User login
Asymptomatic gallstones seldom require surgical intervention
“Most patients with asymptomatic gallstones never develop symptoms and probably don’t need surgical intervention,” lead study author Gareth Morris-Stiff, MD, PhD, said at the annual Digestive Disease Week.
Dr. Morris-Stiff, of the department of general surgery at Cleveland Clinic, said that, while previous studies have evaluated the time to development of gallstone-related complications following identification of asymptomatic gallstones, factors associated with the need for surgical intervention in this population have not been documented. The aims of the current study were to perform a big data analysis to evaluate risk factors associated with intervention in asymptomatic gallstones and to develop a risk stratification tool to aid in patient consultations by predicting individuals likely to need future intervention for their gallstones.
The researchers included Cleveland Clinic patients with CT/US reports containing “cholelithiasis” or “gallstones” between January 1996 and December 2016. Patients were excluded if they had a concurrent or prior event, had an event within 2 months, or lacked follow-up. Data collection included demographic characteristics, comorbid conditions or surgeries, imaging features, and medication use.
Dr. Morris-Stiff and his colleagues constructed Kaplan-Meier curves to analyze time to intervention and calculated cumulative incidence ratios. They used automated forward stepwise competing risk regression to create their model and receiver operating characteristics curves to analyze it.
Of the 49,414 patients identified with asymptomatic gallstones, 22,257 met criteria for analysis. Slightly more than half (51%) were female, their mean age was 61 years, 80% were white, 16% were black, and the rest were from other racial and ethnic groups. The median follow-up was 4.5 years, and the median follow-up of patients undergoing intervention was 3.9 years. This translated to 112,111 total years of observation.
The researchers found that the cumulative incidence of intervention at 15 years was 25% and it increased linearly from the time of initial diagnosis of asymptomatic gallstones. A total of 1,762 patients (7.9%) underwent a surgical procedure, most often cholecystectomy (5.7%). Three factors were associated with a reduced risk for surgical intervention: increasing age (hazard ratio, 0.94; P less than 0.001), male gender (HR, 0.78; P less than 0.001), and statin use (HR, 0.67; P less than 0.001).
Patient variables associated with an increased need for surgical intervention included obesity (HR, 1.44; P less than 0.001) and having a hemolytic disorder (HR, 2.42; P less than 0.001). Gallstone-specific characteristics that increased the need for surgical intervention included a stone size of greater than 9 mm (HR, 1.56; P less than 0.001), the presence of sludge (HR, 1.46; P less than 0.001), the presence of a polyp (HR, 1.68; P less than 0.001), and having multiple stones (HR, 1.69; P less than 0.001).
The analysis enabled Dr. Morris-Stiff and colleagues to generate a Web-based risk score to reliably identify these patients and provide prognostic information for counseling. An app for smartphones based on the score is being developed. The researchers reported having no financial disclosures.
“Most patients with asymptomatic gallstones never develop symptoms and probably don’t need surgical intervention,” lead study author Gareth Morris-Stiff, MD, PhD, said at the annual Digestive Disease Week.
Dr. Morris-Stiff, of the department of general surgery at Cleveland Clinic, said that, while previous studies have evaluated the time to development of gallstone-related complications following identification of asymptomatic gallstones, factors associated with the need for surgical intervention in this population have not been documented. The aims of the current study were to perform a big data analysis to evaluate risk factors associated with intervention in asymptomatic gallstones and to develop a risk stratification tool to aid in patient consultations by predicting individuals likely to need future intervention for their gallstones.
The researchers included Cleveland Clinic patients with CT/US reports containing “cholelithiasis” or “gallstones” between January 1996 and December 2016. Patients were excluded if they had a concurrent or prior event, had an event within 2 months, or lacked follow-up. Data collection included demographic characteristics, comorbid conditions or surgeries, imaging features, and medication use.
Dr. Morris-Stiff and his colleagues constructed Kaplan-Meier curves to analyze time to intervention and calculated cumulative incidence ratios. They used automated forward stepwise competing risk regression to create their model and receiver operating characteristics curves to analyze it.
Of the 49,414 patients identified with asymptomatic gallstones, 22,257 met criteria for analysis. Slightly more than half (51%) were female, their mean age was 61 years, 80% were white, 16% were black, and the rest were from other racial and ethnic groups. The median follow-up was 4.5 years, and the median follow-up of patients undergoing intervention was 3.9 years. This translated to 112,111 total years of observation.
The researchers found that the cumulative incidence of intervention at 15 years was 25% and it increased linearly from the time of initial diagnosis of asymptomatic gallstones. A total of 1,762 patients (7.9%) underwent a surgical procedure, most often cholecystectomy (5.7%). Three factors were associated with a reduced risk for surgical intervention: increasing age (hazard ratio, 0.94; P less than 0.001), male gender (HR, 0.78; P less than 0.001), and statin use (HR, 0.67; P less than 0.001).
Patient variables associated with an increased need for surgical intervention included obesity (HR, 1.44; P less than 0.001) and having a hemolytic disorder (HR, 2.42; P less than 0.001). Gallstone-specific characteristics that increased the need for surgical intervention included a stone size of greater than 9 mm (HR, 1.56; P less than 0.001), the presence of sludge (HR, 1.46; P less than 0.001), the presence of a polyp (HR, 1.68; P less than 0.001), and having multiple stones (HR, 1.69; P less than 0.001).
The analysis enabled Dr. Morris-Stiff and colleagues to generate a Web-based risk score to reliably identify these patients and provide prognostic information for counseling. An app for smartphones based on the score is being developed. The researchers reported having no financial disclosures.
“Most patients with asymptomatic gallstones never develop symptoms and probably don’t need surgical intervention,” lead study author Gareth Morris-Stiff, MD, PhD, said at the annual Digestive Disease Week.
Dr. Morris-Stiff, of the department of general surgery at Cleveland Clinic, said that, while previous studies have evaluated the time to development of gallstone-related complications following identification of asymptomatic gallstones, factors associated with the need for surgical intervention in this population have not been documented. The aims of the current study were to perform a big data analysis to evaluate risk factors associated with intervention in asymptomatic gallstones and to develop a risk stratification tool to aid in patient consultations by predicting individuals likely to need future intervention for their gallstones.
The researchers included Cleveland Clinic patients with CT/US reports containing “cholelithiasis” or “gallstones” between January 1996 and December 2016. Patients were excluded if they had a concurrent or prior event, had an event within 2 months, or lacked follow-up. Data collection included demographic characteristics, comorbid conditions or surgeries, imaging features, and medication use.
Dr. Morris-Stiff and his colleagues constructed Kaplan-Meier curves to analyze time to intervention and calculated cumulative incidence ratios. They used automated forward stepwise competing risk regression to create their model and receiver operating characteristics curves to analyze it.
Of the 49,414 patients identified with asymptomatic gallstones, 22,257 met criteria for analysis. Slightly more than half (51%) were female, their mean age was 61 years, 80% were white, 16% were black, and the rest were from other racial and ethnic groups. The median follow-up was 4.5 years, and the median follow-up of patients undergoing intervention was 3.9 years. This translated to 112,111 total years of observation.
The researchers found that the cumulative incidence of intervention at 15 years was 25% and it increased linearly from the time of initial diagnosis of asymptomatic gallstones. A total of 1,762 patients (7.9%) underwent a surgical procedure, most often cholecystectomy (5.7%). Three factors were associated with a reduced risk for surgical intervention: increasing age (hazard ratio, 0.94; P less than 0.001), male gender (HR, 0.78; P less than 0.001), and statin use (HR, 0.67; P less than 0.001).
Patient variables associated with an increased need for surgical intervention included obesity (HR, 1.44; P less than 0.001) and having a hemolytic disorder (HR, 2.42; P less than 0.001). Gallstone-specific characteristics that increased the need for surgical intervention included a stone size of greater than 9 mm (HR, 1.56; P less than 0.001), the presence of sludge (HR, 1.46; P less than 0.001), the presence of a polyp (HR, 1.68; P less than 0.001), and having multiple stones (HR, 1.69; P less than 0.001).
The analysis enabled Dr. Morris-Stiff and colleagues to generate a Web-based risk score to reliably identify these patients and provide prognostic information for counseling. An app for smartphones based on the score is being developed. The researchers reported having no financial disclosures.
REPORTING FROM DDW 2019
Early cholecystectomy prevents recurrent biliary events
SAN DIEGO – In a retrospective study of 234 patients admitted for gallstone pancreatitis, almost 90% of recurrent biliary events occurred in patients who did not receive a cholecystectomy within 60 days of hospital discharge. The overall rate of recurrence was 19%, and over half of patients (59%) did not receive a cholecystectomy during their index hospitalization.
Additionally, none of the recurrent biliary events occurred in those patients who did receive a cholecystectomy during the index hospitalization or within the first 30 days after discharge. “It really is the case that, ‘if you snooze, you lose,’ ” said Vijay Dalapathi, MD, presenting the findings during an oral presentation at the annual Digestive Disease Week.
Dr. Dalapathi and colleagues had observed that cholecystectomy during an index hospitalization for mild biliary pancreatitis was a far from universal practice, despite guidelines recommending early cholecystectomy.
To delve further into practice patterns, Dr. Dalapathi, first author Mohammed Ullah, MD, and their coauthors at the University of Rochester (N.Y.) conducted a single-site retrospective study of patients who were admitted with gallstone pancreatitis over a 5-year period ending December 2017. Dr. Dalapathi and Dr. Ullah are both second-year gastroenterology fellows.
The study had twin primary outcome measures: cholecystectomy rates performed during an index hospitalization for gallstone pancreatitis and recurrent biliary events after hospitalization. Adult patients were included if they had a diagnosis of acute gallstone pancreatitis, with or without recurrent cholangitis, choledocholithiasis, or acute cholecystitis. Pediatric patients and those with prior cholecystectomy were excluded.
A total of 234 patients were included in the study. Their mean age was 58.3 years, and patients were mostly female (57.3%) and white (91.5%). Mean body mass index was 29.1 kg/m2. A total of 175 patients (74.8%) had endoscopic retrograde cholangiopancreatography.
Out of the entire cohort of patients, 138 (59%) did not have a cholecystectomy during the index hospitalization. Among the patients who did not receive a cholecystectomy, 33 (24%) were deemed unsuitable candidates for the procedure, either because they were critically ill or because they were poor candidates for surgery for other reasons. No reason was provided for the nonperformance of cholecystectomy for an additional 28 patients (20%).
The remaining 75 patients (54%) were deferred to outpatient management. Looking at this subgroup of patients, Dr. Dalapathi and his coinvestigators tracked the amount of time that passed before cholecystectomy.
The researchers found that 19 patients (25%) had not had a cholecystectomy within the study period. A total of 21 patients (28%) had the procedure more than 60 days from hospitalization, and another 23 (31%) had the procedure between 30 and 60 days after hospitalization. Just 12 patients (16%) of this subgroup had their cholecystectomy within 30 days of hospitalization.
Among patients who were discharged without a cholecystectomy, Dr. Dalapathi and his coauthors found 26 recurrent biliary events (19%): 15 were gallstone pancreatitis and 10 were cholecystitis; 1 patient developed cholangitis.
The crux of the study’s findings came when the investigators looked at the association between recurrent events and cholecystectomy timing. They found no recurrent biliary events among those who received cholecystectomy while hospitalized or within the first 30 days after discharge. Of the 26 events, 3 (12%) occurred in those whose cholecystectomies came 30-60 days after discharge. The remaining 23 events (88%) were seen in those receiving a cholecystectomy more than 60 days after discharge, or not at all.
Guidelines from the American Gastroenterological Association, the Society of American Gastrointestinal and Endoscopic Surgeons, and the American College of Gastroenterology all recommend early cholecystectomy after mild acute gallstone pancreatitis, said Dr. Dalapathi.
However, two separate systematic reviews including a total of 22 studies and over 3,000 patients showed that about half (48% and 51%) of patients admitted with mild acute biliary pancreatitis received a cholecystectomy during the index hospitalization or within 14 days of the hospitalization.
Further, he said, previous work had shown recurrent biliary event rates approaching 20% for patients whose biliary pancreatitis bout was not followed by cholecystectomy, a figure in line with the rate seen in the present study.
“Cholecystectomy should be performed during index hospitalization or as soon as possible within 30 days of mild biliary pancreatitis to minimize risk of recurrent biliary events,” said Dr. Dalapathi.
The authors reported no outside sources of funding and no conflicts of interest.
SOURCE: Ullah M. et al. DDW 2019, Abstract 24.
SAN DIEGO – In a retrospective study of 234 patients admitted for gallstone pancreatitis, almost 90% of recurrent biliary events occurred in patients who did not receive a cholecystectomy within 60 days of hospital discharge. The overall rate of recurrence was 19%, and over half of patients (59%) did not receive a cholecystectomy during their index hospitalization.
Additionally, none of the recurrent biliary events occurred in those patients who did receive a cholecystectomy during the index hospitalization or within the first 30 days after discharge. “It really is the case that, ‘if you snooze, you lose,’ ” said Vijay Dalapathi, MD, presenting the findings during an oral presentation at the annual Digestive Disease Week.
Dr. Dalapathi and colleagues had observed that cholecystectomy during an index hospitalization for mild biliary pancreatitis was a far from universal practice, despite guidelines recommending early cholecystectomy.
To delve further into practice patterns, Dr. Dalapathi, first author Mohammed Ullah, MD, and their coauthors at the University of Rochester (N.Y.) conducted a single-site retrospective study of patients who were admitted with gallstone pancreatitis over a 5-year period ending December 2017. Dr. Dalapathi and Dr. Ullah are both second-year gastroenterology fellows.
The study had twin primary outcome measures: cholecystectomy rates performed during an index hospitalization for gallstone pancreatitis and recurrent biliary events after hospitalization. Adult patients were included if they had a diagnosis of acute gallstone pancreatitis, with or without recurrent cholangitis, choledocholithiasis, or acute cholecystitis. Pediatric patients and those with prior cholecystectomy were excluded.
A total of 234 patients were included in the study. Their mean age was 58.3 years, and patients were mostly female (57.3%) and white (91.5%). Mean body mass index was 29.1 kg/m2. A total of 175 patients (74.8%) had endoscopic retrograde cholangiopancreatography.
Out of the entire cohort of patients, 138 (59%) did not have a cholecystectomy during the index hospitalization. Among the patients who did not receive a cholecystectomy, 33 (24%) were deemed unsuitable candidates for the procedure, either because they were critically ill or because they were poor candidates for surgery for other reasons. No reason was provided for the nonperformance of cholecystectomy for an additional 28 patients (20%).
The remaining 75 patients (54%) were deferred to outpatient management. Looking at this subgroup of patients, Dr. Dalapathi and his coinvestigators tracked the amount of time that passed before cholecystectomy.
The researchers found that 19 patients (25%) had not had a cholecystectomy within the study period. A total of 21 patients (28%) had the procedure more than 60 days from hospitalization, and another 23 (31%) had the procedure between 30 and 60 days after hospitalization. Just 12 patients (16%) of this subgroup had their cholecystectomy within 30 days of hospitalization.
Among patients who were discharged without a cholecystectomy, Dr. Dalapathi and his coauthors found 26 recurrent biliary events (19%): 15 were gallstone pancreatitis and 10 were cholecystitis; 1 patient developed cholangitis.
The crux of the study’s findings came when the investigators looked at the association between recurrent events and cholecystectomy timing. They found no recurrent biliary events among those who received cholecystectomy while hospitalized or within the first 30 days after discharge. Of the 26 events, 3 (12%) occurred in those whose cholecystectomies came 30-60 days after discharge. The remaining 23 events (88%) were seen in those receiving a cholecystectomy more than 60 days after discharge, or not at all.
Guidelines from the American Gastroenterological Association, the Society of American Gastrointestinal and Endoscopic Surgeons, and the American College of Gastroenterology all recommend early cholecystectomy after mild acute gallstone pancreatitis, said Dr. Dalapathi.
However, two separate systematic reviews including a total of 22 studies and over 3,000 patients showed that about half (48% and 51%) of patients admitted with mild acute biliary pancreatitis received a cholecystectomy during the index hospitalization or within 14 days of the hospitalization.
Further, he said, previous work had shown recurrent biliary event rates approaching 20% for patients whose biliary pancreatitis bout was not followed by cholecystectomy, a figure in line with the rate seen in the present study.
“Cholecystectomy should be performed during index hospitalization or as soon as possible within 30 days of mild biliary pancreatitis to minimize risk of recurrent biliary events,” said Dr. Dalapathi.
The authors reported no outside sources of funding and no conflicts of interest.
SOURCE: Ullah M. et al. DDW 2019, Abstract 24.
SAN DIEGO – In a retrospective study of 234 patients admitted for gallstone pancreatitis, almost 90% of recurrent biliary events occurred in patients who did not receive a cholecystectomy within 60 days of hospital discharge. The overall rate of recurrence was 19%, and over half of patients (59%) did not receive a cholecystectomy during their index hospitalization.
Additionally, none of the recurrent biliary events occurred in those patients who did receive a cholecystectomy during the index hospitalization or within the first 30 days after discharge. “It really is the case that, ‘if you snooze, you lose,’ ” said Vijay Dalapathi, MD, presenting the findings during an oral presentation at the annual Digestive Disease Week.
Dr. Dalapathi and colleagues had observed that cholecystectomy during an index hospitalization for mild biliary pancreatitis was a far from universal practice, despite guidelines recommending early cholecystectomy.
To delve further into practice patterns, Dr. Dalapathi, first author Mohammed Ullah, MD, and their coauthors at the University of Rochester (N.Y.) conducted a single-site retrospective study of patients who were admitted with gallstone pancreatitis over a 5-year period ending December 2017. Dr. Dalapathi and Dr. Ullah are both second-year gastroenterology fellows.
The study had twin primary outcome measures: cholecystectomy rates performed during an index hospitalization for gallstone pancreatitis and recurrent biliary events after hospitalization. Adult patients were included if they had a diagnosis of acute gallstone pancreatitis, with or without recurrent cholangitis, choledocholithiasis, or acute cholecystitis. Pediatric patients and those with prior cholecystectomy were excluded.
A total of 234 patients were included in the study. Their mean age was 58.3 years, and patients were mostly female (57.3%) and white (91.5%). Mean body mass index was 29.1 kg/m2. A total of 175 patients (74.8%) had endoscopic retrograde cholangiopancreatography.
Out of the entire cohort of patients, 138 (59%) did not have a cholecystectomy during the index hospitalization. Among the patients who did not receive a cholecystectomy, 33 (24%) were deemed unsuitable candidates for the procedure, either because they were critically ill or because they were poor candidates for surgery for other reasons. No reason was provided for the nonperformance of cholecystectomy for an additional 28 patients (20%).
The remaining 75 patients (54%) were deferred to outpatient management. Looking at this subgroup of patients, Dr. Dalapathi and his coinvestigators tracked the amount of time that passed before cholecystectomy.
The researchers found that 19 patients (25%) had not had a cholecystectomy within the study period. A total of 21 patients (28%) had the procedure more than 60 days from hospitalization, and another 23 (31%) had the procedure between 30 and 60 days after hospitalization. Just 12 patients (16%) of this subgroup had their cholecystectomy within 30 days of hospitalization.
Among patients who were discharged without a cholecystectomy, Dr. Dalapathi and his coauthors found 26 recurrent biliary events (19%): 15 were gallstone pancreatitis and 10 were cholecystitis; 1 patient developed cholangitis.
The crux of the study’s findings came when the investigators looked at the association between recurrent events and cholecystectomy timing. They found no recurrent biliary events among those who received cholecystectomy while hospitalized or within the first 30 days after discharge. Of the 26 events, 3 (12%) occurred in those whose cholecystectomies came 30-60 days after discharge. The remaining 23 events (88%) were seen in those receiving a cholecystectomy more than 60 days after discharge, or not at all.
Guidelines from the American Gastroenterological Association, the Society of American Gastrointestinal and Endoscopic Surgeons, and the American College of Gastroenterology all recommend early cholecystectomy after mild acute gallstone pancreatitis, said Dr. Dalapathi.
However, two separate systematic reviews including a total of 22 studies and over 3,000 patients showed that about half (48% and 51%) of patients admitted with mild acute biliary pancreatitis received a cholecystectomy during the index hospitalization or within 14 days of the hospitalization.
Further, he said, previous work had shown recurrent biliary event rates approaching 20% for patients whose biliary pancreatitis bout was not followed by cholecystectomy, a figure in line with the rate seen in the present study.
“Cholecystectomy should be performed during index hospitalization or as soon as possible within 30 days of mild biliary pancreatitis to minimize risk of recurrent biliary events,” said Dr. Dalapathi.
The authors reported no outside sources of funding and no conflicts of interest.
SOURCE: Ullah M. et al. DDW 2019, Abstract 24.
REPORTING FROM DDW 2019
Opioid use associated with common bile duct dilation
SAN DIEGO – Biliary duct dilation in the setting of an intact gallbladder and normal bilirubin levels was more common among those who used opioids, based on the results of a large, retrospective, single-center cohort study.
Patients were included in the study if they had a documented measurement for the diameter of the common bile duct, with no evidence of an obstructive lesion and a normal bilirubin level. The mean common bile duct diameter was significantly higher at 8.67 mm for 867 patients who used opioids, compared with 7.24 mm for 818 similar patients who did not use opioids (P less than .001). The association was strongest among opioid users with an intact gallbladder.
“Opiate use is associated with biliary dilation in the setting of normal bilirubin,” Monique Barakat, MD, a gastroenterologist at Stanford (Calif.) University, reported at the annual Digestive Disease Week. “Known opiate users with normal LFTs [liver function tests] may not require expensive and potentially risky endoscopic evaluation for biliary dilation.”
Dr. Barakat and senior author Subhas Banerjee, MD, professor of gastroenterology and hepatology at Stanford, decided to examine a possible association between biliary duct dilation and opioid use based on previous small clinical studies that found a possible association. Along with opioid status, Dr. Barakat and her coauthor also looked at patient age, cholecystectomy status, ethnicity, weight, and height for possible associations with bile duct diameter.
The researchers took a random 20% sample of adults seen for all causes in the ED at Stanford over a 5-year period. Using a health informatic platform based on the electronic medical record, they identified all patients who had received an abdominal CT or MRI. Patients were included in the study if they had a documented measurement for the diameter of the common bile duct, with no evidence of obstructive lesion and a normal bilirubin level.
Compared with 818 patients who did not use opioids, the 867 patients who used opioids had a significantly larger common bile duct diameter. Using 7 mm as the threshold for biliary duct enlargement, 84% of patients who used opioids had an enlarged common bile duct, compared with 27% of nonopioid users (P less than .001), said Dr. Barakat, recipient of an early investigator award for the study.
“We frequently get referrals for bile duct dilation with concern for more sinister causes of biliary duct dilation – stones, strictures, and malignancy,” said Dr. Barakat. Because of the increase in cross-sectional imaging via CT or MRI, bile duct dilation is being detected at increasingly higher rates.
Dr. Barakat said that about one-third of referrals to the therapeutic endoscopy clinic at Stanford are now for patients with biliary dilation and normal liver function tests. And similar increases are being “seen across all settings – so office, primary care clinic, inpatient, and most markedly, the emergency department. Coupled with this, the population is aging, and patients who present to each of these settings are more likely, if they are older, to undergo cross-sectional imaging.”
Other contributors to higher rates of bile duct dilation include increased rates of obesity and increased prevalence of nonalcoholic steatohepatitis (NASH). About 20% of individuals with NASH will also have abnormal LFTs, she said, and NASH can be the trigger for cross-sectional imaging.
For most of these patients with biliary duct dilation and normal LFTs, no obstructive process was found on endoscopic evaluation.
Although gastroenterology textbooks may say that bile duct diameter increases with age, Dr. Barakat and colleagues didn’t find this to be the case. Among nonopioid users in the study cohort, age did not predict of common bile duct diameter. Among the entire cohort, “Advancing age weakly predicts increased common bile duct diameter,” she said, suggesting that factors other than age along may drive increased bile duct diameter.
Limitations included the retrospective nature of the study, as well as the limitations of information from the electronic medical record. Also, interobserver variability may have come into play, as bile duct diameter measurements were made by multiple radiologists in the course of clinical care.
The study was funded by the National Institutes of Health. Dr. Barakat reported no relevant financial disclosures.
SAN DIEGO – Biliary duct dilation in the setting of an intact gallbladder and normal bilirubin levels was more common among those who used opioids, based on the results of a large, retrospective, single-center cohort study.
Patients were included in the study if they had a documented measurement for the diameter of the common bile duct, with no evidence of an obstructive lesion and a normal bilirubin level. The mean common bile duct diameter was significantly higher at 8.67 mm for 867 patients who used opioids, compared with 7.24 mm for 818 similar patients who did not use opioids (P less than .001). The association was strongest among opioid users with an intact gallbladder.
“Opiate use is associated with biliary dilation in the setting of normal bilirubin,” Monique Barakat, MD, a gastroenterologist at Stanford (Calif.) University, reported at the annual Digestive Disease Week. “Known opiate users with normal LFTs [liver function tests] may not require expensive and potentially risky endoscopic evaluation for biliary dilation.”
Dr. Barakat and senior author Subhas Banerjee, MD, professor of gastroenterology and hepatology at Stanford, decided to examine a possible association between biliary duct dilation and opioid use based on previous small clinical studies that found a possible association. Along with opioid status, Dr. Barakat and her coauthor also looked at patient age, cholecystectomy status, ethnicity, weight, and height for possible associations with bile duct diameter.
The researchers took a random 20% sample of adults seen for all causes in the ED at Stanford over a 5-year period. Using a health informatic platform based on the electronic medical record, they identified all patients who had received an abdominal CT or MRI. Patients were included in the study if they had a documented measurement for the diameter of the common bile duct, with no evidence of obstructive lesion and a normal bilirubin level.
Compared with 818 patients who did not use opioids, the 867 patients who used opioids had a significantly larger common bile duct diameter. Using 7 mm as the threshold for biliary duct enlargement, 84% of patients who used opioids had an enlarged common bile duct, compared with 27% of nonopioid users (P less than .001), said Dr. Barakat, recipient of an early investigator award for the study.
“We frequently get referrals for bile duct dilation with concern for more sinister causes of biliary duct dilation – stones, strictures, and malignancy,” said Dr. Barakat. Because of the increase in cross-sectional imaging via CT or MRI, bile duct dilation is being detected at increasingly higher rates.
Dr. Barakat said that about one-third of referrals to the therapeutic endoscopy clinic at Stanford are now for patients with biliary dilation and normal liver function tests. And similar increases are being “seen across all settings – so office, primary care clinic, inpatient, and most markedly, the emergency department. Coupled with this, the population is aging, and patients who present to each of these settings are more likely, if they are older, to undergo cross-sectional imaging.”
Other contributors to higher rates of bile duct dilation include increased rates of obesity and increased prevalence of nonalcoholic steatohepatitis (NASH). About 20% of individuals with NASH will also have abnormal LFTs, she said, and NASH can be the trigger for cross-sectional imaging.
For most of these patients with biliary duct dilation and normal LFTs, no obstructive process was found on endoscopic evaluation.
Although gastroenterology textbooks may say that bile duct diameter increases with age, Dr. Barakat and colleagues didn’t find this to be the case. Among nonopioid users in the study cohort, age did not predict of common bile duct diameter. Among the entire cohort, “Advancing age weakly predicts increased common bile duct diameter,” she said, suggesting that factors other than age along may drive increased bile duct diameter.
Limitations included the retrospective nature of the study, as well as the limitations of information from the electronic medical record. Also, interobserver variability may have come into play, as bile duct diameter measurements were made by multiple radiologists in the course of clinical care.
The study was funded by the National Institutes of Health. Dr. Barakat reported no relevant financial disclosures.
SAN DIEGO – Biliary duct dilation in the setting of an intact gallbladder and normal bilirubin levels was more common among those who used opioids, based on the results of a large, retrospective, single-center cohort study.
Patients were included in the study if they had a documented measurement for the diameter of the common bile duct, with no evidence of an obstructive lesion and a normal bilirubin level. The mean common bile duct diameter was significantly higher at 8.67 mm for 867 patients who used opioids, compared with 7.24 mm for 818 similar patients who did not use opioids (P less than .001). The association was strongest among opioid users with an intact gallbladder.
“Opiate use is associated with biliary dilation in the setting of normal bilirubin,” Monique Barakat, MD, a gastroenterologist at Stanford (Calif.) University, reported at the annual Digestive Disease Week. “Known opiate users with normal LFTs [liver function tests] may not require expensive and potentially risky endoscopic evaluation for biliary dilation.”
Dr. Barakat and senior author Subhas Banerjee, MD, professor of gastroenterology and hepatology at Stanford, decided to examine a possible association between biliary duct dilation and opioid use based on previous small clinical studies that found a possible association. Along with opioid status, Dr. Barakat and her coauthor also looked at patient age, cholecystectomy status, ethnicity, weight, and height for possible associations with bile duct diameter.
The researchers took a random 20% sample of adults seen for all causes in the ED at Stanford over a 5-year period. Using a health informatic platform based on the electronic medical record, they identified all patients who had received an abdominal CT or MRI. Patients were included in the study if they had a documented measurement for the diameter of the common bile duct, with no evidence of obstructive lesion and a normal bilirubin level.
Compared with 818 patients who did not use opioids, the 867 patients who used opioids had a significantly larger common bile duct diameter. Using 7 mm as the threshold for biliary duct enlargement, 84% of patients who used opioids had an enlarged common bile duct, compared with 27% of nonopioid users (P less than .001), said Dr. Barakat, recipient of an early investigator award for the study.
“We frequently get referrals for bile duct dilation with concern for more sinister causes of biliary duct dilation – stones, strictures, and malignancy,” said Dr. Barakat. Because of the increase in cross-sectional imaging via CT or MRI, bile duct dilation is being detected at increasingly higher rates.
Dr. Barakat said that about one-third of referrals to the therapeutic endoscopy clinic at Stanford are now for patients with biliary dilation and normal liver function tests. And similar increases are being “seen across all settings – so office, primary care clinic, inpatient, and most markedly, the emergency department. Coupled with this, the population is aging, and patients who present to each of these settings are more likely, if they are older, to undergo cross-sectional imaging.”
Other contributors to higher rates of bile duct dilation include increased rates of obesity and increased prevalence of nonalcoholic steatohepatitis (NASH). About 20% of individuals with NASH will also have abnormal LFTs, she said, and NASH can be the trigger for cross-sectional imaging.
For most of these patients with biliary duct dilation and normal LFTs, no obstructive process was found on endoscopic evaluation.
Although gastroenterology textbooks may say that bile duct diameter increases with age, Dr. Barakat and colleagues didn’t find this to be the case. Among nonopioid users in the study cohort, age did not predict of common bile duct diameter. Among the entire cohort, “Advancing age weakly predicts increased common bile duct diameter,” she said, suggesting that factors other than age along may drive increased bile duct diameter.
Limitations included the retrospective nature of the study, as well as the limitations of information from the electronic medical record. Also, interobserver variability may have come into play, as bile duct diameter measurements were made by multiple radiologists in the course of clinical care.
The study was funded by the National Institutes of Health. Dr. Barakat reported no relevant financial disclosures.
REPORTING FROM DDW 2019
Systemic anticoagulation found to benefit acute pancreatitis patients
SAN DIEGO –
“Acute pancreatitis is a very common disease,” lead study author Yan Bi, MD, PhD, a senior associate consultant and assistant professor in the department of gastroenterology and hepatology at Mayo Clinic, Jacksonville, Fla., said in an interview in advance of the annual Digestive Disease Week. “It’s the number one GI cause for hospitalization. Unfortunately, even after decades of basic science and clinical research, there’s still no cure; there’s nothing to prevent it from happening. The only treatment we can offer is supportive care, which includes fluid hydration, pain control, and nutrition support.”
The pathogenesis of acute pancreatitis (AP) is complex, she continued, and represents a sequence of distinct and interconnected pathologic events. “Both animal data and human studies have shown that acute pancreatitis is a hypercoagulable state,” she said. “We hypothesize that coagulation plays important roles in the development of pancreatitis.”
To test their hypothesis, Dr. Bi and associates performed a retrospective study. They drew from the 2014 National Inpatient Sample to evaluate the effect of systemic anticoagulation prior to AP onset on outcomes of the condition. They used ICD-9 codes to identify patients with a primary diagnosis of AP as well as those who were taking systemic anticoagulation. The primary outcome was the odds of AP in patients taking systemic anticoagulation, compared with those who were not. Secondary outcomes were mortality, morbidity, length of hospital stay, and total hospitalization charges and costs. The researchers used propensity score matching to create a 1:1 matching population for sex, age, and Charlson Comorbidity Index, and multivariate regression to adjust for patient ZIP code, income, hospital region, location, size, and teaching status.
Dr. Bi presented results from 442,535 patients with AP. Of these, 12,735 were on systemic anticoagulation prior to AP. Their mean age was 66 and 47% were female. After adjustment for confounders, patients on systemic anticoagulation prior to AP onset displayed a decreased odds of AP occurrence, compared with those who were not on anticoagulation (OR 0.56; P less than .01). In addition, patients on anticoagulation displayed improved outcomes in a number of variables, compared with their counterparts who were not on anticoagulation: mortality (OR 0.65), shock (OR 0.68), acute kidney injury (OR 0.83), ICU admission (OR 0.57), multiorgan failure (OR 0.85), and hospital charges (a mean reduction of $9,275), as well as AP induced by alcohol use (OR 0.26; P less than .01 for all associations). “These data suggest that the majority of AP associated with alcohol was prevented by anticoagulation medication,” Dr. Bi said. “This is very striking. Anticoagulation may hold promise in both the prevention and treatment of AP.”
To further prove their points, Dr. Bi teamed with Baoan Ji, MD, PhD, a basic research scientist at Mayo Clinic, and developed a humanized AP animal model. With this model, they showed that Pradaxa, a Food and Drug Administration–approved anticoagulant, is effective in experimental AP prevention and treatment. “We are currently enrolling patients into a prospective clinical trial to further prove this in humans,” Dr. Bi said. The experimental therapeutic study will be reported at DDW on May 20.
She cautioned against using systemic anticoagulants in this patient population before results of the trial currently underway at Mayo Clinic’s Florida campus are known. “That should be sometime in mid-2020,” she said. “And the bleeding risk should be carefully monitored when using anticoagulants.”
The researchers were supported by funding from the Mayo Clinic and the Department of Defense.
SOURCE: Bi Y et al. DDW 2019, Abstract Sa1381.
SAN DIEGO –
“Acute pancreatitis is a very common disease,” lead study author Yan Bi, MD, PhD, a senior associate consultant and assistant professor in the department of gastroenterology and hepatology at Mayo Clinic, Jacksonville, Fla., said in an interview in advance of the annual Digestive Disease Week. “It’s the number one GI cause for hospitalization. Unfortunately, even after decades of basic science and clinical research, there’s still no cure; there’s nothing to prevent it from happening. The only treatment we can offer is supportive care, which includes fluid hydration, pain control, and nutrition support.”
The pathogenesis of acute pancreatitis (AP) is complex, she continued, and represents a sequence of distinct and interconnected pathologic events. “Both animal data and human studies have shown that acute pancreatitis is a hypercoagulable state,” she said. “We hypothesize that coagulation plays important roles in the development of pancreatitis.”
To test their hypothesis, Dr. Bi and associates performed a retrospective study. They drew from the 2014 National Inpatient Sample to evaluate the effect of systemic anticoagulation prior to AP onset on outcomes of the condition. They used ICD-9 codes to identify patients with a primary diagnosis of AP as well as those who were taking systemic anticoagulation. The primary outcome was the odds of AP in patients taking systemic anticoagulation, compared with those who were not. Secondary outcomes were mortality, morbidity, length of hospital stay, and total hospitalization charges and costs. The researchers used propensity score matching to create a 1:1 matching population for sex, age, and Charlson Comorbidity Index, and multivariate regression to adjust for patient ZIP code, income, hospital region, location, size, and teaching status.
Dr. Bi presented results from 442,535 patients with AP. Of these, 12,735 were on systemic anticoagulation prior to AP. Their mean age was 66 and 47% were female. After adjustment for confounders, patients on systemic anticoagulation prior to AP onset displayed a decreased odds of AP occurrence, compared with those who were not on anticoagulation (OR 0.56; P less than .01). In addition, patients on anticoagulation displayed improved outcomes in a number of variables, compared with their counterparts who were not on anticoagulation: mortality (OR 0.65), shock (OR 0.68), acute kidney injury (OR 0.83), ICU admission (OR 0.57), multiorgan failure (OR 0.85), and hospital charges (a mean reduction of $9,275), as well as AP induced by alcohol use (OR 0.26; P less than .01 for all associations). “These data suggest that the majority of AP associated with alcohol was prevented by anticoagulation medication,” Dr. Bi said. “This is very striking. Anticoagulation may hold promise in both the prevention and treatment of AP.”
To further prove their points, Dr. Bi teamed with Baoan Ji, MD, PhD, a basic research scientist at Mayo Clinic, and developed a humanized AP animal model. With this model, they showed that Pradaxa, a Food and Drug Administration–approved anticoagulant, is effective in experimental AP prevention and treatment. “We are currently enrolling patients into a prospective clinical trial to further prove this in humans,” Dr. Bi said. The experimental therapeutic study will be reported at DDW on May 20.
She cautioned against using systemic anticoagulants in this patient population before results of the trial currently underway at Mayo Clinic’s Florida campus are known. “That should be sometime in mid-2020,” she said. “And the bleeding risk should be carefully monitored when using anticoagulants.”
The researchers were supported by funding from the Mayo Clinic and the Department of Defense.
SOURCE: Bi Y et al. DDW 2019, Abstract Sa1381.
SAN DIEGO –
“Acute pancreatitis is a very common disease,” lead study author Yan Bi, MD, PhD, a senior associate consultant and assistant professor in the department of gastroenterology and hepatology at Mayo Clinic, Jacksonville, Fla., said in an interview in advance of the annual Digestive Disease Week. “It’s the number one GI cause for hospitalization. Unfortunately, even after decades of basic science and clinical research, there’s still no cure; there’s nothing to prevent it from happening. The only treatment we can offer is supportive care, which includes fluid hydration, pain control, and nutrition support.”
The pathogenesis of acute pancreatitis (AP) is complex, she continued, and represents a sequence of distinct and interconnected pathologic events. “Both animal data and human studies have shown that acute pancreatitis is a hypercoagulable state,” she said. “We hypothesize that coagulation plays important roles in the development of pancreatitis.”
To test their hypothesis, Dr. Bi and associates performed a retrospective study. They drew from the 2014 National Inpatient Sample to evaluate the effect of systemic anticoagulation prior to AP onset on outcomes of the condition. They used ICD-9 codes to identify patients with a primary diagnosis of AP as well as those who were taking systemic anticoagulation. The primary outcome was the odds of AP in patients taking systemic anticoagulation, compared with those who were not. Secondary outcomes were mortality, morbidity, length of hospital stay, and total hospitalization charges and costs. The researchers used propensity score matching to create a 1:1 matching population for sex, age, and Charlson Comorbidity Index, and multivariate regression to adjust for patient ZIP code, income, hospital region, location, size, and teaching status.
Dr. Bi presented results from 442,535 patients with AP. Of these, 12,735 were on systemic anticoagulation prior to AP. Their mean age was 66 and 47% were female. After adjustment for confounders, patients on systemic anticoagulation prior to AP onset displayed a decreased odds of AP occurrence, compared with those who were not on anticoagulation (OR 0.56; P less than .01). In addition, patients on anticoagulation displayed improved outcomes in a number of variables, compared with their counterparts who were not on anticoagulation: mortality (OR 0.65), shock (OR 0.68), acute kidney injury (OR 0.83), ICU admission (OR 0.57), multiorgan failure (OR 0.85), and hospital charges (a mean reduction of $9,275), as well as AP induced by alcohol use (OR 0.26; P less than .01 for all associations). “These data suggest that the majority of AP associated with alcohol was prevented by anticoagulation medication,” Dr. Bi said. “This is very striking. Anticoagulation may hold promise in both the prevention and treatment of AP.”
To further prove their points, Dr. Bi teamed with Baoan Ji, MD, PhD, a basic research scientist at Mayo Clinic, and developed a humanized AP animal model. With this model, they showed that Pradaxa, a Food and Drug Administration–approved anticoagulant, is effective in experimental AP prevention and treatment. “We are currently enrolling patients into a prospective clinical trial to further prove this in humans,” Dr. Bi said. The experimental therapeutic study will be reported at DDW on May 20.
She cautioned against using systemic anticoagulants in this patient population before results of the trial currently underway at Mayo Clinic’s Florida campus are known. “That should be sometime in mid-2020,” she said. “And the bleeding risk should be carefully monitored when using anticoagulants.”
The researchers were supported by funding from the Mayo Clinic and the Department of Defense.
SOURCE: Bi Y et al. DDW 2019, Abstract Sa1381.
REPORTING FROM DDW 2019
Key clinical point: Anticoagulation may hold promise in both the prevention and treatment of acute pancreatitis (AP).
Major finding: Patients on systemic anticoagulation prior to AP onset displayed a decreased odds of AP occurrence, compared with those who were not on anticoagulation (OR 0.56; P less than .01).
Study details: A retrospective analysis of 442,535 patients with AP.
Disclosures: The researchers were supported by funding from the Mayo Clinic and the Department of Defense.
Source: Bi Y et al. DDW 2019, Abstract Sa1381.
New concepts in the management of acute pancreatitis
Introduction
Acute pancreatitis (AP) is a major clinical and financial burden in the United States. Several major clinical guidelines provide evidence-based recommendations for the clinical management decisions in AP, including those from the American College of Gastroenterology (ACG; 2013),1 and the International Association of Pancreatology (IAP; 2013).2 More recently, the American Gastroenterological Association (AGA) released their own set of guidelines.3,4 In this update on AP, we review these guidelines and reference recent literature focused on epidemiology, risk factors, etiology, diagnosis, risk stratification, and recent advances in the early medical management of AP. Regarding the latter, we review six treatment interventions (pain management, intravenous fluid resuscitation, feeding, prophylactic antibiotics, probiotics, and timing of endoscopic retrograde cholangiopancreatography (ERCP) in acute biliary pancreatitis) and four preventive interventions (alcohol and smoking cessation, same-admission cholecystectomy for acute biliary pancreatitis, and chemoprevention and fluid administration for post-ERCP pancreatitis [PEP]). Updates on multidisciplinary management of (infected) pancreatic necrosis is beyond the scope of this review. Table 1 summarizes the concepts discussed in this article.

Recent advances in epidemiology and evaluation of AP
Epidemiology
AP is the third most common cause of gastrointestinal-related hospitalizations and fourth most common cause of readmission in 2014.5 Recent epidemiologic studies show conflicting trends for the incidence of AP, both increasing6 and decreasing,7 likely attributable to significant differences in study designs. Importantly, multiple studies have demonstrated that hospital length of stay, costs, and mortality have declined since 2009.6,8-10
Persistent organ failure (POF), defined as organ failure lasting more than 48 hours, is the major cause of death in AP. POF, if only a single organ during AP, is associated with 27%-36% mortality; if it is multiorgan, it is associated with 47% mortality.1,11 Other factors associated with increased hospital mortality include infected pancreatic necrosis,12-14 diabetes mellitus,15 hospital-acquired infection,16 advanced age (70 years and older),17 and obesity.18 Predictive factors of 1-year mortality include readmission within 30 days, higher Charlson Comorbidity Index, and longer hospitalization.19
Risk factors
We briefly highlight recent insights into risk factors for AP (Table 1) and refer to a recent review for further discussion.20 Current and former tobacco use are independent risk factors for AP.21 The dose-response relationship of alcohol to the risk of pancreatitis is complex,22 but five standard drinks per day for 5 years is a commonly used cut-off.1,23 New evidence suggests that the relationship between the dose of alcohol and risk of AP differs by sex, linearly in men but nonlinearly (J-shaped) in women.24 Risk of AP in women was decreased with alcohol consumption of up to 40 g/day (one standard drink contains 14 g of alcohol) and increased above this amount. Cannabis is a possible risk factor for toxin-induced AP and abstinence appears to abolish risk of recurrent attacks.25
Patients with inflammatory bowel disease (IBD) have a 2.9-fold higher risk for AP versus non-IBD cohorts26 with the most common etiologies are from gallstones and medications.27 In patients with end-stage renal disease (ESRD), the risk of AP is higher in those who receive peritoneal dialysis, compared with hemodialysis28-33 and who are women, older, or have cholelithiasis or liver disease.34As recently reviewed,35 pancreatic cancer appears to be associated with first-attack pancreatitis with few exceptions.36 In this setting, the overall incidence of pancreatic cancer is low (1.5%). The risk is greatest within the first year of the attack of AP, negligible below age 40 years but steadily rising through the fifth to eighth decades.37 Pancreatic cancer screening is a conditional recommendation of the ACG guidelines in patients with unexplained AP, particularly those aged 40 years or older.1
Etiology and diagnosis
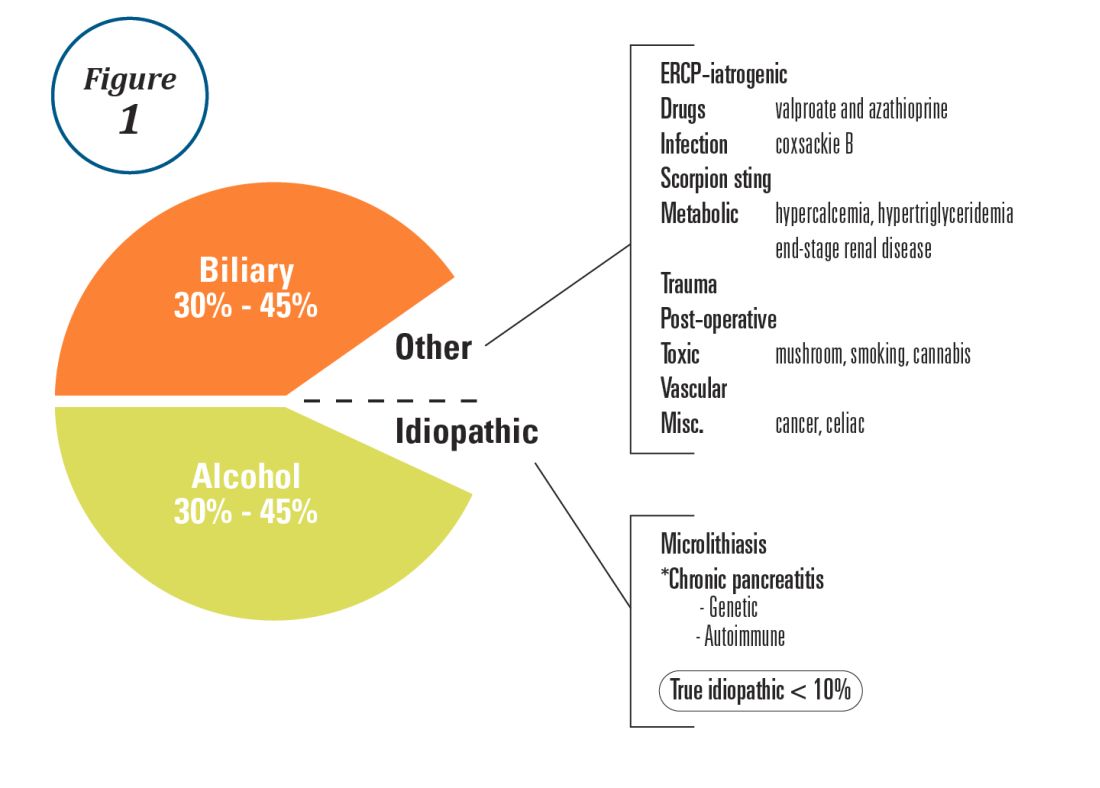
Alcohol and gallstones remain the most prevalent etiologies for AP.1 While hypertriglyceridemia accounted for 9% of AP in a systematic review of acute pancreatitis in 15 different countries,38 it is the second most common cause of acute pancreatitis in Asia (especially China).39 Figure 1 provides a breakdown of the etiologies and risk factors of pancreatitis. Importantly, it remains challenging to assign several toxic-metabolic etiologies as either a cause or risk factor for AP, particularly with regards to alcohol, smoking, and cannabis to name a few.
Guidelines and recent studies of AP raise questions about the threshold above which hypertriglyceridemia causes or poses as an important cofactor for AP. American and European societies define the threshold for triglycerides at 885-1,000 mg/dL.1,42,43 Pedersen et al. provide evidence of a graded risk of AP with hypertriglyceridemia: In multivariable analysis, adjusted hazard ratios for AP were much higher with nonfasting mild to moderately elevated plasma triglycerides (177-885 mg/dL), compared with normal values (below 89 mg/dL).44 Moreover, the risk of severe AP (developing POF) increases in proportion to triglyceride value, independent of the underlying cause of AP.45

Diagnosis of AP is derived from the revised Atlanta classification.46 The recommended timing and indications for offering cross-sectional imaging are after 48-72 hours in patients with no improvement to initial care.1 Endoscopic ultrasonography (EUS) has better diagnostic accuracy and sensitivity, compared with magnetic resonance cholangiopancreatography (MRCP) for choledocholithiasis, and has comparable specificity.47,48 Among noninvasive imaging modalities, MRCP is more sensitive than computed tomography (CT) for diagnosing choledocholithiasis.49 Despite guideline recommendations for more selective use of pancreatic imaging in the early assessment of AP, utilization of early CT or MRCP imaging (within the first 24 hours of care) remained high during 2014-2015, compared with 2006-2007.50
ERCP is not recommended as a pure diagnostic tool, owing to the availability of other diagnostic tests and a complication rate of 5%-10% with risks involving PEP, cholangitis, perforation, and hemorrhage.51 A recent systematic review of EUS and ERCP in acute biliary pancreatitis concluded that EUS had lower failure rates and had no complications, and the use of EUS avoided ERCP in 71.2% of cases.52
Risk stratification
The goals of using risk stratification tools in AP are to identify patients at risk for developing major outcomes, including POF, infected pancreatic necrosis, and death, and to ensure timely triaging of patients to an appropriate level of care. Existing prediction models have only moderate predictive value.53,54 Examples include simple risk stratification tools such as blood urea nitrogen (BUN) and hemoconcentration,55,56 disease-modifying patient variables (age, obesity, etc.), biomarkers (i.e., angiopoietin 2),57 and more complex clinical scoring systems such as Acute Physiology and Chronic Health Evaluation II (APACHE II), BISAP (BUN, impaired mental status, SIRS criteria, age, pleural effusion) score, early warning system (EWS), Glasgow-Imrie score, Japanese severity score, and recently the Pancreatitis Activity Scoring System (PASS).58 Two recent guidelines affirmed the importance of predicting the severity of AP, using one or more predictive tools.1,2 The recent 2018 AGA technical review does not debate this commonsense approach, but does highlight that there is no published observational study or randomized, controlled trial (RCT) investigating whether prediction tools affect clinical outcomes.4
Recent advances in early treatment of AP
Literature review and definitions
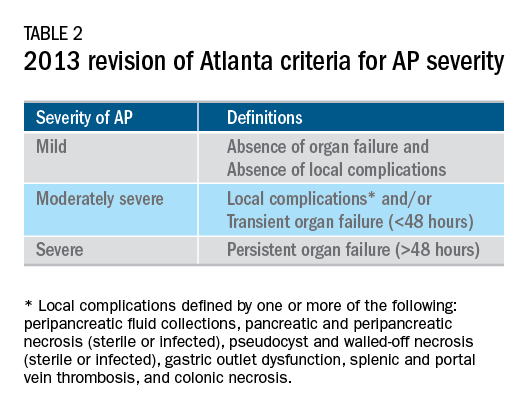
The AP literature contains heterogeneous definitions of severe AP and of what constitutes a major outcome in AP. Based on definitions of the 2013 revised Atlanta Criteria, the 2018 AGA technical review and clinical guidelines emphasized precise definitions of primary outcomes of clinical importance in AP, including death, persistent single organ failure, or persistent multiple organ failure, each requiring a duration of more than 48 hours, and infected pancreatic or peripancreatic necrosis or both (Table 2).3,4
Pain management
Management of pain in AP is complex and requires a detailed discussion beyond the scope of this review, but recent clinical and translational studies raise questions about the current practice of using opioids for pain management in AP. A provocative, multicenter, retrospective cohort study reported lower 30-day mortality among critically ill patients who received epidural analgesia versus standard care without epidural analgesia.59 The possible mechanism of protection and the drugs administered are unclear. An interesting hypothesis is that the epidural cohort may have received lower exposure to morphine, which may increase gut permeability, the risk of infectious complications, and severity of AP, based on a translational study in mice.60
Intravenous fluid administration
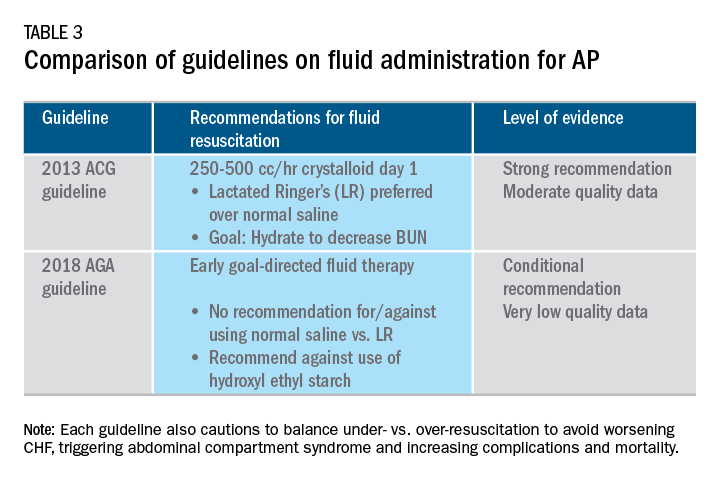
Supportive care with the use of IV fluid hydration is a mainstay of treatment for AP in the first 12-24 hours. Table 3 summarizes the guidelines in regards to IV fluid administration as delineated by the ACG and AGA guidelines on the management of pancreatitis.1,3 Guidelines advocate for early fluid resuscitation to correct intravascular depletion in order to reduce morbidity and mortality associated with AP.1,2,4 The 2018 AGA guidelines endorse a conditional recommendation for using goal-directed therapy for initial fluid management,3 do not recommend for or against normal saline versus lactated Ringer’s (LR), but do advise against the use of hydroxyethyl starch fluids.3 Consistent with these recommendations, two recent RCTs published subsequent to the prespecified time periods of the AGA technical review and guideline, observed no significant differences between LR and normal saline on clinically meaningful outcomes.61,62 The AGA guidelines acknowledge that evidence was of very-low quality in support of goal-directed therapy,3,4 which has not been shown to have a significant reduction in persistent multiple organ failure, mortality, or pancreatic necrosis, compared with usual care. As the authors noted, interpretation of the data was limited by the absence of other critical outcomes in these trials (infected pancreatic necrosis), lack of uniformity of specific outcomes and definitions of transient and POF, few trials, and risk of bias. There is a clear need for a large RCT to provide evidence to guide decision making with fluid resuscitation in AP, particularly in regard to fluid type, volume, rate, duration, endpoints, and clinical outcomes.
Feeding
More recently, the focus of nutrition in the management of AP has shifted away from patients remaining nil per os (NPO). Current guidelines advocate for early oral feeding (within 24 hours) in mild AP,3,4 in order to protect the gut-mucosal barrier. Remaining NPO when compared with early oral feeding has a 2.5-fold higher risk for interventions for necrosis.4 The recently published AGA technical review identified no significant impact on outcomes of early versus delayed oral feeding, which is consistent with observations of a landmark Dutch PYTHON trial entitled “Early versus on-demand nasoenteric tube feeding in acute pancreatitis.”4,63 There is no clear cutoff point for initiating feeding for those with severe AP. A suggested practical approach is to initiate feeding within 24-72 hours and offer enteral nutrition for those intolerant to oral feeds. In severe AP and moderately severe AP, enteral nutrition is recommended over parenteral nutrition.3,4 Enteral nutrition significantly reduces the risk of infected peripancreatic necrosis, single organ failure, and multiorgan failure.4 Finally, the AGA guidelines provide a conditional recommendation for providing enteral nutrition support through either the nasogastric or nasoenteric route.3 Further studies are required to determine the optimal timing, rate, and formulation of enteral nutrition in severe AP.
Antibiotics and probiotics
Current guidelines do not support the use of prophylactic antibiotics to prevent infection in necrotizing AP and severe AP.1-3 The AGA technical review reported that prophylactic antibiotics did not reduce infected pancreatic or peripancreatic necrosis, persistent single organ failure, or mortality.4 Guidelines advocate against the use of probiotics for severe AP, because of increased mortality risk.1
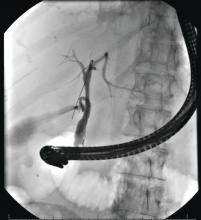
Timing of ERCP in acute biliary pancreatitis
There is universal agreement for offering urgent ERCP (within 24 hours) in biliary AP complicated by cholangitis.1-3,64 Figure 2 demonstrates an example of a cholangiogram completed within 24 hours of presentation of biliary AP complicated by cholangitis.
In the absence of cholangitis, the timing of ERCP for AP with persistent biliary obstruction is less clear.1-3 In line with recent guidelines, the 2018 AGA guidelines advocate against routine use of urgent ERCP for biliary AP without cholangitis,3 a conditional recommendation with overall low quality of data.4 The AGA technical review found that urgent ERCP, compared with conservative management in acute biliary pancreatitis without cholangitis had no significant effect on mortality, organ failure, infected pancreatic necrosis, and total necrotizing pancreatitis, but did significantly shorten hospital length of stay.4 There are limited data to guide decision making of when nonurgent ERCP should be performed in hospitalized patients with biliary AP with persistent obstruction and no cholangitis.3,64
Alcohol and smoking cessation
The AGA technical review advocates for brief alcohol intervention during hospitalization for alcohol-induced AP on the basis of one RCT that addresses the impact of alcohol counseling on recurrent bouts of AP4 plus evidence from a Cochrane review of alcohol-reduction strategies in primary care populations.65 Cessation of smoking – an established independent risk factor of AP – recurrent AP and chronic pancreatitis, should also be recommended as part of the management of AP.
Cholecystectomy
Evidence supports same-admission cholecystectomy for mild gallstone AP, a strong recommendation of published AGA guidelines.3 When compared with delayed cholecystectomy, same-admission cholecystectomy significantly reduced gallstone-related complications, readmissions for recurrent pancreatitis, and pancreaticobiliary complications, without having a significant impact on mortality during a 6-month follow-up period.66 Delaying cholecystectomy 6 weeks in patients with moderate-severe gallstone AP appears to reduce morbidity, including the development of infected collections, and mortality.4 An ongoing RCT, the APEC trial, aims to determine whether early ERCP with biliary sphincterotomy reduces major complications or death when compared with no intervention for biliary AP in patients at high risk of complications.67
Chemoprevention and IV fluid management of post-ERCP pancreatitis
Accumulating data support the effectiveness of chemoprevention, pancreatic stent placement, and fluid administration to prevent post-ERCP pancreatitis. Multiple RCTs, meta-analyses, and systematic reviews indicate that rectal NSAIDs) reduce post-ERCP pancreatitis onset68-71 and moderate-severe post-ERCP pancreatitis. Additionally, placement of a pancreatic duct stent may decrease the risk of severe post-ERCP pancreatitis in high-risk patients.3 Guidelines do not comment on fluid administrations for prevention of post-ERCP pancreatitis, but studies have shown that greater periprocedural IV fluid was an independent protective factor against moderate to severe PEP72 and was associated with shorter hospital length of stay.73 Recent meta-analyses and RCTs support using LR prior to ERCP to prevent PEP.74-77 Interestingly, a recent RCT shows that the combination of rectal indomethacin and LR, compared with combination placebo and normal saline reduced the risk of PEP in high-risk patients.78
Two ongoing multicenter RCTs will clarify the role of combination therapy. The Dutch FLUYT RCT aims to determine the optimal combination of rectal NSAIDs and periprocedural infusion of IV fluids to reduce the incidence of PEP and moderate-severe PEP79 and the Stent vs. Indomethacin (SVI) trial aims to determine the whether combination pancreatic stent placement plus rectal indomethacin is superior to monotherapy indomethacin for preventing post-ERCP pancreatitis in high-risk cases.80
Implications for clinical practice
The diagnosis and optimal management of AP require a systematic approach with multidisciplinary decision making. Morbidity and mortality in AP are driven by early or late POF, and the latter often is triggered by infected necrosis. Risk stratification of these patients at the point of contact is a commonsense approach to enable triaging of patients to the appropriate level of care. Regardless of pancreatitis severity, recommended treatment interventions include goal-directed IV fluid resuscitation, early feeding by mouth or enteral tube when necessary, avoidance of prophylactic antibiotics, avoidance of probiotics, and urgent ERCP for patients with acute biliary pancreatitis complicated by cholangitis. Key measures for preventing hospital readmission and pancreatitis include same-admission cholecystectomy for acute biliary pancreatitis and alcohol and smoking cessation. Preventive measures for post-ERCP pancreatitis in patients undergoing ERCP include rectal indomethacin, prophylactic pancreatic duct stent placement, and periprocedural fluid resuscitation.
Dr. Mandalia is a fellow, gastroenterology, department of internal medicine, division of gastroenterology, Michigan Medicine, Ann Arbor; Dr. DiMagno is associate professor of medicine, director, comprehensive pancreas program, department of internal medicine, division of gastroenterology, University of Michigan, Ann Arbor. Dr. Mandalia reports no conflicts of interest.
References
1. Tenner S et al. Am J Gastroenterol. 2013;108:1400.
2. Besseline M et al. Pancreatology. 2013;13(4, Supplement 2):e1-15.
3. Crockett SD et al. Gastroenterology. 2018;154(4):1096-101.
4. Vege SS et al. Gastroenterology. 2018;154(4):1103-39.
5. Peery AF et al. Gastroenterology. 2019 Jan;156(1):254-72.e11.
6. Krishna SG et al. Pancreas. 2017;46(4):482-8.
7. Sellers ZM et al. Gastroenterology. 2018;155(2):469-78.e1.
8. Brown A et al. JOP. 2008;9(4):408-14.
9. Fagenholz PJ et al. Ann Epidemiol. 2007;17(7):491.e1-.e8.
10. McNabb-Baltar J et al. Pancreas. 2014;43(5):687-91.
11. Johnson CD et al. Gut. 2004;53(9):1340-4.
12. Dellinger EP et al. Ann Surg. 2012;256(6):875-80.
13. Petrov MS et al. Gastroenterology. 2010;139(3):813-20.
14. Sternby H et al. Ann Surg. Apr 18. doi: 10.1097/SLA.0000000000002766.
15. Huh JH et al. J Clin Gastroenterol. 2018;52(2):178-83.
16. Wu BU et al. Gastroenterology. 2008;135(3):816-20.
17. Gardner TB et al. Clin Gastroenterol Hepatol. 2008;6(10):1070-6.
18. Krishna SG et al. Am J Gastroenterol. 2015;110(11):1608-19.
19. Lee PJ et al. Pancreas. 2016;45(4):561-4.
20. Mandalia A et al. F1000Research. 2018 Jun 28;7.
21. Majumder S et al. Pancreas. 2015;44(4):540-6.
22. DiMagno MJ. Clin Gastroenterol Hepatol. 2011;9(11):920-2.
23. Yadav D, Whitcomb DC. Nature Rev Gastroenterol Hepatol. 2010;7(3):131-45.
24. Samokhvalov AV et al. EBioMedicine. 2015;2(12):1996-2002.
25. Barkin JA et al. Pancreas. 2017;46(8):1035-8.
26. Chen Y-T et al. J Gastroenterol Hepatol. 2016;31(4):782-7.
27. Ramos LR et al. J Crohns Colitis. 2016;10(1):95-104.
28. Avram MM. Nephron. 1977;18(1):68-71.
29. Lankisch PG et al. Nephrol Dial Transplant. 2008;23(4):1401-5.
30. Owyang C et al. Mayo Clin Proc. 1979;54(12):769-73.
31. Owyang Cet al. Gut. 1982;23(5):357-61.
32. Quraishi ER et al. Am J Gastroenterol. 2005;100:2288.
33. Vaziri ND et al. Nephron. 1987;46(4):347-9.
34. Chen HJ et al. Nephrol Dial Transplant. 2017;32(10):1731-6.
35. Kirkegard J et al. Gastroenterology. 2018;May;154(6):1729-36.
36. Karlson BM, et al. Gastroenterology. 1997;113(2):587-92.
37. Munigala S et al. Clin Gastroenterol Hepatol. 2014;12(7):1143-50.e1.
38. Carr RA et al. Pancreatology. 2016;16(4):469-76.
39. Li X et al. BMC Gastroenterol. 2018;18(1):89.
40. Ahmed AU et al. Clin Gastroenterol Hepatol. 2016;14(5):738-46.
41. Sankaran SJ et al. Gastroenterology. 2015;149(6):1490-500.e1.
42. Berglund L et al. J Clin Endocrinol Metab. 2012;97(9):2969-89.
43. Catapano AL et al. Atherosclerosis. 2011;217(1):3-46.
44. Pedersen SB et al. JAMA Intern Med. 2016;176(12):1834-42.
45. Nawaz H et al. Am J Gastroenterol. 2015;110(10):1497-503.
46. Banks PA et al. Gut. 2013;62(1):102-11.
47. Kondo S et al. Eur J Radiol. 2005;54(2):271-5.
48. Meeralam Y et al. Gastrointest Endosc. 2017;86(6):986-93.
49. Stimac D et al. Am J Gastroenterol. 2007;102(5):997-1004.
50. Jin DX et al. Dig Dis Sci. 2017;62(10):2894-9.
51. Freeman ML. Gastrointest Endosc Clin N Am. 2012;22(3):567-86.
52. De Lisi S et al. Eur J Gastroenterol Hepatol. 2011;23(5):367-74.
53. Di MY et al. Ann Int Med. 2016;165(7):482-90.
54. Mounzer R et al. Gastroenterology. 2012;142(7):1476-82; quiz e15-6.
55. Koutroumpakis E et al. Am J Gastroenterol. 2015;110(12):1707-16.
56. Wu BU et al. Gastroenterology. 2009;137(1):129-35.
57. Buddingh KT et al. J Am Coll Surg. 2014;218(1):26-32.
58. Buxbaum J et al. Am J Gastroenterol. 2018;113(5):755-64.
59. Jabaudon M et al. Crit Car Med. 2018;46(3):e198-e205.
60. Barlass U et al. Gut. 2018;67(4):600-2.
61. Buxbaum JL et al. Am J Gastroenterol. 2017;112(5):797-803.
62. de-Madaria E et al. United Eur Gastroenterol J. 2018;6(1):63-72.
63. Bakker OJ et al. N Engl J Med. 2014;371(21):1983-93.
64. Tse F et al. Cochrane Database Syst Rev. 2012(5):Cd009779.
65. Kaner EFS et al. Cochrane Database Syst Rev. 2007(2):Cd004148.
66. da Costa DW et al. Lancet. 2015;386(10000):1261-8.
67. Schepers NJ et al. Trials. 2016;17:5.
68. Vadala di Prampero SF et al. Eur J Gastroenterol Hepatol. 2016;28(12):1415-24.
69. Kubiliun NM et al. Clin Gastroenterol Hepatol. 2015;13(7):1231-9; quiz e70-1.
70. Wan J et al. BMC Gastroenterol. 2017;17(1):43.
71. Yang C et al. Pancreatology. 2017;17(5):681-8.
72. DiMagno MJ et al. Pancreas. 2014;43(4):642-7.
73. Sagi SV et al. J Gastroenterol Hepatol. 2014;29(6):1316-20.
74. Choi JH et al. Clin Gastroenterol Hepatol. 2017;15(1):86-92.e1.
75. Wu D et al. J Clin Gastroenterol. 2017;51(8):e68-e76.
76. Zhang ZF et al. J Clin Gastroenterol. 2017;51(3):e17-e26.
77. Park CH et al. Endoscopy 2018 Apr;50(4):378-85.
78. Mok SRS et al. Gastrointest Endosc. 2017;85(5):1005-13.
79. Smeets XJN et al. Trials. 2018;19(1):207.
80. Elmunzer BJ et al. Trials. 2016;17(1):120.
Introduction
Acute pancreatitis (AP) is a major clinical and financial burden in the United States. Several major clinical guidelines provide evidence-based recommendations for the clinical management decisions in AP, including those from the American College of Gastroenterology (ACG; 2013),1 and the International Association of Pancreatology (IAP; 2013).2 More recently, the American Gastroenterological Association (AGA) released their own set of guidelines.3,4 In this update on AP, we review these guidelines and reference recent literature focused on epidemiology, risk factors, etiology, diagnosis, risk stratification, and recent advances in the early medical management of AP. Regarding the latter, we review six treatment interventions (pain management, intravenous fluid resuscitation, feeding, prophylactic antibiotics, probiotics, and timing of endoscopic retrograde cholangiopancreatography (ERCP) in acute biliary pancreatitis) and four preventive interventions (alcohol and smoking cessation, same-admission cholecystectomy for acute biliary pancreatitis, and chemoprevention and fluid administration for post-ERCP pancreatitis [PEP]). Updates on multidisciplinary management of (infected) pancreatic necrosis is beyond the scope of this review. Table 1 summarizes the concepts discussed in this article.

Recent advances in epidemiology and evaluation of AP
Epidemiology
AP is the third most common cause of gastrointestinal-related hospitalizations and fourth most common cause of readmission in 2014.5 Recent epidemiologic studies show conflicting trends for the incidence of AP, both increasing6 and decreasing,7 likely attributable to significant differences in study designs. Importantly, multiple studies have demonstrated that hospital length of stay, costs, and mortality have declined since 2009.6,8-10
Persistent organ failure (POF), defined as organ failure lasting more than 48 hours, is the major cause of death in AP. POF, if only a single organ during AP, is associated with 27%-36% mortality; if it is multiorgan, it is associated with 47% mortality.1,11 Other factors associated with increased hospital mortality include infected pancreatic necrosis,12-14 diabetes mellitus,15 hospital-acquired infection,16 advanced age (70 years and older),17 and obesity.18 Predictive factors of 1-year mortality include readmission within 30 days, higher Charlson Comorbidity Index, and longer hospitalization.19
Risk factors
We briefly highlight recent insights into risk factors for AP (Table 1) and refer to a recent review for further discussion.20 Current and former tobacco use are independent risk factors for AP.21 The dose-response relationship of alcohol to the risk of pancreatitis is complex,22 but five standard drinks per day for 5 years is a commonly used cut-off.1,23 New evidence suggests that the relationship between the dose of alcohol and risk of AP differs by sex, linearly in men but nonlinearly (J-shaped) in women.24 Risk of AP in women was decreased with alcohol consumption of up to 40 g/day (one standard drink contains 14 g of alcohol) and increased above this amount. Cannabis is a possible risk factor for toxin-induced AP and abstinence appears to abolish risk of recurrent attacks.25
Patients with inflammatory bowel disease (IBD) have a 2.9-fold higher risk for AP versus non-IBD cohorts26 with the most common etiologies are from gallstones and medications.27 In patients with end-stage renal disease (ESRD), the risk of AP is higher in those who receive peritoneal dialysis, compared with hemodialysis28-33 and who are women, older, or have cholelithiasis or liver disease.34As recently reviewed,35 pancreatic cancer appears to be associated with first-attack pancreatitis with few exceptions.36 In this setting, the overall incidence of pancreatic cancer is low (1.5%). The risk is greatest within the first year of the attack of AP, negligible below age 40 years but steadily rising through the fifth to eighth decades.37 Pancreatic cancer screening is a conditional recommendation of the ACG guidelines in patients with unexplained AP, particularly those aged 40 years or older.1
Etiology and diagnosis

Alcohol and gallstones remain the most prevalent etiologies for AP.1 While hypertriglyceridemia accounted for 9% of AP in a systematic review of acute pancreatitis in 15 different countries,38 it is the second most common cause of acute pancreatitis in Asia (especially China).39 Figure 1 provides a breakdown of the etiologies and risk factors of pancreatitis. Importantly, it remains challenging to assign several toxic-metabolic etiologies as either a cause or risk factor for AP, particularly with regards to alcohol, smoking, and cannabis to name a few.
Guidelines and recent studies of AP raise questions about the threshold above which hypertriglyceridemia causes or poses as an important cofactor for AP. American and European societies define the threshold for triglycerides at 885-1,000 mg/dL.1,42,43 Pedersen et al. provide evidence of a graded risk of AP with hypertriglyceridemia: In multivariable analysis, adjusted hazard ratios for AP were much higher with nonfasting mild to moderately elevated plasma triglycerides (177-885 mg/dL), compared with normal values (below 89 mg/dL).44 Moreover, the risk of severe AP (developing POF) increases in proportion to triglyceride value, independent of the underlying cause of AP.45

Diagnosis of AP is derived from the revised Atlanta classification.46 The recommended timing and indications for offering cross-sectional imaging are after 48-72 hours in patients with no improvement to initial care.1 Endoscopic ultrasonography (EUS) has better diagnostic accuracy and sensitivity, compared with magnetic resonance cholangiopancreatography (MRCP) for choledocholithiasis, and has comparable specificity.47,48 Among noninvasive imaging modalities, MRCP is more sensitive than computed tomography (CT) for diagnosing choledocholithiasis.49 Despite guideline recommendations for more selective use of pancreatic imaging in the early assessment of AP, utilization of early CT or MRCP imaging (within the first 24 hours of care) remained high during 2014-2015, compared with 2006-2007.50
ERCP is not recommended as a pure diagnostic tool, owing to the availability of other diagnostic tests and a complication rate of 5%-10% with risks involving PEP, cholangitis, perforation, and hemorrhage.51 A recent systematic review of EUS and ERCP in acute biliary pancreatitis concluded that EUS had lower failure rates and had no complications, and the use of EUS avoided ERCP in 71.2% of cases.52
Risk stratification
The goals of using risk stratification tools in AP are to identify patients at risk for developing major outcomes, including POF, infected pancreatic necrosis, and death, and to ensure timely triaging of patients to an appropriate level of care. Existing prediction models have only moderate predictive value.53,54 Examples include simple risk stratification tools such as blood urea nitrogen (BUN) and hemoconcentration,55,56 disease-modifying patient variables (age, obesity, etc.), biomarkers (i.e., angiopoietin 2),57 and more complex clinical scoring systems such as Acute Physiology and Chronic Health Evaluation II (APACHE II), BISAP (BUN, impaired mental status, SIRS criteria, age, pleural effusion) score, early warning system (EWS), Glasgow-Imrie score, Japanese severity score, and recently the Pancreatitis Activity Scoring System (PASS).58 Two recent guidelines affirmed the importance of predicting the severity of AP, using one or more predictive tools.1,2 The recent 2018 AGA technical review does not debate this commonsense approach, but does highlight that there is no published observational study or randomized, controlled trial (RCT) investigating whether prediction tools affect clinical outcomes.4

Recent advances in early treatment of AP
Literature review and definitions
The AP literature contains heterogeneous definitions of severe AP and of what constitutes a major outcome in AP. Based on definitions of the 2013 revised Atlanta Criteria, the 2018 AGA technical review and clinical guidelines emphasized precise definitions of primary outcomes of clinical importance in AP, including death, persistent single organ failure, or persistent multiple organ failure, each requiring a duration of more than 48 hours, and infected pancreatic or peripancreatic necrosis or both (Table 2).3,4
Pain management
Management of pain in AP is complex and requires a detailed discussion beyond the scope of this review, but recent clinical and translational studies raise questions about the current practice of using opioids for pain management in AP. A provocative, multicenter, retrospective cohort study reported lower 30-day mortality among critically ill patients who received epidural analgesia versus standard care without epidural analgesia.59 The possible mechanism of protection and the drugs administered are unclear. An interesting hypothesis is that the epidural cohort may have received lower exposure to morphine, which may increase gut permeability, the risk of infectious complications, and severity of AP, based on a translational study in mice.60
Intravenous fluid administration

Supportive care with the use of IV fluid hydration is a mainstay of treatment for AP in the first 12-24 hours. Table 3 summarizes the guidelines in regards to IV fluid administration as delineated by the ACG and AGA guidelines on the management of pancreatitis.1,3 Guidelines advocate for early fluid resuscitation to correct intravascular depletion in order to reduce morbidity and mortality associated with AP.1,2,4 The 2018 AGA guidelines endorse a conditional recommendation for using goal-directed therapy for initial fluid management,3 do not recommend for or against normal saline versus lactated Ringer’s (LR), but do advise against the use of hydroxyethyl starch fluids.3 Consistent with these recommendations, two recent RCTs published subsequent to the prespecified time periods of the AGA technical review and guideline, observed no significant differences between LR and normal saline on clinically meaningful outcomes.61,62 The AGA guidelines acknowledge that evidence was of very-low quality in support of goal-directed therapy,3,4 which has not been shown to have a significant reduction in persistent multiple organ failure, mortality, or pancreatic necrosis, compared with usual care. As the authors noted, interpretation of the data was limited by the absence of other critical outcomes in these trials (infected pancreatic necrosis), lack of uniformity of specific outcomes and definitions of transient and POF, few trials, and risk of bias. There is a clear need for a large RCT to provide evidence to guide decision making with fluid resuscitation in AP, particularly in regard to fluid type, volume, rate, duration, endpoints, and clinical outcomes.
Feeding
More recently, the focus of nutrition in the management of AP has shifted away from patients remaining nil per os (NPO). Current guidelines advocate for early oral feeding (within 24 hours) in mild AP,3,4 in order to protect the gut-mucosal barrier. Remaining NPO when compared with early oral feeding has a 2.5-fold higher risk for interventions for necrosis.4 The recently published AGA technical review identified no significant impact on outcomes of early versus delayed oral feeding, which is consistent with observations of a landmark Dutch PYTHON trial entitled “Early versus on-demand nasoenteric tube feeding in acute pancreatitis.”4,63 There is no clear cutoff point for initiating feeding for those with severe AP. A suggested practical approach is to initiate feeding within 24-72 hours and offer enteral nutrition for those intolerant to oral feeds. In severe AP and moderately severe AP, enteral nutrition is recommended over parenteral nutrition.3,4 Enteral nutrition significantly reduces the risk of infected peripancreatic necrosis, single organ failure, and multiorgan failure.4 Finally, the AGA guidelines provide a conditional recommendation for providing enteral nutrition support through either the nasogastric or nasoenteric route.3 Further studies are required to determine the optimal timing, rate, and formulation of enteral nutrition in severe AP.
Antibiotics and probiotics
Current guidelines do not support the use of prophylactic antibiotics to prevent infection in necrotizing AP and severe AP.1-3 The AGA technical review reported that prophylactic antibiotics did not reduce infected pancreatic or peripancreatic necrosis, persistent single organ failure, or mortality.4 Guidelines advocate against the use of probiotics for severe AP, because of increased mortality risk.1
Timing of ERCP in acute biliary pancreatitis
There is universal agreement for offering urgent ERCP (within 24 hours) in biliary AP complicated by cholangitis.1-3,64 Figure 2 demonstrates an example of a cholangiogram completed within 24 hours of presentation of biliary AP complicated by cholangitis.
In the absence of cholangitis, the timing of ERCP for AP with persistent biliary obstruction is less clear.1-3 In line with recent guidelines, the 2018 AGA guidelines advocate against routine use of urgent ERCP for biliary AP without cholangitis,3 a conditional recommendation with overall low quality of data.4 The AGA technical review found that urgent ERCP, compared with conservative management in acute biliary pancreatitis without cholangitis had no significant effect on mortality, organ failure, infected pancreatic necrosis, and total necrotizing pancreatitis, but did significantly shorten hospital length of stay.4 There are limited data to guide decision making of when nonurgent ERCP should be performed in hospitalized patients with biliary AP with persistent obstruction and no cholangitis.3,64
Alcohol and smoking cessation
The AGA technical review advocates for brief alcohol intervention during hospitalization for alcohol-induced AP on the basis of one RCT that addresses the impact of alcohol counseling on recurrent bouts of AP4 plus evidence from a Cochrane review of alcohol-reduction strategies in primary care populations.65 Cessation of smoking – an established independent risk factor of AP – recurrent AP and chronic pancreatitis, should also be recommended as part of the management of AP.
Cholecystectomy
Evidence supports same-admission cholecystectomy for mild gallstone AP, a strong recommendation of published AGA guidelines.3 When compared with delayed cholecystectomy, same-admission cholecystectomy significantly reduced gallstone-related complications, readmissions for recurrent pancreatitis, and pancreaticobiliary complications, without having a significant impact on mortality during a 6-month follow-up period.66 Delaying cholecystectomy 6 weeks in patients with moderate-severe gallstone AP appears to reduce morbidity, including the development of infected collections, and mortality.4 An ongoing RCT, the APEC trial, aims to determine whether early ERCP with biliary sphincterotomy reduces major complications or death when compared with no intervention for biliary AP in patients at high risk of complications.67
Chemoprevention and IV fluid management of post-ERCP pancreatitis
Accumulating data support the effectiveness of chemoprevention, pancreatic stent placement, and fluid administration to prevent post-ERCP pancreatitis. Multiple RCTs, meta-analyses, and systematic reviews indicate that rectal NSAIDs) reduce post-ERCP pancreatitis onset68-71 and moderate-severe post-ERCP pancreatitis. Additionally, placement of a pancreatic duct stent may decrease the risk of severe post-ERCP pancreatitis in high-risk patients.3 Guidelines do not comment on fluid administrations for prevention of post-ERCP pancreatitis, but studies have shown that greater periprocedural IV fluid was an independent protective factor against moderate to severe PEP72 and was associated with shorter hospital length of stay.73 Recent meta-analyses and RCTs support using LR prior to ERCP to prevent PEP.74-77 Interestingly, a recent RCT shows that the combination of rectal indomethacin and LR, compared with combination placebo and normal saline reduced the risk of PEP in high-risk patients.78
Two ongoing multicenter RCTs will clarify the role of combination therapy. The Dutch FLUYT RCT aims to determine the optimal combination of rectal NSAIDs and periprocedural infusion of IV fluids to reduce the incidence of PEP and moderate-severe PEP79 and the Stent vs. Indomethacin (SVI) trial aims to determine the whether combination pancreatic stent placement plus rectal indomethacin is superior to monotherapy indomethacin for preventing post-ERCP pancreatitis in high-risk cases.80
Implications for clinical practice
The diagnosis and optimal management of AP require a systematic approach with multidisciplinary decision making. Morbidity and mortality in AP are driven by early or late POF, and the latter often is triggered by infected necrosis. Risk stratification of these patients at the point of contact is a commonsense approach to enable triaging of patients to the appropriate level of care. Regardless of pancreatitis severity, recommended treatment interventions include goal-directed IV fluid resuscitation, early feeding by mouth or enteral tube when necessary, avoidance of prophylactic antibiotics, avoidance of probiotics, and urgent ERCP for patients with acute biliary pancreatitis complicated by cholangitis. Key measures for preventing hospital readmission and pancreatitis include same-admission cholecystectomy for acute biliary pancreatitis and alcohol and smoking cessation. Preventive measures for post-ERCP pancreatitis in patients undergoing ERCP include rectal indomethacin, prophylactic pancreatic duct stent placement, and periprocedural fluid resuscitation.
Dr. Mandalia is a fellow, gastroenterology, department of internal medicine, division of gastroenterology, Michigan Medicine, Ann Arbor; Dr. DiMagno is associate professor of medicine, director, comprehensive pancreas program, department of internal medicine, division of gastroenterology, University of Michigan, Ann Arbor. Dr. Mandalia reports no conflicts of interest.
References
1. Tenner S et al. Am J Gastroenterol. 2013;108:1400.
2. Besseline M et al. Pancreatology. 2013;13(4, Supplement 2):e1-15.
3. Crockett SD et al. Gastroenterology. 2018;154(4):1096-101.
4. Vege SS et al. Gastroenterology. 2018;154(4):1103-39.
5. Peery AF et al. Gastroenterology. 2019 Jan;156(1):254-72.e11.
6. Krishna SG et al. Pancreas. 2017;46(4):482-8.
7. Sellers ZM et al. Gastroenterology. 2018;155(2):469-78.e1.
8. Brown A et al. JOP. 2008;9(4):408-14.
9. Fagenholz PJ et al. Ann Epidemiol. 2007;17(7):491.e1-.e8.
10. McNabb-Baltar J et al. Pancreas. 2014;43(5):687-91.
11. Johnson CD et al. Gut. 2004;53(9):1340-4.
12. Dellinger EP et al. Ann Surg. 2012;256(6):875-80.
13. Petrov MS et al. Gastroenterology. 2010;139(3):813-20.
14. Sternby H et al. Ann Surg. Apr 18. doi: 10.1097/SLA.0000000000002766.
15. Huh JH et al. J Clin Gastroenterol. 2018;52(2):178-83.
16. Wu BU et al. Gastroenterology. 2008;135(3):816-20.
17. Gardner TB et al. Clin Gastroenterol Hepatol. 2008;6(10):1070-6.
18. Krishna SG et al. Am J Gastroenterol. 2015;110(11):1608-19.
19. Lee PJ et al. Pancreas. 2016;45(4):561-4.
20. Mandalia A et al. F1000Research. 2018 Jun 28;7.
21. Majumder S et al. Pancreas. 2015;44(4):540-6.
22. DiMagno MJ. Clin Gastroenterol Hepatol. 2011;9(11):920-2.
23. Yadav D, Whitcomb DC. Nature Rev Gastroenterol Hepatol. 2010;7(3):131-45.
24. Samokhvalov AV et al. EBioMedicine. 2015;2(12):1996-2002.
25. Barkin JA et al. Pancreas. 2017;46(8):1035-8.
26. Chen Y-T et al. J Gastroenterol Hepatol. 2016;31(4):782-7.
27. Ramos LR et al. J Crohns Colitis. 2016;10(1):95-104.
28. Avram MM. Nephron. 1977;18(1):68-71.
29. Lankisch PG et al. Nephrol Dial Transplant. 2008;23(4):1401-5.
30. Owyang C et al. Mayo Clin Proc. 1979;54(12):769-73.
31. Owyang Cet al. Gut. 1982;23(5):357-61.
32. Quraishi ER et al. Am J Gastroenterol. 2005;100:2288.
33. Vaziri ND et al. Nephron. 1987;46(4):347-9.
34. Chen HJ et al. Nephrol Dial Transplant. 2017;32(10):1731-6.
35. Kirkegard J et al. Gastroenterology. 2018;May;154(6):1729-36.
36. Karlson BM, et al. Gastroenterology. 1997;113(2):587-92.
37. Munigala S et al. Clin Gastroenterol Hepatol. 2014;12(7):1143-50.e1.
38. Carr RA et al. Pancreatology. 2016;16(4):469-76.
39. Li X et al. BMC Gastroenterol. 2018;18(1):89.
40. Ahmed AU et al. Clin Gastroenterol Hepatol. 2016;14(5):738-46.
41. Sankaran SJ et al. Gastroenterology. 2015;149(6):1490-500.e1.
42. Berglund L et al. J Clin Endocrinol Metab. 2012;97(9):2969-89.
43. Catapano AL et al. Atherosclerosis. 2011;217(1):3-46.
44. Pedersen SB et al. JAMA Intern Med. 2016;176(12):1834-42.
45. Nawaz H et al. Am J Gastroenterol. 2015;110(10):1497-503.
46. Banks PA et al. Gut. 2013;62(1):102-11.
47. Kondo S et al. Eur J Radiol. 2005;54(2):271-5.
48. Meeralam Y et al. Gastrointest Endosc. 2017;86(6):986-93.
49. Stimac D et al. Am J Gastroenterol. 2007;102(5):997-1004.
50. Jin DX et al. Dig Dis Sci. 2017;62(10):2894-9.
51. Freeman ML. Gastrointest Endosc Clin N Am. 2012;22(3):567-86.
52. De Lisi S et al. Eur J Gastroenterol Hepatol. 2011;23(5):367-74.
53. Di MY et al. Ann Int Med. 2016;165(7):482-90.
54. Mounzer R et al. Gastroenterology. 2012;142(7):1476-82; quiz e15-6.
55. Koutroumpakis E et al. Am J Gastroenterol. 2015;110(12):1707-16.
56. Wu BU et al. Gastroenterology. 2009;137(1):129-35.
57. Buddingh KT et al. J Am Coll Surg. 2014;218(1):26-32.
58. Buxbaum J et al. Am J Gastroenterol. 2018;113(5):755-64.
59. Jabaudon M et al. Crit Car Med. 2018;46(3):e198-e205.
60. Barlass U et al. Gut. 2018;67(4):600-2.
61. Buxbaum JL et al. Am J Gastroenterol. 2017;112(5):797-803.
62. de-Madaria E et al. United Eur Gastroenterol J. 2018;6(1):63-72.
63. Bakker OJ et al. N Engl J Med. 2014;371(21):1983-93.
64. Tse F et al. Cochrane Database Syst Rev. 2012(5):Cd009779.
65. Kaner EFS et al. Cochrane Database Syst Rev. 2007(2):Cd004148.
66. da Costa DW et al. Lancet. 2015;386(10000):1261-8.
67. Schepers NJ et al. Trials. 2016;17:5.
68. Vadala di Prampero SF et al. Eur J Gastroenterol Hepatol. 2016;28(12):1415-24.
69. Kubiliun NM et al. Clin Gastroenterol Hepatol. 2015;13(7):1231-9; quiz e70-1.
70. Wan J et al. BMC Gastroenterol. 2017;17(1):43.
71. Yang C et al. Pancreatology. 2017;17(5):681-8.
72. DiMagno MJ et al. Pancreas. 2014;43(4):642-7.
73. Sagi SV et al. J Gastroenterol Hepatol. 2014;29(6):1316-20.
74. Choi JH et al. Clin Gastroenterol Hepatol. 2017;15(1):86-92.e1.
75. Wu D et al. J Clin Gastroenterol. 2017;51(8):e68-e76.
76. Zhang ZF et al. J Clin Gastroenterol. 2017;51(3):e17-e26.
77. Park CH et al. Endoscopy 2018 Apr;50(4):378-85.
78. Mok SRS et al. Gastrointest Endosc. 2017;85(5):1005-13.
79. Smeets XJN et al. Trials. 2018;19(1):207.
80. Elmunzer BJ et al. Trials. 2016;17(1):120.
Introduction
Acute pancreatitis (AP) is a major clinical and financial burden in the United States. Several major clinical guidelines provide evidence-based recommendations for the clinical management decisions in AP, including those from the American College of Gastroenterology (ACG; 2013),1 and the International Association of Pancreatology (IAP; 2013).2 More recently, the American Gastroenterological Association (AGA) released their own set of guidelines.3,4 In this update on AP, we review these guidelines and reference recent literature focused on epidemiology, risk factors, etiology, diagnosis, risk stratification, and recent advances in the early medical management of AP. Regarding the latter, we review six treatment interventions (pain management, intravenous fluid resuscitation, feeding, prophylactic antibiotics, probiotics, and timing of endoscopic retrograde cholangiopancreatography (ERCP) in acute biliary pancreatitis) and four preventive interventions (alcohol and smoking cessation, same-admission cholecystectomy for acute biliary pancreatitis, and chemoprevention and fluid administration for post-ERCP pancreatitis [PEP]). Updates on multidisciplinary management of (infected) pancreatic necrosis is beyond the scope of this review. Table 1 summarizes the concepts discussed in this article.

Recent advances in epidemiology and evaluation of AP
Epidemiology
AP is the third most common cause of gastrointestinal-related hospitalizations and fourth most common cause of readmission in 2014.5 Recent epidemiologic studies show conflicting trends for the incidence of AP, both increasing6 and decreasing,7 likely attributable to significant differences in study designs. Importantly, multiple studies have demonstrated that hospital length of stay, costs, and mortality have declined since 2009.6,8-10
Persistent organ failure (POF), defined as organ failure lasting more than 48 hours, is the major cause of death in AP. POF, if only a single organ during AP, is associated with 27%-36% mortality; if it is multiorgan, it is associated with 47% mortality.1,11 Other factors associated with increased hospital mortality include infected pancreatic necrosis,12-14 diabetes mellitus,15 hospital-acquired infection,16 advanced age (70 years and older),17 and obesity.18 Predictive factors of 1-year mortality include readmission within 30 days, higher Charlson Comorbidity Index, and longer hospitalization.19
Risk factors
We briefly highlight recent insights into risk factors for AP (Table 1) and refer to a recent review for further discussion.20 Current and former tobacco use are independent risk factors for AP.21 The dose-response relationship of alcohol to the risk of pancreatitis is complex,22 but five standard drinks per day for 5 years is a commonly used cut-off.1,23 New evidence suggests that the relationship between the dose of alcohol and risk of AP differs by sex, linearly in men but nonlinearly (J-shaped) in women.24 Risk of AP in women was decreased with alcohol consumption of up to 40 g/day (one standard drink contains 14 g of alcohol) and increased above this amount. Cannabis is a possible risk factor for toxin-induced AP and abstinence appears to abolish risk of recurrent attacks.25
Patients with inflammatory bowel disease (IBD) have a 2.9-fold higher risk for AP versus non-IBD cohorts26 with the most common etiologies are from gallstones and medications.27 In patients with end-stage renal disease (ESRD), the risk of AP is higher in those who receive peritoneal dialysis, compared with hemodialysis28-33 and who are women, older, or have cholelithiasis or liver disease.34As recently reviewed,35 pancreatic cancer appears to be associated with first-attack pancreatitis with few exceptions.36 In this setting, the overall incidence of pancreatic cancer is low (1.5%). The risk is greatest within the first year of the attack of AP, negligible below age 40 years but steadily rising through the fifth to eighth decades.37 Pancreatic cancer screening is a conditional recommendation of the ACG guidelines in patients with unexplained AP, particularly those aged 40 years or older.1
Etiology and diagnosis

Alcohol and gallstones remain the most prevalent etiologies for AP.1 While hypertriglyceridemia accounted for 9% of AP in a systematic review of acute pancreatitis in 15 different countries,38 it is the second most common cause of acute pancreatitis in Asia (especially China).39 Figure 1 provides a breakdown of the etiologies and risk factors of pancreatitis. Importantly, it remains challenging to assign several toxic-metabolic etiologies as either a cause or risk factor for AP, particularly with regards to alcohol, smoking, and cannabis to name a few.
Guidelines and recent studies of AP raise questions about the threshold above which hypertriglyceridemia causes or poses as an important cofactor for AP. American and European societies define the threshold for triglycerides at 885-1,000 mg/dL.1,42,43 Pedersen et al. provide evidence of a graded risk of AP with hypertriglyceridemia: In multivariable analysis, adjusted hazard ratios for AP were much higher with nonfasting mild to moderately elevated plasma triglycerides (177-885 mg/dL), compared with normal values (below 89 mg/dL).44 Moreover, the risk of severe AP (developing POF) increases in proportion to triglyceride value, independent of the underlying cause of AP.45

Diagnosis of AP is derived from the revised Atlanta classification.46 The recommended timing and indications for offering cross-sectional imaging are after 48-72 hours in patients with no improvement to initial care.1 Endoscopic ultrasonography (EUS) has better diagnostic accuracy and sensitivity, compared with magnetic resonance cholangiopancreatography (MRCP) for choledocholithiasis, and has comparable specificity.47,48 Among noninvasive imaging modalities, MRCP is more sensitive than computed tomography (CT) for diagnosing choledocholithiasis.49 Despite guideline recommendations for more selective use of pancreatic imaging in the early assessment of AP, utilization of early CT or MRCP imaging (within the first 24 hours of care) remained high during 2014-2015, compared with 2006-2007.50
ERCP is not recommended as a pure diagnostic tool, owing to the availability of other diagnostic tests and a complication rate of 5%-10% with risks involving PEP, cholangitis, perforation, and hemorrhage.51 A recent systematic review of EUS and ERCP in acute biliary pancreatitis concluded that EUS had lower failure rates and had no complications, and the use of EUS avoided ERCP in 71.2% of cases.52
Risk stratification
The goals of using risk stratification tools in AP are to identify patients at risk for developing major outcomes, including POF, infected pancreatic necrosis, and death, and to ensure timely triaging of patients to an appropriate level of care. Existing prediction models have only moderate predictive value.53,54 Examples include simple risk stratification tools such as blood urea nitrogen (BUN) and hemoconcentration,55,56 disease-modifying patient variables (age, obesity, etc.), biomarkers (i.e., angiopoietin 2),57 and more complex clinical scoring systems such as Acute Physiology and Chronic Health Evaluation II (APACHE II), BISAP (BUN, impaired mental status, SIRS criteria, age, pleural effusion) score, early warning system (EWS), Glasgow-Imrie score, Japanese severity score, and recently the Pancreatitis Activity Scoring System (PASS).58 Two recent guidelines affirmed the importance of predicting the severity of AP, using one or more predictive tools.1,2 The recent 2018 AGA technical review does not debate this commonsense approach, but does highlight that there is no published observational study or randomized, controlled trial (RCT) investigating whether prediction tools affect clinical outcomes.4

Recent advances in early treatment of AP
Literature review and definitions
The AP literature contains heterogeneous definitions of severe AP and of what constitutes a major outcome in AP. Based on definitions of the 2013 revised Atlanta Criteria, the 2018 AGA technical review and clinical guidelines emphasized precise definitions of primary outcomes of clinical importance in AP, including death, persistent single organ failure, or persistent multiple organ failure, each requiring a duration of more than 48 hours, and infected pancreatic or peripancreatic necrosis or both (Table 2).3,4
Pain management
Management of pain in AP is complex and requires a detailed discussion beyond the scope of this review, but recent clinical and translational studies raise questions about the current practice of using opioids for pain management in AP. A provocative, multicenter, retrospective cohort study reported lower 30-day mortality among critically ill patients who received epidural analgesia versus standard care without epidural analgesia.59 The possible mechanism of protection and the drugs administered are unclear. An interesting hypothesis is that the epidural cohort may have received lower exposure to morphine, which may increase gut permeability, the risk of infectious complications, and severity of AP, based on a translational study in mice.60
Intravenous fluid administration

Supportive care with the use of IV fluid hydration is a mainstay of treatment for AP in the first 12-24 hours. Table 3 summarizes the guidelines in regards to IV fluid administration as delineated by the ACG and AGA guidelines on the management of pancreatitis.1,3 Guidelines advocate for early fluid resuscitation to correct intravascular depletion in order to reduce morbidity and mortality associated with AP.1,2,4 The 2018 AGA guidelines endorse a conditional recommendation for using goal-directed therapy for initial fluid management,3 do not recommend for or against normal saline versus lactated Ringer’s (LR), but do advise against the use of hydroxyethyl starch fluids.3 Consistent with these recommendations, two recent RCTs published subsequent to the prespecified time periods of the AGA technical review and guideline, observed no significant differences between LR and normal saline on clinically meaningful outcomes.61,62 The AGA guidelines acknowledge that evidence was of very-low quality in support of goal-directed therapy,3,4 which has not been shown to have a significant reduction in persistent multiple organ failure, mortality, or pancreatic necrosis, compared with usual care. As the authors noted, interpretation of the data was limited by the absence of other critical outcomes in these trials (infected pancreatic necrosis), lack of uniformity of specific outcomes and definitions of transient and POF, few trials, and risk of bias. There is a clear need for a large RCT to provide evidence to guide decision making with fluid resuscitation in AP, particularly in regard to fluid type, volume, rate, duration, endpoints, and clinical outcomes.
Feeding
More recently, the focus of nutrition in the management of AP has shifted away from patients remaining nil per os (NPO). Current guidelines advocate for early oral feeding (within 24 hours) in mild AP,3,4 in order to protect the gut-mucosal barrier. Remaining NPO when compared with early oral feeding has a 2.5-fold higher risk for interventions for necrosis.4 The recently published AGA technical review identified no significant impact on outcomes of early versus delayed oral feeding, which is consistent with observations of a landmark Dutch PYTHON trial entitled “Early versus on-demand nasoenteric tube feeding in acute pancreatitis.”4,63 There is no clear cutoff point for initiating feeding for those with severe AP. A suggested practical approach is to initiate feeding within 24-72 hours and offer enteral nutrition for those intolerant to oral feeds. In severe AP and moderately severe AP, enteral nutrition is recommended over parenteral nutrition.3,4 Enteral nutrition significantly reduces the risk of infected peripancreatic necrosis, single organ failure, and multiorgan failure.4 Finally, the AGA guidelines provide a conditional recommendation for providing enteral nutrition support through either the nasogastric or nasoenteric route.3 Further studies are required to determine the optimal timing, rate, and formulation of enteral nutrition in severe AP.
Antibiotics and probiotics
Current guidelines do not support the use of prophylactic antibiotics to prevent infection in necrotizing AP and severe AP.1-3 The AGA technical review reported that prophylactic antibiotics did not reduce infected pancreatic or peripancreatic necrosis, persistent single organ failure, or mortality.4 Guidelines advocate against the use of probiotics for severe AP, because of increased mortality risk.1
Timing of ERCP in acute biliary pancreatitis
There is universal agreement for offering urgent ERCP (within 24 hours) in biliary AP complicated by cholangitis.1-3,64 Figure 2 demonstrates an example of a cholangiogram completed within 24 hours of presentation of biliary AP complicated by cholangitis.
In the absence of cholangitis, the timing of ERCP for AP with persistent biliary obstruction is less clear.1-3 In line with recent guidelines, the 2018 AGA guidelines advocate against routine use of urgent ERCP for biliary AP without cholangitis,3 a conditional recommendation with overall low quality of data.4 The AGA technical review found that urgent ERCP, compared with conservative management in acute biliary pancreatitis without cholangitis had no significant effect on mortality, organ failure, infected pancreatic necrosis, and total necrotizing pancreatitis, but did significantly shorten hospital length of stay.4 There are limited data to guide decision making of when nonurgent ERCP should be performed in hospitalized patients with biliary AP with persistent obstruction and no cholangitis.3,64
Alcohol and smoking cessation
The AGA technical review advocates for brief alcohol intervention during hospitalization for alcohol-induced AP on the basis of one RCT that addresses the impact of alcohol counseling on recurrent bouts of AP4 plus evidence from a Cochrane review of alcohol-reduction strategies in primary care populations.65 Cessation of smoking – an established independent risk factor of AP – recurrent AP and chronic pancreatitis, should also be recommended as part of the management of AP.
Cholecystectomy
Evidence supports same-admission cholecystectomy for mild gallstone AP, a strong recommendation of published AGA guidelines.3 When compared with delayed cholecystectomy, same-admission cholecystectomy significantly reduced gallstone-related complications, readmissions for recurrent pancreatitis, and pancreaticobiliary complications, without having a significant impact on mortality during a 6-month follow-up period.66 Delaying cholecystectomy 6 weeks in patients with moderate-severe gallstone AP appears to reduce morbidity, including the development of infected collections, and mortality.4 An ongoing RCT, the APEC trial, aims to determine whether early ERCP with biliary sphincterotomy reduces major complications or death when compared with no intervention for biliary AP in patients at high risk of complications.67
Chemoprevention and IV fluid management of post-ERCP pancreatitis
Accumulating data support the effectiveness of chemoprevention, pancreatic stent placement, and fluid administration to prevent post-ERCP pancreatitis. Multiple RCTs, meta-analyses, and systematic reviews indicate that rectal NSAIDs) reduce post-ERCP pancreatitis onset68-71 and moderate-severe post-ERCP pancreatitis. Additionally, placement of a pancreatic duct stent may decrease the risk of severe post-ERCP pancreatitis in high-risk patients.3 Guidelines do not comment on fluid administrations for prevention of post-ERCP pancreatitis, but studies have shown that greater periprocedural IV fluid was an independent protective factor against moderate to severe PEP72 and was associated with shorter hospital length of stay.73 Recent meta-analyses and RCTs support using LR prior to ERCP to prevent PEP.74-77 Interestingly, a recent RCT shows that the combination of rectal indomethacin and LR, compared with combination placebo and normal saline reduced the risk of PEP in high-risk patients.78
Two ongoing multicenter RCTs will clarify the role of combination therapy. The Dutch FLUYT RCT aims to determine the optimal combination of rectal NSAIDs and periprocedural infusion of IV fluids to reduce the incidence of PEP and moderate-severe PEP79 and the Stent vs. Indomethacin (SVI) trial aims to determine the whether combination pancreatic stent placement plus rectal indomethacin is superior to monotherapy indomethacin for preventing post-ERCP pancreatitis in high-risk cases.80
Implications for clinical practice
The diagnosis and optimal management of AP require a systematic approach with multidisciplinary decision making. Morbidity and mortality in AP are driven by early or late POF, and the latter often is triggered by infected necrosis. Risk stratification of these patients at the point of contact is a commonsense approach to enable triaging of patients to the appropriate level of care. Regardless of pancreatitis severity, recommended treatment interventions include goal-directed IV fluid resuscitation, early feeding by mouth or enteral tube when necessary, avoidance of prophylactic antibiotics, avoidance of probiotics, and urgent ERCP for patients with acute biliary pancreatitis complicated by cholangitis. Key measures for preventing hospital readmission and pancreatitis include same-admission cholecystectomy for acute biliary pancreatitis and alcohol and smoking cessation. Preventive measures for post-ERCP pancreatitis in patients undergoing ERCP include rectal indomethacin, prophylactic pancreatic duct stent placement, and periprocedural fluid resuscitation.
Dr. Mandalia is a fellow, gastroenterology, department of internal medicine, division of gastroenterology, Michigan Medicine, Ann Arbor; Dr. DiMagno is associate professor of medicine, director, comprehensive pancreas program, department of internal medicine, division of gastroenterology, University of Michigan, Ann Arbor. Dr. Mandalia reports no conflicts of interest.
References
1. Tenner S et al. Am J Gastroenterol. 2013;108:1400.
2. Besseline M et al. Pancreatology. 2013;13(4, Supplement 2):e1-15.
3. Crockett SD et al. Gastroenterology. 2018;154(4):1096-101.
4. Vege SS et al. Gastroenterology. 2018;154(4):1103-39.
5. Peery AF et al. Gastroenterology. 2019 Jan;156(1):254-72.e11.
6. Krishna SG et al. Pancreas. 2017;46(4):482-8.
7. Sellers ZM et al. Gastroenterology. 2018;155(2):469-78.e1.
8. Brown A et al. JOP. 2008;9(4):408-14.
9. Fagenholz PJ et al. Ann Epidemiol. 2007;17(7):491.e1-.e8.
10. McNabb-Baltar J et al. Pancreas. 2014;43(5):687-91.
11. Johnson CD et al. Gut. 2004;53(9):1340-4.
12. Dellinger EP et al. Ann Surg. 2012;256(6):875-80.
13. Petrov MS et al. Gastroenterology. 2010;139(3):813-20.
14. Sternby H et al. Ann Surg. Apr 18. doi: 10.1097/SLA.0000000000002766.
15. Huh JH et al. J Clin Gastroenterol. 2018;52(2):178-83.
16. Wu BU et al. Gastroenterology. 2008;135(3):816-20.
17. Gardner TB et al. Clin Gastroenterol Hepatol. 2008;6(10):1070-6.
18. Krishna SG et al. Am J Gastroenterol. 2015;110(11):1608-19.
19. Lee PJ et al. Pancreas. 2016;45(4):561-4.
20. Mandalia A et al. F1000Research. 2018 Jun 28;7.
21. Majumder S et al. Pancreas. 2015;44(4):540-6.
22. DiMagno MJ. Clin Gastroenterol Hepatol. 2011;9(11):920-2.
23. Yadav D, Whitcomb DC. Nature Rev Gastroenterol Hepatol. 2010;7(3):131-45.
24. Samokhvalov AV et al. EBioMedicine. 2015;2(12):1996-2002.
25. Barkin JA et al. Pancreas. 2017;46(8):1035-8.
26. Chen Y-T et al. J Gastroenterol Hepatol. 2016;31(4):782-7.
27. Ramos LR et al. J Crohns Colitis. 2016;10(1):95-104.
28. Avram MM. Nephron. 1977;18(1):68-71.
29. Lankisch PG et al. Nephrol Dial Transplant. 2008;23(4):1401-5.
30. Owyang C et al. Mayo Clin Proc. 1979;54(12):769-73.
31. Owyang Cet al. Gut. 1982;23(5):357-61.
32. Quraishi ER et al. Am J Gastroenterol. 2005;100:2288.
33. Vaziri ND et al. Nephron. 1987;46(4):347-9.
34. Chen HJ et al. Nephrol Dial Transplant. 2017;32(10):1731-6.
35. Kirkegard J et al. Gastroenterology. 2018;May;154(6):1729-36.
36. Karlson BM, et al. Gastroenterology. 1997;113(2):587-92.
37. Munigala S et al. Clin Gastroenterol Hepatol. 2014;12(7):1143-50.e1.
38. Carr RA et al. Pancreatology. 2016;16(4):469-76.
39. Li X et al. BMC Gastroenterol. 2018;18(1):89.
40. Ahmed AU et al. Clin Gastroenterol Hepatol. 2016;14(5):738-46.
41. Sankaran SJ et al. Gastroenterology. 2015;149(6):1490-500.e1.
42. Berglund L et al. J Clin Endocrinol Metab. 2012;97(9):2969-89.
43. Catapano AL et al. Atherosclerosis. 2011;217(1):3-46.
44. Pedersen SB et al. JAMA Intern Med. 2016;176(12):1834-42.
45. Nawaz H et al. Am J Gastroenterol. 2015;110(10):1497-503.
46. Banks PA et al. Gut. 2013;62(1):102-11.
47. Kondo S et al. Eur J Radiol. 2005;54(2):271-5.
48. Meeralam Y et al. Gastrointest Endosc. 2017;86(6):986-93.
49. Stimac D et al. Am J Gastroenterol. 2007;102(5):997-1004.
50. Jin DX et al. Dig Dis Sci. 2017;62(10):2894-9.
51. Freeman ML. Gastrointest Endosc Clin N Am. 2012;22(3):567-86.
52. De Lisi S et al. Eur J Gastroenterol Hepatol. 2011;23(5):367-74.
53. Di MY et al. Ann Int Med. 2016;165(7):482-90.
54. Mounzer R et al. Gastroenterology. 2012;142(7):1476-82; quiz e15-6.
55. Koutroumpakis E et al. Am J Gastroenterol. 2015;110(12):1707-16.
56. Wu BU et al. Gastroenterology. 2009;137(1):129-35.
57. Buddingh KT et al. J Am Coll Surg. 2014;218(1):26-32.
58. Buxbaum J et al. Am J Gastroenterol. 2018;113(5):755-64.
59. Jabaudon M et al. Crit Car Med. 2018;46(3):e198-e205.
60. Barlass U et al. Gut. 2018;67(4):600-2.
61. Buxbaum JL et al. Am J Gastroenterol. 2017;112(5):797-803.
62. de-Madaria E et al. United Eur Gastroenterol J. 2018;6(1):63-72.
63. Bakker OJ et al. N Engl J Med. 2014;371(21):1983-93.
64. Tse F et al. Cochrane Database Syst Rev. 2012(5):Cd009779.
65. Kaner EFS et al. Cochrane Database Syst Rev. 2007(2):Cd004148.
66. da Costa DW et al. Lancet. 2015;386(10000):1261-8.
67. Schepers NJ et al. Trials. 2016;17:5.
68. Vadala di Prampero SF et al. Eur J Gastroenterol Hepatol. 2016;28(12):1415-24.
69. Kubiliun NM et al. Clin Gastroenterol Hepatol. 2015;13(7):1231-9; quiz e70-1.
70. Wan J et al. BMC Gastroenterol. 2017;17(1):43.
71. Yang C et al. Pancreatology. 2017;17(5):681-8.
72. DiMagno MJ et al. Pancreas. 2014;43(4):642-7.
73. Sagi SV et al. J Gastroenterol Hepatol. 2014;29(6):1316-20.
74. Choi JH et al. Clin Gastroenterol Hepatol. 2017;15(1):86-92.e1.
75. Wu D et al. J Clin Gastroenterol. 2017;51(8):e68-e76.
76. Zhang ZF et al. J Clin Gastroenterol. 2017;51(3):e17-e26.
77. Park CH et al. Endoscopy 2018 Apr;50(4):378-85.
78. Mok SRS et al. Gastrointest Endosc. 2017;85(5):1005-13.
79. Smeets XJN et al. Trials. 2018;19(1):207.
80. Elmunzer BJ et al. Trials. 2016;17(1):120.
Four distinct IgG4-related disease groups described in study
IgG4-related disease can be grouped into four distinct clusters based on the distribution of organs involved, according to researchers who analyzed a large, multicenter cohort of patients with this heterogeneous, autoimmune-mediated condition.
The four groups also varied by age, race, sex, time to diagnosis, and concentration of serum IgG4, according to the investigators, led by Zachary S. Wallace, MD, of the division of rheumatology, allergy, and immunology at Massachusetts General Hospital and Harvard Medical School, both in Boston.
“These phenotypes may be used by clinicians to improve recognition of IgG4-related disease,” Dr. Wallace and his coauthors wrote in a report on the study that appears in the Annals of the Rheumatic Diseases.
First described in a Japanese population, IgG4-related disease has been subsequently seen in all racial and ethnic groups, according to the researchers. It is associated with organ failure and can affect nearly any organ or anatomic site, most notably the lungs, kidneys, lymph nodes, salivary glands, pancreatobiliary structures, and retroperitoneum.
In the present study, Dr. Wallace and his coinvestigators used a novel cluster analysis method, called latent class analysis, to categorize 765 cases of IgG4-related disease submitted by 52 investigators from 17 countries. The investigators included 493 of those cases in a primary study population, and the remaining 272 in a smaller cohort used to replicate the results.
In the larger, primary study cohort, about 65% of cases were male, 58% were non-Asian and 40% were white, and the mean age at diagnosis was 59.5 years. The replication cohort had similar characteristics, according to the investigators.
The clustering analysis revealed four distinct subgroups, characterized by pancreato-hepatobiliary, accounting for 31% of cases; retroperitoneal fibrosis and/or aortitis in 24%; disease generally limited to head and neck structures in 24%, and head and neck disease consistent with Mikulicz syndrome plus systemic involvement in 22%.
The highest IgG4 concentrations were seen in the group of patients with Mikulicz syndrome and systemic involvement, according to Dr. Wallace and his coauthors. The serum concentration was 1,170 mg/dL in that group, compared with 445 mg/dL in the group of patients with head and neck-limited disease, 316 mg/dL in the pancreato-hepatobiliary group, and just 178 mg/dL in the retroperitoneal fibrosis/aorta group.
Female and Asian patients were overrepresented in the group characterized by head and neck involvement, investigators also found. Moreover, that group had a significantly lower mean age at diagnosis than did the other groups.
Those variations suggested differences in genetic or environmental risk factors between clusters, according to the investigators.
“Given the similar distribution of subspecialists among investigators in this study practicing in Asian and non-Asian countries, the observed differences are unlikely to be the result of detection or selection biases,” they said in their report.
The findings of this study help to inform subsequent investigations intended to evaluate those factors in more detail, they said.
Dr. Wallace and his coauthors reported no conflicts of interest related to their work, which was previously presented at the American College of Rheumatology annual meeting.
SOURCE: Wallace ZS et al. Ann Rheum Dis. 2019 Jan 5. doi: 10.1136/annrheumdis-2018-214603
IgG4-related disease can be grouped into four distinct clusters based on the distribution of organs involved, according to researchers who analyzed a large, multicenter cohort of patients with this heterogeneous, autoimmune-mediated condition.
The four groups also varied by age, race, sex, time to diagnosis, and concentration of serum IgG4, according to the investigators, led by Zachary S. Wallace, MD, of the division of rheumatology, allergy, and immunology at Massachusetts General Hospital and Harvard Medical School, both in Boston.
“These phenotypes may be used by clinicians to improve recognition of IgG4-related disease,” Dr. Wallace and his coauthors wrote in a report on the study that appears in the Annals of the Rheumatic Diseases.
First described in a Japanese population, IgG4-related disease has been subsequently seen in all racial and ethnic groups, according to the researchers. It is associated with organ failure and can affect nearly any organ or anatomic site, most notably the lungs, kidneys, lymph nodes, salivary glands, pancreatobiliary structures, and retroperitoneum.
In the present study, Dr. Wallace and his coinvestigators used a novel cluster analysis method, called latent class analysis, to categorize 765 cases of IgG4-related disease submitted by 52 investigators from 17 countries. The investigators included 493 of those cases in a primary study population, and the remaining 272 in a smaller cohort used to replicate the results.
In the larger, primary study cohort, about 65% of cases were male, 58% were non-Asian and 40% were white, and the mean age at diagnosis was 59.5 years. The replication cohort had similar characteristics, according to the investigators.
The clustering analysis revealed four distinct subgroups, characterized by pancreato-hepatobiliary, accounting for 31% of cases; retroperitoneal fibrosis and/or aortitis in 24%; disease generally limited to head and neck structures in 24%, and head and neck disease consistent with Mikulicz syndrome plus systemic involvement in 22%.
The highest IgG4 concentrations were seen in the group of patients with Mikulicz syndrome and systemic involvement, according to Dr. Wallace and his coauthors. The serum concentration was 1,170 mg/dL in that group, compared with 445 mg/dL in the group of patients with head and neck-limited disease, 316 mg/dL in the pancreato-hepatobiliary group, and just 178 mg/dL in the retroperitoneal fibrosis/aorta group.
Female and Asian patients were overrepresented in the group characterized by head and neck involvement, investigators also found. Moreover, that group had a significantly lower mean age at diagnosis than did the other groups.
Those variations suggested differences in genetic or environmental risk factors between clusters, according to the investigators.
“Given the similar distribution of subspecialists among investigators in this study practicing in Asian and non-Asian countries, the observed differences are unlikely to be the result of detection or selection biases,” they said in their report.
The findings of this study help to inform subsequent investigations intended to evaluate those factors in more detail, they said.
Dr. Wallace and his coauthors reported no conflicts of interest related to their work, which was previously presented at the American College of Rheumatology annual meeting.
SOURCE: Wallace ZS et al. Ann Rheum Dis. 2019 Jan 5. doi: 10.1136/annrheumdis-2018-214603
IgG4-related disease can be grouped into four distinct clusters based on the distribution of organs involved, according to researchers who analyzed a large, multicenter cohort of patients with this heterogeneous, autoimmune-mediated condition.
The four groups also varied by age, race, sex, time to diagnosis, and concentration of serum IgG4, according to the investigators, led by Zachary S. Wallace, MD, of the division of rheumatology, allergy, and immunology at Massachusetts General Hospital and Harvard Medical School, both in Boston.
“These phenotypes may be used by clinicians to improve recognition of IgG4-related disease,” Dr. Wallace and his coauthors wrote in a report on the study that appears in the Annals of the Rheumatic Diseases.
First described in a Japanese population, IgG4-related disease has been subsequently seen in all racial and ethnic groups, according to the researchers. It is associated with organ failure and can affect nearly any organ or anatomic site, most notably the lungs, kidneys, lymph nodes, salivary glands, pancreatobiliary structures, and retroperitoneum.
In the present study, Dr. Wallace and his coinvestigators used a novel cluster analysis method, called latent class analysis, to categorize 765 cases of IgG4-related disease submitted by 52 investigators from 17 countries. The investigators included 493 of those cases in a primary study population, and the remaining 272 in a smaller cohort used to replicate the results.
In the larger, primary study cohort, about 65% of cases were male, 58% were non-Asian and 40% were white, and the mean age at diagnosis was 59.5 years. The replication cohort had similar characteristics, according to the investigators.
The clustering analysis revealed four distinct subgroups, characterized by pancreato-hepatobiliary, accounting for 31% of cases; retroperitoneal fibrosis and/or aortitis in 24%; disease generally limited to head and neck structures in 24%, and head and neck disease consistent with Mikulicz syndrome plus systemic involvement in 22%.
The highest IgG4 concentrations were seen in the group of patients with Mikulicz syndrome and systemic involvement, according to Dr. Wallace and his coauthors. The serum concentration was 1,170 mg/dL in that group, compared with 445 mg/dL in the group of patients with head and neck-limited disease, 316 mg/dL in the pancreato-hepatobiliary group, and just 178 mg/dL in the retroperitoneal fibrosis/aorta group.
Female and Asian patients were overrepresented in the group characterized by head and neck involvement, investigators also found. Moreover, that group had a significantly lower mean age at diagnosis than did the other groups.
Those variations suggested differences in genetic or environmental risk factors between clusters, according to the investigators.
“Given the similar distribution of subspecialists among investigators in this study practicing in Asian and non-Asian countries, the observed differences are unlikely to be the result of detection or selection biases,” they said in their report.
The findings of this study help to inform subsequent investigations intended to evaluate those factors in more detail, they said.
Dr. Wallace and his coauthors reported no conflicts of interest related to their work, which was previously presented at the American College of Rheumatology annual meeting.
SOURCE: Wallace ZS et al. Ann Rheum Dis. 2019 Jan 5. doi: 10.1136/annrheumdis-2018-214603
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point:
Major finding: The highest IgG4 concentrations (1,170 mg/dL) were seen in a group of patients with Mikulicz syndrome and systemic involvement. Females and Asian patients were overrepresented in a group characterized by head and neck involvement.
Study details: Two cross-sectional studies including a total of 765 cases of IgG4-related disease submitted by 52 investigators in 17 countries.
Disclosures: Authors reported no conflicts of interest.
Source: Wallace ZS et al. Ann Rheum Dis. 2019 Jan 5. doi: 10.1136/annrheumdis-2018-214603.
Acute biliary pancreatitis linked to poor outcomes in elderly
Mortality was almost three times as high in elderly patients after stringent matching for confounding variables, wrote researcher Kishan Patel, MD, of the Ohio State University, Columbus, and coauthors.
These findings represent a “current health care concern,” since the elderly population in the United States is expected to double within the next several decades and the prevalence of acute pancreatitis is on the rise, Dr. Patel and colleagues wrote in a report on the analysis in the Journal of Clinical Gastroenterology.
The analysis is the first, to the investigators’ knowledge, that addresses national-level outcomes associated with acute biliary pancreatitis in elderly patients.
To evaluate clinical outcomes of elderly patients with acute biliary pancreatitis, Dr. Patel and colleagues queried the Nationwide Readmissions Database, which is the largest inpatient readmission database in the United States.
The investigators looked at outcomes associated with index hospitalizations, defined as a patient’s first hospitalization in a calendar year, and found 184,763 adult patients who received a diagnosis of acute biliary pancreatitis between 2011 and 2014. Of those, 41% were elderly.
The mortality rate associated with the index admission was 1.96% (n = 356) for the elderly patients, compared with just 0.32% (n = 1,473) for nonelderly patients (P less than .001), according to the report.
Mortality was increased in the elderly versus nonelderly patients, with an odds ratio of 2.8 (95% CI, 2.2-3.5), according to results of a propensity score matched analysis. Likewise, severe acute pancreatitis was increased in the elderly, with an OR of 1.2 (95% CI: 1.1-1.3) in that analysis.
By contrast, patient age did not impact 30-day readmission rates, according to results of a multivariate analysis that adjusted for confounding factors.
Mortality and severe acute pancreatitis both increased with age within the elderly cohort, further multivariate analysis showed. For example, the ORs for mortality were 1.39 for patients aged 75-84 years and 2.21 for patients aged 85 years and older, the results show.
The elderly population in the United States is expected to almost double by 2050, rising from 48 to 88 million, Dr. Patel and colleagues said. The number of those aged 85 years or older is expected to increase from 5.9 to 18 million by 2050, at which time they will make up nearly 5% of the total U.S. population.
“This specific demographic is more susceptible to common medical ailments, more troubling is acute pancreatitis is one of the most frequent causes of hospitalization in gastroenterology,” Dr. Patel and colleagues wrote.
Dr. Patel and coauthors reported no conflicts of interest related to the analysis.
SOURCE: Patel K et al. J Clin Gastroenterol. 2018 Aug 28. doi: 10.1097/MCG.0000000000001108.
Mortality was almost three times as high in elderly patients after stringent matching for confounding variables, wrote researcher Kishan Patel, MD, of the Ohio State University, Columbus, and coauthors.
These findings represent a “current health care concern,” since the elderly population in the United States is expected to double within the next several decades and the prevalence of acute pancreatitis is on the rise, Dr. Patel and colleagues wrote in a report on the analysis in the Journal of Clinical Gastroenterology.
The analysis is the first, to the investigators’ knowledge, that addresses national-level outcomes associated with acute biliary pancreatitis in elderly patients.
To evaluate clinical outcomes of elderly patients with acute biliary pancreatitis, Dr. Patel and colleagues queried the Nationwide Readmissions Database, which is the largest inpatient readmission database in the United States.
The investigators looked at outcomes associated with index hospitalizations, defined as a patient’s first hospitalization in a calendar year, and found 184,763 adult patients who received a diagnosis of acute biliary pancreatitis between 2011 and 2014. Of those, 41% were elderly.
The mortality rate associated with the index admission was 1.96% (n = 356) for the elderly patients, compared with just 0.32% (n = 1,473) for nonelderly patients (P less than .001), according to the report.
Mortality was increased in the elderly versus nonelderly patients, with an odds ratio of 2.8 (95% CI, 2.2-3.5), according to results of a propensity score matched analysis. Likewise, severe acute pancreatitis was increased in the elderly, with an OR of 1.2 (95% CI: 1.1-1.3) in that analysis.
By contrast, patient age did not impact 30-day readmission rates, according to results of a multivariate analysis that adjusted for confounding factors.
Mortality and severe acute pancreatitis both increased with age within the elderly cohort, further multivariate analysis showed. For example, the ORs for mortality were 1.39 for patients aged 75-84 years and 2.21 for patients aged 85 years and older, the results show.
The elderly population in the United States is expected to almost double by 2050, rising from 48 to 88 million, Dr. Patel and colleagues said. The number of those aged 85 years or older is expected to increase from 5.9 to 18 million by 2050, at which time they will make up nearly 5% of the total U.S. population.
“This specific demographic is more susceptible to common medical ailments, more troubling is acute pancreatitis is one of the most frequent causes of hospitalization in gastroenterology,” Dr. Patel and colleagues wrote.
Dr. Patel and coauthors reported no conflicts of interest related to the analysis.
SOURCE: Patel K et al. J Clin Gastroenterol. 2018 Aug 28. doi: 10.1097/MCG.0000000000001108.
Mortality was almost three times as high in elderly patients after stringent matching for confounding variables, wrote researcher Kishan Patel, MD, of the Ohio State University, Columbus, and coauthors.
These findings represent a “current health care concern,” since the elderly population in the United States is expected to double within the next several decades and the prevalence of acute pancreatitis is on the rise, Dr. Patel and colleagues wrote in a report on the analysis in the Journal of Clinical Gastroenterology.
The analysis is the first, to the investigators’ knowledge, that addresses national-level outcomes associated with acute biliary pancreatitis in elderly patients.
To evaluate clinical outcomes of elderly patients with acute biliary pancreatitis, Dr. Patel and colleagues queried the Nationwide Readmissions Database, which is the largest inpatient readmission database in the United States.
The investigators looked at outcomes associated with index hospitalizations, defined as a patient’s first hospitalization in a calendar year, and found 184,763 adult patients who received a diagnosis of acute biliary pancreatitis between 2011 and 2014. Of those, 41% were elderly.
The mortality rate associated with the index admission was 1.96% (n = 356) for the elderly patients, compared with just 0.32% (n = 1,473) for nonelderly patients (P less than .001), according to the report.
Mortality was increased in the elderly versus nonelderly patients, with an odds ratio of 2.8 (95% CI, 2.2-3.5), according to results of a propensity score matched analysis. Likewise, severe acute pancreatitis was increased in the elderly, with an OR of 1.2 (95% CI: 1.1-1.3) in that analysis.
By contrast, patient age did not impact 30-day readmission rates, according to results of a multivariate analysis that adjusted for confounding factors.
Mortality and severe acute pancreatitis both increased with age within the elderly cohort, further multivariate analysis showed. For example, the ORs for mortality were 1.39 for patients aged 75-84 years and 2.21 for patients aged 85 years and older, the results show.
The elderly population in the United States is expected to almost double by 2050, rising from 48 to 88 million, Dr. Patel and colleagues said. The number of those aged 85 years or older is expected to increase from 5.9 to 18 million by 2050, at which time they will make up nearly 5% of the total U.S. population.
“This specific demographic is more susceptible to common medical ailments, more troubling is acute pancreatitis is one of the most frequent causes of hospitalization in gastroenterology,” Dr. Patel and colleagues wrote.
Dr. Patel and coauthors reported no conflicts of interest related to the analysis.
SOURCE: Patel K et al. J Clin Gastroenterol. 2018 Aug 28. doi: 10.1097/MCG.0000000000001108.
FROM THE JOURNAL OF CLINICAL GASTROENTEROLOGY
Key clinical point: Compared with younger patients, elderly patients admitted for acute biliary pancreatitis have increased rates of adverse outcomes.
Major finding: Elderly patients had increased mortality (odds ratio, 2.8; 95% confidence interval, 2.2-3.5) and severe acute pancreatitis (OR, 1.2; 95% CI: 1.1-1.3).
Study details: A propensity score matched analysis of a large, nationally representative database including nearly 185,000 adults with acute biliary pancreatitis.
Disclosures: The study authors reported no conflicts of interest related to the study.
Source: Patel K et al. J Clin Gastroenterol. 2018 Aug 28. doi: 10.1097/MCG.0000000000001108.
A postgraduate tour through the biliary tree, pancreas, and liver
For the pancreatobiliary session, Michelle Ann Anderson, MD, of the University of Michigan, Ann Arbor, reminded us about appropriate patient selection given the risk of pancreatitis after endoscopic retrograde cholangiopancreatography pancreatitis, also known as post-ERCP pancreatitis. Strategies to prevent post-ERCP pancreatitis include using pancreatic duct stents and using wire rather than contrast for cannulation. She recommended rectal indomethacin for all patients. Because of encouraging data, she recommended 2-3 L of lactated Ringer’s solution during the procedure and recovery.
Katie Morgan, MD, from the Medical University of South Carolina, Charleston, reviewed her group’s experience with 195 total pancreatectomies with islet autotransplants for chronic pancreatitis. Quality of life improved with major reductions in narcotic use, and 25% of patients were insulin free.
Bret Petersen, MD, of Mayo Clinic, Rochester, Minn., discussed multidrug resistant infection in ERCP endoscopes. He reminded us of the risk of lapses in endoscope reprocessing steps and the need for monitoring. He commented on recent Food and Drug Administration’s culture guidance and new technologies in development.
James Scheiman, MD, from the University of Virginia, Charlottesville, discussed pancreatic cysts. He reviewed the controversy between the more conservative American Gastroenterological Association guidelines and the more aggressive International Consensus guidelines. He advised considering patient preferences with a multidisciplinary approach.
For the liver session, Guadalupe García-Tsao, MD, of Yale University, New Haven, Conn., discussed the controversy regarding nonselective beta-blockers. She advised caution if refractory ascites are present because of risk for renal dysfunction, but she also highlighted the benefits including reduced first and recurrent variceal hemorrhage.
Rohit Loomba, MD, from the University of California at San Diego addressed fibrosis assessments in fatty liver. In his algorithm, patients with low Nonalcoholic Fatty Liver Disease Fibrosis Score or Fibrosis-4 scores would have continued observation, while patients with medium or high scores would undergo transient elastography or magnetic resonance elastography.
Patrick Northup, MD, from the University of Virginia discussed anticoagulation for portal vein thrombosis. He also discussed consideration of transjugular intrahepatic portosystemic shunt if there are high-risk varices. Duration of anticoagulation is controversial, but this strategy may prevent decompensation and affect transplant outcomes.
Daryl Lau, MD, MSc, MPH, from Harvard Medical School, Boston, reviewed the hepatitis B virus therapy controversy for e-antigen–negative patients with prolonged viral suppression. She recommended caution in general and emphasized that stage 3-4 fibrosis patients should not discontinue therapy.
The final talk was my review of hepatitis C virus treatment. I emphasized that pretreatment fibrosis assessments are critical given continued risk of hepatocellular carcinoma after cure. Challenges include identifying the remaining patients and supporting them through treatment. HCV therapies demonstrate what is possible when breakthroughs are translated to clinical care, and I was honored to participate in this course that highlighted many advances in our field.
Dr. Muir is a professor of medicine, director of gastroenterology & hepatology research at Duke Clinical Research Institute, and chief of the division of gastroenterology in the department of medicine at Duke University, all in Durham, N.C. He has received research grants from and served on the advisory boards for AbbVie, Gilead Sciences, Merck, and several other pharmaceutical companies. This is a summary provided by the moderator of one of the spring postgraduate course sessions held at DDW 2018.
For the pancreatobiliary session, Michelle Ann Anderson, MD, of the University of Michigan, Ann Arbor, reminded us about appropriate patient selection given the risk of pancreatitis after endoscopic retrograde cholangiopancreatography pancreatitis, also known as post-ERCP pancreatitis. Strategies to prevent post-ERCP pancreatitis include using pancreatic duct stents and using wire rather than contrast for cannulation. She recommended rectal indomethacin for all patients. Because of encouraging data, she recommended 2-3 L of lactated Ringer’s solution during the procedure and recovery.
Katie Morgan, MD, from the Medical University of South Carolina, Charleston, reviewed her group’s experience with 195 total pancreatectomies with islet autotransplants for chronic pancreatitis. Quality of life improved with major reductions in narcotic use, and 25% of patients were insulin free.
Bret Petersen, MD, of Mayo Clinic, Rochester, Minn., discussed multidrug resistant infection in ERCP endoscopes. He reminded us of the risk of lapses in endoscope reprocessing steps and the need for monitoring. He commented on recent Food and Drug Administration’s culture guidance and new technologies in development.
James Scheiman, MD, from the University of Virginia, Charlottesville, discussed pancreatic cysts. He reviewed the controversy between the more conservative American Gastroenterological Association guidelines and the more aggressive International Consensus guidelines. He advised considering patient preferences with a multidisciplinary approach.
For the liver session, Guadalupe García-Tsao, MD, of Yale University, New Haven, Conn., discussed the controversy regarding nonselective beta-blockers. She advised caution if refractory ascites are present because of risk for renal dysfunction, but she also highlighted the benefits including reduced first and recurrent variceal hemorrhage.
Rohit Loomba, MD, from the University of California at San Diego addressed fibrosis assessments in fatty liver. In his algorithm, patients with low Nonalcoholic Fatty Liver Disease Fibrosis Score or Fibrosis-4 scores would have continued observation, while patients with medium or high scores would undergo transient elastography or magnetic resonance elastography.
Patrick Northup, MD, from the University of Virginia discussed anticoagulation for portal vein thrombosis. He also discussed consideration of transjugular intrahepatic portosystemic shunt if there are high-risk varices. Duration of anticoagulation is controversial, but this strategy may prevent decompensation and affect transplant outcomes.
Daryl Lau, MD, MSc, MPH, from Harvard Medical School, Boston, reviewed the hepatitis B virus therapy controversy for e-antigen–negative patients with prolonged viral suppression. She recommended caution in general and emphasized that stage 3-4 fibrosis patients should not discontinue therapy.
The final talk was my review of hepatitis C virus treatment. I emphasized that pretreatment fibrosis assessments are critical given continued risk of hepatocellular carcinoma after cure. Challenges include identifying the remaining patients and supporting them through treatment. HCV therapies demonstrate what is possible when breakthroughs are translated to clinical care, and I was honored to participate in this course that highlighted many advances in our field.
Dr. Muir is a professor of medicine, director of gastroenterology & hepatology research at Duke Clinical Research Institute, and chief of the division of gastroenterology in the department of medicine at Duke University, all in Durham, N.C. He has received research grants from and served on the advisory boards for AbbVie, Gilead Sciences, Merck, and several other pharmaceutical companies. This is a summary provided by the moderator of one of the spring postgraduate course sessions held at DDW 2018.
For the pancreatobiliary session, Michelle Ann Anderson, MD, of the University of Michigan, Ann Arbor, reminded us about appropriate patient selection given the risk of pancreatitis after endoscopic retrograde cholangiopancreatography pancreatitis, also known as post-ERCP pancreatitis. Strategies to prevent post-ERCP pancreatitis include using pancreatic duct stents and using wire rather than contrast for cannulation. She recommended rectal indomethacin for all patients. Because of encouraging data, she recommended 2-3 L of lactated Ringer’s solution during the procedure and recovery.
Katie Morgan, MD, from the Medical University of South Carolina, Charleston, reviewed her group’s experience with 195 total pancreatectomies with islet autotransplants for chronic pancreatitis. Quality of life improved with major reductions in narcotic use, and 25% of patients were insulin free.
Bret Petersen, MD, of Mayo Clinic, Rochester, Minn., discussed multidrug resistant infection in ERCP endoscopes. He reminded us of the risk of lapses in endoscope reprocessing steps and the need for monitoring. He commented on recent Food and Drug Administration’s culture guidance and new technologies in development.
James Scheiman, MD, from the University of Virginia, Charlottesville, discussed pancreatic cysts. He reviewed the controversy between the more conservative American Gastroenterological Association guidelines and the more aggressive International Consensus guidelines. He advised considering patient preferences with a multidisciplinary approach.
For the liver session, Guadalupe García-Tsao, MD, of Yale University, New Haven, Conn., discussed the controversy regarding nonselective beta-blockers. She advised caution if refractory ascites are present because of risk for renal dysfunction, but she also highlighted the benefits including reduced first and recurrent variceal hemorrhage.
Rohit Loomba, MD, from the University of California at San Diego addressed fibrosis assessments in fatty liver. In his algorithm, patients with low Nonalcoholic Fatty Liver Disease Fibrosis Score or Fibrosis-4 scores would have continued observation, while patients with medium or high scores would undergo transient elastography or magnetic resonance elastography.
Patrick Northup, MD, from the University of Virginia discussed anticoagulation for portal vein thrombosis. He also discussed consideration of transjugular intrahepatic portosystemic shunt if there are high-risk varices. Duration of anticoagulation is controversial, but this strategy may prevent decompensation and affect transplant outcomes.
Daryl Lau, MD, MSc, MPH, from Harvard Medical School, Boston, reviewed the hepatitis B virus therapy controversy for e-antigen–negative patients with prolonged viral suppression. She recommended caution in general and emphasized that stage 3-4 fibrosis patients should not discontinue therapy.
The final talk was my review of hepatitis C virus treatment. I emphasized that pretreatment fibrosis assessments are critical given continued risk of hepatocellular carcinoma after cure. Challenges include identifying the remaining patients and supporting them through treatment. HCV therapies demonstrate what is possible when breakthroughs are translated to clinical care, and I was honored to participate in this course that highlighted many advances in our field.
Dr. Muir is a professor of medicine, director of gastroenterology & hepatology research at Duke Clinical Research Institute, and chief of the division of gastroenterology in the department of medicine at Duke University, all in Durham, N.C. He has received research grants from and served on the advisory boards for AbbVie, Gilead Sciences, Merck, and several other pharmaceutical companies. This is a summary provided by the moderator of one of the spring postgraduate course sessions held at DDW 2018.
Pancreatic surveillance identified resectable cancers
Long-term pancreatic surveillance of high-risk patients identified cancers while they were still resectable, and 85% of such patients remained alive 3 years after diagnosis, researchers reported.
“Among individuals undergoing pancreatic surveillance, specific detectable lesions with worrisome features predicted neoplastic progression. The short-term outcomes of patients with screening-detected PDACs [pancreatic ductal adenocarcinomas] improved,” wrote Marcia Irene Canto, MD, MHS, of the Johns Hopkins University, Baltimore, together with her associates in the September issue of Gastroenterology.
The lifetime risk of PDAC is about 1.5%, the researchers noted. Consequently, the U.S. Preventive Services Task Force does not recommend pancreatic surveillance at a population level. However, pancreatic screening is being evaluated for individuals who are at significantly elevated risk of PDAC, including those with at least two first-degree relatives with PDAC or who have germline mutations in BRCA1, BRCA2, PALB2, PRSS1 (hereditary pancreatitis), CDKN2A, MLH1, MSH2, MSH6, PMS2 (Lynch syndrome), or STK11 (Peutz-Jeghers syndrome).
For the study, Dr. Canto and her associates analyzed data from 354 such high-risk individuals enrolled in the CAPS (Cancer of the Pancreas Screening) cohort studies, which were conducted at tertiary care academic centers during 1998-2014. All participants underwent endoscopic ultrasound at baseline, followed by surveillance with endoscopic ultrasound, magnetic resonance imaging, computed tomography, or some combination of these modalities. Patients who developed pancreatic cancer or high-grade dysplasia were offered surgery.
In all, 68 patients (19%) developed pancreatic lesions with worrisome features, such as solid masses, multiple cysts, mural nodules, thickened or enhancing walls, cysts exceeding 3 cm in size or that grew more than 4 mm annually, a greater than 5-mm dilation of the main pancreatic duct, or an abrupt change in duct caliber. The lesions developed over a median of 13.1 months (interquartile range, 0.2-52 months).
A total of 7% of patients had neoplastic progression, including 14 cases of PDAC and 10 cases of high-grade dysplasia. Median times from baseline to detection of PDAC were 4.8 years overall (IQR, 1.6-6.9 years), 1.7 years (IQR, 0.5-4.4 years) among patients aged at least 60 years at baseline, and 5.2 years among younger patients (IQR, 0.4-8 years). Patients developed PDAC at a median of 67 years old.
Among 10 PDACs detected by surveillance, 9 were resectable. Three patients subsequently died of PDAC, while one patient died of complications of gastric cancer surgery. However, 85% of patients survived for at least 3 years after surgical resection of PDAC. The remaining four cases of PDAC were detected outside surveillance or after patients stopped surveillance.
The 10 cases of high-grade dysplasia consisted of intraductal papillary mucinous neoplasm with high-grade dysplasia or high-grade pancreatic intraepithelial neoplasia. Patients whose PDAC or high-grade dysplasia was detected by surveillance survived a median of 5.3 years, while patients whose surveillance was late or stopped and who subsequently developed neoplasia survived a median of only 1.4 years after diagnosis (P less than .0001).
Funders included the Pancreatic Cancer Action Network, Lustgarten Foundation for Pancreatic Cancer Research, the John and Peter Hooven Memorial Endowment, Hugh and Rachel Victor, and ChiRhoClin. Dr. Canto had no disclosures. Three coinvestigators disclosed royalties for licensing of PALB2 as a pancreatic cancer susceptibility gene.
SOURCE: Canto MI et al. Gastroenterology. 2018 May 24. doi: 10.1053/j.gastro.2018.05.035
Long-term pancreatic surveillance of high-risk patients identified cancers while they were still resectable, and 85% of such patients remained alive 3 years after diagnosis, researchers reported.
“Among individuals undergoing pancreatic surveillance, specific detectable lesions with worrisome features predicted neoplastic progression. The short-term outcomes of patients with screening-detected PDACs [pancreatic ductal adenocarcinomas] improved,” wrote Marcia Irene Canto, MD, MHS, of the Johns Hopkins University, Baltimore, together with her associates in the September issue of Gastroenterology.
The lifetime risk of PDAC is about 1.5%, the researchers noted. Consequently, the U.S. Preventive Services Task Force does not recommend pancreatic surveillance at a population level. However, pancreatic screening is being evaluated for individuals who are at significantly elevated risk of PDAC, including those with at least two first-degree relatives with PDAC or who have germline mutations in BRCA1, BRCA2, PALB2, PRSS1 (hereditary pancreatitis), CDKN2A, MLH1, MSH2, MSH6, PMS2 (Lynch syndrome), or STK11 (Peutz-Jeghers syndrome).
For the study, Dr. Canto and her associates analyzed data from 354 such high-risk individuals enrolled in the CAPS (Cancer of the Pancreas Screening) cohort studies, which were conducted at tertiary care academic centers during 1998-2014. All participants underwent endoscopic ultrasound at baseline, followed by surveillance with endoscopic ultrasound, magnetic resonance imaging, computed tomography, or some combination of these modalities. Patients who developed pancreatic cancer or high-grade dysplasia were offered surgery.
In all, 68 patients (19%) developed pancreatic lesions with worrisome features, such as solid masses, multiple cysts, mural nodules, thickened or enhancing walls, cysts exceeding 3 cm in size or that grew more than 4 mm annually, a greater than 5-mm dilation of the main pancreatic duct, or an abrupt change in duct caliber. The lesions developed over a median of 13.1 months (interquartile range, 0.2-52 months).
A total of 7% of patients had neoplastic progression, including 14 cases of PDAC and 10 cases of high-grade dysplasia. Median times from baseline to detection of PDAC were 4.8 years overall (IQR, 1.6-6.9 years), 1.7 years (IQR, 0.5-4.4 years) among patients aged at least 60 years at baseline, and 5.2 years among younger patients (IQR, 0.4-8 years). Patients developed PDAC at a median of 67 years old.
Among 10 PDACs detected by surveillance, 9 were resectable. Three patients subsequently died of PDAC, while one patient died of complications of gastric cancer surgery. However, 85% of patients survived for at least 3 years after surgical resection of PDAC. The remaining four cases of PDAC were detected outside surveillance or after patients stopped surveillance.
The 10 cases of high-grade dysplasia consisted of intraductal papillary mucinous neoplasm with high-grade dysplasia or high-grade pancreatic intraepithelial neoplasia. Patients whose PDAC or high-grade dysplasia was detected by surveillance survived a median of 5.3 years, while patients whose surveillance was late or stopped and who subsequently developed neoplasia survived a median of only 1.4 years after diagnosis (P less than .0001).
Funders included the Pancreatic Cancer Action Network, Lustgarten Foundation for Pancreatic Cancer Research, the John and Peter Hooven Memorial Endowment, Hugh and Rachel Victor, and ChiRhoClin. Dr. Canto had no disclosures. Three coinvestigators disclosed royalties for licensing of PALB2 as a pancreatic cancer susceptibility gene.
SOURCE: Canto MI et al. Gastroenterology. 2018 May 24. doi: 10.1053/j.gastro.2018.05.035
Long-term pancreatic surveillance of high-risk patients identified cancers while they were still resectable, and 85% of such patients remained alive 3 years after diagnosis, researchers reported.
“Among individuals undergoing pancreatic surveillance, specific detectable lesions with worrisome features predicted neoplastic progression. The short-term outcomes of patients with screening-detected PDACs [pancreatic ductal adenocarcinomas] improved,” wrote Marcia Irene Canto, MD, MHS, of the Johns Hopkins University, Baltimore, together with her associates in the September issue of Gastroenterology.
The lifetime risk of PDAC is about 1.5%, the researchers noted. Consequently, the U.S. Preventive Services Task Force does not recommend pancreatic surveillance at a population level. However, pancreatic screening is being evaluated for individuals who are at significantly elevated risk of PDAC, including those with at least two first-degree relatives with PDAC or who have germline mutations in BRCA1, BRCA2, PALB2, PRSS1 (hereditary pancreatitis), CDKN2A, MLH1, MSH2, MSH6, PMS2 (Lynch syndrome), or STK11 (Peutz-Jeghers syndrome).
For the study, Dr. Canto and her associates analyzed data from 354 such high-risk individuals enrolled in the CAPS (Cancer of the Pancreas Screening) cohort studies, which were conducted at tertiary care academic centers during 1998-2014. All participants underwent endoscopic ultrasound at baseline, followed by surveillance with endoscopic ultrasound, magnetic resonance imaging, computed tomography, or some combination of these modalities. Patients who developed pancreatic cancer or high-grade dysplasia were offered surgery.
In all, 68 patients (19%) developed pancreatic lesions with worrisome features, such as solid masses, multiple cysts, mural nodules, thickened or enhancing walls, cysts exceeding 3 cm in size or that grew more than 4 mm annually, a greater than 5-mm dilation of the main pancreatic duct, or an abrupt change in duct caliber. The lesions developed over a median of 13.1 months (interquartile range, 0.2-52 months).
A total of 7% of patients had neoplastic progression, including 14 cases of PDAC and 10 cases of high-grade dysplasia. Median times from baseline to detection of PDAC were 4.8 years overall (IQR, 1.6-6.9 years), 1.7 years (IQR, 0.5-4.4 years) among patients aged at least 60 years at baseline, and 5.2 years among younger patients (IQR, 0.4-8 years). Patients developed PDAC at a median of 67 years old.
Among 10 PDACs detected by surveillance, 9 were resectable. Three patients subsequently died of PDAC, while one patient died of complications of gastric cancer surgery. However, 85% of patients survived for at least 3 years after surgical resection of PDAC. The remaining four cases of PDAC were detected outside surveillance or after patients stopped surveillance.
The 10 cases of high-grade dysplasia consisted of intraductal papillary mucinous neoplasm with high-grade dysplasia or high-grade pancreatic intraepithelial neoplasia. Patients whose PDAC or high-grade dysplasia was detected by surveillance survived a median of 5.3 years, while patients whose surveillance was late or stopped and who subsequently developed neoplasia survived a median of only 1.4 years after diagnosis (P less than .0001).
Funders included the Pancreatic Cancer Action Network, Lustgarten Foundation for Pancreatic Cancer Research, the John and Peter Hooven Memorial Endowment, Hugh and Rachel Victor, and ChiRhoClin. Dr. Canto had no disclosures. Three coinvestigators disclosed royalties for licensing of PALB2 as a pancreatic cancer susceptibility gene.
SOURCE: Canto MI et al. Gastroenterology. 2018 May 24. doi: 10.1053/j.gastro.2018.05.035
FROM GASTROENTEROLOGY
Key clinical point: Pancreatic surveillance of high-risk individuals identified neoplasias when they were still resectable.
Major finding: Nine of ten tumors detected by surveillance were resectable, and 85% of patients remained alive 3 years after surgery, versus 25% of patients who were diagnosed after stopping or delaying surveillance (P less than .001).
Study details: Prospective cohort study of 354 high-risk individuals with 16 years of follow-up.
Disclosures: Funders included the Pancreatic Cancer Action Network, Lustgarten Foundation for Pancreatic Cancer Research, the John and Peter Hooven Memorial Endowment, Hugh and Rachel Victor, and ChiRhoClin. Dr. Canto had no disclosures. Three coinvestigators disclosed royalties for licensing of PALB2 as a pancreatic cancer susceptibility gene.
Source: Canto MI et al. Gastroenterology. 2018 May 24. doi: 10.1053/j.gastro.2018.05.035.
ED visits up for acute pancreatitis linked to younger age, alcohol, chronic disease
, an analysis of a nationally representative database has suggested.
Meanwhile, hospital admissions and length of stay dropped, but ED and inpatient charges increased, according to the analysis by Sushil K. Garg, MD, of the division of gastroenterology and hepatology at the Mayo Clinic, Rochester, Minn., and his coauthors.
“This study identifies important patient populations, specifically young patients with alcohol abuse, to target in order to develop programs to assist in reduction of ED utilization for acute pancreatitis,” Dr. Garg and his colleagues reported in the Journal of Clinical Gastroenterology.
The retrospective analysis was focused on nearly 2.2 million ED visits during 2006-2012 in the National Emergency Department Sample (NEDS) database. The cohort was limited to adults at least 18 years of age with a primary diagnosis of acute pancreatitis.
Overall, there was a nonsignificant 5.5% increase in visits per 10,000 U.S. population during 2006-2012, the researchers found. However, the total number of ED visits in this sample increased significantly – from 292,902 in 2006 to a peak of 326,376, an average rate of increase of 7,213 visits per year (P = .0086), according to the report.
Younger patients had a significant increase in the number of pancreatitis-related ED visits over the study period, while older patients had a significant decrease, according to investigators. Visits were up 9.2% for patients aged 18-44 years and 8.6% for those aged 45-64 but down 13.4% for patients aged 65-84 years and 20.1% for those aged 85 years or older.
The incidence of visits secondary to biliary disease was virtually flat over time, Dr. Garg and his coinvestigators found when looking at visits grouped by the most common presenting etiologies. By contrast, there were significant increases in visits for acute pancreatitis associated with alcohol abuse or chronic pancreatitis.
Specifically, acute pancreatitis associated with biliary disease averaged 20.7% of yearly pancreatitis-related ED visits and did not significantly change over time, the researchers reported.
By contrast, acute pancreatitis associated with alcohol abuse, which accounted for 24.1% of visits on average, increased by 15.9% over the study period, an increase driven by an increase among age groups younger than 65 years.
Acute pancreatitis associated with chronic pancreatitis, which made up 11.5% of visits on average, increased “substantially” in all age groups, according to study authors, with the largest increase in the group aged 45-64 years. Overall, the percentage increase over 7 years was 59.5%.
Rates of hospitalization decreased significantly over time, from 76.2% in 2006 to 72.7% in 2012 (P = .0026), and likewise, the length of stay dropped from 5.36 to 4.64 days (P = .0001), according to the analysis.
Inpatient charges, adjusted for inflation and expressed in 2012 dollars, increased from $32,130.63 to $34,652.00 (P = .0011), an average rate of increase of $489/year.
Predictors of hospitalization included age older than 84 years, alcohol use, smoking, and a Charlson comorbidity score of 1 or greater, according to the results of a multivariate regression analysis.
“Factors which may place patients at higher risk for severe or complicated acute pancreatitis requiring admission, such as obesity, alcohol use, and increasing age, are identified and should be explored in further studies and potentially targeted to improve ED and inpatient care,” Dr. Garg and his coauthors said.
Dr. Garg and his coauthors had no disclosures related to the study.
Help your patients better understand pancreatitis and available tests and treatments by using AGA patient education materials, https://www.gastro.org/practice-guidance/gi-patient-center/topic/pancreatitis.
SOURCE: Garg SK et al. J Clin Gastroenterol. 2018 Apr 6. doi: 10.1097/MCG.0000000000001030.
, an analysis of a nationally representative database has suggested.
Meanwhile, hospital admissions and length of stay dropped, but ED and inpatient charges increased, according to the analysis by Sushil K. Garg, MD, of the division of gastroenterology and hepatology at the Mayo Clinic, Rochester, Minn., and his coauthors.
“This study identifies important patient populations, specifically young patients with alcohol abuse, to target in order to develop programs to assist in reduction of ED utilization for acute pancreatitis,” Dr. Garg and his colleagues reported in the Journal of Clinical Gastroenterology.
The retrospective analysis was focused on nearly 2.2 million ED visits during 2006-2012 in the National Emergency Department Sample (NEDS) database. The cohort was limited to adults at least 18 years of age with a primary diagnosis of acute pancreatitis.
Overall, there was a nonsignificant 5.5% increase in visits per 10,000 U.S. population during 2006-2012, the researchers found. However, the total number of ED visits in this sample increased significantly – from 292,902 in 2006 to a peak of 326,376, an average rate of increase of 7,213 visits per year (P = .0086), according to the report.
Younger patients had a significant increase in the number of pancreatitis-related ED visits over the study period, while older patients had a significant decrease, according to investigators. Visits were up 9.2% for patients aged 18-44 years and 8.6% for those aged 45-64 but down 13.4% for patients aged 65-84 years and 20.1% for those aged 85 years or older.
The incidence of visits secondary to biliary disease was virtually flat over time, Dr. Garg and his coinvestigators found when looking at visits grouped by the most common presenting etiologies. By contrast, there were significant increases in visits for acute pancreatitis associated with alcohol abuse or chronic pancreatitis.
Specifically, acute pancreatitis associated with biliary disease averaged 20.7% of yearly pancreatitis-related ED visits and did not significantly change over time, the researchers reported.
By contrast, acute pancreatitis associated with alcohol abuse, which accounted for 24.1% of visits on average, increased by 15.9% over the study period, an increase driven by an increase among age groups younger than 65 years.
Acute pancreatitis associated with chronic pancreatitis, which made up 11.5% of visits on average, increased “substantially” in all age groups, according to study authors, with the largest increase in the group aged 45-64 years. Overall, the percentage increase over 7 years was 59.5%.
Rates of hospitalization decreased significantly over time, from 76.2% in 2006 to 72.7% in 2012 (P = .0026), and likewise, the length of stay dropped from 5.36 to 4.64 days (P = .0001), according to the analysis.
Inpatient charges, adjusted for inflation and expressed in 2012 dollars, increased from $32,130.63 to $34,652.00 (P = .0011), an average rate of increase of $489/year.
Predictors of hospitalization included age older than 84 years, alcohol use, smoking, and a Charlson comorbidity score of 1 or greater, according to the results of a multivariate regression analysis.
“Factors which may place patients at higher risk for severe or complicated acute pancreatitis requiring admission, such as obesity, alcohol use, and increasing age, are identified and should be explored in further studies and potentially targeted to improve ED and inpatient care,” Dr. Garg and his coauthors said.
Dr. Garg and his coauthors had no disclosures related to the study.
Help your patients better understand pancreatitis and available tests and treatments by using AGA patient education materials, https://www.gastro.org/practice-guidance/gi-patient-center/topic/pancreatitis.
SOURCE: Garg SK et al. J Clin Gastroenterol. 2018 Apr 6. doi: 10.1097/MCG.0000000000001030.
, an analysis of a nationally representative database has suggested.
Meanwhile, hospital admissions and length of stay dropped, but ED and inpatient charges increased, according to the analysis by Sushil K. Garg, MD, of the division of gastroenterology and hepatology at the Mayo Clinic, Rochester, Minn., and his coauthors.
“This study identifies important patient populations, specifically young patients with alcohol abuse, to target in order to develop programs to assist in reduction of ED utilization for acute pancreatitis,” Dr. Garg and his colleagues reported in the Journal of Clinical Gastroenterology.
The retrospective analysis was focused on nearly 2.2 million ED visits during 2006-2012 in the National Emergency Department Sample (NEDS) database. The cohort was limited to adults at least 18 years of age with a primary diagnosis of acute pancreatitis.
Overall, there was a nonsignificant 5.5% increase in visits per 10,000 U.S. population during 2006-2012, the researchers found. However, the total number of ED visits in this sample increased significantly – from 292,902 in 2006 to a peak of 326,376, an average rate of increase of 7,213 visits per year (P = .0086), according to the report.
Younger patients had a significant increase in the number of pancreatitis-related ED visits over the study period, while older patients had a significant decrease, according to investigators. Visits were up 9.2% for patients aged 18-44 years and 8.6% for those aged 45-64 but down 13.4% for patients aged 65-84 years and 20.1% for those aged 85 years or older.
The incidence of visits secondary to biliary disease was virtually flat over time, Dr. Garg and his coinvestigators found when looking at visits grouped by the most common presenting etiologies. By contrast, there were significant increases in visits for acute pancreatitis associated with alcohol abuse or chronic pancreatitis.
Specifically, acute pancreatitis associated with biliary disease averaged 20.7% of yearly pancreatitis-related ED visits and did not significantly change over time, the researchers reported.
By contrast, acute pancreatitis associated with alcohol abuse, which accounted for 24.1% of visits on average, increased by 15.9% over the study period, an increase driven by an increase among age groups younger than 65 years.
Acute pancreatitis associated with chronic pancreatitis, which made up 11.5% of visits on average, increased “substantially” in all age groups, according to study authors, with the largest increase in the group aged 45-64 years. Overall, the percentage increase over 7 years was 59.5%.
Rates of hospitalization decreased significantly over time, from 76.2% in 2006 to 72.7% in 2012 (P = .0026), and likewise, the length of stay dropped from 5.36 to 4.64 days (P = .0001), according to the analysis.
Inpatient charges, adjusted for inflation and expressed in 2012 dollars, increased from $32,130.63 to $34,652.00 (P = .0011), an average rate of increase of $489/year.
Predictors of hospitalization included age older than 84 years, alcohol use, smoking, and a Charlson comorbidity score of 1 or greater, according to the results of a multivariate regression analysis.
“Factors which may place patients at higher risk for severe or complicated acute pancreatitis requiring admission, such as obesity, alcohol use, and increasing age, are identified and should be explored in further studies and potentially targeted to improve ED and inpatient care,” Dr. Garg and his coauthors said.
Dr. Garg and his coauthors had no disclosures related to the study.
Help your patients better understand pancreatitis and available tests and treatments by using AGA patient education materials, https://www.gastro.org/practice-guidance/gi-patient-center/topic/pancreatitis.
SOURCE: Garg SK et al. J Clin Gastroenterol. 2018 Apr 6. doi: 10.1097/MCG.0000000000001030.