User login
Gorham Disease
Take-Home Points
- Gorham disease is a rare condition that manifests as an acute, spontaneous osteolysis.
- There is no clear hereditary pattern of transmission. Bones of any type or location can be affected.
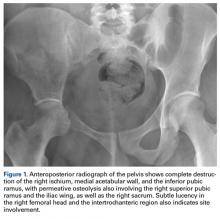
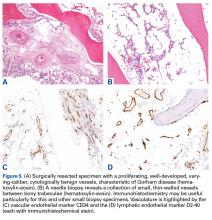
- Imaging studies are nonspecific, but show permeative osteolysis involving the subcortical and intramedullary regions and typically affect regional, contiguous bones, without adjacent sclerosis, somewhat resembling osteoporosis.
- Tissue biopsy is indicated to rule out other potential etiologies of osteolysis, and the histologic findings help confirm a diagnosis of Gorham disease.
- There is no single or combined treatment modality that is considered as the gold standard. Surgical treatment includes resection of the lesion and reconstruction. Also, antiosteoclastic medication can be used.
Gorham disease, a rare condition of unknown etiology, manifests as acute, spontaneous osteolysis associated with benign hemangiomatosis or lymphangiomatosis, which presents as skeletal lucency on radiographs, prompting the classic eponym of vanishing bone disease.1-6 There is no evidence supporting the idea that osteoclasts are present in any meaningful amount in the resorption areas or that local reparative osteogenesis occurs.4,6
Jackson and colleagues first described idiopathic osteolysis in 1838,1,2 and Gorham and Stout3 introduced the syndrome to the orthopedic community in 1955. Since then, few strides have been made in identifying the disease origin.1,2,4 Diagnosis is possible only after meticulous work-up has excluded neoplastic and infectious etiologies.7,8
Clinical Presentation
Gorham disease affects patients ranging widely in age, from 2 months to 78 years, but typically presents in those under 40 years. There is a questionable predilection for males but no correlation with ethnicity or geographic region. There is no clear hereditary pattern of transmission.7 Although the bones of the head, neck, and upper extremities are involved in most cases, bone of any type or location can be affected.6 Pelvic bones seem to be involved least often.6,7
Initial clinical presentation varies considerably but typically involves prolonged soreness in the affected region and, rarely, acute pathologic fracture.1,2,4 The nonspecific nature of complaints, lack of markers of systemic illness, and rarity of the disease contribute to delayed diagnosis.1,2
Imaging
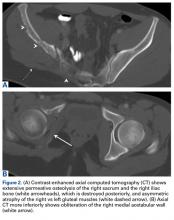
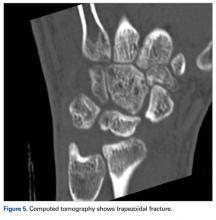
Computed tomography (CT) better defines the severity and extent of these changes.
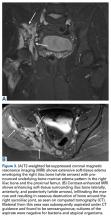
Magnetic resonance imaging shows an infiltrative and irregular T2 hyperintense signal throughout regions of bone affected by osteolysis, but this finding is not characteristic. There is heterogeneous enhancement on postcontrast sequences, and, though masslike enhancement is absent, signal abnormalities may extend into adjacent soft tissues.
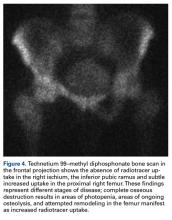
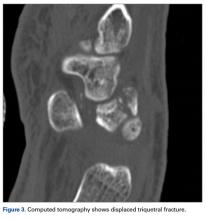
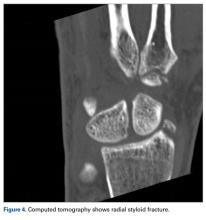
Bone scintigraphy using technetium-99m is similarly nonspecific, typically revealing radiotracer uptake that is consistent with bony reaction to an underlying osteolytic process (Figure 4) but turning negative with ongoing resorption.
Positron emission tomography/CT typically shows foci of increased metabolic activity in the areas of osteolysis.10
Diagnosis
There have been 8 histologic and clinical criteria described to diagnose Gorham disease: (1) biopsy positive for presence of angiomatous tissue, (2) complete absence of any cellular atypia, (3) lack of osteoclastic response and lack of dystrophic calcifications, (4) evidence of progressive resorption of native bone, (5) no evidence of expansive or ulcerative lesion, (6) lack of visceral involvement, (7) osteolytic radiographic pattern, and (8) no concrete diagnosis after hereditary, metabolic, neoplastic, immunologic, and infectious work-up.4-6 These criteria confirm that the diagnosis can be rendered only after exclusion of neoplastic and infectious etiologies through clinical and laboratory work-up, imaging studies, and tissue sampling.
Tissue biopsy is indicated to rule out other potential etiologies of osteolysis, and the histologic findings help confirm a diagnosis of Gorham disease.
The differential diagnosis includes infection (osteomyelitis, Brodie abscess), benign tumors (eosinophilic granuloma/Langerhans cell histiocytosis), malignant tumors (Ewing sarcoma and angiosarcoma), inflammatory conditions (eg, apatite- associated destructive arthritis), endocrine disorders (eg, osteolytic hyperparathyroidism), benign non-neoplastic conditions (venous or venolymphatic malformation), and other syndromes that present with osteolysis.1,2 Nevertheless, progressive and unusually substantial bone destruction without evidence of repair is almost pathognomonic for Gorham disease.9
Treatment
Surgical treatment usually includes lesion resection and subsequent reconstruction using combinations of bone grafts (allogenic) and prostheses. Bone graft alone is quickly resorbed and has not been found to be beneficial.1,2,4,20
1. Saify FY, Gosavi SR. Gorham’s disease: a diagnostic challenge. J Oral Maxillofac Pathol. 2014;18(3):411-414.
2. Patel DV. Gorham’s disease or massive osteolysis. Clin Med Res. 2005;3(2):65-74.
3. Gorham LW, Stout AP. Massive osteolysis (acute spontaneous absorption of bone, phantom bone, disappearing bone); its relation to hemangiomatosis. J Bone Joint Surg Am. 1955;37(5):985-1004.
4. Heffez L, Doku HC, Carter BL, Feeney JE. Perspectives on massive osteolysis. Report of a case and review of the literature. Oral Surg Oral Med Oral Pathol. 1983;55(4):331-343.
5. Gulati U, Mohanty S, Dabas J, Chandra N. “Vanishing bone disease” in maxillofacial region: a review and our experience. J Maxillofac Oral Surg. 2015;14(3):548-557.
6. Nikolaou VS, Chytas D, Korres D, Efstathopoulos N. Vanishing bone disease (Gorham-Stout syndrome): a review of a rare entity. World J Orthop. 2014;5(5):694-698.
7. Möller G, Priemel M, Amling M, Werner M, Kuhlmey AS, Delling G. The Gorham-Stout syndrome (Gorham’s massive osteolysis). A report of six cases with histopathological findings. J Bone Joint Surg Br. 1999;81(3):501-506.
8. Dominguez R, Washowich TL. Gorham’s disease or vanishing bone disease: plain film, CT, and MRI findings of two cases. Pediatr Radiol. 1994;24(5):316-318.
9. Kotecha R, Mascarenhas L, Jackson HA, Venkatramani R. Radiological features of Gorham’s disease. Clin Radiol. 2012;67(8):782-788.
10. Dong A, Bai Y, Wang Y, Zuo C. Bone scan, MRI, and FDG PET/CT findings in composite hemangioendothelioma of the manubrium sterni. Clin Nucl Med. 2014;39(2):e180-e183.
11. Baulieu F, De Pinieux G, Maruani A, Vaillant L, Lorette G. Serial lymphoscintigraphic findings in a patient with Gorham’s disease with lymphedema. Lymphology. 2014;47(3):118-122.
12. Manisali M, Ozaksoy D. Gorham disease: correlation of MR findings with histopathologic changes. Eur Radiol. 1998;8(9):1647-1650.
13. Brodszki N, Länsberg JK, Dictor M, et al. A novel treatment approach for paediatric Gorham-Stout syndrome with chylothorax. Acta Paediatr. 2011;100(11):1448-1453.
14. Nir V, Guralnik L, Livnat G, et al. Propranolol as a treatment option in Gorham-Stout syndrome: a case report. Pediatr Pulmonol. 2014;49(4):417-419.
15. Fontanesi J. Radiation therapy in the treatment of Gorham disease. J Pediatr Hematol. 2003;25(10):816-817.
16. Pfleger A, Schwinger W, Maier A, Tauss J, Popper HH, Zach MS. Gorham-Stout syndrome in a male adolescent—case report and review of the literature. J Pediatr Hematol Oncol. 2006;28(4):231-233.
17. Patrick JH. Massive osteolysis complicated by chylothorax successfully treated by pleurodesis. J Bone Joint Surg Br. 1976;58(3):347-349.
18. Hagberg H, Lamberg K, Åström G. α-2b interferon and oral clodronate for Gorham’s disease. Lancet. 1997;350(9094):1822-1823.
19. Takahashi A, Ogawa C, Kanazawa T, et al. Remission induced by interferon alfa in a patient with massive osteolysis and extension of lymph-hemangiomatosis: a severe case of Gorham-Stout syndrome. J Pediatr Surg. 2005;40(3):E47-E50.
20. Paley MD, Lloyd CJ, Penfold CN. Total mandibular reconstruction for massive osteolysis of the mandible (Gorham-Stout syndrome). Br J Oral Maxillofac Surg. 2005;43(2):166-168.
21. Avelar RL, Martins VB, Antunes AA, de Oliveira Neto PJ, de Souza Andrade ES. Use of zoledronic acid in the treatment of Gorham’s disease. Int J Pediatr Otorhinolaryngol. 2010;74(3):319-322.
22. Holroyd I, Dillon M, Roberts GJ. Gorham’s disease: a case (including dental presentation) of vanishing bone disease. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89(1):125-129.
23. Lee S, Finn L, Sze RW, Perkins JA, Sie KC. Gorham Stout syndrome (disappearing bone disease): two additional case reports and a review of the literature. Arch Otolaryngol Head Neck Surg. 2003;129(12):1340-1343.
Take-Home Points
- Gorham disease is a rare condition that manifests as an acute, spontaneous osteolysis.
- There is no clear hereditary pattern of transmission. Bones of any type or location can be affected.
- Imaging studies are nonspecific, but show permeative osteolysis involving the subcortical and intramedullary regions and typically affect regional, contiguous bones, without adjacent sclerosis, somewhat resembling osteoporosis.
- Tissue biopsy is indicated to rule out other potential etiologies of osteolysis, and the histologic findings help confirm a diagnosis of Gorham disease.
- There is no single or combined treatment modality that is considered as the gold standard. Surgical treatment includes resection of the lesion and reconstruction. Also, antiosteoclastic medication can be used.
Gorham disease, a rare condition of unknown etiology, manifests as acute, spontaneous osteolysis associated with benign hemangiomatosis or lymphangiomatosis, which presents as skeletal lucency on radiographs, prompting the classic eponym of vanishing bone disease.1-6 There is no evidence supporting the idea that osteoclasts are present in any meaningful amount in the resorption areas or that local reparative osteogenesis occurs.4,6
Jackson and colleagues first described idiopathic osteolysis in 1838,1,2 and Gorham and Stout3 introduced the syndrome to the orthopedic community in 1955. Since then, few strides have been made in identifying the disease origin.1,2,4 Diagnosis is possible only after meticulous work-up has excluded neoplastic and infectious etiologies.7,8
Clinical Presentation
Gorham disease affects patients ranging widely in age, from 2 months to 78 years, but typically presents in those under 40 years. There is a questionable predilection for males but no correlation with ethnicity or geographic region. There is no clear hereditary pattern of transmission.7 Although the bones of the head, neck, and upper extremities are involved in most cases, bone of any type or location can be affected.6 Pelvic bones seem to be involved least often.6,7
Initial clinical presentation varies considerably but typically involves prolonged soreness in the affected region and, rarely, acute pathologic fracture.1,2,4 The nonspecific nature of complaints, lack of markers of systemic illness, and rarity of the disease contribute to delayed diagnosis.1,2
Imaging
Computed tomography (CT) better defines the severity and extent of these changes.
Magnetic resonance imaging shows an infiltrative and irregular T2 hyperintense signal throughout regions of bone affected by osteolysis, but this finding is not characteristic. There is heterogeneous enhancement on postcontrast sequences, and, though masslike enhancement is absent, signal abnormalities may extend into adjacent soft tissues.
Bone scintigraphy using technetium-99m is similarly nonspecific, typically revealing radiotracer uptake that is consistent with bony reaction to an underlying osteolytic process (Figure 4) but turning negative with ongoing resorption.
Positron emission tomography/CT typically shows foci of increased metabolic activity in the areas of osteolysis.10
Diagnosis
There have been 8 histologic and clinical criteria described to diagnose Gorham disease: (1) biopsy positive for presence of angiomatous tissue, (2) complete absence of any cellular atypia, (3) lack of osteoclastic response and lack of dystrophic calcifications, (4) evidence of progressive resorption of native bone, (5) no evidence of expansive or ulcerative lesion, (6) lack of visceral involvement, (7) osteolytic radiographic pattern, and (8) no concrete diagnosis after hereditary, metabolic, neoplastic, immunologic, and infectious work-up.4-6 These criteria confirm that the diagnosis can be rendered only after exclusion of neoplastic and infectious etiologies through clinical and laboratory work-up, imaging studies, and tissue sampling.
Tissue biopsy is indicated to rule out other potential etiologies of osteolysis, and the histologic findings help confirm a diagnosis of Gorham disease.
The differential diagnosis includes infection (osteomyelitis, Brodie abscess), benign tumors (eosinophilic granuloma/Langerhans cell histiocytosis), malignant tumors (Ewing sarcoma and angiosarcoma), inflammatory conditions (eg, apatite- associated destructive arthritis), endocrine disorders (eg, osteolytic hyperparathyroidism), benign non-neoplastic conditions (venous or venolymphatic malformation), and other syndromes that present with osteolysis.1,2 Nevertheless, progressive and unusually substantial bone destruction without evidence of repair is almost pathognomonic for Gorham disease.9
Treatment
Surgical treatment usually includes lesion resection and subsequent reconstruction using combinations of bone grafts (allogenic) and prostheses. Bone graft alone is quickly resorbed and has not been found to be beneficial.1,2,4,20
Take-Home Points
- Gorham disease is a rare condition that manifests as an acute, spontaneous osteolysis.
- There is no clear hereditary pattern of transmission. Bones of any type or location can be affected.
- Imaging studies are nonspecific, but show permeative osteolysis involving the subcortical and intramedullary regions and typically affect regional, contiguous bones, without adjacent sclerosis, somewhat resembling osteoporosis.
- Tissue biopsy is indicated to rule out other potential etiologies of osteolysis, and the histologic findings help confirm a diagnosis of Gorham disease.
- There is no single or combined treatment modality that is considered as the gold standard. Surgical treatment includes resection of the lesion and reconstruction. Also, antiosteoclastic medication can be used.
Gorham disease, a rare condition of unknown etiology, manifests as acute, spontaneous osteolysis associated with benign hemangiomatosis or lymphangiomatosis, which presents as skeletal lucency on radiographs, prompting the classic eponym of vanishing bone disease.1-6 There is no evidence supporting the idea that osteoclasts are present in any meaningful amount in the resorption areas or that local reparative osteogenesis occurs.4,6
Jackson and colleagues first described idiopathic osteolysis in 1838,1,2 and Gorham and Stout3 introduced the syndrome to the orthopedic community in 1955. Since then, few strides have been made in identifying the disease origin.1,2,4 Diagnosis is possible only after meticulous work-up has excluded neoplastic and infectious etiologies.7,8
Clinical Presentation
Gorham disease affects patients ranging widely in age, from 2 months to 78 years, but typically presents in those under 40 years. There is a questionable predilection for males but no correlation with ethnicity or geographic region. There is no clear hereditary pattern of transmission.7 Although the bones of the head, neck, and upper extremities are involved in most cases, bone of any type or location can be affected.6 Pelvic bones seem to be involved least often.6,7
Initial clinical presentation varies considerably but typically involves prolonged soreness in the affected region and, rarely, acute pathologic fracture.1,2,4 The nonspecific nature of complaints, lack of markers of systemic illness, and rarity of the disease contribute to delayed diagnosis.1,2
Imaging
Computed tomography (CT) better defines the severity and extent of these changes.
Magnetic resonance imaging shows an infiltrative and irregular T2 hyperintense signal throughout regions of bone affected by osteolysis, but this finding is not characteristic. There is heterogeneous enhancement on postcontrast sequences, and, though masslike enhancement is absent, signal abnormalities may extend into adjacent soft tissues.
Bone scintigraphy using technetium-99m is similarly nonspecific, typically revealing radiotracer uptake that is consistent with bony reaction to an underlying osteolytic process (Figure 4) but turning negative with ongoing resorption.
Positron emission tomography/CT typically shows foci of increased metabolic activity in the areas of osteolysis.10
Diagnosis
There have been 8 histologic and clinical criteria described to diagnose Gorham disease: (1) biopsy positive for presence of angiomatous tissue, (2) complete absence of any cellular atypia, (3) lack of osteoclastic response and lack of dystrophic calcifications, (4) evidence of progressive resorption of native bone, (5) no evidence of expansive or ulcerative lesion, (6) lack of visceral involvement, (7) osteolytic radiographic pattern, and (8) no concrete diagnosis after hereditary, metabolic, neoplastic, immunologic, and infectious work-up.4-6 These criteria confirm that the diagnosis can be rendered only after exclusion of neoplastic and infectious etiologies through clinical and laboratory work-up, imaging studies, and tissue sampling.
Tissue biopsy is indicated to rule out other potential etiologies of osteolysis, and the histologic findings help confirm a diagnosis of Gorham disease.
The differential diagnosis includes infection (osteomyelitis, Brodie abscess), benign tumors (eosinophilic granuloma/Langerhans cell histiocytosis), malignant tumors (Ewing sarcoma and angiosarcoma), inflammatory conditions (eg, apatite- associated destructive arthritis), endocrine disorders (eg, osteolytic hyperparathyroidism), benign non-neoplastic conditions (venous or venolymphatic malformation), and other syndromes that present with osteolysis.1,2 Nevertheless, progressive and unusually substantial bone destruction without evidence of repair is almost pathognomonic for Gorham disease.9
Treatment
Surgical treatment usually includes lesion resection and subsequent reconstruction using combinations of bone grafts (allogenic) and prostheses. Bone graft alone is quickly resorbed and has not been found to be beneficial.1,2,4,20
1. Saify FY, Gosavi SR. Gorham’s disease: a diagnostic challenge. J Oral Maxillofac Pathol. 2014;18(3):411-414.
2. Patel DV. Gorham’s disease or massive osteolysis. Clin Med Res. 2005;3(2):65-74.
3. Gorham LW, Stout AP. Massive osteolysis (acute spontaneous absorption of bone, phantom bone, disappearing bone); its relation to hemangiomatosis. J Bone Joint Surg Am. 1955;37(5):985-1004.
4. Heffez L, Doku HC, Carter BL, Feeney JE. Perspectives on massive osteolysis. Report of a case and review of the literature. Oral Surg Oral Med Oral Pathol. 1983;55(4):331-343.
5. Gulati U, Mohanty S, Dabas J, Chandra N. “Vanishing bone disease” in maxillofacial region: a review and our experience. J Maxillofac Oral Surg. 2015;14(3):548-557.
6. Nikolaou VS, Chytas D, Korres D, Efstathopoulos N. Vanishing bone disease (Gorham-Stout syndrome): a review of a rare entity. World J Orthop. 2014;5(5):694-698.
7. Möller G, Priemel M, Amling M, Werner M, Kuhlmey AS, Delling G. The Gorham-Stout syndrome (Gorham’s massive osteolysis). A report of six cases with histopathological findings. J Bone Joint Surg Br. 1999;81(3):501-506.
8. Dominguez R, Washowich TL. Gorham’s disease or vanishing bone disease: plain film, CT, and MRI findings of two cases. Pediatr Radiol. 1994;24(5):316-318.
9. Kotecha R, Mascarenhas L, Jackson HA, Venkatramani R. Radiological features of Gorham’s disease. Clin Radiol. 2012;67(8):782-788.
10. Dong A, Bai Y, Wang Y, Zuo C. Bone scan, MRI, and FDG PET/CT findings in composite hemangioendothelioma of the manubrium sterni. Clin Nucl Med. 2014;39(2):e180-e183.
11. Baulieu F, De Pinieux G, Maruani A, Vaillant L, Lorette G. Serial lymphoscintigraphic findings in a patient with Gorham’s disease with lymphedema. Lymphology. 2014;47(3):118-122.
12. Manisali M, Ozaksoy D. Gorham disease: correlation of MR findings with histopathologic changes. Eur Radiol. 1998;8(9):1647-1650.
13. Brodszki N, Länsberg JK, Dictor M, et al. A novel treatment approach for paediatric Gorham-Stout syndrome with chylothorax. Acta Paediatr. 2011;100(11):1448-1453.
14. Nir V, Guralnik L, Livnat G, et al. Propranolol as a treatment option in Gorham-Stout syndrome: a case report. Pediatr Pulmonol. 2014;49(4):417-419.
15. Fontanesi J. Radiation therapy in the treatment of Gorham disease. J Pediatr Hematol. 2003;25(10):816-817.
16. Pfleger A, Schwinger W, Maier A, Tauss J, Popper HH, Zach MS. Gorham-Stout syndrome in a male adolescent—case report and review of the literature. J Pediatr Hematol Oncol. 2006;28(4):231-233.
17. Patrick JH. Massive osteolysis complicated by chylothorax successfully treated by pleurodesis. J Bone Joint Surg Br. 1976;58(3):347-349.
18. Hagberg H, Lamberg K, Åström G. α-2b interferon and oral clodronate for Gorham’s disease. Lancet. 1997;350(9094):1822-1823.
19. Takahashi A, Ogawa C, Kanazawa T, et al. Remission induced by interferon alfa in a patient with massive osteolysis and extension of lymph-hemangiomatosis: a severe case of Gorham-Stout syndrome. J Pediatr Surg. 2005;40(3):E47-E50.
20. Paley MD, Lloyd CJ, Penfold CN. Total mandibular reconstruction for massive osteolysis of the mandible (Gorham-Stout syndrome). Br J Oral Maxillofac Surg. 2005;43(2):166-168.
21. Avelar RL, Martins VB, Antunes AA, de Oliveira Neto PJ, de Souza Andrade ES. Use of zoledronic acid in the treatment of Gorham’s disease. Int J Pediatr Otorhinolaryngol. 2010;74(3):319-322.
22. Holroyd I, Dillon M, Roberts GJ. Gorham’s disease: a case (including dental presentation) of vanishing bone disease. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89(1):125-129.
23. Lee S, Finn L, Sze RW, Perkins JA, Sie KC. Gorham Stout syndrome (disappearing bone disease): two additional case reports and a review of the literature. Arch Otolaryngol Head Neck Surg. 2003;129(12):1340-1343.
1. Saify FY, Gosavi SR. Gorham’s disease: a diagnostic challenge. J Oral Maxillofac Pathol. 2014;18(3):411-414.
2. Patel DV. Gorham’s disease or massive osteolysis. Clin Med Res. 2005;3(2):65-74.
3. Gorham LW, Stout AP. Massive osteolysis (acute spontaneous absorption of bone, phantom bone, disappearing bone); its relation to hemangiomatosis. J Bone Joint Surg Am. 1955;37(5):985-1004.
4. Heffez L, Doku HC, Carter BL, Feeney JE. Perspectives on massive osteolysis. Report of a case and review of the literature. Oral Surg Oral Med Oral Pathol. 1983;55(4):331-343.
5. Gulati U, Mohanty S, Dabas J, Chandra N. “Vanishing bone disease” in maxillofacial region: a review and our experience. J Maxillofac Oral Surg. 2015;14(3):548-557.
6. Nikolaou VS, Chytas D, Korres D, Efstathopoulos N. Vanishing bone disease (Gorham-Stout syndrome): a review of a rare entity. World J Orthop. 2014;5(5):694-698.
7. Möller G, Priemel M, Amling M, Werner M, Kuhlmey AS, Delling G. The Gorham-Stout syndrome (Gorham’s massive osteolysis). A report of six cases with histopathological findings. J Bone Joint Surg Br. 1999;81(3):501-506.
8. Dominguez R, Washowich TL. Gorham’s disease or vanishing bone disease: plain film, CT, and MRI findings of two cases. Pediatr Radiol. 1994;24(5):316-318.
9. Kotecha R, Mascarenhas L, Jackson HA, Venkatramani R. Radiological features of Gorham’s disease. Clin Radiol. 2012;67(8):782-788.
10. Dong A, Bai Y, Wang Y, Zuo C. Bone scan, MRI, and FDG PET/CT findings in composite hemangioendothelioma of the manubrium sterni. Clin Nucl Med. 2014;39(2):e180-e183.
11. Baulieu F, De Pinieux G, Maruani A, Vaillant L, Lorette G. Serial lymphoscintigraphic findings in a patient with Gorham’s disease with lymphedema. Lymphology. 2014;47(3):118-122.
12. Manisali M, Ozaksoy D. Gorham disease: correlation of MR findings with histopathologic changes. Eur Radiol. 1998;8(9):1647-1650.
13. Brodszki N, Länsberg JK, Dictor M, et al. A novel treatment approach for paediatric Gorham-Stout syndrome with chylothorax. Acta Paediatr. 2011;100(11):1448-1453.
14. Nir V, Guralnik L, Livnat G, et al. Propranolol as a treatment option in Gorham-Stout syndrome: a case report. Pediatr Pulmonol. 2014;49(4):417-419.
15. Fontanesi J. Radiation therapy in the treatment of Gorham disease. J Pediatr Hematol. 2003;25(10):816-817.
16. Pfleger A, Schwinger W, Maier A, Tauss J, Popper HH, Zach MS. Gorham-Stout syndrome in a male adolescent—case report and review of the literature. J Pediatr Hematol Oncol. 2006;28(4):231-233.
17. Patrick JH. Massive osteolysis complicated by chylothorax successfully treated by pleurodesis. J Bone Joint Surg Br. 1976;58(3):347-349.
18. Hagberg H, Lamberg K, Åström G. α-2b interferon and oral clodronate for Gorham’s disease. Lancet. 1997;350(9094):1822-1823.
19. Takahashi A, Ogawa C, Kanazawa T, et al. Remission induced by interferon alfa in a patient with massive osteolysis and extension of lymph-hemangiomatosis: a severe case of Gorham-Stout syndrome. J Pediatr Surg. 2005;40(3):E47-E50.
20. Paley MD, Lloyd CJ, Penfold CN. Total mandibular reconstruction for massive osteolysis of the mandible (Gorham-Stout syndrome). Br J Oral Maxillofac Surg. 2005;43(2):166-168.
21. Avelar RL, Martins VB, Antunes AA, de Oliveira Neto PJ, de Souza Andrade ES. Use of zoledronic acid in the treatment of Gorham’s disease. Int J Pediatr Otorhinolaryngol. 2010;74(3):319-322.
22. Holroyd I, Dillon M, Roberts GJ. Gorham’s disease: a case (including dental presentation) of vanishing bone disease. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89(1):125-129.
23. Lee S, Finn L, Sze RW, Perkins JA, Sie KC. Gorham Stout syndrome (disappearing bone disease): two additional case reports and a review of the literature. Arch Otolaryngol Head Neck Surg. 2003;129(12):1340-1343.
Intraoperative Use of External Fixator Attachments for Reduction of Lower Extremity Fractures and Dislocations
Take-Home Points
- External fixator attachments are fast and easy to assemble with existing external fixator equipment.
- They allow for multi-directional force application and use of extrinsic power grip.
- They limit radiation exposure and provides unobstructred line of sight to zone of injury.
- The attachments can then be removed once reduction is achieved.
External fixation has a long history both for initial open or closed management of fractures and for definitive management.1 After the introduction of internal fixation constructs using nails or plates, external fixation largely transitioned from a means of definitive management to a temporizing measure taken before definitive internal fixation.
The Delta Frame external fixator (DePuy Synthes), which is used for significantly swollen ankle and pilon fractures, features anteromedially placed tibial shaft pins and a transcalcaneal pin. For distal tibia fractures that are not amenable to urgent internal fixation because of the degree of swelling or soft-tissue injury, it provides ligamentotaxis and traction for reduction of fracture fragments and stabilization.2
Numerous other external fixator configurations, such as knee-spanning or tibia-spanning external fixators, can be used for similar purposes. These stabilization methods are all minimally invasive and thus cause little trauma to the zone of injury3 and give soft-tissue injuries time to heal before definitive internal fixation.
Several different external fixator configurations can be used for a variety of fracture patterns and locations, but we propose using the external fixator as a starting point and adding proximal and distal attachments. These attachments have the potential to create more reduction force, and they provide more control of proximal and distal fracture fragments, continue to be minimally invasive, offer extrinsic grip power, are easily assembled and disassembled for intraoperative fracture reduction, and reduce the surgeon’s radiation exposure.
Materials and Methods
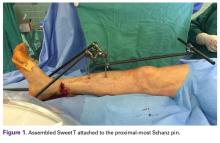
Our institution employs an external fixator system that is often used for high-energy lower extremity pathology. This system facilitates assembly of a Sweet T–Cherry II configuration. For periarticular ankle injuries, a Delta Frame external fixator is applied as described in the AO (Arbeitsgemeinschaft für Osteosynthesefragen) surgical reference. Two diaphyseal Schanz pins are inserted into the tibia anterior to posterior based on the pin-placement guide and confirmed with fluoroscopy. These pins must be positioned close enough to the fracture site to provide stability, but not so close as to enter the zone of injury. A Denham pin is placed in the calcaneus medial to lateral. Care is taken to avoid the posterior tibial neurovascular bundle. Then, with use of pin-carbon fiber rod connectors, rods are attached so the Schanz pins connect with the Denham pin. In Sweet T–Cherry II assembly, a different rod configuration is used; rods are attached to the proximal-most Schanz pin and the Denham pin. In Sweet T assembly, a rod-rod connector is used to attach 2 carbon fiber rods to each other.
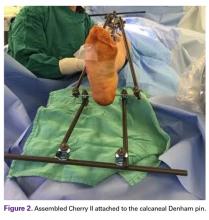
Next, in Cherry II assembly, 2 carbon fiber rods are attached to and locked to the Denham pin, one medial and the other lateral in an orientation orthogonal to the Denham pin extending distally. The Cherry II apparatus is completed with a third rod and is placed parallel to the Denham pin and orthogonal to the first 2 rods.
For knee-spanning external fixators, 2 Sweet T assemblies can be attached to the 2 Schanz pins. Furthermore, if 2 transverse pins are used for tibial external fixation, 2 Cherry II attachments can be used for multidirectional traction. An added benefit is extrinsic grip power, vs the intrinsic grip power provided with use of only the Schanz and Denham pins.
Results
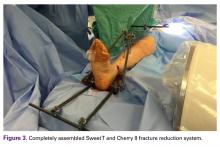
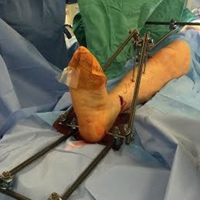
The fully assembled apparatus provides a firm, well-fixed configuration for applying traction in multiple directions. Axial traction can be applied, as can anterior or posterior translation forces, which may be helpful in fracture reduction and joint dislocation. For difficult-to-reduce fractures or dislocations, the surgeon can apply multidirectional traction distally while the assistant applies countertraction proximally. Fracture reduction is confirmed with fluoroscopy, and the external fixator is locked in position to maintain reduction. After reduction is confirmed, Sweet T and Cherry II are easily removed. The end result is a reduced fracture or dislocation that has the typical appearance of the temporizing external fixator.
Discussion
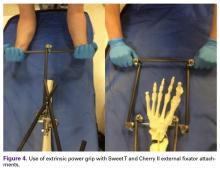
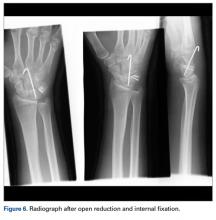
The Sweet T–Cherry II configuration is assembled quickly and can aid the orthopedic surgeon and assistant in managing trauma cases involving difficult-to-reduce fractures. The principle is the same as in any other traction-countertraction model, though the materials required for assembly are already available to the surgeon, and additional equipment is not required. Furthermore, the large size of the attachments has the potential to offer more points of manipulation by the surgeon and assistant, when compared with Schanz and Denham pins alone. The configuration allows full extrinsic grip power as well (Figure 4), whereas only intrinsic hand power is allowed with traction applied through Schanz and Denham pins alone.
These attachments also have the potential to reduce surgeon and assistant radiation exposure. By positioning Sweet T and Cherry II proximally and distally, with the fluoroscopy machine over the zone of injury, the operators of the attachments increase their distance from the source of radiation. This strategic positioning decreases radiation exposure and reduces direct and scatter energy from the fluoroscopy machine and the patient.4 In addition, with the operators of the attachments farther from the zone of injury, the surgeon has a clear and direct view of the procedure, which may otherwise be obstructed with use of tensioning devices or conventional external fixator configurations. Sweet T and Cherry II attachments theoretically could be used for any configuration that uses proximal and distal fixation points. There is also the added benefit that these attachments are not restricted to applying traction axially but can also translate fracture fragments anteriorly, posteriorly, medially, or laterally, which has the potential to aid in reducing fractures and dislocations with varying degrees of translation, not only shortening. Although these attachments have mechanical counterparts, such as femoral distractors and other tensioning devices, the counterparts may take longer to assemble, apply, and activate. Sweet T and Cherry II attachments are included in the external fixator equipment and may generate more force and control while achieving reduction and stabilization similar to those achieved with other traction or tensioning devices. In addition, other tensioning devices may be cumbersome and unwieldy relative to Sweet T and Cherry II. For these attachments, research is needed on forces generated, time of assembly, and ease of use.
Conclusion
The Sweet T and Cherry II external fixator attachments have the potential to aid in managing difficult-to-reduce complex fractures and dislocations. These attachments are quickly assembled and may generate more force than does reduction with Schanz and Denham pins alone. The shape of these attachments may also provide a more comfortable and easier-to-manipulate base for application of traction in multiple directions. An added benefit is extrinsic grip power. Use of these attachments also has the potential to reduce operator radiation exposure and may provide an unobstructed view of the operative field, as the attachment operators are farther from the zone of injury. In addition, the materials used to assemble these attachments are included in almost all external fixation sets. More research comparing standard external fixator configurations with external fixator configurations using Sweet T and Cherry II attachments is needed.
1. Tejwani N, Polonet D, Wolinsky PR. External fixation of tibial fractures. J Am Acad Orthop Surg. 2015;23(2):126-130.
2. Patterson MJ, Cole JD. Two-staged delayed open reduction and internal fixation of severe pilon fractures. J Orthop Trauma. 1999;13(2):85-91.
3. Bible JE, Mir HR. External fixation: principles and applications. J Am Acad Orthop Surg. 2015;23(11):683-690.
4. Giordano BD, Ryder S, Baumhauer JF, DiGiovanni BF. Exposure to direct and scatter radiation with use of mini-c-arm fluoroscopy. J Bone Joint Surg Am. 2007;89(5):948-952.
Take-Home Points
- External fixator attachments are fast and easy to assemble with existing external fixator equipment.
- They allow for multi-directional force application and use of extrinsic power grip.
- They limit radiation exposure and provides unobstructred line of sight to zone of injury.
- The attachments can then be removed once reduction is achieved.
External fixation has a long history both for initial open or closed management of fractures and for definitive management.1 After the introduction of internal fixation constructs using nails or plates, external fixation largely transitioned from a means of definitive management to a temporizing measure taken before definitive internal fixation.
The Delta Frame external fixator (DePuy Synthes), which is used for significantly swollen ankle and pilon fractures, features anteromedially placed tibial shaft pins and a transcalcaneal pin. For distal tibia fractures that are not amenable to urgent internal fixation because of the degree of swelling or soft-tissue injury, it provides ligamentotaxis and traction for reduction of fracture fragments and stabilization.2
Numerous other external fixator configurations, such as knee-spanning or tibia-spanning external fixators, can be used for similar purposes. These stabilization methods are all minimally invasive and thus cause little trauma to the zone of injury3 and give soft-tissue injuries time to heal before definitive internal fixation.
Several different external fixator configurations can be used for a variety of fracture patterns and locations, but we propose using the external fixator as a starting point and adding proximal and distal attachments. These attachments have the potential to create more reduction force, and they provide more control of proximal and distal fracture fragments, continue to be minimally invasive, offer extrinsic grip power, are easily assembled and disassembled for intraoperative fracture reduction, and reduce the surgeon’s radiation exposure.
Materials and Methods
Our institution employs an external fixator system that is often used for high-energy lower extremity pathology. This system facilitates assembly of a Sweet T–Cherry II configuration. For periarticular ankle injuries, a Delta Frame external fixator is applied as described in the AO (Arbeitsgemeinschaft für Osteosynthesefragen) surgical reference. Two diaphyseal Schanz pins are inserted into the tibia anterior to posterior based on the pin-placement guide and confirmed with fluoroscopy. These pins must be positioned close enough to the fracture site to provide stability, but not so close as to enter the zone of injury. A Denham pin is placed in the calcaneus medial to lateral. Care is taken to avoid the posterior tibial neurovascular bundle. Then, with use of pin-carbon fiber rod connectors, rods are attached so the Schanz pins connect with the Denham pin. In Sweet T–Cherry II assembly, a different rod configuration is used; rods are attached to the proximal-most Schanz pin and the Denham pin. In Sweet T assembly, a rod-rod connector is used to attach 2 carbon fiber rods to each other.
Next, in Cherry II assembly, 2 carbon fiber rods are attached to and locked to the Denham pin, one medial and the other lateral in an orientation orthogonal to the Denham pin extending distally. The Cherry II apparatus is completed with a third rod and is placed parallel to the Denham pin and orthogonal to the first 2 rods.
For knee-spanning external fixators, 2 Sweet T assemblies can be attached to the 2 Schanz pins. Furthermore, if 2 transverse pins are used for tibial external fixation, 2 Cherry II attachments can be used for multidirectional traction. An added benefit is extrinsic grip power, vs the intrinsic grip power provided with use of only the Schanz and Denham pins.
Results
The fully assembled apparatus provides a firm, well-fixed configuration for applying traction in multiple directions. Axial traction can be applied, as can anterior or posterior translation forces, which may be helpful in fracture reduction and joint dislocation. For difficult-to-reduce fractures or dislocations, the surgeon can apply multidirectional traction distally while the assistant applies countertraction proximally. Fracture reduction is confirmed with fluoroscopy, and the external fixator is locked in position to maintain reduction. After reduction is confirmed, Sweet T and Cherry II are easily removed. The end result is a reduced fracture or dislocation that has the typical appearance of the temporizing external fixator.
Discussion
The Sweet T–Cherry II configuration is assembled quickly and can aid the orthopedic surgeon and assistant in managing trauma cases involving difficult-to-reduce fractures. The principle is the same as in any other traction-countertraction model, though the materials required for assembly are already available to the surgeon, and additional equipment is not required. Furthermore, the large size of the attachments has the potential to offer more points of manipulation by the surgeon and assistant, when compared with Schanz and Denham pins alone. The configuration allows full extrinsic grip power as well (Figure 4), whereas only intrinsic hand power is allowed with traction applied through Schanz and Denham pins alone.
These attachments also have the potential to reduce surgeon and assistant radiation exposure. By positioning Sweet T and Cherry II proximally and distally, with the fluoroscopy machine over the zone of injury, the operators of the attachments increase their distance from the source of radiation. This strategic positioning decreases radiation exposure and reduces direct and scatter energy from the fluoroscopy machine and the patient.4 In addition, with the operators of the attachments farther from the zone of injury, the surgeon has a clear and direct view of the procedure, which may otherwise be obstructed with use of tensioning devices or conventional external fixator configurations. Sweet T and Cherry II attachments theoretically could be used for any configuration that uses proximal and distal fixation points. There is also the added benefit that these attachments are not restricted to applying traction axially but can also translate fracture fragments anteriorly, posteriorly, medially, or laterally, which has the potential to aid in reducing fractures and dislocations with varying degrees of translation, not only shortening. Although these attachments have mechanical counterparts, such as femoral distractors and other tensioning devices, the counterparts may take longer to assemble, apply, and activate. Sweet T and Cherry II attachments are included in the external fixator equipment and may generate more force and control while achieving reduction and stabilization similar to those achieved with other traction or tensioning devices. In addition, other tensioning devices may be cumbersome and unwieldy relative to Sweet T and Cherry II. For these attachments, research is needed on forces generated, time of assembly, and ease of use.
Conclusion
The Sweet T and Cherry II external fixator attachments have the potential to aid in managing difficult-to-reduce complex fractures and dislocations. These attachments are quickly assembled and may generate more force than does reduction with Schanz and Denham pins alone. The shape of these attachments may also provide a more comfortable and easier-to-manipulate base for application of traction in multiple directions. An added benefit is extrinsic grip power. Use of these attachments also has the potential to reduce operator radiation exposure and may provide an unobstructed view of the operative field, as the attachment operators are farther from the zone of injury. In addition, the materials used to assemble these attachments are included in almost all external fixation sets. More research comparing standard external fixator configurations with external fixator configurations using Sweet T and Cherry II attachments is needed.
Take-Home Points
- External fixator attachments are fast and easy to assemble with existing external fixator equipment.
- They allow for multi-directional force application and use of extrinsic power grip.
- They limit radiation exposure and provides unobstructred line of sight to zone of injury.
- The attachments can then be removed once reduction is achieved.
External fixation has a long history both for initial open or closed management of fractures and for definitive management.1 After the introduction of internal fixation constructs using nails or plates, external fixation largely transitioned from a means of definitive management to a temporizing measure taken before definitive internal fixation.
The Delta Frame external fixator (DePuy Synthes), which is used for significantly swollen ankle and pilon fractures, features anteromedially placed tibial shaft pins and a transcalcaneal pin. For distal tibia fractures that are not amenable to urgent internal fixation because of the degree of swelling or soft-tissue injury, it provides ligamentotaxis and traction for reduction of fracture fragments and stabilization.2
Numerous other external fixator configurations, such as knee-spanning or tibia-spanning external fixators, can be used for similar purposes. These stabilization methods are all minimally invasive and thus cause little trauma to the zone of injury3 and give soft-tissue injuries time to heal before definitive internal fixation.
Several different external fixator configurations can be used for a variety of fracture patterns and locations, but we propose using the external fixator as a starting point and adding proximal and distal attachments. These attachments have the potential to create more reduction force, and they provide more control of proximal and distal fracture fragments, continue to be minimally invasive, offer extrinsic grip power, are easily assembled and disassembled for intraoperative fracture reduction, and reduce the surgeon’s radiation exposure.
Materials and Methods
Our institution employs an external fixator system that is often used for high-energy lower extremity pathology. This system facilitates assembly of a Sweet T–Cherry II configuration. For periarticular ankle injuries, a Delta Frame external fixator is applied as described in the AO (Arbeitsgemeinschaft für Osteosynthesefragen) surgical reference. Two diaphyseal Schanz pins are inserted into the tibia anterior to posterior based on the pin-placement guide and confirmed with fluoroscopy. These pins must be positioned close enough to the fracture site to provide stability, but not so close as to enter the zone of injury. A Denham pin is placed in the calcaneus medial to lateral. Care is taken to avoid the posterior tibial neurovascular bundle. Then, with use of pin-carbon fiber rod connectors, rods are attached so the Schanz pins connect with the Denham pin. In Sweet T–Cherry II assembly, a different rod configuration is used; rods are attached to the proximal-most Schanz pin and the Denham pin. In Sweet T assembly, a rod-rod connector is used to attach 2 carbon fiber rods to each other.
Next, in Cherry II assembly, 2 carbon fiber rods are attached to and locked to the Denham pin, one medial and the other lateral in an orientation orthogonal to the Denham pin extending distally. The Cherry II apparatus is completed with a third rod and is placed parallel to the Denham pin and orthogonal to the first 2 rods.
For knee-spanning external fixators, 2 Sweet T assemblies can be attached to the 2 Schanz pins. Furthermore, if 2 transverse pins are used for tibial external fixation, 2 Cherry II attachments can be used for multidirectional traction. An added benefit is extrinsic grip power, vs the intrinsic grip power provided with use of only the Schanz and Denham pins.
Results
The fully assembled apparatus provides a firm, well-fixed configuration for applying traction in multiple directions. Axial traction can be applied, as can anterior or posterior translation forces, which may be helpful in fracture reduction and joint dislocation. For difficult-to-reduce fractures or dislocations, the surgeon can apply multidirectional traction distally while the assistant applies countertraction proximally. Fracture reduction is confirmed with fluoroscopy, and the external fixator is locked in position to maintain reduction. After reduction is confirmed, Sweet T and Cherry II are easily removed. The end result is a reduced fracture or dislocation that has the typical appearance of the temporizing external fixator.
Discussion
The Sweet T–Cherry II configuration is assembled quickly and can aid the orthopedic surgeon and assistant in managing trauma cases involving difficult-to-reduce fractures. The principle is the same as in any other traction-countertraction model, though the materials required for assembly are already available to the surgeon, and additional equipment is not required. Furthermore, the large size of the attachments has the potential to offer more points of manipulation by the surgeon and assistant, when compared with Schanz and Denham pins alone. The configuration allows full extrinsic grip power as well (Figure 4), whereas only intrinsic hand power is allowed with traction applied through Schanz and Denham pins alone.
These attachments also have the potential to reduce surgeon and assistant radiation exposure. By positioning Sweet T and Cherry II proximally and distally, with the fluoroscopy machine over the zone of injury, the operators of the attachments increase their distance from the source of radiation. This strategic positioning decreases radiation exposure and reduces direct and scatter energy from the fluoroscopy machine and the patient.4 In addition, with the operators of the attachments farther from the zone of injury, the surgeon has a clear and direct view of the procedure, which may otherwise be obstructed with use of tensioning devices or conventional external fixator configurations. Sweet T and Cherry II attachments theoretically could be used for any configuration that uses proximal and distal fixation points. There is also the added benefit that these attachments are not restricted to applying traction axially but can also translate fracture fragments anteriorly, posteriorly, medially, or laterally, which has the potential to aid in reducing fractures and dislocations with varying degrees of translation, not only shortening. Although these attachments have mechanical counterparts, such as femoral distractors and other tensioning devices, the counterparts may take longer to assemble, apply, and activate. Sweet T and Cherry II attachments are included in the external fixator equipment and may generate more force and control while achieving reduction and stabilization similar to those achieved with other traction or tensioning devices. In addition, other tensioning devices may be cumbersome and unwieldy relative to Sweet T and Cherry II. For these attachments, research is needed on forces generated, time of assembly, and ease of use.
Conclusion
The Sweet T and Cherry II external fixator attachments have the potential to aid in managing difficult-to-reduce complex fractures and dislocations. These attachments are quickly assembled and may generate more force than does reduction with Schanz and Denham pins alone. The shape of these attachments may also provide a more comfortable and easier-to-manipulate base for application of traction in multiple directions. An added benefit is extrinsic grip power. Use of these attachments also has the potential to reduce operator radiation exposure and may provide an unobstructed view of the operative field, as the attachment operators are farther from the zone of injury. In addition, the materials used to assemble these attachments are included in almost all external fixation sets. More research comparing standard external fixator configurations with external fixator configurations using Sweet T and Cherry II attachments is needed.
1. Tejwani N, Polonet D, Wolinsky PR. External fixation of tibial fractures. J Am Acad Orthop Surg. 2015;23(2):126-130.
2. Patterson MJ, Cole JD. Two-staged delayed open reduction and internal fixation of severe pilon fractures. J Orthop Trauma. 1999;13(2):85-91.
3. Bible JE, Mir HR. External fixation: principles and applications. J Am Acad Orthop Surg. 2015;23(11):683-690.
4. Giordano BD, Ryder S, Baumhauer JF, DiGiovanni BF. Exposure to direct and scatter radiation with use of mini-c-arm fluoroscopy. J Bone Joint Surg Am. 2007;89(5):948-952.
1. Tejwani N, Polonet D, Wolinsky PR. External fixation of tibial fractures. J Am Acad Orthop Surg. 2015;23(2):126-130.
2. Patterson MJ, Cole JD. Two-staged delayed open reduction and internal fixation of severe pilon fractures. J Orthop Trauma. 1999;13(2):85-91.
3. Bible JE, Mir HR. External fixation: principles and applications. J Am Acad Orthop Surg. 2015;23(11):683-690.
4. Giordano BD, Ryder S, Baumhauer JF, DiGiovanni BF. Exposure to direct and scatter radiation with use of mini-c-arm fluoroscopy. J Bone Joint Surg Am. 2007;89(5):948-952.
Transformation of Benign Giant Cell Tumor of Bone Into Epithelioid Angiosarcoma
Take-Home Points
- Malignant transformation of a benign GCT is extremely rare.
- It is difficult to distinguish between an early malignant transformation and an overlooked malignancy.
- The most common clinical presentation of transformation of GCT into malignancy is pain, often with swelling.
- Interval monitoring of GCTs may be necessary in patients with symptoms concerning for malignant transformation.
- Clinicians should maintain a high clinical suspicion for malignant transformation or late recurrence of GCT in a patient with new pain at the wound site.
Giant cell tumors (GCTs) of bone account for about 5% of all primary bone tumors in adults, with a predominance in the third decade in life.1 Clinically, GCT of bone often presents with pain, pathologic fracture, and/or soft- tissue expansion in the epiphysis of long bones. However, GCT of bone also has been reported in non-long bones, such as the talus and the calcaneus.2,3 Histologically, GCT of bone consists of neoplastic stromal cells, mononuclear histiocytic cells, and multinucleated giant cells that resemble osteoclasts.4 The radiologic appearance of GCT is often described as a lytic, eccentrically located bony lesion that extends near the articular surface in patients with closed physes. Many GCTs have aggressive radiologic features with possible extensive bony destruction and soft-tissue extension.
Although categorized as a benign lesion, GCT can be locally aggressive, with a variable local recurrence rate of 0% to 65%, depending on treatment modality and skeletal location. Given the aggressiveness of GCT of bone, recommendations for operative intervention include intralesional curettage with adjuvant therapy (eg, cryotherapy, phenol, argon beam, electrocautery) and placement of bone void fillers (eg, bone graft polymethylmethacrylate). Wide resection is recommended when the articular surface is no longer viable for reconstruction secondary to extensive destruction. Some authors have reported that surgical margin is the only risk factor in local recurrence,5,6 and thus complete resection may be needed for tumor eradication. In addition, about 3% of GCTs demonstrate benign pulmonary implants, which have been cited as cause of death in 16% to 25% of reported cases of pulmonary spread.7,8
The literature includes few reports of primary or secondary malignant transformation of GCT. Hutter and colleagues9 defined primary malignant GCT as GCT with sarcomatous tissue juxtaposed with zones of typical benign GCT cells. Secondary malignant GCT is a sarcomatous lesion at the site of a previously documented benign GCT. Secondary malignant GCT of bone histologically has been classified as a fibrosarcoma, malignant fibrous histiocytoma, or osteosarcoma transformation.10
Most malignant transformations of GCT of bone have been attributed to previous irradiation of the lesion.11,12 However, there are some case reports of benign bone GCT malignant transformation in situ without any other medical intervention. It was reported that non-radiation-induced secondary transformations occur relatively early after GCT treatment.13 During the early stages of tumor recurrence, however, it is difficult to distinguish between malignant transformation and primary disease overlooked as a result of sampling error.
We report a case of secondary malignant transformation of GCT of bone 11 years after surgical curettage, cryotherapy, and cementation without adjuvant radiation therapy. To our knowledge, this case report is the first to describe transformation of a nonirradiated benign GCT into an aggressive, high-grade epithelioid angiosarcoma, a very rare vascular bone tumor. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
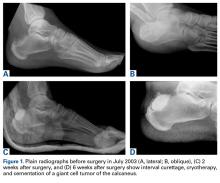
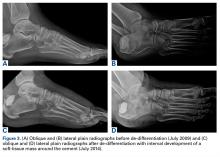
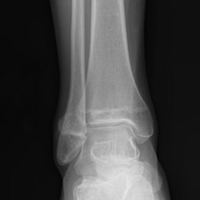
In July 2003, a 46-year-old woman presented with left heel pain of several months’ duration. Plain radiographs showed a nonaggressive-appearing lytic lesion of the superior aspect of the posterior calcaneal tuberosity with a small cortical incongruity along the superior margin of the lesion (Figures 1A-1D).
A postoperative splint was placed, and weight-bearing progressed over 6 weeks. The patient was followed at 2- to 3-month intervals over the first 5 postoperative years. She was able to work and perform activities of daily living, but her postoperative course was complicated by significant chronic pain in multiple extremities and long-term treatment by the chronic pain service. At no time did postoperative imaging—magnetic resonance imaging (MRI) at 6 years, whole-body bone scan at 7 years, plain radiographs at 10 years—show evidence of recurrence.
Radiographs showed stable postoperative changes with a small radiolucent area (with sclerotic rim) surrounding the cement-bone interface. Given its proximity to the Achilles tendon and more motion than usual at the wound site, the radiolucency likely was caused by small movements of the interface. The radiolucent area remained stable over a 15-month period.
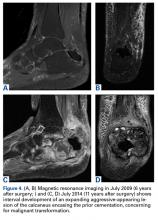
Whole-body bone scan showed a small area of osteoblastic activity in the left calcaneus, consistent with inflammation surrounding the bone- cement interface, but the uptake was minor relative to other areas of signal, and there were no significant inflammatory reactive changes on MRI (Figures 3A, 3B).
Over 11 years, regular 6- to 12-month follow-up examinations revealed no significant changes in the left foot or in plain radiographs of the chest. In addition, physical examinations revealed no evidence of a palpable mass of the left foot.
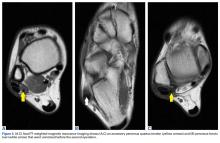
In July 2014 (11 years after curettage and cementation), the patient presented to her pain clinic appointment with severe left foot pain. She said that, over a few weeks, she experienced a significant increase in pain and developed posterolateral foot swelling, which limited her ability to ambulate. Plain radiographs showed a significant soft-tissue prominence around the posterior calcaneus, increased lucency around the bone-cement interface in the calcaneus with elevation, and a cortical break of the superior margin of the posterior calcaneus (Figures 3C, 3D). MRI showed a large lobular mass in the calcaneus and surrounding soft tissue with T1 and T2 signal heterogeneity and enhancement after administration of gadolinium (Figures 4A-4D). There was a large extraosseous extension of the calcaneus-based mass laterally and superiorly with edema in the surrounding hindfoot region (Figure 4).
Physical examination revealed exquisite tenderness along the lateral and posterior aspects of the left hindfoot. The patient was unable to bear weight and had soft-tissue swelling throughout the foot and mid calf as well as a palpable mass in the posterior heel. She was otherwise neurovascularly intact through all distributions of the left lower extremity. It was unclear if the GCT of the calcaneus had recurred or if there was a new, secondary tumor. Given her severe pain and morbidity, the patient decided to proceed with open biopsy and a pathology-pending plan for possible amputation in the near future.
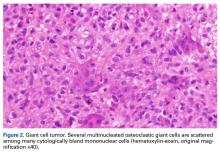
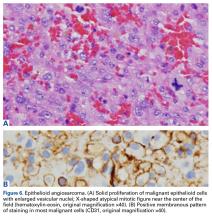
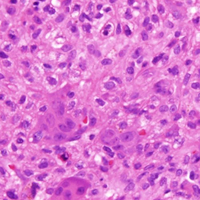
In August 2014, an open biopsy with intraoperative frozen evaluation yielded a diagnosis of malignant neoplasm not otherwise specified. Permanent sections showed a proliferation of malignant epithelioid cells with extensive necrosis, hemorrhage, and hemosiderin deposition but no multinucleated giant cells.
Transformation of the GCT into a high-grade epithelioid angiosarcoma prompted presentation of the patient’s case to a multidisciplinary board of physicians with a focused clinical practice in sarcoma management. The board included board-certified specialists in orthopedic oncology, pathology, musculoskeletal radiology, medical oncology, and radiation oncology. Although discussion included pre-resection use of neoadjuvant chemotherapy to evaluate for disease response, the patient’s severe pain led her to forgo this treatment and proceed directly to below-knee amputation.
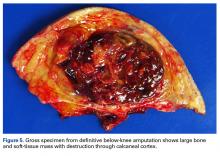
Amputation revealed a 7.7-cm hemorrhagic necrotic mass composed of a highly cellular spindle and epithelioid malignancy with abundant hemosiderin deposition (Figure 5). In addition, several atypical mitotic figures and malignant multinucleated tumor giant cells were randomly scattered throughout the neoplasm.
At first follow-up, the patient reported significant pain relief and asked to begin titrating off her chronic pain medicine. Clinical staging, which involved performing whole-body positron emission tomography/computed tomography, revealed nothing concerning for metastases. When this report was being written, the patient was being monitored for recurrent disease in accordance with National Comprehensive Cancer Network guidelines. In the absence of residual sarcoma, our medical oncology team discussed adjuvant chemotherapy options with her. Subsequently, however, she proceeded only with observation and periodic imaging.
Discussion
Malignant transformation of a benign GCT is extremely rare, especially in cases in which the tumor bed has not previously undergone radiation therapy. Although the literature includes historical case reports, primary and secondary malignant GCTs comprise <9% of all GCTs.11,13,14 Primary bone epithelioid angiosarcoma is also extremely rare, especially in the calcaneus; only 1 case is described in the literature.15 In this article, we report on a benign GCT of bone that transformed into an epithelioid angiosarcoma more than a decade after the GCT was treated with curettage and cementation.
The fact that the malignant areas of a previous tumor may have been missed because of sampling error is important for benign GCT of bone in the early postoperative period, as distinguishing between early malignant transformation and an overlooked malignancy may not be possible. However, transformation is more likely the case when a benign GCT becomes a high-grade malignancy after a long disease-free interval. Several authors have indicated that a benign GCT tumor recurring with a secondary malignancy 2 to 5 years after initial GCT treatment suggests malignant transformation.16 Grote and colleagues10 compiled reports of malignant transformation of GCT of bone and described the clinicopathologic features of secondary malignant transformation of GCTs. The data they compiled and data from several other studies indicate a poor prognosis after malignant transformation of GCT; 4 years after diagnosis, mean survival is 40% to 50%.10,16 The most common clinical presentation of transformation of GCT into malignancy is pain, often with coincident swelling of the native wound bed. However, a few cases have been identified with radiologic imaging alone and without a period of clinical symptoms.16
To our knowledge, this case report is the first to describe a longitudinal assessment of the transformation of a benign GCT of bone into an epithelioid angiosarcoma. Whereas an earlier reported GCT of bone transformed into epithelioid angiosarcoma after irradiation,12 our patient’s GCT of bone transformed without irradiation. GCTs of bone are locally aggressive benign tumors and are relatively rare. Malignant transformation of a benign bone tumor a decade after initial, definitive treatment is concerning, especially given the poor prognosis after malignant transformation in this clinical scenario. Current adjuvant treatments have not changed the prognosis. The literature includes a wide variety of histologic transformations, including high-grade sarcomas, after a long disease-free interval. Although malignant transformation of benign GCTs is rare, clinicians should be aware of the potential. Interval monitoring of GCTs may be necessary in patients with symptoms concerning for malignant transformation—pain or swelling in the wound bed—and patients should know to immediately inform their physician of any changes in pain level or local wound bed. Clinicians should maintain a high clinical suspicion for malignant transformation or late recurrence of GCT in a patient with new pain at the site of a previously treated GCT of bone with a disease-free interval of several years.
1. Unni KK. Dahlin’s Bone Tumors: General Aspects and Data on 11,087 Cases. 5th ed. Philadelphia, PA: Lippincott-Raven; 1996.
2. Errani C, Ruggieri P, Asenzio MA, et al. Giant cell tumor of the extremity: a review of 349 cases from a single institution. Cancer Treat Rev. 2010;36(1):1-7.
3. Campanacci M, Baldini N, Boriani S, Sudanese A. Giant-cell tumor of bone. J Bone Joint Surg Am. 1987;69(1):106-114.
4. Werner M. Giant cell tumour of bone: morphological, biological and histogenetical aspects. Int Orthop. 2006;30(6):484-489.
5 Klenke FM, Wenger DE, Inwards CY, Rose PS, Sim FH. Recurrent giant cell tumor of long bones: analysis of surgical management. Clin Orthop Relat Res. 2011;469(4):1181-1187.
6. McDonald DJ, Sim FH, McLeod RA, Dahlin DC. Giant-cell tumor of bone. J Bone Joint Surg Am. 1986;68(2):235-242.
7. Kay RM, Eckardt JJ, Seeger LL, Mirra JM, Hak DJ. Pulmonary metastasis of benign giant cell tumor of bone. Six histologically confirmed cases, including one of spontaneous regression. Clin Orthop Relat Res. 1994;(302):219-230.
8. Maloney WJ, Vaughan LM, Jones HH, Ross J, Nagel DA. Benign metastasizing giant-cell tumor of bone. Report of three cases and review of the literature. Clin Orthop Relat Res. 1989;(243):208-215.
9. Hutter RV, Worcester JN Jr, Francis KC, Foote FW Jr, Stewart FW. Benign and malignant giant cell tumors of bone. A clinicopathological analysis of the natural history of the disease. Cancer. 1962;15:653-690.
10. Grote HJ, Braun M, Kalinski T, et al. Spontaneous malignant transformation of conventional giant cell tumor. Skeletal Radiol. 2004;33(3):169-175.
11. Rock MG, Sim FH, Unni KK, et al. Secondary malignant giant-cell tumor of bone. Clinicopathological assessment of nineteen patients. J Bone Joint Surg Am. 1986;68(7):1073-1079.
12. Mittal S, Goswami C, Kanoria N, Bhattacharya A. Post-irradiation angiosarcoma of bone. J Cancer Res Ther. 2007;3(2):96-99.
13. Bertoni F, Bacchini P, Staals EL. Malignancy in giant cell tumor of bone. Cancer. 2003;97(10):2520-2529.
14. Dahlin DC, Cupps RE, Johnson EW Jr. Giant-cell tumor: a study of 195 cases. Cancer. 1970;25(5):1061-1070.
15. Balaji GG, Arockiaraj JS, Roy AC, Deepak B. Primary epithelioid angiosarcoma of the calcaneum: a diagnostic dilemma. J Foot Ankle Surg. 2014;53(2):239-242.
16. Anract P, De Pinieux G, Cottias P, Pouillart P, Forest M, Tomeno B. Malignant giant-cell tumours of bone. Clinico-pathological types and prognosis: a review of 29 cases. Int Orthop. 1998;22(1):19-26.
Take-Home Points
- Malignant transformation of a benign GCT is extremely rare.
- It is difficult to distinguish between an early malignant transformation and an overlooked malignancy.
- The most common clinical presentation of transformation of GCT into malignancy is pain, often with swelling.
- Interval monitoring of GCTs may be necessary in patients with symptoms concerning for malignant transformation.
- Clinicians should maintain a high clinical suspicion for malignant transformation or late recurrence of GCT in a patient with new pain at the wound site.
Giant cell tumors (GCTs) of bone account for about 5% of all primary bone tumors in adults, with a predominance in the third decade in life.1 Clinically, GCT of bone often presents with pain, pathologic fracture, and/or soft- tissue expansion in the epiphysis of long bones. However, GCT of bone also has been reported in non-long bones, such as the talus and the calcaneus.2,3 Histologically, GCT of bone consists of neoplastic stromal cells, mononuclear histiocytic cells, and multinucleated giant cells that resemble osteoclasts.4 The radiologic appearance of GCT is often described as a lytic, eccentrically located bony lesion that extends near the articular surface in patients with closed physes. Many GCTs have aggressive radiologic features with possible extensive bony destruction and soft-tissue extension.
Although categorized as a benign lesion, GCT can be locally aggressive, with a variable local recurrence rate of 0% to 65%, depending on treatment modality and skeletal location. Given the aggressiveness of GCT of bone, recommendations for operative intervention include intralesional curettage with adjuvant therapy (eg, cryotherapy, phenol, argon beam, electrocautery) and placement of bone void fillers (eg, bone graft polymethylmethacrylate). Wide resection is recommended when the articular surface is no longer viable for reconstruction secondary to extensive destruction. Some authors have reported that surgical margin is the only risk factor in local recurrence,5,6 and thus complete resection may be needed for tumor eradication. In addition, about 3% of GCTs demonstrate benign pulmonary implants, which have been cited as cause of death in 16% to 25% of reported cases of pulmonary spread.7,8
The literature includes few reports of primary or secondary malignant transformation of GCT. Hutter and colleagues9 defined primary malignant GCT as GCT with sarcomatous tissue juxtaposed with zones of typical benign GCT cells. Secondary malignant GCT is a sarcomatous lesion at the site of a previously documented benign GCT. Secondary malignant GCT of bone histologically has been classified as a fibrosarcoma, malignant fibrous histiocytoma, or osteosarcoma transformation.10
Most malignant transformations of GCT of bone have been attributed to previous irradiation of the lesion.11,12 However, there are some case reports of benign bone GCT malignant transformation in situ without any other medical intervention. It was reported that non-radiation-induced secondary transformations occur relatively early after GCT treatment.13 During the early stages of tumor recurrence, however, it is difficult to distinguish between malignant transformation and primary disease overlooked as a result of sampling error.
We report a case of secondary malignant transformation of GCT of bone 11 years after surgical curettage, cryotherapy, and cementation without adjuvant radiation therapy. To our knowledge, this case report is the first to describe transformation of a nonirradiated benign GCT into an aggressive, high-grade epithelioid angiosarcoma, a very rare vascular bone tumor. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
In July 2003, a 46-year-old woman presented with left heel pain of several months’ duration. Plain radiographs showed a nonaggressive-appearing lytic lesion of the superior aspect of the posterior calcaneal tuberosity with a small cortical incongruity along the superior margin of the lesion (Figures 1A-1D).
A postoperative splint was placed, and weight-bearing progressed over 6 weeks. The patient was followed at 2- to 3-month intervals over the first 5 postoperative years. She was able to work and perform activities of daily living, but her postoperative course was complicated by significant chronic pain in multiple extremities and long-term treatment by the chronic pain service. At no time did postoperative imaging—magnetic resonance imaging (MRI) at 6 years, whole-body bone scan at 7 years, plain radiographs at 10 years—show evidence of recurrence.
Radiographs showed stable postoperative changes with a small radiolucent area (with sclerotic rim) surrounding the cement-bone interface. Given its proximity to the Achilles tendon and more motion than usual at the wound site, the radiolucency likely was caused by small movements of the interface. The radiolucent area remained stable over a 15-month period.
Whole-body bone scan showed a small area of osteoblastic activity in the left calcaneus, consistent with inflammation surrounding the bone- cement interface, but the uptake was minor relative to other areas of signal, and there were no significant inflammatory reactive changes on MRI (Figures 3A, 3B).
Over 11 years, regular 6- to 12-month follow-up examinations revealed no significant changes in the left foot or in plain radiographs of the chest. In addition, physical examinations revealed no evidence of a palpable mass of the left foot.
In July 2014 (11 years after curettage and cementation), the patient presented to her pain clinic appointment with severe left foot pain. She said that, over a few weeks, she experienced a significant increase in pain and developed posterolateral foot swelling, which limited her ability to ambulate. Plain radiographs showed a significant soft-tissue prominence around the posterior calcaneus, increased lucency around the bone-cement interface in the calcaneus with elevation, and a cortical break of the superior margin of the posterior calcaneus (Figures 3C, 3D). MRI showed a large lobular mass in the calcaneus and surrounding soft tissue with T1 and T2 signal heterogeneity and enhancement after administration of gadolinium (Figures 4A-4D). There was a large extraosseous extension of the calcaneus-based mass laterally and superiorly with edema in the surrounding hindfoot region (Figure 4).
Physical examination revealed exquisite tenderness along the lateral and posterior aspects of the left hindfoot. The patient was unable to bear weight and had soft-tissue swelling throughout the foot and mid calf as well as a palpable mass in the posterior heel. She was otherwise neurovascularly intact through all distributions of the left lower extremity. It was unclear if the GCT of the calcaneus had recurred or if there was a new, secondary tumor. Given her severe pain and morbidity, the patient decided to proceed with open biopsy and a pathology-pending plan for possible amputation in the near future.
In August 2014, an open biopsy with intraoperative frozen evaluation yielded a diagnosis of malignant neoplasm not otherwise specified. Permanent sections showed a proliferation of malignant epithelioid cells with extensive necrosis, hemorrhage, and hemosiderin deposition but no multinucleated giant cells.
Transformation of the GCT into a high-grade epithelioid angiosarcoma prompted presentation of the patient’s case to a multidisciplinary board of physicians with a focused clinical practice in sarcoma management. The board included board-certified specialists in orthopedic oncology, pathology, musculoskeletal radiology, medical oncology, and radiation oncology. Although discussion included pre-resection use of neoadjuvant chemotherapy to evaluate for disease response, the patient’s severe pain led her to forgo this treatment and proceed directly to below-knee amputation.
Amputation revealed a 7.7-cm hemorrhagic necrotic mass composed of a highly cellular spindle and epithelioid malignancy with abundant hemosiderin deposition (Figure 5). In addition, several atypical mitotic figures and malignant multinucleated tumor giant cells were randomly scattered throughout the neoplasm.
At first follow-up, the patient reported significant pain relief and asked to begin titrating off her chronic pain medicine. Clinical staging, which involved performing whole-body positron emission tomography/computed tomography, revealed nothing concerning for metastases. When this report was being written, the patient was being monitored for recurrent disease in accordance with National Comprehensive Cancer Network guidelines. In the absence of residual sarcoma, our medical oncology team discussed adjuvant chemotherapy options with her. Subsequently, however, she proceeded only with observation and periodic imaging.
Discussion
Malignant transformation of a benign GCT is extremely rare, especially in cases in which the tumor bed has not previously undergone radiation therapy. Although the literature includes historical case reports, primary and secondary malignant GCTs comprise <9% of all GCTs.11,13,14 Primary bone epithelioid angiosarcoma is also extremely rare, especially in the calcaneus; only 1 case is described in the literature.15 In this article, we report on a benign GCT of bone that transformed into an epithelioid angiosarcoma more than a decade after the GCT was treated with curettage and cementation.
The fact that the malignant areas of a previous tumor may have been missed because of sampling error is important for benign GCT of bone in the early postoperative period, as distinguishing between early malignant transformation and an overlooked malignancy may not be possible. However, transformation is more likely the case when a benign GCT becomes a high-grade malignancy after a long disease-free interval. Several authors have indicated that a benign GCT tumor recurring with a secondary malignancy 2 to 5 years after initial GCT treatment suggests malignant transformation.16 Grote and colleagues10 compiled reports of malignant transformation of GCT of bone and described the clinicopathologic features of secondary malignant transformation of GCTs. The data they compiled and data from several other studies indicate a poor prognosis after malignant transformation of GCT; 4 years after diagnosis, mean survival is 40% to 50%.10,16 The most common clinical presentation of transformation of GCT into malignancy is pain, often with coincident swelling of the native wound bed. However, a few cases have been identified with radiologic imaging alone and without a period of clinical symptoms.16
To our knowledge, this case report is the first to describe a longitudinal assessment of the transformation of a benign GCT of bone into an epithelioid angiosarcoma. Whereas an earlier reported GCT of bone transformed into epithelioid angiosarcoma after irradiation,12 our patient’s GCT of bone transformed without irradiation. GCTs of bone are locally aggressive benign tumors and are relatively rare. Malignant transformation of a benign bone tumor a decade after initial, definitive treatment is concerning, especially given the poor prognosis after malignant transformation in this clinical scenario. Current adjuvant treatments have not changed the prognosis. The literature includes a wide variety of histologic transformations, including high-grade sarcomas, after a long disease-free interval. Although malignant transformation of benign GCTs is rare, clinicians should be aware of the potential. Interval monitoring of GCTs may be necessary in patients with symptoms concerning for malignant transformation—pain or swelling in the wound bed—and patients should know to immediately inform their physician of any changes in pain level or local wound bed. Clinicians should maintain a high clinical suspicion for malignant transformation or late recurrence of GCT in a patient with new pain at the site of a previously treated GCT of bone with a disease-free interval of several years.
Take-Home Points
- Malignant transformation of a benign GCT is extremely rare.
- It is difficult to distinguish between an early malignant transformation and an overlooked malignancy.
- The most common clinical presentation of transformation of GCT into malignancy is pain, often with swelling.
- Interval monitoring of GCTs may be necessary in patients with symptoms concerning for malignant transformation.
- Clinicians should maintain a high clinical suspicion for malignant transformation or late recurrence of GCT in a patient with new pain at the wound site.
Giant cell tumors (GCTs) of bone account for about 5% of all primary bone tumors in adults, with a predominance in the third decade in life.1 Clinically, GCT of bone often presents with pain, pathologic fracture, and/or soft- tissue expansion in the epiphysis of long bones. However, GCT of bone also has been reported in non-long bones, such as the talus and the calcaneus.2,3 Histologically, GCT of bone consists of neoplastic stromal cells, mononuclear histiocytic cells, and multinucleated giant cells that resemble osteoclasts.4 The radiologic appearance of GCT is often described as a lytic, eccentrically located bony lesion that extends near the articular surface in patients with closed physes. Many GCTs have aggressive radiologic features with possible extensive bony destruction and soft-tissue extension.
Although categorized as a benign lesion, GCT can be locally aggressive, with a variable local recurrence rate of 0% to 65%, depending on treatment modality and skeletal location. Given the aggressiveness of GCT of bone, recommendations for operative intervention include intralesional curettage with adjuvant therapy (eg, cryotherapy, phenol, argon beam, electrocautery) and placement of bone void fillers (eg, bone graft polymethylmethacrylate). Wide resection is recommended when the articular surface is no longer viable for reconstruction secondary to extensive destruction. Some authors have reported that surgical margin is the only risk factor in local recurrence,5,6 and thus complete resection may be needed for tumor eradication. In addition, about 3% of GCTs demonstrate benign pulmonary implants, which have been cited as cause of death in 16% to 25% of reported cases of pulmonary spread.7,8
The literature includes few reports of primary or secondary malignant transformation of GCT. Hutter and colleagues9 defined primary malignant GCT as GCT with sarcomatous tissue juxtaposed with zones of typical benign GCT cells. Secondary malignant GCT is a sarcomatous lesion at the site of a previously documented benign GCT. Secondary malignant GCT of bone histologically has been classified as a fibrosarcoma, malignant fibrous histiocytoma, or osteosarcoma transformation.10
Most malignant transformations of GCT of bone have been attributed to previous irradiation of the lesion.11,12 However, there are some case reports of benign bone GCT malignant transformation in situ without any other medical intervention. It was reported that non-radiation-induced secondary transformations occur relatively early after GCT treatment.13 During the early stages of tumor recurrence, however, it is difficult to distinguish between malignant transformation and primary disease overlooked as a result of sampling error.
We report a case of secondary malignant transformation of GCT of bone 11 years after surgical curettage, cryotherapy, and cementation without adjuvant radiation therapy. To our knowledge, this case report is the first to describe transformation of a nonirradiated benign GCT into an aggressive, high-grade epithelioid angiosarcoma, a very rare vascular bone tumor. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
In July 2003, a 46-year-old woman presented with left heel pain of several months’ duration. Plain radiographs showed a nonaggressive-appearing lytic lesion of the superior aspect of the posterior calcaneal tuberosity with a small cortical incongruity along the superior margin of the lesion (Figures 1A-1D).
A postoperative splint was placed, and weight-bearing progressed over 6 weeks. The patient was followed at 2- to 3-month intervals over the first 5 postoperative years. She was able to work and perform activities of daily living, but her postoperative course was complicated by significant chronic pain in multiple extremities and long-term treatment by the chronic pain service. At no time did postoperative imaging—magnetic resonance imaging (MRI) at 6 years, whole-body bone scan at 7 years, plain radiographs at 10 years—show evidence of recurrence.
Radiographs showed stable postoperative changes with a small radiolucent area (with sclerotic rim) surrounding the cement-bone interface. Given its proximity to the Achilles tendon and more motion than usual at the wound site, the radiolucency likely was caused by small movements of the interface. The radiolucent area remained stable over a 15-month period.
Whole-body bone scan showed a small area of osteoblastic activity in the left calcaneus, consistent with inflammation surrounding the bone- cement interface, but the uptake was minor relative to other areas of signal, and there were no significant inflammatory reactive changes on MRI (Figures 3A, 3B).
Over 11 years, regular 6- to 12-month follow-up examinations revealed no significant changes in the left foot or in plain radiographs of the chest. In addition, physical examinations revealed no evidence of a palpable mass of the left foot.
In July 2014 (11 years after curettage and cementation), the patient presented to her pain clinic appointment with severe left foot pain. She said that, over a few weeks, she experienced a significant increase in pain and developed posterolateral foot swelling, which limited her ability to ambulate. Plain radiographs showed a significant soft-tissue prominence around the posterior calcaneus, increased lucency around the bone-cement interface in the calcaneus with elevation, and a cortical break of the superior margin of the posterior calcaneus (Figures 3C, 3D). MRI showed a large lobular mass in the calcaneus and surrounding soft tissue with T1 and T2 signal heterogeneity and enhancement after administration of gadolinium (Figures 4A-4D). There was a large extraosseous extension of the calcaneus-based mass laterally and superiorly with edema in the surrounding hindfoot region (Figure 4).
Physical examination revealed exquisite tenderness along the lateral and posterior aspects of the left hindfoot. The patient was unable to bear weight and had soft-tissue swelling throughout the foot and mid calf as well as a palpable mass in the posterior heel. She was otherwise neurovascularly intact through all distributions of the left lower extremity. It was unclear if the GCT of the calcaneus had recurred or if there was a new, secondary tumor. Given her severe pain and morbidity, the patient decided to proceed with open biopsy and a pathology-pending plan for possible amputation in the near future.
In August 2014, an open biopsy with intraoperative frozen evaluation yielded a diagnosis of malignant neoplasm not otherwise specified. Permanent sections showed a proliferation of malignant epithelioid cells with extensive necrosis, hemorrhage, and hemosiderin deposition but no multinucleated giant cells.
Transformation of the GCT into a high-grade epithelioid angiosarcoma prompted presentation of the patient’s case to a multidisciplinary board of physicians with a focused clinical practice in sarcoma management. The board included board-certified specialists in orthopedic oncology, pathology, musculoskeletal radiology, medical oncology, and radiation oncology. Although discussion included pre-resection use of neoadjuvant chemotherapy to evaluate for disease response, the patient’s severe pain led her to forgo this treatment and proceed directly to below-knee amputation.
Amputation revealed a 7.7-cm hemorrhagic necrotic mass composed of a highly cellular spindle and epithelioid malignancy with abundant hemosiderin deposition (Figure 5). In addition, several atypical mitotic figures and malignant multinucleated tumor giant cells were randomly scattered throughout the neoplasm.
At first follow-up, the patient reported significant pain relief and asked to begin titrating off her chronic pain medicine. Clinical staging, which involved performing whole-body positron emission tomography/computed tomography, revealed nothing concerning for metastases. When this report was being written, the patient was being monitored for recurrent disease in accordance with National Comprehensive Cancer Network guidelines. In the absence of residual sarcoma, our medical oncology team discussed adjuvant chemotherapy options with her. Subsequently, however, she proceeded only with observation and periodic imaging.
Discussion
Malignant transformation of a benign GCT is extremely rare, especially in cases in which the tumor bed has not previously undergone radiation therapy. Although the literature includes historical case reports, primary and secondary malignant GCTs comprise <9% of all GCTs.11,13,14 Primary bone epithelioid angiosarcoma is also extremely rare, especially in the calcaneus; only 1 case is described in the literature.15 In this article, we report on a benign GCT of bone that transformed into an epithelioid angiosarcoma more than a decade after the GCT was treated with curettage and cementation.
The fact that the malignant areas of a previous tumor may have been missed because of sampling error is important for benign GCT of bone in the early postoperative period, as distinguishing between early malignant transformation and an overlooked malignancy may not be possible. However, transformation is more likely the case when a benign GCT becomes a high-grade malignancy after a long disease-free interval. Several authors have indicated that a benign GCT tumor recurring with a secondary malignancy 2 to 5 years after initial GCT treatment suggests malignant transformation.16 Grote and colleagues10 compiled reports of malignant transformation of GCT of bone and described the clinicopathologic features of secondary malignant transformation of GCTs. The data they compiled and data from several other studies indicate a poor prognosis after malignant transformation of GCT; 4 years after diagnosis, mean survival is 40% to 50%.10,16 The most common clinical presentation of transformation of GCT into malignancy is pain, often with coincident swelling of the native wound bed. However, a few cases have been identified with radiologic imaging alone and without a period of clinical symptoms.16
To our knowledge, this case report is the first to describe a longitudinal assessment of the transformation of a benign GCT of bone into an epithelioid angiosarcoma. Whereas an earlier reported GCT of bone transformed into epithelioid angiosarcoma after irradiation,12 our patient’s GCT of bone transformed without irradiation. GCTs of bone are locally aggressive benign tumors and are relatively rare. Malignant transformation of a benign bone tumor a decade after initial, definitive treatment is concerning, especially given the poor prognosis after malignant transformation in this clinical scenario. Current adjuvant treatments have not changed the prognosis. The literature includes a wide variety of histologic transformations, including high-grade sarcomas, after a long disease-free interval. Although malignant transformation of benign GCTs is rare, clinicians should be aware of the potential. Interval monitoring of GCTs may be necessary in patients with symptoms concerning for malignant transformation—pain or swelling in the wound bed—and patients should know to immediately inform their physician of any changes in pain level or local wound bed. Clinicians should maintain a high clinical suspicion for malignant transformation or late recurrence of GCT in a patient with new pain at the site of a previously treated GCT of bone with a disease-free interval of several years.
1. Unni KK. Dahlin’s Bone Tumors: General Aspects and Data on 11,087 Cases. 5th ed. Philadelphia, PA: Lippincott-Raven; 1996.
2. Errani C, Ruggieri P, Asenzio MA, et al. Giant cell tumor of the extremity: a review of 349 cases from a single institution. Cancer Treat Rev. 2010;36(1):1-7.
3. Campanacci M, Baldini N, Boriani S, Sudanese A. Giant-cell tumor of bone. J Bone Joint Surg Am. 1987;69(1):106-114.
4. Werner M. Giant cell tumour of bone: morphological, biological and histogenetical aspects. Int Orthop. 2006;30(6):484-489.
5 Klenke FM, Wenger DE, Inwards CY, Rose PS, Sim FH. Recurrent giant cell tumor of long bones: analysis of surgical management. Clin Orthop Relat Res. 2011;469(4):1181-1187.
6. McDonald DJ, Sim FH, McLeod RA, Dahlin DC. Giant-cell tumor of bone. J Bone Joint Surg Am. 1986;68(2):235-242.
7. Kay RM, Eckardt JJ, Seeger LL, Mirra JM, Hak DJ. Pulmonary metastasis of benign giant cell tumor of bone. Six histologically confirmed cases, including one of spontaneous regression. Clin Orthop Relat Res. 1994;(302):219-230.
8. Maloney WJ, Vaughan LM, Jones HH, Ross J, Nagel DA. Benign metastasizing giant-cell tumor of bone. Report of three cases and review of the literature. Clin Orthop Relat Res. 1989;(243):208-215.
9. Hutter RV, Worcester JN Jr, Francis KC, Foote FW Jr, Stewart FW. Benign and malignant giant cell tumors of bone. A clinicopathological analysis of the natural history of the disease. Cancer. 1962;15:653-690.
10. Grote HJ, Braun M, Kalinski T, et al. Spontaneous malignant transformation of conventional giant cell tumor. Skeletal Radiol. 2004;33(3):169-175.
11. Rock MG, Sim FH, Unni KK, et al. Secondary malignant giant-cell tumor of bone. Clinicopathological assessment of nineteen patients. J Bone Joint Surg Am. 1986;68(7):1073-1079.
12. Mittal S, Goswami C, Kanoria N, Bhattacharya A. Post-irradiation angiosarcoma of bone. J Cancer Res Ther. 2007;3(2):96-99.
13. Bertoni F, Bacchini P, Staals EL. Malignancy in giant cell tumor of bone. Cancer. 2003;97(10):2520-2529.
14. Dahlin DC, Cupps RE, Johnson EW Jr. Giant-cell tumor: a study of 195 cases. Cancer. 1970;25(5):1061-1070.
15. Balaji GG, Arockiaraj JS, Roy AC, Deepak B. Primary epithelioid angiosarcoma of the calcaneum: a diagnostic dilemma. J Foot Ankle Surg. 2014;53(2):239-242.
16. Anract P, De Pinieux G, Cottias P, Pouillart P, Forest M, Tomeno B. Malignant giant-cell tumours of bone. Clinico-pathological types and prognosis: a review of 29 cases. Int Orthop. 1998;22(1):19-26.
1. Unni KK. Dahlin’s Bone Tumors: General Aspects and Data on 11,087 Cases. 5th ed. Philadelphia, PA: Lippincott-Raven; 1996.
2. Errani C, Ruggieri P, Asenzio MA, et al. Giant cell tumor of the extremity: a review of 349 cases from a single institution. Cancer Treat Rev. 2010;36(1):1-7.
3. Campanacci M, Baldini N, Boriani S, Sudanese A. Giant-cell tumor of bone. J Bone Joint Surg Am. 1987;69(1):106-114.
4. Werner M. Giant cell tumour of bone: morphological, biological and histogenetical aspects. Int Orthop. 2006;30(6):484-489.
5 Klenke FM, Wenger DE, Inwards CY, Rose PS, Sim FH. Recurrent giant cell tumor of long bones: analysis of surgical management. Clin Orthop Relat Res. 2011;469(4):1181-1187.
6. McDonald DJ, Sim FH, McLeod RA, Dahlin DC. Giant-cell tumor of bone. J Bone Joint Surg Am. 1986;68(2):235-242.
7. Kay RM, Eckardt JJ, Seeger LL, Mirra JM, Hak DJ. Pulmonary metastasis of benign giant cell tumor of bone. Six histologically confirmed cases, including one of spontaneous regression. Clin Orthop Relat Res. 1994;(302):219-230.
8. Maloney WJ, Vaughan LM, Jones HH, Ross J, Nagel DA. Benign metastasizing giant-cell tumor of bone. Report of three cases and review of the literature. Clin Orthop Relat Res. 1989;(243):208-215.
9. Hutter RV, Worcester JN Jr, Francis KC, Foote FW Jr, Stewart FW. Benign and malignant giant cell tumors of bone. A clinicopathological analysis of the natural history of the disease. Cancer. 1962;15:653-690.
10. Grote HJ, Braun M, Kalinski T, et al. Spontaneous malignant transformation of conventional giant cell tumor. Skeletal Radiol. 2004;33(3):169-175.
11. Rock MG, Sim FH, Unni KK, et al. Secondary malignant giant-cell tumor of bone. Clinicopathological assessment of nineteen patients. J Bone Joint Surg Am. 1986;68(7):1073-1079.
12. Mittal S, Goswami C, Kanoria N, Bhattacharya A. Post-irradiation angiosarcoma of bone. J Cancer Res Ther. 2007;3(2):96-99.
13. Bertoni F, Bacchini P, Staals EL. Malignancy in giant cell tumor of bone. Cancer. 2003;97(10):2520-2529.
14. Dahlin DC, Cupps RE, Johnson EW Jr. Giant-cell tumor: a study of 195 cases. Cancer. 1970;25(5):1061-1070.
15. Balaji GG, Arockiaraj JS, Roy AC, Deepak B. Primary epithelioid angiosarcoma of the calcaneum: a diagnostic dilemma. J Foot Ankle Surg. 2014;53(2):239-242.
16. Anract P, De Pinieux G, Cottias P, Pouillart P, Forest M, Tomeno B. Malignant giant-cell tumours of bone. Clinico-pathological types and prognosis: a review of 29 cases. Int Orthop. 1998;22(1):19-26.
Total Knee Arthroplasty Performed With Long-Acting Liposomal Bupivacaine Versus Femoral Nerve Catheter
Take-Home Points
- At our institution, LALB has shortened our hospital stay.
- There is a trend towards decreased opioid consumption with LALB.
- With the opioid epidemic we face today, LALB can be one of many options in our toolbox towards a solution.
- As stated in prior publications, the effectiveness of LALB is definitely technique dependent.
- Additional clinical studies are warranted to better determine the efficacy and cost-effectiveness of LALB.
Almost 1 million total knee arthroplasties (TKAs) are performed in the United States each year, and the number continues to grow.1.2 For patients about to undergo TKA, a significant concern is postoperative pain.3 Fear of postoperative pain is often cited as a reason for delaying surgery.3 Recent literature suggests that patients with poor pain management during the first 48 hours after surgery have a 50% chance of gaining satisfactory long-term pain relief.4 In addition, inadequate postoperative pain management can interfere with participation in and progression of physical rehabilitation, prolong hospital stay, and increase patient dissatisfaction.5 Poorly controlled pain results in decreased range of motion (ROM), strength, stability, and ambulation thereby prolongs hospital stays, and increases costs and overall dissatisfaction with the procedure.
Post-TKA pain management has received much attention in recent years. A multimodal pain management protocol is now a key component of clinical pathways in TKA. Appropriate postoperative pain control lowers postoperative complications and accelerates recovery.6 Pain-caused loss of function makes surgical patients more susceptible to edema, deep vein thrombosis, and pulmonary embolism.4 Various oral and intravenous medications are used to lessen the pain response during the perioperative period. In addition, regional or neuraxial anesthesia is often added to blunt the immediate surgical pain response.7,8 At our institution, TKA traditionally has been performed with femoral nerve catheters (FNCs) for postoperative pain control. Although effective, this method often results in decreased quadriceps musculature function, which delays rehabilitation and increases the fall risk. Recently, there has been a shift toward using local anesthetic infusions about the knee to provide adequate pain relief and restore motor function, which is often sacrificed with use of regional nerve blocks and continuous catheter infusions.9
Many institutions have started using a new long-acting local anesthetic in their multimodal pain management pathways: Exparel (Pacira Pharmaceuticals), a liposomal membrane-bound bupivacaine with sustained release of approximately 72 hours. Several studies have verified the safety of this medication.10 A systemic review of prospective studies revealed that, compared with bupivacaine, long-acting liposomal bupivacaine (LALB) in therapeutic doses had a higher safety margin and a favorable safety profile.10 However, no study has compared the effectiveness of LALB and FNC in a matched TKA cohort with each patient serving as his or her own control.
We recently reviewed our multimodal pain management protocol for any areas in need of improvement and decided to compare the effects of the indwelling FNC protocol that was in use with the effects of injecting the local anesthetic LALB. We conducted a study to compare the 2 methods with respect to pain control, ROM, ability to ambulate, and hospital length of stay (LOS). We hypothesized that the longer acting local anesthetic would provide comparable post-TKA pain control and post-TKA opioid use but would accelerate post-TKA rehabilitation.
Materials and Methods
This retrospective, longitudinal, repeated- measures study was approved by the Greenville Hospital System Institutional Review Board and conducted at the Steadman Hawkins Clinic of the Carolinas, Greenville Health System.
Interventions
Twenty-three patients underwent separately staged bilateral TKAs between 2010 and 2013. For each TKA, a Genesis II implant (Smith & Nephew) was used, and the surgery was performed with the patient under spinal anesthesia. In each case, FNC was used for pain control after the first TKA, and periarticular injection (PAI) of LALB for pain control after the second TKA.
In the first TKAs, FNC-administered ropivacaine 0.2% (2 mg/mL) was maintained at a standard basal rate of 8 mL/h for 48 hours. In the second TKAs, LALB was administered along with bupivacaine/epinephrine. Twenty milliliters of LALB from a single-use vial was diluted in 40 mL of normal (0.9%) saline to obtain a 60-mL solution, and a 25-gauge needle was used to inject this solution into the periarticular soft tissues; another needle was used for PAI of 30 mL of bupivacaine 0.25% with epinephrine.
Continuous passive motion devices were not used. Most patients began therapy on day of surgery. Knee immobilizers were not used in the FNC group.
The same standardized multimodal pain management protocol was used for all TKAs. Non- narcotic medications, including acetaminophen, ketorolac, and celecoxib, were given on a scheduled basis. Tramadol and opioid medications were administered as needed for pain. The attending physician based patient discharge timing on pain control, ability to safely ambulate, and absence of complications.
Outcome Measures
Outcome measures were LOS; extension and flexion at discharge and 3-week follow-up; total ROM (extension plus flexion) at discharge and 3-week follow-up; per-day and total hospital stay morphine -equivalent doses (MEDs); and per-attempt walking distance during gait training.
ROM was measured with a standard goniometer. Flexion was tested with the patient supine and the hip and knee in neutral rotation. The goniometer axis was along the lateral epicondyle of the femur with the proximal arm of the goniometer parallel to the long axis of the femur and pointing at the greater trochanter and with the distal arm parallel to the long axis of the fibula and pointing at the lateral malleolus. The patient was instructed to flex the hip and knee by moving the heel toward the buttock. Expected normal ROM is 135°. The same landmarks were used for extension. The patient was instructed to push the back of the knee toward the plinth/bed, for maximal active extension. The same ROM assessment strategy was used during the hospitalization and at the 3-week follow-up.
Several opioid medications (eg, hydrocodone, oxycodone, tramadol, hydromorphone, morphine) with different dosages were used during hospitalization. Opioid doses were converted to MEDs to permit FNC–LALB comparisons. For each patient, total MEDs were divided by LOS to determine MEDs per day.
Mean per-attempt walking distance was calculated by dividing the total distance walked during hospitalization—the sum of the number of feet walked during each and every attempt, as measured by the treating physical therapist—by the total number of walking attempts.
Data Analysis
A paired-samples t test was used to calculate differences between all outcome measures: LOS; extension and flexion at discharge and 3-month follow-up; per-day and total MEDs; and mean per-attempt walking distance. P < .05 was considered significant. We elected not to adjust our α for a potential familywise error.
Results
Of the 23 patients, 14 were female and 9 were male, and 19 were white and 4 were black. Mean (SD) age was 64.4 (6.4) years for the FNC group and 66.0 (6.0) years for the LALB group. The age difference was not statistically significant.
Discussion
Poor pain control during the post-TKA period may have a significant impact on recovery rate, standard of living, psychological health, and postoperative complications.10 Inadequate postoperative pain control increases postoperative morbidity, hinders physiotherapy, increases anxiety, disrupts sleep patterns, and decreases patient satisfaction.9 There has been increased interest in PAIs. Local anesthetics are additional sources of pain control at surgical sites. However, the half-life of most local anesthetics is short. Soft-tissue infiltration of LALB into a surgical site extends the duration of active analgesia. Our study found that, compared with patients who received FNC, patients who received LALB had comparable pain control, improved knee ROM, and shorter hospital stays. In addition, the LALB group had no reports of quadriceps weakness or falls, both of which are associated with femoral nerve blocks. The FNC group had no reported falls, either. PAIs have the benefit of avoiding the invasiveness of femoral nerve blocks and possible neuritis.
Many complications are associated with or indirectly related to delayed rehabilitation and immobility during the acute post-TKA period. From prolonged hospitalization to need for manipulation, the consequences of inadequate pain control and decreased function can be numerous and costly for patients and the healthcare system. In the present study, LALB use led to a statistically significant overall decrease in mean LOS (LALB group, 2.3 days; FNC, 2.8 days). With LALB, there was a higher likelihood of discharge the day after surgery; 20% of patients in the LALB group and no patients in the FNC group went home that day.
The implication is that inadequate pain control led to decreased motion and decreased progression during postoperative rehabilitation. Local infiltration resulted in increased total ROM (extension plus flexion) at 3-week follow-up (LALB, 116.3°; FNC, 107.2°). In addition, there was an increase in walking distance per day of hospital stay (LALB, 135.9 feet; FNC, 84.2 feet). Furthermore, patients indicated LALB when asked which anesthetic they preferred. To our knowledge, this is the first study to compare LALB and FNC data in a matched TKA cohort with each patient serving as his or her own control.
Our study had several limitations. First was the retrospective design. Second was the small sample size, which made definitive conclusions difficult. However, the statistically significant differences we noted validated our conclusions. A statistically significant difference favoring LALB over FNC was found for total MEDs during hospitalization, but there was no significant difference in per-day MEDs. A possible reason for this difference is that LALB patients had shorter hospital stays, and therefore received fewer doses overall. Another possible reason is the small sample size; whereas a larger study using our protocol may find a statistically significant difference between LALB and FNC, we found only a trend. In the FNC group, anesthetic infiltration occurred with use of a computerized pump, which was removed on postoperative day 2; most of these patients were discharged home that day or the morning of postoperative day 3. As it is possible that some of these patients could have gone home sooner, our LOS data may have been affected. We do not consider this limitation significant, as one of our discharge criteria was 150 feet of ambulation, and most patients who received FNCs could not ambulate that far until after FNC removal. Furthermore, this study compared LALB only with FNC. It is possible that our improved outcomes could have resulted from the PAIs themselves, irrespective of LALB. In a recent TKA study by Bagsby and colleagues,11 pain was controlled better with the less expensive traditional PAI of ropivacaine, epinephrine, and morphine than with the PAI of liposomal bupivacaine. Last, in our study, the experience of undergoing the first TKA may have increased patients’ confidence going into the second TKA and then helped them make faster progress in rehabilitation. Regardless, the promising results of our study and the firsthand use of LALB at our institution led us to modify our intraoperative pain management protocol for surgeons who perform TKA.
As we continue to use LALB, our study numbers will increase, and we may discover other factors that, though now underpowered, will prove to be statistically significant. Additional clinical studies are needed to better determine the efficacy and cost-effectiveness of LALB and other long-acting local anesthetic formulations.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
2. Ruiz D Jr, Koenig L, Dall TM, et al. The direct and indirect costs to society of treatment for end-stage knee osteoarthritis. J Bone Joint Surg Am. 2013;95(16):1473-1480.
3. Trousdale RT, McGrory BJ, Berry DJ, Becker MW, Harmsen WS. Patients’ concerns prior to undergoing total hip and total knee arthroplasty. Mayo Clin Proc. 1999;74(10):978-982.
4. Wells N, Pasero C, McCaffery M. Improving the quality of care through pain assessment and management. In: Hughes RG, ed. Patient Safety and Quality: An Evidence-Based Handbook for Nurses, Vol 1. Rockville, MD: Agency for Healthcare Research and Quality; 2008:469-497.
5. Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10(4):287-333.
6. Parvizi J, Miller AG, Gandhi K. Multimodal pain management after total joint arthroplasty. J Bone Joint Surg Am. 2011;93(11):1075-1084.
7. Stein BE, Srikumaran U, Tan EW, Freehill MT, Wilckens JH. Lower-extremity peripheral nerve blocks in the perioperative pain management of orthopaedic patients: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(22):e167.
8. Pugely AJ, Martin CT, Gao Y, Mendoza-Lattes S, Callaghan JJ. Differences in short-term complications between spinal and general anesthesia for primary total knee arthroplasty. J Bone Joint Surg Am. 2013;95(3):193-199.
9. Dalury DF, Lieberman JR, MacDonald SJ. Current and innovative pain management techniques in total knee arthroplasty. J Bone Joint Surg Am. 2011;93(20):1938-1943.
10. Portillo J, Kamar N, Melibary S, Quevedo E, Bergese S. Safety of liposome extended-release bupivacaine for postoperative pain control. Front Pharmacol. 2014;5:90.
11. Bagsby DT, Ireland PH, Meneghini RM. Liposomal bupivacaine versus traditional periarticular injection for pain control after total knee arthroplasty. J Arthroplasty. 2014;29(8):1687-1690.
Take-Home Points
- At our institution, LALB has shortened our hospital stay.
- There is a trend towards decreased opioid consumption with LALB.
- With the opioid epidemic we face today, LALB can be one of many options in our toolbox towards a solution.
- As stated in prior publications, the effectiveness of LALB is definitely technique dependent.
- Additional clinical studies are warranted to better determine the efficacy and cost-effectiveness of LALB.
Almost 1 million total knee arthroplasties (TKAs) are performed in the United States each year, and the number continues to grow.1.2 For patients about to undergo TKA, a significant concern is postoperative pain.3 Fear of postoperative pain is often cited as a reason for delaying surgery.3 Recent literature suggests that patients with poor pain management during the first 48 hours after surgery have a 50% chance of gaining satisfactory long-term pain relief.4 In addition, inadequate postoperative pain management can interfere with participation in and progression of physical rehabilitation, prolong hospital stay, and increase patient dissatisfaction.5 Poorly controlled pain results in decreased range of motion (ROM), strength, stability, and ambulation thereby prolongs hospital stays, and increases costs and overall dissatisfaction with the procedure.
Post-TKA pain management has received much attention in recent years. A multimodal pain management protocol is now a key component of clinical pathways in TKA. Appropriate postoperative pain control lowers postoperative complications and accelerates recovery.6 Pain-caused loss of function makes surgical patients more susceptible to edema, deep vein thrombosis, and pulmonary embolism.4 Various oral and intravenous medications are used to lessen the pain response during the perioperative period. In addition, regional or neuraxial anesthesia is often added to blunt the immediate surgical pain response.7,8 At our institution, TKA traditionally has been performed with femoral nerve catheters (FNCs) for postoperative pain control. Although effective, this method often results in decreased quadriceps musculature function, which delays rehabilitation and increases the fall risk. Recently, there has been a shift toward using local anesthetic infusions about the knee to provide adequate pain relief and restore motor function, which is often sacrificed with use of regional nerve blocks and continuous catheter infusions.9
Many institutions have started using a new long-acting local anesthetic in their multimodal pain management pathways: Exparel (Pacira Pharmaceuticals), a liposomal membrane-bound bupivacaine with sustained release of approximately 72 hours. Several studies have verified the safety of this medication.10 A systemic review of prospective studies revealed that, compared with bupivacaine, long-acting liposomal bupivacaine (LALB) in therapeutic doses had a higher safety margin and a favorable safety profile.10 However, no study has compared the effectiveness of LALB and FNC in a matched TKA cohort with each patient serving as his or her own control.
We recently reviewed our multimodal pain management protocol for any areas in need of improvement and decided to compare the effects of the indwelling FNC protocol that was in use with the effects of injecting the local anesthetic LALB. We conducted a study to compare the 2 methods with respect to pain control, ROM, ability to ambulate, and hospital length of stay (LOS). We hypothesized that the longer acting local anesthetic would provide comparable post-TKA pain control and post-TKA opioid use but would accelerate post-TKA rehabilitation.
Materials and Methods
This retrospective, longitudinal, repeated- measures study was approved by the Greenville Hospital System Institutional Review Board and conducted at the Steadman Hawkins Clinic of the Carolinas, Greenville Health System.
Interventions
Twenty-three patients underwent separately staged bilateral TKAs between 2010 and 2013. For each TKA, a Genesis II implant (Smith & Nephew) was used, and the surgery was performed with the patient under spinal anesthesia. In each case, FNC was used for pain control after the first TKA, and periarticular injection (PAI) of LALB for pain control after the second TKA.
In the first TKAs, FNC-administered ropivacaine 0.2% (2 mg/mL) was maintained at a standard basal rate of 8 mL/h for 48 hours. In the second TKAs, LALB was administered along with bupivacaine/epinephrine. Twenty milliliters of LALB from a single-use vial was diluted in 40 mL of normal (0.9%) saline to obtain a 60-mL solution, and a 25-gauge needle was used to inject this solution into the periarticular soft tissues; another needle was used for PAI of 30 mL of bupivacaine 0.25% with epinephrine.
Continuous passive motion devices were not used. Most patients began therapy on day of surgery. Knee immobilizers were not used in the FNC group.
The same standardized multimodal pain management protocol was used for all TKAs. Non- narcotic medications, including acetaminophen, ketorolac, and celecoxib, were given on a scheduled basis. Tramadol and opioid medications were administered as needed for pain. The attending physician based patient discharge timing on pain control, ability to safely ambulate, and absence of complications.
Outcome Measures
Outcome measures were LOS; extension and flexion at discharge and 3-week follow-up; total ROM (extension plus flexion) at discharge and 3-week follow-up; per-day and total hospital stay morphine -equivalent doses (MEDs); and per-attempt walking distance during gait training.
ROM was measured with a standard goniometer. Flexion was tested with the patient supine and the hip and knee in neutral rotation. The goniometer axis was along the lateral epicondyle of the femur with the proximal arm of the goniometer parallel to the long axis of the femur and pointing at the greater trochanter and with the distal arm parallel to the long axis of the fibula and pointing at the lateral malleolus. The patient was instructed to flex the hip and knee by moving the heel toward the buttock. Expected normal ROM is 135°. The same landmarks were used for extension. The patient was instructed to push the back of the knee toward the plinth/bed, for maximal active extension. The same ROM assessment strategy was used during the hospitalization and at the 3-week follow-up.
Several opioid medications (eg, hydrocodone, oxycodone, tramadol, hydromorphone, morphine) with different dosages were used during hospitalization. Opioid doses were converted to MEDs to permit FNC–LALB comparisons. For each patient, total MEDs were divided by LOS to determine MEDs per day.
Mean per-attempt walking distance was calculated by dividing the total distance walked during hospitalization—the sum of the number of feet walked during each and every attempt, as measured by the treating physical therapist—by the total number of walking attempts.
Data Analysis
A paired-samples t test was used to calculate differences between all outcome measures: LOS; extension and flexion at discharge and 3-month follow-up; per-day and total MEDs; and mean per-attempt walking distance. P < .05 was considered significant. We elected not to adjust our α for a potential familywise error.
Results
Of the 23 patients, 14 were female and 9 were male, and 19 were white and 4 were black. Mean (SD) age was 64.4 (6.4) years for the FNC group and 66.0 (6.0) years for the LALB group. The age difference was not statistically significant.
Discussion
Poor pain control during the post-TKA period may have a significant impact on recovery rate, standard of living, psychological health, and postoperative complications.10 Inadequate postoperative pain control increases postoperative morbidity, hinders physiotherapy, increases anxiety, disrupts sleep patterns, and decreases patient satisfaction.9 There has been increased interest in PAIs. Local anesthetics are additional sources of pain control at surgical sites. However, the half-life of most local anesthetics is short. Soft-tissue infiltration of LALB into a surgical site extends the duration of active analgesia. Our study found that, compared with patients who received FNC, patients who received LALB had comparable pain control, improved knee ROM, and shorter hospital stays. In addition, the LALB group had no reports of quadriceps weakness or falls, both of which are associated with femoral nerve blocks. The FNC group had no reported falls, either. PAIs have the benefit of avoiding the invasiveness of femoral nerve blocks and possible neuritis.
Many complications are associated with or indirectly related to delayed rehabilitation and immobility during the acute post-TKA period. From prolonged hospitalization to need for manipulation, the consequences of inadequate pain control and decreased function can be numerous and costly for patients and the healthcare system. In the present study, LALB use led to a statistically significant overall decrease in mean LOS (LALB group, 2.3 days; FNC, 2.8 days). With LALB, there was a higher likelihood of discharge the day after surgery; 20% of patients in the LALB group and no patients in the FNC group went home that day.
The implication is that inadequate pain control led to decreased motion and decreased progression during postoperative rehabilitation. Local infiltration resulted in increased total ROM (extension plus flexion) at 3-week follow-up (LALB, 116.3°; FNC, 107.2°). In addition, there was an increase in walking distance per day of hospital stay (LALB, 135.9 feet; FNC, 84.2 feet). Furthermore, patients indicated LALB when asked which anesthetic they preferred. To our knowledge, this is the first study to compare LALB and FNC data in a matched TKA cohort with each patient serving as his or her own control.
Our study had several limitations. First was the retrospective design. Second was the small sample size, which made definitive conclusions difficult. However, the statistically significant differences we noted validated our conclusions. A statistically significant difference favoring LALB over FNC was found for total MEDs during hospitalization, but there was no significant difference in per-day MEDs. A possible reason for this difference is that LALB patients had shorter hospital stays, and therefore received fewer doses overall. Another possible reason is the small sample size; whereas a larger study using our protocol may find a statistically significant difference between LALB and FNC, we found only a trend. In the FNC group, anesthetic infiltration occurred with use of a computerized pump, which was removed on postoperative day 2; most of these patients were discharged home that day or the morning of postoperative day 3. As it is possible that some of these patients could have gone home sooner, our LOS data may have been affected. We do not consider this limitation significant, as one of our discharge criteria was 150 feet of ambulation, and most patients who received FNCs could not ambulate that far until after FNC removal. Furthermore, this study compared LALB only with FNC. It is possible that our improved outcomes could have resulted from the PAIs themselves, irrespective of LALB. In a recent TKA study by Bagsby and colleagues,11 pain was controlled better with the less expensive traditional PAI of ropivacaine, epinephrine, and morphine than with the PAI of liposomal bupivacaine. Last, in our study, the experience of undergoing the first TKA may have increased patients’ confidence going into the second TKA and then helped them make faster progress in rehabilitation. Regardless, the promising results of our study and the firsthand use of LALB at our institution led us to modify our intraoperative pain management protocol for surgeons who perform TKA.
As we continue to use LALB, our study numbers will increase, and we may discover other factors that, though now underpowered, will prove to be statistically significant. Additional clinical studies are needed to better determine the efficacy and cost-effectiveness of LALB and other long-acting local anesthetic formulations.
Take-Home Points
- At our institution, LALB has shortened our hospital stay.
- There is a trend towards decreased opioid consumption with LALB.
- With the opioid epidemic we face today, LALB can be one of many options in our toolbox towards a solution.
- As stated in prior publications, the effectiveness of LALB is definitely technique dependent.
- Additional clinical studies are warranted to better determine the efficacy and cost-effectiveness of LALB.
Almost 1 million total knee arthroplasties (TKAs) are performed in the United States each year, and the number continues to grow.1.2 For patients about to undergo TKA, a significant concern is postoperative pain.3 Fear of postoperative pain is often cited as a reason for delaying surgery.3 Recent literature suggests that patients with poor pain management during the first 48 hours after surgery have a 50% chance of gaining satisfactory long-term pain relief.4 In addition, inadequate postoperative pain management can interfere with participation in and progression of physical rehabilitation, prolong hospital stay, and increase patient dissatisfaction.5 Poorly controlled pain results in decreased range of motion (ROM), strength, stability, and ambulation thereby prolongs hospital stays, and increases costs and overall dissatisfaction with the procedure.
Post-TKA pain management has received much attention in recent years. A multimodal pain management protocol is now a key component of clinical pathways in TKA. Appropriate postoperative pain control lowers postoperative complications and accelerates recovery.6 Pain-caused loss of function makes surgical patients more susceptible to edema, deep vein thrombosis, and pulmonary embolism.4 Various oral and intravenous medications are used to lessen the pain response during the perioperative period. In addition, regional or neuraxial anesthesia is often added to blunt the immediate surgical pain response.7,8 At our institution, TKA traditionally has been performed with femoral nerve catheters (FNCs) for postoperative pain control. Although effective, this method often results in decreased quadriceps musculature function, which delays rehabilitation and increases the fall risk. Recently, there has been a shift toward using local anesthetic infusions about the knee to provide adequate pain relief and restore motor function, which is often sacrificed with use of regional nerve blocks and continuous catheter infusions.9
Many institutions have started using a new long-acting local anesthetic in their multimodal pain management pathways: Exparel (Pacira Pharmaceuticals), a liposomal membrane-bound bupivacaine with sustained release of approximately 72 hours. Several studies have verified the safety of this medication.10 A systemic review of prospective studies revealed that, compared with bupivacaine, long-acting liposomal bupivacaine (LALB) in therapeutic doses had a higher safety margin and a favorable safety profile.10 However, no study has compared the effectiveness of LALB and FNC in a matched TKA cohort with each patient serving as his or her own control.
We recently reviewed our multimodal pain management protocol for any areas in need of improvement and decided to compare the effects of the indwelling FNC protocol that was in use with the effects of injecting the local anesthetic LALB. We conducted a study to compare the 2 methods with respect to pain control, ROM, ability to ambulate, and hospital length of stay (LOS). We hypothesized that the longer acting local anesthetic would provide comparable post-TKA pain control and post-TKA opioid use but would accelerate post-TKA rehabilitation.
Materials and Methods
This retrospective, longitudinal, repeated- measures study was approved by the Greenville Hospital System Institutional Review Board and conducted at the Steadman Hawkins Clinic of the Carolinas, Greenville Health System.
Interventions
Twenty-three patients underwent separately staged bilateral TKAs between 2010 and 2013. For each TKA, a Genesis II implant (Smith & Nephew) was used, and the surgery was performed with the patient under spinal anesthesia. In each case, FNC was used for pain control after the first TKA, and periarticular injection (PAI) of LALB for pain control after the second TKA.
In the first TKAs, FNC-administered ropivacaine 0.2% (2 mg/mL) was maintained at a standard basal rate of 8 mL/h for 48 hours. In the second TKAs, LALB was administered along with bupivacaine/epinephrine. Twenty milliliters of LALB from a single-use vial was diluted in 40 mL of normal (0.9%) saline to obtain a 60-mL solution, and a 25-gauge needle was used to inject this solution into the periarticular soft tissues; another needle was used for PAI of 30 mL of bupivacaine 0.25% with epinephrine.
Continuous passive motion devices were not used. Most patients began therapy on day of surgery. Knee immobilizers were not used in the FNC group.
The same standardized multimodal pain management protocol was used for all TKAs. Non- narcotic medications, including acetaminophen, ketorolac, and celecoxib, were given on a scheduled basis. Tramadol and opioid medications were administered as needed for pain. The attending physician based patient discharge timing on pain control, ability to safely ambulate, and absence of complications.
Outcome Measures
Outcome measures were LOS; extension and flexion at discharge and 3-week follow-up; total ROM (extension plus flexion) at discharge and 3-week follow-up; per-day and total hospital stay morphine -equivalent doses (MEDs); and per-attempt walking distance during gait training.
ROM was measured with a standard goniometer. Flexion was tested with the patient supine and the hip and knee in neutral rotation. The goniometer axis was along the lateral epicondyle of the femur with the proximal arm of the goniometer parallel to the long axis of the femur and pointing at the greater trochanter and with the distal arm parallel to the long axis of the fibula and pointing at the lateral malleolus. The patient was instructed to flex the hip and knee by moving the heel toward the buttock. Expected normal ROM is 135°. The same landmarks were used for extension. The patient was instructed to push the back of the knee toward the plinth/bed, for maximal active extension. The same ROM assessment strategy was used during the hospitalization and at the 3-week follow-up.
Several opioid medications (eg, hydrocodone, oxycodone, tramadol, hydromorphone, morphine) with different dosages were used during hospitalization. Opioid doses were converted to MEDs to permit FNC–LALB comparisons. For each patient, total MEDs were divided by LOS to determine MEDs per day.
Mean per-attempt walking distance was calculated by dividing the total distance walked during hospitalization—the sum of the number of feet walked during each and every attempt, as measured by the treating physical therapist—by the total number of walking attempts.
Data Analysis
A paired-samples t test was used to calculate differences between all outcome measures: LOS; extension and flexion at discharge and 3-month follow-up; per-day and total MEDs; and mean per-attempt walking distance. P < .05 was considered significant. We elected not to adjust our α for a potential familywise error.
Results
Of the 23 patients, 14 were female and 9 were male, and 19 were white and 4 were black. Mean (SD) age was 64.4 (6.4) years for the FNC group and 66.0 (6.0) years for the LALB group. The age difference was not statistically significant.
Discussion
Poor pain control during the post-TKA period may have a significant impact on recovery rate, standard of living, psychological health, and postoperative complications.10 Inadequate postoperative pain control increases postoperative morbidity, hinders physiotherapy, increases anxiety, disrupts sleep patterns, and decreases patient satisfaction.9 There has been increased interest in PAIs. Local anesthetics are additional sources of pain control at surgical sites. However, the half-life of most local anesthetics is short. Soft-tissue infiltration of LALB into a surgical site extends the duration of active analgesia. Our study found that, compared with patients who received FNC, patients who received LALB had comparable pain control, improved knee ROM, and shorter hospital stays. In addition, the LALB group had no reports of quadriceps weakness or falls, both of which are associated with femoral nerve blocks. The FNC group had no reported falls, either. PAIs have the benefit of avoiding the invasiveness of femoral nerve blocks and possible neuritis.
Many complications are associated with or indirectly related to delayed rehabilitation and immobility during the acute post-TKA period. From prolonged hospitalization to need for manipulation, the consequences of inadequate pain control and decreased function can be numerous and costly for patients and the healthcare system. In the present study, LALB use led to a statistically significant overall decrease in mean LOS (LALB group, 2.3 days; FNC, 2.8 days). With LALB, there was a higher likelihood of discharge the day after surgery; 20% of patients in the LALB group and no patients in the FNC group went home that day.
The implication is that inadequate pain control led to decreased motion and decreased progression during postoperative rehabilitation. Local infiltration resulted in increased total ROM (extension plus flexion) at 3-week follow-up (LALB, 116.3°; FNC, 107.2°). In addition, there was an increase in walking distance per day of hospital stay (LALB, 135.9 feet; FNC, 84.2 feet). Furthermore, patients indicated LALB when asked which anesthetic they preferred. To our knowledge, this is the first study to compare LALB and FNC data in a matched TKA cohort with each patient serving as his or her own control.
Our study had several limitations. First was the retrospective design. Second was the small sample size, which made definitive conclusions difficult. However, the statistically significant differences we noted validated our conclusions. A statistically significant difference favoring LALB over FNC was found for total MEDs during hospitalization, but there was no significant difference in per-day MEDs. A possible reason for this difference is that LALB patients had shorter hospital stays, and therefore received fewer doses overall. Another possible reason is the small sample size; whereas a larger study using our protocol may find a statistically significant difference between LALB and FNC, we found only a trend. In the FNC group, anesthetic infiltration occurred with use of a computerized pump, which was removed on postoperative day 2; most of these patients were discharged home that day or the morning of postoperative day 3. As it is possible that some of these patients could have gone home sooner, our LOS data may have been affected. We do not consider this limitation significant, as one of our discharge criteria was 150 feet of ambulation, and most patients who received FNCs could not ambulate that far until after FNC removal. Furthermore, this study compared LALB only with FNC. It is possible that our improved outcomes could have resulted from the PAIs themselves, irrespective of LALB. In a recent TKA study by Bagsby and colleagues,11 pain was controlled better with the less expensive traditional PAI of ropivacaine, epinephrine, and morphine than with the PAI of liposomal bupivacaine. Last, in our study, the experience of undergoing the first TKA may have increased patients’ confidence going into the second TKA and then helped them make faster progress in rehabilitation. Regardless, the promising results of our study and the firsthand use of LALB at our institution led us to modify our intraoperative pain management protocol for surgeons who perform TKA.
As we continue to use LALB, our study numbers will increase, and we may discover other factors that, though now underpowered, will prove to be statistically significant. Additional clinical studies are needed to better determine the efficacy and cost-effectiveness of LALB and other long-acting local anesthetic formulations.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
2. Ruiz D Jr, Koenig L, Dall TM, et al. The direct and indirect costs to society of treatment for end-stage knee osteoarthritis. J Bone Joint Surg Am. 2013;95(16):1473-1480.
3. Trousdale RT, McGrory BJ, Berry DJ, Becker MW, Harmsen WS. Patients’ concerns prior to undergoing total hip and total knee arthroplasty. Mayo Clin Proc. 1999;74(10):978-982.
4. Wells N, Pasero C, McCaffery M. Improving the quality of care through pain assessment and management. In: Hughes RG, ed. Patient Safety and Quality: An Evidence-Based Handbook for Nurses, Vol 1. Rockville, MD: Agency for Healthcare Research and Quality; 2008:469-497.
5. Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10(4):287-333.
6. Parvizi J, Miller AG, Gandhi K. Multimodal pain management after total joint arthroplasty. J Bone Joint Surg Am. 2011;93(11):1075-1084.
7. Stein BE, Srikumaran U, Tan EW, Freehill MT, Wilckens JH. Lower-extremity peripheral nerve blocks in the perioperative pain management of orthopaedic patients: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(22):e167.
8. Pugely AJ, Martin CT, Gao Y, Mendoza-Lattes S, Callaghan JJ. Differences in short-term complications between spinal and general anesthesia for primary total knee arthroplasty. J Bone Joint Surg Am. 2013;95(3):193-199.
9. Dalury DF, Lieberman JR, MacDonald SJ. Current and innovative pain management techniques in total knee arthroplasty. J Bone Joint Surg Am. 2011;93(20):1938-1943.
10. Portillo J, Kamar N, Melibary S, Quevedo E, Bergese S. Safety of liposome extended-release bupivacaine for postoperative pain control. Front Pharmacol. 2014;5:90.
11. Bagsby DT, Ireland PH, Meneghini RM. Liposomal bupivacaine versus traditional periarticular injection for pain control after total knee arthroplasty. J Arthroplasty. 2014;29(8):1687-1690.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
2. Ruiz D Jr, Koenig L, Dall TM, et al. The direct and indirect costs to society of treatment for end-stage knee osteoarthritis. J Bone Joint Surg Am. 2013;95(16):1473-1480.
3. Trousdale RT, McGrory BJ, Berry DJ, Becker MW, Harmsen WS. Patients’ concerns prior to undergoing total hip and total knee arthroplasty. Mayo Clin Proc. 1999;74(10):978-982.
4. Wells N, Pasero C, McCaffery M. Improving the quality of care through pain assessment and management. In: Hughes RG, ed. Patient Safety and Quality: An Evidence-Based Handbook for Nurses, Vol 1. Rockville, MD: Agency for Healthcare Research and Quality; 2008:469-497.
5. Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10(4):287-333.
6. Parvizi J, Miller AG, Gandhi K. Multimodal pain management after total joint arthroplasty. J Bone Joint Surg Am. 2011;93(11):1075-1084.
7. Stein BE, Srikumaran U, Tan EW, Freehill MT, Wilckens JH. Lower-extremity peripheral nerve blocks in the perioperative pain management of orthopaedic patients: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(22):e167.
8. Pugely AJ, Martin CT, Gao Y, Mendoza-Lattes S, Callaghan JJ. Differences in short-term complications between spinal and general anesthesia for primary total knee arthroplasty. J Bone Joint Surg Am. 2013;95(3):193-199.
9. Dalury DF, Lieberman JR, MacDonald SJ. Current and innovative pain management techniques in total knee arthroplasty. J Bone Joint Surg Am. 2011;93(20):1938-1943.
10. Portillo J, Kamar N, Melibary S, Quevedo E, Bergese S. Safety of liposome extended-release bupivacaine for postoperative pain control. Front Pharmacol. 2014;5:90.
11. Bagsby DT, Ireland PH, Meneghini RM. Liposomal bupivacaine versus traditional periarticular injection for pain control after total knee arthroplasty. J Arthroplasty. 2014;29(8):1687-1690.
Translunate, Transradial, Transtriquetral, Transtrapezoid Perilunate Dislocation With Multiple Metacarpal Neck Fractures
Take-Home Points
- Emergency physicians should be aware of radiological markers to avoid missing perilunate injuries.
- They should have a low threshold to refer a suspected perilunate injury for urgent specialist assessment.
- Although majority of the injuries demonstrate the classical pattern, one should be aware of atypical injuries.
- The principles of early anatomic reduction and stable fixation remain the same.
- Salvage procedures are only indicated in extensive irreparable injuries.
Perilunate fracture-dislocations, rare injuries representing <10% of wrist injuries,1 are part of a wide spectrum of high-energy trauma injuries. The typical mechanism of injury is a fall on a dorsiflexed and ulnar-deviated wrist with forces progressively traversing the scapholunate, lunocapitate, and lunotriquetral ligaments.2
In this article, we report a very unusual case of translunate, transradial, transtriquetral, transtrapezoid perilunate dislocation with multiple metacarpal neck fractures. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A fit and healthy 30-year-old male software professional fell down stairs, landed on his nondominant right hand, and sustained a high-energy wrist injury. The patient also sustained a concussion, without focal neurologic deficit, and was unable to recall the exact mechanism of the wrist injury (there were no other witnesses). Radiographs of the right wrist in the emergency department showed only a nondisplaced fracture of the neck of the second, third, fourth, and fifth metacarpals and a nondisplaced fracture of the radial styloid.
The next day, with the patient under general anesthesia, an attempt to reduce the perilunate dislocation by manipulation was unsuccessful. Open reduction and internal fixation (ORIF) were performed through a dorsal approach; the perilunate dislocation was reduced and stabilized with lunocapitate 1.2-mm Kirschner wire (K-wire). The scapholunate and lunotriquetral ligaments were found to be intact, and the significantly displaced triquetral fracture was treated with internal fixation involving 2 minifragment screws (Figure 6).
Discussion
Perilunate injuries are classified as lesser arc injuries (purely ligamentous) or greater arc injuries (osseoligamentous). Greater arc injuries involve fracture of one or more carpal bones with associated ligamentous injuries.3 The greater or lesser arc injuries described by Mayfield and colleagues2 imply a specific pattern of force transmission with axial loading in a dorsiflexed and ulnar-deviated wrist with intercarpal supination. Graham4 introduced a concept of inferior arc injury with the forces passing through the radiocarpal joint with fracture of the radial styloid or juxta-articular margin. Similarly, lunate fracture in perilunate dislocations was explained by Bain and colleagues5 in the translunate arc concept in which forces pass through the lunate bone. A study involving a literature review of translunate perilunate dislocations noted associated transradial, trans-scaphoid, transcapitate, and transtriquetral fractures in order of decreasing frequency.6 To our knowledge, no case of translunate perilunate dislocation with multiple carpal and metacarpal fractures with radial styloid fracture has been reported in the literature.
Our patient’s associated multiple metacarpal neck fractures can be explained by the peculiar double-impact injury with initial axial loading across the hyperextended metacarpophalangeal joint, followed by axial loading across the hyperextended and ulnar-deviated wrist, causing greater arc perilunate fracture-dislocation. The mechanism of lunate injury in this case seems to be longitudinal impaction of the capitate shearing against the volar lunate in the axial plane causing a volar lip fracture (Teisen type I), and this may be accentuated by tension in the volar radiolunate ligament.6,7 Associated triquetral fracture in perilunate dislocation is well described in the literature.6 However, the trapezoid fracture in our case implies a very atypical pattern of force transmission with the arc probably passing more distally through the trapezoid laterally and the triquetrum medially.
This case, which represents a very rare fracture pattern associated with perilunate dislocation, may have been caused by the variable position of the wrist and the pattern of load transmission at time of impact. Although the majority of cases demonstrate the classical pattern described in the literature, it may not be unusual to find atypical fracture patterns, especially those associated with high-energy trauma.
Perilunate injuries have been missed in busy emergency departments and orthopedic practices. An estimated 25% of such injuries can be missed on initial presentation.8 In the present case, fracture of the radial styloid provided a clue to possible more complex carpal injuries involving the scaphoid, lunate, or scapholunate ligament, as Graham4 suggested with the concept of the “transverse pattern” of force transmission. In this case as well, the injury was initially missed, and its extent became evident only with CT. Therefore, emergency teams should have a very low threshold for suspecting and evaluating high-energy wrist injuries.
The goal in the treatment of perilunate dislocation with multiple carpal fractures is anatomical reduction and restoration of carpal alignment—which frequently require ORIF, though acute salvage procedures like proximal row carpectomy may be considered in irreparable fractures with extensive ligament injuries.9 For open reduction, the approach can be dorsal, volar, or a combination. The approach in our patient’s case was dorsal. His triquetral fracture, his only displaced fracture, was treated with internal fixation. All other fractures were nondisplaced, stable, and did not warrant internal fixation.
A high index of suspicion and urgent specialist consultation are essential in suspected perilunate injuries. The injury and fracture pattern may be atypical, but the principles of early anatomical reduction and stable fixation remain the same.
1. Youssef B, Deshmukh SC. Volar perilunate dislocation: a case report and review of the literature. Open Orthop J. 2008;2:57-58.
2. Mayfield JK, Johnson RP, Kilcoyne RK. Carpal dislocations: pathomechanics and progressive perilunar instability. J Hand Surg Am. 1980;5(3):226-241.
3. Johnson RP. The acutely injured wrist and its residuals. Clin Orthop Relat Res. 1980;(149):33-44.
4. Graham TJ. The inferior arc injury: an addition to the family of complex carpal fracture-dislocation patterns. Am J Orthop. 2003;32(9 suppl):10-19.
5. Bain GI, McLean JM, Turner PC, Sood A, Pourgiezis N. Translunate fracture with associated perilunate injury: 3 case reports with introduction of the translunate arc concept. J Hand Surg Am. 2008;33(10):1770-1776.
6. Bain GI, Pallapati S, Eng K. Translunate perilunate injuries—a spectrum of this uncommon injury. J Wrist Surg. 2013;2(1):63-68.
7. Teisen H, Hjarbaek J. Classification of fresh fractures of the lunate. J Hand Surg Br. 1988;13(4):458-462.
8. Herzberg G, Comtet JJ, Linscheid RL, Amadio PC, Cooney WP, Stalder J. Perilunate dislocations and fracture-dislocations: a multicenter study. J Hand Surg Am. 1993;18(5):768-779.
9. Huish EG Jr, Vitale MA, Shin AY. Acute proximal row carpectomy to treat a transscaphoid, transtriquetral perilunate fracture dislocation: case report and review of the literature. Hand
(N Y). 2013;8(1):105-109.
Take-Home Points
- Emergency physicians should be aware of radiological markers to avoid missing perilunate injuries.
- They should have a low threshold to refer a suspected perilunate injury for urgent specialist assessment.
- Although majority of the injuries demonstrate the classical pattern, one should be aware of atypical injuries.
- The principles of early anatomic reduction and stable fixation remain the same.
- Salvage procedures are only indicated in extensive irreparable injuries.
Perilunate fracture-dislocations, rare injuries representing <10% of wrist injuries,1 are part of a wide spectrum of high-energy trauma injuries. The typical mechanism of injury is a fall on a dorsiflexed and ulnar-deviated wrist with forces progressively traversing the scapholunate, lunocapitate, and lunotriquetral ligaments.2
In this article, we report a very unusual case of translunate, transradial, transtriquetral, transtrapezoid perilunate dislocation with multiple metacarpal neck fractures. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A fit and healthy 30-year-old male software professional fell down stairs, landed on his nondominant right hand, and sustained a high-energy wrist injury. The patient also sustained a concussion, without focal neurologic deficit, and was unable to recall the exact mechanism of the wrist injury (there were no other witnesses). Radiographs of the right wrist in the emergency department showed only a nondisplaced fracture of the neck of the second, third, fourth, and fifth metacarpals and a nondisplaced fracture of the radial styloid.
The next day, with the patient under general anesthesia, an attempt to reduce the perilunate dislocation by manipulation was unsuccessful. Open reduction and internal fixation (ORIF) were performed through a dorsal approach; the perilunate dislocation was reduced and stabilized with lunocapitate 1.2-mm Kirschner wire (K-wire). The scapholunate and lunotriquetral ligaments were found to be intact, and the significantly displaced triquetral fracture was treated with internal fixation involving 2 minifragment screws (Figure 6).
Discussion
Perilunate injuries are classified as lesser arc injuries (purely ligamentous) or greater arc injuries (osseoligamentous). Greater arc injuries involve fracture of one or more carpal bones with associated ligamentous injuries.3 The greater or lesser arc injuries described by Mayfield and colleagues2 imply a specific pattern of force transmission with axial loading in a dorsiflexed and ulnar-deviated wrist with intercarpal supination. Graham4 introduced a concept of inferior arc injury with the forces passing through the radiocarpal joint with fracture of the radial styloid or juxta-articular margin. Similarly, lunate fracture in perilunate dislocations was explained by Bain and colleagues5 in the translunate arc concept in which forces pass through the lunate bone. A study involving a literature review of translunate perilunate dislocations noted associated transradial, trans-scaphoid, transcapitate, and transtriquetral fractures in order of decreasing frequency.6 To our knowledge, no case of translunate perilunate dislocation with multiple carpal and metacarpal fractures with radial styloid fracture has been reported in the literature.
Our patient’s associated multiple metacarpal neck fractures can be explained by the peculiar double-impact injury with initial axial loading across the hyperextended metacarpophalangeal joint, followed by axial loading across the hyperextended and ulnar-deviated wrist, causing greater arc perilunate fracture-dislocation. The mechanism of lunate injury in this case seems to be longitudinal impaction of the capitate shearing against the volar lunate in the axial plane causing a volar lip fracture (Teisen type I), and this may be accentuated by tension in the volar radiolunate ligament.6,7 Associated triquetral fracture in perilunate dislocation is well described in the literature.6 However, the trapezoid fracture in our case implies a very atypical pattern of force transmission with the arc probably passing more distally through the trapezoid laterally and the triquetrum medially.
This case, which represents a very rare fracture pattern associated with perilunate dislocation, may have been caused by the variable position of the wrist and the pattern of load transmission at time of impact. Although the majority of cases demonstrate the classical pattern described in the literature, it may not be unusual to find atypical fracture patterns, especially those associated with high-energy trauma.
Perilunate injuries have been missed in busy emergency departments and orthopedic practices. An estimated 25% of such injuries can be missed on initial presentation.8 In the present case, fracture of the radial styloid provided a clue to possible more complex carpal injuries involving the scaphoid, lunate, or scapholunate ligament, as Graham4 suggested with the concept of the “transverse pattern” of force transmission. In this case as well, the injury was initially missed, and its extent became evident only with CT. Therefore, emergency teams should have a very low threshold for suspecting and evaluating high-energy wrist injuries.
The goal in the treatment of perilunate dislocation with multiple carpal fractures is anatomical reduction and restoration of carpal alignment—which frequently require ORIF, though acute salvage procedures like proximal row carpectomy may be considered in irreparable fractures with extensive ligament injuries.9 For open reduction, the approach can be dorsal, volar, or a combination. The approach in our patient’s case was dorsal. His triquetral fracture, his only displaced fracture, was treated with internal fixation. All other fractures were nondisplaced, stable, and did not warrant internal fixation.
A high index of suspicion and urgent specialist consultation are essential in suspected perilunate injuries. The injury and fracture pattern may be atypical, but the principles of early anatomical reduction and stable fixation remain the same.
Take-Home Points
- Emergency physicians should be aware of radiological markers to avoid missing perilunate injuries.
- They should have a low threshold to refer a suspected perilunate injury for urgent specialist assessment.
- Although majority of the injuries demonstrate the classical pattern, one should be aware of atypical injuries.
- The principles of early anatomic reduction and stable fixation remain the same.
- Salvage procedures are only indicated in extensive irreparable injuries.
Perilunate fracture-dislocations, rare injuries representing <10% of wrist injuries,1 are part of a wide spectrum of high-energy trauma injuries. The typical mechanism of injury is a fall on a dorsiflexed and ulnar-deviated wrist with forces progressively traversing the scapholunate, lunocapitate, and lunotriquetral ligaments.2
In this article, we report a very unusual case of translunate, transradial, transtriquetral, transtrapezoid perilunate dislocation with multiple metacarpal neck fractures. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A fit and healthy 30-year-old male software professional fell down stairs, landed on his nondominant right hand, and sustained a high-energy wrist injury. The patient also sustained a concussion, without focal neurologic deficit, and was unable to recall the exact mechanism of the wrist injury (there were no other witnesses). Radiographs of the right wrist in the emergency department showed only a nondisplaced fracture of the neck of the second, third, fourth, and fifth metacarpals and a nondisplaced fracture of the radial styloid.
The next day, with the patient under general anesthesia, an attempt to reduce the perilunate dislocation by manipulation was unsuccessful. Open reduction and internal fixation (ORIF) were performed through a dorsal approach; the perilunate dislocation was reduced and stabilized with lunocapitate 1.2-mm Kirschner wire (K-wire). The scapholunate and lunotriquetral ligaments were found to be intact, and the significantly displaced triquetral fracture was treated with internal fixation involving 2 minifragment screws (Figure 6).
Discussion
Perilunate injuries are classified as lesser arc injuries (purely ligamentous) or greater arc injuries (osseoligamentous). Greater arc injuries involve fracture of one or more carpal bones with associated ligamentous injuries.3 The greater or lesser arc injuries described by Mayfield and colleagues2 imply a specific pattern of force transmission with axial loading in a dorsiflexed and ulnar-deviated wrist with intercarpal supination. Graham4 introduced a concept of inferior arc injury with the forces passing through the radiocarpal joint with fracture of the radial styloid or juxta-articular margin. Similarly, lunate fracture in perilunate dislocations was explained by Bain and colleagues5 in the translunate arc concept in which forces pass through the lunate bone. A study involving a literature review of translunate perilunate dislocations noted associated transradial, trans-scaphoid, transcapitate, and transtriquetral fractures in order of decreasing frequency.6 To our knowledge, no case of translunate perilunate dislocation with multiple carpal and metacarpal fractures with radial styloid fracture has been reported in the literature.
Our patient’s associated multiple metacarpal neck fractures can be explained by the peculiar double-impact injury with initial axial loading across the hyperextended metacarpophalangeal joint, followed by axial loading across the hyperextended and ulnar-deviated wrist, causing greater arc perilunate fracture-dislocation. The mechanism of lunate injury in this case seems to be longitudinal impaction of the capitate shearing against the volar lunate in the axial plane causing a volar lip fracture (Teisen type I), and this may be accentuated by tension in the volar radiolunate ligament.6,7 Associated triquetral fracture in perilunate dislocation is well described in the literature.6 However, the trapezoid fracture in our case implies a very atypical pattern of force transmission with the arc probably passing more distally through the trapezoid laterally and the triquetrum medially.
This case, which represents a very rare fracture pattern associated with perilunate dislocation, may have been caused by the variable position of the wrist and the pattern of load transmission at time of impact. Although the majority of cases demonstrate the classical pattern described in the literature, it may not be unusual to find atypical fracture patterns, especially those associated with high-energy trauma.
Perilunate injuries have been missed in busy emergency departments and orthopedic practices. An estimated 25% of such injuries can be missed on initial presentation.8 In the present case, fracture of the radial styloid provided a clue to possible more complex carpal injuries involving the scaphoid, lunate, or scapholunate ligament, as Graham4 suggested with the concept of the “transverse pattern” of force transmission. In this case as well, the injury was initially missed, and its extent became evident only with CT. Therefore, emergency teams should have a very low threshold for suspecting and evaluating high-energy wrist injuries.
The goal in the treatment of perilunate dislocation with multiple carpal fractures is anatomical reduction and restoration of carpal alignment—which frequently require ORIF, though acute salvage procedures like proximal row carpectomy may be considered in irreparable fractures with extensive ligament injuries.9 For open reduction, the approach can be dorsal, volar, or a combination. The approach in our patient’s case was dorsal. His triquetral fracture, his only displaced fracture, was treated with internal fixation. All other fractures were nondisplaced, stable, and did not warrant internal fixation.
A high index of suspicion and urgent specialist consultation are essential in suspected perilunate injuries. The injury and fracture pattern may be atypical, but the principles of early anatomical reduction and stable fixation remain the same.
1. Youssef B, Deshmukh SC. Volar perilunate dislocation: a case report and review of the literature. Open Orthop J. 2008;2:57-58.
2. Mayfield JK, Johnson RP, Kilcoyne RK. Carpal dislocations: pathomechanics and progressive perilunar instability. J Hand Surg Am. 1980;5(3):226-241.
3. Johnson RP. The acutely injured wrist and its residuals. Clin Orthop Relat Res. 1980;(149):33-44.
4. Graham TJ. The inferior arc injury: an addition to the family of complex carpal fracture-dislocation patterns. Am J Orthop. 2003;32(9 suppl):10-19.
5. Bain GI, McLean JM, Turner PC, Sood A, Pourgiezis N. Translunate fracture with associated perilunate injury: 3 case reports with introduction of the translunate arc concept. J Hand Surg Am. 2008;33(10):1770-1776.
6. Bain GI, Pallapati S, Eng K. Translunate perilunate injuries—a spectrum of this uncommon injury. J Wrist Surg. 2013;2(1):63-68.
7. Teisen H, Hjarbaek J. Classification of fresh fractures of the lunate. J Hand Surg Br. 1988;13(4):458-462.
8. Herzberg G, Comtet JJ, Linscheid RL, Amadio PC, Cooney WP, Stalder J. Perilunate dislocations and fracture-dislocations: a multicenter study. J Hand Surg Am. 1993;18(5):768-779.
9. Huish EG Jr, Vitale MA, Shin AY. Acute proximal row carpectomy to treat a transscaphoid, transtriquetral perilunate fracture dislocation: case report and review of the literature. Hand
(N Y). 2013;8(1):105-109.
1. Youssef B, Deshmukh SC. Volar perilunate dislocation: a case report and review of the literature. Open Orthop J. 2008;2:57-58.
2. Mayfield JK, Johnson RP, Kilcoyne RK. Carpal dislocations: pathomechanics and progressive perilunar instability. J Hand Surg Am. 1980;5(3):226-241.
3. Johnson RP. The acutely injured wrist and its residuals. Clin Orthop Relat Res. 1980;(149):33-44.
4. Graham TJ. The inferior arc injury: an addition to the family of complex carpal fracture-dislocation patterns. Am J Orthop. 2003;32(9 suppl):10-19.
5. Bain GI, McLean JM, Turner PC, Sood A, Pourgiezis N. Translunate fracture with associated perilunate injury: 3 case reports with introduction of the translunate arc concept. J Hand Surg Am. 2008;33(10):1770-1776.
6. Bain GI, Pallapati S, Eng K. Translunate perilunate injuries—a spectrum of this uncommon injury. J Wrist Surg. 2013;2(1):63-68.
7. Teisen H, Hjarbaek J. Classification of fresh fractures of the lunate. J Hand Surg Br. 1988;13(4):458-462.
8. Herzberg G, Comtet JJ, Linscheid RL, Amadio PC, Cooney WP, Stalder J. Perilunate dislocations and fracture-dislocations: a multicenter study. J Hand Surg Am. 1993;18(5):768-779.
9. Huish EG Jr, Vitale MA, Shin AY. Acute proximal row carpectomy to treat a transscaphoid, transtriquetral perilunate fracture dislocation: case report and review of the literature. Hand
(N Y). 2013;8(1):105-109.
Malpractice Counsel: Don’t Miss Popeye
A 42-year-old man presented to the ED with left arm pain secondary to an injury he sustained at work. The patient stated that he had been helping to lift a heavy steel beam at a construction site when he experienced abrupt onset of pain in his left arm. He further noted that his left arm felt slightly weaker than normal after the injury.
The patient was left-hand dominant, denied any other injury, was otherwise in good health, and on no medications. With the exception of an appendectomy at age 12 years, his medical history was unremarkable. Regarding his social history, he admitted to smoking one pack of cigarettes per day, and to occasional alcohol consumption. He had no known drug allergies.
On physical examination, the patient’s vital signs were: blood pressure, 125/76 mm Hg; heart rate, 78 beats/min; respiratory rate, 16 breaths/min; and temperature, 98.6°F. Oxygen saturation was 99% on room air.
Examination of the patient’s left shoulder revealed no swelling or tenderness; he was able to fully internally/externally rotate the left shoulder, and lift his left hand above his head. The patient did have tenderness along the biceps area of the left arm, but no tenderness in the triceps area. The left elbow was tender in the antecubital fossa, but without swelling. He had full range of motion of the left elbow but with some pain. He likewise had full range of motion in his left wrist, but no tenderness or swelling. The left radial pulse was 2+. The patient had 5/5 grip strength with the left hand and good capillary refill.
The physician assistant (PA) evaluating the patient diagnosed an arm strain. At discharge, he referred the patient to an occupational health physician (OHP) for follow-up. He also instructed the patient to take ibuprofen 400 mg every 6 to 8 hours, and to limit use of his left arm for 3 days.
The patient followed up with the OHP approximately 3 weeks after discharge from the ED. The OHP was concerned the patient had experienced a distal biceps tendon rupture and referred the patient emergently to an orthopedic surgeon. The orthopedic surgeon saw the patient the next day, agreed with the diagnosis of a distal biceps tendon rupture, and attempted surgical repair the following day. The orthopedic surgeon informed the patient prior to the surgery that the delay in the referral and surgery could result in a poor functional outcome. The patient did have a difficult recovery period, and a second surgery was required, which did not result in any significant functional improvement.
The plaintiff sued the treating PA and supervising emergency physician (EP) for failure to properly diagnose the biceps tendon rupture, failure to appreciate the existence of a 3-week window of opportunity to repair the distal biceps tendon rupture, and failure to obtain an urgent orthopedic referral. The experts for the defense argued that the poor outcome was not a consequence of any delay in diagnosis or surgical repair. In addition, the defense disputed the existence of a 3-week window of opportunity for successful repair of a distal biceps tendon rupture. The jury returned a defense verdict.
Discussion
Proximal and Distal Biceps Tendon Ruptures
While both proximal and distal biceps tendon ruptures involve the biceps brachii, they are managed differently and have the potential for very different outcomes.1 At its proximal attachment, the biceps has two distinct tendinous insertions—the long head and the short head. For the distal attachment, the two muscle bellies unite at the midshaft of the humerus and attach as a single tendon on the radial tuberosity. In general, 96% of biceps tendon ruptures involve the long head, 1% involve the short head, and only 3% involve the distal tendon.1 Biceps tendon ruptures occur more commonly in men, patients who use anabolic steroids, cigarette smokers, patient history of tendinopathy, or patients who have a rotator cuff tear.1 Biceps tendon ruptures have not been found to be associated with statin use.2 The mechanism of injury includes heavy-lifting activities, such as weight lifting and rock climbing. However, when associated with a tendinopathy, minimal force may be involved.1
Signs and Symptoms
For proximal biceps tendon rupture, patients usually present with an acute or gradual onset of pain, swelling, and bruising of the upper arm and shoulder. Occasionally, if there is an inciting event, the patient may describe hearing or feeling a “popping” or “snapping” sound. On physical examination, the patient may exhibit a “Popeye” sign—a bulge in the distal biceps area due to the retracted biceps muscle belly. There is also tenderness along the biceps.
On testing, it has been estimated that patients can experience strength loss of approximately 30% with elbow flexion.1 In contrast, patients with distal biceps tendon ruptures usually complain of pain, swelling, and possibly bruising in the antecubital fossa, as was the case with this patient. Similar to proximal ruptures, the patient may admit to hearing or feeling a “popping” sound if there is an inciting event. The patient may exhibit a “reverse Popeye” deformity, with a bulge in the proximal arm secondary to retraction of the biceps muscle belly proximally.1
Diagnosis
There are two tests that can be performed to assist in making the diagnosis—the biceps squeeze test and the hook test.
Biceps Squeeze Test. The first test to assess for distal biceps tendon rupture is the biceps squeeze test, in which the clinician forcefully squeezes the patient’s biceps muscle to observe for forearm flexion/supination. This test is similar in principle to the Thompson test for Achilles tendon rupture. If there is no forearm movement, the injury is suspicious for a complete distal biceps tendon rupture. In one observational study of this test, 21 of 22 patients with a positive biceps squeeze test were found to have a complete distal biceps tendon tear at surgery.3
Hook Test. The second test is the hook test. While the patient actively supinates with the elbow flexed at 900, an intact hook test permits the examiner to “hook” his or her index finger under the intact biceps tendon from the lateral side. The absence of a “hook” means that there is no cord-like structure under which the examiner can hook a finger, indicating distal avulsion.4 In one study comparing the hook test to magnetic resonance imaging (MRI) in 33 patients with this suspected injury, the hook test had 100% sensitivity and specificity, while MRI only demonstrated a 92% sensitivity and 85% specificity.4
Imaging Techniques
The need for diagnostic imaging is based somewhat on the location of the rupture—proximal or distal. Ultrasound has been shown to have a high sensitivity and specificity for identifying normal tendons and complete tears of the long head biceps tendon (ie, proximal). It is not sensitive at identifying proximal partial tears, however. For distal ruptures, ultrasound imaging of the distal biceps tendon is technically difficult and not reliable. For patients with suspected distal biceps tendon ruptures, the EP should consult with orthopedic services prior to ordering an MRI. While MRI is considered the gold standard imaging test, it is neither 100% sensitive nor specific. The bottom line is that the absence of pathologic findings on MRI is not sufficient enough to exclude biceps tendon pathology.5
Treatment and Management
Regarding management, the majority of patients with proximal biceps tendon ruptures tend to do well with conservative management. The exception is for younger, active patients who are less willing to accept the cosmetic deformity, or patients whose occupation makes them unable to tolerate minimal weakness or fatigue cramping (eg, carpenters), in which case referral for a surgical repair (tenodesis) may be appropriate.1 However, multiple systematic reviews examining tenotomy vs tenodesis have not shown any functional improvement, only cosmetic.1,6,7
Distal biceps tendon ruptures are usually treated surgically, since conservative management results in a decrease of 30% to 50% supination strength and 20% flexion strength.1,8 This surgery, however, is not without complications. Approximately 20% of the patients will have a minor complication and 5% will have major complications following surgery on the distal biceps tendon.9 It is preferable to operate on distal ruptures less than 4 weeks from the initial injury; otherwise, these injuries may be more difficult to fix, require a graft, and have less predictable outcomes.1 Nonoperative management should be reserved for the elderly or less active patients with multiple comorbidities, especially if the nondominant arm is involved.10
Summary
The PA clearly missed the correct diagnosis on this patient. A more thorough history and focused physical examination would have led to the correct diagnosis sooner, along with earlier surgical repair. It is impossible, however, to know if the outcome would have been any different in this uncommon injury.
1. Smith D. Proximal versus distal biceps tendon ruptures: when to refer. BCMJ. 2017;59(2):85.
2. Spoendlin J, Layton JB, Mundkur M, Meier C, Jick SS, Meier CR. The risk of achilles or biceps tendon rupture in new statin users: a propensity score-matched sequential cohort study. Drug Safety. 2016;39(12):1229-1237. doi:10.1007/s40264-016-0462-5.
3. Ruland RT, Dunbar RP, Bowen JD. The biceps squeeze test for diagnosis of distal biceps tendon ruptures. Clin Orthop Relat Res. 2005;437:128-131.
4. O’Driscoll SW, Goncalves LBJ, Dietz P. The hook test for distal biceps tendon avulsion. Am J Sports Med. 2007;35(11):1865-1969. doi:10.1177/0363546507305016.
5. Malavolta EA, Assunção JH, Guglielmetti CL, de Souza FF, Gracitelli ME, Ferreira Neto AA. Accuracy of preoperative MRI in the diagnosis of disorders of the long head of the biceps tendon. Eur J Radiol. 2015;84(11):2250-2254. doi:10.1016/j.ejrad.2015.07.031.
6. Tangari M, Carbone S, Gallo M, Campi A. Long head of the biceps tendon rupture in professional wrestlers: treatment with a mini-open tenodesis. J Shoulder Elbow Surg. 2011;20(3):409-413. doi:10.1016/j.jse.2010.08.008.
7. Eakin JL, Bailey JR, Dewing CB, Provencher MT. Subpectoral biceps tenodesis. Oper Tech Sports Med. 2012;20(3):244-252.
8. Thomas JR, Lawton JN. Biceps and triceps ruptures in athletes. Hand Clin. 2017;33(1):35-46. doi:10.1016/j.hcl.2016.08.019.
9. Beks RB, Claessen FM, Oh LS, Ring D, Chen NC. Factors associated with adverse events after distal biceps tendon repair or reconstruction. J Shoulder Elbow Surg. 2016;25(8):1229-1234. doi:10.1016/j.jse.2016.02.032.
10. Savin DD, Watson J, Youderian AR, et al. Surgical management of acute distal biceps tendon ruptures. J Bone Joint Surg. 2017;3(9):785-796. doi:0.2106/JBJS.17.00080.
A 42-year-old man presented to the ED with left arm pain secondary to an injury he sustained at work. The patient stated that he had been helping to lift a heavy steel beam at a construction site when he experienced abrupt onset of pain in his left arm. He further noted that his left arm felt slightly weaker than normal after the injury.
The patient was left-hand dominant, denied any other injury, was otherwise in good health, and on no medications. With the exception of an appendectomy at age 12 years, his medical history was unremarkable. Regarding his social history, he admitted to smoking one pack of cigarettes per day, and to occasional alcohol consumption. He had no known drug allergies.
On physical examination, the patient’s vital signs were: blood pressure, 125/76 mm Hg; heart rate, 78 beats/min; respiratory rate, 16 breaths/min; and temperature, 98.6°F. Oxygen saturation was 99% on room air.
Examination of the patient’s left shoulder revealed no swelling or tenderness; he was able to fully internally/externally rotate the left shoulder, and lift his left hand above his head. The patient did have tenderness along the biceps area of the left arm, but no tenderness in the triceps area. The left elbow was tender in the antecubital fossa, but without swelling. He had full range of motion of the left elbow but with some pain. He likewise had full range of motion in his left wrist, but no tenderness or swelling. The left radial pulse was 2+. The patient had 5/5 grip strength with the left hand and good capillary refill.
The physician assistant (PA) evaluating the patient diagnosed an arm strain. At discharge, he referred the patient to an occupational health physician (OHP) for follow-up. He also instructed the patient to take ibuprofen 400 mg every 6 to 8 hours, and to limit use of his left arm for 3 days.
The patient followed up with the OHP approximately 3 weeks after discharge from the ED. The OHP was concerned the patient had experienced a distal biceps tendon rupture and referred the patient emergently to an orthopedic surgeon. The orthopedic surgeon saw the patient the next day, agreed with the diagnosis of a distal biceps tendon rupture, and attempted surgical repair the following day. The orthopedic surgeon informed the patient prior to the surgery that the delay in the referral and surgery could result in a poor functional outcome. The patient did have a difficult recovery period, and a second surgery was required, which did not result in any significant functional improvement.
The plaintiff sued the treating PA and supervising emergency physician (EP) for failure to properly diagnose the biceps tendon rupture, failure to appreciate the existence of a 3-week window of opportunity to repair the distal biceps tendon rupture, and failure to obtain an urgent orthopedic referral. The experts for the defense argued that the poor outcome was not a consequence of any delay in diagnosis or surgical repair. In addition, the defense disputed the existence of a 3-week window of opportunity for successful repair of a distal biceps tendon rupture. The jury returned a defense verdict.
Discussion
Proximal and Distal Biceps Tendon Ruptures
While both proximal and distal biceps tendon ruptures involve the biceps brachii, they are managed differently and have the potential for very different outcomes.1 At its proximal attachment, the biceps has two distinct tendinous insertions—the long head and the short head. For the distal attachment, the two muscle bellies unite at the midshaft of the humerus and attach as a single tendon on the radial tuberosity. In general, 96% of biceps tendon ruptures involve the long head, 1% involve the short head, and only 3% involve the distal tendon.1 Biceps tendon ruptures occur more commonly in men, patients who use anabolic steroids, cigarette smokers, patient history of tendinopathy, or patients who have a rotator cuff tear.1 Biceps tendon ruptures have not been found to be associated with statin use.2 The mechanism of injury includes heavy-lifting activities, such as weight lifting and rock climbing. However, when associated with a tendinopathy, minimal force may be involved.1
Signs and Symptoms
For proximal biceps tendon rupture, patients usually present with an acute or gradual onset of pain, swelling, and bruising of the upper arm and shoulder. Occasionally, if there is an inciting event, the patient may describe hearing or feeling a “popping” or “snapping” sound. On physical examination, the patient may exhibit a “Popeye” sign—a bulge in the distal biceps area due to the retracted biceps muscle belly. There is also tenderness along the biceps.
On testing, it has been estimated that patients can experience strength loss of approximately 30% with elbow flexion.1 In contrast, patients with distal biceps tendon ruptures usually complain of pain, swelling, and possibly bruising in the antecubital fossa, as was the case with this patient. Similar to proximal ruptures, the patient may admit to hearing or feeling a “popping” sound if there is an inciting event. The patient may exhibit a “reverse Popeye” deformity, with a bulge in the proximal arm secondary to retraction of the biceps muscle belly proximally.1
Diagnosis
There are two tests that can be performed to assist in making the diagnosis—the biceps squeeze test and the hook test.
Biceps Squeeze Test. The first test to assess for distal biceps tendon rupture is the biceps squeeze test, in which the clinician forcefully squeezes the patient’s biceps muscle to observe for forearm flexion/supination. This test is similar in principle to the Thompson test for Achilles tendon rupture. If there is no forearm movement, the injury is suspicious for a complete distal biceps tendon rupture. In one observational study of this test, 21 of 22 patients with a positive biceps squeeze test were found to have a complete distal biceps tendon tear at surgery.3
Hook Test. The second test is the hook test. While the patient actively supinates with the elbow flexed at 900, an intact hook test permits the examiner to “hook” his or her index finger under the intact biceps tendon from the lateral side. The absence of a “hook” means that there is no cord-like structure under which the examiner can hook a finger, indicating distal avulsion.4 In one study comparing the hook test to magnetic resonance imaging (MRI) in 33 patients with this suspected injury, the hook test had 100% sensitivity and specificity, while MRI only demonstrated a 92% sensitivity and 85% specificity.4
Imaging Techniques
The need for diagnostic imaging is based somewhat on the location of the rupture—proximal or distal. Ultrasound has been shown to have a high sensitivity and specificity for identifying normal tendons and complete tears of the long head biceps tendon (ie, proximal). It is not sensitive at identifying proximal partial tears, however. For distal ruptures, ultrasound imaging of the distal biceps tendon is technically difficult and not reliable. For patients with suspected distal biceps tendon ruptures, the EP should consult with orthopedic services prior to ordering an MRI. While MRI is considered the gold standard imaging test, it is neither 100% sensitive nor specific. The bottom line is that the absence of pathologic findings on MRI is not sufficient enough to exclude biceps tendon pathology.5
Treatment and Management
Regarding management, the majority of patients with proximal biceps tendon ruptures tend to do well with conservative management. The exception is for younger, active patients who are less willing to accept the cosmetic deformity, or patients whose occupation makes them unable to tolerate minimal weakness or fatigue cramping (eg, carpenters), in which case referral for a surgical repair (tenodesis) may be appropriate.1 However, multiple systematic reviews examining tenotomy vs tenodesis have not shown any functional improvement, only cosmetic.1,6,7
Distal biceps tendon ruptures are usually treated surgically, since conservative management results in a decrease of 30% to 50% supination strength and 20% flexion strength.1,8 This surgery, however, is not without complications. Approximately 20% of the patients will have a minor complication and 5% will have major complications following surgery on the distal biceps tendon.9 It is preferable to operate on distal ruptures less than 4 weeks from the initial injury; otherwise, these injuries may be more difficult to fix, require a graft, and have less predictable outcomes.1 Nonoperative management should be reserved for the elderly or less active patients with multiple comorbidities, especially if the nondominant arm is involved.10
Summary
The PA clearly missed the correct diagnosis on this patient. A more thorough history and focused physical examination would have led to the correct diagnosis sooner, along with earlier surgical repair. It is impossible, however, to know if the outcome would have been any different in this uncommon injury.
A 42-year-old man presented to the ED with left arm pain secondary to an injury he sustained at work. The patient stated that he had been helping to lift a heavy steel beam at a construction site when he experienced abrupt onset of pain in his left arm. He further noted that his left arm felt slightly weaker than normal after the injury.
The patient was left-hand dominant, denied any other injury, was otherwise in good health, and on no medications. With the exception of an appendectomy at age 12 years, his medical history was unremarkable. Regarding his social history, he admitted to smoking one pack of cigarettes per day, and to occasional alcohol consumption. He had no known drug allergies.
On physical examination, the patient’s vital signs were: blood pressure, 125/76 mm Hg; heart rate, 78 beats/min; respiratory rate, 16 breaths/min; and temperature, 98.6°F. Oxygen saturation was 99% on room air.
Examination of the patient’s left shoulder revealed no swelling or tenderness; he was able to fully internally/externally rotate the left shoulder, and lift his left hand above his head. The patient did have tenderness along the biceps area of the left arm, but no tenderness in the triceps area. The left elbow was tender in the antecubital fossa, but without swelling. He had full range of motion of the left elbow but with some pain. He likewise had full range of motion in his left wrist, but no tenderness or swelling. The left radial pulse was 2+. The patient had 5/5 grip strength with the left hand and good capillary refill.
The physician assistant (PA) evaluating the patient diagnosed an arm strain. At discharge, he referred the patient to an occupational health physician (OHP) for follow-up. He also instructed the patient to take ibuprofen 400 mg every 6 to 8 hours, and to limit use of his left arm for 3 days.
The patient followed up with the OHP approximately 3 weeks after discharge from the ED. The OHP was concerned the patient had experienced a distal biceps tendon rupture and referred the patient emergently to an orthopedic surgeon. The orthopedic surgeon saw the patient the next day, agreed with the diagnosis of a distal biceps tendon rupture, and attempted surgical repair the following day. The orthopedic surgeon informed the patient prior to the surgery that the delay in the referral and surgery could result in a poor functional outcome. The patient did have a difficult recovery period, and a second surgery was required, which did not result in any significant functional improvement.
The plaintiff sued the treating PA and supervising emergency physician (EP) for failure to properly diagnose the biceps tendon rupture, failure to appreciate the existence of a 3-week window of opportunity to repair the distal biceps tendon rupture, and failure to obtain an urgent orthopedic referral. The experts for the defense argued that the poor outcome was not a consequence of any delay in diagnosis or surgical repair. In addition, the defense disputed the existence of a 3-week window of opportunity for successful repair of a distal biceps tendon rupture. The jury returned a defense verdict.
Discussion
Proximal and Distal Biceps Tendon Ruptures
While both proximal and distal biceps tendon ruptures involve the biceps brachii, they are managed differently and have the potential for very different outcomes.1 At its proximal attachment, the biceps has two distinct tendinous insertions—the long head and the short head. For the distal attachment, the two muscle bellies unite at the midshaft of the humerus and attach as a single tendon on the radial tuberosity. In general, 96% of biceps tendon ruptures involve the long head, 1% involve the short head, and only 3% involve the distal tendon.1 Biceps tendon ruptures occur more commonly in men, patients who use anabolic steroids, cigarette smokers, patient history of tendinopathy, or patients who have a rotator cuff tear.1 Biceps tendon ruptures have not been found to be associated with statin use.2 The mechanism of injury includes heavy-lifting activities, such as weight lifting and rock climbing. However, when associated with a tendinopathy, minimal force may be involved.1
Signs and Symptoms
For proximal biceps tendon rupture, patients usually present with an acute or gradual onset of pain, swelling, and bruising of the upper arm and shoulder. Occasionally, if there is an inciting event, the patient may describe hearing or feeling a “popping” or “snapping” sound. On physical examination, the patient may exhibit a “Popeye” sign—a bulge in the distal biceps area due to the retracted biceps muscle belly. There is also tenderness along the biceps.
On testing, it has been estimated that patients can experience strength loss of approximately 30% with elbow flexion.1 In contrast, patients with distal biceps tendon ruptures usually complain of pain, swelling, and possibly bruising in the antecubital fossa, as was the case with this patient. Similar to proximal ruptures, the patient may admit to hearing or feeling a “popping” sound if there is an inciting event. The patient may exhibit a “reverse Popeye” deformity, with a bulge in the proximal arm secondary to retraction of the biceps muscle belly proximally.1
Diagnosis
There are two tests that can be performed to assist in making the diagnosis—the biceps squeeze test and the hook test.
Biceps Squeeze Test. The first test to assess for distal biceps tendon rupture is the biceps squeeze test, in which the clinician forcefully squeezes the patient’s biceps muscle to observe for forearm flexion/supination. This test is similar in principle to the Thompson test for Achilles tendon rupture. If there is no forearm movement, the injury is suspicious for a complete distal biceps tendon rupture. In one observational study of this test, 21 of 22 patients with a positive biceps squeeze test were found to have a complete distal biceps tendon tear at surgery.3
Hook Test. The second test is the hook test. While the patient actively supinates with the elbow flexed at 900, an intact hook test permits the examiner to “hook” his or her index finger under the intact biceps tendon from the lateral side. The absence of a “hook” means that there is no cord-like structure under which the examiner can hook a finger, indicating distal avulsion.4 In one study comparing the hook test to magnetic resonance imaging (MRI) in 33 patients with this suspected injury, the hook test had 100% sensitivity and specificity, while MRI only demonstrated a 92% sensitivity and 85% specificity.4
Imaging Techniques
The need for diagnostic imaging is based somewhat on the location of the rupture—proximal or distal. Ultrasound has been shown to have a high sensitivity and specificity for identifying normal tendons and complete tears of the long head biceps tendon (ie, proximal). It is not sensitive at identifying proximal partial tears, however. For distal ruptures, ultrasound imaging of the distal biceps tendon is technically difficult and not reliable. For patients with suspected distal biceps tendon ruptures, the EP should consult with orthopedic services prior to ordering an MRI. While MRI is considered the gold standard imaging test, it is neither 100% sensitive nor specific. The bottom line is that the absence of pathologic findings on MRI is not sufficient enough to exclude biceps tendon pathology.5
Treatment and Management
Regarding management, the majority of patients with proximal biceps tendon ruptures tend to do well with conservative management. The exception is for younger, active patients who are less willing to accept the cosmetic deformity, or patients whose occupation makes them unable to tolerate minimal weakness or fatigue cramping (eg, carpenters), in which case referral for a surgical repair (tenodesis) may be appropriate.1 However, multiple systematic reviews examining tenotomy vs tenodesis have not shown any functional improvement, only cosmetic.1,6,7
Distal biceps tendon ruptures are usually treated surgically, since conservative management results in a decrease of 30% to 50% supination strength and 20% flexion strength.1,8 This surgery, however, is not without complications. Approximately 20% of the patients will have a minor complication and 5% will have major complications following surgery on the distal biceps tendon.9 It is preferable to operate on distal ruptures less than 4 weeks from the initial injury; otherwise, these injuries may be more difficult to fix, require a graft, and have less predictable outcomes.1 Nonoperative management should be reserved for the elderly or less active patients with multiple comorbidities, especially if the nondominant arm is involved.10
Summary
The PA clearly missed the correct diagnosis on this patient. A more thorough history and focused physical examination would have led to the correct diagnosis sooner, along with earlier surgical repair. It is impossible, however, to know if the outcome would have been any different in this uncommon injury.
1. Smith D. Proximal versus distal biceps tendon ruptures: when to refer. BCMJ. 2017;59(2):85.
2. Spoendlin J, Layton JB, Mundkur M, Meier C, Jick SS, Meier CR. The risk of achilles or biceps tendon rupture in new statin users: a propensity score-matched sequential cohort study. Drug Safety. 2016;39(12):1229-1237. doi:10.1007/s40264-016-0462-5.
3. Ruland RT, Dunbar RP, Bowen JD. The biceps squeeze test for diagnosis of distal biceps tendon ruptures. Clin Orthop Relat Res. 2005;437:128-131.
4. O’Driscoll SW, Goncalves LBJ, Dietz P. The hook test for distal biceps tendon avulsion. Am J Sports Med. 2007;35(11):1865-1969. doi:10.1177/0363546507305016.
5. Malavolta EA, Assunção JH, Guglielmetti CL, de Souza FF, Gracitelli ME, Ferreira Neto AA. Accuracy of preoperative MRI in the diagnosis of disorders of the long head of the biceps tendon. Eur J Radiol. 2015;84(11):2250-2254. doi:10.1016/j.ejrad.2015.07.031.
6. Tangari M, Carbone S, Gallo M, Campi A. Long head of the biceps tendon rupture in professional wrestlers: treatment with a mini-open tenodesis. J Shoulder Elbow Surg. 2011;20(3):409-413. doi:10.1016/j.jse.2010.08.008.
7. Eakin JL, Bailey JR, Dewing CB, Provencher MT. Subpectoral biceps tenodesis. Oper Tech Sports Med. 2012;20(3):244-252.
8. Thomas JR, Lawton JN. Biceps and triceps ruptures in athletes. Hand Clin. 2017;33(1):35-46. doi:10.1016/j.hcl.2016.08.019.
9. Beks RB, Claessen FM, Oh LS, Ring D, Chen NC. Factors associated with adverse events after distal biceps tendon repair or reconstruction. J Shoulder Elbow Surg. 2016;25(8):1229-1234. doi:10.1016/j.jse.2016.02.032.
10. Savin DD, Watson J, Youderian AR, et al. Surgical management of acute distal biceps tendon ruptures. J Bone Joint Surg. 2017;3(9):785-796. doi:0.2106/JBJS.17.00080.
1. Smith D. Proximal versus distal biceps tendon ruptures: when to refer. BCMJ. 2017;59(2):85.
2. Spoendlin J, Layton JB, Mundkur M, Meier C, Jick SS, Meier CR. The risk of achilles or biceps tendon rupture in new statin users: a propensity score-matched sequential cohort study. Drug Safety. 2016;39(12):1229-1237. doi:10.1007/s40264-016-0462-5.
3. Ruland RT, Dunbar RP, Bowen JD. The biceps squeeze test for diagnosis of distal biceps tendon ruptures. Clin Orthop Relat Res. 2005;437:128-131.
4. O’Driscoll SW, Goncalves LBJ, Dietz P. The hook test for distal biceps tendon avulsion. Am J Sports Med. 2007;35(11):1865-1969. doi:10.1177/0363546507305016.
5. Malavolta EA, Assunção JH, Guglielmetti CL, de Souza FF, Gracitelli ME, Ferreira Neto AA. Accuracy of preoperative MRI in the diagnosis of disorders of the long head of the biceps tendon. Eur J Radiol. 2015;84(11):2250-2254. doi:10.1016/j.ejrad.2015.07.031.
6. Tangari M, Carbone S, Gallo M, Campi A. Long head of the biceps tendon rupture in professional wrestlers: treatment with a mini-open tenodesis. J Shoulder Elbow Surg. 2011;20(3):409-413. doi:10.1016/j.jse.2010.08.008.
7. Eakin JL, Bailey JR, Dewing CB, Provencher MT. Subpectoral biceps tenodesis. Oper Tech Sports Med. 2012;20(3):244-252.
8. Thomas JR, Lawton JN. Biceps and triceps ruptures in athletes. Hand Clin. 2017;33(1):35-46. doi:10.1016/j.hcl.2016.08.019.
9. Beks RB, Claessen FM, Oh LS, Ring D, Chen NC. Factors associated with adverse events after distal biceps tendon repair or reconstruction. J Shoulder Elbow Surg. 2016;25(8):1229-1234. doi:10.1016/j.jse.2016.02.032.
10. Savin DD, Watson J, Youderian AR, et al. Surgical management of acute distal biceps tendon ruptures. J Bone Joint Surg. 2017;3(9):785-796. doi:0.2106/JBJS.17.00080.
Peroneus Quartus Muscle
Take-Home Points
- PQ is easily mistaken for a PB tear.
- PQ is best identified on MRI, but commonly missed.
- For symptomatic cases, excision is the best treatment.
- Consider PQ in patients with chronic ankle pain, swelling, and/or instability.
The peroneus quartus (PQ) is an accessory muscle arising from the leg’s lateral compartment, which typically contains the peroneus longus (PL) and the peroneus brevis (PB). The many cadaveric studies that have been conducted indicate a general population prevalence ranging from 6.6% to 23%.1 Radiographic studies, including magnetic resonance imaging (MRI) and ultrasonography, have shown a similar prevalence.2 Although the PQ is asymptomatic in most cases, it may compromise the space of the superior peroneal tunnel and cause problems, including ankle pain, PB tear, subluxation of peroneal tendons, tendinous calcification, painful hypertrophy of retrotrochlear eminence, and recurrent hematomas.1,3-5 Given its differing anatomy, the PQ variously has been referred to as peroneocalcaneus externum, peroneocuboideus, long peroneal tendon, and peroneoperoneolongus.1
Although the PQ’s origin and insertion differ between subjects, the most common origin is the muscle fibers of the PB, and the most common insertion is the retrotrochlear eminence of the calcaneum.3
We report a case of peroneal tendon pathology that was initially thought to be caused primarily by impingement of a large osteochondroma on the tendons, but was later thought to be caused in part by a PQ and a split PB tendon seen only at the time of the second operation. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 16-year-old boy with an osteochondroma of the right distal fibula presented to clinic with the chief complaint of lateral right ankle pain. A “sharp” pain accompanied by audible “popping” occurred with ankle motion. Medical history was significant only for non-Hodgkin lymphoma treated with bone marrow transplantation and whole body radiation at a young age. Physical examination revealed a palpable exostosis of the distal right fibula and associated ankle swelling.
One year after surgery, the patient returned with recurring right ankle pain and audible popping during ankle movement. There was no appreciable peroneal tendon subluxation on physical examination. Repeat imaging of the ankle showed no recurrence of the osteochondroma (Figure 2).
Discussion
Absence of a PQ muscle in simian and prosimian species suggests that the PQ represents an evolutionary adaptation to evert the lateral foot and improve bipedal gait. Although the 3 peroneal (PL, PB, peroneus tertius [PT]) muscles evert the middle part of the lateral border of the foot, the PQ inserts on the retrotrochlear eminence, which everts the posterior part of the lateral border of the foot.1,6 The PQ has often been described as a variation of the PB. The PQ may also stabilize the ankle and reduce the energy required for walking. A similar functional adaptation has been proposed for the PT, which dorsiflexes at the ankle. Although presence of a PT also varies in the population, its occurrence does not correlate with presence of a PQ. In people with PQ muscles, there is an 83% to 95% incidence of also having PT muscles.7
PQ prevalence has ranged from 6.6% to 23% in cadaver studies2 and from 7% to 17% in radiologic studies.1 To better evaluate prevalence, Yammine2 performed a meta-analysis of data from 46 studies (cadaveric dissection, MRI, ultrasonography) and 3928 legs and found an overall incidence of 10.2% and a higher incidence in the Indian population than in other races. Another study found no correlation between PQ presence and sex.7
MRI is the best imaging modality for assessing for PQ but must be performed specifically for this anatomical variation. Axial images may show a fat pad separating the PQ muscle from the PB muscle.8 On imaging, a PQ muscle can be mistaken for a peroneal tendon tear. A feature that helps in distinguishing the 2 is location; the PQ typically is found posterior and medial to the PL and PB tendons, whereas PB tears are anterior to the retromalleolar groove.2 Presence of a PQ muscle may be missed on initial MRI, as occurred in our patient’s case. Zammit and Singh3 reviewed 80 leg MRIs and found 6 PQs. Only 1 of the 6 reports described the PQ as an “atypical appearance of peroneus brevis [that] appears to be made up of more than one tendon.”
Surgical excision is often adequate treatment for a symptomatic PQ. If the PQ muscle is small and symptomatic from pressure to the muscle mass, a short fasciotomy may be performed.9 More commonly, complete excision of the accessory muscle is required. Although the PQ muscle is usually asymptomatic, it should be considered in cases of chronic ankle pain, swelling, or instability; recurrent hematomas; and peroneal tendon subluxation or tears.5,7
Our patient’s diagnosis was initially overlooked because of an osteochondroma in the region of interest. It remains unclear whether his pain was caused by the PQ itself or, more likely, from the PB tear. It is thought that the accessory muscle adds bulk to the peroneal tunnel, predisposing to peroneal pathology, such as muscle tears and tendon subluxation. Regardless, advanced imaging performed before the index procedure, along with a general understanding of the PQ and its classic MRI findings, may have prevented the repeat operation in this case.
The PQ muscle is a rare but sometimes missed potential etiology of ankle pain and tendon subluxation. In our patient’s case, the most obvious abnormality, an osteochondroma, may have masked the true cause.
1. Athavale SA, Gupta V, Kotgirwar S, Singh V. The peroneus quartus muscle: clinical correlation with evolutionary importance. Anat Sci Int. 2012;87(2):106-110.
2. Yammine K. The accessory peroneal (fibular) muscles: peroneus quartus and peroneus digiti quinti. A systematic review and meta-analysis. Surg Radiol Anat. 2015;37(6):617-627.
3. Zammit J, Singh D. The peroneus quartus muscle. Anatomy and clinical relevance. J Bone Joint Surg Br. 2003;85(8):1134-1137.
4. Kulshreshtha R, Kadri S, Rajan DT. A case of unusual combination of injuries around the lateral malleolus. Foot. 2006;16(1):51-53.
5. Donley BG, Leyes M. Peroneus quartus muscle. A rare cause of chronic lateral ankle pain. Am J Sports Med. 2001;29(3):373-375.
6. Hecker P. Study on the peroneus of the tarsus. Anat Rec. 1923;26(1):79-82.
7. Rios Nascimento SR, Watanabe Costa R, Ruiz CR, Wafae N. Analysis on the incidence of the fibularis quartus muscle using magnetic resonance imaging. Anat Res Int. 2012;(2012):485149.
8. Wang XT, Rosenberg ZS, Mechlin MB, Schweitzer ME. Normal variants and diseases of the peroneal tendons and superior peroneal retinaculum: MR imaging features. Radiographics. 2005;25(3):587-602.
9. Martinelli B, Bernobi S. Peroneus quartus muscle and ankle pain. Foot Ankle Surg. 2002;8(3):223-225.
Take-Home Points
- PQ is easily mistaken for a PB tear.
- PQ is best identified on MRI, but commonly missed.
- For symptomatic cases, excision is the best treatment.
- Consider PQ in patients with chronic ankle pain, swelling, and/or instability.
The peroneus quartus (PQ) is an accessory muscle arising from the leg’s lateral compartment, which typically contains the peroneus longus (PL) and the peroneus brevis (PB). The many cadaveric studies that have been conducted indicate a general population prevalence ranging from 6.6% to 23%.1 Radiographic studies, including magnetic resonance imaging (MRI) and ultrasonography, have shown a similar prevalence.2 Although the PQ is asymptomatic in most cases, it may compromise the space of the superior peroneal tunnel and cause problems, including ankle pain, PB tear, subluxation of peroneal tendons, tendinous calcification, painful hypertrophy of retrotrochlear eminence, and recurrent hematomas.1,3-5 Given its differing anatomy, the PQ variously has been referred to as peroneocalcaneus externum, peroneocuboideus, long peroneal tendon, and peroneoperoneolongus.1
Although the PQ’s origin and insertion differ between subjects, the most common origin is the muscle fibers of the PB, and the most common insertion is the retrotrochlear eminence of the calcaneum.3
We report a case of peroneal tendon pathology that was initially thought to be caused primarily by impingement of a large osteochondroma on the tendons, but was later thought to be caused in part by a PQ and a split PB tendon seen only at the time of the second operation. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 16-year-old boy with an osteochondroma of the right distal fibula presented to clinic with the chief complaint of lateral right ankle pain. A “sharp” pain accompanied by audible “popping” occurred with ankle motion. Medical history was significant only for non-Hodgkin lymphoma treated with bone marrow transplantation and whole body radiation at a young age. Physical examination revealed a palpable exostosis of the distal right fibula and associated ankle swelling.
One year after surgery, the patient returned with recurring right ankle pain and audible popping during ankle movement. There was no appreciable peroneal tendon subluxation on physical examination. Repeat imaging of the ankle showed no recurrence of the osteochondroma (Figure 2).
Discussion
Absence of a PQ muscle in simian and prosimian species suggests that the PQ represents an evolutionary adaptation to evert the lateral foot and improve bipedal gait. Although the 3 peroneal (PL, PB, peroneus tertius [PT]) muscles evert the middle part of the lateral border of the foot, the PQ inserts on the retrotrochlear eminence, which everts the posterior part of the lateral border of the foot.1,6 The PQ has often been described as a variation of the PB. The PQ may also stabilize the ankle and reduce the energy required for walking. A similar functional adaptation has been proposed for the PT, which dorsiflexes at the ankle. Although presence of a PT also varies in the population, its occurrence does not correlate with presence of a PQ. In people with PQ muscles, there is an 83% to 95% incidence of also having PT muscles.7
PQ prevalence has ranged from 6.6% to 23% in cadaver studies2 and from 7% to 17% in radiologic studies.1 To better evaluate prevalence, Yammine2 performed a meta-analysis of data from 46 studies (cadaveric dissection, MRI, ultrasonography) and 3928 legs and found an overall incidence of 10.2% and a higher incidence in the Indian population than in other races. Another study found no correlation between PQ presence and sex.7
MRI is the best imaging modality for assessing for PQ but must be performed specifically for this anatomical variation. Axial images may show a fat pad separating the PQ muscle from the PB muscle.8 On imaging, a PQ muscle can be mistaken for a peroneal tendon tear. A feature that helps in distinguishing the 2 is location; the PQ typically is found posterior and medial to the PL and PB tendons, whereas PB tears are anterior to the retromalleolar groove.2 Presence of a PQ muscle may be missed on initial MRI, as occurred in our patient’s case. Zammit and Singh3 reviewed 80 leg MRIs and found 6 PQs. Only 1 of the 6 reports described the PQ as an “atypical appearance of peroneus brevis [that] appears to be made up of more than one tendon.”
Surgical excision is often adequate treatment for a symptomatic PQ. If the PQ muscle is small and symptomatic from pressure to the muscle mass, a short fasciotomy may be performed.9 More commonly, complete excision of the accessory muscle is required. Although the PQ muscle is usually asymptomatic, it should be considered in cases of chronic ankle pain, swelling, or instability; recurrent hematomas; and peroneal tendon subluxation or tears.5,7
Our patient’s diagnosis was initially overlooked because of an osteochondroma in the region of interest. It remains unclear whether his pain was caused by the PQ itself or, more likely, from the PB tear. It is thought that the accessory muscle adds bulk to the peroneal tunnel, predisposing to peroneal pathology, such as muscle tears and tendon subluxation. Regardless, advanced imaging performed before the index procedure, along with a general understanding of the PQ and its classic MRI findings, may have prevented the repeat operation in this case.
The PQ muscle is a rare but sometimes missed potential etiology of ankle pain and tendon subluxation. In our patient’s case, the most obvious abnormality, an osteochondroma, may have masked the true cause.
Take-Home Points
- PQ is easily mistaken for a PB tear.
- PQ is best identified on MRI, but commonly missed.
- For symptomatic cases, excision is the best treatment.
- Consider PQ in patients with chronic ankle pain, swelling, and/or instability.
The peroneus quartus (PQ) is an accessory muscle arising from the leg’s lateral compartment, which typically contains the peroneus longus (PL) and the peroneus brevis (PB). The many cadaveric studies that have been conducted indicate a general population prevalence ranging from 6.6% to 23%.1 Radiographic studies, including magnetic resonance imaging (MRI) and ultrasonography, have shown a similar prevalence.2 Although the PQ is asymptomatic in most cases, it may compromise the space of the superior peroneal tunnel and cause problems, including ankle pain, PB tear, subluxation of peroneal tendons, tendinous calcification, painful hypertrophy of retrotrochlear eminence, and recurrent hematomas.1,3-5 Given its differing anatomy, the PQ variously has been referred to as peroneocalcaneus externum, peroneocuboideus, long peroneal tendon, and peroneoperoneolongus.1
Although the PQ’s origin and insertion differ between subjects, the most common origin is the muscle fibers of the PB, and the most common insertion is the retrotrochlear eminence of the calcaneum.3
We report a case of peroneal tendon pathology that was initially thought to be caused primarily by impingement of a large osteochondroma on the tendons, but was later thought to be caused in part by a PQ and a split PB tendon seen only at the time of the second operation. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 16-year-old boy with an osteochondroma of the right distal fibula presented to clinic with the chief complaint of lateral right ankle pain. A “sharp” pain accompanied by audible “popping” occurred with ankle motion. Medical history was significant only for non-Hodgkin lymphoma treated with bone marrow transplantation and whole body radiation at a young age. Physical examination revealed a palpable exostosis of the distal right fibula and associated ankle swelling.
One year after surgery, the patient returned with recurring right ankle pain and audible popping during ankle movement. There was no appreciable peroneal tendon subluxation on physical examination. Repeat imaging of the ankle showed no recurrence of the osteochondroma (Figure 2).
Discussion
Absence of a PQ muscle in simian and prosimian species suggests that the PQ represents an evolutionary adaptation to evert the lateral foot and improve bipedal gait. Although the 3 peroneal (PL, PB, peroneus tertius [PT]) muscles evert the middle part of the lateral border of the foot, the PQ inserts on the retrotrochlear eminence, which everts the posterior part of the lateral border of the foot.1,6 The PQ has often been described as a variation of the PB. The PQ may also stabilize the ankle and reduce the energy required for walking. A similar functional adaptation has been proposed for the PT, which dorsiflexes at the ankle. Although presence of a PT also varies in the population, its occurrence does not correlate with presence of a PQ. In people with PQ muscles, there is an 83% to 95% incidence of also having PT muscles.7
PQ prevalence has ranged from 6.6% to 23% in cadaver studies2 and from 7% to 17% in radiologic studies.1 To better evaluate prevalence, Yammine2 performed a meta-analysis of data from 46 studies (cadaveric dissection, MRI, ultrasonography) and 3928 legs and found an overall incidence of 10.2% and a higher incidence in the Indian population than in other races. Another study found no correlation between PQ presence and sex.7
MRI is the best imaging modality for assessing for PQ but must be performed specifically for this anatomical variation. Axial images may show a fat pad separating the PQ muscle from the PB muscle.8 On imaging, a PQ muscle can be mistaken for a peroneal tendon tear. A feature that helps in distinguishing the 2 is location; the PQ typically is found posterior and medial to the PL and PB tendons, whereas PB tears are anterior to the retromalleolar groove.2 Presence of a PQ muscle may be missed on initial MRI, as occurred in our patient’s case. Zammit and Singh3 reviewed 80 leg MRIs and found 6 PQs. Only 1 of the 6 reports described the PQ as an “atypical appearance of peroneus brevis [that] appears to be made up of more than one tendon.”
Surgical excision is often adequate treatment for a symptomatic PQ. If the PQ muscle is small and symptomatic from pressure to the muscle mass, a short fasciotomy may be performed.9 More commonly, complete excision of the accessory muscle is required. Although the PQ muscle is usually asymptomatic, it should be considered in cases of chronic ankle pain, swelling, or instability; recurrent hematomas; and peroneal tendon subluxation or tears.5,7
Our patient’s diagnosis was initially overlooked because of an osteochondroma in the region of interest. It remains unclear whether his pain was caused by the PQ itself or, more likely, from the PB tear. It is thought that the accessory muscle adds bulk to the peroneal tunnel, predisposing to peroneal pathology, such as muscle tears and tendon subluxation. Regardless, advanced imaging performed before the index procedure, along with a general understanding of the PQ and its classic MRI findings, may have prevented the repeat operation in this case.
The PQ muscle is a rare but sometimes missed potential etiology of ankle pain and tendon subluxation. In our patient’s case, the most obvious abnormality, an osteochondroma, may have masked the true cause.
1. Athavale SA, Gupta V, Kotgirwar S, Singh V. The peroneus quartus muscle: clinical correlation with evolutionary importance. Anat Sci Int. 2012;87(2):106-110.
2. Yammine K. The accessory peroneal (fibular) muscles: peroneus quartus and peroneus digiti quinti. A systematic review and meta-analysis. Surg Radiol Anat. 2015;37(6):617-627.
3. Zammit J, Singh D. The peroneus quartus muscle. Anatomy and clinical relevance. J Bone Joint Surg Br. 2003;85(8):1134-1137.
4. Kulshreshtha R, Kadri S, Rajan DT. A case of unusual combination of injuries around the lateral malleolus. Foot. 2006;16(1):51-53.
5. Donley BG, Leyes M. Peroneus quartus muscle. A rare cause of chronic lateral ankle pain. Am J Sports Med. 2001;29(3):373-375.
6. Hecker P. Study on the peroneus of the tarsus. Anat Rec. 1923;26(1):79-82.
7. Rios Nascimento SR, Watanabe Costa R, Ruiz CR, Wafae N. Analysis on the incidence of the fibularis quartus muscle using magnetic resonance imaging. Anat Res Int. 2012;(2012):485149.
8. Wang XT, Rosenberg ZS, Mechlin MB, Schweitzer ME. Normal variants and diseases of the peroneal tendons and superior peroneal retinaculum: MR imaging features. Radiographics. 2005;25(3):587-602.
9. Martinelli B, Bernobi S. Peroneus quartus muscle and ankle pain. Foot Ankle Surg. 2002;8(3):223-225.
1. Athavale SA, Gupta V, Kotgirwar S, Singh V. The peroneus quartus muscle: clinical correlation with evolutionary importance. Anat Sci Int. 2012;87(2):106-110.
2. Yammine K. The accessory peroneal (fibular) muscles: peroneus quartus and peroneus digiti quinti. A systematic review and meta-analysis. Surg Radiol Anat. 2015;37(6):617-627.
3. Zammit J, Singh D. The peroneus quartus muscle. Anatomy and clinical relevance. J Bone Joint Surg Br. 2003;85(8):1134-1137.
4. Kulshreshtha R, Kadri S, Rajan DT. A case of unusual combination of injuries around the lateral malleolus. Foot. 2006;16(1):51-53.
5. Donley BG, Leyes M. Peroneus quartus muscle. A rare cause of chronic lateral ankle pain. Am J Sports Med. 2001;29(3):373-375.
6. Hecker P. Study on the peroneus of the tarsus. Anat Rec. 1923;26(1):79-82.
7. Rios Nascimento SR, Watanabe Costa R, Ruiz CR, Wafae N. Analysis on the incidence of the fibularis quartus muscle using magnetic resonance imaging. Anat Res Int. 2012;(2012):485149.
8. Wang XT, Rosenberg ZS, Mechlin MB, Schweitzer ME. Normal variants and diseases of the peroneal tendons and superior peroneal retinaculum: MR imaging features. Radiographics. 2005;25(3):587-602.
9. Martinelli B, Bernobi S. Peroneus quartus muscle and ankle pain. Foot Ankle Surg. 2002;8(3):223-225.
Characterization and Surgical Management of Metastatic Disease of the Tibia
Take-Home Points
- Metastatic disease of the tibia is a rare but significant event in a subset of patients.
- Cancer histologies with historically “acral” spread may not apply to tibial disease.
- Patients with leg pain and any cancer diagnosis should be worked up for tibial metastases.
- Tibial disease is probably a late manifestation, and early detection may indicate late diagnosis of malignancy.
- The ultimate surgical plan for these patients should be a patient-centered multidisciplinary decision making process.
Metastatic dissemination to bones is common in advanced cancer stages and affects the axial and appendicular skeleton.1-4 The appendicular skeleton bones most often involved are the proximal femur and the proximal humerus.5,6 The tibia is involved third most often but is comparatively rarely affected.4-6 Metastatic involvement distal to the knee or elbow is more typical of advanced disease.1,3 Distal appendicular lesions are called acral metastases, but the term is inconsistently used and may refer to lesions either distal to the knee and elbow or distal to the ankle and wrist. Regardless of terminology, tibia lesions are uncommon and not well described.1,4,7,8
The tibia is the primary weight-bearing leg bone. Metastatic tibia lesions may cause pain and instability and impair mobility. Although distal skeletal dissemination often presents late in advanced disease in patients with relatively poor prognoses, in some cases early surgical intervention is indicated for pain relief, increased mobility, and improved quality of life.4,8-10
Materials and Methods
Our Institutional Review Board approved this single-institution retrospective study. We used proprietary research software (Clinical Looking Glass) to identify eligible patients treated between 2000 and 2013. The software was used to search all radiology and pathology reports for the term tibia or any variation (eg, tibial) and metastasis or any variation (eg, metastatic). The software was then used to search by Current Procedural Terminology code for any patients treated with intramedullary nail (IMN) or another tibial fixation method. This list was cross-referenced with the list of patients originally identified to help ensure that all eligible patients were identified.
Inclusion criteria were known malignancy and imaging or biopsy evidence of a metastatic tibia lesion. Treatment strategies for patients with metastatic disease and patients with multiple myeloma are sometimes considered together because of similar goals and methodologies. We specifically excluded patients with multiple myeloma in order to more accurately characterize the natural history of metastatic disease and the timing of metastatic development and to report on a more homogeneous population. Patients were excluded if their electronic medical records were inadequate in establishing a diagnosis.
Demographic and pathology data were collected directly from the institutional electronic medical records system. Dr. Geller and Dr. Greenbaum used Centricity software (General Electric Healthcare) to review all imaging on medical diagnostic display monitors. If their interpretation differed from that in the radiology report, or if clarification was needed, the study was sent to Dr. Thornhill, the institution’s director of musculoskeletal radiology, for review and interpretation. Investigated radiographic characteristics included location, cortical breakthrough, presence of fracture, and size (if advanced imaging was available). Surgical interventions were recorded from reviews of operative reports and postoperative imaging studies.
Time to metastasis was defined as number of days from diagnosis of malignancy to diagnosis of tibial osseous spread. Date of diagnosis of malignancy was the date that a biopsy or other confirmatory test was performed. In cases in which that date was unavailable, an imaging study consistent with disease or a clinical note documenting the known diagnosis date was used instead. When only month and year (ie, not an exact date) of diagnosis were available, the 15th of the month was used as an estimate. Of the 36 patients, 4 had records insufficient for establishing date of diagnosis. The first date of any imaging study confirming (or suggestive of) a metastatic lesion of the tibia was used as the date of tibial metastasis.
Many patients had osseous lesions at sites other than the tibiae. These lesions were noted on review of imaging studies, screening examinations, and physicians’ clinical notes. Widespread disease was defined as including both axial and appendicular lesions, and lesions of the tibiae.
Tibia lesion presentation was recorded as either symptomatic or incidental. If the tibiae were imaged for pain, including posttraumatic pain, the presentation was symptomatic. If a lesion was identified on staging examination (eg, bone or positron emission tomography scan), or if the tibiae were imaged for another reason, the presentation was incidental.
Results
Demographics
Thirty-six patients had 43 affected tibiae. Sixteen male patients (44.4% of the total) had 19 (44.2%) of the affected tibiae, and 20 female patients (55.6%) had the other 24 affected tibiae (55.8%). Mean age was 63.5 years for all patients (range, 6-95 years), 68.1 years for males, and 59.8 years for females. Of the 36 patients, 32 (88.9%) were over age 40 years (Table). All patients had radiographic evidence of ≥1 tibia lesion, and 6 (16.7%) also had biopsy-proven metastatic disease of the tibia.
Tumor Characteristics
There were 12 different primary neoplasms (Table). The most common were prostate cancer (7 patients, 19.4%; 10 tibiae, 23.3%), breast cancer (7 patients, 19.4%; 9 tibiae, 20.9%), and lung cancer (7 patients, 19.4%; 7 tibiae, 16.3%). For males, the most common diagnoses were prostate cancer (7 cases, 43.8% of males) and diffuse large B-cell lymphoma and lung cancer (3 cases and 18.8% of males each). For females, the most common diagnoses were breast cancer (7 cases, 35.0% of females) and lung cancer (4 cases, 20.0% of females).
Most of the lesions were proximal (31 tibiae, 72.1%), followed by diaphyseal (7, 16.3%) and distal (2, 4.7%) (Table). Three tibiae (7.0%) were entirely involved, but 1 of these was more affected at the distal end. One tibia had 2 lesions, 1 proximal and 1 distal.
Time to Metastasis, Other Osseous Disease
Mean time from diagnosis of malignancy to diagnosis of osseous disease of the tibia was 1282 days (range, 0-3708 days) (Table). Of the 36 patients, 32 (88.9%) had other metastatic lesions, 3 (8.3%) had isolated tibia lesions, and 1 (2.8%) had a medical record insufficient for establishing lesion status (isolated or not). Of the 32 patients with known other osseous metastases, 14 (43.8%) had widespread (axial and additional appendicular) disease, and 3 (9.4%) had additional lesions only distal to the identified tibial metastases.
Clinical Presentation
Of the 36 lesions, 18 (50%) were asymptomatic and were found on screening examinations, 17 (47.2%) presented with pain, and 1 (2.8%) had a presentation that could not be determined from the medical record (Table). Of the 17 painful lesions, 3 (17.6%) were found after a trauma brought attention to the site, and the other 14 (82.4%) were atraumatic in origin.
Of the 10 patients with cortical breakthrough, 8 (80%) had painful lesions, 1 (10%) had a lesion that was found on screening examination, and 1 (10%) had a medical record insufficient for establishing clinical presentation. Of the 8 patients who underwent surgical stabilization, 6 (75%) had painful lesions. Only 1 patient with an asymptomatic tibia lesion underwent surgical intervention (total knee arthroplasty).
Surgical Intervention
Two patients (5.6%) with affected tibiae (4.8%) had pathologic fractures. One fracture (non-small cell lung cancer) was treated with open reduction and internal fixation (periarticular locking plate with cement augmentation), and the other (urothelial cancer) was treated with IMN fixation.
Ten patients (27.8%) with affected tibiae (23.8%) had radiographs that showed cortical breakthrough (Table). Two of the 10 cases were managed nonoperatively, and the patients died before surgical stabilization could be attempted. Of the 8 surgically managed cases, 3 were prophylactically stabilized with IMN (2 of these were augmented with cement, and the third with a screw-plate construct), 2 were treated with periarticular resection and reconstruction (total knee megaprosthesis), 1 was treated with an approach undertaken to address a concomitant distal femoral pathologic fracture, and 1 was treated with total knee arthroplasty undertaken to address lesions at the proximal end of the tibia and the distal end of the femur.
Discussion
We have described a retrospective descriptive study conducted to characterize tibial metastases, their histologies, and the circumstances surrounding diagnosis and surgical management. In all cases, general findings confirmed advanced metastatic disease. In only 3 cases, the tibia lesion was an isolated metastatic lesion.
Sex predilection of tibial metastases remains controversial. One study found males had up to twice as many hand and foot metastases as women,11 but this contrasts with the relatively equal sex ratio found in other studies8,10 and in the present study. We found metastatic disease of the tibia was unsurprisingly concentrated in patients over age 40 years, in whom the vast majority of all cancers develop.12,13 Our study agrees with those that have found most tibia lesions develop in patients in the 6th decade of life on average.8,10 Mean age was 8.3 years higher in our male patients than in our female patients.
Tumor Characteristics
The most common primary neoplasms in our cohort were prostate, breast, and lung cancers, which are among the most common cancers in the United States12,13 and which have a predilection for osseous spread.2,6,9,14 Renal cell carcinoma has been reported to spread to distal (or “acral”) skeletal sites,2-4,9,11,14 but the present study did not identify any patients with this diagnosis. Of our patients with a primary lung cancer for whom a histologic description was available (5/7), all had non-small cell lung cancer. Three patients had a primary malignancy of colorectal cancer, which occasionally metastasizes to the distal skeleton.3,8,11 We identified 3 patients with diffuse large B-cell lymphoma, a histology not widely reported to metastasize to distal skeletal sites.
Metastatic disease of the tibia is most common at the proximal end of the bone.1,10,11,14 Other studies8,10 have found the proximal tibia is affected much more commonly than the tibial diaphysis, and even fewer cases develop at the distal end. Our findings agree with theirs: Proximal lesions outnumber all other lesions combined (Table).
Time to Metastasis
Distal metastases are typical of late-stage metastatic disease,1,3 but quantification of the time from diagnosis of malignancy to presentation of a tibia lesion is not well defined. In our study, time to metastasis was <100 days for some patients (Table). As osseous involvement, especially acral disease, was considered a late-stage manifestation of malignancy, this result was unexpected and most likely represents undiagnosed and untreated malignancy. Six patients in this group were diagnosed with tibial metastases within 30 days, essentially at the same time the primary neoplasm was diagnosed. These findings suggest that a tibia lesion found at time of patient presentation should raise concern for late-stage undiagnosed metastatic cancer.
Other Osseous Disease
The patients identified in this study had advanced malignancy, and most had widespread bony dissemination. Those with the lowest disease burden had isolated tibia lesions or additional metastases only distal to the tibia lesion in the ipsilateral lower extremity. Most of these patients had undergone surgery or were scheduled for it (Table). Most of the patients with appendicular metastases proximal to the tibia lesion had disease of the femora, the most common long bones affected by osseous metastatic disease.5,6 In accordance with orthopedic oncology principles, all other osseous disease should be thoroughly identified and staged before any surgical planning for identified tibia lesions. Ipsilateral distal femoral lesions are of particular importance for patients with proximal tibia lesions, as reconstruction with total knee endoprosthesis can potentially provide a functional reconstructive option after resection of both lesions.
Clinical Presentation
Most of the patients who had cortical breakthrough or required surgical stabilization had painful lesions. Although tibial metastasis is rare, its potential occurrence should raise concerns and be investigated in the patient with tibial pain.
Surgical Intervention
General surgical management of metastatic disease of other long bones has been extensively studied,6,7,9,14 but there are fewer published recommendations regarding specific treatments for metastatic lesions of the tibia. In 2003, Kelly and colleagues8 described an algorithm based on the anatomical location of the lesion, with either internal fixation or IMN fixation representing the preferred management for lesions in the metaphyseal or diaphyseal regions. For epiphyseal or extensive proximal metaphyseal lesions, modular oncology endoprostheses are described as the procedure of choice. Piccioli and colleagues10 in 2013 and Beauchamp and Sim1 in 1988 described a similar operative approach.
It is unknown if the algorithm of Kelly and colleagues8 was referenced during clinical decision-making, but it appears operative management mirrored these principles. Deviations from this general approach in the operative management of the patients in the present study included modifications such as the addition of a screw-plate construct to an IMN for better stability.
Surgical management depends largely on the anatomical location within the bone and on remaining bone stock. Generally, extensive proximal disease is managed with total knee endoprosthesis reconstruction, diaphyseal disease with IMN, and distal disease with internal fixation. Construct augmentation, such as the addition of cement or use of additional hardware, is decided case by case on the basis of desired stability and surrounding bone stock.
Study Limitations
Despite being a larger series, this single-institution study had a relatively small sample size, and its patient demographics and primary malignancies may reflect institutional recruitment bias. In addition, the study was limited by its retrospective design and some incomplete medical records. Eleven patients had only a bone or positron emission tomography scan depicting metastatic disease, limiting characterization of these lesions. One patient lacked radiologic images, and characterizations were based on written reports. As multiple physicians were involved in diagnosis and treatment, there were many inconsistencies in clinical decision-making across the group.
Conclusion
Metastasis to the tibia is a rare but significant event in a subset of patients over the course of their treatment and surveillance. Patients may present with pain secondary to either pathologic or impending pathologic fractures, and in such instances surgical intervention is often needed. Despite the historical reports of “acral” histologies, tibia lesions are not indicative of histology, and biopsy should be considered, especially if management will depend on histology. Patients with lower leg pain and known malignancy should be evaluated to rule out tibial metastasis, but screening examinations may be prudent for asymptomatic patients as well. Increased vigilance may be indicated for those with prostate, breast, or lung cancer. These lesions should be surgically managed case by case using fundamental tenets of both orthopedic fracture care and orthopedic oncology. Ideally, patients should be treated by a multidisciplinary team using a patient-centered approach.
1. Beauchamp CP, Sim FH. Lesions of the tibia. In: Sim FH, ed. Diagnosis and Management of Metastatic Bone Disease: A Multidisciplinary Approach. New York, NY: Raven; 1988:201-212.
2. Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12(20 pt 2):6243s-6249s.
3. Healy JH, Turnbull AD, Miedema B, Lane JM. Acrometastases. A study of twenty-nine patients with osseous involvement of the hands and feet. J Bone Joint Surg Am. 1986;68(5):743-746.
4. Leeson MC, Makley JT, Carter JR. Metastatic skeletal disease distal to the elbow and knee. Clin Orthop Relat Res. 1986;(206):94-99.
5. De Geeter K, Reynders P, Samson I, Broos PL. Metastatic fractures of the tibia. Acta Orthop Belg. 2001;67(1):54-59.
6. Kelly M, Lee M, Clarkson P, O’Brien PJ. Metastatic disease of the long bones: a review of the health care burden in a major trauma centre. Can J Surg. 2012;55(2):95-98.
7. Jasmin C. Textbook of Bone Metastases. Chichester, England: Wiley; 2005.
8. Kelly CM, Wilkins RM, Eckardt JJ, Ward WG. Treatment of metastatic disease of the tibia. Clin Orthop Relat Res. 2003;(415 suppl):S219-S229.
9. Nielsen OS, Munro AJ, Tannock IF. Bone metastases: pathophysiology and management policy. J Clin Oncol. 1991;9(3):509-524.
10. Piccioli A, Maccauro G, Scaramuzzo L, Graci C, Spinelli MS. Surgical treatment of impending and pathological fractures of tibia. Injury. 2013;44(8):1092-1096.
11. Flynn CJ, Danjoux C, Wong J, et al. Two cases of acrometastasis to the hands and review of the literature. Curr Oncol. 2008;15(5):51-58.
12. American Cancer Society. Cancer Facts and Figures 2013. Atlanta, GA: American Cancer Society; 2013.
13. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11-30.
14. Capanna R, Campanacci DA. The treatment of metastases in the appendicular skeleton. J Bone Joint Surg Br. 2001;83(4):471-481.
Take-Home Points
- Metastatic disease of the tibia is a rare but significant event in a subset of patients.
- Cancer histologies with historically “acral” spread may not apply to tibial disease.
- Patients with leg pain and any cancer diagnosis should be worked up for tibial metastases.
- Tibial disease is probably a late manifestation, and early detection may indicate late diagnosis of malignancy.
- The ultimate surgical plan for these patients should be a patient-centered multidisciplinary decision making process.
Metastatic dissemination to bones is common in advanced cancer stages and affects the axial and appendicular skeleton.1-4 The appendicular skeleton bones most often involved are the proximal femur and the proximal humerus.5,6 The tibia is involved third most often but is comparatively rarely affected.4-6 Metastatic involvement distal to the knee or elbow is more typical of advanced disease.1,3 Distal appendicular lesions are called acral metastases, but the term is inconsistently used and may refer to lesions either distal to the knee and elbow or distal to the ankle and wrist. Regardless of terminology, tibia lesions are uncommon and not well described.1,4,7,8
The tibia is the primary weight-bearing leg bone. Metastatic tibia lesions may cause pain and instability and impair mobility. Although distal skeletal dissemination often presents late in advanced disease in patients with relatively poor prognoses, in some cases early surgical intervention is indicated for pain relief, increased mobility, and improved quality of life.4,8-10
Materials and Methods
Our Institutional Review Board approved this single-institution retrospective study. We used proprietary research software (Clinical Looking Glass) to identify eligible patients treated between 2000 and 2013. The software was used to search all radiology and pathology reports for the term tibia or any variation (eg, tibial) and metastasis or any variation (eg, metastatic). The software was then used to search by Current Procedural Terminology code for any patients treated with intramedullary nail (IMN) or another tibial fixation method. This list was cross-referenced with the list of patients originally identified to help ensure that all eligible patients were identified.
Inclusion criteria were known malignancy and imaging or biopsy evidence of a metastatic tibia lesion. Treatment strategies for patients with metastatic disease and patients with multiple myeloma are sometimes considered together because of similar goals and methodologies. We specifically excluded patients with multiple myeloma in order to more accurately characterize the natural history of metastatic disease and the timing of metastatic development and to report on a more homogeneous population. Patients were excluded if their electronic medical records were inadequate in establishing a diagnosis.
Demographic and pathology data were collected directly from the institutional electronic medical records system. Dr. Geller and Dr. Greenbaum used Centricity software (General Electric Healthcare) to review all imaging on medical diagnostic display monitors. If their interpretation differed from that in the radiology report, or if clarification was needed, the study was sent to Dr. Thornhill, the institution’s director of musculoskeletal radiology, for review and interpretation. Investigated radiographic characteristics included location, cortical breakthrough, presence of fracture, and size (if advanced imaging was available). Surgical interventions were recorded from reviews of operative reports and postoperative imaging studies.
Time to metastasis was defined as number of days from diagnosis of malignancy to diagnosis of tibial osseous spread. Date of diagnosis of malignancy was the date that a biopsy or other confirmatory test was performed. In cases in which that date was unavailable, an imaging study consistent with disease or a clinical note documenting the known diagnosis date was used instead. When only month and year (ie, not an exact date) of diagnosis were available, the 15th of the month was used as an estimate. Of the 36 patients, 4 had records insufficient for establishing date of diagnosis. The first date of any imaging study confirming (or suggestive of) a metastatic lesion of the tibia was used as the date of tibial metastasis.
Many patients had osseous lesions at sites other than the tibiae. These lesions were noted on review of imaging studies, screening examinations, and physicians’ clinical notes. Widespread disease was defined as including both axial and appendicular lesions, and lesions of the tibiae.
Tibia lesion presentation was recorded as either symptomatic or incidental. If the tibiae were imaged for pain, including posttraumatic pain, the presentation was symptomatic. If a lesion was identified on staging examination (eg, bone or positron emission tomography scan), or if the tibiae were imaged for another reason, the presentation was incidental.
Results
Demographics
Thirty-six patients had 43 affected tibiae. Sixteen male patients (44.4% of the total) had 19 (44.2%) of the affected tibiae, and 20 female patients (55.6%) had the other 24 affected tibiae (55.8%). Mean age was 63.5 years for all patients (range, 6-95 years), 68.1 years for males, and 59.8 years for females. Of the 36 patients, 32 (88.9%) were over age 40 years (Table). All patients had radiographic evidence of ≥1 tibia lesion, and 6 (16.7%) also had biopsy-proven metastatic disease of the tibia.
Tumor Characteristics
There were 12 different primary neoplasms (Table). The most common were prostate cancer (7 patients, 19.4%; 10 tibiae, 23.3%), breast cancer (7 patients, 19.4%; 9 tibiae, 20.9%), and lung cancer (7 patients, 19.4%; 7 tibiae, 16.3%). For males, the most common diagnoses were prostate cancer (7 cases, 43.8% of males) and diffuse large B-cell lymphoma and lung cancer (3 cases and 18.8% of males each). For females, the most common diagnoses were breast cancer (7 cases, 35.0% of females) and lung cancer (4 cases, 20.0% of females).
Most of the lesions were proximal (31 tibiae, 72.1%), followed by diaphyseal (7, 16.3%) and distal (2, 4.7%) (Table). Three tibiae (7.0%) were entirely involved, but 1 of these was more affected at the distal end. One tibia had 2 lesions, 1 proximal and 1 distal.
Time to Metastasis, Other Osseous Disease
Mean time from diagnosis of malignancy to diagnosis of osseous disease of the tibia was 1282 days (range, 0-3708 days) (Table). Of the 36 patients, 32 (88.9%) had other metastatic lesions, 3 (8.3%) had isolated tibia lesions, and 1 (2.8%) had a medical record insufficient for establishing lesion status (isolated or not). Of the 32 patients with known other osseous metastases, 14 (43.8%) had widespread (axial and additional appendicular) disease, and 3 (9.4%) had additional lesions only distal to the identified tibial metastases.
Clinical Presentation
Of the 36 lesions, 18 (50%) were asymptomatic and were found on screening examinations, 17 (47.2%) presented with pain, and 1 (2.8%) had a presentation that could not be determined from the medical record (Table). Of the 17 painful lesions, 3 (17.6%) were found after a trauma brought attention to the site, and the other 14 (82.4%) were atraumatic in origin.
Of the 10 patients with cortical breakthrough, 8 (80%) had painful lesions, 1 (10%) had a lesion that was found on screening examination, and 1 (10%) had a medical record insufficient for establishing clinical presentation. Of the 8 patients who underwent surgical stabilization, 6 (75%) had painful lesions. Only 1 patient with an asymptomatic tibia lesion underwent surgical intervention (total knee arthroplasty).
Surgical Intervention
Two patients (5.6%) with affected tibiae (4.8%) had pathologic fractures. One fracture (non-small cell lung cancer) was treated with open reduction and internal fixation (periarticular locking plate with cement augmentation), and the other (urothelial cancer) was treated with IMN fixation.
Ten patients (27.8%) with affected tibiae (23.8%) had radiographs that showed cortical breakthrough (Table). Two of the 10 cases were managed nonoperatively, and the patients died before surgical stabilization could be attempted. Of the 8 surgically managed cases, 3 were prophylactically stabilized with IMN (2 of these were augmented with cement, and the third with a screw-plate construct), 2 were treated with periarticular resection and reconstruction (total knee megaprosthesis), 1 was treated with an approach undertaken to address a concomitant distal femoral pathologic fracture, and 1 was treated with total knee arthroplasty undertaken to address lesions at the proximal end of the tibia and the distal end of the femur.
Discussion
We have described a retrospective descriptive study conducted to characterize tibial metastases, their histologies, and the circumstances surrounding diagnosis and surgical management. In all cases, general findings confirmed advanced metastatic disease. In only 3 cases, the tibia lesion was an isolated metastatic lesion.
Sex predilection of tibial metastases remains controversial. One study found males had up to twice as many hand and foot metastases as women,11 but this contrasts with the relatively equal sex ratio found in other studies8,10 and in the present study. We found metastatic disease of the tibia was unsurprisingly concentrated in patients over age 40 years, in whom the vast majority of all cancers develop.12,13 Our study agrees with those that have found most tibia lesions develop in patients in the 6th decade of life on average.8,10 Mean age was 8.3 years higher in our male patients than in our female patients.
Tumor Characteristics
The most common primary neoplasms in our cohort were prostate, breast, and lung cancers, which are among the most common cancers in the United States12,13 and which have a predilection for osseous spread.2,6,9,14 Renal cell carcinoma has been reported to spread to distal (or “acral”) skeletal sites,2-4,9,11,14 but the present study did not identify any patients with this diagnosis. Of our patients with a primary lung cancer for whom a histologic description was available (5/7), all had non-small cell lung cancer. Three patients had a primary malignancy of colorectal cancer, which occasionally metastasizes to the distal skeleton.3,8,11 We identified 3 patients with diffuse large B-cell lymphoma, a histology not widely reported to metastasize to distal skeletal sites.
Metastatic disease of the tibia is most common at the proximal end of the bone.1,10,11,14 Other studies8,10 have found the proximal tibia is affected much more commonly than the tibial diaphysis, and even fewer cases develop at the distal end. Our findings agree with theirs: Proximal lesions outnumber all other lesions combined (Table).
Time to Metastasis
Distal metastases are typical of late-stage metastatic disease,1,3 but quantification of the time from diagnosis of malignancy to presentation of a tibia lesion is not well defined. In our study, time to metastasis was <100 days for some patients (Table). As osseous involvement, especially acral disease, was considered a late-stage manifestation of malignancy, this result was unexpected and most likely represents undiagnosed and untreated malignancy. Six patients in this group were diagnosed with tibial metastases within 30 days, essentially at the same time the primary neoplasm was diagnosed. These findings suggest that a tibia lesion found at time of patient presentation should raise concern for late-stage undiagnosed metastatic cancer.
Other Osseous Disease
The patients identified in this study had advanced malignancy, and most had widespread bony dissemination. Those with the lowest disease burden had isolated tibia lesions or additional metastases only distal to the tibia lesion in the ipsilateral lower extremity. Most of these patients had undergone surgery or were scheduled for it (Table). Most of the patients with appendicular metastases proximal to the tibia lesion had disease of the femora, the most common long bones affected by osseous metastatic disease.5,6 In accordance with orthopedic oncology principles, all other osseous disease should be thoroughly identified and staged before any surgical planning for identified tibia lesions. Ipsilateral distal femoral lesions are of particular importance for patients with proximal tibia lesions, as reconstruction with total knee endoprosthesis can potentially provide a functional reconstructive option after resection of both lesions.
Clinical Presentation
Most of the patients who had cortical breakthrough or required surgical stabilization had painful lesions. Although tibial metastasis is rare, its potential occurrence should raise concerns and be investigated in the patient with tibial pain.
Surgical Intervention
General surgical management of metastatic disease of other long bones has been extensively studied,6,7,9,14 but there are fewer published recommendations regarding specific treatments for metastatic lesions of the tibia. In 2003, Kelly and colleagues8 described an algorithm based on the anatomical location of the lesion, with either internal fixation or IMN fixation representing the preferred management for lesions in the metaphyseal or diaphyseal regions. For epiphyseal or extensive proximal metaphyseal lesions, modular oncology endoprostheses are described as the procedure of choice. Piccioli and colleagues10 in 2013 and Beauchamp and Sim1 in 1988 described a similar operative approach.
It is unknown if the algorithm of Kelly and colleagues8 was referenced during clinical decision-making, but it appears operative management mirrored these principles. Deviations from this general approach in the operative management of the patients in the present study included modifications such as the addition of a screw-plate construct to an IMN for better stability.
Surgical management depends largely on the anatomical location within the bone and on remaining bone stock. Generally, extensive proximal disease is managed with total knee endoprosthesis reconstruction, diaphyseal disease with IMN, and distal disease with internal fixation. Construct augmentation, such as the addition of cement or use of additional hardware, is decided case by case on the basis of desired stability and surrounding bone stock.
Study Limitations
Despite being a larger series, this single-institution study had a relatively small sample size, and its patient demographics and primary malignancies may reflect institutional recruitment bias. In addition, the study was limited by its retrospective design and some incomplete medical records. Eleven patients had only a bone or positron emission tomography scan depicting metastatic disease, limiting characterization of these lesions. One patient lacked radiologic images, and characterizations were based on written reports. As multiple physicians were involved in diagnosis and treatment, there were many inconsistencies in clinical decision-making across the group.
Conclusion
Metastasis to the tibia is a rare but significant event in a subset of patients over the course of their treatment and surveillance. Patients may present with pain secondary to either pathologic or impending pathologic fractures, and in such instances surgical intervention is often needed. Despite the historical reports of “acral” histologies, tibia lesions are not indicative of histology, and biopsy should be considered, especially if management will depend on histology. Patients with lower leg pain and known malignancy should be evaluated to rule out tibial metastasis, but screening examinations may be prudent for asymptomatic patients as well. Increased vigilance may be indicated for those with prostate, breast, or lung cancer. These lesions should be surgically managed case by case using fundamental tenets of both orthopedic fracture care and orthopedic oncology. Ideally, patients should be treated by a multidisciplinary team using a patient-centered approach.
Take-Home Points
- Metastatic disease of the tibia is a rare but significant event in a subset of patients.
- Cancer histologies with historically “acral” spread may not apply to tibial disease.
- Patients with leg pain and any cancer diagnosis should be worked up for tibial metastases.
- Tibial disease is probably a late manifestation, and early detection may indicate late diagnosis of malignancy.
- The ultimate surgical plan for these patients should be a patient-centered multidisciplinary decision making process.
Metastatic dissemination to bones is common in advanced cancer stages and affects the axial and appendicular skeleton.1-4 The appendicular skeleton bones most often involved are the proximal femur and the proximal humerus.5,6 The tibia is involved third most often but is comparatively rarely affected.4-6 Metastatic involvement distal to the knee or elbow is more typical of advanced disease.1,3 Distal appendicular lesions are called acral metastases, but the term is inconsistently used and may refer to lesions either distal to the knee and elbow or distal to the ankle and wrist. Regardless of terminology, tibia lesions are uncommon and not well described.1,4,7,8
The tibia is the primary weight-bearing leg bone. Metastatic tibia lesions may cause pain and instability and impair mobility. Although distal skeletal dissemination often presents late in advanced disease in patients with relatively poor prognoses, in some cases early surgical intervention is indicated for pain relief, increased mobility, and improved quality of life.4,8-10
Materials and Methods
Our Institutional Review Board approved this single-institution retrospective study. We used proprietary research software (Clinical Looking Glass) to identify eligible patients treated between 2000 and 2013. The software was used to search all radiology and pathology reports for the term tibia or any variation (eg, tibial) and metastasis or any variation (eg, metastatic). The software was then used to search by Current Procedural Terminology code for any patients treated with intramedullary nail (IMN) or another tibial fixation method. This list was cross-referenced with the list of patients originally identified to help ensure that all eligible patients were identified.
Inclusion criteria were known malignancy and imaging or biopsy evidence of a metastatic tibia lesion. Treatment strategies for patients with metastatic disease and patients with multiple myeloma are sometimes considered together because of similar goals and methodologies. We specifically excluded patients with multiple myeloma in order to more accurately characterize the natural history of metastatic disease and the timing of metastatic development and to report on a more homogeneous population. Patients were excluded if their electronic medical records were inadequate in establishing a diagnosis.
Demographic and pathology data were collected directly from the institutional electronic medical records system. Dr. Geller and Dr. Greenbaum used Centricity software (General Electric Healthcare) to review all imaging on medical diagnostic display monitors. If their interpretation differed from that in the radiology report, or if clarification was needed, the study was sent to Dr. Thornhill, the institution’s director of musculoskeletal radiology, for review and interpretation. Investigated radiographic characteristics included location, cortical breakthrough, presence of fracture, and size (if advanced imaging was available). Surgical interventions were recorded from reviews of operative reports and postoperative imaging studies.
Time to metastasis was defined as number of days from diagnosis of malignancy to diagnosis of tibial osseous spread. Date of diagnosis of malignancy was the date that a biopsy or other confirmatory test was performed. In cases in which that date was unavailable, an imaging study consistent with disease or a clinical note documenting the known diagnosis date was used instead. When only month and year (ie, not an exact date) of diagnosis were available, the 15th of the month was used as an estimate. Of the 36 patients, 4 had records insufficient for establishing date of diagnosis. The first date of any imaging study confirming (or suggestive of) a metastatic lesion of the tibia was used as the date of tibial metastasis.
Many patients had osseous lesions at sites other than the tibiae. These lesions were noted on review of imaging studies, screening examinations, and physicians’ clinical notes. Widespread disease was defined as including both axial and appendicular lesions, and lesions of the tibiae.
Tibia lesion presentation was recorded as either symptomatic or incidental. If the tibiae were imaged for pain, including posttraumatic pain, the presentation was symptomatic. If a lesion was identified on staging examination (eg, bone or positron emission tomography scan), or if the tibiae were imaged for another reason, the presentation was incidental.
Results
Demographics
Thirty-six patients had 43 affected tibiae. Sixteen male patients (44.4% of the total) had 19 (44.2%) of the affected tibiae, and 20 female patients (55.6%) had the other 24 affected tibiae (55.8%). Mean age was 63.5 years for all patients (range, 6-95 years), 68.1 years for males, and 59.8 years for females. Of the 36 patients, 32 (88.9%) were over age 40 years (Table). All patients had radiographic evidence of ≥1 tibia lesion, and 6 (16.7%) also had biopsy-proven metastatic disease of the tibia.
Tumor Characteristics
There were 12 different primary neoplasms (Table). The most common were prostate cancer (7 patients, 19.4%; 10 tibiae, 23.3%), breast cancer (7 patients, 19.4%; 9 tibiae, 20.9%), and lung cancer (7 patients, 19.4%; 7 tibiae, 16.3%). For males, the most common diagnoses were prostate cancer (7 cases, 43.8% of males) and diffuse large B-cell lymphoma and lung cancer (3 cases and 18.8% of males each). For females, the most common diagnoses were breast cancer (7 cases, 35.0% of females) and lung cancer (4 cases, 20.0% of females).
Most of the lesions were proximal (31 tibiae, 72.1%), followed by diaphyseal (7, 16.3%) and distal (2, 4.7%) (Table). Three tibiae (7.0%) were entirely involved, but 1 of these was more affected at the distal end. One tibia had 2 lesions, 1 proximal and 1 distal.
Time to Metastasis, Other Osseous Disease
Mean time from diagnosis of malignancy to diagnosis of osseous disease of the tibia was 1282 days (range, 0-3708 days) (Table). Of the 36 patients, 32 (88.9%) had other metastatic lesions, 3 (8.3%) had isolated tibia lesions, and 1 (2.8%) had a medical record insufficient for establishing lesion status (isolated or not). Of the 32 patients with known other osseous metastases, 14 (43.8%) had widespread (axial and additional appendicular) disease, and 3 (9.4%) had additional lesions only distal to the identified tibial metastases.
Clinical Presentation
Of the 36 lesions, 18 (50%) were asymptomatic and were found on screening examinations, 17 (47.2%) presented with pain, and 1 (2.8%) had a presentation that could not be determined from the medical record (Table). Of the 17 painful lesions, 3 (17.6%) were found after a trauma brought attention to the site, and the other 14 (82.4%) were atraumatic in origin.
Of the 10 patients with cortical breakthrough, 8 (80%) had painful lesions, 1 (10%) had a lesion that was found on screening examination, and 1 (10%) had a medical record insufficient for establishing clinical presentation. Of the 8 patients who underwent surgical stabilization, 6 (75%) had painful lesions. Only 1 patient with an asymptomatic tibia lesion underwent surgical intervention (total knee arthroplasty).
Surgical Intervention
Two patients (5.6%) with affected tibiae (4.8%) had pathologic fractures. One fracture (non-small cell lung cancer) was treated with open reduction and internal fixation (periarticular locking plate with cement augmentation), and the other (urothelial cancer) was treated with IMN fixation.
Ten patients (27.8%) with affected tibiae (23.8%) had radiographs that showed cortical breakthrough (Table). Two of the 10 cases were managed nonoperatively, and the patients died before surgical stabilization could be attempted. Of the 8 surgically managed cases, 3 were prophylactically stabilized with IMN (2 of these were augmented with cement, and the third with a screw-plate construct), 2 were treated with periarticular resection and reconstruction (total knee megaprosthesis), 1 was treated with an approach undertaken to address a concomitant distal femoral pathologic fracture, and 1 was treated with total knee arthroplasty undertaken to address lesions at the proximal end of the tibia and the distal end of the femur.
Discussion
We have described a retrospective descriptive study conducted to characterize tibial metastases, their histologies, and the circumstances surrounding diagnosis and surgical management. In all cases, general findings confirmed advanced metastatic disease. In only 3 cases, the tibia lesion was an isolated metastatic lesion.
Sex predilection of tibial metastases remains controversial. One study found males had up to twice as many hand and foot metastases as women,11 but this contrasts with the relatively equal sex ratio found in other studies8,10 and in the present study. We found metastatic disease of the tibia was unsurprisingly concentrated in patients over age 40 years, in whom the vast majority of all cancers develop.12,13 Our study agrees with those that have found most tibia lesions develop in patients in the 6th decade of life on average.8,10 Mean age was 8.3 years higher in our male patients than in our female patients.
Tumor Characteristics
The most common primary neoplasms in our cohort were prostate, breast, and lung cancers, which are among the most common cancers in the United States12,13 and which have a predilection for osseous spread.2,6,9,14 Renal cell carcinoma has been reported to spread to distal (or “acral”) skeletal sites,2-4,9,11,14 but the present study did not identify any patients with this diagnosis. Of our patients with a primary lung cancer for whom a histologic description was available (5/7), all had non-small cell lung cancer. Three patients had a primary malignancy of colorectal cancer, which occasionally metastasizes to the distal skeleton.3,8,11 We identified 3 patients with diffuse large B-cell lymphoma, a histology not widely reported to metastasize to distal skeletal sites.
Metastatic disease of the tibia is most common at the proximal end of the bone.1,10,11,14 Other studies8,10 have found the proximal tibia is affected much more commonly than the tibial diaphysis, and even fewer cases develop at the distal end. Our findings agree with theirs: Proximal lesions outnumber all other lesions combined (Table).
Time to Metastasis
Distal metastases are typical of late-stage metastatic disease,1,3 but quantification of the time from diagnosis of malignancy to presentation of a tibia lesion is not well defined. In our study, time to metastasis was <100 days for some patients (Table). As osseous involvement, especially acral disease, was considered a late-stage manifestation of malignancy, this result was unexpected and most likely represents undiagnosed and untreated malignancy. Six patients in this group were diagnosed with tibial metastases within 30 days, essentially at the same time the primary neoplasm was diagnosed. These findings suggest that a tibia lesion found at time of patient presentation should raise concern for late-stage undiagnosed metastatic cancer.
Other Osseous Disease
The patients identified in this study had advanced malignancy, and most had widespread bony dissemination. Those with the lowest disease burden had isolated tibia lesions or additional metastases only distal to the tibia lesion in the ipsilateral lower extremity. Most of these patients had undergone surgery or were scheduled for it (Table). Most of the patients with appendicular metastases proximal to the tibia lesion had disease of the femora, the most common long bones affected by osseous metastatic disease.5,6 In accordance with orthopedic oncology principles, all other osseous disease should be thoroughly identified and staged before any surgical planning for identified tibia lesions. Ipsilateral distal femoral lesions are of particular importance for patients with proximal tibia lesions, as reconstruction with total knee endoprosthesis can potentially provide a functional reconstructive option after resection of both lesions.
Clinical Presentation
Most of the patients who had cortical breakthrough or required surgical stabilization had painful lesions. Although tibial metastasis is rare, its potential occurrence should raise concerns and be investigated in the patient with tibial pain.
Surgical Intervention
General surgical management of metastatic disease of other long bones has been extensively studied,6,7,9,14 but there are fewer published recommendations regarding specific treatments for metastatic lesions of the tibia. In 2003, Kelly and colleagues8 described an algorithm based on the anatomical location of the lesion, with either internal fixation or IMN fixation representing the preferred management for lesions in the metaphyseal or diaphyseal regions. For epiphyseal or extensive proximal metaphyseal lesions, modular oncology endoprostheses are described as the procedure of choice. Piccioli and colleagues10 in 2013 and Beauchamp and Sim1 in 1988 described a similar operative approach.
It is unknown if the algorithm of Kelly and colleagues8 was referenced during clinical decision-making, but it appears operative management mirrored these principles. Deviations from this general approach in the operative management of the patients in the present study included modifications such as the addition of a screw-plate construct to an IMN for better stability.
Surgical management depends largely on the anatomical location within the bone and on remaining bone stock. Generally, extensive proximal disease is managed with total knee endoprosthesis reconstruction, diaphyseal disease with IMN, and distal disease with internal fixation. Construct augmentation, such as the addition of cement or use of additional hardware, is decided case by case on the basis of desired stability and surrounding bone stock.
Study Limitations
Despite being a larger series, this single-institution study had a relatively small sample size, and its patient demographics and primary malignancies may reflect institutional recruitment bias. In addition, the study was limited by its retrospective design and some incomplete medical records. Eleven patients had only a bone or positron emission tomography scan depicting metastatic disease, limiting characterization of these lesions. One patient lacked radiologic images, and characterizations were based on written reports. As multiple physicians were involved in diagnosis and treatment, there were many inconsistencies in clinical decision-making across the group.
Conclusion
Metastasis to the tibia is a rare but significant event in a subset of patients over the course of their treatment and surveillance. Patients may present with pain secondary to either pathologic or impending pathologic fractures, and in such instances surgical intervention is often needed. Despite the historical reports of “acral” histologies, tibia lesions are not indicative of histology, and biopsy should be considered, especially if management will depend on histology. Patients with lower leg pain and known malignancy should be evaluated to rule out tibial metastasis, but screening examinations may be prudent for asymptomatic patients as well. Increased vigilance may be indicated for those with prostate, breast, or lung cancer. These lesions should be surgically managed case by case using fundamental tenets of both orthopedic fracture care and orthopedic oncology. Ideally, patients should be treated by a multidisciplinary team using a patient-centered approach.
1. Beauchamp CP, Sim FH. Lesions of the tibia. In: Sim FH, ed. Diagnosis and Management of Metastatic Bone Disease: A Multidisciplinary Approach. New York, NY: Raven; 1988:201-212.
2. Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12(20 pt 2):6243s-6249s.
3. Healy JH, Turnbull AD, Miedema B, Lane JM. Acrometastases. A study of twenty-nine patients with osseous involvement of the hands and feet. J Bone Joint Surg Am. 1986;68(5):743-746.
4. Leeson MC, Makley JT, Carter JR. Metastatic skeletal disease distal to the elbow and knee. Clin Orthop Relat Res. 1986;(206):94-99.
5. De Geeter K, Reynders P, Samson I, Broos PL. Metastatic fractures of the tibia. Acta Orthop Belg. 2001;67(1):54-59.
6. Kelly M, Lee M, Clarkson P, O’Brien PJ. Metastatic disease of the long bones: a review of the health care burden in a major trauma centre. Can J Surg. 2012;55(2):95-98.
7. Jasmin C. Textbook of Bone Metastases. Chichester, England: Wiley; 2005.
8. Kelly CM, Wilkins RM, Eckardt JJ, Ward WG. Treatment of metastatic disease of the tibia. Clin Orthop Relat Res. 2003;(415 suppl):S219-S229.
9. Nielsen OS, Munro AJ, Tannock IF. Bone metastases: pathophysiology and management policy. J Clin Oncol. 1991;9(3):509-524.
10. Piccioli A, Maccauro G, Scaramuzzo L, Graci C, Spinelli MS. Surgical treatment of impending and pathological fractures of tibia. Injury. 2013;44(8):1092-1096.
11. Flynn CJ, Danjoux C, Wong J, et al. Two cases of acrometastasis to the hands and review of the literature. Curr Oncol. 2008;15(5):51-58.
12. American Cancer Society. Cancer Facts and Figures 2013. Atlanta, GA: American Cancer Society; 2013.
13. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11-30.
14. Capanna R, Campanacci DA. The treatment of metastases in the appendicular skeleton. J Bone Joint Surg Br. 2001;83(4):471-481.
1. Beauchamp CP, Sim FH. Lesions of the tibia. In: Sim FH, ed. Diagnosis and Management of Metastatic Bone Disease: A Multidisciplinary Approach. New York, NY: Raven; 1988:201-212.
2. Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12(20 pt 2):6243s-6249s.
3. Healy JH, Turnbull AD, Miedema B, Lane JM. Acrometastases. A study of twenty-nine patients with osseous involvement of the hands and feet. J Bone Joint Surg Am. 1986;68(5):743-746.
4. Leeson MC, Makley JT, Carter JR. Metastatic skeletal disease distal to the elbow and knee. Clin Orthop Relat Res. 1986;(206):94-99.
5. De Geeter K, Reynders P, Samson I, Broos PL. Metastatic fractures of the tibia. Acta Orthop Belg. 2001;67(1):54-59.
6. Kelly M, Lee M, Clarkson P, O’Brien PJ. Metastatic disease of the long bones: a review of the health care burden in a major trauma centre. Can J Surg. 2012;55(2):95-98.
7. Jasmin C. Textbook of Bone Metastases. Chichester, England: Wiley; 2005.
8. Kelly CM, Wilkins RM, Eckardt JJ, Ward WG. Treatment of metastatic disease of the tibia. Clin Orthop Relat Res. 2003;(415 suppl):S219-S229.
9. Nielsen OS, Munro AJ, Tannock IF. Bone metastases: pathophysiology and management policy. J Clin Oncol. 1991;9(3):509-524.
10. Piccioli A, Maccauro G, Scaramuzzo L, Graci C, Spinelli MS. Surgical treatment of impending and pathological fractures of tibia. Injury. 2013;44(8):1092-1096.
11. Flynn CJ, Danjoux C, Wong J, et al. Two cases of acrometastasis to the hands and review of the literature. Curr Oncol. 2008;15(5):51-58.
12. American Cancer Society. Cancer Facts and Figures 2013. Atlanta, GA: American Cancer Society; 2013.
13. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11-30.
14. Capanna R, Campanacci DA. The treatment of metastases in the appendicular skeleton. J Bone Joint Surg Br. 2001;83(4):471-481.
Does Knowledge of Implant Cost Affect Fixation Method Choice in the Management of Stable Intertrochanteric Hip Fractures?
Take-Home Points
- The incidence of geriatric hip fractures is rising nationally.
- Costs associated with hip fracture care have risen significantly.
- CMN and SHS are effective for stable, intertrochanteric hip fractures.
- Current trends show increased utilization of CMN compared to SHS for stable introchanteric hip fractures.
- Surgeon awareness of implant cost is a critical factor in delivering cost-effective, evidence-based surgical care.
The continuing increase in the population of patients older than 65 years in the United States is well known. For orthopedic surgeons, this trend highlights the importance of effective geriatric fracture care, particularly hip fracture care. Hip fractures in the elderly are expected to increase 50% by 2025 and to number 500,000 by 2040.1 The growing burden of hip fracture cases is accompanied by increasing costs of care. In 2012, 88% of the healthcare dollars spent on these patients were for direct fracture care, a significant increase from 60% in 2009.2 Although fewer than 1 in 5 fractures in the elderly are hip fractures, these injuries account for 72% of the total cost of geriatric fracture care, more than the total cost of all other osteoporosis-related injuries combined.1 Currently, the direct cost of hip fracture care ranges from $8358 to $32,195 and is expected, in total, to reach $25 billion by 2025.2,3
About 50% of geriatric hip fractures are extracapsular intertrochanteric or pertrochanteric.4 Several studies have compared sliding hip screws (SHSs) with cephalomedullary nails (CMNs) in the effective management of stable intertrochanteric fractures.5-11 Although these implants have shown an increased risk for peri-implant fracture and subsequent reoperation, markers such as mortality, medical complications, and restoration of prefracture function have all been equivocal relative to SHSs.12 Ultimately, one implant cannot be definitively recommended over the other for management of stable intertrochanteric hip fractures.13,14 Nevertheless, the current trend increasingly favors CMNs over SHSs.4,15 Most orthopedic surgeons are unaware of or underestimate the cost difference between these implants—a fact even more pronounced for newer implants.4,16 Considering the ever growing cost burden of hip fractures in the United States, orthopedists must consider not only the efficacy of care but the cost of delivery.
We conducted a study to determine the effect that surgeon knowledge of implant cost had on rates of use of SHSs and CMNs in the management of stable intertrochanteric hip fractures.
Patients and Methods
On May 1, 2012, all 9 attending orthopedic surgeons in a private practice group serving a suburban level II trauma center met to discuss implant prices and implant-related costs for the $850 Versafix SHS, the $1950 short Gamma3 nail (SGN), and the $2900 long Gamma3 nail (LGN), all manufactured by Stryker. All surgeons denied previous knowledge of the costs of these implants. During the discussion, no particular implant was recommended for management of any specific type of fracture. Surgeons were not directly instructed to consider implant cost in subsequent hip fracture surgeries and were not informed of our upcoming study of implant utilization.
After obtaining Institutional Review Board approval, we performed a retrospective chart and radiologic review of all hip fractures (Current Procedural Terminology [CPT] code 27244 or 27245) managed with fixation at our institution between May 1, 2011 and April 30, 2013. Two hundred six patients were identified (Figure 1).
One year later, surgeons were again shown their respective hip fracture radiographs, with patient identifying data removed. They were asked to reclassify their respective cases using the OTA system and then indicate the implant they would use for operative fixation in each of their cases.
Patient age, sex, injury side, fracture types, and utilization rates of the SHS, SGN, and LGN implants were compared between the groups. For each eligible patient, implant cost and other financial data were obtained from the hospital’s finance department. Statistical analyses were performed with SPSS (Statistical Package for the Social Sciences) Version 20 for Macintosh. Independent 2-sample t test was used for parametric comparisons, and Fisher exact test was used for nonparametric comparisons.
Results
Examination of implant use per fracture classification revealed an interesting change. In the before group, SHS was the implant most commonly used for 31-A1.1 fractures (7/16, 43.8%), 31-A1.2 fractures (8/18, 44.4%), and 31-A2.1 fractures (10/25, 40.0%), and LGN was used in 66.7% (8/12) of 31-A1.3 fractures. By contrast, in the after group, SHS was most commonly used only for 31-A1.2 fractures (7/12, 58.3%), SGN was used in 90% (9/10) of 31-A1.1 fractures, and LGN was used in 42.1% (8/19) of 31-A2.1 fractures. In addition, 85.7% (6/7) of 31-A1.3 fractures were managed with a version of the Gamma nail.
Reclassification resulted in more A2.1 fractures (42.0% vs 37.0%) and fewer A1.3 fractures (10.1% vs 16.0%). About the same numbers of fractures were classified A1.1 (21.0% vs 21.8%) and A1.2 (26.9% vs 25.2%). SHS was favored for A1.1 fractures (92.0%) and A1.2 fractures (65.6%). SGN was favored for A1.3 fractures (75.0%). Gamma nails of both sizes were favored for A2.1 fractures (88.0%).
Discussion
Comparisons of SHS/plate and CMN constructs in the management of stable intertrochanteric hip fractures have long been discussed in the orthopedic literature. The major concern with CMNs (vs SHSs) is a statistically significantly higher rate of revision surgery, most often for peri-implant fracture. Rates of previous revision surgery for peri- implant fracture have ranged from 2.4% to 6% for CMNs and from 0.6% to 4% for SHSs.5-7,9 In a Cochrane review of 22 studies (3749 patients), Parker and Handoll12 compared CMN and SHS outcomes in 23 categories and found a statistically significant difference only in postoperative fracture rate. However, in a meta-analysis of studies conducted between 2000 and 2005, Bhandari and colleagues8 found no statistically significant difference in risk of femoral shaft fracture between CMNs (0.6%) and SHSs (0.1%). In addition, Utrilla and colleagues10 reported no postoperative fractures with use of Gamma3 CMNs. These recent studies may indicate that newer CMN designs can reduce the incidence of postoperative peri-implant fracture.8,10 Other outcome measures, such as 1-year mortality, functional outcome, and medical complication rate, have shown no statistically significant differences between the 2 implants.10-12 Ultimately, the current recommendation for fixation of stable intertrochanteric hip fractures is either SHS or CMN.13,14
Of our study patients, 78.9% (before group) and 64.6% (after group) were female, and 49.3% (before group) and 47.9% (after group) were between 80 and 89 years of age. Similarly, a review of hip fracture Medicare claims made between 1999 and 2002 revealed that >75% of the patients were females and 48% to 49% were octogenarians.4,18 However, our rates of different fracture types differed from those of Adams and colleagues.5 In a 1-year single-institution study, they found that, for both CMNs and SHSs, the most common stable intertrochanteric fractures were 31-A1.1 fractures; in our study’s before and after groups, more than one-third of injuries were 31-A2.1 fractures. Least common were 31-A1.3 fractures, both in the study by Adams and colleagues5 and in our before (16.9%) and after (14.6%) groups. Although our fracture rates differ from those of previous studies, all 4 classification categories fall under the umbrella of stable intertrochanteric hip fracture, which is the sole focus of this study.14
We hypothesized that cost would be a significant driver of implant choice in the management of these injuries. Given that SHS costs $1186.91 less than SGN and $1625.88 less than LGN at our institution, we expected that the before- discussion group’s overall SHS use rate of 38.0% would increase after discussion. Instead, SGN became the favored implant, with use in almost half of all fractures in the after group. Although the change in overall implant use rate was notable, these findings were not statistically significant. Examination of individual fracture patterns revealed 2 areas of interest. First, SHS was assumed to be the implant of choice in the management of the relatively simple 31-A1.1 fractures. Although this assumption was verified in the before group (SHS use in 43.8% of fractures), SGN was used in almost every case (90%) in the after group. However, when surgeons were asked 1 year later to recommend an implant for A1.1 fractures, 92% suggested SHS. The more cost-effective SHS construct may be the most amenable for use in these injury types given all intertrochanteric hip fracture patterns, though this has not been studied.
On the other hand, for 31-A2.1 fractures, perhaps the most complicated of the stable patterns, LGN became the implant of choice (42.1%). Despite surgeons’ awareness of the cost differences, management of these fractures shifted in the after group to the most expensive implant, indicative of surgeon concern about eventual loss of reduction with SHS and surgeon comfort with a particular procedure. This trend held when surgeons were asked to reclassify fractures 1 year later, as CMNs were recommended for 88% of 31-A2.1 fractures. Although both SHS and CMN were acceptable in 97% of the fractures included in this study, SGNs or LGNs were preferred for almost every fracture pattern involving the lesser trochanter. All 9 attending surgeons described involvement of the lesser trochanter as an indicator of posteromedial calcar injury. Surgeons became particularly concerned when this fracture pattern occurred in patients with significant osteopenia; SHS fixation, in their opinion, would be poor in the setting of a combination of greater posteromedial instability and poor bone quality. In a level I prospective, randomized trial, Barton and colleagues7 found no difference in outcomes between LGN and SHS fixation for 31-A2 proximal femur fractures and recommended SHS implants for the cost savings. In the clinical experience of this group, however, A1.3 and A2.1 fractures were at especially high risk for failure with SHS use, which necessitated greater implant stability through CMN fixation. On the other hand, for simpler fracture patterns, most surgeons suggested SHS implants. In their opinion, SGN and LGN implants offered no additional benefit of stability without evidence of posteromedial injury, even in the setting of osteopenia. For A1.2 fractures, posteromedial involvement was judged on the basis of size of the inferomedial spike or the extent of the inferomedial fracture line. Two surgeons preferred CMN for simple fractures, one because of the increased comfort with the implants and the other because of the minimally invasive surgical technique. Overall, our results indicate that knowledge of implant cost is not a strong enough factor to change surgeon behavior in selecting fixation for uncomplicated stable intertrochanteric hip fractures in previously ambulatory elderly patients.
The lack of effect could be a consequence of surgeons’ training and comfort with various implants, especially among younger attending surgeons. Most of the attendings in the practice are under age 50 years, which correlates with a preference for CMN fixation.19 Case loads of >80 intertrochanteric hip fractures per calendar year, as in the after group, also correlates with more CMN use.19 However, the before group had more intertrochanteric hip fractures, and yet SHS was the implant of choice. Resident physician experience and comfort with various implants may play a role too, as teaching hospitals with resident assistance also correlate with CMN use.19 However, no major change in resident physician involvement was undertaken during this period. The institution studied is near a major metropolis in the Northeast, a region that has disfavored SHS in recent years.18 The change from before to after fits an overall trend in changing implant use. Anglen and colleagues15 found a significant decrease in SHS use, from 97% in 1999 to 33% in 2006, for intertrochanteric fracture fixation. Simultaneously, CMN use increased from 3% to 67%.
This study had several limitations. First, its overall sample size was small, and therefore any data fluctuations may be exaggerated. Furthermore, changes in utilization rates were compared over 2 years, which may not be long enough to show a changing trend in implant selection. Post hoc analysis of the sample size determined a power of 0.76 for an α of 0.05 and an effect size of 0.50. Second, radiologic classification was performed in a retrospective review, not officially by the operative surgeon. Fractures that we considered stable may have been considered unstable by the operative surgeon, influencing implant selection. Third, patients were selected from only one hospital, and orthopedic surgeons from other institutions may be more sensitive to cost considerations, changing implant selection more quickly. Fourth, initial selection of patients by CPT code might not have captured all those who satisfied the inclusion criteria. Fifth, only a single intervention was used, and follow-up meetings certainly could have increased the effectiveness of the intervention. Last, this and other retrospective studies are inherently weaker because of possible bias.
Conclusion
Our study results showed that implant cost is not a significant factor in implant selection for uncomplicated stable intertrochanteric hip fractures in previously ambulatory elderly patients. By itself, knowledge of implant cost may not be a strong enough force to change surgeon behavior or preference secondary to consequences of failure or comfort with particular implants. In an economic climate in which healthcare is scrutinized for both its medical effectiveness and cost-effectiveness, further study of forces that could influence orthopedic surgeons to select a more cost-effective implant is warranted.
1. Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22(3):465-475.
2. Kilgore ML, Curtis JR, Delzell E, et al. A close examination of healthcare expenditures related to fractures. J Bone Miner Res. 2013;28(4):816-820.
3. Budhia S, Mikyas Y, Tang M, Badamgarav E. Osteoporotic fractures: a systematic review of U.S. healthcare costs and resource utilization. Pharmacoeconomics. 2012;30(2):147-170.
4. Aros B, Tosteson AN, Gottlieb DJ, Koval KJ. Is a sliding hip screw or IM nail the preferred implant for intertrochanteric fracture fixation? Clin Orthop Relat Res. 2008;466(11):2827-2832.
5. Adams CI, Robinson CM, Court-Brown CM, McQueen MM. Prospective randomized controlled trial of an intramedullary nail versus dynamic screw and plate for intertrochanteric fractures of the femur. J Orthop Trauma. 2001;15(6):394-400.
6. Ahrengart L, Törnkvist H, Fornander P, et al. A randomized study of the compression hip screw and Gamma nail in 426 fractures. Clin Orthop Relat Res. 2002;(401):209-222.
7. Barton TM, Gleeson R, Topliss C, Greenwood R, Harries WJ, Chesser TJ. A comparison of the long Gamma nail with the sliding hip screw for the treatment of AO/OTA 31-A2 fractures of the proximal part of the femur: a prospective randomized trial. J Bone Joint Surg Am. 2010;92(4):792-798.
8. Bhandari M, Schemitsch E, Jönsson A, Zlowodzki M, Haidukewych GJ. Gamma nails revisited: Gamma nails versus compression hip screws in the management of intertrochanteric fractures of the hip: a meta-analysis. J Orthop Trauma. 2009;23(6):460-464.
9. Osnes EK, Lofthus CM, Falch JA, et al. More postoperative femoral fractures with the Gamma nail than the sliding screw plate in the treatment of trochanteric fractures. Acta Orthop Scand. 2001;72(3):252-256.
10. Utrilla AL, Reig JS, Muñoz FM, Tufanisco CB. Trochanteric Gamma nail and compression hip screw for trochanteric fractures. J Orthop Trauma. 2005;19(4):229-233.
11. Verettas DA, Ifantidis P, Chatzipapas CN, et al. Systematic effects of surgical treatment of hip fractures: gliding screw-plating vs intramedullary nailing. Injury. 2010;41(3):279-284.
12. Parker MJ, Handoll HH. Gamma and other cephalocondylic intramedullary nails versus extramedullary implants for extracapsular hip fractures in adults. Cochrane Database Syst Rev. 2010;(9):CD000093.
13. Kaplan K, Miyamoto R, Levine BR, Egol KA, Zuckerman JD. Surgical management of hip fractures: an evidence-based review of the literature. II: intertrochanteric fractures. J Am Acad Orthop Surg. 2008;16(11):665-673.
14. Lindskog DM, Baumgaertner MR. Unstable intertrochanteric hip fractures in the elderly. J Am Acad Orthop Surg. 2004;12(3):179-190.
15. Anglen JO, Weinstein JN; American Board of Orthopaedic Surgery Research Committee. Nail or plate fixation of intertrochanteric hip fractures: changing pattern of practice. A review of the American Board of Orthopaedic Surgery Database. J Bone Joint Surg Am. 2008;90(4):700-707.
16. Streit JJ, Youssef A, Coale RM, Carpenter JE, Marcus RE. Orthopaedic surgeons frequently underestimate the cost of orthopaedic implants. Clin Orthop Relat Res. 2013;471(6):1744-1749.
17. Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium—2007: Orthopaedic Trauma Association classification, database and outcomes committee. J Orthop Trauma. 2007;21(10 suppl):S1-S133.
18. Forte ML, Virnig BA, Kane RL, et al. Geographic variation in device use for intertrochanteric hip fractures. J Bone Joint Surg Am. 2008;90(4):691-699.
19. Forte ML, Virnig BA, Eberly LE, et al. Provider factors associated with intramedullary nail use for intertrochanteric hip fractures. J Bone Joint Surg Am. 2010;92(5):1105-1114.
Take-Home Points
- The incidence of geriatric hip fractures is rising nationally.
- Costs associated with hip fracture care have risen significantly.
- CMN and SHS are effective for stable, intertrochanteric hip fractures.
- Current trends show increased utilization of CMN compared to SHS for stable introchanteric hip fractures.
- Surgeon awareness of implant cost is a critical factor in delivering cost-effective, evidence-based surgical care.
The continuing increase in the population of patients older than 65 years in the United States is well known. For orthopedic surgeons, this trend highlights the importance of effective geriatric fracture care, particularly hip fracture care. Hip fractures in the elderly are expected to increase 50% by 2025 and to number 500,000 by 2040.1 The growing burden of hip fracture cases is accompanied by increasing costs of care. In 2012, 88% of the healthcare dollars spent on these patients were for direct fracture care, a significant increase from 60% in 2009.2 Although fewer than 1 in 5 fractures in the elderly are hip fractures, these injuries account for 72% of the total cost of geriatric fracture care, more than the total cost of all other osteoporosis-related injuries combined.1 Currently, the direct cost of hip fracture care ranges from $8358 to $32,195 and is expected, in total, to reach $25 billion by 2025.2,3
About 50% of geriatric hip fractures are extracapsular intertrochanteric or pertrochanteric.4 Several studies have compared sliding hip screws (SHSs) with cephalomedullary nails (CMNs) in the effective management of stable intertrochanteric fractures.5-11 Although these implants have shown an increased risk for peri-implant fracture and subsequent reoperation, markers such as mortality, medical complications, and restoration of prefracture function have all been equivocal relative to SHSs.12 Ultimately, one implant cannot be definitively recommended over the other for management of stable intertrochanteric hip fractures.13,14 Nevertheless, the current trend increasingly favors CMNs over SHSs.4,15 Most orthopedic surgeons are unaware of or underestimate the cost difference between these implants—a fact even more pronounced for newer implants.4,16 Considering the ever growing cost burden of hip fractures in the United States, orthopedists must consider not only the efficacy of care but the cost of delivery.
We conducted a study to determine the effect that surgeon knowledge of implant cost had on rates of use of SHSs and CMNs in the management of stable intertrochanteric hip fractures.
Patients and Methods
On May 1, 2012, all 9 attending orthopedic surgeons in a private practice group serving a suburban level II trauma center met to discuss implant prices and implant-related costs for the $850 Versafix SHS, the $1950 short Gamma3 nail (SGN), and the $2900 long Gamma3 nail (LGN), all manufactured by Stryker. All surgeons denied previous knowledge of the costs of these implants. During the discussion, no particular implant was recommended for management of any specific type of fracture. Surgeons were not directly instructed to consider implant cost in subsequent hip fracture surgeries and were not informed of our upcoming study of implant utilization.
After obtaining Institutional Review Board approval, we performed a retrospective chart and radiologic review of all hip fractures (Current Procedural Terminology [CPT] code 27244 or 27245) managed with fixation at our institution between May 1, 2011 and April 30, 2013. Two hundred six patients were identified (Figure 1).
One year later, surgeons were again shown their respective hip fracture radiographs, with patient identifying data removed. They were asked to reclassify their respective cases using the OTA system and then indicate the implant they would use for operative fixation in each of their cases.
Patient age, sex, injury side, fracture types, and utilization rates of the SHS, SGN, and LGN implants were compared between the groups. For each eligible patient, implant cost and other financial data were obtained from the hospital’s finance department. Statistical analyses were performed with SPSS (Statistical Package for the Social Sciences) Version 20 for Macintosh. Independent 2-sample t test was used for parametric comparisons, and Fisher exact test was used for nonparametric comparisons.
Results
Examination of implant use per fracture classification revealed an interesting change. In the before group, SHS was the implant most commonly used for 31-A1.1 fractures (7/16, 43.8%), 31-A1.2 fractures (8/18, 44.4%), and 31-A2.1 fractures (10/25, 40.0%), and LGN was used in 66.7% (8/12) of 31-A1.3 fractures. By contrast, in the after group, SHS was most commonly used only for 31-A1.2 fractures (7/12, 58.3%), SGN was used in 90% (9/10) of 31-A1.1 fractures, and LGN was used in 42.1% (8/19) of 31-A2.1 fractures. In addition, 85.7% (6/7) of 31-A1.3 fractures were managed with a version of the Gamma nail.
Reclassification resulted in more A2.1 fractures (42.0% vs 37.0%) and fewer A1.3 fractures (10.1% vs 16.0%). About the same numbers of fractures were classified A1.1 (21.0% vs 21.8%) and A1.2 (26.9% vs 25.2%). SHS was favored for A1.1 fractures (92.0%) and A1.2 fractures (65.6%). SGN was favored for A1.3 fractures (75.0%). Gamma nails of both sizes were favored for A2.1 fractures (88.0%).
Discussion
Comparisons of SHS/plate and CMN constructs in the management of stable intertrochanteric hip fractures have long been discussed in the orthopedic literature. The major concern with CMNs (vs SHSs) is a statistically significantly higher rate of revision surgery, most often for peri-implant fracture. Rates of previous revision surgery for peri- implant fracture have ranged from 2.4% to 6% for CMNs and from 0.6% to 4% for SHSs.5-7,9 In a Cochrane review of 22 studies (3749 patients), Parker and Handoll12 compared CMN and SHS outcomes in 23 categories and found a statistically significant difference only in postoperative fracture rate. However, in a meta-analysis of studies conducted between 2000 and 2005, Bhandari and colleagues8 found no statistically significant difference in risk of femoral shaft fracture between CMNs (0.6%) and SHSs (0.1%). In addition, Utrilla and colleagues10 reported no postoperative fractures with use of Gamma3 CMNs. These recent studies may indicate that newer CMN designs can reduce the incidence of postoperative peri-implant fracture.8,10 Other outcome measures, such as 1-year mortality, functional outcome, and medical complication rate, have shown no statistically significant differences between the 2 implants.10-12 Ultimately, the current recommendation for fixation of stable intertrochanteric hip fractures is either SHS or CMN.13,14
Of our study patients, 78.9% (before group) and 64.6% (after group) were female, and 49.3% (before group) and 47.9% (after group) were between 80 and 89 years of age. Similarly, a review of hip fracture Medicare claims made between 1999 and 2002 revealed that >75% of the patients were females and 48% to 49% were octogenarians.4,18 However, our rates of different fracture types differed from those of Adams and colleagues.5 In a 1-year single-institution study, they found that, for both CMNs and SHSs, the most common stable intertrochanteric fractures were 31-A1.1 fractures; in our study’s before and after groups, more than one-third of injuries were 31-A2.1 fractures. Least common were 31-A1.3 fractures, both in the study by Adams and colleagues5 and in our before (16.9%) and after (14.6%) groups. Although our fracture rates differ from those of previous studies, all 4 classification categories fall under the umbrella of stable intertrochanteric hip fracture, which is the sole focus of this study.14
We hypothesized that cost would be a significant driver of implant choice in the management of these injuries. Given that SHS costs $1186.91 less than SGN and $1625.88 less than LGN at our institution, we expected that the before- discussion group’s overall SHS use rate of 38.0% would increase after discussion. Instead, SGN became the favored implant, with use in almost half of all fractures in the after group. Although the change in overall implant use rate was notable, these findings were not statistically significant. Examination of individual fracture patterns revealed 2 areas of interest. First, SHS was assumed to be the implant of choice in the management of the relatively simple 31-A1.1 fractures. Although this assumption was verified in the before group (SHS use in 43.8% of fractures), SGN was used in almost every case (90%) in the after group. However, when surgeons were asked 1 year later to recommend an implant for A1.1 fractures, 92% suggested SHS. The more cost-effective SHS construct may be the most amenable for use in these injury types given all intertrochanteric hip fracture patterns, though this has not been studied.
On the other hand, for 31-A2.1 fractures, perhaps the most complicated of the stable patterns, LGN became the implant of choice (42.1%). Despite surgeons’ awareness of the cost differences, management of these fractures shifted in the after group to the most expensive implant, indicative of surgeon concern about eventual loss of reduction with SHS and surgeon comfort with a particular procedure. This trend held when surgeons were asked to reclassify fractures 1 year later, as CMNs were recommended for 88% of 31-A2.1 fractures. Although both SHS and CMN were acceptable in 97% of the fractures included in this study, SGNs or LGNs were preferred for almost every fracture pattern involving the lesser trochanter. All 9 attending surgeons described involvement of the lesser trochanter as an indicator of posteromedial calcar injury. Surgeons became particularly concerned when this fracture pattern occurred in patients with significant osteopenia; SHS fixation, in their opinion, would be poor in the setting of a combination of greater posteromedial instability and poor bone quality. In a level I prospective, randomized trial, Barton and colleagues7 found no difference in outcomes between LGN and SHS fixation for 31-A2 proximal femur fractures and recommended SHS implants for the cost savings. In the clinical experience of this group, however, A1.3 and A2.1 fractures were at especially high risk for failure with SHS use, which necessitated greater implant stability through CMN fixation. On the other hand, for simpler fracture patterns, most surgeons suggested SHS implants. In their opinion, SGN and LGN implants offered no additional benefit of stability without evidence of posteromedial injury, even in the setting of osteopenia. For A1.2 fractures, posteromedial involvement was judged on the basis of size of the inferomedial spike or the extent of the inferomedial fracture line. Two surgeons preferred CMN for simple fractures, one because of the increased comfort with the implants and the other because of the minimally invasive surgical technique. Overall, our results indicate that knowledge of implant cost is not a strong enough factor to change surgeon behavior in selecting fixation for uncomplicated stable intertrochanteric hip fractures in previously ambulatory elderly patients.
The lack of effect could be a consequence of surgeons’ training and comfort with various implants, especially among younger attending surgeons. Most of the attendings in the practice are under age 50 years, which correlates with a preference for CMN fixation.19 Case loads of >80 intertrochanteric hip fractures per calendar year, as in the after group, also correlates with more CMN use.19 However, the before group had more intertrochanteric hip fractures, and yet SHS was the implant of choice. Resident physician experience and comfort with various implants may play a role too, as teaching hospitals with resident assistance also correlate with CMN use.19 However, no major change in resident physician involvement was undertaken during this period. The institution studied is near a major metropolis in the Northeast, a region that has disfavored SHS in recent years.18 The change from before to after fits an overall trend in changing implant use. Anglen and colleagues15 found a significant decrease in SHS use, from 97% in 1999 to 33% in 2006, for intertrochanteric fracture fixation. Simultaneously, CMN use increased from 3% to 67%.
This study had several limitations. First, its overall sample size was small, and therefore any data fluctuations may be exaggerated. Furthermore, changes in utilization rates were compared over 2 years, which may not be long enough to show a changing trend in implant selection. Post hoc analysis of the sample size determined a power of 0.76 for an α of 0.05 and an effect size of 0.50. Second, radiologic classification was performed in a retrospective review, not officially by the operative surgeon. Fractures that we considered stable may have been considered unstable by the operative surgeon, influencing implant selection. Third, patients were selected from only one hospital, and orthopedic surgeons from other institutions may be more sensitive to cost considerations, changing implant selection more quickly. Fourth, initial selection of patients by CPT code might not have captured all those who satisfied the inclusion criteria. Fifth, only a single intervention was used, and follow-up meetings certainly could have increased the effectiveness of the intervention. Last, this and other retrospective studies are inherently weaker because of possible bias.
Conclusion
Our study results showed that implant cost is not a significant factor in implant selection for uncomplicated stable intertrochanteric hip fractures in previously ambulatory elderly patients. By itself, knowledge of implant cost may not be a strong enough force to change surgeon behavior or preference secondary to consequences of failure or comfort with particular implants. In an economic climate in which healthcare is scrutinized for both its medical effectiveness and cost-effectiveness, further study of forces that could influence orthopedic surgeons to select a more cost-effective implant is warranted.
Take-Home Points
- The incidence of geriatric hip fractures is rising nationally.
- Costs associated with hip fracture care have risen significantly.
- CMN and SHS are effective for stable, intertrochanteric hip fractures.
- Current trends show increased utilization of CMN compared to SHS for stable introchanteric hip fractures.
- Surgeon awareness of implant cost is a critical factor in delivering cost-effective, evidence-based surgical care.
The continuing increase in the population of patients older than 65 years in the United States is well known. For orthopedic surgeons, this trend highlights the importance of effective geriatric fracture care, particularly hip fracture care. Hip fractures in the elderly are expected to increase 50% by 2025 and to number 500,000 by 2040.1 The growing burden of hip fracture cases is accompanied by increasing costs of care. In 2012, 88% of the healthcare dollars spent on these patients were for direct fracture care, a significant increase from 60% in 2009.2 Although fewer than 1 in 5 fractures in the elderly are hip fractures, these injuries account for 72% of the total cost of geriatric fracture care, more than the total cost of all other osteoporosis-related injuries combined.1 Currently, the direct cost of hip fracture care ranges from $8358 to $32,195 and is expected, in total, to reach $25 billion by 2025.2,3
About 50% of geriatric hip fractures are extracapsular intertrochanteric or pertrochanteric.4 Several studies have compared sliding hip screws (SHSs) with cephalomedullary nails (CMNs) in the effective management of stable intertrochanteric fractures.5-11 Although these implants have shown an increased risk for peri-implant fracture and subsequent reoperation, markers such as mortality, medical complications, and restoration of prefracture function have all been equivocal relative to SHSs.12 Ultimately, one implant cannot be definitively recommended over the other for management of stable intertrochanteric hip fractures.13,14 Nevertheless, the current trend increasingly favors CMNs over SHSs.4,15 Most orthopedic surgeons are unaware of or underestimate the cost difference between these implants—a fact even more pronounced for newer implants.4,16 Considering the ever growing cost burden of hip fractures in the United States, orthopedists must consider not only the efficacy of care but the cost of delivery.
We conducted a study to determine the effect that surgeon knowledge of implant cost had on rates of use of SHSs and CMNs in the management of stable intertrochanteric hip fractures.
Patients and Methods
On May 1, 2012, all 9 attending orthopedic surgeons in a private practice group serving a suburban level II trauma center met to discuss implant prices and implant-related costs for the $850 Versafix SHS, the $1950 short Gamma3 nail (SGN), and the $2900 long Gamma3 nail (LGN), all manufactured by Stryker. All surgeons denied previous knowledge of the costs of these implants. During the discussion, no particular implant was recommended for management of any specific type of fracture. Surgeons were not directly instructed to consider implant cost in subsequent hip fracture surgeries and were not informed of our upcoming study of implant utilization.
After obtaining Institutional Review Board approval, we performed a retrospective chart and radiologic review of all hip fractures (Current Procedural Terminology [CPT] code 27244 or 27245) managed with fixation at our institution between May 1, 2011 and April 30, 2013. Two hundred six patients were identified (Figure 1).
One year later, surgeons were again shown their respective hip fracture radiographs, with patient identifying data removed. They were asked to reclassify their respective cases using the OTA system and then indicate the implant they would use for operative fixation in each of their cases.
Patient age, sex, injury side, fracture types, and utilization rates of the SHS, SGN, and LGN implants were compared between the groups. For each eligible patient, implant cost and other financial data were obtained from the hospital’s finance department. Statistical analyses were performed with SPSS (Statistical Package for the Social Sciences) Version 20 for Macintosh. Independent 2-sample t test was used for parametric comparisons, and Fisher exact test was used for nonparametric comparisons.
Results
Examination of implant use per fracture classification revealed an interesting change. In the before group, SHS was the implant most commonly used for 31-A1.1 fractures (7/16, 43.8%), 31-A1.2 fractures (8/18, 44.4%), and 31-A2.1 fractures (10/25, 40.0%), and LGN was used in 66.7% (8/12) of 31-A1.3 fractures. By contrast, in the after group, SHS was most commonly used only for 31-A1.2 fractures (7/12, 58.3%), SGN was used in 90% (9/10) of 31-A1.1 fractures, and LGN was used in 42.1% (8/19) of 31-A2.1 fractures. In addition, 85.7% (6/7) of 31-A1.3 fractures were managed with a version of the Gamma nail.
Reclassification resulted in more A2.1 fractures (42.0% vs 37.0%) and fewer A1.3 fractures (10.1% vs 16.0%). About the same numbers of fractures were classified A1.1 (21.0% vs 21.8%) and A1.2 (26.9% vs 25.2%). SHS was favored for A1.1 fractures (92.0%) and A1.2 fractures (65.6%). SGN was favored for A1.3 fractures (75.0%). Gamma nails of both sizes were favored for A2.1 fractures (88.0%).
Discussion
Comparisons of SHS/plate and CMN constructs in the management of stable intertrochanteric hip fractures have long been discussed in the orthopedic literature. The major concern with CMNs (vs SHSs) is a statistically significantly higher rate of revision surgery, most often for peri-implant fracture. Rates of previous revision surgery for peri- implant fracture have ranged from 2.4% to 6% for CMNs and from 0.6% to 4% for SHSs.5-7,9 In a Cochrane review of 22 studies (3749 patients), Parker and Handoll12 compared CMN and SHS outcomes in 23 categories and found a statistically significant difference only in postoperative fracture rate. However, in a meta-analysis of studies conducted between 2000 and 2005, Bhandari and colleagues8 found no statistically significant difference in risk of femoral shaft fracture between CMNs (0.6%) and SHSs (0.1%). In addition, Utrilla and colleagues10 reported no postoperative fractures with use of Gamma3 CMNs. These recent studies may indicate that newer CMN designs can reduce the incidence of postoperative peri-implant fracture.8,10 Other outcome measures, such as 1-year mortality, functional outcome, and medical complication rate, have shown no statistically significant differences between the 2 implants.10-12 Ultimately, the current recommendation for fixation of stable intertrochanteric hip fractures is either SHS or CMN.13,14
Of our study patients, 78.9% (before group) and 64.6% (after group) were female, and 49.3% (before group) and 47.9% (after group) were between 80 and 89 years of age. Similarly, a review of hip fracture Medicare claims made between 1999 and 2002 revealed that >75% of the patients were females and 48% to 49% were octogenarians.4,18 However, our rates of different fracture types differed from those of Adams and colleagues.5 In a 1-year single-institution study, they found that, for both CMNs and SHSs, the most common stable intertrochanteric fractures were 31-A1.1 fractures; in our study’s before and after groups, more than one-third of injuries were 31-A2.1 fractures. Least common were 31-A1.3 fractures, both in the study by Adams and colleagues5 and in our before (16.9%) and after (14.6%) groups. Although our fracture rates differ from those of previous studies, all 4 classification categories fall under the umbrella of stable intertrochanteric hip fracture, which is the sole focus of this study.14
We hypothesized that cost would be a significant driver of implant choice in the management of these injuries. Given that SHS costs $1186.91 less than SGN and $1625.88 less than LGN at our institution, we expected that the before- discussion group’s overall SHS use rate of 38.0% would increase after discussion. Instead, SGN became the favored implant, with use in almost half of all fractures in the after group. Although the change in overall implant use rate was notable, these findings were not statistically significant. Examination of individual fracture patterns revealed 2 areas of interest. First, SHS was assumed to be the implant of choice in the management of the relatively simple 31-A1.1 fractures. Although this assumption was verified in the before group (SHS use in 43.8% of fractures), SGN was used in almost every case (90%) in the after group. However, when surgeons were asked 1 year later to recommend an implant for A1.1 fractures, 92% suggested SHS. The more cost-effective SHS construct may be the most amenable for use in these injury types given all intertrochanteric hip fracture patterns, though this has not been studied.
On the other hand, for 31-A2.1 fractures, perhaps the most complicated of the stable patterns, LGN became the implant of choice (42.1%). Despite surgeons’ awareness of the cost differences, management of these fractures shifted in the after group to the most expensive implant, indicative of surgeon concern about eventual loss of reduction with SHS and surgeon comfort with a particular procedure. This trend held when surgeons were asked to reclassify fractures 1 year later, as CMNs were recommended for 88% of 31-A2.1 fractures. Although both SHS and CMN were acceptable in 97% of the fractures included in this study, SGNs or LGNs were preferred for almost every fracture pattern involving the lesser trochanter. All 9 attending surgeons described involvement of the lesser trochanter as an indicator of posteromedial calcar injury. Surgeons became particularly concerned when this fracture pattern occurred in patients with significant osteopenia; SHS fixation, in their opinion, would be poor in the setting of a combination of greater posteromedial instability and poor bone quality. In a level I prospective, randomized trial, Barton and colleagues7 found no difference in outcomes between LGN and SHS fixation for 31-A2 proximal femur fractures and recommended SHS implants for the cost savings. In the clinical experience of this group, however, A1.3 and A2.1 fractures were at especially high risk for failure with SHS use, which necessitated greater implant stability through CMN fixation. On the other hand, for simpler fracture patterns, most surgeons suggested SHS implants. In their opinion, SGN and LGN implants offered no additional benefit of stability without evidence of posteromedial injury, even in the setting of osteopenia. For A1.2 fractures, posteromedial involvement was judged on the basis of size of the inferomedial spike or the extent of the inferomedial fracture line. Two surgeons preferred CMN for simple fractures, one because of the increased comfort with the implants and the other because of the minimally invasive surgical technique. Overall, our results indicate that knowledge of implant cost is not a strong enough factor to change surgeon behavior in selecting fixation for uncomplicated stable intertrochanteric hip fractures in previously ambulatory elderly patients.
The lack of effect could be a consequence of surgeons’ training and comfort with various implants, especially among younger attending surgeons. Most of the attendings in the practice are under age 50 years, which correlates with a preference for CMN fixation.19 Case loads of >80 intertrochanteric hip fractures per calendar year, as in the after group, also correlates with more CMN use.19 However, the before group had more intertrochanteric hip fractures, and yet SHS was the implant of choice. Resident physician experience and comfort with various implants may play a role too, as teaching hospitals with resident assistance also correlate with CMN use.19 However, no major change in resident physician involvement was undertaken during this period. The institution studied is near a major metropolis in the Northeast, a region that has disfavored SHS in recent years.18 The change from before to after fits an overall trend in changing implant use. Anglen and colleagues15 found a significant decrease in SHS use, from 97% in 1999 to 33% in 2006, for intertrochanteric fracture fixation. Simultaneously, CMN use increased from 3% to 67%.
This study had several limitations. First, its overall sample size was small, and therefore any data fluctuations may be exaggerated. Furthermore, changes in utilization rates were compared over 2 years, which may not be long enough to show a changing trend in implant selection. Post hoc analysis of the sample size determined a power of 0.76 for an α of 0.05 and an effect size of 0.50. Second, radiologic classification was performed in a retrospective review, not officially by the operative surgeon. Fractures that we considered stable may have been considered unstable by the operative surgeon, influencing implant selection. Third, patients were selected from only one hospital, and orthopedic surgeons from other institutions may be more sensitive to cost considerations, changing implant selection more quickly. Fourth, initial selection of patients by CPT code might not have captured all those who satisfied the inclusion criteria. Fifth, only a single intervention was used, and follow-up meetings certainly could have increased the effectiveness of the intervention. Last, this and other retrospective studies are inherently weaker because of possible bias.
Conclusion
Our study results showed that implant cost is not a significant factor in implant selection for uncomplicated stable intertrochanteric hip fractures in previously ambulatory elderly patients. By itself, knowledge of implant cost may not be a strong enough force to change surgeon behavior or preference secondary to consequences of failure or comfort with particular implants. In an economic climate in which healthcare is scrutinized for both its medical effectiveness and cost-effectiveness, further study of forces that could influence orthopedic surgeons to select a more cost-effective implant is warranted.
1. Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22(3):465-475.
2. Kilgore ML, Curtis JR, Delzell E, et al. A close examination of healthcare expenditures related to fractures. J Bone Miner Res. 2013;28(4):816-820.
3. Budhia S, Mikyas Y, Tang M, Badamgarav E. Osteoporotic fractures: a systematic review of U.S. healthcare costs and resource utilization. Pharmacoeconomics. 2012;30(2):147-170.
4. Aros B, Tosteson AN, Gottlieb DJ, Koval KJ. Is a sliding hip screw or IM nail the preferred implant for intertrochanteric fracture fixation? Clin Orthop Relat Res. 2008;466(11):2827-2832.
5. Adams CI, Robinson CM, Court-Brown CM, McQueen MM. Prospective randomized controlled trial of an intramedullary nail versus dynamic screw and plate for intertrochanteric fractures of the femur. J Orthop Trauma. 2001;15(6):394-400.
6. Ahrengart L, Törnkvist H, Fornander P, et al. A randomized study of the compression hip screw and Gamma nail in 426 fractures. Clin Orthop Relat Res. 2002;(401):209-222.
7. Barton TM, Gleeson R, Topliss C, Greenwood R, Harries WJ, Chesser TJ. A comparison of the long Gamma nail with the sliding hip screw for the treatment of AO/OTA 31-A2 fractures of the proximal part of the femur: a prospective randomized trial. J Bone Joint Surg Am. 2010;92(4):792-798.
8. Bhandari M, Schemitsch E, Jönsson A, Zlowodzki M, Haidukewych GJ. Gamma nails revisited: Gamma nails versus compression hip screws in the management of intertrochanteric fractures of the hip: a meta-analysis. J Orthop Trauma. 2009;23(6):460-464.
9. Osnes EK, Lofthus CM, Falch JA, et al. More postoperative femoral fractures with the Gamma nail than the sliding screw plate in the treatment of trochanteric fractures. Acta Orthop Scand. 2001;72(3):252-256.
10. Utrilla AL, Reig JS, Muñoz FM, Tufanisco CB. Trochanteric Gamma nail and compression hip screw for trochanteric fractures. J Orthop Trauma. 2005;19(4):229-233.
11. Verettas DA, Ifantidis P, Chatzipapas CN, et al. Systematic effects of surgical treatment of hip fractures: gliding screw-plating vs intramedullary nailing. Injury. 2010;41(3):279-284.
12. Parker MJ, Handoll HH. Gamma and other cephalocondylic intramedullary nails versus extramedullary implants for extracapsular hip fractures in adults. Cochrane Database Syst Rev. 2010;(9):CD000093.
13. Kaplan K, Miyamoto R, Levine BR, Egol KA, Zuckerman JD. Surgical management of hip fractures: an evidence-based review of the literature. II: intertrochanteric fractures. J Am Acad Orthop Surg. 2008;16(11):665-673.
14. Lindskog DM, Baumgaertner MR. Unstable intertrochanteric hip fractures in the elderly. J Am Acad Orthop Surg. 2004;12(3):179-190.
15. Anglen JO, Weinstein JN; American Board of Orthopaedic Surgery Research Committee. Nail or plate fixation of intertrochanteric hip fractures: changing pattern of practice. A review of the American Board of Orthopaedic Surgery Database. J Bone Joint Surg Am. 2008;90(4):700-707.
16. Streit JJ, Youssef A, Coale RM, Carpenter JE, Marcus RE. Orthopaedic surgeons frequently underestimate the cost of orthopaedic implants. Clin Orthop Relat Res. 2013;471(6):1744-1749.
17. Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium—2007: Orthopaedic Trauma Association classification, database and outcomes committee. J Orthop Trauma. 2007;21(10 suppl):S1-S133.
18. Forte ML, Virnig BA, Kane RL, et al. Geographic variation in device use for intertrochanteric hip fractures. J Bone Joint Surg Am. 2008;90(4):691-699.
19. Forte ML, Virnig BA, Eberly LE, et al. Provider factors associated with intramedullary nail use for intertrochanteric hip fractures. J Bone Joint Surg Am. 2010;92(5):1105-1114.
1. Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22(3):465-475.
2. Kilgore ML, Curtis JR, Delzell E, et al. A close examination of healthcare expenditures related to fractures. J Bone Miner Res. 2013;28(4):816-820.
3. Budhia S, Mikyas Y, Tang M, Badamgarav E. Osteoporotic fractures: a systematic review of U.S. healthcare costs and resource utilization. Pharmacoeconomics. 2012;30(2):147-170.
4. Aros B, Tosteson AN, Gottlieb DJ, Koval KJ. Is a sliding hip screw or IM nail the preferred implant for intertrochanteric fracture fixation? Clin Orthop Relat Res. 2008;466(11):2827-2832.
5. Adams CI, Robinson CM, Court-Brown CM, McQueen MM. Prospective randomized controlled trial of an intramedullary nail versus dynamic screw and plate for intertrochanteric fractures of the femur. J Orthop Trauma. 2001;15(6):394-400.
6. Ahrengart L, Törnkvist H, Fornander P, et al. A randomized study of the compression hip screw and Gamma nail in 426 fractures. Clin Orthop Relat Res. 2002;(401):209-222.
7. Barton TM, Gleeson R, Topliss C, Greenwood R, Harries WJ, Chesser TJ. A comparison of the long Gamma nail with the sliding hip screw for the treatment of AO/OTA 31-A2 fractures of the proximal part of the femur: a prospective randomized trial. J Bone Joint Surg Am. 2010;92(4):792-798.
8. Bhandari M, Schemitsch E, Jönsson A, Zlowodzki M, Haidukewych GJ. Gamma nails revisited: Gamma nails versus compression hip screws in the management of intertrochanteric fractures of the hip: a meta-analysis. J Orthop Trauma. 2009;23(6):460-464.
9. Osnes EK, Lofthus CM, Falch JA, et al. More postoperative femoral fractures with the Gamma nail than the sliding screw plate in the treatment of trochanteric fractures. Acta Orthop Scand. 2001;72(3):252-256.
10. Utrilla AL, Reig JS, Muñoz FM, Tufanisco CB. Trochanteric Gamma nail and compression hip screw for trochanteric fractures. J Orthop Trauma. 2005;19(4):229-233.
11. Verettas DA, Ifantidis P, Chatzipapas CN, et al. Systematic effects of surgical treatment of hip fractures: gliding screw-plating vs intramedullary nailing. Injury. 2010;41(3):279-284.
12. Parker MJ, Handoll HH. Gamma and other cephalocondylic intramedullary nails versus extramedullary implants for extracapsular hip fractures in adults. Cochrane Database Syst Rev. 2010;(9):CD000093.
13. Kaplan K, Miyamoto R, Levine BR, Egol KA, Zuckerman JD. Surgical management of hip fractures: an evidence-based review of the literature. II: intertrochanteric fractures. J Am Acad Orthop Surg. 2008;16(11):665-673.
14. Lindskog DM, Baumgaertner MR. Unstable intertrochanteric hip fractures in the elderly. J Am Acad Orthop Surg. 2004;12(3):179-190.
15. Anglen JO, Weinstein JN; American Board of Orthopaedic Surgery Research Committee. Nail or plate fixation of intertrochanteric hip fractures: changing pattern of practice. A review of the American Board of Orthopaedic Surgery Database. J Bone Joint Surg Am. 2008;90(4):700-707.
16. Streit JJ, Youssef A, Coale RM, Carpenter JE, Marcus RE. Orthopaedic surgeons frequently underestimate the cost of orthopaedic implants. Clin Orthop Relat Res. 2013;471(6):1744-1749.
17. Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium—2007: Orthopaedic Trauma Association classification, database and outcomes committee. J Orthop Trauma. 2007;21(10 suppl):S1-S133.
18. Forte ML, Virnig BA, Kane RL, et al. Geographic variation in device use for intertrochanteric hip fractures. J Bone Joint Surg Am. 2008;90(4):691-699.
19. Forte ML, Virnig BA, Eberly LE, et al. Provider factors associated with intramedullary nail use for intertrochanteric hip fractures. J Bone Joint Surg Am. 2010;92(5):1105-1114.
Minimally Invasive Anatomical Reconstruction of Posteromedial Corner of Knee: A Cadaveric Study
Take-Home Points
- Injuries to the medial knee are the most common knee ligament injuries, and often occur in the athletic population.
- Complete posteromedial corner injuries require surgical treatment to restore joint stability and biomechanics.
- Biomechanical evidence has demonstrated an important load-sharing distribution between the sMCL and the POL.
- Valgus instability caused by a medial side injury, can lead to both ACL/posterior cruciate ligament reconstruction graft failure if the medial sided injury is not concurrently repaired or reconstructed.
- Anatomic posteromedial corner reconstruction yields excellent biomechanical and patient-reported outcomes.
Most injuries of the medial structures of the knee are treated conservatively.1-3 In severe acute injuries and chronic symptomatic instabilities, however, surgical treatment is needed to restore knee stability and to prevent degenerative changes secondary to instability.4 Three structures involved in medial stability are the superficial medial collateral ligament (sMCL), which is the primary valgus restraint; the posterior oblique ligament (POL), which is the primary restraint to internal rotation and the secondary valgus restraint; and the semimembranosus.5,6
Surgical techniques for posteromedial knee reconstruction include direct repair,7 repair with augmentation,8,9 advancement of the tibial insertion of the sMCL,10 and transfer of the pes anserine tendons.11 In anatomical reconstruction of the posteromedial corner, which has been described before, the sMCL and the POL are reconstructed to reproduce the native motion and stability of the knee.12 Clinically, repair and reconstruction have similar patient-reported outcomes and medial opening evaluations over the short term.
These approaches require large incisions and extensive dissection of soft tissue on the medial aspect of the knee.5 Given these drawbacks, it is reasonable to consider less invasive options. Minimally invasive surgery has the advantages of reduced scarring and blood loss, less disruption of surrounding tissue, faster recovery, and improved aesthetics.4
We conducted a study of a minimally invasive technique for reconstructing the posteromedial structures of the knee. We compared medial compartment stability measured on valgus stress radiographs in intact, sectioned, and reconstructed states in cadaveric knees. We hypothesized that a minimally invasive technique using autogenous hamstring graft in the appropriate anatomical location would return valgus stability to its nearly native state.
Materials and Methods
This study was conducted at the Buenos Aires British Hospital in Buenos Aires, Argentina, and at the University of Colorado Hospital in Aurora. Ten fresh-frozen cadaveric knees with no evidence of ligamentous injuries, osteoarthritis, or previous surgery were used. Mean donor age was 69.4 years (range, 45-87 years). Each specimen was maintained at room temperature for 24 hours before use. The femur was sectioned 20 cm proximal to the knee joint. The tibia was sectioned 12.5 cm distal to the knee joint.
Identification and Sectioning of Posteromedial Structures
After intact-state evaluation, each knee’s sMCL, dMCL, and POL were sectioned at their tibial insertion. Valgus stress radiograph was repeated and medial compartment gap was remeasured for comparison of the sectioned state with the intact and reconstructed states.
Anatomical Reconstruction With Mini-Invasive Technique
After sectioning of medial stabilizing structures, minimally invasive reconstruction was performed through 2 small incisions on the medial aspect of each of the 10 knees, as follows. First, the semitendinosus tendon was identified through the oblique incision that had been used for sectioning. Then, an open-ended tendon stripper was placed around the circumference of the semitendinosus and was passed proximomedially, transecting the tendon at its musculotendinous junction. While the tendon stripper was being passed, care was taken to maintain the nearby tibial insertion of the sartorius fascia (Figures 1D-1F).
With the semitendinosus tendon looped around the wire, isometricity was tested by pulling the suture within the tendon and moving the knee through a full range of motion. The isometric point was confirmed by tendon migration of <2 mm.13 Migration was measured by marking the graft 2 mm from its insertion; the graft was then pulled to ensure correct isometric point position. An 18-mm cannulated spiked screw and washer (Arthrex) were then passed over the wire and partially secured to the femur—the attachment point for the proximal sMCL portion of the semitendinosus graft. The semitendinosus tendon was then secured beneath the spiked washer with the knee in 20° of flexion with neutral rotation, recreating the sMCL.
Posteriorly, the distal insertion site of the POL was identified at the posteromedial aspect of the tibia through the oblique incision previously described. A 7-mm tunnel was drilled starting posteromedial (10 mm under tibial articular surface) and exiting just distal and medial to the Gerdy tubercle.
After final fixation, the medial knee was openly dissected to assess the inverted-V ligament reconstruction for anatomical placement and avoidance of crucial structures.
Stability Testing
Per International Knee Documentation Committee guidelines for stressing the medial compartment,14 valgus stress radiographs were obtained for all specimens at 0° and 20° of flexion in intact, sectioned, and reconstructed states.
The medial gap formed by the femoral condyle and its corresponding tibial plateau (at site of maximal separation) was tested in all 3 state conditions (intact, sectioned, reconstructed). Distances were digitally measured with a picture archiving and communication system viewer (Imagecast; IDX Systems Corporation). Medial gap was measured by taking the shortest distance between the subchondral bone surface of the most distal aspect of the medial femoral condyle and the corresponding medial tibial plateau. Three independent examiners took all the measurements; each examiner was blinded to the others’ measurements.
Statistics
Paired Student t tests were used to compare the 3 conditions, and the Shapiro-Wilk test was used to check for a normally distributed population. Statistical significance was set at P < .05. Statistical analyses were performed with GraphPad software.
Results
In all 10 specimens, the sMCL, the dMCL, and the POL were successfully identified and sectioned through a medial oblique incision over the distal insertion of the structures.
During all valgus testing states, there was no loss of graft fixation, and there was no gross graft slippage. In addition, all grafts remained in continuity with no evidence of failure, and there were no failures or breakages of the proximal or distal screw.
After posteromedial sectioning, mean medial gap was statistically significantly larger (P = .0002) at full extension (11 mm vs 3.3 mm) and at 20° of flexion (12.6 mm vs 3.8 mm). There was no statistically significant difference between the value of the intact state and the value after minimally invasive reconstruction at 0° (P = .56) or 20° (P = .102) of flexion.
Discussion
In this article, we describe a minimally invasive technique for anatomical posteromedial reconstruction of the knee in a cadaveric model. This technique restores the knee’s native valgus stability without causing extensive damage to the surrounding soft tissues and thereby potentially prevents scar formation and reduces blood loss.
Superficial MCL injury, one of the most common knee ligament injuries, is often associated with POL injury.7 Although most sMCL injuries are treated nonoperatively, with good results,3 surgical treatment is needed for severe (grade III) instabilities, symptomatic chronic instabilities, and knee dislocations.12,17 Most posteromedial reconstruction techniques require an extensive approach that causes damage to surrounding soft tissue,6,7,9,10 which in turn may compromise healing and positive patient outcomes. Surgical techniques include direct repair with sutures or anchors,18 capsular procedures,19 augmentations,9 internal bracing,6 and complete reconstruction of the posteromedial corner.20
LaPrade and Wijdicks12 have previously described anatomical reconstruction of the posteromedial corner. In their technique, a split semitendinosus autograft is used to reconstruct the sMCL and the POL separately, using 4 implants and reproducing each ligament’s anatomical attachment site. In this proposed technique, the distal attachment of the semitendinosus insertion is left intact, and uses 1 attachment point on the distal femur and 1 on the proximal tibia, allowing use of only 2 implants. In addition, it is performed with a minimally invasive approach, reduces cost, limits surgical exposure, and with experience may shorten operative time. To reduce the graft failure rate, the technique of LaPrade and Wijdicks12 positions the sMCL tibial attachment as posterior as possible, which can be performed with this minimally invasive approach as well.
To reduce the graft failure rate, the technique of LaPrade and Wijdicks12 positions the sMCL as posterior as possible. Despite the potential for increased graft stress with an anterior position, as in our modified technique, our group of 10 knees had no graft fixation failures in isolated valgus stress testing in either extension or flexion. Our minimally invasive posteromedial knee reconstruction significantly improved knee stability over the sectioned state as well as medial compartment gapping with valgus stress. There was no significant difference in medial compartment gapping between the intact and reconstructed states.
Our technique was built on open procedures (described by Kim and colleagues13) that carefully identify the isometric point of the graft. In addition, it adopted the modification (proposed by Lind and colleagues21) in which a fixation point is added at the distal insertion of the POL instead of being sutured to the direct arm of the semitendinosus tendon.
Furthermore, our technique, despite being similar to those described by Dong and colleagues22 and Borden and colleagues,23 has the advantages of minimally invasive surgery and reduced disruption of soft tissues. Dong and colleagues22 reported on 64 patients with a mean follow-up of 34 months; patients’ medial opening measurements were significantly decreased at follow-up and fell within the normal range.
The present study had several limitations. First, the age of our specimens was higher than the mean age of patients with knee ligament injury, potentially leading to firmer or more fibrotic tendons less susceptible to elongation. Second, we did not evaluate the knees’ rotational stability, and anterior cruciate ligaments (ACLs) were intact. As most posteromedial injuries co-occur with ACL injuries, a more realistic situation would have been reproduced by assessing rotational stability while performing both ACL reconstruction and the proposed posteromedial reconstruction. Third, static specimen measurements do not reflect the dynamic function of the posteromedial corner. Prospective clinical studies are needed to assess the true effectiveness of the posteromedial corner in the clinical scenario.
Knowledge of the anatomy of the medial aspect of the knee is vital to reconstruction of the medial side of the knee. Our results suggest that a minimally invasive technique can restore valgus stability without the need for extensive dissection and disruption of surrounding soft tissues. More research is needed to determine the results of this technique in vivo.
1. Ellsasser JC, Reynolds FC, Omohundro JR. The non-operative treatment of collateral ligament injuries of the knee in professional football players. An analysis of seventy-four injuries treated non-operatively and twenty-four injuries treated surgically. J Bone Joint Surg Am. 1974;56(6):1185-1190.
2. Indelicato PA. Non-operative treatment of complete tears of the medial collateral ligament of the knee. J Bone Joint Surg Am. 1983;65(3):323-329.
3. Indelicato PA, Hermansdorfer J, Huegel M. Nonoperative management of complete tears of the medial collateral ligament of the knee in intercollegiate football players. Clin Orthop Rel Res. 1990;(256):174-177.
4. Jeng CL, Bluman EM, Myerson MS. Minimally invasive deltoid ligament reconstruction for stage IV flatfoot deformity. Foot Ankle Int. 2011;32(1):21-30.
5. Coobs BR, Wijdicks CA, Armitage BM, et al. An in vitro analysis of an anatomical medial knee reconstruction. Am J Sports Med. 2010;38(2):339-347.
6. Lubowitz JH, MacKay G, Gilmer B. Knee medial collateral ligament and posteromedial corner anatomic repair with internal bracing. Arthrosc Tech. 2014;3(4):e505-e508.
7. Hughston JC, Eilers AF. The role of the posterior oblique ligament in repairs of acute medial (collateral) ligament tears of the knee. J Bone Joint Surg Am. 1973;55(5):923-940.
8. Gorin S, Paul DD, Wilkinson EJ. An anterior cruciate ligament and medial collateral ligament tear in a skeletally immature patient: a new technique to augment primary repair of the medial collateral ligament and an allograft reconstruction of the anterior cruciate ligament. Arthroscopy. 2003;19(10):E21-E26.
Take-Home Points
- Injuries to the medial knee are the most common knee ligament injuries, and often occur in the athletic population.
- Complete posteromedial corner injuries require surgical treatment to restore joint stability and biomechanics.
- Biomechanical evidence has demonstrated an important load-sharing distribution between the sMCL and the POL.
- Valgus instability caused by a medial side injury, can lead to both ACL/posterior cruciate ligament reconstruction graft failure if the medial sided injury is not concurrently repaired or reconstructed.
- Anatomic posteromedial corner reconstruction yields excellent biomechanical and patient-reported outcomes.
Most injuries of the medial structures of the knee are treated conservatively.1-3 In severe acute injuries and chronic symptomatic instabilities, however, surgical treatment is needed to restore knee stability and to prevent degenerative changes secondary to instability.4 Three structures involved in medial stability are the superficial medial collateral ligament (sMCL), which is the primary valgus restraint; the posterior oblique ligament (POL), which is the primary restraint to internal rotation and the secondary valgus restraint; and the semimembranosus.5,6
Surgical techniques for posteromedial knee reconstruction include direct repair,7 repair with augmentation,8,9 advancement of the tibial insertion of the sMCL,10 and transfer of the pes anserine tendons.11 In anatomical reconstruction of the posteromedial corner, which has been described before, the sMCL and the POL are reconstructed to reproduce the native motion and stability of the knee.12 Clinically, repair and reconstruction have similar patient-reported outcomes and medial opening evaluations over the short term.
These approaches require large incisions and extensive dissection of soft tissue on the medial aspect of the knee.5 Given these drawbacks, it is reasonable to consider less invasive options. Minimally invasive surgery has the advantages of reduced scarring and blood loss, less disruption of surrounding tissue, faster recovery, and improved aesthetics.4
We conducted a study of a minimally invasive technique for reconstructing the posteromedial structures of the knee. We compared medial compartment stability measured on valgus stress radiographs in intact, sectioned, and reconstructed states in cadaveric knees. We hypothesized that a minimally invasive technique using autogenous hamstring graft in the appropriate anatomical location would return valgus stability to its nearly native state.
Materials and Methods
This study was conducted at the Buenos Aires British Hospital in Buenos Aires, Argentina, and at the University of Colorado Hospital in Aurora. Ten fresh-frozen cadaveric knees with no evidence of ligamentous injuries, osteoarthritis, or previous surgery were used. Mean donor age was 69.4 years (range, 45-87 years). Each specimen was maintained at room temperature for 24 hours before use. The femur was sectioned 20 cm proximal to the knee joint. The tibia was sectioned 12.5 cm distal to the knee joint.
Identification and Sectioning of Posteromedial Structures
After intact-state evaluation, each knee’s sMCL, dMCL, and POL were sectioned at their tibial insertion. Valgus stress radiograph was repeated and medial compartment gap was remeasured for comparison of the sectioned state with the intact and reconstructed states.
Anatomical Reconstruction With Mini-Invasive Technique
After sectioning of medial stabilizing structures, minimally invasive reconstruction was performed through 2 small incisions on the medial aspect of each of the 10 knees, as follows. First, the semitendinosus tendon was identified through the oblique incision that had been used for sectioning. Then, an open-ended tendon stripper was placed around the circumference of the semitendinosus and was passed proximomedially, transecting the tendon at its musculotendinous junction. While the tendon stripper was being passed, care was taken to maintain the nearby tibial insertion of the sartorius fascia (Figures 1D-1F).
With the semitendinosus tendon looped around the wire, isometricity was tested by pulling the suture within the tendon and moving the knee through a full range of motion. The isometric point was confirmed by tendon migration of <2 mm.13 Migration was measured by marking the graft 2 mm from its insertion; the graft was then pulled to ensure correct isometric point position. An 18-mm cannulated spiked screw and washer (Arthrex) were then passed over the wire and partially secured to the femur—the attachment point for the proximal sMCL portion of the semitendinosus graft. The semitendinosus tendon was then secured beneath the spiked washer with the knee in 20° of flexion with neutral rotation, recreating the sMCL.
Posteriorly, the distal insertion site of the POL was identified at the posteromedial aspect of the tibia through the oblique incision previously described. A 7-mm tunnel was drilled starting posteromedial (10 mm under tibial articular surface) and exiting just distal and medial to the Gerdy tubercle.
After final fixation, the medial knee was openly dissected to assess the inverted-V ligament reconstruction for anatomical placement and avoidance of crucial structures.
Stability Testing
Per International Knee Documentation Committee guidelines for stressing the medial compartment,14 valgus stress radiographs were obtained for all specimens at 0° and 20° of flexion in intact, sectioned, and reconstructed states.
The medial gap formed by the femoral condyle and its corresponding tibial plateau (at site of maximal separation) was tested in all 3 state conditions (intact, sectioned, reconstructed). Distances were digitally measured with a picture archiving and communication system viewer (Imagecast; IDX Systems Corporation). Medial gap was measured by taking the shortest distance between the subchondral bone surface of the most distal aspect of the medial femoral condyle and the corresponding medial tibial plateau. Three independent examiners took all the measurements; each examiner was blinded to the others’ measurements.
Statistics
Paired Student t tests were used to compare the 3 conditions, and the Shapiro-Wilk test was used to check for a normally distributed population. Statistical significance was set at P < .05. Statistical analyses were performed with GraphPad software.
Results
In all 10 specimens, the sMCL, the dMCL, and the POL were successfully identified and sectioned through a medial oblique incision over the distal insertion of the structures.
During all valgus testing states, there was no loss of graft fixation, and there was no gross graft slippage. In addition, all grafts remained in continuity with no evidence of failure, and there were no failures or breakages of the proximal or distal screw.
After posteromedial sectioning, mean medial gap was statistically significantly larger (P = .0002) at full extension (11 mm vs 3.3 mm) and at 20° of flexion (12.6 mm vs 3.8 mm). There was no statistically significant difference between the value of the intact state and the value after minimally invasive reconstruction at 0° (P = .56) or 20° (P = .102) of flexion.
Discussion
In this article, we describe a minimally invasive technique for anatomical posteromedial reconstruction of the knee in a cadaveric model. This technique restores the knee’s native valgus stability without causing extensive damage to the surrounding soft tissues and thereby potentially prevents scar formation and reduces blood loss.
Superficial MCL injury, one of the most common knee ligament injuries, is often associated with POL injury.7 Although most sMCL injuries are treated nonoperatively, with good results,3 surgical treatment is needed for severe (grade III) instabilities, symptomatic chronic instabilities, and knee dislocations.12,17 Most posteromedial reconstruction techniques require an extensive approach that causes damage to surrounding soft tissue,6,7,9,10 which in turn may compromise healing and positive patient outcomes. Surgical techniques include direct repair with sutures or anchors,18 capsular procedures,19 augmentations,9 internal bracing,6 and complete reconstruction of the posteromedial corner.20
LaPrade and Wijdicks12 have previously described anatomical reconstruction of the posteromedial corner. In their technique, a split semitendinosus autograft is used to reconstruct the sMCL and the POL separately, using 4 implants and reproducing each ligament’s anatomical attachment site. In this proposed technique, the distal attachment of the semitendinosus insertion is left intact, and uses 1 attachment point on the distal femur and 1 on the proximal tibia, allowing use of only 2 implants. In addition, it is performed with a minimally invasive approach, reduces cost, limits surgical exposure, and with experience may shorten operative time. To reduce the graft failure rate, the technique of LaPrade and Wijdicks12 positions the sMCL tibial attachment as posterior as possible, which can be performed with this minimally invasive approach as well.
To reduce the graft failure rate, the technique of LaPrade and Wijdicks12 positions the sMCL as posterior as possible. Despite the potential for increased graft stress with an anterior position, as in our modified technique, our group of 10 knees had no graft fixation failures in isolated valgus stress testing in either extension or flexion. Our minimally invasive posteromedial knee reconstruction significantly improved knee stability over the sectioned state as well as medial compartment gapping with valgus stress. There was no significant difference in medial compartment gapping between the intact and reconstructed states.
Our technique was built on open procedures (described by Kim and colleagues13) that carefully identify the isometric point of the graft. In addition, it adopted the modification (proposed by Lind and colleagues21) in which a fixation point is added at the distal insertion of the POL instead of being sutured to the direct arm of the semitendinosus tendon.
Furthermore, our technique, despite being similar to those described by Dong and colleagues22 and Borden and colleagues,23 has the advantages of minimally invasive surgery and reduced disruption of soft tissues. Dong and colleagues22 reported on 64 patients with a mean follow-up of 34 months; patients’ medial opening measurements were significantly decreased at follow-up and fell within the normal range.
The present study had several limitations. First, the age of our specimens was higher than the mean age of patients with knee ligament injury, potentially leading to firmer or more fibrotic tendons less susceptible to elongation. Second, we did not evaluate the knees’ rotational stability, and anterior cruciate ligaments (ACLs) were intact. As most posteromedial injuries co-occur with ACL injuries, a more realistic situation would have been reproduced by assessing rotational stability while performing both ACL reconstruction and the proposed posteromedial reconstruction. Third, static specimen measurements do not reflect the dynamic function of the posteromedial corner. Prospective clinical studies are needed to assess the true effectiveness of the posteromedial corner in the clinical scenario.
Knowledge of the anatomy of the medial aspect of the knee is vital to reconstruction of the medial side of the knee. Our results suggest that a minimally invasive technique can restore valgus stability without the need for extensive dissection and disruption of surrounding soft tissues. More research is needed to determine the results of this technique in vivo.
Take-Home Points
- Injuries to the medial knee are the most common knee ligament injuries, and often occur in the athletic population.
- Complete posteromedial corner injuries require surgical treatment to restore joint stability and biomechanics.
- Biomechanical evidence has demonstrated an important load-sharing distribution between the sMCL and the POL.
- Valgus instability caused by a medial side injury, can lead to both ACL/posterior cruciate ligament reconstruction graft failure if the medial sided injury is not concurrently repaired or reconstructed.
- Anatomic posteromedial corner reconstruction yields excellent biomechanical and patient-reported outcomes.
Most injuries of the medial structures of the knee are treated conservatively.1-3 In severe acute injuries and chronic symptomatic instabilities, however, surgical treatment is needed to restore knee stability and to prevent degenerative changes secondary to instability.4 Three structures involved in medial stability are the superficial medial collateral ligament (sMCL), which is the primary valgus restraint; the posterior oblique ligament (POL), which is the primary restraint to internal rotation and the secondary valgus restraint; and the semimembranosus.5,6
Surgical techniques for posteromedial knee reconstruction include direct repair,7 repair with augmentation,8,9 advancement of the tibial insertion of the sMCL,10 and transfer of the pes anserine tendons.11 In anatomical reconstruction of the posteromedial corner, which has been described before, the sMCL and the POL are reconstructed to reproduce the native motion and stability of the knee.12 Clinically, repair and reconstruction have similar patient-reported outcomes and medial opening evaluations over the short term.
These approaches require large incisions and extensive dissection of soft tissue on the medial aspect of the knee.5 Given these drawbacks, it is reasonable to consider less invasive options. Minimally invasive surgery has the advantages of reduced scarring and blood loss, less disruption of surrounding tissue, faster recovery, and improved aesthetics.4
We conducted a study of a minimally invasive technique for reconstructing the posteromedial structures of the knee. We compared medial compartment stability measured on valgus stress radiographs in intact, sectioned, and reconstructed states in cadaveric knees. We hypothesized that a minimally invasive technique using autogenous hamstring graft in the appropriate anatomical location would return valgus stability to its nearly native state.
Materials and Methods
This study was conducted at the Buenos Aires British Hospital in Buenos Aires, Argentina, and at the University of Colorado Hospital in Aurora. Ten fresh-frozen cadaveric knees with no evidence of ligamentous injuries, osteoarthritis, or previous surgery were used. Mean donor age was 69.4 years (range, 45-87 years). Each specimen was maintained at room temperature for 24 hours before use. The femur was sectioned 20 cm proximal to the knee joint. The tibia was sectioned 12.5 cm distal to the knee joint.
Identification and Sectioning of Posteromedial Structures
After intact-state evaluation, each knee’s sMCL, dMCL, and POL were sectioned at their tibial insertion. Valgus stress radiograph was repeated and medial compartment gap was remeasured for comparison of the sectioned state with the intact and reconstructed states.
Anatomical Reconstruction With Mini-Invasive Technique
After sectioning of medial stabilizing structures, minimally invasive reconstruction was performed through 2 small incisions on the medial aspect of each of the 10 knees, as follows. First, the semitendinosus tendon was identified through the oblique incision that had been used for sectioning. Then, an open-ended tendon stripper was placed around the circumference of the semitendinosus and was passed proximomedially, transecting the tendon at its musculotendinous junction. While the tendon stripper was being passed, care was taken to maintain the nearby tibial insertion of the sartorius fascia (Figures 1D-1F).
With the semitendinosus tendon looped around the wire, isometricity was tested by pulling the suture within the tendon and moving the knee through a full range of motion. The isometric point was confirmed by tendon migration of <2 mm.13 Migration was measured by marking the graft 2 mm from its insertion; the graft was then pulled to ensure correct isometric point position. An 18-mm cannulated spiked screw and washer (Arthrex) were then passed over the wire and partially secured to the femur—the attachment point for the proximal sMCL portion of the semitendinosus graft. The semitendinosus tendon was then secured beneath the spiked washer with the knee in 20° of flexion with neutral rotation, recreating the sMCL.
Posteriorly, the distal insertion site of the POL was identified at the posteromedial aspect of the tibia through the oblique incision previously described. A 7-mm tunnel was drilled starting posteromedial (10 mm under tibial articular surface) and exiting just distal and medial to the Gerdy tubercle.
After final fixation, the medial knee was openly dissected to assess the inverted-V ligament reconstruction for anatomical placement and avoidance of crucial structures.
Stability Testing
Per International Knee Documentation Committee guidelines for stressing the medial compartment,14 valgus stress radiographs were obtained for all specimens at 0° and 20° of flexion in intact, sectioned, and reconstructed states.
The medial gap formed by the femoral condyle and its corresponding tibial plateau (at site of maximal separation) was tested in all 3 state conditions (intact, sectioned, reconstructed). Distances were digitally measured with a picture archiving and communication system viewer (Imagecast; IDX Systems Corporation). Medial gap was measured by taking the shortest distance between the subchondral bone surface of the most distal aspect of the medial femoral condyle and the corresponding medial tibial plateau. Three independent examiners took all the measurements; each examiner was blinded to the others’ measurements.
Statistics
Paired Student t tests were used to compare the 3 conditions, and the Shapiro-Wilk test was used to check for a normally distributed population. Statistical significance was set at P < .05. Statistical analyses were performed with GraphPad software.
Results
In all 10 specimens, the sMCL, the dMCL, and the POL were successfully identified and sectioned through a medial oblique incision over the distal insertion of the structures.
During all valgus testing states, there was no loss of graft fixation, and there was no gross graft slippage. In addition, all grafts remained in continuity with no evidence of failure, and there were no failures or breakages of the proximal or distal screw.
After posteromedial sectioning, mean medial gap was statistically significantly larger (P = .0002) at full extension (11 mm vs 3.3 mm) and at 20° of flexion (12.6 mm vs 3.8 mm). There was no statistically significant difference between the value of the intact state and the value after minimally invasive reconstruction at 0° (P = .56) or 20° (P = .102) of flexion.
Discussion
In this article, we describe a minimally invasive technique for anatomical posteromedial reconstruction of the knee in a cadaveric model. This technique restores the knee’s native valgus stability without causing extensive damage to the surrounding soft tissues and thereby potentially prevents scar formation and reduces blood loss.
Superficial MCL injury, one of the most common knee ligament injuries, is often associated with POL injury.7 Although most sMCL injuries are treated nonoperatively, with good results,3 surgical treatment is needed for severe (grade III) instabilities, symptomatic chronic instabilities, and knee dislocations.12,17 Most posteromedial reconstruction techniques require an extensive approach that causes damage to surrounding soft tissue,6,7,9,10 which in turn may compromise healing and positive patient outcomes. Surgical techniques include direct repair with sutures or anchors,18 capsular procedures,19 augmentations,9 internal bracing,6 and complete reconstruction of the posteromedial corner.20
LaPrade and Wijdicks12 have previously described anatomical reconstruction of the posteromedial corner. In their technique, a split semitendinosus autograft is used to reconstruct the sMCL and the POL separately, using 4 implants and reproducing each ligament’s anatomical attachment site. In this proposed technique, the distal attachment of the semitendinosus insertion is left intact, and uses 1 attachment point on the distal femur and 1 on the proximal tibia, allowing use of only 2 implants. In addition, it is performed with a minimally invasive approach, reduces cost, limits surgical exposure, and with experience may shorten operative time. To reduce the graft failure rate, the technique of LaPrade and Wijdicks12 positions the sMCL tibial attachment as posterior as possible, which can be performed with this minimally invasive approach as well.
To reduce the graft failure rate, the technique of LaPrade and Wijdicks12 positions the sMCL as posterior as possible. Despite the potential for increased graft stress with an anterior position, as in our modified technique, our group of 10 knees had no graft fixation failures in isolated valgus stress testing in either extension or flexion. Our minimally invasive posteromedial knee reconstruction significantly improved knee stability over the sectioned state as well as medial compartment gapping with valgus stress. There was no significant difference in medial compartment gapping between the intact and reconstructed states.
Our technique was built on open procedures (described by Kim and colleagues13) that carefully identify the isometric point of the graft. In addition, it adopted the modification (proposed by Lind and colleagues21) in which a fixation point is added at the distal insertion of the POL instead of being sutured to the direct arm of the semitendinosus tendon.
Furthermore, our technique, despite being similar to those described by Dong and colleagues22 and Borden and colleagues,23 has the advantages of minimally invasive surgery and reduced disruption of soft tissues. Dong and colleagues22 reported on 64 patients with a mean follow-up of 34 months; patients’ medial opening measurements were significantly decreased at follow-up and fell within the normal range.
The present study had several limitations. First, the age of our specimens was higher than the mean age of patients with knee ligament injury, potentially leading to firmer or more fibrotic tendons less susceptible to elongation. Second, we did not evaluate the knees’ rotational stability, and anterior cruciate ligaments (ACLs) were intact. As most posteromedial injuries co-occur with ACL injuries, a more realistic situation would have been reproduced by assessing rotational stability while performing both ACL reconstruction and the proposed posteromedial reconstruction. Third, static specimen measurements do not reflect the dynamic function of the posteromedial corner. Prospective clinical studies are needed to assess the true effectiveness of the posteromedial corner in the clinical scenario.
Knowledge of the anatomy of the medial aspect of the knee is vital to reconstruction of the medial side of the knee. Our results suggest that a minimally invasive technique can restore valgus stability without the need for extensive dissection and disruption of surrounding soft tissues. More research is needed to determine the results of this technique in vivo.
1. Ellsasser JC, Reynolds FC, Omohundro JR. The non-operative treatment of collateral ligament injuries of the knee in professional football players. An analysis of seventy-four injuries treated non-operatively and twenty-four injuries treated surgically. J Bone Joint Surg Am. 1974;56(6):1185-1190.
2. Indelicato PA. Non-operative treatment of complete tears of the medial collateral ligament of the knee. J Bone Joint Surg Am. 1983;65(3):323-329.
3. Indelicato PA, Hermansdorfer J, Huegel M. Nonoperative management of complete tears of the medial collateral ligament of the knee in intercollegiate football players. Clin Orthop Rel Res. 1990;(256):174-177.
4. Jeng CL, Bluman EM, Myerson MS. Minimally invasive deltoid ligament reconstruction for stage IV flatfoot deformity. Foot Ankle Int. 2011;32(1):21-30.
5. Coobs BR, Wijdicks CA, Armitage BM, et al. An in vitro analysis of an anatomical medial knee reconstruction. Am J Sports Med. 2010;38(2):339-347.
6. Lubowitz JH, MacKay G, Gilmer B. Knee medial collateral ligament and posteromedial corner anatomic repair with internal bracing. Arthrosc Tech. 2014;3(4):e505-e508.
7. Hughston JC, Eilers AF. The role of the posterior oblique ligament in repairs of acute medial (collateral) ligament tears of the knee. J Bone Joint Surg Am. 1973;55(5):923-940.
8. Gorin S, Paul DD, Wilkinson EJ. An anterior cruciate ligament and medial collateral ligament tear in a skeletally immature patient: a new technique to augment primary repair of the medial collateral ligament and an allograft reconstruction of the anterior cruciate ligament. Arthroscopy. 2003;19(10):E21-E26.
1. Ellsasser JC, Reynolds FC, Omohundro JR. The non-operative treatment of collateral ligament injuries of the knee in professional football players. An analysis of seventy-four injuries treated non-operatively and twenty-four injuries treated surgically. J Bone Joint Surg Am. 1974;56(6):1185-1190.
2. Indelicato PA. Non-operative treatment of complete tears of the medial collateral ligament of the knee. J Bone Joint Surg Am. 1983;65(3):323-329.
3. Indelicato PA, Hermansdorfer J, Huegel M. Nonoperative management of complete tears of the medial collateral ligament of the knee in intercollegiate football players. Clin Orthop Rel Res. 1990;(256):174-177.
4. Jeng CL, Bluman EM, Myerson MS. Minimally invasive deltoid ligament reconstruction for stage IV flatfoot deformity. Foot Ankle Int. 2011;32(1):21-30.
5. Coobs BR, Wijdicks CA, Armitage BM, et al. An in vitro analysis of an anatomical medial knee reconstruction. Am J Sports Med. 2010;38(2):339-347.
6. Lubowitz JH, MacKay G, Gilmer B. Knee medial collateral ligament and posteromedial corner anatomic repair with internal bracing. Arthrosc Tech. 2014;3(4):e505-e508.
7. Hughston JC, Eilers AF. The role of the posterior oblique ligament in repairs of acute medial (collateral) ligament tears of the knee. J Bone Joint Surg Am. 1973;55(5):923-940.
8. Gorin S, Paul DD, Wilkinson EJ. An anterior cruciate ligament and medial collateral ligament tear in a skeletally immature patient: a new technique to augment primary repair of the medial collateral ligament and an allograft reconstruction of the anterior cruciate ligament. Arthroscopy. 2003;19(10):E21-E26.