User login
Over-the-counter H1-antihistamines
H1-antihistamines are commonly used by pregnant and lactating women. New information suggests that their mechanism of action is different from the initial characterization that they were competitive antagonists of H1-histamine receptors. On the cellular surface, both active and inactive H1-receptors exist in equilibrium, responding to histamines (agonists) and inverse agonists (antihistamines). Antihistamines bind and stabilize the inactive receptors, shifting the equilibrium to the inactive state and preventing or reducing the physiologic effects of histamine (Clin. Exp. Allergy 2002;32:489-98).
Antihistamines can be classified as either first-generation (nonselective) or second-generation (peripherally selective) agents. First-generation antihistamines bind nonselectively to central and peripheral inactive H1-receptors. They have various indications, including allergic rhinitis (hay fever), allergic conjunctivitis, urticaria/angioedema, vasomotor rhinitis, sneezing, asthma, and hypersensitivity reactions. The four first-generation oral agents available over-the-counter (OTC) can be further classified, based on their chemical composition, into two groups: alkylamines (chlorpheniramine) and ethanolamines (clemastine, diphenhydramine, and doxylamine). This latter group has marked sedative properties, as well as anticholinergic and antiemetic actions.
Chlorpheniramine
Brands include Aller-Chlor, Allergy Relief, Chlo-Amine, Chlor-Trimeton, and Efidac 24. More than 1,100 first-trimester exposures to this agent have been reported. In these cases, the number of congenital anomalies was not increased over the expected background risk (Collaborative Perinatal Project 1977 [CPP]; and Michigan Medicaid Data 1993 [MMD]).
Clemastine
Brands include Dayhist-1 and Tavist Allergy. More than 2,800 first-trimester exposures to clemastine have been reported. No increased risk of teratogenicity was noted in one study involving 1,230 exposures (J. Matern. Fetal Neonat. Med. 2002;11:146-52). In contrast, among the 1,617 exposures in the MMD, a possible association with limb reduction defects (5 observed/1.9 expected) was discovered, but a causal association cannot be determined from these data.
Diphenhydramine
Brands include AllerMax, Altaryl Children’s Allergy, Banophen, Benadryl, Diphenhist, Dormin, Genahist, Miles Nervine, Nytol, Siladryl, Sleep-eze 3, Sleepwell 2-nite, and Sominex. Commonly used to promote sleep, as well as to treat nausea and allergies, more than 2,300 first-trimester exposures have been reported in the literature.
Several possible associations with congenital defects have been observed in some of these reports, such as those from the CPP and MMD, but many other studies have not found these associations (Drugs in Pregnancy and Lactation, 9th ed. Riverwoods, Ill.: Wolters Kluwer Health, 2011). Withdrawal was observed in one infant whose mother took diphenhydramine 150 mg/day throughout pregnancy (J. Pediatr. 1974;85:580). A potential drug interaction, resulting in stillbirth, occurred when a mother took 50 mg of the antihistamine for itching and then, 1.5 hours later, a 30-mg dose of temazepam for sleep. Violent fetal movements occurred 3 hours later, followed in 4 hours by the stillbirth of a term female infant. The interaction was confirmed in rabbits with a fetal mortality rate of 81% (N. Engl. J. Med. 1985;313:1417-8).
Doxylamine
Brands include Unisom Nighttime Sleep Aid. This agent is a potent antiemetic and sedative and may be one of the most studied drugs in human pregnancy. Although some studies found associations with various defects, most studies have not (Drugs in Pregnancy and Lactation, 9th ed. Riverwoods, Ill.: Wolters Kluwer Health, 2011). These results suggest that other exposures, conditions, or chance were involved in the positive studies, and doxylamine is considered safe to use in pregnancy. The combination of doxylamine and pyridoxine (vitamin B6) is recommended as a first-line therapy for nausea and vomiting of pregnancy (ACOG Practice Bulletin no. 52. Nausea and vomiting of pregnancy. April 2004. Obstet. Gynecol. 2004;103:803-15). The combination has been available for years as Diclectin in Canada but, in the United States, could only be obtained as individual OTC components. In April 2013, the doxylamine-pyridoxine combination (Diclegis) was approved by the U.S. Food and Drug Administration.
Second-generation antihistamines are selective for peripheral inactive H1-receptors and, as a group, are less sedating. These agents are used for allergic rhinitis, sinusitis, seasonal allergic rhinitis, and chronic idiopathic urticaria, The three OTC agents in this class also can be divided into two subgroups: piperazines (cetirizine) and piperidines (fexofenadine and loratadine).
Cetirizine
It comes in various generic and Zyrtec formulations. Cetirizine is a human metabolite of hydroxyzine. Although the human pregnancy experience is limited (about 120 cases), there is no evidence that it is a significant risk to the embryo and/or fetus. In one report, pregnant women using the drug for allergies had a lower rate of nausea and vomiting than a control group (Ann. Pharmacother. 2000;34:1486-7).
Fexofenadine
There are various generic and Allegra formulations. There are no reports describing the use of this agent in human pregnancy. Animal data suggest moderate risk based on dose-related embryo and fetal toxicity in rats. The use of other antihistamines is recommended.
Loratadine
There are various generic and Claritin and Alavert formulations. The limited human data have generally shown no risk of teratogenicity. A Swedish study reported that exposure during pregnancy doubled the incidence of hypospadias (Int. J. Risk Saf. Med. 2001;14:115-9). However, a later study using data from the U.S. National Birth Defects Prevention Study found no association between loratadine and hypospadias (MMWR 2004;53:219-21).
In summary, the available evidence, both animal and human, indicates that as a class, H1-antihistamines represent a low risk to the embryo and fetus. Because there are no reports for fexofenadine, other antihistamines might be a better choice. Although not discussed here, H1-antihistamines are common components of upper respiratory formulations that contain decongestants, expectorants, or analgesics. Depending on the stage of gestation when used, these combinations may have a risk of maternal or fetal harm.
Breastfeeding
All of the above H1-antihistamines are probably excreted into breast milk. Although published reports are rare, the first-generation agents have caused irritability or drowsiness in nursing infants. Fortunately, the second-generation agents have not been reported to cause these effects in a nursing neonate. Nevertheless, recommended doses of all of these agents are probably compatible with nursing full-term infants, but exposing preterm infants should be avoided.
Mr. Briggs is a pharmacist clinical specialist at the outpatient clinics of Memorial Care Center for Women at Miller Children’s Hospital in Long Beach, Calif.; clinical professor of pharmacy at the University of California, San Francisco; and adjunct professor of pharmacy at the University of Southern California, Los Angeles, and Washington State University, Spokane. He also is coauthor of "Drugs in Pregnancy and Lactation," and coeditor of "Diseases, Complications, and Drug Therapy in Obstetrics." He said he had no relevant financial disclosures. Contact him at [email protected]. View more Drugs, Pregnancy, and Lactation columns by clicking here.
H1-antihistamines are commonly used by pregnant and lactating women. New information suggests that their mechanism of action is different from the initial characterization that they were competitive antagonists of H1-histamine receptors. On the cellular surface, both active and inactive H1-receptors exist in equilibrium, responding to histamines (agonists) and inverse agonists (antihistamines). Antihistamines bind and stabilize the inactive receptors, shifting the equilibrium to the inactive state and preventing or reducing the physiologic effects of histamine (Clin. Exp. Allergy 2002;32:489-98).
Antihistamines can be classified as either first-generation (nonselective) or second-generation (peripherally selective) agents. First-generation antihistamines bind nonselectively to central and peripheral inactive H1-receptors. They have various indications, including allergic rhinitis (hay fever), allergic conjunctivitis, urticaria/angioedema, vasomotor rhinitis, sneezing, asthma, and hypersensitivity reactions. The four first-generation oral agents available over-the-counter (OTC) can be further classified, based on their chemical composition, into two groups: alkylamines (chlorpheniramine) and ethanolamines (clemastine, diphenhydramine, and doxylamine). This latter group has marked sedative properties, as well as anticholinergic and antiemetic actions.
Chlorpheniramine
Brands include Aller-Chlor, Allergy Relief, Chlo-Amine, Chlor-Trimeton, and Efidac 24. More than 1,100 first-trimester exposures to this agent have been reported. In these cases, the number of congenital anomalies was not increased over the expected background risk (Collaborative Perinatal Project 1977 [CPP]; and Michigan Medicaid Data 1993 [MMD]).
Clemastine
Brands include Dayhist-1 and Tavist Allergy. More than 2,800 first-trimester exposures to clemastine have been reported. No increased risk of teratogenicity was noted in one study involving 1,230 exposures (J. Matern. Fetal Neonat. Med. 2002;11:146-52). In contrast, among the 1,617 exposures in the MMD, a possible association with limb reduction defects (5 observed/1.9 expected) was discovered, but a causal association cannot be determined from these data.
Diphenhydramine
Brands include AllerMax, Altaryl Children’s Allergy, Banophen, Benadryl, Diphenhist, Dormin, Genahist, Miles Nervine, Nytol, Siladryl, Sleep-eze 3, Sleepwell 2-nite, and Sominex. Commonly used to promote sleep, as well as to treat nausea and allergies, more than 2,300 first-trimester exposures have been reported in the literature.
Several possible associations with congenital defects have been observed in some of these reports, such as those from the CPP and MMD, but many other studies have not found these associations (Drugs in Pregnancy and Lactation, 9th ed. Riverwoods, Ill.: Wolters Kluwer Health, 2011). Withdrawal was observed in one infant whose mother took diphenhydramine 150 mg/day throughout pregnancy (J. Pediatr. 1974;85:580). A potential drug interaction, resulting in stillbirth, occurred when a mother took 50 mg of the antihistamine for itching and then, 1.5 hours later, a 30-mg dose of temazepam for sleep. Violent fetal movements occurred 3 hours later, followed in 4 hours by the stillbirth of a term female infant. The interaction was confirmed in rabbits with a fetal mortality rate of 81% (N. Engl. J. Med. 1985;313:1417-8).
Doxylamine
Brands include Unisom Nighttime Sleep Aid. This agent is a potent antiemetic and sedative and may be one of the most studied drugs in human pregnancy. Although some studies found associations with various defects, most studies have not (Drugs in Pregnancy and Lactation, 9th ed. Riverwoods, Ill.: Wolters Kluwer Health, 2011). These results suggest that other exposures, conditions, or chance were involved in the positive studies, and doxylamine is considered safe to use in pregnancy. The combination of doxylamine and pyridoxine (vitamin B6) is recommended as a first-line therapy for nausea and vomiting of pregnancy (ACOG Practice Bulletin no. 52. Nausea and vomiting of pregnancy. April 2004. Obstet. Gynecol. 2004;103:803-15). The combination has been available for years as Diclectin in Canada but, in the United States, could only be obtained as individual OTC components. In April 2013, the doxylamine-pyridoxine combination (Diclegis) was approved by the U.S. Food and Drug Administration.
Second-generation antihistamines are selective for peripheral inactive H1-receptors and, as a group, are less sedating. These agents are used for allergic rhinitis, sinusitis, seasonal allergic rhinitis, and chronic idiopathic urticaria, The three OTC agents in this class also can be divided into two subgroups: piperazines (cetirizine) and piperidines (fexofenadine and loratadine).
Cetirizine
It comes in various generic and Zyrtec formulations. Cetirizine is a human metabolite of hydroxyzine. Although the human pregnancy experience is limited (about 120 cases), there is no evidence that it is a significant risk to the embryo and/or fetus. In one report, pregnant women using the drug for allergies had a lower rate of nausea and vomiting than a control group (Ann. Pharmacother. 2000;34:1486-7).
Fexofenadine
There are various generic and Allegra formulations. There are no reports describing the use of this agent in human pregnancy. Animal data suggest moderate risk based on dose-related embryo and fetal toxicity in rats. The use of other antihistamines is recommended.
Loratadine
There are various generic and Claritin and Alavert formulations. The limited human data have generally shown no risk of teratogenicity. A Swedish study reported that exposure during pregnancy doubled the incidence of hypospadias (Int. J. Risk Saf. Med. 2001;14:115-9). However, a later study using data from the U.S. National Birth Defects Prevention Study found no association between loratadine and hypospadias (MMWR 2004;53:219-21).
In summary, the available evidence, both animal and human, indicates that as a class, H1-antihistamines represent a low risk to the embryo and fetus. Because there are no reports for fexofenadine, other antihistamines might be a better choice. Although not discussed here, H1-antihistamines are common components of upper respiratory formulations that contain decongestants, expectorants, or analgesics. Depending on the stage of gestation when used, these combinations may have a risk of maternal or fetal harm.
Breastfeeding
All of the above H1-antihistamines are probably excreted into breast milk. Although published reports are rare, the first-generation agents have caused irritability or drowsiness in nursing infants. Fortunately, the second-generation agents have not been reported to cause these effects in a nursing neonate. Nevertheless, recommended doses of all of these agents are probably compatible with nursing full-term infants, but exposing preterm infants should be avoided.
Mr. Briggs is a pharmacist clinical specialist at the outpatient clinics of Memorial Care Center for Women at Miller Children’s Hospital in Long Beach, Calif.; clinical professor of pharmacy at the University of California, San Francisco; and adjunct professor of pharmacy at the University of Southern California, Los Angeles, and Washington State University, Spokane. He also is coauthor of "Drugs in Pregnancy and Lactation," and coeditor of "Diseases, Complications, and Drug Therapy in Obstetrics." He said he had no relevant financial disclosures. Contact him at [email protected]. View more Drugs, Pregnancy, and Lactation columns by clicking here.
H1-antihistamines are commonly used by pregnant and lactating women. New information suggests that their mechanism of action is different from the initial characterization that they were competitive antagonists of H1-histamine receptors. On the cellular surface, both active and inactive H1-receptors exist in equilibrium, responding to histamines (agonists) and inverse agonists (antihistamines). Antihistamines bind and stabilize the inactive receptors, shifting the equilibrium to the inactive state and preventing or reducing the physiologic effects of histamine (Clin. Exp. Allergy 2002;32:489-98).
Antihistamines can be classified as either first-generation (nonselective) or second-generation (peripherally selective) agents. First-generation antihistamines bind nonselectively to central and peripheral inactive H1-receptors. They have various indications, including allergic rhinitis (hay fever), allergic conjunctivitis, urticaria/angioedema, vasomotor rhinitis, sneezing, asthma, and hypersensitivity reactions. The four first-generation oral agents available over-the-counter (OTC) can be further classified, based on their chemical composition, into two groups: alkylamines (chlorpheniramine) and ethanolamines (clemastine, diphenhydramine, and doxylamine). This latter group has marked sedative properties, as well as anticholinergic and antiemetic actions.
Chlorpheniramine
Brands include Aller-Chlor, Allergy Relief, Chlo-Amine, Chlor-Trimeton, and Efidac 24. More than 1,100 first-trimester exposures to this agent have been reported. In these cases, the number of congenital anomalies was not increased over the expected background risk (Collaborative Perinatal Project 1977 [CPP]; and Michigan Medicaid Data 1993 [MMD]).
Clemastine
Brands include Dayhist-1 and Tavist Allergy. More than 2,800 first-trimester exposures to clemastine have been reported. No increased risk of teratogenicity was noted in one study involving 1,230 exposures (J. Matern. Fetal Neonat. Med. 2002;11:146-52). In contrast, among the 1,617 exposures in the MMD, a possible association with limb reduction defects (5 observed/1.9 expected) was discovered, but a causal association cannot be determined from these data.
Diphenhydramine
Brands include AllerMax, Altaryl Children’s Allergy, Banophen, Benadryl, Diphenhist, Dormin, Genahist, Miles Nervine, Nytol, Siladryl, Sleep-eze 3, Sleepwell 2-nite, and Sominex. Commonly used to promote sleep, as well as to treat nausea and allergies, more than 2,300 first-trimester exposures have been reported in the literature.
Several possible associations with congenital defects have been observed in some of these reports, such as those from the CPP and MMD, but many other studies have not found these associations (Drugs in Pregnancy and Lactation, 9th ed. Riverwoods, Ill.: Wolters Kluwer Health, 2011). Withdrawal was observed in one infant whose mother took diphenhydramine 150 mg/day throughout pregnancy (J. Pediatr. 1974;85:580). A potential drug interaction, resulting in stillbirth, occurred when a mother took 50 mg of the antihistamine for itching and then, 1.5 hours later, a 30-mg dose of temazepam for sleep. Violent fetal movements occurred 3 hours later, followed in 4 hours by the stillbirth of a term female infant. The interaction was confirmed in rabbits with a fetal mortality rate of 81% (N. Engl. J. Med. 1985;313:1417-8).
Doxylamine
Brands include Unisom Nighttime Sleep Aid. This agent is a potent antiemetic and sedative and may be one of the most studied drugs in human pregnancy. Although some studies found associations with various defects, most studies have not (Drugs in Pregnancy and Lactation, 9th ed. Riverwoods, Ill.: Wolters Kluwer Health, 2011). These results suggest that other exposures, conditions, or chance were involved in the positive studies, and doxylamine is considered safe to use in pregnancy. The combination of doxylamine and pyridoxine (vitamin B6) is recommended as a first-line therapy for nausea and vomiting of pregnancy (ACOG Practice Bulletin no. 52. Nausea and vomiting of pregnancy. April 2004. Obstet. Gynecol. 2004;103:803-15). The combination has been available for years as Diclectin in Canada but, in the United States, could only be obtained as individual OTC components. In April 2013, the doxylamine-pyridoxine combination (Diclegis) was approved by the U.S. Food and Drug Administration.
Second-generation antihistamines are selective for peripheral inactive H1-receptors and, as a group, are less sedating. These agents are used for allergic rhinitis, sinusitis, seasonal allergic rhinitis, and chronic idiopathic urticaria, The three OTC agents in this class also can be divided into two subgroups: piperazines (cetirizine) and piperidines (fexofenadine and loratadine).
Cetirizine
It comes in various generic and Zyrtec formulations. Cetirizine is a human metabolite of hydroxyzine. Although the human pregnancy experience is limited (about 120 cases), there is no evidence that it is a significant risk to the embryo and/or fetus. In one report, pregnant women using the drug for allergies had a lower rate of nausea and vomiting than a control group (Ann. Pharmacother. 2000;34:1486-7).
Fexofenadine
There are various generic and Allegra formulations. There are no reports describing the use of this agent in human pregnancy. Animal data suggest moderate risk based on dose-related embryo and fetal toxicity in rats. The use of other antihistamines is recommended.
Loratadine
There are various generic and Claritin and Alavert formulations. The limited human data have generally shown no risk of teratogenicity. A Swedish study reported that exposure during pregnancy doubled the incidence of hypospadias (Int. J. Risk Saf. Med. 2001;14:115-9). However, a later study using data from the U.S. National Birth Defects Prevention Study found no association between loratadine and hypospadias (MMWR 2004;53:219-21).
In summary, the available evidence, both animal and human, indicates that as a class, H1-antihistamines represent a low risk to the embryo and fetus. Because there are no reports for fexofenadine, other antihistamines might be a better choice. Although not discussed here, H1-antihistamines are common components of upper respiratory formulations that contain decongestants, expectorants, or analgesics. Depending on the stage of gestation when used, these combinations may have a risk of maternal or fetal harm.
Breastfeeding
All of the above H1-antihistamines are probably excreted into breast milk. Although published reports are rare, the first-generation agents have caused irritability or drowsiness in nursing infants. Fortunately, the second-generation agents have not been reported to cause these effects in a nursing neonate. Nevertheless, recommended doses of all of these agents are probably compatible with nursing full-term infants, but exposing preterm infants should be avoided.
Mr. Briggs is a pharmacist clinical specialist at the outpatient clinics of Memorial Care Center for Women at Miller Children’s Hospital in Long Beach, Calif.; clinical professor of pharmacy at the University of California, San Francisco; and adjunct professor of pharmacy at the University of Southern California, Los Angeles, and Washington State University, Spokane. He also is coauthor of "Drugs in Pregnancy and Lactation," and coeditor of "Diseases, Complications, and Drug Therapy in Obstetrics." He said he had no relevant financial disclosures. Contact him at [email protected]. View more Drugs, Pregnancy, and Lactation columns by clicking here.
Prenatal exposure to air pollution boosts childhood cancer risk
WASHINGTON – Children whose mothers were exposed to air pollution during pregnancy may have a small, but significant, increase in pediatric cancer risk.
A 9-year case-control study found that children who were exposed to the highest levels of traffic-related pollution prenatally and during the first year of life were 19% more likely to develop bilateral retinoblastoma and 17% more likely to develop germ cell tumors than were children who were exposed to the lowest level.
"This is the first study to report upon traffic pollution in relation to retinoblastoma or germ cell tumors, and since both of these are rare, the findings need to be replicated in other studies," Julia E. Heck, Ph.D., said in an interview at the annual meeting of the American Association for Cancer Research.
Dr. Heck, an epidemiologist at the University of California’s Fielding School of Public Health, Los Angeles, used a state air pollution modeling system to detect the patterns in her study. The California Line Source Dispersion Modeling system, version 4 (CALINE4) uses a test fleet of motor vehicles to predict traffic-related carbon monoxide emissions from vehicles in idle, cruise, acceleration, and deceleration.
Her study cohort comprised 3,590 children with cancer who were included in a California cancer registry. All were born between 1998 and 2007 and were aged younger than 6 years. She compared these children to a random selection of 80,224 children drawn form California birth records. The CALINE4 system generated estimates of local traffic exposure based on where the mothers were living during each trimester of pregnancy.
The model imputed emissions from gasoline and diesel vehicles within a 1,500-meter radius of the address. It included data on traffic volume, roadway geometry, vehicle emission rates, and weather patterns, and divided the cohort into quartiles according to exposure. Dr. Heck looked specifically at carbon monoxide measurements in increments of 53 parts per billion.
Each increase in CO emission raised the risk of acute lymphoblastic leukemia by 4%. Each quartile was associated with an overall 14% increased risk of a retinoblastoma, with the risk significantly elevated for bilateral tumors rather than unilateral tumors (a 19% increase). Dr. Heck also saw a 17% increased risk for germ cell tumors for every quartile increase in CO.
Associations with other cancers, including acute myeloid leukemia, non-Hodgkin’s lymphoma, ependymoma, astrocytoma, neuroblastoma, Wilms tumor, hepatoblastoma, and rhabdomyosarcoma, were nonsignificant.
Because Dr. Heck’s study was a retrospective analysis, it cannot prove causation. "The results need to be confirmed in other studies," she said in an interview. "But our findings do support a link between traffic pollution and some childhood cancers."
The field of childhood cancers and air pollution has not been well studied in recent years, Dr. Heck said, although there were a number of such studies in the late 1990s and early 2000s.
One was a British case-control analysis of birth and death records of 12,018 children in the United Kingdom who had been born and died from leukemia and other cancers from 1955 to 1980. The cancer-related deaths were linked to locations of rail and bus stations, ferry terminals, railways, roads, canals, and rivers (J. Epidemiol. Community Health 2005;59:755-60).
"The most striking result is the extraordinary concentration of cancer births within 0.3 km of bus/coach stations (OR = 12.5)," wrote Dr. E.G. Knox, professor emeritus at the University of Birmingham, England. "This was followed by hospitals (OR = 2.6) and heavy transport centers (OR = 1.6)."
Of the individual pollutants examined, both carbon monoxide and 1,3-butadiene more than doubled the risk of cancer.
Because the study encompassed the child’s location at birth, it demonstrated a link to prenatal exposure. "Childhood cancers are strongly determined by prenatal or early postnatal exposures to oil-based combustion gases, especially from engine exhausts," he noted. "The chief carcinogenic agent is probably 1,3-butadiene."
The National Institute for Occupational Safety and Health has recommended that 1,3-butadiene be treated as a potential occupational carcinogen, teratogen, and as a reproduction hazard.
In 2006, Dr. Knox published another study on the same group, in which he attributed about 24% of the cancers to prenatal or early life proximity to areas of high airborne pollutants. "Child cancer initiations are strongly determined by prenatal or early postnatal exposures to engine exhaust gases, probably through maternal inhalation and accumulation of carcinogens over many months," he noted. (J. Epidemiol. Community Health 2006;60:136-41).
Dr. Heck’s study was funded by the National Institutes of Health. She had no financial disclosures.
WASHINGTON – Children whose mothers were exposed to air pollution during pregnancy may have a small, but significant, increase in pediatric cancer risk.
A 9-year case-control study found that children who were exposed to the highest levels of traffic-related pollution prenatally and during the first year of life were 19% more likely to develop bilateral retinoblastoma and 17% more likely to develop germ cell tumors than were children who were exposed to the lowest level.
"This is the first study to report upon traffic pollution in relation to retinoblastoma or germ cell tumors, and since both of these are rare, the findings need to be replicated in other studies," Julia E. Heck, Ph.D., said in an interview at the annual meeting of the American Association for Cancer Research.
Dr. Heck, an epidemiologist at the University of California’s Fielding School of Public Health, Los Angeles, used a state air pollution modeling system to detect the patterns in her study. The California Line Source Dispersion Modeling system, version 4 (CALINE4) uses a test fleet of motor vehicles to predict traffic-related carbon monoxide emissions from vehicles in idle, cruise, acceleration, and deceleration.
Her study cohort comprised 3,590 children with cancer who were included in a California cancer registry. All were born between 1998 and 2007 and were aged younger than 6 years. She compared these children to a random selection of 80,224 children drawn form California birth records. The CALINE4 system generated estimates of local traffic exposure based on where the mothers were living during each trimester of pregnancy.
The model imputed emissions from gasoline and diesel vehicles within a 1,500-meter radius of the address. It included data on traffic volume, roadway geometry, vehicle emission rates, and weather patterns, and divided the cohort into quartiles according to exposure. Dr. Heck looked specifically at carbon monoxide measurements in increments of 53 parts per billion.
Each increase in CO emission raised the risk of acute lymphoblastic leukemia by 4%. Each quartile was associated with an overall 14% increased risk of a retinoblastoma, with the risk significantly elevated for bilateral tumors rather than unilateral tumors (a 19% increase). Dr. Heck also saw a 17% increased risk for germ cell tumors for every quartile increase in CO.
Associations with other cancers, including acute myeloid leukemia, non-Hodgkin’s lymphoma, ependymoma, astrocytoma, neuroblastoma, Wilms tumor, hepatoblastoma, and rhabdomyosarcoma, were nonsignificant.
Because Dr. Heck’s study was a retrospective analysis, it cannot prove causation. "The results need to be confirmed in other studies," she said in an interview. "But our findings do support a link between traffic pollution and some childhood cancers."
The field of childhood cancers and air pollution has not been well studied in recent years, Dr. Heck said, although there were a number of such studies in the late 1990s and early 2000s.
One was a British case-control analysis of birth and death records of 12,018 children in the United Kingdom who had been born and died from leukemia and other cancers from 1955 to 1980. The cancer-related deaths were linked to locations of rail and bus stations, ferry terminals, railways, roads, canals, and rivers (J. Epidemiol. Community Health 2005;59:755-60).
"The most striking result is the extraordinary concentration of cancer births within 0.3 km of bus/coach stations (OR = 12.5)," wrote Dr. E.G. Knox, professor emeritus at the University of Birmingham, England. "This was followed by hospitals (OR = 2.6) and heavy transport centers (OR = 1.6)."
Of the individual pollutants examined, both carbon monoxide and 1,3-butadiene more than doubled the risk of cancer.
Because the study encompassed the child’s location at birth, it demonstrated a link to prenatal exposure. "Childhood cancers are strongly determined by prenatal or early postnatal exposures to oil-based combustion gases, especially from engine exhausts," he noted. "The chief carcinogenic agent is probably 1,3-butadiene."
The National Institute for Occupational Safety and Health has recommended that 1,3-butadiene be treated as a potential occupational carcinogen, teratogen, and as a reproduction hazard.
In 2006, Dr. Knox published another study on the same group, in which he attributed about 24% of the cancers to prenatal or early life proximity to areas of high airborne pollutants. "Child cancer initiations are strongly determined by prenatal or early postnatal exposures to engine exhaust gases, probably through maternal inhalation and accumulation of carcinogens over many months," he noted. (J. Epidemiol. Community Health 2006;60:136-41).
Dr. Heck’s study was funded by the National Institutes of Health. She had no financial disclosures.
WASHINGTON – Children whose mothers were exposed to air pollution during pregnancy may have a small, but significant, increase in pediatric cancer risk.
A 9-year case-control study found that children who were exposed to the highest levels of traffic-related pollution prenatally and during the first year of life were 19% more likely to develop bilateral retinoblastoma and 17% more likely to develop germ cell tumors than were children who were exposed to the lowest level.
"This is the first study to report upon traffic pollution in relation to retinoblastoma or germ cell tumors, and since both of these are rare, the findings need to be replicated in other studies," Julia E. Heck, Ph.D., said in an interview at the annual meeting of the American Association for Cancer Research.
Dr. Heck, an epidemiologist at the University of California’s Fielding School of Public Health, Los Angeles, used a state air pollution modeling system to detect the patterns in her study. The California Line Source Dispersion Modeling system, version 4 (CALINE4) uses a test fleet of motor vehicles to predict traffic-related carbon monoxide emissions from vehicles in idle, cruise, acceleration, and deceleration.
Her study cohort comprised 3,590 children with cancer who were included in a California cancer registry. All were born between 1998 and 2007 and were aged younger than 6 years. She compared these children to a random selection of 80,224 children drawn form California birth records. The CALINE4 system generated estimates of local traffic exposure based on where the mothers were living during each trimester of pregnancy.
The model imputed emissions from gasoline and diesel vehicles within a 1,500-meter radius of the address. It included data on traffic volume, roadway geometry, vehicle emission rates, and weather patterns, and divided the cohort into quartiles according to exposure. Dr. Heck looked specifically at carbon monoxide measurements in increments of 53 parts per billion.
Each increase in CO emission raised the risk of acute lymphoblastic leukemia by 4%. Each quartile was associated with an overall 14% increased risk of a retinoblastoma, with the risk significantly elevated for bilateral tumors rather than unilateral tumors (a 19% increase). Dr. Heck also saw a 17% increased risk for germ cell tumors for every quartile increase in CO.
Associations with other cancers, including acute myeloid leukemia, non-Hodgkin’s lymphoma, ependymoma, astrocytoma, neuroblastoma, Wilms tumor, hepatoblastoma, and rhabdomyosarcoma, were nonsignificant.
Because Dr. Heck’s study was a retrospective analysis, it cannot prove causation. "The results need to be confirmed in other studies," she said in an interview. "But our findings do support a link between traffic pollution and some childhood cancers."
The field of childhood cancers and air pollution has not been well studied in recent years, Dr. Heck said, although there were a number of such studies in the late 1990s and early 2000s.
One was a British case-control analysis of birth and death records of 12,018 children in the United Kingdom who had been born and died from leukemia and other cancers from 1955 to 1980. The cancer-related deaths were linked to locations of rail and bus stations, ferry terminals, railways, roads, canals, and rivers (J. Epidemiol. Community Health 2005;59:755-60).
"The most striking result is the extraordinary concentration of cancer births within 0.3 km of bus/coach stations (OR = 12.5)," wrote Dr. E.G. Knox, professor emeritus at the University of Birmingham, England. "This was followed by hospitals (OR = 2.6) and heavy transport centers (OR = 1.6)."
Of the individual pollutants examined, both carbon monoxide and 1,3-butadiene more than doubled the risk of cancer.
Because the study encompassed the child’s location at birth, it demonstrated a link to prenatal exposure. "Childhood cancers are strongly determined by prenatal or early postnatal exposures to oil-based combustion gases, especially from engine exhausts," he noted. "The chief carcinogenic agent is probably 1,3-butadiene."
The National Institute for Occupational Safety and Health has recommended that 1,3-butadiene be treated as a potential occupational carcinogen, teratogen, and as a reproduction hazard.
In 2006, Dr. Knox published another study on the same group, in which he attributed about 24% of the cancers to prenatal or early life proximity to areas of high airborne pollutants. "Child cancer initiations are strongly determined by prenatal or early postnatal exposures to engine exhaust gases, probably through maternal inhalation and accumulation of carcinogens over many months," he noted. (J. Epidemiol. Community Health 2006;60:136-41).
Dr. Heck’s study was funded by the National Institutes of Health. She had no financial disclosures.
FROM THE AACR ANNUAL MEETING
Major finding: Children exposed prenatally to high levels of motor vehicle exhaust were 19% more likely to develop bilateral retinoblastoma and 17% more likely to develop germ cell tumors than were children with less exposure.
Data source: Case-control analysis of 3,590 children who developed cancer matched to 80,224 controls.
Disclosures: The study was funded by the National Institutes of Health. Dr. Heck had no financial disclosures.
FDA approves once-popular morning sickness drug
For the first time in 30 years, clinicians will be able to prescribe a Food and Drug Administration-approved treatment for their pregnant patients who are experiencing nausea and vomiting and have not responded to changes in their diet or other conservative measures.
On April 9, the FDA announced the approval of a delayed-release, fixed-dose formulation of 10 mg of doxylamine, an antihistamine; and 10 mg of pyridoxine, a vitamin B6 analog, for "the treatment of nausea and vomiting of pregnancy in women who do not respond to conservative management."
This combination was widely used as an effective treatment for nausea and vomiting of pregnancy (NVP) in the United States and Canada in the 1960s and 1970s, and was marketed as Bendectin. But it was voluntarily taken off the U.S. market by the manufacturer in 1983, amid allegations and lawsuits that the drug caused birth defects – which were never substantiated with scientific data. The combination, which has been available as separate components in the United States, was recommended as a first-line treatment for NVP in a 2004 American College of Obstetricians and Gynecologists practice statement that was reaffirmed in 2011 (Obstet. Gynecol. 2004;103:803-15).
In a clear sign that the issue has been resolved, the FDA has assigned Diclegis a category A pregnancy risk rating, with a statement in the label summarizing the epidemiologic studies indicating that the combination of the two active ingredients has not been associated with increased risks to the fetus.
"Diclegis is now the only FDA-approved treatment for nausea and vomiting due to pregnancy, providing a therapeutic option for pregnant women seeking relief from these symptoms," Dr. Hylton V. Joffe, director of the division of reproductive and urologic products in the FDA’s Center for Drug Evaluation and Research, said in the FDA statement. It is expected to be widely available in the United States by the end of May, according to the Canadian manufacturer, Duchesnay Inc., which markets the same product in Canada.
The approval "is a big victory for American women and health care professionals, especially obstetricians and family physicians, because over the last 3 decades, American women have not had an FDA-approved medication for the most common condition of pregnancy, morning sickness," said Dr. Gideon Koren, the head of the Research Leadership for Better Pharmacotherapy During Pregnancy and Lactation at the Hospital for Sick Children, Toronto. After Bendectin was removed from the market, the rate of hospitalizations for hyperemesis gravidarum markedly increased in the United States, "clearly showing that when you remove a drug that a very large number of women need, a void is created and women suffer," he added.
The FDA approval was based on a study of 261 women with NVP in the United States who were at least 18 years-of-age and who were 7-14 weeks pregnant (mean gestational age was 9.3 weeks). Those randomized to receive treatment with Diclegis for 2 weeks had greater improvements in nausea and vomiting, compared with those who received a placebo, as reflected in changes in a score that incorporated the number of daily episodes of vomiting and heaves, and hours of nausea. The most common side effect associated with Diclegis was drowsiness, which can be severe, according to the FDA.
Diclegis is taken daily and is not used as an as-needed treatment for symptoms. The recommended dosage regimen is an initial dose of two tablets at bedtime on the first day. If symptoms persist through the afternoon of the second day, the woman should take two tablets at bedtime and one tablet the following morning (day 3). If symptoms are still present on the fourth day, she should take one tablet in the morning, one tablet in the middle of the afternoon and two tablets at bedtime. Four tablets are the maximum recommended daily dose. In the study, 19% of the women treated with Diclegis took two tablets a day, 21% took three tablets a day, and 60% took four tablets a day.
The prescribing information includes a statement that the combination of doxylamine and pyridoxine "has been the subject of many epidemiologic studies (cohort, case control, and meta-analyses) designed to detect possible teratogenicity," including studies published between 1963 and 1991, which did not report evidence of fetal abnormalities associated with first trimester exposure.
In an interview, Dr. Koren, professor of pediatrics, pharmacology, pharmacy, medicine, and medical genetics at the University of Toronto, said that the safety studies include data on more than 300,000 mother-child pairs. When Bendectin was taken off the U.S. market, it was already available as a generic in Canada as Diclectin and was never taken off the market. The continuous experience with the product in Canada should also make U.S. practitioners confident with its safety, he added. Dr. Koren was the primary investigator in the U.S. study (Am. J. Obstet. Gynecol. 2010;203:571.e1-7).
Since NVP usually improves after the first trimester, "health care professionals should reassess their patients for continued need for Diclegis as pregnancy progresses," the FDA statement says. In addition, women should not use Diclegis "when engaging in activities requiring mental alertness, such as driving or operating heavy machinery, until cleared to do so by their health care provider."
The label states that Diclegis has not been studied in women with hyperemesis gravidarum and that women should not breastfeed while on the drug.
Dr. Koren has served as a consultant for Duschesnay.
The prescribing information for Diclegis is available here. Adverse events associated with this product should be reported to the FDA’s MedWatch program or by calling 800-332-1088.
For the first time in 30 years, clinicians will be able to prescribe a Food and Drug Administration-approved treatment for their pregnant patients who are experiencing nausea and vomiting and have not responded to changes in their diet or other conservative measures.
On April 9, the FDA announced the approval of a delayed-release, fixed-dose formulation of 10 mg of doxylamine, an antihistamine; and 10 mg of pyridoxine, a vitamin B6 analog, for "the treatment of nausea and vomiting of pregnancy in women who do not respond to conservative management."
This combination was widely used as an effective treatment for nausea and vomiting of pregnancy (NVP) in the United States and Canada in the 1960s and 1970s, and was marketed as Bendectin. But it was voluntarily taken off the U.S. market by the manufacturer in 1983, amid allegations and lawsuits that the drug caused birth defects – which were never substantiated with scientific data. The combination, which has been available as separate components in the United States, was recommended as a first-line treatment for NVP in a 2004 American College of Obstetricians and Gynecologists practice statement that was reaffirmed in 2011 (Obstet. Gynecol. 2004;103:803-15).
In a clear sign that the issue has been resolved, the FDA has assigned Diclegis a category A pregnancy risk rating, with a statement in the label summarizing the epidemiologic studies indicating that the combination of the two active ingredients has not been associated with increased risks to the fetus.
"Diclegis is now the only FDA-approved treatment for nausea and vomiting due to pregnancy, providing a therapeutic option for pregnant women seeking relief from these symptoms," Dr. Hylton V. Joffe, director of the division of reproductive and urologic products in the FDA’s Center for Drug Evaluation and Research, said in the FDA statement. It is expected to be widely available in the United States by the end of May, according to the Canadian manufacturer, Duchesnay Inc., which markets the same product in Canada.
The approval "is a big victory for American women and health care professionals, especially obstetricians and family physicians, because over the last 3 decades, American women have not had an FDA-approved medication for the most common condition of pregnancy, morning sickness," said Dr. Gideon Koren, the head of the Research Leadership for Better Pharmacotherapy During Pregnancy and Lactation at the Hospital for Sick Children, Toronto. After Bendectin was removed from the market, the rate of hospitalizations for hyperemesis gravidarum markedly increased in the United States, "clearly showing that when you remove a drug that a very large number of women need, a void is created and women suffer," he added.
The FDA approval was based on a study of 261 women with NVP in the United States who were at least 18 years-of-age and who were 7-14 weeks pregnant (mean gestational age was 9.3 weeks). Those randomized to receive treatment with Diclegis for 2 weeks had greater improvements in nausea and vomiting, compared with those who received a placebo, as reflected in changes in a score that incorporated the number of daily episodes of vomiting and heaves, and hours of nausea. The most common side effect associated with Diclegis was drowsiness, which can be severe, according to the FDA.
Diclegis is taken daily and is not used as an as-needed treatment for symptoms. The recommended dosage regimen is an initial dose of two tablets at bedtime on the first day. If symptoms persist through the afternoon of the second day, the woman should take two tablets at bedtime and one tablet the following morning (day 3). If symptoms are still present on the fourth day, she should take one tablet in the morning, one tablet in the middle of the afternoon and two tablets at bedtime. Four tablets are the maximum recommended daily dose. In the study, 19% of the women treated with Diclegis took two tablets a day, 21% took three tablets a day, and 60% took four tablets a day.
The prescribing information includes a statement that the combination of doxylamine and pyridoxine "has been the subject of many epidemiologic studies (cohort, case control, and meta-analyses) designed to detect possible teratogenicity," including studies published between 1963 and 1991, which did not report evidence of fetal abnormalities associated with first trimester exposure.
In an interview, Dr. Koren, professor of pediatrics, pharmacology, pharmacy, medicine, and medical genetics at the University of Toronto, said that the safety studies include data on more than 300,000 mother-child pairs. When Bendectin was taken off the U.S. market, it was already available as a generic in Canada as Diclectin and was never taken off the market. The continuous experience with the product in Canada should also make U.S. practitioners confident with its safety, he added. Dr. Koren was the primary investigator in the U.S. study (Am. J. Obstet. Gynecol. 2010;203:571.e1-7).
Since NVP usually improves after the first trimester, "health care professionals should reassess their patients for continued need for Diclegis as pregnancy progresses," the FDA statement says. In addition, women should not use Diclegis "when engaging in activities requiring mental alertness, such as driving or operating heavy machinery, until cleared to do so by their health care provider."
The label states that Diclegis has not been studied in women with hyperemesis gravidarum and that women should not breastfeed while on the drug.
Dr. Koren has served as a consultant for Duschesnay.
The prescribing information for Diclegis is available here. Adverse events associated with this product should be reported to the FDA’s MedWatch program or by calling 800-332-1088.
For the first time in 30 years, clinicians will be able to prescribe a Food and Drug Administration-approved treatment for their pregnant patients who are experiencing nausea and vomiting and have not responded to changes in their diet or other conservative measures.
On April 9, the FDA announced the approval of a delayed-release, fixed-dose formulation of 10 mg of doxylamine, an antihistamine; and 10 mg of pyridoxine, a vitamin B6 analog, for "the treatment of nausea and vomiting of pregnancy in women who do not respond to conservative management."
This combination was widely used as an effective treatment for nausea and vomiting of pregnancy (NVP) in the United States and Canada in the 1960s and 1970s, and was marketed as Bendectin. But it was voluntarily taken off the U.S. market by the manufacturer in 1983, amid allegations and lawsuits that the drug caused birth defects – which were never substantiated with scientific data. The combination, which has been available as separate components in the United States, was recommended as a first-line treatment for NVP in a 2004 American College of Obstetricians and Gynecologists practice statement that was reaffirmed in 2011 (Obstet. Gynecol. 2004;103:803-15).
In a clear sign that the issue has been resolved, the FDA has assigned Diclegis a category A pregnancy risk rating, with a statement in the label summarizing the epidemiologic studies indicating that the combination of the two active ingredients has not been associated with increased risks to the fetus.
"Diclegis is now the only FDA-approved treatment for nausea and vomiting due to pregnancy, providing a therapeutic option for pregnant women seeking relief from these symptoms," Dr. Hylton V. Joffe, director of the division of reproductive and urologic products in the FDA’s Center for Drug Evaluation and Research, said in the FDA statement. It is expected to be widely available in the United States by the end of May, according to the Canadian manufacturer, Duchesnay Inc., which markets the same product in Canada.
The approval "is a big victory for American women and health care professionals, especially obstetricians and family physicians, because over the last 3 decades, American women have not had an FDA-approved medication for the most common condition of pregnancy, morning sickness," said Dr. Gideon Koren, the head of the Research Leadership for Better Pharmacotherapy During Pregnancy and Lactation at the Hospital for Sick Children, Toronto. After Bendectin was removed from the market, the rate of hospitalizations for hyperemesis gravidarum markedly increased in the United States, "clearly showing that when you remove a drug that a very large number of women need, a void is created and women suffer," he added.
The FDA approval was based on a study of 261 women with NVP in the United States who were at least 18 years-of-age and who were 7-14 weeks pregnant (mean gestational age was 9.3 weeks). Those randomized to receive treatment with Diclegis for 2 weeks had greater improvements in nausea and vomiting, compared with those who received a placebo, as reflected in changes in a score that incorporated the number of daily episodes of vomiting and heaves, and hours of nausea. The most common side effect associated with Diclegis was drowsiness, which can be severe, according to the FDA.
Diclegis is taken daily and is not used as an as-needed treatment for symptoms. The recommended dosage regimen is an initial dose of two tablets at bedtime on the first day. If symptoms persist through the afternoon of the second day, the woman should take two tablets at bedtime and one tablet the following morning (day 3). If symptoms are still present on the fourth day, she should take one tablet in the morning, one tablet in the middle of the afternoon and two tablets at bedtime. Four tablets are the maximum recommended daily dose. In the study, 19% of the women treated with Diclegis took two tablets a day, 21% took three tablets a day, and 60% took four tablets a day.
The prescribing information includes a statement that the combination of doxylamine and pyridoxine "has been the subject of many epidemiologic studies (cohort, case control, and meta-analyses) designed to detect possible teratogenicity," including studies published between 1963 and 1991, which did not report evidence of fetal abnormalities associated with first trimester exposure.
In an interview, Dr. Koren, professor of pediatrics, pharmacology, pharmacy, medicine, and medical genetics at the University of Toronto, said that the safety studies include data on more than 300,000 mother-child pairs. When Bendectin was taken off the U.S. market, it was already available as a generic in Canada as Diclectin and was never taken off the market. The continuous experience with the product in Canada should also make U.S. practitioners confident with its safety, he added. Dr. Koren was the primary investigator in the U.S. study (Am. J. Obstet. Gynecol. 2010;203:571.e1-7).
Since NVP usually improves after the first trimester, "health care professionals should reassess their patients for continued need for Diclegis as pregnancy progresses," the FDA statement says. In addition, women should not use Diclegis "when engaging in activities requiring mental alertness, such as driving or operating heavy machinery, until cleared to do so by their health care provider."
The label states that Diclegis has not been studied in women with hyperemesis gravidarum and that women should not breastfeed while on the drug.
Dr. Koren has served as a consultant for Duschesnay.
The prescribing information for Diclegis is available here. Adverse events associated with this product should be reported to the FDA’s MedWatch program or by calling 800-332-1088.
Fetal thigh measurement may predict adiposity
ORLANDO – Fetal thigh measurement at 28 weeks can help identify babies who are at risk of neonatal adiposity, according to a small exploratory study.
The study also showed that abdominal circumference and estimated fetal weight at 28 weeks’ gestation were not correlated with adiposity at birth, nor were prepregnancy body mass index, maternal weight gain, or birth weight.
With a nation facing an obesity epidemic, the study may provide yet another tool to combat the trend by opening the possibility of fetal-based interventions, hence reducing the risk of childhood obesity.
Compared with birth weight, adiposity at birth has been known to be a stronger predictor of childhood obesity, said Dr. Gaea S. Moore of the University of Colorado, Aurora, who presented the abstract at the annual meetingof the Society of Gynecologic Investigation.
But, there’s not yet a good way of measuring how much fat the baby is going to have, said Dr. Anna L. David of University College London Elizabeth Garrett Anderson Institute for Women’s Health, London, who moderated the session. "So this study looks very promising," she said.
While 2D fetal ultrasound has not been a strong tool in measuring fetal adiposity, adding 3D fractional limb volumes (FLV) has helped improve the prediction of both adiposity at birth and macrosomia.
Dr. Moore and her colleagues conducted a prospective cohort study of 18 pregnant women, collecting data from standard 2D ultrasound biometry and mid-thigh cross sectional areas (MTA), and 3D FLV, measuring thigh volume at the 50% of the femur, using a 5-slice technique. Both measurements were analyzed with the 4D View software.
Seven of the 18 subjects were excluded due to poor image quality.
The mean age of mothers was 30 years, with prepregnancy body mass index of 26, parity of 0.83, and the mean gestational age at birth of 39 weeks and 3 days.
Researchers measured neonatal body composition with air displacement plethysmography (Pea Pod) 14 days after birth. The mean birth weight of the infants was 3,383 g, and the mean body fat was 10.68%.
Analyses showed that fetal thigh measurement at 28 weeks correlated with neonatal adiposity. FLV and MTA correlated with adiposity at 28 weeks when normalized for femur length (FL). (FLV/FL, r = 0.77; P = .005. MTA/FL, r = 0.75; P = .007)
The correlation further improved with adjustment of thigh volumes by femur length, and by adjustment for Pea Pod timing and gestational age adjustments.
The study opens the possibility of obtaining data that could lead to a conversation with pregnant moms whose babies might be at risk of neonatal adiposity, Dr. David said. "Maybe there’s an intervention that could reduce the adiposity," she said, whether it’s giving the mother medications that would reduce the sugar going across the placenta, or manipulation of diet.
She also pointed out that larger studies were needed, and that 28 weeks may not be the optimum time for measurement, as seven subjects were excluded due to poor image quality. "At 20 weeks there’s relatively more fluid around the baby, so maybe that might be a better time to take the measurement." She said that the measurement’s accuracy is yet to be determined.
Dr. Moore and her colleagues have obtained measurements at 36 weeks, but have not analyzed the data. She said the group had not evaluated data for earlier than 20 weeks’ gestation.
Dr. Moore and Dr. David reported no disclosures.
On Twitter @naseemsmiller
ORLANDO – Fetal thigh measurement at 28 weeks can help identify babies who are at risk of neonatal adiposity, according to a small exploratory study.
The study also showed that abdominal circumference and estimated fetal weight at 28 weeks’ gestation were not correlated with adiposity at birth, nor were prepregnancy body mass index, maternal weight gain, or birth weight.
With a nation facing an obesity epidemic, the study may provide yet another tool to combat the trend by opening the possibility of fetal-based interventions, hence reducing the risk of childhood obesity.
Compared with birth weight, adiposity at birth has been known to be a stronger predictor of childhood obesity, said Dr. Gaea S. Moore of the University of Colorado, Aurora, who presented the abstract at the annual meetingof the Society of Gynecologic Investigation.
But, there’s not yet a good way of measuring how much fat the baby is going to have, said Dr. Anna L. David of University College London Elizabeth Garrett Anderson Institute for Women’s Health, London, who moderated the session. "So this study looks very promising," she said.
While 2D fetal ultrasound has not been a strong tool in measuring fetal adiposity, adding 3D fractional limb volumes (FLV) has helped improve the prediction of both adiposity at birth and macrosomia.
Dr. Moore and her colleagues conducted a prospective cohort study of 18 pregnant women, collecting data from standard 2D ultrasound biometry and mid-thigh cross sectional areas (MTA), and 3D FLV, measuring thigh volume at the 50% of the femur, using a 5-slice technique. Both measurements were analyzed with the 4D View software.
Seven of the 18 subjects were excluded due to poor image quality.
The mean age of mothers was 30 years, with prepregnancy body mass index of 26, parity of 0.83, and the mean gestational age at birth of 39 weeks and 3 days.
Researchers measured neonatal body composition with air displacement plethysmography (Pea Pod) 14 days after birth. The mean birth weight of the infants was 3,383 g, and the mean body fat was 10.68%.
Analyses showed that fetal thigh measurement at 28 weeks correlated with neonatal adiposity. FLV and MTA correlated with adiposity at 28 weeks when normalized for femur length (FL). (FLV/FL, r = 0.77; P = .005. MTA/FL, r = 0.75; P = .007)
The correlation further improved with adjustment of thigh volumes by femur length, and by adjustment for Pea Pod timing and gestational age adjustments.
The study opens the possibility of obtaining data that could lead to a conversation with pregnant moms whose babies might be at risk of neonatal adiposity, Dr. David said. "Maybe there’s an intervention that could reduce the adiposity," she said, whether it’s giving the mother medications that would reduce the sugar going across the placenta, or manipulation of diet.
She also pointed out that larger studies were needed, and that 28 weeks may not be the optimum time for measurement, as seven subjects were excluded due to poor image quality. "At 20 weeks there’s relatively more fluid around the baby, so maybe that might be a better time to take the measurement." She said that the measurement’s accuracy is yet to be determined.
Dr. Moore and her colleagues have obtained measurements at 36 weeks, but have not analyzed the data. She said the group had not evaluated data for earlier than 20 weeks’ gestation.
Dr. Moore and Dr. David reported no disclosures.
On Twitter @naseemsmiller
ORLANDO – Fetal thigh measurement at 28 weeks can help identify babies who are at risk of neonatal adiposity, according to a small exploratory study.
The study also showed that abdominal circumference and estimated fetal weight at 28 weeks’ gestation were not correlated with adiposity at birth, nor were prepregnancy body mass index, maternal weight gain, or birth weight.
With a nation facing an obesity epidemic, the study may provide yet another tool to combat the trend by opening the possibility of fetal-based interventions, hence reducing the risk of childhood obesity.
Compared with birth weight, adiposity at birth has been known to be a stronger predictor of childhood obesity, said Dr. Gaea S. Moore of the University of Colorado, Aurora, who presented the abstract at the annual meetingof the Society of Gynecologic Investigation.
But, there’s not yet a good way of measuring how much fat the baby is going to have, said Dr. Anna L. David of University College London Elizabeth Garrett Anderson Institute for Women’s Health, London, who moderated the session. "So this study looks very promising," she said.
While 2D fetal ultrasound has not been a strong tool in measuring fetal adiposity, adding 3D fractional limb volumes (FLV) has helped improve the prediction of both adiposity at birth and macrosomia.
Dr. Moore and her colleagues conducted a prospective cohort study of 18 pregnant women, collecting data from standard 2D ultrasound biometry and mid-thigh cross sectional areas (MTA), and 3D FLV, measuring thigh volume at the 50% of the femur, using a 5-slice technique. Both measurements were analyzed with the 4D View software.
Seven of the 18 subjects were excluded due to poor image quality.
The mean age of mothers was 30 years, with prepregnancy body mass index of 26, parity of 0.83, and the mean gestational age at birth of 39 weeks and 3 days.
Researchers measured neonatal body composition with air displacement plethysmography (Pea Pod) 14 days after birth. The mean birth weight of the infants was 3,383 g, and the mean body fat was 10.68%.
Analyses showed that fetal thigh measurement at 28 weeks correlated with neonatal adiposity. FLV and MTA correlated with adiposity at 28 weeks when normalized for femur length (FL). (FLV/FL, r = 0.77; P = .005. MTA/FL, r = 0.75; P = .007)
The correlation further improved with adjustment of thigh volumes by femur length, and by adjustment for Pea Pod timing and gestational age adjustments.
The study opens the possibility of obtaining data that could lead to a conversation with pregnant moms whose babies might be at risk of neonatal adiposity, Dr. David said. "Maybe there’s an intervention that could reduce the adiposity," she said, whether it’s giving the mother medications that would reduce the sugar going across the placenta, or manipulation of diet.
She also pointed out that larger studies were needed, and that 28 weeks may not be the optimum time for measurement, as seven subjects were excluded due to poor image quality. "At 20 weeks there’s relatively more fluid around the baby, so maybe that might be a better time to take the measurement." She said that the measurement’s accuracy is yet to be determined.
Dr. Moore and her colleagues have obtained measurements at 36 weeks, but have not analyzed the data. She said the group had not evaluated data for earlier than 20 weeks’ gestation.
Dr. Moore and Dr. David reported no disclosures.
On Twitter @naseemsmiller
AT THE ANNUAL MEETING OF THE SOCIETY OF GYNECOLOGIC INVESTIGATION
Major Finding: Fetal thigh measurement at 28 weeks correlated with neonatal adiposity. (FLV/FL, r = 0.77; P = .005. MTA/FL, r = 0.75; P = .007).
Data Source: A prospective cohort study of 18 pregnant women, with data from standard 2D ultrasound biometry and mid-thigh cross-sectional areas, and 3D FLV, measuring thigh volume at the 50% of the femur, with a 5-slice technique.
Disclosures: Dr. Moore and Dr. David reported no disclosures.
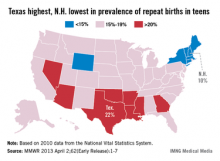
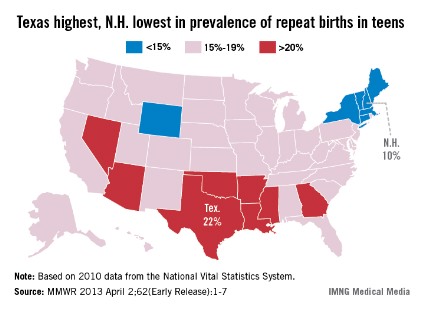
Prevalence of repeat births in teens at 18.3% in 2010
Births to teenage mothers continue to decline, but nearly one in five teen births is a repeat birth, the Centers for Disease Control and Prevention reported.
In 2010, more than 365,000 teens aged 15-19 years gave birth, and 18.3% (66,761) of those were repeat births. Most (85.7%) were second births, but almost 8,400 (12.6%) teens had a third child and more than 1,100 (1.7%) had a fourth-to-sixth child, according to the CDC (MMWR 2013 April 2;62[Early Release];1-7).
The percentage of births that were repeat births has declined 6.2% since 2007, when it was 19.5%.
"Teen birth rates in the United States have declined to a record low, which is good news," CDC director Thomas R. Frieden, M.D., said in a statement. "But rates are still far too high. Repeat births can negatively impact the mother’s education and job opportunities as well as the health of the next generation."
Repeat teen births showed geographic and racial/ethnic disparities. In 2010, prevalence was highest in eight southern and western states and lowest in six northeastern states and Wyoming. The prevalence was highest in American Indian/Alaska natives (21.6%), followed by Hispanics (20.9%), non-Hispanic blacks (20.4%), Asians/Pacific Islanders (17.6%), and whites (14.8%) according to the data from the National Vital Statistics System.
Births to teenage mothers continue to decline, but nearly one in five teen births is a repeat birth, the Centers for Disease Control and Prevention reported.
In 2010, more than 365,000 teens aged 15-19 years gave birth, and 18.3% (66,761) of those were repeat births. Most (85.7%) were second births, but almost 8,400 (12.6%) teens had a third child and more than 1,100 (1.7%) had a fourth-to-sixth child, according to the CDC (MMWR 2013 April 2;62[Early Release];1-7).
The percentage of births that were repeat births has declined 6.2% since 2007, when it was 19.5%.
"Teen birth rates in the United States have declined to a record low, which is good news," CDC director Thomas R. Frieden, M.D., said in a statement. "But rates are still far too high. Repeat births can negatively impact the mother’s education and job opportunities as well as the health of the next generation."
Repeat teen births showed geographic and racial/ethnic disparities. In 2010, prevalence was highest in eight southern and western states and lowest in six northeastern states and Wyoming. The prevalence was highest in American Indian/Alaska natives (21.6%), followed by Hispanics (20.9%), non-Hispanic blacks (20.4%), Asians/Pacific Islanders (17.6%), and whites (14.8%) according to the data from the National Vital Statistics System.
Births to teenage mothers continue to decline, but nearly one in five teen births is a repeat birth, the Centers for Disease Control and Prevention reported.
In 2010, more than 365,000 teens aged 15-19 years gave birth, and 18.3% (66,761) of those were repeat births. Most (85.7%) were second births, but almost 8,400 (12.6%) teens had a third child and more than 1,100 (1.7%) had a fourth-to-sixth child, according to the CDC (MMWR 2013 April 2;62[Early Release];1-7).
The percentage of births that were repeat births has declined 6.2% since 2007, when it was 19.5%.
"Teen birth rates in the United States have declined to a record low, which is good news," CDC director Thomas R. Frieden, M.D., said in a statement. "But rates are still far too high. Repeat births can negatively impact the mother’s education and job opportunities as well as the health of the next generation."
Repeat teen births showed geographic and racial/ethnic disparities. In 2010, prevalence was highest in eight southern and western states and lowest in six northeastern states and Wyoming. The prevalence was highest in American Indian/Alaska natives (21.6%), followed by Hispanics (20.9%), non-Hispanic blacks (20.4%), Asians/Pacific Islanders (17.6%), and whites (14.8%) according to the data from the National Vital Statistics System.
Skin capillary density drop reliably predicts preeclampsia
SAN FRANCISCO – A reduction in skin capillary density during pregnancy constitutes a novel independent and reliable noninvasive predictor of preeclampsia, according to Dr. Tarek Antonios of the University of London.
"Capillary density rarefaction is the most sensitive and specific predictor of preeclampsia to date. Combining capillary density rarefaction and the uterine artery Doppler pulsatility index increases the sensitivity of prediction to 86% and the specificity to 80%, figures that are by far more significant than any other published evidence about the clinical prediction of preeclampsia," he declared at the annual meeting of the American College of Cardiology.
Measurement of skin capillary density changes in pregnancy is inexpensive once the essential equipment – an intravital video microscope suitable for capillaroscopy – has been acquired, he added.
Cardiovascular risk factors known to predispose to preeclampsia include essential hypertension, diabetes, and obesity. Dr. Antonios and coworkers have surmised that the mechanism by which these disorders boost the risk of preeclampsia involves microcirculatory abnormalities and impaired tissue perfusion.
The investigators have developed a reproducible method of measuring skin capillary density on the dorsum of the hand. Further, they have demonstrated that reduced capillary density – which they term "structural capillary rarefaction" – beginning at about 20 weeks of gestation is a harbinger of subsequent onset of preeclampsia.
Dr. Antonios reported on 322 consecutive white women with singleton pregnancies, of whom 13 had a history of preeclampsia, 11 had a history of untreated stage 1 essential hypertension, and the rest were normotensive. They underwent five structured capillaroscopy assessments at 11-16 weeks’ of gestation, 20-24 weeks, 27-32 weeks, 34-38 weeks, and finally at 5-15 weeks post partum. The capillary density measurements were done in a temperature-controlled laboratory with a standardized technique.
Among the 305 women who completed the study, 16 (5%) developed preeclampsia. Four of the 16 (25%) had a history of preeclampsia, compared with just 3% of the 289 (1%) subjects with a normal pregnancy.
The women who became preeclamptic were also set apart by their mean 6.1 capillary/mm2 reduction in density between the weeks 20-24 measurement and the weeks 11-16 baseline. In contrast, the women with normal pregnancies averaged a 1.0 capillary/mm2 decrease during that time frame. Capillary rarefaction further increased over time in women who later developed preeclampsia: their mean reduction in density at the weeks 27-32 measurement was 11.4/mm2, compared with the weeks 11-16 baseline, while the controls averaged a 2.1 capillary/mm2 decrease.
In a multivariate regression analysis, the single strongest predictor of preeclampsia was a history of previous preeclampsia or essential hypertension, which was associated with a 35-fold increase in risk. Each 1 capillary/mm2 reduction in density at 20-24 weeks was associated with a 3% increase in risk, while at 27-32 weeks every 1 capillary/mm2 reduction in density conferred a 26% increase in the risk of preeclampsia.
Significant structural capillary rarefaction at weeks 27-32 had a 77% sensitivity and 77% specificity for subsequent preeclampsia. Combining an increased uterine artery Doppler pulsatility index with a finding of significant capillary density reduction at weeks 27-32 boosted the sensitivity to 86% and the specificity to 80%.
Given that only 16 participants in this study developed preeclampsia, the next step in this research is to conduct a large clinical trial to validate capillaroscopy as a clinical risk prediction tool with an eye toward its eventual integration into routine clinical practice, according to Dr. Antonios.
This study was funded by the British Heart Foundation. Dr. Antonios reported having no financial conflicts.
SAN FRANCISCO – A reduction in skin capillary density during pregnancy constitutes a novel independent and reliable noninvasive predictor of preeclampsia, according to Dr. Tarek Antonios of the University of London.
"Capillary density rarefaction is the most sensitive and specific predictor of preeclampsia to date. Combining capillary density rarefaction and the uterine artery Doppler pulsatility index increases the sensitivity of prediction to 86% and the specificity to 80%, figures that are by far more significant than any other published evidence about the clinical prediction of preeclampsia," he declared at the annual meeting of the American College of Cardiology.
Measurement of skin capillary density changes in pregnancy is inexpensive once the essential equipment – an intravital video microscope suitable for capillaroscopy – has been acquired, he added.
Cardiovascular risk factors known to predispose to preeclampsia include essential hypertension, diabetes, and obesity. Dr. Antonios and coworkers have surmised that the mechanism by which these disorders boost the risk of preeclampsia involves microcirculatory abnormalities and impaired tissue perfusion.
The investigators have developed a reproducible method of measuring skin capillary density on the dorsum of the hand. Further, they have demonstrated that reduced capillary density – which they term "structural capillary rarefaction" – beginning at about 20 weeks of gestation is a harbinger of subsequent onset of preeclampsia.
Dr. Antonios reported on 322 consecutive white women with singleton pregnancies, of whom 13 had a history of preeclampsia, 11 had a history of untreated stage 1 essential hypertension, and the rest were normotensive. They underwent five structured capillaroscopy assessments at 11-16 weeks’ of gestation, 20-24 weeks, 27-32 weeks, 34-38 weeks, and finally at 5-15 weeks post partum. The capillary density measurements were done in a temperature-controlled laboratory with a standardized technique.
Among the 305 women who completed the study, 16 (5%) developed preeclampsia. Four of the 16 (25%) had a history of preeclampsia, compared with just 3% of the 289 (1%) subjects with a normal pregnancy.
The women who became preeclamptic were also set apart by their mean 6.1 capillary/mm2 reduction in density between the weeks 20-24 measurement and the weeks 11-16 baseline. In contrast, the women with normal pregnancies averaged a 1.0 capillary/mm2 decrease during that time frame. Capillary rarefaction further increased over time in women who later developed preeclampsia: their mean reduction in density at the weeks 27-32 measurement was 11.4/mm2, compared with the weeks 11-16 baseline, while the controls averaged a 2.1 capillary/mm2 decrease.
In a multivariate regression analysis, the single strongest predictor of preeclampsia was a history of previous preeclampsia or essential hypertension, which was associated with a 35-fold increase in risk. Each 1 capillary/mm2 reduction in density at 20-24 weeks was associated with a 3% increase in risk, while at 27-32 weeks every 1 capillary/mm2 reduction in density conferred a 26% increase in the risk of preeclampsia.
Significant structural capillary rarefaction at weeks 27-32 had a 77% sensitivity and 77% specificity for subsequent preeclampsia. Combining an increased uterine artery Doppler pulsatility index with a finding of significant capillary density reduction at weeks 27-32 boosted the sensitivity to 86% and the specificity to 80%.
Given that only 16 participants in this study developed preeclampsia, the next step in this research is to conduct a large clinical trial to validate capillaroscopy as a clinical risk prediction tool with an eye toward its eventual integration into routine clinical practice, according to Dr. Antonios.
This study was funded by the British Heart Foundation. Dr. Antonios reported having no financial conflicts.
SAN FRANCISCO – A reduction in skin capillary density during pregnancy constitutes a novel independent and reliable noninvasive predictor of preeclampsia, according to Dr. Tarek Antonios of the University of London.
"Capillary density rarefaction is the most sensitive and specific predictor of preeclampsia to date. Combining capillary density rarefaction and the uterine artery Doppler pulsatility index increases the sensitivity of prediction to 86% and the specificity to 80%, figures that are by far more significant than any other published evidence about the clinical prediction of preeclampsia," he declared at the annual meeting of the American College of Cardiology.
Measurement of skin capillary density changes in pregnancy is inexpensive once the essential equipment – an intravital video microscope suitable for capillaroscopy – has been acquired, he added.
Cardiovascular risk factors known to predispose to preeclampsia include essential hypertension, diabetes, and obesity. Dr. Antonios and coworkers have surmised that the mechanism by which these disorders boost the risk of preeclampsia involves microcirculatory abnormalities and impaired tissue perfusion.
The investigators have developed a reproducible method of measuring skin capillary density on the dorsum of the hand. Further, they have demonstrated that reduced capillary density – which they term "structural capillary rarefaction" – beginning at about 20 weeks of gestation is a harbinger of subsequent onset of preeclampsia.
Dr. Antonios reported on 322 consecutive white women with singleton pregnancies, of whom 13 had a history of preeclampsia, 11 had a history of untreated stage 1 essential hypertension, and the rest were normotensive. They underwent five structured capillaroscopy assessments at 11-16 weeks’ of gestation, 20-24 weeks, 27-32 weeks, 34-38 weeks, and finally at 5-15 weeks post partum. The capillary density measurements were done in a temperature-controlled laboratory with a standardized technique.
Among the 305 women who completed the study, 16 (5%) developed preeclampsia. Four of the 16 (25%) had a history of preeclampsia, compared with just 3% of the 289 (1%) subjects with a normal pregnancy.
The women who became preeclamptic were also set apart by their mean 6.1 capillary/mm2 reduction in density between the weeks 20-24 measurement and the weeks 11-16 baseline. In contrast, the women with normal pregnancies averaged a 1.0 capillary/mm2 decrease during that time frame. Capillary rarefaction further increased over time in women who later developed preeclampsia: their mean reduction in density at the weeks 27-32 measurement was 11.4/mm2, compared with the weeks 11-16 baseline, while the controls averaged a 2.1 capillary/mm2 decrease.
In a multivariate regression analysis, the single strongest predictor of preeclampsia was a history of previous preeclampsia or essential hypertension, which was associated with a 35-fold increase in risk. Each 1 capillary/mm2 reduction in density at 20-24 weeks was associated with a 3% increase in risk, while at 27-32 weeks every 1 capillary/mm2 reduction in density conferred a 26% increase in the risk of preeclampsia.
Significant structural capillary rarefaction at weeks 27-32 had a 77% sensitivity and 77% specificity for subsequent preeclampsia. Combining an increased uterine artery Doppler pulsatility index with a finding of significant capillary density reduction at weeks 27-32 boosted the sensitivity to 86% and the specificity to 80%.
Given that only 16 participants in this study developed preeclampsia, the next step in this research is to conduct a large clinical trial to validate capillaroscopy as a clinical risk prediction tool with an eye toward its eventual integration into routine clinical practice, according to Dr. Antonios.
This study was funded by the British Heart Foundation. Dr. Antonios reported having no financial conflicts.
AT ACC 13
Major finding: A marked reduction in capillary density in the skin on the dorsum of the hand between gestational weeks 11-16 and 27-32 was associated with 77% sensitivity and 77% specificity for subsequent preeclampsia.
Data source: This was a prospective study involving 322 consecutive white women with singleton pregnancies who underwent structured measurement of skin capillary density on four designated occasions during pregnancy and once post partum.
Disclosures: The study was funded by the British Heart Foundation. Dr. Antonios reported having no financial conflicts.
Risk of autism is significantly increased with prenatal exposure to valproate
Pregnancy and epilepsy—managing both, in one patient
Nitin K. Sethi, MD; Amy Wasterlain; Cynthia L. Harden, MD (June 2011)
Guidelines confirm safety of pregnancy in women who have epilepsy—with caveats
Janelle Yates, Senior Editor (September 2009)
Jakob Christensen, PhD, from Aarhus University Hospital in Denmark, and colleagues examined whether prenatal exposure to valproate correlates with the risk of autism in offspring in a population-base study involving 655,615 children born from 1996 through 2006 in Denmark.
Of the cohort, 5,437 were identified with autism spectrum disorder, including 2,067 with childhood autism. After 14 years of follow-up, the researchers found that the estimated absolute risk of autism spectrum disorder was 1.53%, and that of childhood autism was 0.48%. The absolute risks for the 508 children exposed to valproate were 4.42% for autism spectrum disorder (adjusted hazard ratio [aHR], 2.9) and 2.50% for childhood autism (aHR, 5.2). On restriction of the cohort to the 6,584 children born to women with epilepsy, the absolute risk of autism spectrum disorder was 2.44% for those not exposed to valproate (6,152 children) versus 4.15% for those exposed to valproate (432 children; aHR, 1.7); for childhood autism, the corresponding risks were 1.02% and 2.95% (aHR, 2.9).
“Maternal use of valproate during pregnancy was associated with a significantly increased risk of autism in the offspring, even after adjusting for parental psychiatric disease and epilepsy,” the authors write. “For women of childbearing potential who use antiepileptic medications, these findings must be balanced against the treatment benefits for women who require valproate for epilepsy control.”
One author disclosed financial ties to the pharmaceutical industry.
To read the JAMA abstract, click here.
We want to hear from you! Tell us what you think.
More NEWS FOR YOUR PRACTICE…
in pregnancy
Pregnancy and epilepsy—managing both, in one patient
Nitin K. Sethi, MD; Amy Wasterlain; Cynthia L. Harden, MD (June 2011)
Guidelines confirm safety of pregnancy in women who have epilepsy—with caveats
Janelle Yates, Senior Editor (September 2009)
Jakob Christensen, PhD, from Aarhus University Hospital in Denmark, and colleagues examined whether prenatal exposure to valproate correlates with the risk of autism in offspring in a population-base study involving 655,615 children born from 1996 through 2006 in Denmark.
Of the cohort, 5,437 were identified with autism spectrum disorder, including 2,067 with childhood autism. After 14 years of follow-up, the researchers found that the estimated absolute risk of autism spectrum disorder was 1.53%, and that of childhood autism was 0.48%. The absolute risks for the 508 children exposed to valproate were 4.42% for autism spectrum disorder (adjusted hazard ratio [aHR], 2.9) and 2.50% for childhood autism (aHR, 5.2). On restriction of the cohort to the 6,584 children born to women with epilepsy, the absolute risk of autism spectrum disorder was 2.44% for those not exposed to valproate (6,152 children) versus 4.15% for those exposed to valproate (432 children; aHR, 1.7); for childhood autism, the corresponding risks were 1.02% and 2.95% (aHR, 2.9).
“Maternal use of valproate during pregnancy was associated with a significantly increased risk of autism in the offspring, even after adjusting for parental psychiatric disease and epilepsy,” the authors write. “For women of childbearing potential who use antiepileptic medications, these findings must be balanced against the treatment benefits for women who require valproate for epilepsy control.”
One author disclosed financial ties to the pharmaceutical industry.
To read the JAMA abstract, click here.
Pregnancy and epilepsy—managing both, in one patient
Nitin K. Sethi, MD; Amy Wasterlain; Cynthia L. Harden, MD (June 2011)
Guidelines confirm safety of pregnancy in women who have epilepsy—with caveats
Janelle Yates, Senior Editor (September 2009)
Jakob Christensen, PhD, from Aarhus University Hospital in Denmark, and colleagues examined whether prenatal exposure to valproate correlates with the risk of autism in offspring in a population-base study involving 655,615 children born from 1996 through 2006 in Denmark.
Of the cohort, 5,437 were identified with autism spectrum disorder, including 2,067 with childhood autism. After 14 years of follow-up, the researchers found that the estimated absolute risk of autism spectrum disorder was 1.53%, and that of childhood autism was 0.48%. The absolute risks for the 508 children exposed to valproate were 4.42% for autism spectrum disorder (adjusted hazard ratio [aHR], 2.9) and 2.50% for childhood autism (aHR, 5.2). On restriction of the cohort to the 6,584 children born to women with epilepsy, the absolute risk of autism spectrum disorder was 2.44% for those not exposed to valproate (6,152 children) versus 4.15% for those exposed to valproate (432 children; aHR, 1.7); for childhood autism, the corresponding risks were 1.02% and 2.95% (aHR, 2.9).
“Maternal use of valproate during pregnancy was associated with a significantly increased risk of autism in the offspring, even after adjusting for parental psychiatric disease and epilepsy,” the authors write. “For women of childbearing potential who use antiepileptic medications, these findings must be balanced against the treatment benefits for women who require valproate for epilepsy control.”
One author disclosed financial ties to the pharmaceutical industry.
To read the JAMA abstract, click here.
We want to hear from you! Tell us what you think.
More NEWS FOR YOUR PRACTICE…
in pregnancy
We want to hear from you! Tell us what you think.
More NEWS FOR YOUR PRACTICE…
in pregnancy
Copyright © 2013 HealthDay. All rights reserved.
Diclegis now FDA-approved to treat nausea and vomiting in pregnancy
Diclegis (doxylamine succinate and pyridoxine hydrochloride) has been approved by the US Food and Drug Administration to treat nausea and vomiting during pregnancy.
The drug was sanctioned for pregnant women who haven’t responded to other therapies, such as eating smaller meals, eating lower-fat foods, and avoiding smells that can prompt nausea, the agency said in a media release.
Diclegis was evaluated in 261 adult women experiencing nausea and vomiting due to pregnancy who were at least 18 years of age and had been pregnant between 7 and 14 weeks. Women who took the drug had less nausea and vomiting than those who took a placebo. Studies also showed the drug didn’t pose a threat to the fetus, the FDA said.
The starting dose is two pills at bedtime, with an increase to a maximum recommended dose of four tablets daily (one in the morning, one mid-afternoon and two at bedtime) if symptoms are not adequately controlled. The most common side effect of the drug was potentially severe drowsiness. Women who take Diclegis should avoid driving or operating heavy machinery, the agency said.
The drug is marketed by the Canadian pharma firm Duchesnay, based in Quebec.
To read the FDA news release, click here.
We want to hear from you! Tell us what you think.
More NEWS FOR YOUR PRACTICE…
<list type="bullet"> <item><para>Vitamin D supplementation in your pregnant and postmenopausal patients: Recent evidence implies it may not be so important</para></item> <item><para>The newly approved IUD: Which patients is Skyla
appropriate for?</para></item> <item><para>Native tissue is superior to vaginal mesh for prolapse repair, two studies report</para></item> <item><para>Postpartum anxiety more common than depression</para></item> <item><para>Robotic surgery not the best for hysterectomy, ACOG says</para></item> <item><para>Robotically assisted hysterectomy is on the rise for benign gynecologic disorders</para></item> <item><para>Maternal folic acid use linked to reduced autism risk</para></item> </list>
Diclegis (doxylamine succinate and pyridoxine hydrochloride) has been approved by the US Food and Drug Administration to treat nausea and vomiting during pregnancy.
The drug was sanctioned for pregnant women who haven’t responded to other therapies, such as eating smaller meals, eating lower-fat foods, and avoiding smells that can prompt nausea, the agency said in a media release.
Diclegis was evaluated in 261 adult women experiencing nausea and vomiting due to pregnancy who were at least 18 years of age and had been pregnant between 7 and 14 weeks. Women who took the drug had less nausea and vomiting than those who took a placebo. Studies also showed the drug didn’t pose a threat to the fetus, the FDA said.
The starting dose is two pills at bedtime, with an increase to a maximum recommended dose of four tablets daily (one in the morning, one mid-afternoon and two at bedtime) if symptoms are not adequately controlled. The most common side effect of the drug was potentially severe drowsiness. Women who take Diclegis should avoid driving or operating heavy machinery, the agency said.
The drug is marketed by the Canadian pharma firm Duchesnay, based in Quebec.
To read the FDA news release, click here.
Diclegis (doxylamine succinate and pyridoxine hydrochloride) has been approved by the US Food and Drug Administration to treat nausea and vomiting during pregnancy.
The drug was sanctioned for pregnant women who haven’t responded to other therapies, such as eating smaller meals, eating lower-fat foods, and avoiding smells that can prompt nausea, the agency said in a media release.
Diclegis was evaluated in 261 adult women experiencing nausea and vomiting due to pregnancy who were at least 18 years of age and had been pregnant between 7 and 14 weeks. Women who took the drug had less nausea and vomiting than those who took a placebo. Studies also showed the drug didn’t pose a threat to the fetus, the FDA said.
The starting dose is two pills at bedtime, with an increase to a maximum recommended dose of four tablets daily (one in the morning, one mid-afternoon and two at bedtime) if symptoms are not adequately controlled. The most common side effect of the drug was potentially severe drowsiness. Women who take Diclegis should avoid driving or operating heavy machinery, the agency said.
The drug is marketed by the Canadian pharma firm Duchesnay, based in Quebec.
To read the FDA news release, click here.
We want to hear from you! Tell us what you think.
More NEWS FOR YOUR PRACTICE…
<list type="bullet"> <item><para>Vitamin D supplementation in your pregnant and postmenopausal patients: Recent evidence implies it may not be so important</para></item> <item><para>The newly approved IUD: Which patients is Skyla
appropriate for?</para></item> <item><para>Native tissue is superior to vaginal mesh for prolapse repair, two studies report</para></item> <item><para>Postpartum anxiety more common than depression</para></item> <item><para>Robotic surgery not the best for hysterectomy, ACOG says</para></item> <item><para>Robotically assisted hysterectomy is on the rise for benign gynecologic disorders</para></item> <item><para>Maternal folic acid use linked to reduced autism risk</para></item> </list>
We want to hear from you! Tell us what you think.
More NEWS FOR YOUR PRACTICE…
<list type="bullet"> <item><para>Vitamin D supplementation in your pregnant and postmenopausal patients: Recent evidence implies it may not be so important</para></item> <item><para>The newly approved IUD: Which patients is Skyla
appropriate for?</para></item> <item><para>Native tissue is superior to vaginal mesh for prolapse repair, two studies report</para></item> <item><para>Postpartum anxiety more common than depression</para></item> <item><para>Robotic surgery not the best for hysterectomy, ACOG says</para></item> <item><para>Robotically assisted hysterectomy is on the rise for benign gynecologic disorders</para></item> <item><para>Maternal folic acid use linked to reduced autism risk</para></item> </list>
Copyright © 2013 HealthDay. All rights reserved.
Vitamin D supplementation in your pregnant and postmenopausal patients: Recent evidence implies it may not be so important
A study of nearly 4,000 pairs of mothers and their children at the University of Bristol in England found no association between maternal vitamin D levels during pregnancy and the child’s bone health in later life.1
And new guidelines from the US Preventive Services Task Force (USPSTF) recommend against supplementation of vitamin D in amounts up to 400 IU daily and calcium in amounts up to 1,000 mg daily for the primary prevention of fractures in noninstitutionalized postmenopausal women.2
Children of the 90s study is largest so far
In the largest observational study ever published on the effects of maternal vitamin D levels on children’s bone health—the Children of the 90s trial—Lawlor and colleagues assessed vitamin D levels in 3,960 pregnant women, recording data from all three trimesters. When the children of these women reached an average age of 9 years and 11 months, their bone-mineral density (BMD) was assessed using dual-energy x-ray absorptiometry (DXA). Investigators found no significant association between a mother’s vitamin D levels and her child’s BMD.1
On average, the women’s vitamin D levels were lowest during the first trimester and increased as pregnancy progressed. As expected, levels were higher when measured during summer months and lower during winter months. And although nonwhite mothers and those who smoked during pregnancy tended to have lower vitamin D levels overall, this finding appeared to have no effect on their children’s bone health.
Earlier studies of the effects of maternal vitamin D levels on children’s bone health were inconclusive. This study by Lawlor and colleagues is more than 10 times larger than all previous studies combined.
“We believe that there is no strong evidence that pregnant women should receive vitamin D supplementation to prevent low [BMD] in their offspring, although we cannot comment on other possible effects of vitamin D in pregnant women,” said Dr. Debbie Lawlor, lead author of the study. “While excessive vitamin D intake can affect the body’s calcium balance and result in cardiac arrhythmias and muscle problems (both rare), as well as milder problems such as dry mouth and constipation, our study didn’t look at any other potential beneficial or adverse effects of vitamin D supplementation besides the association with children’s bone health.”1
USPSTF mostly found a lack of evidence
The USPSTF commissioned two systematic evidence reviews and a meta-analysis of vitamin D supplementation with or without calcium to assess2:
- the effects of supplementation on bone health in community-dwelling adults
- the association between vitamin D and calcium levels and bone health
- any adverse effects of supplementation.
After reviewing the findings, the Task Force concluded that there is insufficient evidence to determine the true balance of benefits versus risks of supplementation of vitamin D in amounts greater than 400 IU daily and calcium in amounts greater than 1,000 mg daily among postmenopausal women in the community. It also recommended against daily supplementation with 400 IU or less of vitamin D and 1,000 mg or less of calcium in this population, as there is no evidence that this practice prevents bone fracture.
In other words, the USPSTF has no recommendation on doses of these supplements at amounts greater than 400 IU of vitamin D and 1,000 mg of calcium daily among postmenopausal women, and recommends against supplementation at lower amounts in the same population.
Both studies focused on a specific context
Both the USPSTF and Lawlor and colleagues emphasized that their investigations focused only on bone mineral density or the risk of fracture, and they acknowledged that there may be other benefits and risks of vitamin D supplementation in pregnancy—and of supplementation with vitamin D and calcium after menopause.1,2
We want to hear from you! Tell us what you think.
1. Lawlor DA, Wills AK, Fraser A, Sayers A, Fraser WD, Tobias JH. Association of maternal vitamin D status during pregnancy with bone-mineral content in offspring: a prospective cohort study [published online ahead of print March 19, 2013]. Lancet. doi:10.1016/S0140-6736(12)62203-X.
2. Moyer VA. US Preventive Services Task Force. Vitamin D and calcium supplementation to prevent fractures in adults: US Preventive Services Task Force Recommendation Statement [published online ahead of print February 26, 2013]. Ann Intern Med. doi:10.7326/0003-4819-158-9-201305070-00603.
More NEWS FOR YOUR PRACTICE…
<list type="bullet"> <item><para>Native tissue is superior to vaginal mesh for prolapse repair, two studies report</para></item> <item><para>The newly approved IUD: Which patients is Skyla
appropriate for?</para></item> <item><para>Postpartum anxiety more common than depression</para></item> <item><para>Robotic surgery not the best for hysterectomy, ACOG says</para></item> <item><para>Robotically assisted hysterectomy is on the rise for benign gynecologic disorders</para></item> <item><para>Maternal folic acid use linked to reduced autism risk</para></item> <item><para>Support for biennial over annual mammography in older women</para></item> <item><para>Use of emergency contraception has more than doubled</para></item> </list>
A study of nearly 4,000 pairs of mothers and their children at the University of Bristol in England found no association between maternal vitamin D levels during pregnancy and the child’s bone health in later life.1
And new guidelines from the US Preventive Services Task Force (USPSTF) recommend against supplementation of vitamin D in amounts up to 400 IU daily and calcium in amounts up to 1,000 mg daily for the primary prevention of fractures in noninstitutionalized postmenopausal women.2
Children of the 90s study is largest so far
In the largest observational study ever published on the effects of maternal vitamin D levels on children’s bone health—the Children of the 90s trial—Lawlor and colleagues assessed vitamin D levels in 3,960 pregnant women, recording data from all three trimesters. When the children of these women reached an average age of 9 years and 11 months, their bone-mineral density (BMD) was assessed using dual-energy x-ray absorptiometry (DXA). Investigators found no significant association between a mother’s vitamin D levels and her child’s BMD.1
On average, the women’s vitamin D levels were lowest during the first trimester and increased as pregnancy progressed. As expected, levels were higher when measured during summer months and lower during winter months. And although nonwhite mothers and those who smoked during pregnancy tended to have lower vitamin D levels overall, this finding appeared to have no effect on their children’s bone health.
Earlier studies of the effects of maternal vitamin D levels on children’s bone health were inconclusive. This study by Lawlor and colleagues is more than 10 times larger than all previous studies combined.
“We believe that there is no strong evidence that pregnant women should receive vitamin D supplementation to prevent low [BMD] in their offspring, although we cannot comment on other possible effects of vitamin D in pregnant women,” said Dr. Debbie Lawlor, lead author of the study. “While excessive vitamin D intake can affect the body’s calcium balance and result in cardiac arrhythmias and muscle problems (both rare), as well as milder problems such as dry mouth and constipation, our study didn’t look at any other potential beneficial or adverse effects of vitamin D supplementation besides the association with children’s bone health.”1
USPSTF mostly found a lack of evidence
The USPSTF commissioned two systematic evidence reviews and a meta-analysis of vitamin D supplementation with or without calcium to assess2:
- the effects of supplementation on bone health in community-dwelling adults
- the association between vitamin D and calcium levels and bone health
- any adverse effects of supplementation.
After reviewing the findings, the Task Force concluded that there is insufficient evidence to determine the true balance of benefits versus risks of supplementation of vitamin D in amounts greater than 400 IU daily and calcium in amounts greater than 1,000 mg daily among postmenopausal women in the community. It also recommended against daily supplementation with 400 IU or less of vitamin D and 1,000 mg or less of calcium in this population, as there is no evidence that this practice prevents bone fracture.
In other words, the USPSTF has no recommendation on doses of these supplements at amounts greater than 400 IU of vitamin D and 1,000 mg of calcium daily among postmenopausal women, and recommends against supplementation at lower amounts in the same population.
Both studies focused on a specific context
Both the USPSTF and Lawlor and colleagues emphasized that their investigations focused only on bone mineral density or the risk of fracture, and they acknowledged that there may be other benefits and risks of vitamin D supplementation in pregnancy—and of supplementation with vitamin D and calcium after menopause.1,2
We want to hear from you! Tell us what you think.
A study of nearly 4,000 pairs of mothers and their children at the University of Bristol in England found no association between maternal vitamin D levels during pregnancy and the child’s bone health in later life.1
And new guidelines from the US Preventive Services Task Force (USPSTF) recommend against supplementation of vitamin D in amounts up to 400 IU daily and calcium in amounts up to 1,000 mg daily for the primary prevention of fractures in noninstitutionalized postmenopausal women.2
Children of the 90s study is largest so far
In the largest observational study ever published on the effects of maternal vitamin D levels on children’s bone health—the Children of the 90s trial—Lawlor and colleagues assessed vitamin D levels in 3,960 pregnant women, recording data from all three trimesters. When the children of these women reached an average age of 9 years and 11 months, their bone-mineral density (BMD) was assessed using dual-energy x-ray absorptiometry (DXA). Investigators found no significant association between a mother’s vitamin D levels and her child’s BMD.1
On average, the women’s vitamin D levels were lowest during the first trimester and increased as pregnancy progressed. As expected, levels were higher when measured during summer months and lower during winter months. And although nonwhite mothers and those who smoked during pregnancy tended to have lower vitamin D levels overall, this finding appeared to have no effect on their children’s bone health.
Earlier studies of the effects of maternal vitamin D levels on children’s bone health were inconclusive. This study by Lawlor and colleagues is more than 10 times larger than all previous studies combined.
“We believe that there is no strong evidence that pregnant women should receive vitamin D supplementation to prevent low [BMD] in their offspring, although we cannot comment on other possible effects of vitamin D in pregnant women,” said Dr. Debbie Lawlor, lead author of the study. “While excessive vitamin D intake can affect the body’s calcium balance and result in cardiac arrhythmias and muscle problems (both rare), as well as milder problems such as dry mouth and constipation, our study didn’t look at any other potential beneficial or adverse effects of vitamin D supplementation besides the association with children’s bone health.”1
USPSTF mostly found a lack of evidence
The USPSTF commissioned two systematic evidence reviews and a meta-analysis of vitamin D supplementation with or without calcium to assess2:
- the effects of supplementation on bone health in community-dwelling adults
- the association between vitamin D and calcium levels and bone health
- any adverse effects of supplementation.
After reviewing the findings, the Task Force concluded that there is insufficient evidence to determine the true balance of benefits versus risks of supplementation of vitamin D in amounts greater than 400 IU daily and calcium in amounts greater than 1,000 mg daily among postmenopausal women in the community. It also recommended against daily supplementation with 400 IU or less of vitamin D and 1,000 mg or less of calcium in this population, as there is no evidence that this practice prevents bone fracture.
In other words, the USPSTF has no recommendation on doses of these supplements at amounts greater than 400 IU of vitamin D and 1,000 mg of calcium daily among postmenopausal women, and recommends against supplementation at lower amounts in the same population.
Both studies focused on a specific context
Both the USPSTF and Lawlor and colleagues emphasized that their investigations focused only on bone mineral density or the risk of fracture, and they acknowledged that there may be other benefits and risks of vitamin D supplementation in pregnancy—and of supplementation with vitamin D and calcium after menopause.1,2
We want to hear from you! Tell us what you think.
1. Lawlor DA, Wills AK, Fraser A, Sayers A, Fraser WD, Tobias JH. Association of maternal vitamin D status during pregnancy with bone-mineral content in offspring: a prospective cohort study [published online ahead of print March 19, 2013]. Lancet. doi:10.1016/S0140-6736(12)62203-X.
2. Moyer VA. US Preventive Services Task Force. Vitamin D and calcium supplementation to prevent fractures in adults: US Preventive Services Task Force Recommendation Statement [published online ahead of print February 26, 2013]. Ann Intern Med. doi:10.7326/0003-4819-158-9-201305070-00603.
More NEWS FOR YOUR PRACTICE…
<list type="bullet"> <item><para>Native tissue is superior to vaginal mesh for prolapse repair, two studies report</para></item> <item><para>The newly approved IUD: Which patients is Skyla
appropriate for?</para></item> <item><para>Postpartum anxiety more common than depression</para></item> <item><para>Robotic surgery not the best for hysterectomy, ACOG says</para></item> <item><para>Robotically assisted hysterectomy is on the rise for benign gynecologic disorders</para></item> <item><para>Maternal folic acid use linked to reduced autism risk</para></item> <item><para>Support for biennial over annual mammography in older women</para></item> <item><para>Use of emergency contraception has more than doubled</para></item> </list>
1. Lawlor DA, Wills AK, Fraser A, Sayers A, Fraser WD, Tobias JH. Association of maternal vitamin D status during pregnancy with bone-mineral content in offspring: a prospective cohort study [published online ahead of print March 19, 2013]. Lancet. doi:10.1016/S0140-6736(12)62203-X.
2. Moyer VA. US Preventive Services Task Force. Vitamin D and calcium supplementation to prevent fractures in adults: US Preventive Services Task Force Recommendation Statement [published online ahead of print February 26, 2013]. Ann Intern Med. doi:10.7326/0003-4819-158-9-201305070-00603.
More NEWS FOR YOUR PRACTICE…
<list type="bullet"> <item><para>Native tissue is superior to vaginal mesh for prolapse repair, two studies report</para></item> <item><para>The newly approved IUD: Which patients is Skyla
appropriate for?</para></item> <item><para>Postpartum anxiety more common than depression</para></item> <item><para>Robotic surgery not the best for hysterectomy, ACOG says</para></item> <item><para>Robotically assisted hysterectomy is on the rise for benign gynecologic disorders</para></item> <item><para>Maternal folic acid use linked to reduced autism risk</para></item> <item><para>Support for biennial over annual mammography in older women</para></item> <item><para>Use of emergency contraception has more than doubled</para></item> </list>
Which tocolytic agents are most likely to delay delivery and improve neonatal outcomes?
This statistical tour de force on the topic of tocolytic therapy attempts to determine which agents are most effective at delaying delivery. The analysis is not a typical meta-analysis but what the investigators refer to as “network meta-analysis,” the distinction being that the latter makes an assumption of consistency because all relevant data are compared, including treatments that may no longer appear to be effective or relevant. This distinction may be a bit too esoteric for most clinicians, me included.
Details of the trial
Investigators identified 95 randomized, controlled trials of tocolytic therapy.
The probability of delivery being delayed by 48 hours was greatest with prostaglandin inhibitors (odds ratio [OR], 5.39; 95% confidence interval [CI], 2.14–12.34), followed by magnesium sulfate (OR, 2.76; 95% CI, 1.58–4.94), calcium channel blockers (OR, 2.71; 95% CI, 1.17–5.91), beta mimetics (OR, 2.41; 95% CI, 1.27–4.55), and the oxytocin receptor blocker atosiban (OR, 2.02; 95% CI, 1.10–3.80), compared with placebo.
No class of tocolytic was superior to placebo in reducing the incidence of neonatal respiratory distress syndrome.
Side effects that required a change of medication were significantly more common with beta mimetics (OR, 22.68; 95% CI, 7.51–73.67), magnesium sulfate (OR, 8.15; 95% CI, 2.47–27.70), and calcium channel blockers (OR, 3.80; 95% CI, 1.02–16.92), compared with placebo.
Prostaglandin inhibitors and calcium channel blockers were the agents most likely to be ranked in the top three classes of medication for the outcomes of a 48-hour delay in delivery, lower risk of respiratory distress syndrome and neonatal mortality, and fewer maternal side effects.
Strengths and limitations
The major strength of this analysis is its inclusion of trials from the world literature.
Weaknesses include the:
- lack of practice standardization in many trials
- inclusion of many trials that used composite or secondary analysis
- inability to control for the inclusion of magnesium sulfate in more recent trials, when it was classified as a neuroprotective agent and not a tocolytic
- inclusion of studies involving drugs unavailable in the United States.
The investigators also made some decisions about which trials to include and exclude that could have confused the picture. For example, they excluded trials that used combination drug therapy for tocolysis. As a result, the use of magnesium sulfate as a neuroprotective agent could have confounded the results if it was not recorded as a tocolytic when used in conjunction with another tocolytic.
The most recent practice bulletin from the American College of Obstetricians and Gynecologists addresses this point when it cautions practitioners about developing guidelines for concurrent tocolytic use when employing magnesium sulfate for fetal neuroprotection.1 The practice bulletin also confirms Level A evidence for the use of beta-agonists, calcium channel blockers, or nonsteroidal anti-inflammatory drugs for short-term tocolysis, effectively summarizing current clinical practice and agreeing with the conclusions of Haas and colleagues.
Overall, Haas and colleagues affirm current practice, concluding that prostaglandin inhibitors (eg, indomethacin) and calcium channel blockers (eg, nifedipine, nicardipine) delay delivery and improve short-term neonatal outcomes, whereas beta-agonist therapy was not considered to be as preferable, largely due to its maternal side effect profile. In addition, it is difficult to decipher the relative contributions of the drugs described in this investigation to neonatal and maternal side effects.
I plan to continue my current practice of employing indomethacin or nifedipine as first-line, short-term tocolytic therapy in conjunction with administration of magnesium sulfate for fetal neuroprotection, when appropriate.
I give a starting dose of indomethacin of 50 to 100 mg (orally or rectally), with a maintenance dose of 25 mg orally every 4 hours or 50 mg every 6 hours, not to exceed 48 consecutive hours of treatment.
For nifedipine, I give 20 to 30 mg orally, which can be repeated in 2 hours, if necessary. The maintenance dose is 10 to 20 mg orally every 4 to 6 hours, not to exceed 180 mg/day or 72 consecutive hours of treatment.
JOHN T. REPKE, MD
We want to hear from you! Tell us what you think.
ON OBSTETRICS?
Which skin closure technique better reduces the risk of cesarean wound complications: surgical staples or subcuticular suture?
Dhanya Mackeen, MD, MPH; Vincenzo Berghella, MD (February 2013)
Does an unfavorable cervix preclude induction of labor at term in women who have gestational hypertension or mild preeclampsia?
George Macones, MD (December 2012)
Is the rate of progress the same for induced and spontaneous labors?
William F. Rayburn, MD (November 2012)
Does maternal exposure to magnesium sulfate affect fetal
heart-rate patterns?
John M. Thorp, Jr, MD (October 2012)
Is elective delivery at 37 weeks’ gestation safe in uncomplicated
twin pregnancies?
Steven T. Chasen, MD (September 2012)
Does mediolateral episiotomy reduce the risk of anal sphincter injury in operative vaginal delivery?
Errol T. Norwitz, MD, PhD (August 2012)
Does vaginal progesterone reduce preterm delivery among asymptomatic women who have a short cervix in the midtrimester?
John T. Repke, MD (April 2012)
Does weekly progesterone prolong gestation in women who
have PPROM?
Alex C. Vidaeff, MD, MPH (May 2011)
Reference
1. Committee on Practice Bulletin–Obstetrics; American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 127: Management of preterm labor. Obstet Gynecol. 2012;119(6):1307-1317.
This statistical tour de force on the topic of tocolytic therapy attempts to determine which agents are most effective at delaying delivery. The analysis is not a typical meta-analysis but what the investigators refer to as “network meta-analysis,” the distinction being that the latter makes an assumption of consistency because all relevant data are compared, including treatments that may no longer appear to be effective or relevant. This distinction may be a bit too esoteric for most clinicians, me included.
Details of the trial
Investigators identified 95 randomized, controlled trials of tocolytic therapy.
The probability of delivery being delayed by 48 hours was greatest with prostaglandin inhibitors (odds ratio [OR], 5.39; 95% confidence interval [CI], 2.14–12.34), followed by magnesium sulfate (OR, 2.76; 95% CI, 1.58–4.94), calcium channel blockers (OR, 2.71; 95% CI, 1.17–5.91), beta mimetics (OR, 2.41; 95% CI, 1.27–4.55), and the oxytocin receptor blocker atosiban (OR, 2.02; 95% CI, 1.10–3.80), compared with placebo.
No class of tocolytic was superior to placebo in reducing the incidence of neonatal respiratory distress syndrome.
Side effects that required a change of medication were significantly more common with beta mimetics (OR, 22.68; 95% CI, 7.51–73.67), magnesium sulfate (OR, 8.15; 95% CI, 2.47–27.70), and calcium channel blockers (OR, 3.80; 95% CI, 1.02–16.92), compared with placebo.
Prostaglandin inhibitors and calcium channel blockers were the agents most likely to be ranked in the top three classes of medication for the outcomes of a 48-hour delay in delivery, lower risk of respiratory distress syndrome and neonatal mortality, and fewer maternal side effects.
Strengths and limitations
The major strength of this analysis is its inclusion of trials from the world literature.
Weaknesses include the:
- lack of practice standardization in many trials
- inclusion of many trials that used composite or secondary analysis
- inability to control for the inclusion of magnesium sulfate in more recent trials, when it was classified as a neuroprotective agent and not a tocolytic
- inclusion of studies involving drugs unavailable in the United States.
The investigators also made some decisions about which trials to include and exclude that could have confused the picture. For example, they excluded trials that used combination drug therapy for tocolysis. As a result, the use of magnesium sulfate as a neuroprotective agent could have confounded the results if it was not recorded as a tocolytic when used in conjunction with another tocolytic.
The most recent practice bulletin from the American College of Obstetricians and Gynecologists addresses this point when it cautions practitioners about developing guidelines for concurrent tocolytic use when employing magnesium sulfate for fetal neuroprotection.1 The practice bulletin also confirms Level A evidence for the use of beta-agonists, calcium channel blockers, or nonsteroidal anti-inflammatory drugs for short-term tocolysis, effectively summarizing current clinical practice and agreeing with the conclusions of Haas and colleagues.
Overall, Haas and colleagues affirm current practice, concluding that prostaglandin inhibitors (eg, indomethacin) and calcium channel blockers (eg, nifedipine, nicardipine) delay delivery and improve short-term neonatal outcomes, whereas beta-agonist therapy was not considered to be as preferable, largely due to its maternal side effect profile. In addition, it is difficult to decipher the relative contributions of the drugs described in this investigation to neonatal and maternal side effects.
I plan to continue my current practice of employing indomethacin or nifedipine as first-line, short-term tocolytic therapy in conjunction with administration of magnesium sulfate for fetal neuroprotection, when appropriate.
I give a starting dose of indomethacin of 50 to 100 mg (orally or rectally), with a maintenance dose of 25 mg orally every 4 hours or 50 mg every 6 hours, not to exceed 48 consecutive hours of treatment.
For nifedipine, I give 20 to 30 mg orally, which can be repeated in 2 hours, if necessary. The maintenance dose is 10 to 20 mg orally every 4 to 6 hours, not to exceed 180 mg/day or 72 consecutive hours of treatment.
JOHN T. REPKE, MD
We want to hear from you! Tell us what you think.
ON OBSTETRICS?
Which skin closure technique better reduces the risk of cesarean wound complications: surgical staples or subcuticular suture?
Dhanya Mackeen, MD, MPH; Vincenzo Berghella, MD (February 2013)
Does an unfavorable cervix preclude induction of labor at term in women who have gestational hypertension or mild preeclampsia?
George Macones, MD (December 2012)
Is the rate of progress the same for induced and spontaneous labors?
William F. Rayburn, MD (November 2012)
Does maternal exposure to magnesium sulfate affect fetal
heart-rate patterns?
John M. Thorp, Jr, MD (October 2012)
Is elective delivery at 37 weeks’ gestation safe in uncomplicated
twin pregnancies?
Steven T. Chasen, MD (September 2012)
Does mediolateral episiotomy reduce the risk of anal sphincter injury in operative vaginal delivery?
Errol T. Norwitz, MD, PhD (August 2012)
Does vaginal progesterone reduce preterm delivery among asymptomatic women who have a short cervix in the midtrimester?
John T. Repke, MD (April 2012)
Does weekly progesterone prolong gestation in women who
have PPROM?
Alex C. Vidaeff, MD, MPH (May 2011)
This statistical tour de force on the topic of tocolytic therapy attempts to determine which agents are most effective at delaying delivery. The analysis is not a typical meta-analysis but what the investigators refer to as “network meta-analysis,” the distinction being that the latter makes an assumption of consistency because all relevant data are compared, including treatments that may no longer appear to be effective or relevant. This distinction may be a bit too esoteric for most clinicians, me included.
Details of the trial
Investigators identified 95 randomized, controlled trials of tocolytic therapy.
The probability of delivery being delayed by 48 hours was greatest with prostaglandin inhibitors (odds ratio [OR], 5.39; 95% confidence interval [CI], 2.14–12.34), followed by magnesium sulfate (OR, 2.76; 95% CI, 1.58–4.94), calcium channel blockers (OR, 2.71; 95% CI, 1.17–5.91), beta mimetics (OR, 2.41; 95% CI, 1.27–4.55), and the oxytocin receptor blocker atosiban (OR, 2.02; 95% CI, 1.10–3.80), compared with placebo.
No class of tocolytic was superior to placebo in reducing the incidence of neonatal respiratory distress syndrome.
Side effects that required a change of medication were significantly more common with beta mimetics (OR, 22.68; 95% CI, 7.51–73.67), magnesium sulfate (OR, 8.15; 95% CI, 2.47–27.70), and calcium channel blockers (OR, 3.80; 95% CI, 1.02–16.92), compared with placebo.
Prostaglandin inhibitors and calcium channel blockers were the agents most likely to be ranked in the top three classes of medication for the outcomes of a 48-hour delay in delivery, lower risk of respiratory distress syndrome and neonatal mortality, and fewer maternal side effects.
Strengths and limitations
The major strength of this analysis is its inclusion of trials from the world literature.
Weaknesses include the:
- lack of practice standardization in many trials
- inclusion of many trials that used composite or secondary analysis
- inability to control for the inclusion of magnesium sulfate in more recent trials, when it was classified as a neuroprotective agent and not a tocolytic
- inclusion of studies involving drugs unavailable in the United States.
The investigators also made some decisions about which trials to include and exclude that could have confused the picture. For example, they excluded trials that used combination drug therapy for tocolysis. As a result, the use of magnesium sulfate as a neuroprotective agent could have confounded the results if it was not recorded as a tocolytic when used in conjunction with another tocolytic.
The most recent practice bulletin from the American College of Obstetricians and Gynecologists addresses this point when it cautions practitioners about developing guidelines for concurrent tocolytic use when employing magnesium sulfate for fetal neuroprotection.1 The practice bulletin also confirms Level A evidence for the use of beta-agonists, calcium channel blockers, or nonsteroidal anti-inflammatory drugs for short-term tocolysis, effectively summarizing current clinical practice and agreeing with the conclusions of Haas and colleagues.
Overall, Haas and colleagues affirm current practice, concluding that prostaglandin inhibitors (eg, indomethacin) and calcium channel blockers (eg, nifedipine, nicardipine) delay delivery and improve short-term neonatal outcomes, whereas beta-agonist therapy was not considered to be as preferable, largely due to its maternal side effect profile. In addition, it is difficult to decipher the relative contributions of the drugs described in this investigation to neonatal and maternal side effects.
I plan to continue my current practice of employing indomethacin or nifedipine as first-line, short-term tocolytic therapy in conjunction with administration of magnesium sulfate for fetal neuroprotection, when appropriate.
I give a starting dose of indomethacin of 50 to 100 mg (orally or rectally), with a maintenance dose of 25 mg orally every 4 hours or 50 mg every 6 hours, not to exceed 48 consecutive hours of treatment.
For nifedipine, I give 20 to 30 mg orally, which can be repeated in 2 hours, if necessary. The maintenance dose is 10 to 20 mg orally every 4 to 6 hours, not to exceed 180 mg/day or 72 consecutive hours of treatment.
JOHN T. REPKE, MD
We want to hear from you! Tell us what you think.
ON OBSTETRICS?
Which skin closure technique better reduces the risk of cesarean wound complications: surgical staples or subcuticular suture?
Dhanya Mackeen, MD, MPH; Vincenzo Berghella, MD (February 2013)
Does an unfavorable cervix preclude induction of labor at term in women who have gestational hypertension or mild preeclampsia?
George Macones, MD (December 2012)
Is the rate of progress the same for induced and spontaneous labors?
William F. Rayburn, MD (November 2012)
Does maternal exposure to magnesium sulfate affect fetal
heart-rate patterns?
John M. Thorp, Jr, MD (October 2012)
Is elective delivery at 37 weeks’ gestation safe in uncomplicated
twin pregnancies?
Steven T. Chasen, MD (September 2012)
Does mediolateral episiotomy reduce the risk of anal sphincter injury in operative vaginal delivery?
Errol T. Norwitz, MD, PhD (August 2012)
Does vaginal progesterone reduce preterm delivery among asymptomatic women who have a short cervix in the midtrimester?
John T. Repke, MD (April 2012)
Does weekly progesterone prolong gestation in women who
have PPROM?
Alex C. Vidaeff, MD, MPH (May 2011)
Reference
1. Committee on Practice Bulletin–Obstetrics; American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 127: Management of preterm labor. Obstet Gynecol. 2012;119(6):1307-1317.
Reference
1. Committee on Practice Bulletin–Obstetrics; American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 127: Management of preterm labor. Obstet Gynecol. 2012;119(6):1307-1317.