User login
Shoulder Arthroplasty in Cases of Significant Bone Loss: An Overview
Over the past few decades, there has been a dramatic increase in the number of shoulder arthroplasties performed around the world. This increase is the result of an aging and increasingly more active population, better implant technology, and the advent of reverse shoulder arthroplasty (RSA) for rotator cuff arthropathy. Additionally, as the indications for RSA have expanded to include pathologies such as rotator cuff insufficiency, chronic instabilities, trauma, and tumors, the number of arthroplasties will continue to increase. Although the results of most arthroplasties are good and predictable, any glenoid and/or humeral bone deficiencies can have detrimental effects on the clinical outcomes of these procedures. Bone loss becomes more of a problem in revision cases, and, as the number of primary arthroplasties increases, it follows that the number of revision procedures will also increase.
Many of the disease- or procedure-specific processes indicated for shoulder arthroplasty have predictable patterns of bone loss, especially on the glenoid side. Walch and colleagues1 and Bercik and colleagues2 made us aware that many patients with primary osteoarthritis have significant glenoid bone deformity. Similarly, there have been a number of first- and second-generation classification systems for delineating glenoid deformity in rotator cuff tear arthropathy and in revision settings. In revision settings, both glenoid and humeral bone deficiencies can occur as a result of implant removal, iatrogenic fracture, and even infection. Each of these bone loss patterns must be recognized and treated appropriately for the best surgical outcome.
The articles in this month of The American Journal of Orthopedics address the most up-to-date concepts and solutions regarding both humeral and glenoid bone loss in shoulder arthroplasty of all types.
HUMERAL BONE LOSS
Humeral bone loss is typically encountered in proximal humerus fractures, in revision surgery necessitating humeral component removal, and, less commonly, in tumors and infection.
In many displaced proximal humeral fractures indicated for shoulder arthroplasty, the bone is comminuted with displacement of the lesser and greater tuberosities. In these situations, failure of tuberosity healing may result in loss of rotator cuff function with loss of elevation, rotation, and even instability. Humeral shortening can also occur as a result of bone loss and can compromise deltoid function by loss of proper muscle tension, leading to instability, dysfunction, or both. In addition to possible instability, humeral shortening with metaphyseal bone loss can adversely affect long-term fixation of the humeral component, leading to stem loosening or failure. Cuff and colleagues3 showed significantly more rotational micromotion in cases lacking metaphyseal support, leading to aseptic loosening of the humeral stem.
Humeral bone loss can also result from humeral stem component removal in revision shoulder arthroplasty for infection, component failure or loosening, and even periprosthetic fracture resulting from surgery or trauma.
For the surgeon, humeral bone loss can create a complex set of circumstances related to rotator cuff attachment failure, soft-tissue balancing effects, and component fixation issues. Any such issue must be recognized and addressed for best outcomes. Best results can be obtained with preoperative imaging, planning, use of bone graft techniques, proximal humeral allografts, and, more recently, modular and patient-specific implants. All of these issues are discussed comprehensively in the articles this month.
Continue to: GLENOID BONE LOSS
GLENOID BONE LOSS
Proper glenoid component placement with durable fixation is crucial for success in anatomical total shoulder arthroplasty and RSA. Glenoid bone deformity and loss can result from intrinsic deformity characteristics seen in primary osteoarthritis, cuff tear arthropathy, or glenoid component removal in revision situations and infection. These bone deformity complications can be extremely difficult to treat and in some cases lead to catastrophic failure of the index arthroplasty.
We are now aware that one key to success in the face of moderate to severe deformity is proper recognition. Newer imaging techniques, including 2-dimensional (2-D) computed tomography (CT) and 3-dimensional (3-D) modeling and surgical planning software tools, which are outlined in an upcoming article, have given surgeons important new instruments that can help in treating these difficult cases.
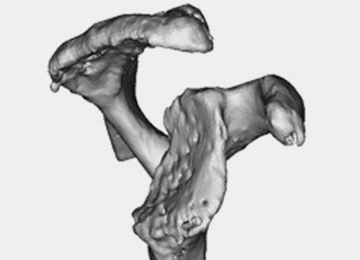
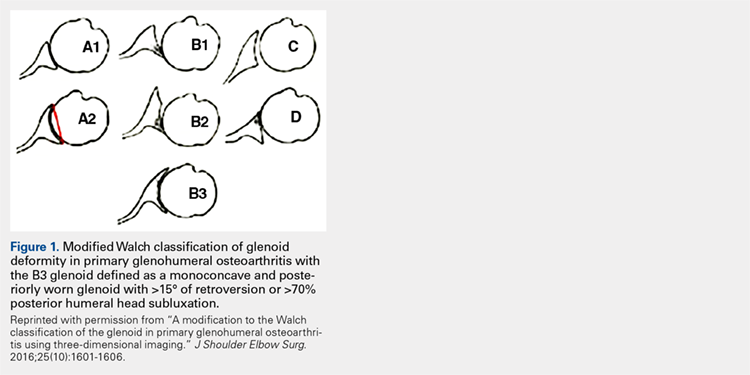
Glenoid bone deformity in primary osteoarthritis was well delineated in the 1999 seminal study of CT changes by Walch and colleagues.1 The Walch classification system, which characterized glenoid morphology based on 2-D CT findings, was recently upgraded, based on 3-D imaging technology, to include Walch B3 and D patterns (Figure 1).2 Recognition of certain primary deformities in osteoarthritis has led to increased use of RSA in some cases of Walch B2, B3, and C deformities with substantial glenoid retroversion and/or humeral head subluxation.4
In cases of rotator cuff tear arthropathy, glenoid bone deformities are well described with several classification systems based on degree and dimension of bone insufficiency. The Hamada classification system defines the degree of medial glenoid erosion and superior bone loss, as well as acetabularization of the acromion in 5 grades; 5 Rispoli and colleagues6 defined and graded the degree of medicalization of the glenohumeral joint based on degree of subchondral plate erosion; and Visotsky and colleagues7 based their classification system on wear patterns of bone loss, alignment, and concomitant soft-tissue insufficiencies leading to instability and rotation loss.
In severe glenoid bone deficiency after glenoid component removal, Antuna and colleagues8 described the classic findings related to medial bone loss, anterior and posterior wall failure, and combinations thereof.
Continue to: All these classification systems...
All these classification systems are based on the 2-D appearance of the glenoid and should be considered cautiously. The glenoid is a complex 3-D structure that can be affected by any number of disease processes, trauma, and surgical intervention. Using more modern CT techniques and 3-D imaging, we now know that many deformities previously classified as unidirectional are, instead, complex and multidirectional.
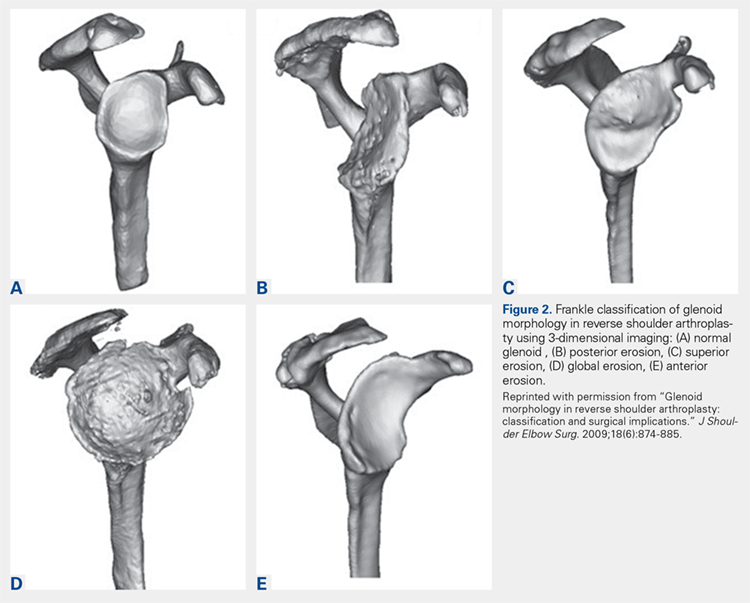
Frankle and colleagues9 developed a classification based more 3-D CT models which has further classified severe glenoid vault deformities in relation to direction and degree of bone loss (Figures 2A-2E). Using this system, they were better able to determine degree and direction of deformity than in previous 2-D evaluations, and they were able to determine the amount of glenoid vault bone available for baseplate fixation. Scalise and colleagues10 further defined the influence of such 3-D planning in total shoulder arthroplasty.
With knowledge of these classification systems and use of contemporary imaging systems, shoulder arthroplasty in cases of severe glenoid deficiency can be more successful. Potentially, we can improve outcomes even more in the more severe cases of bone loss with use of patient-specific planning tools, including the guides and patient-specific implants that are now readily available with many implant systems.11
Preoperative planning tools, bone-grafting techniques, augmented and specialized glenoid and humeral implants, and patient-specific implants are discussed this month to give our readers a comprehensive review of the latest concepts in shoulder arthroplasty in cases of significant bone loss or deformity.
This month of The American Journal of Orthopedics presents the most current and cutting-edge solutions for humeral and glenoid bone deformities and deficiencies in contemporary shoulder arthroplasties.
1. Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty. 1999;14(6):756-760.
2. Bercik MJ, Kruse K 2nd, Yalizis M, Gauci MO, Chaoui J, Walch G. A modification to the Walch classification of the glenoid in primary glenohumeral osteoarthritis using three-dimensional imaging. J Shoulder Elbow Surg. 2016;25(10):1601-1606.
3. Cuff D, Levy JC, Gutiérrez S, Frankle M. Torsional stability of modular and non-modular reverse shoulder humeral components in a proximal humeral bone loss model. J Shoulder Elbow Surg. 2011;20(4):646-651.
4. Denard PJ, Walch G. Current concepts in the surgical management of primary glenohumeral arthritis with a biconcave glenoid. J Shoulder Elbow Surg. 2013;22(11):1589-1598.
5. Hamada K, Fukuda H, Mikasa M, Kobayashi Y. Roentgenographic findings in massive rotator cuff tears. A long-term observation. Clin Orthop Relat Res. 1990;(254):92-96.
6. Rispoli D, Sperling JW, Athwal GS, Schleck CD, Cofield RH. Humeral head replacement for the treatment of osteoarthritis. J Bone Joint Surg Am. 2006;88(12):2637-2644.
7. Visotsky JL, Basamania C, Seebauer L, Rockwood CA, Jensen KL. Cuff tear arthropathy: pathogenesis, classification, and algorithm for treatment. J Bone Joint Surg Am. 2004;86(suppl 2):35-40.
8. Antuna SA, Sperling JW, Cofield RH, Rowland CM. Glenoid revision surgery after total shoulder arthroplasty. J Shoulder Elbow Surg. 2001;10(3):217-224.
9. Frankle MA, Teramoto A, Luo ZP, Levy JC, Pupello D. Glenoid morphology in reverse shoulder arthroplasty: classification and surgical implications. J Shoulder Elbow Surg. 2009;18(6):874-885.
10. Scalise JJ, Codsi MJ, Bryan J, Brems JJ, Iannotti JP. The influence of three-dimensional computed tomography images of the shoulder in preoperative planning for total shoulder arthroplasty. J Bone Joint Surg Am. 2008;90(11):2438-2445.
11. Dines DM, Gulotta L, Craig EV, Dines JS. Novel solution for massive glenoid defects in shoulder arthroplasty: a patient-specific glenoid vault reconstruction system. Am J Orthop. 2017;46(2):104-108.
Over the past few decades, there has been a dramatic increase in the number of shoulder arthroplasties performed around the world. This increase is the result of an aging and increasingly more active population, better implant technology, and the advent of reverse shoulder arthroplasty (RSA) for rotator cuff arthropathy. Additionally, as the indications for RSA have expanded to include pathologies such as rotator cuff insufficiency, chronic instabilities, trauma, and tumors, the number of arthroplasties will continue to increase. Although the results of most arthroplasties are good and predictable, any glenoid and/or humeral bone deficiencies can have detrimental effects on the clinical outcomes of these procedures. Bone loss becomes more of a problem in revision cases, and, as the number of primary arthroplasties increases, it follows that the number of revision procedures will also increase.
Many of the disease- or procedure-specific processes indicated for shoulder arthroplasty have predictable patterns of bone loss, especially on the glenoid side. Walch and colleagues1 and Bercik and colleagues2 made us aware that many patients with primary osteoarthritis have significant glenoid bone deformity. Similarly, there have been a number of first- and second-generation classification systems for delineating glenoid deformity in rotator cuff tear arthropathy and in revision settings. In revision settings, both glenoid and humeral bone deficiencies can occur as a result of implant removal, iatrogenic fracture, and even infection. Each of these bone loss patterns must be recognized and treated appropriately for the best surgical outcome.
The articles in this month of The American Journal of Orthopedics address the most up-to-date concepts and solutions regarding both humeral and glenoid bone loss in shoulder arthroplasty of all types.
HUMERAL BONE LOSS
Humeral bone loss is typically encountered in proximal humerus fractures, in revision surgery necessitating humeral component removal, and, less commonly, in tumors and infection.
In many displaced proximal humeral fractures indicated for shoulder arthroplasty, the bone is comminuted with displacement of the lesser and greater tuberosities. In these situations, failure of tuberosity healing may result in loss of rotator cuff function with loss of elevation, rotation, and even instability. Humeral shortening can also occur as a result of bone loss and can compromise deltoid function by loss of proper muscle tension, leading to instability, dysfunction, or both. In addition to possible instability, humeral shortening with metaphyseal bone loss can adversely affect long-term fixation of the humeral component, leading to stem loosening or failure. Cuff and colleagues3 showed significantly more rotational micromotion in cases lacking metaphyseal support, leading to aseptic loosening of the humeral stem.
Humeral bone loss can also result from humeral stem component removal in revision shoulder arthroplasty for infection, component failure or loosening, and even periprosthetic fracture resulting from surgery or trauma.
For the surgeon, humeral bone loss can create a complex set of circumstances related to rotator cuff attachment failure, soft-tissue balancing effects, and component fixation issues. Any such issue must be recognized and addressed for best outcomes. Best results can be obtained with preoperative imaging, planning, use of bone graft techniques, proximal humeral allografts, and, more recently, modular and patient-specific implants. All of these issues are discussed comprehensively in the articles this month.
Continue to: GLENOID BONE LOSS
GLENOID BONE LOSS
Proper glenoid component placement with durable fixation is crucial for success in anatomical total shoulder arthroplasty and RSA. Glenoid bone deformity and loss can result from intrinsic deformity characteristics seen in primary osteoarthritis, cuff tear arthropathy, or glenoid component removal in revision situations and infection. These bone deformity complications can be extremely difficult to treat and in some cases lead to catastrophic failure of the index arthroplasty.
We are now aware that one key to success in the face of moderate to severe deformity is proper recognition. Newer imaging techniques, including 2-dimensional (2-D) computed tomography (CT) and 3-dimensional (3-D) modeling and surgical planning software tools, which are outlined in an upcoming article, have given surgeons important new instruments that can help in treating these difficult cases.
Glenoid bone deformity in primary osteoarthritis was well delineated in the 1999 seminal study of CT changes by Walch and colleagues.1 The Walch classification system, which characterized glenoid morphology based on 2-D CT findings, was recently upgraded, based on 3-D imaging technology, to include Walch B3 and D patterns (Figure 1).2 Recognition of certain primary deformities in osteoarthritis has led to increased use of RSA in some cases of Walch B2, B3, and C deformities with substantial glenoid retroversion and/or humeral head subluxation.4

In cases of rotator cuff tear arthropathy, glenoid bone deformities are well described with several classification systems based on degree and dimension of bone insufficiency. The Hamada classification system defines the degree of medial glenoid erosion and superior bone loss, as well as acetabularization of the acromion in 5 grades; 5 Rispoli and colleagues6 defined and graded the degree of medicalization of the glenohumeral joint based on degree of subchondral plate erosion; and Visotsky and colleagues7 based their classification system on wear patterns of bone loss, alignment, and concomitant soft-tissue insufficiencies leading to instability and rotation loss.
In severe glenoid bone deficiency after glenoid component removal, Antuna and colleagues8 described the classic findings related to medial bone loss, anterior and posterior wall failure, and combinations thereof.
Continue to: All these classification systems...
All these classification systems are based on the 2-D appearance of the glenoid and should be considered cautiously. The glenoid is a complex 3-D structure that can be affected by any number of disease processes, trauma, and surgical intervention. Using more modern CT techniques and 3-D imaging, we now know that many deformities previously classified as unidirectional are, instead, complex and multidirectional.
Frankle and colleagues9 developed a classification based more 3-D CT models which has further classified severe glenoid vault deformities in relation to direction and degree of bone loss (Figures 2A-2E). Using this system, they were better able to determine degree and direction of deformity than in previous 2-D evaluations, and they were able to determine the amount of glenoid vault bone available for baseplate fixation. Scalise and colleagues10 further defined the influence of such 3-D planning in total shoulder arthroplasty.

With knowledge of these classification systems and use of contemporary imaging systems, shoulder arthroplasty in cases of severe glenoid deficiency can be more successful. Potentially, we can improve outcomes even more in the more severe cases of bone loss with use of patient-specific planning tools, including the guides and patient-specific implants that are now readily available with many implant systems.11
Preoperative planning tools, bone-grafting techniques, augmented and specialized glenoid and humeral implants, and patient-specific implants are discussed this month to give our readers a comprehensive review of the latest concepts in shoulder arthroplasty in cases of significant bone loss or deformity.
This month of The American Journal of Orthopedics presents the most current and cutting-edge solutions for humeral and glenoid bone deformities and deficiencies in contemporary shoulder arthroplasties.
Over the past few decades, there has been a dramatic increase in the number of shoulder arthroplasties performed around the world. This increase is the result of an aging and increasingly more active population, better implant technology, and the advent of reverse shoulder arthroplasty (RSA) for rotator cuff arthropathy. Additionally, as the indications for RSA have expanded to include pathologies such as rotator cuff insufficiency, chronic instabilities, trauma, and tumors, the number of arthroplasties will continue to increase. Although the results of most arthroplasties are good and predictable, any glenoid and/or humeral bone deficiencies can have detrimental effects on the clinical outcomes of these procedures. Bone loss becomes more of a problem in revision cases, and, as the number of primary arthroplasties increases, it follows that the number of revision procedures will also increase.
Many of the disease- or procedure-specific processes indicated for shoulder arthroplasty have predictable patterns of bone loss, especially on the glenoid side. Walch and colleagues1 and Bercik and colleagues2 made us aware that many patients with primary osteoarthritis have significant glenoid bone deformity. Similarly, there have been a number of first- and second-generation classification systems for delineating glenoid deformity in rotator cuff tear arthropathy and in revision settings. In revision settings, both glenoid and humeral bone deficiencies can occur as a result of implant removal, iatrogenic fracture, and even infection. Each of these bone loss patterns must be recognized and treated appropriately for the best surgical outcome.
The articles in this month of The American Journal of Orthopedics address the most up-to-date concepts and solutions regarding both humeral and glenoid bone loss in shoulder arthroplasty of all types.
HUMERAL BONE LOSS
Humeral bone loss is typically encountered in proximal humerus fractures, in revision surgery necessitating humeral component removal, and, less commonly, in tumors and infection.
In many displaced proximal humeral fractures indicated for shoulder arthroplasty, the bone is comminuted with displacement of the lesser and greater tuberosities. In these situations, failure of tuberosity healing may result in loss of rotator cuff function with loss of elevation, rotation, and even instability. Humeral shortening can also occur as a result of bone loss and can compromise deltoid function by loss of proper muscle tension, leading to instability, dysfunction, or both. In addition to possible instability, humeral shortening with metaphyseal bone loss can adversely affect long-term fixation of the humeral component, leading to stem loosening or failure. Cuff and colleagues3 showed significantly more rotational micromotion in cases lacking metaphyseal support, leading to aseptic loosening of the humeral stem.
Humeral bone loss can also result from humeral stem component removal in revision shoulder arthroplasty for infection, component failure or loosening, and even periprosthetic fracture resulting from surgery or trauma.
For the surgeon, humeral bone loss can create a complex set of circumstances related to rotator cuff attachment failure, soft-tissue balancing effects, and component fixation issues. Any such issue must be recognized and addressed for best outcomes. Best results can be obtained with preoperative imaging, planning, use of bone graft techniques, proximal humeral allografts, and, more recently, modular and patient-specific implants. All of these issues are discussed comprehensively in the articles this month.
Continue to: GLENOID BONE LOSS
GLENOID BONE LOSS
Proper glenoid component placement with durable fixation is crucial for success in anatomical total shoulder arthroplasty and RSA. Glenoid bone deformity and loss can result from intrinsic deformity characteristics seen in primary osteoarthritis, cuff tear arthropathy, or glenoid component removal in revision situations and infection. These bone deformity complications can be extremely difficult to treat and in some cases lead to catastrophic failure of the index arthroplasty.
We are now aware that one key to success in the face of moderate to severe deformity is proper recognition. Newer imaging techniques, including 2-dimensional (2-D) computed tomography (CT) and 3-dimensional (3-D) modeling and surgical planning software tools, which are outlined in an upcoming article, have given surgeons important new instruments that can help in treating these difficult cases.
Glenoid bone deformity in primary osteoarthritis was well delineated in the 1999 seminal study of CT changes by Walch and colleagues.1 The Walch classification system, which characterized glenoid morphology based on 2-D CT findings, was recently upgraded, based on 3-D imaging technology, to include Walch B3 and D patterns (Figure 1).2 Recognition of certain primary deformities in osteoarthritis has led to increased use of RSA in some cases of Walch B2, B3, and C deformities with substantial glenoid retroversion and/or humeral head subluxation.4

In cases of rotator cuff tear arthropathy, glenoid bone deformities are well described with several classification systems based on degree and dimension of bone insufficiency. The Hamada classification system defines the degree of medial glenoid erosion and superior bone loss, as well as acetabularization of the acromion in 5 grades; 5 Rispoli and colleagues6 defined and graded the degree of medicalization of the glenohumeral joint based on degree of subchondral plate erosion; and Visotsky and colleagues7 based their classification system on wear patterns of bone loss, alignment, and concomitant soft-tissue insufficiencies leading to instability and rotation loss.
In severe glenoid bone deficiency after glenoid component removal, Antuna and colleagues8 described the classic findings related to medial bone loss, anterior and posterior wall failure, and combinations thereof.
Continue to: All these classification systems...
All these classification systems are based on the 2-D appearance of the glenoid and should be considered cautiously. The glenoid is a complex 3-D structure that can be affected by any number of disease processes, trauma, and surgical intervention. Using more modern CT techniques and 3-D imaging, we now know that many deformities previously classified as unidirectional are, instead, complex and multidirectional.
Frankle and colleagues9 developed a classification based more 3-D CT models which has further classified severe glenoid vault deformities in relation to direction and degree of bone loss (Figures 2A-2E). Using this system, they were better able to determine degree and direction of deformity than in previous 2-D evaluations, and they were able to determine the amount of glenoid vault bone available for baseplate fixation. Scalise and colleagues10 further defined the influence of such 3-D planning in total shoulder arthroplasty.

With knowledge of these classification systems and use of contemporary imaging systems, shoulder arthroplasty in cases of severe glenoid deficiency can be more successful. Potentially, we can improve outcomes even more in the more severe cases of bone loss with use of patient-specific planning tools, including the guides and patient-specific implants that are now readily available with many implant systems.11
Preoperative planning tools, bone-grafting techniques, augmented and specialized glenoid and humeral implants, and patient-specific implants are discussed this month to give our readers a comprehensive review of the latest concepts in shoulder arthroplasty in cases of significant bone loss or deformity.
This month of The American Journal of Orthopedics presents the most current and cutting-edge solutions for humeral and glenoid bone deformities and deficiencies in contemporary shoulder arthroplasties.
1. Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty. 1999;14(6):756-760.
2. Bercik MJ, Kruse K 2nd, Yalizis M, Gauci MO, Chaoui J, Walch G. A modification to the Walch classification of the glenoid in primary glenohumeral osteoarthritis using three-dimensional imaging. J Shoulder Elbow Surg. 2016;25(10):1601-1606.
3. Cuff D, Levy JC, Gutiérrez S, Frankle M. Torsional stability of modular and non-modular reverse shoulder humeral components in a proximal humeral bone loss model. J Shoulder Elbow Surg. 2011;20(4):646-651.
4. Denard PJ, Walch G. Current concepts in the surgical management of primary glenohumeral arthritis with a biconcave glenoid. J Shoulder Elbow Surg. 2013;22(11):1589-1598.
5. Hamada K, Fukuda H, Mikasa M, Kobayashi Y. Roentgenographic findings in massive rotator cuff tears. A long-term observation. Clin Orthop Relat Res. 1990;(254):92-96.
6. Rispoli D, Sperling JW, Athwal GS, Schleck CD, Cofield RH. Humeral head replacement for the treatment of osteoarthritis. J Bone Joint Surg Am. 2006;88(12):2637-2644.
7. Visotsky JL, Basamania C, Seebauer L, Rockwood CA, Jensen KL. Cuff tear arthropathy: pathogenesis, classification, and algorithm for treatment. J Bone Joint Surg Am. 2004;86(suppl 2):35-40.
8. Antuna SA, Sperling JW, Cofield RH, Rowland CM. Glenoid revision surgery after total shoulder arthroplasty. J Shoulder Elbow Surg. 2001;10(3):217-224.
9. Frankle MA, Teramoto A, Luo ZP, Levy JC, Pupello D. Glenoid morphology in reverse shoulder arthroplasty: classification and surgical implications. J Shoulder Elbow Surg. 2009;18(6):874-885.
10. Scalise JJ, Codsi MJ, Bryan J, Brems JJ, Iannotti JP. The influence of three-dimensional computed tomography images of the shoulder in preoperative planning for total shoulder arthroplasty. J Bone Joint Surg Am. 2008;90(11):2438-2445.
11. Dines DM, Gulotta L, Craig EV, Dines JS. Novel solution for massive glenoid defects in shoulder arthroplasty: a patient-specific glenoid vault reconstruction system. Am J Orthop. 2017;46(2):104-108.
1. Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty. 1999;14(6):756-760.
2. Bercik MJ, Kruse K 2nd, Yalizis M, Gauci MO, Chaoui J, Walch G. A modification to the Walch classification of the glenoid in primary glenohumeral osteoarthritis using three-dimensional imaging. J Shoulder Elbow Surg. 2016;25(10):1601-1606.
3. Cuff D, Levy JC, Gutiérrez S, Frankle M. Torsional stability of modular and non-modular reverse shoulder humeral components in a proximal humeral bone loss model. J Shoulder Elbow Surg. 2011;20(4):646-651.
4. Denard PJ, Walch G. Current concepts in the surgical management of primary glenohumeral arthritis with a biconcave glenoid. J Shoulder Elbow Surg. 2013;22(11):1589-1598.
5. Hamada K, Fukuda H, Mikasa M, Kobayashi Y. Roentgenographic findings in massive rotator cuff tears. A long-term observation. Clin Orthop Relat Res. 1990;(254):92-96.
6. Rispoli D, Sperling JW, Athwal GS, Schleck CD, Cofield RH. Humeral head replacement for the treatment of osteoarthritis. J Bone Joint Surg Am. 2006;88(12):2637-2644.
7. Visotsky JL, Basamania C, Seebauer L, Rockwood CA, Jensen KL. Cuff tear arthropathy: pathogenesis, classification, and algorithm for treatment. J Bone Joint Surg Am. 2004;86(suppl 2):35-40.
8. Antuna SA, Sperling JW, Cofield RH, Rowland CM. Glenoid revision surgery after total shoulder arthroplasty. J Shoulder Elbow Surg. 2001;10(3):217-224.
9. Frankle MA, Teramoto A, Luo ZP, Levy JC, Pupello D. Glenoid morphology in reverse shoulder arthroplasty: classification and surgical implications. J Shoulder Elbow Surg. 2009;18(6):874-885.
10. Scalise JJ, Codsi MJ, Bryan J, Brems JJ, Iannotti JP. The influence of three-dimensional computed tomography images of the shoulder in preoperative planning for total shoulder arthroplasty. J Bone Joint Surg Am. 2008;90(11):2438-2445.
11. Dines DM, Gulotta L, Craig EV, Dines JS. Novel solution for massive glenoid defects in shoulder arthroplasty: a patient-specific glenoid vault reconstruction system. Am J Orthop. 2017;46(2):104-108.
Pseudo-Pedicle Heterotopic Ossification From Use of Recombinant Human Bone Morphogenetic Protein 2 (rhBMP-2) in Transforaminal Lumbar Interbody Fusion Cages
ABSTRACT
We conducted a study to determine the common characteristics of patients who developed radiculopathy symptoms and corresponding heterotopic ossification (HO) from transforaminal lumbar interbody fusions (TLIF) using recombinant human bone morphogenetic protein 2 (rhBMP-2). HO can arise from a disk space with rhBMP-2 use in TLIF. Formation of bone around nerve roots or the thecal sac can cause a radiculopathy with a consistent pattern of symptoms.
We identified 38 patients (26 males, 12 females) with a mean (SD) age of 50.8 (7.5) years who developed radiculopathy symptoms and corresponding HO from TLIF with rhBMP-2 in the disk space between 2002 and 2015. To document this complication and improve its recognition, we recorded common patterns of symptom development and radiologic findings: specifically, time from implantation of rhBMP-2 to symptom development, consistency with side of TLIF placement, and radiologic findings.
Radicular pain generally developed a mean (SD) of 3.8 (1.0) months after TLIF with rhBMP-2. Development of radiculopathy symptoms corresponded to consistent “pseudo-pedicle”-like HO. In all 38 patients, HO arising from the annulotomy site showed a distinct pseudo-pedicle pattern encompassing nerve roots and the thecal sac. In addition, development of radiculopathy symptoms and corresponding HO appear to be independent of amount of rhBMP-2. HO resulting from TLIF with rhBMP-2 in the disk space is a pain generator and a recognizable complication that can be diagnosed by assessment of symptoms and computed tomography characteristics.
Continue to: Bone morphogenetic proteins...
Bone morphogenetic proteins (BMPs), first isolated by Urist in 19641, are a family of growth factors that stimulate the cascade of bone formation. Recombinant human BMP (rhBMP), specifically rhBMP-2 and rhBMP-7 (also known as osteogenic protein 1 [OP-1]), was developed in the 1990s after the advent of gene splicing. Then, in 2002, the US Food and Drug Administration (FDA) approved use of rhBMP to stimulate fusion in the human spine. Specifically, rhBMP-2 (Medtronic) was approved for use in combination with a specific brand of interbody cage in 1-level anterior lumbar interbody fusion.2 Over the past decade, off-label use of rhBMP-2 to achieve osseous union has increased dramatically, particularly in spinal surgery: transforaminal lumbar interbody fusion (TLIF), posterior lumbar interbody fusion, and posterolateral lumbar fusion.3-9 However, this widespread off-label use for posterior spinal fusion began despite FDA data indicating that specific complications were underreported in the peer-reviewed literature.10,11 Although rhBMP-2 is very effective in increasing osteoblast formation and improving osteogenesis and subsequent bone healing in spinal surgery,12,13 its use in TLIF resulted in significant adverse side effects, including radiculopathy with and without neuroforaminal heterotopic ossification (HO); 14-24 complications in the FDA studies; 14,22,25-27 and osteolysis causing intervertebral cage subsidence, inflammatory radiculitis, genitourinary complications, infections, possible systemic effects, and significant HO complications.10,28-30 Of these, HO complications involved rhBMP leakage through the annulotomy to the disk space that led to HO. Specifically, rhBMP leaked directly out of the disk space and formed a pillar of bone that encased the nerve roots and dura, which led to occlusion of the foramen and symptoms of radiculopathy.10,28-30
Despite this frequent finding of HO in the intervertebral space outside the target fusion area, use of rhBMP-2 with intervertebral cages increased so rapidly that rhBMP-2 was used more often than autologous bone.5,11,17,31 In this study, we reviewed the common characteristics of patients who developed HO and subsequent radiculopathy from TLIF with rhBMP.
METHODS
After this study received Institutional Review Board approval, we retrospectively reviewed cases of radiculopathy symptoms that developed after TLIF with rhBMP between January 2002 and January 2015. During this period, 38 patients (26 males, 12 females) with a mean (SD) age of 50.8 (7.5) years and radiculopathy symptoms arising from TLIF with rhBMP-2 were identified to determine commonalities and defining characteristics that will help facilitate diagnosis.
Inclusion criteria were computed tomography (CT)–documented HO arising from the TLIF annulotomy site in continuity with bone in the disk space or ectopic bone forming a distinctive shell with contouring around the thecal sac or nerve roots, as well as recurrence or initial occurrence of radiculopathy with signs and symptoms corresponding to the CT site of aberrant bone growth in terms of laterality and particular nerve root(s) involved. Exclusion criteria were malplacement of interbody cage or pedicle screws, disk herniation, systemic neuropathic disease, and new or unresolved radiculopathy immediately after index surgery.
To improve recognition of this complication, we also documented the amount of BMP used, common patterns of radiculopathy symptom development, and radiologic findings. Type and timing of radiculopathy symptom onset and consistency with side of TLIF placement were documented as well. Radiculopathy symptoms included shooting pain in the legs, incontinence, sexual dysfunction, and severe paralysis. Radiologic findings were specific to bone formation from the disk space (detected with CT).
Continue to: RESULTS
RESULTS
All 38 selected patients had radiculopathy symptoms from HO out of the intervertebral space. The Table lists the patients’ overall characteristics. The left side had the most radiculopathy symptoms (31/38 patients), followed by the right side (5/38) and both sides (2/38). Radiculopathy symptoms began a mean (SD) of 3.8 (1.0) months (range, 2-6 months) after index surgery. The 38 patients had 4 characteristics in common:
Table. Transforaminal Lumbar Interbody Fusion With Recombinant Human Bone Morphogenetic Protein 2: Onset Time for Radiculopathy Symptoms, Surgery Level, Side of Pseudo-Pedicle Bone Formation, and Subsequent Complications
| Pt | Sympton Onset, mo | Surgery Level(s) | Side(s) | Complication(s) |
| 1 | 3 | L3-L5 (2) | Both | Radiculopathy, pseudo-pedicle, urine |
| 2 | 3 | L4-L5 (2) | R | Radiculopathy, pseudo-pedicle |
| 3 | 4 | L5-S1 (1) | R | Radiculopathy, pseudo-pedicle |
| 4 | 5 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 5 | 4 | L4-S1 (2) | L | Radiculopathy, pseudo-pedicle, subsidence |
| 6 | 5 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 7 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 8 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 9 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 10 | 2 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 11 | 2 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle, subsidence, neurologic |
| 12 | 6 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 13 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle, neurologic |
| 14 | 2 | L2-L3 (1) | R | Radiculopathy, pseudo-pedicle |
| 15 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 16 | 3 | L4-L5 (1) | L | Radiculopathy, pseudo-pedicle |
| 17 | 3 | L2-L3, L4-L5 (2) | L | Radiculopathy, pseudo-pedicle |
| 18 | 3 | L4-L5, L2-L3 (1) | L | Radiculopathy, pseudo-pedicle, nonunion |
| 19 | 4 | L4-L5 (1) | R | Radiculopathy, pseudo-pedicle |
| 20 | 5 | L4-L5 (1) | L | Radiculopathy, pseudo-pedicle |
| 21 | 5 | L5-S1 (1) | R | Radiculopathy, pseudo-pedicle |
| 22 | 3 | L3-L4, L5-S1 (2) | Both | Radiculopathy, pseudo-pedicle |
| 23 | 4 | L4-L5 (1) | L | Radiculopathy, pseudo-pedicle |
| 24 | 6 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 25 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 26 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle, urine, bowel |
| 27 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 28 | 4 | L4-L5 (1) | L | Radiculopathy, pseudo-pedicle |
| 29 | 6 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 30 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 31 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 32 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 33 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 34 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 35 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 36 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 37 | 4 | L4-L5 (1) | L | Radiculopathy, pseudo-pedicle |
| 38 | 4 | L4-L5 (1) | L | Radiculopathy, pseudo-pedicle |
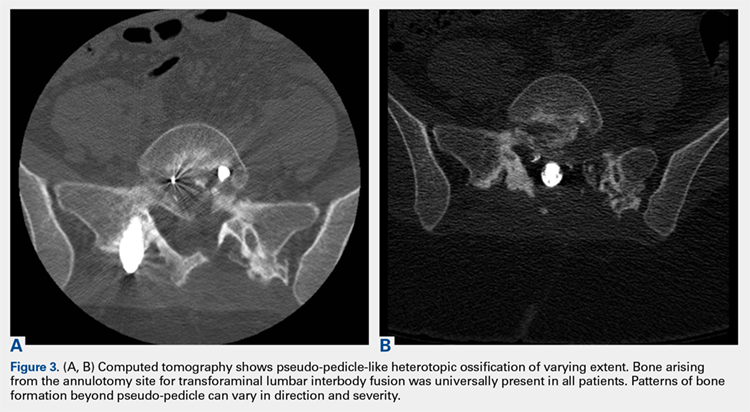
1. Bone growing out of the annulotomy site for TLIF cage placement was present and in continuity with the disk space in 33 (87%) of the 38 cases. In the other 5 cases (13%), HO was present around the neural tissue, but not necessarily in continuity with the disk space. This bone appeared ectopic and not osteophytic and facet-related, as it formed a shell around either the nerve root or the thecal sac, contouring to the structure.
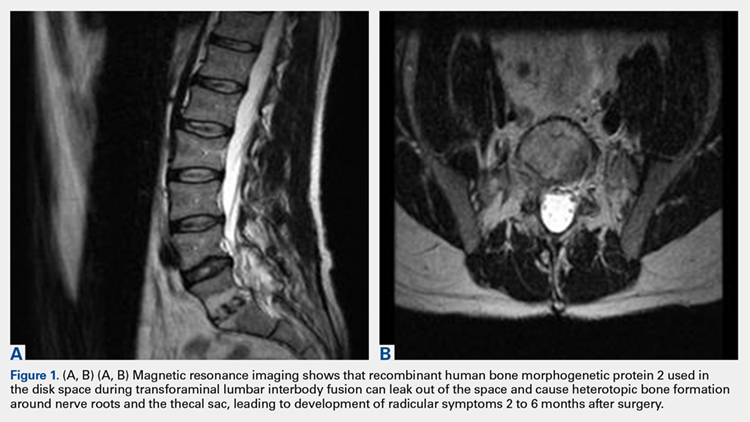
2. The common, novel finding on CT was a “pseudo-pedicle” (Figures 1A, 1B), which appeared as ectopic growth from the disk space—a solid piece of bone in the same direction as the anatomical pedicle. Confusing similarity to the anatomical pedicle is present on axial cuts and during surgery. The pseudo-pedicle varied in thickness and extent out of the disk space, but was always presented as a bar of bone arising from the annulotomy site. After arising from the disk space, the HO could disperse in any direction, further calcifying neural structures or the facet joints above or below. There was no apparent distinguishable repeating pattern, given the variable nature of arthritic facet changes, scoliotic deformities, size of annulotomies, amount of rhBMP used, and placement in cage and disk space or only in cage.
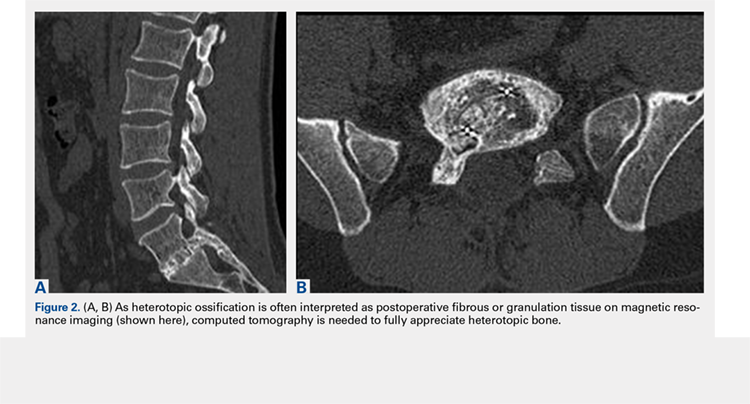
3. In 36 (95%) of the 38 cases, the initial interpretation of HO on magnetic resonance imaging (MRI) was of tissue other than bone, such as fibrous tissue, granulation tissue, recurrent disk herniation, or postoperative changes. However, this tissue was later determined to be bone from HO complications, which we confirmed with CT in all 38 cases. It is important to note that HO on MRI (Figures 2A, 2B) was initially interpreted by a radiologist as fibrous tissue, but same-level CT of the same case (Figures 3A, 3B) showed clear HO.
4. The radiculopathy symptoms caused by HO were independent of the amount of rhBMP-2 used in TLIF. Of the 38 patients, 19 had 1 rhBMP-2 sponge placed in the cage, 12 had a small kit sponge (1.05 mg), 5 had 1 sponge placed in the cage and 1 sponge placed directly in the disk space before cage placement (no notation of precise size or amount of rhBMP-2), and 2 had 1 sponge placed in the cage (no notation of rhBMP-2 amount). The data showed that HO can occur with even a small amount of rhBMP-2.
Continue to: Bone formation with rhBMP-2...
Bone formation with rhBMP-2 is robust and beneficial, but HO-related complications are significant, and identifiable on assessment of radiculopathy symptoms and CT characteristics.
DISCUSSION
We identified 38 patients with a recognizable and consistent pattern of complications of off-label use of rhBMP-2 in TLIF performed at our institution between 2002 and 2015. This pattern included consistent radiculopathy symptoms with corresponding HO at the annulotomy site in continuity with bone in the disk space or ectopic bone forming a distinctive shell around the thecal sac or nerve roots, as well as showing a distinct pseudo-pedicle pattern encompassing nerve roots and the thecal sac. Our finding differs from other findings of similar complication characteristics, but with much larger variations without consistency within the patient population.19,20,22,24 Specifically, previous studies found an association between off-label rhBMP-2 use in the posterior spine and radiculopathy with and without neuroforaminal HO. However, our study found consistent radiculopathy symptoms with pseudo-pedicle-like HO complications in all its 38 patients a mean (SD) of 3.8 (1.0) months after surgery.
In this study, consistent radiculopathy symptoms with pseudo-pedicle-like HO complications were independent of the amount of rhBMP-2 used, as some complications occurred with use of small pack rhBMP-2 with TLIF. It is well understood that high doses of rhBMP-2 may be required to improve fusion rates, but to our knowledge an optimal dosing strategy for TLIF has not been reported, particularly with respect to potential complications.8,20,31-33 For anterior lumbar interbody fusion surgery, the FDA-approved use of rhBMP-2 appears to have a significantly decreased risk of neuroforaminal HO complications. This may be attributable to the protective presence of the intact posterior annulus and longitudinal ligament for this procedure.20,33 For TLIF, it has been suggested that rhBMP-2 should be placed only along the anterior annulus with a posterior strut and morselized bone allograft barricade,33 and that fibrin glue should be used to limit BMP diffusion through the annulotomy site31 to prevent this complication.
Our study results suggest that radiculopathy symptoms with pseudo-pedicle-like HO complications appear to be caused by leakage of rhBMP-2 from the disk space through the annulotomy site. This was often initially interpreted incorrectly on MRI in the first year after surgery as being fibrous or granulation tissue, or even postoperative changes that the heterotopic tissue was bone was obvious only on CT. Even then the tissue may be incorrectly identified, as the encasing nerve roots in bone are similar to the scar tissue having no compressive effect. HO may compress, but it also has an inflammatory component that the scars lack. Additionally, the HO from the disk space, caused by leakage of the BMP placed in or around the fusion cage, can create a pseudo-pedicle of varying size and extent. This was present in all 38 of our cases.
This retrospective case series had its limitations. Its clinical and radiographic findings were not blinded. Confounding variables cannot be isolated for causal relationships, if any, to the complication in a case series such as this.
Bone formation with rhBMP-2 is robust and beneficial, but HO-related complications are significant, and identifiable on assessment of radiculopathy symptoms and CT characteristics.
1. Urist MR. Bone: formation by autoinduction. Science. 1965;150(3698):893-899.
2. Burkus JK, Gornet MF, Schuler TC, Kleeman TJ, Zdeblick TA. Six-year outcomes of anterior lumbar interbody arthrodesis with use of interbody fusion cages and recombinant human bone morphogenetic protein-2. J Bone Joint Surg Am. 2009;91(5):1181-1189.
3. Boden SD, Kang J, Sandhu H, Heller JG. Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans: a prospective, randomized clinical pilot trial: 2002 Volvo award in clinical studies. Spine. 2002;27(23):2662-2673.
4. Boden SD, Zdeblick TA, Sandhu HS, Heim SE. The use of rhBMP-2 in interbody fusion cages. Definitive evidence of osteoinduction in humans: a preliminary report. Spine. 2000;25(3):376-381.
5. Haid RW Jr, Branch CL Jr, Alexander JT, Burkus JK. Posterior lumbar interbody fusion using recombinant human bone morphogenetic protein type 2 with cylindrical interbody cages. Spine J. 2004;4(5):527-538.
6. Meisel HJ, Schnöring M, Hohaus C, et al. Posterior lumbar interbody fusion using rhBMP-2. Eur Spine J. 2008;17(12):1735-1744.
7. Mummaneni PV, Pan J, Haid RW, Rodts GE. Contribution of recombinant human bone morphogenetic protein-2 to the rapid creation of interbody fusion when used in transforaminal lumbar interbody fusion: a preliminary report. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2004. J Neurosurg Spine. 2004;1(1):19-23.
8. Shimer AL, Oner FC, Vaccaro AR. Spinal reconstruction and bone morphogenetic proteins: open questions. Injury. 2009;40(suppl 3):S32-S38.
9. Slosar PJ, Josey R, Reynolds J. Accelerating lumbar fusions by combining rhBMP-2 with allograft bone: a prospective analysis of interbody fusion rates and clinical outcomes. Spine J. 2007;7(3):301-307.
10. Knox JB, Dai JM 3rd, Orchowski J. Osteolysis in transforaminal lumbar interbody fusion with bone morphogenetic protein-2. Spine. 2011;36(8):672-676.
11. Owens K, Glassman SD, Howard JM, Djurasovic M, Witten JL, Carreon LY. Perioperative complications with rhBMP-2 in transforaminal lumbar interbody fusion. Eur Spine J. 2011;20(4):612-617.
12. Mindea SA, Shih P, Song JK. Recombinant human bone morphogenetic protein-2-induced radiculitis in elective minimally invasive transforaminal lumbar interbody fusions: a series review. Spine. 2009;34(14):1480-1484.
13. Yoon ST, Park JS, Kim KS, et al. ISSLS prize winner: LMP-1 upregulates intervertebral disc cell production of proteoglycans and BMPs in vitro and in vivo. Spine. 2004;29(23):2603-2611.
14. Cahill KS, Chi JH, Day A, Claus EB. Prevalence, complications, and hospital charges associated with use of bone-morphogenetic proteins in spinal fusion procedures. JAMA. 2009;302(1):58-66.
15. Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 2011;11(6):471-491.
16. Chen NF, Smith ZA, Stiner E, Armin S, Sheikh H, Khoo LT. Symptomatic ectopic bone formation after off-label use of recombinant human bone morphogenetic protein-2 in transforaminal lumbar interbody fusion. J Neurosurg Spine. 2010;12(1):40-46.
17. Joseph V, Rampersaud YR. Heterotopic bone formation with the use of rhBMP2 in posterior minimal access interbody fusion: a CT analysis. Spine. 2007;32(25):2885-2890.
18. McClellan JW, Mulconrey DS, Forbes RJ, Fullmer N. Vertebral bone resorption after transforaminal lumbar interbody fusion with bone morphogenetic protein (rhBMP-2). J Spinal Disord Tech. 2006;19(7):483-486.
19. Mroz TE, Wang JC, Hashimoto R, Norvell DC. Complications related to osteobiologics use in spine surgery: a systematic review. Spine. 2010;35(9 suppl):S86-S104.
20. Muchow RD, Hsu WK, Anderson PA. Histopathologic inflammatory response induced by recombinant bone morphogenetic protein-2 causing radiculopathy after transforaminal lumbar interbody fusion. Spine J. 2010;10(9):e1-e6.
21. Ong KL, Villarraga ML, Lau E, Carreon LY, Kurtz SM, Glassman SD. Off-label use of bone morphogenetic proteins in the United States using administrative data. Spine. 2010;35(19):1794-1800.
22. Rihn JA, Patel R, Makda J, et al. Complications associated with single-level transforaminal lumbar interbody fusion. Spine J. 2009;9(8):623-629.
23. Vaidya R, Sethi A, Bartol S, Jacobson M, Coe C, Craig JG. Complications in the use of rhBMP-2 in PEEK cages for interbody spinal fusions. J Spinal Disord Tech. 2008;21(8):557-562.
24. Wong DA, Kumar A, Jatana S, Ghiselli G, Wong K. Neurologic impairment from ectopic bone in the lumbar canal: a potential complication of off-label PLIF/TLIF use of bone morphogenetic protein-2 (BMP-2). Spine J. 2008;8(6):1011-1018.
25. Delawi D, Dhert WJ, Rillardon L, et al. A prospective, randomized, controlled, multicenter study of osteogenic protein-1 in instrumented posterolateral fusions: report on safety and feasibility. Spine. 2010;35(12):1185-1191.
26. Vaccaro AR, Patel T, Fischgrund J, et al. A pilot study evaluating the safety and efficacy of OP-1 putty (rhBMP-7) as a replacement for iliac crest autograft in posterolateral lumbar arthrodesis for degenerative spondylolisthesis. Spine. 2004;29(17):1885-1892.
27. Vaidya R, Weir R, Sethi A, Meisterling S, Hakeos W, Wybo CD. Interbody fusion with allograft and rhBMP-2 leads to consistent fusion but early subsidence. J Bone Joint Surg Br. 2007;89(3):342-345.
28. Glassman SD, Howard J, Dimar J, Sweet A, Wilson G, Carreon L. Complications with recombinant human bone morphogenic protein-2 in posterolateral spine fusion: a consecutive series of 1037 cases. Spine. 2011;36(22):1849-1854.
29. Helgeson MD, Lehman RA Jr, Patzkowski JC, Dmitriev AE, Rosner MK, Mack AW. Adjacent vertebral body osteolysis with bone morphogenetic protein use in transforaminal lumbar interbody fusion. Spine J. 2011;11(6):507-510.
30. Hoffmann MF, Jones CB, Sietsema DL. Adjuncts in posterior lumbar spine fusion: comparison of complications and efficacy. Arch Orthop Trauma Surg. 2012;132(8):1105-1110.
31. Villavicencio AT, Burneikiene S, Nelson EL, Bulsara KR, Favors M, Thramann J. Safety of transforaminal lumbar interbody fusion and intervertebral recombinant human bone morphogenetic protein-2. J Neurosurg Spine. 2005;3(6):436-443.
32. Patel VV, Zhao L, Wong P, et al. Controlling bone morphogenetic protein diffusion and bone morphogenetic protein-stimulated bone growth using fibrin glue. Spine. 2006;31(11):1201-1206.
33. Zhang H, Sucato DJ, Welch RD. Recombinant human bone morphogenic protein-2-enhanced anterior spine fusion without bone encroachment into the spinal canal: a histomorphometric study in a thoracoscopically instrumented porcine model. Spine. 2005;30(5):512-518.
ABSTRACT
We conducted a study to determine the common characteristics of patients who developed radiculopathy symptoms and corresponding heterotopic ossification (HO) from transforaminal lumbar interbody fusions (TLIF) using recombinant human bone morphogenetic protein 2 (rhBMP-2). HO can arise from a disk space with rhBMP-2 use in TLIF. Formation of bone around nerve roots or the thecal sac can cause a radiculopathy with a consistent pattern of symptoms.
We identified 38 patients (26 males, 12 females) with a mean (SD) age of 50.8 (7.5) years who developed radiculopathy symptoms and corresponding HO from TLIF with rhBMP-2 in the disk space between 2002 and 2015. To document this complication and improve its recognition, we recorded common patterns of symptom development and radiologic findings: specifically, time from implantation of rhBMP-2 to symptom development, consistency with side of TLIF placement, and radiologic findings.
Radicular pain generally developed a mean (SD) of 3.8 (1.0) months after TLIF with rhBMP-2. Development of radiculopathy symptoms corresponded to consistent “pseudo-pedicle”-like HO. In all 38 patients, HO arising from the annulotomy site showed a distinct pseudo-pedicle pattern encompassing nerve roots and the thecal sac. In addition, development of radiculopathy symptoms and corresponding HO appear to be independent of amount of rhBMP-2. HO resulting from TLIF with rhBMP-2 in the disk space is a pain generator and a recognizable complication that can be diagnosed by assessment of symptoms and computed tomography characteristics.
Continue to: Bone morphogenetic proteins...
Bone morphogenetic proteins (BMPs), first isolated by Urist in 19641, are a family of growth factors that stimulate the cascade of bone formation. Recombinant human BMP (rhBMP), specifically rhBMP-2 and rhBMP-7 (also known as osteogenic protein 1 [OP-1]), was developed in the 1990s after the advent of gene splicing. Then, in 2002, the US Food and Drug Administration (FDA) approved use of rhBMP to stimulate fusion in the human spine. Specifically, rhBMP-2 (Medtronic) was approved for use in combination with a specific brand of interbody cage in 1-level anterior lumbar interbody fusion.2 Over the past decade, off-label use of rhBMP-2 to achieve osseous union has increased dramatically, particularly in spinal surgery: transforaminal lumbar interbody fusion (TLIF), posterior lumbar interbody fusion, and posterolateral lumbar fusion.3-9 However, this widespread off-label use for posterior spinal fusion began despite FDA data indicating that specific complications were underreported in the peer-reviewed literature.10,11 Although rhBMP-2 is very effective in increasing osteoblast formation and improving osteogenesis and subsequent bone healing in spinal surgery,12,13 its use in TLIF resulted in significant adverse side effects, including radiculopathy with and without neuroforaminal heterotopic ossification (HO); 14-24 complications in the FDA studies; 14,22,25-27 and osteolysis causing intervertebral cage subsidence, inflammatory radiculitis, genitourinary complications, infections, possible systemic effects, and significant HO complications.10,28-30 Of these, HO complications involved rhBMP leakage through the annulotomy to the disk space that led to HO. Specifically, rhBMP leaked directly out of the disk space and formed a pillar of bone that encased the nerve roots and dura, which led to occlusion of the foramen and symptoms of radiculopathy.10,28-30
Despite this frequent finding of HO in the intervertebral space outside the target fusion area, use of rhBMP-2 with intervertebral cages increased so rapidly that rhBMP-2 was used more often than autologous bone.5,11,17,31 In this study, we reviewed the common characteristics of patients who developed HO and subsequent radiculopathy from TLIF with rhBMP.
METHODS
After this study received Institutional Review Board approval, we retrospectively reviewed cases of radiculopathy symptoms that developed after TLIF with rhBMP between January 2002 and January 2015. During this period, 38 patients (26 males, 12 females) with a mean (SD) age of 50.8 (7.5) years and radiculopathy symptoms arising from TLIF with rhBMP-2 were identified to determine commonalities and defining characteristics that will help facilitate diagnosis.
Inclusion criteria were computed tomography (CT)–documented HO arising from the TLIF annulotomy site in continuity with bone in the disk space or ectopic bone forming a distinctive shell with contouring around the thecal sac or nerve roots, as well as recurrence or initial occurrence of radiculopathy with signs and symptoms corresponding to the CT site of aberrant bone growth in terms of laterality and particular nerve root(s) involved. Exclusion criteria were malplacement of interbody cage or pedicle screws, disk herniation, systemic neuropathic disease, and new or unresolved radiculopathy immediately after index surgery.
To improve recognition of this complication, we also documented the amount of BMP used, common patterns of radiculopathy symptom development, and radiologic findings. Type and timing of radiculopathy symptom onset and consistency with side of TLIF placement were documented as well. Radiculopathy symptoms included shooting pain in the legs, incontinence, sexual dysfunction, and severe paralysis. Radiologic findings were specific to bone formation from the disk space (detected with CT).
Continue to: RESULTS
RESULTS
All 38 selected patients had radiculopathy symptoms from HO out of the intervertebral space. The Table lists the patients’ overall characteristics. The left side had the most radiculopathy symptoms (31/38 patients), followed by the right side (5/38) and both sides (2/38). Radiculopathy symptoms began a mean (SD) of 3.8 (1.0) months (range, 2-6 months) after index surgery. The 38 patients had 4 characteristics in common:
Table. Transforaminal Lumbar Interbody Fusion With Recombinant Human Bone Morphogenetic Protein 2: Onset Time for Radiculopathy Symptoms, Surgery Level, Side of Pseudo-Pedicle Bone Formation, and Subsequent Complications
| Pt | Sympton Onset, mo | Surgery Level(s) | Side(s) | Complication(s) |
| 1 | 3 | L3-L5 (2) | Both | Radiculopathy, pseudo-pedicle, urine |
| 2 | 3 | L4-L5 (2) | R | Radiculopathy, pseudo-pedicle |
| 3 | 4 | L5-S1 (1) | R | Radiculopathy, pseudo-pedicle |
| 4 | 5 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 5 | 4 | L4-S1 (2) | L | Radiculopathy, pseudo-pedicle, subsidence |
| 6 | 5 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 7 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 8 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 9 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 10 | 2 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 11 | 2 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle, subsidence, neurologic |
| 12 | 6 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 13 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle, neurologic |
| 14 | 2 | L2-L3 (1) | R | Radiculopathy, pseudo-pedicle |
| 15 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 16 | 3 | L4-L5 (1) | L | Radiculopathy, pseudo-pedicle |
| 17 | 3 | L2-L3, L4-L5 (2) | L | Radiculopathy, pseudo-pedicle |
| 18 | 3 | L4-L5, L2-L3 (1) | L | Radiculopathy, pseudo-pedicle, nonunion |
| 19 | 4 | L4-L5 (1) | R | Radiculopathy, pseudo-pedicle |
| 20 | 5 | L4-L5 (1) | L | Radiculopathy, pseudo-pedicle |
| 21 | 5 | L5-S1 (1) | R | Radiculopathy, pseudo-pedicle |
| 22 | 3 | L3-L4, L5-S1 (2) | Both | Radiculopathy, pseudo-pedicle |
| 23 | 4 | L4-L5 (1) | L | Radiculopathy, pseudo-pedicle |
| 24 | 6 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 25 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 26 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle, urine, bowel |
| 27 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 28 | 4 | L4-L5 (1) | L | Radiculopathy, pseudo-pedicle |
| 29 | 6 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 30 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 31 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 32 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 33 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 34 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 35 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 36 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 37 | 4 | L4-L5 (1) | L | Radiculopathy, pseudo-pedicle |
| 38 | 4 | L4-L5 (1) | L | Radiculopathy, pseudo-pedicle |
1. Bone growing out of the annulotomy site for TLIF cage placement was present and in continuity with the disk space in 33 (87%) of the 38 cases. In the other 5 cases (13%), HO was present around the neural tissue, but not necessarily in continuity with the disk space. This bone appeared ectopic and not osteophytic and facet-related, as it formed a shell around either the nerve root or the thecal sac, contouring to the structure.

2. The common, novel finding on CT was a “pseudo-pedicle” (Figures 1A, 1B), which appeared as ectopic growth from the disk space—a solid piece of bone in the same direction as the anatomical pedicle. Confusing similarity to the anatomical pedicle is present on axial cuts and during surgery. The pseudo-pedicle varied in thickness and extent out of the disk space, but was always presented as a bar of bone arising from the annulotomy site. After arising from the disk space, the HO could disperse in any direction, further calcifying neural structures or the facet joints above or below. There was no apparent distinguishable repeating pattern, given the variable nature of arthritic facet changes, scoliotic deformities, size of annulotomies, amount of rhBMP used, and placement in cage and disk space or only in cage.

3. In 36 (95%) of the 38 cases, the initial interpretation of HO on magnetic resonance imaging (MRI) was of tissue other than bone, such as fibrous tissue, granulation tissue, recurrent disk herniation, or postoperative changes. However, this tissue was later determined to be bone from HO complications, which we confirmed with CT in all 38 cases. It is important to note that HO on MRI (Figures 2A, 2B) was initially interpreted by a radiologist as fibrous tissue, but same-level CT of the same case (Figures 3A, 3B) showed clear HO.

4. The radiculopathy symptoms caused by HO were independent of the amount of rhBMP-2 used in TLIF. Of the 38 patients, 19 had 1 rhBMP-2 sponge placed in the cage, 12 had a small kit sponge (1.05 mg), 5 had 1 sponge placed in the cage and 1 sponge placed directly in the disk space before cage placement (no notation of precise size or amount of rhBMP-2), and 2 had 1 sponge placed in the cage (no notation of rhBMP-2 amount). The data showed that HO can occur with even a small amount of rhBMP-2.
Continue to: Bone formation with rhBMP-2...
Bone formation with rhBMP-2 is robust and beneficial, but HO-related complications are significant, and identifiable on assessment of radiculopathy symptoms and CT characteristics.
DISCUSSION
We identified 38 patients with a recognizable and consistent pattern of complications of off-label use of rhBMP-2 in TLIF performed at our institution between 2002 and 2015. This pattern included consistent radiculopathy symptoms with corresponding HO at the annulotomy site in continuity with bone in the disk space or ectopic bone forming a distinctive shell around the thecal sac or nerve roots, as well as showing a distinct pseudo-pedicle pattern encompassing nerve roots and the thecal sac. Our finding differs from other findings of similar complication characteristics, but with much larger variations without consistency within the patient population.19,20,22,24 Specifically, previous studies found an association between off-label rhBMP-2 use in the posterior spine and radiculopathy with and without neuroforaminal HO. However, our study found consistent radiculopathy symptoms with pseudo-pedicle-like HO complications in all its 38 patients a mean (SD) of 3.8 (1.0) months after surgery.
In this study, consistent radiculopathy symptoms with pseudo-pedicle-like HO complications were independent of the amount of rhBMP-2 used, as some complications occurred with use of small pack rhBMP-2 with TLIF. It is well understood that high doses of rhBMP-2 may be required to improve fusion rates, but to our knowledge an optimal dosing strategy for TLIF has not been reported, particularly with respect to potential complications.8,20,31-33 For anterior lumbar interbody fusion surgery, the FDA-approved use of rhBMP-2 appears to have a significantly decreased risk of neuroforaminal HO complications. This may be attributable to the protective presence of the intact posterior annulus and longitudinal ligament for this procedure.20,33 For TLIF, it has been suggested that rhBMP-2 should be placed only along the anterior annulus with a posterior strut and morselized bone allograft barricade,33 and that fibrin glue should be used to limit BMP diffusion through the annulotomy site31 to prevent this complication.
Our study results suggest that radiculopathy symptoms with pseudo-pedicle-like HO complications appear to be caused by leakage of rhBMP-2 from the disk space through the annulotomy site. This was often initially interpreted incorrectly on MRI in the first year after surgery as being fibrous or granulation tissue, or even postoperative changes that the heterotopic tissue was bone was obvious only on CT. Even then the tissue may be incorrectly identified, as the encasing nerve roots in bone are similar to the scar tissue having no compressive effect. HO may compress, but it also has an inflammatory component that the scars lack. Additionally, the HO from the disk space, caused by leakage of the BMP placed in or around the fusion cage, can create a pseudo-pedicle of varying size and extent. This was present in all 38 of our cases.
This retrospective case series had its limitations. Its clinical and radiographic findings were not blinded. Confounding variables cannot be isolated for causal relationships, if any, to the complication in a case series such as this.
Bone formation with rhBMP-2 is robust and beneficial, but HO-related complications are significant, and identifiable on assessment of radiculopathy symptoms and CT characteristics.
ABSTRACT
We conducted a study to determine the common characteristics of patients who developed radiculopathy symptoms and corresponding heterotopic ossification (HO) from transforaminal lumbar interbody fusions (TLIF) using recombinant human bone morphogenetic protein 2 (rhBMP-2). HO can arise from a disk space with rhBMP-2 use in TLIF. Formation of bone around nerve roots or the thecal sac can cause a radiculopathy with a consistent pattern of symptoms.
We identified 38 patients (26 males, 12 females) with a mean (SD) age of 50.8 (7.5) years who developed radiculopathy symptoms and corresponding HO from TLIF with rhBMP-2 in the disk space between 2002 and 2015. To document this complication and improve its recognition, we recorded common patterns of symptom development and radiologic findings: specifically, time from implantation of rhBMP-2 to symptom development, consistency with side of TLIF placement, and radiologic findings.
Radicular pain generally developed a mean (SD) of 3.8 (1.0) months after TLIF with rhBMP-2. Development of radiculopathy symptoms corresponded to consistent “pseudo-pedicle”-like HO. In all 38 patients, HO arising from the annulotomy site showed a distinct pseudo-pedicle pattern encompassing nerve roots and the thecal sac. In addition, development of radiculopathy symptoms and corresponding HO appear to be independent of amount of rhBMP-2. HO resulting from TLIF with rhBMP-2 in the disk space is a pain generator and a recognizable complication that can be diagnosed by assessment of symptoms and computed tomography characteristics.
Continue to: Bone morphogenetic proteins...
Bone morphogenetic proteins (BMPs), first isolated by Urist in 19641, are a family of growth factors that stimulate the cascade of bone formation. Recombinant human BMP (rhBMP), specifically rhBMP-2 and rhBMP-7 (also known as osteogenic protein 1 [OP-1]), was developed in the 1990s after the advent of gene splicing. Then, in 2002, the US Food and Drug Administration (FDA) approved use of rhBMP to stimulate fusion in the human spine. Specifically, rhBMP-2 (Medtronic) was approved for use in combination with a specific brand of interbody cage in 1-level anterior lumbar interbody fusion.2 Over the past decade, off-label use of rhBMP-2 to achieve osseous union has increased dramatically, particularly in spinal surgery: transforaminal lumbar interbody fusion (TLIF), posterior lumbar interbody fusion, and posterolateral lumbar fusion.3-9 However, this widespread off-label use for posterior spinal fusion began despite FDA data indicating that specific complications were underreported in the peer-reviewed literature.10,11 Although rhBMP-2 is very effective in increasing osteoblast formation and improving osteogenesis and subsequent bone healing in spinal surgery,12,13 its use in TLIF resulted in significant adverse side effects, including radiculopathy with and without neuroforaminal heterotopic ossification (HO); 14-24 complications in the FDA studies; 14,22,25-27 and osteolysis causing intervertebral cage subsidence, inflammatory radiculitis, genitourinary complications, infections, possible systemic effects, and significant HO complications.10,28-30 Of these, HO complications involved rhBMP leakage through the annulotomy to the disk space that led to HO. Specifically, rhBMP leaked directly out of the disk space and formed a pillar of bone that encased the nerve roots and dura, which led to occlusion of the foramen and symptoms of radiculopathy.10,28-30
Despite this frequent finding of HO in the intervertebral space outside the target fusion area, use of rhBMP-2 with intervertebral cages increased so rapidly that rhBMP-2 was used more often than autologous bone.5,11,17,31 In this study, we reviewed the common characteristics of patients who developed HO and subsequent radiculopathy from TLIF with rhBMP.
METHODS
After this study received Institutional Review Board approval, we retrospectively reviewed cases of radiculopathy symptoms that developed after TLIF with rhBMP between January 2002 and January 2015. During this period, 38 patients (26 males, 12 females) with a mean (SD) age of 50.8 (7.5) years and radiculopathy symptoms arising from TLIF with rhBMP-2 were identified to determine commonalities and defining characteristics that will help facilitate diagnosis.
Inclusion criteria were computed tomography (CT)–documented HO arising from the TLIF annulotomy site in continuity with bone in the disk space or ectopic bone forming a distinctive shell with contouring around the thecal sac or nerve roots, as well as recurrence or initial occurrence of radiculopathy with signs and symptoms corresponding to the CT site of aberrant bone growth in terms of laterality and particular nerve root(s) involved. Exclusion criteria were malplacement of interbody cage or pedicle screws, disk herniation, systemic neuropathic disease, and new or unresolved radiculopathy immediately after index surgery.
To improve recognition of this complication, we also documented the amount of BMP used, common patterns of radiculopathy symptom development, and radiologic findings. Type and timing of radiculopathy symptom onset and consistency with side of TLIF placement were documented as well. Radiculopathy symptoms included shooting pain in the legs, incontinence, sexual dysfunction, and severe paralysis. Radiologic findings were specific to bone formation from the disk space (detected with CT).
Continue to: RESULTS
RESULTS
All 38 selected patients had radiculopathy symptoms from HO out of the intervertebral space. The Table lists the patients’ overall characteristics. The left side had the most radiculopathy symptoms (31/38 patients), followed by the right side (5/38) and both sides (2/38). Radiculopathy symptoms began a mean (SD) of 3.8 (1.0) months (range, 2-6 months) after index surgery. The 38 patients had 4 characteristics in common:
Table. Transforaminal Lumbar Interbody Fusion With Recombinant Human Bone Morphogenetic Protein 2: Onset Time for Radiculopathy Symptoms, Surgery Level, Side of Pseudo-Pedicle Bone Formation, and Subsequent Complications
| Pt | Sympton Onset, mo | Surgery Level(s) | Side(s) | Complication(s) |
| 1 | 3 | L3-L5 (2) | Both | Radiculopathy, pseudo-pedicle, urine |
| 2 | 3 | L4-L5 (2) | R | Radiculopathy, pseudo-pedicle |
| 3 | 4 | L5-S1 (1) | R | Radiculopathy, pseudo-pedicle |
| 4 | 5 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 5 | 4 | L4-S1 (2) | L | Radiculopathy, pseudo-pedicle, subsidence |
| 6 | 5 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 7 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 8 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 9 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 10 | 2 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 11 | 2 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle, subsidence, neurologic |
| 12 | 6 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 13 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle, neurologic |
| 14 | 2 | L2-L3 (1) | R | Radiculopathy, pseudo-pedicle |
| 15 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 16 | 3 | L4-L5 (1) | L | Radiculopathy, pseudo-pedicle |
| 17 | 3 | L2-L3, L4-L5 (2) | L | Radiculopathy, pseudo-pedicle |
| 18 | 3 | L4-L5, L2-L3 (1) | L | Radiculopathy, pseudo-pedicle, nonunion |
| 19 | 4 | L4-L5 (1) | R | Radiculopathy, pseudo-pedicle |
| 20 | 5 | L4-L5 (1) | L | Radiculopathy, pseudo-pedicle |
| 21 | 5 | L5-S1 (1) | R | Radiculopathy, pseudo-pedicle |
| 22 | 3 | L3-L4, L5-S1 (2) | Both | Radiculopathy, pseudo-pedicle |
| 23 | 4 | L4-L5 (1) | L | Radiculopathy, pseudo-pedicle |
| 24 | 6 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 25 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 26 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle, urine, bowel |
| 27 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 28 | 4 | L4-L5 (1) | L | Radiculopathy, pseudo-pedicle |
| 29 | 6 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 30 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 31 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 32 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 33 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 34 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 35 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 36 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 37 | 4 | L4-L5 (1) | L | Radiculopathy, pseudo-pedicle |
| 38 | 4 | L4-L5 (1) | L | Radiculopathy, pseudo-pedicle |
1. Bone growing out of the annulotomy site for TLIF cage placement was present and in continuity with the disk space in 33 (87%) of the 38 cases. In the other 5 cases (13%), HO was present around the neural tissue, but not necessarily in continuity with the disk space. This bone appeared ectopic and not osteophytic and facet-related, as it formed a shell around either the nerve root or the thecal sac, contouring to the structure.

2. The common, novel finding on CT was a “pseudo-pedicle” (Figures 1A, 1B), which appeared as ectopic growth from the disk space—a solid piece of bone in the same direction as the anatomical pedicle. Confusing similarity to the anatomical pedicle is present on axial cuts and during surgery. The pseudo-pedicle varied in thickness and extent out of the disk space, but was always presented as a bar of bone arising from the annulotomy site. After arising from the disk space, the HO could disperse in any direction, further calcifying neural structures or the facet joints above or below. There was no apparent distinguishable repeating pattern, given the variable nature of arthritic facet changes, scoliotic deformities, size of annulotomies, amount of rhBMP used, and placement in cage and disk space or only in cage.

3. In 36 (95%) of the 38 cases, the initial interpretation of HO on magnetic resonance imaging (MRI) was of tissue other than bone, such as fibrous tissue, granulation tissue, recurrent disk herniation, or postoperative changes. However, this tissue was later determined to be bone from HO complications, which we confirmed with CT in all 38 cases. It is important to note that HO on MRI (Figures 2A, 2B) was initially interpreted by a radiologist as fibrous tissue, but same-level CT of the same case (Figures 3A, 3B) showed clear HO.

4. The radiculopathy symptoms caused by HO were independent of the amount of rhBMP-2 used in TLIF. Of the 38 patients, 19 had 1 rhBMP-2 sponge placed in the cage, 12 had a small kit sponge (1.05 mg), 5 had 1 sponge placed in the cage and 1 sponge placed directly in the disk space before cage placement (no notation of precise size or amount of rhBMP-2), and 2 had 1 sponge placed in the cage (no notation of rhBMP-2 amount). The data showed that HO can occur with even a small amount of rhBMP-2.
Continue to: Bone formation with rhBMP-2...
Bone formation with rhBMP-2 is robust and beneficial, but HO-related complications are significant, and identifiable on assessment of radiculopathy symptoms and CT characteristics.
DISCUSSION
We identified 38 patients with a recognizable and consistent pattern of complications of off-label use of rhBMP-2 in TLIF performed at our institution between 2002 and 2015. This pattern included consistent radiculopathy symptoms with corresponding HO at the annulotomy site in continuity with bone in the disk space or ectopic bone forming a distinctive shell around the thecal sac or nerve roots, as well as showing a distinct pseudo-pedicle pattern encompassing nerve roots and the thecal sac. Our finding differs from other findings of similar complication characteristics, but with much larger variations without consistency within the patient population.19,20,22,24 Specifically, previous studies found an association between off-label rhBMP-2 use in the posterior spine and radiculopathy with and without neuroforaminal HO. However, our study found consistent radiculopathy symptoms with pseudo-pedicle-like HO complications in all its 38 patients a mean (SD) of 3.8 (1.0) months after surgery.
In this study, consistent radiculopathy symptoms with pseudo-pedicle-like HO complications were independent of the amount of rhBMP-2 used, as some complications occurred with use of small pack rhBMP-2 with TLIF. It is well understood that high doses of rhBMP-2 may be required to improve fusion rates, but to our knowledge an optimal dosing strategy for TLIF has not been reported, particularly with respect to potential complications.8,20,31-33 For anterior lumbar interbody fusion surgery, the FDA-approved use of rhBMP-2 appears to have a significantly decreased risk of neuroforaminal HO complications. This may be attributable to the protective presence of the intact posterior annulus and longitudinal ligament for this procedure.20,33 For TLIF, it has been suggested that rhBMP-2 should be placed only along the anterior annulus with a posterior strut and morselized bone allograft barricade,33 and that fibrin glue should be used to limit BMP diffusion through the annulotomy site31 to prevent this complication.
Our study results suggest that radiculopathy symptoms with pseudo-pedicle-like HO complications appear to be caused by leakage of rhBMP-2 from the disk space through the annulotomy site. This was often initially interpreted incorrectly on MRI in the first year after surgery as being fibrous or granulation tissue, or even postoperative changes that the heterotopic tissue was bone was obvious only on CT. Even then the tissue may be incorrectly identified, as the encasing nerve roots in bone are similar to the scar tissue having no compressive effect. HO may compress, but it also has an inflammatory component that the scars lack. Additionally, the HO from the disk space, caused by leakage of the BMP placed in or around the fusion cage, can create a pseudo-pedicle of varying size and extent. This was present in all 38 of our cases.
This retrospective case series had its limitations. Its clinical and radiographic findings were not blinded. Confounding variables cannot be isolated for causal relationships, if any, to the complication in a case series such as this.
Bone formation with rhBMP-2 is robust and beneficial, but HO-related complications are significant, and identifiable on assessment of radiculopathy symptoms and CT characteristics.
1. Urist MR. Bone: formation by autoinduction. Science. 1965;150(3698):893-899.
2. Burkus JK, Gornet MF, Schuler TC, Kleeman TJ, Zdeblick TA. Six-year outcomes of anterior lumbar interbody arthrodesis with use of interbody fusion cages and recombinant human bone morphogenetic protein-2. J Bone Joint Surg Am. 2009;91(5):1181-1189.
3. Boden SD, Kang J, Sandhu H, Heller JG. Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans: a prospective, randomized clinical pilot trial: 2002 Volvo award in clinical studies. Spine. 2002;27(23):2662-2673.
4. Boden SD, Zdeblick TA, Sandhu HS, Heim SE. The use of rhBMP-2 in interbody fusion cages. Definitive evidence of osteoinduction in humans: a preliminary report. Spine. 2000;25(3):376-381.
5. Haid RW Jr, Branch CL Jr, Alexander JT, Burkus JK. Posterior lumbar interbody fusion using recombinant human bone morphogenetic protein type 2 with cylindrical interbody cages. Spine J. 2004;4(5):527-538.
6. Meisel HJ, Schnöring M, Hohaus C, et al. Posterior lumbar interbody fusion using rhBMP-2. Eur Spine J. 2008;17(12):1735-1744.
7. Mummaneni PV, Pan J, Haid RW, Rodts GE. Contribution of recombinant human bone morphogenetic protein-2 to the rapid creation of interbody fusion when used in transforaminal lumbar interbody fusion: a preliminary report. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2004. J Neurosurg Spine. 2004;1(1):19-23.
8. Shimer AL, Oner FC, Vaccaro AR. Spinal reconstruction and bone morphogenetic proteins: open questions. Injury. 2009;40(suppl 3):S32-S38.
9. Slosar PJ, Josey R, Reynolds J. Accelerating lumbar fusions by combining rhBMP-2 with allograft bone: a prospective analysis of interbody fusion rates and clinical outcomes. Spine J. 2007;7(3):301-307.
10. Knox JB, Dai JM 3rd, Orchowski J. Osteolysis in transforaminal lumbar interbody fusion with bone morphogenetic protein-2. Spine. 2011;36(8):672-676.
11. Owens K, Glassman SD, Howard JM, Djurasovic M, Witten JL, Carreon LY. Perioperative complications with rhBMP-2 in transforaminal lumbar interbody fusion. Eur Spine J. 2011;20(4):612-617.
12. Mindea SA, Shih P, Song JK. Recombinant human bone morphogenetic protein-2-induced radiculitis in elective minimally invasive transforaminal lumbar interbody fusions: a series review. Spine. 2009;34(14):1480-1484.
13. Yoon ST, Park JS, Kim KS, et al. ISSLS prize winner: LMP-1 upregulates intervertebral disc cell production of proteoglycans and BMPs in vitro and in vivo. Spine. 2004;29(23):2603-2611.
14. Cahill KS, Chi JH, Day A, Claus EB. Prevalence, complications, and hospital charges associated with use of bone-morphogenetic proteins in spinal fusion procedures. JAMA. 2009;302(1):58-66.
15. Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 2011;11(6):471-491.
16. Chen NF, Smith ZA, Stiner E, Armin S, Sheikh H, Khoo LT. Symptomatic ectopic bone formation after off-label use of recombinant human bone morphogenetic protein-2 in transforaminal lumbar interbody fusion. J Neurosurg Spine. 2010;12(1):40-46.
17. Joseph V, Rampersaud YR. Heterotopic bone formation with the use of rhBMP2 in posterior minimal access interbody fusion: a CT analysis. Spine. 2007;32(25):2885-2890.
18. McClellan JW, Mulconrey DS, Forbes RJ, Fullmer N. Vertebral bone resorption after transforaminal lumbar interbody fusion with bone morphogenetic protein (rhBMP-2). J Spinal Disord Tech. 2006;19(7):483-486.
19. Mroz TE, Wang JC, Hashimoto R, Norvell DC. Complications related to osteobiologics use in spine surgery: a systematic review. Spine. 2010;35(9 suppl):S86-S104.
20. Muchow RD, Hsu WK, Anderson PA. Histopathologic inflammatory response induced by recombinant bone morphogenetic protein-2 causing radiculopathy after transforaminal lumbar interbody fusion. Spine J. 2010;10(9):e1-e6.
21. Ong KL, Villarraga ML, Lau E, Carreon LY, Kurtz SM, Glassman SD. Off-label use of bone morphogenetic proteins in the United States using administrative data. Spine. 2010;35(19):1794-1800.
22. Rihn JA, Patel R, Makda J, et al. Complications associated with single-level transforaminal lumbar interbody fusion. Spine J. 2009;9(8):623-629.
23. Vaidya R, Sethi A, Bartol S, Jacobson M, Coe C, Craig JG. Complications in the use of rhBMP-2 in PEEK cages for interbody spinal fusions. J Spinal Disord Tech. 2008;21(8):557-562.
24. Wong DA, Kumar A, Jatana S, Ghiselli G, Wong K. Neurologic impairment from ectopic bone in the lumbar canal: a potential complication of off-label PLIF/TLIF use of bone morphogenetic protein-2 (BMP-2). Spine J. 2008;8(6):1011-1018.
25. Delawi D, Dhert WJ, Rillardon L, et al. A prospective, randomized, controlled, multicenter study of osteogenic protein-1 in instrumented posterolateral fusions: report on safety and feasibility. Spine. 2010;35(12):1185-1191.
26. Vaccaro AR, Patel T, Fischgrund J, et al. A pilot study evaluating the safety and efficacy of OP-1 putty (rhBMP-7) as a replacement for iliac crest autograft in posterolateral lumbar arthrodesis for degenerative spondylolisthesis. Spine. 2004;29(17):1885-1892.
27. Vaidya R, Weir R, Sethi A, Meisterling S, Hakeos W, Wybo CD. Interbody fusion with allograft and rhBMP-2 leads to consistent fusion but early subsidence. J Bone Joint Surg Br. 2007;89(3):342-345.
28. Glassman SD, Howard J, Dimar J, Sweet A, Wilson G, Carreon L. Complications with recombinant human bone morphogenic protein-2 in posterolateral spine fusion: a consecutive series of 1037 cases. Spine. 2011;36(22):1849-1854.
29. Helgeson MD, Lehman RA Jr, Patzkowski JC, Dmitriev AE, Rosner MK, Mack AW. Adjacent vertebral body osteolysis with bone morphogenetic protein use in transforaminal lumbar interbody fusion. Spine J. 2011;11(6):507-510.
30. Hoffmann MF, Jones CB, Sietsema DL. Adjuncts in posterior lumbar spine fusion: comparison of complications and efficacy. Arch Orthop Trauma Surg. 2012;132(8):1105-1110.
31. Villavicencio AT, Burneikiene S, Nelson EL, Bulsara KR, Favors M, Thramann J. Safety of transforaminal lumbar interbody fusion and intervertebral recombinant human bone morphogenetic protein-2. J Neurosurg Spine. 2005;3(6):436-443.
32. Patel VV, Zhao L, Wong P, et al. Controlling bone morphogenetic protein diffusion and bone morphogenetic protein-stimulated bone growth using fibrin glue. Spine. 2006;31(11):1201-1206.
33. Zhang H, Sucato DJ, Welch RD. Recombinant human bone morphogenic protein-2-enhanced anterior spine fusion without bone encroachment into the spinal canal: a histomorphometric study in a thoracoscopically instrumented porcine model. Spine. 2005;30(5):512-518.
1. Urist MR. Bone: formation by autoinduction. Science. 1965;150(3698):893-899.
2. Burkus JK, Gornet MF, Schuler TC, Kleeman TJ, Zdeblick TA. Six-year outcomes of anterior lumbar interbody arthrodesis with use of interbody fusion cages and recombinant human bone morphogenetic protein-2. J Bone Joint Surg Am. 2009;91(5):1181-1189.
3. Boden SD, Kang J, Sandhu H, Heller JG. Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans: a prospective, randomized clinical pilot trial: 2002 Volvo award in clinical studies. Spine. 2002;27(23):2662-2673.
4. Boden SD, Zdeblick TA, Sandhu HS, Heim SE. The use of rhBMP-2 in interbody fusion cages. Definitive evidence of osteoinduction in humans: a preliminary report. Spine. 2000;25(3):376-381.
5. Haid RW Jr, Branch CL Jr, Alexander JT, Burkus JK. Posterior lumbar interbody fusion using recombinant human bone morphogenetic protein type 2 with cylindrical interbody cages. Spine J. 2004;4(5):527-538.
6. Meisel HJ, Schnöring M, Hohaus C, et al. Posterior lumbar interbody fusion using rhBMP-2. Eur Spine J. 2008;17(12):1735-1744.
7. Mummaneni PV, Pan J, Haid RW, Rodts GE. Contribution of recombinant human bone morphogenetic protein-2 to the rapid creation of interbody fusion when used in transforaminal lumbar interbody fusion: a preliminary report. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2004. J Neurosurg Spine. 2004;1(1):19-23.
8. Shimer AL, Oner FC, Vaccaro AR. Spinal reconstruction and bone morphogenetic proteins: open questions. Injury. 2009;40(suppl 3):S32-S38.
9. Slosar PJ, Josey R, Reynolds J. Accelerating lumbar fusions by combining rhBMP-2 with allograft bone: a prospective analysis of interbody fusion rates and clinical outcomes. Spine J. 2007;7(3):301-307.
10. Knox JB, Dai JM 3rd, Orchowski J. Osteolysis in transforaminal lumbar interbody fusion with bone morphogenetic protein-2. Spine. 2011;36(8):672-676.
11. Owens K, Glassman SD, Howard JM, Djurasovic M, Witten JL, Carreon LY. Perioperative complications with rhBMP-2 in transforaminal lumbar interbody fusion. Eur Spine J. 2011;20(4):612-617.
12. Mindea SA, Shih P, Song JK. Recombinant human bone morphogenetic protein-2-induced radiculitis in elective minimally invasive transforaminal lumbar interbody fusions: a series review. Spine. 2009;34(14):1480-1484.
13. Yoon ST, Park JS, Kim KS, et al. ISSLS prize winner: LMP-1 upregulates intervertebral disc cell production of proteoglycans and BMPs in vitro and in vivo. Spine. 2004;29(23):2603-2611.
14. Cahill KS, Chi JH, Day A, Claus EB. Prevalence, complications, and hospital charges associated with use of bone-morphogenetic proteins in spinal fusion procedures. JAMA. 2009;302(1):58-66.
15. Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 2011;11(6):471-491.
16. Chen NF, Smith ZA, Stiner E, Armin S, Sheikh H, Khoo LT. Symptomatic ectopic bone formation after off-label use of recombinant human bone morphogenetic protein-2 in transforaminal lumbar interbody fusion. J Neurosurg Spine. 2010;12(1):40-46.
17. Joseph V, Rampersaud YR. Heterotopic bone formation with the use of rhBMP2 in posterior minimal access interbody fusion: a CT analysis. Spine. 2007;32(25):2885-2890.
18. McClellan JW, Mulconrey DS, Forbes RJ, Fullmer N. Vertebral bone resorption after transforaminal lumbar interbody fusion with bone morphogenetic protein (rhBMP-2). J Spinal Disord Tech. 2006;19(7):483-486.
19. Mroz TE, Wang JC, Hashimoto R, Norvell DC. Complications related to osteobiologics use in spine surgery: a systematic review. Spine. 2010;35(9 suppl):S86-S104.
20. Muchow RD, Hsu WK, Anderson PA. Histopathologic inflammatory response induced by recombinant bone morphogenetic protein-2 causing radiculopathy after transforaminal lumbar interbody fusion. Spine J. 2010;10(9):e1-e6.
21. Ong KL, Villarraga ML, Lau E, Carreon LY, Kurtz SM, Glassman SD. Off-label use of bone morphogenetic proteins in the United States using administrative data. Spine. 2010;35(19):1794-1800.
22. Rihn JA, Patel R, Makda J, et al. Complications associated with single-level transforaminal lumbar interbody fusion. Spine J. 2009;9(8):623-629.
23. Vaidya R, Sethi A, Bartol S, Jacobson M, Coe C, Craig JG. Complications in the use of rhBMP-2 in PEEK cages for interbody spinal fusions. J Spinal Disord Tech. 2008;21(8):557-562.
24. Wong DA, Kumar A, Jatana S, Ghiselli G, Wong K. Neurologic impairment from ectopic bone in the lumbar canal: a potential complication of off-label PLIF/TLIF use of bone morphogenetic protein-2 (BMP-2). Spine J. 2008;8(6):1011-1018.
25. Delawi D, Dhert WJ, Rillardon L, et al. A prospective, randomized, controlled, multicenter study of osteogenic protein-1 in instrumented posterolateral fusions: report on safety and feasibility. Spine. 2010;35(12):1185-1191.
26. Vaccaro AR, Patel T, Fischgrund J, et al. A pilot study evaluating the safety and efficacy of OP-1 putty (rhBMP-7) as a replacement for iliac crest autograft in posterolateral lumbar arthrodesis for degenerative spondylolisthesis. Spine. 2004;29(17):1885-1892.
27. Vaidya R, Weir R, Sethi A, Meisterling S, Hakeos W, Wybo CD. Interbody fusion with allograft and rhBMP-2 leads to consistent fusion but early subsidence. J Bone Joint Surg Br. 2007;89(3):342-345.
28. Glassman SD, Howard J, Dimar J, Sweet A, Wilson G, Carreon L. Complications with recombinant human bone morphogenic protein-2 in posterolateral spine fusion: a consecutive series of 1037 cases. Spine. 2011;36(22):1849-1854.
29. Helgeson MD, Lehman RA Jr, Patzkowski JC, Dmitriev AE, Rosner MK, Mack AW. Adjacent vertebral body osteolysis with bone morphogenetic protein use in transforaminal lumbar interbody fusion. Spine J. 2011;11(6):507-510.
30. Hoffmann MF, Jones CB, Sietsema DL. Adjuncts in posterior lumbar spine fusion: comparison of complications and efficacy. Arch Orthop Trauma Surg. 2012;132(8):1105-1110.
31. Villavicencio AT, Burneikiene S, Nelson EL, Bulsara KR, Favors M, Thramann J. Safety of transforaminal lumbar interbody fusion and intervertebral recombinant human bone morphogenetic protein-2. J Neurosurg Spine. 2005;3(6):436-443.
32. Patel VV, Zhao L, Wong P, et al. Controlling bone morphogenetic protein diffusion and bone morphogenetic protein-stimulated bone growth using fibrin glue. Spine. 2006;31(11):1201-1206.
33. Zhang H, Sucato DJ, Welch RD. Recombinant human bone morphogenic protein-2-enhanced anterior spine fusion without bone encroachment into the spinal canal: a histomorphometric study in a thoracoscopically instrumented porcine model. Spine. 2005;30(5):512-518.
TAKE-HOME POINTS
- Use of rhBMP-2 in TLIF cages can result in HO out of the cage into the spinal canal.
- HO from rhBMP-2 in TLIF cages can result in a radiculopathy from compression or inflammatory reaction.
- HO out of the cage into the spinal canal resulting from use of rhBMP-2 in TLIF cages can be adequately diagnosed only with CT.
- HO can appear as a pedicle or pseudo-pedicle.
- Consider potential HO when using rhBMP-2 in TLIF cages.
Arthroscopic Anterior Ankle Decompression Is Successful in National Football League Players
ABSTRACT
Anterior ankle impingement is a frequent cause of pain and disability in athletes with impingement of soft-tissue or osseous structures along the anterior margin of the tibiotalar joint during dorsiflexion.
In this study, we hypothesized that arthroscopic decompression of anterior ankle impingement would result in significant, reliable, and durable improvement in pain and range of motion (ROM), and would allow National Football League (NFL) players to return to their preoperative level of play.
We reviewed 29 arthroscopic ankle débridements performed by a single surgeon. Each NFL player underwent arthroscopic débridement of pathologic soft tissue and of tibial and talar osteophytes in the anterior ankle. Preoperative and postoperative visual analog scale (VAS) pain scores, American Orthopaedic Foot and Ankle Society (AOFAS) hindfoot scores, and ankle ROM were compared; time to return to play (RTP), events missed secondary to surgery, and complications were recorded.
All athletes returned to the same level of NFL play at a mean (SD) of 8.4 (4.1) weeks after surgery and continued playing for a mean (SD) of 3.43 (2.57) years after surgery. Mean (SD) VAS pain scores decreased significantly (P < .001), to 0.38 (0.89) from 4.21 (1.52). Mean (SD) active ankle dorsiflexion increased significantly (P < .001), to 18.86° (2.62°) from 8.28° (4.14°). Mean (SD) AOFAS hindfoot scores increased significantly (P < .001), to 97.45 (4.72) from 70.62 (10.39). Degree of arthritis (r = 0.305) and age (r = 0.106) were poorly correlated to time to RTP.
In all cases, arthroscopic débridement of anterior ankle impingement resulted in RTP at the same level at a mean of 2 months after surgery. There were significant improvements in VAS pain scores, AOFAS hindfoot scores, and ROM.
Arthroscopic débridement of anterior ankle impingement relieves pain, restores ROM and function, and results in reliable RTP in professional football players.
Continue to: Anterior ankle impingement...
Anterior ankle impingement is a frequent cause of disability in athletes.1 This condition results from repetitive trauma over time, which leads to osseous and soft-tissue impingement, pain, and decreased ankle range of motion (ROM).
First termed footballer’s ankle, this condition is linked to repeated, forceful plantarflexion,2 though later studies attributed the phenomenon to repeated dorsiflexion resulting in periosteal hemorrhage.3 Both osseous and soft-tissue structures can cause impingement at the tibiotalar joint, often with osteophytes anteromedially at the tibial talar joint. Soft-tissue structures, including hypertrophic synovium, meniscoid lesions, and a thickened anterior talofibular ligament, more often cause anterolateral impingement.4-6 This process results in pain in extreme dorsiflexion, which comes into play in almost all football maneuvers, including sprinting, back-peddling, and offensive and defensive stances. Therefore, maintenance of pain-free dorsiflexion is required for high-level football. Decreased ROM can lead to decreased ability to perform these high-level athletic functions and can limit performance.
Arthroscopic débridement improves functional outcomes and functional motion in both athletes and nonathletes.7,8 In addition, findings of a recent systematic review provide support for arthroscopic treatment of ankle impingement.9 Although arthroscopic treatment is effective in nonathletes and recreational athletes,10 there is a paucity of data on the efficacy of this procedure and on time to return to play (RTP) in professional football players.
We conducted a study to evaluate the outcomes (pain, ROM, RTP) of arthroscopic débridement for anterior ankle impingement in National Football League (NFL) players. We hypothesized that arthroscopic decompression of anterior ankle impingement would result in significant, reliable, and durable improvement in pain and ROM, and would allow NFL players to return to their preoperative level of play.
METHODS
After this study was granted Institutional Review Board approval, we retrospectively reviewed a consecutive series of arthroscopically treated anterior ankle impingement athletes by a single surgeon (JPB). Indications for surgery were anterior ankle impingement resulting in ankle pain and decreased ROM that interfered with sport. Active NFL players who underwent ankle arthroscopy for symptomatic anterior ankle impingement were included. Excluded were players who underwent surgery after retirement or who retired before returning to play for reasons unrelated to the ankle. Medical records, operative reports, and rehabilitation reports were reviewed.
Continue to: Preoperative and postoperative...
Preoperative and postoperative visual analog scale (VAS) pain scores, American Orthopaedic Foot and Ankle Society (AOFAS) hindfoot scores, and ankle ROM were compared; time to RTP, events missed secondary to surgery, and complications were recorded. These preoperative and postoperative variables were compared with paired Student 2-way t tests for continuous variables. Pearson correlation coefficients were calculated.
PROCEDURE
Ankle arthroscopy was performed with the patient supine after spinal or general anesthesia was induced. Prophylactic antibiotics were given in each case. Arthroscopy was performed with standard anterolateral and anteromedial portals. First, an incision was made through skin only, followed by blunt subcutaneous dissection down to the ankle capsule. A capsulotomy was then made bluntly. Care was taken to avoid all neurovascular structures. Posterior portals were not used. A 2.7-mm arthroscope was inserted and alternated between the anteromedial and anterolateral portals to maximally visualize the ankle joint. Diagnostic arthroscopy was performed to document synovitis, chondral injury, osseous, and soft-tissue impingement and any other noted pathology (Figures 1A-1C).
A full radius resector was then used to perform a synovectomy and débridement of impinging soft tissue from the anterior talofibular ligament or anterior inferior talofibular ligament. All patients underwent arthroscopic débridement of pathologic soft tissue and of tibial and talar osteophytes in the anterior ankle. A small burr was used to débride and remove the osteophytes on the talus and/or tibia. Soft-tissue and osseous structures were resected until the contours of the talus and tibia were normal. Any unstable articular defects were débrided and loose bodies were removed. Ankle ROM was checked to confirm complete resolution of impingement (Figures 2A-2D). Patients were not immobilized and were allowed progressive weight-bearing as tolerated. Crutches were used for assisted ambulation the first 3 to 5 postoperative days.
Physical therapy progressed through 3 phases: (1) inflammation control and ROM restoration, (2) initiation of ankle strengthening, including eversion and inversion, and (3) agility, proprioception, and functional rehabilitation.
RESULTS
Twenty-five NFL players (29 surgeries) were included in the study. Two players were excluded because they had retired at the end of the season before the surgery for reasons unrelated to the operative ankle. Mean (SD) age was 28.1 (2.9) years. Six included players had a history of ankle sprains, 1 had a history of ipsilateral ankle fracture, and 1 had a history of ipsilateral ankle dislocation. Table 1 lists the positions of players who underwent ankle arthroscopic decompression.
Table 1. Positions of National Football League Players Who Underwent Ankle Arthroscopic Decompression for Anterior Ankle Impingement
Position | Surgeries, n |
| Offensive line | 8 |
| Defensive line | 8 |
| Wide receiver | 4 |
| Running back | 4 |
| Linebacker | 3 |
| Quarterback | 1 |
| Defensive back | 1 |
Continue to: During diagnostic arthroscopy...
During diagnostic arthroscopy, changes to the articular cartilage were noted: grade 0 in 38% of patients, grade 1 in 17%, grade 2 in 21%, grade 3 in 21%, and grade 4 in 3%. Four patients had an osteochondral lesion (<1 cm in each case), which was treated with chondroplasty without microfracture.
Each included patient returned to NFL play. Mean (SD) time to RTP without restrictions was 8.4 (4.1) weeks after surgery (range, 2-20 weeks). There was a poor correlation between degree of chondrosis and time to RTP (r = 0.305). In addition, there was a poor correlation between age and time to RTP (r = 0.106).
Dorsiflexion improved significantly (P < .001), patients had significantly less pain after surgery (P < .001), and AOFAS hindfoot scores improved significantly (P < .001) (Table 2).
Table 2. Preoperative and Postoperative Dorsiflexion, Pain, and AOFAS Score Before and After Arthroscopic Débridement of Anterior Ankle Impingementa
| Mean (SD) | ||
|---|---|---|
| Preoperative | Postoperative | |
| Dorsiflexion | 8.28º (4.14º) | 18.86° (2.62°) |
| VAS pain score | 4.21 (1.52) | 0.38 (0.89) |
| AOFAS score | 70.62 (10.39) | 97.45 (4.72) |
aAll values were significantly improved after surgery (P < .001).
Abbreviations: AOFAS, American Orthopaedic Foot and Ankle Society; VAS, visual analog scale.
The athletes played in the NFL for a mean (SD) of 3.43 (2.57) years after surgery (range, 1-10 seasons). These players included 6 who were still active at time of publication. No patient required revision surgery or additional surgery on the ipsilateral ankle. The one patient who was treated for superficial thrombophlebitis after surgery reported symptoms before surgery as well.
DISCUSSION
Arthroscopic decompression of anterior ankle impingement is safe and significantly improves pain and ROM in professional American football players. The procedure results in reliable RTP at an elite level, with durable results over the time remaining in their NFL careers.
Continue to: before the 1988 study by Hawkins...
Before the 1988 study by Hawkins,11 ankle spurs were removed with open procedures. Hawkins11 used arthroscopy for better and safer visualization of the ankle joint and used a burr for less painful removal of spurs from the tibia and the talus. In 2002, a series of 105 patients (median age, 35 years) had reduced pain and improved function a minimum of 2 years after arthroscopic débridement.12 These patients had a mix of pathology, including soft-tissue impingement, bony impingement, chondral lesions, loose bodies, and osteoarthritis.
For many elite athletes, anterior ankle impingement can cause significant limitation. Reduced ankle dorsiflexion can alter all limb mechanics and predispose athletes to injury.13 In addition, because NFL players’ ankle ROM often approaches or exceeds normal physiologic limits,14 an ankle ROM limitation will often hinder their performance.
Miyamoto and colleagues15 studied a series of 9 professional athletes (6 soccer players, 1 baseball pitcher, 1 mixed martial artist, 1 golfer) who underwent decompression of both anterior and posterior impingement. With regard to anterior impingement, they found anterior osteophytes in all the ankles, as was seen in the present study. Furthermore, they noted that mean dorsiflexion improved from 10° before surgery to 15° after surgery and that their athletes returned to play 12 to 15 weeks after surgery. Their results are similar to ours, though we noted more improvement in dorsiflexion, from 8.28° before surgery to 18.86° after surgery.
One of the most important metrics in evaluating treatment options for professional athletes is time from surgery to RTP without restrictions. Mean time to full RTP was shorter in our study (8.4 weeks) than in the study by Miyamoto and colleagues15 (up to 20 weeks). However, many of their procedures were performed during the off-season, when there was no need to expeditiously clear patients for full sports participation. In addition, the patients in their study had both anterior and posterior pathology.
Faster return to high-level athletics was supported in a study of 11 elite ballet dancers,16 whose pain and dance performance improved after arthroscopic débridement. Of the 11 patients, 9 returned to dance at a mean of 7 weeks after surgery; the other 2 required reoperation. Although the pathology differed in their study of elite professional soccer players, Calder and colleagues17 found that mean time to RTP after ankle arthroscopy for posterior impingement was 5 weeks.
Continue to: For the NFL players in our study...
For the NFL players in our study, RTP at their elite level was 100% after arthroscopic débridement of anterior ankle impingement. In the literature, time to RTP varies. Table 3 lists RTP rates for recreational athletes in published studies.18-27 In their recent systematic literature review, Zwiers and colleagues10 noted that 24% to 96.4% of recreational athletes returned to play after arthroscopic treatment for anterior ankle impingement. The percentage was significantly higher for the professional athletes in our study. Historical comparison supports an evolution in the indications and techniques for this procedure, with more recent literature suggesting a RTP rate much higher than earlier rates. In addition, compared with recreational athletes, professional athletes have strong financial incentives to return to their sports. Furthermore, our professional cohort was significantly younger than the recreational cohorts in those studies.
Table 3. Frequency of Recreational Athletes’ Return to Play After Arthroscopic Débridement of Anterior Ankle Impingement, as Reported in the Literature
| Study | Year | Journal | Return to Play | |
|---|---|---|---|---|
| n/N | % | |||
| Akseki et al18 | 1999 | Acta Orthop Scand | 10/11 | 91 |
| Baums et al19 | 2006 | Knee Surg Sports Traumatol Arthrosc | 25/26 | 96 |
| Branca et al20 | 1997 | Foot Ankle Int | 13/27 | 48 |
| Di Palma et al21 | 1999 | J Sports Traumatol Relat Res | 21/32 | 66 |
| Ferkel et al22 | 1991 | Am J Sports Med | 27/31 | 87.1 |
| Hassan23 | 2007 | Knee Surg Sports Traumatol Arthrosc | 9/11 | 82 |
| Jerosch et al24 | 1994 | Knee Surg Sports Traumatol Arthrosc | 9/38 | 24 |
| Murawski & Kennedy25 | 2010 | Am J Sports Med | 27/28 | 96.4 |
| Ogilvie-Harris et al26 | 1993 | J Bone Joint Surg Br | 21/28 | 75 |
| Rouvillain et al27 | 2014 | Eur J Orthop Surg Traumatol | 10/11 | 90 |
Total | 172/243 | 70 | ||
Current recommendations for recreational athletes include initial conservative treatment with rest, ankle bracing, and avoidance of jumping and other repetitive dorsiflexing activities. Physical therapy should include joint mobilization and work along the entire kinetic chain. Night splints or a removable walking boot can be used temporarily, as can a single intra-articular corticosteroid injection to reduce inflammation and evaluate improvement in more refractory cases.28 Commonly, conservative treatments fail if patients remain active, and soft tissue and/or osteophytes can be resected, though resection typically is reserved for recreational athletes for whom nonoperative treatments have been exhausted.29,30
This study had several limitations, including its retrospective nature and lack of control group. In addition, follow-up was relatively short, and we did not use more recently described outcome measures, such as the Sports subscale of the Foot and Ankle Ability Measure, which may be more sensitive in describing function in elite athletes. However, many of the cases in our study predated these measures, but the rate of RTP at the NFL level requires a very high degree of postoperative ankle function, making this outcome the most meaningful. In the context of professional athletes, specifically the length of their careers, our study results provide valuable information regarding expectations about RTP and the durability of arthroscopic débridement of anterior ankle impingement in a high-demand setting.
CONCLUSION
For all the NFL players in this study, arthroscopic débridement of anterior ankle impingement resulted in return to preoperative level of play at a mean of 2 months after surgery. There were significant improvements in VAS pain scores, AOFAS hindfoot scores, and ROM. Arthroscopic débridement of anterior ankle impingement relieves pain, restores ROM and function, and results in reliable RTP in professional football players.
1. Lubowitz JH. Editorial commentary: ankle anterior impingement is common in athletes and could be under-recognized. Arthroscopy. 2015;31(8):1597.
2. Mcdougall A. Footballer’s ankle. Lancet. 1955;269(6902):1219-1220.
3. Kleiger B. Anterior tibiotalar impingement syndromes in dancers. Foot Ankle. 1982;3(2):69-73.
4. Bassett FH 3rd, Gates HS 3rd, Billys JB, Morris HB, Nikolaou PK. Talar impingement by the anteroinferior tibiofibular ligament. A cause of chronic pain in the ankle after inversion sprain. J Bone Joint Surg Am. 1990;72(1):55-59.
5. Liu SH, Raskin A, Osti L, et al. Arthroscopic treatment of anterolateral ankle impingement. Arthroscopy. 1994;10(2):215-218.
6. Thein R, Eichenblat M. Arthroscopic treatment of sports-related synovitis of the ankle. Am J Sports Med. 1992;20(5):496-498.
7. Arnold H. Posttraumatic impingement syndrome of the ankle—indication and results of arthroscopic therapy. Foot Ankle Surg. 2011;17(2):85-88.
8. Walsh SJ, Twaddle BC, Rosenfeldt MP, Boyle MJ. Arthroscopic treatment of anterior ankle impingement: a prospective study of 46 patients with 5-year follow-up. Am J Sports Med. 2014;42(11):2722-2726.
9. Glazebrook MA, Ganapathy V, Bridge MA, Stone JW, Allard JP. Evidence-based indications for ankle arthroscopy. Arthroscopy. 2009;25(12):1478-1490.
10. Zwiers R, Wiegerinck JI, Murawski CD, Fraser EJ, Kennedy JG, van Dijk CN. Arthroscopic treatment for anterior ankle impingement: a systematic review of the current literature. Arthroscopy. 2015;31(8):1585-1596.
11. Hawkins RB. Arthroscopic treatment of sports-related anterior osteophytes in the ankle. Foot Ankle. 1988;9(2):87-90.
12. Rasmussen S, Hjorth Jensen C. Arthroscopic treatment of impingement of the ankle reduces pain and enhances function. Scand J Med Sci Sports. 2002;12(2):69-72.
13. Mason-Mackay AR, Whatman C, Reid D. The effect of reduced ankle dorsiflexion on lower extremity mechanics during landing: a systematic review. J Sci Med Sport. 2017;20(5):451-458.
14. Riley PO, Kent RW, Dierks TA, Lievers WB, Frimenko RE, Crandall JR. Foot kinematics and loading of professional athletes in American football-specific tasks. Gait Posture. 2013;38(4):563-569.
15. Miyamoto W, Takao M, Matsui K, Matsushita T. Simultaneous ankle arthroscopy and hindfoot endoscopy for combined anterior and posterior ankle impingement syndrome in professional athletes. J Orthop Sci. 2015;20(4):642-648.
16. Nihal A, Rose DJ, Trepman E. Arthroscopic treatment of anterior ankle impingement syndrome in dancers. Foot Ankle Int. 2005;26(11):908-912.
17. Calder JD, Sexton SA, Pearce CJ. Return to training and playing after posterior ankle arthroscopy for posterior impingement in elite professional soccer. Am J Sports Med. 2010;38(1):120-124.
18. Akseki D, Pinar H, Bozkurt M, Yaldiz K, Arag S. The distal fascicle of the anterior inferior tibiofibular ligament as a cause of anterolateral ankle impingement: results of arthroscopic resection. Acta Orthop Scand. 1999;70(5):478-482.
19. Baums MH, Kahl E, Schultz W, Klinger HM. Clinical outcome of the arthroscopic management of sports-related “anterior ankle pain”: a prospective study. Knee Surg Sports Traumatol Arthrosc. 2006;14(5):482-486.
20. Branca A, Di Palma L, Bucca C, Visconti CS, Di Mille M. Arthroscopic treatment of anterior ankle impingement. Foot Ankle Int. 1997;18(7):418-423.
21. Di Palma L, Bucca C, Di Mille M, Branca A. Diagnosis and arthroscopic treatment of fibrous impingement of the ankle. J Sports Traumatol Relat Res. 1999;21:170-177.
22. Ferkel RD, Karzel RP, Del Pizzo W, Friedman MJ, Fischer SP. Arthroscopic treatment of anterolateral impingement of the ankle. Am J Sports Med. 1991;19(5):440-446.
23. Hassan AH. Treatment of anterolateral impingements of the ankle joint by arthroscopy. Knee Surg Sports Traumatol Arthrosc. 2007;15(9):1150-1154.
24. Jerosch J, Steinbeck J, Schröder M, Halm H. Arthroscopic treatment of anterior synovitis of the ankle in athletes. Knee Surg Sports Traumatol Arthrosc. 1994;2(3):176-181.
25. Murawski CD, Kennedy JG. Anteromedial impingement in the ankle joint: outcomes following arthroscopy. Am J Sports Med. 2010;38(10):2017-2024.
26. Ogilvie-Harris DJ, Mahomed N, Demazière A. Anterior impingement of the ankle treated by arthroscopic removal of bony spurs. J Bone Joint Surg Br. 1993;75(3):437-440.
27. Rouvillain JL, Daoud W, Donica A, Garron E, Uzel AP. Distraction-free ankle arthroscopy for anterolateral impingement. Eur J Orthop Surg Traumatol. 2014;24(6):1019-1023.
28. O’Kane JW, Kadel N. Anterior impingement syndrome in dancers. Curr Rev Musculoskelet Med. 2008;1(1):12-16.
29. Lavery KP, McHale KJ, Rossy WH, Theodore G. Ankle impingement. J Orthop Surg Res. 2016;11(1):97.
30. Talusan PG, Toy J, Perez JL, Milewski MD, Reach JS. Anterior ankle impingement: diagnosis and treatment. J Am Acad Orthop Surg. 2014;22(5):333-339.
ABSTRACT
Anterior ankle impingement is a frequent cause of pain and disability in athletes with impingement of soft-tissue or osseous structures along the anterior margin of the tibiotalar joint during dorsiflexion.
In this study, we hypothesized that arthroscopic decompression of anterior ankle impingement would result in significant, reliable, and durable improvement in pain and range of motion (ROM), and would allow National Football League (NFL) players to return to their preoperative level of play.
We reviewed 29 arthroscopic ankle débridements performed by a single surgeon. Each NFL player underwent arthroscopic débridement of pathologic soft tissue and of tibial and talar osteophytes in the anterior ankle. Preoperative and postoperative visual analog scale (VAS) pain scores, American Orthopaedic Foot and Ankle Society (AOFAS) hindfoot scores, and ankle ROM were compared; time to return to play (RTP), events missed secondary to surgery, and complications were recorded.
All athletes returned to the same level of NFL play at a mean (SD) of 8.4 (4.1) weeks after surgery and continued playing for a mean (SD) of 3.43 (2.57) years after surgery. Mean (SD) VAS pain scores decreased significantly (P < .001), to 0.38 (0.89) from 4.21 (1.52). Mean (SD) active ankle dorsiflexion increased significantly (P < .001), to 18.86° (2.62°) from 8.28° (4.14°). Mean (SD) AOFAS hindfoot scores increased significantly (P < .001), to 97.45 (4.72) from 70.62 (10.39). Degree of arthritis (r = 0.305) and age (r = 0.106) were poorly correlated to time to RTP.
In all cases, arthroscopic débridement of anterior ankle impingement resulted in RTP at the same level at a mean of 2 months after surgery. There were significant improvements in VAS pain scores, AOFAS hindfoot scores, and ROM.
Arthroscopic débridement of anterior ankle impingement relieves pain, restores ROM and function, and results in reliable RTP in professional football players.
Continue to: Anterior ankle impingement...
Anterior ankle impingement is a frequent cause of disability in athletes.1 This condition results from repetitive trauma over time, which leads to osseous and soft-tissue impingement, pain, and decreased ankle range of motion (ROM).
First termed footballer’s ankle, this condition is linked to repeated, forceful plantarflexion,2 though later studies attributed the phenomenon to repeated dorsiflexion resulting in periosteal hemorrhage.3 Both osseous and soft-tissue structures can cause impingement at the tibiotalar joint, often with osteophytes anteromedially at the tibial talar joint. Soft-tissue structures, including hypertrophic synovium, meniscoid lesions, and a thickened anterior talofibular ligament, more often cause anterolateral impingement.4-6 This process results in pain in extreme dorsiflexion, which comes into play in almost all football maneuvers, including sprinting, back-peddling, and offensive and defensive stances. Therefore, maintenance of pain-free dorsiflexion is required for high-level football. Decreased ROM can lead to decreased ability to perform these high-level athletic functions and can limit performance.
Arthroscopic débridement improves functional outcomes and functional motion in both athletes and nonathletes.7,8 In addition, findings of a recent systematic review provide support for arthroscopic treatment of ankle impingement.9 Although arthroscopic treatment is effective in nonathletes and recreational athletes,10 there is a paucity of data on the efficacy of this procedure and on time to return to play (RTP) in professional football players.
We conducted a study to evaluate the outcomes (pain, ROM, RTP) of arthroscopic débridement for anterior ankle impingement in National Football League (NFL) players. We hypothesized that arthroscopic decompression of anterior ankle impingement would result in significant, reliable, and durable improvement in pain and ROM, and would allow NFL players to return to their preoperative level of play.
METHODS
After this study was granted Institutional Review Board approval, we retrospectively reviewed a consecutive series of arthroscopically treated anterior ankle impingement athletes by a single surgeon (JPB). Indications for surgery were anterior ankle impingement resulting in ankle pain and decreased ROM that interfered with sport. Active NFL players who underwent ankle arthroscopy for symptomatic anterior ankle impingement were included. Excluded were players who underwent surgery after retirement or who retired before returning to play for reasons unrelated to the ankle. Medical records, operative reports, and rehabilitation reports were reviewed.
Continue to: Preoperative and postoperative...
Preoperative and postoperative visual analog scale (VAS) pain scores, American Orthopaedic Foot and Ankle Society (AOFAS) hindfoot scores, and ankle ROM were compared; time to RTP, events missed secondary to surgery, and complications were recorded. These preoperative and postoperative variables were compared with paired Student 2-way t tests for continuous variables. Pearson correlation coefficients were calculated.
PROCEDURE
Ankle arthroscopy was performed with the patient supine after spinal or general anesthesia was induced. Prophylactic antibiotics were given in each case. Arthroscopy was performed with standard anterolateral and anteromedial portals. First, an incision was made through skin only, followed by blunt subcutaneous dissection down to the ankle capsule. A capsulotomy was then made bluntly. Care was taken to avoid all neurovascular structures. Posterior portals were not used. A 2.7-mm arthroscope was inserted and alternated between the anteromedial and anterolateral portals to maximally visualize the ankle joint. Diagnostic arthroscopy was performed to document synovitis, chondral injury, osseous, and soft-tissue impingement and any other noted pathology (Figures 1A-1C).

A full radius resector was then used to perform a synovectomy and débridement of impinging soft tissue from the anterior talofibular ligament or anterior inferior talofibular ligament. All patients underwent arthroscopic débridement of pathologic soft tissue and of tibial and talar osteophytes in the anterior ankle. A small burr was used to débride and remove the osteophytes on the talus and/or tibia. Soft-tissue and osseous structures were resected until the contours of the talus and tibia were normal. Any unstable articular defects were débrided and loose bodies were removed. Ankle ROM was checked to confirm complete resolution of impingement (Figures 2A-2D). Patients were not immobilized and were allowed progressive weight-bearing as tolerated. Crutches were used for assisted ambulation the first 3 to 5 postoperative days.

Physical therapy progressed through 3 phases: (1) inflammation control and ROM restoration, (2) initiation of ankle strengthening, including eversion and inversion, and (3) agility, proprioception, and functional rehabilitation.
RESULTS
Twenty-five NFL players (29 surgeries) were included in the study. Two players were excluded because they had retired at the end of the season before the surgery for reasons unrelated to the operative ankle. Mean (SD) age was 28.1 (2.9) years. Six included players had a history of ankle sprains, 1 had a history of ipsilateral ankle fracture, and 1 had a history of ipsilateral ankle dislocation. Table 1 lists the positions of players who underwent ankle arthroscopic decompression.
Table 1. Positions of National Football League Players Who Underwent Ankle Arthroscopic Decompression for Anterior Ankle Impingement
Position | Surgeries, n |
| Offensive line | 8 |
| Defensive line | 8 |
| Wide receiver | 4 |
| Running back | 4 |
| Linebacker | 3 |
| Quarterback | 1 |
| Defensive back | 1 |
Continue to: During diagnostic arthroscopy...
During diagnostic arthroscopy, changes to the articular cartilage were noted: grade 0 in 38% of patients, grade 1 in 17%, grade 2 in 21%, grade 3 in 21%, and grade 4 in 3%. Four patients had an osteochondral lesion (<1 cm in each case), which was treated with chondroplasty without microfracture.
Each included patient returned to NFL play. Mean (SD) time to RTP without restrictions was 8.4 (4.1) weeks after surgery (range, 2-20 weeks). There was a poor correlation between degree of chondrosis and time to RTP (r = 0.305). In addition, there was a poor correlation between age and time to RTP (r = 0.106).
Dorsiflexion improved significantly (P < .001), patients had significantly less pain after surgery (P < .001), and AOFAS hindfoot scores improved significantly (P < .001) (Table 2).
Table 2. Preoperative and Postoperative Dorsiflexion, Pain, and AOFAS Score Before and After Arthroscopic Débridement of Anterior Ankle Impingementa
| Mean (SD) | ||
|---|---|---|
| Preoperative | Postoperative | |
| Dorsiflexion | 8.28º (4.14º) | 18.86° (2.62°) |
| VAS pain score | 4.21 (1.52) | 0.38 (0.89) |
| AOFAS score | 70.62 (10.39) | 97.45 (4.72) |
aAll values were significantly improved after surgery (P < .001).
Abbreviations: AOFAS, American Orthopaedic Foot and Ankle Society; VAS, visual analog scale.
The athletes played in the NFL for a mean (SD) of 3.43 (2.57) years after surgery (range, 1-10 seasons). These players included 6 who were still active at time of publication. No patient required revision surgery or additional surgery on the ipsilateral ankle. The one patient who was treated for superficial thrombophlebitis after surgery reported symptoms before surgery as well.
DISCUSSION
Arthroscopic decompression of anterior ankle impingement is safe and significantly improves pain and ROM in professional American football players. The procedure results in reliable RTP at an elite level, with durable results over the time remaining in their NFL careers.
Continue to: before the 1988 study by Hawkins...
Before the 1988 study by Hawkins,11 ankle spurs were removed with open procedures. Hawkins11 used arthroscopy for better and safer visualization of the ankle joint and used a burr for less painful removal of spurs from the tibia and the talus. In 2002, a series of 105 patients (median age, 35 years) had reduced pain and improved function a minimum of 2 years after arthroscopic débridement.12 These patients had a mix of pathology, including soft-tissue impingement, bony impingement, chondral lesions, loose bodies, and osteoarthritis.
For many elite athletes, anterior ankle impingement can cause significant limitation. Reduced ankle dorsiflexion can alter all limb mechanics and predispose athletes to injury.13 In addition, because NFL players’ ankle ROM often approaches or exceeds normal physiologic limits,14 an ankle ROM limitation will often hinder their performance.
Miyamoto and colleagues15 studied a series of 9 professional athletes (6 soccer players, 1 baseball pitcher, 1 mixed martial artist, 1 golfer) who underwent decompression of both anterior and posterior impingement. With regard to anterior impingement, they found anterior osteophytes in all the ankles, as was seen in the present study. Furthermore, they noted that mean dorsiflexion improved from 10° before surgery to 15° after surgery and that their athletes returned to play 12 to 15 weeks after surgery. Their results are similar to ours, though we noted more improvement in dorsiflexion, from 8.28° before surgery to 18.86° after surgery.
One of the most important metrics in evaluating treatment options for professional athletes is time from surgery to RTP without restrictions. Mean time to full RTP was shorter in our study (8.4 weeks) than in the study by Miyamoto and colleagues15 (up to 20 weeks). However, many of their procedures were performed during the off-season, when there was no need to expeditiously clear patients for full sports participation. In addition, the patients in their study had both anterior and posterior pathology.
Faster return to high-level athletics was supported in a study of 11 elite ballet dancers,16 whose pain and dance performance improved after arthroscopic débridement. Of the 11 patients, 9 returned to dance at a mean of 7 weeks after surgery; the other 2 required reoperation. Although the pathology differed in their study of elite professional soccer players, Calder and colleagues17 found that mean time to RTP after ankle arthroscopy for posterior impingement was 5 weeks.
Continue to: For the NFL players in our study...
For the NFL players in our study, RTP at their elite level was 100% after arthroscopic débridement of anterior ankle impingement. In the literature, time to RTP varies. Table 3 lists RTP rates for recreational athletes in published studies.18-27 In their recent systematic literature review, Zwiers and colleagues10 noted that 24% to 96.4% of recreational athletes returned to play after arthroscopic treatment for anterior ankle impingement. The percentage was significantly higher for the professional athletes in our study. Historical comparison supports an evolution in the indications and techniques for this procedure, with more recent literature suggesting a RTP rate much higher than earlier rates. In addition, compared with recreational athletes, professional athletes have strong financial incentives to return to their sports. Furthermore, our professional cohort was significantly younger than the recreational cohorts in those studies.
Table 3. Frequency of Recreational Athletes’ Return to Play After Arthroscopic Débridement of Anterior Ankle Impingement, as Reported in the Literature
| Study | Year | Journal | Return to Play | |
|---|---|---|---|---|
| n/N | % | |||
| Akseki et al18 | 1999 | Acta Orthop Scand | 10/11 | 91 |
| Baums et al19 | 2006 | Knee Surg Sports Traumatol Arthrosc | 25/26 | 96 |
| Branca et al20 | 1997 | Foot Ankle Int | 13/27 | 48 |
| Di Palma et al21 | 1999 | J Sports Traumatol Relat Res | 21/32 | 66 |
| Ferkel et al22 | 1991 | Am J Sports Med | 27/31 | 87.1 |
| Hassan23 | 2007 | Knee Surg Sports Traumatol Arthrosc | 9/11 | 82 |
| Jerosch et al24 | 1994 | Knee Surg Sports Traumatol Arthrosc | 9/38 | 24 |
| Murawski & Kennedy25 | 2010 | Am J Sports Med | 27/28 | 96.4 |
| Ogilvie-Harris et al26 | 1993 | J Bone Joint Surg Br | 21/28 | 75 |
| Rouvillain et al27 | 2014 | Eur J Orthop Surg Traumatol | 10/11 | 90 |
Total | 172/243 | 70 | ||
Current recommendations for recreational athletes include initial conservative treatment with rest, ankle bracing, and avoidance of jumping and other repetitive dorsiflexing activities. Physical therapy should include joint mobilization and work along the entire kinetic chain. Night splints or a removable walking boot can be used temporarily, as can a single intra-articular corticosteroid injection to reduce inflammation and evaluate improvement in more refractory cases.28 Commonly, conservative treatments fail if patients remain active, and soft tissue and/or osteophytes can be resected, though resection typically is reserved for recreational athletes for whom nonoperative treatments have been exhausted.29,30
This study had several limitations, including its retrospective nature and lack of control group. In addition, follow-up was relatively short, and we did not use more recently described outcome measures, such as the Sports subscale of the Foot and Ankle Ability Measure, which may be more sensitive in describing function in elite athletes. However, many of the cases in our study predated these measures, but the rate of RTP at the NFL level requires a very high degree of postoperative ankle function, making this outcome the most meaningful. In the context of professional athletes, specifically the length of their careers, our study results provide valuable information regarding expectations about RTP and the durability of arthroscopic débridement of anterior ankle impingement in a high-demand setting.
CONCLUSION
For all the NFL players in this study, arthroscopic débridement of anterior ankle impingement resulted in return to preoperative level of play at a mean of 2 months after surgery. There were significant improvements in VAS pain scores, AOFAS hindfoot scores, and ROM. Arthroscopic débridement of anterior ankle impingement relieves pain, restores ROM and function, and results in reliable RTP in professional football players.
ABSTRACT
Anterior ankle impingement is a frequent cause of pain and disability in athletes with impingement of soft-tissue or osseous structures along the anterior margin of the tibiotalar joint during dorsiflexion.
In this study, we hypothesized that arthroscopic decompression of anterior ankle impingement would result in significant, reliable, and durable improvement in pain and range of motion (ROM), and would allow National Football League (NFL) players to return to their preoperative level of play.
We reviewed 29 arthroscopic ankle débridements performed by a single surgeon. Each NFL player underwent arthroscopic débridement of pathologic soft tissue and of tibial and talar osteophytes in the anterior ankle. Preoperative and postoperative visual analog scale (VAS) pain scores, American Orthopaedic Foot and Ankle Society (AOFAS) hindfoot scores, and ankle ROM were compared; time to return to play (RTP), events missed secondary to surgery, and complications were recorded.
All athletes returned to the same level of NFL play at a mean (SD) of 8.4 (4.1) weeks after surgery and continued playing for a mean (SD) of 3.43 (2.57) years after surgery. Mean (SD) VAS pain scores decreased significantly (P < .001), to 0.38 (0.89) from 4.21 (1.52). Mean (SD) active ankle dorsiflexion increased significantly (P < .001), to 18.86° (2.62°) from 8.28° (4.14°). Mean (SD) AOFAS hindfoot scores increased significantly (P < .001), to 97.45 (4.72) from 70.62 (10.39). Degree of arthritis (r = 0.305) and age (r = 0.106) were poorly correlated to time to RTP.
In all cases, arthroscopic débridement of anterior ankle impingement resulted in RTP at the same level at a mean of 2 months after surgery. There were significant improvements in VAS pain scores, AOFAS hindfoot scores, and ROM.
Arthroscopic débridement of anterior ankle impingement relieves pain, restores ROM and function, and results in reliable RTP in professional football players.
Continue to: Anterior ankle impingement...
Anterior ankle impingement is a frequent cause of disability in athletes.1 This condition results from repetitive trauma over time, which leads to osseous and soft-tissue impingement, pain, and decreased ankle range of motion (ROM).
First termed footballer’s ankle, this condition is linked to repeated, forceful plantarflexion,2 though later studies attributed the phenomenon to repeated dorsiflexion resulting in periosteal hemorrhage.3 Both osseous and soft-tissue structures can cause impingement at the tibiotalar joint, often with osteophytes anteromedially at the tibial talar joint. Soft-tissue structures, including hypertrophic synovium, meniscoid lesions, and a thickened anterior talofibular ligament, more often cause anterolateral impingement.4-6 This process results in pain in extreme dorsiflexion, which comes into play in almost all football maneuvers, including sprinting, back-peddling, and offensive and defensive stances. Therefore, maintenance of pain-free dorsiflexion is required for high-level football. Decreased ROM can lead to decreased ability to perform these high-level athletic functions and can limit performance.
Arthroscopic débridement improves functional outcomes and functional motion in both athletes and nonathletes.7,8 In addition, findings of a recent systematic review provide support for arthroscopic treatment of ankle impingement.9 Although arthroscopic treatment is effective in nonathletes and recreational athletes,10 there is a paucity of data on the efficacy of this procedure and on time to return to play (RTP) in professional football players.
We conducted a study to evaluate the outcomes (pain, ROM, RTP) of arthroscopic débridement for anterior ankle impingement in National Football League (NFL) players. We hypothesized that arthroscopic decompression of anterior ankle impingement would result in significant, reliable, and durable improvement in pain and ROM, and would allow NFL players to return to their preoperative level of play.
METHODS
After this study was granted Institutional Review Board approval, we retrospectively reviewed a consecutive series of arthroscopically treated anterior ankle impingement athletes by a single surgeon (JPB). Indications for surgery were anterior ankle impingement resulting in ankle pain and decreased ROM that interfered with sport. Active NFL players who underwent ankle arthroscopy for symptomatic anterior ankle impingement were included. Excluded were players who underwent surgery after retirement or who retired before returning to play for reasons unrelated to the ankle. Medical records, operative reports, and rehabilitation reports were reviewed.
Continue to: Preoperative and postoperative...
Preoperative and postoperative visual analog scale (VAS) pain scores, American Orthopaedic Foot and Ankle Society (AOFAS) hindfoot scores, and ankle ROM were compared; time to RTP, events missed secondary to surgery, and complications were recorded. These preoperative and postoperative variables were compared with paired Student 2-way t tests for continuous variables. Pearson correlation coefficients were calculated.
PROCEDURE
Ankle arthroscopy was performed with the patient supine after spinal or general anesthesia was induced. Prophylactic antibiotics were given in each case. Arthroscopy was performed with standard anterolateral and anteromedial portals. First, an incision was made through skin only, followed by blunt subcutaneous dissection down to the ankle capsule. A capsulotomy was then made bluntly. Care was taken to avoid all neurovascular structures. Posterior portals were not used. A 2.7-mm arthroscope was inserted and alternated between the anteromedial and anterolateral portals to maximally visualize the ankle joint. Diagnostic arthroscopy was performed to document synovitis, chondral injury, osseous, and soft-tissue impingement and any other noted pathology (Figures 1A-1C).

A full radius resector was then used to perform a synovectomy and débridement of impinging soft tissue from the anterior talofibular ligament or anterior inferior talofibular ligament. All patients underwent arthroscopic débridement of pathologic soft tissue and of tibial and talar osteophytes in the anterior ankle. A small burr was used to débride and remove the osteophytes on the talus and/or tibia. Soft-tissue and osseous structures were resected until the contours of the talus and tibia were normal. Any unstable articular defects were débrided and loose bodies were removed. Ankle ROM was checked to confirm complete resolution of impingement (Figures 2A-2D). Patients were not immobilized and were allowed progressive weight-bearing as tolerated. Crutches were used for assisted ambulation the first 3 to 5 postoperative days.

Physical therapy progressed through 3 phases: (1) inflammation control and ROM restoration, (2) initiation of ankle strengthening, including eversion and inversion, and (3) agility, proprioception, and functional rehabilitation.
RESULTS
Twenty-five NFL players (29 surgeries) were included in the study. Two players were excluded because they had retired at the end of the season before the surgery for reasons unrelated to the operative ankle. Mean (SD) age was 28.1 (2.9) years. Six included players had a history of ankle sprains, 1 had a history of ipsilateral ankle fracture, and 1 had a history of ipsilateral ankle dislocation. Table 1 lists the positions of players who underwent ankle arthroscopic decompression.
Table 1. Positions of National Football League Players Who Underwent Ankle Arthroscopic Decompression for Anterior Ankle Impingement
Position | Surgeries, n |
| Offensive line | 8 |
| Defensive line | 8 |
| Wide receiver | 4 |
| Running back | 4 |
| Linebacker | 3 |
| Quarterback | 1 |
| Defensive back | 1 |
Continue to: During diagnostic arthroscopy...
During diagnostic arthroscopy, changes to the articular cartilage were noted: grade 0 in 38% of patients, grade 1 in 17%, grade 2 in 21%, grade 3 in 21%, and grade 4 in 3%. Four patients had an osteochondral lesion (<1 cm in each case), which was treated with chondroplasty without microfracture.
Each included patient returned to NFL play. Mean (SD) time to RTP without restrictions was 8.4 (4.1) weeks after surgery (range, 2-20 weeks). There was a poor correlation between degree of chondrosis and time to RTP (r = 0.305). In addition, there was a poor correlation between age and time to RTP (r = 0.106).
Dorsiflexion improved significantly (P < .001), patients had significantly less pain after surgery (P < .001), and AOFAS hindfoot scores improved significantly (P < .001) (Table 2).
Table 2. Preoperative and Postoperative Dorsiflexion, Pain, and AOFAS Score Before and After Arthroscopic Débridement of Anterior Ankle Impingementa
| Mean (SD) | ||
|---|---|---|
| Preoperative | Postoperative | |
| Dorsiflexion | 8.28º (4.14º) | 18.86° (2.62°) |
| VAS pain score | 4.21 (1.52) | 0.38 (0.89) |
| AOFAS score | 70.62 (10.39) | 97.45 (4.72) |
aAll values were significantly improved after surgery (P < .001).
Abbreviations: AOFAS, American Orthopaedic Foot and Ankle Society; VAS, visual analog scale.
The athletes played in the NFL for a mean (SD) of 3.43 (2.57) years after surgery (range, 1-10 seasons). These players included 6 who were still active at time of publication. No patient required revision surgery or additional surgery on the ipsilateral ankle. The one patient who was treated for superficial thrombophlebitis after surgery reported symptoms before surgery as well.
DISCUSSION
Arthroscopic decompression of anterior ankle impingement is safe and significantly improves pain and ROM in professional American football players. The procedure results in reliable RTP at an elite level, with durable results over the time remaining in their NFL careers.
Continue to: before the 1988 study by Hawkins...
Before the 1988 study by Hawkins,11 ankle spurs were removed with open procedures. Hawkins11 used arthroscopy for better and safer visualization of the ankle joint and used a burr for less painful removal of spurs from the tibia and the talus. In 2002, a series of 105 patients (median age, 35 years) had reduced pain and improved function a minimum of 2 years after arthroscopic débridement.12 These patients had a mix of pathology, including soft-tissue impingement, bony impingement, chondral lesions, loose bodies, and osteoarthritis.
For many elite athletes, anterior ankle impingement can cause significant limitation. Reduced ankle dorsiflexion can alter all limb mechanics and predispose athletes to injury.13 In addition, because NFL players’ ankle ROM often approaches or exceeds normal physiologic limits,14 an ankle ROM limitation will often hinder their performance.
Miyamoto and colleagues15 studied a series of 9 professional athletes (6 soccer players, 1 baseball pitcher, 1 mixed martial artist, 1 golfer) who underwent decompression of both anterior and posterior impingement. With regard to anterior impingement, they found anterior osteophytes in all the ankles, as was seen in the present study. Furthermore, they noted that mean dorsiflexion improved from 10° before surgery to 15° after surgery and that their athletes returned to play 12 to 15 weeks after surgery. Their results are similar to ours, though we noted more improvement in dorsiflexion, from 8.28° before surgery to 18.86° after surgery.
One of the most important metrics in evaluating treatment options for professional athletes is time from surgery to RTP without restrictions. Mean time to full RTP was shorter in our study (8.4 weeks) than in the study by Miyamoto and colleagues15 (up to 20 weeks). However, many of their procedures were performed during the off-season, when there was no need to expeditiously clear patients for full sports participation. In addition, the patients in their study had both anterior and posterior pathology.
Faster return to high-level athletics was supported in a study of 11 elite ballet dancers,16 whose pain and dance performance improved after arthroscopic débridement. Of the 11 patients, 9 returned to dance at a mean of 7 weeks after surgery; the other 2 required reoperation. Although the pathology differed in their study of elite professional soccer players, Calder and colleagues17 found that mean time to RTP after ankle arthroscopy for posterior impingement was 5 weeks.
Continue to: For the NFL players in our study...
For the NFL players in our study, RTP at their elite level was 100% after arthroscopic débridement of anterior ankle impingement. In the literature, time to RTP varies. Table 3 lists RTP rates for recreational athletes in published studies.18-27 In their recent systematic literature review, Zwiers and colleagues10 noted that 24% to 96.4% of recreational athletes returned to play after arthroscopic treatment for anterior ankle impingement. The percentage was significantly higher for the professional athletes in our study. Historical comparison supports an evolution in the indications and techniques for this procedure, with more recent literature suggesting a RTP rate much higher than earlier rates. In addition, compared with recreational athletes, professional athletes have strong financial incentives to return to their sports. Furthermore, our professional cohort was significantly younger than the recreational cohorts in those studies.
Table 3. Frequency of Recreational Athletes’ Return to Play After Arthroscopic Débridement of Anterior Ankle Impingement, as Reported in the Literature
| Study | Year | Journal | Return to Play | |
|---|---|---|---|---|
| n/N | % | |||
| Akseki et al18 | 1999 | Acta Orthop Scand | 10/11 | 91 |
| Baums et al19 | 2006 | Knee Surg Sports Traumatol Arthrosc | 25/26 | 96 |
| Branca et al20 | 1997 | Foot Ankle Int | 13/27 | 48 |
| Di Palma et al21 | 1999 | J Sports Traumatol Relat Res | 21/32 | 66 |
| Ferkel et al22 | 1991 | Am J Sports Med | 27/31 | 87.1 |
| Hassan23 | 2007 | Knee Surg Sports Traumatol Arthrosc | 9/11 | 82 |
| Jerosch et al24 | 1994 | Knee Surg Sports Traumatol Arthrosc | 9/38 | 24 |
| Murawski & Kennedy25 | 2010 | Am J Sports Med | 27/28 | 96.4 |
| Ogilvie-Harris et al26 | 1993 | J Bone Joint Surg Br | 21/28 | 75 |
| Rouvillain et al27 | 2014 | Eur J Orthop Surg Traumatol | 10/11 | 90 |
Total | 172/243 | 70 | ||
Current recommendations for recreational athletes include initial conservative treatment with rest, ankle bracing, and avoidance of jumping and other repetitive dorsiflexing activities. Physical therapy should include joint mobilization and work along the entire kinetic chain. Night splints or a removable walking boot can be used temporarily, as can a single intra-articular corticosteroid injection to reduce inflammation and evaluate improvement in more refractory cases.28 Commonly, conservative treatments fail if patients remain active, and soft tissue and/or osteophytes can be resected, though resection typically is reserved for recreational athletes for whom nonoperative treatments have been exhausted.29,30
This study had several limitations, including its retrospective nature and lack of control group. In addition, follow-up was relatively short, and we did not use more recently described outcome measures, such as the Sports subscale of the Foot and Ankle Ability Measure, which may be more sensitive in describing function in elite athletes. However, many of the cases in our study predated these measures, but the rate of RTP at the NFL level requires a very high degree of postoperative ankle function, making this outcome the most meaningful. In the context of professional athletes, specifically the length of their careers, our study results provide valuable information regarding expectations about RTP and the durability of arthroscopic débridement of anterior ankle impingement in a high-demand setting.
CONCLUSION
For all the NFL players in this study, arthroscopic débridement of anterior ankle impingement resulted in return to preoperative level of play at a mean of 2 months after surgery. There were significant improvements in VAS pain scores, AOFAS hindfoot scores, and ROM. Arthroscopic débridement of anterior ankle impingement relieves pain, restores ROM and function, and results in reliable RTP in professional football players.
1. Lubowitz JH. Editorial commentary: ankle anterior impingement is common in athletes and could be under-recognized. Arthroscopy. 2015;31(8):1597.
2. Mcdougall A. Footballer’s ankle. Lancet. 1955;269(6902):1219-1220.
3. Kleiger B. Anterior tibiotalar impingement syndromes in dancers. Foot Ankle. 1982;3(2):69-73.
4. Bassett FH 3rd, Gates HS 3rd, Billys JB, Morris HB, Nikolaou PK. Talar impingement by the anteroinferior tibiofibular ligament. A cause of chronic pain in the ankle after inversion sprain. J Bone Joint Surg Am. 1990;72(1):55-59.
5. Liu SH, Raskin A, Osti L, et al. Arthroscopic treatment of anterolateral ankle impingement. Arthroscopy. 1994;10(2):215-218.
6. Thein R, Eichenblat M. Arthroscopic treatment of sports-related synovitis of the ankle. Am J Sports Med. 1992;20(5):496-498.
7. Arnold H. Posttraumatic impingement syndrome of the ankle—indication and results of arthroscopic therapy. Foot Ankle Surg. 2011;17(2):85-88.
8. Walsh SJ, Twaddle BC, Rosenfeldt MP, Boyle MJ. Arthroscopic treatment of anterior ankle impingement: a prospective study of 46 patients with 5-year follow-up. Am J Sports Med. 2014;42(11):2722-2726.
9. Glazebrook MA, Ganapathy V, Bridge MA, Stone JW, Allard JP. Evidence-based indications for ankle arthroscopy. Arthroscopy. 2009;25(12):1478-1490.
10. Zwiers R, Wiegerinck JI, Murawski CD, Fraser EJ, Kennedy JG, van Dijk CN. Arthroscopic treatment for anterior ankle impingement: a systematic review of the current literature. Arthroscopy. 2015;31(8):1585-1596.
11. Hawkins RB. Arthroscopic treatment of sports-related anterior osteophytes in the ankle. Foot Ankle. 1988;9(2):87-90.
12. Rasmussen S, Hjorth Jensen C. Arthroscopic treatment of impingement of the ankle reduces pain and enhances function. Scand J Med Sci Sports. 2002;12(2):69-72.
13. Mason-Mackay AR, Whatman C, Reid D. The effect of reduced ankle dorsiflexion on lower extremity mechanics during landing: a systematic review. J Sci Med Sport. 2017;20(5):451-458.
14. Riley PO, Kent RW, Dierks TA, Lievers WB, Frimenko RE, Crandall JR. Foot kinematics and loading of professional athletes in American football-specific tasks. Gait Posture. 2013;38(4):563-569.
15. Miyamoto W, Takao M, Matsui K, Matsushita T. Simultaneous ankle arthroscopy and hindfoot endoscopy for combined anterior and posterior ankle impingement syndrome in professional athletes. J Orthop Sci. 2015;20(4):642-648.
16. Nihal A, Rose DJ, Trepman E. Arthroscopic treatment of anterior ankle impingement syndrome in dancers. Foot Ankle Int. 2005;26(11):908-912.
17. Calder JD, Sexton SA, Pearce CJ. Return to training and playing after posterior ankle arthroscopy for posterior impingement in elite professional soccer. Am J Sports Med. 2010;38(1):120-124.
18. Akseki D, Pinar H, Bozkurt M, Yaldiz K, Arag S. The distal fascicle of the anterior inferior tibiofibular ligament as a cause of anterolateral ankle impingement: results of arthroscopic resection. Acta Orthop Scand. 1999;70(5):478-482.
19. Baums MH, Kahl E, Schultz W, Klinger HM. Clinical outcome of the arthroscopic management of sports-related “anterior ankle pain”: a prospective study. Knee Surg Sports Traumatol Arthrosc. 2006;14(5):482-486.
20. Branca A, Di Palma L, Bucca C, Visconti CS, Di Mille M. Arthroscopic treatment of anterior ankle impingement. Foot Ankle Int. 1997;18(7):418-423.
21. Di Palma L, Bucca C, Di Mille M, Branca A. Diagnosis and arthroscopic treatment of fibrous impingement of the ankle. J Sports Traumatol Relat Res. 1999;21:170-177.
22. Ferkel RD, Karzel RP, Del Pizzo W, Friedman MJ, Fischer SP. Arthroscopic treatment of anterolateral impingement of the ankle. Am J Sports Med. 1991;19(5):440-446.
23. Hassan AH. Treatment of anterolateral impingements of the ankle joint by arthroscopy. Knee Surg Sports Traumatol Arthrosc. 2007;15(9):1150-1154.
24. Jerosch J, Steinbeck J, Schröder M, Halm H. Arthroscopic treatment of anterior synovitis of the ankle in athletes. Knee Surg Sports Traumatol Arthrosc. 1994;2(3):176-181.
25. Murawski CD, Kennedy JG. Anteromedial impingement in the ankle joint: outcomes following arthroscopy. Am J Sports Med. 2010;38(10):2017-2024.
26. Ogilvie-Harris DJ, Mahomed N, Demazière A. Anterior impingement of the ankle treated by arthroscopic removal of bony spurs. J Bone Joint Surg Br. 1993;75(3):437-440.
27. Rouvillain JL, Daoud W, Donica A, Garron E, Uzel AP. Distraction-free ankle arthroscopy for anterolateral impingement. Eur J Orthop Surg Traumatol. 2014;24(6):1019-1023.
28. O’Kane JW, Kadel N. Anterior impingement syndrome in dancers. Curr Rev Musculoskelet Med. 2008;1(1):12-16.
29. Lavery KP, McHale KJ, Rossy WH, Theodore G. Ankle impingement. J Orthop Surg Res. 2016;11(1):97.
30. Talusan PG, Toy J, Perez JL, Milewski MD, Reach JS. Anterior ankle impingement: diagnosis and treatment. J Am Acad Orthop Surg. 2014;22(5):333-339.
1. Lubowitz JH. Editorial commentary: ankle anterior impingement is common in athletes and could be under-recognized. Arthroscopy. 2015;31(8):1597.
2. Mcdougall A. Footballer’s ankle. Lancet. 1955;269(6902):1219-1220.
3. Kleiger B. Anterior tibiotalar impingement syndromes in dancers. Foot Ankle. 1982;3(2):69-73.
4. Bassett FH 3rd, Gates HS 3rd, Billys JB, Morris HB, Nikolaou PK. Talar impingement by the anteroinferior tibiofibular ligament. A cause of chronic pain in the ankle after inversion sprain. J Bone Joint Surg Am. 1990;72(1):55-59.
5. Liu SH, Raskin A, Osti L, et al. Arthroscopic treatment of anterolateral ankle impingement. Arthroscopy. 1994;10(2):215-218.
6. Thein R, Eichenblat M. Arthroscopic treatment of sports-related synovitis of the ankle. Am J Sports Med. 1992;20(5):496-498.
7. Arnold H. Posttraumatic impingement syndrome of the ankle—indication and results of arthroscopic therapy. Foot Ankle Surg. 2011;17(2):85-88.
8. Walsh SJ, Twaddle BC, Rosenfeldt MP, Boyle MJ. Arthroscopic treatment of anterior ankle impingement: a prospective study of 46 patients with 5-year follow-up. Am J Sports Med. 2014;42(11):2722-2726.
9. Glazebrook MA, Ganapathy V, Bridge MA, Stone JW, Allard JP. Evidence-based indications for ankle arthroscopy. Arthroscopy. 2009;25(12):1478-1490.
10. Zwiers R, Wiegerinck JI, Murawski CD, Fraser EJ, Kennedy JG, van Dijk CN. Arthroscopic treatment for anterior ankle impingement: a systematic review of the current literature. Arthroscopy. 2015;31(8):1585-1596.
11. Hawkins RB. Arthroscopic treatment of sports-related anterior osteophytes in the ankle. Foot Ankle. 1988;9(2):87-90.
12. Rasmussen S, Hjorth Jensen C. Arthroscopic treatment of impingement of the ankle reduces pain and enhances function. Scand J Med Sci Sports. 2002;12(2):69-72.
13. Mason-Mackay AR, Whatman C, Reid D. The effect of reduced ankle dorsiflexion on lower extremity mechanics during landing: a systematic review. J Sci Med Sport. 2017;20(5):451-458.
14. Riley PO, Kent RW, Dierks TA, Lievers WB, Frimenko RE, Crandall JR. Foot kinematics and loading of professional athletes in American football-specific tasks. Gait Posture. 2013;38(4):563-569.
15. Miyamoto W, Takao M, Matsui K, Matsushita T. Simultaneous ankle arthroscopy and hindfoot endoscopy for combined anterior and posterior ankle impingement syndrome in professional athletes. J Orthop Sci. 2015;20(4):642-648.
16. Nihal A, Rose DJ, Trepman E. Arthroscopic treatment of anterior ankle impingement syndrome in dancers. Foot Ankle Int. 2005;26(11):908-912.
17. Calder JD, Sexton SA, Pearce CJ. Return to training and playing after posterior ankle arthroscopy for posterior impingement in elite professional soccer. Am J Sports Med. 2010;38(1):120-124.
18. Akseki D, Pinar H, Bozkurt M, Yaldiz K, Arag S. The distal fascicle of the anterior inferior tibiofibular ligament as a cause of anterolateral ankle impingement: results of arthroscopic resection. Acta Orthop Scand. 1999;70(5):478-482.
19. Baums MH, Kahl E, Schultz W, Klinger HM. Clinical outcome of the arthroscopic management of sports-related “anterior ankle pain”: a prospective study. Knee Surg Sports Traumatol Arthrosc. 2006;14(5):482-486.
20. Branca A, Di Palma L, Bucca C, Visconti CS, Di Mille M. Arthroscopic treatment of anterior ankle impingement. Foot Ankle Int. 1997;18(7):418-423.
21. Di Palma L, Bucca C, Di Mille M, Branca A. Diagnosis and arthroscopic treatment of fibrous impingement of the ankle. J Sports Traumatol Relat Res. 1999;21:170-177.
22. Ferkel RD, Karzel RP, Del Pizzo W, Friedman MJ, Fischer SP. Arthroscopic treatment of anterolateral impingement of the ankle. Am J Sports Med. 1991;19(5):440-446.
23. Hassan AH. Treatment of anterolateral impingements of the ankle joint by arthroscopy. Knee Surg Sports Traumatol Arthrosc. 2007;15(9):1150-1154.
24. Jerosch J, Steinbeck J, Schröder M, Halm H. Arthroscopic treatment of anterior synovitis of the ankle in athletes. Knee Surg Sports Traumatol Arthrosc. 1994;2(3):176-181.
25. Murawski CD, Kennedy JG. Anteromedial impingement in the ankle joint: outcomes following arthroscopy. Am J Sports Med. 2010;38(10):2017-2024.
26. Ogilvie-Harris DJ, Mahomed N, Demazière A. Anterior impingement of the ankle treated by arthroscopic removal of bony spurs. J Bone Joint Surg Br. 1993;75(3):437-440.
27. Rouvillain JL, Daoud W, Donica A, Garron E, Uzel AP. Distraction-free ankle arthroscopy for anterolateral impingement. Eur J Orthop Surg Traumatol. 2014;24(6):1019-1023.
28. O’Kane JW, Kadel N. Anterior impingement syndrome in dancers. Curr Rev Musculoskelet Med. 2008;1(1):12-16.
29. Lavery KP, McHale KJ, Rossy WH, Theodore G. Ankle impingement. J Orthop Surg Res. 2016;11(1):97.
30. Talusan PG, Toy J, Perez JL, Milewski MD, Reach JS. Anterior ankle impingement: diagnosis and treatment. J Am Acad Orthop Surg. 2014;22(5):333-339.
TAKE-HOME POINTS
- Anterior ankle impingement can be very debilitating in elite athletes and may lead to significantly decreased performance.
- First line treatment for anterior ankle impingement is conservative which includes rest, ankle bracing, and avoidance of repetitive dorsiflexing activities such as jumping.
- Arthroscopic débridement of anterior ankle impingement reliably relieves pain, and restores ROM and function.
- Arthroscopic débridement of anterior ankle impingement results in reliable RTP in professional football players.
- RTP after arthroscopic anterior ankle débridement for impingement averaged 2 months in professional football players.
Knotless Tape Suture Fixation of Quadriceps Tendon Rupture: A Novel Technique
ABSTRACT
Quadriceps tendon ruptures disrupt the extensor mechanism of the knee and require urgent surgical management. Traditional repair techniques have had mixed biomechanical and clinical results risking weakness and extensor lag. We describe a novel technique using tape suture and knotless anchors, which has performed superiorly during biomechanical testing and yielded terrific early clinical results.
Continue to: Quadriceps tendon rupture...
Quadriceps tendon rupture is an uncommon yet potentially devastating knee injury with an estimated incidence of 1.37 in 100,000.1 It most often occurs in male, middle-aged or older patients with degenerative tendon changes and serious systemic diseases, such as chronic renal failure, diabetes mellitus, rheumatoid arthritis, and disorders requiring long-term steroid use (tissue quality is often compromised by patient age and comorbidities).2-10 Whereas partial tears with an intact extensor mechanism may be managed nonoperatively, prompt operative intervention is indicated in cases of complete tear or an incompetent extensor mechanism to facilitate early range of motion (ROM) and return of knee function.2-4,8,9
The standard of care is repair with a nonabsorbable suture passed through transosseous patellar tunnels, often with several weeks of postoperative immobilization to protect the repair.3,4,7,10-12 Reported complications of this method include significant extension lag, decreased strength, and ROM compared with the contralateral knee, chronic pain, and iatrogenic patellar fracture.8,13-18 Repair techniques using suture anchors have been proposed as viable alternatives, but biomechanical studies comparing them with standard transosseous repair have reported mixed results.7,10-12,18-20 Two studies found improved biomechanical characteristics with suture anchors,10,21 but 2 others found the characteristics of suture anchor fixation equal to11 or worse than12 those of transosseous fixation. In light of the controversy regarding strength and clinical outcomes of suture anchor repair compared with transosseous repair, new and potentially superior surgical interventions should be considered.
We recently completed a cadaveric study comparing the biomechanical properties of a novel quadriceps tendon repair technique using 4.75-mm biocomposite knotless suture anchors with suture tape and the properties of conventional techniques using either transosseous or suture anchor repair alone.22 In the cadaveric model, compared with transosseous and fully threaded suture anchor techniques, repair of quadriceps tendon ruptures with this knotless suture anchor with suture tape technique was biomechanically superior in cyclic displacement, construct stiffness, and ultimate load to failure.22 Additionally, this method allows for less extensive dissection, shorter operative times, and the potential for earlier and more aggressive rehabilitation protocols.22 We propose this technique, presented in this article, as a superior alternative to traditional quadriceps tendon repair techniques.
TECHNIQUE
The patient is placed in supine position with a tourniquet placed on the proximal thigh. A midline incision is made from the proximal pole of the patella, proximally by 5 cm. A combination of sharp and blunt dissection is performed through skin and subcutaneous tissues down to the extensor mechanism, exposing the proximal pole of the patella and the torn quadriceps tendon.
The distal aspect of the quadriceps tendon is then débrided of any devitalized tissue and secured with an Allis clamp. A long tape suture (FiberTape; Arthrex) is then used to place a locking Krackow stitch in a distal-to-proximal and then proximal-to-distal direction for 5 throws in each direction within the quadriceps tendon, with the tails exiting distally at the tear site. Care is taken with each pass to ensure that there is no slack within the system.
Continue to: The proximal pole of the patella...
The proximal pole of the patella is then prepared by débriding any remaining soft tissue back to an area of exposed subcortical bone, which is débrided to a bleeding bony bed. Holes are drilled in the medial and lateral thirds of the patella at the proximal pole using the drill for 4.75-mm biocomposite knotless suture anchors (SwiveLock; Arthrex). The tap for the 4.75-mm anchors is then passed at each guide hole. In hard bone, double-tapping is recommended.
Next, the medial strand of tape suture is loaded within a 4.75-mm biocomposite knotless suture anchor eyelet and reduced to the patella. The medial anchor is malleted and screwed into place, while tension is kept on the lateral strand with the knee in full extension. The lateral strand is then placed into its 4.75-mm biocomposite knotless suture anchor, reduced to the patella, and then malleted and screwed into place in the lateral hole, thereby completing the core portion of the repair (Figures A-D). The core strands from the 4.75-mm biocomposite knotless suture anchors are then back-passed in mattress fashion and tied, and medial and lateral retinacular repairs are then performed using supersuture tape (SutureTape or FiberWire; Arthrex).
After surgery, the patient is placed in a knee brace locked in full extension and allowed to weight-bear as tolerated using crutches. During the first week, knee ROM is allowed up to 30°. During weeks 2 to 6 passive ROM is gradually increased to 90°, and use of crutches is tapered. At week 6 the brace is unlocked for ambulation; it may be discontinued after 7 to 8 weeks or when determined safe. Light activity is permitted from month 4 to month 6. A patient who achieves satisfactory strength, is clinically examined, and progresses through rehabilitation is allowed to return to fully unrestricted sport.
DISCUSSION
Quadriceps tendon rupture is an uncommon clinical entity that requires early surgical management.1-5,12,17,19 The standard of care is passage of nonabsorbable sutures through transosseous patellar bone tunnels, but repair with suture anchors has been studied as an alternative that allows for less tissue trauma, decreased operative time, safe early initiation of rehabilitation protocols, and reduced risk of patella fracture or damage.3,7,10-12,18-20,21,23 Despite these potential advantages, biomechanical studies have yielded inconsistent results regarding the superiority of suture anchor repair over repair with transosseous tunnels.7,10-12,18-20 We propose quadriceps tendon repair using the 4.75-mm biocomposite knotless suture anchor with tape suture technique as a biomechanically superior alternative to either transosseous tunnels or suture anchor repair alone, with significant advantages both in and out of the operating room.
Results of biomechanical studies comparing transosseous tunnel repair and standard suture anchor repair have been mixed, though the heterogeneity of their study methods and endpoints makes direct comparisons difficult.7,10-12,18-20 Petri and colleagues10 and Sherman and colleagues21 reported statistically significant higher load to failure10 and reduced gapping during cyclic loading10,21 with suture anchor repair relative to transosseous repair. However, Hart and colleagues12 found that repair with suture anchors had lower ultimate tensile load, and they concluded that transosseous repair is superior. Lighthart and colleagues11 found no significant difference in displacement between the 2 repairs.
Continue to: In our cadaveric biomechanical study...
In our cadaveric biomechanical study, a novel 4.75-mm biocomposite knotless suture anchor with suture tape repair was compared with traditional 3-tunnel transosseous repair and with standard 2-anchor suture anchor repair.22 Statistically significant superiority was found across multiple parameters, including initial tendon displacement, stiffness, and ultimate load to failure (vs 5.5-mm biocomposite fully threaded suture anchor repair), as well as initial and late tendon displacement, stiffness, and ultimate load to failure (vs transosseous repair).22 Although definitive conclusions are difficult to draw on the basis of prior cadaveric studies comparing standard suture anchor repair and transosseous repair, our results decidedly favor the biomechanical characteristics of this 4.75-mm biocomposite knotless suture anchor with suture tape repair and make it a potentially superior repair technique based on biomechanics alone.22
Similarly to standard repair with suture anchors, repair using a 4.75-mm biocomposite knotless suture anchor with tape suture eliminates the need to expose the distal pole of the patella.7,10-12,21 This allows for a smaller surgical incision, less extensive dissection, and prevents possible interference with the patellar tendon.7,10-12,21 Additionally, it eliminates the risk of iatrogenic patellar fracture and damage to the articular surface from drilling the transpatellar tunnels.17,18 Both our own review of cases repaired with our 4.75-mm biocomposite knotless suture anchor with suture tape technique as well as studies of suture anchor repair have consistently found operative times of <1 hour.21 Shorter operative times and smaller surgical wounds are advantageous given that many of these patients have medical comorbidities that predispose them to intraoperative and wound-healing complications.12,19-22
Optimal rehabilitation protocols for quadriceps tendon repair are a matter of controversy. Multiple studies of repair with transosseous patellar tunnels describe immobilization for 6 weeks after surgery, but there has been a recent push toward early motion.7,13,23,24 Reported complications of extended immobilization include limited flexion, pain, weakness, decreased patellar mobility, and patella baja.14 Studies have suggested that, while excessive loading can cause gap formation and weaken the repair, some controlled motion is necessary to heal the tendon23,25 and reduce the risks of stiffness and atrophy.14 The improved biomechanical characteristics of the 4.75-mm biocomposite knotless suture anchor with tape suture technique allow for safe early initiation of ROM exercises and accelerated rehabilitation protocols.
In our early experience with this technique, functional outcomes have been excellent. A formal 2-year outcome study of patients who have undergone quadriceps tendon repair with this 4.75-mm biocomposite knotless suture anchor with tape suture technique is under way.
1. Clayton RA, Court-Brown CM. The epidemiology of musculoskeletal tendinous and ligamentous injuries. Injury. 2008;39(12):1338-1344.
2. Rasul AT Jr, Fischer DA. Primary repair of quadriceps tendon ruptures. Clin Orthop Relat Res. 1993;(289):205-207.
3. Ilan DI, Tejwani N, Keschner M, Leibman M. Quadriceps tendon rupture. J Am Acad Orthop Surg. 2003;11(3):192-200.
4. Ramseier LE, Werner CM, Heinzelmann M. Quadriceps and patellar tendon rupture. Injury. 2006;37(6):516-519.
5. Ciriello V, Gudipati S, Tosounidis T, Soucacos PN, Giannoudis PV. Clinical outcomes after repair of quadriceps tendon rupture: a systematic review. Injury. 2012;43(11):1931-1938.
6. O’Shea K, Kenny P, Donovan J, Condon F, McElwain JP. Outcomes following quadriceps tendon ruptures. Injury. 2002;33(3):257-260.
7. Richards DP, Barber FA. Repair of quadriceps tendon ruptures using suture anchors. Arthroscopy. 2002;18(5):556-559.
8. Wenzl ME, Kirchner R, Seide K, Strametz S, Jürgens C. Quadriceps tendon ruptures—is there a complete functional restitution? Injury. 2004;35(9):922-926.
9. Boudissa M, Roudet A, Rubens-Duval B, Chaussard C, Saragaglia D. Acute quadriceps tendon ruptures: a series of 50 knees with an average follow-up of more than 6 years. Orthop Traumatol Surg Res. 2014;100(2):213-216.
10. Petri M, Dratzidis A, Brand S, et al. Suture anchor repair yields better biomechanical properties than transosseous sutures in ruptured quadriceps tendons. Knee Surg Sports Traumatol Arthrosc. 2015;23(4):1039-1045.
11. Lighthart WC, Cohen DA, Levine RG, Parks BG, Boucher HR. Suture anchor versus suture through tunnel fixation for quadriceps tendon rupture: a biomechanical study. Orthopedics. 2008;31(5):441.
12. Hart ND, Wallace MK, Scovell JF, Krupp RJ, Cook C, Wyland DJ. Quadriceps tendon rupture: a biomechanical comparison of transosseous equivalent double-row suture anchor versus transosseous tunnel repair. J Knee Surg. 2012;25(4):335-339.
13. Rougraff BT, Reeck CC, Essenmacher J. Complete quadriceps tendon ruptures. Orthopedics. 1996;19(6):509-514.
14. West JL, Keene JS, Kaplan LD. Early motion after quadriceps and patellar tendon repairs: outcomes with single-suture augmentation. Am J Sports Med. 2008;36(2):316-323.
15. De Baere T, Geulette B, Manche E, Barras L. Functional results after surgical repair of quadriceps tendon rupture. Acta Orthop Belg. 2002;68(2):146-149.
16. Konrath GA, Chen D, Lock T, et al. Outcomes following repair of quadriceps tendon ruptures. J Orthop Trauma. 1998;12(4):273-279.
17. Gregory JM, Sherman SL, Mather R, Bach BR Jr. Patellar stress fracture after transosseous extensor mechanism repair: report of 3 cases. Am J Sports Med. 2012;40(7):1668-1672.
18. Bushnell BD, Whitener GB, Rubright JH, Creighton RA, Logel KJ, Wood ML. The use of suture anchors to repair the ruptured quadriceps tendon. J Orthop Trauma. 2007;21(6):407-413.
19. Harris JD, Abrams GD, Yanke AB, Hellman MD, Erickson BJ, Bach BR Jr. Suture anchor repair of quadriceps tendon rupture. Orthopedics. 2014;37(3):183-186.
20. Maniscalco P, Bertone C, Rivera F, Bocchi L. A new method of repair for quadriceps tendon ruptures. A case report. Panminerva Med. 2000;42(3):223-225.
21. Sherman SL, Copeland ME, Milles JL, Flood DA, Pfeiffer FM. Biomechanical evaluation of suture anchor versus transosseous tunnel quadriceps tendon repair techniques. Arthroscopy. 2016;32(6):1117-1124.
22. Kindya MC, Konicek J, Rizzi A, Komatsu DE, Paci JM. Knotless suture anchor with suture tape quadriceps tendon repair is biomechanically superior to transosseous and traditional suture anchor-based repairs in a cadaveric model. Arthroscopy. 2017;33(1):190-198.
23. Brossard P, Le Roux G, Vasse B; Orthopedics, Traumatology Society of Western France (SOO). Acute quadriceps tendon rupture repaired by suture anchors: outcomes at 7 years’ follow-up in 25 cases. Orthop Traumatol Surg Res. 2017;103(4):597-601.
24. Langenhan R, Baumann M, Ricart P, et al. Postoperative functional rehabilitation after repair of quadriceps tendon ruptures: a comparison of two different protocols. Knee Surg Sports Traumatol Arthrosc. 2012;20(11):2275-2278.
25. Killian ML, Cavinatto L, Galatz LM, Thomopoulos S. The role of mechanobiology in tendon healing. J Shoulder Elbow Surg. 2012;21(2):228-237.
ABSTRACT
Quadriceps tendon ruptures disrupt the extensor mechanism of the knee and require urgent surgical management. Traditional repair techniques have had mixed biomechanical and clinical results risking weakness and extensor lag. We describe a novel technique using tape suture and knotless anchors, which has performed superiorly during biomechanical testing and yielded terrific early clinical results.
Continue to: Quadriceps tendon rupture...
Quadriceps tendon rupture is an uncommon yet potentially devastating knee injury with an estimated incidence of 1.37 in 100,000.1 It most often occurs in male, middle-aged or older patients with degenerative tendon changes and serious systemic diseases, such as chronic renal failure, diabetes mellitus, rheumatoid arthritis, and disorders requiring long-term steroid use (tissue quality is often compromised by patient age and comorbidities).2-10 Whereas partial tears with an intact extensor mechanism may be managed nonoperatively, prompt operative intervention is indicated in cases of complete tear or an incompetent extensor mechanism to facilitate early range of motion (ROM) and return of knee function.2-4,8,9
The standard of care is repair with a nonabsorbable suture passed through transosseous patellar tunnels, often with several weeks of postoperative immobilization to protect the repair.3,4,7,10-12 Reported complications of this method include significant extension lag, decreased strength, and ROM compared with the contralateral knee, chronic pain, and iatrogenic patellar fracture.8,13-18 Repair techniques using suture anchors have been proposed as viable alternatives, but biomechanical studies comparing them with standard transosseous repair have reported mixed results.7,10-12,18-20 Two studies found improved biomechanical characteristics with suture anchors,10,21 but 2 others found the characteristics of suture anchor fixation equal to11 or worse than12 those of transosseous fixation. In light of the controversy regarding strength and clinical outcomes of suture anchor repair compared with transosseous repair, new and potentially superior surgical interventions should be considered.
We recently completed a cadaveric study comparing the biomechanical properties of a novel quadriceps tendon repair technique using 4.75-mm biocomposite knotless suture anchors with suture tape and the properties of conventional techniques using either transosseous or suture anchor repair alone.22 In the cadaveric model, compared with transosseous and fully threaded suture anchor techniques, repair of quadriceps tendon ruptures with this knotless suture anchor with suture tape technique was biomechanically superior in cyclic displacement, construct stiffness, and ultimate load to failure.22 Additionally, this method allows for less extensive dissection, shorter operative times, and the potential for earlier and more aggressive rehabilitation protocols.22 We propose this technique, presented in this article, as a superior alternative to traditional quadriceps tendon repair techniques.
TECHNIQUE
The patient is placed in supine position with a tourniquet placed on the proximal thigh. A midline incision is made from the proximal pole of the patella, proximally by 5 cm. A combination of sharp and blunt dissection is performed through skin and subcutaneous tissues down to the extensor mechanism, exposing the proximal pole of the patella and the torn quadriceps tendon.
The distal aspect of the quadriceps tendon is then débrided of any devitalized tissue and secured with an Allis clamp. A long tape suture (FiberTape; Arthrex) is then used to place a locking Krackow stitch in a distal-to-proximal and then proximal-to-distal direction for 5 throws in each direction within the quadriceps tendon, with the tails exiting distally at the tear site. Care is taken with each pass to ensure that there is no slack within the system.
Continue to: The proximal pole of the patella...
The proximal pole of the patella is then prepared by débriding any remaining soft tissue back to an area of exposed subcortical bone, which is débrided to a bleeding bony bed. Holes are drilled in the medial and lateral thirds of the patella at the proximal pole using the drill for 4.75-mm biocomposite knotless suture anchors (SwiveLock; Arthrex). The tap for the 4.75-mm anchors is then passed at each guide hole. In hard bone, double-tapping is recommended.
Next, the medial strand of tape suture is loaded within a 4.75-mm biocomposite knotless suture anchor eyelet and reduced to the patella. The medial anchor is malleted and screwed into place, while tension is kept on the lateral strand with the knee in full extension. The lateral strand is then placed into its 4.75-mm biocomposite knotless suture anchor, reduced to the patella, and then malleted and screwed into place in the lateral hole, thereby completing the core portion of the repair (Figures A-D). The core strands from the 4.75-mm biocomposite knotless suture anchors are then back-passed in mattress fashion and tied, and medial and lateral retinacular repairs are then performed using supersuture tape (SutureTape or FiberWire; Arthrex).

After surgery, the patient is placed in a knee brace locked in full extension and allowed to weight-bear as tolerated using crutches. During the first week, knee ROM is allowed up to 30°. During weeks 2 to 6 passive ROM is gradually increased to 90°, and use of crutches is tapered. At week 6 the brace is unlocked for ambulation; it may be discontinued after 7 to 8 weeks or when determined safe. Light activity is permitted from month 4 to month 6. A patient who achieves satisfactory strength, is clinically examined, and progresses through rehabilitation is allowed to return to fully unrestricted sport.
DISCUSSION
Quadriceps tendon rupture is an uncommon clinical entity that requires early surgical management.1-5,12,17,19 The standard of care is passage of nonabsorbable sutures through transosseous patellar bone tunnels, but repair with suture anchors has been studied as an alternative that allows for less tissue trauma, decreased operative time, safe early initiation of rehabilitation protocols, and reduced risk of patella fracture or damage.3,7,10-12,18-20,21,23 Despite these potential advantages, biomechanical studies have yielded inconsistent results regarding the superiority of suture anchor repair over repair with transosseous tunnels.7,10-12,18-20 We propose quadriceps tendon repair using the 4.75-mm biocomposite knotless suture anchor with tape suture technique as a biomechanically superior alternative to either transosseous tunnels or suture anchor repair alone, with significant advantages both in and out of the operating room.
Results of biomechanical studies comparing transosseous tunnel repair and standard suture anchor repair have been mixed, though the heterogeneity of their study methods and endpoints makes direct comparisons difficult.7,10-12,18-20 Petri and colleagues10 and Sherman and colleagues21 reported statistically significant higher load to failure10 and reduced gapping during cyclic loading10,21 with suture anchor repair relative to transosseous repair. However, Hart and colleagues12 found that repair with suture anchors had lower ultimate tensile load, and they concluded that transosseous repair is superior. Lighthart and colleagues11 found no significant difference in displacement between the 2 repairs.
Continue to: In our cadaveric biomechanical study...
In our cadaveric biomechanical study, a novel 4.75-mm biocomposite knotless suture anchor with suture tape repair was compared with traditional 3-tunnel transosseous repair and with standard 2-anchor suture anchor repair.22 Statistically significant superiority was found across multiple parameters, including initial tendon displacement, stiffness, and ultimate load to failure (vs 5.5-mm biocomposite fully threaded suture anchor repair), as well as initial and late tendon displacement, stiffness, and ultimate load to failure (vs transosseous repair).22 Although definitive conclusions are difficult to draw on the basis of prior cadaveric studies comparing standard suture anchor repair and transosseous repair, our results decidedly favor the biomechanical characteristics of this 4.75-mm biocomposite knotless suture anchor with suture tape repair and make it a potentially superior repair technique based on biomechanics alone.22
Similarly to standard repair with suture anchors, repair using a 4.75-mm biocomposite knotless suture anchor with tape suture eliminates the need to expose the distal pole of the patella.7,10-12,21 This allows for a smaller surgical incision, less extensive dissection, and prevents possible interference with the patellar tendon.7,10-12,21 Additionally, it eliminates the risk of iatrogenic patellar fracture and damage to the articular surface from drilling the transpatellar tunnels.17,18 Both our own review of cases repaired with our 4.75-mm biocomposite knotless suture anchor with suture tape technique as well as studies of suture anchor repair have consistently found operative times of <1 hour.21 Shorter operative times and smaller surgical wounds are advantageous given that many of these patients have medical comorbidities that predispose them to intraoperative and wound-healing complications.12,19-22
Optimal rehabilitation protocols for quadriceps tendon repair are a matter of controversy. Multiple studies of repair with transosseous patellar tunnels describe immobilization for 6 weeks after surgery, but there has been a recent push toward early motion.7,13,23,24 Reported complications of extended immobilization include limited flexion, pain, weakness, decreased patellar mobility, and patella baja.14 Studies have suggested that, while excessive loading can cause gap formation and weaken the repair, some controlled motion is necessary to heal the tendon23,25 and reduce the risks of stiffness and atrophy.14 The improved biomechanical characteristics of the 4.75-mm biocomposite knotless suture anchor with tape suture technique allow for safe early initiation of ROM exercises and accelerated rehabilitation protocols.
In our early experience with this technique, functional outcomes have been excellent. A formal 2-year outcome study of patients who have undergone quadriceps tendon repair with this 4.75-mm biocomposite knotless suture anchor with tape suture technique is under way.
ABSTRACT
Quadriceps tendon ruptures disrupt the extensor mechanism of the knee and require urgent surgical management. Traditional repair techniques have had mixed biomechanical and clinical results risking weakness and extensor lag. We describe a novel technique using tape suture and knotless anchors, which has performed superiorly during biomechanical testing and yielded terrific early clinical results.
Continue to: Quadriceps tendon rupture...
Quadriceps tendon rupture is an uncommon yet potentially devastating knee injury with an estimated incidence of 1.37 in 100,000.1 It most often occurs in male, middle-aged or older patients with degenerative tendon changes and serious systemic diseases, such as chronic renal failure, diabetes mellitus, rheumatoid arthritis, and disorders requiring long-term steroid use (tissue quality is often compromised by patient age and comorbidities).2-10 Whereas partial tears with an intact extensor mechanism may be managed nonoperatively, prompt operative intervention is indicated in cases of complete tear or an incompetent extensor mechanism to facilitate early range of motion (ROM) and return of knee function.2-4,8,9
The standard of care is repair with a nonabsorbable suture passed through transosseous patellar tunnels, often with several weeks of postoperative immobilization to protect the repair.3,4,7,10-12 Reported complications of this method include significant extension lag, decreased strength, and ROM compared with the contralateral knee, chronic pain, and iatrogenic patellar fracture.8,13-18 Repair techniques using suture anchors have been proposed as viable alternatives, but biomechanical studies comparing them with standard transosseous repair have reported mixed results.7,10-12,18-20 Two studies found improved biomechanical characteristics with suture anchors,10,21 but 2 others found the characteristics of suture anchor fixation equal to11 or worse than12 those of transosseous fixation. In light of the controversy regarding strength and clinical outcomes of suture anchor repair compared with transosseous repair, new and potentially superior surgical interventions should be considered.
We recently completed a cadaveric study comparing the biomechanical properties of a novel quadriceps tendon repair technique using 4.75-mm biocomposite knotless suture anchors with suture tape and the properties of conventional techniques using either transosseous or suture anchor repair alone.22 In the cadaveric model, compared with transosseous and fully threaded suture anchor techniques, repair of quadriceps tendon ruptures with this knotless suture anchor with suture tape technique was biomechanically superior in cyclic displacement, construct stiffness, and ultimate load to failure.22 Additionally, this method allows for less extensive dissection, shorter operative times, and the potential for earlier and more aggressive rehabilitation protocols.22 We propose this technique, presented in this article, as a superior alternative to traditional quadriceps tendon repair techniques.
TECHNIQUE
The patient is placed in supine position with a tourniquet placed on the proximal thigh. A midline incision is made from the proximal pole of the patella, proximally by 5 cm. A combination of sharp and blunt dissection is performed through skin and subcutaneous tissues down to the extensor mechanism, exposing the proximal pole of the patella and the torn quadriceps tendon.
The distal aspect of the quadriceps tendon is then débrided of any devitalized tissue and secured with an Allis clamp. A long tape suture (FiberTape; Arthrex) is then used to place a locking Krackow stitch in a distal-to-proximal and then proximal-to-distal direction for 5 throws in each direction within the quadriceps tendon, with the tails exiting distally at the tear site. Care is taken with each pass to ensure that there is no slack within the system.
Continue to: The proximal pole of the patella...
The proximal pole of the patella is then prepared by débriding any remaining soft tissue back to an area of exposed subcortical bone, which is débrided to a bleeding bony bed. Holes are drilled in the medial and lateral thirds of the patella at the proximal pole using the drill for 4.75-mm biocomposite knotless suture anchors (SwiveLock; Arthrex). The tap for the 4.75-mm anchors is then passed at each guide hole. In hard bone, double-tapping is recommended.
Next, the medial strand of tape suture is loaded within a 4.75-mm biocomposite knotless suture anchor eyelet and reduced to the patella. The medial anchor is malleted and screwed into place, while tension is kept on the lateral strand with the knee in full extension. The lateral strand is then placed into its 4.75-mm biocomposite knotless suture anchor, reduced to the patella, and then malleted and screwed into place in the lateral hole, thereby completing the core portion of the repair (Figures A-D). The core strands from the 4.75-mm biocomposite knotless suture anchors are then back-passed in mattress fashion and tied, and medial and lateral retinacular repairs are then performed using supersuture tape (SutureTape or FiberWire; Arthrex).

After surgery, the patient is placed in a knee brace locked in full extension and allowed to weight-bear as tolerated using crutches. During the first week, knee ROM is allowed up to 30°. During weeks 2 to 6 passive ROM is gradually increased to 90°, and use of crutches is tapered. At week 6 the brace is unlocked for ambulation; it may be discontinued after 7 to 8 weeks or when determined safe. Light activity is permitted from month 4 to month 6. A patient who achieves satisfactory strength, is clinically examined, and progresses through rehabilitation is allowed to return to fully unrestricted sport.
DISCUSSION
Quadriceps tendon rupture is an uncommon clinical entity that requires early surgical management.1-5,12,17,19 The standard of care is passage of nonabsorbable sutures through transosseous patellar bone tunnels, but repair with suture anchors has been studied as an alternative that allows for less tissue trauma, decreased operative time, safe early initiation of rehabilitation protocols, and reduced risk of patella fracture or damage.3,7,10-12,18-20,21,23 Despite these potential advantages, biomechanical studies have yielded inconsistent results regarding the superiority of suture anchor repair over repair with transosseous tunnels.7,10-12,18-20 We propose quadriceps tendon repair using the 4.75-mm biocomposite knotless suture anchor with tape suture technique as a biomechanically superior alternative to either transosseous tunnels or suture anchor repair alone, with significant advantages both in and out of the operating room.
Results of biomechanical studies comparing transosseous tunnel repair and standard suture anchor repair have been mixed, though the heterogeneity of their study methods and endpoints makes direct comparisons difficult.7,10-12,18-20 Petri and colleagues10 and Sherman and colleagues21 reported statistically significant higher load to failure10 and reduced gapping during cyclic loading10,21 with suture anchor repair relative to transosseous repair. However, Hart and colleagues12 found that repair with suture anchors had lower ultimate tensile load, and they concluded that transosseous repair is superior. Lighthart and colleagues11 found no significant difference in displacement between the 2 repairs.
Continue to: In our cadaveric biomechanical study...
In our cadaveric biomechanical study, a novel 4.75-mm biocomposite knotless suture anchor with suture tape repair was compared with traditional 3-tunnel transosseous repair and with standard 2-anchor suture anchor repair.22 Statistically significant superiority was found across multiple parameters, including initial tendon displacement, stiffness, and ultimate load to failure (vs 5.5-mm biocomposite fully threaded suture anchor repair), as well as initial and late tendon displacement, stiffness, and ultimate load to failure (vs transosseous repair).22 Although definitive conclusions are difficult to draw on the basis of prior cadaveric studies comparing standard suture anchor repair and transosseous repair, our results decidedly favor the biomechanical characteristics of this 4.75-mm biocomposite knotless suture anchor with suture tape repair and make it a potentially superior repair technique based on biomechanics alone.22
Similarly to standard repair with suture anchors, repair using a 4.75-mm biocomposite knotless suture anchor with tape suture eliminates the need to expose the distal pole of the patella.7,10-12,21 This allows for a smaller surgical incision, less extensive dissection, and prevents possible interference with the patellar tendon.7,10-12,21 Additionally, it eliminates the risk of iatrogenic patellar fracture and damage to the articular surface from drilling the transpatellar tunnels.17,18 Both our own review of cases repaired with our 4.75-mm biocomposite knotless suture anchor with suture tape technique as well as studies of suture anchor repair have consistently found operative times of <1 hour.21 Shorter operative times and smaller surgical wounds are advantageous given that many of these patients have medical comorbidities that predispose them to intraoperative and wound-healing complications.12,19-22
Optimal rehabilitation protocols for quadriceps tendon repair are a matter of controversy. Multiple studies of repair with transosseous patellar tunnels describe immobilization for 6 weeks after surgery, but there has been a recent push toward early motion.7,13,23,24 Reported complications of extended immobilization include limited flexion, pain, weakness, decreased patellar mobility, and patella baja.14 Studies have suggested that, while excessive loading can cause gap formation and weaken the repair, some controlled motion is necessary to heal the tendon23,25 and reduce the risks of stiffness and atrophy.14 The improved biomechanical characteristics of the 4.75-mm biocomposite knotless suture anchor with tape suture technique allow for safe early initiation of ROM exercises and accelerated rehabilitation protocols.
In our early experience with this technique, functional outcomes have been excellent. A formal 2-year outcome study of patients who have undergone quadriceps tendon repair with this 4.75-mm biocomposite knotless suture anchor with tape suture technique is under way.
1. Clayton RA, Court-Brown CM. The epidemiology of musculoskeletal tendinous and ligamentous injuries. Injury. 2008;39(12):1338-1344.
2. Rasul AT Jr, Fischer DA. Primary repair of quadriceps tendon ruptures. Clin Orthop Relat Res. 1993;(289):205-207.
3. Ilan DI, Tejwani N, Keschner M, Leibman M. Quadriceps tendon rupture. J Am Acad Orthop Surg. 2003;11(3):192-200.
4. Ramseier LE, Werner CM, Heinzelmann M. Quadriceps and patellar tendon rupture. Injury. 2006;37(6):516-519.
5. Ciriello V, Gudipati S, Tosounidis T, Soucacos PN, Giannoudis PV. Clinical outcomes after repair of quadriceps tendon rupture: a systematic review. Injury. 2012;43(11):1931-1938.
6. O’Shea K, Kenny P, Donovan J, Condon F, McElwain JP. Outcomes following quadriceps tendon ruptures. Injury. 2002;33(3):257-260.
7. Richards DP, Barber FA. Repair of quadriceps tendon ruptures using suture anchors. Arthroscopy. 2002;18(5):556-559.
8. Wenzl ME, Kirchner R, Seide K, Strametz S, Jürgens C. Quadriceps tendon ruptures—is there a complete functional restitution? Injury. 2004;35(9):922-926.
9. Boudissa M, Roudet A, Rubens-Duval B, Chaussard C, Saragaglia D. Acute quadriceps tendon ruptures: a series of 50 knees with an average follow-up of more than 6 years. Orthop Traumatol Surg Res. 2014;100(2):213-216.
10. Petri M, Dratzidis A, Brand S, et al. Suture anchor repair yields better biomechanical properties than transosseous sutures in ruptured quadriceps tendons. Knee Surg Sports Traumatol Arthrosc. 2015;23(4):1039-1045.
11. Lighthart WC, Cohen DA, Levine RG, Parks BG, Boucher HR. Suture anchor versus suture through tunnel fixation for quadriceps tendon rupture: a biomechanical study. Orthopedics. 2008;31(5):441.
12. Hart ND, Wallace MK, Scovell JF, Krupp RJ, Cook C, Wyland DJ. Quadriceps tendon rupture: a biomechanical comparison of transosseous equivalent double-row suture anchor versus transosseous tunnel repair. J Knee Surg. 2012;25(4):335-339.
13. Rougraff BT, Reeck CC, Essenmacher J. Complete quadriceps tendon ruptures. Orthopedics. 1996;19(6):509-514.
14. West JL, Keene JS, Kaplan LD. Early motion after quadriceps and patellar tendon repairs: outcomes with single-suture augmentation. Am J Sports Med. 2008;36(2):316-323.
15. De Baere T, Geulette B, Manche E, Barras L. Functional results after surgical repair of quadriceps tendon rupture. Acta Orthop Belg. 2002;68(2):146-149.
16. Konrath GA, Chen D, Lock T, et al. Outcomes following repair of quadriceps tendon ruptures. J Orthop Trauma. 1998;12(4):273-279.
17. Gregory JM, Sherman SL, Mather R, Bach BR Jr. Patellar stress fracture after transosseous extensor mechanism repair: report of 3 cases. Am J Sports Med. 2012;40(7):1668-1672.
18. Bushnell BD, Whitener GB, Rubright JH, Creighton RA, Logel KJ, Wood ML. The use of suture anchors to repair the ruptured quadriceps tendon. J Orthop Trauma. 2007;21(6):407-413.
19. Harris JD, Abrams GD, Yanke AB, Hellman MD, Erickson BJ, Bach BR Jr. Suture anchor repair of quadriceps tendon rupture. Orthopedics. 2014;37(3):183-186.
20. Maniscalco P, Bertone C, Rivera F, Bocchi L. A new method of repair for quadriceps tendon ruptures. A case report. Panminerva Med. 2000;42(3):223-225.
21. Sherman SL, Copeland ME, Milles JL, Flood DA, Pfeiffer FM. Biomechanical evaluation of suture anchor versus transosseous tunnel quadriceps tendon repair techniques. Arthroscopy. 2016;32(6):1117-1124.
22. Kindya MC, Konicek J, Rizzi A, Komatsu DE, Paci JM. Knotless suture anchor with suture tape quadriceps tendon repair is biomechanically superior to transosseous and traditional suture anchor-based repairs in a cadaveric model. Arthroscopy. 2017;33(1):190-198.
23. Brossard P, Le Roux G, Vasse B; Orthopedics, Traumatology Society of Western France (SOO). Acute quadriceps tendon rupture repaired by suture anchors: outcomes at 7 years’ follow-up in 25 cases. Orthop Traumatol Surg Res. 2017;103(4):597-601.
24. Langenhan R, Baumann M, Ricart P, et al. Postoperative functional rehabilitation after repair of quadriceps tendon ruptures: a comparison of two different protocols. Knee Surg Sports Traumatol Arthrosc. 2012;20(11):2275-2278.
25. Killian ML, Cavinatto L, Galatz LM, Thomopoulos S. The role of mechanobiology in tendon healing. J Shoulder Elbow Surg. 2012;21(2):228-237.
1. Clayton RA, Court-Brown CM. The epidemiology of musculoskeletal tendinous and ligamentous injuries. Injury. 2008;39(12):1338-1344.
2. Rasul AT Jr, Fischer DA. Primary repair of quadriceps tendon ruptures. Clin Orthop Relat Res. 1993;(289):205-207.
3. Ilan DI, Tejwani N, Keschner M, Leibman M. Quadriceps tendon rupture. J Am Acad Orthop Surg. 2003;11(3):192-200.
4. Ramseier LE, Werner CM, Heinzelmann M. Quadriceps and patellar tendon rupture. Injury. 2006;37(6):516-519.
5. Ciriello V, Gudipati S, Tosounidis T, Soucacos PN, Giannoudis PV. Clinical outcomes after repair of quadriceps tendon rupture: a systematic review. Injury. 2012;43(11):1931-1938.
6. O’Shea K, Kenny P, Donovan J, Condon F, McElwain JP. Outcomes following quadriceps tendon ruptures. Injury. 2002;33(3):257-260.
7. Richards DP, Barber FA. Repair of quadriceps tendon ruptures using suture anchors. Arthroscopy. 2002;18(5):556-559.
8. Wenzl ME, Kirchner R, Seide K, Strametz S, Jürgens C. Quadriceps tendon ruptures—is there a complete functional restitution? Injury. 2004;35(9):922-926.
9. Boudissa M, Roudet A, Rubens-Duval B, Chaussard C, Saragaglia D. Acute quadriceps tendon ruptures: a series of 50 knees with an average follow-up of more than 6 years. Orthop Traumatol Surg Res. 2014;100(2):213-216.
10. Petri M, Dratzidis A, Brand S, et al. Suture anchor repair yields better biomechanical properties than transosseous sutures in ruptured quadriceps tendons. Knee Surg Sports Traumatol Arthrosc. 2015;23(4):1039-1045.
11. Lighthart WC, Cohen DA, Levine RG, Parks BG, Boucher HR. Suture anchor versus suture through tunnel fixation for quadriceps tendon rupture: a biomechanical study. Orthopedics. 2008;31(5):441.
12. Hart ND, Wallace MK, Scovell JF, Krupp RJ, Cook C, Wyland DJ. Quadriceps tendon rupture: a biomechanical comparison of transosseous equivalent double-row suture anchor versus transosseous tunnel repair. J Knee Surg. 2012;25(4):335-339.
13. Rougraff BT, Reeck CC, Essenmacher J. Complete quadriceps tendon ruptures. Orthopedics. 1996;19(6):509-514.
14. West JL, Keene JS, Kaplan LD. Early motion after quadriceps and patellar tendon repairs: outcomes with single-suture augmentation. Am J Sports Med. 2008;36(2):316-323.
15. De Baere T, Geulette B, Manche E, Barras L. Functional results after surgical repair of quadriceps tendon rupture. Acta Orthop Belg. 2002;68(2):146-149.
16. Konrath GA, Chen D, Lock T, et al. Outcomes following repair of quadriceps tendon ruptures. J Orthop Trauma. 1998;12(4):273-279.
17. Gregory JM, Sherman SL, Mather R, Bach BR Jr. Patellar stress fracture after transosseous extensor mechanism repair: report of 3 cases. Am J Sports Med. 2012;40(7):1668-1672.
18. Bushnell BD, Whitener GB, Rubright JH, Creighton RA, Logel KJ, Wood ML. The use of suture anchors to repair the ruptured quadriceps tendon. J Orthop Trauma. 2007;21(6):407-413.
19. Harris JD, Abrams GD, Yanke AB, Hellman MD, Erickson BJ, Bach BR Jr. Suture anchor repair of quadriceps tendon rupture. Orthopedics. 2014;37(3):183-186.
20. Maniscalco P, Bertone C, Rivera F, Bocchi L. A new method of repair for quadriceps tendon ruptures. A case report. Panminerva Med. 2000;42(3):223-225.
21. Sherman SL, Copeland ME, Milles JL, Flood DA, Pfeiffer FM. Biomechanical evaluation of suture anchor versus transosseous tunnel quadriceps tendon repair techniques. Arthroscopy. 2016;32(6):1117-1124.
22. Kindya MC, Konicek J, Rizzi A, Komatsu DE, Paci JM. Knotless suture anchor with suture tape quadriceps tendon repair is biomechanically superior to transosseous and traditional suture anchor-based repairs in a cadaveric model. Arthroscopy. 2017;33(1):190-198.
23. Brossard P, Le Roux G, Vasse B; Orthopedics, Traumatology Society of Western France (SOO). Acute quadriceps tendon rupture repaired by suture anchors: outcomes at 7 years’ follow-up in 25 cases. Orthop Traumatol Surg Res. 2017;103(4):597-601.
24. Langenhan R, Baumann M, Ricart P, et al. Postoperative functional rehabilitation after repair of quadriceps tendon ruptures: a comparison of two different protocols. Knee Surg Sports Traumatol Arthrosc. 2012;20(11):2275-2278.
25. Killian ML, Cavinatto L, Galatz LM, Thomopoulos S. The role of mechanobiology in tendon healing. J Shoulder Elbow Surg. 2012;21(2):228-237.
TAKE-HOME POINTS
- Knotless tape suture fixation of the quadriceps tendon is biomechanically superior to traditional fixation techniques.
- When passing locking Krackow stitches, be sure to take all slack out with each pass.
- Consider double tapping the patella pilot holes prior to placing anchors, as the bone is very hard.
- Palpate the articular surface of the patella when drilling pilot holes for safe placement.
- Perform an adequate retinacular repair to complete the repair.
Diagnosing Multiple Myeloma in Primary Care
IN THIS ARTICLE
- Presenting symptoms
- Diagnostic tests
- Differential diagnostic criteria
Multiple myeloma (MM) is a fatal, malignant neoplasm that originates in the plasma cells of bone marrow. A genetic mutation in the plasma cells creates myeloma cells, which replicate and produce monoclonal protein (M-protein). This accumulation of cells and abnormal protein can result in destruction and eventual marrow failure.1,2
MM’s insidious nature means it often goes undetected or misdiagnosed in its early stages; this delayed diagnosis can cause sequelae that limit quality of life. Furthermore, the five-year survival rate for myeloma varies by stage at which the disease is diagnosed: from 48% for distant (metastasized) myeloma to 71% for localized disease.3 It has also been noted that, in the past two decades, improvements in available treatment options and supportive care have contributed to a doubling of median survival time (from three years to six years).4 It is therefore paramount that providers be aware of MM and its signs to facilitate early diagnosis and treatment.
INCIDENCE AND EPIDEMIOLOGY
MM accounts for 1% of all cancers and about 10% of all hematologic malignancies.5 In 2017, the American Cancer Society estimated that more than 30,000 new cases of MM would be diagnosed in the United States.6 Additionally, MM was expected to cause more than 12,000 deaths last year.6
Median age at diagnosis is 69.3 In fact, 75% of men are older than 75 and 79% of women are older than 70 at diagnosis.1
Apart from age, other risk factors for MM have been identified but not fully explicated. For example, the disease is more common in men than in women (with men comprising two-thirds of new cases per year).3 MM is also two to three times more common in black than in white persons, making it the most common hematologic malignancy in this demographic group.3,7
The possibility of a genetic predisposition has also been studied. Several analyses have indicated an increased risk for MM in patients with a family history of the disease—as much as four times higher in those with an affected first-degree relative. This risk was further elevated in black compared with white patients (odds ratios, 17.4 and 1.5, respectively).7 However, many patients with MM have no relatives with this disorder.6,8
DISEASE PROGRESSION
Almost all patients who develop MM also experience an asymptomatic premalignant stage called monoclonal gammopathy of undetermined significance (MGUS). MGUS is present in 3% to 4% of the general population older than 50 and is often an incidental finding. This stage almost always precedes MM—but because it is asymptomatic, only 10% of individuals diagnosed with MM have a known history of MGUS.8
In some patients, an asymptomatic intermediate stage called smoldering multiple myeloma (SMM) can be identified. SMM progresses to MM at a rate of 10% per year for the first five years; the rate decreases to 3% per year over the following five years, and 1% per year after that.8
MM is not curable, but as noted, the survival rate is steadily increasing due to rapidly evolving treatment regimens. Discussion of treatment is outside the scope of this article, but early diagnosis can improve quality of life and clinical outcomes and prolong life expectancy.
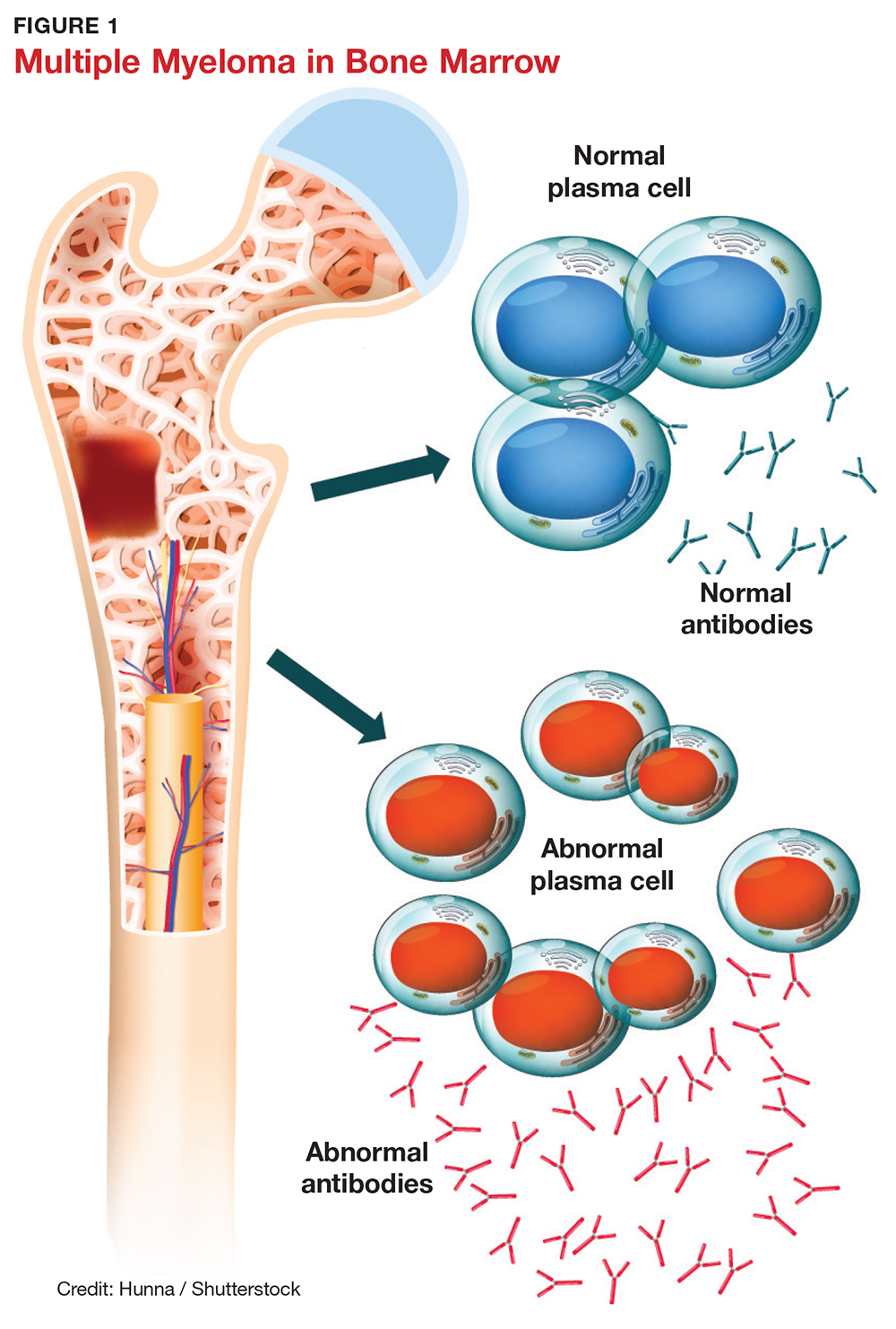
SYMPTOMS
The initial symptoms of MM can be nonspecific and may lead the provider to suspect a host of other conditions.2,6 (Those for advanced disease are also vague but tend to be more pronounced.) These may include fatigue, weakness, easy bruising or bleeding, and bone pain. Other common clinical manifestations of MM are anemia, chronic infection, bone disease, and/or renal failure.1,4 Patients may also experience loss of appetite, nausea, vomiting, increased thirst, and increased urination.9
Recent studies have shown that patients with SMM and/or MGUS also exhibit early signs of bone disease and increased risk for fracture.10 Eighty percent of patients who progress to MM have evidence of pathologic bone fractures.10 It is also possible for bones in the spine to weaken and collapse, pressing on the spinal nerves. This is known as spinal cord compression, which can manifest with sudden, severe back pain or numbness and/or muscle weakness (most often in the legs).6
MM must be included in the differential diagnosis, particularly when symptoms do not point to one specific disease process. Without early diagnosis, disease progression can result in complications such as bone fracture and osteoporosis, reduced kidney function, peripheral neuropathy, chronic anemia, and ultimately, death.2,6 The presence of bone fractures increases mortality risk by 20%.10
DIAGNOSTIC WORKUP
Evidence of MM may be discovered during routine bloodwork and screening tests, while presenting symptoms or subtle changes in lab results can raise suspicion for the disease. Initial bloodwork abnormalities include anemia, elevated calcium levels, renal insufficiency, and/or elevated protein levels.8
A combination of abnormalities in the complete blood count (CBC) and complete metabolic panel (CMP), along with symptoms, should alert the provider to the possibility of MM, prompting additional workup. Table 1 outlines suggested diagnostic tests; the possible findings are discussed below.
CBC. The CBC may reveal abnormalities including anemia (which occurs in 75% of patients with MM), thrombocytopenia, and leukopenia.1,8 These findings can contribute to fatigue, increased incidence of infection, and abnormal bruising of the skin.2,8
CMP. A CMP may show increases in serum calcium or protein. Hypercalcemia occurs in 15% of patients with MM, leading to symptoms such as loss of appetite, nausea, vomiting, increased urination, weakness, and confusion.8 An increase in protein may alter the albumin/globulin ratio, which should raise suspicion for MM. A decrease in albumin can signify disease severity. Also, the CMP may show worsening renal function and elevated serum creatinine, which occurs in 20% of patients with MM.8
Serum protein electrophoresis (SPEP). Suspicion of MM should prompt the clinician to evaluate proteins via SPEP. This test may be indicated for patients with anemia, hypercalcemia, bone pain, and unexplained neuropathy.9 The electrophoresis separates proteins based on their physical properties. This identifies the presence and amount of M-protein, which can determine the extent of the disease.1 M-protein is identified in approximately 82% of patients with MM using this test.8
Serum free light chain (FLC) assay. This diagnostic test can identify MM in individuals with high clinical suspicion for the disease but no discernible M-protein on SPEP; it increases sensitivity to 97%.8 The serum FLC assay evaluates for presence and ratio of free light chains—proteins produced by plasma cells. This test is also useful for monitoring treatment response and disease progression.1
Urine protein electrophoresis (UPEP). The UPEP separates proteins according to charge, which is helpful for classifying renal injury. Protein patterns are interpreted and may be reported as glomerular, tubular, or mixed. UPEP also tests for M-protein in the urine.1,11
24-hour urine. The 24-h urine test quantifies the amount and type of protein excreted in the urine and helps determine the extent of kidney disease.1
Skeletal survey. MM causes significant bone changes that can be identified with radiographic studies. The most common locations for fractures are the vertebral, pelvic, and clavicular areas.10 Currently, the skeletal survey is the gold standard for detecting fractures and osteolytic lesions associated with MM.10 Radiographic films ordered for other purposes may uncover abnormalities in bones.
Bone mineral density (BMD) test. Most often, BMD testing is used to evaluate treatment and progression of bone involvement. Because it can uncover osteopenia or osteoporosis, however, it can also be used to corroborate the diagnosis of MM.10
Once the presence of M-protein is identified, patients are referred for specialty care. At that time, further workup will include a bone marrow biopsy and imaging studies, such as additional radiographic films, CT scans (without contrast, as contrast dye can damage frail kidneys), and MRI.1,8 These diagnostic tests provide useful information for the classification of the disease and guide initiation of treatment.
CLASSIFICATION OF DISEASE
MM can be classified into three stages—MGUS, SMM, and MM—based on recommendations from the International Myeloma Working Group.12 Table 2 outlines the diagnostic criteria for each stage.
Individuals with MGUS and SMM are considered asymptomatic; guidelines do not recommend treatment for these patients. Those who are diagnosed with MM are referred to oncologists and treated based on current clinical practice guidelines.1
CONCLUSION
Multiple myeloma is a malignant neoplasm without a cure. Presenting symptoms may include anemia, bone pain, elevated creatinine or serum protein, fatigue, and hypercalcemia. Early diagnosis is key to early intervention and treatment, which can improve quality of life and clinical outcomes for those affected. Primary care providers play a major role in recognizing the subtle symptoms and ordering the appropriate diagnostic tests.
1. National Comprehensive Cancer Network. Multiple myeloma. NCCN clinical practice guidelines in oncology version 2.2015.
2. Rajkumar VS. Multiple myeloma symptoms, diagnosis, and staging. www.uptodate.com/contents/clinical-features-laboratory-manifestations-and-diagnosis-of-multiple-myeloma?source=machineLearning&search=multiple+myeloma&selectedTitle=1%7E150§ionRank=1&anchor=H25#H26. Accessed October 16, 2017.
3. National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer stat facts: myeloma. https://seer.cancer.gov/statfacts/html/mulmy.html. Accessed October 26, 2017.
4. Röllig C, Knop S, Bornhäuser M. Multiple myeloma. Lancet. 2015;385(9983):2197-2208.
5. Moreau P, San Miguel J, Sonneveld M, et al. Multiple myeloma: ESMO clinical practice guidelines. Ann Oncol. 2017;28(4):iv52-iv61.
6. American Cancer Society. Multiple myeloma. www.cancer.org/cancer/multiplemyeloma/detailedguide. Accessed October 16, 2017.
7. Koura DT, Langston AA. Inherited predisposition to multiple myeloma. Ther Adv Hematol. 2013;4(4):291-297.
8. Rajkumar SV, Kumar S. Multiple myeloma: diagnosis and treatment. Mayo Clin Proc. 2016;91:101-119.
9. O’Connell T, Horita TJ, Kasravi B. Understanding and interpreting serum electrophoresis. Am Fam Physician. 2005; 71(1):105-112.
10. Kristinsson SY, Minter AR, Korde N, et al. Bone disease in multiple myeloma and precursor disease; novel diagnostic approaches and implications on clinical management. Expert Rev Mol Diagn. 2011;11(6):593-603.
11. Jacobs D, DeMott W, Oxley D. Laboratory Test Handbook: Concise With Disease Index. Hudson, OH: Lexi-Comp; 2004.
12. Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia. 2009;23(1):3-9.
IN THIS ARTICLE
- Presenting symptoms
- Diagnostic tests
- Differential diagnostic criteria
Multiple myeloma (MM) is a fatal, malignant neoplasm that originates in the plasma cells of bone marrow. A genetic mutation in the plasma cells creates myeloma cells, which replicate and produce monoclonal protein (M-protein). This accumulation of cells and abnormal protein can result in destruction and eventual marrow failure.1,2
MM’s insidious nature means it often goes undetected or misdiagnosed in its early stages; this delayed diagnosis can cause sequelae that limit quality of life. Furthermore, the five-year survival rate for myeloma varies by stage at which the disease is diagnosed: from 48% for distant (metastasized) myeloma to 71% for localized disease.3 It has also been noted that, in the past two decades, improvements in available treatment options and supportive care have contributed to a doubling of median survival time (from three years to six years).4 It is therefore paramount that providers be aware of MM and its signs to facilitate early diagnosis and treatment.
INCIDENCE AND EPIDEMIOLOGY
MM accounts for 1% of all cancers and about 10% of all hematologic malignancies.5 In 2017, the American Cancer Society estimated that more than 30,000 new cases of MM would be diagnosed in the United States.6 Additionally, MM was expected to cause more than 12,000 deaths last year.6
Median age at diagnosis is 69.3 In fact, 75% of men are older than 75 and 79% of women are older than 70 at diagnosis.1
Apart from age, other risk factors for MM have been identified but not fully explicated. For example, the disease is more common in men than in women (with men comprising two-thirds of new cases per year).3 MM is also two to three times more common in black than in white persons, making it the most common hematologic malignancy in this demographic group.3,7
The possibility of a genetic predisposition has also been studied. Several analyses have indicated an increased risk for MM in patients with a family history of the disease—as much as four times higher in those with an affected first-degree relative. This risk was further elevated in black compared with white patients (odds ratios, 17.4 and 1.5, respectively).7 However, many patients with MM have no relatives with this disorder.6,8
DISEASE PROGRESSION
Almost all patients who develop MM also experience an asymptomatic premalignant stage called monoclonal gammopathy of undetermined significance (MGUS). MGUS is present in 3% to 4% of the general population older than 50 and is often an incidental finding. This stage almost always precedes MM—but because it is asymptomatic, only 10% of individuals diagnosed with MM have a known history of MGUS.8
In some patients, an asymptomatic intermediate stage called smoldering multiple myeloma (SMM) can be identified. SMM progresses to MM at a rate of 10% per year for the first five years; the rate decreases to 3% per year over the following five years, and 1% per year after that.8
MM is not curable, but as noted, the survival rate is steadily increasing due to rapidly evolving treatment regimens. Discussion of treatment is outside the scope of this article, but early diagnosis can improve quality of life and clinical outcomes and prolong life expectancy.

SYMPTOMS
The initial symptoms of MM can be nonspecific and may lead the provider to suspect a host of other conditions.2,6 (Those for advanced disease are also vague but tend to be more pronounced.) These may include fatigue, weakness, easy bruising or bleeding, and bone pain. Other common clinical manifestations of MM are anemia, chronic infection, bone disease, and/or renal failure.1,4 Patients may also experience loss of appetite, nausea, vomiting, increased thirst, and increased urination.9
Recent studies have shown that patients with SMM and/or MGUS also exhibit early signs of bone disease and increased risk for fracture.10 Eighty percent of patients who progress to MM have evidence of pathologic bone fractures.10 It is also possible for bones in the spine to weaken and collapse, pressing on the spinal nerves. This is known as spinal cord compression, which can manifest with sudden, severe back pain or numbness and/or muscle weakness (most often in the legs).6
MM must be included in the differential diagnosis, particularly when symptoms do not point to one specific disease process. Without early diagnosis, disease progression can result in complications such as bone fracture and osteoporosis, reduced kidney function, peripheral neuropathy, chronic anemia, and ultimately, death.2,6 The presence of bone fractures increases mortality risk by 20%.10
DIAGNOSTIC WORKUP
Evidence of MM may be discovered during routine bloodwork and screening tests, while presenting symptoms or subtle changes in lab results can raise suspicion for the disease. Initial bloodwork abnormalities include anemia, elevated calcium levels, renal insufficiency, and/or elevated protein levels.8

A combination of abnormalities in the complete blood count (CBC) and complete metabolic panel (CMP), along with symptoms, should alert the provider to the possibility of MM, prompting additional workup. Table 1 outlines suggested diagnostic tests; the possible findings are discussed below.
CBC. The CBC may reveal abnormalities including anemia (which occurs in 75% of patients with MM), thrombocytopenia, and leukopenia.1,8 These findings can contribute to fatigue, increased incidence of infection, and abnormal bruising of the skin.2,8
CMP. A CMP may show increases in serum calcium or protein. Hypercalcemia occurs in 15% of patients with MM, leading to symptoms such as loss of appetite, nausea, vomiting, increased urination, weakness, and confusion.8 An increase in protein may alter the albumin/globulin ratio, which should raise suspicion for MM. A decrease in albumin can signify disease severity. Also, the CMP may show worsening renal function and elevated serum creatinine, which occurs in 20% of patients with MM.8
Serum protein electrophoresis (SPEP). Suspicion of MM should prompt the clinician to evaluate proteins via SPEP. This test may be indicated for patients with anemia, hypercalcemia, bone pain, and unexplained neuropathy.9 The electrophoresis separates proteins based on their physical properties. This identifies the presence and amount of M-protein, which can determine the extent of the disease.1 M-protein is identified in approximately 82% of patients with MM using this test.8
Serum free light chain (FLC) assay. This diagnostic test can identify MM in individuals with high clinical suspicion for the disease but no discernible M-protein on SPEP; it increases sensitivity to 97%.8 The serum FLC assay evaluates for presence and ratio of free light chains—proteins produced by plasma cells. This test is also useful for monitoring treatment response and disease progression.1
Urine protein electrophoresis (UPEP). The UPEP separates proteins according to charge, which is helpful for classifying renal injury. Protein patterns are interpreted and may be reported as glomerular, tubular, or mixed. UPEP also tests for M-protein in the urine.1,11
24-hour urine. The 24-h urine test quantifies the amount and type of protein excreted in the urine and helps determine the extent of kidney disease.1
Skeletal survey. MM causes significant bone changes that can be identified with radiographic studies. The most common locations for fractures are the vertebral, pelvic, and clavicular areas.10 Currently, the skeletal survey is the gold standard for detecting fractures and osteolytic lesions associated with MM.10 Radiographic films ordered for other purposes may uncover abnormalities in bones.
Bone mineral density (BMD) test. Most often, BMD testing is used to evaluate treatment and progression of bone involvement. Because it can uncover osteopenia or osteoporosis, however, it can also be used to corroborate the diagnosis of MM.10
Once the presence of M-protein is identified, patients are referred for specialty care. At that time, further workup will include a bone marrow biopsy and imaging studies, such as additional radiographic films, CT scans (without contrast, as contrast dye can damage frail kidneys), and MRI.1,8 These diagnostic tests provide useful information for the classification of the disease and guide initiation of treatment.
CLASSIFICATION OF DISEASE
MM can be classified into three stages—MGUS, SMM, and MM—based on recommendations from the International Myeloma Working Group.12 Table 2 outlines the diagnostic criteria for each stage.

Individuals with MGUS and SMM are considered asymptomatic; guidelines do not recommend treatment for these patients. Those who are diagnosed with MM are referred to oncologists and treated based on current clinical practice guidelines.1
CONCLUSION
Multiple myeloma is a malignant neoplasm without a cure. Presenting symptoms may include anemia, bone pain, elevated creatinine or serum protein, fatigue, and hypercalcemia. Early diagnosis is key to early intervention and treatment, which can improve quality of life and clinical outcomes for those affected. Primary care providers play a major role in recognizing the subtle symptoms and ordering the appropriate diagnostic tests.
IN THIS ARTICLE
- Presenting symptoms
- Diagnostic tests
- Differential diagnostic criteria
Multiple myeloma (MM) is a fatal, malignant neoplasm that originates in the plasma cells of bone marrow. A genetic mutation in the plasma cells creates myeloma cells, which replicate and produce monoclonal protein (M-protein). This accumulation of cells and abnormal protein can result in destruction and eventual marrow failure.1,2
MM’s insidious nature means it often goes undetected or misdiagnosed in its early stages; this delayed diagnosis can cause sequelae that limit quality of life. Furthermore, the five-year survival rate for myeloma varies by stage at which the disease is diagnosed: from 48% for distant (metastasized) myeloma to 71% for localized disease.3 It has also been noted that, in the past two decades, improvements in available treatment options and supportive care have contributed to a doubling of median survival time (from three years to six years).4 It is therefore paramount that providers be aware of MM and its signs to facilitate early diagnosis and treatment.
INCIDENCE AND EPIDEMIOLOGY
MM accounts for 1% of all cancers and about 10% of all hematologic malignancies.5 In 2017, the American Cancer Society estimated that more than 30,000 new cases of MM would be diagnosed in the United States.6 Additionally, MM was expected to cause more than 12,000 deaths last year.6
Median age at diagnosis is 69.3 In fact, 75% of men are older than 75 and 79% of women are older than 70 at diagnosis.1
Apart from age, other risk factors for MM have been identified but not fully explicated. For example, the disease is more common in men than in women (with men comprising two-thirds of new cases per year).3 MM is also two to three times more common in black than in white persons, making it the most common hematologic malignancy in this demographic group.3,7
The possibility of a genetic predisposition has also been studied. Several analyses have indicated an increased risk for MM in patients with a family history of the disease—as much as four times higher in those with an affected first-degree relative. This risk was further elevated in black compared with white patients (odds ratios, 17.4 and 1.5, respectively).7 However, many patients with MM have no relatives with this disorder.6,8
DISEASE PROGRESSION
Almost all patients who develop MM also experience an asymptomatic premalignant stage called monoclonal gammopathy of undetermined significance (MGUS). MGUS is present in 3% to 4% of the general population older than 50 and is often an incidental finding. This stage almost always precedes MM—but because it is asymptomatic, only 10% of individuals diagnosed with MM have a known history of MGUS.8
In some patients, an asymptomatic intermediate stage called smoldering multiple myeloma (SMM) can be identified. SMM progresses to MM at a rate of 10% per year for the first five years; the rate decreases to 3% per year over the following five years, and 1% per year after that.8
MM is not curable, but as noted, the survival rate is steadily increasing due to rapidly evolving treatment regimens. Discussion of treatment is outside the scope of this article, but early diagnosis can improve quality of life and clinical outcomes and prolong life expectancy.

SYMPTOMS
The initial symptoms of MM can be nonspecific and may lead the provider to suspect a host of other conditions.2,6 (Those for advanced disease are also vague but tend to be more pronounced.) These may include fatigue, weakness, easy bruising or bleeding, and bone pain. Other common clinical manifestations of MM are anemia, chronic infection, bone disease, and/or renal failure.1,4 Patients may also experience loss of appetite, nausea, vomiting, increased thirst, and increased urination.9
Recent studies have shown that patients with SMM and/or MGUS also exhibit early signs of bone disease and increased risk for fracture.10 Eighty percent of patients who progress to MM have evidence of pathologic bone fractures.10 It is also possible for bones in the spine to weaken and collapse, pressing on the spinal nerves. This is known as spinal cord compression, which can manifest with sudden, severe back pain or numbness and/or muscle weakness (most often in the legs).6
MM must be included in the differential diagnosis, particularly when symptoms do not point to one specific disease process. Without early diagnosis, disease progression can result in complications such as bone fracture and osteoporosis, reduced kidney function, peripheral neuropathy, chronic anemia, and ultimately, death.2,6 The presence of bone fractures increases mortality risk by 20%.10
DIAGNOSTIC WORKUP
Evidence of MM may be discovered during routine bloodwork and screening tests, while presenting symptoms or subtle changes in lab results can raise suspicion for the disease. Initial bloodwork abnormalities include anemia, elevated calcium levels, renal insufficiency, and/or elevated protein levels.8

A combination of abnormalities in the complete blood count (CBC) and complete metabolic panel (CMP), along with symptoms, should alert the provider to the possibility of MM, prompting additional workup. Table 1 outlines suggested diagnostic tests; the possible findings are discussed below.
CBC. The CBC may reveal abnormalities including anemia (which occurs in 75% of patients with MM), thrombocytopenia, and leukopenia.1,8 These findings can contribute to fatigue, increased incidence of infection, and abnormal bruising of the skin.2,8
CMP. A CMP may show increases in serum calcium or protein. Hypercalcemia occurs in 15% of patients with MM, leading to symptoms such as loss of appetite, nausea, vomiting, increased urination, weakness, and confusion.8 An increase in protein may alter the albumin/globulin ratio, which should raise suspicion for MM. A decrease in albumin can signify disease severity. Also, the CMP may show worsening renal function and elevated serum creatinine, which occurs in 20% of patients with MM.8
Serum protein electrophoresis (SPEP). Suspicion of MM should prompt the clinician to evaluate proteins via SPEP. This test may be indicated for patients with anemia, hypercalcemia, bone pain, and unexplained neuropathy.9 The electrophoresis separates proteins based on their physical properties. This identifies the presence and amount of M-protein, which can determine the extent of the disease.1 M-protein is identified in approximately 82% of patients with MM using this test.8
Serum free light chain (FLC) assay. This diagnostic test can identify MM in individuals with high clinical suspicion for the disease but no discernible M-protein on SPEP; it increases sensitivity to 97%.8 The serum FLC assay evaluates for presence and ratio of free light chains—proteins produced by plasma cells. This test is also useful for monitoring treatment response and disease progression.1
Urine protein electrophoresis (UPEP). The UPEP separates proteins according to charge, which is helpful for classifying renal injury. Protein patterns are interpreted and may be reported as glomerular, tubular, or mixed. UPEP also tests for M-protein in the urine.1,11
24-hour urine. The 24-h urine test quantifies the amount and type of protein excreted in the urine and helps determine the extent of kidney disease.1
Skeletal survey. MM causes significant bone changes that can be identified with radiographic studies. The most common locations for fractures are the vertebral, pelvic, and clavicular areas.10 Currently, the skeletal survey is the gold standard for detecting fractures and osteolytic lesions associated with MM.10 Radiographic films ordered for other purposes may uncover abnormalities in bones.
Bone mineral density (BMD) test. Most often, BMD testing is used to evaluate treatment and progression of bone involvement. Because it can uncover osteopenia or osteoporosis, however, it can also be used to corroborate the diagnosis of MM.10
Once the presence of M-protein is identified, patients are referred for specialty care. At that time, further workup will include a bone marrow biopsy and imaging studies, such as additional radiographic films, CT scans (without contrast, as contrast dye can damage frail kidneys), and MRI.1,8 These diagnostic tests provide useful information for the classification of the disease and guide initiation of treatment.
CLASSIFICATION OF DISEASE
MM can be classified into three stages—MGUS, SMM, and MM—based on recommendations from the International Myeloma Working Group.12 Table 2 outlines the diagnostic criteria for each stage.

Individuals with MGUS and SMM are considered asymptomatic; guidelines do not recommend treatment for these patients. Those who are diagnosed with MM are referred to oncologists and treated based on current clinical practice guidelines.1
CONCLUSION
Multiple myeloma is a malignant neoplasm without a cure. Presenting symptoms may include anemia, bone pain, elevated creatinine or serum protein, fatigue, and hypercalcemia. Early diagnosis is key to early intervention and treatment, which can improve quality of life and clinical outcomes for those affected. Primary care providers play a major role in recognizing the subtle symptoms and ordering the appropriate diagnostic tests.
1. National Comprehensive Cancer Network. Multiple myeloma. NCCN clinical practice guidelines in oncology version 2.2015.
2. Rajkumar VS. Multiple myeloma symptoms, diagnosis, and staging. www.uptodate.com/contents/clinical-features-laboratory-manifestations-and-diagnosis-of-multiple-myeloma?source=machineLearning&search=multiple+myeloma&selectedTitle=1%7E150§ionRank=1&anchor=H25#H26. Accessed October 16, 2017.
3. National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer stat facts: myeloma. https://seer.cancer.gov/statfacts/html/mulmy.html. Accessed October 26, 2017.
4. Röllig C, Knop S, Bornhäuser M. Multiple myeloma. Lancet. 2015;385(9983):2197-2208.
5. Moreau P, San Miguel J, Sonneveld M, et al. Multiple myeloma: ESMO clinical practice guidelines. Ann Oncol. 2017;28(4):iv52-iv61.
6. American Cancer Society. Multiple myeloma. www.cancer.org/cancer/multiplemyeloma/detailedguide. Accessed October 16, 2017.
7. Koura DT, Langston AA. Inherited predisposition to multiple myeloma. Ther Adv Hematol. 2013;4(4):291-297.
8. Rajkumar SV, Kumar S. Multiple myeloma: diagnosis and treatment. Mayo Clin Proc. 2016;91:101-119.
9. O’Connell T, Horita TJ, Kasravi B. Understanding and interpreting serum electrophoresis. Am Fam Physician. 2005; 71(1):105-112.
10. Kristinsson SY, Minter AR, Korde N, et al. Bone disease in multiple myeloma and precursor disease; novel diagnostic approaches and implications on clinical management. Expert Rev Mol Diagn. 2011;11(6):593-603.
11. Jacobs D, DeMott W, Oxley D. Laboratory Test Handbook: Concise With Disease Index. Hudson, OH: Lexi-Comp; 2004.
12. Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia. 2009;23(1):3-9.
1. National Comprehensive Cancer Network. Multiple myeloma. NCCN clinical practice guidelines in oncology version 2.2015.
2. Rajkumar VS. Multiple myeloma symptoms, diagnosis, and staging. www.uptodate.com/contents/clinical-features-laboratory-manifestations-and-diagnosis-of-multiple-myeloma?source=machineLearning&search=multiple+myeloma&selectedTitle=1%7E150§ionRank=1&anchor=H25#H26. Accessed October 16, 2017.
3. National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer stat facts: myeloma. https://seer.cancer.gov/statfacts/html/mulmy.html. Accessed October 26, 2017.
4. Röllig C, Knop S, Bornhäuser M. Multiple myeloma. Lancet. 2015;385(9983):2197-2208.
5. Moreau P, San Miguel J, Sonneveld M, et al. Multiple myeloma: ESMO clinical practice guidelines. Ann Oncol. 2017;28(4):iv52-iv61.
6. American Cancer Society. Multiple myeloma. www.cancer.org/cancer/multiplemyeloma/detailedguide. Accessed October 16, 2017.
7. Koura DT, Langston AA. Inherited predisposition to multiple myeloma. Ther Adv Hematol. 2013;4(4):291-297.
8. Rajkumar SV, Kumar S. Multiple myeloma: diagnosis and treatment. Mayo Clin Proc. 2016;91:101-119.
9. O’Connell T, Horita TJ, Kasravi B. Understanding and interpreting serum electrophoresis. Am Fam Physician. 2005; 71(1):105-112.
10. Kristinsson SY, Minter AR, Korde N, et al. Bone disease in multiple myeloma and precursor disease; novel diagnostic approaches and implications on clinical management. Expert Rev Mol Diagn. 2011;11(6):593-603.
11. Jacobs D, DeMott W, Oxley D. Laboratory Test Handbook: Concise With Disease Index. Hudson, OH: Lexi-Comp; 2004.
12. Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia. 2009;23(1):3-9.
Biomechanical Evaluation of a Novel Suture Augment in Patella Fixation
Take-Home Points
- Suture augmentation improves construct strength for patella fixation.
- Krackow sutures may be placed in the quadriceps and patella tendons, then secured over the anterior patella (much like an anterior tension band).
- The Krackow technique described was superior to the suture cerclage technique based on mean load values, but did not reach statistical significance.
- The Krackow suture technique is a viable and easily applied technique for suture augmentation of patella fixation constructs.
Patella fractures are relatively uncommon, accounting for only 1% of skeletal injuries.1 Restoration of the function of the patella and the extensor mechanism is vital for knee extension and gait. However, patella fractures have an inherently high rate of complications, making these injuries challenging to treat.2-4 In patients with intact extensor function, displacement of <4 mm, and articular step-off of <3 mm, nonoperative management is extremely effective, with 99% of patients reporting favorable results.5 However, for fractures in which the extensor mechanism is disrupted, surgical intervention typically is indicated.6
Authors have reported various surgical interventions, one of the most commonly used being the anterior tension band (ATB) technique, first described by the AO (Arbeitsgemeinschaft für Osteosynthesefragen) group in the 1950s.7 By converting distractive anterior force during knee flexion to compressive force at the fracture site, the ATB technique provides a repair stronger than the previously used cerclage repair.8 Although initially considered standard of care, the ATB technique was soon found to be associated with implant failure and subcutaneous irritation prompting implant removal.9
To address these issues, Berg10 and Carpenter and colleagues11 evaluated an ATB technique that used cannulated screws instead of Kirschner wires (K-wires). This variation on the ATB technique reduced the implant-related issues while maintaining the mechanical advantage of the tension band. The more rigid design also permitted earlier postoperative rehabilitation, which significantly reduced development of arthrofibrosis.6,7,10 This modified ATB (MATB) technique has since been investigated for additional augments, mainly focusing on use of different tension band materials, including polyester suture and braided composite suture.12-14
However, there is little research on augments that incorporate the surrounding soft tissue, specifically the quadriceps and patellar tendons. In a recent retrospective clinical study, Oh and colleagues15 found positive clinical results with use of Krackow sutures, though 2 or 3 vertically oriented stainless steel wires were used instead of cannulated screws.
We conducted a study to determine the biomechanical efficacy of using a cerclage suture augment and a Krackow suture augment coupled with and compared with conventional MATB repair. If effective, this technique may represent another strategy for increasing repair strength and thereby improve postoperative outcomes.
Materials and Methods
Specimen Preparation
Fresh-frozen cadaver extensor mechanisms (quadriceps tendon, patella, surrounding retinaculum, patellar tendon) were kept frozen at –4°C until preparation. Fifteen specimens were selected. Mean (SD) age at death was 68 (10) years (range, 51-85 years). One specimen was excluded for a short patella tendon, which precluded adequate attachment for testing. All specimens were free of overt osseous pathology.
After specimens were thawed overnight, the patellae were transversely osteotomized with an osteotome at the junction of the middle and distal thirds of the patella. Sharp dissection was performed to carry the division through the medial and lateral retinaculum at the same level. All 14 specimens were then repaired using the MATB technique. First, the transverse fracture was reduced with a reduction clamp. Then, two 4-mm cannulated screws (DePuy Synthes) were inserted parallel to each other and perpendicular to the fracture. An 18-gauge stainless steel wire was then passed through each screw, crossed anteriorly, and tightened to create a figure-of-8 ATB. The specimens were then randomly divided into 3 groups—MATB; MATB with cerclage suture augment; MATB with Krackow suture augment—while ensuring specimens from a single cadaver were placed in different groups to avoid confounding based on bone density differences.
Experimental Setup
Repaired specimens were secured with tissue clamps at the quadriceps and patellar tendons on an MTS Bionix 858 (MTS Systems) hydraulic arm.
Each patella was secured for cyclic testing. Initially it was placed under 10 N of tension. Then it underwent tensile loading from 10 N to 300 N at 50 N/s for 10 cycles. These parameters were based on previous biomechanical patella studies.10,11 Load was measured with the MTS load cell and displacement with the displacement transducer. Fracture displacement associated with 300-N cyclic tension was recorded. Displacement was calculated as the difference between 10th cycle and 2nd cycle values, which accounted for any degree of initial tissue slippage. After cyclic testing, the patella was placed back in 10 N of tensile loading and subjected to maximum force loading to determine ultimate repair strength. For maximum loading, the patella was stretched progressively at 50 N/s until failure. Again, load and displacement were measured with MTS.
Statistical Analysis
After testing, fracture displacement and maximum load force data were compiled for analysis. One-way analysis of variance with Bonferroni correction was used to determine if there were significant differences between groups. Significance level was set at P < .05.
Results
For cyclic testing, mean total displacement was measured over 10 cycles for each group. Again, displacement was determined by taking the difference between 10th cycle and 2nd cycle values, allowing for system stabilization.
Discussion
Our main objective was to compare the efficacy of a novel suture augment technique with that of other patella fracture repair techniques. Our hypothesis—that adding a Krackow suture augment would increase strength in both cyclic and maximum loading—was supported. Although testing results were not statistically significant because of the small sample size, we think this novel technique has clinically relevant descriptive significance and warrants further investigation.
Proper anatomical reduction and postoperative stabilization are of utmost importance in clinical approaches to patella fractures. In addition, regardless of which technical procedure is used, open reduction should also allow for early range of motion to prevent joint arthrofibrosis. Ever since the ATB technique was first described by the AO group, postoperative outcomes have improved significantly. In a retrospective study by Levack and colleagues,17 30 of 64 patients with patella fractures underwent internal fixation. Mean follow-up was 6.2 years. By both objective and subjective measures, the best functional outcomes were associated with internal tension band fixation (vs cerclage repair). Lotke and Ecker18 also documented the efficacy of the tension band technique. Sixteen patients with patella fractures underwent anterior tension banding; those with a comminuted fracture also underwent cerclage repair for patella stabilization during tension banding. At 6-week follow-up, all patients had good range of motion (≥90° flexion), relatively few symptoms, and no implant failures. Results were similar to those of Levack and colleagues.17
Although it improves stability and functional outcomes over conventional patellectomy and cerclage wiring, the ATB technique has been associated with subcutaneous irritation caused by the K-wires used to secure the band. Hung and colleagues9 followed up 68 patients with patellar fractures. Five of these patients underwent tension banding. Although there was a high level of adequate functional outcomes, implant irritation was found to be “quite frequent.”
To address this issue, Carpenter and colleagues11 evaluated an ATB technique that uses K-wires instead of cannulated screws. Biomechanical testing in a cadaver model revealed less fracture displacement and overall more repair strength through cyclic and maximum load testing. Clinically, these results were supported by Berg,10 who followed up 10 patients with transverse patella fractures repaired with the MATB technique. At a mean follow-up of 24 months, 7 of the 10 patients had good to excellent outcomes, and there were no implant failures.
Further investigation into patella repairs has mainly focused on improving the MATB technique and experimenting with different tension band materials. Rabalais and colleagues13 biomechanically tested high-strength polyethylene suture as a replacement for standard 18-gauge wire, and Bryant and colleagues14 tested a braided composite suture (FiberWire; Arthrex) as a replacement for standard 18-gauge stainless steel wire. Both found no significant difference with use of augmented tension band material, but Rabalais and colleagues13 did find more advantages with a parallel tension band construct than with a standard figure-of-8 arrangement.
In developing our novel technique, we considered that Krackow sutures are routinely used in both quadriceps tendon repair and patellar tendon repair, including partial patellectomy for distal patella fracture. With a suture placed in both tendons, the augment could be expected to resist longitudinal gapping and augment the tension band across the anterior patella. First described by Krackow and colleagues,19 the Krackow suture is widely used for tendon reconstruction. In an interlocking system of sutures, the Krackow suture provides a repair that is more stable than repair with conventional suture techniques, specifically in the context of tendon repair.20 Given the sesamoidal nature of the patella, its repair shares the goal of gap prevention with other tendon repairs. In theory, anchoring the supporting structures that are above and below the patella provides support for the intervening patella and ultimately improves fracture fixation strength.
Oh and colleagues15 reported on the clinical efficacy of a Krackow augment in distal pole patella repairs. Similarly, we found a Krackow augment to be efficacious, supporting its potential in clinical approaches to patella repairs. Our results indicate this augment can be a useful clinical adjunct in biomechanical evaluation.
Limitations of this study include its use of dissected extensor mechanisms, which may have less biofidelity than whole-knee specimens. In our model, specimens were secured at the patellar tendon and the quadriceps tendons, as opposed to the quadriceps tendon and the tibia distally. Use of this model could have led to an increase in early displacement during cyclic testing as a result of tissue slippage. Furthermore, our small sample size could have affected our ability to demonstrate a difference between these techniques.
Given its increased strength as demonstrated by mean displacement during cyclic loading and mean load to failure, as well as the early clinical data recently published, the Krackow suture augment represents a feasible technique for patella fixation. It likely will be most useful in cases in which conventional techniques are prone to failure or cannot be applied, such as severe distal comminution or poor bone density. Further biomechanical testing with a larger number of specimens may be required for statistical significance.
Conclusion
In patella fracture repair strategies, the Krackow suture augment increased strength when used with a MATB technique. Failure to reach statistical significance likely resulted from our small sample size. Further biomechanical testing and clinical studies are needed for more complete evaluation of this technique. We think it will be most useful in the setting of poor bone quality or severe comminution, which can limit fixation options. As increased repair strength allows earlier postoperative rehabilitation and maintains fracture reduction, patient outcomes should improve. This novel technique represents another strategy for managing challenging patella fractures.
1. Boström Å. Fracture of the patella. A study of 422 patellar fractures. Acta Orthop Scand Suppl. 1972;143:1-80.
2. Hungerford DS, Barry M. Biomechanics of the patellofemoral joint. Clin Orthop Relat Res. 1979;(144):9-15.
3. LeBrun CT, Langford JR, Sagi HC. Functional outcomes after operatively treated patella fractures. J Orthop Trauma. 2012;26(7):422-426.
4. Kaufer H. Mechanical function of the patella. J Bone Joint Surg Am. 1971;53(8):1551-1560.
5. Braun W, Wiedemann M, Rüter A, Kundel K, Kolbinger S. Indications and results of nonoperative treatment of patellar fractures. Clin Orthop Relat Res. 1993;(289):197-201.
6. Melvin JS, Mehta S. Patellar fractures in adults. J Am Acad Orthop Surg. 2011;19(4):198-207.
7. Müller M, Allgöwer M, Schneider R, Willeneger H. Manual of Internal Fixation: Techniques Recommended by the AO Group. Berlin, Germany: Springer; 1979.
8. Weber MJ, Janecki CJ, McLeod P, Nelson CL, Thompson JA. Efficacy of various forms of fixation of transverse fractures of the patella. J Bone Joint Surg Am. 1980;62(2):215-220.
9. Hung LK, Chan KM, Chow YN, Leung PC. Fractured patella: operative treatment using the tension band principle. Injury. 1985;16(5):343-347.
10. Berg EE. Open reduction internal fixation of displaced transverse patella fractures with figure-eight wiring through parallel cannulated compression screws. J Orthop Trauma. 1997;11(8):573-576.
11. Carpenter JE, Kasman RA, Patel N, Lee ML, Goldstein SA. Biomechanical evaluation of current patella fracture fixation techniques. J Orthop Trauma. 1997;11(5):351-356.
12. Hughes SC, Stott PM, Hearnden AJ, Ripley LG. A new and effective tension-band braided polyester suture technique for transverse patellar fracture fixation. Injury. 2007;38(2):212-222.
13. Rabalais RD, Burger E, Lu Y, Mansour A, Baratta RV. Comparison of two tension-band fixation materials and techniques in transverse patella fractures: a biomechanical study. Orthopedics. 2008;31(2):128.
14. Bryant TL, Anderson CL, Stevens CG, Conrad BP, Vincent HK, Sadasivan KK. Comparison of cannulated screws with FiberWire or stainless steel wire for patella fracture fixation: a pilot study. J Orthop. 2014;12(2):92-96.
15. Oh HK, Choo SK, Kim JW, Lee M. Internal fixation of displaced inferior pole of the patella fractures using vertical wiring augmented with Krachow suturing. Injury. 2015;46(12):2512-2515.
16. Goodfellow J, Hungerford DS, Zindel M. Patello-femoral joint mechanics and pathology. 1. Functional anatomy of the patello-femoral joint. J Bone Joint Surg Br. 1976;58(3):287-290.
17. Levack B, Flannagan JP, Hobbs S. Results of surgical treatment of patellar fractures. J Bone Joint Surg Br. 1985;67(3):416-419.
18. Lotke PA, Ecker ML. Transverse fractures of the patella. Clin Orthop Relat Res. 1981;(158):180-184.
19. Krackow KA, Thomas SC, Jones LC. Ligament-tendon fixation: analysis of a new stitch and comparison with standard techniques. Orthopedics. 1988;11(6):909-917.
20. Hahn JM, Inceoğlu S, Wongworawat MD. Biomechanical comparison of Krackow locking stitch versus nonlocking loop stitch with varying number of throws. Am J Sports Med. 2014;42(12):3003-3008.
Take-Home Points
- Suture augmentation improves construct strength for patella fixation.
- Krackow sutures may be placed in the quadriceps and patella tendons, then secured over the anterior patella (much like an anterior tension band).
- The Krackow technique described was superior to the suture cerclage technique based on mean load values, but did not reach statistical significance.
- The Krackow suture technique is a viable and easily applied technique for suture augmentation of patella fixation constructs.
Patella fractures are relatively uncommon, accounting for only 1% of skeletal injuries.1 Restoration of the function of the patella and the extensor mechanism is vital for knee extension and gait. However, patella fractures have an inherently high rate of complications, making these injuries challenging to treat.2-4 In patients with intact extensor function, displacement of <4 mm, and articular step-off of <3 mm, nonoperative management is extremely effective, with 99% of patients reporting favorable results.5 However, for fractures in which the extensor mechanism is disrupted, surgical intervention typically is indicated.6
Authors have reported various surgical interventions, one of the most commonly used being the anterior tension band (ATB) technique, first described by the AO (Arbeitsgemeinschaft für Osteosynthesefragen) group in the 1950s.7 By converting distractive anterior force during knee flexion to compressive force at the fracture site, the ATB technique provides a repair stronger than the previously used cerclage repair.8 Although initially considered standard of care, the ATB technique was soon found to be associated with implant failure and subcutaneous irritation prompting implant removal.9
To address these issues, Berg10 and Carpenter and colleagues11 evaluated an ATB technique that used cannulated screws instead of Kirschner wires (K-wires). This variation on the ATB technique reduced the implant-related issues while maintaining the mechanical advantage of the tension band. The more rigid design also permitted earlier postoperative rehabilitation, which significantly reduced development of arthrofibrosis.6,7,10 This modified ATB (MATB) technique has since been investigated for additional augments, mainly focusing on use of different tension band materials, including polyester suture and braided composite suture.12-14
However, there is little research on augments that incorporate the surrounding soft tissue, specifically the quadriceps and patellar tendons. In a recent retrospective clinical study, Oh and colleagues15 found positive clinical results with use of Krackow sutures, though 2 or 3 vertically oriented stainless steel wires were used instead of cannulated screws.
We conducted a study to determine the biomechanical efficacy of using a cerclage suture augment and a Krackow suture augment coupled with and compared with conventional MATB repair. If effective, this technique may represent another strategy for increasing repair strength and thereby improve postoperative outcomes.
Materials and Methods
Specimen Preparation
Fresh-frozen cadaver extensor mechanisms (quadriceps tendon, patella, surrounding retinaculum, patellar tendon) were kept frozen at –4°C until preparation. Fifteen specimens were selected. Mean (SD) age at death was 68 (10) years (range, 51-85 years). One specimen was excluded for a short patella tendon, which precluded adequate attachment for testing. All specimens were free of overt osseous pathology.
After specimens were thawed overnight, the patellae were transversely osteotomized with an osteotome at the junction of the middle and distal thirds of the patella. Sharp dissection was performed to carry the division through the medial and lateral retinaculum at the same level. All 14 specimens were then repaired using the MATB technique. First, the transverse fracture was reduced with a reduction clamp. Then, two 4-mm cannulated screws (DePuy Synthes) were inserted parallel to each other and perpendicular to the fracture. An 18-gauge stainless steel wire was then passed through each screw, crossed anteriorly, and tightened to create a figure-of-8 ATB. The specimens were then randomly divided into 3 groups—MATB; MATB with cerclage suture augment; MATB with Krackow suture augment—while ensuring specimens from a single cadaver were placed in different groups to avoid confounding based on bone density differences.
Experimental Setup
Repaired specimens were secured with tissue clamps at the quadriceps and patellar tendons on an MTS Bionix 858 (MTS Systems) hydraulic arm.
Each patella was secured for cyclic testing. Initially it was placed under 10 N of tension. Then it underwent tensile loading from 10 N to 300 N at 50 N/s for 10 cycles. These parameters were based on previous biomechanical patella studies.10,11 Load was measured with the MTS load cell and displacement with the displacement transducer. Fracture displacement associated with 300-N cyclic tension was recorded. Displacement was calculated as the difference between 10th cycle and 2nd cycle values, which accounted for any degree of initial tissue slippage. After cyclic testing, the patella was placed back in 10 N of tensile loading and subjected to maximum force loading to determine ultimate repair strength. For maximum loading, the patella was stretched progressively at 50 N/s until failure. Again, load and displacement were measured with MTS.
Statistical Analysis
After testing, fracture displacement and maximum load force data were compiled for analysis. One-way analysis of variance with Bonferroni correction was used to determine if there were significant differences between groups. Significance level was set at P < .05.
Results
For cyclic testing, mean total displacement was measured over 10 cycles for each group. Again, displacement was determined by taking the difference between 10th cycle and 2nd cycle values, allowing for system stabilization.
Discussion
Our main objective was to compare the efficacy of a novel suture augment technique with that of other patella fracture repair techniques. Our hypothesis—that adding a Krackow suture augment would increase strength in both cyclic and maximum loading—was supported. Although testing results were not statistically significant because of the small sample size, we think this novel technique has clinically relevant descriptive significance and warrants further investigation.
Proper anatomical reduction and postoperative stabilization are of utmost importance in clinical approaches to patella fractures. In addition, regardless of which technical procedure is used, open reduction should also allow for early range of motion to prevent joint arthrofibrosis. Ever since the ATB technique was first described by the AO group, postoperative outcomes have improved significantly. In a retrospective study by Levack and colleagues,17 30 of 64 patients with patella fractures underwent internal fixation. Mean follow-up was 6.2 years. By both objective and subjective measures, the best functional outcomes were associated with internal tension band fixation (vs cerclage repair). Lotke and Ecker18 also documented the efficacy of the tension band technique. Sixteen patients with patella fractures underwent anterior tension banding; those with a comminuted fracture also underwent cerclage repair for patella stabilization during tension banding. At 6-week follow-up, all patients had good range of motion (≥90° flexion), relatively few symptoms, and no implant failures. Results were similar to those of Levack and colleagues.17
Although it improves stability and functional outcomes over conventional patellectomy and cerclage wiring, the ATB technique has been associated with subcutaneous irritation caused by the K-wires used to secure the band. Hung and colleagues9 followed up 68 patients with patellar fractures. Five of these patients underwent tension banding. Although there was a high level of adequate functional outcomes, implant irritation was found to be “quite frequent.”
To address this issue, Carpenter and colleagues11 evaluated an ATB technique that uses K-wires instead of cannulated screws. Biomechanical testing in a cadaver model revealed less fracture displacement and overall more repair strength through cyclic and maximum load testing. Clinically, these results were supported by Berg,10 who followed up 10 patients with transverse patella fractures repaired with the MATB technique. At a mean follow-up of 24 months, 7 of the 10 patients had good to excellent outcomes, and there were no implant failures.
Further investigation into patella repairs has mainly focused on improving the MATB technique and experimenting with different tension band materials. Rabalais and colleagues13 biomechanically tested high-strength polyethylene suture as a replacement for standard 18-gauge wire, and Bryant and colleagues14 tested a braided composite suture (FiberWire; Arthrex) as a replacement for standard 18-gauge stainless steel wire. Both found no significant difference with use of augmented tension band material, but Rabalais and colleagues13 did find more advantages with a parallel tension band construct than with a standard figure-of-8 arrangement.
In developing our novel technique, we considered that Krackow sutures are routinely used in both quadriceps tendon repair and patellar tendon repair, including partial patellectomy for distal patella fracture. With a suture placed in both tendons, the augment could be expected to resist longitudinal gapping and augment the tension band across the anterior patella. First described by Krackow and colleagues,19 the Krackow suture is widely used for tendon reconstruction. In an interlocking system of sutures, the Krackow suture provides a repair that is more stable than repair with conventional suture techniques, specifically in the context of tendon repair.20 Given the sesamoidal nature of the patella, its repair shares the goal of gap prevention with other tendon repairs. In theory, anchoring the supporting structures that are above and below the patella provides support for the intervening patella and ultimately improves fracture fixation strength.
Oh and colleagues15 reported on the clinical efficacy of a Krackow augment in distal pole patella repairs. Similarly, we found a Krackow augment to be efficacious, supporting its potential in clinical approaches to patella repairs. Our results indicate this augment can be a useful clinical adjunct in biomechanical evaluation.
Limitations of this study include its use of dissected extensor mechanisms, which may have less biofidelity than whole-knee specimens. In our model, specimens were secured at the patellar tendon and the quadriceps tendons, as opposed to the quadriceps tendon and the tibia distally. Use of this model could have led to an increase in early displacement during cyclic testing as a result of tissue slippage. Furthermore, our small sample size could have affected our ability to demonstrate a difference between these techniques.
Given its increased strength as demonstrated by mean displacement during cyclic loading and mean load to failure, as well as the early clinical data recently published, the Krackow suture augment represents a feasible technique for patella fixation. It likely will be most useful in cases in which conventional techniques are prone to failure or cannot be applied, such as severe distal comminution or poor bone density. Further biomechanical testing with a larger number of specimens may be required for statistical significance.
Conclusion
In patella fracture repair strategies, the Krackow suture augment increased strength when used with a MATB technique. Failure to reach statistical significance likely resulted from our small sample size. Further biomechanical testing and clinical studies are needed for more complete evaluation of this technique. We think it will be most useful in the setting of poor bone quality or severe comminution, which can limit fixation options. As increased repair strength allows earlier postoperative rehabilitation and maintains fracture reduction, patient outcomes should improve. This novel technique represents another strategy for managing challenging patella fractures.
Take-Home Points
- Suture augmentation improves construct strength for patella fixation.
- Krackow sutures may be placed in the quadriceps and patella tendons, then secured over the anterior patella (much like an anterior tension band).
- The Krackow technique described was superior to the suture cerclage technique based on mean load values, but did not reach statistical significance.
- The Krackow suture technique is a viable and easily applied technique for suture augmentation of patella fixation constructs.
Patella fractures are relatively uncommon, accounting for only 1% of skeletal injuries.1 Restoration of the function of the patella and the extensor mechanism is vital for knee extension and gait. However, patella fractures have an inherently high rate of complications, making these injuries challenging to treat.2-4 In patients with intact extensor function, displacement of <4 mm, and articular step-off of <3 mm, nonoperative management is extremely effective, with 99% of patients reporting favorable results.5 However, for fractures in which the extensor mechanism is disrupted, surgical intervention typically is indicated.6
Authors have reported various surgical interventions, one of the most commonly used being the anterior tension band (ATB) technique, first described by the AO (Arbeitsgemeinschaft für Osteosynthesefragen) group in the 1950s.7 By converting distractive anterior force during knee flexion to compressive force at the fracture site, the ATB technique provides a repair stronger than the previously used cerclage repair.8 Although initially considered standard of care, the ATB technique was soon found to be associated with implant failure and subcutaneous irritation prompting implant removal.9
To address these issues, Berg10 and Carpenter and colleagues11 evaluated an ATB technique that used cannulated screws instead of Kirschner wires (K-wires). This variation on the ATB technique reduced the implant-related issues while maintaining the mechanical advantage of the tension band. The more rigid design also permitted earlier postoperative rehabilitation, which significantly reduced development of arthrofibrosis.6,7,10 This modified ATB (MATB) technique has since been investigated for additional augments, mainly focusing on use of different tension band materials, including polyester suture and braided composite suture.12-14
However, there is little research on augments that incorporate the surrounding soft tissue, specifically the quadriceps and patellar tendons. In a recent retrospective clinical study, Oh and colleagues15 found positive clinical results with use of Krackow sutures, though 2 or 3 vertically oriented stainless steel wires were used instead of cannulated screws.
We conducted a study to determine the biomechanical efficacy of using a cerclage suture augment and a Krackow suture augment coupled with and compared with conventional MATB repair. If effective, this technique may represent another strategy for increasing repair strength and thereby improve postoperative outcomes.
Materials and Methods
Specimen Preparation
Fresh-frozen cadaver extensor mechanisms (quadriceps tendon, patella, surrounding retinaculum, patellar tendon) were kept frozen at –4°C until preparation. Fifteen specimens were selected. Mean (SD) age at death was 68 (10) years (range, 51-85 years). One specimen was excluded for a short patella tendon, which precluded adequate attachment for testing. All specimens were free of overt osseous pathology.
After specimens were thawed overnight, the patellae were transversely osteotomized with an osteotome at the junction of the middle and distal thirds of the patella. Sharp dissection was performed to carry the division through the medial and lateral retinaculum at the same level. All 14 specimens were then repaired using the MATB technique. First, the transverse fracture was reduced with a reduction clamp. Then, two 4-mm cannulated screws (DePuy Synthes) were inserted parallel to each other and perpendicular to the fracture. An 18-gauge stainless steel wire was then passed through each screw, crossed anteriorly, and tightened to create a figure-of-8 ATB. The specimens were then randomly divided into 3 groups—MATB; MATB with cerclage suture augment; MATB with Krackow suture augment—while ensuring specimens from a single cadaver were placed in different groups to avoid confounding based on bone density differences.
Experimental Setup
Repaired specimens were secured with tissue clamps at the quadriceps and patellar tendons on an MTS Bionix 858 (MTS Systems) hydraulic arm.
Each patella was secured for cyclic testing. Initially it was placed under 10 N of tension. Then it underwent tensile loading from 10 N to 300 N at 50 N/s for 10 cycles. These parameters were based on previous biomechanical patella studies.10,11 Load was measured with the MTS load cell and displacement with the displacement transducer. Fracture displacement associated with 300-N cyclic tension was recorded. Displacement was calculated as the difference between 10th cycle and 2nd cycle values, which accounted for any degree of initial tissue slippage. After cyclic testing, the patella was placed back in 10 N of tensile loading and subjected to maximum force loading to determine ultimate repair strength. For maximum loading, the patella was stretched progressively at 50 N/s until failure. Again, load and displacement were measured with MTS.
Statistical Analysis
After testing, fracture displacement and maximum load force data were compiled for analysis. One-way analysis of variance with Bonferroni correction was used to determine if there were significant differences between groups. Significance level was set at P < .05.
Results
For cyclic testing, mean total displacement was measured over 10 cycles for each group. Again, displacement was determined by taking the difference between 10th cycle and 2nd cycle values, allowing for system stabilization.
Discussion
Our main objective was to compare the efficacy of a novel suture augment technique with that of other patella fracture repair techniques. Our hypothesis—that adding a Krackow suture augment would increase strength in both cyclic and maximum loading—was supported. Although testing results were not statistically significant because of the small sample size, we think this novel technique has clinically relevant descriptive significance and warrants further investigation.
Proper anatomical reduction and postoperative stabilization are of utmost importance in clinical approaches to patella fractures. In addition, regardless of which technical procedure is used, open reduction should also allow for early range of motion to prevent joint arthrofibrosis. Ever since the ATB technique was first described by the AO group, postoperative outcomes have improved significantly. In a retrospective study by Levack and colleagues,17 30 of 64 patients with patella fractures underwent internal fixation. Mean follow-up was 6.2 years. By both objective and subjective measures, the best functional outcomes were associated with internal tension band fixation (vs cerclage repair). Lotke and Ecker18 also documented the efficacy of the tension band technique. Sixteen patients with patella fractures underwent anterior tension banding; those with a comminuted fracture also underwent cerclage repair for patella stabilization during tension banding. At 6-week follow-up, all patients had good range of motion (≥90° flexion), relatively few symptoms, and no implant failures. Results were similar to those of Levack and colleagues.17
Although it improves stability and functional outcomes over conventional patellectomy and cerclage wiring, the ATB technique has been associated with subcutaneous irritation caused by the K-wires used to secure the band. Hung and colleagues9 followed up 68 patients with patellar fractures. Five of these patients underwent tension banding. Although there was a high level of adequate functional outcomes, implant irritation was found to be “quite frequent.”
To address this issue, Carpenter and colleagues11 evaluated an ATB technique that uses K-wires instead of cannulated screws. Biomechanical testing in a cadaver model revealed less fracture displacement and overall more repair strength through cyclic and maximum load testing. Clinically, these results were supported by Berg,10 who followed up 10 patients with transverse patella fractures repaired with the MATB technique. At a mean follow-up of 24 months, 7 of the 10 patients had good to excellent outcomes, and there were no implant failures.
Further investigation into patella repairs has mainly focused on improving the MATB technique and experimenting with different tension band materials. Rabalais and colleagues13 biomechanically tested high-strength polyethylene suture as a replacement for standard 18-gauge wire, and Bryant and colleagues14 tested a braided composite suture (FiberWire; Arthrex) as a replacement for standard 18-gauge stainless steel wire. Both found no significant difference with use of augmented tension band material, but Rabalais and colleagues13 did find more advantages with a parallel tension band construct than with a standard figure-of-8 arrangement.
In developing our novel technique, we considered that Krackow sutures are routinely used in both quadriceps tendon repair and patellar tendon repair, including partial patellectomy for distal patella fracture. With a suture placed in both tendons, the augment could be expected to resist longitudinal gapping and augment the tension band across the anterior patella. First described by Krackow and colleagues,19 the Krackow suture is widely used for tendon reconstruction. In an interlocking system of sutures, the Krackow suture provides a repair that is more stable than repair with conventional suture techniques, specifically in the context of tendon repair.20 Given the sesamoidal nature of the patella, its repair shares the goal of gap prevention with other tendon repairs. In theory, anchoring the supporting structures that are above and below the patella provides support for the intervening patella and ultimately improves fracture fixation strength.
Oh and colleagues15 reported on the clinical efficacy of a Krackow augment in distal pole patella repairs. Similarly, we found a Krackow augment to be efficacious, supporting its potential in clinical approaches to patella repairs. Our results indicate this augment can be a useful clinical adjunct in biomechanical evaluation.
Limitations of this study include its use of dissected extensor mechanisms, which may have less biofidelity than whole-knee specimens. In our model, specimens were secured at the patellar tendon and the quadriceps tendons, as opposed to the quadriceps tendon and the tibia distally. Use of this model could have led to an increase in early displacement during cyclic testing as a result of tissue slippage. Furthermore, our small sample size could have affected our ability to demonstrate a difference between these techniques.
Given its increased strength as demonstrated by mean displacement during cyclic loading and mean load to failure, as well as the early clinical data recently published, the Krackow suture augment represents a feasible technique for patella fixation. It likely will be most useful in cases in which conventional techniques are prone to failure or cannot be applied, such as severe distal comminution or poor bone density. Further biomechanical testing with a larger number of specimens may be required for statistical significance.
Conclusion
In patella fracture repair strategies, the Krackow suture augment increased strength when used with a MATB technique. Failure to reach statistical significance likely resulted from our small sample size. Further biomechanical testing and clinical studies are needed for more complete evaluation of this technique. We think it will be most useful in the setting of poor bone quality or severe comminution, which can limit fixation options. As increased repair strength allows earlier postoperative rehabilitation and maintains fracture reduction, patient outcomes should improve. This novel technique represents another strategy for managing challenging patella fractures.
1. Boström Å. Fracture of the patella. A study of 422 patellar fractures. Acta Orthop Scand Suppl. 1972;143:1-80.
2. Hungerford DS, Barry M. Biomechanics of the patellofemoral joint. Clin Orthop Relat Res. 1979;(144):9-15.
3. LeBrun CT, Langford JR, Sagi HC. Functional outcomes after operatively treated patella fractures. J Orthop Trauma. 2012;26(7):422-426.
4. Kaufer H. Mechanical function of the patella. J Bone Joint Surg Am. 1971;53(8):1551-1560.
5. Braun W, Wiedemann M, Rüter A, Kundel K, Kolbinger S. Indications and results of nonoperative treatment of patellar fractures. Clin Orthop Relat Res. 1993;(289):197-201.
6. Melvin JS, Mehta S. Patellar fractures in adults. J Am Acad Orthop Surg. 2011;19(4):198-207.
7. Müller M, Allgöwer M, Schneider R, Willeneger H. Manual of Internal Fixation: Techniques Recommended by the AO Group. Berlin, Germany: Springer; 1979.
8. Weber MJ, Janecki CJ, McLeod P, Nelson CL, Thompson JA. Efficacy of various forms of fixation of transverse fractures of the patella. J Bone Joint Surg Am. 1980;62(2):215-220.
9. Hung LK, Chan KM, Chow YN, Leung PC. Fractured patella: operative treatment using the tension band principle. Injury. 1985;16(5):343-347.
10. Berg EE. Open reduction internal fixation of displaced transverse patella fractures with figure-eight wiring through parallel cannulated compression screws. J Orthop Trauma. 1997;11(8):573-576.
11. Carpenter JE, Kasman RA, Patel N, Lee ML, Goldstein SA. Biomechanical evaluation of current patella fracture fixation techniques. J Orthop Trauma. 1997;11(5):351-356.
12. Hughes SC, Stott PM, Hearnden AJ, Ripley LG. A new and effective tension-band braided polyester suture technique for transverse patellar fracture fixation. Injury. 2007;38(2):212-222.
13. Rabalais RD, Burger E, Lu Y, Mansour A, Baratta RV. Comparison of two tension-band fixation materials and techniques in transverse patella fractures: a biomechanical study. Orthopedics. 2008;31(2):128.
14. Bryant TL, Anderson CL, Stevens CG, Conrad BP, Vincent HK, Sadasivan KK. Comparison of cannulated screws with FiberWire or stainless steel wire for patella fracture fixation: a pilot study. J Orthop. 2014;12(2):92-96.
15. Oh HK, Choo SK, Kim JW, Lee M. Internal fixation of displaced inferior pole of the patella fractures using vertical wiring augmented with Krachow suturing. Injury. 2015;46(12):2512-2515.
16. Goodfellow J, Hungerford DS, Zindel M. Patello-femoral joint mechanics and pathology. 1. Functional anatomy of the patello-femoral joint. J Bone Joint Surg Br. 1976;58(3):287-290.
17. Levack B, Flannagan JP, Hobbs S. Results of surgical treatment of patellar fractures. J Bone Joint Surg Br. 1985;67(3):416-419.
18. Lotke PA, Ecker ML. Transverse fractures of the patella. Clin Orthop Relat Res. 1981;(158):180-184.
19. Krackow KA, Thomas SC, Jones LC. Ligament-tendon fixation: analysis of a new stitch and comparison with standard techniques. Orthopedics. 1988;11(6):909-917.
20. Hahn JM, Inceoğlu S, Wongworawat MD. Biomechanical comparison of Krackow locking stitch versus nonlocking loop stitch with varying number of throws. Am J Sports Med. 2014;42(12):3003-3008.
1. Boström Å. Fracture of the patella. A study of 422 patellar fractures. Acta Orthop Scand Suppl. 1972;143:1-80.
2. Hungerford DS, Barry M. Biomechanics of the patellofemoral joint. Clin Orthop Relat Res. 1979;(144):9-15.
3. LeBrun CT, Langford JR, Sagi HC. Functional outcomes after operatively treated patella fractures. J Orthop Trauma. 2012;26(7):422-426.
4. Kaufer H. Mechanical function of the patella. J Bone Joint Surg Am. 1971;53(8):1551-1560.
5. Braun W, Wiedemann M, Rüter A, Kundel K, Kolbinger S. Indications and results of nonoperative treatment of patellar fractures. Clin Orthop Relat Res. 1993;(289):197-201.
6. Melvin JS, Mehta S. Patellar fractures in adults. J Am Acad Orthop Surg. 2011;19(4):198-207.
7. Müller M, Allgöwer M, Schneider R, Willeneger H. Manual of Internal Fixation: Techniques Recommended by the AO Group. Berlin, Germany: Springer; 1979.
8. Weber MJ, Janecki CJ, McLeod P, Nelson CL, Thompson JA. Efficacy of various forms of fixation of transverse fractures of the patella. J Bone Joint Surg Am. 1980;62(2):215-220.
9. Hung LK, Chan KM, Chow YN, Leung PC. Fractured patella: operative treatment using the tension band principle. Injury. 1985;16(5):343-347.
10. Berg EE. Open reduction internal fixation of displaced transverse patella fractures with figure-eight wiring through parallel cannulated compression screws. J Orthop Trauma. 1997;11(8):573-576.
11. Carpenter JE, Kasman RA, Patel N, Lee ML, Goldstein SA. Biomechanical evaluation of current patella fracture fixation techniques. J Orthop Trauma. 1997;11(5):351-356.
12. Hughes SC, Stott PM, Hearnden AJ, Ripley LG. A new and effective tension-band braided polyester suture technique for transverse patellar fracture fixation. Injury. 2007;38(2):212-222.
13. Rabalais RD, Burger E, Lu Y, Mansour A, Baratta RV. Comparison of two tension-band fixation materials and techniques in transverse patella fractures: a biomechanical study. Orthopedics. 2008;31(2):128.
14. Bryant TL, Anderson CL, Stevens CG, Conrad BP, Vincent HK, Sadasivan KK. Comparison of cannulated screws with FiberWire or stainless steel wire for patella fracture fixation: a pilot study. J Orthop. 2014;12(2):92-96.
15. Oh HK, Choo SK, Kim JW, Lee M. Internal fixation of displaced inferior pole of the patella fractures using vertical wiring augmented with Krachow suturing. Injury. 2015;46(12):2512-2515.
16. Goodfellow J, Hungerford DS, Zindel M. Patello-femoral joint mechanics and pathology. 1. Functional anatomy of the patello-femoral joint. J Bone Joint Surg Br. 1976;58(3):287-290.
17. Levack B, Flannagan JP, Hobbs S. Results of surgical treatment of patellar fractures. J Bone Joint Surg Br. 1985;67(3):416-419.
18. Lotke PA, Ecker ML. Transverse fractures of the patella. Clin Orthop Relat Res. 1981;(158):180-184.
19. Krackow KA, Thomas SC, Jones LC. Ligament-tendon fixation: analysis of a new stitch and comparison with standard techniques. Orthopedics. 1988;11(6):909-917.
20. Hahn JM, Inceoğlu S, Wongworawat MD. Biomechanical comparison of Krackow locking stitch versus nonlocking loop stitch with varying number of throws. Am J Sports Med. 2014;42(12):3003-3008.
Brace for Impact
ANSWER
The radiograph shows an oblique fracture through the radial styloid process. The patient was placed in a splint and referred to outpatient orthopedics for follow-up.

ANSWER
The radiograph shows an oblique fracture through the radial styloid process. The patient was placed in a splint and referred to outpatient orthopedics for follow-up.

ANSWER
The radiograph shows an oblique fracture through the radial styloid process. The patient was placed in a splint and referred to outpatient orthopedics for follow-up.
A 35-year-old woman arrives at the emergency department following a motor vehicle accident. She was a restrained driver who was crossing an intersection when another vehicle pulled out in front of her. She recalls gripping the steering wheel in anticipation of impact. No air bags deployed. She complains of wrist pain, but denies any other ailment.
Medical history is unremarkable. Vital signs are normal. Physical examination of the patient’s left wrist shows no obvious deformity. There is mild soft-tissue swelling, decreased range of motion, and moderate point tenderness along the radial aspect of the wrist. The nailbeds have good capillary refill. Strong pulses are present, as well.
Triage has already obtained a radiograph of the left wrist (shown). What is your impression?
Total Hip Arthroplasty and Hemiarthroplasty: US National Trends in the Treatment of Femoral Neck Fractures
Take-Home Points
- An increasing number of THAs and HAs were performed over time for FNF.
- HA patients tended to be older.
- Hospitalization and blood transfusion rates were higher for THA.
- Hospital size affected the rate of HAs, while hospital location affected the rate of THAs.
- A larger proportion of THA patients had private insurance.
Femoral neck fractures (FNFs) are a common source of morbidity and mortality worldwide. The increasing number of FNFs in the United States is attributed to increases in number of US residents >65 years old, the average life span, and the incidence of osteoporosis.1 Three hundred forty thousand hip fractures occurred in the United States in 1996, and the number is expected to double by 2050.2 By that year, an estimated 6.3 million hip fractures will occur worldwide.3 Given the 1-year mortality rate of 14% to 36%, optimizing the management of these fractures is an important public health issue that must be addressed.4
Treatment is based on preoperative ambulatory status, cognitive function, comorbidities, fracture type and displacement, and other factors. In physiologically elderly patients with displaced fractures, surgical treatment usually involves either hemiarthroplasty (HA) or total hip arthroplasty (THA). There is controversy regarding which modality is the preferred treatment.
Proponents of HA point to a higher rate of dislocation for FNFs treated with THAs,5,6 attributed to increased range of motion.7 Proponents of THA point to superior short-term clinical results and fewer complications, especially in mobile, independent patients.8
We conducted a study to assess recent US national trends in performing THA and HA for FNFs and to evaluate perioperative outcomes for each treatment group.
Materials and Methods
Data for this study were obtained from the National Center for Health Statistics (NCHS) National Hospital Discharge Survey (NHDS) and were imported into Microsoft Office Excel 2010.9 The NHDS examines patient discharges from various hospitals across the US, including federal, military, and Veterans Administration hospitals.9 Only short-stay hospitals (mean stay, <30 days) and hospitals with a general specialty are included in the survey. Each year, about 1% of all hospital admissions from across the US are abstracted and weighted to provide nationwide estimates. The information collected from each hospital record includes age, sex, race, marital status, discharge month, discharge status, days of care, hospital location, hospital size (number of beds), hospital type (proprietary or for-profit, government, nonprofit/church), and up to 15 discharge diagnoses and 8 procedures performed during admission.9
International Classification of Diseases, Ninth Revision (ICD-9) procedure codes were used to search the NHDS for patients admitted after FNF for each year from 2001 through 2010. These codes were then used to identify patients within this group who underwent THA or HA. We also collected data on patient demographics, hospitalization duration, discharge disposition, in-hospital adverse events (deep vein thrombosis [DVT], pulmonary embolism [PE], blood transfusion, mortality), form of primary medical insurance, number of hospital beds (0-99, 100-199, 200-299, 300-499, ≥500), hospital type (proprietary, government, nonprofit/church), and hospital region (Northeast, Midwest, South, West).
Trends were evaluated by linear regression with the Pearson correlation coefficient (r). Statistical comparisons were made using the Student t test for continuous data, and both the Fisher exact test and the χ2 test for categorical variables. Significance level was set at P < .05. All analyses were performed with IBM SPSS Statistics 22.
Results
Hospital stay was longer (P < .01) for THA patients (7.7 days; range, 1-312 days) than for HA patients (6.7 days; range, 1-118 days), and blood transfusion rate was higher (P = .02) for THA patients (30.4%) than for HA patients (25.7%), but the groups did not differ in their rates of DVT (THA, 1.2%; HA, 0.80%, P = .50), PE (THA, 0.52%; HA, 0.72%, P = .52), or mortality (THA, 1.8%; HA, 2.9%; P = .16). Discharge disposition varied with surgical status (P < .01): 23.2% of THA patients and 11.6% of HA patients were discharged directly home after their inpatient stay, and 76.8% of THA patients and 88.4% of HA patients were discharged or transferred to a short- or long-term care facility.
Private medical insurance provided coverage for 14.3% of THAs and 9.1% of HAs, and Medicare provided coverage for 80.9% of THAs and 86.0% of HAs (P < .01).
Discussion
The NHDS data showed a preference for HA over THA in the treatment of FNFs and suggested THA was favored for younger, healthier patients while HA was reserved for older patients with more comorbidities. Despite being younger and healthier, the THA group had higher transfusion rates and longer hospitalizations, possibly because of the increased complexity of THA procedures, which generally involve more operative time and increased blood loss. The resultant higher transfusion rate for THAs likely contributed to longer hospitalizations for FNFs. However, the THA and HA groups did not differ in their rates of DVT, PE, or mortality.
Multiple studies have noted no differences in mortality, infection, or general complications between THA and HA for FNF.8,10,11 THA patients have better functional outcomes, including Harris and Oxford hip scores and walking distance, but higher dislocation rates,8,10-12 and HA patients are at higher risk for reoperation because of progressive acetabular erosion.8,10,11
We noted an increase in use of both THA and HA for FNF over the study period (2001-2010). In a review of operative treatment for FNF by surgeons applying for the American Board of Orthopaedic Surgery certification between 1999 and 2011, Miller and colleagues13 found a similar increase in the THA rate over time, but decreases in the HA and internal fixation rates, with candidates in the “adult reconstruction” subspecialty showing a particularly strong trend toward THA use.
These findings reflect a general propensity toward femoral head replacement rather than preservation through open reduction and internal fixation (ORIF). Recent studies have found that ORIF carries a 39% to 43% rate of fixation failure and need for secondary revision, as well as risks of avascular necrosis, malunion, and nonunion.1,14-16 This need for secondary surgery makes ORIF ultimately less cost-effective than either THA or HA.16,17 Most authors would recommend arthroplasty for FNF in elderly patients with normal mental function1,16,18 and would reserve ORIF for young patients with good bone stock, joint space preservation, and reducible noncomminuted fractures.1,19
Our study results suggest that smaller hospitals (<100 beds) tend to have lower rates of HA (P < .01, significant) and THA (P = .10, not significant; Table), possibly because FNF patients who present to these hospitals may be referred elsewhere because of regional differences in the availability of orthopedic traumatologists and arthroplasty subspecialists. Surgeon volume affects postoperative outcomes and may play a role in referral patterns.20 Ames and colleagues20 found that HA performed for FNF by surgeons with high-volume THA experience (vs non-hip-arthroplasty surgeons) had lower rates of dislocation, superficial infection, and mortality.
Regional differences were significant for THA alone, with the highest THA rates in the South (5.2%) and the lowest in the West (3.3%; Figure 5). There were no clear regional trends for HA. Possible explanations include a propensity toward a more aggressive approach in these regions, increased regional prevalence of acetabular disease, regional surgeon preferences, and regional differences in patient characteristics (eg, increased prevalence of obesity in the South).21
HA rates were highest for nonprofit/church hospitals and lowest for proprietary hospitals, whereas THA rates did not differ by hospital type. Possible explanations include an older, less mobile nonprofit/church patient cohort that is more amenable to HA, and surgeon preference.
THA patients were more likely to be covered by private medical insurance than by Medicare—a finding in agreement with Hochfelder and colleagues,22 who found that, compared with federal insurance and self-pay patients, private insurance patients were 41% more likely to undergo THA than HA or internal fixation for FNF. We think that the age difference between our THA and HA groups contributed to the insurance variability in our study.
Our study had several limitations. It was conducted to examine the rates of THA and HA after FNF, not to survey treatment types, including ORIF and nonoperative management. The NHDS database does not provide information on HA implant type (unipolar, bipolar), use or nonuse of cement with HA, or surgical approach. Surgical approach could influence the rate of postoperative dislocation, an outcome measure that was not examined in this study. Last, the NHDS database tracks admissions and discharges, not patients. When a patient is discharged, collection of information on the patient’s postoperative course stops; a patient who returns even only 1 day later is recorded as a new or unique patient. Therefore, intermediate or long-term outcome information is unavailable, which likely led to an underrepresentation of DVT, PE, and mortality after these THA and HA procedures.
There was a trend toward femoral head replacement rather than ORIF in the treatment of FNF. Cognitively functional and independent elderly patients, and patients with osteoarthritis or rheumatoid arthritis, may benefit from THA, whereas HA may be better suited to cognitively dysfunctional patients.23,24 The NHDS reflects an increasing trend toward arthroplasty over ORIF, but the exact treatment choice is affected by hospital type, size, location and surgeon preference, training, and subspecialization.
1. Macaulay W, Pagnotto MR, Iorio R, Mont MA, Saleh KJ. Displaced femoral neck fractures in the elderly: hemiarthroplasty versus total hip arthroplasty. J Am Acad Orthop Surg. 2006;14(5):287-293.
2. Miyamoto RG, Kaplan KM, Levine BR, Egol KA, Zuckerman JD. Surgical management of hip fractures: an evidence-based review of the literature. I: femoral neck fractures. J Am Acad Orthop Surg. 2008;16(10):596-607.
3. Kannus P, Parkkari J, Sievänen H, Heinonen A, Vuori I, Järvinen M. Epidemiology of hip fractures. Bone. 1996;18(1 suppl):57S-63S.
4. Zuckerman JD. Hip fracture. N Engl J Med. 1996;334(23):1519-1525.
5. Papandrea RF, Froimson MI. Total hip arthroplasty after acute displaced femoral neck fractures. Am J Orthop. 1996;25(2):85-88.
6. Burgers PT, Van Geene AR, Van den Bekerom MP, et al. Total hip arthroplasty versus hemiarthroplasty for displaced femoral neck fractures in the healthy elderly: a meta-analysis and systematic review of randomized trials. Int Orthop. 2012;36(8):1549-1560.
7. Skinner P, Riley D, Ellery J, Beaumont A, Coumine R, Shafighian B. Displaced subcapital fractures of the femur: a prospective randomized comparison of internal fixation, hemiarthroplasty and total hip replacement. Injury. 1989;20(5):291-293.
8. Baker RP, Squires B, Gargan MF, Bannister GC. Total hip arthroplasty and hemiarthroplasty in mobile, independent patients with a displaced intracapsular fracture of the femoral neck. A randomized, controlled trial. J Bone Joint Surg Am. 2006;88(12):2583-2589.
9. Centers for Disease Control and Prevention, National Center for Health Statistics. National Hospital Discharge Survey. http://www.cdc.gov/nchs/nhds/about_nhds.htm. Last updated December 6, 2011. Accessed December 10, 2013.
10. Zi-Sheng A, You-Shui G, Zhi-Zhen J, Ting Y, Chang-Qing Z. Hemiarthroplasty vs primary total hip arthroplasty for displaced fractures of the femoral neck in the elderly: a meta-analysis. J Arthroplasty. 2012;27(4):583-590.
11. Yu L, Wang Y, Chen J. Total hip arthroplasty versus hemiarthroplasty for displaced femoral neck fractures: meta-analysis of randomized trials. Clin Orthop Relat Res. 2012;470(8):2235-2243.
12. Hopley C, Stengel D, Ekkernkamp A, Wich M. Primary total hip arthroplasty versus hemiarthroplasty for displaced intracapsular hip fractures in older patients: systematic review. BMJ. 2010;340:c2332.
13. Miller BJ, Callaghan JJ, Cram P, Karam M, Marsh JL, Noiseux NO. Changing trends in the treatment of femoral neck fractures: a review of the American Board of Orthopaedic Surgery database. J Bone Joint Surg Am. 2014;96(17):e149.
14. Rogmark C, Carlsson A, Johnell O, Sernbo I. A prospective randomised trial of internal fixation versus arthroplasty for displaced fractures of the neck of the femur. Functional outcome for 450 patients at two years. J Bone Joint Surg Br. 2002;84(2):183-188.
15. Bhandari M, Devereaux PJ, Swiontkowski MF, et al. Internal fixation compared with arthroplasty for displaced fractures of the femoral neck. A meta-analysis. J Bone Joint Surg Am. 2003;85(9):1673-1681.
16. Keating JF, Grant A, Masson M, Scott NW, Forbes JF. Randomized comparison of reduction and fixation, bipolar hemiarthroplasty, and total hip arthroplasty. Treatment of displaced intracapsular hip fractures in healthy older patients. J Bone Joint Surg Am. 2006;88(2):249-260.
17. Iorio R, Healy WL, Lemos DW, Appleby D, Lucchesi CA, Saleh KJ. Displaced femoral neck fractures in the elderly: outcomes and cost effectiveness. Clin Orthop Relat Res. 2001;(383):229-242.
18. Johansson T, Jacobsson SA, Ivarsson I, Knutsson A, Wahlström O. Internal fixation versus total hip arthroplasty in the treatment of displaced femoral neck fractures: a prospective randomized study of 100 hips. Acta Orthop Scand. 2000;71(6):597-602.
19. Shah AK, Eissler J, Radomisli T. Algorithms for the treatment of femoral neck fractures. Clin Orthop Relat Res. 2002;(399):28-34.
20. Ames JB, Lurie JD, Tomek IM, Zhou W, Koval KJ. Does surgeon volume for total hip arthroplasty affect outcomes after hemiarthroplasty for femoral neck fracture? Am J Orthop. 2010;39(8):E84-E89.
21. Le A, Judd SE, Allison DB, et al. The geographic distribution of obesity in the US and the potential regional differences in misreporting of obesity. Obesity. 2014;22(1):300-306.
22. Hochfelder JP, Khatib ON, Glait SA, Slover JD. Femoral neck fractures in New York state. Is the rate of THA increasing, and do race or payer influence decision making? J Orthop Trauma. 2014;28(7):422-426.
23. Lowe JA, Crist BD, Bhandari M, Ferguson TA. Optimal treatment of femoral neck fractures according to patient’s physiologic age: an evidence-based review. Orthop Clin North Am. 2010;41(2):157-166.
24. Callaghan JJ, Liu SS, Haidukewych GJ. Subcapital fractures: a changing paradigm. J Bone Joint Surg Br. 2012;94(11 suppl A):19-21.
Take-Home Points
- An increasing number of THAs and HAs were performed over time for FNF.
- HA patients tended to be older.
- Hospitalization and blood transfusion rates were higher for THA.
- Hospital size affected the rate of HAs, while hospital location affected the rate of THAs.
- A larger proportion of THA patients had private insurance.
Femoral neck fractures (FNFs) are a common source of morbidity and mortality worldwide. The increasing number of FNFs in the United States is attributed to increases in number of US residents >65 years old, the average life span, and the incidence of osteoporosis.1 Three hundred forty thousand hip fractures occurred in the United States in 1996, and the number is expected to double by 2050.2 By that year, an estimated 6.3 million hip fractures will occur worldwide.3 Given the 1-year mortality rate of 14% to 36%, optimizing the management of these fractures is an important public health issue that must be addressed.4
Treatment is based on preoperative ambulatory status, cognitive function, comorbidities, fracture type and displacement, and other factors. In physiologically elderly patients with displaced fractures, surgical treatment usually involves either hemiarthroplasty (HA) or total hip arthroplasty (THA). There is controversy regarding which modality is the preferred treatment.
Proponents of HA point to a higher rate of dislocation for FNFs treated with THAs,5,6 attributed to increased range of motion.7 Proponents of THA point to superior short-term clinical results and fewer complications, especially in mobile, independent patients.8
We conducted a study to assess recent US national trends in performing THA and HA for FNFs and to evaluate perioperative outcomes for each treatment group.
Materials and Methods
Data for this study were obtained from the National Center for Health Statistics (NCHS) National Hospital Discharge Survey (NHDS) and were imported into Microsoft Office Excel 2010.9 The NHDS examines patient discharges from various hospitals across the US, including federal, military, and Veterans Administration hospitals.9 Only short-stay hospitals (mean stay, <30 days) and hospitals with a general specialty are included in the survey. Each year, about 1% of all hospital admissions from across the US are abstracted and weighted to provide nationwide estimates. The information collected from each hospital record includes age, sex, race, marital status, discharge month, discharge status, days of care, hospital location, hospital size (number of beds), hospital type (proprietary or for-profit, government, nonprofit/church), and up to 15 discharge diagnoses and 8 procedures performed during admission.9
International Classification of Diseases, Ninth Revision (ICD-9) procedure codes were used to search the NHDS for patients admitted after FNF for each year from 2001 through 2010. These codes were then used to identify patients within this group who underwent THA or HA. We also collected data on patient demographics, hospitalization duration, discharge disposition, in-hospital adverse events (deep vein thrombosis [DVT], pulmonary embolism [PE], blood transfusion, mortality), form of primary medical insurance, number of hospital beds (0-99, 100-199, 200-299, 300-499, ≥500), hospital type (proprietary, government, nonprofit/church), and hospital region (Northeast, Midwest, South, West).
Trends were evaluated by linear regression with the Pearson correlation coefficient (r). Statistical comparisons were made using the Student t test for continuous data, and both the Fisher exact test and the χ2 test for categorical variables. Significance level was set at P < .05. All analyses were performed with IBM SPSS Statistics 22.
Results
Hospital stay was longer (P < .01) for THA patients (7.7 days; range, 1-312 days) than for HA patients (6.7 days; range, 1-118 days), and blood transfusion rate was higher (P = .02) for THA patients (30.4%) than for HA patients (25.7%), but the groups did not differ in their rates of DVT (THA, 1.2%; HA, 0.80%, P = .50), PE (THA, 0.52%; HA, 0.72%, P = .52), or mortality (THA, 1.8%; HA, 2.9%; P = .16). Discharge disposition varied with surgical status (P < .01): 23.2% of THA patients and 11.6% of HA patients were discharged directly home after their inpatient stay, and 76.8% of THA patients and 88.4% of HA patients were discharged or transferred to a short- or long-term care facility.
Private medical insurance provided coverage for 14.3% of THAs and 9.1% of HAs, and Medicare provided coverage for 80.9% of THAs and 86.0% of HAs (P < .01).
Discussion
The NHDS data showed a preference for HA over THA in the treatment of FNFs and suggested THA was favored for younger, healthier patients while HA was reserved for older patients with more comorbidities. Despite being younger and healthier, the THA group had higher transfusion rates and longer hospitalizations, possibly because of the increased complexity of THA procedures, which generally involve more operative time and increased blood loss. The resultant higher transfusion rate for THAs likely contributed to longer hospitalizations for FNFs. However, the THA and HA groups did not differ in their rates of DVT, PE, or mortality.
Multiple studies have noted no differences in mortality, infection, or general complications between THA and HA for FNF.8,10,11 THA patients have better functional outcomes, including Harris and Oxford hip scores and walking distance, but higher dislocation rates,8,10-12 and HA patients are at higher risk for reoperation because of progressive acetabular erosion.8,10,11
We noted an increase in use of both THA and HA for FNF over the study period (2001-2010). In a review of operative treatment for FNF by surgeons applying for the American Board of Orthopaedic Surgery certification between 1999 and 2011, Miller and colleagues13 found a similar increase in the THA rate over time, but decreases in the HA and internal fixation rates, with candidates in the “adult reconstruction” subspecialty showing a particularly strong trend toward THA use.
These findings reflect a general propensity toward femoral head replacement rather than preservation through open reduction and internal fixation (ORIF). Recent studies have found that ORIF carries a 39% to 43% rate of fixation failure and need for secondary revision, as well as risks of avascular necrosis, malunion, and nonunion.1,14-16 This need for secondary surgery makes ORIF ultimately less cost-effective than either THA or HA.16,17 Most authors would recommend arthroplasty for FNF in elderly patients with normal mental function1,16,18 and would reserve ORIF for young patients with good bone stock, joint space preservation, and reducible noncomminuted fractures.1,19
Our study results suggest that smaller hospitals (<100 beds) tend to have lower rates of HA (P < .01, significant) and THA (P = .10, not significant; Table), possibly because FNF patients who present to these hospitals may be referred elsewhere because of regional differences in the availability of orthopedic traumatologists and arthroplasty subspecialists. Surgeon volume affects postoperative outcomes and may play a role in referral patterns.20 Ames and colleagues20 found that HA performed for FNF by surgeons with high-volume THA experience (vs non-hip-arthroplasty surgeons) had lower rates of dislocation, superficial infection, and mortality.
Regional differences were significant for THA alone, with the highest THA rates in the South (5.2%) and the lowest in the West (3.3%; Figure 5). There were no clear regional trends for HA. Possible explanations include a propensity toward a more aggressive approach in these regions, increased regional prevalence of acetabular disease, regional surgeon preferences, and regional differences in patient characteristics (eg, increased prevalence of obesity in the South).21
HA rates were highest for nonprofit/church hospitals and lowest for proprietary hospitals, whereas THA rates did not differ by hospital type. Possible explanations include an older, less mobile nonprofit/church patient cohort that is more amenable to HA, and surgeon preference.
THA patients were more likely to be covered by private medical insurance than by Medicare—a finding in agreement with Hochfelder and colleagues,22 who found that, compared with federal insurance and self-pay patients, private insurance patients were 41% more likely to undergo THA than HA or internal fixation for FNF. We think that the age difference between our THA and HA groups contributed to the insurance variability in our study.
Our study had several limitations. It was conducted to examine the rates of THA and HA after FNF, not to survey treatment types, including ORIF and nonoperative management. The NHDS database does not provide information on HA implant type (unipolar, bipolar), use or nonuse of cement with HA, or surgical approach. Surgical approach could influence the rate of postoperative dislocation, an outcome measure that was not examined in this study. Last, the NHDS database tracks admissions and discharges, not patients. When a patient is discharged, collection of information on the patient’s postoperative course stops; a patient who returns even only 1 day later is recorded as a new or unique patient. Therefore, intermediate or long-term outcome information is unavailable, which likely led to an underrepresentation of DVT, PE, and mortality after these THA and HA procedures.
There was a trend toward femoral head replacement rather than ORIF in the treatment of FNF. Cognitively functional and independent elderly patients, and patients with osteoarthritis or rheumatoid arthritis, may benefit from THA, whereas HA may be better suited to cognitively dysfunctional patients.23,24 The NHDS reflects an increasing trend toward arthroplasty over ORIF, but the exact treatment choice is affected by hospital type, size, location and surgeon preference, training, and subspecialization.
Take-Home Points
- An increasing number of THAs and HAs were performed over time for FNF.
- HA patients tended to be older.
- Hospitalization and blood transfusion rates were higher for THA.
- Hospital size affected the rate of HAs, while hospital location affected the rate of THAs.
- A larger proportion of THA patients had private insurance.
Femoral neck fractures (FNFs) are a common source of morbidity and mortality worldwide. The increasing number of FNFs in the United States is attributed to increases in number of US residents >65 years old, the average life span, and the incidence of osteoporosis.1 Three hundred forty thousand hip fractures occurred in the United States in 1996, and the number is expected to double by 2050.2 By that year, an estimated 6.3 million hip fractures will occur worldwide.3 Given the 1-year mortality rate of 14% to 36%, optimizing the management of these fractures is an important public health issue that must be addressed.4
Treatment is based on preoperative ambulatory status, cognitive function, comorbidities, fracture type and displacement, and other factors. In physiologically elderly patients with displaced fractures, surgical treatment usually involves either hemiarthroplasty (HA) or total hip arthroplasty (THA). There is controversy regarding which modality is the preferred treatment.
Proponents of HA point to a higher rate of dislocation for FNFs treated with THAs,5,6 attributed to increased range of motion.7 Proponents of THA point to superior short-term clinical results and fewer complications, especially in mobile, independent patients.8
We conducted a study to assess recent US national trends in performing THA and HA for FNFs and to evaluate perioperative outcomes for each treatment group.
Materials and Methods
Data for this study were obtained from the National Center for Health Statistics (NCHS) National Hospital Discharge Survey (NHDS) and were imported into Microsoft Office Excel 2010.9 The NHDS examines patient discharges from various hospitals across the US, including federal, military, and Veterans Administration hospitals.9 Only short-stay hospitals (mean stay, <30 days) and hospitals with a general specialty are included in the survey. Each year, about 1% of all hospital admissions from across the US are abstracted and weighted to provide nationwide estimates. The information collected from each hospital record includes age, sex, race, marital status, discharge month, discharge status, days of care, hospital location, hospital size (number of beds), hospital type (proprietary or for-profit, government, nonprofit/church), and up to 15 discharge diagnoses and 8 procedures performed during admission.9
International Classification of Diseases, Ninth Revision (ICD-9) procedure codes were used to search the NHDS for patients admitted after FNF for each year from 2001 through 2010. These codes were then used to identify patients within this group who underwent THA or HA. We also collected data on patient demographics, hospitalization duration, discharge disposition, in-hospital adverse events (deep vein thrombosis [DVT], pulmonary embolism [PE], blood transfusion, mortality), form of primary medical insurance, number of hospital beds (0-99, 100-199, 200-299, 300-499, ≥500), hospital type (proprietary, government, nonprofit/church), and hospital region (Northeast, Midwest, South, West).
Trends were evaluated by linear regression with the Pearson correlation coefficient (r). Statistical comparisons were made using the Student t test for continuous data, and both the Fisher exact test and the χ2 test for categorical variables. Significance level was set at P < .05. All analyses were performed with IBM SPSS Statistics 22.
Results
Hospital stay was longer (P < .01) for THA patients (7.7 days; range, 1-312 days) than for HA patients (6.7 days; range, 1-118 days), and blood transfusion rate was higher (P = .02) for THA patients (30.4%) than for HA patients (25.7%), but the groups did not differ in their rates of DVT (THA, 1.2%; HA, 0.80%, P = .50), PE (THA, 0.52%; HA, 0.72%, P = .52), or mortality (THA, 1.8%; HA, 2.9%; P = .16). Discharge disposition varied with surgical status (P < .01): 23.2% of THA patients and 11.6% of HA patients were discharged directly home after their inpatient stay, and 76.8% of THA patients and 88.4% of HA patients were discharged or transferred to a short- or long-term care facility.
Private medical insurance provided coverage for 14.3% of THAs and 9.1% of HAs, and Medicare provided coverage for 80.9% of THAs and 86.0% of HAs (P < .01).
Discussion
The NHDS data showed a preference for HA over THA in the treatment of FNFs and suggested THA was favored for younger, healthier patients while HA was reserved for older patients with more comorbidities. Despite being younger and healthier, the THA group had higher transfusion rates and longer hospitalizations, possibly because of the increased complexity of THA procedures, which generally involve more operative time and increased blood loss. The resultant higher transfusion rate for THAs likely contributed to longer hospitalizations for FNFs. However, the THA and HA groups did not differ in their rates of DVT, PE, or mortality.
Multiple studies have noted no differences in mortality, infection, or general complications between THA and HA for FNF.8,10,11 THA patients have better functional outcomes, including Harris and Oxford hip scores and walking distance, but higher dislocation rates,8,10-12 and HA patients are at higher risk for reoperation because of progressive acetabular erosion.8,10,11
We noted an increase in use of both THA and HA for FNF over the study period (2001-2010). In a review of operative treatment for FNF by surgeons applying for the American Board of Orthopaedic Surgery certification between 1999 and 2011, Miller and colleagues13 found a similar increase in the THA rate over time, but decreases in the HA and internal fixation rates, with candidates in the “adult reconstruction” subspecialty showing a particularly strong trend toward THA use.
These findings reflect a general propensity toward femoral head replacement rather than preservation through open reduction and internal fixation (ORIF). Recent studies have found that ORIF carries a 39% to 43% rate of fixation failure and need for secondary revision, as well as risks of avascular necrosis, malunion, and nonunion.1,14-16 This need for secondary surgery makes ORIF ultimately less cost-effective than either THA or HA.16,17 Most authors would recommend arthroplasty for FNF in elderly patients with normal mental function1,16,18 and would reserve ORIF for young patients with good bone stock, joint space preservation, and reducible noncomminuted fractures.1,19
Our study results suggest that smaller hospitals (<100 beds) tend to have lower rates of HA (P < .01, significant) and THA (P = .10, not significant; Table), possibly because FNF patients who present to these hospitals may be referred elsewhere because of regional differences in the availability of orthopedic traumatologists and arthroplasty subspecialists. Surgeon volume affects postoperative outcomes and may play a role in referral patterns.20 Ames and colleagues20 found that HA performed for FNF by surgeons with high-volume THA experience (vs non-hip-arthroplasty surgeons) had lower rates of dislocation, superficial infection, and mortality.
Regional differences were significant for THA alone, with the highest THA rates in the South (5.2%) and the lowest in the West (3.3%; Figure 5). There were no clear regional trends for HA. Possible explanations include a propensity toward a more aggressive approach in these regions, increased regional prevalence of acetabular disease, regional surgeon preferences, and regional differences in patient characteristics (eg, increased prevalence of obesity in the South).21
HA rates were highest for nonprofit/church hospitals and lowest for proprietary hospitals, whereas THA rates did not differ by hospital type. Possible explanations include an older, less mobile nonprofit/church patient cohort that is more amenable to HA, and surgeon preference.
THA patients were more likely to be covered by private medical insurance than by Medicare—a finding in agreement with Hochfelder and colleagues,22 who found that, compared with federal insurance and self-pay patients, private insurance patients were 41% more likely to undergo THA than HA or internal fixation for FNF. We think that the age difference between our THA and HA groups contributed to the insurance variability in our study.
Our study had several limitations. It was conducted to examine the rates of THA and HA after FNF, not to survey treatment types, including ORIF and nonoperative management. The NHDS database does not provide information on HA implant type (unipolar, bipolar), use or nonuse of cement with HA, or surgical approach. Surgical approach could influence the rate of postoperative dislocation, an outcome measure that was not examined in this study. Last, the NHDS database tracks admissions and discharges, not patients. When a patient is discharged, collection of information on the patient’s postoperative course stops; a patient who returns even only 1 day later is recorded as a new or unique patient. Therefore, intermediate or long-term outcome information is unavailable, which likely led to an underrepresentation of DVT, PE, and mortality after these THA and HA procedures.
There was a trend toward femoral head replacement rather than ORIF in the treatment of FNF. Cognitively functional and independent elderly patients, and patients with osteoarthritis or rheumatoid arthritis, may benefit from THA, whereas HA may be better suited to cognitively dysfunctional patients.23,24 The NHDS reflects an increasing trend toward arthroplasty over ORIF, but the exact treatment choice is affected by hospital type, size, location and surgeon preference, training, and subspecialization.
1. Macaulay W, Pagnotto MR, Iorio R, Mont MA, Saleh KJ. Displaced femoral neck fractures in the elderly: hemiarthroplasty versus total hip arthroplasty. J Am Acad Orthop Surg. 2006;14(5):287-293.
2. Miyamoto RG, Kaplan KM, Levine BR, Egol KA, Zuckerman JD. Surgical management of hip fractures: an evidence-based review of the literature. I: femoral neck fractures. J Am Acad Orthop Surg. 2008;16(10):596-607.
3. Kannus P, Parkkari J, Sievänen H, Heinonen A, Vuori I, Järvinen M. Epidemiology of hip fractures. Bone. 1996;18(1 suppl):57S-63S.
4. Zuckerman JD. Hip fracture. N Engl J Med. 1996;334(23):1519-1525.
5. Papandrea RF, Froimson MI. Total hip arthroplasty after acute displaced femoral neck fractures. Am J Orthop. 1996;25(2):85-88.
6. Burgers PT, Van Geene AR, Van den Bekerom MP, et al. Total hip arthroplasty versus hemiarthroplasty for displaced femoral neck fractures in the healthy elderly: a meta-analysis and systematic review of randomized trials. Int Orthop. 2012;36(8):1549-1560.
7. Skinner P, Riley D, Ellery J, Beaumont A, Coumine R, Shafighian B. Displaced subcapital fractures of the femur: a prospective randomized comparison of internal fixation, hemiarthroplasty and total hip replacement. Injury. 1989;20(5):291-293.
8. Baker RP, Squires B, Gargan MF, Bannister GC. Total hip arthroplasty and hemiarthroplasty in mobile, independent patients with a displaced intracapsular fracture of the femoral neck. A randomized, controlled trial. J Bone Joint Surg Am. 2006;88(12):2583-2589.
9. Centers for Disease Control and Prevention, National Center for Health Statistics. National Hospital Discharge Survey. http://www.cdc.gov/nchs/nhds/about_nhds.htm. Last updated December 6, 2011. Accessed December 10, 2013.
10. Zi-Sheng A, You-Shui G, Zhi-Zhen J, Ting Y, Chang-Qing Z. Hemiarthroplasty vs primary total hip arthroplasty for displaced fractures of the femoral neck in the elderly: a meta-analysis. J Arthroplasty. 2012;27(4):583-590.
11. Yu L, Wang Y, Chen J. Total hip arthroplasty versus hemiarthroplasty for displaced femoral neck fractures: meta-analysis of randomized trials. Clin Orthop Relat Res. 2012;470(8):2235-2243.
12. Hopley C, Stengel D, Ekkernkamp A, Wich M. Primary total hip arthroplasty versus hemiarthroplasty for displaced intracapsular hip fractures in older patients: systematic review. BMJ. 2010;340:c2332.
13. Miller BJ, Callaghan JJ, Cram P, Karam M, Marsh JL, Noiseux NO. Changing trends in the treatment of femoral neck fractures: a review of the American Board of Orthopaedic Surgery database. J Bone Joint Surg Am. 2014;96(17):e149.
14. Rogmark C, Carlsson A, Johnell O, Sernbo I. A prospective randomised trial of internal fixation versus arthroplasty for displaced fractures of the neck of the femur. Functional outcome for 450 patients at two years. J Bone Joint Surg Br. 2002;84(2):183-188.
15. Bhandari M, Devereaux PJ, Swiontkowski MF, et al. Internal fixation compared with arthroplasty for displaced fractures of the femoral neck. A meta-analysis. J Bone Joint Surg Am. 2003;85(9):1673-1681.
16. Keating JF, Grant A, Masson M, Scott NW, Forbes JF. Randomized comparison of reduction and fixation, bipolar hemiarthroplasty, and total hip arthroplasty. Treatment of displaced intracapsular hip fractures in healthy older patients. J Bone Joint Surg Am. 2006;88(2):249-260.
17. Iorio R, Healy WL, Lemos DW, Appleby D, Lucchesi CA, Saleh KJ. Displaced femoral neck fractures in the elderly: outcomes and cost effectiveness. Clin Orthop Relat Res. 2001;(383):229-242.
18. Johansson T, Jacobsson SA, Ivarsson I, Knutsson A, Wahlström O. Internal fixation versus total hip arthroplasty in the treatment of displaced femoral neck fractures: a prospective randomized study of 100 hips. Acta Orthop Scand. 2000;71(6):597-602.
19. Shah AK, Eissler J, Radomisli T. Algorithms for the treatment of femoral neck fractures. Clin Orthop Relat Res. 2002;(399):28-34.
20. Ames JB, Lurie JD, Tomek IM, Zhou W, Koval KJ. Does surgeon volume for total hip arthroplasty affect outcomes after hemiarthroplasty for femoral neck fracture? Am J Orthop. 2010;39(8):E84-E89.
21. Le A, Judd SE, Allison DB, et al. The geographic distribution of obesity in the US and the potential regional differences in misreporting of obesity. Obesity. 2014;22(1):300-306.
22. Hochfelder JP, Khatib ON, Glait SA, Slover JD. Femoral neck fractures in New York state. Is the rate of THA increasing, and do race or payer influence decision making? J Orthop Trauma. 2014;28(7):422-426.
23. Lowe JA, Crist BD, Bhandari M, Ferguson TA. Optimal treatment of femoral neck fractures according to patient’s physiologic age: an evidence-based review. Orthop Clin North Am. 2010;41(2):157-166.
24. Callaghan JJ, Liu SS, Haidukewych GJ. Subcapital fractures: a changing paradigm. J Bone Joint Surg Br. 2012;94(11 suppl A):19-21.
1. Macaulay W, Pagnotto MR, Iorio R, Mont MA, Saleh KJ. Displaced femoral neck fractures in the elderly: hemiarthroplasty versus total hip arthroplasty. J Am Acad Orthop Surg. 2006;14(5):287-293.
2. Miyamoto RG, Kaplan KM, Levine BR, Egol KA, Zuckerman JD. Surgical management of hip fractures: an evidence-based review of the literature. I: femoral neck fractures. J Am Acad Orthop Surg. 2008;16(10):596-607.
3. Kannus P, Parkkari J, Sievänen H, Heinonen A, Vuori I, Järvinen M. Epidemiology of hip fractures. Bone. 1996;18(1 suppl):57S-63S.
4. Zuckerman JD. Hip fracture. N Engl J Med. 1996;334(23):1519-1525.
5. Papandrea RF, Froimson MI. Total hip arthroplasty after acute displaced femoral neck fractures. Am J Orthop. 1996;25(2):85-88.
6. Burgers PT, Van Geene AR, Van den Bekerom MP, et al. Total hip arthroplasty versus hemiarthroplasty for displaced femoral neck fractures in the healthy elderly: a meta-analysis and systematic review of randomized trials. Int Orthop. 2012;36(8):1549-1560.
7. Skinner P, Riley D, Ellery J, Beaumont A, Coumine R, Shafighian B. Displaced subcapital fractures of the femur: a prospective randomized comparison of internal fixation, hemiarthroplasty and total hip replacement. Injury. 1989;20(5):291-293.
8. Baker RP, Squires B, Gargan MF, Bannister GC. Total hip arthroplasty and hemiarthroplasty in mobile, independent patients with a displaced intracapsular fracture of the femoral neck. A randomized, controlled trial. J Bone Joint Surg Am. 2006;88(12):2583-2589.
9. Centers for Disease Control and Prevention, National Center for Health Statistics. National Hospital Discharge Survey. http://www.cdc.gov/nchs/nhds/about_nhds.htm. Last updated December 6, 2011. Accessed December 10, 2013.
10. Zi-Sheng A, You-Shui G, Zhi-Zhen J, Ting Y, Chang-Qing Z. Hemiarthroplasty vs primary total hip arthroplasty for displaced fractures of the femoral neck in the elderly: a meta-analysis. J Arthroplasty. 2012;27(4):583-590.
11. Yu L, Wang Y, Chen J. Total hip arthroplasty versus hemiarthroplasty for displaced femoral neck fractures: meta-analysis of randomized trials. Clin Orthop Relat Res. 2012;470(8):2235-2243.
12. Hopley C, Stengel D, Ekkernkamp A, Wich M. Primary total hip arthroplasty versus hemiarthroplasty for displaced intracapsular hip fractures in older patients: systematic review. BMJ. 2010;340:c2332.
13. Miller BJ, Callaghan JJ, Cram P, Karam M, Marsh JL, Noiseux NO. Changing trends in the treatment of femoral neck fractures: a review of the American Board of Orthopaedic Surgery database. J Bone Joint Surg Am. 2014;96(17):e149.
14. Rogmark C, Carlsson A, Johnell O, Sernbo I. A prospective randomised trial of internal fixation versus arthroplasty for displaced fractures of the neck of the femur. Functional outcome for 450 patients at two years. J Bone Joint Surg Br. 2002;84(2):183-188.
15. Bhandari M, Devereaux PJ, Swiontkowski MF, et al. Internal fixation compared with arthroplasty for displaced fractures of the femoral neck. A meta-analysis. J Bone Joint Surg Am. 2003;85(9):1673-1681.
16. Keating JF, Grant A, Masson M, Scott NW, Forbes JF. Randomized comparison of reduction and fixation, bipolar hemiarthroplasty, and total hip arthroplasty. Treatment of displaced intracapsular hip fractures in healthy older patients. J Bone Joint Surg Am. 2006;88(2):249-260.
17. Iorio R, Healy WL, Lemos DW, Appleby D, Lucchesi CA, Saleh KJ. Displaced femoral neck fractures in the elderly: outcomes and cost effectiveness. Clin Orthop Relat Res. 2001;(383):229-242.
18. Johansson T, Jacobsson SA, Ivarsson I, Knutsson A, Wahlström O. Internal fixation versus total hip arthroplasty in the treatment of displaced femoral neck fractures: a prospective randomized study of 100 hips. Acta Orthop Scand. 2000;71(6):597-602.
19. Shah AK, Eissler J, Radomisli T. Algorithms for the treatment of femoral neck fractures. Clin Orthop Relat Res. 2002;(399):28-34.
20. Ames JB, Lurie JD, Tomek IM, Zhou W, Koval KJ. Does surgeon volume for total hip arthroplasty affect outcomes after hemiarthroplasty for femoral neck fracture? Am J Orthop. 2010;39(8):E84-E89.
21. Le A, Judd SE, Allison DB, et al. The geographic distribution of obesity in the US and the potential regional differences in misreporting of obesity. Obesity. 2014;22(1):300-306.
22. Hochfelder JP, Khatib ON, Glait SA, Slover JD. Femoral neck fractures in New York state. Is the rate of THA increasing, and do race or payer influence decision making? J Orthop Trauma. 2014;28(7):422-426.
23. Lowe JA, Crist BD, Bhandari M, Ferguson TA. Optimal treatment of femoral neck fractures according to patient’s physiologic age: an evidence-based review. Orthop Clin North Am. 2010;41(2):157-166.
24. Callaghan JJ, Liu SS, Haidukewych GJ. Subcapital fractures: a changing paradigm. J Bone Joint Surg Br. 2012;94(11 suppl A):19-21.
Hypercalcemia, Parathyroid Disease, Vitamin D Deficiency
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
This video was filmed at Metabolic & Endocrine Disease Summit (MEDS). Click here to learn more.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
This video was filmed at Metabolic & Endocrine Disease Summit (MEDS). Click here to learn more.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
This video was filmed at Metabolic & Endocrine Disease Summit (MEDS). Click here to learn more.
Management of Isolated Greater Tuberosity Fractures: A Systematic Review
Take-Home Points
- Fractures of the greater tuberosity are often mismanaged.
- Comprehension of greater tuberosity fractures involves classification into nonoperative and operative treatment, displacement >5mm or <5 mm, and open vs arthroscopic surgery.
- Nearly a third of patients may suffer concomitant anterior glenohumeral instability.
- Stiffness is the most common postoperative complication.
- Surgery is associated with high patient satisfaction and low rates of complications and reoperations.
Although proximal humerus fractures are common in the elderly, isolated fractures of the greater tuberosity occur less often. Management depends on several factors, including fracture pattern and displacement.1,2 Nondisplaced fractures are often successfully managed with sling immobilization and early range of motion.3,4 Although surgical intervention improves outcomes in displaced greater tuberosity fractures, the ideal surgical treatment is less clear.5
Displaced greater tuberosity fractures may require surgery for prevention of subacromial impingement and range-of-motion deficits.2 Superior fracture displacement results in decreased shoulder abduction, and posterior displacement can limit external rotation.6 Although the greater tuberosity can displace in any direction, posterosuperior displacement has the worst outcomes.1 The exact surgery-warranting displacement amount ranges from 3 mm to 10 mm but is yet to be clearly elucidated.5,6 Less displacement is tolerated by young overhead athletes, and more displacement by older less active patients.5,7,8 Surgical options for isolated greater tuberosity fractures include fragment excision, open reduction and internal fixation (ORIF), closed reduction with percutaneous fixation, and arthroscopically assisted reduction with internal fixation.3,9,10
We conducted a study to determine the management patterns for isolated greater tuberosity fractures. We hypothesized that greater tuberosity fractures displaced <5 mm may be managed nonoperatively and that greater tuberosity fractures displaced >5 mm require surgical fixation.
Methods
Search Strategy
We performed this systematic review according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) checklist11 and registered it (CRD42014010691) with the PROSPERO international prospective register of systematic reviews. Literature searches using the PubMed/Medline database and the Cochrane Central Register of Clinical Trials were completed in August 2014. There were no date or year restrictions. Key words were used to capture all English- language studies with level I to IV evidence (Oxford Centre for Evidence-Based Medicine) and reported clinical or radiographic outcomes. Initial exclusion criteria were cadaveric, biomechanical, histologic, and kinematic results. An electronic search algorithm with key words and a series of NOT phrases was designed to match our exclusion criteria:
((((((((((((((((((((((((((((((((((((((((((((((((((greater[Title/Abstract]) AND tuberosity [Title/Abstract] OR tubercle [Title/Abstract]) AND fracture[Title/Abstract]) AND proximal[Title/Abstract] AND (English[lang]))) NOT intramedullary[Title] AND (English[lang]))) NOT nonunion[Title] AND (English[lang]))) NOT malunion[Title] AND (English[lang]))) NOT biomechanical[Title/Abstract] AND (English[lang]))) NOT cadaveric[Title/Abstract] AND (English[lang]))) NOT cadaver[Title/Abstract] AND (English[lang]))) NOT ((basic[Title/Abstract]) AND science[Title/Abstract] AND (English[lang])) AND (English[lang]))) NOT revision[Title] AND (English[lang]))) NOT pediatric[Title] AND (English[lang]))) NOT physeal[Title] AND (English[lang]))) NOT children[Title] AND (English[lang]))) NOT instability[Title] AND (English[lang]))) NOT imaging[Title])) NOT salter[Title])) NOT physis[Title])) NOT shaft[Title])) NOT distal[Title])) NOT clavicle[Title])) NOT scapula[Title])) NOT ((diaphysis[Title]) AND diaphyseal[Title]))) NOT infection[Title])) NOT laboratory[Title/Abstract])) NOT metastatic[Title/Abstract])) NOT (((((((malignancy[Title/Abstract]) OR malignant[Title/Abstract]) OR tumor[Title/Abstract]) OR oncologic[Title/Abstract]) OR cyst[Title/Abstract]) OR aneurysmal[Title/Abstract]) OR unicameral[Title/Abstract]).
Study Selection
Data Extraction
We extracted data from the 13 studies that met the eligibility criteria. Details of study design, sample size, and patient demographics, including age, sex, and hand dominance, were recorded, as were mechanism of injury and concomitant anterior shoulder instability. To capture the most patients, we noted radiographic fracture displacement categorically rather than continuously; patients were divided into 2 displacement groups (<5 mm, >5 mm). Most studies did not define degree of comminution or specific direction of displacement per fracture, so these variables were not included in the data analysis. Nonoperative management and operative management were studied. We abstracted surgical factors, such as approach, method, fixation type (screws or sutures), and technique (suture anchors or transosseous tunnels). Clinical outcomes included physical examination findings, functional assessment results (patient satisfaction; Constant and University of California Los Angeles [UCLA] shoulder scores), and the number of revisions. Radiologic outcomes, retrieved from radiographs or computed tomography scans, focused on loss of reduction (as determined by the respective authors), malunion, nonunion, and heterotopic ossification. Each study’s methodologic quality and bias were evaluated with the 15-item Modified Coleman Methodology Score (MCMS), which was described by Cowan and colleagues.23 The MCMS has been used to assess randomized and nonrandomized patient trials.24,25 Its scaled potential score ranges from 0 to 100 (85-100, excellent; 70-84, good; 55-69, fair; <55, poor).
Statistical Analysis
We report our data as weighted means (SDs). A mean was calculated for each study that reported a respective data point, and each mean was then weighed according to its study sample size. This calculation was performed by multiplying a study’s individual mean by the number of patients enrolled in that study and dividing the sum of these weighted data points by the number of eligible patients in all relevant studies. The result was that the nonweighted means from studies with smaller sample sizes did not carry as much weight as the nonweighted means from larger studies. We compared 3 paired groups: treatment type (nonoperative vs operative), fracture displacement amount (<5 mm vs >5 mm), and surgery type (open vs arthroscopic). Regarding all patient, surgery, and outcomes data, unpaired Student t tests were used for continuous variables and 2-tailed Fisher exact tests for categorical variables with α = 0.05 (SPSS Version 18; IBM).
Results
Postoperative physical examination findings were underreported so that surgical groups could be compared. Of all the surgical studies, 4 reported postoperative forward elevation (mean, 160°; SD, 9.8°) and external rotation (mean, 46.4°; SD 26.3°).14,15,18,22 No malunions and only 1 nonunion were reported in all 13 studies. No deaths or other serious medical complications were reported. Patients with anterior instability more often underwent surgery than were treated nonoperatively (39.2% vs 12.0%; P < .01) and more often had fractures displaced >5 mm than <5 mm (44.3% vs 14.5%; P < .01).
Fisher exact tests were used to perform isolated comparisons of screws and sutures as well as suture anchors and transosseous tunnels. Patients with screw fixation were significantly (P = .051) less likely to require reoperation (0/56; 0%) than patients with suture fixation (8/100; 8.0%). Screw fixation also led to significantly less stiffness (0% vs 12.0%; P < .01) but trended toward a higher rate of superficial infection (3.6% vs 0%; P = .13). There was no statistical difference in nerve injury rates between screws and sutures (1.8% vs 3.0%; P = 1.0). There were no significant differences in reoperations, stiffness, superficial infections, or nerve injuries between suture anchor and transosseous tunnel constructs.
For all 13 studies, mean (SD) MCMS was 41.1 (8.6).
Discussion
Five percent of all fractures involve the proximal humerus, and 20% of proximal humerus fractures are isolated greater tuberosity fractures.26,27 In his classic 1970 article, Neer6 formulated the 4-part proximal humerus fracture classification and defined greater tuberosity fracture “parts” using the same criteria as for other fracture “parts.” Neer6 recommended nonoperative management for isolated greater tuberosity fractures displaced <1 cm but did not present evidence corroborating his recommendation. More recent cutoffs for nonoperative management include 5 mm (general population) and 3 mm (athletes).7,17
In the present systematic review of greater tuberosity fractures, 3 separate comparisons were made: treatment type (nonoperative vs operative), fracture displacement amount (<5 mm vs >5 mm), and surgery type (open vs arthroscopic).
Treatment Type. Only 4 studies reported data on nonoperative treatment outcomes.5,12,16,17 Of these 4 studies, 2 found successful outcomes for fractures displaced <5 mm.12,17 Platzer and colleagues17 found good or excellent results in 97% of 135 shoulders after 4 years. Good results were defined with shoulder scores of ≥80 (Constant), <8 (Vienna), and >28 (UCLA), and excellent results were defined with maximum scores on 2 of the 3 systems. Platzer and colleagues17 also found nonsignificantly worse shoulder scores with superior displacement of 3 mm to 5 mm and recommended surgery for overhead athletes in this group. Rath and colleagues12 described a successful 3-phase rehabilitation protocol of sling immobilization for 3 weeks, pendulum exercises for 3 weeks, and active exercises thereafter. By an average of 31 months, patient satisfaction scores improved to 9.5 from 4.2 (10-point scale), though the authors cautioned that pain and decreased motion lasted 8 months on average. Conservative treatment was far less successful in the 2 studies of fractures displaced >5 mm.5,16 Keene and colleagues16 reported unsatisfactory results in all 4 patients with fractures displaced >1.5 cm. In a study separate from their 2005 analysis,17 Platzer and colleagues5 in 2008 evaluated displaced fractures and found function and patient satisfaction were inferior after nonoperative treatment than after surgery. The studies by Keene and colleagues16 and Platzer and colleagues5 support the finding of an overall lower patient satisfaction rate in nonoperative patients.
Fracture Displacement Amount. Only 2 arthroscopic studies and no open studies addressed surgery for fractures displaced <5 mm. Fewer than 16% of these fractures were managed operatively, and <1% required reoperation. By contrast, almost all fractures displaced >5 mm were managed operatively, and 3.6% required reoperation. Radiographic loss of reduction was more common in fractures displaced <5 mm, primarily because they were managed without fixation. Radiographic loss of reduction was reported in only 9 operatively treated patients, none of whom was symptomatic enough to require another surgery.5 Reoperations were most commonly performed for stiffness, which itself was significantly more common in fractures displaced >5 mm. Bhatia and colleagues14 reported the highest reoperation rate (14.3%; 3/21), but they studied more complex, comminuted fractures of the greater tuberosity. Two of their 3 reoperations were biceps tenodeses for inflamed, stiff tenosynovitis, and the third patient had a foreign body giant cell reaction to suture material. Fewer than 1% of patients with operatively managed displaced fractures required revision ORIF, and <2% developed a superficial infection or postoperative nerve palsy.19,22 For displaced greater tuberosity fractures, surgery is highly successful overall, complication rates are very low, and 90% of patients report being satisfied.
Surgery Type. Patients were divided into 2 groups. In the nonarthroscopic group, open and percutaneous approaches were used. All studies that described a percutaneous approach used screw fixation5,21; in addition, 32 patients were treated with screws through an open approach.2,5 The other open and arthroscopic studies used suture fixation. Interestingly, no studies reported on clinical outcomes of fragment excision. There were no statistically significant differences in rates of reoperation, stiffness, infection, or neurologic injury between the arthroscopic and nonarthroscopic groups. Patient satisfaction scores were slightly higher in the nonarthroscopic group (91.0% vs 87.8%), but the difference was not statistically significant.
With surgical techniques isolated, there were no significant differences between suture anchors and transosseous tunnel constructs, but screws performed significantly better than suture techniques. Compared with suture fixation, screw fixation led to significantly fewer cases of stiffness and reoperation, which suggests surgeons need to give screws more consideration in the operative management of these fractures. However, the number of patients treated with screws was smaller than the number treated with suture fixation; it is possible the differences between these cohorts would be eliminated if there were more patients in the screw cohort. In addition, screw fixation was universally performed with an open or percutaneous approach and trended toward a higher infection rate. As screw and suture techniques have low rates of complications and reoperations, we recommend leaving fixation choice to the surgeon.
Anterior shoulder instability has been associated with greater tuberosity fractures.1,8,19 The supraspinatus, infraspinatus, and teres minor muscles all insert into the greater tuberosity and resist anterior translation of the proximal humerus. Loss of this dynamic muscle stabilization is amplified by tuberosity fracture displacement: Anterior shoulder instability was significantly more common in fractures displaced >5 mm (44.3%) vs <5 mm (14.5%). In turn, glenohumeral instability was more common in patients treated with surgery, specifically open surgery, because displaced fractures may not be as easily accessed with arthroscopic techniques. No studies reported concomitant labral repair or capsular plication techniques.
This systematic review was limited by the studies analyzed. All but 1 study5 had level IV evidence. Mean (SD) MCMS was 41.8 (8.6). Any MCMS score <54 indicates a poor methodology level, but this scoring system is designed for randomized controlled trials,23 and there were none in this study. Physical examination findings, such as range of motion, were underreported. In addition, radiographic parameters were not consistently described but rather were determined by the respective authors’ subjective interpretations of malunion, nonunion, and loss of reduction. Publication bias is present in that we excluded non- English language studies and medical conference abstracts and may have omitted potentially eligible studies not discoverable with our search methodology. Performance bias is a factor in any systematic review with multiple surgeons and wide variation in surgical technique.
Conclusion
Greater tuberosity fractures displaced <5 mm may be safely managed nonoperatively, as there are no reports of nonoperatively managed fractures that subsequently required surgery. Nonoperative treatment was initially associated with low patient satisfaction, but only because displaced fractures were conservatively managed in early studies.5,16 Fractures displaced >5 mm respond well to operative fixation with screws, suture anchors, or transosseous suture tunnels. Stiffness is the most common postoperative complication (<6%), followed by heterotopic ossification, transient neurapraxias, and superficial infection. There are no discernible differences in outcome between open and arthroscopic techniques, but screw fixation may lead to significantly fewer cases of stiffness and reoperation in comparison with suture constructs.
1. Verdano MA, Aliani D, Pellegrini A, Baudi P, Pedrazzi G, Ceccarelli F. Isolated fractures of the greater tuberosity in proximal humerus: does the direction of displacement influence functional outcome? An analysis of displacement in greater tuberosity fractures. Acta Biomed. 2013;84(3):219-228.
2. Yin B, Moen TC, Thompson SA, Bigliani LU, Ahmad CS, Levine WN. Operative treatment of isolated greater tuberosity fractures: retrospective review of clinical and functional outcomes. Orthopedics. 2012;35(6):e807-e814.
3. Green A, Izzi J. Isolated fractures of the greater tuberosity of the proximal humerus. J Shoulder Elbow Surg. 2003;12(6):641-649.
4. Norouzi M, Naderi MN, Komasi MH, Sharifzadeh SR, Shahrezaei M, Eajazi A. Clinical results of using the proximal humeral internal locking system plate for internal fixation of displaced proximal humeral fractures. Am J Orthop. 2012;41(5):E64-E68.
5. Platzer P, Thalhammer G, Oberleitner G, et al. Displaced fractures of the greater tuberosity: a comparison of operative and nonoperative treatment. J Trauma. 2008;65(4):843-848.
6. Neer CS. Displaced proximal humeral fractures. I. Classification and evaluation. J Bone Joint Surg Am. 1970;52(6):1077-1089.
7. Park TS, Choi IY, Kim YH, Park MR, Shon JH, Kim SI. A new suggestion for the treatment of minimally displaced fractures of the greater tuberosity of the proximal humerus. Bull Hosp Jt Dis. 1997;56(3):171-176.
8. McLaughlin HL. Dislocation of the shoulder with tuberosity fracture. Surg Clin North Am. 1963;43:1615-1620.
9. DeBottis D, Anavian J, Green A. Surgical management of isolated greater tuberosity fractures of the proximal humerus. Orthop Clin North Am. 2014;45(2):207-218.
10. Monga P, Verma R, Sharma VK. Closed reduction and external fixation for displaced proximal humeral fractures. J Orthop Surg (Hong Kong). 2009;17(2):142-145.
11. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006-1012.
12. Rath E, Alkrinawi N, Levy O, Debbi R, Amar E, Atoun E. Minimally displaced fractures of the greater tuberosity: outcome of non-operative treatment. J Shoulder Elbow Surg. 2013;22(10):e8-e11.
13. Dimakopoulos P, Panagopoulos A, Kasimatis G. Transosseous suture fixation of proximal humeral fractures. J Bone Joint Surg Am. 2007;89(8):1700-1709.
14. Bhatia DN, van Rooyen KS, Toit du DF, de Beer JF. Surgical treatment of comminuted, displaced fractures of the greater tuberosity of the proximal humerus: a new technique of double-row suture-anchor fixation and long-term results. Injury. 2006;37(10):946-952.
15. Flatow EL, Cuomo F, Maday MG, Miller SR, McIlveen SJ, Bigliani LU. Open reduction and internal fixation of two-part displaced fractures of the greater tuberosity of the proximal part of the humerus. J Bone Joint Surg Am. 1991;73(8):1213-1218.
16. Keene JS, Huizenga RE, Engber WD, Rogers SC. Proximal humeral fractures: a correlation of residual deformity with long-term function. Orthopedics. 1983;6(2):173-178.
17. Platzer P, Kutscha-Lissberg F, Lehr S, Vecsei V, Gaebler C. The influence of displacement on shoulder function in patients with minimally displaced fractures of the greater tuberosity. Injury. 2005;36(10):1185-1189.
18. Park SE, Ji JH, Shafi M, Jung JJ, Gil HJ, Lee HH. Arthroscopic management of occult greater tuberosity fracture of the shoulder. Eur J Orthop Surg Traumatol. 2014;24(4):475-482.
19. Dimakopoulos P, Panagopoulos A, Kasimatis G, Syggelos SA, Lambiris E. Anterior traumatic shoulder dislocation associated with displaced greater tuberosity fracture: the necessity of operative treatment. J Orthop Trauma. 2007;21(2):104-112.
20. Kim SH, Ha KI. Arthroscopic treatment of symptomatic shoulders with minimally displaced greater tuberosity fracture. Arthroscopy. 2000;16(7):695-700.
21. Chen CY, Chao EK, Tu YK, Ueng SW, Shih CH. Closed management and percutaneous fixation of unstable proximal humerus fractures. J Trauma. 1998;45(6):1039-1045.
22. Ji JH, Shafi M, Song IS, Kim YY, McFarland EG, Moon CY. Arthroscopic fixation technique for comminuted, displaced greater tuberosity fracture. Arthroscopy. 2010;26(5):600-609.
23. Cowan J, Lozano-Calderón S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699.
24. Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010;92(12):2220-2233.
25. Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation—a systematic review. Osteoarthritis Cartilage. 2011;19(7):779-791.
26. Chun JM, Groh GI, Rockwood CA. Two-part fractures of the proximal humerus. J Shoulder Elbow Surg. 1994;3(5):273-287.
27. Gruson KI, Ruchelsman DE, Tejwani NC. Isolated tuberosity fractures of the proximal humeral: current concepts. Injury. 2008;39(3):284-298.
Take-Home Points
- Fractures of the greater tuberosity are often mismanaged.
- Comprehension of greater tuberosity fractures involves classification into nonoperative and operative treatment, displacement >5mm or <5 mm, and open vs arthroscopic surgery.
- Nearly a third of patients may suffer concomitant anterior glenohumeral instability.
- Stiffness is the most common postoperative complication.
- Surgery is associated with high patient satisfaction and low rates of complications and reoperations.
Although proximal humerus fractures are common in the elderly, isolated fractures of the greater tuberosity occur less often. Management depends on several factors, including fracture pattern and displacement.1,2 Nondisplaced fractures are often successfully managed with sling immobilization and early range of motion.3,4 Although surgical intervention improves outcomes in displaced greater tuberosity fractures, the ideal surgical treatment is less clear.5
Displaced greater tuberosity fractures may require surgery for prevention of subacromial impingement and range-of-motion deficits.2 Superior fracture displacement results in decreased shoulder abduction, and posterior displacement can limit external rotation.6 Although the greater tuberosity can displace in any direction, posterosuperior displacement has the worst outcomes.1 The exact surgery-warranting displacement amount ranges from 3 mm to 10 mm but is yet to be clearly elucidated.5,6 Less displacement is tolerated by young overhead athletes, and more displacement by older less active patients.5,7,8 Surgical options for isolated greater tuberosity fractures include fragment excision, open reduction and internal fixation (ORIF), closed reduction with percutaneous fixation, and arthroscopically assisted reduction with internal fixation.3,9,10
We conducted a study to determine the management patterns for isolated greater tuberosity fractures. We hypothesized that greater tuberosity fractures displaced <5 mm may be managed nonoperatively and that greater tuberosity fractures displaced >5 mm require surgical fixation.
Methods
Search Strategy
We performed this systematic review according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) checklist11 and registered it (CRD42014010691) with the PROSPERO international prospective register of systematic reviews. Literature searches using the PubMed/Medline database and the Cochrane Central Register of Clinical Trials were completed in August 2014. There were no date or year restrictions. Key words were used to capture all English- language studies with level I to IV evidence (Oxford Centre for Evidence-Based Medicine) and reported clinical or radiographic outcomes. Initial exclusion criteria were cadaveric, biomechanical, histologic, and kinematic results. An electronic search algorithm with key words and a series of NOT phrases was designed to match our exclusion criteria:
((((((((((((((((((((((((((((((((((((((((((((((((((greater[Title/Abstract]) AND tuberosity [Title/Abstract] OR tubercle [Title/Abstract]) AND fracture[Title/Abstract]) AND proximal[Title/Abstract] AND (English[lang]))) NOT intramedullary[Title] AND (English[lang]))) NOT nonunion[Title] AND (English[lang]))) NOT malunion[Title] AND (English[lang]))) NOT biomechanical[Title/Abstract] AND (English[lang]))) NOT cadaveric[Title/Abstract] AND (English[lang]))) NOT cadaver[Title/Abstract] AND (English[lang]))) NOT ((basic[Title/Abstract]) AND science[Title/Abstract] AND (English[lang])) AND (English[lang]))) NOT revision[Title] AND (English[lang]))) NOT pediatric[Title] AND (English[lang]))) NOT physeal[Title] AND (English[lang]))) NOT children[Title] AND (English[lang]))) NOT instability[Title] AND (English[lang]))) NOT imaging[Title])) NOT salter[Title])) NOT physis[Title])) NOT shaft[Title])) NOT distal[Title])) NOT clavicle[Title])) NOT scapula[Title])) NOT ((diaphysis[Title]) AND diaphyseal[Title]))) NOT infection[Title])) NOT laboratory[Title/Abstract])) NOT metastatic[Title/Abstract])) NOT (((((((malignancy[Title/Abstract]) OR malignant[Title/Abstract]) OR tumor[Title/Abstract]) OR oncologic[Title/Abstract]) OR cyst[Title/Abstract]) OR aneurysmal[Title/Abstract]) OR unicameral[Title/Abstract]).
Study Selection
Data Extraction
We extracted data from the 13 studies that met the eligibility criteria. Details of study design, sample size, and patient demographics, including age, sex, and hand dominance, were recorded, as were mechanism of injury and concomitant anterior shoulder instability. To capture the most patients, we noted radiographic fracture displacement categorically rather than continuously; patients were divided into 2 displacement groups (<5 mm, >5 mm). Most studies did not define degree of comminution or specific direction of displacement per fracture, so these variables were not included in the data analysis. Nonoperative management and operative management were studied. We abstracted surgical factors, such as approach, method, fixation type (screws or sutures), and technique (suture anchors or transosseous tunnels). Clinical outcomes included physical examination findings, functional assessment results (patient satisfaction; Constant and University of California Los Angeles [UCLA] shoulder scores), and the number of revisions. Radiologic outcomes, retrieved from radiographs or computed tomography scans, focused on loss of reduction (as determined by the respective authors), malunion, nonunion, and heterotopic ossification. Each study’s methodologic quality and bias were evaluated with the 15-item Modified Coleman Methodology Score (MCMS), which was described by Cowan and colleagues.23 The MCMS has been used to assess randomized and nonrandomized patient trials.24,25 Its scaled potential score ranges from 0 to 100 (85-100, excellent; 70-84, good; 55-69, fair; <55, poor).
Statistical Analysis
We report our data as weighted means (SDs). A mean was calculated for each study that reported a respective data point, and each mean was then weighed according to its study sample size. This calculation was performed by multiplying a study’s individual mean by the number of patients enrolled in that study and dividing the sum of these weighted data points by the number of eligible patients in all relevant studies. The result was that the nonweighted means from studies with smaller sample sizes did not carry as much weight as the nonweighted means from larger studies. We compared 3 paired groups: treatment type (nonoperative vs operative), fracture displacement amount (<5 mm vs >5 mm), and surgery type (open vs arthroscopic). Regarding all patient, surgery, and outcomes data, unpaired Student t tests were used for continuous variables and 2-tailed Fisher exact tests for categorical variables with α = 0.05 (SPSS Version 18; IBM).
Results
Postoperative physical examination findings were underreported so that surgical groups could be compared. Of all the surgical studies, 4 reported postoperative forward elevation (mean, 160°; SD, 9.8°) and external rotation (mean, 46.4°; SD 26.3°).14,15,18,22 No malunions and only 1 nonunion were reported in all 13 studies. No deaths or other serious medical complications were reported. Patients with anterior instability more often underwent surgery than were treated nonoperatively (39.2% vs 12.0%; P < .01) and more often had fractures displaced >5 mm than <5 mm (44.3% vs 14.5%; P < .01).
Fisher exact tests were used to perform isolated comparisons of screws and sutures as well as suture anchors and transosseous tunnels. Patients with screw fixation were significantly (P = .051) less likely to require reoperation (0/56; 0%) than patients with suture fixation (8/100; 8.0%). Screw fixation also led to significantly less stiffness (0% vs 12.0%; P < .01) but trended toward a higher rate of superficial infection (3.6% vs 0%; P = .13). There was no statistical difference in nerve injury rates between screws and sutures (1.8% vs 3.0%; P = 1.0). There were no significant differences in reoperations, stiffness, superficial infections, or nerve injuries between suture anchor and transosseous tunnel constructs.
For all 13 studies, mean (SD) MCMS was 41.1 (8.6).
Discussion
Five percent of all fractures involve the proximal humerus, and 20% of proximal humerus fractures are isolated greater tuberosity fractures.26,27 In his classic 1970 article, Neer6 formulated the 4-part proximal humerus fracture classification and defined greater tuberosity fracture “parts” using the same criteria as for other fracture “parts.” Neer6 recommended nonoperative management for isolated greater tuberosity fractures displaced <1 cm but did not present evidence corroborating his recommendation. More recent cutoffs for nonoperative management include 5 mm (general population) and 3 mm (athletes).7,17
In the present systematic review of greater tuberosity fractures, 3 separate comparisons were made: treatment type (nonoperative vs operative), fracture displacement amount (<5 mm vs >5 mm), and surgery type (open vs arthroscopic).
Treatment Type. Only 4 studies reported data on nonoperative treatment outcomes.5,12,16,17 Of these 4 studies, 2 found successful outcomes for fractures displaced <5 mm.12,17 Platzer and colleagues17 found good or excellent results in 97% of 135 shoulders after 4 years. Good results were defined with shoulder scores of ≥80 (Constant), <8 (Vienna), and >28 (UCLA), and excellent results were defined with maximum scores on 2 of the 3 systems. Platzer and colleagues17 also found nonsignificantly worse shoulder scores with superior displacement of 3 mm to 5 mm and recommended surgery for overhead athletes in this group. Rath and colleagues12 described a successful 3-phase rehabilitation protocol of sling immobilization for 3 weeks, pendulum exercises for 3 weeks, and active exercises thereafter. By an average of 31 months, patient satisfaction scores improved to 9.5 from 4.2 (10-point scale), though the authors cautioned that pain and decreased motion lasted 8 months on average. Conservative treatment was far less successful in the 2 studies of fractures displaced >5 mm.5,16 Keene and colleagues16 reported unsatisfactory results in all 4 patients with fractures displaced >1.5 cm. In a study separate from their 2005 analysis,17 Platzer and colleagues5 in 2008 evaluated displaced fractures and found function and patient satisfaction were inferior after nonoperative treatment than after surgery. The studies by Keene and colleagues16 and Platzer and colleagues5 support the finding of an overall lower patient satisfaction rate in nonoperative patients.
Fracture Displacement Amount. Only 2 arthroscopic studies and no open studies addressed surgery for fractures displaced <5 mm. Fewer than 16% of these fractures were managed operatively, and <1% required reoperation. By contrast, almost all fractures displaced >5 mm were managed operatively, and 3.6% required reoperation. Radiographic loss of reduction was more common in fractures displaced <5 mm, primarily because they were managed without fixation. Radiographic loss of reduction was reported in only 9 operatively treated patients, none of whom was symptomatic enough to require another surgery.5 Reoperations were most commonly performed for stiffness, which itself was significantly more common in fractures displaced >5 mm. Bhatia and colleagues14 reported the highest reoperation rate (14.3%; 3/21), but they studied more complex, comminuted fractures of the greater tuberosity. Two of their 3 reoperations were biceps tenodeses for inflamed, stiff tenosynovitis, and the third patient had a foreign body giant cell reaction to suture material. Fewer than 1% of patients with operatively managed displaced fractures required revision ORIF, and <2% developed a superficial infection or postoperative nerve palsy.19,22 For displaced greater tuberosity fractures, surgery is highly successful overall, complication rates are very low, and 90% of patients report being satisfied.
Surgery Type. Patients were divided into 2 groups. In the nonarthroscopic group, open and percutaneous approaches were used. All studies that described a percutaneous approach used screw fixation5,21; in addition, 32 patients were treated with screws through an open approach.2,5 The other open and arthroscopic studies used suture fixation. Interestingly, no studies reported on clinical outcomes of fragment excision. There were no statistically significant differences in rates of reoperation, stiffness, infection, or neurologic injury between the arthroscopic and nonarthroscopic groups. Patient satisfaction scores were slightly higher in the nonarthroscopic group (91.0% vs 87.8%), but the difference was not statistically significant.
With surgical techniques isolated, there were no significant differences between suture anchors and transosseous tunnel constructs, but screws performed significantly better than suture techniques. Compared with suture fixation, screw fixation led to significantly fewer cases of stiffness and reoperation, which suggests surgeons need to give screws more consideration in the operative management of these fractures. However, the number of patients treated with screws was smaller than the number treated with suture fixation; it is possible the differences between these cohorts would be eliminated if there were more patients in the screw cohort. In addition, screw fixation was universally performed with an open or percutaneous approach and trended toward a higher infection rate. As screw and suture techniques have low rates of complications and reoperations, we recommend leaving fixation choice to the surgeon.
Anterior shoulder instability has been associated with greater tuberosity fractures.1,8,19 The supraspinatus, infraspinatus, and teres minor muscles all insert into the greater tuberosity and resist anterior translation of the proximal humerus. Loss of this dynamic muscle stabilization is amplified by tuberosity fracture displacement: Anterior shoulder instability was significantly more common in fractures displaced >5 mm (44.3%) vs <5 mm (14.5%). In turn, glenohumeral instability was more common in patients treated with surgery, specifically open surgery, because displaced fractures may not be as easily accessed with arthroscopic techniques. No studies reported concomitant labral repair or capsular plication techniques.
This systematic review was limited by the studies analyzed. All but 1 study5 had level IV evidence. Mean (SD) MCMS was 41.8 (8.6). Any MCMS score <54 indicates a poor methodology level, but this scoring system is designed for randomized controlled trials,23 and there were none in this study. Physical examination findings, such as range of motion, were underreported. In addition, radiographic parameters were not consistently described but rather were determined by the respective authors’ subjective interpretations of malunion, nonunion, and loss of reduction. Publication bias is present in that we excluded non- English language studies and medical conference abstracts and may have omitted potentially eligible studies not discoverable with our search methodology. Performance bias is a factor in any systematic review with multiple surgeons and wide variation in surgical technique.
Conclusion
Greater tuberosity fractures displaced <5 mm may be safely managed nonoperatively, as there are no reports of nonoperatively managed fractures that subsequently required surgery. Nonoperative treatment was initially associated with low patient satisfaction, but only because displaced fractures were conservatively managed in early studies.5,16 Fractures displaced >5 mm respond well to operative fixation with screws, suture anchors, or transosseous suture tunnels. Stiffness is the most common postoperative complication (<6%), followed by heterotopic ossification, transient neurapraxias, and superficial infection. There are no discernible differences in outcome between open and arthroscopic techniques, but screw fixation may lead to significantly fewer cases of stiffness and reoperation in comparison with suture constructs.
Take-Home Points
- Fractures of the greater tuberosity are often mismanaged.
- Comprehension of greater tuberosity fractures involves classification into nonoperative and operative treatment, displacement >5mm or <5 mm, and open vs arthroscopic surgery.
- Nearly a third of patients may suffer concomitant anterior glenohumeral instability.
- Stiffness is the most common postoperative complication.
- Surgery is associated with high patient satisfaction and low rates of complications and reoperations.
Although proximal humerus fractures are common in the elderly, isolated fractures of the greater tuberosity occur less often. Management depends on several factors, including fracture pattern and displacement.1,2 Nondisplaced fractures are often successfully managed with sling immobilization and early range of motion.3,4 Although surgical intervention improves outcomes in displaced greater tuberosity fractures, the ideal surgical treatment is less clear.5
Displaced greater tuberosity fractures may require surgery for prevention of subacromial impingement and range-of-motion deficits.2 Superior fracture displacement results in decreased shoulder abduction, and posterior displacement can limit external rotation.6 Although the greater tuberosity can displace in any direction, posterosuperior displacement has the worst outcomes.1 The exact surgery-warranting displacement amount ranges from 3 mm to 10 mm but is yet to be clearly elucidated.5,6 Less displacement is tolerated by young overhead athletes, and more displacement by older less active patients.5,7,8 Surgical options for isolated greater tuberosity fractures include fragment excision, open reduction and internal fixation (ORIF), closed reduction with percutaneous fixation, and arthroscopically assisted reduction with internal fixation.3,9,10
We conducted a study to determine the management patterns for isolated greater tuberosity fractures. We hypothesized that greater tuberosity fractures displaced <5 mm may be managed nonoperatively and that greater tuberosity fractures displaced >5 mm require surgical fixation.
Methods
Search Strategy
We performed this systematic review according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) checklist11 and registered it (CRD42014010691) with the PROSPERO international prospective register of systematic reviews. Literature searches using the PubMed/Medline database and the Cochrane Central Register of Clinical Trials were completed in August 2014. There were no date or year restrictions. Key words were used to capture all English- language studies with level I to IV evidence (Oxford Centre for Evidence-Based Medicine) and reported clinical or radiographic outcomes. Initial exclusion criteria were cadaveric, biomechanical, histologic, and kinematic results. An electronic search algorithm with key words and a series of NOT phrases was designed to match our exclusion criteria:
((((((((((((((((((((((((((((((((((((((((((((((((((greater[Title/Abstract]) AND tuberosity [Title/Abstract] OR tubercle [Title/Abstract]) AND fracture[Title/Abstract]) AND proximal[Title/Abstract] AND (English[lang]))) NOT intramedullary[Title] AND (English[lang]))) NOT nonunion[Title] AND (English[lang]))) NOT malunion[Title] AND (English[lang]))) NOT biomechanical[Title/Abstract] AND (English[lang]))) NOT cadaveric[Title/Abstract] AND (English[lang]))) NOT cadaver[Title/Abstract] AND (English[lang]))) NOT ((basic[Title/Abstract]) AND science[Title/Abstract] AND (English[lang])) AND (English[lang]))) NOT revision[Title] AND (English[lang]))) NOT pediatric[Title] AND (English[lang]))) NOT physeal[Title] AND (English[lang]))) NOT children[Title] AND (English[lang]))) NOT instability[Title] AND (English[lang]))) NOT imaging[Title])) NOT salter[Title])) NOT physis[Title])) NOT shaft[Title])) NOT distal[Title])) NOT clavicle[Title])) NOT scapula[Title])) NOT ((diaphysis[Title]) AND diaphyseal[Title]))) NOT infection[Title])) NOT laboratory[Title/Abstract])) NOT metastatic[Title/Abstract])) NOT (((((((malignancy[Title/Abstract]) OR malignant[Title/Abstract]) OR tumor[Title/Abstract]) OR oncologic[Title/Abstract]) OR cyst[Title/Abstract]) OR aneurysmal[Title/Abstract]) OR unicameral[Title/Abstract]).
Study Selection
Data Extraction
We extracted data from the 13 studies that met the eligibility criteria. Details of study design, sample size, and patient demographics, including age, sex, and hand dominance, were recorded, as were mechanism of injury and concomitant anterior shoulder instability. To capture the most patients, we noted radiographic fracture displacement categorically rather than continuously; patients were divided into 2 displacement groups (<5 mm, >5 mm). Most studies did not define degree of comminution or specific direction of displacement per fracture, so these variables were not included in the data analysis. Nonoperative management and operative management were studied. We abstracted surgical factors, such as approach, method, fixation type (screws or sutures), and technique (suture anchors or transosseous tunnels). Clinical outcomes included physical examination findings, functional assessment results (patient satisfaction; Constant and University of California Los Angeles [UCLA] shoulder scores), and the number of revisions. Radiologic outcomes, retrieved from radiographs or computed tomography scans, focused on loss of reduction (as determined by the respective authors), malunion, nonunion, and heterotopic ossification. Each study’s methodologic quality and bias were evaluated with the 15-item Modified Coleman Methodology Score (MCMS), which was described by Cowan and colleagues.23 The MCMS has been used to assess randomized and nonrandomized patient trials.24,25 Its scaled potential score ranges from 0 to 100 (85-100, excellent; 70-84, good; 55-69, fair; <55, poor).
Statistical Analysis
We report our data as weighted means (SDs). A mean was calculated for each study that reported a respective data point, and each mean was then weighed according to its study sample size. This calculation was performed by multiplying a study’s individual mean by the number of patients enrolled in that study and dividing the sum of these weighted data points by the number of eligible patients in all relevant studies. The result was that the nonweighted means from studies with smaller sample sizes did not carry as much weight as the nonweighted means from larger studies. We compared 3 paired groups: treatment type (nonoperative vs operative), fracture displacement amount (<5 mm vs >5 mm), and surgery type (open vs arthroscopic). Regarding all patient, surgery, and outcomes data, unpaired Student t tests were used for continuous variables and 2-tailed Fisher exact tests for categorical variables with α = 0.05 (SPSS Version 18; IBM).
Results
Postoperative physical examination findings were underreported so that surgical groups could be compared. Of all the surgical studies, 4 reported postoperative forward elevation (mean, 160°; SD, 9.8°) and external rotation (mean, 46.4°; SD 26.3°).14,15,18,22 No malunions and only 1 nonunion were reported in all 13 studies. No deaths or other serious medical complications were reported. Patients with anterior instability more often underwent surgery than were treated nonoperatively (39.2% vs 12.0%; P < .01) and more often had fractures displaced >5 mm than <5 mm (44.3% vs 14.5%; P < .01).
Fisher exact tests were used to perform isolated comparisons of screws and sutures as well as suture anchors and transosseous tunnels. Patients with screw fixation were significantly (P = .051) less likely to require reoperation (0/56; 0%) than patients with suture fixation (8/100; 8.0%). Screw fixation also led to significantly less stiffness (0% vs 12.0%; P < .01) but trended toward a higher rate of superficial infection (3.6% vs 0%; P = .13). There was no statistical difference in nerve injury rates between screws and sutures (1.8% vs 3.0%; P = 1.0). There were no significant differences in reoperations, stiffness, superficial infections, or nerve injuries between suture anchor and transosseous tunnel constructs.
For all 13 studies, mean (SD) MCMS was 41.1 (8.6).
Discussion
Five percent of all fractures involve the proximal humerus, and 20% of proximal humerus fractures are isolated greater tuberosity fractures.26,27 In his classic 1970 article, Neer6 formulated the 4-part proximal humerus fracture classification and defined greater tuberosity fracture “parts” using the same criteria as for other fracture “parts.” Neer6 recommended nonoperative management for isolated greater tuberosity fractures displaced <1 cm but did not present evidence corroborating his recommendation. More recent cutoffs for nonoperative management include 5 mm (general population) and 3 mm (athletes).7,17
In the present systematic review of greater tuberosity fractures, 3 separate comparisons were made: treatment type (nonoperative vs operative), fracture displacement amount (<5 mm vs >5 mm), and surgery type (open vs arthroscopic).
Treatment Type. Only 4 studies reported data on nonoperative treatment outcomes.5,12,16,17 Of these 4 studies, 2 found successful outcomes for fractures displaced <5 mm.12,17 Platzer and colleagues17 found good or excellent results in 97% of 135 shoulders after 4 years. Good results were defined with shoulder scores of ≥80 (Constant), <8 (Vienna), and >28 (UCLA), and excellent results were defined with maximum scores on 2 of the 3 systems. Platzer and colleagues17 also found nonsignificantly worse shoulder scores with superior displacement of 3 mm to 5 mm and recommended surgery for overhead athletes in this group. Rath and colleagues12 described a successful 3-phase rehabilitation protocol of sling immobilization for 3 weeks, pendulum exercises for 3 weeks, and active exercises thereafter. By an average of 31 months, patient satisfaction scores improved to 9.5 from 4.2 (10-point scale), though the authors cautioned that pain and decreased motion lasted 8 months on average. Conservative treatment was far less successful in the 2 studies of fractures displaced >5 mm.5,16 Keene and colleagues16 reported unsatisfactory results in all 4 patients with fractures displaced >1.5 cm. In a study separate from their 2005 analysis,17 Platzer and colleagues5 in 2008 evaluated displaced fractures and found function and patient satisfaction were inferior after nonoperative treatment than after surgery. The studies by Keene and colleagues16 and Platzer and colleagues5 support the finding of an overall lower patient satisfaction rate in nonoperative patients.
Fracture Displacement Amount. Only 2 arthroscopic studies and no open studies addressed surgery for fractures displaced <5 mm. Fewer than 16% of these fractures were managed operatively, and <1% required reoperation. By contrast, almost all fractures displaced >5 mm were managed operatively, and 3.6% required reoperation. Radiographic loss of reduction was more common in fractures displaced <5 mm, primarily because they were managed without fixation. Radiographic loss of reduction was reported in only 9 operatively treated patients, none of whom was symptomatic enough to require another surgery.5 Reoperations were most commonly performed for stiffness, which itself was significantly more common in fractures displaced >5 mm. Bhatia and colleagues14 reported the highest reoperation rate (14.3%; 3/21), but they studied more complex, comminuted fractures of the greater tuberosity. Two of their 3 reoperations were biceps tenodeses for inflamed, stiff tenosynovitis, and the third patient had a foreign body giant cell reaction to suture material. Fewer than 1% of patients with operatively managed displaced fractures required revision ORIF, and <2% developed a superficial infection or postoperative nerve palsy.19,22 For displaced greater tuberosity fractures, surgery is highly successful overall, complication rates are very low, and 90% of patients report being satisfied.
Surgery Type. Patients were divided into 2 groups. In the nonarthroscopic group, open and percutaneous approaches were used. All studies that described a percutaneous approach used screw fixation5,21; in addition, 32 patients were treated with screws through an open approach.2,5 The other open and arthroscopic studies used suture fixation. Interestingly, no studies reported on clinical outcomes of fragment excision. There were no statistically significant differences in rates of reoperation, stiffness, infection, or neurologic injury between the arthroscopic and nonarthroscopic groups. Patient satisfaction scores were slightly higher in the nonarthroscopic group (91.0% vs 87.8%), but the difference was not statistically significant.
With surgical techniques isolated, there were no significant differences between suture anchors and transosseous tunnel constructs, but screws performed significantly better than suture techniques. Compared with suture fixation, screw fixation led to significantly fewer cases of stiffness and reoperation, which suggests surgeons need to give screws more consideration in the operative management of these fractures. However, the number of patients treated with screws was smaller than the number treated with suture fixation; it is possible the differences between these cohorts would be eliminated if there were more patients in the screw cohort. In addition, screw fixation was universally performed with an open or percutaneous approach and trended toward a higher infection rate. As screw and suture techniques have low rates of complications and reoperations, we recommend leaving fixation choice to the surgeon.
Anterior shoulder instability has been associated with greater tuberosity fractures.1,8,19 The supraspinatus, infraspinatus, and teres minor muscles all insert into the greater tuberosity and resist anterior translation of the proximal humerus. Loss of this dynamic muscle stabilization is amplified by tuberosity fracture displacement: Anterior shoulder instability was significantly more common in fractures displaced >5 mm (44.3%) vs <5 mm (14.5%). In turn, glenohumeral instability was more common in patients treated with surgery, specifically open surgery, because displaced fractures may not be as easily accessed with arthroscopic techniques. No studies reported concomitant labral repair or capsular plication techniques.
This systematic review was limited by the studies analyzed. All but 1 study5 had level IV evidence. Mean (SD) MCMS was 41.8 (8.6). Any MCMS score <54 indicates a poor methodology level, but this scoring system is designed for randomized controlled trials,23 and there were none in this study. Physical examination findings, such as range of motion, were underreported. In addition, radiographic parameters were not consistently described but rather were determined by the respective authors’ subjective interpretations of malunion, nonunion, and loss of reduction. Publication bias is present in that we excluded non- English language studies and medical conference abstracts and may have omitted potentially eligible studies not discoverable with our search methodology. Performance bias is a factor in any systematic review with multiple surgeons and wide variation in surgical technique.
Conclusion
Greater tuberosity fractures displaced <5 mm may be safely managed nonoperatively, as there are no reports of nonoperatively managed fractures that subsequently required surgery. Nonoperative treatment was initially associated with low patient satisfaction, but only because displaced fractures were conservatively managed in early studies.5,16 Fractures displaced >5 mm respond well to operative fixation with screws, suture anchors, or transosseous suture tunnels. Stiffness is the most common postoperative complication (<6%), followed by heterotopic ossification, transient neurapraxias, and superficial infection. There are no discernible differences in outcome between open and arthroscopic techniques, but screw fixation may lead to significantly fewer cases of stiffness and reoperation in comparison with suture constructs.
1. Verdano MA, Aliani D, Pellegrini A, Baudi P, Pedrazzi G, Ceccarelli F. Isolated fractures of the greater tuberosity in proximal humerus: does the direction of displacement influence functional outcome? An analysis of displacement in greater tuberosity fractures. Acta Biomed. 2013;84(3):219-228.
2. Yin B, Moen TC, Thompson SA, Bigliani LU, Ahmad CS, Levine WN. Operative treatment of isolated greater tuberosity fractures: retrospective review of clinical and functional outcomes. Orthopedics. 2012;35(6):e807-e814.
3. Green A, Izzi J. Isolated fractures of the greater tuberosity of the proximal humerus. J Shoulder Elbow Surg. 2003;12(6):641-649.
4. Norouzi M, Naderi MN, Komasi MH, Sharifzadeh SR, Shahrezaei M, Eajazi A. Clinical results of using the proximal humeral internal locking system plate for internal fixation of displaced proximal humeral fractures. Am J Orthop. 2012;41(5):E64-E68.
5. Platzer P, Thalhammer G, Oberleitner G, et al. Displaced fractures of the greater tuberosity: a comparison of operative and nonoperative treatment. J Trauma. 2008;65(4):843-848.
6. Neer CS. Displaced proximal humeral fractures. I. Classification and evaluation. J Bone Joint Surg Am. 1970;52(6):1077-1089.
7. Park TS, Choi IY, Kim YH, Park MR, Shon JH, Kim SI. A new suggestion for the treatment of minimally displaced fractures of the greater tuberosity of the proximal humerus. Bull Hosp Jt Dis. 1997;56(3):171-176.
8. McLaughlin HL. Dislocation of the shoulder with tuberosity fracture. Surg Clin North Am. 1963;43:1615-1620.
9. DeBottis D, Anavian J, Green A. Surgical management of isolated greater tuberosity fractures of the proximal humerus. Orthop Clin North Am. 2014;45(2):207-218.
10. Monga P, Verma R, Sharma VK. Closed reduction and external fixation for displaced proximal humeral fractures. J Orthop Surg (Hong Kong). 2009;17(2):142-145.
11. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006-1012.
12. Rath E, Alkrinawi N, Levy O, Debbi R, Amar E, Atoun E. Minimally displaced fractures of the greater tuberosity: outcome of non-operative treatment. J Shoulder Elbow Surg. 2013;22(10):e8-e11.
13. Dimakopoulos P, Panagopoulos A, Kasimatis G. Transosseous suture fixation of proximal humeral fractures. J Bone Joint Surg Am. 2007;89(8):1700-1709.
14. Bhatia DN, van Rooyen KS, Toit du DF, de Beer JF. Surgical treatment of comminuted, displaced fractures of the greater tuberosity of the proximal humerus: a new technique of double-row suture-anchor fixation and long-term results. Injury. 2006;37(10):946-952.
15. Flatow EL, Cuomo F, Maday MG, Miller SR, McIlveen SJ, Bigliani LU. Open reduction and internal fixation of two-part displaced fractures of the greater tuberosity of the proximal part of the humerus. J Bone Joint Surg Am. 1991;73(8):1213-1218.
16. Keene JS, Huizenga RE, Engber WD, Rogers SC. Proximal humeral fractures: a correlation of residual deformity with long-term function. Orthopedics. 1983;6(2):173-178.
17. Platzer P, Kutscha-Lissberg F, Lehr S, Vecsei V, Gaebler C. The influence of displacement on shoulder function in patients with minimally displaced fractures of the greater tuberosity. Injury. 2005;36(10):1185-1189.
18. Park SE, Ji JH, Shafi M, Jung JJ, Gil HJ, Lee HH. Arthroscopic management of occult greater tuberosity fracture of the shoulder. Eur J Orthop Surg Traumatol. 2014;24(4):475-482.
19. Dimakopoulos P, Panagopoulos A, Kasimatis G, Syggelos SA, Lambiris E. Anterior traumatic shoulder dislocation associated with displaced greater tuberosity fracture: the necessity of operative treatment. J Orthop Trauma. 2007;21(2):104-112.
20. Kim SH, Ha KI. Arthroscopic treatment of symptomatic shoulders with minimally displaced greater tuberosity fracture. Arthroscopy. 2000;16(7):695-700.
21. Chen CY, Chao EK, Tu YK, Ueng SW, Shih CH. Closed management and percutaneous fixation of unstable proximal humerus fractures. J Trauma. 1998;45(6):1039-1045.
22. Ji JH, Shafi M, Song IS, Kim YY, McFarland EG, Moon CY. Arthroscopic fixation technique for comminuted, displaced greater tuberosity fracture. Arthroscopy. 2010;26(5):600-609.
23. Cowan J, Lozano-Calderón S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699.
24. Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010;92(12):2220-2233.
25. Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation—a systematic review. Osteoarthritis Cartilage. 2011;19(7):779-791.
26. Chun JM, Groh GI, Rockwood CA. Two-part fractures of the proximal humerus. J Shoulder Elbow Surg. 1994;3(5):273-287.
27. Gruson KI, Ruchelsman DE, Tejwani NC. Isolated tuberosity fractures of the proximal humeral: current concepts. Injury. 2008;39(3):284-298.
1. Verdano MA, Aliani D, Pellegrini A, Baudi P, Pedrazzi G, Ceccarelli F. Isolated fractures of the greater tuberosity in proximal humerus: does the direction of displacement influence functional outcome? An analysis of displacement in greater tuberosity fractures. Acta Biomed. 2013;84(3):219-228.
2. Yin B, Moen TC, Thompson SA, Bigliani LU, Ahmad CS, Levine WN. Operative treatment of isolated greater tuberosity fractures: retrospective review of clinical and functional outcomes. Orthopedics. 2012;35(6):e807-e814.
3. Green A, Izzi J. Isolated fractures of the greater tuberosity of the proximal humerus. J Shoulder Elbow Surg. 2003;12(6):641-649.
4. Norouzi M, Naderi MN, Komasi MH, Sharifzadeh SR, Shahrezaei M, Eajazi A. Clinical results of using the proximal humeral internal locking system plate for internal fixation of displaced proximal humeral fractures. Am J Orthop. 2012;41(5):E64-E68.
5. Platzer P, Thalhammer G, Oberleitner G, et al. Displaced fractures of the greater tuberosity: a comparison of operative and nonoperative treatment. J Trauma. 2008;65(4):843-848.
6. Neer CS. Displaced proximal humeral fractures. I. Classification and evaluation. J Bone Joint Surg Am. 1970;52(6):1077-1089.
7. Park TS, Choi IY, Kim YH, Park MR, Shon JH, Kim SI. A new suggestion for the treatment of minimally displaced fractures of the greater tuberosity of the proximal humerus. Bull Hosp Jt Dis. 1997;56(3):171-176.
8. McLaughlin HL. Dislocation of the shoulder with tuberosity fracture. Surg Clin North Am. 1963;43:1615-1620.
9. DeBottis D, Anavian J, Green A. Surgical management of isolated greater tuberosity fractures of the proximal humerus. Orthop Clin North Am. 2014;45(2):207-218.
10. Monga P, Verma R, Sharma VK. Closed reduction and external fixation for displaced proximal humeral fractures. J Orthop Surg (Hong Kong). 2009;17(2):142-145.
11. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006-1012.
12. Rath E, Alkrinawi N, Levy O, Debbi R, Amar E, Atoun E. Minimally displaced fractures of the greater tuberosity: outcome of non-operative treatment. J Shoulder Elbow Surg. 2013;22(10):e8-e11.
13. Dimakopoulos P, Panagopoulos A, Kasimatis G. Transosseous suture fixation of proximal humeral fractures. J Bone Joint Surg Am. 2007;89(8):1700-1709.
14. Bhatia DN, van Rooyen KS, Toit du DF, de Beer JF. Surgical treatment of comminuted, displaced fractures of the greater tuberosity of the proximal humerus: a new technique of double-row suture-anchor fixation and long-term results. Injury. 2006;37(10):946-952.
15. Flatow EL, Cuomo F, Maday MG, Miller SR, McIlveen SJ, Bigliani LU. Open reduction and internal fixation of two-part displaced fractures of the greater tuberosity of the proximal part of the humerus. J Bone Joint Surg Am. 1991;73(8):1213-1218.
16. Keene JS, Huizenga RE, Engber WD, Rogers SC. Proximal humeral fractures: a correlation of residual deformity with long-term function. Orthopedics. 1983;6(2):173-178.
17. Platzer P, Kutscha-Lissberg F, Lehr S, Vecsei V, Gaebler C. The influence of displacement on shoulder function in patients with minimally displaced fractures of the greater tuberosity. Injury. 2005;36(10):1185-1189.
18. Park SE, Ji JH, Shafi M, Jung JJ, Gil HJ, Lee HH. Arthroscopic management of occult greater tuberosity fracture of the shoulder. Eur J Orthop Surg Traumatol. 2014;24(4):475-482.
19. Dimakopoulos P, Panagopoulos A, Kasimatis G, Syggelos SA, Lambiris E. Anterior traumatic shoulder dislocation associated with displaced greater tuberosity fracture: the necessity of operative treatment. J Orthop Trauma. 2007;21(2):104-112.
20. Kim SH, Ha KI. Arthroscopic treatment of symptomatic shoulders with minimally displaced greater tuberosity fracture. Arthroscopy. 2000;16(7):695-700.
21. Chen CY, Chao EK, Tu YK, Ueng SW, Shih CH. Closed management and percutaneous fixation of unstable proximal humerus fractures. J Trauma. 1998;45(6):1039-1045.
22. Ji JH, Shafi M, Song IS, Kim YY, McFarland EG, Moon CY. Arthroscopic fixation technique for comminuted, displaced greater tuberosity fracture. Arthroscopy. 2010;26(5):600-609.
23. Cowan J, Lozano-Calderón S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699.
24. Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010;92(12):2220-2233.
25. Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation—a systematic review. Osteoarthritis Cartilage. 2011;19(7):779-791.
26. Chun JM, Groh GI, Rockwood CA. Two-part fractures of the proximal humerus. J Shoulder Elbow Surg. 1994;3(5):273-287.
27. Gruson KI, Ruchelsman DE, Tejwani NC. Isolated tuberosity fractures of the proximal humeral: current concepts. Injury. 2008;39(3):284-298.