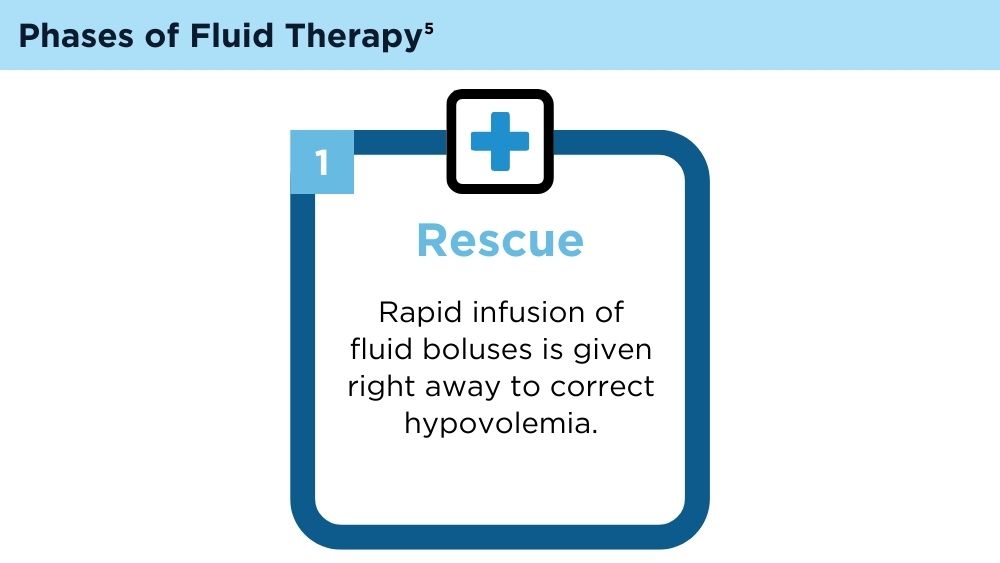
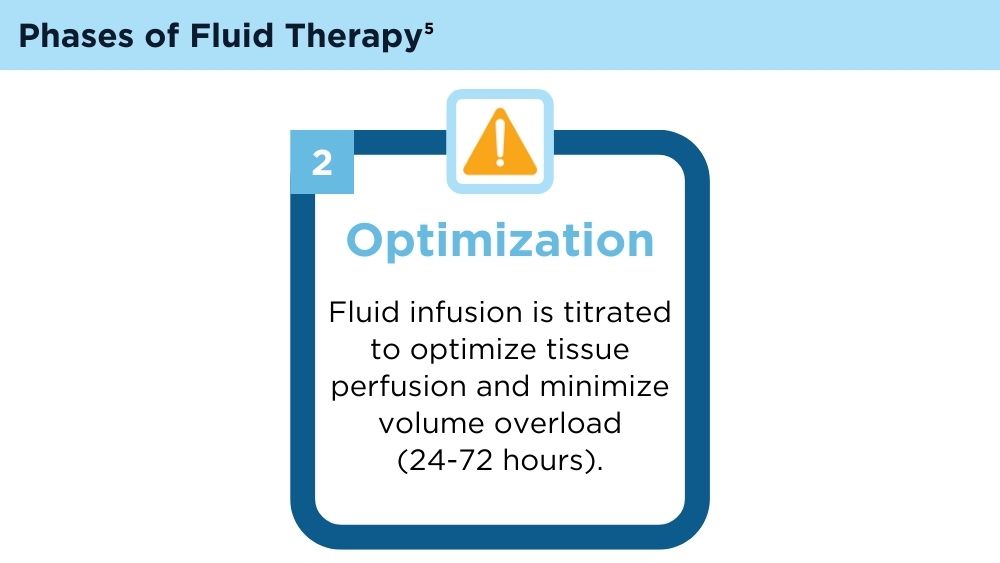
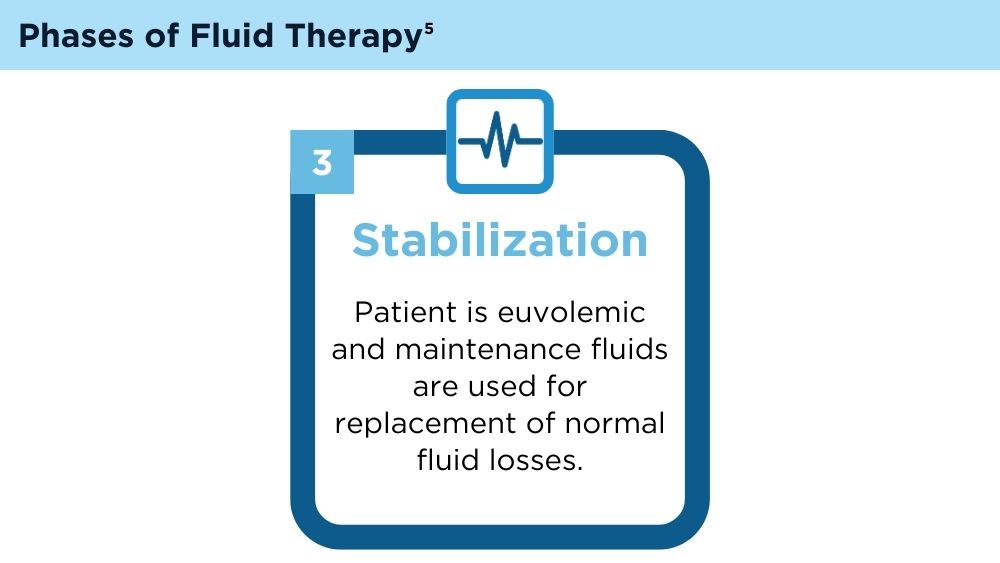
User login
Reassuring Data on GLP-1 RAs and Pancreatic Cancer Risk
PHILADELPHIA —
Instead, the large electronic health record (EHR) analysis of patients with type 2 diabetes (T2D) found those taking GLP-1 RAs had a significantly lower risk for pancreatic cancer than peers on other antidiabetic medications.
“Although there were previous reports suggesting possible association between pancreatic cancer and GLP-1 receptor agonist medications, this study provides reassurance that there is no observed increased incidence of pancreatic cancer in patients prescribed these medications,” said Khaled Alsabbagh Alchirazi, MD, a gastroenterology fellow with Aurora Healthcare in Brookfield, Wisconsin.
He presented the study findings at the American College of Gastroenterology (ACG) 2024 Annual Scientific Meeting.
Important Topic
Patients with T2D are at increased risk for several malignancies, including pancreatic cancer. Given the unique mechanism of action of GLP-1 RAs in the pancreas, it was important to investigate the relationship between use of these drugs and incidence of pancreatic cancer, he explained.
Using the TriNetX database, the study team identified 4.95 million antidiabetic drug naive T2D patients who were prescribed antidiabetic medications for the first time between 2005 and 2020. None had a history of pancreatic cancer.
A total of 245,532 were prescribed a GLP-1 RA. The researchers compared GLP-1 RAs users to users of other antidiabetic medications — namely, insulin, metformin, alpha-glucosidase inhibitors, dipeptidyl-peptidase 4 inhibitors (DPP-4i), sodium-glucose cotransporter-2 inhibitors (SGLT2i), sulfonylureas, and thiazolidinediones.
Patients were propensity score-matched based on demographics, health determinants, lifestyle factors, medical history, family history of cancers, and acute/chronic pancreatitis.
The risk for pancreatic cancer was significantly lower among patients on GLP-1 RAs vs insulin (hazard ratio [HR], 0.47; 95% CI, 0.40-0.55), DPP-4i (HR, 0.80; 95% CI, 0.73-0.89), SGLT2i (HR, 0.78; 95% CI, 0.69-0.89), and sulfonylureas (HR, 0.84; 95% CI, 0.74-0.95), Alchirazi reported.
The results were consistent across different groups, including patients with obesity/ overweight on GLP-1 RAs vs insulin (HR, 0.53; 95% CI, 0.43-0.65) and SGLT2i (HR, 0.81; 95% CI, 0.69-0.96).
Strengths of the analysis included the large and diverse cohort of propensity score-matched patients. Limitations included the retrospective design and use of claims data that did not provide granular data on pathology reports.
The study by Alchirazi and colleagues aligns with a large population-based cohort study from Israel that found no evidence that GLP-1 RAs increase risk for pancreatic cancer over 7 years following initiation.
Separately, a study of more than 1.6 million patients with T2D found that treatment with a GLP-1 RA (vs insulin or metformin) was associated with lower risks for specific types of obesity-related cancers, including pancreatic cancer.
The study had no specific funding. Alchirazi had no relevant disclosures.
A version of this article appeared on Medscape.com.
PHILADELPHIA —
Instead, the large electronic health record (EHR) analysis of patients with type 2 diabetes (T2D) found those taking GLP-1 RAs had a significantly lower risk for pancreatic cancer than peers on other antidiabetic medications.
“Although there were previous reports suggesting possible association between pancreatic cancer and GLP-1 receptor agonist medications, this study provides reassurance that there is no observed increased incidence of pancreatic cancer in patients prescribed these medications,” said Khaled Alsabbagh Alchirazi, MD, a gastroenterology fellow with Aurora Healthcare in Brookfield, Wisconsin.
He presented the study findings at the American College of Gastroenterology (ACG) 2024 Annual Scientific Meeting.
Important Topic
Patients with T2D are at increased risk for several malignancies, including pancreatic cancer. Given the unique mechanism of action of GLP-1 RAs in the pancreas, it was important to investigate the relationship between use of these drugs and incidence of pancreatic cancer, he explained.
Using the TriNetX database, the study team identified 4.95 million antidiabetic drug naive T2D patients who were prescribed antidiabetic medications for the first time between 2005 and 2020. None had a history of pancreatic cancer.
A total of 245,532 were prescribed a GLP-1 RA. The researchers compared GLP-1 RAs users to users of other antidiabetic medications — namely, insulin, metformin, alpha-glucosidase inhibitors, dipeptidyl-peptidase 4 inhibitors (DPP-4i), sodium-glucose cotransporter-2 inhibitors (SGLT2i), sulfonylureas, and thiazolidinediones.
Patients were propensity score-matched based on demographics, health determinants, lifestyle factors, medical history, family history of cancers, and acute/chronic pancreatitis.
The risk for pancreatic cancer was significantly lower among patients on GLP-1 RAs vs insulin (hazard ratio [HR], 0.47; 95% CI, 0.40-0.55), DPP-4i (HR, 0.80; 95% CI, 0.73-0.89), SGLT2i (HR, 0.78; 95% CI, 0.69-0.89), and sulfonylureas (HR, 0.84; 95% CI, 0.74-0.95), Alchirazi reported.
The results were consistent across different groups, including patients with obesity/ overweight on GLP-1 RAs vs insulin (HR, 0.53; 95% CI, 0.43-0.65) and SGLT2i (HR, 0.81; 95% CI, 0.69-0.96).
Strengths of the analysis included the large and diverse cohort of propensity score-matched patients. Limitations included the retrospective design and use of claims data that did not provide granular data on pathology reports.
The study by Alchirazi and colleagues aligns with a large population-based cohort study from Israel that found no evidence that GLP-1 RAs increase risk for pancreatic cancer over 7 years following initiation.
Separately, a study of more than 1.6 million patients with T2D found that treatment with a GLP-1 RA (vs insulin or metformin) was associated with lower risks for specific types of obesity-related cancers, including pancreatic cancer.
The study had no specific funding. Alchirazi had no relevant disclosures.
A version of this article appeared on Medscape.com.
PHILADELPHIA —
Instead, the large electronic health record (EHR) analysis of patients with type 2 diabetes (T2D) found those taking GLP-1 RAs had a significantly lower risk for pancreatic cancer than peers on other antidiabetic medications.
“Although there were previous reports suggesting possible association between pancreatic cancer and GLP-1 receptor agonist medications, this study provides reassurance that there is no observed increased incidence of pancreatic cancer in patients prescribed these medications,” said Khaled Alsabbagh Alchirazi, MD, a gastroenterology fellow with Aurora Healthcare in Brookfield, Wisconsin.
He presented the study findings at the American College of Gastroenterology (ACG) 2024 Annual Scientific Meeting.
Important Topic
Patients with T2D are at increased risk for several malignancies, including pancreatic cancer. Given the unique mechanism of action of GLP-1 RAs in the pancreas, it was important to investigate the relationship between use of these drugs and incidence of pancreatic cancer, he explained.
Using the TriNetX database, the study team identified 4.95 million antidiabetic drug naive T2D patients who were prescribed antidiabetic medications for the first time between 2005 and 2020. None had a history of pancreatic cancer.
A total of 245,532 were prescribed a GLP-1 RA. The researchers compared GLP-1 RAs users to users of other antidiabetic medications — namely, insulin, metformin, alpha-glucosidase inhibitors, dipeptidyl-peptidase 4 inhibitors (DPP-4i), sodium-glucose cotransporter-2 inhibitors (SGLT2i), sulfonylureas, and thiazolidinediones.
Patients were propensity score-matched based on demographics, health determinants, lifestyle factors, medical history, family history of cancers, and acute/chronic pancreatitis.
The risk for pancreatic cancer was significantly lower among patients on GLP-1 RAs vs insulin (hazard ratio [HR], 0.47; 95% CI, 0.40-0.55), DPP-4i (HR, 0.80; 95% CI, 0.73-0.89), SGLT2i (HR, 0.78; 95% CI, 0.69-0.89), and sulfonylureas (HR, 0.84; 95% CI, 0.74-0.95), Alchirazi reported.
The results were consistent across different groups, including patients with obesity/ overweight on GLP-1 RAs vs insulin (HR, 0.53; 95% CI, 0.43-0.65) and SGLT2i (HR, 0.81; 95% CI, 0.69-0.96).
Strengths of the analysis included the large and diverse cohort of propensity score-matched patients. Limitations included the retrospective design and use of claims data that did not provide granular data on pathology reports.
The study by Alchirazi and colleagues aligns with a large population-based cohort study from Israel that found no evidence that GLP-1 RAs increase risk for pancreatic cancer over 7 years following initiation.
Separately, a study of more than 1.6 million patients with T2D found that treatment with a GLP-1 RA (vs insulin or metformin) was associated with lower risks for specific types of obesity-related cancers, including pancreatic cancer.
The study had no specific funding. Alchirazi had no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM ACG 2024
Screening Options for Rare Malignancies
Dear colleagues,
As gastroenterologists and endoscopists, we spend significant time preventing and diagnosing GI malignancies.
For instance, is it worthwhile screening for pancreatic cancer, and, if so, how should this be done? Likewise, diagnosing cholangiocarcinoma is challenging; how best should one evaluate for this in higher risk populations, such as primary sclerosing cholangitis? And what about the costs, financial and otherwise, associated with screening?
In this issue of Perspectives, Dr. Darshan Kothari and Dr. Daniel Bernstein discuss their approach to pancreatic cancer screening, including who is eligible, the preferred screening modalities, and the barriers to screening. In the accompanying perspective, Dr. Aparna Goel and Dr. Judah Kupferman focus on cholangiocarcinoma screening, identifying high-risk populations and discussing some of the concerns with screening, necessitating shared decision-making.
We welcome your thoughts on this issue. Share with us on X at @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, and chief of endoscopy at West Haven VA Medical Center, both in Connecticut. He is an associate editor for GI & Hepatology News.
An Approach to Pancreatic Cancer Screening
BY DANIEL A. BERNSTEIN, MD, AND DARSHAN KOTHARI, MD
Pancreatic cancer carries a dismal prognosis, now accounting for the third-most cancer-related mortality in the United States. A small proportion of patients are diagnosed at a local stage of disease, with over half found to have metastatic disease at presentation. Given the low overall incidence and lifetime risk in the general population, population-based screening is not justified.
About 10% of cases of pancreas cancer are associated with germ-line mutations and/or with a strong family history of pancreatic cancer. Several academic societies and expert committees now recommend regular screening for pancreatic cancer in patients who are considered high-risk individuals, as they carry a fivefold relative risk for pancreatic cancer. Moreover, studies suggest that screening has the potential to identify early-stage resectable disease and decrease mortality in this patient population.
Patients who benefit from pancreatic cancer screening are those who carry an increased lifetime risk (in excess of 5%) of pancreatic cancer. High-risk individuals include those with germ-line mutations and/or those with a family history of pancreatic cancer in first-degree relatives. Consensus guidelines by the International Cancer of the Pancreas Screening Consortium and the American Society for Gastrointestinal Endoscopy provide medical centers with detailed recommendations on who and when to start screening.
High-risk individuals fall into three categories:
- Patients with high-risk germline mutations including: familial atypical multiple mole melanoma syndrome (CDKN2A), hereditary breast and ovarian cancer syndromes (BRCA1, BRCA2, and PALB2), Peutz-Jeghers syndrome (STK11), and hereditary pancreatitis (PRSS1 and SPINK1)
- Patients with low- to moderate-risk germ-line mutations with at least one first-degree relative with pancreatic cancer: Lynch Syndrome (particularly MLH1 mutation), ataxia-telangiectasia (ATM), or Li-Fraumeni syndrome (p53)
- Patients with one first-degree relative with pancreatic cancer who in turn has one first-degree relative with pancreatic cancer (eg, a patient’s mother and maternal aunt or a patient’s father and patient’s sister)
Consistent with established guidelines, we recommend screening for high-risk patients beginning at age 50, or 10 years before the youngest age at which pancreas cancer was diagnosed in an affected relative. Screening is recommended earlier in patients with particularly high risk: at age 40 for patients with CDKN2A and STKI11 mutations and age 40 for patients with PRSS1 mutation or 20 years after the first attack of acute pancreatitis. For patients with a strong family history of pancreas cancer, we recommend comprehensive evaluation by a certified genetic counselor at a high-volume cancer center.
In practice, patients at our institution who are identified as high risk based on the above criteria are referred for an initial consultation at our pancreas center. In most cases, this should occur no sooner than 5 years prior to the recommended starting age for screening. All patients who are identified as high risk should be screened annually for diabetes given the growing evidence base supporting an association between new-onset diabetes and pancreatic cancer.
After an initial visit and discussion of the risks and benefits of screening, most screening protocols start with a baseline endoscopic ultrasound (EUS) and contrast-enhanced magnetic resonance abdomen with magnetic resonance cholangiopancreatography (MRI/MRCP), which will be repeated annually or sooner as the clinical condition warrants. A sooner-interval EUS should be considered for patients already undergoing screening who are newly found to have diabetes.
At our institution, we start with an in-person clinic evaluation followed by EUS. Thereafter, patients undergo MRI/MRCP (synchronized with a same-day clinic visit) alternating with EUS every 6 months to ensure patients are seen twice a year, though there is no specific data to support this approach. Non-diabetics also undergo yearly diabetes screening which will trigger an EUS if patients become diabetic.
We engage in shared decision-making with our high-risk individuals undergoing pancreatic cancer screening and at each visit we review their concurrent medical conditions and suitability to continue screening. We consider discontinuing screening after age 75, at the onset of any life-limiting illness, or after a discussion of risks and benefits if comorbidities lead to a substantial deterioration in a patient’s overall health status.
While a growing body of evidence exists to support the application of pancreatic cancer screening in high-risk individuals, this preventive service remains underutilized. Recent analysis of the screening cohort at our institution showed a demographically homogeneous group of mostly highly educated, high-income White females. These findings are consistent with the patient cohorts described in other pancreatic cancer screening programs and represent only a fraction of people who would qualify for pancreatic cancer screening.
A survey of patients undergoing screening at our institution identified cost, travel, and time associated with pancreatic cancer screening to be frequent challenges to participation. Further studies are needed to fully explore the barriers and psychological burden of pancreas cancer screening in high-risk individuals, and to identify ways to enrich the cohort of patients undergoing screening. This may involve novel methods to identify family members of patients with a new diagnosis of pancreas cancer and increasing health literacy around pancreatic cancer screening among patients and providers.
Pancreatic cancer screening has the potential to identify early-stage disease in patients who are at high risk because of germ-line mutations and/or family history. We recommend that patients engage in pancreatic cancer screening at high-volume centers with well-supported oncology, genetics, and research infrastructure.
Dr. Bernstein is a gastroenterology fellow at Duke University School of Medicine, Durham, North Carolina. Dr. Kothari is an associate professor of medicine in gastroenterology and hepatology at Duke University School of Medicine.
Screening for Cholangiocarcinoma
BY JUDAH KUPFERMAN, MD, AND APARNA GOEL, MD
Cholangiocarcinoma is a rare but aggressive cancer of the bile ducts that poses many diagnostic challenges. Approximately 3% of gastrointestinal cancers are attributed to cholangiocarcinoma, and while the annual incidence of disease in the United States is about 1.26 per 100,000 people, the incidence of intrahepatic disease has been rising considerably.1,2 Screening for cholangiocarcinoma is reserved for high-risk individuals — such as those with primary sclerosing cholangitis (PSC), secondary sclerosing cholangitis (SSC), and biliary tract disorders such as choledochal cysts or Caroli’s disease. The goal is to balance the benefits of early diagnosis with the costs and risks associated with screening, particularly given the limitations of available tools like MRI with cholangiopancreatography (MRCP), which has a sensitivity of 70%-85%. In general, we recommend annual cholangiocarcinoma screening for high-risk individuals with MRI and MRCP as well as with cancer antigen (CA) 19-9. .
Screening in Patients with Primary Sclerosing Cholangitis
The lifetime risk of cholangiocarcinoma in patients with PSC is 10%-15% with an annual risk of 0.5%-1.5%. In our experience, this is often the most feared complication for PSC patients, even more so than the risk of liver transplantation. We recommend annual MRI with MRCP in addition to CA 19-9 for patients with PSC in the first decade of their diagnosis, as most cancers are diagnosed during this period. If a patient’s imaging has remained stable for over a decade and there is minimal hepatic fibrosis, we discuss the option of reducing screening frequency to every 2 years to minimize costs and exposure to MRI contrast risks.
If MRI reveals a concerning new large duct stricture, we will evaluate this with an endoscopic retrograde cholangiopancreatography (ERCP), as differentiating benign and malignant strictures is quite challenging with MRI. We generally recommend ERCP with brush cytology and fluorescence in situ hybridization to improve diagnostic yield. Depending on imaging findings and location of the new large duct stricture, we may consider cholangioscopy during ERCP for direct visualization of the bile duct and directed tissue biopsies. Unfortunately, even in young, asymptomatic patients who undergo regular screening, cholangiocarcinoma is frequently diagnosed at an advanced stage.
Screening in Patients with Secondary Sclerosing Cholangitis
Patients with SSC may develop cholangiocarcinoma because of chronic inflammatory and fibrotic processes, such as IgG4-associated cholangiopathy, sarcoidosis, ischemic cholangiopathy, cystic fibrosis, recurrent pyogenic cholangitis, severe sepsis (as recently seen from SARS-CoV-2), surgical complications, or other etiologies. When the condition is reversible, such as with IgG4-associated cholangiopathy, cancer screening may not be necessary. However, when irreversible damage occurs, the cancer risk increases, though it varies by disease type and severity. In most cases, we recommend routine screening for cholangiocarcinoma with MRI and CA 19-9 in this population.
Screening in Patients with Biliary Tract Disorders
Biliary tract disorders such as choledochal cysts and Caroli’s disease also harbor an increased risk of cholangiocarcinoma. Choledochal cysts are congenital cystic dilations of the bile duct that have a 10%-30% lifetime risk of malignant transformation to cholangiocarcinoma. Surgical intervention to remove the cyst is often recommended because of this high risk. However, some patients may be unable or unwilling to undergo this surgery or they may have residual cysts. We recommend ongoing screening with MRI and CA 19-9 for these patients. Similarly, Caroli’s disease is a congenital disease associated with intrahepatic and extrahepatic bile duct cysts and associated with a 5%-15% lifetime risk of cholangiocarcinoma. MRI with MRCP and CA 19-9 should be performed routinely for patients with Caroli’s disease and syndrome.
Risks and Challenges in Cholangiocarcinoma Screening
While MRI with MRCP is the gold standard for cholangiocarcinoma screening, its limitations must be carefully considered. One growing concern is the potential for gadolinium retention in the brain, bones, or skin following repeated MRI scans. Though the long-term effects of gadolinium retention are not fully understood, we factor this into screening decisions, particularly for younger patients who may undergo decades of regular imaging.
MRI is not always feasible for certain patients, including those with metal implants, on hemodialysis, or with severe allergic reactions. In such cases, CT or ultrasound may serve as alternatives, though with lower sensitivity for detecting cholangiocarcinoma. Additionally, claustrophobia during MRI can be addressed with sedation, but this underscores the importance of shared decision-making.
From our perspective, cholangiocarcinoma screening in high-risk patients is crucial but not without challenges. Our current screening methods, while essential, are far from perfect, often missing early cancers or leading to unnecessary interventions. Because of these limitations, the window for treatment of localized disease can easily be missed. In our practice, we tailor screening strategies to each patient’s specific needs, weighing the potential benefits against the risks, costs, and the inherent uncertainty of early detection tools. We believe it is essential to involve patients in this decision-making process to provide a balanced, individualized approach that considers both clinical evidence and the personal preferences of each person.
Dr. Kupferman is a gastroenterology fellow at Stanford University School of Medicine in California. Dr. Goel is a transplant hepatologist and a clinical associate professor in gastroenterology & hepatology at Stanford.
References
1. Vithayathil M and Khan SA. J Hepatol. 2022 Dec. doi: 10.1016/j.jhep.2022.07.022.
2. Patel N and Benipal B. Cureus. 2019 Jan. doi: 10.7759/cureus.3962.
Dear colleagues,
As gastroenterologists and endoscopists, we spend significant time preventing and diagnosing GI malignancies.
For instance, is it worthwhile screening for pancreatic cancer, and, if so, how should this be done? Likewise, diagnosing cholangiocarcinoma is challenging; how best should one evaluate for this in higher risk populations, such as primary sclerosing cholangitis? And what about the costs, financial and otherwise, associated with screening?
In this issue of Perspectives, Dr. Darshan Kothari and Dr. Daniel Bernstein discuss their approach to pancreatic cancer screening, including who is eligible, the preferred screening modalities, and the barriers to screening. In the accompanying perspective, Dr. Aparna Goel and Dr. Judah Kupferman focus on cholangiocarcinoma screening, identifying high-risk populations and discussing some of the concerns with screening, necessitating shared decision-making.
We welcome your thoughts on this issue. Share with us on X at @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, and chief of endoscopy at West Haven VA Medical Center, both in Connecticut. He is an associate editor for GI & Hepatology News.
An Approach to Pancreatic Cancer Screening
BY DANIEL A. BERNSTEIN, MD, AND DARSHAN KOTHARI, MD
Pancreatic cancer carries a dismal prognosis, now accounting for the third-most cancer-related mortality in the United States. A small proportion of patients are diagnosed at a local stage of disease, with over half found to have metastatic disease at presentation. Given the low overall incidence and lifetime risk in the general population, population-based screening is not justified.
About 10% of cases of pancreas cancer are associated with germ-line mutations and/or with a strong family history of pancreatic cancer. Several academic societies and expert committees now recommend regular screening for pancreatic cancer in patients who are considered high-risk individuals, as they carry a fivefold relative risk for pancreatic cancer. Moreover, studies suggest that screening has the potential to identify early-stage resectable disease and decrease mortality in this patient population.
Patients who benefit from pancreatic cancer screening are those who carry an increased lifetime risk (in excess of 5%) of pancreatic cancer. High-risk individuals include those with germ-line mutations and/or those with a family history of pancreatic cancer in first-degree relatives. Consensus guidelines by the International Cancer of the Pancreas Screening Consortium and the American Society for Gastrointestinal Endoscopy provide medical centers with detailed recommendations on who and when to start screening.
High-risk individuals fall into three categories:
- Patients with high-risk germline mutations including: familial atypical multiple mole melanoma syndrome (CDKN2A), hereditary breast and ovarian cancer syndromes (BRCA1, BRCA2, and PALB2), Peutz-Jeghers syndrome (STK11), and hereditary pancreatitis (PRSS1 and SPINK1)
- Patients with low- to moderate-risk germ-line mutations with at least one first-degree relative with pancreatic cancer: Lynch Syndrome (particularly MLH1 mutation), ataxia-telangiectasia (ATM), or Li-Fraumeni syndrome (p53)
- Patients with one first-degree relative with pancreatic cancer who in turn has one first-degree relative with pancreatic cancer (eg, a patient’s mother and maternal aunt or a patient’s father and patient’s sister)
Consistent with established guidelines, we recommend screening for high-risk patients beginning at age 50, or 10 years before the youngest age at which pancreas cancer was diagnosed in an affected relative. Screening is recommended earlier in patients with particularly high risk: at age 40 for patients with CDKN2A and STKI11 mutations and age 40 for patients with PRSS1 mutation or 20 years after the first attack of acute pancreatitis. For patients with a strong family history of pancreas cancer, we recommend comprehensive evaluation by a certified genetic counselor at a high-volume cancer center.
In practice, patients at our institution who are identified as high risk based on the above criteria are referred for an initial consultation at our pancreas center. In most cases, this should occur no sooner than 5 years prior to the recommended starting age for screening. All patients who are identified as high risk should be screened annually for diabetes given the growing evidence base supporting an association between new-onset diabetes and pancreatic cancer.
After an initial visit and discussion of the risks and benefits of screening, most screening protocols start with a baseline endoscopic ultrasound (EUS) and contrast-enhanced magnetic resonance abdomen with magnetic resonance cholangiopancreatography (MRI/MRCP), which will be repeated annually or sooner as the clinical condition warrants. A sooner-interval EUS should be considered for patients already undergoing screening who are newly found to have diabetes.
At our institution, we start with an in-person clinic evaluation followed by EUS. Thereafter, patients undergo MRI/MRCP (synchronized with a same-day clinic visit) alternating with EUS every 6 months to ensure patients are seen twice a year, though there is no specific data to support this approach. Non-diabetics also undergo yearly diabetes screening which will trigger an EUS if patients become diabetic.
We engage in shared decision-making with our high-risk individuals undergoing pancreatic cancer screening and at each visit we review their concurrent medical conditions and suitability to continue screening. We consider discontinuing screening after age 75, at the onset of any life-limiting illness, or after a discussion of risks and benefits if comorbidities lead to a substantial deterioration in a patient’s overall health status.
While a growing body of evidence exists to support the application of pancreatic cancer screening in high-risk individuals, this preventive service remains underutilized. Recent analysis of the screening cohort at our institution showed a demographically homogeneous group of mostly highly educated, high-income White females. These findings are consistent with the patient cohorts described in other pancreatic cancer screening programs and represent only a fraction of people who would qualify for pancreatic cancer screening.
A survey of patients undergoing screening at our institution identified cost, travel, and time associated with pancreatic cancer screening to be frequent challenges to participation. Further studies are needed to fully explore the barriers and psychological burden of pancreas cancer screening in high-risk individuals, and to identify ways to enrich the cohort of patients undergoing screening. This may involve novel methods to identify family members of patients with a new diagnosis of pancreas cancer and increasing health literacy around pancreatic cancer screening among patients and providers.
Pancreatic cancer screening has the potential to identify early-stage disease in patients who are at high risk because of germ-line mutations and/or family history. We recommend that patients engage in pancreatic cancer screening at high-volume centers with well-supported oncology, genetics, and research infrastructure.
Dr. Bernstein is a gastroenterology fellow at Duke University School of Medicine, Durham, North Carolina. Dr. Kothari is an associate professor of medicine in gastroenterology and hepatology at Duke University School of Medicine.
Screening for Cholangiocarcinoma
BY JUDAH KUPFERMAN, MD, AND APARNA GOEL, MD
Cholangiocarcinoma is a rare but aggressive cancer of the bile ducts that poses many diagnostic challenges. Approximately 3% of gastrointestinal cancers are attributed to cholangiocarcinoma, and while the annual incidence of disease in the United States is about 1.26 per 100,000 people, the incidence of intrahepatic disease has been rising considerably.1,2 Screening for cholangiocarcinoma is reserved for high-risk individuals — such as those with primary sclerosing cholangitis (PSC), secondary sclerosing cholangitis (SSC), and biliary tract disorders such as choledochal cysts or Caroli’s disease. The goal is to balance the benefits of early diagnosis with the costs and risks associated with screening, particularly given the limitations of available tools like MRI with cholangiopancreatography (MRCP), which has a sensitivity of 70%-85%. In general, we recommend annual cholangiocarcinoma screening for high-risk individuals with MRI and MRCP as well as with cancer antigen (CA) 19-9. .
Screening in Patients with Primary Sclerosing Cholangitis
The lifetime risk of cholangiocarcinoma in patients with PSC is 10%-15% with an annual risk of 0.5%-1.5%. In our experience, this is often the most feared complication for PSC patients, even more so than the risk of liver transplantation. We recommend annual MRI with MRCP in addition to CA 19-9 for patients with PSC in the first decade of their diagnosis, as most cancers are diagnosed during this period. If a patient’s imaging has remained stable for over a decade and there is minimal hepatic fibrosis, we discuss the option of reducing screening frequency to every 2 years to minimize costs and exposure to MRI contrast risks.
If MRI reveals a concerning new large duct stricture, we will evaluate this with an endoscopic retrograde cholangiopancreatography (ERCP), as differentiating benign and malignant strictures is quite challenging with MRI. We generally recommend ERCP with brush cytology and fluorescence in situ hybridization to improve diagnostic yield. Depending on imaging findings and location of the new large duct stricture, we may consider cholangioscopy during ERCP for direct visualization of the bile duct and directed tissue biopsies. Unfortunately, even in young, asymptomatic patients who undergo regular screening, cholangiocarcinoma is frequently diagnosed at an advanced stage.
Screening in Patients with Secondary Sclerosing Cholangitis
Patients with SSC may develop cholangiocarcinoma because of chronic inflammatory and fibrotic processes, such as IgG4-associated cholangiopathy, sarcoidosis, ischemic cholangiopathy, cystic fibrosis, recurrent pyogenic cholangitis, severe sepsis (as recently seen from SARS-CoV-2), surgical complications, or other etiologies. When the condition is reversible, such as with IgG4-associated cholangiopathy, cancer screening may not be necessary. However, when irreversible damage occurs, the cancer risk increases, though it varies by disease type and severity. In most cases, we recommend routine screening for cholangiocarcinoma with MRI and CA 19-9 in this population.
Screening in Patients with Biliary Tract Disorders
Biliary tract disorders such as choledochal cysts and Caroli’s disease also harbor an increased risk of cholangiocarcinoma. Choledochal cysts are congenital cystic dilations of the bile duct that have a 10%-30% lifetime risk of malignant transformation to cholangiocarcinoma. Surgical intervention to remove the cyst is often recommended because of this high risk. However, some patients may be unable or unwilling to undergo this surgery or they may have residual cysts. We recommend ongoing screening with MRI and CA 19-9 for these patients. Similarly, Caroli’s disease is a congenital disease associated with intrahepatic and extrahepatic bile duct cysts and associated with a 5%-15% lifetime risk of cholangiocarcinoma. MRI with MRCP and CA 19-9 should be performed routinely for patients with Caroli’s disease and syndrome.
Risks and Challenges in Cholangiocarcinoma Screening
While MRI with MRCP is the gold standard for cholangiocarcinoma screening, its limitations must be carefully considered. One growing concern is the potential for gadolinium retention in the brain, bones, or skin following repeated MRI scans. Though the long-term effects of gadolinium retention are not fully understood, we factor this into screening decisions, particularly for younger patients who may undergo decades of regular imaging.
MRI is not always feasible for certain patients, including those with metal implants, on hemodialysis, or with severe allergic reactions. In such cases, CT or ultrasound may serve as alternatives, though with lower sensitivity for detecting cholangiocarcinoma. Additionally, claustrophobia during MRI can be addressed with sedation, but this underscores the importance of shared decision-making.
From our perspective, cholangiocarcinoma screening in high-risk patients is crucial but not without challenges. Our current screening methods, while essential, are far from perfect, often missing early cancers or leading to unnecessary interventions. Because of these limitations, the window for treatment of localized disease can easily be missed. In our practice, we tailor screening strategies to each patient’s specific needs, weighing the potential benefits against the risks, costs, and the inherent uncertainty of early detection tools. We believe it is essential to involve patients in this decision-making process to provide a balanced, individualized approach that considers both clinical evidence and the personal preferences of each person.
Dr. Kupferman is a gastroenterology fellow at Stanford University School of Medicine in California. Dr. Goel is a transplant hepatologist and a clinical associate professor in gastroenterology & hepatology at Stanford.
References
1. Vithayathil M and Khan SA. J Hepatol. 2022 Dec. doi: 10.1016/j.jhep.2022.07.022.
2. Patel N and Benipal B. Cureus. 2019 Jan. doi: 10.7759/cureus.3962.
Dear colleagues,
As gastroenterologists and endoscopists, we spend significant time preventing and diagnosing GI malignancies.
For instance, is it worthwhile screening for pancreatic cancer, and, if so, how should this be done? Likewise, diagnosing cholangiocarcinoma is challenging; how best should one evaluate for this in higher risk populations, such as primary sclerosing cholangitis? And what about the costs, financial and otherwise, associated with screening?
In this issue of Perspectives, Dr. Darshan Kothari and Dr. Daniel Bernstein discuss their approach to pancreatic cancer screening, including who is eligible, the preferred screening modalities, and the barriers to screening. In the accompanying perspective, Dr. Aparna Goel and Dr. Judah Kupferman focus on cholangiocarcinoma screening, identifying high-risk populations and discussing some of the concerns with screening, necessitating shared decision-making.
We welcome your thoughts on this issue. Share with us on X at @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, and chief of endoscopy at West Haven VA Medical Center, both in Connecticut. He is an associate editor for GI & Hepatology News.
An Approach to Pancreatic Cancer Screening
BY DANIEL A. BERNSTEIN, MD, AND DARSHAN KOTHARI, MD
Pancreatic cancer carries a dismal prognosis, now accounting for the third-most cancer-related mortality in the United States. A small proportion of patients are diagnosed at a local stage of disease, with over half found to have metastatic disease at presentation. Given the low overall incidence and lifetime risk in the general population, population-based screening is not justified.
About 10% of cases of pancreas cancer are associated with germ-line mutations and/or with a strong family history of pancreatic cancer. Several academic societies and expert committees now recommend regular screening for pancreatic cancer in patients who are considered high-risk individuals, as they carry a fivefold relative risk for pancreatic cancer. Moreover, studies suggest that screening has the potential to identify early-stage resectable disease and decrease mortality in this patient population.
Patients who benefit from pancreatic cancer screening are those who carry an increased lifetime risk (in excess of 5%) of pancreatic cancer. High-risk individuals include those with germ-line mutations and/or those with a family history of pancreatic cancer in first-degree relatives. Consensus guidelines by the International Cancer of the Pancreas Screening Consortium and the American Society for Gastrointestinal Endoscopy provide medical centers with detailed recommendations on who and when to start screening.
High-risk individuals fall into three categories:
- Patients with high-risk germline mutations including: familial atypical multiple mole melanoma syndrome (CDKN2A), hereditary breast and ovarian cancer syndromes (BRCA1, BRCA2, and PALB2), Peutz-Jeghers syndrome (STK11), and hereditary pancreatitis (PRSS1 and SPINK1)
- Patients with low- to moderate-risk germ-line mutations with at least one first-degree relative with pancreatic cancer: Lynch Syndrome (particularly MLH1 mutation), ataxia-telangiectasia (ATM), or Li-Fraumeni syndrome (p53)
- Patients with one first-degree relative with pancreatic cancer who in turn has one first-degree relative with pancreatic cancer (eg, a patient’s mother and maternal aunt or a patient’s father and patient’s sister)
Consistent with established guidelines, we recommend screening for high-risk patients beginning at age 50, or 10 years before the youngest age at which pancreas cancer was diagnosed in an affected relative. Screening is recommended earlier in patients with particularly high risk: at age 40 for patients with CDKN2A and STKI11 mutations and age 40 for patients with PRSS1 mutation or 20 years after the first attack of acute pancreatitis. For patients with a strong family history of pancreas cancer, we recommend comprehensive evaluation by a certified genetic counselor at a high-volume cancer center.
In practice, patients at our institution who are identified as high risk based on the above criteria are referred for an initial consultation at our pancreas center. In most cases, this should occur no sooner than 5 years prior to the recommended starting age for screening. All patients who are identified as high risk should be screened annually for diabetes given the growing evidence base supporting an association between new-onset diabetes and pancreatic cancer.
After an initial visit and discussion of the risks and benefits of screening, most screening protocols start with a baseline endoscopic ultrasound (EUS) and contrast-enhanced magnetic resonance abdomen with magnetic resonance cholangiopancreatography (MRI/MRCP), which will be repeated annually or sooner as the clinical condition warrants. A sooner-interval EUS should be considered for patients already undergoing screening who are newly found to have diabetes.
At our institution, we start with an in-person clinic evaluation followed by EUS. Thereafter, patients undergo MRI/MRCP (synchronized with a same-day clinic visit) alternating with EUS every 6 months to ensure patients are seen twice a year, though there is no specific data to support this approach. Non-diabetics also undergo yearly diabetes screening which will trigger an EUS if patients become diabetic.
We engage in shared decision-making with our high-risk individuals undergoing pancreatic cancer screening and at each visit we review their concurrent medical conditions and suitability to continue screening. We consider discontinuing screening after age 75, at the onset of any life-limiting illness, or after a discussion of risks and benefits if comorbidities lead to a substantial deterioration in a patient’s overall health status.
While a growing body of evidence exists to support the application of pancreatic cancer screening in high-risk individuals, this preventive service remains underutilized. Recent analysis of the screening cohort at our institution showed a demographically homogeneous group of mostly highly educated, high-income White females. These findings are consistent with the patient cohorts described in other pancreatic cancer screening programs and represent only a fraction of people who would qualify for pancreatic cancer screening.
A survey of patients undergoing screening at our institution identified cost, travel, and time associated with pancreatic cancer screening to be frequent challenges to participation. Further studies are needed to fully explore the barriers and psychological burden of pancreas cancer screening in high-risk individuals, and to identify ways to enrich the cohort of patients undergoing screening. This may involve novel methods to identify family members of patients with a new diagnosis of pancreas cancer and increasing health literacy around pancreatic cancer screening among patients and providers.
Pancreatic cancer screening has the potential to identify early-stage disease in patients who are at high risk because of germ-line mutations and/or family history. We recommend that patients engage in pancreatic cancer screening at high-volume centers with well-supported oncology, genetics, and research infrastructure.
Dr. Bernstein is a gastroenterology fellow at Duke University School of Medicine, Durham, North Carolina. Dr. Kothari is an associate professor of medicine in gastroenterology and hepatology at Duke University School of Medicine.
Screening for Cholangiocarcinoma
BY JUDAH KUPFERMAN, MD, AND APARNA GOEL, MD
Cholangiocarcinoma is a rare but aggressive cancer of the bile ducts that poses many diagnostic challenges. Approximately 3% of gastrointestinal cancers are attributed to cholangiocarcinoma, and while the annual incidence of disease in the United States is about 1.26 per 100,000 people, the incidence of intrahepatic disease has been rising considerably.1,2 Screening for cholangiocarcinoma is reserved for high-risk individuals — such as those with primary sclerosing cholangitis (PSC), secondary sclerosing cholangitis (SSC), and biliary tract disorders such as choledochal cysts or Caroli’s disease. The goal is to balance the benefits of early diagnosis with the costs and risks associated with screening, particularly given the limitations of available tools like MRI with cholangiopancreatography (MRCP), which has a sensitivity of 70%-85%. In general, we recommend annual cholangiocarcinoma screening for high-risk individuals with MRI and MRCP as well as with cancer antigen (CA) 19-9. .
Screening in Patients with Primary Sclerosing Cholangitis
The lifetime risk of cholangiocarcinoma in patients with PSC is 10%-15% with an annual risk of 0.5%-1.5%. In our experience, this is often the most feared complication for PSC patients, even more so than the risk of liver transplantation. We recommend annual MRI with MRCP in addition to CA 19-9 for patients with PSC in the first decade of their diagnosis, as most cancers are diagnosed during this period. If a patient’s imaging has remained stable for over a decade and there is minimal hepatic fibrosis, we discuss the option of reducing screening frequency to every 2 years to minimize costs and exposure to MRI contrast risks.
If MRI reveals a concerning new large duct stricture, we will evaluate this with an endoscopic retrograde cholangiopancreatography (ERCP), as differentiating benign and malignant strictures is quite challenging with MRI. We generally recommend ERCP with brush cytology and fluorescence in situ hybridization to improve diagnostic yield. Depending on imaging findings and location of the new large duct stricture, we may consider cholangioscopy during ERCP for direct visualization of the bile duct and directed tissue biopsies. Unfortunately, even in young, asymptomatic patients who undergo regular screening, cholangiocarcinoma is frequently diagnosed at an advanced stage.
Screening in Patients with Secondary Sclerosing Cholangitis
Patients with SSC may develop cholangiocarcinoma because of chronic inflammatory and fibrotic processes, such as IgG4-associated cholangiopathy, sarcoidosis, ischemic cholangiopathy, cystic fibrosis, recurrent pyogenic cholangitis, severe sepsis (as recently seen from SARS-CoV-2), surgical complications, or other etiologies. When the condition is reversible, such as with IgG4-associated cholangiopathy, cancer screening may not be necessary. However, when irreversible damage occurs, the cancer risk increases, though it varies by disease type and severity. In most cases, we recommend routine screening for cholangiocarcinoma with MRI and CA 19-9 in this population.
Screening in Patients with Biliary Tract Disorders
Biliary tract disorders such as choledochal cysts and Caroli’s disease also harbor an increased risk of cholangiocarcinoma. Choledochal cysts are congenital cystic dilations of the bile duct that have a 10%-30% lifetime risk of malignant transformation to cholangiocarcinoma. Surgical intervention to remove the cyst is often recommended because of this high risk. However, some patients may be unable or unwilling to undergo this surgery or they may have residual cysts. We recommend ongoing screening with MRI and CA 19-9 for these patients. Similarly, Caroli’s disease is a congenital disease associated with intrahepatic and extrahepatic bile duct cysts and associated with a 5%-15% lifetime risk of cholangiocarcinoma. MRI with MRCP and CA 19-9 should be performed routinely for patients with Caroli’s disease and syndrome.
Risks and Challenges in Cholangiocarcinoma Screening
While MRI with MRCP is the gold standard for cholangiocarcinoma screening, its limitations must be carefully considered. One growing concern is the potential for gadolinium retention in the brain, bones, or skin following repeated MRI scans. Though the long-term effects of gadolinium retention are not fully understood, we factor this into screening decisions, particularly for younger patients who may undergo decades of regular imaging.
MRI is not always feasible for certain patients, including those with metal implants, on hemodialysis, or with severe allergic reactions. In such cases, CT or ultrasound may serve as alternatives, though with lower sensitivity for detecting cholangiocarcinoma. Additionally, claustrophobia during MRI can be addressed with sedation, but this underscores the importance of shared decision-making.
From our perspective, cholangiocarcinoma screening in high-risk patients is crucial but not without challenges. Our current screening methods, while essential, are far from perfect, often missing early cancers or leading to unnecessary interventions. Because of these limitations, the window for treatment of localized disease can easily be missed. In our practice, we tailor screening strategies to each patient’s specific needs, weighing the potential benefits against the risks, costs, and the inherent uncertainty of early detection tools. We believe it is essential to involve patients in this decision-making process to provide a balanced, individualized approach that considers both clinical evidence and the personal preferences of each person.
Dr. Kupferman is a gastroenterology fellow at Stanford University School of Medicine in California. Dr. Goel is a transplant hepatologist and a clinical associate professor in gastroenterology & hepatology at Stanford.
References
1. Vithayathil M and Khan SA. J Hepatol. 2022 Dec. doi: 10.1016/j.jhep.2022.07.022.
2. Patel N and Benipal B. Cureus. 2019 Jan. doi: 10.7759/cureus.3962.
AI Tool Helps Detect, Differentiate Pancreatic Lesions During Endoscopic Ultrasound
PHILADELPHIA —
This was a transatlantic collaborative effort involving researchers in Portugal, Spain, the United States, and Brazil, and the AI tool “works on different platforms and different devices,” Miguel Mascarenhas, MD, PhD, with Centro Hospitalar Universitário de São João, Porto, Portugal, said in a presentation at the annual meeting of the American College of Gastroenterology.
Mascarenhas noted that pancreatic cystic lesions (PCLs) are a common incidental finding during imaging and are differentiated by whether they’re mucinous PCLs (M-PCLs) or non-mucinous PCLs (NM-PCLs). The malignancy risk is almost exclusive of PCL with a mucinous phenotype.
Pancreatic solid lesions are also prevalent, and differentiation is challenging. Pancreatic ductal adenocarcinoma (P-DAC) is the most common pancreatic solid lesion and has a poor prognosis because of late-stage disease at diagnosis. Pancreatic neuroendocrine tumors (P-NETs) are less common but have malignant potential.
EUS is the “gold standard” for pancreatic lesion evaluation, but its diagnostic accuracy is suboptimal, particularly for lesions < 10 mm, Mascarenhas noted.
With an eye toward improving diagnostic accuracy, he and colleagues developed a convolutional neural network for detecting and differentiating cystic (M-PCL and NM-PCL) and solid (P-DAC and P-NET) pancreatic lesions.
They leveraged data from 378 EUS exams with 126,000 still images — 19,528 M-PCL, 8175 NM-PCL, 64,286 P-DAC, 29,153 P-NET, and 4858 normal pancreas images.
The AI tool demonstrated 99.1% accuracy for identifying normal pancreatic tissue, and it showed 99% and 99.8% accuracy for M-PCL and NM-PCL, respectively.
For pancreatic solid lesions, P-DAC and P-NET were distinguished with 94% accuracy, with 98.7% and 83.6% sensitivity for P-DAC and P-NET, respectively.
Real-Time Validation Next
“AI is delivering promising results throughout medicine, but particularly in gastroenterology, which is one of the most fertile areas of AI research. This comes mostly from the deployment of deep-learning models, most of them convolutional neural networks, which are highly efficient for image analysis,” Mascarenhas told attendees.
This is the “first worldwide convolutional neural network” capable of detecting and differentiating both cystic and solid pancreatic lesions. The use of a large dataset from four centers in two continents helps minimize the impact of demographic bias, Mascarenhas added.
The study is based on still images, not full videos, he noted. As a next step, the team is conducting a multicenter study focused on real-time clinical validation of the model during EUS procedures.
“AI has the potential to improve the diagnostic accuracy of endoscopic ultrasound. We’re just on the tip of the iceberg. There is enormous potential to harness AI, and we welcome all the groups that might want to join our research,” Mascarenhas said.
Brennan Spiegel, MD, MSHS, AGAF, director of Health Services Research at Cedars-Sinai Medical Center, Los Angeles, who wasn’t involved in the study, is optimistic about emerging applications for AI.
“AI holds incredible promise in gastroenterology, especially for diagnosing complex pancreatic lesions where early, accurate differentiation can be lifesaving,” Spiegel said in an interview.
“This study’s high accuracy across diverse datasets is encouraging; however, as a retrospective analysis, it leaves the real-time clinical impact still to be proven. Prospective studies will be essential to confirm AI’s role in enhancing our diagnostic capabilities,” Spiegel cautioned.
“More generally, AI is rapidly transforming gastroenterology by enhancing our ability to detect, differentiate, and monitor conditions with unprecedented precision. From improving early cancer detection to guiding complex diagnostic procedures, AI stands to become an invaluable tool that complements clinical expertise. As we refine these technologies, the potential for AI to elevate both diagnostic accuracy and patient outcomes in GI is truly remarkable,” Spiegel said.
The study had no specific funding. Mascarenhas and Spiegel have declared no conflicts of interest.
A version of this article appeared on Medscape.com.
PHILADELPHIA —
This was a transatlantic collaborative effort involving researchers in Portugal, Spain, the United States, and Brazil, and the AI tool “works on different platforms and different devices,” Miguel Mascarenhas, MD, PhD, with Centro Hospitalar Universitário de São João, Porto, Portugal, said in a presentation at the annual meeting of the American College of Gastroenterology.
Mascarenhas noted that pancreatic cystic lesions (PCLs) are a common incidental finding during imaging and are differentiated by whether they’re mucinous PCLs (M-PCLs) or non-mucinous PCLs (NM-PCLs). The malignancy risk is almost exclusive of PCL with a mucinous phenotype.
Pancreatic solid lesions are also prevalent, and differentiation is challenging. Pancreatic ductal adenocarcinoma (P-DAC) is the most common pancreatic solid lesion and has a poor prognosis because of late-stage disease at diagnosis. Pancreatic neuroendocrine tumors (P-NETs) are less common but have malignant potential.
EUS is the “gold standard” for pancreatic lesion evaluation, but its diagnostic accuracy is suboptimal, particularly for lesions < 10 mm, Mascarenhas noted.
With an eye toward improving diagnostic accuracy, he and colleagues developed a convolutional neural network for detecting and differentiating cystic (M-PCL and NM-PCL) and solid (P-DAC and P-NET) pancreatic lesions.
They leveraged data from 378 EUS exams with 126,000 still images — 19,528 M-PCL, 8175 NM-PCL, 64,286 P-DAC, 29,153 P-NET, and 4858 normal pancreas images.
The AI tool demonstrated 99.1% accuracy for identifying normal pancreatic tissue, and it showed 99% and 99.8% accuracy for M-PCL and NM-PCL, respectively.
For pancreatic solid lesions, P-DAC and P-NET were distinguished with 94% accuracy, with 98.7% and 83.6% sensitivity for P-DAC and P-NET, respectively.
Real-Time Validation Next
“AI is delivering promising results throughout medicine, but particularly in gastroenterology, which is one of the most fertile areas of AI research. This comes mostly from the deployment of deep-learning models, most of them convolutional neural networks, which are highly efficient for image analysis,” Mascarenhas told attendees.
This is the “first worldwide convolutional neural network” capable of detecting and differentiating both cystic and solid pancreatic lesions. The use of a large dataset from four centers in two continents helps minimize the impact of demographic bias, Mascarenhas added.
The study is based on still images, not full videos, he noted. As a next step, the team is conducting a multicenter study focused on real-time clinical validation of the model during EUS procedures.
“AI has the potential to improve the diagnostic accuracy of endoscopic ultrasound. We’re just on the tip of the iceberg. There is enormous potential to harness AI, and we welcome all the groups that might want to join our research,” Mascarenhas said.
Brennan Spiegel, MD, MSHS, AGAF, director of Health Services Research at Cedars-Sinai Medical Center, Los Angeles, who wasn’t involved in the study, is optimistic about emerging applications for AI.
“AI holds incredible promise in gastroenterology, especially for diagnosing complex pancreatic lesions where early, accurate differentiation can be lifesaving,” Spiegel said in an interview.
“This study’s high accuracy across diverse datasets is encouraging; however, as a retrospective analysis, it leaves the real-time clinical impact still to be proven. Prospective studies will be essential to confirm AI’s role in enhancing our diagnostic capabilities,” Spiegel cautioned.
“More generally, AI is rapidly transforming gastroenterology by enhancing our ability to detect, differentiate, and monitor conditions with unprecedented precision. From improving early cancer detection to guiding complex diagnostic procedures, AI stands to become an invaluable tool that complements clinical expertise. As we refine these technologies, the potential for AI to elevate both diagnostic accuracy and patient outcomes in GI is truly remarkable,” Spiegel said.
The study had no specific funding. Mascarenhas and Spiegel have declared no conflicts of interest.
A version of this article appeared on Medscape.com.
PHILADELPHIA —
This was a transatlantic collaborative effort involving researchers in Portugal, Spain, the United States, and Brazil, and the AI tool “works on different platforms and different devices,” Miguel Mascarenhas, MD, PhD, with Centro Hospitalar Universitário de São João, Porto, Portugal, said in a presentation at the annual meeting of the American College of Gastroenterology.
Mascarenhas noted that pancreatic cystic lesions (PCLs) are a common incidental finding during imaging and are differentiated by whether they’re mucinous PCLs (M-PCLs) or non-mucinous PCLs (NM-PCLs). The malignancy risk is almost exclusive of PCL with a mucinous phenotype.
Pancreatic solid lesions are also prevalent, and differentiation is challenging. Pancreatic ductal adenocarcinoma (P-DAC) is the most common pancreatic solid lesion and has a poor prognosis because of late-stage disease at diagnosis. Pancreatic neuroendocrine tumors (P-NETs) are less common but have malignant potential.
EUS is the “gold standard” for pancreatic lesion evaluation, but its diagnostic accuracy is suboptimal, particularly for lesions < 10 mm, Mascarenhas noted.
With an eye toward improving diagnostic accuracy, he and colleagues developed a convolutional neural network for detecting and differentiating cystic (M-PCL and NM-PCL) and solid (P-DAC and P-NET) pancreatic lesions.
They leveraged data from 378 EUS exams with 126,000 still images — 19,528 M-PCL, 8175 NM-PCL, 64,286 P-DAC, 29,153 P-NET, and 4858 normal pancreas images.
The AI tool demonstrated 99.1% accuracy for identifying normal pancreatic tissue, and it showed 99% and 99.8% accuracy for M-PCL and NM-PCL, respectively.
For pancreatic solid lesions, P-DAC and P-NET were distinguished with 94% accuracy, with 98.7% and 83.6% sensitivity for P-DAC and P-NET, respectively.
Real-Time Validation Next
“AI is delivering promising results throughout medicine, but particularly in gastroenterology, which is one of the most fertile areas of AI research. This comes mostly from the deployment of deep-learning models, most of them convolutional neural networks, which are highly efficient for image analysis,” Mascarenhas told attendees.
This is the “first worldwide convolutional neural network” capable of detecting and differentiating both cystic and solid pancreatic lesions. The use of a large dataset from four centers in two continents helps minimize the impact of demographic bias, Mascarenhas added.
The study is based on still images, not full videos, he noted. As a next step, the team is conducting a multicenter study focused on real-time clinical validation of the model during EUS procedures.
“AI has the potential to improve the diagnostic accuracy of endoscopic ultrasound. We’re just on the tip of the iceberg. There is enormous potential to harness AI, and we welcome all the groups that might want to join our research,” Mascarenhas said.
Brennan Spiegel, MD, MSHS, AGAF, director of Health Services Research at Cedars-Sinai Medical Center, Los Angeles, who wasn’t involved in the study, is optimistic about emerging applications for AI.
“AI holds incredible promise in gastroenterology, especially for diagnosing complex pancreatic lesions where early, accurate differentiation can be lifesaving,” Spiegel said in an interview.
“This study’s high accuracy across diverse datasets is encouraging; however, as a retrospective analysis, it leaves the real-time clinical impact still to be proven. Prospective studies will be essential to confirm AI’s role in enhancing our diagnostic capabilities,” Spiegel cautioned.
“More generally, AI is rapidly transforming gastroenterology by enhancing our ability to detect, differentiate, and monitor conditions with unprecedented precision. From improving early cancer detection to guiding complex diagnostic procedures, AI stands to become an invaluable tool that complements clinical expertise. As we refine these technologies, the potential for AI to elevate both diagnostic accuracy and patient outcomes in GI is truly remarkable,” Spiegel said.
The study had no specific funding. Mascarenhas and Spiegel have declared no conflicts of interest.
A version of this article appeared on Medscape.com.
FROM ACG 2024
Exocrine Pancreatic Insufficiency: Optimal PERT Dose Varies by Primary Pancreatic Disease
VIENNA, AUSTRIA — according to results of a prospective study using European registry data.
Specifically, patients with EPI caused by pancreatic cancer or pancreatectomy need significantly more enzyme replacement than patients with insufficiency caused by chronic pancreatitis and acute pancreatitis. The need to add a proton pump inhibitor (PPI) to achieve the therapeutic goal also varies by condition, the study showed.
One of the main symptoms of EPI is malnutrition, and successful PERT is defined as the resolution of nutritional deficiencies and relief of symptoms and signs associated with insufficiency, said study lead Enrique Domínguez Muñoz, MD, director of the department of gastroenterology and hepatology at University Hospital of Santiago de Compostela, Spain.
Our findings show that, “in order to achieve this, enzyme dose escalation and sometimes additional treatment with a [PPI] should be applied as required by the individual”, he reported in a presentation at the United European Gastroenterology (UEG) Week 2024.
Therefore, having dose recommendations for PERT for different causes of EPI is very helpful, said Domínguez Muñoz.
Pancreatic enzyme preparations, specifically pancreatin, are the recommended first-line treatment for EPI, but the initial doses of PERT vary depending on the patient’s age (whether adult or child), the severity of the insufficiency, and the fat content of the meal eaten.
Domínguez Muñoz and colleagues wanted to explore whether — and how — the severity of EPI varied with different diseases, therefore varying the optimal dose of PERT.
Optimal Dosing to Achieve Therapeutic Goal
The prospective study drew on data from a European multicenter registry of patients diagnosed with EPI being treated with PERT in expert centers.
The researchers evaluated the dose of PERT required to achieve symptom relief and normalization of the nutritional status in adult patients with EPI secondary to different pancreatic diseases and conditions. The percentage of patients who required the addition of a PPI to PERT to achieve the therapeutic goal was also determined.
Decisions on the initial enzyme dose (including the addition of a PPI) and any necessary adjustments during follow-up to achieve the therapeutic goal were made by the participants’ clinicians.
A total of 678 patients (mean age, 61.2 ± 13.8 years; 63.6% male) were stratified according to disease: 50% had chronic pancreatitis, 10% had acute pancreatitis, 17% had undergone pancreaticoduodenectomy, 15% had pancreatic cancer, and 8% had another pancreatic condition.
To achieve the therapeutic goal, the median optimal enzyme doses with the main meal for patients with acute pancreatitis, chronic pancreatitis, pancreatic cancer, and pancreaticoduodenectomy, were 40,000, 50,000, 70,000, and 75,000 Ph.U, respectively. The respective optimal daily enzyme doses were 100,000, 150,000, 210,000, and 225,000 Ph.U.
The highest enzyme doses required with the main meal to achieve the therapeutic goal for patients with acute pancreatitis, chronic pancreatitis, pancreatic cancer, and pancreaticoduodenectomy were 125,000, 210,000, 175,000, and 210,000 Ph.U, respectively. The respective highest daily enzyme doses were 400,000, 625,000, 675,000, and 750,000 Ph.U.
The need for additional therapy with twice-daily PPI to achieve the therapeutic goal also varied according to the underlying disease. It was administered to 44.1% of patients with acute pancreatitis, 37.2% of patients with chronic pancreatitis, 78.8% of patients with pancreatic cancer, and 74.1% of patients who had undergone pancreaticoduodenectomy.
“This shows us that sometimes we really do need to significantly increase the dose of pancreatic enzyme replacement therapy,” reported Domínguez Muñoz.
Clear Direction on Where to Start
Comoderator Kasper Overbeek, MD, from the department of gastroenterology and hepatology, Erasmus MC Cancer Institute, University Medical Center, the Netherlands, commented: “It’s a useful study because it gives us practical advice on what to do in specific cases.”
Until now, we’ve done the same thing for everyone, he said, “but these data clearly show that this is not optimal.”
In addition, “it is often the case with enzyme replacement therapy that doctors under-dose so it is necessary to increase the dose,” he said.
“This work gives us a clearer direction on where to start,” Overbeek said. “For example, with patients who have cancer, because they do not have time to start low and titrate up, they need a higher dose than patients with chronic pancreatitis.”
This pragmatic and novel guidance will “help us in our clinical practice,” he added.
Domínguez Muñoz reports receiving speaking and consultancy fees from Viatris, Abbott Pharmaceuticals, and Boston Scientific. Overbeek reports no relevant disclosures.
A version of this article first appeared on Medscape.com.
VIENNA, AUSTRIA — according to results of a prospective study using European registry data.
Specifically, patients with EPI caused by pancreatic cancer or pancreatectomy need significantly more enzyme replacement than patients with insufficiency caused by chronic pancreatitis and acute pancreatitis. The need to add a proton pump inhibitor (PPI) to achieve the therapeutic goal also varies by condition, the study showed.
One of the main symptoms of EPI is malnutrition, and successful PERT is defined as the resolution of nutritional deficiencies and relief of symptoms and signs associated with insufficiency, said study lead Enrique Domínguez Muñoz, MD, director of the department of gastroenterology and hepatology at University Hospital of Santiago de Compostela, Spain.
Our findings show that, “in order to achieve this, enzyme dose escalation and sometimes additional treatment with a [PPI] should be applied as required by the individual”, he reported in a presentation at the United European Gastroenterology (UEG) Week 2024.
Therefore, having dose recommendations for PERT for different causes of EPI is very helpful, said Domínguez Muñoz.
Pancreatic enzyme preparations, specifically pancreatin, are the recommended first-line treatment for EPI, but the initial doses of PERT vary depending on the patient’s age (whether adult or child), the severity of the insufficiency, and the fat content of the meal eaten.
Domínguez Muñoz and colleagues wanted to explore whether — and how — the severity of EPI varied with different diseases, therefore varying the optimal dose of PERT.
Optimal Dosing to Achieve Therapeutic Goal
The prospective study drew on data from a European multicenter registry of patients diagnosed with EPI being treated with PERT in expert centers.
The researchers evaluated the dose of PERT required to achieve symptom relief and normalization of the nutritional status in adult patients with EPI secondary to different pancreatic diseases and conditions. The percentage of patients who required the addition of a PPI to PERT to achieve the therapeutic goal was also determined.
Decisions on the initial enzyme dose (including the addition of a PPI) and any necessary adjustments during follow-up to achieve the therapeutic goal were made by the participants’ clinicians.
A total of 678 patients (mean age, 61.2 ± 13.8 years; 63.6% male) were stratified according to disease: 50% had chronic pancreatitis, 10% had acute pancreatitis, 17% had undergone pancreaticoduodenectomy, 15% had pancreatic cancer, and 8% had another pancreatic condition.
To achieve the therapeutic goal, the median optimal enzyme doses with the main meal for patients with acute pancreatitis, chronic pancreatitis, pancreatic cancer, and pancreaticoduodenectomy, were 40,000, 50,000, 70,000, and 75,000 Ph.U, respectively. The respective optimal daily enzyme doses were 100,000, 150,000, 210,000, and 225,000 Ph.U.
The highest enzyme doses required with the main meal to achieve the therapeutic goal for patients with acute pancreatitis, chronic pancreatitis, pancreatic cancer, and pancreaticoduodenectomy were 125,000, 210,000, 175,000, and 210,000 Ph.U, respectively. The respective highest daily enzyme doses were 400,000, 625,000, 675,000, and 750,000 Ph.U.
The need for additional therapy with twice-daily PPI to achieve the therapeutic goal also varied according to the underlying disease. It was administered to 44.1% of patients with acute pancreatitis, 37.2% of patients with chronic pancreatitis, 78.8% of patients with pancreatic cancer, and 74.1% of patients who had undergone pancreaticoduodenectomy.
“This shows us that sometimes we really do need to significantly increase the dose of pancreatic enzyme replacement therapy,” reported Domínguez Muñoz.
Clear Direction on Where to Start
Comoderator Kasper Overbeek, MD, from the department of gastroenterology and hepatology, Erasmus MC Cancer Institute, University Medical Center, the Netherlands, commented: “It’s a useful study because it gives us practical advice on what to do in specific cases.”
Until now, we’ve done the same thing for everyone, he said, “but these data clearly show that this is not optimal.”
In addition, “it is often the case with enzyme replacement therapy that doctors under-dose so it is necessary to increase the dose,” he said.
“This work gives us a clearer direction on where to start,” Overbeek said. “For example, with patients who have cancer, because they do not have time to start low and titrate up, they need a higher dose than patients with chronic pancreatitis.”
This pragmatic and novel guidance will “help us in our clinical practice,” he added.
Domínguez Muñoz reports receiving speaking and consultancy fees from Viatris, Abbott Pharmaceuticals, and Boston Scientific. Overbeek reports no relevant disclosures.
A version of this article first appeared on Medscape.com.
VIENNA, AUSTRIA — according to results of a prospective study using European registry data.
Specifically, patients with EPI caused by pancreatic cancer or pancreatectomy need significantly more enzyme replacement than patients with insufficiency caused by chronic pancreatitis and acute pancreatitis. The need to add a proton pump inhibitor (PPI) to achieve the therapeutic goal also varies by condition, the study showed.
One of the main symptoms of EPI is malnutrition, and successful PERT is defined as the resolution of nutritional deficiencies and relief of symptoms and signs associated with insufficiency, said study lead Enrique Domínguez Muñoz, MD, director of the department of gastroenterology and hepatology at University Hospital of Santiago de Compostela, Spain.
Our findings show that, “in order to achieve this, enzyme dose escalation and sometimes additional treatment with a [PPI] should be applied as required by the individual”, he reported in a presentation at the United European Gastroenterology (UEG) Week 2024.
Therefore, having dose recommendations for PERT for different causes of EPI is very helpful, said Domínguez Muñoz.
Pancreatic enzyme preparations, specifically pancreatin, are the recommended first-line treatment for EPI, but the initial doses of PERT vary depending on the patient’s age (whether adult or child), the severity of the insufficiency, and the fat content of the meal eaten.
Domínguez Muñoz and colleagues wanted to explore whether — and how — the severity of EPI varied with different diseases, therefore varying the optimal dose of PERT.
Optimal Dosing to Achieve Therapeutic Goal
The prospective study drew on data from a European multicenter registry of patients diagnosed with EPI being treated with PERT in expert centers.
The researchers evaluated the dose of PERT required to achieve symptom relief and normalization of the nutritional status in adult patients with EPI secondary to different pancreatic diseases and conditions. The percentage of patients who required the addition of a PPI to PERT to achieve the therapeutic goal was also determined.
Decisions on the initial enzyme dose (including the addition of a PPI) and any necessary adjustments during follow-up to achieve the therapeutic goal were made by the participants’ clinicians.
A total of 678 patients (mean age, 61.2 ± 13.8 years; 63.6% male) were stratified according to disease: 50% had chronic pancreatitis, 10% had acute pancreatitis, 17% had undergone pancreaticoduodenectomy, 15% had pancreatic cancer, and 8% had another pancreatic condition.
To achieve the therapeutic goal, the median optimal enzyme doses with the main meal for patients with acute pancreatitis, chronic pancreatitis, pancreatic cancer, and pancreaticoduodenectomy, were 40,000, 50,000, 70,000, and 75,000 Ph.U, respectively. The respective optimal daily enzyme doses were 100,000, 150,000, 210,000, and 225,000 Ph.U.
The highest enzyme doses required with the main meal to achieve the therapeutic goal for patients with acute pancreatitis, chronic pancreatitis, pancreatic cancer, and pancreaticoduodenectomy were 125,000, 210,000, 175,000, and 210,000 Ph.U, respectively. The respective highest daily enzyme doses were 400,000, 625,000, 675,000, and 750,000 Ph.U.
The need for additional therapy with twice-daily PPI to achieve the therapeutic goal also varied according to the underlying disease. It was administered to 44.1% of patients with acute pancreatitis, 37.2% of patients with chronic pancreatitis, 78.8% of patients with pancreatic cancer, and 74.1% of patients who had undergone pancreaticoduodenectomy.
“This shows us that sometimes we really do need to significantly increase the dose of pancreatic enzyme replacement therapy,” reported Domínguez Muñoz.
Clear Direction on Where to Start
Comoderator Kasper Overbeek, MD, from the department of gastroenterology and hepatology, Erasmus MC Cancer Institute, University Medical Center, the Netherlands, commented: “It’s a useful study because it gives us practical advice on what to do in specific cases.”
Until now, we’ve done the same thing for everyone, he said, “but these data clearly show that this is not optimal.”
In addition, “it is often the case with enzyme replacement therapy that doctors under-dose so it is necessary to increase the dose,” he said.
“This work gives us a clearer direction on where to start,” Overbeek said. “For example, with patients who have cancer, because they do not have time to start low and titrate up, they need a higher dose than patients with chronic pancreatitis.”
This pragmatic and novel guidance will “help us in our clinical practice,” he added.
Domínguez Muñoz reports receiving speaking and consultancy fees from Viatris, Abbott Pharmaceuticals, and Boston Scientific. Overbeek reports no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM UEG 2024
Automated ERCP Report Card Offers High Accuracy, Minimal Work
offering a real-time gauge of both individual- and institutional-level quality indicators, according to the developers.
The tool boasts an accuracy level exceeding 96%, integrates with multiple electronic health records, and requires minimal additional work time, reported Anmol Singh, MD, of TriStar Centennial Medical Center, Nashville, Tennessee, and colleagues.
“Implementation of quality indicator tracking remains difficult due to the complexity of ERCP as compared with other endoscopic procedures, resulting in significant limitations in the extraction and synthesis of these data,” the investigators wrote in Techniques and Innovations in Gastrointestinal Endoscopy. “Manual extraction methods such as self-assessment forms and chart reviews are both time intensive and error prone, and current automated extraction methods, such as natural language processing, can require substantial resources to implement and undesirably complicate the endoscopy work flow.”
To overcome these challenges, Dr. Singh and colleagues designed an analytics tool that automatically collects ERCP quality indicators from endoscopy reports with “minimal input” from the endoscopist, and is compatible with “any electronic reporting system.”
Development relied upon endoscopy records from 2,146 ERCPs performed by 12 endoscopists at four facilities. The most common reason for ERCP was choledocholithiasis, followed by malignant and benign biliary stricture. Most common procedures were stent placement and sphincterotomy.
Data were aggregated in a Health Level–7 (HL-7) interface, an international standard system that enables compatibility between different types of electronic health records. Some inputs were entered by the performing endoscopist via drop-down menus.
Next, data were shifted into an analytics suite, which evaluated quality indicators, including cannulation difficulty and success rate, and administration of post-ERCP pancreatitis prophylaxis.
Manual review showed that this approach yielded an accuracy of 96.5%-100%.
Beyond this high level of accuracy, Dr. Singh and colleagues described several reasons why their tool may be superior to previous attempts at an automated ERCP report card.
“Our HL-7–based tool offers several advantages, including versatility via compatibility with multiple types of commercial reporting software and flexibility in customizing the type and aesthetic of the data displayed,” they wrote. “These features improve the user interface, keep costs down, and allow for integration into smaller or nonacademic practice settings.”
They also highlighted how the tool measures quality in relation to procedure indication and difficulty at the provider level.
“Unlike in colonoscopy, where metrics such as adenoma detection rate can be ubiquitously applied to all screening procedures, the difficulty and risk profile of ERCP is inextricably dependent on patient and procedural factors such as indication of the procedure, history of interventions, or history of altered anatomy,” Dr. Singh and colleagues wrote. “Prior studies have shown that both the cost-effectiveness and complication rates of procedures are influenced by procedural indication and complexity. As such, benchmarking an individual provider’s performance necessarily requires the correct procedural context.”
With further optimization, this tool can be integrated into various types of existing endoscopy reporting software at a reasonable cost, and with minimal impact on routine work flow, the investigators concluded.
The investigators disclosed relationships with AbbVie, Boston Scientific, Organon, and others.
offering a real-time gauge of both individual- and institutional-level quality indicators, according to the developers.
The tool boasts an accuracy level exceeding 96%, integrates with multiple electronic health records, and requires minimal additional work time, reported Anmol Singh, MD, of TriStar Centennial Medical Center, Nashville, Tennessee, and colleagues.
“Implementation of quality indicator tracking remains difficult due to the complexity of ERCP as compared with other endoscopic procedures, resulting in significant limitations in the extraction and synthesis of these data,” the investigators wrote in Techniques and Innovations in Gastrointestinal Endoscopy. “Manual extraction methods such as self-assessment forms and chart reviews are both time intensive and error prone, and current automated extraction methods, such as natural language processing, can require substantial resources to implement and undesirably complicate the endoscopy work flow.”
To overcome these challenges, Dr. Singh and colleagues designed an analytics tool that automatically collects ERCP quality indicators from endoscopy reports with “minimal input” from the endoscopist, and is compatible with “any electronic reporting system.”
Development relied upon endoscopy records from 2,146 ERCPs performed by 12 endoscopists at four facilities. The most common reason for ERCP was choledocholithiasis, followed by malignant and benign biliary stricture. Most common procedures were stent placement and sphincterotomy.
Data were aggregated in a Health Level–7 (HL-7) interface, an international standard system that enables compatibility between different types of electronic health records. Some inputs were entered by the performing endoscopist via drop-down menus.
Next, data were shifted into an analytics suite, which evaluated quality indicators, including cannulation difficulty and success rate, and administration of post-ERCP pancreatitis prophylaxis.
Manual review showed that this approach yielded an accuracy of 96.5%-100%.
Beyond this high level of accuracy, Dr. Singh and colleagues described several reasons why their tool may be superior to previous attempts at an automated ERCP report card.
“Our HL-7–based tool offers several advantages, including versatility via compatibility with multiple types of commercial reporting software and flexibility in customizing the type and aesthetic of the data displayed,” they wrote. “These features improve the user interface, keep costs down, and allow for integration into smaller or nonacademic practice settings.”
They also highlighted how the tool measures quality in relation to procedure indication and difficulty at the provider level.
“Unlike in colonoscopy, where metrics such as adenoma detection rate can be ubiquitously applied to all screening procedures, the difficulty and risk profile of ERCP is inextricably dependent on patient and procedural factors such as indication of the procedure, history of interventions, or history of altered anatomy,” Dr. Singh and colleagues wrote. “Prior studies have shown that both the cost-effectiveness and complication rates of procedures are influenced by procedural indication and complexity. As such, benchmarking an individual provider’s performance necessarily requires the correct procedural context.”
With further optimization, this tool can be integrated into various types of existing endoscopy reporting software at a reasonable cost, and with minimal impact on routine work flow, the investigators concluded.
The investigators disclosed relationships with AbbVie, Boston Scientific, Organon, and others.
offering a real-time gauge of both individual- and institutional-level quality indicators, according to the developers.
The tool boasts an accuracy level exceeding 96%, integrates with multiple electronic health records, and requires minimal additional work time, reported Anmol Singh, MD, of TriStar Centennial Medical Center, Nashville, Tennessee, and colleagues.
“Implementation of quality indicator tracking remains difficult due to the complexity of ERCP as compared with other endoscopic procedures, resulting in significant limitations in the extraction and synthesis of these data,” the investigators wrote in Techniques and Innovations in Gastrointestinal Endoscopy. “Manual extraction methods such as self-assessment forms and chart reviews are both time intensive and error prone, and current automated extraction methods, such as natural language processing, can require substantial resources to implement and undesirably complicate the endoscopy work flow.”
To overcome these challenges, Dr. Singh and colleagues designed an analytics tool that automatically collects ERCP quality indicators from endoscopy reports with “minimal input” from the endoscopist, and is compatible with “any electronic reporting system.”
Development relied upon endoscopy records from 2,146 ERCPs performed by 12 endoscopists at four facilities. The most common reason for ERCP was choledocholithiasis, followed by malignant and benign biliary stricture. Most common procedures were stent placement and sphincterotomy.
Data were aggregated in a Health Level–7 (HL-7) interface, an international standard system that enables compatibility between different types of electronic health records. Some inputs were entered by the performing endoscopist via drop-down menus.
Next, data were shifted into an analytics suite, which evaluated quality indicators, including cannulation difficulty and success rate, and administration of post-ERCP pancreatitis prophylaxis.
Manual review showed that this approach yielded an accuracy of 96.5%-100%.
Beyond this high level of accuracy, Dr. Singh and colleagues described several reasons why their tool may be superior to previous attempts at an automated ERCP report card.
“Our HL-7–based tool offers several advantages, including versatility via compatibility with multiple types of commercial reporting software and flexibility in customizing the type and aesthetic of the data displayed,” they wrote. “These features improve the user interface, keep costs down, and allow for integration into smaller or nonacademic practice settings.”
They also highlighted how the tool measures quality in relation to procedure indication and difficulty at the provider level.
“Unlike in colonoscopy, where metrics such as adenoma detection rate can be ubiquitously applied to all screening procedures, the difficulty and risk profile of ERCP is inextricably dependent on patient and procedural factors such as indication of the procedure, history of interventions, or history of altered anatomy,” Dr. Singh and colleagues wrote. “Prior studies have shown that both the cost-effectiveness and complication rates of procedures are influenced by procedural indication and complexity. As such, benchmarking an individual provider’s performance necessarily requires the correct procedural context.”
With further optimization, this tool can be integrated into various types of existing endoscopy reporting software at a reasonable cost, and with minimal impact on routine work flow, the investigators concluded.
The investigators disclosed relationships with AbbVie, Boston Scientific, Organon, and others.
FROM TECHNIQUES AND INNOVATIONS IN GASTROINTESTINAL ENDOSCOPY
Late ERCP After Cholecystectomy Linked with Worse Outcomes
, according to investigators.
These findings suggest a need for more careful patient selection with ERCP, and greater reliance upon noninvasive imaging prior to considering the procedure, reported lead author Nikhil R. Thiruvengadam, MD, of Loma Linda University Health, Loma Linda, California, and colleagues.
“It is assumed that cholecystectomy is a definitive procedure for symptomatic gallstone disease in patients without concomitant choledocholithiasis,” the investigators wrote in Clinical Gastroenterology and Hepatology. “This is because the development of primary choledocholithiasis is rare. Despite this, many patients have persistent or new gastrointestinal symptoms post cholecystectomy.”
Symptoms such as a dilated bile duct or abnormal liver function tests may suggest choledocholithiasis or sphincter of Oddi disorders (SOD), they noted, but recent data supporting ERCP for SOD show no significant benefit for patients with normal-sized ducts.
“Guidelines advocate for confirming the presence of choledocholithiasis using magnetic resonance cholangiopancreatography (MRCP) or endoscopic ultrasound (EUS) given the substantial risks associated with ERCP,” Dr. Thiruvengadam and colleagues wrote.
Real-world implementation of this and associated strategies, however, remain unclear, prompting the present study.
The dataset, drawn from the Optum Clinformatics Data Mart, included 583,712 adults who had undergone cholecystectomy from 2004 to 2019, focusing on 4274 individuals who had their first ERCP more than one year post surgery. The investigators assessed the incidence, characteristics, and outcomes of these late ERCP procedures, exploring their association with patient comorbidities and the use of biliary imaging techniques such as MRCP and EUS.
From 2004 to 2021, use of noninvasive biliary imaging approximately doubled from 35.9% to 65.5% (P < .001). Yet incidence of first-time ERCP more than 1 year after cholecystectomy increased much more — by eightfold — from 0.5 to 4.2 per 1000 person-years (P < .001). Less than half (44%) of these late ERCP procedures involved gallstone removal.
Patients undergoing late ERCP were more likely to have higher baseline comorbidities, including disorders of gut-brain interaction (DGBI) and metabolic dysfunction-associated steatotic liver disease. They were also more likely to be taking an antispasmodic, anxiolytic, or chronic opioid medication.
“Late ERCP is more common and associated with worse outcomes, presumably because of higher baseline comorbidities that overlap with DGBI and mimickers of choledocholithiasis,” the investigators noted. “These highly symptomatic individuals are more likely to undergo noninvasive biliary imaging, which seems to be prompting more late ERCP.”
In turn, late ERCP is incurring more adverse events, including post-ERCP pancreatitis (7.1%), hospitalization (13.1%), and new chronic opioid use (9.7%).
“Given the known risks of ERCP, especially in this context, there remains a need to be more restrictive with offering ERCP in this setting,” Dr. Thiruvengadam and colleagues concluded. “ERCP should be used sparingly for patients who do not have confirmed choledocholithiasis until future studies ... can define which patients with a remote history of cholecystectomy respond to ERCP interventions.”
The investigators disclosed relationships with Olympus, Medtronic, ACI, and others.
, according to investigators.
These findings suggest a need for more careful patient selection with ERCP, and greater reliance upon noninvasive imaging prior to considering the procedure, reported lead author Nikhil R. Thiruvengadam, MD, of Loma Linda University Health, Loma Linda, California, and colleagues.
“It is assumed that cholecystectomy is a definitive procedure for symptomatic gallstone disease in patients without concomitant choledocholithiasis,” the investigators wrote in Clinical Gastroenterology and Hepatology. “This is because the development of primary choledocholithiasis is rare. Despite this, many patients have persistent or new gastrointestinal symptoms post cholecystectomy.”
Symptoms such as a dilated bile duct or abnormal liver function tests may suggest choledocholithiasis or sphincter of Oddi disorders (SOD), they noted, but recent data supporting ERCP for SOD show no significant benefit for patients with normal-sized ducts.
“Guidelines advocate for confirming the presence of choledocholithiasis using magnetic resonance cholangiopancreatography (MRCP) or endoscopic ultrasound (EUS) given the substantial risks associated with ERCP,” Dr. Thiruvengadam and colleagues wrote.
Real-world implementation of this and associated strategies, however, remain unclear, prompting the present study.
The dataset, drawn from the Optum Clinformatics Data Mart, included 583,712 adults who had undergone cholecystectomy from 2004 to 2019, focusing on 4274 individuals who had their first ERCP more than one year post surgery. The investigators assessed the incidence, characteristics, and outcomes of these late ERCP procedures, exploring their association with patient comorbidities and the use of biliary imaging techniques such as MRCP and EUS.
From 2004 to 2021, use of noninvasive biliary imaging approximately doubled from 35.9% to 65.5% (P < .001). Yet incidence of first-time ERCP more than 1 year after cholecystectomy increased much more — by eightfold — from 0.5 to 4.2 per 1000 person-years (P < .001). Less than half (44%) of these late ERCP procedures involved gallstone removal.
Patients undergoing late ERCP were more likely to have higher baseline comorbidities, including disorders of gut-brain interaction (DGBI) and metabolic dysfunction-associated steatotic liver disease. They were also more likely to be taking an antispasmodic, anxiolytic, or chronic opioid medication.
“Late ERCP is more common and associated with worse outcomes, presumably because of higher baseline comorbidities that overlap with DGBI and mimickers of choledocholithiasis,” the investigators noted. “These highly symptomatic individuals are more likely to undergo noninvasive biliary imaging, which seems to be prompting more late ERCP.”
In turn, late ERCP is incurring more adverse events, including post-ERCP pancreatitis (7.1%), hospitalization (13.1%), and new chronic opioid use (9.7%).
“Given the known risks of ERCP, especially in this context, there remains a need to be more restrictive with offering ERCP in this setting,” Dr. Thiruvengadam and colleagues concluded. “ERCP should be used sparingly for patients who do not have confirmed choledocholithiasis until future studies ... can define which patients with a remote history of cholecystectomy respond to ERCP interventions.”
The investigators disclosed relationships with Olympus, Medtronic, ACI, and others.
, according to investigators.
These findings suggest a need for more careful patient selection with ERCP, and greater reliance upon noninvasive imaging prior to considering the procedure, reported lead author Nikhil R. Thiruvengadam, MD, of Loma Linda University Health, Loma Linda, California, and colleagues.
“It is assumed that cholecystectomy is a definitive procedure for symptomatic gallstone disease in patients without concomitant choledocholithiasis,” the investigators wrote in Clinical Gastroenterology and Hepatology. “This is because the development of primary choledocholithiasis is rare. Despite this, many patients have persistent or new gastrointestinal symptoms post cholecystectomy.”
Symptoms such as a dilated bile duct or abnormal liver function tests may suggest choledocholithiasis or sphincter of Oddi disorders (SOD), they noted, but recent data supporting ERCP for SOD show no significant benefit for patients with normal-sized ducts.
“Guidelines advocate for confirming the presence of choledocholithiasis using magnetic resonance cholangiopancreatography (MRCP) or endoscopic ultrasound (EUS) given the substantial risks associated with ERCP,” Dr. Thiruvengadam and colleagues wrote.
Real-world implementation of this and associated strategies, however, remain unclear, prompting the present study.
The dataset, drawn from the Optum Clinformatics Data Mart, included 583,712 adults who had undergone cholecystectomy from 2004 to 2019, focusing on 4274 individuals who had their first ERCP more than one year post surgery. The investigators assessed the incidence, characteristics, and outcomes of these late ERCP procedures, exploring their association with patient comorbidities and the use of biliary imaging techniques such as MRCP and EUS.
From 2004 to 2021, use of noninvasive biliary imaging approximately doubled from 35.9% to 65.5% (P < .001). Yet incidence of first-time ERCP more than 1 year after cholecystectomy increased much more — by eightfold — from 0.5 to 4.2 per 1000 person-years (P < .001). Less than half (44%) of these late ERCP procedures involved gallstone removal.
Patients undergoing late ERCP were more likely to have higher baseline comorbidities, including disorders of gut-brain interaction (DGBI) and metabolic dysfunction-associated steatotic liver disease. They were also more likely to be taking an antispasmodic, anxiolytic, or chronic opioid medication.
“Late ERCP is more common and associated with worse outcomes, presumably because of higher baseline comorbidities that overlap with DGBI and mimickers of choledocholithiasis,” the investigators noted. “These highly symptomatic individuals are more likely to undergo noninvasive biliary imaging, which seems to be prompting more late ERCP.”
In turn, late ERCP is incurring more adverse events, including post-ERCP pancreatitis (7.1%), hospitalization (13.1%), and new chronic opioid use (9.7%).
“Given the known risks of ERCP, especially in this context, there remains a need to be more restrictive with offering ERCP in this setting,” Dr. Thiruvengadam and colleagues concluded. “ERCP should be used sparingly for patients who do not have confirmed choledocholithiasis until future studies ... can define which patients with a remote history of cholecystectomy respond to ERCP interventions.”
The investigators disclosed relationships with Olympus, Medtronic, ACI, and others.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
August 2024 – ICYMI
Gastroenterology
April 2024
Shah I, et al. Disparities in Colorectal Cancer Screening Among Asian American Populations and Strategies to Address These Disparities. Gastroenterology. 2024 Apr;166(4):549-552. doi: 10.1053/j.gastro.2024.02.009. PMID: 38521575.
Shiha MG, et al. Accuracy of the No-Biopsy Approach for the Diagnosis of Celiac Disease in Adults: A Systematic Review and Meta-Analysis. Gastroenterology. 2024 Apr;166(4):620-630. doi: 10.1053/j.gastro.2023.12.023. Epub 2024 Jan 2. PMID: 38176661.
Goltstein LCMJ, et al. Standard of Care Versus Octreotide in Angiodysplasia-Related Bleeding (the OCEAN Study): A Multicenter Randomized Controlled Trial. Gastroenterology. 2024 Apr;166(4):690-703. doi: 10.1053/j.gastro.2023.12.020. Epub 2023 Dec 28. PMID: 38158089.
May 2024
Robertson DJ, et al. Colonoscopy vs the Fecal Immunochemical Test: Which is Best? Gastroenterology. 2024 May;166(5):758-771. doi: 10.1053/j.gastro.2023.12.027. Epub 2024 Feb 9. PMID: 38342196.
Mårild K, et al. Histologic Remission in Inflammatory Bowel Disease and Female Fertility: A Nationwide Study. Gastroenterology. 2024 May;166(5):802-814.e18. doi: 10.1053/j.gastro.2024.01.018. Epub 2024 Feb 6. PMID: 38331202.
June 2024
Trivedi PJ, et al. Immunopathogenesis of Primary Biliary Cholangitis, Primary Sclerosing Cholangitis and Autoimmune Hepatitis: Themes and Concepts. Gastroenterology. 2024 Jun;166(6):995-1019. doi: 10.1053/j.gastro.2024.01.049. Epub 2024 Feb 10. PMID: 38342195.
Rubenstein JH, et al. AGA Clinical Practice Guideline on Endoscopic Eradication Therapy of Barrett’s Esophagus and Related Neoplasia. Gastroenterology. 2024 Jun;166(6):1020-1055. doi: 10.1053/j.gastro.2024.03.019. PMID: 38763697.
Ridtitid W, et al. Endoscopic Gallbladder Stenting to Prevent Recurrent Cholecystitis in Deferred Cholecystectomy: A Randomized Trial. Gastroenterology. 2024 Jun;166(6):1145-1155. doi: 10.1053/j.gastro.2024.02.007. Epub 2024 Feb 14. PMID: 38360274.
Clinical Gastroenterology and Hepatology
April 2024
Berwald G, et al. The Diagnostic Performance of Fecal Immunochemical Tests for Detecting Advanced Neoplasia at Surveillance Colonoscopy. Clin Gastroenterol Hepatol. 2024 Apr;22(4):878-885.e2. doi: 10.1016/j.cgh.2023.09.016. Epub 2023 Sep 22. PMID: 37743036.
Hashash JG, et al. AGA Rapid Clinical Practice Update on the Management of Patients Taking GLP-1 Receptor Agonists Prior to Endoscopy: Communication. Clin Gastroenterol Hepatol. 2024 Apr;22(4):705-707. doi: 10.1016/j.cgh.2023.11.002. Epub 2023 Nov 7. PMID: 37944573.
Sharma R, et al. Statins Are Associated With a Decreased Risk of Severe Liver Disease in Individuals With Noncirrhotic Chronic Liver Disease. Clin Gastroenterol Hepatol. 2024 Apr;22(4):749-759.e19. doi: 10.1016/j.cgh.2023.04.017. Epub 2023 Apr 28. PMID: 37121528.
May 2024
Overbeek KA, et al; PrescrAIP Study Group. Type 1 Autoimmune Pancreatitis in Europe: Clinical Profile and Response to Treatment. Clin Gastroenterol Hepatol. 2024 May;22(5):994-1004.e10. doi: 10.1016/j.cgh.2023.12.010. Epub 2024 Jan 5. Erratum in: Clin Gastroenterol Hepatol. 2024 Jun 1:S1542-3565(24)00446-4. doi: 10.1016/j.cgh.2024.05.005. PMID: 38184096.
Jairath V, et al. ENTERPRET: A Randomized Controlled Trial of Vedolizumab Dose Optimization in Patients With Ulcerative Colitis Who Have Early Nonresponse. Clin Gastroenterol Hepatol. 2024 May;22(5):1077-1086.e13. doi: 10.1016/j.cgh.2023.10.029. Epub 2023 Nov 10. PMID: 37951560.
Gunby SA, et al. Smoking and Alcohol Consumption and Risk of Incident Diverticulitis in Women. Clin Gastroenterol Hepatol. 2024 May;22(5):1108-1116. doi: 10.1016/j.cgh.2023.11.036. Epub 2023 Dec 19. PMID: 38122959; PMCID: PMC11045313.
June 2024
Krause AJ, et al. Validated Clinical Score to Predict Gastroesophageal Reflux in Patients With Chronic Laryngeal Symptoms: COuGH RefluX. Clin Gastroenterol Hepatol. 2024 Jun;22(6):1200-1209.e1. doi: 10.1016/j.cgh.2024.01.021. Epub 2024 Feb 2. PMID: 38309491; PMCID: PMC11128352.
Peng X, et al. Efficacy and Safety of Vonoprazan-Amoxicillin Dual Regimen With Varying Dose and Duration for Helicobacter pylori Eradication: A Multicenter, Prospective, Randomized Study. Clin Gastroenterol Hepatol. 2024 Jun;22(6):1210-1216. doi: 10.1016/j.cgh.2024.01.022. Epub 2024 Feb 1. PMID: 38309492.
Kedia S, et al. Coconut Water Induces Clinical Remission in Mild to Moderate Ulcerative Colitis: Double-blind Placebo-controlled Trial. Clin Gastroenterol Hepatol. 2024 Jun;22(6):1295-1306.e7. doi: 10.1016/j.cgh.2024.01.013. Epub 2024 Jan 24. PMID: 38278200.
Techniques and Innovations in Gastrointestinal Endoscopy
Ogura T, et al. Step-Up Strategy for Endoscopic Hemostasis Using PuraStat After Endoscopic Sphincterotomy Bleeding (STOP Trial). Tech Innov Gastrointest Endosc. 2024 March 16. doi: 10.1016/j.tige.2024.03.005.
Nakai Y, et al. Cyst Detection Rate: A Quality Indicator in the Era of Pancreatic Screening Endoscopic Ultrasonography. Tech Innov Gastrointest Endosc. 2024 May. doi: 10.1016/j.tige.2024.04.001.
Gastro Hep Advances
Kimura Y, et al. Early Sonographic Improvement Predicts Clinical Remission and Mucosal Healing With Molecular-Targeted Drugs in Ulcerative Colitis. Gastro Hep Adv. 2024 April 22. doi: 10.1016/j.gastha.2024.04.007.
Hunaut T, et al. Long-Term Neoplastic Risk Associated With Colorectal Strictures in Crohn’s Disease: A Multicenter Study. Gastro Hep Adv. 2024 May 15. doi: 10.1016/j.gastha.2024.05.003.
Gastroenterology
April 2024
Shah I, et al. Disparities in Colorectal Cancer Screening Among Asian American Populations and Strategies to Address These Disparities. Gastroenterology. 2024 Apr;166(4):549-552. doi: 10.1053/j.gastro.2024.02.009. PMID: 38521575.
Shiha MG, et al. Accuracy of the No-Biopsy Approach for the Diagnosis of Celiac Disease in Adults: A Systematic Review and Meta-Analysis. Gastroenterology. 2024 Apr;166(4):620-630. doi: 10.1053/j.gastro.2023.12.023. Epub 2024 Jan 2. PMID: 38176661.
Goltstein LCMJ, et al. Standard of Care Versus Octreotide in Angiodysplasia-Related Bleeding (the OCEAN Study): A Multicenter Randomized Controlled Trial. Gastroenterology. 2024 Apr;166(4):690-703. doi: 10.1053/j.gastro.2023.12.020. Epub 2023 Dec 28. PMID: 38158089.
May 2024
Robertson DJ, et al. Colonoscopy vs the Fecal Immunochemical Test: Which is Best? Gastroenterology. 2024 May;166(5):758-771. doi: 10.1053/j.gastro.2023.12.027. Epub 2024 Feb 9. PMID: 38342196.
Mårild K, et al. Histologic Remission in Inflammatory Bowel Disease and Female Fertility: A Nationwide Study. Gastroenterology. 2024 May;166(5):802-814.e18. doi: 10.1053/j.gastro.2024.01.018. Epub 2024 Feb 6. PMID: 38331202.
June 2024
Trivedi PJ, et al. Immunopathogenesis of Primary Biliary Cholangitis, Primary Sclerosing Cholangitis and Autoimmune Hepatitis: Themes and Concepts. Gastroenterology. 2024 Jun;166(6):995-1019. doi: 10.1053/j.gastro.2024.01.049. Epub 2024 Feb 10. PMID: 38342195.
Rubenstein JH, et al. AGA Clinical Practice Guideline on Endoscopic Eradication Therapy of Barrett’s Esophagus and Related Neoplasia. Gastroenterology. 2024 Jun;166(6):1020-1055. doi: 10.1053/j.gastro.2024.03.019. PMID: 38763697.
Ridtitid W, et al. Endoscopic Gallbladder Stenting to Prevent Recurrent Cholecystitis in Deferred Cholecystectomy: A Randomized Trial. Gastroenterology. 2024 Jun;166(6):1145-1155. doi: 10.1053/j.gastro.2024.02.007. Epub 2024 Feb 14. PMID: 38360274.
Clinical Gastroenterology and Hepatology
April 2024
Berwald G, et al. The Diagnostic Performance of Fecal Immunochemical Tests for Detecting Advanced Neoplasia at Surveillance Colonoscopy. Clin Gastroenterol Hepatol. 2024 Apr;22(4):878-885.e2. doi: 10.1016/j.cgh.2023.09.016. Epub 2023 Sep 22. PMID: 37743036.
Hashash JG, et al. AGA Rapid Clinical Practice Update on the Management of Patients Taking GLP-1 Receptor Agonists Prior to Endoscopy: Communication. Clin Gastroenterol Hepatol. 2024 Apr;22(4):705-707. doi: 10.1016/j.cgh.2023.11.002. Epub 2023 Nov 7. PMID: 37944573.
Sharma R, et al. Statins Are Associated With a Decreased Risk of Severe Liver Disease in Individuals With Noncirrhotic Chronic Liver Disease. Clin Gastroenterol Hepatol. 2024 Apr;22(4):749-759.e19. doi: 10.1016/j.cgh.2023.04.017. Epub 2023 Apr 28. PMID: 37121528.
May 2024
Overbeek KA, et al; PrescrAIP Study Group. Type 1 Autoimmune Pancreatitis in Europe: Clinical Profile and Response to Treatment. Clin Gastroenterol Hepatol. 2024 May;22(5):994-1004.e10. doi: 10.1016/j.cgh.2023.12.010. Epub 2024 Jan 5. Erratum in: Clin Gastroenterol Hepatol. 2024 Jun 1:S1542-3565(24)00446-4. doi: 10.1016/j.cgh.2024.05.005. PMID: 38184096.
Jairath V, et al. ENTERPRET: A Randomized Controlled Trial of Vedolizumab Dose Optimization in Patients With Ulcerative Colitis Who Have Early Nonresponse. Clin Gastroenterol Hepatol. 2024 May;22(5):1077-1086.e13. doi: 10.1016/j.cgh.2023.10.029. Epub 2023 Nov 10. PMID: 37951560.
Gunby SA, et al. Smoking and Alcohol Consumption and Risk of Incident Diverticulitis in Women. Clin Gastroenterol Hepatol. 2024 May;22(5):1108-1116. doi: 10.1016/j.cgh.2023.11.036. Epub 2023 Dec 19. PMID: 38122959; PMCID: PMC11045313.
June 2024
Krause AJ, et al. Validated Clinical Score to Predict Gastroesophageal Reflux in Patients With Chronic Laryngeal Symptoms: COuGH RefluX. Clin Gastroenterol Hepatol. 2024 Jun;22(6):1200-1209.e1. doi: 10.1016/j.cgh.2024.01.021. Epub 2024 Feb 2. PMID: 38309491; PMCID: PMC11128352.
Peng X, et al. Efficacy and Safety of Vonoprazan-Amoxicillin Dual Regimen With Varying Dose and Duration for Helicobacter pylori Eradication: A Multicenter, Prospective, Randomized Study. Clin Gastroenterol Hepatol. 2024 Jun;22(6):1210-1216. doi: 10.1016/j.cgh.2024.01.022. Epub 2024 Feb 1. PMID: 38309492.
Kedia S, et al. Coconut Water Induces Clinical Remission in Mild to Moderate Ulcerative Colitis: Double-blind Placebo-controlled Trial. Clin Gastroenterol Hepatol. 2024 Jun;22(6):1295-1306.e7. doi: 10.1016/j.cgh.2024.01.013. Epub 2024 Jan 24. PMID: 38278200.
Techniques and Innovations in Gastrointestinal Endoscopy
Ogura T, et al. Step-Up Strategy for Endoscopic Hemostasis Using PuraStat After Endoscopic Sphincterotomy Bleeding (STOP Trial). Tech Innov Gastrointest Endosc. 2024 March 16. doi: 10.1016/j.tige.2024.03.005.
Nakai Y, et al. Cyst Detection Rate: A Quality Indicator in the Era of Pancreatic Screening Endoscopic Ultrasonography. Tech Innov Gastrointest Endosc. 2024 May. doi: 10.1016/j.tige.2024.04.001.
Gastro Hep Advances
Kimura Y, et al. Early Sonographic Improvement Predicts Clinical Remission and Mucosal Healing With Molecular-Targeted Drugs in Ulcerative Colitis. Gastro Hep Adv. 2024 April 22. doi: 10.1016/j.gastha.2024.04.007.
Hunaut T, et al. Long-Term Neoplastic Risk Associated With Colorectal Strictures in Crohn’s Disease: A Multicenter Study. Gastro Hep Adv. 2024 May 15. doi: 10.1016/j.gastha.2024.05.003.
Gastroenterology
April 2024
Shah I, et al. Disparities in Colorectal Cancer Screening Among Asian American Populations and Strategies to Address These Disparities. Gastroenterology. 2024 Apr;166(4):549-552. doi: 10.1053/j.gastro.2024.02.009. PMID: 38521575.
Shiha MG, et al. Accuracy of the No-Biopsy Approach for the Diagnosis of Celiac Disease in Adults: A Systematic Review and Meta-Analysis. Gastroenterology. 2024 Apr;166(4):620-630. doi: 10.1053/j.gastro.2023.12.023. Epub 2024 Jan 2. PMID: 38176661.
Goltstein LCMJ, et al. Standard of Care Versus Octreotide in Angiodysplasia-Related Bleeding (the OCEAN Study): A Multicenter Randomized Controlled Trial. Gastroenterology. 2024 Apr;166(4):690-703. doi: 10.1053/j.gastro.2023.12.020. Epub 2023 Dec 28. PMID: 38158089.
May 2024
Robertson DJ, et al. Colonoscopy vs the Fecal Immunochemical Test: Which is Best? Gastroenterology. 2024 May;166(5):758-771. doi: 10.1053/j.gastro.2023.12.027. Epub 2024 Feb 9. PMID: 38342196.
Mårild K, et al. Histologic Remission in Inflammatory Bowel Disease and Female Fertility: A Nationwide Study. Gastroenterology. 2024 May;166(5):802-814.e18. doi: 10.1053/j.gastro.2024.01.018. Epub 2024 Feb 6. PMID: 38331202.
June 2024
Trivedi PJ, et al. Immunopathogenesis of Primary Biliary Cholangitis, Primary Sclerosing Cholangitis and Autoimmune Hepatitis: Themes and Concepts. Gastroenterology. 2024 Jun;166(6):995-1019. doi: 10.1053/j.gastro.2024.01.049. Epub 2024 Feb 10. PMID: 38342195.
Rubenstein JH, et al. AGA Clinical Practice Guideline on Endoscopic Eradication Therapy of Barrett’s Esophagus and Related Neoplasia. Gastroenterology. 2024 Jun;166(6):1020-1055. doi: 10.1053/j.gastro.2024.03.019. PMID: 38763697.
Ridtitid W, et al. Endoscopic Gallbladder Stenting to Prevent Recurrent Cholecystitis in Deferred Cholecystectomy: A Randomized Trial. Gastroenterology. 2024 Jun;166(6):1145-1155. doi: 10.1053/j.gastro.2024.02.007. Epub 2024 Feb 14. PMID: 38360274.
Clinical Gastroenterology and Hepatology
April 2024
Berwald G, et al. The Diagnostic Performance of Fecal Immunochemical Tests for Detecting Advanced Neoplasia at Surveillance Colonoscopy. Clin Gastroenterol Hepatol. 2024 Apr;22(4):878-885.e2. doi: 10.1016/j.cgh.2023.09.016. Epub 2023 Sep 22. PMID: 37743036.
Hashash JG, et al. AGA Rapid Clinical Practice Update on the Management of Patients Taking GLP-1 Receptor Agonists Prior to Endoscopy: Communication. Clin Gastroenterol Hepatol. 2024 Apr;22(4):705-707. doi: 10.1016/j.cgh.2023.11.002. Epub 2023 Nov 7. PMID: 37944573.
Sharma R, et al. Statins Are Associated With a Decreased Risk of Severe Liver Disease in Individuals With Noncirrhotic Chronic Liver Disease. Clin Gastroenterol Hepatol. 2024 Apr;22(4):749-759.e19. doi: 10.1016/j.cgh.2023.04.017. Epub 2023 Apr 28. PMID: 37121528.
May 2024
Overbeek KA, et al; PrescrAIP Study Group. Type 1 Autoimmune Pancreatitis in Europe: Clinical Profile and Response to Treatment. Clin Gastroenterol Hepatol. 2024 May;22(5):994-1004.e10. doi: 10.1016/j.cgh.2023.12.010. Epub 2024 Jan 5. Erratum in: Clin Gastroenterol Hepatol. 2024 Jun 1:S1542-3565(24)00446-4. doi: 10.1016/j.cgh.2024.05.005. PMID: 38184096.
Jairath V, et al. ENTERPRET: A Randomized Controlled Trial of Vedolizumab Dose Optimization in Patients With Ulcerative Colitis Who Have Early Nonresponse. Clin Gastroenterol Hepatol. 2024 May;22(5):1077-1086.e13. doi: 10.1016/j.cgh.2023.10.029. Epub 2023 Nov 10. PMID: 37951560.
Gunby SA, et al. Smoking and Alcohol Consumption and Risk of Incident Diverticulitis in Women. Clin Gastroenterol Hepatol. 2024 May;22(5):1108-1116. doi: 10.1016/j.cgh.2023.11.036. Epub 2023 Dec 19. PMID: 38122959; PMCID: PMC11045313.
June 2024
Krause AJ, et al. Validated Clinical Score to Predict Gastroesophageal Reflux in Patients With Chronic Laryngeal Symptoms: COuGH RefluX. Clin Gastroenterol Hepatol. 2024 Jun;22(6):1200-1209.e1. doi: 10.1016/j.cgh.2024.01.021. Epub 2024 Feb 2. PMID: 38309491; PMCID: PMC11128352.
Peng X, et al. Efficacy and Safety of Vonoprazan-Amoxicillin Dual Regimen With Varying Dose and Duration for Helicobacter pylori Eradication: A Multicenter, Prospective, Randomized Study. Clin Gastroenterol Hepatol. 2024 Jun;22(6):1210-1216. doi: 10.1016/j.cgh.2024.01.022. Epub 2024 Feb 1. PMID: 38309492.
Kedia S, et al. Coconut Water Induces Clinical Remission in Mild to Moderate Ulcerative Colitis: Double-blind Placebo-controlled Trial. Clin Gastroenterol Hepatol. 2024 Jun;22(6):1295-1306.e7. doi: 10.1016/j.cgh.2024.01.013. Epub 2024 Jan 24. PMID: 38278200.
Techniques and Innovations in Gastrointestinal Endoscopy
Ogura T, et al. Step-Up Strategy for Endoscopic Hemostasis Using PuraStat After Endoscopic Sphincterotomy Bleeding (STOP Trial). Tech Innov Gastrointest Endosc. 2024 March 16. doi: 10.1016/j.tige.2024.03.005.
Nakai Y, et al. Cyst Detection Rate: A Quality Indicator in the Era of Pancreatic Screening Endoscopic Ultrasonography. Tech Innov Gastrointest Endosc. 2024 May. doi: 10.1016/j.tige.2024.04.001.
Gastro Hep Advances
Kimura Y, et al. Early Sonographic Improvement Predicts Clinical Remission and Mucosal Healing With Molecular-Targeted Drugs in Ulcerative Colitis. Gastro Hep Adv. 2024 April 22. doi: 10.1016/j.gastha.2024.04.007.
Hunaut T, et al. Long-Term Neoplastic Risk Associated With Colorectal Strictures in Crohn’s Disease: A Multicenter Study. Gastro Hep Adv. 2024 May 15. doi: 10.1016/j.gastha.2024.05.003.
FDA OKs Iqirvo, First-in-Class PPAR Treatment for Primary Biliary Cholangitis
in combination with ursodeoxycholic acid (UDCA) in adults who do not respond adequately to UDCA or as monotherapy in patients unable to tolerate UDCA.
PBC is a rare, chronic cholestatic liver disease that destroys interlobular bile ducts and leads to cholestasis and liver fibrosis. Left untreated, the disease can worsen over time, leading to cirrhosis and liver transplant and, in some cases, premature death. PBC also harms quality of life, with patients often experiencing severe fatigue and pruritus.
Iqirvo, an oral dual peroxisome proliferator–activated receptor (PPAR) alpha and delta agonist, is the first new drug approved in nearly a decade for treatment of PBC.
Accelerated approval of Iqirvo for PBC was based on data from the phase 3 ELATIVE trial published last year in The New England Journal of Medicine.
The trial randomly assigned patients with PBC who had an inadequate response to or unacceptable side effects with UDCA to receive either once-daily elafibranor (80 mg) or placebo.
The primary endpoint was a biochemical response, defined as an alkaline phosphatase (ALP) level < 1.67 times the upper limit of the normal range, with a reduction ≥ 15% from baseline, as well as normal total bilirubin levels.
Among 161 patients, a biochemical response was seen in 55 of 108 (51%) who received elafibranor vs 2 of 53 (4%) who received placebo.
At week 52, the ALP level normalized in 15% of patients in the elafibranor group and none of the patients in the placebo group.
In a news release announcing approval of Iqirvo, the company notes that improvement in survival and prevention of liver decompensation events have not been demonstrated and that continued approval for PBC may be contingent upon verification and description of clinical benefit in confirmatory trials.
The most common adverse effects with Iqirvo, reported in ≥ 10% of study participants, were weight gain, abdominal pain, diarrhea, nausea, and vomiting. Iqirvo is not recommended for people who have or develop decompensated cirrhosis. Full prescribing information is available online.
The data show that Iqirvo is “an effective second-line treatment for patients with PBC with favorable benefit and risk data,” Kris Kowdley, MD, AGAF, director of the Liver Institute Northwest in Seattle, Washington, and a primary investigator on the ELATIVE study, said in the news release.
The approval of Iqirvo “will allow healthcare providers in the US to address an unmet need with the potential to significantly reduce ALP levels for our patients with PBC,” Dr. Kowdley said.
A version of this article appeared on Medscape.com.
in combination with ursodeoxycholic acid (UDCA) in adults who do not respond adequately to UDCA or as monotherapy in patients unable to tolerate UDCA.
PBC is a rare, chronic cholestatic liver disease that destroys interlobular bile ducts and leads to cholestasis and liver fibrosis. Left untreated, the disease can worsen over time, leading to cirrhosis and liver transplant and, in some cases, premature death. PBC also harms quality of life, with patients often experiencing severe fatigue and pruritus.
Iqirvo, an oral dual peroxisome proliferator–activated receptor (PPAR) alpha and delta agonist, is the first new drug approved in nearly a decade for treatment of PBC.
Accelerated approval of Iqirvo for PBC was based on data from the phase 3 ELATIVE trial published last year in The New England Journal of Medicine.
The trial randomly assigned patients with PBC who had an inadequate response to or unacceptable side effects with UDCA to receive either once-daily elafibranor (80 mg) or placebo.
The primary endpoint was a biochemical response, defined as an alkaline phosphatase (ALP) level < 1.67 times the upper limit of the normal range, with a reduction ≥ 15% from baseline, as well as normal total bilirubin levels.
Among 161 patients, a biochemical response was seen in 55 of 108 (51%) who received elafibranor vs 2 of 53 (4%) who received placebo.
At week 52, the ALP level normalized in 15% of patients in the elafibranor group and none of the patients in the placebo group.
In a news release announcing approval of Iqirvo, the company notes that improvement in survival and prevention of liver decompensation events have not been demonstrated and that continued approval for PBC may be contingent upon verification and description of clinical benefit in confirmatory trials.
The most common adverse effects with Iqirvo, reported in ≥ 10% of study participants, were weight gain, abdominal pain, diarrhea, nausea, and vomiting. Iqirvo is not recommended for people who have or develop decompensated cirrhosis. Full prescribing information is available online.
The data show that Iqirvo is “an effective second-line treatment for patients with PBC with favorable benefit and risk data,” Kris Kowdley, MD, AGAF, director of the Liver Institute Northwest in Seattle, Washington, and a primary investigator on the ELATIVE study, said in the news release.
The approval of Iqirvo “will allow healthcare providers in the US to address an unmet need with the potential to significantly reduce ALP levels for our patients with PBC,” Dr. Kowdley said.
A version of this article appeared on Medscape.com.
in combination with ursodeoxycholic acid (UDCA) in adults who do not respond adequately to UDCA or as monotherapy in patients unable to tolerate UDCA.
PBC is a rare, chronic cholestatic liver disease that destroys interlobular bile ducts and leads to cholestasis and liver fibrosis. Left untreated, the disease can worsen over time, leading to cirrhosis and liver transplant and, in some cases, premature death. PBC also harms quality of life, with patients often experiencing severe fatigue and pruritus.
Iqirvo, an oral dual peroxisome proliferator–activated receptor (PPAR) alpha and delta agonist, is the first new drug approved in nearly a decade for treatment of PBC.
Accelerated approval of Iqirvo for PBC was based on data from the phase 3 ELATIVE trial published last year in The New England Journal of Medicine.
The trial randomly assigned patients with PBC who had an inadequate response to or unacceptable side effects with UDCA to receive either once-daily elafibranor (80 mg) or placebo.
The primary endpoint was a biochemical response, defined as an alkaline phosphatase (ALP) level < 1.67 times the upper limit of the normal range, with a reduction ≥ 15% from baseline, as well as normal total bilirubin levels.
Among 161 patients, a biochemical response was seen in 55 of 108 (51%) who received elafibranor vs 2 of 53 (4%) who received placebo.
At week 52, the ALP level normalized in 15% of patients in the elafibranor group and none of the patients in the placebo group.
In a news release announcing approval of Iqirvo, the company notes that improvement in survival and prevention of liver decompensation events have not been demonstrated and that continued approval for PBC may be contingent upon verification and description of clinical benefit in confirmatory trials.
The most common adverse effects with Iqirvo, reported in ≥ 10% of study participants, were weight gain, abdominal pain, diarrhea, nausea, and vomiting. Iqirvo is not recommended for people who have or develop decompensated cirrhosis. Full prescribing information is available online.
The data show that Iqirvo is “an effective second-line treatment for patients with PBC with favorable benefit and risk data,” Kris Kowdley, MD, AGAF, director of the Liver Institute Northwest in Seattle, Washington, and a primary investigator on the ELATIVE study, said in the news release.
The approval of Iqirvo “will allow healthcare providers in the US to address an unmet need with the potential to significantly reduce ALP levels for our patients with PBC,” Dr. Kowdley said.
A version of this article appeared on Medscape.com.
Fluid Management in Acute Pancreatitis
Tenner S, Baillie J, DeWitt J, Vege SS; American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis [published correction appears in Am J Gastroenterol. 2014;109(2):302]. Am J Gastroenterol. 2013;108(9):1400-1415. doi:10.1038/ajg.2013.218
de-Madaria E, Buxbaum JL, Maisonneuve P, et al. Aggressive or moderate fluid resuscitation in acute pancreatitis. N Engl J Med. 2022;387(11):989-1000. doi:10.1056/NEJMoa2202884
Zhao G, Zhang JG, Wu HS, et al. Effects of different resuscitation fluid on severe acute pancreatitis. World J Gastroenterol. 2013;19(13):2044-2052. doi:10.3748/wjg.v19.i13.2044
Guzmán-Calderón E, Diaz-Arocutipa C, Monge E. Lactate Ringer's versus normal saline in the management of acute pancreatitis: a systematic review and meta-analysis of randomized controlled trials. Dig Dis Sci. 2022;67(8):4131-4139. doi:10.1007/s10620-021-07269-8
Hoste EA, Maitland K, Brudney CS, et al; ADQI XII Investigators Group. Four phases of intravenous fluid therapy: a conceptual model. Br J Anaesth. 2014;113(5):740-747. doi:10.1093/bja/aeu300
Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13(4 suppl 2):e1-e15. doi:10.1016/j.pan.2013.07.063
Machicado JD, Papachristou GI. Pharmacologic management and prevention of acute pancreatitis. Curr Opin Gastroenterol. 2019;35(5):460-467. doi:10.1097/MOG.0000000000000563
Tenner S, Baillie J, DeWitt J, Vege SS; American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis [published correction appears in Am J Gastroenterol. 2014;109(2):302]. Am J Gastroenterol. 2013;108(9):1400-1415. doi:10.1038/ajg.2013.218
de-Madaria E, Buxbaum JL, Maisonneuve P, et al. Aggressive or moderate fluid resuscitation in acute pancreatitis. N Engl J Med. 2022;387(11):989-1000. doi:10.1056/NEJMoa2202884
Zhao G, Zhang JG, Wu HS, et al. Effects of different resuscitation fluid on severe acute pancreatitis. World J Gastroenterol. 2013;19(13):2044-2052. doi:10.3748/wjg.v19.i13.2044
Guzmán-Calderón E, Diaz-Arocutipa C, Monge E. Lactate Ringer's versus normal saline in the management of acute pancreatitis: a systematic review and meta-analysis of randomized controlled trials. Dig Dis Sci. 2022;67(8):4131-4139. doi:10.1007/s10620-021-07269-8
Hoste EA, Maitland K, Brudney CS, et al; ADQI XII Investigators Group. Four phases of intravenous fluid therapy: a conceptual model. Br J Anaesth. 2014;113(5):740-747. doi:10.1093/bja/aeu300
Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13(4 suppl 2):e1-e15. doi:10.1016/j.pan.2013.07.063
Machicado JD, Papachristou GI. Pharmacologic management and prevention of acute pancreatitis. Curr Opin Gastroenterol. 2019;35(5):460-467. doi:10.1097/MOG.0000000000000563
Tenner S, Baillie J, DeWitt J, Vege SS; American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis [published correction appears in Am J Gastroenterol. 2014;109(2):302]. Am J Gastroenterol. 2013;108(9):1400-1415. doi:10.1038/ajg.2013.218
de-Madaria E, Buxbaum JL, Maisonneuve P, et al. Aggressive or moderate fluid resuscitation in acute pancreatitis. N Engl J Med. 2022;387(11):989-1000. doi:10.1056/NEJMoa2202884
Zhao G, Zhang JG, Wu HS, et al. Effects of different resuscitation fluid on severe acute pancreatitis. World J Gastroenterol. 2013;19(13):2044-2052. doi:10.3748/wjg.v19.i13.2044
Guzmán-Calderón E, Diaz-Arocutipa C, Monge E. Lactate Ringer's versus normal saline in the management of acute pancreatitis: a systematic review and meta-analysis of randomized controlled trials. Dig Dis Sci. 2022;67(8):4131-4139. doi:10.1007/s10620-021-07269-8
Hoste EA, Maitland K, Brudney CS, et al; ADQI XII Investigators Group. Four phases of intravenous fluid therapy: a conceptual model. Br J Anaesth. 2014;113(5):740-747. doi:10.1093/bja/aeu300
Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13(4 suppl 2):e1-e15. doi:10.1016/j.pan.2013.07.063
Machicado JD, Papachristou GI. Pharmacologic management and prevention of acute pancreatitis. Curr Opin Gastroenterol. 2019;35(5):460-467. doi:10.1097/MOG.0000000000000563
In IBD Patients, Statin Use Associated with Lower Risk of Developing PSC
WASHINGTON — , according to a study presented at Digestive Disease Week® (DDW) 2024.
Statin use was associated with an 86% risk reduction, and only .09% of IBD patients who took statins developed PSC.
“We all take care of patients with liver disease, and we know what a significant burden PSC is. These patients have a significantly elevated risk of enhanced fibrosis and cirrhosis, multiple cancers, and cholangitis and sepsis,” said lead author Chiraag Kulkarni, MD, a gastroenterology fellow at Stanford (California) University Medical School.
“Despite this, we have to date no proven effective medical care for PSC,” he said. “However, over the last decade, there is growing evidence that statins may be beneficial in liver disease, and we see this evidence base stretching from basic science to clinical data.”
Dr. Kulkarni pointed to numerous studies that indicate statins may slow disease progression in steatotic liver disease, viral hepatitis, and cirrhosis. But could statins prevent the onset of PSC?
Because PSC incidence is low, Dr. Kulkarni and colleagues focused on a patient population with higher prevalence — those with IBD, who have an overall lifetime risk of 2% to 7%. The research team followed patients from the date of IBD diagnosis.
Among 33,813 patients with IBD in a national dataset from 2018 onward, 8813 used statins. Statin users tended to be older than non–statin users.
Overall, 181 patients developed new onset PSC during a median follow-up of about 45 months after initial IBD diagnosis. Only eight statin users (.09%) developed PSC, compared with 173 patients (.69%) in the control group.
In a propensity score-matched analysis, statin therapy was associated with a significantly lower risk of developing PSC (HR .14, P < .001). The associated E-value was 5.5, which suggested a robust finding and unlikely to be due to non-visible confounding.
The findings were consistent across secondary and sensitivity analyses, including by age, duration of statin use, and type of statin. For instance, for patients under age 50 where PSC is more likely to occur, statins were associated with a 90% reduction in PSC risk.
“We take away two things from this. First, it’s suggested that a protective effect occurs at ages where PSC is most likely to occur,” Dr. Kulkarni said. “Second, in combination with our propensity score-matched analysis, the results we are observing are not due to a survival bias, where the patients who survive to an age where statins are prescribed simply have a biologically different predilection for developing PSC.”
Statins also protected against PSC in both ulcerative colitis (HR .21) and Crohn’s disease (HR .15), as well as both women (HR .16) and men (HR .22).
Given the uncertainty about the optimal duration of statin therapy for a protective effect, Dr. Kulkarni and colleagues looked at a lag time of 12 months. They found statins were associated with an 84% risk reduction (HR .16), which was similar to the primary analysis.
The study was limited by the inability to capture dosage data or medication adherence. The findings raised several questions, Dr. Kulkarni said, such as the underlying mechanisms and clinical implications. For instance, the underlying mechanisms appear to be related to the pleiotropic effect of statins, modulation of gut inflammation, and alterations in bile acid profiles.
“This is really fascinating and interesting. I wonder about this as a primary prevention strategy in those who have normal cholesterol. Could this work or not?” said Gyongyi Szabo, MD, AGAF, chief academic officer at Beth Israel Deaconess Medical Center, Boston, who was a moderator for the Liver & Biliary Section Distinguished Abstract Plenary Session.
Dr. Kulkarni noted that these findings wouldn’t change clinical practice alone, but based on existing literature around statin hesitancy among patients with cardiovascular disease, the risk reduction for PSC could provide another reason to encourage patients to take them.
“To move this to a place where you can actually think about primary prevention, I think the biological mechanisms need to be teased out a little bit more,” Dr. Kulkarni said. “Then I think you probably still need to identify a higher-risk group than IBD alone.”
Dr. Kulkarni declared no disclosures.
WASHINGTON — , according to a study presented at Digestive Disease Week® (DDW) 2024.
Statin use was associated with an 86% risk reduction, and only .09% of IBD patients who took statins developed PSC.
“We all take care of patients with liver disease, and we know what a significant burden PSC is. These patients have a significantly elevated risk of enhanced fibrosis and cirrhosis, multiple cancers, and cholangitis and sepsis,” said lead author Chiraag Kulkarni, MD, a gastroenterology fellow at Stanford (California) University Medical School.
“Despite this, we have to date no proven effective medical care for PSC,” he said. “However, over the last decade, there is growing evidence that statins may be beneficial in liver disease, and we see this evidence base stretching from basic science to clinical data.”
Dr. Kulkarni pointed to numerous studies that indicate statins may slow disease progression in steatotic liver disease, viral hepatitis, and cirrhosis. But could statins prevent the onset of PSC?
Because PSC incidence is low, Dr. Kulkarni and colleagues focused on a patient population with higher prevalence — those with IBD, who have an overall lifetime risk of 2% to 7%. The research team followed patients from the date of IBD diagnosis.
Among 33,813 patients with IBD in a national dataset from 2018 onward, 8813 used statins. Statin users tended to be older than non–statin users.
Overall, 181 patients developed new onset PSC during a median follow-up of about 45 months after initial IBD diagnosis. Only eight statin users (.09%) developed PSC, compared with 173 patients (.69%) in the control group.
In a propensity score-matched analysis, statin therapy was associated with a significantly lower risk of developing PSC (HR .14, P < .001). The associated E-value was 5.5, which suggested a robust finding and unlikely to be due to non-visible confounding.
The findings were consistent across secondary and sensitivity analyses, including by age, duration of statin use, and type of statin. For instance, for patients under age 50 where PSC is more likely to occur, statins were associated with a 90% reduction in PSC risk.
“We take away two things from this. First, it’s suggested that a protective effect occurs at ages where PSC is most likely to occur,” Dr. Kulkarni said. “Second, in combination with our propensity score-matched analysis, the results we are observing are not due to a survival bias, where the patients who survive to an age where statins are prescribed simply have a biologically different predilection for developing PSC.”
Statins also protected against PSC in both ulcerative colitis (HR .21) and Crohn’s disease (HR .15), as well as both women (HR .16) and men (HR .22).
Given the uncertainty about the optimal duration of statin therapy for a protective effect, Dr. Kulkarni and colleagues looked at a lag time of 12 months. They found statins were associated with an 84% risk reduction (HR .16), which was similar to the primary analysis.
The study was limited by the inability to capture dosage data or medication adherence. The findings raised several questions, Dr. Kulkarni said, such as the underlying mechanisms and clinical implications. For instance, the underlying mechanisms appear to be related to the pleiotropic effect of statins, modulation of gut inflammation, and alterations in bile acid profiles.
“This is really fascinating and interesting. I wonder about this as a primary prevention strategy in those who have normal cholesterol. Could this work or not?” said Gyongyi Szabo, MD, AGAF, chief academic officer at Beth Israel Deaconess Medical Center, Boston, who was a moderator for the Liver & Biliary Section Distinguished Abstract Plenary Session.
Dr. Kulkarni noted that these findings wouldn’t change clinical practice alone, but based on existing literature around statin hesitancy among patients with cardiovascular disease, the risk reduction for PSC could provide another reason to encourage patients to take them.
“To move this to a place where you can actually think about primary prevention, I think the biological mechanisms need to be teased out a little bit more,” Dr. Kulkarni said. “Then I think you probably still need to identify a higher-risk group than IBD alone.”
Dr. Kulkarni declared no disclosures.
WASHINGTON — , according to a study presented at Digestive Disease Week® (DDW) 2024.
Statin use was associated with an 86% risk reduction, and only .09% of IBD patients who took statins developed PSC.
“We all take care of patients with liver disease, and we know what a significant burden PSC is. These patients have a significantly elevated risk of enhanced fibrosis and cirrhosis, multiple cancers, and cholangitis and sepsis,” said lead author Chiraag Kulkarni, MD, a gastroenterology fellow at Stanford (California) University Medical School.
“Despite this, we have to date no proven effective medical care for PSC,” he said. “However, over the last decade, there is growing evidence that statins may be beneficial in liver disease, and we see this evidence base stretching from basic science to clinical data.”
Dr. Kulkarni pointed to numerous studies that indicate statins may slow disease progression in steatotic liver disease, viral hepatitis, and cirrhosis. But could statins prevent the onset of PSC?
Because PSC incidence is low, Dr. Kulkarni and colleagues focused on a patient population with higher prevalence — those with IBD, who have an overall lifetime risk of 2% to 7%. The research team followed patients from the date of IBD diagnosis.
Among 33,813 patients with IBD in a national dataset from 2018 onward, 8813 used statins. Statin users tended to be older than non–statin users.
Overall, 181 patients developed new onset PSC during a median follow-up of about 45 months after initial IBD diagnosis. Only eight statin users (.09%) developed PSC, compared with 173 patients (.69%) in the control group.
In a propensity score-matched analysis, statin therapy was associated with a significantly lower risk of developing PSC (HR .14, P < .001). The associated E-value was 5.5, which suggested a robust finding and unlikely to be due to non-visible confounding.
The findings were consistent across secondary and sensitivity analyses, including by age, duration of statin use, and type of statin. For instance, for patients under age 50 where PSC is more likely to occur, statins were associated with a 90% reduction in PSC risk.
“We take away two things from this. First, it’s suggested that a protective effect occurs at ages where PSC is most likely to occur,” Dr. Kulkarni said. “Second, in combination with our propensity score-matched analysis, the results we are observing are not due to a survival bias, where the patients who survive to an age where statins are prescribed simply have a biologically different predilection for developing PSC.”
Statins also protected against PSC in both ulcerative colitis (HR .21) and Crohn’s disease (HR .15), as well as both women (HR .16) and men (HR .22).
Given the uncertainty about the optimal duration of statin therapy for a protective effect, Dr. Kulkarni and colleagues looked at a lag time of 12 months. They found statins were associated with an 84% risk reduction (HR .16), which was similar to the primary analysis.
The study was limited by the inability to capture dosage data or medication adherence. The findings raised several questions, Dr. Kulkarni said, such as the underlying mechanisms and clinical implications. For instance, the underlying mechanisms appear to be related to the pleiotropic effect of statins, modulation of gut inflammation, and alterations in bile acid profiles.
“This is really fascinating and interesting. I wonder about this as a primary prevention strategy in those who have normal cholesterol. Could this work or not?” said Gyongyi Szabo, MD, AGAF, chief academic officer at Beth Israel Deaconess Medical Center, Boston, who was a moderator for the Liver & Biliary Section Distinguished Abstract Plenary Session.
Dr. Kulkarni noted that these findings wouldn’t change clinical practice alone, but based on existing literature around statin hesitancy among patients with cardiovascular disease, the risk reduction for PSC could provide another reason to encourage patients to take them.
“To move this to a place where you can actually think about primary prevention, I think the biological mechanisms need to be teased out a little bit more,” Dr. Kulkarni said. “Then I think you probably still need to identify a higher-risk group than IBD alone.”
Dr. Kulkarni declared no disclosures.
FROM DDW 2024