User login
USPSTF: Insufficient evidence to judge vitamin supplements
Current evidence is still insufficient to adequately assess whether taking vitamin supplements to prevent cardiovascular disease or cancer is beneficial or harmful, according to a recommendation statement issued by the U.S. Preventive Services Task Force and published online Feb. 24 in Annals of Internal Medicine.
This update of the 2003 USPSTF recommendation on vitamin supplementation to prevent CVD or cancer is based on a close review of 4 randomized controlled trials and 1 cohort study of multivitamins, as well as 24 studies of individual vitamins, minerals, or nutrient pairs. The USPSTF concluded that for the general adult population, there still isn’t enough evidence either for or against multivitamins, individual vitamins and minerals, or nutrient pairs. However, there are two exceptions: The task force clearly recommends against the use of beta-carotene and vitamin E as preventives, said Dr. Virginia A. Moyer, chair of the task force at the time this recommendation was finalized and a vice president of the American Board of Pediatrics, and her associates.
The recommendation applies to healthy adults only, not to children, women who are pregnant or may become pregnant, or people who are hospitalized, have chronic illness, or have known nutritional deficiency.
The statement noted that the use of vitamin supplements is common among American adults, with annual sales reaching $28.1 billion in 2010. Many supplements are promoted as preventing heart disease and cancer, and industry-sponsored surveys indicate that many physicians and nurses recommend them to patients (Ann. Intern. Med. 2014 Feb. 24).
Like the USPSTF, the National Institutes of Health also has concluded that the evidence is insufficient to recommend for or against the use of multivitamins to prevent chronic disease. In addition, the Academy of Nutrition and Dietetics has stated that there is no evidence that vitamin supplements are effective at preventing chronic disease, and neither the American Cancer Society nor the American Institute for Cancer Research supports their use. The position of the American Heart Association and the American Academy of Family Physicians also is consistent with that of the USPSTF, Dr. Moyer and her associates said.
No financial conflicts of interest were reported.
Current evidence is still insufficient to adequately assess whether taking vitamin supplements to prevent cardiovascular disease or cancer is beneficial or harmful, according to a recommendation statement issued by the U.S. Preventive Services Task Force and published online Feb. 24 in Annals of Internal Medicine.
This update of the 2003 USPSTF recommendation on vitamin supplementation to prevent CVD or cancer is based on a close review of 4 randomized controlled trials and 1 cohort study of multivitamins, as well as 24 studies of individual vitamins, minerals, or nutrient pairs. The USPSTF concluded that for the general adult population, there still isn’t enough evidence either for or against multivitamins, individual vitamins and minerals, or nutrient pairs. However, there are two exceptions: The task force clearly recommends against the use of beta-carotene and vitamin E as preventives, said Dr. Virginia A. Moyer, chair of the task force at the time this recommendation was finalized and a vice president of the American Board of Pediatrics, and her associates.
The recommendation applies to healthy adults only, not to children, women who are pregnant or may become pregnant, or people who are hospitalized, have chronic illness, or have known nutritional deficiency.
The statement noted that the use of vitamin supplements is common among American adults, with annual sales reaching $28.1 billion in 2010. Many supplements are promoted as preventing heart disease and cancer, and industry-sponsored surveys indicate that many physicians and nurses recommend them to patients (Ann. Intern. Med. 2014 Feb. 24).
Like the USPSTF, the National Institutes of Health also has concluded that the evidence is insufficient to recommend for or against the use of multivitamins to prevent chronic disease. In addition, the Academy of Nutrition and Dietetics has stated that there is no evidence that vitamin supplements are effective at preventing chronic disease, and neither the American Cancer Society nor the American Institute for Cancer Research supports their use. The position of the American Heart Association and the American Academy of Family Physicians also is consistent with that of the USPSTF, Dr. Moyer and her associates said.
No financial conflicts of interest were reported.
Current evidence is still insufficient to adequately assess whether taking vitamin supplements to prevent cardiovascular disease or cancer is beneficial or harmful, according to a recommendation statement issued by the U.S. Preventive Services Task Force and published online Feb. 24 in Annals of Internal Medicine.
This update of the 2003 USPSTF recommendation on vitamin supplementation to prevent CVD or cancer is based on a close review of 4 randomized controlled trials and 1 cohort study of multivitamins, as well as 24 studies of individual vitamins, minerals, or nutrient pairs. The USPSTF concluded that for the general adult population, there still isn’t enough evidence either for or against multivitamins, individual vitamins and minerals, or nutrient pairs. However, there are two exceptions: The task force clearly recommends against the use of beta-carotene and vitamin E as preventives, said Dr. Virginia A. Moyer, chair of the task force at the time this recommendation was finalized and a vice president of the American Board of Pediatrics, and her associates.
The recommendation applies to healthy adults only, not to children, women who are pregnant or may become pregnant, or people who are hospitalized, have chronic illness, or have known nutritional deficiency.
The statement noted that the use of vitamin supplements is common among American adults, with annual sales reaching $28.1 billion in 2010. Many supplements are promoted as preventing heart disease and cancer, and industry-sponsored surveys indicate that many physicians and nurses recommend them to patients (Ann. Intern. Med. 2014 Feb. 24).
Like the USPSTF, the National Institutes of Health also has concluded that the evidence is insufficient to recommend for or against the use of multivitamins to prevent chronic disease. In addition, the Academy of Nutrition and Dietetics has stated that there is no evidence that vitamin supplements are effective at preventing chronic disease, and neither the American Cancer Society nor the American Institute for Cancer Research supports their use. The position of the American Heart Association and the American Academy of Family Physicians also is consistent with that of the USPSTF, Dr. Moyer and her associates said.
No financial conflicts of interest were reported.
FROM ANNALS OF INTERNAL MEDICINE
Major Finding: Current evidence is still insufficient to recommend for or against the use of multivitamins, individual vitamins and minerals, or pairs of nutrients to prevent cardiovascular disease or cancer, except that beta-carotene and vitamin E are not recommended for this purpose.
Data Source: A detailed review of the literature and a recommendation statement regarding the use of vitamin supplements to prevent cardiovascular disease or cancer.
Disclosures: No financial conflicts of interest were reported.
Vitamin D deficiency common in trauma ICU patients
NAPLES, FLA. – Vitamin D deficiency is common in critically ill trauma patients and portends worse outcomes, a retrospective study suggests.
Among 200 trauma patients with available vitamin D levels, 26% were vitamin D deficient on ICU admission.
"These patients have a higher APACHE II score, have a longer ICU stay, and will likely be hospitalized greater than 2 weeks," Dr. Joseph Ibrahim reported at the annual scientific assembly of the Eastern Association for the Surgery of Trauma.
Long known to be essential for bone development and wound healing, recent studies have demonstrated that vitamin D deficiency is a significant predictor of 30- and 90-day all-cause mortality in critically ill patients, even after adjustment for such factors as age, Charlson/Deyo index, sepsis, and season (Crit. Care. Med. 2012;40:63-72). It also has been shown to significantly predict acute kidney injury in the critically ill (Crit. Care Med. 2012;40:3170-9).
For the current analysis, vitamin D levels were drawn upon ICU admission, at 72 hours, and every 7 days until hospital discharge in 200 of 234 consecutive adult trauma patients admitted to the ICU at the Level 1 Orlando Regional Medical Center during a 4-month period. Deficiency was defined as 25-hydroxyvitamin D 20 ng/mL or less. All patients received nutritional support using a standard protocol, but not vitamin D supplementation.
Median vitamin D ICU admission levels in the 51 vitamin D–deficient patients were significantly lower than for nondeficient patients (16 ng/mL vs. 28 ng/mL; P less than .001). Levels decreased a median of 4 ng/mL at 72 hours in both groups, but only the sufficient group returned to admission baseline levels at week 2, reported Dr. Ibrahim, a critical care surgeon with the medical center.
"This demonstrates that if we wish to obtain normal vitamin D levels in these patients, we will have to supplement them with much higher doses than what we are providing with standard enteral formulas," he said in an interview.
Patients with vitamin D deficiency spent more time than did nondeficient patients in the ICU (median 3 days vs. 2.7 days) and hospital (median 8.4 days vs. 7.1 days), but these trends did not reach statistical significance.
Significantly more deficient patients, however, remained in the hospital for at least 2 weeks (37% vs. 20%; P = .01).
The investigators were unable to show a difference in mortality between the deficient and nondeficient groups (16% vs. 12%; P = .51), possibly because the study was underpowered, he said.
Deficient and sufficient patients did not differ in age (median 48 years vs. 44 years), body mass index (26.2 kg/m2 vs. 25.7 kg/m2), admission ionized calcium (1.06 mmol/L for both), or Injury Severity Score (14 vs. 13). Only APACHE II scores were significantly higher in deficient patients (20 vs. 15).
"It makes sense that with the significant difference in APACHE II score, one would expect to see a similar difference in mortality, but again we were unable to show this with this study," Dr. Ibrahim said.
Prehospital factors significantly associated with low vitamin D status were African American race, diabetes, and lack of vitamin D supplementation.
Vitamin D supplementation may be helpful in critically ill trauma patients during hospitalization, but more research is needed, Dr. Ibrahim said. The group is planning a supplementation study, looking at vitamin D dosing and frequency of testing.
"Our first goal was to demonstrate a significant incidence, which we did," he said. "It should be noted that the incidence was in a location with probably one of the highest amounts of sunshine in the country and that the findings may underestimate what one would find in other areas of the United States."
Dr. Oscar Guillamondegui, of Vanderbilt University Medical Center in Nashville, Tenn., who proctored the poster session, said he would expect vitamin D levels to be lower in acutely sick patients requiring ICU management because production of vitamin D–binding protein, a subprotein in the albumin family of proteins involved in vitamin D transport and storage, is decreased in high stress situations to allow for the increase in acute phase protein production.
"Although the data are intriguing, as a retrospective study, it is too early to suggest that supplementation is essential," he said. "I do believe this is great work and will stimulate several studies to prove the need for supplementation and for that, I commend Dr. Ibrahim and his group in their efforts."
Dr. Ibrahim and Dr. Guillamondegui reported having no financial disclosures.
NAPLES, FLA. – Vitamin D deficiency is common in critically ill trauma patients and portends worse outcomes, a retrospective study suggests.
Among 200 trauma patients with available vitamin D levels, 26% were vitamin D deficient on ICU admission.
"These patients have a higher APACHE II score, have a longer ICU stay, and will likely be hospitalized greater than 2 weeks," Dr. Joseph Ibrahim reported at the annual scientific assembly of the Eastern Association for the Surgery of Trauma.
Long known to be essential for bone development and wound healing, recent studies have demonstrated that vitamin D deficiency is a significant predictor of 30- and 90-day all-cause mortality in critically ill patients, even after adjustment for such factors as age, Charlson/Deyo index, sepsis, and season (Crit. Care. Med. 2012;40:63-72). It also has been shown to significantly predict acute kidney injury in the critically ill (Crit. Care Med. 2012;40:3170-9).
For the current analysis, vitamin D levels were drawn upon ICU admission, at 72 hours, and every 7 days until hospital discharge in 200 of 234 consecutive adult trauma patients admitted to the ICU at the Level 1 Orlando Regional Medical Center during a 4-month period. Deficiency was defined as 25-hydroxyvitamin D 20 ng/mL or less. All patients received nutritional support using a standard protocol, but not vitamin D supplementation.
Median vitamin D ICU admission levels in the 51 vitamin D–deficient patients were significantly lower than for nondeficient patients (16 ng/mL vs. 28 ng/mL; P less than .001). Levels decreased a median of 4 ng/mL at 72 hours in both groups, but only the sufficient group returned to admission baseline levels at week 2, reported Dr. Ibrahim, a critical care surgeon with the medical center.
"This demonstrates that if we wish to obtain normal vitamin D levels in these patients, we will have to supplement them with much higher doses than what we are providing with standard enteral formulas," he said in an interview.
Patients with vitamin D deficiency spent more time than did nondeficient patients in the ICU (median 3 days vs. 2.7 days) and hospital (median 8.4 days vs. 7.1 days), but these trends did not reach statistical significance.
Significantly more deficient patients, however, remained in the hospital for at least 2 weeks (37% vs. 20%; P = .01).
The investigators were unable to show a difference in mortality between the deficient and nondeficient groups (16% vs. 12%; P = .51), possibly because the study was underpowered, he said.
Deficient and sufficient patients did not differ in age (median 48 years vs. 44 years), body mass index (26.2 kg/m2 vs. 25.7 kg/m2), admission ionized calcium (1.06 mmol/L for both), or Injury Severity Score (14 vs. 13). Only APACHE II scores were significantly higher in deficient patients (20 vs. 15).
"It makes sense that with the significant difference in APACHE II score, one would expect to see a similar difference in mortality, but again we were unable to show this with this study," Dr. Ibrahim said.
Prehospital factors significantly associated with low vitamin D status were African American race, diabetes, and lack of vitamin D supplementation.
Vitamin D supplementation may be helpful in critically ill trauma patients during hospitalization, but more research is needed, Dr. Ibrahim said. The group is planning a supplementation study, looking at vitamin D dosing and frequency of testing.
"Our first goal was to demonstrate a significant incidence, which we did," he said. "It should be noted that the incidence was in a location with probably one of the highest amounts of sunshine in the country and that the findings may underestimate what one would find in other areas of the United States."
Dr. Oscar Guillamondegui, of Vanderbilt University Medical Center in Nashville, Tenn., who proctored the poster session, said he would expect vitamin D levels to be lower in acutely sick patients requiring ICU management because production of vitamin D–binding protein, a subprotein in the albumin family of proteins involved in vitamin D transport and storage, is decreased in high stress situations to allow for the increase in acute phase protein production.
"Although the data are intriguing, as a retrospective study, it is too early to suggest that supplementation is essential," he said. "I do believe this is great work and will stimulate several studies to prove the need for supplementation and for that, I commend Dr. Ibrahim and his group in their efforts."
Dr. Ibrahim and Dr. Guillamondegui reported having no financial disclosures.
NAPLES, FLA. – Vitamin D deficiency is common in critically ill trauma patients and portends worse outcomes, a retrospective study suggests.
Among 200 trauma patients with available vitamin D levels, 26% were vitamin D deficient on ICU admission.
"These patients have a higher APACHE II score, have a longer ICU stay, and will likely be hospitalized greater than 2 weeks," Dr. Joseph Ibrahim reported at the annual scientific assembly of the Eastern Association for the Surgery of Trauma.
Long known to be essential for bone development and wound healing, recent studies have demonstrated that vitamin D deficiency is a significant predictor of 30- and 90-day all-cause mortality in critically ill patients, even after adjustment for such factors as age, Charlson/Deyo index, sepsis, and season (Crit. Care. Med. 2012;40:63-72). It also has been shown to significantly predict acute kidney injury in the critically ill (Crit. Care Med. 2012;40:3170-9).
For the current analysis, vitamin D levels were drawn upon ICU admission, at 72 hours, and every 7 days until hospital discharge in 200 of 234 consecutive adult trauma patients admitted to the ICU at the Level 1 Orlando Regional Medical Center during a 4-month period. Deficiency was defined as 25-hydroxyvitamin D 20 ng/mL or less. All patients received nutritional support using a standard protocol, but not vitamin D supplementation.
Median vitamin D ICU admission levels in the 51 vitamin D–deficient patients were significantly lower than for nondeficient patients (16 ng/mL vs. 28 ng/mL; P less than .001). Levels decreased a median of 4 ng/mL at 72 hours in both groups, but only the sufficient group returned to admission baseline levels at week 2, reported Dr. Ibrahim, a critical care surgeon with the medical center.
"This demonstrates that if we wish to obtain normal vitamin D levels in these patients, we will have to supplement them with much higher doses than what we are providing with standard enteral formulas," he said in an interview.
Patients with vitamin D deficiency spent more time than did nondeficient patients in the ICU (median 3 days vs. 2.7 days) and hospital (median 8.4 days vs. 7.1 days), but these trends did not reach statistical significance.
Significantly more deficient patients, however, remained in the hospital for at least 2 weeks (37% vs. 20%; P = .01).
The investigators were unable to show a difference in mortality between the deficient and nondeficient groups (16% vs. 12%; P = .51), possibly because the study was underpowered, he said.
Deficient and sufficient patients did not differ in age (median 48 years vs. 44 years), body mass index (26.2 kg/m2 vs. 25.7 kg/m2), admission ionized calcium (1.06 mmol/L for both), or Injury Severity Score (14 vs. 13). Only APACHE II scores were significantly higher in deficient patients (20 vs. 15).
"It makes sense that with the significant difference in APACHE II score, one would expect to see a similar difference in mortality, but again we were unable to show this with this study," Dr. Ibrahim said.
Prehospital factors significantly associated with low vitamin D status were African American race, diabetes, and lack of vitamin D supplementation.
Vitamin D supplementation may be helpful in critically ill trauma patients during hospitalization, but more research is needed, Dr. Ibrahim said. The group is planning a supplementation study, looking at vitamin D dosing and frequency of testing.
"Our first goal was to demonstrate a significant incidence, which we did," he said. "It should be noted that the incidence was in a location with probably one of the highest amounts of sunshine in the country and that the findings may underestimate what one would find in other areas of the United States."
Dr. Oscar Guillamondegui, of Vanderbilt University Medical Center in Nashville, Tenn., who proctored the poster session, said he would expect vitamin D levels to be lower in acutely sick patients requiring ICU management because production of vitamin D–binding protein, a subprotein in the albumin family of proteins involved in vitamin D transport and storage, is decreased in high stress situations to allow for the increase in acute phase protein production.
"Although the data are intriguing, as a retrospective study, it is too early to suggest that supplementation is essential," he said. "I do believe this is great work and will stimulate several studies to prove the need for supplementation and for that, I commend Dr. Ibrahim and his group in their efforts."
Dr. Ibrahim and Dr. Guillamondegui reported having no financial disclosures.
AT THE EAST SCIENTIFIC ASSEMBLY
Major finding: In all, 26% of patients were vitamin D deficient on ICU admission.
Data source: A retrospective study of 200 ICU trauma patients.
Disclosures: Dr. Ibrahim and Dr. Guillamondegui reported having no financial disclosures.
Romosozumab boosts bone density while cutting resorption
Romosozumab, a monoclonal antibody that targets the osteoblast inhibitor sclerostin, increased bone mineral density and bone formation while decreasing bone resorption in a phase II clinical trial in 419 postmenopausal women with low bone mass, according to a report published online Jan. 13 in the New England Journal of Medicine.
"The consequence of these divergent effects on bone formation and bone resorption ... is a strongly positive balance in bone turnover, accounting for the rapid and large increases in bone mineral density that we observed," said Dr. Michael R. McClung of the Oregon Osteoporosis Center, Portland, and his associates.
After 1 year of periodic subcutaneous injections of romosozumab, BMD at the lumbar spine was significantly greater than it was with placebo injections, regardless of the dose frequency or dose level of the active drug. Total hip and femoral neck BMD also were significantly greater with romosozumab.
These improvements in BMD also were significantly greater than those obtained with two comparator drugs used open-label in this trial, alendronate and teriparatide, Dr. McClung and his colleagues noted.
Sclerostin is a glycoprotein secreted by osteocytes that is known to be a key regulator of bone formation, capable of impeding osteoblast proliferation and function. Expression of the gene that encodes sclerostin is confined to skeletal tissue, which suggests that a drug that targets sclerostin should have minimal effects on other tissues.
Patients who have a genetic deficiency of sclerostin have greater than average bone mass, with corresponding bone strength and resistance to fractures. And, in animal models of estrogen deficiency, treatment with antisclerostin antibodies restored bone mass and bone strength to higher than normal levels.
In a previous phase I study, single injections of the humanized monoclonal antisclerostin antibody increased BMD, stimulated bone formation, and decreased bone resorption. Dr. McClung and his associates now report the results of their phase II study assessing the efficacy and safety of a variety of doses of romosozumab in postmenopausal women aged 55-85 years who had low bone mass.
The study participants were treated and followed at 28 medical centers in Europe, five dosing regimens of romosozumab (70 mg, 140 mg, or 210 mg injected once monthly; or 140 mg or 210 mg injected once every 3 months); or to 70 mg oral alendronate weekly; or to 20 mcg teriparatide injected daily; or to placebo injections that mirrored the dosing schedules of romosozumab.
A total of 383 women (91%) completed the 1-year study; 86% were white, and the mean T scores were –2.29 at the lumbar spine, –1.53 at the total hip, and –1.93 at the femoral neck.
The primary endpoint was change in BMD at the lumbar spine at 1 year. Participants in the pooled romosozumab groups showed a significant increase in this measure, compared with those pooled in the placebo groups, regardless of dose frequency or dose level.
Similarly, each of the romosozumab groups showed a significant increase in this measure when compared with the pooled placebo groups. Women who received romosozumab also showed significantly greater increases in BMD at the total hip and the femoral neck, but not at the wrist.
The greatest improvements were noted among women who received the highest monthly dose of romosozumab (210 mg), who showed a mean increase of 11.3% at the lumbar spine, 4.1% at the total hip, and 3.7% at the femoral neck at 1 year, the investigators said (N. Engl. J. Med. 2014 [doi:10.1056/NEJMoa1305224]).
The improvements in BMD with romosozumab also were significantly greater than those with alendronate and teriparatide.
The onset of action for romosozumab was swift. "The increase in BMD at the lumbar spine and proximal femur was rapid and substantial with romosozumab by 3 months, and by 6 months, the increase was greater with the 210-mg monthly dose of romosozumab than with either active comparator," Dr. McClung and his associates said.
The increase in BMD was accompanied by a significant decrease in bone resorption. This dual action differs markedly from the effects of bisphosphonates and other agents, which reduce both bone formation and bone resorption, they noted.
Overall, romosozumab’s effects on bone formation were strong but transitory, diminishing after 6 months even though the participants continued taking the drug. In contrast, romosozumab’s effects on bone resorption were more moderate but more sustained, continuing throughout the study period.
The study population was too small to allow adequate assessment of the drug’s safety, the investigators noted.
The overall incidence of adverse events and of serious adverse events was similar among all the study groups, except that mild injection-site reactions were more common with romosozumab. No serious adverse events were considered to be related to any of the treatments, and none of the study subjects showed any notable changes in vital signs, laboratory values, or ECG factors.
This study was funded by Amgen and UCB Pharma, makers of romosozumab. Dr. McClung reported receiving fees and honoraria from Amgen, Eli Lilly, Merck, Novartis, and Warner Chilcott, and his associates reported ties to numerous industry sources.
The "impressive" findings reported by McClung et al. "represent a potential breakthrough in osteoporosis therapeutics," said Dr. Carolyn B. Becker.
"The pattern of brief stimulation [of bone formation], coupled with chronic suppression of bone resorption ... is unprecedented among current therapies for osteoporosis," she noted.
However, many questions remain. Further study must establish whether the improvements in BMD do in fact translate into decreased fractures and whether the drug is safe enough to be taken long term. A phase III clinical trial is now under way to address such issues.
Dr. Carolyn B. Becker is an endocrinologist at Brigham and Women’s Hospital, Boston. She reported no potential financial conflicts of interest. These remarks were taken from her editorial accompanying Dr. McClung’s report (N. Engl. J. Med. 2014 [doi:10.1056/NEJMe1315500]).
The "impressive" findings reported by McClung et al. "represent a potential breakthrough in osteoporosis therapeutics," said Dr. Carolyn B. Becker.
"The pattern of brief stimulation [of bone formation], coupled with chronic suppression of bone resorption ... is unprecedented among current therapies for osteoporosis," she noted.
However, many questions remain. Further study must establish whether the improvements in BMD do in fact translate into decreased fractures and whether the drug is safe enough to be taken long term. A phase III clinical trial is now under way to address such issues.
Dr. Carolyn B. Becker is an endocrinologist at Brigham and Women’s Hospital, Boston. She reported no potential financial conflicts of interest. These remarks were taken from her editorial accompanying Dr. McClung’s report (N. Engl. J. Med. 2014 [doi:10.1056/NEJMe1315500]).
The "impressive" findings reported by McClung et al. "represent a potential breakthrough in osteoporosis therapeutics," said Dr. Carolyn B. Becker.
"The pattern of brief stimulation [of bone formation], coupled with chronic suppression of bone resorption ... is unprecedented among current therapies for osteoporosis," she noted.
However, many questions remain. Further study must establish whether the improvements in BMD do in fact translate into decreased fractures and whether the drug is safe enough to be taken long term. A phase III clinical trial is now under way to address such issues.
Dr. Carolyn B. Becker is an endocrinologist at Brigham and Women’s Hospital, Boston. She reported no potential financial conflicts of interest. These remarks were taken from her editorial accompanying Dr. McClung’s report (N. Engl. J. Med. 2014 [doi:10.1056/NEJMe1315500]).
Romosozumab, a monoclonal antibody that targets the osteoblast inhibitor sclerostin, increased bone mineral density and bone formation while decreasing bone resorption in a phase II clinical trial in 419 postmenopausal women with low bone mass, according to a report published online Jan. 13 in the New England Journal of Medicine.
"The consequence of these divergent effects on bone formation and bone resorption ... is a strongly positive balance in bone turnover, accounting for the rapid and large increases in bone mineral density that we observed," said Dr. Michael R. McClung of the Oregon Osteoporosis Center, Portland, and his associates.
After 1 year of periodic subcutaneous injections of romosozumab, BMD at the lumbar spine was significantly greater than it was with placebo injections, regardless of the dose frequency or dose level of the active drug. Total hip and femoral neck BMD also were significantly greater with romosozumab.
These improvements in BMD also were significantly greater than those obtained with two comparator drugs used open-label in this trial, alendronate and teriparatide, Dr. McClung and his colleagues noted.
Sclerostin is a glycoprotein secreted by osteocytes that is known to be a key regulator of bone formation, capable of impeding osteoblast proliferation and function. Expression of the gene that encodes sclerostin is confined to skeletal tissue, which suggests that a drug that targets sclerostin should have minimal effects on other tissues.
Patients who have a genetic deficiency of sclerostin have greater than average bone mass, with corresponding bone strength and resistance to fractures. And, in animal models of estrogen deficiency, treatment with antisclerostin antibodies restored bone mass and bone strength to higher than normal levels.
In a previous phase I study, single injections of the humanized monoclonal antisclerostin antibody increased BMD, stimulated bone formation, and decreased bone resorption. Dr. McClung and his associates now report the results of their phase II study assessing the efficacy and safety of a variety of doses of romosozumab in postmenopausal women aged 55-85 years who had low bone mass.
The study participants were treated and followed at 28 medical centers in Europe, five dosing regimens of romosozumab (70 mg, 140 mg, or 210 mg injected once monthly; or 140 mg or 210 mg injected once every 3 months); or to 70 mg oral alendronate weekly; or to 20 mcg teriparatide injected daily; or to placebo injections that mirrored the dosing schedules of romosozumab.
A total of 383 women (91%) completed the 1-year study; 86% were white, and the mean T scores were –2.29 at the lumbar spine, –1.53 at the total hip, and –1.93 at the femoral neck.
The primary endpoint was change in BMD at the lumbar spine at 1 year. Participants in the pooled romosozumab groups showed a significant increase in this measure, compared with those pooled in the placebo groups, regardless of dose frequency or dose level.
Similarly, each of the romosozumab groups showed a significant increase in this measure when compared with the pooled placebo groups. Women who received romosozumab also showed significantly greater increases in BMD at the total hip and the femoral neck, but not at the wrist.
The greatest improvements were noted among women who received the highest monthly dose of romosozumab (210 mg), who showed a mean increase of 11.3% at the lumbar spine, 4.1% at the total hip, and 3.7% at the femoral neck at 1 year, the investigators said (N. Engl. J. Med. 2014 [doi:10.1056/NEJMoa1305224]).
The improvements in BMD with romosozumab also were significantly greater than those with alendronate and teriparatide.
The onset of action for romosozumab was swift. "The increase in BMD at the lumbar spine and proximal femur was rapid and substantial with romosozumab by 3 months, and by 6 months, the increase was greater with the 210-mg monthly dose of romosozumab than with either active comparator," Dr. McClung and his associates said.
The increase in BMD was accompanied by a significant decrease in bone resorption. This dual action differs markedly from the effects of bisphosphonates and other agents, which reduce both bone formation and bone resorption, they noted.
Overall, romosozumab’s effects on bone formation were strong but transitory, diminishing after 6 months even though the participants continued taking the drug. In contrast, romosozumab’s effects on bone resorption were more moderate but more sustained, continuing throughout the study period.
The study population was too small to allow adequate assessment of the drug’s safety, the investigators noted.
The overall incidence of adverse events and of serious adverse events was similar among all the study groups, except that mild injection-site reactions were more common with romosozumab. No serious adverse events were considered to be related to any of the treatments, and none of the study subjects showed any notable changes in vital signs, laboratory values, or ECG factors.
This study was funded by Amgen and UCB Pharma, makers of romosozumab. Dr. McClung reported receiving fees and honoraria from Amgen, Eli Lilly, Merck, Novartis, and Warner Chilcott, and his associates reported ties to numerous industry sources.
Romosozumab, a monoclonal antibody that targets the osteoblast inhibitor sclerostin, increased bone mineral density and bone formation while decreasing bone resorption in a phase II clinical trial in 419 postmenopausal women with low bone mass, according to a report published online Jan. 13 in the New England Journal of Medicine.
"The consequence of these divergent effects on bone formation and bone resorption ... is a strongly positive balance in bone turnover, accounting for the rapid and large increases in bone mineral density that we observed," said Dr. Michael R. McClung of the Oregon Osteoporosis Center, Portland, and his associates.
After 1 year of periodic subcutaneous injections of romosozumab, BMD at the lumbar spine was significantly greater than it was with placebo injections, regardless of the dose frequency or dose level of the active drug. Total hip and femoral neck BMD also were significantly greater with romosozumab.
These improvements in BMD also were significantly greater than those obtained with two comparator drugs used open-label in this trial, alendronate and teriparatide, Dr. McClung and his colleagues noted.
Sclerostin is a glycoprotein secreted by osteocytes that is known to be a key regulator of bone formation, capable of impeding osteoblast proliferation and function. Expression of the gene that encodes sclerostin is confined to skeletal tissue, which suggests that a drug that targets sclerostin should have minimal effects on other tissues.
Patients who have a genetic deficiency of sclerostin have greater than average bone mass, with corresponding bone strength and resistance to fractures. And, in animal models of estrogen deficiency, treatment with antisclerostin antibodies restored bone mass and bone strength to higher than normal levels.
In a previous phase I study, single injections of the humanized monoclonal antisclerostin antibody increased BMD, stimulated bone formation, and decreased bone resorption. Dr. McClung and his associates now report the results of their phase II study assessing the efficacy and safety of a variety of doses of romosozumab in postmenopausal women aged 55-85 years who had low bone mass.
The study participants were treated and followed at 28 medical centers in Europe, five dosing regimens of romosozumab (70 mg, 140 mg, or 210 mg injected once monthly; or 140 mg or 210 mg injected once every 3 months); or to 70 mg oral alendronate weekly; or to 20 mcg teriparatide injected daily; or to placebo injections that mirrored the dosing schedules of romosozumab.
A total of 383 women (91%) completed the 1-year study; 86% were white, and the mean T scores were –2.29 at the lumbar spine, –1.53 at the total hip, and –1.93 at the femoral neck.
The primary endpoint was change in BMD at the lumbar spine at 1 year. Participants in the pooled romosozumab groups showed a significant increase in this measure, compared with those pooled in the placebo groups, regardless of dose frequency or dose level.
Similarly, each of the romosozumab groups showed a significant increase in this measure when compared with the pooled placebo groups. Women who received romosozumab also showed significantly greater increases in BMD at the total hip and the femoral neck, but not at the wrist.
The greatest improvements were noted among women who received the highest monthly dose of romosozumab (210 mg), who showed a mean increase of 11.3% at the lumbar spine, 4.1% at the total hip, and 3.7% at the femoral neck at 1 year, the investigators said (N. Engl. J. Med. 2014 [doi:10.1056/NEJMoa1305224]).
The improvements in BMD with romosozumab also were significantly greater than those with alendronate and teriparatide.
The onset of action for romosozumab was swift. "The increase in BMD at the lumbar spine and proximal femur was rapid and substantial with romosozumab by 3 months, and by 6 months, the increase was greater with the 210-mg monthly dose of romosozumab than with either active comparator," Dr. McClung and his associates said.
The increase in BMD was accompanied by a significant decrease in bone resorption. This dual action differs markedly from the effects of bisphosphonates and other agents, which reduce both bone formation and bone resorption, they noted.
Overall, romosozumab’s effects on bone formation were strong but transitory, diminishing after 6 months even though the participants continued taking the drug. In contrast, romosozumab’s effects on bone resorption were more moderate but more sustained, continuing throughout the study period.
The study population was too small to allow adequate assessment of the drug’s safety, the investigators noted.
The overall incidence of adverse events and of serious adverse events was similar among all the study groups, except that mild injection-site reactions were more common with romosozumab. No serious adverse events were considered to be related to any of the treatments, and none of the study subjects showed any notable changes in vital signs, laboratory values, or ECG factors.
This study was funded by Amgen and UCB Pharma, makers of romosozumab. Dr. McClung reported receiving fees and honoraria from Amgen, Eli Lilly, Merck, Novartis, and Warner Chilcott, and his associates reported ties to numerous industry sources.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Major finding: Women who received the highest monthly dose of romosozumab showed a mean increase in BMD of 11.3% at the lumbar spine, 4.1% at the total hip, and 3.7% at the femoral neck at 1 year.
Data source: An international randomized, controlled, phase II clinical trial comparing 1 year of romosozumab therapy against alendronate, teriparatide, and placebo in 419 postmenopausal women with low BMD.
Disclosures: This study was funded by Amgen and UCB Pharma, makers of romosozumab. Dr. McClung reported receiving fees and honoraria from Amgen, Eli Lilly, Merck, Novartis, and Warner Chilcott, and his associates reported ties to numerous industry sources.
Bisphosphonate safe, effective in IBD
Bisphosphonates are safe and effective for treating low bone mineral density in inflammatory bowel disease, according to a meta-analysis of 19 randomized controlled studies published in the January issue of Clinical Gastroenterology and Hepatology.
On the other hand, alternative therapies such as calcium plus vitamin D, calcitonin, and low-impact exercise demonstrated questionable efficacy, leading the authors to conclude that bisphosphonates alone "should be more aggressively considered" in this population.
Dr. John Melek of Mercy Hospital and Medical Center, Chicago, and Dr. Atsushi Sakuraba of the Inflammatory Bowel Disease Center at the University of Chicago Medicine, searched the MEDLINE and EMBASE databases as well as Google scholar, the UMIN Clinical Trials Registry, and the Cochrane Central Register for randomized controlled trials conducted between 1981 and 2011 assessing treatment for low BMD in IBD.
Overall, 11 of the 19 included studies evaluated bisphosphonates versus placebo or no treatment, while 4 looked at sodium fluoride versus placebo/no treatment, and 2 assessed calcium plus vitamin D versus placebo/no treatment.
The remaining analyses tested calcitonin versus placebo (1); low-impact exercise versus habitual physical activity (1); and bisphosphonates versus vitamin D (1) and fluoride (1). (Three studies compared multiple arms within the same study.)
Among all data on bisphosphonate efficacy, the authors found that the pooled overall effect by mixed-effect analysis revealed bisphosphonates to be significantly superior to control therapies in improving lumbar spine BMD, with a standard difference in means (SDm) of 0.51 (95% confidence interval [CI], 0.29-0.72; P less than .01).
Indeed, among the seven studies that reported improvements in the hip BMD, for example, a pooled overall effect by mixed-effect analysis showed that bisphosphonates were significantly superior to controls (both other treatments and no treatment; SDm, 0.26; 95% CI, 0.04-0.49; P = .02).
Moreover, among studies which reported the incidences of nonvertebral and vertebral fractures, the pooled ORs were 0.35 (95% CI, 0.06-1.95; P = .23) and 0.38 (95% CI, 0.15-0.96; P = .04), respectively.
Looking at adverse effects, meanwhile, the pooled odds ratio of adverse effects was a nonsignificant 1.24 (95% CI, 0.83-1.85; P = .29), "demonstrating that bisphosphonate treatment was not associated with an increased incidence of adverse effects."
Sodium fluoride, meanwhile, showed some efficacy: The four studies that assessed this treatment showed it was superior to placebo/no treatment in improving lumbar spine BMD (SDm, 1.18; 95% CI, 0.10-2.26; P = .03).
Fluoride did not, however, significantly improve hip BMD, compared with placebo, nor did it reduce the incidence of vertebral or nonvertebral fractures.
Similarly, the one study that looked at calcitonin (and assessed only children and adolescents with active or quiescent Crohn’s disease or ulcerative colitis, plus osteopenia or osteoporosis) found that this treatment was not superior to placebo in improving BMD at the lumbar spine. It did not find changes in hip BMD or the incidence of fractures.
Nor was low-impact exercise superior to control (habitual physical activity) in improving BMD at the hip or lumbar spine; fracture incidence was not assessed.
The authors conceded several limitations, the greatest of which was the presence of marked heterogeneity among studies, which necessitated use of the random-effects model or mixed-effect analysis. Indeed, much of this heterogeneity was due to the fact that some studies aimed to prevent bone loss, whereas others treated established osteopenia, they wrote.
Additionally, although many IBD patients are today treated with biologic therapies, few studies evaluated BMD regimens in IBD patients who underwent biologic treatment.
The authors disclosed no conflicts of interest related to this analysis. Dr. Sakuraba was supported by the Foreign Clinical Pharmacology Training Program of the Japanese Society of Clinical Pharmacology and Therapeutics.

|
| Dr. Stephen B. Hanauer |
My colleague, Atsushi Sakuraba, is the senior author of the meta-analysis evaluating the efficacy and safety of medical therapies to prevent or treat osteoporosis in a wide spectrum of inflammatory bowel disorder patients. Both ulcerative colitis and Crohn's disease have numerous risk factors for the development of osteoporosis and the younger ages of IBD patients create a longer duration of risk such that the AGA considers monitoring of bone mineral density and treatment with calcium and vitamin D in patients exposed to corticosteroids for greater than 3 months as important indicators of quality care. Active inflammation, malabsorption, vitamin D deficiency, and treatment with glucocorticoids all contribute to the risk of decreased bone density and fractures. Because of the heterogeneity of risk factors and many small, individual clinical trials, a meta-analytic approach was required to substantiate benefits in the diverse patient subpopulations (active vs. quiescent ulcerative colitis or Crohn's disease) and in patients taking glucocorticoids. The most significant finding is that bisphosphonates, whether oral or parenteral, were the singular therapeutic drug class that provided benefits across the disease states; calcium plus vitamin D alone in very small patient samples, fluoride, or calcitonin were not effective. It is reassuring that, even in the presence of IBD, these agents were well tolerated. Of course, while bisphosphonates may be the "mortar," adequate replacement of calcium and vitamin D, are the necessary "bricks" in the wall.
Dr. Stephen B. Hanauer is the Joseph B. Kirsner Professor of Medicine and Clinical Pharmacology, University of Chicago. He has no relevant conflicts of interest.

|
| Dr. Stephen B. Hanauer |
My colleague, Atsushi Sakuraba, is the senior author of the meta-analysis evaluating the efficacy and safety of medical therapies to prevent or treat osteoporosis in a wide spectrum of inflammatory bowel disorder patients. Both ulcerative colitis and Crohn's disease have numerous risk factors for the development of osteoporosis and the younger ages of IBD patients create a longer duration of risk such that the AGA considers monitoring of bone mineral density and treatment with calcium and vitamin D in patients exposed to corticosteroids for greater than 3 months as important indicators of quality care. Active inflammation, malabsorption, vitamin D deficiency, and treatment with glucocorticoids all contribute to the risk of decreased bone density and fractures. Because of the heterogeneity of risk factors and many small, individual clinical trials, a meta-analytic approach was required to substantiate benefits in the diverse patient subpopulations (active vs. quiescent ulcerative colitis or Crohn's disease) and in patients taking glucocorticoids. The most significant finding is that bisphosphonates, whether oral or parenteral, were the singular therapeutic drug class that provided benefits across the disease states; calcium plus vitamin D alone in very small patient samples, fluoride, or calcitonin were not effective. It is reassuring that, even in the presence of IBD, these agents were well tolerated. Of course, while bisphosphonates may be the "mortar," adequate replacement of calcium and vitamin D, are the necessary "bricks" in the wall.
Dr. Stephen B. Hanauer is the Joseph B. Kirsner Professor of Medicine and Clinical Pharmacology, University of Chicago. He has no relevant conflicts of interest.

|
| Dr. Stephen B. Hanauer |
My colleague, Atsushi Sakuraba, is the senior author of the meta-analysis evaluating the efficacy and safety of medical therapies to prevent or treat osteoporosis in a wide spectrum of inflammatory bowel disorder patients. Both ulcerative colitis and Crohn's disease have numerous risk factors for the development of osteoporosis and the younger ages of IBD patients create a longer duration of risk such that the AGA considers monitoring of bone mineral density and treatment with calcium and vitamin D in patients exposed to corticosteroids for greater than 3 months as important indicators of quality care. Active inflammation, malabsorption, vitamin D deficiency, and treatment with glucocorticoids all contribute to the risk of decreased bone density and fractures. Because of the heterogeneity of risk factors and many small, individual clinical trials, a meta-analytic approach was required to substantiate benefits in the diverse patient subpopulations (active vs. quiescent ulcerative colitis or Crohn's disease) and in patients taking glucocorticoids. The most significant finding is that bisphosphonates, whether oral or parenteral, were the singular therapeutic drug class that provided benefits across the disease states; calcium plus vitamin D alone in very small patient samples, fluoride, or calcitonin were not effective. It is reassuring that, even in the presence of IBD, these agents were well tolerated. Of course, while bisphosphonates may be the "mortar," adequate replacement of calcium and vitamin D, are the necessary "bricks" in the wall.
Dr. Stephen B. Hanauer is the Joseph B. Kirsner Professor of Medicine and Clinical Pharmacology, University of Chicago. He has no relevant conflicts of interest.
Bisphosphonates are safe and effective for treating low bone mineral density in inflammatory bowel disease, according to a meta-analysis of 19 randomized controlled studies published in the January issue of Clinical Gastroenterology and Hepatology.
On the other hand, alternative therapies such as calcium plus vitamin D, calcitonin, and low-impact exercise demonstrated questionable efficacy, leading the authors to conclude that bisphosphonates alone "should be more aggressively considered" in this population.
Dr. John Melek of Mercy Hospital and Medical Center, Chicago, and Dr. Atsushi Sakuraba of the Inflammatory Bowel Disease Center at the University of Chicago Medicine, searched the MEDLINE and EMBASE databases as well as Google scholar, the UMIN Clinical Trials Registry, and the Cochrane Central Register for randomized controlled trials conducted between 1981 and 2011 assessing treatment for low BMD in IBD.
Overall, 11 of the 19 included studies evaluated bisphosphonates versus placebo or no treatment, while 4 looked at sodium fluoride versus placebo/no treatment, and 2 assessed calcium plus vitamin D versus placebo/no treatment.
The remaining analyses tested calcitonin versus placebo (1); low-impact exercise versus habitual physical activity (1); and bisphosphonates versus vitamin D (1) and fluoride (1). (Three studies compared multiple arms within the same study.)
Among all data on bisphosphonate efficacy, the authors found that the pooled overall effect by mixed-effect analysis revealed bisphosphonates to be significantly superior to control therapies in improving lumbar spine BMD, with a standard difference in means (SDm) of 0.51 (95% confidence interval [CI], 0.29-0.72; P less than .01).
Indeed, among the seven studies that reported improvements in the hip BMD, for example, a pooled overall effect by mixed-effect analysis showed that bisphosphonates were significantly superior to controls (both other treatments and no treatment; SDm, 0.26; 95% CI, 0.04-0.49; P = .02).
Moreover, among studies which reported the incidences of nonvertebral and vertebral fractures, the pooled ORs were 0.35 (95% CI, 0.06-1.95; P = .23) and 0.38 (95% CI, 0.15-0.96; P = .04), respectively.
Looking at adverse effects, meanwhile, the pooled odds ratio of adverse effects was a nonsignificant 1.24 (95% CI, 0.83-1.85; P = .29), "demonstrating that bisphosphonate treatment was not associated with an increased incidence of adverse effects."
Sodium fluoride, meanwhile, showed some efficacy: The four studies that assessed this treatment showed it was superior to placebo/no treatment in improving lumbar spine BMD (SDm, 1.18; 95% CI, 0.10-2.26; P = .03).
Fluoride did not, however, significantly improve hip BMD, compared with placebo, nor did it reduce the incidence of vertebral or nonvertebral fractures.
Similarly, the one study that looked at calcitonin (and assessed only children and adolescents with active or quiescent Crohn’s disease or ulcerative colitis, plus osteopenia or osteoporosis) found that this treatment was not superior to placebo in improving BMD at the lumbar spine. It did not find changes in hip BMD or the incidence of fractures.
Nor was low-impact exercise superior to control (habitual physical activity) in improving BMD at the hip or lumbar spine; fracture incidence was not assessed.
The authors conceded several limitations, the greatest of which was the presence of marked heterogeneity among studies, which necessitated use of the random-effects model or mixed-effect analysis. Indeed, much of this heterogeneity was due to the fact that some studies aimed to prevent bone loss, whereas others treated established osteopenia, they wrote.
Additionally, although many IBD patients are today treated with biologic therapies, few studies evaluated BMD regimens in IBD patients who underwent biologic treatment.
The authors disclosed no conflicts of interest related to this analysis. Dr. Sakuraba was supported by the Foreign Clinical Pharmacology Training Program of the Japanese Society of Clinical Pharmacology and Therapeutics.
Bisphosphonates are safe and effective for treating low bone mineral density in inflammatory bowel disease, according to a meta-analysis of 19 randomized controlled studies published in the January issue of Clinical Gastroenterology and Hepatology.
On the other hand, alternative therapies such as calcium plus vitamin D, calcitonin, and low-impact exercise demonstrated questionable efficacy, leading the authors to conclude that bisphosphonates alone "should be more aggressively considered" in this population.
Dr. John Melek of Mercy Hospital and Medical Center, Chicago, and Dr. Atsushi Sakuraba of the Inflammatory Bowel Disease Center at the University of Chicago Medicine, searched the MEDLINE and EMBASE databases as well as Google scholar, the UMIN Clinical Trials Registry, and the Cochrane Central Register for randomized controlled trials conducted between 1981 and 2011 assessing treatment for low BMD in IBD.
Overall, 11 of the 19 included studies evaluated bisphosphonates versus placebo or no treatment, while 4 looked at sodium fluoride versus placebo/no treatment, and 2 assessed calcium plus vitamin D versus placebo/no treatment.
The remaining analyses tested calcitonin versus placebo (1); low-impact exercise versus habitual physical activity (1); and bisphosphonates versus vitamin D (1) and fluoride (1). (Three studies compared multiple arms within the same study.)
Among all data on bisphosphonate efficacy, the authors found that the pooled overall effect by mixed-effect analysis revealed bisphosphonates to be significantly superior to control therapies in improving lumbar spine BMD, with a standard difference in means (SDm) of 0.51 (95% confidence interval [CI], 0.29-0.72; P less than .01).
Indeed, among the seven studies that reported improvements in the hip BMD, for example, a pooled overall effect by mixed-effect analysis showed that bisphosphonates were significantly superior to controls (both other treatments and no treatment; SDm, 0.26; 95% CI, 0.04-0.49; P = .02).
Moreover, among studies which reported the incidences of nonvertebral and vertebral fractures, the pooled ORs were 0.35 (95% CI, 0.06-1.95; P = .23) and 0.38 (95% CI, 0.15-0.96; P = .04), respectively.
Looking at adverse effects, meanwhile, the pooled odds ratio of adverse effects was a nonsignificant 1.24 (95% CI, 0.83-1.85; P = .29), "demonstrating that bisphosphonate treatment was not associated with an increased incidence of adverse effects."
Sodium fluoride, meanwhile, showed some efficacy: The four studies that assessed this treatment showed it was superior to placebo/no treatment in improving lumbar spine BMD (SDm, 1.18; 95% CI, 0.10-2.26; P = .03).
Fluoride did not, however, significantly improve hip BMD, compared with placebo, nor did it reduce the incidence of vertebral or nonvertebral fractures.
Similarly, the one study that looked at calcitonin (and assessed only children and adolescents with active or quiescent Crohn’s disease or ulcerative colitis, plus osteopenia or osteoporosis) found that this treatment was not superior to placebo in improving BMD at the lumbar spine. It did not find changes in hip BMD or the incidence of fractures.
Nor was low-impact exercise superior to control (habitual physical activity) in improving BMD at the hip or lumbar spine; fracture incidence was not assessed.
The authors conceded several limitations, the greatest of which was the presence of marked heterogeneity among studies, which necessitated use of the random-effects model or mixed-effect analysis. Indeed, much of this heterogeneity was due to the fact that some studies aimed to prevent bone loss, whereas others treated established osteopenia, they wrote.
Additionally, although many IBD patients are today treated with biologic therapies, few studies evaluated BMD regimens in IBD patients who underwent biologic treatment.
The authors disclosed no conflicts of interest related to this analysis. Dr. Sakuraba was supported by the Foreign Clinical Pharmacology Training Program of the Japanese Society of Clinical Pharmacology and Therapeutics.
Major finding: Bisphosphonate treatment beat sodium fluoride, calcium plus vitamin D, and low-impact exercise in improving bone mineral density and reducing fractures.
Data source: A meta-analysis of 19 randomized controlled trials.
Disclosures: The authors disclosed no conflicts of interest related to this analysis. Dr. Sakuraba was supported by the Foreign Clinical Pharmacology Training Program of the Japanese Society of Clinical Pharmacology and Therapeutics.
No survival benefit to bisphosphonate in chemoresistant breast cancer
The bisphosphonate zolendronate didn't improve survival in patients with chemoresistant breast cancer, according to results from the phase III NATAN trial.
In an interview at the San Antonio Breast Cancer Symposium, Dr. Gunter von Minckwitz discusses the trial's results and clinical implications, and whether a role remains for bisphosphonates in postmenopausal patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The bisphosphonate zolendronate didn't improve survival in patients with chemoresistant breast cancer, according to results from the phase III NATAN trial.
In an interview at the San Antonio Breast Cancer Symposium, Dr. Gunter von Minckwitz discusses the trial's results and clinical implications, and whether a role remains for bisphosphonates in postmenopausal patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The bisphosphonate zolendronate didn't improve survival in patients with chemoresistant breast cancer, according to results from the phase III NATAN trial.
In an interview at the San Antonio Breast Cancer Symposium, Dr. Gunter von Minckwitz discusses the trial's results and clinical implications, and whether a role remains for bisphosphonates in postmenopausal patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Bone mineral density identifies fracture risk in women over 65
Bone mineral density testing is an effective means of identifying women aged 65 years and older who are at high risk of fracture, according to Jane A. Cauley, Dr.P.H., an epidemiologist at the University of Pittsburgh.
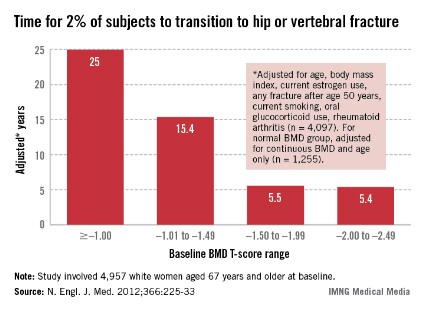
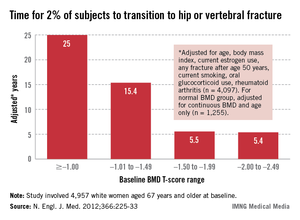
All women aged 65 and over should have a BMD test. In a presentation at the annual meeting of the North American Menopause Society, she related the findings of a 2012 study conducted to determine when the test should be repeated. In that study of 4,957 women aged 67 years and older at baseline who underwent baseline T-score testing, the mean interval until 2% of participants had a hip or clinical vertebral fracture was 25 years for women whose baseline bone mineral density (BMD) T-score was greater than or equal to –1.00, 15.4 years for those whose T-score range was –1.01 to –1.49, 5.5 years for those whose T-score range was –1.50 to –1.99, and 5.4 years for those whose T-score was –2.00 to –2.49 (N. Engl. J. Med. 2012;366:225-33).
Also, in women with BMD T-scores greater than –1.50, osteoporosis developed in less than 10% during 15 years of follow-up.
Based on these findings, Dr. Cauley suggested that an initial BMD test should be recommended for all women aged 65 years and older, and that physicians should reassess the testing interval based on the initial result. If the T-score declines, so should the length of the testing interval.
Bone mineral density testing is an effective means of identifying women aged 65 years and older who are at high risk of fracture, according to Jane A. Cauley, Dr.P.H., an epidemiologist at the University of Pittsburgh.
All women aged 65 and over should have a BMD test. In a presentation at the annual meeting of the North American Menopause Society, she related the findings of a 2012 study conducted to determine when the test should be repeated. In that study of 4,957 women aged 67 years and older at baseline who underwent baseline T-score testing, the mean interval until 2% of participants had a hip or clinical vertebral fracture was 25 years for women whose baseline bone mineral density (BMD) T-score was greater than or equal to –1.00, 15.4 years for those whose T-score range was –1.01 to –1.49, 5.5 years for those whose T-score range was –1.50 to –1.99, and 5.4 years for those whose T-score was –2.00 to –2.49 (N. Engl. J. Med. 2012;366:225-33).
Also, in women with BMD T-scores greater than –1.50, osteoporosis developed in less than 10% during 15 years of follow-up.
Based on these findings, Dr. Cauley suggested that an initial BMD test should be recommended for all women aged 65 years and older, and that physicians should reassess the testing interval based on the initial result. If the T-score declines, so should the length of the testing interval.

Bone mineral density testing is an effective means of identifying women aged 65 years and older who are at high risk of fracture, according to Jane A. Cauley, Dr.P.H., an epidemiologist at the University of Pittsburgh.
All women aged 65 and over should have a BMD test. In a presentation at the annual meeting of the North American Menopause Society, she related the findings of a 2012 study conducted to determine when the test should be repeated. In that study of 4,957 women aged 67 years and older at baseline who underwent baseline T-score testing, the mean interval until 2% of participants had a hip or clinical vertebral fracture was 25 years for women whose baseline bone mineral density (BMD) T-score was greater than or equal to –1.00, 15.4 years for those whose T-score range was –1.01 to –1.49, 5.5 years for those whose T-score range was –1.50 to –1.99, and 5.4 years for those whose T-score was –2.00 to –2.49 (N. Engl. J. Med. 2012;366:225-33).
Also, in women with BMD T-scores greater than –1.50, osteoporosis developed in less than 10% during 15 years of follow-up.
Based on these findings, Dr. Cauley suggested that an initial BMD test should be recommended for all women aged 65 years and older, and that physicians should reassess the testing interval based on the initial result. If the T-score declines, so should the length of the testing interval.

Endocrine societies release 'Choosing Wisely' recommendations
The Endocrine Society and the American Association of Clinical Endocrinologists have released a series of recommendations that advise against unnecessary tests and procedures in patient diagnosis and treatment.
More than 30 health organizations have released or will release treatment guidelines as part of the American Board of Internal Medicine Foundation’s "Choosing Wisely" campaign, which is meant to educate patients and physicians about unnecessary and potentially harmful testing and treatment. The recommendation lists, expected to be completed by early 2014, are part of a series that hopes to "spark conversations between patients and physicians about what care is really necessary for specific conditions."
The Endocrine Society and AACE recommended the following:
• Avoid routine multiple daily self-glucose monitoring in adults with stable type 2 diabetes on agents that do not cause hypoglycemia. Such testing can be excessive for patients who are doing a good job of maintaining glycemic control, and testing results can become quite predictable. There are a number of exceptions, such as when a patient is sick or losing weight, or when new medications are added or hemoglobin A1c values stray from targets.
• Don’t routinely measure 1,25-dihydroxyvitamin D unless the patient has hypercalcemia or decreased kidney function. In vitamin D deficiency, 1,25-dihydroxyvitamin D levels go up, not down. Serum levels of 1,25-dihyroxyvitamin D are regulated primarily by parathyroid hormone levels and have little connection to vitamin D stores. When trying to assess vitamin D stores or diagnose vitamin D deficiency (or toxicity), 25-hydroxyvitamin D is the correct test.
• Don’t routinely order a thyroid ultrasound in patients with abnormal thyroid function tests if there is no palpable abnormality of the thyroid gland. Nodules detected via ultrasound are usually unrelated to the abnormal thyroid function and may divert the clinical evaluation to assess the nodules, rather than the thyroid dysfunction.
• Don’t order a total or free T3 level when assessing levothyroxine (T4) dose in hypothyroid patients, as the blood level of total or free T3 may be misleading. With most patients, a normal TSH indicates a correct dose of T4.
• Don’t prescribe testosterone therapy unless there is strong biochemical evidence of testosterone deficiency. Many of the symptoms attributed to male hypogonadism are common in the normal male aging process or are the result of a comorbid condition. In addition, testosterone therapy is expensive and has the potential for serious side effects in some patients. Total testosterone levels should be taken in the morning, and a low level should be confirmed on a different day.
The Endocrine Society and the American Association of Clinical Endocrinologists have released a series of recommendations that advise against unnecessary tests and procedures in patient diagnosis and treatment.
More than 30 health organizations have released or will release treatment guidelines as part of the American Board of Internal Medicine Foundation’s "Choosing Wisely" campaign, which is meant to educate patients and physicians about unnecessary and potentially harmful testing and treatment. The recommendation lists, expected to be completed by early 2014, are part of a series that hopes to "spark conversations between patients and physicians about what care is really necessary for specific conditions."
The Endocrine Society and AACE recommended the following:
• Avoid routine multiple daily self-glucose monitoring in adults with stable type 2 diabetes on agents that do not cause hypoglycemia. Such testing can be excessive for patients who are doing a good job of maintaining glycemic control, and testing results can become quite predictable. There are a number of exceptions, such as when a patient is sick or losing weight, or when new medications are added or hemoglobin A1c values stray from targets.
• Don’t routinely measure 1,25-dihydroxyvitamin D unless the patient has hypercalcemia or decreased kidney function. In vitamin D deficiency, 1,25-dihydroxyvitamin D levels go up, not down. Serum levels of 1,25-dihyroxyvitamin D are regulated primarily by parathyroid hormone levels and have little connection to vitamin D stores. When trying to assess vitamin D stores or diagnose vitamin D deficiency (or toxicity), 25-hydroxyvitamin D is the correct test.
• Don’t routinely order a thyroid ultrasound in patients with abnormal thyroid function tests if there is no palpable abnormality of the thyroid gland. Nodules detected via ultrasound are usually unrelated to the abnormal thyroid function and may divert the clinical evaluation to assess the nodules, rather than the thyroid dysfunction.
• Don’t order a total or free T3 level when assessing levothyroxine (T4) dose in hypothyroid patients, as the blood level of total or free T3 may be misleading. With most patients, a normal TSH indicates a correct dose of T4.
• Don’t prescribe testosterone therapy unless there is strong biochemical evidence of testosterone deficiency. Many of the symptoms attributed to male hypogonadism are common in the normal male aging process or are the result of a comorbid condition. In addition, testosterone therapy is expensive and has the potential for serious side effects in some patients. Total testosterone levels should be taken in the morning, and a low level should be confirmed on a different day.
The Endocrine Society and the American Association of Clinical Endocrinologists have released a series of recommendations that advise against unnecessary tests and procedures in patient diagnosis and treatment.
More than 30 health organizations have released or will release treatment guidelines as part of the American Board of Internal Medicine Foundation’s "Choosing Wisely" campaign, which is meant to educate patients and physicians about unnecessary and potentially harmful testing and treatment. The recommendation lists, expected to be completed by early 2014, are part of a series that hopes to "spark conversations between patients and physicians about what care is really necessary for specific conditions."
The Endocrine Society and AACE recommended the following:
• Avoid routine multiple daily self-glucose monitoring in adults with stable type 2 diabetes on agents that do not cause hypoglycemia. Such testing can be excessive for patients who are doing a good job of maintaining glycemic control, and testing results can become quite predictable. There are a number of exceptions, such as when a patient is sick or losing weight, or when new medications are added or hemoglobin A1c values stray from targets.
• Don’t routinely measure 1,25-dihydroxyvitamin D unless the patient has hypercalcemia or decreased kidney function. In vitamin D deficiency, 1,25-dihydroxyvitamin D levels go up, not down. Serum levels of 1,25-dihyroxyvitamin D are regulated primarily by parathyroid hormone levels and have little connection to vitamin D stores. When trying to assess vitamin D stores or diagnose vitamin D deficiency (or toxicity), 25-hydroxyvitamin D is the correct test.
• Don’t routinely order a thyroid ultrasound in patients with abnormal thyroid function tests if there is no palpable abnormality of the thyroid gland. Nodules detected via ultrasound are usually unrelated to the abnormal thyroid function and may divert the clinical evaluation to assess the nodules, rather than the thyroid dysfunction.
• Don’t order a total or free T3 level when assessing levothyroxine (T4) dose in hypothyroid patients, as the blood level of total or free T3 may be misleading. With most patients, a normal TSH indicates a correct dose of T4.
• Don’t prescribe testosterone therapy unless there is strong biochemical evidence of testosterone deficiency. Many of the symptoms attributed to male hypogonadism are common in the normal male aging process or are the result of a comorbid condition. In addition, testosterone therapy is expensive and has the potential for serious side effects in some patients. Total testosterone levels should be taken in the morning, and a low level should be confirmed on a different day.
Follow-up bone mineral density didn’t sharpen fracture risk assessment
Performing a second bone mineral density measurement 4 years after an initial measurement was "of little value" in refining the estimation of fracture risk in osteoporosis, according to a report published online Sept. 24 in JAMA.
The finding calls into question the current practice of performing serial bone mineral density (BMD) tests at even shorter 2-year intervals to enhance fracture risk assessment, the study investigators noted.
In a secondary analysis of data from the Framingham Osteoporosis Study, BMD change during a 4-year interval "provided little additional information beyond baseline BMD for the clinical management of osteoporosis," said Dr. Sarah D. Berry of the Institute for Aging Research, Hebrew SeniorLife, Boston, and her associates (JAMA 2013;310:1256-62).
The appropriate interval between BMD assessments remains controversial, and studies of the issue have yielded mixed results.
Studies that reported a strong association between BMD loss during a short interval and subsequent fractures typically focused on the small subgroup of patients who had accelerated bone deterioration. Other studies primarily involving patients with low or normal bone loss have reported only a weak association between BMD loss and later fractures, the investigators said.
To determine whether repeat BMD testing is useful, Dr. Berry and her colleagues examined the rate of hip and major osteoporotic fracture among 310 men and 492 women in the prospective, population-based Framingham cohort.
The study patients underwent an initial BMD test of the femoral neck in 1987-1999, at a mean age of 74.8 years. None was receiving treatment for osteoporosis at that time. The patients then had at least one repeat BMD test a mean of 3.7 years later (range, 2.4-6.0 years later). The study participants then were followed for approximately 12 years or until they died.
During follow-up, 113 study patients (14.1%) had one or more major osteoporotic fractures. There were 88 hip, 24 spine, 5 shoulder, and 33 forearm fractures.
BMD loss during the interval between the first and second BMD assessments was associated with subsequent fracture. However, assessment of such loss provided little clinical value beyond that of the initial BMD test.
"The second BMD measure resulted in a small proportion of individuals [being] reclassified as [at] high risk of hip or major osteoporotic fracture," Dr. Berry and her associates said. But it remains unclear whether such a small number of reclassifications justifies the current practice of performing repeat BMD tests every 2 years, they added.
"We conclude that repeating a BMD test after 4 years would rarely change the clinical management of osteoporosis based on risk scores of hip fracture," the researchers explained. "Individuals with the greatest changes in risk scores were those who would have already been classified at high risk based on [the initial] BMD and [their] clinical characteristics."
Although some experts suggest that short rescreening intervals are warranted in high-risk patients, "we found no difference in the association between BMD change and fracture when stratified by sex, age, BMI, weight loss, T score, or fracture risk score," the study authors noted.
"Despite our findings, we recognize that detecting BMD loss would have been paramount for the small numbers of individuals reclassified by a second BMD test who went on to experience a fracture," Dr. Berry and her colleagues said. For those patients, a repeat screening test would allow physicians to give osteoporosis medications and reduce fracture risk, even among patients 75 years or older.
However, for the clear majority of patients who show normal or only mild bone loss at an initial screening, further study is needed to predict which patients are likely to transition to high risk of fracture and would therefore benefit from repeat BMD testing.
The study was limited in that almost all the patients were white. The usefulness of repeated BMD screening might be different in other racial and ethnic populations, the investigators said.
The National Institutes of Health, the National Heart, Lung, and Blood Institute, and the Friends of Hebrew SeniorLife supported the study. Dr. Berry reported no relevant financial conflicts of interest; one of her associates reported ties to Amgen, Ammonett Pharma, Eli Lilly, Hologic, Merck Sharpe & Dohme, Novartis, and Roche.
Performing a second bone mineral density measurement 4 years after an initial measurement was "of little value" in refining the estimation of fracture risk in osteoporosis, according to a report published online Sept. 24 in JAMA.
The finding calls into question the current practice of performing serial bone mineral density (BMD) tests at even shorter 2-year intervals to enhance fracture risk assessment, the study investigators noted.
In a secondary analysis of data from the Framingham Osteoporosis Study, BMD change during a 4-year interval "provided little additional information beyond baseline BMD for the clinical management of osteoporosis," said Dr. Sarah D. Berry of the Institute for Aging Research, Hebrew SeniorLife, Boston, and her associates (JAMA 2013;310:1256-62).
The appropriate interval between BMD assessments remains controversial, and studies of the issue have yielded mixed results.
Studies that reported a strong association between BMD loss during a short interval and subsequent fractures typically focused on the small subgroup of patients who had accelerated bone deterioration. Other studies primarily involving patients with low or normal bone loss have reported only a weak association between BMD loss and later fractures, the investigators said.
To determine whether repeat BMD testing is useful, Dr. Berry and her colleagues examined the rate of hip and major osteoporotic fracture among 310 men and 492 women in the prospective, population-based Framingham cohort.
The study patients underwent an initial BMD test of the femoral neck in 1987-1999, at a mean age of 74.8 years. None was receiving treatment for osteoporosis at that time. The patients then had at least one repeat BMD test a mean of 3.7 years later (range, 2.4-6.0 years later). The study participants then were followed for approximately 12 years or until they died.
During follow-up, 113 study patients (14.1%) had one or more major osteoporotic fractures. There were 88 hip, 24 spine, 5 shoulder, and 33 forearm fractures.
BMD loss during the interval between the first and second BMD assessments was associated with subsequent fracture. However, assessment of such loss provided little clinical value beyond that of the initial BMD test.
"The second BMD measure resulted in a small proportion of individuals [being] reclassified as [at] high risk of hip or major osteoporotic fracture," Dr. Berry and her associates said. But it remains unclear whether such a small number of reclassifications justifies the current practice of performing repeat BMD tests every 2 years, they added.
"We conclude that repeating a BMD test after 4 years would rarely change the clinical management of osteoporosis based on risk scores of hip fracture," the researchers explained. "Individuals with the greatest changes in risk scores were those who would have already been classified at high risk based on [the initial] BMD and [their] clinical characteristics."
Although some experts suggest that short rescreening intervals are warranted in high-risk patients, "we found no difference in the association between BMD change and fracture when stratified by sex, age, BMI, weight loss, T score, or fracture risk score," the study authors noted.
"Despite our findings, we recognize that detecting BMD loss would have been paramount for the small numbers of individuals reclassified by a second BMD test who went on to experience a fracture," Dr. Berry and her colleagues said. For those patients, a repeat screening test would allow physicians to give osteoporosis medications and reduce fracture risk, even among patients 75 years or older.
However, for the clear majority of patients who show normal or only mild bone loss at an initial screening, further study is needed to predict which patients are likely to transition to high risk of fracture and would therefore benefit from repeat BMD testing.
The study was limited in that almost all the patients were white. The usefulness of repeated BMD screening might be different in other racial and ethnic populations, the investigators said.
The National Institutes of Health, the National Heart, Lung, and Blood Institute, and the Friends of Hebrew SeniorLife supported the study. Dr. Berry reported no relevant financial conflicts of interest; one of her associates reported ties to Amgen, Ammonett Pharma, Eli Lilly, Hologic, Merck Sharpe & Dohme, Novartis, and Roche.
Performing a second bone mineral density measurement 4 years after an initial measurement was "of little value" in refining the estimation of fracture risk in osteoporosis, according to a report published online Sept. 24 in JAMA.
The finding calls into question the current practice of performing serial bone mineral density (BMD) tests at even shorter 2-year intervals to enhance fracture risk assessment, the study investigators noted.
In a secondary analysis of data from the Framingham Osteoporosis Study, BMD change during a 4-year interval "provided little additional information beyond baseline BMD for the clinical management of osteoporosis," said Dr. Sarah D. Berry of the Institute for Aging Research, Hebrew SeniorLife, Boston, and her associates (JAMA 2013;310:1256-62).
The appropriate interval between BMD assessments remains controversial, and studies of the issue have yielded mixed results.
Studies that reported a strong association between BMD loss during a short interval and subsequent fractures typically focused on the small subgroup of patients who had accelerated bone deterioration. Other studies primarily involving patients with low or normal bone loss have reported only a weak association between BMD loss and later fractures, the investigators said.
To determine whether repeat BMD testing is useful, Dr. Berry and her colleagues examined the rate of hip and major osteoporotic fracture among 310 men and 492 women in the prospective, population-based Framingham cohort.
The study patients underwent an initial BMD test of the femoral neck in 1987-1999, at a mean age of 74.8 years. None was receiving treatment for osteoporosis at that time. The patients then had at least one repeat BMD test a mean of 3.7 years later (range, 2.4-6.0 years later). The study participants then were followed for approximately 12 years or until they died.
During follow-up, 113 study patients (14.1%) had one or more major osteoporotic fractures. There were 88 hip, 24 spine, 5 shoulder, and 33 forearm fractures.
BMD loss during the interval between the first and second BMD assessments was associated with subsequent fracture. However, assessment of such loss provided little clinical value beyond that of the initial BMD test.
"The second BMD measure resulted in a small proportion of individuals [being] reclassified as [at] high risk of hip or major osteoporotic fracture," Dr. Berry and her associates said. But it remains unclear whether such a small number of reclassifications justifies the current practice of performing repeat BMD tests every 2 years, they added.
"We conclude that repeating a BMD test after 4 years would rarely change the clinical management of osteoporosis based on risk scores of hip fracture," the researchers explained. "Individuals with the greatest changes in risk scores were those who would have already been classified at high risk based on [the initial] BMD and [their] clinical characteristics."
Although some experts suggest that short rescreening intervals are warranted in high-risk patients, "we found no difference in the association between BMD change and fracture when stratified by sex, age, BMI, weight loss, T score, or fracture risk score," the study authors noted.
"Despite our findings, we recognize that detecting BMD loss would have been paramount for the small numbers of individuals reclassified by a second BMD test who went on to experience a fracture," Dr. Berry and her colleagues said. For those patients, a repeat screening test would allow physicians to give osteoporosis medications and reduce fracture risk, even among patients 75 years or older.
However, for the clear majority of patients who show normal or only mild bone loss at an initial screening, further study is needed to predict which patients are likely to transition to high risk of fracture and would therefore benefit from repeat BMD testing.
The study was limited in that almost all the patients were white. The usefulness of repeated BMD screening might be different in other racial and ethnic populations, the investigators said.
The National Institutes of Health, the National Heart, Lung, and Blood Institute, and the Friends of Hebrew SeniorLife supported the study. Dr. Berry reported no relevant financial conflicts of interest; one of her associates reported ties to Amgen, Ammonett Pharma, Eli Lilly, Hologic, Merck Sharpe & Dohme, Novartis, and Roche.
FROM JAMA
Major finding: A second bone mineral density test 4 years after an initial test was not useful in refining the prediction of major fractures and didn't change the clinical management of osteoporosis.
Data source: A secondary analysis of data from the prospective, population-based Framingham Osteoporosis Study involving 802 older men and women who underwent serial BMD testing and were followed for 12 years.
Disclosures: The National Institutes of Health, the National Heart, Lung, and Blood Institute, and the Friends of Hebrew SeniorLife supported the study. Dr. Berry reported no relevant financial conflicts of interest; one of her associates reported ties to Amgen, Ammonett Pharma, Eli Lilly, Hologic, Merck Sharpe & Dohme, Novartis, and Roche.
Switching nonadherent to denosumab improved bone density
SAN FRANCISCO – Switching to denosumab boosted bone mineral density more than did switching to ibandronate or risedronate in high-risk osteoporosis patients who were not adherent to oral bisphosphonate therapy, an assessment of data from 1,576 patients in two studies found.
The open-label studies included postmenopausal women who previously had been prescribed oral bisphosphonate therapy for osteoporosis but had either stopped the drug or were insufficiently adherent to therapy, by a score of less than six on the Osteoporosis-Specific Morisky Medication Adherence Scale.
One study randomized patients to denosumab (Prolia) 60 mg subcutaneously every 6 months or ibandronate (Boniva) 150 mg orally every month.
The other study randomized patients to the same denosumab regimen or risedronate (Actonel) 150 mg orally per month, taken as a 75-mg tablet on each of two consecutive days.
BMD measurements on 1,576 women in the studies showed significantly greater increases at 12 months at the hip, femoral neck, and spine in patients receiving denosumab, compared with either of the oral bisphosphonates, Dr. Christopher Recknor and his associates reported at the annual meeting of the Endocrine Society.
Bone density in patients on denosumab increased at the hip by about 2.3% in one study and 2% in the other, increased at the femoral neck by roughly 1.7% in one study and 1.5% in the other, and increased at the spine by 4% in one study and 3.5% in the other. In comparison, BMDs in patients on ibandronate increased by roughly 1.1% at the hip, 0.7% at the femoral neck, and 2% at the spine. BMDs in patients on risedronate increased by about 0.4% at the hip and 1% at the spine but decreased by 0.1% at the femoral neck.
The changes in bone density did not differ significantly between the 33% of patients who had had a prior fragility fracture and patients with no history of fragility fracture, with one exception: The gains in femoral neck BMD from denosumab compared with ibandronate were significantly larger in patients with a prior fracture, compared with those with no prior fracture, reported Dr. Recknor, medical director of the United Osteoporosis Centers, Gainesville, Ga.
In patients with a prior fragility fracture, femoral neck BMD increased by about 2.2% on denosumab and by 0.1% on ibandronate, compared with gains in patients with no prior fracture of 1.5% on denosumab and 1% on ibandronate.
The study defined fragility fractures as those not involving the skull, facial bones, fingers, and toes and not associated with severe trauma or pathological fractures.
In the lower-risk patients without a prior fracture, "that is where Boniva does not seem to work very well" in comparison with switching to another oral bisphosphonate, Dr. Recknor said in an interview. For high-risk patients with a prior fracture, however, "I’m even more convinced that if I’ve got somebody who’s at high risk and they’re noncompliant" with bisphosphonate therapy, "giving Prolia would be the way to go."
Even if the bisphosphonate regimen is switched to monthly dosing to try to improve adherence, "the bone density increases are just not going to be that great," he said.
BMDs at baseline did not differ significantly between treatment groups or between patients with or without a prior fragility fracture.
Infections, though not serious ones, were reported in 24% of patients on denosumab in each study and in 19% of patients on ibandronate and 21% of patients on risedronate. There were no cases of atypical femoral fracture, delayed fracture healing, or osteonecrosis of the jaw.
The study was funded by Amgen, which markets denosumab, and by GlaxoSmithKline. Dr. Recknor disclosed ties with Amgen, Novartis Pharmaceuticals, Ion Med Systems, Eli Lilly, Merck, and UCB. Some coinvestigators were employees of Amgen and others reported financial ties with these companies and/or Warner Chilcott, which markets risedronate; Roche Pharmaceuticals, which markets ibandronate through Genentech; Tarsa, Wyeth, Takeda, Nycomed, Servier, and Pfizer.
On Twitter @sherryboschert
SAN FRANCISCO – Switching to denosumab boosted bone mineral density more than did switching to ibandronate or risedronate in high-risk osteoporosis patients who were not adherent to oral bisphosphonate therapy, an assessment of data from 1,576 patients in two studies found.
The open-label studies included postmenopausal women who previously had been prescribed oral bisphosphonate therapy for osteoporosis but had either stopped the drug or were insufficiently adherent to therapy, by a score of less than six on the Osteoporosis-Specific Morisky Medication Adherence Scale.
One study randomized patients to denosumab (Prolia) 60 mg subcutaneously every 6 months or ibandronate (Boniva) 150 mg orally every month.
The other study randomized patients to the same denosumab regimen or risedronate (Actonel) 150 mg orally per month, taken as a 75-mg tablet on each of two consecutive days.
BMD measurements on 1,576 women in the studies showed significantly greater increases at 12 months at the hip, femoral neck, and spine in patients receiving denosumab, compared with either of the oral bisphosphonates, Dr. Christopher Recknor and his associates reported at the annual meeting of the Endocrine Society.
Bone density in patients on denosumab increased at the hip by about 2.3% in one study and 2% in the other, increased at the femoral neck by roughly 1.7% in one study and 1.5% in the other, and increased at the spine by 4% in one study and 3.5% in the other. In comparison, BMDs in patients on ibandronate increased by roughly 1.1% at the hip, 0.7% at the femoral neck, and 2% at the spine. BMDs in patients on risedronate increased by about 0.4% at the hip and 1% at the spine but decreased by 0.1% at the femoral neck.
The changes in bone density did not differ significantly between the 33% of patients who had had a prior fragility fracture and patients with no history of fragility fracture, with one exception: The gains in femoral neck BMD from denosumab compared with ibandronate were significantly larger in patients with a prior fracture, compared with those with no prior fracture, reported Dr. Recknor, medical director of the United Osteoporosis Centers, Gainesville, Ga.
In patients with a prior fragility fracture, femoral neck BMD increased by about 2.2% on denosumab and by 0.1% on ibandronate, compared with gains in patients with no prior fracture of 1.5% on denosumab and 1% on ibandronate.
The study defined fragility fractures as those not involving the skull, facial bones, fingers, and toes and not associated with severe trauma or pathological fractures.
In the lower-risk patients without a prior fracture, "that is where Boniva does not seem to work very well" in comparison with switching to another oral bisphosphonate, Dr. Recknor said in an interview. For high-risk patients with a prior fracture, however, "I’m even more convinced that if I’ve got somebody who’s at high risk and they’re noncompliant" with bisphosphonate therapy, "giving Prolia would be the way to go."
Even if the bisphosphonate regimen is switched to monthly dosing to try to improve adherence, "the bone density increases are just not going to be that great," he said.
BMDs at baseline did not differ significantly between treatment groups or between patients with or without a prior fragility fracture.
Infections, though not serious ones, were reported in 24% of patients on denosumab in each study and in 19% of patients on ibandronate and 21% of patients on risedronate. There were no cases of atypical femoral fracture, delayed fracture healing, or osteonecrosis of the jaw.
The study was funded by Amgen, which markets denosumab, and by GlaxoSmithKline. Dr. Recknor disclosed ties with Amgen, Novartis Pharmaceuticals, Ion Med Systems, Eli Lilly, Merck, and UCB. Some coinvestigators were employees of Amgen and others reported financial ties with these companies and/or Warner Chilcott, which markets risedronate; Roche Pharmaceuticals, which markets ibandronate through Genentech; Tarsa, Wyeth, Takeda, Nycomed, Servier, and Pfizer.
On Twitter @sherryboschert
SAN FRANCISCO – Switching to denosumab boosted bone mineral density more than did switching to ibandronate or risedronate in high-risk osteoporosis patients who were not adherent to oral bisphosphonate therapy, an assessment of data from 1,576 patients in two studies found.
The open-label studies included postmenopausal women who previously had been prescribed oral bisphosphonate therapy for osteoporosis but had either stopped the drug or were insufficiently adherent to therapy, by a score of less than six on the Osteoporosis-Specific Morisky Medication Adherence Scale.
One study randomized patients to denosumab (Prolia) 60 mg subcutaneously every 6 months or ibandronate (Boniva) 150 mg orally every month.
The other study randomized patients to the same denosumab regimen or risedronate (Actonel) 150 mg orally per month, taken as a 75-mg tablet on each of two consecutive days.
BMD measurements on 1,576 women in the studies showed significantly greater increases at 12 months at the hip, femoral neck, and spine in patients receiving denosumab, compared with either of the oral bisphosphonates, Dr. Christopher Recknor and his associates reported at the annual meeting of the Endocrine Society.
Bone density in patients on denosumab increased at the hip by about 2.3% in one study and 2% in the other, increased at the femoral neck by roughly 1.7% in one study and 1.5% in the other, and increased at the spine by 4% in one study and 3.5% in the other. In comparison, BMDs in patients on ibandronate increased by roughly 1.1% at the hip, 0.7% at the femoral neck, and 2% at the spine. BMDs in patients on risedronate increased by about 0.4% at the hip and 1% at the spine but decreased by 0.1% at the femoral neck.
The changes in bone density did not differ significantly between the 33% of patients who had had a prior fragility fracture and patients with no history of fragility fracture, with one exception: The gains in femoral neck BMD from denosumab compared with ibandronate were significantly larger in patients with a prior fracture, compared with those with no prior fracture, reported Dr. Recknor, medical director of the United Osteoporosis Centers, Gainesville, Ga.
In patients with a prior fragility fracture, femoral neck BMD increased by about 2.2% on denosumab and by 0.1% on ibandronate, compared with gains in patients with no prior fracture of 1.5% on denosumab and 1% on ibandronate.
The study defined fragility fractures as those not involving the skull, facial bones, fingers, and toes and not associated with severe trauma or pathological fractures.
In the lower-risk patients without a prior fracture, "that is where Boniva does not seem to work very well" in comparison with switching to another oral bisphosphonate, Dr. Recknor said in an interview. For high-risk patients with a prior fracture, however, "I’m even more convinced that if I’ve got somebody who’s at high risk and they’re noncompliant" with bisphosphonate therapy, "giving Prolia would be the way to go."
Even if the bisphosphonate regimen is switched to monthly dosing to try to improve adherence, "the bone density increases are just not going to be that great," he said.
BMDs at baseline did not differ significantly between treatment groups or between patients with or without a prior fragility fracture.
Infections, though not serious ones, were reported in 24% of patients on denosumab in each study and in 19% of patients on ibandronate and 21% of patients on risedronate. There were no cases of atypical femoral fracture, delayed fracture healing, or osteonecrosis of the jaw.
The study was funded by Amgen, which markets denosumab, and by GlaxoSmithKline. Dr. Recknor disclosed ties with Amgen, Novartis Pharmaceuticals, Ion Med Systems, Eli Lilly, Merck, and UCB. Some coinvestigators were employees of Amgen and others reported financial ties with these companies and/or Warner Chilcott, which markets risedronate; Roche Pharmaceuticals, which markets ibandronate through Genentech; Tarsa, Wyeth, Takeda, Nycomed, Servier, and Pfizer.
On Twitter @sherryboschert
AT ENDO 2013
Major finding: Bone density increased at 1 year by 2.3% and 2% in the hip, 1.7% and 1.5% at the femoral neck, and 4% and 3.5% at the spine on denosumab compared with 1.1% at the hip, 0.7% at the femoral neck, and 2% at the spine on ibandronate and 0.4% at the hip and 0.4% at the spine with slightly decreased density at the femoral neck on risedronate.
Data source: Secondary analysis of data from two open-label, randomized studies of 1,576 postmenopausal women who had been insufficiently adherent to oral bisphosphonate therapy.
Disclosures: The study was funded by Amgen, which markets denosumab, and by GlaxoSmithKline. Dr. Recknor disclosed ties with Amgen, Novartis Pharmaceuticals, Ion Med Systems, Eli Lilly, Merck, and UCB. Some coinvestigators were employees of Amgen and others reported financial ties with these companies and/or Warner Chilcott, which markets risedronate; Roche Pharmaceuticals, which markets ibandronate through Genentech; Tarsa, Wyeth, Takeda, Nycomed, Servier, and Pfizer.
Vitamin D deficiency in elderly linked to functional limitations
Older adults with vitamin D deficiency are more likely than those with adequate vitamin D levels to have functional limitations, findings from the Longitudinal Aging Study Amsterdam suggest.
These limitations became apparent within 3 years in a cohort aged 65-88 years, compared with 6 years in one aged 55-65 years.
In a cohort of 1,237 adults who were aged 65-88 years at baseline, 56% had at least one functional limitation, and in a second cohort of 725 adults aged 55-65 years at baseline, 30% had at least 1 functional limitation. Those in the older cohort who had vitamin D deficiency, defined as a 25-hydroxyvitamin D (25[OH]D) level of less than 20 ng/mL, had a nearly twofold increased risk of having a functional limitation, compared with those who had a 25(OH)D level greater than 30 ng/mL (odds ratio, 1.7), and those in the younger cohort who had vitamin D deficiency had more than a twofold increased risk (OR, 2.1), according to Dr. Evelien Sohl and her colleagues from the VU University Medical Center, Amsterdam.
Vitamin D status also was associated with the number of functional limitations cross-sectionally, the investigators reported online July 17 in the Journal of Clinical Endocrinology and Metabolism.
"In the fully adjusted models, participants in the older cohort with serum 25(OH)D levels of less than 20 ng/mL had a 1.6 times higher odds for having 1 more functional limitation than participants in the reference category. In the younger cohort, this odds ratio was 1.9," they said (J. Clin. Endocrinol. Metab. 2013 July 17 [doi: 10.1210/jc.2013-1698]).
The investigators also found that vitamin D deficiency was associated with an increase of two or more additional limitations at 3 years in the older cohort (OR, 2.0), but not at 6 years, while the association between vitamin D deficiency and an increase of two or more limitations did not reach significance until after 6 years in the younger cohort (OR, 3.3).
"Age and sex did not significantly modify the associations between serum 25(OH)D and functional status. Relevant confounders were age, sex, body mass index, number of chronic diseases, level of education, and level of urbanization," they said.
Specific functional limitations associated with vitamin D status in the older cohort included walking stairs (OR, 1.8), cutting toenails (OR, 1.5), and walking outside (OR, 2.1). Statistical power was insufficient for analyzing functional limitations in the younger cohort separately because of the low number of limitations in that group.
The Longitudinal Aging Study Amsterdam is an ongoing prospective cohort study of the older Dutch population. Functional limitations were assessed by an interviewer-administered questionnaire. Participants were asked about their ability and degree of difficulty with respect to walking up and down a staircase of 15 steps without resting; dressing and undressing oneself; sitting down and standing up from a chair; cutting one’s toenails; walking outside for 5 minutes without resting; and using one’s own or public transportation. Limitations were defined as any difficulty with performing a specific activity.
Although research on the association between vitamin D status and functional limitations is "scarce and not conclusive," according to the investigators, the findings "are in line with the results of most studies on vitamin D status and physical performance."
"Because functional limitations are a predictor of adverse outcomes, further research is necessary to explore underlying mechanisms and the potential benefits of vitamin D supplements on functional status," they concluded.
The study authors reported having no disclosures.
The findings by Dr. Sohl and colleagues provide "one more piece of evidence that vitamin D deficiency has adverse consequences, especially for an aging population whose reserves are dwindling," said Dr. Robert P. Heaney.
The findings extend upon those from prior studies, which, taken together, leave little doubt that vitamin D status and functional ability have a dose-dependent relationship, and that improving vitamin D levels in those with vitamin D deficiency would be of benefit, he said in an interview.
"The only real question is not if it works, but what level is necessary to achieve the desired effect," he said, noting the importance of striving for ancestral intake levels. In the Dutch population in this study, the ancestral level – the level that preagricultural ancestors obtained from the environment (and a good indicator of the level that would be optimal today) – was probably in the 50-ng/mL range, which is much higher than the 30-ng/mL level considered adequate by the investigators.
During his participation a few years ago on a panel convened by the American Geriatric Society and the Centers for Disease Control and Prevention to develop guidelines for supplementation in the elderly, Dr. Heaney polled the nine scientists on the panel about their own vitamin D supplementation and found that on average, their intake was about 5,500 IU/day, which is sufficient to support ancestral intake, he said.
Existing data support that level of intake, and those panelists felt the evidence was quite strong, he noted, adding: "I think it’s useful for clinicians to know that’s where the working scientific community is on this topic."
The recommendations developed by that panel are pending publication, he noted.
Dr. Heaney, a clinical endocrinologist, holds the John A. Creighton University Professorship at Creighton University, Omaha, Neb. He reported having no disclosures.
The findings by Dr. Sohl and colleagues provide "one more piece of evidence that vitamin D deficiency has adverse consequences, especially for an aging population whose reserves are dwindling," said Dr. Robert P. Heaney.
The findings extend upon those from prior studies, which, taken together, leave little doubt that vitamin D status and functional ability have a dose-dependent relationship, and that improving vitamin D levels in those with vitamin D deficiency would be of benefit, he said in an interview.
"The only real question is not if it works, but what level is necessary to achieve the desired effect," he said, noting the importance of striving for ancestral intake levels. In the Dutch population in this study, the ancestral level – the level that preagricultural ancestors obtained from the environment (and a good indicator of the level that would be optimal today) – was probably in the 50-ng/mL range, which is much higher than the 30-ng/mL level considered adequate by the investigators.
During his participation a few years ago on a panel convened by the American Geriatric Society and the Centers for Disease Control and Prevention to develop guidelines for supplementation in the elderly, Dr. Heaney polled the nine scientists on the panel about their own vitamin D supplementation and found that on average, their intake was about 5,500 IU/day, which is sufficient to support ancestral intake, he said.
Existing data support that level of intake, and those panelists felt the evidence was quite strong, he noted, adding: "I think it’s useful for clinicians to know that’s where the working scientific community is on this topic."
The recommendations developed by that panel are pending publication, he noted.
Dr. Heaney, a clinical endocrinologist, holds the John A. Creighton University Professorship at Creighton University, Omaha, Neb. He reported having no disclosures.
The findings by Dr. Sohl and colleagues provide "one more piece of evidence that vitamin D deficiency has adverse consequences, especially for an aging population whose reserves are dwindling," said Dr. Robert P. Heaney.
The findings extend upon those from prior studies, which, taken together, leave little doubt that vitamin D status and functional ability have a dose-dependent relationship, and that improving vitamin D levels in those with vitamin D deficiency would be of benefit, he said in an interview.
"The only real question is not if it works, but what level is necessary to achieve the desired effect," he said, noting the importance of striving for ancestral intake levels. In the Dutch population in this study, the ancestral level – the level that preagricultural ancestors obtained from the environment (and a good indicator of the level that would be optimal today) – was probably in the 50-ng/mL range, which is much higher than the 30-ng/mL level considered adequate by the investigators.
During his participation a few years ago on a panel convened by the American Geriatric Society and the Centers for Disease Control and Prevention to develop guidelines for supplementation in the elderly, Dr. Heaney polled the nine scientists on the panel about their own vitamin D supplementation and found that on average, their intake was about 5,500 IU/day, which is sufficient to support ancestral intake, he said.
Existing data support that level of intake, and those panelists felt the evidence was quite strong, he noted, adding: "I think it’s useful for clinicians to know that’s where the working scientific community is on this topic."
The recommendations developed by that panel are pending publication, he noted.
Dr. Heaney, a clinical endocrinologist, holds the John A. Creighton University Professorship at Creighton University, Omaha, Neb. He reported having no disclosures.
Older adults with vitamin D deficiency are more likely than those with adequate vitamin D levels to have functional limitations, findings from the Longitudinal Aging Study Amsterdam suggest.
These limitations became apparent within 3 years in a cohort aged 65-88 years, compared with 6 years in one aged 55-65 years.
In a cohort of 1,237 adults who were aged 65-88 years at baseline, 56% had at least one functional limitation, and in a second cohort of 725 adults aged 55-65 years at baseline, 30% had at least 1 functional limitation. Those in the older cohort who had vitamin D deficiency, defined as a 25-hydroxyvitamin D (25[OH]D) level of less than 20 ng/mL, had a nearly twofold increased risk of having a functional limitation, compared with those who had a 25(OH)D level greater than 30 ng/mL (odds ratio, 1.7), and those in the younger cohort who had vitamin D deficiency had more than a twofold increased risk (OR, 2.1), according to Dr. Evelien Sohl and her colleagues from the VU University Medical Center, Amsterdam.
Vitamin D status also was associated with the number of functional limitations cross-sectionally, the investigators reported online July 17 in the Journal of Clinical Endocrinology and Metabolism.
"In the fully adjusted models, participants in the older cohort with serum 25(OH)D levels of less than 20 ng/mL had a 1.6 times higher odds for having 1 more functional limitation than participants in the reference category. In the younger cohort, this odds ratio was 1.9," they said (J. Clin. Endocrinol. Metab. 2013 July 17 [doi: 10.1210/jc.2013-1698]).
The investigators also found that vitamin D deficiency was associated with an increase of two or more additional limitations at 3 years in the older cohort (OR, 2.0), but not at 6 years, while the association between vitamin D deficiency and an increase of two or more limitations did not reach significance until after 6 years in the younger cohort (OR, 3.3).
"Age and sex did not significantly modify the associations between serum 25(OH)D and functional status. Relevant confounders were age, sex, body mass index, number of chronic diseases, level of education, and level of urbanization," they said.
Specific functional limitations associated with vitamin D status in the older cohort included walking stairs (OR, 1.8), cutting toenails (OR, 1.5), and walking outside (OR, 2.1). Statistical power was insufficient for analyzing functional limitations in the younger cohort separately because of the low number of limitations in that group.
The Longitudinal Aging Study Amsterdam is an ongoing prospective cohort study of the older Dutch population. Functional limitations were assessed by an interviewer-administered questionnaire. Participants were asked about their ability and degree of difficulty with respect to walking up and down a staircase of 15 steps without resting; dressing and undressing oneself; sitting down and standing up from a chair; cutting one’s toenails; walking outside for 5 minutes without resting; and using one’s own or public transportation. Limitations were defined as any difficulty with performing a specific activity.
Although research on the association between vitamin D status and functional limitations is "scarce and not conclusive," according to the investigators, the findings "are in line with the results of most studies on vitamin D status and physical performance."
"Because functional limitations are a predictor of adverse outcomes, further research is necessary to explore underlying mechanisms and the potential benefits of vitamin D supplements on functional status," they concluded.
The study authors reported having no disclosures.
Older adults with vitamin D deficiency are more likely than those with adequate vitamin D levels to have functional limitations, findings from the Longitudinal Aging Study Amsterdam suggest.
These limitations became apparent within 3 years in a cohort aged 65-88 years, compared with 6 years in one aged 55-65 years.
In a cohort of 1,237 adults who were aged 65-88 years at baseline, 56% had at least one functional limitation, and in a second cohort of 725 adults aged 55-65 years at baseline, 30% had at least 1 functional limitation. Those in the older cohort who had vitamin D deficiency, defined as a 25-hydroxyvitamin D (25[OH]D) level of less than 20 ng/mL, had a nearly twofold increased risk of having a functional limitation, compared with those who had a 25(OH)D level greater than 30 ng/mL (odds ratio, 1.7), and those in the younger cohort who had vitamin D deficiency had more than a twofold increased risk (OR, 2.1), according to Dr. Evelien Sohl and her colleagues from the VU University Medical Center, Amsterdam.
Vitamin D status also was associated with the number of functional limitations cross-sectionally, the investigators reported online July 17 in the Journal of Clinical Endocrinology and Metabolism.
"In the fully adjusted models, participants in the older cohort with serum 25(OH)D levels of less than 20 ng/mL had a 1.6 times higher odds for having 1 more functional limitation than participants in the reference category. In the younger cohort, this odds ratio was 1.9," they said (J. Clin. Endocrinol. Metab. 2013 July 17 [doi: 10.1210/jc.2013-1698]).
The investigators also found that vitamin D deficiency was associated with an increase of two or more additional limitations at 3 years in the older cohort (OR, 2.0), but not at 6 years, while the association between vitamin D deficiency and an increase of two or more limitations did not reach significance until after 6 years in the younger cohort (OR, 3.3).
"Age and sex did not significantly modify the associations between serum 25(OH)D and functional status. Relevant confounders were age, sex, body mass index, number of chronic diseases, level of education, and level of urbanization," they said.
Specific functional limitations associated with vitamin D status in the older cohort included walking stairs (OR, 1.8), cutting toenails (OR, 1.5), and walking outside (OR, 2.1). Statistical power was insufficient for analyzing functional limitations in the younger cohort separately because of the low number of limitations in that group.
The Longitudinal Aging Study Amsterdam is an ongoing prospective cohort study of the older Dutch population. Functional limitations were assessed by an interviewer-administered questionnaire. Participants were asked about their ability and degree of difficulty with respect to walking up and down a staircase of 15 steps without resting; dressing and undressing oneself; sitting down and standing up from a chair; cutting one’s toenails; walking outside for 5 minutes without resting; and using one’s own or public transportation. Limitations were defined as any difficulty with performing a specific activity.
Although research on the association between vitamin D status and functional limitations is "scarce and not conclusive," according to the investigators, the findings "are in line with the results of most studies on vitamin D status and physical performance."
"Because functional limitations are a predictor of adverse outcomes, further research is necessary to explore underlying mechanisms and the potential benefits of vitamin D supplements on functional status," they concluded.
The study authors reported having no disclosures.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY AND METABOLISM
Major finding: Older adults with vitamin D deficiency had about a twofold increase in the risk of functional limitations.
Data source: Two cohorts from the prospective Longitudinal Aging Study Amsterdam, including a total of 1,962 older adults.
Disclosures: The authors reported having no disclosures.