User login
Thigh Injuries in American Football
American football has the highest injury rate of any team sport in the United States at the high school, collegiate, and professional levels.1-3 Muscle strains and contusions constitute a large proportion of football injuries. For example, at the high school level, muscle strains comprise 12% to 24% of all injuries;2 at the collegiate level, they account for approximately 20% of all practice injuries, with nearly half of all strains occurring within the thigh.1,4 Among a single National Football League (NFL) team, Feeley and colleagues5 reported that muscle strains accounted for 46% of practice and 22% of preseason game injuries. The hamstrings, followed by the quadriceps, are the most commonly strained muscle groups among both professional and amateur athletes,5,6 with hamstring and quadriceps injuries making up approximately 13% of all injuries among NFL players.7 Given the relatively large surface area and muscle volume of the anterior and posterior thigh, as well as the activities and maneuvers necessitated by the various football positions, it is not surprising that the thigh is frequently involved in football-related injuries.
The purpose of this review is to describe the clinical manifestations of thigh-related soft-tissue injuries seen in football players. Two of these conditions—muscle strains and contusions—are relatively common, while a third condition—the Morel-Lavallée lesion—is a rare, yet relevant injury that warrants discussion.
Quadriceps Contusion
Pathophysiology
Contusion to the quadriceps muscle is a common injury in contact sports generally resulting from a direct blow from a helmet, knee, or shoulder.8 Bleeding within the musculature causes swelling, pain, stiffness, and limitation of quadriceps excursion, ultimately resulting in loss of knee flexion and an inability to run or squat. The injury is typically confined to a single quadriceps muscle.8 The use of thigh padding, though helpful, does not completely eliminate the risk of this injury.
History and Physical Examination
Immediately after injury, the athlete may complain only of thigh pain. However, swelling, pain, and diminished range of knee motion may develop within the first 24 hours depending on the severity of injury and how quickly treatment is instituted.8 Jackson and Feagin9 developed an injury grading system for quadriceps contusions based on the limitation of knee flexion observed (Table 1).
Imaging
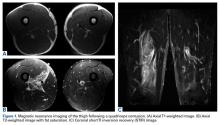
A quadriceps contusion is a clinical diagnosis based on a typical history and physical examination; therefore, advanced imaging usually does not need to be obtained except to gauge the severity of injury, to rule out concurrent injuries (ie, tendon rupture), and to identify the presence of a hematoma that may necessitate aspiration. Plain radiographs are typically unremarkable in the acute setting. Appearance on magnetic resonance imaging (MRI) varies by injury severity, with increased signal throughout the affected muscle belly and a diffuse, feathery appearance centered at the point of impact on short TI inversion recovery (STIR) and T2-weighted images reflecting edema and possibly hematoma (Figures 1A-1C).8,11
Treatment
Treatment of a quadriceps contusion is nonoperative and consists of a 3-phase recovery.10 The first phase lasts approximately 2 days and consists of rest, ice, compression, and elevation (RICE) to limit hemorrhage. The knee should be rested in a flexed position to maintain quadriceps muscle fiber length in order to promote muscle compression and limit knee stiffness. For severe contusions in which there is a question of an acute thigh compartment syndrome, compression should be avoided with appropriate treatment based on typical symptoms and intra-compartmental pressure measurement.12 Nonsteroidal anti-inflammatory drugs (NSAIDs) may be administered to diminish pain as well as the risk of myositis ossificans. While there is no data on the efficacy of NSAIDs in preventing myositis ossificans following quadriceps contusions, both COX-2 selective (ie, celecoxib) and nonselective (ie, naproxen, indomethacin) COX inhibitors have been demonstrated to significantly reduce the incidence of heterotopic ossification following hip surgery—a condition occurring from a similar pathophysiologic process as myositis ossificans.13-17 However, this class of drugs should not be given any sooner than 48 to 72 hours after injury to decrease further bleeding risk, given its inhibitory effect on platelet function.18 Narcotic pain medications are rarely required.
The second phase focuses on restoring active and passive knee and hip flexion and begins when permitted by pain.8 Icing, pain control, and physical therapy modalities are also continued in order to reduce pain and swelling as knee motion is progressed. The third phase begins once full range of knee and hip motion is restored and consists of quadriceps strengthening and functional rehabilitation of the lower extremity.8,19 Return to athletic activities and eventually competition should take place when a full, painless range of motion is restored and strength returns to baseline. Isokinetic strength testing may be utilized to more accurately assess strength and endurance. Noncontact, position-specific drills are incorporated as clinical improvement allows. A full recovery should be expected within 4 weeks of injury, with faster resolution and return to play seen in less severe contusions depending on the athlete’s position.8 Continued quadriceps stretching is recommended to prevent recurrence once the athlete returns to play. A protective hard shell may also be utilized both during rehabilitation as well as once the athlete returns to play in order to protect the thigh from reinjury, which may increase the risk of myositis ossificans.8
Complications
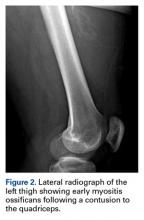
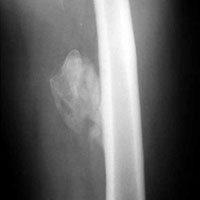
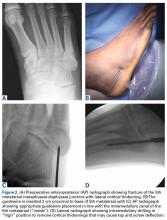
A prolonged recovery or persistent symptoms should alert the treating physician to the possibility of complications, including myositis ossificans.8,20 Myositis ossificans typically results from moderate to severe contusions, which may present initially as a painful, indurated mass that later becomes quite firm. This mass may be seen on plain radiographs as early as 2 to 4 weeks following injury if the athlete complains of persistent pain or a palpable thigh mass (Figure 2).9
Mani-Babu and colleagues23 reported a case of a 14-year-old male football player who sustained a quadriceps contusion after a direct blow from an opponent’s helmet to the lateral thigh. Persistent pain and limitation of motion at 2 months follow-up prompted imaging studies that demonstrated myositis ossificans. The patient was treated with intravenous pamidronate (a bisphosphonate) twice over a 3-month period and demonstrated a full recovery within 5 months.
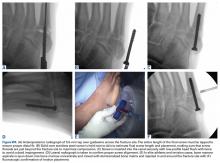
Acute compartment syndrome of the thigh has also been reported following severe quadriceps contusions, with the majority occurring in the anterior compartment.12,24-28 When injury from blunt trauma extends into and disrupts the muscular layer adjacent to the femur, vascular disruption can cause hematoma formation, muscle edema, and significant swelling, thereby increasing intracompartmental pressure. The relatively large volume of the anterior thigh compartment and lack of a rigid deep fascial envelope may be protective from the development of compartment syndrome compared to other sites.28 It can be difficult to distinguish a severe contusion from a compartment syndrome, as both can occur from the same mechanism and have similar presenting signs and symptoms. Signs of a compartment syndrome include pain out of proportion to the injury that is aggravated by passive stretch of the quadriceps muscles, an increasingly firm muscle compartment to palpation, and neurovascular deficits.29 Both acute compartment syndrome and a severe contusion may present with significant pain, inability to bear weight, tense swelling, tenderness to palpation, and pain with passive knee flexion.24 While the successful conservative treatment of athletes with acute compartment syndrome of the thigh has been reported, it is important to closely monitor the patient’s condition and consider intracompartmental pressure monitoring if the patient’s clinical condition deteriorates.12 An acute fasciotomy should be strongly considered when intracompartmental pressures are within 30 mm Hg of diastolic pressure.24-27 Fortunately, it is highly uncommon for thigh compartment pressure to rise to this level. Percutaneous compartment decompression using liposuction equipment or a large cannula has been described to decrease intracompartmental pressure, potentially expediting recovery and minimizing morbidity.18 Interestingly, reports of fasciotomies for acute thigh compartment syndrome following closed athletic injuries have not described necrotic or non-contractile muscle typical of an acute compartment syndrome, calling into question the need for fasciotomy following closed blunt athletic trauma to the thigh.18
Quadriceps Strain
Pathophysiology
Acute quadriceps strains occur during sudden forceful eccentric contraction of the extensor mechanism. Occasionally, in the absence of a clear mechanism, these injuries mistakenly appear as a contusion resulting from a direct blow to the thigh.30,31 The rectus femoris is the most frequently strained quadriceps muscle due, in part, to its superficial location and predominance of type II muscle fibers, which are more likely to be strained.11,32 Although classically described as occurring along the distal portion of the rectus femoris at the musculotendinous junction, quadriceps strains most commonly occur at the mid to proximal aspect of the rectus femoris.30,33 The quadriceps muscle complex crosses 2 joints and, as a result, is more predisposed to eccentric injury than mono-articular muscles.34 We have had a subset of complete myotendinous tears of the rectus femoris that occur in the plant leg of placekickers that result in significant disability.
Risk Factors
Quadriceps and thigh injuries comprise approximately 4.5% of injuries among NFL players.7 Several risk factors for quadriceps strains have been described. In a study of Australian Rules football players, Orchard35 demonstrated that for all muscle strains, the strongest risk factor was a recent history of the same injury, with the next strongest risk factor being a past history of the same injury. Increasing age was found to be a risk factor for hamstring strains but not quadriceps strains. Muscle fatigue may also contribute to injury susceptibility.36
History and Physical Examination
Injuries typically occur during kicking, jumping, or a sudden change in direction while running.30 Athletes may localize pain anywhere along the quadriceps muscle, although strains most commonly occur at the proximal to mid portion of the rectus femoris.30,33 The grading system for quadriceps strains described by Kary30 is based on level of pain, quadriceps strength, and the presence or absence of a palpable defect (Table 2).
The athlete typically walks with an antalgic gait. Visible swelling and/or ecchymosis may be present depending on when the athlete is seen, as ecchymosis may develop within the first 24 hours of injury. The examiner should palpate along the entire length of the injured muscle. High-grade strains or complete tears may present with a bulge or defect in the muscle belly, but in most cases no defect will be palpable. There may be loss of knee flexion similar to a quadriceps contusion. Strength testing should be performed in both the sitting and prone position with the hip both flexed and extended to assess resisted knee extension strength.30 Loss of strength is proportional to the degree of injury.
Imaging
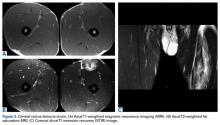
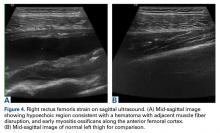
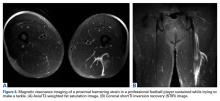
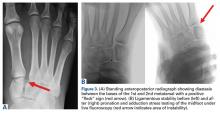
While most quadriceps strains are adequately diagnosed clinically without the need for imaging studies, ultrasound or MRI can be used to evaluate for partial or complete rupture.30,33 In milder cases, MRI usually demonstrates interstitial edema and hemorrhage with a feathery appearance on STIR and T2-weighted imaging (Figures 3A-3C).11
Treatment
Acute treatment of quadriceps strains focuses on minimizing bleeding using the principles of RICE treatment.37 NSAIDs may be used immediately to assist with pain control.30 COX-2-specific NSAIDs are preferred due to their lack of any inhibitory effect on platelet function in order to reduce the risk of further bleeding within the muscle compartment. For the first 24 to 72 hours following injury, the quadriceps should be maintained relatively immobilized to prevent further injury.38 High-grade injuries might necessitate crutches for ambulatory assistance.
Depending on injury severity, the active phase of treatment usually begins within 5 days of injury and consists of stretching and knee/hip range of motion. An active warm-up should precede rehabilitation exercises to activate neural pathways within the muscle and improve muscle elasticity.38 Ballistic stretching should be avoided to prevent additional injury to the muscle fibers. Strengthening should proceed when the athlete recovers a pain-free range of motion. When isometric exercises can be completed at increasing degrees of knee flexion, isotonic exercises may be implemented into the rehabilitation program.30 Return to football can be considered when the athlete has recovered knee and hip range of motion, is pain-free, and has near-normal strength compared to the contralateral side. The athlete should also perform satisfactorily in simulated position-specific activities in a noncontact fashion prior to return to full competition.30
Hamstring Strain
Pathophysiology
Hamstring strains are the most common noncontact injuries in football resulting from excessive muscle stretching during eccentric contraction generally occurring at the musculotendinous junction.5,39 Because the hamstrings cross both the hip and knee, simultaneous hip flexion and knee extension results in maximal lengthening, making them most vulnerable to injury at the terminal swing phase of gait just prior to heel strike.39-42 The long head of the biceps femoris undergoes the greatest stretch, reaching 110% of resting length during terminal swing phase and is the most commonly injured hamstring muscle.43,44 Injury occurs when the force of eccentric contraction, and resulting muscle strain, exceeds the mechanical limits of the tissue.42,45 It remains to be shown whether hamstring strains occur as a result of accumulated microscopic muscle damage or secondary to a single event that exceeds the mechanical limits of the muscle.42
Epidemiology and Risk Factors
The majority of hamstring strains are sustained during noncontact activities, with most athletes citing sprinting as the activity at the time of injury.3 Approximately 93% of injuries occur during noncontact activities among defensive backs and wide receivers.3 Hamstring strains are the second-most common injury among NFL players, comprising approximately 9% of all injuries,5,7 with 16% to 31% of these injuries associated with recurrence.3,5,35,46 Using the NFL’s Injury Surveillance System, Elliott and colleagues3 reported 1716 hamstring strains over a 10-year period (1989-1998). Fifty-one percent of hamstring strains occurred during the 7-week preseason, with a greater than 4-fold increased injury rate noted during the preseason compared to the 16-week regular season. An increased incidence in the preseason is partially attributable to relative deconditioning over the offseason. Defensive backs, wide receivers, and special teams players accounted for the majority of injured players, suggesting that speed position players and those who must “backpedal” (run backwards) are at an increased risk for injury.
Several risk factors for hamstring strain have been described, including prior injury, older age, quadriceps-hamstring strength imbalances, limited hip and knee flexibility, and fatigue.39,42,47 Inadequate rehabilitation and premature return to competition are also likely important factors predisposing to recurrent injury.39,48
History and Physical Examination
The majority of hamstring strains occur in the acute setting when the player experiences the sudden onset of pain in the posterior thigh during strenuous exercise, most commonly while sprinting.39 The injury typically occurs in the early or late stage of practice or competition due, in part, to inadequate warm-up or fatigue. The athlete may describe an audible pop and an inability to continue play, depending on injury severity.
Physical examination may demonstrate palpable induration and tenderness immediately or shortly after injury. In the setting of severe strains, there can be significant thigh swelling and ecchymosis, and in complete ruptures, a palpable defect.39 The affected muscle should be palpated along its entire length, and is best performed prone with the knee flexed to 90° as well as with the knee partially extended to place it under mild tension. Injury severity can be assessed by determining the restriction of passive knee extension while the athlete is lying supine with the hip flexed to 90°. The severity of hamstring strains varies from minor damage of a few myofibers without loss of structural integrity to complete muscle rupture.
Imaging
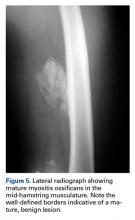
Similar to other muscle strains, hamstring strains are a clinical diagnosis and generally do not necessitate advanced imaging studies except to assess the degree of damage (ie, partial vs complete rupture) and to rule out other injuries, especially if the athlete fails to respond to treatment. Plain radiographs in acute cases are usually unremarkable. However, more severe injuries may go on to develop myositis ossificans similar to quadriceps soft tissue injuries (Figure 5).
Treatment
Most hamstring strains respond to conservative treatment, with operative intervention rarely indicated except for proximal or distal tendon avulsions.39 Like other muscle strains, initial management consists of RICE. COX-2-selective NSAIDs are preferred initially following injury. During a brief period of immobilization, the leg should be extended as much as tolerated to maximize muscle length, limit hematoma formation, and reduce the risk of contracture.39 Controlled mobilization should begin as soon as tolerated by the athlete.39 Isometric exercises and a stretching program should be started early in the rehabilitation period, with isotonic exercises added as motion and pain improve. Active stretching should be initiated and progressed to passive, static stretching as guided by pain.
The late phase of rehabilitation and long-term conditioning protocols should incorporate eccentric training once the athlete is pain-free, performing isotonic and isokinetic exercises. Eccentric exercises best strengthen the hamstrings at their most susceptible point, prepares the athlete for functional activities, and minimizes the risk of reinjury,3,50,51 Elliot and colleagues3 reported an order of magnitude decrease in hamstring injuries in high-risk athletes with identifiable hamstring muscle weakness after implementing an eccentric strengthening program and progressive sprint training. Similarly, in a large cohort of elite soccer players, correction of strength deficits in players with prior hamstring injuries led to similar rates of injury compared to athletes without strength deficits or prior injury.52 Those athletes with persistent weakness who did not undergo rehabilitation had significantly higher rates of reinjury.
Various injections containing local anesthetics, corticosteroids, platelet-rich plasma (PRP), and other substances have been administered to football players following acute muscle strains in an effort to alleviate pain and safely return the athlete to competition. Some practitioners have been reluctant to administer injections (especially those containing corticosteroids) due to a potentially increased risk of tendinopathy or rupture.31 Drakos and colleagues53 reported their outcomes following muscle and ligament strains treated with combined corticosteroid and local anesthetic injections on one NFL team. While quadriceps and hamstring strains were associated with the most missed games among all muscle strains, these injections resulted in no adverse events or progression of injury severity. Similarly, Levine and colleagues 51 administered intramuscular corticosteroid injections to 58 NFL players with high-grade hamstring injuries that had a palpable defect within the muscle belly. They reported no complications or strength deficits at final examination. In a case-control study, Rettig and colleagues46 administered PRP injections under ultrasound guidance in 5 NFL players with hamstring injuries. Compared to players treated with a focused rehabilitation program only, there were no significant differences in recovery or return to play.
The decision to return to play should be based on a clinical assessment considering pain, strength, motion, and flexibility. Player position should also be considered. Return-to-play guidelines describing the appropriate progression through rehabilitation and return to sport have been described and can be used as a template for the rehabilitation of football players.54 It should be noted that primary hamstring strains are associated with decreased athletic performance and an increased risk of more severe reinjury after return to sport.55,56
Morel-Lavallée Lesion
Pathophysiology
Morel-Lavallée lesions (MLLs) are uncommon football injuries, but often occur in the thigh.57,58 An MLL is a posttraumatic soft tissue injury in which deforming forces of pressure and shear cause a closed, soft tissue degloving injury; in this injury, the skin and subcutaneous tissues are separated from the underlying fascia, disrupting perforating blood vessels. The resulting space between the fascia and subcutaneous tissue fills with blood, lymphatics, and necrotic fat, resulting in a hematoma/seroma that can be a nidus for bacterial infection.58 The most common anatomic regions are the anterior distal thigh and lateral hip. Both of these areas are commonly involved in both direct contact and shear forces following a fall to the ground.
History and Physical Examination
Athletes with MLLs typically present with the insidious onset of a fluid collection within the thigh following a fall to the ground, usually while sliding or diving on the playing surface.57,58 The fluid collection can be associated with thigh tightness and may extend distally into the suprapatellar region or proximally over the greater trochanter. Thigh swelling, ecchymosis, and palpable fluctuance are seen in most cases. Progressive increases in pain and thigh swelling may be seen in severe injuries, but thigh compartments generally remain soft and nontender. Signs and symptoms of an MLL do not typically manifest immediately following the athletic event. Tejwani and colleagues58 reported a case series of MLLs of the knee in 27 NFL players from a single team over a 14-year period, with an average of 3 days between injury and evaluation by the medical staff. The mechanism of injury was a shearing blow from the knee striking the playing surface in 81% of cases and direct contact to the knee from another player in 19% of cases; all cases occurred in game situations. No affected players were wearing kneepads at the time of injury.
Imaging
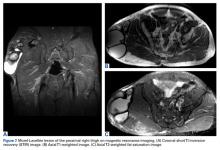
Plain radiography may reveal a noncalcified soft tissue mass over the involved area and is not usually helpful except to rule out an underlying fracture. The appearance of an MLL on ultrasound is nonspecific and variable, often described as anechoic, hypoechoic, or hyperechoic depending on the presence of hemolymphatic fluid sedimentation and varying amounts of internal fat debris. MRI is the imaging modality of choice and typically shows a well-defined oval or fusiform, fluid-filled mass with tapering margins blending with adjacent fascial planes.
Treatment
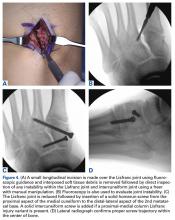
Similar to quadriceps contusions, treatment goals for MLLs are evacuation of the fluid collection, prevention of fluid recurrence, a full range of active knee flexion, and prompt return to play.57,58 Initial treatment for smaller lesions consists of cryotherapy, compression wrapping of the involved area, and immediate active and passive range of motion of the hip and knee. While MLLs were traditionally treated with serial open debridements, less invasive approaches—including elastic compression, aspiration, percutaneous irrigation with debridement and suction drainage, or liposuction and drainage followed by suction therapy—have been recently described.57,58,60,61 Less invasive approaches aim to minimize soft tissue dissection and disruption of the vascular supply while accelerating rehabilitation. The presence of a surrounding capsule on MRI makes conservative or minimally invasive approaches less likely to be successful and may necessitate an open procedure.62 Antibiotics should be used preoperatively due to the presence of a dead space containing necrotic debris that makes infection a potential complication. While elite contact athletes can expect to return to competition long before complete resolution of an MLL, there is a risk of further delamination and lesion expansion due to re-injury prior to compete healing.
Tejwani and colleagues58 performed aspiration at the area of palpable fluctuance in the thigh or suprapatellar region using a 14-gauge needle in those athletes who failed to improve with conservative treatments alone. Mean time to resolution of the fluid collection was 16 days following aspiration. Fifty-two percent of the athletes were successfully treated with cryotherapy, compression, and motion exercises alone; 48% were treated with at least one aspiration, with a mean of 2.7 aspirations per knee. In 11% of cases that failed to resolve after multiple aspirations, doxycycline sclerodesis was performed immediately following an aspiration. Patients treated with sclerodesis had no return of the fluid collection and returned to play the following day.
Matava and colleagues57 described the case of an NFL player who sustained a closed MLL of the lateral hip while diving onto an artificial turf surface attempting to catch a pass. Despite immediate thigh pain and swelling, he was able to continue play. Immediately following the game, the player was examined and had a tense thigh with ecchymosis extending into the trochanteric region. Aspiration of the fluctuant area was unsuccessful. Progressive increases in pain and thigh swelling prompted hospital admission. Percutaneous irrigation and debridement was performed as described by Tseng and Tornetta.61 A suction drain was placed within the residual dead space, and constant wall suction was applied in addition to hip compression using a spica
Conclusion
Quadriceps and hamstring injuries occur frequently in football and are generally treated conservatively. While return to competition following hamstring strains is relatively quick, a high rate of injury recurrence highlights the importance of targeted rehabilitation and conditioning. Rarely, complications from quadriceps contusions, including acute compartment syndrome and myositis ossificans, may require operative intervention if unresponsive to conservative treatment. MLLs are rare in sports, but usually involve the thigh when they occur in football players. Team physicians must maintain a heightened degree of awareness of this injury as it may require operative intervention.
Acknowledgements: The authors would like to thank Jonathon Baker, MD and David Rubin, MD for their assistance in providing radiographic images for this paper.
Am J Orthop. 2016;45(6):E308-E318. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Hootman JM, Dick R, Agel J. Epidemiology of collegiate injuries for 15 sports: summary and recommendations for injury prevention initiatives. J Athl Train. 2007;42(2):311-319.
2. Rechel JA, Yard EE, Comstock RD. An epidemiologic comparison of high school sports injuries sustained in practice and competition. J Athl Train. 2008;43(2):197-204.
3. Elliott MC, Zarins B, Powell JW, Kenyon CD. Hamstring muscle strains in professional football players: a 10-year review. Am J Sports Med. 2011;39(4):843-850.
4. Dick R, Ferrara MS, Agel J, et al. Descriptive epidemiology of collegiate men’s football injuries: National Collegiate Athletic Association Injury Surveillance System, 1988-1989 through 2003-2004. J Athl Train. 2007;42(2):221-233.
5. Feeley BT, Kennelly S, Barnes RP, et al. Epidemiology of National Football League training camp injuries from 1998 to 2007. Am J Sports Med. 2008;36(8):1597-1603.
6. Garrett WE Jr. Muscle strain injuries. Am J Sports Med. 1996;24(6 Suppl):S2-S8.
7. Lawrence DW, Hutchison MG, Comper P. Descriptive epidemiology of musculoskeletal injuries and concussions in the National Football League, 2012-2014. Orthop J Sports Med. 2015;3(5):2325967115583653.
8. Diaz JA, Fischer DA, Rettig AC, Davis TJ, Shelbourne KD. Severe quadriceps muscle contusions in athletes. A report of three cases. Am J Sports Med. 2003;31(2):289-293.
9. Jackson DW, Feagin JA. Quadriceps contusions in young athletes. Relation of severity of injury to treatment and prognosis. J Bone Joint Surg Am. 1973;55(1):95-105.
10. Ryan JB, Wheeler JH, Hopkinson WJ, Arciero RA, Kolakowski KR. Quadriceps contusions. West Point update. Am J Sports Med. 1991;19(3):299-304.
11. Bencardino JT, Rosenberg ZS, Brown RR, Hassankhani A, Lustrin ES, Beltran J. Traumatic musculotendinous injuries of the knee: diagnosis with MR imaging. Radiographics. 2000;20 Spec No:S103-S120.
12. Robinson D, On E, Halperin N. Anterior compartment syndrome of the thigh in athletes--indications for conservative treatment. J Trauma. 1992;32(2):183-186.
13. Beckmann JT, Wylie JD, Kapron AL, Hanson JA, Maak TG, Aoki SK. The effect of NSAID prophylaxis and operative variables on heterotopic ossification after hip arthroscopy. Am J Sports Med. 2014;42(6):1359-1364.
14. Shehab D, Elgazzar AH, Collier BD. Heterotopic ossification. J Nucl Med. 2002;43(3):346-353.
15. Beckmann JT, Wylie JD, Potter MQ, Maak TG, Greene TH, Aoki SK. Effect of naproxen prophylaxis on heterotopic ossification following hip arthroscopy: a double-blind randomized placebo-controlled trial. J Bone Joint Surg Am. 2015;97(24):2032-2037.
16. Yeung M, Jamshidi S, Horner N, Simunovic N, Karlsson J, Ayeni OR. Efficacy of nonsteroidal anti-inflammatory drug prophylaxis for heterotrophic ossification in hip arthroscopy: a systematic review. Arthroscopy. 2016;32(3):519-525.
17. Goyal K, Pettis CR, Bancroft AE, Wasyliw CW, Scherer KF. Myositis ossificans in the thigh of a lacrosse player. Orthopedics. 2015;38(8):468,515-518.
18. Cooper DE. Severe quadriceps muscle contusions in athletes. Am J Sports Med. 2004;32(3):820.
19. Bonsell S, Freudigman PT, Moore HA. Quadriceps muscle contusion resulting in osteomyelitis of the femur in a high school football player. A case report. Am J Sports Med. 2001;29(6):818-820.
20. Rothwell AG. Quadriceps hematoma. A prospective clinical study. Clin Orthop Relat Res. 1982;(171):97-103.
21. Armfield DR, Kim DH, Towers JD, Bradley JP, Robertson DD. Sports-related muscle injury in the lower extremity. Clin Sports Med. 2006;25(4):803-842.
22. Lipscomb AB, Thomas ED, Johnston RK. Treatment of myositis ossificans traumatica in athletes. Am J Sports Med. 1976;4(3):111-120.
23. Mani-Babu S, Wolman R, Keen R. Quadriceps traumatic myositis ossificans in a football player: management with intravenous pamidronate. Clin J Sport Med. 2014;24(5):e56-e58.
24. McCaffrey DD, Clarke J, Bunn J, McCormack MJ. Acute compartment syndrome of the anterior thigh in the absence of fracture secondary to sporting trauma. J Trauma. 2009;66(4):1238-1242.
25. Klasson SC, Vander Schilden JL. Acute anterior thigh compartment syndrome complicating quadriceps hematoma. Two case reports and review of the literature. Orthop Rev. 1990;19(5):421-427.
26. Rooser B. Quadriceps contusion with compartment syndrome. Evacuation of hematoma in 2 cases. Acta Orthop Scand. 1987;58(2):170-172.
27. Rooser B, Bengtson S, Hagglund G. Acute compartment syndrome from anterior thigh muscle contusion: a report of eight cases. J Orthop Trauma. 1991;5(1):57-59.
28. Schwartz JT Jr, Brumback RJ, Lakatos R, Poka A, Bathon GH, Burgess AR. Acute compartment syndrome of the thigh. A spectrum of injury. J Bone Joint Surg Am. 1989;71(3):392-400.
29. Elliott KG, Johnstone AJ. Diagnosing acute compartment syndrome. J Bone Joint Surg Br. 2003;85(5):625-632.
30. Kary JM. Diagnosis and management of quadriceps strains and contusions. Curr Rev Musculoskelet Med. 2010;3(1-4):26-31.
31. Boublik M, Schlegel TF, Koonce RC, Genuario JW, Kinkartz JD. Quadriceps tendon injuries in national football league players. Am J Sports Med. 2013;41(8):1841-1846.
32. Palmer WE, Kuong SJ, Elmadbouh HM. MR imaging of myotendinous strain. AJR Am J Roentgenol. 1999;173(3):703-709.
33. Cross TM, Gibbs N, Houang MT, Cameron M. Acute quadriceps muscle strains: magnetic resonance imaging features and prognosis. Am J Sports Med. 2004;32(3):710-719.
34. Hughes C 4th, Hasselman CT, Best TM, Martinez S, Garrett WE Jr. Incomplete, intrasubstance strain injuries of the rectus femoris muscle. Am J Sports Med. 1995;23(4):500-506.
35. Orchard JW. Intrinsic and extrinsic risk factors for muscle strains in Australian football. Am J Sports Med. 2001;29(3):300-303.36. Mair SD, Seaber AV, Glisson RR, Garrett WE, Jr. The role of fatigue in susceptibility to acute muscle strain injury. Am J Sports Med. 1996;24(2):137-143.
37. Bleakley C, McDonough S, MacAuley D. The use of ice in the treatment of acute soft-tissue injury: a systematic review of randomized controlled trials. Am J Sports Med. 2004;32(1):251-261.
38. Jarvinen TA, Jarvinen TL, Kaariainen M, Kalimo H, Jarvinen M. Muscle injuries: biology and treatment. Am J Sports Med. 2005;33(5):745-764.
39. Clanton TO, Coupe KJ. Hamstring strains in athletes: diagnosis and treatment. J Am Acad Orthop Surg. 1998;6(4):237-248.
40. Novacheck TF. The biomechanics of running. Gait Posture. 1998;7(1):77-95.
41. Yu B, Queen RM, Abbey AN, Liu Y, Moorman CT, Garrett WE. Hamstring muscle kinematics and activation during overground sprinting. J Biomech. 2008;41(15):3121-3126.
42. Opar DA, Williams MD, Shield AJ. Hamstring strain injuries: factors that lead to injury and re-injury. Sports Med. 2012;42(3):209-226.
43. Askling CM, Tengvar M, Saartok T, Thorstensson A. Acute first-time hamstring strains during high-speed running: a longitudinal study including clinical and magnetic resonance imaging findings. Am J Sports Med. 2007;35(2):197-206.
44. Thelen DG, Chumanov ES, Hoerth DM, et al. Hamstring muscle kinematics during treadmill sprinting. Med Sci Sports Exerc. 2005;37(1):108-114.
45. Chumanov ES, Heiderscheit BC, Thelen DG. The effect of speed and influence of individual muscles on hamstring mechanics during the swing phase of sprinting. J Biomech. 2007;40(16):3555-3562.
46. Rettig AC, Meyer S, Bhadra AK. Platelet-rich plasma in addition to rehabilitation for acute hamstring injuries in NFL players: clinical effects and time to return to play. Orthop J Sports Med. 2013;1(1):2325967113494354.
47. Zvijac JE, Toriscelli TA, Merrick S, Kiebzak GM. Isokinetic concentric quadriceps and hamstring strength variables from the NFL Scouting Combine are not predictive of hamstring injury in first-year professional football players. Am J Sports Med. 2013;41(7):1511-1518.
48. Arnason A, Sigurdsson SB, Gudmundsson A, Holme I, Engebretsen L, Bahr R. Risk factors for injuries in football. Am J Sports Med. 2004;32(1 Suppl):5S-16S.
49. Zarins B, Ciullo JV. Acute muscle and tendon injuries in athletes. Clin Sports Med. 1983;2(1):167-182.
50. Arnason A, Andersen TE, Holme I, Engebretsen L, Bahr R. Prevention of hamstring strains in elite soccer: an intervention study. Scand J Med Sci Sports. 2008;18(1):40-48.
51. Levine WN, Bergfeld JA, Tessendorf W, Moorman CT 3rd. Intramuscular corticosteroid injection for hamstring injuries. A 13-year experience in the National Football League. Am J Sports Med. 2000;28(3):297-300.
52. Croisier JL, Ganteaume S, Binet J, Genty M, Ferret JM. Strength imbalances and prevention of hamstring injury in professional soccer players: a prospective study. Am J Sports Med. 2008;36(8):1469-1475.
53. Drakos M, Birmingham P, Delos D, et al. Corticosteroid and anesthetic injections for muscle strains and ligament sprains in the NFL. HSS J. 2014;10(2):136-142.
54. Worrell TW. Factors associated with hamstring injuries. An approach to treatment and preventative measures. Sports Med. 1994;17(5):338-345.
55. Brooks JH, Fuller CW, Kemp SP, Reddin DB. Incidence, risk, and prevention of hamstring muscle injuries in professional rugby union. Am J Sports Med. 2006;34(8):1297-1306.
56. Verrall GM, Kalairajah Y, Slavotinek JP, Spriggins AJ. Assessment of player performance following return to sport after hamstring muscle strain injury. J Sci Med Sport. 2006;9(1-2):87-90.
57. Matava MJ, Ellis E, Shah NR, Pogue D, Williams T. Morel-lavallee lesion in a professional american football player. Am J Orthop. 2010;39(3):144-147.
58. Tejwani SG, Cohen SB, Bradley JP. Management of Morel-Lavallee lesion of the knee: twenty-seven cases in the national football league. Am J Sports Med. 2007;35(7):1162-1167.
59. Mellado JM, Bencardino JT. Morel-Lavallee lesion: review with emphasis on MR imaging. Magn Reson Imaging Clin N Am. 2005;13(4):775-782.
60. Harma A, Inan M, Ertem K. [The Morel-Lavallee lesion: a conservative approach to closed degloving injuries]. Acta Orthop Traumatol Turc. 2004;38(4):270-273.
61. Tseng S, Tornetta P 3rd. Percutaneous management of Morel-Lavallee lesions. J Bone Joint Surg Am. 2006;88(1):92-96.
62. Gilbert BC, Bui-Mansfield LT, Dejong S. MRI of a Morel-Lavellee lesion. AJR Am J Roentgenol. 2004;182(5):1347-1348.
American football has the highest injury rate of any team sport in the United States at the high school, collegiate, and professional levels.1-3 Muscle strains and contusions constitute a large proportion of football injuries. For example, at the high school level, muscle strains comprise 12% to 24% of all injuries;2 at the collegiate level, they account for approximately 20% of all practice injuries, with nearly half of all strains occurring within the thigh.1,4 Among a single National Football League (NFL) team, Feeley and colleagues5 reported that muscle strains accounted for 46% of practice and 22% of preseason game injuries. The hamstrings, followed by the quadriceps, are the most commonly strained muscle groups among both professional and amateur athletes,5,6 with hamstring and quadriceps injuries making up approximately 13% of all injuries among NFL players.7 Given the relatively large surface area and muscle volume of the anterior and posterior thigh, as well as the activities and maneuvers necessitated by the various football positions, it is not surprising that the thigh is frequently involved in football-related injuries.
The purpose of this review is to describe the clinical manifestations of thigh-related soft-tissue injuries seen in football players. Two of these conditions—muscle strains and contusions—are relatively common, while a third condition—the Morel-Lavallée lesion—is a rare, yet relevant injury that warrants discussion.
Quadriceps Contusion
Pathophysiology
Contusion to the quadriceps muscle is a common injury in contact sports generally resulting from a direct blow from a helmet, knee, or shoulder.8 Bleeding within the musculature causes swelling, pain, stiffness, and limitation of quadriceps excursion, ultimately resulting in loss of knee flexion and an inability to run or squat. The injury is typically confined to a single quadriceps muscle.8 The use of thigh padding, though helpful, does not completely eliminate the risk of this injury.
History and Physical Examination
Immediately after injury, the athlete may complain only of thigh pain. However, swelling, pain, and diminished range of knee motion may develop within the first 24 hours depending on the severity of injury and how quickly treatment is instituted.8 Jackson and Feagin9 developed an injury grading system for quadriceps contusions based on the limitation of knee flexion observed (Table 1).
Imaging
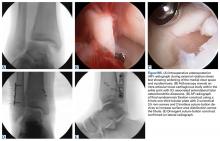
A quadriceps contusion is a clinical diagnosis based on a typical history and physical examination; therefore, advanced imaging usually does not need to be obtained except to gauge the severity of injury, to rule out concurrent injuries (ie, tendon rupture), and to identify the presence of a hematoma that may necessitate aspiration. Plain radiographs are typically unremarkable in the acute setting. Appearance on magnetic resonance imaging (MRI) varies by injury severity, with increased signal throughout the affected muscle belly and a diffuse, feathery appearance centered at the point of impact on short TI inversion recovery (STIR) and T2-weighted images reflecting edema and possibly hematoma (Figures 1A-1C).8,11
Treatment
Treatment of a quadriceps contusion is nonoperative and consists of a 3-phase recovery.10 The first phase lasts approximately 2 days and consists of rest, ice, compression, and elevation (RICE) to limit hemorrhage. The knee should be rested in a flexed position to maintain quadriceps muscle fiber length in order to promote muscle compression and limit knee stiffness. For severe contusions in which there is a question of an acute thigh compartment syndrome, compression should be avoided with appropriate treatment based on typical symptoms and intra-compartmental pressure measurement.12 Nonsteroidal anti-inflammatory drugs (NSAIDs) may be administered to diminish pain as well as the risk of myositis ossificans. While there is no data on the efficacy of NSAIDs in preventing myositis ossificans following quadriceps contusions, both COX-2 selective (ie, celecoxib) and nonselective (ie, naproxen, indomethacin) COX inhibitors have been demonstrated to significantly reduce the incidence of heterotopic ossification following hip surgery—a condition occurring from a similar pathophysiologic process as myositis ossificans.13-17 However, this class of drugs should not be given any sooner than 48 to 72 hours after injury to decrease further bleeding risk, given its inhibitory effect on platelet function.18 Narcotic pain medications are rarely required.
The second phase focuses on restoring active and passive knee and hip flexion and begins when permitted by pain.8 Icing, pain control, and physical therapy modalities are also continued in order to reduce pain and swelling as knee motion is progressed. The third phase begins once full range of knee and hip motion is restored and consists of quadriceps strengthening and functional rehabilitation of the lower extremity.8,19 Return to athletic activities and eventually competition should take place when a full, painless range of motion is restored and strength returns to baseline. Isokinetic strength testing may be utilized to more accurately assess strength and endurance. Noncontact, position-specific drills are incorporated as clinical improvement allows. A full recovery should be expected within 4 weeks of injury, with faster resolution and return to play seen in less severe contusions depending on the athlete’s position.8 Continued quadriceps stretching is recommended to prevent recurrence once the athlete returns to play. A protective hard shell may also be utilized both during rehabilitation as well as once the athlete returns to play in order to protect the thigh from reinjury, which may increase the risk of myositis ossificans.8
Complications
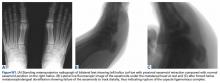
A prolonged recovery or persistent symptoms should alert the treating physician to the possibility of complications, including myositis ossificans.8,20 Myositis ossificans typically results from moderate to severe contusions, which may present initially as a painful, indurated mass that later becomes quite firm. This mass may be seen on plain radiographs as early as 2 to 4 weeks following injury if the athlete complains of persistent pain or a palpable thigh mass (Figure 2).9
Mani-Babu and colleagues23 reported a case of a 14-year-old male football player who sustained a quadriceps contusion after a direct blow from an opponent’s helmet to the lateral thigh. Persistent pain and limitation of motion at 2 months follow-up prompted imaging studies that demonstrated myositis ossificans. The patient was treated with intravenous pamidronate (a bisphosphonate) twice over a 3-month period and demonstrated a full recovery within 5 months.
Acute compartment syndrome of the thigh has also been reported following severe quadriceps contusions, with the majority occurring in the anterior compartment.12,24-28 When injury from blunt trauma extends into and disrupts the muscular layer adjacent to the femur, vascular disruption can cause hematoma formation, muscle edema, and significant swelling, thereby increasing intracompartmental pressure. The relatively large volume of the anterior thigh compartment and lack of a rigid deep fascial envelope may be protective from the development of compartment syndrome compared to other sites.28 It can be difficult to distinguish a severe contusion from a compartment syndrome, as both can occur from the same mechanism and have similar presenting signs and symptoms. Signs of a compartment syndrome include pain out of proportion to the injury that is aggravated by passive stretch of the quadriceps muscles, an increasingly firm muscle compartment to palpation, and neurovascular deficits.29 Both acute compartment syndrome and a severe contusion may present with significant pain, inability to bear weight, tense swelling, tenderness to palpation, and pain with passive knee flexion.24 While the successful conservative treatment of athletes with acute compartment syndrome of the thigh has been reported, it is important to closely monitor the patient’s condition and consider intracompartmental pressure monitoring if the patient’s clinical condition deteriorates.12 An acute fasciotomy should be strongly considered when intracompartmental pressures are within 30 mm Hg of diastolic pressure.24-27 Fortunately, it is highly uncommon for thigh compartment pressure to rise to this level. Percutaneous compartment decompression using liposuction equipment or a large cannula has been described to decrease intracompartmental pressure, potentially expediting recovery and minimizing morbidity.18 Interestingly, reports of fasciotomies for acute thigh compartment syndrome following closed athletic injuries have not described necrotic or non-contractile muscle typical of an acute compartment syndrome, calling into question the need for fasciotomy following closed blunt athletic trauma to the thigh.18
Quadriceps Strain
Pathophysiology
Acute quadriceps strains occur during sudden forceful eccentric contraction of the extensor mechanism. Occasionally, in the absence of a clear mechanism, these injuries mistakenly appear as a contusion resulting from a direct blow to the thigh.30,31 The rectus femoris is the most frequently strained quadriceps muscle due, in part, to its superficial location and predominance of type II muscle fibers, which are more likely to be strained.11,32 Although classically described as occurring along the distal portion of the rectus femoris at the musculotendinous junction, quadriceps strains most commonly occur at the mid to proximal aspect of the rectus femoris.30,33 The quadriceps muscle complex crosses 2 joints and, as a result, is more predisposed to eccentric injury than mono-articular muscles.34 We have had a subset of complete myotendinous tears of the rectus femoris that occur in the plant leg of placekickers that result in significant disability.
Risk Factors
Quadriceps and thigh injuries comprise approximately 4.5% of injuries among NFL players.7 Several risk factors for quadriceps strains have been described. In a study of Australian Rules football players, Orchard35 demonstrated that for all muscle strains, the strongest risk factor was a recent history of the same injury, with the next strongest risk factor being a past history of the same injury. Increasing age was found to be a risk factor for hamstring strains but not quadriceps strains. Muscle fatigue may also contribute to injury susceptibility.36
History and Physical Examination
Injuries typically occur during kicking, jumping, or a sudden change in direction while running.30 Athletes may localize pain anywhere along the quadriceps muscle, although strains most commonly occur at the proximal to mid portion of the rectus femoris.30,33 The grading system for quadriceps strains described by Kary30 is based on level of pain, quadriceps strength, and the presence or absence of a palpable defect (Table 2).
The athlete typically walks with an antalgic gait. Visible swelling and/or ecchymosis may be present depending on when the athlete is seen, as ecchymosis may develop within the first 24 hours of injury. The examiner should palpate along the entire length of the injured muscle. High-grade strains or complete tears may present with a bulge or defect in the muscle belly, but in most cases no defect will be palpable. There may be loss of knee flexion similar to a quadriceps contusion. Strength testing should be performed in both the sitting and prone position with the hip both flexed and extended to assess resisted knee extension strength.30 Loss of strength is proportional to the degree of injury.
Imaging
While most quadriceps strains are adequately diagnosed clinically without the need for imaging studies, ultrasound or MRI can be used to evaluate for partial or complete rupture.30,33 In milder cases, MRI usually demonstrates interstitial edema and hemorrhage with a feathery appearance on STIR and T2-weighted imaging (Figures 3A-3C).11
Treatment
Acute treatment of quadriceps strains focuses on minimizing bleeding using the principles of RICE treatment.37 NSAIDs may be used immediately to assist with pain control.30 COX-2-specific NSAIDs are preferred due to their lack of any inhibitory effect on platelet function in order to reduce the risk of further bleeding within the muscle compartment. For the first 24 to 72 hours following injury, the quadriceps should be maintained relatively immobilized to prevent further injury.38 High-grade injuries might necessitate crutches for ambulatory assistance.
Depending on injury severity, the active phase of treatment usually begins within 5 days of injury and consists of stretching and knee/hip range of motion. An active warm-up should precede rehabilitation exercises to activate neural pathways within the muscle and improve muscle elasticity.38 Ballistic stretching should be avoided to prevent additional injury to the muscle fibers. Strengthening should proceed when the athlete recovers a pain-free range of motion. When isometric exercises can be completed at increasing degrees of knee flexion, isotonic exercises may be implemented into the rehabilitation program.30 Return to football can be considered when the athlete has recovered knee and hip range of motion, is pain-free, and has near-normal strength compared to the contralateral side. The athlete should also perform satisfactorily in simulated position-specific activities in a noncontact fashion prior to return to full competition.30
Hamstring Strain
Pathophysiology
Hamstring strains are the most common noncontact injuries in football resulting from excessive muscle stretching during eccentric contraction generally occurring at the musculotendinous junction.5,39 Because the hamstrings cross both the hip and knee, simultaneous hip flexion and knee extension results in maximal lengthening, making them most vulnerable to injury at the terminal swing phase of gait just prior to heel strike.39-42 The long head of the biceps femoris undergoes the greatest stretch, reaching 110% of resting length during terminal swing phase and is the most commonly injured hamstring muscle.43,44 Injury occurs when the force of eccentric contraction, and resulting muscle strain, exceeds the mechanical limits of the tissue.42,45 It remains to be shown whether hamstring strains occur as a result of accumulated microscopic muscle damage or secondary to a single event that exceeds the mechanical limits of the muscle.42
Epidemiology and Risk Factors
The majority of hamstring strains are sustained during noncontact activities, with most athletes citing sprinting as the activity at the time of injury.3 Approximately 93% of injuries occur during noncontact activities among defensive backs and wide receivers.3 Hamstring strains are the second-most common injury among NFL players, comprising approximately 9% of all injuries,5,7 with 16% to 31% of these injuries associated with recurrence.3,5,35,46 Using the NFL’s Injury Surveillance System, Elliott and colleagues3 reported 1716 hamstring strains over a 10-year period (1989-1998). Fifty-one percent of hamstring strains occurred during the 7-week preseason, with a greater than 4-fold increased injury rate noted during the preseason compared to the 16-week regular season. An increased incidence in the preseason is partially attributable to relative deconditioning over the offseason. Defensive backs, wide receivers, and special teams players accounted for the majority of injured players, suggesting that speed position players and those who must “backpedal” (run backwards) are at an increased risk for injury.
Several risk factors for hamstring strain have been described, including prior injury, older age, quadriceps-hamstring strength imbalances, limited hip and knee flexibility, and fatigue.39,42,47 Inadequate rehabilitation and premature return to competition are also likely important factors predisposing to recurrent injury.39,48
History and Physical Examination
The majority of hamstring strains occur in the acute setting when the player experiences the sudden onset of pain in the posterior thigh during strenuous exercise, most commonly while sprinting.39 The injury typically occurs in the early or late stage of practice or competition due, in part, to inadequate warm-up or fatigue. The athlete may describe an audible pop and an inability to continue play, depending on injury severity.
Physical examination may demonstrate palpable induration and tenderness immediately or shortly after injury. In the setting of severe strains, there can be significant thigh swelling and ecchymosis, and in complete ruptures, a palpable defect.39 The affected muscle should be palpated along its entire length, and is best performed prone with the knee flexed to 90° as well as with the knee partially extended to place it under mild tension. Injury severity can be assessed by determining the restriction of passive knee extension while the athlete is lying supine with the hip flexed to 90°. The severity of hamstring strains varies from minor damage of a few myofibers without loss of structural integrity to complete muscle rupture.
Imaging
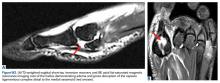
Similar to other muscle strains, hamstring strains are a clinical diagnosis and generally do not necessitate advanced imaging studies except to assess the degree of damage (ie, partial vs complete rupture) and to rule out other injuries, especially if the athlete fails to respond to treatment. Plain radiographs in acute cases are usually unremarkable. However, more severe injuries may go on to develop myositis ossificans similar to quadriceps soft tissue injuries (Figure 5).
Treatment
Most hamstring strains respond to conservative treatment, with operative intervention rarely indicated except for proximal or distal tendon avulsions.39 Like other muscle strains, initial management consists of RICE. COX-2-selective NSAIDs are preferred initially following injury. During a brief period of immobilization, the leg should be extended as much as tolerated to maximize muscle length, limit hematoma formation, and reduce the risk of contracture.39 Controlled mobilization should begin as soon as tolerated by the athlete.39 Isometric exercises and a stretching program should be started early in the rehabilitation period, with isotonic exercises added as motion and pain improve. Active stretching should be initiated and progressed to passive, static stretching as guided by pain.
The late phase of rehabilitation and long-term conditioning protocols should incorporate eccentric training once the athlete is pain-free, performing isotonic and isokinetic exercises. Eccentric exercises best strengthen the hamstrings at their most susceptible point, prepares the athlete for functional activities, and minimizes the risk of reinjury,3,50,51 Elliot and colleagues3 reported an order of magnitude decrease in hamstring injuries in high-risk athletes with identifiable hamstring muscle weakness after implementing an eccentric strengthening program and progressive sprint training. Similarly, in a large cohort of elite soccer players, correction of strength deficits in players with prior hamstring injuries led to similar rates of injury compared to athletes without strength deficits or prior injury.52 Those athletes with persistent weakness who did not undergo rehabilitation had significantly higher rates of reinjury.
Various injections containing local anesthetics, corticosteroids, platelet-rich plasma (PRP), and other substances have been administered to football players following acute muscle strains in an effort to alleviate pain and safely return the athlete to competition. Some practitioners have been reluctant to administer injections (especially those containing corticosteroids) due to a potentially increased risk of tendinopathy or rupture.31 Drakos and colleagues53 reported their outcomes following muscle and ligament strains treated with combined corticosteroid and local anesthetic injections on one NFL team. While quadriceps and hamstring strains were associated with the most missed games among all muscle strains, these injections resulted in no adverse events or progression of injury severity. Similarly, Levine and colleagues 51 administered intramuscular corticosteroid injections to 58 NFL players with high-grade hamstring injuries that had a palpable defect within the muscle belly. They reported no complications or strength deficits at final examination. In a case-control study, Rettig and colleagues46 administered PRP injections under ultrasound guidance in 5 NFL players with hamstring injuries. Compared to players treated with a focused rehabilitation program only, there were no significant differences in recovery or return to play.
The decision to return to play should be based on a clinical assessment considering pain, strength, motion, and flexibility. Player position should also be considered. Return-to-play guidelines describing the appropriate progression through rehabilitation and return to sport have been described and can be used as a template for the rehabilitation of football players.54 It should be noted that primary hamstring strains are associated with decreased athletic performance and an increased risk of more severe reinjury after return to sport.55,56
Morel-Lavallée Lesion
Pathophysiology
Morel-Lavallée lesions (MLLs) are uncommon football injuries, but often occur in the thigh.57,58 An MLL is a posttraumatic soft tissue injury in which deforming forces of pressure and shear cause a closed, soft tissue degloving injury; in this injury, the skin and subcutaneous tissues are separated from the underlying fascia, disrupting perforating blood vessels. The resulting space between the fascia and subcutaneous tissue fills with blood, lymphatics, and necrotic fat, resulting in a hematoma/seroma that can be a nidus for bacterial infection.58 The most common anatomic regions are the anterior distal thigh and lateral hip. Both of these areas are commonly involved in both direct contact and shear forces following a fall to the ground.
History and Physical Examination
Athletes with MLLs typically present with the insidious onset of a fluid collection within the thigh following a fall to the ground, usually while sliding or diving on the playing surface.57,58 The fluid collection can be associated with thigh tightness and may extend distally into the suprapatellar region or proximally over the greater trochanter. Thigh swelling, ecchymosis, and palpable fluctuance are seen in most cases. Progressive increases in pain and thigh swelling may be seen in severe injuries, but thigh compartments generally remain soft and nontender. Signs and symptoms of an MLL do not typically manifest immediately following the athletic event. Tejwani and colleagues58 reported a case series of MLLs of the knee in 27 NFL players from a single team over a 14-year period, with an average of 3 days between injury and evaluation by the medical staff. The mechanism of injury was a shearing blow from the knee striking the playing surface in 81% of cases and direct contact to the knee from another player in 19% of cases; all cases occurred in game situations. No affected players were wearing kneepads at the time of injury.
Imaging
Plain radiography may reveal a noncalcified soft tissue mass over the involved area and is not usually helpful except to rule out an underlying fracture. The appearance of an MLL on ultrasound is nonspecific and variable, often described as anechoic, hypoechoic, or hyperechoic depending on the presence of hemolymphatic fluid sedimentation and varying amounts of internal fat debris. MRI is the imaging modality of choice and typically shows a well-defined oval or fusiform, fluid-filled mass with tapering margins blending with adjacent fascial planes.
Treatment
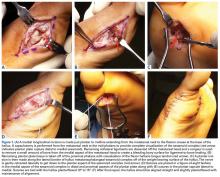
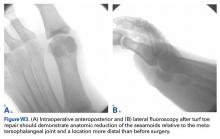
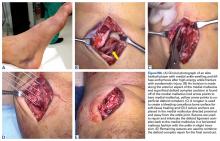
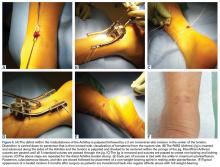
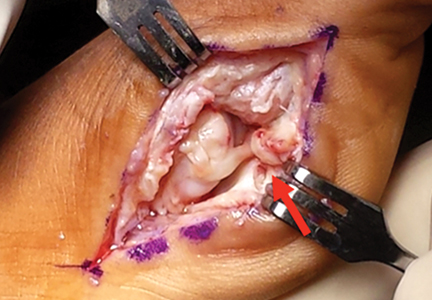
Similar to quadriceps contusions, treatment goals for MLLs are evacuation of the fluid collection, prevention of fluid recurrence, a full range of active knee flexion, and prompt return to play.57,58 Initial treatment for smaller lesions consists of cryotherapy, compression wrapping of the involved area, and immediate active and passive range of motion of the hip and knee. While MLLs were traditionally treated with serial open debridements, less invasive approaches—including elastic compression, aspiration, percutaneous irrigation with debridement and suction drainage, or liposuction and drainage followed by suction therapy—have been recently described.57,58,60,61 Less invasive approaches aim to minimize soft tissue dissection and disruption of the vascular supply while accelerating rehabilitation. The presence of a surrounding capsule on MRI makes conservative or minimally invasive approaches less likely to be successful and may necessitate an open procedure.62 Antibiotics should be used preoperatively due to the presence of a dead space containing necrotic debris that makes infection a potential complication. While elite contact athletes can expect to return to competition long before complete resolution of an MLL, there is a risk of further delamination and lesion expansion due to re-injury prior to compete healing.
Tejwani and colleagues58 performed aspiration at the area of palpable fluctuance in the thigh or suprapatellar region using a 14-gauge needle in those athletes who failed to improve with conservative treatments alone. Mean time to resolution of the fluid collection was 16 days following aspiration. Fifty-two percent of the athletes were successfully treated with cryotherapy, compression, and motion exercises alone; 48% were treated with at least one aspiration, with a mean of 2.7 aspirations per knee. In 11% of cases that failed to resolve after multiple aspirations, doxycycline sclerodesis was performed immediately following an aspiration. Patients treated with sclerodesis had no return of the fluid collection and returned to play the following day.
Matava and colleagues57 described the case of an NFL player who sustained a closed MLL of the lateral hip while diving onto an artificial turf surface attempting to catch a pass. Despite immediate thigh pain and swelling, he was able to continue play. Immediately following the game, the player was examined and had a tense thigh with ecchymosis extending into the trochanteric region. Aspiration of the fluctuant area was unsuccessful. Progressive increases in pain and thigh swelling prompted hospital admission. Percutaneous irrigation and debridement was performed as described by Tseng and Tornetta.61 A suction drain was placed within the residual dead space, and constant wall suction was applied in addition to hip compression using a spica
Conclusion
Quadriceps and hamstring injuries occur frequently in football and are generally treated conservatively. While return to competition following hamstring strains is relatively quick, a high rate of injury recurrence highlights the importance of targeted rehabilitation and conditioning. Rarely, complications from quadriceps contusions, including acute compartment syndrome and myositis ossificans, may require operative intervention if unresponsive to conservative treatment. MLLs are rare in sports, but usually involve the thigh when they occur in football players. Team physicians must maintain a heightened degree of awareness of this injury as it may require operative intervention.
Acknowledgements: The authors would like to thank Jonathon Baker, MD and David Rubin, MD for their assistance in providing radiographic images for this paper.
Am J Orthop. 2016;45(6):E308-E318. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
American football has the highest injury rate of any team sport in the United States at the high school, collegiate, and professional levels.1-3 Muscle strains and contusions constitute a large proportion of football injuries. For example, at the high school level, muscle strains comprise 12% to 24% of all injuries;2 at the collegiate level, they account for approximately 20% of all practice injuries, with nearly half of all strains occurring within the thigh.1,4 Among a single National Football League (NFL) team, Feeley and colleagues5 reported that muscle strains accounted for 46% of practice and 22% of preseason game injuries. The hamstrings, followed by the quadriceps, are the most commonly strained muscle groups among both professional and amateur athletes,5,6 with hamstring and quadriceps injuries making up approximately 13% of all injuries among NFL players.7 Given the relatively large surface area and muscle volume of the anterior and posterior thigh, as well as the activities and maneuvers necessitated by the various football positions, it is not surprising that the thigh is frequently involved in football-related injuries.
The purpose of this review is to describe the clinical manifestations of thigh-related soft-tissue injuries seen in football players. Two of these conditions—muscle strains and contusions—are relatively common, while a third condition—the Morel-Lavallée lesion—is a rare, yet relevant injury that warrants discussion.
Quadriceps Contusion
Pathophysiology
Contusion to the quadriceps muscle is a common injury in contact sports generally resulting from a direct blow from a helmet, knee, or shoulder.8 Bleeding within the musculature causes swelling, pain, stiffness, and limitation of quadriceps excursion, ultimately resulting in loss of knee flexion and an inability to run or squat. The injury is typically confined to a single quadriceps muscle.8 The use of thigh padding, though helpful, does not completely eliminate the risk of this injury.
History and Physical Examination
Immediately after injury, the athlete may complain only of thigh pain. However, swelling, pain, and diminished range of knee motion may develop within the first 24 hours depending on the severity of injury and how quickly treatment is instituted.8 Jackson and Feagin9 developed an injury grading system for quadriceps contusions based on the limitation of knee flexion observed (Table 1).
Imaging
A quadriceps contusion is a clinical diagnosis based on a typical history and physical examination; therefore, advanced imaging usually does not need to be obtained except to gauge the severity of injury, to rule out concurrent injuries (ie, tendon rupture), and to identify the presence of a hematoma that may necessitate aspiration. Plain radiographs are typically unremarkable in the acute setting. Appearance on magnetic resonance imaging (MRI) varies by injury severity, with increased signal throughout the affected muscle belly and a diffuse, feathery appearance centered at the point of impact on short TI inversion recovery (STIR) and T2-weighted images reflecting edema and possibly hematoma (Figures 1A-1C).8,11
Treatment
Treatment of a quadriceps contusion is nonoperative and consists of a 3-phase recovery.10 The first phase lasts approximately 2 days and consists of rest, ice, compression, and elevation (RICE) to limit hemorrhage. The knee should be rested in a flexed position to maintain quadriceps muscle fiber length in order to promote muscle compression and limit knee stiffness. For severe contusions in which there is a question of an acute thigh compartment syndrome, compression should be avoided with appropriate treatment based on typical symptoms and intra-compartmental pressure measurement.12 Nonsteroidal anti-inflammatory drugs (NSAIDs) may be administered to diminish pain as well as the risk of myositis ossificans. While there is no data on the efficacy of NSAIDs in preventing myositis ossificans following quadriceps contusions, both COX-2 selective (ie, celecoxib) and nonselective (ie, naproxen, indomethacin) COX inhibitors have been demonstrated to significantly reduce the incidence of heterotopic ossification following hip surgery—a condition occurring from a similar pathophysiologic process as myositis ossificans.13-17 However, this class of drugs should not be given any sooner than 48 to 72 hours after injury to decrease further bleeding risk, given its inhibitory effect on platelet function.18 Narcotic pain medications are rarely required.
The second phase focuses on restoring active and passive knee and hip flexion and begins when permitted by pain.8 Icing, pain control, and physical therapy modalities are also continued in order to reduce pain and swelling as knee motion is progressed. The third phase begins once full range of knee and hip motion is restored and consists of quadriceps strengthening and functional rehabilitation of the lower extremity.8,19 Return to athletic activities and eventually competition should take place when a full, painless range of motion is restored and strength returns to baseline. Isokinetic strength testing may be utilized to more accurately assess strength and endurance. Noncontact, position-specific drills are incorporated as clinical improvement allows. A full recovery should be expected within 4 weeks of injury, with faster resolution and return to play seen in less severe contusions depending on the athlete’s position.8 Continued quadriceps stretching is recommended to prevent recurrence once the athlete returns to play. A protective hard shell may also be utilized both during rehabilitation as well as once the athlete returns to play in order to protect the thigh from reinjury, which may increase the risk of myositis ossificans.8
Complications
A prolonged recovery or persistent symptoms should alert the treating physician to the possibility of complications, including myositis ossificans.8,20 Myositis ossificans typically results from moderate to severe contusions, which may present initially as a painful, indurated mass that later becomes quite firm. This mass may be seen on plain radiographs as early as 2 to 4 weeks following injury if the athlete complains of persistent pain or a palpable thigh mass (Figure 2).9
Mani-Babu and colleagues23 reported a case of a 14-year-old male football player who sustained a quadriceps contusion after a direct blow from an opponent’s helmet to the lateral thigh. Persistent pain and limitation of motion at 2 months follow-up prompted imaging studies that demonstrated myositis ossificans. The patient was treated with intravenous pamidronate (a bisphosphonate) twice over a 3-month period and demonstrated a full recovery within 5 months.
Acute compartment syndrome of the thigh has also been reported following severe quadriceps contusions, with the majority occurring in the anterior compartment.12,24-28 When injury from blunt trauma extends into and disrupts the muscular layer adjacent to the femur, vascular disruption can cause hematoma formation, muscle edema, and significant swelling, thereby increasing intracompartmental pressure. The relatively large volume of the anterior thigh compartment and lack of a rigid deep fascial envelope may be protective from the development of compartment syndrome compared to other sites.28 It can be difficult to distinguish a severe contusion from a compartment syndrome, as both can occur from the same mechanism and have similar presenting signs and symptoms. Signs of a compartment syndrome include pain out of proportion to the injury that is aggravated by passive stretch of the quadriceps muscles, an increasingly firm muscle compartment to palpation, and neurovascular deficits.29 Both acute compartment syndrome and a severe contusion may present with significant pain, inability to bear weight, tense swelling, tenderness to palpation, and pain with passive knee flexion.24 While the successful conservative treatment of athletes with acute compartment syndrome of the thigh has been reported, it is important to closely monitor the patient’s condition and consider intracompartmental pressure monitoring if the patient’s clinical condition deteriorates.12 An acute fasciotomy should be strongly considered when intracompartmental pressures are within 30 mm Hg of diastolic pressure.24-27 Fortunately, it is highly uncommon for thigh compartment pressure to rise to this level. Percutaneous compartment decompression using liposuction equipment or a large cannula has been described to decrease intracompartmental pressure, potentially expediting recovery and minimizing morbidity.18 Interestingly, reports of fasciotomies for acute thigh compartment syndrome following closed athletic injuries have not described necrotic or non-contractile muscle typical of an acute compartment syndrome, calling into question the need for fasciotomy following closed blunt athletic trauma to the thigh.18
Quadriceps Strain
Pathophysiology
Acute quadriceps strains occur during sudden forceful eccentric contraction of the extensor mechanism. Occasionally, in the absence of a clear mechanism, these injuries mistakenly appear as a contusion resulting from a direct blow to the thigh.30,31 The rectus femoris is the most frequently strained quadriceps muscle due, in part, to its superficial location and predominance of type II muscle fibers, which are more likely to be strained.11,32 Although classically described as occurring along the distal portion of the rectus femoris at the musculotendinous junction, quadriceps strains most commonly occur at the mid to proximal aspect of the rectus femoris.30,33 The quadriceps muscle complex crosses 2 joints and, as a result, is more predisposed to eccentric injury than mono-articular muscles.34 We have had a subset of complete myotendinous tears of the rectus femoris that occur in the plant leg of placekickers that result in significant disability.
Risk Factors
Quadriceps and thigh injuries comprise approximately 4.5% of injuries among NFL players.7 Several risk factors for quadriceps strains have been described. In a study of Australian Rules football players, Orchard35 demonstrated that for all muscle strains, the strongest risk factor was a recent history of the same injury, with the next strongest risk factor being a past history of the same injury. Increasing age was found to be a risk factor for hamstring strains but not quadriceps strains. Muscle fatigue may also contribute to injury susceptibility.36
History and Physical Examination
Injuries typically occur during kicking, jumping, or a sudden change in direction while running.30 Athletes may localize pain anywhere along the quadriceps muscle, although strains most commonly occur at the proximal to mid portion of the rectus femoris.30,33 The grading system for quadriceps strains described by Kary30 is based on level of pain, quadriceps strength, and the presence or absence of a palpable defect (Table 2).
The athlete typically walks with an antalgic gait. Visible swelling and/or ecchymosis may be present depending on when the athlete is seen, as ecchymosis may develop within the first 24 hours of injury. The examiner should palpate along the entire length of the injured muscle. High-grade strains or complete tears may present with a bulge or defect in the muscle belly, but in most cases no defect will be palpable. There may be loss of knee flexion similar to a quadriceps contusion. Strength testing should be performed in both the sitting and prone position with the hip both flexed and extended to assess resisted knee extension strength.30 Loss of strength is proportional to the degree of injury.
Imaging
While most quadriceps strains are adequately diagnosed clinically without the need for imaging studies, ultrasound or MRI can be used to evaluate for partial or complete rupture.30,33 In milder cases, MRI usually demonstrates interstitial edema and hemorrhage with a feathery appearance on STIR and T2-weighted imaging (Figures 3A-3C).11
Treatment
Acute treatment of quadriceps strains focuses on minimizing bleeding using the principles of RICE treatment.37 NSAIDs may be used immediately to assist with pain control.30 COX-2-specific NSAIDs are preferred due to their lack of any inhibitory effect on platelet function in order to reduce the risk of further bleeding within the muscle compartment. For the first 24 to 72 hours following injury, the quadriceps should be maintained relatively immobilized to prevent further injury.38 High-grade injuries might necessitate crutches for ambulatory assistance.
Depending on injury severity, the active phase of treatment usually begins within 5 days of injury and consists of stretching and knee/hip range of motion. An active warm-up should precede rehabilitation exercises to activate neural pathways within the muscle and improve muscle elasticity.38 Ballistic stretching should be avoided to prevent additional injury to the muscle fibers. Strengthening should proceed when the athlete recovers a pain-free range of motion. When isometric exercises can be completed at increasing degrees of knee flexion, isotonic exercises may be implemented into the rehabilitation program.30 Return to football can be considered when the athlete has recovered knee and hip range of motion, is pain-free, and has near-normal strength compared to the contralateral side. The athlete should also perform satisfactorily in simulated position-specific activities in a noncontact fashion prior to return to full competition.30
Hamstring Strain
Pathophysiology
Hamstring strains are the most common noncontact injuries in football resulting from excessive muscle stretching during eccentric contraction generally occurring at the musculotendinous junction.5,39 Because the hamstrings cross both the hip and knee, simultaneous hip flexion and knee extension results in maximal lengthening, making them most vulnerable to injury at the terminal swing phase of gait just prior to heel strike.39-42 The long head of the biceps femoris undergoes the greatest stretch, reaching 110% of resting length during terminal swing phase and is the most commonly injured hamstring muscle.43,44 Injury occurs when the force of eccentric contraction, and resulting muscle strain, exceeds the mechanical limits of the tissue.42,45 It remains to be shown whether hamstring strains occur as a result of accumulated microscopic muscle damage or secondary to a single event that exceeds the mechanical limits of the muscle.42
Epidemiology and Risk Factors
The majority of hamstring strains are sustained during noncontact activities, with most athletes citing sprinting as the activity at the time of injury.3 Approximately 93% of injuries occur during noncontact activities among defensive backs and wide receivers.3 Hamstring strains are the second-most common injury among NFL players, comprising approximately 9% of all injuries,5,7 with 16% to 31% of these injuries associated with recurrence.3,5,35,46 Using the NFL’s Injury Surveillance System, Elliott and colleagues3 reported 1716 hamstring strains over a 10-year period (1989-1998). Fifty-one percent of hamstring strains occurred during the 7-week preseason, with a greater than 4-fold increased injury rate noted during the preseason compared to the 16-week regular season. An increased incidence in the preseason is partially attributable to relative deconditioning over the offseason. Defensive backs, wide receivers, and special teams players accounted for the majority of injured players, suggesting that speed position players and those who must “backpedal” (run backwards) are at an increased risk for injury.
Several risk factors for hamstring strain have been described, including prior injury, older age, quadriceps-hamstring strength imbalances, limited hip and knee flexibility, and fatigue.39,42,47 Inadequate rehabilitation and premature return to competition are also likely important factors predisposing to recurrent injury.39,48
History and Physical Examination
The majority of hamstring strains occur in the acute setting when the player experiences the sudden onset of pain in the posterior thigh during strenuous exercise, most commonly while sprinting.39 The injury typically occurs in the early or late stage of practice or competition due, in part, to inadequate warm-up or fatigue. The athlete may describe an audible pop and an inability to continue play, depending on injury severity.
Physical examination may demonstrate palpable induration and tenderness immediately or shortly after injury. In the setting of severe strains, there can be significant thigh swelling and ecchymosis, and in complete ruptures, a palpable defect.39 The affected muscle should be palpated along its entire length, and is best performed prone with the knee flexed to 90° as well as with the knee partially extended to place it under mild tension. Injury severity can be assessed by determining the restriction of passive knee extension while the athlete is lying supine with the hip flexed to 90°. The severity of hamstring strains varies from minor damage of a few myofibers without loss of structural integrity to complete muscle rupture.
Imaging
Similar to other muscle strains, hamstring strains are a clinical diagnosis and generally do not necessitate advanced imaging studies except to assess the degree of damage (ie, partial vs complete rupture) and to rule out other injuries, especially if the athlete fails to respond to treatment. Plain radiographs in acute cases are usually unremarkable. However, more severe injuries may go on to develop myositis ossificans similar to quadriceps soft tissue injuries (Figure 5).
Treatment
Most hamstring strains respond to conservative treatment, with operative intervention rarely indicated except for proximal or distal tendon avulsions.39 Like other muscle strains, initial management consists of RICE. COX-2-selective NSAIDs are preferred initially following injury. During a brief period of immobilization, the leg should be extended as much as tolerated to maximize muscle length, limit hematoma formation, and reduce the risk of contracture.39 Controlled mobilization should begin as soon as tolerated by the athlete.39 Isometric exercises and a stretching program should be started early in the rehabilitation period, with isotonic exercises added as motion and pain improve. Active stretching should be initiated and progressed to passive, static stretching as guided by pain.
The late phase of rehabilitation and long-term conditioning protocols should incorporate eccentric training once the athlete is pain-free, performing isotonic and isokinetic exercises. Eccentric exercises best strengthen the hamstrings at their most susceptible point, prepares the athlete for functional activities, and minimizes the risk of reinjury,3,50,51 Elliot and colleagues3 reported an order of magnitude decrease in hamstring injuries in high-risk athletes with identifiable hamstring muscle weakness after implementing an eccentric strengthening program and progressive sprint training. Similarly, in a large cohort of elite soccer players, correction of strength deficits in players with prior hamstring injuries led to similar rates of injury compared to athletes without strength deficits or prior injury.52 Those athletes with persistent weakness who did not undergo rehabilitation had significantly higher rates of reinjury.
Various injections containing local anesthetics, corticosteroids, platelet-rich plasma (PRP), and other substances have been administered to football players following acute muscle strains in an effort to alleviate pain and safely return the athlete to competition. Some practitioners have been reluctant to administer injections (especially those containing corticosteroids) due to a potentially increased risk of tendinopathy or rupture.31 Drakos and colleagues53 reported their outcomes following muscle and ligament strains treated with combined corticosteroid and local anesthetic injections on one NFL team. While quadriceps and hamstring strains were associated with the most missed games among all muscle strains, these injections resulted in no adverse events or progression of injury severity. Similarly, Levine and colleagues 51 administered intramuscular corticosteroid injections to 58 NFL players with high-grade hamstring injuries that had a palpable defect within the muscle belly. They reported no complications or strength deficits at final examination. In a case-control study, Rettig and colleagues46 administered PRP injections under ultrasound guidance in 5 NFL players with hamstring injuries. Compared to players treated with a focused rehabilitation program only, there were no significant differences in recovery or return to play.
The decision to return to play should be based on a clinical assessment considering pain, strength, motion, and flexibility. Player position should also be considered. Return-to-play guidelines describing the appropriate progression through rehabilitation and return to sport have been described and can be used as a template for the rehabilitation of football players.54 It should be noted that primary hamstring strains are associated with decreased athletic performance and an increased risk of more severe reinjury after return to sport.55,56
Morel-Lavallée Lesion
Pathophysiology
Morel-Lavallée lesions (MLLs) are uncommon football injuries, but often occur in the thigh.57,58 An MLL is a posttraumatic soft tissue injury in which deforming forces of pressure and shear cause a closed, soft tissue degloving injury; in this injury, the skin and subcutaneous tissues are separated from the underlying fascia, disrupting perforating blood vessels. The resulting space between the fascia and subcutaneous tissue fills with blood, lymphatics, and necrotic fat, resulting in a hematoma/seroma that can be a nidus for bacterial infection.58 The most common anatomic regions are the anterior distal thigh and lateral hip. Both of these areas are commonly involved in both direct contact and shear forces following a fall to the ground.
History and Physical Examination
Athletes with MLLs typically present with the insidious onset of a fluid collection within the thigh following a fall to the ground, usually while sliding or diving on the playing surface.57,58 The fluid collection can be associated with thigh tightness and may extend distally into the suprapatellar region or proximally over the greater trochanter. Thigh swelling, ecchymosis, and palpable fluctuance are seen in most cases. Progressive increases in pain and thigh swelling may be seen in severe injuries, but thigh compartments generally remain soft and nontender. Signs and symptoms of an MLL do not typically manifest immediately following the athletic event. Tejwani and colleagues58 reported a case series of MLLs of the knee in 27 NFL players from a single team over a 14-year period, with an average of 3 days between injury and evaluation by the medical staff. The mechanism of injury was a shearing blow from the knee striking the playing surface in 81% of cases and direct contact to the knee from another player in 19% of cases; all cases occurred in game situations. No affected players were wearing kneepads at the time of injury.
Imaging
Plain radiography may reveal a noncalcified soft tissue mass over the involved area and is not usually helpful except to rule out an underlying fracture. The appearance of an MLL on ultrasound is nonspecific and variable, often described as anechoic, hypoechoic, or hyperechoic depending on the presence of hemolymphatic fluid sedimentation and varying amounts of internal fat debris. MRI is the imaging modality of choice and typically shows a well-defined oval or fusiform, fluid-filled mass with tapering margins blending with adjacent fascial planes.
Treatment
Similar to quadriceps contusions, treatment goals for MLLs are evacuation of the fluid collection, prevention of fluid recurrence, a full range of active knee flexion, and prompt return to play.57,58 Initial treatment for smaller lesions consists of cryotherapy, compression wrapping of the involved area, and immediate active and passive range of motion of the hip and knee. While MLLs were traditionally treated with serial open debridements, less invasive approaches—including elastic compression, aspiration, percutaneous irrigation with debridement and suction drainage, or liposuction and drainage followed by suction therapy—have been recently described.57,58,60,61 Less invasive approaches aim to minimize soft tissue dissection and disruption of the vascular supply while accelerating rehabilitation. The presence of a surrounding capsule on MRI makes conservative or minimally invasive approaches less likely to be successful and may necessitate an open procedure.62 Antibiotics should be used preoperatively due to the presence of a dead space containing necrotic debris that makes infection a potential complication. While elite contact athletes can expect to return to competition long before complete resolution of an MLL, there is a risk of further delamination and lesion expansion due to re-injury prior to compete healing.
Tejwani and colleagues58 performed aspiration at the area of palpable fluctuance in the thigh or suprapatellar region using a 14-gauge needle in those athletes who failed to improve with conservative treatments alone. Mean time to resolution of the fluid collection was 16 days following aspiration. Fifty-two percent of the athletes were successfully treated with cryotherapy, compression, and motion exercises alone; 48% were treated with at least one aspiration, with a mean of 2.7 aspirations per knee. In 11% of cases that failed to resolve after multiple aspirations, doxycycline sclerodesis was performed immediately following an aspiration. Patients treated with sclerodesis had no return of the fluid collection and returned to play the following day.
Matava and colleagues57 described the case of an NFL player who sustained a closed MLL of the lateral hip while diving onto an artificial turf surface attempting to catch a pass. Despite immediate thigh pain and swelling, he was able to continue play. Immediately following the game, the player was examined and had a tense thigh with ecchymosis extending into the trochanteric region. Aspiration of the fluctuant area was unsuccessful. Progressive increases in pain and thigh swelling prompted hospital admission. Percutaneous irrigation and debridement was performed as described by Tseng and Tornetta.61 A suction drain was placed within the residual dead space, and constant wall suction was applied in addition to hip compression using a spica
Conclusion
Quadriceps and hamstring injuries occur frequently in football and are generally treated conservatively. While return to competition following hamstring strains is relatively quick, a high rate of injury recurrence highlights the importance of targeted rehabilitation and conditioning. Rarely, complications from quadriceps contusions, including acute compartment syndrome and myositis ossificans, may require operative intervention if unresponsive to conservative treatment. MLLs are rare in sports, but usually involve the thigh when they occur in football players. Team physicians must maintain a heightened degree of awareness of this injury as it may require operative intervention.
Acknowledgements: The authors would like to thank Jonathon Baker, MD and David Rubin, MD for their assistance in providing radiographic images for this paper.
Am J Orthop. 2016;45(6):E308-E318. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Hootman JM, Dick R, Agel J. Epidemiology of collegiate injuries for 15 sports: summary and recommendations for injury prevention initiatives. J Athl Train. 2007;42(2):311-319.
2. Rechel JA, Yard EE, Comstock RD. An epidemiologic comparison of high school sports injuries sustained in practice and competition. J Athl Train. 2008;43(2):197-204.
3. Elliott MC, Zarins B, Powell JW, Kenyon CD. Hamstring muscle strains in professional football players: a 10-year review. Am J Sports Med. 2011;39(4):843-850.
4. Dick R, Ferrara MS, Agel J, et al. Descriptive epidemiology of collegiate men’s football injuries: National Collegiate Athletic Association Injury Surveillance System, 1988-1989 through 2003-2004. J Athl Train. 2007;42(2):221-233.
5. Feeley BT, Kennelly S, Barnes RP, et al. Epidemiology of National Football League training camp injuries from 1998 to 2007. Am J Sports Med. 2008;36(8):1597-1603.
6. Garrett WE Jr. Muscle strain injuries. Am J Sports Med. 1996;24(6 Suppl):S2-S8.
7. Lawrence DW, Hutchison MG, Comper P. Descriptive epidemiology of musculoskeletal injuries and concussions in the National Football League, 2012-2014. Orthop J Sports Med. 2015;3(5):2325967115583653.
8. Diaz JA, Fischer DA, Rettig AC, Davis TJ, Shelbourne KD. Severe quadriceps muscle contusions in athletes. A report of three cases. Am J Sports Med. 2003;31(2):289-293.
9. Jackson DW, Feagin JA. Quadriceps contusions in young athletes. Relation of severity of injury to treatment and prognosis. J Bone Joint Surg Am. 1973;55(1):95-105.
10. Ryan JB, Wheeler JH, Hopkinson WJ, Arciero RA, Kolakowski KR. Quadriceps contusions. West Point update. Am J Sports Med. 1991;19(3):299-304.
11. Bencardino JT, Rosenberg ZS, Brown RR, Hassankhani A, Lustrin ES, Beltran J. Traumatic musculotendinous injuries of the knee: diagnosis with MR imaging. Radiographics. 2000;20 Spec No:S103-S120.
12. Robinson D, On E, Halperin N. Anterior compartment syndrome of the thigh in athletes--indications for conservative treatment. J Trauma. 1992;32(2):183-186.
13. Beckmann JT, Wylie JD, Kapron AL, Hanson JA, Maak TG, Aoki SK. The effect of NSAID prophylaxis and operative variables on heterotopic ossification after hip arthroscopy. Am J Sports Med. 2014;42(6):1359-1364.
14. Shehab D, Elgazzar AH, Collier BD. Heterotopic ossification. J Nucl Med. 2002;43(3):346-353.
15. Beckmann JT, Wylie JD, Potter MQ, Maak TG, Greene TH, Aoki SK. Effect of naproxen prophylaxis on heterotopic ossification following hip arthroscopy: a double-blind randomized placebo-controlled trial. J Bone Joint Surg Am. 2015;97(24):2032-2037.
16. Yeung M, Jamshidi S, Horner N, Simunovic N, Karlsson J, Ayeni OR. Efficacy of nonsteroidal anti-inflammatory drug prophylaxis for heterotrophic ossification in hip arthroscopy: a systematic review. Arthroscopy. 2016;32(3):519-525.
17. Goyal K, Pettis CR, Bancroft AE, Wasyliw CW, Scherer KF. Myositis ossificans in the thigh of a lacrosse player. Orthopedics. 2015;38(8):468,515-518.
18. Cooper DE. Severe quadriceps muscle contusions in athletes. Am J Sports Med. 2004;32(3):820.
19. Bonsell S, Freudigman PT, Moore HA. Quadriceps muscle contusion resulting in osteomyelitis of the femur in a high school football player. A case report. Am J Sports Med. 2001;29(6):818-820.
20. Rothwell AG. Quadriceps hematoma. A prospective clinical study. Clin Orthop Relat Res. 1982;(171):97-103.
21. Armfield DR, Kim DH, Towers JD, Bradley JP, Robertson DD. Sports-related muscle injury in the lower extremity. Clin Sports Med. 2006;25(4):803-842.
22. Lipscomb AB, Thomas ED, Johnston RK. Treatment of myositis ossificans traumatica in athletes. Am J Sports Med. 1976;4(3):111-120.
23. Mani-Babu S, Wolman R, Keen R. Quadriceps traumatic myositis ossificans in a football player: management with intravenous pamidronate. Clin J Sport Med. 2014;24(5):e56-e58.
24. McCaffrey DD, Clarke J, Bunn J, McCormack MJ. Acute compartment syndrome of the anterior thigh in the absence of fracture secondary to sporting trauma. J Trauma. 2009;66(4):1238-1242.
25. Klasson SC, Vander Schilden JL. Acute anterior thigh compartment syndrome complicating quadriceps hematoma. Two case reports and review of the literature. Orthop Rev. 1990;19(5):421-427.
26. Rooser B. Quadriceps contusion with compartment syndrome. Evacuation of hematoma in 2 cases. Acta Orthop Scand. 1987;58(2):170-172.
27. Rooser B, Bengtson S, Hagglund G. Acute compartment syndrome from anterior thigh muscle contusion: a report of eight cases. J Orthop Trauma. 1991;5(1):57-59.
28. Schwartz JT Jr, Brumback RJ, Lakatos R, Poka A, Bathon GH, Burgess AR. Acute compartment syndrome of the thigh. A spectrum of injury. J Bone Joint Surg Am. 1989;71(3):392-400.
29. Elliott KG, Johnstone AJ. Diagnosing acute compartment syndrome. J Bone Joint Surg Br. 2003;85(5):625-632.
30. Kary JM. Diagnosis and management of quadriceps strains and contusions. Curr Rev Musculoskelet Med. 2010;3(1-4):26-31.
31. Boublik M, Schlegel TF, Koonce RC, Genuario JW, Kinkartz JD. Quadriceps tendon injuries in national football league players. Am J Sports Med. 2013;41(8):1841-1846.
32. Palmer WE, Kuong SJ, Elmadbouh HM. MR imaging of myotendinous strain. AJR Am J Roentgenol. 1999;173(3):703-709.
33. Cross TM, Gibbs N, Houang MT, Cameron M. Acute quadriceps muscle strains: magnetic resonance imaging features and prognosis. Am J Sports Med. 2004;32(3):710-719.
34. Hughes C 4th, Hasselman CT, Best TM, Martinez S, Garrett WE Jr. Incomplete, intrasubstance strain injuries of the rectus femoris muscle. Am J Sports Med. 1995;23(4):500-506.
35. Orchard JW. Intrinsic and extrinsic risk factors for muscle strains in Australian football. Am J Sports Med. 2001;29(3):300-303.36. Mair SD, Seaber AV, Glisson RR, Garrett WE, Jr. The role of fatigue in susceptibility to acute muscle strain injury. Am J Sports Med. 1996;24(2):137-143.
37. Bleakley C, McDonough S, MacAuley D. The use of ice in the treatment of acute soft-tissue injury: a systematic review of randomized controlled trials. Am J Sports Med. 2004;32(1):251-261.
38. Jarvinen TA, Jarvinen TL, Kaariainen M, Kalimo H, Jarvinen M. Muscle injuries: biology and treatment. Am J Sports Med. 2005;33(5):745-764.
39. Clanton TO, Coupe KJ. Hamstring strains in athletes: diagnosis and treatment. J Am Acad Orthop Surg. 1998;6(4):237-248.
40. Novacheck TF. The biomechanics of running. Gait Posture. 1998;7(1):77-95.
41. Yu B, Queen RM, Abbey AN, Liu Y, Moorman CT, Garrett WE. Hamstring muscle kinematics and activation during overground sprinting. J Biomech. 2008;41(15):3121-3126.
42. Opar DA, Williams MD, Shield AJ. Hamstring strain injuries: factors that lead to injury and re-injury. Sports Med. 2012;42(3):209-226.
43. Askling CM, Tengvar M, Saartok T, Thorstensson A. Acute first-time hamstring strains during high-speed running: a longitudinal study including clinical and magnetic resonance imaging findings. Am J Sports Med. 2007;35(2):197-206.
44. Thelen DG, Chumanov ES, Hoerth DM, et al. Hamstring muscle kinematics during treadmill sprinting. Med Sci Sports Exerc. 2005;37(1):108-114.
45. Chumanov ES, Heiderscheit BC, Thelen DG. The effect of speed and influence of individual muscles on hamstring mechanics during the swing phase of sprinting. J Biomech. 2007;40(16):3555-3562.
46. Rettig AC, Meyer S, Bhadra AK. Platelet-rich plasma in addition to rehabilitation for acute hamstring injuries in NFL players: clinical effects and time to return to play. Orthop J Sports Med. 2013;1(1):2325967113494354.
47. Zvijac JE, Toriscelli TA, Merrick S, Kiebzak GM. Isokinetic concentric quadriceps and hamstring strength variables from the NFL Scouting Combine are not predictive of hamstring injury in first-year professional football players. Am J Sports Med. 2013;41(7):1511-1518.
48. Arnason A, Sigurdsson SB, Gudmundsson A, Holme I, Engebretsen L, Bahr R. Risk factors for injuries in football. Am J Sports Med. 2004;32(1 Suppl):5S-16S.
49. Zarins B, Ciullo JV. Acute muscle and tendon injuries in athletes. Clin Sports Med. 1983;2(1):167-182.
50. Arnason A, Andersen TE, Holme I, Engebretsen L, Bahr R. Prevention of hamstring strains in elite soccer: an intervention study. Scand J Med Sci Sports. 2008;18(1):40-48.
51. Levine WN, Bergfeld JA, Tessendorf W, Moorman CT 3rd. Intramuscular corticosteroid injection for hamstring injuries. A 13-year experience in the National Football League. Am J Sports Med. 2000;28(3):297-300.
52. Croisier JL, Ganteaume S, Binet J, Genty M, Ferret JM. Strength imbalances and prevention of hamstring injury in professional soccer players: a prospective study. Am J Sports Med. 2008;36(8):1469-1475.
53. Drakos M, Birmingham P, Delos D, et al. Corticosteroid and anesthetic injections for muscle strains and ligament sprains in the NFL. HSS J. 2014;10(2):136-142.
54. Worrell TW. Factors associated with hamstring injuries. An approach to treatment and preventative measures. Sports Med. 1994;17(5):338-345.
55. Brooks JH, Fuller CW, Kemp SP, Reddin DB. Incidence, risk, and prevention of hamstring muscle injuries in professional rugby union. Am J Sports Med. 2006;34(8):1297-1306.
56. Verrall GM, Kalairajah Y, Slavotinek JP, Spriggins AJ. Assessment of player performance following return to sport after hamstring muscle strain injury. J Sci Med Sport. 2006;9(1-2):87-90.
57. Matava MJ, Ellis E, Shah NR, Pogue D, Williams T. Morel-lavallee lesion in a professional american football player. Am J Orthop. 2010;39(3):144-147.
58. Tejwani SG, Cohen SB, Bradley JP. Management of Morel-Lavallee lesion of the knee: twenty-seven cases in the national football league. Am J Sports Med. 2007;35(7):1162-1167.
59. Mellado JM, Bencardino JT. Morel-Lavallee lesion: review with emphasis on MR imaging. Magn Reson Imaging Clin N Am. 2005;13(4):775-782.
60. Harma A, Inan M, Ertem K. [The Morel-Lavallee lesion: a conservative approach to closed degloving injuries]. Acta Orthop Traumatol Turc. 2004;38(4):270-273.
61. Tseng S, Tornetta P 3rd. Percutaneous management of Morel-Lavallee lesions. J Bone Joint Surg Am. 2006;88(1):92-96.
62. Gilbert BC, Bui-Mansfield LT, Dejong S. MRI of a Morel-Lavellee lesion. AJR Am J Roentgenol. 2004;182(5):1347-1348.
1. Hootman JM, Dick R, Agel J. Epidemiology of collegiate injuries for 15 sports: summary and recommendations for injury prevention initiatives. J Athl Train. 2007;42(2):311-319.
2. Rechel JA, Yard EE, Comstock RD. An epidemiologic comparison of high school sports injuries sustained in practice and competition. J Athl Train. 2008;43(2):197-204.
3. Elliott MC, Zarins B, Powell JW, Kenyon CD. Hamstring muscle strains in professional football players: a 10-year review. Am J Sports Med. 2011;39(4):843-850.
4. Dick R, Ferrara MS, Agel J, et al. Descriptive epidemiology of collegiate men’s football injuries: National Collegiate Athletic Association Injury Surveillance System, 1988-1989 through 2003-2004. J Athl Train. 2007;42(2):221-233.
5. Feeley BT, Kennelly S, Barnes RP, et al. Epidemiology of National Football League training camp injuries from 1998 to 2007. Am J Sports Med. 2008;36(8):1597-1603.
6. Garrett WE Jr. Muscle strain injuries. Am J Sports Med. 1996;24(6 Suppl):S2-S8.
7. Lawrence DW, Hutchison MG, Comper P. Descriptive epidemiology of musculoskeletal injuries and concussions in the National Football League, 2012-2014. Orthop J Sports Med. 2015;3(5):2325967115583653.
8. Diaz JA, Fischer DA, Rettig AC, Davis TJ, Shelbourne KD. Severe quadriceps muscle contusions in athletes. A report of three cases. Am J Sports Med. 2003;31(2):289-293.
9. Jackson DW, Feagin JA. Quadriceps contusions in young athletes. Relation of severity of injury to treatment and prognosis. J Bone Joint Surg Am. 1973;55(1):95-105.
10. Ryan JB, Wheeler JH, Hopkinson WJ, Arciero RA, Kolakowski KR. Quadriceps contusions. West Point update. Am J Sports Med. 1991;19(3):299-304.
11. Bencardino JT, Rosenberg ZS, Brown RR, Hassankhani A, Lustrin ES, Beltran J. Traumatic musculotendinous injuries of the knee: diagnosis with MR imaging. Radiographics. 2000;20 Spec No:S103-S120.
12. Robinson D, On E, Halperin N. Anterior compartment syndrome of the thigh in athletes--indications for conservative treatment. J Trauma. 1992;32(2):183-186.
13. Beckmann JT, Wylie JD, Kapron AL, Hanson JA, Maak TG, Aoki SK. The effect of NSAID prophylaxis and operative variables on heterotopic ossification after hip arthroscopy. Am J Sports Med. 2014;42(6):1359-1364.
14. Shehab D, Elgazzar AH, Collier BD. Heterotopic ossification. J Nucl Med. 2002;43(3):346-353.
15. Beckmann JT, Wylie JD, Potter MQ, Maak TG, Greene TH, Aoki SK. Effect of naproxen prophylaxis on heterotopic ossification following hip arthroscopy: a double-blind randomized placebo-controlled trial. J Bone Joint Surg Am. 2015;97(24):2032-2037.
16. Yeung M, Jamshidi S, Horner N, Simunovic N, Karlsson J, Ayeni OR. Efficacy of nonsteroidal anti-inflammatory drug prophylaxis for heterotrophic ossification in hip arthroscopy: a systematic review. Arthroscopy. 2016;32(3):519-525.
17. Goyal K, Pettis CR, Bancroft AE, Wasyliw CW, Scherer KF. Myositis ossificans in the thigh of a lacrosse player. Orthopedics. 2015;38(8):468,515-518.
18. Cooper DE. Severe quadriceps muscle contusions in athletes. Am J Sports Med. 2004;32(3):820.
19. Bonsell S, Freudigman PT, Moore HA. Quadriceps muscle contusion resulting in osteomyelitis of the femur in a high school football player. A case report. Am J Sports Med. 2001;29(6):818-820.
20. Rothwell AG. Quadriceps hematoma. A prospective clinical study. Clin Orthop Relat Res. 1982;(171):97-103.
21. Armfield DR, Kim DH, Towers JD, Bradley JP, Robertson DD. Sports-related muscle injury in the lower extremity. Clin Sports Med. 2006;25(4):803-842.
22. Lipscomb AB, Thomas ED, Johnston RK. Treatment of myositis ossificans traumatica in athletes. Am J Sports Med. 1976;4(3):111-120.
23. Mani-Babu S, Wolman R, Keen R. Quadriceps traumatic myositis ossificans in a football player: management with intravenous pamidronate. Clin J Sport Med. 2014;24(5):e56-e58.
24. McCaffrey DD, Clarke J, Bunn J, McCormack MJ. Acute compartment syndrome of the anterior thigh in the absence of fracture secondary to sporting trauma. J Trauma. 2009;66(4):1238-1242.
25. Klasson SC, Vander Schilden JL. Acute anterior thigh compartment syndrome complicating quadriceps hematoma. Two case reports and review of the literature. Orthop Rev. 1990;19(5):421-427.
26. Rooser B. Quadriceps contusion with compartment syndrome. Evacuation of hematoma in 2 cases. Acta Orthop Scand. 1987;58(2):170-172.
27. Rooser B, Bengtson S, Hagglund G. Acute compartment syndrome from anterior thigh muscle contusion: a report of eight cases. J Orthop Trauma. 1991;5(1):57-59.
28. Schwartz JT Jr, Brumback RJ, Lakatos R, Poka A, Bathon GH, Burgess AR. Acute compartment syndrome of the thigh. A spectrum of injury. J Bone Joint Surg Am. 1989;71(3):392-400.
29. Elliott KG, Johnstone AJ. Diagnosing acute compartment syndrome. J Bone Joint Surg Br. 2003;85(5):625-632.
30. Kary JM. Diagnosis and management of quadriceps strains and contusions. Curr Rev Musculoskelet Med. 2010;3(1-4):26-31.
31. Boublik M, Schlegel TF, Koonce RC, Genuario JW, Kinkartz JD. Quadriceps tendon injuries in national football league players. Am J Sports Med. 2013;41(8):1841-1846.
32. Palmer WE, Kuong SJ, Elmadbouh HM. MR imaging of myotendinous strain. AJR Am J Roentgenol. 1999;173(3):703-709.
33. Cross TM, Gibbs N, Houang MT, Cameron M. Acute quadriceps muscle strains: magnetic resonance imaging features and prognosis. Am J Sports Med. 2004;32(3):710-719.
34. Hughes C 4th, Hasselman CT, Best TM, Martinez S, Garrett WE Jr. Incomplete, intrasubstance strain injuries of the rectus femoris muscle. Am J Sports Med. 1995;23(4):500-506.
35. Orchard JW. Intrinsic and extrinsic risk factors for muscle strains in Australian football. Am J Sports Med. 2001;29(3):300-303.36. Mair SD, Seaber AV, Glisson RR, Garrett WE, Jr. The role of fatigue in susceptibility to acute muscle strain injury. Am J Sports Med. 1996;24(2):137-143.
37. Bleakley C, McDonough S, MacAuley D. The use of ice in the treatment of acute soft-tissue injury: a systematic review of randomized controlled trials. Am J Sports Med. 2004;32(1):251-261.
38. Jarvinen TA, Jarvinen TL, Kaariainen M, Kalimo H, Jarvinen M. Muscle injuries: biology and treatment. Am J Sports Med. 2005;33(5):745-764.
39. Clanton TO, Coupe KJ. Hamstring strains in athletes: diagnosis and treatment. J Am Acad Orthop Surg. 1998;6(4):237-248.
40. Novacheck TF. The biomechanics of running. Gait Posture. 1998;7(1):77-95.
41. Yu B, Queen RM, Abbey AN, Liu Y, Moorman CT, Garrett WE. Hamstring muscle kinematics and activation during overground sprinting. J Biomech. 2008;41(15):3121-3126.
42. Opar DA, Williams MD, Shield AJ. Hamstring strain injuries: factors that lead to injury and re-injury. Sports Med. 2012;42(3):209-226.
43. Askling CM, Tengvar M, Saartok T, Thorstensson A. Acute first-time hamstring strains during high-speed running: a longitudinal study including clinical and magnetic resonance imaging findings. Am J Sports Med. 2007;35(2):197-206.
44. Thelen DG, Chumanov ES, Hoerth DM, et al. Hamstring muscle kinematics during treadmill sprinting. Med Sci Sports Exerc. 2005;37(1):108-114.
45. Chumanov ES, Heiderscheit BC, Thelen DG. The effect of speed and influence of individual muscles on hamstring mechanics during the swing phase of sprinting. J Biomech. 2007;40(16):3555-3562.
46. Rettig AC, Meyer S, Bhadra AK. Platelet-rich plasma in addition to rehabilitation for acute hamstring injuries in NFL players: clinical effects and time to return to play. Orthop J Sports Med. 2013;1(1):2325967113494354.
47. Zvijac JE, Toriscelli TA, Merrick S, Kiebzak GM. Isokinetic concentric quadriceps and hamstring strength variables from the NFL Scouting Combine are not predictive of hamstring injury in first-year professional football players. Am J Sports Med. 2013;41(7):1511-1518.
48. Arnason A, Sigurdsson SB, Gudmundsson A, Holme I, Engebretsen L, Bahr R. Risk factors for injuries in football. Am J Sports Med. 2004;32(1 Suppl):5S-16S.
49. Zarins B, Ciullo JV. Acute muscle and tendon injuries in athletes. Clin Sports Med. 1983;2(1):167-182.
50. Arnason A, Andersen TE, Holme I, Engebretsen L, Bahr R. Prevention of hamstring strains in elite soccer: an intervention study. Scand J Med Sci Sports. 2008;18(1):40-48.
51. Levine WN, Bergfeld JA, Tessendorf W, Moorman CT 3rd. Intramuscular corticosteroid injection for hamstring injuries. A 13-year experience in the National Football League. Am J Sports Med. 2000;28(3):297-300.
52. Croisier JL, Ganteaume S, Binet J, Genty M, Ferret JM. Strength imbalances and prevention of hamstring injury in professional soccer players: a prospective study. Am J Sports Med. 2008;36(8):1469-1475.
53. Drakos M, Birmingham P, Delos D, et al. Corticosteroid and anesthetic injections for muscle strains and ligament sprains in the NFL. HSS J. 2014;10(2):136-142.
54. Worrell TW. Factors associated with hamstring injuries. An approach to treatment and preventative measures. Sports Med. 1994;17(5):338-345.
55. Brooks JH, Fuller CW, Kemp SP, Reddin DB. Incidence, risk, and prevention of hamstring muscle injuries in professional rugby union. Am J Sports Med. 2006;34(8):1297-1306.
56. Verrall GM, Kalairajah Y, Slavotinek JP, Spriggins AJ. Assessment of player performance following return to sport after hamstring muscle strain injury. J Sci Med Sport. 2006;9(1-2):87-90.
57. Matava MJ, Ellis E, Shah NR, Pogue D, Williams T. Morel-lavallee lesion in a professional american football player. Am J Orthop. 2010;39(3):144-147.
58. Tejwani SG, Cohen SB, Bradley JP. Management of Morel-Lavallee lesion of the knee: twenty-seven cases in the national football league. Am J Sports Med. 2007;35(7):1162-1167.
59. Mellado JM, Bencardino JT. Morel-Lavallee lesion: review with emphasis on MR imaging. Magn Reson Imaging Clin N Am. 2005;13(4):775-782.
60. Harma A, Inan M, Ertem K. [The Morel-Lavallee lesion: a conservative approach to closed degloving injuries]. Acta Orthop Traumatol Turc. 2004;38(4):270-273.
61. Tseng S, Tornetta P 3rd. Percutaneous management of Morel-Lavallee lesions. J Bone Joint Surg Am. 2006;88(1):92-96.
62. Gilbert BC, Bui-Mansfield LT, Dejong S. MRI of a Morel-Lavellee lesion. AJR Am J Roentgenol. 2004;182(5):1347-1348.
Back to Basics: The Role of the Team Physician
Editor’s Note: AJO Deputy Editor-in-Chief Robin West, MD, is the Head Team Physician for the Washington Redskins and the Washington Nationals. She has previously served as a team physician for 2 Super Bowl-winning Pittsburgh Steelers teams. I am pleased to “hand off” this issue to her.
—Bryan T. Hanypsiak, MD
The summer is over, football season has begun, and team physicians are busy trying to manage and treat the plethora of injuries that come with the game. Football is one of the most popular sports played by young athletes. Youth participation (ages 6-14 years) in tackle football was 2.169 million in 2015, according to a study conducted by the Physical Activity Council and presented by USA Football. There were 1.084 million boys (and 1500 girls) playing high school football in the 2014-2015 season, nearly twice the number of the next most popular sport, track and field, according to the National Federation of State High School Associations.Due to the sheer volume of athletes and high-impact nature of the game, football leads all other sports in the number of sustained injuries.
Team physicians have the leadership role in the organization, management, and provision of care of the athletes on the team. The roles and responsibilities of the team physician are ever-evolving. The team physician has to meet certain medical qualifications and education requirements, and understand the ethical and medicolegal issues.
The American Academy of Orthopaedic Surgeons and several other medical associations have put together a Team Physician Consensus Statement (available at http://bit.ly/2b8rOzS). All team physicians, coaches, and athletic trainers should read and understand this statement, as it delineates the qualifications, duties, and responsibilities of the team physician.
Our Football Issue focuses on the most common injuries that the team physician will encounter during the season. Our goal is to create a comprehensive guide for the team physician on the acute management of these injuries. As team physicians, we have to make quick return-to-play decisions that are often difficult, as we are dealing with extremely competitive athletes and coaches in the heat of the moment. Since we can’t control the high levels of adrenalin, loud stadium, or rapid speed of the game, we need to be prepared to perform a comprehensive evaluation and diagnosis under these circumstances. This return-to-play decision should be based solely on the severity of the injury and safety of the player. As a team physician, you are responsible for making the “final call” on when the player is safe to return to the game.
This issue includes a section on the most common medical issues (ophthalmology, dental, and dermatology), concussion, exertional heat stroke, knee injuries, and foot and ankle injuries. We also have a special list of the most common items to include in the athletic trainer’s medical bag when covering a high school or collegiate football game (see page 376). Our prominent contributing authors all have extensive experience covering high school, collegiate, and professional teams.
I hope that our Football Issue helps you to keep your athletes safe and injury-free, which is necessary to have a successful season. Remember, as the team physician, your primary focus is the well being of the players. The success of the team only comes when the players are healthy. A cohesive, well-organized medical team, led by the head athletic trainer and team physician, is a key component to the care of the athletes. It truly takes a village to provide top-notch medical care to a football team.
Am J Orthop. 2016;45(6):338. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Editor’s Note: AJO Deputy Editor-in-Chief Robin West, MD, is the Head Team Physician for the Washington Redskins and the Washington Nationals. She has previously served as a team physician for 2 Super Bowl-winning Pittsburgh Steelers teams. I am pleased to “hand off” this issue to her.
—Bryan T. Hanypsiak, MD
The summer is over, football season has begun, and team physicians are busy trying to manage and treat the plethora of injuries that come with the game. Football is one of the most popular sports played by young athletes. Youth participation (ages 6-14 years) in tackle football was 2.169 million in 2015, according to a study conducted by the Physical Activity Council and presented by USA Football. There were 1.084 million boys (and 1500 girls) playing high school football in the 2014-2015 season, nearly twice the number of the next most popular sport, track and field, according to the National Federation of State High School Associations.Due to the sheer volume of athletes and high-impact nature of the game, football leads all other sports in the number of sustained injuries.
Team physicians have the leadership role in the organization, management, and provision of care of the athletes on the team. The roles and responsibilities of the team physician are ever-evolving. The team physician has to meet certain medical qualifications and education requirements, and understand the ethical and medicolegal issues.
The American Academy of Orthopaedic Surgeons and several other medical associations have put together a Team Physician Consensus Statement (available at http://bit.ly/2b8rOzS). All team physicians, coaches, and athletic trainers should read and understand this statement, as it delineates the qualifications, duties, and responsibilities of the team physician.
Our Football Issue focuses on the most common injuries that the team physician will encounter during the season. Our goal is to create a comprehensive guide for the team physician on the acute management of these injuries. As team physicians, we have to make quick return-to-play decisions that are often difficult, as we are dealing with extremely competitive athletes and coaches in the heat of the moment. Since we can’t control the high levels of adrenalin, loud stadium, or rapid speed of the game, we need to be prepared to perform a comprehensive evaluation and diagnosis under these circumstances. This return-to-play decision should be based solely on the severity of the injury and safety of the player. As a team physician, you are responsible for making the “final call” on when the player is safe to return to the game.
This issue includes a section on the most common medical issues (ophthalmology, dental, and dermatology), concussion, exertional heat stroke, knee injuries, and foot and ankle injuries. We also have a special list of the most common items to include in the athletic trainer’s medical bag when covering a high school or collegiate football game (see page 376). Our prominent contributing authors all have extensive experience covering high school, collegiate, and professional teams.
I hope that our Football Issue helps you to keep your athletes safe and injury-free, which is necessary to have a successful season. Remember, as the team physician, your primary focus is the well being of the players. The success of the team only comes when the players are healthy. A cohesive, well-organized medical team, led by the head athletic trainer and team physician, is a key component to the care of the athletes. It truly takes a village to provide top-notch medical care to a football team.
Am J Orthop. 2016;45(6):338. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Editor’s Note: AJO Deputy Editor-in-Chief Robin West, MD, is the Head Team Physician for the Washington Redskins and the Washington Nationals. She has previously served as a team physician for 2 Super Bowl-winning Pittsburgh Steelers teams. I am pleased to “hand off” this issue to her.
—Bryan T. Hanypsiak, MD
The summer is over, football season has begun, and team physicians are busy trying to manage and treat the plethora of injuries that come with the game. Football is one of the most popular sports played by young athletes. Youth participation (ages 6-14 years) in tackle football was 2.169 million in 2015, according to a study conducted by the Physical Activity Council and presented by USA Football. There were 1.084 million boys (and 1500 girls) playing high school football in the 2014-2015 season, nearly twice the number of the next most popular sport, track and field, according to the National Federation of State High School Associations.Due to the sheer volume of athletes and high-impact nature of the game, football leads all other sports in the number of sustained injuries.
Team physicians have the leadership role in the organization, management, and provision of care of the athletes on the team. The roles and responsibilities of the team physician are ever-evolving. The team physician has to meet certain medical qualifications and education requirements, and understand the ethical and medicolegal issues.
The American Academy of Orthopaedic Surgeons and several other medical associations have put together a Team Physician Consensus Statement (available at http://bit.ly/2b8rOzS). All team physicians, coaches, and athletic trainers should read and understand this statement, as it delineates the qualifications, duties, and responsibilities of the team physician.
Our Football Issue focuses on the most common injuries that the team physician will encounter during the season. Our goal is to create a comprehensive guide for the team physician on the acute management of these injuries. As team physicians, we have to make quick return-to-play decisions that are often difficult, as we are dealing with extremely competitive athletes and coaches in the heat of the moment. Since we can’t control the high levels of adrenalin, loud stadium, or rapid speed of the game, we need to be prepared to perform a comprehensive evaluation and diagnosis under these circumstances. This return-to-play decision should be based solely on the severity of the injury and safety of the player. As a team physician, you are responsible for making the “final call” on when the player is safe to return to the game.
This issue includes a section on the most common medical issues (ophthalmology, dental, and dermatology), concussion, exertional heat stroke, knee injuries, and foot and ankle injuries. We also have a special list of the most common items to include in the athletic trainer’s medical bag when covering a high school or collegiate football game (see page 376). Our prominent contributing authors all have extensive experience covering high school, collegiate, and professional teams.
I hope that our Football Issue helps you to keep your athletes safe and injury-free, which is necessary to have a successful season. Remember, as the team physician, your primary focus is the well being of the players. The success of the team only comes when the players are healthy. A cohesive, well-organized medical team, led by the head athletic trainer and team physician, is a key component to the care of the athletes. It truly takes a village to provide top-notch medical care to a football team.
Am J Orthop. 2016;45(6):338. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Collagen Meniscus Implant
Ivy Sports Medicine (http://www.ivysportsmed.com/en)
Collagen Meniscus Implant
The number of patients undergoing arthroscopic partial meniscectomy has continued to increase. However, this is potentially not a benign procedure, as there are increased contact pressures on the articular cartilage even with the removal of only a segment of the meniscus.
The Collagen Meniscus Implant (CMI, Ivy Sports Medicine) is a resorbable and biocompatible Type I collagen matrix that was developed to restore the segmental loss of meniscal tissue in the knee. It consists of a porous cross-linked matrix scaffold that allows for the ingrowth of the body’s own cells. The CMI is the only meniscal implant composed of purely biological materials and is available in an off-the-shelf supply.
The CMI is available in the United States for use in the restoration of segmental loss of the medial meniscus. The CMI can be utilized in either an acute or chronic situation. In the acute case, it would be indicated when the medial meniscus is irreparable, and that segment must be removed. In the chronic case, the patient would have had a previous partial meniscectomy and/or failed meniscus repair and had developed either pain or signs of early articular cartilage wear in the compartment. The procedure can be done arthroscopically and as an outpatient. The CMI can be kept on the shelf to be available as needed; it has a 2-year shelf life. There are specialized instruments for measuring the length of implant needed and for delivery of the implant.
The CMI has been utilized clinically for 18 years with excellent clinical results. Patients treated with CMI have benefited in over 80% of cases. Studies have demonstrated improved knee function, activity levels, and pain values from the pre- to postoperative periods.1,2 In addition, functional improvements have been maintained for over 10 years. The reoperation rate has been demonstrated to be 10% to 20%, which is comparable to the reoperation rate after meniscal repair.
Surgical pearl: The surgical technique for insertion of the CMI is relatively uncomplicated (Figures A, B).
The second step is to measure the length of your meniscus defect with the measuring rod.
Once measured, you want to oversize the implant 10% to 15% (ie, if you measure 30 mm, you will cut at least 34 mm). Use the measuring rod to measure the length of the CMI and mark your length. Use a new scalpel blade to cut the CMI.
Place the measured CMI into the delivery clamp and insert through a mini-arthrotomy into the meniscal defect. The fixation technique of the CMI is entirely up to the implanting surgeon. Most surgeons have used a combination of all-inside and inside-out meniscus repair techniques. It is recommended to start fixing the CMI first posteriorly. The posterior stitch is usually an all-inside horizontal mattress stitch. Coming 1 cm anteriorly, place a vertical mattress stitch. Continue this method sequentially while moving anteriorly. The anterior suture is the surgeon’s choice for device, but it should be a horizontal mattress like the most posterior stitch. It is important while tightening your suture tension to apply the concept of “approximated and not strangulated.” Once completed, close wounds in typical fashion.
1. Zaffagnini S, Marcheggiani Muccioli GM, Lopomo N, et al. Prospective long-term outcomes of the medial collagen meniscus implant versus partial medial meniscectomy: a minimum 10-year follow-up study. Am J Sports Med. 2011;39(5):977-985
2. Bulgheroni P, Murena L, Ratti C, Bulgheroni E, Ronga M, Cherubino P. Follow-up of collagen meniscus implant patients: clinical, radiological, and magnetic resonance imaging results at 5 years. Knee. 2010;17(3):224-229.
Ivy Sports Medicine (http://www.ivysportsmed.com/en)
Collagen Meniscus Implant
The number of patients undergoing arthroscopic partial meniscectomy has continued to increase. However, this is potentially not a benign procedure, as there are increased contact pressures on the articular cartilage even with the removal of only a segment of the meniscus.
The Collagen Meniscus Implant (CMI, Ivy Sports Medicine) is a resorbable and biocompatible Type I collagen matrix that was developed to restore the segmental loss of meniscal tissue in the knee. It consists of a porous cross-linked matrix scaffold that allows for the ingrowth of the body’s own cells. The CMI is the only meniscal implant composed of purely biological materials and is available in an off-the-shelf supply.
The CMI is available in the United States for use in the restoration of segmental loss of the medial meniscus. The CMI can be utilized in either an acute or chronic situation. In the acute case, it would be indicated when the medial meniscus is irreparable, and that segment must be removed. In the chronic case, the patient would have had a previous partial meniscectomy and/or failed meniscus repair and had developed either pain or signs of early articular cartilage wear in the compartment. The procedure can be done arthroscopically and as an outpatient. The CMI can be kept on the shelf to be available as needed; it has a 2-year shelf life. There are specialized instruments for measuring the length of implant needed and for delivery of the implant.
The CMI has been utilized clinically for 18 years with excellent clinical results. Patients treated with CMI have benefited in over 80% of cases. Studies have demonstrated improved knee function, activity levels, and pain values from the pre- to postoperative periods.1,2 In addition, functional improvements have been maintained for over 10 years. The reoperation rate has been demonstrated to be 10% to 20%, which is comparable to the reoperation rate after meniscal repair.
Surgical pearl: The surgical technique for insertion of the CMI is relatively uncomplicated (Figures A, B).
The second step is to measure the length of your meniscus defect with the measuring rod.
Once measured, you want to oversize the implant 10% to 15% (ie, if you measure 30 mm, you will cut at least 34 mm). Use the measuring rod to measure the length of the CMI and mark your length. Use a new scalpel blade to cut the CMI.
Place the measured CMI into the delivery clamp and insert through a mini-arthrotomy into the meniscal defect. The fixation technique of the CMI is entirely up to the implanting surgeon. Most surgeons have used a combination of all-inside and inside-out meniscus repair techniques. It is recommended to start fixing the CMI first posteriorly. The posterior stitch is usually an all-inside horizontal mattress stitch. Coming 1 cm anteriorly, place a vertical mattress stitch. Continue this method sequentially while moving anteriorly. The anterior suture is the surgeon’s choice for device, but it should be a horizontal mattress like the most posterior stitch. It is important while tightening your suture tension to apply the concept of “approximated and not strangulated.” Once completed, close wounds in typical fashion.
Ivy Sports Medicine (http://www.ivysportsmed.com/en)
Collagen Meniscus Implant
The number of patients undergoing arthroscopic partial meniscectomy has continued to increase. However, this is potentially not a benign procedure, as there are increased contact pressures on the articular cartilage even with the removal of only a segment of the meniscus.
The Collagen Meniscus Implant (CMI, Ivy Sports Medicine) is a resorbable and biocompatible Type I collagen matrix that was developed to restore the segmental loss of meniscal tissue in the knee. It consists of a porous cross-linked matrix scaffold that allows for the ingrowth of the body’s own cells. The CMI is the only meniscal implant composed of purely biological materials and is available in an off-the-shelf supply.
The CMI is available in the United States for use in the restoration of segmental loss of the medial meniscus. The CMI can be utilized in either an acute or chronic situation. In the acute case, it would be indicated when the medial meniscus is irreparable, and that segment must be removed. In the chronic case, the patient would have had a previous partial meniscectomy and/or failed meniscus repair and had developed either pain or signs of early articular cartilage wear in the compartment. The procedure can be done arthroscopically and as an outpatient. The CMI can be kept on the shelf to be available as needed; it has a 2-year shelf life. There are specialized instruments for measuring the length of implant needed and for delivery of the implant.
The CMI has been utilized clinically for 18 years with excellent clinical results. Patients treated with CMI have benefited in over 80% of cases. Studies have demonstrated improved knee function, activity levels, and pain values from the pre- to postoperative periods.1,2 In addition, functional improvements have been maintained for over 10 years. The reoperation rate has been demonstrated to be 10% to 20%, which is comparable to the reoperation rate after meniscal repair.
Surgical pearl: The surgical technique for insertion of the CMI is relatively uncomplicated (Figures A, B).
The second step is to measure the length of your meniscus defect with the measuring rod.
Once measured, you want to oversize the implant 10% to 15% (ie, if you measure 30 mm, you will cut at least 34 mm). Use the measuring rod to measure the length of the CMI and mark your length. Use a new scalpel blade to cut the CMI.
Place the measured CMI into the delivery clamp and insert through a mini-arthrotomy into the meniscal defect. The fixation technique of the CMI is entirely up to the implanting surgeon. Most surgeons have used a combination of all-inside and inside-out meniscus repair techniques. It is recommended to start fixing the CMI first posteriorly. The posterior stitch is usually an all-inside horizontal mattress stitch. Coming 1 cm anteriorly, place a vertical mattress stitch. Continue this method sequentially while moving anteriorly. The anterior suture is the surgeon’s choice for device, but it should be a horizontal mattress like the most posterior stitch. It is important while tightening your suture tension to apply the concept of “approximated and not strangulated.” Once completed, close wounds in typical fashion.
1. Zaffagnini S, Marcheggiani Muccioli GM, Lopomo N, et al. Prospective long-term outcomes of the medial collagen meniscus implant versus partial medial meniscectomy: a minimum 10-year follow-up study. Am J Sports Med. 2011;39(5):977-985
2. Bulgheroni P, Murena L, Ratti C, Bulgheroni E, Ronga M, Cherubino P. Follow-up of collagen meniscus implant patients: clinical, radiological, and magnetic resonance imaging results at 5 years. Knee. 2010;17(3):224-229.
1. Zaffagnini S, Marcheggiani Muccioli GM, Lopomo N, et al. Prospective long-term outcomes of the medial collagen meniscus implant versus partial medial meniscectomy: a minimum 10-year follow-up study. Am J Sports Med. 2011;39(5):977-985
2. Bulgheroni P, Murena L, Ratti C, Bulgheroni E, Ronga M, Cherubino P. Follow-up of collagen meniscus implant patients: clinical, radiological, and magnetic resonance imaging results at 5 years. Knee. 2010;17(3):224-229.
Setting Up Your New Physician for Success
Practices and hospitals invest significant time and money in recruiting a new physician. From phone interviews to site visits to contract negotiations, it’s a long and involved process.
Beyond setting up a new physician’s office and appointment schedule, completing human resources paperwork, and ordering business cards, what does your practice do to support new physicians to ensure they are successful? Although a new colleague may arrive with excellent clinical skills, even the most promising surgeon can fall short if not provided with the right expectations, training, and collegial support. Here’s how to fast track your new physician to professional heights.
Credentialing Is Key
At the crux of a new physician’s success is credentialing him or her with hospitals and insurance plans before the official start date to see patients.
“A state medical license is the first domino,” says orthopedic surgeon Michael R. Marks, MD, MBA, consultant and coding educator with KarenZupko & Associates, Inc. Marks has led or participated in physician recruitment in orthopedic and multispecialty groups. The firm has developed a comprehensive New Physician Onboarding Checklist, available at https://www.karenzupko.com/new-physician-onboarding-checklist/.
“Without a medical license,” Marks continues, “you can’t get the new physician hospital privileges and you can’t get him or her credentialed with plans. Without being credentialed, the physician can’t bill for patients treated.” Because commercial carriers won’t allow retrospective billing for services already rendered, “even a 3-month delay in credentialing could cost an orthopedic practice $60,000 to $180,000 in lost revenue.”
And if you think you can bill the new physician’s services under another partner’s name, you are incorrect. “The billing physician will have signed the note, but not have treated the patient,” warns Marks. “This is improper billing. Don’t do it.”
The remedy for ensuring that the new physician is credentialed is simple: get organized and plan ahead.
“When I first started participating in recruitment, I remember telling physicians, ‘I need you tomorrow!’” admits Amon T. Ferry, MD, a practicing orthopedist who leads recruitment efforts at IMS Orthopedics, a division of Integrated Medical Specialists in Phoenix, Arizona. “So they’d get hired before the practice was prepared and before credentialing was completed. Now, I set more realistic expectations,” he says, noting that in Arizona it takes 3 months to get a medical license, 6 months to contract with the hospital, and 9 months to get on insurance plans. And even after a plan has credentialed a new physician, “sometimes it still takes 4 to 6 weeks before the physician’s data is loaded into the plan’s computer systems.”
“The way to do credentialing right is to get all departments communicating,” Marks says. “If you keep everyone siloed, staff don’t understand that a lack of timeliness on their part impacts other areas of the practice.”
Ferry agrees, and says his group learned to organize its multiple departments after making mistakes and missing deadlines. “We now have an 8-page pre-employment application for new physicians,” he explains. “In addition to asking for contact information and everything we need to know in order to get the physician credentialed, we ask questions about malpractice suit history and whether there are issues with the medical board. We also ask about gaps in employment and details about where the physician has practiced in the past.” All of this is done to identify early whether credentialing will require more time and effort. Ferry says that the application has solved a number of processing problems the practice had in the past.
And whether credentialing is done within the practice or outsourced, Ferry says that it pays to be persistent. “Don’t sit back and assume it will get done. Even if you have outsourced credentialing to a company, someone must check with payers and hospitals weekly and provide the practice a status update.”
In one case, when getting a new physician contracted at a hospital was taking forever, Ferry directed the staff to call. “Turns out, they had been trying to reach us and had the wrong phone number,” he says. “When people are processing thousands of physician renewals, things get lost. You have to be proactive and be your own advocate. Don’t be afraid to be the squeaky wheel.”
Staff Relationships and Operational Wisdom
Marks points out that in many practices, the new physician is shown the examination rooms and his or her office, gets electronic health record (EHR) training, and that’s it. To be successful, Marks insists that the new physician must build relationships with personnel and understand operational basics. “In other business industries, successful leaders understand at least the basics of what everyone does. Part of how they do this is by getting to know the employees.”
Ideally, Marks advises that new physicians spend time with each staff member. “The best time to do this is in the first few weeks of employment,” he suggests. “Odds are, the new orthopedist doesn’t have 40 patients a day on the schedule. So schedule conversations within the first few weeks or month, and schedule observation time as well. When a patient complains about check-in, the physician will have an understanding of how things work up there if he or she knows the basic processes.” The new doctor should also spend time in the billing office getting to know the challenges faced by staff, and sit with the surgery coordinator to understand the process of getting cases booked and scheduled.
Plan for an initial and then periodic meetings with the practice administrator and other supervisors. Transparency about business operations, data, and strategy will help the new physician get up to speed faster.
“The executive director of our group was an absolutely invaluable information resource,” says Kathryn J. McCarthy, MD, an orthopedic spine surgeon with Arkansas Specialty Orthopaedics in Little Rock, Arkansas. McCarthy has been with the group for 3 years.
The practice’s executive director developed and presented a PowerPoint (Microsoft) explaining general business procedures, expectations for the coding and billing process, and pertinent compliance and risk issues. She had also developed an interactive model of the compensation formula and buy-in program, using Excel (Microsoft). McCarthy met with the executive director at 3 months, 6 months, and 9 months to review her patient and case volumes and how they were trending against the estimates made about her income, bonus, and buy-in status.
From the new physician’s perspective, McCarthy says having the new physician understand the complexities of certain business systems helps them understand things better. “If you sit in the business meetings long enough, you figure it out,” she says, “but it would have made some of the growing pains less painful if I understood what my overhead charge was going to, or more about the workflow of the clinic.” She adds that an overview of hospital relationships and any overlapping ownership interests will benefit new physicians as well.
“I think it’s useful to provide new physicians with a history of the practice and the vision of where things are going,” McCarthy says. “It’s important to outline the business vision, especially for subspecialties. If you explain to the new physician where you want to grow and when the practice plans on bringing on the next physician, it could really drive someone to grow their practice.”
Don’t Underestimate the Need for Coding Training
“When fellows come out of training, they are comfortable with clinical activity but uncomfortable with business administration,” Marks says. “And we know they don’t get training on coding and billing.”
Marks cites a recent conversation at an American Academy of Orthopaedic Surgeons (AAOS) coding workshop. “A surgeon new in practice told me, ‘I’ve been in practice for 4 months. I understand the clinical side but nobody educated me about coding and billing before this course.’” Practices must provide new physicians with coding and documentation training, and coach them to make sure they feel up to speed and comfortable. “The practice’s future revenue depends on it,” Marks says.
McCarthy agrees. “Having an administrative mentorship for coding is incredibly valuable. They don’t teach it in school.”
So from a practical standpoint, purchase AAOS’ Orthopaedic Code-X, a software tool that will help the new physician navigate and integrate Current Procedural Terminology (CPT), ICD-10 (International Classification of Diseases, Tenth Revision), and other coding data easily and accurately. Send him or her to one of the Academy’s regional coding and reimbursement workshops as well. “It will behoove the practice to send them even before they start seeing patients,” Marks says.
And don’t just stop there. High-performing groups conduct peer reviews of evaluation and management (E/M) and operative notes, blinding the codes billed and discussing which CPT and ICD-10 codes are appropriate for the visit or case. “It will take time for the new physician to completely integrate coding with their clinical care,” says Marks. “Peer review sessions, as well as having a partner review codes before they go to the billing office, can help speed learning.”
Collegial Coaching Counts
The week before her official start day, Mc-Carthy scrubbed in as a first assist with each of her new partners. “It was a great way to start ramping up,” she says. “I could see what kind of equipment was present in the hospitals, and got a touch point for hospital logistics. Plus, as a young surgeon it’s great to see how your skill sets match up with your new partners, and which best practices are being deployed by the group.”
This kind of “collegial coaching” is a vital part of the clinical and cultural integration to the practice. Beyond providing clinical support, it builds relationships and trust among the group, and fosters collaboration.
Arkansas Specialty Orthopaedics organized McCarthy’s clinic and operating room (OR) schedules so that a partner was always present. “There was also someone I could bounce ideas off of,” McCarthy explains. “Every day in the OR, there was a partner there at the same time. If I got into a sticky situation, one of my colleagues was willing to come in and scrub in the OR.”
McCarthy says that patients responded favorably when she told them her plan was developed in conjunction with her partners. “Patients find comfort in knowing that several people’s opinions were considered,” she says. “And as a young surgeon, knowing that you have backup, even if you don’t use it, when caring for high-risk and complex cases really means a lot,” she says.
And although her group didn’t offer a formal mentoring program, McCarthy found that an informal mentorship grew organically when a friendship developed with one of her new partners. “In the first 6 months, every single weekend we sat by the pool and rolled through a ton of cases,” she says. “That was fabulous and it alleviated so much stress for me.” And when it was time for McCarthy to move into board case selection, this colleague and another were instrumental in her board preparation because, “they knew my style and where I would need to focus.”
IMS Orthopedics’ approach is to provide the staff and systems that allow new physicians to step up and take responsibility. “If they want to scrub in with me, that’s great. If they’d like to visit additional facilities and get the lay of the land, we encourage it. But we don’t do a lot of handholding. We set them up for success and make sure people are in place to help them,” says Ferry.
A Marketing Plan Is a Must
“The vast majority of practices do very little when it comes to thinking about how to market and build the practice of their new physician,” Marks says. “Practice-building is more of a challenge for surgical specialists today than it was in the old days when new surgeons could easily meet internists as they were rounding at the hospital. Now, a new physician and the practice must come up with a game plan.”
That game plan starts with the easy things: order business cards, schedule a photo shoot, and update the practice’s Web site pages with the physician’s biography and an introductory video. But with social media, online reviews, and subspecialty competition, Marks says practices must think beyond the basics. Think through each element of marketing, from online to outreach to developing referral relationships.
“I tell practices to draft a written marketing plan,” he says. “Not only does it provide a roadmap for the new physician, but also indicates that the practice has put some thought into how he or she can build a practice. It can make the new physician feel less overwhelmed knowing that he or she doesn’t have to do the marketing alone.” Once you’ve developed a list of actions, Marks suggests creating a spreadsheet with deadlines, and ensuring each action is completed.
McCarthy was scheduled to visit family practice clinics, and joined by the administrator who “handed out cookies and cards while I talked,” she says. Arkansas Specialty Orthopaedics also hired an external marketing firm to develop promotional opportunities for her. For example, “I was scheduled to appear on news channels, where I discussed new and interesting procedures,” she says. “It got my name out into the community.”
If your practice is too small to hire an outside firm, Marks suggests reaching out to agencies such as nursing homes, fitness centers, or the YMCA, which frequently offers educational programs for members. “Contact the administrators or medical directors in these organizations. A few minutes on the phone or a short visit can go a long way to building these relationships and getting your new physician on the map.”
As the old saying goes, an ounce of prevention is worth a pound of cure. Scheduling time for orientation, training, staff integration, and collegial coaching will speed up a new physician’s integration into the practice, and increase his or her opportunity for success.
Practices and hospitals invest significant time and money in recruiting a new physician. From phone interviews to site visits to contract negotiations, it’s a long and involved process.
Beyond setting up a new physician’s office and appointment schedule, completing human resources paperwork, and ordering business cards, what does your practice do to support new physicians to ensure they are successful? Although a new colleague may arrive with excellent clinical skills, even the most promising surgeon can fall short if not provided with the right expectations, training, and collegial support. Here’s how to fast track your new physician to professional heights.
Credentialing Is Key
At the crux of a new physician’s success is credentialing him or her with hospitals and insurance plans before the official start date to see patients.
“A state medical license is the first domino,” says orthopedic surgeon Michael R. Marks, MD, MBA, consultant and coding educator with KarenZupko & Associates, Inc. Marks has led or participated in physician recruitment in orthopedic and multispecialty groups. The firm has developed a comprehensive New Physician Onboarding Checklist, available at https://www.karenzupko.com/new-physician-onboarding-checklist/.
“Without a medical license,” Marks continues, “you can’t get the new physician hospital privileges and you can’t get him or her credentialed with plans. Without being credentialed, the physician can’t bill for patients treated.” Because commercial carriers won’t allow retrospective billing for services already rendered, “even a 3-month delay in credentialing could cost an orthopedic practice $60,000 to $180,000 in lost revenue.”
And if you think you can bill the new physician’s services under another partner’s name, you are incorrect. “The billing physician will have signed the note, but not have treated the patient,” warns Marks. “This is improper billing. Don’t do it.”
The remedy for ensuring that the new physician is credentialed is simple: get organized and plan ahead.
“When I first started participating in recruitment, I remember telling physicians, ‘I need you tomorrow!’” admits Amon T. Ferry, MD, a practicing orthopedist who leads recruitment efforts at IMS Orthopedics, a division of Integrated Medical Specialists in Phoenix, Arizona. “So they’d get hired before the practice was prepared and before credentialing was completed. Now, I set more realistic expectations,” he says, noting that in Arizona it takes 3 months to get a medical license, 6 months to contract with the hospital, and 9 months to get on insurance plans. And even after a plan has credentialed a new physician, “sometimes it still takes 4 to 6 weeks before the physician’s data is loaded into the plan’s computer systems.”
“The way to do credentialing right is to get all departments communicating,” Marks says. “If you keep everyone siloed, staff don’t understand that a lack of timeliness on their part impacts other areas of the practice.”
Ferry agrees, and says his group learned to organize its multiple departments after making mistakes and missing deadlines. “We now have an 8-page pre-employment application for new physicians,” he explains. “In addition to asking for contact information and everything we need to know in order to get the physician credentialed, we ask questions about malpractice suit history and whether there are issues with the medical board. We also ask about gaps in employment and details about where the physician has practiced in the past.” All of this is done to identify early whether credentialing will require more time and effort. Ferry says that the application has solved a number of processing problems the practice had in the past.
And whether credentialing is done within the practice or outsourced, Ferry says that it pays to be persistent. “Don’t sit back and assume it will get done. Even if you have outsourced credentialing to a company, someone must check with payers and hospitals weekly and provide the practice a status update.”
In one case, when getting a new physician contracted at a hospital was taking forever, Ferry directed the staff to call. “Turns out, they had been trying to reach us and had the wrong phone number,” he says. “When people are processing thousands of physician renewals, things get lost. You have to be proactive and be your own advocate. Don’t be afraid to be the squeaky wheel.”
Staff Relationships and Operational Wisdom
Marks points out that in many practices, the new physician is shown the examination rooms and his or her office, gets electronic health record (EHR) training, and that’s it. To be successful, Marks insists that the new physician must build relationships with personnel and understand operational basics. “In other business industries, successful leaders understand at least the basics of what everyone does. Part of how they do this is by getting to know the employees.”
Ideally, Marks advises that new physicians spend time with each staff member. “The best time to do this is in the first few weeks of employment,” he suggests. “Odds are, the new orthopedist doesn’t have 40 patients a day on the schedule. So schedule conversations within the first few weeks or month, and schedule observation time as well. When a patient complains about check-in, the physician will have an understanding of how things work up there if he or she knows the basic processes.” The new doctor should also spend time in the billing office getting to know the challenges faced by staff, and sit with the surgery coordinator to understand the process of getting cases booked and scheduled.
Plan for an initial and then periodic meetings with the practice administrator and other supervisors. Transparency about business operations, data, and strategy will help the new physician get up to speed faster.
“The executive director of our group was an absolutely invaluable information resource,” says Kathryn J. McCarthy, MD, an orthopedic spine surgeon with Arkansas Specialty Orthopaedics in Little Rock, Arkansas. McCarthy has been with the group for 3 years.
The practice’s executive director developed and presented a PowerPoint (Microsoft) explaining general business procedures, expectations for the coding and billing process, and pertinent compliance and risk issues. She had also developed an interactive model of the compensation formula and buy-in program, using Excel (Microsoft). McCarthy met with the executive director at 3 months, 6 months, and 9 months to review her patient and case volumes and how they were trending against the estimates made about her income, bonus, and buy-in status.
From the new physician’s perspective, McCarthy says having the new physician understand the complexities of certain business systems helps them understand things better. “If you sit in the business meetings long enough, you figure it out,” she says, “but it would have made some of the growing pains less painful if I understood what my overhead charge was going to, or more about the workflow of the clinic.” She adds that an overview of hospital relationships and any overlapping ownership interests will benefit new physicians as well.
“I think it’s useful to provide new physicians with a history of the practice and the vision of where things are going,” McCarthy says. “It’s important to outline the business vision, especially for subspecialties. If you explain to the new physician where you want to grow and when the practice plans on bringing on the next physician, it could really drive someone to grow their practice.”
Don’t Underestimate the Need for Coding Training
“When fellows come out of training, they are comfortable with clinical activity but uncomfortable with business administration,” Marks says. “And we know they don’t get training on coding and billing.”
Marks cites a recent conversation at an American Academy of Orthopaedic Surgeons (AAOS) coding workshop. “A surgeon new in practice told me, ‘I’ve been in practice for 4 months. I understand the clinical side but nobody educated me about coding and billing before this course.’” Practices must provide new physicians with coding and documentation training, and coach them to make sure they feel up to speed and comfortable. “The practice’s future revenue depends on it,” Marks says.
McCarthy agrees. “Having an administrative mentorship for coding is incredibly valuable. They don’t teach it in school.”
So from a practical standpoint, purchase AAOS’ Orthopaedic Code-X, a software tool that will help the new physician navigate and integrate Current Procedural Terminology (CPT), ICD-10 (International Classification of Diseases, Tenth Revision), and other coding data easily and accurately. Send him or her to one of the Academy’s regional coding and reimbursement workshops as well. “It will behoove the practice to send them even before they start seeing patients,” Marks says.
And don’t just stop there. High-performing groups conduct peer reviews of evaluation and management (E/M) and operative notes, blinding the codes billed and discussing which CPT and ICD-10 codes are appropriate for the visit or case. “It will take time for the new physician to completely integrate coding with their clinical care,” says Marks. “Peer review sessions, as well as having a partner review codes before they go to the billing office, can help speed learning.”
Collegial Coaching Counts
The week before her official start day, Mc-Carthy scrubbed in as a first assist with each of her new partners. “It was a great way to start ramping up,” she says. “I could see what kind of equipment was present in the hospitals, and got a touch point for hospital logistics. Plus, as a young surgeon it’s great to see how your skill sets match up with your new partners, and which best practices are being deployed by the group.”
This kind of “collegial coaching” is a vital part of the clinical and cultural integration to the practice. Beyond providing clinical support, it builds relationships and trust among the group, and fosters collaboration.
Arkansas Specialty Orthopaedics organized McCarthy’s clinic and operating room (OR) schedules so that a partner was always present. “There was also someone I could bounce ideas off of,” McCarthy explains. “Every day in the OR, there was a partner there at the same time. If I got into a sticky situation, one of my colleagues was willing to come in and scrub in the OR.”
McCarthy says that patients responded favorably when she told them her plan was developed in conjunction with her partners. “Patients find comfort in knowing that several people’s opinions were considered,” she says. “And as a young surgeon, knowing that you have backup, even if you don’t use it, when caring for high-risk and complex cases really means a lot,” she says.
And although her group didn’t offer a formal mentoring program, McCarthy found that an informal mentorship grew organically when a friendship developed with one of her new partners. “In the first 6 months, every single weekend we sat by the pool and rolled through a ton of cases,” she says. “That was fabulous and it alleviated so much stress for me.” And when it was time for McCarthy to move into board case selection, this colleague and another were instrumental in her board preparation because, “they knew my style and where I would need to focus.”
IMS Orthopedics’ approach is to provide the staff and systems that allow new physicians to step up and take responsibility. “If they want to scrub in with me, that’s great. If they’d like to visit additional facilities and get the lay of the land, we encourage it. But we don’t do a lot of handholding. We set them up for success and make sure people are in place to help them,” says Ferry.
A Marketing Plan Is a Must
“The vast majority of practices do very little when it comes to thinking about how to market and build the practice of their new physician,” Marks says. “Practice-building is more of a challenge for surgical specialists today than it was in the old days when new surgeons could easily meet internists as they were rounding at the hospital. Now, a new physician and the practice must come up with a game plan.”
That game plan starts with the easy things: order business cards, schedule a photo shoot, and update the practice’s Web site pages with the physician’s biography and an introductory video. But with social media, online reviews, and subspecialty competition, Marks says practices must think beyond the basics. Think through each element of marketing, from online to outreach to developing referral relationships.
“I tell practices to draft a written marketing plan,” he says. “Not only does it provide a roadmap for the new physician, but also indicates that the practice has put some thought into how he or she can build a practice. It can make the new physician feel less overwhelmed knowing that he or she doesn’t have to do the marketing alone.” Once you’ve developed a list of actions, Marks suggests creating a spreadsheet with deadlines, and ensuring each action is completed.
McCarthy was scheduled to visit family practice clinics, and joined by the administrator who “handed out cookies and cards while I talked,” she says. Arkansas Specialty Orthopaedics also hired an external marketing firm to develop promotional opportunities for her. For example, “I was scheduled to appear on news channels, where I discussed new and interesting procedures,” she says. “It got my name out into the community.”
If your practice is too small to hire an outside firm, Marks suggests reaching out to agencies such as nursing homes, fitness centers, or the YMCA, which frequently offers educational programs for members. “Contact the administrators or medical directors in these organizations. A few minutes on the phone or a short visit can go a long way to building these relationships and getting your new physician on the map.”
As the old saying goes, an ounce of prevention is worth a pound of cure. Scheduling time for orientation, training, staff integration, and collegial coaching will speed up a new physician’s integration into the practice, and increase his or her opportunity for success.
Practices and hospitals invest significant time and money in recruiting a new physician. From phone interviews to site visits to contract negotiations, it’s a long and involved process.
Beyond setting up a new physician’s office and appointment schedule, completing human resources paperwork, and ordering business cards, what does your practice do to support new physicians to ensure they are successful? Although a new colleague may arrive with excellent clinical skills, even the most promising surgeon can fall short if not provided with the right expectations, training, and collegial support. Here’s how to fast track your new physician to professional heights.
Credentialing Is Key
At the crux of a new physician’s success is credentialing him or her with hospitals and insurance plans before the official start date to see patients.
“A state medical license is the first domino,” says orthopedic surgeon Michael R. Marks, MD, MBA, consultant and coding educator with KarenZupko & Associates, Inc. Marks has led or participated in physician recruitment in orthopedic and multispecialty groups. The firm has developed a comprehensive New Physician Onboarding Checklist, available at https://www.karenzupko.com/new-physician-onboarding-checklist/.
“Without a medical license,” Marks continues, “you can’t get the new physician hospital privileges and you can’t get him or her credentialed with plans. Without being credentialed, the physician can’t bill for patients treated.” Because commercial carriers won’t allow retrospective billing for services already rendered, “even a 3-month delay in credentialing could cost an orthopedic practice $60,000 to $180,000 in lost revenue.”
And if you think you can bill the new physician’s services under another partner’s name, you are incorrect. “The billing physician will have signed the note, but not have treated the patient,” warns Marks. “This is improper billing. Don’t do it.”
The remedy for ensuring that the new physician is credentialed is simple: get organized and plan ahead.
“When I first started participating in recruitment, I remember telling physicians, ‘I need you tomorrow!’” admits Amon T. Ferry, MD, a practicing orthopedist who leads recruitment efforts at IMS Orthopedics, a division of Integrated Medical Specialists in Phoenix, Arizona. “So they’d get hired before the practice was prepared and before credentialing was completed. Now, I set more realistic expectations,” he says, noting that in Arizona it takes 3 months to get a medical license, 6 months to contract with the hospital, and 9 months to get on insurance plans. And even after a plan has credentialed a new physician, “sometimes it still takes 4 to 6 weeks before the physician’s data is loaded into the plan’s computer systems.”
“The way to do credentialing right is to get all departments communicating,” Marks says. “If you keep everyone siloed, staff don’t understand that a lack of timeliness on their part impacts other areas of the practice.”
Ferry agrees, and says his group learned to organize its multiple departments after making mistakes and missing deadlines. “We now have an 8-page pre-employment application for new physicians,” he explains. “In addition to asking for contact information and everything we need to know in order to get the physician credentialed, we ask questions about malpractice suit history and whether there are issues with the medical board. We also ask about gaps in employment and details about where the physician has practiced in the past.” All of this is done to identify early whether credentialing will require more time and effort. Ferry says that the application has solved a number of processing problems the practice had in the past.
And whether credentialing is done within the practice or outsourced, Ferry says that it pays to be persistent. “Don’t sit back and assume it will get done. Even if you have outsourced credentialing to a company, someone must check with payers and hospitals weekly and provide the practice a status update.”
In one case, when getting a new physician contracted at a hospital was taking forever, Ferry directed the staff to call. “Turns out, they had been trying to reach us and had the wrong phone number,” he says. “When people are processing thousands of physician renewals, things get lost. You have to be proactive and be your own advocate. Don’t be afraid to be the squeaky wheel.”
Staff Relationships and Operational Wisdom
Marks points out that in many practices, the new physician is shown the examination rooms and his or her office, gets electronic health record (EHR) training, and that’s it. To be successful, Marks insists that the new physician must build relationships with personnel and understand operational basics. “In other business industries, successful leaders understand at least the basics of what everyone does. Part of how they do this is by getting to know the employees.”
Ideally, Marks advises that new physicians spend time with each staff member. “The best time to do this is in the first few weeks of employment,” he suggests. “Odds are, the new orthopedist doesn’t have 40 patients a day on the schedule. So schedule conversations within the first few weeks or month, and schedule observation time as well. When a patient complains about check-in, the physician will have an understanding of how things work up there if he or she knows the basic processes.” The new doctor should also spend time in the billing office getting to know the challenges faced by staff, and sit with the surgery coordinator to understand the process of getting cases booked and scheduled.
Plan for an initial and then periodic meetings with the practice administrator and other supervisors. Transparency about business operations, data, and strategy will help the new physician get up to speed faster.
“The executive director of our group was an absolutely invaluable information resource,” says Kathryn J. McCarthy, MD, an orthopedic spine surgeon with Arkansas Specialty Orthopaedics in Little Rock, Arkansas. McCarthy has been with the group for 3 years.
The practice’s executive director developed and presented a PowerPoint (Microsoft) explaining general business procedures, expectations for the coding and billing process, and pertinent compliance and risk issues. She had also developed an interactive model of the compensation formula and buy-in program, using Excel (Microsoft). McCarthy met with the executive director at 3 months, 6 months, and 9 months to review her patient and case volumes and how they were trending against the estimates made about her income, bonus, and buy-in status.
From the new physician’s perspective, McCarthy says having the new physician understand the complexities of certain business systems helps them understand things better. “If you sit in the business meetings long enough, you figure it out,” she says, “but it would have made some of the growing pains less painful if I understood what my overhead charge was going to, or more about the workflow of the clinic.” She adds that an overview of hospital relationships and any overlapping ownership interests will benefit new physicians as well.
“I think it’s useful to provide new physicians with a history of the practice and the vision of where things are going,” McCarthy says. “It’s important to outline the business vision, especially for subspecialties. If you explain to the new physician where you want to grow and when the practice plans on bringing on the next physician, it could really drive someone to grow their practice.”
Don’t Underestimate the Need for Coding Training
“When fellows come out of training, they are comfortable with clinical activity but uncomfortable with business administration,” Marks says. “And we know they don’t get training on coding and billing.”
Marks cites a recent conversation at an American Academy of Orthopaedic Surgeons (AAOS) coding workshop. “A surgeon new in practice told me, ‘I’ve been in practice for 4 months. I understand the clinical side but nobody educated me about coding and billing before this course.’” Practices must provide new physicians with coding and documentation training, and coach them to make sure they feel up to speed and comfortable. “The practice’s future revenue depends on it,” Marks says.
McCarthy agrees. “Having an administrative mentorship for coding is incredibly valuable. They don’t teach it in school.”
So from a practical standpoint, purchase AAOS’ Orthopaedic Code-X, a software tool that will help the new physician navigate and integrate Current Procedural Terminology (CPT), ICD-10 (International Classification of Diseases, Tenth Revision), and other coding data easily and accurately. Send him or her to one of the Academy’s regional coding and reimbursement workshops as well. “It will behoove the practice to send them even before they start seeing patients,” Marks says.
And don’t just stop there. High-performing groups conduct peer reviews of evaluation and management (E/M) and operative notes, blinding the codes billed and discussing which CPT and ICD-10 codes are appropriate for the visit or case. “It will take time for the new physician to completely integrate coding with their clinical care,” says Marks. “Peer review sessions, as well as having a partner review codes before they go to the billing office, can help speed learning.”
Collegial Coaching Counts
The week before her official start day, Mc-Carthy scrubbed in as a first assist with each of her new partners. “It was a great way to start ramping up,” she says. “I could see what kind of equipment was present in the hospitals, and got a touch point for hospital logistics. Plus, as a young surgeon it’s great to see how your skill sets match up with your new partners, and which best practices are being deployed by the group.”
This kind of “collegial coaching” is a vital part of the clinical and cultural integration to the practice. Beyond providing clinical support, it builds relationships and trust among the group, and fosters collaboration.
Arkansas Specialty Orthopaedics organized McCarthy’s clinic and operating room (OR) schedules so that a partner was always present. “There was also someone I could bounce ideas off of,” McCarthy explains. “Every day in the OR, there was a partner there at the same time. If I got into a sticky situation, one of my colleagues was willing to come in and scrub in the OR.”
McCarthy says that patients responded favorably when she told them her plan was developed in conjunction with her partners. “Patients find comfort in knowing that several people’s opinions were considered,” she says. “And as a young surgeon, knowing that you have backup, even if you don’t use it, when caring for high-risk and complex cases really means a lot,” she says.
And although her group didn’t offer a formal mentoring program, McCarthy found that an informal mentorship grew organically when a friendship developed with one of her new partners. “In the first 6 months, every single weekend we sat by the pool and rolled through a ton of cases,” she says. “That was fabulous and it alleviated so much stress for me.” And when it was time for McCarthy to move into board case selection, this colleague and another were instrumental in her board preparation because, “they knew my style and where I would need to focus.”
IMS Orthopedics’ approach is to provide the staff and systems that allow new physicians to step up and take responsibility. “If they want to scrub in with me, that’s great. If they’d like to visit additional facilities and get the lay of the land, we encourage it. But we don’t do a lot of handholding. We set them up for success and make sure people are in place to help them,” says Ferry.
A Marketing Plan Is a Must
“The vast majority of practices do very little when it comes to thinking about how to market and build the practice of their new physician,” Marks says. “Practice-building is more of a challenge for surgical specialists today than it was in the old days when new surgeons could easily meet internists as they were rounding at the hospital. Now, a new physician and the practice must come up with a game plan.”
That game plan starts with the easy things: order business cards, schedule a photo shoot, and update the practice’s Web site pages with the physician’s biography and an introductory video. But with social media, online reviews, and subspecialty competition, Marks says practices must think beyond the basics. Think through each element of marketing, from online to outreach to developing referral relationships.
“I tell practices to draft a written marketing plan,” he says. “Not only does it provide a roadmap for the new physician, but also indicates that the practice has put some thought into how he or she can build a practice. It can make the new physician feel less overwhelmed knowing that he or she doesn’t have to do the marketing alone.” Once you’ve developed a list of actions, Marks suggests creating a spreadsheet with deadlines, and ensuring each action is completed.
McCarthy was scheduled to visit family practice clinics, and joined by the administrator who “handed out cookies and cards while I talked,” she says. Arkansas Specialty Orthopaedics also hired an external marketing firm to develop promotional opportunities for her. For example, “I was scheduled to appear on news channels, where I discussed new and interesting procedures,” she says. “It got my name out into the community.”
If your practice is too small to hire an outside firm, Marks suggests reaching out to agencies such as nursing homes, fitness centers, or the YMCA, which frequently offers educational programs for members. “Contact the administrators or medical directors in these organizations. A few minutes on the phone or a short visit can go a long way to building these relationships and getting your new physician on the map.”
As the old saying goes, an ounce of prevention is worth a pound of cure. Scheduling time for orientation, training, staff integration, and collegial coaching will speed up a new physician’s integration into the practice, and increase his or her opportunity for success.
Surgical Pearls in Total Knee Arthroplasty: A Lifetime of Lessons Learned
After over 4 decades of experience with total knee arthroplasty (TKA), I have learned many lessons regarding surgical technique. These include exposure issues, alignment methods, bone preparation, correction of deformity, and implantation techniques. Most of these lessons have been self-taught, but some have been suggested by or modified from colleague and student interaction. Attribution is given when possible.
The Incision
The skin incision should be marked in flexion rather than extension because the skin moves approximately 1 cm laterally from extension to flexion.1 This occurs because the tibia internally rotates beneath the skin as the knee is flexed and externally rotates as full extension is achieved. This lateral movement of the skin could bring an incision marked in extension on top of the tibial tubercle when the knee is flexed and may result in pain and dysfunction when the patient attempts to kneel. A review of kneeling ability after TKA showed that most patients are hesitant to kneel initially after their arthroplasty, but gain confidence and improved comfort and ability as their scar matures.2
Exposure
Patellar eversion can be difficult in a markedly obese or ankylosed knee, especially when the patella is difficult to grasp. This is facilitated by the use of a standard patellar clamp that is normally used to compress the patella during component cementation (Figure 1).3
Exposing the Ankylosed Knee and Protecting the Patellar Tendon From Avulsion
A tibial tubercle osteotomy is often recommended in the ankylosed knee but can be avoided by making a short inverted “V” incision in the proximal quadriceps tendon (Figure 2).4
Protecting the Soft Tissues During Surgery
Moist wound towels sewn into the joint capsule protect the underlying soft tissues from debris and desiccation during the procedure and will intuitively lower the chance of wound infection from contamination and tissue injury (Figures 4A, 4B).
Locating and Coagulating the Lateral Inferior Genicular Vessels
The lateral inferior genicular artery and vein can be easily located and coagulated just outside the posterior rim of the lateral meniscus near the popliteus hiatus. This will minimize both intraoperative and postoperative blood loss.
Determining the Entry Point in the Distal Femur for Intramedullary Alignment Devices
Templating the femoral entry point for insertion of an intramedullary alignment device on a preoperative radiograph will help avoid inadvertent excessive distal femoral valgus resection. This is especially important in valgus knees that have a valgus metaphyseal bow (Figure 5).
Avoiding Notching of the Anterior Femoral Cortex
Notching the anterior femoral cortex when in-between femoral sizes or when there is a preexisting dysplastic or shallow trochlea (Figure 6)
Obtaining a Medial Release by Removing Peripheral Medial Tibial Bone
Varus deformities can be corrected without performing a formal medial collateral ligament (MCL) release by a so-called reduction tibial osteotomy.5,6 In mild varus deformity, sufficient medial release can be achieved by removing medial femoral and tibial peripheral osteophytes that tent up the MCL and medial capsule. When this is insufficient, removal of additional peripheral tibial bone further shortens the distance between the origin and insertion of the MCL, effectively lengthening the ligament (Figure 7).
An Inverted Cruciform Lateral Retinacular Release to Correct Severe Valgus Deformity
An inverted cruciform lateral retinacular release effectively corrects a severe valgus deformity and avoids the need for a lateral collateral ligament (LCL) release.7
Relieving Posterior Femoral Impingement
Uncapped posterior condylar bone or retained posterior osteophytes can limit both flexion and extension and cause impingement. Trimming the posterior femoral condyles and removing posterior osteophytes is best accomplished using a trial femoral component as a template.4 A curved osteotome is passed tangential to the metallic condyles to define the bone requiring resection. After removal of the trial, the outlined bone can be easily and accurately resected.
Minimizing Postoperative Posterior Condylar Bone-Cement Radiolucencies
Zone 4 femoral bone-cement radiolucencies8 can be minimized using the “smear” technique.4 These radiolucencies are common because most prosthetic femoral components have posterior condyles that are parallel to the femoral fixation lugs and do not allow for compression of this interface during implantation. Most surgeons put no cement on the posterior condylar bone but place it on the inside of the prosthetic condyle instead. The lack of compression upon insertion leads to a poor interface and the resultant lucencies. In the long term, these lucencies could allow access of wear debris to the posterior condylar bone, with the potential for osteolysis and loosening. To improve this interface, cement can be smeared or packed into the posterior condyles and also placed on the posterior condyles of the prosthesis. This could lead to posterior extrusion of some cement during polymerization, so a removable trial insert should be utilized to allow access posteriorly after polymerization is complete.
Predicting Potential Postoperative Flexion
The best indicator of potential postoperative flexion for any individual patient is not preoperative flexion but is intraoperative flexion against gravity measured after capsular closure.9 Surgeons should measure and record this value for reference if a patient has difficulty regaining flexion during their recovery (Figure 9).
Summary
The short- and long-term success of TKA is highly dependent on surgical technique that allows proper and safe exposure under all circumstances, correction of deformity, and accurate component implantation while minimizing intraoperative and postoperative complications. The surgical pearls shared above will hopefully aid in achieving these goals.
Am J Orthop. 2016;45(6):384-388. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Yacoubian SV, Scott RD. Skin incision translation in total knee arthroplasty: the difference between flexion and extension. J Arthroplasty. 2007;22(3):353-355.
2. Schai PA, Gibbon AJ, Scott RD. Kneeling ability after total knee arthroplasty. Perception and reality. Clin Orthop Relat Res. 1999;367:195-200.
3. Springorum HP, Scott RD. A technique to facilitate everting the patella in stiff or obese knees in total knee arthroplasty. Am J Orthop. 2009;38(10):507-508.
4. Scott RD. Total Knee Arthroplasty. 2nd ed. Philadelphia, PA: Elsevier; 2014.
5. Dixon MC, Parsch D, Brown RR, Scott RD. The correction of severe varus deformity in total knee arthroplasty by tibial component downsizing and resection of uncapped proximal medial bone. J Arthroplasty. 2004;19(1):19-22.
6. Mullaji AB, Padmanabhan V, Jindal G. Total knee arthroplasty for profound varus deformity: technique and radiological results in 173 knees with varus of more than 20 degrees. J Arthroplasty. 2005;20(5):550-561.
7. Politi J, Scott RD. Balancing severe valgus deformity in total knee arthroplasty using a lateral cruciform retinacular release. J Arthroplasty. 2004;19(5):553-557.
8. Huddleston JI, Wiley JW, Scott RD. Zone 4 femoral radiolucent lines in hybrid versus cemented total knee arthroplasties: are they clinically significant? Clin Orthop Relat Res. 2005;441:334-339.
9. Lee DC, Kim DH, Scott RD, Suthers K. Intraoperative flexion against gravity as an indication of ultimate range of motion in individual cases after total knee arthroplasty. J Arthroplasty. 1998;13(5):500-503.
After over 4 decades of experience with total knee arthroplasty (TKA), I have learned many lessons regarding surgical technique. These include exposure issues, alignment methods, bone preparation, correction of deformity, and implantation techniques. Most of these lessons have been self-taught, but some have been suggested by or modified from colleague and student interaction. Attribution is given when possible.
The Incision
The skin incision should be marked in flexion rather than extension because the skin moves approximately 1 cm laterally from extension to flexion.1 This occurs because the tibia internally rotates beneath the skin as the knee is flexed and externally rotates as full extension is achieved. This lateral movement of the skin could bring an incision marked in extension on top of the tibial tubercle when the knee is flexed and may result in pain and dysfunction when the patient attempts to kneel. A review of kneeling ability after TKA showed that most patients are hesitant to kneel initially after their arthroplasty, but gain confidence and improved comfort and ability as their scar matures.2
Exposure
Patellar eversion can be difficult in a markedly obese or ankylosed knee, especially when the patella is difficult to grasp. This is facilitated by the use of a standard patellar clamp that is normally used to compress the patella during component cementation (Figure 1).3
Exposing the Ankylosed Knee and Protecting the Patellar Tendon From Avulsion
A tibial tubercle osteotomy is often recommended in the ankylosed knee but can be avoided by making a short inverted “V” incision in the proximal quadriceps tendon (Figure 2).4
Protecting the Soft Tissues During Surgery
Moist wound towels sewn into the joint capsule protect the underlying soft tissues from debris and desiccation during the procedure and will intuitively lower the chance of wound infection from contamination and tissue injury (Figures 4A, 4B).
Locating and Coagulating the Lateral Inferior Genicular Vessels
The lateral inferior genicular artery and vein can be easily located and coagulated just outside the posterior rim of the lateral meniscus near the popliteus hiatus. This will minimize both intraoperative and postoperative blood loss.
Determining the Entry Point in the Distal Femur for Intramedullary Alignment Devices
Templating the femoral entry point for insertion of an intramedullary alignment device on a preoperative radiograph will help avoid inadvertent excessive distal femoral valgus resection. This is especially important in valgus knees that have a valgus metaphyseal bow (Figure 5).
Avoiding Notching of the Anterior Femoral Cortex
Notching the anterior femoral cortex when in-between femoral sizes or when there is a preexisting dysplastic or shallow trochlea (Figure 6)
Obtaining a Medial Release by Removing Peripheral Medial Tibial Bone
Varus deformities can be corrected without performing a formal medial collateral ligament (MCL) release by a so-called reduction tibial osteotomy.5,6 In mild varus deformity, sufficient medial release can be achieved by removing medial femoral and tibial peripheral osteophytes that tent up the MCL and medial capsule. When this is insufficient, removal of additional peripheral tibial bone further shortens the distance between the origin and insertion of the MCL, effectively lengthening the ligament (Figure 7).
An Inverted Cruciform Lateral Retinacular Release to Correct Severe Valgus Deformity
An inverted cruciform lateral retinacular release effectively corrects a severe valgus deformity and avoids the need for a lateral collateral ligament (LCL) release.7
Relieving Posterior Femoral Impingement
Uncapped posterior condylar bone or retained posterior osteophytes can limit both flexion and extension and cause impingement. Trimming the posterior femoral condyles and removing posterior osteophytes is best accomplished using a trial femoral component as a template.4 A curved osteotome is passed tangential to the metallic condyles to define the bone requiring resection. After removal of the trial, the outlined bone can be easily and accurately resected.
Minimizing Postoperative Posterior Condylar Bone-Cement Radiolucencies
Zone 4 femoral bone-cement radiolucencies8 can be minimized using the “smear” technique.4 These radiolucencies are common because most prosthetic femoral components have posterior condyles that are parallel to the femoral fixation lugs and do not allow for compression of this interface during implantation. Most surgeons put no cement on the posterior condylar bone but place it on the inside of the prosthetic condyle instead. The lack of compression upon insertion leads to a poor interface and the resultant lucencies. In the long term, these lucencies could allow access of wear debris to the posterior condylar bone, with the potential for osteolysis and loosening. To improve this interface, cement can be smeared or packed into the posterior condyles and also placed on the posterior condyles of the prosthesis. This could lead to posterior extrusion of some cement during polymerization, so a removable trial insert should be utilized to allow access posteriorly after polymerization is complete.
Predicting Potential Postoperative Flexion
The best indicator of potential postoperative flexion for any individual patient is not preoperative flexion but is intraoperative flexion against gravity measured after capsular closure.9 Surgeons should measure and record this value for reference if a patient has difficulty regaining flexion during their recovery (Figure 9).
Summary
The short- and long-term success of TKA is highly dependent on surgical technique that allows proper and safe exposure under all circumstances, correction of deformity, and accurate component implantation while minimizing intraoperative and postoperative complications. The surgical pearls shared above will hopefully aid in achieving these goals.
Am J Orthop. 2016;45(6):384-388. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
After over 4 decades of experience with total knee arthroplasty (TKA), I have learned many lessons regarding surgical technique. These include exposure issues, alignment methods, bone preparation, correction of deformity, and implantation techniques. Most of these lessons have been self-taught, but some have been suggested by or modified from colleague and student interaction. Attribution is given when possible.
The Incision
The skin incision should be marked in flexion rather than extension because the skin moves approximately 1 cm laterally from extension to flexion.1 This occurs because the tibia internally rotates beneath the skin as the knee is flexed and externally rotates as full extension is achieved. This lateral movement of the skin could bring an incision marked in extension on top of the tibial tubercle when the knee is flexed and may result in pain and dysfunction when the patient attempts to kneel. A review of kneeling ability after TKA showed that most patients are hesitant to kneel initially after their arthroplasty, but gain confidence and improved comfort and ability as their scar matures.2
Exposure
Patellar eversion can be difficult in a markedly obese or ankylosed knee, especially when the patella is difficult to grasp. This is facilitated by the use of a standard patellar clamp that is normally used to compress the patella during component cementation (Figure 1).3
Exposing the Ankylosed Knee and Protecting the Patellar Tendon From Avulsion
A tibial tubercle osteotomy is often recommended in the ankylosed knee but can be avoided by making a short inverted “V” incision in the proximal quadriceps tendon (Figure 2).4
Protecting the Soft Tissues During Surgery
Moist wound towels sewn into the joint capsule protect the underlying soft tissues from debris and desiccation during the procedure and will intuitively lower the chance of wound infection from contamination and tissue injury (Figures 4A, 4B).
Locating and Coagulating the Lateral Inferior Genicular Vessels
The lateral inferior genicular artery and vein can be easily located and coagulated just outside the posterior rim of the lateral meniscus near the popliteus hiatus. This will minimize both intraoperative and postoperative blood loss.
Determining the Entry Point in the Distal Femur for Intramedullary Alignment Devices
Templating the femoral entry point for insertion of an intramedullary alignment device on a preoperative radiograph will help avoid inadvertent excessive distal femoral valgus resection. This is especially important in valgus knees that have a valgus metaphyseal bow (Figure 5).
Avoiding Notching of the Anterior Femoral Cortex
Notching the anterior femoral cortex when in-between femoral sizes or when there is a preexisting dysplastic or shallow trochlea (Figure 6)
Obtaining a Medial Release by Removing Peripheral Medial Tibial Bone
Varus deformities can be corrected without performing a formal medial collateral ligament (MCL) release by a so-called reduction tibial osteotomy.5,6 In mild varus deformity, sufficient medial release can be achieved by removing medial femoral and tibial peripheral osteophytes that tent up the MCL and medial capsule. When this is insufficient, removal of additional peripheral tibial bone further shortens the distance between the origin and insertion of the MCL, effectively lengthening the ligament (Figure 7).
An Inverted Cruciform Lateral Retinacular Release to Correct Severe Valgus Deformity
An inverted cruciform lateral retinacular release effectively corrects a severe valgus deformity and avoids the need for a lateral collateral ligament (LCL) release.7
Relieving Posterior Femoral Impingement
Uncapped posterior condylar bone or retained posterior osteophytes can limit both flexion and extension and cause impingement. Trimming the posterior femoral condyles and removing posterior osteophytes is best accomplished using a trial femoral component as a template.4 A curved osteotome is passed tangential to the metallic condyles to define the bone requiring resection. After removal of the trial, the outlined bone can be easily and accurately resected.
Minimizing Postoperative Posterior Condylar Bone-Cement Radiolucencies
Zone 4 femoral bone-cement radiolucencies8 can be minimized using the “smear” technique.4 These radiolucencies are common because most prosthetic femoral components have posterior condyles that are parallel to the femoral fixation lugs and do not allow for compression of this interface during implantation. Most surgeons put no cement on the posterior condylar bone but place it on the inside of the prosthetic condyle instead. The lack of compression upon insertion leads to a poor interface and the resultant lucencies. In the long term, these lucencies could allow access of wear debris to the posterior condylar bone, with the potential for osteolysis and loosening. To improve this interface, cement can be smeared or packed into the posterior condyles and also placed on the posterior condyles of the prosthesis. This could lead to posterior extrusion of some cement during polymerization, so a removable trial insert should be utilized to allow access posteriorly after polymerization is complete.
Predicting Potential Postoperative Flexion
The best indicator of potential postoperative flexion for any individual patient is not preoperative flexion but is intraoperative flexion against gravity measured after capsular closure.9 Surgeons should measure and record this value for reference if a patient has difficulty regaining flexion during their recovery (Figure 9).
Summary
The short- and long-term success of TKA is highly dependent on surgical technique that allows proper and safe exposure under all circumstances, correction of deformity, and accurate component implantation while minimizing intraoperative and postoperative complications. The surgical pearls shared above will hopefully aid in achieving these goals.
Am J Orthop. 2016;45(6):384-388. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Yacoubian SV, Scott RD. Skin incision translation in total knee arthroplasty: the difference between flexion and extension. J Arthroplasty. 2007;22(3):353-355.
2. Schai PA, Gibbon AJ, Scott RD. Kneeling ability after total knee arthroplasty. Perception and reality. Clin Orthop Relat Res. 1999;367:195-200.
3. Springorum HP, Scott RD. A technique to facilitate everting the patella in stiff or obese knees in total knee arthroplasty. Am J Orthop. 2009;38(10):507-508.
4. Scott RD. Total Knee Arthroplasty. 2nd ed. Philadelphia, PA: Elsevier; 2014.
5. Dixon MC, Parsch D, Brown RR, Scott RD. The correction of severe varus deformity in total knee arthroplasty by tibial component downsizing and resection of uncapped proximal medial bone. J Arthroplasty. 2004;19(1):19-22.
6. Mullaji AB, Padmanabhan V, Jindal G. Total knee arthroplasty for profound varus deformity: technique and radiological results in 173 knees with varus of more than 20 degrees. J Arthroplasty. 2005;20(5):550-561.
7. Politi J, Scott RD. Balancing severe valgus deformity in total knee arthroplasty using a lateral cruciform retinacular release. J Arthroplasty. 2004;19(5):553-557.
8. Huddleston JI, Wiley JW, Scott RD. Zone 4 femoral radiolucent lines in hybrid versus cemented total knee arthroplasties: are they clinically significant? Clin Orthop Relat Res. 2005;441:334-339.
9. Lee DC, Kim DH, Scott RD, Suthers K. Intraoperative flexion against gravity as an indication of ultimate range of motion in individual cases after total knee arthroplasty. J Arthroplasty. 1998;13(5):500-503.
1. Yacoubian SV, Scott RD. Skin incision translation in total knee arthroplasty: the difference between flexion and extension. J Arthroplasty. 2007;22(3):353-355.
2. Schai PA, Gibbon AJ, Scott RD. Kneeling ability after total knee arthroplasty. Perception and reality. Clin Orthop Relat Res. 1999;367:195-200.
3. Springorum HP, Scott RD. A technique to facilitate everting the patella in stiff or obese knees in total knee arthroplasty. Am J Orthop. 2009;38(10):507-508.
4. Scott RD. Total Knee Arthroplasty. 2nd ed. Philadelphia, PA: Elsevier; 2014.
5. Dixon MC, Parsch D, Brown RR, Scott RD. The correction of severe varus deformity in total knee arthroplasty by tibial component downsizing and resection of uncapped proximal medial bone. J Arthroplasty. 2004;19(1):19-22.
6. Mullaji AB, Padmanabhan V, Jindal G. Total knee arthroplasty for profound varus deformity: technique and radiological results in 173 knees with varus of more than 20 degrees. J Arthroplasty. 2005;20(5):550-561.
7. Politi J, Scott RD. Balancing severe valgus deformity in total knee arthroplasty using a lateral cruciform retinacular release. J Arthroplasty. 2004;19(5):553-557.
8. Huddleston JI, Wiley JW, Scott RD. Zone 4 femoral radiolucent lines in hybrid versus cemented total knee arthroplasties: are they clinically significant? Clin Orthop Relat Res. 2005;441:334-339.
9. Lee DC, Kim DH, Scott RD, Suthers K. Intraoperative flexion against gravity as an indication of ultimate range of motion in individual cases after total knee arthroplasty. J Arthroplasty. 1998;13(5):500-503.
Medical Issues in American Football: Eyes, Teeth, and Skin
Orthopedic conditions are only one of the many medical issues football team physicians may face. In this review, we cover the management of a few of the most common nonorthopedic medical issues football team physicians are likely to encounter, including eye injuries, dental concerns, and skin conditions.
Eye Injuries
More than 2.5 million eye injuries occur each year, with 50,000 people permanently losing part or all of their vision.1 Eye injuries account for over 600,000 yearly emergency department visits; over 30% of these eye injuries were attributed to a sports injury.1 Football is classified as high risk for eye injury, along with baseball, hockey, basketball, and lacrosse.2 Common eye injury mechanisms are categorized as blunt, penetrating, and radiating. Blunt injuries are most common.2 When evaluating an athlete on the sideline, relevant history would include the size of the object, the level of force, and the direction from which the impact occurred. An examination should include best-corrected visual acuity using an eye chart, confrontational visual fields, assessment of extraocular movements, assessment of red reflex, and pupil evaluation with a light source.2
Cornea Injuries
The outermost layer of the eye, the cornea, can be subject to blunt and penetrating injuries. Corneal abrasions often occur from mechanical trauma, such as one from the fingernail of an opposing player, that disrupts the integrity of the corneal epithelium. A corneal abrasion can be identified by applying fluorescein strips after application of a topical anesthetic. Abrasions appear fluorescent green when viewed with a cobalt blue light. If an abrasion is identified, management includes preventing infection and treating pain. Prophylactic topical antibiotics can be applied, particularly for contact lens wearers. Patching has not shown benefit in treatment of pain.3 The physician can consider using topical nonsteroidal anti-inflammatory drugs, such as diclofenac or ketorolac, with a soft contact lens to treat the pain.4 The patient should follow up frequently for monitoring for infection and healing.
Orbital Fractures
Orbital fractures should be considered when an object larger than the orbital opening, such as an elbow or knee, causes blunt trauma to the surrounding bony structures, or a digital poke occurs to the globe.5 The floor of the orbit and medial wall are thin bones that often break sacrificially to protect the globe from rupture. Examination findings may include diplopia, sunken globe, numbness in the distribution of infraorbital nerve, or periorbital emphysema.6 Urgent evaluation should be considered to rule out associated intraocular damage. Imaging and a physical examination can help guide surgical management, if indicated. The most common outcome after this injury is diplopia with upper field gaze.5
Retina Issues
Trauma to the face or head may result in a separation of the retina from the underlying retinal pigment epithelium and allow vitreous fluid to seep in and further separate the layers, causing a retinal detachment. Symptoms may include flashes of light (photopsia), floaters, and visual field defects. Emergent referral is indicated, as the outcomes from this condition are time-sensitive. Consider placing an eye shield to prevent any further pressure on the globe.
Globe Injuries and Rupture
Another emergent ophthalmologic condition that can occur in football is globe rupture. Clinical findings usually prompt the clinician to consider this diagnosis. Hyphema (the collection of blood in the anterior chamber) may be seen in globe injuries. The most common clinical finding of athletes requiring hospitalization after an ocular injury is macroscopic hyphema (Figure 1).7-9
Prompt referral is warranted when there is a sudden decrease or change in vision, pain during movements, photophobia, and floaters and/or flashes.2 Consideration of return to play should take into account the patient’s vision and comfort level, which should not be masked by topical analgesics. Protective eyewear has been mandated in several sports, and has decreased the rate of eye injuries.10 Polycarbonate lenses of 3-mm thickness are recommended due to the significant comparable strength and impact-resistance.2 During the preparticipation physical for high-risk sports, the utilization of protective eyewear should be discussed.
Dental Concerns
Dental injuries may present a challenge for the sports medicine clinician. Contact injuries from elbows, fists, and other nonprojectile objects typically result in low-speed, lower-energy injuries, such as soft tissue lacerations and contusions. On the other hand, high-speed injuries occurring from balls, pucks, and sticks may result in more significant trauma. Baseball accounts for the highest percentage of sports-related dental injuries (40.2%), while basketball was second (20.2%) and football third (12.5%). Over 75% of these injuries occurred in males.11
On-field management of dental injuries should always start with the primary trauma survey, including assessment of the athlete’s airway, breathing, and circulatory function, as well as a targeted cervical spine evaluation. When obtaining a history, one should recognize the mechanism of injury and assess for signs of concomitant injuries, ie, respiratory compromise, concussion, leakage of cerebrospinal fluid, and teeth alignment. Findings from this initial evaluation may reveal critical conditions that will require management in addition to the dental injury.
Of central concern in managing dental trauma is preserving the viability of the injured structures. Therefore, much attention is paid to the pulpal and root vitality of injured teeth. The International Association of Dental Traumology Dental Trauma Guidelines recommend a biological approach to the urgent care of dental injuries:12
1. Stabilize the injury by carefully repositioning displaced entities and suturing soft tissue lacerations.
2. Eliminate or reduce the complications from bacterial contamination by rinsing and flushing with available liquids and use of chlorhexidine when possible.
3. Promote the opportunity for healing by replanting avulsed teeth and repositioning displaced teeth.
4. Make every effort to allow continued development of alveolar ridges in children.
Mouth guards are the single most effective prevention strategy for most contact sport dental injuries. One meta-analysis demonstrated a pooled 86% increased risk of orofacial injuries in nonusers.13
To review the anatomy (and injuries) of the tooth, one must consider the Ellis classification of enamel, dentin, and pulp injuries (Figure 2).
Tooth Subluxation
Tooth subluxations usually occur secondary to trauma and cause loosening of the tooth in its alveolar socket. A root fracture should be suspected in the setting of a subluxation. On exam, the tooth may be excessively mobile with gentle pressure. If unstable, immobilization with gauze packing or aluminum foil with dental follow-up is recommended.
Fractures
Ellis class I fractures are small chips in the enamel. There should be uniform color at the fracture site. A dental referral may be warranted to smooth rough enamel edges, but if no other injuries are present, these athletes may continue playing with some protection of the fractured surface. A mouth guard may be helpful to avoid mucosal lacerations.
Ellis class II fractures often present with sensitivity to inhaled air and to hot and cold temperatures. Yellow dentin is visible at the fracture site (Figure 3).
Ellis class III fractures may also present with air and temperature sensitivity. Finger pressure may expose a large fracture. Pink or red pulp is visible at the fracture site. Wiping the fracture site with sterile gauze may reveal bleeding from the pulp. This is considered a dental emergency. Immediate restriction from contact sports participation and urgent dental evaluation is indicated for root canal and capping and to prevent abscess formation.
Tooth Avulsion
Tooth avulsions occur when a tooth is completely displaced from the socket (Figure 4).
Skin Issues
Dermatological issues are some of the most common medical conditions faced by a football team physician. Skin infections in particular can pose a significant challenge both diagnostically as well as from a clearance-to-play perspective, given the potential for infections to affect other participants, such as other members of the team. Skin infection rates vary by sport and age group, with one study reporting 28.56 infections per 100,000 athletic exposures in high school wrestlers, which was more than 10 times that of football.14 Still, football players are at a higher risk of skin infections given the contact nature of the sport and close person-to-person proximity. A precise diagnosis may be difficult early in the course of a skin eruption, and with differing guidelines from various professional societies, it may be best suited for medical personnel familiar with these conditions, such as a sports medicine physician or dermatologist, to manage these athletes. A thorough and systematic evaluation is recommended, as athletes are often treated with unnecessary antibiotics, which contributes to antibiotic resistance. Previous antibiotic use may also be a risk factor for developing community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA).15
Two terms sports medicine clinicians must be familiar with are “adequately protected” and “properly covered.” The National Collegiate Athletic Association (NCAA) defines a wound or skin condition as adequately protected when the condition is considered noninfectious, adequately treated by a healthcare provider, and is able to be properly covered. A skin infection is considered properly covered when the lesion is covered by a securely attached bandage or dressing that will contain all drainage and remain intact throughout the sport activity.16
Impetigo
Impetigo is often caused by Staphylococcus and Streptococcus subspecies. The classic presentation is a dry, honey-crusted lesion with an erythematous base. Culture or gram stain may be helpful, but treatment may be initiated on a clinical basis without these studies. Topical antibiotics may be used, but in the setting of multiple lesions or an outbreak, systemic (eg, oral) antibiotics are preferred. Oral antibiotics may also shorten the time to return to play. If not responsive to the initial treatment, MRSA should be considered. No new lesions for 48 hours and a minimum of 72 hours of therapy with no moist, exudative, or draining lesions are required prior to return to play. These lesions cannot be covered as the sole means of return to play.
Methicillin-Resistant Staphylococcus aureus
MRSA is one of the most challenging skin infections for the sports medicine clinician to manage. Several outbreaks have been reported in the high school, college, and professional settings.17-20 Standardized precautions and a proactive approach are key in preventing MRSA outbreaks. It appears that different activities within a given sport may contribute to MRSA risk. One study reported football linemen had the highest attack rate, while another study reported cornerbacks and wide receivers to have the highest rate of MRSA infections.17,20 The elbow area was the most common site infected in both studies.
Abscesses are best initially managed by incision and drainage as well as obtaining wound cultures (Figure 5).
Preventative measures are thought to be useful, especially in the management of teams. The Centers for Disease Control and Prevention has published guidelines for both clinicians and patients. Precautions including hand washing; encouraging good overall hygiene; avoiding whirlpools; discouraging the sharing of towels, razors, and athletic gear; maintaining clean equipment/facilities; and encouraging early reporting of skin lesions.14,17,21,22 Isolated cases of MRSA do not need to be reported, but if more than one athlete is infected, one should notify the athletic training and team coaching staff. In the setting of an outbreak, the physician may need to notify local or state health agencies. No new lesions for 48 hours and a minimum of 72 hours of therapy with no moist, exudative, or draining lesions are required prior to returning to play. These lesions cannot be covered as the sole means of return to play.
Tinea Pedis
Tinea pedis is a common dermatophyte infection involving the feet and is most commonly caused by Trichophyton rubrum. Its distribution is usually interdigital or along the plantar surface of the foot. Topical antifungals with either allylamines or azoles are usually sufficient. Terbinafine has been shown to have a shorter duration of treatment. Athletes with tinea pedis are not restricted from sports participation during treatment, as long as the lesions are properly covered.
Tinea Corporis
Tinea corporis is a common superficial fungal infection of the body. It classically presents as pruritic, annular lesions, with well-demarcated borders and central clearing (
Tinea Cruris
Commonly known as “jock-itch,” this fungal infection is often very pruritic and involves the groin or genital region. The area is also inflamed and scaly. Treatment usually consists of topical allylamines or azoles. Allylamines amines are often preferred, as they require a shorter duration of treatment. There are no specific guidelines on the return to play with these athletes. Clearance is at the team physician’s discretion, but usually there are no restrictions. Athletes with extensive lesions may need to be disqualified from contact sports activities.
Am J Orthop. 2016;45(6):377-382. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Owens PL, Mutter R. Emergency Department Visits Related to Eye Injuries, 2008. Agency for Healthcare Research and Quality Web site. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb112.pdf. Published May 2011. Accessed August 18, 2016.
2. Rodriguez JO, Lavina AM, Agarwai A. Prevention and treatment of common eye injuries in sports. Am Fam Physician. 2003;67(7):1481-1496.
3. Lim CH, Turner A, Lim BX. Patching for corneal abrasion. Cochrane Database Syst Rev. 2016;7:CD004764.
4. Weaver CS, Terrell KM. Evidence-based emergency medicine. Update: do ophthalmic nonsteroidal anti-inflammatory drugs reduce the pain associated with simple corneal abrasion without delaying healing? Ann Emerg Med. 2003;41(1):134-140.
5. Williams RJ 3rd, Marx RG, Barnes R, O’Brien SJ, Warren RF. Fractures about the orbit in professional American football players. Am J Sports Med. 2001;29(1):55-57.
6. Forrest LA, Schuller DE, Strauss RH. Management of orbital blow-out fractures. Case reports and discussion. Am J Sports Med. 1989;17(2):217-220.
7. Barr A, Baines PS, Desai P, MacEwen CJ. Ocular sports injuries: the current picture. Br J Sports Med. 2000;34(6):456-458.
8. Pokhrel PK, Loftus SA. Ocular emergencies. Am Fam Physician. 2007;76(6):829-836.
9. Usatine RP, Smith MA, Mayeaux EJ Jr, Chumley H. Eye Trauma—Hyphema. The Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013.
10. Lincoln AE, Caswell SV, Almquist JL, et al. Effectiveness of the women’s lacrosse protective eyewear mandate in the reduction of eye injuries. Am J Sports Med. 2012;40(3):611-614.
11. Stewart GB, Shields BJ, Fields S, Comstock RD, Smith GA. Consumer products and activities associated with dental injuries to children treated in United States emergency departments, 1990-2003. Dental Traumatol. 2009;25(4):399-405.
12. Bakland LK. Dental trauma guidelines. Pediatric Dent. 2013;35(2):106-108.
13. Knapik J, Marshall SW, Lee RB, et al. Mouthguards in sport activities: history, physical properties and Injury prevention effectiveness. Sports Med. 2007;37(2):117-144.
14. Ashack KA, Burton KA, Johnson TR, Currie DW, Comstock RD, Dellavalle RP. Skin infections among US high school athletes: a national survey. J Am Acad Dermatol. 2016;74(4):679-684.e1.
15. Ellis MW, Hospenthal DR, Dooley DP, Gray PJ, Murray CK. Natural history of community-acquired methicillin-resistant Staphylococcus aureus colonization and infection in soldiers. Clin Infect Dis. 2004;39(7):971-979.
16. The National Collegiate Athletic Association. 2014-15 NCAA Sports Medicine Handbook. http://www.ncaapublications.com/productdownloads/MD15.pdf. Revised June 2008. Accessed August 18, 2016.
17. Anderson BJ. The effectiveness of valacyclovir in preventing reactivation of herpes gladiatorum in wrestlers. Clin J Sport Med. 1999;9(2):86-90.
18. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18-e55.
19. Jeffords MD, Batts K. Dermatology. In: O’Connor FG, Casa DJ, Davis BA, Pierre PS, Sallis RE, Wilder RP, eds. ACSM’s Sports Medicine: A Comprehensive Review. Riverwoods, IL: Wolters Kluwer; 2016:181-188.
20. Kazakova SV, Hageman JC, Matava M, et al. A clone of methicillin-resistant Staphylococcus aureus among professional football players. N Engl J Med. 2005;352(5):468-475.
21. Begier EM, Frenette K, Barrett NL, et al. A high-morbidity outbreak of methicillin-resistant Staphylococcus aureus among players on a college football team, facilitated by cosmetic body shaving and turf burns. Clin Infect Dis. 2004;39(10):1446-1453.
22. Geissler KE, Borchers JR. More than meets the eye: a rapidly progressive skin infection in a football player. Clin J Sport Med. 2015;25(3):e54-e56.
Orthopedic conditions are only one of the many medical issues football team physicians may face. In this review, we cover the management of a few of the most common nonorthopedic medical issues football team physicians are likely to encounter, including eye injuries, dental concerns, and skin conditions.
Eye Injuries
More than 2.5 million eye injuries occur each year, with 50,000 people permanently losing part or all of their vision.1 Eye injuries account for over 600,000 yearly emergency department visits; over 30% of these eye injuries were attributed to a sports injury.1 Football is classified as high risk for eye injury, along with baseball, hockey, basketball, and lacrosse.2 Common eye injury mechanisms are categorized as blunt, penetrating, and radiating. Blunt injuries are most common.2 When evaluating an athlete on the sideline, relevant history would include the size of the object, the level of force, and the direction from which the impact occurred. An examination should include best-corrected visual acuity using an eye chart, confrontational visual fields, assessment of extraocular movements, assessment of red reflex, and pupil evaluation with a light source.2
Cornea Injuries
The outermost layer of the eye, the cornea, can be subject to blunt and penetrating injuries. Corneal abrasions often occur from mechanical trauma, such as one from the fingernail of an opposing player, that disrupts the integrity of the corneal epithelium. A corneal abrasion can be identified by applying fluorescein strips after application of a topical anesthetic. Abrasions appear fluorescent green when viewed with a cobalt blue light. If an abrasion is identified, management includes preventing infection and treating pain. Prophylactic topical antibiotics can be applied, particularly for contact lens wearers. Patching has not shown benefit in treatment of pain.3 The physician can consider using topical nonsteroidal anti-inflammatory drugs, such as diclofenac or ketorolac, with a soft contact lens to treat the pain.4 The patient should follow up frequently for monitoring for infection and healing.
Orbital Fractures
Orbital fractures should be considered when an object larger than the orbital opening, such as an elbow or knee, causes blunt trauma to the surrounding bony structures, or a digital poke occurs to the globe.5 The floor of the orbit and medial wall are thin bones that often break sacrificially to protect the globe from rupture. Examination findings may include diplopia, sunken globe, numbness in the distribution of infraorbital nerve, or periorbital emphysema.6 Urgent evaluation should be considered to rule out associated intraocular damage. Imaging and a physical examination can help guide surgical management, if indicated. The most common outcome after this injury is diplopia with upper field gaze.5
Retina Issues
Trauma to the face or head may result in a separation of the retina from the underlying retinal pigment epithelium and allow vitreous fluid to seep in and further separate the layers, causing a retinal detachment. Symptoms may include flashes of light (photopsia), floaters, and visual field defects. Emergent referral is indicated, as the outcomes from this condition are time-sensitive. Consider placing an eye shield to prevent any further pressure on the globe.
Globe Injuries and Rupture
Another emergent ophthalmologic condition that can occur in football is globe rupture. Clinical findings usually prompt the clinician to consider this diagnosis. Hyphema (the collection of blood in the anterior chamber) may be seen in globe injuries. The most common clinical finding of athletes requiring hospitalization after an ocular injury is macroscopic hyphema (Figure 1).7-9
Prompt referral is warranted when there is a sudden decrease or change in vision, pain during movements, photophobia, and floaters and/or flashes.2 Consideration of return to play should take into account the patient’s vision and comfort level, which should not be masked by topical analgesics. Protective eyewear has been mandated in several sports, and has decreased the rate of eye injuries.10 Polycarbonate lenses of 3-mm thickness are recommended due to the significant comparable strength and impact-resistance.2 During the preparticipation physical for high-risk sports, the utilization of protective eyewear should be discussed.
Dental Concerns
Dental injuries may present a challenge for the sports medicine clinician. Contact injuries from elbows, fists, and other nonprojectile objects typically result in low-speed, lower-energy injuries, such as soft tissue lacerations and contusions. On the other hand, high-speed injuries occurring from balls, pucks, and sticks may result in more significant trauma. Baseball accounts for the highest percentage of sports-related dental injuries (40.2%), while basketball was second (20.2%) and football third (12.5%). Over 75% of these injuries occurred in males.11
On-field management of dental injuries should always start with the primary trauma survey, including assessment of the athlete’s airway, breathing, and circulatory function, as well as a targeted cervical spine evaluation. When obtaining a history, one should recognize the mechanism of injury and assess for signs of concomitant injuries, ie, respiratory compromise, concussion, leakage of cerebrospinal fluid, and teeth alignment. Findings from this initial evaluation may reveal critical conditions that will require management in addition to the dental injury.
Of central concern in managing dental trauma is preserving the viability of the injured structures. Therefore, much attention is paid to the pulpal and root vitality of injured teeth. The International Association of Dental Traumology Dental Trauma Guidelines recommend a biological approach to the urgent care of dental injuries:12
1. Stabilize the injury by carefully repositioning displaced entities and suturing soft tissue lacerations.
2. Eliminate or reduce the complications from bacterial contamination by rinsing and flushing with available liquids and use of chlorhexidine when possible.
3. Promote the opportunity for healing by replanting avulsed teeth and repositioning displaced teeth.
4. Make every effort to allow continued development of alveolar ridges in children.
Mouth guards are the single most effective prevention strategy for most contact sport dental injuries. One meta-analysis demonstrated a pooled 86% increased risk of orofacial injuries in nonusers.13
To review the anatomy (and injuries) of the tooth, one must consider the Ellis classification of enamel, dentin, and pulp injuries (Figure 2).
Tooth Subluxation
Tooth subluxations usually occur secondary to trauma and cause loosening of the tooth in its alveolar socket. A root fracture should be suspected in the setting of a subluxation. On exam, the tooth may be excessively mobile with gentle pressure. If unstable, immobilization with gauze packing or aluminum foil with dental follow-up is recommended.
Fractures
Ellis class I fractures are small chips in the enamel. There should be uniform color at the fracture site. A dental referral may be warranted to smooth rough enamel edges, but if no other injuries are present, these athletes may continue playing with some protection of the fractured surface. A mouth guard may be helpful to avoid mucosal lacerations.
Ellis class II fractures often present with sensitivity to inhaled air and to hot and cold temperatures. Yellow dentin is visible at the fracture site (Figure 3).
Ellis class III fractures may also present with air and temperature sensitivity. Finger pressure may expose a large fracture. Pink or red pulp is visible at the fracture site. Wiping the fracture site with sterile gauze may reveal bleeding from the pulp. This is considered a dental emergency. Immediate restriction from contact sports participation and urgent dental evaluation is indicated for root canal and capping and to prevent abscess formation.
Tooth Avulsion
Tooth avulsions occur when a tooth is completely displaced from the socket (Figure 4).
Skin Issues
Dermatological issues are some of the most common medical conditions faced by a football team physician. Skin infections in particular can pose a significant challenge both diagnostically as well as from a clearance-to-play perspective, given the potential for infections to affect other participants, such as other members of the team. Skin infection rates vary by sport and age group, with one study reporting 28.56 infections per 100,000 athletic exposures in high school wrestlers, which was more than 10 times that of football.14 Still, football players are at a higher risk of skin infections given the contact nature of the sport and close person-to-person proximity. A precise diagnosis may be difficult early in the course of a skin eruption, and with differing guidelines from various professional societies, it may be best suited for medical personnel familiar with these conditions, such as a sports medicine physician or dermatologist, to manage these athletes. A thorough and systematic evaluation is recommended, as athletes are often treated with unnecessary antibiotics, which contributes to antibiotic resistance. Previous antibiotic use may also be a risk factor for developing community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA).15
Two terms sports medicine clinicians must be familiar with are “adequately protected” and “properly covered.” The National Collegiate Athletic Association (NCAA) defines a wound or skin condition as adequately protected when the condition is considered noninfectious, adequately treated by a healthcare provider, and is able to be properly covered. A skin infection is considered properly covered when the lesion is covered by a securely attached bandage or dressing that will contain all drainage and remain intact throughout the sport activity.16
Impetigo
Impetigo is often caused by Staphylococcus and Streptococcus subspecies. The classic presentation is a dry, honey-crusted lesion with an erythematous base. Culture or gram stain may be helpful, but treatment may be initiated on a clinical basis without these studies. Topical antibiotics may be used, but in the setting of multiple lesions or an outbreak, systemic (eg, oral) antibiotics are preferred. Oral antibiotics may also shorten the time to return to play. If not responsive to the initial treatment, MRSA should be considered. No new lesions for 48 hours and a minimum of 72 hours of therapy with no moist, exudative, or draining lesions are required prior to return to play. These lesions cannot be covered as the sole means of return to play.
Methicillin-Resistant Staphylococcus aureus
MRSA is one of the most challenging skin infections for the sports medicine clinician to manage. Several outbreaks have been reported in the high school, college, and professional settings.17-20 Standardized precautions and a proactive approach are key in preventing MRSA outbreaks. It appears that different activities within a given sport may contribute to MRSA risk. One study reported football linemen had the highest attack rate, while another study reported cornerbacks and wide receivers to have the highest rate of MRSA infections.17,20 The elbow area was the most common site infected in both studies.
Abscesses are best initially managed by incision and drainage as well as obtaining wound cultures (Figure 5).
Preventative measures are thought to be useful, especially in the management of teams. The Centers for Disease Control and Prevention has published guidelines for both clinicians and patients. Precautions including hand washing; encouraging good overall hygiene; avoiding whirlpools; discouraging the sharing of towels, razors, and athletic gear; maintaining clean equipment/facilities; and encouraging early reporting of skin lesions.14,17,21,22 Isolated cases of MRSA do not need to be reported, but if more than one athlete is infected, one should notify the athletic training and team coaching staff. In the setting of an outbreak, the physician may need to notify local or state health agencies. No new lesions for 48 hours and a minimum of 72 hours of therapy with no moist, exudative, or draining lesions are required prior to returning to play. These lesions cannot be covered as the sole means of return to play.
Tinea Pedis
Tinea pedis is a common dermatophyte infection involving the feet and is most commonly caused by Trichophyton rubrum. Its distribution is usually interdigital or along the plantar surface of the foot. Topical antifungals with either allylamines or azoles are usually sufficient. Terbinafine has been shown to have a shorter duration of treatment. Athletes with tinea pedis are not restricted from sports participation during treatment, as long as the lesions are properly covered.
Tinea Corporis
Tinea corporis is a common superficial fungal infection of the body. It classically presents as pruritic, annular lesions, with well-demarcated borders and central clearing (
Tinea Cruris
Commonly known as “jock-itch,” this fungal infection is often very pruritic and involves the groin or genital region. The area is also inflamed and scaly. Treatment usually consists of topical allylamines or azoles. Allylamines amines are often preferred, as they require a shorter duration of treatment. There are no specific guidelines on the return to play with these athletes. Clearance is at the team physician’s discretion, but usually there are no restrictions. Athletes with extensive lesions may need to be disqualified from contact sports activities.
Am J Orthop. 2016;45(6):377-382. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Orthopedic conditions are only one of the many medical issues football team physicians may face. In this review, we cover the management of a few of the most common nonorthopedic medical issues football team physicians are likely to encounter, including eye injuries, dental concerns, and skin conditions.
Eye Injuries
More than 2.5 million eye injuries occur each year, with 50,000 people permanently losing part or all of their vision.1 Eye injuries account for over 600,000 yearly emergency department visits; over 30% of these eye injuries were attributed to a sports injury.1 Football is classified as high risk for eye injury, along with baseball, hockey, basketball, and lacrosse.2 Common eye injury mechanisms are categorized as blunt, penetrating, and radiating. Blunt injuries are most common.2 When evaluating an athlete on the sideline, relevant history would include the size of the object, the level of force, and the direction from which the impact occurred. An examination should include best-corrected visual acuity using an eye chart, confrontational visual fields, assessment of extraocular movements, assessment of red reflex, and pupil evaluation with a light source.2
Cornea Injuries
The outermost layer of the eye, the cornea, can be subject to blunt and penetrating injuries. Corneal abrasions often occur from mechanical trauma, such as one from the fingernail of an opposing player, that disrupts the integrity of the corneal epithelium. A corneal abrasion can be identified by applying fluorescein strips after application of a topical anesthetic. Abrasions appear fluorescent green when viewed with a cobalt blue light. If an abrasion is identified, management includes preventing infection and treating pain. Prophylactic topical antibiotics can be applied, particularly for contact lens wearers. Patching has not shown benefit in treatment of pain.3 The physician can consider using topical nonsteroidal anti-inflammatory drugs, such as diclofenac or ketorolac, with a soft contact lens to treat the pain.4 The patient should follow up frequently for monitoring for infection and healing.
Orbital Fractures
Orbital fractures should be considered when an object larger than the orbital opening, such as an elbow or knee, causes blunt trauma to the surrounding bony structures, or a digital poke occurs to the globe.5 The floor of the orbit and medial wall are thin bones that often break sacrificially to protect the globe from rupture. Examination findings may include diplopia, sunken globe, numbness in the distribution of infraorbital nerve, or periorbital emphysema.6 Urgent evaluation should be considered to rule out associated intraocular damage. Imaging and a physical examination can help guide surgical management, if indicated. The most common outcome after this injury is diplopia with upper field gaze.5
Retina Issues
Trauma to the face or head may result in a separation of the retina from the underlying retinal pigment epithelium and allow vitreous fluid to seep in and further separate the layers, causing a retinal detachment. Symptoms may include flashes of light (photopsia), floaters, and visual field defects. Emergent referral is indicated, as the outcomes from this condition are time-sensitive. Consider placing an eye shield to prevent any further pressure on the globe.
Globe Injuries and Rupture
Another emergent ophthalmologic condition that can occur in football is globe rupture. Clinical findings usually prompt the clinician to consider this diagnosis. Hyphema (the collection of blood in the anterior chamber) may be seen in globe injuries. The most common clinical finding of athletes requiring hospitalization after an ocular injury is macroscopic hyphema (Figure 1).7-9
Prompt referral is warranted when there is a sudden decrease or change in vision, pain during movements, photophobia, and floaters and/or flashes.2 Consideration of return to play should take into account the patient’s vision and comfort level, which should not be masked by topical analgesics. Protective eyewear has been mandated in several sports, and has decreased the rate of eye injuries.10 Polycarbonate lenses of 3-mm thickness are recommended due to the significant comparable strength and impact-resistance.2 During the preparticipation physical for high-risk sports, the utilization of protective eyewear should be discussed.
Dental Concerns
Dental injuries may present a challenge for the sports medicine clinician. Contact injuries from elbows, fists, and other nonprojectile objects typically result in low-speed, lower-energy injuries, such as soft tissue lacerations and contusions. On the other hand, high-speed injuries occurring from balls, pucks, and sticks may result in more significant trauma. Baseball accounts for the highest percentage of sports-related dental injuries (40.2%), while basketball was second (20.2%) and football third (12.5%). Over 75% of these injuries occurred in males.11
On-field management of dental injuries should always start with the primary trauma survey, including assessment of the athlete’s airway, breathing, and circulatory function, as well as a targeted cervical spine evaluation. When obtaining a history, one should recognize the mechanism of injury and assess for signs of concomitant injuries, ie, respiratory compromise, concussion, leakage of cerebrospinal fluid, and teeth alignment. Findings from this initial evaluation may reveal critical conditions that will require management in addition to the dental injury.
Of central concern in managing dental trauma is preserving the viability of the injured structures. Therefore, much attention is paid to the pulpal and root vitality of injured teeth. The International Association of Dental Traumology Dental Trauma Guidelines recommend a biological approach to the urgent care of dental injuries:12
1. Stabilize the injury by carefully repositioning displaced entities and suturing soft tissue lacerations.
2. Eliminate or reduce the complications from bacterial contamination by rinsing and flushing with available liquids and use of chlorhexidine when possible.
3. Promote the opportunity for healing by replanting avulsed teeth and repositioning displaced teeth.
4. Make every effort to allow continued development of alveolar ridges in children.
Mouth guards are the single most effective prevention strategy for most contact sport dental injuries. One meta-analysis demonstrated a pooled 86% increased risk of orofacial injuries in nonusers.13
To review the anatomy (and injuries) of the tooth, one must consider the Ellis classification of enamel, dentin, and pulp injuries (Figure 2).
Tooth Subluxation
Tooth subluxations usually occur secondary to trauma and cause loosening of the tooth in its alveolar socket. A root fracture should be suspected in the setting of a subluxation. On exam, the tooth may be excessively mobile with gentle pressure. If unstable, immobilization with gauze packing or aluminum foil with dental follow-up is recommended.
Fractures
Ellis class I fractures are small chips in the enamel. There should be uniform color at the fracture site. A dental referral may be warranted to smooth rough enamel edges, but if no other injuries are present, these athletes may continue playing with some protection of the fractured surface. A mouth guard may be helpful to avoid mucosal lacerations.
Ellis class II fractures often present with sensitivity to inhaled air and to hot and cold temperatures. Yellow dentin is visible at the fracture site (Figure 3).
Ellis class III fractures may also present with air and temperature sensitivity. Finger pressure may expose a large fracture. Pink or red pulp is visible at the fracture site. Wiping the fracture site with sterile gauze may reveal bleeding from the pulp. This is considered a dental emergency. Immediate restriction from contact sports participation and urgent dental evaluation is indicated for root canal and capping and to prevent abscess formation.
Tooth Avulsion
Tooth avulsions occur when a tooth is completely displaced from the socket (Figure 4).
Skin Issues
Dermatological issues are some of the most common medical conditions faced by a football team physician. Skin infections in particular can pose a significant challenge both diagnostically as well as from a clearance-to-play perspective, given the potential for infections to affect other participants, such as other members of the team. Skin infection rates vary by sport and age group, with one study reporting 28.56 infections per 100,000 athletic exposures in high school wrestlers, which was more than 10 times that of football.14 Still, football players are at a higher risk of skin infections given the contact nature of the sport and close person-to-person proximity. A precise diagnosis may be difficult early in the course of a skin eruption, and with differing guidelines from various professional societies, it may be best suited for medical personnel familiar with these conditions, such as a sports medicine physician or dermatologist, to manage these athletes. A thorough and systematic evaluation is recommended, as athletes are often treated with unnecessary antibiotics, which contributes to antibiotic resistance. Previous antibiotic use may also be a risk factor for developing community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA).15
Two terms sports medicine clinicians must be familiar with are “adequately protected” and “properly covered.” The National Collegiate Athletic Association (NCAA) defines a wound or skin condition as adequately protected when the condition is considered noninfectious, adequately treated by a healthcare provider, and is able to be properly covered. A skin infection is considered properly covered when the lesion is covered by a securely attached bandage or dressing that will contain all drainage and remain intact throughout the sport activity.16
Impetigo
Impetigo is often caused by Staphylococcus and Streptococcus subspecies. The classic presentation is a dry, honey-crusted lesion with an erythematous base. Culture or gram stain may be helpful, but treatment may be initiated on a clinical basis without these studies. Topical antibiotics may be used, but in the setting of multiple lesions or an outbreak, systemic (eg, oral) antibiotics are preferred. Oral antibiotics may also shorten the time to return to play. If not responsive to the initial treatment, MRSA should be considered. No new lesions for 48 hours and a minimum of 72 hours of therapy with no moist, exudative, or draining lesions are required prior to return to play. These lesions cannot be covered as the sole means of return to play.
Methicillin-Resistant Staphylococcus aureus
MRSA is one of the most challenging skin infections for the sports medicine clinician to manage. Several outbreaks have been reported in the high school, college, and professional settings.17-20 Standardized precautions and a proactive approach are key in preventing MRSA outbreaks. It appears that different activities within a given sport may contribute to MRSA risk. One study reported football linemen had the highest attack rate, while another study reported cornerbacks and wide receivers to have the highest rate of MRSA infections.17,20 The elbow area was the most common site infected in both studies.
Abscesses are best initially managed by incision and drainage as well as obtaining wound cultures (Figure 5).
Preventative measures are thought to be useful, especially in the management of teams. The Centers for Disease Control and Prevention has published guidelines for both clinicians and patients. Precautions including hand washing; encouraging good overall hygiene; avoiding whirlpools; discouraging the sharing of towels, razors, and athletic gear; maintaining clean equipment/facilities; and encouraging early reporting of skin lesions.14,17,21,22 Isolated cases of MRSA do not need to be reported, but if more than one athlete is infected, one should notify the athletic training and team coaching staff. In the setting of an outbreak, the physician may need to notify local or state health agencies. No new lesions for 48 hours and a minimum of 72 hours of therapy with no moist, exudative, or draining lesions are required prior to returning to play. These lesions cannot be covered as the sole means of return to play.
Tinea Pedis
Tinea pedis is a common dermatophyte infection involving the feet and is most commonly caused by Trichophyton rubrum. Its distribution is usually interdigital or along the plantar surface of the foot. Topical antifungals with either allylamines or azoles are usually sufficient. Terbinafine has been shown to have a shorter duration of treatment. Athletes with tinea pedis are not restricted from sports participation during treatment, as long as the lesions are properly covered.
Tinea Corporis
Tinea corporis is a common superficial fungal infection of the body. It classically presents as pruritic, annular lesions, with well-demarcated borders and central clearing (
Tinea Cruris
Commonly known as “jock-itch,” this fungal infection is often very pruritic and involves the groin or genital region. The area is also inflamed and scaly. Treatment usually consists of topical allylamines or azoles. Allylamines amines are often preferred, as they require a shorter duration of treatment. There are no specific guidelines on the return to play with these athletes. Clearance is at the team physician’s discretion, but usually there are no restrictions. Athletes with extensive lesions may need to be disqualified from contact sports activities.
Am J Orthop. 2016;45(6):377-382. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Owens PL, Mutter R. Emergency Department Visits Related to Eye Injuries, 2008. Agency for Healthcare Research and Quality Web site. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb112.pdf. Published May 2011. Accessed August 18, 2016.
2. Rodriguez JO, Lavina AM, Agarwai A. Prevention and treatment of common eye injuries in sports. Am Fam Physician. 2003;67(7):1481-1496.
3. Lim CH, Turner A, Lim BX. Patching for corneal abrasion. Cochrane Database Syst Rev. 2016;7:CD004764.
4. Weaver CS, Terrell KM. Evidence-based emergency medicine. Update: do ophthalmic nonsteroidal anti-inflammatory drugs reduce the pain associated with simple corneal abrasion without delaying healing? Ann Emerg Med. 2003;41(1):134-140.
5. Williams RJ 3rd, Marx RG, Barnes R, O’Brien SJ, Warren RF. Fractures about the orbit in professional American football players. Am J Sports Med. 2001;29(1):55-57.
6. Forrest LA, Schuller DE, Strauss RH. Management of orbital blow-out fractures. Case reports and discussion. Am J Sports Med. 1989;17(2):217-220.
7. Barr A, Baines PS, Desai P, MacEwen CJ. Ocular sports injuries: the current picture. Br J Sports Med. 2000;34(6):456-458.
8. Pokhrel PK, Loftus SA. Ocular emergencies. Am Fam Physician. 2007;76(6):829-836.
9. Usatine RP, Smith MA, Mayeaux EJ Jr, Chumley H. Eye Trauma—Hyphema. The Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013.
10. Lincoln AE, Caswell SV, Almquist JL, et al. Effectiveness of the women’s lacrosse protective eyewear mandate in the reduction of eye injuries. Am J Sports Med. 2012;40(3):611-614.
11. Stewart GB, Shields BJ, Fields S, Comstock RD, Smith GA. Consumer products and activities associated with dental injuries to children treated in United States emergency departments, 1990-2003. Dental Traumatol. 2009;25(4):399-405.
12. Bakland LK. Dental trauma guidelines. Pediatric Dent. 2013;35(2):106-108.
13. Knapik J, Marshall SW, Lee RB, et al. Mouthguards in sport activities: history, physical properties and Injury prevention effectiveness. Sports Med. 2007;37(2):117-144.
14. Ashack KA, Burton KA, Johnson TR, Currie DW, Comstock RD, Dellavalle RP. Skin infections among US high school athletes: a national survey. J Am Acad Dermatol. 2016;74(4):679-684.e1.
15. Ellis MW, Hospenthal DR, Dooley DP, Gray PJ, Murray CK. Natural history of community-acquired methicillin-resistant Staphylococcus aureus colonization and infection in soldiers. Clin Infect Dis. 2004;39(7):971-979.
16. The National Collegiate Athletic Association. 2014-15 NCAA Sports Medicine Handbook. http://www.ncaapublications.com/productdownloads/MD15.pdf. Revised June 2008. Accessed August 18, 2016.
17. Anderson BJ. The effectiveness of valacyclovir in preventing reactivation of herpes gladiatorum in wrestlers. Clin J Sport Med. 1999;9(2):86-90.
18. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18-e55.
19. Jeffords MD, Batts K. Dermatology. In: O’Connor FG, Casa DJ, Davis BA, Pierre PS, Sallis RE, Wilder RP, eds. ACSM’s Sports Medicine: A Comprehensive Review. Riverwoods, IL: Wolters Kluwer; 2016:181-188.
20. Kazakova SV, Hageman JC, Matava M, et al. A clone of methicillin-resistant Staphylococcus aureus among professional football players. N Engl J Med. 2005;352(5):468-475.
21. Begier EM, Frenette K, Barrett NL, et al. A high-morbidity outbreak of methicillin-resistant Staphylococcus aureus among players on a college football team, facilitated by cosmetic body shaving and turf burns. Clin Infect Dis. 2004;39(10):1446-1453.
22. Geissler KE, Borchers JR. More than meets the eye: a rapidly progressive skin infection in a football player. Clin J Sport Med. 2015;25(3):e54-e56.
1. Owens PL, Mutter R. Emergency Department Visits Related to Eye Injuries, 2008. Agency for Healthcare Research and Quality Web site. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb112.pdf. Published May 2011. Accessed August 18, 2016.
2. Rodriguez JO, Lavina AM, Agarwai A. Prevention and treatment of common eye injuries in sports. Am Fam Physician. 2003;67(7):1481-1496.
3. Lim CH, Turner A, Lim BX. Patching for corneal abrasion. Cochrane Database Syst Rev. 2016;7:CD004764.
4. Weaver CS, Terrell KM. Evidence-based emergency medicine. Update: do ophthalmic nonsteroidal anti-inflammatory drugs reduce the pain associated with simple corneal abrasion without delaying healing? Ann Emerg Med. 2003;41(1):134-140.
5. Williams RJ 3rd, Marx RG, Barnes R, O’Brien SJ, Warren RF. Fractures about the orbit in professional American football players. Am J Sports Med. 2001;29(1):55-57.
6. Forrest LA, Schuller DE, Strauss RH. Management of orbital blow-out fractures. Case reports and discussion. Am J Sports Med. 1989;17(2):217-220.
7. Barr A, Baines PS, Desai P, MacEwen CJ. Ocular sports injuries: the current picture. Br J Sports Med. 2000;34(6):456-458.
8. Pokhrel PK, Loftus SA. Ocular emergencies. Am Fam Physician. 2007;76(6):829-836.
9. Usatine RP, Smith MA, Mayeaux EJ Jr, Chumley H. Eye Trauma—Hyphema. The Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013.
10. Lincoln AE, Caswell SV, Almquist JL, et al. Effectiveness of the women’s lacrosse protective eyewear mandate in the reduction of eye injuries. Am J Sports Med. 2012;40(3):611-614.
11. Stewart GB, Shields BJ, Fields S, Comstock RD, Smith GA. Consumer products and activities associated with dental injuries to children treated in United States emergency departments, 1990-2003. Dental Traumatol. 2009;25(4):399-405.
12. Bakland LK. Dental trauma guidelines. Pediatric Dent. 2013;35(2):106-108.
13. Knapik J, Marshall SW, Lee RB, et al. Mouthguards in sport activities: history, physical properties and Injury prevention effectiveness. Sports Med. 2007;37(2):117-144.
14. Ashack KA, Burton KA, Johnson TR, Currie DW, Comstock RD, Dellavalle RP. Skin infections among US high school athletes: a national survey. J Am Acad Dermatol. 2016;74(4):679-684.e1.
15. Ellis MW, Hospenthal DR, Dooley DP, Gray PJ, Murray CK. Natural history of community-acquired methicillin-resistant Staphylococcus aureus colonization and infection in soldiers. Clin Infect Dis. 2004;39(7):971-979.
16. The National Collegiate Athletic Association. 2014-15 NCAA Sports Medicine Handbook. http://www.ncaapublications.com/productdownloads/MD15.pdf. Revised June 2008. Accessed August 18, 2016.
17. Anderson BJ. The effectiveness of valacyclovir in preventing reactivation of herpes gladiatorum in wrestlers. Clin J Sport Med. 1999;9(2):86-90.
18. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18-e55.
19. Jeffords MD, Batts K. Dermatology. In: O’Connor FG, Casa DJ, Davis BA, Pierre PS, Sallis RE, Wilder RP, eds. ACSM’s Sports Medicine: A Comprehensive Review. Riverwoods, IL: Wolters Kluwer; 2016:181-188.
20. Kazakova SV, Hageman JC, Matava M, et al. A clone of methicillin-resistant Staphylococcus aureus among professional football players. N Engl J Med. 2005;352(5):468-475.
21. Begier EM, Frenette K, Barrett NL, et al. A high-morbidity outbreak of methicillin-resistant Staphylococcus aureus among players on a college football team, facilitated by cosmetic body shaving and turf burns. Clin Infect Dis. 2004;39(10):1446-1453.
22. Geissler KE, Borchers JR. More than meets the eye: a rapidly progressive skin infection in a football player. Clin J Sport Med. 2015;25(3):e54-e56.
In My Athletic Trainer’s Bag
Editor’s Note: Doug Quon, MAT, ATC, PES, is the Assistant Athletic Trainer for the Washington Redskins. Click the PDF button below to view and download his list of the essential components of an athletic trainer’s bag for high school football
Editor’s Note: Doug Quon, MAT, ATC, PES, is the Assistant Athletic Trainer for the Washington Redskins. Click the PDF button below to view and download his list of the essential components of an athletic trainer’s bag for high school football
Editor’s Note: Doug Quon, MAT, ATC, PES, is the Assistant Athletic Trainer for the Washington Redskins. Click the PDF button below to view and download his list of the essential components of an athletic trainer’s bag for high school football
Knee Injuries in American Football: An Epidemiological Review
Football is one of the most popular sports in the United States. Every year more than 1 million high school males and over 60,000 collegiate males participate in organized football. The number of males who play football is greater than the combined number of males and females who participate in track and field or basketball.1 Football has the highest injury rate amongst popular American sports.2 From 2001 to 2005, there was an estimated 1.1 million emergency room visits as a direct result of football.3 Injuries are more likely to occur during games,1,2,4,5 more likely to require surgery,4 and more likely to end the player’s season or career when compared to other sports.6 Of those injuries that end seasons or careers, the knee is the most common culprit.6 This is of particular concern because knee injuries are most common in football.1,2,5,7 This article reviews the epidemiology of 4 of the most common knee injuries in American football: tears of the anterior cruciate ligament (ACL), medial collateral ligament (MCL), medial patellofemoral ligament (MPFL), and posterior cruciate ligament (PCL).
Anterior Cruciate Ligament
The ACL is the primary structure preventing anterior tibial translation. It is composed of 2 anatomic bundles: the anteromedial and posterolateral bundles. The ACL originates from the posteromedial portion of the lateral femoral condyle and inserts between and slightly anterior to the tibial intercondylar eminence. The bundles are named for their relative insertions onto the tibia.
Injury to the ACL occurs both through noncontact and contact mechanisms. Typical noncontact mechanism is a forceful valgus collapse with the knee close to full extension with combined external or internal rotation of the tibia.8 This is often the result of a sudden deceleration prior to a change in direction.9 Contact injuries to the ACL are the result of a direct blow to the knee causing valgus collapse.9 The majority of ACL injuries amongst all sports are a result of a noncontact mechanism. However, Dragoo and colleagues10 found the majority of football ACL injuries (55%-60%) were from contact. As a result, football players are 4 times more likely to sustain ACL injuries than in other sports.11
ACL injuries are associated with significant time loss from sport. At the high school level, they are the most likely injury to end a season or career.6 Because these are higher-energy injuries, they are frequently associated with damage to additional structures. ACL injuries that occur in football are associated with increased rates of meniscus, chondral, and multi-ligamentous injuries.12,13
The incidence of ACL injuries increases with level of competition. In high school athletes it is 11.1 per 100,000 athlete exposures (AE).1,11 In collegiate football, the rate increases to 14.2 to 18 per 100,000 AE.2,14 Though no incidence data per AE was found in our review of the literature, there were 219 ACL injuries in the National Football League (NFL) from 2010 to 2013.15 In addition, 14.2% of retired NFL athletes in one survey reported a history of ACL injury.16
The most common high-risk positions are running backs and linebackers. Brophy and colleagues17 found that 9.7% of running backs and 8.9% of linebackers participating in the NFL Combine had a history of ACL injury. This may be because both the running back and linebacker are involved in frequent high-energy collisions and often quickly change direction. Other studies have also identified running backs and linebackers as high risk, in addition to tight ends, wide receivers, and interior linemen.13,15,18
Treatment of choice for elite level athletes with ACL injury is reconstruction.19 Of those who undergo ACL reconstruction, the rate of return to play ranges from 63% to 80%.20-22 The average time to return to play is 9 to 13 months. The odds of making a successful return hinges on how successful the athlete was prior to injury. Factors such as prior game experience, position on depth chart, being on scholarship, and draft position for NFL athletes have all been shown to have a positive predictive value on a patient’s chance of returning from ACL reconstruction.20,21
Players who return have variable levels of success afterwards. In a study of NFL quarterbacks who sustained ACL injuries, 12 out of 13 were able to return to game action with no appreciable dropoff in performance based on in-game production.23 Carey and colleagues24 looked specifically at NFL wide receivers and running backs and found an 80% return to play rate but with an approximate decrease in production of one-third upon return. Furthermore, in the Multicenter Orthopaedic Outcomes Network (MOON) cohort study, only 43% of participants felt they returned to their preoperative level.22
Medial Collateral Ligament
The MCL consists of superficial and deep components. The superficial MCL is the primary restraint to valgus laxity at the knee. The superficial MCL has 1 femoral and 2 tibial attachments. The deep MCL is a thickening of the medial joint capsule and runs deep and parallel to the superficial MCL. The amount of medial joint gapping with a valgus force on examination is used to grade severity of MCL injuries. Grade I is a <5-mm opening; Grade II, 5- to 10-mm opening; and grade III, >10-mm opening.
The MCL is the most common knee injury in high school, collegiate, and professional football.1,18,25-28 Injuries are typically due to contact when a valgus force is applied to the knee.29 The annual incidence of MCL injuries amongst high school football players is 24.2 per 100,000 AE.1 The positions that appear to be at greatest risk for MCL injuries are offensive and defensive linemen.18,30-32 In a review of 5047 collegiate athletes participating in the NFL Combine from 1987 to 2000, 23% of offensive linemen had a history of MCL injury, compared to the overall rate of 16%.33 In a similar study, Bradley and colleagues18 performed medical histories on athletes invited to the 2005 NFL Combine and also found offensive linemen had the highest rate of MCL injury at 33%, compared to the overall rate of 22%. They reasonably hypothesized that “chop blocks” and other players “rolling up” on the outside of linemen’s knees were responsible for these injuries. Albright and colleagues32 found that prophylactic knee braces decreased the incidence of MCL injuries in collegiate offensive lineman. However, additional studies have not been able to reproduce these results and the use of prophylactic knee braces remains controversial.26
Treatment of MCL injuries depends upon the grade of injury, associated injuries, and anatomical location of injury. Management of MCL injuries is for the most part nonsurgical. In 1974, Ellsasser and colleagues34 were the first to publish data on nonoperative management of Grade I and Grade II injuries with immediate motion and rehabilitation instead of cast immobilization. They found 93% of patients returned to football in 3 to 8 weeks.34 Derscheid and Garrick27 observed nonoperative treatment of Grade I and II sprains in collegiate football players, with a time loss of 10.6 days and 19.5 days for Grade I and II injuries, respectively. Holden and colleagues35 evaluated nonoperative management of Grade I and II MCL injuries in collegiate football players and found an average return to play of 21 days.
Grade III injury treatment is more controversial. Indelicato and colleagues36 demonstrated successful nonoperative management of Grade III MCL injuries in collegiate football players, with an average return to play of 64.4 days. Jones and colleagues37 had similar success with high school football players, with an average return to play of 34 days. However, isolated Grade III injuries are rare and therefore treatment is likely to be dictated by concomitant injuries. Fetto and Marshall38 found that 78% of Grade III injuries were associated with an additional ligamentous injury. Of those additional injuries, 95% were ACL tears.
Finally, one must consider the location of the MCL injury. Injuries of the distal MCL at its tibial insertion may result in poor healing, as the ligament is displaced away from its insertion. Therefore, some authors recommend surgical management for these injuries.39,40
The patellofemoral joint is a complex structure in which the patella is stabilized within the trochlear groove of the femur by both bony and soft tissue structures. The MPFL is one of the most important soft tissue stabilizers. The MPFL is the primary restraint to lateral patellar translation within the first 20° of knee flexion, contributing to 60% of the total restraining force.41 The MPFL originates on the medial femoral condyle and inserts on the superomedial aspect of the patella.
Patellar instability is the subluxation or dislocation of the patella out of the trochlear groove. Patellar subluxation and dislocation account for approximately 3% of all knee injuries.42 Patella dislocations are more common in younger populations43-45 with the majority (52%-63%) occurring during sports.43,44,46 Mitchell and colleagues47 reported an incidence of 4.1 patellar subluxations/dislocations per 100,000 AE in high school football players.
Dislocation is most commonly the result of knee flexion with the tibia in a valgus position.44,48 The majority of patellar dislocations occur via a noncontact mechanism.44,48 However, the majority of these injuries in football are from contact (63%).47
Acute patellar dislocations are associated with more soft tissue damage than those with recurrent dislocations.46 In acute patella dislocations, the MPFL is almost always ruptured.44 In contrast, Fithian and colleagues46 found only 38% of recurrent dislocators had MPFL injury. As a result, it is thought that those with recurrent instability dislocate without trauma and do not have the same characteristics as those who dislocate from high-energy trauma in sport. Risk factors for atraumatic dislocation are numerous and have been well described in the literature.49 However, traumatic dislocators usually do not have risk factors.50
Traumatic patella dislocations are higher energy and are associated with chondral injury in up to 95%of cases 51 and osteochondral injury 58% to 76% of the time.52,53 In contrast, people with “articular hypermobility” are less likely to sustain articular damage.54 This concept is important when considering risk for recurrent patella dislocation. The literature reports a 17% to 50% rate of recurrent instability after acute patella dislocation.46,55,56 However, most studies do not distinguish between traumatic and atraumatic injuries. Because the majority of patellar dislocations in football occur through contact mechanisms, the rate of recurrent instability in these athletes may in fact be less than what is reported in the literature.
First-time patella dislocations are generally treated nonoperatively. Mitchell and colleagues47 reported that 72.6% of high school athletes with patella subluxation treated conservatively were able to return to sports within 3 weeks, compared to only 34.1% of those with patellar dislocations. In the same study, patellar dislocations were season-ending 37% of the time.47 Atkin and colleagues50 followed 74 patients treated conservatively for first-time patellar dislocation and noted 58% at 6 months still had difficulty in squatting, jumping, or cutting.
Those who have failed conservative management and have an additional dislocation are 7 times more likely to redislocate.46 Therefore, they are usually treated operatively with MPFL reconstruction. Return to sport ranges from 3 to 6 months,57 with 53% to 77.3% reporting return to their previous functionality.57-59 Overall, 84.1% of patients are able to return to sport with 1.2% risk of recurrent dislocation.60
Posterior Cruciate Ligament
The PCL is the primary posterior stabilizer of the knee.61,62 It consists of the anterolateral and posteromedial bundles, named by their insertion on the posterior tibial plateau. The larger, stronger anterolateral bundle is the primary restraint to posterior tibial translation.63
Due to the relative infrequency of PCL injuries, there is a paucity of epidemiological data on sports-related PCL injuries. These injuries in the literature are commonly found due to traffic accidents (45%-57%) or from sports (33%-40%).64,65 According to Swensen and colleagues,1 PCL injuries account for 2.4% of all high school sport knee injuries. In a cohort of 62 knees with PCL injuries, Patel and colleagues66 found football was the most common cause of injury (19.3%).
The most common mechanism of injury in athletes is knee hyperflexion or a direct blow to the tibia in a flexed knee.67 In football, contact mechanisms are the most common. In a 16-year review of the National Collegiate Athletic Association (NCAA) injury surveillance system, the incidence of contact PCL injuries during games were 7.3 times higher than noncontact.68 The most common activity was being tackled, which accounted for 22.9% of all PCL injuries.68
Due to the high energy of these injuries, isolated PCL injuries are rare. In one trauma center’s experience, 96.5% of PCL injuries had an additional ligament injury.64 In that study, injuries to the PCL were associated with posterolateral corner, ACL, and MCL injuries 62%, 46%, and 31% of the time, respectively.64,69
Because isolated PCL injuries are rare, clinicians must rely on a thorough history and physical examination when evaluating athletes with knee injuries. Classification of PCL injuries is based on the amount of posterior tibial translation in relation to the femur with the knee bent to 90°. Grade I is 1 to 5 mm; Grade II, 6 to 10 mm; and Grade III, >10 mm. If there is suspicion of a PCL injury, there should be a very low threshold for magnetic resonance imaging, given the high association with additional injuries.
Natural history of Grade I and II isolated PCL injuries is generally favorable compared to Grade III and multi-ligamentous injuries.70 As a result, isolated Grade I and II PCL injuries are generally treated nonoperatively. Treatment consists of physical therapy with emphasis on quadriceps strengthening. Return to play can be considered as early as 2 to 4 weeks from injury.71 Recent long-term data have shown successful conservative management of Grade I and II injuries with quadriceps strength to 97% of contralateral leg and full range of motion.72 However, there was 11% moderate to severe osteoarthritis in these patients at a mean follow-up of 14.3 years.72 Fowler and Messieh67 managed athletes with 7 isolated complete PCL tears and 5 partial tears nonoperatively, all of whom were able to return to sport without limitation. Parolie and Bergfeld73 managed 25 athletes with isolated PCL tears conservatively. In this study, 80% of athletes reported satisfaction and 68% returned to previous level of play.73 Neither of the aforementioned studies specify the grades of the injuries. Finally, Patel and colleagues66 managed 6 NFL athletes with Grade I and II injuries nonoperatively, and all were able to return to sport.
Treatment of isolated Grade III PCL injuries is more controversial, and no consensus exists in the literature. In an epidemiological study, Dick and colleagues68 found that only 39% of NCAA football athletes underwent surgery for their torn PCLs, compared to 79% of ACL injuries. However, their study makes no mention to the severity of these injuries. Numerous options exist for PCL reconstruction, with no consensus on the preferred method.
Conclusion
Knee injuries are the most common injury in football. Knowledge of the natural history of these injuries, as well as treatment options and expected outcomes, will help treating physicians educate their patients on the optimal treatment and manage return to play expectations.
Am J Orthop. 2016;45(6):368-373. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Swenson DM, Collins CL, Best TM, Flanigan DC, Fields SK, Comstock RD. Epidemiology of knee injuries among U.S. high school athletes, 2005/2006-2010/2011. Med Sci Sports Exerc. 2013;45(3):462-469.
2. Hootman JM, Dick R, Agel J. Epidemiology of collegiate injuries for 15 sports: summary and recommendations for injury prevention initiatives. J Athl Train. 2007;42(2):311-319.
3. Mello MJ, Myers R, Christian JB, Palmisciano L, Linakis JG. Injuries in youth football: national emergency department visits during 2001-2005 for young and adolescent players. Acad Emerg Med. 2009;16(3):243-248.
4. Rechel JA, Collins CL, Comstock RD. Epidemiology of injuries requiring surgery among high school athletes in the United States, 2005 to 2010. J Trauma. 2011;71(4):982-989.
5. Ingram JG, Fields SK, Yard EE, Comstock RD. Epidemiology of knee injuries among boys and girls in US high school athletics. Am J Sports Med. 2008;36(6):1116-1122.
6. Tirabassi J, Brou L, Khodaee M, Lefort R, Fields SK, Comstock RD. Epidemiology of high school sports-related injuries resulting in medical disqualification: 2005-2006 through 2013-2014 academic years. Am J Sports Med. 2016 May 10. [Epub ahead of print]
7. Fernandez WG, Yard EE, Comstock RD. Epidemiology of lower extremity injuries among U.S. high school athletes. Acad Emerg Med. 2007;14(7):641-645.
8. Olsen OE, Myklebust G, Engebretsen L, Bahr R. Injury mechanisms for anterior cruciate ligament injuries in team handball: a systematic video analysis. Am J Sports Med. 2004;32(4):1002-1012.
9. Boden BP, Dean GS, Feagin JA Jr, Garrett WE Jr. Mechanisms of anterior cruciate ligament injury. Orthopedics. 2000;23(6):573-578.
10. Dragoo JL, Braun HJ, Harris AH. The effect of playing surface on the incidence of ACL injuries in National Collegiate Athletic Association American Football. Knee. 2013;20(3):191-195.
11. Joseph AM, Collins CL, Henke NM, Yard EE, Fields SK, Comstock RD. A multisport epidemiologic comparison of anterior cruciate ligament injuries in high school athletics. J Athl Train. 2013;48(6):810-817.
12. Granan LP, Inacio MC, Maletis GB, Funahashi TT, Engebretsen L. Sport-specific injury pattern recorded during anterior cruciate ligament reconstruction. Am J Sports Med. 2013;41(12):2814-2818.
13. Bradley JP, Klimkiewicz JJ, Rytel MJ, Powell JW. Anterior cruciate ligament injuries in the National Football League: epidemiology and current treatment trends among team physicians. Arthroscopy. 2002;18(5):502-509.
14. Dragoo JL, Braun HJ, Durham JL, Chen MR, Harris AH. Incidence and risk factors for injuries to the anterior cruciate ligament in National Collegiate Athletic Association football: data from the 2004-2005 through 2008-2009 National Collegiate Athletic Association Injury Surveillance System. Am J Sports Med. 2012;40(5):990-995.
15. Dodson CC, Secrist ES, Bhat SB, Woods DP, Deluca PF. Anterior cruciate ligamenti in National Football League athletes from 2010 to 2013: a descriptive epidemiology study. Orthop J Sports Med. 2016;4(3):2325967116631949.
16. Golightly YM, Marshall SW, Callahan LF, Guskiewicz K. Early-onset arthritis in retired National Football League players. J Phys Act Health. 2009;6(5):638-643.
17. Brophy RH, Lyman S, Chehab EL, Barnes RP, Rodeo SA, Warren RF. Predictive value of prior injury on career in professional American football is affected by player position. Am J Sports Med. 2009;37(4):768-775.
18. Bradley J, Honkamp NJ, Jost P, West R, Norwig J, Kaplan LD. Incidence and variance of knee injuries in elite college football players. Am J Orthop. 2008;37(6):310-314.
19. Erickson BJ, Harris JD, Fillingham YA, et al. Anterior cruciate ligament reconstruction practice patterns by NFL and NCAA football team physicians. Arthroscopy. 2014;30(6):731-738.
20. Daruawalla JH, Greis PE, Hancock R; ASP Collaborative Group, Xerogeanes JW. Rates and determinants of return to play after anterior cruciate ligament reconstruction in NCAA Division 1 college football athletes: a study of the ACC, SEC, and PAC-12 conferences. Orthop J Sports Med. 2014;2(8):2325967114543901.
21. Shah VM, Andrews JR, Fleisig GS, McMichael CS, Lemak LJ. Return to play after anterior cruciate ligament reconstruction in National Football League athletes. Am J Sports Med. 2010;38(11):2233-2239.
22. McCullough KA, Phelps KD, Spindler KP, et al. Return to high school- and college-level football after anterior cruciate ligament reconstruction: a Multicenter Orthopaedic Outcomes Network (MOON) cohort study. Am J Sports Med. 2012;40(11):2523-2529.
23. Erickson BJ, Harris JD, Heninger JR, et al. Performance and return-to-sport after ACL reconstruction in NFL quarterbacks. Orthopedics. 2014;37(8):e728-e734.
24. Carey JL, Huffman GR, Parekh SG, Sennett BJ. Outcomes of anterior cruciate ligament injuries to running backs and wide receivers in the National Football League. Am J Sports Med. 2006;34(12):1911-1917.
25. Hershman EB, Anderson R, Bergfeld JA, et al. An analysis of specific lower extremity injury rates on grass and FieldTurf playing surfaces in National Football League Games: 2000-2009 seasons. Am J Sports Med. 2012;40(10):2200-2205.
26. Salata MJ, Gibbs AE, Sekiya JK. The effectiveness of prophylactic knee bracing in American football: a systematic review. Sports Health. 2010;2(5):375-379.
27. Derscheid GL, Garrick JG. Medial collateral ligament injuries in football. Nonoperative management of grade I and grade II sprains. Am J Sports Med. 1981;9(6):365-368.
28. Meyers MC, Barnhill BS. Incidence, causes, and severity of high school football injuries on FieldTurf versus natural grass: a 5-year prospective study. Am J Sports Med. 2004;32(7):1626-1638.
29. Lundblad M, Waldén M, Magnusson H, Karlsson J, Ekstrand J. The UEFA injury study: 11-year data concerning 346 MCL injuries and time to return to play. Br J Sports Med. 2013;47(12):759-762.
30. Hewson GF Jr, Mendini RA, Wang JB. Prophylactic knee bracing in college football. Am J Sports Med. 1986;14(4):262-266.
31. Rovere GD, Haupt HA, Yates CS. Prophylactic knee bracing in college football. Am J Sports Med. 1987;15(2):111-116.
32. Albright JP, Powell JW, Smith W, et al. Medial collateral ligament knee sprains in college football. Brace wear preferences and injury risk. Am J Sports Med. 1994;22(1):2-11.
33. Brophy RH, Barnes R, Rodeo SA, Warren RF. Prevalence of musculoskeletal disorders at the NFL Combine--trends from 1987 to 2000. Med Sci Sports Exerc. 2007;39(1):22-27.
34. Ellsasser JC, Reynolds FC, Omohundro JR. The non-operative treatment of collateral ligament injuries of the knee in professional football players. An analysis of seventy-four injuries treated non-operatively and twenty-four injuries treated surgically. J Bone Joint Surg Am. 1974;56(6):1185-1190.
35. Holden DL, Eggert AW, Butler JE. The nonoperative treatment of grade I and II medial collateral ligament injuries to the knee. Am J Sports Med. 1983;11(5):340-344.
36. Indelicato PA, Hermansdorfer J, Huegel M. Nonoperative management of complete tears of the medial collateral ligament of the knee in intercollegiate football players. Clin Orthop Relat Res. 1990;(256):174-177.
37. Jones RE, Henley MB, Francis P. Nonoperative management of isolated grade III collateral ligament injury in high school football players. Clin Orthop Relat Res. 1986;(213):137-140.
38. Fetto JF, Marshall JL. Medial collateral ligament injuries of the knee: a rationale for treatment. Clin Orthop Relat Res. 1978;(132):206-218.
39. Corten K, Hoser C, Fink C, Bellemans J. Case reports: a Stener-like lesion of the medial collateral ligament of the knee. Clin Orthop Relat Res. 2010;468(1):289-293.
40. Marchant MH Jr, Tibor LM, Sekiya JK, Hardaker WT Jr, Garrett WE Jr, Taylor DC. Management of medial-sided knee injuries, part 1: medial collateral ligament. Am J Sports Med. 2011;39(5):1102-1113.
41. Desio SM, Burks RT, Bachus KN. Soft tissue restraints to lateral patellar translation in the human knee. Am J Sports Med. 1998;26(1):59-65.
42. Casteleyn PP, Handelberg F. Arthroscopy in the diagnosis of occult dislocation of the patella. Acta Orthop Belg. 1989;55(3):381-383.
43. Waterman BR, Belmont PJ Jr, Owens BD. Patellar dislocation in the United States: role of sex, age, race, and athletic participation. J Knee Surg. 2012;25(1):51-57.
44. Sillanpää P, Mattila VM, Iivonen T, Visuri T, Pihlajamäki H. Incidence and risk factors of acute traumatic primary patellar dislocation. Med Sci Sports Exerc. 2008;40(4):606-611.
45. Hsiao M, Owens BD, Burks R, Sturdivant RX, Cameron KL. Incidence of acute traumatic patellar dislocation among active-duty United States military service members. Am J Sports Med. 2010;38(10):1997-2004.
46. Fithian DC, Paxton EW, Stone ML, et al. Epidemiology and natural history of acute patellar dislocation. Am J Sports Med. 2004;32(5):1114-1121.
47. Mitchell J, Magnussen RA, Collins CL, et al. Epidemiology of patellofemoral instability injuries among high school athletes in the United States. Am J Sports Med. 2015;43(7):1676-1682.
48. Nikku R, Nietosvaara Y, Aalto K, Kallio PE. The mechanism of primary patellar dislocation: trauma history of 126 patients. Acta Orthop. 2009;80(4):432-434.
49. Tsai CH, Hsu CJ, Hung CH, Hsu HC. Primary traumatic patellar dislocation. J Orthop Surg Res. 2012;7:21.
50. Atkin DM, Fithian DC, Marangi KS, Stone ML, Dobson BE, Mendelsohn C. Characteristics of patients with primary acute lateral patellar dislocation and their recovery within the first 6 months of injury. Am J Sports Med. 2000;28(4):472-479.
51. Nomura E, Inoue M, Kurimura M. Chondral and osteochondral injuries associated with acute patellar dislocation. Arthroscopy. 2003;19(7):717-721.
52. Kirsch MD, Fitzgerald SW, Friedman H, Rogers LF. Transient lateral patellar dislocation: diagnosis with MR imaging. AJR Am J Roentgenol. 1993;161(1):109-113.
53. Virolainen H, Visuri T, Kuusela T. Acute dislocation of the patella: MR findings. Radiology. 1993;189(1):243-246.
54. Stanitski CL. Articular hypermobility and chondral injury in patients with acute patellar dislocation. Am J Sports Med. 1995;23(2):146-150.
55. Mäenpää H, Huhtala H, Lehto MU. Recurrence after patellar dislocation. Redislocation in 37/75 patients followed for 6-24 years. Acta Orthop Scand. 1997;68(5):424-426.
56. Buchner M, Baudendistel B, Sabo D, Schmitt H. Acute traumatic primary patellar dislocation: long-term results comparing conservative and surgical treatment. Clin J Sport Med. 2005;15(2):62-66.
57. Fisher B, Nyland J, Brand E, Curtin B. Medial patellofemoral ligament reconstruction for recurrent patellar dislocation: a systematic review including rehabilitation and return-to-sports efficacy. Arthroscopy. 2010;26(10):1384-1394.
58. Lippacher S, Dreyhaupt J, Williams SR, Reichel H, Nelitz M. Reconstruction of the medial patellofemoral ligament: clinical outcomes and return to sports. Am J Sports Med. 2014;42(7):1661-1668.
59. Panni AS, Alam M, Cerciello S, Vasso M, Maffulli N. Medial patellofemoral ligament reconstruction with a divergent patellar transverse 2-tunnel technique. Am J Sports Med. 2011;39(12):2647-1655.
60. Schneider DK, Grawe B, Magnussen RA, et al. Outcomes after isolated medial patellofemoral ligament reconstruction for the treatment of recurrent lateral patellar dislocations: a systematic review and meta-analysis. Am J Sports Med. 2016 Feb 12. [Epub ahead of print]
61. Amis AA, Bull AM, Gupte CM, Hijazi I, Race A, Robinson JR. Biomechanics of the PCL and related structures: posterolateral, posteromedial and meniscofemoral ligaments. Knee Surg Sports Traumatol Arthrosc. 2003;11(5):271-281.
62. Fu FH, Harner CD, Johnson DL, Miller MD, Woo SL. Biomechanics of knee ligaments: basic concepts and clinical application. Instr Course Lect. 1994;43:137-148.
63. Markolf KL, Feeley BT, Tejwani SG, Martin DE, McAllister DR. Changes in knee laxity and ligament force after sectioning the posteromedial bundle of the posterior cruciate ligament. Arthroscopy. 2006; 22(10):1100-1106.
64. Ganelli GC, Edson CJ. Posterior cruciate ligament injuries in trauma patients: Part II. Arthroscopy. 1995;11(5):526-529.
65. Schulz MS, Russe K, Weiler A, Eichhorn HJ, Strobel MJ. Epidemiology of posterior cruciate ligament injuries. Arch Orthop Trauma Surg. 2003;123(4):186-191.
66. Patel DV, Allen AA, Warren RF, Wickiewicz TL, Simonian PT. The nonoperative treatment of acute, isolated (partial or complete) posterior cruciate ligament-deficient knees: an intermediate-term follow-up study. HSS J. 2007;3(2):137-146.
67. Fowler PJ, Messieh SS. Isolated posterior cruciate ligament injuries in athletes. Am J Sports Med. 1987;15(6):553-557.
68. Dick R, Ferrara MS, Agel J, et al. Descriptive epidemiology of collegiate men’s football injuries: National Collegiate Athletic Association Injury Surveillance System, 1988-1989 through 2003-2004. J Athl Train. 2007;42(2):221-233.
69. LaPrade CM, Civitarese DM, Rasmussen MT, LaPrade RF. Emerging updates on the posterior cruciate ligament: a review of the current literature. Am J Sports Med. 2015;43(12):3077-3092.
70. Torg JS, Barton TM, Pavlov H, Stine R. Natural history of the posterior cruciate ligament-deficient knee. Clin Orthop Relat Res. 1989(246):208-216.
71. Miller MD. Orthopaedic Knowledge Update: Sports Medicine 5. Rosemont, IL; American Academy of Orthopaedic Surgeons; 2016.
72. Shelbourne KD, Clark M, Gray T. Minimum 10-year follow-up of patients after an acute, isolated posterior cruciate ligament injury treated nonoperatively. Am J Sports Med. 2013;41(7):1526-1533.
73. Parolie JM, Bergfeld JA. Long-term results of nonoperative treatment of isolated posterior cruciate ligament injuries in the athlete. Am J Sports Med. 1986;14(1):35-38.
Football is one of the most popular sports in the United States. Every year more than 1 million high school males and over 60,000 collegiate males participate in organized football. The number of males who play football is greater than the combined number of males and females who participate in track and field or basketball.1 Football has the highest injury rate amongst popular American sports.2 From 2001 to 2005, there was an estimated 1.1 million emergency room visits as a direct result of football.3 Injuries are more likely to occur during games,1,2,4,5 more likely to require surgery,4 and more likely to end the player’s season or career when compared to other sports.6 Of those injuries that end seasons or careers, the knee is the most common culprit.6 This is of particular concern because knee injuries are most common in football.1,2,5,7 This article reviews the epidemiology of 4 of the most common knee injuries in American football: tears of the anterior cruciate ligament (ACL), medial collateral ligament (MCL), medial patellofemoral ligament (MPFL), and posterior cruciate ligament (PCL).
Anterior Cruciate Ligament
The ACL is the primary structure preventing anterior tibial translation. It is composed of 2 anatomic bundles: the anteromedial and posterolateral bundles. The ACL originates from the posteromedial portion of the lateral femoral condyle and inserts between and slightly anterior to the tibial intercondylar eminence. The bundles are named for their relative insertions onto the tibia.
Injury to the ACL occurs both through noncontact and contact mechanisms. Typical noncontact mechanism is a forceful valgus collapse with the knee close to full extension with combined external or internal rotation of the tibia.8 This is often the result of a sudden deceleration prior to a change in direction.9 Contact injuries to the ACL are the result of a direct blow to the knee causing valgus collapse.9 The majority of ACL injuries amongst all sports are a result of a noncontact mechanism. However, Dragoo and colleagues10 found the majority of football ACL injuries (55%-60%) were from contact. As a result, football players are 4 times more likely to sustain ACL injuries than in other sports.11
ACL injuries are associated with significant time loss from sport. At the high school level, they are the most likely injury to end a season or career.6 Because these are higher-energy injuries, they are frequently associated with damage to additional structures. ACL injuries that occur in football are associated with increased rates of meniscus, chondral, and multi-ligamentous injuries.12,13
The incidence of ACL injuries increases with level of competition. In high school athletes it is 11.1 per 100,000 athlete exposures (AE).1,11 In collegiate football, the rate increases to 14.2 to 18 per 100,000 AE.2,14 Though no incidence data per AE was found in our review of the literature, there were 219 ACL injuries in the National Football League (NFL) from 2010 to 2013.15 In addition, 14.2% of retired NFL athletes in one survey reported a history of ACL injury.16
The most common high-risk positions are running backs and linebackers. Brophy and colleagues17 found that 9.7% of running backs and 8.9% of linebackers participating in the NFL Combine had a history of ACL injury. This may be because both the running back and linebacker are involved in frequent high-energy collisions and often quickly change direction. Other studies have also identified running backs and linebackers as high risk, in addition to tight ends, wide receivers, and interior linemen.13,15,18
Treatment of choice for elite level athletes with ACL injury is reconstruction.19 Of those who undergo ACL reconstruction, the rate of return to play ranges from 63% to 80%.20-22 The average time to return to play is 9 to 13 months. The odds of making a successful return hinges on how successful the athlete was prior to injury. Factors such as prior game experience, position on depth chart, being on scholarship, and draft position for NFL athletes have all been shown to have a positive predictive value on a patient’s chance of returning from ACL reconstruction.20,21
Players who return have variable levels of success afterwards. In a study of NFL quarterbacks who sustained ACL injuries, 12 out of 13 were able to return to game action with no appreciable dropoff in performance based on in-game production.23 Carey and colleagues24 looked specifically at NFL wide receivers and running backs and found an 80% return to play rate but with an approximate decrease in production of one-third upon return. Furthermore, in the Multicenter Orthopaedic Outcomes Network (MOON) cohort study, only 43% of participants felt they returned to their preoperative level.22
Medial Collateral Ligament
The MCL consists of superficial and deep components. The superficial MCL is the primary restraint to valgus laxity at the knee. The superficial MCL has 1 femoral and 2 tibial attachments. The deep MCL is a thickening of the medial joint capsule and runs deep and parallel to the superficial MCL. The amount of medial joint gapping with a valgus force on examination is used to grade severity of MCL injuries. Grade I is a <5-mm opening; Grade II, 5- to 10-mm opening; and grade III, >10-mm opening.
The MCL is the most common knee injury in high school, collegiate, and professional football.1,18,25-28 Injuries are typically due to contact when a valgus force is applied to the knee.29 The annual incidence of MCL injuries amongst high school football players is 24.2 per 100,000 AE.1 The positions that appear to be at greatest risk for MCL injuries are offensive and defensive linemen.18,30-32 In a review of 5047 collegiate athletes participating in the NFL Combine from 1987 to 2000, 23% of offensive linemen had a history of MCL injury, compared to the overall rate of 16%.33 In a similar study, Bradley and colleagues18 performed medical histories on athletes invited to the 2005 NFL Combine and also found offensive linemen had the highest rate of MCL injury at 33%, compared to the overall rate of 22%. They reasonably hypothesized that “chop blocks” and other players “rolling up” on the outside of linemen’s knees were responsible for these injuries. Albright and colleagues32 found that prophylactic knee braces decreased the incidence of MCL injuries in collegiate offensive lineman. However, additional studies have not been able to reproduce these results and the use of prophylactic knee braces remains controversial.26
Treatment of MCL injuries depends upon the grade of injury, associated injuries, and anatomical location of injury. Management of MCL injuries is for the most part nonsurgical. In 1974, Ellsasser and colleagues34 were the first to publish data on nonoperative management of Grade I and Grade II injuries with immediate motion and rehabilitation instead of cast immobilization. They found 93% of patients returned to football in 3 to 8 weeks.34 Derscheid and Garrick27 observed nonoperative treatment of Grade I and II sprains in collegiate football players, with a time loss of 10.6 days and 19.5 days for Grade I and II injuries, respectively. Holden and colleagues35 evaluated nonoperative management of Grade I and II MCL injuries in collegiate football players and found an average return to play of 21 days.
Grade III injury treatment is more controversial. Indelicato and colleagues36 demonstrated successful nonoperative management of Grade III MCL injuries in collegiate football players, with an average return to play of 64.4 days. Jones and colleagues37 had similar success with high school football players, with an average return to play of 34 days. However, isolated Grade III injuries are rare and therefore treatment is likely to be dictated by concomitant injuries. Fetto and Marshall38 found that 78% of Grade III injuries were associated with an additional ligamentous injury. Of those additional injuries, 95% were ACL tears.
Finally, one must consider the location of the MCL injury. Injuries of the distal MCL at its tibial insertion may result in poor healing, as the ligament is displaced away from its insertion. Therefore, some authors recommend surgical management for these injuries.39,40
The patellofemoral joint is a complex structure in which the patella is stabilized within the trochlear groove of the femur by both bony and soft tissue structures. The MPFL is one of the most important soft tissue stabilizers. The MPFL is the primary restraint to lateral patellar translation within the first 20° of knee flexion, contributing to 60% of the total restraining force.41 The MPFL originates on the medial femoral condyle and inserts on the superomedial aspect of the patella.
Patellar instability is the subluxation or dislocation of the patella out of the trochlear groove. Patellar subluxation and dislocation account for approximately 3% of all knee injuries.42 Patella dislocations are more common in younger populations43-45 with the majority (52%-63%) occurring during sports.43,44,46 Mitchell and colleagues47 reported an incidence of 4.1 patellar subluxations/dislocations per 100,000 AE in high school football players.
Dislocation is most commonly the result of knee flexion with the tibia in a valgus position.44,48 The majority of patellar dislocations occur via a noncontact mechanism.44,48 However, the majority of these injuries in football are from contact (63%).47
Acute patellar dislocations are associated with more soft tissue damage than those with recurrent dislocations.46 In acute patella dislocations, the MPFL is almost always ruptured.44 In contrast, Fithian and colleagues46 found only 38% of recurrent dislocators had MPFL injury. As a result, it is thought that those with recurrent instability dislocate without trauma and do not have the same characteristics as those who dislocate from high-energy trauma in sport. Risk factors for atraumatic dislocation are numerous and have been well described in the literature.49 However, traumatic dislocators usually do not have risk factors.50
Traumatic patella dislocations are higher energy and are associated with chondral injury in up to 95%of cases 51 and osteochondral injury 58% to 76% of the time.52,53 In contrast, people with “articular hypermobility” are less likely to sustain articular damage.54 This concept is important when considering risk for recurrent patella dislocation. The literature reports a 17% to 50% rate of recurrent instability after acute patella dislocation.46,55,56 However, most studies do not distinguish between traumatic and atraumatic injuries. Because the majority of patellar dislocations in football occur through contact mechanisms, the rate of recurrent instability in these athletes may in fact be less than what is reported in the literature.
First-time patella dislocations are generally treated nonoperatively. Mitchell and colleagues47 reported that 72.6% of high school athletes with patella subluxation treated conservatively were able to return to sports within 3 weeks, compared to only 34.1% of those with patellar dislocations. In the same study, patellar dislocations were season-ending 37% of the time.47 Atkin and colleagues50 followed 74 patients treated conservatively for first-time patellar dislocation and noted 58% at 6 months still had difficulty in squatting, jumping, or cutting.
Those who have failed conservative management and have an additional dislocation are 7 times more likely to redislocate.46 Therefore, they are usually treated operatively with MPFL reconstruction. Return to sport ranges from 3 to 6 months,57 with 53% to 77.3% reporting return to their previous functionality.57-59 Overall, 84.1% of patients are able to return to sport with 1.2% risk of recurrent dislocation.60
Posterior Cruciate Ligament
The PCL is the primary posterior stabilizer of the knee.61,62 It consists of the anterolateral and posteromedial bundles, named by their insertion on the posterior tibial plateau. The larger, stronger anterolateral bundle is the primary restraint to posterior tibial translation.63
Due to the relative infrequency of PCL injuries, there is a paucity of epidemiological data on sports-related PCL injuries. These injuries in the literature are commonly found due to traffic accidents (45%-57%) or from sports (33%-40%).64,65 According to Swensen and colleagues,1 PCL injuries account for 2.4% of all high school sport knee injuries. In a cohort of 62 knees with PCL injuries, Patel and colleagues66 found football was the most common cause of injury (19.3%).
The most common mechanism of injury in athletes is knee hyperflexion or a direct blow to the tibia in a flexed knee.67 In football, contact mechanisms are the most common. In a 16-year review of the National Collegiate Athletic Association (NCAA) injury surveillance system, the incidence of contact PCL injuries during games were 7.3 times higher than noncontact.68 The most common activity was being tackled, which accounted for 22.9% of all PCL injuries.68
Due to the high energy of these injuries, isolated PCL injuries are rare. In one trauma center’s experience, 96.5% of PCL injuries had an additional ligament injury.64 In that study, injuries to the PCL were associated with posterolateral corner, ACL, and MCL injuries 62%, 46%, and 31% of the time, respectively.64,69
Because isolated PCL injuries are rare, clinicians must rely on a thorough history and physical examination when evaluating athletes with knee injuries. Classification of PCL injuries is based on the amount of posterior tibial translation in relation to the femur with the knee bent to 90°. Grade I is 1 to 5 mm; Grade II, 6 to 10 mm; and Grade III, >10 mm. If there is suspicion of a PCL injury, there should be a very low threshold for magnetic resonance imaging, given the high association with additional injuries.
Natural history of Grade I and II isolated PCL injuries is generally favorable compared to Grade III and multi-ligamentous injuries.70 As a result, isolated Grade I and II PCL injuries are generally treated nonoperatively. Treatment consists of physical therapy with emphasis on quadriceps strengthening. Return to play can be considered as early as 2 to 4 weeks from injury.71 Recent long-term data have shown successful conservative management of Grade I and II injuries with quadriceps strength to 97% of contralateral leg and full range of motion.72 However, there was 11% moderate to severe osteoarthritis in these patients at a mean follow-up of 14.3 years.72 Fowler and Messieh67 managed athletes with 7 isolated complete PCL tears and 5 partial tears nonoperatively, all of whom were able to return to sport without limitation. Parolie and Bergfeld73 managed 25 athletes with isolated PCL tears conservatively. In this study, 80% of athletes reported satisfaction and 68% returned to previous level of play.73 Neither of the aforementioned studies specify the grades of the injuries. Finally, Patel and colleagues66 managed 6 NFL athletes with Grade I and II injuries nonoperatively, and all were able to return to sport.
Treatment of isolated Grade III PCL injuries is more controversial, and no consensus exists in the literature. In an epidemiological study, Dick and colleagues68 found that only 39% of NCAA football athletes underwent surgery for their torn PCLs, compared to 79% of ACL injuries. However, their study makes no mention to the severity of these injuries. Numerous options exist for PCL reconstruction, with no consensus on the preferred method.
Conclusion
Knee injuries are the most common injury in football. Knowledge of the natural history of these injuries, as well as treatment options and expected outcomes, will help treating physicians educate their patients on the optimal treatment and manage return to play expectations.
Am J Orthop. 2016;45(6):368-373. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Football is one of the most popular sports in the United States. Every year more than 1 million high school males and over 60,000 collegiate males participate in organized football. The number of males who play football is greater than the combined number of males and females who participate in track and field or basketball.1 Football has the highest injury rate amongst popular American sports.2 From 2001 to 2005, there was an estimated 1.1 million emergency room visits as a direct result of football.3 Injuries are more likely to occur during games,1,2,4,5 more likely to require surgery,4 and more likely to end the player’s season or career when compared to other sports.6 Of those injuries that end seasons or careers, the knee is the most common culprit.6 This is of particular concern because knee injuries are most common in football.1,2,5,7 This article reviews the epidemiology of 4 of the most common knee injuries in American football: tears of the anterior cruciate ligament (ACL), medial collateral ligament (MCL), medial patellofemoral ligament (MPFL), and posterior cruciate ligament (PCL).
Anterior Cruciate Ligament
The ACL is the primary structure preventing anterior tibial translation. It is composed of 2 anatomic bundles: the anteromedial and posterolateral bundles. The ACL originates from the posteromedial portion of the lateral femoral condyle and inserts between and slightly anterior to the tibial intercondylar eminence. The bundles are named for their relative insertions onto the tibia.
Injury to the ACL occurs both through noncontact and contact mechanisms. Typical noncontact mechanism is a forceful valgus collapse with the knee close to full extension with combined external or internal rotation of the tibia.8 This is often the result of a sudden deceleration prior to a change in direction.9 Contact injuries to the ACL are the result of a direct blow to the knee causing valgus collapse.9 The majority of ACL injuries amongst all sports are a result of a noncontact mechanism. However, Dragoo and colleagues10 found the majority of football ACL injuries (55%-60%) were from contact. As a result, football players are 4 times more likely to sustain ACL injuries than in other sports.11
ACL injuries are associated with significant time loss from sport. At the high school level, they are the most likely injury to end a season or career.6 Because these are higher-energy injuries, they are frequently associated with damage to additional structures. ACL injuries that occur in football are associated with increased rates of meniscus, chondral, and multi-ligamentous injuries.12,13
The incidence of ACL injuries increases with level of competition. In high school athletes it is 11.1 per 100,000 athlete exposures (AE).1,11 In collegiate football, the rate increases to 14.2 to 18 per 100,000 AE.2,14 Though no incidence data per AE was found in our review of the literature, there were 219 ACL injuries in the National Football League (NFL) from 2010 to 2013.15 In addition, 14.2% of retired NFL athletes in one survey reported a history of ACL injury.16
The most common high-risk positions are running backs and linebackers. Brophy and colleagues17 found that 9.7% of running backs and 8.9% of linebackers participating in the NFL Combine had a history of ACL injury. This may be because both the running back and linebacker are involved in frequent high-energy collisions and often quickly change direction. Other studies have also identified running backs and linebackers as high risk, in addition to tight ends, wide receivers, and interior linemen.13,15,18
Treatment of choice for elite level athletes with ACL injury is reconstruction.19 Of those who undergo ACL reconstruction, the rate of return to play ranges from 63% to 80%.20-22 The average time to return to play is 9 to 13 months. The odds of making a successful return hinges on how successful the athlete was prior to injury. Factors such as prior game experience, position on depth chart, being on scholarship, and draft position for NFL athletes have all been shown to have a positive predictive value on a patient’s chance of returning from ACL reconstruction.20,21
Players who return have variable levels of success afterwards. In a study of NFL quarterbacks who sustained ACL injuries, 12 out of 13 were able to return to game action with no appreciable dropoff in performance based on in-game production.23 Carey and colleagues24 looked specifically at NFL wide receivers and running backs and found an 80% return to play rate but with an approximate decrease in production of one-third upon return. Furthermore, in the Multicenter Orthopaedic Outcomes Network (MOON) cohort study, only 43% of participants felt they returned to their preoperative level.22
Medial Collateral Ligament
The MCL consists of superficial and deep components. The superficial MCL is the primary restraint to valgus laxity at the knee. The superficial MCL has 1 femoral and 2 tibial attachments. The deep MCL is a thickening of the medial joint capsule and runs deep and parallel to the superficial MCL. The amount of medial joint gapping with a valgus force on examination is used to grade severity of MCL injuries. Grade I is a <5-mm opening; Grade II, 5- to 10-mm opening; and grade III, >10-mm opening.
The MCL is the most common knee injury in high school, collegiate, and professional football.1,18,25-28 Injuries are typically due to contact when a valgus force is applied to the knee.29 The annual incidence of MCL injuries amongst high school football players is 24.2 per 100,000 AE.1 The positions that appear to be at greatest risk for MCL injuries are offensive and defensive linemen.18,30-32 In a review of 5047 collegiate athletes participating in the NFL Combine from 1987 to 2000, 23% of offensive linemen had a history of MCL injury, compared to the overall rate of 16%.33 In a similar study, Bradley and colleagues18 performed medical histories on athletes invited to the 2005 NFL Combine and also found offensive linemen had the highest rate of MCL injury at 33%, compared to the overall rate of 22%. They reasonably hypothesized that “chop blocks” and other players “rolling up” on the outside of linemen’s knees were responsible for these injuries. Albright and colleagues32 found that prophylactic knee braces decreased the incidence of MCL injuries in collegiate offensive lineman. However, additional studies have not been able to reproduce these results and the use of prophylactic knee braces remains controversial.26
Treatment of MCL injuries depends upon the grade of injury, associated injuries, and anatomical location of injury. Management of MCL injuries is for the most part nonsurgical. In 1974, Ellsasser and colleagues34 were the first to publish data on nonoperative management of Grade I and Grade II injuries with immediate motion and rehabilitation instead of cast immobilization. They found 93% of patients returned to football in 3 to 8 weeks.34 Derscheid and Garrick27 observed nonoperative treatment of Grade I and II sprains in collegiate football players, with a time loss of 10.6 days and 19.5 days for Grade I and II injuries, respectively. Holden and colleagues35 evaluated nonoperative management of Grade I and II MCL injuries in collegiate football players and found an average return to play of 21 days.
Grade III injury treatment is more controversial. Indelicato and colleagues36 demonstrated successful nonoperative management of Grade III MCL injuries in collegiate football players, with an average return to play of 64.4 days. Jones and colleagues37 had similar success with high school football players, with an average return to play of 34 days. However, isolated Grade III injuries are rare and therefore treatment is likely to be dictated by concomitant injuries. Fetto and Marshall38 found that 78% of Grade III injuries were associated with an additional ligamentous injury. Of those additional injuries, 95% were ACL tears.
Finally, one must consider the location of the MCL injury. Injuries of the distal MCL at its tibial insertion may result in poor healing, as the ligament is displaced away from its insertion. Therefore, some authors recommend surgical management for these injuries.39,40
The patellofemoral joint is a complex structure in which the patella is stabilized within the trochlear groove of the femur by both bony and soft tissue structures. The MPFL is one of the most important soft tissue stabilizers. The MPFL is the primary restraint to lateral patellar translation within the first 20° of knee flexion, contributing to 60% of the total restraining force.41 The MPFL originates on the medial femoral condyle and inserts on the superomedial aspect of the patella.
Patellar instability is the subluxation or dislocation of the patella out of the trochlear groove. Patellar subluxation and dislocation account for approximately 3% of all knee injuries.42 Patella dislocations are more common in younger populations43-45 with the majority (52%-63%) occurring during sports.43,44,46 Mitchell and colleagues47 reported an incidence of 4.1 patellar subluxations/dislocations per 100,000 AE in high school football players.
Dislocation is most commonly the result of knee flexion with the tibia in a valgus position.44,48 The majority of patellar dislocations occur via a noncontact mechanism.44,48 However, the majority of these injuries in football are from contact (63%).47
Acute patellar dislocations are associated with more soft tissue damage than those with recurrent dislocations.46 In acute patella dislocations, the MPFL is almost always ruptured.44 In contrast, Fithian and colleagues46 found only 38% of recurrent dislocators had MPFL injury. As a result, it is thought that those with recurrent instability dislocate without trauma and do not have the same characteristics as those who dislocate from high-energy trauma in sport. Risk factors for atraumatic dislocation are numerous and have been well described in the literature.49 However, traumatic dislocators usually do not have risk factors.50
Traumatic patella dislocations are higher energy and are associated with chondral injury in up to 95%of cases 51 and osteochondral injury 58% to 76% of the time.52,53 In contrast, people with “articular hypermobility” are less likely to sustain articular damage.54 This concept is important when considering risk for recurrent patella dislocation. The literature reports a 17% to 50% rate of recurrent instability after acute patella dislocation.46,55,56 However, most studies do not distinguish between traumatic and atraumatic injuries. Because the majority of patellar dislocations in football occur through contact mechanisms, the rate of recurrent instability in these athletes may in fact be less than what is reported in the literature.
First-time patella dislocations are generally treated nonoperatively. Mitchell and colleagues47 reported that 72.6% of high school athletes with patella subluxation treated conservatively were able to return to sports within 3 weeks, compared to only 34.1% of those with patellar dislocations. In the same study, patellar dislocations were season-ending 37% of the time.47 Atkin and colleagues50 followed 74 patients treated conservatively for first-time patellar dislocation and noted 58% at 6 months still had difficulty in squatting, jumping, or cutting.
Those who have failed conservative management and have an additional dislocation are 7 times more likely to redislocate.46 Therefore, they are usually treated operatively with MPFL reconstruction. Return to sport ranges from 3 to 6 months,57 with 53% to 77.3% reporting return to their previous functionality.57-59 Overall, 84.1% of patients are able to return to sport with 1.2% risk of recurrent dislocation.60
Posterior Cruciate Ligament
The PCL is the primary posterior stabilizer of the knee.61,62 It consists of the anterolateral and posteromedial bundles, named by their insertion on the posterior tibial plateau. The larger, stronger anterolateral bundle is the primary restraint to posterior tibial translation.63
Due to the relative infrequency of PCL injuries, there is a paucity of epidemiological data on sports-related PCL injuries. These injuries in the literature are commonly found due to traffic accidents (45%-57%) or from sports (33%-40%).64,65 According to Swensen and colleagues,1 PCL injuries account for 2.4% of all high school sport knee injuries. In a cohort of 62 knees with PCL injuries, Patel and colleagues66 found football was the most common cause of injury (19.3%).
The most common mechanism of injury in athletes is knee hyperflexion or a direct blow to the tibia in a flexed knee.67 In football, contact mechanisms are the most common. In a 16-year review of the National Collegiate Athletic Association (NCAA) injury surveillance system, the incidence of contact PCL injuries during games were 7.3 times higher than noncontact.68 The most common activity was being tackled, which accounted for 22.9% of all PCL injuries.68
Due to the high energy of these injuries, isolated PCL injuries are rare. In one trauma center’s experience, 96.5% of PCL injuries had an additional ligament injury.64 In that study, injuries to the PCL were associated with posterolateral corner, ACL, and MCL injuries 62%, 46%, and 31% of the time, respectively.64,69
Because isolated PCL injuries are rare, clinicians must rely on a thorough history and physical examination when evaluating athletes with knee injuries. Classification of PCL injuries is based on the amount of posterior tibial translation in relation to the femur with the knee bent to 90°. Grade I is 1 to 5 mm; Grade II, 6 to 10 mm; and Grade III, >10 mm. If there is suspicion of a PCL injury, there should be a very low threshold for magnetic resonance imaging, given the high association with additional injuries.
Natural history of Grade I and II isolated PCL injuries is generally favorable compared to Grade III and multi-ligamentous injuries.70 As a result, isolated Grade I and II PCL injuries are generally treated nonoperatively. Treatment consists of physical therapy with emphasis on quadriceps strengthening. Return to play can be considered as early as 2 to 4 weeks from injury.71 Recent long-term data have shown successful conservative management of Grade I and II injuries with quadriceps strength to 97% of contralateral leg and full range of motion.72 However, there was 11% moderate to severe osteoarthritis in these patients at a mean follow-up of 14.3 years.72 Fowler and Messieh67 managed athletes with 7 isolated complete PCL tears and 5 partial tears nonoperatively, all of whom were able to return to sport without limitation. Parolie and Bergfeld73 managed 25 athletes with isolated PCL tears conservatively. In this study, 80% of athletes reported satisfaction and 68% returned to previous level of play.73 Neither of the aforementioned studies specify the grades of the injuries. Finally, Patel and colleagues66 managed 6 NFL athletes with Grade I and II injuries nonoperatively, and all were able to return to sport.
Treatment of isolated Grade III PCL injuries is more controversial, and no consensus exists in the literature. In an epidemiological study, Dick and colleagues68 found that only 39% of NCAA football athletes underwent surgery for their torn PCLs, compared to 79% of ACL injuries. However, their study makes no mention to the severity of these injuries. Numerous options exist for PCL reconstruction, with no consensus on the preferred method.
Conclusion
Knee injuries are the most common injury in football. Knowledge of the natural history of these injuries, as well as treatment options and expected outcomes, will help treating physicians educate their patients on the optimal treatment and manage return to play expectations.
Am J Orthop. 2016;45(6):368-373. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Swenson DM, Collins CL, Best TM, Flanigan DC, Fields SK, Comstock RD. Epidemiology of knee injuries among U.S. high school athletes, 2005/2006-2010/2011. Med Sci Sports Exerc. 2013;45(3):462-469.
2. Hootman JM, Dick R, Agel J. Epidemiology of collegiate injuries for 15 sports: summary and recommendations for injury prevention initiatives. J Athl Train. 2007;42(2):311-319.
3. Mello MJ, Myers R, Christian JB, Palmisciano L, Linakis JG. Injuries in youth football: national emergency department visits during 2001-2005 for young and adolescent players. Acad Emerg Med. 2009;16(3):243-248.
4. Rechel JA, Collins CL, Comstock RD. Epidemiology of injuries requiring surgery among high school athletes in the United States, 2005 to 2010. J Trauma. 2011;71(4):982-989.
5. Ingram JG, Fields SK, Yard EE, Comstock RD. Epidemiology of knee injuries among boys and girls in US high school athletics. Am J Sports Med. 2008;36(6):1116-1122.
6. Tirabassi J, Brou L, Khodaee M, Lefort R, Fields SK, Comstock RD. Epidemiology of high school sports-related injuries resulting in medical disqualification: 2005-2006 through 2013-2014 academic years. Am J Sports Med. 2016 May 10. [Epub ahead of print]
7. Fernandez WG, Yard EE, Comstock RD. Epidemiology of lower extremity injuries among U.S. high school athletes. Acad Emerg Med. 2007;14(7):641-645.
8. Olsen OE, Myklebust G, Engebretsen L, Bahr R. Injury mechanisms for anterior cruciate ligament injuries in team handball: a systematic video analysis. Am J Sports Med. 2004;32(4):1002-1012.
9. Boden BP, Dean GS, Feagin JA Jr, Garrett WE Jr. Mechanisms of anterior cruciate ligament injury. Orthopedics. 2000;23(6):573-578.
10. Dragoo JL, Braun HJ, Harris AH. The effect of playing surface on the incidence of ACL injuries in National Collegiate Athletic Association American Football. Knee. 2013;20(3):191-195.
11. Joseph AM, Collins CL, Henke NM, Yard EE, Fields SK, Comstock RD. A multisport epidemiologic comparison of anterior cruciate ligament injuries in high school athletics. J Athl Train. 2013;48(6):810-817.
12. Granan LP, Inacio MC, Maletis GB, Funahashi TT, Engebretsen L. Sport-specific injury pattern recorded during anterior cruciate ligament reconstruction. Am J Sports Med. 2013;41(12):2814-2818.
13. Bradley JP, Klimkiewicz JJ, Rytel MJ, Powell JW. Anterior cruciate ligament injuries in the National Football League: epidemiology and current treatment trends among team physicians. Arthroscopy. 2002;18(5):502-509.
14. Dragoo JL, Braun HJ, Durham JL, Chen MR, Harris AH. Incidence and risk factors for injuries to the anterior cruciate ligament in National Collegiate Athletic Association football: data from the 2004-2005 through 2008-2009 National Collegiate Athletic Association Injury Surveillance System. Am J Sports Med. 2012;40(5):990-995.
15. Dodson CC, Secrist ES, Bhat SB, Woods DP, Deluca PF. Anterior cruciate ligamenti in National Football League athletes from 2010 to 2013: a descriptive epidemiology study. Orthop J Sports Med. 2016;4(3):2325967116631949.
16. Golightly YM, Marshall SW, Callahan LF, Guskiewicz K. Early-onset arthritis in retired National Football League players. J Phys Act Health. 2009;6(5):638-643.
17. Brophy RH, Lyman S, Chehab EL, Barnes RP, Rodeo SA, Warren RF. Predictive value of prior injury on career in professional American football is affected by player position. Am J Sports Med. 2009;37(4):768-775.
18. Bradley J, Honkamp NJ, Jost P, West R, Norwig J, Kaplan LD. Incidence and variance of knee injuries in elite college football players. Am J Orthop. 2008;37(6):310-314.
19. Erickson BJ, Harris JD, Fillingham YA, et al. Anterior cruciate ligament reconstruction practice patterns by NFL and NCAA football team physicians. Arthroscopy. 2014;30(6):731-738.
20. Daruawalla JH, Greis PE, Hancock R; ASP Collaborative Group, Xerogeanes JW. Rates and determinants of return to play after anterior cruciate ligament reconstruction in NCAA Division 1 college football athletes: a study of the ACC, SEC, and PAC-12 conferences. Orthop J Sports Med. 2014;2(8):2325967114543901.
21. Shah VM, Andrews JR, Fleisig GS, McMichael CS, Lemak LJ. Return to play after anterior cruciate ligament reconstruction in National Football League athletes. Am J Sports Med. 2010;38(11):2233-2239.
22. McCullough KA, Phelps KD, Spindler KP, et al. Return to high school- and college-level football after anterior cruciate ligament reconstruction: a Multicenter Orthopaedic Outcomes Network (MOON) cohort study. Am J Sports Med. 2012;40(11):2523-2529.
23. Erickson BJ, Harris JD, Heninger JR, et al. Performance and return-to-sport after ACL reconstruction in NFL quarterbacks. Orthopedics. 2014;37(8):e728-e734.
24. Carey JL, Huffman GR, Parekh SG, Sennett BJ. Outcomes of anterior cruciate ligament injuries to running backs and wide receivers in the National Football League. Am J Sports Med. 2006;34(12):1911-1917.
25. Hershman EB, Anderson R, Bergfeld JA, et al. An analysis of specific lower extremity injury rates on grass and FieldTurf playing surfaces in National Football League Games: 2000-2009 seasons. Am J Sports Med. 2012;40(10):2200-2205.
26. Salata MJ, Gibbs AE, Sekiya JK. The effectiveness of prophylactic knee bracing in American football: a systematic review. Sports Health. 2010;2(5):375-379.
27. Derscheid GL, Garrick JG. Medial collateral ligament injuries in football. Nonoperative management of grade I and grade II sprains. Am J Sports Med. 1981;9(6):365-368.
28. Meyers MC, Barnhill BS. Incidence, causes, and severity of high school football injuries on FieldTurf versus natural grass: a 5-year prospective study. Am J Sports Med. 2004;32(7):1626-1638.
29. Lundblad M, Waldén M, Magnusson H, Karlsson J, Ekstrand J. The UEFA injury study: 11-year data concerning 346 MCL injuries and time to return to play. Br J Sports Med. 2013;47(12):759-762.
30. Hewson GF Jr, Mendini RA, Wang JB. Prophylactic knee bracing in college football. Am J Sports Med. 1986;14(4):262-266.
31. Rovere GD, Haupt HA, Yates CS. Prophylactic knee bracing in college football. Am J Sports Med. 1987;15(2):111-116.
32. Albright JP, Powell JW, Smith W, et al. Medial collateral ligament knee sprains in college football. Brace wear preferences and injury risk. Am J Sports Med. 1994;22(1):2-11.
33. Brophy RH, Barnes R, Rodeo SA, Warren RF. Prevalence of musculoskeletal disorders at the NFL Combine--trends from 1987 to 2000. Med Sci Sports Exerc. 2007;39(1):22-27.
34. Ellsasser JC, Reynolds FC, Omohundro JR. The non-operative treatment of collateral ligament injuries of the knee in professional football players. An analysis of seventy-four injuries treated non-operatively and twenty-four injuries treated surgically. J Bone Joint Surg Am. 1974;56(6):1185-1190.
35. Holden DL, Eggert AW, Butler JE. The nonoperative treatment of grade I and II medial collateral ligament injuries to the knee. Am J Sports Med. 1983;11(5):340-344.
36. Indelicato PA, Hermansdorfer J, Huegel M. Nonoperative management of complete tears of the medial collateral ligament of the knee in intercollegiate football players. Clin Orthop Relat Res. 1990;(256):174-177.
37. Jones RE, Henley MB, Francis P. Nonoperative management of isolated grade III collateral ligament injury in high school football players. Clin Orthop Relat Res. 1986;(213):137-140.
38. Fetto JF, Marshall JL. Medial collateral ligament injuries of the knee: a rationale for treatment. Clin Orthop Relat Res. 1978;(132):206-218.
39. Corten K, Hoser C, Fink C, Bellemans J. Case reports: a Stener-like lesion of the medial collateral ligament of the knee. Clin Orthop Relat Res. 2010;468(1):289-293.
40. Marchant MH Jr, Tibor LM, Sekiya JK, Hardaker WT Jr, Garrett WE Jr, Taylor DC. Management of medial-sided knee injuries, part 1: medial collateral ligament. Am J Sports Med. 2011;39(5):1102-1113.
41. Desio SM, Burks RT, Bachus KN. Soft tissue restraints to lateral patellar translation in the human knee. Am J Sports Med. 1998;26(1):59-65.
42. Casteleyn PP, Handelberg F. Arthroscopy in the diagnosis of occult dislocation of the patella. Acta Orthop Belg. 1989;55(3):381-383.
43. Waterman BR, Belmont PJ Jr, Owens BD. Patellar dislocation in the United States: role of sex, age, race, and athletic participation. J Knee Surg. 2012;25(1):51-57.
44. Sillanpää P, Mattila VM, Iivonen T, Visuri T, Pihlajamäki H. Incidence and risk factors of acute traumatic primary patellar dislocation. Med Sci Sports Exerc. 2008;40(4):606-611.
45. Hsiao M, Owens BD, Burks R, Sturdivant RX, Cameron KL. Incidence of acute traumatic patellar dislocation among active-duty United States military service members. Am J Sports Med. 2010;38(10):1997-2004.
46. Fithian DC, Paxton EW, Stone ML, et al. Epidemiology and natural history of acute patellar dislocation. Am J Sports Med. 2004;32(5):1114-1121.
47. Mitchell J, Magnussen RA, Collins CL, et al. Epidemiology of patellofemoral instability injuries among high school athletes in the United States. Am J Sports Med. 2015;43(7):1676-1682.
48. Nikku R, Nietosvaara Y, Aalto K, Kallio PE. The mechanism of primary patellar dislocation: trauma history of 126 patients. Acta Orthop. 2009;80(4):432-434.
49. Tsai CH, Hsu CJ, Hung CH, Hsu HC. Primary traumatic patellar dislocation. J Orthop Surg Res. 2012;7:21.
50. Atkin DM, Fithian DC, Marangi KS, Stone ML, Dobson BE, Mendelsohn C. Characteristics of patients with primary acute lateral patellar dislocation and their recovery within the first 6 months of injury. Am J Sports Med. 2000;28(4):472-479.
51. Nomura E, Inoue M, Kurimura M. Chondral and osteochondral injuries associated with acute patellar dislocation. Arthroscopy. 2003;19(7):717-721.
52. Kirsch MD, Fitzgerald SW, Friedman H, Rogers LF. Transient lateral patellar dislocation: diagnosis with MR imaging. AJR Am J Roentgenol. 1993;161(1):109-113.
53. Virolainen H, Visuri T, Kuusela T. Acute dislocation of the patella: MR findings. Radiology. 1993;189(1):243-246.
54. Stanitski CL. Articular hypermobility and chondral injury in patients with acute patellar dislocation. Am J Sports Med. 1995;23(2):146-150.
55. Mäenpää H, Huhtala H, Lehto MU. Recurrence after patellar dislocation. Redislocation in 37/75 patients followed for 6-24 years. Acta Orthop Scand. 1997;68(5):424-426.
56. Buchner M, Baudendistel B, Sabo D, Schmitt H. Acute traumatic primary patellar dislocation: long-term results comparing conservative and surgical treatment. Clin J Sport Med. 2005;15(2):62-66.
57. Fisher B, Nyland J, Brand E, Curtin B. Medial patellofemoral ligament reconstruction for recurrent patellar dislocation: a systematic review including rehabilitation and return-to-sports efficacy. Arthroscopy. 2010;26(10):1384-1394.
58. Lippacher S, Dreyhaupt J, Williams SR, Reichel H, Nelitz M. Reconstruction of the medial patellofemoral ligament: clinical outcomes and return to sports. Am J Sports Med. 2014;42(7):1661-1668.
59. Panni AS, Alam M, Cerciello S, Vasso M, Maffulli N. Medial patellofemoral ligament reconstruction with a divergent patellar transverse 2-tunnel technique. Am J Sports Med. 2011;39(12):2647-1655.
60. Schneider DK, Grawe B, Magnussen RA, et al. Outcomes after isolated medial patellofemoral ligament reconstruction for the treatment of recurrent lateral patellar dislocations: a systematic review and meta-analysis. Am J Sports Med. 2016 Feb 12. [Epub ahead of print]
61. Amis AA, Bull AM, Gupte CM, Hijazi I, Race A, Robinson JR. Biomechanics of the PCL and related structures: posterolateral, posteromedial and meniscofemoral ligaments. Knee Surg Sports Traumatol Arthrosc. 2003;11(5):271-281.
62. Fu FH, Harner CD, Johnson DL, Miller MD, Woo SL. Biomechanics of knee ligaments: basic concepts and clinical application. Instr Course Lect. 1994;43:137-148.
63. Markolf KL, Feeley BT, Tejwani SG, Martin DE, McAllister DR. Changes in knee laxity and ligament force after sectioning the posteromedial bundle of the posterior cruciate ligament. Arthroscopy. 2006; 22(10):1100-1106.
64. Ganelli GC, Edson CJ. Posterior cruciate ligament injuries in trauma patients: Part II. Arthroscopy. 1995;11(5):526-529.
65. Schulz MS, Russe K, Weiler A, Eichhorn HJ, Strobel MJ. Epidemiology of posterior cruciate ligament injuries. Arch Orthop Trauma Surg. 2003;123(4):186-191.
66. Patel DV, Allen AA, Warren RF, Wickiewicz TL, Simonian PT. The nonoperative treatment of acute, isolated (partial or complete) posterior cruciate ligament-deficient knees: an intermediate-term follow-up study. HSS J. 2007;3(2):137-146.
67. Fowler PJ, Messieh SS. Isolated posterior cruciate ligament injuries in athletes. Am J Sports Med. 1987;15(6):553-557.
68. Dick R, Ferrara MS, Agel J, et al. Descriptive epidemiology of collegiate men’s football injuries: National Collegiate Athletic Association Injury Surveillance System, 1988-1989 through 2003-2004. J Athl Train. 2007;42(2):221-233.
69. LaPrade CM, Civitarese DM, Rasmussen MT, LaPrade RF. Emerging updates on the posterior cruciate ligament: a review of the current literature. Am J Sports Med. 2015;43(12):3077-3092.
70. Torg JS, Barton TM, Pavlov H, Stine R. Natural history of the posterior cruciate ligament-deficient knee. Clin Orthop Relat Res. 1989(246):208-216.
71. Miller MD. Orthopaedic Knowledge Update: Sports Medicine 5. Rosemont, IL; American Academy of Orthopaedic Surgeons; 2016.
72. Shelbourne KD, Clark M, Gray T. Minimum 10-year follow-up of patients after an acute, isolated posterior cruciate ligament injury treated nonoperatively. Am J Sports Med. 2013;41(7):1526-1533.
73. Parolie JM, Bergfeld JA. Long-term results of nonoperative treatment of isolated posterior cruciate ligament injuries in the athlete. Am J Sports Med. 1986;14(1):35-38.
1. Swenson DM, Collins CL, Best TM, Flanigan DC, Fields SK, Comstock RD. Epidemiology of knee injuries among U.S. high school athletes, 2005/2006-2010/2011. Med Sci Sports Exerc. 2013;45(3):462-469.
2. Hootman JM, Dick R, Agel J. Epidemiology of collegiate injuries for 15 sports: summary and recommendations for injury prevention initiatives. J Athl Train. 2007;42(2):311-319.
3. Mello MJ, Myers R, Christian JB, Palmisciano L, Linakis JG. Injuries in youth football: national emergency department visits during 2001-2005 for young and adolescent players. Acad Emerg Med. 2009;16(3):243-248.
4. Rechel JA, Collins CL, Comstock RD. Epidemiology of injuries requiring surgery among high school athletes in the United States, 2005 to 2010. J Trauma. 2011;71(4):982-989.
5. Ingram JG, Fields SK, Yard EE, Comstock RD. Epidemiology of knee injuries among boys and girls in US high school athletics. Am J Sports Med. 2008;36(6):1116-1122.
6. Tirabassi J, Brou L, Khodaee M, Lefort R, Fields SK, Comstock RD. Epidemiology of high school sports-related injuries resulting in medical disqualification: 2005-2006 through 2013-2014 academic years. Am J Sports Med. 2016 May 10. [Epub ahead of print]
7. Fernandez WG, Yard EE, Comstock RD. Epidemiology of lower extremity injuries among U.S. high school athletes. Acad Emerg Med. 2007;14(7):641-645.
8. Olsen OE, Myklebust G, Engebretsen L, Bahr R. Injury mechanisms for anterior cruciate ligament injuries in team handball: a systematic video analysis. Am J Sports Med. 2004;32(4):1002-1012.
9. Boden BP, Dean GS, Feagin JA Jr, Garrett WE Jr. Mechanisms of anterior cruciate ligament injury. Orthopedics. 2000;23(6):573-578.
10. Dragoo JL, Braun HJ, Harris AH. The effect of playing surface on the incidence of ACL injuries in National Collegiate Athletic Association American Football. Knee. 2013;20(3):191-195.
11. Joseph AM, Collins CL, Henke NM, Yard EE, Fields SK, Comstock RD. A multisport epidemiologic comparison of anterior cruciate ligament injuries in high school athletics. J Athl Train. 2013;48(6):810-817.
12. Granan LP, Inacio MC, Maletis GB, Funahashi TT, Engebretsen L. Sport-specific injury pattern recorded during anterior cruciate ligament reconstruction. Am J Sports Med. 2013;41(12):2814-2818.
13. Bradley JP, Klimkiewicz JJ, Rytel MJ, Powell JW. Anterior cruciate ligament injuries in the National Football League: epidemiology and current treatment trends among team physicians. Arthroscopy. 2002;18(5):502-509.
14. Dragoo JL, Braun HJ, Durham JL, Chen MR, Harris AH. Incidence and risk factors for injuries to the anterior cruciate ligament in National Collegiate Athletic Association football: data from the 2004-2005 through 2008-2009 National Collegiate Athletic Association Injury Surveillance System. Am J Sports Med. 2012;40(5):990-995.
15. Dodson CC, Secrist ES, Bhat SB, Woods DP, Deluca PF. Anterior cruciate ligamenti in National Football League athletes from 2010 to 2013: a descriptive epidemiology study. Orthop J Sports Med. 2016;4(3):2325967116631949.
16. Golightly YM, Marshall SW, Callahan LF, Guskiewicz K. Early-onset arthritis in retired National Football League players. J Phys Act Health. 2009;6(5):638-643.
17. Brophy RH, Lyman S, Chehab EL, Barnes RP, Rodeo SA, Warren RF. Predictive value of prior injury on career in professional American football is affected by player position. Am J Sports Med. 2009;37(4):768-775.
18. Bradley J, Honkamp NJ, Jost P, West R, Norwig J, Kaplan LD. Incidence and variance of knee injuries in elite college football players. Am J Orthop. 2008;37(6):310-314.
19. Erickson BJ, Harris JD, Fillingham YA, et al. Anterior cruciate ligament reconstruction practice patterns by NFL and NCAA football team physicians. Arthroscopy. 2014;30(6):731-738.
20. Daruawalla JH, Greis PE, Hancock R; ASP Collaborative Group, Xerogeanes JW. Rates and determinants of return to play after anterior cruciate ligament reconstruction in NCAA Division 1 college football athletes: a study of the ACC, SEC, and PAC-12 conferences. Orthop J Sports Med. 2014;2(8):2325967114543901.
21. Shah VM, Andrews JR, Fleisig GS, McMichael CS, Lemak LJ. Return to play after anterior cruciate ligament reconstruction in National Football League athletes. Am J Sports Med. 2010;38(11):2233-2239.
22. McCullough KA, Phelps KD, Spindler KP, et al. Return to high school- and college-level football after anterior cruciate ligament reconstruction: a Multicenter Orthopaedic Outcomes Network (MOON) cohort study. Am J Sports Med. 2012;40(11):2523-2529.
23. Erickson BJ, Harris JD, Heninger JR, et al. Performance and return-to-sport after ACL reconstruction in NFL quarterbacks. Orthopedics. 2014;37(8):e728-e734.
24. Carey JL, Huffman GR, Parekh SG, Sennett BJ. Outcomes of anterior cruciate ligament injuries to running backs and wide receivers in the National Football League. Am J Sports Med. 2006;34(12):1911-1917.
25. Hershman EB, Anderson R, Bergfeld JA, et al. An analysis of specific lower extremity injury rates on grass and FieldTurf playing surfaces in National Football League Games: 2000-2009 seasons. Am J Sports Med. 2012;40(10):2200-2205.
26. Salata MJ, Gibbs AE, Sekiya JK. The effectiveness of prophylactic knee bracing in American football: a systematic review. Sports Health. 2010;2(5):375-379.
27. Derscheid GL, Garrick JG. Medial collateral ligament injuries in football. Nonoperative management of grade I and grade II sprains. Am J Sports Med. 1981;9(6):365-368.
28. Meyers MC, Barnhill BS. Incidence, causes, and severity of high school football injuries on FieldTurf versus natural grass: a 5-year prospective study. Am J Sports Med. 2004;32(7):1626-1638.
29. Lundblad M, Waldén M, Magnusson H, Karlsson J, Ekstrand J. The UEFA injury study: 11-year data concerning 346 MCL injuries and time to return to play. Br J Sports Med. 2013;47(12):759-762.
30. Hewson GF Jr, Mendini RA, Wang JB. Prophylactic knee bracing in college football. Am J Sports Med. 1986;14(4):262-266.
31. Rovere GD, Haupt HA, Yates CS. Prophylactic knee bracing in college football. Am J Sports Med. 1987;15(2):111-116.
32. Albright JP, Powell JW, Smith W, et al. Medial collateral ligament knee sprains in college football. Brace wear preferences and injury risk. Am J Sports Med. 1994;22(1):2-11.
33. Brophy RH, Barnes R, Rodeo SA, Warren RF. Prevalence of musculoskeletal disorders at the NFL Combine--trends from 1987 to 2000. Med Sci Sports Exerc. 2007;39(1):22-27.
34. Ellsasser JC, Reynolds FC, Omohundro JR. The non-operative treatment of collateral ligament injuries of the knee in professional football players. An analysis of seventy-four injuries treated non-operatively and twenty-four injuries treated surgically. J Bone Joint Surg Am. 1974;56(6):1185-1190.
35. Holden DL, Eggert AW, Butler JE. The nonoperative treatment of grade I and II medial collateral ligament injuries to the knee. Am J Sports Med. 1983;11(5):340-344.
36. Indelicato PA, Hermansdorfer J, Huegel M. Nonoperative management of complete tears of the medial collateral ligament of the knee in intercollegiate football players. Clin Orthop Relat Res. 1990;(256):174-177.
37. Jones RE, Henley MB, Francis P. Nonoperative management of isolated grade III collateral ligament injury in high school football players. Clin Orthop Relat Res. 1986;(213):137-140.
38. Fetto JF, Marshall JL. Medial collateral ligament injuries of the knee: a rationale for treatment. Clin Orthop Relat Res. 1978;(132):206-218.
39. Corten K, Hoser C, Fink C, Bellemans J. Case reports: a Stener-like lesion of the medial collateral ligament of the knee. Clin Orthop Relat Res. 2010;468(1):289-293.
40. Marchant MH Jr, Tibor LM, Sekiya JK, Hardaker WT Jr, Garrett WE Jr, Taylor DC. Management of medial-sided knee injuries, part 1: medial collateral ligament. Am J Sports Med. 2011;39(5):1102-1113.
41. Desio SM, Burks RT, Bachus KN. Soft tissue restraints to lateral patellar translation in the human knee. Am J Sports Med. 1998;26(1):59-65.
42. Casteleyn PP, Handelberg F. Arthroscopy in the diagnosis of occult dislocation of the patella. Acta Orthop Belg. 1989;55(3):381-383.
43. Waterman BR, Belmont PJ Jr, Owens BD. Patellar dislocation in the United States: role of sex, age, race, and athletic participation. J Knee Surg. 2012;25(1):51-57.
44. Sillanpää P, Mattila VM, Iivonen T, Visuri T, Pihlajamäki H. Incidence and risk factors of acute traumatic primary patellar dislocation. Med Sci Sports Exerc. 2008;40(4):606-611.
45. Hsiao M, Owens BD, Burks R, Sturdivant RX, Cameron KL. Incidence of acute traumatic patellar dislocation among active-duty United States military service members. Am J Sports Med. 2010;38(10):1997-2004.
46. Fithian DC, Paxton EW, Stone ML, et al. Epidemiology and natural history of acute patellar dislocation. Am J Sports Med. 2004;32(5):1114-1121.
47. Mitchell J, Magnussen RA, Collins CL, et al. Epidemiology of patellofemoral instability injuries among high school athletes in the United States. Am J Sports Med. 2015;43(7):1676-1682.
48. Nikku R, Nietosvaara Y, Aalto K, Kallio PE. The mechanism of primary patellar dislocation: trauma history of 126 patients. Acta Orthop. 2009;80(4):432-434.
49. Tsai CH, Hsu CJ, Hung CH, Hsu HC. Primary traumatic patellar dislocation. J Orthop Surg Res. 2012;7:21.
50. Atkin DM, Fithian DC, Marangi KS, Stone ML, Dobson BE, Mendelsohn C. Characteristics of patients with primary acute lateral patellar dislocation and their recovery within the first 6 months of injury. Am J Sports Med. 2000;28(4):472-479.
51. Nomura E, Inoue M, Kurimura M. Chondral and osteochondral injuries associated with acute patellar dislocation. Arthroscopy. 2003;19(7):717-721.
52. Kirsch MD, Fitzgerald SW, Friedman H, Rogers LF. Transient lateral patellar dislocation: diagnosis with MR imaging. AJR Am J Roentgenol. 1993;161(1):109-113.
53. Virolainen H, Visuri T, Kuusela T. Acute dislocation of the patella: MR findings. Radiology. 1993;189(1):243-246.
54. Stanitski CL. Articular hypermobility and chondral injury in patients with acute patellar dislocation. Am J Sports Med. 1995;23(2):146-150.
55. Mäenpää H, Huhtala H, Lehto MU. Recurrence after patellar dislocation. Redislocation in 37/75 patients followed for 6-24 years. Acta Orthop Scand. 1997;68(5):424-426.
56. Buchner M, Baudendistel B, Sabo D, Schmitt H. Acute traumatic primary patellar dislocation: long-term results comparing conservative and surgical treatment. Clin J Sport Med. 2005;15(2):62-66.
57. Fisher B, Nyland J, Brand E, Curtin B. Medial patellofemoral ligament reconstruction for recurrent patellar dislocation: a systematic review including rehabilitation and return-to-sports efficacy. Arthroscopy. 2010;26(10):1384-1394.
58. Lippacher S, Dreyhaupt J, Williams SR, Reichel H, Nelitz M. Reconstruction of the medial patellofemoral ligament: clinical outcomes and return to sports. Am J Sports Med. 2014;42(7):1661-1668.
59. Panni AS, Alam M, Cerciello S, Vasso M, Maffulli N. Medial patellofemoral ligament reconstruction with a divergent patellar transverse 2-tunnel technique. Am J Sports Med. 2011;39(12):2647-1655.
60. Schneider DK, Grawe B, Magnussen RA, et al. Outcomes after isolated medial patellofemoral ligament reconstruction for the treatment of recurrent lateral patellar dislocations: a systematic review and meta-analysis. Am J Sports Med. 2016 Feb 12. [Epub ahead of print]
61. Amis AA, Bull AM, Gupte CM, Hijazi I, Race A, Robinson JR. Biomechanics of the PCL and related structures: posterolateral, posteromedial and meniscofemoral ligaments. Knee Surg Sports Traumatol Arthrosc. 2003;11(5):271-281.
62. Fu FH, Harner CD, Johnson DL, Miller MD, Woo SL. Biomechanics of knee ligaments: basic concepts and clinical application. Instr Course Lect. 1994;43:137-148.
63. Markolf KL, Feeley BT, Tejwani SG, Martin DE, McAllister DR. Changes in knee laxity and ligament force after sectioning the posteromedial bundle of the posterior cruciate ligament. Arthroscopy. 2006; 22(10):1100-1106.
64. Ganelli GC, Edson CJ. Posterior cruciate ligament injuries in trauma patients: Part II. Arthroscopy. 1995;11(5):526-529.
65. Schulz MS, Russe K, Weiler A, Eichhorn HJ, Strobel MJ. Epidemiology of posterior cruciate ligament injuries. Arch Orthop Trauma Surg. 2003;123(4):186-191.
66. Patel DV, Allen AA, Warren RF, Wickiewicz TL, Simonian PT. The nonoperative treatment of acute, isolated (partial or complete) posterior cruciate ligament-deficient knees: an intermediate-term follow-up study. HSS J. 2007;3(2):137-146.
67. Fowler PJ, Messieh SS. Isolated posterior cruciate ligament injuries in athletes. Am J Sports Med. 1987;15(6):553-557.
68. Dick R, Ferrara MS, Agel J, et al. Descriptive epidemiology of collegiate men’s football injuries: National Collegiate Athletic Association Injury Surveillance System, 1988-1989 through 2003-2004. J Athl Train. 2007;42(2):221-233.
69. LaPrade CM, Civitarese DM, Rasmussen MT, LaPrade RF. Emerging updates on the posterior cruciate ligament: a review of the current literature. Am J Sports Med. 2015;43(12):3077-3092.
70. Torg JS, Barton TM, Pavlov H, Stine R. Natural history of the posterior cruciate ligament-deficient knee. Clin Orthop Relat Res. 1989(246):208-216.
71. Miller MD. Orthopaedic Knowledge Update: Sports Medicine 5. Rosemont, IL; American Academy of Orthopaedic Surgeons; 2016.
72. Shelbourne KD, Clark M, Gray T. Minimum 10-year follow-up of patients after an acute, isolated posterior cruciate ligament injury treated nonoperatively. Am J Sports Med. 2013;41(7):1526-1533.
73. Parolie JM, Bergfeld JA. Long-term results of nonoperative treatment of isolated posterior cruciate ligament injuries in the athlete. Am J Sports Med. 1986;14(1):35-38.
Foot and Ankle Injuries in American Football
Foot and ankle injuries are common in American football, with injury rates significantly increasing over the past decade.1-5 Epidemiologic studies of collegiate football players have shown an annual incidence of foot and ankle injuries ranging from 9% to 39%,3,6 with as many as 72% of all collegiate players presenting to the National Football League (NFL) Combine with a history of a foot or ankle injury and 13% undergoing surgical treatment.5 Player position influences the rate and type of foot and ankle injury. Offensive and “skill position” players, including linemen, running backs, and wide receivers, are particularly susceptible to foot and ankle injuries due to high levels of force and torque placed on the distal extremity during running, cutting, and tackling. Shoe wear changes, playing field conditions, increasing player size and speed, and improved reporting of injuries are also contributing to increasing injury rates.
The interaction between player cleats and the playing surface is a central issue of foot and ankle injuries in football. Improved traction relates to performance, but increased subsequent torque on the lower extremity is associated with injury. While lateral ankle sprains are the most common foot and ankle injury experienced by football players,7 numerous other injuries can occur, including turf toe, Jones fractures, Lisfranc injuries, syndesmotic disruption, deltoid complex avulsion, and Achilles ruptures. It is important for physicians to be able to recognize, diagnose, and appropriately treat these injuries in players in order to expedite recovery, restore function, and help prevent future injury and long-term sequelae. This review focuses on updated treatment principles, surgical advances, and rehabilitation protocols for common football foot and ankle injuries.
Turf Toe
The term “turf toe” was first used in 1976 to refer to hyperextension injuries and plantar capsule-ligament sprains of the hallux metatarsophalangeal (MTP) joint that can lead to progressive cock-up deformity.8 While these injuries can occur on any surface and disrupt soft tissue balance with functional implications, predisposing factors include increasing playing surface hardness and decreasing shoe stiffness. In a classic scenario, the foot is fixed in equinus as an axial load is placed on the back of the heel, resulting in forced dorsiflexion of the hallux MTP joint.9 As the proximal phalanx extends, the sesamoids are drawn distally and the more dorsal portion of the metatarsal head articular surface bears the majority of the load, causing partial or complete tearing of the plantar plate with or without hallux MTP dislocation. Osteochondral lesions of the MTP joint and subchondral edema of the metatarsal head can occur concurrently as the proximal phalanx impacts or shears across the metatarsal head articular surface.
Clinical examination should focus on hallux swelling, alignment, and flexor hallucis longus (FHL) function along with vertical instability of the hallux MTP joint using a Lachman test. Radiographs should be evaluated for proximal migration of the sesamoids or diastasis (Figures W1A-W1C).
Indications for surgical intervention include loss of push-off strength, gross MTP instability, proximal migration of the sesamoids, and progressive hallux malalignment or clawing after immobilization. Cases can involve one or a combination of the following: (1) large capsular avulsion with unstable MTP joint; (2) diastasis of bipartite sesamoid; (3) diastasis of sesamoid fracture; (4) retraction of sesamoid; (5) traumatic hallux valgus deformity; (6) vertical instability (positive Lachman test); (7) loose body in MTP joint; or (8) chondral injury in MTP joint. The goal of surgery is the restoration of anatomy in order to restore normal function of the hallux MTP joint.
We have found that using dual medial and plantar incisions places less traction on the plantar medial cutaneous nerve, improves lateral exposure, and provides better wound healing. The medial capsulotomy extends from the metatarsal neck to the mid-phalanx to provide complete visualization of the sesamoid complex (Figures 1A-1F).
It is important to recognize that not all turf toe injuries involve pure hyperextension on artificial playing surfaces. In recent years, we have found an increasing rate of medial variant turf toe injuries in which a forceful valgus stress on the hallux leads to rupture of the medial collateral ligament, medial or plantar-medial capsule, and/or abductor halluces. Medial variant turf toe can lead to progressive hallux valgus and a traumatic bunion with a significant loss of push-off strength and difficulty with cutting maneuvers. Surgical treatment requires a modified McBride bunionectomy with adductor tenotomy and direct repair of the medial soft tissue defect.
Postoperative management is just as important as proper surgical technique for these injuries and involves a delicate balance between protecting the repair and starting early range of motion (ROM). Patients are immobilized non-weight-bearing (NWB) for 5 to 7 days maximum followed immediately with the initiation of passive hallux plantarflexion to keep the sesamoids moving. Active hallux plantarflexion is started at 4 weeks after surgery with active dorsiflexion from 6 to 8 weeks. Patients are transitioned to an accommodative shoe with stiff hallux insert 8 weeks postoperative with continued therapy focusing on hallux ROM. Running is initiated at 12 weeks and return to play (RTP) is typically allowed 4 months after surgery.
Jones Fractures
Jones fractures are fractures of the 5th metatarsal at the metaphyseal-diaphyseal junction, where there is a watershed area of decreased vascularity between the intramedullary nutrient and metaphyseal arteries. Current thought is that the rising rate of Jones fractures among football players is partially caused by the use of flexible, narrow cleats that do not provide enough stiffness and lateral support for the 5th metatarsal during running and cutting. Additionally, lateral overload from a baseline cavovarus foot posture with or without metatarsus adductus and/or skewfoot is thought to contribute to Jones fractures.10 Preoperative radiographs should be evaluated for fracture location, orientation, amount of cortical thickening, and overall geometry of the foot and 5th metatarsal. In elite athletes, the threshold for surgical intervention is decreasing in order to expedite healing and recovery and decrease re-fracture risk. This rationale is based on delayed union rates of 25% to 66%, nonunion rates of 7% to 28%,11 and re-fracture rates of up to 33% associated with nonoperative treatment.12 Nonoperative management is usually not feasible in the competitive athlete, as it typically involves a period of protected weight-bearing in a tall controlled ankle motion (CAM) boot for 6 to 8 weeks with serial radiographs to evaluate healing.
Our preference for surgical intervention involves percutaneous screw fixation with a “high and inside” starting point on fluoroscopy (Figures 2A-2D).
In career athletes, we augment the fracture site using a mixture of bone marrow aspirate concentrate (BMA) (Magellan, Arteriocyte Medical Systems) and demineralized bone matrix (DBM) (Mini Ignite, Wright Medical Technology) injected percutaneously in and around the fracture site under fluoroscopic guidance. Using this technique in a cohort of 25 NFL players treated operatively for Jones fractures, we found that 100% of athletes were able to RTP in the NFL in an average of 9.5 weeks.14 Two patients (7.5%) suffered re-fractures requiring revision surgery with iliac crest bone graft and repeat screw placement with a RTP after 15 weeks. We did not find an association between RTP and re-fracture rate.
The appropriate rehabilitation protocol for Jones fractures after surgery remains controversial and dependent on individual needs and abilities.15,16 For athletes in-season, we recommend a brief period of NWB for 1 to 2 weeks followed by toe-touch weight-bearing in a tall CAM boot for 2 to 4 weeks. After 4 weeks, patients begin gentle exercises on a stationary bike and pool therapy to reduce impact on the fracture site. Low-intensity pulsed ultrasound bone stimulators (Exogen, Bioventus) are frequently used directly over fracture site throughout the postoperative protocol as an adjuvant therapy. If clinically nontender over the fracture site, patients are allowed to begin running in modified protective shoe wear 4 weeks after surgery with an average RTP of 6 to 8 weeks. RTP is determined clinically, as radiographic union may not be evident for 12 to 16 weeks. Useful custom orthoses include turf toe plates with a cushioned lateral column and lateral heel wedge if hindfoot varus is present preoperatively.10 The solid intramedullary screw is left in place permanently.
In our experience, we have found the average re-fracture and nonunion rate to be approximately 8% across all athletes. Our observation that union rates do not appear to be related to RTP times suggests that underlying biology such as Vitamin D deficiency may play a larger role in union rates than previously thought. We have found that most Jones re-fractures occur in the first year after the initial injury, but can occur up to 2.5 years afterwards as well.14 For the management of symptomatic re-fractures and nonunions, the previous screw must be first removed. This can be difficult if the screw is bent or broken, and may require a lateral corticotomy of the metatarsal.
After hardware removal, we advocate open bone grafting of the fracture site using bone from the iliac crest retrieved with a small, percutaneous trephine. Re-fixation should be achieved using a larger, solid screw and postoperative adjuvants may include bone stimulators, Vitamin D and calcium supplemention, and possible teriparatide use (Forteo, Eli Lilly), depending on individual patient profile. In a cohort of 21 elite athletes treated for Jones fracture revision surgery with screw exchange and bone grafting, we found that 100% of patients had computed tomography (CT) evidence of union, with an average RTP of 12.3 weeks.17
Lisfranc Injuries
Lisfranc injuries include any bony or ligamentous damage that involves the tarsometatarsal (TMT) joints. While axial loading of a fixed, plantarflexed foot has traditionally been thought of as the most common mechanism of Lisfranc injury, we have found that noncontact twisting injuries leading to Lisfranc disruption are actually more common among NFL players. This mechanism is similar to noncontact turf toe and results in a purely ligamentous injury. We have found this to be particularly true in the case of defensive ends engaged with offensive linemen in which no axial loading or contact of the foot occurs. Clinically, patients often have painful weight-bearing, inability to perform a single limb heel rise, plantar ecchymosis, and swelling and point tenderness across the bases of the 1st and 2nd metatarsals.
It is critical to obtain comparison weight-bearing radiographs of both feet during initial work-up to look for evidence of instability. Subtle radiographic findings of Lisfranc injury include a bony “fleck” sign, compression fracture of the cuboid, and diastasis between the base of the 1st and 2nd metatarsals and/or medial and middle cuneiforms (Figures 3A, 3B).
The goal of surgical intervention is to obtain and maintain anatomic reduction of all unstable joints in order to restore a normal foot posture. One of the difficulties with Lisfranc injuries is that there are no exact diastasis parameters and individuals should be treated based on symptoms, functional needs, and degree of instability. It has been shown that 5 mm of displacement can have good long-term clinical results in select cases without surgery.18 For surgery, we recommend open reduction to remove interposed soft tissue debris and directly assess the articular surfaces (Figures 4A-4D).
Proximal-medial column Lisfranc injury variants are increasingly common among football players.20 In these injuries, the force of injury extends through the intercuneiform joint and exits out the naviculocuneiform joint, thus causing instability at multiple joints and an unstable 1st ray. Patients often have minimal clinical findings and normal radiographs and stress radiographs. MRI of the foot often reveals edema at the naviculocuneiform joint. Often patients fail to improve with nonoperative immobilization with continued inability to push off from the hallux. Unrecognized or untreated instability will lead to rapid deterioration of the naviculocuneiform joint. Surgical intervention requires a homerun screw and intercuneiform screw. We do not recommend primary arthrodesis in athletes due to significant risk of malunion and nonunion unless severe articular damage is present.
Patients are typically kept NWB in a splint for 2 weeks after surgery followed by NWB in a tall CAM from 3 to 4 weeks postoperative. Progressive weight-bearing and ROM exercises are initiated from 4 to 8 weeks, followed by return to accommodative shoe wear from 10 to 12 weeks. Hardware removal is performed 4 to 6 months after surgery, typically in the off-season to allow for 6 to 8 weeks or protected recovery afterwards. Premature hardware removal can lead to loss of reduction, particularly at the intercuneiform joints. All hardware crossing the TMT joints should be removed, while the homerun screw can be left in place in addition to the intercuneiform screw. RTP in football typically occurs 6 to 7 months after surgery. Final functional outcome is related to the adequacy of initial reduction and severity of the initial injury.21
Syndesmotic Disruption
Syndesmotic injuries comprise 1% to 18% of ankle sprains in the general population, but occur at much higher rates in football due to the increased rotation forces placed on the ankle during cutting and tackling. RTP after syndesmotic injury often takes twice as long when compared to isolated lateral ankle ligamentous injury.22 Missed injuries are common and if not treated properly can lead to chronic ankle instability and posttraumatic ankle arthritis.23 Syndesmotic injury can occur in isolation or with concomitant adjacent bony, cartilaginous, or ligamentous injuries. Therefore, clinical examination and imaging work-up are critical to successful management.
Syndesmotic injuries often result from an external rotation force applied to a hyperdorsiflexed ankle while the foot is planted. This mechanism causes the fibula to externally rotate while translating posteriorly and laterally, resulting in rupture of the anterior inferior tibiofibular ligament (AITFL) first, followed by the deep deltoid ligament, interosseous ligament (IOL), and lastly posterior talofibular ligament.24 Most syndesmotic injuries involve rupture of only the AITFL and IOL.25 Multiple clinical stress tests have been designed to assess syndesmotic stability, including the squeeze test, external rotation stress test, crossed-leg test, and fibula-translation test.26-29 However, no physical examination maneuver has been shown to reliably predict the presence or degree of syndesmotic injury and therefore imaging studies are necessary.30
Initial imaging should include standing radiographs of the affected ankle. An increase in the medial clear space between the medial malleolus and talus can occur with a combined syndesmotic and deltoid disruption. In the case of subtle syndesmotic injuries, contralateral comparison weight-bearing radiographs are recommended. CT and MRI can provide additional information, but these static imaging tests cannot identify instability. Fluoroscopic stress evaluation is beneficial but has a high false-negative rate in low-grade injuries and may not detect partial rupture of the AITFL and IOL.31 It has been shown that malrotation of as much as 30° of external rotation can occur if relying on intraoperative fluoroscopy alone.32 It has been our practice to recommend surgical reduction and stabilization for any syndesmotic injury with documented diastasis or instability seen on imaging and/or arthroscopy.
Nonoperative treatment is indicated for truly stable grade I syndesmotic injuries. This involves rest and immobilization followed by a progressive rehabilitation program consisting of stretching, strengthening, and proprioceptive exercises.33 After 1 week of protected weight-bearing in a cast or tall CAM boot, progression to full weight-bearing should occur over the following week. Active-assisted ankle ROM exercises and light proprioceptive training should then be initiated followed by sport-specific exercises 2 to 3 weeks after injury.
Arthroscopy can be a valuable diagnostic tool in the setting of subtle syndesmotic injury with negative radiographs, positive MRI for edema, and a protracted recovery course with vague pain (Figures W5A-W5E).
Implants are placed above the true syndesmotic joint (at least 15 mm above the tibial plafond) angled 30° posterior to anterior to follow the normal relationship of the fibula to the distal tibia in the incisura. Typically 2 suture-buttons are used, with the devices placed in a divergent fashion. We highly recommend the use of a fibular buttress plate with button placement in individuals returning to contact activity. This construct increases surface area distribution while preventing stress risers and the risk of fibula fractures. In a cadaver model with deliberate syndesmotic malreduction, suture-button stabilization resulted in decreased postoperative displacement as opposed to conventional screw fixation.34 Therefore, dynamic syndesmotic fixation may help to decrease the negative sequelae of iatrogenic clamp malreduction.
Postoperative rehabilitation involves NWB in a cast or tall CAM boot for 4 weeks followed by ankle ROM exercises and progressive weight-bearing and physical therapy. Patients are transitioned to a lace-up ankle brace and athletic shoe from 6 to 12 weeks postoperative with increasing activity. Running and jumping is permitted 4 months after surgery with RTP typically at 6 to 7 months. Athletes who have had surgical stabilization for documented instability without any diastasis may engage in a more rapid recovery and RTP as symptoms and function allow.
Deltoid Complex Avulsion
Missed or neglected deltoid ligament injuries can lead to progressive chondral injury and joint degeneration. These injuries are often subtle and difficult to diagnose. An inability to perform a single limb heel rise, persistent pain with activity, and lack of normal functional improvement despite appropriate care are indicators of subtle ligament instability. These injuries often require an examination under anesthesia with combined ankle arthroscopy. Valgus stress testing of the ankle while directly visualizing the deltoid ligament from the anterolateral portal can reveal medial laxity in addition to potential osteochondral lesions along the anterolateral talar dome.
In American football players, we have observed that infolding and retraction of an avulsed superficial deltoid ligament complex after an ankle fracture, Maisonneuve injury, or severe high ankle sprain can be a source of persistent increased medial clear space, malreduction, and postoperative pain and medial instability. We have found that there is often complete avulsion of the superficial deltoid complex off the proximal aspect of the medial malleolus during high-energy ankle fractures in football players that is amenable to direct repair to bone (Figures W6A-W6E).
During surgical repair, an incision is made along the anterior aspect of the medial malleolus and the superficial deltoid ligament complex can often be found flipped and interposed in the medial gutter. A rongeur is used to create a bleeding cancellous bone surface for soft-tissue healing and 1 to 2 suture anchors are used to repair and imbricate the deltoid ligament complex back to the medial malleolus. The goal of these sutures is to repair the tibionavicular and tibial spring ligaments back to the medial malleolus. We believe that superficial deltoid complex avulsion during high-energy ankle fractures is a distinct injury pattern that should be recognized and may benefit from primary open repair.
We currently open explore every deltoid ligament complex in athletes with unstable syndesmotic injuries, as we believe that deltoid avulsion injuries are underrecognized and do not heal in an anatomic fashion if left alone. Postoperative recovery follows the same immobilization, progressive weight-bearing, and physical therapy protocol as that for syndesmotic disruption.
Achilles Ruptures
Acute midsubstance Achilles tendon ruptures are an increasingly common injury in patients 30 to 50 years of age, with more than 50% of all injuries occurring during basketball.36,37 Among NFL players, we have found that Achilles ruptures tend to occur at a higher rate during training camp, when athletes are deconditioned and quickly returning to explosive push-off activities. Physical examination should include a Thompson test, palpation of a gap within the tendon, and evaluation of resting ankle dorsiflexion in the affected extremity in the prone position with the knees bent. Lateral radiographs should be analyzed for the presence of a bony avulsion fragment indicative of an insertional avulsion injury or midsubstance calcium deposition reflecting chronic Achilles tendinosis, as both of these conditions will change surgical management. MRI is not recommended with acute midsubstance ruptures but may be helpful in the case of chronic ruptures or more proximal tears of the musculotendinous junction.
The management of acute midsubstance Achilles tendon ruptures is controversial, with no general consensus in the literature regarding nonoperative treatment, surgical repair, and ideal repair technique.36,38-42 American Academy of Orthopaedic Surgeons clinical practice guidelines report moderate evidence that nonoperative treatment of Achilles tendon ruptures has lower wound healing complications but higher rates of re-rupture.38,39 Additionally, limited incision approaches have been found to have fewer overall complications compared with traditional open repair. In an effort to reduce the incidence of postoperative wound complications while improving functional recovery, modern repair techniques focus on a limited incision repair using percutaneous suture insertion and management (PARS Achilles Jig System, Arthrex).36 The limited incision technique utilizes a 2-cm transverse incision and non-disposable jig with divergent needle passes and locking suture fixation options to secure and fixate both tendon ends with minimal dissection of skin, subcutaneous tissue, and paratenon. Limited incision repair is ideally performed within 2 weeks of the injury to ensure that both tendon ends are easy to identify, mobilize, and repair. An open repair is generally recommended for midsubstance ruptures more than 4 weeks old and cases of insertional rupture and Achilles tendinopathy.
In a cohort of 9 NFL players treated for midsubstance Achilles ruptures using the PARS technique, we found no re-ruptures, no wound complications, and no sural nerve issues after surgery.43 A comparative review of 270 cases of operatively treated Achilles tendon ruptures (101 PARS, 169 traditional open repair) showed that the PARS group had significantly shorter operative times and a higher number of patients able to return to baseline physical activities by 5 months compared to open repair.36 Although not statistically significant, the overall PARS complication rate was 5% while the open complication rate was 11%. The PARS group had no cases of sural neuritis or deep infection requiring reoperation. We currently use a limited incision technique for all acute midsubstance Achilles ruptures in athletes regardless of sport, patient size, or position played.
During surgery, a 2-cm transverse incision is made over the gap in the Achilles tendon and dissection is carried down to the rupture site with minimal manipulation of the skin (Figures 5A-5F).
A key aspect of postoperative recovery is avoiding excessive ankle dorsiflexion while the tendon is healing during the first 4 weeks after surgery, as this can lead to an elongated tendon with loss of push-off strength. Patients are kept in a plantarflexion splint NWB for 2 weeks after surgery. If the incision is healed at 2 weeks, sutures are removed and patients are transitioned into a NWB tall CAM boot for 2 weeks with gentle ankle ROM exercises. If there is any concern regarding wound healing status, sutures are maintained for an additional 1 to 2 weeks.
From 4 to 8 weeks after surgery, progressive weight-bearing with continued ankle ROM exercises is initiated with peel-away heel lifts (~2 cm thick total, 3 layers). Each layer of the heel lift is gradually removed as pain allows every 2 to 3 days with the goal of being full weight-bearing with the foot flat at 6 weeks postoperative. Physical therapy focusing on ankle ROM and gentle Achilles stretching and strengthening is also started 6 weeks after surgery. From 8 to 12 weeks postoperative, patients are transitioned out of the tall CAM boot into normal, accommodative shoe wear with full weight-bearing. We avoid ankle dorsiflexion past neutral until 12 weeks after surgery, as overlengthening of the Achilles complex and the subsequent loss of push-off power can be devastating to running athletes. Activity levels are increased as tolerated, with no running or jumping from 12 to 16 weeks with full release to all activities after 16 weeks. RTP often takes 5 to 6 months after surgery, depending on the position played.
Am J Orthop. 2016;45(6):358-367. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Canale ST, Cantler ED Jr, Sisk TD, Freeman BL 3rd. A chronicle of injuries of an American intercollegiate football team. Am J Sports Med. 1981;9(6):384-389.2. Robey JM, Blyth CS, Mueller FO. Athletic injuries. Application of epidemiologic methods. JAMA. 1971;217(2):184-189.
3. Saal JA. Common American football injuries. Sports Med. 1991;12(2):132-147.
4. Thompson N, Halpern B, Curl WW, et al. High school football injuries: evaluation. Am J Sports Med. 1987;15(2):117-124.
5. Kaplan LD, Jost PW, Honkamp N, Norwig J, West R, Bradley JP. Incidence and variance of foot and ankle injuries in elite college football players. Am J Orthop. 2011;40(1):40-44.
6. DeLee JC, Farney WC. Incidence of injury in Texas high school football. Am J Sports Med. 1992;20(5):575-580.
7. Brophy RH, Barnes R, Rodeo SA, Warren RF. Prevalence of musculoskeletal disorders at the NFL Combine--trends from 1987 to 2000. Med Sci Sports Exerc. 2007;39(1):22-27.
8. Bowers KD Jr, Martin RB. Turf-toe: a shoe-surface related football injury. Med Sci Sports. 1976;8(2):81-83.
9. McCormick JJ, Anderson RB. Turf toe: anatomy, diagnosis, and treatment. Sports Health. 2010;2(6):487-494.
10. Raikin SM, Slenker N, Ratigan B. The association of a varus hindfoot and fracture of the fifth metatarsal metaphyseal-diaphyseal junction: the Jones fracture. Am J Sports Med. 2008;36(7):1367-1372.
11. Title CI, Katchis SD. Traumatic foot and ankle injuries in the athlete. Orthop Clin North Am. 2002;33(3):587-598.
12. Quill GE Jr. Fractures of the proximal fifth metatarsal. Orthop Clin North Am. 1995;26(2):353-361.
13. Nunley JA, Glisson RR. A new option for intramedullary fixation of Jones fractures: the Charlotte Carolina Jones Fracture System. Foot Ankle Int. 2008;29(12):1216-1221.
14. Lareau CR, Hsu AR, Anderson RB. Return to play in National Football League players after operative Jones fracture treatment. Foot Ankle Int. 2016;37(1):8-16.
15. Larson CM, Almekinders LC, Taft TN, Garrett WE. Intramedullary screw fixation of Jones fractures. Analysis of failure. Am J Sports Med. 2002;30(1):55-60.
16. Portland G, Kelikian A, Kodros S. Acute surgical management of Jones’ fractures. Foot Ankle Int. 2003;24(11):829-833.
17. Hunt KJ, Anderson RB. Treatment of Jones fracture nonunions and refractures in the elite athlete: outcomes of intramedullary screw fixation with bone grafting. Am J Sports Med. 2011;39(9):1948-1954.
18. Nunley JA, Vertullo CJ. Classification, investigation, and management of midfoot sprains: Lisfranc injuries in the athlete. Am J Sports Med. 2002;30(6):871-878.
19. Alberta FG, Aronow MS, Barrero M, Diaz-Doran V, Sullivan RJ, Adams DJ. Ligamentous Lisfranc joint injuries: a biomechanical comparison of dorsal plate and transarticular screw fixation. Foot Ankle Int. 2005;26(6):462-473.
20. Ardoin GT, Anderson RB. Subtle Lisfranc injury. Tech Foot Ankle Surg. 2010;9(3):100-106.
21. Kuo RS, Tejwani NC, Digiovanni CW, et al. Outcome after open reduction and internal fixation of Lisfranc joint injuries. J Bone Joint Surg Am. 2000;82-A(11):1609-1618.
22. Wright RW, Barile RJ, Surprenant DA, Matava MJ. Ankle syndesmosis sprains in national hockey league players. Am J Sports Med. 2004;32(8):1941-1945.
23. Williams GN, Jones MH, Amendola A. Syndesmotic ankle sprains in athletes. Am J Sports Med. 2007;35(7):1197-1207.
24. Beumer A, Valstar ER, Garling EH, et al. Effects of ligament sectioning on the kinematics of the distal tibiofibular syndesmosis: a radiostereometric study of 10 cadaveric specimens based on presumed trauma mechanisms with suggestions for treatment. Acta Orthop. 2006;77(3):531-540.
25. McCollum GA, van den Bekerom MP, Kerkhoffs GM, Calder JD, van Dijk CN. Syndesmosis and deltoid ligament injuries in the athlete. Knee Surg Sports Traumatol Arthrosc. 2013;21(6):1328-1337.
26. Boytim MJ, Fischer DA, Neumann L. Syndesmotic ankle sprains. Am J Sports Med. 1991;19(3):294-298.
27. Nussbaum ED, Hosea TM, Sieler SD, Incremona BR, Kessler DE. Prospective evaluation of syndesmotic ankle sprains without diastasis. Am J Sports Med. 2001;29(1):31-35.
28. Kiter E, Bozkurt M. The crossed-leg test for examination of ankle syndesmosis injuries. Foot Ankle Int. 2005;26(2):187-188.
29. Beumer A, van Hemert WL, Swierstra BA, Jasper LE, Belkoff SM. A biomechanical evaluation of clinical stress tests for syndesmotic ankle instability. Foot Ankle Int. 2003;24(4):358-363.
30. Amendola A, Williams G, Foster D. Evidence-based approach to treatment of acute traumatic syndesmosis (high ankle) sprains. Sports Med Arthrosc. 2006;14(4):232-236.
31. Beumer A, Valstar ER, Garling EH, et al. External rotation stress imaging in syndesmotic injuries of the ankle: comparison of lateral radiography and radiostereometry in a cadaveric model. Acta Orthop Scand. 2003;74(2):201-205.
32. Marmor M, Hansen E, Han HK, Buckley J, Matityahu A. Limitations of standard fluoroscopy in detecting rotational malreduction of the syndesmosis in an ankle fracture model. Foot Ankle Int. 2011;32(6):616-622.
33. Williams GN, Allen EJ. Rehabilitation of syndesmotic (high) ankle sprains. Sports Health. 2010;2(6):460-470.
34. Westermann RW, Rungprai C, Goetz JE, Femino J, Amendola A, Phisitkul P. The effect of suture-button fixation on simulated syndesmotic malreduction: a cadaveric study. J Bone Joint Surg Am. 2014;96(20):1732-1738.
35. Hsu AR, Lareau CR, Anderson RB. Repair of acute superficial deltoid complex avulsion during ankle fracture fixation in National Football League players. Foot Ankle Int. 2015;36(11):1272-1278.
36. Hsu AR, Jones CP, Cohen BE, Davis WH, Ellington JK, Anderson RB. Clinical outcomes and complications of percutaneous Achilles repair system versus open technique for acute achilles tendon ruptures. Foot Ankle Int. 2015;36(11):1279-1286.
37. Raikin SM, Garras DN, Krapchev PV. Achilles tendon injuries in a United States population. Foot Ankle Int. 2013;34(4):475-480.
38. Chiodo CP, Glazebrook M, Bluman EM, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on treatment of achilles tendon rupture. J Bone Joint Surg Am. 2010;92(14):2466-2468.
39. Chiodo CP, Glazebrook M, Bluman EM, et al. Diagnosis and treatment of acute achilles tendon rupture. J Am Acad Orthop Surg. 2010;18(8):503-510.
40. Khan RJ, Fick D, Keogh A, Crawford J, Brammar T, Parker M. Treatment of acute achilles tendon ruptures. A meta-analysis of randomized, controlled trials. J Bone Joint Surg Am. 2005;87(10):2202-2210.
41. Renninger CH, Kuhn K, Fellars T, Youngblood S, Bellamy J. Operative and nonoperative management of achilles tendon ruptures in active duty military population. Foot Ankle Int. 2016;37(3):269-273.
42. Khan RJ, Carey Smith RL. Surgical interventions for treating acute achilles tendon ruptures. Cochrane Database Syst Rev. 2010;(9):CD003674.
43. McCullough KA, Shaw CM, Anderson RB. Mini-open repair of achilles rupture in the national football league. J Surg Orthop Adv. 2014;23(4):179-183.
Foot and ankle injuries are common in American football, with injury rates significantly increasing over the past decade.1-5 Epidemiologic studies of collegiate football players have shown an annual incidence of foot and ankle injuries ranging from 9% to 39%,3,6 with as many as 72% of all collegiate players presenting to the National Football League (NFL) Combine with a history of a foot or ankle injury and 13% undergoing surgical treatment.5 Player position influences the rate and type of foot and ankle injury. Offensive and “skill position” players, including linemen, running backs, and wide receivers, are particularly susceptible to foot and ankle injuries due to high levels of force and torque placed on the distal extremity during running, cutting, and tackling. Shoe wear changes, playing field conditions, increasing player size and speed, and improved reporting of injuries are also contributing to increasing injury rates.
The interaction between player cleats and the playing surface is a central issue of foot and ankle injuries in football. Improved traction relates to performance, but increased subsequent torque on the lower extremity is associated with injury. While lateral ankle sprains are the most common foot and ankle injury experienced by football players,7 numerous other injuries can occur, including turf toe, Jones fractures, Lisfranc injuries, syndesmotic disruption, deltoid complex avulsion, and Achilles ruptures. It is important for physicians to be able to recognize, diagnose, and appropriately treat these injuries in players in order to expedite recovery, restore function, and help prevent future injury and long-term sequelae. This review focuses on updated treatment principles, surgical advances, and rehabilitation protocols for common football foot and ankle injuries.
Turf Toe
The term “turf toe” was first used in 1976 to refer to hyperextension injuries and plantar capsule-ligament sprains of the hallux metatarsophalangeal (MTP) joint that can lead to progressive cock-up deformity.8 While these injuries can occur on any surface and disrupt soft tissue balance with functional implications, predisposing factors include increasing playing surface hardness and decreasing shoe stiffness. In a classic scenario, the foot is fixed in equinus as an axial load is placed on the back of the heel, resulting in forced dorsiflexion of the hallux MTP joint.9 As the proximal phalanx extends, the sesamoids are drawn distally and the more dorsal portion of the metatarsal head articular surface bears the majority of the load, causing partial or complete tearing of the plantar plate with or without hallux MTP dislocation. Osteochondral lesions of the MTP joint and subchondral edema of the metatarsal head can occur concurrently as the proximal phalanx impacts or shears across the metatarsal head articular surface.
Clinical examination should focus on hallux swelling, alignment, and flexor hallucis longus (FHL) function along with vertical instability of the hallux MTP joint using a Lachman test. Radiographs should be evaluated for proximal migration of the sesamoids or diastasis (Figures W1A-W1C).
Indications for surgical intervention include loss of push-off strength, gross MTP instability, proximal migration of the sesamoids, and progressive hallux malalignment or clawing after immobilization. Cases can involve one or a combination of the following: (1) large capsular avulsion with unstable MTP joint; (2) diastasis of bipartite sesamoid; (3) diastasis of sesamoid fracture; (4) retraction of sesamoid; (5) traumatic hallux valgus deformity; (6) vertical instability (positive Lachman test); (7) loose body in MTP joint; or (8) chondral injury in MTP joint. The goal of surgery is the restoration of anatomy in order to restore normal function of the hallux MTP joint.
We have found that using dual medial and plantar incisions places less traction on the plantar medial cutaneous nerve, improves lateral exposure, and provides better wound healing. The medial capsulotomy extends from the metatarsal neck to the mid-phalanx to provide complete visualization of the sesamoid complex (Figures 1A-1F).
It is important to recognize that not all turf toe injuries involve pure hyperextension on artificial playing surfaces. In recent years, we have found an increasing rate of medial variant turf toe injuries in which a forceful valgus stress on the hallux leads to rupture of the medial collateral ligament, medial or plantar-medial capsule, and/or abductor halluces. Medial variant turf toe can lead to progressive hallux valgus and a traumatic bunion with a significant loss of push-off strength and difficulty with cutting maneuvers. Surgical treatment requires a modified McBride bunionectomy with adductor tenotomy and direct repair of the medial soft tissue defect.
Postoperative management is just as important as proper surgical technique for these injuries and involves a delicate balance between protecting the repair and starting early range of motion (ROM). Patients are immobilized non-weight-bearing (NWB) for 5 to 7 days maximum followed immediately with the initiation of passive hallux plantarflexion to keep the sesamoids moving. Active hallux plantarflexion is started at 4 weeks after surgery with active dorsiflexion from 6 to 8 weeks. Patients are transitioned to an accommodative shoe with stiff hallux insert 8 weeks postoperative with continued therapy focusing on hallux ROM. Running is initiated at 12 weeks and return to play (RTP) is typically allowed 4 months after surgery.
Jones Fractures
Jones fractures are fractures of the 5th metatarsal at the metaphyseal-diaphyseal junction, where there is a watershed area of decreased vascularity between the intramedullary nutrient and metaphyseal arteries. Current thought is that the rising rate of Jones fractures among football players is partially caused by the use of flexible, narrow cleats that do not provide enough stiffness and lateral support for the 5th metatarsal during running and cutting. Additionally, lateral overload from a baseline cavovarus foot posture with or without metatarsus adductus and/or skewfoot is thought to contribute to Jones fractures.10 Preoperative radiographs should be evaluated for fracture location, orientation, amount of cortical thickening, and overall geometry of the foot and 5th metatarsal. In elite athletes, the threshold for surgical intervention is decreasing in order to expedite healing and recovery and decrease re-fracture risk. This rationale is based on delayed union rates of 25% to 66%, nonunion rates of 7% to 28%,11 and re-fracture rates of up to 33% associated with nonoperative treatment.12 Nonoperative management is usually not feasible in the competitive athlete, as it typically involves a period of protected weight-bearing in a tall controlled ankle motion (CAM) boot for 6 to 8 weeks with serial radiographs to evaluate healing.
Our preference for surgical intervention involves percutaneous screw fixation with a “high and inside” starting point on fluoroscopy (Figures 2A-2D).
In career athletes, we augment the fracture site using a mixture of bone marrow aspirate concentrate (BMA) (Magellan, Arteriocyte Medical Systems) and demineralized bone matrix (DBM) (Mini Ignite, Wright Medical Technology) injected percutaneously in and around the fracture site under fluoroscopic guidance. Using this technique in a cohort of 25 NFL players treated operatively for Jones fractures, we found that 100% of athletes were able to RTP in the NFL in an average of 9.5 weeks.14 Two patients (7.5%) suffered re-fractures requiring revision surgery with iliac crest bone graft and repeat screw placement with a RTP after 15 weeks. We did not find an association between RTP and re-fracture rate.
The appropriate rehabilitation protocol for Jones fractures after surgery remains controversial and dependent on individual needs and abilities.15,16 For athletes in-season, we recommend a brief period of NWB for 1 to 2 weeks followed by toe-touch weight-bearing in a tall CAM boot for 2 to 4 weeks. After 4 weeks, patients begin gentle exercises on a stationary bike and pool therapy to reduce impact on the fracture site. Low-intensity pulsed ultrasound bone stimulators (Exogen, Bioventus) are frequently used directly over fracture site throughout the postoperative protocol as an adjuvant therapy. If clinically nontender over the fracture site, patients are allowed to begin running in modified protective shoe wear 4 weeks after surgery with an average RTP of 6 to 8 weeks. RTP is determined clinically, as radiographic union may not be evident for 12 to 16 weeks. Useful custom orthoses include turf toe plates with a cushioned lateral column and lateral heel wedge if hindfoot varus is present preoperatively.10 The solid intramedullary screw is left in place permanently.
In our experience, we have found the average re-fracture and nonunion rate to be approximately 8% across all athletes. Our observation that union rates do not appear to be related to RTP times suggests that underlying biology such as Vitamin D deficiency may play a larger role in union rates than previously thought. We have found that most Jones re-fractures occur in the first year after the initial injury, but can occur up to 2.5 years afterwards as well.14 For the management of symptomatic re-fractures and nonunions, the previous screw must be first removed. This can be difficult if the screw is bent or broken, and may require a lateral corticotomy of the metatarsal.
After hardware removal, we advocate open bone grafting of the fracture site using bone from the iliac crest retrieved with a small, percutaneous trephine. Re-fixation should be achieved using a larger, solid screw and postoperative adjuvants may include bone stimulators, Vitamin D and calcium supplemention, and possible teriparatide use (Forteo, Eli Lilly), depending on individual patient profile. In a cohort of 21 elite athletes treated for Jones fracture revision surgery with screw exchange and bone grafting, we found that 100% of patients had computed tomography (CT) evidence of union, with an average RTP of 12.3 weeks.17
Lisfranc Injuries
Lisfranc injuries include any bony or ligamentous damage that involves the tarsometatarsal (TMT) joints. While axial loading of a fixed, plantarflexed foot has traditionally been thought of as the most common mechanism of Lisfranc injury, we have found that noncontact twisting injuries leading to Lisfranc disruption are actually more common among NFL players. This mechanism is similar to noncontact turf toe and results in a purely ligamentous injury. We have found this to be particularly true in the case of defensive ends engaged with offensive linemen in which no axial loading or contact of the foot occurs. Clinically, patients often have painful weight-bearing, inability to perform a single limb heel rise, plantar ecchymosis, and swelling and point tenderness across the bases of the 1st and 2nd metatarsals.
It is critical to obtain comparison weight-bearing radiographs of both feet during initial work-up to look for evidence of instability. Subtle radiographic findings of Lisfranc injury include a bony “fleck” sign, compression fracture of the cuboid, and diastasis between the base of the 1st and 2nd metatarsals and/or medial and middle cuneiforms (Figures 3A, 3B).
The goal of surgical intervention is to obtain and maintain anatomic reduction of all unstable joints in order to restore a normal foot posture. One of the difficulties with Lisfranc injuries is that there are no exact diastasis parameters and individuals should be treated based on symptoms, functional needs, and degree of instability. It has been shown that 5 mm of displacement can have good long-term clinical results in select cases without surgery.18 For surgery, we recommend open reduction to remove interposed soft tissue debris and directly assess the articular surfaces (Figures 4A-4D).
Proximal-medial column Lisfranc injury variants are increasingly common among football players.20 In these injuries, the force of injury extends through the intercuneiform joint and exits out the naviculocuneiform joint, thus causing instability at multiple joints and an unstable 1st ray. Patients often have minimal clinical findings and normal radiographs and stress radiographs. MRI of the foot often reveals edema at the naviculocuneiform joint. Often patients fail to improve with nonoperative immobilization with continued inability to push off from the hallux. Unrecognized or untreated instability will lead to rapid deterioration of the naviculocuneiform joint. Surgical intervention requires a homerun screw and intercuneiform screw. We do not recommend primary arthrodesis in athletes due to significant risk of malunion and nonunion unless severe articular damage is present.
Patients are typically kept NWB in a splint for 2 weeks after surgery followed by NWB in a tall CAM from 3 to 4 weeks postoperative. Progressive weight-bearing and ROM exercises are initiated from 4 to 8 weeks, followed by return to accommodative shoe wear from 10 to 12 weeks. Hardware removal is performed 4 to 6 months after surgery, typically in the off-season to allow for 6 to 8 weeks or protected recovery afterwards. Premature hardware removal can lead to loss of reduction, particularly at the intercuneiform joints. All hardware crossing the TMT joints should be removed, while the homerun screw can be left in place in addition to the intercuneiform screw. RTP in football typically occurs 6 to 7 months after surgery. Final functional outcome is related to the adequacy of initial reduction and severity of the initial injury.21
Syndesmotic Disruption
Syndesmotic injuries comprise 1% to 18% of ankle sprains in the general population, but occur at much higher rates in football due to the increased rotation forces placed on the ankle during cutting and tackling. RTP after syndesmotic injury often takes twice as long when compared to isolated lateral ankle ligamentous injury.22 Missed injuries are common and if not treated properly can lead to chronic ankle instability and posttraumatic ankle arthritis.23 Syndesmotic injury can occur in isolation or with concomitant adjacent bony, cartilaginous, or ligamentous injuries. Therefore, clinical examination and imaging work-up are critical to successful management.
Syndesmotic injuries often result from an external rotation force applied to a hyperdorsiflexed ankle while the foot is planted. This mechanism causes the fibula to externally rotate while translating posteriorly and laterally, resulting in rupture of the anterior inferior tibiofibular ligament (AITFL) first, followed by the deep deltoid ligament, interosseous ligament (IOL), and lastly posterior talofibular ligament.24 Most syndesmotic injuries involve rupture of only the AITFL and IOL.25 Multiple clinical stress tests have been designed to assess syndesmotic stability, including the squeeze test, external rotation stress test, crossed-leg test, and fibula-translation test.26-29 However, no physical examination maneuver has been shown to reliably predict the presence or degree of syndesmotic injury and therefore imaging studies are necessary.30
Initial imaging should include standing radiographs of the affected ankle. An increase in the medial clear space between the medial malleolus and talus can occur with a combined syndesmotic and deltoid disruption. In the case of subtle syndesmotic injuries, contralateral comparison weight-bearing radiographs are recommended. CT and MRI can provide additional information, but these static imaging tests cannot identify instability. Fluoroscopic stress evaluation is beneficial but has a high false-negative rate in low-grade injuries and may not detect partial rupture of the AITFL and IOL.31 It has been shown that malrotation of as much as 30° of external rotation can occur if relying on intraoperative fluoroscopy alone.32 It has been our practice to recommend surgical reduction and stabilization for any syndesmotic injury with documented diastasis or instability seen on imaging and/or arthroscopy.
Nonoperative treatment is indicated for truly stable grade I syndesmotic injuries. This involves rest and immobilization followed by a progressive rehabilitation program consisting of stretching, strengthening, and proprioceptive exercises.33 After 1 week of protected weight-bearing in a cast or tall CAM boot, progression to full weight-bearing should occur over the following week. Active-assisted ankle ROM exercises and light proprioceptive training should then be initiated followed by sport-specific exercises 2 to 3 weeks after injury.
Arthroscopy can be a valuable diagnostic tool in the setting of subtle syndesmotic injury with negative radiographs, positive MRI for edema, and a protracted recovery course with vague pain (Figures W5A-W5E).
Implants are placed above the true syndesmotic joint (at least 15 mm above the tibial plafond) angled 30° posterior to anterior to follow the normal relationship of the fibula to the distal tibia in the incisura. Typically 2 suture-buttons are used, with the devices placed in a divergent fashion. We highly recommend the use of a fibular buttress plate with button placement in individuals returning to contact activity. This construct increases surface area distribution while preventing stress risers and the risk of fibula fractures. In a cadaver model with deliberate syndesmotic malreduction, suture-button stabilization resulted in decreased postoperative displacement as opposed to conventional screw fixation.34 Therefore, dynamic syndesmotic fixation may help to decrease the negative sequelae of iatrogenic clamp malreduction.
Postoperative rehabilitation involves NWB in a cast or tall CAM boot for 4 weeks followed by ankle ROM exercises and progressive weight-bearing and physical therapy. Patients are transitioned to a lace-up ankle brace and athletic shoe from 6 to 12 weeks postoperative with increasing activity. Running and jumping is permitted 4 months after surgery with RTP typically at 6 to 7 months. Athletes who have had surgical stabilization for documented instability without any diastasis may engage in a more rapid recovery and RTP as symptoms and function allow.
Deltoid Complex Avulsion
Missed or neglected deltoid ligament injuries can lead to progressive chondral injury and joint degeneration. These injuries are often subtle and difficult to diagnose. An inability to perform a single limb heel rise, persistent pain with activity, and lack of normal functional improvement despite appropriate care are indicators of subtle ligament instability. These injuries often require an examination under anesthesia with combined ankle arthroscopy. Valgus stress testing of the ankle while directly visualizing the deltoid ligament from the anterolateral portal can reveal medial laxity in addition to potential osteochondral lesions along the anterolateral talar dome.
In American football players, we have observed that infolding and retraction of an avulsed superficial deltoid ligament complex after an ankle fracture, Maisonneuve injury, or severe high ankle sprain can be a source of persistent increased medial clear space, malreduction, and postoperative pain and medial instability. We have found that there is often complete avulsion of the superficial deltoid complex off the proximal aspect of the medial malleolus during high-energy ankle fractures in football players that is amenable to direct repair to bone (Figures W6A-W6E).
During surgical repair, an incision is made along the anterior aspect of the medial malleolus and the superficial deltoid ligament complex can often be found flipped and interposed in the medial gutter. A rongeur is used to create a bleeding cancellous bone surface for soft-tissue healing and 1 to 2 suture anchors are used to repair and imbricate the deltoid ligament complex back to the medial malleolus. The goal of these sutures is to repair the tibionavicular and tibial spring ligaments back to the medial malleolus. We believe that superficial deltoid complex avulsion during high-energy ankle fractures is a distinct injury pattern that should be recognized and may benefit from primary open repair.
We currently open explore every deltoid ligament complex in athletes with unstable syndesmotic injuries, as we believe that deltoid avulsion injuries are underrecognized and do not heal in an anatomic fashion if left alone. Postoperative recovery follows the same immobilization, progressive weight-bearing, and physical therapy protocol as that for syndesmotic disruption.
Achilles Ruptures
Acute midsubstance Achilles tendon ruptures are an increasingly common injury in patients 30 to 50 years of age, with more than 50% of all injuries occurring during basketball.36,37 Among NFL players, we have found that Achilles ruptures tend to occur at a higher rate during training camp, when athletes are deconditioned and quickly returning to explosive push-off activities. Physical examination should include a Thompson test, palpation of a gap within the tendon, and evaluation of resting ankle dorsiflexion in the affected extremity in the prone position with the knees bent. Lateral radiographs should be analyzed for the presence of a bony avulsion fragment indicative of an insertional avulsion injury or midsubstance calcium deposition reflecting chronic Achilles tendinosis, as both of these conditions will change surgical management. MRI is not recommended with acute midsubstance ruptures but may be helpful in the case of chronic ruptures or more proximal tears of the musculotendinous junction.
The management of acute midsubstance Achilles tendon ruptures is controversial, with no general consensus in the literature regarding nonoperative treatment, surgical repair, and ideal repair technique.36,38-42 American Academy of Orthopaedic Surgeons clinical practice guidelines report moderate evidence that nonoperative treatment of Achilles tendon ruptures has lower wound healing complications but higher rates of re-rupture.38,39 Additionally, limited incision approaches have been found to have fewer overall complications compared with traditional open repair. In an effort to reduce the incidence of postoperative wound complications while improving functional recovery, modern repair techniques focus on a limited incision repair using percutaneous suture insertion and management (PARS Achilles Jig System, Arthrex).36 The limited incision technique utilizes a 2-cm transverse incision and non-disposable jig with divergent needle passes and locking suture fixation options to secure and fixate both tendon ends with minimal dissection of skin, subcutaneous tissue, and paratenon. Limited incision repair is ideally performed within 2 weeks of the injury to ensure that both tendon ends are easy to identify, mobilize, and repair. An open repair is generally recommended for midsubstance ruptures more than 4 weeks old and cases of insertional rupture and Achilles tendinopathy.
In a cohort of 9 NFL players treated for midsubstance Achilles ruptures using the PARS technique, we found no re-ruptures, no wound complications, and no sural nerve issues after surgery.43 A comparative review of 270 cases of operatively treated Achilles tendon ruptures (101 PARS, 169 traditional open repair) showed that the PARS group had significantly shorter operative times and a higher number of patients able to return to baseline physical activities by 5 months compared to open repair.36 Although not statistically significant, the overall PARS complication rate was 5% while the open complication rate was 11%. The PARS group had no cases of sural neuritis or deep infection requiring reoperation. We currently use a limited incision technique for all acute midsubstance Achilles ruptures in athletes regardless of sport, patient size, or position played.
During surgery, a 2-cm transverse incision is made over the gap in the Achilles tendon and dissection is carried down to the rupture site with minimal manipulation of the skin (Figures 5A-5F).
A key aspect of postoperative recovery is avoiding excessive ankle dorsiflexion while the tendon is healing during the first 4 weeks after surgery, as this can lead to an elongated tendon with loss of push-off strength. Patients are kept in a plantarflexion splint NWB for 2 weeks after surgery. If the incision is healed at 2 weeks, sutures are removed and patients are transitioned into a NWB tall CAM boot for 2 weeks with gentle ankle ROM exercises. If there is any concern regarding wound healing status, sutures are maintained for an additional 1 to 2 weeks.
From 4 to 8 weeks after surgery, progressive weight-bearing with continued ankle ROM exercises is initiated with peel-away heel lifts (~2 cm thick total, 3 layers). Each layer of the heel lift is gradually removed as pain allows every 2 to 3 days with the goal of being full weight-bearing with the foot flat at 6 weeks postoperative. Physical therapy focusing on ankle ROM and gentle Achilles stretching and strengthening is also started 6 weeks after surgery. From 8 to 12 weeks postoperative, patients are transitioned out of the tall CAM boot into normal, accommodative shoe wear with full weight-bearing. We avoid ankle dorsiflexion past neutral until 12 weeks after surgery, as overlengthening of the Achilles complex and the subsequent loss of push-off power can be devastating to running athletes. Activity levels are increased as tolerated, with no running or jumping from 12 to 16 weeks with full release to all activities after 16 weeks. RTP often takes 5 to 6 months after surgery, depending on the position played.
Am J Orthop. 2016;45(6):358-367. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Foot and ankle injuries are common in American football, with injury rates significantly increasing over the past decade.1-5 Epidemiologic studies of collegiate football players have shown an annual incidence of foot and ankle injuries ranging from 9% to 39%,3,6 with as many as 72% of all collegiate players presenting to the National Football League (NFL) Combine with a history of a foot or ankle injury and 13% undergoing surgical treatment.5 Player position influences the rate and type of foot and ankle injury. Offensive and “skill position” players, including linemen, running backs, and wide receivers, are particularly susceptible to foot and ankle injuries due to high levels of force and torque placed on the distal extremity during running, cutting, and tackling. Shoe wear changes, playing field conditions, increasing player size and speed, and improved reporting of injuries are also contributing to increasing injury rates.
The interaction between player cleats and the playing surface is a central issue of foot and ankle injuries in football. Improved traction relates to performance, but increased subsequent torque on the lower extremity is associated with injury. While lateral ankle sprains are the most common foot and ankle injury experienced by football players,7 numerous other injuries can occur, including turf toe, Jones fractures, Lisfranc injuries, syndesmotic disruption, deltoid complex avulsion, and Achilles ruptures. It is important for physicians to be able to recognize, diagnose, and appropriately treat these injuries in players in order to expedite recovery, restore function, and help prevent future injury and long-term sequelae. This review focuses on updated treatment principles, surgical advances, and rehabilitation protocols for common football foot and ankle injuries.
Turf Toe
The term “turf toe” was first used in 1976 to refer to hyperextension injuries and plantar capsule-ligament sprains of the hallux metatarsophalangeal (MTP) joint that can lead to progressive cock-up deformity.8 While these injuries can occur on any surface and disrupt soft tissue balance with functional implications, predisposing factors include increasing playing surface hardness and decreasing shoe stiffness. In a classic scenario, the foot is fixed in equinus as an axial load is placed on the back of the heel, resulting in forced dorsiflexion of the hallux MTP joint.9 As the proximal phalanx extends, the sesamoids are drawn distally and the more dorsal portion of the metatarsal head articular surface bears the majority of the load, causing partial or complete tearing of the plantar plate with or without hallux MTP dislocation. Osteochondral lesions of the MTP joint and subchondral edema of the metatarsal head can occur concurrently as the proximal phalanx impacts or shears across the metatarsal head articular surface.
Clinical examination should focus on hallux swelling, alignment, and flexor hallucis longus (FHL) function along with vertical instability of the hallux MTP joint using a Lachman test. Radiographs should be evaluated for proximal migration of the sesamoids or diastasis (Figures W1A-W1C).
Indications for surgical intervention include loss of push-off strength, gross MTP instability, proximal migration of the sesamoids, and progressive hallux malalignment or clawing after immobilization. Cases can involve one or a combination of the following: (1) large capsular avulsion with unstable MTP joint; (2) diastasis of bipartite sesamoid; (3) diastasis of sesamoid fracture; (4) retraction of sesamoid; (5) traumatic hallux valgus deformity; (6) vertical instability (positive Lachman test); (7) loose body in MTP joint; or (8) chondral injury in MTP joint. The goal of surgery is the restoration of anatomy in order to restore normal function of the hallux MTP joint.
We have found that using dual medial and plantar incisions places less traction on the plantar medial cutaneous nerve, improves lateral exposure, and provides better wound healing. The medial capsulotomy extends from the metatarsal neck to the mid-phalanx to provide complete visualization of the sesamoid complex (Figures 1A-1F).
It is important to recognize that not all turf toe injuries involve pure hyperextension on artificial playing surfaces. In recent years, we have found an increasing rate of medial variant turf toe injuries in which a forceful valgus stress on the hallux leads to rupture of the medial collateral ligament, medial or plantar-medial capsule, and/or abductor halluces. Medial variant turf toe can lead to progressive hallux valgus and a traumatic bunion with a significant loss of push-off strength and difficulty with cutting maneuvers. Surgical treatment requires a modified McBride bunionectomy with adductor tenotomy and direct repair of the medial soft tissue defect.
Postoperative management is just as important as proper surgical technique for these injuries and involves a delicate balance between protecting the repair and starting early range of motion (ROM). Patients are immobilized non-weight-bearing (NWB) for 5 to 7 days maximum followed immediately with the initiation of passive hallux plantarflexion to keep the sesamoids moving. Active hallux plantarflexion is started at 4 weeks after surgery with active dorsiflexion from 6 to 8 weeks. Patients are transitioned to an accommodative shoe with stiff hallux insert 8 weeks postoperative with continued therapy focusing on hallux ROM. Running is initiated at 12 weeks and return to play (RTP) is typically allowed 4 months after surgery.
Jones Fractures
Jones fractures are fractures of the 5th metatarsal at the metaphyseal-diaphyseal junction, where there is a watershed area of decreased vascularity between the intramedullary nutrient and metaphyseal arteries. Current thought is that the rising rate of Jones fractures among football players is partially caused by the use of flexible, narrow cleats that do not provide enough stiffness and lateral support for the 5th metatarsal during running and cutting. Additionally, lateral overload from a baseline cavovarus foot posture with or without metatarsus adductus and/or skewfoot is thought to contribute to Jones fractures.10 Preoperative radiographs should be evaluated for fracture location, orientation, amount of cortical thickening, and overall geometry of the foot and 5th metatarsal. In elite athletes, the threshold for surgical intervention is decreasing in order to expedite healing and recovery and decrease re-fracture risk. This rationale is based on delayed union rates of 25% to 66%, nonunion rates of 7% to 28%,11 and re-fracture rates of up to 33% associated with nonoperative treatment.12 Nonoperative management is usually not feasible in the competitive athlete, as it typically involves a period of protected weight-bearing in a tall controlled ankle motion (CAM) boot for 6 to 8 weeks with serial radiographs to evaluate healing.
Our preference for surgical intervention involves percutaneous screw fixation with a “high and inside” starting point on fluoroscopy (Figures 2A-2D).
In career athletes, we augment the fracture site using a mixture of bone marrow aspirate concentrate (BMA) (Magellan, Arteriocyte Medical Systems) and demineralized bone matrix (DBM) (Mini Ignite, Wright Medical Technology) injected percutaneously in and around the fracture site under fluoroscopic guidance. Using this technique in a cohort of 25 NFL players treated operatively for Jones fractures, we found that 100% of athletes were able to RTP in the NFL in an average of 9.5 weeks.14 Two patients (7.5%) suffered re-fractures requiring revision surgery with iliac crest bone graft and repeat screw placement with a RTP after 15 weeks. We did not find an association between RTP and re-fracture rate.
The appropriate rehabilitation protocol for Jones fractures after surgery remains controversial and dependent on individual needs and abilities.15,16 For athletes in-season, we recommend a brief period of NWB for 1 to 2 weeks followed by toe-touch weight-bearing in a tall CAM boot for 2 to 4 weeks. After 4 weeks, patients begin gentle exercises on a stationary bike and pool therapy to reduce impact on the fracture site. Low-intensity pulsed ultrasound bone stimulators (Exogen, Bioventus) are frequently used directly over fracture site throughout the postoperative protocol as an adjuvant therapy. If clinically nontender over the fracture site, patients are allowed to begin running in modified protective shoe wear 4 weeks after surgery with an average RTP of 6 to 8 weeks. RTP is determined clinically, as radiographic union may not be evident for 12 to 16 weeks. Useful custom orthoses include turf toe plates with a cushioned lateral column and lateral heel wedge if hindfoot varus is present preoperatively.10 The solid intramedullary screw is left in place permanently.
In our experience, we have found the average re-fracture and nonunion rate to be approximately 8% across all athletes. Our observation that union rates do not appear to be related to RTP times suggests that underlying biology such as Vitamin D deficiency may play a larger role in union rates than previously thought. We have found that most Jones re-fractures occur in the first year after the initial injury, but can occur up to 2.5 years afterwards as well.14 For the management of symptomatic re-fractures and nonunions, the previous screw must be first removed. This can be difficult if the screw is bent or broken, and may require a lateral corticotomy of the metatarsal.
After hardware removal, we advocate open bone grafting of the fracture site using bone from the iliac crest retrieved with a small, percutaneous trephine. Re-fixation should be achieved using a larger, solid screw and postoperative adjuvants may include bone stimulators, Vitamin D and calcium supplemention, and possible teriparatide use (Forteo, Eli Lilly), depending on individual patient profile. In a cohort of 21 elite athletes treated for Jones fracture revision surgery with screw exchange and bone grafting, we found that 100% of patients had computed tomography (CT) evidence of union, with an average RTP of 12.3 weeks.17
Lisfranc Injuries
Lisfranc injuries include any bony or ligamentous damage that involves the tarsometatarsal (TMT) joints. While axial loading of a fixed, plantarflexed foot has traditionally been thought of as the most common mechanism of Lisfranc injury, we have found that noncontact twisting injuries leading to Lisfranc disruption are actually more common among NFL players. This mechanism is similar to noncontact turf toe and results in a purely ligamentous injury. We have found this to be particularly true in the case of defensive ends engaged with offensive linemen in which no axial loading or contact of the foot occurs. Clinically, patients often have painful weight-bearing, inability to perform a single limb heel rise, plantar ecchymosis, and swelling and point tenderness across the bases of the 1st and 2nd metatarsals.
It is critical to obtain comparison weight-bearing radiographs of both feet during initial work-up to look for evidence of instability. Subtle radiographic findings of Lisfranc injury include a bony “fleck” sign, compression fracture of the cuboid, and diastasis between the base of the 1st and 2nd metatarsals and/or medial and middle cuneiforms (Figures 3A, 3B).
The goal of surgical intervention is to obtain and maintain anatomic reduction of all unstable joints in order to restore a normal foot posture. One of the difficulties with Lisfranc injuries is that there are no exact diastasis parameters and individuals should be treated based on symptoms, functional needs, and degree of instability. It has been shown that 5 mm of displacement can have good long-term clinical results in select cases without surgery.18 For surgery, we recommend open reduction to remove interposed soft tissue debris and directly assess the articular surfaces (Figures 4A-4D).
Proximal-medial column Lisfranc injury variants are increasingly common among football players.20 In these injuries, the force of injury extends through the intercuneiform joint and exits out the naviculocuneiform joint, thus causing instability at multiple joints and an unstable 1st ray. Patients often have minimal clinical findings and normal radiographs and stress radiographs. MRI of the foot often reveals edema at the naviculocuneiform joint. Often patients fail to improve with nonoperative immobilization with continued inability to push off from the hallux. Unrecognized or untreated instability will lead to rapid deterioration of the naviculocuneiform joint. Surgical intervention requires a homerun screw and intercuneiform screw. We do not recommend primary arthrodesis in athletes due to significant risk of malunion and nonunion unless severe articular damage is present.
Patients are typically kept NWB in a splint for 2 weeks after surgery followed by NWB in a tall CAM from 3 to 4 weeks postoperative. Progressive weight-bearing and ROM exercises are initiated from 4 to 8 weeks, followed by return to accommodative shoe wear from 10 to 12 weeks. Hardware removal is performed 4 to 6 months after surgery, typically in the off-season to allow for 6 to 8 weeks or protected recovery afterwards. Premature hardware removal can lead to loss of reduction, particularly at the intercuneiform joints. All hardware crossing the TMT joints should be removed, while the homerun screw can be left in place in addition to the intercuneiform screw. RTP in football typically occurs 6 to 7 months after surgery. Final functional outcome is related to the adequacy of initial reduction and severity of the initial injury.21
Syndesmotic Disruption
Syndesmotic injuries comprise 1% to 18% of ankle sprains in the general population, but occur at much higher rates in football due to the increased rotation forces placed on the ankle during cutting and tackling. RTP after syndesmotic injury often takes twice as long when compared to isolated lateral ankle ligamentous injury.22 Missed injuries are common and if not treated properly can lead to chronic ankle instability and posttraumatic ankle arthritis.23 Syndesmotic injury can occur in isolation or with concomitant adjacent bony, cartilaginous, or ligamentous injuries. Therefore, clinical examination and imaging work-up are critical to successful management.
Syndesmotic injuries often result from an external rotation force applied to a hyperdorsiflexed ankle while the foot is planted. This mechanism causes the fibula to externally rotate while translating posteriorly and laterally, resulting in rupture of the anterior inferior tibiofibular ligament (AITFL) first, followed by the deep deltoid ligament, interosseous ligament (IOL), and lastly posterior talofibular ligament.24 Most syndesmotic injuries involve rupture of only the AITFL and IOL.25 Multiple clinical stress tests have been designed to assess syndesmotic stability, including the squeeze test, external rotation stress test, crossed-leg test, and fibula-translation test.26-29 However, no physical examination maneuver has been shown to reliably predict the presence or degree of syndesmotic injury and therefore imaging studies are necessary.30
Initial imaging should include standing radiographs of the affected ankle. An increase in the medial clear space between the medial malleolus and talus can occur with a combined syndesmotic and deltoid disruption. In the case of subtle syndesmotic injuries, contralateral comparison weight-bearing radiographs are recommended. CT and MRI can provide additional information, but these static imaging tests cannot identify instability. Fluoroscopic stress evaluation is beneficial but has a high false-negative rate in low-grade injuries and may not detect partial rupture of the AITFL and IOL.31 It has been shown that malrotation of as much as 30° of external rotation can occur if relying on intraoperative fluoroscopy alone.32 It has been our practice to recommend surgical reduction and stabilization for any syndesmotic injury with documented diastasis or instability seen on imaging and/or arthroscopy.
Nonoperative treatment is indicated for truly stable grade I syndesmotic injuries. This involves rest and immobilization followed by a progressive rehabilitation program consisting of stretching, strengthening, and proprioceptive exercises.33 After 1 week of protected weight-bearing in a cast or tall CAM boot, progression to full weight-bearing should occur over the following week. Active-assisted ankle ROM exercises and light proprioceptive training should then be initiated followed by sport-specific exercises 2 to 3 weeks after injury.
Arthroscopy can be a valuable diagnostic tool in the setting of subtle syndesmotic injury with negative radiographs, positive MRI for edema, and a protracted recovery course with vague pain (Figures W5A-W5E).
Implants are placed above the true syndesmotic joint (at least 15 mm above the tibial plafond) angled 30° posterior to anterior to follow the normal relationship of the fibula to the distal tibia in the incisura. Typically 2 suture-buttons are used, with the devices placed in a divergent fashion. We highly recommend the use of a fibular buttress plate with button placement in individuals returning to contact activity. This construct increases surface area distribution while preventing stress risers and the risk of fibula fractures. In a cadaver model with deliberate syndesmotic malreduction, suture-button stabilization resulted in decreased postoperative displacement as opposed to conventional screw fixation.34 Therefore, dynamic syndesmotic fixation may help to decrease the negative sequelae of iatrogenic clamp malreduction.
Postoperative rehabilitation involves NWB in a cast or tall CAM boot for 4 weeks followed by ankle ROM exercises and progressive weight-bearing and physical therapy. Patients are transitioned to a lace-up ankle brace and athletic shoe from 6 to 12 weeks postoperative with increasing activity. Running and jumping is permitted 4 months after surgery with RTP typically at 6 to 7 months. Athletes who have had surgical stabilization for documented instability without any diastasis may engage in a more rapid recovery and RTP as symptoms and function allow.
Deltoid Complex Avulsion
Missed or neglected deltoid ligament injuries can lead to progressive chondral injury and joint degeneration. These injuries are often subtle and difficult to diagnose. An inability to perform a single limb heel rise, persistent pain with activity, and lack of normal functional improvement despite appropriate care are indicators of subtle ligament instability. These injuries often require an examination under anesthesia with combined ankle arthroscopy. Valgus stress testing of the ankle while directly visualizing the deltoid ligament from the anterolateral portal can reveal medial laxity in addition to potential osteochondral lesions along the anterolateral talar dome.
In American football players, we have observed that infolding and retraction of an avulsed superficial deltoid ligament complex after an ankle fracture, Maisonneuve injury, or severe high ankle sprain can be a source of persistent increased medial clear space, malreduction, and postoperative pain and medial instability. We have found that there is often complete avulsion of the superficial deltoid complex off the proximal aspect of the medial malleolus during high-energy ankle fractures in football players that is amenable to direct repair to bone (Figures W6A-W6E).
During surgical repair, an incision is made along the anterior aspect of the medial malleolus and the superficial deltoid ligament complex can often be found flipped and interposed in the medial gutter. A rongeur is used to create a bleeding cancellous bone surface for soft-tissue healing and 1 to 2 suture anchors are used to repair and imbricate the deltoid ligament complex back to the medial malleolus. The goal of these sutures is to repair the tibionavicular and tibial spring ligaments back to the medial malleolus. We believe that superficial deltoid complex avulsion during high-energy ankle fractures is a distinct injury pattern that should be recognized and may benefit from primary open repair.
We currently open explore every deltoid ligament complex in athletes with unstable syndesmotic injuries, as we believe that deltoid avulsion injuries are underrecognized and do not heal in an anatomic fashion if left alone. Postoperative recovery follows the same immobilization, progressive weight-bearing, and physical therapy protocol as that for syndesmotic disruption.
Achilles Ruptures
Acute midsubstance Achilles tendon ruptures are an increasingly common injury in patients 30 to 50 years of age, with more than 50% of all injuries occurring during basketball.36,37 Among NFL players, we have found that Achilles ruptures tend to occur at a higher rate during training camp, when athletes are deconditioned and quickly returning to explosive push-off activities. Physical examination should include a Thompson test, palpation of a gap within the tendon, and evaluation of resting ankle dorsiflexion in the affected extremity in the prone position with the knees bent. Lateral radiographs should be analyzed for the presence of a bony avulsion fragment indicative of an insertional avulsion injury or midsubstance calcium deposition reflecting chronic Achilles tendinosis, as both of these conditions will change surgical management. MRI is not recommended with acute midsubstance ruptures but may be helpful in the case of chronic ruptures or more proximal tears of the musculotendinous junction.
The management of acute midsubstance Achilles tendon ruptures is controversial, with no general consensus in the literature regarding nonoperative treatment, surgical repair, and ideal repair technique.36,38-42 American Academy of Orthopaedic Surgeons clinical practice guidelines report moderate evidence that nonoperative treatment of Achilles tendon ruptures has lower wound healing complications but higher rates of re-rupture.38,39 Additionally, limited incision approaches have been found to have fewer overall complications compared with traditional open repair. In an effort to reduce the incidence of postoperative wound complications while improving functional recovery, modern repair techniques focus on a limited incision repair using percutaneous suture insertion and management (PARS Achilles Jig System, Arthrex).36 The limited incision technique utilizes a 2-cm transverse incision and non-disposable jig with divergent needle passes and locking suture fixation options to secure and fixate both tendon ends with minimal dissection of skin, subcutaneous tissue, and paratenon. Limited incision repair is ideally performed within 2 weeks of the injury to ensure that both tendon ends are easy to identify, mobilize, and repair. An open repair is generally recommended for midsubstance ruptures more than 4 weeks old and cases of insertional rupture and Achilles tendinopathy.
In a cohort of 9 NFL players treated for midsubstance Achilles ruptures using the PARS technique, we found no re-ruptures, no wound complications, and no sural nerve issues after surgery.43 A comparative review of 270 cases of operatively treated Achilles tendon ruptures (101 PARS, 169 traditional open repair) showed that the PARS group had significantly shorter operative times and a higher number of patients able to return to baseline physical activities by 5 months compared to open repair.36 Although not statistically significant, the overall PARS complication rate was 5% while the open complication rate was 11%. The PARS group had no cases of sural neuritis or deep infection requiring reoperation. We currently use a limited incision technique for all acute midsubstance Achilles ruptures in athletes regardless of sport, patient size, or position played.
During surgery, a 2-cm transverse incision is made over the gap in the Achilles tendon and dissection is carried down to the rupture site with minimal manipulation of the skin (Figures 5A-5F).
A key aspect of postoperative recovery is avoiding excessive ankle dorsiflexion while the tendon is healing during the first 4 weeks after surgery, as this can lead to an elongated tendon with loss of push-off strength. Patients are kept in a plantarflexion splint NWB for 2 weeks after surgery. If the incision is healed at 2 weeks, sutures are removed and patients are transitioned into a NWB tall CAM boot for 2 weeks with gentle ankle ROM exercises. If there is any concern regarding wound healing status, sutures are maintained for an additional 1 to 2 weeks.
From 4 to 8 weeks after surgery, progressive weight-bearing with continued ankle ROM exercises is initiated with peel-away heel lifts (~2 cm thick total, 3 layers). Each layer of the heel lift is gradually removed as pain allows every 2 to 3 days with the goal of being full weight-bearing with the foot flat at 6 weeks postoperative. Physical therapy focusing on ankle ROM and gentle Achilles stretching and strengthening is also started 6 weeks after surgery. From 8 to 12 weeks postoperative, patients are transitioned out of the tall CAM boot into normal, accommodative shoe wear with full weight-bearing. We avoid ankle dorsiflexion past neutral until 12 weeks after surgery, as overlengthening of the Achilles complex and the subsequent loss of push-off power can be devastating to running athletes. Activity levels are increased as tolerated, with no running or jumping from 12 to 16 weeks with full release to all activities after 16 weeks. RTP often takes 5 to 6 months after surgery, depending on the position played.
Am J Orthop. 2016;45(6):358-367. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Canale ST, Cantler ED Jr, Sisk TD, Freeman BL 3rd. A chronicle of injuries of an American intercollegiate football team. Am J Sports Med. 1981;9(6):384-389.2. Robey JM, Blyth CS, Mueller FO. Athletic injuries. Application of epidemiologic methods. JAMA. 1971;217(2):184-189.
3. Saal JA. Common American football injuries. Sports Med. 1991;12(2):132-147.
4. Thompson N, Halpern B, Curl WW, et al. High school football injuries: evaluation. Am J Sports Med. 1987;15(2):117-124.
5. Kaplan LD, Jost PW, Honkamp N, Norwig J, West R, Bradley JP. Incidence and variance of foot and ankle injuries in elite college football players. Am J Orthop. 2011;40(1):40-44.
6. DeLee JC, Farney WC. Incidence of injury in Texas high school football. Am J Sports Med. 1992;20(5):575-580.
7. Brophy RH, Barnes R, Rodeo SA, Warren RF. Prevalence of musculoskeletal disorders at the NFL Combine--trends from 1987 to 2000. Med Sci Sports Exerc. 2007;39(1):22-27.
8. Bowers KD Jr, Martin RB. Turf-toe: a shoe-surface related football injury. Med Sci Sports. 1976;8(2):81-83.
9. McCormick JJ, Anderson RB. Turf toe: anatomy, diagnosis, and treatment. Sports Health. 2010;2(6):487-494.
10. Raikin SM, Slenker N, Ratigan B. The association of a varus hindfoot and fracture of the fifth metatarsal metaphyseal-diaphyseal junction: the Jones fracture. Am J Sports Med. 2008;36(7):1367-1372.
11. Title CI, Katchis SD. Traumatic foot and ankle injuries in the athlete. Orthop Clin North Am. 2002;33(3):587-598.
12. Quill GE Jr. Fractures of the proximal fifth metatarsal. Orthop Clin North Am. 1995;26(2):353-361.
13. Nunley JA, Glisson RR. A new option for intramedullary fixation of Jones fractures: the Charlotte Carolina Jones Fracture System. Foot Ankle Int. 2008;29(12):1216-1221.
14. Lareau CR, Hsu AR, Anderson RB. Return to play in National Football League players after operative Jones fracture treatment. Foot Ankle Int. 2016;37(1):8-16.
15. Larson CM, Almekinders LC, Taft TN, Garrett WE. Intramedullary screw fixation of Jones fractures. Analysis of failure. Am J Sports Med. 2002;30(1):55-60.
16. Portland G, Kelikian A, Kodros S. Acute surgical management of Jones’ fractures. Foot Ankle Int. 2003;24(11):829-833.
17. Hunt KJ, Anderson RB. Treatment of Jones fracture nonunions and refractures in the elite athlete: outcomes of intramedullary screw fixation with bone grafting. Am J Sports Med. 2011;39(9):1948-1954.
18. Nunley JA, Vertullo CJ. Classification, investigation, and management of midfoot sprains: Lisfranc injuries in the athlete. Am J Sports Med. 2002;30(6):871-878.
19. Alberta FG, Aronow MS, Barrero M, Diaz-Doran V, Sullivan RJ, Adams DJ. Ligamentous Lisfranc joint injuries: a biomechanical comparison of dorsal plate and transarticular screw fixation. Foot Ankle Int. 2005;26(6):462-473.
20. Ardoin GT, Anderson RB. Subtle Lisfranc injury. Tech Foot Ankle Surg. 2010;9(3):100-106.
21. Kuo RS, Tejwani NC, Digiovanni CW, et al. Outcome after open reduction and internal fixation of Lisfranc joint injuries. J Bone Joint Surg Am. 2000;82-A(11):1609-1618.
22. Wright RW, Barile RJ, Surprenant DA, Matava MJ. Ankle syndesmosis sprains in national hockey league players. Am J Sports Med. 2004;32(8):1941-1945.
23. Williams GN, Jones MH, Amendola A. Syndesmotic ankle sprains in athletes. Am J Sports Med. 2007;35(7):1197-1207.
24. Beumer A, Valstar ER, Garling EH, et al. Effects of ligament sectioning on the kinematics of the distal tibiofibular syndesmosis: a radiostereometric study of 10 cadaveric specimens based on presumed trauma mechanisms with suggestions for treatment. Acta Orthop. 2006;77(3):531-540.
25. McCollum GA, van den Bekerom MP, Kerkhoffs GM, Calder JD, van Dijk CN. Syndesmosis and deltoid ligament injuries in the athlete. Knee Surg Sports Traumatol Arthrosc. 2013;21(6):1328-1337.
26. Boytim MJ, Fischer DA, Neumann L. Syndesmotic ankle sprains. Am J Sports Med. 1991;19(3):294-298.
27. Nussbaum ED, Hosea TM, Sieler SD, Incremona BR, Kessler DE. Prospective evaluation of syndesmotic ankle sprains without diastasis. Am J Sports Med. 2001;29(1):31-35.
28. Kiter E, Bozkurt M. The crossed-leg test for examination of ankle syndesmosis injuries. Foot Ankle Int. 2005;26(2):187-188.
29. Beumer A, van Hemert WL, Swierstra BA, Jasper LE, Belkoff SM. A biomechanical evaluation of clinical stress tests for syndesmotic ankle instability. Foot Ankle Int. 2003;24(4):358-363.
30. Amendola A, Williams G, Foster D. Evidence-based approach to treatment of acute traumatic syndesmosis (high ankle) sprains. Sports Med Arthrosc. 2006;14(4):232-236.
31. Beumer A, Valstar ER, Garling EH, et al. External rotation stress imaging in syndesmotic injuries of the ankle: comparison of lateral radiography and radiostereometry in a cadaveric model. Acta Orthop Scand. 2003;74(2):201-205.
32. Marmor M, Hansen E, Han HK, Buckley J, Matityahu A. Limitations of standard fluoroscopy in detecting rotational malreduction of the syndesmosis in an ankle fracture model. Foot Ankle Int. 2011;32(6):616-622.
33. Williams GN, Allen EJ. Rehabilitation of syndesmotic (high) ankle sprains. Sports Health. 2010;2(6):460-470.
34. Westermann RW, Rungprai C, Goetz JE, Femino J, Amendola A, Phisitkul P. The effect of suture-button fixation on simulated syndesmotic malreduction: a cadaveric study. J Bone Joint Surg Am. 2014;96(20):1732-1738.
35. Hsu AR, Lareau CR, Anderson RB. Repair of acute superficial deltoid complex avulsion during ankle fracture fixation in National Football League players. Foot Ankle Int. 2015;36(11):1272-1278.
36. Hsu AR, Jones CP, Cohen BE, Davis WH, Ellington JK, Anderson RB. Clinical outcomes and complications of percutaneous Achilles repair system versus open technique for acute achilles tendon ruptures. Foot Ankle Int. 2015;36(11):1279-1286.
37. Raikin SM, Garras DN, Krapchev PV. Achilles tendon injuries in a United States population. Foot Ankle Int. 2013;34(4):475-480.
38. Chiodo CP, Glazebrook M, Bluman EM, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on treatment of achilles tendon rupture. J Bone Joint Surg Am. 2010;92(14):2466-2468.
39. Chiodo CP, Glazebrook M, Bluman EM, et al. Diagnosis and treatment of acute achilles tendon rupture. J Am Acad Orthop Surg. 2010;18(8):503-510.
40. Khan RJ, Fick D, Keogh A, Crawford J, Brammar T, Parker M. Treatment of acute achilles tendon ruptures. A meta-analysis of randomized, controlled trials. J Bone Joint Surg Am. 2005;87(10):2202-2210.
41. Renninger CH, Kuhn K, Fellars T, Youngblood S, Bellamy J. Operative and nonoperative management of achilles tendon ruptures in active duty military population. Foot Ankle Int. 2016;37(3):269-273.
42. Khan RJ, Carey Smith RL. Surgical interventions for treating acute achilles tendon ruptures. Cochrane Database Syst Rev. 2010;(9):CD003674.
43. McCullough KA, Shaw CM, Anderson RB. Mini-open repair of achilles rupture in the national football league. J Surg Orthop Adv. 2014;23(4):179-183.
1. Canale ST, Cantler ED Jr, Sisk TD, Freeman BL 3rd. A chronicle of injuries of an American intercollegiate football team. Am J Sports Med. 1981;9(6):384-389.2. Robey JM, Blyth CS, Mueller FO. Athletic injuries. Application of epidemiologic methods. JAMA. 1971;217(2):184-189.
3. Saal JA. Common American football injuries. Sports Med. 1991;12(2):132-147.
4. Thompson N, Halpern B, Curl WW, et al. High school football injuries: evaluation. Am J Sports Med. 1987;15(2):117-124.
5. Kaplan LD, Jost PW, Honkamp N, Norwig J, West R, Bradley JP. Incidence and variance of foot and ankle injuries in elite college football players. Am J Orthop. 2011;40(1):40-44.
6. DeLee JC, Farney WC. Incidence of injury in Texas high school football. Am J Sports Med. 1992;20(5):575-580.
7. Brophy RH, Barnes R, Rodeo SA, Warren RF. Prevalence of musculoskeletal disorders at the NFL Combine--trends from 1987 to 2000. Med Sci Sports Exerc. 2007;39(1):22-27.
8. Bowers KD Jr, Martin RB. Turf-toe: a shoe-surface related football injury. Med Sci Sports. 1976;8(2):81-83.
9. McCormick JJ, Anderson RB. Turf toe: anatomy, diagnosis, and treatment. Sports Health. 2010;2(6):487-494.
10. Raikin SM, Slenker N, Ratigan B. The association of a varus hindfoot and fracture of the fifth metatarsal metaphyseal-diaphyseal junction: the Jones fracture. Am J Sports Med. 2008;36(7):1367-1372.
11. Title CI, Katchis SD. Traumatic foot and ankle injuries in the athlete. Orthop Clin North Am. 2002;33(3):587-598.
12. Quill GE Jr. Fractures of the proximal fifth metatarsal. Orthop Clin North Am. 1995;26(2):353-361.
13. Nunley JA, Glisson RR. A new option for intramedullary fixation of Jones fractures: the Charlotte Carolina Jones Fracture System. Foot Ankle Int. 2008;29(12):1216-1221.
14. Lareau CR, Hsu AR, Anderson RB. Return to play in National Football League players after operative Jones fracture treatment. Foot Ankle Int. 2016;37(1):8-16.
15. Larson CM, Almekinders LC, Taft TN, Garrett WE. Intramedullary screw fixation of Jones fractures. Analysis of failure. Am J Sports Med. 2002;30(1):55-60.
16. Portland G, Kelikian A, Kodros S. Acute surgical management of Jones’ fractures. Foot Ankle Int. 2003;24(11):829-833.
17. Hunt KJ, Anderson RB. Treatment of Jones fracture nonunions and refractures in the elite athlete: outcomes of intramedullary screw fixation with bone grafting. Am J Sports Med. 2011;39(9):1948-1954.
18. Nunley JA, Vertullo CJ. Classification, investigation, and management of midfoot sprains: Lisfranc injuries in the athlete. Am J Sports Med. 2002;30(6):871-878.
19. Alberta FG, Aronow MS, Barrero M, Diaz-Doran V, Sullivan RJ, Adams DJ. Ligamentous Lisfranc joint injuries: a biomechanical comparison of dorsal plate and transarticular screw fixation. Foot Ankle Int. 2005;26(6):462-473.
20. Ardoin GT, Anderson RB. Subtle Lisfranc injury. Tech Foot Ankle Surg. 2010;9(3):100-106.
21. Kuo RS, Tejwani NC, Digiovanni CW, et al. Outcome after open reduction and internal fixation of Lisfranc joint injuries. J Bone Joint Surg Am. 2000;82-A(11):1609-1618.
22. Wright RW, Barile RJ, Surprenant DA, Matava MJ. Ankle syndesmosis sprains in national hockey league players. Am J Sports Med. 2004;32(8):1941-1945.
23. Williams GN, Jones MH, Amendola A. Syndesmotic ankle sprains in athletes. Am J Sports Med. 2007;35(7):1197-1207.
24. Beumer A, Valstar ER, Garling EH, et al. Effects of ligament sectioning on the kinematics of the distal tibiofibular syndesmosis: a radiostereometric study of 10 cadaveric specimens based on presumed trauma mechanisms with suggestions for treatment. Acta Orthop. 2006;77(3):531-540.
25. McCollum GA, van den Bekerom MP, Kerkhoffs GM, Calder JD, van Dijk CN. Syndesmosis and deltoid ligament injuries in the athlete. Knee Surg Sports Traumatol Arthrosc. 2013;21(6):1328-1337.
26. Boytim MJ, Fischer DA, Neumann L. Syndesmotic ankle sprains. Am J Sports Med. 1991;19(3):294-298.
27. Nussbaum ED, Hosea TM, Sieler SD, Incremona BR, Kessler DE. Prospective evaluation of syndesmotic ankle sprains without diastasis. Am J Sports Med. 2001;29(1):31-35.
28. Kiter E, Bozkurt M. The crossed-leg test for examination of ankle syndesmosis injuries. Foot Ankle Int. 2005;26(2):187-188.
29. Beumer A, van Hemert WL, Swierstra BA, Jasper LE, Belkoff SM. A biomechanical evaluation of clinical stress tests for syndesmotic ankle instability. Foot Ankle Int. 2003;24(4):358-363.
30. Amendola A, Williams G, Foster D. Evidence-based approach to treatment of acute traumatic syndesmosis (high ankle) sprains. Sports Med Arthrosc. 2006;14(4):232-236.
31. Beumer A, Valstar ER, Garling EH, et al. External rotation stress imaging in syndesmotic injuries of the ankle: comparison of lateral radiography and radiostereometry in a cadaveric model. Acta Orthop Scand. 2003;74(2):201-205.
32. Marmor M, Hansen E, Han HK, Buckley J, Matityahu A. Limitations of standard fluoroscopy in detecting rotational malreduction of the syndesmosis in an ankle fracture model. Foot Ankle Int. 2011;32(6):616-622.
33. Williams GN, Allen EJ. Rehabilitation of syndesmotic (high) ankle sprains. Sports Health. 2010;2(6):460-470.
34. Westermann RW, Rungprai C, Goetz JE, Femino J, Amendola A, Phisitkul P. The effect of suture-button fixation on simulated syndesmotic malreduction: a cadaveric study. J Bone Joint Surg Am. 2014;96(20):1732-1738.
35. Hsu AR, Lareau CR, Anderson RB. Repair of acute superficial deltoid complex avulsion during ankle fracture fixation in National Football League players. Foot Ankle Int. 2015;36(11):1272-1278.
36. Hsu AR, Jones CP, Cohen BE, Davis WH, Ellington JK, Anderson RB. Clinical outcomes and complications of percutaneous Achilles repair system versus open technique for acute achilles tendon ruptures. Foot Ankle Int. 2015;36(11):1279-1286.
37. Raikin SM, Garras DN, Krapchev PV. Achilles tendon injuries in a United States population. Foot Ankle Int. 2013;34(4):475-480.
38. Chiodo CP, Glazebrook M, Bluman EM, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on treatment of achilles tendon rupture. J Bone Joint Surg Am. 2010;92(14):2466-2468.
39. Chiodo CP, Glazebrook M, Bluman EM, et al. Diagnosis and treatment of acute achilles tendon rupture. J Am Acad Orthop Surg. 2010;18(8):503-510.
40. Khan RJ, Fick D, Keogh A, Crawford J, Brammar T, Parker M. Treatment of acute achilles tendon ruptures. A meta-analysis of randomized, controlled trials. J Bone Joint Surg Am. 2005;87(10):2202-2210.
41. Renninger CH, Kuhn K, Fellars T, Youngblood S, Bellamy J. Operative and nonoperative management of achilles tendon ruptures in active duty military population. Foot Ankle Int. 2016;37(3):269-273.
42. Khan RJ, Carey Smith RL. Surgical interventions for treating acute achilles tendon ruptures. Cochrane Database Syst Rev. 2010;(9):CD003674.
43. McCullough KA, Shaw CM, Anderson RB. Mini-open repair of achilles rupture in the national football league. J Surg Orthop Adv. 2014;23(4):179-183.
Concussions in American Football
Football is an important component of American culture, with approximately 3 million youth athletes, 1.1 million high school athletes, and 100,000 college athletes participating each year.1 Participation in football provides athletes with physical, social, psychological, and academic benefits. Despite these benefits, widespread focus has been placed on the safety of football due to the risk for sport-related concussion (SRC) and potentially long-term effects; however, little recognition has been given to the advancements in concussion management across time and occurrence of concussions during most life activities. Although it is reasonable for concerns to be presented, it is important to better understand SRC and the current factors leading to prolonged recoveries, increased risk for injury, and potentially long-term effects.
What Is a Concussion?
Concussions occur after sustaining direct or indirect injury to the head or other parts of the body, as long as the injury force is transmitted to the head. Athletes often experience physical, cognitive, emotional, and sleep-related symptoms post-concussion secondary to an “energy crisis” within the brain.2 The energy crisis occurs as the result of transient neurological dysfunction triggered by changes in the brain (eg, release of neurotransmitters, impaired axonal function).2,3 Concussion is undetectable with traditional imaging; however, advanced imaging techniques (eg, diffuse tensor imaging) have shown progress in assessing axonal injury.3 Symptom duration post-concussion is highly variable due to individual differences; a recent study showed recovery took 3 to 4 weeks for memory and symptoms.4,5
Previous Concussion Management
Identification techniques and return-to-play guidelines for concussion have significantly changed across time. In the past, concussion grading scales were utilized for diagnosis and return to play was possible within the same contest.6,7 It has since been recognized that initial concussion severity makes it difficult to predict recovery.3 For example, research revealed memory decline and increased symptoms 36 hours post-injury for athletes with a grade 1 concussion (ie, transient confusion, no loss of consciousness, concussion symptoms or mental status changes that resolve within 15 minutes of injury) compared to baseline.7 Another study found duration of mental status changes to be related to slower symptom resolution and memory impairment 36 hours to 7 days post-injury.6 Consequently, return to play within the same contest was likely too liberal. Guidelines today recommend immediate removal from play with suspected SRC. Nevertheless, the “play through pain” culture has led athletes to continue playing after SRC, contributing to prolonged recoveries and potentially long-term effects.
Current Concussion Management: Continued Concerns and Areas of Improvement
Despite increased awareness of concussions, recent estimates revealed high rates (ie, 27:1 ratio for general players) of underreporting in college football, particularly amongst offensive linemen.8 Researchers have studied recovery implications for remaining in play, with one study revealing a 2.2 times greater risk for prolonged recovery in college athletes with delayed vs immediate removal.9 Another similar study discovered an 8.8 times greater risk for prolonged recovery in adolescent and young adult athletes not removed vs removed from play.10 Further analysis found remaining in play to be the greatest risk factor for prolonged recovery compared to other previously studied risk factors (eg, age, sex, posttraumatic migraine).10 Additionally, significant differences in neurocognitive data were seen between the “removed” and “not removed” groups for verbal memory, visual memory, processing speed, and reaction time at 1 to 7 days and 8 to 30 days.10 The recovery implications of remaining in play and the additional risk for second impact syndrome (SIS), or repeat concussion when recovering from another injury, emphasizes the need for further education efforts amongst athletes to encourage immediate reporting of injury.11
Sideline Assessment
Sideline assessment has become a vital component of concussion management to rule out concussion and/or significant injury other than concussion. Assessment should include observation, cognitive/balance testing, neurologic examination, and possible exertion testing to ensure a comprehensive evaluation of all areas of potential dysfunction.12 Indications for emergency department evaluation include suspicion for cervical spine injury, intracranial hemorrhage, or skull fracture as well as prolonged loss of consciousness, high-risk mechanisms, posttraumatic seizure(s), and/or significant worsening of symptoms.12
Observation
On the sideline, it is important to identify any immediate signs of injury (ie, loss of consciousness, anterograde/retrograde amnesia, and disorientation/confusion). Since immediate signs are not always present, it is important to be aware of the most commonly reported symptoms, including headache, difficulty concentrating, fatigue, drowsiness, and dizziness.13 If symptoms are not reported by the athlete, balance problems, lack of coordination, increased emotionality, and difficulty following instructions may be observed during play.12
On-Field Assessment
Cognitive and balance testing are essential in determining if an athlete has sustained a concussion. Immediate declines in memory, concentration abilities, and balance abilities are common. Given limitations in administering long testing batteries on the sideline, brief standardized tests such as the Standardized Assessment of Concussion (SAC), Balance Error Scoring System (BESS), and Sport Concussion Assessment Tool (SCAT) are commonly utilized. Identification of cognitive and/or balance abnormalities can help the athlete recognize deficits following injury.12 Balance problems are experienced due to abnormalities in sensory organization and generally resolve during the acute recovery period.14,15 Cognitive difficulties typically persist longer than balance problems, though duration varies widely.
Neurologic Evaluation
A neurologic evaluation including cranial nerve testing and evaluation of motor-sensory function (ie, assessment for the strength and sensation of upper and lower extremities) is important to identify focal deficits (ie, sensation changes, loss of fine motor control) indicative of serious intracranial pathology.12 Additionally, clinicians have suggested inclusion of vestibular and oculomotor assessments due to frequent dysfunction post-concussion.12,15,16 Examination of the vestibular/oculomotor systems through tools such as the Vestibular/Ocular Motor Screening (VOMS) assessment (assesses both the vestibular and oculomotor systems) and King-Devick Test (primarily assesses saccadic eye movements) can elicit symptoms that may not present immediately. If assessment appears normal, exertion testing can be utilized to determine if symptoms are provoked through physical exercise that should include cardio, dynamic, and sport-specific activities to stress the vestibular system.12
Risk Factors for Injury and Prolonged Recovery
Medical professionals must consider the presence of risk factors when managing concussion in order to make appropriate treatment recommendations and return-to-play decisions. Research has demonstrated the role of female gender, learning disability, attention-deficit/hyperactivity disorder, psychiatric history, young age, motion sickness, sleep problems, somatization, concussion history, on-field dizziness, posttraumatic migraine, and fogginess in increased risk for injury and/or prolonged recovery.17-25 Additionally, athletes with ongoing symptoms from a previous injury are at risk for sustaining another injury.
Acute Home Concussion Management
Although strict rest has been recommended post-concussion, recent research evaluating strict rest vs usual care for adolescents revealed greater symptom reports and longer recovery periods for the strict rest group.26 Based on these findings and emphasis for regulation within the migraine literature (due to the common pathophysiology between migraine and concussion27), we recommend that athletes follow a regulated daily schedule post-concussion including: 1) regular sleep-wake schedule with avoidance of naps, 2) regular meals, 3) adequate fluid hydration, 4) light noncontact physical activity (ie, walking, with progressions recommended by a physician), and 5) stress management techniques. Use of these strategies immediately can help in preventing against increased symptoms and stress, and decreases the need for medication in select cases. Additionally, over-the-counter medications should be limited to 2 to 3 doses per week to avoid rebound headaches.28
In-Office Concussion Management
Athletes diagnosed with SRC will experience different symptoms based on the injury mechanism, risk factors, and management approach. Comprehensive evaluation should include assessment of risk factors, injury details, symptoms, neurocognitive functioning, vestibular/oculomotor dysfunction, tolerance of physical exertion, balance functioning, and cervical spine integrity (if necessary).29,30 Due to individual differences and the heterogeneous symptom profiles, concussion management must move beyond a “one size fits all” approach to avoid nonspecific treatment strategies and consequently prolonged recoveries.29 Clinicians and researchers at University of Pittsburgh Medical Center have identified 6 concussion clinical profiles (ie, vestibular, ocular, posttraumatic migraine, cervical, anxiety/mood, and cognitive/fatigue) that are generally identifiable 48 hours after injury.29,30 Identification of the clinical profile(s) through a comprehensive evaluation guides the development of individualized treatment plans and targeted rehabilitation strategies.29,30
Vestibular. The vestibular system is responsible for stabilizing vision while the head moves and balance control.15 Athletes can experience central and/or peripheral vestibular dysfunction to include benign paroxysmal positional vertigo (BPPV), visual motion sensitivity, vestibular ocular reflex impairment, and balance impairment.30,31 Symptoms typically include dizziness, impaired balance, blurry vision, difficulty focusing, and environmental sensitivity.15,29,30 Potential treatment options include vestibular rehabilitation, exertion therapy, and school/work accommodations.
Ocular. The oculomotor system is responsible for control of eye movements. Athletes can experience many different posttraumatic vision changes, including convergence problems, eye-tracking difficulties, refractive error, difficulty with pursuits/saccades, and accommodation insufficiency. Symptoms typically include light sensitivity, blurred vision, double vision, headaches, fatigue, and memory difficulties.15,29,30 Potential treatment options include vision therapy, vestibular rehabilitation, and school/work accommodations.32
Posttraumatic Migraine. Headache, the most common post-concussion symptom, can persist and meet criteria for posttraumatic migraine (ie, unilateral headache with accompanying nausea and/or photophobia and phonophobia).29,30,33 Implementation of a routine schedule, daily physical activity, exertion therapy, pharmacologic intervention, and school/work accommodations are potential treatment options.
Cervical. The cervical spine can be injured during whiplash-type injuries. Therefore, determining the location, onset, and typical exacerbations of pain can be helpful in identifying cervical involvement.29,30 Symptoms typically include headaches, neck pain, numbness, and tingling. Evaluation and therapy by a certified physical therapist and pharmacologic intervention (eg, muscle relaxants) are potential treatment options. 29,30
Anxiety/Mood. Anxiety, or worry and fear about everyday situations, is common post-concussion and can sometimes be related to ongoing vestibular impairment. Symptoms typically include ruminative thoughts, avoidance of specific situations, hypervigilance, feelings of being overwhelmed, and difficulty falling asleep.29,30 Potential treatment options include implementation of a routine schedule, exposure to provocative situations, psychotherapy, pharmacologic intervention, and school/work accommodations.34
Cognitive/Fatigue. A global concussion factor (including cognitive, fatigue, and migraine symptoms) has been identified within 1 to 7 days of injury. Although this factor of symptoms generally resolves during the acute recovery period, it persists in select cases.13 Symptoms typically include fatigue, decreased energy levels, nonspecific headaches, potential sleep disruption, increased symptoms towards the end of the day, difficulty concentrating, and increased headache with cognitive activities.29,30,35 Routine schedule, daily physical activity, exertion therapy, pharmacologic intervention (eg, amantadine), and school/work accommodations are potential treatment options.30
Conclusion
Advancements in SRC management warrant change in the conversations regarding concussion in football. Specifically, conversations should address the current understanding of concussion and improvements in the safety of football through stricter concussion guidelines, detailed sideline evaluations, recognition of risk factors, improved acute management, and identification of concussion profiles that help to direct individualized treatment plans and targeted rehabilitation strategies. The biggest concerns related to concussions in football include underreporting of injury, premature return to play, and receiving routine rather than individualized treatment. Therefore, to further improve the safety of football and management of concussion it is essential that future efforts focus on the following 6 areas:
Education: Improved understanding of concussion is imperative to reducing poor outcomes and widespread concerns.
Immediate reporting: Reporting of concussion must be expected and encouraged through consistent responses by coaches to reduce underreporting and fear of reporting in athletes.
Prevention techniques: Athletes must be taught proper form and playing techniques to reduce the risk for concussion. Proper form and technique should be incentivized.
Targeted treatment: Individualized treatment plans and targeted rehabilitation strategies must be developed based on the identified clinical profile(s) to avoid nonspecific treatment recommendations.
Multidisciplinary treatment teams: Given the heterogeneous symptoms profiles and need for care provided by different medical specialties, multidisciplinary teams are essential.
Remain current: With the progress in understanding concussion, providers must remain vigilant of future advances in concussion management to further improve the safety of football.
Am J Orthop. 2016;45(6):352-356. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Dompier TP, Kerr ZY, Marshall SW, et al. Incidence of concussion during practice and games in youth, high school, and collegiate American football players. JAMA Pediatrics. 2015;169(7):659-665.
2. Giza C, Hovda D. The new neurometabolic cascade of concussion
3. Barkhoudarian G, Hovda DA, Giza CC. The molecular pathophysiology of concussive brain injury - an update. Phys Med Rehabil Clin N Am. 2016;27:373-393.
4. Henry L, Elbin R, Collins M, Marchetti G, Kontos A. Examining recovery trajectories after sport-related concussion with a multimodal clinical assessment approach. Neurosurgery. 2016;78(2):232-241.
5. McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Brit J Sports Med. 2013;47(5):250-258.
6. Lovell MR, Collins MW, Iverson GL, et al. Recovery from mild concussion in high school athletes. J Neurosurg. 2003;98(2):296-301.
7. Lovell MR, Collins MW, Iverson GL, Johnston KM, Bradley JP. Grade 1 or “ding” concussions in high school athletes. Am J Sports Med. 2004;32(1):47-54.
8. Baugh CM, Kroshus E, Daneshvar DH, Filali NA, Hiscox MJ, Glantz LH. Concussion management in United States college sports: compliance with National Collegiate Athletic Association concussion policy and areas for improvement. Am J Sports Med. 2015;43(1):47-56.
9. Asken BM, McCrea MA, Clugston JR, Snyder AR, Houck ZM, Bauer RM. “Playing through it”: Delayed reporting and removal from athletic activity after concussion predicts prolonged recovery. J Athl Train. 2016;51(4):329-335.
10. Elbin RJ, Sufrinko A, Schatz P, et al. Athletes that continue to play with concussion demonstrate worse recovery outcomes than athletes immediately removed from play. J Pediatr. In press.
11. Signoretti S, Lazzarino G, Tavazzi B, Vagnozzi R. The pathophysiology of concussion. PM R. 2011;3(10 Suppl 2):S359-S368.
12. Bloom J, Blount JG. Sideline evaluation of concussion. UpToDate. 2016. http://www.uptodate.com/contents/sideline-evaluation-of-concussion. Accessed July 13, 2016.
13. Kontos AP, Elbin RJ, Schatz P, et al. A revised factor structure for the post-concussion symptom scale: baseline and postconcussion factors. Am J Sports Med. 2012;40(10):2375-2384.
14. Guskiewicz KM, Ross SE, Marshall SW. Postural stability and neuropsychological deficits after concussion in collegiate athletes. J Athl Train. 2001;36(3):263.
15. Mucha A, Collins MW, Elbin R, et al. A brief Vestibular/Ocular Motor Screening (VOMS) assessment to evaluate concussions preliminary findings. Am J Sports Med. 2014;42(10):2479-2486.
16. Bloom J. Vestibular and ocular motor assessments: Important pieces to the concussion puzzle. Athletic Training and Sports Health Care. 2013;5(6):246-248.
17. Covassin T, Elbin R, Harris W, Parker T, Kontos A. The role of age and sex in symptoms, neurocognitive performance, and postural stability in athletes after concussion. Am J Sports Med. 2012;40(6):1303-1312.
18. Kontos A, Sufrinko A, Elbin R, Puskar A, Collins M. Reliability and associated risk factors for performance on the Vestibular/Ocular Motor Screening (VOMS) tool in healthy collegiate athletes. Am J Sports Med. 2016;44(6):1400-1406.
19. Guskiewicz KM, McCrea M, Marshall SW, et al. Cumulative effects associated with recurrent concussion in collegiate football players: the NCAA Concussion Study. JAMA. 2003;290(19):2549-2555.
20. Lau B, Lovell MR, Collins MW, Pardini J. Neurocognitive and symptom predictors of recovery in high school athletes. Clin J Sport Med. 2009;19(3):216-221.
21. Lau BC, Kontos AP, Collins MW, Mucha A, Lovell MR. Which on-field signs/symptoms predict protracted recovery from sport-related concussion among high school football players? Am J Sports Med. 2011;39(11):2311-2318.
22. Mihalik JP, Register-Mihalik J, Kerr ZY, Marshall SW, McCrea MC, Guskiewicz KM. Recovery of posttraumatic migraine characteristics in patients after mild traumatic brain injury. Am J Sports Med. 2013;41(7):1490-1496.
23. Covassin T, Moran R, Elbin RJ. Sex differences in reported concussion injury rates and time loss from participation: An update of the National Collegiate Athletic Association injury surveillance program from 2004-2005 through 2008-2009. J Athl Train. 2016;51(3):189-194.
24. Root JM, Zuckerbraun NS, Wang L, et al. History of somatization is associated with prolonged recovery from concussion. J Pediatr. 2016;174:39-44.
25. Sufrinko A, Pearce K, Elbin RJ, et al. The effect of preinjury sleep difficulties on neurocognitive impairment and symptoms after sport-related concussion. Am J Sports Med. 2015;43(4):830-838.
26. Thomas DG, Apps JN, Hoffmann RG, McCrea M, Hammeke T. Benefits of strict rest after acute concussion: a randomized controlled trial. Pediatrics. 2015;135(2):213-223.
27. Choe M, Blume H. Pediatric posttraumatic Headache: a review. J Child Neurol. 2016;31(1):76-85.
28. Tepper SJ, Tepper DE. Breaking the cycle of medication overuse. Cleve Clin J Med. 2010;77(4):236-242.
29. Collins M, Kontos A, Reynolds E, Murawski C, Fu F. A comprehensive, targeted approach to the clinical care of athletes following sport-related concussion. Knee Surg Sports Traumatol Arthrosc. 2014;22(2):235-246.
30. Reynolds E, Collins MW, Mucha A, Troutman-Ensecki C. Establishing a clinical service for the management of sports-related concussions. Neurosurgery. 2014;75 Suppl 4:S71-S81.
31. Broglio SP, Collins MW, Williams RM, Mucha A, Kontos AP. Current and emerging rehabilitation for concussion: a review of the evidence. Clin Sports Med. 2015;34(2):213-231.
32. Master C, Scheiman M, Gallaway M, et al. Vision diagnoses are common after concussion in adolescents. Clin Pediatr (Phila). 2016;55(3):260-267.
33. Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition (beta version). Cephalalgia. 2013;33(9):629-808.
34. Kontos A, Deitrick JM, Reynolds E. Mental health implication and consequences following sport-related concussion. Brit J Sports Med. 2016;50(3):139-140.
35. Kontos AP, Covassin T, Elbin R, Parker T. Depression and neurocognitive performance after concussion among male and female high school and collegiate athletes. Arch Phys Med Rehabil. 2012;93(10):1751-1756.
Football is an important component of American culture, with approximately 3 million youth athletes, 1.1 million high school athletes, and 100,000 college athletes participating each year.1 Participation in football provides athletes with physical, social, psychological, and academic benefits. Despite these benefits, widespread focus has been placed on the safety of football due to the risk for sport-related concussion (SRC) and potentially long-term effects; however, little recognition has been given to the advancements in concussion management across time and occurrence of concussions during most life activities. Although it is reasonable for concerns to be presented, it is important to better understand SRC and the current factors leading to prolonged recoveries, increased risk for injury, and potentially long-term effects.
What Is a Concussion?
Concussions occur after sustaining direct or indirect injury to the head or other parts of the body, as long as the injury force is transmitted to the head. Athletes often experience physical, cognitive, emotional, and sleep-related symptoms post-concussion secondary to an “energy crisis” within the brain.2 The energy crisis occurs as the result of transient neurological dysfunction triggered by changes in the brain (eg, release of neurotransmitters, impaired axonal function).2,3 Concussion is undetectable with traditional imaging; however, advanced imaging techniques (eg, diffuse tensor imaging) have shown progress in assessing axonal injury.3 Symptom duration post-concussion is highly variable due to individual differences; a recent study showed recovery took 3 to 4 weeks for memory and symptoms.4,5
Previous Concussion Management
Identification techniques and return-to-play guidelines for concussion have significantly changed across time. In the past, concussion grading scales were utilized for diagnosis and return to play was possible within the same contest.6,7 It has since been recognized that initial concussion severity makes it difficult to predict recovery.3 For example, research revealed memory decline and increased symptoms 36 hours post-injury for athletes with a grade 1 concussion (ie, transient confusion, no loss of consciousness, concussion symptoms or mental status changes that resolve within 15 minutes of injury) compared to baseline.7 Another study found duration of mental status changes to be related to slower symptom resolution and memory impairment 36 hours to 7 days post-injury.6 Consequently, return to play within the same contest was likely too liberal. Guidelines today recommend immediate removal from play with suspected SRC. Nevertheless, the “play through pain” culture has led athletes to continue playing after SRC, contributing to prolonged recoveries and potentially long-term effects.
Current Concussion Management: Continued Concerns and Areas of Improvement
Despite increased awareness of concussions, recent estimates revealed high rates (ie, 27:1 ratio for general players) of underreporting in college football, particularly amongst offensive linemen.8 Researchers have studied recovery implications for remaining in play, with one study revealing a 2.2 times greater risk for prolonged recovery in college athletes with delayed vs immediate removal.9 Another similar study discovered an 8.8 times greater risk for prolonged recovery in adolescent and young adult athletes not removed vs removed from play.10 Further analysis found remaining in play to be the greatest risk factor for prolonged recovery compared to other previously studied risk factors (eg, age, sex, posttraumatic migraine).10 Additionally, significant differences in neurocognitive data were seen between the “removed” and “not removed” groups for verbal memory, visual memory, processing speed, and reaction time at 1 to 7 days and 8 to 30 days.10 The recovery implications of remaining in play and the additional risk for second impact syndrome (SIS), or repeat concussion when recovering from another injury, emphasizes the need for further education efforts amongst athletes to encourage immediate reporting of injury.11
Sideline Assessment
Sideline assessment has become a vital component of concussion management to rule out concussion and/or significant injury other than concussion. Assessment should include observation, cognitive/balance testing, neurologic examination, and possible exertion testing to ensure a comprehensive evaluation of all areas of potential dysfunction.12 Indications for emergency department evaluation include suspicion for cervical spine injury, intracranial hemorrhage, or skull fracture as well as prolonged loss of consciousness, high-risk mechanisms, posttraumatic seizure(s), and/or significant worsening of symptoms.12
Observation
On the sideline, it is important to identify any immediate signs of injury (ie, loss of consciousness, anterograde/retrograde amnesia, and disorientation/confusion). Since immediate signs are not always present, it is important to be aware of the most commonly reported symptoms, including headache, difficulty concentrating, fatigue, drowsiness, and dizziness.13 If symptoms are not reported by the athlete, balance problems, lack of coordination, increased emotionality, and difficulty following instructions may be observed during play.12
On-Field Assessment
Cognitive and balance testing are essential in determining if an athlete has sustained a concussion. Immediate declines in memory, concentration abilities, and balance abilities are common. Given limitations in administering long testing batteries on the sideline, brief standardized tests such as the Standardized Assessment of Concussion (SAC), Balance Error Scoring System (BESS), and Sport Concussion Assessment Tool (SCAT) are commonly utilized. Identification of cognitive and/or balance abnormalities can help the athlete recognize deficits following injury.12 Balance problems are experienced due to abnormalities in sensory organization and generally resolve during the acute recovery period.14,15 Cognitive difficulties typically persist longer than balance problems, though duration varies widely.
Neurologic Evaluation
A neurologic evaluation including cranial nerve testing and evaluation of motor-sensory function (ie, assessment for the strength and sensation of upper and lower extremities) is important to identify focal deficits (ie, sensation changes, loss of fine motor control) indicative of serious intracranial pathology.12 Additionally, clinicians have suggested inclusion of vestibular and oculomotor assessments due to frequent dysfunction post-concussion.12,15,16 Examination of the vestibular/oculomotor systems through tools such as the Vestibular/Ocular Motor Screening (VOMS) assessment (assesses both the vestibular and oculomotor systems) and King-Devick Test (primarily assesses saccadic eye movements) can elicit symptoms that may not present immediately. If assessment appears normal, exertion testing can be utilized to determine if symptoms are provoked through physical exercise that should include cardio, dynamic, and sport-specific activities to stress the vestibular system.12
Risk Factors for Injury and Prolonged Recovery
Medical professionals must consider the presence of risk factors when managing concussion in order to make appropriate treatment recommendations and return-to-play decisions. Research has demonstrated the role of female gender, learning disability, attention-deficit/hyperactivity disorder, psychiatric history, young age, motion sickness, sleep problems, somatization, concussion history, on-field dizziness, posttraumatic migraine, and fogginess in increased risk for injury and/or prolonged recovery.17-25 Additionally, athletes with ongoing symptoms from a previous injury are at risk for sustaining another injury.
Acute Home Concussion Management
Although strict rest has been recommended post-concussion, recent research evaluating strict rest vs usual care for adolescents revealed greater symptom reports and longer recovery periods for the strict rest group.26 Based on these findings and emphasis for regulation within the migraine literature (due to the common pathophysiology between migraine and concussion27), we recommend that athletes follow a regulated daily schedule post-concussion including: 1) regular sleep-wake schedule with avoidance of naps, 2) regular meals, 3) adequate fluid hydration, 4) light noncontact physical activity (ie, walking, with progressions recommended by a physician), and 5) stress management techniques. Use of these strategies immediately can help in preventing against increased symptoms and stress, and decreases the need for medication in select cases. Additionally, over-the-counter medications should be limited to 2 to 3 doses per week to avoid rebound headaches.28
In-Office Concussion Management
Athletes diagnosed with SRC will experience different symptoms based on the injury mechanism, risk factors, and management approach. Comprehensive evaluation should include assessment of risk factors, injury details, symptoms, neurocognitive functioning, vestibular/oculomotor dysfunction, tolerance of physical exertion, balance functioning, and cervical spine integrity (if necessary).29,30 Due to individual differences and the heterogeneous symptom profiles, concussion management must move beyond a “one size fits all” approach to avoid nonspecific treatment strategies and consequently prolonged recoveries.29 Clinicians and researchers at University of Pittsburgh Medical Center have identified 6 concussion clinical profiles (ie, vestibular, ocular, posttraumatic migraine, cervical, anxiety/mood, and cognitive/fatigue) that are generally identifiable 48 hours after injury.29,30 Identification of the clinical profile(s) through a comprehensive evaluation guides the development of individualized treatment plans and targeted rehabilitation strategies.29,30
Vestibular. The vestibular system is responsible for stabilizing vision while the head moves and balance control.15 Athletes can experience central and/or peripheral vestibular dysfunction to include benign paroxysmal positional vertigo (BPPV), visual motion sensitivity, vestibular ocular reflex impairment, and balance impairment.30,31 Symptoms typically include dizziness, impaired balance, blurry vision, difficulty focusing, and environmental sensitivity.15,29,30 Potential treatment options include vestibular rehabilitation, exertion therapy, and school/work accommodations.
Ocular. The oculomotor system is responsible for control of eye movements. Athletes can experience many different posttraumatic vision changes, including convergence problems, eye-tracking difficulties, refractive error, difficulty with pursuits/saccades, and accommodation insufficiency. Symptoms typically include light sensitivity, blurred vision, double vision, headaches, fatigue, and memory difficulties.15,29,30 Potential treatment options include vision therapy, vestibular rehabilitation, and school/work accommodations.32
Posttraumatic Migraine. Headache, the most common post-concussion symptom, can persist and meet criteria for posttraumatic migraine (ie, unilateral headache with accompanying nausea and/or photophobia and phonophobia).29,30,33 Implementation of a routine schedule, daily physical activity, exertion therapy, pharmacologic intervention, and school/work accommodations are potential treatment options.
Cervical. The cervical spine can be injured during whiplash-type injuries. Therefore, determining the location, onset, and typical exacerbations of pain can be helpful in identifying cervical involvement.29,30 Symptoms typically include headaches, neck pain, numbness, and tingling. Evaluation and therapy by a certified physical therapist and pharmacologic intervention (eg, muscle relaxants) are potential treatment options. 29,30
Anxiety/Mood. Anxiety, or worry and fear about everyday situations, is common post-concussion and can sometimes be related to ongoing vestibular impairment. Symptoms typically include ruminative thoughts, avoidance of specific situations, hypervigilance, feelings of being overwhelmed, and difficulty falling asleep.29,30 Potential treatment options include implementation of a routine schedule, exposure to provocative situations, psychotherapy, pharmacologic intervention, and school/work accommodations.34
Cognitive/Fatigue. A global concussion factor (including cognitive, fatigue, and migraine symptoms) has been identified within 1 to 7 days of injury. Although this factor of symptoms generally resolves during the acute recovery period, it persists in select cases.13 Symptoms typically include fatigue, decreased energy levels, nonspecific headaches, potential sleep disruption, increased symptoms towards the end of the day, difficulty concentrating, and increased headache with cognitive activities.29,30,35 Routine schedule, daily physical activity, exertion therapy, pharmacologic intervention (eg, amantadine), and school/work accommodations are potential treatment options.30
Conclusion
Advancements in SRC management warrant change in the conversations regarding concussion in football. Specifically, conversations should address the current understanding of concussion and improvements in the safety of football through stricter concussion guidelines, detailed sideline evaluations, recognition of risk factors, improved acute management, and identification of concussion profiles that help to direct individualized treatment plans and targeted rehabilitation strategies. The biggest concerns related to concussions in football include underreporting of injury, premature return to play, and receiving routine rather than individualized treatment. Therefore, to further improve the safety of football and management of concussion it is essential that future efforts focus on the following 6 areas:
Education: Improved understanding of concussion is imperative to reducing poor outcomes and widespread concerns.
Immediate reporting: Reporting of concussion must be expected and encouraged through consistent responses by coaches to reduce underreporting and fear of reporting in athletes.
Prevention techniques: Athletes must be taught proper form and playing techniques to reduce the risk for concussion. Proper form and technique should be incentivized.
Targeted treatment: Individualized treatment plans and targeted rehabilitation strategies must be developed based on the identified clinical profile(s) to avoid nonspecific treatment recommendations.
Multidisciplinary treatment teams: Given the heterogeneous symptoms profiles and need for care provided by different medical specialties, multidisciplinary teams are essential.
Remain current: With the progress in understanding concussion, providers must remain vigilant of future advances in concussion management to further improve the safety of football.
Am J Orthop. 2016;45(6):352-356. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Football is an important component of American culture, with approximately 3 million youth athletes, 1.1 million high school athletes, and 100,000 college athletes participating each year.1 Participation in football provides athletes with physical, social, psychological, and academic benefits. Despite these benefits, widespread focus has been placed on the safety of football due to the risk for sport-related concussion (SRC) and potentially long-term effects; however, little recognition has been given to the advancements in concussion management across time and occurrence of concussions during most life activities. Although it is reasonable for concerns to be presented, it is important to better understand SRC and the current factors leading to prolonged recoveries, increased risk for injury, and potentially long-term effects.
What Is a Concussion?
Concussions occur after sustaining direct or indirect injury to the head or other parts of the body, as long as the injury force is transmitted to the head. Athletes often experience physical, cognitive, emotional, and sleep-related symptoms post-concussion secondary to an “energy crisis” within the brain.2 The energy crisis occurs as the result of transient neurological dysfunction triggered by changes in the brain (eg, release of neurotransmitters, impaired axonal function).2,3 Concussion is undetectable with traditional imaging; however, advanced imaging techniques (eg, diffuse tensor imaging) have shown progress in assessing axonal injury.3 Symptom duration post-concussion is highly variable due to individual differences; a recent study showed recovery took 3 to 4 weeks for memory and symptoms.4,5
Previous Concussion Management
Identification techniques and return-to-play guidelines for concussion have significantly changed across time. In the past, concussion grading scales were utilized for diagnosis and return to play was possible within the same contest.6,7 It has since been recognized that initial concussion severity makes it difficult to predict recovery.3 For example, research revealed memory decline and increased symptoms 36 hours post-injury for athletes with a grade 1 concussion (ie, transient confusion, no loss of consciousness, concussion symptoms or mental status changes that resolve within 15 minutes of injury) compared to baseline.7 Another study found duration of mental status changes to be related to slower symptom resolution and memory impairment 36 hours to 7 days post-injury.6 Consequently, return to play within the same contest was likely too liberal. Guidelines today recommend immediate removal from play with suspected SRC. Nevertheless, the “play through pain” culture has led athletes to continue playing after SRC, contributing to prolonged recoveries and potentially long-term effects.
Current Concussion Management: Continued Concerns and Areas of Improvement
Despite increased awareness of concussions, recent estimates revealed high rates (ie, 27:1 ratio for general players) of underreporting in college football, particularly amongst offensive linemen.8 Researchers have studied recovery implications for remaining in play, with one study revealing a 2.2 times greater risk for prolonged recovery in college athletes with delayed vs immediate removal.9 Another similar study discovered an 8.8 times greater risk for prolonged recovery in adolescent and young adult athletes not removed vs removed from play.10 Further analysis found remaining in play to be the greatest risk factor for prolonged recovery compared to other previously studied risk factors (eg, age, sex, posttraumatic migraine).10 Additionally, significant differences in neurocognitive data were seen between the “removed” and “not removed” groups for verbal memory, visual memory, processing speed, and reaction time at 1 to 7 days and 8 to 30 days.10 The recovery implications of remaining in play and the additional risk for second impact syndrome (SIS), or repeat concussion when recovering from another injury, emphasizes the need for further education efforts amongst athletes to encourage immediate reporting of injury.11
Sideline Assessment
Sideline assessment has become a vital component of concussion management to rule out concussion and/or significant injury other than concussion. Assessment should include observation, cognitive/balance testing, neurologic examination, and possible exertion testing to ensure a comprehensive evaluation of all areas of potential dysfunction.12 Indications for emergency department evaluation include suspicion for cervical spine injury, intracranial hemorrhage, or skull fracture as well as prolonged loss of consciousness, high-risk mechanisms, posttraumatic seizure(s), and/or significant worsening of symptoms.12
Observation
On the sideline, it is important to identify any immediate signs of injury (ie, loss of consciousness, anterograde/retrograde amnesia, and disorientation/confusion). Since immediate signs are not always present, it is important to be aware of the most commonly reported symptoms, including headache, difficulty concentrating, fatigue, drowsiness, and dizziness.13 If symptoms are not reported by the athlete, balance problems, lack of coordination, increased emotionality, and difficulty following instructions may be observed during play.12
On-Field Assessment
Cognitive and balance testing are essential in determining if an athlete has sustained a concussion. Immediate declines in memory, concentration abilities, and balance abilities are common. Given limitations in administering long testing batteries on the sideline, brief standardized tests such as the Standardized Assessment of Concussion (SAC), Balance Error Scoring System (BESS), and Sport Concussion Assessment Tool (SCAT) are commonly utilized. Identification of cognitive and/or balance abnormalities can help the athlete recognize deficits following injury.12 Balance problems are experienced due to abnormalities in sensory organization and generally resolve during the acute recovery period.14,15 Cognitive difficulties typically persist longer than balance problems, though duration varies widely.
Neurologic Evaluation
A neurologic evaluation including cranial nerve testing and evaluation of motor-sensory function (ie, assessment for the strength and sensation of upper and lower extremities) is important to identify focal deficits (ie, sensation changes, loss of fine motor control) indicative of serious intracranial pathology.12 Additionally, clinicians have suggested inclusion of vestibular and oculomotor assessments due to frequent dysfunction post-concussion.12,15,16 Examination of the vestibular/oculomotor systems through tools such as the Vestibular/Ocular Motor Screening (VOMS) assessment (assesses both the vestibular and oculomotor systems) and King-Devick Test (primarily assesses saccadic eye movements) can elicit symptoms that may not present immediately. If assessment appears normal, exertion testing can be utilized to determine if symptoms are provoked through physical exercise that should include cardio, dynamic, and sport-specific activities to stress the vestibular system.12
Risk Factors for Injury and Prolonged Recovery
Medical professionals must consider the presence of risk factors when managing concussion in order to make appropriate treatment recommendations and return-to-play decisions. Research has demonstrated the role of female gender, learning disability, attention-deficit/hyperactivity disorder, psychiatric history, young age, motion sickness, sleep problems, somatization, concussion history, on-field dizziness, posttraumatic migraine, and fogginess in increased risk for injury and/or prolonged recovery.17-25 Additionally, athletes with ongoing symptoms from a previous injury are at risk for sustaining another injury.
Acute Home Concussion Management
Although strict rest has been recommended post-concussion, recent research evaluating strict rest vs usual care for adolescents revealed greater symptom reports and longer recovery periods for the strict rest group.26 Based on these findings and emphasis for regulation within the migraine literature (due to the common pathophysiology between migraine and concussion27), we recommend that athletes follow a regulated daily schedule post-concussion including: 1) regular sleep-wake schedule with avoidance of naps, 2) regular meals, 3) adequate fluid hydration, 4) light noncontact physical activity (ie, walking, with progressions recommended by a physician), and 5) stress management techniques. Use of these strategies immediately can help in preventing against increased symptoms and stress, and decreases the need for medication in select cases. Additionally, over-the-counter medications should be limited to 2 to 3 doses per week to avoid rebound headaches.28
In-Office Concussion Management
Athletes diagnosed with SRC will experience different symptoms based on the injury mechanism, risk factors, and management approach. Comprehensive evaluation should include assessment of risk factors, injury details, symptoms, neurocognitive functioning, vestibular/oculomotor dysfunction, tolerance of physical exertion, balance functioning, and cervical spine integrity (if necessary).29,30 Due to individual differences and the heterogeneous symptom profiles, concussion management must move beyond a “one size fits all” approach to avoid nonspecific treatment strategies and consequently prolonged recoveries.29 Clinicians and researchers at University of Pittsburgh Medical Center have identified 6 concussion clinical profiles (ie, vestibular, ocular, posttraumatic migraine, cervical, anxiety/mood, and cognitive/fatigue) that are generally identifiable 48 hours after injury.29,30 Identification of the clinical profile(s) through a comprehensive evaluation guides the development of individualized treatment plans and targeted rehabilitation strategies.29,30
Vestibular. The vestibular system is responsible for stabilizing vision while the head moves and balance control.15 Athletes can experience central and/or peripheral vestibular dysfunction to include benign paroxysmal positional vertigo (BPPV), visual motion sensitivity, vestibular ocular reflex impairment, and balance impairment.30,31 Symptoms typically include dizziness, impaired balance, blurry vision, difficulty focusing, and environmental sensitivity.15,29,30 Potential treatment options include vestibular rehabilitation, exertion therapy, and school/work accommodations.
Ocular. The oculomotor system is responsible for control of eye movements. Athletes can experience many different posttraumatic vision changes, including convergence problems, eye-tracking difficulties, refractive error, difficulty with pursuits/saccades, and accommodation insufficiency. Symptoms typically include light sensitivity, blurred vision, double vision, headaches, fatigue, and memory difficulties.15,29,30 Potential treatment options include vision therapy, vestibular rehabilitation, and school/work accommodations.32
Posttraumatic Migraine. Headache, the most common post-concussion symptom, can persist and meet criteria for posttraumatic migraine (ie, unilateral headache with accompanying nausea and/or photophobia and phonophobia).29,30,33 Implementation of a routine schedule, daily physical activity, exertion therapy, pharmacologic intervention, and school/work accommodations are potential treatment options.
Cervical. The cervical spine can be injured during whiplash-type injuries. Therefore, determining the location, onset, and typical exacerbations of pain can be helpful in identifying cervical involvement.29,30 Symptoms typically include headaches, neck pain, numbness, and tingling. Evaluation and therapy by a certified physical therapist and pharmacologic intervention (eg, muscle relaxants) are potential treatment options. 29,30
Anxiety/Mood. Anxiety, or worry and fear about everyday situations, is common post-concussion and can sometimes be related to ongoing vestibular impairment. Symptoms typically include ruminative thoughts, avoidance of specific situations, hypervigilance, feelings of being overwhelmed, and difficulty falling asleep.29,30 Potential treatment options include implementation of a routine schedule, exposure to provocative situations, psychotherapy, pharmacologic intervention, and school/work accommodations.34
Cognitive/Fatigue. A global concussion factor (including cognitive, fatigue, and migraine symptoms) has been identified within 1 to 7 days of injury. Although this factor of symptoms generally resolves during the acute recovery period, it persists in select cases.13 Symptoms typically include fatigue, decreased energy levels, nonspecific headaches, potential sleep disruption, increased symptoms towards the end of the day, difficulty concentrating, and increased headache with cognitive activities.29,30,35 Routine schedule, daily physical activity, exertion therapy, pharmacologic intervention (eg, amantadine), and school/work accommodations are potential treatment options.30
Conclusion
Advancements in SRC management warrant change in the conversations regarding concussion in football. Specifically, conversations should address the current understanding of concussion and improvements in the safety of football through stricter concussion guidelines, detailed sideline evaluations, recognition of risk factors, improved acute management, and identification of concussion profiles that help to direct individualized treatment plans and targeted rehabilitation strategies. The biggest concerns related to concussions in football include underreporting of injury, premature return to play, and receiving routine rather than individualized treatment. Therefore, to further improve the safety of football and management of concussion it is essential that future efforts focus on the following 6 areas:
Education: Improved understanding of concussion is imperative to reducing poor outcomes and widespread concerns.
Immediate reporting: Reporting of concussion must be expected and encouraged through consistent responses by coaches to reduce underreporting and fear of reporting in athletes.
Prevention techniques: Athletes must be taught proper form and playing techniques to reduce the risk for concussion. Proper form and technique should be incentivized.
Targeted treatment: Individualized treatment plans and targeted rehabilitation strategies must be developed based on the identified clinical profile(s) to avoid nonspecific treatment recommendations.
Multidisciplinary treatment teams: Given the heterogeneous symptoms profiles and need for care provided by different medical specialties, multidisciplinary teams are essential.
Remain current: With the progress in understanding concussion, providers must remain vigilant of future advances in concussion management to further improve the safety of football.
Am J Orthop. 2016;45(6):352-356. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Dompier TP, Kerr ZY, Marshall SW, et al. Incidence of concussion during practice and games in youth, high school, and collegiate American football players. JAMA Pediatrics. 2015;169(7):659-665.
2. Giza C, Hovda D. The new neurometabolic cascade of concussion
3. Barkhoudarian G, Hovda DA, Giza CC. The molecular pathophysiology of concussive brain injury - an update. Phys Med Rehabil Clin N Am. 2016;27:373-393.
4. Henry L, Elbin R, Collins M, Marchetti G, Kontos A. Examining recovery trajectories after sport-related concussion with a multimodal clinical assessment approach. Neurosurgery. 2016;78(2):232-241.
5. McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Brit J Sports Med. 2013;47(5):250-258.
6. Lovell MR, Collins MW, Iverson GL, et al. Recovery from mild concussion in high school athletes. J Neurosurg. 2003;98(2):296-301.
7. Lovell MR, Collins MW, Iverson GL, Johnston KM, Bradley JP. Grade 1 or “ding” concussions in high school athletes. Am J Sports Med. 2004;32(1):47-54.
8. Baugh CM, Kroshus E, Daneshvar DH, Filali NA, Hiscox MJ, Glantz LH. Concussion management in United States college sports: compliance with National Collegiate Athletic Association concussion policy and areas for improvement. Am J Sports Med. 2015;43(1):47-56.
9. Asken BM, McCrea MA, Clugston JR, Snyder AR, Houck ZM, Bauer RM. “Playing through it”: Delayed reporting and removal from athletic activity after concussion predicts prolonged recovery. J Athl Train. 2016;51(4):329-335.
10. Elbin RJ, Sufrinko A, Schatz P, et al. Athletes that continue to play with concussion demonstrate worse recovery outcomes than athletes immediately removed from play. J Pediatr. In press.
11. Signoretti S, Lazzarino G, Tavazzi B, Vagnozzi R. The pathophysiology of concussion. PM R. 2011;3(10 Suppl 2):S359-S368.
12. Bloom J, Blount JG. Sideline evaluation of concussion. UpToDate. 2016. http://www.uptodate.com/contents/sideline-evaluation-of-concussion. Accessed July 13, 2016.
13. Kontos AP, Elbin RJ, Schatz P, et al. A revised factor structure for the post-concussion symptom scale: baseline and postconcussion factors. Am J Sports Med. 2012;40(10):2375-2384.
14. Guskiewicz KM, Ross SE, Marshall SW. Postural stability and neuropsychological deficits after concussion in collegiate athletes. J Athl Train. 2001;36(3):263.
15. Mucha A, Collins MW, Elbin R, et al. A brief Vestibular/Ocular Motor Screening (VOMS) assessment to evaluate concussions preliminary findings. Am J Sports Med. 2014;42(10):2479-2486.
16. Bloom J. Vestibular and ocular motor assessments: Important pieces to the concussion puzzle. Athletic Training and Sports Health Care. 2013;5(6):246-248.
17. Covassin T, Elbin R, Harris W, Parker T, Kontos A. The role of age and sex in symptoms, neurocognitive performance, and postural stability in athletes after concussion. Am J Sports Med. 2012;40(6):1303-1312.
18. Kontos A, Sufrinko A, Elbin R, Puskar A, Collins M. Reliability and associated risk factors for performance on the Vestibular/Ocular Motor Screening (VOMS) tool in healthy collegiate athletes. Am J Sports Med. 2016;44(6):1400-1406.
19. Guskiewicz KM, McCrea M, Marshall SW, et al. Cumulative effects associated with recurrent concussion in collegiate football players: the NCAA Concussion Study. JAMA. 2003;290(19):2549-2555.
20. Lau B, Lovell MR, Collins MW, Pardini J. Neurocognitive and symptom predictors of recovery in high school athletes. Clin J Sport Med. 2009;19(3):216-221.
21. Lau BC, Kontos AP, Collins MW, Mucha A, Lovell MR. Which on-field signs/symptoms predict protracted recovery from sport-related concussion among high school football players? Am J Sports Med. 2011;39(11):2311-2318.
22. Mihalik JP, Register-Mihalik J, Kerr ZY, Marshall SW, McCrea MC, Guskiewicz KM. Recovery of posttraumatic migraine characteristics in patients after mild traumatic brain injury. Am J Sports Med. 2013;41(7):1490-1496.
23. Covassin T, Moran R, Elbin RJ. Sex differences in reported concussion injury rates and time loss from participation: An update of the National Collegiate Athletic Association injury surveillance program from 2004-2005 through 2008-2009. J Athl Train. 2016;51(3):189-194.
24. Root JM, Zuckerbraun NS, Wang L, et al. History of somatization is associated with prolonged recovery from concussion. J Pediatr. 2016;174:39-44.
25. Sufrinko A, Pearce K, Elbin RJ, et al. The effect of preinjury sleep difficulties on neurocognitive impairment and symptoms after sport-related concussion. Am J Sports Med. 2015;43(4):830-838.
26. Thomas DG, Apps JN, Hoffmann RG, McCrea M, Hammeke T. Benefits of strict rest after acute concussion: a randomized controlled trial. Pediatrics. 2015;135(2):213-223.
27. Choe M, Blume H. Pediatric posttraumatic Headache: a review. J Child Neurol. 2016;31(1):76-85.
28. Tepper SJ, Tepper DE. Breaking the cycle of medication overuse. Cleve Clin J Med. 2010;77(4):236-242.
29. Collins M, Kontos A, Reynolds E, Murawski C, Fu F. A comprehensive, targeted approach to the clinical care of athletes following sport-related concussion. Knee Surg Sports Traumatol Arthrosc. 2014;22(2):235-246.
30. Reynolds E, Collins MW, Mucha A, Troutman-Ensecki C. Establishing a clinical service for the management of sports-related concussions. Neurosurgery. 2014;75 Suppl 4:S71-S81.
31. Broglio SP, Collins MW, Williams RM, Mucha A, Kontos AP. Current and emerging rehabilitation for concussion: a review of the evidence. Clin Sports Med. 2015;34(2):213-231.
32. Master C, Scheiman M, Gallaway M, et al. Vision diagnoses are common after concussion in adolescents. Clin Pediatr (Phila). 2016;55(3):260-267.
33. Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition (beta version). Cephalalgia. 2013;33(9):629-808.
34. Kontos A, Deitrick JM, Reynolds E. Mental health implication and consequences following sport-related concussion. Brit J Sports Med. 2016;50(3):139-140.
35. Kontos AP, Covassin T, Elbin R, Parker T. Depression and neurocognitive performance after concussion among male and female high school and collegiate athletes. Arch Phys Med Rehabil. 2012;93(10):1751-1756.
1. Dompier TP, Kerr ZY, Marshall SW, et al. Incidence of concussion during practice and games in youth, high school, and collegiate American football players. JAMA Pediatrics. 2015;169(7):659-665.
2. Giza C, Hovda D. The new neurometabolic cascade of concussion
3. Barkhoudarian G, Hovda DA, Giza CC. The molecular pathophysiology of concussive brain injury - an update. Phys Med Rehabil Clin N Am. 2016;27:373-393.
4. Henry L, Elbin R, Collins M, Marchetti G, Kontos A. Examining recovery trajectories after sport-related concussion with a multimodal clinical assessment approach. Neurosurgery. 2016;78(2):232-241.
5. McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Brit J Sports Med. 2013;47(5):250-258.
6. Lovell MR, Collins MW, Iverson GL, et al. Recovery from mild concussion in high school athletes. J Neurosurg. 2003;98(2):296-301.
7. Lovell MR, Collins MW, Iverson GL, Johnston KM, Bradley JP. Grade 1 or “ding” concussions in high school athletes. Am J Sports Med. 2004;32(1):47-54.
8. Baugh CM, Kroshus E, Daneshvar DH, Filali NA, Hiscox MJ, Glantz LH. Concussion management in United States college sports: compliance with National Collegiate Athletic Association concussion policy and areas for improvement. Am J Sports Med. 2015;43(1):47-56.
9. Asken BM, McCrea MA, Clugston JR, Snyder AR, Houck ZM, Bauer RM. “Playing through it”: Delayed reporting and removal from athletic activity after concussion predicts prolonged recovery. J Athl Train. 2016;51(4):329-335.
10. Elbin RJ, Sufrinko A, Schatz P, et al. Athletes that continue to play with concussion demonstrate worse recovery outcomes than athletes immediately removed from play. J Pediatr. In press.
11. Signoretti S, Lazzarino G, Tavazzi B, Vagnozzi R. The pathophysiology of concussion. PM R. 2011;3(10 Suppl 2):S359-S368.
12. Bloom J, Blount JG. Sideline evaluation of concussion. UpToDate. 2016. http://www.uptodate.com/contents/sideline-evaluation-of-concussion. Accessed July 13, 2016.
13. Kontos AP, Elbin RJ, Schatz P, et al. A revised factor structure for the post-concussion symptom scale: baseline and postconcussion factors. Am J Sports Med. 2012;40(10):2375-2384.
14. Guskiewicz KM, Ross SE, Marshall SW. Postural stability and neuropsychological deficits after concussion in collegiate athletes. J Athl Train. 2001;36(3):263.
15. Mucha A, Collins MW, Elbin R, et al. A brief Vestibular/Ocular Motor Screening (VOMS) assessment to evaluate concussions preliminary findings. Am J Sports Med. 2014;42(10):2479-2486.
16. Bloom J. Vestibular and ocular motor assessments: Important pieces to the concussion puzzle. Athletic Training and Sports Health Care. 2013;5(6):246-248.
17. Covassin T, Elbin R, Harris W, Parker T, Kontos A. The role of age and sex in symptoms, neurocognitive performance, and postural stability in athletes after concussion. Am J Sports Med. 2012;40(6):1303-1312.
18. Kontos A, Sufrinko A, Elbin R, Puskar A, Collins M. Reliability and associated risk factors for performance on the Vestibular/Ocular Motor Screening (VOMS) tool in healthy collegiate athletes. Am J Sports Med. 2016;44(6):1400-1406.
19. Guskiewicz KM, McCrea M, Marshall SW, et al. Cumulative effects associated with recurrent concussion in collegiate football players: the NCAA Concussion Study. JAMA. 2003;290(19):2549-2555.
20. Lau B, Lovell MR, Collins MW, Pardini J. Neurocognitive and symptom predictors of recovery in high school athletes. Clin J Sport Med. 2009;19(3):216-221.
21. Lau BC, Kontos AP, Collins MW, Mucha A, Lovell MR. Which on-field signs/symptoms predict protracted recovery from sport-related concussion among high school football players? Am J Sports Med. 2011;39(11):2311-2318.
22. Mihalik JP, Register-Mihalik J, Kerr ZY, Marshall SW, McCrea MC, Guskiewicz KM. Recovery of posttraumatic migraine characteristics in patients after mild traumatic brain injury. Am J Sports Med. 2013;41(7):1490-1496.
23. Covassin T, Moran R, Elbin RJ. Sex differences in reported concussion injury rates and time loss from participation: An update of the National Collegiate Athletic Association injury surveillance program from 2004-2005 through 2008-2009. J Athl Train. 2016;51(3):189-194.
24. Root JM, Zuckerbraun NS, Wang L, et al. History of somatization is associated with prolonged recovery from concussion. J Pediatr. 2016;174:39-44.
25. Sufrinko A, Pearce K, Elbin RJ, et al. The effect of preinjury sleep difficulties on neurocognitive impairment and symptoms after sport-related concussion. Am J Sports Med. 2015;43(4):830-838.
26. Thomas DG, Apps JN, Hoffmann RG, McCrea M, Hammeke T. Benefits of strict rest after acute concussion: a randomized controlled trial. Pediatrics. 2015;135(2):213-223.
27. Choe M, Blume H. Pediatric posttraumatic Headache: a review. J Child Neurol. 2016;31(1):76-85.
28. Tepper SJ, Tepper DE. Breaking the cycle of medication overuse. Cleve Clin J Med. 2010;77(4):236-242.
29. Collins M, Kontos A, Reynolds E, Murawski C, Fu F. A comprehensive, targeted approach to the clinical care of athletes following sport-related concussion. Knee Surg Sports Traumatol Arthrosc. 2014;22(2):235-246.
30. Reynolds E, Collins MW, Mucha A, Troutman-Ensecki C. Establishing a clinical service for the management of sports-related concussions. Neurosurgery. 2014;75 Suppl 4:S71-S81.
31. Broglio SP, Collins MW, Williams RM, Mucha A, Kontos AP. Current and emerging rehabilitation for concussion: a review of the evidence. Clin Sports Med. 2015;34(2):213-231.
32. Master C, Scheiman M, Gallaway M, et al. Vision diagnoses are common after concussion in adolescents. Clin Pediatr (Phila). 2016;55(3):260-267.
33. Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition (beta version). Cephalalgia. 2013;33(9):629-808.
34. Kontos A, Deitrick JM, Reynolds E. Mental health implication and consequences following sport-related concussion. Brit J Sports Med. 2016;50(3):139-140.
35. Kontos AP, Covassin T, Elbin R, Parker T. Depression and neurocognitive performance after concussion among male and female high school and collegiate athletes. Arch Phys Med Rehabil. 2012;93(10):1751-1756.