User login
New studies inform best practices for pelvic organ prolapse
“Approximately one in five women will undergo surgery for prolapse and/or urinary incontinence by the age of 80, which is more likely than the risk of developing breast cancer,” said David D. Rahn, MD, corresponding author of the study on perioperative vaginal estrogen, in an interview.
“About 13% of women will specifically undergo surgery to repair pelvic organ prolapse,” said Dr. Rahn, of the department of obstetrics and gynecology, University of Texas Southwestern Medical Center, Dallas. Reoperation for recurrent prolapse is not uncommon.
In their study, Dr. Rahn and colleagues examined whether the addition of perioperative vaginal estrogen cream in postmenopausal women with prolapse planning surgical correction could both strengthen the repair and lessen the likelihood of recurrence. The researchers randomized 206 postmenopausal women who were seeking surgical repair for bothersome anterior and apical vaginal prolapse to 1 gram of conjugated estrogen cream or a placebo for nightly vaginal insertion for 2 weeks, then twice weekly for at least 5 weeks of preoperative use. The treatment continued twice weekly for 12 months following surgery.
The primary outcome was the time to a failed prolapse repair by 12 months after surgery. Failure was defined by at least one of three criteria, “anatomical/objective prolapse of anterior or posterior walls beyond the hymen or the apex descending more than one-third of the vaginal length, subjective vaginal bulge symptoms, or repeated prolapse treatment,” the researchers wrote. The mean age of the patients was 65 years, and 90% and 92% of patients in the treatment and placebo groups, respectively, were White; 10% and 5%, respectively, were Black. Other baseline characteristics were similar between the groups.
After 12 months, the surgical failure incidence was not significantly different between the vaginal estrogen and placebo groups (19% vs. 9%, respectively; adjusted hazard ratio, 1.97).
Overall, anatomic recurrence was the most common outcome associated with surgical failure.
However, vaginal atrophy scores for most bothersome symptom was significantly better at 12 months in the vaginal estrogen group, compared with the placebo group, in a subset of 109 patients who reported vaginal atrophy that was at least “moderately bothersome,” the researchers said.
The findings were limited by several factors including the use of a nonvalidated instrument to assess secondary outcomes, the potentially short time period to the primary outcome, and the inclusion of the apex descending below one third total vaginal length as a criterion for surgical failure (which could be considered conservative), the researchers noted.
Unexpected results
“This work followed logically from a pilot study that similarly randomized postmenopausal women with prolapse planning surgical repair to vaginal estrogen cream versus placebo,” Dr. Rahn said. “In that smaller study, full thickness vaginal wall biopsies were collected at the time of surgery. Those participants who received the estrogen had a thicker vaginal epithelium, thicker underlying muscularis, and appeared to have a more robust concentration of strong connective tissue (i.e., type I collagen) with less of the proteases that break down connective tissue.”
This suggested that preoperative estrogen might optimize the vaginal tissue at the time of the repair. Dr. Rahn said. However, “despite evidence that the application of vaginal estrogen cream decreased the symptoms and signs of atrophic vaginal tissues, this did not lessen the likelihood of pelvic organ prolapse recurrence 12 months after surgical repair.”
The current study “would argue against routine prescription of vaginal estrogen to optimize vaginal tissue for prolapse repair, a practice that is recommended by some experts and commonly prescribed anecdotally,” said Dr. Rahn. “However, in those patients with prolapse and bothersome atrophy-related complaints such as vaginal dryness and pain with intercourse, vaginal estrogen may still be appropriate,” and vaginal estrogen also could be useful for postoperatively for patients prone to recurrent urinary tract infections.
Additional research from the study is underway, said Dr. Rahn. “All participants have now been followed to 3 years after surgery, and those clinical results are now being analyzed. In addition, full-thickness vaginal wall biopsies were collected at the time of all 186 surgeries; these are being analyzed and may yield important information regarding how biomarkers for connective tissue health could point to increased (or decreased) risk for prolapse recurrence.”
Manchester technique surpasses sacrospinous hysteropexy
In the second JAMA study, sacrospinous hysteropexy for uterine-sparing surgical management of uterine prolapse was less effective than the older Manchester procedure, based on data from nearly 400 individuals.
“Until now, the optimal uterus-sparing procedure for the treatment of uterine descent remained uncertain,” lead author Rosa Enklaar, MD, of Radboud (the Netherlands) University Medical Center, said in an interview.
“Globally, there has been a lack of scientific evidence comparing the efficacy of these two techniques, and this study aims to bridge that gap,” she said.
In their study, Dr. Enklaar and colleagues randomized 215 women to sacrospinous hysteropexy and 215 to the Manchester procedure. The mean age of the participants was 61.7 years.
The Manchester procedure involves “extraperitoneal plication of the uterosacral ligaments at the posterior side of the uterus and amputation of the cervix,” and “the cardinal ligaments are plicated on the anterior side of the cervix, “ the researchers wrote.
The primary outcome was a composite outcome of surgical success at 2 years after surgery, defined as the absence of three elements: absence of vaginal prolapse beyond the hymen, absence of bothersome bulge symptoms, and absence of retreatment of current prolapse.
Overall, 87.3% of patients in the Manchester group and 77.0% in the sacrospinous hysteropexy group met the primary outcome. At the end of the 2-year follow-up period, perioperative and patient-reported outcomes were not significantly different between the groups.
Dr. Enklaar said she was surprised by the findings. “At the start of this study, we hypothesized that there would be no difference between the two techniques,” as both have been used for a long period of time.
However, “based on the composite outcome of success at 2-year follow-up after the primary uterus-sparing surgery for uterine descent in patients with pelvic organ prolapse, these findings indicate that the sacrospinous hysteropexy is inferior to the Manchester procedure,” she said.
The study findings were limited by several factors including the lack of blinding and the applicability of the results only to women without uterine prolapse past the hymen, as well as the exclusion of patients with higher-stage prolapse, the researchers said. However, the results suggest that sacrospinous hysteropexy is inferior to the Manchester technique for uterine-sparing pelvic organ prolapse surgery.
As for additional research, few studies of prolapse surgery with long-term follow-up data are available, Dr. Enklaar said. “It is important that this current study will be continued to see the results after a longer follow-up period. Personalized health care is increasingly important, and we need to provide adequate information when counselling patients. With studies such as this one, we hope to improve the choices regarding surgical treatment of uterine descent.”
Studies challenge current prolapse protocols
The study by Dr. Rahn and colleagues contradicts the common clinical practice of preoperative vaginal estrogen to reduce recurrence of prolapse, wrote Charles W. Nager, MD, of the University of California San Diego Health, La Jolla, in an accompanying editorial that addressed both studies.
The results suggest that use of perioperative intravaginal estrogen had no impact on outcomes, “despite the surgeon assessment of less atrophy and better vaginal apex tissue in the estrogen group,” he noted. Although vaginal estrogen has other benefits in terms of patient symptoms and effects on the vaginal epithelium, “surgeons should not prescribe vaginal estrogen with the expectation that it will improve surgical success.”
The study by Dr. Enklaar and colleagues reflects the growing interest in uterine-conserving procedures, Dr. Nager wrote. The modified Manchester procedure conforms to professional society guidelines, and the composite outcome conforms to current standards for the treatment of pelvic organ prolapse.
Although suspension of the vaginal apex was quite successful, the researchers interpreted their noninferiority findings with caution, said Dr. Nager. However, they suggested that the modified Manchester procedure as performed in their study “has a role in modern prolapse surgical repair for women with uterine descent that does not protrude beyond the hymen.”
The vaginal estrogen study was supported by the National Institute on Aging, a Bridge Award from the American Board of Obstetrics & Gynecology and the American Association of Obstetricians and Gynecologists Foundation. Dr. Rahn disclosed grants from the National Institute on Aging, the American Board of Obstetrics & Gynecology, and the AAOGF bridge award, as well as nonfinancial support from National Center for Advancing Translational Sciences and Pfizer during the study. The uterine prolapse study was supported by the Netherlands Organisation for Health Research and Development. The researchers had no financial conflicts to disclose. Dr. Nager had no financial conflicts to disclose.
“Approximately one in five women will undergo surgery for prolapse and/or urinary incontinence by the age of 80, which is more likely than the risk of developing breast cancer,” said David D. Rahn, MD, corresponding author of the study on perioperative vaginal estrogen, in an interview.
“About 13% of women will specifically undergo surgery to repair pelvic organ prolapse,” said Dr. Rahn, of the department of obstetrics and gynecology, University of Texas Southwestern Medical Center, Dallas. Reoperation for recurrent prolapse is not uncommon.
In their study, Dr. Rahn and colleagues examined whether the addition of perioperative vaginal estrogen cream in postmenopausal women with prolapse planning surgical correction could both strengthen the repair and lessen the likelihood of recurrence. The researchers randomized 206 postmenopausal women who were seeking surgical repair for bothersome anterior and apical vaginal prolapse to 1 gram of conjugated estrogen cream or a placebo for nightly vaginal insertion for 2 weeks, then twice weekly for at least 5 weeks of preoperative use. The treatment continued twice weekly for 12 months following surgery.
The primary outcome was the time to a failed prolapse repair by 12 months after surgery. Failure was defined by at least one of three criteria, “anatomical/objective prolapse of anterior or posterior walls beyond the hymen or the apex descending more than one-third of the vaginal length, subjective vaginal bulge symptoms, or repeated prolapse treatment,” the researchers wrote. The mean age of the patients was 65 years, and 90% and 92% of patients in the treatment and placebo groups, respectively, were White; 10% and 5%, respectively, were Black. Other baseline characteristics were similar between the groups.
After 12 months, the surgical failure incidence was not significantly different between the vaginal estrogen and placebo groups (19% vs. 9%, respectively; adjusted hazard ratio, 1.97).
Overall, anatomic recurrence was the most common outcome associated with surgical failure.
However, vaginal atrophy scores for most bothersome symptom was significantly better at 12 months in the vaginal estrogen group, compared with the placebo group, in a subset of 109 patients who reported vaginal atrophy that was at least “moderately bothersome,” the researchers said.
The findings were limited by several factors including the use of a nonvalidated instrument to assess secondary outcomes, the potentially short time period to the primary outcome, and the inclusion of the apex descending below one third total vaginal length as a criterion for surgical failure (which could be considered conservative), the researchers noted.
Unexpected results
“This work followed logically from a pilot study that similarly randomized postmenopausal women with prolapse planning surgical repair to vaginal estrogen cream versus placebo,” Dr. Rahn said. “In that smaller study, full thickness vaginal wall biopsies were collected at the time of surgery. Those participants who received the estrogen had a thicker vaginal epithelium, thicker underlying muscularis, and appeared to have a more robust concentration of strong connective tissue (i.e., type I collagen) with less of the proteases that break down connective tissue.”
This suggested that preoperative estrogen might optimize the vaginal tissue at the time of the repair. Dr. Rahn said. However, “despite evidence that the application of vaginal estrogen cream decreased the symptoms and signs of atrophic vaginal tissues, this did not lessen the likelihood of pelvic organ prolapse recurrence 12 months after surgical repair.”
The current study “would argue against routine prescription of vaginal estrogen to optimize vaginal tissue for prolapse repair, a practice that is recommended by some experts and commonly prescribed anecdotally,” said Dr. Rahn. “However, in those patients with prolapse and bothersome atrophy-related complaints such as vaginal dryness and pain with intercourse, vaginal estrogen may still be appropriate,” and vaginal estrogen also could be useful for postoperatively for patients prone to recurrent urinary tract infections.
Additional research from the study is underway, said Dr. Rahn. “All participants have now been followed to 3 years after surgery, and those clinical results are now being analyzed. In addition, full-thickness vaginal wall biopsies were collected at the time of all 186 surgeries; these are being analyzed and may yield important information regarding how biomarkers for connective tissue health could point to increased (or decreased) risk for prolapse recurrence.”
Manchester technique surpasses sacrospinous hysteropexy
In the second JAMA study, sacrospinous hysteropexy for uterine-sparing surgical management of uterine prolapse was less effective than the older Manchester procedure, based on data from nearly 400 individuals.
“Until now, the optimal uterus-sparing procedure for the treatment of uterine descent remained uncertain,” lead author Rosa Enklaar, MD, of Radboud (the Netherlands) University Medical Center, said in an interview.
“Globally, there has been a lack of scientific evidence comparing the efficacy of these two techniques, and this study aims to bridge that gap,” she said.
In their study, Dr. Enklaar and colleagues randomized 215 women to sacrospinous hysteropexy and 215 to the Manchester procedure. The mean age of the participants was 61.7 years.
The Manchester procedure involves “extraperitoneal plication of the uterosacral ligaments at the posterior side of the uterus and amputation of the cervix,” and “the cardinal ligaments are plicated on the anterior side of the cervix, “ the researchers wrote.
The primary outcome was a composite outcome of surgical success at 2 years after surgery, defined as the absence of three elements: absence of vaginal prolapse beyond the hymen, absence of bothersome bulge symptoms, and absence of retreatment of current prolapse.
Overall, 87.3% of patients in the Manchester group and 77.0% in the sacrospinous hysteropexy group met the primary outcome. At the end of the 2-year follow-up period, perioperative and patient-reported outcomes were not significantly different between the groups.
Dr. Enklaar said she was surprised by the findings. “At the start of this study, we hypothesized that there would be no difference between the two techniques,” as both have been used for a long period of time.
However, “based on the composite outcome of success at 2-year follow-up after the primary uterus-sparing surgery for uterine descent in patients with pelvic organ prolapse, these findings indicate that the sacrospinous hysteropexy is inferior to the Manchester procedure,” she said.
The study findings were limited by several factors including the lack of blinding and the applicability of the results only to women without uterine prolapse past the hymen, as well as the exclusion of patients with higher-stage prolapse, the researchers said. However, the results suggest that sacrospinous hysteropexy is inferior to the Manchester technique for uterine-sparing pelvic organ prolapse surgery.
As for additional research, few studies of prolapse surgery with long-term follow-up data are available, Dr. Enklaar said. “It is important that this current study will be continued to see the results after a longer follow-up period. Personalized health care is increasingly important, and we need to provide adequate information when counselling patients. With studies such as this one, we hope to improve the choices regarding surgical treatment of uterine descent.”
Studies challenge current prolapse protocols
The study by Dr. Rahn and colleagues contradicts the common clinical practice of preoperative vaginal estrogen to reduce recurrence of prolapse, wrote Charles W. Nager, MD, of the University of California San Diego Health, La Jolla, in an accompanying editorial that addressed both studies.
The results suggest that use of perioperative intravaginal estrogen had no impact on outcomes, “despite the surgeon assessment of less atrophy and better vaginal apex tissue in the estrogen group,” he noted. Although vaginal estrogen has other benefits in terms of patient symptoms and effects on the vaginal epithelium, “surgeons should not prescribe vaginal estrogen with the expectation that it will improve surgical success.”
The study by Dr. Enklaar and colleagues reflects the growing interest in uterine-conserving procedures, Dr. Nager wrote. The modified Manchester procedure conforms to professional society guidelines, and the composite outcome conforms to current standards for the treatment of pelvic organ prolapse.
Although suspension of the vaginal apex was quite successful, the researchers interpreted their noninferiority findings with caution, said Dr. Nager. However, they suggested that the modified Manchester procedure as performed in their study “has a role in modern prolapse surgical repair for women with uterine descent that does not protrude beyond the hymen.”
The vaginal estrogen study was supported by the National Institute on Aging, a Bridge Award from the American Board of Obstetrics & Gynecology and the American Association of Obstetricians and Gynecologists Foundation. Dr. Rahn disclosed grants from the National Institute on Aging, the American Board of Obstetrics & Gynecology, and the AAOGF bridge award, as well as nonfinancial support from National Center for Advancing Translational Sciences and Pfizer during the study. The uterine prolapse study was supported by the Netherlands Organisation for Health Research and Development. The researchers had no financial conflicts to disclose. Dr. Nager had no financial conflicts to disclose.
“Approximately one in five women will undergo surgery for prolapse and/or urinary incontinence by the age of 80, which is more likely than the risk of developing breast cancer,” said David D. Rahn, MD, corresponding author of the study on perioperative vaginal estrogen, in an interview.
“About 13% of women will specifically undergo surgery to repair pelvic organ prolapse,” said Dr. Rahn, of the department of obstetrics and gynecology, University of Texas Southwestern Medical Center, Dallas. Reoperation for recurrent prolapse is not uncommon.
In their study, Dr. Rahn and colleagues examined whether the addition of perioperative vaginal estrogen cream in postmenopausal women with prolapse planning surgical correction could both strengthen the repair and lessen the likelihood of recurrence. The researchers randomized 206 postmenopausal women who were seeking surgical repair for bothersome anterior and apical vaginal prolapse to 1 gram of conjugated estrogen cream or a placebo for nightly vaginal insertion for 2 weeks, then twice weekly for at least 5 weeks of preoperative use. The treatment continued twice weekly for 12 months following surgery.
The primary outcome was the time to a failed prolapse repair by 12 months after surgery. Failure was defined by at least one of three criteria, “anatomical/objective prolapse of anterior or posterior walls beyond the hymen or the apex descending more than one-third of the vaginal length, subjective vaginal bulge symptoms, or repeated prolapse treatment,” the researchers wrote. The mean age of the patients was 65 years, and 90% and 92% of patients in the treatment and placebo groups, respectively, were White; 10% and 5%, respectively, were Black. Other baseline characteristics were similar between the groups.
After 12 months, the surgical failure incidence was not significantly different between the vaginal estrogen and placebo groups (19% vs. 9%, respectively; adjusted hazard ratio, 1.97).
Overall, anatomic recurrence was the most common outcome associated with surgical failure.
However, vaginal atrophy scores for most bothersome symptom was significantly better at 12 months in the vaginal estrogen group, compared with the placebo group, in a subset of 109 patients who reported vaginal atrophy that was at least “moderately bothersome,” the researchers said.
The findings were limited by several factors including the use of a nonvalidated instrument to assess secondary outcomes, the potentially short time period to the primary outcome, and the inclusion of the apex descending below one third total vaginal length as a criterion for surgical failure (which could be considered conservative), the researchers noted.
Unexpected results
“This work followed logically from a pilot study that similarly randomized postmenopausal women with prolapse planning surgical repair to vaginal estrogen cream versus placebo,” Dr. Rahn said. “In that smaller study, full thickness vaginal wall biopsies were collected at the time of surgery. Those participants who received the estrogen had a thicker vaginal epithelium, thicker underlying muscularis, and appeared to have a more robust concentration of strong connective tissue (i.e., type I collagen) with less of the proteases that break down connective tissue.”
This suggested that preoperative estrogen might optimize the vaginal tissue at the time of the repair. Dr. Rahn said. However, “despite evidence that the application of vaginal estrogen cream decreased the symptoms and signs of atrophic vaginal tissues, this did not lessen the likelihood of pelvic organ prolapse recurrence 12 months after surgical repair.”
The current study “would argue against routine prescription of vaginal estrogen to optimize vaginal tissue for prolapse repair, a practice that is recommended by some experts and commonly prescribed anecdotally,” said Dr. Rahn. “However, in those patients with prolapse and bothersome atrophy-related complaints such as vaginal dryness and pain with intercourse, vaginal estrogen may still be appropriate,” and vaginal estrogen also could be useful for postoperatively for patients prone to recurrent urinary tract infections.
Additional research from the study is underway, said Dr. Rahn. “All participants have now been followed to 3 years after surgery, and those clinical results are now being analyzed. In addition, full-thickness vaginal wall biopsies were collected at the time of all 186 surgeries; these are being analyzed and may yield important information regarding how biomarkers for connective tissue health could point to increased (or decreased) risk for prolapse recurrence.”
Manchester technique surpasses sacrospinous hysteropexy
In the second JAMA study, sacrospinous hysteropexy for uterine-sparing surgical management of uterine prolapse was less effective than the older Manchester procedure, based on data from nearly 400 individuals.
“Until now, the optimal uterus-sparing procedure for the treatment of uterine descent remained uncertain,” lead author Rosa Enklaar, MD, of Radboud (the Netherlands) University Medical Center, said in an interview.
“Globally, there has been a lack of scientific evidence comparing the efficacy of these two techniques, and this study aims to bridge that gap,” she said.
In their study, Dr. Enklaar and colleagues randomized 215 women to sacrospinous hysteropexy and 215 to the Manchester procedure. The mean age of the participants was 61.7 years.
The Manchester procedure involves “extraperitoneal plication of the uterosacral ligaments at the posterior side of the uterus and amputation of the cervix,” and “the cardinal ligaments are plicated on the anterior side of the cervix, “ the researchers wrote.
The primary outcome was a composite outcome of surgical success at 2 years after surgery, defined as the absence of three elements: absence of vaginal prolapse beyond the hymen, absence of bothersome bulge symptoms, and absence of retreatment of current prolapse.
Overall, 87.3% of patients in the Manchester group and 77.0% in the sacrospinous hysteropexy group met the primary outcome. At the end of the 2-year follow-up period, perioperative and patient-reported outcomes were not significantly different between the groups.
Dr. Enklaar said she was surprised by the findings. “At the start of this study, we hypothesized that there would be no difference between the two techniques,” as both have been used for a long period of time.
However, “based on the composite outcome of success at 2-year follow-up after the primary uterus-sparing surgery for uterine descent in patients with pelvic organ prolapse, these findings indicate that the sacrospinous hysteropexy is inferior to the Manchester procedure,” she said.
The study findings were limited by several factors including the lack of blinding and the applicability of the results only to women without uterine prolapse past the hymen, as well as the exclusion of patients with higher-stage prolapse, the researchers said. However, the results suggest that sacrospinous hysteropexy is inferior to the Manchester technique for uterine-sparing pelvic organ prolapse surgery.
As for additional research, few studies of prolapse surgery with long-term follow-up data are available, Dr. Enklaar said. “It is important that this current study will be continued to see the results after a longer follow-up period. Personalized health care is increasingly important, and we need to provide adequate information when counselling patients. With studies such as this one, we hope to improve the choices regarding surgical treatment of uterine descent.”
Studies challenge current prolapse protocols
The study by Dr. Rahn and colleagues contradicts the common clinical practice of preoperative vaginal estrogen to reduce recurrence of prolapse, wrote Charles W. Nager, MD, of the University of California San Diego Health, La Jolla, in an accompanying editorial that addressed both studies.
The results suggest that use of perioperative intravaginal estrogen had no impact on outcomes, “despite the surgeon assessment of less atrophy and better vaginal apex tissue in the estrogen group,” he noted. Although vaginal estrogen has other benefits in terms of patient symptoms and effects on the vaginal epithelium, “surgeons should not prescribe vaginal estrogen with the expectation that it will improve surgical success.”
The study by Dr. Enklaar and colleagues reflects the growing interest in uterine-conserving procedures, Dr. Nager wrote. The modified Manchester procedure conforms to professional society guidelines, and the composite outcome conforms to current standards for the treatment of pelvic organ prolapse.
Although suspension of the vaginal apex was quite successful, the researchers interpreted their noninferiority findings with caution, said Dr. Nager. However, they suggested that the modified Manchester procedure as performed in their study “has a role in modern prolapse surgical repair for women with uterine descent that does not protrude beyond the hymen.”
The vaginal estrogen study was supported by the National Institute on Aging, a Bridge Award from the American Board of Obstetrics & Gynecology and the American Association of Obstetricians and Gynecologists Foundation. Dr. Rahn disclosed grants from the National Institute on Aging, the American Board of Obstetrics & Gynecology, and the AAOGF bridge award, as well as nonfinancial support from National Center for Advancing Translational Sciences and Pfizer during the study. The uterine prolapse study was supported by the Netherlands Organisation for Health Research and Development. The researchers had no financial conflicts to disclose. Dr. Nager had no financial conflicts to disclose.
FROM JAMA
Vulvodynia: A little-known and treatable condition
Vulvodynia is a little-known condition that, according to some U.S. studies, affects 3%-14% of the female population. It is defined as chronic pain, present for at least 3 months, that generally involves the vulva or some of its specific areas such as the clitoris or vestibule and is not attributable to causes of an infectious, inflammatory, oncologic, or endocrine nature; skin trauma; or damage to nerve fibers.
“There are probably many more women who suffer from it who don’t talk about it out of shame, because they feel ‘wrong,’ ” said gynecologist Pina Belfiore, MD, chair of the Italian Interdisciplinary Society of Vulvology, at the annual conference of the Italian Society of Gender Medicine in Neurosciences. “It is a treatable condition, or at the very least, a patient’s quality of life can be significantly improved with a personalized therapeutic approach.”
The correct diagnosis
The first step for setting the patient on the right course toward recovery is to offer welcome and empathy, recognizing that the suffering, which can have psychological causes, is not imaginary. “We need to explain to patients that their condition has a name, that they are not alone in this situation, and, above all, that there is hope for solving the problem. They can get through it,” said Dr. Belfiore.
First, an accurate history of the pain is needed to correctly diagnose vulvodynia. How long has the pain been going on? Is it continuous or is it triggered by an environmental factor, for example by sexual intercourse or contact with underwear? Is it a burning or stinging sensation? Did it first occur after an infection or after a physical or psychological trauma? Does the patient suffer from other forms of chronic pain such as recurring headaches or fibromyalgia?
“It is then necessary to inspect the vulva to exclude other systematic conditions or injuries that may be responsible for the pain, as well as to locate hypersensitive areas and evaluate the intensity of the symptoms,” said Dr. Belfiore.” A swab test is performed for this purpose, which is carried out by applying light pressure on different points of the vulva with a cotton swab.”
CNS dysfunction
, which confuses signals coming from the peripheral area, interpreting signals of a different nature as painful stimuli.
“The origin of this dysfunction is an individual predisposition. In fact, often the women who suffer from it are also affected by other forms of chronic pain,” said Dr. Belfiore. “Triggers for vulvodynia can be bacterial infections, candidiasis, or traumatic events such as surgically assisted birth or psychological trauma.”
Because inflammatory mechanisms are not involved, anti-inflammatory drugs are not helpful in treating the problem. “Instead, it is necessary to reduce the sensitivity of the CNS. For this purpose, low-dose antidepressant or antiepileptic drugs are used,” said Dr. Belfiore. “Pelvic floor rehabilitation is another treatment that can be beneficial when combined with pharmacologic treatment. This should be conducted by a professional with specific experience in vulvodynia, because an excessive increase in the tone of the levator ani muscle can make the situation worse. Psychotherapy and the adoption of certain hygienic and behavioral measures can also help, such as using lubricant during sexual intercourse, wearing pure cotton underwear, and using gentle intimate body washes.”
“It is important that family doctors who see women with this problem refer them to an experienced specialist,” said Dr. Belfiore.
A version of this article first appeared on Medscape.com.
This article was translated from Univadis Italy, which is part of the Medscape Professional Network.
Vulvodynia is a little-known condition that, according to some U.S. studies, affects 3%-14% of the female population. It is defined as chronic pain, present for at least 3 months, that generally involves the vulva or some of its specific areas such as the clitoris or vestibule and is not attributable to causes of an infectious, inflammatory, oncologic, or endocrine nature; skin trauma; or damage to nerve fibers.
“There are probably many more women who suffer from it who don’t talk about it out of shame, because they feel ‘wrong,’ ” said gynecologist Pina Belfiore, MD, chair of the Italian Interdisciplinary Society of Vulvology, at the annual conference of the Italian Society of Gender Medicine in Neurosciences. “It is a treatable condition, or at the very least, a patient’s quality of life can be significantly improved with a personalized therapeutic approach.”
The correct diagnosis
The first step for setting the patient on the right course toward recovery is to offer welcome and empathy, recognizing that the suffering, which can have psychological causes, is not imaginary. “We need to explain to patients that their condition has a name, that they are not alone in this situation, and, above all, that there is hope for solving the problem. They can get through it,” said Dr. Belfiore.
First, an accurate history of the pain is needed to correctly diagnose vulvodynia. How long has the pain been going on? Is it continuous or is it triggered by an environmental factor, for example by sexual intercourse or contact with underwear? Is it a burning or stinging sensation? Did it first occur after an infection or after a physical or psychological trauma? Does the patient suffer from other forms of chronic pain such as recurring headaches or fibromyalgia?
“It is then necessary to inspect the vulva to exclude other systematic conditions or injuries that may be responsible for the pain, as well as to locate hypersensitive areas and evaluate the intensity of the symptoms,” said Dr. Belfiore.” A swab test is performed for this purpose, which is carried out by applying light pressure on different points of the vulva with a cotton swab.”
CNS dysfunction
, which confuses signals coming from the peripheral area, interpreting signals of a different nature as painful stimuli.
“The origin of this dysfunction is an individual predisposition. In fact, often the women who suffer from it are also affected by other forms of chronic pain,” said Dr. Belfiore. “Triggers for vulvodynia can be bacterial infections, candidiasis, or traumatic events such as surgically assisted birth or psychological trauma.”
Because inflammatory mechanisms are not involved, anti-inflammatory drugs are not helpful in treating the problem. “Instead, it is necessary to reduce the sensitivity of the CNS. For this purpose, low-dose antidepressant or antiepileptic drugs are used,” said Dr. Belfiore. “Pelvic floor rehabilitation is another treatment that can be beneficial when combined with pharmacologic treatment. This should be conducted by a professional with specific experience in vulvodynia, because an excessive increase in the tone of the levator ani muscle can make the situation worse. Psychotherapy and the adoption of certain hygienic and behavioral measures can also help, such as using lubricant during sexual intercourse, wearing pure cotton underwear, and using gentle intimate body washes.”
“It is important that family doctors who see women with this problem refer them to an experienced specialist,” said Dr. Belfiore.
A version of this article first appeared on Medscape.com.
This article was translated from Univadis Italy, which is part of the Medscape Professional Network.
Vulvodynia is a little-known condition that, according to some U.S. studies, affects 3%-14% of the female population. It is defined as chronic pain, present for at least 3 months, that generally involves the vulva or some of its specific areas such as the clitoris or vestibule and is not attributable to causes of an infectious, inflammatory, oncologic, or endocrine nature; skin trauma; or damage to nerve fibers.
“There are probably many more women who suffer from it who don’t talk about it out of shame, because they feel ‘wrong,’ ” said gynecologist Pina Belfiore, MD, chair of the Italian Interdisciplinary Society of Vulvology, at the annual conference of the Italian Society of Gender Medicine in Neurosciences. “It is a treatable condition, or at the very least, a patient’s quality of life can be significantly improved with a personalized therapeutic approach.”
The correct diagnosis
The first step for setting the patient on the right course toward recovery is to offer welcome and empathy, recognizing that the suffering, which can have psychological causes, is not imaginary. “We need to explain to patients that their condition has a name, that they are not alone in this situation, and, above all, that there is hope for solving the problem. They can get through it,” said Dr. Belfiore.
First, an accurate history of the pain is needed to correctly diagnose vulvodynia. How long has the pain been going on? Is it continuous or is it triggered by an environmental factor, for example by sexual intercourse or contact with underwear? Is it a burning or stinging sensation? Did it first occur after an infection or after a physical or psychological trauma? Does the patient suffer from other forms of chronic pain such as recurring headaches or fibromyalgia?
“It is then necessary to inspect the vulva to exclude other systematic conditions or injuries that may be responsible for the pain, as well as to locate hypersensitive areas and evaluate the intensity of the symptoms,” said Dr. Belfiore.” A swab test is performed for this purpose, which is carried out by applying light pressure on different points of the vulva with a cotton swab.”
CNS dysfunction
, which confuses signals coming from the peripheral area, interpreting signals of a different nature as painful stimuli.
“The origin of this dysfunction is an individual predisposition. In fact, often the women who suffer from it are also affected by other forms of chronic pain,” said Dr. Belfiore. “Triggers for vulvodynia can be bacterial infections, candidiasis, or traumatic events such as surgically assisted birth or psychological trauma.”
Because inflammatory mechanisms are not involved, anti-inflammatory drugs are not helpful in treating the problem. “Instead, it is necessary to reduce the sensitivity of the CNS. For this purpose, low-dose antidepressant or antiepileptic drugs are used,” said Dr. Belfiore. “Pelvic floor rehabilitation is another treatment that can be beneficial when combined with pharmacologic treatment. This should be conducted by a professional with specific experience in vulvodynia, because an excessive increase in the tone of the levator ani muscle can make the situation worse. Psychotherapy and the adoption of certain hygienic and behavioral measures can also help, such as using lubricant during sexual intercourse, wearing pure cotton underwear, and using gentle intimate body washes.”
“It is important that family doctors who see women with this problem refer them to an experienced specialist,” said Dr. Belfiore.
A version of this article first appeared on Medscape.com.
This article was translated from Univadis Italy, which is part of the Medscape Professional Network.
Best practices document outlines genitourinary applications of lasers and energy-based devices
PHOENIX –
“Even a cursory review of PubMed today yields over 100,000 results” on this topic, Macrene R. Alexiades, MD, PhD, associate clinical professor of dermatology at Yale University, New Haven, Conn., said at the annual conference of the American Society for Laser Medicine and Surgery. “Add to that radiofrequency and various diagnoses, the number of publications has skyrocketed, particularly over the last 10 years.”
What has been missing from this hot research topic all these years, she continued, is that no one has distilled this pile of data into a practical guide for office-based clinicians who use lasers and energy-based devices for genitourinary conditions – until now. Working with experts in gynecology and urogynecology, Dr. Alexiades spearheaded a 2-year-long effort to assemble a document on optimal protocols and best practices for genitourinary application of lasers and energy-based devices. The document, published soon after the ASLMS meeting in Lasers in Medicine and Surgery, includes a table that lists the current Food and Drug Administration approval status of devices in genitourinary applications, as well as individual sections dedicated to fractional lasers, radiofrequency (RF) devices, and high-intensity focused electromagnetic technology. It concludes with a section on the current status of clearances and future pathways.
“The work we did was exhaustive,” said Dr. Alexiades, who is also founder and director of Dermatology & Laser Surgery Center of New York. “We went through all the clinical trial data and compiled the parameters that, as a consensus, we agree are best practices for each technology for which we had rigorous published data.”
The document contains a brief background on the history of the devices used for genitourinary issues and it addresses core topics for each technology, such as conditions treated, contraindications, preoperative physical assessment and preparation, perioperative protocols, and postoperative care.
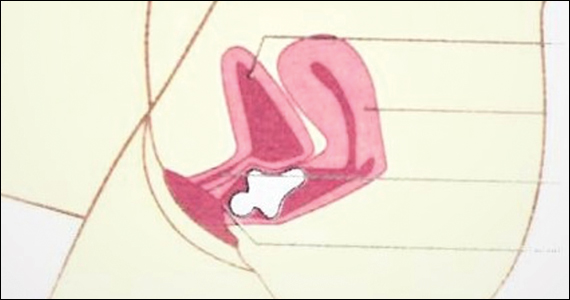
Contraindications to the genitourinary use of lasers and energy-based devices are numerous and include use of an intrauterine device, active urinary tract or genital infection, vaginal bleeding, current pregnancy, active or recent malignancy, having an electrical implant anywhere in the body, significant concurrent illness, and an anticoagulative or thromboembolic condition or taking anticoagulant medications 1 week prior to the procedure. Another condition to screen for is advanced prolapse, which was considered a contraindication in all clinical trials, she added. “It’s important that you’re able to do the speculum exam and stage the prolapse” so that a patient with this contraindication is not treated.
Dr. Alexiades shared the following highlights from the document’s section related to the use of fractional CO2 lasers:
Preoperative management. Schedule the treatment one week after the patient’s menstrual period. Patients should avoid blood thinners for 7 days and avoid intercourse the night before the procedure. Reschedule in the case of fever, chills, or vaginal bleeding or discharge.
Preoperative physical exam and testing. A normal speculum exam and a recent negative PAP smear are required. For those of child-bearing potential, a pregnancy test is warranted. Obtain written and verbal consent, including discussion of all treatment options, risks, and benefits. No topical or local anesthesia is necessary internally. “Externally, we sometimes apply topical lidocaine gel, but I have found that’s not necessary in most cases,” Dr. Alexiades said. “The treatment is so quick.”
Peri-operative management. In general, device settings are provided by the manufacturer. “For most of the studies that had successful outcomes and no adverse events, researchers adhered to the mild or moderate settings on the technology,” she said. Energy settings were between 15 and 30 watts, delivered at a laser fluence of about 250-300 mJ/cm2 with a spacing of microbeams 1 mm apart. Typically, three treatments are done at 1-month intervals and maintenance treatments are recommended at 6 and 12 months based on duration of the outcomes.
Vulvovaginal postoperative management. A 3-day recovery time is recommended with avoidance of intercourse during this period, because “re-epithelialization is usually complete in 3 days, so we want to give the opportunity for the lining to heal prior to introducing any friction, Dr. Alexiades said.” Rarely, spotting or discharge may occur and there should be no discomfort. “Any severe discomfort or burning may potentially signify infection and should prompt evaluation and possibly vaginal cultures. The patient can shower, but we recommend avoiding seated baths to decrease any introduction of infectious agents.”
Patients should be followed up monthly until three treatments are completed, and a maintenance treatment is considered appropriate between 6 and 12 months. “I do recommend doing a 1-month follow-up following the final treatment, unless it’s a patient who has already had a series of three treatments and is coming in for maintenance,” she said.
In a study from her own practice, Dr. Alexiades evaluated a series of three fractional CO2 laser treatments to the vulva and vagina with a 1-year follow-up in postmenopausal patients. She used the Vaginal Health Index (VHI) to assess changes in vaginal elasticity, fluid volume, vaginal pH, epithelial integrity, and moisture. She and her colleagues discovered that there was improvement in every VHI category after treatment and during the follow-up interval up to 6 months.
“Between 6 and 12 months, we started to see a return a bit toward baseline on all of these parameters,” she said. “The serendipitous discovery that I made during the course of that study was that early intervention improves outcomes. I observed that the younger, most recently postmenopausal cohort seemed to attain normal or near normal VHI quicker than the more extended postmenopausal cohorts.”
In an editorial published in 2020, Dr. Alexiades reviewed the effects of fractional CO2 laser treatment of vulvar skin on vaginal pH and referred to a study she conducted that found that the mean baseline pH pretreatment was 6.32 in the cohort of postmenopausal patients, and was reduced after 3 treatments. “Postmenopausally, the normal acidic pH becomes alkaline,” she said. But she did not expect to see an additional reduction in pH following the treatment out to 6 months. “This indicates that, whatever the wound healing and other restorative effects of these devices are, they seem to continue out to 6 months, at which point it turns around and moves toward baseline [levels].”
Dr. Alexiades highlighted two published meta-analyses of studies related to the genitourinary use of lasers and energy-based devices. One included 59 studies of 3,609 women treated for vaginal rejuvenation using either radiofrequency or fractional ablative laser therapy. The studies reported improvements in symptoms of GSM/VVA and sexual function, high patient satisfaction, with minor adverse events, including treatment-associated vaginal swelling or vaginal discharge.
“Further research needs to be completed to determine which specific pathologies can be treated, if maintenance treatment is necessary, and long-term safety concerns,” the authors concluded.
In another review, researchers analyzed 64 studies related to vaginal laser therapy for GSM. Of these, 47 were before and after studies without a control group, 10 were controlled intervention studies, and 7 were observational cohort and cross-sectional studies.
Vaginal laser treatment “seems to improve scores on the visual analogue scale, Female Sexual Function Index, and the Vaginal Health Index over the short term,” the authors wrote. “Safety outcomes are underreported and short term. Further well-designed clinical trials with sham-laser control groups and evaluating objective variables are needed to provide the best evidence on efficacy.”
“Lasers and energy-based devices are now considered alternative therapeutic modalities for genitourinary conditions,” Dr. Alexiades concluded. “The shortcomings in the literature with respect to lasers and device treatments demonstrate the need for the consensus on best practices and protocols.”
During a separate presentation at the meeting, Michael Gold, MD, highlighted data from Grand View Research, a market research database, which estimated that the global women’s health and wellness market is valued at more than $31 billion globally and is expected to grow at a compound annual growth rate of 4.8% from 2022 to 2030.
“Sales of women’s health energy-based devices continue to grow as new technologies are developed,” said Dr. Gold, a Nashville, Tenn.–based dermatologist and cosmetic surgeon who is also editor-in-chief of the Journal of Cosmetic Dermatology. “Evolving societal norms have made discussions about feminine health issues acceptable. Suffering in silence is no longer necessary or advocated.”
Dr. Alexiades disclosed that she has conducted research for Candela Lasers, Lumenis, Allergan/AbbVie, InMode, and Endymed. She is also the founder and CEO of Macrene Actives. Dr. Gold disclosed that he is a consultant to and/or an investigator and a speaker for Joylux, InMode, and Alma Lasers.
PHOENIX –
“Even a cursory review of PubMed today yields over 100,000 results” on this topic, Macrene R. Alexiades, MD, PhD, associate clinical professor of dermatology at Yale University, New Haven, Conn., said at the annual conference of the American Society for Laser Medicine and Surgery. “Add to that radiofrequency and various diagnoses, the number of publications has skyrocketed, particularly over the last 10 years.”
What has been missing from this hot research topic all these years, she continued, is that no one has distilled this pile of data into a practical guide for office-based clinicians who use lasers and energy-based devices for genitourinary conditions – until now. Working with experts in gynecology and urogynecology, Dr. Alexiades spearheaded a 2-year-long effort to assemble a document on optimal protocols and best practices for genitourinary application of lasers and energy-based devices. The document, published soon after the ASLMS meeting in Lasers in Medicine and Surgery, includes a table that lists the current Food and Drug Administration approval status of devices in genitourinary applications, as well as individual sections dedicated to fractional lasers, radiofrequency (RF) devices, and high-intensity focused electromagnetic technology. It concludes with a section on the current status of clearances and future pathways.
“The work we did was exhaustive,” said Dr. Alexiades, who is also founder and director of Dermatology & Laser Surgery Center of New York. “We went through all the clinical trial data and compiled the parameters that, as a consensus, we agree are best practices for each technology for which we had rigorous published data.”
The document contains a brief background on the history of the devices used for genitourinary issues and it addresses core topics for each technology, such as conditions treated, contraindications, preoperative physical assessment and preparation, perioperative protocols, and postoperative care.
Contraindications to the genitourinary use of lasers and energy-based devices are numerous and include use of an intrauterine device, active urinary tract or genital infection, vaginal bleeding, current pregnancy, active or recent malignancy, having an electrical implant anywhere in the body, significant concurrent illness, and an anticoagulative or thromboembolic condition or taking anticoagulant medications 1 week prior to the procedure. Another condition to screen for is advanced prolapse, which was considered a contraindication in all clinical trials, she added. “It’s important that you’re able to do the speculum exam and stage the prolapse” so that a patient with this contraindication is not treated.
Dr. Alexiades shared the following highlights from the document’s section related to the use of fractional CO2 lasers:
Preoperative management. Schedule the treatment one week after the patient’s menstrual period. Patients should avoid blood thinners for 7 days and avoid intercourse the night before the procedure. Reschedule in the case of fever, chills, or vaginal bleeding or discharge.
Preoperative physical exam and testing. A normal speculum exam and a recent negative PAP smear are required. For those of child-bearing potential, a pregnancy test is warranted. Obtain written and verbal consent, including discussion of all treatment options, risks, and benefits. No topical or local anesthesia is necessary internally. “Externally, we sometimes apply topical lidocaine gel, but I have found that’s not necessary in most cases,” Dr. Alexiades said. “The treatment is so quick.”
Peri-operative management. In general, device settings are provided by the manufacturer. “For most of the studies that had successful outcomes and no adverse events, researchers adhered to the mild or moderate settings on the technology,” she said. Energy settings were between 15 and 30 watts, delivered at a laser fluence of about 250-300 mJ/cm2 with a spacing of microbeams 1 mm apart. Typically, three treatments are done at 1-month intervals and maintenance treatments are recommended at 6 and 12 months based on duration of the outcomes.
Vulvovaginal postoperative management. A 3-day recovery time is recommended with avoidance of intercourse during this period, because “re-epithelialization is usually complete in 3 days, so we want to give the opportunity for the lining to heal prior to introducing any friction, Dr. Alexiades said.” Rarely, spotting or discharge may occur and there should be no discomfort. “Any severe discomfort or burning may potentially signify infection and should prompt evaluation and possibly vaginal cultures. The patient can shower, but we recommend avoiding seated baths to decrease any introduction of infectious agents.”
Patients should be followed up monthly until three treatments are completed, and a maintenance treatment is considered appropriate between 6 and 12 months. “I do recommend doing a 1-month follow-up following the final treatment, unless it’s a patient who has already had a series of three treatments and is coming in for maintenance,” she said.
In a study from her own practice, Dr. Alexiades evaluated a series of three fractional CO2 laser treatments to the vulva and vagina with a 1-year follow-up in postmenopausal patients. She used the Vaginal Health Index (VHI) to assess changes in vaginal elasticity, fluid volume, vaginal pH, epithelial integrity, and moisture. She and her colleagues discovered that there was improvement in every VHI category after treatment and during the follow-up interval up to 6 months.
“Between 6 and 12 months, we started to see a return a bit toward baseline on all of these parameters,” she said. “The serendipitous discovery that I made during the course of that study was that early intervention improves outcomes. I observed that the younger, most recently postmenopausal cohort seemed to attain normal or near normal VHI quicker than the more extended postmenopausal cohorts.”
In an editorial published in 2020, Dr. Alexiades reviewed the effects of fractional CO2 laser treatment of vulvar skin on vaginal pH and referred to a study she conducted that found that the mean baseline pH pretreatment was 6.32 in the cohort of postmenopausal patients, and was reduced after 3 treatments. “Postmenopausally, the normal acidic pH becomes alkaline,” she said. But she did not expect to see an additional reduction in pH following the treatment out to 6 months. “This indicates that, whatever the wound healing and other restorative effects of these devices are, they seem to continue out to 6 months, at which point it turns around and moves toward baseline [levels].”
Dr. Alexiades highlighted two published meta-analyses of studies related to the genitourinary use of lasers and energy-based devices. One included 59 studies of 3,609 women treated for vaginal rejuvenation using either radiofrequency or fractional ablative laser therapy. The studies reported improvements in symptoms of GSM/VVA and sexual function, high patient satisfaction, with minor adverse events, including treatment-associated vaginal swelling or vaginal discharge.
“Further research needs to be completed to determine which specific pathologies can be treated, if maintenance treatment is necessary, and long-term safety concerns,” the authors concluded.
In another review, researchers analyzed 64 studies related to vaginal laser therapy for GSM. Of these, 47 were before and after studies without a control group, 10 were controlled intervention studies, and 7 were observational cohort and cross-sectional studies.
Vaginal laser treatment “seems to improve scores on the visual analogue scale, Female Sexual Function Index, and the Vaginal Health Index over the short term,” the authors wrote. “Safety outcomes are underreported and short term. Further well-designed clinical trials with sham-laser control groups and evaluating objective variables are needed to provide the best evidence on efficacy.”
“Lasers and energy-based devices are now considered alternative therapeutic modalities for genitourinary conditions,” Dr. Alexiades concluded. “The shortcomings in the literature with respect to lasers and device treatments demonstrate the need for the consensus on best practices and protocols.”
During a separate presentation at the meeting, Michael Gold, MD, highlighted data from Grand View Research, a market research database, which estimated that the global women’s health and wellness market is valued at more than $31 billion globally and is expected to grow at a compound annual growth rate of 4.8% from 2022 to 2030.
“Sales of women’s health energy-based devices continue to grow as new technologies are developed,” said Dr. Gold, a Nashville, Tenn.–based dermatologist and cosmetic surgeon who is also editor-in-chief of the Journal of Cosmetic Dermatology. “Evolving societal norms have made discussions about feminine health issues acceptable. Suffering in silence is no longer necessary or advocated.”
Dr. Alexiades disclosed that she has conducted research for Candela Lasers, Lumenis, Allergan/AbbVie, InMode, and Endymed. She is also the founder and CEO of Macrene Actives. Dr. Gold disclosed that he is a consultant to and/or an investigator and a speaker for Joylux, InMode, and Alma Lasers.
PHOENIX –
“Even a cursory review of PubMed today yields over 100,000 results” on this topic, Macrene R. Alexiades, MD, PhD, associate clinical professor of dermatology at Yale University, New Haven, Conn., said at the annual conference of the American Society for Laser Medicine and Surgery. “Add to that radiofrequency and various diagnoses, the number of publications has skyrocketed, particularly over the last 10 years.”
What has been missing from this hot research topic all these years, she continued, is that no one has distilled this pile of data into a practical guide for office-based clinicians who use lasers and energy-based devices for genitourinary conditions – until now. Working with experts in gynecology and urogynecology, Dr. Alexiades spearheaded a 2-year-long effort to assemble a document on optimal protocols and best practices for genitourinary application of lasers and energy-based devices. The document, published soon after the ASLMS meeting in Lasers in Medicine and Surgery, includes a table that lists the current Food and Drug Administration approval status of devices in genitourinary applications, as well as individual sections dedicated to fractional lasers, radiofrequency (RF) devices, and high-intensity focused electromagnetic technology. It concludes with a section on the current status of clearances and future pathways.
“The work we did was exhaustive,” said Dr. Alexiades, who is also founder and director of Dermatology & Laser Surgery Center of New York. “We went through all the clinical trial data and compiled the parameters that, as a consensus, we agree are best practices for each technology for which we had rigorous published data.”
The document contains a brief background on the history of the devices used for genitourinary issues and it addresses core topics for each technology, such as conditions treated, contraindications, preoperative physical assessment and preparation, perioperative protocols, and postoperative care.
Contraindications to the genitourinary use of lasers and energy-based devices are numerous and include use of an intrauterine device, active urinary tract or genital infection, vaginal bleeding, current pregnancy, active or recent malignancy, having an electrical implant anywhere in the body, significant concurrent illness, and an anticoagulative or thromboembolic condition or taking anticoagulant medications 1 week prior to the procedure. Another condition to screen for is advanced prolapse, which was considered a contraindication in all clinical trials, she added. “It’s important that you’re able to do the speculum exam and stage the prolapse” so that a patient with this contraindication is not treated.
Dr. Alexiades shared the following highlights from the document’s section related to the use of fractional CO2 lasers:
Preoperative management. Schedule the treatment one week after the patient’s menstrual period. Patients should avoid blood thinners for 7 days and avoid intercourse the night before the procedure. Reschedule in the case of fever, chills, or vaginal bleeding or discharge.
Preoperative physical exam and testing. A normal speculum exam and a recent negative PAP smear are required. For those of child-bearing potential, a pregnancy test is warranted. Obtain written and verbal consent, including discussion of all treatment options, risks, and benefits. No topical or local anesthesia is necessary internally. “Externally, we sometimes apply topical lidocaine gel, but I have found that’s not necessary in most cases,” Dr. Alexiades said. “The treatment is so quick.”
Peri-operative management. In general, device settings are provided by the manufacturer. “For most of the studies that had successful outcomes and no adverse events, researchers adhered to the mild or moderate settings on the technology,” she said. Energy settings were between 15 and 30 watts, delivered at a laser fluence of about 250-300 mJ/cm2 with a spacing of microbeams 1 mm apart. Typically, three treatments are done at 1-month intervals and maintenance treatments are recommended at 6 and 12 months based on duration of the outcomes.
Vulvovaginal postoperative management. A 3-day recovery time is recommended with avoidance of intercourse during this period, because “re-epithelialization is usually complete in 3 days, so we want to give the opportunity for the lining to heal prior to introducing any friction, Dr. Alexiades said.” Rarely, spotting or discharge may occur and there should be no discomfort. “Any severe discomfort or burning may potentially signify infection and should prompt evaluation and possibly vaginal cultures. The patient can shower, but we recommend avoiding seated baths to decrease any introduction of infectious agents.”
Patients should be followed up monthly until three treatments are completed, and a maintenance treatment is considered appropriate between 6 and 12 months. “I do recommend doing a 1-month follow-up following the final treatment, unless it’s a patient who has already had a series of three treatments and is coming in for maintenance,” she said.
In a study from her own practice, Dr. Alexiades evaluated a series of three fractional CO2 laser treatments to the vulva and vagina with a 1-year follow-up in postmenopausal patients. She used the Vaginal Health Index (VHI) to assess changes in vaginal elasticity, fluid volume, vaginal pH, epithelial integrity, and moisture. She and her colleagues discovered that there was improvement in every VHI category after treatment and during the follow-up interval up to 6 months.
“Between 6 and 12 months, we started to see a return a bit toward baseline on all of these parameters,” she said. “The serendipitous discovery that I made during the course of that study was that early intervention improves outcomes. I observed that the younger, most recently postmenopausal cohort seemed to attain normal or near normal VHI quicker than the more extended postmenopausal cohorts.”
In an editorial published in 2020, Dr. Alexiades reviewed the effects of fractional CO2 laser treatment of vulvar skin on vaginal pH and referred to a study she conducted that found that the mean baseline pH pretreatment was 6.32 in the cohort of postmenopausal patients, and was reduced after 3 treatments. “Postmenopausally, the normal acidic pH becomes alkaline,” she said. But she did not expect to see an additional reduction in pH following the treatment out to 6 months. “This indicates that, whatever the wound healing and other restorative effects of these devices are, they seem to continue out to 6 months, at which point it turns around and moves toward baseline [levels].”
Dr. Alexiades highlighted two published meta-analyses of studies related to the genitourinary use of lasers and energy-based devices. One included 59 studies of 3,609 women treated for vaginal rejuvenation using either radiofrequency or fractional ablative laser therapy. The studies reported improvements in symptoms of GSM/VVA and sexual function, high patient satisfaction, with minor adverse events, including treatment-associated vaginal swelling or vaginal discharge.
“Further research needs to be completed to determine which specific pathologies can be treated, if maintenance treatment is necessary, and long-term safety concerns,” the authors concluded.
In another review, researchers analyzed 64 studies related to vaginal laser therapy for GSM. Of these, 47 were before and after studies without a control group, 10 were controlled intervention studies, and 7 were observational cohort and cross-sectional studies.
Vaginal laser treatment “seems to improve scores on the visual analogue scale, Female Sexual Function Index, and the Vaginal Health Index over the short term,” the authors wrote. “Safety outcomes are underreported and short term. Further well-designed clinical trials with sham-laser control groups and evaluating objective variables are needed to provide the best evidence on efficacy.”
“Lasers and energy-based devices are now considered alternative therapeutic modalities for genitourinary conditions,” Dr. Alexiades concluded. “The shortcomings in the literature with respect to lasers and device treatments demonstrate the need for the consensus on best practices and protocols.”
During a separate presentation at the meeting, Michael Gold, MD, highlighted data from Grand View Research, a market research database, which estimated that the global women’s health and wellness market is valued at more than $31 billion globally and is expected to grow at a compound annual growth rate of 4.8% from 2022 to 2030.
“Sales of women’s health energy-based devices continue to grow as new technologies are developed,” said Dr. Gold, a Nashville, Tenn.–based dermatologist and cosmetic surgeon who is also editor-in-chief of the Journal of Cosmetic Dermatology. “Evolving societal norms have made discussions about feminine health issues acceptable. Suffering in silence is no longer necessary or advocated.”
Dr. Alexiades disclosed that she has conducted research for Candela Lasers, Lumenis, Allergan/AbbVie, InMode, and Endymed. She is also the founder and CEO of Macrene Actives. Dr. Gold disclosed that he is a consultant to and/or an investigator and a speaker for Joylux, InMode, and Alma Lasers.
AT ASLMS 2023
Wireless neurostimulation safe for urge incontinence
CHICAGO – , according to new findings presented at the 2023 annual meeting of the American Urological Association.
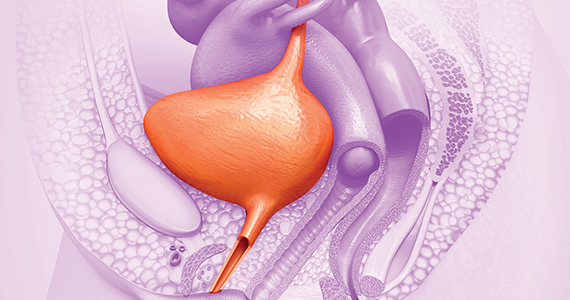
As many as half of women in the United States aged 60 and older will experience urinary incontinence. Of those, roughly one in four experience urge urinary incontinence, marked by a sudden need to void that cannot be fully suppressed.
Researchers studied the benefits of the RENOVA iStim (BlueWind Medical) implantable tibial neuromodulation system for the treatment of overactive bladder in the OASIS trial.
Study investigator Roger R. Dmochowski, MD, MMHC, professor of urology and surgery and associate surgeon-in-chief at Vanderbilt University Medical Center, Nashville, Tenn., said the first-line treatment of urinary incontinence is lifestyle changes to retrain the bladder or physical therapy, including pelvic floor and Kegel exercises, per AUA guidelines. He said the success rate is about 30% and is not sustained. Second-line treatments include medications, which most (60%) patients stop taking by 6 months.
More than three-quarters of the 151 women who received the device responded to therapy at 1 year, and 84.6% of the patients showed improvement, according to Dr. Dmochowski.
The participants (mean age, 58.8) demonstrated a mean baseline of 4.8 urge incidents per day (standard deviation, 2.9) and 10 voids/day (SD, 3.3). No device or procedure-related serious adverse events were reported at 12 months. Half of the women no longer had symptoms on three consecutive days, Dr. Dmochowski said.
Because urge urinary incontinence is a chronic condition, “treatment with the BlueWind System will be ongoing, with frequency determined based on the patient’s response,” Dr. Dmochowski said. “The patient is then empowered to control when and where they perform therapy.”
“The device is activated by the external wearable. It’s like an on-off switch. It has a receiver within it that basically has the capacity to be turned on and off by the wearable, which is the control device. The device is in an off-position until the wearable is applied,” he said.
He said the device should be worn twice a day for about 20 minutes, with many patients using it less.
Only one implanted tibial neuromodulation device has been approved by the Food and Drug Administration – eCOIN (Valencia Technologies). The RENOVA iStim is an investigational device under review by the FDA, Dr. Dmochowski said.
In installing the device, Dr. Dmochowski said urologists use a subfascial technique to enable direct visualization of the tibial nerve and suture fixation that increases the possibility of a predictable placement. Patients use an external wearable, which activates the implant, without concern for battery longevity or replacement.
“This therapy is not associated with any adverse effects and may be beneficial for patients who do not respond to other treatments for OAB such as medications or Botox,” said Carol E. Bretschneider, MD, a urogynecologic and pelvic surgeon at Northwestern Medicine Central DuPage Hospital, outside Chicago. “Neurostimulators can be a great advanced therapy option for patients who do not respond to more conservative treatments or cannot take or tolerate a medication.”
The devices do not stimulate or strengthen muscles but act by modulating the reflexes that influence the bladder, sphincter, and pelvic floor, added Dr. Bretschneider, who was not involved in the study.
Other treatments for urge incontinence can include acupuncture, or percutaneous tibial nerve stimulation, to target the posterior tibial nerve in the ankle, which shares the same nerve root that controls the bladder, according to Aron Liaw, MD, a reconstructive urologist and assistant professor of urology at Wayne State University in Detroit. This treatment has been shown to be at least as effective as available medications, but with fewer side effects, he said.
But regular stimulation is necessary to achieve and preserve efficacy, he said.
Dr. Liaw, who was not involved in the neuromodulation study, said the benefits of a device like Renova iStim are that implantation is relatively easy and can be performed in office settings, and patients can then treat themselves at home. However, because the new study did not compare the device to other treatments or a placebo device, its relative benefits are unclear, he said,
Other treatments for urge urinary incontinence, such as bladder Botox and sacral neuromodulation, also are minimally invasive and have proven benefit, “so a device like this could well be less effective with little other advantage,” he said.
“Lifestyle changes can make a big difference, but making big lifestyle changes is not always easy,” added Dr. Liaw. “I have found neuromodulation [to be] very effective, especially in conjunction with lifestyle changes.”
BlueWind Medical funds the OASIS trial. Dr. Dmochowski reported he received no grants nor has any relevant financial relationships. Dr. Bretschneider and Dr. Liaw report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CHICAGO – , according to new findings presented at the 2023 annual meeting of the American Urological Association.
As many as half of women in the United States aged 60 and older will experience urinary incontinence. Of those, roughly one in four experience urge urinary incontinence, marked by a sudden need to void that cannot be fully suppressed.
Researchers studied the benefits of the RENOVA iStim (BlueWind Medical) implantable tibial neuromodulation system for the treatment of overactive bladder in the OASIS trial.
Study investigator Roger R. Dmochowski, MD, MMHC, professor of urology and surgery and associate surgeon-in-chief at Vanderbilt University Medical Center, Nashville, Tenn., said the first-line treatment of urinary incontinence is lifestyle changes to retrain the bladder or physical therapy, including pelvic floor and Kegel exercises, per AUA guidelines. He said the success rate is about 30% and is not sustained. Second-line treatments include medications, which most (60%) patients stop taking by 6 months.
More than three-quarters of the 151 women who received the device responded to therapy at 1 year, and 84.6% of the patients showed improvement, according to Dr. Dmochowski.
The participants (mean age, 58.8) demonstrated a mean baseline of 4.8 urge incidents per day (standard deviation, 2.9) and 10 voids/day (SD, 3.3). No device or procedure-related serious adverse events were reported at 12 months. Half of the women no longer had symptoms on three consecutive days, Dr. Dmochowski said.
Because urge urinary incontinence is a chronic condition, “treatment with the BlueWind System will be ongoing, with frequency determined based on the patient’s response,” Dr. Dmochowski said. “The patient is then empowered to control when and where they perform therapy.”
“The device is activated by the external wearable. It’s like an on-off switch. It has a receiver within it that basically has the capacity to be turned on and off by the wearable, which is the control device. The device is in an off-position until the wearable is applied,” he said.
He said the device should be worn twice a day for about 20 minutes, with many patients using it less.
Only one implanted tibial neuromodulation device has been approved by the Food and Drug Administration – eCOIN (Valencia Technologies). The RENOVA iStim is an investigational device under review by the FDA, Dr. Dmochowski said.
In installing the device, Dr. Dmochowski said urologists use a subfascial technique to enable direct visualization of the tibial nerve and suture fixation that increases the possibility of a predictable placement. Patients use an external wearable, which activates the implant, without concern for battery longevity or replacement.
“This therapy is not associated with any adverse effects and may be beneficial for patients who do not respond to other treatments for OAB such as medications or Botox,” said Carol E. Bretschneider, MD, a urogynecologic and pelvic surgeon at Northwestern Medicine Central DuPage Hospital, outside Chicago. “Neurostimulators can be a great advanced therapy option for patients who do not respond to more conservative treatments or cannot take or tolerate a medication.”
The devices do not stimulate or strengthen muscles but act by modulating the reflexes that influence the bladder, sphincter, and pelvic floor, added Dr. Bretschneider, who was not involved in the study.
Other treatments for urge incontinence can include acupuncture, or percutaneous tibial nerve stimulation, to target the posterior tibial nerve in the ankle, which shares the same nerve root that controls the bladder, according to Aron Liaw, MD, a reconstructive urologist and assistant professor of urology at Wayne State University in Detroit. This treatment has been shown to be at least as effective as available medications, but with fewer side effects, he said.
But regular stimulation is necessary to achieve and preserve efficacy, he said.
Dr. Liaw, who was not involved in the neuromodulation study, said the benefits of a device like Renova iStim are that implantation is relatively easy and can be performed in office settings, and patients can then treat themselves at home. However, because the new study did not compare the device to other treatments or a placebo device, its relative benefits are unclear, he said,
Other treatments for urge urinary incontinence, such as bladder Botox and sacral neuromodulation, also are minimally invasive and have proven benefit, “so a device like this could well be less effective with little other advantage,” he said.
“Lifestyle changes can make a big difference, but making big lifestyle changes is not always easy,” added Dr. Liaw. “I have found neuromodulation [to be] very effective, especially in conjunction with lifestyle changes.”
BlueWind Medical funds the OASIS trial. Dr. Dmochowski reported he received no grants nor has any relevant financial relationships. Dr. Bretschneider and Dr. Liaw report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CHICAGO – , according to new findings presented at the 2023 annual meeting of the American Urological Association.
As many as half of women in the United States aged 60 and older will experience urinary incontinence. Of those, roughly one in four experience urge urinary incontinence, marked by a sudden need to void that cannot be fully suppressed.
Researchers studied the benefits of the RENOVA iStim (BlueWind Medical) implantable tibial neuromodulation system for the treatment of overactive bladder in the OASIS trial.
Study investigator Roger R. Dmochowski, MD, MMHC, professor of urology and surgery and associate surgeon-in-chief at Vanderbilt University Medical Center, Nashville, Tenn., said the first-line treatment of urinary incontinence is lifestyle changes to retrain the bladder or physical therapy, including pelvic floor and Kegel exercises, per AUA guidelines. He said the success rate is about 30% and is not sustained. Second-line treatments include medications, which most (60%) patients stop taking by 6 months.
More than three-quarters of the 151 women who received the device responded to therapy at 1 year, and 84.6% of the patients showed improvement, according to Dr. Dmochowski.
The participants (mean age, 58.8) demonstrated a mean baseline of 4.8 urge incidents per day (standard deviation, 2.9) and 10 voids/day (SD, 3.3). No device or procedure-related serious adverse events were reported at 12 months. Half of the women no longer had symptoms on three consecutive days, Dr. Dmochowski said.
Because urge urinary incontinence is a chronic condition, “treatment with the BlueWind System will be ongoing, with frequency determined based on the patient’s response,” Dr. Dmochowski said. “The patient is then empowered to control when and where they perform therapy.”
“The device is activated by the external wearable. It’s like an on-off switch. It has a receiver within it that basically has the capacity to be turned on and off by the wearable, which is the control device. The device is in an off-position until the wearable is applied,” he said.
He said the device should be worn twice a day for about 20 minutes, with many patients using it less.
Only one implanted tibial neuromodulation device has been approved by the Food and Drug Administration – eCOIN (Valencia Technologies). The RENOVA iStim is an investigational device under review by the FDA, Dr. Dmochowski said.
In installing the device, Dr. Dmochowski said urologists use a subfascial technique to enable direct visualization of the tibial nerve and suture fixation that increases the possibility of a predictable placement. Patients use an external wearable, which activates the implant, without concern for battery longevity or replacement.
“This therapy is not associated with any adverse effects and may be beneficial for patients who do not respond to other treatments for OAB such as medications or Botox,” said Carol E. Bretschneider, MD, a urogynecologic and pelvic surgeon at Northwestern Medicine Central DuPage Hospital, outside Chicago. “Neurostimulators can be a great advanced therapy option for patients who do not respond to more conservative treatments or cannot take or tolerate a medication.”
The devices do not stimulate or strengthen muscles but act by modulating the reflexes that influence the bladder, sphincter, and pelvic floor, added Dr. Bretschneider, who was not involved in the study.
Other treatments for urge incontinence can include acupuncture, or percutaneous tibial nerve stimulation, to target the posterior tibial nerve in the ankle, which shares the same nerve root that controls the bladder, according to Aron Liaw, MD, a reconstructive urologist and assistant professor of urology at Wayne State University in Detroit. This treatment has been shown to be at least as effective as available medications, but with fewer side effects, he said.
But regular stimulation is necessary to achieve and preserve efficacy, he said.
Dr. Liaw, who was not involved in the neuromodulation study, said the benefits of a device like Renova iStim are that implantation is relatively easy and can be performed in office settings, and patients can then treat themselves at home. However, because the new study did not compare the device to other treatments or a placebo device, its relative benefits are unclear, he said,
Other treatments for urge urinary incontinence, such as bladder Botox and sacral neuromodulation, also are minimally invasive and have proven benefit, “so a device like this could well be less effective with little other advantage,” he said.
“Lifestyle changes can make a big difference, but making big lifestyle changes is not always easy,” added Dr. Liaw. “I have found neuromodulation [to be] very effective, especially in conjunction with lifestyle changes.”
BlueWind Medical funds the OASIS trial. Dr. Dmochowski reported he received no grants nor has any relevant financial relationships. Dr. Bretschneider and Dr. Liaw report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT AUA 2023
Little evidence to support lasers for ‘vaginal rejuvenation’
Laser devices licensed in Canada to treat genitourinary syndrome of menopause (GSM) are often marketed for vaginal rejuvenation with claims that they will tighten the vagina and improve sexual function, despite lack of evidence, a new commentary reveals.
Vaginal lasers heat the vaginal epithelium and cause thermal necrosis. This intervention induces collagen remodeling and synthesis, neovascularization, and elastin formation and may result in improved vaginal elasticity and restoration of premenopausal epithelial function, according to coauthors Blayne Welk, MD, MSc, an associate professor of urologic surgery at Western University, London, Ont., and Erin Kelly, MD, a lecturer in obstetrics and gynecology at the University of Alberta, Edmonton.
Their patients’ questions and experiences with the laser devices prompted the commentary, they told this news organization.
“A large part of my practice involves addressing GSM and urinary incontinence,” said Dr. Kelly. “Many women present to the clinic having heard of vaginal laser procedures, having had vaginal laser procedures, or having been told they need vaginal laser procedures. My impression has been that these procedures are being marketed to women … without rigorous study.”
“Many women are reluctant to have mesh slings for stress incontinence due to some of the potential risks,” and they are looking for less invasive options, said Dr. Welk. Over the past few years, he has had increasing questions from patients about the use of lasers to improve this condition.
The commentary was published online in the Canadian Medical Association Journal.
Transparency needed
The first vaginal energy device was licensed by Health Canada in 2015 to treat GSM. That meant the device was deemed to have met basic safety, effectiveness, and quality criteria. But no controlled studies are required for regulatory approval of such devices, and after licensing, some providers rebranded the device indication from GSM to vaginal rejuvenation, said Dr. Kelly and Dr. Welk.
Vaginal laser therapies are offered throughout Canada, with at least one provider of vaginal rejuvenation procedures in the 10 most populous cities. Under the current system, the number of patients who pay for these procedures and the amount that they pay cannot be tracked. Nor can the number of vaginal laser systems active in Canada be tracked. Patients can refer themselves for the service, and providers’ publicly quoted costs (on websites, for example) are thousands of dollars for treatment.
The rebranding for vaginal rejuvenation “represents a difference between the licensing of a medical device by Health Canada and the way that these devices are used and marketed,” according to the commentary. “A procedure with limited high-quality evidence supporting its efficacy and a potential financial conflict of interest for providers may not be serving the best interests of people in Canada, even if the risk of adverse events is low.”
Updates to Canada’s medical devices action plan, including mandatory reporting of serious incidents and the ability to compel manufacturers to provide information on safety and effectiveness, “represent important progress,” according to Dr. Kelly and Dr. Welk. However, problems persist, including lack of a requirement for peer-reviewed, controlled studies.
Furthermore, women who undergo laser treatment for GSM, urinary incontinence, or vaginal rejuvenation may not receive a proper medical evaluation and standard treatments, the authors noted.
“I would like to see more transparency and public-facing information available on approved medical devices,” said Dr. Welk. “Health Canada has an online database of approved devices, but no information around the evidence submitted during the approval process is available, nor are the indications for the various devices.”
In addition, he said, many devices in the registry are listed by a serial number rather than the name that would be familiar to the public, “making it hard to match up information.”
Dr. Kelly added the “encouraging” news that the Canadian Society for Pelvic Medicine is working with Health Canada to “improve knowledge translation when it comes to transparency regarding medical devices.”
Medicine before marketing
“The commentary provides an accurate and evidence-based assessment of the use of vaginal laser treatments,” Jason Abbott, B Med (Hons), PhD, professor of gynecology at the University of New South Wales, Sydney, told this news organization. “The marketing of this device is a case of putting the cart before the horse. It is essential that strong, scientific, and reproducible studies be available on efficacy and safety before there is a direct-to-consumer marketing approach.”
Clinicians should advise patients when the treatment effect is likely to be minimal or risky, especially when there is a financial incentive to the clinician, he said. “Governments, regulators, and medical societies have a duty of care to the public to make sure that the medicine comes before the marketing. Otherwise, we are no better than snake oil sellers.
“Given the size of studies to date, the improvement in symptoms following treatment may be less than a few percent,” he noted. “That may be acceptable to some women. We don’t know.”
Dr. Abbott’s team is conducting research to define what women would want as a minimal level of improvement, the maximum cost, and the maximum risk from the laser procedure.
“In cancer … the benefit of a new treatment may only be a few percent for survival,” he said. “That may be completely acceptable for some or even many patients. What we cannot do, however, is extrapolate those same expectations to a treatment for a benign condition where quality of life is compromised.”
Echoing Dr. Kelly and Dr. Welk, Dr. Abbott said, “It is important that there be transparency in the clinical communication. Patients should be told that the best scientific studies that are judged based on their quality show there is no benefit to laser treatment for GSM or urinary incontinence.”
Although the medical risks may be low, he added, “financial risk also needs to be discussed. Patients should be encouraged to participate in clinical trials where there is no cost to them to gain the information first, before wholesale uptake of the treatment. … Should patients still wish to undergo the procedure once the risks and an honest account of the evidence is given to them, that of course is their choice.” Dr. Kelly, Dr. Welk, and Dr. Abbott had no commercial funding or relevant financial relationships to report.
A version of this article first appeared on Medscape.com.
Laser devices licensed in Canada to treat genitourinary syndrome of menopause (GSM) are often marketed for vaginal rejuvenation with claims that they will tighten the vagina and improve sexual function, despite lack of evidence, a new commentary reveals.
Vaginal lasers heat the vaginal epithelium and cause thermal necrosis. This intervention induces collagen remodeling and synthesis, neovascularization, and elastin formation and may result in improved vaginal elasticity and restoration of premenopausal epithelial function, according to coauthors Blayne Welk, MD, MSc, an associate professor of urologic surgery at Western University, London, Ont., and Erin Kelly, MD, a lecturer in obstetrics and gynecology at the University of Alberta, Edmonton.
Their patients’ questions and experiences with the laser devices prompted the commentary, they told this news organization.
“A large part of my practice involves addressing GSM and urinary incontinence,” said Dr. Kelly. “Many women present to the clinic having heard of vaginal laser procedures, having had vaginal laser procedures, or having been told they need vaginal laser procedures. My impression has been that these procedures are being marketed to women … without rigorous study.”
“Many women are reluctant to have mesh slings for stress incontinence due to some of the potential risks,” and they are looking for less invasive options, said Dr. Welk. Over the past few years, he has had increasing questions from patients about the use of lasers to improve this condition.
The commentary was published online in the Canadian Medical Association Journal.
Transparency needed
The first vaginal energy device was licensed by Health Canada in 2015 to treat GSM. That meant the device was deemed to have met basic safety, effectiveness, and quality criteria. But no controlled studies are required for regulatory approval of such devices, and after licensing, some providers rebranded the device indication from GSM to vaginal rejuvenation, said Dr. Kelly and Dr. Welk.
Vaginal laser therapies are offered throughout Canada, with at least one provider of vaginal rejuvenation procedures in the 10 most populous cities. Under the current system, the number of patients who pay for these procedures and the amount that they pay cannot be tracked. Nor can the number of vaginal laser systems active in Canada be tracked. Patients can refer themselves for the service, and providers’ publicly quoted costs (on websites, for example) are thousands of dollars for treatment.
The rebranding for vaginal rejuvenation “represents a difference between the licensing of a medical device by Health Canada and the way that these devices are used and marketed,” according to the commentary. “A procedure with limited high-quality evidence supporting its efficacy and a potential financial conflict of interest for providers may not be serving the best interests of people in Canada, even if the risk of adverse events is low.”
Updates to Canada’s medical devices action plan, including mandatory reporting of serious incidents and the ability to compel manufacturers to provide information on safety and effectiveness, “represent important progress,” according to Dr. Kelly and Dr. Welk. However, problems persist, including lack of a requirement for peer-reviewed, controlled studies.
Furthermore, women who undergo laser treatment for GSM, urinary incontinence, or vaginal rejuvenation may not receive a proper medical evaluation and standard treatments, the authors noted.
“I would like to see more transparency and public-facing information available on approved medical devices,” said Dr. Welk. “Health Canada has an online database of approved devices, but no information around the evidence submitted during the approval process is available, nor are the indications for the various devices.”
In addition, he said, many devices in the registry are listed by a serial number rather than the name that would be familiar to the public, “making it hard to match up information.”
Dr. Kelly added the “encouraging” news that the Canadian Society for Pelvic Medicine is working with Health Canada to “improve knowledge translation when it comes to transparency regarding medical devices.”
Medicine before marketing
“The commentary provides an accurate and evidence-based assessment of the use of vaginal laser treatments,” Jason Abbott, B Med (Hons), PhD, professor of gynecology at the University of New South Wales, Sydney, told this news organization. “The marketing of this device is a case of putting the cart before the horse. It is essential that strong, scientific, and reproducible studies be available on efficacy and safety before there is a direct-to-consumer marketing approach.”
Clinicians should advise patients when the treatment effect is likely to be minimal or risky, especially when there is a financial incentive to the clinician, he said. “Governments, regulators, and medical societies have a duty of care to the public to make sure that the medicine comes before the marketing. Otherwise, we are no better than snake oil sellers.
“Given the size of studies to date, the improvement in symptoms following treatment may be less than a few percent,” he noted. “That may be acceptable to some women. We don’t know.”
Dr. Abbott’s team is conducting research to define what women would want as a minimal level of improvement, the maximum cost, and the maximum risk from the laser procedure.
“In cancer … the benefit of a new treatment may only be a few percent for survival,” he said. “That may be completely acceptable for some or even many patients. What we cannot do, however, is extrapolate those same expectations to a treatment for a benign condition where quality of life is compromised.”
Echoing Dr. Kelly and Dr. Welk, Dr. Abbott said, “It is important that there be transparency in the clinical communication. Patients should be told that the best scientific studies that are judged based on their quality show there is no benefit to laser treatment for GSM or urinary incontinence.”
Although the medical risks may be low, he added, “financial risk also needs to be discussed. Patients should be encouraged to participate in clinical trials where there is no cost to them to gain the information first, before wholesale uptake of the treatment. … Should patients still wish to undergo the procedure once the risks and an honest account of the evidence is given to them, that of course is their choice.” Dr. Kelly, Dr. Welk, and Dr. Abbott had no commercial funding or relevant financial relationships to report.
A version of this article first appeared on Medscape.com.
Laser devices licensed in Canada to treat genitourinary syndrome of menopause (GSM) are often marketed for vaginal rejuvenation with claims that they will tighten the vagina and improve sexual function, despite lack of evidence, a new commentary reveals.
Vaginal lasers heat the vaginal epithelium and cause thermal necrosis. This intervention induces collagen remodeling and synthesis, neovascularization, and elastin formation and may result in improved vaginal elasticity and restoration of premenopausal epithelial function, according to coauthors Blayne Welk, MD, MSc, an associate professor of urologic surgery at Western University, London, Ont., and Erin Kelly, MD, a lecturer in obstetrics and gynecology at the University of Alberta, Edmonton.
Their patients’ questions and experiences with the laser devices prompted the commentary, they told this news organization.
“A large part of my practice involves addressing GSM and urinary incontinence,” said Dr. Kelly. “Many women present to the clinic having heard of vaginal laser procedures, having had vaginal laser procedures, or having been told they need vaginal laser procedures. My impression has been that these procedures are being marketed to women … without rigorous study.”
“Many women are reluctant to have mesh slings for stress incontinence due to some of the potential risks,” and they are looking for less invasive options, said Dr. Welk. Over the past few years, he has had increasing questions from patients about the use of lasers to improve this condition.
The commentary was published online in the Canadian Medical Association Journal.
Transparency needed
The first vaginal energy device was licensed by Health Canada in 2015 to treat GSM. That meant the device was deemed to have met basic safety, effectiveness, and quality criteria. But no controlled studies are required for regulatory approval of such devices, and after licensing, some providers rebranded the device indication from GSM to vaginal rejuvenation, said Dr. Kelly and Dr. Welk.
Vaginal laser therapies are offered throughout Canada, with at least one provider of vaginal rejuvenation procedures in the 10 most populous cities. Under the current system, the number of patients who pay for these procedures and the amount that they pay cannot be tracked. Nor can the number of vaginal laser systems active in Canada be tracked. Patients can refer themselves for the service, and providers’ publicly quoted costs (on websites, for example) are thousands of dollars for treatment.
The rebranding for vaginal rejuvenation “represents a difference between the licensing of a medical device by Health Canada and the way that these devices are used and marketed,” according to the commentary. “A procedure with limited high-quality evidence supporting its efficacy and a potential financial conflict of interest for providers may not be serving the best interests of people in Canada, even if the risk of adverse events is low.”
Updates to Canada’s medical devices action plan, including mandatory reporting of serious incidents and the ability to compel manufacturers to provide information on safety and effectiveness, “represent important progress,” according to Dr. Kelly and Dr. Welk. However, problems persist, including lack of a requirement for peer-reviewed, controlled studies.
Furthermore, women who undergo laser treatment for GSM, urinary incontinence, or vaginal rejuvenation may not receive a proper medical evaluation and standard treatments, the authors noted.
“I would like to see more transparency and public-facing information available on approved medical devices,” said Dr. Welk. “Health Canada has an online database of approved devices, but no information around the evidence submitted during the approval process is available, nor are the indications for the various devices.”
In addition, he said, many devices in the registry are listed by a serial number rather than the name that would be familiar to the public, “making it hard to match up information.”
Dr. Kelly added the “encouraging” news that the Canadian Society for Pelvic Medicine is working with Health Canada to “improve knowledge translation when it comes to transparency regarding medical devices.”
Medicine before marketing
“The commentary provides an accurate and evidence-based assessment of the use of vaginal laser treatments,” Jason Abbott, B Med (Hons), PhD, professor of gynecology at the University of New South Wales, Sydney, told this news organization. “The marketing of this device is a case of putting the cart before the horse. It is essential that strong, scientific, and reproducible studies be available on efficacy and safety before there is a direct-to-consumer marketing approach.”
Clinicians should advise patients when the treatment effect is likely to be minimal or risky, especially when there is a financial incentive to the clinician, he said. “Governments, regulators, and medical societies have a duty of care to the public to make sure that the medicine comes before the marketing. Otherwise, we are no better than snake oil sellers.
“Given the size of studies to date, the improvement in symptoms following treatment may be less than a few percent,” he noted. “That may be acceptable to some women. We don’t know.”
Dr. Abbott’s team is conducting research to define what women would want as a minimal level of improvement, the maximum cost, and the maximum risk from the laser procedure.
“In cancer … the benefit of a new treatment may only be a few percent for survival,” he said. “That may be completely acceptable for some or even many patients. What we cannot do, however, is extrapolate those same expectations to a treatment for a benign condition where quality of life is compromised.”
Echoing Dr. Kelly and Dr. Welk, Dr. Abbott said, “It is important that there be transparency in the clinical communication. Patients should be told that the best scientific studies that are judged based on their quality show there is no benefit to laser treatment for GSM or urinary incontinence.”
Although the medical risks may be low, he added, “financial risk also needs to be discussed. Patients should be encouraged to participate in clinical trials where there is no cost to them to gain the information first, before wholesale uptake of the treatment. … Should patients still wish to undergo the procedure once the risks and an honest account of the evidence is given to them, that of course is their choice.” Dr. Kelly, Dr. Welk, and Dr. Abbott had no commercial funding or relevant financial relationships to report.
A version of this article first appeared on Medscape.com.
Findings question value of pessary for pelvic organ prolapse
The standard nonsurgical treatment for pelvic organ prolapse does not appear to work as well as surgery to correct the problem, Dutch researchers have found.
Pelvic organ prolapse is an uncomfortable condition, causing a troublesome vaginal bulge, often accompanied by urinary, bowel, or sexual dysfunction. Between 3% and 6% of women develop symptomatic prolapse, with the highest incidence in women aged 60-69 years – a fast-growing demographic.
Although many women choose surgical treatment, the American College of Obstetricians and Gynecologists recommends that women be offered a vaginal pessary as a noninvasive alternative, despite inconsistent data from observational studies on their effectiveness.
Lisa van der Vaart, MD, a doctoral student in ob.gyn. at the University of Amsterdam and the lead author of the new study, published in JAMA, said that differences in outcome measures, small sample size, and lack of long-term follow-up have bedeviled previous comparisons of the two techniques.
“We thought it was very important to perform a randomized control trial on this subject to improve counseling to women who suffer from symptomatic pelvic organ prolapse,” Dr. van der Vaart said.
She and her colleagues conducted a noninferiority randomized clinical trial that recruited 1,605 women with stage II or higher prolapse who were referred to specialty care at 21 hospitals in the Netherlands between 2015 and 2019. Of the 440 women who agreed to participate in the trial, 218 received a pessary, a device inserted into the vagina that provides support to tissues displaced by prolapse, and 222 underwent surgery.
The primary outcome was subjective improvement using a standardized questionnaire at 24 months; women were asked to rank their symptoms on a seven-point scale, and subjective improvement was defined as a response of much better or very much better.
“We saw a substantial amount of improvement in both groups,” Dr. van der Vaart said in an interview.
After 24 months of follow-up, outcome data were available for 173 women in the pessary group and 162 in the surgery group. For this intention-to treat population, 76.3% in the pessary group and 81.5% in the surgery group reported improvement.
Results were similar for the smaller group of participants who completed the study per protocol, without crossing over to a treatment to which they had not been allocated.
However, neither the intention-to-treat nor per-protocol analysis met the prespecified criteria for noninferiority, suggesting that use of a vaginal pessary is not equivalent to surgery.
The study also found differences in adverse events. Among women randomly assigned to surgery, 9% suffered a postoperative urinary tract infection, and 5.4% underwent additional therapy, such as pessary or repeat operation.
But use of a pessary also had downsides. The most common adverse event was discomfort (42.7%), and by 24 months, 60% of the participants in the pessary group had discontinued use.
Dr. van der Vaart said that she was surprised by the high number of women assigned to the pessary group who later elected to undergo surgery. “Women should be told that their chance of crossing over to a surgical intervention is quite high – more than 50% do eventually end up having surgery.”
Cheryl Iglesia, MD, director of the National Center for Advanced Pelvic Surgery at MedStar Health and professor of obstetrics and gynecology and urology at Georgetown University, both in Washington, was also struck by the high crossover rate. “We’ve had the same pessaries probably for the last 100 years,” she said. “We need to get better.”
Dr. Iglesia welcomed new approaches to making vaginal pessaries that are custom designed for each woman’s unique anatomy using 3D printing and pointed to promising initial clinical trials of disposable pessaries. With the aging of the population and demand for treatment of prolapse increasing, she cited a need for better nonsurgical alternatives: “We have a work-force issue and may not have enough adequately trained urogynecologists to meet the demand for prolapse repairs as our population ages.”
The study was funded by a grant from ZonMW, a Dutch governmental health care organization. Dr. van der Vaart reported grants from ZonMW during the conduct of the study.
A version of this article first appeared on Medscape.com.
The standard nonsurgical treatment for pelvic organ prolapse does not appear to work as well as surgery to correct the problem, Dutch researchers have found.
Pelvic organ prolapse is an uncomfortable condition, causing a troublesome vaginal bulge, often accompanied by urinary, bowel, or sexual dysfunction. Between 3% and 6% of women develop symptomatic prolapse, with the highest incidence in women aged 60-69 years – a fast-growing demographic.
Although many women choose surgical treatment, the American College of Obstetricians and Gynecologists recommends that women be offered a vaginal pessary as a noninvasive alternative, despite inconsistent data from observational studies on their effectiveness.
Lisa van der Vaart, MD, a doctoral student in ob.gyn. at the University of Amsterdam and the lead author of the new study, published in JAMA, said that differences in outcome measures, small sample size, and lack of long-term follow-up have bedeviled previous comparisons of the two techniques.
“We thought it was very important to perform a randomized control trial on this subject to improve counseling to women who suffer from symptomatic pelvic organ prolapse,” Dr. van der Vaart said.
She and her colleagues conducted a noninferiority randomized clinical trial that recruited 1,605 women with stage II or higher prolapse who were referred to specialty care at 21 hospitals in the Netherlands between 2015 and 2019. Of the 440 women who agreed to participate in the trial, 218 received a pessary, a device inserted into the vagina that provides support to tissues displaced by prolapse, and 222 underwent surgery.
The primary outcome was subjective improvement using a standardized questionnaire at 24 months; women were asked to rank their symptoms on a seven-point scale, and subjective improvement was defined as a response of much better or very much better.
“We saw a substantial amount of improvement in both groups,” Dr. van der Vaart said in an interview.
After 24 months of follow-up, outcome data were available for 173 women in the pessary group and 162 in the surgery group. For this intention-to treat population, 76.3% in the pessary group and 81.5% in the surgery group reported improvement.
Results were similar for the smaller group of participants who completed the study per protocol, without crossing over to a treatment to which they had not been allocated.
However, neither the intention-to-treat nor per-protocol analysis met the prespecified criteria for noninferiority, suggesting that use of a vaginal pessary is not equivalent to surgery.
The study also found differences in adverse events. Among women randomly assigned to surgery, 9% suffered a postoperative urinary tract infection, and 5.4% underwent additional therapy, such as pessary or repeat operation.
But use of a pessary also had downsides. The most common adverse event was discomfort (42.7%), and by 24 months, 60% of the participants in the pessary group had discontinued use.
Dr. van der Vaart said that she was surprised by the high number of women assigned to the pessary group who later elected to undergo surgery. “Women should be told that their chance of crossing over to a surgical intervention is quite high – more than 50% do eventually end up having surgery.”
Cheryl Iglesia, MD, director of the National Center for Advanced Pelvic Surgery at MedStar Health and professor of obstetrics and gynecology and urology at Georgetown University, both in Washington, was also struck by the high crossover rate. “We’ve had the same pessaries probably for the last 100 years,” she said. “We need to get better.”
Dr. Iglesia welcomed new approaches to making vaginal pessaries that are custom designed for each woman’s unique anatomy using 3D printing and pointed to promising initial clinical trials of disposable pessaries. With the aging of the population and demand for treatment of prolapse increasing, she cited a need for better nonsurgical alternatives: “We have a work-force issue and may not have enough adequately trained urogynecologists to meet the demand for prolapse repairs as our population ages.”
The study was funded by a grant from ZonMW, a Dutch governmental health care organization. Dr. van der Vaart reported grants from ZonMW during the conduct of the study.
A version of this article first appeared on Medscape.com.
The standard nonsurgical treatment for pelvic organ prolapse does not appear to work as well as surgery to correct the problem, Dutch researchers have found.
Pelvic organ prolapse is an uncomfortable condition, causing a troublesome vaginal bulge, often accompanied by urinary, bowel, or sexual dysfunction. Between 3% and 6% of women develop symptomatic prolapse, with the highest incidence in women aged 60-69 years – a fast-growing demographic.
Although many women choose surgical treatment, the American College of Obstetricians and Gynecologists recommends that women be offered a vaginal pessary as a noninvasive alternative, despite inconsistent data from observational studies on their effectiveness.
Lisa van der Vaart, MD, a doctoral student in ob.gyn. at the University of Amsterdam and the lead author of the new study, published in JAMA, said that differences in outcome measures, small sample size, and lack of long-term follow-up have bedeviled previous comparisons of the two techniques.
“We thought it was very important to perform a randomized control trial on this subject to improve counseling to women who suffer from symptomatic pelvic organ prolapse,” Dr. van der Vaart said.
She and her colleagues conducted a noninferiority randomized clinical trial that recruited 1,605 women with stage II or higher prolapse who were referred to specialty care at 21 hospitals in the Netherlands between 2015 and 2019. Of the 440 women who agreed to participate in the trial, 218 received a pessary, a device inserted into the vagina that provides support to tissues displaced by prolapse, and 222 underwent surgery.
The primary outcome was subjective improvement using a standardized questionnaire at 24 months; women were asked to rank their symptoms on a seven-point scale, and subjective improvement was defined as a response of much better or very much better.
“We saw a substantial amount of improvement in both groups,” Dr. van der Vaart said in an interview.
After 24 months of follow-up, outcome data were available for 173 women in the pessary group and 162 in the surgery group. For this intention-to treat population, 76.3% in the pessary group and 81.5% in the surgery group reported improvement.
Results were similar for the smaller group of participants who completed the study per protocol, without crossing over to a treatment to which they had not been allocated.
However, neither the intention-to-treat nor per-protocol analysis met the prespecified criteria for noninferiority, suggesting that use of a vaginal pessary is not equivalent to surgery.
The study also found differences in adverse events. Among women randomly assigned to surgery, 9% suffered a postoperative urinary tract infection, and 5.4% underwent additional therapy, such as pessary or repeat operation.
But use of a pessary also had downsides. The most common adverse event was discomfort (42.7%), and by 24 months, 60% of the participants in the pessary group had discontinued use.
Dr. van der Vaart said that she was surprised by the high number of women assigned to the pessary group who later elected to undergo surgery. “Women should be told that their chance of crossing over to a surgical intervention is quite high – more than 50% do eventually end up having surgery.”
Cheryl Iglesia, MD, director of the National Center for Advanced Pelvic Surgery at MedStar Health and professor of obstetrics and gynecology and urology at Georgetown University, both in Washington, was also struck by the high crossover rate. “We’ve had the same pessaries probably for the last 100 years,” she said. “We need to get better.”
Dr. Iglesia welcomed new approaches to making vaginal pessaries that are custom designed for each woman’s unique anatomy using 3D printing and pointed to promising initial clinical trials of disposable pessaries. With the aging of the population and demand for treatment of prolapse increasing, she cited a need for better nonsurgical alternatives: “We have a work-force issue and may not have enough adequately trained urogynecologists to meet the demand for prolapse repairs as our population ages.”
The study was funded by a grant from ZonMW, a Dutch governmental health care organization. Dr. van der Vaart reported grants from ZonMW during the conduct of the study.
A version of this article first appeared on Medscape.com.
FROM JAMA
2022 Update on pelvic floor dysfunction
Knowledge of the latest evidence on the management of pelvic floor disorders is essential for all practicing ObGyns. In this Update, we review long-term outcomes for a polyacrylamide hydrogel urethral bulking agent for the treatment of stress urinary incontinence (SUI) that presents a viable alternative to the gold standard, midurethral sling. We review the new recommendations from the American Urogynecologic Society (AUGS) regarding the administration of anticholinergics, highlighting a paradigm shift in the management of overactive bladder (OAB). In addition, we present data on a proposed threshold glycosylated hemoglobin A1c (HbA1c) level for patients undergoing pelvic organ prolapse (POP) surgery that may help reduce the risk of perioperative complications. Finally, we consider new evidence on the long-term efficacy and safety of transvaginal mesh for repair of POP.
Periurethral injection with polyacrylamide hydrogel is a long-term durable and safe option for women with SUI
Brosche T, Kuhn A, Lobodasch K, et al. Seven-year efficacy and safety outcomes of Bulkamid for the treatment of stress urinary incontinence. Neurourol Urodyn. 2021;40:502-508. doi:10.1002/nau.24589.
Urethral bulking agents are a less invasive management option for women with SUI compared with the gold standard, midurethral sling. Treatment with a polyacrylamide hydrogel (PAHG; Bulkamid)—a nonparticulate hydrogel bulking agent—showed long-term efficacy and a favorable safety profile at 7 years’ follow-up.
Study details
Brosche and colleagues conducted a retrospective cohort study that included women with SUI or stress-predominant mixed urinary incontinence (MUI) who underwent transurethral PAHG injections for primary treatment of their incontinence symptoms. The study objective was to evaluate the long-term efficacy of PAHG based on patient satisfaction. Treatment safety was a secondary outcome.
Pad counts and validated questionnaires were used to determine treatment effectiveness. Additional data on reinjection rates, perioperative complications, and postoperative complications also were collected.
Long-term outcomes favorable
During the study time period, 1,200 patients were treated with PAHG, and 7-year data were available for 553 women. Of the 553 patients, 67% reported improvement or cure of their SUI symptoms when PAHG was performed as a primary procedure, consistent with previously published 12-month data. There were no perioperative complications. Postoperative complications were transient. Short-term subjective prolonged bladder emptying was the most common complication and occurred in 15% of patients.
PAHG injection is a durable and safe alternative for the treatment of stress urinary incontinence in women who are not candidates for or who decline treatment with alternative methods, such as a midurethral sling.
Continue to: New society guidance...
New society guidance on the use of anticholinergic medications for the treatment of OAB
AUGS Clinical Consensus Statement: Association of anticholinergic medication use and cognition in women with overactive bladder. Female Pelvic Med Reconstr Surg. 2021;27:69-71. doi:10.1097/ SPV.0000000000001008.
In 2021, AUGS updated its consensus statement on the use of anticholinergic medications for the treatment of OAB. This action was in response to growing evidence that supports the association of anticholinergic medications with long-term cognitive adverse effects, including cognitive impairment, dementia, and Alzheimer disease.
Here, we summarize the most recent modifications, which differentiate the updated statement from the preceding consensus document published in 2017.
Updated AUGS recommendations
- If considering anticholinergic medications, counsel patients about the risk of cognitive adverse effects and weigh these risks against the potential benefits to their quality of life and overall health.
- Use the lowest possible dose when prescribing anticholinergics and consider alternatives such as β3 agonists (for example, mirabegron or vibegron).
- Avoid using anticholinergic medications in women older than age 70. However, if an anticholinergic must be used, consider a medication that has low potential to cross the blood-brain barrier (for example, trospium).
For patients who are unresponsive to behavioral therapies for OAB, medical management may be considered. However, the risks of anticholinergic medications may outweigh the benefits—especially for older women—and these medications should be prescribed with caution after discussing the potential cognitive adverse effects with patients. β3 agonists should be preferentially prescribed when appropriate. Consider referral to a urogynecologist for discussion of third-line therapies in patients who prefer to forego or may not be candidates for medical management of their OAB symptoms.
HbA1c levels > 8% may increase complications risk in urogyn surgery
Ringel NE, de Winter KL, Siddique M, et al. Surgical outcomes in urogynecology—assessment of perioperative and postoperative complications relative to preoperative hemoglobin A1c—a Fellows Pelvic Research Network study. Female Pelvic Med Reconstr Surg. 2022;28:7-13. doi:10.1097/ SPV.0000000000001057.
Diabetes mellitus is a known risk factor for complications following surgery. Adoption of an HbA1c level threshold for risk stratification before urogynecologic surgery may help improve patient outcomes.
Study details
Ringel and colleagues conducted a multicenter retrospective cohort study that included women with diabetes mellitus who underwent prolapse and/or SUI surgery between 2013 and 2018. The aim of the study was to identify a hemoglobin A1C threshold that would help predict increased risk for perioperative complications in women undergoing pelvic reconstructive surgery. Demographics, preoperative HbA1c levels, and surgical data were collected.
Complication risks correlated with higher HbA1c threshold
The study included 807 women with HbA1c values that ranged from 5% to 12%. The overall complication rate was 44%. Sensitivity analysis was performed to compare complication rates between patients with varying HbA1c levels and determine a threshold HbA1c value with the greatest difference in complication rates.
The authors concluded that women with an HbA1c level ≥ 8% showed the greatest increase of perioperative complications. Patients with an HbA1c ≥ 8%, compared with those who had an HbA1c < 8%, had a statistically significantly increased rate of overall (58% vs 42%, P = .002) and severe (27% vs 13%, P< .001) perioperative complications.
After multivariate logistic regression, the risk of overall complications remained elevated, with a 1.9-times higher risk of perioperative complications for women with an HbA1c ≥ 8%.
Women should be medically optimized before undergoing surgery and, while this study was restricted to urogynecologic surgery patients, it seems reasonable to assume that a similar HbA1c threshold would be beneficial for women undergoing other gynecologic procedures. Appropriately screening patients and referring them for early intervention with their primary care clinician or endocrinologist may improve surgical outcomes, especially in women with an HbA1c level > 8%.
Continue to: Success is similar for TV mesh and native tissue repair...
Success is similar for TV mesh and native tissue repair
Kahn B, Varner RE, Murphy M, et al. Transvaginal mesh compared with native tissue repair for pelvic organ prolapse. Obstet Gynecol. 2022;139:975-985. doi:10.1097/AOG.0000000000004794.
The distribution of vaginal mesh kits for the repair of POP was halted by the US Food and Drug Administration (FDA) in 2019. However, concerns have been raised about the measures used by the FDA to justify pulling these devices from the market. A cohort study compared 36-month outcomes between women who underwent prolapse repair with newer generation transvaginal mesh versus native tissue repair.
Study details
In a nonrandomized prospective multicenter cohort study, Kahn and colleagues compared outcomes in women with POP who underwent native tissue repair or transvaginal mesh repair with the Uphold LITE vaginal support system. The study’s objective was to compare the safety and efficacy of native tissue and transvaginal mesh prolapse repairs at 36 months postoperatively.
Treatment success was measured based on composite and individual measures of anatomic and subjective success, need for retreatment, and the occurrence of adverse events. Quality of life (QoL) measures also were obtained using validated questionnaires. Intention-to-treat and per-protocol analyses were performed.
Composite success rate was higher for mesh repair
A total of 710 patients were screened for eligibility (225 received transvaginal mesh and 485 received native tissue repair). Transvaginal mesh placement was found to be significantly superior to native tissue repair for composite success (84% vs 73%, P = .009) when prolapse within the hymen (that is, Ba and/or C < 0 on the Pelvic Organ Prolapse Quantification System) was used to define anatomic success.
Adverse events were similar between transvaginal mesh and native tissue repair groups, with most adverse events occurring within the first 6 months. The mesh exposure rate was 4.9%. Of the 13 incidents of mesh exposure, 4 patients required surgical intervention and 1 incident was considered a serious adverse event. QoL measures demonstrated improvement without any statistically significant differences between the treatment cohorts. ●
This study established the superiority and safety of newer generation transvaginal mesh used for the treatment of pelvic organ prolapse. Women who received newer generation transvaginal mesh can be reassured that the prolapse recurrence rates are low and that adverse events related to their mesh are rare—even when compared with those of native tissue repair. Patients also may be reassured that most adverse events would have occurred within 6 months of the initial prolapse repair surgery
Knowledge of the latest evidence on the management of pelvic floor disorders is essential for all practicing ObGyns. In this Update, we review long-term outcomes for a polyacrylamide hydrogel urethral bulking agent for the treatment of stress urinary incontinence (SUI) that presents a viable alternative to the gold standard, midurethral sling. We review the new recommendations from the American Urogynecologic Society (AUGS) regarding the administration of anticholinergics, highlighting a paradigm shift in the management of overactive bladder (OAB). In addition, we present data on a proposed threshold glycosylated hemoglobin A1c (HbA1c) level for patients undergoing pelvic organ prolapse (POP) surgery that may help reduce the risk of perioperative complications. Finally, we consider new evidence on the long-term efficacy and safety of transvaginal mesh for repair of POP.
Periurethral injection with polyacrylamide hydrogel is a long-term durable and safe option for women with SUI
Brosche T, Kuhn A, Lobodasch K, et al. Seven-year efficacy and safety outcomes of Bulkamid for the treatment of stress urinary incontinence. Neurourol Urodyn. 2021;40:502-508. doi:10.1002/nau.24589.
Urethral bulking agents are a less invasive management option for women with SUI compared with the gold standard, midurethral sling. Treatment with a polyacrylamide hydrogel (PAHG; Bulkamid)—a nonparticulate hydrogel bulking agent—showed long-term efficacy and a favorable safety profile at 7 years’ follow-up.
Study details
Brosche and colleagues conducted a retrospective cohort study that included women with SUI or stress-predominant mixed urinary incontinence (MUI) who underwent transurethral PAHG injections for primary treatment of their incontinence symptoms. The study objective was to evaluate the long-term efficacy of PAHG based on patient satisfaction. Treatment safety was a secondary outcome.
Pad counts and validated questionnaires were used to determine treatment effectiveness. Additional data on reinjection rates, perioperative complications, and postoperative complications also were collected.
Long-term outcomes favorable
During the study time period, 1,200 patients were treated with PAHG, and 7-year data were available for 553 women. Of the 553 patients, 67% reported improvement or cure of their SUI symptoms when PAHG was performed as a primary procedure, consistent with previously published 12-month data. There were no perioperative complications. Postoperative complications were transient. Short-term subjective prolonged bladder emptying was the most common complication and occurred in 15% of patients.
PAHG injection is a durable and safe alternative for the treatment of stress urinary incontinence in women who are not candidates for or who decline treatment with alternative methods, such as a midurethral sling.
Continue to: New society guidance...
New society guidance on the use of anticholinergic medications for the treatment of OAB
AUGS Clinical Consensus Statement: Association of anticholinergic medication use and cognition in women with overactive bladder. Female Pelvic Med Reconstr Surg. 2021;27:69-71. doi:10.1097/ SPV.0000000000001008.
In 2021, AUGS updated its consensus statement on the use of anticholinergic medications for the treatment of OAB. This action was in response to growing evidence that supports the association of anticholinergic medications with long-term cognitive adverse effects, including cognitive impairment, dementia, and Alzheimer disease.
Here, we summarize the most recent modifications, which differentiate the updated statement from the preceding consensus document published in 2017.
Updated AUGS recommendations
- If considering anticholinergic medications, counsel patients about the risk of cognitive adverse effects and weigh these risks against the potential benefits to their quality of life and overall health.
- Use the lowest possible dose when prescribing anticholinergics and consider alternatives such as β3 agonists (for example, mirabegron or vibegron).
- Avoid using anticholinergic medications in women older than age 70. However, if an anticholinergic must be used, consider a medication that has low potential to cross the blood-brain barrier (for example, trospium).
For patients who are unresponsive to behavioral therapies for OAB, medical management may be considered. However, the risks of anticholinergic medications may outweigh the benefits—especially for older women—and these medications should be prescribed with caution after discussing the potential cognitive adverse effects with patients. β3 agonists should be preferentially prescribed when appropriate. Consider referral to a urogynecologist for discussion of third-line therapies in patients who prefer to forego or may not be candidates for medical management of their OAB symptoms.
HbA1c levels > 8% may increase complications risk in urogyn surgery
Ringel NE, de Winter KL, Siddique M, et al. Surgical outcomes in urogynecology—assessment of perioperative and postoperative complications relative to preoperative hemoglobin A1c—a Fellows Pelvic Research Network study. Female Pelvic Med Reconstr Surg. 2022;28:7-13. doi:10.1097/ SPV.0000000000001057.
Diabetes mellitus is a known risk factor for complications following surgery. Adoption of an HbA1c level threshold for risk stratification before urogynecologic surgery may help improve patient outcomes.
Study details
Ringel and colleagues conducted a multicenter retrospective cohort study that included women with diabetes mellitus who underwent prolapse and/or SUI surgery between 2013 and 2018. The aim of the study was to identify a hemoglobin A1C threshold that would help predict increased risk for perioperative complications in women undergoing pelvic reconstructive surgery. Demographics, preoperative HbA1c levels, and surgical data were collected.
Complication risks correlated with higher HbA1c threshold
The study included 807 women with HbA1c values that ranged from 5% to 12%. The overall complication rate was 44%. Sensitivity analysis was performed to compare complication rates between patients with varying HbA1c levels and determine a threshold HbA1c value with the greatest difference in complication rates.
The authors concluded that women with an HbA1c level ≥ 8% showed the greatest increase of perioperative complications. Patients with an HbA1c ≥ 8%, compared with those who had an HbA1c < 8%, had a statistically significantly increased rate of overall (58% vs 42%, P = .002) and severe (27% vs 13%, P< .001) perioperative complications.
After multivariate logistic regression, the risk of overall complications remained elevated, with a 1.9-times higher risk of perioperative complications for women with an HbA1c ≥ 8%.
Women should be medically optimized before undergoing surgery and, while this study was restricted to urogynecologic surgery patients, it seems reasonable to assume that a similar HbA1c threshold would be beneficial for women undergoing other gynecologic procedures. Appropriately screening patients and referring them for early intervention with their primary care clinician or endocrinologist may improve surgical outcomes, especially in women with an HbA1c level > 8%.
Continue to: Success is similar for TV mesh and native tissue repair...
Success is similar for TV mesh and native tissue repair
Kahn B, Varner RE, Murphy M, et al. Transvaginal mesh compared with native tissue repair for pelvic organ prolapse. Obstet Gynecol. 2022;139:975-985. doi:10.1097/AOG.0000000000004794.
The distribution of vaginal mesh kits for the repair of POP was halted by the US Food and Drug Administration (FDA) in 2019. However, concerns have been raised about the measures used by the FDA to justify pulling these devices from the market. A cohort study compared 36-month outcomes between women who underwent prolapse repair with newer generation transvaginal mesh versus native tissue repair.
Study details
In a nonrandomized prospective multicenter cohort study, Kahn and colleagues compared outcomes in women with POP who underwent native tissue repair or transvaginal mesh repair with the Uphold LITE vaginal support system. The study’s objective was to compare the safety and efficacy of native tissue and transvaginal mesh prolapse repairs at 36 months postoperatively.
Treatment success was measured based on composite and individual measures of anatomic and subjective success, need for retreatment, and the occurrence of adverse events. Quality of life (QoL) measures also were obtained using validated questionnaires. Intention-to-treat and per-protocol analyses were performed.
Composite success rate was higher for mesh repair
A total of 710 patients were screened for eligibility (225 received transvaginal mesh and 485 received native tissue repair). Transvaginal mesh placement was found to be significantly superior to native tissue repair for composite success (84% vs 73%, P = .009) when prolapse within the hymen (that is, Ba and/or C < 0 on the Pelvic Organ Prolapse Quantification System) was used to define anatomic success.
Adverse events were similar between transvaginal mesh and native tissue repair groups, with most adverse events occurring within the first 6 months. The mesh exposure rate was 4.9%. Of the 13 incidents of mesh exposure, 4 patients required surgical intervention and 1 incident was considered a serious adverse event. QoL measures demonstrated improvement without any statistically significant differences between the treatment cohorts. ●
This study established the superiority and safety of newer generation transvaginal mesh used for the treatment of pelvic organ prolapse. Women who received newer generation transvaginal mesh can be reassured that the prolapse recurrence rates are low and that adverse events related to their mesh are rare—even when compared with those of native tissue repair. Patients also may be reassured that most adverse events would have occurred within 6 months of the initial prolapse repair surgery
Knowledge of the latest evidence on the management of pelvic floor disorders is essential for all practicing ObGyns. In this Update, we review long-term outcomes for a polyacrylamide hydrogel urethral bulking agent for the treatment of stress urinary incontinence (SUI) that presents a viable alternative to the gold standard, midurethral sling. We review the new recommendations from the American Urogynecologic Society (AUGS) regarding the administration of anticholinergics, highlighting a paradigm shift in the management of overactive bladder (OAB). In addition, we present data on a proposed threshold glycosylated hemoglobin A1c (HbA1c) level for patients undergoing pelvic organ prolapse (POP) surgery that may help reduce the risk of perioperative complications. Finally, we consider new evidence on the long-term efficacy and safety of transvaginal mesh for repair of POP.
Periurethral injection with polyacrylamide hydrogel is a long-term durable and safe option for women with SUI
Brosche T, Kuhn A, Lobodasch K, et al. Seven-year efficacy and safety outcomes of Bulkamid for the treatment of stress urinary incontinence. Neurourol Urodyn. 2021;40:502-508. doi:10.1002/nau.24589.
Urethral bulking agents are a less invasive management option for women with SUI compared with the gold standard, midurethral sling. Treatment with a polyacrylamide hydrogel (PAHG; Bulkamid)—a nonparticulate hydrogel bulking agent—showed long-term efficacy and a favorable safety profile at 7 years’ follow-up.
Study details
Brosche and colleagues conducted a retrospective cohort study that included women with SUI or stress-predominant mixed urinary incontinence (MUI) who underwent transurethral PAHG injections for primary treatment of their incontinence symptoms. The study objective was to evaluate the long-term efficacy of PAHG based on patient satisfaction. Treatment safety was a secondary outcome.
Pad counts and validated questionnaires were used to determine treatment effectiveness. Additional data on reinjection rates, perioperative complications, and postoperative complications also were collected.
Long-term outcomes favorable
During the study time period, 1,200 patients were treated with PAHG, and 7-year data were available for 553 women. Of the 553 patients, 67% reported improvement or cure of their SUI symptoms when PAHG was performed as a primary procedure, consistent with previously published 12-month data. There were no perioperative complications. Postoperative complications were transient. Short-term subjective prolonged bladder emptying was the most common complication and occurred in 15% of patients.
PAHG injection is a durable and safe alternative for the treatment of stress urinary incontinence in women who are not candidates for or who decline treatment with alternative methods, such as a midurethral sling.
Continue to: New society guidance...
New society guidance on the use of anticholinergic medications for the treatment of OAB
AUGS Clinical Consensus Statement: Association of anticholinergic medication use and cognition in women with overactive bladder. Female Pelvic Med Reconstr Surg. 2021;27:69-71. doi:10.1097/ SPV.0000000000001008.
In 2021, AUGS updated its consensus statement on the use of anticholinergic medications for the treatment of OAB. This action was in response to growing evidence that supports the association of anticholinergic medications with long-term cognitive adverse effects, including cognitive impairment, dementia, and Alzheimer disease.
Here, we summarize the most recent modifications, which differentiate the updated statement from the preceding consensus document published in 2017.
Updated AUGS recommendations
- If considering anticholinergic medications, counsel patients about the risk of cognitive adverse effects and weigh these risks against the potential benefits to their quality of life and overall health.
- Use the lowest possible dose when prescribing anticholinergics and consider alternatives such as β3 agonists (for example, mirabegron or vibegron).
- Avoid using anticholinergic medications in women older than age 70. However, if an anticholinergic must be used, consider a medication that has low potential to cross the blood-brain barrier (for example, trospium).
For patients who are unresponsive to behavioral therapies for OAB, medical management may be considered. However, the risks of anticholinergic medications may outweigh the benefits—especially for older women—and these medications should be prescribed with caution after discussing the potential cognitive adverse effects with patients. β3 agonists should be preferentially prescribed when appropriate. Consider referral to a urogynecologist for discussion of third-line therapies in patients who prefer to forego or may not be candidates for medical management of their OAB symptoms.
HbA1c levels > 8% may increase complications risk in urogyn surgery
Ringel NE, de Winter KL, Siddique M, et al. Surgical outcomes in urogynecology—assessment of perioperative and postoperative complications relative to preoperative hemoglobin A1c—a Fellows Pelvic Research Network study. Female Pelvic Med Reconstr Surg. 2022;28:7-13. doi:10.1097/ SPV.0000000000001057.
Diabetes mellitus is a known risk factor for complications following surgery. Adoption of an HbA1c level threshold for risk stratification before urogynecologic surgery may help improve patient outcomes.
Study details
Ringel and colleagues conducted a multicenter retrospective cohort study that included women with diabetes mellitus who underwent prolapse and/or SUI surgery between 2013 and 2018. The aim of the study was to identify a hemoglobin A1C threshold that would help predict increased risk for perioperative complications in women undergoing pelvic reconstructive surgery. Demographics, preoperative HbA1c levels, and surgical data were collected.
Complication risks correlated with higher HbA1c threshold
The study included 807 women with HbA1c values that ranged from 5% to 12%. The overall complication rate was 44%. Sensitivity analysis was performed to compare complication rates between patients with varying HbA1c levels and determine a threshold HbA1c value with the greatest difference in complication rates.
The authors concluded that women with an HbA1c level ≥ 8% showed the greatest increase of perioperative complications. Patients with an HbA1c ≥ 8%, compared with those who had an HbA1c < 8%, had a statistically significantly increased rate of overall (58% vs 42%, P = .002) and severe (27% vs 13%, P< .001) perioperative complications.
After multivariate logistic regression, the risk of overall complications remained elevated, with a 1.9-times higher risk of perioperative complications for women with an HbA1c ≥ 8%.
Women should be medically optimized before undergoing surgery and, while this study was restricted to urogynecologic surgery patients, it seems reasonable to assume that a similar HbA1c threshold would be beneficial for women undergoing other gynecologic procedures. Appropriately screening patients and referring them for early intervention with their primary care clinician or endocrinologist may improve surgical outcomes, especially in women with an HbA1c level > 8%.
Continue to: Success is similar for TV mesh and native tissue repair...
Success is similar for TV mesh and native tissue repair
Kahn B, Varner RE, Murphy M, et al. Transvaginal mesh compared with native tissue repair for pelvic organ prolapse. Obstet Gynecol. 2022;139:975-985. doi:10.1097/AOG.0000000000004794.
The distribution of vaginal mesh kits for the repair of POP was halted by the US Food and Drug Administration (FDA) in 2019. However, concerns have been raised about the measures used by the FDA to justify pulling these devices from the market. A cohort study compared 36-month outcomes between women who underwent prolapse repair with newer generation transvaginal mesh versus native tissue repair.
Study details
In a nonrandomized prospective multicenter cohort study, Kahn and colleagues compared outcomes in women with POP who underwent native tissue repair or transvaginal mesh repair with the Uphold LITE vaginal support system. The study’s objective was to compare the safety and efficacy of native tissue and transvaginal mesh prolapse repairs at 36 months postoperatively.
Treatment success was measured based on composite and individual measures of anatomic and subjective success, need for retreatment, and the occurrence of adverse events. Quality of life (QoL) measures also were obtained using validated questionnaires. Intention-to-treat and per-protocol analyses were performed.
Composite success rate was higher for mesh repair
A total of 710 patients were screened for eligibility (225 received transvaginal mesh and 485 received native tissue repair). Transvaginal mesh placement was found to be significantly superior to native tissue repair for composite success (84% vs 73%, P = .009) when prolapse within the hymen (that is, Ba and/or C < 0 on the Pelvic Organ Prolapse Quantification System) was used to define anatomic success.
Adverse events were similar between transvaginal mesh and native tissue repair groups, with most adverse events occurring within the first 6 months. The mesh exposure rate was 4.9%. Of the 13 incidents of mesh exposure, 4 patients required surgical intervention and 1 incident was considered a serious adverse event. QoL measures demonstrated improvement without any statistically significant differences between the treatment cohorts. ●
This study established the superiority and safety of newer generation transvaginal mesh used for the treatment of pelvic organ prolapse. Women who received newer generation transvaginal mesh can be reassured that the prolapse recurrence rates are low and that adverse events related to their mesh are rare—even when compared with those of native tissue repair. Patients also may be reassured that most adverse events would have occurred within 6 months of the initial prolapse repair surgery
Nonsurgical treatments for patients with urinary incontinence
CASE Patient has urine leakage that worsens with exercise
At her annual preventative health visit, a 39-year-old woman reports that she has leakage of urine. She states that she drinks “a gallon of water daily” to help her lose the 20 lb she gained during the COVID-19 pandemic. She wants to resume Zumba fitness classes, but exercise makes her urine leakage worse. She started wearing protective pads because she finds herself often leaking urine on the way to the bathroom.
What nonsurgical treatment options are available for this patient?
Nearly half of all women experience urinary incontinence (UI), the involuntary loss of urine, and the condition increases with age.1 This common condition negatively impacts physical and psychological health and has been associated with social isolation, sexual dysfunction, and reduced independence.2,3 Symptoms of UI are underreported, and therefore universal screening is recommended for women of all ages.4 The diversity of available treatments (TABLE 1) provides patients and clinicians an opportunity to develop a plan that aligns with their symptom severity, goals, preferences, and resources.
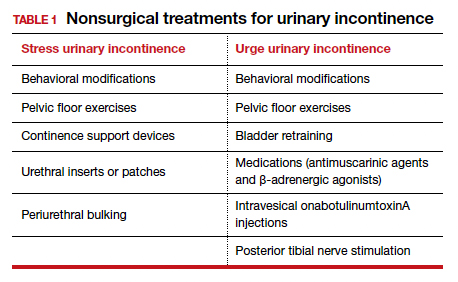
Types of UI
The most common types of UI are stress urinary incontinence (SUI) and urgency urinary incontinence (UUI). Mixed urinary incontinence (MUI) occurs when symptoms of both SUI and UUI are present. Although the mechanisms that lead to urine leakage vary by the type of incontinence, many primary interventions improve both types of leakage, so a clinical diagnosis is sufficient to initiate treatment.
Stress urinary incontinence results from an impaired or weakened sphincter, which leads to involuntary, yet predictable, urine loss during increased abdominal pressure, such as coughing, laughing, sneezing, lifting, or physical activity.5 In UUI, involuntary loss of urine often accompanies the sudden urge to void. UUI is associated with overactive bladder (OAB), defined as urinary urgency, with or without urinary incontinence, usually accompanied by urinary frequency and/or nocturia (urination that interrupts sleep).6
In OAB, the detrusor muscle contracts randomly, leading to a sudden urge to void. When bladder pressure exceeds urethral sphincter closure pressure, urine leakage occurs. Women describe the urgency episodes as unpredictable, the urine leakage as prolonged with large volumes, and often occurring as they seek the toilet. Risk factors include age, obesity, parity, history of vaginal delivery, family history, ethnicity/race, medical comorbidities, menopausal status, and tobacco use.5
Making a diagnosis
A basic office evaluation is the most key step for diagnostic accuracy that leads to treatment success. This includes a detailed history, assessment of symptom severity, physical exam, pelvic exam, urinalysis, postvoid residual (to rule out urinary retention), and a cough stress test (to demonstrate SUI). The goal is to assess symptom severity, determine the type of UI, and identify contributing and potentially reversible factors, such as a urinary tract infection, medications, pelvic organ prolapse, incomplete bladder emptying, or impaired neurologic status. In the absence of the latter, advanced diagnostic tests, such as urodynamics, contribute little toward discerning the type of incontinence or changing first-line treatment plans.7
During the COVID-19 pandemic, abbreviated, virtual assessments for urinary symptoms were associated with high degrees of satisfaction (91% for fulfillment of personal needs, 94% overall satisfaction).8 This highlights the value of validated symptom questionnaires that help establish a working diagnosis and treatment plan in the absence of a physical exam. Questionnaire-based diagnoses have acceptable accuracy for classifying UUI and SUI among women with uncomplicated medical and surgical histories and for initiating low-risk therapies for defined intervals.
The 3 incontinence questions (3IQ) screen is an example of a useful, quick diagnostic tool designed for the primary care setting (FIGURE 1).9 It has been used in pharmaceutical treatment trials for UUI, with low frequency of misdiagnosis (1%–4%), resulting in no harm by the drug treatment prescribed or by the delay in appropriate care.10 Due to the limitations of an abbreviated remote evaluation, however, clinicians should assess patient response to primary interventions in a timely window. Patients who fail to experience satisfactory symptom reduction within 6 to 12 weeks should complete their evaluation in person or through a referral to a urogynecology program.

Continue to: Primary therapies for UI...
Primary therapies for UI
Primary therapies for UUI and SUI target strength training of the pelvic floor muscles, moderation of fluid intake, and adjustment in voiding behaviors and medications. Any functional barriers to continence also should be identified and addressed. Simple interventions, including a daily bowel regimen to address constipation, a bedside commode, and scheduled voiding, may reduce incontinence episodes without incurring significant cost or risk. For women suspected of having MUI, the treatment plan should prioritize their most bothersome symptoms.
Lifestyle and behavioral modifications
Everyday habits, medical comorbidities, and medications may exacerbate the severity of both SUI and UUI. Behavioral therapy alone or in combination with other interventions effectively reduces both SUI and UUI symptoms and has been shown to improve the efficacy of continence surgery.11 Information gained from a 3-day bladder diary (FIGURE 2)12 can guide clinicians on personalized patient recommendations, such as reducing excessive consumption of fluids and bladder irritants, limiting late evening drinking in the setting of bothersome nocturia, and scheduling voids (every 2–3 hours) to preempt incontinence episodes.

Weight loss
Obesity is a strong, independent, modifiable risk factor for both SUI and UUI. Each 5 kg/m2 increase in body mass index (BMI) has been associated with a 20% to 70% increased risk of UI, while weight loss of 5% or greater in overweight or obese women can lead to at least a 50% decrease in UI frequency.13
Reducing fluid intake and bladder irritants
Overactive bladder symptoms often respond to moderation of excessive fluid intake and reduction of bladder irritants (caffeine, carbonated beverages, diet beverages, and alcohol). While there is no established definition of excess caffeine intake, one study categorized high caffeine intake as greater than 400 mg/day (approximately four 8-oz cups of coffee).14
Information provided in a bladder diary can guide individualized recommendations for reducing fluid intake, particularly when 24-hour urine production exceeds the normative range (> 50–60 oz or 1.5-1.8 L/day).15 Hydration needs vary by activity, environment, and food; some general guidelines suggest 48 to 64 oz/day.5,16
Continue to: Pelvic floor muscle training...
Pelvic floor muscle training
An effective treatment for both UUI and SUI symptoms, pelvic floor muscle training (PFMT) leads to high degrees of patient satisfaction and improvement in quality of life.17 The presumed mechanisms of action of PFMT include improved urethral closure pressure and inhibition of detrusor muscle contractions.
Common exercise protocols recommend 3 sets of 10 contractions, held for 6 to 10 seconds per day, in varying positions of sitting, standing, and lying. While many women may be familiar with Kegel exercises, poor technique with straining and recruitment of gluteal and abdominal muscles can undermine the effect of PFMT. Clinicians can confirm successful pelvic muscle contractions by placing a finger in the vagina to appreciate contraction around and elevation of the finger toward the pubic symphysis in the absence of pushing.
Referral to supervised physical therapy and use of such teaching aid tools as booklets, mobile applications, and biofeedback can improve exercise adherence and outcomes.18,19 Systematic reviews report initial cure or improvement of incontinence symptoms as high as 74%, although little information is available about the long-term duration of effect.17
Vaginal pessaries
Vaginal continence support pessaries and devices work by stabilizing urethral mobility and compression of the bladder neck. Continence devices are particularly effective for situational SUI (such as during exercise).
The reusable medical grade silicone pessaries are available in numerous shapes and sizes and are fitted by a health care clinician (FIGURE 3). Uresta is a self-fitted intravaginal device that women can purchase online with a prescription. The Poise Impressa bladder support is a disposable intravaginal device marketed for incontinence and available over-the-counter, without a prescription (FIGURE 4). Anecdotally, many women find that menstrual tampons provide a similar effect, but outcome data are lacking.
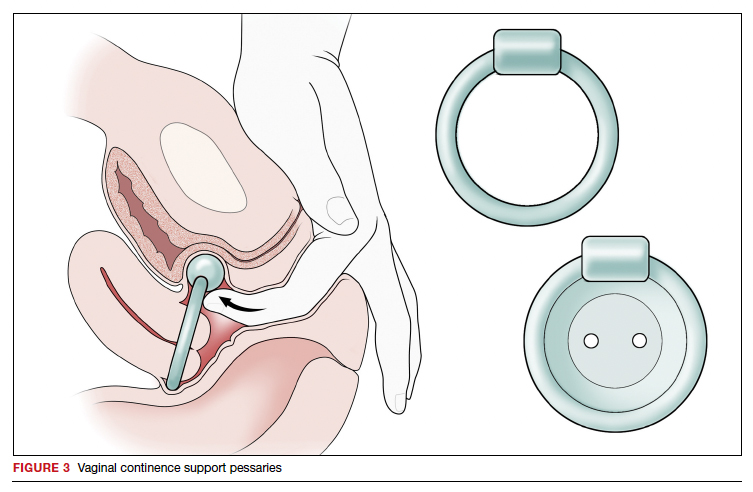
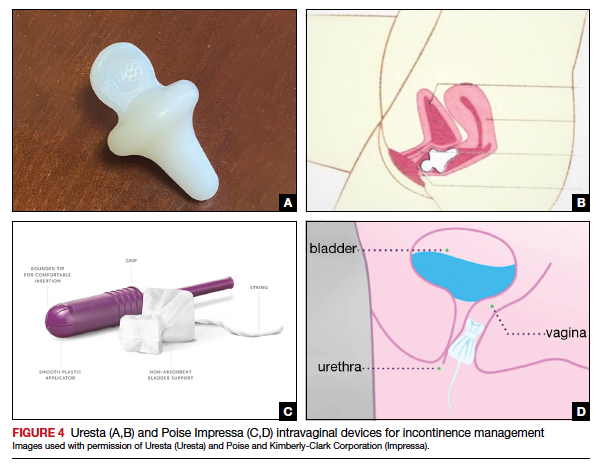
In a comparative effectiveness trial of a continence pessary and behavior therapy, behavioral therapy was more likely to result in no bothersome incontinence symptoms (49% vs 33%, P = .006) and greater treatment satisfaction at 3 months.20 However, these short-term group differences did not persist at 12 months, presumably due to waning adherence.
UUI-specific nonsurgical treatments
Drug therapy
All medications approved by the US Food and Drug Administration (FDA) for UI are for the indications of OAB or UUI. These second-line treatments are most effective as adjuncts to behavioral modifications and PFMT.
A multicenter randomized trial that evaluated the efficacy of drug therapy alone compared with drug therapy in combination with behavioral modification, PFMT, urge suppression strategies, timed voiding, and fluid management for UUI found that combined therapy was more successful in achieving greater than 70% reduction in incontinence episodes (58% for drug therapy vs 69% for combined therapy).21
Of the 8 medications currently marketed in the United States for OAB or UUI, 6 are anticholinergic agents that block muscarinic receptors in the smooth muscle of the bladder, leading to inhibition of detrusor contractions, and 2 are β-adrenergic receptor agonists that promote bladder storage capacity by relaxing the detrusor muscle (TABLE 2). Similar efficacies lead most clinicians to initiate drug therapy based on formulary coverage and tolerance for adverse effects. Patients can expect a 53% to 80% reduction in UUI episodes and a 12% to 32% reduction in urinary frequency.22
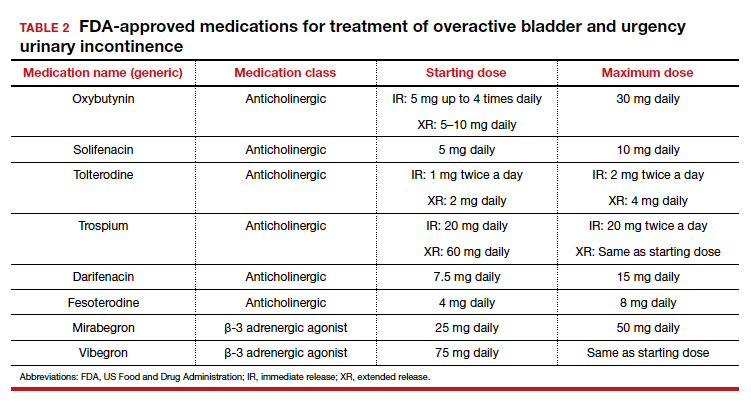
Extended-release formulations are associated with reduced anticholinergic side effects (dry mouth, constipation, somnolence, dry eyes), leading to improved adherence. Notably, the anticholinergic medications are contraindicated in patients with untreated narrow-angle glaucoma, gastric retention, and supraventricular tachycardia. Mirabegron should be used with caution in patients with poorly controlled hypertension. 5 Due to concerns regarding the association between cumulative anticholinergic burden and the development of dementia, clinicians may consider avoiding the anticholinergic medications in older and at-risk patients.23
Continue to: UUI office-based procedure treatments...
UUI office-based procedure treatments
If behavioral therapies and medications are ineffective, contraindicated, or not the patient’s preference, additional FDA-approved therapies for UUI are available, typically through referral to a urogynecologist, urologist, or continence center.
Posterior tibial nerve stimulation (PTNS) is a nondrug treatment that delivers electrical stimulation using an acupuncture needle for 12 weekly 30-minute sessions followed by monthly maintenance for responders. The time commitment for this treatment plan can be a barrier for some patients. However, patients who adhere to the recommended protocol can expect a 60% improvement in symptoms, with minimal adverse events. Treatment efficacy is comparable to that of anticholinergic medication.24
OnabotulinumtoxinA injections into the bladder muscle are performed cystoscopically under local anesthetic. The toxin blocks the presynaptic release of acetylcholine at the neuromuscular junction, resulting in temporary muscle paralysis. This treatment is associated with high satisfaction. Efficacy varies by study population and outcome measure.
In one US comparative effectiveness trial, 67% of study participants with UUI symptoms refractory to oral medication reported a greater than 50% reduction in OAB symptoms at 6 months, 20% reported complete resolution of UUI, and 72% requested a second injection within 24 months.25 The interval between the first and second injection was nearly 1 year (350 days).Risks include urinary tract infection (12% within 1 month of the procedure and 35% through 6 months); urinary retention requiring catheterization has decreased to 6% with recognition that most moderate retention is tolerated by patients.
Some insurers limit onabotulinumtoxinA treatment coverage to patients who have failed to achieve symptom control with first- and second-line treatments.
SUI-specific nonsurgical treatments
Cystoscopic injection of urethral bulking agents into the urethral submucosa is designed to improve urethral coaptation. It is a minor procedure that can be performed in an ambulatory setting under local anesthetic with or without sedation.
Various bulking agents have been approved for use in the United States, some of which have been withdrawn due to complications of migration, erosion, and pseudoabscess formation. Cure or improvement after bulking agent injection was found to be superior to a home pelvic floor exercise program but inferior to a midurethral sling procedure for cure (9% vs 89%).26
The durability of currently available urethral bulking agents beyond 1 year is unknown. Complications are typically minor and transient and include pain at the injection site, urinary retention, de novo urgency, and implant leakage. The advantages include no postprocedure activity restrictions.
CASE Symptom presentation guides treatment plan
Our patient described symptoms of stress-predominant MUI. She was counseled to moderate her fluid intake to 2 L per day and to strategically time voids (before exercise, and at least every 4 hours). The patient was fitted with an incontinence pessary, and she elected to pursue a course of supervised physical therapy for pelvic floor muscle strengthening. Her follow-up visit is scheduled in 3 months to determine if other interventions are warranted. ●
1. Lee UJ, Feinstein L, Ward JB, et al. Prevalence of urinary incontinence among a nationally representative sample of women, 2005–2016: findings from the Urologic Diseases in America Project. J Urol. 2021;205:1718-1724. doi:10.1097 /JU.0000000000001634
2. Sims J, Browning C, Lundgren-Lindquist B, et al. Urinary incontinence in a community sample of older adults: prevalence and impact on quality of life. Disabil Rehabil. 2011;33:1389-1398. doi:10.3109/09638288.2010.532284
3. Sarikaya S, Yildiz FG, Senocak C, et al. Urinary incontinence as a cause of depression and sexual dysfunction: questionnaire-based study. Rev Int Androl. 2020:18:50-54. doi:10.1016 /j.androl.2018.08.003
4. O’Reilly N, Nelson HD, Conry JM, et al; Women’s Preventive Services Initiative. Screening for urinary incontinence in women: a recommendation from the Women’s Preventive Services Initiative. Ann Intern Med. 2018;169(5):320-328. doi:10.7326/M18-0595
5. Barber MD, Walters MD, Karram MM, et al. Walters & Karram Urogynecology and Reconstructive Pelvic Surgery. 5th ed. Elsevier Saunders; 2021.
6. Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J. 2010;21: 5-26. doi:10.1007/s00192-009-0976-9
7. ACOG practice bulletin no. 155. Urinary incontinence in women. Obstet Gynecol. 2015;126:e66-e81. doi:10.1097 /AOG.0000000000001148
8. Sansone S, Lu J, Drangsholt S, et al. No pelvic exam, no problem: patient satisfaction following the integration of comprehensive urogynecology telemedicine. Int Urogynecol J. 2022;1:3. doi:10.1007/s00192-022-05104-w
9. Brown JS, Bradley CS, Subak LL, et al; Diagnostic Aspects of Incontinence Study (DAISy) Research Group. The sensitivity and specificity of a simple test to distinguish between urge and stress urinary incontinence. Ann Intern Med. 2006;144:715723. doi:10.7326/0003-4819-144-10-200605160-00005
10. Hess R, Huang AJ, Richter HE, et al. Long-term efficacy and safety of questionnaire-based initiation of urgency urinary incontinence treatment. Am J Obstet Gynecol. 2013;209:244. e1-9. doi:10.1016/j.ajog.2013.05.008
11. Sung VW, Borello-France D, Newman DK, et al; NICHD Pelvic Floor Disorders Network. Effect of behavioral and pelvic floor muscle therapy combined with surgery vs surgery alone on incontinence symptoms among women with mixed urinary incontinence. JAMA. 2019;322:1066-1076. doi:10.1001 /jama.2019.12467
12. American Urogynecologic Society. Voices for PFD: intake and voiding diary. Accessed August 11, 2022. https://www .voicesforpfd.org/assets/2/6/Voiding_Diary.pdf
13. Subak LL, Richter HE, Hunskaar S. Obesity and urinary incontinence: epidemiology and clinical research update. J Urol. 2009;182(6 suppl):S2-7. doi:10.1016/j.juro.2009.08.071
14. Arya LA, Myers DL, Jackson ND. Dietary caffeine intake and the risk for detrusor instability: a case-control study. Obstet Gynecol. 2000;96:85-89. doi:10.1016/s0029-7844(00)00808-5
15. Wyman JF, Zhou J, LaCoursiere DY, et al. Normative noninvasive bladder function measurements in healthy women: a systematic review and meta-analysis. Neurourol Urodyn. 2020;39:507-522. doi:10.1002/nau.24265
16. Hashim H, Al Mousa R. Management of fluid intake in patients with overactive bladder. Curr Urol Rep. 2009;10: 428-433. doi:10.1007/s11934-009-0068-x
17. Dumoulin C, Cacciari LP, Hay-Smith EJC. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women. Cochrane Database Syst Rev. 2018;10:CD005654. doi:10.1002/14651858.CD005654.pub4
18. Araujo CC, de A Marques A, Juliato CRT. The adherence of home pelvic floor muscles training using a mobile device application for women with urinary incontinence: a randomized controlled trial. Female Pelvic Med Reconstr Surg. 2020;26:697-703. doi:10.1097/SPV.0000000000000670
19. Sjöström M, Umefjord G, Stenlund H, et al. Internet-based treatment of stress urinary incontinence: a randomized controlled study with focus on pelvic floor muscle training. BJU Int. 2013;112:362-372. doi:10.1111/j.1464 -410X.2012.11713.x
20. Richter HE, Burgio KL, Brubaker L, et al; Pelvic Floor Disorders Network. Continence pessary compared with behavioral therapy or combined therapy for stress incontinence: a randomized controlled trial. Obstet Gynecol. 2010;115:609617. doi:10.1097/AOG.0b013e3181d055d4
21. Burgio KL, Kraus SR, Menefee S, et al. Behavioral therapy to enable women with urge incontinence to discontinue drug treatment: a randomized trial. Ann Intern Med. 2008;149(3): 161-169. doi:10.7326/0003-4819-149-3-200808050 -00005
22. Lukacz ES, Santiago-Lastra Y, Albo ME, et al. Urinary incontinence in women: a review. JAMA. 2017;318:1592-1604. doi:10.1001/jama.2017.12137
23. Welk B, Richardson K, Panicker JN. The cognitive effect of anticholinergics for patients with overactive bladder. Nat Rev Urol. 2021;18:686-700. doi:10.1038/s41585-021-00504-x
24. Burton C, Sajja A, Latthe PM. Effectiveness of percutaneous posterior tibial nerve stimulation for overactive bladder: a systematic review and meta-analysis. Neurourol Urodyn. 2012;31:1206-1216. doi:10.1002/nau.22251
25. Amundsen CL, Richter HE, Menefee SA, et al. OnabotulinumtoxinA vs sacral neuromodulation on refractory urgency urinary incontinence in women: A randomized clinical trial. JAMA. 2016;316:1366-1374. doi:10.1001/jama.2016.14617
26. Kirchin V, Page T, Keegan PE, et al. Urethral injection therapy for urinary incontinence in women. Cochrane Database Syst Rev. 2017;7:CD003881. doi:10.1002/14651858.CD003881.pub4
CASE Patient has urine leakage that worsens with exercise
At her annual preventative health visit, a 39-year-old woman reports that she has leakage of urine. She states that she drinks “a gallon of water daily” to help her lose the 20 lb she gained during the COVID-19 pandemic. She wants to resume Zumba fitness classes, but exercise makes her urine leakage worse. She started wearing protective pads because she finds herself often leaking urine on the way to the bathroom.
What nonsurgical treatment options are available for this patient?
Nearly half of all women experience urinary incontinence (UI), the involuntary loss of urine, and the condition increases with age.1 This common condition negatively impacts physical and psychological health and has been associated with social isolation, sexual dysfunction, and reduced independence.2,3 Symptoms of UI are underreported, and therefore universal screening is recommended for women of all ages.4 The diversity of available treatments (TABLE 1) provides patients and clinicians an opportunity to develop a plan that aligns with their symptom severity, goals, preferences, and resources.

Types of UI
The most common types of UI are stress urinary incontinence (SUI) and urgency urinary incontinence (UUI). Mixed urinary incontinence (MUI) occurs when symptoms of both SUI and UUI are present. Although the mechanisms that lead to urine leakage vary by the type of incontinence, many primary interventions improve both types of leakage, so a clinical diagnosis is sufficient to initiate treatment.
Stress urinary incontinence results from an impaired or weakened sphincter, which leads to involuntary, yet predictable, urine loss during increased abdominal pressure, such as coughing, laughing, sneezing, lifting, or physical activity.5 In UUI, involuntary loss of urine often accompanies the sudden urge to void. UUI is associated with overactive bladder (OAB), defined as urinary urgency, with or without urinary incontinence, usually accompanied by urinary frequency and/or nocturia (urination that interrupts sleep).6
In OAB, the detrusor muscle contracts randomly, leading to a sudden urge to void. When bladder pressure exceeds urethral sphincter closure pressure, urine leakage occurs. Women describe the urgency episodes as unpredictable, the urine leakage as prolonged with large volumes, and often occurring as they seek the toilet. Risk factors include age, obesity, parity, history of vaginal delivery, family history, ethnicity/race, medical comorbidities, menopausal status, and tobacco use.5
Making a diagnosis
A basic office evaluation is the most key step for diagnostic accuracy that leads to treatment success. This includes a detailed history, assessment of symptom severity, physical exam, pelvic exam, urinalysis, postvoid residual (to rule out urinary retention), and a cough stress test (to demonstrate SUI). The goal is to assess symptom severity, determine the type of UI, and identify contributing and potentially reversible factors, such as a urinary tract infection, medications, pelvic organ prolapse, incomplete bladder emptying, or impaired neurologic status. In the absence of the latter, advanced diagnostic tests, such as urodynamics, contribute little toward discerning the type of incontinence or changing first-line treatment plans.7
During the COVID-19 pandemic, abbreviated, virtual assessments for urinary symptoms were associated with high degrees of satisfaction (91% for fulfillment of personal needs, 94% overall satisfaction).8 This highlights the value of validated symptom questionnaires that help establish a working diagnosis and treatment plan in the absence of a physical exam. Questionnaire-based diagnoses have acceptable accuracy for classifying UUI and SUI among women with uncomplicated medical and surgical histories and for initiating low-risk therapies for defined intervals.
The 3 incontinence questions (3IQ) screen is an example of a useful, quick diagnostic tool designed for the primary care setting (FIGURE 1).9 It has been used in pharmaceutical treatment trials for UUI, with low frequency of misdiagnosis (1%–4%), resulting in no harm by the drug treatment prescribed or by the delay in appropriate care.10 Due to the limitations of an abbreviated remote evaluation, however, clinicians should assess patient response to primary interventions in a timely window. Patients who fail to experience satisfactory symptom reduction within 6 to 12 weeks should complete their evaluation in person or through a referral to a urogynecology program.

Continue to: Primary therapies for UI...
Primary therapies for UI
Primary therapies for UUI and SUI target strength training of the pelvic floor muscles, moderation of fluid intake, and adjustment in voiding behaviors and medications. Any functional barriers to continence also should be identified and addressed. Simple interventions, including a daily bowel regimen to address constipation, a bedside commode, and scheduled voiding, may reduce incontinence episodes without incurring significant cost or risk. For women suspected of having MUI, the treatment plan should prioritize their most bothersome symptoms.
Lifestyle and behavioral modifications
Everyday habits, medical comorbidities, and medications may exacerbate the severity of both SUI and UUI. Behavioral therapy alone or in combination with other interventions effectively reduces both SUI and UUI symptoms and has been shown to improve the efficacy of continence surgery.11 Information gained from a 3-day bladder diary (FIGURE 2)12 can guide clinicians on personalized patient recommendations, such as reducing excessive consumption of fluids and bladder irritants, limiting late evening drinking in the setting of bothersome nocturia, and scheduling voids (every 2–3 hours) to preempt incontinence episodes.

Weight loss
Obesity is a strong, independent, modifiable risk factor for both SUI and UUI. Each 5 kg/m2 increase in body mass index (BMI) has been associated with a 20% to 70% increased risk of UI, while weight loss of 5% or greater in overweight or obese women can lead to at least a 50% decrease in UI frequency.13
Reducing fluid intake and bladder irritants
Overactive bladder symptoms often respond to moderation of excessive fluid intake and reduction of bladder irritants (caffeine, carbonated beverages, diet beverages, and alcohol). While there is no established definition of excess caffeine intake, one study categorized high caffeine intake as greater than 400 mg/day (approximately four 8-oz cups of coffee).14
Information provided in a bladder diary can guide individualized recommendations for reducing fluid intake, particularly when 24-hour urine production exceeds the normative range (> 50–60 oz or 1.5-1.8 L/day).15 Hydration needs vary by activity, environment, and food; some general guidelines suggest 48 to 64 oz/day.5,16
Continue to: Pelvic floor muscle training...
Pelvic floor muscle training
An effective treatment for both UUI and SUI symptoms, pelvic floor muscle training (PFMT) leads to high degrees of patient satisfaction and improvement in quality of life.17 The presumed mechanisms of action of PFMT include improved urethral closure pressure and inhibition of detrusor muscle contractions.
Common exercise protocols recommend 3 sets of 10 contractions, held for 6 to 10 seconds per day, in varying positions of sitting, standing, and lying. While many women may be familiar with Kegel exercises, poor technique with straining and recruitment of gluteal and abdominal muscles can undermine the effect of PFMT. Clinicians can confirm successful pelvic muscle contractions by placing a finger in the vagina to appreciate contraction around and elevation of the finger toward the pubic symphysis in the absence of pushing.
Referral to supervised physical therapy and use of such teaching aid tools as booklets, mobile applications, and biofeedback can improve exercise adherence and outcomes.18,19 Systematic reviews report initial cure or improvement of incontinence symptoms as high as 74%, although little information is available about the long-term duration of effect.17
Vaginal pessaries
Vaginal continence support pessaries and devices work by stabilizing urethral mobility and compression of the bladder neck. Continence devices are particularly effective for situational SUI (such as during exercise).
The reusable medical grade silicone pessaries are available in numerous shapes and sizes and are fitted by a health care clinician (FIGURE 3). Uresta is a self-fitted intravaginal device that women can purchase online with a prescription. The Poise Impressa bladder support is a disposable intravaginal device marketed for incontinence and available over-the-counter, without a prescription (FIGURE 4). Anecdotally, many women find that menstrual tampons provide a similar effect, but outcome data are lacking.


In a comparative effectiveness trial of a continence pessary and behavior therapy, behavioral therapy was more likely to result in no bothersome incontinence symptoms (49% vs 33%, P = .006) and greater treatment satisfaction at 3 months.20 However, these short-term group differences did not persist at 12 months, presumably due to waning adherence.
UUI-specific nonsurgical treatments
Drug therapy
All medications approved by the US Food and Drug Administration (FDA) for UI are for the indications of OAB or UUI. These second-line treatments are most effective as adjuncts to behavioral modifications and PFMT.
A multicenter randomized trial that evaluated the efficacy of drug therapy alone compared with drug therapy in combination with behavioral modification, PFMT, urge suppression strategies, timed voiding, and fluid management for UUI found that combined therapy was more successful in achieving greater than 70% reduction in incontinence episodes (58% for drug therapy vs 69% for combined therapy).21
Of the 8 medications currently marketed in the United States for OAB or UUI, 6 are anticholinergic agents that block muscarinic receptors in the smooth muscle of the bladder, leading to inhibition of detrusor contractions, and 2 are β-adrenergic receptor agonists that promote bladder storage capacity by relaxing the detrusor muscle (TABLE 2). Similar efficacies lead most clinicians to initiate drug therapy based on formulary coverage and tolerance for adverse effects. Patients can expect a 53% to 80% reduction in UUI episodes and a 12% to 32% reduction in urinary frequency.22

Extended-release formulations are associated with reduced anticholinergic side effects (dry mouth, constipation, somnolence, dry eyes), leading to improved adherence. Notably, the anticholinergic medications are contraindicated in patients with untreated narrow-angle glaucoma, gastric retention, and supraventricular tachycardia. Mirabegron should be used with caution in patients with poorly controlled hypertension. 5 Due to concerns regarding the association between cumulative anticholinergic burden and the development of dementia, clinicians may consider avoiding the anticholinergic medications in older and at-risk patients.23
Continue to: UUI office-based procedure treatments...
UUI office-based procedure treatments
If behavioral therapies and medications are ineffective, contraindicated, or not the patient’s preference, additional FDA-approved therapies for UUI are available, typically through referral to a urogynecologist, urologist, or continence center.
Posterior tibial nerve stimulation (PTNS) is a nondrug treatment that delivers electrical stimulation using an acupuncture needle for 12 weekly 30-minute sessions followed by monthly maintenance for responders. The time commitment for this treatment plan can be a barrier for some patients. However, patients who adhere to the recommended protocol can expect a 60% improvement in symptoms, with minimal adverse events. Treatment efficacy is comparable to that of anticholinergic medication.24
OnabotulinumtoxinA injections into the bladder muscle are performed cystoscopically under local anesthetic. The toxin blocks the presynaptic release of acetylcholine at the neuromuscular junction, resulting in temporary muscle paralysis. This treatment is associated with high satisfaction. Efficacy varies by study population and outcome measure.
In one US comparative effectiveness trial, 67% of study participants with UUI symptoms refractory to oral medication reported a greater than 50% reduction in OAB symptoms at 6 months, 20% reported complete resolution of UUI, and 72% requested a second injection within 24 months.25 The interval between the first and second injection was nearly 1 year (350 days).Risks include urinary tract infection (12% within 1 month of the procedure and 35% through 6 months); urinary retention requiring catheterization has decreased to 6% with recognition that most moderate retention is tolerated by patients.
Some insurers limit onabotulinumtoxinA treatment coverage to patients who have failed to achieve symptom control with first- and second-line treatments.
SUI-specific nonsurgical treatments
Cystoscopic injection of urethral bulking agents into the urethral submucosa is designed to improve urethral coaptation. It is a minor procedure that can be performed in an ambulatory setting under local anesthetic with or without sedation.
Various bulking agents have been approved for use in the United States, some of which have been withdrawn due to complications of migration, erosion, and pseudoabscess formation. Cure or improvement after bulking agent injection was found to be superior to a home pelvic floor exercise program but inferior to a midurethral sling procedure for cure (9% vs 89%).26
The durability of currently available urethral bulking agents beyond 1 year is unknown. Complications are typically minor and transient and include pain at the injection site, urinary retention, de novo urgency, and implant leakage. The advantages include no postprocedure activity restrictions.
CASE Symptom presentation guides treatment plan
Our patient described symptoms of stress-predominant MUI. She was counseled to moderate her fluid intake to 2 L per day and to strategically time voids (before exercise, and at least every 4 hours). The patient was fitted with an incontinence pessary, and she elected to pursue a course of supervised physical therapy for pelvic floor muscle strengthening. Her follow-up visit is scheduled in 3 months to determine if other interventions are warranted. ●
CASE Patient has urine leakage that worsens with exercise
At her annual preventative health visit, a 39-year-old woman reports that she has leakage of urine. She states that she drinks “a gallon of water daily” to help her lose the 20 lb she gained during the COVID-19 pandemic. She wants to resume Zumba fitness classes, but exercise makes her urine leakage worse. She started wearing protective pads because she finds herself often leaking urine on the way to the bathroom.
What nonsurgical treatment options are available for this patient?
Nearly half of all women experience urinary incontinence (UI), the involuntary loss of urine, and the condition increases with age.1 This common condition negatively impacts physical and psychological health and has been associated with social isolation, sexual dysfunction, and reduced independence.2,3 Symptoms of UI are underreported, and therefore universal screening is recommended for women of all ages.4 The diversity of available treatments (TABLE 1) provides patients and clinicians an opportunity to develop a plan that aligns with their symptom severity, goals, preferences, and resources.

Types of UI
The most common types of UI are stress urinary incontinence (SUI) and urgency urinary incontinence (UUI). Mixed urinary incontinence (MUI) occurs when symptoms of both SUI and UUI are present. Although the mechanisms that lead to urine leakage vary by the type of incontinence, many primary interventions improve both types of leakage, so a clinical diagnosis is sufficient to initiate treatment.
Stress urinary incontinence results from an impaired or weakened sphincter, which leads to involuntary, yet predictable, urine loss during increased abdominal pressure, such as coughing, laughing, sneezing, lifting, or physical activity.5 In UUI, involuntary loss of urine often accompanies the sudden urge to void. UUI is associated with overactive bladder (OAB), defined as urinary urgency, with or without urinary incontinence, usually accompanied by urinary frequency and/or nocturia (urination that interrupts sleep).6
In OAB, the detrusor muscle contracts randomly, leading to a sudden urge to void. When bladder pressure exceeds urethral sphincter closure pressure, urine leakage occurs. Women describe the urgency episodes as unpredictable, the urine leakage as prolonged with large volumes, and often occurring as they seek the toilet. Risk factors include age, obesity, parity, history of vaginal delivery, family history, ethnicity/race, medical comorbidities, menopausal status, and tobacco use.5
Making a diagnosis
A basic office evaluation is the most key step for diagnostic accuracy that leads to treatment success. This includes a detailed history, assessment of symptom severity, physical exam, pelvic exam, urinalysis, postvoid residual (to rule out urinary retention), and a cough stress test (to demonstrate SUI). The goal is to assess symptom severity, determine the type of UI, and identify contributing and potentially reversible factors, such as a urinary tract infection, medications, pelvic organ prolapse, incomplete bladder emptying, or impaired neurologic status. In the absence of the latter, advanced diagnostic tests, such as urodynamics, contribute little toward discerning the type of incontinence or changing first-line treatment plans.7
During the COVID-19 pandemic, abbreviated, virtual assessments for urinary symptoms were associated with high degrees of satisfaction (91% for fulfillment of personal needs, 94% overall satisfaction).8 This highlights the value of validated symptom questionnaires that help establish a working diagnosis and treatment plan in the absence of a physical exam. Questionnaire-based diagnoses have acceptable accuracy for classifying UUI and SUI among women with uncomplicated medical and surgical histories and for initiating low-risk therapies for defined intervals.
The 3 incontinence questions (3IQ) screen is an example of a useful, quick diagnostic tool designed for the primary care setting (FIGURE 1).9 It has been used in pharmaceutical treatment trials for UUI, with low frequency of misdiagnosis (1%–4%), resulting in no harm by the drug treatment prescribed or by the delay in appropriate care.10 Due to the limitations of an abbreviated remote evaluation, however, clinicians should assess patient response to primary interventions in a timely window. Patients who fail to experience satisfactory symptom reduction within 6 to 12 weeks should complete their evaluation in person or through a referral to a urogynecology program.

Continue to: Primary therapies for UI...
Primary therapies for UI
Primary therapies for UUI and SUI target strength training of the pelvic floor muscles, moderation of fluid intake, and adjustment in voiding behaviors and medications. Any functional barriers to continence also should be identified and addressed. Simple interventions, including a daily bowel regimen to address constipation, a bedside commode, and scheduled voiding, may reduce incontinence episodes without incurring significant cost or risk. For women suspected of having MUI, the treatment plan should prioritize their most bothersome symptoms.
Lifestyle and behavioral modifications
Everyday habits, medical comorbidities, and medications may exacerbate the severity of both SUI and UUI. Behavioral therapy alone or in combination with other interventions effectively reduces both SUI and UUI symptoms and has been shown to improve the efficacy of continence surgery.11 Information gained from a 3-day bladder diary (FIGURE 2)12 can guide clinicians on personalized patient recommendations, such as reducing excessive consumption of fluids and bladder irritants, limiting late evening drinking in the setting of bothersome nocturia, and scheduling voids (every 2–3 hours) to preempt incontinence episodes.

Weight loss
Obesity is a strong, independent, modifiable risk factor for both SUI and UUI. Each 5 kg/m2 increase in body mass index (BMI) has been associated with a 20% to 70% increased risk of UI, while weight loss of 5% or greater in overweight or obese women can lead to at least a 50% decrease in UI frequency.13
Reducing fluid intake and bladder irritants
Overactive bladder symptoms often respond to moderation of excessive fluid intake and reduction of bladder irritants (caffeine, carbonated beverages, diet beverages, and alcohol). While there is no established definition of excess caffeine intake, one study categorized high caffeine intake as greater than 400 mg/day (approximately four 8-oz cups of coffee).14
Information provided in a bladder diary can guide individualized recommendations for reducing fluid intake, particularly when 24-hour urine production exceeds the normative range (> 50–60 oz or 1.5-1.8 L/day).15 Hydration needs vary by activity, environment, and food; some general guidelines suggest 48 to 64 oz/day.5,16
Continue to: Pelvic floor muscle training...
Pelvic floor muscle training
An effective treatment for both UUI and SUI symptoms, pelvic floor muscle training (PFMT) leads to high degrees of patient satisfaction and improvement in quality of life.17 The presumed mechanisms of action of PFMT include improved urethral closure pressure and inhibition of detrusor muscle contractions.
Common exercise protocols recommend 3 sets of 10 contractions, held for 6 to 10 seconds per day, in varying positions of sitting, standing, and lying. While many women may be familiar with Kegel exercises, poor technique with straining and recruitment of gluteal and abdominal muscles can undermine the effect of PFMT. Clinicians can confirm successful pelvic muscle contractions by placing a finger in the vagina to appreciate contraction around and elevation of the finger toward the pubic symphysis in the absence of pushing.
Referral to supervised physical therapy and use of such teaching aid tools as booklets, mobile applications, and biofeedback can improve exercise adherence and outcomes.18,19 Systematic reviews report initial cure or improvement of incontinence symptoms as high as 74%, although little information is available about the long-term duration of effect.17
Vaginal pessaries
Vaginal continence support pessaries and devices work by stabilizing urethral mobility and compression of the bladder neck. Continence devices are particularly effective for situational SUI (such as during exercise).
The reusable medical grade silicone pessaries are available in numerous shapes and sizes and are fitted by a health care clinician (FIGURE 3). Uresta is a self-fitted intravaginal device that women can purchase online with a prescription. The Poise Impressa bladder support is a disposable intravaginal device marketed for incontinence and available over-the-counter, without a prescription (FIGURE 4). Anecdotally, many women find that menstrual tampons provide a similar effect, but outcome data are lacking.


In a comparative effectiveness trial of a continence pessary and behavior therapy, behavioral therapy was more likely to result in no bothersome incontinence symptoms (49% vs 33%, P = .006) and greater treatment satisfaction at 3 months.20 However, these short-term group differences did not persist at 12 months, presumably due to waning adherence.
UUI-specific nonsurgical treatments
Drug therapy
All medications approved by the US Food and Drug Administration (FDA) for UI are for the indications of OAB or UUI. These second-line treatments are most effective as adjuncts to behavioral modifications and PFMT.
A multicenter randomized trial that evaluated the efficacy of drug therapy alone compared with drug therapy in combination with behavioral modification, PFMT, urge suppression strategies, timed voiding, and fluid management for UUI found that combined therapy was more successful in achieving greater than 70% reduction in incontinence episodes (58% for drug therapy vs 69% for combined therapy).21
Of the 8 medications currently marketed in the United States for OAB or UUI, 6 are anticholinergic agents that block muscarinic receptors in the smooth muscle of the bladder, leading to inhibition of detrusor contractions, and 2 are β-adrenergic receptor agonists that promote bladder storage capacity by relaxing the detrusor muscle (TABLE 2). Similar efficacies lead most clinicians to initiate drug therapy based on formulary coverage and tolerance for adverse effects. Patients can expect a 53% to 80% reduction in UUI episodes and a 12% to 32% reduction in urinary frequency.22

Extended-release formulations are associated with reduced anticholinergic side effects (dry mouth, constipation, somnolence, dry eyes), leading to improved adherence. Notably, the anticholinergic medications are contraindicated in patients with untreated narrow-angle glaucoma, gastric retention, and supraventricular tachycardia. Mirabegron should be used with caution in patients with poorly controlled hypertension. 5 Due to concerns regarding the association between cumulative anticholinergic burden and the development of dementia, clinicians may consider avoiding the anticholinergic medications in older and at-risk patients.23
Continue to: UUI office-based procedure treatments...
UUI office-based procedure treatments
If behavioral therapies and medications are ineffective, contraindicated, or not the patient’s preference, additional FDA-approved therapies for UUI are available, typically through referral to a urogynecologist, urologist, or continence center.
Posterior tibial nerve stimulation (PTNS) is a nondrug treatment that delivers electrical stimulation using an acupuncture needle for 12 weekly 30-minute sessions followed by monthly maintenance for responders. The time commitment for this treatment plan can be a barrier for some patients. However, patients who adhere to the recommended protocol can expect a 60% improvement in symptoms, with minimal adverse events. Treatment efficacy is comparable to that of anticholinergic medication.24
OnabotulinumtoxinA injections into the bladder muscle are performed cystoscopically under local anesthetic. The toxin blocks the presynaptic release of acetylcholine at the neuromuscular junction, resulting in temporary muscle paralysis. This treatment is associated with high satisfaction. Efficacy varies by study population and outcome measure.
In one US comparative effectiveness trial, 67% of study participants with UUI symptoms refractory to oral medication reported a greater than 50% reduction in OAB symptoms at 6 months, 20% reported complete resolution of UUI, and 72% requested a second injection within 24 months.25 The interval between the first and second injection was nearly 1 year (350 days).Risks include urinary tract infection (12% within 1 month of the procedure and 35% through 6 months); urinary retention requiring catheterization has decreased to 6% with recognition that most moderate retention is tolerated by patients.
Some insurers limit onabotulinumtoxinA treatment coverage to patients who have failed to achieve symptom control with first- and second-line treatments.
SUI-specific nonsurgical treatments
Cystoscopic injection of urethral bulking agents into the urethral submucosa is designed to improve urethral coaptation. It is a minor procedure that can be performed in an ambulatory setting under local anesthetic with or without sedation.
Various bulking agents have been approved for use in the United States, some of which have been withdrawn due to complications of migration, erosion, and pseudoabscess formation. Cure or improvement after bulking agent injection was found to be superior to a home pelvic floor exercise program but inferior to a midurethral sling procedure for cure (9% vs 89%).26
The durability of currently available urethral bulking agents beyond 1 year is unknown. Complications are typically minor and transient and include pain at the injection site, urinary retention, de novo urgency, and implant leakage. The advantages include no postprocedure activity restrictions.
CASE Symptom presentation guides treatment plan
Our patient described symptoms of stress-predominant MUI. She was counseled to moderate her fluid intake to 2 L per day and to strategically time voids (before exercise, and at least every 4 hours). The patient was fitted with an incontinence pessary, and she elected to pursue a course of supervised physical therapy for pelvic floor muscle strengthening. Her follow-up visit is scheduled in 3 months to determine if other interventions are warranted. ●
1. Lee UJ, Feinstein L, Ward JB, et al. Prevalence of urinary incontinence among a nationally representative sample of women, 2005–2016: findings from the Urologic Diseases in America Project. J Urol. 2021;205:1718-1724. doi:10.1097 /JU.0000000000001634
2. Sims J, Browning C, Lundgren-Lindquist B, et al. Urinary incontinence in a community sample of older adults: prevalence and impact on quality of life. Disabil Rehabil. 2011;33:1389-1398. doi:10.3109/09638288.2010.532284
3. Sarikaya S, Yildiz FG, Senocak C, et al. Urinary incontinence as a cause of depression and sexual dysfunction: questionnaire-based study. Rev Int Androl. 2020:18:50-54. doi:10.1016 /j.androl.2018.08.003
4. O’Reilly N, Nelson HD, Conry JM, et al; Women’s Preventive Services Initiative. Screening for urinary incontinence in women: a recommendation from the Women’s Preventive Services Initiative. Ann Intern Med. 2018;169(5):320-328. doi:10.7326/M18-0595
5. Barber MD, Walters MD, Karram MM, et al. Walters & Karram Urogynecology and Reconstructive Pelvic Surgery. 5th ed. Elsevier Saunders; 2021.
6. Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J. 2010;21: 5-26. doi:10.1007/s00192-009-0976-9
7. ACOG practice bulletin no. 155. Urinary incontinence in women. Obstet Gynecol. 2015;126:e66-e81. doi:10.1097 /AOG.0000000000001148
8. Sansone S, Lu J, Drangsholt S, et al. No pelvic exam, no problem: patient satisfaction following the integration of comprehensive urogynecology telemedicine. Int Urogynecol J. 2022;1:3. doi:10.1007/s00192-022-05104-w
9. Brown JS, Bradley CS, Subak LL, et al; Diagnostic Aspects of Incontinence Study (DAISy) Research Group. The sensitivity and specificity of a simple test to distinguish between urge and stress urinary incontinence. Ann Intern Med. 2006;144:715723. doi:10.7326/0003-4819-144-10-200605160-00005
10. Hess R, Huang AJ, Richter HE, et al. Long-term efficacy and safety of questionnaire-based initiation of urgency urinary incontinence treatment. Am J Obstet Gynecol. 2013;209:244. e1-9. doi:10.1016/j.ajog.2013.05.008
11. Sung VW, Borello-France D, Newman DK, et al; NICHD Pelvic Floor Disorders Network. Effect of behavioral and pelvic floor muscle therapy combined with surgery vs surgery alone on incontinence symptoms among women with mixed urinary incontinence. JAMA. 2019;322:1066-1076. doi:10.1001 /jama.2019.12467
12. American Urogynecologic Society. Voices for PFD: intake and voiding diary. Accessed August 11, 2022. https://www .voicesforpfd.org/assets/2/6/Voiding_Diary.pdf
13. Subak LL, Richter HE, Hunskaar S. Obesity and urinary incontinence: epidemiology and clinical research update. J Urol. 2009;182(6 suppl):S2-7. doi:10.1016/j.juro.2009.08.071
14. Arya LA, Myers DL, Jackson ND. Dietary caffeine intake and the risk for detrusor instability: a case-control study. Obstet Gynecol. 2000;96:85-89. doi:10.1016/s0029-7844(00)00808-5
15. Wyman JF, Zhou J, LaCoursiere DY, et al. Normative noninvasive bladder function measurements in healthy women: a systematic review and meta-analysis. Neurourol Urodyn. 2020;39:507-522. doi:10.1002/nau.24265
16. Hashim H, Al Mousa R. Management of fluid intake in patients with overactive bladder. Curr Urol Rep. 2009;10: 428-433. doi:10.1007/s11934-009-0068-x
17. Dumoulin C, Cacciari LP, Hay-Smith EJC. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women. Cochrane Database Syst Rev. 2018;10:CD005654. doi:10.1002/14651858.CD005654.pub4
18. Araujo CC, de A Marques A, Juliato CRT. The adherence of home pelvic floor muscles training using a mobile device application for women with urinary incontinence: a randomized controlled trial. Female Pelvic Med Reconstr Surg. 2020;26:697-703. doi:10.1097/SPV.0000000000000670
19. Sjöström M, Umefjord G, Stenlund H, et al. Internet-based treatment of stress urinary incontinence: a randomized controlled study with focus on pelvic floor muscle training. BJU Int. 2013;112:362-372. doi:10.1111/j.1464 -410X.2012.11713.x
20. Richter HE, Burgio KL, Brubaker L, et al; Pelvic Floor Disorders Network. Continence pessary compared with behavioral therapy or combined therapy for stress incontinence: a randomized controlled trial. Obstet Gynecol. 2010;115:609617. doi:10.1097/AOG.0b013e3181d055d4
21. Burgio KL, Kraus SR, Menefee S, et al. Behavioral therapy to enable women with urge incontinence to discontinue drug treatment: a randomized trial. Ann Intern Med. 2008;149(3): 161-169. doi:10.7326/0003-4819-149-3-200808050 -00005
22. Lukacz ES, Santiago-Lastra Y, Albo ME, et al. Urinary incontinence in women: a review. JAMA. 2017;318:1592-1604. doi:10.1001/jama.2017.12137
23. Welk B, Richardson K, Panicker JN. The cognitive effect of anticholinergics for patients with overactive bladder. Nat Rev Urol. 2021;18:686-700. doi:10.1038/s41585-021-00504-x
24. Burton C, Sajja A, Latthe PM. Effectiveness of percutaneous posterior tibial nerve stimulation for overactive bladder: a systematic review and meta-analysis. Neurourol Urodyn. 2012;31:1206-1216. doi:10.1002/nau.22251
25. Amundsen CL, Richter HE, Menefee SA, et al. OnabotulinumtoxinA vs sacral neuromodulation on refractory urgency urinary incontinence in women: A randomized clinical trial. JAMA. 2016;316:1366-1374. doi:10.1001/jama.2016.14617
26. Kirchin V, Page T, Keegan PE, et al. Urethral injection therapy for urinary incontinence in women. Cochrane Database Syst Rev. 2017;7:CD003881. doi:10.1002/14651858.CD003881.pub4
1. Lee UJ, Feinstein L, Ward JB, et al. Prevalence of urinary incontinence among a nationally representative sample of women, 2005–2016: findings from the Urologic Diseases in America Project. J Urol. 2021;205:1718-1724. doi:10.1097 /JU.0000000000001634
2. Sims J, Browning C, Lundgren-Lindquist B, et al. Urinary incontinence in a community sample of older adults: prevalence and impact on quality of life. Disabil Rehabil. 2011;33:1389-1398. doi:10.3109/09638288.2010.532284
3. Sarikaya S, Yildiz FG, Senocak C, et al. Urinary incontinence as a cause of depression and sexual dysfunction: questionnaire-based study. Rev Int Androl. 2020:18:50-54. doi:10.1016 /j.androl.2018.08.003
4. O’Reilly N, Nelson HD, Conry JM, et al; Women’s Preventive Services Initiative. Screening for urinary incontinence in women: a recommendation from the Women’s Preventive Services Initiative. Ann Intern Med. 2018;169(5):320-328. doi:10.7326/M18-0595
5. Barber MD, Walters MD, Karram MM, et al. Walters & Karram Urogynecology and Reconstructive Pelvic Surgery. 5th ed. Elsevier Saunders; 2021.
6. Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J. 2010;21: 5-26. doi:10.1007/s00192-009-0976-9
7. ACOG practice bulletin no. 155. Urinary incontinence in women. Obstet Gynecol. 2015;126:e66-e81. doi:10.1097 /AOG.0000000000001148
8. Sansone S, Lu J, Drangsholt S, et al. No pelvic exam, no problem: patient satisfaction following the integration of comprehensive urogynecology telemedicine. Int Urogynecol J. 2022;1:3. doi:10.1007/s00192-022-05104-w
9. Brown JS, Bradley CS, Subak LL, et al; Diagnostic Aspects of Incontinence Study (DAISy) Research Group. The sensitivity and specificity of a simple test to distinguish between urge and stress urinary incontinence. Ann Intern Med. 2006;144:715723. doi:10.7326/0003-4819-144-10-200605160-00005
10. Hess R, Huang AJ, Richter HE, et al. Long-term efficacy and safety of questionnaire-based initiation of urgency urinary incontinence treatment. Am J Obstet Gynecol. 2013;209:244. e1-9. doi:10.1016/j.ajog.2013.05.008
11. Sung VW, Borello-France D, Newman DK, et al; NICHD Pelvic Floor Disorders Network. Effect of behavioral and pelvic floor muscle therapy combined with surgery vs surgery alone on incontinence symptoms among women with mixed urinary incontinence. JAMA. 2019;322:1066-1076. doi:10.1001 /jama.2019.12467
12. American Urogynecologic Society. Voices for PFD: intake and voiding diary. Accessed August 11, 2022. https://www .voicesforpfd.org/assets/2/6/Voiding_Diary.pdf
13. Subak LL, Richter HE, Hunskaar S. Obesity and urinary incontinence: epidemiology and clinical research update. J Urol. 2009;182(6 suppl):S2-7. doi:10.1016/j.juro.2009.08.071
14. Arya LA, Myers DL, Jackson ND. Dietary caffeine intake and the risk for detrusor instability: a case-control study. Obstet Gynecol. 2000;96:85-89. doi:10.1016/s0029-7844(00)00808-5
15. Wyman JF, Zhou J, LaCoursiere DY, et al. Normative noninvasive bladder function measurements in healthy women: a systematic review and meta-analysis. Neurourol Urodyn. 2020;39:507-522. doi:10.1002/nau.24265
16. Hashim H, Al Mousa R. Management of fluid intake in patients with overactive bladder. Curr Urol Rep. 2009;10: 428-433. doi:10.1007/s11934-009-0068-x
17. Dumoulin C, Cacciari LP, Hay-Smith EJC. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women. Cochrane Database Syst Rev. 2018;10:CD005654. doi:10.1002/14651858.CD005654.pub4
18. Araujo CC, de A Marques A, Juliato CRT. The adherence of home pelvic floor muscles training using a mobile device application for women with urinary incontinence: a randomized controlled trial. Female Pelvic Med Reconstr Surg. 2020;26:697-703. doi:10.1097/SPV.0000000000000670
19. Sjöström M, Umefjord G, Stenlund H, et al. Internet-based treatment of stress urinary incontinence: a randomized controlled study with focus on pelvic floor muscle training. BJU Int. 2013;112:362-372. doi:10.1111/j.1464 -410X.2012.11713.x
20. Richter HE, Burgio KL, Brubaker L, et al; Pelvic Floor Disorders Network. Continence pessary compared with behavioral therapy or combined therapy for stress incontinence: a randomized controlled trial. Obstet Gynecol. 2010;115:609617. doi:10.1097/AOG.0b013e3181d055d4
21. Burgio KL, Kraus SR, Menefee S, et al. Behavioral therapy to enable women with urge incontinence to discontinue drug treatment: a randomized trial. Ann Intern Med. 2008;149(3): 161-169. doi:10.7326/0003-4819-149-3-200808050 -00005
22. Lukacz ES, Santiago-Lastra Y, Albo ME, et al. Urinary incontinence in women: a review. JAMA. 2017;318:1592-1604. doi:10.1001/jama.2017.12137
23. Welk B, Richardson K, Panicker JN. The cognitive effect of anticholinergics for patients with overactive bladder. Nat Rev Urol. 2021;18:686-700. doi:10.1038/s41585-021-00504-x
24. Burton C, Sajja A, Latthe PM. Effectiveness of percutaneous posterior tibial nerve stimulation for overactive bladder: a systematic review and meta-analysis. Neurourol Urodyn. 2012;31:1206-1216. doi:10.1002/nau.22251
25. Amundsen CL, Richter HE, Menefee SA, et al. OnabotulinumtoxinA vs sacral neuromodulation on refractory urgency urinary incontinence in women: A randomized clinical trial. JAMA. 2016;316:1366-1374. doi:10.1001/jama.2016.14617
26. Kirchin V, Page T, Keegan PE, et al. Urethral injection therapy for urinary incontinence in women. Cochrane Database Syst Rev. 2017;7:CD003881. doi:10.1002/14651858.CD003881.pub4
Is yoga the answer to pelvic floor woes?
After New York–based yoga instructor Erin Conley’s two sisters gave birth, Ms. Conley suggested a few advanced poses to help strengthen their pelvic floor.
“With one of my sisters, she said, ‘Honestly right now, I just can’t even stand up,’ ” she recalled.
Ms. Conley’s other sister could do slightly more advanced poses – leading Ms. Conley to recognize that after delivery, women’s ability to practice yoga varied widely.
“Post-birth is certainly a progression for each woman,” she said. “You can’t just go into these advance postures.”
Ms. Conley tailored a slow sequence of 30-second poses that each sister could start with, and they eventually reported an improvement of pelvic floor issues. Ms. Conley’s suggestions to her sisters are backed by a small but growing body of research. One study published in August in the journal Urology suggests that yoga may be a way to help treat multiple types of pelvic floor disorders.
More than 1 in 4 women in the United States experience pelvic floor disorders such as bowel or urinary incontinence or pelvic organ prolapse, many as a result of giving birth. But less than 15% of these women seek medical treatment for their symptoms, according to Hari Tunuguntla, MD, associate professor of urologic surgery at Rutgers University’s Robert Wood Johnson Medical School, New Brunswick.
For those who do seek medical help, many patients have trouble complying with initial lifestyle-based recommendations, such as refraining from drinking caffeinated and carbonated beverages, Dr. Tunuguntla said.
“It requires a lot of persistence and knowledge and compliance,” he said.
Medication and physical therapy are routes doctors can order before considering surgery, but some patients find clinical-based interventions to be costly. The cost of the interventions can add up depending on what a person’s insurance policy covers, Dr. Tunuguntla said.
With those struggles in mind, he and his colleagues set out to study the efficacy of the mobile app Yoga of Immortals, which offers a holistic form of yoga that includes postures, breathing exercises, sound therapy, and meditation.
“It includes sound therapy, summative breathing exercise,” Dr. Tunuguntla said. “These are useful not just for the condition but for general well-being.”
For the study, Dr. Tunuguntla and his colleagues emailed surveys to 420 people between ages 18 and 74 years in 23 countries who reported having any type of urinary incontinence, regardless of severity. The participants, most of whom were women, used the yoga app for 30 minutes a day for 8 weeks.
More than three-quarters of participants reported that the frequency and severity of their incontinence improved after 8 weeks of practice, compared with when they started, without having to visit their health care provider. Most participants also said that they felt “very much better” after 8 weeks, compared with when they began the yoga regimen, the researchers found.
The study did not compare the effectiveness of the approach with other standard treatments for incontinence, like physical therapy, medication, or surgery.
Ms. Conley, an instructor since 2010, said that one of the benefits of yoga is building strength and flexibility slowly and simultaneously. She uses yoga poses that focus less on movement and more on holding positions for longer periods of time.
“I’ll do sequences of a mountain pose with a block to activate the core in the most basic ways and really focus on the breathing,” she said.
Another benefit of slower forms of yoga is that they can help participants become more aware of the structures of their pelvic floor, according to Alison Huang, MD, professor of medicine, urology and epidemiology, and biostatistics at University of California, San Francisco.
“In some ways we can think of it as a complementary substitute for rehabilitation therapy,” Dr. Huang said.
Dr. Huang and her colleagues published a short report recently in The Journal of Integrative and Complementary Medicine, showing that even telehealth-based yoga programs for older women with urinary incontinence can offer an accessible way for women of any background to take advantage of yoga’s benefits.
An estimated 93% of 66 participants who practiced yoga through planned telehealth appointments reported feeling “very or moderately satisfied” with their practice. Dr. Huang said that the study is not yet complete but offers a glimpse into some of the advantages of yoga for women with urinary incontinence.
“Any kind of treatment that relies on intensive one-on-one visits with specialists is going to be harder to access for some women,” Dr. Huang told this news organization. “Yoga is typically practiced in a community setting, outside of traditional health care settings.”
The accessibility of yoga and its community-based practice may help eliminate any obstacles to care and compliance that clinicians like she and Dr. Tunuguntla at times experience. Mounting studies have also indicated that yoga may help improve overall wellness, manage stress, promote healthier eating, and benefit a person’s mental and emotional health.
Despite emerging research on the link between yoga and pelvic floor disorders, Dr. Huang said that it’s still early for clinicians to recommend the exercise form for every patient.
“We just don’t have the [solid] evidence to show your pelvic floor will improve,” she said.
“For any woman who is starting out more sedentary, I think there are benefits to practic[ing] yoga for overall health,” Dr. Huang said. “Most clinicians would say there are opportunities to practice yoga regularly in a way that is safe, with a knowledgeable instructor.”
According to Ms. Conley, yoga is only as beneficial as a person’s level of consistency in the practice.
“The dedication to yoga is your willingness to showing up,” she said. “I think depending on your commitment to the practice, if you’re really committed to the practice – just like you show up to physical therapy every day – you will improve,” said Ms. Conley.
“Being gentle and patient with the process is important too,” she said.
Dr. Tunuguntla and coauthors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
After New York–based yoga instructor Erin Conley’s two sisters gave birth, Ms. Conley suggested a few advanced poses to help strengthen their pelvic floor.
“With one of my sisters, she said, ‘Honestly right now, I just can’t even stand up,’ ” she recalled.
Ms. Conley’s other sister could do slightly more advanced poses – leading Ms. Conley to recognize that after delivery, women’s ability to practice yoga varied widely.
“Post-birth is certainly a progression for each woman,” she said. “You can’t just go into these advance postures.”
Ms. Conley tailored a slow sequence of 30-second poses that each sister could start with, and they eventually reported an improvement of pelvic floor issues. Ms. Conley’s suggestions to her sisters are backed by a small but growing body of research. One study published in August in the journal Urology suggests that yoga may be a way to help treat multiple types of pelvic floor disorders.
More than 1 in 4 women in the United States experience pelvic floor disorders such as bowel or urinary incontinence or pelvic organ prolapse, many as a result of giving birth. But less than 15% of these women seek medical treatment for their symptoms, according to Hari Tunuguntla, MD, associate professor of urologic surgery at Rutgers University’s Robert Wood Johnson Medical School, New Brunswick.
For those who do seek medical help, many patients have trouble complying with initial lifestyle-based recommendations, such as refraining from drinking caffeinated and carbonated beverages, Dr. Tunuguntla said.
“It requires a lot of persistence and knowledge and compliance,” he said.
Medication and physical therapy are routes doctors can order before considering surgery, but some patients find clinical-based interventions to be costly. The cost of the interventions can add up depending on what a person’s insurance policy covers, Dr. Tunuguntla said.
With those struggles in mind, he and his colleagues set out to study the efficacy of the mobile app Yoga of Immortals, which offers a holistic form of yoga that includes postures, breathing exercises, sound therapy, and meditation.
“It includes sound therapy, summative breathing exercise,” Dr. Tunuguntla said. “These are useful not just for the condition but for general well-being.”
For the study, Dr. Tunuguntla and his colleagues emailed surveys to 420 people between ages 18 and 74 years in 23 countries who reported having any type of urinary incontinence, regardless of severity. The participants, most of whom were women, used the yoga app for 30 minutes a day for 8 weeks.
More than three-quarters of participants reported that the frequency and severity of their incontinence improved after 8 weeks of practice, compared with when they started, without having to visit their health care provider. Most participants also said that they felt “very much better” after 8 weeks, compared with when they began the yoga regimen, the researchers found.
The study did not compare the effectiveness of the approach with other standard treatments for incontinence, like physical therapy, medication, or surgery.
Ms. Conley, an instructor since 2010, said that one of the benefits of yoga is building strength and flexibility slowly and simultaneously. She uses yoga poses that focus less on movement and more on holding positions for longer periods of time.
“I’ll do sequences of a mountain pose with a block to activate the core in the most basic ways and really focus on the breathing,” she said.
Another benefit of slower forms of yoga is that they can help participants become more aware of the structures of their pelvic floor, according to Alison Huang, MD, professor of medicine, urology and epidemiology, and biostatistics at University of California, San Francisco.
“In some ways we can think of it as a complementary substitute for rehabilitation therapy,” Dr. Huang said.
Dr. Huang and her colleagues published a short report recently in The Journal of Integrative and Complementary Medicine, showing that even telehealth-based yoga programs for older women with urinary incontinence can offer an accessible way for women of any background to take advantage of yoga’s benefits.
An estimated 93% of 66 participants who practiced yoga through planned telehealth appointments reported feeling “very or moderately satisfied” with their practice. Dr. Huang said that the study is not yet complete but offers a glimpse into some of the advantages of yoga for women with urinary incontinence.
“Any kind of treatment that relies on intensive one-on-one visits with specialists is going to be harder to access for some women,” Dr. Huang told this news organization. “Yoga is typically practiced in a community setting, outside of traditional health care settings.”
The accessibility of yoga and its community-based practice may help eliminate any obstacles to care and compliance that clinicians like she and Dr. Tunuguntla at times experience. Mounting studies have also indicated that yoga may help improve overall wellness, manage stress, promote healthier eating, and benefit a person’s mental and emotional health.
Despite emerging research on the link between yoga and pelvic floor disorders, Dr. Huang said that it’s still early for clinicians to recommend the exercise form for every patient.
“We just don’t have the [solid] evidence to show your pelvic floor will improve,” she said.
“For any woman who is starting out more sedentary, I think there are benefits to practic[ing] yoga for overall health,” Dr. Huang said. “Most clinicians would say there are opportunities to practice yoga regularly in a way that is safe, with a knowledgeable instructor.”
According to Ms. Conley, yoga is only as beneficial as a person’s level of consistency in the practice.
“The dedication to yoga is your willingness to showing up,” she said. “I think depending on your commitment to the practice, if you’re really committed to the practice – just like you show up to physical therapy every day – you will improve,” said Ms. Conley.
“Being gentle and patient with the process is important too,” she said.
Dr. Tunuguntla and coauthors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
After New York–based yoga instructor Erin Conley’s two sisters gave birth, Ms. Conley suggested a few advanced poses to help strengthen their pelvic floor.
“With one of my sisters, she said, ‘Honestly right now, I just can’t even stand up,’ ” she recalled.
Ms. Conley’s other sister could do slightly more advanced poses – leading Ms. Conley to recognize that after delivery, women’s ability to practice yoga varied widely.
“Post-birth is certainly a progression for each woman,” she said. “You can’t just go into these advance postures.”
Ms. Conley tailored a slow sequence of 30-second poses that each sister could start with, and they eventually reported an improvement of pelvic floor issues. Ms. Conley’s suggestions to her sisters are backed by a small but growing body of research. One study published in August in the journal Urology suggests that yoga may be a way to help treat multiple types of pelvic floor disorders.
More than 1 in 4 women in the United States experience pelvic floor disorders such as bowel or urinary incontinence or pelvic organ prolapse, many as a result of giving birth. But less than 15% of these women seek medical treatment for their symptoms, according to Hari Tunuguntla, MD, associate professor of urologic surgery at Rutgers University’s Robert Wood Johnson Medical School, New Brunswick.
For those who do seek medical help, many patients have trouble complying with initial lifestyle-based recommendations, such as refraining from drinking caffeinated and carbonated beverages, Dr. Tunuguntla said.
“It requires a lot of persistence and knowledge and compliance,” he said.
Medication and physical therapy are routes doctors can order before considering surgery, but some patients find clinical-based interventions to be costly. The cost of the interventions can add up depending on what a person’s insurance policy covers, Dr. Tunuguntla said.
With those struggles in mind, he and his colleagues set out to study the efficacy of the mobile app Yoga of Immortals, which offers a holistic form of yoga that includes postures, breathing exercises, sound therapy, and meditation.
“It includes sound therapy, summative breathing exercise,” Dr. Tunuguntla said. “These are useful not just for the condition but for general well-being.”
For the study, Dr. Tunuguntla and his colleagues emailed surveys to 420 people between ages 18 and 74 years in 23 countries who reported having any type of urinary incontinence, regardless of severity. The participants, most of whom were women, used the yoga app for 30 minutes a day for 8 weeks.
More than three-quarters of participants reported that the frequency and severity of their incontinence improved after 8 weeks of practice, compared with when they started, without having to visit their health care provider. Most participants also said that they felt “very much better” after 8 weeks, compared with when they began the yoga regimen, the researchers found.
The study did not compare the effectiveness of the approach with other standard treatments for incontinence, like physical therapy, medication, or surgery.
Ms. Conley, an instructor since 2010, said that one of the benefits of yoga is building strength and flexibility slowly and simultaneously. She uses yoga poses that focus less on movement and more on holding positions for longer periods of time.
“I’ll do sequences of a mountain pose with a block to activate the core in the most basic ways and really focus on the breathing,” she said.
Another benefit of slower forms of yoga is that they can help participants become more aware of the structures of their pelvic floor, according to Alison Huang, MD, professor of medicine, urology and epidemiology, and biostatistics at University of California, San Francisco.
“In some ways we can think of it as a complementary substitute for rehabilitation therapy,” Dr. Huang said.
Dr. Huang and her colleagues published a short report recently in The Journal of Integrative and Complementary Medicine, showing that even telehealth-based yoga programs for older women with urinary incontinence can offer an accessible way for women of any background to take advantage of yoga’s benefits.
An estimated 93% of 66 participants who practiced yoga through planned telehealth appointments reported feeling “very or moderately satisfied” with their practice. Dr. Huang said that the study is not yet complete but offers a glimpse into some of the advantages of yoga for women with urinary incontinence.
“Any kind of treatment that relies on intensive one-on-one visits with specialists is going to be harder to access for some women,” Dr. Huang told this news organization. “Yoga is typically practiced in a community setting, outside of traditional health care settings.”
The accessibility of yoga and its community-based practice may help eliminate any obstacles to care and compliance that clinicians like she and Dr. Tunuguntla at times experience. Mounting studies have also indicated that yoga may help improve overall wellness, manage stress, promote healthier eating, and benefit a person’s mental and emotional health.
Despite emerging research on the link between yoga and pelvic floor disorders, Dr. Huang said that it’s still early for clinicians to recommend the exercise form for every patient.
“We just don’t have the [solid] evidence to show your pelvic floor will improve,” she said.
“For any woman who is starting out more sedentary, I think there are benefits to practic[ing] yoga for overall health,” Dr. Huang said. “Most clinicians would say there are opportunities to practice yoga regularly in a way that is safe, with a knowledgeable instructor.”
According to Ms. Conley, yoga is only as beneficial as a person’s level of consistency in the practice.
“The dedication to yoga is your willingness to showing up,” she said. “I think depending on your commitment to the practice, if you’re really committed to the practice – just like you show up to physical therapy every day – you will improve,” said Ms. Conley.
“Being gentle and patient with the process is important too,” she said.
Dr. Tunuguntla and coauthors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Are single-incision mini-slings the new gold standard for stress urinary incontinence?
Abdel-Fattah M, Cooper D, Davidson T, et al. Single-incision mini-slings for stress urinary incontinence in women. N Engl J Med. 2022;386:1230-1243.
EXPERT COMMENTARY
A joint society position statement by the American Urogynecologic Society and the Society of Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction published in December 2021 declared synthetic midurethral slings, first cleared for use in the United States in the early 1990s, the most extensively studied anti-incontinence operation and the standard of care for the treatment of female stress urinary incontinence.1 Full-length retropubic and transobturator (out-in and in-out) slings have been extensively evaluated for safety and efficacy in well-conducted randomized trials.2 Single-incision mini-slings (SIMS) were first cleared for use in 2006, but they lack the long-term safety and comparative effectiveness data of full-length standard midurethral slings (SMUS).3 Furthermore, several iterations of the mini-slings have come to market but have been withdrawn or modified to allow for adjustability.
The SIMS trial by Abdel-Fattah and colleagues, published recently in the New England Journal of Medicine, is one of the few randomized trials with long-term (3 year) subjective and objective outcome data based on comparison of adjustable single-incision mini-slings versus standard full-length midurethral slings.
Details of the study
The SIMS trial is a noninferiority multicenter randomized controlled trial funded by the National Institute for Health Research at 21 hospitals in the United Kingdom that compared adjustable mini-sling procedures performed under local anesthesia with full-length retrotropubic and transobturator sling procedures performed under general anesthesia. Patients and surgeons were not masked to study group assignment because of the differences in anesthesia, and patients with greater than stage 2 prolapse were excluded from the trial.
The primary outcome was Patient Global Impression of Improvement (PGI-I) based on a 7-point Likert scale, with success defined as very much improved or much improved at 15 months and failure defined as all other responses (improved, same, worse, much worse, and very much worse). A noninferiority margin was set at 10 percentage points at 15 months.
Secondary outcomes and adverse events at 36 months included postoperative pain, return to normal activities, objective success based on a 24-hour pad test weight of less than 8 g, and tape exposure, organ injury, new or worsening urinary urgency, dyspareunia, and need for prolonged catheterization.
A total of 596 women were enrolled in the study, 298 in the mini-sling arm and 298 in the standard midurethral sling arm. Baseline characteristics were similar in both groups with most sling procedures being performed by general consultant gynecologists (>60%) versus subspecialist urogynecologists.
Results. Success at 15 months, based on the PGI-I responses of very much better or much better, was noted in 79.1% of patients in the mini-sling group (212/268) versus 75.6% in the full-length sling group (189/250). The authors deemed mini-slings noninferior to standard full-length slings (adjusted risk difference, 4.6 percentage points; 95% confidence interval [CI], -2.7 to 11.8; P<.001 for noninferiority). Success rates declined but remained similar in both groups at 36 months: 72% in the mini-sling group (177/246) and 66.8% (157/235) in the full-length sling group.
More than 70% of mini-incision slings were Altis (Coloplast) and 22% were Ajust (CR Bard; since withdrawn from the market). The majority of standard midurethral full-length slings were transobturator slings (52.9%) versus retropubic slings (35.6%).
While blood loss, organ injury, and 36-month objective 24-hour pad test did not differ between groups, there were significant differences in other secondary outcomes. Dyspareunia and coital incontinence were more common with mini-slings at 15 and 36 months, reported in 11.7% of the mini-sling group and 4.8% of the full-length group (P<.01). Groin or thigh pain did not differ significantly between groups at 36 months (14.1% in mini-sling and 14.9% in full-length sling group, P = .61). Mesh exposure was noted in 3.3% of those with mini-slings and 1.9% of those with standard midurethral slings. The need for surgical intervention to treat recurrent stress incontinence or mesh removal for voiding dysfunction, pain, or mesh exposure also did not differ between groups (8.7% of the mini-sling group and 4.6% of the midurethral sling group; P = .12).
Study strengths and limitations
The strengths of this pragmatic randomized trial are in the use of clinically important and validated patient-reported subjective and objective outcomes in an adequately powered multisite trial of long duration (36 months). This study is important in demonstrating noninferiority of the mini-sling procedure compared with full-length slings, especially given this trial’s timing when there was a pause or suspension of sling mesh use in the United Kingdom beginning in 2018.
Study limitations include the loss to follow-up with diminished response rate of 87.1% at 15 months and 81.4% at 36 months and the inability to adequately assess for the uncommon outcomes, such as mesh-related complications and groin pain.
Further analysis needed
The high rate of dyspareunia (11.7%) with mini-slings deserves further analysis and consideration of whether or not to implant them in patients who are sexually active. Groin or thigh pain did not differ at 36 months but reported pain coincided with the higher percentage of transobturator slings placed over retropubic slings. Prior randomized trials of transobturator versus retropubic midurethral slings have demonstrated this same phenomenon of increased groin pain with the transobturator approach.2 Furthermore, this study by Abdel-Fattah and colleagues excluded patients with advanced anterior or apical prolapse, but one trial is currently underway in the United States.4
In conclusion, this trial suggests some advantages of single-incision mini-slings—ability to perform the procedure under local anesthesia, less synthetic mesh implantation with theoretically decreased risk of bladder perforation or bowel injury, and potential for easier removal compared with full-length slings. Disadvantages include dyspareunia and mesh exposure, which could be significant trade-offs for patients. ●
In the IDEAL framework for evaluating new surgical innovations, the recommended process begins with an idea, followed by development by a few surgeons in a few patients, then exploration in a feasibility randomized controlled trial, an assessment in larger trials by many surgeons, and long-term follow-up.5 The SIMS trial falls under the assessment tab of the IDEAL framework and represents a much-needed study prior to widespread adoption of single-incision mini-slings. The higher dyspareunia rate in women undergoing single-incision mini-slings deserves further evaluation.
CHERYL B. IGLESIA, MD
- Joint position statement on midurethral slings for stress urinary incontinence. Female Pelvic Med Reconstr Surg. 2021;27:707-710. doi: 10.1097/SPV.0000000000001096.
- Richter HE, Albo ME, Zyczynski HM, et al; Urinary Incontinence Treatment Network. Retropubic versus transobturator midurethral slings for stress incontinence. N Engl J Med. 2010;362:2066-2076.
- Nambiar A, Cody JD, Jeffery ST. Single-incision sling operations for urinary incontinence in women. Cochrane Database Syst Rev. 2014;6:CD008709.
- National Institutes of Health. Retropubic vs single-incision mid-urethral sling for stress urinary incontinence. ClinicalTrials.gov identifier NCT03520114. Accessed July16, 2022. https://www.clinicaltrials.gov/ct2/show/NCT0352011 4?cond=altis+sling&draw=2&rank=6
- McCulloch P, Altman DG, Campbell WB, et al. No surgical innovation without evaluation: the IDEAL recommendations. Lancet. 2009;374:1105-1111.
Abdel-Fattah M, Cooper D, Davidson T, et al. Single-incision mini-slings for stress urinary incontinence in women. N Engl J Med. 2022;386:1230-1243.
EXPERT COMMENTARY
A joint society position statement by the American Urogynecologic Society and the Society of Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction published in December 2021 declared synthetic midurethral slings, first cleared for use in the United States in the early 1990s, the most extensively studied anti-incontinence operation and the standard of care for the treatment of female stress urinary incontinence.1 Full-length retropubic and transobturator (out-in and in-out) slings have been extensively evaluated for safety and efficacy in well-conducted randomized trials.2 Single-incision mini-slings (SIMS) were first cleared for use in 2006, but they lack the long-term safety and comparative effectiveness data of full-length standard midurethral slings (SMUS).3 Furthermore, several iterations of the mini-slings have come to market but have been withdrawn or modified to allow for adjustability.
The SIMS trial by Abdel-Fattah and colleagues, published recently in the New England Journal of Medicine, is one of the few randomized trials with long-term (3 year) subjective and objective outcome data based on comparison of adjustable single-incision mini-slings versus standard full-length midurethral slings.
Details of the study
The SIMS trial is a noninferiority multicenter randomized controlled trial funded by the National Institute for Health Research at 21 hospitals in the United Kingdom that compared adjustable mini-sling procedures performed under local anesthesia with full-length retrotropubic and transobturator sling procedures performed under general anesthesia. Patients and surgeons were not masked to study group assignment because of the differences in anesthesia, and patients with greater than stage 2 prolapse were excluded from the trial.
The primary outcome was Patient Global Impression of Improvement (PGI-I) based on a 7-point Likert scale, with success defined as very much improved or much improved at 15 months and failure defined as all other responses (improved, same, worse, much worse, and very much worse). A noninferiority margin was set at 10 percentage points at 15 months.
Secondary outcomes and adverse events at 36 months included postoperative pain, return to normal activities, objective success based on a 24-hour pad test weight of less than 8 g, and tape exposure, organ injury, new or worsening urinary urgency, dyspareunia, and need for prolonged catheterization.
A total of 596 women were enrolled in the study, 298 in the mini-sling arm and 298 in the standard midurethral sling arm. Baseline characteristics were similar in both groups with most sling procedures being performed by general consultant gynecologists (>60%) versus subspecialist urogynecologists.
Results. Success at 15 months, based on the PGI-I responses of very much better or much better, was noted in 79.1% of patients in the mini-sling group (212/268) versus 75.6% in the full-length sling group (189/250). The authors deemed mini-slings noninferior to standard full-length slings (adjusted risk difference, 4.6 percentage points; 95% confidence interval [CI], -2.7 to 11.8; P<.001 for noninferiority). Success rates declined but remained similar in both groups at 36 months: 72% in the mini-sling group (177/246) and 66.8% (157/235) in the full-length sling group.
More than 70% of mini-incision slings were Altis (Coloplast) and 22% were Ajust (CR Bard; since withdrawn from the market). The majority of standard midurethral full-length slings were transobturator slings (52.9%) versus retropubic slings (35.6%).
While blood loss, organ injury, and 36-month objective 24-hour pad test did not differ between groups, there were significant differences in other secondary outcomes. Dyspareunia and coital incontinence were more common with mini-slings at 15 and 36 months, reported in 11.7% of the mini-sling group and 4.8% of the full-length group (P<.01). Groin or thigh pain did not differ significantly between groups at 36 months (14.1% in mini-sling and 14.9% in full-length sling group, P = .61). Mesh exposure was noted in 3.3% of those with mini-slings and 1.9% of those with standard midurethral slings. The need for surgical intervention to treat recurrent stress incontinence or mesh removal for voiding dysfunction, pain, or mesh exposure also did not differ between groups (8.7% of the mini-sling group and 4.6% of the midurethral sling group; P = .12).
Study strengths and limitations
The strengths of this pragmatic randomized trial are in the use of clinically important and validated patient-reported subjective and objective outcomes in an adequately powered multisite trial of long duration (36 months). This study is important in demonstrating noninferiority of the mini-sling procedure compared with full-length slings, especially given this trial’s timing when there was a pause or suspension of sling mesh use in the United Kingdom beginning in 2018.
Study limitations include the loss to follow-up with diminished response rate of 87.1% at 15 months and 81.4% at 36 months and the inability to adequately assess for the uncommon outcomes, such as mesh-related complications and groin pain.
Further analysis needed
The high rate of dyspareunia (11.7%) with mini-slings deserves further analysis and consideration of whether or not to implant them in patients who are sexually active. Groin or thigh pain did not differ at 36 months but reported pain coincided with the higher percentage of transobturator slings placed over retropubic slings. Prior randomized trials of transobturator versus retropubic midurethral slings have demonstrated this same phenomenon of increased groin pain with the transobturator approach.2 Furthermore, this study by Abdel-Fattah and colleagues excluded patients with advanced anterior or apical prolapse, but one trial is currently underway in the United States.4
In conclusion, this trial suggests some advantages of single-incision mini-slings—ability to perform the procedure under local anesthesia, less synthetic mesh implantation with theoretically decreased risk of bladder perforation or bowel injury, and potential for easier removal compared with full-length slings. Disadvantages include dyspareunia and mesh exposure, which could be significant trade-offs for patients. ●
In the IDEAL framework for evaluating new surgical innovations, the recommended process begins with an idea, followed by development by a few surgeons in a few patients, then exploration in a feasibility randomized controlled trial, an assessment in larger trials by many surgeons, and long-term follow-up.5 The SIMS trial falls under the assessment tab of the IDEAL framework and represents a much-needed study prior to widespread adoption of single-incision mini-slings. The higher dyspareunia rate in women undergoing single-incision mini-slings deserves further evaluation.
CHERYL B. IGLESIA, MD
Abdel-Fattah M, Cooper D, Davidson T, et al. Single-incision mini-slings for stress urinary incontinence in women. N Engl J Med. 2022;386:1230-1243.
EXPERT COMMENTARY
A joint society position statement by the American Urogynecologic Society and the Society of Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction published in December 2021 declared synthetic midurethral slings, first cleared for use in the United States in the early 1990s, the most extensively studied anti-incontinence operation and the standard of care for the treatment of female stress urinary incontinence.1 Full-length retropubic and transobturator (out-in and in-out) slings have been extensively evaluated for safety and efficacy in well-conducted randomized trials.2 Single-incision mini-slings (SIMS) were first cleared for use in 2006, but they lack the long-term safety and comparative effectiveness data of full-length standard midurethral slings (SMUS).3 Furthermore, several iterations of the mini-slings have come to market but have been withdrawn or modified to allow for adjustability.
The SIMS trial by Abdel-Fattah and colleagues, published recently in the New England Journal of Medicine, is one of the few randomized trials with long-term (3 year) subjective and objective outcome data based on comparison of adjustable single-incision mini-slings versus standard full-length midurethral slings.
Details of the study
The SIMS trial is a noninferiority multicenter randomized controlled trial funded by the National Institute for Health Research at 21 hospitals in the United Kingdom that compared adjustable mini-sling procedures performed under local anesthesia with full-length retrotropubic and transobturator sling procedures performed under general anesthesia. Patients and surgeons were not masked to study group assignment because of the differences in anesthesia, and patients with greater than stage 2 prolapse were excluded from the trial.
The primary outcome was Patient Global Impression of Improvement (PGI-I) based on a 7-point Likert scale, with success defined as very much improved or much improved at 15 months and failure defined as all other responses (improved, same, worse, much worse, and very much worse). A noninferiority margin was set at 10 percentage points at 15 months.
Secondary outcomes and adverse events at 36 months included postoperative pain, return to normal activities, objective success based on a 24-hour pad test weight of less than 8 g, and tape exposure, organ injury, new or worsening urinary urgency, dyspareunia, and need for prolonged catheterization.
A total of 596 women were enrolled in the study, 298 in the mini-sling arm and 298 in the standard midurethral sling arm. Baseline characteristics were similar in both groups with most sling procedures being performed by general consultant gynecologists (>60%) versus subspecialist urogynecologists.
Results. Success at 15 months, based on the PGI-I responses of very much better or much better, was noted in 79.1% of patients in the mini-sling group (212/268) versus 75.6% in the full-length sling group (189/250). The authors deemed mini-slings noninferior to standard full-length slings (adjusted risk difference, 4.6 percentage points; 95% confidence interval [CI], -2.7 to 11.8; P<.001 for noninferiority). Success rates declined but remained similar in both groups at 36 months: 72% in the mini-sling group (177/246) and 66.8% (157/235) in the full-length sling group.
More than 70% of mini-incision slings were Altis (Coloplast) and 22% were Ajust (CR Bard; since withdrawn from the market). The majority of standard midurethral full-length slings were transobturator slings (52.9%) versus retropubic slings (35.6%).
While blood loss, organ injury, and 36-month objective 24-hour pad test did not differ between groups, there were significant differences in other secondary outcomes. Dyspareunia and coital incontinence were more common with mini-slings at 15 and 36 months, reported in 11.7% of the mini-sling group and 4.8% of the full-length group (P<.01). Groin or thigh pain did not differ significantly between groups at 36 months (14.1% in mini-sling and 14.9% in full-length sling group, P = .61). Mesh exposure was noted in 3.3% of those with mini-slings and 1.9% of those with standard midurethral slings. The need for surgical intervention to treat recurrent stress incontinence or mesh removal for voiding dysfunction, pain, or mesh exposure also did not differ between groups (8.7% of the mini-sling group and 4.6% of the midurethral sling group; P = .12).
Study strengths and limitations
The strengths of this pragmatic randomized trial are in the use of clinically important and validated patient-reported subjective and objective outcomes in an adequately powered multisite trial of long duration (36 months). This study is important in demonstrating noninferiority of the mini-sling procedure compared with full-length slings, especially given this trial’s timing when there was a pause or suspension of sling mesh use in the United Kingdom beginning in 2018.
Study limitations include the loss to follow-up with diminished response rate of 87.1% at 15 months and 81.4% at 36 months and the inability to adequately assess for the uncommon outcomes, such as mesh-related complications and groin pain.
Further analysis needed
The high rate of dyspareunia (11.7%) with mini-slings deserves further analysis and consideration of whether or not to implant them in patients who are sexually active. Groin or thigh pain did not differ at 36 months but reported pain coincided with the higher percentage of transobturator slings placed over retropubic slings. Prior randomized trials of transobturator versus retropubic midurethral slings have demonstrated this same phenomenon of increased groin pain with the transobturator approach.2 Furthermore, this study by Abdel-Fattah and colleagues excluded patients with advanced anterior or apical prolapse, but one trial is currently underway in the United States.4
In conclusion, this trial suggests some advantages of single-incision mini-slings—ability to perform the procedure under local anesthesia, less synthetic mesh implantation with theoretically decreased risk of bladder perforation or bowel injury, and potential for easier removal compared with full-length slings. Disadvantages include dyspareunia and mesh exposure, which could be significant trade-offs for patients. ●
In the IDEAL framework for evaluating new surgical innovations, the recommended process begins with an idea, followed by development by a few surgeons in a few patients, then exploration in a feasibility randomized controlled trial, an assessment in larger trials by many surgeons, and long-term follow-up.5 The SIMS trial falls under the assessment tab of the IDEAL framework and represents a much-needed study prior to widespread adoption of single-incision mini-slings. The higher dyspareunia rate in women undergoing single-incision mini-slings deserves further evaluation.
CHERYL B. IGLESIA, MD
- Joint position statement on midurethral slings for stress urinary incontinence. Female Pelvic Med Reconstr Surg. 2021;27:707-710. doi: 10.1097/SPV.0000000000001096.
- Richter HE, Albo ME, Zyczynski HM, et al; Urinary Incontinence Treatment Network. Retropubic versus transobturator midurethral slings for stress incontinence. N Engl J Med. 2010;362:2066-2076.
- Nambiar A, Cody JD, Jeffery ST. Single-incision sling operations for urinary incontinence in women. Cochrane Database Syst Rev. 2014;6:CD008709.
- National Institutes of Health. Retropubic vs single-incision mid-urethral sling for stress urinary incontinence. ClinicalTrials.gov identifier NCT03520114. Accessed July16, 2022. https://www.clinicaltrials.gov/ct2/show/NCT0352011 4?cond=altis+sling&draw=2&rank=6
- McCulloch P, Altman DG, Campbell WB, et al. No surgical innovation without evaluation: the IDEAL recommendations. Lancet. 2009;374:1105-1111.
- Joint position statement on midurethral slings for stress urinary incontinence. Female Pelvic Med Reconstr Surg. 2021;27:707-710. doi: 10.1097/SPV.0000000000001096.
- Richter HE, Albo ME, Zyczynski HM, et al; Urinary Incontinence Treatment Network. Retropubic versus transobturator midurethral slings for stress incontinence. N Engl J Med. 2010;362:2066-2076.
- Nambiar A, Cody JD, Jeffery ST. Single-incision sling operations for urinary incontinence in women. Cochrane Database Syst Rev. 2014;6:CD008709.
- National Institutes of Health. Retropubic vs single-incision mid-urethral sling for stress urinary incontinence. ClinicalTrials.gov identifier NCT03520114. Accessed July16, 2022. https://www.clinicaltrials.gov/ct2/show/NCT0352011 4?cond=altis+sling&draw=2&rank=6
- McCulloch P, Altman DG, Campbell WB, et al. No surgical innovation without evaluation: the IDEAL recommendations. Lancet. 2009;374:1105-1111.