of peripartum cardiomyopathy.16
Preterm delivery is more common
Cleary-Goodman and colleagues noted an increased incidence of preterm delivery in women aged 40 and older, compared with women younger than age 35, but no increase in spontaneous preterm labor.4 Advanced maternal age appears to be associated with an increased risk of preterm birth largely as a consequence of underlying complications of fetal growth restriction and maternal disease, including hypertension. Because preterm birth is an important contributor to perinatal morbidity and mortality, steroids should be administered for fetal lung maturity whenever preterm labor is diagnosed before 34 weeks’ gestation.
Risk of placenta previa is 1.1%
Joseph and colleagues found the risk of placenta previa to be 1.1% in women aged 40 and older, compared with 0.3% in women aged 25 to 29 years.17 This increased risk likely is a consequence not only of maternal age but increased parity and a history of prior uterine surgery. If transabdominal US results are suspicious for placenta previa, transvaginal US is indicated for confirmation. Additional US assessment of the cord insertion site to the placenta also should be performed to rule out vasa previa.
Look for neonatal complications
Ziadeh and colleagues found that, although maternal morbidity was increased in older women, the overall neonatal outcome did not appear to be affected.18 However, we noted a higher rate of neonatal complications in women aged 40 or older, including higher NICU admission rates and more low-birth–weight infants.11
In addition, Odibo and colleagues found advanced maternal age to be an independent risk factor for intrauterine growth restriction (IUGR).19 In that study, the odds ratio for IUGR was 3.2 (95% confidence interval [CI], 1.9–5.4) for a maternal age of 40 years or older, compared with a control group. For that reason, they recommend routine screening for IUGR in all pregnant women of advanced age.
Stillbirth risk peaks at 37 to 41 weeks
In a review of more than 5.4 million singleton pregnancies without reported congenital anomalies, Reddy and colleagues found an association between advanced maternal age and stillbirth, with a higher risk of stillbirth at 37 to 41 weeks’ gestation.6 This effect of maternal age persisted despite adjusting for medical disease, parity, and race/ethnicity.
Many women older than age 40 have independent medical or fetal indications for antenatal testing. Some experts have suggested antepartum surveillance starting at 37 weeks for women of advanced maternal age; they argue that the risk of stillbirth at this gestational age is similar in frequency to other high-risk conditions for which testing is performed routinely. However, the National Institute of Child Health and Human Development (NICHD) workshop on antepartum fetal monitoring found insufficient evidence that antenatal testing for the sole indication of advanced maternal age reduces stillbirth or improves perinatal outcomes.20
If increased antenatal testing is indicated for a high-risk condition or electively chosen given advanced age, it should include electronic fetal monitoring as well as amniotic fluid volume assessment. Because the risk of fetal loss sharply increased at 40 weeks’ gestation in the study by Reddy and colleagues,6 women older than age 40 should be considered for delivery by 40 weeks’ gestation in the presence of good dating criteria.
Some clinicians also would consider delivery by 39 weeks’ gestation with good dating criteria if the Bishop score is favorable.
Risks of labor and delivery
Multiple variables contribute to a higher cesarean delivery rate
The risk of cesarean delivery increases with advancing maternal age.5,11 This increased risk is a consequence of multiple variables, including the rate of previous cesarean delivery, malpresentation, underlying complications such as preeclampsia and diabetes, and a higher prevalence of dysfunctional labor.21 Further, Vaughn and colleagues noted that the cesarean delivery rate increases in direct proportion to age, with a rate of 54.4% in women older than age 40.5
As Cohen pointed out in a commentary accompanying a study of dysfunctional labor in women of advancing age, “the notion of a premium baby (ie, that the fetus of a woman with a reduced likelihood of having another pregnancy is somehow more deserving of being spared the rigours of labour than the fetus of a young woman) may play a role” in the high rate of cesarean delivery.21,22
Postpartum hemorrhage risk may be lower in older women
Advanced maternal age is assumed to be a risk factor for postpartum hemorrhage.23 The increased risk was thought to be related to the increased incidence of multiple underlying factors, such as cesarean delivery, multiple gestation, and hypertensive disorders of pregnancy.
However, in a retrospective cohort study, Lao and colleagues found that advanced maternal age (≥35 years) served only as a surrogate factor for postpartum hemorrhage due to associated risk factors, obstetric complications, and interventions.24 After multivariate analysis, aging was associated with a decreased rate of postpartum hemorrhage, which declined progressively from ages 25 to 40 years and older, compared with women aged 20 to 24.24
Nevertheless, medical interventions should be readily available at the time of delivery for treatment of uterine atony, especially with multiple gestation and grand multiparity.
Case: Resolved
The patient is admitted to the hospital, where she is given IV magnesium sulfate (6-g load followed by an infusion of 3 g/hr) and betamethasone for fetal lung maturity enhancement. She continues to receive IV fluids as well (125 mL/hr lactated Ringers solution). Uterine activity abates.
IV magnesium sulfate is continued for 36 hours, but urine protein output is not monitored. Her heart rate ranges from 105 to 115 bpm, and blood pressure from 130/80 mm Hg to 138/88 mm Hg. Forty-eight hours after admission, she reports a gradual onset of tightness of the chest and breathlessness. She is agitated, with a pulse of 130 bpm, 30 respirations/min, and room air pulse oximetry of 90%. Rales are noted upon auscultation of both lungs. A radiograph of the chest demonstrates bilateral air-space disease consistent with pulmonary edema. IV furosemide and oxygen (by mask) are provided, with some respiratory improvement.
The patient then reports leakage of amniotic fluid, and preterm rupture of membranes is confirmed on examination. Because steroids for fetal lung maturity have been administered, and given improvement in her pulmonary edema and a footling breech presentation for Twin A, cesarean delivery is performed.
The patient’s immediate postoperative course is uncomplicated. On postoperative day 2, however, she develops recurrent pulmonary edema, as confirmed by physical examination and chest radiograph. She also reports headache, and her blood pressure rises to 164/114 mm Hg—findings consistent with postpartum preeclampsia. Magnesium sulfate and antihypertensive therapy are initiated, along with IV furosemide and oxygen, which improves her respiratory status.
An echocardiogram to rule out peripartum dilated cardiomyopathy finds no evidence of a dilated left ventricle, and the calculated left ventricular ejection fraction (55%) is normal.
After diuresis and improvement in her blood pressure, she is discharged home. By the time of her follow-up office visit 7 days later, her blood pressure has normalized on labetalol therapy.
For an overview of evaluation and management of pregnant women aged 40 or older, see TABLE 4.
Preconception
- Identify risk factors (ie, diabetes, obesity, hypertension, cardiac dysfunction, family history
- Review outcome of previous pregnancy, if applicable
- Review risks (multiple gestation, birth defects) associated with assisted reproductive technologies if they were needed to achieve pregnancy
- Optimize maternal health
- Begin folic acid supplementation
- Encourage smoking cessation
- If the patient is ≥45 years old:
– Electrocardiogram
– Glucose screening (fasting plasma glucose or hemoglobin A1c)
– Echocardiogram for patients with chronic hypertension
First trimester
- Ultrasonography for dating and to assess fetal number and chorionicity
- Baseline metabolic profile and complete blood count
- Baseline urinalysis
- Continue folic acid supplementation
- Offer first-trimester genetic testing or other genetic screening
Second trimester
- If first-trimester genetic testing is declined, offer second-trimester testing or screening
- Detailed fetal anomaly evaluation by ultrasound
- Fetal echocardiogram if pregnancy was achieved by in vitro fertilization or if it is a monochorionic twin gestation
- Screen for gestational diabetes
Third trimester
- Increased antenatal testing for routine indications, including hypertension, diabetes, and lupus
- Ultrasonography for growth and later ultrasonographic findings of fetal aneuploidy
- Consider delivery
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Mathews TJ, Hamilton BE. First births to older women continue to rise. National Center for Health Statistics. NCHS Data Brief No. 152. May 2014. http://www.cdc.gov/nchs/data/databriefs/db152.pdf. Accessed October 3, 2014.
2. Mills M, Rindfuss RR, McDonald P, te Velde E. Why do people postpone parenthood? Reasons and social policy incentives. Hum Reprod Update. 2011;17(6):848–860.
3. Ziadeh SM. Maternal and perinatal outcome in nulliparous women aged 35 and older. Gynecol Obstet Invest. 2002;54(1):6–10.
4. Cleary-Goldman J, Malone FD, Vidaver J, et al; FASTER Consortium. Impact of maternal age on obstetric outcome. Obstet Gynecol. 2005;105(5 pt 1):983–990.
5. Vaughn DA, Cleary BJ, Murphy DJ. Delivery outcomes for nulliparous women at the extremes of maternal age—a cohort study. BJOG. 2014;121(3):261–268.
6. Reddy UM, Ko CW, Willinger M. Maternal age and the risk of stillbirth through pregnancy in the United States. Am J Obstet Gynecol. 2006;195(3):764–770.
7. Meyers C, Adam R, Dungan J, Prenger V. Aneuploidy in twin gestations: when is maternal age advanced? Obstet Gynecol. 1997;89(2):248–251.
8. Nelson SM, Telfer EE, Anderson RA. The ageing ovary and uterus: new biological insights. Hum Reprod Update. 2013;19(1):67–83.
9. Johnson JA, Tough S. Delayed child-bearing. J Obstet Gynaecol Can. 2012;34(1):80–93.
10. Goriely A, Wilkie AO. Paternal age effect mutations and selfish spermatogonial selection: causes and consequences for human disease. Am J Hum Genet. 2012;90(2):175–200.
11. Barton JR, Sibai AJ, Istwan NB, Rhea DJ, Desch CN, Sibai BM. Spontaneously conceived pregnancy after 40: influence of age and obesity on outcome. Am J Perinatol. 2014;31(9):795–798.
12. Roberts JM, August PA, Bakris JR, et al. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122–1131.
13. Jahromi BN, Husseini Z. Pregnancy outcome at maternal age 40 and older. Taiwan J Obstet Gynecol. 2008;47(3):318–321.
14. Le Ray C, Scherier S, Anselem O, et al. Association between oocyte donation and maternal and perinatal outcomes in women aged 43 years or older. Hum Reprod. 2012;27(3):896–901.
15. Barton JR, Bergauer NK, Jacques DL, Coleman SK, Stanziano GJ, Sibai BM. Does advanced maternal age affect pregnancy outcome in women with mild hypertension remote from term? Am J Obstet Gynecol. 1997;176(6):1236–1243.
16. Habli M, O’Brien T, Nowack E, et al. Peripartum cardiomyopathy: prognostic factors for long-term maternal outcome. Am J Obstet Gynecol. 2008;199(4):415.e1–e5.
17. Joseph KS, Allen AC, Dodds L, Turner LA, Scott H, Liston R. The perinatal effects of delayed childbearing. Obstet Gynecol. 2005;105(6):1410–1418.
18. Ziadeh S, Yahaya A. Pregnancy outcome at age 40 and older. Arch Gynecol Obstet. 2001;265(1):30–33.
19. Odibo AO, Nelson D, Stamilio DM, Sehdev HM, Macones GA. Advanced maternal age is an independent risk factor for intrauterine growth restriction. Am J Perinatol. 2006;23(5):325–328.
20. Signore C, Freeman RK, Spong CY. Antenatal testing—a reevaluation: executive summary of a Eunice Kennedy Shriver National Institute of Child Health and Human Development workshop. Obstet Gynecol. 2009;113(3):687–701.
21. Cohen WR, Newman L, Friedman EA. Risk of labor abnormalities with advancing maternal age. Obstet Gynecol. 1980;55(4):414–416.
22. Cohen WR. Does maternal age affect pregnancy outcome? BJOG. 2014;121(3):252–254.
23. Bateman BT, Berman MF, Riley LE, Leffert LR. The epidemiology of postpartum hemorrhage in a large, nationwide sample of deliveries. Anesth Analg. 2010;110(5):1368–1373.
24. Lao TT, Sahota DS, Cheng YK, Law LW, Leung TY. Advanced maternal age and postpartum hemorrhage—risk factor or red herring? J Matern Fetal Neonatal Med. 2014;27(3):243–246.
CASE: Preterm labor in an older woman
G.S. is a 41-year-old G1P0 with a several-year history of infertility and a medical history of chronic hypertension. She undergoes in vitro fertilization (IVF) using her own oocytes, with transfer of two embryos. Early ultrasonography (US) confirms a diamniotic/dichorionic twin gestation. She undergoes chorionic villus sampling (CVS) during the first trimester, with normal fetal karyotypes noted.
For her chronic hypertension, the patient is treated with oral labetalol 200 mg twice daily, beginning in the first trimester. Results of a baseline comprehensive metabolic profile and complete blood count, and electrocardiogram are normal. Baseline 24-hour urine study results reveal no significant proteinuria and a normal creatinine clearance.
At 18 weeks’ gestation, US results show normal growth and amniotic fluid volume for each fetus, with no anomalies detected. Because of a gradual increase in the patient’s blood pressure, her labetalol dose is increased to 400 mg orally thrice daily. Her urine protein output remains negative on dipstick, and US every 4 weeks until 28 weeks’ gestation continues to show normal fetal growth and amniotic fluid volume.
At 33 weeks’ gestation, the patient presents with regular uterine activity. Nonstress tests for both fetuses are reactive. She is given a 1-L intravenous (IV) fluid bolus of lactated Ringers solution, as well as subcutaneous terbutaline sulfate every 15 minutes for four doses, without resolution of the uterine contractions. Her pulse has increased to 120 bpm.
How do you manage this patient’s care?
Nine times as many women aged 35 and older gave birth to their first child in 2012 than did women of the same age 40 years ago, according to the most recent data from the National Center for Health Statistics.1 The rate of first births for women aged 40 to 44 remained essentially stable during the 1970s and early 1980s but increased more than fourfold from 1985 through 2012—from 0.5 to 2.3 per 1,000 women.1 Clearly, more women are delaying childbearing to a later age by personal choice for reasons such as completion of education and career advancement.2
The path to late motherhood is not without thorns, however. Heightened risks associated with increasing maternal age include:
- fetal aneuploidy
- fetal malformation
- gestational diabetes
- chronic and gestational hypertension
- antepartum hemorrhage
- placenta previa
- prelabor rupture of membranes
- preterm labor.3,4
Women with advanced age at conception also are more likely to have a multifetal gestation because of the need for assisted reproduction and are more likely to require cesarean delivery5 as a result of abnormal placentation, fetal malpresentation, an abnormal pattern of labor, or increased use of oxytocin in labor. In addition, they are more likely to experience rupture of the sphincter, postpartum hemorrhage, and thromboembolism.3 Advanced maternal age also is associated with a higher risk of stillbirth throughout gestation, with the peak risk period reported to occur at 37 to 41 weeks.6
Maternal age-related risks of autosomal trisomies (especially Down syndrome) are well understood and have been quantified for singleton and twin gestations. TABLE 1 shows the risks at term for singleton and twin gestations for at least one chromosomally abnormal fetus by maternal age (40–46 years) and race.7
Preconception considerations
Aging and fertility
These combined result of aging of the ovary and uterus and an escalating risk of underlying medical comorbidities has a detrimental effect on fertility.8 Although assisted reproductive technologies are helpful, they cannot guarantee a live birth or completely compensate for an age-related decline in fertility.9
Many IVF programs refuse infertility treatment to women over age 43 or 44 who want to use their own oocytes. The reason: low pregnancy rates. The use of donor oocytes, however, increases the risks of gestational hypertension and preeclampsia. And if assisted reproductive technologies are needed, the risk for multifetal pregnancy increases.
Women of advanced maternal age are likely to have an older spouse or partner. There is no clearly accepted definition of advanced paternal age, but it is most often defined as an age of 40 years or older at the time of conception. Advanced paternal age has been associated with a higher risk for autism spectrum disorder and schizophrenia, as well as mutations in the FGFR2 and FGFR3 genes that result in skeletal dysplasias and craniosynostosis syndromes.10
Medical conditions are more common
Women of advanced maternal age have an increased rate of such prepregnancy chronic medical complications as diabetes, chronic hypertension, obesity, and renal and cardiac disease. Therefore, it is best to optimize control of these chronic illnesses prior to conception to minimize the risks of miscarriage, fetal anomalies, and gestational hypertension and preeclampsia.
Preeclampsia. Although daily low-dose (60–81 mg) aspirin has been used to reduce the risk of preeclampsia, current recommendations from the American College of Obstetricians and Gynecologists (ACOG) suggest that this therapy be reserved for women with a medical history of early-onset preeclampsia or those who have had preeclampsia in more than one pregnancy.11
Impact of obesity. We recently examined the influence of age and obesity on pregnancy outcomes of nulliparous women aged 40 or older at delivery.12 The study included women aged 20 to 29 years (n = 52,249) and 40 or older (n = 1,231) who delivered singleton infants. Women who reported medical disorders, tobacco use, or conception with assisted reproductive technology were excluded.
In the older age group (≥40 years), obese women had significantly higher rates of cesarean delivery, gestational hypertension, preeclampsia, gestational diabetes, preterm delivery before 37 weeks’ gestation, and preterm delivery before 28 weeks, and their infants had higher rates of admission to the neonatal intensive care unit (NICU), compared with nonobese women (FIGURE).
It would appear, however, that healthy, obese women who delay pregnancy until the age of 40 or later may modify their risk for cesarean delivery, gestational diabetes mellitus, and gestational hypertension and preeclampsia by reducing their body mass index to nonobese levels prior to conception.
In addition to maternal risks for women of advanced maternal age, there are risks to the fetus and neonate, as well as a risk of placental abnormalities. These risks are summarized in TABLE 2.
Placental
- Molar or partial molar pregnancy
- Fetus or twins with a complete mole
- Placenta previa, vasa previa
Fetal/neonatal
- Aneuploidy
- Selective fetal growth restriction in twin gestation
- Twin-twin transfusion syndrome
- Preterm birth
- Perinatal death
Antepartum
- Gestational diabetes
- Insulin-dependent diabetes
- Gestational hypertension and preeclampsia
- Cholestasis of pregnancy
- Acute fatty liver of pregnancy
- Venous thromboembolism
- Preterm labor, preterm premature rupture
of membranes
Intrapartum
- Dysfunctional labor
- Malpresentation
- Cesarean delivery
Postpartum
- Venous thromboembolism
- Postpartum hemorrhage
Folic acid supplementation can reduce some risks
The potential benefit of folic acid supplementation to reduce the risk of fetal open neural tube defects is well documented. More recent data suggest that folic acid also is associated with a reduction in the risks of congenital heart defects, abdominal wall defects, cleft lip and palate, and spontaneous abortion. Supplementation should be initiated at least 3 months prior to conception and continued through the first trimester.
The first trimester
Early pregnancy loss is a risk
Women of advanced maternal age are more likely than younger women to experience early pregnancy loss. This risk is due to higher rates of fetal aneuploidy as well as declining ovarian and uterine function and a higher rate of ectopic pregnancy.
In the First and Second Trimester Evaluation of Risk (FASTER) trial, in which investigators reported pregnancy outcomes by maternal age for 36,056 pregnancies, the rate of spontaneous abortion after 10 weeks of gestation was 0.8% among women younger than 35 years, compared with 2.2% for women aged 40 or older.4
The likelihood of multiple gestation increases
The background risk of multiple births is higher in women of advanced maternal age, compared with younger women. This risk increases further with fertility treatment.
Multiple gestations at any age are associated with increased risks for preterm birth and very-low–birthweight infants. Potential maternal risks are listed in TABLE 3.
- Hypertension (2.5 times the risk of a singleton gestation)
- Abruption (3.0 times the risk)
- Anemia (2.5 times the risk)
- Urinary tract infection (1.5 times the risk)
- Preeclampsia (risk of 26%–75%) (occurs at earlier gestation) — HELLP syndrome (risk of 9%)
- Abruption (20%) (10 times the risk of a singleton gestation)
- Anemia (24%)
- Preterm premature rupture of membranes (24%)
- Gestational diabetes (14%)
- Acute fatty liver (4%) (1 in 10,000 singletons)
- Postpartum hemorrhage (9%)
To reduce the number of multiple gestations with assisted reproduction, consider elective single embryo transfer, especially if the mother has significant underlying medical complications.
Multiple gestations present difficult management issues in older women. Strategies shown to prevent preterm delivery in singleton gestations, including weekly 17-hydroxyprogesterone injections and cervical cerclage, are not effective in multiple gestations. Moreover, many of these therapies—including bed rest—increase the risk of thromboembolic events in multiple gestations, particularly when the mother is of advanced age.
Maternal adaptations in multiple gestations also may be poorly tolerated by older patients, particularly cardiac changes that markedly increase stroke volume, heart rate, cardiac output, and plasma volume.
The range of genetic screening and testing options has broadened
Options include first-trimester CVS, which provides information about the fetal chromosomal complement but not the presence of a fetal open neural tube defect. The procedure-related rate of fetal loss with CVS is quoted as 1%.
Options for genetic testing in the second trimester include transabdominal amniocentesis. A procedure-related fetal loss rate of 1 in 500 to 1 in 1,600 is quoted for midtrimester amniocentesis.
A relatively new screening option is analysis of cell-free fetal DNA in maternal blood, which can be performed after 10 weeks’ gestation in singleton and multiple gestations. This directed analysis measures the relative proportions of chromosomes. The detection rate for fetal Down syndrome using cell-free fetal DNA is greater than 98%, with a false-positive rate of less than 0.5%. However, this screening is unreliable in triplet gestations.
Other screening options include US and biochemical screening to detect fetal aneuploidy and open neural tube defects during the second trimester. These options should be included in counseling of the patient.
Second and third trimesters
Gestational hypertension and preeclampsia are significant risks
Older pregnant women have an incidence of gestational hypertension and preeclampsia 2 to 4 times as high as that of patients younger than 30 years.13 The underlying risk for preeclampsia is further increased if coexisting medical disorders such as diabetes or chronic hypertension are present. Moreover, the risk for preeclampsia increases to 10% to 20% in twin gestations and 25% to 60% in triplet gestations. Le Ray and colleagues reported that, if oocyte donation is used with IVF in women older than age 43, the risk for preeclampsia triples.14
We previously studied 379 women aged 35 and older who had mild gestational hypertension remote from term, comparing them with their younger adult counterparts in a matched cohort design.15 Outpatient management produced similar maternal outcomes in both groups, but older women had a statistically insignificant increase in the rate of stillbirth (5 vs 0; P = .063).15
Gestational diabetes risk doubles
The rates of both diabetes mellitus and gestational diabetes increase with advanced maternal age. Data from the FASTER consortium included an adjusted odds ratio of 2.4 for gestational diabetes in women aged 40 or older, compared with a younger control group.4 This increased risk may be a consequence of greater maternal habitus as well as declining insulin sensitivity.
Diabetes increases the risks of macrosomia, cesarean birth, and gestational hypertension. Among women with pregestational diabetes, the risks of congenital heart disease and fetal neural tube defects increase threefold. Because of this increased risk, perinatal screening is indicated for both anomalies in older women.
Pulmonary complications increase
Another risk facing women of advanced maternal age—particularly those carrying a multiple gestation—is pulmonary edema, owing to the increased cardiac output, heart rate, and blood volume, the decreased systemic vascular resistance, and the physiologic anemia of pregnancy. These risks rise further in women who develop preterm labor that requires therapy and in those who develop gestational hypertension and/or preeclampsia. Judicious use of IV fluids, particularly those with lower sodium concentrations, can reduce the risk of pulmonary complications.
Women who develop pulmonary edema have an increased risk of peripartum cardiomyopathy.16
Preterm delivery is more common
Cleary-Goodman and colleagues noted an increased incidence of preterm delivery in women aged 40 and older, compared with women younger than age 35, but no increase in spontaneous preterm labor.4 Advanced maternal age appears to be associated with an increased risk of preterm birth largely as a consequence of underlying complications of fetal growth restriction and maternal disease, including hypertension. Because preterm birth is an important contributor to perinatal morbidity and mortality, steroids should be administered for fetal lung maturity whenever preterm labor is diagnosed before 34 weeks’ gestation.
Risk of placenta previa is 1.1%
Joseph and colleagues found the risk of placenta previa to be 1.1% in women aged 40 and older, compared with 0.3% in women aged 25 to 29 years.17 This increased risk likely is a consequence not only of maternal age but increased parity and a history of prior uterine surgery. If transabdominal US results are suspicious for placenta previa, transvaginal US is indicated for confirmation. Additional US assessment of the cord insertion site to the placenta also should be performed to rule out vasa previa.
Look for neonatal complications
Ziadeh and colleagues found that, although maternal morbidity was increased in older women, the overall neonatal outcome did not appear to be affected.18 However, we noted a higher rate of neonatal complications in women aged 40 or older, including higher NICU admission rates and more low-birth–weight infants.11
In addition, Odibo and colleagues found advanced maternal age to be an independent risk factor for intrauterine growth restriction (IUGR).19 In that study, the odds ratio for IUGR was 3.2 (95% confidence interval [CI], 1.9–5.4) for a maternal age of 40 years or older, compared with a control group. For that reason, they recommend routine screening for IUGR in all pregnant women of advanced age.
Stillbirth risk peaks at 37 to 41 weeks
In a review of more than 5.4 million singleton pregnancies without reported congenital anomalies, Reddy and colleagues found an association between advanced maternal age and stillbirth, with a higher risk of stillbirth at 37 to 41 weeks’ gestation.6 This effect of maternal age persisted despite adjusting for medical disease, parity, and race/ethnicity.
Many women older than age 40 have independent medical or fetal indications for antenatal testing. Some experts have suggested antepartum surveillance starting at 37 weeks for women of advanced maternal age; they argue that the risk of stillbirth at this gestational age is similar in frequency to other high-risk conditions for which testing is performed routinely. However, the National Institute of Child Health and Human Development (NICHD) workshop on antepartum fetal monitoring found insufficient evidence that antenatal testing for the sole indication of advanced maternal age reduces stillbirth or improves perinatal outcomes.20
If increased antenatal testing is indicated for a high-risk condition or electively chosen given advanced age, it should include electronic fetal monitoring as well as amniotic fluid volume assessment. Because the risk of fetal loss sharply increased at 40 weeks’ gestation in the study by Reddy and colleagues,6 women older than age 40 should be considered for delivery by 40 weeks’ gestation in the presence of good dating criteria.
Some clinicians also would consider delivery by 39 weeks’ gestation with good dating criteria if the Bishop score is favorable.
Risks of labor and delivery
Multiple variables contribute to a higher cesarean delivery rate
The risk of cesarean delivery increases with advancing maternal age.5,11 This increased risk is a consequence of multiple variables, including the rate of previous cesarean delivery, malpresentation, underlying complications such as preeclampsia and diabetes, and a higher prevalence of dysfunctional labor.21 Further, Vaughn and colleagues noted that the cesarean delivery rate increases in direct proportion to age, with a rate of 54.4% in women older than age 40.5
As Cohen pointed out in a commentary accompanying a study of dysfunctional labor in women of advancing age, “the notion of a premium baby (ie, that the fetus of a woman with a reduced likelihood of having another pregnancy is somehow more deserving of being spared the rigours of labour than the fetus of a young woman) may play a role” in the high rate of cesarean delivery.21,22
Postpartum hemorrhage risk may be lower in older women
Advanced maternal age is assumed to be a risk factor for postpartum hemorrhage.23 The increased risk was thought to be related to the increased incidence of multiple underlying factors, such as cesarean delivery, multiple gestation, and hypertensive disorders of pregnancy.
However, in a retrospective cohort study, Lao and colleagues found that advanced maternal age (≥35 years) served only as a surrogate factor for postpartum hemorrhage due to associated risk factors, obstetric complications, and interventions.24 After multivariate analysis, aging was associated with a decreased rate of postpartum hemorrhage, which declined progressively from ages 25 to 40 years and older, compared with women aged 20 to 24.24
Nevertheless, medical interventions should be readily available at the time of delivery for treatment of uterine atony, especially with multiple gestation and grand multiparity.
Case: Resolved
The patient is admitted to the hospital, where she is given IV magnesium sulfate (6-g load followed by an infusion of 3 g/hr) and betamethasone for fetal lung maturity enhancement. She continues to receive IV fluids as well (125 mL/hr lactated Ringers solution). Uterine activity abates.
IV magnesium sulfate is continued for 36 hours, but urine protein output is not monitored. Her heart rate ranges from 105 to 115 bpm, and blood pressure from 130/80 mm Hg to 138/88 mm Hg. Forty-eight hours after admission, she reports a gradual onset of tightness of the chest and breathlessness. She is agitated, with a pulse of 130 bpm, 30 respirations/min, and room air pulse oximetry of 90%. Rales are noted upon auscultation of both lungs. A radiograph of the chest demonstrates bilateral air-space disease consistent with pulmonary edema. IV furosemide and oxygen (by mask) are provided, with some respiratory improvement.
The patient then reports leakage of amniotic fluid, and preterm rupture of membranes is confirmed on examination. Because steroids for fetal lung maturity have been administered, and given improvement in her pulmonary edema and a footling breech presentation for Twin A, cesarean delivery is performed.
The patient’s immediate postoperative course is uncomplicated. On postoperative day 2, however, she develops recurrent pulmonary edema, as confirmed by physical examination and chest radiograph. She also reports headache, and her blood pressure rises to 164/114 mm Hg—findings consistent with postpartum preeclampsia. Magnesium sulfate and antihypertensive therapy are initiated, along with IV furosemide and oxygen, which improves her respiratory status.
An echocardiogram to rule out peripartum dilated cardiomyopathy finds no evidence of a dilated left ventricle, and the calculated left ventricular ejection fraction (55%) is normal.
After diuresis and improvement in her blood pressure, she is discharged home. By the time of her follow-up office visit 7 days later, her blood pressure has normalized on labetalol therapy.
For an overview of evaluation and management of pregnant women aged 40 or older, see TABLE 4.
Preconception
- Identify risk factors (ie, diabetes, obesity, hypertension, cardiac dysfunction, family history
- Review outcome of previous pregnancy, if applicable
- Review risks (multiple gestation, birth defects) associated with assisted reproductive technologies if they were needed to achieve pregnancy
- Optimize maternal health
- Begin folic acid supplementation
- Encourage smoking cessation
- If the patient is ≥45 years old:
– Electrocardiogram
– Glucose screening (fasting plasma glucose or hemoglobin A1c)
– Echocardiogram for patients with chronic hypertension
First trimester
- Ultrasonography for dating and to assess fetal number and chorionicity
- Baseline metabolic profile and complete blood count
- Baseline urinalysis
- Continue folic acid supplementation
- Offer first-trimester genetic testing or other genetic screening
Second trimester
- If first-trimester genetic testing is declined, offer second-trimester testing or screening
- Detailed fetal anomaly evaluation by ultrasound
- Fetal echocardiogram if pregnancy was achieved by in vitro fertilization or if it is a monochorionic twin gestation
- Screen for gestational diabetes
Third trimester
- Increased antenatal testing for routine indications, including hypertension, diabetes, and lupus
- Ultrasonography for growth and later ultrasonographic findings of fetal aneuploidy
- Consider delivery
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
CASE: Preterm labor in an older woman
G.S. is a 41-year-old G1P0 with a several-year history of infertility and a medical history of chronic hypertension. She undergoes in vitro fertilization (IVF) using her own oocytes, with transfer of two embryos. Early ultrasonography (US) confirms a diamniotic/dichorionic twin gestation. She undergoes chorionic villus sampling (CVS) during the first trimester, with normal fetal karyotypes noted.
For her chronic hypertension, the patient is treated with oral labetalol 200 mg twice daily, beginning in the first trimester. Results of a baseline comprehensive metabolic profile and complete blood count, and electrocardiogram are normal. Baseline 24-hour urine study results reveal no significant proteinuria and a normal creatinine clearance.
At 18 weeks’ gestation, US results show normal growth and amniotic fluid volume for each fetus, with no anomalies detected. Because of a gradual increase in the patient’s blood pressure, her labetalol dose is increased to 400 mg orally thrice daily. Her urine protein output remains negative on dipstick, and US every 4 weeks until 28 weeks’ gestation continues to show normal fetal growth and amniotic fluid volume.
At 33 weeks’ gestation, the patient presents with regular uterine activity. Nonstress tests for both fetuses are reactive. She is given a 1-L intravenous (IV) fluid bolus of lactated Ringers solution, as well as subcutaneous terbutaline sulfate every 15 minutes for four doses, without resolution of the uterine contractions. Her pulse has increased to 120 bpm.
How do you manage this patient’s care?
Nine times as many women aged 35 and older gave birth to their first child in 2012 than did women of the same age 40 years ago, according to the most recent data from the National Center for Health Statistics.1 The rate of first births for women aged 40 to 44 remained essentially stable during the 1970s and early 1980s but increased more than fourfold from 1985 through 2012—from 0.5 to 2.3 per 1,000 women.1 Clearly, more women are delaying childbearing to a later age by personal choice for reasons such as completion of education and career advancement.2
The path to late motherhood is not without thorns, however. Heightened risks associated with increasing maternal age include:
- fetal aneuploidy
- fetal malformation
- gestational diabetes
- chronic and gestational hypertension
- antepartum hemorrhage
- placenta previa
- prelabor rupture of membranes
- preterm labor.3,4
Women with advanced age at conception also are more likely to have a multifetal gestation because of the need for assisted reproduction and are more likely to require cesarean delivery5 as a result of abnormal placentation, fetal malpresentation, an abnormal pattern of labor, or increased use of oxytocin in labor. In addition, they are more likely to experience rupture of the sphincter, postpartum hemorrhage, and thromboembolism.3 Advanced maternal age also is associated with a higher risk of stillbirth throughout gestation, with the peak risk period reported to occur at 37 to 41 weeks.6
Maternal age-related risks of autosomal trisomies (especially Down syndrome) are well understood and have been quantified for singleton and twin gestations. TABLE 1 shows the risks at term for singleton and twin gestations for at least one chromosomally abnormal fetus by maternal age (40–46 years) and race.7
Preconception considerations
Aging and fertility
These combined result of aging of the ovary and uterus and an escalating risk of underlying medical comorbidities has a detrimental effect on fertility.8 Although assisted reproductive technologies are helpful, they cannot guarantee a live birth or completely compensate for an age-related decline in fertility.9
Many IVF programs refuse infertility treatment to women over age 43 or 44 who want to use their own oocytes. The reason: low pregnancy rates. The use of donor oocytes, however, increases the risks of gestational hypertension and preeclampsia. And if assisted reproductive technologies are needed, the risk for multifetal pregnancy increases.
Women of advanced maternal age are likely to have an older spouse or partner. There is no clearly accepted definition of advanced paternal age, but it is most often defined as an age of 40 years or older at the time of conception. Advanced paternal age has been associated with a higher risk for autism spectrum disorder and schizophrenia, as well as mutations in the FGFR2 and FGFR3 genes that result in skeletal dysplasias and craniosynostosis syndromes.10
Medical conditions are more common
Women of advanced maternal age have an increased rate of such prepregnancy chronic medical complications as diabetes, chronic hypertension, obesity, and renal and cardiac disease. Therefore, it is best to optimize control of these chronic illnesses prior to conception to minimize the risks of miscarriage, fetal anomalies, and gestational hypertension and preeclampsia.
Preeclampsia. Although daily low-dose (60–81 mg) aspirin has been used to reduce the risk of preeclampsia, current recommendations from the American College of Obstetricians and Gynecologists (ACOG) suggest that this therapy be reserved for women with a medical history of early-onset preeclampsia or those who have had preeclampsia in more than one pregnancy.11
Impact of obesity. We recently examined the influence of age and obesity on pregnancy outcomes of nulliparous women aged 40 or older at delivery.12 The study included women aged 20 to 29 years (n = 52,249) and 40 or older (n = 1,231) who delivered singleton infants. Women who reported medical disorders, tobacco use, or conception with assisted reproductive technology were excluded.
In the older age group (≥40 years), obese women had significantly higher rates of cesarean delivery, gestational hypertension, preeclampsia, gestational diabetes, preterm delivery before 37 weeks’ gestation, and preterm delivery before 28 weeks, and their infants had higher rates of admission to the neonatal intensive care unit (NICU), compared with nonobese women (FIGURE).
It would appear, however, that healthy, obese women who delay pregnancy until the age of 40 or later may modify their risk for cesarean delivery, gestational diabetes mellitus, and gestational hypertension and preeclampsia by reducing their body mass index to nonobese levels prior to conception.
In addition to maternal risks for women of advanced maternal age, there are risks to the fetus and neonate, as well as a risk of placental abnormalities. These risks are summarized in TABLE 2.
Placental
- Molar or partial molar pregnancy
- Fetus or twins with a complete mole
- Placenta previa, vasa previa
Fetal/neonatal
- Aneuploidy
- Selective fetal growth restriction in twin gestation
- Twin-twin transfusion syndrome
- Preterm birth
- Perinatal death
Antepartum
- Gestational diabetes
- Insulin-dependent diabetes
- Gestational hypertension and preeclampsia
- Cholestasis of pregnancy
- Acute fatty liver of pregnancy
- Venous thromboembolism
- Preterm labor, preterm premature rupture
of membranes
Intrapartum
- Dysfunctional labor
- Malpresentation
- Cesarean delivery
Postpartum
- Venous thromboembolism
- Postpartum hemorrhage
Folic acid supplementation can reduce some risks
The potential benefit of folic acid supplementation to reduce the risk of fetal open neural tube defects is well documented. More recent data suggest that folic acid also is associated with a reduction in the risks of congenital heart defects, abdominal wall defects, cleft lip and palate, and spontaneous abortion. Supplementation should be initiated at least 3 months prior to conception and continued through the first trimester.
The first trimester
Early pregnancy loss is a risk
Women of advanced maternal age are more likely than younger women to experience early pregnancy loss. This risk is due to higher rates of fetal aneuploidy as well as declining ovarian and uterine function and a higher rate of ectopic pregnancy.
In the First and Second Trimester Evaluation of Risk (FASTER) trial, in which investigators reported pregnancy outcomes by maternal age for 36,056 pregnancies, the rate of spontaneous abortion after 10 weeks of gestation was 0.8% among women younger than 35 years, compared with 2.2% for women aged 40 or older.4
The likelihood of multiple gestation increases
The background risk of multiple births is higher in women of advanced maternal age, compared with younger women. This risk increases further with fertility treatment.
Multiple gestations at any age are associated with increased risks for preterm birth and very-low–birthweight infants. Potential maternal risks are listed in TABLE 3.
- Hypertension (2.5 times the risk of a singleton gestation)
- Abruption (3.0 times the risk)
- Anemia (2.5 times the risk)
- Urinary tract infection (1.5 times the risk)
- Preeclampsia (risk of 26%–75%) (occurs at earlier gestation) — HELLP syndrome (risk of 9%)
- Abruption (20%) (10 times the risk of a singleton gestation)
- Anemia (24%)
- Preterm premature rupture of membranes (24%)
- Gestational diabetes (14%)
- Acute fatty liver (4%) (1 in 10,000 singletons)
- Postpartum hemorrhage (9%)
To reduce the number of multiple gestations with assisted reproduction, consider elective single embryo transfer, especially if the mother has significant underlying medical complications.
Multiple gestations present difficult management issues in older women. Strategies shown to prevent preterm delivery in singleton gestations, including weekly 17-hydroxyprogesterone injections and cervical cerclage, are not effective in multiple gestations. Moreover, many of these therapies—including bed rest—increase the risk of thromboembolic events in multiple gestations, particularly when the mother is of advanced age.
Maternal adaptations in multiple gestations also may be poorly tolerated by older patients, particularly cardiac changes that markedly increase stroke volume, heart rate, cardiac output, and plasma volume.
The range of genetic screening and testing options has broadened
Options include first-trimester CVS, which provides information about the fetal chromosomal complement but not the presence of a fetal open neural tube defect. The procedure-related rate of fetal loss with CVS is quoted as 1%.
Options for genetic testing in the second trimester include transabdominal amniocentesis. A procedure-related fetal loss rate of 1 in 500 to 1 in 1,600 is quoted for midtrimester amniocentesis.
A relatively new screening option is analysis of cell-free fetal DNA in maternal blood, which can be performed after 10 weeks’ gestation in singleton and multiple gestations. This directed analysis measures the relative proportions of chromosomes. The detection rate for fetal Down syndrome using cell-free fetal DNA is greater than 98%, with a false-positive rate of less than 0.5%. However, this screening is unreliable in triplet gestations.
Other screening options include US and biochemical screening to detect fetal aneuploidy and open neural tube defects during the second trimester. These options should be included in counseling of the patient.
Second and third trimesters
Gestational hypertension and preeclampsia are significant risks
Older pregnant women have an incidence of gestational hypertension and preeclampsia 2 to 4 times as high as that of patients younger than 30 years.13 The underlying risk for preeclampsia is further increased if coexisting medical disorders such as diabetes or chronic hypertension are present. Moreover, the risk for preeclampsia increases to 10% to 20% in twin gestations and 25% to 60% in triplet gestations. Le Ray and colleagues reported that, if oocyte donation is used with IVF in women older than age 43, the risk for preeclampsia triples.14
We previously studied 379 women aged 35 and older who had mild gestational hypertension remote from term, comparing them with their younger adult counterparts in a matched cohort design.15 Outpatient management produced similar maternal outcomes in both groups, but older women had a statistically insignificant increase in the rate of stillbirth (5 vs 0; P = .063).15
Gestational diabetes risk doubles
The rates of both diabetes mellitus and gestational diabetes increase with advanced maternal age. Data from the FASTER consortium included an adjusted odds ratio of 2.4 for gestational diabetes in women aged 40 or older, compared with a younger control group.4 This increased risk may be a consequence of greater maternal habitus as well as declining insulin sensitivity.
Diabetes increases the risks of macrosomia, cesarean birth, and gestational hypertension. Among women with pregestational diabetes, the risks of congenital heart disease and fetal neural tube defects increase threefold. Because of this increased risk, perinatal screening is indicated for both anomalies in older women.
Pulmonary complications increase
Another risk facing women of advanced maternal age—particularly those carrying a multiple gestation—is pulmonary edema, owing to the increased cardiac output, heart rate, and blood volume, the decreased systemic vascular resistance, and the physiologic anemia of pregnancy. These risks rise further in women who develop preterm labor that requires therapy and in those who develop gestational hypertension and/or preeclampsia. Judicious use of IV fluids, particularly those with lower sodium concentrations, can reduce the risk of pulmonary complications.
Women who develop pulmonary edema have an increased risk of peripartum cardiomyopathy.16
Preterm delivery is more common
Cleary-Goodman and colleagues noted an increased incidence of preterm delivery in women aged 40 and older, compared with women younger than age 35, but no increase in spontaneous preterm labor.4 Advanced maternal age appears to be associated with an increased risk of preterm birth largely as a consequence of underlying complications of fetal growth restriction and maternal disease, including hypertension. Because preterm birth is an important contributor to perinatal morbidity and mortality, steroids should be administered for fetal lung maturity whenever preterm labor is diagnosed before 34 weeks’ gestation.
Risk of placenta previa is 1.1%
Joseph and colleagues found the risk of placenta previa to be 1.1% in women aged 40 and older, compared with 0.3% in women aged 25 to 29 years.17 This increased risk likely is a consequence not only of maternal age but increased parity and a history of prior uterine surgery. If transabdominal US results are suspicious for placenta previa, transvaginal US is indicated for confirmation. Additional US assessment of the cord insertion site to the placenta also should be performed to rule out vasa previa.
Look for neonatal complications
Ziadeh and colleagues found that, although maternal morbidity was increased in older women, the overall neonatal outcome did not appear to be affected.18 However, we noted a higher rate of neonatal complications in women aged 40 or older, including higher NICU admission rates and more low-birth–weight infants.11
In addition, Odibo and colleagues found advanced maternal age to be an independent risk factor for intrauterine growth restriction (IUGR).19 In that study, the odds ratio for IUGR was 3.2 (95% confidence interval [CI], 1.9–5.4) for a maternal age of 40 years or older, compared with a control group. For that reason, they recommend routine screening for IUGR in all pregnant women of advanced age.
Stillbirth risk peaks at 37 to 41 weeks
In a review of more than 5.4 million singleton pregnancies without reported congenital anomalies, Reddy and colleagues found an association between advanced maternal age and stillbirth, with a higher risk of stillbirth at 37 to 41 weeks’ gestation.6 This effect of maternal age persisted despite adjusting for medical disease, parity, and race/ethnicity.
Many women older than age 40 have independent medical or fetal indications for antenatal testing. Some experts have suggested antepartum surveillance starting at 37 weeks for women of advanced maternal age; they argue that the risk of stillbirth at this gestational age is similar in frequency to other high-risk conditions for which testing is performed routinely. However, the National Institute of Child Health and Human Development (NICHD) workshop on antepartum fetal monitoring found insufficient evidence that antenatal testing for the sole indication of advanced maternal age reduces stillbirth or improves perinatal outcomes.20
If increased antenatal testing is indicated for a high-risk condition or electively chosen given advanced age, it should include electronic fetal monitoring as well as amniotic fluid volume assessment. Because the risk of fetal loss sharply increased at 40 weeks’ gestation in the study by Reddy and colleagues,6 women older than age 40 should be considered for delivery by 40 weeks’ gestation in the presence of good dating criteria.
Some clinicians also would consider delivery by 39 weeks’ gestation with good dating criteria if the Bishop score is favorable.
Risks of labor and delivery
Multiple variables contribute to a higher cesarean delivery rate
The risk of cesarean delivery increases with advancing maternal age.5,11 This increased risk is a consequence of multiple variables, including the rate of previous cesarean delivery, malpresentation, underlying complications such as preeclampsia and diabetes, and a higher prevalence of dysfunctional labor.21 Further, Vaughn and colleagues noted that the cesarean delivery rate increases in direct proportion to age, with a rate of 54.4% in women older than age 40.5
As Cohen pointed out in a commentary accompanying a study of dysfunctional labor in women of advancing age, “the notion of a premium baby (ie, that the fetus of a woman with a reduced likelihood of having another pregnancy is somehow more deserving of being spared the rigours of labour than the fetus of a young woman) may play a role” in the high rate of cesarean delivery.21,22
Postpartum hemorrhage risk may be lower in older women
Advanced maternal age is assumed to be a risk factor for postpartum hemorrhage.23 The increased risk was thought to be related to the increased incidence of multiple underlying factors, such as cesarean delivery, multiple gestation, and hypertensive disorders of pregnancy.
However, in a retrospective cohort study, Lao and colleagues found that advanced maternal age (≥35 years) served only as a surrogate factor for postpartum hemorrhage due to associated risk factors, obstetric complications, and interventions.24 After multivariate analysis, aging was associated with a decreased rate of postpartum hemorrhage, which declined progressively from ages 25 to 40 years and older, compared with women aged 20 to 24.24
Nevertheless, medical interventions should be readily available at the time of delivery for treatment of uterine atony, especially with multiple gestation and grand multiparity.
Case: Resolved
The patient is admitted to the hospital, where she is given IV magnesium sulfate (6-g load followed by an infusion of 3 g/hr) and betamethasone for fetal lung maturity enhancement. She continues to receive IV fluids as well (125 mL/hr lactated Ringers solution). Uterine activity abates.
IV magnesium sulfate is continued for 36 hours, but urine protein output is not monitored. Her heart rate ranges from 105 to 115 bpm, and blood pressure from 130/80 mm Hg to 138/88 mm Hg. Forty-eight hours after admission, she reports a gradual onset of tightness of the chest and breathlessness. She is agitated, with a pulse of 130 bpm, 30 respirations/min, and room air pulse oximetry of 90%. Rales are noted upon auscultation of both lungs. A radiograph of the chest demonstrates bilateral air-space disease consistent with pulmonary edema. IV furosemide and oxygen (by mask) are provided, with some respiratory improvement.
The patient then reports leakage of amniotic fluid, and preterm rupture of membranes is confirmed on examination. Because steroids for fetal lung maturity have been administered, and given improvement in her pulmonary edema and a footling breech presentation for Twin A, cesarean delivery is performed.
The patient’s immediate postoperative course is uncomplicated. On postoperative day 2, however, she develops recurrent pulmonary edema, as confirmed by physical examination and chest radiograph. She also reports headache, and her blood pressure rises to 164/114 mm Hg—findings consistent with postpartum preeclampsia. Magnesium sulfate and antihypertensive therapy are initiated, along with IV furosemide and oxygen, which improves her respiratory status.
An echocardiogram to rule out peripartum dilated cardiomyopathy finds no evidence of a dilated left ventricle, and the calculated left ventricular ejection fraction (55%) is normal.
After diuresis and improvement in her blood pressure, she is discharged home. By the time of her follow-up office visit 7 days later, her blood pressure has normalized on labetalol therapy.
For an overview of evaluation and management of pregnant women aged 40 or older, see TABLE 4.
Preconception
- Identify risk factors (ie, diabetes, obesity, hypertension, cardiac dysfunction, family history
- Review outcome of previous pregnancy, if applicable
- Review risks (multiple gestation, birth defects) associated with assisted reproductive technologies if they were needed to achieve pregnancy
- Optimize maternal health
- Begin folic acid supplementation
- Encourage smoking cessation
- If the patient is ≥45 years old:
– Electrocardiogram
– Glucose screening (fasting plasma glucose or hemoglobin A1c)
– Echocardiogram for patients with chronic hypertension
First trimester
- Ultrasonography for dating and to assess fetal number and chorionicity
- Baseline metabolic profile and complete blood count
- Baseline urinalysis
- Continue folic acid supplementation
- Offer first-trimester genetic testing or other genetic screening
Second trimester
- If first-trimester genetic testing is declined, offer second-trimester testing or screening
- Detailed fetal anomaly evaluation by ultrasound
- Fetal echocardiogram if pregnancy was achieved by in vitro fertilization or if it is a monochorionic twin gestation
- Screen for gestational diabetes
Third trimester
- Increased antenatal testing for routine indications, including hypertension, diabetes, and lupus
- Ultrasonography for growth and later ultrasonographic findings of fetal aneuploidy
- Consider delivery
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Mathews TJ, Hamilton BE. First births to older women continue to rise. National Center for Health Statistics. NCHS Data Brief No. 152. May 2014. http://www.cdc.gov/nchs/data/databriefs/db152.pdf. Accessed October 3, 2014.
2. Mills M, Rindfuss RR, McDonald P, te Velde E. Why do people postpone parenthood? Reasons and social policy incentives. Hum Reprod Update. 2011;17(6):848–860.
3. Ziadeh SM. Maternal and perinatal outcome in nulliparous women aged 35 and older. Gynecol Obstet Invest. 2002;54(1):6–10.
4. Cleary-Goldman J, Malone FD, Vidaver J, et al; FASTER Consortium. Impact of maternal age on obstetric outcome. Obstet Gynecol. 2005;105(5 pt 1):983–990.
5. Vaughn DA, Cleary BJ, Murphy DJ. Delivery outcomes for nulliparous women at the extremes of maternal age—a cohort study. BJOG. 2014;121(3):261–268.
6. Reddy UM, Ko CW, Willinger M. Maternal age and the risk of stillbirth through pregnancy in the United States. Am J Obstet Gynecol. 2006;195(3):764–770.
7. Meyers C, Adam R, Dungan J, Prenger V. Aneuploidy in twin gestations: when is maternal age advanced? Obstet Gynecol. 1997;89(2):248–251.
8. Nelson SM, Telfer EE, Anderson RA. The ageing ovary and uterus: new biological insights. Hum Reprod Update. 2013;19(1):67–83.
9. Johnson JA, Tough S. Delayed child-bearing. J Obstet Gynaecol Can. 2012;34(1):80–93.
10. Goriely A, Wilkie AO. Paternal age effect mutations and selfish spermatogonial selection: causes and consequences for human disease. Am J Hum Genet. 2012;90(2):175–200.
11. Barton JR, Sibai AJ, Istwan NB, Rhea DJ, Desch CN, Sibai BM. Spontaneously conceived pregnancy after 40: influence of age and obesity on outcome. Am J Perinatol. 2014;31(9):795–798.
12. Roberts JM, August PA, Bakris JR, et al. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122–1131.
13. Jahromi BN, Husseini Z. Pregnancy outcome at maternal age 40 and older. Taiwan J Obstet Gynecol. 2008;47(3):318–321.
14. Le Ray C, Scherier S, Anselem O, et al. Association between oocyte donation and maternal and perinatal outcomes in women aged 43 years or older. Hum Reprod. 2012;27(3):896–901.
15. Barton JR, Bergauer NK, Jacques DL, Coleman SK, Stanziano GJ, Sibai BM. Does advanced maternal age affect pregnancy outcome in women with mild hypertension remote from term? Am J Obstet Gynecol. 1997;176(6):1236–1243.
16. Habli M, O’Brien T, Nowack E, et al. Peripartum cardiomyopathy: prognostic factors for long-term maternal outcome. Am J Obstet Gynecol. 2008;199(4):415.e1–e5.
17. Joseph KS, Allen AC, Dodds L, Turner LA, Scott H, Liston R. The perinatal effects of delayed childbearing. Obstet Gynecol. 2005;105(6):1410–1418.
18. Ziadeh S, Yahaya A. Pregnancy outcome at age 40 and older. Arch Gynecol Obstet. 2001;265(1):30–33.
19. Odibo AO, Nelson D, Stamilio DM, Sehdev HM, Macones GA. Advanced maternal age is an independent risk factor for intrauterine growth restriction. Am J Perinatol. 2006;23(5):325–328.
20. Signore C, Freeman RK, Spong CY. Antenatal testing—a reevaluation: executive summary of a Eunice Kennedy Shriver National Institute of Child Health and Human Development workshop. Obstet Gynecol. 2009;113(3):687–701.
21. Cohen WR, Newman L, Friedman EA. Risk of labor abnormalities with advancing maternal age. Obstet Gynecol. 1980;55(4):414–416.
22. Cohen WR. Does maternal age affect pregnancy outcome? BJOG. 2014;121(3):252–254.
23. Bateman BT, Berman MF, Riley LE, Leffert LR. The epidemiology of postpartum hemorrhage in a large, nationwide sample of deliveries. Anesth Analg. 2010;110(5):1368–1373.
24. Lao TT, Sahota DS, Cheng YK, Law LW, Leung TY. Advanced maternal age and postpartum hemorrhage—risk factor or red herring? J Matern Fetal Neonatal Med. 2014;27(3):243–246.
1. Mathews TJ, Hamilton BE. First births to older women continue to rise. National Center for Health Statistics. NCHS Data Brief No. 152. May 2014. http://www.cdc.gov/nchs/data/databriefs/db152.pdf. Accessed October 3, 2014.
2. Mills M, Rindfuss RR, McDonald P, te Velde E. Why do people postpone parenthood? Reasons and social policy incentives. Hum Reprod Update. 2011;17(6):848–860.
3. Ziadeh SM. Maternal and perinatal outcome in nulliparous women aged 35 and older. Gynecol Obstet Invest. 2002;54(1):6–10.
4. Cleary-Goldman J, Malone FD, Vidaver J, et al; FASTER Consortium. Impact of maternal age on obstetric outcome. Obstet Gynecol. 2005;105(5 pt 1):983–990.
5. Vaughn DA, Cleary BJ, Murphy DJ. Delivery outcomes for nulliparous women at the extremes of maternal age—a cohort study. BJOG. 2014;121(3):261–268.
6. Reddy UM, Ko CW, Willinger M. Maternal age and the risk of stillbirth through pregnancy in the United States. Am J Obstet Gynecol. 2006;195(3):764–770.
7. Meyers C, Adam R, Dungan J, Prenger V. Aneuploidy in twin gestations: when is maternal age advanced? Obstet Gynecol. 1997;89(2):248–251.
8. Nelson SM, Telfer EE, Anderson RA. The ageing ovary and uterus: new biological insights. Hum Reprod Update. 2013;19(1):67–83.
9. Johnson JA, Tough S. Delayed child-bearing. J Obstet Gynaecol Can. 2012;34(1):80–93.
10. Goriely A, Wilkie AO. Paternal age effect mutations and selfish spermatogonial selection: causes and consequences for human disease. Am J Hum Genet. 2012;90(2):175–200.
11. Barton JR, Sibai AJ, Istwan NB, Rhea DJ, Desch CN, Sibai BM. Spontaneously conceived pregnancy after 40: influence of age and obesity on outcome. Am J Perinatol. 2014;31(9):795–798.
12. Roberts JM, August PA, Bakris JR, et al. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122–1131.
13. Jahromi BN, Husseini Z. Pregnancy outcome at maternal age 40 and older. Taiwan J Obstet Gynecol. 2008;47(3):318–321.
14. Le Ray C, Scherier S, Anselem O, et al. Association between oocyte donation and maternal and perinatal outcomes in women aged 43 years or older. Hum Reprod. 2012;27(3):896–901.
15. Barton JR, Bergauer NK, Jacques DL, Coleman SK, Stanziano GJ, Sibai BM. Does advanced maternal age affect pregnancy outcome in women with mild hypertension remote from term? Am J Obstet Gynecol. 1997;176(6):1236–1243.
16. Habli M, O’Brien T, Nowack E, et al. Peripartum cardiomyopathy: prognostic factors for long-term maternal outcome. Am J Obstet Gynecol. 2008;199(4):415.e1–e5.
17. Joseph KS, Allen AC, Dodds L, Turner LA, Scott H, Liston R. The perinatal effects of delayed childbearing. Obstet Gynecol. 2005;105(6):1410–1418.
18. Ziadeh S, Yahaya A. Pregnancy outcome at age 40 and older. Arch Gynecol Obstet. 2001;265(1):30–33.
19. Odibo AO, Nelson D, Stamilio DM, Sehdev HM, Macones GA. Advanced maternal age is an independent risk factor for intrauterine growth restriction. Am J Perinatol. 2006;23(5):325–328.
20. Signore C, Freeman RK, Spong CY. Antenatal testing—a reevaluation: executive summary of a Eunice Kennedy Shriver National Institute of Child Health and Human Development workshop. Obstet Gynecol. 2009;113(3):687–701.
21. Cohen WR, Newman L, Friedman EA. Risk of labor abnormalities with advancing maternal age. Obstet Gynecol. 1980;55(4):414–416.
22. Cohen WR. Does maternal age affect pregnancy outcome? BJOG. 2014;121(3):252–254.
23. Bateman BT, Berman MF, Riley LE, Leffert LR. The epidemiology of postpartum hemorrhage in a large, nationwide sample of deliveries. Anesth Analg. 2010;110(5):1368–1373.
24. Lao TT, Sahota DS, Cheng YK, Law LW, Leung TY. Advanced maternal age and postpartum hemorrhage—risk factor or red herring? J Matern Fetal Neonatal Med. 2014;27(3):243–246.
Does stage of labor at time of cesarean affect the risk of subsequent preterm birth?
Recent policy changes in the United States have led to a modest reduction in the incidence of late PTB (34–37 weeks), but the rate of PTB before 34 weeks’ gestation has changed little over the past 40 years. One reason: It has taken us more than 30 years to recognize that uterine contractions are a downstream consequence—not a cause—of preterm labor. By the time a woman presents to labor and delivery with regular phasic uterine contractions and cervical change, it is too late to alter the course of events; the pathogenic processes leading to this clinical presentation have been active for weeks—probably months. Efforts to suppress myometrial contractility at this point using standard tocolytic medications have little or no effect. At best, they delay delivery for 24 to 48 hours, just time enough to transfer the patient to a tertiary care center, administer antenatal corticosteroids, and, possibly, administer magnesium sulfate for neuroprotection.
There is mounting evidence that the cervix plays a central role in spontaneous PTB pathogenesis. The task of the cervix is to remain functionally intact (long and closed) throughout gestation even as the fetus grows and the uterus expands, and then to efface and dilate in the days and hours before labor. PTB ensues if this process of cervical remodeling occurs prematurely.
In support of this hypothesis, cervical shortening on transvaginal ultrasound in the mid second trimester is a major risk factor for spontaneous PTB that is independent of parity and obstetric history.1 This risk can be abrogated by interventions that artificially “strengthen” the cervix (such as placement of a cervical cerclage or pessary) or interfere with the biochemical changes within the cervical stroma that promote cervical effacement (by progesterone supplementation). This analysis by Levine and colleagues provides additional evidence in support of the role of the cervix in spontaneous PTB.
As the investigators themselves hypothesize: “… there may be an inherent biologic risk in achieving complete dilation, regardless of mode of delivery, or perhaps something protective about not achieving complete dilation. This could be attributed to changes in cervical stroma that occur with complete dilation that causes the cervix to be more susceptible to premature dilation in a future pregnancy.”
Although compelling, the “cervical trauma” hypothesis remains to be confirmed. Additional studies are needed to confirm these observations, and it would be preferable to limit these studies to the risk of subsequent spontaneous PTB with intact membranes only, rather than including women with preterm premature rupture of membranes, as in the current study.
What this evidence means for practice
This study suggests that the attainment of full dilation during the course of labor may damage the fibrous tissues that make up the cervical stroma, leading to a persistent functional defect that manifests as spontaneous PTB in a future pregnancy. If that is true, could it also be true that a normal vaginal delivery at term is a “risk factor” for spontaneous PTB in a future pregnancy? There is one clinical variable that could account for this apparent contradiction—the interpregnancy interval. It is well known that a short interval (<6 months) is a risk factor for adverse pregnancy outcomes, including PTB. It is possible that, having been injured at the time of delivery, the cervical stroma needs time to heal. Similar observations have been made when investigating the association between cervical conization and PTB.2 This is a testable hypothesis that I hope these investigators will pursue. In the meantime, we should continue to advise our patients to allow for an appropriate interval between pregnancies.
Errol R. Norwitz, MD, PHD
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Iams JD, Goldenberg RL, Meis PJ, et al. The length of the cervix and the risk of spontaneous premature delivery. N Engl J Med. 1996;334(9):567–572.
2.Himes KP, Simhan HN. Time from cervical conization to pregnancy and preterm birth. Obstet Gynecol. 2007;109(2 pt 1):314–319.
Recent policy changes in the United States have led to a modest reduction in the incidence of late PTB (34–37 weeks), but the rate of PTB before 34 weeks’ gestation has changed little over the past 40 years. One reason: It has taken us more than 30 years to recognize that uterine contractions are a downstream consequence—not a cause—of preterm labor. By the time a woman presents to labor and delivery with regular phasic uterine contractions and cervical change, it is too late to alter the course of events; the pathogenic processes leading to this clinical presentation have been active for weeks—probably months. Efforts to suppress myometrial contractility at this point using standard tocolytic medications have little or no effect. At best, they delay delivery for 24 to 48 hours, just time enough to transfer the patient to a tertiary care center, administer antenatal corticosteroids, and, possibly, administer magnesium sulfate for neuroprotection.
There is mounting evidence that the cervix plays a central role in spontaneous PTB pathogenesis. The task of the cervix is to remain functionally intact (long and closed) throughout gestation even as the fetus grows and the uterus expands, and then to efface and dilate in the days and hours before labor. PTB ensues if this process of cervical remodeling occurs prematurely.
In support of this hypothesis, cervical shortening on transvaginal ultrasound in the mid second trimester is a major risk factor for spontaneous PTB that is independent of parity and obstetric history.1 This risk can be abrogated by interventions that artificially “strengthen” the cervix (such as placement of a cervical cerclage or pessary) or interfere with the biochemical changes within the cervical stroma that promote cervical effacement (by progesterone supplementation). This analysis by Levine and colleagues provides additional evidence in support of the role of the cervix in spontaneous PTB.
As the investigators themselves hypothesize: “… there may be an inherent biologic risk in achieving complete dilation, regardless of mode of delivery, or perhaps something protective about not achieving complete dilation. This could be attributed to changes in cervical stroma that occur with complete dilation that causes the cervix to be more susceptible to premature dilation in a future pregnancy.”
Although compelling, the “cervical trauma” hypothesis remains to be confirmed. Additional studies are needed to confirm these observations, and it would be preferable to limit these studies to the risk of subsequent spontaneous PTB with intact membranes only, rather than including women with preterm premature rupture of membranes, as in the current study.
What this evidence means for practice
This study suggests that the attainment of full dilation during the course of labor may damage the fibrous tissues that make up the cervical stroma, leading to a persistent functional defect that manifests as spontaneous PTB in a future pregnancy. If that is true, could it also be true that a normal vaginal delivery at term is a “risk factor” for spontaneous PTB in a future pregnancy? There is one clinical variable that could account for this apparent contradiction—the interpregnancy interval. It is well known that a short interval (<6 months) is a risk factor for adverse pregnancy outcomes, including PTB. It is possible that, having been injured at the time of delivery, the cervical stroma needs time to heal. Similar observations have been made when investigating the association between cervical conization and PTB.2 This is a testable hypothesis that I hope these investigators will pursue. In the meantime, we should continue to advise our patients to allow for an appropriate interval between pregnancies.
Errol R. Norwitz, MD, PHD
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Recent policy changes in the United States have led to a modest reduction in the incidence of late PTB (34–37 weeks), but the rate of PTB before 34 weeks’ gestation has changed little over the past 40 years. One reason: It has taken us more than 30 years to recognize that uterine contractions are a downstream consequence—not a cause—of preterm labor. By the time a woman presents to labor and delivery with regular phasic uterine contractions and cervical change, it is too late to alter the course of events; the pathogenic processes leading to this clinical presentation have been active for weeks—probably months. Efforts to suppress myometrial contractility at this point using standard tocolytic medications have little or no effect. At best, they delay delivery for 24 to 48 hours, just time enough to transfer the patient to a tertiary care center, administer antenatal corticosteroids, and, possibly, administer magnesium sulfate for neuroprotection.
There is mounting evidence that the cervix plays a central role in spontaneous PTB pathogenesis. The task of the cervix is to remain functionally intact (long and closed) throughout gestation even as the fetus grows and the uterus expands, and then to efface and dilate in the days and hours before labor. PTB ensues if this process of cervical remodeling occurs prematurely.
In support of this hypothesis, cervical shortening on transvaginal ultrasound in the mid second trimester is a major risk factor for spontaneous PTB that is independent of parity and obstetric history.1 This risk can be abrogated by interventions that artificially “strengthen” the cervix (such as placement of a cervical cerclage or pessary) or interfere with the biochemical changes within the cervical stroma that promote cervical effacement (by progesterone supplementation). This analysis by Levine and colleagues provides additional evidence in support of the role of the cervix in spontaneous PTB.
As the investigators themselves hypothesize: “… there may be an inherent biologic risk in achieving complete dilation, regardless of mode of delivery, or perhaps something protective about not achieving complete dilation. This could be attributed to changes in cervical stroma that occur with complete dilation that causes the cervix to be more susceptible to premature dilation in a future pregnancy.”
Although compelling, the “cervical trauma” hypothesis remains to be confirmed. Additional studies are needed to confirm these observations, and it would be preferable to limit these studies to the risk of subsequent spontaneous PTB with intact membranes only, rather than including women with preterm premature rupture of membranes, as in the current study.
What this evidence means for practice
This study suggests that the attainment of full dilation during the course of labor may damage the fibrous tissues that make up the cervical stroma, leading to a persistent functional defect that manifests as spontaneous PTB in a future pregnancy. If that is true, could it also be true that a normal vaginal delivery at term is a “risk factor” for spontaneous PTB in a future pregnancy? There is one clinical variable that could account for this apparent contradiction—the interpregnancy interval. It is well known that a short interval (<6 months) is a risk factor for adverse pregnancy outcomes, including PTB. It is possible that, having been injured at the time of delivery, the cervical stroma needs time to heal. Similar observations have been made when investigating the association between cervical conization and PTB.2 This is a testable hypothesis that I hope these investigators will pursue. In the meantime, we should continue to advise our patients to allow for an appropriate interval between pregnancies.
Errol R. Norwitz, MD, PHD
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Iams JD, Goldenberg RL, Meis PJ, et al. The length of the cervix and the risk of spontaneous premature delivery. N Engl J Med. 1996;334(9):567–572.
2.Himes KP, Simhan HN. Time from cervical conization to pregnancy and preterm birth. Obstet Gynecol. 2007;109(2 pt 1):314–319.
1. Iams JD, Goldenberg RL, Meis PJ, et al. The length of the cervix and the risk of spontaneous premature delivery. N Engl J Med. 1996;334(9):567–572.
2.Himes KP, Simhan HN. Time from cervical conization to pregnancy and preterm birth. Obstet Gynecol. 2007;109(2 pt 1):314–319.
Premature infant has CP: $14.5M verdict
Premature infant has CP: $14.5M verdict
After learning that, 14 years earlier, a 36-year-old woman had undergone an emergency cesarean delivery at 32 weeks’ gestation, her health-care providers planned a cesarean delivery for the new pregnancy. The woman was admitted to the hospital in preterm labor. Three days later, she was discharged, but readmitted twice more over a 2-week period. At each admission, preterm labor was halted using medication and bed rest.
The patient’s water broke and she was admitted to the hospital at 25 weeks’ gestation, about a week after the previous admission. Shortly after admission, the patient asked about a cesarean delivery, but no action was taken. When her ObGyn arrived at the hospital 5 hours later, the patient asked for a cesarean delivery; the ObGyn said he wanted to wait to see how her labor was progressing. After 3 hours, the fetus showed signs of distress, and an emergency cesarean delivery was undertaken. The infant experienced a massive brain hemorrhage, resulting in cerebral palsy (CP). The child has cognitive delays, visual impairment, and additional problems; he will require lifelong care.
PARENTS’ CLAIM The ObGyn and hospital were negligent in discharging the woman from admission for preterm labor. Cesarean delivery should have been performed much earlier due to nonreassuring fetal heart tones. Severe variable decelerations caused cerebral blood flow fluctuations that led to the hemorrhage.
DEFENDANTS’ DEFENSE The child’s prematurity and a severe placental infection led to the injuries. Nothing would have changed the outcome.
VERDICT A $14.5 million Ohio verdict was returned, including $1.5 million for the mother.
_______________
Costs returned afterverdict for the defense
A 65-year-old woman underwent a hysterectomy for treatment of uterine cancer performed by a gynecologic oncologist. Postoperatively, the patient developed an infection. A small-bowel injury was surgically repaired. The patient was hospitalized for 4 months for treatment of sepsis.
PARENTS’ CLAIM The physician was negligent for injuring the patient’s bowel and then failing to identify and repair the injury during surgery.
PHYSICIAN’S DEFENSE There was no negligence. The patient had significant adhesions from prior surgeries. The physician noted minor serosal tears of the bowel, several of which were repaired during surgery. He checked the length of the bowel for tears/perforations several times during the procedure, but found none. The patient had areas of weakness in her bowel, one of which broke down after surgery. The perforation was repaired in a timely manner.
VERDICT A Michigan defense verdict was returned. The physician was awarded $14,535 in costs.
_______________
Colon injury after cystectomy
A 21-year-old woman underwent laparoscopic ovarian cystectomy, performed by her gynecologist, and was discharged the next day. Eight days later, the patient went to the emergency department (ED) with pelvic pain. Testing revealed a perforated colon with peritonitis. She underwent repair by laparotomy, including bowel resection and colostomy, which was reversed several months later. She has not regained regular bowel function, cannot digest food that has not been finely sliced, and constantly uses laxatives.
PARENTS’ CLAIM The colon injury occurred during cystectomy because the gynecologist was negligent in failing to maintain proper anatomical landmarks. The injury should have been recognized at the time of surgery by injecting saline solution into the colon. She had not been informed of the risk of colon injury.
DEFENDANTS’ DEFENSE Colon injury is a known complication of cystectomy. The injury could have occurred after surgery due to a minor nick of the colon that was undetectable during surgery. Proper informed consent was acquired.
VERDICT A $340,000 New York settlement was reached.
_______________
Mother hemorrhages, dies after delivery: $1M settlement
A 19-year-old woman presented at full term to a community hospital. After several hours of labor, an emergency cesarean delivery was performed due to arrested descent.
Fifteen minutes after delivery, the mother exhibited moderate bleeding with decreasing blood pressure and tachycardia. The post-anesthesia care unit nurse assessed the patient’s uterus as “boggy,” and alerted the ObGyn, who immediately reacted by expressing clots from the uterus. He noted that the fundus was firm. He ordered intravenous (IV) oxytocin, but the patient continued to hemorrhage. Fifteen minutes later, the patient’s vital signs worsened. The ObGyn ordered blood products, uterotonics, and an additional IV line for fluid resuscitation. He began to massage the fundus and expressed clots.
When the patient did not stabilize, she was returned to the OR. After attempting to stop the bleeding with O’Leary stitches, the ObGyn performed a hysterectomy. Six hours after surgery, and after transfusion of a total of 12 units of blood, the woman coded multiple times. She died 14 hours after delivery. Cause of death was disseminated intravascular coagulopathy caused by an atonic uterus.
ESTATE’S CLAIM The ObGyn failed to recognize the extent of the postpartum hemorrhage and should have acted more aggressively with resuscitation. He should have returned her to the OR earlier. The ObGyn was negligent in waiting 45 minutes for cross-matched blood rather than using universal donor O-negative blood that was readily available.
PHYSICIAN’S DEFENSE The ObGyn denied negligence and maintained that he had acted properly. He returned the patient to the OR within 90 minutes of first learning of the hemorrhage.
VERDICT A $1 million Virginia settlement was reached.
_______________
Infant born with broken arms, collarbone, facial bones
A 23-year-old woman had gestational diabetes. She is 5’9” tall and weighed 300 lb while pregnant. She went to the hospital in labor.
During delivery, shoulder dystocia was encountered. The ObGyn performed a variety of techniques, including the McRobert’s maneuver. Forceps were eventually used for delivery.
Both of the newborn’s arms were broken, and she had a broken collarbone and facial fractures. The mother also suffered significant vaginal lacerations and required an episiotomy. She continues to complain of bladder and bowel problems.
PARENTS’ CLAIM A vaginal delivery should not have been attempted due to the mother’s gestational diabetes and the risk of having a macrosomic baby. A cesarean delivery should have been performed. The ObGyn did not use the proper techniques when delivering the child after shoulder dystocia was encountered.
PHYSICIAN’S DEFENSE The ObGyn denied negligence. He claimed that the baby recovered well from her injuries. The mother underwent surgery and now has excellent bladder and bowel control.
VERDICT A confidential Louisiana settlement was reached with the hospital before trial. A defense verdict was returned for the ObGyn.
_______________
Protein found in urine at 39 weeks’ gestation: mother and child die
At 39 weeks' gestation, a woman saw her ObGyn for a prenatal visit. During the examination, the ObGyn found high levels of protein in the woman’s urine, an accumulation of fluid in her ankles, and the highest blood pressure (BP) reading of the woman’s pregnancy. However, because the BP reading was lower than that required to diagnose preeclampsia, the ObGyn sent the patient home and scheduled the next prenatal visit for the following week. The woman and her unborn child died 5 days later.
ESTATE’S CLAIM The ObGyn was negligent in failing to order a urine study and more closely monitor the mother’s symptoms when signs of preeclampsia were evident at 39 weeks’ gestation. Delivery of the child would have resolved the problem and saved both lives.
PHYSICIAN’S DEFENSE The case was settled during the trial.
VERDICT A $3 million Illinois settlement was reached.
_______________
Baby dies from group B strep
A 16-year-old woman planned delivery at a local hospital. Her ObGyn’s practice regularly sends the hospital its patients’ prenatal records, starting at 25 weeks’ gestation. At 33 weeks, the ObGyn took a vaginal culture to test for group B Streptococcus (GBS) bacteria. The laboratory reported positive GBS results to a computer in the ObGyn’s office, but the results were not entered into the patient’s chart.
The mother went to the ED in labor a week later; she was evaluated and discharged. Several days later, she returned to the ED, but was again discharged. She returned the next day, now in gestational week 36. An on-call ObGyn admitted her. A labor and delivery nurse claimed that the ObGyn’s office reported that the mother was GBS negative, so the nurse placed a negative sign in the prenatal record in the chart. When the patient’s ObGyn arrived at the hospital, he noticed the negative sign in the chart.
At birth, the baby’s Apgar scores were 7 at 1 minute and 7 at 5 minutes. She appeared limp and was grunting. A pediatrician diagnosed transient respiratory problems related to prematurity. The baby continued to deteriorate; antibiotics were ordered 7 hours after birth. After the child was transported to another facility, she died. The cause of death was GBS sepsis and pneumonia.
PARENTS’ CLAIM The ObGyn was negligent in failing to properly and timely note the positive GBS test result in the mother’s chart. The ObGyn’s office staff was negligent in miscommunicating the GBS status to the nurse.
DEFENDANTS’ DEFENSE The ObGyn usually noted laboratory results at the next prenatal visit, but the mother gave birth before that occurred. The on-call ObGyn failed to give antibiotics when the mother presented in preterm labor with unknown GBS status. The hospital did not have a protocol that required the on-call ObGyn to prescribe prophylactic antibiotics in this context. The nurse was negligent for failing to verify the oral telephone report of GBS-negative status with a written or faxed laboratory report.
The ObGyn surmised that the infection had occurred in utero, not during birth; antibiotics would not have changed the outcome.
VERDICT The parents settled with the hospital for a confidential amount. An Arizona defense verdict was returned for the ObGyn.
_______________
Child has quadraparetic CP after oxytocin-augmented delivery
A pregnant woman was hospitalized for 23-hour observation with blood work and obstetric ultrasonography. The admitting nurse noted that the patient was having mild contractions and that fetal heart tones were 130 bpm with moderate variability. The mother’s cervix was dilated to 2.5 cm, 70% effaced, at –1 station, with intact and bulging membranes and normal maternal vital signs. The ObGyn ordered intravenous ampicillin and sent the mother to labor and delivery. He prescribed oxytocin (6 mU/min), but, after its initiation, oxytocin was discontinued for almost 2 hours. When the mother had five contractions in 10 minutes, oxytocin was restarted at 8 mU/min. The oxytocin dosage was later increased to 10 mU/min, and then to 12 mU/min.
When shoulder dystocia was encountered, various maneuvers were performed. The baby was delivered using vacuum extraction. The newborn was immediately sent to the neonatal intensive care unit (NICU) with a suspected humerus fracture and poor respiration. Mechanical ventilation and treatment for hypoperfusion were initiated. She had persistently low Apgar scores, intracranial hemorrhaging, seizures, severe metabolic acidosis, and hypoxic ischemic encephalopathy. She has quadraparetic cerebral palsy with related disabilities.
PARENTS’ CLAIM The ObGyn and hospital were negligent in the treatment of the mother during labor and delivery, causing the child to be born with serious injuries.
DEFENDANTS’ DEFENSE The case was settled during the trial.
VERDICT A $4,250,000 Texas settlement was reached, including $75,000 for the parents, and the remainder placed into a trust for the child.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Premature infant has CP: $14.5M verdict
After learning that, 14 years earlier, a 36-year-old woman had undergone an emergency cesarean delivery at 32 weeks’ gestation, her health-care providers planned a cesarean delivery for the new pregnancy. The woman was admitted to the hospital in preterm labor. Three days later, she was discharged, but readmitted twice more over a 2-week period. At each admission, preterm labor was halted using medication and bed rest.
The patient’s water broke and she was admitted to the hospital at 25 weeks’ gestation, about a week after the previous admission. Shortly after admission, the patient asked about a cesarean delivery, but no action was taken. When her ObGyn arrived at the hospital 5 hours later, the patient asked for a cesarean delivery; the ObGyn said he wanted to wait to see how her labor was progressing. After 3 hours, the fetus showed signs of distress, and an emergency cesarean delivery was undertaken. The infant experienced a massive brain hemorrhage, resulting in cerebral palsy (CP). The child has cognitive delays, visual impairment, and additional problems; he will require lifelong care.
PARENTS’ CLAIM The ObGyn and hospital were negligent in discharging the woman from admission for preterm labor. Cesarean delivery should have been performed much earlier due to nonreassuring fetal heart tones. Severe variable decelerations caused cerebral blood flow fluctuations that led to the hemorrhage.
DEFENDANTS’ DEFENSE The child’s prematurity and a severe placental infection led to the injuries. Nothing would have changed the outcome.
VERDICT A $14.5 million Ohio verdict was returned, including $1.5 million for the mother.
_______________
Costs returned afterverdict for the defense
A 65-year-old woman underwent a hysterectomy for treatment of uterine cancer performed by a gynecologic oncologist. Postoperatively, the patient developed an infection. A small-bowel injury was surgically repaired. The patient was hospitalized for 4 months for treatment of sepsis.
PARENTS’ CLAIM The physician was negligent for injuring the patient’s bowel and then failing to identify and repair the injury during surgery.
PHYSICIAN’S DEFENSE There was no negligence. The patient had significant adhesions from prior surgeries. The physician noted minor serosal tears of the bowel, several of which were repaired during surgery. He checked the length of the bowel for tears/perforations several times during the procedure, but found none. The patient had areas of weakness in her bowel, one of which broke down after surgery. The perforation was repaired in a timely manner.
VERDICT A Michigan defense verdict was returned. The physician was awarded $14,535 in costs.
_______________
Colon injury after cystectomy
A 21-year-old woman underwent laparoscopic ovarian cystectomy, performed by her gynecologist, and was discharged the next day. Eight days later, the patient went to the emergency department (ED) with pelvic pain. Testing revealed a perforated colon with peritonitis. She underwent repair by laparotomy, including bowel resection and colostomy, which was reversed several months later. She has not regained regular bowel function, cannot digest food that has not been finely sliced, and constantly uses laxatives.
PARENTS’ CLAIM The colon injury occurred during cystectomy because the gynecologist was negligent in failing to maintain proper anatomical landmarks. The injury should have been recognized at the time of surgery by injecting saline solution into the colon. She had not been informed of the risk of colon injury.
DEFENDANTS’ DEFENSE Colon injury is a known complication of cystectomy. The injury could have occurred after surgery due to a minor nick of the colon that was undetectable during surgery. Proper informed consent was acquired.
VERDICT A $340,000 New York settlement was reached.
_______________
Mother hemorrhages, dies after delivery: $1M settlement
A 19-year-old woman presented at full term to a community hospital. After several hours of labor, an emergency cesarean delivery was performed due to arrested descent.
Fifteen minutes after delivery, the mother exhibited moderate bleeding with decreasing blood pressure and tachycardia. The post-anesthesia care unit nurse assessed the patient’s uterus as “boggy,” and alerted the ObGyn, who immediately reacted by expressing clots from the uterus. He noted that the fundus was firm. He ordered intravenous (IV) oxytocin, but the patient continued to hemorrhage. Fifteen minutes later, the patient’s vital signs worsened. The ObGyn ordered blood products, uterotonics, and an additional IV line for fluid resuscitation. He began to massage the fundus and expressed clots.
When the patient did not stabilize, she was returned to the OR. After attempting to stop the bleeding with O’Leary stitches, the ObGyn performed a hysterectomy. Six hours after surgery, and after transfusion of a total of 12 units of blood, the woman coded multiple times. She died 14 hours after delivery. Cause of death was disseminated intravascular coagulopathy caused by an atonic uterus.
ESTATE’S CLAIM The ObGyn failed to recognize the extent of the postpartum hemorrhage and should have acted more aggressively with resuscitation. He should have returned her to the OR earlier. The ObGyn was negligent in waiting 45 minutes for cross-matched blood rather than using universal donor O-negative blood that was readily available.
PHYSICIAN’S DEFENSE The ObGyn denied negligence and maintained that he had acted properly. He returned the patient to the OR within 90 minutes of first learning of the hemorrhage.
VERDICT A $1 million Virginia settlement was reached.
_______________
Infant born with broken arms, collarbone, facial bones
A 23-year-old woman had gestational diabetes. She is 5’9” tall and weighed 300 lb while pregnant. She went to the hospital in labor.
During delivery, shoulder dystocia was encountered. The ObGyn performed a variety of techniques, including the McRobert’s maneuver. Forceps were eventually used for delivery.
Both of the newborn’s arms were broken, and she had a broken collarbone and facial fractures. The mother also suffered significant vaginal lacerations and required an episiotomy. She continues to complain of bladder and bowel problems.
PARENTS’ CLAIM A vaginal delivery should not have been attempted due to the mother’s gestational diabetes and the risk of having a macrosomic baby. A cesarean delivery should have been performed. The ObGyn did not use the proper techniques when delivering the child after shoulder dystocia was encountered.
PHYSICIAN’S DEFENSE The ObGyn denied negligence. He claimed that the baby recovered well from her injuries. The mother underwent surgery and now has excellent bladder and bowel control.
VERDICT A confidential Louisiana settlement was reached with the hospital before trial. A defense verdict was returned for the ObGyn.
_______________
Protein found in urine at 39 weeks’ gestation: mother and child die
At 39 weeks' gestation, a woman saw her ObGyn for a prenatal visit. During the examination, the ObGyn found high levels of protein in the woman’s urine, an accumulation of fluid in her ankles, and the highest blood pressure (BP) reading of the woman’s pregnancy. However, because the BP reading was lower than that required to diagnose preeclampsia, the ObGyn sent the patient home and scheduled the next prenatal visit for the following week. The woman and her unborn child died 5 days later.
ESTATE’S CLAIM The ObGyn was negligent in failing to order a urine study and more closely monitor the mother’s symptoms when signs of preeclampsia were evident at 39 weeks’ gestation. Delivery of the child would have resolved the problem and saved both lives.
PHYSICIAN’S DEFENSE The case was settled during the trial.
VERDICT A $3 million Illinois settlement was reached.
_______________
Baby dies from group B strep
A 16-year-old woman planned delivery at a local hospital. Her ObGyn’s practice regularly sends the hospital its patients’ prenatal records, starting at 25 weeks’ gestation. At 33 weeks, the ObGyn took a vaginal culture to test for group B Streptococcus (GBS) bacteria. The laboratory reported positive GBS results to a computer in the ObGyn’s office, but the results were not entered into the patient’s chart.
The mother went to the ED in labor a week later; she was evaluated and discharged. Several days later, she returned to the ED, but was again discharged. She returned the next day, now in gestational week 36. An on-call ObGyn admitted her. A labor and delivery nurse claimed that the ObGyn’s office reported that the mother was GBS negative, so the nurse placed a negative sign in the prenatal record in the chart. When the patient’s ObGyn arrived at the hospital, he noticed the negative sign in the chart.
At birth, the baby’s Apgar scores were 7 at 1 minute and 7 at 5 minutes. She appeared limp and was grunting. A pediatrician diagnosed transient respiratory problems related to prematurity. The baby continued to deteriorate; antibiotics were ordered 7 hours after birth. After the child was transported to another facility, she died. The cause of death was GBS sepsis and pneumonia.
PARENTS’ CLAIM The ObGyn was negligent in failing to properly and timely note the positive GBS test result in the mother’s chart. The ObGyn’s office staff was negligent in miscommunicating the GBS status to the nurse.
DEFENDANTS’ DEFENSE The ObGyn usually noted laboratory results at the next prenatal visit, but the mother gave birth before that occurred. The on-call ObGyn failed to give antibiotics when the mother presented in preterm labor with unknown GBS status. The hospital did not have a protocol that required the on-call ObGyn to prescribe prophylactic antibiotics in this context. The nurse was negligent for failing to verify the oral telephone report of GBS-negative status with a written or faxed laboratory report.
The ObGyn surmised that the infection had occurred in utero, not during birth; antibiotics would not have changed the outcome.
VERDICT The parents settled with the hospital for a confidential amount. An Arizona defense verdict was returned for the ObGyn.
_______________
Child has quadraparetic CP after oxytocin-augmented delivery
A pregnant woman was hospitalized for 23-hour observation with blood work and obstetric ultrasonography. The admitting nurse noted that the patient was having mild contractions and that fetal heart tones were 130 bpm with moderate variability. The mother’s cervix was dilated to 2.5 cm, 70% effaced, at –1 station, with intact and bulging membranes and normal maternal vital signs. The ObGyn ordered intravenous ampicillin and sent the mother to labor and delivery. He prescribed oxytocin (6 mU/min), but, after its initiation, oxytocin was discontinued for almost 2 hours. When the mother had five contractions in 10 minutes, oxytocin was restarted at 8 mU/min. The oxytocin dosage was later increased to 10 mU/min, and then to 12 mU/min.
When shoulder dystocia was encountered, various maneuvers were performed. The baby was delivered using vacuum extraction. The newborn was immediately sent to the neonatal intensive care unit (NICU) with a suspected humerus fracture and poor respiration. Mechanical ventilation and treatment for hypoperfusion were initiated. She had persistently low Apgar scores, intracranial hemorrhaging, seizures, severe metabolic acidosis, and hypoxic ischemic encephalopathy. She has quadraparetic cerebral palsy with related disabilities.
PARENTS’ CLAIM The ObGyn and hospital were negligent in the treatment of the mother during labor and delivery, causing the child to be born with serious injuries.
DEFENDANTS’ DEFENSE The case was settled during the trial.
VERDICT A $4,250,000 Texas settlement was reached, including $75,000 for the parents, and the remainder placed into a trust for the child.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Premature infant has CP: $14.5M verdict
After learning that, 14 years earlier, a 36-year-old woman had undergone an emergency cesarean delivery at 32 weeks’ gestation, her health-care providers planned a cesarean delivery for the new pregnancy. The woman was admitted to the hospital in preterm labor. Three days later, she was discharged, but readmitted twice more over a 2-week period. At each admission, preterm labor was halted using medication and bed rest.
The patient’s water broke and she was admitted to the hospital at 25 weeks’ gestation, about a week after the previous admission. Shortly after admission, the patient asked about a cesarean delivery, but no action was taken. When her ObGyn arrived at the hospital 5 hours later, the patient asked for a cesarean delivery; the ObGyn said he wanted to wait to see how her labor was progressing. After 3 hours, the fetus showed signs of distress, and an emergency cesarean delivery was undertaken. The infant experienced a massive brain hemorrhage, resulting in cerebral palsy (CP). The child has cognitive delays, visual impairment, and additional problems; he will require lifelong care.
PARENTS’ CLAIM The ObGyn and hospital were negligent in discharging the woman from admission for preterm labor. Cesarean delivery should have been performed much earlier due to nonreassuring fetal heart tones. Severe variable decelerations caused cerebral blood flow fluctuations that led to the hemorrhage.
DEFENDANTS’ DEFENSE The child’s prematurity and a severe placental infection led to the injuries. Nothing would have changed the outcome.
VERDICT A $14.5 million Ohio verdict was returned, including $1.5 million for the mother.
_______________
Costs returned afterverdict for the defense
A 65-year-old woman underwent a hysterectomy for treatment of uterine cancer performed by a gynecologic oncologist. Postoperatively, the patient developed an infection. A small-bowel injury was surgically repaired. The patient was hospitalized for 4 months for treatment of sepsis.
PARENTS’ CLAIM The physician was negligent for injuring the patient’s bowel and then failing to identify and repair the injury during surgery.
PHYSICIAN’S DEFENSE There was no negligence. The patient had significant adhesions from prior surgeries. The physician noted minor serosal tears of the bowel, several of which were repaired during surgery. He checked the length of the bowel for tears/perforations several times during the procedure, but found none. The patient had areas of weakness in her bowel, one of which broke down after surgery. The perforation was repaired in a timely manner.
VERDICT A Michigan defense verdict was returned. The physician was awarded $14,535 in costs.
_______________
Colon injury after cystectomy
A 21-year-old woman underwent laparoscopic ovarian cystectomy, performed by her gynecologist, and was discharged the next day. Eight days later, the patient went to the emergency department (ED) with pelvic pain. Testing revealed a perforated colon with peritonitis. She underwent repair by laparotomy, including bowel resection and colostomy, which was reversed several months later. She has not regained regular bowel function, cannot digest food that has not been finely sliced, and constantly uses laxatives.
PARENTS’ CLAIM The colon injury occurred during cystectomy because the gynecologist was negligent in failing to maintain proper anatomical landmarks. The injury should have been recognized at the time of surgery by injecting saline solution into the colon. She had not been informed of the risk of colon injury.
DEFENDANTS’ DEFENSE Colon injury is a known complication of cystectomy. The injury could have occurred after surgery due to a minor nick of the colon that was undetectable during surgery. Proper informed consent was acquired.
VERDICT A $340,000 New York settlement was reached.
_______________
Mother hemorrhages, dies after delivery: $1M settlement
A 19-year-old woman presented at full term to a community hospital. After several hours of labor, an emergency cesarean delivery was performed due to arrested descent.
Fifteen minutes after delivery, the mother exhibited moderate bleeding with decreasing blood pressure and tachycardia. The post-anesthesia care unit nurse assessed the patient’s uterus as “boggy,” and alerted the ObGyn, who immediately reacted by expressing clots from the uterus. He noted that the fundus was firm. He ordered intravenous (IV) oxytocin, but the patient continued to hemorrhage. Fifteen minutes later, the patient’s vital signs worsened. The ObGyn ordered blood products, uterotonics, and an additional IV line for fluid resuscitation. He began to massage the fundus and expressed clots.
When the patient did not stabilize, she was returned to the OR. After attempting to stop the bleeding with O’Leary stitches, the ObGyn performed a hysterectomy. Six hours after surgery, and after transfusion of a total of 12 units of blood, the woman coded multiple times. She died 14 hours after delivery. Cause of death was disseminated intravascular coagulopathy caused by an atonic uterus.
ESTATE’S CLAIM The ObGyn failed to recognize the extent of the postpartum hemorrhage and should have acted more aggressively with resuscitation. He should have returned her to the OR earlier. The ObGyn was negligent in waiting 45 minutes for cross-matched blood rather than using universal donor O-negative blood that was readily available.
PHYSICIAN’S DEFENSE The ObGyn denied negligence and maintained that he had acted properly. He returned the patient to the OR within 90 minutes of first learning of the hemorrhage.
VERDICT A $1 million Virginia settlement was reached.
_______________
Infant born with broken arms, collarbone, facial bones
A 23-year-old woman had gestational diabetes. She is 5’9” tall and weighed 300 lb while pregnant. She went to the hospital in labor.
During delivery, shoulder dystocia was encountered. The ObGyn performed a variety of techniques, including the McRobert’s maneuver. Forceps were eventually used for delivery.
Both of the newborn’s arms were broken, and she had a broken collarbone and facial fractures. The mother also suffered significant vaginal lacerations and required an episiotomy. She continues to complain of bladder and bowel problems.
PARENTS’ CLAIM A vaginal delivery should not have been attempted due to the mother’s gestational diabetes and the risk of having a macrosomic baby. A cesarean delivery should have been performed. The ObGyn did not use the proper techniques when delivering the child after shoulder dystocia was encountered.
PHYSICIAN’S DEFENSE The ObGyn denied negligence. He claimed that the baby recovered well from her injuries. The mother underwent surgery and now has excellent bladder and bowel control.
VERDICT A confidential Louisiana settlement was reached with the hospital before trial. A defense verdict was returned for the ObGyn.
_______________
Protein found in urine at 39 weeks’ gestation: mother and child die
At 39 weeks' gestation, a woman saw her ObGyn for a prenatal visit. During the examination, the ObGyn found high levels of protein in the woman’s urine, an accumulation of fluid in her ankles, and the highest blood pressure (BP) reading of the woman’s pregnancy. However, because the BP reading was lower than that required to diagnose preeclampsia, the ObGyn sent the patient home and scheduled the next prenatal visit for the following week. The woman and her unborn child died 5 days later.
ESTATE’S CLAIM The ObGyn was negligent in failing to order a urine study and more closely monitor the mother’s symptoms when signs of preeclampsia were evident at 39 weeks’ gestation. Delivery of the child would have resolved the problem and saved both lives.
PHYSICIAN’S DEFENSE The case was settled during the trial.
VERDICT A $3 million Illinois settlement was reached.
_______________
Baby dies from group B strep
A 16-year-old woman planned delivery at a local hospital. Her ObGyn’s practice regularly sends the hospital its patients’ prenatal records, starting at 25 weeks’ gestation. At 33 weeks, the ObGyn took a vaginal culture to test for group B Streptococcus (GBS) bacteria. The laboratory reported positive GBS results to a computer in the ObGyn’s office, but the results were not entered into the patient’s chart.
The mother went to the ED in labor a week later; she was evaluated and discharged. Several days later, she returned to the ED, but was again discharged. She returned the next day, now in gestational week 36. An on-call ObGyn admitted her. A labor and delivery nurse claimed that the ObGyn’s office reported that the mother was GBS negative, so the nurse placed a negative sign in the prenatal record in the chart. When the patient’s ObGyn arrived at the hospital, he noticed the negative sign in the chart.
At birth, the baby’s Apgar scores were 7 at 1 minute and 7 at 5 minutes. She appeared limp and was grunting. A pediatrician diagnosed transient respiratory problems related to prematurity. The baby continued to deteriorate; antibiotics were ordered 7 hours after birth. After the child was transported to another facility, she died. The cause of death was GBS sepsis and pneumonia.
PARENTS’ CLAIM The ObGyn was negligent in failing to properly and timely note the positive GBS test result in the mother’s chart. The ObGyn’s office staff was negligent in miscommunicating the GBS status to the nurse.
DEFENDANTS’ DEFENSE The ObGyn usually noted laboratory results at the next prenatal visit, but the mother gave birth before that occurred. The on-call ObGyn failed to give antibiotics when the mother presented in preterm labor with unknown GBS status. The hospital did not have a protocol that required the on-call ObGyn to prescribe prophylactic antibiotics in this context. The nurse was negligent for failing to verify the oral telephone report of GBS-negative status with a written or faxed laboratory report.
The ObGyn surmised that the infection had occurred in utero, not during birth; antibiotics would not have changed the outcome.
VERDICT The parents settled with the hospital for a confidential amount. An Arizona defense verdict was returned for the ObGyn.
_______________
Child has quadraparetic CP after oxytocin-augmented delivery
A pregnant woman was hospitalized for 23-hour observation with blood work and obstetric ultrasonography. The admitting nurse noted that the patient was having mild contractions and that fetal heart tones were 130 bpm with moderate variability. The mother’s cervix was dilated to 2.5 cm, 70% effaced, at –1 station, with intact and bulging membranes and normal maternal vital signs. The ObGyn ordered intravenous ampicillin and sent the mother to labor and delivery. He prescribed oxytocin (6 mU/min), but, after its initiation, oxytocin was discontinued for almost 2 hours. When the mother had five contractions in 10 minutes, oxytocin was restarted at 8 mU/min. The oxytocin dosage was later increased to 10 mU/min, and then to 12 mU/min.
When shoulder dystocia was encountered, various maneuvers were performed. The baby was delivered using vacuum extraction. The newborn was immediately sent to the neonatal intensive care unit (NICU) with a suspected humerus fracture and poor respiration. Mechanical ventilation and treatment for hypoperfusion were initiated. She had persistently low Apgar scores, intracranial hemorrhaging, seizures, severe metabolic acidosis, and hypoxic ischemic encephalopathy. She has quadraparetic cerebral palsy with related disabilities.
PARENTS’ CLAIM The ObGyn and hospital were negligent in the treatment of the mother during labor and delivery, causing the child to be born with serious injuries.
DEFENDANTS’ DEFENSE The case was settled during the trial.
VERDICT A $4,250,000 Texas settlement was reached, including $75,000 for the parents, and the remainder placed into a trust for the child.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Low IgG1/high IgG4 ratios seen in pregnancy may alter flu vaccine response
PHILADELPHIA – Low IgG1/high IgG4 ratios appear to be more common in pregnant women and may be associated with a diminished response to influenza vaccination, according to Dr. Elizabeth P. Schlaudecker.
“Basically, we know that at the maternal-fetal interface, there are lots of cytokine changes going on and lots of immunologic changes going on, but does this immune milieu of pregnancy actually influence systemic response? Obviously we think it does, but does it really affect the pregnant woman’s response to immunization? In turn, does it really influence the magnitude and character of the antibody response to flu vaccine?” Dr. Schlaudecker, of Cincinnati Children’s Hospital Medical Center, said at an annual scientific meeting on infectious diseases.
These questions prompted her research, and the answers are important because pregnant women don’t do well during flu seasons, she said, noting that “this was especially brought to light during the H1N1 pandemic,” when pregnant women had higher rates of hospital admission, more medical encounters with confirmed or suspected influenza, and greater severity of disease during late pregnancy than nonpregnant women and others.
These effects, which also occur during regular flu seasons, are more pronounced during the second and third trimesters, she said.
Because of this, influenza vaccine is recommended universally in pregnancy with the goal of preventing infection in both mothers and infants.
To determine whether the effects of pregnancy that worsen the outcome of influenza infection also suppress the response to flu vaccine, Dr. Schlaudecker and her colleagues reviewed reports about the immunogenicity of influenza vaccine in pregnancy.
Most studies and reports show that vaccinated pregnant women are likely to have seroprotective responses, but few have compared pregnant and nonpregnant women, so she recruited 70 pregnant women and 65 nonpregnant women, aged 18-39 years, and compared sera before and 28 days after influenza immunization during the 2011-2012 and 2012-2013 flu seasons.
Hemagglutination inhibition (HAI), as expected, was reduced during pregnancy. The pregnant women, who were in either their second or third trimester, had significantly lower HAI titers for anti-influenza H3N2 (154.55 vs. 242.51), and the differences approached significance for both H1N1 (129.96 vs. 181.84) and B antigens (24.91 v. 35.20). She reported these findings at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
“So I took this information and realized that women may have a decreased antibody response when they are pregnant, and hypothesized that during this time of pregnancy, there are cytokines associated with this maternal-fetal interface that suppress the IgG1 and IgG3 response and promote an IgG4 response to influenza vaccination, particularly during the second and third trimesters, when production of these cytokines should be highest,” she said.
This matters, because the four subclasses of IgG have functional differences. The effector functions of the IgG subclasses are usually opsonization and complement activation, and activation of inflammatory cells through Fc-gamma receptors. IgG1 and IgG3 are more effective than IgG2 or IgG4 in binding stimulatory Fc-gamma receptors and activating complement, she explained.
“IgG4 is also functionally monovalent, so it doesn’t aggregate antigens very well, and it makes it less protective against viruses,” she said, explaining that in normal, nonpregnant women, IgG1 and IgG3 are the predominant responders to viral infection, and they are most likely to be involved in flu virus protection.
“So the question is, although we know [down-regulation of IgG1 and IgG3, and up-regulation of IgG4,] is taking place around the placenta, are these cytokine effects actually circulating systemically enough to affect the flu vaccine?” she asked.
In the study participants, there was a general trend for most pregnant women to have responses that had high IgG4s and low IgG1s, which is not protective, and for nonpregnant women to have high IgG1s and low IgG4s.
The difference between the groups in this regard was not statistically significant, but there were significantly more pregnant women than nonpregnant women with high IgG4 and low IgG1 (10% vs. 0%), and there were significantly fewer pregnant women than nonpregnant women with low IgG4 and high IgG1 (3% vs. 15%), she said.
Both anti-H1N1 HAI and IgG1 titers were significantly lower in pregnant vs. nonpregnant women, but for anti-H1N1 IgG4 titers, the levels were much higher in pregnant vs. nonpregnant women, she said.
“These correlated with each other, suggesting that when you have a high HAI titer, you have a high IgG1 response, which goes along with IgG1 being the predominant IgG isotype and the one most associated with protecting against viral disease,” she said.
“A subset of these women had this very high IgG4 and low IgG1 response, and this suggests a Th2/Treg influence. This unique isotype profile was not found in any nonpregnant women,” she said, adding that very few pregnant women in their second or third trimester make the high IgG1/low IgG4 that should provide a good response to flu vaccine.
Although Dr. Schlaudecker acknowledged that 80%-90% of the pregnant women in the study had protective HAI titers after immunization, she said the findings have important implications.
“I’m concerned that these low IgG1/high IgG4 ratios seen in pregnant women might actually be giving poor protection against flu infection, which brings us back to the pediatric patients. If pregnant women are not protected well, we are not protecting the babies as well. This suggests that we might need to reconsider approaches to timing of flu vaccine or actually the particular vaccines that we give to pregnant women, and it also shows that pregnancy likely effects systemic responses to things like flu vaccine and other vaccines,” she said.
Dr. Schlaudecker reported having no disclosures.
PHILADELPHIA – Low IgG1/high IgG4 ratios appear to be more common in pregnant women and may be associated with a diminished response to influenza vaccination, according to Dr. Elizabeth P. Schlaudecker.
“Basically, we know that at the maternal-fetal interface, there are lots of cytokine changes going on and lots of immunologic changes going on, but does this immune milieu of pregnancy actually influence systemic response? Obviously we think it does, but does it really affect the pregnant woman’s response to immunization? In turn, does it really influence the magnitude and character of the antibody response to flu vaccine?” Dr. Schlaudecker, of Cincinnati Children’s Hospital Medical Center, said at an annual scientific meeting on infectious diseases.
These questions prompted her research, and the answers are important because pregnant women don’t do well during flu seasons, she said, noting that “this was especially brought to light during the H1N1 pandemic,” when pregnant women had higher rates of hospital admission, more medical encounters with confirmed or suspected influenza, and greater severity of disease during late pregnancy than nonpregnant women and others.
These effects, which also occur during regular flu seasons, are more pronounced during the second and third trimesters, she said.
Because of this, influenza vaccine is recommended universally in pregnancy with the goal of preventing infection in both mothers and infants.
To determine whether the effects of pregnancy that worsen the outcome of influenza infection also suppress the response to flu vaccine, Dr. Schlaudecker and her colleagues reviewed reports about the immunogenicity of influenza vaccine in pregnancy.
Most studies and reports show that vaccinated pregnant women are likely to have seroprotective responses, but few have compared pregnant and nonpregnant women, so she recruited 70 pregnant women and 65 nonpregnant women, aged 18-39 years, and compared sera before and 28 days after influenza immunization during the 2011-2012 and 2012-2013 flu seasons.
Hemagglutination inhibition (HAI), as expected, was reduced during pregnancy. The pregnant women, who were in either their second or third trimester, had significantly lower HAI titers for anti-influenza H3N2 (154.55 vs. 242.51), and the differences approached significance for both H1N1 (129.96 vs. 181.84) and B antigens (24.91 v. 35.20). She reported these findings at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
“So I took this information and realized that women may have a decreased antibody response when they are pregnant, and hypothesized that during this time of pregnancy, there are cytokines associated with this maternal-fetal interface that suppress the IgG1 and IgG3 response and promote an IgG4 response to influenza vaccination, particularly during the second and third trimesters, when production of these cytokines should be highest,” she said.
This matters, because the four subclasses of IgG have functional differences. The effector functions of the IgG subclasses are usually opsonization and complement activation, and activation of inflammatory cells through Fc-gamma receptors. IgG1 and IgG3 are more effective than IgG2 or IgG4 in binding stimulatory Fc-gamma receptors and activating complement, she explained.
“IgG4 is also functionally monovalent, so it doesn’t aggregate antigens very well, and it makes it less protective against viruses,” she said, explaining that in normal, nonpregnant women, IgG1 and IgG3 are the predominant responders to viral infection, and they are most likely to be involved in flu virus protection.
“So the question is, although we know [down-regulation of IgG1 and IgG3, and up-regulation of IgG4,] is taking place around the placenta, are these cytokine effects actually circulating systemically enough to affect the flu vaccine?” she asked.
In the study participants, there was a general trend for most pregnant women to have responses that had high IgG4s and low IgG1s, which is not protective, and for nonpregnant women to have high IgG1s and low IgG4s.
The difference between the groups in this regard was not statistically significant, but there were significantly more pregnant women than nonpregnant women with high IgG4 and low IgG1 (10% vs. 0%), and there were significantly fewer pregnant women than nonpregnant women with low IgG4 and high IgG1 (3% vs. 15%), she said.
Both anti-H1N1 HAI and IgG1 titers were significantly lower in pregnant vs. nonpregnant women, but for anti-H1N1 IgG4 titers, the levels were much higher in pregnant vs. nonpregnant women, she said.
“These correlated with each other, suggesting that when you have a high HAI titer, you have a high IgG1 response, which goes along with IgG1 being the predominant IgG isotype and the one most associated with protecting against viral disease,” she said.
“A subset of these women had this very high IgG4 and low IgG1 response, and this suggests a Th2/Treg influence. This unique isotype profile was not found in any nonpregnant women,” she said, adding that very few pregnant women in their second or third trimester make the high IgG1/low IgG4 that should provide a good response to flu vaccine.
Although Dr. Schlaudecker acknowledged that 80%-90% of the pregnant women in the study had protective HAI titers after immunization, she said the findings have important implications.
“I’m concerned that these low IgG1/high IgG4 ratios seen in pregnant women might actually be giving poor protection against flu infection, which brings us back to the pediatric patients. If pregnant women are not protected well, we are not protecting the babies as well. This suggests that we might need to reconsider approaches to timing of flu vaccine or actually the particular vaccines that we give to pregnant women, and it also shows that pregnancy likely effects systemic responses to things like flu vaccine and other vaccines,” she said.
Dr. Schlaudecker reported having no disclosures.
PHILADELPHIA – Low IgG1/high IgG4 ratios appear to be more common in pregnant women and may be associated with a diminished response to influenza vaccination, according to Dr. Elizabeth P. Schlaudecker.
“Basically, we know that at the maternal-fetal interface, there are lots of cytokine changes going on and lots of immunologic changes going on, but does this immune milieu of pregnancy actually influence systemic response? Obviously we think it does, but does it really affect the pregnant woman’s response to immunization? In turn, does it really influence the magnitude and character of the antibody response to flu vaccine?” Dr. Schlaudecker, of Cincinnati Children’s Hospital Medical Center, said at an annual scientific meeting on infectious diseases.
These questions prompted her research, and the answers are important because pregnant women don’t do well during flu seasons, she said, noting that “this was especially brought to light during the H1N1 pandemic,” when pregnant women had higher rates of hospital admission, more medical encounters with confirmed or suspected influenza, and greater severity of disease during late pregnancy than nonpregnant women and others.
These effects, which also occur during regular flu seasons, are more pronounced during the second and third trimesters, she said.
Because of this, influenza vaccine is recommended universally in pregnancy with the goal of preventing infection in both mothers and infants.
To determine whether the effects of pregnancy that worsen the outcome of influenza infection also suppress the response to flu vaccine, Dr. Schlaudecker and her colleagues reviewed reports about the immunogenicity of influenza vaccine in pregnancy.
Most studies and reports show that vaccinated pregnant women are likely to have seroprotective responses, but few have compared pregnant and nonpregnant women, so she recruited 70 pregnant women and 65 nonpregnant women, aged 18-39 years, and compared sera before and 28 days after influenza immunization during the 2011-2012 and 2012-2013 flu seasons.
Hemagglutination inhibition (HAI), as expected, was reduced during pregnancy. The pregnant women, who were in either their second or third trimester, had significantly lower HAI titers for anti-influenza H3N2 (154.55 vs. 242.51), and the differences approached significance for both H1N1 (129.96 vs. 181.84) and B antigens (24.91 v. 35.20). She reported these findings at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
“So I took this information and realized that women may have a decreased antibody response when they are pregnant, and hypothesized that during this time of pregnancy, there are cytokines associated with this maternal-fetal interface that suppress the IgG1 and IgG3 response and promote an IgG4 response to influenza vaccination, particularly during the second and third trimesters, when production of these cytokines should be highest,” she said.
This matters, because the four subclasses of IgG have functional differences. The effector functions of the IgG subclasses are usually opsonization and complement activation, and activation of inflammatory cells through Fc-gamma receptors. IgG1 and IgG3 are more effective than IgG2 or IgG4 in binding stimulatory Fc-gamma receptors and activating complement, she explained.
“IgG4 is also functionally monovalent, so it doesn’t aggregate antigens very well, and it makes it less protective against viruses,” she said, explaining that in normal, nonpregnant women, IgG1 and IgG3 are the predominant responders to viral infection, and they are most likely to be involved in flu virus protection.
“So the question is, although we know [down-regulation of IgG1 and IgG3, and up-regulation of IgG4,] is taking place around the placenta, are these cytokine effects actually circulating systemically enough to affect the flu vaccine?” she asked.
In the study participants, there was a general trend for most pregnant women to have responses that had high IgG4s and low IgG1s, which is not protective, and for nonpregnant women to have high IgG1s and low IgG4s.
The difference between the groups in this regard was not statistically significant, but there were significantly more pregnant women than nonpregnant women with high IgG4 and low IgG1 (10% vs. 0%), and there were significantly fewer pregnant women than nonpregnant women with low IgG4 and high IgG1 (3% vs. 15%), she said.
Both anti-H1N1 HAI and IgG1 titers were significantly lower in pregnant vs. nonpregnant women, but for anti-H1N1 IgG4 titers, the levels were much higher in pregnant vs. nonpregnant women, she said.
“These correlated with each other, suggesting that when you have a high HAI titer, you have a high IgG1 response, which goes along with IgG1 being the predominant IgG isotype and the one most associated with protecting against viral disease,” she said.
“A subset of these women had this very high IgG4 and low IgG1 response, and this suggests a Th2/Treg influence. This unique isotype profile was not found in any nonpregnant women,” she said, adding that very few pregnant women in their second or third trimester make the high IgG1/low IgG4 that should provide a good response to flu vaccine.
Although Dr. Schlaudecker acknowledged that 80%-90% of the pregnant women in the study had protective HAI titers after immunization, she said the findings have important implications.
“I’m concerned that these low IgG1/high IgG4 ratios seen in pregnant women might actually be giving poor protection against flu infection, which brings us back to the pediatric patients. If pregnant women are not protected well, we are not protecting the babies as well. This suggests that we might need to reconsider approaches to timing of flu vaccine or actually the particular vaccines that we give to pregnant women, and it also shows that pregnancy likely effects systemic responses to things like flu vaccine and other vaccines,” she said.
Dr. Schlaudecker reported having no disclosures.
Key clinical point: Changes during pregnancy may diminish the effects of influenza vaccine, requiring a new approach to vaccination in this population.
Major finding: Significantly more pregnant women than nonpregnant women had high IgG4 and low IgG1 (10% vs. 0%), and significantly fewer pregnant women than nonpregnant women had low IgG4 and high IgG1 (3% vs. 15%).
Data source: An observational study of 70 pregnant and 65 nonpregnant women.
Disclosures: Dr. Schlaudecker reported having no disclosures.
Timing of lifestyle interventions for obesity
Obesity has become so pervasive that it is now considered a major health concern during pregnancy. Almost 56% of women aged 20-39 years in the United States are overweight or obese, based on the World Health Organization’s criteria for body mass index (BMI) and data from the 2009-2010 National Health and Nutrition Examination Survey (NHANES). Moreover, 7.5% of women in this age group are morbidly obese, with a body mass index (BMI) greater than 40 kg/m2 (JAMA 2012;307:491-7).
Obesity in pregnancy not only increases the risk of spontaneous abortions and congenital anomalies, it also increases the risk of gestational diabetes (GDM), hypertensive disorders, and other metabolic complications that affect both the mother and fetus.
Of much concern is the increased risk of fetal overgrowth and long-term health consequences for children of obese mothers. Obesity in early pregnancy has been shown to more than double the risk of obesity in the offspring, which in turn puts these children at risk for developing the metabolic syndrome – and, as Dr. Thomas Moore pointed out in September’s Master Class – appears to program these offspring for downstream cardiovascular risk in adulthood.
Mean term birth weights have risen in the United States during the past several decades. In Cleveland, we have seen a significant 116 g increase in mean term birth weight since 1975; this increase encompasses weights from the 5th to the 95th percentiles. Even more concerning is our finding that the ponderal index in our neonatal population has increased because of decreased fetal length over the last decade.
Some recent studies have suggested that the increase in birth weight in the United States has reached a plateau, but our analyses of national trends suggest that such change is secondary to factors such as earlier gestational age of delivery. Concurrently, an alarming number of children and adolescents – 17% of those aged 2-19 years, according to the 2009-2010 NHANES data – are overweight or obese (JAMA 2012;307:483-90).
How to best treat obesity for improved maternal and fetal health has thus become a focus of research. Studies on lifestyle interventions for obese women during pregnancy have aimed to prevent excessive gestational weight gain and decrease adverse perinatal outcomes – mainly macrosomia, GDM, and hypertensive disorders.
However, the results of this recent body of research have been disappointing. Lifestyle interventions initiated during pregnancy have had only limited success in improving perinatal outcomes. The research tells us that while we may be able to reduce excessive gestational weight gain, it is unlikely that we will be successful in reducing fetal overgrowth, GDM, or preeclampsia in obese women.
Moreover, other studies show that it is a high pregravid BMI – not excessive gestational weight gain or the development of GDM – that plays the biggest role in fetal overgrowth and fetal adiposity.
A paradigm shift is in order. We must think about lifestyle intervention and weight loss before pregnancy, when the woman’s metabolic condition can be improved in time to minimize adverse perinatal metabolic outcomes and to maximize metabolic benefits relating to fetal body composition and metabolism.
Role of prepregnancy BMI
In 2008, the Institute of Medicine (IOM) and National Research Council reexamined 1990 guidelines for gestational weight gain. They concluded that excessive weight gain in pregnancy was a primary contributor to the development of obesity in women. In fact, according to the 2009 IOM report, “Weight Gain During Pregnancy: Reexamining the Guidelines” (Washington: National Academy Press, 2009), 38% of normal weight, 63% of overweight, and 46% of obese women had gained weight in excess of the earlier guidelines.
Helping our patients to gain within the guidelines is important. Excessive gestational weight gain is a primary risk factor for maternal postpartum weight retention, which increases the risk for maternal obesity in a subsequent pregnancy. It also has been associated with a modest increased risk of preterm birth and development of type 2 diabetes.
Interestingly, however, high gestational weight gain has not been related to an increased risk of fetal overgrowth or macrosomia in many obese women. Increased gestational weight gain is a greater risk for fetal overgrowth in women who are of normal weight prior to pregnancy (J. Clin. Endocrinol. Metab. 2012;97:3648-54).
Our research has found that in overweight and obese women, it is maternal pregravid BMI – and not gestational weight gain – that presents the greatest risk for fetal macrosomia, and more specifically, the greatest risk for fetal obesity. Even when glucose tolerance levels are normal, overweight and obese women have neonates who are heavier and who have significant increases in the percentage of body fat and fat mass (Am. J. Obstet. Gynecol. 2006;195:1100-3).
In an 8-year prospective study of the perinatal risk factors associated with childhood obesity, we similarly found that maternal pregravid BMI – independent of maternal glucose status or gestational weight gain – was the strongest predictor of childhood obesity and metabolic dysfunction (Am. J. Clin. Nutr. 2009;90:1303-13).
Other studies have teased apart the roles of maternal obesity and GDM in long-term health of offspring. This work has found that maternal obesity during pregnancy is associated with metabolic syndrome in the offspring and an increased risk of type 2 diabetes in youth, independent of maternal diabetes during pregnancy. A recent meta-analysis also reported that, although maternal diabetes is associated with an increased BMI z score, this was no longer significant after adjustments were made for prepregnancy BMI (Diabetologia 2011;54:1957-66).
Maternal pregravid obesity, therefore, is not only a risk factor for neonatal adiposity at birth, but also for the longer-term risk of obesity and metabolic dysfunction in the offspring – independent of maternal GDM or excessive gestational weight gain.
Interventions in Pregnancy
Numerous prospective trials have examined lifestyle interventions for obese women during pregnancy. One randomized controlled study of a low glycemic index diet in pregnancy (coined the ROLO study) involved 800 women in Ireland who had previously delivered an infant weighting greater than 4,000 g. Women were randomized to receive the restricted diet or no intervention at 13 weeks. Despite a decrease in gestational weight gain in the intervention group, there were no differences in birth weight, birth weight percentile, ponderal index, or macrosomia between the two groups (BMJ 2012;345:e5605).
Another randomized controlled trial reported by a Danish group involved an intervention that consisted of dietary guidance, free membership in a fitness center, and personal coaching initiated between 10 and 14 weeks of gestation. There was a decrease in gestational weight gain in the intervention group, but paradoxically, the infants in the intervention group also had significantly higher birth weight, compared with controls (Diabetes Care 2011;34:2502-7).
Additionally, there have been at least five meta-analyses published in the past 2 years looking at lifestyle interventions during pregnancy. All have concluded that interventions initiated during pregnancy have limited success in reducing excessive gestational weight gain but not necessarily to within the IOM guidelines. The literature contains scant evidence to support further benefits for infant or maternal health (in other words, fetal overgrowth, GDM, or hypertensive disorders).
A recent Cochrane review also concluded that the results of several randomized controlled trials suggest no significant difference in GDM incidence between women receiving exercise intervention versus routine care.
Just this year, three additional randomized controlled trials of lifestyle interventions during pregnancy were published. Only one, the Treatment of Obese Pregnant Women (TOP) study, showed a modest effect in decreasing gestational weight gain. None found a reduction in GDM or fetal overgrowth.
Focus on prepregnancy
Obesity is an inflammatory condition that increases the risk of insulin resistance, impaired beta-cell function, and abnormal adiponectin concentrations. In pregnancy, maternal obesity and hyperinsulinemia can affect placental growth and gene expression.
We have studied lean and obese women recruited prior to a planned pregnancy, as well as lean and obese women scheduled for elective pregnancy termination in the first trimester. Our research, some of which we reported recently in the American Journal of Physiology , has shown increased expression of lipogenic and inflammatory genes in maternal adipose tissue and in the placenta of obese women in the early first trimester, before any phenotypic change becomes apparent (Am. J. Physiol. Endocrinol. Metab. 2012;303:e832-40).
Specifically, hyperinsulinemia and/or defective insulin action in obese women appears to affect the placental programming of genes relating to adipokine expression and lipid metabolism, as well as mitrochondrial function. Altered inflammatory and lipid pathways affect the availability of nutrients for the fetus and, consequently, the size and body composition of the fetus. Fetal overgrowth and neonatal adiposity can result.
In addition, our research has shown that obese women have decreased insulin suppression of lipolysis in white adipose tissue, which during pregnancy results in improved lipid availability for fetal fat accretion and lipotoxicity.
When interventions aimed at weight loss and improved insulin sensitivity are undertaken before pregnancy or in the period between pregnancies, we have the opportunity to increase fat oxidation and reduce oxidative stress in early pregnancy. We also may be able to limit placental inflammation and favorably affect placental growth and gene expression. By the second trimester, our research suggests, gene expression in the placenta and early molecular changes in the white adipose tissue have already been programmed and cannot be reversed (Am. J. Physiol. Endocrinol. Metab. 2012;303:e832-40).
In studies by our group and others of interpregnancy weight loss or gain, interpregnancy weight loss has been associated with a lower risk of large-for-gestational-age (LGA) infants, whereas interpregnancy weight gain has been associated with an increased risk of LGA. Preliminary work from our group shows that the decrease in birth weight involves primarily fat and not lean mass.
The 2009 IOM guidelines support weight loss before pregnancy and state that overweight women should receive individual preconceptional counseling to improve diet quality, increase physical activity, and normalize weight. Multifaceted interventions do work: In obese nonpregnant individuals, lifestyle interventions, which include an exercise program, diet, and behavioral modification have been shown to be successful in improving insulin sensitivity, inflammation, and overall metabolic function.
According to the IOM report, preconceptional services aimed at achieving a healthy weight before conceiving will represent “a radical change to the care provided to obese women of childbearing age.” With continuing research and accumulating data, however, the concept is gaining traction as a viable paradigm for improving perinatal outcomes, with long-term benefits for both the mother and her baby.
Dr. Catalano reports that he has no disclosures relevant to this Master Class.
Obesity has become so pervasive that it is now considered a major health concern during pregnancy. Almost 56% of women aged 20-39 years in the United States are overweight or obese, based on the World Health Organization’s criteria for body mass index (BMI) and data from the 2009-2010 National Health and Nutrition Examination Survey (NHANES). Moreover, 7.5% of women in this age group are morbidly obese, with a body mass index (BMI) greater than 40 kg/m2 (JAMA 2012;307:491-7).
Obesity in pregnancy not only increases the risk of spontaneous abortions and congenital anomalies, it also increases the risk of gestational diabetes (GDM), hypertensive disorders, and other metabolic complications that affect both the mother and fetus.
Of much concern is the increased risk of fetal overgrowth and long-term health consequences for children of obese mothers. Obesity in early pregnancy has been shown to more than double the risk of obesity in the offspring, which in turn puts these children at risk for developing the metabolic syndrome – and, as Dr. Thomas Moore pointed out in September’s Master Class – appears to program these offspring for downstream cardiovascular risk in adulthood.
Mean term birth weights have risen in the United States during the past several decades. In Cleveland, we have seen a significant 116 g increase in mean term birth weight since 1975; this increase encompasses weights from the 5th to the 95th percentiles. Even more concerning is our finding that the ponderal index in our neonatal population has increased because of decreased fetal length over the last decade.
Some recent studies have suggested that the increase in birth weight in the United States has reached a plateau, but our analyses of national trends suggest that such change is secondary to factors such as earlier gestational age of delivery. Concurrently, an alarming number of children and adolescents – 17% of those aged 2-19 years, according to the 2009-2010 NHANES data – are overweight or obese (JAMA 2012;307:483-90).
How to best treat obesity for improved maternal and fetal health has thus become a focus of research. Studies on lifestyle interventions for obese women during pregnancy have aimed to prevent excessive gestational weight gain and decrease adverse perinatal outcomes – mainly macrosomia, GDM, and hypertensive disorders.
However, the results of this recent body of research have been disappointing. Lifestyle interventions initiated during pregnancy have had only limited success in improving perinatal outcomes. The research tells us that while we may be able to reduce excessive gestational weight gain, it is unlikely that we will be successful in reducing fetal overgrowth, GDM, or preeclampsia in obese women.
Moreover, other studies show that it is a high pregravid BMI – not excessive gestational weight gain or the development of GDM – that plays the biggest role in fetal overgrowth and fetal adiposity.
A paradigm shift is in order. We must think about lifestyle intervention and weight loss before pregnancy, when the woman’s metabolic condition can be improved in time to minimize adverse perinatal metabolic outcomes and to maximize metabolic benefits relating to fetal body composition and metabolism.
Role of prepregnancy BMI
In 2008, the Institute of Medicine (IOM) and National Research Council reexamined 1990 guidelines for gestational weight gain. They concluded that excessive weight gain in pregnancy was a primary contributor to the development of obesity in women. In fact, according to the 2009 IOM report, “Weight Gain During Pregnancy: Reexamining the Guidelines” (Washington: National Academy Press, 2009), 38% of normal weight, 63% of overweight, and 46% of obese women had gained weight in excess of the earlier guidelines.
Helping our patients to gain within the guidelines is important. Excessive gestational weight gain is a primary risk factor for maternal postpartum weight retention, which increases the risk for maternal obesity in a subsequent pregnancy. It also has been associated with a modest increased risk of preterm birth and development of type 2 diabetes.
Interestingly, however, high gestational weight gain has not been related to an increased risk of fetal overgrowth or macrosomia in many obese women. Increased gestational weight gain is a greater risk for fetal overgrowth in women who are of normal weight prior to pregnancy (J. Clin. Endocrinol. Metab. 2012;97:3648-54).
Our research has found that in overweight and obese women, it is maternal pregravid BMI – and not gestational weight gain – that presents the greatest risk for fetal macrosomia, and more specifically, the greatest risk for fetal obesity. Even when glucose tolerance levels are normal, overweight and obese women have neonates who are heavier and who have significant increases in the percentage of body fat and fat mass (Am. J. Obstet. Gynecol. 2006;195:1100-3).
In an 8-year prospective study of the perinatal risk factors associated with childhood obesity, we similarly found that maternal pregravid BMI – independent of maternal glucose status or gestational weight gain – was the strongest predictor of childhood obesity and metabolic dysfunction (Am. J. Clin. Nutr. 2009;90:1303-13).
Other studies have teased apart the roles of maternal obesity and GDM in long-term health of offspring. This work has found that maternal obesity during pregnancy is associated with metabolic syndrome in the offspring and an increased risk of type 2 diabetes in youth, independent of maternal diabetes during pregnancy. A recent meta-analysis also reported that, although maternal diabetes is associated with an increased BMI z score, this was no longer significant after adjustments were made for prepregnancy BMI (Diabetologia 2011;54:1957-66).
Maternal pregravid obesity, therefore, is not only a risk factor for neonatal adiposity at birth, but also for the longer-term risk of obesity and metabolic dysfunction in the offspring – independent of maternal GDM or excessive gestational weight gain.
Interventions in Pregnancy
Numerous prospective trials have examined lifestyle interventions for obese women during pregnancy. One randomized controlled study of a low glycemic index diet in pregnancy (coined the ROLO study) involved 800 women in Ireland who had previously delivered an infant weighting greater than 4,000 g. Women were randomized to receive the restricted diet or no intervention at 13 weeks. Despite a decrease in gestational weight gain in the intervention group, there were no differences in birth weight, birth weight percentile, ponderal index, or macrosomia between the two groups (BMJ 2012;345:e5605).
Another randomized controlled trial reported by a Danish group involved an intervention that consisted of dietary guidance, free membership in a fitness center, and personal coaching initiated between 10 and 14 weeks of gestation. There was a decrease in gestational weight gain in the intervention group, but paradoxically, the infants in the intervention group also had significantly higher birth weight, compared with controls (Diabetes Care 2011;34:2502-7).
Additionally, there have been at least five meta-analyses published in the past 2 years looking at lifestyle interventions during pregnancy. All have concluded that interventions initiated during pregnancy have limited success in reducing excessive gestational weight gain but not necessarily to within the IOM guidelines. The literature contains scant evidence to support further benefits for infant or maternal health (in other words, fetal overgrowth, GDM, or hypertensive disorders).
A recent Cochrane review also concluded that the results of several randomized controlled trials suggest no significant difference in GDM incidence between women receiving exercise intervention versus routine care.
Just this year, three additional randomized controlled trials of lifestyle interventions during pregnancy were published. Only one, the Treatment of Obese Pregnant Women (TOP) study, showed a modest effect in decreasing gestational weight gain. None found a reduction in GDM or fetal overgrowth.
Focus on prepregnancy
Obesity is an inflammatory condition that increases the risk of insulin resistance, impaired beta-cell function, and abnormal adiponectin concentrations. In pregnancy, maternal obesity and hyperinsulinemia can affect placental growth and gene expression.
We have studied lean and obese women recruited prior to a planned pregnancy, as well as lean and obese women scheduled for elective pregnancy termination in the first trimester. Our research, some of which we reported recently in the American Journal of Physiology , has shown increased expression of lipogenic and inflammatory genes in maternal adipose tissue and in the placenta of obese women in the early first trimester, before any phenotypic change becomes apparent (Am. J. Physiol. Endocrinol. Metab. 2012;303:e832-40).
Specifically, hyperinsulinemia and/or defective insulin action in obese women appears to affect the placental programming of genes relating to adipokine expression and lipid metabolism, as well as mitrochondrial function. Altered inflammatory and lipid pathways affect the availability of nutrients for the fetus and, consequently, the size and body composition of the fetus. Fetal overgrowth and neonatal adiposity can result.
In addition, our research has shown that obese women have decreased insulin suppression of lipolysis in white adipose tissue, which during pregnancy results in improved lipid availability for fetal fat accretion and lipotoxicity.
When interventions aimed at weight loss and improved insulin sensitivity are undertaken before pregnancy or in the period between pregnancies, we have the opportunity to increase fat oxidation and reduce oxidative stress in early pregnancy. We also may be able to limit placental inflammation and favorably affect placental growth and gene expression. By the second trimester, our research suggests, gene expression in the placenta and early molecular changes in the white adipose tissue have already been programmed and cannot be reversed (Am. J. Physiol. Endocrinol. Metab. 2012;303:e832-40).
In studies by our group and others of interpregnancy weight loss or gain, interpregnancy weight loss has been associated with a lower risk of large-for-gestational-age (LGA) infants, whereas interpregnancy weight gain has been associated with an increased risk of LGA. Preliminary work from our group shows that the decrease in birth weight involves primarily fat and not lean mass.
The 2009 IOM guidelines support weight loss before pregnancy and state that overweight women should receive individual preconceptional counseling to improve diet quality, increase physical activity, and normalize weight. Multifaceted interventions do work: In obese nonpregnant individuals, lifestyle interventions, which include an exercise program, diet, and behavioral modification have been shown to be successful in improving insulin sensitivity, inflammation, and overall metabolic function.
According to the IOM report, preconceptional services aimed at achieving a healthy weight before conceiving will represent “a radical change to the care provided to obese women of childbearing age.” With continuing research and accumulating data, however, the concept is gaining traction as a viable paradigm for improving perinatal outcomes, with long-term benefits for both the mother and her baby.
Dr. Catalano reports that he has no disclosures relevant to this Master Class.
Obesity has become so pervasive that it is now considered a major health concern during pregnancy. Almost 56% of women aged 20-39 years in the United States are overweight or obese, based on the World Health Organization’s criteria for body mass index (BMI) and data from the 2009-2010 National Health and Nutrition Examination Survey (NHANES). Moreover, 7.5% of women in this age group are morbidly obese, with a body mass index (BMI) greater than 40 kg/m2 (JAMA 2012;307:491-7).
Obesity in pregnancy not only increases the risk of spontaneous abortions and congenital anomalies, it also increases the risk of gestational diabetes (GDM), hypertensive disorders, and other metabolic complications that affect both the mother and fetus.
Of much concern is the increased risk of fetal overgrowth and long-term health consequences for children of obese mothers. Obesity in early pregnancy has been shown to more than double the risk of obesity in the offspring, which in turn puts these children at risk for developing the metabolic syndrome – and, as Dr. Thomas Moore pointed out in September’s Master Class – appears to program these offspring for downstream cardiovascular risk in adulthood.
Mean term birth weights have risen in the United States during the past several decades. In Cleveland, we have seen a significant 116 g increase in mean term birth weight since 1975; this increase encompasses weights from the 5th to the 95th percentiles. Even more concerning is our finding that the ponderal index in our neonatal population has increased because of decreased fetal length over the last decade.
Some recent studies have suggested that the increase in birth weight in the United States has reached a plateau, but our analyses of national trends suggest that such change is secondary to factors such as earlier gestational age of delivery. Concurrently, an alarming number of children and adolescents – 17% of those aged 2-19 years, according to the 2009-2010 NHANES data – are overweight or obese (JAMA 2012;307:483-90).
How to best treat obesity for improved maternal and fetal health has thus become a focus of research. Studies on lifestyle interventions for obese women during pregnancy have aimed to prevent excessive gestational weight gain and decrease adverse perinatal outcomes – mainly macrosomia, GDM, and hypertensive disorders.
However, the results of this recent body of research have been disappointing. Lifestyle interventions initiated during pregnancy have had only limited success in improving perinatal outcomes. The research tells us that while we may be able to reduce excessive gestational weight gain, it is unlikely that we will be successful in reducing fetal overgrowth, GDM, or preeclampsia in obese women.
Moreover, other studies show that it is a high pregravid BMI – not excessive gestational weight gain or the development of GDM – that plays the biggest role in fetal overgrowth and fetal adiposity.
A paradigm shift is in order. We must think about lifestyle intervention and weight loss before pregnancy, when the woman’s metabolic condition can be improved in time to minimize adverse perinatal metabolic outcomes and to maximize metabolic benefits relating to fetal body composition and metabolism.
Role of prepregnancy BMI
In 2008, the Institute of Medicine (IOM) and National Research Council reexamined 1990 guidelines for gestational weight gain. They concluded that excessive weight gain in pregnancy was a primary contributor to the development of obesity in women. In fact, according to the 2009 IOM report, “Weight Gain During Pregnancy: Reexamining the Guidelines” (Washington: National Academy Press, 2009), 38% of normal weight, 63% of overweight, and 46% of obese women had gained weight in excess of the earlier guidelines.
Helping our patients to gain within the guidelines is important. Excessive gestational weight gain is a primary risk factor for maternal postpartum weight retention, which increases the risk for maternal obesity in a subsequent pregnancy. It also has been associated with a modest increased risk of preterm birth and development of type 2 diabetes.
Interestingly, however, high gestational weight gain has not been related to an increased risk of fetal overgrowth or macrosomia in many obese women. Increased gestational weight gain is a greater risk for fetal overgrowth in women who are of normal weight prior to pregnancy (J. Clin. Endocrinol. Metab. 2012;97:3648-54).
Our research has found that in overweight and obese women, it is maternal pregravid BMI – and not gestational weight gain – that presents the greatest risk for fetal macrosomia, and more specifically, the greatest risk for fetal obesity. Even when glucose tolerance levels are normal, overweight and obese women have neonates who are heavier and who have significant increases in the percentage of body fat and fat mass (Am. J. Obstet. Gynecol. 2006;195:1100-3).
In an 8-year prospective study of the perinatal risk factors associated with childhood obesity, we similarly found that maternal pregravid BMI – independent of maternal glucose status or gestational weight gain – was the strongest predictor of childhood obesity and metabolic dysfunction (Am. J. Clin. Nutr. 2009;90:1303-13).
Other studies have teased apart the roles of maternal obesity and GDM in long-term health of offspring. This work has found that maternal obesity during pregnancy is associated with metabolic syndrome in the offspring and an increased risk of type 2 diabetes in youth, independent of maternal diabetes during pregnancy. A recent meta-analysis also reported that, although maternal diabetes is associated with an increased BMI z score, this was no longer significant after adjustments were made for prepregnancy BMI (Diabetologia 2011;54:1957-66).
Maternal pregravid obesity, therefore, is not only a risk factor for neonatal adiposity at birth, but also for the longer-term risk of obesity and metabolic dysfunction in the offspring – independent of maternal GDM or excessive gestational weight gain.
Interventions in Pregnancy
Numerous prospective trials have examined lifestyle interventions for obese women during pregnancy. One randomized controlled study of a low glycemic index diet in pregnancy (coined the ROLO study) involved 800 women in Ireland who had previously delivered an infant weighting greater than 4,000 g. Women were randomized to receive the restricted diet or no intervention at 13 weeks. Despite a decrease in gestational weight gain in the intervention group, there were no differences in birth weight, birth weight percentile, ponderal index, or macrosomia between the two groups (BMJ 2012;345:e5605).
Another randomized controlled trial reported by a Danish group involved an intervention that consisted of dietary guidance, free membership in a fitness center, and personal coaching initiated between 10 and 14 weeks of gestation. There was a decrease in gestational weight gain in the intervention group, but paradoxically, the infants in the intervention group also had significantly higher birth weight, compared with controls (Diabetes Care 2011;34:2502-7).
Additionally, there have been at least five meta-analyses published in the past 2 years looking at lifestyle interventions during pregnancy. All have concluded that interventions initiated during pregnancy have limited success in reducing excessive gestational weight gain but not necessarily to within the IOM guidelines. The literature contains scant evidence to support further benefits for infant or maternal health (in other words, fetal overgrowth, GDM, or hypertensive disorders).
A recent Cochrane review also concluded that the results of several randomized controlled trials suggest no significant difference in GDM incidence between women receiving exercise intervention versus routine care.
Just this year, three additional randomized controlled trials of lifestyle interventions during pregnancy were published. Only one, the Treatment of Obese Pregnant Women (TOP) study, showed a modest effect in decreasing gestational weight gain. None found a reduction in GDM or fetal overgrowth.
Focus on prepregnancy
Obesity is an inflammatory condition that increases the risk of insulin resistance, impaired beta-cell function, and abnormal adiponectin concentrations. In pregnancy, maternal obesity and hyperinsulinemia can affect placental growth and gene expression.
We have studied lean and obese women recruited prior to a planned pregnancy, as well as lean and obese women scheduled for elective pregnancy termination in the first trimester. Our research, some of which we reported recently in the American Journal of Physiology , has shown increased expression of lipogenic and inflammatory genes in maternal adipose tissue and in the placenta of obese women in the early first trimester, before any phenotypic change becomes apparent (Am. J. Physiol. Endocrinol. Metab. 2012;303:e832-40).
Specifically, hyperinsulinemia and/or defective insulin action in obese women appears to affect the placental programming of genes relating to adipokine expression and lipid metabolism, as well as mitrochondrial function. Altered inflammatory and lipid pathways affect the availability of nutrients for the fetus and, consequently, the size and body composition of the fetus. Fetal overgrowth and neonatal adiposity can result.
In addition, our research has shown that obese women have decreased insulin suppression of lipolysis in white adipose tissue, which during pregnancy results in improved lipid availability for fetal fat accretion and lipotoxicity.
When interventions aimed at weight loss and improved insulin sensitivity are undertaken before pregnancy or in the period between pregnancies, we have the opportunity to increase fat oxidation and reduce oxidative stress in early pregnancy. We also may be able to limit placental inflammation and favorably affect placental growth and gene expression. By the second trimester, our research suggests, gene expression in the placenta and early molecular changes in the white adipose tissue have already been programmed and cannot be reversed (Am. J. Physiol. Endocrinol. Metab. 2012;303:e832-40).
In studies by our group and others of interpregnancy weight loss or gain, interpregnancy weight loss has been associated with a lower risk of large-for-gestational-age (LGA) infants, whereas interpregnancy weight gain has been associated with an increased risk of LGA. Preliminary work from our group shows that the decrease in birth weight involves primarily fat and not lean mass.
The 2009 IOM guidelines support weight loss before pregnancy and state that overweight women should receive individual preconceptional counseling to improve diet quality, increase physical activity, and normalize weight. Multifaceted interventions do work: In obese nonpregnant individuals, lifestyle interventions, which include an exercise program, diet, and behavioral modification have been shown to be successful in improving insulin sensitivity, inflammation, and overall metabolic function.
According to the IOM report, preconceptional services aimed at achieving a healthy weight before conceiving will represent “a radical change to the care provided to obese women of childbearing age.” With continuing research and accumulating data, however, the concept is gaining traction as a viable paradigm for improving perinatal outcomes, with long-term benefits for both the mother and her baby.
Dr. Catalano reports that he has no disclosures relevant to this Master Class.