User login
Marijuana habit not linked to lung cancer
WASHINGTON – Regular cannabis smokers are no more likely to develop lung cancer than are people who indulge occasionally.
The finding of no significant increased risk held true whether the smokers imbibed once or twice – or more – each day, and regardless of how many years they had smoked, Dr. Li Rita Zhang reported at the annual meeting of the American Association of Cancer Research.
The study included data from six case-control studies conducted from 1999 to 2012 in the United States, Canada, the United Kingdom, and New Zealand, with a subject pool of 2,159 lung cancer cases and 2,985 controls. All of the studies were part of the International Lung Cancer Consortium (ILCCO), an international group of lung cancer researchers with the aim of sharing comparable data from ongoing and recently completed lung cancer studies from different geographical areas and ethnicities.
Dr. Zhang of the University of California, Los Angeles, performed two analyses. One compared all lung cancer cases and all controls, regardless of concurrent or past tobacco use. Then, to reduce confounding by tobacco, she restricted the analysis to those who had never smoked tobacco. That group comprised 370 cancer cases and 1,358 controls. The models were also adjusted for age, sex, sociodemographic factors, and tobacco pack-years. Habitual use was defined as one joint per day per year.
When compared with cannabis smokers who also used tobacco, habitual pot smokers had no significant increase in cancer risk.
In an analysis of marijuana smokers that excluded tobacco smokers, there were no significant differences in any of the comparisons, including habitual vs. nonhabitual use; number of joints smoked per day; duration of up to 20 years or duration of more than 20 years.
Other literature has shown a link between cannabis smoking and lung cancer, pulmonologist Michael Alberts said in an interview. However, he said, "The conventional wisdom is that cannabis smoking is not as dangerous as cigarette smoking."
The difference in risk is likely related to chemical additives in commercial cigarettes that aren’t present in most methods of inhaling marijuana smoke.
As a general recommendation, smoking anything isn’t good for the respiratory system, said Dr. Alberts, chief medical officer of the Moffitt Cancer Center, Tampa. But for patients using medical marijuana, the benefit could outweigh the risks.
"You can think of it as similar to a CT scan. Radiation isn’t good, but if the scan is something beneficial and the risk is low, you take it. If cannabis is indicated, and if it’s legal, and if there’s literature backing up the indication for use, then you weigh the risk of smoking and the benefit it could bring, and make the decision."
Dr. Zhang declined to comment on the study. In her poster presentation she noted that, "Our results cannot preclude the possibility that cannabis may exhibit an association with lung cancer risk at extremely high dosage over long periods of continued exposure."
Dr. Zhang did not disclosure any financial relationships. Dr. Alberts said he had no disclosures.
WASHINGTON – Regular cannabis smokers are no more likely to develop lung cancer than are people who indulge occasionally.
The finding of no significant increased risk held true whether the smokers imbibed once or twice – or more – each day, and regardless of how many years they had smoked, Dr. Li Rita Zhang reported at the annual meeting of the American Association of Cancer Research.
The study included data from six case-control studies conducted from 1999 to 2012 in the United States, Canada, the United Kingdom, and New Zealand, with a subject pool of 2,159 lung cancer cases and 2,985 controls. All of the studies were part of the International Lung Cancer Consortium (ILCCO), an international group of lung cancer researchers with the aim of sharing comparable data from ongoing and recently completed lung cancer studies from different geographical areas and ethnicities.
Dr. Zhang of the University of California, Los Angeles, performed two analyses. One compared all lung cancer cases and all controls, regardless of concurrent or past tobacco use. Then, to reduce confounding by tobacco, she restricted the analysis to those who had never smoked tobacco. That group comprised 370 cancer cases and 1,358 controls. The models were also adjusted for age, sex, sociodemographic factors, and tobacco pack-years. Habitual use was defined as one joint per day per year.
When compared with cannabis smokers who also used tobacco, habitual pot smokers had no significant increase in cancer risk.
In an analysis of marijuana smokers that excluded tobacco smokers, there were no significant differences in any of the comparisons, including habitual vs. nonhabitual use; number of joints smoked per day; duration of up to 20 years or duration of more than 20 years.
Other literature has shown a link between cannabis smoking and lung cancer, pulmonologist Michael Alberts said in an interview. However, he said, "The conventional wisdom is that cannabis smoking is not as dangerous as cigarette smoking."
The difference in risk is likely related to chemical additives in commercial cigarettes that aren’t present in most methods of inhaling marijuana smoke.
As a general recommendation, smoking anything isn’t good for the respiratory system, said Dr. Alberts, chief medical officer of the Moffitt Cancer Center, Tampa. But for patients using medical marijuana, the benefit could outweigh the risks.
"You can think of it as similar to a CT scan. Radiation isn’t good, but if the scan is something beneficial and the risk is low, you take it. If cannabis is indicated, and if it’s legal, and if there’s literature backing up the indication for use, then you weigh the risk of smoking and the benefit it could bring, and make the decision."
Dr. Zhang declined to comment on the study. In her poster presentation she noted that, "Our results cannot preclude the possibility that cannabis may exhibit an association with lung cancer risk at extremely high dosage over long periods of continued exposure."
Dr. Zhang did not disclosure any financial relationships. Dr. Alberts said he had no disclosures.
WASHINGTON – Regular cannabis smokers are no more likely to develop lung cancer than are people who indulge occasionally.
The finding of no significant increased risk held true whether the smokers imbibed once or twice – or more – each day, and regardless of how many years they had smoked, Dr. Li Rita Zhang reported at the annual meeting of the American Association of Cancer Research.
The study included data from six case-control studies conducted from 1999 to 2012 in the United States, Canada, the United Kingdom, and New Zealand, with a subject pool of 2,159 lung cancer cases and 2,985 controls. All of the studies were part of the International Lung Cancer Consortium (ILCCO), an international group of lung cancer researchers with the aim of sharing comparable data from ongoing and recently completed lung cancer studies from different geographical areas and ethnicities.
Dr. Zhang of the University of California, Los Angeles, performed two analyses. One compared all lung cancer cases and all controls, regardless of concurrent or past tobacco use. Then, to reduce confounding by tobacco, she restricted the analysis to those who had never smoked tobacco. That group comprised 370 cancer cases and 1,358 controls. The models were also adjusted for age, sex, sociodemographic factors, and tobacco pack-years. Habitual use was defined as one joint per day per year.
When compared with cannabis smokers who also used tobacco, habitual pot smokers had no significant increase in cancer risk.
In an analysis of marijuana smokers that excluded tobacco smokers, there were no significant differences in any of the comparisons, including habitual vs. nonhabitual use; number of joints smoked per day; duration of up to 20 years or duration of more than 20 years.
Other literature has shown a link between cannabis smoking and lung cancer, pulmonologist Michael Alberts said in an interview. However, he said, "The conventional wisdom is that cannabis smoking is not as dangerous as cigarette smoking."
The difference in risk is likely related to chemical additives in commercial cigarettes that aren’t present in most methods of inhaling marijuana smoke.
As a general recommendation, smoking anything isn’t good for the respiratory system, said Dr. Alberts, chief medical officer of the Moffitt Cancer Center, Tampa. But for patients using medical marijuana, the benefit could outweigh the risks.
"You can think of it as similar to a CT scan. Radiation isn’t good, but if the scan is something beneficial and the risk is low, you take it. If cannabis is indicated, and if it’s legal, and if there’s literature backing up the indication for use, then you weigh the risk of smoking and the benefit it could bring, and make the decision."
Dr. Zhang declined to comment on the study. In her poster presentation she noted that, "Our results cannot preclude the possibility that cannabis may exhibit an association with lung cancer risk at extremely high dosage over long periods of continued exposure."
Dr. Zhang did not disclosure any financial relationships. Dr. Alberts said he had no disclosures.
AT THE AACR ANNUAL MEETING
Major finding: People who habitually smoked marijuana were no more likely to develop lung cancer than those who didn’t, whether or not they also smoked cigarettes.
Data source: The metaanalysis included 2,159 lung cancer cases and 2,985 controls extracted from six studies.
Disclosures: Neither Dr. Zhang nor Dr. Alberts reported any financial disclosures.
Study suggests statin use decreases breast cancer mortality
WASHINGTON – Statin use – both before and after a diagnosis of breast cancer – was associated with up to a 66% reduction in the risk of dying from that cancer, a large database study has concluded.
Investigators reported that the risk reduction was consistent even after controlling for age, tumor stage and morphology, and primary treatment choice, Dr. Teemu Murtola said during an interview at the annual meeting of the American Association for Cancer Research.
"We saw that there was clearly a dose-dependent reduction in the risk of breast cancer mortality among statin users," who comprised 13% of the more than 31,000 women in the study, said Dr. Murtola, an epidemiologist at Johns Hopkins University in Baltimore. "The association held for both localized and metastatic tumors. There really appears to be something going on here, and it’s certainly a reason for further study."
During the follow-up, 6,011 women died; 3,169 deaths were due to breast cancer. The death rate among users was significantly lower than it was among nonusers (7.5% vs. 21%). In the adjusted analysis, statin users were 67% less likely to die than were nonusers with localized disease (hazard ratio, 0.33). Among those with metastatic disease, statins conferred a 48% decreased risk of death (HR, 0.52).
All of the statins investigated in the study cohort were associated with a significantly decreased risk of breast cancer death, including simvastatin (HR, 0.47), atorvastatin (HR, 0.27), fluvastatin (HR, 0.35), and pravastatin (HR, 0.50).
The retrospective study looked at statin use and breast cancer mortality among 31,114 women with breast cancer who were diagnosed in Finland during 1995-2003. All of the patients were included in the Finnish Cancer Registry. The country’s national health database also contained detailed information on statin use and other health indicators. The data on statins included the date of each prescription purchase, the numbers of refills, the dosage, and the number of pills in each refill.
Breast cancer data included the date of diagnosis, stage (local, lymph-node positive or metastatic), morphology, primary treatment choice (surgery, radiation, chemotherapy, hormonal therapy, or other), and the date and cause of death.
Follow-up started at the time of diagnosis and continued until death or the common closing date of Dec. 31, 2003, whichever came first. Median follow-up was about 3 years, but ranged from less than 1 year to 9 years.
Statin users accounted for 13% of the cohort (4,169); the remainder never used the drugs. Women who took the medications were significantly older than nonusers (64 years vs. 58 years), and more likely to have had surgery as a treatment (96% vs. 92%). They were also more likely to have undergone radiation (55% vs. 54%), but significantly less likely to have had chemotherapy (15% vs. 24%) or hormonal therapy (20% vs. 25%). Metastatic cancer was significantly less common among statin users – 4% vs. 7%. These differences may reflect an early identification of breast cancer among users, perhaps because they were visiting a physician more frequently for lipid monitoring, Dr. Murtola said. However, the multivariate analysis controlled for all these differences, including metastatic and local disease.
Dr. Murtola then investigated the mortality effects of both pre- and postdiagnostic statin use.
Compliance was high among women who started the drugs before their breast cancer diagnosis, with 85% continuing until the end of follow-up. Compared with nonusers, women who used statins for up to 490 days were 46% less likely to die of local disease and 30% less likely to die from metastatic disease.
Those who took the drugs for 491 days or more before diagnosis were 66% less likely to die from local disease and 40% less likely to die from metastatic disease.
There was no such clear, dose-dependent relationship between mortality risk and statins taken after diagnosis, Dr. Murtola said. But, he added, low compliance could have masked any benefit. "Only 14% of those who started statins after diagnosis continued to take them until the end of the follow-up period," he said. "When we limited the analysis to those who were compliant until the end of their follow-up, we saw a very similar dose-dependent risk reduction in breast cancer mortality."
In vitro studies suggest that statins could have a direct effect on breast cancer cells, he said. They seem to block the mevalonate pathway – a process involved in the manufacture of cholesterol, and also key to cell metabolism. Cells with abnormally high metabolic rates – like breast cancer cells – could be particularly vulnerable to this action, he said.
"I think that because the drugs target this pathway their impact extends beyond cholesterol. It’s certainly worth further study."
Dr. Murtola had no financial disclosures.
WASHINGTON – Statin use – both before and after a diagnosis of breast cancer – was associated with up to a 66% reduction in the risk of dying from that cancer, a large database study has concluded.
Investigators reported that the risk reduction was consistent even after controlling for age, tumor stage and morphology, and primary treatment choice, Dr. Teemu Murtola said during an interview at the annual meeting of the American Association for Cancer Research.
"We saw that there was clearly a dose-dependent reduction in the risk of breast cancer mortality among statin users," who comprised 13% of the more than 31,000 women in the study, said Dr. Murtola, an epidemiologist at Johns Hopkins University in Baltimore. "The association held for both localized and metastatic tumors. There really appears to be something going on here, and it’s certainly a reason for further study."
During the follow-up, 6,011 women died; 3,169 deaths were due to breast cancer. The death rate among users was significantly lower than it was among nonusers (7.5% vs. 21%). In the adjusted analysis, statin users were 67% less likely to die than were nonusers with localized disease (hazard ratio, 0.33). Among those with metastatic disease, statins conferred a 48% decreased risk of death (HR, 0.52).
All of the statins investigated in the study cohort were associated with a significantly decreased risk of breast cancer death, including simvastatin (HR, 0.47), atorvastatin (HR, 0.27), fluvastatin (HR, 0.35), and pravastatin (HR, 0.50).
The retrospective study looked at statin use and breast cancer mortality among 31,114 women with breast cancer who were diagnosed in Finland during 1995-2003. All of the patients were included in the Finnish Cancer Registry. The country’s national health database also contained detailed information on statin use and other health indicators. The data on statins included the date of each prescription purchase, the numbers of refills, the dosage, and the number of pills in each refill.
Breast cancer data included the date of diagnosis, stage (local, lymph-node positive or metastatic), morphology, primary treatment choice (surgery, radiation, chemotherapy, hormonal therapy, or other), and the date and cause of death.
Follow-up started at the time of diagnosis and continued until death or the common closing date of Dec. 31, 2003, whichever came first. Median follow-up was about 3 years, but ranged from less than 1 year to 9 years.
Statin users accounted for 13% of the cohort (4,169); the remainder never used the drugs. Women who took the medications were significantly older than nonusers (64 years vs. 58 years), and more likely to have had surgery as a treatment (96% vs. 92%). They were also more likely to have undergone radiation (55% vs. 54%), but significantly less likely to have had chemotherapy (15% vs. 24%) or hormonal therapy (20% vs. 25%). Metastatic cancer was significantly less common among statin users – 4% vs. 7%. These differences may reflect an early identification of breast cancer among users, perhaps because they were visiting a physician more frequently for lipid monitoring, Dr. Murtola said. However, the multivariate analysis controlled for all these differences, including metastatic and local disease.
Dr. Murtola then investigated the mortality effects of both pre- and postdiagnostic statin use.
Compliance was high among women who started the drugs before their breast cancer diagnosis, with 85% continuing until the end of follow-up. Compared with nonusers, women who used statins for up to 490 days were 46% less likely to die of local disease and 30% less likely to die from metastatic disease.
Those who took the drugs for 491 days or more before diagnosis were 66% less likely to die from local disease and 40% less likely to die from metastatic disease.
There was no such clear, dose-dependent relationship between mortality risk and statins taken after diagnosis, Dr. Murtola said. But, he added, low compliance could have masked any benefit. "Only 14% of those who started statins after diagnosis continued to take them until the end of the follow-up period," he said. "When we limited the analysis to those who were compliant until the end of their follow-up, we saw a very similar dose-dependent risk reduction in breast cancer mortality."
In vitro studies suggest that statins could have a direct effect on breast cancer cells, he said. They seem to block the mevalonate pathway – a process involved in the manufacture of cholesterol, and also key to cell metabolism. Cells with abnormally high metabolic rates – like breast cancer cells – could be particularly vulnerable to this action, he said.
"I think that because the drugs target this pathway their impact extends beyond cholesterol. It’s certainly worth further study."
Dr. Murtola had no financial disclosures.
WASHINGTON – Statin use – both before and after a diagnosis of breast cancer – was associated with up to a 66% reduction in the risk of dying from that cancer, a large database study has concluded.
Investigators reported that the risk reduction was consistent even after controlling for age, tumor stage and morphology, and primary treatment choice, Dr. Teemu Murtola said during an interview at the annual meeting of the American Association for Cancer Research.
"We saw that there was clearly a dose-dependent reduction in the risk of breast cancer mortality among statin users," who comprised 13% of the more than 31,000 women in the study, said Dr. Murtola, an epidemiologist at Johns Hopkins University in Baltimore. "The association held for both localized and metastatic tumors. There really appears to be something going on here, and it’s certainly a reason for further study."
During the follow-up, 6,011 women died; 3,169 deaths were due to breast cancer. The death rate among users was significantly lower than it was among nonusers (7.5% vs. 21%). In the adjusted analysis, statin users were 67% less likely to die than were nonusers with localized disease (hazard ratio, 0.33). Among those with metastatic disease, statins conferred a 48% decreased risk of death (HR, 0.52).
All of the statins investigated in the study cohort were associated with a significantly decreased risk of breast cancer death, including simvastatin (HR, 0.47), atorvastatin (HR, 0.27), fluvastatin (HR, 0.35), and pravastatin (HR, 0.50).
The retrospective study looked at statin use and breast cancer mortality among 31,114 women with breast cancer who were diagnosed in Finland during 1995-2003. All of the patients were included in the Finnish Cancer Registry. The country’s national health database also contained detailed information on statin use and other health indicators. The data on statins included the date of each prescription purchase, the numbers of refills, the dosage, and the number of pills in each refill.
Breast cancer data included the date of diagnosis, stage (local, lymph-node positive or metastatic), morphology, primary treatment choice (surgery, radiation, chemotherapy, hormonal therapy, or other), and the date and cause of death.
Follow-up started at the time of diagnosis and continued until death or the common closing date of Dec. 31, 2003, whichever came first. Median follow-up was about 3 years, but ranged from less than 1 year to 9 years.
Statin users accounted for 13% of the cohort (4,169); the remainder never used the drugs. Women who took the medications were significantly older than nonusers (64 years vs. 58 years), and more likely to have had surgery as a treatment (96% vs. 92%). They were also more likely to have undergone radiation (55% vs. 54%), but significantly less likely to have had chemotherapy (15% vs. 24%) or hormonal therapy (20% vs. 25%). Metastatic cancer was significantly less common among statin users – 4% vs. 7%. These differences may reflect an early identification of breast cancer among users, perhaps because they were visiting a physician more frequently for lipid monitoring, Dr. Murtola said. However, the multivariate analysis controlled for all these differences, including metastatic and local disease.
Dr. Murtola then investigated the mortality effects of both pre- and postdiagnostic statin use.
Compliance was high among women who started the drugs before their breast cancer diagnosis, with 85% continuing until the end of follow-up. Compared with nonusers, women who used statins for up to 490 days were 46% less likely to die of local disease and 30% less likely to die from metastatic disease.
Those who took the drugs for 491 days or more before diagnosis were 66% less likely to die from local disease and 40% less likely to die from metastatic disease.
There was no such clear, dose-dependent relationship between mortality risk and statins taken after diagnosis, Dr. Murtola said. But, he added, low compliance could have masked any benefit. "Only 14% of those who started statins after diagnosis continued to take them until the end of the follow-up period," he said. "When we limited the analysis to those who were compliant until the end of their follow-up, we saw a very similar dose-dependent risk reduction in breast cancer mortality."
In vitro studies suggest that statins could have a direct effect on breast cancer cells, he said. They seem to block the mevalonate pathway – a process involved in the manufacture of cholesterol, and also key to cell metabolism. Cells with abnormally high metabolic rates – like breast cancer cells – could be particularly vulnerable to this action, he said.
"I think that because the drugs target this pathway their impact extends beyond cholesterol. It’s certainly worth further study."
Dr. Murtola had no financial disclosures.
AT THE AACR ANNUAL MEETING
Major finding: Women who took statins before a diagnosis of breast cancer were up to 67% less likely to die from their disease than were those who never took the medications.
Data source: The database study comprised 31,114 women with breast cancer included in the Finnish Cancer Registry.
Disclosures: Dr. Murtola had no financial disclosures.
Prenatal exposure to air pollution boosts childhood cancer risk
WASHINGTON – Children whose mothers were exposed to air pollution during pregnancy may have a small, but significant, increase in pediatric cancer risk.
A 9-year case-control study found that children who were exposed to the highest levels of traffic-related pollution prenatally and during the first year of life were 19% more likely to develop bilateral retinoblastoma and 17% more likely to develop germ cell tumors than were children who were exposed to the lowest level.
"This is the first study to report upon traffic pollution in relation to retinoblastoma or germ cell tumors, and since both of these are rare, the findings need to be replicated in other studies," Julia E. Heck, Ph.D., said in an interview at the annual meeting of the American Association for Cancer Research.
Dr. Heck, an epidemiologist at the University of California’s Fielding School of Public Health, Los Angeles, used a state air pollution modeling system to detect the patterns in her study. The California Line Source Dispersion Modeling system, version 4 (CALINE4) uses a test fleet of motor vehicles to predict traffic-related carbon monoxide emissions from vehicles in idle, cruise, acceleration, and deceleration.
Her study cohort comprised 3,590 children with cancer who were included in a California cancer registry. All were born between 1998 and 2007 and were aged younger than 6 years. She compared these children to a random selection of 80,224 children drawn form California birth records. The CALINE4 system generated estimates of local traffic exposure based on where the mothers were living during each trimester of pregnancy.
The model imputed emissions from gasoline and diesel vehicles within a 1,500-meter radius of the address. It included data on traffic volume, roadway geometry, vehicle emission rates, and weather patterns, and divided the cohort into quartiles according to exposure. Dr. Heck looked specifically at carbon monoxide measurements in increments of 53 parts per billion.
Each increase in CO emission raised the risk of acute lymphoblastic leukemia by 4%. Each quartile was associated with an overall 14% increased risk of a retinoblastoma, with the risk significantly elevated for bilateral tumors rather than unilateral tumors (a 19% increase). Dr. Heck also saw a 17% increased risk for germ cell tumors for every quartile increase in CO.
Associations with other cancers, including acute myeloid leukemia, non-Hodgkin’s lymphoma, ependymoma, astrocytoma, neuroblastoma, Wilms tumor, hepatoblastoma, and rhabdomyosarcoma, were nonsignificant.
Because Dr. Heck’s study was a retrospective analysis, it cannot prove causation. "The results need to be confirmed in other studies," she said in an interview. "But our findings do support a link between traffic pollution and some childhood cancers."
The field of childhood cancers and air pollution has not been well studied in recent years, Dr. Heck said, although there were a number of such studies in the late 1990s and early 2000s.
One was a British case-control analysis of birth and death records of 12,018 children in the United Kingdom who had been born and died from leukemia and other cancers from 1955 to 1980. The cancer-related deaths were linked to locations of rail and bus stations, ferry terminals, railways, roads, canals, and rivers (J. Epidemiol. Community Health 2005;59:755-60).
"The most striking result is the extraordinary concentration of cancer births within 0.3 km of bus/coach stations (OR = 12.5)," wrote Dr. E.G. Knox, professor emeritus at the University of Birmingham, England. "This was followed by hospitals (OR = 2.6) and heavy transport centers (OR = 1.6)."
Of the individual pollutants examined, both carbon monoxide and 1,3-butadiene more than doubled the risk of cancer.
Because the study encompassed the child’s location at birth, it demonstrated a link to prenatal exposure. "Childhood cancers are strongly determined by prenatal or early postnatal exposures to oil-based combustion gases, especially from engine exhausts," he noted. "The chief carcinogenic agent is probably 1,3-butadiene."
The National Institute for Occupational Safety and Health has recommended that 1,3-butadiene be treated as a potential occupational carcinogen, teratogen, and as a reproduction hazard.
In 2006, Dr. Knox published another study on the same group, in which he attributed about 24% of the cancers to prenatal or early life proximity to areas of high airborne pollutants. "Child cancer initiations are strongly determined by prenatal or early postnatal exposures to engine exhaust gases, probably through maternal inhalation and accumulation of carcinogens over many months," he noted. (J. Epidemiol. Community Health 2006;60:136-41).
Dr. Heck’s study was funded by the National Institutes of Health. She had no financial disclosures.
WASHINGTON – Children whose mothers were exposed to air pollution during pregnancy may have a small, but significant, increase in pediatric cancer risk.
A 9-year case-control study found that children who were exposed to the highest levels of traffic-related pollution prenatally and during the first year of life were 19% more likely to develop bilateral retinoblastoma and 17% more likely to develop germ cell tumors than were children who were exposed to the lowest level.
"This is the first study to report upon traffic pollution in relation to retinoblastoma or germ cell tumors, and since both of these are rare, the findings need to be replicated in other studies," Julia E. Heck, Ph.D., said in an interview at the annual meeting of the American Association for Cancer Research.
Dr. Heck, an epidemiologist at the University of California’s Fielding School of Public Health, Los Angeles, used a state air pollution modeling system to detect the patterns in her study. The California Line Source Dispersion Modeling system, version 4 (CALINE4) uses a test fleet of motor vehicles to predict traffic-related carbon monoxide emissions from vehicles in idle, cruise, acceleration, and deceleration.
Her study cohort comprised 3,590 children with cancer who were included in a California cancer registry. All were born between 1998 and 2007 and were aged younger than 6 years. She compared these children to a random selection of 80,224 children drawn form California birth records. The CALINE4 system generated estimates of local traffic exposure based on where the mothers were living during each trimester of pregnancy.
The model imputed emissions from gasoline and diesel vehicles within a 1,500-meter radius of the address. It included data on traffic volume, roadway geometry, vehicle emission rates, and weather patterns, and divided the cohort into quartiles according to exposure. Dr. Heck looked specifically at carbon monoxide measurements in increments of 53 parts per billion.
Each increase in CO emission raised the risk of acute lymphoblastic leukemia by 4%. Each quartile was associated with an overall 14% increased risk of a retinoblastoma, with the risk significantly elevated for bilateral tumors rather than unilateral tumors (a 19% increase). Dr. Heck also saw a 17% increased risk for germ cell tumors for every quartile increase in CO.
Associations with other cancers, including acute myeloid leukemia, non-Hodgkin’s lymphoma, ependymoma, astrocytoma, neuroblastoma, Wilms tumor, hepatoblastoma, and rhabdomyosarcoma, were nonsignificant.
Because Dr. Heck’s study was a retrospective analysis, it cannot prove causation. "The results need to be confirmed in other studies," she said in an interview. "But our findings do support a link between traffic pollution and some childhood cancers."
The field of childhood cancers and air pollution has not been well studied in recent years, Dr. Heck said, although there were a number of such studies in the late 1990s and early 2000s.
One was a British case-control analysis of birth and death records of 12,018 children in the United Kingdom who had been born and died from leukemia and other cancers from 1955 to 1980. The cancer-related deaths were linked to locations of rail and bus stations, ferry terminals, railways, roads, canals, and rivers (J. Epidemiol. Community Health 2005;59:755-60).
"The most striking result is the extraordinary concentration of cancer births within 0.3 km of bus/coach stations (OR = 12.5)," wrote Dr. E.G. Knox, professor emeritus at the University of Birmingham, England. "This was followed by hospitals (OR = 2.6) and heavy transport centers (OR = 1.6)."
Of the individual pollutants examined, both carbon monoxide and 1,3-butadiene more than doubled the risk of cancer.
Because the study encompassed the child’s location at birth, it demonstrated a link to prenatal exposure. "Childhood cancers are strongly determined by prenatal or early postnatal exposures to oil-based combustion gases, especially from engine exhausts," he noted. "The chief carcinogenic agent is probably 1,3-butadiene."
The National Institute for Occupational Safety and Health has recommended that 1,3-butadiene be treated as a potential occupational carcinogen, teratogen, and as a reproduction hazard.
In 2006, Dr. Knox published another study on the same group, in which he attributed about 24% of the cancers to prenatal or early life proximity to areas of high airborne pollutants. "Child cancer initiations are strongly determined by prenatal or early postnatal exposures to engine exhaust gases, probably through maternal inhalation and accumulation of carcinogens over many months," he noted. (J. Epidemiol. Community Health 2006;60:136-41).
Dr. Heck’s study was funded by the National Institutes of Health. She had no financial disclosures.
WASHINGTON – Children whose mothers were exposed to air pollution during pregnancy may have a small, but significant, increase in pediatric cancer risk.
A 9-year case-control study found that children who were exposed to the highest levels of traffic-related pollution prenatally and during the first year of life were 19% more likely to develop bilateral retinoblastoma and 17% more likely to develop germ cell tumors than were children who were exposed to the lowest level.
"This is the first study to report upon traffic pollution in relation to retinoblastoma or germ cell tumors, and since both of these are rare, the findings need to be replicated in other studies," Julia E. Heck, Ph.D., said in an interview at the annual meeting of the American Association for Cancer Research.
Dr. Heck, an epidemiologist at the University of California’s Fielding School of Public Health, Los Angeles, used a state air pollution modeling system to detect the patterns in her study. The California Line Source Dispersion Modeling system, version 4 (CALINE4) uses a test fleet of motor vehicles to predict traffic-related carbon monoxide emissions from vehicles in idle, cruise, acceleration, and deceleration.
Her study cohort comprised 3,590 children with cancer who were included in a California cancer registry. All were born between 1998 and 2007 and were aged younger than 6 years. She compared these children to a random selection of 80,224 children drawn form California birth records. The CALINE4 system generated estimates of local traffic exposure based on where the mothers were living during each trimester of pregnancy.
The model imputed emissions from gasoline and diesel vehicles within a 1,500-meter radius of the address. It included data on traffic volume, roadway geometry, vehicle emission rates, and weather patterns, and divided the cohort into quartiles according to exposure. Dr. Heck looked specifically at carbon monoxide measurements in increments of 53 parts per billion.
Each increase in CO emission raised the risk of acute lymphoblastic leukemia by 4%. Each quartile was associated with an overall 14% increased risk of a retinoblastoma, with the risk significantly elevated for bilateral tumors rather than unilateral tumors (a 19% increase). Dr. Heck also saw a 17% increased risk for germ cell tumors for every quartile increase in CO.
Associations with other cancers, including acute myeloid leukemia, non-Hodgkin’s lymphoma, ependymoma, astrocytoma, neuroblastoma, Wilms tumor, hepatoblastoma, and rhabdomyosarcoma, were nonsignificant.
Because Dr. Heck’s study was a retrospective analysis, it cannot prove causation. "The results need to be confirmed in other studies," she said in an interview. "But our findings do support a link between traffic pollution and some childhood cancers."
The field of childhood cancers and air pollution has not been well studied in recent years, Dr. Heck said, although there were a number of such studies in the late 1990s and early 2000s.
One was a British case-control analysis of birth and death records of 12,018 children in the United Kingdom who had been born and died from leukemia and other cancers from 1955 to 1980. The cancer-related deaths were linked to locations of rail and bus stations, ferry terminals, railways, roads, canals, and rivers (J. Epidemiol. Community Health 2005;59:755-60).
"The most striking result is the extraordinary concentration of cancer births within 0.3 km of bus/coach stations (OR = 12.5)," wrote Dr. E.G. Knox, professor emeritus at the University of Birmingham, England. "This was followed by hospitals (OR = 2.6) and heavy transport centers (OR = 1.6)."
Of the individual pollutants examined, both carbon monoxide and 1,3-butadiene more than doubled the risk of cancer.
Because the study encompassed the child’s location at birth, it demonstrated a link to prenatal exposure. "Childhood cancers are strongly determined by prenatal or early postnatal exposures to oil-based combustion gases, especially from engine exhausts," he noted. "The chief carcinogenic agent is probably 1,3-butadiene."
The National Institute for Occupational Safety and Health has recommended that 1,3-butadiene be treated as a potential occupational carcinogen, teratogen, and as a reproduction hazard.
In 2006, Dr. Knox published another study on the same group, in which he attributed about 24% of the cancers to prenatal or early life proximity to areas of high airborne pollutants. "Child cancer initiations are strongly determined by prenatal or early postnatal exposures to engine exhaust gases, probably through maternal inhalation and accumulation of carcinogens over many months," he noted. (J. Epidemiol. Community Health 2006;60:136-41).
Dr. Heck’s study was funded by the National Institutes of Health. She had no financial disclosures.
FROM THE AACR ANNUAL MEETING
Major finding: Children exposed prenatally to high levels of motor vehicle exhaust were 19% more likely to develop bilateral retinoblastoma and 17% more likely to develop germ cell tumors than were children with less exposure.
Data source: Case-control analysis of 3,590 children who developed cancer matched to 80,224 controls.
Disclosures: The study was funded by the National Institutes of Health. Dr. Heck had no financial disclosures.
Androgen deprivation decreases lung tumors in men with prostate cancer
WASHINGTON – Men who received androgen blockade therapy as part of prostate cancer treatment were less likely to develop a secondary lung cancer than were those who didn’t receive the hormone regimen.
The significant finding suggests that estrogen may fuel non–small cell lung cancers in both men and women, Dr. Hyunseok Kang reported at the annual meeting of the American Association for Cancer Research.
"Recent literature suggests estrogen receptors and aromatase play a role in the development of non–small cell lung cancers in women with breast cancer," Dr. Kang said. "Aromatase can convert testosterone to estrogen – which could increase the same risk in men."
This recently observed association prompted Dr. Kang, a fellow at Emory University, Atlanta, and his colleagues to investigate whether a similar hormone-mediated link might exist for men with prostate cancer – and whether blocking it could also block lung cancer.
The retrospective observational study pulled data from the Veterans Affairs Central Cancer Registry. It comprised 62,589 men who were diagnosed with prostate cancer between 1999 and 2008, with follow-up through 2010.
There were some significant – and important – baseline differences between the 20,378 men who underwent androgen blockade and those who did not. Men who received the treatment were significantly older (mean age, 71 years vs. 66 years) and slightly more likely to have ever smoked tobacco (73% vs. 71%).
Prostate cancer stage at diagnosis was also significantly different, with fewer of the hormone therapy group presenting at stage II (77% vs. 92%) and more presenting at stage IV (17% vs. 2%).
Men from the VA database were significantly more likely than the general population to develop lung cancer, whether they underwent androgen blockade therapy or not (1.8/100,000 men who received treatment and 2.4/100,000 men who did not receive treatment vs. 0.80/100,000 in the general population).
Of men in the VA database who received androgen treatment, 258 developed secondary lung cancers, compared with 508 men who did not receive treatment.
In an unadjusted model, men who had treatment were 18% less likely to develop a secondary lung cancer (hazard ratio, 0.82). That finding held in a multivariate analysis that adjusted for smoking and age (HR, 0.83).
The study was sponsored by the Veterans Health Administration. Dr. Kang had no financial disclosures.
WASHINGTON – Men who received androgen blockade therapy as part of prostate cancer treatment were less likely to develop a secondary lung cancer than were those who didn’t receive the hormone regimen.
The significant finding suggests that estrogen may fuel non–small cell lung cancers in both men and women, Dr. Hyunseok Kang reported at the annual meeting of the American Association for Cancer Research.
"Recent literature suggests estrogen receptors and aromatase play a role in the development of non–small cell lung cancers in women with breast cancer," Dr. Kang said. "Aromatase can convert testosterone to estrogen – which could increase the same risk in men."
This recently observed association prompted Dr. Kang, a fellow at Emory University, Atlanta, and his colleagues to investigate whether a similar hormone-mediated link might exist for men with prostate cancer – and whether blocking it could also block lung cancer.
The retrospective observational study pulled data from the Veterans Affairs Central Cancer Registry. It comprised 62,589 men who were diagnosed with prostate cancer between 1999 and 2008, with follow-up through 2010.
There were some significant – and important – baseline differences between the 20,378 men who underwent androgen blockade and those who did not. Men who received the treatment were significantly older (mean age, 71 years vs. 66 years) and slightly more likely to have ever smoked tobacco (73% vs. 71%).
Prostate cancer stage at diagnosis was also significantly different, with fewer of the hormone therapy group presenting at stage II (77% vs. 92%) and more presenting at stage IV (17% vs. 2%).
Men from the VA database were significantly more likely than the general population to develop lung cancer, whether they underwent androgen blockade therapy or not (1.8/100,000 men who received treatment and 2.4/100,000 men who did not receive treatment vs. 0.80/100,000 in the general population).
Of men in the VA database who received androgen treatment, 258 developed secondary lung cancers, compared with 508 men who did not receive treatment.
In an unadjusted model, men who had treatment were 18% less likely to develop a secondary lung cancer (hazard ratio, 0.82). That finding held in a multivariate analysis that adjusted for smoking and age (HR, 0.83).
The study was sponsored by the Veterans Health Administration. Dr. Kang had no financial disclosures.
WASHINGTON – Men who received androgen blockade therapy as part of prostate cancer treatment were less likely to develop a secondary lung cancer than were those who didn’t receive the hormone regimen.
The significant finding suggests that estrogen may fuel non–small cell lung cancers in both men and women, Dr. Hyunseok Kang reported at the annual meeting of the American Association for Cancer Research.
"Recent literature suggests estrogen receptors and aromatase play a role in the development of non–small cell lung cancers in women with breast cancer," Dr. Kang said. "Aromatase can convert testosterone to estrogen – which could increase the same risk in men."
This recently observed association prompted Dr. Kang, a fellow at Emory University, Atlanta, and his colleagues to investigate whether a similar hormone-mediated link might exist for men with prostate cancer – and whether blocking it could also block lung cancer.
The retrospective observational study pulled data from the Veterans Affairs Central Cancer Registry. It comprised 62,589 men who were diagnosed with prostate cancer between 1999 and 2008, with follow-up through 2010.
There were some significant – and important – baseline differences between the 20,378 men who underwent androgen blockade and those who did not. Men who received the treatment were significantly older (mean age, 71 years vs. 66 years) and slightly more likely to have ever smoked tobacco (73% vs. 71%).
Prostate cancer stage at diagnosis was also significantly different, with fewer of the hormone therapy group presenting at stage II (77% vs. 92%) and more presenting at stage IV (17% vs. 2%).
Men from the VA database were significantly more likely than the general population to develop lung cancer, whether they underwent androgen blockade therapy or not (1.8/100,000 men who received treatment and 2.4/100,000 men who did not receive treatment vs. 0.80/100,000 in the general population).
Of men in the VA database who received androgen treatment, 258 developed secondary lung cancers, compared with 508 men who did not receive treatment.
In an unadjusted model, men who had treatment were 18% less likely to develop a secondary lung cancer (hazard ratio, 0.82). That finding held in a multivariate analysis that adjusted for smoking and age (HR, 0.83).
The study was sponsored by the Veterans Health Administration. Dr. Kang had no financial disclosures.
FROM THE AACR ANNUAL MEETING
Major finding: Men with prostate cancer who underwent androgen blockade were 17% less likely to develop secondary non–small cell lung cancer than were those who didn’t have the hormone treatment.
Data source: The observational study comprised 62,589 men who were diagnosed with prostate cancer during 1998-2008, with follow-up through 2010.
Disclosures: The Veterans Health Administration sponsored the study. Dr. Kang had no financial disclosures.
One in four melanoma patients shun sunscreen
WASHINGTON – While melanoma survivors appear to be more aware of sun safety than does the general public, more than a quarter do not regularly use sunscreen.
"We know that melanoma is a malignancy prevalent in our population, and we know that for many people with melanoma, sun exposure is a major risk factor for recurrence; and sun protection may reduce their chances of getting melanoma again," Dr. Anees B. Chagpar said at the annual meeting of the American Association for Cancer Research. "Although we found that melanoma survivors did better than the general public at protecting their skin from the sun, we also found that more than a quarter of melanoma survivors never wear sunscreen. That blew my mind."
A few survivors – about 2% – even frequent tanning salons, said Dr. Chagpar, an associate professor of surgery at Yale University, New Haven, Conn.
Dr. Chagpar and her colleagues based their findings on data extracted from the 2010 National Health Interview Survey. They focused on self-reported history of melanoma, sun protection practices, and indoor tanning.
The 2010 survey included information on 27,120 adults; 171 had a history of melanoma. Of the adults included in the survey, most (55%) were men; 10% were younger than 40 years.
Compared with the general population, melanoma survivors demonstrated an overall increased rate of sun awareness. Significantly more melanoma survivors reported that they always stay in the shade, compared with the general population (16% vs. 10%, respectively). They were significantly more likely to always wear a baseball cap or visor (31% vs. 18%), a wide-brimmed hat (20% vs. 6%), and a long-sleeved shirt (12% vs. 5%) when going outside on a warm, sunny day for more than 1 hour. They were significantly more likely to report always using sunscreen (32% vs. 17%).
However, Dr. Chagpar said, a good proportion of melanoma survivors are not adequately protecting themselves from sun exposure. About 15% reported rarely or never staying in the shade, and 27% reported never wearing sunscreen.
"The bright spot in this story is that melanoma survivors are more likely to use sunscreen than nonmelanoma survivors," she said in an interview. "But when over a quarter of melanoma survivors admit that they never use sunscreen, we have considerable work to do in educating people about the importance of sun protective behaviors."
And while melanoma survivors overall were significantly less likely to use indoor tanning devices, 2% still reported having done so in the past 12 moths.
"It is distressing to know that melanoma survivors continue to tan," she said. "We have to do a better job in educating our patients about the risks of UV irradiation and the risk of developing melanoma – particularly in those who have survived the disease once already."
Dr. Chagpar had no financial disclosures.
WASHINGTON – While melanoma survivors appear to be more aware of sun safety than does the general public, more than a quarter do not regularly use sunscreen.
"We know that melanoma is a malignancy prevalent in our population, and we know that for many people with melanoma, sun exposure is a major risk factor for recurrence; and sun protection may reduce their chances of getting melanoma again," Dr. Anees B. Chagpar said at the annual meeting of the American Association for Cancer Research. "Although we found that melanoma survivors did better than the general public at protecting their skin from the sun, we also found that more than a quarter of melanoma survivors never wear sunscreen. That blew my mind."
A few survivors – about 2% – even frequent tanning salons, said Dr. Chagpar, an associate professor of surgery at Yale University, New Haven, Conn.
Dr. Chagpar and her colleagues based their findings on data extracted from the 2010 National Health Interview Survey. They focused on self-reported history of melanoma, sun protection practices, and indoor tanning.
The 2010 survey included information on 27,120 adults; 171 had a history of melanoma. Of the adults included in the survey, most (55%) were men; 10% were younger than 40 years.
Compared with the general population, melanoma survivors demonstrated an overall increased rate of sun awareness. Significantly more melanoma survivors reported that they always stay in the shade, compared with the general population (16% vs. 10%, respectively). They were significantly more likely to always wear a baseball cap or visor (31% vs. 18%), a wide-brimmed hat (20% vs. 6%), and a long-sleeved shirt (12% vs. 5%) when going outside on a warm, sunny day for more than 1 hour. They were significantly more likely to report always using sunscreen (32% vs. 17%).
However, Dr. Chagpar said, a good proportion of melanoma survivors are not adequately protecting themselves from sun exposure. About 15% reported rarely or never staying in the shade, and 27% reported never wearing sunscreen.
"The bright spot in this story is that melanoma survivors are more likely to use sunscreen than nonmelanoma survivors," she said in an interview. "But when over a quarter of melanoma survivors admit that they never use sunscreen, we have considerable work to do in educating people about the importance of sun protective behaviors."
And while melanoma survivors overall were significantly less likely to use indoor tanning devices, 2% still reported having done so in the past 12 moths.
"It is distressing to know that melanoma survivors continue to tan," she said. "We have to do a better job in educating our patients about the risks of UV irradiation and the risk of developing melanoma – particularly in those who have survived the disease once already."
Dr. Chagpar had no financial disclosures.
WASHINGTON – While melanoma survivors appear to be more aware of sun safety than does the general public, more than a quarter do not regularly use sunscreen.
"We know that melanoma is a malignancy prevalent in our population, and we know that for many people with melanoma, sun exposure is a major risk factor for recurrence; and sun protection may reduce their chances of getting melanoma again," Dr. Anees B. Chagpar said at the annual meeting of the American Association for Cancer Research. "Although we found that melanoma survivors did better than the general public at protecting their skin from the sun, we also found that more than a quarter of melanoma survivors never wear sunscreen. That blew my mind."
A few survivors – about 2% – even frequent tanning salons, said Dr. Chagpar, an associate professor of surgery at Yale University, New Haven, Conn.
Dr. Chagpar and her colleagues based their findings on data extracted from the 2010 National Health Interview Survey. They focused on self-reported history of melanoma, sun protection practices, and indoor tanning.
The 2010 survey included information on 27,120 adults; 171 had a history of melanoma. Of the adults included in the survey, most (55%) were men; 10% were younger than 40 years.
Compared with the general population, melanoma survivors demonstrated an overall increased rate of sun awareness. Significantly more melanoma survivors reported that they always stay in the shade, compared with the general population (16% vs. 10%, respectively). They were significantly more likely to always wear a baseball cap or visor (31% vs. 18%), a wide-brimmed hat (20% vs. 6%), and a long-sleeved shirt (12% vs. 5%) when going outside on a warm, sunny day for more than 1 hour. They were significantly more likely to report always using sunscreen (32% vs. 17%).
However, Dr. Chagpar said, a good proportion of melanoma survivors are not adequately protecting themselves from sun exposure. About 15% reported rarely or never staying in the shade, and 27% reported never wearing sunscreen.
"The bright spot in this story is that melanoma survivors are more likely to use sunscreen than nonmelanoma survivors," she said in an interview. "But when over a quarter of melanoma survivors admit that they never use sunscreen, we have considerable work to do in educating people about the importance of sun protective behaviors."
And while melanoma survivors overall were significantly less likely to use indoor tanning devices, 2% still reported having done so in the past 12 moths.
"It is distressing to know that melanoma survivors continue to tan," she said. "We have to do a better job in educating our patients about the risks of UV irradiation and the risk of developing melanoma – particularly in those who have survived the disease once already."
Dr. Chagpar had no financial disclosures.
FROM THE AACR ANNUAL MEETING
Major finding: About 27% of melanoma survivors reported never wearing sunscreen, and 2% reported using a tanning bed.
Data source: A database review comparing sun safety behaviors between 171 melanoma survivors and almost 27,000 people who never had the cancer.
Disclosures: Dr. Chagpar had no financial disclosures.
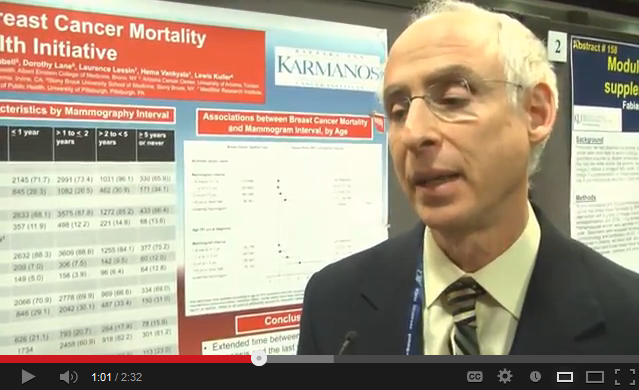
Mammography screening at 75 may have value
WASHINGTON – For women aged 75 years and older, a span of 5 or more years between mammograms translated into a threefold increased risk of death from breast cancer, compared with women in the same age group who had yearly exams.
While the exact reasons for the finding can’t be pinpointed, it should at least reawaken the discussion about whether older women should get a regular mammogram, Dr. Michael Simon noted at the annual meeting of the American Association for Cancer Research.
In 2009, the U.S. Preventive Services Task Force changed its recommendation on screening exams for women aged 75 and older, noting that there was insufficient evidence to recommend for or against breast cancer screening in this population (Ann. Intern. Med. 2009;151:716-26).
However, the absence of evidence does not automatically translate into absence of benefit, Dr. Simon said in an interview. "You can’t assume that at 75, it’s not worth screening anymore. Yes, there’s no evidence from randomized trials of women older than 75, and the recommendations are based on this kind of evidence. But just because there are no trials doesn’t mean there is no benefit."
Dr. Simon and his coinvestigators extracted data from the 15-year Women’s Health Initiative study. The cohort comprised 9,057 women who had been diagnosed with breast cancer over a 12-year period.
The multivariate analysis examined the relationship between death from breast cancer and mammography screening intervals. The screening intervals studied were from 6 months to 1 year, 1 to 2 years, 2 to 5 years, and more than 5 years or never. The study cohort was divided into women by age: 50-74 years (6,497) and 75 years or older (2,560).
Baseline characteristics showed that the older group of women presented with dangerous breast cancers almost as often as did the younger group, including moderately differentiated (22% vs. 21%, respectively) and poorly differentiated tumors (28% vs. 31%). Older women were more likely to have hormone receptor–positive tumors (75% vs. 67%), and just as likely to have hormone receptor–negative tumors (12% of each group).
Stage was also similar between the older and younger groups: in situ (18% vs. 20%, respectively); localized (62% vs. 59%); regional (17% vs. 19%); and distant (1% in both groups).
Significant differences occurred when the investigators broke the group of more than 9,000 women out by mammogram intervals.
Overall, an interval of 5 or more years between a woman’s last mammogram and breast cancer diagnosis was associated with advanced-stage disease in 23%, compared with 20% in women with an interval of 1 year or less – a statistically significant difference, which could affect large numbers of women, said Dr. Simon, head of the breast multidisciplinary team at Barbara Ann Karmanos Cancer Institute in Detroit.
"We also saw significantly more aggressive cancers that were hormone receptor negative in the group with the longest interval between mammograms," he said (22% vs. 16%).
The multivariate analysis adjusted for age at enrollment in the WHI study and the trial component in which subjects enrolled, age at diagnosis, race and ethnicity, insurance and marital status, comorbidities, and body mass index at baseline.
In this analysis, women aged 75 years and older who went at least 5 years between mammograms were 67% more likely to die from a breast cancer than were those who had yearly exams. Older women who went 5 or more years between mammograms were three times more likely to die than were those who had yearly screening, Dr. Simon said.
The results show a need for stronger emphasis on regular mammograms for older women, he said. "Women are living longer and living vitally longer. If we have a test that can identify an early cancer ... we should take advantage of it."
For women of any age, mammograms have their downsides, including anxiety while waiting for results, overdiagnosis, and treatment of indolent, in situ tumors that may never progress to serious cancers – especially in women with a shorter lifespan ahead of them. Dr. Simon said those risks are small compared with the benefit of finding and treating a potentially lethal tumor.
Cost, however, is the sticking point, admitted Dr. Simon, who is also a public health expert.
"Resource allocation is something we can’t ignore. We know that it takes 1,700 mammograms to benefit one woman. That sounds like a lot, but when you think about it in a global perspective – how many women there are in this country – you see it in a different light. What we’re paying for mammograms is small potatoes."
Dr. Simon had no financial disclosures.
WASHINGTON – For women aged 75 years and older, a span of 5 or more years between mammograms translated into a threefold increased risk of death from breast cancer, compared with women in the same age group who had yearly exams.
While the exact reasons for the finding can’t be pinpointed, it should at least reawaken the discussion about whether older women should get a regular mammogram, Dr. Michael Simon noted at the annual meeting of the American Association for Cancer Research.
In 2009, the U.S. Preventive Services Task Force changed its recommendation on screening exams for women aged 75 and older, noting that there was insufficient evidence to recommend for or against breast cancer screening in this population (Ann. Intern. Med. 2009;151:716-26).
However, the absence of evidence does not automatically translate into absence of benefit, Dr. Simon said in an interview. "You can’t assume that at 75, it’s not worth screening anymore. Yes, there’s no evidence from randomized trials of women older than 75, and the recommendations are based on this kind of evidence. But just because there are no trials doesn’t mean there is no benefit."
Dr. Simon and his coinvestigators extracted data from the 15-year Women’s Health Initiative study. The cohort comprised 9,057 women who had been diagnosed with breast cancer over a 12-year period.
The multivariate analysis examined the relationship between death from breast cancer and mammography screening intervals. The screening intervals studied were from 6 months to 1 year, 1 to 2 years, 2 to 5 years, and more than 5 years or never. The study cohort was divided into women by age: 50-74 years (6,497) and 75 years or older (2,560).
Baseline characteristics showed that the older group of women presented with dangerous breast cancers almost as often as did the younger group, including moderately differentiated (22% vs. 21%, respectively) and poorly differentiated tumors (28% vs. 31%). Older women were more likely to have hormone receptor–positive tumors (75% vs. 67%), and just as likely to have hormone receptor–negative tumors (12% of each group).
Stage was also similar between the older and younger groups: in situ (18% vs. 20%, respectively); localized (62% vs. 59%); regional (17% vs. 19%); and distant (1% in both groups).
Significant differences occurred when the investigators broke the group of more than 9,000 women out by mammogram intervals.
Overall, an interval of 5 or more years between a woman’s last mammogram and breast cancer diagnosis was associated with advanced-stage disease in 23%, compared with 20% in women with an interval of 1 year or less – a statistically significant difference, which could affect large numbers of women, said Dr. Simon, head of the breast multidisciplinary team at Barbara Ann Karmanos Cancer Institute in Detroit.
"We also saw significantly more aggressive cancers that were hormone receptor negative in the group with the longest interval between mammograms," he said (22% vs. 16%).
The multivariate analysis adjusted for age at enrollment in the WHI study and the trial component in which subjects enrolled, age at diagnosis, race and ethnicity, insurance and marital status, comorbidities, and body mass index at baseline.
In this analysis, women aged 75 years and older who went at least 5 years between mammograms were 67% more likely to die from a breast cancer than were those who had yearly exams. Older women who went 5 or more years between mammograms were three times more likely to die than were those who had yearly screening, Dr. Simon said.
The results show a need for stronger emphasis on regular mammograms for older women, he said. "Women are living longer and living vitally longer. If we have a test that can identify an early cancer ... we should take advantage of it."
For women of any age, mammograms have their downsides, including anxiety while waiting for results, overdiagnosis, and treatment of indolent, in situ tumors that may never progress to serious cancers – especially in women with a shorter lifespan ahead of them. Dr. Simon said those risks are small compared with the benefit of finding and treating a potentially lethal tumor.
Cost, however, is the sticking point, admitted Dr. Simon, who is also a public health expert.
"Resource allocation is something we can’t ignore. We know that it takes 1,700 mammograms to benefit one woman. That sounds like a lot, but when you think about it in a global perspective – how many women there are in this country – you see it in a different light. What we’re paying for mammograms is small potatoes."
Dr. Simon had no financial disclosures.
WASHINGTON – For women aged 75 years and older, a span of 5 or more years between mammograms translated into a threefold increased risk of death from breast cancer, compared with women in the same age group who had yearly exams.
While the exact reasons for the finding can’t be pinpointed, it should at least reawaken the discussion about whether older women should get a regular mammogram, Dr. Michael Simon noted at the annual meeting of the American Association for Cancer Research.
In 2009, the U.S. Preventive Services Task Force changed its recommendation on screening exams for women aged 75 and older, noting that there was insufficient evidence to recommend for or against breast cancer screening in this population (Ann. Intern. Med. 2009;151:716-26).
However, the absence of evidence does not automatically translate into absence of benefit, Dr. Simon said in an interview. "You can’t assume that at 75, it’s not worth screening anymore. Yes, there’s no evidence from randomized trials of women older than 75, and the recommendations are based on this kind of evidence. But just because there are no trials doesn’t mean there is no benefit."
Dr. Simon and his coinvestigators extracted data from the 15-year Women’s Health Initiative study. The cohort comprised 9,057 women who had been diagnosed with breast cancer over a 12-year period.
The multivariate analysis examined the relationship between death from breast cancer and mammography screening intervals. The screening intervals studied were from 6 months to 1 year, 1 to 2 years, 2 to 5 years, and more than 5 years or never. The study cohort was divided into women by age: 50-74 years (6,497) and 75 years or older (2,560).
Baseline characteristics showed that the older group of women presented with dangerous breast cancers almost as often as did the younger group, including moderately differentiated (22% vs. 21%, respectively) and poorly differentiated tumors (28% vs. 31%). Older women were more likely to have hormone receptor–positive tumors (75% vs. 67%), and just as likely to have hormone receptor–negative tumors (12% of each group).
Stage was also similar between the older and younger groups: in situ (18% vs. 20%, respectively); localized (62% vs. 59%); regional (17% vs. 19%); and distant (1% in both groups).
Significant differences occurred when the investigators broke the group of more than 9,000 women out by mammogram intervals.
Overall, an interval of 5 or more years between a woman’s last mammogram and breast cancer diagnosis was associated with advanced-stage disease in 23%, compared with 20% in women with an interval of 1 year or less – a statistically significant difference, which could affect large numbers of women, said Dr. Simon, head of the breast multidisciplinary team at Barbara Ann Karmanos Cancer Institute in Detroit.
"We also saw significantly more aggressive cancers that were hormone receptor negative in the group with the longest interval between mammograms," he said (22% vs. 16%).
The multivariate analysis adjusted for age at enrollment in the WHI study and the trial component in which subjects enrolled, age at diagnosis, race and ethnicity, insurance and marital status, comorbidities, and body mass index at baseline.
In this analysis, women aged 75 years and older who went at least 5 years between mammograms were 67% more likely to die from a breast cancer than were those who had yearly exams. Older women who went 5 or more years between mammograms were three times more likely to die than were those who had yearly screening, Dr. Simon said.
The results show a need for stronger emphasis on regular mammograms for older women, he said. "Women are living longer and living vitally longer. If we have a test that can identify an early cancer ... we should take advantage of it."
For women of any age, mammograms have their downsides, including anxiety while waiting for results, overdiagnosis, and treatment of indolent, in situ tumors that may never progress to serious cancers – especially in women with a shorter lifespan ahead of them. Dr. Simon said those risks are small compared with the benefit of finding and treating a potentially lethal tumor.
Cost, however, is the sticking point, admitted Dr. Simon, who is also a public health expert.
"Resource allocation is something we can’t ignore. We know that it takes 1,700 mammograms to benefit one woman. That sounds like a lot, but when you think about it in a global perspective – how many women there are in this country – you see it in a different light. What we’re paying for mammograms is small potatoes."
Dr. Simon had no financial disclosures.
AT THE AACR ANNUAL MEETING
Major finding: Women aged 75 years and older were 3 times more likely to die from breast cancer if they went 5 or more years between mammograms, compared with those who had a yearly screening.
Data source: Women’s Health Initiative data on more than 9,000 women diagnosed with breast cancer.
Disclosures: Dr. Simon had no financial disclosures.
Rally seeks to restore funding for biomedical research
WASHINGTON – Flat funding over the last decade combined with inflation has effectively cut 20% out of the research budget for the National Institutes of Health – and now another 5% is gone thanks to the federal budget sequester.
Cancer researchers, practicing oncologists, advocates, and congressional supporters took a break from the annual meeting of the American Association for Cancer Research to protest the long and lingering decline in funding. They were joined at the Rally for Medical Research by representatives from at least 200 partner organizations involved in research, patient care, or advocacy for diabetes, heart disease, stroke, Alzheimer’s disease, and AIDS, among other conditions.
Even President Obama chimed in via a message read by Dr. Margaret Foti, AACR chief executive officer.
"By investing in the best ideas and supporting the work of our scientists, we will improve health and change lives in ways we could have never imagined," read Dr. Foti from the president’s remarks.
In her own remarks, Dr. Foti said that just showing up was important to the greater goal. "By participating in today’s rally, you are taking a very important step to ensure that America will continue to lead the world in medical research," she said.
The rally focused on the cuts to funding for the 27 institutes and centers at the National Institutes of Health. The NIH is currently funded at $30.6 billion, which makes it biggest supporter of medical research in the world, according to rally organizers. But, they said, NIH appropriations have been flat since 2003. Factoring in biomedical inflation, the agency has effectively lost approximately $6 billion, or 20% of its purchasing power over that same time period. Sequestration threatens another 5%, or $5 billion cut.
Rep. Rosa DeLauro (D-Conn.), an ovarian cancer survivor, said at the rally that biomedical research helps grow the economy. "Every dollar that goes to the NIH results in $2 of business activity," she said, calling on rally participants to visit their congressional delegations and urge them to take money from other programs to balance the budget.
Rep. Chris Van Hollen (D-Md.), is whose district NIH is headquartered, said that continued cuts to biomedical funding could deter young people from pursuing careers in science.
"We should not be retreating on medical research, we should be redoubling our efforts," said Rep. Van Hollen, who serves as ranking member on the House Budget Committee.
Rep. Van Hollen said he hoped the rally would bring a sharper focus to the impact of the sequester. "The more public attention we draw to this issue, the better chance we’ll have at replacing the sequester," he said in an interview. "This is one area that has consequences for people of every political persuasion."
The sequester cuts are being felt already, according to Candace Johnson, Ph.D., deputy director of the Roswell Park Cancer Institute, Buffalo, N.Y. The institute could lose at least $8 million from its overall budget of $550 million, but the cuts will fall disproportionately on the research programs, Dr. Johnson said in an interview. And there’s continued uncertainty with the sequester – it is possible that some grants will be cut even more than anticipated. "We’re in the dark here," she said.
Cutting research will also have an effect on clinical care, Dr. Johnson said. "When you cut basic science, you cut that road to better and more innovative clinical care. If you don’t have basic science, you don’t have cancer centers doing basic discovery, then you don’t have a phase I program."
Dr. Sandra Swain, president of the American Society of Clinical Oncology, said that sustained and predictable funding increases were crucial to progress. And she noted, there’s no substitute for federal funds. "While private industry is a strong partner in cancer research, public funding is essential to pursue the types of research in which industry may have no interest," she said, in a statement. "This is particularly true for rare diseases, combinations of different companies’ products, therapies with multiple treatment modalities (such as surgery, radiation, and chemotherapy), and direct comparisons of company products," Dr. Swain said.
ASCO also sounded a note of caution about the budget for the Food and Drug Administration, which is also cut under sequestration. ASCO "is concerned that sequestration will cause slower approval of new and potentially life-saving drugs, difficulty in meeting challenges in monitoring of food and drug safety, and an inability to keep up with advancing science and technology," Dr. Swain said.
On Twitter @aliciaault
WASHINGTON – Flat funding over the last decade combined with inflation has effectively cut 20% out of the research budget for the National Institutes of Health – and now another 5% is gone thanks to the federal budget sequester.
Cancer researchers, practicing oncologists, advocates, and congressional supporters took a break from the annual meeting of the American Association for Cancer Research to protest the long and lingering decline in funding. They were joined at the Rally for Medical Research by representatives from at least 200 partner organizations involved in research, patient care, or advocacy for diabetes, heart disease, stroke, Alzheimer’s disease, and AIDS, among other conditions.
Even President Obama chimed in via a message read by Dr. Margaret Foti, AACR chief executive officer.
"By investing in the best ideas and supporting the work of our scientists, we will improve health and change lives in ways we could have never imagined," read Dr. Foti from the president’s remarks.
In her own remarks, Dr. Foti said that just showing up was important to the greater goal. "By participating in today’s rally, you are taking a very important step to ensure that America will continue to lead the world in medical research," she said.
The rally focused on the cuts to funding for the 27 institutes and centers at the National Institutes of Health. The NIH is currently funded at $30.6 billion, which makes it biggest supporter of medical research in the world, according to rally organizers. But, they said, NIH appropriations have been flat since 2003. Factoring in biomedical inflation, the agency has effectively lost approximately $6 billion, or 20% of its purchasing power over that same time period. Sequestration threatens another 5%, or $5 billion cut.
Rep. Rosa DeLauro (D-Conn.), an ovarian cancer survivor, said at the rally that biomedical research helps grow the economy. "Every dollar that goes to the NIH results in $2 of business activity," she said, calling on rally participants to visit their congressional delegations and urge them to take money from other programs to balance the budget.
Rep. Chris Van Hollen (D-Md.), is whose district NIH is headquartered, said that continued cuts to biomedical funding could deter young people from pursuing careers in science.
"We should not be retreating on medical research, we should be redoubling our efforts," said Rep. Van Hollen, who serves as ranking member on the House Budget Committee.
Rep. Van Hollen said he hoped the rally would bring a sharper focus to the impact of the sequester. "The more public attention we draw to this issue, the better chance we’ll have at replacing the sequester," he said in an interview. "This is one area that has consequences for people of every political persuasion."
The sequester cuts are being felt already, according to Candace Johnson, Ph.D., deputy director of the Roswell Park Cancer Institute, Buffalo, N.Y. The institute could lose at least $8 million from its overall budget of $550 million, but the cuts will fall disproportionately on the research programs, Dr. Johnson said in an interview. And there’s continued uncertainty with the sequester – it is possible that some grants will be cut even more than anticipated. "We’re in the dark here," she said.
Cutting research will also have an effect on clinical care, Dr. Johnson said. "When you cut basic science, you cut that road to better and more innovative clinical care. If you don’t have basic science, you don’t have cancer centers doing basic discovery, then you don’t have a phase I program."
Dr. Sandra Swain, president of the American Society of Clinical Oncology, said that sustained and predictable funding increases were crucial to progress. And she noted, there’s no substitute for federal funds. "While private industry is a strong partner in cancer research, public funding is essential to pursue the types of research in which industry may have no interest," she said, in a statement. "This is particularly true for rare diseases, combinations of different companies’ products, therapies with multiple treatment modalities (such as surgery, radiation, and chemotherapy), and direct comparisons of company products," Dr. Swain said.
ASCO also sounded a note of caution about the budget for the Food and Drug Administration, which is also cut under sequestration. ASCO "is concerned that sequestration will cause slower approval of new and potentially life-saving drugs, difficulty in meeting challenges in monitoring of food and drug safety, and an inability to keep up with advancing science and technology," Dr. Swain said.
On Twitter @aliciaault
WASHINGTON – Flat funding over the last decade combined with inflation has effectively cut 20% out of the research budget for the National Institutes of Health – and now another 5% is gone thanks to the federal budget sequester.
Cancer researchers, practicing oncologists, advocates, and congressional supporters took a break from the annual meeting of the American Association for Cancer Research to protest the long and lingering decline in funding. They were joined at the Rally for Medical Research by representatives from at least 200 partner organizations involved in research, patient care, or advocacy for diabetes, heart disease, stroke, Alzheimer’s disease, and AIDS, among other conditions.
Even President Obama chimed in via a message read by Dr. Margaret Foti, AACR chief executive officer.
"By investing in the best ideas and supporting the work of our scientists, we will improve health and change lives in ways we could have never imagined," read Dr. Foti from the president’s remarks.
In her own remarks, Dr. Foti said that just showing up was important to the greater goal. "By participating in today’s rally, you are taking a very important step to ensure that America will continue to lead the world in medical research," she said.
The rally focused on the cuts to funding for the 27 institutes and centers at the National Institutes of Health. The NIH is currently funded at $30.6 billion, which makes it biggest supporter of medical research in the world, according to rally organizers. But, they said, NIH appropriations have been flat since 2003. Factoring in biomedical inflation, the agency has effectively lost approximately $6 billion, or 20% of its purchasing power over that same time period. Sequestration threatens another 5%, or $5 billion cut.
Rep. Rosa DeLauro (D-Conn.), an ovarian cancer survivor, said at the rally that biomedical research helps grow the economy. "Every dollar that goes to the NIH results in $2 of business activity," she said, calling on rally participants to visit their congressional delegations and urge them to take money from other programs to balance the budget.
Rep. Chris Van Hollen (D-Md.), is whose district NIH is headquartered, said that continued cuts to biomedical funding could deter young people from pursuing careers in science.
"We should not be retreating on medical research, we should be redoubling our efforts," said Rep. Van Hollen, who serves as ranking member on the House Budget Committee.
Rep. Van Hollen said he hoped the rally would bring a sharper focus to the impact of the sequester. "The more public attention we draw to this issue, the better chance we’ll have at replacing the sequester," he said in an interview. "This is one area that has consequences for people of every political persuasion."
The sequester cuts are being felt already, according to Candace Johnson, Ph.D., deputy director of the Roswell Park Cancer Institute, Buffalo, N.Y. The institute could lose at least $8 million from its overall budget of $550 million, but the cuts will fall disproportionately on the research programs, Dr. Johnson said in an interview. And there’s continued uncertainty with the sequester – it is possible that some grants will be cut even more than anticipated. "We’re in the dark here," she said.
Cutting research will also have an effect on clinical care, Dr. Johnson said. "When you cut basic science, you cut that road to better and more innovative clinical care. If you don’t have basic science, you don’t have cancer centers doing basic discovery, then you don’t have a phase I program."
Dr. Sandra Swain, president of the American Society of Clinical Oncology, said that sustained and predictable funding increases were crucial to progress. And she noted, there’s no substitute for federal funds. "While private industry is a strong partner in cancer research, public funding is essential to pursue the types of research in which industry may have no interest," she said, in a statement. "This is particularly true for rare diseases, combinations of different companies’ products, therapies with multiple treatment modalities (such as surgery, radiation, and chemotherapy), and direct comparisons of company products," Dr. Swain said.
ASCO also sounded a note of caution about the budget for the Food and Drug Administration, which is also cut under sequestration. ASCO "is concerned that sequestration will cause slower approval of new and potentially life-saving drugs, difficulty in meeting challenges in monitoring of food and drug safety, and an inability to keep up with advancing science and technology," Dr. Swain said.
On Twitter @aliciaault
AT THE AACR ANNUAL MEETING
Immunotherapy boosts response in recurrent ovarian cancer
WASHINGTON – A novel ovarian cancer treatment that uses the patient’s own tumor to stimulate her immune system provoked a positive response in 66% of women with advanced stage disease – including 20 patients who had no obvious disease at the end of treatment, and one who is still in complete remission nearly 4 years later.
When researchers added a second step of T-cell reprogramming for patients with residual disease, clinical benefit was seen in about 75% of patients, Lana Kandalaft, Ph.D., said during a press briefing at the annual meeting of the American Association of Cancer Research.
"This is the first time such a combination immunotherapy approach has been used for patients with ovarian cancer," said Dr. Kandalaft of the University of Pennsylvania. "Most patients with ovarian cancer are diagnosed at an advanced stage and many of those relapse within 2 years; most die within 5 years. Given these grim outcomes, there is definitely a vast unmet need for the development of novel, alternate therapies."
The team reported on its first 6 patients in January (Oncoimmunology. 2013;2: e22664). Four patients achieved clinical benefits, defined as tumor shrinkage of at least 30%. Two had a partial response; one with a partial response had previously progressed on chemotherapy alone but improved after the vaccine was added to her treatment, suggesting that immunomodulation conferred an additional therapeutic benefit.
Two other subjects exhibited stable disease. One of these still in remission. The last two patients continued to progress.
At the press briefing, Dr. Kandalaft reported on 31 patients – the original cohort plus 25 more women. All had recurrent progressive stage III or IV ovarian cancer and had undergone surgical reduction with tumor cryopreservation. The immunotherapy regimen began after each patient completed chemotherapy.
At that point, Dr. Kandalaft and her colleagues prepared a tumor lysate and used it to prime harvested dendritic cells. Patients received this individualized vaccine through intranodal injections in the groin, along with bevacizumab and cyclophosphamide, every 2 weeks for 3 months.
Eleven patients who responded to the vaccine treatment but still had residual disease moved to a second step: 3 months of adoptive T-cell therapy. Dr. Kandalaft and her colleagues removed T-cells, which had been educated in the body by the tumor-stimulated dendritic cells, and expanded these in culture. They then transferred the T-cells back to the patient, creating a population completely primed to attack the tumor.
Of these 11 patients, seven showed stable disease and one had a complete response.
Dr. Kandalaft noted that there is set length for this treatment, because each patient’s vaccine amount is limited by her tumor’s size and characteristics. During the follow-up period, patients receive the treatment until their supply is exhausted. The woman who remains in remission has been without vaccine for about a year, Dr. Kandalaft noted.
The team continues to refine the protocol. Some patients are now taking aspirin in addition to bevacizumab and cyclophosphamide. "We have some evidence from the lab that aspirin opens the endothelial barrier on the tumor and makes it easier for the T cells to attack."
Side effects are common, but so far have been mild or moderate; most were flu-like symptoms as well as the adverse events usually associated with bevacizumab and cyclophosphamide.
The study continues to accrue patients, Dr. Kandalaft said. Although other facilities are not approved to administer the vaccine, women with recurrent stage III or IV ovarian cancer who meet the eligibility criteria can still participate.
"These women would have their surgery at their own facility, and have the tumor cryopreserved according to our specifications, and then sent to us," Dr. Kandalaft said. "Then we would prepare the lysate and the woman would come to us for the remainder of her treatment.
She hopes to include a new set of patients as well – those who are in early remission after their primary treatment. "The immune system is healthier in this setting," she said. "For these women we will be trying to boost that system to prevent a recurrence in the first place. We think that is population who would get the biggest benefit from this treatment."
The study was funded by a National Cancer Institute Ovarian Specialized Program of Research Excellence grant, the National Institutes of Health and the Ovarian Cancer Immunotherapy Initiative.
WASHINGTON – A novel ovarian cancer treatment that uses the patient’s own tumor to stimulate her immune system provoked a positive response in 66% of women with advanced stage disease – including 20 patients who had no obvious disease at the end of treatment, and one who is still in complete remission nearly 4 years later.
When researchers added a second step of T-cell reprogramming for patients with residual disease, clinical benefit was seen in about 75% of patients, Lana Kandalaft, Ph.D., said during a press briefing at the annual meeting of the American Association of Cancer Research.
"This is the first time such a combination immunotherapy approach has been used for patients with ovarian cancer," said Dr. Kandalaft of the University of Pennsylvania. "Most patients with ovarian cancer are diagnosed at an advanced stage and many of those relapse within 2 years; most die within 5 years. Given these grim outcomes, there is definitely a vast unmet need for the development of novel, alternate therapies."
The team reported on its first 6 patients in January (Oncoimmunology. 2013;2: e22664). Four patients achieved clinical benefits, defined as tumor shrinkage of at least 30%. Two had a partial response; one with a partial response had previously progressed on chemotherapy alone but improved after the vaccine was added to her treatment, suggesting that immunomodulation conferred an additional therapeutic benefit.
Two other subjects exhibited stable disease. One of these still in remission. The last two patients continued to progress.
At the press briefing, Dr. Kandalaft reported on 31 patients – the original cohort plus 25 more women. All had recurrent progressive stage III or IV ovarian cancer and had undergone surgical reduction with tumor cryopreservation. The immunotherapy regimen began after each patient completed chemotherapy.
At that point, Dr. Kandalaft and her colleagues prepared a tumor lysate and used it to prime harvested dendritic cells. Patients received this individualized vaccine through intranodal injections in the groin, along with bevacizumab and cyclophosphamide, every 2 weeks for 3 months.
Eleven patients who responded to the vaccine treatment but still had residual disease moved to a second step: 3 months of adoptive T-cell therapy. Dr. Kandalaft and her colleagues removed T-cells, which had been educated in the body by the tumor-stimulated dendritic cells, and expanded these in culture. They then transferred the T-cells back to the patient, creating a population completely primed to attack the tumor.
Of these 11 patients, seven showed stable disease and one had a complete response.
Dr. Kandalaft noted that there is set length for this treatment, because each patient’s vaccine amount is limited by her tumor’s size and characteristics. During the follow-up period, patients receive the treatment until their supply is exhausted. The woman who remains in remission has been without vaccine for about a year, Dr. Kandalaft noted.
The team continues to refine the protocol. Some patients are now taking aspirin in addition to bevacizumab and cyclophosphamide. "We have some evidence from the lab that aspirin opens the endothelial barrier on the tumor and makes it easier for the T cells to attack."
Side effects are common, but so far have been mild or moderate; most were flu-like symptoms as well as the adverse events usually associated with bevacizumab and cyclophosphamide.
The study continues to accrue patients, Dr. Kandalaft said. Although other facilities are not approved to administer the vaccine, women with recurrent stage III or IV ovarian cancer who meet the eligibility criteria can still participate.
"These women would have their surgery at their own facility, and have the tumor cryopreserved according to our specifications, and then sent to us," Dr. Kandalaft said. "Then we would prepare the lysate and the woman would come to us for the remainder of her treatment.
She hopes to include a new set of patients as well – those who are in early remission after their primary treatment. "The immune system is healthier in this setting," she said. "For these women we will be trying to boost that system to prevent a recurrence in the first place. We think that is population who would get the biggest benefit from this treatment."
The study was funded by a National Cancer Institute Ovarian Specialized Program of Research Excellence grant, the National Institutes of Health and the Ovarian Cancer Immunotherapy Initiative.
WASHINGTON – A novel ovarian cancer treatment that uses the patient’s own tumor to stimulate her immune system provoked a positive response in 66% of women with advanced stage disease – including 20 patients who had no obvious disease at the end of treatment, and one who is still in complete remission nearly 4 years later.
When researchers added a second step of T-cell reprogramming for patients with residual disease, clinical benefit was seen in about 75% of patients, Lana Kandalaft, Ph.D., said during a press briefing at the annual meeting of the American Association of Cancer Research.
"This is the first time such a combination immunotherapy approach has been used for patients with ovarian cancer," said Dr. Kandalaft of the University of Pennsylvania. "Most patients with ovarian cancer are diagnosed at an advanced stage and many of those relapse within 2 years; most die within 5 years. Given these grim outcomes, there is definitely a vast unmet need for the development of novel, alternate therapies."
The team reported on its first 6 patients in January (Oncoimmunology. 2013;2: e22664). Four patients achieved clinical benefits, defined as tumor shrinkage of at least 30%. Two had a partial response; one with a partial response had previously progressed on chemotherapy alone but improved after the vaccine was added to her treatment, suggesting that immunomodulation conferred an additional therapeutic benefit.
Two other subjects exhibited stable disease. One of these still in remission. The last two patients continued to progress.
At the press briefing, Dr. Kandalaft reported on 31 patients – the original cohort plus 25 more women. All had recurrent progressive stage III or IV ovarian cancer and had undergone surgical reduction with tumor cryopreservation. The immunotherapy regimen began after each patient completed chemotherapy.
At that point, Dr. Kandalaft and her colleagues prepared a tumor lysate and used it to prime harvested dendritic cells. Patients received this individualized vaccine through intranodal injections in the groin, along with bevacizumab and cyclophosphamide, every 2 weeks for 3 months.
Eleven patients who responded to the vaccine treatment but still had residual disease moved to a second step: 3 months of adoptive T-cell therapy. Dr. Kandalaft and her colleagues removed T-cells, which had been educated in the body by the tumor-stimulated dendritic cells, and expanded these in culture. They then transferred the T-cells back to the patient, creating a population completely primed to attack the tumor.
Of these 11 patients, seven showed stable disease and one had a complete response.
Dr. Kandalaft noted that there is set length for this treatment, because each patient’s vaccine amount is limited by her tumor’s size and characteristics. During the follow-up period, patients receive the treatment until their supply is exhausted. The woman who remains in remission has been without vaccine for about a year, Dr. Kandalaft noted.
The team continues to refine the protocol. Some patients are now taking aspirin in addition to bevacizumab and cyclophosphamide. "We have some evidence from the lab that aspirin opens the endothelial barrier on the tumor and makes it easier for the T cells to attack."
Side effects are common, but so far have been mild or moderate; most were flu-like symptoms as well as the adverse events usually associated with bevacizumab and cyclophosphamide.
The study continues to accrue patients, Dr. Kandalaft said. Although other facilities are not approved to administer the vaccine, women with recurrent stage III or IV ovarian cancer who meet the eligibility criteria can still participate.
"These women would have their surgery at their own facility, and have the tumor cryopreserved according to our specifications, and then sent to us," Dr. Kandalaft said. "Then we would prepare the lysate and the woman would come to us for the remainder of her treatment.
She hopes to include a new set of patients as well – those who are in early remission after their primary treatment. "The immune system is healthier in this setting," she said. "For these women we will be trying to boost that system to prevent a recurrence in the first place. We think that is population who would get the biggest benefit from this treatment."
The study was funded by a National Cancer Institute Ovarian Specialized Program of Research Excellence grant, the National Institutes of Health and the Ovarian Cancer Immunotherapy Initiative.
AT THE AACR ANNUAL MEETING
Major finding: A two-step immunotherapy regimen combined with chemotherapy had up to a 75% response rate for women with recurrent stage III or IV ovarian cancer.
Data source: The study comprised 31 women who underwent dendritic cell priming with tumor lysate; 11 of these also underwent T-cell reprogramming.
Disclosures: The study was funded by a National Cancer Institute Ovarian Specialized Program of Research Excellence grant, the National Institutes of Health, and the Ovarian Cancer Immunotherapy Initiative.